User login
Yeast infection in pregnancy? Think twice about fluconazole
PRACTICE CHANGER
Avoid prescribing oral fluconazole in early pregnancy because it is associated with a higher rate of spontaneous abortion than is topical azole therapy.1
Strength of recommendation
B: Based on large cohort study performed in Denmark.
Mølgaard-Nielsen D, Svanström H, Melbye M, et al. Association between use of oral fluconazole during pregnancy and risk of spontaneous abortion and stillbirth. JAMA. 2016;315:58-67.
Illustrative Case
A 25-year-old woman who is 16 weeks pregnant with her first child is experiencing increased vaginal discharge associated with vaginal itching. A microscopic examination of the discharge confirms your suspicions of vaginal candidiasis. Is oral fluconazole or a topical azole your treatment of choice?
Because of the increased production of sex hormones, vaginal candidiasis is common during pregnancy, affecting up to 10% of pregnant women in the United States.1,2 Treatment options include oral fluconazole and a variety of topical azoles. Although topical azoles are recommended as first-line therapy,3 the ease of oral therapy makes it an attractive treatment option.4 The safety of oral fluconazole during pregnancy, however, has recently come under scrutiny.
Case reports have linked high-dose fluconazole use during pregnancy with congenital malformations.5,6 These case reports led to epidemiological studies evaluating fluconazole’s safety, but, in these studies, no association with congenital malformations was found.7,8
A large cohort study involving 1079 fluconazole-exposed pregnancies and 170,453 unexposed pregnancies found no increased risk of congenital malformations or stillbirth; rates of spontaneous abortion and miscarriage were not evaluated.9 A prospective cohort study of 226 pregnant women found no association between fluconazole use during the first trimester and miscarriages.10 However, the validity of both studies’ findings was limited by small numbers of participants. The current study is the largest to date to evaluate whether use of fluconazole compared to that of topical azoles in early pregnancy is associated with increased rates of spontaneous abortion and stillbirth.
Study Summary
Fluconazole significantly increases risk of miscarriage, but not stillbirth
This nationwide cohort study, conducted using the Medical Birth Register in Denmark, evaluated more than 1.4 million pregnancies occurring from 1997 to 2013 for exposure to oral fluconazole between 7 and 22 weeks’ gestation. Each oral fluconazole-exposed pregnancy was matched with up to 4 unexposed pregnancies (based on propensity score, maternal age, calendar year, and gestational age) and to pregnancies exposed to intravaginal formulations of topical azoles. Exposure to fluconazole was documented based on filled prescriptions from the National Prescription Register. Primary outcomes were rates of spontaneous abortion (loss before 22 weeks) and stillbirth (loss after 23 weeks).
Rates of spontaneous abortion. From the total cohort of more than 1.4 million pregnancies, 3315 were exposed to oral fluconazole between 7 and 22 weeks’ gestation. Spontaneous abortions occurred in 147 of the 3315 fluconazole-exposed pregnancies and in 563 of 13,246 unexposed, matched pregnancies (hazard ratio [HR]=1.48; 95% confidence interval [CI], 1.23-1.77).
Rates of stillbirth. Of 5382 pregnancies exposed to fluconazole from week 7 to birth, 21 resulted in stillbirth; 77 stillbirths occurred in the 21,506 unexposed matched pregnancies (HR=1.32; 95% CI, 0.82-2.14). In a sensitivity analysis, however, higher doses of fluconazole (350 mg) were 4 times more likely to be associated with stillbirth (HR=4.10; 95% CI, 1.89-8.90) than lower doses (150 mg) (HR= 0.99; 95% CI, 0.56-1.74).
Oral fluconazole vs topical azole. Use of oral fluconazole in pregnancy was associated with an increased risk of spontaneous abortion when compared to topical azole use: 130 of 2823 pregnancies vs 118 of 2823 pregnancies, respectively (HR=1.62; 95% CI, 1.26-2.07), but not an increased risk of stillbirths: 20 of 4301 pregnancies vs 22 of 4301 pregnancies, respectively (HR=1.18; 95% CI, 0.64-2.16).
What’s New
A sizeable study with a treatment comparison
The authors found that exposure in early pregnancy to oral fluconazole, as compared to topical azoles, increases the risk of spontaneous abortion. By comparing treatments in a sensitivity analysis, the confounder of Candida infections causing spontaneous abortion was removed. In addition, when considering the ease of dosing of fluconazole as compared with topical imidazoles, this study challenges the balance of ease of use with safety.
Caveats
A skewed population and limited generalizability?
This large cohort study using the National Patient Register in Denmark may not be generalizable to a larger, non-Scandinavian population. Since a hospital registry was used, those not seeking care through the hospital were likely missed. If patients
In addition, the study focused on women exposed from 7 to 22 weeks’ gestation; the findings may not be generalizable to fluconazole exposure prior to 7 weeks. Likewise, the registry is unlikely to capture very early spontaneous abortions that are not recognized clinically. In all, given the large sample size and the care taken to match each exposed pregnancy with up to 4 unexposed pregnancies, these limitations are likely to have had little influence on the overall findings of the study.
Challenges to Implementation
Balancing ease of use with safety
Given the ease of using oral fluconazole vs daily topical azole therapy, many physicians and patients may still opt for oral treatment.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Mølgaard-Nielsen D, Svanström H, Melbye M, et al. Association between use of oral fluconazole during pregnancy and risk of spontaneous abortion and stillbirth. JAMA. 2016;315:58-67.
2. Cotch MF, Hillier SL, Gibbs RS, et al. Epidemiology and outcomes associated with moderate to heavy Candida colonization during pregnancy. Vaginal Infections and Prematurity Study Group. Am J Obstet Gynecol. 1998;178:374-380.
3. Workowski KA, Bolan GA, Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64:1-137.
4. Tooley PJ. Patient and doctor preferences in the treatment of vaginal candidosis. Practitioner. 1985;229: 655-660.
5. Aleck KA, Bartley DL. Multiple malformation syndrome following fluconazole use in pregnancy: report of an additional patient. Am J Med Genet. 1997;72:253-256.
6. Lee BE, Feinberg M, Abraham JJ, et al. Congenital malformations in an infant born to a woman treated with fluconazole. Pediatr Infect Dis J. 1992;11:1062-1064.
7. Jick SS. Pregnancy outcomes after maternal exposure to fluconazole. Pharmacotherapy. 1999;19:221-222.
8. Mølgaard-Nielsen D, Pasternak B, Hviid A. Use of oral fluconazole during pregnancy and the risk of birth defects. N Engl J Med. 2013;369:830-839.
9. Nørgaard M, Pedersen L, Gislum M, et al. Maternal use of fluconazole and risk of congenital malformations: a Danish population-based cohort study. J Antimicrob Chemother. 2008;62:172-176.
10. Mastroiacovo P, Mazzone T, Botto LD, et al. Prospective assessment of pregnancy outcomes after first-trimester exposure to fluconazole. Am J Obstet Gynecol. 1996;175:1645-1650.
PRACTICE CHANGER
Avoid prescribing oral fluconazole in early pregnancy because it is associated with a higher rate of spontaneous abortion than is topical azole therapy.1
Strength of recommendation
B: Based on large cohort study performed in Denmark.
Mølgaard-Nielsen D, Svanström H, Melbye M, et al. Association between use of oral fluconazole during pregnancy and risk of spontaneous abortion and stillbirth. JAMA. 2016;315:58-67.
Illustrative Case
A 25-year-old woman who is 16 weeks pregnant with her first child is experiencing increased vaginal discharge associated with vaginal itching. A microscopic examination of the discharge confirms your suspicions of vaginal candidiasis. Is oral fluconazole or a topical azole your treatment of choice?
Because of the increased production of sex hormones, vaginal candidiasis is common during pregnancy, affecting up to 10% of pregnant women in the United States.1,2 Treatment options include oral fluconazole and a variety of topical azoles. Although topical azoles are recommended as first-line therapy,3 the ease of oral therapy makes it an attractive treatment option.4 The safety of oral fluconazole during pregnancy, however, has recently come under scrutiny.
Case reports have linked high-dose fluconazole use during pregnancy with congenital malformations.5,6 These case reports led to epidemiological studies evaluating fluconazole’s safety, but, in these studies, no association with congenital malformations was found.7,8
A large cohort study involving 1079 fluconazole-exposed pregnancies and 170,453 unexposed pregnancies found no increased risk of congenital malformations or stillbirth; rates of spontaneous abortion and miscarriage were not evaluated.9 A prospective cohort study of 226 pregnant women found no association between fluconazole use during the first trimester and miscarriages.10 However, the validity of both studies’ findings was limited by small numbers of participants. The current study is the largest to date to evaluate whether use of fluconazole compared to that of topical azoles in early pregnancy is associated with increased rates of spontaneous abortion and stillbirth.
Study Summary
Fluconazole significantly increases risk of miscarriage, but not stillbirth
This nationwide cohort study, conducted using the Medical Birth Register in Denmark, evaluated more than 1.4 million pregnancies occurring from 1997 to 2013 for exposure to oral fluconazole between 7 and 22 weeks’ gestation. Each oral fluconazole-exposed pregnancy was matched with up to 4 unexposed pregnancies (based on propensity score, maternal age, calendar year, and gestational age) and to pregnancies exposed to intravaginal formulations of topical azoles. Exposure to fluconazole was documented based on filled prescriptions from the National Prescription Register. Primary outcomes were rates of spontaneous abortion (loss before 22 weeks) and stillbirth (loss after 23 weeks).
Rates of spontaneous abortion. From the total cohort of more than 1.4 million pregnancies, 3315 were exposed to oral fluconazole between 7 and 22 weeks’ gestation. Spontaneous abortions occurred in 147 of the 3315 fluconazole-exposed pregnancies and in 563 of 13,246 unexposed, matched pregnancies (hazard ratio [HR]=1.48; 95% confidence interval [CI], 1.23-1.77).
Rates of stillbirth. Of 5382 pregnancies exposed to fluconazole from week 7 to birth, 21 resulted in stillbirth; 77 stillbirths occurred in the 21,506 unexposed matched pregnancies (HR=1.32; 95% CI, 0.82-2.14). In a sensitivity analysis, however, higher doses of fluconazole (350 mg) were 4 times more likely to be associated with stillbirth (HR=4.10; 95% CI, 1.89-8.90) than lower doses (150 mg) (HR= 0.99; 95% CI, 0.56-1.74).
Oral fluconazole vs topical azole. Use of oral fluconazole in pregnancy was associated with an increased risk of spontaneous abortion when compared to topical azole use: 130 of 2823 pregnancies vs 118 of 2823 pregnancies, respectively (HR=1.62; 95% CI, 1.26-2.07), but not an increased risk of stillbirths: 20 of 4301 pregnancies vs 22 of 4301 pregnancies, respectively (HR=1.18; 95% CI, 0.64-2.16).
What’s New
A sizeable study with a treatment comparison
The authors found that exposure in early pregnancy to oral fluconazole, as compared to topical azoles, increases the risk of spontaneous abortion. By comparing treatments in a sensitivity analysis, the confounder of Candida infections causing spontaneous abortion was removed. In addition, when considering the ease of dosing of fluconazole as compared with topical imidazoles, this study challenges the balance of ease of use with safety.
Caveats
A skewed population and limited generalizability?
This large cohort study using the National Patient Register in Denmark may not be generalizable to a larger, non-Scandinavian population. Since a hospital registry was used, those not seeking care through the hospital were likely missed. If patients
In addition, the study focused on women exposed from 7 to 22 weeks’ gestation; the findings may not be generalizable to fluconazole exposure prior to 7 weeks. Likewise, the registry is unlikely to capture very early spontaneous abortions that are not recognized clinically. In all, given the large sample size and the care taken to match each exposed pregnancy with up to 4 unexposed pregnancies, these limitations are likely to have had little influence on the overall findings of the study.
Challenges to Implementation
Balancing ease of use with safety
Given the ease of using oral fluconazole vs daily topical azole therapy, many physicians and patients may still opt for oral treatment.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
PRACTICE CHANGER
Avoid prescribing oral fluconazole in early pregnancy because it is associated with a higher rate of spontaneous abortion than is topical azole therapy.1
Strength of recommendation
B: Based on large cohort study performed in Denmark.
Mølgaard-Nielsen D, Svanström H, Melbye M, et al. Association between use of oral fluconazole during pregnancy and risk of spontaneous abortion and stillbirth. JAMA. 2016;315:58-67.
Illustrative Case
A 25-year-old woman who is 16 weeks pregnant with her first child is experiencing increased vaginal discharge associated with vaginal itching. A microscopic examination of the discharge confirms your suspicions of vaginal candidiasis. Is oral fluconazole or a topical azole your treatment of choice?
Because of the increased production of sex hormones, vaginal candidiasis is common during pregnancy, affecting up to 10% of pregnant women in the United States.1,2 Treatment options include oral fluconazole and a variety of topical azoles. Although topical azoles are recommended as first-line therapy,3 the ease of oral therapy makes it an attractive treatment option.4 The safety of oral fluconazole during pregnancy, however, has recently come under scrutiny.
Case reports have linked high-dose fluconazole use during pregnancy with congenital malformations.5,6 These case reports led to epidemiological studies evaluating fluconazole’s safety, but, in these studies, no association with congenital malformations was found.7,8
A large cohort study involving 1079 fluconazole-exposed pregnancies and 170,453 unexposed pregnancies found no increased risk of congenital malformations or stillbirth; rates of spontaneous abortion and miscarriage were not evaluated.9 A prospective cohort study of 226 pregnant women found no association between fluconazole use during the first trimester and miscarriages.10 However, the validity of both studies’ findings was limited by small numbers of participants. The current study is the largest to date to evaluate whether use of fluconazole compared to that of topical azoles in early pregnancy is associated with increased rates of spontaneous abortion and stillbirth.
Study Summary
Fluconazole significantly increases risk of miscarriage, but not stillbirth
This nationwide cohort study, conducted using the Medical Birth Register in Denmark, evaluated more than 1.4 million pregnancies occurring from 1997 to 2013 for exposure to oral fluconazole between 7 and 22 weeks’ gestation. Each oral fluconazole-exposed pregnancy was matched with up to 4 unexposed pregnancies (based on propensity score, maternal age, calendar year, and gestational age) and to pregnancies exposed to intravaginal formulations of topical azoles. Exposure to fluconazole was documented based on filled prescriptions from the National Prescription Register. Primary outcomes were rates of spontaneous abortion (loss before 22 weeks) and stillbirth (loss after 23 weeks).
Rates of spontaneous abortion. From the total cohort of more than 1.4 million pregnancies, 3315 were exposed to oral fluconazole between 7 and 22 weeks’ gestation. Spontaneous abortions occurred in 147 of the 3315 fluconazole-exposed pregnancies and in 563 of 13,246 unexposed, matched pregnancies (hazard ratio [HR]=1.48; 95% confidence interval [CI], 1.23-1.77).
