User login
When Heart Problems Crop Up
ANSWER
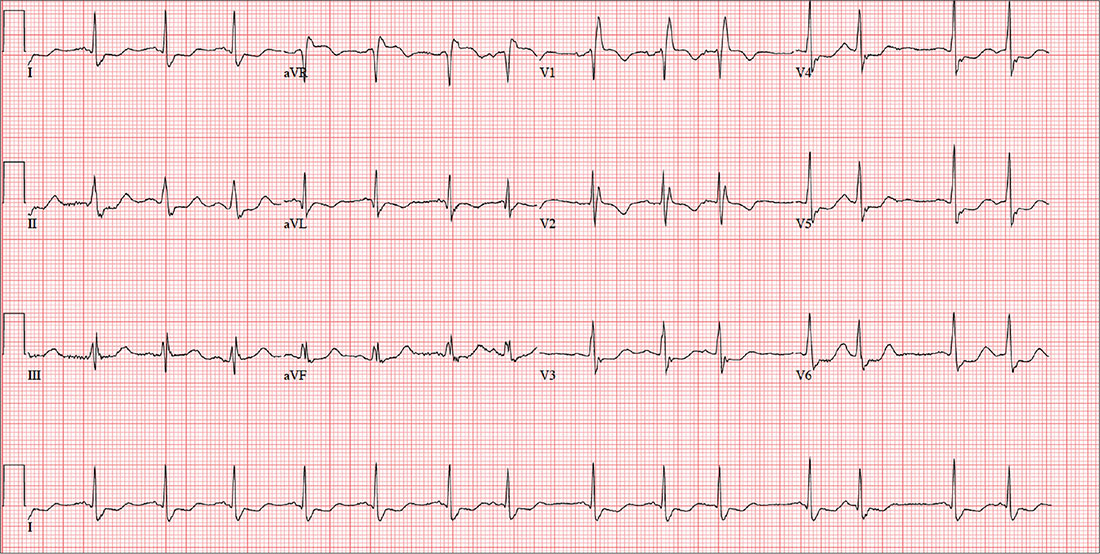
This ECG shows a sinus rhythm with premature atrial complexes, a normal axis, a right bundle branch block, and T-wave abnormalities consistent with lateral ischemia.
Sinus rhythm is evidenced by the P waves’ rate of ≥ 60 beats/min and ≤ 100 beats/min. The P waves that indicate premature atrial contractions have a different appearance than normal P waves, a result of the atrial activation from a site other than the sinus node. Because both the normal P wave and the premature atrial contraction conduct through the normal ventricular conduction system, the associated QRS complexes are identica
A right bundle branch block is identified by a QRS duration > 120 ms with a terminal broad S wave in lead I and an RSR’ complex in leads V1 and V2. The ST segment depressions in precordial leads V3 through V6 propagate the diagnosis of lateral ischemia.
The patient was not found to have atrial fibrillation, and his palpitations and evidence of lateral ischemia resolved when he resumed taking his ß-blocker.
ANSWER
This ECG shows a sinus rhythm with premature atrial complexes, a normal axis, a right bundle branch block, and T-wave abnormalities consistent with lateral ischemia.
Sinus rhythm is evidenced by the P waves’ rate of ≥ 60 beats/min and ≤ 100 beats/min. The P waves that indicate premature atrial contractions have a different appearance than normal P waves, a result of the atrial activation from a site other than the sinus node. Because both the normal P wave and the premature atrial contraction conduct through the normal ventricular conduction system, the associated QRS complexes are identica
A right bundle branch block is identified by a QRS duration > 120 ms with a terminal broad S wave in lead I and an RSR’ complex in leads V1 and V2. The ST segment depressions in precordial leads V3 through V6 propagate the diagnosis of lateral ischemia.
The patient was not found to have atrial fibrillation, and his palpitations and evidence of lateral ischemia resolved when he resumed taking his ß-blocker.
ANSWER
This ECG shows a sinus rhythm with premature atrial complexes, a normal axis, a right bundle branch block, and T-wave abnormalities consistent with lateral ischemia.
Sinus rhythm is evidenced by the P waves’ rate of ≥ 60 beats/min and ≤ 100 beats/min. The P waves that indicate premature atrial contractions have a different appearance than normal P waves, a result of the atrial activation from a site other than the sinus node. Because both the normal P wave and the premature atrial contraction conduct through the normal ventricular conduction system, the associated QRS complexes are identica
A right bundle branch block is identified by a QRS duration > 120 ms with a terminal broad S wave in lead I and an RSR’ complex in leads V1 and V2. The ST segment depressions in precordial leads V3 through V6 propagate the diagnosis of lateral ischemia.
The patient was not found to have atrial fibrillation, and his palpitations and evidence of lateral ischemia resolved when he resumed taking his ß-blocker.
A 72-year-old farmer presents with heart palpitations he has had for the past three weeks. He fears he may be in atrial fibrillation and at risk for a stroke, stating that a few months ago his neighbor complained of palpitations (later attributed to atrial fibrillation) and had an embolic stroke due to a clot in the left atrium. The patient denies any history of cardiac arrhythmias, chest pain, syncope, or near-syncope. He does report occasional bouts of lightheadedness, which have increased in frequency. He is more concerned, though, by the strong, intensified heartbeats that he can feel in his throat.
Past medical and surgical histories are positive for type 2 diabetes, hypertension, and hyperlipidemia, as well as an appendectomy and cholecystectomy. He has also had several upper extremity fractures in the past, all of which have healed well.
The patient’s wife died recently from complications following a hip replacement surgery, but he and his two sons continue to work on the 460-acre family farm he has owned all his life. A chronic smoker, he has smoked one to two packs of cigarettes per day since he was 15. He does not drink alcohol but does consume one to two pots of coffee per day.
His medication list includes metformin, atorvastatin, and metoprolol. He hasn’t taken his metoprolol for two months, because he switched pharmacies and the pills he received were a different color so he didn’t believe they were the right drug (despite the label on the bottle). He has no known drug allergies, but sulfa drugs induce nausea.
The review of systems is remarkable for arthritic pain in his hands, shoulders, hips, knees, and ankles. The patient complains of gastric reflux and occasional diarrhea. He states his mood is still down from the loss of his wife, but he denies being depressed.
His weight is 232 lb and his height, 70 in. Vital signs include a blood pressure of 168/92 mm Hg; pulse, 84 beats/min; respiratory rate, 14 breaths/min-1; and temperature, 99.2°F.
Physical exam reveals a weathered but otherwise healthy-looking male in no distress. He wears corrective lenses. The HEENT exam reveals surprisingly good dentition for a man his age. There are no carotid bruits. The lungs have expiratory crackles in both bases that change with coughing.
The cardiac exam reveals an irregular rate of 88 beats/min with a grade II/VI early systolic murmur at the left upper sternal border. There are no extra heart sounds or rubs. The abdomen is soft and nontender with old, well-healed cholecystectomy and appendectomy incision scars. The peripheral pulses are strong and equal bilaterally. Arthritic changes are evident in the hands and feet. The neurologic exam is grossly intact.
An ECG is performed; it reveals a ventricular rate of 87 beats/min; PR interval, 156 ms; QRS duration, 138 ms; QT/QTc interval, 440/529 ms; P axis, 58°; R axis, 26°; and T axis, 105°. What is your interpretation of this ECG?
Sneak Peek: The Hospital Leader Blog
Editor’s note: This article first appeared on “The Hospital Leader” blog. Read the full post at hospitalleader.org.
In December, I wrote a letter to hospital executives, urging them to deliberately invest their own personal time and effort in fostering hospitalist well-being. I suggested several actions that leaders can take to enhance hospitalist job satisfaction and reduce the risk of burnout and turnover.
Following publication of that post, I heard from several hospital executives and was pleasantly surprised that they all responded positively to my message. Several execs told me that they gained valuable new insights about their hospitalists’ challenges and needs; others said they planned to take action on one or more of my suggestions that had never occurred to them before.
Their feedback reinforced my belief that most hospital leaders actually do care a lot about promoting healthy, stable, and sustainable hospitalist programs, but the hospital leaders I talked with also had some messages for their hospitalist colleagues, and I think it’s important to share them in the spirit of fostering a healthy exchange of perspectives. Your hospital’s leaders would be delighted and encouraged if you engaged them in dialogue about these issues.
Help us help you
Several hospital leaders told me that their hospitalists grumble about being treated by the medical staff (and even nurses) like second-class citizens or glorified residents. Those same hospitalists, however, routinely show up for work dressed in scrubs and tennis shoes rather than professional attire. They rarely come in early when it’s busy or invest more time than is absolutely needed to see the patients on their list, making it easy for others to dismiss them as shift workers.
Hospitalists, they say, are unwilling to come in on their own time to attend a medical staff meeting, something other doctors do as a matter of course. And instead of interacting as social peers with other physicians when opportunity arises (i.e., in the cafeteria or doctors’ lounge), the hospitalists just grab food and head back to eat together in their work room.
The executives said they want to help enhance the stature of their hospitalists within the medical staff, but the Here’s a typical comment:
“[Hospitalists] also need to be willing to participate in hospital and system committees. Although this may require them to interrupt their workflow and stay late on some days they are working or come in on days off, they will never garner the respect of their colleagues if they are unwilling to do so.”
Read the full post at hospitalleader.org.
Leslie Flores is a hospital medicine consultant and member of SHM’s Practice Analysis Committee.
Also on The Hospital Leader. . .
• Creating Value through Crowdsourcing & Finding ‘Value’ in the New Year, by Vineet Arora, MD, MPP, FHM
• BREAKING NEWS: “Physicians Deemed Unnecessary”; Social Worker Promoted to Hospital CEO, by Jordan Messler, MD, SFHM
• ER Docs and Out-of-Network Billing: Are We in the Same Boat?, by Brad Flansbaum, DO, MPH, MHM
• The Best Way to Die?, by David Brabeck, MD
Editor’s note: This article first appeared on “The Hospital Leader” blog. Read the full post at hospitalleader.org.
In December, I wrote a letter to hospital executives, urging them to deliberately invest their own personal time and effort in fostering hospitalist well-being. I suggested several actions that leaders can take to enhance hospitalist job satisfaction and reduce the risk of burnout and turnover.
Following publication of that post, I heard from several hospital executives and was pleasantly surprised that they all responded positively to my message. Several execs told me that they gained valuable new insights about their hospitalists’ challenges and needs; others said they planned to take action on one or more of my suggestions that had never occurred to them before.
Their feedback reinforced my belief that most hospital leaders actually do care a lot about promoting healthy, stable, and sustainable hospitalist programs, but the hospital leaders I talked with also had some messages for their hospitalist colleagues, and I think it’s important to share them in the spirit of fostering a healthy exchange of perspectives. Your hospital’s leaders would be delighted and encouraged if you engaged them in dialogue about these issues.
Help us help you
Several hospital leaders told me that their hospitalists grumble about being treated by the medical staff (and even nurses) like second-class citizens or glorified residents. Those same hospitalists, however, routinely show up for work dressed in scrubs and tennis shoes rather than professional attire. They rarely come in early when it’s busy or invest more time than is absolutely needed to see the patients on their list, making it easy for others to dismiss them as shift workers.
Hospitalists, they say, are unwilling to come in on their own time to attend a medical staff meeting, something other doctors do as a matter of course. And instead of interacting as social peers with other physicians when opportunity arises (i.e., in the cafeteria or doctors’ lounge), the hospitalists just grab food and head back to eat together in their work room.
