User login
Screening for Symptomatic Mefloquine Exposure Among Veterans With Chronic Psychiatric Symptoms
Mefloquine is an antimalarial drug that is associated with a significant risk of chronic neuropsychiatric adverse effects (AEs). The drug was licensed by the FDA in 1989 after development by scientists affiliated with Walter Reed Army Institute of Research (WRAIR). By the early 1990s, mefloquine had become the U.S. military’s drug of choice both for treatment of uncomplicated malaria and for antimalarial prophylaxis and was administered as a convenient weekly dose. Mefloquine was prescribed widely to U.S. military personnel beginning with operations in Somalia in 1992 and over the next 2 decades during certain deployments to Iraq and Afghanistan and to other malaria-endemic areas.1
In 2013, following a decline of U.S. military use, the FDA added a boxed warning to the mefloquine product documentation to caution that neuropsychiatric AEs from the drug could last years after use and even be permanent. The U.S. military subsequently deemed mefloquine to be a prophylactic “drug of last resort.”2 Recently, researchers at WRAIR have acknowledged that chronic neuropsychiatric AEs attributable to mefloquine, including nightmares, insomnia, anxiety, irritability, and cognitive dysfunction, may confound the diagnosis of posttraumatic stress disorder (PTSD).3 The VA has awarded at least 1 disability claim for service-connected psychiatric conditions that it attributed to mefloquine exposure, and it is likely that in the coming years such claims will increase.2
Susceptibility to Chronic Neuropsychiatric AEs
Why mefloquine seems to cause chronic neuropsychiatric AEs in only certain individuals is unclear, although genetic susceptibility to drug-induced toxic encephalopathy and neurotoxicity are suspected.1 There is no screening test for susceptibility to AEs before mefloquine use, so the current U.S. product documentation cautiously warns that when used for prophylaxis, mefloquine should be discontinued at the onset of any neurologic or psychiatric symptom, many of which are considered prodromal to more serious AEs that may occur with continued dosing.4
Although chronic neuropsychiatric AEs have been reported to develop after only a single weekly dose, most clinically significant chronic AEs seem to occur among those who developed at least 1 prodromal neuropsychiatric symptom during early use but who continued weekly use despite these symptoms in a manner contrary to current product documentation guidance.4 In contrast, when mefloquine is administered for the treatment of malaria, typically at 5 times the weekly prophylactic dose and commonly in split doses over 8 to 12 hours, dosing is often complete by the time prodromal symptoms develop. Consequently, when mefloquine is used for treatment of malaria, the risks of more serious AEs are significantly higher than when the drug is used as directed in prophylaxis.5
Screening for Symptomatic Mefloquine Exposure
As the boxed warning indicates, certain psychiatric symptoms that occur with mefloquine use may become chronic and may confound psychiatric diagnosis. Particularly among veterans, these symptoms risk being misattributed, potentially affecting treatment decisions.6 Clinicians caring for veterans with persistent psychiatric symptoms should therefore screen for prior symptomatic mefloquine exposure and consider the possible AEs of the drug when formulating a differential diagnosis and treatment plan.
For example, a veteran with a history of symptomatic mefloquine exposure who later is diagnosed with PTSD may experience 1 or more symptoms, such as insomnia or cognitive dysfunction, which may be primarily attributable to the chronic AEs of the drug. The origins of the symptoms may be distinct from exposure to trauma and may not respond as effectively to certain conventional therapies for PTSD, requiring consideration of alternate therapies.3 The confounding role of psychiatric symptoms attributable to mefloquine exposure may explain failed response to medications and psychotherapy. Multidisciplinary evaluation and management may be appropriate for such patients.
If symptomatic mefloquine exposure is suspected, a clinician must establish evidence of exposure to the drug and the veteran’s development of neuropsychiatric symptoms associated with such exposure. The following sections provide guidance to aid in screening both for exposure to the drug and for the development of specific neuropsychiatric symptoms during prophylaxis or following the treatment of malaria.
Mefloquine Exposure
Mefloquine was licensed in the U.S. as a branded medication (Lariam) from 1989 to 2011, and the drug also has been available in a variety of generic equivalents from 2003 to the present. All versions of mefloquine approved in the U.S. have been formulated as a white/slightly-off-white, smaller than dime-sized round tablet, containing 250 mg of mefloquine hydrochloride.
When used for prophylaxis in military settings, the drug was often dispensed informally without documentation, sometimes including directly observed therapy under command direction.1,2 Therefore, even in the absence of prescribing documentation, a veteran who endorses a consistent history of malaria prophylaxis with mefloquine should be considered as having evidence of exposure.2
Exposure to mefloquine is unlikely if the veteran reports taking a daily antimalarial medication—more likely it was doxycycline or atovaquone/proguanil (marketed as Malarone). In rare cases, the drug may have been erroneously prescribed or been mistakenly taken daily for prophylaxis or, in more common cases, a prophylactic “loading dose” (typically 1 tablet daily for 3 days prior to weekly dosing) was used.7,8
Exposure also was unlikely if the veteran reports taking an antimalarial that was dosed weekly with a tablet that was not of the appropriate color, shape, and size. More likely that drug was chloroquine. Although most prophylactic use of mefloquine among U.S. veterans followed its licensing by the FDA in 1989, the drug is known to have been administered to a small number of U.S. military personnel prior to its licensing during clinical trials, including personnel deployed on certain operations during the 1980s.1
For treatment of malaria, mefloquine was used widely until better-tolerated drugs became available, beginning in the early 2000s, although some use of mefloquine in the military continues to this day. In most cases, clinicians should rely on records of hospitalization to identify whether mefloquine was administered. In rare cases where documentation is unavailable, exposure should be assumed if the veteran reports a reliable history of taking about 5 tablets (corresponding to the usual treatment dose of 1,250 mg) of appropriate color, shape, and size in response to confirmed or suspected malaria infection, either in 1 dose, or in split doses over 8 to 12 hours.
Symptoms During Prophylaxis
If prophylactic exposure to the drug has been established, the clinician should confirm the presence of neuropsychiatric symptoms during the exposure. Particularly among veterans deploying to malaria-endemic combat areas, such symptoms may have occurred during a period of heightened stress coincident with their initial deployment, and the veterans may have misattributed these symptoms to nonmefloquine factors. The clinician should therefore take a careful history to identify specific symptoms listed in the mefloquine product documentation. Many AEs will commonly manifest following the first 3 doses, and the clinician may find that focusing on this period is useful.9
When mefloquine is used for prophylaxis, anxiety and depression each affect between 1% and 10% of users. Other AEs that may develop include panic attacks; severe mood swings; behavioral AEs, including agitation, aggression, restlessness, and mania; symptoms of psychosis, including paranoia, delusions, and hallucinations; dissociative symptoms, including depersonalization; suicidal ideation; and cognitive AEs, including confusion.
The common symptoms of insomnia and abnormal dreaming affect > 10% of users. Particularly if multiple symptoms occur or if any of these symptoms occur following or coincident with symptoms of disturbed sleep, these should be considered strong evidence of symptomatic exposure.4 Veterans who report a history of continued mefloquine use despite the onset of such symptoms may be at particularly increased risk of chronic AEs.
The clinician should consider as evidence of symptomatic exposure information provided by others, including reports of obvious signs of nightmares or psychosis affecting the veteran. Clinicians should be aware that confusion and other psychiatric AEs caused by mefloquine during prophylactic use may limit the validity of self-reported history. Similarly, a history of seizure with mefloquine use or of the development of specific neurologic symptoms, particularly visual disturbances, dizziness, vertigo, disequilibrium, and paresthesias, also should be considered strong evidence of symptomatic exposure and indication of an increased risk of chronic psychiatric AEs.4
Posttreatment Adverse Effects
Although chronic psychiatric AEs following malaria infection have long been attributed to cerebral involvement, recognition that mefloquine may independently cause chronic neuropsychiatric AEs may require that individual cases be reexamined to properly assign causation.10 Particularly in uncomplicated cases of malaria, neuropsychiatric symptoms that develop only after treatment with mefloquine should be considered plausibly to be due to the drug and as evidence of symptomatic exposure.
As with use of mefloquine in prophylaxis, these neuropsychiatric symptoms may evolve in the weeks to months following exposure. They also may contribute to lasting and significant changes in personality, mood, cognition, thought, sleep, and behavior.6
Conclusion
Chronic AEs from mefloquine may provide a parsimonious explanation for the onset and persistence of a veteran’s psychiatric symptoms, particularly in cases where these may have failed to respond to treatment. Clinicians evaluating veterans who are seeking care for lasting psychiatric symptoms should ensure that they screen for prior symptomatic mefloquine exposure. As recognition grows of the drug’s chronic AEs, symptomatic mefloquine exposure is likely to emerge as a significant known confounder in the diagnosis of psychiatric disorders, including PTSD, among the current generation of U.S. veterans.
1. Nevin RL. Mefloquine and posttraumatic stress disorder. In: Ritchie EC, ed. Textbook of Military Medicine. Forensic and Ethical Issues in Military Behavioral Health. Washington, DC: Borden Institute; 2015:277-296.
2. Nevin RL, Ritchie EC. FDA black box, VA red ink? A successful service-connected disability claim for chronic neuropsychiatric adverse effects from mefloquine. Fed Pract. 2016;33(10):20-24.
3. Livezey J, Oliver T, Cantilena L. Prolonged neuropsychiatric symptoms in a military service member exposed to mefloquine. Drug Saf Case Rep. 2016;3(1):7.
4. Nevin RL, Byrd AM. Neuropsychiatric adverse reactions to mefloquine: a systematic comparison of prescribing and patient safety guidance in the US, UK, Ireland, Australia, New Zealand, and Canada. Neurol Ther. 2016;5(1):69-83.
5. Rendi-Wagner P, Noedl H, Wernsdorfer WH, Wiedermann G, Mikolasek A, Kollaritsch H. Unexpected frequency, duration and spectrum of adverse events after therapeutic dose of mefloquine in healthy adults. Acta Trop. 2002;81(2):167-173.
6. Nevin RL, Ritchie E. The mefloquine intoxication syndrome: a significant potential confounder in the diagnosis and management of PTSD and other chronic deployment-related neuropsychiatric disorders. In: Ritchie EC, ed. Posttraumatic Stress Disorder and Related Diseases in Combat Veterans. Cham, Switzerland: Springer International Publishing; 2015:257-278.
7. Cohen MR, Smetzer JL. FDA advise-ERR: mefloquine—not the same as Malarone; zoster vaccine is not for the immunosuppressed; TXA mistaken as tenecteplase; guidelines for adult IV push medications. Hosp Pharm. 2015;50(11):961-964.
8. Boudreau E, Schuster B, Sanchez J, et al. Tolerability of prophylactic Lariam regimens. Trop Med Parasitol. 1993;44(3):257-265.
9. Stürchler D, Handschin J, Kaiser D, et al. Neuropsychiatric side effects of mefloquine. N Engl J Med. 1990;322(24):1752-1753.
10. Nevin RL, Croft AM. Psychiatric effects of malaria and anti-malarial drugs: historical and modern perspectives. Malar J. 2016;15:332.
Mefloquine is an antimalarial drug that is associated with a significant risk of chronic neuropsychiatric adverse effects (AEs). The drug was licensed by the FDA in 1989 after development by scientists affiliated with Walter Reed Army Institute of Research (WRAIR). By the early 1990s, mefloquine had become the U.S. military’s drug of choice both for treatment of uncomplicated malaria and for antimalarial prophylaxis and was administered as a convenient weekly dose. Mefloquine was prescribed widely to U.S. military personnel beginning with operations in Somalia in 1992 and over the next 2 decades during certain deployments to Iraq and Afghanistan and to other malaria-endemic areas.1
In 2013, following a decline of U.S. military use, the FDA added a boxed warning to the mefloquine product documentation to caution that neuropsychiatric AEs from the drug could last years after use and even be permanent. The U.S. military subsequently deemed mefloquine to be a prophylactic “drug of last resort.”2 Recently, researchers at WRAIR have acknowledged that chronic neuropsychiatric AEs attributable to mefloquine, including nightmares, insomnia, anxiety, irritability, and cognitive dysfunction, may confound the diagnosis of posttraumatic stress disorder (PTSD).3 The VA has awarded at least 1 disability claim for service-connected psychiatric conditions that it attributed to mefloquine exposure, and it is likely that in the coming years such claims will increase.2
Susceptibility to Chronic Neuropsychiatric AEs
Why mefloquine seems to cause chronic neuropsychiatric AEs in only certain individuals is unclear, although genetic susceptibility to drug-induced toxic encephalopathy and neurotoxicity are suspected.1 There is no screening test for susceptibility to AEs before mefloquine use, so the current U.S. product documentation cautiously warns that when used for prophylaxis, mefloquine should be discontinued at the onset of any neurologic or psychiatric symptom, many of which are considered prodromal to more serious AEs that may occur with continued dosing.4
Although chronic neuropsychiatric AEs have been reported to develop after only a single weekly dose, most clinically significant chronic AEs seem to occur among those who developed at least 1 prodromal neuropsychiatric symptom during early use but who continued weekly use despite these symptoms in a manner contrary to current product documentation guidance.4 In contrast, when mefloquine is administered for the treatment of malaria, typically at 5 times the weekly prophylactic dose and commonly in split doses over 8 to 12 hours, dosing is often complete by the time prodromal symptoms develop. Consequently, when mefloquine is used for treatment of malaria, the risks of more serious AEs are significantly higher than when the drug is used as directed in prophylaxis.5
Screening for Symptomatic Mefloquine Exposure
As the boxed warning indicates, certain psychiatric symptoms that occur with mefloquine use may become chronic and may confound psychiatric diagnosis. Particularly among veterans, these symptoms risk being misattributed, potentially affecting treatment decisions.6 Clinicians caring for veterans with persistent psychiatric symptoms should therefore screen for prior symptomatic mefloquine exposure and consider the possible AEs of the drug when formulating a differential diagnosis and treatment plan.
For example, a veteran with a history of symptomatic mefloquine exposure who later is diagnosed with PTSD may experience 1 or more symptoms, such as insomnia or cognitive dysfunction, which may be primarily attributable to the chronic AEs of the drug. The origins of the symptoms may be distinct from exposure to trauma and may not respond as effectively to certain conventional therapies for PTSD, requiring consideration of alternate therapies.3 The confounding role of psychiatric symptoms attributable to mefloquine exposure may explain failed response to medications and psychotherapy. Multidisciplinary evaluation and management may be appropriate for such patients.
If symptomatic mefloquine exposure is suspected, a clinician must establish evidence of exposure to the drug and the veteran’s development of neuropsychiatric symptoms associated with such exposure. The following sections provide guidance to aid in screening both for exposure to the drug and for the development of specific neuropsychiatric symptoms during prophylaxis or following the treatment of malaria.
Mefloquine Exposure
Mefloquine was licensed in the U.S. as a branded medication (Lariam) from 1989 to 2011, and the drug also has been available in a variety of generic equivalents from 2003 to the present. All versions of mefloquine approved in the U.S. have been formulated as a white/slightly-off-white, smaller than dime-sized round tablet, containing 250 mg of mefloquine hydrochloride.
When used for prophylaxis in military settings, the drug was often dispensed informally without documentation, sometimes including directly observed therapy under command direction.1,2 Therefore, even in the absence of prescribing documentation, a veteran who endorses a consistent history of malaria prophylaxis with mefloquine should be considered as having evidence of exposure.2
Exposure to mefloquine is unlikely if the veteran reports taking a daily antimalarial medication—more likely it was doxycycline or atovaquone/proguanil (marketed as Malarone). In rare cases, the drug may have been erroneously prescribed or been mistakenly taken daily for prophylaxis or, in more common cases, a prophylactic “loading dose” (typically 1 tablet daily for 3 days prior to weekly dosing) was used.7,8
Exposure also was unlikely if the veteran reports taking an antimalarial that was dosed weekly with a tablet that was not of the appropriate color, shape, and size. More likely that drug was chloroquine. Although most prophylactic use of mefloquine among U.S. veterans followed its licensing by the FDA in 1989, the drug is known to have been administered to a small number of U.S. military personnel prior to its licensing during clinical trials, including personnel deployed on certain operations during the 1980s.1
For treatment of malaria, mefloquine was used widely until better-tolerated drugs became available, beginning in the early 2000s, although some use of mefloquine in the military continues to this day. In most cases, clinicians should rely on records of hospitalization to identify whether mefloquine was administered. In rare cases where documentation is unavailable, exposure should be assumed if the veteran reports a reliable history of taking about 5 tablets (corresponding to the usual treatment dose of 1,250 mg) of appropriate color, shape, and size in response to confirmed or suspected malaria infection, either in 1 dose, or in split doses over 8 to 12 hours.
Symptoms During Prophylaxis
If prophylactic exposure to the drug has been established, the clinician should confirm the presence of neuropsychiatric symptoms during the exposure. Particularly among veterans deploying to malaria-endemic combat areas, such symptoms may have occurred during a period of heightened stress coincident with their initial deployment, and the veterans may have misattributed these symptoms to nonmefloquine factors. The clinician should therefore take a careful history to identify specific symptoms listed in the mefloquine product documentation. Many AEs will commonly manifest following the first 3 doses, and the clinician may find that focusing on this period is useful.9
When mefloquine is used for prophylaxis, anxiety and depression each affect between 1% and 10% of users. Other AEs that may develop include panic attacks; severe mood swings; behavioral AEs, including agitation, aggression, restlessness, and mania; symptoms of psychosis, including paranoia, delusions, and hallucinations; dissociative symptoms, including depersonalization; suicidal ideation; and cognitive AEs, including confusion.
The common symptoms of insomnia and abnormal dreaming affect > 10% of users. Particularly if multiple symptoms occur or if any of these symptoms occur following or coincident with symptoms of disturbed sleep, these should be considered strong evidence of symptomatic exposure.4 Veterans who report a history of continued mefloquine use despite the onset of such symptoms may be at particularly increased risk of chronic AEs.
The clinician should consider as evidence of symptomatic exposure information provided by others, including reports of obvious signs of nightmares or psychosis affecting the veteran. Clinicians should be aware that confusion and other psychiatric AEs caused by mefloquine during prophylactic use may limit the validity of self-reported history. Similarly, a history of seizure with mefloquine use or of the development of specific neurologic symptoms, particularly visual disturbances, dizziness, vertigo, disequilibrium, and paresthesias, also should be considered strong evidence of symptomatic exposure and indication of an increased risk of chronic psychiatric AEs.4
Posttreatment Adverse Effects
Although chronic psychiatric AEs following malaria infection have long been attributed to cerebral involvement, recognition that mefloquine may independently cause chronic neuropsychiatric AEs may require that individual cases be reexamined to properly assign causation.10 Particularly in uncomplicated cases of malaria, neuropsychiatric symptoms that develop only after treatment with mefloquine should be considered plausibly to be due to the drug and as evidence of symptomatic exposure.
As with use of mefloquine in prophylaxis, these neuropsychiatric symptoms may evolve in the weeks to months following exposure. They also may contribute to lasting and significant changes in personality, mood, cognition, thought, sleep, and behavior.6
Conclusion
Chronic AEs from mefloquine may provide a parsimonious explanation for the onset and persistence of a veteran’s psychiatric symptoms, particularly in cases where these may have failed to respond to treatment. Clinicians evaluating veterans who are seeking care for lasting psychiatric symptoms should ensure that they screen for prior symptomatic mefloquine exposure. As recognition grows of the drug’s chronic AEs, symptomatic mefloquine exposure is likely to emerge as a significant known confounder in the diagnosis of psychiatric disorders, including PTSD, among the current generation of U.S. veterans.
Mefloquine is an antimalarial drug that is associated with a significant risk of chronic neuropsychiatric adverse effects (AEs). The drug was licensed by the FDA in 1989 after development by scientists affiliated with Walter Reed Army Institute of Research (WRAIR). By the early 1990s, mefloquine had become the U.S. military’s drug of choice both for treatment of uncomplicated malaria and for antimalarial prophylaxis and was administered as a convenient weekly dose. Mefloquine was prescribed widely to U.S. military personnel beginning with operations in Somalia in 1992 and over the next 2 decades during certain deployments to Iraq and Afghanistan and to other malaria-endemic areas.1
In 2013, following a decline of U.S. military use, the FDA added a boxed warning to the mefloquine product documentation to caution that neuropsychiatric AEs from the drug could last years after use and even be permanent. The U.S. military subsequently deemed mefloquine to be a prophylactic “drug of last resort.”2 Recently, researchers at WRAIR have acknowledged that chronic neuropsychiatric AEs attributable to mefloquine, including nightmares, insomnia, anxiety, irritability, and cognitive dysfunction, may confound the diagnosis of posttraumatic stress disorder (PTSD).3 The VA has awarded at least 1 disability claim for service-connected psychiatric conditions that it attributed to mefloquine exposure, and it is likely that in the coming years such claims will increase.2
Susceptibility to Chronic Neuropsychiatric AEs
Why mefloquine seems to cause chronic neuropsychiatric AEs in only certain individuals is unclear, although genetic susceptibility to drug-induced toxic encephalopathy and neurotoxicity are suspected.1 There is no screening test for susceptibility to AEs before mefloquine use, so the current U.S. product documentation cautiously warns that when used for prophylaxis, mefloquine should be discontinued at the onset of any neurologic or psychiatric symptom, many of which are considered prodromal to more serious AEs that may occur with continued dosing.4
Although chronic neuropsychiatric AEs have been reported to develop after only a single weekly dose, most clinically significant chronic AEs seem to occur among those who developed at least 1 prodromal neuropsychiatric symptom during early use but who continued weekly use despite these symptoms in a manner contrary to current product documentation guidance.4 In contrast, when mefloquine is administered for the treatment of malaria, typically at 5 times the weekly prophylactic dose and commonly in split doses over 8 to 12 hours, dosing is often complete by the time prodromal symptoms develop. Consequently, when mefloquine is used for treatment of malaria, the risks of more serious AEs are significantly higher than when the drug is used as directed in prophylaxis.5
Screening for Symptomatic Mefloquine Exposure
As the boxed warning indicates, certain psychiatric symptoms that occur with mefloquine use may become chronic and may confound psychiatric diagnosis. Particularly among veterans, these symptoms risk being misattributed, potentially affecting treatment decisions.6 Clinicians caring for veterans with persistent psychiatric symptoms should therefore screen for prior symptomatic mefloquine exposure and consider the possible AEs of the drug when formulating a differential diagnosis and treatment plan.
For example, a veteran with a history of symptomatic mefloquine exposure who later is diagnosed with PTSD may experience 1 or more symptoms, such as insomnia or cognitive dysfunction, which may be primarily attributable to the chronic AEs of the drug. The origins of the symptoms may be distinct from exposure to trauma and may not respond as effectively to certain conventional therapies for PTSD, requiring consideration of alternate therapies.3 The confounding role of psychiatric symptoms attributable to mefloquine exposure may explain failed response to medications and psychotherapy. Multidisciplinary evaluation and management may be appropriate for such patients.
If symptomatic mefloquine exposure is suspected, a clinician must establish evidence of exposure to the drug and the veteran’s development of neuropsychiatric symptoms associated with such exposure. The following sections provide guidance to aid in screening both for exposure to the drug and for the development of specific neuropsychiatric symptoms during prophylaxis or following the treatment of malaria.
Mefloquine Exposure
Mefloquine was licensed in the U.S. as a branded medication (Lariam) from 1989 to 2011, and the drug also has been available in a variety of generic equivalents from 2003 to the present. All versions of mefloquine approved in the U.S. have been formulated as a white/slightly-off-white, smaller than dime-sized round tablet, containing 250 mg of mefloquine hydrochloride.
When used for prophylaxis in military settings, the drug was often dispensed informally without documentation, sometimes including directly observed therapy under command direction.1,2 Therefore, even in the absence of prescribing documentation, a veteran who endorses a consistent history of malaria prophylaxis with mefloquine should be considered as having evidence of exposure.2
Exposure to mefloquine is unlikely if the veteran reports taking a daily antimalarial medication—more likely it was doxycycline or atovaquone/proguanil (marketed as Malarone). In rare cases, the drug may have been erroneously prescribed or been mistakenly taken daily for prophylaxis or, in more common cases, a prophylactic “loading dose” (typically 1 tablet daily for 3 days prior to weekly dosing) was used.7,8
Exposure also was unlikely if the veteran reports taking an antimalarial that was dosed weekly with a tablet that was not of the appropriate color, shape, and size. More likely that drug was chloroquine. Although most prophylactic use of mefloquine among U.S. veterans followed its licensing by the FDA in 1989, the drug is known to have been administered to a small number of U.S. military personnel prior to its licensing during clinical trials, including personnel deployed on certain operations during the 1980s.1
For treatment of malaria, mefloquine was used widely until better-tolerated drugs became available, beginning in the early 2000s, although some use of mefloquine in the military continues to this day. In most cases, clinicians should rely on records of hospitalization to identify whether mefloquine was administered. In rare cases where documentation is unavailable, exposure should be assumed if the veteran reports a reliable history of taking about 5 tablets (corresponding to the usual treatment dose of 1,250 mg) of appropriate color, shape, and size in response to confirmed or suspected malaria infection, either in 1 dose, or in split doses over 8 to 12 hours.
Symptoms During Prophylaxis
If prophylactic exposure to the drug has been established, the clinician should confirm the presence of neuropsychiatric symptoms during the exposure. Particularly among veterans deploying to malaria-endemic combat areas, such symptoms may have occurred during a period of heightened stress coincident with their initial deployment, and the veterans may have misattributed these symptoms to nonmefloquine factors. The clinician should therefore take a careful history to identify specific symptoms listed in the mefloquine product documentation. Many AEs will commonly manifest following the first 3 doses, and the clinician may find that focusing on this period is useful.9
When mefloquine is used for prophylaxis, anxiety and depression each affect between 1% and 10% of users. Other AEs that may develop include panic attacks; severe mood swings; behavioral AEs, including agitation, aggression, restlessness, and mania; symptoms of psychosis, including paranoia, delusions, and hallucinations; dissociative symptoms, including depersonalization; suicidal ideation; and cognitive AEs, including confusion.
The common symptoms of insomnia and abnormal dreaming affect > 10% of users. Particularly if multiple symptoms occur or if any of these symptoms occur following or coincident with symptoms of disturbed sleep, these should be considered strong evidence of symptomatic exposure.4 Veterans who report a history of continued mefloquine use despite the onset of such symptoms may be at particularly increased risk of chronic AEs.
