User login
FDA: CT scans safe for patients with electronic medical devices
There’s no need to let fear of electronic interference between computed tomography and electronic medical devices preclude the ordering of such scans for patients with insulin pumps, cardiac implantable electronic devices, or neurostimulators, the Food and Drug Administration said in a written notification.
“The probability of an adverse event being caused by exposing these devices to CT irradiation is extremely low, and it is greatly outweighed by the clinical benefit of a medically indicated CT examination,” according to the new notification, which updates and replaces a preliminary health notification released on July 14, 2008.
The preliminary notification said there was a “possibility that the x-rays used during CT examinations may cause some implanted and external electronic medical devices to malfunction.” It also included recommendations to reduce the potential risk of such events from occurring and cited adverse events experienced by a few patients with medical devices who had undergone CT scanning, including unintended shocks from neurostimulators, malfunctions of insulin infusion pumps, and transient changes in pacemaker output pulse rate.
The new notification says there is an extremely low probability that a CT scanner directly irradiating the circuitry of certain implantable or wearable electronic medical devices can cause sufficient electronic interference to affect the function and operation of the medical device, and this probability is even lower when the radiation dose and the radiation dose rate are reduced. The FDA also notes that the interference is completely avoided when the medical device is outside of the primary x-ray beam of the CT scanner.
The update, which provides additional reports of adverse events by patients with electronic medical devices who had CT scans, states that the number of such events was small, compared with the number of patients with insulin pumps, cardiac implantable electronic devices, and neurostimulators who were scanned without adverse effects.
The FDA encourages health care providers and patients who suspect a problem with a medical imaging device to file a voluntary report through MedWatch, the FDA Safety Information and Adverse Event Reporting Program.
There’s no need to let fear of electronic interference between computed tomography and electronic medical devices preclude the ordering of such scans for patients with insulin pumps, cardiac implantable electronic devices, or neurostimulators, the Food and Drug Administration said in a written notification.
“The probability of an adverse event being caused by exposing these devices to CT irradiation is extremely low, and it is greatly outweighed by the clinical benefit of a medically indicated CT examination,” according to the new notification, which updates and replaces a preliminary health notification released on July 14, 2008.
The preliminary notification said there was a “possibility that the x-rays used during CT examinations may cause some implanted and external electronic medical devices to malfunction.” It also included recommendations to reduce the potential risk of such events from occurring and cited adverse events experienced by a few patients with medical devices who had undergone CT scanning, including unintended shocks from neurostimulators, malfunctions of insulin infusion pumps, and transient changes in pacemaker output pulse rate.
The new notification says there is an extremely low probability that a CT scanner directly irradiating the circuitry of certain implantable or wearable electronic medical devices can cause sufficient electronic interference to affect the function and operation of the medical device, and this probability is even lower when the radiation dose and the radiation dose rate are reduced. The FDA also notes that the interference is completely avoided when the medical device is outside of the primary x-ray beam of the CT scanner.
The update, which provides additional reports of adverse events by patients with electronic medical devices who had CT scans, states that the number of such events was small, compared with the number of patients with insulin pumps, cardiac implantable electronic devices, and neurostimulators who were scanned without adverse effects.
The FDA encourages health care providers and patients who suspect a problem with a medical imaging device to file a voluntary report through MedWatch, the FDA Safety Information and Adverse Event Reporting Program.
There’s no need to let fear of electronic interference between computed tomography and electronic medical devices preclude the ordering of such scans for patients with insulin pumps, cardiac implantable electronic devices, or neurostimulators, the Food and Drug Administration said in a written notification.
“The probability of an adverse event being caused by exposing these devices to CT irradiation is extremely low, and it is greatly outweighed by the clinical benefit of a medically indicated CT examination,” according to the new notification, which updates and replaces a preliminary health notification released on July 14, 2008.
The preliminary notification said there was a “possibility that the x-rays used during CT examinations may cause some implanted and external electronic medical devices to malfunction.” It also included recommendations to reduce the potential risk of such events from occurring and cited adverse events experienced by a few patients with medical devices who had undergone CT scanning, including unintended shocks from neurostimulators, malfunctions of insulin infusion pumps, and transient changes in pacemaker output pulse rate.
The new notification says there is an extremely low probability that a CT scanner directly irradiating the circuitry of certain implantable or wearable electronic medical devices can cause sufficient electronic interference to affect the function and operation of the medical device, and this probability is even lower when the radiation dose and the radiation dose rate are reduced. The FDA also notes that the interference is completely avoided when the medical device is outside of the primary x-ray beam of the CT scanner.
The update, which provides additional reports of adverse events by patients with electronic medical devices who had CT scans, states that the number of such events was small, compared with the number of patients with insulin pumps, cardiac implantable electronic devices, and neurostimulators who were scanned without adverse effects.