The executives said they want to help enhance the stature of their hospitalists within the medical staff, but the Here’s a typical comment:
“[Hospitalists] also need to be willing to participate in hospital and system committees. Although this may require them to interrupt their workflow and stay late on some days they are working or come in on days off, they will never garner the respect of their colleagues if they are unwilling to do so.”
Read the full post at hospitalleader.org.
Leslie Flores is a hospital medicine consultant and member of SHM’s Practice Analysis Committee.
Also on The Hospital Leader. . .
• Creating Value through Crowdsourcing & Finding ‘Value’ in the New Year, by Vineet Arora, MD, MPP, FHM
• BREAKING NEWS: “Physicians Deemed Unnecessary”; Social Worker Promoted to Hospital CEO, by Jordan Messler, MD, SFHM
• ER Docs and Out-of-Network Billing: Are We in the Same Boat?, by Brad Flansbaum, DO, MPH, MHM
• The Best Way to Die?, by David Brabeck, MD
Editor’s note: This article first appeared on “The Hospital Leader” blog. Read the full post at hospitalleader.org.
In December, I wrote a letter to hospital executives, urging them to deliberately invest their own personal time and effort in fostering hospitalist well-being. I suggested several actions that leaders can take to enhance hospitalist job satisfaction and reduce the risk of burnout and turnover.
Following publication of that post, I heard from several hospital executives and was pleasantly surprised that they all responded positively to my message. Several execs told me that they gained valuable new insights about their hospitalists’ challenges and needs; others said they planned to take action on one or more of my suggestions that had never occurred to them before.
Their feedback reinforced my belief that most hospital leaders actually do care a lot about promoting healthy, stable, and sustainable hospitalist programs, but the hospital leaders I talked with also had some messages for their hospitalist colleagues, and I think it’s important to share them in the spirit of fostering a healthy exchange of perspectives. Your hospital’s leaders would be delighted and encouraged if you engaged them in dialogue about these issues.
Help us help you
Several hospital leaders told me that their hospitalists grumble about being treated by the medical staff (and even nurses) like second-class citizens or glorified residents. Those same hospitalists, however, routinely show up for work dressed in scrubs and tennis shoes rather than professional attire. They rarely come in early when it’s busy or invest more time than is absolutely needed to see the patients on their list, making it easy for others to dismiss them as shift workers.
Hospitalists, they say, are unwilling to come in on their own time to attend a medical staff meeting, something other doctors do as a matter of course. And instead of interacting as social peers with other physicians when opportunity arises (i.e., in the cafeteria or doctors’ lounge), the hospitalists just grab food and head back to eat together in their work room.
The executives said they want to help enhance the stature of their hospitalists within the medical staff, but the Here’s a typical comment:
“[Hospitalists] also need to be willing to participate in hospital and system committees. Although this may require them to interrupt their workflow and stay late on some days they are working or come in on days off, they will never garner the respect of their colleagues if they are unwilling to do so.”
Read the full post at hospitalleader.org.
Leslie Flores is a hospital medicine consultant and member of SHM’s Practice Analysis Committee.
Also on The Hospital Leader. . .
• Creating Value through Crowdsourcing & Finding ‘Value’ in the New Year, by Vineet Arora, MD, MPP, FHM
• BREAKING NEWS: “Physicians Deemed Unnecessary”; Social Worker Promoted to Hospital CEO, by Jordan Messler, MD, SFHM
• ER Docs and Out-of-Network Billing: Are We in the Same Boat?, by Brad Flansbaum, DO, MPH, MHM
• The Best Way to Die?, by David Brabeck, MD
New Trump travel order could disrupt meetings, trainees
President’s Trump’s revised executive order blocking travelers from six Muslim-majority countries from entering the United States could land a damaging blow to global cooperation in scientific research and impede assemblies of the world’s top medical experts.
The executive order, signed March 6, bars citizens of Iran, Libya, Somalia, Sudan, Syria, and Yemen from obtaining visas for 90 days and blocks refugees from the affected countries from entering the U.S. for 120 days. The new executive measure, which takes effect March 16, supersedes President Trump’s original Jan. 27 travel ban that has been blocked by federal courts.
The new order clarifies that citizens of the six countries who are legal permanent U.S. residents or who have current visas to enter the country are exempt from the travel prohibition.
The revised travel ban could disrupt the exchange of medical knowledge by barring foreign experts from traveling to medical and scientific conferences in the United States, and leaves the status of medical trainees from those countries in limbo, according to American Medical Association President Andrew W. Gurman, MD.
“Hundreds of physicians from six countries are subject to the revised executive order and have applied to U.S. training programs and requested visa sponsorship,” he said in a statement. “The new executive order leaves them in limbo and without an explicit waiver, these foreign physicians will be unable to provide care in the U.S. when training programs begin on July 1.”
The new order is already being challenged in court. On March 7, the state of Hawaii filed a lawsuit seeking to block the order, saying that it subjects a portion of Hawaii’s population to “discrimination and second-class treatment.”
When the original ban took effect, thousands of academics from around the world, including physicians, researchers, and professors, vowed to boycott U.S.-based conferences. A Google Docs petition started shortly after the ban was announced garnered more than 5,000 signatures by professionals acting in solidarity with those affected by the travel restrictions. The academicians who signed the petition said they would not attend international conferences in the United States until those restricted from participating could rejoin their colleagues and freely share their ideas.
The new executive order comes nearly 2 months after President Trump’s original travel ban caused nationwide protests and led to a series of legal challenges. The states of Washington and Minnesota, which sued President Trump over his original ban, argued that such a ban harms the teaching and research missions of their universities and prevents students and faculty from traveling for research and academic collaboration. In addition, the executive order restricts universities from hiring attractive candidates from countries affected by the ban, state officials said.
A federal court temporarily blocked the original travel ban on Feb. 3, a decision upheld by the 9th U.S. Circuit Court of Appeals on Feb. 9.
The new executive order excludes Iraq this time around and also removes language that had indefinitely banned Syrian refugees. In a March 6 memorandum, the White House said the purpose of the ban is to prevent “foreign nationals who may aid, support, or commit violent, criminal, or terrorist acts,” while the administration enhances the screening and vetting protocols and procedures for granting visas and admission to the United States.
“This nation cannot delay the immediate implementation of additional heightened screening and vetting protocols and procedures for issuing visas to ensure that we strengthen the safety and security of our country,” the memo states.
This article was updated March 8, 2017.
agallegos@frontlinemedcom.com
On Twitter @legal_med
President’s Trump’s revised executive order blocking travelers from six Muslim-majority countries from entering the United States could land a damaging blow to global cooperation in scientific research and impede assemblies of the world’s top medical experts.
The executive order, signed March 6, bars citizens of Iran, Libya, Somalia, Sudan, Syria, and Yemen from obtaining visas for 90 days and blocks refugees from the affected countries from entering the U.S. for 120 days. The new executive measure, which takes effect March 16, supersedes President Trump’s original Jan. 27 travel ban that has been blocked by federal courts.
The new order clarifies that citizens of the six countries who are legal permanent U.S. residents or who have current visas to enter the country are exempt from the travel prohibition.
The revised travel ban could disrupt the exchange of medical knowledge by barring foreign experts from traveling to medical and scientific conferences in the United States, and leaves the status of medical trainees from those countries in limbo, according to American Medical Association President Andrew W. Gurman, MD.
“Hundreds of physicians from six countries are subject to the revised executive order and have applied to U.S. training programs and requested visa sponsorship,” he said in a statement. “The new executive order leaves them in limbo and without an explicit waiver, these foreign physicians will be unable to provide care in the U.S. when training programs begin on July 1.”
The new order is already being challenged in court. On March 7, the state of Hawaii filed a lawsuit seeking to block the order, saying that it subjects a portion of Hawaii’s population to “discrimination and second-class treatment.”
When the original ban took effect, thousands of academics from around the world, including physicians, researchers, and professors, vowed to boycott U.S.-based conferences. A Google Docs petition started shortly after the ban was announced garnered more than 5,000 signatures by professionals acting in solidarity with those affected by the travel restrictions. The academicians who signed the petition said they would not attend international conferences in the United States until those restricted from participating could rejoin their colleagues and freely share their ideas.
The new executive order comes nearly 2 months after President Trump’s original travel ban caused nationwide protests and led to a series of legal challenges. The states of Washington and Minnesota, which sued President Trump over his original ban, argued that such a ban harms the teaching and research missions of their universities and prevents students and faculty from traveling for research and academic collaboration. In addition, the executive order restricts universities from hiring attractive candidates from countries affected by the ban, state officials said.
A federal court temporarily blocked the original travel ban on Feb. 3, a decision upheld by the 9th U.S. Circuit Court of Appeals on Feb. 9.
The new executive order excludes Iraq this time around and also removes language that had indefinitely banned Syrian refugees. In a March 6 memorandum, the White House said the purpose of the ban is to prevent “foreign nationals who may aid, support, or commit violent, criminal, or terrorist acts,” while the administration enhances the screening and vetting protocols and procedures for granting visas and admission to the United States.
“This nation cannot delay the immediate implementation of additional heightened screening and vetting protocols and procedures for issuing visas to ensure that we strengthen the safety and security of our country,” the memo states.
This article was updated March 8, 2017.
agallegos@frontlinemedcom.com
On Twitter @legal_med
President’s Trump’s revised executive order blocking travelers from six Muslim-majority countries from entering the United States could land a damaging blow to global cooperation in scientific research and impede assemblies of the world’s top medical experts.
The executive order, signed March 6, bars citizens of Iran, Libya, Somalia, Sudan, Syria, and Yemen from obtaining visas for 90 days and blocks refugees from the affected countries from entering the U.S. for 120 days. The new executive measure, which takes effect March 16, supersedes President Trump’s original Jan. 27 travel ban that has been blocked by federal courts.
The new order clarifies that citizens of the six countries who are legal permanent U.S. residents or who have current visas to enter the country are exempt from the travel prohibition.
The revised travel ban could disrupt the exchange of medical knowledge by barring foreign experts from traveling to medical and scientific conferences in the United States, and leaves the status of medical trainees from those countries in limbo, according to American Medical Association President Andrew W. Gurman, MD.
“Hundreds of physicians from six countries are subject to the revised executive order and have applied to U.S. training programs and requested visa sponsorship,” he said in a statement. “The new executive order leaves them in limbo and without an explicit waiver, these foreign physicians will be unable to provide care in the U.S. when training programs begin on July 1.”
The new order is already being challenged in court. On March 7, the state of Hawaii filed a lawsuit seeking to block the order, saying that it subjects a portion of Hawaii’s population to “discrimination and second-class treatment.”
When the original ban took effect, thousands of academics from around the world, including physicians, researchers, and professors, vowed to boycott U.S.-based conferences. A Google Docs petition started shortly after the ban was announced garnered more than 5,000 signatures by professionals acting in solidarity with those affected by the travel restrictions. The academicians who signed the petition said they would not attend international conferences in the United States until those restricted from participating could rejoin their colleagues and freely share their ideas.
The new executive order comes nearly 2 months after President Trump’s original travel ban caused nationwide protests and led to a series of legal challenges. The states of Washington and Minnesota, which sued President Trump over his original ban, argued that such a ban harms the teaching and research missions of their universities and prevents students and faculty from traveling for research and academic collaboration. In addition, the executive order restricts universities from hiring attractive candidates from countries affected by the ban, state officials said.
A federal court temporarily blocked the original travel ban on Feb. 3, a decision upheld by the 9th U.S. Circuit Court of Appeals on Feb. 9.
