User login
Rare Cancer Gets Timely Right Treatment
Be careful not to assume multiple cancerous lesions are advanced stage metastatic cancer, caution clinicians from H Plus Yangji Hospital in Seoul, and Inje University Haeundae Paik Hospital in Busan, both in the Republic of Korea. They came close to making that mistake.
Related: A Mysterious Massive Hemorrhage
The researchers reported on a patient who was on the verge of getting only palliative care for advanced laryngeal cancer with multiple lung metastases, when in fact he was a candidate for curative-aim chemotherapy. Thanks to a “meticulous approach,” the researchers switched their plan in time.
The patient was referred to their hospital because of symptoms such as “foreign body sensation” and voice change. A smoker for 45 years, he stopped 5 years before. A physical examination revealed that lymph nodes in the neck, axillary, and inguinal areas were not enlarged. Blood count and laboratory data were all normal, but a computed tomography (CT) scan and MRI revealed that the patient had an unusual constellation of simultaneous triple primary cancers: laryngeal supraglottic cancer, small cell lung cancer (SCLC), and squamous cell lung cancer.
Head and neck cancer with synchronous or metachronous lung cancers during follow-up isn’t rare, the clinicians say. But their patient’s case is uncommon because the coexistence of SCLC and squamous cell lung cancer has rarely been reported.
Related: Finding Synchronous Cancers
Supraglottic laryngeal cancer is usually diagnosed in the advanced stages with cervical lymph node metastasis because of its nonspecific presenting symptoms and its anatomic characteristics, including a rich lymphatic network, the researchers say. That was why at the initial presentation they presumed the patient had advanced metastatic cancer. If there had not been “high suspicion” or effort to confirm the 2 distinct lung masses by invasive diagnostic procedures, the patient would have received the expected palliative treatment and not the curative-aim treatment.
After the patient received concurrent chemoradiation therapy with weekly cisplatin, the supraglottic laryngeal cancer was “markedly decreased” without newly developed cervical lymph node metastasis. A follow-up chest CT showed partial response for the SCLC in the left upper lobe; the squamous cell lung cancer in the right lower lobe remained stable. The patient was given additional chemotherapy with etoposide and cisplatin. He has survived without recurrence.
Related: Timeliness of Lung Cancer Diagnosis and Treatment
Source:
Kim EK, Kim JY, Kim BM, Lim SN. BMJ Case Rep. 2017;2017.
doi: 10.1136/bcr-2016-216305.
Be careful not to assume multiple cancerous lesions are advanced stage metastatic cancer, caution clinicians from H Plus Yangji Hospital in Seoul, and Inje University Haeundae Paik Hospital in Busan, both in the Republic of Korea. They came close to making that mistake.
Related: A Mysterious Massive Hemorrhage
The researchers reported on a patient who was on the verge of getting only palliative care for advanced laryngeal cancer with multiple lung metastases, when in fact he was a candidate for curative-aim chemotherapy. Thanks to a “meticulous approach,” the researchers switched their plan in time.
The patient was referred to their hospital because of symptoms such as “foreign body sensation” and voice change. A smoker for 45 years, he stopped 5 years before. A physical examination revealed that lymph nodes in the neck, axillary, and inguinal areas were not enlarged. Blood count and laboratory data were all normal, but a computed tomography (CT) scan and MRI revealed that the patient had an unusual constellation of simultaneous triple primary cancers: laryngeal supraglottic cancer, small cell lung cancer (SCLC), and squamous cell lung cancer.
Head and neck cancer with synchronous or metachronous lung cancers during follow-up isn’t rare, the clinicians say. But their patient’s case is uncommon because the coexistence of SCLC and squamous cell lung cancer has rarely been reported.
Related: Finding Synchronous Cancers
Supraglottic laryngeal cancer is usually diagnosed in the advanced stages with cervical lymph node metastasis because of its nonspecific presenting symptoms and its anatomic characteristics, including a rich lymphatic network, the researchers say. That was why at the initial presentation they presumed the patient had advanced metastatic cancer. If there had not been “high suspicion” or effort to confirm the 2 distinct lung masses by invasive diagnostic procedures, the patient would have received the expected palliative treatment and not the curative-aim treatment.
After the patient received concurrent chemoradiation therapy with weekly cisplatin, the supraglottic laryngeal cancer was “markedly decreased” without newly developed cervical lymph node metastasis. A follow-up chest CT showed partial response for the SCLC in the left upper lobe; the squamous cell lung cancer in the right lower lobe remained stable. The patient was given additional chemotherapy with etoposide and cisplatin. He has survived without recurrence.
Related: Timeliness of Lung Cancer Diagnosis and Treatment
Source:
Kim EK, Kim JY, Kim BM, Lim SN. BMJ Case Rep. 2017;2017.
doi: 10.1136/bcr-2016-216305.
Be careful not to assume multiple cancerous lesions are advanced stage metastatic cancer, caution clinicians from H Plus Yangji Hospital in Seoul, and Inje University Haeundae Paik Hospital in Busan, both in the Republic of Korea. They came close to making that mistake.
Related: A Mysterious Massive Hemorrhage
The researchers reported on a patient who was on the verge of getting only palliative care for advanced laryngeal cancer with multiple lung metastases, when in fact he was a candidate for curative-aim chemotherapy. Thanks to a “meticulous approach,” the researchers switched their plan in time.
The patient was referred to their hospital because of symptoms such as “foreign body sensation” and voice change. A smoker for 45 years, he stopped 5 years before. A physical examination revealed that lymph nodes in the neck, axillary, and inguinal areas were not enlarged. Blood count and laboratory data were all normal, but a computed tomography (CT) scan and MRI revealed that the patient had an unusual constellation of simultaneous triple primary cancers: laryngeal supraglottic cancer, small cell lung cancer (SCLC), and squamous cell lung cancer.
Head and neck cancer with synchronous or metachronous lung cancers during follow-up isn’t rare, the clinicians say. But their patient’s case is uncommon because the coexistence of SCLC and squamous cell lung cancer has rarely been reported.
Related: Finding Synchronous Cancers
Supraglottic laryngeal cancer is usually diagnosed in the advanced stages with cervical lymph node metastasis because of its nonspecific presenting symptoms and its anatomic characteristics, including a rich lymphatic network, the researchers say. That was why at the initial presentation they presumed the patient had advanced metastatic cancer. If there had not been “high suspicion” or effort to confirm the 2 distinct lung masses by invasive diagnostic procedures, the patient would have received the expected palliative treatment and not the curative-aim treatment.
After the patient received concurrent chemoradiation therapy with weekly cisplatin, the supraglottic laryngeal cancer was “markedly decreased” without newly developed cervical lymph node metastasis. A follow-up chest CT showed partial response for the SCLC in the left upper lobe; the squamous cell lung cancer in the right lower lobe remained stable. The patient was given additional chemotherapy with etoposide and cisplatin. He has survived without recurrence.
Related: Timeliness of Lung Cancer Diagnosis and Treatment
Source:
Kim EK, Kim JY, Kim BM, Lim SN. BMJ Case Rep. 2017;2017.
doi: 10.1136/bcr-2016-216305.
Warfarin, aspirin linked to higher risk of ICH in AFib
An analysis of data from the ARISTOTLE trial suggests patients with atrial fibrillation (AFib) have a higher risk of intracranial hemorrhage (ICH) when they receive warfarin as opposed to apixaban.