The FDA encourages health care providers and patients who suspect a problem with a medical imaging device to file a voluntary report through MedWatch, the FDA Safety Information and Adverse Event Reporting Program.
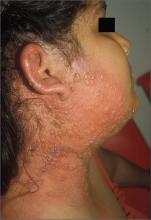
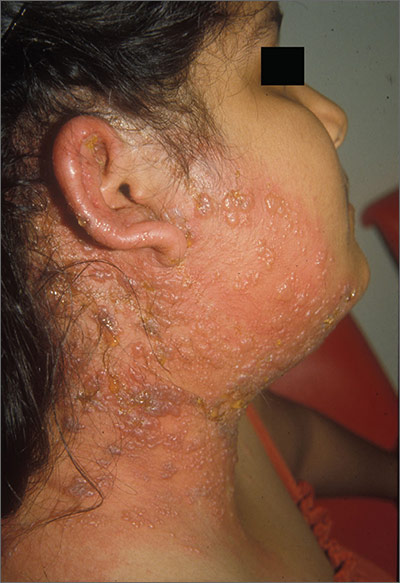
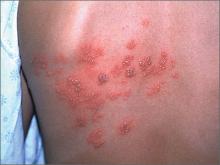
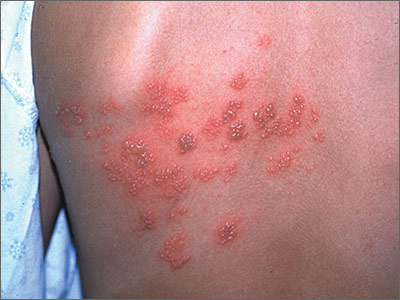
Blisters on face and ear
The FP diagnosed herpes zoster with possible Ramsay Hunt syndrome in this patient. The zoster also appeared superinfected by a bacterial infection, which was causing the extensive erythema, weeping of yellow fluid, and some honey crusting.
Herpes zoster oticus (Ramsay Hunt syndrome) includes the triad of ipsilateral facial paralysis, ear pain, and vesicles in the auditory canal and auricle. Disturbances in taste perception, hearing (tinnitus, hyperacusis), lacrimation, and vestibular function (vertigo) may occur. Fortunately, the child did not have facial paralysis, but did have ear pain and vesicles on the auricle. The child also admitted to some ringing in the right ear.
The FP prescribed oral acyclovir and cultured the weeping fluid to determine if this was truly a bacterial superinfection. The child was started on oral cephalexin 3 times a day to cover Streptococcus pyogenes and Staphylococcus aureus. Oral acetaminophen was prescribed for the pain and fever. The physician called his ear, nose, and throat (ENT) colleague who agreed to see the patient later that day.
The FP and ENT physician decided to follow her without immediate hospitalization since she was taking fluids well and did not appear systemically ill. The following day, the patient was already improving and later that week the culture grew out Staphylococcus aureus that was methicillin sensitive. The child fully recovered from the superinfected herpes zoster without any sequelae. The tinnitus resolved, as well.
Photo courtesy of UTHSCSA Dermatology division. Text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux EJ, Usatine R. Zoster. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:712-717.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
The FP diagnosed herpes zoster with possible Ramsay Hunt syndrome in this patient. The zoster also appeared superinfected by a bacterial infection, which was causing the extensive erythema, weeping of yellow fluid, and some honey crusting.
Herpes zoster oticus (Ramsay Hunt syndrome) includes the triad of ipsilateral facial paralysis, ear pain, and vesicles in the auditory canal and auricle. Disturbances in taste perception, hearing (tinnitus, hyperacusis), lacrimation, and vestibular function (vertigo) may occur. Fortunately, the child did not have facial paralysis, but did have ear pain and vesicles on the auricle. The child also admitted to some ringing in the right ear.
The FP prescribed oral acyclovir and cultured the weeping fluid to determine if this was truly a bacterial superinfection. The child was started on oral cephalexin 3 times a day to cover Streptococcus pyogenes and Staphylococcus aureus. Oral acetaminophen was prescribed for the pain and fever. The physician called his ear, nose, and throat (ENT) colleague who agreed to see the patient later that day.
The FP and ENT physician decided to follow her without immediate hospitalization since she was taking fluids well and did not appear systemically ill. The following day, the patient was already improving and later that week the culture grew out Staphylococcus aureus that was methicillin sensitive. The child fully recovered from the superinfected herpes zoster without any sequelae. The tinnitus resolved, as well.
Photo courtesy of UTHSCSA Dermatology division. Text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux EJ, Usatine R. Zoster. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:712-717.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
The FP diagnosed herpes zoster with possible Ramsay Hunt syndrome in this patient. The zoster also appeared superinfected by a bacterial infection, which was causing the extensive erythema, weeping of yellow fluid, and some honey crusting.