Rates of stillbirth. Of 5382 pregnancies exposed to fluconazole from week 7 to birth, 21 resulted in stillbirth; 77 stillbirths occurred in the 21,506 unexposed matched pregnancies (HR=1.32; 95% CI, 0.82-2.14). In a sensitivity analysis, however, higher doses of fluconazole (350 mg) were 4 times more likely to be associated with stillbirth (HR=4.10; 95% CI, 1.89-8.90) than lower doses (150 mg) (HR= 0.99; 95% CI, 0.56-1.74).
Oral fluconazole vs topical azole. Use of oral fluconazole in pregnancy was associated with an increased risk of spontaneous abortion when compared to topical azole use: 130 of 2823 pregnancies vs 118 of 2823 pregnancies, respectively (HR=1.62; 95% CI, 1.26-2.07), but not an increased risk of stillbirths: 20 of 4301 pregnancies vs 22 of 4301 pregnancies, respectively (HR=1.18; 95% CI, 0.64-2.16).
What’s New
A sizeable study with a treatment comparison
The authors found that exposure in early pregnancy to oral fluconazole, as compared to topical azoles, increases the risk of spontaneous abortion. By comparing treatments in a sensitivity analysis, the confounder of Candida infections causing spontaneous abortion was removed. In addition, when considering the ease of dosing of fluconazole as compared with topical imidazoles, this study challenges the balance of ease of use with safety.
Caveats
A skewed population and limited generalizability?
This large cohort study using the National Patient Register in Denmark may not be generalizable to a larger, non-Scandinavian population. Since a hospital registry was used, those not seeking care through the hospital were likely missed. If patients
In addition, the study focused on women exposed from 7 to 22 weeks’ gestation; the findings may not be generalizable to fluconazole exposure prior to 7 weeks. Likewise, the registry is unlikely to capture very early spontaneous abortions that are not recognized clinically. In all, given the large sample size and the care taken to match each exposed pregnancy with up to 4 unexposed pregnancies, these limitations are likely to have had little influence on the overall findings of the study.
Challenges to Implementation
Balancing ease of use with safety
Given the ease of using oral fluconazole vs daily topical azole therapy, many physicians and patients may still opt for oral treatment.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Mølgaard-Nielsen D, Svanström H, Melbye M, et al. Association between use of oral fluconazole during pregnancy and risk of spontaneous abortion and stillbirth. JAMA. 2016;315:58-67.
2. Cotch MF, Hillier SL, Gibbs RS, et al. Epidemiology and outcomes associated with moderate to heavy Candida colonization during pregnancy. Vaginal Infections and Prematurity Study Group. Am J Obstet Gynecol. 1998;178:374-380.
3. Workowski KA, Bolan GA, Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64:1-137.
4. Tooley PJ. Patient and doctor preferences in the treatment of vaginal candidosis. Practitioner. 1985;229: 655-660.
5. Aleck KA, Bartley DL. Multiple malformation syndrome following fluconazole use in pregnancy: report of an additional patient. Am J Med Genet. 1997;72:253-256.
6. Lee BE, Feinberg M, Abraham JJ, et al. Congenital malformations in an infant born to a woman treated with fluconazole. Pediatr Infect Dis J. 1992;11:1062-1064.
7. Jick SS. Pregnancy outcomes after maternal exposure to fluconazole. Pharmacotherapy. 1999;19:221-222.
8. Mølgaard-Nielsen D, Pasternak B, Hviid A. Use of oral fluconazole during pregnancy and the risk of birth defects. N Engl J Med. 2013;369:830-839.
9. Nørgaard M, Pedersen L, Gislum M, et al. Maternal use of fluconazole and risk of congenital malformations: a Danish population-based cohort study. J Antimicrob Chemother. 2008;62:172-176.
10. Mastroiacovo P, Mazzone T, Botto LD, et al. Prospective assessment of pregnancy outcomes after first-trimester exposure to fluconazole. Am J Obstet Gynecol. 1996;175:1645-1650.
1. Mølgaard-Nielsen D, Svanström H, Melbye M, et al. Association between use of oral fluconazole during pregnancy and risk of spontaneous abortion and stillbirth. JAMA. 2016;315:58-67.
2. Cotch MF, Hillier SL, Gibbs RS, et al. Epidemiology and outcomes associated with moderate to heavy Candida colonization during pregnancy. Vaginal Infections and Prematurity Study Group. Am J Obstet Gynecol. 1998;178:374-380.
3. Workowski KA, Bolan GA, Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64:1-137.
4. Tooley PJ. Patient and doctor preferences in the treatment of vaginal candidosis. Practitioner. 1985;229: 655-660.
5. Aleck KA, Bartley DL. Multiple malformation syndrome following fluconazole use in pregnancy: report of an additional patient. Am J Med Genet. 1997;72:253-256.
6. Lee BE, Feinberg M, Abraham JJ, et al. Congenital malformations in an infant born to a woman treated with fluconazole. Pediatr Infect Dis J. 1992;11:1062-1064.
7. Jick SS. Pregnancy outcomes after maternal exposure to fluconazole. Pharmacotherapy. 1999;19:221-222.
8. Mølgaard-Nielsen D, Pasternak B, Hviid A. Use of oral fluconazole during pregnancy and the risk of birth defects. N Engl J Med. 2013;369:830-839.
9. Nørgaard M, Pedersen L, Gislum M, et al. Maternal use of fluconazole and risk of congenital malformations: a Danish population-based cohort study. J Antimicrob Chemother. 2008;62:172-176.
10. Mastroiacovo P, Mazzone T, Botto LD, et al. Prospective assessment of pregnancy outcomes after first-trimester exposure to fluconazole. Am J Obstet Gynecol. 1996;175:1645-1650.
Copyright © 2016. The Family Physicians Inquiries Network. All rights reserved.
Taking hysteroscopy to the office
Along with global endometrial ablation, diagnostic and minor operative hysteroscopy are excellent procedures to bring into your office environment. These operations are generally of short duration and provide little risk to the patient. Moreover, reimbursement exceeds that for the hospital setting. A constant revenue stream can be created after an initial moderate expenditure.
The key to a successful office procedure is patient comfort; this begins with minimizing pain and trauma. In our practice, we note decreased pain when performing vaginoscopy and hysteroscopy without the use of a speculum or tenaculum. This is well substantiated in literature by Professor Stefano Bettocchi, who immediately preceded me as president of the International Society for Gynecologic Endoscopy (ISGE).
In this issue of Master Class in Gynecologic Surgery, I have asked my partner, Aarathi Cholkeri-Singh, MD, to discuss vaginoscopy. Dr. Cholkeri-Singh is clinical assistant professor at the University of Illinois at Chicago, lecturer at Rosalind Franklin University of Medicine and Science, and associate director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.
She also serves as codirector of the AAGL/Society of Reproductive Surgeons fellowship in minimally invasive gynecologic surgery and director of gynecologic surgical education at Advocate Lutheran, and is chair for a postgraduate course on hysteroscopy at the upcoming AAGL 45th Annual Global Congress. Among her publications is a recent review in the Journal of Minimally Invasive Gynecology on hysteroscopy for infertile women (doi:10.1016/j.jmig.2014.12.163).
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, and past president of the AAGL and the International Society for Gynecologic Endoscopy. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville and Schaumburg, Ill.; director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. He reported having no financial disclosures relevant to this column. Email him at obnews@frontlinemedcom.com.
Along with global endometrial ablation, diagnostic and minor operative hysteroscopy are excellent procedures to bring into your office environment. These operations are generally of short duration and provide little risk to the patient. Moreover, reimbursement exceeds that for the hospital setting. A constant revenue stream can be created after an initial moderate expenditure.
The key to a successful office procedure is patient comfort; this begins with minimizing pain and trauma. In our practice, we note decreased pain when performing vaginoscopy and hysteroscopy without the use of a speculum or tenaculum. This is well substantiated in literature by Professor Stefano Bettocchi, who immediately preceded me as president of the International Society for Gynecologic Endoscopy (ISGE).
In this issue of Master Class in Gynecologic Surgery, I have asked my partner, Aarathi Cholkeri-Singh, MD, to discuss vaginoscopy. Dr. Cholkeri-Singh is clinical assistant professor at the University of Illinois at Chicago, lecturer at Rosalind Franklin University of Medicine and Science, and associate director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.
She also serves as codirector of the AAGL/Society of Reproductive Surgeons fellowship in minimally invasive gynecologic surgery and director of gynecologic surgical education at Advocate Lutheran, and is chair for a postgraduate course on hysteroscopy at the upcoming AAGL 45th Annual Global Congress. Among her publications is a recent review in the Journal of Minimally Invasive Gynecology on hysteroscopy for infertile women (doi:10.1016/j.jmig.2014.12.163).
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, and past president of the AAGL and the International Society for Gynecologic Endoscopy. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville and Schaumburg, Ill.; director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. He reported having no financial disclosures relevant to this column. Email him at obnews@frontlinemedcom.com.
Along with global endometrial ablation, diagnostic and minor operative hysteroscopy are excellent procedures to bring into your office environment. These operations are generally of short duration and provide little risk to the patient. Moreover, reimbursement exceeds that for the hospital setting. A constant revenue stream can be created after an initial moderate expenditure.
The key to a successful office procedure is patient comfort; this begins with minimizing pain and trauma. In our practice, we note decreased pain when performing vaginoscopy and hysteroscopy without the use of a speculum or tenaculum. This is well substantiated in literature by Professor Stefano Bettocchi, who immediately preceded me as president of the International Society for Gynecologic Endoscopy (ISGE).
In this issue of Master Class in Gynecologic Surgery, I have asked my partner, Aarathi Cholkeri-Singh, MD, to discuss vaginoscopy. Dr. Cholkeri-Singh is clinical assistant professor at the University of Illinois at Chicago, lecturer at Rosalind Franklin University of Medicine and Science, and associate director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.
She also serves as codirector of the AAGL/Society of Reproductive Surgeons fellowship in minimally invasive gynecologic surgery and director of gynecologic surgical education at Advocate Lutheran, and is chair for a postgraduate course on hysteroscopy at the upcoming AAGL 45th Annual Global Congress. Among her publications is a recent review in the Journal of Minimally Invasive Gynecology on hysteroscopy for infertile women (doi:10.1016/j.jmig.2014.12.163).
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, and past president of the AAGL and the International Society for Gynecologic Endoscopy. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville and Schaumburg, Ill.; director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. He reported having no financial disclosures relevant to this column. Email him at obnews@frontlinemedcom.com.
The benefits of integrating in-office hysteroscopy
The benefits of integrating hysteroscopy into office practice are compelling. Most importantly, patients appreciate the comfort and convenience of having hysteroscopic procedures done in a familiar setting. Patients can generally be in and out of the office in less than 30 minutes for a diagnostic procedure, and in less than 1-2 hours for an operative procedure.
Not only is an in-office approach patient centered and clinically valuable, but it is more efficient and economically favorable for the gynecologic surgeon. Physicians earn higher reimbursement for diagnostic hysteroscopies, as well as many therapeutic and operative hysteroscopies, when these procedures are done in the office rather than when they’re performed in a hospital or an outpatient center.
Transitioning to in-office hysteroscopy need not be daunting: The setup is relatively simple and does not require an operating suite, just a dedicated exam room. And the need for premedication and local anesthesia can be low, particularly when a vaginoscopic approach to hysteroscopy is employed. For most gynecologic surgeons, the necessary skills and comfort levels fall into place after only a few vaginoscopic procedures.
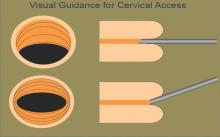
A vaginoscopic approach avoids the use of a vaginal speculum or cervical tenaculum, significantly decreasing discomfort or pain. Not using these instruments is the only difference between this and traditional hysteroscopy. It is a less invasive approach that is much more tolerable for patients. And for the surgeon, it can be easier and quicker and provides equally good visualization without any impairment in cervical passage.
Described in the literature as far back as the 1950s, vaginoscopy has its roots in the pediatric/adolescent population, where it was used for the removal of foreign bodies and evaluation of the vagina and external cervical os.
More recently, Stefano Bettocchi, MD, and Luigi Selvaggi, MD, in Italy were the first to describe a vaginoscopic approach to hysteroscopy for evaluating the endocervical canal and uterine cavity.
In a series of papers from 1997 to 2004, Dr. Bettocchi and Dr. Selvaggi documented their efforts to improve patient tolerance during diagnostic hysteroscopies. When they used both the speculum and tenaculum in 163 patients, with local anesthesia, 8% reported severe pain, 11% reported moderate pain, and 69% reported mild pain. Only 12% reported no discomfort. With speculum use only, and no anesthesia, in 308 patients, none reported severe pain, 2% reported moderate pain, 32% reported mild pain, and 66% reported no discomfort. When neither instrument was used (again, no anesthesia), patient discomfort was nearly eliminated: In 680 procedures, patients had a 96% no-discomfort rate (J Am Assoc Gynecol Laparosc. 1997 Feb;4[2]:255-8; Curr Opin Obstet Gynecol. 2003 Aug;15[4]:303-8; Obstet Gynecol Clin North Am. 2004 Sep;31[3]:641-54, xi).
Since then, research has affirmed the differences in patient tolerance and has shown that there is no significant difference between traditional and vaginoscopic hysteroscopy in the rate of procedure failure (0%-10%).
In my practice, in addition to vaginal or cervical examination and evaluation of the uterine cavity, I utilize a vaginoscopic approach to perform minor therapeutic and operative procedures such as biopsies, polypectomies, tubal occlusion using the Essure system, and removal of lost intrauterine devices. I can assess infertility, trauma, abnormal uterine bleeding, and mesh erosion, and provide pre- and postsurgical evaluations. In all of these cases, I use minimal premedication and only rarely need any local anesthetic and/or sedation.
Instrumentation and technique
There are a variety of hysteroscopes available on the market, from single-channel flexible diagnostic hysteroscopes that are 3 mm to 4 mm in diameter, to “see-and-treat” operative hysteroscopes that are rigid and have various diameters and camera lens angles.
A hysteroscope with a 5.5-mm outer diameter works well for a vaginoscopic approach that avoids cervical dilation. Accessory instrumentation includes semirigid 5 Fr 35-cm–long biopsy forceps, scissors, and alligator forceps.
In timing the procedure, our main goal is a thin uterine lining. This can be achieved by scheduling the procedure during the early proliferative phase of the menstrual cycle or by using a gonadotropin-releasing hormone agonist or a transdermal or transvaginal contraceptive medication.