The clinician should consider as evidence of symptomatic exposure information provided by others, including reports of obvious signs of nightmares or psychosis affecting the veteran. Clinicians should be aware that confusion and other psychiatric AEs caused by mefloquine during prophylactic use may limit the validity of self-reported history. Similarly, a history of seizure with mefloquine use or of the development of specific neurologic symptoms, particularly visual disturbances, dizziness, vertigo, disequilibrium, and paresthesias, also should be considered strong evidence of symptomatic exposure and indication of an increased risk of chronic psychiatric AEs.4
Posttreatment Adverse Effects
Although chronic psychiatric AEs following malaria infection have long been attributed to cerebral involvement, recognition that mefloquine may independently cause chronic neuropsychiatric AEs may require that individual cases be reexamined to properly assign causation.10 Particularly in uncomplicated cases of malaria, neuropsychiatric symptoms that develop only after treatment with mefloquine should be considered plausibly to be due to the drug and as evidence of symptomatic exposure.
As with use of mefloquine in prophylaxis, these neuropsychiatric symptoms may evolve in the weeks to months following exposure. They also may contribute to lasting and significant changes in personality, mood, cognition, thought, sleep, and behavior.6
Conclusion
Chronic AEs from mefloquine may provide a parsimonious explanation for the onset and persistence of a veteran’s psychiatric symptoms, particularly in cases where these may have failed to respond to treatment. Clinicians evaluating veterans who are seeking care for lasting psychiatric symptoms should ensure that they screen for prior symptomatic mefloquine exposure. As recognition grows of the drug’s chronic AEs, symptomatic mefloquine exposure is likely to emerge as a significant known confounder in the diagnosis of psychiatric disorders, including PTSD, among the current generation of U.S. veterans.
1. Nevin RL. Mefloquine and posttraumatic stress disorder. In: Ritchie EC, ed. Textbook of Military Medicine. Forensic and Ethical Issues in Military Behavioral Health. Washington, DC: Borden Institute; 2015:277-296.
2. Nevin RL, Ritchie EC. FDA black box, VA red ink? A successful service-connected disability claim for chronic neuropsychiatric adverse effects from mefloquine. Fed Pract. 2016;33(10):20-24.
3. Livezey J, Oliver T, Cantilena L. Prolonged neuropsychiatric symptoms in a military service member exposed to mefloquine. Drug Saf Case Rep. 2016;3(1):7.
4. Nevin RL, Byrd AM. Neuropsychiatric adverse reactions to mefloquine: a systematic comparison of prescribing and patient safety guidance in the US, UK, Ireland, Australia, New Zealand, and Canada. Neurol Ther. 2016;5(1):69-83.
5. Rendi-Wagner P, Noedl H, Wernsdorfer WH, Wiedermann G, Mikolasek A, Kollaritsch H. Unexpected frequency, duration and spectrum of adverse events after therapeutic dose of mefloquine in healthy adults. Acta Trop. 2002;81(2):167-173.
6. Nevin RL, Ritchie E. The mefloquine intoxication syndrome: a significant potential confounder in the diagnosis and management of PTSD and other chronic deployment-related neuropsychiatric disorders. In: Ritchie EC, ed. Posttraumatic Stress Disorder and Related Diseases in Combat Veterans. Cham, Switzerland: Springer International Publishing; 2015:257-278.
7. Cohen MR, Smetzer JL. FDA advise-ERR: mefloquine—not the same as Malarone; zoster vaccine is not for the immunosuppressed; TXA mistaken as tenecteplase; guidelines for adult IV push medications. Hosp Pharm. 2015;50(11):961-964.
8. Boudreau E, Schuster B, Sanchez J, et al. Tolerability of prophylactic Lariam regimens. Trop Med Parasitol. 1993;44(3):257-265.
9. Stürchler D, Handschin J, Kaiser D, et al. Neuropsychiatric side effects of mefloquine. N Engl J Med. 1990;322(24):1752-1753.
10. Nevin RL, Croft AM. Psychiatric effects of malaria and anti-malarial drugs: historical and modern perspectives. Malar J. 2016;15:332.
1. Nevin RL. Mefloquine and posttraumatic stress disorder. In: Ritchie EC, ed. Textbook of Military Medicine. Forensic and Ethical Issues in Military Behavioral Health. Washington, DC: Borden Institute; 2015:277-296.
2. Nevin RL, Ritchie EC. FDA black box, VA red ink? A successful service-connected disability claim for chronic neuropsychiatric adverse effects from mefloquine. Fed Pract. 2016;33(10):20-24.
3. Livezey J, Oliver T, Cantilena L. Prolonged neuropsychiatric symptoms in a military service member exposed to mefloquine. Drug Saf Case Rep. 2016;3(1):7.
4. Nevin RL, Byrd AM. Neuropsychiatric adverse reactions to mefloquine: a systematic comparison of prescribing and patient safety guidance in the US, UK, Ireland, Australia, New Zealand, and Canada. Neurol Ther. 2016;5(1):69-83.
5. Rendi-Wagner P, Noedl H, Wernsdorfer WH, Wiedermann G, Mikolasek A, Kollaritsch H. Unexpected frequency, duration and spectrum of adverse events after therapeutic dose of mefloquine in healthy adults. Acta Trop. 2002;81(2):167-173.
6. Nevin RL, Ritchie E. The mefloquine intoxication syndrome: a significant potential confounder in the diagnosis and management of PTSD and other chronic deployment-related neuropsychiatric disorders. In: Ritchie EC, ed. Posttraumatic Stress Disorder and Related Diseases in Combat Veterans. Cham, Switzerland: Springer International Publishing; 2015:257-278.
7. Cohen MR, Smetzer JL. FDA advise-ERR: mefloquine—not the same as Malarone; zoster vaccine is not for the immunosuppressed; TXA mistaken as tenecteplase; guidelines for adult IV push medications. Hosp Pharm. 2015;50(11):961-964.
8. Boudreau E, Schuster B, Sanchez J, et al. Tolerability of prophylactic Lariam regimens. Trop Med Parasitol. 1993;44(3):257-265.
9. Stürchler D, Handschin J, Kaiser D, et al. Neuropsychiatric side effects of mefloquine. N Engl J Med. 1990;322(24):1752-1753.
10. Nevin RL, Croft AM. Psychiatric effects of malaria and anti-malarial drugs: historical and modern perspectives. Malar J. 2016;15:332.
The Link Between Low-Density Lipoproteins and Chronic Lymphocytic Leukemia
Researchers from the University of Toronto had found in earlier studies that low-density lipoprotein (LDL) levels are elevated in up to 75% of patients with chronic lymphocytic leukemia (CLL). They also found that statins delayed the need for chemotherapy in those patients by nearly 3 years. They furthered their research using data on 2,124 patients with CLL and 7,935 controls in a population-based, case-control study. The researchers found a significantly higher incidence of hypercholesterolemia before CLL was diagnosed and a survival benefit of 3.7 years for patients taking statins.
In a new study to understand why hypercholesterolemia is apparently a tumor promoter for CLL, researchers first used an in vitro model of the “microenvironments” called pseudofollicles, where CLL cells proliferate. The researchers purified CLL cells from patients and activated them in lipid-poor conditions with interleukin-2 and resiquimod to represent stimulatory signals in pseudofollicles.
Related: New Treatments for Chronic Lymphocytic Leukemia
Adding LDLs increased viable cell numbers and signal transduction in molecules that mediate growth and proliferation of CLL cells.
To determine whether the effects were unique to CLL, the researchers conducted a similar experiment using peripheral blood mononuclear cells from donors without CLL. Their results suggested that normal blood cells handled cholesterol from LDLs in a different way than did CLL cells.
In another experiment, the researchers used circulating CLL cells from an additional cohort of 30 patients, including 11 who had been taking statins for at least 6 months. Cholesterol content of circulating CLL cells correlated directly with blood LDL levels, suggesting that LDLs may enhance proliferative responses of CLL cells to inflammatory signals. Statin use was associated with lower cholesterol in CLL cells, fewer circulating leukemia cells, and longer doubling times in vivo.
Researchers from the University of Toronto had found in earlier studies that low-density lipoprotein (LDL) levels are elevated in up to 75% of patients with chronic lymphocytic leukemia (CLL). They also found that statins delayed the need for chemotherapy in those patients by nearly 3 years. They furthered their research using data on 2,124 patients with CLL and 7,935 controls in a population-based, case-control study. The researchers found a significantly higher incidence of hypercholesterolemia before CLL was diagnosed and a survival benefit of 3.7 years for patients taking statins.
In a new study to understand why hypercholesterolemia is apparently a tumor promoter for CLL, researchers first used an in vitro model of the “microenvironments” called pseudofollicles, where CLL cells proliferate. The researchers purified CLL cells from patients and activated them in lipid-poor conditions with interleukin-2 and resiquimod to represent stimulatory signals in pseudofollicles.
Related: New Treatments for Chronic Lymphocytic Leukemia
Adding LDLs increased viable cell numbers and signal transduction in molecules that mediate growth and proliferation of CLL cells.
To determine whether the effects were unique to CLL, the researchers conducted a similar experiment using peripheral blood mononuclear cells from donors without CLL. Their results suggested that normal blood cells handled cholesterol from LDLs in a different way than did CLL cells.
In another experiment, the researchers used circulating CLL cells from an additional cohort of 30 patients, including 11 who had been taking statins for at least 6 months. Cholesterol content of circulating CLL cells correlated directly with blood LDL levels, suggesting that LDLs may enhance proliferative responses of CLL cells to inflammatory signals. Statin use was associated with lower cholesterol in CLL cells, fewer circulating leukemia cells, and longer doubling times in vivo.
Researchers from the University of Toronto had found in earlier studies that low-density lipoprotein (LDL) levels are elevated in up to 75% of patients with chronic lymphocytic leukemia (CLL). They also found that statins delayed the need for chemotherapy in those patients by nearly 3 years. They furthered their research using data on 2,124 patients with CLL and 7,935 controls in a population-based, case-control study. The researchers found a significantly higher incidence of hypercholesterolemia before CLL was diagnosed and a survival benefit of 3.7 years for patients taking statins.
In a new study to understand why hypercholesterolemia is apparently a tumor promoter for CLL, researchers first used an in vitro model of the “microenvironments” called pseudofollicles, where CLL cells proliferate. The researchers purified CLL cells from patients and activated them in lipid-poor conditions with interleukin-2 and resiquimod to represent stimulatory signals in pseudofollicles.
Related: New Treatments for Chronic Lymphocytic Leukemia
Adding LDLs increased viable cell numbers and signal transduction in molecules that mediate growth and proliferation of CLL cells.
To determine whether the effects were unique to CLL, the researchers conducted a similar experiment using peripheral blood mononuclear cells from donors without CLL. Their results suggested that normal blood cells handled cholesterol from LDLs in a different way than did CLL cells.
In another experiment, the researchers used circulating CLL cells from an additional cohort of 30 patients, including 11 who had been taking statins for at least 6 months. Cholesterol content of circulating CLL cells correlated directly with blood LDL levels, suggesting that LDLs may enhance proliferative responses of CLL cells to inflammatory signals. Statin use was associated with lower cholesterol in CLL cells, fewer circulating leukemia cells, and longer doubling times in vivo.
Applying a Time-Out and Standardized Report Form in Anesthesia Handoffs
Improving health care safety is one of the top priorities of the U.S. health care system. A key element for health care safety is the elimination of sentinel events—unexpected occurrences involving death or serious physical or psychological injury, such as loss of limb or function—or even the risk.1 Problems in communication, continuity of care, and planning have been identified as the root cause in more than 80% of documented sentinel events.2 As a direct result, The Joint Commission (JC) added National Patient Safety Goal 2E, which instructs each organization to implement a standardized approach to patient handoff.1 According to the JC, the objective of a handoff is to “provide accurate information about a patient’s care, treatment, and services, current condition, and any recent or anticipated changesand must include open communication and opportunities for questions.”1,3 The JC identified the patient handoff from anesthesia providers to the Surgical Intensive Care Unit (SICU) and Postanesthesia Care Unit (PACU) an opportunity for an improvement.1,3
At the Memphis VAMC in Tennessee, there was no established protocol for patient handoff from anesthesia providers to the SICU and PACU. The Anesthesia and SICU staffs were frustrated by inconsistent and incomplete postsurgical handoffs. Issues identified by the anesthesia team included difficulty contacting SICU staff to give a report and inconsistent availability of staff on first arrival to SICU. The SICU staff felt communication was rushed and there were inconsistencies in length and quality of the reports, resulting in incomplete postsurgical handoffs.
A baseline survey showed only 75% of staff felt the handoff report was thorough, and 67% “felt like a team.” In response, a multidisciplinary safe patient handoff committee (SPHOC) was formed by representatives from the involved units to discuss issues and offer solutions. The SPHOC efforts were aided by the VA National Center for Patient Safety (NCPS).
This quality improvement project was implemented as part of the U.S. Army Graduate Program in Anesthesia Nursing (USAGPAN) and the Northeastern University doctorate of nursing practice curriculum. The goal was to develop a simple, reliable, easily trainable handoff protocol for implemententation. This goal aligned with the priorites of the Memphis VAMC, USAGPAN, and VA to establish a culture based on patient safety and continuity of care.4
Methods
Standardization of handoffs began with JC National Patient Safety Goal 2E. There has been a wealth of medical literature on the need for standardization of handoffs and the implementation of specific handoff protocols in the postoperative setting. The SPHOC completed a review of the literature supporting standardization of handoff protocols. After completion, a second literature search was completed to identify the concepts for the implementation phase of the project. A critical appraisal of the evidence was completed using the method described by Melnyk and Fineout-Overholt.5 Literature from January 2005 through March 2015 was obtained via the Cumulative Index to Nursing and Allied Health Literature (CINAHL) and Google Scholar. The search methods included the keywords handover, handoff, transfer, and safety combined with anesthesia, PACU, surgery, operating room, and intensive care. Articles about handoffs not originating in the operating room (OR) were excluded.
The 13 articles found in the literature review established an overall need for standardization of handoffs outside the OR. Four articles identified a correlation between adverse events and poor or incomplete handoffs.3,6-8 Multiple articles discussed the need to develop a standardized handoff protocol in order to increase team work and quality of care.3,6,7,9,10 Petrovic and colleagues reported a 10% decrease in missed information and a boost in staff satisfaction from 61% to 81% with a standardized handoff.9 Additionally, a decrease in handoff time by > 1 minute was noted.8 Two articles identified an increase in quality of care after the implementation of a standardized handoff protocol.8,10
The second phase of the literature review examined relevant handoff information, best practices for participation in the handoff, and established staff buy-in for the process. Segall and colleagues created a table with handoff strategies consistently identified in the literature.10 The most relevant of these were using a structured written checklist to guide communication, using protocols to standardize the process, and providing formal team training.10
Six articles identified a written checklist and standardized handoff process as successful strategies used to improve patient safety.11-16 Zavalkoff and colleagues discussed the use of a template sheet filled out by the anesthesia provider prior to the handoff for consistency and accuracy of report.16 Catchpole and colleagues drew correlations between a Formula 1 pit stop and anesthesia handoffs and discussed the teamwork portion of the handoff protocol relating to staff buy-in.14 After delegating roles and making a set protocol for the handoff process, the study group was able to meet their objectives of efficient and safe handoff.14
With the information provided from the literature review, the SPHOC established a standardized handoff for the postsurgical patient. The committee created a handoff sheet for the anesthesia provider to use for report. This also included standardizing the handoff process and delineating specific roles for each provider.
After completing a NCPS training workshop, goals were identified at a SPHOC meeting. The SPHOC discussed current barriers to safe patient transfer and suggestions to overcome the barriers. Initial interventions planned by SPHOC focused on the problems of unsafe handoffs and delays in transfer. First, SICU identified the best phone number to call, which was distributed to the anesthesia and OR staffs. Additionally, the committee began tracking the number of attempted calls to reach SICU and availability of the nurse to take the report.
Implementation
A standardized handoff form was created by SPHOC, and anesthesia providers began to call time-out after the patient was deemed stable. After time-out was called, the SICU nurse provided his or her undivided attention and received the report. When SPHOC deemed the process successful, it was implemented in PACU as well. The entire OR, PACU, and anesthesia staffs were updated regarding the progress of the SPHOC on a monthly basis.
The implementation phase involved SPHOC tracking compliance of handoff sheets and time-outs. Compliance was tracked by counting the number of handoff sheets collected at the end of the day vs the total number of cases on the OR schedule. Tracking compliance with SICU transfers was monitored by the SICU members of the SPHOC through a tracking form. Initially a high level of SICU weekly compliance (93%) was noted.
Building on this success, SPHOC extended use of the handoff sheets and time-out to the PACU. Student registered nurse anesthetists (SRNAs) were tasked with education of the anesthesia and PACU staffs. Education continued via individual teaching, presentation at staff meetings, and e-mail reminders. To prevent confusion, no additional changes were made to the handoff sheet for an extended trial.
Despite these interventions, PACU compliance began to lag, averaging 33% over 3 weeks. Encouraging staff buy-in and a change in culture were identified as strategies to improve compliance. The third month of the trial started with 71% compliance. Interventions regarding staff buy-in emphasized individual accountability. Names were attached to handoff sheets, and those found with < 80% of sheets completed were provided with additional education. Those participants with ≥ 80% compliance were praised for their efforts.
Fostering a culture change proved to be more challenging. Interviews and discussions with anesthesia staff identified forgetting to fill out the sheet as the most common reason for noncompliance. Laminated copies of the handoff sheet were affixed to all anesthesia machines as a visual reminder. A sign denoting where to place the completed handoff sheets was placed in the PACU as a visual cue. The SPHOC stocked each anesthesia machine with handoff sheets on a daily basis.
To strengthen the culture of change, the PACU and SICU RNs were encouraged to ask for a time-out from the anesthesia provider. Handoff sheets were printed on yellow card stock to encourage anesthesia staff to “slow down for patient safety.” With these interventions, compliance increased to 98% by the end of the month.
Survey
An anonymous and voluntary survey was created and distributed to all staff involved in the handoff process. The 5-question survey was based on a 5-point Likert scale from 1 for strongly disagree to 5 for strongly disagree. The survey included the following questions: The new surgery report is very thorough; I feel more comfortable when assuming care of the postoperative patient; staff is more attentive when listening to the surgery report when a time-out is called; I feel the new surgery report is more effective and efficient; I feel I am more of a team with the OR with our changes in handoff of care process.
The survey was used as a baseline and to evaluate further changes in the process. Medical literature has shown that improper handoff communication was the leading cause of adverse events in the postsurgical patient.3,6
Results
Surgery to SICU transfers using the Handoff card increased from 33% in the first month to an average of 98% after interventions. In the 10-month intervention period,
After compliance initially increased, SPHOC focused on the more complex aspects of the handoff process—staff satisfaction, which was chosen based on an area of weakness identified in the initial survey results. Overall, staff was satisfied with the handoff sheets; however, only 67% of SICU staff reported that they felt part of the team with the OR as a result of the handoff of care process.
To address this issue, the team delineated roles for providers when a new surgical patient arrived in the SICU. This was dubbed the ABCs of safe handoff with roles for the anesthesia provider or respiratory therapist, the circulating nurse and SICU nurse, and the anesthesia provider. A graphic representation explains the mnemonic, the roles created, and laminated copies were distributed throughout the OR and SICU (Figure). Subsequent surveys showed 80% of staff felt more like a team with the new process.
Conclusion
The overall impact of the project has been to further promote a culture of patient safety at the Memphis VAMC and establish continuity of care as an institutional priority. The existing handoff sheet, time-out, and cross-check have been adapted to all hospital-wide transfers. With the SPHOC guidance and expertise, PACU began using a handoff sheet and time-out when transferring patients to the medical/surgical floors. The handoff sheet has also been adapted to fit the needs of transfers from the emergency department to the medical/surgical floors.
The framework of a standardized handoff is adaptable for other units to customize and has been adopted hospital-wide. The project is sustainable as it requires almost no money to create and sustain. The primary weakness of the process is the requirement of sustained staff participation and buy-in. Each unit and hospital invariably comes with a different culture and priorities; therefore, the process developed at Memphis VAMC may not meet the needs of other facilities. ˜
Acknowledgments
Special thanks to Susan Baldwin, RN; Bianca Mathews, MSN, RN; Wendy Regel, RN; Armance White, CRNA; Reginald Witt, MD; Odie Powell, RN; Alma Farris, RN; Clarisa Reed, RN; Susan Baily, RN; Linda Sueing, RN; Teresa Nguyen, RN; and John Craig, DNP, CRNA.
1. The Joint Commission. Topic library item: sentinel event policy and procedures.. http://www .jointcommission.org/Sentinel_Event_Policy _and_Procedures. Updated October 14, 2016. Accessed February 14, 2017.
2. Streitenberger K, Breen-Reid K, Harris C. Handoffs in care—can we make them safer? Pediatr Clin N Am. 2006;53(6):1185-1195.
3. Petrovic MA, Aboumatar H, Baumgartner WA, et al. Pilot implementation of a perioperative protocol to guide operating room-to-intensive care unit patient handoffs. J Cardiothorac Vasc Anesth. 2012;26(1):11-16.
4. U.S. Department of Veteran Affairs. VA national center for patient safety. http://www.patientsafety .va.gov/about/index.asp . Updated June 3, 2015. Accessed February 14, 2017.
5. Melnyk BM, Fineout-Overholt E. Evidence-Based Practice in Nursing & Healthcare: A Guide to Best Practice. Philadelphia, PA: Lippincott Williams & Wilkins; 2011.
6. Hudson CC, McDonald B, Hudson JK, Tran D, Boodhwani M. Impact of anesthetic handover on mortality and morbidity in cardiac surgery: a cohort study. J Cardiothorac Vasc Anesth. 2015;29(1):11-16.
7. Lane-Fall MB, Beidas RS, Pascual JL, et al. Handoffs and transitions in critical care (HATRICC): protocol for a mixed methods study of operating room to intensive care unit handoffs. BMC Surg. 2012;14:96.
8. Nagpal K, Arora S, Abboudi M, et al. Postoperative handover: problems, pitfalls, and prevention of error. Ann Surg. 2010;252(1):171-176.
9. Petrovic MA, Martinez EA, Aboumatar H. Implementing a perioperative handoff tool to improve postprocedural patient transfers. Jt Comm J Qual Patient Saf. 2012;38(3):135-142.
10. Segall N, Bonifacio AS, Schroeder RA, et al; Durham VA Patient Safety Center of Inquiry. Can we make postoperative patient handovers safer? A systematic review of the literature. Anesth Analg. 2012;115(1):102-115.
11. Riesenberg LA, Leitzsch J, Little BW. Systematic review of handoff mnemonics literature. Am J Med Qual. 2009;24(3):196-204.
12. Arora V, Johnson J. A model for building a standardized hand-off protocol. Jt Comm J Qual Patient Saf. 206;32(11):646-655.
13. Riesenberg LA, Leitzsch J, Cunningham JM. Nursing handoffs: a systematic review of the literature. Am J Nurse. 2010;110(4):24-34.
14. Catchpole KR, de Leval MR, McEwan A, et al. Patient handover from surgery to intensive care: using Formula 1 pit‐stop and aviation models to improve safety and quality. Paediatr Anesth. 2007;17(5):470-478.
15. Wahr JA, Prager RL, Abernathy JH III, et al; American Heart Association Council on Cardiovascular Surgery and Anesthesia, Council on Cardiovascular and Stroke Nursing, and Council on Quality of Care and Outcomes Research. Patient safety in the cardiac operating room: human factors and teamwork: a scientific statement from the American Heart Association. Circulation. 2013;128(10):1139-1169.
16. Zavalkoff SR, Razack SI, Lavoie J, Dancea AB. Handover after pediatric heart surgery: a simple tool improves information exchange. Pediatr Crit Care Med. 2011;12(3):309-313.
Improving health care safety is one of the top priorities of the U.S. health care system. A key element for health care safety is the elimination of sentinel events—unexpected occurrences involving death or serious physical or psychological injury, such as loss of limb or function—or even the risk.1 Problems in communication, continuity of care, and planning have been identified as the root cause in more than 80% of documented sentinel events.2 As a direct result, The Joint Commission (JC) added National Patient Safety Goal 2E, which instructs each organization to implement a standardized approach to patient handoff.1 According to the JC, the objective of a handoff is to “provide accurate information about a patient’s care, treatment, and services, current condition, and any recent or anticipated changesand must include open communication and opportunities for questions.”1,3 The JC identified the patient handoff from anesthesia providers to the Surgical Intensive Care Unit (SICU) and Postanesthesia Care Unit (PACU) an opportunity for an improvement.1,3
At the Memphis VAMC in Tennessee, there was no established protocol for patient handoff from anesthesia providers to the SICU and PACU. The Anesthesia and SICU staffs were frustrated by inconsistent and incomplete postsurgical handoffs. Issues identified by the anesthesia team included difficulty contacting SICU staff to give a report and inconsistent availability of staff on first arrival to SICU. The SICU staff felt communication was rushed and there were inconsistencies in length and quality of the reports, resulting in incomplete postsurgical handoffs.
A baseline survey showed only 75% of staff felt the handoff report was thorough, and 67% “felt like a team.” In response, a multidisciplinary safe patient handoff committee (SPHOC) was formed by representatives from the involved units to discuss issues and offer solutions. The SPHOC efforts were aided by the VA National Center for Patient Safety (NCPS).
This quality improvement project was implemented as part of the U.S. Army Graduate Program in Anesthesia Nursing (USAGPAN) and the Northeastern University doctorate of nursing practice curriculum. The goal was to develop a simple, reliable, easily trainable handoff protocol for implemententation. This goal aligned with the priorites of the Memphis VAMC, USAGPAN, and VA to establish a culture based on patient safety and continuity of care.4
Methods
Standardization of handoffs began with JC National Patient Safety Goal 2E. There has been a wealth of medical literature on the need for standardization of handoffs and the implementation of specific handoff protocols in the postoperative setting. The SPHOC completed a review of the literature supporting standardization of handoff protocols. After completion, a second literature search was completed to identify the concepts for the implementation phase of the project. A critical appraisal of the evidence was completed using the method described by Melnyk and Fineout-Overholt.5 Literature from January 2005 through March 2015 was obtained via the Cumulative Index to Nursing and Allied Health Literature (CINAHL) and Google Scholar. The search methods included the keywords handover, handoff, transfer, and safety combined with anesthesia, PACU, surgery, operating room, and intensive care. Articles about handoffs not originating in the operating room (OR) were excluded.
