User login
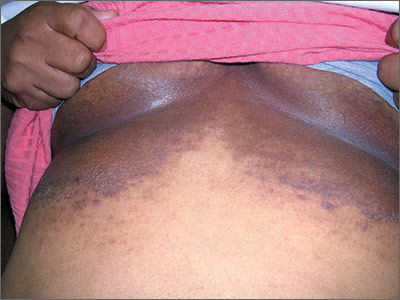
Dark rash under breasts
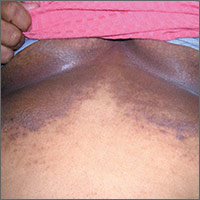
The family physician (FP) suspected that the patient had some type of fungal infection under her breasts. The FP scraped the edge and performed a potassium hydroxide (KOH) preparation that showed pseudohyphae and budding yeast forms, indicating a candida infection. (See video on how to perform a KOH preparation.) The FP also noted that there appeared to be some satellite lesions around the edge of the hyperpigmented area. Under the left breast was a small fissure and some white coloration at the skinfold. The differential diagnosis included tinea corporis and inverse psoriasis.
The FP prescribed topical clotrimazole cream 1% over the counter to be applied twice daily until at least one week after the rash resolved. While nystatin would be another option, it required a prescription and would not cover any possible dermatophyte in the area. The FP also explained that the dark pigmentation is the result of a longstanding inflammatory process related to the infection and would not go away immediately with treatment. In fact, the postinflammatory hyperpigmentation might remain for life.
The FP also spent time counseling the patient on an improved diet, increased exercise, and adherence to her diabetes medicines. A follow-up appointment for one month was set to review the patient's general health and to see if the candida infection resolved.
In cases where intertriginous fungal infections are treated without KOH or fungal culture confirmation, be suspicious for inverse psoriasis if the patient does not respond to antifungal medications. With such a large area involved, an oral antifungal such as fluconazole might occasionally be needed. Terbinafine is not an effective medicine for cutaneous candidiasis. If psoriasis is suspected, it helps to look for clues, such as nail pitting or cutaneous involvement with plaques on the elbows, knees, or scalp. If all else fails, a punch biopsy can be used to determine the correct diagnosis.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Usatine R. Candidiasis. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:777-781.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
The family physician (FP) suspected that the patient had some type of fungal infection under her breasts. The FP scraped the edge and performed a potassium hydroxide (KOH) preparation that showed pseudohyphae and budding yeast forms, indicating a candida infection. (See video on how to perform a KOH preparation.) The FP also noted that there appeared to be some satellite lesions around the edge of the hyperpigmented area. Under the left breast was a small fissure and some white coloration at the skinfold. The differential diagnosis included tinea corporis and inverse psoriasis.
The FP prescribed topical clotrimazole cream 1% over the counter to be applied twice daily until at least one week after the rash resolved. While nystatin would be another option, it required a prescription and would not cover any possible dermatophyte in the area. The FP also explained that the dark pigmentation is the result of a longstanding inflammatory process related to the infection and would not go away immediately with treatment. In fact, the postinflammatory hyperpigmentation might remain for life.
The FP also spent time counseling the patient on an improved diet, increased exercise, and adherence to her diabetes medicines. A follow-up appointment for one month was set to review the patient's general health and to see if the candida infection resolved.
In cases where intertriginous fungal infections are treated without KOH or fungal culture confirmation, be suspicious for inverse psoriasis if the patient does not respond to antifungal medications. With such a large area involved, an oral antifungal such as fluconazole might occasionally be needed. Terbinafine is not an effective medicine for cutaneous candidiasis. If psoriasis is suspected, it helps to look for clues, such as nail pitting or cutaneous involvement with plaques on the elbows, knees, or scalp. If all else fails, a punch biopsy can be used to determine the correct diagnosis.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Usatine R. Candidiasis. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:777-781.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
The family physician (FP) suspected that the patient had some type of fungal infection under her breasts. The FP scraped the edge and performed a potassium hydroxide (KOH) preparation that showed pseudohyphae and budding yeast forms, indicating a candida infection. (See video on how to perform a KOH preparation.) The FP also noted that there appeared to be some satellite lesions around the edge of the hyperpigmented area. Under the left breast was a small fissure and some white coloration at the skinfold. The differential diagnosis included tinea corporis and inverse psoriasis.
The FP prescribed topical clotrimazole cream 1% over the counter to be applied twice daily until at least one week after the rash resolved. While nystatin would be another option, it required a prescription and would not cover any possible dermatophyte in the area. The FP also explained that the dark pigmentation is the result of a longstanding inflammatory process related to the infection and would not go away immediately with treatment. In fact, the postinflammatory hyperpigmentation might remain for life.
The FP also spent time counseling the patient on an improved diet, increased exercise, and adherence to her diabetes medicines. A follow-up appointment for one month was set to review the patient's general health and to see if the candida infection resolved.
In cases where intertriginous fungal infections are treated without KOH or fungal culture confirmation, be suspicious for inverse psoriasis if the patient does not respond to antifungal medications. With such a large area involved, an oral antifungal such as fluconazole might occasionally be needed. Terbinafine is not an effective medicine for cutaneous candidiasis. If psoriasis is suspected, it helps to look for clues, such as nail pitting or cutaneous involvement with plaques on the elbows, knees, or scalp. If all else fails, a punch biopsy can be used to determine the correct diagnosis.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Usatine R. Candidiasis. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:777-781.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
ICS/LABA exacerbation benefit outweighs pneumonia risk
LONDON – The benefit of a fixed-dose inhaled corticosteroid (ICS) and long-acting beta-agonist (LABA) combination in reducing exacerbations of chronic obstructive pulmonary disease (COPD) far outweighed any risk for pneumonia in a post hoc analysis of the 48-week FORWARD study.
Although there were 13 extra pneumonia events when a fixed-dose combination of beclometasone diproprionate and formoterol fumarate (Foster, Chiesi Farmaceutici SpA) was used as compared to formoterol fumarate alone, there were 123 fewer moderate to severe COPD exacerbations over a 342-day analysis period.
“Analysis of pneumonia and exacerbation cumulative number of events shows that the number of incident pneumonia remains very small relative to that of moderate to severe exacerbations,” Massimo Corradi, MD, of the University of Parma (Italy), reported at the annual congress of the European Respiratory Society.
Dr. Corradi added that the new analysis confirms that the ICS/LABA combination has a “positive risk-benefit balance over LABA monotherapy, supporting [the argument that] the benefits of adding an ICS to a bronchodilator significantly outweigh potential risks.”
The FORWARD study was a two-arm trial designed to compare the efficacy and safety of fixed-dose treatment with beclometasone diproprionate and formoterol fumarate versus formoterol fumarate alone in 1,199 patients with severe COPD.
For inclusion in the study, patients had to have a post-bronchodilator forced expiratory volume in 1 second below 50% of predicted and a forced vital capacity ratio of less than 0.7. They also had to have a smoking history of 10 pack-years or more, and a history of at least one COPD exacerbation in the previous 12 months that had required treatment or hospitalization (Eur Respir J. 2013;41[1]:12-7).
After a 2-week run-in period, where all patients received a 24-mcg dose of formoterol fumarate, patients were randomized to continue treatment with formoterol fumarate or to receive the fixed-dose combination of beclometasone diproprionate 400 mcg and beclometasone diproprionate 24 mcg for 48 weeks.
A total of 1,186 patients, most of whom were male (69%) with a mean age of 64 years, formed the intention-to-treat population.
Published results (Respir Med. 2014;108[8]:1153-62) showed that the combination of the ICS beclometasone diproprionate and the LABA formoterol fumarate (Chiesi Farmaceutici SpA) was associated with a 28% reduction in the annual rate of moderate to severe exacerbations versus the LABA alone.
The adjusted rate of exacerbations per patient per year was 0.80 in patients treated with the ICS/LABA combination versus 1.12 for those treated with just the LABA, with an adjusted rate ratio of 0.72 (P less than .001).
The published data also showed that pneumonia was reported by 23 patients (3.8%) treated with the ICS/LABA and by 11 (1.8%) treated with the LABA only.
For the new analysis, Dr. Corradi and his coinvestigators looked at the cases of pneumonia and COPD exacerbations in more detail, plotting out the cumulative number of events over time and also characterizing the types of pneumonia in more detail.
All patients had a chest x-ray to confirm the presence of pneumonia, he said, noting that overall there were 35 cases of pneumonia, 24 occurring in patients treated with the fixed-dose beclometasone diproprionate and formoterol fumarate combination and 11 in patients treated only with formoterol fumarate.
Of these cases, 25 required in-hospital treatment – 16 patients in the ICS/LABA arm and 9 in the LABA-only arm. There were three instances of patients acquiring pneumonia in hospital – two in the ICS/LABA and one in the LABA-only arm.
There were also two fatal cases of pneumonia – one in each treatment group. Neither were thought to be related to either of the treatments.
These findings are in line with a recent review of the use of ICS for COPD by the European Medicines Agency (EMA/488280/2016), which noted that “overall the benefits of inhaled corticosteroid medicines in treating COPD continue to outweigh their risks and there should be no change to the way in which these medicines are used.”
The European Medicines Agency advised that patients and clinicians need to “be alert for signs and symptoms of pneumonia, bearing in mind that the clinical features of pneumonia overlap with those of a worsening (exacerbation) of the underlying disease.”
Dr. Corradi has received speaker fees from Chiesi Farmaceutici SpA, which funded the FORWARD study, and his coauthors are employees of the company.
LONDON – The benefit of a fixed-dose inhaled corticosteroid (ICS) and long-acting beta-agonist (LABA) combination in reducing exacerbations of chronic obstructive pulmonary disease (COPD) far outweighed any risk for pneumonia in a post hoc analysis of the 48-week FORWARD study.
Although there were 13 extra pneumonia events when a fixed-dose combination of beclometasone diproprionate and formoterol fumarate (Foster, Chiesi Farmaceutici SpA) was used as compared to formoterol fumarate alone, there were 123 fewer moderate to severe COPD exacerbations over a 342-day analysis period.
“Analysis of pneumonia and exacerbation cumulative number of events shows that the number of incident pneumonia remains very small relative to that of moderate to severe exacerbations,” Massimo Corradi, MD, of the University of Parma (Italy), reported at the annual congress of the European Respiratory Society.
Dr. Corradi added that the new analysis confirms that the ICS/LABA combination has a “positive risk-benefit balance over LABA monotherapy, supporting [the argument that] the benefits of adding an ICS to a bronchodilator significantly outweigh potential risks.”
The FORWARD study was a two-arm trial designed to compare the efficacy and safety of fixed-dose treatment with beclometasone diproprionate and formoterol fumarate versus formoterol fumarate alone in 1,199 patients with severe COPD.
For inclusion in the study, patients had to have a post-bronchodilator forced expiratory volume in 1 second below 50% of predicted and a forced vital capacity ratio of less than 0.7. They also had to have a smoking history of 10 pack-years or more, and a history of at least one COPD exacerbation in the previous 12 months that had required treatment or hospitalization (Eur Respir J. 2013;41[1]:12-7).
After a 2-week run-in period, where all patients received a 24-mcg dose of formoterol fumarate, patients were randomized to continue treatment with formoterol fumarate or to receive the fixed-dose combination of beclometasone diproprionate 400 mcg and beclometasone diproprionate 24 mcg for 48 weeks.