The new executive order excludes Iraq this time around and also removes language that had indefinitely banned Syrian refugees. In a March 6 memorandum, the White House said the purpose of the ban is to prevent “foreign nationals who may aid, support, or commit violent, criminal, or terrorist acts,” while the administration enhances the screening and vetting protocols and procedures for granting visas and admission to the United States.
“This nation cannot delay the immediate implementation of additional heightened screening and vetting protocols and procedures for issuing visas to ensure that we strengthen the safety and security of our country,” the memo states.
This article was updated March 8, 2017.
agallegos@frontlinemedcom.com
On Twitter @legal_med
House Republicans start work on ACA repeal/replace
House Republicans have formally begun their efforts to repeal and replace the Affordable Care Act, but a group of four GOP senators could present a significant hurdle to getting the budget reconciliation package to the president’s desk.
The budget reconciliation language – introduced March 6 – represents the first of three phases in their planned effort to quash the ACA.
“Second are all the regulatory modifications and changes that can be put into place,” Dr. Price said, noting that the Obama administration made 192 specific rules related to the ACA and published more than 5,000 letters of guidance. “We are going to go through every single one of those and ... if they help patients, we need to continue them. If they harm patients or increase costs, they need to be addressed.”
He added that additional legislation could be needed to address changes that cannot be made through the reconciliation process, such as addressing prescription drug costs and allowing health insurance to be sold across state lines.
Sections of the reconciliation package were introduced in the House Energy and Commerce and House Ways and Means Committees, covering areas relative to each committee’s jurisdiction. Taken together, they put forth the plan to repeal and replace certain revenue aspects of the ACA.
Provisions to repeal Medicaid expansion likely will prove the most problematic. The Energy and Commerce language calls for the repeal of Medicaid expansion provisions by 2020. It also repeals a requirement that state Medicaid plans offer the same minimum essential health benefits that are required by plans on the exchanges.
Starting in fiscal 2020, states would begin to receive a per capita allotment to fund their Medicaid programs, a fixed amount per Medicaid enrollee that will adjust over time for inflation using the consumer price index. States spending more than their annual allotment per beneficiary would be penalized with reduced funding in the following year.
That provision has run afoul of certain Republican senators whose votes are needed to gain a simple majority and pass the budget reconciliation package. Because of the slim nature of the Republican majority, any defection from the party line could endanger passage.
“We are concerned that any poorly implemented or poorly timed change in the current funding structure in Medicaid could result in a reduction in access to life-saving health care services,” Sen. Rob Portman (R-Ohio), Sen. Shelley Moore Capito (R-W.Va.), Sen. Cory Gardner (R-Colo.), and Sen. Lisa Murkowski (R-Alaska) said in a letter to Senate Majority Leader Mitch McConnell (R-Ky.). The letter was based on an earlier draft of the legislative language but remains valid as little substantive change has occurred in the formal, introduced language.
“We believe Medicaid needs to be reformed, but reform should not come at the cost of disruption in access to health care for our country’s most vulnerable and sickest individuals,” the senators continued. “Any changes made to how Medicaid is financed through the state and federal governments should be coupled with significant new flexibility so they can efficiently and effectively manage their Medicaid programs to best meet their own needs. ... [The House proposal] does not meet the test of stability for individuals currently enrolled in the program, and we will not support a plan that does not include stability for Medicaid expansion populations or flexibility for states.”
The Energy and Commerce language also includes provisions for block grants to states that would allow them to be used in a manner they see fit within a defined list, including providing financial assistance to high-risk individuals; arrangements that help stabilize premiums in the individual market; reduce the cost of providing insurance in the small group and individual markets; promoting participation in the small group and individual markets; and promoting access to preventive, dental, and vision services as well as treatment for mental and substance abuse disorders. Funds could also be used to provide payments for care or assistance to reduce out-of-pocket costs.
The House proposal also would repeal the premium cost sharing subsidies in the ACA, which would be replaced with refundable tax credits, according to the language released by the Ways and Means Committee.
The credit can be used to purchase a state-approved, major health insurance or unsubsidized COBRA (the Consolidated Omnibus Budget Reconciliation Act) coverage and increases based on age. Individuals younger than age 30 years would get $2,000, and the credit increases in $500 increments per 10-year age block, plateauing at $4,000 for those aged 60 years and older. The caps will adjust over time, based on inflation, and are available to those making up to $75,000 ($150,000 for joint filers); the credit phases out by $100 for every $1,000 over those thresholds.
The Ways and Means language also repeals a number of revenue provisions, including the individual mandate and associated penalties; the employer mandate and associated penalties; taxes on high-cost health plans (Cadillac tax); over-the-counter and prescription medications; health savings accounts; tanning; investment; and on health insurers.
In place of the individual mandate, the language will allow insurers to raise premiums by up to 30%.
The bill does not repeal the provisions regarding young adults being able to stay on their parents’ policies to the age of 26 years, nor does it allow insurers to deny coverage for preexisting conditions.
The House committees will begin consideration of their respective languages on March 8.
House Republicans have formally begun their efforts to repeal and replace the Affordable Care Act, but a group of four GOP senators could present a significant hurdle to getting the budget reconciliation package to the president’s desk.
The budget reconciliation language – introduced March 6 – represents the first of three phases in their planned effort to quash the ACA.
“Second are all the regulatory modifications and changes that can be put into place,” Dr. Price said, noting that the Obama administration made 192 specific rules related to the ACA and published more than 5,000 letters of guidance. “We are going to go through every single one of those and ... if they help patients, we need to continue them. If they harm patients or increase costs, they need to be addressed.”
He added that additional legislation could be needed to address changes that cannot be made through the reconciliation process, such as addressing prescription drug costs and allowing health insurance to be sold across state lines.
Sections of the reconciliation package were introduced in the House Energy and Commerce and House Ways and Means Committees, covering areas relative to each committee’s jurisdiction. Taken together, they put forth the plan to repeal and replace certain revenue aspects of the ACA.
Provisions to repeal Medicaid expansion likely will prove the most problematic. The Energy and Commerce language calls for the repeal of Medicaid expansion provisions by 2020. It also repeals a requirement that state Medicaid plans offer the same minimum essential health benefits that are required by plans on the exchanges.
Starting in fiscal 2020, states would begin to receive a per capita allotment to fund their Medicaid programs, a fixed amount per Medicaid enrollee that will adjust over time for inflation using the consumer price index. States spending more than their annual allotment per beneficiary would be penalized with reduced funding in the following year.
That provision has run afoul of certain Republican senators whose votes are needed to gain a simple majority and pass the budget reconciliation package. Because of the slim nature of the Republican majority, any defection from the party line could endanger passage.
“We are concerned that any poorly implemented or poorly timed change in the current funding structure in Medicaid could result in a reduction in access to life-saving health care services,” Sen. Rob Portman (R-Ohio), Sen. Shelley Moore Capito (R-W.Va.), Sen. Cory Gardner (R-Colo.), and Sen. Lisa Murkowski (R-Alaska) said in a letter to Senate Majority Leader Mitch McConnell (R-Ky.). The letter was based on an earlier draft of the legislative language but remains valid as little substantive change has occurred in the formal, introduced language.
“We believe Medicaid needs to be reformed, but reform should not come at the cost of disruption in access to health care for our country’s most vulnerable and sickest individuals,” the senators continued. “Any changes made to how Medicaid is financed through the state and federal governments should be coupled with significant new flexibility so they can efficiently and effectively manage their Medicaid programs to best meet their own needs. ... [The House proposal] does not meet the test of stability for individuals currently enrolled in the program, and we will not support a plan that does not include stability for Medicaid expansion populations or flexibility for states.”
The Energy and Commerce language also includes provisions for block grants to states that would allow them to be used in a manner they see fit within a defined list, including providing financial assistance to high-risk individuals; arrangements that help stabilize premiums in the individual market; reduce the cost of providing insurance in the small group and individual markets; promoting participation in the small group and individual markets; and promoting access to preventive, dental, and vision services as well as treatment for mental and substance abuse disorders. Funds could also be used to provide payments for care or assistance to reduce out-of-pocket costs.
The House proposal also would repeal the premium cost sharing subsidies in the ACA, which would be replaced with refundable tax credits, according to the language released by the Ways and Means Committee.
The credit can be used to purchase a state-approved, major health insurance or unsubsidized COBRA (the Consolidated Omnibus Budget Reconciliation Act) coverage and increases based on age. Individuals younger than age 30 years would get $2,000, and the credit increases in $500 increments per 10-year age block, plateauing at $4,000 for those aged 60 years and older. The caps will adjust over time, based on inflation, and are available to those making up to $75,000 ($150,000 for joint filers); the credit phases out by $100 for every $1,000 over those thresholds.
The Ways and Means language also repeals a number of revenue provisions, including the individual mandate and associated penalties; the employer mandate and associated penalties; taxes on high-cost health plans (Cadillac tax); over-the-counter and prescription medications; health savings accounts; tanning; investment; and on health insurers.
In place of the individual mandate, the language will allow insurers to raise premiums by up to 30%.
The bill does not repeal the provisions regarding young adults being able to stay on their parents’ policies to the age of 26 years, nor does it allow insurers to deny coverage for preexisting conditions.
The House committees will begin consideration of their respective languages on March 8.
House Republicans have formally begun their efforts to repeal and replace the Affordable Care Act, but a group of four GOP senators could present a significant hurdle to getting the budget reconciliation package to the president’s desk.
The budget reconciliation language – introduced March 6 – represents the first of three phases in their planned effort to quash the ACA.
“Second are all the regulatory modifications and changes that can be put into place,” Dr. Price said, noting that the Obama administration made 192 specific rules related to the ACA and published more than 5,000 letters of guidance. “We are going to go through every single one of those and ... if they help patients, we need to continue them. If they harm patients or increase costs, they need to be addressed.”
He added that additional legislation could be needed to address changes that cannot be made through the reconciliation process, such as addressing prescription drug costs and allowing health insurance to be sold across state lines.
Sections of the reconciliation package were introduced in the House Energy and Commerce and House Ways and Means Committees, covering areas relative to each committee’s jurisdiction. Taken together, they put forth the plan to repeal and replace certain revenue aspects of the ACA.
Provisions to repeal Medicaid expansion likely will prove the most problematic. The Energy and Commerce language calls for the repeal of Medicaid expansion provisions by 2020. It also repeals a requirement that state Medicaid plans offer the same minimum essential health benefits that are required by plans on the exchanges.
Starting in fiscal 2020, states would begin to receive a per capita allotment to fund their Medicaid programs, a fixed amount per Medicaid enrollee that will adjust over time for inflation using the consumer price index. States spending more than their annual allotment per beneficiary would be penalized with reduced funding in the following year.
That provision has run afoul of certain Republican senators whose votes are needed to gain a simple majority and pass the budget reconciliation package. Because of the slim nature of the Republican majority, any defection from the party line could endanger passage.
“We are concerned that any poorly implemented or poorly timed change in the current funding structure in Medicaid could result in a reduction in access to life-saving health care services,” Sen. Rob Portman (R-Ohio), Sen. Shelley Moore Capito (R-W.Va.), Sen. Cory Gardner (R-Colo.), and Sen. Lisa Murkowski (R-Alaska) said in a letter to Senate Majority Leader Mitch McConnell (R-Ky.). The letter was based on an earlier draft of the legislative language but remains valid as little substantive change has occurred in the formal, introduced language.