Herpes zoster oticus (Ramsay Hunt syndrome) includes the triad of ipsilateral facial paralysis, ear pain, and vesicles in the auditory canal and auricle. Disturbances in taste perception, hearing (tinnitus, hyperacusis), lacrimation, and vestibular function (vertigo) may occur. Fortunately, the child did not have facial paralysis, but did have ear pain and vesicles on the auricle. The child also admitted to some ringing in the right ear.
The FP prescribed oral acyclovir and cultured the weeping fluid to determine if this was truly a bacterial superinfection. The child was started on oral cephalexin 3 times a day to cover Streptococcus pyogenes and Staphylococcus aureus. Oral acetaminophen was prescribed for the pain and fever. The physician called his ear, nose, and throat (ENT) colleague who agreed to see the patient later that day.
The FP and ENT physician decided to follow her without immediate hospitalization since she was taking fluids well and did not appear systemically ill. The following day, the patient was already improving and later that week the culture grew out Staphylococcus aureus that was methicillin sensitive. The child fully recovered from the superinfected herpes zoster without any sequelae. The tinnitus resolved, as well.
Photo courtesy of UTHSCSA Dermatology division. Text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux EJ, Usatine R. Zoster. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:712-717.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
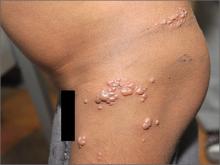
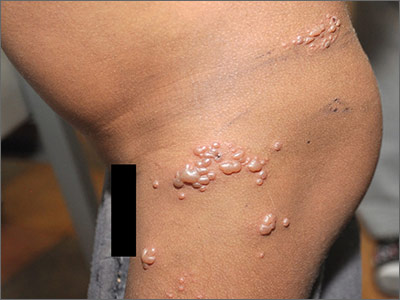
Blisters on thigh
The FP diagnosed the boy with herpes zoster that was found in multiple dermatomes. He did not think it was disseminated because it was still localized to one area and there were not 20 or more lesions outside the primary zoster. However, the FP was very concerned about HIV and the boy was tested. A rapid HIV test came back positive.
The FP discussed the results of his findings with the child's grandmother, who was now caring for the child. An attempt was made to obtain oral or intravenous acyclovir but it was not available in the village or local health center. The child was given oral liquid acetaminophen for the pain and was added to the list for the local HIV clinic. Fortunately, the zoster resolved without antiviral medications and the child began to receive care for his HIV infection.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux EJ, Usatine R. Zoster. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:712-717.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
The FP diagnosed the boy with herpes zoster that was found in multiple dermatomes. He did not think it was disseminated because it was still localized to one area and there were not 20 or more lesions outside the primary zoster. However, the FP was very concerned about HIV and the boy was tested. A rapid HIV test came back positive.
The FP discussed the results of his findings with the child's grandmother, who was now caring for the child. An attempt was made to obtain oral or intravenous acyclovir but it was not available in the village or local health center. The child was given oral liquid acetaminophen for the pain and was added to the list for the local HIV clinic. Fortunately, the zoster resolved without antiviral medications and the child began to receive care for his HIV infection.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux EJ, Usatine R. Zoster. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:712-717.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
The FP diagnosed the boy with herpes zoster that was found in multiple dermatomes. He did not think it was disseminated because it was still localized to one area and there were not 20 or more lesions outside the primary zoster. However, the FP was very concerned about HIV and the boy was tested. A rapid HIV test came back positive.
The FP discussed the results of his findings with the child's grandmother, who was now caring for the child. An attempt was made to obtain oral or intravenous acyclovir but it was not available in the village or local health center. The child was given oral liquid acetaminophen for the pain and was added to the list for the local HIV clinic. Fortunately, the zoster resolved without antiviral medications and the child began to receive care for his HIV infection.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux EJ, Usatine R. Zoster. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:712-717.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
Pruritic rash on 16-year-old girl
Despite the fact that some of the vesicles crossed the midline (which is suggestive of disseminated zoster), the FP felt confident in diagnosing herpes zoster (shingles) in this patient. The FP knew that the varicella-zoster virus (VZV) leaves the dorsal root ganglion to travel down the spinal nerves to the cutaneous nerves of the skin. But she also knew that the vesicles could cross the midline by a few centimeters because the posterior primary ramus of the spinal nerve includes a small cutaneous medial branch that reaches across the midline.1
After a primary infection with either chickenpox or vaccine-type VZV, a latent infection is established in the sensory dorsal root ganglia. Reactivation of this latent VZV infection results in herpes zoster. Both sensory ganglia neurons and satellite cells surrounding the neurons serve as sites of VZV latent infection. Once reactivated, the virus spreads to other cells within the ganglion. The dermatomal distribution of the rash corresponds to the sensory fields of the infected neurons within the specific ganglion.