A total of 1,186 patients, most of whom were male (69%) with a mean age of 64 years, formed the intention-to-treat population.
Published results (Respir Med. 2014;108[8]:1153-62) showed that the combination of the ICS beclometasone diproprionate and the LABA formoterol fumarate (Chiesi Farmaceutici SpA) was associated with a 28% reduction in the annual rate of moderate to severe exacerbations versus the LABA alone.
The adjusted rate of exacerbations per patient per year was 0.80 in patients treated with the ICS/LABA combination versus 1.12 for those treated with just the LABA, with an adjusted rate ratio of 0.72 (P less than .001).
The published data also showed that pneumonia was reported by 23 patients (3.8%) treated with the ICS/LABA and by 11 (1.8%) treated with the LABA only.
For the new analysis, Dr. Corradi and his coinvestigators looked at the cases of pneumonia and COPD exacerbations in more detail, plotting out the cumulative number of events over time and also characterizing the types of pneumonia in more detail.
All patients had a chest x-ray to confirm the presence of pneumonia, he said, noting that overall there were 35 cases of pneumonia, 24 occurring in patients treated with the fixed-dose beclometasone diproprionate and formoterol fumarate combination and 11 in patients treated only with formoterol fumarate.
Of these cases, 25 required in-hospital treatment – 16 patients in the ICS/LABA arm and 9 in the LABA-only arm. There were three instances of patients acquiring pneumonia in hospital – two in the ICS/LABA and one in the LABA-only arm.
There were also two fatal cases of pneumonia – one in each treatment group. Neither were thought to be related to either of the treatments.
These findings are in line with a recent review of the use of ICS for COPD by the European Medicines Agency (EMA/488280/2016), which noted that “overall the benefits of inhaled corticosteroid medicines in treating COPD continue to outweigh their risks and there should be no change to the way in which these medicines are used.”
The European Medicines Agency advised that patients and clinicians need to “be alert for signs and symptoms of pneumonia, bearing in mind that the clinical features of pneumonia overlap with those of a worsening (exacerbation) of the underlying disease.”
Dr. Corradi has received speaker fees from Chiesi Farmaceutici SpA, which funded the FORWARD study, and his coauthors are employees of the company.
LONDON – The benefit of a fixed-dose inhaled corticosteroid (ICS) and long-acting beta-agonist (LABA) combination in reducing exacerbations of chronic obstructive pulmonary disease (COPD) far outweighed any risk for pneumonia in a post hoc analysis of the 48-week FORWARD study.
Although there were 13 extra pneumonia events when a fixed-dose combination of beclometasone diproprionate and formoterol fumarate (Foster, Chiesi Farmaceutici SpA) was used as compared to formoterol fumarate alone, there were 123 fewer moderate to severe COPD exacerbations over a 342-day analysis period.
“Analysis of pneumonia and exacerbation cumulative number of events shows that the number of incident pneumonia remains very small relative to that of moderate to severe exacerbations,” Massimo Corradi, MD, of the University of Parma (Italy), reported at the annual congress of the European Respiratory Society.
Dr. Corradi added that the new analysis confirms that the ICS/LABA combination has a “positive risk-benefit balance over LABA monotherapy, supporting [the argument that] the benefits of adding an ICS to a bronchodilator significantly outweigh potential risks.”
The FORWARD study was a two-arm trial designed to compare the efficacy and safety of fixed-dose treatment with beclometasone diproprionate and formoterol fumarate versus formoterol fumarate alone in 1,199 patients with severe COPD.
For inclusion in the study, patients had to have a post-bronchodilator forced expiratory volume in 1 second below 50% of predicted and a forced vital capacity ratio of less than 0.7. They also had to have a smoking history of 10 pack-years or more, and a history of at least one COPD exacerbation in the previous 12 months that had required treatment or hospitalization (Eur Respir J. 2013;41[1]:12-7).
After a 2-week run-in period, where all patients received a 24-mcg dose of formoterol fumarate, patients were randomized to continue treatment with formoterol fumarate or to receive the fixed-dose combination of beclometasone diproprionate 400 mcg and beclometasone diproprionate 24 mcg for 48 weeks.
A total of 1,186 patients, most of whom were male (69%) with a mean age of 64 years, formed the intention-to-treat population.
Published results (Respir Med. 2014;108[8]:1153-62) showed that the combination of the ICS beclometasone diproprionate and the LABA formoterol fumarate (Chiesi Farmaceutici SpA) was associated with a 28% reduction in the annual rate of moderate to severe exacerbations versus the LABA alone.
The adjusted rate of exacerbations per patient per year was 0.80 in patients treated with the ICS/LABA combination versus 1.12 for those treated with just the LABA, with an adjusted rate ratio of 0.72 (P less than .001).
The published data also showed that pneumonia was reported by 23 patients (3.8%) treated with the ICS/LABA and by 11 (1.8%) treated with the LABA only.
For the new analysis, Dr. Corradi and his coinvestigators looked at the cases of pneumonia and COPD exacerbations in more detail, plotting out the cumulative number of events over time and also characterizing the types of pneumonia in more detail.
All patients had a chest x-ray to confirm the presence of pneumonia, he said, noting that overall there were 35 cases of pneumonia, 24 occurring in patients treated with the fixed-dose beclometasone diproprionate and formoterol fumarate combination and 11 in patients treated only with formoterol fumarate.
Of these cases, 25 required in-hospital treatment – 16 patients in the ICS/LABA arm and 9 in the LABA-only arm. There were three instances of patients acquiring pneumonia in hospital – two in the ICS/LABA and one in the LABA-only arm.
There were also two fatal cases of pneumonia – one in each treatment group. Neither were thought to be related to either of the treatments.
These findings are in line with a recent review of the use of ICS for COPD by the European Medicines Agency (EMA/488280/2016), which noted that “overall the benefits of inhaled corticosteroid medicines in treating COPD continue to outweigh their risks and there should be no change to the way in which these medicines are used.”
The European Medicines Agency advised that patients and clinicians need to “be alert for signs and symptoms of pneumonia, bearing in mind that the clinical features of pneumonia overlap with those of a worsening (exacerbation) of the underlying disease.”
Dr. Corradi has received speaker fees from Chiesi Farmaceutici SpA, which funded the FORWARD study, and his coauthors are employees of the company.
AT THE ERS CONGRESS 2016
Key clinical point: The risk for pneumonia associated with drugs containing inhaled corticosteroids (ICS) is outweighed by the reduction in chronic obstructive pulmonary disease (COPD) exacerbations that can be achieved.
Major finding: There were 13 extra pneumonia events but 123 fewer COPD exacerbations when a fixed dose ICS/long-acting beta-agonist (LABA) combination was used versus a LABA alone.
Data source: Post hoc analysis of the FORWARD study, a multicenter, randomized, double-blind, active-controlled 48-week trial of a fixed-dose ICS/LABA combination versus LABA for reducing exacerbations in 1,186 patients with COPD.
Disclosures: Dr. Corradi has received speaker fees from Chiesi Farmaceutici SpA, which funded the FORWARD study, and his coauthors are employees of the company.
How Do Diet, Exercise, and Supplements Affect Parkinson’s Disease Progression?
Prior studies have found that people who consume green tea, coffee, and blueberries and avoid dairy may have a lower risk of Parkinson’s disease. Whether nutrition is associated with rate of disease progression in patients with Parkinson’s disease, however, is not known.
To evaluate whether diet, exercise, and supplements are associated with rate of Parkinson’s disease progression, Laurie Mischley, ND, PhD, MPH, Assistant Research Scientist at Bastyr University Research Institute in Kenmore, Washington, and Richard Lau, MPH, a PhD student in the College of Public Health and Human Sciences at Oregon State University in Corvalis conducted an Internet-based natural history study. A total of 1,024 patients participated in the study. Participants had a mean age of 60.7 and had been diagnosed with Parkinson’s disease for an average of 6.7 years.
The researchers used the Patient-Reported Outcomes in Parkinson’s Disease (PRO-PD) scale to assess Parkinson’s disease severity. Disease progression was defined as PRO-PD score adjusted for age and years since diagnosis. They used baseline food frequency questionnaires to quantify dietary intake in the cross-sectional analysis.
Fresh fruit, fresh vegetables, nuts and seeds, olive oil, fish (non-fried), wine, eggs, and fresh herbs were associated with a statistically significant improvement in PRO-PD score, the researchers said. Fried foods, beef, diet soda, canned fruits, and canned vegetables were associated with more severe disease. Dairy consumption was not associated with disease severity.
Of the supplements and pharmaceuticals studied, oral glutathione, rasagiline, and coenzyme Q10 were associated with improved PRO-PD scores, whereas iron was associated with more severe disease. The effect of melatonin was not significant, however, when the researchers considered poor sleep.The researchers observed a dose response curve with exercise. Exercising at least 30 minutes daily was associated with the greatest reduction in disease severity.
“Whether iron, fried foods, diet soda, or canned goods provide environmental insults that accelerate disease progression warrants immediate attention,” the researchers concluded. “This pragmatic natural history study offers the first evidence base for prescribing lifestyle modification (beyond exercise) to patients with Parkinson’s disease. Patients should be empowered to know that they can make choices that affect outcomes."
—Jake Remaly
Prior studies have found that people who consume green tea, coffee, and blueberries and avoid dairy may have a lower risk of Parkinson’s disease. Whether nutrition is associated with rate of disease progression in patients with Parkinson’s disease, however, is not known.
To evaluate whether diet, exercise, and supplements are associated with rate of Parkinson’s disease progression, Laurie Mischley, ND, PhD, MPH, Assistant Research Scientist at Bastyr University Research Institute in Kenmore, Washington, and Richard Lau, MPH, a PhD student in the College of Public Health and Human Sciences at Oregon State University in Corvalis conducted an Internet-based natural history study. A total of 1,024 patients participated in the study. Participants had a mean age of 60.7 and had been diagnosed with Parkinson’s disease for an average of 6.7 years.
The researchers used the Patient-Reported Outcomes in Parkinson’s Disease (PRO-PD) scale to assess Parkinson’s disease severity. Disease progression was defined as PRO-PD score adjusted for age and years since diagnosis. They used baseline food frequency questionnaires to quantify dietary intake in the cross-sectional analysis.
Fresh fruit, fresh vegetables, nuts and seeds, olive oil, fish (non-fried), wine, eggs, and fresh herbs were associated with a statistically significant improvement in PRO-PD score, the researchers said. Fried foods, beef, diet soda, canned fruits, and canned vegetables were associated with more severe disease. Dairy consumption was not associated with disease severity.
Of the supplements and pharmaceuticals studied, oral glutathione, rasagiline, and coenzyme Q10 were associated with improved PRO-PD scores, whereas iron was associated with more severe disease. The effect of melatonin was not significant, however, when the researchers considered poor sleep.The researchers observed a dose response curve with exercise. Exercising at least 30 minutes daily was associated with the greatest reduction in disease severity.
“Whether iron, fried foods, diet soda, or canned goods provide environmental insults that accelerate disease progression warrants immediate attention,” the researchers concluded. “This pragmatic natural history study offers the first evidence base for prescribing lifestyle modification (beyond exercise) to patients with Parkinson’s disease. Patients should be empowered to know that they can make choices that affect outcomes."
