User login
Big Health Policy Implications Loom with 2016 Election
Editor's Note: Listen to Robert Blendon talk more about the health policy implications of the 2016 election.
One thing is certain: The outcome of this year’s election will usher in profound change for the American healthcare system. It also means a great deal of uncertainty for physicians, hospital systems, insurers, patients, and healthcare providers more broadly for weeks, months, or even years to come.
The Policy Proposals
Democratic presidential nominee Hillary Clinton has vowed to keep, strengthen, and “fix” the ACA, with proposals that include allowing people to begin buying into Medicare at age 55 and eliminating the Cadillac tax, plus a vow to defend access to reproductive healthcare. Republican nominee Donald Trump has the seven-point “Healthcare Reform to Make America Great Again,” which has as its first pillar to “completely replace Obamacare.”
While Clinton’s platform is highly detailed, Trump has offered few specifics with regard to its replacement, “just a set of general principles,” says Robert Blendon, the Richard L. Menschel Professor of Public Health at Harvard T.H. Chan School of Public Health (HSPH) and a professor of health policy and political analysis at HSPH and the Harvard Kennedy School of Government. “His supporters are just not focused on what the healthcare bill of the future would look like,” he adds.
Under majority Republican leadership, “it’s absolutely clear,” Blendon says, that the party would attempt to repeal the ACA. That would mean millions of people could lose insurance coverage or face higher levels of cost-sharing, benefits would be less comprehensive, and government regulation would decrease, leading to fewer directives for physicians and providers, he says.
A Democratic sweep of the executive and legislative branches would likely bring more funding for the National Institutes of Health and the Centers for Disease Control and Prevention. It might also lead to the introduction of a government alternative insurance plan that would compete with private insurance for those under age 65, Blendon explains.
“There’d be more money spent, but there’d be much more government regulation, including discussions of Medicare price limits on certain types of drugs,” he says.
Healthcare, though, has been caught in the middle of a host of broader issues, Blendon says.
“Put very simply, you almost have three parties that are running,” he says. “You have Democratic, which is [the] more liberal-moderate party, which is basically running on a health platform that is continuing Obama’s eight years but enlarging it in a number of areas. You have the party of the Republicans strictly in the Congress, which are running as a conservative party, which is to get rid of part of the ACA, to slow Medicare costs, and very concerned with a tax cut broadly and restraining federal optional expenditures in the future.
“The third is Mr. Trump, but it’s not widely understood unless you follow European political situations a lot,” Blendon says. “Mr. Trump is actually running what would be called in Europe a nationalist party. Their issues are a bit different.”
Key components of Trump’s seven-point healthcare plan embrace some historical or current Republican policy ideas. These include using tax-free health savings accounts, allowing tax deductions for insurance premiums, and providing Medicaid block grants to states (though he has vowed not to cut overall Medicaid spending).
But Trump also breaks with the party, promising not to alter Medicare, proposing, like Clinton, to allow Medicare to negotiate pharmaceutical drug prices, and considering the idea of allowing pharmaceuticals to be imported from overseas, also like his Democratic opponent.
“I believe on the healthcare issue, he will be somewhat deferential to what the Republican leaders want their healthcare bill to look like in the future … not necessarily because that’s his particular choice but because he has a whole other agenda, which he says over and over is really important to him, and he needs the Republican leadership [to support it],” Blendon says.
How Will Things Get Done?
According to a Brookings Institution policy document published earlier this year, anyone proposing healthcare policy changes will confront “a daunting negotiation with powerful stakeholders to defend and enhance their varied interests” following the 2016 election.1
Three possible scenarios include a full Democratic president and Congress, a full Republican president and Congress, or a split presidency and Congress (including the two houses going each to the other party).
“If there is a split in the House and Senate, will things get done?” says Bradley Flansbaum, DO, MPH, MHM, a member of SHM’s Public Policy Committee. “Democrats don’t want to indicate the law has flaws and needs fixes. That admission invites the GOP to say, ‘See, it’s broken.’ Conversely, if Republicans do try to work with anyone on the other side of the aisle, they will be branded a pariah.”
One hospitalist sees Congress as the main force behind whether the ACA is kept intact.
“Congress holds the purse strings and has the control to chip away at the financial underpinnings until those toothpicks that hold up the Obamacare elephant break and it comes crashing down,” says Joshua Lenchus, DO, RPh, FACP, SFHM, a hospitalist at the University of Miami/Jackson Memorial Hospital in Florida and a member of SHM’s Public Policy Committee.
ACA Fixes?
One option Clinton has proposed is a federally administered public alternative to private insurers in the ACA marketplace, particularly as more companies leave exchanges across the country. Blendon says there is some concern over the idea’s viability since, while it could help keep pricing competitive, it might just “attract some of the sickest people because they’ll feel it provides more financial security.”
“A very high priority for a Clinton administration and a Democratic Congress [is] to get in there with a rescue team, and this is an issue of providing wraparound protection for [insurance] companies that basically end up with either older or sicker people than they had at all anticipated and some sort of a financial cushion to carry them into other years,” Blendon says.
In its policy paper, the Brookings Institution says any serious Republican idea to repeal the ACA should offer an alternative to replace the healthcare bill’s spending reductions, particularly since the Congressional Budget Office estimates repeal of the ACA would increase direct Medicare spending by $802 billion over the next decade, possibly accelerating the depletion of the program’s trust fund.1
“I think what would happen would be some amount of what the Republican leadership has talked about, some sort of a partial alternative to the ACA, and it would cover less people and less benefits, but there would be an absolute plan that they would try to have in place,” Blendon says.
But only time will tell how the election will affect hospitalists in their day-to-day work.
“Unfortunately, we’re still not at a stage that you could say to somebody, ‘This is what the next five years are going to look like; that’s how you should think about what your hospital and practice should be thinking,’” Blendon says. “You’re much more stuck with, ‘There is uncertainty here.’” TH
Kelly April Tyrrell is a freelance writer in Madison, Wis.
Reference
- Rivlin AM, Reischauer RD. Health policy issues and the 2016 presidential election. Brookings Institution website. Accessed August 31, 2016.
Editor's Note: Listen to Robert Blendon talk more about the health policy implications of the 2016 election.
One thing is certain: The outcome of this year’s election will usher in profound change for the American healthcare system. It also means a great deal of uncertainty for physicians, hospital systems, insurers, patients, and healthcare providers more broadly for weeks, months, or even years to come.
The Policy Proposals
Democratic presidential nominee Hillary Clinton has vowed to keep, strengthen, and “fix” the ACA, with proposals that include allowing people to begin buying into Medicare at age 55 and eliminating the Cadillac tax, plus a vow to defend access to reproductive healthcare. Republican nominee Donald Trump has the seven-point “Healthcare Reform to Make America Great Again,” which has as its first pillar to “completely replace Obamacare.”
While Clinton’s platform is highly detailed, Trump has offered few specifics with regard to its replacement, “just a set of general principles,” says Robert Blendon, the Richard L. Menschel Professor of Public Health at Harvard T.H. Chan School of Public Health (HSPH) and a professor of health policy and political analysis at HSPH and the Harvard Kennedy School of Government. “His supporters are just not focused on what the healthcare bill of the future would look like,” he adds.
Under majority Republican leadership, “it’s absolutely clear,” Blendon says, that the party would attempt to repeal the ACA. That would mean millions of people could lose insurance coverage or face higher levels of cost-sharing, benefits would be less comprehensive, and government regulation would decrease, leading to fewer directives for physicians and providers, he says.
A Democratic sweep of the executive and legislative branches would likely bring more funding for the National Institutes of Health and the Centers for Disease Control and Prevention. It might also lead to the introduction of a government alternative insurance plan that would compete with private insurance for those under age 65, Blendon explains.
“There’d be more money spent, but there’d be much more government regulation, including discussions of Medicare price limits on certain types of drugs,” he says.
Healthcare, though, has been caught in the middle of a host of broader issues, Blendon says.
“Put very simply, you almost have three parties that are running,” he says. “You have Democratic, which is [the] more liberal-moderate party, which is basically running on a health platform that is continuing Obama’s eight years but enlarging it in a number of areas. You have the party of the Republicans strictly in the Congress, which are running as a conservative party, which is to get rid of part of the ACA, to slow Medicare costs, and very concerned with a tax cut broadly and restraining federal optional expenditures in the future.
“The third is Mr. Trump, but it’s not widely understood unless you follow European political situations a lot,” Blendon says. “Mr. Trump is actually running what would be called in Europe a nationalist party. Their issues are a bit different.”
Key components of Trump’s seven-point healthcare plan embrace some historical or current Republican policy ideas. These include using tax-free health savings accounts, allowing tax deductions for insurance premiums, and providing Medicaid block grants to states (though he has vowed not to cut overall Medicaid spending).
But Trump also breaks with the party, promising not to alter Medicare, proposing, like Clinton, to allow Medicare to negotiate pharmaceutical drug prices, and considering the idea of allowing pharmaceuticals to be imported from overseas, also like his Democratic opponent.
“I believe on the healthcare issue, he will be somewhat deferential to what the Republican leaders want their healthcare bill to look like in the future … not necessarily because that’s his particular choice but because he has a whole other agenda, which he says over and over is really important to him, and he needs the Republican leadership [to support it],” Blendon says.
How Will Things Get Done?
According to a Brookings Institution policy document published earlier this year, anyone proposing healthcare policy changes will confront “a daunting negotiation with powerful stakeholders to defend and enhance their varied interests” following the 2016 election.1
Three possible scenarios include a full Democratic president and Congress, a full Republican president and Congress, or a split presidency and Congress (including the two houses going each to the other party).
“If there is a split in the House and Senate, will things get done?” says Bradley Flansbaum, DO, MPH, MHM, a member of SHM’s Public Policy Committee. “Democrats don’t want to indicate the law has flaws and needs fixes. That admission invites the GOP to say, ‘See, it’s broken.’ Conversely, if Republicans do try to work with anyone on the other side of the aisle, they will be branded a pariah.”
One hospitalist sees Congress as the main force behind whether the ACA is kept intact.
“Congress holds the purse strings and has the control to chip away at the financial underpinnings until those toothpicks that hold up the Obamacare elephant break and it comes crashing down,” says Joshua Lenchus, DO, RPh, FACP, SFHM, a hospitalist at the University of Miami/Jackson Memorial Hospital in Florida and a member of SHM’s Public Policy Committee.
ACA Fixes?
One option Clinton has proposed is a federally administered public alternative to private insurers in the ACA marketplace, particularly as more companies leave exchanges across the country. Blendon says there is some concern over the idea’s viability since, while it could help keep pricing competitive, it might just “attract some of the sickest people because they’ll feel it provides more financial security.”
“A very high priority for a Clinton administration and a Democratic Congress [is] to get in there with a rescue team, and this is an issue of providing wraparound protection for [insurance] companies that basically end up with either older or sicker people than they had at all anticipated and some sort of a financial cushion to carry them into other years,” Blendon says.
In its policy paper, the Brookings Institution says any serious Republican idea to repeal the ACA should offer an alternative to replace the healthcare bill’s spending reductions, particularly since the Congressional Budget Office estimates repeal of the ACA would increase direct Medicare spending by $802 billion over the next decade, possibly accelerating the depletion of the program’s trust fund.1
“I think what would happen would be some amount of what the Republican leadership has talked about, some sort of a partial alternative to the ACA, and it would cover less people and less benefits, but there would be an absolute plan that they would try to have in place,” Blendon says.
But only time will tell how the election will affect hospitalists in their day-to-day work.
“Unfortunately, we’re still not at a stage that you could say to somebody, ‘This is what the next five years are going to look like; that’s how you should think about what your hospital and practice should be thinking,’” Blendon says. “You’re much more stuck with, ‘There is uncertainty here.’” TH
Kelly April Tyrrell is a freelance writer in Madison, Wis.
Reference
- Rivlin AM, Reischauer RD. Health policy issues and the 2016 presidential election. Brookings Institution website. Accessed August 31, 2016.
Editor's Note: Listen to Robert Blendon talk more about the health policy implications of the 2016 election.
One thing is certain: The outcome of this year’s election will usher in profound change for the American healthcare system. It also means a great deal of uncertainty for physicians, hospital systems, insurers, patients, and healthcare providers more broadly for weeks, months, or even years to come.
The Policy Proposals
Democratic presidential nominee Hillary Clinton has vowed to keep, strengthen, and “fix” the ACA, with proposals that include allowing people to begin buying into Medicare at age 55 and eliminating the Cadillac tax, plus a vow to defend access to reproductive healthcare. Republican nominee Donald Trump has the seven-point “Healthcare Reform to Make America Great Again,” which has as its first pillar to “completely replace Obamacare.”
While Clinton’s platform is highly detailed, Trump has offered few specifics with regard to its replacement, “just a set of general principles,” says Robert Blendon, the Richard L. Menschel Professor of Public Health at Harvard T.H. Chan School of Public Health (HSPH) and a professor of health policy and political analysis at HSPH and the Harvard Kennedy School of Government. “His supporters are just not focused on what the healthcare bill of the future would look like,” he adds.
Under majority Republican leadership, “it’s absolutely clear,” Blendon says, that the party would attempt to repeal the ACA. That would mean millions of people could lose insurance coverage or face higher levels of cost-sharing, benefits would be less comprehensive, and government regulation would decrease, leading to fewer directives for physicians and providers, he says.
A Democratic sweep of the executive and legislative branches would likely bring more funding for the National Institutes of Health and the Centers for Disease Control and Prevention. It might also lead to the introduction of a government alternative insurance plan that would compete with private insurance for those under age 65, Blendon explains.
“There’d be more money spent, but there’d be much more government regulation, including discussions of Medicare price limits on certain types of drugs,” he says.
Healthcare, though, has been caught in the middle of a host of broader issues, Blendon says.
“Put very simply, you almost have three parties that are running,” he says. “You have Democratic, which is [the] more liberal-moderate party, which is basically running on a health platform that is continuing Obama’s eight years but enlarging it in a number of areas. You have the party of the Republicans strictly in the Congress, which are running as a conservative party, which is to get rid of part of the ACA, to slow Medicare costs, and very concerned with a tax cut broadly and restraining federal optional expenditures in the future.
“The third is Mr. Trump, but it’s not widely understood unless you follow European political situations a lot,” Blendon says. “Mr. Trump is actually running what would be called in Europe a nationalist party. Their issues are a bit different.”
Key components of Trump’s seven-point healthcare plan embrace some historical or current Republican policy ideas. These include using tax-free health savings accounts, allowing tax deductions for insurance premiums, and providing Medicaid block grants to states (though he has vowed not to cut overall Medicaid spending).
But Trump also breaks with the party, promising not to alter Medicare, proposing, like Clinton, to allow Medicare to negotiate pharmaceutical drug prices, and considering the idea of allowing pharmaceuticals to be imported from overseas, also like his Democratic opponent.
“I believe on the healthcare issue, he will be somewhat deferential to what the Republican leaders want their healthcare bill to look like in the future … not necessarily because that’s his particular choice but because he has a whole other agenda, which he says over and over is really important to him, and he needs the Republican leadership [to support it],” Blendon says.
How Will Things Get Done?
According to a Brookings Institution policy document published earlier this year, anyone proposing healthcare policy changes will confront “a daunting negotiation with powerful stakeholders to defend and enhance their varied interests” following the 2016 election.1
Three possible scenarios include a full Democratic president and Congress, a full Republican president and Congress, or a split presidency and Congress (including the two houses going each to the other party).