The pain associated with zoster infections and postherpetic neuralgia (PHN) is thought to result from injury to the peripheral nerves and altered central nervous system processing. PHN occurs more commonly in individuals older than age 60 and in immunosuppressed individuals. The lesions typically crust in approximately a week, with complete resolution within 3 to 4 weeks. If there are more than 20 lesions distributed outside the affected dermatome, the patient has disseminated zoster.
The treatment of herpes zoster includes antiviral agents, such as acyclovir, famciclovir, and valacyclovir. The evidence only supports their use if started within 72 hours of rash onset. Pain can be managed with nonprescription analgesics or narcotics and should be treated aggressively. This may actually prevent or lessen the severity of PHN.
The FP in this case was also concerned about whether the patient might be positive for human immunodeficiency virus (HIV) or have some type of immunosuppression. The patient denied sexual activity and use of intravenous drugs (even when her mom wasn’t in the room). She was otherwise healthy, so no further workup for HIV or immunosuppression was ordered. The FP told the teen to stay home from school until the lesions crusted over. A follow-up visit in one month was scheduled. The zoster resolved and the girl returned to her usual state of good health.
1. Usatine RP, Clemente C. Is herpes zoster unilateral? West J Med. 1999;170:263.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux EJ, Usatine R. Zoster. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:712-717.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
Despite the fact that some of the vesicles crossed the midline (which is suggestive of disseminated zoster), the FP felt confident in diagnosing herpes zoster (shingles) in this patient. The FP knew that the varicella-zoster virus (VZV) leaves the dorsal root ganglion to travel down the spinal nerves to the cutaneous nerves of the skin. But she also knew that the vesicles could cross the midline by a few centimeters because the posterior primary ramus of the spinal nerve includes a small cutaneous medial branch that reaches across the midline.1
After a primary infection with either chickenpox or vaccine-type VZV, a latent infection is established in the sensory dorsal root ganglia. Reactivation of this latent VZV infection results in herpes zoster. Both sensory ganglia neurons and satellite cells surrounding the neurons serve as sites of VZV latent infection. Once reactivated, the virus spreads to other cells within the ganglion. The dermatomal distribution of the rash corresponds to the sensory fields of the infected neurons within the specific ganglion.
The pain associated with zoster infections and postherpetic neuralgia (PHN) is thought to result from injury to the peripheral nerves and altered central nervous system processing. PHN occurs more commonly in individuals older than age 60 and in immunosuppressed individuals. The lesions typically crust in approximately a week, with complete resolution within 3 to 4 weeks. If there are more than 20 lesions distributed outside the affected dermatome, the patient has disseminated zoster.
The treatment of herpes zoster includes antiviral agents, such as acyclovir, famciclovir, and valacyclovir. The evidence only supports their use if started within 72 hours of rash onset. Pain can be managed with nonprescription analgesics or narcotics and should be treated aggressively. This may actually prevent or lessen the severity of PHN.
The FP in this case was also concerned about whether the patient might be positive for human immunodeficiency virus (HIV) or have some type of immunosuppression. The patient denied sexual activity and use of intravenous drugs (even when her mom wasn’t in the room). She was otherwise healthy, so no further workup for HIV or immunosuppression was ordered. The FP told the teen to stay home from school until the lesions crusted over. A follow-up visit in one month was scheduled. The zoster resolved and the girl returned to her usual state of good health.
1. Usatine RP, Clemente C. Is herpes zoster unilateral? West J Med. 1999;170:263.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux EJ, Usatine R. Zoster. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:712-717.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
Despite the fact that some of the vesicles crossed the midline (which is suggestive of disseminated zoster), the FP felt confident in diagnosing herpes zoster (shingles) in this patient. The FP knew that the varicella-zoster virus (VZV) leaves the dorsal root ganglion to travel down the spinal nerves to the cutaneous nerves of the skin. But she also knew that the vesicles could cross the midline by a few centimeters because the posterior primary ramus of the spinal nerve includes a small cutaneous medial branch that reaches across the midline.1
After a primary infection with either chickenpox or vaccine-type VZV, a latent infection is established in the sensory dorsal root ganglia. Reactivation of this latent VZV infection results in herpes zoster. Both sensory ganglia neurons and satellite cells surrounding the neurons serve as sites of VZV latent infection. Once reactivated, the virus spreads to other cells within the ganglion. The dermatomal distribution of the rash corresponds to the sensory fields of the infected neurons within the specific ganglion.
