User login
The Use of IVC Filters in Cancer Patients: A 15-Year Experience at a Single Veterans Affairs Medical Center
Background: Cancer and chemotherapy are both associated with an increased risk of thrombosis. Venous thromboembolism (VTE) is the leading cause of death in cancer patients. Inferior vena cava (IVC) filter use is recommended in patients with VTE and contraindication to anticoagulation (AC) or recurrent VTE despite treatment. The use of IVC filters in cancer patients outside the Veterans Affairs Medical Center (VAMC) has been described; no such study exists within the VAMC.
Purpose: Descriptive analysis of IVC filters use in cancer patients at the Washington DC VAMC.
Methods: Retrospective study utilizing data from the Washington DC VA Cancer Registry and the electronic health records (EHR). Current Procedural Terminology (CPT) codes for IVC filter placement were used to identify subjects in the cancer registry who received an IVC filter. Demographics, cancer date of diagnosis, VTE date of diagnosis, type of filter, indication, placement date, procedural complications, and AC medication use at time of filter placement were collected. Cancer subjects (n = 6,678) were identified from October 1999 – May 2015 and 64 patients met inclusion criteria.
Data Analysis: Percentages were calculated for the aforementioned data points.
Results: Characteristics of the 64 cancer patients with IVC filter placement include: 100% male, 75% black or African American, 15.6% white, 1.6% Hispanic or Latino, 7.8% unknown. The average age at cancer diagnosis was 65.3 years. Date of cancer diagnosis to VTE diagnosis is currently being analyzed.
VTE diagnosis with an immediate contraindication to AC medication led the list of IVC filter indications (59%). An additional 16% of patients received filters due to subsequent development of AC contraindication. The remaining patients (25%) received filters for prophylaxis, AC failure, or in combination with AC medications. For subjects whose filter type was captured from chart review, 51.8% were given permanent filters versus 48.1% who were given retrievable filters. Overall, there were no complications associated with the procedure.
Implications: Contraindication to immediate AC is the leading indication for IVC filter use in our cancer patients with VTE, consistent with current guidelines. Overall, rates of permanent versus retrievable filter use were similar.
Background: Cancer and chemotherapy are both associated with an increased risk of thrombosis. Venous thromboembolism (VTE) is the leading cause of death in cancer patients. Inferior vena cava (IVC) filter use is recommended in patients with VTE and contraindication to anticoagulation (AC) or recurrent VTE despite treatment. The use of IVC filters in cancer patients outside the Veterans Affairs Medical Center (VAMC) has been described; no such study exists within the VAMC.
Purpose: Descriptive analysis of IVC filters use in cancer patients at the Washington DC VAMC.
Methods: Retrospective study utilizing data from the Washington DC VA Cancer Registry and the electronic health records (EHR). Current Procedural Terminology (CPT) codes for IVC filter placement were used to identify subjects in the cancer registry who received an IVC filter. Demographics, cancer date of diagnosis, VTE date of diagnosis, type of filter, indication, placement date, procedural complications, and AC medication use at time of filter placement were collected. Cancer subjects (n = 6,678) were identified from October 1999 – May 2015 and 64 patients met inclusion criteria.
Data Analysis: Percentages were calculated for the aforementioned data points.
Results: Characteristics of the 64 cancer patients with IVC filter placement include: 100% male, 75% black or African American, 15.6% white, 1.6% Hispanic or Latino, 7.8% unknown. The average age at cancer diagnosis was 65.3 years. Date of cancer diagnosis to VTE diagnosis is currently being analyzed.
VTE diagnosis with an immediate contraindication to AC medication led the list of IVC filter indications (59%). An additional 16% of patients received filters due to subsequent development of AC contraindication. The remaining patients (25%) received filters for prophylaxis, AC failure, or in combination with AC medications. For subjects whose filter type was captured from chart review, 51.8% were given permanent filters versus 48.1% who were given retrievable filters. Overall, there were no complications associated with the procedure.
Implications: Contraindication to immediate AC is the leading indication for IVC filter use in our cancer patients with VTE, consistent with current guidelines. Overall, rates of permanent versus retrievable filter use were similar.
Background: Cancer and chemotherapy are both associated with an increased risk of thrombosis. Venous thromboembolism (VTE) is the leading cause of death in cancer patients. Inferior vena cava (IVC) filter use is recommended in patients with VTE and contraindication to anticoagulation (AC) or recurrent VTE despite treatment. The use of IVC filters in cancer patients outside the Veterans Affairs Medical Center (VAMC) has been described; no such study exists within the VAMC.
Purpose: Descriptive analysis of IVC filters use in cancer patients at the Washington DC VAMC.
Methods: Retrospective study utilizing data from the Washington DC VA Cancer Registry and the electronic health records (EHR). Current Procedural Terminology (CPT) codes for IVC filter placement were used to identify subjects in the cancer registry who received an IVC filter. Demographics, cancer date of diagnosis, VTE date of diagnosis, type of filter, indication, placement date, procedural complications, and AC medication use at time of filter placement were collected. Cancer subjects (n = 6,678) were identified from October 1999 – May 2015 and 64 patients met inclusion criteria.
Data Analysis: Percentages were calculated for the aforementioned data points.
Results: Characteristics of the 64 cancer patients with IVC filter placement include: 100% male, 75% black or African American, 15.6% white, 1.6% Hispanic or Latino, 7.8% unknown. The average age at cancer diagnosis was 65.3 years. Date of cancer diagnosis to VTE diagnosis is currently being analyzed.
VTE diagnosis with an immediate contraindication to AC medication led the list of IVC filter indications (59%). An additional 16% of patients received filters due to subsequent development of AC contraindication. The remaining patients (25%) received filters for prophylaxis, AC failure, or in combination with AC medications. For subjects whose filter type was captured from chart review, 51.8% were given permanent filters versus 48.1% who were given retrievable filters. Overall, there were no complications associated with the procedure.
Implications: Contraindication to immediate AC is the leading indication for IVC filter use in our cancer patients with VTE, consistent with current guidelines. Overall, rates of permanent versus retrievable filter use were similar.
Lean Six Sigma Applied to Tracking Head/Neck Cancer Patients
Purpose: The ENT Clinic will provide safe and quality care to its head/neck cancer (HNC) patients with optimal treatment interventions and cancer surveillance through regularly scheduled follow-up visits by preventing patients from being inadvertently lost to follow-up care.
Background/Problem: ~ 400,000 new cases of HNC are diagnosed and reported each year. A study reported a 47.3% disease recurrence in the first year post-treatment. HNC patients require frequent follow-up care due to the high percentage of potential disease recurrence.
Before activation of a HNC Cancer Surveillance Program a record review in 2012 showed 31.1% of HNC patients in the ENT clinic were lost to follow-up care when appointments were canceled or missed by patients and did not get a rescheduled appointment.
Methods: Vigorous Lean Six Sigma methodological tools were used to carefully assess the problem and to improve outcomes encompassing such tools as root-cause analysis, defining waste barriers, Plan, Do, Study, Act (PDSA).
In Phase I, an Excel spreadsheet was created to manually track and monitor HNC patients for cancer surveillance. Monthly reports thereafter proved that tracking HNC patients using an Excel spreadsheet was successful, and 100% of HNC patients had received follow-up appointments. However, the manual process of tracking HNC patients on an Excel spreadsheet was time consuming with limited functionality.
Phase II – A robust automated electronic identification system was implemented for tracking HNC patients which included additional features that far exceeded the capabilities of manual tracking.
Data Analysis: During the first 8 months of its operation (February 2014 – September 2014) 25 newly diagnosed HNC patients were identified electronically; patients that manual tracking might have missed.
Results: FY15 and FY16 targeted goal was achieved. 100% of HNC patient appointments were recaptured for cancer surveillance that otherwise might have been lost to follow-up using the automated electronic tracking system.
Implications: The automated HNC Dashboard has proved to be a vital tool providing improved access to care. It can be used and customized for tracking other cancer types.