The analysis also indicates that concomitant aspirin use increases the risk of ICH among AFib patients taking either warfarin or apixaban as stroke prophylaxis.
“We know that aspirin has only a modest effect in preventing stroke in atrial fibrillation patients, yet it was one of the top predictors of intracranial hemorrhage,” said study author Renato D. Lopes, MD, PhD, of Duke Clinical Research Institute in Durham, North Carolina.
“Our finding demonstrates that aspirin is not as safe as one might think.”
Dr Lopes and his colleagues reported this finding in Blood.
Previous results from the ARISTOTLE trial, published in 2011, suggested that apixaban was superior to warfarin in terms of preventing stroke and systemic embolism, reducing bleeding, and reducing mortality in AFib patients.
However, a report published in 2013 suggested the results of this trial may have been affected by patients at 1 or more clinical trial sites receiving the wrong medication or dose, adverse events going unreported, and records being changed.
Bristol-Myers Squibb and Pfizer (funders of ARISTOTLE) said these issues likely had a negligible effect on the trial results. And the US Food and Drug Administration agreed when it approved apixaban for use in AFib patients in 2013.
Current analysis
For the current analysis, researchers used ARISTOTLE data to investigate the frequency and characteristics of ICH. They analyzed data from 18,140 AFib patients treated with warfarin or apixaban at sites in North America, Latin America, Europe, and Asia.
In all, 174 patients developed ICH. Most of these bleeds were spontaneous (71.2%) rather than traumatic (28.8%).
Apixaban vs warfarin
Patients randomized to receive apixaban had a lower incidence of ICH than those randomized to warfarin—0.33% per year and 0.80% per year, respectively.
The risk of ICH was significantly lower in the apixaban arm than the warfarin arm (hazard ratio [HR]=0.42, 95% CI 0.30–0.58; P<0.0001). The same was true for spontaneous ICH (HR=0.52, 95% CI 190 0.35–0.75; P=0.0006) and traumatic ICH (HR=0.26, 95% CI 0.13–0.53; P=0.0002).
In the warfarin arm, the median time from the most recent international normalized ratio (INR) test to ICH was 13 days (range, 6-21).
Most patients (78.5%) in the warfarin arm who developed ICH had a pre-ICH INR below 3.0. The median INR was 2.6 (range, 2.1-3.0).
Other risk factors
The researchers found that several factors other than study treatment were associated with an increased risk of ICH.
Patients treated at trial sites in Asia (HR=3.19) or Latin America (HR=1.57) had an increased risk of ICH compared to patients treated in Europe.
Each 5-year increase in patient age was associated with an increased risk of ICH (HR=1.25.)
Patients with a prior stroke or transient ischemic attack (HR=1.83) had an increased risk of ICH, as did patients receiving aspirin at baseline (HR=1.37).
The researchers said the increased risk of ICH associated with aspirin use was particularly pronounced in older patients. ![]()
An analysis of data from the ARISTOTLE trial suggests patients with atrial fibrillation (AFib) have a higher risk of intracranial hemorrhage (ICH) when they receive warfarin as opposed to apixaban.
The analysis also indicates that concomitant aspirin use increases the risk of ICH among AFib patients taking either warfarin or apixaban as stroke prophylaxis.
“We know that aspirin has only a modest effect in preventing stroke in atrial fibrillation patients, yet it was one of the top predictors of intracranial hemorrhage,” said study author Renato D. Lopes, MD, PhD, of Duke Clinical Research Institute in Durham, North Carolina.
“Our finding demonstrates that aspirin is not as safe as one might think.”
Dr Lopes and his colleagues reported this finding in Blood.
Previous results from the ARISTOTLE trial, published in 2011, suggested that apixaban was superior to warfarin in terms of preventing stroke and systemic embolism, reducing bleeding, and reducing mortality in AFib patients.
However, a report published in 2013 suggested the results of this trial may have been affected by patients at 1 or more clinical trial sites receiving the wrong medication or dose, adverse events going unreported, and records being changed.
Bristol-Myers Squibb and Pfizer (funders of ARISTOTLE) said these issues likely had a negligible effect on the trial results. And the US Food and Drug Administration agreed when it approved apixaban for use in AFib patients in 2013.
Current analysis
For the current analysis, researchers used ARISTOTLE data to investigate the frequency and characteristics of ICH. They analyzed data from 18,140 AFib patients treated with warfarin or apixaban at sites in North America, Latin America, Europe, and Asia.
In all, 174 patients developed ICH. Most of these bleeds were spontaneous (71.2%) rather than traumatic (28.8%).
Apixaban vs warfarin
Patients randomized to receive apixaban had a lower incidence of ICH than those randomized to warfarin—0.33% per year and 0.80% per year, respectively.
The risk of ICH was significantly lower in the apixaban arm than the warfarin arm (hazard ratio [HR]=0.42, 95% CI 0.30–0.58; P<0.0001). The same was true for spontaneous ICH (HR=0.52, 95% CI 190 0.35–0.75; P=0.0006) and traumatic ICH (HR=0.26, 95% CI 0.13–0.53; P=0.0002).
In the warfarin arm, the median time from the most recent international normalized ratio (INR) test to ICH was 13 days (range, 6-21).
Most patients (78.5%) in the warfarin arm who developed ICH had a pre-ICH INR below 3.0. The median INR was 2.6 (range, 2.1-3.0).
Other risk factors
The researchers found that several factors other than study treatment were associated with an increased risk of ICH.
Patients treated at trial sites in Asia (HR=3.19) or Latin America (HR=1.57) had an increased risk of ICH compared to patients treated in Europe.
Each 5-year increase in patient age was associated with an increased risk of ICH (HR=1.25.)
Patients with a prior stroke or transient ischemic attack (HR=1.83) had an increased risk of ICH, as did patients receiving aspirin at baseline (HR=1.37).
The researchers said the increased risk of ICH associated with aspirin use was particularly pronounced in older patients. ![]()
An analysis of data from the ARISTOTLE trial suggests patients with atrial fibrillation (AFib) have a higher risk of intracranial hemorrhage (ICH) when they receive warfarin as opposed to apixaban.
The analysis also indicates that concomitant aspirin use increases the risk of ICH among AFib patients taking either warfarin or apixaban as stroke prophylaxis.
“We know that aspirin has only a modest effect in preventing stroke in atrial fibrillation patients, yet it was one of the top predictors of intracranial hemorrhage,” said study author Renato D. Lopes, MD, PhD, of Duke Clinical Research Institute in Durham, North Carolina.
“Our finding demonstrates that aspirin is not as safe as one might think.”
Dr Lopes and his colleagues reported this finding in Blood.
Previous results from the ARISTOTLE trial, published in 2011, suggested that apixaban was superior to warfarin in terms of preventing stroke and systemic embolism, reducing bleeding, and reducing mortality in AFib patients.
However, a report published in 2013 suggested the results of this trial may have been affected by patients at 1 or more clinical trial sites receiving the wrong medication or dose, adverse events going unreported, and records being changed.
Bristol-Myers Squibb and Pfizer (funders of ARISTOTLE) said these issues likely had a negligible effect on the trial results. And the US Food and Drug Administration agreed when it approved apixaban for use in AFib patients in 2013.
Current analysis
For the current analysis, researchers used ARISTOTLE data to investigate the frequency and characteristics of ICH. They analyzed data from 18,140 AFib patients treated with warfarin or apixaban at sites in North America, Latin America, Europe, and Asia.
