User login
VIDEO: Inflammation’s role in atherosclerosis confirmed in CANTOS
BARCELONA – The results of the Canakinumab Anti-Inflammatory Thrombosis Outcomes Study (CANTOS) mark the validation of many years of research on inflammation for Peter Libby, MD, Mallinckrodt Professor of Medicine, Harvard Medical School, Boston.
The CANTOS investigator said that, although some trials, most notably JUPITER, have linked reduced markers of inflammation with reduced cardiovascular events, none have been able to separate the effects of lowering LDL cholesterol from those of lowering the inflammatory marker interleukin-1B.
But using the monoclonal antibody canakinumab to target only interleukin-1B in CANTOS reduced the composite endpoint of nonfatal MI, nonfatal stroke, or cardiovascular death by 15% at the highest dosage tested, compared with placebo, while lowering high-sensitivity C-reactive protein by 39 percentage points.
Dr. Libby has been studying interleukin-1B since the 1980s. “Now, today, for the first time, in a rigorous trial, we can show that an anti-inflammatory agent that is neutral for lipids (that doesn’t lower LDL) can provide a benefit for our patients, and that’s a real step forward,” Dr. Libby said in a video interview at the annual congress of the European Society of Cardiology.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Importantly, a dividend of the investigation was that “we found a decrease in fatal cancers, particularly lung cancer. So this again opens the door toward a whole new therapeutic window in patients not just in the cardiovascular space, but also in oncology. So it’s a doubly exciting day for us.”
CANTOS was presented at the meeting by Paul Ridker, MD, also of Harvard Medical School; the results were also published online (N Engl J Med. 2017 Aug 27. doi: 10.1056/NEJMoa1707914).
BARCELONA – The results of the Canakinumab Anti-Inflammatory Thrombosis Outcomes Study (CANTOS) mark the validation of many years of research on inflammation for Peter Libby, MD, Mallinckrodt Professor of Medicine, Harvard Medical School, Boston.
The CANTOS investigator said that, although some trials, most notably JUPITER, have linked reduced markers of inflammation with reduced cardiovascular events, none have been able to separate the effects of lowering LDL cholesterol from those of lowering the inflammatory marker interleukin-1B.
But using the monoclonal antibody canakinumab to target only interleukin-1B in CANTOS reduced the composite endpoint of nonfatal MI, nonfatal stroke, or cardiovascular death by 15% at the highest dosage tested, compared with placebo, while lowering high-sensitivity C-reactive protein by 39 percentage points.
Dr. Libby has been studying interleukin-1B since the 1980s. “Now, today, for the first time, in a rigorous trial, we can show that an anti-inflammatory agent that is neutral for lipids (that doesn’t lower LDL) can provide a benefit for our patients, and that’s a real step forward,” Dr. Libby said in a video interview at the annual congress of the European Society of Cardiology.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Importantly, a dividend of the investigation was that “we found a decrease in fatal cancers, particularly lung cancer. So this again opens the door toward a whole new therapeutic window in patients not just in the cardiovascular space, but also in oncology. So it’s a doubly exciting day for us.”
CANTOS was presented at the meeting by Paul Ridker, MD, also of Harvard Medical School; the results were also published online (N Engl J Med. 2017 Aug 27. doi: 10.1056/NEJMoa1707914).
BARCELONA – The results of the Canakinumab Anti-Inflammatory Thrombosis Outcomes Study (CANTOS) mark the validation of many years of research on inflammation for Peter Libby, MD, Mallinckrodt Professor of Medicine, Harvard Medical School, Boston.
The CANTOS investigator said that, although some trials, most notably JUPITER, have linked reduced markers of inflammation with reduced cardiovascular events, none have been able to separate the effects of lowering LDL cholesterol from those of lowering the inflammatory marker interleukin-1B.
But using the monoclonal antibody canakinumab to target only interleukin-1B in CANTOS reduced the composite endpoint of nonfatal MI, nonfatal stroke, or cardiovascular death by 15% at the highest dosage tested, compared with placebo, while lowering high-sensitivity C-reactive protein by 39 percentage points.
Dr. Libby has been studying interleukin-1B since the 1980s. “Now, today, for the first time, in a rigorous trial, we can show that an anti-inflammatory agent that is neutral for lipids (that doesn’t lower LDL) can provide a benefit for our patients, and that’s a real step forward,” Dr. Libby said in a video interview at the annual congress of the European Society of Cardiology.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Importantly, a dividend of the investigation was that “we found a decrease in fatal cancers, particularly lung cancer. So this again opens the door toward a whole new therapeutic window in patients not just in the cardiovascular space, but also in oncology. So it’s a doubly exciting day for us.”
CANTOS was presented at the meeting by Paul Ridker, MD, also of Harvard Medical School; the results were also published online (N Engl J Med. 2017 Aug 27. doi: 10.1056/NEJMoa1707914).
AT THE ESC CONGRESS 2017
Make the Diagnosis - August 2017
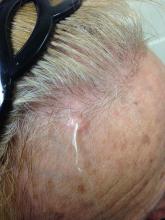
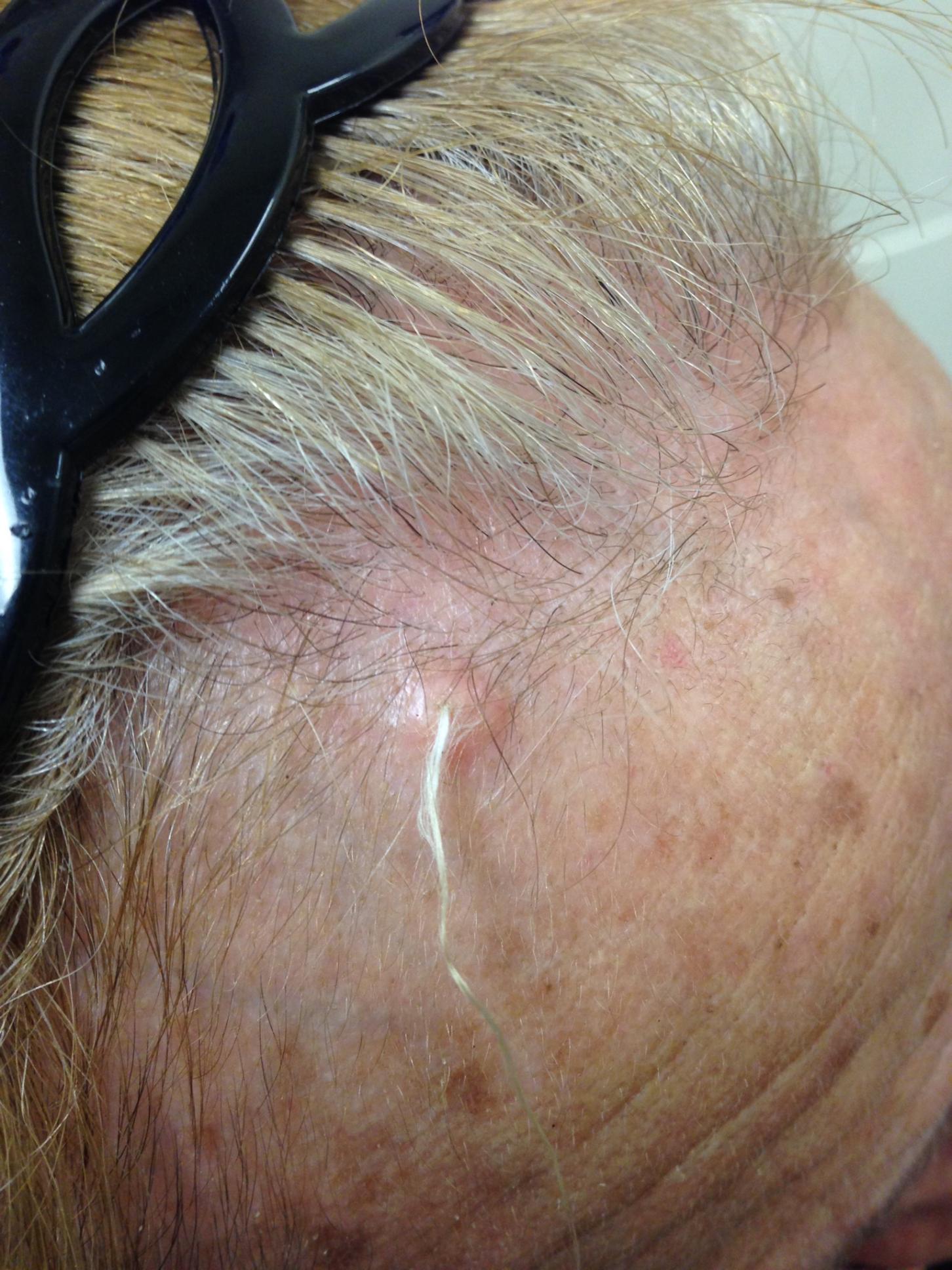
Trichofolliculoma
Trichofolliculoma is a rare, benign skin lesion that was first described in 1944. While the etiology is largely known, it is considered to be a hamartoma with follicular differentiation that results when the pluripotent skin cells cease to differentiate into the hair follicle. The lesion lacks specific predilection for gender or race but most commonly occurs on the face of adults. Clinically, trichofolliculoma presents as a solitary, skin-colored papule or nodule. It may contain a central sebum-producing pore or have a tuft of white hair protruding from the central pore, although neither of these manifestations are mandatory. Trichofolliculomas generally are asymptomatic and are not associated with any systemic or other cutaneous diseases. Therefore, no treatment is required. However, surgical excision or curettage and electrodesiccation may be performed for cosmetic reasons.
Definitive diagnosis of trichofolliculoma requires a biopsy. The histopathology shows a primary cystic structure within the dermis that may or may not be connected to the epidermis. There are multiple abnormal secondary follicles that radiate from the primary cystic structure, and the entire apparatus is surrounded by a well-circumscribed dense connective tissue. Immunohistochemical studies have revealed that trichofolliculoma is characterized by activated, aberrant cytokeratin-15 (CK15)–positive follicle stem cells that show outer root sheath differentiation while attempting to make hair. This results in disorder of the normal hair cycle.
The clinical differential diagnosis for trichofolliculoma includes dermal nevus, basal cell carcinoma, and pilar sheath acanthoma. These cutaneous entities may be ruled out by histopathology.
The histopathology of basal cell carcinoma consists of tumors of basaloid cells with little cytoplasm and hyperchromatic nuclei. There is palisading around the periphery of the tumor with stromal retraction that leads to a clefting appearance, as well as mucinous change in the stroma.
The histopathology of dermal nevus reveals small nests of melanocytes within the upper dermis that commonly localize around the pilosebaceous units. There is variability in pigmentation and cellularity of the melanocytes, and there is no junctional component.
The histopathology of pilar sheath acanthoma shows dermal proliferation of lobules comprising benign squamous epithelium that arrange themselves around small cystic spaces. The lobules themselves are surrounded by eosinophilic basement membrane.
The case and photo were submitted by Victoria Billero, MS; Adam Wulkan, MD; and Caroline Winslow, MD, all of the department of dermatology, University of Miami (Fla.).
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit a case for possible publication, send an email to dermnews@frontlinemedcom.com.