“If there is a split in the House and Senate, will things get done?” says Bradley Flansbaum, DO, MPH, MHM, a member of SHM’s Public Policy Committee. “Democrats don’t want to indicate the law has flaws and needs fixes. That admission invites the GOP to say, ‘See, it’s broken.’ Conversely, if Republicans do try to work with anyone on the other side of the aisle, they will be branded a pariah.”
One hospitalist sees Congress as the main force behind whether the ACA is kept intact.
“Congress holds the purse strings and has the control to chip away at the financial underpinnings until those toothpicks that hold up the Obamacare elephant break and it comes crashing down,” says Joshua Lenchus, DO, RPh, FACP, SFHM, a hospitalist at the University of Miami/Jackson Memorial Hospital in Florida and a member of SHM’s Public Policy Committee.
ACA Fixes?
One option Clinton has proposed is a federally administered public alternative to private insurers in the ACA marketplace, particularly as more companies leave exchanges across the country. Blendon says there is some concern over the idea’s viability since, while it could help keep pricing competitive, it might just “attract some of the sickest people because they’ll feel it provides more financial security.”
“A very high priority for a Clinton administration and a Democratic Congress [is] to get in there with a rescue team, and this is an issue of providing wraparound protection for [insurance] companies that basically end up with either older or sicker people than they had at all anticipated and some sort of a financial cushion to carry them into other years,” Blendon says.
In its policy paper, the Brookings Institution says any serious Republican idea to repeal the ACA should offer an alternative to replace the healthcare bill’s spending reductions, particularly since the Congressional Budget Office estimates repeal of the ACA would increase direct Medicare spending by $802 billion over the next decade, possibly accelerating the depletion of the program’s trust fund.1
“I think what would happen would be some amount of what the Republican leadership has talked about, some sort of a partial alternative to the ACA, and it would cover less people and less benefits, but there would be an absolute plan that they would try to have in place,” Blendon says.
But only time will tell how the election will affect hospitalists in their day-to-day work.
“Unfortunately, we’re still not at a stage that you could say to somebody, ‘This is what the next five years are going to look like; that’s how you should think about what your hospital and practice should be thinking,’” Blendon says. “You’re much more stuck with, ‘There is uncertainty here.’” TH
Kelly April Tyrrell is a freelance writer in Madison, Wis.
Reference
- Rivlin AM, Reischauer RD. Health policy issues and the 2016 presidential election. Brookings Institution website. Accessed August 31, 2016.
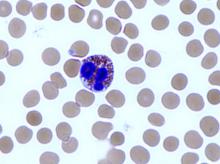
Team explains how MM cells alter BM to thrive

New research helps explain how multiple myeloma (MM) cells manipulate the bone marrow (BM) environment to promote disease progression.
The researchers knew that mesenchymal stem cells (MSCs) are often altered in MM in a way that favors disease progression, but the mechanisms behind this phenomenon weren’t well understood.
So the team set out to determine how and when normal MSCs evolve into tumor-promoting MSCs.
Mahmoud Dabbah, of Meir Medical Center in Kfar Saba, Israel, and his colleagues conducted this research and reported the results in the Journal of Leukocyte Biology.
The researchers cultured the MM cell lines U266 and ARP1 with MSCs from healthy donors, looking for changes in the MSCs. The team observed changes in migration and protein translation initiation.
Specifically, they found that co-culturing MSCs with MM cell lines induced an elevation in translation initiation factors eIF4E and eIF4GI as well as their regulators and targets. But these changes were reversible.
MM-conditioned MSCs had significantly elevated levels of peIF4E, total eIF4E, peIF4GI, and total eIF4GI after 3 days of co-culture with both MM cell lines (all increased about 200%, P<0.05).
In fact, levels of peIF4GI and peIF4E were significantly elevated in the MSCs within 1.5 hours of co-culture (both increased more than 250%, P<0.05).
However, when the MSCs were removed from co-culture, the levels returned to baseline within 3 to 6 hours.
MM-conditioned MSCs also showed a significant increase in migration. When cultured for 16 hours, MSC migration increased more than 400% (P<0.05).
Accordingly, the MM-conditioned MSCs expressed lower levels of microRNAs with established roles in cell migration.
The MSCs showed decreased levels of MIR-125a-5p and MIR-199b-3p after 12 hours of co-culture (a decrease of 160% to 250%, P<0.05). And this effect was maintained as long as the co-culture continued.
The researchers said their findings suggest a dynamic interaction between MM cells and the BM niche that causes profound changes in non-malignant BM constituents. They hope that future studies will reveal clinically relevant means of blocking this crosstalk to improve MM therapy.
“Our research should help identify therapeutic targets that may be used to minimize the collateral damage,” Dabbah said. “The identification of the translation initiation phase as a dialogue platform affords a potential new therapeutic target to be explored.” ![]()

New research helps explain how multiple myeloma (MM) cells manipulate the bone marrow (BM) environment to promote disease progression.
The researchers knew that mesenchymal stem cells (MSCs) are often altered in MM in a way that favors disease progression, but the mechanisms behind this phenomenon weren’t well understood.
So the team set out to determine how and when normal MSCs evolve into tumor-promoting MSCs.
Mahmoud Dabbah, of Meir Medical Center in Kfar Saba, Israel, and his colleagues conducted this research and reported the results in the Journal of Leukocyte Biology.
The researchers cultured the MM cell lines U266 and ARP1 with MSCs from healthy donors, looking for changes in the MSCs. The team observed changes in migration and protein translation initiation.
Specifically, they found that co-culturing MSCs with MM cell lines induced an elevation in translation initiation factors eIF4E and eIF4GI as well as their regulators and targets. But these changes were reversible.
MM-conditioned MSCs had significantly elevated levels of peIF4E, total eIF4E, peIF4GI, and total eIF4GI after 3 days of co-culture with both MM cell lines (all increased about 200%, P<0.05).
In fact, levels of peIF4GI and peIF4E were significantly elevated in the MSCs within 1.5 hours of co-culture (both increased more than 250%, P<0.05).
However, when the MSCs were removed from co-culture, the levels returned to baseline within 3 to 6 hours.
MM-conditioned MSCs also showed a significant increase in migration. When cultured for 16 hours, MSC migration increased more than 400% (P<0.05).
Accordingly, the MM-conditioned MSCs expressed lower levels of microRNAs with established roles in cell migration.
The MSCs showed decreased levels of MIR-125a-5p and MIR-199b-3p after 12 hours of co-culture (a decrease of 160% to 250%, P<0.05). And this effect was maintained as long as the co-culture continued.
The researchers said their findings suggest a dynamic interaction between MM cells and the BM niche that causes profound changes in non-malignant BM constituents. They hope that future studies will reveal clinically relevant means of blocking this crosstalk to improve MM therapy.
“Our research should help identify therapeutic targets that may be used to minimize the collateral damage,” Dabbah said. “The identification of the translation initiation phase as a dialogue platform affords a potential new therapeutic target to be explored.” ![]()

New research helps explain how multiple myeloma (MM) cells manipulate the bone marrow (BM) environment to promote disease progression.
The researchers knew that mesenchymal stem cells (MSCs) are often altered in MM in a way that favors disease progression, but the mechanisms behind this phenomenon weren’t well understood.
So the team set out to determine how and when normal MSCs evolve into tumor-promoting MSCs.
Mahmoud Dabbah, of Meir Medical Center in Kfar Saba, Israel, and his colleagues conducted this research and reported the results in the Journal of Leukocyte Biology.
The researchers cultured the MM cell lines U266 and ARP1 with MSCs from healthy donors, looking for changes in the MSCs. The team observed changes in migration and protein translation initiation.
Specifically, they found that co-culturing MSCs with MM cell lines induced an elevation in translation initiation factors eIF4E and eIF4GI as well as their regulators and targets. But these changes were reversible.
MM-conditioned MSCs had significantly elevated levels of peIF4E, total eIF4E, peIF4GI, and total eIF4GI after 3 days of co-culture with both MM cell lines (all increased about 200%, P<0.05).
In fact, levels of peIF4GI and peIF4E were significantly elevated in the MSCs within 1.5 hours of co-culture (both increased more than 250%, P<0.05).
However, when the MSCs were removed from co-culture, the levels returned to baseline within 3 to 6 hours.
MM-conditioned MSCs also showed a significant increase in migration. When cultured for 16 hours, MSC migration increased more than 400% (P<0.05).
Accordingly, the MM-conditioned MSCs expressed lower levels of microRNAs with established roles in cell migration.
The MSCs showed decreased levels of MIR-125a-5p and MIR-199b-3p after 12 hours of co-culture (a decrease of 160% to 250%, P<0.05). And this effect was maintained as long as the co-culture continued.
The researchers said their findings suggest a dynamic interaction between MM cells and the BM niche that causes profound changes in non-malignant BM constituents. They hope that future studies will reveal clinically relevant means of blocking this crosstalk to improve MM therapy.
“Our research should help identify therapeutic targets that may be used to minimize the collateral damage,” Dabbah said. “The identification of the translation initiation phase as a dialogue platform affords a potential new therapeutic target to be explored.” ![]()
Trial of hemophilia therapy back on

Image courtesy of NIGMS
A UK regulatory agency has lifted the hold placed on a phase 1/2 study of the gene therapy BMN 270 in patients with hemophilia A.
Dosing was suspended in this trial after the first 9 patients were enrolled, but the Medicines and Healthcare Products Regulatory Agency (MHRA) has decided the study can continue.
BioMarin Pharmaceutical Inc., the company developing BMN 270, said the trial should resume enrollment by the end of this year.
BMN 270 is a recombinant adeno-associated virus vector coding for human coagulation factor VIII (FVIII).
The phase 1/2 study of BMN 270 was designed to evaluate the safety and efficacy of the therapy in up to 15 patients with severe hemophilia A.
Results in 7 patients on this study were recently presented at the World Federation of Hemophilia 2016 World Congress.
However, after the study had enrolled 9 patients, dosing of BMN 270 was suspended due to increases in alanine aminotransferase levels that exceeded a pre-specified threshold.
Following the suspension, BioMarin reviewed safety and efficacy data on the 9 patients with the MHRA. Based on this review, the MHRA approved resumption of the study.
The agency also approved the company’s proposed amendments to the study, which included eliminating the requirement for prophylactic corticosteroids and increasing potential additional enrollment from up to 3 additional patients to up to 6 additional patients.
BioMarin said it intends to resume enrollment in the study before the end of 2016. Based on protocol amendments, 3 patients will be enrolled at a dose of 4 x 1013 vg/kg, and an additional 3 patients may be enrolled at this dose or the previously tested high dose of 6 x 1013 vg/kg.
In the up to 6 additional patients, the requirement for prophylactic corticosteroids has been removed, and the threshold for starting therapeutic corticosteroids has been increased.
BioMarin said safety and efficacy data from these patients will inform the phase 2b study expected to begin in the second half of 2017.
“We are pleased that MHRA has approved the resumption of enrollment of the BMN 270 study, as well as the study amendments,” said Hank Fuchs, MD, chief medical officer at BioMarin.
“We believe that the amendments will allow us to optimize the design of a robust phase 2b clinical trial, which potentially could support an accelerated approval by health authorities. We are grateful to the patients who are participating in this current study and are encouraged by the results so far for this phase 1/2 trial.” ![]()

Image courtesy of NIGMS
A UK regulatory agency has lifted the hold placed on a phase 1/2 study of the gene therapy BMN 270 in patients with hemophilia A.
Dosing was suspended in this trial after the first 9 patients were enrolled, but the Medicines and Healthcare Products Regulatory Agency (MHRA) has decided the study can continue.
BioMarin Pharmaceutical Inc., the company developing BMN 270, said the trial should resume enrollment by the end of this year.
BMN 270 is a recombinant adeno-associated virus vector coding for human coagulation factor VIII (FVIII).
The phase 1/2 study of BMN 270 was designed to evaluate the safety and efficacy of the therapy in up to 15 patients with severe hemophilia A.
Results in 7 patients on this study were recently presented at the World Federation of Hemophilia 2016 World Congress.
However, after the study had enrolled 9 patients, dosing of BMN 270 was suspended due to increases in alanine aminotransferase levels that exceeded a pre-specified threshold.
Following the suspension, BioMarin reviewed safety and efficacy data on the 9 patients with the MHRA. Based on this review, the MHRA approved resumption of the study.
The agency also approved the company’s proposed amendments to the study, which included eliminating the requirement for prophylactic corticosteroids and increasing potential additional enrollment from up to 3 additional patients to up to 6 additional patients.
BioMarin said it intends to resume enrollment in the study before the end of 2016. Based on protocol amendments, 3 patients will be enrolled at a dose of 4 x 1013 vg/kg, and an additional 3 patients may be enrolled at this dose or the previously tested high dose of 6 x 1013 vg/kg.
In the up to 6 additional patients, the requirement for prophylactic corticosteroids has been removed, and the threshold for starting therapeutic corticosteroids has been increased.
BioMarin said safety and efficacy data from these patients will inform the phase 2b study expected to begin in the second half of 2017.
“We are pleased that MHRA has approved the resumption of enrollment of the BMN 270 study, as well as the study amendments,” said Hank Fuchs, MD, chief medical officer at BioMarin.
“We believe that the amendments will allow us to optimize the design of a robust phase 2b clinical trial, which potentially could support an accelerated approval by health authorities. We are grateful to the patients who are participating in this current study and are encouraged by the results so far for this phase 1/2 trial.” ![]()

Image courtesy of NIGMS
A UK regulatory agency has lifted the hold placed on a phase 1/2 study of the gene therapy BMN 270 in patients with hemophilia A.
Dosing was suspended in this trial after the first 9 patients were enrolled, but the Medicines and Healthcare Products Regulatory Agency (MHRA) has decided the study can continue.
BioMarin Pharmaceutical Inc., the company developing BMN 270, said the trial should resume enrollment by the end of this year.
BMN 270 is a recombinant adeno-associated virus vector coding for human coagulation factor VIII (FVIII).
The phase 1/2 study of BMN 270 was designed to evaluate the safety and efficacy of the therapy in up to 15 patients with severe hemophilia A.
Results in 7 patients on this study were recently presented at the World Federation of Hemophilia 2016 World Congress.
However, after the study had enrolled 9 patients, dosing of BMN 270 was suspended due to increases in alanine aminotransferase levels that exceeded a pre-specified threshold.
Following the suspension, BioMarin reviewed safety and efficacy data on the 9 patients with the MHRA. Based on this review, the MHRA approved resumption of the study.
The agency also approved the company’s proposed amendments to the study, which included eliminating the requirement for prophylactic corticosteroids and increasing potential additional enrollment from up to 3 additional patients to up to 6 additional patients.
BioMarin said it intends to resume enrollment in the study before the end of 2016. Based on protocol amendments, 3 patients will be enrolled at a dose of 4 x 1013 vg/kg, and an additional 3 patients may be enrolled at this dose or the previously tested high dose of 6 x 1013 vg/kg.
In the up to 6 additional patients, the requirement for prophylactic corticosteroids has been removed, and the threshold for starting therapeutic corticosteroids has been increased.
BioMarin said safety and efficacy data from these patients will inform the phase 2b study expected to begin in the second half of 2017.
“We are pleased that MHRA has approved the resumption of enrollment of the BMN 270 study, as well as the study amendments,” said Hank Fuchs, MD, chief medical officer at BioMarin.