—Jake Remaly
Prior studies have found that people who consume green tea, coffee, and blueberries and avoid dairy may have a lower risk of Parkinson’s disease. Whether nutrition is associated with rate of disease progression in patients with Parkinson’s disease, however, is not known.
To evaluate whether diet, exercise, and supplements are associated with rate of Parkinson’s disease progression, Laurie Mischley, ND, PhD, MPH, Assistant Research Scientist at Bastyr University Research Institute in Kenmore, Washington, and Richard Lau, MPH, a PhD student in the College of Public Health and Human Sciences at Oregon State University in Corvalis conducted an Internet-based natural history study. A total of 1,024 patients participated in the study. Participants had a mean age of 60.7 and had been diagnosed with Parkinson’s disease for an average of 6.7 years.
The researchers used the Patient-Reported Outcomes in Parkinson’s Disease (PRO-PD) scale to assess Parkinson’s disease severity. Disease progression was defined as PRO-PD score adjusted for age and years since diagnosis. They used baseline food frequency questionnaires to quantify dietary intake in the cross-sectional analysis.
Fresh fruit, fresh vegetables, nuts and seeds, olive oil, fish (non-fried), wine, eggs, and fresh herbs were associated with a statistically significant improvement in PRO-PD score, the researchers said. Fried foods, beef, diet soda, canned fruits, and canned vegetables were associated with more severe disease. Dairy consumption was not associated with disease severity.
Of the supplements and pharmaceuticals studied, oral glutathione, rasagiline, and coenzyme Q10 were associated with improved PRO-PD scores, whereas iron was associated with more severe disease. The effect of melatonin was not significant, however, when the researchers considered poor sleep.The researchers observed a dose response curve with exercise. Exercising at least 30 minutes daily was associated with the greatest reduction in disease severity.
“Whether iron, fried foods, diet soda, or canned goods provide environmental insults that accelerate disease progression warrants immediate attention,” the researchers concluded. “This pragmatic natural history study offers the first evidence base for prescribing lifestyle modification (beyond exercise) to patients with Parkinson’s disease. Patients should be empowered to know that they can make choices that affect outcomes."
—Jake Remaly
Can Treating Neuroinflammation in REM Sleep Behavior Disorder Delay Parkinson’s Disease Onset?
PORTLAND, OR—In patients with idiopathic REM sleep behavior disorder, microglial activation is increased in the substantia nigra, compared with controls, and microglial activation correlates with putamenal dopaminergic dysfunction, according to research presented at the Fourth World Parkinson Congress. These findings suggest that “anti-inflammatory agents could possibly delay progression to a manifest synucleinopathy in subjects with idiopathic REM sleep behavior disorder,” researchers said.
Longitudinal studies have found that patients with idiopathic REM sleep behavior disorder have an increased risk of Parkinson’s disease and related Lewy body disorders. “This implies that, in idiopathic REM sleep behavior disorder, the underlying pathology of developing neurodegenerative disorders can be investigated years prior to the development of manifest symptoms,” said Morten Gersel Stokholm, MD, a researcher in the Department of Clinical Medicine at Aarhus University and the Department of Nuclear Medicine & PET-Centre at Aarhus University Hospital, Denmark, and his research colleagues.
Chronic activation of microglial cells may have a detrimental effect on neurons and contribute to the development of neurodegenerative disorders. Studies have found that uptake of an in vivo marker of microglial activation, 11C-PK11195, is increased in Parkinson’s disease and other neurodegenerative disorders.
To investigate the in vivo occurrence of neuroinflammation in the brains of patients with idiopathic REM sleep behavior disorder and neuroinflammation’s temporal relationship with striatal dopamine dysfunction, Dr. Stokholm and colleagues conducted a multitracer PET study of patients with idiopathic REM sleep behavior disorder.
The investigators enrolled 15 patients with polysomnography-confirmed idiopathic REM sleep behavior disorder at Aarhus University Hospital and Hospital Clínic de Barcelona. They also enrolled 19 matched controls. Participants underwent two PET scans with 18F-DOPA and 11C- PK11195 and a structural T1 MRI scan. Parametric images of specific tracer uptake (ie, F-dopa Ki-values and PK11195 binding potential) were constructed at voxel level using Patlak graphical analysis and a supervised cluster-analysis with compartmental modeling, respectively. A region of interest analysis was performed on a priori defined regions.
Compared with controls, patients with idiopathic REM sleep behavior disorder showed significantly reduced 18F-DOPA tracer uptake in the substantia nigra. Patients with higher substantia nigra11C-PK11195 binding also had increased binding in the ipsilateral putamen. Patients with more severe reductions in putaminal 18F-DOPA uptake had significantly higher 11C-PK11195 binding in the putamen and substantia nigra.
—Jake Remaly
PORTLAND, OR—In patients with idiopathic REM sleep behavior disorder, microglial activation is increased in the substantia nigra, compared with controls, and microglial activation correlates with putamenal dopaminergic dysfunction, according to research presented at the Fourth World Parkinson Congress. These findings suggest that “anti-inflammatory agents could possibly delay progression to a manifest synucleinopathy in subjects with idiopathic REM sleep behavior disorder,” researchers said.
Longitudinal studies have found that patients with idiopathic REM sleep behavior disorder have an increased risk of Parkinson’s disease and related Lewy body disorders. “This implies that, in idiopathic REM sleep behavior disorder, the underlying pathology of developing neurodegenerative disorders can be investigated years prior to the development of manifest symptoms,” said Morten Gersel Stokholm, MD, a researcher in the Department of Clinical Medicine at Aarhus University and the Department of Nuclear Medicine & PET-Centre at Aarhus University Hospital, Denmark, and his research colleagues.
Chronic activation of microglial cells may have a detrimental effect on neurons and contribute to the development of neurodegenerative disorders. Studies have found that uptake of an in vivo marker of microglial activation, 11C-PK11195, is increased in Parkinson’s disease and other neurodegenerative disorders.
To investigate the in vivo occurrence of neuroinflammation in the brains of patients with idiopathic REM sleep behavior disorder and neuroinflammation’s temporal relationship with striatal dopamine dysfunction, Dr. Stokholm and colleagues conducted a multitracer PET study of patients with idiopathic REM sleep behavior disorder.
The investigators enrolled 15 patients with polysomnography-confirmed idiopathic REM sleep behavior disorder at Aarhus University Hospital and Hospital Clínic de Barcelona. They also enrolled 19 matched controls. Participants underwent two PET scans with 18F-DOPA and 11C- PK11195 and a structural T1 MRI scan. Parametric images of specific tracer uptake (ie, F-dopa Ki-values and PK11195 binding potential) were constructed at voxel level using Patlak graphical analysis and a supervised cluster-analysis with compartmental modeling, respectively. A region of interest analysis was performed on a priori defined regions.
Compared with controls, patients with idiopathic REM sleep behavior disorder showed significantly reduced 18F-DOPA tracer uptake in the substantia nigra. Patients with higher substantia nigra11C-PK11195 binding also had increased binding in the ipsilateral putamen. Patients with more severe reductions in putaminal 18F-DOPA uptake had significantly higher 11C-PK11195 binding in the putamen and substantia nigra.
—Jake Remaly
PORTLAND, OR—In patients with idiopathic REM sleep behavior disorder, microglial activation is increased in the substantia nigra, compared with controls, and microglial activation correlates with putamenal dopaminergic dysfunction, according to research presented at the Fourth World Parkinson Congress. These findings suggest that “anti-inflammatory agents could possibly delay progression to a manifest synucleinopathy in subjects with idiopathic REM sleep behavior disorder,” researchers said.
Longitudinal studies have found that patients with idiopathic REM sleep behavior disorder have an increased risk of Parkinson’s disease and related Lewy body disorders. “This implies that, in idiopathic REM sleep behavior disorder, the underlying pathology of developing neurodegenerative disorders can be investigated years prior to the development of manifest symptoms,” said Morten Gersel Stokholm, MD, a researcher in the Department of Clinical Medicine at Aarhus University and the Department of Nuclear Medicine & PET-Centre at Aarhus University Hospital, Denmark, and his research colleagues.
Chronic activation of microglial cells may have a detrimental effect on neurons and contribute to the development of neurodegenerative disorders. Studies have found that uptake of an in vivo marker of microglial activation, 11C-PK11195, is increased in Parkinson’s disease and other neurodegenerative disorders.
To investigate the in vivo occurrence of neuroinflammation in the brains of patients with idiopathic REM sleep behavior disorder and neuroinflammation’s temporal relationship with striatal dopamine dysfunction, Dr. Stokholm and colleagues conducted a multitracer PET study of patients with idiopathic REM sleep behavior disorder.
The investigators enrolled 15 patients with polysomnography-confirmed idiopathic REM sleep behavior disorder at Aarhus University Hospital and Hospital Clínic de Barcelona. They also enrolled 19 matched controls. Participants underwent two PET scans with 18F-DOPA and 11C- PK11195 and a structural T1 MRI scan. Parametric images of specific tracer uptake (ie, F-dopa Ki-values and PK11195 binding potential) were constructed at voxel level using Patlak graphical analysis and a supervised cluster-analysis with compartmental modeling, respectively. A region of interest analysis was performed on a priori defined regions.
Compared with controls, patients with idiopathic REM sleep behavior disorder showed significantly reduced 18F-DOPA tracer uptake in the substantia nigra. Patients with higher substantia nigra11C-PK11195 binding also had increased binding in the ipsilateral putamen. Patients with more severe reductions in putaminal 18F-DOPA uptake had significantly higher 11C-PK11195 binding in the putamen and substantia nigra.
—Jake Remaly
NOACs outpace warfarin for afib anticoagulation
The NOAC revolution has happened.
New oral anticoagulants (NOACs) now claim the majority of the oral anticoagulant market in patients with new atrial fibrillation, wresting the advantage from warfarin in the U.S. and globally. This is the promise NOACs held even while still in development, the potential to whittle warfarin use down to a shadow of what it was. Despite a sputtering reception when the first NOAC, dabigatran (Pradaxa), came onto the U.S. market in late 2010, the four NOACs (also apixaban [Eliquis], edoxaban [Savaysa], and rivaroxaban [Xarelto]) now available in the United States and elsewhere gradually gained traction and today they collectively form the most widely used oral anticoagulant option for patients newly diagnosed with atrial fibrillation.
Data reported in August at the annual congress of the European Society of Cardiology showed that in Denmark during the first half of 2015, NOACs accounted for 73% of oral anticoagulant prescriptions for patients with newly diagnosed atrial fibrillation, Kasper Gadsboll, MD, reported. He and his associates studied NOAC and warfarin use in a total of 108,410 atrial fibrillation patients newly diagnosed starting in 2005. Through 2010, warfarin was the only option, but starting in early 2011 when the first NOAC became available in Denmark, use of the class rose sharply with a corresponding plummet in warfarin prescriptions. Not only did the NOACs largely supplant warfarin during 2011-2015, but they also powered an overall rise in the percentage of atrial fibrillation patients treated with an oral anticoagulant, boosting the rate by a relative 75% between the end of 2009 and mid-2015.
“People were reluctant to start an oral anticoagulant because they feared bleeding. Now with NOACs they are not as fearful,” said Dr. Gadsboll, a cardiology researcher at Gentofte Hospital in Hellerup, Denmark. He also noted that in Denmark the national health system pays for prescribed drugs, so the increased direct cost for NOAC treatment in place of warfarin is covered by the Danish government.