Trichofolliculoma
Trichofolliculoma is a rare, benign skin lesion that was first described in 1944. While the etiology is largely known, it is considered to be a hamartoma with follicular differentiation that results when the pluripotent skin cells cease to differentiate into the hair follicle. The lesion lacks specific predilection for gender or race but most commonly occurs on the face of adults. Clinically, trichofolliculoma presents as a solitary, skin-colored papule or nodule. It may contain a central sebum-producing pore or have a tuft of white hair protruding from the central pore, although neither of these manifestations are mandatory. Trichofolliculomas generally are asymptomatic and are not associated with any systemic or other cutaneous diseases. Therefore, no treatment is required. However, surgical excision or curettage and electrodesiccation may be performed for cosmetic reasons.
Definitive diagnosis of trichofolliculoma requires a biopsy. The histopathology shows a primary cystic structure within the dermis that may or may not be connected to the epidermis. There are multiple abnormal secondary follicles that radiate from the primary cystic structure, and the entire apparatus is surrounded by a well-circumscribed dense connective tissue. Immunohistochemical studies have revealed that trichofolliculoma is characterized by activated, aberrant cytokeratin-15 (CK15)–positive follicle stem cells that show outer root sheath differentiation while attempting to make hair. This results in disorder of the normal hair cycle.
The clinical differential diagnosis for trichofolliculoma includes dermal nevus, basal cell carcinoma, and pilar sheath acanthoma. These cutaneous entities may be ruled out by histopathology.
The histopathology of basal cell carcinoma consists of tumors of basaloid cells with little cytoplasm and hyperchromatic nuclei. There is palisading around the periphery of the tumor with stromal retraction that leads to a clefting appearance, as well as mucinous change in the stroma.
The histopathology of dermal nevus reveals small nests of melanocytes within the upper dermis that commonly localize around the pilosebaceous units. There is variability in pigmentation and cellularity of the melanocytes, and there is no junctional component.
The histopathology of pilar sheath acanthoma shows dermal proliferation of lobules comprising benign squamous epithelium that arrange themselves around small cystic spaces. The lobules themselves are surrounded by eosinophilic basement membrane.
The case and photo were submitted by Victoria Billero, MS; Adam Wulkan, MD; and Caroline Winslow, MD, all of the department of dermatology, University of Miami (Fla.).
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit a case for possible publication, send an email to dermnews@frontlinemedcom.com.
Trichofolliculoma
Trichofolliculoma is a rare, benign skin lesion that was first described in 1944. While the etiology is largely known, it is considered to be a hamartoma with follicular differentiation that results when the pluripotent skin cells cease to differentiate into the hair follicle. The lesion lacks specific predilection for gender or race but most commonly occurs on the face of adults. Clinically, trichofolliculoma presents as a solitary, skin-colored papule or nodule. It may contain a central sebum-producing pore or have a tuft of white hair protruding from the central pore, although neither of these manifestations are mandatory. Trichofolliculomas generally are asymptomatic and are not associated with any systemic or other cutaneous diseases. Therefore, no treatment is required. However, surgical excision or curettage and electrodesiccation may be performed for cosmetic reasons.
Definitive diagnosis of trichofolliculoma requires a biopsy. The histopathology shows a primary cystic structure within the dermis that may or may not be connected to the epidermis. There are multiple abnormal secondary follicles that radiate from the primary cystic structure, and the entire apparatus is surrounded by a well-circumscribed dense connective tissue. Immunohistochemical studies have revealed that trichofolliculoma is characterized by activated, aberrant cytokeratin-15 (CK15)–positive follicle stem cells that show outer root sheath differentiation while attempting to make hair. This results in disorder of the normal hair cycle.
The clinical differential diagnosis for trichofolliculoma includes dermal nevus, basal cell carcinoma, and pilar sheath acanthoma. These cutaneous entities may be ruled out by histopathology.
The histopathology of basal cell carcinoma consists of tumors of basaloid cells with little cytoplasm and hyperchromatic nuclei. There is palisading around the periphery of the tumor with stromal retraction that leads to a clefting appearance, as well as mucinous change in the stroma.
The histopathology of dermal nevus reveals small nests of melanocytes within the upper dermis that commonly localize around the pilosebaceous units. There is variability in pigmentation and cellularity of the melanocytes, and there is no junctional component.
The histopathology of pilar sheath acanthoma shows dermal proliferation of lobules comprising benign squamous epithelium that arrange themselves around small cystic spaces. The lobules themselves are surrounded by eosinophilic basement membrane.
The case and photo were submitted by Victoria Billero, MS; Adam Wulkan, MD; and Caroline Winslow, MD, all of the department of dermatology, University of Miami (Fla.).
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit a case for possible publication, send an email to dermnews@frontlinemedcom.com.
A 67-year-old white female with no significant past medical history presented as a first-time patient to clinic for a full skin check. An incidental finding of a solitary, asymptomatic, non-tender 8 mm flesh-colored papule with a tuft of long white hair protruding through a central pore was observed on her right lateral forehead that had been present for longer than 10 years.
Orsiro coronary DES outperforms Xience
BARCELONA – A new model of drug-eluting coronary stent outperformed the reigning benchmark Xience stent in a head-to-head, pivotal comparison with 1,334 patients.
The results “advance a new standard for drug eluting stents,” David E. Kandzari, MD, said at the annual congress of the European Society of Cardiology. The trial tested the Orsiro stent, which features very thin, 60-micron-thick cobalt chromium struts, a bioresorbable polymer, and sirolimus as the antiproliferative drug that’s released during the first 90 days of stent placement.
“To our knowledge, this is the only trial that has demonstrated superiority [of a new stent] to the Xience drug-eluting stent in a large, randomized trial. This is a landmark in interventional cardiology that raises the bar for future comparisons” of drug eluting coronary stents, Dr. Kandzari said in an interview.
The study’s primary endpoint of target lesion failure after 12 months of follow-up – a rate that combined the incidence of cardiovascular death, target-vessel related MI, and ischemia-driven target-lesion revascularization – stood at 6.2% for the 884 patients treated with the Orsiro stent and 9.6% of the 450 randomized to treatment with the Xience stent, which has struts that are 81 microns wide, and a durable polymer that releases everolimus as the antiproliferative drug.
The difference in the primary endpoint was driven primarily by a 3.6% absolute difference in the rate of target-vessel related MIs, a statistically significant difference, plus the Orsiro-treated patients showed numerically smaller rates of cardiac death and ischemia-driven target lesion revascularization, although the between-group differences for each of these two endpoints were not statistically significant. The Orsiro stent also showed a significantly reduced rate of late stent thrombosis, occurring during day 31 through 1 year, a 0.1% rate in the Orsiro-treated patients and a 0.9% rate in those who received Xience stents.
“These are remarkable results,” said Michael Haude, MD, an interventional cardiologist at Lukas Hospital in Neuss, Germany, and a cochair of the session in which Dr. Kandzari gave his report.
These results from the Safety and Effectiveness of the Orsiro Sirolimus Eluting Coronary Stent System in Subjects With Coronary Artery Lesions (BIOFLOW-V) trial will be the centerpiece of an application for U.S. marketing approval for the Orsiro stent, Dr. Kandzari said. The BIOFLOW-V trial enrolled patients at 90 centers in 13 countries including the United States.
Concurrently with his report, the results were published online (Lancet. 2017 Aug 26. doi: 10.1016/S0140-6736[17]32249-3).
The potential advantage of a thinner-strut drug eluting stent showed up during the initial treatment phase, with a procedural success rate of 94% using the Orsiro stent and 90% with the Xience stent. This difference in procedural success seemed largely the result of an increased rate of periprocedural MIs, a difference that might be explained by the difference in strut thickness, said Dr. Kandzari, of Piedmont Heart Institute, Atlanta. Based on this success, designers of future drug-eluting stents may focus on thinner-strut models, he suggested.
An additional analysis that Dr. Kandzari reported combined results from two prior randomized comparisons of the Orsiro and Xience stents, creating a pooled analysis with 2,208 patients. This Bayesian analysis calculated a 100% probability of noninferiority of the Orsiro stent compared with the Xience stent, and a 97% probability of superiority. This 97% probability of superiority fell just short of the 97.5% threshold for establishing superiority that Dr. Kandzari and his associates had prespecified for this analysis.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
BARCELONA – A new model of drug-eluting coronary stent outperformed the reigning benchmark Xience stent in a head-to-head, pivotal comparison with 1,334 patients.
The results “advance a new standard for drug eluting stents,” David E. Kandzari, MD, said at the annual congress of the European Society of Cardiology. The trial tested the Orsiro stent, which features very thin, 60-micron-thick cobalt chromium struts, a bioresorbable polymer, and sirolimus as the antiproliferative drug that’s released during the first 90 days of stent placement.
“To our knowledge, this is the only trial that has demonstrated superiority [of a new stent] to the Xience drug-eluting stent in a large, randomized trial. This is a landmark in interventional cardiology that raises the bar for future comparisons” of drug eluting coronary stents, Dr. Kandzari said in an interview.
The study’s primary endpoint of target lesion failure after 12 months of follow-up – a rate that combined the incidence of cardiovascular death, target-vessel related MI, and ischemia-driven target-lesion revascularization – stood at 6.2% for the 884 patients treated with the Orsiro stent and 9.6% of the 450 randomized to treatment with the Xience stent, which has struts that are 81 microns wide, and a durable polymer that releases everolimus as the antiproliferative drug.
The difference in the primary endpoint was driven primarily by a 3.6% absolute difference in the rate of target-vessel related MIs, a statistically significant difference, plus the Orsiro-treated patients showed numerically smaller rates of cardiac death and ischemia-driven target lesion revascularization, although the between-group differences for each of these two endpoints were not statistically significant. The Orsiro stent also showed a significantly reduced rate of late stent thrombosis, occurring during day 31 through 1 year, a 0.1% rate in the Orsiro-treated patients and a 0.9% rate in those who received Xience stents.
“These are remarkable results,” said Michael Haude, MD, an interventional cardiologist at Lukas Hospital in Neuss, Germany, and a cochair of the session in which Dr. Kandzari gave his report.
These results from the Safety and Effectiveness of the Orsiro Sirolimus Eluting Coronary Stent System in Subjects With Coronary Artery Lesions (BIOFLOW-V) trial will be the centerpiece of an application for U.S. marketing approval for the Orsiro stent, Dr. Kandzari said. The BIOFLOW-V trial enrolled patients at 90 centers in 13 countries including the United States.
Concurrently with his report, the results were published online (Lancet. 2017 Aug 26. doi: 10.1016/S0140-6736[17]32249-3).
The potential advantage of a thinner-strut drug eluting stent showed up during the initial treatment phase, with a procedural success rate of 94% using the Orsiro stent and 90% with the Xience stent. This difference in procedural success seemed largely the result of an increased rate of periprocedural MIs, a difference that might be explained by the difference in strut thickness, said Dr. Kandzari, of Piedmont Heart Institute, Atlanta. Based on this success, designers of future drug-eluting stents may focus on thinner-strut models, he suggested.
An additional analysis that Dr. Kandzari reported combined results from two prior randomized comparisons of the Orsiro and Xience stents, creating a pooled analysis with 2,208 patients. This Bayesian analysis calculated a 100% probability of noninferiority of the Orsiro stent compared with the Xience stent, and a 97% probability of superiority. This 97% probability of superiority fell just short of the 97.5% threshold for establishing superiority that Dr. Kandzari and his associates had prespecified for this analysis.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
BARCELONA – A new model of drug-eluting coronary stent outperformed the reigning benchmark Xience stent in a head-to-head, pivotal comparison with 1,334 patients.