In all, 174 patients developed ICH. Most of these bleeds were spontaneous (71.2%) rather than traumatic (28.8%).
Apixaban vs warfarin
Patients randomized to receive apixaban had a lower incidence of ICH than those randomized to warfarin—0.33% per year and 0.80% per year, respectively.
The risk of ICH was significantly lower in the apixaban arm than the warfarin arm (hazard ratio [HR]=0.42, 95% CI 0.30–0.58; P<0.0001). The same was true for spontaneous ICH (HR=0.52, 95% CI 190 0.35–0.75; P=0.0006) and traumatic ICH (HR=0.26, 95% CI 0.13–0.53; P=0.0002).
In the warfarin arm, the median time from the most recent international normalized ratio (INR) test to ICH was 13 days (range, 6-21).
Most patients (78.5%) in the warfarin arm who developed ICH had a pre-ICH INR below 3.0. The median INR was 2.6 (range, 2.1-3.0).
Other risk factors
The researchers found that several factors other than study treatment were associated with an increased risk of ICH.
Patients treated at trial sites in Asia (HR=3.19) or Latin America (HR=1.57) had an increased risk of ICH compared to patients treated in Europe.
Each 5-year increase in patient age was associated with an increased risk of ICH (HR=1.25.)
Patients with a prior stroke or transient ischemic attack (HR=1.83) had an increased risk of ICH, as did patients receiving aspirin at baseline (HR=1.37).
The researchers said the increased risk of ICH associated with aspirin use was particularly pronounced in older patients. ![]()
Studies support Zika screening in entire US blood supply
Results from a pair of studies suggest it is necessary to test for Zika virus in all blood donated in the US, even blood collected outside areas of active Zika transmission.
Last year, the US Food and Drug Administration recommended that all states and US territories screen donated whole blood and blood components for the Zika virus.
Two studies published in Transfusion support that recommendation by revealing the presence of blood donors who tested positive for Zika and may have acquired the infection via travel or sexual contact.
In the first study, researchers screened donor plasma samples using the cobas Zika test. Some of the researchers are employees/contractors of Roche Molecular Systems, Inc., which developed the test.
The study included 358,786 blood donations made in US states. Plasma samples from 23 of the donors were reactive on the first test.
For these cases, the testing lab performed repeat tests with cobas Zika. The lab also simulated minipool testing by diluting a donor sample 1:6 with Zika-negative human plasma. In addition, the reactive samples were sent out for alternate nucleic acid testing and serology testing.
The additional tests suggested 14 of the samples were positive for the Zika virus.
Ten of the 14 donors said they had traveled to an area of active Zika transmission within 90 days of their donation, and 3 of the 10 donors also had a sexual exposure risk. The median time from the end of the donors’ travel to their donation was 25 days (range, 6-71).
Three donors had not traveled to an area of active Zika transmission outside the US, but they lived in Miami-Dade County and were thought to have contracted the virus there.
For the remaining donor, there was no information on travel or sexual exposure risk.
The researchers said minipool testing likely would have identified half of the Zika-positive donations, as only 7 of the 14 donations with probable Zika virus infection were detectable via the simulated minipool testing.
The team also said the estimated specificity of the cobas Zika test was 99.997%.
In the second study, researchers screened donor plasma samples using the Procleix Zika virus assay. Some of the researchers are employees/contractors of Hologic, Inc., and Grifols Diagnostic Solutions, Inc., the companies that co-developed the assay.
The study included 466,834 blood donations in the US (outside of Puerto Rico and Florida). Twenty donor samples were reactive on the initial test.
These 20 samples (and additional samples from these donors) underwent subsequent testing with the Procleix Zika virus assay, real-time polymerase chain reaction, and Zika virus IgG and IgM capture ELISAs.
According to subsequent tests, 5 donors were reactive for Zika virus RNA. All of these donations were collected outside areas of active Zika transmission, but all 5 donors had traveled to areas of active transmission.
The researchers said the estimated specificity of the Procleix Zika virus assay was 99.997%.
The team also reported transfusion of an apheresis platelet donation from 1 of the 5 Zika-positive donors. The recipient of this product did not develop Zika infection, which suggests these units may not be infectious.
However, other researchers previously reported what they believed to be transmission of the Zika virus via platelet transfusion. ![]()
Results from a pair of studies suggest it is necessary to test for Zika virus in all blood donated in the US, even blood collected outside areas of active Zika transmission.
Last year, the US Food and Drug Administration recommended that all states and US territories screen donated whole blood and blood components for the Zika virus.
Two studies published in Transfusion support that recommendation by revealing the presence of blood donors who tested positive for Zika and may have acquired the infection via travel or sexual contact.
In the first study, researchers screened donor plasma samples using the cobas Zika test. Some of the researchers are employees/contractors of Roche Molecular Systems, Inc., which developed the test.
The study included 358,786 blood donations made in US states. Plasma samples from 23 of the donors were reactive on the first test.
For these cases, the testing lab performed repeat tests with cobas Zika. The lab also simulated minipool testing by diluting a donor sample 1:6 with Zika-negative human plasma. In addition, the reactive samples were sent out for alternate nucleic acid testing and serology testing.
The additional tests suggested 14 of the samples were positive for the Zika virus.
Ten of the 14 donors said they had traveled to an area of active Zika transmission within 90 days of their donation, and 3 of the 10 donors also had a sexual exposure risk. The median time from the end of the donors’ travel to their donation was 25 days (range, 6-71).
Three donors had not traveled to an area of active Zika transmission outside the US, but they lived in Miami-Dade County and were thought to have contracted the virus there.
For the remaining donor, there was no information on travel or sexual exposure risk.
The researchers said minipool testing likely would have identified half of the Zika-positive donations, as only 7 of the 14 donations with probable Zika virus infection were detectable via the simulated minipool testing.
The team also said the estimated specificity of the cobas Zika test was 99.997%.
In the second study, researchers screened donor plasma samples using the Procleix Zika virus assay. Some of the researchers are employees/contractors of Hologic, Inc., and Grifols Diagnostic Solutions, Inc., the companies that co-developed the assay.
The study included 466,834 blood donations in the US (outside of Puerto Rico and Florida). Twenty donor samples were reactive on the initial test.
These 20 samples (and additional samples from these donors) underwent subsequent testing with the Procleix Zika virus assay, real-time polymerase chain reaction, and Zika virus IgG and IgM capture ELISAs.
According to subsequent tests, 5 donors were reactive for Zika virus RNA. All of these donations were collected outside areas of active Zika transmission, but all 5 donors had traveled to areas of active transmission.
The researchers said the estimated specificity of the Procleix Zika virus assay was 99.997%.
The team also reported transfusion of an apheresis platelet donation from 1 of the 5 Zika-positive donors. The recipient of this product did not develop Zika infection, which suggests these units may not be infectious.
However, other researchers previously reported what they believed to be transmission of the Zika virus via platelet transfusion. ![]()
Results from a pair of studies suggest it is necessary to test for Zika virus in all blood donated in the US, even blood collected outside areas of active Zika transmission.
Last year, the US Food and Drug Administration recommended that all states and US territories screen donated whole blood and blood components for the Zika virus.
Two studies published in Transfusion support that recommendation by revealing the presence of blood donors who tested positive for Zika and may have acquired the infection via travel or sexual contact.