By far the most important element of pain control and analgesia is the time spent with each patient to thoroughly discuss the experience of hysteroscopy and to set expectations about what she will hear, see, and feel. An unexpected experience can worsen anxiety, which in turn can worsen pain. If everything is as familiar and relaxed as possible, there will be little need for analgesia.
I tell patients in preprocedure counseling that the distention of the uterine walls usually causes some cramping, and that NSAIDs can minimize this cramping. In rare cases, when a patient is very worried about her pain tolerance, I will prescribe diazepam. However, many of my patients opt to do nothing other than take ibuprofen. On a case-by-case basis, you can determine with your patient what type and level of analgesia and preprocedure medication will be best.
Paracervical blocks are an option for some surgical patients, but I advise my patients to move forward without the block and assure them that it can be administered later if needed. Thus far, I’ve never proceeded with a paracervical block. There are other methods and sites for introducing local anesthesia, including intracervical, by injection or topical, or topical intracavitary techniques. Nevertheless, it is unclear from randomized controlled trials whether local anesthesia is effective. Trials of paracervical blocks similarly have had inconsistent outcomes.
I do commonly premedicate patients – mainly nulliparous patients and postmenopausal patients – with misoprostol, which softens the cervix and facilitates an easier entry of the hysteroscope into the cervix.
Published studies on misoprostol administration before hysteroscopy have had mixed results. A Cochrane review from 2015 concluded there is moderate-quality evidence in support of preoperative ripening with the agent, while another meta-analysis also published in 2015 concluded that data are poor and do not support its use. Recently, however, there appear to be more supportive studies demonstrating or suggesting that misoprostol is effective in reducing discomfort.
Patient discomfort is also minimized when there is little manipulation of the hysteroscope. Scopes that are angled (12, 25, or 30 degrees) allow optimal visualization with minimal movement; the scope can be brought to the midline of the uterine cavity and the light cord rotated to the 3:00 and 9:00 o’clock positions to enable visualization of the cornu. A 0-degree scope, on the other hand, must be manipulated quite a bit for the same degree of visualization, potentially increasing patient discomfort.
Prior to hysteroscopy, the cervix and vagina are cleaned with a small-diameter swab dipped in povidone-iodine or chlorhexidine gluconate in the case of allergies. One or two 1,000-cc bags of saline inserted into pressure bags are attached to Y-type tubing. (A diagnostic procedure rarely will require two bags.) I spread the labia initially while guiding the scope into the posterior fornix of the vagina. If the leakage of fluid causes inadequate distension of the vaginal walls, I will gently pinch the labia together with gauze.
I then gently pull back the scope and manipulate it posteriorly to visualize the external cervical os anteriorly. The hysteroscope may then be introduced through the cervical os, endocervical canal, and uterine cavity, with care taken so that the instrument does not rub against the cervix or the uterine tissue and cause trauma, pain, and bleeding. The uterus will progressively align with the cervix and vagina, thereby eliminating the need for a tenaculum to straighten the uterine axis.
Fluid monitoring is important, especially during operative hysteroscopy. In my practice, a nurse watches inflow and outflow amounts while I explain what I am doing and visualizing. Some patients like to be able to view the surgery, so I am always ready to tilt the screen accordingly.
The economics
How do you know if office hysteroscopy is right for you? Your own surgical skill and the skills of your staff, who must be trained to handle and sterilize equipment and to consistently assist you, are major factors, as is ensurance of a return on your investment.
One manufacturer contacted for this Master Class lists the price of a complete office tower (light source, camera, and monitor) at approximately $9,700 and the price of a rigid hysteroscope, sheath, and hand instruments at about $6,300. A complete setup for office hysteroscopy, including a standard operative (rigid) hysteroscope, should therefore cost between $15,000 and $17,000. Companies also offer leasing options for about $300-400/month.
Flexible hysteroscopes cost about $6,000 more, which prompts many gynecologic surgeons to focus their investment on a rigid scope that can be used for both diagnostic and therapeutic procedures. Disposables cost $10 or less, and $40-50 or less, for each diagnostic and operative hysteroscopy, respectively.
A look at the Medicare Relative Value Units (RVUs) – a key component of the Medicare reimbursement system and a standard for many payers in determining compensation – shows higher reimbursement for quite a few hysteroscopic codes when these procedures are performed in the office.
Total RVUs have three components:
1. Physician work, including time and the technical skill and intensity of effort it takes to perform a service or procedure.
2. Practice expenses, such as rent, equipment and supplies, and nonphysician salaries.
3. Malpractice insurance premiums.
Each component is multiplied by a factor that accounts for geographic cost variations, and each total RVU is multiplied by a dollar amount known as the conversion factor.
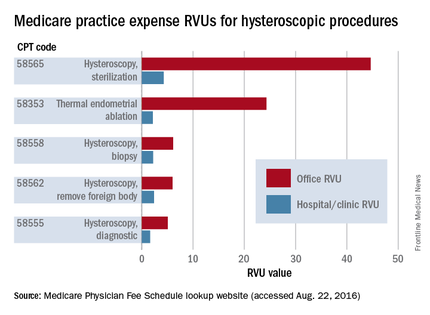
Practice expense (PE) RVUs for services provided in a “facility” (e.g., hospital or outpatient clinic) are often lower than office-based PE RVUs for the same services. Hysteroscopy is no exception. The PE RVU value for diagnostic hysteroscopy performed in the office, for instance, is approximately 5 units, compared with 1.64 units for diagnostic hysteroscopy performed in a facility.
Information on hysteroscopic procedures, and their associated RVUs, on geographic practice cost indices and on pricing, can be accessed using Medicare’s Physician Fee Schedule lookup tool (www.cms.gov/apps/physician-fee-schedule/overview.aspx).
This tool is useful for calculating returns on investment. According to national payment amounts listed in August, a diagnostic hysteroscopy performed in the office will earn an average of $315.08 vs. $192.27 for each case performed in the hospital. If you perform 12 such procedures a year, that’s about $3,781 in the office, compared with $2,307 in the hospital.
This difference alone might not be worth an investment of $15,000 or more, but if you anticipate performing additional procedures with higher margins and higher reimbursement, such as 12 thermal endometrial ablations a year in combination with diagnostic hysteroscopy (which, according to the Medicare national fee schedule averages would earn $15,962 in the office vs. $4,971 in the hospital), or 12 Essure tubal occlusions ($22,595 vs. $5,263), the investment will look more favorable.
And if your patients are largely privately insured, your return on investment will occur much more quickly. In metropolitan Chicago, Blue Cross Blue Shield is reimbursing in-office diagnostic hysteroscopy at approximately $568, hysteroscopic ablations at $3,844, and Essure tubal occlusions at $3,885.
In addition to reimbursement levels, it’s important to consider the efficiencies of in-office hysteroscopy. You can perform an annual exam while the assistant sets up the room and greets each patient, for instance, or see another established patient while the assistant discharges your patient and turns the room over. Our patients, in turn, benefit from increased accessibility, with less time spent away from work or family, as well as more familiarity and comfort and reduced out-of-pocket expenses.
Dr. Cholkeri-Singh is clinical assistant professor at the University of Illinois in Chicago and is director of gynecologic surgical education and associate director of minimally invasive gynecology at Advocate Lutheran General Hospital. She is in private practice with Dr. Charles Miller and Dr. Kristen Sasaki at the Advanced Gynecologic Surgical Institute in Chicago. She is a consultant for DySIS Medical, Hologic, and Bayer HealthCare.
The benefits of integrating hysteroscopy into office practice are compelling. Most importantly, patients appreciate the comfort and convenience of having hysteroscopic procedures done in a familiar setting. Patients can generally be in and out of the office in less than 30 minutes for a diagnostic procedure, and in less than 1-2 hours for an operative procedure.
Not only is an in-office approach patient centered and clinically valuable, but it is more efficient and economically favorable for the gynecologic surgeon. Physicians earn higher reimbursement for diagnostic hysteroscopies, as well as many therapeutic and operative hysteroscopies, when these procedures are done in the office rather than when they’re performed in a hospital or an outpatient center.
Transitioning to in-office hysteroscopy need not be daunting: The setup is relatively simple and does not require an operating suite, just a dedicated exam room. And the need for premedication and local anesthesia can be low, particularly when a vaginoscopic approach to hysteroscopy is employed. For most gynecologic surgeons, the necessary skills and comfort levels fall into place after only a few vaginoscopic procedures.
A vaginoscopic approach avoids the use of a vaginal speculum or cervical tenaculum, significantly decreasing discomfort or pain. Not using these instruments is the only difference between this and traditional hysteroscopy. It is a less invasive approach that is much more tolerable for patients. And for the surgeon, it can be easier and quicker and provides equally good visualization without any impairment in cervical passage.
Described in the literature as far back as the 1950s, vaginoscopy has its roots in the pediatric/adolescent population, where it was used for the removal of foreign bodies and evaluation of the vagina and external cervical os.
More recently, Stefano Bettocchi, MD, and Luigi Selvaggi, MD, in Italy were the first to describe a vaginoscopic approach to hysteroscopy for evaluating the endocervical canal and uterine cavity.
In a series of papers from 1997 to 2004, Dr. Bettocchi and Dr. Selvaggi documented their efforts to improve patient tolerance during diagnostic hysteroscopies. When they used both the speculum and tenaculum in 163 patients, with local anesthesia, 8% reported severe pain, 11% reported moderate pain, and 69% reported mild pain. Only 12% reported no discomfort. With speculum use only, and no anesthesia, in 308 patients, none reported severe pain, 2% reported moderate pain, 32% reported mild pain, and 66% reported no discomfort. When neither instrument was used (again, no anesthesia), patient discomfort was nearly eliminated: In 680 procedures, patients had a 96% no-discomfort rate (J Am Assoc Gynecol Laparosc. 1997 Feb;4[2]:255-8; Curr Opin Obstet Gynecol. 2003 Aug;15[4]:303-8; Obstet Gynecol Clin North Am. 2004 Sep;31[3]:641-54, xi).
Since then, research has affirmed the differences in patient tolerance and has shown that there is no significant difference between traditional and vaginoscopic hysteroscopy in the rate of procedure failure (0%-10%).
In my practice, in addition to vaginal or cervical examination and evaluation of the uterine cavity, I utilize a vaginoscopic approach to perform minor therapeutic and operative procedures such as biopsies, polypectomies, tubal occlusion using the Essure system, and removal of lost intrauterine devices. I can assess infertility, trauma, abnormal uterine bleeding, and mesh erosion, and provide pre- and postsurgical evaluations. In all of these cases, I use minimal premedication and only rarely need any local anesthetic and/or sedation.
Instrumentation and technique
There are a variety of hysteroscopes available on the market, from single-channel flexible diagnostic hysteroscopes that are 3 mm to 4 mm in diameter, to “see-and-treat” operative hysteroscopes that are rigid and have various diameters and camera lens angles.
A hysteroscope with a 5.5-mm outer diameter works well for a vaginoscopic approach that avoids cervical dilation. Accessory instrumentation includes semirigid 5 Fr 35-cm–long biopsy forceps, scissors, and alligator forceps.
In timing the procedure, our main goal is a thin uterine lining. This can be achieved by scheduling the procedure during the early proliferative phase of the menstrual cycle or by using a gonadotropin-releasing hormone agonist or a transdermal or transvaginal contraceptive medication.
By far the most important element of pain control and analgesia is the time spent with each patient to thoroughly discuss the experience of hysteroscopy and to set expectations about what she will hear, see, and feel. An unexpected experience can worsen anxiety, which in turn can worsen pain. If everything is as familiar and relaxed as possible, there will be little need for analgesia.
I tell patients in preprocedure counseling that the distention of the uterine walls usually causes some cramping, and that NSAIDs can minimize this cramping. In rare cases, when a patient is very worried about her pain tolerance, I will prescribe diazepam. However, many of my patients opt to do nothing other than take ibuprofen. On a case-by-case basis, you can determine with your patient what type and level of analgesia and preprocedure medication will be best.
Paracervical blocks are an option for some surgical patients, but I advise my patients to move forward without the block and assure them that it can be administered later if needed. Thus far, I’ve never proceeded with a paracervical block. There are other methods and sites for introducing local anesthesia, including intracervical, by injection or topical, or topical intracavitary techniques. Nevertheless, it is unclear from randomized controlled trials whether local anesthesia is effective. Trials of paracervical blocks similarly have had inconsistent outcomes.
I do commonly premedicate patients – mainly nulliparous patients and postmenopausal patients – with misoprostol, which softens the cervix and facilitates an easier entry of the hysteroscope into the cervix.
Published studies on misoprostol administration before hysteroscopy have had mixed results. A Cochrane review from 2015 concluded there is moderate-quality evidence in support of preoperative ripening with the agent, while another meta-analysis also published in 2015 concluded that data are poor and do not support its use. Recently, however, there appear to be more supportive studies demonstrating or suggesting that misoprostol is effective in reducing discomfort.
Patient discomfort is also minimized when there is little manipulation of the hysteroscope. Scopes that are angled (12, 25, or 30 degrees) allow optimal visualization with minimal movement; the scope can be brought to the midline of the uterine cavity and the light cord rotated to the 3:00 and 9:00 o’clock positions to enable visualization of the cornu. A 0-degree scope, on the other hand, must be manipulated quite a bit for the same degree of visualization, potentially increasing patient discomfort.
Prior to hysteroscopy, the cervix and vagina are cleaned with a small-diameter swab dipped in povidone-iodine or chlorhexidine gluconate in the case of allergies. One or two 1,000-cc bags of saline inserted into pressure bags are attached to Y-type tubing. (A diagnostic procedure rarely will require two bags.) I spread the labia initially while guiding the scope into the posterior fornix of the vagina. If the leakage of fluid causes inadequate distension of the vaginal walls, I will gently pinch the labia together with gauze.
I then gently pull back the scope and manipulate it posteriorly to visualize the external cervical os anteriorly. The hysteroscope may then be introduced through the cervical os, endocervical canal, and uterine cavity, with care taken so that the instrument does not rub against the cervix or the uterine tissue and cause trauma, pain, and bleeding. The uterus will progressively align with the cervix and vagina, thereby eliminating the need for a tenaculum to straighten the uterine axis.
Fluid monitoring is important, especially during operative hysteroscopy. In my practice, a nurse watches inflow and outflow amounts while I explain what I am doing and visualizing. Some patients like to be able to view the surgery, so I am always ready to tilt the screen accordingly.
The economics
How do you know if office hysteroscopy is right for you? Your own surgical skill and the skills of your staff, who must be trained to handle and sterilize equipment and to consistently assist you, are major factors, as is ensurance of a return on your investment.
One manufacturer contacted for this Master Class lists the price of a complete office tower (light source, camera, and monitor) at approximately $9,700 and the price of a rigid hysteroscope, sheath, and hand instruments at about $6,300. A complete setup for office hysteroscopy, including a standard operative (rigid) hysteroscope, should therefore cost between $15,000 and $17,000. Companies also offer leasing options for about $300-400/month.