The pain associated with zoster infections and postherpetic neuralgia (PHN) is thought to result from injury to the peripheral nerves and altered central nervous system processing. PHN occurs more commonly in individuals older than age 60 and in immunosuppressed individuals. The lesions typically crust in approximately a week, with complete resolution within 3 to 4 weeks. If there are more than 20 lesions distributed outside the affected dermatome, the patient has disseminated zoster.
The treatment of herpes zoster includes antiviral agents, such as acyclovir, famciclovir, and valacyclovir. The evidence only supports their use if started within 72 hours of rash onset. Pain can be managed with nonprescription analgesics or narcotics and should be treated aggressively. This may actually prevent or lessen the severity of PHN.
The FP in this case was also concerned about whether the patient might be positive for human immunodeficiency virus (HIV) or have some type of immunosuppression. The patient denied sexual activity and use of intravenous drugs (even when her mom wasn’t in the room). She was otherwise healthy, so no further workup for HIV or immunosuppression was ordered. The FP told the teen to stay home from school until the lesions crusted over. A follow-up visit in one month was scheduled. The zoster resolved and the girl returned to her usual state of good health.
1. Usatine RP, Clemente C. Is herpes zoster unilateral? West J Med. 1999;170:263.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux EJ, Usatine R. Zoster. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:712-717.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
Early biopsy predicts levonorgestrel IUD response in endometrial cancer
SAN DIEGO – Endometrial pathology findings at 3 months predicted response to levonorgestrel-releasing IUD treatment for complex atypical hyperplasia or grade 1 endometrial cancer at the MD Anderson Cancer Center in Houston.
Twenty-nine of 32 women (91%) who responded by 12 months showed stromal, glandular, or other endometrial changes indicating an effect at 3 months, vs. only 3 of 9 nonresponders (33%) (P less than .001). There were no differences in responders versus nonresponders in median age (47 vs. 56 years, P = .2) or body mass index (45 vs. 55 kg/m2, P = .16).
The finding addresses an “unmet need” for markers of response to levonorgestrel-releasing IUD therapy. “You can look at [early] pathology” and have an idea how patients will do, Dr. Shannon Westin, a study investigator who is with the department of gynecologic oncology at MD Anderson, said at the annual meeting of the Society of Gynecologic Oncology.
Twenty-seven of 29 women (93%) with complex atypical hyperplasia (CAH) responded completely to the IUD, meaning they had normal endometrium or hyperplasia without atypia at 12 months. The response rate for endometrial cancer was 67%; 7 of 12 women had a complete response, and an 8th was diagnosed at 12 months with CAH, indicating a partial response. The rest of the patients remained stable or progressed.
Endometrial biopsies were performed every 3 months; the team also did molecular testing on tumors from 20 patients. Baseline protein Ki67 – a marker of proliferation – was significantly higher in nonresponders. Expression of several estrogen-induced genes was higher in responders.
Patients opted for the IUD to retain fertility or because obesity or comorbidities precluded surgery. Exclusion criteria included prior treatment for CAH or endometrial cancer, evidence of extrauterine spread, or levonorgestrel IUD contraindications, such as uterine infection.
Adverse events – primarily irregular bleeding and cramping – were mild and tended to resolve by 12 months. Treatment had little effect on measures of social, mental, and physical function. About half of the patients were white, a third were Hispanic, and most of the remaining patients were black.
There was no external funding for the work. Dr. Westin is a consultant for AstraZeneca, Medivation, Roche, Ovation, and Vermillion, and reported receiving research funding from AstraZeneca, Critical Outcomes Technologies, and Novartis.
SAN DIEGO – Endometrial pathology findings at 3 months predicted response to levonorgestrel-releasing IUD treatment for complex atypical hyperplasia or grade 1 endometrial cancer at the MD Anderson Cancer Center in Houston.
Twenty-nine of 32 women (91%) who responded by 12 months showed stromal, glandular, or other endometrial changes indicating an effect at 3 months, vs. only 3 of 9 nonresponders (33%) (P less than .001). There were no differences in responders versus nonresponders in median age (47 vs. 56 years, P = .2) or body mass index (45 vs. 55 kg/m2, P = .16).
The finding addresses an “unmet need” for markers of response to levonorgestrel-releasing IUD therapy. “You can look at [early] pathology” and have an idea how patients will do, Dr. Shannon Westin, a study investigator who is with the department of gynecologic oncology at MD Anderson, said at the annual meeting of the Society of Gynecologic Oncology.
Twenty-seven of 29 women (93%) with complex atypical hyperplasia (CAH) responded completely to the IUD, meaning they had normal endometrium or hyperplasia without atypia at 12 months. The response rate for endometrial cancer was 67%; 7 of 12 women had a complete response, and an 8th was diagnosed at 12 months with CAH, indicating a partial response. The rest of the patients remained stable or progressed.