The results “advance a new standard for drug eluting stents,” David E. Kandzari, MD, said at the annual congress of the European Society of Cardiology. The trial tested the Orsiro stent, which features very thin, 60-micron-thick cobalt chromium struts, a bioresorbable polymer, and sirolimus as the antiproliferative drug that’s released during the first 90 days of stent placement.
“To our knowledge, this is the only trial that has demonstrated superiority [of a new stent] to the Xience drug-eluting stent in a large, randomized trial. This is a landmark in interventional cardiology that raises the bar for future comparisons” of drug eluting coronary stents, Dr. Kandzari said in an interview.
The study’s primary endpoint of target lesion failure after 12 months of follow-up – a rate that combined the incidence of cardiovascular death, target-vessel related MI, and ischemia-driven target-lesion revascularization – stood at 6.2% for the 884 patients treated with the Orsiro stent and 9.6% of the 450 randomized to treatment with the Xience stent, which has struts that are 81 microns wide, and a durable polymer that releases everolimus as the antiproliferative drug.
The difference in the primary endpoint was driven primarily by a 3.6% absolute difference in the rate of target-vessel related MIs, a statistically significant difference, plus the Orsiro-treated patients showed numerically smaller rates of cardiac death and ischemia-driven target lesion revascularization, although the between-group differences for each of these two endpoints were not statistically significant. The Orsiro stent also showed a significantly reduced rate of late stent thrombosis, occurring during day 31 through 1 year, a 0.1% rate in the Orsiro-treated patients and a 0.9% rate in those who received Xience stents.
“These are remarkable results,” said Michael Haude, MD, an interventional cardiologist at Lukas Hospital in Neuss, Germany, and a cochair of the session in which Dr. Kandzari gave his report.
These results from the Safety and Effectiveness of the Orsiro Sirolimus Eluting Coronary Stent System in Subjects With Coronary Artery Lesions (BIOFLOW-V) trial will be the centerpiece of an application for U.S. marketing approval for the Orsiro stent, Dr. Kandzari said. The BIOFLOW-V trial enrolled patients at 90 centers in 13 countries including the United States.
Concurrently with his report, the results were published online (Lancet. 2017 Aug 26. doi: 10.1016/S0140-6736[17]32249-3).
The potential advantage of a thinner-strut drug eluting stent showed up during the initial treatment phase, with a procedural success rate of 94% using the Orsiro stent and 90% with the Xience stent. This difference in procedural success seemed largely the result of an increased rate of periprocedural MIs, a difference that might be explained by the difference in strut thickness, said Dr. Kandzari, of Piedmont Heart Institute, Atlanta. Based on this success, designers of future drug-eluting stents may focus on thinner-strut models, he suggested.
An additional analysis that Dr. Kandzari reported combined results from two prior randomized comparisons of the Orsiro and Xience stents, creating a pooled analysis with 2,208 patients. This Bayesian analysis calculated a 100% probability of noninferiority of the Orsiro stent compared with the Xience stent, and a 97% probability of superiority. This 97% probability of superiority fell just short of the 97.5% threshold for establishing superiority that Dr. Kandzari and his associates had prespecified for this analysis.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
AT THE ESC CONGRESS 2017
Key clinical point:
Major finding: The target-lesion failure rate after 12 months was 6.2% with the Orsiro stent and 9.6% with the Xience stent.
Data source: BIOFLOW-V, a multicenter, randomized trial with 1,334 patients.
Disclosures: BIOFLOW-V was sponsored by Biotronik, the company that markets the Orsiro stent. Dr. Kandzari has been a consultant to and/or has received research funding from Biotronik, Boston Scientific, Medtronic, Micell Technologies, Abbott Vascular, St. Jude, Medinol, and OrbusNeich. Dr. Haude has been a consultant to and has received honoraria from Biotronik and from several other device and drug companies.
Big risk of serious falls after first episode of syncope
BARCELONA – Patients have an exorbitant 80% increased risk of hospitalization for falls resulting in fracture or head injury in the first year after discharge following a first-ever episode of syncope, according to a Danish national cohort study. One in five patients who sustained a fall resulting in hospitalization experienced a hip fracture, according to Anna-Karin Nume, MD, of the University of Copenhagen.
In this interview at the annual congress of the European Society of Cardiology, Dr. Nume highlights findings from her study, which included 125,763 Danish adults with first-time syncope.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
BARCELONA – Patients have an exorbitant 80% increased risk of hospitalization for falls resulting in fracture or head injury in the first year after discharge following a first-ever episode of syncope, according to a Danish national cohort study. One in five patients who sustained a fall resulting in hospitalization experienced a hip fracture, according to Anna-Karin Nume, MD, of the University of Copenhagen.
In this interview at the annual congress of the European Society of Cardiology, Dr. Nume highlights findings from her study, which included 125,763 Danish adults with first-time syncope.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
BARCELONA – Patients have an exorbitant 80% increased risk of hospitalization for falls resulting in fracture or head injury in the first year after discharge following a first-ever episode of syncope, according to a Danish national cohort study. One in five patients who sustained a fall resulting in hospitalization experienced a hip fracture, according to Anna-Karin Nume, MD, of the University of Copenhagen.
In this interview at the annual congress of the European Society of Cardiology, Dr. Nume highlights findings from her study, which included 125,763 Danish adults with first-time syncope.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE ESC CONGRESS 2017
Addition of daratumumab improves PFS in MM, company says
Interim results of a phase 3 study suggest adding daratumumab to a 3-drug regimen can improve progression-free survival (PFS) in patients with newly diagnosed multiple myeloma (MM).
In the phase 3 ALCYONE study, researchers are comparing daratumumab in combination with bortezomib, melphalan, and prednisone (VMP) to VMP alone in patients with newly diagnosed MM who are not considered candidates for autologous stem cell transplant.
Genmab A/S recently announced interim results from this trial and said additional data are expected to be submitted for presentation at an upcoming medical conference and for publication in a peer-reviewed journal.
The ALCYONE trial enrolled 706 patients who were randomized to receive 9 cycles of daratumumab plus VMP or VMP alone.
In the daratumumab arm, patients received 16 mg/kg of daratumumab once weekly for 6 weeks (cycle 1; 1 cycle = 42 days), followed by once every 3 weeks (cycles 2-9). After that, patients continued to receive 16 mg/kg of daratumumab once every 4 weeks until disease progression.
At the pre-planned interim analysis, the study’s primary endpoint was met. Treatment with daratumumab reduced the risk of disease progression or death by 50% when compared to VMP alone (hazard ratio=0.50; 95% CI 0.38-0.65; P<0.0001).
The median PFS has not been reached in the daratumumab arm but was an estimated 18.1 months for patients who received VMP alone.
Genmab said that, overall, the safety profile of daratumumab in combination with VMP is consistent with the known safety profile of the VMP regimen and the known safety profile of daratumumab.
Based on these results, an independent data monitoring committee recommended the data be unblinded. All patients will continue to be monitored for safety and overall survival.
Janssen Biotech, Inc., which licensed daratumumab from Genmab in 2012, said it will discuss with health authorities the potential for a regulatory submission for daratumumab in combination with VMP as a treatment for newly diagnosed MM.
Daratumumab is already approved in the US (as DARZALEX®) for use in combination with lenalidomide and dexamethasone, or bortezomib and dexamethasone, to treat MM patients who have received at least 1 prior therapy.
Daratumumab is also approved for use in combination with pomalidomide and dexamethasone to treat MM patients who have received at least 2 prior therapies, including lenalidomide and a proteasome inhibitor (PI).
And daratumumab is approved as monotherapy for MM patients who have received at least 3 prior lines of therapy, including a PI and an immunomodulatory agent, or who are double-refractory to a PI and an immunomodulatory agent. ![]()
Interim results of a phase 3 study suggest adding daratumumab to a 3-drug regimen can improve progression-free survival (PFS) in patients with newly diagnosed multiple myeloma (MM).
In the phase 3 ALCYONE study, researchers are comparing daratumumab in combination with bortezomib, melphalan, and prednisone (VMP) to VMP alone in patients with newly diagnosed MM who are not considered candidates for autologous stem cell transplant.
Genmab A/S recently announced interim results from this trial and said additional data are expected to be submitted for presentation at an upcoming medical conference and for publication in a peer-reviewed journal.
The ALCYONE trial enrolled 706 patients who were randomized to receive 9 cycles of daratumumab plus VMP or VMP alone.
In the daratumumab arm, patients received 16 mg/kg of daratumumab once weekly for 6 weeks (cycle 1; 1 cycle = 42 days), followed by once every 3 weeks (cycles 2-9). After that, patients continued to receive 16 mg/kg of daratumumab once every 4 weeks until disease progression.
At the pre-planned interim analysis, the study’s primary endpoint was met. Treatment with daratumumab reduced the risk of disease progression or death by 50% when compared to VMP alone (hazard ratio=0.50; 95% CI 0.38-0.65; P<0.0001).
The median PFS has not been reached in the daratumumab arm but was an estimated 18.1 months for patients who received VMP alone.
Genmab said that, overall, the safety profile of daratumumab in combination with VMP is consistent with the known safety profile of the VMP regimen and the known safety profile of daratumumab.
Based on these results, an independent data monitoring committee recommended the data be unblinded. All patients will continue to be monitored for safety and overall survival.
Janssen Biotech, Inc., which licensed daratumumab from Genmab in 2012, said it will discuss with health authorities the potential for a regulatory submission for daratumumab in combination with VMP as a treatment for newly diagnosed MM.
Daratumumab is already approved in the US (as DARZALEX®) for use in combination with lenalidomide and dexamethasone, or bortezomib and dexamethasone, to treat MM patients who have received at least 1 prior therapy.
Daratumumab is also approved for use in combination with pomalidomide and dexamethasone to treat MM patients who have received at least 2 prior therapies, including lenalidomide and a proteasome inhibitor (PI).
And daratumumab is approved as monotherapy for MM patients who have received at least 3 prior lines of therapy, including a PI and an immunomodulatory agent, or who are double-refractory to a PI and an immunomodulatory agent. ![]()
Interim results of a phase 3 study suggest adding daratumumab to a 3-drug regimen can improve progression-free survival (PFS) in patients with newly diagnosed multiple myeloma (MM).
In the phase 3 ALCYONE study, researchers are comparing daratumumab in combination with bortezomib, melphalan, and prednisone (VMP) to VMP alone in patients with newly diagnosed MM who are not considered candidates for autologous stem cell transplant.
Genmab A/S recently announced interim results from this trial and said additional data are expected to be submitted for presentation at an upcoming medical conference and for publication in a peer-reviewed journal.
The ALCYONE trial enrolled 706 patients who were randomized to receive 9 cycles of daratumumab plus VMP or VMP alone.
In the daratumumab arm, patients received 16 mg/kg of daratumumab once weekly for 6 weeks (cycle 1; 1 cycle = 42 days), followed by once every 3 weeks (cycles 2-9). After that, patients continued to receive 16 mg/kg of daratumumab once every 4 weeks until disease progression.
At the pre-planned interim analysis, the study’s primary endpoint was met. Treatment with daratumumab reduced the risk of disease progression or death by 50% when compared to VMP alone (hazard ratio=0.50; 95% CI 0.38-0.65; P<0.0001).
The median PFS has not been reached in the daratumumab arm but was an estimated 18.1 months for patients who received VMP alone.
Genmab said that, overall, the safety profile of daratumumab in combination with VMP is consistent with the known safety profile of the VMP regimen and the known safety profile of daratumumab.
Based on these results, an independent data monitoring committee recommended the data be unblinded. All patients will continue to be monitored for safety and overall survival.