In the first study, researchers screened donor plasma samples using the cobas Zika test. Some of the researchers are employees/contractors of Roche Molecular Systems, Inc., which developed the test.
The study included 358,786 blood donations made in US states. Plasma samples from 23 of the donors were reactive on the first test.
For these cases, the testing lab performed repeat tests with cobas Zika. The lab also simulated minipool testing by diluting a donor sample 1:6 with Zika-negative human plasma. In addition, the reactive samples were sent out for alternate nucleic acid testing and serology testing.
The additional tests suggested 14 of the samples were positive for the Zika virus.
Ten of the 14 donors said they had traveled to an area of active Zika transmission within 90 days of their donation, and 3 of the 10 donors also had a sexual exposure risk. The median time from the end of the donors’ travel to their donation was 25 days (range, 6-71).
Three donors had not traveled to an area of active Zika transmission outside the US, but they lived in Miami-Dade County and were thought to have contracted the virus there.
For the remaining donor, there was no information on travel or sexual exposure risk.
The researchers said minipool testing likely would have identified half of the Zika-positive donations, as only 7 of the 14 donations with probable Zika virus infection were detectable via the simulated minipool testing.
The team also said the estimated specificity of the cobas Zika test was 99.997%.
In the second study, researchers screened donor plasma samples using the Procleix Zika virus assay. Some of the researchers are employees/contractors of Hologic, Inc., and Grifols Diagnostic Solutions, Inc., the companies that co-developed the assay.
The study included 466,834 blood donations in the US (outside of Puerto Rico and Florida). Twenty donor samples were reactive on the initial test.
These 20 samples (and additional samples from these donors) underwent subsequent testing with the Procleix Zika virus assay, real-time polymerase chain reaction, and Zika virus IgG and IgM capture ELISAs.
According to subsequent tests, 5 donors were reactive for Zika virus RNA. All of these donations were collected outside areas of active Zika transmission, but all 5 donors had traveled to areas of active transmission.
The researchers said the estimated specificity of the Procleix Zika virus assay was 99.997%.
The team also reported transfusion of an apheresis platelet donation from 1 of the 5 Zika-positive donors. The recipient of this product did not develop Zika infection, which suggests these units may not be infectious.
However, other researchers previously reported what they believed to be transmission of the Zika virus via platelet transfusion. ![]()
FDA grants priority review for BLA of CAR T-cell therapy
The US Food and Drug Administration (FDA) has granted priority review for a biologics license application (BLA) for CTL019 (tisagenlecleucel-T), an investigational chimeric antigen receptor (CAR) T-cell therapy.
The BLA is for CTL019 as a treatment for pediatric patients and young adults with relapsed or refractory B-cell acute lymphoblastic leukemia (ALL).
The FDA grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
The agency’s goal is to take action on a priority review application within 6 months of receiving it, rather than the standard 10 months.
The FDA accepted the BLA for CTL019 yesterday, according to Novartis.
About CTL019
CTL019 consists of autologous T cells expressing a CD19-specific CAR. The therapy was first developed by the University of Pennsylvania.
In 2012, the university and Novartis entered into a global collaboration to further research, develop, and commercialize CAR-T cell therapies, including CTL019. Novartis holds the worldwide rights to CARs developed through the collaboration.
CTL019 already has breakthrough therapy designation for the treatment of adults and children with relapsed/refractory ALL.
Novartis said it is planning additional filings for CTL019 in the US and European Union later this year, including a BLA with the FDA for the treatment of adults with relapsed/refractory diffuse large B-cell lymphoma (DLBCL) and applications for marketing authorization with the European Medicines Agency in relapsed/refractory B-cell ALL and relapsed/refractory DLBCL.
Trials of CTL019 in ALL
The priority review designation for CTL019 is based on results from the Novartis-sponsored ELIANA study (NCT02435849). Results from this international, phase 2 trial were presented at ASH 2016.
The trial enrolled patients who had CD19-positive B-ALL with morphologic marrow tumor involvement at registration (>5% blasts) and were primary refractory, were chemo-refractory after first relapse, had relapsed after second-line therapy, or were ineligible for allogeneic hematopoietic stem cell transplant.
Most patients received fludarabine/cyclophosphamide lymphodepleting chemotherapy followed by a single dose of CTL019.
Three months post-infusion, 82% of patients (41/50) had achieved a complete response or complete response with incomplete blood count recovery.
Nearly half of the patients in the trial (48%) experienced grade 3/4 cytokine release syndrome (CRS), though there were no deaths due to CRS. Fifteen percent of patients experienced grade 3 neurological and psychiatric events, including confusion, delirium, encephalopathy, agitation, and seizure.
The BLA for CTL019 is also supported by results from the phase 2 ENSIGN trial, which were presented at ASH 2016, and results of a pilot study in patients with relapsed/refractory ALL, which were presented at ASH 2015. ![]()
The US Food and Drug Administration (FDA) has granted priority review for a biologics license application (BLA) for CTL019 (tisagenlecleucel-T), an investigational chimeric antigen receptor (CAR) T-cell therapy.
The BLA is for CTL019 as a treatment for pediatric patients and young adults with relapsed or refractory B-cell acute lymphoblastic leukemia (ALL).
The FDA grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
The agency’s goal is to take action on a priority review application within 6 months of receiving it, rather than the standard 10 months.
The FDA accepted the BLA for CTL019 yesterday, according to Novartis.
About CTL019
CTL019 consists of autologous T cells expressing a CD19-specific CAR. The therapy was first developed by the University of Pennsylvania.
In 2012, the university and Novartis entered into a global collaboration to further research, develop, and commercialize CAR-T cell therapies, including CTL019. Novartis holds the worldwide rights to CARs developed through the collaboration.
CTL019 already has breakthrough therapy designation for the treatment of adults and children with relapsed/refractory ALL.
Novartis said it is planning additional filings for CTL019 in the US and European Union later this year, including a BLA with the FDA for the treatment of adults with relapsed/refractory diffuse large B-cell lymphoma (DLBCL) and applications for marketing authorization with the European Medicines Agency in relapsed/refractory B-cell ALL and relapsed/refractory DLBCL.
Trials of CTL019 in ALL
The priority review designation for CTL019 is based on results from the Novartis-sponsored ELIANA study (NCT02435849). Results from this international, phase 2 trial were presented at ASH 2016.
The trial enrolled patients who had CD19-positive B-ALL with morphologic marrow tumor involvement at registration (>5% blasts) and were primary refractory, were chemo-refractory after first relapse, had relapsed after second-line therapy, or were ineligible for allogeneic hematopoietic stem cell transplant.
Most patients received fludarabine/cyclophosphamide lymphodepleting chemotherapy followed by a single dose of CTL019.
Three months post-infusion, 82% of patients (41/50) had achieved a complete response or complete response with incomplete blood count recovery.
Nearly half of the patients in the trial (48%) experienced grade 3/4 cytokine release syndrome (CRS), though there were no deaths due to CRS. Fifteen percent of patients experienced grade 3 neurological and psychiatric events, including confusion, delirium, encephalopathy, agitation, and seizure.
The BLA for CTL019 is also supported by results from the phase 2 ENSIGN trial, which were presented at ASH 2016, and results of a pilot study in patients with relapsed/refractory ALL, which were presented at ASH 2015. ![]()
The US Food and Drug Administration (FDA) has granted priority review for a biologics license application (BLA) for CTL019 (tisagenlecleucel-T), an investigational chimeric antigen receptor (CAR) T-cell therapy.