“We believe that the amendments will allow us to optimize the design of a robust phase 2b clinical trial, which potentially could support an accelerated approval by health authorities. We are grateful to the patients who are participating in this current study and are encouraged by the results so far for this phase 1/2 trial.” ![]()
COMP recommends orphan designation for CMV-CTLs

The European Medicines Agency’s Committee for Orphan Medicinal Products (COMP) is recommending orphan designation for a cytomegalovirus-specific cytotoxic T-lymphocyte product (CMV-CTLs) intended to treat CMV infection in patients with impaired cell-mediated immunity.
The CMV-CTLs are designed to find and kill cells expressing CMV.
To create CMV-CTLs, T cells are collected from the blood of third-party donors and then exposed to CMV antigens.
The resulting activated T cells are then expanded, characterized, and stored for future use in a partially HLA-matched patient.
The CMV-CTLs are being developed by Atara Biotherapeutics, Inc.
The cells are currently under investigation in a pair of phase 2 trials (NCT01646645 and NCT02136797).
Results of a phase 1 trial (published in Biology of Blood and Marrow Transplantation in 2015) suggested CMV-CTLs are safe and can clear CMV infection in patients who have undergone allogeneic hematopoietic stem cell transplant.
The trial included 17 transplant recipients with CMV viremia or clinical infection that persisted despite prolonged treatment with antiviral drugs. Fourteen of the patients had received T-cell-depleted transplants without graft-versus-host disease (GVHD) prophylaxis.
Sixteen of the patients received CMV-CTLs created using cells derived from their transplant donor, and 1 patient received cells from a third-party donor.
Fifteen patients achieved clearance of CMV viremia, including 3 of the 5 patients with overt disease and the patient who received cells from a third-party donor.
In addition, the researchers said CMV-CTLs were well-tolerated. None of the patients experienced fever, alterations in vital signs, or other toxicities during the first 48 hours of observation.
None of the patients developed manifestations of de novo acute GVHD, and GHVD did not worsen in either of the 2 patients who had GVHD prior to infusion.
About orphan designation
The COMP adopts an opinion on the granting of orphan drug designation, and that opinion is submitted to the European Commission for a final decision.
Orphan designation provides regulatory and financial incentives for companies to develop and market therapies that treat life-threatening or chronically debilitating conditions affecting no more than 5 in 10,000 people in the European Union, and where no satisfactory treatment is available.
Orphan designation provides a 10-year period of marketing exclusivity if the drug receives regulatory approval.
The designation also provides incentives for companies seeking protocol assistance from the European Medicines Agency during the product development phase and direct access to the centralized authorization procedure. ![]()

The European Medicines Agency’s Committee for Orphan Medicinal Products (COMP) is recommending orphan designation for a cytomegalovirus-specific cytotoxic T-lymphocyte product (CMV-CTLs) intended to treat CMV infection in patients with impaired cell-mediated immunity.
The CMV-CTLs are designed to find and kill cells expressing CMV.
To create CMV-CTLs, T cells are collected from the blood of third-party donors and then exposed to CMV antigens.
The resulting activated T cells are then expanded, characterized, and stored for future use in a partially HLA-matched patient.
The CMV-CTLs are being developed by Atara Biotherapeutics, Inc.
The cells are currently under investigation in a pair of phase 2 trials (NCT01646645 and NCT02136797).
Results of a phase 1 trial (published in Biology of Blood and Marrow Transplantation in 2015) suggested CMV-CTLs are safe and can clear CMV infection in patients who have undergone allogeneic hematopoietic stem cell transplant.
The trial included 17 transplant recipients with CMV viremia or clinical infection that persisted despite prolonged treatment with antiviral drugs. Fourteen of the patients had received T-cell-depleted transplants without graft-versus-host disease (GVHD) prophylaxis.
Sixteen of the patients received CMV-CTLs created using cells derived from their transplant donor, and 1 patient received cells from a third-party donor.
Fifteen patients achieved clearance of CMV viremia, including 3 of the 5 patients with overt disease and the patient who received cells from a third-party donor.
In addition, the researchers said CMV-CTLs were well-tolerated. None of the patients experienced fever, alterations in vital signs, or other toxicities during the first 48 hours of observation.
None of the patients developed manifestations of de novo acute GVHD, and GHVD did not worsen in either of the 2 patients who had GVHD prior to infusion.
About orphan designation
The COMP adopts an opinion on the granting of orphan drug designation, and that opinion is submitted to the European Commission for a final decision.
Orphan designation provides regulatory and financial incentives for companies to develop and market therapies that treat life-threatening or chronically debilitating conditions affecting no more than 5 in 10,000 people in the European Union, and where no satisfactory treatment is available.
Orphan designation provides a 10-year period of marketing exclusivity if the drug receives regulatory approval.
The designation also provides incentives for companies seeking protocol assistance from the European Medicines Agency during the product development phase and direct access to the centralized authorization procedure. ![]()

The European Medicines Agency’s Committee for Orphan Medicinal Products (COMP) is recommending orphan designation for a cytomegalovirus-specific cytotoxic T-lymphocyte product (CMV-CTLs) intended to treat CMV infection in patients with impaired cell-mediated immunity.
The CMV-CTLs are designed to find and kill cells expressing CMV.
To create CMV-CTLs, T cells are collected from the blood of third-party donors and then exposed to CMV antigens.
The resulting activated T cells are then expanded, characterized, and stored for future use in a partially HLA-matched patient.
The CMV-CTLs are being developed by Atara Biotherapeutics, Inc.
The cells are currently under investigation in a pair of phase 2 trials (NCT01646645 and NCT02136797).
Results of a phase 1 trial (published in Biology of Blood and Marrow Transplantation in 2015) suggested CMV-CTLs are safe and can clear CMV infection in patients who have undergone allogeneic hematopoietic stem cell transplant.
The trial included 17 transplant recipients with CMV viremia or clinical infection that persisted despite prolonged treatment with antiviral drugs. Fourteen of the patients had received T-cell-depleted transplants without graft-versus-host disease (GVHD) prophylaxis.
Sixteen of the patients received CMV-CTLs created using cells derived from their transplant donor, and 1 patient received cells from a third-party donor.
Fifteen patients achieved clearance of CMV viremia, including 3 of the 5 patients with overt disease and the patient who received cells from a third-party donor.
In addition, the researchers said CMV-CTLs were well-tolerated. None of the patients experienced fever, alterations in vital signs, or other toxicities during the first 48 hours of observation.
None of the patients developed manifestations of de novo acute GVHD, and GHVD did not worsen in either of the 2 patients who had GVHD prior to infusion.
About orphan designation
The COMP adopts an opinion on the granting of orphan drug designation, and that opinion is submitted to the European Commission for a final decision.
Orphan designation provides regulatory and financial incentives for companies to develop and market therapies that treat life-threatening or chronically debilitating conditions affecting no more than 5 in 10,000 people in the European Union, and where no satisfactory treatment is available.
Orphan designation provides a 10-year period of marketing exclusivity if the drug receives regulatory approval.
The designation also provides incentives for companies seeking protocol assistance from the European Medicines Agency during the product development phase and direct access to the centralized authorization procedure. ![]()
Findings could aid treatment of resistant T-ALL
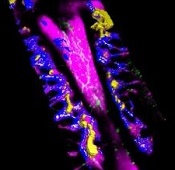
(blue and purple) invaded
by leukemia cells (yellow).
Image courtesy of Edwin
Hawkins, Delfim Duarte,
and Imperial College London
Preclinical research has shed light on how certain leukemia cells survive treatment and could pave the way for better therapeutic targeting of these resistant cells.
Researchers have speculated that some leukemia cells survive treatment by hiding out in specific niches in the bone marrow.
Results of the new research, conducted in mouse models and human samples of T-cell acute lymphoblastic leukemia (T-ALL), contradict that theory.
The experiments showed that resistant T-ALL cells move rapidly through the bone marrow before, during, and after treatment, interacting with—and sometimes killing—healthy cells.
“We expected the cells that survived treatment to be sat in particular niches, but, instead, they are very active throughout the bone marrow,” said Cristina Lo Celso, PhD, of Imperial College London in the UK.
“We now know that it would be ineffective to target particular niches in the bone marrow to tackle treatment-resistant leukemia. Now that we know that the cells don’t hide, we can explore why that is and how their movement helps them to survive. Ultimately, we want to find out whether we can stop the movement and whether this could kill the treatment-resistant cells.”
Dr Lo Celso and her colleagues described these findings in a letter to Nature.
The researchers used intravital microscopy to track the movement of T-ALL cells in mice—before, during, and after treatment. Treatment consisted of dexamethasone alone, vincristine alone, or combination dexamethasone, vincristine, and L-asparaginase.
The team found that T-ALL cells moved around rapidly, not showing any preference for bone marrow subcompartments. The cells’ behavior was consistent over time—from the earliest bone marrow seeding through to treatment response and resistance.
However, the researchers noted that surviving T-ALL cells were “highly migratory” and travelled at significantly faster speeds than early infiltrating cells. In addition, resistant T-ALL cells were still capable of undergoing division at times when other T-ALL cells were dying.
The team suggested that the act of moving may help T-ALL cells to survive, possibly through short-lived interactions with other cells.
This theory was supported by the discovery that T-ALL cells actively attack osteoblasts. The researchers noted that osteoblasts are associated with hematopoietic fitness. So the loss of osteoblasts may contribute to the loss of healthy hematopoiesis observed in leukemia patients.
The team believes this insight could aid the development of treatments to safeguard the production of healthy blood cells in T-ALL patients.
“Our study supports the idea that, at least in this leukemia, new therapies should target the cancer cells themselves instead of the surrounding normal stromal cells to better eradicate the disease,” said study author Delfim Duarte, MD, a PhD student at Imperial College London.
“Our work also suggests that protecting normal stromal bone cells from the attack of leukemia cells can have wide implications in the support of healthy blood cell production,” said Edwin Hawkins, PhD, of the Walter and Eliza Hall Institute of Medical Research in Melbourne, Victoria, Australia.
“Keeping blood cell levels up would prevent anemia, infection, and bleeding.” ![]()

(blue and purple) invaded
by leukemia cells (yellow).
Image courtesy of Edwin
Hawkins, Delfim Duarte,
and Imperial College London
Preclinical research has shed light on how certain leukemia cells survive treatment and could pave the way for better therapeutic targeting of these resistant cells.
Researchers have speculated that some leukemia cells survive treatment by hiding out in specific niches in the bone marrow.
Results of the new research, conducted in mouse models and human samples of T-cell acute lymphoblastic leukemia (T-ALL), contradict that theory.
The experiments showed that resistant T-ALL cells move rapidly through the bone marrow before, during, and after treatment, interacting with—and sometimes killing—healthy cells.
“We expected the cells that survived treatment to be sat in particular niches, but, instead, they are very active throughout the bone marrow,” said Cristina Lo Celso, PhD, of Imperial College London in the UK.
“We now know that it would be ineffective to target particular niches in the bone marrow to tackle treatment-resistant leukemia. Now that we know that the cells don’t hide, we can explore why that is and how their movement helps them to survive. Ultimately, we want to find out whether we can stop the movement and whether this could kill the treatment-resistant cells.”
Dr Lo Celso and her colleagues described these findings in a letter to Nature.
The researchers used intravital microscopy to track the movement of T-ALL cells in mice—before, during, and after treatment. Treatment consisted of dexamethasone alone, vincristine alone, or combination dexamethasone, vincristine, and L-asparaginase.
The team found that T-ALL cells moved around rapidly, not showing any preference for bone marrow subcompartments. The cells’ behavior was consistent over time—from the earliest bone marrow seeding through to treatment response and resistance.
However, the researchers noted that surviving T-ALL cells were “highly migratory” and travelled at significantly faster speeds than early infiltrating cells. In addition, resistant T-ALL cells were still capable of undergoing division at times when other T-ALL cells were dying.
The team suggested that the act of moving may help T-ALL cells to survive, possibly through short-lived interactions with other cells.
This theory was supported by the discovery that T-ALL cells actively attack osteoblasts. The researchers noted that osteoblasts are associated with hematopoietic fitness. So the loss of osteoblasts may contribute to the loss of healthy hematopoiesis observed in leukemia patients.
The team believes this insight could aid the development of treatments to safeguard the production of healthy blood cells in T-ALL patients.
“Our study supports the idea that, at least in this leukemia, new therapies should target the cancer cells themselves instead of the surrounding normal stromal cells to better eradicate the disease,” said study author Delfim Duarte, MD, a PhD student at Imperial College London.
“Our work also suggests that protecting normal stromal bone cells from the attack of leukemia cells can have wide implications in the support of healthy blood cell production,” said Edwin Hawkins, PhD, of the Walter and Eliza Hall Institute of Medical Research in Melbourne, Victoria, Australia.
“Keeping blood cell levels up would prevent anemia, infection, and bleeding.” ![]()

(blue and purple) invaded
by leukemia cells (yellow).
Image courtesy of Edwin
Hawkins, Delfim Duarte,
and Imperial College London
Preclinical research has shed light on how certain leukemia cells survive treatment and could pave the way for better therapeutic targeting of these resistant cells.
Researchers have speculated that some leukemia cells survive treatment by hiding out in specific niches in the bone marrow.
Results of the new research, conducted in mouse models and human samples of T-cell acute lymphoblastic leukemia (T-ALL), contradict that theory.
The experiments showed that resistant T-ALL cells move rapidly through the bone marrow before, during, and after treatment, interacting with—and sometimes killing—healthy cells.
“We expected the cells that survived treatment to be sat in particular niches, but, instead, they are very active throughout the bone marrow,” said Cristina Lo Celso, PhD, of Imperial College London in the UK.
“We now know that it would be ineffective to target particular niches in the bone marrow to tackle treatment-resistant leukemia. Now that we know that the cells don’t hide, we can explore why that is and how their movement helps them to survive. Ultimately, we want to find out whether we can stop the movement and whether this could kill the treatment-resistant cells.”
Dr Lo Celso and her colleagues described these findings in a letter to Nature.
The researchers used intravital microscopy to track the movement of T-ALL cells in mice—before, during, and after treatment. Treatment consisted of dexamethasone alone, vincristine alone, or combination dexamethasone, vincristine, and L-asparaginase.
The team found that T-ALL cells moved around rapidly, not showing any preference for bone marrow subcompartments. The cells’ behavior was consistent over time—from the earliest bone marrow seeding through to treatment response and resistance.
However, the researchers noted that surviving T-ALL cells were “highly migratory” and travelled at significantly faster speeds than early infiltrating cells. In addition, resistant T-ALL cells were still capable of undergoing division at times when other T-ALL cells were dying.
The team suggested that the act of moving may help T-ALL cells to survive, possibly through short-lived interactions with other cells.
This theory was supported by the discovery that T-ALL cells actively attack osteoblasts. The researchers noted that osteoblasts are associated with hematopoietic fitness. So the loss of osteoblasts may contribute to the loss of healthy hematopoiesis observed in leukemia patients.
The team believes this insight could aid the development of treatments to safeguard the production of healthy blood cells in T-ALL patients.
“Our study supports the idea that, at least in this leukemia, new therapies should target the cancer cells themselves instead of the surrounding normal stromal cells to better eradicate the disease,” said study author Delfim Duarte, MD, a PhD student at Imperial College London.
“Our work also suggests that protecting normal stromal bone cells from the attack of leukemia cells can have wide implications in the support of healthy blood cell production,” said Edwin Hawkins, PhD, of the Walter and Eliza Hall Institute of Medical Research in Melbourne, Victoria, Australia.