Janssen Biotech, Inc., which licensed daratumumab from Genmab in 2012, said it will discuss with health authorities the potential for a regulatory submission for daratumumab in combination with VMP as a treatment for newly diagnosed MM.
Daratumumab is already approved in the US (as DARZALEX®) for use in combination with lenalidomide and dexamethasone, or bortezomib and dexamethasone, to treat MM patients who have received at least 1 prior therapy.
Daratumumab is also approved for use in combination with pomalidomide and dexamethasone to treat MM patients who have received at least 2 prior therapies, including lenalidomide and a proteasome inhibitor (PI).
And daratumumab is approved as monotherapy for MM patients who have received at least 3 prior lines of therapy, including a PI and an immunomodulatory agent, or who are double-refractory to a PI and an immunomodulatory agent. ![]()
In T1 diabetes, CABG seems better than PCI
In patients with type 1 diabetes in need of multivessel revascularization, coronary artery bypass graft (CABG) may be a better choice than percutaneous coronary intervention (PCI), according to results from a new comparative study presented at the annual congress of the European Society of Cardiology by Martin J. Holzmann, PhD, of the Karolinska Institute, Stockholm.
The two procedures had similar mortality rates, but PCI patients fared worse with respect to mortality due to myocardial infarction and several cardiovascular outcomes.
Previous studies had also suggested better outcomes with CABG than with PCI, but they lumped together patients with type 1 and type 2 diabetes, while the current study focused only on patients with type 1 diabetes.
The study included patients in Sweden with type 1 diabetes who underwent CABG (683 patients) or PCI (1,863 patients) between 1995 and 2013. During follow-up, 44.6% of patients in the PCI group died, compared with 53.3% in the CABG group. After adjustment for between-group differences, however, there was no significant difference in mortality risk between the two groups.
However, assessments of cause-specific mortality told a different story. Subjects in the PCI group had a greater risk of death from coronary artery disease (hazard ratio, 1.45; 95% confidence interval, 1.21-1.74).
Subjects in the PCI group were also more likely to suffer myocardial infarction (HR, 1.47; 95% CI, 1.21-1.77) and were more than five times more likely to undergo repeat vascularization (adjusted HR, 5.64; 95% CI, 4.67-6.82). The CABG group had a higher 30-day stroke risk (1.9% vs. 0.8%), but there was no difference in long-term risk.
The two groups had similar risks of hospitalization for heart failure.
The researchers noted a large difference between the two groups with respect to risk during the first year of follow-up, which suggests that some patients underwent PCI because they were too ill to undergo CABG. This limitation is also suggested by the greater proportion of previous stroke, heart failure, active cancer, and end-stage renal disease in the PCI group. The researchers adjusted for these differences, but it remains possible that there were residual confounders.
No source of funding was disclosed. One of the authors has received consultancy honoraria from Actelion and Pfizer. Dr. Domanski and Dr. Farkouh report no relevant financial relationships.
In patients with aggressive multivessel CAD and stable symptoms associated with diabetes or high SYNTAX score, the mechanisms of benefit of PCI and CABG are different, and this difference likely explains the superior results of CABG.
Better stents alone cannot change the superiority of CABG, compared with PCI for patients with aggressive CAD (diabetes or high SYNTAX score), because PCI addresses only a small portion of the coronary anatomy. This does not diminish the importance of continuing advances in stent technology, but rather, it puts into appropriate perspective what can be expected from these advances.
The findings of this important study help to better inform practice, and should influence decision-making for revascularization in patients with T1DM.
These remarks were taken from an editorial by Michael J. Domanski, MD, and Michael E. Farkouh, MD (J Am Coll Cardiol. 2017. doi: 10.1016/j.jacc.2017.07.781). Dr. Domanski is with the Peter Munk Cardiac Centre, Toronto, and the Heart and Stroke Richard Lewar Centre, University of Toronto. Dr. Farokouh is the director of clinical trials at the Peter Munk Cardiac Centre, University of Toronto.
In patients with aggressive multivessel CAD and stable symptoms associated with diabetes or high SYNTAX score, the mechanisms of benefit of PCI and CABG are different, and this difference likely explains the superior results of CABG.
Better stents alone cannot change the superiority of CABG, compared with PCI for patients with aggressive CAD (diabetes or high SYNTAX score), because PCI addresses only a small portion of the coronary anatomy. This does not diminish the importance of continuing advances in stent technology, but rather, it puts into appropriate perspective what can be expected from these advances.
The findings of this important study help to better inform practice, and should influence decision-making for revascularization in patients with T1DM.
These remarks were taken from an editorial by Michael J. Domanski, MD, and Michael E. Farkouh, MD (J Am Coll Cardiol. 2017. doi: 10.1016/j.jacc.2017.07.781). Dr. Domanski is with the Peter Munk Cardiac Centre, Toronto, and the Heart and Stroke Richard Lewar Centre, University of Toronto. Dr. Farokouh is the director of clinical trials at the Peter Munk Cardiac Centre, University of Toronto.
In patients with aggressive multivessel CAD and stable symptoms associated with diabetes or high SYNTAX score, the mechanisms of benefit of PCI and CABG are different, and this difference likely explains the superior results of CABG.
Better stents alone cannot change the superiority of CABG, compared with PCI for patients with aggressive CAD (diabetes or high SYNTAX score), because PCI addresses only a small portion of the coronary anatomy. This does not diminish the importance of continuing advances in stent technology, but rather, it puts into appropriate perspective what can be expected from these advances.
The findings of this important study help to better inform practice, and should influence decision-making for revascularization in patients with T1DM.
These remarks were taken from an editorial by Michael J. Domanski, MD, and Michael E. Farkouh, MD (J Am Coll Cardiol. 2017. doi: 10.1016/j.jacc.2017.07.781). Dr. Domanski is with the Peter Munk Cardiac Centre, Toronto, and the Heart and Stroke Richard Lewar Centre, University of Toronto. Dr. Farokouh is the director of clinical trials at the Peter Munk Cardiac Centre, University of Toronto.
In patients with type 1 diabetes in need of multivessel revascularization, coronary artery bypass graft (CABG) may be a better choice than percutaneous coronary intervention (PCI), according to results from a new comparative study presented at the annual congress of the European Society of Cardiology by Martin J. Holzmann, PhD, of the Karolinska Institute, Stockholm.
The two procedures had similar mortality rates, but PCI patients fared worse with respect to mortality due to myocardial infarction and several cardiovascular outcomes.
Previous studies had also suggested better outcomes with CABG than with PCI, but they lumped together patients with type 1 and type 2 diabetes, while the current study focused only on patients with type 1 diabetes.
The study included patients in Sweden with type 1 diabetes who underwent CABG (683 patients) or PCI (1,863 patients) between 1995 and 2013. During follow-up, 44.6% of patients in the PCI group died, compared with 53.3% in the CABG group. After adjustment for between-group differences, however, there was no significant difference in mortality risk between the two groups.
However, assessments of cause-specific mortality told a different story. Subjects in the PCI group had a greater risk of death from coronary artery disease (hazard ratio, 1.45; 95% confidence interval, 1.21-1.74).
Subjects in the PCI group were also more likely to suffer myocardial infarction (HR, 1.47; 95% CI, 1.21-1.77) and were more than five times more likely to undergo repeat vascularization (adjusted HR, 5.64; 95% CI, 4.67-6.82). The CABG group had a higher 30-day stroke risk (1.9% vs. 0.8%), but there was no difference in long-term risk.
The two groups had similar risks of hospitalization for heart failure.
The researchers noted a large difference between the two groups with respect to risk during the first year of follow-up, which suggests that some patients underwent PCI because they were too ill to undergo CABG. This limitation is also suggested by the greater proportion of previous stroke, heart failure, active cancer, and end-stage renal disease in the PCI group. The researchers adjusted for these differences, but it remains possible that there were residual confounders.
No source of funding was disclosed. One of the authors has received consultancy honoraria from Actelion and Pfizer. Dr. Domanski and Dr. Farkouh report no relevant financial relationships.
In patients with type 1 diabetes in need of multivessel revascularization, coronary artery bypass graft (CABG) may be a better choice than percutaneous coronary intervention (PCI), according to results from a new comparative study presented at the annual congress of the European Society of Cardiology by Martin J. Holzmann, PhD, of the Karolinska Institute, Stockholm.
The two procedures had similar mortality rates, but PCI patients fared worse with respect to mortality due to myocardial infarction and several cardiovascular outcomes.
Previous studies had also suggested better outcomes with CABG than with PCI, but they lumped together patients with type 1 and type 2 diabetes, while the current study focused only on patients with type 1 diabetes.
The study included patients in Sweden with type 1 diabetes who underwent CABG (683 patients) or PCI (1,863 patients) between 1995 and 2013. During follow-up, 44.6% of patients in the PCI group died, compared with 53.3% in the CABG group. After adjustment for between-group differences, however, there was no significant difference in mortality risk between the two groups.
However, assessments of cause-specific mortality told a different story. Subjects in the PCI group had a greater risk of death from coronary artery disease (hazard ratio, 1.45; 95% confidence interval, 1.21-1.74).
Subjects in the PCI group were also more likely to suffer myocardial infarction (HR, 1.47; 95% CI, 1.21-1.77) and were more than five times more likely to undergo repeat vascularization (adjusted HR, 5.64; 95% CI, 4.67-6.82). The CABG group had a higher 30-day stroke risk (1.9% vs. 0.8%), but there was no difference in long-term risk.
The two groups had similar risks of hospitalization for heart failure.
The researchers noted a large difference between the two groups with respect to risk during the first year of follow-up, which suggests that some patients underwent PCI because they were too ill to undergo CABG. This limitation is also suggested by the greater proportion of previous stroke, heart failure, active cancer, and end-stage renal disease in the PCI group. The researchers adjusted for these differences, but it remains possible that there were residual confounders.
No source of funding was disclosed. One of the authors has received consultancy honoraria from Actelion and Pfizer. Dr. Domanski and Dr. Farkouh report no relevant financial relationships.
FROM THE ESC CONGRESS 2017
Key clinical point: Patients undergoing PCI had worse cardiovascular outcomes than those receiving CABG.
Major finding: The PCI group had a 45% increased risk of death due to myocardial infarction.
Data source: Observational study (n = 2,546).
Disclosures: No source of funding was disclosed. One of the authors has received consultancy honoraria from Actelion and Pfizer. Dr. Domanski and Dr. Farkouh report no relevant financial relationships.
Undiagnosed AF common in higher-risk patients
Over an 18-month period, small, insertable cardiac monitors detected atrial fibrillation in 29% of previously undiagnosed patients who were at high risk of both AF and stroke, and in 40% of patients over 30 months, according to investigators. The study was presented at the annual congress of the European Society of Cardiology and simultaneously published in JAMA Cardiology.
More than half (56%) of patients consequently started oral anticoagulation therapy, noted James A. Reiffel, MD, of Columbia University College of Physicians and Surgeons, New York, with his associates, for the REVEAL AF investigators.
“The incidence of previously undiagnosed atrial fibrillation may be substantial in patients with risk factors for AF and stroke,” they concluded. “Atrial fibrillation would have gone undetected in most patients had monitoring been limited to 30 days. Further trials regarding the value of detecting subclinical AF and of prophylactic therapies are warranted.”