The 13 articles found in the literature review established an overall need for standardization of handoffs outside the OR. Four articles identified a correlation between adverse events and poor or incomplete handoffs.3,6-8 Multiple articles discussed the need to develop a standardized handoff protocol in order to increase team work and quality of care.3,6,7,9,10 Petrovic and colleagues reported a 10% decrease in missed information and a boost in staff satisfaction from 61% to 81% with a standardized handoff.9 Additionally, a decrease in handoff time by > 1 minute was noted.8 Two articles identified an increase in quality of care after the implementation of a standardized handoff protocol.8,10
The second phase of the literature review examined relevant handoff information, best practices for participation in the handoff, and established staff buy-in for the process. Segall and colleagues created a table with handoff strategies consistently identified in the literature.10 The most relevant of these were using a structured written checklist to guide communication, using protocols to standardize the process, and providing formal team training.10
Six articles identified a written checklist and standardized handoff process as successful strategies used to improve patient safety.11-16 Zavalkoff and colleagues discussed the use of a template sheet filled out by the anesthesia provider prior to the handoff for consistency and accuracy of report.16 Catchpole and colleagues drew correlations between a Formula 1 pit stop and anesthesia handoffs and discussed the teamwork portion of the handoff protocol relating to staff buy-in.14 After delegating roles and making a set protocol for the handoff process, the study group was able to meet their objectives of efficient and safe handoff.14
With the information provided from the literature review, the SPHOC established a standardized handoff for the postsurgical patient. The committee created a handoff sheet for the anesthesia provider to use for report. This also included standardizing the handoff process and delineating specific roles for each provider.
After completing a NCPS training workshop, goals were identified at a SPHOC meeting. The SPHOC discussed current barriers to safe patient transfer and suggestions to overcome the barriers. Initial interventions planned by SPHOC focused on the problems of unsafe handoffs and delays in transfer. First, SICU identified the best phone number to call, which was distributed to the anesthesia and OR staffs. Additionally, the committee began tracking the number of attempted calls to reach SICU and availability of the nurse to take the report.
Implementation
A standardized handoff form was created by SPHOC, and anesthesia providers began to call time-out after the patient was deemed stable. After time-out was called, the SICU nurse provided his or her undivided attention and received the report. When SPHOC deemed the process successful, it was implemented in PACU as well. The entire OR, PACU, and anesthesia staffs were updated regarding the progress of the SPHOC on a monthly basis.
The implementation phase involved SPHOC tracking compliance of handoff sheets and time-outs. Compliance was tracked by counting the number of handoff sheets collected at the end of the day vs the total number of cases on the OR schedule. Tracking compliance with SICU transfers was monitored by the SICU members of the SPHOC through a tracking form. Initially a high level of SICU weekly compliance (93%) was noted.
Building on this success, SPHOC extended use of the handoff sheets and time-out to the PACU. Student registered nurse anesthetists (SRNAs) were tasked with education of the anesthesia and PACU staffs. Education continued via individual teaching, presentation at staff meetings, and e-mail reminders. To prevent confusion, no additional changes were made to the handoff sheet for an extended trial.
Despite these interventions, PACU compliance began to lag, averaging 33% over 3 weeks. Encouraging staff buy-in and a change in culture were identified as strategies to improve compliance. The third month of the trial started with 71% compliance. Interventions regarding staff buy-in emphasized individual accountability. Names were attached to handoff sheets, and those found with < 80% of sheets completed were provided with additional education. Those participants with ≥ 80% compliance were praised for their efforts.
Fostering a culture change proved to be more challenging. Interviews and discussions with anesthesia staff identified forgetting to fill out the sheet as the most common reason for noncompliance. Laminated copies of the handoff sheet were affixed to all anesthesia machines as a visual reminder. A sign denoting where to place the completed handoff sheets was placed in the PACU as a visual cue. The SPHOC stocked each anesthesia machine with handoff sheets on a daily basis.
To strengthen the culture of change, the PACU and SICU RNs were encouraged to ask for a time-out from the anesthesia provider. Handoff sheets were printed on yellow card stock to encourage anesthesia staff to “slow down for patient safety.” With these interventions, compliance increased to 98% by the end of the month.
Survey
An anonymous and voluntary survey was created and distributed to all staff involved in the handoff process. The 5-question survey was based on a 5-point Likert scale from 1 for strongly disagree to 5 for strongly disagree. The survey included the following questions: The new surgery report is very thorough; I feel more comfortable when assuming care of the postoperative patient; staff is more attentive when listening to the surgery report when a time-out is called; I feel the new surgery report is more effective and efficient; I feel I am more of a team with the OR with our changes in handoff of care process.
The survey was used as a baseline and to evaluate further changes in the process. Medical literature has shown that improper handoff communication was the leading cause of adverse events in the postsurgical patient.3,6
Results
Surgery to SICU transfers using the Handoff card increased from 33% in the first month to an average of 98% after interventions. In the 10-month intervention period,
After compliance initially increased, SPHOC focused on the more complex aspects of the handoff process—staff satisfaction, which was chosen based on an area of weakness identified in the initial survey results. Overall, staff was satisfied with the handoff sheets; however, only 67% of SICU staff reported that they felt part of the team with the OR as a result of the handoff of care process.
To address this issue, the team delineated roles for providers when a new surgical patient arrived in the SICU. This was dubbed the ABCs of safe handoff with roles for the anesthesia provider or respiratory therapist, the circulating nurse and SICU nurse, and the anesthesia provider. A graphic representation explains the mnemonic, the roles created, and laminated copies were distributed throughout the OR and SICU (Figure). Subsequent surveys showed 80% of staff felt more like a team with the new process.
Conclusion
The overall impact of the project has been to further promote a culture of patient safety at the Memphis VAMC and establish continuity of care as an institutional priority. The existing handoff sheet, time-out, and cross-check have been adapted to all hospital-wide transfers. With the SPHOC guidance and expertise, PACU began using a handoff sheet and time-out when transferring patients to the medical/surgical floors. The handoff sheet has also been adapted to fit the needs of transfers from the emergency department to the medical/surgical floors.
The framework of a standardized handoff is adaptable for other units to customize and has been adopted hospital-wide. The project is sustainable as it requires almost no money to create and sustain. The primary weakness of the process is the requirement of sustained staff participation and buy-in. Each unit and hospital invariably comes with a different culture and priorities; therefore, the process developed at Memphis VAMC may not meet the needs of other facilities. ˜
Acknowledgments
Special thanks to Susan Baldwin, RN; Bianca Mathews, MSN, RN; Wendy Regel, RN; Armance White, CRNA; Reginald Witt, MD; Odie Powell, RN; Alma Farris, RN; Clarisa Reed, RN; Susan Baily, RN; Linda Sueing, RN; Teresa Nguyen, RN; and John Craig, DNP, CRNA.
Improving health care safety is one of the top priorities of the U.S. health care system. A key element for health care safety is the elimination of sentinel events—unexpected occurrences involving death or serious physical or psychological injury, such as loss of limb or function—or even the risk.1 Problems in communication, continuity of care, and planning have been identified as the root cause in more than 80% of documented sentinel events.2 As a direct result, The Joint Commission (JC) added National Patient Safety Goal 2E, which instructs each organization to implement a standardized approach to patient handoff.1 According to the JC, the objective of a handoff is to “provide accurate information about a patient’s care, treatment, and services, current condition, and any recent or anticipated changesand must include open communication and opportunities for questions.”1,3 The JC identified the patient handoff from anesthesia providers to the Surgical Intensive Care Unit (SICU) and Postanesthesia Care Unit (PACU) an opportunity for an improvement.1,3
At the Memphis VAMC in Tennessee, there was no established protocol for patient handoff from anesthesia providers to the SICU and PACU. The Anesthesia and SICU staffs were frustrated by inconsistent and incomplete postsurgical handoffs. Issues identified by the anesthesia team included difficulty contacting SICU staff to give a report and inconsistent availability of staff on first arrival to SICU. The SICU staff felt communication was rushed and there were inconsistencies in length and quality of the reports, resulting in incomplete postsurgical handoffs.
A baseline survey showed only 75% of staff felt the handoff report was thorough, and 67% “felt like a team.” In response, a multidisciplinary safe patient handoff committee (SPHOC) was formed by representatives from the involved units to discuss issues and offer solutions. The SPHOC efforts were aided by the VA National Center for Patient Safety (NCPS).
This quality improvement project was implemented as part of the U.S. Army Graduate Program in Anesthesia Nursing (USAGPAN) and the Northeastern University doctorate of nursing practice curriculum. The goal was to develop a simple, reliable, easily trainable handoff protocol for implemententation. This goal aligned with the priorites of the Memphis VAMC, USAGPAN, and VA to establish a culture based on patient safety and continuity of care.4
Methods
Standardization of handoffs began with JC National Patient Safety Goal 2E. There has been a wealth of medical literature on the need for standardization of handoffs and the implementation of specific handoff protocols in the postoperative setting. The SPHOC completed a review of the literature supporting standardization of handoff protocols. After completion, a second literature search was completed to identify the concepts for the implementation phase of the project. A critical appraisal of the evidence was completed using the method described by Melnyk and Fineout-Overholt.5 Literature from January 2005 through March 2015 was obtained via the Cumulative Index to Nursing and Allied Health Literature (CINAHL) and Google Scholar. The search methods included the keywords handover, handoff, transfer, and safety combined with anesthesia, PACU, surgery, operating room, and intensive care. Articles about handoffs not originating in the operating room (OR) were excluded.
The 13 articles found in the literature review established an overall need for standardization of handoffs outside the OR. Four articles identified a correlation between adverse events and poor or incomplete handoffs.3,6-8 Multiple articles discussed the need to develop a standardized handoff protocol in order to increase team work and quality of care.3,6,7,9,10 Petrovic and colleagues reported a 10% decrease in missed information and a boost in staff satisfaction from 61% to 81% with a standardized handoff.9 Additionally, a decrease in handoff time by > 1 minute was noted.8 Two articles identified an increase in quality of care after the implementation of a standardized handoff protocol.8,10
The second phase of the literature review examined relevant handoff information, best practices for participation in the handoff, and established staff buy-in for the process. Segall and colleagues created a table with handoff strategies consistently identified in the literature.10 The most relevant of these were using a structured written checklist to guide communication, using protocols to standardize the process, and providing formal team training.10
Six articles identified a written checklist and standardized handoff process as successful strategies used to improve patient safety.11-16 Zavalkoff and colleagues discussed the use of a template sheet filled out by the anesthesia provider prior to the handoff for consistency and accuracy of report.16 Catchpole and colleagues drew correlations between a Formula 1 pit stop and anesthesia handoffs and discussed the teamwork portion of the handoff protocol relating to staff buy-in.14 After delegating roles and making a set protocol for the handoff process, the study group was able to meet their objectives of efficient and safe handoff.14
With the information provided from the literature review, the SPHOC established a standardized handoff for the postsurgical patient. The committee created a handoff sheet for the anesthesia provider to use for report. This also included standardizing the handoff process and delineating specific roles for each provider.
After completing a NCPS training workshop, goals were identified at a SPHOC meeting. The SPHOC discussed current barriers to safe patient transfer and suggestions to overcome the barriers. Initial interventions planned by SPHOC focused on the problems of unsafe handoffs and delays in transfer. First, SICU identified the best phone number to call, which was distributed to the anesthesia and OR staffs. Additionally, the committee began tracking the number of attempted calls to reach SICU and availability of the nurse to take the report.
Implementation
A standardized handoff form was created by SPHOC, and anesthesia providers began to call time-out after the patient was deemed stable. After time-out was called, the SICU nurse provided his or her undivided attention and received the report. When SPHOC deemed the process successful, it was implemented in PACU as well. The entire OR, PACU, and anesthesia staffs were updated regarding the progress of the SPHOC on a monthly basis.
The implementation phase involved SPHOC tracking compliance of handoff sheets and time-outs. Compliance was tracked by counting the number of handoff sheets collected at the end of the day vs the total number of cases on the OR schedule. Tracking compliance with SICU transfers was monitored by the SICU members of the SPHOC through a tracking form. Initially a high level of SICU weekly compliance (93%) was noted.
Building on this success, SPHOC extended use of the handoff sheets and time-out to the PACU. Student registered nurse anesthetists (SRNAs) were tasked with education of the anesthesia and PACU staffs. Education continued via individual teaching, presentation at staff meetings, and e-mail reminders. To prevent confusion, no additional changes were made to the handoff sheet for an extended trial.
Despite these interventions, PACU compliance began to lag, averaging 33% over 3 weeks. Encouraging staff buy-in and a change in culture were identified as strategies to improve compliance. The third month of the trial started with 71% compliance. Interventions regarding staff buy-in emphasized individual accountability. Names were attached to handoff sheets, and those found with < 80% of sheets completed were provided with additional education. Those participants with ≥ 80% compliance were praised for their efforts.
Fostering a culture change proved to be more challenging. Interviews and discussions with anesthesia staff identified forgetting to fill out the sheet as the most common reason for noncompliance. Laminated copies of the handoff sheet were affixed to all anesthesia machines as a visual reminder. A sign denoting where to place the completed handoff sheets was placed in the PACU as a visual cue. The SPHOC stocked each anesthesia machine with handoff sheets on a daily basis.
To strengthen the culture of change, the PACU and SICU RNs were encouraged to ask for a time-out from the anesthesia provider. Handoff sheets were printed on yellow card stock to encourage anesthesia staff to “slow down for patient safety.” With these interventions, compliance increased to 98% by the end of the month.
Survey
An anonymous and voluntary survey was created and distributed to all staff involved in the handoff process. The 5-question survey was based on a 5-point Likert scale from 1 for strongly disagree to 5 for strongly disagree. The survey included the following questions: The new surgery report is very thorough; I feel more comfortable when assuming care of the postoperative patient; staff is more attentive when listening to the surgery report when a time-out is called; I feel the new surgery report is more effective and efficient; I feel I am more of a team with the OR with our changes in handoff of care process.
The survey was used as a baseline and to evaluate further changes in the process. Medical literature has shown that improper handoff communication was the leading cause of adverse events in the postsurgical patient.3,6
Results
Surgery to SICU transfers using the Handoff card increased from 33% in the first month to an average of 98% after interventions. In the 10-month intervention period,
After compliance initially increased, SPHOC focused on the more complex aspects of the handoff process—staff satisfaction, which was chosen based on an area of weakness identified in the initial survey results. Overall, staff was satisfied with the handoff sheets; however, only 67% of SICU staff reported that they felt part of the team with the OR as a result of the handoff of care process.
To address this issue, the team delineated roles for providers when a new surgical patient arrived in the SICU. This was dubbed the ABCs of safe handoff with roles for the anesthesia provider or respiratory therapist, the circulating nurse and SICU nurse, and the anesthesia provider. A graphic representation explains the mnemonic, the roles created, and laminated copies were distributed throughout the OR and SICU (Figure). Subsequent surveys showed 80% of staff felt more like a team with the new process.
Conclusion
The overall impact of the project has been to further promote a culture of patient safety at the Memphis VAMC and establish continuity of care as an institutional priority. The existing handoff sheet, time-out, and cross-check have been adapted to all hospital-wide transfers. With the SPHOC guidance and expertise, PACU began using a handoff sheet and time-out when transferring patients to the medical/surgical floors. The handoff sheet has also been adapted to fit the needs of transfers from the emergency department to the medical/surgical floors.
The framework of a standardized handoff is adaptable for other units to customize and has been adopted hospital-wide. The project is sustainable as it requires almost no money to create and sustain. The primary weakness of the process is the requirement of sustained staff participation and buy-in. Each unit and hospital invariably comes with a different culture and priorities; therefore, the process developed at Memphis VAMC may not meet the needs of other facilities. ˜
Acknowledgments
Special thanks to Susan Baldwin, RN; Bianca Mathews, MSN, RN; Wendy Regel, RN; Armance White, CRNA; Reginald Witt, MD; Odie Powell, RN; Alma Farris, RN; Clarisa Reed, RN; Susan Baily, RN; Linda Sueing, RN; Teresa Nguyen, RN; and John Craig, DNP, CRNA.
1. The Joint Commission. Topic library item: sentinel event policy and procedures.. http://www .jointcommission.org/Sentinel_Event_Policy _and_Procedures. Updated October 14, 2016. Accessed February 14, 2017.
2. Streitenberger K, Breen-Reid K, Harris C. Handoffs in care—can we make them safer? Pediatr Clin N Am. 2006;53(6):1185-1195.
3. Petrovic MA, Aboumatar H, Baumgartner WA, et al. Pilot implementation of a perioperative protocol to guide operating room-to-intensive care unit patient handoffs. J Cardiothorac Vasc Anesth. 2012;26(1):11-16.
4. U.S. Department of Veteran Affairs. VA national center for patient safety. http://www.patientsafety .va.gov/about/index.asp . Updated June 3, 2015. Accessed February 14, 2017.
5. Melnyk BM, Fineout-Overholt E. Evidence-Based Practice in Nursing & Healthcare: A Guide to Best Practice. Philadelphia, PA: Lippincott Williams & Wilkins; 2011.
6. Hudson CC, McDonald B, Hudson JK, Tran D, Boodhwani M. Impact of anesthetic handover on mortality and morbidity in cardiac surgery: a cohort study. J Cardiothorac Vasc Anesth. 2015;29(1):11-16.
7. Lane-Fall MB, Beidas RS, Pascual JL, et al. Handoffs and transitions in critical care (HATRICC): protocol for a mixed methods study of operating room to intensive care unit handoffs. BMC Surg. 2012;14:96.
8. Nagpal K, Arora S, Abboudi M, et al. Postoperative handover: problems, pitfalls, and prevention of error. Ann Surg. 2010;252(1):171-176.
9. Petrovic MA, Martinez EA, Aboumatar H. Implementing a perioperative handoff tool to improve postprocedural patient transfers. Jt Comm J Qual Patient Saf. 2012;38(3):135-142.
10. Segall N, Bonifacio AS, Schroeder RA, et al; Durham VA Patient Safety Center of Inquiry. Can we make postoperative patient handovers safer? A systematic review of the literature. Anesth Analg. 2012;115(1):102-115.
11. Riesenberg LA, Leitzsch J, Little BW. Systematic review of handoff mnemonics literature. Am J Med Qual. 2009;24(3):196-204.
12. Arora V, Johnson J. A model for building a standardized hand-off protocol. Jt Comm J Qual Patient Saf. 206;32(11):646-655.
13. Riesenberg LA, Leitzsch J, Cunningham JM. Nursing handoffs: a systematic review of the literature. Am J Nurse. 2010;110(4):24-34.
14. Catchpole KR, de Leval MR, McEwan A, et al. Patient handover from surgery to intensive care: using Formula 1 pit‐stop and aviation models to improve safety and quality. Paediatr Anesth. 2007;17(5):470-478.
15. Wahr JA, Prager RL, Abernathy JH III, et al; American Heart Association Council on Cardiovascular Surgery and Anesthesia, Council on Cardiovascular and Stroke Nursing, and Council on Quality of Care and Outcomes Research. Patient safety in the cardiac operating room: human factors and teamwork: a scientific statement from the American Heart Association. Circulation. 2013;128(10):1139-1169.
16. Zavalkoff SR, Razack SI, Lavoie J, Dancea AB. Handover after pediatric heart surgery: a simple tool improves information exchange. Pediatr Crit Care Med. 2011;12(3):309-313.
1. The Joint Commission. Topic library item: sentinel event policy and procedures.. http://www .jointcommission.org/Sentinel_Event_Policy _and_Procedures. Updated October 14, 2016. Accessed February 14, 2017.
2. Streitenberger K, Breen-Reid K, Harris C. Handoffs in care—can we make them safer? Pediatr Clin N Am. 2006;53(6):1185-1195.
3. Petrovic MA, Aboumatar H, Baumgartner WA, et al. Pilot implementation of a perioperative protocol to guide operating room-to-intensive care unit patient handoffs. J Cardiothorac Vasc Anesth. 2012;26(1):11-16.
4. U.S. Department of Veteran Affairs. VA national center for patient safety. http://www.patientsafety .va.gov/about/index.asp . Updated June 3, 2015. Accessed February 14, 2017.
5. Melnyk BM, Fineout-Overholt E. Evidence-Based Practice in Nursing & Healthcare: A Guide to Best Practice. Philadelphia, PA: Lippincott Williams & Wilkins; 2011.
6. Hudson CC, McDonald B, Hudson JK, Tran D, Boodhwani M. Impact of anesthetic handover on mortality and morbidity in cardiac surgery: a cohort study. J Cardiothorac Vasc Anesth. 2015;29(1):11-16.
7. Lane-Fall MB, Beidas RS, Pascual JL, et al. Handoffs and transitions in critical care (HATRICC): protocol for a mixed methods study of operating room to intensive care unit handoffs. BMC Surg. 2012;14:96.
8. Nagpal K, Arora S, Abboudi M, et al. Postoperative handover: problems, pitfalls, and prevention of error. Ann Surg. 2010;252(1):171-176.
9. Petrovic MA, Martinez EA, Aboumatar H. Implementing a perioperative handoff tool to improve postprocedural patient transfers. Jt Comm J Qual Patient Saf. 2012;38(3):135-142.
10. Segall N, Bonifacio AS, Schroeder RA, et al; Durham VA Patient Safety Center of Inquiry. Can we make postoperative patient handovers safer? A systematic review of the literature. Anesth Analg. 2012;115(1):102-115.
11. Riesenberg LA, Leitzsch J, Little BW. Systematic review of handoff mnemonics literature. Am J Med Qual. 2009;24(3):196-204.
12. Arora V, Johnson J. A model for building a standardized hand-off protocol. Jt Comm J Qual Patient Saf. 206;32(11):646-655.
13. Riesenberg LA, Leitzsch J, Cunningham JM. Nursing handoffs: a systematic review of the literature. Am J Nurse. 2010;110(4):24-34.
14. Catchpole KR, de Leval MR, McEwan A, et al. Patient handover from surgery to intensive care: using Formula 1 pit‐stop and aviation models to improve safety and quality. Paediatr Anesth. 2007;17(5):470-478.
15. Wahr JA, Prager RL, Abernathy JH III, et al; American Heart Association Council on Cardiovascular Surgery and Anesthesia, Council on Cardiovascular and Stroke Nursing, and Council on Quality of Care and Outcomes Research. Patient safety in the cardiac operating room: human factors and teamwork: a scientific statement from the American Heart Association. Circulation. 2013;128(10):1139-1169.
16. Zavalkoff SR, Razack SI, Lavoie J, Dancea AB. Handover after pediatric heart surgery: a simple tool improves information exchange. Pediatr Crit Care Med. 2011;12(3):309-313.
Pathogenesis of breast-implant-associated ALCL
SAN FRANCISCO—A small study suggests an abnormal immune response characterized by the production of interleukin-13 (IL-13) underlies the pathogenesis of breast implant-associated anaplastic large-cell lymphoma (BIA-ALCL).
The immune response appears to be directed toward bacterial antigens on the surface of the breast implants.
Marshall E. Kadin, MD, of Roger Williams Medical Center in Providence, Rhode Island, presented these findings at the 9th Annual T-cell Lymphoma Forum.
Dr Kadin noted that BIA-ALCL is a rare type of CD30+ T-cell ALCL that has been reported in more than 200 women worldwide.
Although a cause-and-effect relationship between breast implants and BIA-ALCL has been suggested, the underlying pathogenesis of this malignancy is unclear.
A bacterial biofilm containing gram-negative bacilli has been detected in breast implants from patients with BIA-ALCL.
Therefore, Dr Kadin and his colleagues hypothesized that an immune response toward the bacterial antigens may mediate the pathogenesis of BIA-ALCL.
The researchers studied 13 clinical samples of breast implant capsules and regional lymph nodes from 4 patients with BIA-ALCL, 7 patients with systemic ALCL, and 1 patient with peripheral T-cell lymphoma-not otherwise specified (PTCL-NOS).
Immunohistochemistry was used to determine the presence of IL-13, IL-4, GATA3, and immunoglobulin E (IgE) in these samples.
All clinical samples of anaplastic cells from breast implant capsules tested positive for the presence of IL-13 (13/13). GATA3 was expressed in most anaplastic cell samples (12/13), and IL-4 expression was found in some anaplastic cell samples (6/13). IL-13 and GATA3 expression were observed in some intra-capsular small lymphocytes.
While IL-13 was also detected in BIA-ALCL cell lines, it was not found in 4 of the 7 systemic ALCL cases or the PTCL-NOS case.
Dr Kadin said the lack of IL-13 receptor expression in BIA-ALCL cell lines suggests that IL-13 is not an autocrine growth factor for BIA-ALCL, and its expression is most likely associated with an allergic immune response.
IL-13 is known to induce immunoglobulin class switching in plasma cells to produce IgE. H&E and Giemsa staining of the BIA-ALCL tumor tissue and involved regional lymph nodes revealed IgE and eosinophils on the surface of mast cells and follicular dendritic cells.
Taken together, these data point to an allergic reaction in breast implant capsules of BIA-ALCL.
Dr Kadin was hopeful that these findings could be extrapolated to prevent BIA-ALCL by identifying individuals at higher risk for developing the disease.
The next step for this research is to decipher the role of bacterial antigens in mediating the immune response and whether women who develop BIA-ALCL have a significant increase in other atopic conditions. ![]()
SAN FRANCISCO—A small study suggests an abnormal immune response characterized by the production of interleukin-13 (IL-13) underlies the pathogenesis of breast implant-associated anaplastic large-cell lymphoma (BIA-ALCL).
The immune response appears to be directed toward bacterial antigens on the surface of the breast implants.
Marshall E. Kadin, MD, of Roger Williams Medical Center in Providence, Rhode Island, presented these findings at the 9th Annual T-cell Lymphoma Forum.
Dr Kadin noted that BIA-ALCL is a rare type of CD30+ T-cell ALCL that has been reported in more than 200 women worldwide.
Although a cause-and-effect relationship between breast implants and BIA-ALCL has been suggested, the underlying pathogenesis of this malignancy is unclear.
A bacterial biofilm containing gram-negative bacilli has been detected in breast implants from patients with BIA-ALCL.