The BLA is for CTL019 as a treatment for pediatric patients and young adults with relapsed or refractory B-cell acute lymphoblastic leukemia (ALL).
The FDA grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
The agency’s goal is to take action on a priority review application within 6 months of receiving it, rather than the standard 10 months.
The FDA accepted the BLA for CTL019 yesterday, according to Novartis.
About CTL019
CTL019 consists of autologous T cells expressing a CD19-specific CAR. The therapy was first developed by the University of Pennsylvania.
In 2012, the university and Novartis entered into a global collaboration to further research, develop, and commercialize CAR-T cell therapies, including CTL019. Novartis holds the worldwide rights to CARs developed through the collaboration.
CTL019 already has breakthrough therapy designation for the treatment of adults and children with relapsed/refractory ALL.
Novartis said it is planning additional filings for CTL019 in the US and European Union later this year, including a BLA with the FDA for the treatment of adults with relapsed/refractory diffuse large B-cell lymphoma (DLBCL) and applications for marketing authorization with the European Medicines Agency in relapsed/refractory B-cell ALL and relapsed/refractory DLBCL.
Trials of CTL019 in ALL
The priority review designation for CTL019 is based on results from the Novartis-sponsored ELIANA study (NCT02435849). Results from this international, phase 2 trial were presented at ASH 2016.
The trial enrolled patients who had CD19-positive B-ALL with morphologic marrow tumor involvement at registration (>5% blasts) and were primary refractory, were chemo-refractory after first relapse, had relapsed after second-line therapy, or were ineligible for allogeneic hematopoietic stem cell transplant.
Most patients received fludarabine/cyclophosphamide lymphodepleting chemotherapy followed by a single dose of CTL019.
Three months post-infusion, 82% of patients (41/50) had achieved a complete response or complete response with incomplete blood count recovery.
Nearly half of the patients in the trial (48%) experienced grade 3/4 cytokine release syndrome (CRS), though there were no deaths due to CRS. Fifteen percent of patients experienced grade 3 neurological and psychiatric events, including confusion, delirium, encephalopathy, agitation, and seizure.
The BLA for CTL019 is also supported by results from the phase 2 ENSIGN trial, which were presented at ASH 2016, and results of a pilot study in patients with relapsed/refractory ALL, which were presented at ASH 2015. ![]()
Team identifies proteins that give malaria parasites ‘superpower’
Researchers say they have identified proteins that enable the malaria parasite Plasmodium falciparum to “walk through cell walls.”
The team believes the proteins—SPECT and PLP1—could be targeted to develop antimalarial drugs or vaccines.
Justin Boddey, PhD, of the Walter and Eliza Hall Institute of Medical Research in Parkville, Victoria, Australia, and his colleagues described this research in Cell Reports.
“The malaria infection cycle begins with a mosquito bite, when parasites are injected into the skin, and then rapidly move to the liver,” Dr Boddey explained. “We have shown that P falciparum employs a technique called cell traversal to quickly move through host cells in their path as they seek out liver cells to infect.”
“Our study identified that P falciparum parasites traverse human cells—effectively walking through cell walls—using 2 proteins called SPECT and PLP1 to achieve this superpower. This allows parasites to get from the skin to the liver very quickly following a mosquito bite.”
Dr Boddey said pinpointing these proteins was a good avenue for new therapies.
“Our long-term goal is to eradicate malaria, so we have to look at ways of breaking the cycle of infection,” he said. “A vaccine or treatment that halts the liver-stage infection offers the best chance of eradication because it stops parasites before they take hold.” ![]()
Researchers say they have identified proteins that enable the malaria parasite Plasmodium falciparum to “walk through cell walls.”
The team believes the proteins—SPECT and PLP1—could be targeted to develop antimalarial drugs or vaccines.
Justin Boddey, PhD, of the Walter and Eliza Hall Institute of Medical Research in Parkville, Victoria, Australia, and his colleagues described this research in Cell Reports.
“The malaria infection cycle begins with a mosquito bite, when parasites are injected into the skin, and then rapidly move to the liver,” Dr Boddey explained. “We have shown that P falciparum employs a technique called cell traversal to quickly move through host cells in their path as they seek out liver cells to infect.”
“Our study identified that P falciparum parasites traverse human cells—effectively walking through cell walls—using 2 proteins called SPECT and PLP1 to achieve this superpower. This allows parasites to get from the skin to the liver very quickly following a mosquito bite.”
Dr Boddey said pinpointing these proteins was a good avenue for new therapies.
“Our long-term goal is to eradicate malaria, so we have to look at ways of breaking the cycle of infection,” he said. “A vaccine or treatment that halts the liver-stage infection offers the best chance of eradication because it stops parasites before they take hold.” ![]()
Researchers say they have identified proteins that enable the malaria parasite Plasmodium falciparum to “walk through cell walls.”
The team believes the proteins—SPECT and PLP1—could be targeted to develop antimalarial drugs or vaccines.
Justin Boddey, PhD, of the Walter and Eliza Hall Institute of Medical Research in Parkville, Victoria, Australia, and his colleagues described this research in Cell Reports.
“The malaria infection cycle begins with a mosquito bite, when parasites are injected into the skin, and then rapidly move to the liver,” Dr Boddey explained. “We have shown that P falciparum employs a technique called cell traversal to quickly move through host cells in their path as they seek out liver cells to infect.”
“Our study identified that P falciparum parasites traverse human cells—effectively walking through cell walls—using 2 proteins called SPECT and PLP1 to achieve this superpower. This allows parasites to get from the skin to the liver very quickly following a mosquito bite.”
Dr Boddey said pinpointing these proteins was a good avenue for new therapies.
“Our long-term goal is to eradicate malaria, so we have to look at ways of breaking the cycle of infection,” he said. “A vaccine or treatment that halts the liver-stage infection offers the best chance of eradication because it stops parasites before they take hold.” ![]()
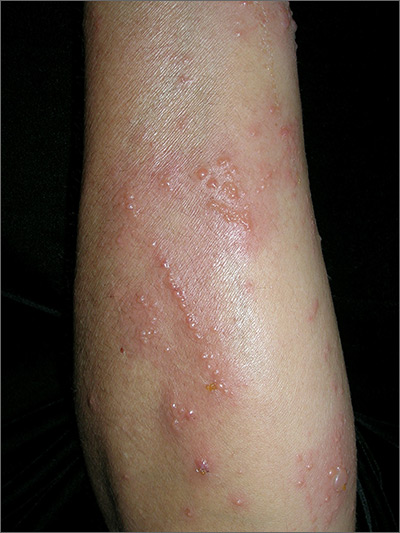
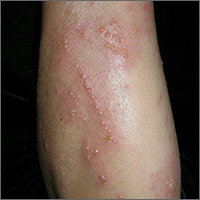
Intensely pruritic rash
The linear pattern of the vesicles and their distribution on the patient’s arms prompted the family physician (FP) to suspect that this was a case of allergic contact dermatitis (ACD) caused by exposure to a plant. While drug eruptions can cause all kinds of rashes—including vesicular eruptions—it would be rare for them to cause a perfect linear pattern. Upon further questioning, the FP learned that the patient had been gardening in her backyard a few days before the eruption started. This additional information supported a diagnosis of Rhus dermatitis from poison ivy. (Depending on the plants growing in the region, it could also have been poison oak.)