Atrial fibrillation affects millions worldwide and is associated with older age, hypertension, diabetes, and heart failure, all of which also independently increase the risk of stroke. Minimally invasive prolonged electrocardiographic monitoring with insertable cardiac monitors might help hasten detection and treatment of AF, but diagnostic yield in high-risk patients has been unclear.
In this single-arm, multicenter, prospective study, researchers inserted Reveal XT or Reveal LINQ (Medtronic) cardiac monitors in 385 adults who had either CHAD2 scores of 3, or CHAD2 scores of 2 and one additional risk factor for AF, such as coronary artery disease, sleep apnea, chronic obstructive pulmonary disease, or renal insufficiency. The primary endpoint was AF lasting at least 6 minutes (JAMA Cardiol. 2017 Aug 26. doi: 10.1001/jamacardio.2017.3180). Median follow-up time was 22.5 months. Rates of detecting AF were 6% at 30 days compared with 20% at 6 months, 27% at 12 months, 34% at 24 months, and 40% at 30 months. Patients typically had their first AF episode about 4 months (median, 123 days) after the device was inserted. Among patients who had experienced AF by 18 months, 10% had one or more episodes lasting at least 24 hours, and 72 (56%) were prescribed oral anticoagulation therapy.
The recent PREDATE AF and ASSERT-II studies also found that previously undiagnosed AF was common among high-risk patients, the researchers noted. However, whether anticoagulating patients who have only brief episodes of AF significantly reduces their risk of stroke remains unclear, they added. Three trials (ARTESiA, NOAH, and LOOP) are underway to assess whether oral anticoagulation therapy improves outcomes in patients with device-detected AF.
Medtronic funded the study. Dr. Reiffel and five coinvestigators disclosed consulting for and receiving “modest honoraria” from Medtronic. Two other coinvestigators reported employment with and stock ownership in Medtronic.
The availability of safe and effective oral anticoagulant therapy makes the findings of REVEAL AF highly relevant. This high rate of incident AF makes ICM-based screenings of high-risk individuals a potentially attractive stroke prevention strategy. More detailed subgroup analyses may help identify a patient population with an even higher risk of developing AF. It is also conceivable that this population could have a sufficiently high risk of AF and stroke that a strategy of empiric oral anticoagulation, without the need for AF monitoring, could prove beneficial.
The REVEAL AF study has shown that AF is extremely common among older individuals with stroke risk factors. Over the next 3-4 years, subgroup analyses, economic evaluations, and randomized clinical trials will help determine if this insight can be translated into a cost-effective stroke prevention strategy for high-risk individuals.
Jeff S. Healey, MD, MSc, is at the Population Health Research Institute, McMaster University, Hamilton, Ont. He is the principal investigator of the ASSERT-II and ARTESiA trials, and had no other relevant disclosures. These comments are from his editorial (JAMA Cardiol. 2017 Aug 26. doi: 10.1001/jamacardio.2017.3203).
The availability of safe and effective oral anticoagulant therapy makes the findings of REVEAL AF highly relevant. This high rate of incident AF makes ICM-based screenings of high-risk individuals a potentially attractive stroke prevention strategy. More detailed subgroup analyses may help identify a patient population with an even higher risk of developing AF. It is also conceivable that this population could have a sufficiently high risk of AF and stroke that a strategy of empiric oral anticoagulation, without the need for AF monitoring, could prove beneficial.
The REVEAL AF study has shown that AF is extremely common among older individuals with stroke risk factors. Over the next 3-4 years, subgroup analyses, economic evaluations, and randomized clinical trials will help determine if this insight can be translated into a cost-effective stroke prevention strategy for high-risk individuals.
Jeff S. Healey, MD, MSc, is at the Population Health Research Institute, McMaster University, Hamilton, Ont. He is the principal investigator of the ASSERT-II and ARTESiA trials, and had no other relevant disclosures. These comments are from his editorial (JAMA Cardiol. 2017 Aug 26. doi: 10.1001/jamacardio.2017.3203).
The availability of safe and effective oral anticoagulant therapy makes the findings of REVEAL AF highly relevant. This high rate of incident AF makes ICM-based screenings of high-risk individuals a potentially attractive stroke prevention strategy. More detailed subgroup analyses may help identify a patient population with an even higher risk of developing AF. It is also conceivable that this population could have a sufficiently high risk of AF and stroke that a strategy of empiric oral anticoagulation, without the need for AF monitoring, could prove beneficial.
The REVEAL AF study has shown that AF is extremely common among older individuals with stroke risk factors. Over the next 3-4 years, subgroup analyses, economic evaluations, and randomized clinical trials will help determine if this insight can be translated into a cost-effective stroke prevention strategy for high-risk individuals.
Jeff S. Healey, MD, MSc, is at the Population Health Research Institute, McMaster University, Hamilton, Ont. He is the principal investigator of the ASSERT-II and ARTESiA trials, and had no other relevant disclosures. These comments are from his editorial (JAMA Cardiol. 2017 Aug 26. doi: 10.1001/jamacardio.2017.3203).
Over an 18-month period, small, insertable cardiac monitors detected atrial fibrillation in 29% of previously undiagnosed patients who were at high risk of both AF and stroke, and in 40% of patients over 30 months, according to investigators. The study was presented at the annual congress of the European Society of Cardiology and simultaneously published in JAMA Cardiology.
More than half (56%) of patients consequently started oral anticoagulation therapy, noted James A. Reiffel, MD, of Columbia University College of Physicians and Surgeons, New York, with his associates, for the REVEAL AF investigators.
“The incidence of previously undiagnosed atrial fibrillation may be substantial in patients with risk factors for AF and stroke,” they concluded. “Atrial fibrillation would have gone undetected in most patients had monitoring been limited to 30 days. Further trials regarding the value of detecting subclinical AF and of prophylactic therapies are warranted.”
Atrial fibrillation affects millions worldwide and is associated with older age, hypertension, diabetes, and heart failure, all of which also independently increase the risk of stroke. Minimally invasive prolonged electrocardiographic monitoring with insertable cardiac monitors might help hasten detection and treatment of AF, but diagnostic yield in high-risk patients has been unclear.
In this single-arm, multicenter, prospective study, researchers inserted Reveal XT or Reveal LINQ (Medtronic) cardiac monitors in 385 adults who had either CHAD2 scores of 3, or CHAD2 scores of 2 and one additional risk factor for AF, such as coronary artery disease, sleep apnea, chronic obstructive pulmonary disease, or renal insufficiency. The primary endpoint was AF lasting at least 6 minutes (JAMA Cardiol. 2017 Aug 26. doi: 10.1001/jamacardio.2017.3180). Median follow-up time was 22.5 months. Rates of detecting AF were 6% at 30 days compared with 20% at 6 months, 27% at 12 months, 34% at 24 months, and 40% at 30 months. Patients typically had their first AF episode about 4 months (median, 123 days) after the device was inserted. Among patients who had experienced AF by 18 months, 10% had one or more episodes lasting at least 24 hours, and 72 (56%) were prescribed oral anticoagulation therapy.
The recent PREDATE AF and ASSERT-II studies also found that previously undiagnosed AF was common among high-risk patients, the researchers noted. However, whether anticoagulating patients who have only brief episodes of AF significantly reduces their risk of stroke remains unclear, they added. Three trials (ARTESiA, NOAH, and LOOP) are underway to assess whether oral anticoagulation therapy improves outcomes in patients with device-detected AF.
Medtronic funded the study. Dr. Reiffel and five coinvestigators disclosed consulting for and receiving “modest honoraria” from Medtronic. Two other coinvestigators reported employment with and stock ownership in Medtronic.
Over an 18-month period, small, insertable cardiac monitors detected atrial fibrillation in 29% of previously undiagnosed patients who were at high risk of both AF and stroke, and in 40% of patients over 30 months, according to investigators. The study was presented at the annual congress of the European Society of Cardiology and simultaneously published in JAMA Cardiology.
More than half (56%) of patients consequently started oral anticoagulation therapy, noted James A. Reiffel, MD, of Columbia University College of Physicians and Surgeons, New York, with his associates, for the REVEAL AF investigators.
“The incidence of previously undiagnosed atrial fibrillation may be substantial in patients with risk factors for AF and stroke,” they concluded. “Atrial fibrillation would have gone undetected in most patients had monitoring been limited to 30 days. Further trials regarding the value of detecting subclinical AF and of prophylactic therapies are warranted.”
Atrial fibrillation affects millions worldwide and is associated with older age, hypertension, diabetes, and heart failure, all of which also independently increase the risk of stroke. Minimally invasive prolonged electrocardiographic monitoring with insertable cardiac monitors might help hasten detection and treatment of AF, but diagnostic yield in high-risk patients has been unclear.
In this single-arm, multicenter, prospective study, researchers inserted Reveal XT or Reveal LINQ (Medtronic) cardiac monitors in 385 adults who had either CHAD2 scores of 3, or CHAD2 scores of 2 and one additional risk factor for AF, such as coronary artery disease, sleep apnea, chronic obstructive pulmonary disease, or renal insufficiency. The primary endpoint was AF lasting at least 6 minutes (JAMA Cardiol. 2017 Aug 26. doi: 10.1001/jamacardio.2017.3180). Median follow-up time was 22.5 months. Rates of detecting AF were 6% at 30 days compared with 20% at 6 months, 27% at 12 months, 34% at 24 months, and 40% at 30 months. Patients typically had their first AF episode about 4 months (median, 123 days) after the device was inserted. Among patients who had experienced AF by 18 months, 10% had one or more episodes lasting at least 24 hours, and 72 (56%) were prescribed oral anticoagulation therapy.
The recent PREDATE AF and ASSERT-II studies also found that previously undiagnosed AF was common among high-risk patients, the researchers noted. However, whether anticoagulating patients who have only brief episodes of AF significantly reduces their risk of stroke remains unclear, they added. Three trials (ARTESiA, NOAH, and LOOP) are underway to assess whether oral anticoagulation therapy improves outcomes in patients with device-detected AF.
Medtronic funded the study. Dr. Reiffel and five coinvestigators disclosed consulting for and receiving “modest honoraria” from Medtronic. Two other coinvestigators reported employment with and stock ownership in Medtronic.
FROM THE ESC CONGRESS 2017
Key clinical point: Undiagnosed atrial fibrillation is common in high-risk patients.
Major finding: At 18 months, 29% of previously undiagnosed, high-risk patients had experienced atrial fibrillation lasting 6 or more minutes.
Data source: A single-arm, prospective, multicenter study of 446 patients with a CHADS2 score of at least 3, or a CHADS2 score of at least 2 plus at least one other risk factor (coronary artery disease, sleep apnea, chronic obstructive pulmonary disease, or renal insufficiency).
Disclosures: Medtronic funded the study. Dr. Reiffel and five coinvestigators disclosed consulting for and receiving “modest honoraria” from Medtronic. Two other coinvestigators reported employment with and stock ownership in Medtronic.
Drug granted breakthrough designation for CTCL
The US Food and Drug Administration (FDA) has granted breakthrough therapy designation to mogamulizumab for the treatment of adults with cutaneous T-cell lymphoma (CTCL) who have received at least 1 prior systemic therapy.
Mogamulizumab is a humanized monoclonal antibody directed against CCR4. It is being developed by Kyowa Hakko Kirin Co., Ltd.
The breakthrough designation for mogamulizumab in CTCL is based on data from the phase 3 MAVORIC study, the largest randomized trial in CTCL.
In MAVORIC, researchers are comparing mogamulizumab and vorinostat in patients with CTCL (both mycosis fungoides and Sézary syndrome) who have failed at least 1 prior systemic treatment.