“Keeping blood cell levels up would prevent anemia, infection, and bleeding.” ![]()
The mesh tradeoff: Lower recurrence risk vs. complicaitons
Among patients undergoing elective incisional repair of abdominal wall hernias, the use of mesh reinforcement decreases the short-term recurrence rate by 5% but increases major complications by approximately the same amount over the subsequent 5 years, Dunja Kokotovic, MB, reported at the annual clinical congress of the American College of Surgeons.
“Given the continuously increasing incidence of mesh-related complications with time, it is expected that, with even longer follow-up up than the 5 years observed in this study, mesh-related complications continue to accrue,” said Dr. Kokotovic of the Center for Surgical Science, Zealand University Hospital, Koge, Denmark. The findings of this observational registry-based cohort study were presented at the congress and simultaneously published online in JAMA (2016 Oct 17. doi: 10.1001/jama.2016.15217).
These results highlight the need to assess the long-term safety of interventions before making definitive conclusions about their benefits and widely adopting them. In the United States, manufacturers are required to demonstrate the long-term safety of drugs but not of some devices, including hernia meshes. There were approximately 190,000 such hernia repairs performed in the United States alone during the most recent year for which data are available, and mesh is estimated to have been used in at least half, Dr. Kokotovic noted.
She and her associates analyzed the 5-year outcomes for virtually all incisional hernia repairs performed in Denmark from 2007 through 2010 using data in a mandatory national registry. Their analysis included 3,242 patients (mean age, 58 years): 1,119 (34.5%) who had open mesh repair, 1,757 (54.2%) who had laparoscopic mesh repair, and 366 (11.3%) who had nonmesh repair.
The cumulative risk of reoperation for hernia recurrence was significantly lower for patients who had open mesh repair (12.3%) or laparoscopic mesh repair (10.6%) than for those who had nonmesh repair (17.1%). However, this benefit was offset by the rate of major mesh-related complications requiring surgical intervention – including surgical site infection, formation of a chronic sinus tract, late-onset intra-abdominal abscess, enterocutaneous fistula, bowel obstruction, and bowel perforation – which progressively increased over time. The cumulative incidence of such complications was 5.6% for open mesh repair and 3.7% for laparoscopic mesh repair.
This study was limited in that it was observational rather than being based on randomized data, so selection bias and imbalances among the study groups couldn’t be fully controlled for. However, two strengths of this study were that it reflects the real-world experience of an entire country and all the surgeons performing hernia repairs there, and it had 100% follow-up, the researchers said.
This study was partly funded by the Edgar Schnohr and Wife Gilberte Schnohr’s Foundation supporting independent surgical and anesthesiological research. Dr. Kokotovic reported having no relevant financial disclosures; one investigator reported receiving personal fees from Bard and Etichon for educational presentations.
These study findings suggest that the risk-benefit ratio of mesh reinforcement is not as clear as previously thought and call into question the current widespread use of mesh, even for repair of small hernias, when mesh is the norm for all incisional hernia repairs of any size.
Although the study by Kokotovic and colleagues is one of the most comprehensive long-term studies in hernia surgery, many questions remain about the optimal approach for repairing ventral hernia. To provide more rigorous data to better understand optimal approaches to this common clinical problem, surgeons will need to design large multicenter pragmatic trials with long-term follow-up.
Kamal M. F. Itani, MD, is at the VA Boston Health Care System, Boston University, West Roxbury, Mass., and Harvard Medical School in Boston. He reported having served as a research consultant to Davol 4 years ago regarding an antibiotic-coated mesh product. These remarks are excerpted from an editorial accompanying Dr. Kokotovic’s report (JAMA 2016 Oct 17. doi: 10.1001/jama.2016.15722).
These study findings suggest that the risk-benefit ratio of mesh reinforcement is not as clear as previously thought and call into question the current widespread use of mesh, even for repair of small hernias, when mesh is the norm for all incisional hernia repairs of any size.
Although the study by Kokotovic and colleagues is one of the most comprehensive long-term studies in hernia surgery, many questions remain about the optimal approach for repairing ventral hernia. To provide more rigorous data to better understand optimal approaches to this common clinical problem, surgeons will need to design large multicenter pragmatic trials with long-term follow-up.
Kamal M. F. Itani, MD, is at the VA Boston Health Care System, Boston University, West Roxbury, Mass., and Harvard Medical School in Boston. He reported having served as a research consultant to Davol 4 years ago regarding an antibiotic-coated mesh product. These remarks are excerpted from an editorial accompanying Dr. Kokotovic’s report (JAMA 2016 Oct 17. doi: 10.1001/jama.2016.15722).
These study findings suggest that the risk-benefit ratio of mesh reinforcement is not as clear as previously thought and call into question the current widespread use of mesh, even for repair of small hernias, when mesh is the norm for all incisional hernia repairs of any size.
Although the study by Kokotovic and colleagues is one of the most comprehensive long-term studies in hernia surgery, many questions remain about the optimal approach for repairing ventral hernia. To provide more rigorous data to better understand optimal approaches to this common clinical problem, surgeons will need to design large multicenter pragmatic trials with long-term follow-up.
Kamal M. F. Itani, MD, is at the VA Boston Health Care System, Boston University, West Roxbury, Mass., and Harvard Medical School in Boston. He reported having served as a research consultant to Davol 4 years ago regarding an antibiotic-coated mesh product. These remarks are excerpted from an editorial accompanying Dr. Kokotovic’s report (JAMA 2016 Oct 17. doi: 10.1001/jama.2016.15722).
Among patients undergoing elective incisional repair of abdominal wall hernias, the use of mesh reinforcement decreases the short-term recurrence rate by 5% but increases major complications by approximately the same amount over the subsequent 5 years, Dunja Kokotovic, MB, reported at the annual clinical congress of the American College of Surgeons.
“Given the continuously increasing incidence of mesh-related complications with time, it is expected that, with even longer follow-up up than the 5 years observed in this study, mesh-related complications continue to accrue,” said Dr. Kokotovic of the Center for Surgical Science, Zealand University Hospital, Koge, Denmark. The findings of this observational registry-based cohort study were presented at the congress and simultaneously published online in JAMA (2016 Oct 17. doi: 10.1001/jama.2016.15217).
These results highlight the need to assess the long-term safety of interventions before making definitive conclusions about their benefits and widely adopting them. In the United States, manufacturers are required to demonstrate the long-term safety of drugs but not of some devices, including hernia meshes. There were approximately 190,000 such hernia repairs performed in the United States alone during the most recent year for which data are available, and mesh is estimated to have been used in at least half, Dr. Kokotovic noted.
She and her associates analyzed the 5-year outcomes for virtually all incisional hernia repairs performed in Denmark from 2007 through 2010 using data in a mandatory national registry. Their analysis included 3,242 patients (mean age, 58 years): 1,119 (34.5%) who had open mesh repair, 1,757 (54.2%) who had laparoscopic mesh repair, and 366 (11.3%) who had nonmesh repair.
The cumulative risk of reoperation for hernia recurrence was significantly lower for patients who had open mesh repair (12.3%) or laparoscopic mesh repair (10.6%) than for those who had nonmesh repair (17.1%). However, this benefit was offset by the rate of major mesh-related complications requiring surgical intervention – including surgical site infection, formation of a chronic sinus tract, late-onset intra-abdominal abscess, enterocutaneous fistula, bowel obstruction, and bowel perforation – which progressively increased over time. The cumulative incidence of such complications was 5.6% for open mesh repair and 3.7% for laparoscopic mesh repair.
This study was limited in that it was observational rather than being based on randomized data, so selection bias and imbalances among the study groups couldn’t be fully controlled for. However, two strengths of this study were that it reflects the real-world experience of an entire country and all the surgeons performing hernia repairs there, and it had 100% follow-up, the researchers said.
This study was partly funded by the Edgar Schnohr and Wife Gilberte Schnohr’s Foundation supporting independent surgical and anesthesiological research. Dr. Kokotovic reported having no relevant financial disclosures; one investigator reported receiving personal fees from Bard and Etichon for educational presentations.
Among patients undergoing elective incisional repair of abdominal wall hernias, the use of mesh reinforcement decreases the short-term recurrence rate by 5% but increases major complications by approximately the same amount over the subsequent 5 years, Dunja Kokotovic, MB, reported at the annual clinical congress of the American College of Surgeons.
“Given the continuously increasing incidence of mesh-related complications with time, it is expected that, with even longer follow-up up than the 5 years observed in this study, mesh-related complications continue to accrue,” said Dr. Kokotovic of the Center for Surgical Science, Zealand University Hospital, Koge, Denmark. The findings of this observational registry-based cohort study were presented at the congress and simultaneously published online in JAMA (2016 Oct 17. doi: 10.1001/jama.2016.15217).
These results highlight the need to assess the long-term safety of interventions before making definitive conclusions about their benefits and widely adopting them. In the United States, manufacturers are required to demonstrate the long-term safety of drugs but not of some devices, including hernia meshes. There were approximately 190,000 such hernia repairs performed in the United States alone during the most recent year for which data are available, and mesh is estimated to have been used in at least half, Dr. Kokotovic noted.
She and her associates analyzed the 5-year outcomes for virtually all incisional hernia repairs performed in Denmark from 2007 through 2010 using data in a mandatory national registry. Their analysis included 3,242 patients (mean age, 58 years): 1,119 (34.5%) who had open mesh repair, 1,757 (54.2%) who had laparoscopic mesh repair, and 366 (11.3%) who had nonmesh repair.
The cumulative risk of reoperation for hernia recurrence was significantly lower for patients who had open mesh repair (12.3%) or laparoscopic mesh repair (10.6%) than for those who had nonmesh repair (17.1%). However, this benefit was offset by the rate of major mesh-related complications requiring surgical intervention – including surgical site infection, formation of a chronic sinus tract, late-onset intra-abdominal abscess, enterocutaneous fistula, bowel obstruction, and bowel perforation – which progressively increased over time. The cumulative incidence of such complications was 5.6% for open mesh repair and 3.7% for laparoscopic mesh repair.
This study was limited in that it was observational rather than being based on randomized data, so selection bias and imbalances among the study groups couldn’t be fully controlled for. However, two strengths of this study were that it reflects the real-world experience of an entire country and all the surgeons performing hernia repairs there, and it had 100% follow-up, the researchers said.
This study was partly funded by the Edgar Schnohr and Wife Gilberte Schnohr’s Foundation supporting independent surgical and anesthesiological research. Dr. Kokotovic reported having no relevant financial disclosures; one investigator reported receiving personal fees from Bard and Etichon for educational presentations.
FROM THE ACS CLINICAL CONGRESS
Key clinical point: Among patients undergoing elective incisional repair of abdominal wall hernias, the use of mesh reinforcement decreases the short-term recurrence rate by 5% but increases major complications by 5.6% over the subsequent 5 years.
Major finding: The cumulative rate of major mesh-related complications requiring surgical intervention was 5.6% for open mesh repair and 3.7% for laparoscopic mesh repair, compared with 0% for nonmesh repair.
Data source: A nationwide observational registry-based cohort study involving virtually all incisional hernia repairs (3,242) performed in Denmark from 2007 through 2010.
Disclosures: This study was partly funded by the Edgar Schnohr and Wife Gilberte Schnohr’s Foundation supporting independent surgical and anesthesiological research. Dr. Kokotovic reported having no relevant financial disclosures; one investigator reported receiving personal fees from Bard and Etichon for educational presentations.
Hepatitis infection raises non-Hodgkin lymphoma risk in HIV patients
HIV-infected individuals on antiretroviral therapy who also have chronic coinfection with hepatitis B or C virus have an increased risk of developing non-Hodgkin lymphoma, according to new research published in Annals of Internal Medicine.
Lead author Qing Wang, PhD, of the Basel Institute for Clinical Epidemiology & Biostatistics at University Hospital Basel, Switzerland, and her coauthors said there is growing evidence of an association between both chronic hepatitis B virus infection (HBV) and chronic hepatitis C virus infection (HCV), and non-Hodgkin lymphoma, with chronic immune activation and B cell proliferation suggested as potential mechanisms. However, the impact of chronic coinfection in individuals with HIV is unclear.
Researchers undertook a cohort study of 52,479 treatment-naive individuals with HIV infection, using 18 of 33 cohorts from the Collaboration of Observational HIV Epidemiological Research Europe. Of these participants, 1,336 had chronic HBV and 7,506 had chronic HCV infection, and more than three-quarters (77%) later started treatment with antiretroviral therapy.
After 13 months of follow-up in the treatment-naive group and 50 months in the antiretroviral group, there were 252 cases of non-Hodgkin lymphoma in the treatment-naive group and 310 cases in the treated group (Ann Intern Med. 2016 Oct 17. doi: 10.7326/M16-0240).
Antiretroviral-treated patients with chronic hepatitis B showed a significant 74% greater risk (95% confidence interval, 1.08-2.82) and those with hepatitis C showed a 73% greater risk (95% CI, 1.21-2.46) of non-Hodgkin lymphoma compared to treated individuals with neither coinfection. However, the differences in non-Hodgkin lymphoma rates in treatment-naive HBV and HCV coinfected individuals were not significant, which the authors suggested could be due to lower numbers of events and limited follow-up.
“The median CD4 count at the time of NHL diagnosis was less than 0.250 x 109 cells/L in both ART-naive and treated patients coinfected with HBV and HCV, indicating that coinfected patients with NHL initiate ART late or have insufficient HIV viral control and immune recovery that may be due to multiple reasons,” the authors wrote. “This unfavorable constellation is aggravated by the fact that chronic HBV infection attenuates immune recovery in ART-treated patients; whether this is also the case for chronic HCV infection is less clear.”
The authors said routine screening for chronic HBV and HCV infection, in conjunction with early diagnosis and treatment of HIV infection, was essential to reduce morbidity and mortality from non-Hodgkin lymphoma.
“Our findings provide strong evidence that HCV coinfected patients with poor immune status or restoration (CD4 count lower than 0.250 x 109 cells/L) are at high risk for NHL and death and deserve high priority for access to well-tolerated, interferon-free, direct-acting antiviral treatment programs similar to those for patients with advanced liver fibrosis or cirrhosis.”
The study was supported by the European Union Seventh Framework Programme, Schweizerische Krebsliga, Agence Nationale de Recherches sur le SIDA et les Hepatites Virales (ANRS), Paris; the HIV Monitoring Foundation, Amsterdam; and the Augustinus Foundation, Copenhagen. Eleven authors declared grants, personal fees, and other support from pharmaceutical companies including those involved in the manufacture of HIV and hepatitis drugs. No other conflicts of interest were reported.
HIV-infected individuals on antiretroviral therapy who also have chronic coinfection with hepatitis B or C virus have an increased risk of developing non-Hodgkin lymphoma, according to new research published in Annals of Internal Medicine.
Lead author Qing Wang, PhD, of the Basel Institute for Clinical Epidemiology & Biostatistics at University Hospital Basel, Switzerland, and her coauthors said there is growing evidence of an association between both chronic hepatitis B virus infection (HBV) and chronic hepatitis C virus infection (HCV), and non-Hodgkin lymphoma, with chronic immune activation and B cell proliferation suggested as potential mechanisms. However, the impact of chronic coinfection in individuals with HIV is unclear.
Researchers undertook a cohort study of 52,479 treatment-naive individuals with HIV infection, using 18 of 33 cohorts from the Collaboration of Observational HIV Epidemiological Research Europe. Of these participants, 1,336 had chronic HBV and 7,506 had chronic HCV infection, and more than three-quarters (77%) later started treatment with antiretroviral therapy.
After 13 months of follow-up in the treatment-naive group and 50 months in the antiretroviral group, there were 252 cases of non-Hodgkin lymphoma in the treatment-naive group and 310 cases in the treated group (Ann Intern Med. 2016 Oct 17. doi: 10.7326/M16-0240).