Therefore, Dr Kadin and his colleagues hypothesized that an immune response toward the bacterial antigens may mediate the pathogenesis of BIA-ALCL.
The researchers studied 13 clinical samples of breast implant capsules and regional lymph nodes from 4 patients with BIA-ALCL, 7 patients with systemic ALCL, and 1 patient with peripheral T-cell lymphoma-not otherwise specified (PTCL-NOS).
Immunohistochemistry was used to determine the presence of IL-13, IL-4, GATA3, and immunoglobulin E (IgE) in these samples.
All clinical samples of anaplastic cells from breast implant capsules tested positive for the presence of IL-13 (13/13). GATA3 was expressed in most anaplastic cell samples (12/13), and IL-4 expression was found in some anaplastic cell samples (6/13). IL-13 and GATA3 expression were observed in some intra-capsular small lymphocytes.
While IL-13 was also detected in BIA-ALCL cell lines, it was not found in 4 of the 7 systemic ALCL cases or the PTCL-NOS case.
Dr Kadin said the lack of IL-13 receptor expression in BIA-ALCL cell lines suggests that IL-13 is not an autocrine growth factor for BIA-ALCL, and its expression is most likely associated with an allergic immune response.
IL-13 is known to induce immunoglobulin class switching in plasma cells to produce IgE. H&E and Giemsa staining of the BIA-ALCL tumor tissue and involved regional lymph nodes revealed IgE and eosinophils on the surface of mast cells and follicular dendritic cells.
Taken together, these data point to an allergic reaction in breast implant capsules of BIA-ALCL.
Dr Kadin was hopeful that these findings could be extrapolated to prevent BIA-ALCL by identifying individuals at higher risk for developing the disease.
The next step for this research is to decipher the role of bacterial antigens in mediating the immune response and whether women who develop BIA-ALCL have a significant increase in other atopic conditions. ![]()
SAN FRANCISCO—A small study suggests an abnormal immune response characterized by the production of interleukin-13 (IL-13) underlies the pathogenesis of breast implant-associated anaplastic large-cell lymphoma (BIA-ALCL).
The immune response appears to be directed toward bacterial antigens on the surface of the breast implants.
Marshall E. Kadin, MD, of Roger Williams Medical Center in Providence, Rhode Island, presented these findings at the 9th Annual T-cell Lymphoma Forum.
Dr Kadin noted that BIA-ALCL is a rare type of CD30+ T-cell ALCL that has been reported in more than 200 women worldwide.
Although a cause-and-effect relationship between breast implants and BIA-ALCL has been suggested, the underlying pathogenesis of this malignancy is unclear.
A bacterial biofilm containing gram-negative bacilli has been detected in breast implants from patients with BIA-ALCL.
Therefore, Dr Kadin and his colleagues hypothesized that an immune response toward the bacterial antigens may mediate the pathogenesis of BIA-ALCL.
The researchers studied 13 clinical samples of breast implant capsules and regional lymph nodes from 4 patients with BIA-ALCL, 7 patients with systemic ALCL, and 1 patient with peripheral T-cell lymphoma-not otherwise specified (PTCL-NOS).
Immunohistochemistry was used to determine the presence of IL-13, IL-4, GATA3, and immunoglobulin E (IgE) in these samples.
All clinical samples of anaplastic cells from breast implant capsules tested positive for the presence of IL-13 (13/13). GATA3 was expressed in most anaplastic cell samples (12/13), and IL-4 expression was found in some anaplastic cell samples (6/13). IL-13 and GATA3 expression were observed in some intra-capsular small lymphocytes.
While IL-13 was also detected in BIA-ALCL cell lines, it was not found in 4 of the 7 systemic ALCL cases or the PTCL-NOS case.
Dr Kadin said the lack of IL-13 receptor expression in BIA-ALCL cell lines suggests that IL-13 is not an autocrine growth factor for BIA-ALCL, and its expression is most likely associated with an allergic immune response.
IL-13 is known to induce immunoglobulin class switching in plasma cells to produce IgE. H&E and Giemsa staining of the BIA-ALCL tumor tissue and involved regional lymph nodes revealed IgE and eosinophils on the surface of mast cells and follicular dendritic cells.
Taken together, these data point to an allergic reaction in breast implant capsules of BIA-ALCL.
Dr Kadin was hopeful that these findings could be extrapolated to prevent BIA-ALCL by identifying individuals at higher risk for developing the disease.
The next step for this research is to decipher the role of bacterial antigens in mediating the immune response and whether women who develop BIA-ALCL have a significant increase in other atopic conditions. ![]()
Study reveals higher rate of early death in kids with cancer
New research suggests that, in the US, early deaths from childhood cancer may be more common than clinical trials suggest.
Researchers found the rate of death within 1 month of diagnosis was higher among patients included in a large national database than among patients enrolled in phase 3 trials.
The data also indicated that early death is more likely in cancer patients under the age of 1 and those belonging to minority racial and ethnic groups.
Adam Green, MD, of the University of Colorado Anschutz Medical Campus in Aurora, and his colleagues conducted this research and reported the results in the Journal of Clinical Oncology.
The researchers analyzed data from the Surveillance, Epidemiology and End Results (SEER) database, which collects about 15% of all cancer outcomes across the US (representing a geographic and socioeconomic cross-section).
The team identified 36,337 patients with pediatric cancer (ages 0 to 19) diagnosed between 1992 and 2011. Of these patients, 555 (1.5%) died within 1 month of diagnosis.
Young age
Overall, the strongest predictor of early death was age below 1 year. The odds ratio (OR) was 4.36 for these patients (P<0.001), 0.75 for patients age 1 to 4, 0.78 for patients age 5 to 9, 1.00 (reference) for those age 10 to 14, and 1.69 for those age 15 to 19.
For hematologic malignancies, the adjusted OR (adjusted by poverty, unemployment, education, and year of diagnosis) was 4.32 for patients younger than 1 (P<0.001), 0.76 for patients age 1 to 4, 0.79 for patients age 5 to 9, 1.00 for those age 10 to 14, and 1.76 for those age 15 to 19 (P=0.002).
“In general, babies are just challenging, clinically, because they can’t tell you what they’re feeling,” Dr Green noted. “Parents and physicians have to pick the ones with cancer from the ones with a cold, without the patient being able to tell you about symptoms that could be diagnostic.”
“Babies tend to get aggressive cancers, it’s hard to tell when they’re getting sick, and some are even born with cancers that have already progressed. These factors combine to make very young age the strongest predictor of early death in our study.”
Race/ethnicity
Black race and Hispanic ethnicity also predicted early death. The OR was 1.48 for black race, 1.00 for white race (reference), and 1.09 for “other” races (P=0.102). The OR was 1.39 for Hispanic patients and 1.00 (reference) for non-Hispanic patients (P=0.007).
For hematologic malignancies, the adjusted OR was 1.68 for black patients (P=0.01) and 1.44 for patients of other, non-white races. The adjusted OR was 1.48 for Hispanic patients (P=0.009).
Dr Green said he hopes future studies will be able to determine the factors responsible for these disparities.
Higher death rates
Dr Green and his colleagues also found the rate of early death due to pediatric cancers is higher than reported in clinical trials, and this was true for all cancer subtypes assessed.
“Most of what we know about outcomes for cancer patients come from clinical trials, which have much more thorough reporting rules than cancer treated outside trials,” Dr Green said. “However, these kids in our study aren’t surviving long enough to join clinical trials.”
The researchers looked at a phase 3 trial (COG AAML0531) of pediatric patients with acute myeloid leukemia (AML) who were studied from August 2006 to June 2010. The early death rate in this trial was 1.6% (16/1022).
In contrast, the SEER database showed an early death rate for pediatric AML patients of 5.9% (15/256) during the same period as the trial and 6.2% (106/1698) for the period from 1992 to 2011. This is almost 4 times as high as the death rate in the trial.
The same effect was observed for acute lymphoblastic leukemia (ALL).
The early death rate for non-infant ALL was 0.7% (13/1790) in a trial (POG 9900) conducted from April 2000 to April 2005, 1.6% (30/1823) in the SEER data covering the same time period, and 1.3% (94/7353) in the SEER data from 1992 to 2011.
For infant ALL, the rates were 2.0% (3/149) in a trial (COG AALL0631) conducted from January 2008 to June 2014, 5.0% (2/40) in the SEER data during the trial period, and 5.4% (12/223) in the SEER data from 1992 to 2011.
“I had a hunch this was a bigger problem than we thought,” Dr Green said. “Now we see that is indeed the case.” ![]()
New research suggests that, in the US, early deaths from childhood cancer may be more common than clinical trials suggest.
Researchers found the rate of death within 1 month of diagnosis was higher among patients included in a large national database than among patients enrolled in phase 3 trials.
The data also indicated that early death is more likely in cancer patients under the age of 1 and those belonging to minority racial and ethnic groups.
Adam Green, MD, of the University of Colorado Anschutz Medical Campus in Aurora, and his colleagues conducted this research and reported the results in the Journal of Clinical Oncology.
The researchers analyzed data from the Surveillance, Epidemiology and End Results (SEER) database, which collects about 15% of all cancer outcomes across the US (representing a geographic and socioeconomic cross-section).
The team identified 36,337 patients with pediatric cancer (ages 0 to 19) diagnosed between 1992 and 2011. Of these patients, 555 (1.5%) died within 1 month of diagnosis.
Young age
Overall, the strongest predictor of early death was age below 1 year. The odds ratio (OR) was 4.36 for these patients (P<0.001), 0.75 for patients age 1 to 4, 0.78 for patients age 5 to 9, 1.00 (reference) for those age 10 to 14, and 1.69 for those age 15 to 19.
For hematologic malignancies, the adjusted OR (adjusted by poverty, unemployment, education, and year of diagnosis) was 4.32 for patients younger than 1 (P<0.001), 0.76 for patients age 1 to 4, 0.79 for patients age 5 to 9, 1.00 for those age 10 to 14, and 1.76 for those age 15 to 19 (P=0.002).
“In general, babies are just challenging, clinically, because they can’t tell you what they’re feeling,” Dr Green noted. “Parents and physicians have to pick the ones with cancer from the ones with a cold, without the patient being able to tell you about symptoms that could be diagnostic.”
“Babies tend to get aggressive cancers, it’s hard to tell when they’re getting sick, and some are even born with cancers that have already progressed. These factors combine to make very young age the strongest predictor of early death in our study.”
Race/ethnicity
Black race and Hispanic ethnicity also predicted early death. The OR was 1.48 for black race, 1.00 for white race (reference), and 1.09 for “other” races (P=0.102). The OR was 1.39 for Hispanic patients and 1.00 (reference) for non-Hispanic patients (P=0.007).
For hematologic malignancies, the adjusted OR was 1.68 for black patients (P=0.01) and 1.44 for patients of other, non-white races. The adjusted OR was 1.48 for Hispanic patients (P=0.009).
Dr Green said he hopes future studies will be able to determine the factors responsible for these disparities.
Higher death rates
Dr Green and his colleagues also found the rate of early death due to pediatric cancers is higher than reported in clinical trials, and this was true for all cancer subtypes assessed.
“Most of what we know about outcomes for cancer patients come from clinical trials, which have much more thorough reporting rules than cancer treated outside trials,” Dr Green said. “However, these kids in our study aren’t surviving long enough to join clinical trials.”
The researchers looked at a phase 3 trial (COG AAML0531) of pediatric patients with acute myeloid leukemia (AML) who were studied from August 2006 to June 2010. The early death rate in this trial was 1.6% (16/1022).
In contrast, the SEER database showed an early death rate for pediatric AML patients of 5.9% (15/256) during the same period as the trial and 6.2% (106/1698) for the period from 1992 to 2011. This is almost 4 times as high as the death rate in the trial.
The same effect was observed for acute lymphoblastic leukemia (ALL).
The early death rate for non-infant ALL was 0.7% (13/1790) in a trial (POG 9900) conducted from April 2000 to April 2005, 1.6% (30/1823) in the SEER data covering the same time period, and 1.3% (94/7353) in the SEER data from 1992 to 2011.
For infant ALL, the rates were 2.0% (3/149) in a trial (COG AALL0631) conducted from January 2008 to June 2014, 5.0% (2/40) in the SEER data during the trial period, and 5.4% (12/223) in the SEER data from 1992 to 2011.
“I had a hunch this was a bigger problem than we thought,” Dr Green said. “Now we see that is indeed the case.” ![]()
New research suggests that, in the US, early deaths from childhood cancer may be more common than clinical trials suggest.
Researchers found the rate of death within 1 month of diagnosis was higher among patients included in a large national database than among patients enrolled in phase 3 trials.
The data also indicated that early death is more likely in cancer patients under the age of 1 and those belonging to minority racial and ethnic groups.
Adam Green, MD, of the University of Colorado Anschutz Medical Campus in Aurora, and his colleagues conducted this research and reported the results in the Journal of Clinical Oncology.
The researchers analyzed data from the Surveillance, Epidemiology and End Results (SEER) database, which collects about 15% of all cancer outcomes across the US (representing a geographic and socioeconomic cross-section).
The team identified 36,337 patients with pediatric cancer (ages 0 to 19) diagnosed between 1992 and 2011. Of these patients, 555 (1.5%) died within 1 month of diagnosis.
Young age
Overall, the strongest predictor of early death was age below 1 year. The odds ratio (OR) was 4.36 for these patients (P<0.001), 0.75 for patients age 1 to 4, 0.78 for patients age 5 to 9, 1.00 (reference) for those age 10 to 14, and 1.69 for those age 15 to 19.
For hematologic malignancies, the adjusted OR (adjusted by poverty, unemployment, education, and year of diagnosis) was 4.32 for patients younger than 1 (P<0.001), 0.76 for patients age 1 to 4, 0.79 for patients age 5 to 9, 1.00 for those age 10 to 14, and 1.76 for those age 15 to 19 (P=0.002).
“In general, babies are just challenging, clinically, because they can’t tell you what they’re feeling,” Dr Green noted. “Parents and physicians have to pick the ones with cancer from the ones with a cold, without the patient being able to tell you about symptoms that could be diagnostic.”
“Babies tend to get aggressive cancers, it’s hard to tell when they’re getting sick, and some are even born with cancers that have already progressed. These factors combine to make very young age the strongest predictor of early death in our study.”
Race/ethnicity
Black race and Hispanic ethnicity also predicted early death. The OR was 1.48 for black race, 1.00 for white race (reference), and 1.09 for “other” races (P=0.102). The OR was 1.39 for Hispanic patients and 1.00 (reference) for non-Hispanic patients (P=0.007).
For hematologic malignancies, the adjusted OR was 1.68 for black patients (P=0.01) and 1.44 for patients of other, non-white races. The adjusted OR was 1.48 for Hispanic patients (P=0.009).
Dr Green said he hopes future studies will be able to determine the factors responsible for these disparities.
Higher death rates
Dr Green and his colleagues also found the rate of early death due to pediatric cancers is higher than reported in clinical trials, and this was true for all cancer subtypes assessed.
“Most of what we know about outcomes for cancer patients come from clinical trials, which have much more thorough reporting rules than cancer treated outside trials,” Dr Green said. “However, these kids in our study aren’t surviving long enough to join clinical trials.”
The researchers looked at a phase 3 trial (COG AAML0531) of pediatric patients with acute myeloid leukemia (AML) who were studied from August 2006 to June 2010. The early death rate in this trial was 1.6% (16/1022).
In contrast, the SEER database showed an early death rate for pediatric AML patients of 5.9% (15/256) during the same period as the trial and 6.2% (106/1698) for the period from 1992 to 2011. This is almost 4 times as high as the death rate in the trial.
The same effect was observed for acute lymphoblastic leukemia (ALL).
The early death rate for non-infant ALL was 0.7% (13/1790) in a trial (POG 9900) conducted from April 2000 to April 2005, 1.6% (30/1823) in the SEER data covering the same time period, and 1.3% (94/7353) in the SEER data from 1992 to 2011.
For infant ALL, the rates were 2.0% (3/149) in a trial (COG AALL0631) conducted from January 2008 to June 2014, 5.0% (2/40) in the SEER data during the trial period, and 5.4% (12/223) in the SEER data from 1992 to 2011.
“I had a hunch this was a bigger problem than we thought,” Dr Green said. “Now we see that is indeed the case.” ![]()
Gene therapy proves effective in SCD patient
Researchers have reported a favorable outcome in the first patient with severe sickle cell disease (SCD) to receive gene therapy in the HGB-205 study.
The subject, known as Patient 1204, was treated with LentiGlobin BB305, a product consisting of his own manipulated hematopoietic stem cells (HSCs).
A functional human β-globin gene was inserted into the patient’s HSCs ex vivo, and the cells were returned to him via transplant.
Fifteen months after receiving this treatment, Patient 1204 had high levels of anti-sickling hemoglobin (HbAT87Q), and there were no adverse events thought to be related to LentiGlobin BB305.
These results were published in NEJM. The research was supported by bluebird bio, the company developing LentiGlobin BB305.
Patient 1204 is a male with βS/βS genotype. In May 2014, at 13 years of age, the patient was enrolled in the HGB-205 study at Hôpital Necker-Enfants Malades in Paris, France.
The patient had received hydroxyurea from age 2 to 9 and had both a cholecystectomy and a splenectomy. He received regular transfusions (plus iron chelation with deferasirox) for 4 years prior to this study.
The patient had an average of 1.6 SCD-related events annually in the 9 years prior to starting transfusions. His complications from SCD included vaso-occlusive crises, acute-chest syndrome, bilateral hip osteonecrosis, and cerebral vasculopathy.
The patient underwent 2 bone marrow harvests to collect HSCs for gene transfer and back-up (6.2×108 and 5.4×108 total nucleated cells/kg harvested).
CD34+ cells were enriched from the harvested marrow and then transduced with LentiGlobin BB305 lentiviral vector.
The patient underwent myeloablation with intravenous busulfan (2.3 to 4.8 mg/kg per day for 4 days) with daily pharmacokinetic studies and dose adjustment. Total busulfan area under the curve was 19,363 μmol/min.
After a 2-day washout, the patient received LentiGlobin BB305 in October 2014 at a post-thaw total dose of 5.6×106 CD34+ cells/kg. Neutrophil and platelet engraftment were achieved on day 38 and day 91 post-transplant, respectively.
Red blood cell transfusions were to be continued after transplant until a sufficient proportion of HbAT87Q (25% to 30% of total hemoglobin) was detected. Transfusions were discontinued after day 88 post-transplant.
HbAT87Q reached 5.5 g/dL (46% of total hemoglobin) at month 9 and continued to increase to 5.7 g/dL at month 15 (48%). Hemoglobin S levels were 5.5 g/dL (46%) at month 9 and 5.8 g/dL (49%) at month 15.
Total hemoglobin levels were stable, between 10.6 and 12.0 g/dL, from months 6 to 15. Fetal hemoglobin levels remained below 1.0 g/dL.
No adverse events related to LentiGlobin BB305 were reported. There were, however, adverse events related to busulfan conditioning (grade 3 anemia, thrombocytopenia, and infection; grade 4 neutropenia).
During 15 months of follow-up, there were no SCD-related clinical events or hospitalizations. The patient was able to stop all medications, including pain medication.
The patient resumed regular school attendance and reported full participation in normal physical activities.
“We have managed this patient at Necker for more than 10 years, and standard treatments were not able to control his SCD symptoms,” said Marina Cavazzana, MD, PhD, of Hôpital Necker-Enfants Malades.
“He had to receive blood transfusions every month to prevent severe pain crises. Since receiving the autologous stem cell transplant with LentiGlobin, he has been free from severe symptoms and has resumed normal activities, without the need for further transfusions.” ![]()
Researchers have reported a favorable outcome in the first patient with severe sickle cell disease (SCD) to receive gene therapy in the HGB-205 study.
The subject, known as Patient 1204, was treated with LentiGlobin BB305, a product consisting of his own manipulated hematopoietic stem cells (HSCs).
A functional human β-globin gene was inserted into the patient’s HSCs ex vivo, and the cells were returned to him via transplant.
Fifteen months after receiving this treatment, Patient 1204 had high levels of anti-sickling hemoglobin (HbAT87Q), and there were no adverse events thought to be related to LentiGlobin BB305.
These results were published in NEJM. The research was supported by bluebird bio, the company developing LentiGlobin BB305.
Patient 1204 is a male with βS/βS genotype. In May 2014, at 13 years of age, the patient was enrolled in the HGB-205 study at Hôpital Necker-Enfants Malades in Paris, France.
The patient had received hydroxyurea from age 2 to 9 and had both a cholecystectomy and a splenectomy. He received regular transfusions (plus iron chelation with deferasirox) for 4 years prior to this study.
The patient had an average of 1.6 SCD-related events annually in the 9 years prior to starting transfusions. His complications from SCD included vaso-occlusive crises, acute-chest syndrome, bilateral hip osteonecrosis, and cerebral vasculopathy.
The patient underwent 2 bone marrow harvests to collect HSCs for gene transfer and back-up (6.2×108 and 5.4×108 total nucleated cells/kg harvested).
CD34+ cells were enriched from the harvested marrow and then transduced with LentiGlobin BB305 lentiviral vector.
The patient underwent myeloablation with intravenous busulfan (2.3 to 4.8 mg/kg per day for 4 days) with daily pharmacokinetic studies and dose adjustment. Total busulfan area under the curve was 19,363 μmol/min.
After a 2-day washout, the patient received LentiGlobin BB305 in October 2014 at a post-thaw total dose of 5.6×106 CD34+ cells/kg. Neutrophil and platelet engraftment were achieved on day 38 and day 91 post-transplant, respectively.
Red blood cell transfusions were to be continued after transplant until a sufficient proportion of HbAT87Q (25% to 30% of total hemoglobin) was detected. Transfusions were discontinued after day 88 post-transplant.
HbAT87Q reached 5.5 g/dL (46% of total hemoglobin) at month 9 and continued to increase to 5.7 g/dL at month 15 (48%). Hemoglobin S levels were 5.5 g/dL (46%) at month 9 and 5.8 g/dL (49%) at month 15.
Total hemoglobin levels were stable, between 10.6 and 12.0 g/dL, from months 6 to 15. Fetal hemoglobin levels remained below 1.0 g/dL.
No adverse events related to LentiGlobin BB305 were reported. There were, however, adverse events related to busulfan conditioning (grade 3 anemia, thrombocytopenia, and infection; grade 4 neutropenia).
During 15 months of follow-up, there were no SCD-related clinical events or hospitalizations. The patient was able to stop all medications, including pain medication.
The patient resumed regular school attendance and reported full participation in normal physical activities.
“We have managed this patient at Necker for more than 10 years, and standard treatments were not able to control his SCD symptoms,” said Marina Cavazzana, MD, PhD, of Hôpital Necker-Enfants Malades.
“He had to receive blood transfusions every month to prevent severe pain crises. Since receiving the autologous stem cell transplant with LentiGlobin, he has been free from severe symptoms and has resumed normal activities, without the need for further transfusions.” ![]()
Researchers have reported a favorable outcome in the first patient with severe sickle cell disease (SCD) to receive gene therapy in the HGB-205 study.
The subject, known as Patient 1204, was treated with LentiGlobin BB305, a product consisting of his own manipulated hematopoietic stem cells (HSCs).
A functional human β-globin gene was inserted into the patient’s HSCs ex vivo, and the cells were returned to him via transplant.
Fifteen months after receiving this treatment, Patient 1204 had high levels of anti-sickling hemoglobin (HbAT87Q), and there were no adverse events thought to be related to LentiGlobin BB305.
These results were published in NEJM. The research was supported by bluebird bio, the company developing LentiGlobin BB305.
Patient 1204 is a male with βS/βS genotype. In May 2014, at 13 years of age, the patient was enrolled in the HGB-205 study at Hôpital Necker-Enfants Malades in Paris, France.
The patient had received hydroxyurea from age 2 to 9 and had both a cholecystectomy and a splenectomy. He received regular transfusions (plus iron chelation with deferasirox) for 4 years prior to this study.
The patient had an average of 1.6 SCD-related events annually in the 9 years prior to starting transfusions. His complications from SCD included vaso-occlusive crises, acute-chest syndrome, bilateral hip osteonecrosis, and cerebral vasculopathy.
The patient underwent 2 bone marrow harvests to collect HSCs for gene transfer and back-up (6.2×108 and 5.4×108 total nucleated cells/kg harvested).
CD34+ cells were enriched from the harvested marrow and then transduced with LentiGlobin BB305 lentiviral vector.
The patient underwent myeloablation with intravenous busulfan (2.3 to 4.8 mg/kg per day for 4 days) with daily pharmacokinetic studies and dose adjustment. Total busulfan area under the curve was 19,363 μmol/min.
After a 2-day washout, the patient received LentiGlobin BB305 in October 2014 at a post-thaw total dose of 5.6×106 CD34+ cells/kg. Neutrophil and platelet engraftment were achieved on day 38 and day 91 post-transplant, respectively.
Red blood cell transfusions were to be continued after transplant until a sufficient proportion of HbAT87Q (25% to 30% of total hemoglobin) was detected. Transfusions were discontinued after day 88 post-transplant.
HbAT87Q reached 5.5 g/dL (46% of total hemoglobin) at month 9 and continued to increase to 5.7 g/dL at month 15 (48%). Hemoglobin S levels were 5.5 g/dL (46%) at month 9 and 5.8 g/dL (49%) at month 15.
Total hemoglobin levels were stable, between 10.6 and 12.0 g/dL, from months 6 to 15. Fetal hemoglobin levels remained below 1.0 g/dL.
No adverse events related to LentiGlobin BB305 were reported. There were, however, adverse events related to busulfan conditioning (grade 3 anemia, thrombocytopenia, and infection; grade 4 neutropenia).
During 15 months of follow-up, there were no SCD-related clinical events or hospitalizations. The patient was able to stop all medications, including pain medication.
The patient resumed regular school attendance and reported full participation in normal physical activities.
“We have managed this patient at Necker for more than 10 years, and standard treatments were not able to control his SCD symptoms,” said Marina Cavazzana, MD, PhD, of Hôpital Necker-Enfants Malades.
“He had to receive blood transfusions every month to prevent severe pain crises. Since receiving the autologous stem cell transplant with LentiGlobin, he has been free from severe symptoms and has resumed normal activities, without the need for further transfusions.” ![]()
Hemophilia B therapy granted access to PRIME program
SPK-9001, an investigational therapy intended to treat hemophilia B, has been granted access to the European Medicines Agency’s (EMA) PRIority MEdicines (PRIME) program.