Endometrial biopsies were performed every 3 months; the team also did molecular testing on tumors from 20 patients. Baseline protein Ki67 – a marker of proliferation – was significantly higher in nonresponders. Expression of several estrogen-induced genes was higher in responders.
Patients opted for the IUD to retain fertility or because obesity or comorbidities precluded surgery. Exclusion criteria included prior treatment for CAH or endometrial cancer, evidence of extrauterine spread, or levonorgestrel IUD contraindications, such as uterine infection.
Adverse events – primarily irregular bleeding and cramping – were mild and tended to resolve by 12 months. Treatment had little effect on measures of social, mental, and physical function. About half of the patients were white, a third were Hispanic, and most of the remaining patients were black.
There was no external funding for the work. Dr. Westin is a consultant for AstraZeneca, Medivation, Roche, Ovation, and Vermillion, and reported receiving research funding from AstraZeneca, Critical Outcomes Technologies, and Novartis.
SAN DIEGO – Endometrial pathology findings at 3 months predicted response to levonorgestrel-releasing IUD treatment for complex atypical hyperplasia or grade 1 endometrial cancer at the MD Anderson Cancer Center in Houston.
Twenty-nine of 32 women (91%) who responded by 12 months showed stromal, glandular, or other endometrial changes indicating an effect at 3 months, vs. only 3 of 9 nonresponders (33%) (P less than .001). There were no differences in responders versus nonresponders in median age (47 vs. 56 years, P = .2) or body mass index (45 vs. 55 kg/m2, P = .16).
The finding addresses an “unmet need” for markers of response to levonorgestrel-releasing IUD therapy. “You can look at [early] pathology” and have an idea how patients will do, Dr. Shannon Westin, a study investigator who is with the department of gynecologic oncology at MD Anderson, said at the annual meeting of the Society of Gynecologic Oncology.
Twenty-seven of 29 women (93%) with complex atypical hyperplasia (CAH) responded completely to the IUD, meaning they had normal endometrium or hyperplasia without atypia at 12 months. The response rate for endometrial cancer was 67%; 7 of 12 women had a complete response, and an 8th was diagnosed at 12 months with CAH, indicating a partial response. The rest of the patients remained stable or progressed.
Endometrial biopsies were performed every 3 months; the team also did molecular testing on tumors from 20 patients. Baseline protein Ki67 – a marker of proliferation – was significantly higher in nonresponders. Expression of several estrogen-induced genes was higher in responders.
Patients opted for the IUD to retain fertility or because obesity or comorbidities precluded surgery. Exclusion criteria included prior treatment for CAH or endometrial cancer, evidence of extrauterine spread, or levonorgestrel IUD contraindications, such as uterine infection.
Adverse events – primarily irregular bleeding and cramping – were mild and tended to resolve by 12 months. Treatment had little effect on measures of social, mental, and physical function. About half of the patients were white, a third were Hispanic, and most of the remaining patients were black.
There was no external funding for the work. Dr. Westin is a consultant for AstraZeneca, Medivation, Roche, Ovation, and Vermillion, and reported receiving research funding from AstraZeneca, Critical Outcomes Technologies, and Novartis.
AT THE ANNUAL MEETING ON WOMEN’S CANCER
Key clinical point: Pathology and molecular findings identify good candidates for levonorgestrel IUD therapy.
Major finding: Twenty-nine of 32 women (91%) who responded by 12 months showed stromal, glandular, or other endometrial changes indicating an effect at 3 months, versus only 3 of 9 nonresponders (33%) (P less than .001).
Data source: A prospective investigation of 41 women.
Disclosures: There was no external funding for the work. Dr. Westin is a consultant for and receives research funding from AstraZeneca and several other companies.
Women with suspected CAD classified as lower risk than men
Women with suspected coronary artery disease had similar symptoms and more heart disease risk factors, compared with men, but were assessed as lower risk by their providers and on all standard risk scores, according to a secondary analysis of the PROMISE trial.
The results “highlight the need for sex-specific approaches to coronary artery disease evaluation and testing,” said Kshipra Hemal at Duke Clinical Research Institute in Durham, N.C., and her associates. The findings will be presented April 3 at the annual meeting of the American College of Cardiology and were published online March 23 in the Journal of the American College of Cardiology: Cardiovascular Imaging.