Purpose: The ENT Clinic will provide safe and quality care to its head/neck cancer (HNC) patients with optimal treatment interventions and cancer surveillance through regularly scheduled follow-up visits by preventing patients from being inadvertently lost to follow-up care.
Background/Problem: ~ 400,000 new cases of HNC are diagnosed and reported each year. A study reported a 47.3% disease recurrence in the first year post-treatment. HNC patients require frequent follow-up care due to the high percentage of potential disease recurrence.
Before activation of a HNC Cancer Surveillance Program a record review in 2012 showed 31.1% of HNC patients in the ENT clinic were lost to follow-up care when appointments were canceled or missed by patients and did not get a rescheduled appointment.
Methods: Vigorous Lean Six Sigma methodological tools were used to carefully assess the problem and to improve outcomes encompassing such tools as root-cause analysis, defining waste barriers, Plan, Do, Study, Act (PDSA).
In Phase I, an Excel spreadsheet was created to manually track and monitor HNC patients for cancer surveillance. Monthly reports thereafter proved that tracking HNC patients using an Excel spreadsheet was successful, and 100% of HNC patients had received follow-up appointments. However, the manual process of tracking HNC patients on an Excel spreadsheet was time consuming with limited functionality.
Phase II – A robust automated electronic identification system was implemented for tracking HNC patients which included additional features that far exceeded the capabilities of manual tracking.
Data Analysis: During the first 8 months of its operation (February 2014 – September 2014) 25 newly diagnosed HNC patients were identified electronically; patients that manual tracking might have missed.
Results: FY15 and FY16 targeted goal was achieved. 100% of HNC patient appointments were recaptured for cancer surveillance that otherwise might have been lost to follow-up using the automated electronic tracking system.
Implications: The automated HNC Dashboard has proved to be a vital tool providing improved access to care. It can be used and customized for tracking other cancer types.
Purpose: The ENT Clinic will provide safe and quality care to its head/neck cancer (HNC) patients with optimal treatment interventions and cancer surveillance through regularly scheduled follow-up visits by preventing patients from being inadvertently lost to follow-up care.
Background/Problem: ~ 400,000 new cases of HNC are diagnosed and reported each year. A study reported a 47.3% disease recurrence in the first year post-treatment. HNC patients require frequent follow-up care due to the high percentage of potential disease recurrence.
Before activation of a HNC Cancer Surveillance Program a record review in 2012 showed 31.1% of HNC patients in the ENT clinic were lost to follow-up care when appointments were canceled or missed by patients and did not get a rescheduled appointment.
Methods: Vigorous Lean Six Sigma methodological tools were used to carefully assess the problem and to improve outcomes encompassing such tools as root-cause analysis, defining waste barriers, Plan, Do, Study, Act (PDSA).
In Phase I, an Excel spreadsheet was created to manually track and monitor HNC patients for cancer surveillance. Monthly reports thereafter proved that tracking HNC patients using an Excel spreadsheet was successful, and 100% of HNC patients had received follow-up appointments. However, the manual process of tracking HNC patients on an Excel spreadsheet was time consuming with limited functionality.
Phase II – A robust automated electronic identification system was implemented for tracking HNC patients which included additional features that far exceeded the capabilities of manual tracking.
Data Analysis: During the first 8 months of its operation (February 2014 – September 2014) 25 newly diagnosed HNC patients were identified electronically; patients that manual tracking might have missed.
Results: FY15 and FY16 targeted goal was achieved. 100% of HNC patient appointments were recaptured for cancer surveillance that otherwise might have been lost to follow-up using the automated electronic tracking system.
Implications: The automated HNC Dashboard has proved to be a vital tool providing improved access to care. It can be used and customized for tracking other cancer types.
Implementing Psychosocial Distress Screening at the VA Palo Alto Health Care System: Lessons Learned and Future Directions
Purpose: To create and evaluate psychosocial distress screening procedures for patients diagnosed with cancer at the VA Palo Alto Health Care System (VAPAHCS).
Relevant Background/Problem: A program development and evaluation project was conducted at VAPAHCS in order to implement psychosocial distress screening according to the Cancer Program Standards (Standard 3.2) of the American College of Surgeon’s Commission on Cancer.
Methods: Veterans recently diagnosed with cancer were screened for distress by a psychologist, social worker, or psychology trainee during one of their regularly scheduled oncology appointments using the National Comprehensive Cancer Network Distress Thermometer and Symptom Checklist. Data were abstracted from CPRS for distress screens conducted from January 2015-April 2016. The number of new cancer cases at VAPAHCS during the same time period was obtained from the cancer registry.
Data Analysis: The number of distress screens conducted was compared to cancer registry data of total new cases of cancer. Descriptive statistics were used to explore the level of distress and frequency of biopsychosocial symptoms endorsed by veterans in this sample.
Results: A total of 372 veterans completed distress screening during the program evaluation period. During the same time period, 920 veterans were newly diagnosed with cancer at VAPAHCS. Average level of distress was 3.3 (0 = no distress, 10 = extreme distress). Forty-one percent (n = 152) of veterans scored above the clinical cut-off for significant distress (4/10). The most commonly endorsed symptoms were fatigue (n = 97; 26.0%), worry (n = 93; 25.0%), pain (n = 92; 24.7%), sleep (n = 78; 21.0%), and skin dry/itchy (n = 78; 21.0%).
Implications: The discrepancy between number of new cases of cancer and number of distress screenings conducted during the program evaluation period suggests that modifications to current procedures are necessary to ensure that all cancer patients at VAPAHCS are screened for distress. Over 40% of veterans screened endorsed clinically significant levels of distress and over 20% of veterans endorsed problems with fatigue, worry, pain, sleep and/or skin issues. Future directions for care include introducing psychosocial interventions in the oncology clinic to reduce distress and cope with commonly reported symptoms/side effects.
Purpose: To create and evaluate psychosocial distress screening procedures for patients diagnosed with cancer at the VA Palo Alto Health Care System (VAPAHCS).
Relevant Background/Problem: A program development and evaluation project was conducted at VAPAHCS in order to implement psychosocial distress screening according to the Cancer Program Standards (Standard 3.2) of the American College of Surgeon’s Commission on Cancer.
Methods: Veterans recently diagnosed with cancer were screened for distress by a psychologist, social worker, or psychology trainee during one of their regularly scheduled oncology appointments using the National Comprehensive Cancer Network Distress Thermometer and Symptom Checklist. Data were abstracted from CPRS for distress screens conducted from January 2015-April 2016. The number of new cancer cases at VAPAHCS during the same time period was obtained from the cancer registry.
Data Analysis: The number of distress screens conducted was compared to cancer registry data of total new cases of cancer. Descriptive statistics were used to explore the level of distress and frequency of biopsychosocial symptoms endorsed by veterans in this sample.
Results: A total of 372 veterans completed distress screening during the program evaluation period. During the same time period, 920 veterans were newly diagnosed with cancer at VAPAHCS. Average level of distress was 3.3 (0 = no distress, 10 = extreme distress). Forty-one percent (n = 152) of veterans scored above the clinical cut-off for significant distress (4/10). The most commonly endorsed symptoms were fatigue (n = 97; 26.0%), worry (n = 93; 25.0%), pain (n = 92; 24.7%), sleep (n = 78; 21.0%), and skin dry/itchy (n = 78; 21.0%).
Implications: The discrepancy between number of new cases of cancer and number of distress screenings conducted during the program evaluation period suggests that modifications to current procedures are necessary to ensure that all cancer patients at VAPAHCS are screened for distress. Over 40% of veterans screened endorsed clinically significant levels of distress and over 20% of veterans endorsed problems with fatigue, worry, pain, sleep and/or skin issues. Future directions for care include introducing psychosocial interventions in the oncology clinic to reduce distress and cope with commonly reported symptoms/side effects.
Purpose: To create and evaluate psychosocial distress screening procedures for patients diagnosed with cancer at the VA Palo Alto Health Care System (VAPAHCS).
Relevant Background/Problem: A program development and evaluation project was conducted at VAPAHCS in order to implement psychosocial distress screening according to the Cancer Program Standards (Standard 3.2) of the American College of Surgeon’s Commission on Cancer.