The goal of the EMA’s PRIME program is to accelerate the development of therapies that may offer a major advantage over existing treatments or benefit patients with no treatment options.
Through PRIME, the EMA offers early and enhanced support to developers in order to optimize development plans and speed regulatory evaluations to potentially bring therapies to patients more quickly.
To be accepted for PRIME, a therapy must demonstrate the potential to benefit patients with unmet medical need through early clinical or nonclinical data.
About SPK-9001
SPK-9001 is a bio-engineered adeno-associated virus capsid expressing a codon-optimized, high-activity human factor IX variant enabling endogenous production of factor IX. The therapy is being developed by Spark Therapeutics and Pfizer Inc.
SPK-9001 is under investigation in a phase 1/2 trial. Results from this trial were presented at the 2016 ASH Annual Meeting.
The presentation included data on 9 patients who received a single intravenous infusion of SPK-9001 at 5 x 1011 vg/kg. The patients were followed for 52 weeks, with a long-term follow-up of 14 years.
Before their SPK-9001 infusion, the patients required anywhere from 0 to 159 factor IX infusions each year. Three patients had annualized bleeding rates (ABRs) of 0, and the remaining 6 patients had ABRs of 1, 2, 4, 10, 12, and 49.
After SPK-9001, 8 patients were able to stop receiving factor IX infusions and achieved an ABR of 0. One patient had an ABR of 2 and required on-demand factor IX therapy. (All patients stopped receiving factor IX prophylaxis).
The researchers said there were no unexpected vector or procedure-related adverse events, and none of the patients developed factor IX alloinhibitory antibodies. ![]()
SPK-9001, an investigational therapy intended to treat hemophilia B, has been granted access to the European Medicines Agency’s (EMA) PRIority MEdicines (PRIME) program.
The goal of the EMA’s PRIME program is to accelerate the development of therapies that may offer a major advantage over existing treatments or benefit patients with no treatment options.
Through PRIME, the EMA offers early and enhanced support to developers in order to optimize development plans and speed regulatory evaluations to potentially bring therapies to patients more quickly.
To be accepted for PRIME, a therapy must demonstrate the potential to benefit patients with unmet medical need through early clinical or nonclinical data.
About SPK-9001
SPK-9001 is a bio-engineered adeno-associated virus capsid expressing a codon-optimized, high-activity human factor IX variant enabling endogenous production of factor IX. The therapy is being developed by Spark Therapeutics and Pfizer Inc.
SPK-9001 is under investigation in a phase 1/2 trial. Results from this trial were presented at the 2016 ASH Annual Meeting.
The presentation included data on 9 patients who received a single intravenous infusion of SPK-9001 at 5 x 1011 vg/kg. The patients were followed for 52 weeks, with a long-term follow-up of 14 years.
Before their SPK-9001 infusion, the patients required anywhere from 0 to 159 factor IX infusions each year. Three patients had annualized bleeding rates (ABRs) of 0, and the remaining 6 patients had ABRs of 1, 2, 4, 10, 12, and 49.
After SPK-9001, 8 patients were able to stop receiving factor IX infusions and achieved an ABR of 0. One patient had an ABR of 2 and required on-demand factor IX therapy. (All patients stopped receiving factor IX prophylaxis).
The researchers said there were no unexpected vector or procedure-related adverse events, and none of the patients developed factor IX alloinhibitory antibodies. ![]()
SPK-9001, an investigational therapy intended to treat hemophilia B, has been granted access to the European Medicines Agency’s (EMA) PRIority MEdicines (PRIME) program.
The goal of the EMA’s PRIME program is to accelerate the development of therapies that may offer a major advantage over existing treatments or benefit patients with no treatment options.
Through PRIME, the EMA offers early and enhanced support to developers in order to optimize development plans and speed regulatory evaluations to potentially bring therapies to patients more quickly.
To be accepted for PRIME, a therapy must demonstrate the potential to benefit patients with unmet medical need through early clinical or nonclinical data.
About SPK-9001
SPK-9001 is a bio-engineered adeno-associated virus capsid expressing a codon-optimized, high-activity human factor IX variant enabling endogenous production of factor IX. The therapy is being developed by Spark Therapeutics and Pfizer Inc.
SPK-9001 is under investigation in a phase 1/2 trial. Results from this trial were presented at the 2016 ASH Annual Meeting.
The presentation included data on 9 patients who received a single intravenous infusion of SPK-9001 at 5 x 1011 vg/kg. The patients were followed for 52 weeks, with a long-term follow-up of 14 years.
Before their SPK-9001 infusion, the patients required anywhere from 0 to 159 factor IX infusions each year. Three patients had annualized bleeding rates (ABRs) of 0, and the remaining 6 patients had ABRs of 1, 2, 4, 10, 12, and 49.
After SPK-9001, 8 patients were able to stop receiving factor IX infusions and achieved an ABR of 0. One patient had an ABR of 2 and required on-demand factor IX therapy. (All patients stopped receiving factor IX prophylaxis).
The researchers said there were no unexpected vector or procedure-related adverse events, and none of the patients developed factor IX alloinhibitory antibodies. ![]()
Cigarette Smoking: Modifiable Risk Factor for MS
Q) What impact does cigarette smoking have on multiple sclerosis?
The development and disease course of multiple sclerosis (MS) are influenced by a variety of factors. Some—such as genetics, environmental exposure to viruses, and place of residence at an early age—cannot be modified. There are, however, other factors that can be modified, one of which is cigarette smoking.
Between 15% and 17% of patients with MS smoke cigarettes, a rate comparable to that of the general United States population; among US veterans with MS, prevalence can reach as high as 28.5%.1-4 Factors that correlate with smoking in patients with MS include younger age, lower economic/educational background, being single, and lack of available or affordable cessation strategies.1,2,4
Studies have found that in addition to contributing to the development of diseases (eg, cardiovascular and pulmonary) and certain cancers, smoking cigarettes may put individuals at higher risk for MS.5-7 Data also show that increased duration of smoking and/or increased quantity of cigarettes smoked may exacerbate this risk.5 While the mechanisms are not well understood, there appears to be a higher prevalence of MS in male smokers and in current smokers (compared with those who have already quit).6 Other studies have also suggested an increased risk with passive exposure to cigarette smoke.6,7
Current cigarette smoking accelerates the conversion from a relapsing to a progressive form of MS.8-11 One study demonstrated that after the diagnosis of MS had been made, continued smoking increased the rate of acceleration to a progressive form by 5% per year.9 Current smokers also had a higher disability rate attributable to their MS, but smoking cessation may improve disability outcomes.9-11
Current smokers, with or without other risk factors, therefore have incentive to quit smoking to reduce risk for MS. Patients with MS who smoke should be counseled on the increased risk associated with the combination of passive smoke exposure and other genetic and environmental factors, which may increase risk for MS in first-degree family members.3
While there is no one-size-fits-all strategy for smoking cessation in patients with MS, traditional behavioral and/or medication therapies should be offered. Some factors involved in cigarette smoking are variable and difficult to address, in addition to the physical dependence on nicotine. Many patients will require interventions to address related poor health behaviors (eg, lack of exercise) and comorbid factors (eg, depression).5,7 —BW
Bryan Walker, MHS, PA-C
Department of Neurology, Division of MS and Neuroimmunology, Duke University Medical Center, Durham, North Carolina
1. Friend KB, Mernoff ST, Block P, Reeve G. Smoking rates and smoking cessation among individuals with multiple sclerosis. Disabil Rehabil. 2006;28(18):1135-1141.
2. Marrie R, Horwitz R, Cutter G, et al. High frequency of adverse health behaviors in multiple sclerosis. Mult Scler. 2009;15(1): 105-113.
3. CDC. Current cigarette smoking among adults in the United States. www.cdc.gov/tobacco/data_statistics/fact_sheets/adult_data/cig_smoking. Accessed January 13, 2017.
4. Turner AP, Kivlahan DR, Kazis LE, Haselkorn JK. Smoking among veterans with multiple sclerosis: prevalence correlates, quit attempts, and unmet need for services. Arch Phys Med Rehabil. 2007;88(11):1394-1399.
5. Ascherio A, Munger K. Epidemiology of multiple sclerosis: from risk factors to prevention—an update. Semin Neurol. 2016; 36(2):103-114.
6. Zhang P, Wang R, Li Z, et al. The risk of smoking on multiple sclerosis: a meta-analysis based on 20,626 cases from case-control and cohort studies. PeerJ. 2016;4:e1797.
7. Hedström AK, Olsson T, Alfredsson L. Smoking is a major preventable risk factor for multiple sclerosis. Mult Scler. 2016; 22(8):1021-1026.
8. Healy BC, Ali EN, Guttmann CR, et al. Smoking and disease progression in multiple sclerosis. Arch Neurol. 2009;66(7):858-864.
9. Goldman MD, Stüve O. Smoking beyond multiple sclerosis diagnosis: a risk factor still worth modifying. JAMA Neurol. 2015;72(10):1105-1106.
10. Ramanujam R, Hedström A, Manouchehrinia A, et al. Effect of smoking cessation on multiple sclerosis prognosis. JAMA Neurol. 2015;72(10):1117-1123.
11. Ben-Zacharia A. The effect of modifiable risk factors on multiple sclerosis progression. Neurology. 2016;86(16):Supplement P1.387.
Q) What impact does cigarette smoking have on multiple sclerosis?
The development and disease course of multiple sclerosis (MS) are influenced by a variety of factors. Some—such as genetics, environmental exposure to viruses, and place of residence at an early age—cannot be modified. There are, however, other factors that can be modified, one of which is cigarette smoking.
Between 15% and 17% of patients with MS smoke cigarettes, a rate comparable to that of the general United States population; among US veterans with MS, prevalence can reach as high as 28.5%.1-4 Factors that correlate with smoking in patients with MS include younger age, lower economic/educational background, being single, and lack of available or affordable cessation strategies.1,2,4
Studies have found that in addition to contributing to the development of diseases (eg, cardiovascular and pulmonary) and certain cancers, smoking cigarettes may put individuals at higher risk for MS.5-7 Data also show that increased duration of smoking and/or increased quantity of cigarettes smoked may exacerbate this risk.5 While the mechanisms are not well understood, there appears to be a higher prevalence of MS in male smokers and in current smokers (compared with those who have already quit).6 Other studies have also suggested an increased risk with passive exposure to cigarette smoke.6,7
Current cigarette smoking accelerates the conversion from a relapsing to a progressive form of MS.8-11 One study demonstrated that after the diagnosis of MS had been made, continued smoking increased the rate of acceleration to a progressive form by 5% per year.9 Current smokers also had a higher disability rate attributable to their MS, but smoking cessation may improve disability outcomes.9-11
Current smokers, with or without other risk factors, therefore have incentive to quit smoking to reduce risk for MS. Patients with MS who smoke should be counseled on the increased risk associated with the combination of passive smoke exposure and other genetic and environmental factors, which may increase risk for MS in first-degree family members.3
While there is no one-size-fits-all strategy for smoking cessation in patients with MS, traditional behavioral and/or medication therapies should be offered. Some factors involved in cigarette smoking are variable and difficult to address, in addition to the physical dependence on nicotine. Many patients will require interventions to address related poor health behaviors (eg, lack of exercise) and comorbid factors (eg, depression).5,7 —BW
Bryan Walker, MHS, PA-C
Department of Neurology, Division of MS and Neuroimmunology, Duke University Medical Center, Durham, North Carolina
Q) What impact does cigarette smoking have on multiple sclerosis?
The development and disease course of multiple sclerosis (MS) are influenced by a variety of factors. Some—such as genetics, environmental exposure to viruses, and place of residence at an early age—cannot be modified. There are, however, other factors that can be modified, one of which is cigarette smoking.
Between 15% and 17% of patients with MS smoke cigarettes, a rate comparable to that of the general United States population; among US veterans with MS, prevalence can reach as high as 28.5%.1-4 Factors that correlate with smoking in patients with MS include younger age, lower economic/educational background, being single, and lack of available or affordable cessation strategies.1,2,4
Studies have found that in addition to contributing to the development of diseases (eg, cardiovascular and pulmonary) and certain cancers, smoking cigarettes may put individuals at higher risk for MS.5-7 Data also show that increased duration of smoking and/or increased quantity of cigarettes smoked may exacerbate this risk.5 While the mechanisms are not well understood, there appears to be a higher prevalence of MS in male smokers and in current smokers (compared with those who have already quit).6 Other studies have also suggested an increased risk with passive exposure to cigarette smoke.6,7
Current cigarette smoking accelerates the conversion from a relapsing to a progressive form of MS.8-11 One study demonstrated that after the diagnosis of MS had been made, continued smoking increased the rate of acceleration to a progressive form by 5% per year.9 Current smokers also had a higher disability rate attributable to their MS, but smoking cessation may improve disability outcomes.9-11
Current smokers, with or without other risk factors, therefore have incentive to quit smoking to reduce risk for MS. Patients with MS who smoke should be counseled on the increased risk associated with the combination of passive smoke exposure and other genetic and environmental factors, which may increase risk for MS in first-degree family members.3
While there is no one-size-fits-all strategy for smoking cessation in patients with MS, traditional behavioral and/or medication therapies should be offered. Some factors involved in cigarette smoking are variable and difficult to address, in addition to the physical dependence on nicotine. Many patients will require interventions to address related poor health behaviors (eg, lack of exercise) and comorbid factors (eg, depression).5,7 —BW
Bryan Walker, MHS, PA-C
Department of Neurology, Division of MS and Neuroimmunology, Duke University Medical Center, Durham, North Carolina
1. Friend KB, Mernoff ST, Block P, Reeve G. Smoking rates and smoking cessation among individuals with multiple sclerosis. Disabil Rehabil. 2006;28(18):1135-1141.
2. Marrie R, Horwitz R, Cutter G, et al. High frequency of adverse health behaviors in multiple sclerosis. Mult Scler. 2009;15(1): 105-113.
3. CDC. Current cigarette smoking among adults in the United States. www.cdc.gov/tobacco/data_statistics/fact_sheets/adult_data/cig_smoking. Accessed January 13, 2017.
4. Turner AP, Kivlahan DR, Kazis LE, Haselkorn JK. Smoking among veterans with multiple sclerosis: prevalence correlates, quit attempts, and unmet need for services. Arch Phys Med Rehabil. 2007;88(11):1394-1399.
5. Ascherio A, Munger K. Epidemiology of multiple sclerosis: from risk factors to prevention—an update. Semin Neurol. 2016; 36(2):103-114.
6. Zhang P, Wang R, Li Z, et al. The risk of smoking on multiple sclerosis: a meta-analysis based on 20,626 cases from case-control and cohort studies. PeerJ. 2016;4:e1797.
7. Hedström AK, Olsson T, Alfredsson L. Smoking is a major preventable risk factor for multiple sclerosis. Mult Scler. 2016; 22(8):1021-1026.
8. Healy BC, Ali EN, Guttmann CR, et al. Smoking and disease progression in multiple sclerosis. Arch Neurol. 2009;66(7):858-864.
9. Goldman MD, Stüve O. Smoking beyond multiple sclerosis diagnosis: a risk factor still worth modifying. JAMA Neurol. 2015;72(10):1105-1106.
10. Ramanujam R, Hedström A, Manouchehrinia A, et al. Effect of smoking cessation on multiple sclerosis prognosis. JAMA Neurol. 2015;72(10):1117-1123.
11. Ben-Zacharia A. The effect of modifiable risk factors on multiple sclerosis progression. Neurology. 2016;86(16):Supplement P1.387.
1. Friend KB, Mernoff ST, Block P, Reeve G. Smoking rates and smoking cessation among individuals with multiple sclerosis. Disabil Rehabil. 2006;28(18):1135-1141.
2. Marrie R, Horwitz R, Cutter G, et al. High frequency of adverse health behaviors in multiple sclerosis. Mult Scler. 2009;15(1): 105-113.
3. CDC. Current cigarette smoking among adults in the United States. www.cdc.gov/tobacco/data_statistics/fact_sheets/adult_data/cig_smoking. Accessed January 13, 2017.
4. Turner AP, Kivlahan DR, Kazis LE, Haselkorn JK. Smoking among veterans with multiple sclerosis: prevalence correlates, quit attempts, and unmet need for services. Arch Phys Med Rehabil. 2007;88(11):1394-1399.
5. Ascherio A, Munger K. Epidemiology of multiple sclerosis: from risk factors to prevention—an update. Semin Neurol. 2016; 36(2):103-114.
6. Zhang P, Wang R, Li Z, et al. The risk of smoking on multiple sclerosis: a meta-analysis based on 20,626 cases from case-control and cohort studies. PeerJ. 2016;4:e1797.
7. Hedström AK, Olsson T, Alfredsson L. Smoking is a major preventable risk factor for multiple sclerosis. Mult Scler. 2016; 22(8):1021-1026.
8. Healy BC, Ali EN, Guttmann CR, et al. Smoking and disease progression in multiple sclerosis. Arch Neurol. 2009;66(7):858-864.
9. Goldman MD, Stüve O. Smoking beyond multiple sclerosis diagnosis: a risk factor still worth modifying. JAMA Neurol. 2015;72(10):1105-1106.
10. Ramanujam R, Hedström A, Manouchehrinia A, et al. Effect of smoking cessation on multiple sclerosis prognosis. JAMA Neurol. 2015;72(10):1117-1123.
11. Ben-Zacharia A. The effect of modifiable risk factors on multiple sclerosis progression. Neurology. 2016;86(16):Supplement P1.387.
Early Interferon Treatment Delays Time to Clinically Definite MS
In patients presenting with a first clinical demyelinating event, early treatment with subcutaneous interferon beta-1a three times per week over five years prolonged the time to clinically definite multiple sclerosis (MS), compared with delayed treatment, according to research published online ahead of print December 30, 2016 in the Journal of Neurology, Neurosurgery and Psychiatry. Compared with delayed treatment, early treatment also prolonged the time to McDonald MS conversion.
Giancarlo Comi, MD, Professor of Neurology at the Scientific Institute H.S. Raffaele in Milan, and colleagues conducted REFLEXION, a preplanned extension of the REFLEX study, to compare the effect of more frequent and early dosing with that of delayed treatment. Eligible patients were between ages 18 and 50, had an Expanded Disability Status Scale (EDSS) score of 5.0 or lower, and a single clinical event suggestive of MS within 60 days of enrollment. In REFLEX, patients had been randomized to interferon beta-1a (44 μg) thrice weekly or once weekly, or placebo. Treatment lasted for 24 months or until a patient developed clinically definite MS.
In REFLEXION, patients first randomized to interferon beta-1a who had not converted to clinically definite MS continued their original dosing regimen. Patients originally receiving placebo who had not converted to clinically definite MS were switched to interferon beta-1a (44 μg thrice weekly). These patients became the delayed-treatment group. Patients who converted to clinically definite MS during REFLEX or REFLEXION received open-label interferon beta-1a 44 μg thrice weekly from then on.
EDSS scores and clinically definite MS assessments were recorded at extension baseline (ie, month 24) and every six months thereafter. The primary end point was time to conversion to clinically definite MS from first randomization to month 36. Time to clinically definite MS to month 60 was a secondary end point.
The extension study included 127 participants originally randomized to interferon beta-1a thrice weekly, 142 participants originally randomized to interferon beta-1a once weekly, and 133 patients originally randomized to placebo. At month 36, the proportion of patients who had converted to clinically definite MS was lower among patients receiving interferon thrice weekly or weekly, compared with the delayed-treatment group (25.1%, 25.7%, and 38.6%, respectively). The risk of conversion to clinically definite MS was significantly reduced for patients receiving early treatment, compared with delayed treatment. The researchers found no significant difference between the early-treatment groups.
The analysis for month 60 also found that both early-treatment groups had longer times to clinically definite MS, compared with delayed treatment. The proportion of patients who converted to clinically definite MS increased at month 60, but was still lower among participants receiving thrice-weekly or weekly interferon than among patients on delayed treatment (32.2%, 36.0%, and 40.4%, respectively). Cumulative probability of conversion was lower with thrice-weekly and weekly early treatment than with delayed treatment (39.2%, 40.7%, and 44.6%, respectively).
—Erik Greb
Suggested Reading
Comi G, De Stefano N, Freedman MS, et al. Subcutaneous interferon β-1a in the treatment of clinically isolated syndromes: 3-year and 5-year results of the phase III dosing frequency-blind multicentre REFLEXION study. J Neurol Neurosurg Psychiatry. 2016 Dec 30 [Epub ahead of print].
In patients presenting with a first clinical demyelinating event, early treatment with subcutaneous interferon beta-1a three times per week over five years prolonged the time to clinically definite multiple sclerosis (MS), compared with delayed treatment, according to research published online ahead of print December 30, 2016 in the Journal of Neurology, Neurosurgery and Psychiatry. Compared with delayed treatment, early treatment also prolonged the time to McDonald MS conversion.
Giancarlo Comi, MD, Professor of Neurology at the Scientific Institute H.S. Raffaele in Milan, and colleagues conducted REFLEXION, a preplanned extension of the REFLEX study, to compare the effect of more frequent and early dosing with that of delayed treatment. Eligible patients were between ages 18 and 50, had an Expanded Disability Status Scale (EDSS) score of 5.0 or lower, and a single clinical event suggestive of MS within 60 days of enrollment. In REFLEX, patients had been randomized to interferon beta-1a (44 μg) thrice weekly or once weekly, or placebo. Treatment lasted for 24 months or until a patient developed clinically definite MS.
In REFLEXION, patients first randomized to interferon beta-1a who had not converted to clinically definite MS continued their original dosing regimen. Patients originally receiving placebo who had not converted to clinically definite MS were switched to interferon beta-1a (44 μg thrice weekly). These patients became the delayed-treatment group. Patients who converted to clinically definite MS during REFLEX or REFLEXION received open-label interferon beta-1a 44 μg thrice weekly from then on.
EDSS scores and clinically definite MS assessments were recorded at extension baseline (ie, month 24) and every six months thereafter. The primary end point was time to conversion to clinically definite MS from first randomization to month 36. Time to clinically definite MS to month 60 was a secondary end point.
The extension study included 127 participants originally randomized to interferon beta-1a thrice weekly, 142 participants originally randomized to interferon beta-1a once weekly, and 133 patients originally randomized to placebo. At month 36, the proportion of patients who had converted to clinically definite MS was lower among patients receiving interferon thrice weekly or weekly, compared with the delayed-treatment group (25.1%, 25.7%, and 38.6%, respectively). The risk of conversion to clinically definite MS was significantly reduced for patients receiving early treatment, compared with delayed treatment. The researchers found no significant difference between the early-treatment groups.
The analysis for month 60 also found that both early-treatment groups had longer times to clinically definite MS, compared with delayed treatment. The proportion of patients who converted to clinically definite MS increased at month 60, but was still lower among participants receiving thrice-weekly or weekly interferon than among patients on delayed treatment (32.2%, 36.0%, and 40.4%, respectively). Cumulative probability of conversion was lower with thrice-weekly and weekly early treatment than with delayed treatment (39.2%, 40.7%, and 44.6%, respectively).
—Erik Greb
Suggested Reading
Comi G, De Stefano N, Freedman MS, et al. Subcutaneous interferon β-1a in the treatment of clinically isolated syndromes: 3-year and 5-year results of the phase III dosing frequency-blind multicentre REFLEXION study. J Neurol Neurosurg Psychiatry. 2016 Dec 30 [Epub ahead of print].
In patients presenting with a first clinical demyelinating event, early treatment with subcutaneous interferon beta-1a three times per week over five years prolonged the time to clinically definite multiple sclerosis (MS), compared with delayed treatment, according to research published online ahead of print December 30, 2016 in the Journal of Neurology, Neurosurgery and Psychiatry. Compared with delayed treatment, early treatment also prolonged the time to McDonald MS conversion.
Giancarlo Comi, MD, Professor of Neurology at the Scientific Institute H.S. Raffaele in Milan, and colleagues conducted REFLEXION, a preplanned extension of the REFLEX study, to compare the effect of more frequent and early dosing with that of delayed treatment. Eligible patients were between ages 18 and 50, had an Expanded Disability Status Scale (EDSS) score of 5.0 or lower, and a single clinical event suggestive of MS within 60 days of enrollment. In REFLEX, patients had been randomized to interferon beta-1a (44 μg) thrice weekly or once weekly, or placebo. Treatment lasted for 24 months or until a patient developed clinically definite MS.
In REFLEXION, patients first randomized to interferon beta-1a who had not converted to clinically definite MS continued their original dosing regimen. Patients originally receiving placebo who had not converted to clinically definite MS were switched to interferon beta-1a (44 μg thrice weekly). These patients became the delayed-treatment group. Patients who converted to clinically definite MS during REFLEX or REFLEXION received open-label interferon beta-1a 44 μg thrice weekly from then on.
EDSS scores and clinically definite MS assessments were recorded at extension baseline (ie, month 24) and every six months thereafter. The primary end point was time to conversion to clinically definite MS from first randomization to month 36. Time to clinically definite MS to month 60 was a secondary end point.
The extension study included 127 participants originally randomized to interferon beta-1a thrice weekly, 142 participants originally randomized to interferon beta-1a once weekly, and 133 patients originally randomized to placebo. At month 36, the proportion of patients who had converted to clinically definite MS was lower among patients receiving interferon thrice weekly or weekly, compared with the delayed-treatment group (25.1%, 25.7%, and 38.6%, respectively). The risk of conversion to clinically definite MS was significantly reduced for patients receiving early treatment, compared with delayed treatment. The researchers found no significant difference between the early-treatment groups.
The analysis for month 60 also found that both early-treatment groups had longer times to clinically definite MS, compared with delayed treatment. The proportion of patients who converted to clinically definite MS increased at month 60, but was still lower among participants receiving thrice-weekly or weekly interferon than among patients on delayed treatment (32.2%, 36.0%, and 40.4%, respectively). Cumulative probability of conversion was lower with thrice-weekly and weekly early treatment than with delayed treatment (39.2%, 40.7%, and 44.6%, respectively).
—Erik Greb
Suggested Reading
Comi G, De Stefano N, Freedman MS, et al. Subcutaneous interferon β-1a in the treatment of clinically isolated syndromes: 3-year and 5-year results of the phase III dosing frequency-blind multicentre REFLEXION study. J Neurol Neurosurg Psychiatry. 2016 Dec 30 [Epub ahead of print].