“We believe Medicaid needs to be reformed, but reform should not come at the cost of disruption in access to health care for our country’s most vulnerable and sickest individuals,” the senators continued. “Any changes made to how Medicaid is financed through the state and federal governments should be coupled with significant new flexibility so they can efficiently and effectively manage their Medicaid programs to best meet their own needs. ... [The House proposal] does not meet the test of stability for individuals currently enrolled in the program, and we will not support a plan that does not include stability for Medicaid expansion populations or flexibility for states.”
The Energy and Commerce language also includes provisions for block grants to states that would allow them to be used in a manner they see fit within a defined list, including providing financial assistance to high-risk individuals; arrangements that help stabilize premiums in the individual market; reduce the cost of providing insurance in the small group and individual markets; promoting participation in the small group and individual markets; and promoting access to preventive, dental, and vision services as well as treatment for mental and substance abuse disorders. Funds could also be used to provide payments for care or assistance to reduce out-of-pocket costs.
The House proposal also would repeal the premium cost sharing subsidies in the ACA, which would be replaced with refundable tax credits, according to the language released by the Ways and Means Committee.
The credit can be used to purchase a state-approved, major health insurance or unsubsidized COBRA (the Consolidated Omnibus Budget Reconciliation Act) coverage and increases based on age. Individuals younger than age 30 years would get $2,000, and the credit increases in $500 increments per 10-year age block, plateauing at $4,000 for those aged 60 years and older. The caps will adjust over time, based on inflation, and are available to those making up to $75,000 ($150,000 for joint filers); the credit phases out by $100 for every $1,000 over those thresholds.
The Ways and Means language also repeals a number of revenue provisions, including the individual mandate and associated penalties; the employer mandate and associated penalties; taxes on high-cost health plans (Cadillac tax); over-the-counter and prescription medications; health savings accounts; tanning; investment; and on health insurers.
In place of the individual mandate, the language will allow insurers to raise premiums by up to 30%.
The bill does not repeal the provisions regarding young adults being able to stay on their parents’ policies to the age of 26 years, nor does it allow insurers to deny coverage for preexisting conditions.
The House committees will begin consideration of their respective languages on March 8.
CDC: Greater activity limitations accompany rising arthritis prevalence
The number of adults with arthritis in the United States continues to rise, with the number projected to climb as high as 78 million by the year 2040. Some of the keys to stemming this rising tide are exercise, along with greater knowledge of how to manage symptoms, according to a new report from the Centers for Disease Control and Prevention.
“Arthritis is at an all-time high: more than 54 million people report a diagnosis of it, and alarmingly, more people with arthritis are suffering from it,” said Anne Schuchat, MD, acting director of the CDC, during a conference call regarding the agency’s latest Vital Signs report (MMWR Morb Mortal Wkly Rep. 2017 Mar 7. doi: org/10.15585/mmwr.mm6609e1). “Among adults with arthritis, the percentage whose lives are particularly limited has increased by about 20% since 2002, from about 36% in 2002 to 43% in 2015. We’re seeing this increase independent of aging of the population.”
“Physical activity can be the antidote for many people [and] can actually decrease pain and improve function by almost 40%,” Dr. Schuchat explained. “Right now, one in three adults with arthritis report being inactive [because of] pain or fear of pain or not knowing what exercise is safe for their joints.”
This inactivity can lead to arthritis patients developing other serious chronic conditions, such as heart disease, diabetes and obesity – conditions that all require physical activity in order to properly manage them. Arthritis alone puts an extraordinary financial burden on the domestic health care industry, as direct medical costs associated with the condition total roughly $81 billion per year, according to the CDC. Additionally, about half of all adults with heart disease or diabetes, and about one-third of obese adults, also have arthritis.
In addition to engaging in regular physical activity, the Vital Signs report also recommends that arthritis patients attend disease management education programs, which are available regionally but often go underutilized, largely due to lack of awareness about them or trepidation regarding how effective the programs really are. To combat this, the CDC is calling on health care providers to help them educate patients about these classes and spread the word about the steps that can be taken to manage arthritis. In 2017, the CDC is funding arthritis programs in 12 states (California, Kansas, Kentucky, Michigan, Missouri, Montana, New York, Oregon, Pennsylvania, Rhode Island, South Carolina, and Utah) to disseminate arthritis-appropriate evidence-based physical activity and self-management education interventions.
“Men or women with arthritis can reduce their symptoms by 10%-20% by participating in disease management education programs to acquire skills to better manage their symptoms. Right now, these programs are only reaching about 1 in 10 people with arthritis, but the classes are available in many community settings,” Dr. Schuchat said. “We know that adults with arthritis are significantly more likely to attend a disease management education program when a health care provider recommends it to them.”
When seeing patients with arthritis, Dr. Schuchat advised health care providers to recommend routine physical activity, such as walking, biking, swimming, and physical activity programs offered by local parks and recreation centers, as well as weight loss, in order to ease joint pain. The American College of Rheumatology and other professional organizations provide guidelines for discussing treatment options with patients. Providing treatment or additional services for depression or anxiety, which occur in about one-third of adult arthritis patients, may help individuals to better manage their arthritis symptoms.
The agency’s report derives from its analysis of 2013-2015 data from the National Health Interview Survey, which comprised a nationally representative sample of about 36,000 in-person interviews. The survey classifies individuals with physician-diagnosed arthritis as those who answered “yes” to the question “Have you ever been told by a doctor or other health professional that you have some form of arthritis, rheumatoid arthritis, gout, lupus, or fibromyalgia?”
The number of adults with arthritis in the United States continues to rise, with the number projected to climb as high as 78 million by the year 2040. Some of the keys to stemming this rising tide are exercise, along with greater knowledge of how to manage symptoms, according to a new report from the Centers for Disease Control and Prevention.
“Arthritis is at an all-time high: more than 54 million people report a diagnosis of it, and alarmingly, more people with arthritis are suffering from it,” said Anne Schuchat, MD, acting director of the CDC, during a conference call regarding the agency’s latest Vital Signs report (MMWR Morb Mortal Wkly Rep. 2017 Mar 7. doi: org/10.15585/mmwr.mm6609e1). “Among adults with arthritis, the percentage whose lives are particularly limited has increased by about 20% since 2002, from about 36% in 2002 to 43% in 2015. We’re seeing this increase independent of aging of the population.”
“Physical activity can be the antidote for many people [and] can actually decrease pain and improve function by almost 40%,” Dr. Schuchat explained. “Right now, one in three adults with arthritis report being inactive [because of] pain or fear of pain or not knowing what exercise is safe for their joints.”
This inactivity can lead to arthritis patients developing other serious chronic conditions, such as heart disease, diabetes and obesity – conditions that all require physical activity in order to properly manage them. Arthritis alone puts an extraordinary financial burden on the domestic health care industry, as direct medical costs associated with the condition total roughly $81 billion per year, according to the CDC. Additionally, about half of all adults with heart disease or diabetes, and about one-third of obese adults, also have arthritis.
In addition to engaging in regular physical activity, the Vital Signs report also recommends that arthritis patients attend disease management education programs, which are available regionally but often go underutilized, largely due to lack of awareness about them or trepidation regarding how effective the programs really are. To combat this, the CDC is calling on health care providers to help them educate patients about these classes and spread the word about the steps that can be taken to manage arthritis. In 2017, the CDC is funding arthritis programs in 12 states (California, Kansas, Kentucky, Michigan, Missouri, Montana, New York, Oregon, Pennsylvania, Rhode Island, South Carolina, and Utah) to disseminate arthritis-appropriate evidence-based physical activity and self-management education interventions.
“Men or women with arthritis can reduce their symptoms by 10%-20% by participating in disease management education programs to acquire skills to better manage their symptoms. Right now, these programs are only reaching about 1 in 10 people with arthritis, but the classes are available in many community settings,” Dr. Schuchat said. “We know that adults with arthritis are significantly more likely to attend a disease management education program when a health care provider recommends it to them.”
When seeing patients with arthritis, Dr. Schuchat advised health care providers to recommend routine physical activity, such as walking, biking, swimming, and physical activity programs offered by local parks and recreation centers, as well as weight loss, in order to ease joint pain. The American College of Rheumatology and other professional organizations provide guidelines for discussing treatment options with patients. Providing treatment or additional services for depression or anxiety, which occur in about one-third of adult arthritis patients, may help individuals to better manage their arthritis symptoms.
The agency’s report derives from its analysis of 2013-2015 data from the National Health Interview Survey, which comprised a nationally representative sample of about 36,000 in-person interviews. The survey classifies individuals with physician-diagnosed arthritis as those who answered “yes” to the question “Have you ever been told by a doctor or other health professional that you have some form of arthritis, rheumatoid arthritis, gout, lupus, or fibromyalgia?”
The number of adults with arthritis in the United States continues to rise, with the number projected to climb as high as 78 million by the year 2040. Some of the keys to stemming this rising tide are exercise, along with greater knowledge of how to manage symptoms, according to a new report from the Centers for Disease Control and Prevention.
“Arthritis is at an all-time high: more than 54 million people report a diagnosis of it, and alarmingly, more people with arthritis are suffering from it,” said Anne Schuchat, MD, acting director of the CDC, during a conference call regarding the agency’s latest Vital Signs report (MMWR Morb Mortal Wkly Rep. 2017 Mar 7. doi: org/10.15585/mmwr.mm6609e1). “Among adults with arthritis, the percentage whose lives are particularly limited has increased by about 20% since 2002, from about 36% in 2002 to 43% in 2015. We’re seeing this increase independent of aging of the population.”
“Physical activity can be the antidote for many people [and] can actually decrease pain and improve function by almost 40%,” Dr. Schuchat explained. “Right now, one in three adults with arthritis report being inactive [because of] pain or fear of pain or not knowing what exercise is safe for their joints.”
This inactivity can lead to arthritis patients developing other serious chronic conditions, such as heart disease, diabetes and obesity – conditions that all require physical activity in order to properly manage them. Arthritis alone puts an extraordinary financial burden on the domestic health care industry, as direct medical costs associated with the condition total roughly $81 billion per year, according to the CDC. Additionally, about half of all adults with heart disease or diabetes, and about one-third of obese adults, also have arthritis.
In addition to engaging in regular physical activity, the Vital Signs report also recommends that arthritis patients attend disease management education programs, which are available regionally but often go underutilized, largely due to lack of awareness about them or trepidation regarding how effective the programs really are. To combat this, the CDC is calling on health care providers to help them educate patients about these classes and spread the word about the steps that can be taken to manage arthritis. In 2017, the CDC is funding arthritis programs in 12 states (California, Kansas, Kentucky, Michigan, Missouri, Montana, New York, Oregon, Pennsylvania, Rhode Island, South Carolina, and Utah) to disseminate arthritis-appropriate evidence-based physical activity and self-management education interventions.
“Men or women with arthritis can reduce their symptoms by 10%-20% by participating in disease management education programs to acquire skills to better manage their symptoms. Right now, these programs are only reaching about 1 in 10 people with arthritis, but the classes are available in many community settings,” Dr. Schuchat said. “We know that adults with arthritis are significantly more likely to attend a disease management education program when a health care provider recommends it to them.”
When seeing patients with arthritis, Dr. Schuchat advised health care providers to recommend routine physical activity, such as walking, biking, swimming, and physical activity programs offered by local parks and recreation centers, as well as weight loss, in order to ease joint pain. The American College of Rheumatology and other professional organizations provide guidelines for discussing treatment options with patients. Providing treatment or additional services for depression or anxiety, which occur in about one-third of adult arthritis patients, may help individuals to better manage their arthritis symptoms.