“NOAC uptake is gathering steam,” commented Stuart J. Connolly, MD, an electrophysiologist and professor of medicine at McMaster University in Hamilton, Ontario. “When the government pays for it, people prescribe it more, but increased NOAC use is happening everywhere,” said Dr. Connolly, who led major trials that assessed dabigatran and apixaban in atrial fibrillation patients. “I’ve seen Canadian data that show warfarin use is falling and NOAC use is increasing. People are more willing to prescribe NOACs because they are safer,” he said in an interview.
The most current U.S. data I found came from the IMS Health National Disease and Therapeutic Index in 2014, based on a survey of a representative sample of about 4,800 U.S. office-based physicians. The results showed that the NOAC share of oral anticoagulant use in patients who had office visits for atrial fibrillation jumped from 6% of patients in early 2011 to roughly half of all atrial fibrillation patients, 48%, during 2014 (Am J Med. 2015 Dec;128[12]:1300-5). That trajectory makes it likely that by now NOACs have a clear lead.
It’s a similar story in several European countries, such as in Belgium where NOACs now account for about 70% of new prescriptions for atrial fibrillation patients, commented Freek W.A. Verheugt, MD. In the Netherlands, however, NOACs have not taken off, in large part because a popular and entrenched thrombosis clinic system exists that employs thousands of Dutch workers, thereby making choice of an anticoagulant drug a social and political question as well as a medical one, he said. “Warfarin is still indicated for patients with an artificial heart valve or poor kidney function,” noted Dr. Verheugt, professor of cardiology at the University of Nijmegen in the Netherlands, “but a majority of atrial fibrillation patients will eventually change to a NOAC. Even when treatment with warfarin has a good time-in-therapeutic-range, NOACs are still better. They are so much safer.”
On Twitter @mitchelzoler
The NOAC revolution has happened.
New oral anticoagulants (NOACs) now claim the majority of the oral anticoagulant market in patients with new atrial fibrillation, wresting the advantage from warfarin in the U.S. and globally. This is the promise NOACs held even while still in development, the potential to whittle warfarin use down to a shadow of what it was. Despite a sputtering reception when the first NOAC, dabigatran (Pradaxa), came onto the U.S. market in late 2010, the four NOACs (also apixaban [Eliquis], edoxaban [Savaysa], and rivaroxaban [Xarelto]) now available in the United States and elsewhere gradually gained traction and today they collectively form the most widely used oral anticoagulant option for patients newly diagnosed with atrial fibrillation.
Data reported in August at the annual congress of the European Society of Cardiology showed that in Denmark during the first half of 2015, NOACs accounted for 73% of oral anticoagulant prescriptions for patients with newly diagnosed atrial fibrillation, Kasper Gadsboll, MD, reported. He and his associates studied NOAC and warfarin use in a total of 108,410 atrial fibrillation patients newly diagnosed starting in 2005. Through 2010, warfarin was the only option, but starting in early 2011 when the first NOAC became available in Denmark, use of the class rose sharply with a corresponding plummet in warfarin prescriptions. Not only did the NOACs largely supplant warfarin during 2011-2015, but they also powered an overall rise in the percentage of atrial fibrillation patients treated with an oral anticoagulant, boosting the rate by a relative 75% between the end of 2009 and mid-2015.
“People were reluctant to start an oral anticoagulant because they feared bleeding. Now with NOACs they are not as fearful,” said Dr. Gadsboll, a cardiology researcher at Gentofte Hospital in Hellerup, Denmark. He also noted that in Denmark the national health system pays for prescribed drugs, so the increased direct cost for NOAC treatment in place of warfarin is covered by the Danish government.
“NOAC uptake is gathering steam,” commented Stuart J. Connolly, MD, an electrophysiologist and professor of medicine at McMaster University in Hamilton, Ontario. “When the government pays for it, people prescribe it more, but increased NOAC use is happening everywhere,” said Dr. Connolly, who led major trials that assessed dabigatran and apixaban in atrial fibrillation patients. “I’ve seen Canadian data that show warfarin use is falling and NOAC use is increasing. People are more willing to prescribe NOACs because they are safer,” he said in an interview.
The most current U.S. data I found came from the IMS Health National Disease and Therapeutic Index in 2014, based on a survey of a representative sample of about 4,800 U.S. office-based physicians. The results showed that the NOAC share of oral anticoagulant use in patients who had office visits for atrial fibrillation jumped from 6% of patients in early 2011 to roughly half of all atrial fibrillation patients, 48%, during 2014 (Am J Med. 2015 Dec;128[12]:1300-5). That trajectory makes it likely that by now NOACs have a clear lead.
It’s a similar story in several European countries, such as in Belgium where NOACs now account for about 70% of new prescriptions for atrial fibrillation patients, commented Freek W.A. Verheugt, MD. In the Netherlands, however, NOACs have not taken off, in large part because a popular and entrenched thrombosis clinic system exists that employs thousands of Dutch workers, thereby making choice of an anticoagulant drug a social and political question as well as a medical one, he said. “Warfarin is still indicated for patients with an artificial heart valve or poor kidney function,” noted Dr. Verheugt, professor of cardiology at the University of Nijmegen in the Netherlands, “but a majority of atrial fibrillation patients will eventually change to a NOAC. Even when treatment with warfarin has a good time-in-therapeutic-range, NOACs are still better. They are so much safer.”
On Twitter @mitchelzoler
The NOAC revolution has happened.
New oral anticoagulants (NOACs) now claim the majority of the oral anticoagulant market in patients with new atrial fibrillation, wresting the advantage from warfarin in the U.S. and globally. This is the promise NOACs held even while still in development, the potential to whittle warfarin use down to a shadow of what it was. Despite a sputtering reception when the first NOAC, dabigatran (Pradaxa), came onto the U.S. market in late 2010, the four NOACs (also apixaban [Eliquis], edoxaban [Savaysa], and rivaroxaban [Xarelto]) now available in the United States and elsewhere gradually gained traction and today they collectively form the most widely used oral anticoagulant option for patients newly diagnosed with atrial fibrillation.
Data reported in August at the annual congress of the European Society of Cardiology showed that in Denmark during the first half of 2015, NOACs accounted for 73% of oral anticoagulant prescriptions for patients with newly diagnosed atrial fibrillation, Kasper Gadsboll, MD, reported. He and his associates studied NOAC and warfarin use in a total of 108,410 atrial fibrillation patients newly diagnosed starting in 2005. Through 2010, warfarin was the only option, but starting in early 2011 when the first NOAC became available in Denmark, use of the class rose sharply with a corresponding plummet in warfarin prescriptions. Not only did the NOACs largely supplant warfarin during 2011-2015, but they also powered an overall rise in the percentage of atrial fibrillation patients treated with an oral anticoagulant, boosting the rate by a relative 75% between the end of 2009 and mid-2015.
“People were reluctant to start an oral anticoagulant because they feared bleeding. Now with NOACs they are not as fearful,” said Dr. Gadsboll, a cardiology researcher at Gentofte Hospital in Hellerup, Denmark. He also noted that in Denmark the national health system pays for prescribed drugs, so the increased direct cost for NOAC treatment in place of warfarin is covered by the Danish government.
“NOAC uptake is gathering steam,” commented Stuart J. Connolly, MD, an electrophysiologist and professor of medicine at McMaster University in Hamilton, Ontario. “When the government pays for it, people prescribe it more, but increased NOAC use is happening everywhere,” said Dr. Connolly, who led major trials that assessed dabigatran and apixaban in atrial fibrillation patients. “I’ve seen Canadian data that show warfarin use is falling and NOAC use is increasing. People are more willing to prescribe NOACs because they are safer,” he said in an interview.
The most current U.S. data I found came from the IMS Health National Disease and Therapeutic Index in 2014, based on a survey of a representative sample of about 4,800 U.S. office-based physicians. The results showed that the NOAC share of oral anticoagulant use in patients who had office visits for atrial fibrillation jumped from 6% of patients in early 2011 to roughly half of all atrial fibrillation patients, 48%, during 2014 (Am J Med. 2015 Dec;128[12]:1300-5). That trajectory makes it likely that by now NOACs have a clear lead.
It’s a similar story in several European countries, such as in Belgium where NOACs now account for about 70% of new prescriptions for atrial fibrillation patients, commented Freek W.A. Verheugt, MD. In the Netherlands, however, NOACs have not taken off, in large part because a popular and entrenched thrombosis clinic system exists that employs thousands of Dutch workers, thereby making choice of an anticoagulant drug a social and political question as well as a medical one, he said. “Warfarin is still indicated for patients with an artificial heart valve or poor kidney function,” noted Dr. Verheugt, professor of cardiology at the University of Nijmegen in the Netherlands, “but a majority of atrial fibrillation patients will eventually change to a NOAC. Even when treatment with warfarin has a good time-in-therapeutic-range, NOACs are still better. They are so much safer.”
On Twitter @mitchelzoler
Direct Immunofluorescence Staining Patterns in Blistering Disorders
Review the PDF of the fact sheet on Direct Immunofluorescence Staining Patterns in Blistering Disorders with board-relevant, easy-to-review material. This fact sheet reviews the dermatologic conditions that typically have positive immunofluorescence staining patterns.
Practice Questions
1. Which autoimmune blistering disease shows deposition of immunoglobulin on the floor of salt-split skin?
a. BP
b. dermatitis herpetiformis
c. epidermolysis bullosa acquisita
d. paraneoplastic pemphigus
e. PV
2. What medicine is commonly implicated in drug-induced pemphigus?
a. acetaminophen
b. amoxicillin
c. naproxen
d. penicillamine
e. penicillin
3. Which autoimmune blistering disease predominantly shows deposition of IgG on DIF?
a. dermatitis herpetiformis
b. IgA pemphigus
c. linear IgA bullous dermatosis
d. paraneoplastic pemphigus
e. porphyria cutanea tarda
4. Which of the following diseases has a negative direct immunofluorescence?
a. dermatitis herpetiformis
b. herpes gestationis
c. pemphigus vulgaris
d. porphyria cutanea tarda
e. transient acantholytic dermatosis
5. Which of the following diseases shows a linear deposition of IgG and C3 along the dermoepidermal junction?
a. CP
b. IgA pemphigus
c. PF
d. porphyria cutanea tarda
e. PV
Answers to practice questions provided on next page
Practice Question Answers
1. Which autoimmune blistering disease shows deposition of immunoglobulin on the floor of salt-split skin?
a. BP
b. dermatitis herpetiformis
c. epidermolysis bullosa acquisita
d. paraneoplastic pemphigus
e. PV
2. What medicine is commonly implicated in drug-induced pemphigus?
a. acetaminophen
b. amoxicillin
c. naproxen
d. penicillamine
e. penicillin
3. Which autoimmune blistering disease predominantly shows deposition of IgG on DIF?
a. dermatitis herpetiformis
b. IgA pemphigus
c. linear IgA bullous dermatosis
d. paraneoplastic pemphigus
e. porphyria cutanea tarda
4. Which of the following diseases has a negative direct immunofluorescence?
a. dermatitis herpetiformis
b. herpes gestationis
c. pemphigus vulgaris
d. porphyria cutanea tarda
e. transient acantholytic dermatosis
5. Which of the following diseases shows a linear deposition of IgG and C3 along the dermoepidermal junction?
a. CP
b. IgA pemphigus
c. PF
d. porphyria cutanea tarda
e. PV
Review the PDF of the fact sheet on Direct Immunofluorescence Staining Patterns in Blistering Disorders with board-relevant, easy-to-review material. This fact sheet reviews the dermatologic conditions that typically have positive immunofluorescence staining patterns.