Methods: Veterans recently diagnosed with cancer were screened for distress by a psychologist, social worker, or psychology trainee during one of their regularly scheduled oncology appointments using the National Comprehensive Cancer Network Distress Thermometer and Symptom Checklist. Data were abstracted from CPRS for distress screens conducted from January 2015-April 2016. The number of new cancer cases at VAPAHCS during the same time period was obtained from the cancer registry.
Data Analysis: The number of distress screens conducted was compared to cancer registry data of total new cases of cancer. Descriptive statistics were used to explore the level of distress and frequency of biopsychosocial symptoms endorsed by veterans in this sample.
Results: A total of 372 veterans completed distress screening during the program evaluation period. During the same time period, 920 veterans were newly diagnosed with cancer at VAPAHCS. Average level of distress was 3.3 (0 = no distress, 10 = extreme distress). Forty-one percent (n = 152) of veterans scored above the clinical cut-off for significant distress (4/10). The most commonly endorsed symptoms were fatigue (n = 97; 26.0%), worry (n = 93; 25.0%), pain (n = 92; 24.7%), sleep (n = 78; 21.0%), and skin dry/itchy (n = 78; 21.0%).
Implications: The discrepancy between number of new cases of cancer and number of distress screenings conducted during the program evaluation period suggests that modifications to current procedures are necessary to ensure that all cancer patients at VAPAHCS are screened for distress. Over 40% of veterans screened endorsed clinically significant levels of distress and over 20% of veterans endorsed problems with fatigue, worry, pain, sleep and/or skin issues. Future directions for care include introducing psychosocial interventions in the oncology clinic to reduce distress and cope with commonly reported symptoms/side effects.
Use of the Community Needs Assessment (CNA) to Identify Barriers and Improve Access to Cancer Care for Veterans
Purpose: To disseminate information regarding The American College of Surgeons Commission on Cancer (ACOS COC) requirements of Patient Navigation that can be used across VAMCs.
Background: The ACOS COC requires that each facility determine a patient navigation process. The process must focus on a barrier to care identified within a Community Needs Assessment (CNA) that is administered at least every 3 years. From the results of the CNA, a patient navigation process can be developed to address patient, provider, or system barriers to care. These results are also presented to the Cancer Committee (CC) to compile a report summarizing the findings and implementing a plan to improve the quality of cancer care delivered.
Methods: A CNA questionnaire was reviewed by an interdisciplinary group consisting of oncology social worker, oncology psychologist, medical oncologist, survivorship advanced practice nurse, 3 oncology nurse care coordinators and the Cancer Center Program Administrator. The questionnaire was formatted for ease of reading and comprehension. The group presented the CNA questionnaire to the CC for review and approval. The questionnaire was distributed and completed by Veteran’s at varying stages along the cancer trajectory.
Data Analysis: The questionnaire was distributed and completed by 50 Veterans from Feb 2014-Sept 2014. The questionnaires were distributed and collected in the chemotherapy infusion clinic, during outpatient clinic visits, and during the Louis Stokes Cleveland VAMC (LSCVAMC) annual Cancer Fair.
Results: The top rated concern was found to be travel. According the National Cancer Data Base (NCDB) generated in May 2015 shows that from the years of 2007-2012, 34% of Veterans receiving their care at the LSCVAMC traveled between 50-99 miles to receive their cancer care. The data were presented to the CC, and plans were made to further look at travel resources and community services available to our Veterans. A comprehensive report addressing resources was compiled and presented to the CC.
Implications: Identifying and breaking down barriers to transportation is vital to improving access to Veteran’s cancer care.
Purpose: To disseminate information regarding The American College of Surgeons Commission on Cancer (ACOS COC) requirements of Patient Navigation that can be used across VAMCs.
Background: The ACOS COC requires that each facility determine a patient navigation process. The process must focus on a barrier to care identified within a Community Needs Assessment (CNA) that is administered at least every 3 years. From the results of the CNA, a patient navigation process can be developed to address patient, provider, or system barriers to care. These results are also presented to the Cancer Committee (CC) to compile a report summarizing the findings and implementing a plan to improve the quality of cancer care delivered.
Methods: A CNA questionnaire was reviewed by an interdisciplinary group consisting of oncology social worker, oncology psychologist, medical oncologist, survivorship advanced practice nurse, 3 oncology nurse care coordinators and the Cancer Center Program Administrator. The questionnaire was formatted for ease of reading and comprehension. The group presented the CNA questionnaire to the CC for review and approval. The questionnaire was distributed and completed by Veteran’s at varying stages along the cancer trajectory.
Data Analysis: The questionnaire was distributed and completed by 50 Veterans from Feb 2014-Sept 2014. The questionnaires were distributed and collected in the chemotherapy infusion clinic, during outpatient clinic visits, and during the Louis Stokes Cleveland VAMC (LSCVAMC) annual Cancer Fair.
Results: The top rated concern was found to be travel. According the National Cancer Data Base (NCDB) generated in May 2015 shows that from the years of 2007-2012, 34% of Veterans receiving their care at the LSCVAMC traveled between 50-99 miles to receive their cancer care. The data were presented to the CC, and plans were made to further look at travel resources and community services available to our Veterans. A comprehensive report addressing resources was compiled and presented to the CC.
Implications: Identifying and breaking down barriers to transportation is vital to improving access to Veteran’s cancer care.
Purpose: To disseminate information regarding The American College of Surgeons Commission on Cancer (ACOS COC) requirements of Patient Navigation that can be used across VAMCs.
Background: The ACOS COC requires that each facility determine a patient navigation process. The process must focus on a barrier to care identified within a Community Needs Assessment (CNA) that is administered at least every 3 years. From the results of the CNA, a patient navigation process can be developed to address patient, provider, or system barriers to care. These results are also presented to the Cancer Committee (CC) to compile a report summarizing the findings and implementing a plan to improve the quality of cancer care delivered.
Methods: A CNA questionnaire was reviewed by an interdisciplinary group consisting of oncology social worker, oncology psychologist, medical oncologist, survivorship advanced practice nurse, 3 oncology nurse care coordinators and the Cancer Center Program Administrator. The questionnaire was formatted for ease of reading and comprehension. The group presented the CNA questionnaire to the CC for review and approval. The questionnaire was distributed and completed by Veteran’s at varying stages along the cancer trajectory.
Data Analysis: The questionnaire was distributed and completed by 50 Veterans from Feb 2014-Sept 2014. The questionnaires were distributed and collected in the chemotherapy infusion clinic, during outpatient clinic visits, and during the Louis Stokes Cleveland VAMC (LSCVAMC) annual Cancer Fair.
Results: The top rated concern was found to be travel. According the National Cancer Data Base (NCDB) generated in May 2015 shows that from the years of 2007-2012, 34% of Veterans receiving their care at the LSCVAMC traveled between 50-99 miles to receive their cancer care. The data were presented to the CC, and plans were made to further look at travel resources and community services available to our Veterans. A comprehensive report addressing resources was compiled and presented to the CC.
Implications: Identifying and breaking down barriers to transportation is vital to improving access to Veteran’s cancer care.
The VA Precision Oncology Research Program
The VA recently launched the Precision Oncology Program (POP), a clinical program that provides a turnkey operation for targeted sequencing of tumor samples and return of annotated results to the patient record. The Program recommends available clinical trials and consultative advice to clinicians and patients. We describe here the accompanying Research Program (Re-POP) that leverages the artifacts of the POP.
Lung cancer patients whose tumors are sequenced as part of the clinical POP will be given the opportunity to participate in the research Program. The goals of the Re-POP include: 1) creation of a network of VA sites to participate as a consortium in clinical trials; 2) provision of a research data repository containing information regarding tumor features including mutational status, patient demographics, and cancer treatments and outcomes; and 3) re-use of residual patient tumor tissue for expanded analysis.