Flexible hysteroscopes cost about $6,000 more, which prompts many gynecologic surgeons to focus their investment on a rigid scope that can be used for both diagnostic and therapeutic procedures. Disposables cost $10 or less, and $40-50 or less, for each diagnostic and operative hysteroscopy, respectively.
A look at the Medicare Relative Value Units (RVUs) – a key component of the Medicare reimbursement system and a standard for many payers in determining compensation – shows higher reimbursement for quite a few hysteroscopic codes when these procedures are performed in the office.
Total RVUs have three components:
1. Physician work, including time and the technical skill and intensity of effort it takes to perform a service or procedure.
2. Practice expenses, such as rent, equipment and supplies, and nonphysician salaries.
3. Malpractice insurance premiums.
Each component is multiplied by a factor that accounts for geographic cost variations, and each total RVU is multiplied by a dollar amount known as the conversion factor.
Practice expense (PE) RVUs for services provided in a “facility” (e.g., hospital or outpatient clinic) are often lower than office-based PE RVUs for the same services. Hysteroscopy is no exception. The PE RVU value for diagnostic hysteroscopy performed in the office, for instance, is approximately 5 units, compared with 1.64 units for diagnostic hysteroscopy performed in a facility.
Information on hysteroscopic procedures, and their associated RVUs, on geographic practice cost indices and on pricing, can be accessed using Medicare’s Physician Fee Schedule lookup tool (www.cms.gov/apps/physician-fee-schedule/overview.aspx).
This tool is useful for calculating returns on investment. According to national payment amounts listed in August, a diagnostic hysteroscopy performed in the office will earn an average of $315.08 vs. $192.27 for each case performed in the hospital. If you perform 12 such procedures a year, that’s about $3,781 in the office, compared with $2,307 in the hospital.
This difference alone might not be worth an investment of $15,000 or more, but if you anticipate performing additional procedures with higher margins and higher reimbursement, such as 12 thermal endometrial ablations a year in combination with diagnostic hysteroscopy (which, according to the Medicare national fee schedule averages would earn $15,962 in the office vs. $4,971 in the hospital), or 12 Essure tubal occlusions ($22,595 vs. $5,263), the investment will look more favorable.
And if your patients are largely privately insured, your return on investment will occur much more quickly. In metropolitan Chicago, Blue Cross Blue Shield is reimbursing in-office diagnostic hysteroscopy at approximately $568, hysteroscopic ablations at $3,844, and Essure tubal occlusions at $3,885.
In addition to reimbursement levels, it’s important to consider the efficiencies of in-office hysteroscopy. You can perform an annual exam while the assistant sets up the room and greets each patient, for instance, or see another established patient while the assistant discharges your patient and turns the room over. Our patients, in turn, benefit from increased accessibility, with less time spent away from work or family, as well as more familiarity and comfort and reduced out-of-pocket expenses.
Dr. Cholkeri-Singh is clinical assistant professor at the University of Illinois in Chicago and is director of gynecologic surgical education and associate director of minimally invasive gynecology at Advocate Lutheran General Hospital. She is in private practice with Dr. Charles Miller and Dr. Kristen Sasaki at the Advanced Gynecologic Surgical Institute in Chicago. She is a consultant for DySIS Medical, Hologic, and Bayer HealthCare.
The benefits of integrating hysteroscopy into office practice are compelling. Most importantly, patients appreciate the comfort and convenience of having hysteroscopic procedures done in a familiar setting. Patients can generally be in and out of the office in less than 30 minutes for a diagnostic procedure, and in less than 1-2 hours for an operative procedure.
Not only is an in-office approach patient centered and clinically valuable, but it is more efficient and economically favorable for the gynecologic surgeon. Physicians earn higher reimbursement for diagnostic hysteroscopies, as well as many therapeutic and operative hysteroscopies, when these procedures are done in the office rather than when they’re performed in a hospital or an outpatient center.
Transitioning to in-office hysteroscopy need not be daunting: The setup is relatively simple and does not require an operating suite, just a dedicated exam room. And the need for premedication and local anesthesia can be low, particularly when a vaginoscopic approach to hysteroscopy is employed. For most gynecologic surgeons, the necessary skills and comfort levels fall into place after only a few vaginoscopic procedures.
A vaginoscopic approach avoids the use of a vaginal speculum or cervical tenaculum, significantly decreasing discomfort or pain. Not using these instruments is the only difference between this and traditional hysteroscopy. It is a less invasive approach that is much more tolerable for patients. And for the surgeon, it can be easier and quicker and provides equally good visualization without any impairment in cervical passage.
Described in the literature as far back as the 1950s, vaginoscopy has its roots in the pediatric/adolescent population, where it was used for the removal of foreign bodies and evaluation of the vagina and external cervical os.
More recently, Stefano Bettocchi, MD, and Luigi Selvaggi, MD, in Italy were the first to describe a vaginoscopic approach to hysteroscopy for evaluating the endocervical canal and uterine cavity.
In a series of papers from 1997 to 2004, Dr. Bettocchi and Dr. Selvaggi documented their efforts to improve patient tolerance during diagnostic hysteroscopies. When they used both the speculum and tenaculum in 163 patients, with local anesthesia, 8% reported severe pain, 11% reported moderate pain, and 69% reported mild pain. Only 12% reported no discomfort. With speculum use only, and no anesthesia, in 308 patients, none reported severe pain, 2% reported moderate pain, 32% reported mild pain, and 66% reported no discomfort. When neither instrument was used (again, no anesthesia), patient discomfort was nearly eliminated: In 680 procedures, patients had a 96% no-discomfort rate (J Am Assoc Gynecol Laparosc. 1997 Feb;4[2]:255-8; Curr Opin Obstet Gynecol. 2003 Aug;15[4]:303-8; Obstet Gynecol Clin North Am. 2004 Sep;31[3]:641-54, xi).
Since then, research has affirmed the differences in patient tolerance and has shown that there is no significant difference between traditional and vaginoscopic hysteroscopy in the rate of procedure failure (0%-10%).
In my practice, in addition to vaginal or cervical examination and evaluation of the uterine cavity, I utilize a vaginoscopic approach to perform minor therapeutic and operative procedures such as biopsies, polypectomies, tubal occlusion using the Essure system, and removal of lost intrauterine devices. I can assess infertility, trauma, abnormal uterine bleeding, and mesh erosion, and provide pre- and postsurgical evaluations. In all of these cases, I use minimal premedication and only rarely need any local anesthetic and/or sedation.
Instrumentation and technique
There are a variety of hysteroscopes available on the market, from single-channel flexible diagnostic hysteroscopes that are 3 mm to 4 mm in diameter, to “see-and-treat” operative hysteroscopes that are rigid and have various diameters and camera lens angles.
A hysteroscope with a 5.5-mm outer diameter works well for a vaginoscopic approach that avoids cervical dilation. Accessory instrumentation includes semirigid 5 Fr 35-cm–long biopsy forceps, scissors, and alligator forceps.
In timing the procedure, our main goal is a thin uterine lining. This can be achieved by scheduling the procedure during the early proliferative phase of the menstrual cycle or by using a gonadotropin-releasing hormone agonist or a transdermal or transvaginal contraceptive medication.
By far the most important element of pain control and analgesia is the time spent with each patient to thoroughly discuss the experience of hysteroscopy and to set expectations about what she will hear, see, and feel. An unexpected experience can worsen anxiety, which in turn can worsen pain. If everything is as familiar and relaxed as possible, there will be little need for analgesia.
I tell patients in preprocedure counseling that the distention of the uterine walls usually causes some cramping, and that NSAIDs can minimize this cramping. In rare cases, when a patient is very worried about her pain tolerance, I will prescribe diazepam. However, many of my patients opt to do nothing other than take ibuprofen. On a case-by-case basis, you can determine with your patient what type and level of analgesia and preprocedure medication will be best.
Paracervical blocks are an option for some surgical patients, but I advise my patients to move forward without the block and assure them that it can be administered later if needed. Thus far, I’ve never proceeded with a paracervical block. There are other methods and sites for introducing local anesthesia, including intracervical, by injection or topical, or topical intracavitary techniques. Nevertheless, it is unclear from randomized controlled trials whether local anesthesia is effective. Trials of paracervical blocks similarly have had inconsistent outcomes.
I do commonly premedicate patients – mainly nulliparous patients and postmenopausal patients – with misoprostol, which softens the cervix and facilitates an easier entry of the hysteroscope into the cervix.
Published studies on misoprostol administration before hysteroscopy have had mixed results. A Cochrane review from 2015 concluded there is moderate-quality evidence in support of preoperative ripening with the agent, while another meta-analysis also published in 2015 concluded that data are poor and do not support its use. Recently, however, there appear to be more supportive studies demonstrating or suggesting that misoprostol is effective in reducing discomfort.
Patient discomfort is also minimized when there is little manipulation of the hysteroscope. Scopes that are angled (12, 25, or 30 degrees) allow optimal visualization with minimal movement; the scope can be brought to the midline of the uterine cavity and the light cord rotated to the 3:00 and 9:00 o’clock positions to enable visualization of the cornu. A 0-degree scope, on the other hand, must be manipulated quite a bit for the same degree of visualization, potentially increasing patient discomfort.
Prior to hysteroscopy, the cervix and vagina are cleaned with a small-diameter swab dipped in povidone-iodine or chlorhexidine gluconate in the case of allergies. One or two 1,000-cc bags of saline inserted into pressure bags are attached to Y-type tubing. (A diagnostic procedure rarely will require two bags.) I spread the labia initially while guiding the scope into the posterior fornix of the vagina. If the leakage of fluid causes inadequate distension of the vaginal walls, I will gently pinch the labia together with gauze.
I then gently pull back the scope and manipulate it posteriorly to visualize the external cervical os anteriorly. The hysteroscope may then be introduced through the cervical os, endocervical canal, and uterine cavity, with care taken so that the instrument does not rub against the cervix or the uterine tissue and cause trauma, pain, and bleeding. The uterus will progressively align with the cervix and vagina, thereby eliminating the need for a tenaculum to straighten the uterine axis.
Fluid monitoring is important, especially during operative hysteroscopy. In my practice, a nurse watches inflow and outflow amounts while I explain what I am doing and visualizing. Some patients like to be able to view the surgery, so I am always ready to tilt the screen accordingly.
The economics
How do you know if office hysteroscopy is right for you? Your own surgical skill and the skills of your staff, who must be trained to handle and sterilize equipment and to consistently assist you, are major factors, as is ensurance of a return on your investment.
One manufacturer contacted for this Master Class lists the price of a complete office tower (light source, camera, and monitor) at approximately $9,700 and the price of a rigid hysteroscope, sheath, and hand instruments at about $6,300. A complete setup for office hysteroscopy, including a standard operative (rigid) hysteroscope, should therefore cost between $15,000 and $17,000. Companies also offer leasing options for about $300-400/month.

Flexible hysteroscopes cost about $6,000 more, which prompts many gynecologic surgeons to focus their investment on a rigid scope that can be used for both diagnostic and therapeutic procedures. Disposables cost $10 or less, and $40-50 or less, for each diagnostic and operative hysteroscopy, respectively.
A look at the Medicare Relative Value Units (RVUs) – a key component of the Medicare reimbursement system and a standard for many payers in determining compensation – shows higher reimbursement for quite a few hysteroscopic codes when these procedures are performed in the office.
Total RVUs have three components:
1. Physician work, including time and the technical skill and intensity of effort it takes to perform a service or procedure.
2. Practice expenses, such as rent, equipment and supplies, and nonphysician salaries.
3. Malpractice insurance premiums.
Each component is multiplied by a factor that accounts for geographic cost variations, and each total RVU is multiplied by a dollar amount known as the conversion factor.
Practice expense (PE) RVUs for services provided in a “facility” (e.g., hospital or outpatient clinic) are often lower than office-based PE RVUs for the same services. Hysteroscopy is no exception. The PE RVU value for diagnostic hysteroscopy performed in the office, for instance, is approximately 5 units, compared with 1.64 units for diagnostic hysteroscopy performed in a facility.
Information on hysteroscopic procedures, and their associated RVUs, on geographic practice cost indices and on pricing, can be accessed using Medicare’s Physician Fee Schedule lookup tool (www.cms.gov/apps/physician-fee-schedule/overview.aspx).
This tool is useful for calculating returns on investment. According to national payment amounts listed in August, a diagnostic hysteroscopy performed in the office will earn an average of $315.08 vs. $192.27 for each case performed in the hospital. If you perform 12 such procedures a year, that’s about $3,781 in the office, compared with $2,307 in the hospital.
This difference alone might not be worth an investment of $15,000 or more, but if you anticipate performing additional procedures with higher margins and higher reimbursement, such as 12 thermal endometrial ablations a year in combination with diagnostic hysteroscopy (which, according to the Medicare national fee schedule averages would earn $15,962 in the office vs. $4,971 in the hospital), or 12 Essure tubal occlusions ($22,595 vs. $5,263), the investment will look more favorable.
And if your patients are largely privately insured, your return on investment will occur much more quickly. In metropolitan Chicago, Blue Cross Blue Shield is reimbursing in-office diagnostic hysteroscopy at approximately $568, hysteroscopic ablations at $3,844, and Essure tubal occlusions at $3,885.
In addition to reimbursement levels, it’s important to consider the efficiencies of in-office hysteroscopy. You can perform an annual exam while the assistant sets up the room and greets each patient, for instance, or see another established patient while the assistant discharges your patient and turns the room over. Our patients, in turn, benefit from increased accessibility, with less time spent away from work or family, as well as more familiarity and comfort and reduced out-of-pocket expenses.
Dr. Cholkeri-Singh is clinical assistant professor at the University of Illinois in Chicago and is director of gynecologic surgical education and associate director of minimally invasive gynecology at Advocate Lutheran General Hospital. She is in private practice with Dr. Charles Miller and Dr. Kristen Sasaki at the Advanced Gynecologic Surgical Institute in Chicago. She is a consultant for DySIS Medical, Hologic, and Bayer HealthCare.
The Clinical Lab Information Retrieval (CLIR) Framework—An R Framework for CDW Clinical Lab Data Extraction and Retrieval
Purpose: Extract, retrieve, and validate clinical lab information from the VA Corporate Data Warehouse (CDW).
Background: CDW clinical lab information provide a unique opportunity to assess real world cancer treatment effectiveness and safety with higher granularity and validity compared to administrative data. Unfortunately, there is significant heterogeneity in how this information is encoded across time and geography. Various efforts have been made to clean these data and provide a consistent and reliable mapping; however, the availability and validity of these efforts also vary across lab concepts. This presents a significant barrier to utilization of CDW clinical lab information in comparative effectiveness research.