In April, Kyowa Hakko Kirin announced results from this trial, which showed that patients treated with mogamulizumab have significantly better progression-free survival than patients treated with vorinostat. The company also said mogamulizumab has a tolerable safety profile.
Kyowa Hakko Kirin has not provided any data from MAVORIC but is working with investigators on the future presentation and publication of results from this trial.
Results of a phase 1/2 study of mogamulizumab in previously treated CTCL patients were published in Blood in March 2015.
About breakthrough designation
The FDA’s breakthrough designation is intended to expedite the development and review of new treatments for serious or life-threatening conditions.
The designation entitles the company developing a therapy to more intensive FDA guidance on an efficient and accelerated development program, as well as eligibility for other actions to expedite FDA review, such as rolling submission and priority review.
To earn breakthrough designation, a treatment must show encouraging early clinical results demonstrating substantial improvement over available therapies with regard to a clinically significant endpoint, or it must fulfill an unmet need. ![]()
The US Food and Drug Administration (FDA) has granted breakthrough therapy designation to mogamulizumab for the treatment of adults with cutaneous T-cell lymphoma (CTCL) who have received at least 1 prior systemic therapy.
Mogamulizumab is a humanized monoclonal antibody directed against CCR4. It is being developed by Kyowa Hakko Kirin Co., Ltd.
The breakthrough designation for mogamulizumab in CTCL is based on data from the phase 3 MAVORIC study, the largest randomized trial in CTCL.
In MAVORIC, researchers are comparing mogamulizumab and vorinostat in patients with CTCL (both mycosis fungoides and Sézary syndrome) who have failed at least 1 prior systemic treatment.
In April, Kyowa Hakko Kirin announced results from this trial, which showed that patients treated with mogamulizumab have significantly better progression-free survival than patients treated with vorinostat. The company also said mogamulizumab has a tolerable safety profile.
Kyowa Hakko Kirin has not provided any data from MAVORIC but is working with investigators on the future presentation and publication of results from this trial.
Results of a phase 1/2 study of mogamulizumab in previously treated CTCL patients were published in Blood in March 2015.
About breakthrough designation
The FDA’s breakthrough designation is intended to expedite the development and review of new treatments for serious or life-threatening conditions.
The designation entitles the company developing a therapy to more intensive FDA guidance on an efficient and accelerated development program, as well as eligibility for other actions to expedite FDA review, such as rolling submission and priority review.
To earn breakthrough designation, a treatment must show encouraging early clinical results demonstrating substantial improvement over available therapies with regard to a clinically significant endpoint, or it must fulfill an unmet need. ![]()
The US Food and Drug Administration (FDA) has granted breakthrough therapy designation to mogamulizumab for the treatment of adults with cutaneous T-cell lymphoma (CTCL) who have received at least 1 prior systemic therapy.
Mogamulizumab is a humanized monoclonal antibody directed against CCR4. It is being developed by Kyowa Hakko Kirin Co., Ltd.
The breakthrough designation for mogamulizumab in CTCL is based on data from the phase 3 MAVORIC study, the largest randomized trial in CTCL.
In MAVORIC, researchers are comparing mogamulizumab and vorinostat in patients with CTCL (both mycosis fungoides and Sézary syndrome) who have failed at least 1 prior systemic treatment.
In April, Kyowa Hakko Kirin announced results from this trial, which showed that patients treated with mogamulizumab have significantly better progression-free survival than patients treated with vorinostat. The company also said mogamulizumab has a tolerable safety profile.
Kyowa Hakko Kirin has not provided any data from MAVORIC but is working with investigators on the future presentation and publication of results from this trial.
Results of a phase 1/2 study of mogamulizumab in previously treated CTCL patients were published in Blood in March 2015.
About breakthrough designation
The FDA’s breakthrough designation is intended to expedite the development and review of new treatments for serious or life-threatening conditions.
The designation entitles the company developing a therapy to more intensive FDA guidance on an efficient and accelerated development program, as well as eligibility for other actions to expedite FDA review, such as rolling submission and priority review.
To earn breakthrough designation, a treatment must show encouraging early clinical results demonstrating substantial improvement over available therapies with regard to a clinically significant endpoint, or it must fulfill an unmet need. ![]()
Think beyond BMI to optimize bariatric patients presurgery
NEW YORK – A structured, four-pronged approach to get patients as fit and healthy as possible prior to bariatric surgery holds the potential to improve postoperative outcomes. In general, bariatric surgery patients are in a better position than most surgery candidates because of a longer preoperative period. During this time, surgeons can work with a multidisciplinary team to optimize any medical, nutritional, exercise-related, and mental health concerns.
“People focus on the size of our patients and the weight of our patients, but [body mass index] is only one factor. They can have many other comorbidities that are significant,” Dr. LaMasters said. Patients can present with cardiac and pulmonary issues, hypertension, sleep apnea, diabetes, asthma, reflux and “a very high incidence of anxiety and depression.”
“So we have a lot of challenges,” she added. “We take care of complex, high-risk patients, and our goal is to improve outcomes. Using presurgery optimization can be a key to that.”
Maximizing medical readiness
Multiple providers drive the medical intervention, Dr. LaMasters said, including surgeons and primary care doctors, as well as advanced practice providers, medical weight loss providers, and other specialists. “We do try to get patients to lose weight before surgery, but that’s not an absolute requirement. More important is adjustment of other risk factors like pulmonary risk factors, control of hypertension, treatment of sleep apnea, and control of hyperglycemia. We’d like to have their A1c [test results to be] under 8%. We want to start [proton pump inhibitors] early because there is a very high prevalence of reflux and gastritis in this population.”
Bariatric surgery patients “are uniquely positioned to have a substantial benefit from that ‘prehabilitation,’ but this only works if you have a multidisciplinary team,” Dr. LaMasters said at the American College of Surgeons Quality and Safety Conference. “Think of this as down-staging disease, like in a cancer model.”
“The message from this is there is an opportunity if we build it into the prehab phase of care. It’s a new way of thinking in surgery. You can change your results,” said session moderator David B. Hoyt, MD, FACS, Executive Director of the American College of Surgeons.
Nutritional know-how
Dietitians determine the second component – how to optimize nutrition before surgery. They focus on education, evaluation, setting goals, “and very importantly, supporting patients to attain those goals,” Dr. LaMasters said. Goals include increasing protein intake prior to surgery to a recommended 1.5 g/kg/day and starting nutritional supplements ahead of time.
Even though they typically consume an excess amount of calories, “many of our patients have baseline malnutrition,” Dr. LaMasters said. Establishing mindful behavior for meal planning, preparation, and eating is a potential solution, as is addressing any socioeconomic factors that can present challenges to healthy eating.
Emphasizing exercise
“The exercise piece is really key for our patients,” Dr. LaMasters said. Many candidates for bariatric surgery have mobility issues. “The first thing many say is ‘I can’t exercise.’ We instruct them that they can exercise. Our job is to find out what they can do – there are many different exercise modalities.”
A good baseline assessment is a 6-minute walk test to assess their distance limits, oxygen level, and any resulting symptoms.
“Our goal is to get them to walking – even those who can barely walk with a walker – for 5-10 minutes, six times a day,” Dr. LaMasters said. “We feel that is a minimum threshold to prevent blood clots after surgery.” Another recommendation is to get surgical candidates to do some activity 30 minutes a day, four times a week, at a minimum. “Eventually, after surgery and when they’ve lost weight and are healthier, the goal is going to be 1 hour, five days a week.”
Start the exercise program at least 4-8 weeks prior to surgery. Most studies show significant benefit if you start at least 4 weeks prior to surgery, Dr. LaMasters suggested. “In our own practice, we’ve seen if you can start a daily walking program even just 2 weeks prior to surgery, we see a significant benefit.”
Addressing anxiety or depression
The mental health piece is very important and should be guided by mental health providers on the multidisciplinary team, Dr. LaMasters said.
“Our patients have a high degree of stress in their lives, especially related to socioeconomic factors. A patient who does not have their anxiety or depression under control will not do as well after surgery.”
Optimization in other specialties
The benefits of a prehabilitation exercise program have been demonstrated across many other specialties, especially in colorectal surgery, cardiovascular surgery, and orthopedic surgery, Dr. LaMasters said. In randomized, controlled studies, this optimization is associated with decreased complications, mortality, and length of hospital stay.
“There is actually way less data from bariatric studies. I suggest to you that our bariatric surgery patients have similar comorbidities when compared with those other specialties – specialties that refer their patients to us for treatment,” Dr. LaMasters said.
In a study of cardiorespiratory fitness before bariatric surgery, other researchers found that the most serious postoperative complications occurred more often among patients who were less fit preoperatively (Chest. 2006 Aug;130[2]:517-25). These investigators measured peak oxygen consumption (VO2) preoperatively in 109 patients. “Each unit increase in peak VO2 rate was associated with 61% decrease in overall complications,” Dr. LaMasters said. “So a small increase in fitness led to a big decrease in complications.”
Other researchers compared optimization of exercise, nutrition, and psychological factors before and after surgery in 185 patients with colorectal cancer (Acta Oncol. 2017 Feb;56[2]:295-300). A control group received the interventions postoperatively. “They found a statistically significant difference in the prehabilitation group in increased functional capacity, with more than a 30-meter improvement in 6-minute walk test before surgery,” Dr. LaMasters said. Although the 6-minute walk test results decreased 4 weeks after surgery, as might be expected, by 8 weeks the prehabilitation patients performed better than controls – and even better than their own baseline, she added. “This model of optimization can be very well applied in bariatric surgery.”
“The goal is safe surgery with outstanding long-term outcomes,” Dr. LaMasters said. “It is really not enough in this era to ‘get a patient through surgery.’ We really need to optimize the risk factors we can and identify any areas where they will have additional needs after surgery,” she added. “This will allow us to have excellent outcomes in this complex patient population.”
Dr. LaMasters and Dr. Hoyt had no relevant financial disclosures.
NEW YORK – A structured, four-pronged approach to get patients as fit and healthy as possible prior to bariatric surgery holds the potential to improve postoperative outcomes. In general, bariatric surgery patients are in a better position than most surgery candidates because of a longer preoperative period. During this time, surgeons can work with a multidisciplinary team to optimize any medical, nutritional, exercise-related, and mental health concerns.
“People focus on the size of our patients and the weight of our patients, but [body mass index] is only one factor. They can have many other comorbidities that are significant,” Dr. LaMasters said. Patients can present with cardiac and pulmonary issues, hypertension, sleep apnea, diabetes, asthma, reflux and “a very high incidence of anxiety and depression.”
“So we have a lot of challenges,” she added. “We take care of complex, high-risk patients, and our goal is to improve outcomes. Using presurgery optimization can be a key to that.”
Maximizing medical readiness
Multiple providers drive the medical intervention, Dr. LaMasters said, including surgeons and primary care doctors, as well as advanced practice providers, medical weight loss providers, and other specialists. “We do try to get patients to lose weight before surgery, but that’s not an absolute requirement. More important is adjustment of other risk factors like pulmonary risk factors, control of hypertension, treatment of sleep apnea, and control of hyperglycemia. We’d like to have their A1c [test results to be] under 8%. We want to start [proton pump inhibitors] early because there is a very high prevalence of reflux and gastritis in this population.”
Bariatric surgery patients “are uniquely positioned to have a substantial benefit from that ‘prehabilitation,’ but this only works if you have a multidisciplinary team,” Dr. LaMasters said at the American College of Surgeons Quality and Safety Conference. “Think of this as down-staging disease, like in a cancer model.”