Through collaborative efforts with research groups Re-POP will open large trials on a national level throughout the VA. Use of a centralized IRB and coordinating center (at Massachusetts Veterans Epidemiology Research and Information Center) will facilitate opening studies at any VA site that wishes to participate. The VA Cooperative Studies Program will support these activities. Preliminary data suggest that the Program can make available protocols offering either experimental or off-label therapies for approximately half of all nonsmall cell lung cancer patients.
The Re-POP data repository will reside at the NCI Genomic Data Commons and is available to both academic and industry researchers. Predictive analytics of these data support learning healthcare system activities (predicting individual patient outcomes) and the production of population-level generalizable knowledge.
Residual tissue and clinical data from Re-POP will be made available to researchers to identify new biomarkers that will enhance understanding of response to therapies as well as identify new therapeutic targets. An NCI-VA-DoD collaboration to study the value of proteomics in predicting response to targeted therapies in lung cancer was recently announced as part of the White House’s cancer moonshot initiative.
The VA recently launched the Precision Oncology Program (POP), a clinical program that provides a turnkey operation for targeted sequencing of tumor samples and return of annotated results to the patient record. The Program recommends available clinical trials and consultative advice to clinicians and patients. We describe here the accompanying Research Program (Re-POP) that leverages the artifacts of the POP.
Lung cancer patients whose tumors are sequenced as part of the clinical POP will be given the opportunity to participate in the research Program. The goals of the Re-POP include: 1) creation of a network of VA sites to participate as a consortium in clinical trials; 2) provision of a research data repository containing information regarding tumor features including mutational status, patient demographics, and cancer treatments and outcomes; and 3) re-use of residual patient tumor tissue for expanded analysis.
Through collaborative efforts with research groups Re-POP will open large trials on a national level throughout the VA. Use of a centralized IRB and coordinating center (at Massachusetts Veterans Epidemiology Research and Information Center) will facilitate opening studies at any VA site that wishes to participate. The VA Cooperative Studies Program will support these activities. Preliminary data suggest that the Program can make available protocols offering either experimental or off-label therapies for approximately half of all nonsmall cell lung cancer patients.
The Re-POP data repository will reside at the NCI Genomic Data Commons and is available to both academic and industry researchers. Predictive analytics of these data support learning healthcare system activities (predicting individual patient outcomes) and the production of population-level generalizable knowledge.
Residual tissue and clinical data from Re-POP will be made available to researchers to identify new biomarkers that will enhance understanding of response to therapies as well as identify new therapeutic targets. An NCI-VA-DoD collaboration to study the value of proteomics in predicting response to targeted therapies in lung cancer was recently announced as part of the White House’s cancer moonshot initiative.
The VA recently launched the Precision Oncology Program (POP), a clinical program that provides a turnkey operation for targeted sequencing of tumor samples and return of annotated results to the patient record. The Program recommends available clinical trials and consultative advice to clinicians and patients. We describe here the accompanying Research Program (Re-POP) that leverages the artifacts of the POP.
Lung cancer patients whose tumors are sequenced as part of the clinical POP will be given the opportunity to participate in the research Program. The goals of the Re-POP include: 1) creation of a network of VA sites to participate as a consortium in clinical trials; 2) provision of a research data repository containing information regarding tumor features including mutational status, patient demographics, and cancer treatments and outcomes; and 3) re-use of residual patient tumor tissue for expanded analysis.
Through collaborative efforts with research groups Re-POP will open large trials on a national level throughout the VA. Use of a centralized IRB and coordinating center (at Massachusetts Veterans Epidemiology Research and Information Center) will facilitate opening studies at any VA site that wishes to participate. The VA Cooperative Studies Program will support these activities. Preliminary data suggest that the Program can make available protocols offering either experimental or off-label therapies for approximately half of all nonsmall cell lung cancer patients.
The Re-POP data repository will reside at the NCI Genomic Data Commons and is available to both academic and industry researchers. Predictive analytics of these data support learning healthcare system activities (predicting individual patient outcomes) and the production of population-level generalizable knowledge.
Residual tissue and clinical data from Re-POP will be made available to researchers to identify new biomarkers that will enhance understanding of response to therapies as well as identify new therapeutic targets. An NCI-VA-DoD collaboration to study the value of proteomics in predicting response to targeted therapies in lung cancer was recently announced as part of the White House’s cancer moonshot initiative.
Longer DAPT better for PAD, study suggests

Photo by Sage Ross
ROME—A subanalysis of the PRODIGY study suggests a longer duration of dual antiplatelet therapy (DAPT) improves outcomes after percutaneous coronary intervention (PCI) for patients with peripheral arterial disease (PAD).
Receiving long-term DAPT after PCI reduced the risk of atherothrombotic events and death in patients with PAD, without increasing the risk of actionable bleeding episodes.
However, patients without PAD fared better with short-term DAPT.
These results were presented at ESC Congress 2016 (abstract 5154) and published in JAMA Cardiology.
Marco Valgimigli, MD, PhD, of Bern University Hospital in Bern, Switzerland, and his colleagues performed this analysis of PRODIGY data.
The study included patients from tertiary care hospitals who had stable coronary artery disease or acute coronary syndromes, with or without concomitant PAD, and were undergoing PCI.
There were 246 patients with PAD—118 who were randomized to receive DAPT for 24 months after PCI and 128 who were randomized to DAPT for 6 months or less.
There were 1724 patients without PAD—869 who were randomized to receive DAPT for 24 months after PCI and 855 who were randomized to DAPT for 6 months or less.
The patients with PAD were older and more frequently underwent multivessel intervention. They were also more likely to have hypertension, type 1 or 2 diabetes, previous myocardial infarction, previous coronary artery bypass grafting, non-ST-segment elevation myocardial infarction, and more complex coronary artery disease.
At 30 days, patients with PAD were more often taking diuretics, and patients without PAD were more often taking beta-blockers and statins.
Patients with PAD who were randomized to long-term DAPT were younger, had a higher body mass index, and less frequently underwent PCI of the left main coronary artery than PAD patients randomized to short-term DAPT.
Having PAD was associated with a higher risk of death and ischemic events, with a hazard ratio (HR) of 2.80 (95% CI, 2.05-3.83; P<0.001).
Results
The primary efficacy endpoint of this study was a composite of death, myocardial infarction, and cerebrovascular accidents.
Among patients with PAD, those who received long-term DAPT had a lower risk of this endpoint than those who received short-term DAPT—16.1% and 27.3%, respectively. The HR was 0.54 (95% CI, 0.31-0.95; P=0.03).
Among patients without PAD, there was no significant difference in the incidence of the primary endpoint according to DAPT duration. It occurred in 9.3% of patients who received long-term DAPT and 7.4% of patients who received short-term DAPT. The HR was 1.28 (95% CI, 0.92-1.77; P=0.15).
The key safety endpoint was a composite of Bleeding Academic Research Consortium (BARC) type 2, 3, or 5 bleeding.
There was no significant difference in this endpoint according to DAPT duration for patients with PAD, but long-term DAPT was associated with a significant increase in this endpoint for patients without PAD.
Among patients with PAD, BARC type 2, 3, or 5 bleeding occurred in 5.2% of those receiving long-term DAPT and 6.9% of those receiving short-term DAPT. The HR was 0.77 (95% CI, 0.27-2.21; P=0.62).
Among patients without PAD, BARC type 2, 3, or 5 bleeding occurred in 8% of those receiving long-term DAPT and 3.1% of those receiving short-term DAPT. The HR was 2.61 (95% CI, 0.27-2.21; P<0.001).
The researchers said the apparent neutral effect of long-term DAPT on bleeding risk in PAD patients requires further evaluation in adequately powered studies, but this research suggests patients with PAD will benefit from prolonged DAPT after PCI. ![]()

Photo by Sage Ross
ROME—A subanalysis of the PRODIGY study suggests a longer duration of dual antiplatelet therapy (DAPT) improves outcomes after percutaneous coronary intervention (PCI) for patients with peripheral arterial disease (PAD).
Receiving long-term DAPT after PCI reduced the risk of atherothrombotic events and death in patients with PAD, without increasing the risk of actionable bleeding episodes.
However, patients without PAD fared better with short-term DAPT.