The agency’s report derives from its analysis of 2013-2015 data from the National Health Interview Survey, which comprised a nationally representative sample of about 36,000 in-person interviews. The survey classifies individuals with physician-diagnosed arthritis as those who answered “yes” to the question “Have you ever been told by a doctor or other health professional that you have some form of arthritis, rheumatoid arthritis, gout, lupus, or fibromyalgia?”
FROM MMWR
Key clinical point:
Major finding: About 24 million American adults report being significantly limited due to their arthritis.
Data source: 2013-2015 data from the National Health Interview Survey.
Disclosures: No disclosures were reported.
Depression and deep brain stimulation: ‘Furor therapeuticus redux’
Looking back after a long and distinguished career, Leon Eisenberg, MD, invoked the term “furor therapeuticus” to describe overzealous treatment by doctors who became frustrated with therapeutic limitations or motivated by professional enthusiasm.1
With this in mind, Dr. Eisenberg criticized expansive marketing and prescribing of psychotropic drugs in an editorial published exactly 10 years ago. He might also have questioned the current interest in deep brain stimulation (DBS) as a treatment for depression and a growing list of behavioral disorders. Initial studies of DBS in depression were promising, but recent setbacks have brought research to a scientific and ethical crossroads that compels broader discussion.
Besides uncertainties over the right targets to stimulate, identification of the right candidates for DBS treatment can be difficult. Trials of DBS recruited highly selected depressed subjects with no consensus on symptoms or biomarkers that could be used to predict who might respond. Doctors still rely on clinical symptoms to distinguish patients with melancholic depression, who respond to medications or electroconvulsive therapy and might also respond to DBS, from patients with depressed mood because of psychosocial problems, who respond to psychotherapy or social interventions.
Evidence on the efficacy and safety of DBS in depression is mixed. Initial open trials were promising, with dramatic and sustained recovery in some patients, but they were limited by small numbers of subjects and a lack of randomized controls and standardized methods.4,5
DBS is not without serious side effects, and substantial maintenance costs are not always covered by insurance. So, two recent industry trials were eagerly anticipated but showed no significant differences between active and sham stimulations in depression.6,7 These disappointing results prompted soul-searching among investigators, who presented ingenious ideas for correcting shortcomings that could be tested in future trials but also raised doubts as to the prospects of DBS in depression.4,5
Given that DBS devices already are marketed for neurological disorders, regulation of practice is crucial to prevent off-label misuse in behavioral disorders.8 Federal agencies enforce rules governing DBS devices but rely on investigators and local review boards in research and on voluntary postmarketing reports by individual practitioners to monitor compliance and safety. Unscrupulous commercial interests could expand the market for these devices, as demonstrated by the proliferation of psychotropic drug prescribing decried by Dr. Eisenberg. DBS also must be restricted to specialized teams and medical centers to prevent inappropriate implantation by poorly trained providers.
Because behavioral disorders exact an enormous toll on patients, families, and society, better access to effective care and the search for better treatments must remain public health priorities.
Transformative, breakthrough discoveries in brain research will undoubtedly lead to improvements in treatment, including surgical devices in some cases, but, DBS is at risk of being exaggerated and oversold. Adverse consequences of misuse could provoke a public backlash that would have a chilling effect on vital brain research.
One possible way to prevent this is the risk evaluation and mitigation strategy established by the Food and Drug Administration to manage high-risk pharmaceuticals. The FDA mandates that certain high-risk drugs can be prescribed only if doctors are certified and only if patients are enrolled in a national registry where eligibility, course, and outcome are monitored. A similar mechanism should apply to high-risk surgical devices when used for behavioral disorders.9,10
People with behavioral disorders deserve the right to volunteer for experimental programs that offer hope of recovery for themselves and future generations, but they also deserve to be treated with the utmost scientific rigor and protection that society can provide.
Dr. Caroff is emeritus professor of psychiatry at the University of Pennsylvania, Philadelphia. He has received research grant funding from Sunovion Pharmaceuticals and serves as a consultant to Neurocrine Biosciences and TEVA.
References
1. Am J Psychiatry. 2007;164(4):552-5
2. Curr Behav Neurosci Rep. 2014;1(2):55-63
3. J Affect Disord. 2014;156:1-7
4. JAMA Psychiatry. 2016;739(5):439-40
5. Biol Psychiatry. 2016;79(4):e9-10
6. Stereotact Funct Neurosurg. 2015;93:366-9
7. Neurotherapeutics. 2014;11(3):475-84
8. J Neurol Neurosurg Psychiatry. 2014;85(9):1003-8
9. Brain Stimul. 2012;5(4):653-5
10. Fed Reg. 1977 May 23;42(99):26318-32
Looking back after a long and distinguished career, Leon Eisenberg, MD, invoked the term “furor therapeuticus” to describe overzealous treatment by doctors who became frustrated with therapeutic limitations or motivated by professional enthusiasm.1
With this in mind, Dr. Eisenberg criticized expansive marketing and prescribing of psychotropic drugs in an editorial published exactly 10 years ago. He might also have questioned the current interest in deep brain stimulation (DBS) as a treatment for depression and a growing list of behavioral disorders. Initial studies of DBS in depression were promising, but recent setbacks have brought research to a scientific and ethical crossroads that compels broader discussion.
Besides uncertainties over the right targets to stimulate, identification of the right candidates for DBS treatment can be difficult. Trials of DBS recruited highly selected depressed subjects with no consensus on symptoms or biomarkers that could be used to predict who might respond. Doctors still rely on clinical symptoms to distinguish patients with melancholic depression, who respond to medications or electroconvulsive therapy and might also respond to DBS, from patients with depressed mood because of psychosocial problems, who respond to psychotherapy or social interventions.
Evidence on the efficacy and safety of DBS in depression is mixed. Initial open trials were promising, with dramatic and sustained recovery in some patients, but they were limited by small numbers of subjects and a lack of randomized controls and standardized methods.4,5
DBS is not without serious side effects, and substantial maintenance costs are not always covered by insurance. So, two recent industry trials were eagerly anticipated but showed no significant differences between active and sham stimulations in depression.6,7 These disappointing results prompted soul-searching among investigators, who presented ingenious ideas for correcting shortcomings that could be tested in future trials but also raised doubts as to the prospects of DBS in depression.4,5
Given that DBS devices already are marketed for neurological disorders, regulation of practice is crucial to prevent off-label misuse in behavioral disorders.8 Federal agencies enforce rules governing DBS devices but rely on investigators and local review boards in research and on voluntary postmarketing reports by individual practitioners to monitor compliance and safety. Unscrupulous commercial interests could expand the market for these devices, as demonstrated by the proliferation of psychotropic drug prescribing decried by Dr. Eisenberg. DBS also must be restricted to specialized teams and medical centers to prevent inappropriate implantation by poorly trained providers.
Because behavioral disorders exact an enormous toll on patients, families, and society, better access to effective care and the search for better treatments must remain public health priorities.
Transformative, breakthrough discoveries in brain research will undoubtedly lead to improvements in treatment, including surgical devices in some cases, but, DBS is at risk of being exaggerated and oversold. Adverse consequences of misuse could provoke a public backlash that would have a chilling effect on vital brain research.
One possible way to prevent this is the risk evaluation and mitigation strategy established by the Food and Drug Administration to manage high-risk pharmaceuticals. The FDA mandates that certain high-risk drugs can be prescribed only if doctors are certified and only if patients are enrolled in a national registry where eligibility, course, and outcome are monitored. A similar mechanism should apply to high-risk surgical devices when used for behavioral disorders.9,10
People with behavioral disorders deserve the right to volunteer for experimental programs that offer hope of recovery for themselves and future generations, but they also deserve to be treated with the utmost scientific rigor and protection that society can provide.
Dr. Caroff is emeritus professor of psychiatry at the University of Pennsylvania, Philadelphia. He has received research grant funding from Sunovion Pharmaceuticals and serves as a consultant to Neurocrine Biosciences and TEVA.
References
1. Am J Psychiatry. 2007;164(4):552-5
2. Curr Behav Neurosci Rep. 2014;1(2):55-63
3. J Affect Disord. 2014;156:1-7
4. JAMA Psychiatry. 2016;739(5):439-40
5. Biol Psychiatry. 2016;79(4):e9-10
6. Stereotact Funct Neurosurg. 2015;93:366-9
7. Neurotherapeutics. 2014;11(3):475-84
8. J Neurol Neurosurg Psychiatry. 2014;85(9):1003-8
9. Brain Stimul. 2012;5(4):653-5
10. Fed Reg. 1977 May 23;42(99):26318-32
Looking back after a long and distinguished career, Leon Eisenberg, MD, invoked the term “furor therapeuticus” to describe overzealous treatment by doctors who became frustrated with therapeutic limitations or motivated by professional enthusiasm.1
With this in mind, Dr. Eisenberg criticized expansive marketing and prescribing of psychotropic drugs in an editorial published exactly 10 years ago. He might also have questioned the current interest in deep brain stimulation (DBS) as a treatment for depression and a growing list of behavioral disorders. Initial studies of DBS in depression were promising, but recent setbacks have brought research to a scientific and ethical crossroads that compels broader discussion.
Besides uncertainties over the right targets to stimulate, identification of the right candidates for DBS treatment can be difficult. Trials of DBS recruited highly selected depressed subjects with no consensus on symptoms or biomarkers that could be used to predict who might respond. Doctors still rely on clinical symptoms to distinguish patients with melancholic depression, who respond to medications or electroconvulsive therapy and might also respond to DBS, from patients with depressed mood because of psychosocial problems, who respond to psychotherapy or social interventions.
Evidence on the efficacy and safety of DBS in depression is mixed. Initial open trials were promising, with dramatic and sustained recovery in some patients, but they were limited by small numbers of subjects and a lack of randomized controls and standardized methods.4,5
DBS is not without serious side effects, and substantial maintenance costs are not always covered by insurance. So, two recent industry trials were eagerly anticipated but showed no significant differences between active and sham stimulations in depression.6,7 These disappointing results prompted soul-searching among investigators, who presented ingenious ideas for correcting shortcomings that could be tested in future trials but also raised doubts as to the prospects of DBS in depression.4,5
Given that DBS devices already are marketed for neurological disorders, regulation of practice is crucial to prevent off-label misuse in behavioral disorders.8 Federal agencies enforce rules governing DBS devices but rely on investigators and local review boards in research and on voluntary postmarketing reports by individual practitioners to monitor compliance and safety. Unscrupulous commercial interests could expand the market for these devices, as demonstrated by the proliferation of psychotropic drug prescribing decried by Dr. Eisenberg. DBS also must be restricted to specialized teams and medical centers to prevent inappropriate implantation by poorly trained providers.
Because behavioral disorders exact an enormous toll on patients, families, and society, better access to effective care and the search for better treatments must remain public health priorities.
Transformative, breakthrough discoveries in brain research will undoubtedly lead to improvements in treatment, including surgical devices in some cases, but, DBS is at risk of being exaggerated and oversold. Adverse consequences of misuse could provoke a public backlash that would have a chilling effect on vital brain research.
One possible way to prevent this is the risk evaluation and mitigation strategy established by the Food and Drug Administration to manage high-risk pharmaceuticals. The FDA mandates that certain high-risk drugs can be prescribed only if doctors are certified and only if patients are enrolled in a national registry where eligibility, course, and outcome are monitored. A similar mechanism should apply to high-risk surgical devices when used for behavioral disorders.9,10
People with behavioral disorders deserve the right to volunteer for experimental programs that offer hope of recovery for themselves and future generations, but they also deserve to be treated with the utmost scientific rigor and protection that society can provide.