Methods: We defined a conceptual framework for retrieval of lab information 5 features: Logical Observation Identifiers Names and Codes (LOINC) codes, test names, topography, unit, and unit reference ranges. This was then implemented as a framework in R comprised of 7 discrete modules. Each module corresponds to a defined task in the conceptual framework: Concept -> LOINC/test name -> cleaned LOINC/test name -> LOINC/test name internal identifier -> fact information retrieval -> topography selection -> unit and reference range cleaning and harmonization. Each module has a defined input and output allowing implementation transparency, reproducibility, and flexibility.
Results: Using the CLIR framework, we retrieved peripheral blood total white count of patients with hematologic malignancies. In a cohort of about 300,000 patients diagnosed and or treated for a hematologic malignancy in the VHA between 2001-2016, we identified ~ 11x10^6 potential total WBC count based on LOINC codes and lab test name. Of those, ~ 9x106 were mappable to the correct topography, and the overwhelming majority of which (99%) were mappable to a harmonized unit and reference range.
Conclusion: The CLIR framework provides a conceptual framework and an implementation in R for clinical lab information retrieval from the VA CDW. Future efforts will entail refining the methodology across multiple data domains and comparing CLIR output with other ongoing efforts aimed at cleaning and harmonization of clinical lab data in the CDW.
Purpose: Extract, retrieve, and validate clinical lab information from the VA Corporate Data Warehouse (CDW).
Background: CDW clinical lab information provide a unique opportunity to assess real world cancer treatment effectiveness and safety with higher granularity and validity compared to administrative data. Unfortunately, there is significant heterogeneity in how this information is encoded across time and geography. Various efforts have been made to clean these data and provide a consistent and reliable mapping; however, the availability and validity of these efforts also vary across lab concepts. This presents a significant barrier to utilization of CDW clinical lab information in comparative effectiveness research.
Methods: We defined a conceptual framework for retrieval of lab information 5 features: Logical Observation Identifiers Names and Codes (LOINC) codes, test names, topography, unit, and unit reference ranges. This was then implemented as a framework in R comprised of 7 discrete modules. Each module corresponds to a defined task in the conceptual framework: Concept -> LOINC/test name -> cleaned LOINC/test name -> LOINC/test name internal identifier -> fact information retrieval -> topography selection -> unit and reference range cleaning and harmonization. Each module has a defined input and output allowing implementation transparency, reproducibility, and flexibility.
Results: Using the CLIR framework, we retrieved peripheral blood total white count of patients with hematologic malignancies. In a cohort of about 300,000 patients diagnosed and or treated for a hematologic malignancy in the VHA between 2001-2016, we identified ~ 11x10^6 potential total WBC count based on LOINC codes and lab test name. Of those, ~ 9x106 were mappable to the correct topography, and the overwhelming majority of which (99%) were mappable to a harmonized unit and reference range.
Conclusion: The CLIR framework provides a conceptual framework and an implementation in R for clinical lab information retrieval from the VA CDW. Future efforts will entail refining the methodology across multiple data domains and comparing CLIR output with other ongoing efforts aimed at cleaning and harmonization of clinical lab data in the CDW.
Purpose: Extract, retrieve, and validate clinical lab information from the VA Corporate Data Warehouse (CDW).
Background: CDW clinical lab information provide a unique opportunity to assess real world cancer treatment effectiveness and safety with higher granularity and validity compared to administrative data. Unfortunately, there is significant heterogeneity in how this information is encoded across time and geography. Various efforts have been made to clean these data and provide a consistent and reliable mapping; however, the availability and validity of these efforts also vary across lab concepts. This presents a significant barrier to utilization of CDW clinical lab information in comparative effectiveness research.
Methods: We defined a conceptual framework for retrieval of lab information 5 features: Logical Observation Identifiers Names and Codes (LOINC) codes, test names, topography, unit, and unit reference ranges. This was then implemented as a framework in R comprised of 7 discrete modules. Each module corresponds to a defined task in the conceptual framework: Concept -> LOINC/test name -> cleaned LOINC/test name -> LOINC/test name internal identifier -> fact information retrieval -> topography selection -> unit and reference range cleaning and harmonization. Each module has a defined input and output allowing implementation transparency, reproducibility, and flexibility.
Results: Using the CLIR framework, we retrieved peripheral blood total white count of patients with hematologic malignancies. In a cohort of about 300,000 patients diagnosed and or treated for a hematologic malignancy in the VHA between 2001-2016, we identified ~ 11x10^6 potential total WBC count based on LOINC codes and lab test name. Of those, ~ 9x106 were mappable to the correct topography, and the overwhelming majority of which (99%) were mappable to a harmonized unit and reference range.
Conclusion: The CLIR framework provides a conceptual framework and an implementation in R for clinical lab information retrieval from the VA CDW. Future efforts will entail refining the methodology across multiple data domains and comparing CLIR output with other ongoing efforts aimed at cleaning and harmonization of clinical lab data in the CDW.
BRAF Inhibitor Resistance Reprograms Metabolic and Survival Pathways to Sensitize Melanoma Cells to Arginine Deprivation
BRAF inhibitor (BRAFi) combined with MEK inhibitor (MEKi) are used to treat melanomas harboring (V600E) mutation. While response is high, the majority of them relapsed. We found novel mechanisms to treat BRAFi resistant (BR) patients.
Five BR cells were established from known BRAF mutant cell lines A375, MEL-1220, A2058, UACC-62, and SKMEL28 by long-term exposure to BRAFi. These BR cells are hypersensitive to ADI-PEG20, an enzyme which degrades arginine to citrulline. ADI-PEG20 treatment resulted in 10-30% increase in apoptosis (Annexin V/PI) in BR cells compared to their paternal cells. The mechanisms involved are as follows: All BR cells express very low levels or do not express argininosuccinate synthetase (ASS, a key enzyme to generate arginine from citrulline) and also have attenuated glucose uptake. Thus, these BR cells rely primarily on exogenous arginine. Importantly, their ability to undergo autophagy upon nutritional stress is also impaired. The underlying mechanism for low levels of ASS is due to diminished c-Myc expression,a positive regulator for ASS. Additionally, AMPK-α 1, which governed autophagy and glucose uptake, was attenuated in BR cells. Overexpression of AMPK-α 1 using plasmid transfection in A2058BR and MEL-1220BR cells rescued 8-28% ADI-PEG20-induced apoptosis and enhanced autophagosome formation. Conversely, knockdown of AMPK-α 1 significantly enhanced ADI-PEG20-reduced cell viability in A2058 and MEL-1220 cells through attenuation of autophagy and glucose uptake. This is evidenced by decreased GLUT1 and LC3-II expression, and low activity of 2-NBDG uptake seen in BR cells. BMR (BRAF and MEK inhibitor) resistant cells also shared similar biochemical changes. Immunohistochemical staining further confirmed lower levels of ASS and AMPK-α 1 in A2058BR, BMR and in tumor tissues from 10 BR patients relative to their parental counterparts (average H-scores of ASS and AMPK in parental tissues vs. BR tissues are 58.2 vs. 7.8, and 146 vs. 78.3, respectively, P < 0.03). Importantly, these findings also apply to cell lines and tumor samples from patients who failed both BRAF and MEK inhibitor.
In summary, our data suggest that attenuated AMPK-α 1 mediated autophagy and glucose uptake and decreased c-Myc mediated ASS re-expression sensitize BR cells to arginine deprivation, and can be used as salvage therapy for BR patients.
Supported by 1RO1CA152197 and VA Merit Review BLR&D11860649.
BRAF inhibitor (BRAFi) combined with MEK inhibitor (MEKi) are used to treat melanomas harboring (V600E) mutation. While response is high, the majority of them relapsed. We found novel mechanisms to treat BRAFi resistant (BR) patients.
Five BR cells were established from known BRAF mutant cell lines A375, MEL-1220, A2058, UACC-62, and SKMEL28 by long-term exposure to BRAFi. These BR cells are hypersensitive to ADI-PEG20, an enzyme which degrades arginine to citrulline. ADI-PEG20 treatment resulted in 10-30% increase in apoptosis (Annexin V/PI) in BR cells compared to their paternal cells. The mechanisms involved are as follows: All BR cells express very low levels or do not express argininosuccinate synthetase (ASS, a key enzyme to generate arginine from citrulline) and also have attenuated glucose uptake. Thus, these BR cells rely primarily on exogenous arginine. Importantly, their ability to undergo autophagy upon nutritional stress is also impaired. The underlying mechanism for low levels of ASS is due to diminished c-Myc expression,a positive regulator for ASS. Additionally, AMPK-α 1, which governed autophagy and glucose uptake, was attenuated in BR cells. Overexpression of AMPK-α 1 using plasmid transfection in A2058BR and MEL-1220BR cells rescued 8-28% ADI-PEG20-induced apoptosis and enhanced autophagosome formation. Conversely, knockdown of AMPK-α 1 significantly enhanced ADI-PEG20-reduced cell viability in A2058 and MEL-1220 cells through attenuation of autophagy and glucose uptake. This is evidenced by decreased GLUT1 and LC3-II expression, and low activity of 2-NBDG uptake seen in BR cells. BMR (BRAF and MEK inhibitor) resistant cells also shared similar biochemical changes. Immunohistochemical staining further confirmed lower levels of ASS and AMPK-α 1 in A2058BR, BMR and in tumor tissues from 10 BR patients relative to their parental counterparts (average H-scores of ASS and AMPK in parental tissues vs. BR tissues are 58.2 vs. 7.8, and 146 vs. 78.3, respectively, P < 0.03). Importantly, these findings also apply to cell lines and tumor samples from patients who failed both BRAF and MEK inhibitor.
In summary, our data suggest that attenuated AMPK-α 1 mediated autophagy and glucose uptake and decreased c-Myc mediated ASS re-expression sensitize BR cells to arginine deprivation, and can be used as salvage therapy for BR patients.
Supported by 1RO1CA152197 and VA Merit Review BLR&D11860649.
BRAF inhibitor (BRAFi) combined with MEK inhibitor (MEKi) are used to treat melanomas harboring (V600E) mutation. While response is high, the majority of them relapsed. We found novel mechanisms to treat BRAFi resistant (BR) patients.
Five BR cells were established from known BRAF mutant cell lines A375, MEL-1220, A2058, UACC-62, and SKMEL28 by long-term exposure to BRAFi. These BR cells are hypersensitive to ADI-PEG20, an enzyme which degrades arginine to citrulline. ADI-PEG20 treatment resulted in 10-30% increase in apoptosis (Annexin V/PI) in BR cells compared to their paternal cells. The mechanisms involved are as follows: All BR cells express very low levels or do not express argininosuccinate synthetase (ASS, a key enzyme to generate arginine from citrulline) and also have attenuated glucose uptake. Thus, these BR cells rely primarily on exogenous arginine. Importantly, their ability to undergo autophagy upon nutritional stress is also impaired. The underlying mechanism for low levels of ASS is due to diminished c-Myc expression,a positive regulator for ASS. Additionally, AMPK-α 1, which governed autophagy and glucose uptake, was attenuated in BR cells. Overexpression of AMPK-α 1 using plasmid transfection in A2058BR and MEL-1220BR cells rescued 8-28% ADI-PEG20-induced apoptosis and enhanced autophagosome formation. Conversely, knockdown of AMPK-α 1 significantly enhanced ADI-PEG20-reduced cell viability in A2058 and MEL-1220 cells through attenuation of autophagy and glucose uptake. This is evidenced by decreased GLUT1 and LC3-II expression, and low activity of 2-NBDG uptake seen in BR cells. BMR (BRAF and MEK inhibitor) resistant cells also shared similar biochemical changes. Immunohistochemical staining further confirmed lower levels of ASS and AMPK-α 1 in A2058BR, BMR and in tumor tissues from 10 BR patients relative to their parental counterparts (average H-scores of ASS and AMPK in parental tissues vs. BR tissues are 58.2 vs. 7.8, and 146 vs. 78.3, respectively, P < 0.03). Importantly, these findings also apply to cell lines and tumor samples from patients who failed both BRAF and MEK inhibitor.
In summary, our data suggest that attenuated AMPK-α 1 mediated autophagy and glucose uptake and decreased c-Myc mediated ASS re-expression sensitize BR cells to arginine deprivation, and can be used as salvage therapy for BR patients.
Supported by 1RO1CA152197 and VA Merit Review BLR&D11860649.
September 2016: Click for Credit
Here are 5 articles in the August issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Women With BRCA1 Mutations at Higher Risk for Endometrial Cancers
To take the posttest, go to: http://bit.ly/2t6SPIY
Expires June 30, 2017
VITALSKey clinical point: Clinicians may wish to discuss the option of hysterectomy at the time of salpingo-oophorectomy in women with deleterious BRCA1 mutations.
Major finding: Among women with BRCA1 but not BRCA2 mutations there was increased risk for serous/serous-like endometrial carcinomas.
Data source: Prospective multicenter follow-up study of 1,083 women with BRCA mutations who underwent salpingo-oophorectomy without hysterectomy.
Disclosures: The study was supported by grants from the Department of Defense, National Institutes of Health, and public and private foundations. Coauthor Robert Soslow, MD, disclosed consulting for EMD Serono. No others reported conflicts of interest. The editorialists reported no conflicts of interest related to the study.
2. Cochrane Review: Topical Steroid—Vitamin D Combo Best for Scalp Psoriasis
To take the posttest, go to: http://bit.ly/2sIyLNI
Expires July 14, 2017
VITALSKey clinical point: The combination of a topical steroid and topical vitamin D is marginally better but with a similar safety profile to steroids alone as a treatment for psoriasis on the scalp.
Major finding: The combination of a topical steroid and vitamin D showed a small but statistically significant advantage over steroids alone, and a greater advantage over vitamin D alone.
Data source: A systematic review of 59 randomized controlled studies in 11,561 patients.
Disclosures: The study was supported by the Universidade Federal de São Paulo, Brazil; the Universidade Federal do Rio Grande do Norte, Brazil; and the National Institute for Health Research, United Kingdom. Six authors and one clinical referee declared speakers' fees, research grants, and funding from the pharmaceutical industry. One author had no conflicts of interest to disclose.
3. Study Finds Emergence of Azithromycin-resistant Gonorrhea
To take the posttest, go to: http://bit.ly/2u1nMmb
Expires July 16, 2017
VITALSKey clinical point:Resistance to azithromycin is emerging among patients diagnosed with gonorrhea.
Major finding: Among patients with gonorrhea, resistance to azithromycin increased from 0.6% in 2013 to 2.5% in 2014, predominantly in the Midwest.
Data source: An analysis of 5,093 Neisseria gonorrhoeae isolates from 27 clinics as part of the CDC's Gonococcal Isolate Surveillance Project.
Disclosures: The researchers had no financial disclosures.