Posttreatment survivorship care needs of Spanish-speaking Latinas with breast cancer
After treatment, cancer patients transition to a survivorship phase, often with little information or support. Cancer survivors are at increased risk of recurrence, secondary cancers, comorbid conditions, and late treatment effects.1,2 However, many remain unaware of these risks and the options for managing them3 and face numerous unmet medical, psychosocial, and informational needs that can be addressed through survivorship care programs.4 Anxiety may increase as they lose their treatment team’s support while attempting to reestablish their lives.2 Patients need to know the long-term risks of cancer treatments, probabilities of recurrence and second cancers, effectiveness of surveillance and interventions for managing late effects and psychosocial concerns, and benefits of healthy lifestyles.2
Due to sociocultural and economic factors, Spanish-speaking Latina breast cancer survivors (SSBCS) suffer worse posttreatment health-related quality of life and more pain, fatigue, depressive symptoms, body image issues, and distress than their white counterparts.5-7 However, they are less likely to receive necessary cancer treatment, symptom management, and surveillance. For example, compared with whites, Latina breast cancer survivors receive less guideline-adherent treatment8 and follow-up care, including survivorship information.3,9 SSBCS, in particular have less access to survivorship information.10 Consequently, SSBCS are more likely to report unmet symptom management needs.11
Several breast cancer survivorship program trials have included Latinas,12,13 but their effectiveness has been demonstrated only for depressive symptoms or health worry. A comprehensive assessment of the posttreatment needs of SSBCS would provide a foundation for designing tailored survivorship interventions for this vulnerable group. This study aimed to identify the symptom management, psychosocial, and informational needs of SSBCS during the transition to survivorship from the perspectives of SSBCS and their cancer support providers and cancer physicians.
Methods
We sampled respondents within a 5-county area in Northern California to obtain multiple perspectives of the survivorship care needs of SSBCS using structured and in-depth methods: a telephone survey of SSBCS; semistructured interviews with SSBCS; semistructured interviews with cancer support providers serving SSBCS; and semistructured interviews with physicians providing cancer care for SSBCS. The study protocol was approved by the University of California San Francisco Committee on Human Research.
Sample and procedures
Structured telephone survey with SSBCS. The sample was drawn evenly from San Francisco General Hospital-University of California San Francisco primary care practices and SSBCS from a previous study who agreed to be re-contacted.14 The inclusion criteria were: completed active treatment (except adjuvant hormonal therapy) for nonmetastatic breast cancer within 10 years; living in one of the five counties; primarily Spanish-speaking; and self-identified as Latina. The exclusion criteria were: previous cancer except nonmelanoma skin cancer; terminal illness; or metastatic breast cancer. Study staff mailed potential participants a bilingual letter and information sheet, and bilingual opt-out postcard (6th grade reading level assessed by Flesch-Kincaid grade level statistic). Female bilingual-bicultural research associates conducted interviews of 20-30 minutes in Spanish after obtaining verbal consent. Participants were mailed $20. Surveys were conducted during March-November 2014.
Semistructured in-person interviews with SSBCS. Four community-based organizations (CBOs) in the targeted area providing cancer support services to Latinos agreed to recruit SSBCS for interviews. Inclusion criteria were identical to the survey. Patient navigators or support providers from CBOs contacted women by phone or in-person to invite them to an interview to assess their cancer survivorship needs. Women could choose a focus group or individual interview. With permission, names and contact information were given to study interviewers who called, explained the study, screened for eligibility, and scheduled an interview.
Recruitment was stratified by age (under or over age 50). We sampled women until saturation was achieved (no new themes emerged). Focus groups (90 minutes) were conducted at the CBOs. Individual interviews (45 minutes) were conducted in participants’ homes. Written informed consent was obtained. Participants were paid $50. Interviews were conducted during August-November, 2014, audiotaped, and transcribed.
Semistructured in-person interviews with cancer support providers and physicians. Investigators invited five cancer support providers (three patient navigators from three county hospitals, and two CBO directors of cancer psychosocial support services) and four physicians (three oncologists and one breast cancer surgeon from three county hospitals) to an in-person interview to identify SSBCS’ survivorship care needs. All agreed to participate. No further candidates were approached because saturation was achieved. We obtained written informed consent and 30-minute interviews were conducted in participants’ offices during August-October, 2014. Interviews were audiotaped and transcribed. Participants were paid $50.
Ethical approval. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent. Informed consent was obtained from all individual participants included in the study.
Measures
Structured telephone survey. Based on cancer survivor needs assessments,15 we assessed: physical and emotional symptoms; problems with sleep and memory/concentration; concerns about mortality, family, social isolation, intimacy, appearance; and healthy lifestyles. Items were adapted and translated into Spanish if needed, using forward/backward translation with team reconciliation. These questions used the introduction, “Now I am going to ask you if you have had any problems because of your cancer. In the past month, how much have you been bothered by …” with responses rated on a scale of 1-5 (1, Not at all; 5, A lot). For example, we asked, “In the past month, how much have you been bothered by fatigue?”
Regarding healthy lifestyles, we used the introduction, “Here are some changes women sometimes want to make after cancer. Would you like help with …?” For example, we asked, “Would you like help with getting more exercise?” We asked if they wanted help getting more exercise, eating healthier, managing stress, and doing meditation or yoga (Yes/No).
Semistructured interview guide for SSBCS. Participants were asked about their emotional and physical concerns when treatment ended, current cancer needs, symptoms or late effects, and issues related to relationships, family, employment, insurance, financial hardships, barriers to follow-up care, health behaviors, and survivorship program content. Sample questions are, “Have you had any symptoms or side effects related to your cancer or treatment?” and “What kinds of information do you feel you need now about your cancer or treatment?” A brief questionnaire assessed demographics.
Semistructured interview guide for cancer support providers and physicians. Support providers and physicians were asked about informational, psychosocial, and symptom management needs of SSBCS and recommended self-management content and formats. Sample questions are, “What kinds of information and support do you wish was available to help Spanish-speaking women take care of their health after treatment ends?” and “What do you think are the most pressing emotional needs of Spanish-speaking women after breast cancer treatment ends?” A brief questionnaire assessed demographics.
Analysis
Frequencies are reported for survey items. For questions about symptoms/concerns, we report the frequency of responding that they were bothered Somewhat/Quite a bit/A lot. For healthy lifestyles, we present the frequency answering Yes.
Verbatim semistructured interview transcripts were verified against audiotapes. Using QSR NVIVO software, transcripts were coded independently by two bilingual-bicultural investigators using a constant comparative method to generate coding categories for cancer survivorship needs.16 Coders started with themes specified by the interview guides, expanded them to represent the data, and discussed and reconciled coding discrepancies. Coding was compared by type of interview participant (survivor, support provider, or physician). Triangulation of survey and semistructured interviews occurred through team discussions to verify codes, themes, and implications for interventions.
Results
Telephone survey of SSBCS
Of the telephone survey sampling frame (N = 231), 118 individuals (51%) completed the interview, 37 (16%) were ineligible, 31 (13%) could not be reached, 22 (10%) had incorrect contact information, 19 (8%) refused to participate, and 4 (2%) were deceased. Mean age of the participants was 54.9 years (SD, 12.3); all were foreign-born, with more than half of Mexican origin; and most had less than a high-school education (Table 1). All had completed active treatment, and most (68%) were within 2 years of diagnosis.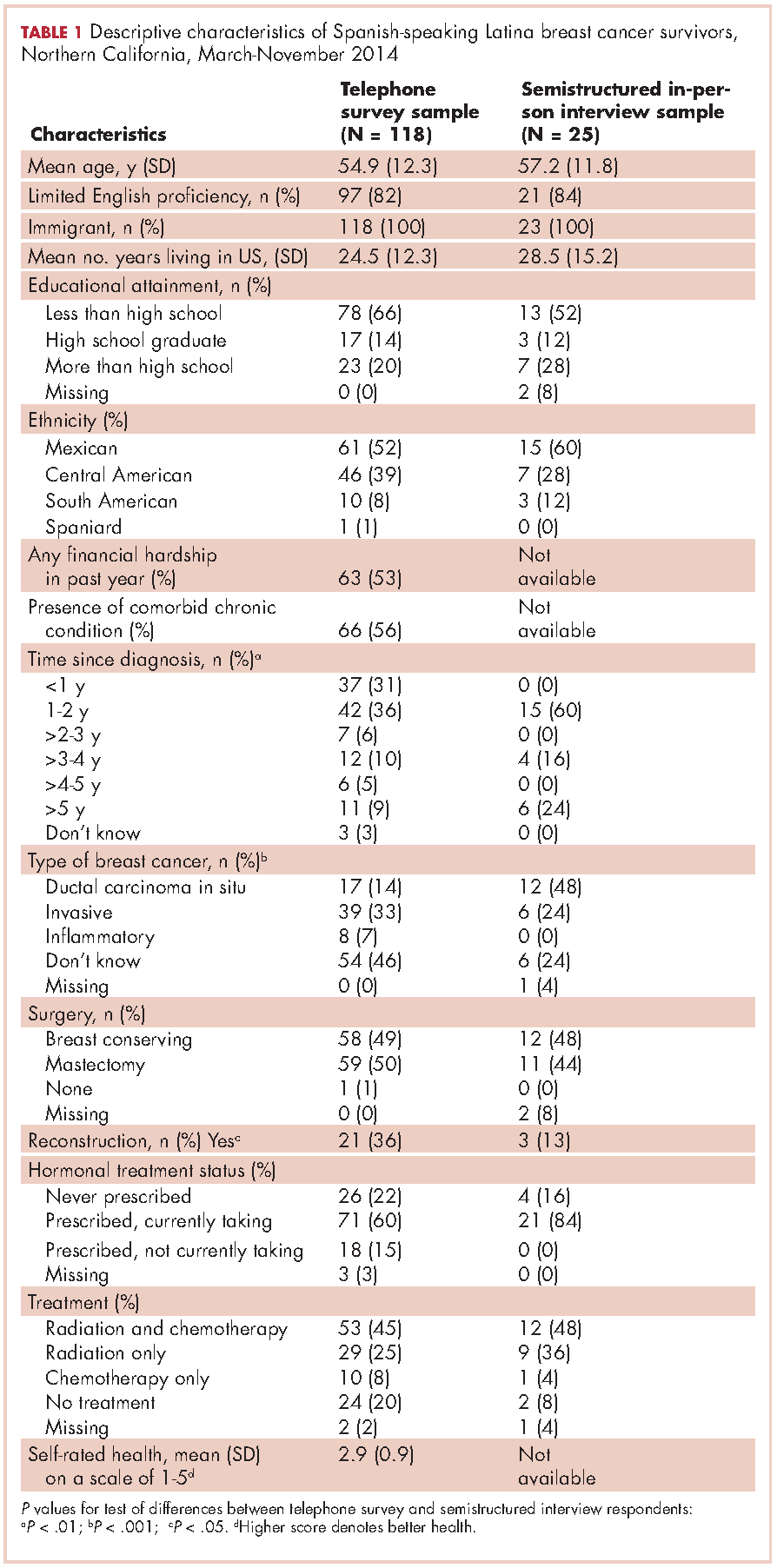
For symptom management needs (Table 2), the most prevalent (bothersome) symptoms (reported by more than 30%, in rank order) were joint pain, sleep problems, fatigue, hot flashes, numbness/tingling of extremities, and memory. Next most prevalent (reported by 20%-30%) were vaginal dryness, dry/itchy skin, dry nose/mouth, inability to concentrate, constipation, changes in urination, and shortness of breath.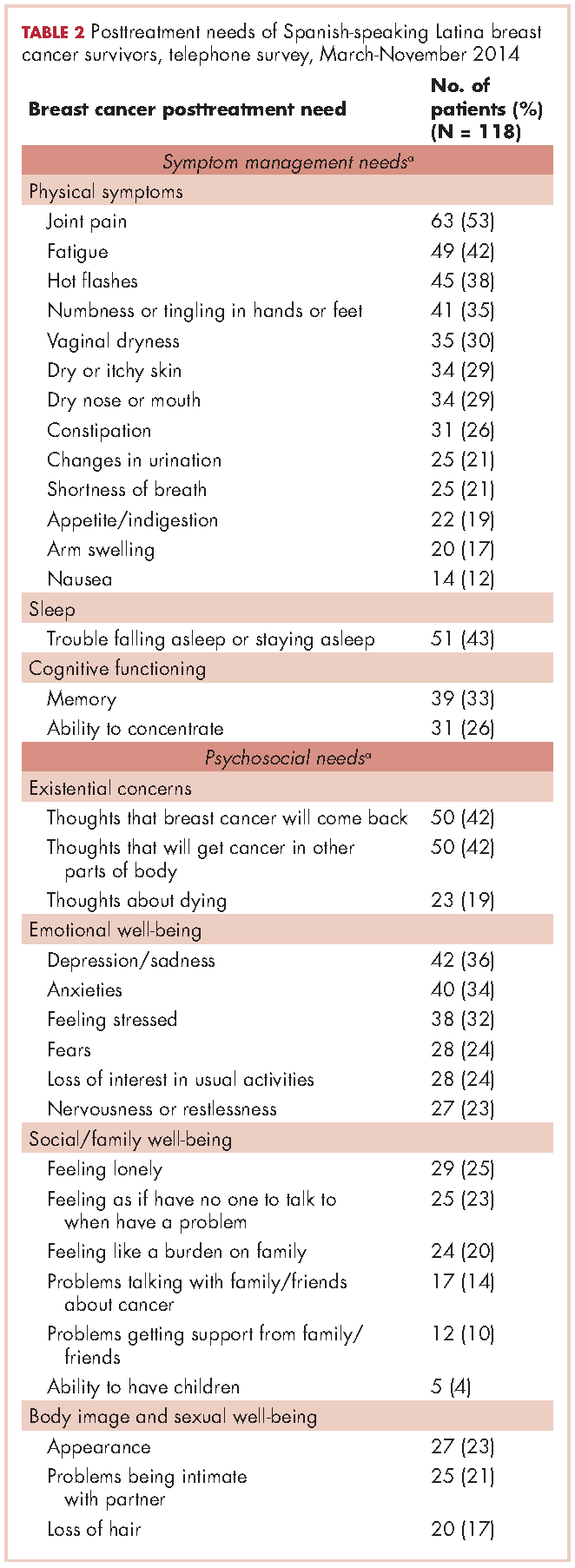

For psychosocial needs, fears of recurrence or new cancers were reported by 42%. Emotional symptoms reported by more than 30% were depression/sadness, anxieties, and feeling stressed. Next most prevalent (20%-30%) were fears, loss of interest in usual activities, and nervousness/restlessness. Social well-being concerns reported by 20%-30% of survivors were loneliness, having no one to talk to, and being a burden to their families. Body image and sexual problems reported by 20%-30% of survivors included appearance and problems being intimate with partners.
Regarding lifestyle, most of the participants said they wanted help with eating a healthier diet (74%), getting more exercise (69%), managing stress (63%), and doing yoga or meditation (55%).
Semistructured interviews
Twenty-five SSBCS completed semistructured interviews, 10 in individual interviews and 15 in one of two focus groups (one of 9 women older than 50 years; one of 6 women younger than 50). The telephone survey respondents were similar to semistructured interview respondents on all sociodemographic characteristics, but differed slightly on some clinical characteristics (Table 1). The telephone survey women had been more recently diagnosed (P < .01), were less likely to have ductal carcinoma in situ (P < .001), and more likely to have had reconstructive surgery
(P < .05).
Five cancer support providers and 4 physicians were interviewed. All support providers were Spanish-speaking Latinas with at least some college education. Cancer physicians were board certified. Two were men; two were white and two Asian; one was a breast surgeon and three were hematologists/oncologists; three spoke Spanish poorly/not at all and one spoke it fairly well.
Seven themes emerged from interviews: unmet physical symptom management needs; social support often ends when treatment ends; challenges resuming roles; sense of abandonment by health care system when treatment ends; need for formal transition from active treatment to follow-up care; fear of recurrence especially when obtaining follow-up care; and desire for information on late effects of initial treatments and side effects of hormonal treatments. We summarize results according to these themes.
Unmet physical symptom management needs. The main physical symptoms reported by survivors and physicians in interviews were arthralgia, menopausal symptoms, and neuropathy. Fatigue was reported only by survivors. Many survivors and several support providers expressed that symptoms were poorly managed and often ignored. One stated,
I have a lot of pain where I had surgery, it burns. I worry a lot about my arm because I have sacs of fluid. My doctor only says, ‘They will dissolve over the years.’ So, I don’t feel any support. (FocGrp1#6)
Survivors reported side effects of hormonal therapies, and felt that physicians downplayed these to prevent them from discontinuing medications.
Social support from family and friends often ends when treatment ends. Many survivors described a loss of support from family and friends who expected them to get back to “normal” once treatment ended. One said,
My sisters have told me to my face that there’s nothing wrong with me. So now when people ask me, I say, ‘I’m fine, thank God, I have nothing,’ even though I’m dying of pain and have all these pills to take. (Survivor#1025)
A support provider related,
The client was telling me that as she was getting closer to finishing her treatment, her husband was upset because he felt like all she was doing was focusing on the cancer. I think caregivers, family, spouses, and children out of their own sort of selfishness want this person to be well. (SuppProv#104)
A few survivors said that family bonds were strengthened after cancer and several reported lacking support because their families were in their home countries.
Challenges resuming roles, especially returning to work. Survivors, support providers, and physicians described challenges and few resources as women transition back to their normal roles. Survivors questioned their ability to return to work due to physically demanding occupations. One stated,
I would like information on how to take care of myself, how working can affect this side if I don’t take care of it. I clean houses and I need both hands. (Survivor#3012)
Survivors described how changes in memory affected daily chores and work performance. Support providers and physicians described the need for resources to aid with return to work and household responsibilities. One physician noted,
There are usually questions about how to go ahead and live their lives from that point forward. It’s a sort of reverse shock: going back to life as they know it. (Physician#004)
Support providers and physicians mentioned that women needed help with resuming intimate partner relations.
Sense of abandonment by health care system once active treatment ends. Survivors, support providers, and physicians reported a loss of support and sense of abandonment by the patients’ oncology team at the end of active treatment. One survivor stated,
Once they tell you to stop the pills, ‘You’re cured, there’s nothing wrong with you,’ the truth is that one feels, ‘Now what do I do? I have no one to help me.’ I felt very abandoned. (FocGrp1#5)
A provider said,
The support system falls apart once women complete treatment. They lose their entire support system at the medical level. They no longer have nurses checking in about symptoms and addressing anything that’s come up. They won’t have access to doctors unless they’re doing their screening. (SuppProv#101)
An oncologist, noting that this loss of support occurs when women face pressures of transitioning back to work or family obligations, commented,
So here’s a woman whose marriage is in turmoil, whose husband may even have left her during this, and now her clinic is leaving her and she’s on her own … that must be scary as hell because there’s nobody out there to support her. (Physician#002)
Need for formal transition from active treatment to follow-up care. Two themes emerged about transitioning from active treatment: transferring care from oncologists to primary care physicians (PCP); and issues of follow-up care (with oncologists or PCPs). Survivors felt lost in transitioning from specialty to primary care, or expressed apprehension seeing a PCP rather than a cancer specialist. One stated,
I have my doctor but she is not a specialist. She does what I tell her to and orders a mammogram every year. But, I don’t go to the oncologist anymore, and so I worry. With the specialists, I feel protected. (FocGrp1#5)
Physicians acknowledged the lack of a formal transition to primary care such as a survivorship care program.
Follow-up care issues were common. Physicians stressed that women needed to know how often to return for follow-up once active treatment ends and about recommended examinations and tests, especially when receiving hormonal therapy. Physicians indicated the need for patient education materials specific to patients’ treatments, for example, elevated risk of heart disease with certain chemotherapy agents. An oncologist expressed concern that PCPs are not prepared adequately about late effects and hormonal treatment side effects, and suggested providing summary notes for PCPs detailing these.
Survivors identified several barriers to follow-up care: lacking information on which symptoms merited a call to physicians; financial burden/limited health insurance; lacking appointment reminders; fear of examinations; and limited English proficiency. A survivor stated,
If you have insurance, you can make your appointment, see the doctor, and have your mammogram. I stopped taking my pills because I didn’t have insurance. I tried to get them again but they told me they would cost me a thousand dollars. (FocGrp1#5)
One oncologist suggested scheduling a follow-up appointment before patients leave treatment and calling patients who miss appointments.
Facilitators of regular follow-up care identified by survivors were physicians informing them about symptom monitoring and reporting, having a clinic contact person/navigator, being given a follow-up appointment, being assertive about one’s care, and physicians’ reinforcement of adherence to hormonal treatment and follow-up. According to support providers, a key facilitator was having a clinic contact person/navigator. Once treatment ended, support providers often served as the liaison between the patient and the physician, making them the first point of contact for symptom reporting.
Fear of recurrence especially when obtaining follow-up care. Fear of recurrence dominated survivor interviews. This fear was heightened at the time of follow-up examinations or when they experienced unusual pain. A survivor commented,
Every time I’m due for my mammogram, I can’t sleep, worrying. I lose sleep until I get the letter with my results. Then I feel at peace again. (FocGrp1#9)
Support providers discussed the need to provide reassurance to SSBCS to help them cope with fears of recurrence. Physicians expressed challenges in allaying fears of recurrence among SSBCS, requiring a lot of time when recommending follow-up mammograms.
Desire for information on late effects of treatments and side effects of hormonal therapies. All survivors expressed receiving insufficient information on potential symptoms and side effects. One stated,
Doctors only have five minutes. There has never been someone who gave me guidance like, ‘From now on you have to do this or you might get these symptoms now or in the future. (Survivor#6019)
They indicated uncertainty about what symptoms were “normal” and when symptoms merited a call to the physician. Several survivors reported being unaware that fatigue, arthralgia and neuropathy were side effects of breast cancer treatments until they reported these to physicians.
Physicians stressed the importance of women knowing about the elevated risk of future cancers, symptoms of recurrence, and seeking follow-up care if they experience symptoms that are out of the ordinary. Support providers felt that it was important to provide SSBCS with information on signs of recurrence and when to report these. However, providers expressed concern that giving women too much information might elevate their anxiety. A physician suggested,
It’s probably better to have a symptom list that’s short and relevant for the most common and catastrophic things, same thing with side effects … short to avoid overwhelming the patient. (Physician#001)
Hormonal treatments were of special concern. Survivors expressed a need for information on hormonal treatments and support providers stressed that this information is needed in simple Spanish. Several survivors indicated they stopped taking hormonal treatments due to side effects. One woman experienced severe headaches and heart palpitations, stopped taking the hormonal medication, felt better, and did not inform her physician until her next appointment. A support provider stated,
What I hear from a lot of women is that if side effects are too uncomfortable, they just stop it (hormonal treatment) without saying anything to the doctor. So more information about why they have to take it and that there is a good chance of recurrence is really important. (SuppProv#101)
Likewise, physicians indicated that SSBCS’ lack of information on hormonal treatments often resulted in nonadherence, emphasizing the need to reinforce adherence to prevent recurrence.
Conceptual framework of interventions
Based on triangulation of survey and interview results, we compiled a conceptual framework that includes needs identified, suggested components of a survivorship care intervention to address these needs, potential mediators by which such interventions could improve outcomes, and relevant outcomes (Figure). Survivorship care needs fell into four categories: symptom management, psychosocial, sense of abandonment by health care team, and healthy lifestyles. Survivorship care programs would provide skills training in symptom and stress management, and communicating with providers, family, friends, and coworkers. Mediators include increased self-efficacy, knowledge and perceived social support, ultimately leading to reduced distress (anxiety and depressive symptoms) and stress, and improved health-related quality of life.
Conclusions
Our study aimed to identify the most critical needs of SSBCS in the posttreatment survivorship phase to facilitate the design of survivorship interventions for this vulnerable group. SSBCS, cancer support providers, and cancer physicians reported substantial symptom management, psychosocial, and informational needs among this population. Results from surveys and open-ended interviews were remarkably consistent. Survivors, physicians, and support providers viewed transition out of active treatment as a time of increased psychosocial need and heightened vulnerability.
Our findings are consistent with needs assessments conducted in other breast cancer survivors. Similar to a study of rural white women with breast cancer, fear of recurrence was among the most common psychosocial concerns.17 Results of two studies that included white, African American and Latina breast cancer survivors were consistent with ours in finding that pain and fatigue were among the most persistent symptoms; in both studies, Latinas were more likely to report pain and a higher number of symptoms.7,18 The prevalence of sleep problems in our sample was identical to that reported in a sample of African American breast cancer survivors.19 Our findings of a high need for symptom management information and support, social support from family and friends, and self-management resources were similar to studies of other vulnerable breast cancer survivors.18,20
Our results suggest that it is critical for health care professionals to provide assistance with managing side effects and information to alleviate fears, and reinforce behaviors of symptom monitoring and reporting, and adherence to follow-up care and hormonal therapies. Yet this information is not being conveyed effectively and is complicated by the need to balance women’s need for information with minimizing anxiety when providing such information.
A limitation of our study is that most of our sample was Mexican origin and may not reflect experiences of Spanish-speaking Latinas of other national origin groups or outside of Northern California. Another limitation is the lack of an English-speaking comparison group, which would have permitted the identification of similarities and differences across language groups. Finally, we did not interview radiation oncologists who may have had opinions that are not represented here.
Survivorship care programs offer great promise for meeting patients’ informational and symptom management needs and improving well-being and communication with clinicians.21 Due to limited access to survivorship care information, financial hardships, and pressures from their families to resume their social roles, concerted efforts are needed to develop appropriate survivorship programs for SSBCS.22 Unique language, cultural and socioeconomic factors of Spanish-speaking Latinas require tailoring of cancer survivorship programs to best meet their needs.23 These programs need to provide psychosocial stress and symptom management assistance, simple information on recommended follow-up care, and healthy lifestyle and role reintegration strategies that account for their unique sociocultural contexts.
1. Danese MD, O’Malley C, Lindquist K, Gleeson M, Griffiths RI. An observational study of the prevalence and incidence of comorbid conditions in older women with breast cancer. Ann Oncol. 2012;23(7):1756-1765.
2. Hewitt M, Greenfield S, Stovall E, eds. From cancer patient to cancer survivor: lost in transition. Washington, DC: National Academy of Sciences; 2006.