“The message from this is there is an opportunity if we build it into the prehab phase of care. It’s a new way of thinking in surgery. You can change your results,” said session moderator David B. Hoyt, MD, FACS, Executive Director of the American College of Surgeons.
Nutritional know-how
Dietitians determine the second component – how to optimize nutrition before surgery. They focus on education, evaluation, setting goals, “and very importantly, supporting patients to attain those goals,” Dr. LaMasters said. Goals include increasing protein intake prior to surgery to a recommended 1.5 g/kg/day and starting nutritional supplements ahead of time.
Even though they typically consume an excess amount of calories, “many of our patients have baseline malnutrition,” Dr. LaMasters said. Establishing mindful behavior for meal planning, preparation, and eating is a potential solution, as is addressing any socioeconomic factors that can present challenges to healthy eating.
Emphasizing exercise
“The exercise piece is really key for our patients,” Dr. LaMasters said. Many candidates for bariatric surgery have mobility issues. “The first thing many say is ‘I can’t exercise.’ We instruct them that they can exercise. Our job is to find out what they can do – there are many different exercise modalities.”
A good baseline assessment is a 6-minute walk test to assess their distance limits, oxygen level, and any resulting symptoms.
“Our goal is to get them to walking – even those who can barely walk with a walker – for 5-10 minutes, six times a day,” Dr. LaMasters said. “We feel that is a minimum threshold to prevent blood clots after surgery.” Another recommendation is to get surgical candidates to do some activity 30 minutes a day, four times a week, at a minimum. “Eventually, after surgery and when they’ve lost weight and are healthier, the goal is going to be 1 hour, five days a week.”
Start the exercise program at least 4-8 weeks prior to surgery. Most studies show significant benefit if you start at least 4 weeks prior to surgery, Dr. LaMasters suggested. “In our own practice, we’ve seen if you can start a daily walking program even just 2 weeks prior to surgery, we see a significant benefit.”
Addressing anxiety or depression
The mental health piece is very important and should be guided by mental health providers on the multidisciplinary team, Dr. LaMasters said.
“Our patients have a high degree of stress in their lives, especially related to socioeconomic factors. A patient who does not have their anxiety or depression under control will not do as well after surgery.”
Optimization in other specialties
The benefits of a prehabilitation exercise program have been demonstrated across many other specialties, especially in colorectal surgery, cardiovascular surgery, and orthopedic surgery, Dr. LaMasters said. In randomized, controlled studies, this optimization is associated with decreased complications, mortality, and length of hospital stay.
“There is actually way less data from bariatric studies. I suggest to you that our bariatric surgery patients have similar comorbidities when compared with those other specialties – specialties that refer their patients to us for treatment,” Dr. LaMasters said.
In a study of cardiorespiratory fitness before bariatric surgery, other researchers found that the most serious postoperative complications occurred more often among patients who were less fit preoperatively (Chest. 2006 Aug;130[2]:517-25). These investigators measured peak oxygen consumption (VO2) preoperatively in 109 patients. “Each unit increase in peak VO2 rate was associated with 61% decrease in overall complications,” Dr. LaMasters said. “So a small increase in fitness led to a big decrease in complications.”
Other researchers compared optimization of exercise, nutrition, and psychological factors before and after surgery in 185 patients with colorectal cancer (Acta Oncol. 2017 Feb;56[2]:295-300). A control group received the interventions postoperatively. “They found a statistically significant difference in the prehabilitation group in increased functional capacity, with more than a 30-meter improvement in 6-minute walk test before surgery,” Dr. LaMasters said. Although the 6-minute walk test results decreased 4 weeks after surgery, as might be expected, by 8 weeks the prehabilitation patients performed better than controls – and even better than their own baseline, she added. “This model of optimization can be very well applied in bariatric surgery.”
“The goal is safe surgery with outstanding long-term outcomes,” Dr. LaMasters said. “It is really not enough in this era to ‘get a patient through surgery.’ We really need to optimize the risk factors we can and identify any areas where they will have additional needs after surgery,” she added. “This will allow us to have excellent outcomes in this complex patient population.”
Dr. LaMasters and Dr. Hoyt had no relevant financial disclosures.
NEW YORK – A structured, four-pronged approach to get patients as fit and healthy as possible prior to bariatric surgery holds the potential to improve postoperative outcomes. In general, bariatric surgery patients are in a better position than most surgery candidates because of a longer preoperative period. During this time, surgeons can work with a multidisciplinary team to optimize any medical, nutritional, exercise-related, and mental health concerns.
“People focus on the size of our patients and the weight of our patients, but [body mass index] is only one factor. They can have many other comorbidities that are significant,” Dr. LaMasters said. Patients can present with cardiac and pulmonary issues, hypertension, sleep apnea, diabetes, asthma, reflux and “a very high incidence of anxiety and depression.”
“So we have a lot of challenges,” she added. “We take care of complex, high-risk patients, and our goal is to improve outcomes. Using presurgery optimization can be a key to that.”
Maximizing medical readiness
Multiple providers drive the medical intervention, Dr. LaMasters said, including surgeons and primary care doctors, as well as advanced practice providers, medical weight loss providers, and other specialists. “We do try to get patients to lose weight before surgery, but that’s not an absolute requirement. More important is adjustment of other risk factors like pulmonary risk factors, control of hypertension, treatment of sleep apnea, and control of hyperglycemia. We’d like to have their A1c [test results to be] under 8%. We want to start [proton pump inhibitors] early because there is a very high prevalence of reflux and gastritis in this population.”
Bariatric surgery patients “are uniquely positioned to have a substantial benefit from that ‘prehabilitation,’ but this only works if you have a multidisciplinary team,” Dr. LaMasters said at the American College of Surgeons Quality and Safety Conference. “Think of this as down-staging disease, like in a cancer model.”
“The message from this is there is an opportunity if we build it into the prehab phase of care. It’s a new way of thinking in surgery. You can change your results,” said session moderator David B. Hoyt, MD, FACS, Executive Director of the American College of Surgeons.
Nutritional know-how
Dietitians determine the second component – how to optimize nutrition before surgery. They focus on education, evaluation, setting goals, “and very importantly, supporting patients to attain those goals,” Dr. LaMasters said. Goals include increasing protein intake prior to surgery to a recommended 1.5 g/kg/day and starting nutritional supplements ahead of time.
Even though they typically consume an excess amount of calories, “many of our patients have baseline malnutrition,” Dr. LaMasters said. Establishing mindful behavior for meal planning, preparation, and eating is a potential solution, as is addressing any socioeconomic factors that can present challenges to healthy eating.
Emphasizing exercise
“The exercise piece is really key for our patients,” Dr. LaMasters said. Many candidates for bariatric surgery have mobility issues. “The first thing many say is ‘I can’t exercise.’ We instruct them that they can exercise. Our job is to find out what they can do – there are many different exercise modalities.”
A good baseline assessment is a 6-minute walk test to assess their distance limits, oxygen level, and any resulting symptoms.
“Our goal is to get them to walking – even those who can barely walk with a walker – for 5-10 minutes, six times a day,” Dr. LaMasters said. “We feel that is a minimum threshold to prevent blood clots after surgery.” Another recommendation is to get surgical candidates to do some activity 30 minutes a day, four times a week, at a minimum. “Eventually, after surgery and when they’ve lost weight and are healthier, the goal is going to be 1 hour, five days a week.”
Start the exercise program at least 4-8 weeks prior to surgery. Most studies show significant benefit if you start at least 4 weeks prior to surgery, Dr. LaMasters suggested. “In our own practice, we’ve seen if you can start a daily walking program even just 2 weeks prior to surgery, we see a significant benefit.”
Addressing anxiety or depression
The mental health piece is very important and should be guided by mental health providers on the multidisciplinary team, Dr. LaMasters said.
“Our patients have a high degree of stress in their lives, especially related to socioeconomic factors. A patient who does not have their anxiety or depression under control will not do as well after surgery.”
Optimization in other specialties
The benefits of a prehabilitation exercise program have been demonstrated across many other specialties, especially in colorectal surgery, cardiovascular surgery, and orthopedic surgery, Dr. LaMasters said. In randomized, controlled studies, this optimization is associated with decreased complications, mortality, and length of hospital stay.
“There is actually way less data from bariatric studies. I suggest to you that our bariatric surgery patients have similar comorbidities when compared with those other specialties – specialties that refer their patients to us for treatment,” Dr. LaMasters said.
In a study of cardiorespiratory fitness before bariatric surgery, other researchers found that the most serious postoperative complications occurred more often among patients who were less fit preoperatively (Chest. 2006 Aug;130[2]:517-25). These investigators measured peak oxygen consumption (VO2) preoperatively in 109 patients. “Each unit increase in peak VO2 rate was associated with 61% decrease in overall complications,” Dr. LaMasters said. “So a small increase in fitness led to a big decrease in complications.”
Other researchers compared optimization of exercise, nutrition, and psychological factors before and after surgery in 185 patients with colorectal cancer (Acta Oncol. 2017 Feb;56[2]:295-300). A control group received the interventions postoperatively. “They found a statistically significant difference in the prehabilitation group in increased functional capacity, with more than a 30-meter improvement in 6-minute walk test before surgery,” Dr. LaMasters said. Although the 6-minute walk test results decreased 4 weeks after surgery, as might be expected, by 8 weeks the prehabilitation patients performed better than controls – and even better than their own baseline, she added. “This model of optimization can be very well applied in bariatric surgery.”
“The goal is safe surgery with outstanding long-term outcomes,” Dr. LaMasters said. “It is really not enough in this era to ‘get a patient through surgery.’ We really need to optimize the risk factors we can and identify any areas where they will have additional needs after surgery,” she added. “This will allow us to have excellent outcomes in this complex patient population.”
Dr. LaMasters and Dr. Hoyt had no relevant financial disclosures.
AT THE ACS QUALITY & SAFETY CONFERENCE
Questions plague platelet-rich plasma’s promise
NEW YORK – If platelet-rich plasma is good enough for Kim Kardashian, what more do you need to know?
Turns out, there’s plenty to know, and plenty more that remains unknown about the procedure, which is sometimes referred to as PRP or, in Kardashian’s case, as a “vampire facial,” according to Terrence Keaney, MD, of the department of dermatology, George Washington University, Washington.
It’s easy to make: draw blood, centrifuge it, and then deliver it. The platelets themselves are not the active substances. For that, you have to look at what the platelets release from their alpha granules. They include a wealth of growth factors, including platelet-derived growth factor, transforming growth factor, vascular endothelial growth factor, epidermal growth factor, fibroblast growth factor, and connective tissue growth factor.
That’s not all. “There are 800 other bioactive molecules secreted by platelets,” including cell adhesion molecules, cytokines, antimicrobial peptides, and anti-inflammatory molecules, said Dr. Keaney, founder and director of SkinDC, in Arlington, Va. “You bring it all together, what is PRP? A growth factor/cytokine cocktail.”
But, like the cocktails one can find in a college dorm, compared with the ones found at a bar at an upscale hotel, there can be big differences – depending on who’s doing the mixing.
Still, its reputation as an all-natural, safe product has made it appealing to the public, as well as to doctors in fields beyond dermatology, he said, citing sports medicine, dentistry, otolaryngology, ophthalmology, urology, wound healing, cosmetic medicine, and cardiothoracic and maxillofacial medicine.