Antiretroviral-treated patients with chronic hepatitis B showed a significant 74% greater risk (95% confidence interval, 1.08-2.82) and those with hepatitis C showed a 73% greater risk (95% CI, 1.21-2.46) of non-Hodgkin lymphoma compared to treated individuals with neither coinfection. However, the differences in non-Hodgkin lymphoma rates in treatment-naive HBV and HCV coinfected individuals were not significant, which the authors suggested could be due to lower numbers of events and limited follow-up.
“The median CD4 count at the time of NHL diagnosis was less than 0.250 x 109 cells/L in both ART-naive and treated patients coinfected with HBV and HCV, indicating that coinfected patients with NHL initiate ART late or have insufficient HIV viral control and immune recovery that may be due to multiple reasons,” the authors wrote. “This unfavorable constellation is aggravated by the fact that chronic HBV infection attenuates immune recovery in ART-treated patients; whether this is also the case for chronic HCV infection is less clear.”
The authors said routine screening for chronic HBV and HCV infection, in conjunction with early diagnosis and treatment of HIV infection, was essential to reduce morbidity and mortality from non-Hodgkin lymphoma.
“Our findings provide strong evidence that HCV coinfected patients with poor immune status or restoration (CD4 count lower than 0.250 x 109 cells/L) are at high risk for NHL and death and deserve high priority for access to well-tolerated, interferon-free, direct-acting antiviral treatment programs similar to those for patients with advanced liver fibrosis or cirrhosis.”
The study was supported by the European Union Seventh Framework Programme, Schweizerische Krebsliga, Agence Nationale de Recherches sur le SIDA et les Hepatites Virales (ANRS), Paris; the HIV Monitoring Foundation, Amsterdam; and the Augustinus Foundation, Copenhagen. Eleven authors declared grants, personal fees, and other support from pharmaceutical companies including those involved in the manufacture of HIV and hepatitis drugs. No other conflicts of interest were reported.
HIV-infected individuals on antiretroviral therapy who also have chronic coinfection with hepatitis B or C virus have an increased risk of developing non-Hodgkin lymphoma, according to new research published in Annals of Internal Medicine.
Lead author Qing Wang, PhD, of the Basel Institute for Clinical Epidemiology & Biostatistics at University Hospital Basel, Switzerland, and her coauthors said there is growing evidence of an association between both chronic hepatitis B virus infection (HBV) and chronic hepatitis C virus infection (HCV), and non-Hodgkin lymphoma, with chronic immune activation and B cell proliferation suggested as potential mechanisms. However, the impact of chronic coinfection in individuals with HIV is unclear.
Researchers undertook a cohort study of 52,479 treatment-naive individuals with HIV infection, using 18 of 33 cohorts from the Collaboration of Observational HIV Epidemiological Research Europe. Of these participants, 1,336 had chronic HBV and 7,506 had chronic HCV infection, and more than three-quarters (77%) later started treatment with antiretroviral therapy.
After 13 months of follow-up in the treatment-naive group and 50 months in the antiretroviral group, there were 252 cases of non-Hodgkin lymphoma in the treatment-naive group and 310 cases in the treated group (Ann Intern Med. 2016 Oct 17. doi: 10.7326/M16-0240).
Antiretroviral-treated patients with chronic hepatitis B showed a significant 74% greater risk (95% confidence interval, 1.08-2.82) and those with hepatitis C showed a 73% greater risk (95% CI, 1.21-2.46) of non-Hodgkin lymphoma compared to treated individuals with neither coinfection. However, the differences in non-Hodgkin lymphoma rates in treatment-naive HBV and HCV coinfected individuals were not significant, which the authors suggested could be due to lower numbers of events and limited follow-up.
“The median CD4 count at the time of NHL diagnosis was less than 0.250 x 109 cells/L in both ART-naive and treated patients coinfected with HBV and HCV, indicating that coinfected patients with NHL initiate ART late or have insufficient HIV viral control and immune recovery that may be due to multiple reasons,” the authors wrote. “This unfavorable constellation is aggravated by the fact that chronic HBV infection attenuates immune recovery in ART-treated patients; whether this is also the case for chronic HCV infection is less clear.”
The authors said routine screening for chronic HBV and HCV infection, in conjunction with early diagnosis and treatment of HIV infection, was essential to reduce morbidity and mortality from non-Hodgkin lymphoma.
“Our findings provide strong evidence that HCV coinfected patients with poor immune status or restoration (CD4 count lower than 0.250 x 109 cells/L) are at high risk for NHL and death and deserve high priority for access to well-tolerated, interferon-free, direct-acting antiviral treatment programs similar to those for patients with advanced liver fibrosis or cirrhosis.”
The study was supported by the European Union Seventh Framework Programme, Schweizerische Krebsliga, Agence Nationale de Recherches sur le SIDA et les Hepatites Virales (ANRS), Paris; the HIV Monitoring Foundation, Amsterdam; and the Augustinus Foundation, Copenhagen. Eleven authors declared grants, personal fees, and other support from pharmaceutical companies including those involved in the manufacture of HIV and hepatitis drugs. No other conflicts of interest were reported.
FROM ANNALS OF INTERNAL MEDICINE
Key clinical point: HIV-infected individuals on antiretroviral therapy who also have chronic coinfection with hepatitis B or C virus have an increased risk of developing non-Hodgkin lymphoma.
Major finding: Antiretroviral-treated patients with chronic hepatitis B showed a significant 74% greater risk and those with hepatitis C showed a 73% greater risk of non-Hodgkin lymphoma, compared to treated individuals with neither coinfection.
Data source: A cohort study of 52,479 treatment-naive individuals with HIV infection, using 18 of 33 cohorts from the Collaboration of Observational HIV Epidemiological Research Europe.
Disclosures: The study was supported by the European Union Seventh Framework Programme, Schweizerische Krebsliga, Agence Nationale de Recherches sur le SIDA et les Hepatites Virales (ANRS), Paris; the HIV Monitoring Foundation, Amsterdam; and the Augustinus Foundation, Copenhagen. Eleven authors declared grants, personal fees, and other support from pharmaceutical companies including those involved in the manufacture of HIV and hepatitis drugs. No other conflicts of interest were reported.
Reslizumab performance tied to eosinophil count
Reslizumab was most effective in patients with high baseline eosinophil counts, two randomized, placebo-controlled studies have determined. The companion studies were simultaneously published in the October issue of Chest.
The drug reslizumab, an anti–interleukin-5 monoclonal antibody, is made by Teva Branded Pharmaceutical Products R&D, who sponsored both studies.
The larger study, comprising 492 patients, found no significant benefit of reslizumab over placebo, Jonathan Corren, MD, and his colleagues wrote (Chest. 2016;150:799-810). But this study didn’t stratify patients by baseline eosinophil levels; a post-hoc subanalysis found a significant benefit in forced expiratory volume in 69 of the patients who had at least 400 eosinophils/microliter (mcL) when treatment began.
In these patients, the drug significantly improved not only lung function, but asthma symptoms and asthma-related quality of life scores.
“These efficacy findings are consistent with results from other reslizumab trials and combined with the favorable safety profile observed, support the use of reslizumab in patients with asthma and elevated blood eosinophils, uncontrolled by an inhaled corticosteroid-based regimen,” Dr. Bjermer and his coauthors wrote.
The unstratified trial was conducted at 66 sites in the U.S. All of the patients had poorly controlled asthma despite using at least a medium-dosed inhaled corticosteroid. They were randomized to infusions of reslizumab 3.0 mg/kg or placebo given once every 4 weeks for 16 weeks.*
The primary endpoint was the change in forced expiratory volume in one second (FEV1); secondary endpoints included quality of life scores; the need for rescue medication; forced vital capacity; and eosinophil count.
Patients in the placebo and reslizumab groups were an average age of 45.1 years and 44.9 years, respectively. The mean disease duration of patients in both groups was 26 years.
At week 16, the mean change in FEV1 from baseline was 255 ml in the active group and 187 ml in the placebo group – not a significant difference.
The team performed a post-hoc subgroup analysis that dichotomized the cohort based on baseline eosinophil levels. For the 343 with counts of less than 400 cells/mcL, there was no difference in FEV1 at 16 weeks, between the patients who received treatment and the patients who received a placebo. The FEV1s of these two groups were separated by just 33 mL.
The story was different for the 82 patients with at least 400 cells/mcL, with 69 of such patients receiving the drug and 13 of such patients receiving the placebo. At 16 weeks, the difference in FEV1 change was 270 mL, in favor of the active group. The strength of these findings may be weakened by the large difference in size between the treatment and placebo groups and the “near complete lack of response in the small number of placebo-treated patients.”
“Interpretation of the results in the [400 or more cells/mcL] subgroup is limited as the study was not designed or statistically powered to specifically test this group of patients,” the team wrote. Nevertheless, they concluded that reslizumab is a reasonable treatment option for this group. “These findings support an acceptable benefit-risk profile for reslizumab in asthma patients with a blood eosinophil threshold” of at least 400 cells/mcL.
The stratified study examined more endpoints: pre-bronchodilator spirometry (forced expiratory volume in one second FEV1, forced vital capacity, and forced expiratory flow), asthma symptoms, quality of life, rescue inhaler use, and blood eosinophil levels.
The 315 patients in this study all had a baseline eosinophil count of at least 400 cells/mcL. They were randomized to placebo or to 0.3 or 3.0 mg/kg reslizumab dose once every 4 weeks for 16 weeks. The mean ages for the patients taking the placebo, reslizumab 0.3 mg/kg, and reslizumab 3.0 mg/kg were 44.2, 44.5, and 43.0, respectively. The range of average disease durations for patients in the placebo group and two reslizumab groups was 20 to 20.7 years.
The final FEV1 was significantly improved over placebo in both active groups, although the change was much more pronounced in those taking 3.0 mg/kg, compared with those taking 0.3 mg/kg (160 mL and 115 mL, respectively, relative to placebo). Forced vital capacity also improved significantly in the 3.0 mg/kg dose group (130 mL relative to placebo).
Reslizumab was generally well tolerated in both studies. The most frequent adverse events in the stratified study were asthma worsening, headache, nasopharyngitis, upper respiratory infections, and sinusitis.
In the unstratified study, there were two anaphylactic reactions, but only one was related to the study drug. No deaths occurred in either treatment group of this study.
Both trials were sponsored by Teva. Dr. Bjermer has served on advisory boards or provided lectures for Aerocrine, Airsonett, ALK, Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, Genentech, GlaxoSmithKline, Meda, Mundipharma, Nigaard, Novartis, Regeneron, Sanofi-Aventis, Takeda, and Teva. Dr. Corren has been involved in speaker bureau activities for Genentech and Merck; he has served on advisory boards for Genentech, Merck, Novartis, and Vectura.
*CORRECTION 12/9/16: An earlier version of this article misstated the infusion frequency.
msullivan@frontlinemedcom.com
On Twitter @Alz_Gal
Persistent eosinophilic inflammation is present in about half of patients with severe asthma, and the studies by Corren and Bjermer represent important advances in learning how to target this inflammatory pathway, Richard Russell, MBBS, MRCP, and Christopher Brightling, PhD, FCCP, wrote in an accompanying editorial (Chest. 2016;150:766-8).
“The most advanced therapeutic target is IL-5, which is an attractive target because it is an obligate cytokine for eosinophil maturation and survival. Its inhibition is thus predicted to reduce bone marrow production of eosinophils and promote apoptosis,” wrote Dr. Russell, a clinical research fellow, and Dr. Brightling, a professor, both at the University of Leicester (England).
“These findings support the view that an elevated blood eosinophil count is associated with a good clinical response [to the antibody reslizumab] but [the studies] did not find a clear correlation between the intensity of the baseline eosinophil count and response,” the colleagues wrote. “Thus, the best cut-off for the blood eosinophil count to apply clinically remains uncertain.”
Clinicians and patients may find a different answer to this riddle than do payers.
“From a patient perspective there is an argument to select a low or no cut-off as there is some benefit even with low baseline eosinophil counts, whereas from a payer’s perspective the health economic benefit is better with a higher cut-off.”
As is often the case, more study will help clarify these new concerns.
“We are moving into a new era of Type-2 immunity-mediated therapies that will bring new opportunities for clinicians and our patients, but with this opportunity comes new challenges. Biomarkers will increase in importance to help drive precision medicine, but we need to understand how to use them, and, in particular, what cut-points to apply. For anti-IL-5 approaches, we probably need to look towards elevated blood eosinophil counts, as aiming for a high cut-off is most likely the best way we shall achieve success.”
Dr. Russell had no financial disclosures. Mr. Brightling reported financial relationships with several pharmaceutical companies, but not with Teva.
Persistent eosinophilic inflammation is present in about half of patients with severe asthma, and the studies by Corren and Bjermer represent important advances in learning how to target this inflammatory pathway, Richard Russell, MBBS, MRCP, and Christopher Brightling, PhD, FCCP, wrote in an accompanying editorial (Chest. 2016;150:766-8).
“The most advanced therapeutic target is IL-5, which is an attractive target because it is an obligate cytokine for eosinophil maturation and survival. Its inhibition is thus predicted to reduce bone marrow production of eosinophils and promote apoptosis,” wrote Dr. Russell, a clinical research fellow, and Dr. Brightling, a professor, both at the University of Leicester (England).
“These findings support the view that an elevated blood eosinophil count is associated with a good clinical response [to the antibody reslizumab] but [the studies] did not find a clear correlation between the intensity of the baseline eosinophil count and response,” the colleagues wrote. “Thus, the best cut-off for the blood eosinophil count to apply clinically remains uncertain.”
Clinicians and patients may find a different answer to this riddle than do payers.
“From a patient perspective there is an argument to select a low or no cut-off as there is some benefit even with low baseline eosinophil counts, whereas from a payer’s perspective the health economic benefit is better with a higher cut-off.”
As is often the case, more study will help clarify these new concerns.
“We are moving into a new era of Type-2 immunity-mediated therapies that will bring new opportunities for clinicians and our patients, but with this opportunity comes new challenges. Biomarkers will increase in importance to help drive precision medicine, but we need to understand how to use them, and, in particular, what cut-points to apply. For anti-IL-5 approaches, we probably need to look towards elevated blood eosinophil counts, as aiming for a high cut-off is most likely the best way we shall achieve success.”
Dr. Russell had no financial disclosures. Mr. Brightling reported financial relationships with several pharmaceutical companies, but not with Teva.
Persistent eosinophilic inflammation is present in about half of patients with severe asthma, and the studies by Corren and Bjermer represent important advances in learning how to target this inflammatory pathway, Richard Russell, MBBS, MRCP, and Christopher Brightling, PhD, FCCP, wrote in an accompanying editorial (Chest. 2016;150:766-8).
“The most advanced therapeutic target is IL-5, which is an attractive target because it is an obligate cytokine for eosinophil maturation and survival. Its inhibition is thus predicted to reduce bone marrow production of eosinophils and promote apoptosis,” wrote Dr. Russell, a clinical research fellow, and Dr. Brightling, a professor, both at the University of Leicester (England).
“These findings support the view that an elevated blood eosinophil count is associated with a good clinical response [to the antibody reslizumab] but [the studies] did not find a clear correlation between the intensity of the baseline eosinophil count and response,” the colleagues wrote. “Thus, the best cut-off for the blood eosinophil count to apply clinically remains uncertain.”
Clinicians and patients may find a different answer to this riddle than do payers.