The PROMISE (Prospective Multicenter Imaging Study for the Evaluation of Chest Pain) trial is one of the largest contemporary trials of symptomatic, nonacute suspected CAD. The study included 10,003 stable outpatients, nearly half of whom were women. The researchers calculated the 2008 Framingham score, 2013 Atherosclerotic Cardiovascular Disease score, 1979 Diamond and Forrester score, modified 2011 Diamond and Forrester score, and 2012 combined Diamond-Forrester and Coronary Artery Surgery Study scores for all patients. Patients also were randomly assigned to either anatomical testing with CT angiography or to functional testing with exercise electrocardiogram, stress nuclear imaging, or stress echocardiogram (J Am Coll Cardiol Img. 2016 Mar 23. doi: 10.1016/j.jcmg.2016.02.001).
Women in the study were an average of 3 years older than the men and were significantly more likely to be hypertensive (67% vs. 63%), dyslipidemic (69% vs. 66%), and to have a family history of premature CAD (35% vs. 29%; P less than .01 for all comparisons), the researchers reported. Nonetheless, all five risk scores characterized women as lower risk than men (P less than .001 for mean differences). Moreover, before testing, providers characterized 41% of women having a low (less than 30%) likelihood of CAD, compared with 34% of men (P less than .001).
Women were more likely than men to be referred for stress echocardiography or nuclear stress test, but only 9.7% had a positive noninvasive test, compared with 15% of men (P less than .001), the researchers also reported. “A number of characteristics predicted positive test results, and many characteristics were similar between the sexes,” they added. “However, in multivariable models, key predictors of test positivity were few and varied by sex.” Body mass index and Framingham risk score predicted a positive test for women, while both the Framingham and modified Diamond-Forrester risk scores predicted a positive test for men.
Chest pain was the most common primary symptom reported by nearly three-quarters of women and men and was described as “crushing/pressure/squeezing/tightness” 53% and 46% of the time, respectively (P less than .001). Dyspnea was the second most frequent primary symptom at 15% for both sexes. Women were more likely than men to describe back pain, neck or jaw pain, or palpitations, but only 0.6% to 2.7% of patients ranked these among their main symptoms.
“Further studies are warranted to examine the underlying pathophysiology and implications for clinical care of the sex-based clinical differences observed along the entire diagnostic pathway of suspected CAD, including risk factor burden, presenting symptoms, and testing results,” the researchers concluded.
The PROMISE study was funded by the National Heart, Lung, and Blood Institute. Ms. Hemal had no disclosures. Senior author Dr. Pamela S. Douglas disclosed grant support from HeartFlow and having served on a data and safety monitoring board for General Electric Healthcare. Two of the other 15 coinvestigators also disclosed relationships with industry; the rest had no disclosures.
Despite symptomatic presentation, greater family history of premature coronary artery disease, and higher risk factor burden, including older age and greater prevalence of hypertension and dyslipidemia, the women in PROMISE were more likely to be characterized as low risk based on standard cardiovascular risk assessment scores and thus, not surprisingly, also were considered to be at lower risk by their providers. These findings add credence to the ongoing concerns that women are preferentially likely to receive less intensive management of CAD than their male counterparts.
The 2014 American Heart Association Consensus Statement on noninvasive diagnostic testing in women with suspected ischemic heart disease highlighted the development of novel diagnostic tools that have an expanded role in the evaluation of symptomatic female patients to detect not only focal epicardial coronary stenosis, but also nonobstructive atherosclerosis as well as the identification of ischemia resulting from microvascular dysfunction. Such methods using advanced imaging are making steady progress in the understanding of microvascular disease and its consequences.
We agree with the PROMISE investigators that focused sex-specific diagnostic strategies are needed to reduce the cardiovascular mortality and morbidity in women. With emerging data on the full pathophysiologic spectrum of ischemic heart disease in women, diagnostic algorithms must include functional and anatomic cardiac tests as well as physiologic assessments of endothelial and microvascular function, for accurately establishing the diagnosis and prognosis of women with suspected IHD.
Dr. Jennifer H. Mieres is with Hofstra University, Hempstead, N.Y. Dr. Robert O. Bonow is with Northwestern University, Chicago. They had no disclosures. These comments are from their editorial (J Am Coll Cardiol Img. 2016 Mar 23. doi: 10.1016/j.jcmg.2016.02.0089).
Despite symptomatic presentation, greater family history of premature coronary artery disease, and higher risk factor burden, including older age and greater prevalence of hypertension and dyslipidemia, the women in PROMISE were more likely to be characterized as low risk based on standard cardiovascular risk assessment scores and thus, not surprisingly, also were considered to be at lower risk by their providers. These findings add credence to the ongoing concerns that women are preferentially likely to receive less intensive management of CAD than their male counterparts.
The 2014 American Heart Association Consensus Statement on noninvasive diagnostic testing in women with suspected ischemic heart disease highlighted the development of novel diagnostic tools that have an expanded role in the evaluation of symptomatic female patients to detect not only focal epicardial coronary stenosis, but also nonobstructive atherosclerosis as well as the identification of ischemia resulting from microvascular dysfunction. Such methods using advanced imaging are making steady progress in the understanding of microvascular disease and its consequences.