These results were presented at ESC Congress 2016 (abstract 5154) and published in JAMA Cardiology.
Marco Valgimigli, MD, PhD, of Bern University Hospital in Bern, Switzerland, and his colleagues performed this analysis of PRODIGY data.
The study included patients from tertiary care hospitals who had stable coronary artery disease or acute coronary syndromes, with or without concomitant PAD, and were undergoing PCI.
There were 246 patients with PAD—118 who were randomized to receive DAPT for 24 months after PCI and 128 who were randomized to DAPT for 6 months or less.
There were 1724 patients without PAD—869 who were randomized to receive DAPT for 24 months after PCI and 855 who were randomized to DAPT for 6 months or less.
The patients with PAD were older and more frequently underwent multivessel intervention. They were also more likely to have hypertension, type 1 or 2 diabetes, previous myocardial infarction, previous coronary artery bypass grafting, non-ST-segment elevation myocardial infarction, and more complex coronary artery disease.
At 30 days, patients with PAD were more often taking diuretics, and patients without PAD were more often taking beta-blockers and statins.
Patients with PAD who were randomized to long-term DAPT were younger, had a higher body mass index, and less frequently underwent PCI of the left main coronary artery than PAD patients randomized to short-term DAPT.
Having PAD was associated with a higher risk of death and ischemic events, with a hazard ratio (HR) of 2.80 (95% CI, 2.05-3.83; P<0.001).
Results
The primary efficacy endpoint of this study was a composite of death, myocardial infarction, and cerebrovascular accidents.
Among patients with PAD, those who received long-term DAPT had a lower risk of this endpoint than those who received short-term DAPT—16.1% and 27.3%, respectively. The HR was 0.54 (95% CI, 0.31-0.95; P=0.03).
Among patients without PAD, there was no significant difference in the incidence of the primary endpoint according to DAPT duration. It occurred in 9.3% of patients who received long-term DAPT and 7.4% of patients who received short-term DAPT. The HR was 1.28 (95% CI, 0.92-1.77; P=0.15).
The key safety endpoint was a composite of Bleeding Academic Research Consortium (BARC) type 2, 3, or 5 bleeding.
There was no significant difference in this endpoint according to DAPT duration for patients with PAD, but long-term DAPT was associated with a significant increase in this endpoint for patients without PAD.
Among patients with PAD, BARC type 2, 3, or 5 bleeding occurred in 5.2% of those receiving long-term DAPT and 6.9% of those receiving short-term DAPT. The HR was 0.77 (95% CI, 0.27-2.21; P=0.62).
Among patients without PAD, BARC type 2, 3, or 5 bleeding occurred in 8% of those receiving long-term DAPT and 3.1% of those receiving short-term DAPT. The HR was 2.61 (95% CI, 0.27-2.21; P<0.001).
The researchers said the apparent neutral effect of long-term DAPT on bleeding risk in PAD patients requires further evaluation in adequately powered studies, but this research suggests patients with PAD will benefit from prolonged DAPT after PCI. ![]()

Photo by Sage Ross
ROME—A subanalysis of the PRODIGY study suggests a longer duration of dual antiplatelet therapy (DAPT) improves outcomes after percutaneous coronary intervention (PCI) for patients with peripheral arterial disease (PAD).
Receiving long-term DAPT after PCI reduced the risk of atherothrombotic events and death in patients with PAD, without increasing the risk of actionable bleeding episodes.
However, patients without PAD fared better with short-term DAPT.
These results were presented at ESC Congress 2016 (abstract 5154) and published in JAMA Cardiology.
Marco Valgimigli, MD, PhD, of Bern University Hospital in Bern, Switzerland, and his colleagues performed this analysis of PRODIGY data.
The study included patients from tertiary care hospitals who had stable coronary artery disease or acute coronary syndromes, with or without concomitant PAD, and were undergoing PCI.
There were 246 patients with PAD—118 who were randomized to receive DAPT for 24 months after PCI and 128 who were randomized to DAPT for 6 months or less.
There were 1724 patients without PAD—869 who were randomized to receive DAPT for 24 months after PCI and 855 who were randomized to DAPT for 6 months or less.
The patients with PAD were older and more frequently underwent multivessel intervention. They were also more likely to have hypertension, type 1 or 2 diabetes, previous myocardial infarction, previous coronary artery bypass grafting, non-ST-segment elevation myocardial infarction, and more complex coronary artery disease.
At 30 days, patients with PAD were more often taking diuretics, and patients without PAD were more often taking beta-blockers and statins.
Patients with PAD who were randomized to long-term DAPT were younger, had a higher body mass index, and less frequently underwent PCI of the left main coronary artery than PAD patients randomized to short-term DAPT.
Having PAD was associated with a higher risk of death and ischemic events, with a hazard ratio (HR) of 2.80 (95% CI, 2.05-3.83; P<0.001).
Results
The primary efficacy endpoint of this study was a composite of death, myocardial infarction, and cerebrovascular accidents.
Among patients with PAD, those who received long-term DAPT had a lower risk of this endpoint than those who received short-term DAPT—16.1% and 27.3%, respectively. The HR was 0.54 (95% CI, 0.31-0.95; P=0.03).
Among patients without PAD, there was no significant difference in the incidence of the primary endpoint according to DAPT duration. It occurred in 9.3% of patients who received long-term DAPT and 7.4% of patients who received short-term DAPT. The HR was 1.28 (95% CI, 0.92-1.77; P=0.15).
The key safety endpoint was a composite of Bleeding Academic Research Consortium (BARC) type 2, 3, or 5 bleeding.
There was no significant difference in this endpoint according to DAPT duration for patients with PAD, but long-term DAPT was associated with a significant increase in this endpoint for patients without PAD.
Among patients with PAD, BARC type 2, 3, or 5 bleeding occurred in 5.2% of those receiving long-term DAPT and 6.9% of those receiving short-term DAPT. The HR was 0.77 (95% CI, 0.27-2.21; P=0.62).
Among patients without PAD, BARC type 2, 3, or 5 bleeding occurred in 8% of those receiving long-term DAPT and 3.1% of those receiving short-term DAPT. The HR was 2.61 (95% CI, 0.27-2.21; P<0.001).
The researchers said the apparent neutral effect of long-term DAPT on bleeding risk in PAD patients requires further evaluation in adequately powered studies, but this research suggests patients with PAD will benefit from prolonged DAPT after PCI. ![]()
MRI measurements reveal effects of sleep deprivation
Lack of sleep had a significant impact on brain responses to an attention task, and circadian rhythms played a role, according to functional magnetic resonance imaging data from 33 healthy adults. The findings were published online Aug. 11 in Science.
Despite the data showing that acute sleep loss impacts cognition, “human performance remains remarkably well preserved until wakefulness is extended into the biological night,” wrote Vincenzo Muto of the University of Liège, Belgium, and his colleagues (Science 2016;353:687-90. doi: 10.1126/science.aad2993).
Study participants stayed awake for 42 hours, beginning in the morning and covering 2 biological days, 1 biological night, and part of a second night. They periodically performed the psychomotor vigilance task (PVT), a visual reaction time task designed to measure attention; and an auditory n-back task, and the researchers collected functional and structural MRI data across 13 sessions. The average age of the participants (17 men and 16 women) was 21 years.
Overall, PVT performance was stable during the first day, but decreased significantly after sleep deprivation, then recovered during the second day, and returned to baseline after a period of recovery sleep, the researchers said.
Brain responses to the n-back task were “significantly modulated by a circadian oscillation, synchronous to the melatonin rhythm,” they noted. “This finding rules out a global task-independent circadian influence and suggest the influence of a local, region-specific task-dependent circadian signal,” they added.
Although more research is needed on how different cognitive tasks are affected by sleep deprivation, the findings may help in “understanding of the brain mechanisms underlying the maintenance of daytime cognitive performance and its deterioration, as observed in shift work, jet lag, sleep disorders, aging, and neurodegenerative diseases,” the researchers wrote.
They had no financial conflicts to disclose.