4. Statins Improve Ovarian Cancer Survival
To take the posttest, go to: http://bit.ly/2t6swCF
Expires June 16, 2017
VITALSKey clinical point: The risk of all-cause mortality in ovarian cancer patients on statin therapy was reduced by one-third.
Major finding: Mean survival in a large cohort of women with stage III ovarian cancer was 5.8 months longer among those on statin therapy.
Data source: A retrospective study of 1,510 women diagnosed with epithelial ovarian cancer during 2007-2009.
Disclosures: Dr. Vogel reported having no financial conflicts regarding this study, conducted without commercial support.
5. Common Surgeries Linked to Chronic Opioid Use Among Opioid-naive Patients
To take the posttest, go to: http://bit.ly/2ub9fFg
Expires June 18, 2017
VITALSKey clinical point: Common surgeries increase the risk of chronic opioid use in opioid-naive adults, especially among those using antidepressants or benzodiazepines before their operations, and those with substance abuse histories.
Major finding: After adjustment for potential confounders, knee replacement increased the risk fivefold; open cholecystectomy almost fourfold; and total hip replacement and simple mastectomy almost threefold.
Data source: Insurance claims of more than 18 million people.
Disclosures: The authors had no disclosures. The work was funded in part by the Foundation for Anesthesia Education and Research and the Anesthesia Quality Institute. Claims data came from MarketScan (Truven Health Analytics).
Here are 5 articles in the August issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Women With BRCA1 Mutations at Higher Risk for Endometrial Cancers
To take the posttest, go to: http://bit.ly/2t6SPIY
Expires June 30, 2017
VITALSKey clinical point: Clinicians may wish to discuss the option of hysterectomy at the time of salpingo-oophorectomy in women with deleterious BRCA1 mutations.
Major finding: Among women with BRCA1 but not BRCA2 mutations there was increased risk for serous/serous-like endometrial carcinomas.
Data source: Prospective multicenter follow-up study of 1,083 women with BRCA mutations who underwent salpingo-oophorectomy without hysterectomy.
Disclosures: The study was supported by grants from the Department of Defense, National Institutes of Health, and public and private foundations. Coauthor Robert Soslow, MD, disclosed consulting for EMD Serono. No others reported conflicts of interest. The editorialists reported no conflicts of interest related to the study.
2. Cochrane Review: Topical Steroid—Vitamin D Combo Best for Scalp Psoriasis
To take the posttest, go to: http://bit.ly/2sIyLNI
Expires July 14, 2017
VITALSKey clinical point: The combination of a topical steroid and topical vitamin D is marginally better but with a similar safety profile to steroids alone as a treatment for psoriasis on the scalp.
Major finding: The combination of a topical steroid and vitamin D showed a small but statistically significant advantage over steroids alone, and a greater advantage over vitamin D alone.
Data source: A systematic review of 59 randomized controlled studies in 11,561 patients.
Disclosures: The study was supported by the Universidade Federal de São Paulo, Brazil; the Universidade Federal do Rio Grande do Norte, Brazil; and the National Institute for Health Research, United Kingdom. Six authors and one clinical referee declared speakers' fees, research grants, and funding from the pharmaceutical industry. One author had no conflicts of interest to disclose.
3. Study Finds Emergence of Azithromycin-resistant Gonorrhea
To take the posttest, go to: http://bit.ly/2u1nMmb
Expires July 16, 2017
VITALSKey clinical point:Resistance to azithromycin is emerging among patients diagnosed with gonorrhea.
Major finding: Among patients with gonorrhea, resistance to azithromycin increased from 0.6% in 2013 to 2.5% in 2014, predominantly in the Midwest.
Data source: An analysis of 5,093 Neisseria gonorrhoeae isolates from 27 clinics as part of the CDC's Gonococcal Isolate Surveillance Project.
Disclosures: The researchers had no financial disclosures.
4. Statins Improve Ovarian Cancer Survival
To take the posttest, go to: http://bit.ly/2t6swCF
Expires June 16, 2017
VITALSKey clinical point: The risk of all-cause mortality in ovarian cancer patients on statin therapy was reduced by one-third.
Major finding: Mean survival in a large cohort of women with stage III ovarian cancer was 5.8 months longer among those on statin therapy.
Data source: A retrospective study of 1,510 women diagnosed with epithelial ovarian cancer during 2007-2009.
Disclosures: Dr. Vogel reported having no financial conflicts regarding this study, conducted without commercial support.
5. Common Surgeries Linked to Chronic Opioid Use Among Opioid-naive Patients
To take the posttest, go to: http://bit.ly/2ub9fFg
Expires June 18, 2017
VITALSKey clinical point: Common surgeries increase the risk of chronic opioid use in opioid-naive adults, especially among those using antidepressants or benzodiazepines before their operations, and those with substance abuse histories.
Major finding: After adjustment for potential confounders, knee replacement increased the risk fivefold; open cholecystectomy almost fourfold; and total hip replacement and simple mastectomy almost threefold.
Data source: Insurance claims of more than 18 million people.
Disclosures: The authors had no disclosures. The work was funded in part by the Foundation for Anesthesia Education and Research and the Anesthesia Quality Institute. Claims data came from MarketScan (Truven Health Analytics).
Here are 5 articles in the August issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Women With BRCA1 Mutations at Higher Risk for Endometrial Cancers
To take the posttest, go to: http://bit.ly/2t6SPIY
Expires June 30, 2017
VITALSKey clinical point: Clinicians may wish to discuss the option of hysterectomy at the time of salpingo-oophorectomy in women with deleterious BRCA1 mutations.
Major finding: Among women with BRCA1 but not BRCA2 mutations there was increased risk for serous/serous-like endometrial carcinomas.
Data source: Prospective multicenter follow-up study of 1,083 women with BRCA mutations who underwent salpingo-oophorectomy without hysterectomy.
Disclosures: The study was supported by grants from the Department of Defense, National Institutes of Health, and public and private foundations. Coauthor Robert Soslow, MD, disclosed consulting for EMD Serono. No others reported conflicts of interest. The editorialists reported no conflicts of interest related to the study.
2. Cochrane Review: Topical Steroid—Vitamin D Combo Best for Scalp Psoriasis
To take the posttest, go to: http://bit.ly/2sIyLNI
Expires July 14, 2017
VITALSKey clinical point: The combination of a topical steroid and topical vitamin D is marginally better but with a similar safety profile to steroids alone as a treatment for psoriasis on the scalp.
Major finding: The combination of a topical steroid and vitamin D showed a small but statistically significant advantage over steroids alone, and a greater advantage over vitamin D alone.
Data source: A systematic review of 59 randomized controlled studies in 11,561 patients.
Disclosures: The study was supported by the Universidade Federal de São Paulo, Brazil; the Universidade Federal do Rio Grande do Norte, Brazil; and the National Institute for Health Research, United Kingdom. Six authors and one clinical referee declared speakers' fees, research grants, and funding from the pharmaceutical industry. One author had no conflicts of interest to disclose.
3. Study Finds Emergence of Azithromycin-resistant Gonorrhea
To take the posttest, go to: http://bit.ly/2u1nMmb
Expires July 16, 2017
VITALSKey clinical point:Resistance to azithromycin is emerging among patients diagnosed with gonorrhea.
Major finding: Among patients with gonorrhea, resistance to azithromycin increased from 0.6% in 2013 to 2.5% in 2014, predominantly in the Midwest.
Data source: An analysis of 5,093 Neisseria gonorrhoeae isolates from 27 clinics as part of the CDC's Gonococcal Isolate Surveillance Project.
Disclosures: The researchers had no financial disclosures.
4. Statins Improve Ovarian Cancer Survival
To take the posttest, go to: http://bit.ly/2t6swCF
Expires June 16, 2017
VITALSKey clinical point: The risk of all-cause mortality in ovarian cancer patients on statin therapy was reduced by one-third.
Major finding: Mean survival in a large cohort of women with stage III ovarian cancer was 5.8 months longer among those on statin therapy.
Data source: A retrospective study of 1,510 women diagnosed with epithelial ovarian cancer during 2007-2009.
Disclosures: Dr. Vogel reported having no financial conflicts regarding this study, conducted without commercial support.
5. Common Surgeries Linked to Chronic Opioid Use Among Opioid-naive Patients
To take the posttest, go to: http://bit.ly/2ub9fFg
Expires June 18, 2017
VITALSKey clinical point: Common surgeries increase the risk of chronic opioid use in opioid-naive adults, especially among those using antidepressants or benzodiazepines before their operations, and those with substance abuse histories.
Major finding: After adjustment for potential confounders, knee replacement increased the risk fivefold; open cholecystectomy almost fourfold; and total hip replacement and simple mastectomy almost threefold.
Data source: Insurance claims of more than 18 million people.
Disclosures: The authors had no disclosures. The work was funded in part by the Foundation for Anesthesia Education and Research and the Anesthesia Quality Institute. Claims data came from MarketScan (Truven Health Analytics).
Spreading Innovation among Hospitalists
“Increasingly, we are not having faculty who are going up for promotion and reliably running into challenges around mentorship, national reputation, and having a network outside of their local hospital that is critical for advancement,” says lead author Ethan Cumbler, MD, FHM, FACP, of the Department of Medicine at the University of Colorado School of Medicine. “Hospital medicine as a movement is built on a foundation of innovation, and so as a specialty, we have a mandate to not only innovate but to disseminate those innovations.”
The model of the visiting professorship described in the paper takes midcareer academic hospitalists and provides an infrastructure for reciprocal faculty exchanges. This provides a forum to increase professional networks.
“We found that both junior faculty and our visiting professors saw value in advancing those goals,” Dr. Cumbler says. “We also saw evidence of the spread of ideas and new shared scholarship derived from having these reciprocal visits.”
This has model relevance for nonacademic hospitals, too. For example, it’d be useful for hospital medicine groups to share ideas with one another, Dr. Cumbler says.
“This is a simple structure, but it’s just like a small pebble thrown into a large body of water can create ripples which affect distant shores—sometimes it’s very simple concepts that are worth pursuing,” he says.
Reference
- Cumbler E, Herzke C, Smalligan R, Glasheen JJ, O’Malley C, Pierce JR Jr. Visiting professorship in hospital medicine: an innovative twist for a growing specialty [published online ahead of print June 23, 2016]. J Hosp Med. doi:10.1002/jhm.2625.
“Increasingly, we are not having faculty who are going up for promotion and reliably running into challenges around mentorship, national reputation, and having a network outside of their local hospital that is critical for advancement,” says lead author Ethan Cumbler, MD, FHM, FACP, of the Department of Medicine at the University of Colorado School of Medicine. “Hospital medicine as a movement is built on a foundation of innovation, and so as a specialty, we have a mandate to not only innovate but to disseminate those innovations.”
The model of the visiting professorship described in the paper takes midcareer academic hospitalists and provides an infrastructure for reciprocal faculty exchanges. This provides a forum to increase professional networks.
“We found that both junior faculty and our visiting professors saw value in advancing those goals,” Dr. Cumbler says. “We also saw evidence of the spread of ideas and new shared scholarship derived from having these reciprocal visits.”
This has model relevance for nonacademic hospitals, too. For example, it’d be useful for hospital medicine groups to share ideas with one another, Dr. Cumbler says.
“This is a simple structure, but it’s just like a small pebble thrown into a large body of water can create ripples which affect distant shores—sometimes it’s very simple concepts that are worth pursuing,” he says.
Reference
- Cumbler E, Herzke C, Smalligan R, Glasheen JJ, O’Malley C, Pierce JR Jr. Visiting professorship in hospital medicine: an innovative twist for a growing specialty [published online ahead of print June 23, 2016]. J Hosp Med. doi:10.1002/jhm.2625.
“Increasingly, we are not having faculty who are going up for promotion and reliably running into challenges around mentorship, national reputation, and having a network outside of their local hospital that is critical for advancement,” says lead author Ethan Cumbler, MD, FHM, FACP, of the Department of Medicine at the University of Colorado School of Medicine. “Hospital medicine as a movement is built on a foundation of innovation, and so as a specialty, we have a mandate to not only innovate but to disseminate those innovations.”
The model of the visiting professorship described in the paper takes midcareer academic hospitalists and provides an infrastructure for reciprocal faculty exchanges. This provides a forum to increase professional networks.
“We found that both junior faculty and our visiting professors saw value in advancing those goals,” Dr. Cumbler says. “We also saw evidence of the spread of ideas and new shared scholarship derived from having these reciprocal visits.”
This has model relevance for nonacademic hospitals, too. For example, it’d be useful for hospital medicine groups to share ideas with one another, Dr. Cumbler says.
“This is a simple structure, but it’s just like a small pebble thrown into a large body of water can create ripples which affect distant shores—sometimes it’s very simple concepts that are worth pursuing,” he says.
Reference
- Cumbler E, Herzke C, Smalligan R, Glasheen JJ, O’Malley C, Pierce JR Jr. Visiting professorship in hospital medicine: an innovative twist for a growing specialty [published online ahead of print June 23, 2016]. J Hosp Med. doi:10.1002/jhm.2625.
PAs Should Focus on Patient Care, Not Unnecessary Testing
PAs are highly educated, trusted health care providers who lead patient-centered medical teams. Trained as experts in general medicine, we often pursue multiple specialties over the course of our careers—typically in three or four. PAs can decide to work in surgery, emergency care, orthopedics, oncology, pediatrics, dermatology, and many other areas. Moving among and between specialties is a hallmark of our profession. Unfortunately, a new proposal would alter how PAs are tested in order to maintain their certification—and, in 20 states, potentially jeopardize their license to practice.
The National Commission on Certification of PAs (NCCPA) has proposed significant changes to how PAs are recertified by requiring multiple exams, including a proctored, closed-book exam in a specialty area and two or three take-home exams during every 10-year recertification cycle. The proposal would in effect force PAs to choose a specialty and as a result undermine their ability to fill care gaps in hospitals, health systems, and communities.
The new requirements are cumbersome and unnecessary. PAs already undergo rigorous medical training and have initial licensing requirements that are similar to those of our physician, nurse practitioner, and pharmacist colleagues—none of whom are required to retest. PAs must graduate from an accredited program and take a test in general medicine in order to be licensed and certified in the first place. Throughout their careers, they have to complete extensive continuing medical education (CME). They also practice in clinical settings that continually inform and enhance their experience and base of knowledge.
Additional testing would take valuable time away from patients and could even discourage PAs from staying in a profession that is in high demand. More to the point, NCCPA has pursued its proposal even though studies have shown that recertification testing is not related to improvements in patient outcomes or safety.
AAPA recently received a message from longtime PA Peter Schuman, who is as passionate about patient care as he is leery of NCCPA’s recertification plan. “[NCCPA has] no significant, scientifically valid evidence to support their claims. I can honestly say that their testing requirements have not helped me care for patients better or become more knowledgeable in my field of practice/expertise one bit,” he wrote. “The PANRE is a waste of time and effort and is a source of great stress, taking time away from my patients, practice, and family. Enough is enough, NCCPA!”