Toxicodendron (Rhus) dermatitis (poison ivy, poison oak, and poison sumac) is caused by urushiol, which is found in the sap of this plant family. Clinically, a line of vesicles can occur from brushing against one of the plants. The linear pattern can also occur from scratching and dragging the urushiol across pruritic skin with the fingernails. If ACD involves extensive skin areas (>20%), systemic steroid therapy is often required and offers relief within 12 to 24 hours. Severe poison ivy/oak is treated with oral prednisone for 2 to 3 weeks. Methylprednisolone should be avoided because the dose and duration are insufficient and can lead to a rebound contact dermatitis at the end of the short course.
In this case, the patient didn’t need an oral steroid. The patient was happy knowing the diagnosis and that the eruption would go away spontaneously. The FP suggested over-the-counter calamine lotion to sooth the itching and also asked the patient if she wanted a prescription for a topical steroid. The patient said that she would like one as a backup in case the over-the-counter lotion didn’t work, so the FP gave her a prescription for 0.1% triamcinolone cream to be applied once to twice daily.
While the evidence for topical steroids in Rhus dermatitis isn’t strong, the risk of adverse effects from topical steroids is much less than the risks associated with weeks of an oral steroid. During a future visit for her hypertension, the patient indicated that the poison ivy had gone away uneventfully.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Usatine R. Contact dermatitis. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:591-596.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
The linear pattern of the vesicles and their distribution on the patient’s arms prompted the family physician (FP) to suspect that this was a case of allergic contact dermatitis (ACD) caused by exposure to a plant. While drug eruptions can cause all kinds of rashes—including vesicular eruptions—it would be rare for them to cause a perfect linear pattern. Upon further questioning, the FP learned that the patient had been gardening in her backyard a few days before the eruption started. This additional information supported a diagnosis of Rhus dermatitis from poison ivy. (Depending on the plants growing in the region, it could also have been poison oak.)
Toxicodendron (Rhus) dermatitis (poison ivy, poison oak, and poison sumac) is caused by urushiol, which is found in the sap of this plant family. Clinically, a line of vesicles can occur from brushing against one of the plants. The linear pattern can also occur from scratching and dragging the urushiol across pruritic skin with the fingernails. If ACD involves extensive skin areas (>20%), systemic steroid therapy is often required and offers relief within 12 to 24 hours. Severe poison ivy/oak is treated with oral prednisone for 2 to 3 weeks. Methylprednisolone should be avoided because the dose and duration are insufficient and can lead to a rebound contact dermatitis at the end of the short course.
In this case, the patient didn’t need an oral steroid. The patient was happy knowing the diagnosis and that the eruption would go away spontaneously. The FP suggested over-the-counter calamine lotion to sooth the itching and also asked the patient if she wanted a prescription for a topical steroid. The patient said that she would like one as a backup in case the over-the-counter lotion didn’t work, so the FP gave her a prescription for 0.1% triamcinolone cream to be applied once to twice daily.
While the evidence for topical steroids in Rhus dermatitis isn’t strong, the risk of adverse effects from topical steroids is much less than the risks associated with weeks of an oral steroid. During a future visit for her hypertension, the patient indicated that the poison ivy had gone away uneventfully.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Usatine R. Contact dermatitis. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:591-596.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
The linear pattern of the vesicles and their distribution on the patient’s arms prompted the family physician (FP) to suspect that this was a case of allergic contact dermatitis (ACD) caused by exposure to a plant. While drug eruptions can cause all kinds of rashes—including vesicular eruptions—it would be rare for them to cause a perfect linear pattern. Upon further questioning, the FP learned that the patient had been gardening in her backyard a few days before the eruption started. This additional information supported a diagnosis of Rhus dermatitis from poison ivy. (Depending on the plants growing in the region, it could also have been poison oak.)
Toxicodendron (Rhus) dermatitis (poison ivy, poison oak, and poison sumac) is caused by urushiol, which is found in the sap of this plant family. Clinically, a line of vesicles can occur from brushing against one of the plants. The linear pattern can also occur from scratching and dragging the urushiol across pruritic skin with the fingernails. If ACD involves extensive skin areas (>20%), systemic steroid therapy is often required and offers relief within 12 to 24 hours. Severe poison ivy/oak is treated with oral prednisone for 2 to 3 weeks. Methylprednisolone should be avoided because the dose and duration are insufficient and can lead to a rebound contact dermatitis at the end of the short course.
In this case, the patient didn’t need an oral steroid. The patient was happy knowing the diagnosis and that the eruption would go away spontaneously. The FP suggested over-the-counter calamine lotion to sooth the itching and also asked the patient if she wanted a prescription for a topical steroid. The patient said that she would like one as a backup in case the over-the-counter lotion didn’t work, so the FP gave her a prescription for 0.1% triamcinolone cream to be applied once to twice daily.
While the evidence for topical steroids in Rhus dermatitis isn’t strong, the risk of adverse effects from topical steroids is much less than the risks associated with weeks of an oral steroid. During a future visit for her hypertension, the patient indicated that the poison ivy had gone away uneventfully.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Usatine R. Contact dermatitis. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:591-596.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
When one patient decompensates, others on the ward may follow
Clinical question: How does the clinical decompensation of a ward patient affect the likelihood of another patient’s decompensation?
Background: Previous research has attempted to identify patient-specific characteristics for risk of decompensation, but there may be environmental factors that contribute.
Setting: Thirteen geographically distinct, adult medical-surgical wards at an academic medical center.
Synopsis: Of 83,723 admissions to medical-surgical wards, 4,286 patients experienced cardiac arrest (179) or were transferred to the ICU (4,107). When one or more of these events occurred, other patients on the same ward had an increased risk for either event over the subsequent 6 (OR 1.18; CI, 1.07-1.31) and 12 hours and the risk was higher if more than one event occurred. Importantly, for patients exposed to other patients on the same ward decompensating, there were no differences in the severity of illness for patients transferred to ICU or in overall mortality.
Intuitively, when one patient becomes critically ill, other patients receive less attention. Though the effect was small, this study highlights that diversion of resources may increase other patients to greater risk. Surprisingly, the increased risk was not significant during night-time hours, when resources are more limited, but data collection may have been also affected.
Bottom Line: When a patient becomes critically ill, another patient on the same ward is more likely to decompensate within the next 6-12 hours, but the effect is small.
Citations: Volchenboum SL, Mayampurath A, Göksu-Gürsoy G, et al. Association between in-hospital critical illness events and outcomes in patients on the same ward. JAMA. 2016;316(24):2674-5.
Dr. Anstett is Hospital Medicine Fellow in Quality and Systems Leadership, Division of Hospital Medicine, University of Colorado School of Medicine, Aurora.
Clinical question: How does the clinical decompensation of a ward patient affect the likelihood of another patient’s decompensation?
Background: Previous research has attempted to identify patient-specific characteristics for risk of decompensation, but there may be environmental factors that contribute.
Setting: Thirteen geographically distinct, adult medical-surgical wards at an academic medical center.
Synopsis: Of 83,723 admissions to medical-surgical wards, 4,286 patients experienced cardiac arrest (179) or were transferred to the ICU (4,107). When one or more of these events occurred, other patients on the same ward had an increased risk for either event over the subsequent 6 (OR 1.18; CI, 1.07-1.31) and 12 hours and the risk was higher if more than one event occurred. Importantly, for patients exposed to other patients on the same ward decompensating, there were no differences in the severity of illness for patients transferred to ICU or in overall mortality.