Lack of sleep had a significant impact on brain responses to an attention task, and circadian rhythms played a role, according to functional magnetic resonance imaging data from 33 healthy adults. The findings were published online Aug. 11 in Science.
Despite the data showing that acute sleep loss impacts cognition, “human performance remains remarkably well preserved until wakefulness is extended into the biological night,” wrote Vincenzo Muto of the University of Liège, Belgium, and his colleagues (Science 2016;353:687-90. doi: 10.1126/science.aad2993).
Study participants stayed awake for 42 hours, beginning in the morning and covering 2 biological days, 1 biological night, and part of a second night. They periodically performed the psychomotor vigilance task (PVT), a visual reaction time task designed to measure attention; and an auditory n-back task, and the researchers collected functional and structural MRI data across 13 sessions. The average age of the participants (17 men and 16 women) was 21 years.
Overall, PVT performance was stable during the first day, but decreased significantly after sleep deprivation, then recovered during the second day, and returned to baseline after a period of recovery sleep, the researchers said.
Brain responses to the n-back task were “significantly modulated by a circadian oscillation, synchronous to the melatonin rhythm,” they noted. “This finding rules out a global task-independent circadian influence and suggest the influence of a local, region-specific task-dependent circadian signal,” they added.
Although more research is needed on how different cognitive tasks are affected by sleep deprivation, the findings may help in “understanding of the brain mechanisms underlying the maintenance of daytime cognitive performance and its deterioration, as observed in shift work, jet lag, sleep disorders, aging, and neurodegenerative diseases,” the researchers wrote.
They had no financial conflicts to disclose.
Lack of sleep had a significant impact on brain responses to an attention task, and circadian rhythms played a role, according to functional magnetic resonance imaging data from 33 healthy adults. The findings were published online Aug. 11 in Science.
Despite the data showing that acute sleep loss impacts cognition, “human performance remains remarkably well preserved until wakefulness is extended into the biological night,” wrote Vincenzo Muto of the University of Liège, Belgium, and his colleagues (Science 2016;353:687-90. doi: 10.1126/science.aad2993).
Study participants stayed awake for 42 hours, beginning in the morning and covering 2 biological days, 1 biological night, and part of a second night. They periodically performed the psychomotor vigilance task (PVT), a visual reaction time task designed to measure attention; and an auditory n-back task, and the researchers collected functional and structural MRI data across 13 sessions. The average age of the participants (17 men and 16 women) was 21 years.
Overall, PVT performance was stable during the first day, but decreased significantly after sleep deprivation, then recovered during the second day, and returned to baseline after a period of recovery sleep, the researchers said.
Brain responses to the n-back task were “significantly modulated by a circadian oscillation, synchronous to the melatonin rhythm,” they noted. “This finding rules out a global task-independent circadian influence and suggest the influence of a local, region-specific task-dependent circadian signal,” they added.
Although more research is needed on how different cognitive tasks are affected by sleep deprivation, the findings may help in “understanding of the brain mechanisms underlying the maintenance of daytime cognitive performance and its deterioration, as observed in shift work, jet lag, sleep disorders, aging, and neurodegenerative diseases,” the researchers wrote.
They had no financial conflicts to disclose.
FROM SCIENCE
Key clinical point: Brain responses to sustained-attention tasks deteriorated with sleep deprivation and varied according to circadian rhythms, according to functional MRI data.
Major finding: MRI data collected over 42 hours of wakefulness and after recovery sleep showed a significant (P less than .05) impact of circadian rhythms on participants’ abilities to perform visual and auditory tasks.
Data source: A sleep study that used functional MRI to measure changes in brain response in 33 healthy adults.
Disclosures: The researchers had no financial conflicts to disclose.
FDA rule will pull many consumer antibacterial soaps from market
Over-the-counter consumer antiseptic wash products with active ingredients such as triclosan and triclocarban will be pulled from the market, following a final rule issued Sept. 2 by the Food and Drug Administration.
Companies will no longer be able to sell antibacterial washes with those ingredients, the FDA said, because manufacturers failed to show the ingredients are safe for long-term daily use and are better than plain soap and water at preventing illness and the spread of infections.
The final rule targets consumer antiseptic wash products containing 1 or more of 19 active ingredients, including the 2 most commonly used ingredients, triclosan and triclocarban. Companies have 1 year to comply with the new rule.
The FDA’s rule does not apply to hand sanitizers, wipes, or antibacterial products used in health care settings.
The agency has deferred for 1 year a decision on the continued use of three other ingredients in consumer wash products: benzalkonium chloride, benzethonium chloride, and chloroxylenol.
The FDA’s decision was driven in part by concerns about the risks posed by long-term exposure to such products, including bacterial resistance or hormonal effects.
“Consumers may think antibacterial washes are more effective at preventing the spread of germs, but we have no scientific evidence that they are any better than plain soap and water,” said Janet Woodcock, MD, director of the FDA’s Center for Drug Evaluation and Research, in a statement. “In fact, some data suggest that antibacterial ingredients may do more harm than good over the long term.”
Washing with plain soap and water remains one of the most important steps consumers can take to prevent illness and the spread of infection, the FDA advised. The agency also recommended use of alcohol-based hand sanitizer with at least 60% alcohol.
Read the full press release on the FDA website.
Over-the-counter consumer antiseptic wash products with active ingredients such as triclosan and triclocarban will be pulled from the market, following a final rule issued Sept. 2 by the Food and Drug Administration.
Companies will no longer be able to sell antibacterial washes with those ingredients, the FDA said, because manufacturers failed to show the ingredients are safe for long-term daily use and are better than plain soap and water at preventing illness and the spread of infections.
The final rule targets consumer antiseptic wash products containing 1 or more of 19 active ingredients, including the 2 most commonly used ingredients, triclosan and triclocarban. Companies have 1 year to comply with the new rule.
The FDA’s rule does not apply to hand sanitizers, wipes, or antibacterial products used in health care settings.
The agency has deferred for 1 year a decision on the continued use of three other ingredients in consumer wash products: benzalkonium chloride, benzethonium chloride, and chloroxylenol.
The FDA’s decision was driven in part by concerns about the risks posed by long-term exposure to such products, including bacterial resistance or hormonal effects.
“Consumers may think antibacterial washes are more effective at preventing the spread of germs, but we have no scientific evidence that they are any better than plain soap and water,” said Janet Woodcock, MD, director of the FDA’s Center for Drug Evaluation and Research, in a statement. “In fact, some data suggest that antibacterial ingredients may do more harm than good over the long term.”
Washing with plain soap and water remains one of the most important steps consumers can take to prevent illness and the spread of infection, the FDA advised. The agency also recommended use of alcohol-based hand sanitizer with at least 60% alcohol.
Read the full press release on the FDA website.
Over-the-counter consumer antiseptic wash products with active ingredients such as triclosan and triclocarban will be pulled from the market, following a final rule issued Sept. 2 by the Food and Drug Administration.
Companies will no longer be able to sell antibacterial washes with those ingredients, the FDA said, because manufacturers failed to show the ingredients are safe for long-term daily use and are better than plain soap and water at preventing illness and the spread of infections.
The final rule targets consumer antiseptic wash products containing 1 or more of 19 active ingredients, including the 2 most commonly used ingredients, triclosan and triclocarban. Companies have 1 year to comply with the new rule.
The FDA’s rule does not apply to hand sanitizers, wipes, or antibacterial products used in health care settings.
The agency has deferred for 1 year a decision on the continued use of three other ingredients in consumer wash products: benzalkonium chloride, benzethonium chloride, and chloroxylenol.
The FDA’s decision was driven in part by concerns about the risks posed by long-term exposure to such products, including bacterial resistance or hormonal effects.
“Consumers may think antibacterial washes are more effective at preventing the spread of germs, but we have no scientific evidence that they are any better than plain soap and water,” said Janet Woodcock, MD, director of the FDA’s Center for Drug Evaluation and Research, in a statement. “In fact, some data suggest that antibacterial ingredients may do more harm than good over the long term.”
Washing with plain soap and water remains one of the most important steps consumers can take to prevent illness and the spread of infection, the FDA advised. The agency also recommended use of alcohol-based hand sanitizer with at least 60% alcohol.