Dr. Caroff is emeritus professor of psychiatry at the University of Pennsylvania, Philadelphia. He has received research grant funding from Sunovion Pharmaceuticals and serves as a consultant to Neurocrine Biosciences and TEVA.
References
1. Am J Psychiatry. 2007;164(4):552-5
2. Curr Behav Neurosci Rep. 2014;1(2):55-63
3. J Affect Disord. 2014;156:1-7
4. JAMA Psychiatry. 2016;739(5):439-40
5. Biol Psychiatry. 2016;79(4):e9-10
6. Stereotact Funct Neurosurg. 2015;93:366-9
7. Neurotherapeutics. 2014;11(3):475-84
8. J Neurol Neurosurg Psychiatry. 2014;85(9):1003-8
9. Brain Stimul. 2012;5(4):653-5
10. Fed Reg. 1977 May 23;42(99):26318-32
Small study: Watchful waiting better for pediatric IBS
Children with irritable bowel syndrome (IBS) may recover more successfully with reassurance than with medication, according to a study from Federico II University in Naples, Italy.
Of 83 children in this single-center observational study, 30 of the 48 children (62.8%) who reported symptom resolution were not put on medication, according to Eleonora Giannetti, MD, and her colleagues (J Pediatr. 2017 Jan 18. doi: 10.1016/j.jpeds.2016.12.036).
Researchers assessed children using a symptom survey, having patients and their parents report “how often days off school or interruption of daily activities because of IBS were needed,” as well as a scale of disruption ranging from 0 to 4.
A total of 47 children received verbal reassurance only, with no medication, while 9 received polyethylene glycol, 24 received probiotics, and 3 received trimebutine (not available in the United States), according to Dr. Giannetti.
Dr. Giannetti and her colleagues argued the data showed not only a positive correlation between decreased symptoms and lack of pharmaceutical intervention, but a negative impact of medication on children with IBS.
“Despite larger interventional studies being needed, our results also seem to suggest that traditionally prescribed medications, particularly probiotics and [polyethlene glycol], poorly affect the progression of IBS symptoms,” Dr. Giannetti wrote. “There was even a trend toward worse outcome of patients receiving probiotics.”
Limitations included a small sample size and medication not being randomly allocated.
Researchers said they also were limited by a short time frame, which, in regards to the “recurrent nature” of IBS, makes it difficult for researchers to know if and when IBS is completely resolved.
ezimmerman@frontlinemedcom.com
On Twitter @EAZtweets
Children with irritable bowel syndrome (IBS) may recover more successfully with reassurance than with medication, according to a study from Federico II University in Naples, Italy.
Of 83 children in this single-center observational study, 30 of the 48 children (62.8%) who reported symptom resolution were not put on medication, according to Eleonora Giannetti, MD, and her colleagues (J Pediatr. 2017 Jan 18. doi: 10.1016/j.jpeds.2016.12.036).
Researchers assessed children using a symptom survey, having patients and their parents report “how often days off school or interruption of daily activities because of IBS were needed,” as well as a scale of disruption ranging from 0 to 4.
A total of 47 children received verbal reassurance only, with no medication, while 9 received polyethylene glycol, 24 received probiotics, and 3 received trimebutine (not available in the United States), according to Dr. Giannetti.
Dr. Giannetti and her colleagues argued the data showed not only a positive correlation between decreased symptoms and lack of pharmaceutical intervention, but a negative impact of medication on children with IBS.
“Despite larger interventional studies being needed, our results also seem to suggest that traditionally prescribed medications, particularly probiotics and [polyethlene glycol], poorly affect the progression of IBS symptoms,” Dr. Giannetti wrote. “There was even a trend toward worse outcome of patients receiving probiotics.”
Limitations included a small sample size and medication not being randomly allocated.
Researchers said they also were limited by a short time frame, which, in regards to the “recurrent nature” of IBS, makes it difficult for researchers to know if and when IBS is completely resolved.
ezimmerman@frontlinemedcom.com
On Twitter @EAZtweets
Children with irritable bowel syndrome (IBS) may recover more successfully with reassurance than with medication, according to a study from Federico II University in Naples, Italy.
Of 83 children in this single-center observational study, 30 of the 48 children (62.8%) who reported symptom resolution were not put on medication, according to Eleonora Giannetti, MD, and her colleagues (J Pediatr. 2017 Jan 18. doi: 10.1016/j.jpeds.2016.12.036).
Researchers assessed children using a symptom survey, having patients and their parents report “how often days off school or interruption of daily activities because of IBS were needed,” as well as a scale of disruption ranging from 0 to 4.
A total of 47 children received verbal reassurance only, with no medication, while 9 received polyethylene glycol, 24 received probiotics, and 3 received trimebutine (not available in the United States), according to Dr. Giannetti.
Dr. Giannetti and her colleagues argued the data showed not only a positive correlation between decreased symptoms and lack of pharmaceutical intervention, but a negative impact of medication on children with IBS.
“Despite larger interventional studies being needed, our results also seem to suggest that traditionally prescribed medications, particularly probiotics and [polyethlene glycol], poorly affect the progression of IBS symptoms,” Dr. Giannetti wrote. “There was even a trend toward worse outcome of patients receiving probiotics.”
Limitations included a small sample size and medication not being randomly allocated.
Researchers said they also were limited by a short time frame, which, in regards to the “recurrent nature” of IBS, makes it difficult for researchers to know if and when IBS is completely resolved.
ezimmerman@frontlinemedcom.com
On Twitter @EAZtweets
FROM THE JOURNAL OF PEDIATRICS
Key clinical point:
Major finding: Thirty of 48 children who reported IBS symptom resolution received no medical intervention; 18 were treated with one of three drugs.
Data Source: An observational, single-center study of 83 children.
Disclosures: The investigators reported no relevant conflicts of interest.
Vulvovaginal disorders: When should you biopsy a suspicious lesion?
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Also from PAGS 2016:
- Dr. Tommaso Falcone offers Top 3 things I learned at the PAGS 2016 symposium
- Visit PAGS
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Also from PAGS 2016:
- Dr. Tommaso Falcone offers Top 3 things I learned at the PAGS 2016 symposium
- Visit PAGS
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Also from PAGS 2016:
- Dr. Tommaso Falcone offers Top 3 things I learned at the PAGS 2016 symposium
- Visit PAGS
Biosimilars: No big dollar savings, but are clinically ‘dead on’
SNOWMASS, COLO. – If you thought biosimilars would bring sharply reduced pricing compared with their parent agents, with resultant greater patient access to highly effective therapies for rheumatic diseases ... think again.
“The promise to our patients of biosimilars – greater access to treatments – is something I think we’re just not going to see, at least not here in the U.S.,” Michael E. Weinblatt, MD, declared at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
In contrast, the safety and efficacy of the biosimilars, as well as their interchangeability with their reference products, appear to be as hoped for. At the 2016 annual meeting of the American College of Rheumatology, Dr. Weinblatt presented the week 24 results of a phase III, randomized trial involving rheumatoid arthritis patients on background methotrexate plus either adalimumab (Humira) or its biosimilar SB5.
“Essentially, they’re dead on in clinical response, they’re dead on in antibody levels, and they’re dead on in toxicity. And, you can put any of the biosimilars up there and the results are the same. If they get approved, this is what you’re going to see,” the rheumatologist said.
Also at the 2016 ACR annual meeting, he noted, Danish investigators presented reassuring 1-year follow-up data on 802 Danes with inflammatory rheumatic diseases who switched from infliximab (Remicade) to its biosimilar Remsima. Disease activity and flare rates in the year following the switch were similar to those in the year before. The 1-year rate of adherence to Remsima was 84%, similar to the historical 86% 1-year rate with infliximab.
“So, I’m pretty comfortable with the biosimilars,” Dr. Weinblatt continued.
He observed that, of all the systemic rheumatic diseases, the greatest progress has occurred in the treatment of rheumatoid arthritis.
“We have made great advances in the treatment of this disease, unlike many of our other diseases. Methotrexate and combination therapies with small molecules and biologics has dramatically changed the course of the disease,” he noted. “The greatest challenge we have now as rheumatologists is access barriers for our patients.”
Dr. Weinblatt reported receiving research grants from half a dozen companies and serving as a consultant to more than two dozen.
SNOWMASS, COLO. – If you thought biosimilars would bring sharply reduced pricing compared with their parent agents, with resultant greater patient access to highly effective therapies for rheumatic diseases ... think again.
“The promise to our patients of biosimilars – greater access to treatments – is something I think we’re just not going to see, at least not here in the U.S.,” Michael E. Weinblatt, MD, declared at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
In contrast, the safety and efficacy of the biosimilars, as well as their interchangeability with their reference products, appear to be as hoped for. At the 2016 annual meeting of the American College of Rheumatology, Dr. Weinblatt presented the week 24 results of a phase III, randomized trial involving rheumatoid arthritis patients on background methotrexate plus either adalimumab (Humira) or its biosimilar SB5.
“Essentially, they’re dead on in clinical response, they’re dead on in antibody levels, and they’re dead on in toxicity. And, you can put any of the biosimilars up there and the results are the same. If they get approved, this is what you’re going to see,” the rheumatologist said.
Also at the 2016 ACR annual meeting, he noted, Danish investigators presented reassuring 1-year follow-up data on 802 Danes with inflammatory rheumatic diseases who switched from infliximab (Remicade) to its biosimilar Remsima. Disease activity and flare rates in the year following the switch were similar to those in the year before. The 1-year rate of adherence to Remsima was 84%, similar to the historical 86% 1-year rate with infliximab.
“So, I’m pretty comfortable with the biosimilars,” Dr. Weinblatt continued.
He observed that, of all the systemic rheumatic diseases, the greatest progress has occurred in the treatment of rheumatoid arthritis.
“We have made great advances in the treatment of this disease, unlike many of our other diseases. Methotrexate and combination therapies with small molecules and biologics has dramatically changed the course of the disease,” he noted. “The greatest challenge we have now as rheumatologists is access barriers for our patients.”
Dr. Weinblatt reported receiving research grants from half a dozen companies and serving as a consultant to more than two dozen.
SNOWMASS, COLO. – If you thought biosimilars would bring sharply reduced pricing compared with their parent agents, with resultant greater patient access to highly effective therapies for rheumatic diseases ... think again.
“The promise to our patients of biosimilars – greater access to treatments – is something I think we’re just not going to see, at least not here in the U.S.,” Michael E. Weinblatt, MD, declared at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
In contrast, the safety and efficacy of the biosimilars, as well as their interchangeability with their reference products, appear to be as hoped for. At the 2016 annual meeting of the American College of Rheumatology, Dr. Weinblatt presented the week 24 results of a phase III, randomized trial involving rheumatoid arthritis patients on background methotrexate plus either adalimumab (Humira) or its biosimilar SB5.
“Essentially, they’re dead on in clinical response, they’re dead on in antibody levels, and they’re dead on in toxicity. And, you can put any of the biosimilars up there and the results are the same. If they get approved, this is what you’re going to see,” the rheumatologist said.
Also at the 2016 ACR annual meeting, he noted, Danish investigators presented reassuring 1-year follow-up data on 802 Danes with inflammatory rheumatic diseases who switched from infliximab (Remicade) to its biosimilar Remsima. Disease activity and flare rates in the year following the switch were similar to those in the year before. The 1-year rate of adherence to Remsima was 84%, similar to the historical 86% 1-year rate with infliximab.
“So, I’m pretty comfortable with the biosimilars,” Dr. Weinblatt continued.
He observed that, of all the systemic rheumatic diseases, the greatest progress has occurred in the treatment of rheumatoid arthritis.