Intuitively, when one patient becomes critically ill, other patients receive less attention. Though the effect was small, this study highlights that diversion of resources may increase other patients to greater risk. Surprisingly, the increased risk was not significant during night-time hours, when resources are more limited, but data collection may have been also affected.
Bottom Line: When a patient becomes critically ill, another patient on the same ward is more likely to decompensate within the next 6-12 hours, but the effect is small.
Citations: Volchenboum SL, Mayampurath A, Göksu-Gürsoy G, et al. Association between in-hospital critical illness events and outcomes in patients on the same ward. JAMA. 2016;316(24):2674-5.
Dr. Anstett is Hospital Medicine Fellow in Quality and Systems Leadership, Division of Hospital Medicine, University of Colorado School of Medicine, Aurora.
Clinical question: How does the clinical decompensation of a ward patient affect the likelihood of another patient’s decompensation?
Background: Previous research has attempted to identify patient-specific characteristics for risk of decompensation, but there may be environmental factors that contribute.
Setting: Thirteen geographically distinct, adult medical-surgical wards at an academic medical center.
Synopsis: Of 83,723 admissions to medical-surgical wards, 4,286 patients experienced cardiac arrest (179) or were transferred to the ICU (4,107). When one or more of these events occurred, other patients on the same ward had an increased risk for either event over the subsequent 6 (OR 1.18; CI, 1.07-1.31) and 12 hours and the risk was higher if more than one event occurred. Importantly, for patients exposed to other patients on the same ward decompensating, there were no differences in the severity of illness for patients transferred to ICU or in overall mortality.
Intuitively, when one patient becomes critically ill, other patients receive less attention. Though the effect was small, this study highlights that diversion of resources may increase other patients to greater risk. Surprisingly, the increased risk was not significant during night-time hours, when resources are more limited, but data collection may have been also affected.
Bottom Line: When a patient becomes critically ill, another patient on the same ward is more likely to decompensate within the next 6-12 hours, but the effect is small.
Citations: Volchenboum SL, Mayampurath A, Göksu-Gürsoy G, et al. Association between in-hospital critical illness events and outcomes in patients on the same ward. JAMA. 2016;316(24):2674-5.
Dr. Anstett is Hospital Medicine Fellow in Quality and Systems Leadership, Division of Hospital Medicine, University of Colorado School of Medicine, Aurora.
Principles learned from a successful improvement program can increase compliance and reduce hospital acquired VTEs (HA-VTEs) across multiple institutions
Clinical question: Can a single institution’s VTE prophylaxis program be scaled to increase prophylaxis and reduce HA-VTEs across multiple institutions?
Study design: prospective, unblinded, open-intervention study
Setting: Inpatient medical and surgical services at five independent, cooperating academic hospitals
Synopsis: Each site used common principles to develop their own multi-pronged VTE prophylaxis program including structured order-sets, simplified risk-assessment, feedback to providers, and education programs.
306,906 inpatient discharges were evaluated with average VTE prophylaxis bundle compliance reaching 89% across all institutions. HA-VTE rates declined from 0.90% to 0.69% (RR, 0.76; CI, 0.68-0.85) – equivalent to averting 81 pulmonary emboli and 89 deep venous thrombi. Of note, HA-VTE rates only declined at three of the five institutions with the greatest improvement at those with the highest baseline rates. Further, while HA-VTE rates improved across all patient populations, the incidence reduction was statistically significant in Oncologic and Surgical populations.
Bottom Line: Hospital systems can reduce HA-VTE and increase VTE prophylaxis by implementing a bundle of interventions and these efforts are highest yield for Oncologic and Surgical populations.
Citations: Jenkins IH, White RH, Amin AN, et al. Reducing the incidence of hospital-associated venous thromboembolism within a network of academic hospitals: findings from five University of California medical centers. J Hosp Med. 2016;11:S22-8.
Dr. Anstett is Hospital Medicine Fellow in Quality and Systems Leadership, Division of Hospital Medicine, University of Colorado School of Medicine, Aurora.
Clinical question: Can a single institution’s VTE prophylaxis program be scaled to increase prophylaxis and reduce HA-VTEs across multiple institutions?
Study design: prospective, unblinded, open-intervention study
Setting: Inpatient medical and surgical services at five independent, cooperating academic hospitals
Synopsis: Each site used common principles to develop their own multi-pronged VTE prophylaxis program including structured order-sets, simplified risk-assessment, feedback to providers, and education programs.
306,906 inpatient discharges were evaluated with average VTE prophylaxis bundle compliance reaching 89% across all institutions. HA-VTE rates declined from 0.90% to 0.69% (RR, 0.76; CI, 0.68-0.85) – equivalent to averting 81 pulmonary emboli and 89 deep venous thrombi. Of note, HA-VTE rates only declined at three of the five institutions with the greatest improvement at those with the highest baseline rates. Further, while HA-VTE rates improved across all patient populations, the incidence reduction was statistically significant in Oncologic and Surgical populations.
Bottom Line: Hospital systems can reduce HA-VTE and increase VTE prophylaxis by implementing a bundle of interventions and these efforts are highest yield for Oncologic and Surgical populations.
Citations: Jenkins IH, White RH, Amin AN, et al. Reducing the incidence of hospital-associated venous thromboembolism within a network of academic hospitals: findings from five University of California medical centers. J Hosp Med. 2016;11:S22-8.
Dr. Anstett is Hospital Medicine Fellow in Quality and Systems Leadership, Division of Hospital Medicine, University of Colorado School of Medicine, Aurora.
Clinical question: Can a single institution’s VTE prophylaxis program be scaled to increase prophylaxis and reduce HA-VTEs across multiple institutions?
Study design: prospective, unblinded, open-intervention study
Setting: Inpatient medical and surgical services at five independent, cooperating academic hospitals
Synopsis: Each site used common principles to develop their own multi-pronged VTE prophylaxis program including structured order-sets, simplified risk-assessment, feedback to providers, and education programs.
306,906 inpatient discharges were evaluated with average VTE prophylaxis bundle compliance reaching 89% across all institutions. HA-VTE rates declined from 0.90% to 0.69% (RR, 0.76; CI, 0.68-0.85) – equivalent to averting 81 pulmonary emboli and 89 deep venous thrombi. Of note, HA-VTE rates only declined at three of the five institutions with the greatest improvement at those with the highest baseline rates. Further, while HA-VTE rates improved across all patient populations, the incidence reduction was statistically significant in Oncologic and Surgical populations.
Bottom Line: Hospital systems can reduce HA-VTE and increase VTE prophylaxis by implementing a bundle of interventions and these efforts are highest yield for Oncologic and Surgical populations.
Citations: Jenkins IH, White RH, Amin AN, et al. Reducing the incidence of hospital-associated venous thromboembolism within a network of academic hospitals: findings from five University of California medical centers. J Hosp Med. 2016;11:S22-8.
Dr. Anstett is Hospital Medicine Fellow in Quality and Systems Leadership, Division of Hospital Medicine, University of Colorado School of Medicine, Aurora.
Quick Poll: Cosmetic Procedures
[polldaddy:9711250]
[polldaddy:9711250]
[polldaddy:9711250]
What happens when a baked egg oral challenge is negative?