3. Beckjord EB, Arora NK, McLaughlin W, Oakley-Girvan I, Hamilton AS, Hesse BW. Health-related information needs in a large and diverse sample of adult cancer survivors: implications for cancer care. J Cancer Surviv. 2008;2(3):179-189.
4. Hewitt ME, Bamundo A, Day R, Harvey C. Perspectives on posttreatment cancer care: qualitative research with survivors, nurses, and physicians. J Clin Oncol. 2007;25(16):2270-2273.
5. Ashing-Giwa KT, Tejero JS, Kim J, Padilla GV, Hellemann G. Examining predictive models of HRQOL in a population-based, multiethnic sample of women with breast carcinoma. Qual Life Res. 2007;16(3):413-428.
6. Clauser SB, Arora NK, Bellizzi KM, Haffer SC, Topor M, Hays RD. Disparities in HRQOL of cancer survivors and non-cancer managed care enrollees. Health Care Financ Rev. 2008;29(4):23-40.
7. Eversley R, Estrin D, Dibble S, Wardlaw L, Pedrosa M, Favila-Penney W. Posttreatment symptoms among ethnic minority breast cancer survivors. Oncol Nurs Forum. 2005;32(2):250-256.
8. Bickell NA, Wang JJ, Oluwole S, et al. Missed opportunities: racial disparities in adjuvant breast cancer treatment. J Clin Oncol. 2006;24(9):1357-1362.
9. Arora NK, Reeve BB, Hays RD, Clauser SB, Oakley-Girvan I. Assessment of quality of cancer-related follow-up care from the cancer survivor’s perspective. J Clin Oncol. 2011;29(10):1280-1289.
10. Janz NK, Mujahid MS, Hawley ST, Griggs JJ, Hamilton AS, Katz SJ. Racial/ethnic differences in adequacy of information and support for women with breast cancer. Cancer. 2008;113(5):1058-1067.
11. Yoon J, Malin JL, Tisnado DM, et al. Symptom management after breast cancer treatment: is it influenced by patient characteristics? Breast Cancer Res Treat. 2008;108(1):69-77.
12. Ashing K, Rosales M. A telephonic-based trial to reduce depressive symptoms among Latina breast cancer survivors. Psychooncology. 2014;23(5):507-515.
13. Hershman DL, Greenlee H, Awad D, et al. Randomized controlled trial of a clinic-based survivorship intervention following adjuvant therapy in breast cancer survivors. Breast Cancer Res Treat. 2013;138(3):795-806.
14. Napoles AM, Ortiz C, Santoyo-Olsson J, et al. Nuevo Amanecer: results of a randomized controlled trial of a community-based, peer-delivered stress management intervention to improve quality of life in Latinas with breast cancer. Am J Public Health. 2015;105(suppl 3):e55-63.
15. Rechis R, Reynolds KA, Beckjord EB, Nutt S, Burns RM, Schaefer JS. ‘I learned to live with it’ is not good enough: challenges reported by posttreatment cancer survivors in the Livestrong surveys. Austin, TX: Livestrong;2011.
16. Glaser BG, Strauss AL. The discovery of grounded theory: strategies for qualitative research. Hawthorne: Aldine Publishing Company; 1967.
17. Befort CA, Klemp J. Sequelae of breast cancer and the influence of menopausal status at diagnosis among rural breast cancer survivors. J Womens Health (Larchmt). 2011;20(9):1307-1313.
18. Fu OS, Crew KD, Jacobson JS, et al. Ethnicity and persistent symptom burden in breast cancer survivors. J Cancer Surviv. 2009;3(4):241-250.
19. Taylor TR, Huntley ED, Makambi K, et al. Understanding sleep disturbances in African-American breast cancer survivors: a pilot study. Psychooncology. 2012;21(8):896-902.
20. Adams N, Gisiger-Camata S, Hardy CM, Thomas TF, Jukkala A, Meneses K. Evaluating survivorship experiences and needs among rural African American breast cancer survivors. J Cancer Educ. October 24, 2015 [Epub ahead of print].
21. Blinder VS, Patil S, Thind A, et al. Return to work in low-income Latina and non-Latina white breast cancer survivors: a 3-year longitudinal study. Cancer. 2012;118(6):1664-1674.
22. Lopez-Class M, Perret-Gentil M, Kreling B, Caicedo L, Mandelblatt J, Graves KD. Quality of life among immigrant Latina breast cancer survivors: realities of culture and enhancing cancer care. J Cancer Educ. 2011;26(4):724-733.
23. Napoles-Springer AM, Ortiz C, O’Brien H, Diaz-Mendez M. Developing a culturally competent peer support intervention for Spanish-speaking Latinas with breast cancer. J Immigr Minor Health. 2009;11(4):268-280
After treatment, cancer patients transition to a survivorship phase, often with little information or support. Cancer survivors are at increased risk of recurrence, secondary cancers, comorbid conditions, and late treatment effects.1,2 However, many remain unaware of these risks and the options for managing them3 and face numerous unmet medical, psychosocial, and informational needs that can be addressed through survivorship care programs.4 Anxiety may increase as they lose their treatment team’s support while attempting to reestablish their lives.2 Patients need to know the long-term risks of cancer treatments, probabilities of recurrence and second cancers, effectiveness of surveillance and interventions for managing late effects and psychosocial concerns, and benefits of healthy lifestyles.2
Due to sociocultural and economic factors, Spanish-speaking Latina breast cancer survivors (SSBCS) suffer worse posttreatment health-related quality of life and more pain, fatigue, depressive symptoms, body image issues, and distress than their white counterparts.5-7 However, they are less likely to receive necessary cancer treatment, symptom management, and surveillance. For example, compared with whites, Latina breast cancer survivors receive less guideline-adherent treatment8 and follow-up care, including survivorship information.3,9 SSBCS, in particular have less access to survivorship information.10 Consequently, SSBCS are more likely to report unmet symptom management needs.11
Several breast cancer survivorship program trials have included Latinas,12,13 but their effectiveness has been demonstrated only for depressive symptoms or health worry. A comprehensive assessment of the posttreatment needs of SSBCS would provide a foundation for designing tailored survivorship interventions for this vulnerable group. This study aimed to identify the symptom management, psychosocial, and informational needs of SSBCS during the transition to survivorship from the perspectives of SSBCS and their cancer support providers and cancer physicians.
Methods
We sampled respondents within a 5-county area in Northern California to obtain multiple perspectives of the survivorship care needs of SSBCS using structured and in-depth methods: a telephone survey of SSBCS; semistructured interviews with SSBCS; semistructured interviews with cancer support providers serving SSBCS; and semistructured interviews with physicians providing cancer care for SSBCS. The study protocol was approved by the University of California San Francisco Committee on Human Research.
Sample and procedures
Structured telephone survey with SSBCS. The sample was drawn evenly from San Francisco General Hospital-University of California San Francisco primary care practices and SSBCS from a previous study who agreed to be re-contacted.14 The inclusion criteria were: completed active treatment (except adjuvant hormonal therapy) for nonmetastatic breast cancer within 10 years; living in one of the five counties; primarily Spanish-speaking; and self-identified as Latina. The exclusion criteria were: previous cancer except nonmelanoma skin cancer; terminal illness; or metastatic breast cancer. Study staff mailed potential participants a bilingual letter and information sheet, and bilingual opt-out postcard (6th grade reading level assessed by Flesch-Kincaid grade level statistic). Female bilingual-bicultural research associates conducted interviews of 20-30 minutes in Spanish after obtaining verbal consent. Participants were mailed $20. Surveys were conducted during March-November 2014.
Semistructured in-person interviews with SSBCS. Four community-based organizations (CBOs) in the targeted area providing cancer support services to Latinos agreed to recruit SSBCS for interviews. Inclusion criteria were identical to the survey. Patient navigators or support providers from CBOs contacted women by phone or in-person to invite them to an interview to assess their cancer survivorship needs. Women could choose a focus group or individual interview. With permission, names and contact information were given to study interviewers who called, explained the study, screened for eligibility, and scheduled an interview.
Recruitment was stratified by age (under or over age 50). We sampled women until saturation was achieved (no new themes emerged). Focus groups (90 minutes) were conducted at the CBOs. Individual interviews (45 minutes) were conducted in participants’ homes. Written informed consent was obtained. Participants were paid $50. Interviews were conducted during August-November, 2014, audiotaped, and transcribed.
Semistructured in-person interviews with cancer support providers and physicians. Investigators invited five cancer support providers (three patient navigators from three county hospitals, and two CBO directors of cancer psychosocial support services) and four physicians (three oncologists and one breast cancer surgeon from three county hospitals) to an in-person interview to identify SSBCS’ survivorship care needs. All agreed to participate. No further candidates were approached because saturation was achieved. We obtained written informed consent and 30-minute interviews were conducted in participants’ offices during August-October, 2014. Interviews were audiotaped and transcribed. Participants were paid $50.
Ethical approval. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent. Informed consent was obtained from all individual participants included in the study.
Measures
Structured telephone survey. Based on cancer survivor needs assessments,15 we assessed: physical and emotional symptoms; problems with sleep and memory/concentration; concerns about mortality, family, social isolation, intimacy, appearance; and healthy lifestyles. Items were adapted and translated into Spanish if needed, using forward/backward translation with team reconciliation. These questions used the introduction, “Now I am going to ask you if you have had any problems because of your cancer. In the past month, how much have you been bothered by …” with responses rated on a scale of 1-5 (1, Not at all; 5, A lot). For example, we asked, “In the past month, how much have you been bothered by fatigue?”
Regarding healthy lifestyles, we used the introduction, “Here are some changes women sometimes want to make after cancer. Would you like help with …?” For example, we asked, “Would you like help with getting more exercise?” We asked if they wanted help getting more exercise, eating healthier, managing stress, and doing meditation or yoga (Yes/No).
Semistructured interview guide for SSBCS. Participants were asked about their emotional and physical concerns when treatment ended, current cancer needs, symptoms or late effects, and issues related to relationships, family, employment, insurance, financial hardships, barriers to follow-up care, health behaviors, and survivorship program content. Sample questions are, “Have you had any symptoms or side effects related to your cancer or treatment?” and “What kinds of information do you feel you need now about your cancer or treatment?” A brief questionnaire assessed demographics.
Semistructured interview guide for cancer support providers and physicians. Support providers and physicians were asked about informational, psychosocial, and symptom management needs of SSBCS and recommended self-management content and formats. Sample questions are, “What kinds of information and support do you wish was available to help Spanish-speaking women take care of their health after treatment ends?” and “What do you think are the most pressing emotional needs of Spanish-speaking women after breast cancer treatment ends?” A brief questionnaire assessed demographics.
Analysis
Frequencies are reported for survey items. For questions about symptoms/concerns, we report the frequency of responding that they were bothered Somewhat/Quite a bit/A lot. For healthy lifestyles, we present the frequency answering Yes.
Verbatim semistructured interview transcripts were verified against audiotapes. Using QSR NVIVO software, transcripts were coded independently by two bilingual-bicultural investigators using a constant comparative method to generate coding categories for cancer survivorship needs.16 Coders started with themes specified by the interview guides, expanded them to represent the data, and discussed and reconciled coding discrepancies. Coding was compared by type of interview participant (survivor, support provider, or physician). Triangulation of survey and semistructured interviews occurred through team discussions to verify codes, themes, and implications for interventions.
Results
Telephone survey of SSBCS
Of the telephone survey sampling frame (N = 231), 118 individuals (51%) completed the interview, 37 (16%) were ineligible, 31 (13%) could not be reached, 22 (10%) had incorrect contact information, 19 (8%) refused to participate, and 4 (2%) were deceased. Mean age of the participants was 54.9 years (SD, 12.3); all were foreign-born, with more than half of Mexican origin; and most had less than a high-school education (Table 1). All had completed active treatment, and most (68%) were within 2 years of diagnosis.
For symptom management needs (Table 2), the most prevalent (bothersome) symptoms (reported by more than 30%, in rank order) were joint pain, sleep problems, fatigue, hot flashes, numbness/tingling of extremities, and memory. Next most prevalent (reported by 20%-30%) were vaginal dryness, dry/itchy skin, dry nose/mouth, inability to concentrate, constipation, changes in urination, and shortness of breath.

For psychosocial needs, fears of recurrence or new cancers were reported by 42%. Emotional symptoms reported by more than 30% were depression/sadness, anxieties, and feeling stressed. Next most prevalent (20%-30%) were fears, loss of interest in usual activities, and nervousness/restlessness. Social well-being concerns reported by 20%-30% of survivors were loneliness, having no one to talk to, and being a burden to their families. Body image and sexual problems reported by 20%-30% of survivors included appearance and problems being intimate with partners.
Regarding lifestyle, most of the participants said they wanted help with eating a healthier diet (74%), getting more exercise (69%), managing stress (63%), and doing yoga or meditation (55%).
Semistructured interviews
Twenty-five SSBCS completed semistructured interviews, 10 in individual interviews and 15 in one of two focus groups (one of 9 women older than 50 years; one of 6 women younger than 50). The telephone survey respondents were similar to semistructured interview respondents on all sociodemographic characteristics, but differed slightly on some clinical characteristics (Table 1). The telephone survey women had been more recently diagnosed (P < .01), were less likely to have ductal carcinoma in situ (P < .001), and more likely to have had reconstructive surgery
(P < .05).
Five cancer support providers and 4 physicians were interviewed. All support providers were Spanish-speaking Latinas with at least some college education. Cancer physicians were board certified. Two were men; two were white and two Asian; one was a breast surgeon and three were hematologists/oncologists; three spoke Spanish poorly/not at all and one spoke it fairly well.
Seven themes emerged from interviews: unmet physical symptom management needs; social support often ends when treatment ends; challenges resuming roles; sense of abandonment by health care system when treatment ends; need for formal transition from active treatment to follow-up care; fear of recurrence especially when obtaining follow-up care; and desire for information on late effects of initial treatments and side effects of hormonal treatments. We summarize results according to these themes.
Unmet physical symptom management needs. The main physical symptoms reported by survivors and physicians in interviews were arthralgia, menopausal symptoms, and neuropathy. Fatigue was reported only by survivors. Many survivors and several support providers expressed that symptoms were poorly managed and often ignored. One stated,
I have a lot of pain where I had surgery, it burns. I worry a lot about my arm because I have sacs of fluid. My doctor only says, ‘They will dissolve over the years.’ So, I don’t feel any support. (FocGrp1#6)
Survivors reported side effects of hormonal therapies, and felt that physicians downplayed these to prevent them from discontinuing medications.
Social support from family and friends often ends when treatment ends. Many survivors described a loss of support from family and friends who expected them to get back to “normal” once treatment ended. One said,
My sisters have told me to my face that there’s nothing wrong with me. So now when people ask me, I say, ‘I’m fine, thank God, I have nothing,’ even though I’m dying of pain and have all these pills to take. (Survivor#1025)
A support provider related,
The client was telling me that as she was getting closer to finishing her treatment, her husband was upset because he felt like all she was doing was focusing on the cancer. I think caregivers, family, spouses, and children out of their own sort of selfishness want this person to be well. (SuppProv#104)
A few survivors said that family bonds were strengthened after cancer and several reported lacking support because their families were in their home countries.
Challenges resuming roles, especially returning to work. Survivors, support providers, and physicians described challenges and few resources as women transition back to their normal roles. Survivors questioned their ability to return to work due to physically demanding occupations. One stated,
I would like information on how to take care of myself, how working can affect this side if I don’t take care of it. I clean houses and I need both hands. (Survivor#3012)
Survivors described how changes in memory affected daily chores and work performance. Support providers and physicians described the need for resources to aid with return to work and household responsibilities. One physician noted,
There are usually questions about how to go ahead and live their lives from that point forward. It’s a sort of reverse shock: going back to life as they know it. (Physician#004)
Support providers and physicians mentioned that women needed help with resuming intimate partner relations.
Sense of abandonment by health care system once active treatment ends. Survivors, support providers, and physicians reported a loss of support and sense of abandonment by the patients’ oncology team at the end of active treatment. One survivor stated,
Once they tell you to stop the pills, ‘You’re cured, there’s nothing wrong with you,’ the truth is that one feels, ‘Now what do I do? I have no one to help me.’ I felt very abandoned. (FocGrp1#5)
A provider said,
The support system falls apart once women complete treatment. They lose their entire support system at the medical level. They no longer have nurses checking in about symptoms and addressing anything that’s come up. They won’t have access to doctors unless they’re doing their screening. (SuppProv#101)
An oncologist, noting that this loss of support occurs when women face pressures of transitioning back to work or family obligations, commented,
So here’s a woman whose marriage is in turmoil, whose husband may even have left her during this, and now her clinic is leaving her and she’s on her own … that must be scary as hell because there’s nobody out there to support her. (Physician#002)
Need for formal transition from active treatment to follow-up care. Two themes emerged about transitioning from active treatment: transferring care from oncologists to primary care physicians (PCP); and issues of follow-up care (with oncologists or PCPs). Survivors felt lost in transitioning from specialty to primary care, or expressed apprehension seeing a PCP rather than a cancer specialist. One stated,
I have my doctor but she is not a specialist. She does what I tell her to and orders a mammogram every year. But, I don’t go to the oncologist anymore, and so I worry. With the specialists, I feel protected. (FocGrp1#5)
Physicians acknowledged the lack of a formal transition to primary care such as a survivorship care program.
Follow-up care issues were common. Physicians stressed that women needed to know how often to return for follow-up once active treatment ends and about recommended examinations and tests, especially when receiving hormonal therapy. Physicians indicated the need for patient education materials specific to patients’ treatments, for example, elevated risk of heart disease with certain chemotherapy agents. An oncologist expressed concern that PCPs are not prepared adequately about late effects and hormonal treatment side effects, and suggested providing summary notes for PCPs detailing these.
Survivors identified several barriers to follow-up care: lacking information on which symptoms merited a call to physicians; financial burden/limited health insurance; lacking appointment reminders; fear of examinations; and limited English proficiency. A survivor stated,
If you have insurance, you can make your appointment, see the doctor, and have your mammogram. I stopped taking my pills because I didn’t have insurance. I tried to get them again but they told me they would cost me a thousand dollars. (FocGrp1#5)
One oncologist suggested scheduling a follow-up appointment before patients leave treatment and calling patients who miss appointments.
Facilitators of regular follow-up care identified by survivors were physicians informing them about symptom monitoring and reporting, having a clinic contact person/navigator, being given a follow-up appointment, being assertive about one’s care, and physicians’ reinforcement of adherence to hormonal treatment and follow-up. According to support providers, a key facilitator was having a clinic contact person/navigator. Once treatment ended, support providers often served as the liaison between the patient and the physician, making them the first point of contact for symptom reporting.
Fear of recurrence especially when obtaining follow-up care. Fear of recurrence dominated survivor interviews. This fear was heightened at the time of follow-up examinations or when they experienced unusual pain. A survivor commented,
Every time I’m due for my mammogram, I can’t sleep, worrying. I lose sleep until I get the letter with my results. Then I feel at peace again. (FocGrp1#9)
Support providers discussed the need to provide reassurance to SSBCS to help them cope with fears of recurrence. Physicians expressed challenges in allaying fears of recurrence among SSBCS, requiring a lot of time when recommending follow-up mammograms.
Desire for information on late effects of treatments and side effects of hormonal therapies. All survivors expressed receiving insufficient information on potential symptoms and side effects. One stated,
Doctors only have five minutes. There has never been someone who gave me guidance like, ‘From now on you have to do this or you might get these symptoms now or in the future. (Survivor#6019)
They indicated uncertainty about what symptoms were “normal” and when symptoms merited a call to the physician. Several survivors reported being unaware that fatigue, arthralgia and neuropathy were side effects of breast cancer treatments until they reported these to physicians.
Physicians stressed the importance of women knowing about the elevated risk of future cancers, symptoms of recurrence, and seeking follow-up care if they experience symptoms that are out of the ordinary. Support providers felt that it was important to provide SSBCS with information on signs of recurrence and when to report these. However, providers expressed concern that giving women too much information might elevate their anxiety. A physician suggested,
It’s probably better to have a symptom list that’s short and relevant for the most common and catastrophic things, same thing with side effects … short to avoid overwhelming the patient. (Physician#001)
Hormonal treatments were of special concern. Survivors expressed a need for information on hormonal treatments and support providers stressed that this information is needed in simple Spanish. Several survivors indicated they stopped taking hormonal treatments due to side effects. One woman experienced severe headaches and heart palpitations, stopped taking the hormonal medication, felt better, and did not inform her physician until her next appointment. A support provider stated,
What I hear from a lot of women is that if side effects are too uncomfortable, they just stop it (hormonal treatment) without saying anything to the doctor. So more information about why they have to take it and that there is a good chance of recurrence is really important. (SuppProv#101)
Likewise, physicians indicated that SSBCS’ lack of information on hormonal treatments often resulted in nonadherence, emphasizing the need to reinforce adherence to prevent recurrence.
Conceptual framework of interventions
Based on triangulation of survey and interview results, we compiled a conceptual framework that includes needs identified, suggested components of a survivorship care intervention to address these needs, potential mediators by which such interventions could improve outcomes, and relevant outcomes (Figure). Survivorship care needs fell into four categories: symptom management, psychosocial, sense of abandonment by health care team, and healthy lifestyles. Survivorship care programs would provide skills training in symptom and stress management, and communicating with providers, family, friends, and coworkers. Mediators include increased self-efficacy, knowledge and perceived social support, ultimately leading to reduced distress (anxiety and depressive symptoms) and stress, and improved health-related quality of life.
Conclusions
Our study aimed to identify the most critical needs of SSBCS in the posttreatment survivorship phase to facilitate the design of survivorship interventions for this vulnerable group. SSBCS, cancer support providers, and cancer physicians reported substantial symptom management, psychosocial, and informational needs among this population. Results from surveys and open-ended interviews were remarkably consistent. Survivors, physicians, and support providers viewed transition out of active treatment as a time of increased psychosocial need and heightened vulnerability.
Our findings are consistent with needs assessments conducted in other breast cancer survivors. Similar to a study of rural white women with breast cancer, fear of recurrence was among the most common psychosocial concerns.17 Results of two studies that included white, African American and Latina breast cancer survivors were consistent with ours in finding that pain and fatigue were among the most persistent symptoms; in both studies, Latinas were more likely to report pain and a higher number of symptoms.7,18 The prevalence of sleep problems in our sample was identical to that reported in a sample of African American breast cancer survivors.19 Our findings of a high need for symptom management information and support, social support from family and friends, and self-management resources were similar to studies of other vulnerable breast cancer survivors.18,20
Our results suggest that it is critical for health care professionals to provide assistance with managing side effects and information to alleviate fears, and reinforce behaviors of symptom monitoring and reporting, and adherence to follow-up care and hormonal therapies. Yet this information is not being conveyed effectively and is complicated by the need to balance women’s need for information with minimizing anxiety when providing such information.
A limitation of our study is that most of our sample was Mexican origin and may not reflect experiences of Spanish-speaking Latinas of other national origin groups or outside of Northern California. Another limitation is the lack of an English-speaking comparison group, which would have permitted the identification of similarities and differences across language groups. Finally, we did not interview radiation oncologists who may have had opinions that are not represented here.
Survivorship care programs offer great promise for meeting patients’ informational and symptom management needs and improving well-being and communication with clinicians.21 Due to limited access to survivorship care information, financial hardships, and pressures from their families to resume their social roles, concerted efforts are needed to develop appropriate survivorship programs for SSBCS.22 Unique language, cultural and socioeconomic factors of Spanish-speaking Latinas require tailoring of cancer survivorship programs to best meet their needs.23 These programs need to provide psychosocial stress and symptom management assistance, simple information on recommended follow-up care, and healthy lifestyle and role reintegration strategies that account for their unique sociocultural contexts.
After treatment, cancer patients transition to a survivorship phase, often with little information or support. Cancer survivors are at increased risk of recurrence, secondary cancers, comorbid conditions, and late treatment effects.1,2 However, many remain unaware of these risks and the options for managing them3 and face numerous unmet medical, psychosocial, and informational needs that can be addressed through survivorship care programs.4 Anxiety may increase as they lose their treatment team’s support while attempting to reestablish their lives.2 Patients need to know the long-term risks of cancer treatments, probabilities of recurrence and second cancers, effectiveness of surveillance and interventions for managing late effects and psychosocial concerns, and benefits of healthy lifestyles.2
Due to sociocultural and economic factors, Spanish-speaking Latina breast cancer survivors (SSBCS) suffer worse posttreatment health-related quality of life and more pain, fatigue, depressive symptoms, body image issues, and distress than their white counterparts.5-7 However, they are less likely to receive necessary cancer treatment, symptom management, and surveillance. For example, compared with whites, Latina breast cancer survivors receive less guideline-adherent treatment8 and follow-up care, including survivorship information.3,9 SSBCS, in particular have less access to survivorship information.10 Consequently, SSBCS are more likely to report unmet symptom management needs.11
Several breast cancer survivorship program trials have included Latinas,12,13 but their effectiveness has been demonstrated only for depressive symptoms or health worry. A comprehensive assessment of the posttreatment needs of SSBCS would provide a foundation for designing tailored survivorship interventions for this vulnerable group. This study aimed to identify the symptom management, psychosocial, and informational needs of SSBCS during the transition to survivorship from the perspectives of SSBCS and their cancer support providers and cancer physicians.
Methods
We sampled respondents within a 5-county area in Northern California to obtain multiple perspectives of the survivorship care needs of SSBCS using structured and in-depth methods: a telephone survey of SSBCS; semistructured interviews with SSBCS; semistructured interviews with cancer support providers serving SSBCS; and semistructured interviews with physicians providing cancer care for SSBCS. The study protocol was approved by the University of California San Francisco Committee on Human Research.
Sample and procedures
Structured telephone survey with SSBCS. The sample was drawn evenly from San Francisco General Hospital-University of California San Francisco primary care practices and SSBCS from a previous study who agreed to be re-contacted.14 The inclusion criteria were: completed active treatment (except adjuvant hormonal therapy) for nonmetastatic breast cancer within 10 years; living in one of the five counties; primarily Spanish-speaking; and self-identified as Latina. The exclusion criteria were: previous cancer except nonmelanoma skin cancer; terminal illness; or metastatic breast cancer. Study staff mailed potential participants a bilingual letter and information sheet, and bilingual opt-out postcard (6th grade reading level assessed by Flesch-Kincaid grade level statistic). Female bilingual-bicultural research associates conducted interviews of 20-30 minutes in Spanish after obtaining verbal consent. Participants were mailed $20. Surveys were conducted during March-November 2014.