Practice Questions
1. Which autoimmune blistering disease shows deposition of immunoglobulin on the floor of salt-split skin?
a. BP
b. dermatitis herpetiformis
c. epidermolysis bullosa acquisita
d. paraneoplastic pemphigus
e. PV
2. What medicine is commonly implicated in drug-induced pemphigus?
a. acetaminophen
b. amoxicillin
c. naproxen
d. penicillamine
e. penicillin
3. Which autoimmune blistering disease predominantly shows deposition of IgG on DIF?
a. dermatitis herpetiformis
b. IgA pemphigus
c. linear IgA bullous dermatosis
d. paraneoplastic pemphigus
e. porphyria cutanea tarda
4. Which of the following diseases has a negative direct immunofluorescence?
a. dermatitis herpetiformis
b. herpes gestationis
c. pemphigus vulgaris
d. porphyria cutanea tarda
e. transient acantholytic dermatosis
5. Which of the following diseases shows a linear deposition of IgG and C3 along the dermoepidermal junction?
a. CP
b. IgA pemphigus
c. PF
d. porphyria cutanea tarda
e. PV
Answers to practice questions provided on next page
Practice Question Answers
1. Which autoimmune blistering disease shows deposition of immunoglobulin on the floor of salt-split skin?
a. BP
b. dermatitis herpetiformis
c. epidermolysis bullosa acquisita
d. paraneoplastic pemphigus
e. PV
2. What medicine is commonly implicated in drug-induced pemphigus?
a. acetaminophen
b. amoxicillin
c. naproxen
d. penicillamine
e. penicillin
3. Which autoimmune blistering disease predominantly shows deposition of IgG on DIF?
a. dermatitis herpetiformis
b. IgA pemphigus
c. linear IgA bullous dermatosis
d. paraneoplastic pemphigus
e. porphyria cutanea tarda
4. Which of the following diseases has a negative direct immunofluorescence?
a. dermatitis herpetiformis
b. herpes gestationis
c. pemphigus vulgaris
d. porphyria cutanea tarda
e. transient acantholytic dermatosis
5. Which of the following diseases shows a linear deposition of IgG and C3 along the dermoepidermal junction?
a. CP
b. IgA pemphigus
c. PF
d. porphyria cutanea tarda
e. PV
Review the PDF of the fact sheet on Direct Immunofluorescence Staining Patterns in Blistering Disorders with board-relevant, easy-to-review material. This fact sheet reviews the dermatologic conditions that typically have positive immunofluorescence staining patterns.
Practice Questions
1. Which autoimmune blistering disease shows deposition of immunoglobulin on the floor of salt-split skin?
a. BP
b. dermatitis herpetiformis
c. epidermolysis bullosa acquisita
d. paraneoplastic pemphigus
e. PV
2. What medicine is commonly implicated in drug-induced pemphigus?
a. acetaminophen
b. amoxicillin
c. naproxen
d. penicillamine
e. penicillin
3. Which autoimmune blistering disease predominantly shows deposition of IgG on DIF?
a. dermatitis herpetiformis
b. IgA pemphigus
c. linear IgA bullous dermatosis
d. paraneoplastic pemphigus
e. porphyria cutanea tarda
4. Which of the following diseases has a negative direct immunofluorescence?
a. dermatitis herpetiformis
b. herpes gestationis
c. pemphigus vulgaris
d. porphyria cutanea tarda
e. transient acantholytic dermatosis
5. Which of the following diseases shows a linear deposition of IgG and C3 along the dermoepidermal junction?
a. CP
b. IgA pemphigus
c. PF
d. porphyria cutanea tarda
e. PV
Answers to practice questions provided on next page
Practice Question Answers
1. Which autoimmune blistering disease shows deposition of immunoglobulin on the floor of salt-split skin?
a. BP
b. dermatitis herpetiformis
c. epidermolysis bullosa acquisita
d. paraneoplastic pemphigus
e. PV
2. What medicine is commonly implicated in drug-induced pemphigus?
a. acetaminophen
b. amoxicillin
c. naproxen
d. penicillamine
e. penicillin
3. Which autoimmune blistering disease predominantly shows deposition of IgG on DIF?
a. dermatitis herpetiformis
b. IgA pemphigus
c. linear IgA bullous dermatosis
d. paraneoplastic pemphigus
e. porphyria cutanea tarda
4. Which of the following diseases has a negative direct immunofluorescence?
a. dermatitis herpetiformis
b. herpes gestationis
c. pemphigus vulgaris
d. porphyria cutanea tarda
e. transient acantholytic dermatosis
5. Which of the following diseases shows a linear deposition of IgG and C3 along the dermoepidermal junction?
a. CP
b. IgA pemphigus
c. PF
d. porphyria cutanea tarda
e. PV
CardioMEMS shows real-world heart failure benefit
ORLANDO – Pulmonary artery pressure monitoring using an implanted device was even more effective for controlling pulmonary artery pressures in 2,000 real-world U.S. heart failure patients than it was in the pivotal trial that led to the device’s regulatory approval.
Data from the first 2,000 U.S. heart failure patients to receive the CardioMEMS pulmonary artery (PA) pressure monitoring device and have at least 6 months of follow-up data since the device received Food and Drug Administration approval in 2014 showed that cumulative PA pressure reductions in these patients during the first 6 months of use averaged 434 mm Hg per patient when compared with their baseline PA pressure when they first received the device. This was nearly threefold better than the average 150–mm Hg cumulative reduction in PA pressure per patient during 6 months of use seen in the CHAMPION trial, Dr. William T. Abraham reported at the annual scientific meeting of the Heart Failure Society of America.
Although this analysis of data from the registry maintained for U.S. patients who receive the CardioMEMS device does not yet include information on how these patients fared clinically, and specifically how often they required rehospitalization for heart failure, the strikingly high level of PA pressure control seen in the first 2,000 U.S. patients bodes well for what the clinical findings will show once they are available.
“In our experience with PA pressure monitoring, there is almost a linear relationship between reduced PA pressures and reduced numbers of events” in the form of rehospitalizations for heart failure, said Dr. Abraham, professor of medicine and director of cardiovascular medicine at Ohio State University in Columbus. Once data on outcomes are analyzed for the registry patients, “I think they will be even better than they were in the trial,” he said in an interview.
The PA pressure data in these initial patients “are very important because they tell us that in general use, clinicians – many of whom are at community hospitals – are very capable of using the CardioMEMS data to control patient pressures, and in CHAMPION we showed that there is a relationship between controlled pressures and improved outcomes,” he said. The findings also help allay a key concern about the potential benefit from implanting a device to monitor PA pressure, which is that clinicians must respond to the information and tweak a patient’s diuretic and vasodilator treatments in order for pressure monitoring to have an effect on heart failure outcomes.
“These data clearly refute that concern,” Dr. Abraham said.
He expressed some surprise that PA pressure control with monitoring was so much more effective in real-world use than in the CHAMPION pivotal trial. “In the trial, it was a paradigm shift to manage heart failure patients based on their PA pressures and not according to their symptoms,” he said. With CardioMEMS pressure monitoring, clinicians are supposed to treat high PA pressure with dose adjustments even if the patient feels okay. The new data suggest that clinicians now using the device “have gotten the message that if you don’t do something with the data the patients won’t improve.”
The registry patients came from 47 states and 427 unique physicians who worked in a range of settings including large and small centers, and academic and nonacademic community centers. The patients averaged 70 years, 40% were women, a third had a left ventricular ejection fraction at or above 40%, and their average PA pressure at the time they had their device implanted was 34.9 mm Hg. This pressure was notably higher than the average 31.6 mm Hg pressure among patients enrolled in CHAMPION, a fact that also helps explain why the registry patients received a larger pressure-reduction benefit: They started from a higher level than the trial patients, and during follow-up, their achieved pressures were always compared back to their high baseline pressures.
The registry patients were also substantially older than the trial patients, who had averaged 62 years, and the registry included substantially more women and more patients with higher ejection fractions. Dr. Abraham did not report data on their New York Heart Association class at entry, but labeling for CardioMEMS specifies that patients should have class III heart failure as well as a recent heart failure hospitalization.
Dr. Abraham’s analysis also showed that the greatest degree of PA pressure control occurred in the patients who began device-based treatment with the highest PA pressures. Nearly half the 2,000 registry patients had an entry PA pressure at or above 35 mm Hg, and over a period of 6 months, they averaged a cumulative 876–mm Hg reduction in their PA pressure relative to their baseline level. The third of patients who began with a PA pressure of 25-34 mm Hg had an average 169–mm Hg cumulative pressure reduction over the 6 month period, and the 18% of patients who began with a PA pressure of less than 25 mm Hg actually had an average cumulative increase in the PA pressure of 163 mm Hg. Target PA pressures are usually in the normal range of 18-25 mm Hg.
The analyses also showed that the impact of PA pressure monitoring on pressure was roughly similar regardless of the left ventricular ejection fraction patients had at baseline, and regardless of their sex.
The registry data were collected by St. Jude, the company that markets the CardioMEMS device. Dr. Abraham is a consultant to St. Jude and was lead investigator for the CHAMPION pivotal trial.
On Twitter @mitchelzoler
ORLANDO – Pulmonary artery pressure monitoring using an implanted device was even more effective for controlling pulmonary artery pressures in 2,000 real-world U.S. heart failure patients than it was in the pivotal trial that led to the device’s regulatory approval.
Data from the first 2,000 U.S. heart failure patients to receive the CardioMEMS pulmonary artery (PA) pressure monitoring device and have at least 6 months of follow-up data since the device received Food and Drug Administration approval in 2014 showed that cumulative PA pressure reductions in these patients during the first 6 months of use averaged 434 mm Hg per patient when compared with their baseline PA pressure when they first received the device. This was nearly threefold better than the average 150–mm Hg cumulative reduction in PA pressure per patient during 6 months of use seen in the CHAMPION trial, Dr. William T. Abraham reported at the annual scientific meeting of the Heart Failure Society of America.
Although this analysis of data from the registry maintained for U.S. patients who receive the CardioMEMS device does not yet include information on how these patients fared clinically, and specifically how often they required rehospitalization for heart failure, the strikingly high level of PA pressure control seen in the first 2,000 U.S. patients bodes well for what the clinical findings will show once they are available.
“In our experience with PA pressure monitoring, there is almost a linear relationship between reduced PA pressures and reduced numbers of events” in the form of rehospitalizations for heart failure, said Dr. Abraham, professor of medicine and director of cardiovascular medicine at Ohio State University in Columbus. Once data on outcomes are analyzed for the registry patients, “I think they will be even better than they were in the trial,” he said in an interview.
The PA pressure data in these initial patients “are very important because they tell us that in general use, clinicians – many of whom are at community hospitals – are very capable of using the CardioMEMS data to control patient pressures, and in CHAMPION we showed that there is a relationship between controlled pressures and improved outcomes,” he said. The findings also help allay a key concern about the potential benefit from implanting a device to monitor PA pressure, which is that clinicians must respond to the information and tweak a patient’s diuretic and vasodilator treatments in order for pressure monitoring to have an effect on heart failure outcomes.