“From a patient perspective there is an argument to select a low or no cut-off as there is some benefit even with low baseline eosinophil counts, whereas from a payer’s perspective the health economic benefit is better with a higher cut-off.”
As is often the case, more study will help clarify these new concerns.
“We are moving into a new era of Type-2 immunity-mediated therapies that will bring new opportunities for clinicians and our patients, but with this opportunity comes new challenges. Biomarkers will increase in importance to help drive precision medicine, but we need to understand how to use them, and, in particular, what cut-points to apply. For anti-IL-5 approaches, we probably need to look towards elevated blood eosinophil counts, as aiming for a high cut-off is most likely the best way we shall achieve success.”
Dr. Russell had no financial disclosures. Mr. Brightling reported financial relationships with several pharmaceutical companies, but not with Teva.
Reslizumab was most effective in patients with high baseline eosinophil counts, two randomized, placebo-controlled studies have determined. The companion studies were simultaneously published in the October issue of Chest.
The drug reslizumab, an anti–interleukin-5 monoclonal antibody, is made by Teva Branded Pharmaceutical Products R&D, who sponsored both studies.
The larger study, comprising 492 patients, found no significant benefit of reslizumab over placebo, Jonathan Corren, MD, and his colleagues wrote (Chest. 2016;150:799-810). But this study didn’t stratify patients by baseline eosinophil levels; a post-hoc subanalysis found a significant benefit in forced expiratory volume in 69 of the patients who had at least 400 eosinophils/microliter (mcL) when treatment began.
In these patients, the drug significantly improved not only lung function, but asthma symptoms and asthma-related quality of life scores.
“These efficacy findings are consistent with results from other reslizumab trials and combined with the favorable safety profile observed, support the use of reslizumab in patients with asthma and elevated blood eosinophils, uncontrolled by an inhaled corticosteroid-based regimen,” Dr. Bjermer and his coauthors wrote.
The unstratified trial was conducted at 66 sites in the U.S. All of the patients had poorly controlled asthma despite using at least a medium-dosed inhaled corticosteroid. They were randomized to infusions of reslizumab 3.0 mg/kg or placebo given once every 4 weeks for 16 weeks.*
The primary endpoint was the change in forced expiratory volume in one second (FEV1); secondary endpoints included quality of life scores; the need for rescue medication; forced vital capacity; and eosinophil count.
Patients in the placebo and reslizumab groups were an average age of 45.1 years and 44.9 years, respectively. The mean disease duration of patients in both groups was 26 years.
At week 16, the mean change in FEV1 from baseline was 255 ml in the active group and 187 ml in the placebo group – not a significant difference.
The team performed a post-hoc subgroup analysis that dichotomized the cohort based on baseline eosinophil levels. For the 343 with counts of less than 400 cells/mcL, there was no difference in FEV1 at 16 weeks, between the patients who received treatment and the patients who received a placebo. The FEV1s of these two groups were separated by just 33 mL.
The story was different for the 82 patients with at least 400 cells/mcL, with 69 of such patients receiving the drug and 13 of such patients receiving the placebo. At 16 weeks, the difference in FEV1 change was 270 mL, in favor of the active group. The strength of these findings may be weakened by the large difference in size between the treatment and placebo groups and the “near complete lack of response in the small number of placebo-treated patients.”
“Interpretation of the results in the [400 or more cells/mcL] subgroup is limited as the study was not designed or statistically powered to specifically test this group of patients,” the team wrote. Nevertheless, they concluded that reslizumab is a reasonable treatment option for this group. “These findings support an acceptable benefit-risk profile for reslizumab in asthma patients with a blood eosinophil threshold” of at least 400 cells/mcL.
The stratified study examined more endpoints: pre-bronchodilator spirometry (forced expiratory volume in one second FEV1, forced vital capacity, and forced expiratory flow), asthma symptoms, quality of life, rescue inhaler use, and blood eosinophil levels.
The 315 patients in this study all had a baseline eosinophil count of at least 400 cells/mcL. They were randomized to placebo or to 0.3 or 3.0 mg/kg reslizumab dose once every 4 weeks for 16 weeks. The mean ages for the patients taking the placebo, reslizumab 0.3 mg/kg, and reslizumab 3.0 mg/kg were 44.2, 44.5, and 43.0, respectively. The range of average disease durations for patients in the placebo group and two reslizumab groups was 20 to 20.7 years.
The final FEV1 was significantly improved over placebo in both active groups, although the change was much more pronounced in those taking 3.0 mg/kg, compared with those taking 0.3 mg/kg (160 mL and 115 mL, respectively, relative to placebo). Forced vital capacity also improved significantly in the 3.0 mg/kg dose group (130 mL relative to placebo).
Reslizumab was generally well tolerated in both studies. The most frequent adverse events in the stratified study were asthma worsening, headache, nasopharyngitis, upper respiratory infections, and sinusitis.
In the unstratified study, there were two anaphylactic reactions, but only one was related to the study drug. No deaths occurred in either treatment group of this study.
Both trials were sponsored by Teva. Dr. Bjermer has served on advisory boards or provided lectures for Aerocrine, Airsonett, ALK, Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, Genentech, GlaxoSmithKline, Meda, Mundipharma, Nigaard, Novartis, Regeneron, Sanofi-Aventis, Takeda, and Teva. Dr. Corren has been involved in speaker bureau activities for Genentech and Merck; he has served on advisory boards for Genentech, Merck, Novartis, and Vectura.
*CORRECTION 12/9/16: An earlier version of this article misstated the infusion frequency.
msullivan@frontlinemedcom.com
On Twitter @Alz_Gal
Reslizumab was most effective in patients with high baseline eosinophil counts, two randomized, placebo-controlled studies have determined. The companion studies were simultaneously published in the October issue of Chest.
The drug reslizumab, an anti–interleukin-5 monoclonal antibody, is made by Teva Branded Pharmaceutical Products R&D, who sponsored both studies.
The larger study, comprising 492 patients, found no significant benefit of reslizumab over placebo, Jonathan Corren, MD, and his colleagues wrote (Chest. 2016;150:799-810). But this study didn’t stratify patients by baseline eosinophil levels; a post-hoc subanalysis found a significant benefit in forced expiratory volume in 69 of the patients who had at least 400 eosinophils/microliter (mcL) when treatment began.
In these patients, the drug significantly improved not only lung function, but asthma symptoms and asthma-related quality of life scores.
“These efficacy findings are consistent with results from other reslizumab trials and combined with the favorable safety profile observed, support the use of reslizumab in patients with asthma and elevated blood eosinophils, uncontrolled by an inhaled corticosteroid-based regimen,” Dr. Bjermer and his coauthors wrote.
The unstratified trial was conducted at 66 sites in the U.S. All of the patients had poorly controlled asthma despite using at least a medium-dosed inhaled corticosteroid. They were randomized to infusions of reslizumab 3.0 mg/kg or placebo given once every 4 weeks for 16 weeks.*
The primary endpoint was the change in forced expiratory volume in one second (FEV1); secondary endpoints included quality of life scores; the need for rescue medication; forced vital capacity; and eosinophil count.
Patients in the placebo and reslizumab groups were an average age of 45.1 years and 44.9 years, respectively. The mean disease duration of patients in both groups was 26 years.
At week 16, the mean change in FEV1 from baseline was 255 ml in the active group and 187 ml in the placebo group – not a significant difference.
The team performed a post-hoc subgroup analysis that dichotomized the cohort based on baseline eosinophil levels. For the 343 with counts of less than 400 cells/mcL, there was no difference in FEV1 at 16 weeks, between the patients who received treatment and the patients who received a placebo. The FEV1s of these two groups were separated by just 33 mL.
The story was different for the 82 patients with at least 400 cells/mcL, with 69 of such patients receiving the drug and 13 of such patients receiving the placebo. At 16 weeks, the difference in FEV1 change was 270 mL, in favor of the active group. The strength of these findings may be weakened by the large difference in size between the treatment and placebo groups and the “near complete lack of response in the small number of placebo-treated patients.”
“Interpretation of the results in the [400 or more cells/mcL] subgroup is limited as the study was not designed or statistically powered to specifically test this group of patients,” the team wrote. Nevertheless, they concluded that reslizumab is a reasonable treatment option for this group. “These findings support an acceptable benefit-risk profile for reslizumab in asthma patients with a blood eosinophil threshold” of at least 400 cells/mcL.
The stratified study examined more endpoints: pre-bronchodilator spirometry (forced expiratory volume in one second FEV1, forced vital capacity, and forced expiratory flow), asthma symptoms, quality of life, rescue inhaler use, and blood eosinophil levels.
The 315 patients in this study all had a baseline eosinophil count of at least 400 cells/mcL. They were randomized to placebo or to 0.3 or 3.0 mg/kg reslizumab dose once every 4 weeks for 16 weeks. The mean ages for the patients taking the placebo, reslizumab 0.3 mg/kg, and reslizumab 3.0 mg/kg were 44.2, 44.5, and 43.0, respectively. The range of average disease durations for patients in the placebo group and two reslizumab groups was 20 to 20.7 years.
The final FEV1 was significantly improved over placebo in both active groups, although the change was much more pronounced in those taking 3.0 mg/kg, compared with those taking 0.3 mg/kg (160 mL and 115 mL, respectively, relative to placebo). Forced vital capacity also improved significantly in the 3.0 mg/kg dose group (130 mL relative to placebo).
Reslizumab was generally well tolerated in both studies. The most frequent adverse events in the stratified study were asthma worsening, headache, nasopharyngitis, upper respiratory infections, and sinusitis.
In the unstratified study, there were two anaphylactic reactions, but only one was related to the study drug. No deaths occurred in either treatment group of this study.
Both trials were sponsored by Teva. Dr. Bjermer has served on advisory boards or provided lectures for Aerocrine, Airsonett, ALK, Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, Genentech, GlaxoSmithKline, Meda, Mundipharma, Nigaard, Novartis, Regeneron, Sanofi-Aventis, Takeda, and Teva. Dr. Corren has been involved in speaker bureau activities for Genentech and Merck; he has served on advisory boards for Genentech, Merck, Novartis, and Vectura.
*CORRECTION 12/9/16: An earlier version of this article misstated the infusion frequency.
msullivan@frontlinemedcom.com
On Twitter @Alz_Gal
FROM CHEST
Key clinical point:
Major finding: Forced expiratory volume in 1 second (FEV1) improved by 160 mL with reslizumab 3.0 mg/kg relative to placebo.
Data source: The two randomized, placebo-controlled studies comprised about 800 patients.
Disclosures: Both trials were sponsored by Teva Branded Pharmaceutical Products R&D. Dr. Bjermer has served on an advisory board or provided lectures for Teva. Dr. Corren has served on advisory boards and has been involved in speaker bureau activities for various pharmaceutical companies.
Pembrolizumab ‘new standard of care’ in advanced PD-L1-rich NSCLC
COPENHAGEN – Chalk up another one for immunotherapy: the PD-1 checkpoint inhibitor pembrolizumab cut the risk of disease progression or death in half among select patients with non–small cell lung cancer (NSCLC), compared with standard platinum doublet chemotherapy, in the first-line setting.
Among 305 patients with non–small cell lung cancers with 50% or greater expression of the programmed death ligand 1 (PD-L1), median progression-free survival (PFS) for patients treated with pembrolizumab (Keytruda) was 10.3 months, compared with 6 months for patients assigned to receive platinum-based chemotherapy at the investigators discretion (hazard ratio, 0.50, P less than .001), reported Martin Reck, MD, from the department of thoracic oncology at the Lung Clinic Grosshansdorf, in Germany.
Results of the KEYNOTE-024 study were also published online in the New England Journal of Medicine (2016 Oct 9. doi: 10.1056/NEJMoa1606774). Approximately 23%-30% of patients with advanced non–small cell lung cancers have tumors that express PD-L1 on the membrane of at least 50% of tumor cells, making them attractive targets for pembrolizumab, which is a monoclonal antibody directed against programmed death 1 (PD-1). Pembrolizumab disengages the brake on the immune system caused by the interaction of receptor PD-1 with the PD-L1 and PD-L2 ligands.
The study was conducted to compare upfront pembrolizumab with platinum-based chemotherapy in patients with newly diagnosed advanced NSCLC that did not carry targetable EGFR-activating mutations or ALK translocations.
A total of 305 patients from 16 countries with untreated stage IV NSCLC, good performance status, and tumors with a 50% or greater expression of PD-L1 were enrolled and randomized to either pembrolizumab 200 mg intravenously every 3 weeks for up to 2 years. Or four to six cycles of platinum-doublet chemotherapy at the investigator’s discretion. The combinations included carboplatin plus pemetrexed, cisplatin plus pemetrexed, carboplatin plus gemcitabine, cisplatin plus gemcitabine, or carboplatin plus paclitaxel.
At a median follow-up of 11.2 months, 48.1% of patients assigned to pembrolizumab were still on treatment, as were 10% of those assigned to standard chemotherapy.
As noted before, PFS, the primary endpoint, was significantly better with pembrolizumab, as was the secondary endpoint of overall survival at 6 months. In all, 80% of patients treated with pembrolizumab were still alive at 6 months, compared with 72% of patients on chemotherapy (HR, 0.60; P = .005).
The confirmed response rate was also higher in the pembrolizumab arm, at 44.8% vs. 27.8%(P = .0011), and the median duration of response was longer (not reached vs. 6.3 months). There were six complete responses in the pembrolizumab arm.
Pembrolizumab also demonstrated a generally more favorable safety profile, with adverse events of any grade occurring in 73.4% of patients, compared with 90% of those treated with chemotherapy.
Grade 3 or 4 adverse events and treatment-related deaths were also lower in the pembrolizumab arm, at 26.6% vs. 53.3%.
Jean-Charles Soria, MD, chair of drug development at Gustave Roussy Cancer Center in Paris, the invited discussant, noted that the “45% objective response rate in first-line non–small cell lung cancer is unheard of, and is achieved with a monotherapy.”
“Pembrolizumab clearly leads to a higher objective response, a longer duration of response, a lower frequency of adverse events, better PFS, better OS, compared to chemotherapy.”
“We have, probably, a new standard of care” for patients with high PD-L1 expression and no targetable mutations,” he said.
COPENHAGEN – Chalk up another one for immunotherapy: the PD-1 checkpoint inhibitor pembrolizumab cut the risk of disease progression or death in half among select patients with non–small cell lung cancer (NSCLC), compared with standard platinum doublet chemotherapy, in the first-line setting.
Among 305 patients with non–small cell lung cancers with 50% or greater expression of the programmed death ligand 1 (PD-L1), median progression-free survival (PFS) for patients treated with pembrolizumab (Keytruda) was 10.3 months, compared with 6 months for patients assigned to receive platinum-based chemotherapy at the investigators discretion (hazard ratio, 0.50, P less than .001), reported Martin Reck, MD, from the department of thoracic oncology at the Lung Clinic Grosshansdorf, in Germany.
Results of the KEYNOTE-024 study were also published online in the New England Journal of Medicine (2016 Oct 9. doi: 10.1056/NEJMoa1606774). Approximately 23%-30% of patients with advanced non–small cell lung cancers have tumors that express PD-L1 on the membrane of at least 50% of tumor cells, making them attractive targets for pembrolizumab, which is a monoclonal antibody directed against programmed death 1 (PD-1). Pembrolizumab disengages the brake on the immune system caused by the interaction of receptor PD-1 with the PD-L1 and PD-L2 ligands.
The study was conducted to compare upfront pembrolizumab with platinum-based chemotherapy in patients with newly diagnosed advanced NSCLC that did not carry targetable EGFR-activating mutations or ALK translocations.