ATLANTA – The majority of patients who had cooked egg exposure following a negative physician-supervised baked egg oral challenge are tolerating cooked egg, according to a retrospective study.
However, no correlation between results and development of tolerance was identified with skin prick testing or serum IgE testing.
To find out, Dr. Peng, a second-year fellow in the department of pediatrics at the University of California, Los Angeles, and her associates identified 22 patients who underwent negative physician-supervised baked egg oral challenges from July 2011 until June 2016. They reviewed medical charts to obtain data on age, clinical history, skin prick test results, and results of serum IgE testing to egg and its components. Next, the researchers contacted patients and their families and invited them to participate in a telephone survey about patterns of baked, cooked, and raw egg exposure and associated reactions, following their negative baked challenge. The patients ranged in age from 10 months to 44 years and their mean age was 7 years.
Dr. Peng presented results from 18 of the 22 patients who were successfully contacted. A mean of 26 months had passed since their baked egg oral challenge. Of these patients, 17 (94%) have had continued exposure to egg while 15 (83%) have shown tolerance to cooked egg. The researchers observed variable patterns of baked egg intake following the negative physician-supervised baked egg challenge. “Some patients are able to tolerate cooked egg rapidly but are not interested in continuing frequent consumption,” Dr. Peng said. “They may say, ‘My 3-year-old doesn’t like scrambled eggs, so I’m not going to keep pushing them.’ They’re not considering themselves egg allergic so their quality of life is much better. I understand that tolerating baked egg is a big deal, as an allergist I want to see them do more, such as tolerating cooked egg.”
When patients were asked about adverse reactions to egg consumption, three (17%) described gastrointestinal symptoms, five (28%) described cutaneous symptoms, and three (17%) described respiratory reactions. Dr. Peng noted that of the three patients who have not achieved tolerance to cooked egg, one patient reported mild reaction to baked egg 2 weeks after the baked egg challenge, while the other two continue to have baked egg exposure but have not yet introduced cooked egg into their diets. “I hope that most pediatricians and family practice physicians consider referral to an allergist if they’re not comfortable introducing a baked egg oral challenge.”
The researchers could not identify any correlation between skin prick or serum IgE test results and development of tolerance to cooked egg. “Data in the literature suggests that serum testing is predictive [of tolerance], but it’s not 100%,” Dr. Peng said.
She reported having no relevant financial disclosures.
ATLANTA – The majority of patients who had cooked egg exposure following a negative physician-supervised baked egg oral challenge are tolerating cooked egg, according to a retrospective study.
However, no correlation between results and development of tolerance was identified with skin prick testing or serum IgE testing.
To find out, Dr. Peng, a second-year fellow in the department of pediatrics at the University of California, Los Angeles, and her associates identified 22 patients who underwent negative physician-supervised baked egg oral challenges from July 2011 until June 2016. They reviewed medical charts to obtain data on age, clinical history, skin prick test results, and results of serum IgE testing to egg and its components. Next, the researchers contacted patients and their families and invited them to participate in a telephone survey about patterns of baked, cooked, and raw egg exposure and associated reactions, following their negative baked challenge. The patients ranged in age from 10 months to 44 years and their mean age was 7 years.
Dr. Peng presented results from 18 of the 22 patients who were successfully contacted. A mean of 26 months had passed since their baked egg oral challenge. Of these patients, 17 (94%) have had continued exposure to egg while 15 (83%) have shown tolerance to cooked egg. The researchers observed variable patterns of baked egg intake following the negative physician-supervised baked egg challenge. “Some patients are able to tolerate cooked egg rapidly but are not interested in continuing frequent consumption,” Dr. Peng said. “They may say, ‘My 3-year-old doesn’t like scrambled eggs, so I’m not going to keep pushing them.’ They’re not considering themselves egg allergic so their quality of life is much better. I understand that tolerating baked egg is a big deal, as an allergist I want to see them do more, such as tolerating cooked egg.”
When patients were asked about adverse reactions to egg consumption, three (17%) described gastrointestinal symptoms, five (28%) described cutaneous symptoms, and three (17%) described respiratory reactions. Dr. Peng noted that of the three patients who have not achieved tolerance to cooked egg, one patient reported mild reaction to baked egg 2 weeks after the baked egg challenge, while the other two continue to have baked egg exposure but have not yet introduced cooked egg into their diets. “I hope that most pediatricians and family practice physicians consider referral to an allergist if they’re not comfortable introducing a baked egg oral challenge.”
The researchers could not identify any correlation between skin prick or serum IgE test results and development of tolerance to cooked egg. “Data in the literature suggests that serum testing is predictive [of tolerance], but it’s not 100%,” Dr. Peng said.
She reported having no relevant financial disclosures.
ATLANTA – The majority of patients who had cooked egg exposure following a negative physician-supervised baked egg oral challenge are tolerating cooked egg, according to a retrospective study.
However, no correlation between results and development of tolerance was identified with skin prick testing or serum IgE testing.
To find out, Dr. Peng, a second-year fellow in the department of pediatrics at the University of California, Los Angeles, and her associates identified 22 patients who underwent negative physician-supervised baked egg oral challenges from July 2011 until June 2016. They reviewed medical charts to obtain data on age, clinical history, skin prick test results, and results of serum IgE testing to egg and its components. Next, the researchers contacted patients and their families and invited them to participate in a telephone survey about patterns of baked, cooked, and raw egg exposure and associated reactions, following their negative baked challenge. The patients ranged in age from 10 months to 44 years and their mean age was 7 years.
Dr. Peng presented results from 18 of the 22 patients who were successfully contacted. A mean of 26 months had passed since their baked egg oral challenge. Of these patients, 17 (94%) have had continued exposure to egg while 15 (83%) have shown tolerance to cooked egg. The researchers observed variable patterns of baked egg intake following the negative physician-supervised baked egg challenge. “Some patients are able to tolerate cooked egg rapidly but are not interested in continuing frequent consumption,” Dr. Peng said. “They may say, ‘My 3-year-old doesn’t like scrambled eggs, so I’m not going to keep pushing them.’ They’re not considering themselves egg allergic so their quality of life is much better. I understand that tolerating baked egg is a big deal, as an allergist I want to see them do more, such as tolerating cooked egg.”
When patients were asked about adverse reactions to egg consumption, three (17%) described gastrointestinal symptoms, five (28%) described cutaneous symptoms, and three (17%) described respiratory reactions. Dr. Peng noted that of the three patients who have not achieved tolerance to cooked egg, one patient reported mild reaction to baked egg 2 weeks after the baked egg challenge, while the other two continue to have baked egg exposure but have not yet introduced cooked egg into their diets. “I hope that most pediatricians and family practice physicians consider referral to an allergist if they’re not comfortable introducing a baked egg oral challenge.”
The researchers could not identify any correlation between skin prick or serum IgE test results and development of tolerance to cooked egg. “Data in the literature suggests that serum testing is predictive [of tolerance], but it’s not 100%,” Dr. Peng said.
She reported having no relevant financial disclosures.
AT THE 2017 AAAAI ANNUAL MEETING
Key clinical point:
Major finding: Following a negative physician-supervised baked egg oral challenge 94% of patients have had continued exposure to egg while 83% have shown tolerance to cooked egg.
Data source: A retrospective review of 22 patients who underwent a physician-supervised negative oral baked egg challenge.
Disclosures: Dr. Peng reported having no relevant financial disclosures.