“We have made great advances in the treatment of this disease, unlike many of our other diseases. Methotrexate and combination therapies with small molecules and biologics has dramatically changed the course of the disease,” he noted. “The greatest challenge we have now as rheumatologists is access barriers for our patients.”
Dr. Weinblatt reported receiving research grants from half a dozen companies and serving as a consultant to more than two dozen.
EXPERT ANALYSIS FROM THE WINTER RHEUMATOLOGY SYMPOSIUM
How Can Neurologists Diagnose and Manage Sport-Related Concussion?
RIVIERA BEACH, FL—If a neurologist is at a sporting event during which a player sustains a head injury, audience members or officials may look to him or her for guidance, according to an overview delivered at the 44th Annual Meeting of the Southern Clinical Neurological Society. Understanding how to diagnose and manage concussion may be a vital skill for neurologists, regardless of specialty.
What Is Concussion?
Loss of consciousness previously was considered necessary for a diagnosis of concussion. Later, it was taken as a marker of serious injury. Neither of these principles is accepted any longer. Data indicate that at least 90% of concussions are not associated with loss of consciousness, and studies have not shown that loss of consciousness portends a worse prognosis or protracted recovery from the injury, said Dr. Kosa.
The pathophysiology of concussion is not certain. The current proposal is that concussion entails disruption of neuronal cell membranes resulting from disruption of normal ion channels (eg, calcium, potassium, and sodium), leading to a loss of normal neuronal homeostasis. This situation can cause a cascade of events, including mitochondrial dysfunction that causes neuronal energy failure, loss of normal glucose metabolism, activation of NMDA receptors from increased levels of glutamate, production of lactic acid, and generation of free radicals, all of which damage the neurons and supporting cells. Most cells survive the concussive injury, but can be functionally compromised. Severe injuries can lead to neuronal cell death.
What Are the Possible Sequelae of Concussion?
Concussion increases the risk of second impact syndrome, which can occur if the patient sustains another injury at between 24 hours and 10 days after a concussion. Research on second impact syndrome is limited, but the syndrome is understood to entail rapid and massive brain edema that leads to brain herniation and likely death or severe disability. The syndrome occurs within minutes of the second impact and is thought to be enabled by the period of vulnerability that follows an initial concussion. The syndrome occurs mostly in young patients, but has been described in boxers. For this reason, neurologists should be especially cautious when deciding whether to let a child with concussion return to play, said Dr. Kosa. The Centers for Disease Control and Prevention (CDC) estimate that second impact syndrome causes four to six deaths in patients under age 18 annually.
Concussion may be accompanied by traumatic brain injury (TBI). In 2010, the CDC reported 2.5 million hospital encounters related to TBI. Among these encounters, 87% of patients were treated in the emergency department and released, 11% were hospitalized and discharged, and 2% died. The highest incidence of TBI is in young children, and causes include sports accidents, bicycle accidents, skateboard accidents, vehicular accidents, and falls. The CDC estimates that between 3.2 million and 5.3 million people in the United States have permanent TBI-related disability, which results in great economic, physical, and emotional burdens.
Patients with repeated mild TBI may be at risk of chronic traumatic encephalopathy (CTE). This disorder has been described in football players, veterans, and boxers. Symptoms develop later in the patient’s life, and four stages have been described. The first stage includes headaches, inattention, and poor concentration. Stage two consists of significant mood disturbance with depression, along with explosive bouts of anger and short-term memory impairment. The third stage includes further cognitive or memory impairment that manifests as prominent executive dysfunction, where reasoning and organization or planning are most affected. In stage four, the patient has dementia; the cognitive and memory impairment has progressed to the point where the patient depends on others for activities of daily living.
McKee et al observed that CTE was associated with cerebral atrophy, mammillary body atrophy, dilation of the lateral ventricles, fenestrations of the septum pellucidum, and tau deposition. Researchers and clinicians, however, have not arrived at a consensus about the pathologic and clinical criteria for CTE. Furthermore, Cantu et al stated that it is not yet possible to determine the causality or risk factors of CTE with certainty. The hypothesis that repeated concussion or subconcussive impacts leads to the development of CTE has not been scientifically proven to date, they added.
What Should Be Done on the Field?
If a player at a sporting event sustains a head injury, he or she should be removed from play immediately and not allowed to return to the game. If he or she has not directly observed the injury, the neurologist should get information about it from witnesses. The neurologist should perform a focused physical examination, searching for evidence of decreased level of consciousness, confusion, focal weakness or incoordination, visual disturbance, cervical spine injury, or facial fractures. The Sport Concussion Assessment Tool (SCAT) can assist the clinician in concussion evaluation and treatment in a standardized and methodical way to determine whether and when a player can safely return to play.
A patient with an abnormal examination may need to be transferred to the local emergency department for further testing. CT imaging should not be performed automatically, because it may expose the patient to radiation unnecessarily. Two sets of criteria offer guidance about CT imaging. The New Orleans criteria state that a patient should undergo CT if he or she has a headache, has vomited, is older than 60, had been using alcohol or other drugs, had a seizure, has visible trauma above the clavicle, or has a short-term memory deficit. The Canadian CT Head Rule lists similar criteria, including a Glasgow Coma Scale score at two hours of less than 15, any sign of a basal skull fracture, and amnesia for events that took place 30 minutes before the injury.
Anticoagulants increase the risk of immediate or delayed hemorrhage after head injury. If a patient has intracranial hemorrhage on CT and has been using anticoagulants, the clinician should rapidly reverse the anticoagulant effect with the appropriate agent. A repeat head CT 24 hours later should be considered in those thought to be at high risk for intracranial hemorrhage and whose initial CT imaging is negative for bleed. “Err on the side of admitting these patients, at least for observation,” said Dr. Kosa. Before the patient is discharged from the emergency room, he or she should receive education about postconcussion symptoms that should prompt another visit to the emergency department. Educational materials are available on the CDC’s website.
When Can a Patient Return to Play?
The consensus statement on concussion in sport adopted at the Third International Conference on Concussion in Sport includes guidelines for graduated return to play. At first, the patient should undergo symptom-limited physical and cognitive rest until he or she recovers. Next, the patient may start light aerobic exercise such as walking, swimming, or cycling. The goal is to increase heart rate, but the patient should reduce activity if symptoms occur. Then, the patient may engage in sport-specific exercise. If recovery proceeds well, the patient may begin noncontact training drills and, later, full contact practice. Only when the patient has full confidence and coaching or training staff has assessed his or her functional skills can the athlete return to play.
What Concussion Research Is Under Way?
Investigations currently under way aim to improve understanding of concussion, as well as to aid diagnosis and treatment. Researchers are looking for a reliable biomarker of concussion that can be detected with an easy, cost-effective, and preferably noninvasive test. Saliva, tears, urine, blood, and CSF are among the candidate samples being studied. CSF is the most reliable fluid to test because of its proximity to the brain and its low susceptibility to extracerebral confounders, but it is the most invasive option. Groups are examining potential serum biomarkers such as S100b, neuron-specific enolase, myelin basic protein, glial fibrillary acidic protein, and cleaved tau.
In addition, McKee and colleagues are working to define clear pathologic criteria defining the various stages of CTE. They also are seeking a way of distinguishing CTE from Alzheimer’s disease, amyotrophic lateral sclerosis, and other neurodegenerative diseases in postmortem brain tissue. The group’s ultimate goal is to identify features that may assist in the diagnosis of CTE in living people using advanced neuroimaging.
—Erik Greb
Suggested Reading
Giza CC, Kutcher JS, Ashwal S, et al. Summary of evidence-based guideline update: evaluation and management of concussion in sports: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;80(24):2250-2257.
McCrory P, Meeuwisse W, Johnston K, et al. Consensus statement on concussion in sport--the 3rd International Conference on concussion in sport, held in Zurich, November 2008. J Clin Neurosci. 2009;16(6):755-763.
Omalu BI, Hamilton RL, Kamboh MI, et al. Chronic traumatic encephalopathy (CTE) in a National Football League Player: Case report and emerging medicolegal practice questions. J Forensic Nurs. 2010;6(1):40-46.
RIVIERA BEACH, FL—If a neurologist is at a sporting event during which a player sustains a head injury, audience members or officials may look to him or her for guidance, according to an overview delivered at the 44th Annual Meeting of the Southern Clinical Neurological Society. Understanding how to diagnose and manage concussion may be a vital skill for neurologists, regardless of specialty.
What Is Concussion?
Loss of consciousness previously was considered necessary for a diagnosis of concussion. Later, it was taken as a marker of serious injury. Neither of these principles is accepted any longer. Data indicate that at least 90% of concussions are not associated with loss of consciousness, and studies have not shown that loss of consciousness portends a worse prognosis or protracted recovery from the injury, said Dr. Kosa.
The pathophysiology of concussion is not certain. The current proposal is that concussion entails disruption of neuronal cell membranes resulting from disruption of normal ion channels (eg, calcium, potassium, and sodium), leading to a loss of normal neuronal homeostasis. This situation can cause a cascade of events, including mitochondrial dysfunction that causes neuronal energy failure, loss of normal glucose metabolism, activation of NMDA receptors from increased levels of glutamate, production of lactic acid, and generation of free radicals, all of which damage the neurons and supporting cells. Most cells survive the concussive injury, but can be functionally compromised. Severe injuries can lead to neuronal cell death.
What Are the Possible Sequelae of Concussion?
Concussion increases the risk of second impact syndrome, which can occur if the patient sustains another injury at between 24 hours and 10 days after a concussion. Research on second impact syndrome is limited, but the syndrome is understood to entail rapid and massive brain edema that leads to brain herniation and likely death or severe disability. The syndrome occurs within minutes of the second impact and is thought to be enabled by the period of vulnerability that follows an initial concussion. The syndrome occurs mostly in young patients, but has been described in boxers. For this reason, neurologists should be especially cautious when deciding whether to let a child with concussion return to play, said Dr. Kosa. The Centers for Disease Control and Prevention (CDC) estimate that second impact syndrome causes four to six deaths in patients under age 18 annually.
Concussion may be accompanied by traumatic brain injury (TBI). In 2010, the CDC reported 2.5 million hospital encounters related to TBI. Among these encounters, 87% of patients were treated in the emergency department and released, 11% were hospitalized and discharged, and 2% died. The highest incidence of TBI is in young children, and causes include sports accidents, bicycle accidents, skateboard accidents, vehicular accidents, and falls. The CDC estimates that between 3.2 million and 5.3 million people in the United States have permanent TBI-related disability, which results in great economic, physical, and emotional burdens.
Patients with repeated mild TBI may be at risk of chronic traumatic encephalopathy (CTE). This disorder has been described in football players, veterans, and boxers. Symptoms develop later in the patient’s life, and four stages have been described. The first stage includes headaches, inattention, and poor concentration. Stage two consists of significant mood disturbance with depression, along with explosive bouts of anger and short-term memory impairment. The third stage includes further cognitive or memory impairment that manifests as prominent executive dysfunction, where reasoning and organization or planning are most affected. In stage four, the patient has dementia; the cognitive and memory impairment has progressed to the point where the patient depends on others for activities of daily living.
McKee et al observed that CTE was associated with cerebral atrophy, mammillary body atrophy, dilation of the lateral ventricles, fenestrations of the septum pellucidum, and tau deposition. Researchers and clinicians, however, have not arrived at a consensus about the pathologic and clinical criteria for CTE. Furthermore, Cantu et al stated that it is not yet possible to determine the causality or risk factors of CTE with certainty. The hypothesis that repeated concussion or subconcussive impacts leads to the development of CTE has not been scientifically proven to date, they added.