A total of 305 patients from 16 countries with untreated stage IV NSCLC, good performance status, and tumors with a 50% or greater expression of PD-L1 were enrolled and randomized to either pembrolizumab 200 mg intravenously every 3 weeks for up to 2 years. Or four to six cycles of platinum-doublet chemotherapy at the investigator’s discretion. The combinations included carboplatin plus pemetrexed, cisplatin plus pemetrexed, carboplatin plus gemcitabine, cisplatin plus gemcitabine, or carboplatin plus paclitaxel.
At a median follow-up of 11.2 months, 48.1% of patients assigned to pembrolizumab were still on treatment, as were 10% of those assigned to standard chemotherapy.
As noted before, PFS, the primary endpoint, was significantly better with pembrolizumab, as was the secondary endpoint of overall survival at 6 months. In all, 80% of patients treated with pembrolizumab were still alive at 6 months, compared with 72% of patients on chemotherapy (HR, 0.60; P = .005).
The confirmed response rate was also higher in the pembrolizumab arm, at 44.8% vs. 27.8%(P = .0011), and the median duration of response was longer (not reached vs. 6.3 months). There were six complete responses in the pembrolizumab arm.
Pembrolizumab also demonstrated a generally more favorable safety profile, with adverse events of any grade occurring in 73.4% of patients, compared with 90% of those treated with chemotherapy.
Grade 3 or 4 adverse events and treatment-related deaths were also lower in the pembrolizumab arm, at 26.6% vs. 53.3%.
Jean-Charles Soria, MD, chair of drug development at Gustave Roussy Cancer Center in Paris, the invited discussant, noted that the “45% objective response rate in first-line non–small cell lung cancer is unheard of, and is achieved with a monotherapy.”
“Pembrolizumab clearly leads to a higher objective response, a longer duration of response, a lower frequency of adverse events, better PFS, better OS, compared to chemotherapy.”
“We have, probably, a new standard of care” for patients with high PD-L1 expression and no targetable mutations,” he said.
COPENHAGEN – Chalk up another one for immunotherapy: the PD-1 checkpoint inhibitor pembrolizumab cut the risk of disease progression or death in half among select patients with non–small cell lung cancer (NSCLC), compared with standard platinum doublet chemotherapy, in the first-line setting.
Among 305 patients with non–small cell lung cancers with 50% or greater expression of the programmed death ligand 1 (PD-L1), median progression-free survival (PFS) for patients treated with pembrolizumab (Keytruda) was 10.3 months, compared with 6 months for patients assigned to receive platinum-based chemotherapy at the investigators discretion (hazard ratio, 0.50, P less than .001), reported Martin Reck, MD, from the department of thoracic oncology at the Lung Clinic Grosshansdorf, in Germany.
Results of the KEYNOTE-024 study were also published online in the New England Journal of Medicine (2016 Oct 9. doi: 10.1056/NEJMoa1606774). Approximately 23%-30% of patients with advanced non–small cell lung cancers have tumors that express PD-L1 on the membrane of at least 50% of tumor cells, making them attractive targets for pembrolizumab, which is a monoclonal antibody directed against programmed death 1 (PD-1). Pembrolizumab disengages the brake on the immune system caused by the interaction of receptor PD-1 with the PD-L1 and PD-L2 ligands.
The study was conducted to compare upfront pembrolizumab with platinum-based chemotherapy in patients with newly diagnosed advanced NSCLC that did not carry targetable EGFR-activating mutations or ALK translocations.
A total of 305 patients from 16 countries with untreated stage IV NSCLC, good performance status, and tumors with a 50% or greater expression of PD-L1 were enrolled and randomized to either pembrolizumab 200 mg intravenously every 3 weeks for up to 2 years. Or four to six cycles of platinum-doublet chemotherapy at the investigator’s discretion. The combinations included carboplatin plus pemetrexed, cisplatin plus pemetrexed, carboplatin plus gemcitabine, cisplatin plus gemcitabine, or carboplatin plus paclitaxel.
At a median follow-up of 11.2 months, 48.1% of patients assigned to pembrolizumab were still on treatment, as were 10% of those assigned to standard chemotherapy.
As noted before, PFS, the primary endpoint, was significantly better with pembrolizumab, as was the secondary endpoint of overall survival at 6 months. In all, 80% of patients treated with pembrolizumab were still alive at 6 months, compared with 72% of patients on chemotherapy (HR, 0.60; P = .005).
The confirmed response rate was also higher in the pembrolizumab arm, at 44.8% vs. 27.8%(P = .0011), and the median duration of response was longer (not reached vs. 6.3 months). There were six complete responses in the pembrolizumab arm.
Pembrolizumab also demonstrated a generally more favorable safety profile, with adverse events of any grade occurring in 73.4% of patients, compared with 90% of those treated with chemotherapy.
Grade 3 or 4 adverse events and treatment-related deaths were also lower in the pembrolizumab arm, at 26.6% vs. 53.3%.
Jean-Charles Soria, MD, chair of drug development at Gustave Roussy Cancer Center in Paris, the invited discussant, noted that the “45% objective response rate in first-line non–small cell lung cancer is unheard of, and is achieved with a monotherapy.”
“Pembrolizumab clearly leads to a higher objective response, a longer duration of response, a lower frequency of adverse events, better PFS, better OS, compared to chemotherapy.”
“We have, probably, a new standard of care” for patients with high PD-L1 expression and no targetable mutations,” he said.
AT ESMO 2016
Key clinical point: Pembrolizumab was superior to chemotherapy in stage IV NSCLC with PD-L1 expression of 50% or more.
Major finding: The hazard ratio for progression-free survival was 0.50 for pembrolizumab vs. platinum-based chemotherapy.
Data source: Randomized phase III trial in 305 patients with untreated stage IV NSCLC with 50% or more of tumor cells expressing PD-L1
Disclosures: The study was sponsored by Merck, Sharp & Dohme. Dr. Reck and Dr. Soria disclosed financial relationships (consulting/honoraria, research funding, etc.) with several companies, but not Merck.
Tooth Decay Is the Most Prevalent Disease
There are 2 leading types of dental diseases: tooth decay (dental caries or cavities) and gum disease (periodontal disease). Tooth decay is by far the more prevalent of the 2, causing a greater, needless loss in the quality of life.
Prevalence of Tooth Decay
Dental caries is a major public health problem. Even though in the past 50 years there has been a significant decline in dental caries, it is the most common ailment in the U.S., and large segments of the population experience various barriers to care. Among children and adolescents, dental caries is 4 to 5 times more common than asthma.1,2 Data from the National Health and Nutrition Examination Survey, 2011-2012, show that among children aged 2 to 8 years, 37% had dental caries in their primary teeth. Among adolescents aged 12 to 19 years, the prevalence of dental caries in the permanent teeth was 58%. About 90% of adults aged ≥ 20 years had dental caries.3,4
Unmet Dental Needs
The real disease burden of dental caries is the amount of unmet needs or untreated decay. In recent years, there has been a shift in the peak prevalence of untreated tooth decay from children to adults, possibly attributable to socioecologic factors.3,5 The prevalence of untreated decay in children and adolescents was about 15%. However, the prevalence of untreated decay among adults aged 20 to 64 years was higher: 27% of the adults had decayed teeth that were left untreated.3,4
The rates of untreated decay among these adults were higher among Hispanics (36%) and non-Hispanic blacks (42%) than among non-Hispanic whites (22%) and non-Hispanic Asians (17%).3,4 Despite the decline in dental caries, disparities persist among different racial, ethnic, educational, and income groups; it remains a modern curse for large segments of the population.
Basics of Tooth Decay
Tooth decay is caused by Streptococcus mutans (S mutans) bacterial infection. The cariogenic bacteria are transmissible from mother or caregiver to children.6 After tooth eruption, the mouth is quickly colonized by S mutans. Even though the bacteria are ubiquitous, the disease burden is more concentrated in some populations.
What makes some people more susceptible to tooth decay? Contrary to popular belief, it is not caused by childbirth or low dietary intake of calcium.7,8 Tooth decay typically starts on the chewing surfaces or proximal contacts of teeth.
S mutans is introduced into the mouth principally from another person. The bacteria colonize the mouth and with the formation of plaques, adhere to the teeth. Plaque is the soft, sticky film formed on teeth from food degradation. The biofilm is conducive to bacterial proliferation and teeming with bacteria.
The S mutans bacteria break down sugars and produce lactic acids, which cause tooth decay—a process of demineralization, or loss of calcium phosphate, from the tooth structure.2,9 As a result, the tooth “softens” and eventually collapses on itself, forming a cavity. Tooth decay most commonly occurs at the occlusal (chewing) surfaces and the proximal contacts of teeth. The occlusal aspects of teeth have a natural pit-and-fissure morphology that facilitates bacterial adherence and colonization. The proximal contacts of teeth also facilitate adherence of plaque and bacteria.
A tooth is made up of 3 layers. The outermost layer of the tooth crown is the enamel, which is the hardest or most mineralized part of the tooth—it is harder than bone. The next layer is the dentin, which has a higher percentage of organic material (collagen) and water than the enamel has and is softer. In the center of the tooth is the pulp (consisting of nerves and blood vessels), which keeps the tooth alive and provides sensation to the tooth. The outermost layer of the tooth root is cementum (instead of enamel), followed by dentin, which encases the pulp tissues contained within the root canals (Figure).
Process of Tooth Loss
At the early stage of tooth decay, when the decay is confined to the enamel, the tooth is asymptomatic, and the damage is reversible. When the decay extends into the dentin, restorations are a consideration. The further the decay extends toward the pulp, the greater the risk of tooth sensitivity and pain. Decay naturally progresses faster in the (less mineralized) dentin than when the decay is still confined to the enamel. If left unchecked, the decay may progress, and the bacteria eventually invade the pulp. At that point, there is risk of not only pain, but also swelling and tooth loss.
At the earlier stage of decay, a tooth may be treated with restorations, which are typically made of dental amalgam, resin (composite), glass ionomer, porcelain, or gold. When the bacteria have invaded the pulp, a dental restoration will not treat the pain and infection. Root canal therapy is needed to remove the infected tissues in the pulp chamber and root canals. Further, if the tooth is too compromised with extensive destruction from the decay or if there are financial or other barriers to root canal therapy, extraction is likely the only option.
Key to Oral Health
Personal daily oral hygiene (brushing and flossing) helps remove plaque from accumulating on tooth surfaces—especially the occlusal surfaces and proximal contacts, which are most prone to decay. Brushing teeth is of limited benefit without the use of fluoride toothpaste.8 The most important time for brushing is before bedtime, because less salivation occurs during sleep. Saliva helps clear fermented bacterial products, buffers the drop in pH, prevents demineralization, and enhances remineralizaton.10
Fluoride benefits children and adults of all ages, and comes in the form of fluoridated tap water, toothpaste, mouth rinse, and professionally applied gel and varnish. Fluoride occurs naturally in the environment, too. Its effect is mainly topical—fluoride gets incorporated into the tooth as fluorapatite, replacing the hydroxyapatite. Fluorapatite is harder than the hydroxyapatite; hence, the tooth is better able to resist demineralization by bacterial acid attacks.11 Professionally applied fluoride is generally recommended for high-risk patients, typically patients with high caries burden, ie, many decayed teeth. The best predictor of future caries is the past caries experience.
Sealants are thin, plastic coatings that are painted on to tooth occlusal surfaces, obliterating the pits and fissures normally found on posterior teeth. The smooth sealants effectively inhibit the colonization of S mutans on the occlusal aspects of teeth.12 Sealants are applied in dental offices (by the dentists or dental hygienists) or in school-based programs.
1. U.S. Department of Health and Human Services. Oral health in America: a report of the Surgeon General. http://www.nidcr.nih.gov/DataStatistics/SurgeonGeneral/Report/ExecutiveSummary.htm. Updated March 7, 2014. Accessed August 22, 2016.
2. Centers for Disease Control and Prevention. Hygiene-related diseases. http://www.cdc.gov/healthywater/hygiene/disease/dental_caries.html. Updated December 16, 2014. Accessed August 22, 2016.
3. Dye BA, Thornton-Evan G, Xianfen L, Iafolla TJ. Dental caries and sealant prevalence in children and adolescents in the United States, 2011-2012. http://www.cdc.gov/nchs/data/databriefs/db191.pdf. Published March 2015. NCHS Data Brief. Accessed August 22, 2016.
4. Dye BA, Thornton-Evan G, Xianfen L, Iafolla TJ. Dental caries and tooth loss in adults in the United States, 2011-2012. http://www.cdc.gov/nchs/data/databriefs/db197.pdf. NCHS Data Brief. Published May 2015. Accessed August 22, 2016.
5. Kassebaum NJ, Bernabé E, Dahiya M, Bhandari B, Murray CJ, Marcenes W. Global burden of untreated caries: a systematic review and metaregression. J Dent Res. 2015;94(5):650-658.
6. Smith RE, Badner VM, Morse DE, Freeman K. Maternal risk indicators for childhood caries in an inner city population. Community Dent Oral Epidemiol. 2002;30(3):176-181.
7. Boggess KA, Urlaub DM, Moos MK, Polinkovsky M, El-Khorazaty J, Lorenz C. Knowledge and beliefs regarding oral health among pregnant women. J Am Dent Assoc. 2011;142(11):1275-1282.
8. Roberts-Thomson KF, Spencer AJ. Public knowledge of the prevention of dental decay and gum diseases. Aust Dent J. 1999;44(4):253-258.
9. Cochrane NJ, Cai F, Hug NL, Burrow MF, Reynolds EC. New approaches to enhanced remineralization of tooth enamel. J Dent Res. 2010;89(11):1187-1197.
10. Prabhakar AR, Dodawad R, Os R. Evaluation of flow Rate, pH, buffering capacity, calcium, total protein and total antioxidant levels of saliva in caries free and caries active children—an in vivo study. Int J Clin Pediatr Dent. 2009;2(1):9-12.
11. Hicks J, Garcia-Godoy F, Flaitz C. Biological factors in dental caries: role of remineralization and fluoride in the dynamic process of demineralization and remuneration (part 3). J Clin Pediatr Dent. 2004;28(3):203-214.
12. Azarpazhooh A, Main PA. Pit and fissure sealants in the prevention of dental caries in children and adolescents: a systematic review. J Can Dent Assoc. 2008;74(2):171-177.
There are 2 leading types of dental diseases: tooth decay (dental caries or cavities) and gum disease (periodontal disease). Tooth decay is by far the more prevalent of the 2, causing a greater, needless loss in the quality of life.
Prevalence of Tooth Decay
Dental caries is a major public health problem. Even though in the past 50 years there has been a significant decline in dental caries, it is the most common ailment in the U.S., and large segments of the population experience various barriers to care. Among children and adolescents, dental caries is 4 to 5 times more common than asthma.1,2 Data from the National Health and Nutrition Examination Survey, 2011-2012, show that among children aged 2 to 8 years, 37% had dental caries in their primary teeth. Among adolescents aged 12 to 19 years, the prevalence of dental caries in the permanent teeth was 58%. About 90% of adults aged ≥ 20 years had dental caries.3,4
Unmet Dental Needs
The real disease burden of dental caries is the amount of unmet needs or untreated decay. In recent years, there has been a shift in the peak prevalence of untreated tooth decay from children to adults, possibly attributable to socioecologic factors.3,5 The prevalence of untreated decay in children and adolescents was about 15%. However, the prevalence of untreated decay among adults aged 20 to 64 years was higher: 27% of the adults had decayed teeth that were left untreated.3,4
The rates of untreated decay among these adults were higher among Hispanics (36%) and non-Hispanic blacks (42%) than among non-Hispanic whites (22%) and non-Hispanic Asians (17%).3,4 Despite the decline in dental caries, disparities persist among different racial, ethnic, educational, and income groups; it remains a modern curse for large segments of the population.