Semistructured in-person interviews with SSBCS. Four community-based organizations (CBOs) in the targeted area providing cancer support services to Latinos agreed to recruit SSBCS for interviews. Inclusion criteria were identical to the survey. Patient navigators or support providers from CBOs contacted women by phone or in-person to invite them to an interview to assess their cancer survivorship needs. Women could choose a focus group or individual interview. With permission, names and contact information were given to study interviewers who called, explained the study, screened for eligibility, and scheduled an interview.
Recruitment was stratified by age (under or over age 50). We sampled women until saturation was achieved (no new themes emerged). Focus groups (90 minutes) were conducted at the CBOs. Individual interviews (45 minutes) were conducted in participants’ homes. Written informed consent was obtained. Participants were paid $50. Interviews were conducted during August-November, 2014, audiotaped, and transcribed.
Semistructured in-person interviews with cancer support providers and physicians. Investigators invited five cancer support providers (three patient navigators from three county hospitals, and two CBO directors of cancer psychosocial support services) and four physicians (three oncologists and one breast cancer surgeon from three county hospitals) to an in-person interview to identify SSBCS’ survivorship care needs. All agreed to participate. No further candidates were approached because saturation was achieved. We obtained written informed consent and 30-minute interviews were conducted in participants’ offices during August-October, 2014. Interviews were audiotaped and transcribed. Participants were paid $50.
Ethical approval. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent. Informed consent was obtained from all individual participants included in the study.
Measures
Structured telephone survey. Based on cancer survivor needs assessments,15 we assessed: physical and emotional symptoms; problems with sleep and memory/concentration; concerns about mortality, family, social isolation, intimacy, appearance; and healthy lifestyles. Items were adapted and translated into Spanish if needed, using forward/backward translation with team reconciliation. These questions used the introduction, “Now I am going to ask you if you have had any problems because of your cancer. In the past month, how much have you been bothered by …” with responses rated on a scale of 1-5 (1, Not at all; 5, A lot). For example, we asked, “In the past month, how much have you been bothered by fatigue?”
Regarding healthy lifestyles, we used the introduction, “Here are some changes women sometimes want to make after cancer. Would you like help with …?” For example, we asked, “Would you like help with getting more exercise?” We asked if they wanted help getting more exercise, eating healthier, managing stress, and doing meditation or yoga (Yes/No).
Semistructured interview guide for SSBCS. Participants were asked about their emotional and physical concerns when treatment ended, current cancer needs, symptoms or late effects, and issues related to relationships, family, employment, insurance, financial hardships, barriers to follow-up care, health behaviors, and survivorship program content. Sample questions are, “Have you had any symptoms or side effects related to your cancer or treatment?” and “What kinds of information do you feel you need now about your cancer or treatment?” A brief questionnaire assessed demographics.
Semistructured interview guide for cancer support providers and physicians. Support providers and physicians were asked about informational, psychosocial, and symptom management needs of SSBCS and recommended self-management content and formats. Sample questions are, “What kinds of information and support do you wish was available to help Spanish-speaking women take care of their health after treatment ends?” and “What do you think are the most pressing emotional needs of Spanish-speaking women after breast cancer treatment ends?” A brief questionnaire assessed demographics.
Analysis
Frequencies are reported for survey items. For questions about symptoms/concerns, we report the frequency of responding that they were bothered Somewhat/Quite a bit/A lot. For healthy lifestyles, we present the frequency answering Yes.
Verbatim semistructured interview transcripts were verified against audiotapes. Using QSR NVIVO software, transcripts were coded independently by two bilingual-bicultural investigators using a constant comparative method to generate coding categories for cancer survivorship needs.16 Coders started with themes specified by the interview guides, expanded them to represent the data, and discussed and reconciled coding discrepancies. Coding was compared by type of interview participant (survivor, support provider, or physician). Triangulation of survey and semistructured interviews occurred through team discussions to verify codes, themes, and implications for interventions.
Results
Telephone survey of SSBCS
Of the telephone survey sampling frame (N = 231), 118 individuals (51%) completed the interview, 37 (16%) were ineligible, 31 (13%) could not be reached, 22 (10%) had incorrect contact information, 19 (8%) refused to participate, and 4 (2%) were deceased. Mean age of the participants was 54.9 years (SD, 12.3); all were foreign-born, with more than half of Mexican origin; and most had less than a high-school education (Table 1). All had completed active treatment, and most (68%) were within 2 years of diagnosis.
For symptom management needs (Table 2), the most prevalent (bothersome) symptoms (reported by more than 30%, in rank order) were joint pain, sleep problems, fatigue, hot flashes, numbness/tingling of extremities, and memory. Next most prevalent (reported by 20%-30%) were vaginal dryness, dry/itchy skin, dry nose/mouth, inability to concentrate, constipation, changes in urination, and shortness of breath.

For psychosocial needs, fears of recurrence or new cancers were reported by 42%. Emotional symptoms reported by more than 30% were depression/sadness, anxieties, and feeling stressed. Next most prevalent (20%-30%) were fears, loss of interest in usual activities, and nervousness/restlessness. Social well-being concerns reported by 20%-30% of survivors were loneliness, having no one to talk to, and being a burden to their families. Body image and sexual problems reported by 20%-30% of survivors included appearance and problems being intimate with partners.
Regarding lifestyle, most of the participants said they wanted help with eating a healthier diet (74%), getting more exercise (69%), managing stress (63%), and doing yoga or meditation (55%).
Semistructured interviews
Twenty-five SSBCS completed semistructured interviews, 10 in individual interviews and 15 in one of two focus groups (one of 9 women older than 50 years; one of 6 women younger than 50). The telephone survey respondents were similar to semistructured interview respondents on all sociodemographic characteristics, but differed slightly on some clinical characteristics (Table 1). The telephone survey women had been more recently diagnosed (P < .01), were less likely to have ductal carcinoma in situ (P < .001), and more likely to have had reconstructive surgery
(P < .05).
Five cancer support providers and 4 physicians were interviewed. All support providers were Spanish-speaking Latinas with at least some college education. Cancer physicians were board certified. Two were men; two were white and two Asian; one was a breast surgeon and three were hematologists/oncologists; three spoke Spanish poorly/not at all and one spoke it fairly well.
Seven themes emerged from interviews: unmet physical symptom management needs; social support often ends when treatment ends; challenges resuming roles; sense of abandonment by health care system when treatment ends; need for formal transition from active treatment to follow-up care; fear of recurrence especially when obtaining follow-up care; and desire for information on late effects of initial treatments and side effects of hormonal treatments. We summarize results according to these themes.
Unmet physical symptom management needs. The main physical symptoms reported by survivors and physicians in interviews were arthralgia, menopausal symptoms, and neuropathy. Fatigue was reported only by survivors. Many survivors and several support providers expressed that symptoms were poorly managed and often ignored. One stated,
I have a lot of pain where I had surgery, it burns. I worry a lot about my arm because I have sacs of fluid. My doctor only says, ‘They will dissolve over the years.’ So, I don’t feel any support. (FocGrp1#6)
Survivors reported side effects of hormonal therapies, and felt that physicians downplayed these to prevent them from discontinuing medications.
Social support from family and friends often ends when treatment ends. Many survivors described a loss of support from family and friends who expected them to get back to “normal” once treatment ended. One said,
My sisters have told me to my face that there’s nothing wrong with me. So now when people ask me, I say, ‘I’m fine, thank God, I have nothing,’ even though I’m dying of pain and have all these pills to take. (Survivor#1025)
A support provider related,
The client was telling me that as she was getting closer to finishing her treatment, her husband was upset because he felt like all she was doing was focusing on the cancer. I think caregivers, family, spouses, and children out of their own sort of selfishness want this person to be well. (SuppProv#104)
A few survivors said that family bonds were strengthened after cancer and several reported lacking support because their families were in their home countries.
Challenges resuming roles, especially returning to work. Survivors, support providers, and physicians described challenges and few resources as women transition back to their normal roles. Survivors questioned their ability to return to work due to physically demanding occupations. One stated,
I would like information on how to take care of myself, how working can affect this side if I don’t take care of it. I clean houses and I need both hands. (Survivor#3012)
Survivors described how changes in memory affected daily chores and work performance. Support providers and physicians described the need for resources to aid with return to work and household responsibilities. One physician noted,
There are usually questions about how to go ahead and live their lives from that point forward. It’s a sort of reverse shock: going back to life as they know it. (Physician#004)
Support providers and physicians mentioned that women needed help with resuming intimate partner relations.
Sense of abandonment by health care system once active treatment ends. Survivors, support providers, and physicians reported a loss of support and sense of abandonment by the patients’ oncology team at the end of active treatment. One survivor stated,
Once they tell you to stop the pills, ‘You’re cured, there’s nothing wrong with you,’ the truth is that one feels, ‘Now what do I do? I have no one to help me.’ I felt very abandoned. (FocGrp1#5)
A provider said,
The support system falls apart once women complete treatment. They lose their entire support system at the medical level. They no longer have nurses checking in about symptoms and addressing anything that’s come up. They won’t have access to doctors unless they’re doing their screening. (SuppProv#101)
An oncologist, noting that this loss of support occurs when women face pressures of transitioning back to work or family obligations, commented,
So here’s a woman whose marriage is in turmoil, whose husband may even have left her during this, and now her clinic is leaving her and she’s on her own … that must be scary as hell because there’s nobody out there to support her. (Physician#002)
Need for formal transition from active treatment to follow-up care. Two themes emerged about transitioning from active treatment: transferring care from oncologists to primary care physicians (PCP); and issues of follow-up care (with oncologists or PCPs). Survivors felt lost in transitioning from specialty to primary care, or expressed apprehension seeing a PCP rather than a cancer specialist. One stated,
I have my doctor but she is not a specialist. She does what I tell her to and orders a mammogram every year. But, I don’t go to the oncologist anymore, and so I worry. With the specialists, I feel protected. (FocGrp1#5)
Physicians acknowledged the lack of a formal transition to primary care such as a survivorship care program.
Follow-up care issues were common. Physicians stressed that women needed to know how often to return for follow-up once active treatment ends and about recommended examinations and tests, especially when receiving hormonal therapy. Physicians indicated the need for patient education materials specific to patients’ treatments, for example, elevated risk of heart disease with certain chemotherapy agents. An oncologist expressed concern that PCPs are not prepared adequately about late effects and hormonal treatment side effects, and suggested providing summary notes for PCPs detailing these.
Survivors identified several barriers to follow-up care: lacking information on which symptoms merited a call to physicians; financial burden/limited health insurance; lacking appointment reminders; fear of examinations; and limited English proficiency. A survivor stated,
If you have insurance, you can make your appointment, see the doctor, and have your mammogram. I stopped taking my pills because I didn’t have insurance. I tried to get them again but they told me they would cost me a thousand dollars. (FocGrp1#5)
One oncologist suggested scheduling a follow-up appointment before patients leave treatment and calling patients who miss appointments.
Facilitators of regular follow-up care identified by survivors were physicians informing them about symptom monitoring and reporting, having a clinic contact person/navigator, being given a follow-up appointment, being assertive about one’s care, and physicians’ reinforcement of adherence to hormonal treatment and follow-up. According to support providers, a key facilitator was having a clinic contact person/navigator. Once treatment ended, support providers often served as the liaison between the patient and the physician, making them the first point of contact for symptom reporting.
Fear of recurrence especially when obtaining follow-up care. Fear of recurrence dominated survivor interviews. This fear was heightened at the time of follow-up examinations or when they experienced unusual pain. A survivor commented,
Every time I’m due for my mammogram, I can’t sleep, worrying. I lose sleep until I get the letter with my results. Then I feel at peace again. (FocGrp1#9)
Support providers discussed the need to provide reassurance to SSBCS to help them cope with fears of recurrence. Physicians expressed challenges in allaying fears of recurrence among SSBCS, requiring a lot of time when recommending follow-up mammograms.
Desire for information on late effects of treatments and side effects of hormonal therapies. All survivors expressed receiving insufficient information on potential symptoms and side effects. One stated,
Doctors only have five minutes. There has never been someone who gave me guidance like, ‘From now on you have to do this or you might get these symptoms now or in the future. (Survivor#6019)
They indicated uncertainty about what symptoms were “normal” and when symptoms merited a call to the physician. Several survivors reported being unaware that fatigue, arthralgia and neuropathy were side effects of breast cancer treatments until they reported these to physicians.
Physicians stressed the importance of women knowing about the elevated risk of future cancers, symptoms of recurrence, and seeking follow-up care if they experience symptoms that are out of the ordinary. Support providers felt that it was important to provide SSBCS with information on signs of recurrence and when to report these. However, providers expressed concern that giving women too much information might elevate their anxiety. A physician suggested,
It’s probably better to have a symptom list that’s short and relevant for the most common and catastrophic things, same thing with side effects … short to avoid overwhelming the patient. (Physician#001)
Hormonal treatments were of special concern. Survivors expressed a need for information on hormonal treatments and support providers stressed that this information is needed in simple Spanish. Several survivors indicated they stopped taking hormonal treatments due to side effects. One woman experienced severe headaches and heart palpitations, stopped taking the hormonal medication, felt better, and did not inform her physician until her next appointment. A support provider stated,
What I hear from a lot of women is that if side effects are too uncomfortable, they just stop it (hormonal treatment) without saying anything to the doctor. So more information about why they have to take it and that there is a good chance of recurrence is really important. (SuppProv#101)
Likewise, physicians indicated that SSBCS’ lack of information on hormonal treatments often resulted in nonadherence, emphasizing the need to reinforce adherence to prevent recurrence.
Conceptual framework of interventions
Based on triangulation of survey and interview results, we compiled a conceptual framework that includes needs identified, suggested components of a survivorship care intervention to address these needs, potential mediators by which such interventions could improve outcomes, and relevant outcomes (Figure). Survivorship care needs fell into four categories: symptom management, psychosocial, sense of abandonment by health care team, and healthy lifestyles. Survivorship care programs would provide skills training in symptom and stress management, and communicating with providers, family, friends, and coworkers. Mediators include increased self-efficacy, knowledge and perceived social support, ultimately leading to reduced distress (anxiety and depressive symptoms) and stress, and improved health-related quality of life.
Conclusions
Our study aimed to identify the most critical needs of SSBCS in the posttreatment survivorship phase to facilitate the design of survivorship interventions for this vulnerable group. SSBCS, cancer support providers, and cancer physicians reported substantial symptom management, psychosocial, and informational needs among this population. Results from surveys and open-ended interviews were remarkably consistent. Survivors, physicians, and support providers viewed transition out of active treatment as a time of increased psychosocial need and heightened vulnerability.
Our findings are consistent with needs assessments conducted in other breast cancer survivors. Similar to a study of rural white women with breast cancer, fear of recurrence was among the most common psychosocial concerns.17 Results of two studies that included white, African American and Latina breast cancer survivors were consistent with ours in finding that pain and fatigue were among the most persistent symptoms; in both studies, Latinas were more likely to report pain and a higher number of symptoms.7,18 The prevalence of sleep problems in our sample was identical to that reported in a sample of African American breast cancer survivors.19 Our findings of a high need for symptom management information and support, social support from family and friends, and self-management resources were similar to studies of other vulnerable breast cancer survivors.18,20
Our results suggest that it is critical for health care professionals to provide assistance with managing side effects and information to alleviate fears, and reinforce behaviors of symptom monitoring and reporting, and adherence to follow-up care and hormonal therapies. Yet this information is not being conveyed effectively and is complicated by the need to balance women’s need for information with minimizing anxiety when providing such information.
A limitation of our study is that most of our sample was Mexican origin and may not reflect experiences of Spanish-speaking Latinas of other national origin groups or outside of Northern California. Another limitation is the lack of an English-speaking comparison group, which would have permitted the identification of similarities and differences across language groups. Finally, we did not interview radiation oncologists who may have had opinions that are not represented here.
Survivorship care programs offer great promise for meeting patients’ informational and symptom management needs and improving well-being and communication with clinicians.21 Due to limited access to survivorship care information, financial hardships, and pressures from their families to resume their social roles, concerted efforts are needed to develop appropriate survivorship programs for SSBCS.22 Unique language, cultural and socioeconomic factors of Spanish-speaking Latinas require tailoring of cancer survivorship programs to best meet their needs.23 These programs need to provide psychosocial stress and symptom management assistance, simple information on recommended follow-up care, and healthy lifestyle and role reintegration strategies that account for their unique sociocultural contexts.
1. Danese MD, O’Malley C, Lindquist K, Gleeson M, Griffiths RI. An observational study of the prevalence and incidence of comorbid conditions in older women with breast cancer. Ann Oncol. 2012;23(7):1756-1765.
2. Hewitt M, Greenfield S, Stovall E, eds. From cancer patient to cancer survivor: lost in transition. Washington, DC: National Academy of Sciences; 2006.
3. Beckjord EB, Arora NK, McLaughlin W, Oakley-Girvan I, Hamilton AS, Hesse BW. Health-related information needs in a large and diverse sample of adult cancer survivors: implications for cancer care. J Cancer Surviv. 2008;2(3):179-189.
4. Hewitt ME, Bamundo A, Day R, Harvey C. Perspectives on posttreatment cancer care: qualitative research with survivors, nurses, and physicians. J Clin Oncol. 2007;25(16):2270-2273.
5. Ashing-Giwa KT, Tejero JS, Kim J, Padilla GV, Hellemann G. Examining predictive models of HRQOL in a population-based, multiethnic sample of women with breast carcinoma. Qual Life Res. 2007;16(3):413-428.
6. Clauser SB, Arora NK, Bellizzi KM, Haffer SC, Topor M, Hays RD. Disparities in HRQOL of cancer survivors and non-cancer managed care enrollees. Health Care Financ Rev. 2008;29(4):23-40.
7. Eversley R, Estrin D, Dibble S, Wardlaw L, Pedrosa M, Favila-Penney W. Posttreatment symptoms among ethnic minority breast cancer survivors. Oncol Nurs Forum. 2005;32(2):250-256.
8. Bickell NA, Wang JJ, Oluwole S, et al. Missed opportunities: racial disparities in adjuvant breast cancer treatment. J Clin Oncol. 2006;24(9):1357-1362.
9. Arora NK, Reeve BB, Hays RD, Clauser SB, Oakley-Girvan I. Assessment of quality of cancer-related follow-up care from the cancer survivor’s perspective. J Clin Oncol. 2011;29(10):1280-1289.
10. Janz NK, Mujahid MS, Hawley ST, Griggs JJ, Hamilton AS, Katz SJ. Racial/ethnic differences in adequacy of information and support for women with breast cancer. Cancer. 2008;113(5):1058-1067.
11. Yoon J, Malin JL, Tisnado DM, et al. Symptom management after breast cancer treatment: is it influenced by patient characteristics? Breast Cancer Res Treat. 2008;108(1):69-77.
12. Ashing K, Rosales M. A telephonic-based trial to reduce depressive symptoms among Latina breast cancer survivors. Psychooncology. 2014;23(5):507-515.
13. Hershman DL, Greenlee H, Awad D, et al. Randomized controlled trial of a clinic-based survivorship intervention following adjuvant therapy in breast cancer survivors. Breast Cancer Res Treat. 2013;138(3):795-806.
14. Napoles AM, Ortiz C, Santoyo-Olsson J, et al. Nuevo Amanecer: results of a randomized controlled trial of a community-based, peer-delivered stress management intervention to improve quality of life in Latinas with breast cancer. Am J Public Health. 2015;105(suppl 3):e55-63.
15. Rechis R, Reynolds KA, Beckjord EB, Nutt S, Burns RM, Schaefer JS. ‘I learned to live with it’ is not good enough: challenges reported by posttreatment cancer survivors in the Livestrong surveys. Austin, TX: Livestrong;2011.
16. Glaser BG, Strauss AL. The discovery of grounded theory: strategies for qualitative research. Hawthorne: Aldine Publishing Company; 1967.
17. Befort CA, Klemp J. Sequelae of breast cancer and the influence of menopausal status at diagnosis among rural breast cancer survivors. J Womens Health (Larchmt). 2011;20(9):1307-1313.
18. Fu OS, Crew KD, Jacobson JS, et al. Ethnicity and persistent symptom burden in breast cancer survivors. J Cancer Surviv. 2009;3(4):241-250.
19. Taylor TR, Huntley ED, Makambi K, et al. Understanding sleep disturbances in African-American breast cancer survivors: a pilot study. Psychooncology. 2012;21(8):896-902.
20. Adams N, Gisiger-Camata S, Hardy CM, Thomas TF, Jukkala A, Meneses K. Evaluating survivorship experiences and needs among rural African American breast cancer survivors. J Cancer Educ. October 24, 2015 [Epub ahead of print].
21. Blinder VS, Patil S, Thind A, et al. Return to work in low-income Latina and non-Latina white breast cancer survivors: a 3-year longitudinal study. Cancer. 2012;118(6):1664-1674.
22. Lopez-Class M, Perret-Gentil M, Kreling B, Caicedo L, Mandelblatt J, Graves KD. Quality of life among immigrant Latina breast cancer survivors: realities of culture and enhancing cancer care. J Cancer Educ. 2011;26(4):724-733.
23. Napoles-Springer AM, Ortiz C, O’Brien H, Diaz-Mendez M. Developing a culturally competent peer support intervention for Spanish-speaking Latinas with breast cancer. J Immigr Minor Health. 2009;11(4):268-280
1. Danese MD, O’Malley C, Lindquist K, Gleeson M, Griffiths RI. An observational study of the prevalence and incidence of comorbid conditions in older women with breast cancer. Ann Oncol. 2012;23(7):1756-1765.
2. Hewitt M, Greenfield S, Stovall E, eds. From cancer patient to cancer survivor: lost in transition. Washington, DC: National Academy of Sciences; 2006.
3. Beckjord EB, Arora NK, McLaughlin W, Oakley-Girvan I, Hamilton AS, Hesse BW. Health-related information needs in a large and diverse sample of adult cancer survivors: implications for cancer care. J Cancer Surviv. 2008;2(3):179-189.
4. Hewitt ME, Bamundo A, Day R, Harvey C. Perspectives on posttreatment cancer care: qualitative research with survivors, nurses, and physicians. J Clin Oncol. 2007;25(16):2270-2273.
5. Ashing-Giwa KT, Tejero JS, Kim J, Padilla GV, Hellemann G. Examining predictive models of HRQOL in a population-based, multiethnic sample of women with breast carcinoma. Qual Life Res. 2007;16(3):413-428.
6. Clauser SB, Arora NK, Bellizzi KM, Haffer SC, Topor M, Hays RD. Disparities in HRQOL of cancer survivors and non-cancer managed care enrollees. Health Care Financ Rev. 2008;29(4):23-40.
7. Eversley R, Estrin D, Dibble S, Wardlaw L, Pedrosa M, Favila-Penney W. Posttreatment symptoms among ethnic minority breast cancer survivors. Oncol Nurs Forum. 2005;32(2):250-256.
8. Bickell NA, Wang JJ, Oluwole S, et al. Missed opportunities: racial disparities in adjuvant breast cancer treatment. J Clin Oncol. 2006;24(9):1357-1362.
9. Arora NK, Reeve BB, Hays RD, Clauser SB, Oakley-Girvan I. Assessment of quality of cancer-related follow-up care from the cancer survivor’s perspective. J Clin Oncol. 2011;29(10):1280-1289.
10. Janz NK, Mujahid MS, Hawley ST, Griggs JJ, Hamilton AS, Katz SJ. Racial/ethnic differences in adequacy of information and support for women with breast cancer. Cancer. 2008;113(5):1058-1067.
11. Yoon J, Malin JL, Tisnado DM, et al. Symptom management after breast cancer treatment: is it influenced by patient characteristics? Breast Cancer Res Treat. 2008;108(1):69-77.
12. Ashing K, Rosales M. A telephonic-based trial to reduce depressive symptoms among Latina breast cancer survivors. Psychooncology. 2014;23(5):507-515.
13. Hershman DL, Greenlee H, Awad D, et al. Randomized controlled trial of a clinic-based survivorship intervention following adjuvant therapy in breast cancer survivors. Breast Cancer Res Treat. 2013;138(3):795-806.
14. Napoles AM, Ortiz C, Santoyo-Olsson J, et al. Nuevo Amanecer: results of a randomized controlled trial of a community-based, peer-delivered stress management intervention to improve quality of life in Latinas with breast cancer. Am J Public Health. 2015;105(suppl 3):e55-63.
15. Rechis R, Reynolds KA, Beckjord EB, Nutt S, Burns RM, Schaefer JS. ‘I learned to live with it’ is not good enough: challenges reported by posttreatment cancer survivors in the Livestrong surveys. Austin, TX: Livestrong;2011.
16. Glaser BG, Strauss AL. The discovery of grounded theory: strategies for qualitative research. Hawthorne: Aldine Publishing Company; 1967.
17. Befort CA, Klemp J. Sequelae of breast cancer and the influence of menopausal status at diagnosis among rural breast cancer survivors. J Womens Health (Larchmt). 2011;20(9):1307-1313.
18. Fu OS, Crew KD, Jacobson JS, et al. Ethnicity and persistent symptom burden in breast cancer survivors. J Cancer Surviv. 2009;3(4):241-250.
19. Taylor TR, Huntley ED, Makambi K, et al. Understanding sleep disturbances in African-American breast cancer survivors: a pilot study. Psychooncology. 2012;21(8):896-902.
20. Adams N, Gisiger-Camata S, Hardy CM, Thomas TF, Jukkala A, Meneses K. Evaluating survivorship experiences and needs among rural African American breast cancer survivors. J Cancer Educ. October 24, 2015 [Epub ahead of print].
21. Blinder VS, Patil S, Thind A, et al. Return to work in low-income Latina and non-Latina white breast cancer survivors: a 3-year longitudinal study. Cancer. 2012;118(6):1664-1674.
22. Lopez-Class M, Perret-Gentil M, Kreling B, Caicedo L, Mandelblatt J, Graves KD. Quality of life among immigrant Latina breast cancer survivors: realities of culture and enhancing cancer care. J Cancer Educ. 2011;26(4):724-733.
23. Napoles-Springer AM, Ortiz C, O’Brien H, Diaz-Mendez M. Developing a culturally competent peer support intervention for Spanish-speaking Latinas with breast cancer. J Immigr Minor Health. 2009;11(4):268-280