Read the full press release on the FDA website.
Treat bed bug bites with topical steroids
BOSTON – Preventing repeated occurrences of bed bug bites means eliminating infestations or – if the bites occur during travel – avoiding bringing the bugs home, according to Theodore Rosen, MD.
Bed bugs are “very, very, very hardy,” and can live months or maybe even years between blood meals, Dr. Rosen, professor of dermatology at Baylor College of Medicine, Houston, said at the American Academy of Dermatology summer meeting.
“They lay lots of eggs and have lots of offspring,” he said, adding that bed bugs have evolved and developed mutations that make them resistant to insecticides, and can survive up to a week at a continuous temperature of 10 degrees Fahrenheit.
Advise patients who will be traveling to check whether the area they are traveling to has known bed bug infestations. Recommend that they always check areas within 3 feet or so of hotel beds for evidence of bed bugs, he said.
Bed bugs like right angles, so they may be found on headboards, bed frames, mattress piping, drawers, and windows. They can also be found hiding under peeling paint, and or in the piping or under surfaces of furniture in hotel rooms.
Dr. Rosen is often asked by patients if he really looks for bed bugs when he travels. “I do look – and I’ve found them,” he said, noting that they can be found in the nicest of hotels. He found some lurking beneath the drawer of a nightstand in one luxury hotel.
Bugs can be seen, but droppings are another telltale sign of infestation; bed bug excrement stains fabrics, so if round brown splotches are seen on mattress or other fabric, bed bugs have been there and probably still are there, Dr. Rosen said.
“I pull up the sheets, look at the piping on the mattress, pull up the mattress and look at the frame, look at the coils, look at the night stand,” he said.
A pricey monitoring and trapping device called the NightWatch can be purchased to help in the event of an infestation. The device emits heated carbon dioxide and pheromones that attract and trap bed bugs, but the $300-$400 price tag can be prohibitive, he noted.
The best bet is to avoid bringing home bugs by keeping all bags and clothes off the floor. Bags should be kept on a dresser or stand, clothes should be hung.
If infestation occurs, let patients know that the bites aren’t dangerous. There is no convincing evidence that bed bugs transmit any diseases. Anemia with heavy infestation and repeated bites could theoretically be a concern, but other than itching and looking bad, bites are rarely an issue.
Bed bug bites usually respond well to topical steroid treatment, and only in rare cases are oral steroids or antihistamines needed. Home bites will recur if there is infestation, and the problem can be a source of depression and anxiety, which in some patients can be severe and lead to suicidal ideation.
Infestation should be addressed by heavy vacuuming and steaming, which kills bed bugs if he temperatures are high enough. Mattress encasement can also help, but insecticides are rarely effective. Freezing smaller objects can be tried, but bed bugs can withstand freezing temperatures for long periods of time, he said.
“If you bring bed bugs home, you have to treat the whole environment,” he said.
One treatment that has shown promise is ivermectin in the host. Studies suggest this approach kills some bugs, but it is not 100% effective and is probably best reserved for widespread infestation, such as in a nursing home, Dr. Rosen said.
He disclosed financial or other relationships with Anacor Pharmaceuticals; Innocutis; Sandoz, a Novartis company; and Valeant Pharmaceuticals International.
BOSTON – Preventing repeated occurrences of bed bug bites means eliminating infestations or – if the bites occur during travel – avoiding bringing the bugs home, according to Theodore Rosen, MD.
Bed bugs are “very, very, very hardy,” and can live months or maybe even years between blood meals, Dr. Rosen, professor of dermatology at Baylor College of Medicine, Houston, said at the American Academy of Dermatology summer meeting.
“They lay lots of eggs and have lots of offspring,” he said, adding that bed bugs have evolved and developed mutations that make them resistant to insecticides, and can survive up to a week at a continuous temperature of 10 degrees Fahrenheit.
Advise patients who will be traveling to check whether the area they are traveling to has known bed bug infestations. Recommend that they always check areas within 3 feet or so of hotel beds for evidence of bed bugs, he said.
Bed bugs like right angles, so they may be found on headboards, bed frames, mattress piping, drawers, and windows. They can also be found hiding under peeling paint, and or in the piping or under surfaces of furniture in hotel rooms.
Dr. Rosen is often asked by patients if he really looks for bed bugs when he travels. “I do look – and I’ve found them,” he said, noting that they can be found in the nicest of hotels. He found some lurking beneath the drawer of a nightstand in one luxury hotel.
Bugs can be seen, but droppings are another telltale sign of infestation; bed bug excrement stains fabrics, so if round brown splotches are seen on mattress or other fabric, bed bugs have been there and probably still are there, Dr. Rosen said.
“I pull up the sheets, look at the piping on the mattress, pull up the mattress and look at the frame, look at the coils, look at the night stand,” he said.
A pricey monitoring and trapping device called the NightWatch can be purchased to help in the event of an infestation. The device emits heated carbon dioxide and pheromones that attract and trap bed bugs, but the $300-$400 price tag can be prohibitive, he noted.
The best bet is to avoid bringing home bugs by keeping all bags and clothes off the floor. Bags should be kept on a dresser or stand, clothes should be hung.
If infestation occurs, let patients know that the bites aren’t dangerous. There is no convincing evidence that bed bugs transmit any diseases. Anemia with heavy infestation and repeated bites could theoretically be a concern, but other than itching and looking bad, bites are rarely an issue.
Bed bug bites usually respond well to topical steroid treatment, and only in rare cases are oral steroids or antihistamines needed. Home bites will recur if there is infestation, and the problem can be a source of depression and anxiety, which in some patients can be severe and lead to suicidal ideation.
Infestation should be addressed by heavy vacuuming and steaming, which kills bed bugs if he temperatures are high enough. Mattress encasement can also help, but insecticides are rarely effective. Freezing smaller objects can be tried, but bed bugs can withstand freezing temperatures for long periods of time, he said.
“If you bring bed bugs home, you have to treat the whole environment,” he said.
One treatment that has shown promise is ivermectin in the host. Studies suggest this approach kills some bugs, but it is not 100% effective and is probably best reserved for widespread infestation, such as in a nursing home, Dr. Rosen said.
He disclosed financial or other relationships with Anacor Pharmaceuticals; Innocutis; Sandoz, a Novartis company; and Valeant Pharmaceuticals International.
BOSTON – Preventing repeated occurrences of bed bug bites means eliminating infestations or – if the bites occur during travel – avoiding bringing the bugs home, according to Theodore Rosen, MD.
Bed bugs are “very, very, very hardy,” and can live months or maybe even years between blood meals, Dr. Rosen, professor of dermatology at Baylor College of Medicine, Houston, said at the American Academy of Dermatology summer meeting.
“They lay lots of eggs and have lots of offspring,” he said, adding that bed bugs have evolved and developed mutations that make them resistant to insecticides, and can survive up to a week at a continuous temperature of 10 degrees Fahrenheit.
Advise patients who will be traveling to check whether the area they are traveling to has known bed bug infestations. Recommend that they always check areas within 3 feet or so of hotel beds for evidence of bed bugs, he said.
Bed bugs like right angles, so they may be found on headboards, bed frames, mattress piping, drawers, and windows. They can also be found hiding under peeling paint, and or in the piping or under surfaces of furniture in hotel rooms.
Dr. Rosen is often asked by patients if he really looks for bed bugs when he travels. “I do look – and I’ve found them,” he said, noting that they can be found in the nicest of hotels. He found some lurking beneath the drawer of a nightstand in one luxury hotel.
Bugs can be seen, but droppings are another telltale sign of infestation; bed bug excrement stains fabrics, so if round brown splotches are seen on mattress or other fabric, bed bugs have been there and probably still are there, Dr. Rosen said.
“I pull up the sheets, look at the piping on the mattress, pull up the mattress and look at the frame, look at the coils, look at the night stand,” he said.
A pricey monitoring and trapping device called the NightWatch can be purchased to help in the event of an infestation. The device emits heated carbon dioxide and pheromones that attract and trap bed bugs, but the $300-$400 price tag can be prohibitive, he noted.