“These data clearly refute that concern,” Dr. Abraham said.
He expressed some surprise that PA pressure control with monitoring was so much more effective in real-world use than in the CHAMPION pivotal trial. “In the trial, it was a paradigm shift to manage heart failure patients based on their PA pressures and not according to their symptoms,” he said. With CardioMEMS pressure monitoring, clinicians are supposed to treat high PA pressure with dose adjustments even if the patient feels okay. The new data suggest that clinicians now using the device “have gotten the message that if you don’t do something with the data the patients won’t improve.”
The registry patients came from 47 states and 427 unique physicians who worked in a range of settings including large and small centers, and academic and nonacademic community centers. The patients averaged 70 years, 40% were women, a third had a left ventricular ejection fraction at or above 40%, and their average PA pressure at the time they had their device implanted was 34.9 mm Hg. This pressure was notably higher than the average 31.6 mm Hg pressure among patients enrolled in CHAMPION, a fact that also helps explain why the registry patients received a larger pressure-reduction benefit: They started from a higher level than the trial patients, and during follow-up, their achieved pressures were always compared back to their high baseline pressures.
The registry patients were also substantially older than the trial patients, who had averaged 62 years, and the registry included substantially more women and more patients with higher ejection fractions. Dr. Abraham did not report data on their New York Heart Association class at entry, but labeling for CardioMEMS specifies that patients should have class III heart failure as well as a recent heart failure hospitalization.
Dr. Abraham’s analysis also showed that the greatest degree of PA pressure control occurred in the patients who began device-based treatment with the highest PA pressures. Nearly half the 2,000 registry patients had an entry PA pressure at or above 35 mm Hg, and over a period of 6 months, they averaged a cumulative 876–mm Hg reduction in their PA pressure relative to their baseline level. The third of patients who began with a PA pressure of 25-34 mm Hg had an average 169–mm Hg cumulative pressure reduction over the 6 month period, and the 18% of patients who began with a PA pressure of less than 25 mm Hg actually had an average cumulative increase in the PA pressure of 163 mm Hg. Target PA pressures are usually in the normal range of 18-25 mm Hg.
The analyses also showed that the impact of PA pressure monitoring on pressure was roughly similar regardless of the left ventricular ejection fraction patients had at baseline, and regardless of their sex.
The registry data were collected by St. Jude, the company that markets the CardioMEMS device. Dr. Abraham is a consultant to St. Jude and was lead investigator for the CHAMPION pivotal trial.
On Twitter @mitchelzoler
ORLANDO – Pulmonary artery pressure monitoring using an implanted device was even more effective for controlling pulmonary artery pressures in 2,000 real-world U.S. heart failure patients than it was in the pivotal trial that led to the device’s regulatory approval.
Data from the first 2,000 U.S. heart failure patients to receive the CardioMEMS pulmonary artery (PA) pressure monitoring device and have at least 6 months of follow-up data since the device received Food and Drug Administration approval in 2014 showed that cumulative PA pressure reductions in these patients during the first 6 months of use averaged 434 mm Hg per patient when compared with their baseline PA pressure when they first received the device. This was nearly threefold better than the average 150–mm Hg cumulative reduction in PA pressure per patient during 6 months of use seen in the CHAMPION trial, Dr. William T. Abraham reported at the annual scientific meeting of the Heart Failure Society of America.
Although this analysis of data from the registry maintained for U.S. patients who receive the CardioMEMS device does not yet include information on how these patients fared clinically, and specifically how often they required rehospitalization for heart failure, the strikingly high level of PA pressure control seen in the first 2,000 U.S. patients bodes well for what the clinical findings will show once they are available.
“In our experience with PA pressure monitoring, there is almost a linear relationship between reduced PA pressures and reduced numbers of events” in the form of rehospitalizations for heart failure, said Dr. Abraham, professor of medicine and director of cardiovascular medicine at Ohio State University in Columbus. Once data on outcomes are analyzed for the registry patients, “I think they will be even better than they were in the trial,” he said in an interview.
The PA pressure data in these initial patients “are very important because they tell us that in general use, clinicians – many of whom are at community hospitals – are very capable of using the CardioMEMS data to control patient pressures, and in CHAMPION we showed that there is a relationship between controlled pressures and improved outcomes,” he said. The findings also help allay a key concern about the potential benefit from implanting a device to monitor PA pressure, which is that clinicians must respond to the information and tweak a patient’s diuretic and vasodilator treatments in order for pressure monitoring to have an effect on heart failure outcomes.
“These data clearly refute that concern,” Dr. Abraham said.
He expressed some surprise that PA pressure control with monitoring was so much more effective in real-world use than in the CHAMPION pivotal trial. “In the trial, it was a paradigm shift to manage heart failure patients based on their PA pressures and not according to their symptoms,” he said. With CardioMEMS pressure monitoring, clinicians are supposed to treat high PA pressure with dose adjustments even if the patient feels okay. The new data suggest that clinicians now using the device “have gotten the message that if you don’t do something with the data the patients won’t improve.”
The registry patients came from 47 states and 427 unique physicians who worked in a range of settings including large and small centers, and academic and nonacademic community centers. The patients averaged 70 years, 40% were women, a third had a left ventricular ejection fraction at or above 40%, and their average PA pressure at the time they had their device implanted was 34.9 mm Hg. This pressure was notably higher than the average 31.6 mm Hg pressure among patients enrolled in CHAMPION, a fact that also helps explain why the registry patients received a larger pressure-reduction benefit: They started from a higher level than the trial patients, and during follow-up, their achieved pressures were always compared back to their high baseline pressures.
The registry patients were also substantially older than the trial patients, who had averaged 62 years, and the registry included substantially more women and more patients with higher ejection fractions. Dr. Abraham did not report data on their New York Heart Association class at entry, but labeling for CardioMEMS specifies that patients should have class III heart failure as well as a recent heart failure hospitalization.
Dr. Abraham’s analysis also showed that the greatest degree of PA pressure control occurred in the patients who began device-based treatment with the highest PA pressures. Nearly half the 2,000 registry patients had an entry PA pressure at or above 35 mm Hg, and over a period of 6 months, they averaged a cumulative 876–mm Hg reduction in their PA pressure relative to their baseline level. The third of patients who began with a PA pressure of 25-34 mm Hg had an average 169–mm Hg cumulative pressure reduction over the 6 month period, and the 18% of patients who began with a PA pressure of less than 25 mm Hg actually had an average cumulative increase in the PA pressure of 163 mm Hg. Target PA pressures are usually in the normal range of 18-25 mm Hg.
The analyses also showed that the impact of PA pressure monitoring on pressure was roughly similar regardless of the left ventricular ejection fraction patients had at baseline, and regardless of their sex.
The registry data were collected by St. Jude, the company that markets the CardioMEMS device. Dr. Abraham is a consultant to St. Jude and was lead investigator for the CHAMPION pivotal trial.
On Twitter @mitchelzoler
AT THE HFSA ANNUAL SCIENTIFIC MEETING
Key clinical point: Registry data from the first 2,000 U.S. heart failure patients who received an implanted pulmonary artery pressure monitor showed a level of pressure control over a period of 6 months nearly triple that seen in the pivotal trial.
Major finding: Cumulative pulmonary artery pressure reductions averaged 434 mm Hg over a period of 6 months, compared with an average reduction of 150 mm Hg in the pivotal trial.
Data source: The first 2,000 U.S. patients who received a CardioMEMS device and were followed for at least 6 months.
Disclosures: The registry data were collected by St. Jude, the company that markets the CardioMEMS device. Dr. Abraham is a consultant to St. Jude and was lead investigator for the CHAMPION pivotal trial.
ACOS definitions under fire
LONDON – A study comparing patient data with six definitions of the Asthma-COPD Overlap Syndrome (ACOS) found only one of the patients analyzed met all definitions. This provoked an animated discussion at the annual congress of the European Respiratory Society about the utility of ACOS as a clinical entity.
Of 864 patients diagnosed with chronic obstructive pulmonary disease (COPD) or asthma drawn from the Netherlands Epidemiology of Obesity cohort (a population-based study with 5,784 patients), 39.1% (338 patients) met at least one of the definitions of ACOS, while 0.1% (one patient) met the criteria for all six definitions.
When this finding was presented, the ERS audience first laughed and then applauded. At the end of the presentation, long lines formed at the microphones. Every comment made was hostile to the concept of ACOS.
“Let us bring ACOS to an honorable death,” said one audience member. His point, reiterated by all who commented subsequently, was that ACOS confuses efforts to treat the underlying respiratory symptoms. Even in those who have both asthma and COPD, the speaker, like other members of the audience, said he considered the diagnosis of ACOS unhelpful.
The six definitions in the study included the latest and just published consensus definition from the ERS (Eur Respir J. 2016;48[3]:664-73). According to the ERS definition, the key features of ACOS are age greater than 40 years, long-term history of asthma (since childhood or early adulthood), and significant exposure to cigarette or biomass smoke.
The other definitions analyzed included a medical history of both asthma and COPD, a self-reported history of both asthma and COPD, and a record of the proportion of a person’s vital capacity that he/she is able to expire in 1 second of forced expiration of less than 0.7 plus a record of fractionated nitric oxide concentration in exhaled breath of greater than or equal to 45 parts per billion.
Although attempted, a Venn diagram that would show overlapping subsets of patients that fell into these definitions “was not possible,” according to Tobias Bonten, MD, University of Leiden, the Netherlands.
Asthma duration was just over 10 years in those identified as having ACOS by medical history alone (registry-based definitions), just over 20 years in those with a medical history and objective evidence of impaired lung function, but about 40 years in those with a self-report of both asthma and COPD.
One area that all groups created by the ACOS definitions did have in common was demographic variables, such as median age, proportion of patients defined as overweight or obese by body mass index, and proportion who were current smokers.
Members of the audience acknowledged the importance of considering the coexistence of asthma and COPD, but expressed skepticism about the value of ACOS as a separate entity in the clinic.
“ACOS is something like the emperor’s new clothes,” one audience member said during the discussion. “It is important to identify asthma patients with obstruction because they have reduced lung function that should be treated more actively, but I find the definition [of ACOS] unnecessary,” he said.
A similar conclusion was drawn in a review article devoted to ACOS published last year (N Engl J Med. 2015;373[13]:1241-9). “It is premature to recommend the designation of ACOS as a disease entity,” the authors wrote.
This is a position widely shared by clinicians, judging from audience comments provoked by this demonstration.
For the sake of time, the moderators were forced to end the discussion with significant lines of clinicians at the microphone.
“It is quite clear that ACOS should die,” said one of the last speakers given a chance to voice an opinion. He suggested that the coexistence of asthma and COPD is something that “quite clearly can happen,” but he objected to definitions he said are unhelpful for clinical care.
Dr. Bonten reported no relevant financial relationships.
LONDON – A study comparing patient data with six definitions of the Asthma-COPD Overlap Syndrome (ACOS) found only one of the patients analyzed met all definitions. This provoked an animated discussion at the annual congress of the European Respiratory Society about the utility of ACOS as a clinical entity.
Of 864 patients diagnosed with chronic obstructive pulmonary disease (COPD) or asthma drawn from the Netherlands Epidemiology of Obesity cohort (a population-based study with 5,784 patients), 39.1% (338 patients) met at least one of the definitions of ACOS, while 0.1% (one patient) met the criteria for all six definitions.