Basics of Tooth Decay
Tooth decay is caused by Streptococcus mutans (S mutans) bacterial infection. The cariogenic bacteria are transmissible from mother or caregiver to children.6 After tooth eruption, the mouth is quickly colonized by S mutans. Even though the bacteria are ubiquitous, the disease burden is more concentrated in some populations.
What makes some people more susceptible to tooth decay? Contrary to popular belief, it is not caused by childbirth or low dietary intake of calcium.7,8 Tooth decay typically starts on the chewing surfaces or proximal contacts of teeth.
S mutans is introduced into the mouth principally from another person. The bacteria colonize the mouth and with the formation of plaques, adhere to the teeth. Plaque is the soft, sticky film formed on teeth from food degradation. The biofilm is conducive to bacterial proliferation and teeming with bacteria.
The S mutans bacteria break down sugars and produce lactic acids, which cause tooth decay—a process of demineralization, or loss of calcium phosphate, from the tooth structure.2,9 As a result, the tooth “softens” and eventually collapses on itself, forming a cavity. Tooth decay most commonly occurs at the occlusal (chewing) surfaces and the proximal contacts of teeth. The occlusal aspects of teeth have a natural pit-and-fissure morphology that facilitates bacterial adherence and colonization. The proximal contacts of teeth also facilitate adherence of plaque and bacteria.
A tooth is made up of 3 layers. The outermost layer of the tooth crown is the enamel, which is the hardest or most mineralized part of the tooth—it is harder than bone. The next layer is the dentin, which has a higher percentage of organic material (collagen) and water than the enamel has and is softer. In the center of the tooth is the pulp (consisting of nerves and blood vessels), which keeps the tooth alive and provides sensation to the tooth. The outermost layer of the tooth root is cementum (instead of enamel), followed by dentin, which encases the pulp tissues contained within the root canals (Figure).
Process of Tooth Loss
At the early stage of tooth decay, when the decay is confined to the enamel, the tooth is asymptomatic, and the damage is reversible. When the decay extends into the dentin, restorations are a consideration. The further the decay extends toward the pulp, the greater the risk of tooth sensitivity and pain. Decay naturally progresses faster in the (less mineralized) dentin than when the decay is still confined to the enamel. If left unchecked, the decay may progress, and the bacteria eventually invade the pulp. At that point, there is risk of not only pain, but also swelling and tooth loss.
At the earlier stage of decay, a tooth may be treated with restorations, which are typically made of dental amalgam, resin (composite), glass ionomer, porcelain, or gold. When the bacteria have invaded the pulp, a dental restoration will not treat the pain and infection. Root canal therapy is needed to remove the infected tissues in the pulp chamber and root canals. Further, if the tooth is too compromised with extensive destruction from the decay or if there are financial or other barriers to root canal therapy, extraction is likely the only option.
Key to Oral Health
Personal daily oral hygiene (brushing and flossing) helps remove plaque from accumulating on tooth surfaces—especially the occlusal surfaces and proximal contacts, which are most prone to decay. Brushing teeth is of limited benefit without the use of fluoride toothpaste.8 The most important time for brushing is before bedtime, because less salivation occurs during sleep. Saliva helps clear fermented bacterial products, buffers the drop in pH, prevents demineralization, and enhances remineralizaton.10
Fluoride benefits children and adults of all ages, and comes in the form of fluoridated tap water, toothpaste, mouth rinse, and professionally applied gel and varnish. Fluoride occurs naturally in the environment, too. Its effect is mainly topical—fluoride gets incorporated into the tooth as fluorapatite, replacing the hydroxyapatite. Fluorapatite is harder than the hydroxyapatite; hence, the tooth is better able to resist demineralization by bacterial acid attacks.11 Professionally applied fluoride is generally recommended for high-risk patients, typically patients with high caries burden, ie, many decayed teeth. The best predictor of future caries is the past caries experience.
Sealants are thin, plastic coatings that are painted on to tooth occlusal surfaces, obliterating the pits and fissures normally found on posterior teeth. The smooth sealants effectively inhibit the colonization of S mutans on the occlusal aspects of teeth.12 Sealants are applied in dental offices (by the dentists or dental hygienists) or in school-based programs.
There are 2 leading types of dental diseases: tooth decay (dental caries or cavities) and gum disease (periodontal disease). Tooth decay is by far the more prevalent of the 2, causing a greater, needless loss in the quality of life.
Prevalence of Tooth Decay
Dental caries is a major public health problem. Even though in the past 50 years there has been a significant decline in dental caries, it is the most common ailment in the U.S., and large segments of the population experience various barriers to care. Among children and adolescents, dental caries is 4 to 5 times more common than asthma.1,2 Data from the National Health and Nutrition Examination Survey, 2011-2012, show that among children aged 2 to 8 years, 37% had dental caries in their primary teeth. Among adolescents aged 12 to 19 years, the prevalence of dental caries in the permanent teeth was 58%. About 90% of adults aged ≥ 20 years had dental caries.3,4
Unmet Dental Needs
The real disease burden of dental caries is the amount of unmet needs or untreated decay. In recent years, there has been a shift in the peak prevalence of untreated tooth decay from children to adults, possibly attributable to socioecologic factors.3,5 The prevalence of untreated decay in children and adolescents was about 15%. However, the prevalence of untreated decay among adults aged 20 to 64 years was higher: 27% of the adults had decayed teeth that were left untreated.3,4
The rates of untreated decay among these adults were higher among Hispanics (36%) and non-Hispanic blacks (42%) than among non-Hispanic whites (22%) and non-Hispanic Asians (17%).3,4 Despite the decline in dental caries, disparities persist among different racial, ethnic, educational, and income groups; it remains a modern curse for large segments of the population.
Basics of Tooth Decay
Tooth decay is caused by Streptococcus mutans (S mutans) bacterial infection. The cariogenic bacteria are transmissible from mother or caregiver to children.6 After tooth eruption, the mouth is quickly colonized by S mutans. Even though the bacteria are ubiquitous, the disease burden is more concentrated in some populations.
What makes some people more susceptible to tooth decay? Contrary to popular belief, it is not caused by childbirth or low dietary intake of calcium.7,8 Tooth decay typically starts on the chewing surfaces or proximal contacts of teeth.
S mutans is introduced into the mouth principally from another person. The bacteria colonize the mouth and with the formation of plaques, adhere to the teeth. Plaque is the soft, sticky film formed on teeth from food degradation. The biofilm is conducive to bacterial proliferation and teeming with bacteria.
The S mutans bacteria break down sugars and produce lactic acids, which cause tooth decay—a process of demineralization, or loss of calcium phosphate, from the tooth structure.2,9 As a result, the tooth “softens” and eventually collapses on itself, forming a cavity. Tooth decay most commonly occurs at the occlusal (chewing) surfaces and the proximal contacts of teeth. The occlusal aspects of teeth have a natural pit-and-fissure morphology that facilitates bacterial adherence and colonization. The proximal contacts of teeth also facilitate adherence of plaque and bacteria.
A tooth is made up of 3 layers. The outermost layer of the tooth crown is the enamel, which is the hardest or most mineralized part of the tooth—it is harder than bone. The next layer is the dentin, which has a higher percentage of organic material (collagen) and water than the enamel has and is softer. In the center of the tooth is the pulp (consisting of nerves and blood vessels), which keeps the tooth alive and provides sensation to the tooth. The outermost layer of the tooth root is cementum (instead of enamel), followed by dentin, which encases the pulp tissues contained within the root canals (Figure).
Process of Tooth Loss
At the early stage of tooth decay, when the decay is confined to the enamel, the tooth is asymptomatic, and the damage is reversible. When the decay extends into the dentin, restorations are a consideration. The further the decay extends toward the pulp, the greater the risk of tooth sensitivity and pain. Decay naturally progresses faster in the (less mineralized) dentin than when the decay is still confined to the enamel. If left unchecked, the decay may progress, and the bacteria eventually invade the pulp. At that point, there is risk of not only pain, but also swelling and tooth loss.
At the earlier stage of decay, a tooth may be treated with restorations, which are typically made of dental amalgam, resin (composite), glass ionomer, porcelain, or gold. When the bacteria have invaded the pulp, a dental restoration will not treat the pain and infection. Root canal therapy is needed to remove the infected tissues in the pulp chamber and root canals. Further, if the tooth is too compromised with extensive destruction from the decay or if there are financial or other barriers to root canal therapy, extraction is likely the only option.
Key to Oral Health
Personal daily oral hygiene (brushing and flossing) helps remove plaque from accumulating on tooth surfaces—especially the occlusal surfaces and proximal contacts, which are most prone to decay. Brushing teeth is of limited benefit without the use of fluoride toothpaste.8 The most important time for brushing is before bedtime, because less salivation occurs during sleep. Saliva helps clear fermented bacterial products, buffers the drop in pH, prevents demineralization, and enhances remineralizaton.10
Fluoride benefits children and adults of all ages, and comes in the form of fluoridated tap water, toothpaste, mouth rinse, and professionally applied gel and varnish. Fluoride occurs naturally in the environment, too. Its effect is mainly topical—fluoride gets incorporated into the tooth as fluorapatite, replacing the hydroxyapatite. Fluorapatite is harder than the hydroxyapatite; hence, the tooth is better able to resist demineralization by bacterial acid attacks.11 Professionally applied fluoride is generally recommended for high-risk patients, typically patients with high caries burden, ie, many decayed teeth. The best predictor of future caries is the past caries experience.
Sealants are thin, plastic coatings that are painted on to tooth occlusal surfaces, obliterating the pits and fissures normally found on posterior teeth. The smooth sealants effectively inhibit the colonization of S mutans on the occlusal aspects of teeth.12 Sealants are applied in dental offices (by the dentists or dental hygienists) or in school-based programs.
1. U.S. Department of Health and Human Services. Oral health in America: a report of the Surgeon General. http://www.nidcr.nih.gov/DataStatistics/SurgeonGeneral/Report/ExecutiveSummary.htm. Updated March 7, 2014. Accessed August 22, 2016.
2. Centers for Disease Control and Prevention. Hygiene-related diseases. http://www.cdc.gov/healthywater/hygiene/disease/dental_caries.html. Updated December 16, 2014. Accessed August 22, 2016.
3. Dye BA, Thornton-Evan G, Xianfen L, Iafolla TJ. Dental caries and sealant prevalence in children and adolescents in the United States, 2011-2012. http://www.cdc.gov/nchs/data/databriefs/db191.pdf. Published March 2015. NCHS Data Brief. Accessed August 22, 2016.
4. Dye BA, Thornton-Evan G, Xianfen L, Iafolla TJ. Dental caries and tooth loss in adults in the United States, 2011-2012. http://www.cdc.gov/nchs/data/databriefs/db197.pdf. NCHS Data Brief. Published May 2015. Accessed August 22, 2016.
5. Kassebaum NJ, Bernabé E, Dahiya M, Bhandari B, Murray CJ, Marcenes W. Global burden of untreated caries: a systematic review and metaregression. J Dent Res. 2015;94(5):650-658.
6. Smith RE, Badner VM, Morse DE, Freeman K. Maternal risk indicators for childhood caries in an inner city population. Community Dent Oral Epidemiol. 2002;30(3):176-181.
7. Boggess KA, Urlaub DM, Moos MK, Polinkovsky M, El-Khorazaty J, Lorenz C. Knowledge and beliefs regarding oral health among pregnant women. J Am Dent Assoc. 2011;142(11):1275-1282.
8. Roberts-Thomson KF, Spencer AJ. Public knowledge of the prevention of dental decay and gum diseases. Aust Dent J. 1999;44(4):253-258.
9. Cochrane NJ, Cai F, Hug NL, Burrow MF, Reynolds EC. New approaches to enhanced remineralization of tooth enamel. J Dent Res. 2010;89(11):1187-1197.
10. Prabhakar AR, Dodawad R, Os R. Evaluation of flow Rate, pH, buffering capacity, calcium, total protein and total antioxidant levels of saliva in caries free and caries active children—an in vivo study. Int J Clin Pediatr Dent. 2009;2(1):9-12.
11. Hicks J, Garcia-Godoy F, Flaitz C. Biological factors in dental caries: role of remineralization and fluoride in the dynamic process of demineralization and remuneration (part 3). J Clin Pediatr Dent. 2004;28(3):203-214.
12. Azarpazhooh A, Main PA. Pit and fissure sealants in the prevention of dental caries in children and adolescents: a systematic review. J Can Dent Assoc. 2008;74(2):171-177.
1. U.S. Department of Health and Human Services. Oral health in America: a report of the Surgeon General. http://www.nidcr.nih.gov/DataStatistics/SurgeonGeneral/Report/ExecutiveSummary.htm. Updated March 7, 2014. Accessed August 22, 2016.
2. Centers for Disease Control and Prevention. Hygiene-related diseases. http://www.cdc.gov/healthywater/hygiene/disease/dental_caries.html. Updated December 16, 2014. Accessed August 22, 2016.
3. Dye BA, Thornton-Evan G, Xianfen L, Iafolla TJ. Dental caries and sealant prevalence in children and adolescents in the United States, 2011-2012. http://www.cdc.gov/nchs/data/databriefs/db191.pdf. Published March 2015. NCHS Data Brief. Accessed August 22, 2016.
4. Dye BA, Thornton-Evan G, Xianfen L, Iafolla TJ. Dental caries and tooth loss in adults in the United States, 2011-2012. http://www.cdc.gov/nchs/data/databriefs/db197.pdf. NCHS Data Brief. Published May 2015. Accessed August 22, 2016.
5. Kassebaum NJ, Bernabé E, Dahiya M, Bhandari B, Murray CJ, Marcenes W. Global burden of untreated caries: a systematic review and metaregression. J Dent Res. 2015;94(5):650-658.
6. Smith RE, Badner VM, Morse DE, Freeman K. Maternal risk indicators for childhood caries in an inner city population. Community Dent Oral Epidemiol. 2002;30(3):176-181.
7. Boggess KA, Urlaub DM, Moos MK, Polinkovsky M, El-Khorazaty J, Lorenz C. Knowledge and beliefs regarding oral health among pregnant women. J Am Dent Assoc. 2011;142(11):1275-1282.
8. Roberts-Thomson KF, Spencer AJ. Public knowledge of the prevention of dental decay and gum diseases. Aust Dent J. 1999;44(4):253-258.
9. Cochrane NJ, Cai F, Hug NL, Burrow MF, Reynolds EC. New approaches to enhanced remineralization of tooth enamel. J Dent Res. 2010;89(11):1187-1197.
10. Prabhakar AR, Dodawad R, Os R. Evaluation of flow Rate, pH, buffering capacity, calcium, total protein and total antioxidant levels of saliva in caries free and caries active children—an in vivo study. Int J Clin Pediatr Dent. 2009;2(1):9-12.
11. Hicks J, Garcia-Godoy F, Flaitz C. Biological factors in dental caries: role of remineralization and fluoride in the dynamic process of demineralization and remuneration (part 3). J Clin Pediatr Dent. 2004;28(3):203-214.
12. Azarpazhooh A, Main PA. Pit and fissure sealants in the prevention of dental caries in children and adolescents: a systematic review. J Can Dent Assoc. 2008;74(2):171-177.