The best bet is to avoid bringing home bugs by keeping all bags and clothes off the floor. Bags should be kept on a dresser or stand, clothes should be hung.
If infestation occurs, let patients know that the bites aren’t dangerous. There is no convincing evidence that bed bugs transmit any diseases. Anemia with heavy infestation and repeated bites could theoretically be a concern, but other than itching and looking bad, bites are rarely an issue.
Bed bug bites usually respond well to topical steroid treatment, and only in rare cases are oral steroids or antihistamines needed. Home bites will recur if there is infestation, and the problem can be a source of depression and anxiety, which in some patients can be severe and lead to suicidal ideation.
Infestation should be addressed by heavy vacuuming and steaming, which kills bed bugs if he temperatures are high enough. Mattress encasement can also help, but insecticides are rarely effective. Freezing smaller objects can be tried, but bed bugs can withstand freezing temperatures for long periods of time, he said.
“If you bring bed bugs home, you have to treat the whole environment,” he said.
One treatment that has shown promise is ivermectin in the host. Studies suggest this approach kills some bugs, but it is not 100% effective and is probably best reserved for widespread infestation, such as in a nursing home, Dr. Rosen said.
He disclosed financial or other relationships with Anacor Pharmaceuticals; Innocutis; Sandoz, a Novartis company; and Valeant Pharmaceuticals International.
EXPERT ANALYSIS FROM AAD Summer Academy 2016
Another 199 pregnant women with Zika
Zika virus shows no signs of slowing down, as the number of pregnant women with laboratory evidence of possible infection in the United States and its territories took its largest jump yet during the week ending Aug. 25, according to the Centers for Disease Control and Prevention.
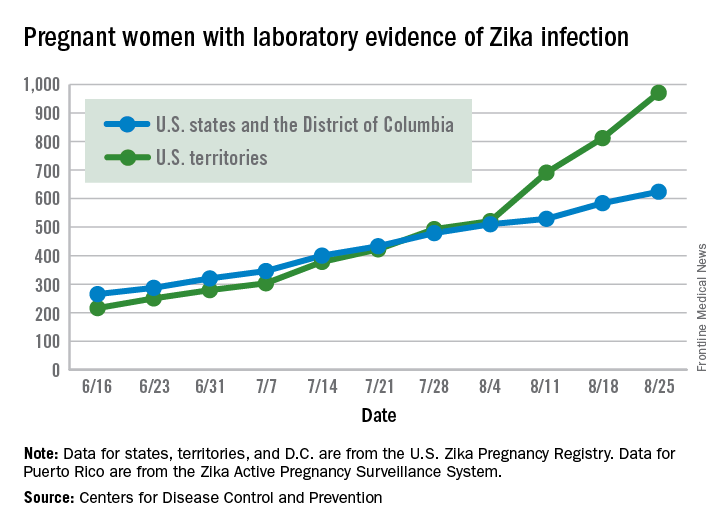
There were 199 new cases of Zika that week: 159 in the U.S. territories and 40 in the 50 states and the District of Columbia. The previous high had been 189 for the week ending Aug. 11. Cases in pregnant women for 2016 so far number 971 in the territories and 624 in the states and D.C. – a total of 1,595, the CDC reported Sept. 1.
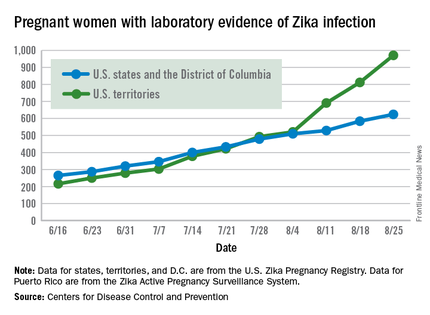
The number of poor outcomes among pregnant women with Zika virus infection did not change for the week ending Aug. 25. The number of live-born infants with Zika-related birth defects remained at 17 – 16 in the states/D.C. and 1 in the territories – and the number of pregnancy losses with birth defects was still 6 – 5 in the states/D.C. and 1 in the territories, the CDC said. State- or territorial-level data are not being reported to protect the privacy of affected women and children.
Among the entire U.S. population, 16,832 cases of Zika have been reported to the CDC Arboviral Disease Branch in 2015-2016, with 5,304 reported for the week ending Aug. 31 (Puerto Rico retroactively reported 5,000 cases that had been identified between June 4 and Aug. 6). The states/D.C. account for 2,722 of total cases, and the territories have reported 14,110 cases, of which Puerto Rico accounts for 13,791, the CDC noted.
The figures for states, territories, and D.C. reflect reporting to the U.S. Zika Pregnancy Registry; data for Puerto Rico are reported to the U.S. Zika Active Pregnancy Surveillance System.
Zika-related birth defects recorded by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, and termination with evidence of birth defects.
Zika virus shows no signs of slowing down, as the number of pregnant women with laboratory evidence of possible infection in the United States and its territories took its largest jump yet during the week ending Aug. 25, according to the Centers for Disease Control and Prevention.
There were 199 new cases of Zika that week: 159 in the U.S. territories and 40 in the 50 states and the District of Columbia. The previous high had been 189 for the week ending Aug. 11. Cases in pregnant women for 2016 so far number 971 in the territories and 624 in the states and D.C. – a total of 1,595, the CDC reported Sept. 1.

The number of poor outcomes among pregnant women with Zika virus infection did not change for the week ending Aug. 25. The number of live-born infants with Zika-related birth defects remained at 17 – 16 in the states/D.C. and 1 in the territories – and the number of pregnancy losses with birth defects was still 6 – 5 in the states/D.C. and 1 in the territories, the CDC said. State- or territorial-level data are not being reported to protect the privacy of affected women and children.
Among the entire U.S. population, 16,832 cases of Zika have been reported to the CDC Arboviral Disease Branch in 2015-2016, with 5,304 reported for the week ending Aug. 31 (Puerto Rico retroactively reported 5,000 cases that had been identified between June 4 and Aug. 6). The states/D.C. account for 2,722 of total cases, and the territories have reported 14,110 cases, of which Puerto Rico accounts for 13,791, the CDC noted.
The figures for states, territories, and D.C. reflect reporting to the U.S. Zika Pregnancy Registry; data for Puerto Rico are reported to the U.S. Zika Active Pregnancy Surveillance System.
Zika-related birth defects recorded by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, and termination with evidence of birth defects.
Zika virus shows no signs of slowing down, as the number of pregnant women with laboratory evidence of possible infection in the United States and its territories took its largest jump yet during the week ending Aug. 25, according to the Centers for Disease Control and Prevention.
There were 199 new cases of Zika that week: 159 in the U.S. territories and 40 in the 50 states and the District of Columbia. The previous high had been 189 for the week ending Aug. 11. Cases in pregnant women for 2016 so far number 971 in the territories and 624 in the states and D.C. – a total of 1,595, the CDC reported Sept. 1.

The number of poor outcomes among pregnant women with Zika virus infection did not change for the week ending Aug. 25. The number of live-born infants with Zika-related birth defects remained at 17 – 16 in the states/D.C. and 1 in the territories – and the number of pregnancy losses with birth defects was still 6 – 5 in the states/D.C. and 1 in the territories, the CDC said. State- or territorial-level data are not being reported to protect the privacy of affected women and children.
Among the entire U.S. population, 16,832 cases of Zika have been reported to the CDC Arboviral Disease Branch in 2015-2016, with 5,304 reported for the week ending Aug. 31 (Puerto Rico retroactively reported 5,000 cases that had been identified between June 4 and Aug. 6). The states/D.C. account for 2,722 of total cases, and the territories have reported 14,110 cases, of which Puerto Rico accounts for 13,791, the CDC noted.
The figures for states, territories, and D.C. reflect reporting to the U.S. Zika Pregnancy Registry; data for Puerto Rico are reported to the U.S. Zika Active Pregnancy Surveillance System.
Zika-related birth defects recorded by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, and termination with evidence of birth defects.