What Should Be Done on the Field?
If a player at a sporting event sustains a head injury, he or she should be removed from play immediately and not allowed to return to the game. If he or she has not directly observed the injury, the neurologist should get information about it from witnesses. The neurologist should perform a focused physical examination, searching for evidence of decreased level of consciousness, confusion, focal weakness or incoordination, visual disturbance, cervical spine injury, or facial fractures. The Sport Concussion Assessment Tool (SCAT) can assist the clinician in concussion evaluation and treatment in a standardized and methodical way to determine whether and when a player can safely return to play.
A patient with an abnormal examination may need to be transferred to the local emergency department for further testing. CT imaging should not be performed automatically, because it may expose the patient to radiation unnecessarily. Two sets of criteria offer guidance about CT imaging. The New Orleans criteria state that a patient should undergo CT if he or she has a headache, has vomited, is older than 60, had been using alcohol or other drugs, had a seizure, has visible trauma above the clavicle, or has a short-term memory deficit. The Canadian CT Head Rule lists similar criteria, including a Glasgow Coma Scale score at two hours of less than 15, any sign of a basal skull fracture, and amnesia for events that took place 30 minutes before the injury.
Anticoagulants increase the risk of immediate or delayed hemorrhage after head injury. If a patient has intracranial hemorrhage on CT and has been using anticoagulants, the clinician should rapidly reverse the anticoagulant effect with the appropriate agent. A repeat head CT 24 hours later should be considered in those thought to be at high risk for intracranial hemorrhage and whose initial CT imaging is negative for bleed. “Err on the side of admitting these patients, at least for observation,” said Dr. Kosa. Before the patient is discharged from the emergency room, he or she should receive education about postconcussion symptoms that should prompt another visit to the emergency department. Educational materials are available on the CDC’s website.
When Can a Patient Return to Play?
The consensus statement on concussion in sport adopted at the Third International Conference on Concussion in Sport includes guidelines for graduated return to play. At first, the patient should undergo symptom-limited physical and cognitive rest until he or she recovers. Next, the patient may start light aerobic exercise such as walking, swimming, or cycling. The goal is to increase heart rate, but the patient should reduce activity if symptoms occur. Then, the patient may engage in sport-specific exercise. If recovery proceeds well, the patient may begin noncontact training drills and, later, full contact practice. Only when the patient has full confidence and coaching or training staff has assessed his or her functional skills can the athlete return to play.
What Concussion Research Is Under Way?
Investigations currently under way aim to improve understanding of concussion, as well as to aid diagnosis and treatment. Researchers are looking for a reliable biomarker of concussion that can be detected with an easy, cost-effective, and preferably noninvasive test. Saliva, tears, urine, blood, and CSF are among the candidate samples being studied. CSF is the most reliable fluid to test because of its proximity to the brain and its low susceptibility to extracerebral confounders, but it is the most invasive option. Groups are examining potential serum biomarkers such as S100b, neuron-specific enolase, myelin basic protein, glial fibrillary acidic protein, and cleaved tau.
In addition, McKee and colleagues are working to define clear pathologic criteria defining the various stages of CTE. They also are seeking a way of distinguishing CTE from Alzheimer’s disease, amyotrophic lateral sclerosis, and other neurodegenerative diseases in postmortem brain tissue. The group’s ultimate goal is to identify features that may assist in the diagnosis of CTE in living people using advanced neuroimaging.
—Erik Greb
Suggested Reading
Giza CC, Kutcher JS, Ashwal S, et al. Summary of evidence-based guideline update: evaluation and management of concussion in sports: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;80(24):2250-2257.
McCrory P, Meeuwisse W, Johnston K, et al. Consensus statement on concussion in sport--the 3rd International Conference on concussion in sport, held in Zurich, November 2008. J Clin Neurosci. 2009;16(6):755-763.
Omalu BI, Hamilton RL, Kamboh MI, et al. Chronic traumatic encephalopathy (CTE) in a National Football League Player: Case report and emerging medicolegal practice questions. J Forensic Nurs. 2010;6(1):40-46.
RIVIERA BEACH, FL—If a neurologist is at a sporting event during which a player sustains a head injury, audience members or officials may look to him or her for guidance, according to an overview delivered at the 44th Annual Meeting of the Southern Clinical Neurological Society. Understanding how to diagnose and manage concussion may be a vital skill for neurologists, regardless of specialty.
What Is Concussion?
Loss of consciousness previously was considered necessary for a diagnosis of concussion. Later, it was taken as a marker of serious injury. Neither of these principles is accepted any longer. Data indicate that at least 90% of concussions are not associated with loss of consciousness, and studies have not shown that loss of consciousness portends a worse prognosis or protracted recovery from the injury, said Dr. Kosa.
The pathophysiology of concussion is not certain. The current proposal is that concussion entails disruption of neuronal cell membranes resulting from disruption of normal ion channels (eg, calcium, potassium, and sodium), leading to a loss of normal neuronal homeostasis. This situation can cause a cascade of events, including mitochondrial dysfunction that causes neuronal energy failure, loss of normal glucose metabolism, activation of NMDA receptors from increased levels of glutamate, production of lactic acid, and generation of free radicals, all of which damage the neurons and supporting cells. Most cells survive the concussive injury, but can be functionally compromised. Severe injuries can lead to neuronal cell death.
What Are the Possible Sequelae of Concussion?
Concussion increases the risk of second impact syndrome, which can occur if the patient sustains another injury at between 24 hours and 10 days after a concussion. Research on second impact syndrome is limited, but the syndrome is understood to entail rapid and massive brain edema that leads to brain herniation and likely death or severe disability. The syndrome occurs within minutes of the second impact and is thought to be enabled by the period of vulnerability that follows an initial concussion. The syndrome occurs mostly in young patients, but has been described in boxers. For this reason, neurologists should be especially cautious when deciding whether to let a child with concussion return to play, said Dr. Kosa. The Centers for Disease Control and Prevention (CDC) estimate that second impact syndrome causes four to six deaths in patients under age 18 annually.
Concussion may be accompanied by traumatic brain injury (TBI). In 2010, the CDC reported 2.5 million hospital encounters related to TBI. Among these encounters, 87% of patients were treated in the emergency department and released, 11% were hospitalized and discharged, and 2% died. The highest incidence of TBI is in young children, and causes include sports accidents, bicycle accidents, skateboard accidents, vehicular accidents, and falls. The CDC estimates that between 3.2 million and 5.3 million people in the United States have permanent TBI-related disability, which results in great economic, physical, and emotional burdens.
Patients with repeated mild TBI may be at risk of chronic traumatic encephalopathy (CTE). This disorder has been described in football players, veterans, and boxers. Symptoms develop later in the patient’s life, and four stages have been described. The first stage includes headaches, inattention, and poor concentration. Stage two consists of significant mood disturbance with depression, along with explosive bouts of anger and short-term memory impairment. The third stage includes further cognitive or memory impairment that manifests as prominent executive dysfunction, where reasoning and organization or planning are most affected. In stage four, the patient has dementia; the cognitive and memory impairment has progressed to the point where the patient depends on others for activities of daily living.
McKee et al observed that CTE was associated with cerebral atrophy, mammillary body atrophy, dilation of the lateral ventricles, fenestrations of the septum pellucidum, and tau deposition. Researchers and clinicians, however, have not arrived at a consensus about the pathologic and clinical criteria for CTE. Furthermore, Cantu et al stated that it is not yet possible to determine the causality or risk factors of CTE with certainty. The hypothesis that repeated concussion or subconcussive impacts leads to the development of CTE has not been scientifically proven to date, they added.
What Should Be Done on the Field?
If a player at a sporting event sustains a head injury, he or she should be removed from play immediately and not allowed to return to the game. If he or she has not directly observed the injury, the neurologist should get information about it from witnesses. The neurologist should perform a focused physical examination, searching for evidence of decreased level of consciousness, confusion, focal weakness or incoordination, visual disturbance, cervical spine injury, or facial fractures. The Sport Concussion Assessment Tool (SCAT) can assist the clinician in concussion evaluation and treatment in a standardized and methodical way to determine whether and when a player can safely return to play.
A patient with an abnormal examination may need to be transferred to the local emergency department for further testing. CT imaging should not be performed automatically, because it may expose the patient to radiation unnecessarily. Two sets of criteria offer guidance about CT imaging. The New Orleans criteria state that a patient should undergo CT if he or she has a headache, has vomited, is older than 60, had been using alcohol or other drugs, had a seizure, has visible trauma above the clavicle, or has a short-term memory deficit. The Canadian CT Head Rule lists similar criteria, including a Glasgow Coma Scale score at two hours of less than 15, any sign of a basal skull fracture, and amnesia for events that took place 30 minutes before the injury.
Anticoagulants increase the risk of immediate or delayed hemorrhage after head injury. If a patient has intracranial hemorrhage on CT and has been using anticoagulants, the clinician should rapidly reverse the anticoagulant effect with the appropriate agent. A repeat head CT 24 hours later should be considered in those thought to be at high risk for intracranial hemorrhage and whose initial CT imaging is negative for bleed. “Err on the side of admitting these patients, at least for observation,” said Dr. Kosa. Before the patient is discharged from the emergency room, he or she should receive education about postconcussion symptoms that should prompt another visit to the emergency department. Educational materials are available on the CDC’s website.
When Can a Patient Return to Play?
The consensus statement on concussion in sport adopted at the Third International Conference on Concussion in Sport includes guidelines for graduated return to play. At first, the patient should undergo symptom-limited physical and cognitive rest until he or she recovers. Next, the patient may start light aerobic exercise such as walking, swimming, or cycling. The goal is to increase heart rate, but the patient should reduce activity if symptoms occur. Then, the patient may engage in sport-specific exercise. If recovery proceeds well, the patient may begin noncontact training drills and, later, full contact practice. Only when the patient has full confidence and coaching or training staff has assessed his or her functional skills can the athlete return to play.
What Concussion Research Is Under Way?
Investigations currently under way aim to improve understanding of concussion, as well as to aid diagnosis and treatment. Researchers are looking for a reliable biomarker of concussion that can be detected with an easy, cost-effective, and preferably noninvasive test. Saliva, tears, urine, blood, and CSF are among the candidate samples being studied. CSF is the most reliable fluid to test because of its proximity to the brain and its low susceptibility to extracerebral confounders, but it is the most invasive option. Groups are examining potential serum biomarkers such as S100b, neuron-specific enolase, myelin basic protein, glial fibrillary acidic protein, and cleaved tau.
In addition, McKee and colleagues are working to define clear pathologic criteria defining the various stages of CTE. They also are seeking a way of distinguishing CTE from Alzheimer’s disease, amyotrophic lateral sclerosis, and other neurodegenerative diseases in postmortem brain tissue. The group’s ultimate goal is to identify features that may assist in the diagnosis of CTE in living people using advanced neuroimaging.
—Erik Greb
Suggested Reading
Giza CC, Kutcher JS, Ashwal S, et al. Summary of evidence-based guideline update: evaluation and management of concussion in sports: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;80(24):2250-2257.
McCrory P, Meeuwisse W, Johnston K, et al. Consensus statement on concussion in sport--the 3rd International Conference on concussion in sport, held in Zurich, November 2008. J Clin Neurosci. 2009;16(6):755-763.
Omalu BI, Hamilton RL, Kamboh MI, et al. Chronic traumatic encephalopathy (CTE) in a National Football League Player: Case report and emerging medicolegal practice questions. J Forensic Nurs. 2010;6(1):40-46.