When this finding was presented, the ERS audience first laughed and then applauded. At the end of the presentation, long lines formed at the microphones. Every comment made was hostile to the concept of ACOS.
“Let us bring ACOS to an honorable death,” said one audience member. His point, reiterated by all who commented subsequently, was that ACOS confuses efforts to treat the underlying respiratory symptoms. Even in those who have both asthma and COPD, the speaker, like other members of the audience, said he considered the diagnosis of ACOS unhelpful.
The six definitions in the study included the latest and just published consensus definition from the ERS (Eur Respir J. 2016;48[3]:664-73). According to the ERS definition, the key features of ACOS are age greater than 40 years, long-term history of asthma (since childhood or early adulthood), and significant exposure to cigarette or biomass smoke.
The other definitions analyzed included a medical history of both asthma and COPD, a self-reported history of both asthma and COPD, and a record of the proportion of a person’s vital capacity that he/she is able to expire in 1 second of forced expiration of less than 0.7 plus a record of fractionated nitric oxide concentration in exhaled breath of greater than or equal to 45 parts per billion.
Although attempted, a Venn diagram that would show overlapping subsets of patients that fell into these definitions “was not possible,” according to Tobias Bonten, MD, University of Leiden, the Netherlands.
Asthma duration was just over 10 years in those identified as having ACOS by medical history alone (registry-based definitions), just over 20 years in those with a medical history and objective evidence of impaired lung function, but about 40 years in those with a self-report of both asthma and COPD.
One area that all groups created by the ACOS definitions did have in common was demographic variables, such as median age, proportion of patients defined as overweight or obese by body mass index, and proportion who were current smokers.
Members of the audience acknowledged the importance of considering the coexistence of asthma and COPD, but expressed skepticism about the value of ACOS as a separate entity in the clinic.
“ACOS is something like the emperor’s new clothes,” one audience member said during the discussion. “It is important to identify asthma patients with obstruction because they have reduced lung function that should be treated more actively, but I find the definition [of ACOS] unnecessary,” he said.
A similar conclusion was drawn in a review article devoted to ACOS published last year (N Engl J Med. 2015;373[13]:1241-9). “It is premature to recommend the designation of ACOS as a disease entity,” the authors wrote.
This is a position widely shared by clinicians, judging from audience comments provoked by this demonstration.
For the sake of time, the moderators were forced to end the discussion with significant lines of clinicians at the microphone.
“It is quite clear that ACOS should die,” said one of the last speakers given a chance to voice an opinion. He suggested that the coexistence of asthma and COPD is something that “quite clearly can happen,” but he objected to definitions he said are unhelpful for clinical care.
Dr. Bonten reported no relevant financial relationships.
LONDON – A study comparing patient data with six definitions of the Asthma-COPD Overlap Syndrome (ACOS) found only one of the patients analyzed met all definitions. This provoked an animated discussion at the annual congress of the European Respiratory Society about the utility of ACOS as a clinical entity.
Of 864 patients diagnosed with chronic obstructive pulmonary disease (COPD) or asthma drawn from the Netherlands Epidemiology of Obesity cohort (a population-based study with 5,784 patients), 39.1% (338 patients) met at least one of the definitions of ACOS, while 0.1% (one patient) met the criteria for all six definitions.
When this finding was presented, the ERS audience first laughed and then applauded. At the end of the presentation, long lines formed at the microphones. Every comment made was hostile to the concept of ACOS.
“Let us bring ACOS to an honorable death,” said one audience member. His point, reiterated by all who commented subsequently, was that ACOS confuses efforts to treat the underlying respiratory symptoms. Even in those who have both asthma and COPD, the speaker, like other members of the audience, said he considered the diagnosis of ACOS unhelpful.
The six definitions in the study included the latest and just published consensus definition from the ERS (Eur Respir J. 2016;48[3]:664-73). According to the ERS definition, the key features of ACOS are age greater than 40 years, long-term history of asthma (since childhood or early adulthood), and significant exposure to cigarette or biomass smoke.
The other definitions analyzed included a medical history of both asthma and COPD, a self-reported history of both asthma and COPD, and a record of the proportion of a person’s vital capacity that he/she is able to expire in 1 second of forced expiration of less than 0.7 plus a record of fractionated nitric oxide concentration in exhaled breath of greater than or equal to 45 parts per billion.
Although attempted, a Venn diagram that would show overlapping subsets of patients that fell into these definitions “was not possible,” according to Tobias Bonten, MD, University of Leiden, the Netherlands.
Asthma duration was just over 10 years in those identified as having ACOS by medical history alone (registry-based definitions), just over 20 years in those with a medical history and objective evidence of impaired lung function, but about 40 years in those with a self-report of both asthma and COPD.
One area that all groups created by the ACOS definitions did have in common was demographic variables, such as median age, proportion of patients defined as overweight or obese by body mass index, and proportion who were current smokers.
Members of the audience acknowledged the importance of considering the coexistence of asthma and COPD, but expressed skepticism about the value of ACOS as a separate entity in the clinic.
“ACOS is something like the emperor’s new clothes,” one audience member said during the discussion. “It is important to identify asthma patients with obstruction because they have reduced lung function that should be treated more actively, but I find the definition [of ACOS] unnecessary,” he said.
A similar conclusion was drawn in a review article devoted to ACOS published last year (N Engl J Med. 2015;373[13]:1241-9). “It is premature to recommend the designation of ACOS as a disease entity,” the authors wrote.
This is a position widely shared by clinicians, judging from audience comments provoked by this demonstration.
For the sake of time, the moderators were forced to end the discussion with significant lines of clinicians at the microphone.
“It is quite clear that ACOS should die,” said one of the last speakers given a chance to voice an opinion. He suggested that the coexistence of asthma and COPD is something that “quite clearly can happen,” but he objected to definitions he said are unhelpful for clinical care.
Dr. Bonten reported no relevant financial relationships.
AT THE ERS CONGRESS 2016
Key clinical point: A study comparing current definitions of Asthma-COPD Overlap Syndrome (ACOS) provoked criticism of the very concept.
Major finding: When six definitions of ACOS were compared in a population-based study, only 1 (0.1%) of 864 possible candidates met all criteria of all definitions.
Data source: Population-based cohort study.
Disclosures: Dr. Bonten reported no relevant financial relationships.
Evaluating PD-1 Inhibitors
PD-1 antibodies are being touted as a promising immunotherapeutic approach for treating malignant melanoma among other cancers. The antibodies target proteins that promote programmed cell death and activate the immune system to attack tumors.
To find out more about the efficacy and safety of PD-1 antibody treatment, researchers from Xiamen University, Chinese Academy of Medical Sciences, and Peking Union Medical College, China, reviewed data from 5 multicenter, randomized clinical trials involving 2,828 patients. In 2 trials, patients were previously untreated; in the other 3, patients had progression after anti-CTLA-4 treatment or had received no more than 1 previous systemic therapy. Patients in the experimental groups received nivolumab or pembrolizumab; patients in the control groups received ipilimumab or chemotherapy.
In all 5 trials, the researchers noted significant differences between the anti-PD-1 groups and the control groups. The PD-1 antibody treatment was associated with a significantly better overall response rate (ORR): 40.0% in patients on nivolumab 3 mg/kg IV every 2 weeks as front-line therapy and 31.6% in those who received nivolumab at the same dosage after progression from anti-CTLA-4 treatment. Response was improved whether the drug was used as first-line treatment or for refractory/relapsed melanoma.
Related: Nivolumab Approved for Expanded Indication
Patients in the PD-1 groups also had a significantly greater rate of progression-free survival (PFS) compared with those who received other treatments, such as chemotherapy and ipilimumab. The median PFS was > 4.7 months in the nivolumab group and > 3.7 months in the pembrolizumab group. In 2 trials, the ORR among patients receiving pembrolizumab was between 23.3% and 33.2%; different dosages improved the overall response rate in both untreated and relapsed/refractory patients.
The most common adverse events (AEs) were fatigue, diarrhea, pruritus, rash, and nausea. Patients treated with nivolumab reported significantly fewer AEs. Although a high dosage or short intermission of pembrolizumab extended the median PFS, a subgroup analysis of different doses revealed a significant dose-dependent increase in AEs.
Source:
Lin Z, Chen X, Li Z, et al. PLoS One. 2016;11(8):e0160485.
doi: 10.1371/journal.pone.0160485.
PD-1 antibodies are being touted as a promising immunotherapeutic approach for treating malignant melanoma among other cancers. The antibodies target proteins that promote programmed cell death and activate the immune system to attack tumors.
To find out more about the efficacy and safety of PD-1 antibody treatment, researchers from Xiamen University, Chinese Academy of Medical Sciences, and Peking Union Medical College, China, reviewed data from 5 multicenter, randomized clinical trials involving 2,828 patients. In 2 trials, patients were previously untreated; in the other 3, patients had progression after anti-CTLA-4 treatment or had received no more than 1 previous systemic therapy. Patients in the experimental groups received nivolumab or pembrolizumab; patients in the control groups received ipilimumab or chemotherapy.
In all 5 trials, the researchers noted significant differences between the anti-PD-1 groups and the control groups. The PD-1 antibody treatment was associated with a significantly better overall response rate (ORR): 40.0% in patients on nivolumab 3 mg/kg IV every 2 weeks as front-line therapy and 31.6% in those who received nivolumab at the same dosage after progression from anti-CTLA-4 treatment. Response was improved whether the drug was used as first-line treatment or for refractory/relapsed melanoma.
Related: Nivolumab Approved for Expanded Indication
Patients in the PD-1 groups also had a significantly greater rate of progression-free survival (PFS) compared with those who received other treatments, such as chemotherapy and ipilimumab. The median PFS was > 4.7 months in the nivolumab group and > 3.7 months in the pembrolizumab group. In 2 trials, the ORR among patients receiving pembrolizumab was between 23.3% and 33.2%; different dosages improved the overall response rate in both untreated and relapsed/refractory patients.
The most common adverse events (AEs) were fatigue, diarrhea, pruritus, rash, and nausea. Patients treated with nivolumab reported significantly fewer AEs. Although a high dosage or short intermission of pembrolizumab extended the median PFS, a subgroup analysis of different doses revealed a significant dose-dependent increase in AEs.
Source:
Lin Z, Chen X, Li Z, et al. PLoS One. 2016;11(8):e0160485.
doi: 10.1371/journal.pone.0160485.
PD-1 antibodies are being touted as a promising immunotherapeutic approach for treating malignant melanoma among other cancers. The antibodies target proteins that promote programmed cell death and activate the immune system to attack tumors.
To find out more about the efficacy and safety of PD-1 antibody treatment, researchers from Xiamen University, Chinese Academy of Medical Sciences, and Peking Union Medical College, China, reviewed data from 5 multicenter, randomized clinical trials involving 2,828 patients. In 2 trials, patients were previously untreated; in the other 3, patients had progression after anti-CTLA-4 treatment or had received no more than 1 previous systemic therapy. Patients in the experimental groups received nivolumab or pembrolizumab; patients in the control groups received ipilimumab or chemotherapy.