The Food and Drug Administration considers it a blood product, which means that it is exempt from the FDA’s traditional regulatory pathways, which would require animal studies and clinical trials. Instead, oversight falls to the FDA’s Center for Biologics Evaluation and Research, which is responsible for regulating human cells, tissues, and cellular- and tissue-based products.
A number of device makers have used the 510(k) application to bring PRP preparation systems to market. Under the application, devices that are “substantially equivalent” to a currently marketed device gain FDA clearance (J Knee Surg. 2015 Feb;28[1]:29-34). The result is that many such systems are available.
Nearly all of the devices have received clearance to produce PRP for use with bone graft materials in platelet-rich products for use by orthopedic surgeons. Other uses of the product, like stimulating hair growth, would be considered off-label.
Nevertheless, the purveyors of PRP have found people willing to part with their money in exchange for the hope that they may be able to hold on to their hair. That is not surprising, given the “pretty meager” therapeutic armamentarium available to them, Dr. Keaney said, citing minoxidil and finasteride – each of which was approved more than 20 years ago.
He bemoaned the lack of standardization for everything from platelet preparation technique to potential applications, which include facial rejuvenation, wound healing, and hair loss. “PRP has hype and it has hope, but it needs help,” he said. “There are lots of clinical questions that need to be answered.”
He added that the data remain thin. “Unfortunately, our clinical data does not match the hype around PRP,” he said, citing a recently published meta-analysis of six studies involving 177 patients (J Cosmet Dermatol. 2017 Mar 13. doi: 10.1111/jocd.12331).
Its conclusion was measured: “Platelet-rich plasma injection for local hair restoration in patients with androgenetic alopecia seems to increase hair’s number and thickness with minimal or no collateral effects. However, the current evidence does not support this treatment’s modality over hair transplantation due to the lack of established protocols,” the authors wrote. The meta-analysis results, they added, “should be interpreted with caution because it consists of pooling many small studies and larger randomized studies should be performed to verify this perception.”
Questions include how to determine the proper concentration and how many times PRP should be centrifuged, Dr. Keaney said. And it is not clear how or how often to deliver PRP. Subdermally? Via microneedle? Both? After traumatizing the skin to increase endogenous activators? Daily? Weekly? Monthly?
“We don’t know,” he said.
And, Dr. Keaney acknowledged, that may not change. “There is little incentive for industry to do a large-scale study,” he said. “If the results aren’t what they look for then you’ve killed your golden goose.”
Still, he has not been dissuaded. “From my standpoint, there’s a good scientific rationale, a proposed mechanism of action, molecular pathways.”
Though the clinical data have been variable, the studies small, and the study designs inconsistent, “there is a trend towards clinical effect,” he said. “If this is done appropriately, using appropriate systems and protocols in your office, this can be a very safe procedure – with injection site discomfort,” he said.
Dr. Keaney has spoken on behalf of a PRP preparation manufacturer.
dermnews@frontlinemedcom.com
NEW YORK – If platelet-rich plasma is good enough for Kim Kardashian, what more do you need to know?
Turns out, there’s plenty to know, and plenty more that remains unknown about the procedure, which is sometimes referred to as PRP or, in Kardashian’s case, as a “vampire facial,” according to Terrence Keaney, MD, of the department of dermatology, George Washington University, Washington.
It’s easy to make: draw blood, centrifuge it, and then deliver it. The platelets themselves are not the active substances. For that, you have to look at what the platelets release from their alpha granules. They include a wealth of growth factors, including platelet-derived growth factor, transforming growth factor, vascular endothelial growth factor, epidermal growth factor, fibroblast growth factor, and connective tissue growth factor.
That’s not all. “There are 800 other bioactive molecules secreted by platelets,” including cell adhesion molecules, cytokines, antimicrobial peptides, and anti-inflammatory molecules, said Dr. Keaney, founder and director of SkinDC, in Arlington, Va. “You bring it all together, what is PRP? A growth factor/cytokine cocktail.”
But, like the cocktails one can find in a college dorm, compared with the ones found at a bar at an upscale hotel, there can be big differences – depending on who’s doing the mixing.
Still, its reputation as an all-natural, safe product has made it appealing to the public, as well as to doctors in fields beyond dermatology, he said, citing sports medicine, dentistry, otolaryngology, ophthalmology, urology, wound healing, cosmetic medicine, and cardiothoracic and maxillofacial medicine.
The Food and Drug Administration considers it a blood product, which means that it is exempt from the FDA’s traditional regulatory pathways, which would require animal studies and clinical trials. Instead, oversight falls to the FDA’s Center for Biologics Evaluation and Research, which is responsible for regulating human cells, tissues, and cellular- and tissue-based products.
A number of device makers have used the 510(k) application to bring PRP preparation systems to market. Under the application, devices that are “substantially equivalent” to a currently marketed device gain FDA clearance (J Knee Surg. 2015 Feb;28[1]:29-34). The result is that many such systems are available.
Nearly all of the devices have received clearance to produce PRP for use with bone graft materials in platelet-rich products for use by orthopedic surgeons. Other uses of the product, like stimulating hair growth, would be considered off-label.
Nevertheless, the purveyors of PRP have found people willing to part with their money in exchange for the hope that they may be able to hold on to their hair. That is not surprising, given the “pretty meager” therapeutic armamentarium available to them, Dr. Keaney said, citing minoxidil and finasteride – each of which was approved more than 20 years ago.
He bemoaned the lack of standardization for everything from platelet preparation technique to potential applications, which include facial rejuvenation, wound healing, and hair loss. “PRP has hype and it has hope, but it needs help,” he said. “There are lots of clinical questions that need to be answered.”
He added that the data remain thin. “Unfortunately, our clinical data does not match the hype around PRP,” he said, citing a recently published meta-analysis of six studies involving 177 patients (J Cosmet Dermatol. 2017 Mar 13. doi: 10.1111/jocd.12331).
Its conclusion was measured: “Platelet-rich plasma injection for local hair restoration in patients with androgenetic alopecia seems to increase hair’s number and thickness with minimal or no collateral effects. However, the current evidence does not support this treatment’s modality over hair transplantation due to the lack of established protocols,” the authors wrote. The meta-analysis results, they added, “should be interpreted with caution because it consists of pooling many small studies and larger randomized studies should be performed to verify this perception.”
Questions include how to determine the proper concentration and how many times PRP should be centrifuged, Dr. Keaney said. And it is not clear how or how often to deliver PRP. Subdermally? Via microneedle? Both? After traumatizing the skin to increase endogenous activators? Daily? Weekly? Monthly?
“We don’t know,” he said.
And, Dr. Keaney acknowledged, that may not change. “There is little incentive for industry to do a large-scale study,” he said. “If the results aren’t what they look for then you’ve killed your golden goose.”
Still, he has not been dissuaded. “From my standpoint, there’s a good scientific rationale, a proposed mechanism of action, molecular pathways.”
Though the clinical data have been variable, the studies small, and the study designs inconsistent, “there is a trend towards clinical effect,” he said. “If this is done appropriately, using appropriate systems and protocols in your office, this can be a very safe procedure – with injection site discomfort,” he said.
Dr. Keaney has spoken on behalf of a PRP preparation manufacturer.
dermnews@frontlinemedcom.com
NEW YORK – If platelet-rich plasma is good enough for Kim Kardashian, what more do you need to know?
Turns out, there’s plenty to know, and plenty more that remains unknown about the procedure, which is sometimes referred to as PRP or, in Kardashian’s case, as a “vampire facial,” according to Terrence Keaney, MD, of the department of dermatology, George Washington University, Washington.
It’s easy to make: draw blood, centrifuge it, and then deliver it. The platelets themselves are not the active substances. For that, you have to look at what the platelets release from their alpha granules. They include a wealth of growth factors, including platelet-derived growth factor, transforming growth factor, vascular endothelial growth factor, epidermal growth factor, fibroblast growth factor, and connective tissue growth factor.
That’s not all. “There are 800 other bioactive molecules secreted by platelets,” including cell adhesion molecules, cytokines, antimicrobial peptides, and anti-inflammatory molecules, said Dr. Keaney, founder and director of SkinDC, in Arlington, Va. “You bring it all together, what is PRP? A growth factor/cytokine cocktail.”
But, like the cocktails one can find in a college dorm, compared with the ones found at a bar at an upscale hotel, there can be big differences – depending on who’s doing the mixing.
Still, its reputation as an all-natural, safe product has made it appealing to the public, as well as to doctors in fields beyond dermatology, he said, citing sports medicine, dentistry, otolaryngology, ophthalmology, urology, wound healing, cosmetic medicine, and cardiothoracic and maxillofacial medicine.
The Food and Drug Administration considers it a blood product, which means that it is exempt from the FDA’s traditional regulatory pathways, which would require animal studies and clinical trials. Instead, oversight falls to the FDA’s Center for Biologics Evaluation and Research, which is responsible for regulating human cells, tissues, and cellular- and tissue-based products.
A number of device makers have used the 510(k) application to bring PRP preparation systems to market. Under the application, devices that are “substantially equivalent” to a currently marketed device gain FDA clearance (J Knee Surg. 2015 Feb;28[1]:29-34). The result is that many such systems are available.
Nearly all of the devices have received clearance to produce PRP for use with bone graft materials in platelet-rich products for use by orthopedic surgeons. Other uses of the product, like stimulating hair growth, would be considered off-label.
Nevertheless, the purveyors of PRP have found people willing to part with their money in exchange for the hope that they may be able to hold on to their hair. That is not surprising, given the “pretty meager” therapeutic armamentarium available to them, Dr. Keaney said, citing minoxidil and finasteride – each of which was approved more than 20 years ago.
He bemoaned the lack of standardization for everything from platelet preparation technique to potential applications, which include facial rejuvenation, wound healing, and hair loss. “PRP has hype and it has hope, but it needs help,” he said. “There are lots of clinical questions that need to be answered.”
He added that the data remain thin. “Unfortunately, our clinical data does not match the hype around PRP,” he said, citing a recently published meta-analysis of six studies involving 177 patients (J Cosmet Dermatol. 2017 Mar 13. doi: 10.1111/jocd.12331).
Its conclusion was measured: “Platelet-rich plasma injection for local hair restoration in patients with androgenetic alopecia seems to increase hair’s number and thickness with minimal or no collateral effects. However, the current evidence does not support this treatment’s modality over hair transplantation due to the lack of established protocols,” the authors wrote. The meta-analysis results, they added, “should be interpreted with caution because it consists of pooling many small studies and larger randomized studies should be performed to verify this perception.”
Questions include how to determine the proper concentration and how many times PRP should be centrifuged, Dr. Keaney said. And it is not clear how or how often to deliver PRP. Subdermally? Via microneedle? Both? After traumatizing the skin to increase endogenous activators? Daily? Weekly? Monthly?
“We don’t know,” he said.
And, Dr. Keaney acknowledged, that may not change. “There is little incentive for industry to do a large-scale study,” he said. “If the results aren’t what they look for then you’ve killed your golden goose.”
Still, he has not been dissuaded. “From my standpoint, there’s a good scientific rationale, a proposed mechanism of action, molecular pathways.”
Though the clinical data have been variable, the studies small, and the study designs inconsistent, “there is a trend towards clinical effect,” he said. “If this is done appropriately, using appropriate systems and protocols in your office, this can be a very safe procedure – with injection site discomfort,” he said.
Dr. Keaney has spoken on behalf of a PRP preparation manufacturer.
dermnews@frontlinemedcom.com
AT THE 2017 AAD SUMMER MEETING