The AAPA board has reached out to NCCPA and still hopes that it will engage in substantive discussions. Given the seriousness of our concerns, however, the AAPA Board voted recently to take steps toward the creation of an alternative certifying body for PAs. This vote came after careful deliberation and in response to the concerns of the thousands of professionals that AAPA represents. The decision was not made lightly and it reflects the priority of PAs to put patient care ahead of unnecessary testing.
AAPA is not alone in its opposition to recertification testing. A growing number of medical associations, including the American Medical Association (AMA), reject it as unnecessary and overly burdensome. AMA has rightly identified these exams as high-stakes tests because, it said, “failure to pass can result in a physician’s loss of privileges or employment.” Every PA would face similar consequences and, in 20 states, put their license at risk. At least 19 state medical associations have adopted similar resolutions in opposition to unnecessary retesting.
To be clear, AAPA does not oppose initial testing for certification and licensing and embraces the value of an exam in the licensing process. AAPA also strongly supports extensive CME and the benefits it provides.
We have not yet decided whether to establish a new certification organization. But we do know that there is no reason PAs should be singled out for additional testing when the extra requirement does not help patients and when other medical professionals are not required to do the same. Let PAs focus on patient care, not unwarranted test-taking.
PAs are highly educated, trusted health care providers who lead patient-centered medical teams. Trained as experts in general medicine, we often pursue multiple specialties over the course of our careers—typically in three or four. PAs can decide to work in surgery, emergency care, orthopedics, oncology, pediatrics, dermatology, and many other areas. Moving among and between specialties is a hallmark of our profession. Unfortunately, a new proposal would alter how PAs are tested in order to maintain their certification—and, in 20 states, potentially jeopardize their license to practice.
The National Commission on Certification of PAs (NCCPA) has proposed significant changes to how PAs are recertified by requiring multiple exams, including a proctored, closed-book exam in a specialty area and two or three take-home exams during every 10-year recertification cycle. The proposal would in effect force PAs to choose a specialty and as a result undermine their ability to fill care gaps in hospitals, health systems, and communities.
The new requirements are cumbersome and unnecessary. PAs already undergo rigorous medical training and have initial licensing requirements that are similar to those of our physician, nurse practitioner, and pharmacist colleagues—none of whom are required to retest. PAs must graduate from an accredited program and take a test in general medicine in order to be licensed and certified in the first place. Throughout their careers, they have to complete extensive continuing medical education (CME). They also practice in clinical settings that continually inform and enhance their experience and base of knowledge.
Additional testing would take valuable time away from patients and could even discourage PAs from staying in a profession that is in high demand. More to the point, NCCPA has pursued its proposal even though studies have shown that recertification testing is not related to improvements in patient outcomes or safety.
AAPA recently received a message from longtime PA Peter Schuman, who is as passionate about patient care as he is leery of NCCPA’s recertification plan. “[NCCPA has] no significant, scientifically valid evidence to support their claims. I can honestly say that their testing requirements have not helped me care for patients better or become more knowledgeable in my field of practice/expertise one bit,” he wrote. “The PANRE is a waste of time and effort and is a source of great stress, taking time away from my patients, practice, and family. Enough is enough, NCCPA!”
The AAPA board has reached out to NCCPA and still hopes that it will engage in substantive discussions. Given the seriousness of our concerns, however, the AAPA Board voted recently to take steps toward the creation of an alternative certifying body for PAs. This vote came after careful deliberation and in response to the concerns of the thousands of professionals that AAPA represents. The decision was not made lightly and it reflects the priority of PAs to put patient care ahead of unnecessary testing.
AAPA is not alone in its opposition to recertification testing. A growing number of medical associations, including the American Medical Association (AMA), reject it as unnecessary and overly burdensome. AMA has rightly identified these exams as high-stakes tests because, it said, “failure to pass can result in a physician’s loss of privileges or employment.” Every PA would face similar consequences and, in 20 states, put their license at risk. At least 19 state medical associations have adopted similar resolutions in opposition to unnecessary retesting.
To be clear, AAPA does not oppose initial testing for certification and licensing and embraces the value of an exam in the licensing process. AAPA also strongly supports extensive CME and the benefits it provides.
We have not yet decided whether to establish a new certification organization. But we do know that there is no reason PAs should be singled out for additional testing when the extra requirement does not help patients and when other medical professionals are not required to do the same. Let PAs focus on patient care, not unwarranted test-taking.
PAs are highly educated, trusted health care providers who lead patient-centered medical teams. Trained as experts in general medicine, we often pursue multiple specialties over the course of our careers—typically in three or four. PAs can decide to work in surgery, emergency care, orthopedics, oncology, pediatrics, dermatology, and many other areas. Moving among and between specialties is a hallmark of our profession. Unfortunately, a new proposal would alter how PAs are tested in order to maintain their certification—and, in 20 states, potentially jeopardize their license to practice.
The National Commission on Certification of PAs (NCCPA) has proposed significant changes to how PAs are recertified by requiring multiple exams, including a proctored, closed-book exam in a specialty area and two or three take-home exams during every 10-year recertification cycle. The proposal would in effect force PAs to choose a specialty and as a result undermine their ability to fill care gaps in hospitals, health systems, and communities.
The new requirements are cumbersome and unnecessary. PAs already undergo rigorous medical training and have initial licensing requirements that are similar to those of our physician, nurse practitioner, and pharmacist colleagues—none of whom are required to retest. PAs must graduate from an accredited program and take a test in general medicine in order to be licensed and certified in the first place. Throughout their careers, they have to complete extensive continuing medical education (CME). They also practice in clinical settings that continually inform and enhance their experience and base of knowledge.
Additional testing would take valuable time away from patients and could even discourage PAs from staying in a profession that is in high demand. More to the point, NCCPA has pursued its proposal even though studies have shown that recertification testing is not related to improvements in patient outcomes or safety.
AAPA recently received a message from longtime PA Peter Schuman, who is as passionate about patient care as he is leery of NCCPA’s recertification plan. “[NCCPA has] no significant, scientifically valid evidence to support their claims. I can honestly say that their testing requirements have not helped me care for patients better or become more knowledgeable in my field of practice/expertise one bit,” he wrote. “The PANRE is a waste of time and effort and is a source of great stress, taking time away from my patients, practice, and family. Enough is enough, NCCPA!”
The AAPA board has reached out to NCCPA and still hopes that it will engage in substantive discussions. Given the seriousness of our concerns, however, the AAPA Board voted recently to take steps toward the creation of an alternative certifying body for PAs. This vote came after careful deliberation and in response to the concerns of the thousands of professionals that AAPA represents. The decision was not made lightly and it reflects the priority of PAs to put patient care ahead of unnecessary testing.
AAPA is not alone in its opposition to recertification testing. A growing number of medical associations, including the American Medical Association (AMA), reject it as unnecessary and overly burdensome. AMA has rightly identified these exams as high-stakes tests because, it said, “failure to pass can result in a physician’s loss of privileges or employment.” Every PA would face similar consequences and, in 20 states, put their license at risk. At least 19 state medical associations have adopted similar resolutions in opposition to unnecessary retesting.
To be clear, AAPA does not oppose initial testing for certification and licensing and embraces the value of an exam in the licensing process. AAPA also strongly supports extensive CME and the benefits it provides.
We have not yet decided whether to establish a new certification organization. But we do know that there is no reason PAs should be singled out for additional testing when the extra requirement does not help patients and when other medical professionals are not required to do the same. Let PAs focus on patient care, not unwarranted test-taking.
Antidote to factor Xa inhibitors exhibits efficacy in patients with major bleeding

ROME—Preliminary results from the ANNEXA-4 study suggest that andexanet alfa, an investigational antidote to factor Xa inhibitors, can be effective in patients with acute major bleeding.
The drug reversed the anticoagulant effects of rivaroxaban, apixaban, and enoxaparin in this study, providing “excellent” or “good” hemostatic efficacy in 79% of patients over 12 hours.
Thrombotic events occurred in 18% of patients, and 15% died during the 30-day follow-up period.
According to investigators, these events occurred within the range expected in this patient population, given the severity of their bleeding, their underlying thrombotic risk, and the low percentage of patients who restarted anticoagulant therapy following their bleeding episode.
Stuart J. Connolly, MD, of McMaster University in Hamilton, Ontario, Canada, presented results from ANNEXA-4 at ESC Congress 2016 (abstract 5718).
Results were also published in NEJM. The study was funded by Portola Pharmaceuticals Inc.
Patients and treatment
The preliminary analysis of the phase 3/4 ANNEXA-4 trial included 67 patients. All of these patients were evaluated for safety, and 47 were evaluated for efficacy. The mean age of both populations was 77.1, and slightly more than half of the patients were male.
All patients received andexanet alfa given as a bolus dose over 30 minutes, followed by a 2-hour infusion. Patients received a low or high dose depending on which factor Xa inhibitor they received and the time they received it. The patients were evaluated for 30 days following andexanet alfa administration.
The co-primary efficacy endpoints are the percent change in anti-factor Xa activity at 2 hours and the assessment of hemostasis over 12 hours following the infusion. Hemostatic efficacy is assessed by an independent endpoint adjudication committee as excellent, good, or poor/none.
Efficacy
“In this preliminary analysis, [andexanet alfa] was effective in rapidly reversing anti-factor Xa inhibitor activity and restoring normal blood clotting in real-world patients with factor Xa inhibitor-related bleeding,” Dr Connolly said.
Of the 47 patients evaluable for efficacy, 32 were receiving an anticoagulant due to atrial fibrillation, 12 had venous thromboembolism (VTE), and 3 had both atrial fibrillation and VTE.
Twenty-six patients were receiving rivaroxaban, 20 were receiving apixaban, and 1 was receiving enoxaparin. Twenty-five patients had gastrointestinal bleeding, 20 had intracranial bleeding, and 2 had bleeding at other sites.
Forty-two patients received a low dose of andexanet alfa, and 5 received a high dose. The mean time from presentation to the emergency department and the administration of the andexanet alfa bolus was 4.8 ± 1.8 hours.
After the bolus, the median anti-factor Xa activity decreased by 89% from baseline among patients receiving rivaroxaban and by 93% among those receiving apixaban. At the end of the 2-hour infusion, the decrease from baseline was 86% and 92%, respectively.
Twelve hours after the infusion ended, the median anti-factor Xa activity had decreased 64% from baseline among patients receiving rivaroxaban and 31% among those receiving apixaban.
Overall, at 12 hours, clinical hemostasis was rated excellent or good in 79% of patients. Hemostatic efficacy was rated as excellent or good in 81% of the patients on rivaroxaban, 75% of the patients on apixaban, and in the 1 patient on edoxaban.
Safety
Of the 67 patients in the safety population, 47 were receiving an anticoagulant due to atrial fibrillation, 15 had VTE, and 5 had both atrial fibrillation and VTE.
Thirty-two patients were receiving rivaroxaban, 31 were receiving apixaban, and 4 were receiving edoxaban. Thirty-eight patients had gastrointestinal bleeding, 28 had intracranial bleeding, and 6 had bleeding at other sites.
There were no infusion reactions, no antibodies to factors Xa or X, and no neutralizing antibodies to andexanet alfa.
Twelve patients (18%) experienced thrombotic events—1 with myocardial infarction, 5 with stroke, 7 with deep-vein thrombosis, and 1 with pulmonary embolism. (Some patients had more than 1 event.)
“This rate of events is not unexpected, considering the thrombotic potential of the patients and the fact that, in most of them, anticoagulation was discontinued at the time of bleeding and not restarted,” Dr Connolly said.
Four patients had a thrombotic event within 3 days of andexanet alfa treatment, and the rest occurred between 4 days and 30 days.
Eighteen patients (27%) resumed anticoagulant therapy within 30 days. One of the 12 patients with a thrombotic event restarted anticoagulation at a therapeutic dose before the event. One other patient received prophylactic doses of enoxaparin before developing a deep-vein thrombosis.
There were 10 deaths (15%), 6 due to cardiovascular events.
Andexanet alfa development
Andexanet alfa is being developed as a reversal agent for apixaban, rivaroxaban, edoxaban, and enoxaparin. Andexanet alfa is intended to be used when reversal of anticoagulation is needed due to life-threatening or uncontrolled bleeding.
The drug is under review by the US Food and Drug Administration (FDA) and the European Medicines Agency. The FDA recently issued a complete response letter regarding the biologics license application for andexanet alfa.
Portola said it plans to meet with the FDA as soon as possible to resolve the outstanding questions in the letter and determine the appropriate next steps. ![]()

ROME—Preliminary results from the ANNEXA-4 study suggest that andexanet alfa, an investigational antidote to factor Xa inhibitors, can be effective in patients with acute major bleeding.
The drug reversed the anticoagulant effects of rivaroxaban, apixaban, and enoxaparin in this study, providing “excellent” or “good” hemostatic efficacy in 79% of patients over 12 hours.
Thrombotic events occurred in 18% of patients, and 15% died during the 30-day follow-up period.
According to investigators, these events occurred within the range expected in this patient population, given the severity of their bleeding, their underlying thrombotic risk, and the low percentage of patients who restarted anticoagulant therapy following their bleeding episode.
Stuart J. Connolly, MD, of McMaster University in Hamilton, Ontario, Canada, presented results from ANNEXA-4 at ESC Congress 2016 (abstract 5718).
Results were also published in NEJM. The study was funded by Portola Pharmaceuticals Inc.
Patients and treatment
The preliminary analysis of the phase 3/4 ANNEXA-4 trial included 67 patients. All of these patients were evaluated for safety, and 47 were evaluated for efficacy. The mean age of both populations was 77.1, and slightly more than half of the patients were male.
All patients received andexanet alfa given as a bolus dose over 30 minutes, followed by a 2-hour infusion. Patients received a low or high dose depending on which factor Xa inhibitor they received and the time they received it. The patients were evaluated for 30 days following andexanet alfa administration.
The co-primary efficacy endpoints are the percent change in anti-factor Xa activity at 2 hours and the assessment of hemostasis over 12 hours following the infusion. Hemostatic efficacy is assessed by an independent endpoint adjudication committee as excellent, good, or poor/none.
Efficacy
“In this preliminary analysis, [andexanet alfa] was effective in rapidly reversing anti-factor Xa inhibitor activity and restoring normal blood clotting in real-world patients with factor Xa inhibitor-related bleeding,” Dr Connolly said.
Of the 47 patients evaluable for efficacy, 32 were receiving an anticoagulant due to atrial fibrillation, 12 had venous thromboembolism (VTE), and 3 had both atrial fibrillation and VTE.