In all 5 trials, the researchers noted significant differences between the anti-PD-1 groups and the control groups. The PD-1 antibody treatment was associated with a significantly better overall response rate (ORR): 40.0% in patients on nivolumab 3 mg/kg IV every 2 weeks as front-line therapy and 31.6% in those who received nivolumab at the same dosage after progression from anti-CTLA-4 treatment. Response was improved whether the drug was used as first-line treatment or for refractory/relapsed melanoma.
Related: Nivolumab Approved for Expanded Indication
Patients in the PD-1 groups also had a significantly greater rate of progression-free survival (PFS) compared with those who received other treatments, such as chemotherapy and ipilimumab. The median PFS was > 4.7 months in the nivolumab group and > 3.7 months in the pembrolizumab group. In 2 trials, the ORR among patients receiving pembrolizumab was between 23.3% and 33.2%; different dosages improved the overall response rate in both untreated and relapsed/refractory patients.
The most common adverse events (AEs) were fatigue, diarrhea, pruritus, rash, and nausea. Patients treated with nivolumab reported significantly fewer AEs. Although a high dosage or short intermission of pembrolizumab extended the median PFS, a subgroup analysis of different doses revealed a significant dose-dependent increase in AEs.
Source:
Lin Z, Chen X, Li Z, et al. PLoS One. 2016;11(8):e0160485.
doi: 10.1371/journal.pone.0160485.
VHA Touts Best Practices to Senate
In a rare turn, the Senate Veterans Affairs Committee recently focused on what the VA is doing right. “We are changing culture and doing so by celebrating the people who have dedicated their careers to serving veterans,” testified Carolyn Clancy, MD, deputy under secretary for health for organizational excellence. “We are breaking down cultural barriers, like competition, by creating systematic incentives to share what has worked with others in the system.”
Dr. Clancy outlined some of the successes from the VA’s newly developed Diffusion of Excellence Initiative. The goal of the initiative has been to “identify clinical and administrative best practices, disseminate these practices to other sites of care, and encourage standardization of practices that deliver positive outcomes for veterans and their families,” Dr. Clancy reported. So far the initiative has generated more than 260 ongoing innovations at 70 facilities.
According to Dr. Clancy, the Diffusion of Excellence Initiative uses an internal VA social media platform to identify promising practices that align with strategic priorities, offer efficient resource use, can be implemented in diverse care environments, and can be implemented within 6 to 12 months. The initiative also sought to identify local champions or early adopters who can begin to implement the best practice locally. Once it is proven practice, the initiative seeks to find additional locations to further test the practice in a different setting. Best practices are chosen for national standardization if they have had relative success with an initial implementation and similar outcomes when replicated elsewhere.
So far 50 best practices have been replicated and are ready for widespread dissemination. “Identifying and spreading best practices can be a major driver of consistent, high-quality health care for veterans,” Dr. Clancy told the Senate panel. “This is restoring trust in the system. It offers a model for other health systems.”
Examples of best practices include the following:
Improving Same-Day Access Using Registered Nurse (RN) Care Manager “Chair” Visits: At the Boise VAMC in Idaho, the primary care team created a process where same-day appointment requests are triaged and scribed by RN Care Managers, saving primary care providers’ time when they see patients between appointments to assess and confirm the care plan.
Access Data Dashboard to Improve Clinic Management: The data analysis team at Harry S. Truman Memorial Veterans’ Hospital (Columbia, Missouri) implemented a dashboard for clinic access metrics (no-shows, completed appointment wait times, clinic utilization, etc). These metrics are posted monthly on an accessible dashboard that can be used by staff to solve problems and make key decisions that help veterans get timely access to care.
Planning for Future Medical Decision via Group Visits: This initiative presents advance care directives and other care planning for future medical decisions in interactive and patient-centered group visits.
Increasing Access to Primary Care With Pharmacists: The William S. Middleton Memorial Veterans’ Hospital (Madison, Wisconsin) matched clinical pharmacy specialists with multiple patient aligned care teams to conduct new patient intake calls 1 week before a new patient has his or her first appointment with a provider, collecting medications, noting any formulary conversions, and orienting the patient to VA.
eScreening: The eScreening Program is a mobile technology developed to facilitate the screening process and improve care coordination and measurement-based care for veterans. It offers veteran-directed screening, real-time scoring, individualized patient feedback, instantaneous medical record clinical documentation, immediate alerts to clinicians for evaluation and triage, and monitoring of treatment outcomes.
Regional Liver Tumor Board: The hepatology team at the Philadelphia VAMC combined a regional telehealth-supported liver cancer tumor board model, a web-based submission process, and a consolidated database to manage and track communications for patients with liver cancer. This practice has shortened the time for veterans with liver cancer to receive their evaluation and first treatment as well as reduced unnecessary biopsies—easing the minds and experiences of patients and their families in an incredibly stressful time.
In a rare turn, the Senate Veterans Affairs Committee recently focused on what the VA is doing right. “We are changing culture and doing so by celebrating the people who have dedicated their careers to serving veterans,” testified Carolyn Clancy, MD, deputy under secretary for health for organizational excellence. “We are breaking down cultural barriers, like competition, by creating systematic incentives to share what has worked with others in the system.”
Dr. Clancy outlined some of the successes from the VA’s newly developed Diffusion of Excellence Initiative. The goal of the initiative has been to “identify clinical and administrative best practices, disseminate these practices to other sites of care, and encourage standardization of practices that deliver positive outcomes for veterans and their families,” Dr. Clancy reported. So far the initiative has generated more than 260 ongoing innovations at 70 facilities.
According to Dr. Clancy, the Diffusion of Excellence Initiative uses an internal VA social media platform to identify promising practices that align with strategic priorities, offer efficient resource use, can be implemented in diverse care environments, and can be implemented within 6 to 12 months. The initiative also sought to identify local champions or early adopters who can begin to implement the best practice locally. Once it is proven practice, the initiative seeks to find additional locations to further test the practice in a different setting. Best practices are chosen for national standardization if they have had relative success with an initial implementation and similar outcomes when replicated elsewhere.
So far 50 best practices have been replicated and are ready for widespread dissemination. “Identifying and spreading best practices can be a major driver of consistent, high-quality health care for veterans,” Dr. Clancy told the Senate panel. “This is restoring trust in the system. It offers a model for other health systems.”
Examples of best practices include the following:
Improving Same-Day Access Using Registered Nurse (RN) Care Manager “Chair” Visits: At the Boise VAMC in Idaho, the primary care team created a process where same-day appointment requests are triaged and scribed by RN Care Managers, saving primary care providers’ time when they see patients between appointments to assess and confirm the care plan.
Access Data Dashboard to Improve Clinic Management: The data analysis team at Harry S. Truman Memorial Veterans’ Hospital (Columbia, Missouri) implemented a dashboard for clinic access metrics (no-shows, completed appointment wait times, clinic utilization, etc). These metrics are posted monthly on an accessible dashboard that can be used by staff to solve problems and make key decisions that help veterans get timely access to care.
Planning for Future Medical Decision via Group Visits: This initiative presents advance care directives and other care planning for future medical decisions in interactive and patient-centered group visits.
Increasing Access to Primary Care With Pharmacists: The William S. Middleton Memorial Veterans’ Hospital (Madison, Wisconsin) matched clinical pharmacy specialists with multiple patient aligned care teams to conduct new patient intake calls 1 week before a new patient has his or her first appointment with a provider, collecting medications, noting any formulary conversions, and orienting the patient to VA.
eScreening: The eScreening Program is a mobile technology developed to facilitate the screening process and improve care coordination and measurement-based care for veterans. It offers veteran-directed screening, real-time scoring, individualized patient feedback, instantaneous medical record clinical documentation, immediate alerts to clinicians for evaluation and triage, and monitoring of treatment outcomes.
Regional Liver Tumor Board: The hepatology team at the Philadelphia VAMC combined a regional telehealth-supported liver cancer tumor board model, a web-based submission process, and a consolidated database to manage and track communications for patients with liver cancer. This practice has shortened the time for veterans with liver cancer to receive their evaluation and first treatment as well as reduced unnecessary biopsies—easing the minds and experiences of patients and their families in an incredibly stressful time.
In a rare turn, the Senate Veterans Affairs Committee recently focused on what the VA is doing right. “We are changing culture and doing so by celebrating the people who have dedicated their careers to serving veterans,” testified Carolyn Clancy, MD, deputy under secretary for health for organizational excellence. “We are breaking down cultural barriers, like competition, by creating systematic incentives to share what has worked with others in the system.”
Dr. Clancy outlined some of the successes from the VA’s newly developed Diffusion of Excellence Initiative. The goal of the initiative has been to “identify clinical and administrative best practices, disseminate these practices to other sites of care, and encourage standardization of practices that deliver positive outcomes for veterans and their families,” Dr. Clancy reported. So far the initiative has generated more than 260 ongoing innovations at 70 facilities.
According to Dr. Clancy, the Diffusion of Excellence Initiative uses an internal VA social media platform to identify promising practices that align with strategic priorities, offer efficient resource use, can be implemented in diverse care environments, and can be implemented within 6 to 12 months. The initiative also sought to identify local champions or early adopters who can begin to implement the best practice locally. Once it is proven practice, the initiative seeks to find additional locations to further test the practice in a different setting. Best practices are chosen for national standardization if they have had relative success with an initial implementation and similar outcomes when replicated elsewhere.
So far 50 best practices have been replicated and are ready for widespread dissemination. “Identifying and spreading best practices can be a major driver of consistent, high-quality health care for veterans,” Dr. Clancy told the Senate panel. “This is restoring trust in the system. It offers a model for other health systems.”
Examples of best practices include the following:
Improving Same-Day Access Using Registered Nurse (RN) Care Manager “Chair” Visits: At the Boise VAMC in Idaho, the primary care team created a process where same-day appointment requests are triaged and scribed by RN Care Managers, saving primary care providers’ time when they see patients between appointments to assess and confirm the care plan.
Access Data Dashboard to Improve Clinic Management: The data analysis team at Harry S. Truman Memorial Veterans’ Hospital (Columbia, Missouri) implemented a dashboard for clinic access metrics (no-shows, completed appointment wait times, clinic utilization, etc). These metrics are posted monthly on an accessible dashboard that can be used by staff to solve problems and make key decisions that help veterans get timely access to care.
Planning for Future Medical Decision via Group Visits: This initiative presents advance care directives and other care planning for future medical decisions in interactive and patient-centered group visits.
Increasing Access to Primary Care With Pharmacists: The William S. Middleton Memorial Veterans’ Hospital (Madison, Wisconsin) matched clinical pharmacy specialists with multiple patient aligned care teams to conduct new patient intake calls 1 week before a new patient has his or her first appointment with a provider, collecting medications, noting any formulary conversions, and orienting the patient to VA.
eScreening: The eScreening Program is a mobile technology developed to facilitate the screening process and improve care coordination and measurement-based care for veterans. It offers veteran-directed screening, real-time scoring, individualized patient feedback, instantaneous medical record clinical documentation, immediate alerts to clinicians for evaluation and triage, and monitoring of treatment outcomes.
Regional Liver Tumor Board: The hepatology team at the Philadelphia VAMC combined a regional telehealth-supported liver cancer tumor board model, a web-based submission process, and a consolidated database to manage and track communications for patients with liver cancer. This practice has shortened the time for veterans with liver cancer to receive their evaluation and first treatment as well as reduced unnecessary biopsies—easing the minds and experiences of patients and their families in an incredibly stressful time.