Twenty-six patients were receiving rivaroxaban, 20 were receiving apixaban, and 1 was receiving enoxaparin. Twenty-five patients had gastrointestinal bleeding, 20 had intracranial bleeding, and 2 had bleeding at other sites.
Forty-two patients received a low dose of andexanet alfa, and 5 received a high dose. The mean time from presentation to the emergency department and the administration of the andexanet alfa bolus was 4.8 ± 1.8 hours.
After the bolus, the median anti-factor Xa activity decreased by 89% from baseline among patients receiving rivaroxaban and by 93% among those receiving apixaban. At the end of the 2-hour infusion, the decrease from baseline was 86% and 92%, respectively.
Twelve hours after the infusion ended, the median anti-factor Xa activity had decreased 64% from baseline among patients receiving rivaroxaban and 31% among those receiving apixaban.
Overall, at 12 hours, clinical hemostasis was rated excellent or good in 79% of patients. Hemostatic efficacy was rated as excellent or good in 81% of the patients on rivaroxaban, 75% of the patients on apixaban, and in the 1 patient on edoxaban.
Safety
Of the 67 patients in the safety population, 47 were receiving an anticoagulant due to atrial fibrillation, 15 had VTE, and 5 had both atrial fibrillation and VTE.
Thirty-two patients were receiving rivaroxaban, 31 were receiving apixaban, and 4 were receiving edoxaban. Thirty-eight patients had gastrointestinal bleeding, 28 had intracranial bleeding, and 6 had bleeding at other sites.
There were no infusion reactions, no antibodies to factors Xa or X, and no neutralizing antibodies to andexanet alfa.
Twelve patients (18%) experienced thrombotic events—1 with myocardial infarction, 5 with stroke, 7 with deep-vein thrombosis, and 1 with pulmonary embolism. (Some patients had more than 1 event.)
“This rate of events is not unexpected, considering the thrombotic potential of the patients and the fact that, in most of them, anticoagulation was discontinued at the time of bleeding and not restarted,” Dr Connolly said.
Four patients had a thrombotic event within 3 days of andexanet alfa treatment, and the rest occurred between 4 days and 30 days.
Eighteen patients (27%) resumed anticoagulant therapy within 30 days. One of the 12 patients with a thrombotic event restarted anticoagulation at a therapeutic dose before the event. One other patient received prophylactic doses of enoxaparin before developing a deep-vein thrombosis.
There were 10 deaths (15%), 6 due to cardiovascular events.
Andexanet alfa development
Andexanet alfa is being developed as a reversal agent for apixaban, rivaroxaban, edoxaban, and enoxaparin. Andexanet alfa is intended to be used when reversal of anticoagulation is needed due to life-threatening or uncontrolled bleeding.
The drug is under review by the US Food and Drug Administration (FDA) and the European Medicines Agency. The FDA recently issued a complete response letter regarding the biologics license application for andexanet alfa.
Portola said it plans to meet with the FDA as soon as possible to resolve the outstanding questions in the letter and determine the appropriate next steps. ![]()

ROME—Preliminary results from the ANNEXA-4 study suggest that andexanet alfa, an investigational antidote to factor Xa inhibitors, can be effective in patients with acute major bleeding.
The drug reversed the anticoagulant effects of rivaroxaban, apixaban, and enoxaparin in this study, providing “excellent” or “good” hemostatic efficacy in 79% of patients over 12 hours.
Thrombotic events occurred in 18% of patients, and 15% died during the 30-day follow-up period.
According to investigators, these events occurred within the range expected in this patient population, given the severity of their bleeding, their underlying thrombotic risk, and the low percentage of patients who restarted anticoagulant therapy following their bleeding episode.
Stuart J. Connolly, MD, of McMaster University in Hamilton, Ontario, Canada, presented results from ANNEXA-4 at ESC Congress 2016 (abstract 5718).
Results were also published in NEJM. The study was funded by Portola Pharmaceuticals Inc.
Patients and treatment
The preliminary analysis of the phase 3/4 ANNEXA-4 trial included 67 patients. All of these patients were evaluated for safety, and 47 were evaluated for efficacy. The mean age of both populations was 77.1, and slightly more than half of the patients were male.
All patients received andexanet alfa given as a bolus dose over 30 minutes, followed by a 2-hour infusion. Patients received a low or high dose depending on which factor Xa inhibitor they received and the time they received it. The patients were evaluated for 30 days following andexanet alfa administration.
The co-primary efficacy endpoints are the percent change in anti-factor Xa activity at 2 hours and the assessment of hemostasis over 12 hours following the infusion. Hemostatic efficacy is assessed by an independent endpoint adjudication committee as excellent, good, or poor/none.
Efficacy
“In this preliminary analysis, [andexanet alfa] was effective in rapidly reversing anti-factor Xa inhibitor activity and restoring normal blood clotting in real-world patients with factor Xa inhibitor-related bleeding,” Dr Connolly said.
Of the 47 patients evaluable for efficacy, 32 were receiving an anticoagulant due to atrial fibrillation, 12 had venous thromboembolism (VTE), and 3 had both atrial fibrillation and VTE.
Twenty-six patients were receiving rivaroxaban, 20 were receiving apixaban, and 1 was receiving enoxaparin. Twenty-five patients had gastrointestinal bleeding, 20 had intracranial bleeding, and 2 had bleeding at other sites.
Forty-two patients received a low dose of andexanet alfa, and 5 received a high dose. The mean time from presentation to the emergency department and the administration of the andexanet alfa bolus was 4.8 ± 1.8 hours.
After the bolus, the median anti-factor Xa activity decreased by 89% from baseline among patients receiving rivaroxaban and by 93% among those receiving apixaban. At the end of the 2-hour infusion, the decrease from baseline was 86% and 92%, respectively.
Twelve hours after the infusion ended, the median anti-factor Xa activity had decreased 64% from baseline among patients receiving rivaroxaban and 31% among those receiving apixaban.
Overall, at 12 hours, clinical hemostasis was rated excellent or good in 79% of patients. Hemostatic efficacy was rated as excellent or good in 81% of the patients on rivaroxaban, 75% of the patients on apixaban, and in the 1 patient on edoxaban.
Safety
Of the 67 patients in the safety population, 47 were receiving an anticoagulant due to atrial fibrillation, 15 had VTE, and 5 had both atrial fibrillation and VTE.
Thirty-two patients were receiving rivaroxaban, 31 were receiving apixaban, and 4 were receiving edoxaban. Thirty-eight patients had gastrointestinal bleeding, 28 had intracranial bleeding, and 6 had bleeding at other sites.
There were no infusion reactions, no antibodies to factors Xa or X, and no neutralizing antibodies to andexanet alfa.
Twelve patients (18%) experienced thrombotic events—1 with myocardial infarction, 5 with stroke, 7 with deep-vein thrombosis, and 1 with pulmonary embolism. (Some patients had more than 1 event.)
“This rate of events is not unexpected, considering the thrombotic potential of the patients and the fact that, in most of them, anticoagulation was discontinued at the time of bleeding and not restarted,” Dr Connolly said.
Four patients had a thrombotic event within 3 days of andexanet alfa treatment, and the rest occurred between 4 days and 30 days.
Eighteen patients (27%) resumed anticoagulant therapy within 30 days. One of the 12 patients with a thrombotic event restarted anticoagulation at a therapeutic dose before the event. One other patient received prophylactic doses of enoxaparin before developing a deep-vein thrombosis.
There were 10 deaths (15%), 6 due to cardiovascular events.
Andexanet alfa development
Andexanet alfa is being developed as a reversal agent for apixaban, rivaroxaban, edoxaban, and enoxaparin. Andexanet alfa is intended to be used when reversal of anticoagulation is needed due to life-threatening or uncontrolled bleeding.
The drug is under review by the US Food and Drug Administration (FDA) and the European Medicines Agency. The FDA recently issued a complete response letter regarding the biologics license application for andexanet alfa.
Portola said it plans to meet with the FDA as soon as possible to resolve the outstanding questions in the letter and determine the appropriate next steps. ![]()
Antiplatelet drugs comparable in patients with AMI

Photo courtesy of AstraZeneca
ROME—The antiplatelet drugs prasugrel and ticagrelor produce similar early results in patients with acute myocardial infarction (AMI) treated with percutaneous coronary intervention (PCI), according to PRAGUE-18, the first randomized, head-to-head comparison of the drugs.
“Our findings confirm previous indirect—non-randomized—comparisons of these 2 drugs, based on analyses of various registries,” said study investigator Petr Widimsky MD, DSc, of Charles University in Prague, Czech Republic.
“Thus, both drugs are very effective and safe and significantly contribute to the excellent outcomes of patients with acute myocardial infarction in modern cardiology.”
Dr Widimsky presented results from PRAGUE-18 at ESC Congress 2016 (abstract 5028). This was an investigator-initiated study, so there was no industry support.
The trial included 1230 AMI patients who were randomized to receive prasugrel (n=634) or ticagrelor (n=596) prior to PCI. There were no significant differences in baseline characteristics between the treatment arms.
Randomization took place immediately after a patient’s arrival to the PCI center. Patients received prasugrel at 60 mg, followed by 10 mg per day (5 mg per day if they were older than 75 or weighed less than 60 kg) for 1 year. Patients received ticagrelor at 180 mg, followed by 90 mg twice a day for 1 year.
The study’s primary endpoint was the occurrence of death, re-infarction, urgent target vessel revascularization, stroke, prolonged hospitalization, or serious bleeding requiring transfusion at 7 days (or discharge if earlier).
The trial was halted prematurely, after an interim analysis showed no significant difference in the rate of the primary endpoint between the prasugrel and ticagrelor arms—4.0% and 4.1%, respectively (odds ratio=0.98, P=0.939).
Likewise, there was no significant difference between the treatment arms for any of the components of the primary endpoint.
The key secondary endpoint was a composite of cardiovascular death, non-fatal myocardial infarction, and stroke within 30 days. There was no significant difference in the rate of this endpoint between the prasugrel and ticagrelor arms—2.7% and 2.5%, respectively (odds ratio=1.06, P=0.864).
“This study did not show any difference between ticagrelor and prasugrel in the early phase of acute myocardial infarction treated by primary PCI,” Dr Widimsky concluded.
He and his colleagues are planning the final follow-up of this study at 1 year, which will be completed in 2017. ![]()

Photo courtesy of AstraZeneca
ROME—The antiplatelet drugs prasugrel and ticagrelor produce similar early results in patients with acute myocardial infarction (AMI) treated with percutaneous coronary intervention (PCI), according to PRAGUE-18, the first randomized, head-to-head comparison of the drugs.
“Our findings confirm previous indirect—non-randomized—comparisons of these 2 drugs, based on analyses of various registries,” said study investigator Petr Widimsky MD, DSc, of Charles University in Prague, Czech Republic.
“Thus, both drugs are very effective and safe and significantly contribute to the excellent outcomes of patients with acute myocardial infarction in modern cardiology.”
Dr Widimsky presented results from PRAGUE-18 at ESC Congress 2016 (abstract 5028). This was an investigator-initiated study, so there was no industry support.
The trial included 1230 AMI patients who were randomized to receive prasugrel (n=634) or ticagrelor (n=596) prior to PCI. There were no significant differences in baseline characteristics between the treatment arms.
Randomization took place immediately after a patient’s arrival to the PCI center. Patients received prasugrel at 60 mg, followed by 10 mg per day (5 mg per day if they were older than 75 or weighed less than 60 kg) for 1 year. Patients received ticagrelor at 180 mg, followed by 90 mg twice a day for 1 year.
The study’s primary endpoint was the occurrence of death, re-infarction, urgent target vessel revascularization, stroke, prolonged hospitalization, or serious bleeding requiring transfusion at 7 days (or discharge if earlier).
The trial was halted prematurely, after an interim analysis showed no significant difference in the rate of the primary endpoint between the prasugrel and ticagrelor arms—4.0% and 4.1%, respectively (odds ratio=0.98, P=0.939).
Likewise, there was no significant difference between the treatment arms for any of the components of the primary endpoint.
The key secondary endpoint was a composite of cardiovascular death, non-fatal myocardial infarction, and stroke within 30 days. There was no significant difference in the rate of this endpoint between the prasugrel and ticagrelor arms—2.7% and 2.5%, respectively (odds ratio=1.06, P=0.864).
“This study did not show any difference between ticagrelor and prasugrel in the early phase of acute myocardial infarction treated by primary PCI,” Dr Widimsky concluded.
He and his colleagues are planning the final follow-up of this study at 1 year, which will be completed in 2017. ![]()

Photo courtesy of AstraZeneca
ROME—The antiplatelet drugs prasugrel and ticagrelor produce similar early results in patients with acute myocardial infarction (AMI) treated with percutaneous coronary intervention (PCI), according to PRAGUE-18, the first randomized, head-to-head comparison of the drugs.
“Our findings confirm previous indirect—non-randomized—comparisons of these 2 drugs, based on analyses of various registries,” said study investigator Petr Widimsky MD, DSc, of Charles University in Prague, Czech Republic.
“Thus, both drugs are very effective and safe and significantly contribute to the excellent outcomes of patients with acute myocardial infarction in modern cardiology.”
Dr Widimsky presented results from PRAGUE-18 at ESC Congress 2016 (abstract 5028). This was an investigator-initiated study, so there was no industry support.
The trial included 1230 AMI patients who were randomized to receive prasugrel (n=634) or ticagrelor (n=596) prior to PCI. There were no significant differences in baseline characteristics between the treatment arms.
Randomization took place immediately after a patient’s arrival to the PCI center. Patients received prasugrel at 60 mg, followed by 10 mg per day (5 mg per day if they were older than 75 or weighed less than 60 kg) for 1 year. Patients received ticagrelor at 180 mg, followed by 90 mg twice a day for 1 year.
The study’s primary endpoint was the occurrence of death, re-infarction, urgent target vessel revascularization, stroke, prolonged hospitalization, or serious bleeding requiring transfusion at 7 days (or discharge if earlier).
The trial was halted prematurely, after an interim analysis showed no significant difference in the rate of the primary endpoint between the prasugrel and ticagrelor arms—4.0% and 4.1%, respectively (odds ratio=0.98, P=0.939).
Likewise, there was no significant difference between the treatment arms for any of the components of the primary endpoint.
The key secondary endpoint was a composite of cardiovascular death, non-fatal myocardial infarction, and stroke within 30 days. There was no significant difference in the rate of this endpoint between the prasugrel and ticagrelor arms—2.7% and 2.5%, respectively (odds ratio=1.06, P=0.864).
“This study did not show any difference between ticagrelor and prasugrel in the early phase of acute myocardial infarction treated by primary PCI,” Dr Widimsky concluded.
He and his colleagues are planning the final follow-up of this study at 1 year, which will be completed in 2017. ![]()