We agree with the PROMISE investigators that focused sex-specific diagnostic strategies are needed to reduce the cardiovascular mortality and morbidity in women. With emerging data on the full pathophysiologic spectrum of ischemic heart disease in women, diagnostic algorithms must include functional and anatomic cardiac tests as well as physiologic assessments of endothelial and microvascular function, for accurately establishing the diagnosis and prognosis of women with suspected IHD.
Dr. Jennifer H. Mieres is with Hofstra University, Hempstead, N.Y. Dr. Robert O. Bonow is with Northwestern University, Chicago. They had no disclosures. These comments are from their editorial (J Am Coll Cardiol Img. 2016 Mar 23. doi: 10.1016/j.jcmg.2016.02.0089).
Despite symptomatic presentation, greater family history of premature coronary artery disease, and higher risk factor burden, including older age and greater prevalence of hypertension and dyslipidemia, the women in PROMISE were more likely to be characterized as low risk based on standard cardiovascular risk assessment scores and thus, not surprisingly, also were considered to be at lower risk by their providers. These findings add credence to the ongoing concerns that women are preferentially likely to receive less intensive management of CAD than their male counterparts.
The 2014 American Heart Association Consensus Statement on noninvasive diagnostic testing in women with suspected ischemic heart disease highlighted the development of novel diagnostic tools that have an expanded role in the evaluation of symptomatic female patients to detect not only focal epicardial coronary stenosis, but also nonobstructive atherosclerosis as well as the identification of ischemia resulting from microvascular dysfunction. Such methods using advanced imaging are making steady progress in the understanding of microvascular disease and its consequences.
We agree with the PROMISE investigators that focused sex-specific diagnostic strategies are needed to reduce the cardiovascular mortality and morbidity in women. With emerging data on the full pathophysiologic spectrum of ischemic heart disease in women, diagnostic algorithms must include functional and anatomic cardiac tests as well as physiologic assessments of endothelial and microvascular function, for accurately establishing the diagnosis and prognosis of women with suspected IHD.
Dr. Jennifer H. Mieres is with Hofstra University, Hempstead, N.Y. Dr. Robert O. Bonow is with Northwestern University, Chicago. They had no disclosures. These comments are from their editorial (J Am Coll Cardiol Img. 2016 Mar 23. doi: 10.1016/j.jcmg.2016.02.0089).
Women with suspected coronary artery disease had similar symptoms and more heart disease risk factors, compared with men, but were assessed as lower risk by their providers and on all standard risk scores, according to a secondary analysis of the PROMISE trial.
The results “highlight the need for sex-specific approaches to coronary artery disease evaluation and testing,” said Kshipra Hemal at Duke Clinical Research Institute in Durham, N.C., and her associates. The findings will be presented April 3 at the annual meeting of the American College of Cardiology and were published online March 23 in the Journal of the American College of Cardiology: Cardiovascular Imaging.
The PROMISE (Prospective Multicenter Imaging Study for the Evaluation of Chest Pain) trial is one of the largest contemporary trials of symptomatic, nonacute suspected CAD. The study included 10,003 stable outpatients, nearly half of whom were women. The researchers calculated the 2008 Framingham score, 2013 Atherosclerotic Cardiovascular Disease score, 1979 Diamond and Forrester score, modified 2011 Diamond and Forrester score, and 2012 combined Diamond-Forrester and Coronary Artery Surgery Study scores for all patients. Patients also were randomly assigned to either anatomical testing with CT angiography or to functional testing with exercise electrocardiogram, stress nuclear imaging, or stress echocardiogram (J Am Coll Cardiol Img. 2016 Mar 23. doi: 10.1016/j.jcmg.2016.02.001).
Women in the study were an average of 3 years older than the men and were significantly more likely to be hypertensive (67% vs. 63%), dyslipidemic (69% vs. 66%), and to have a family history of premature CAD (35% vs. 29%; P less than .01 for all comparisons), the researchers reported. Nonetheless, all five risk scores characterized women as lower risk than men (P less than .001 for mean differences). Moreover, before testing, providers characterized 41% of women having a low (less than 30%) likelihood of CAD, compared with 34% of men (P less than .001).
Women were more likely than men to be referred for stress echocardiography or nuclear stress test, but only 9.7% had a positive noninvasive test, compared with 15% of men (P less than .001), the researchers also reported. “A number of characteristics predicted positive test results, and many characteristics were similar between the sexes,” they added. “However, in multivariable models, key predictors of test positivity were few and varied by sex.” Body mass index and Framingham risk score predicted a positive test for women, while both the Framingham and modified Diamond-Forrester risk scores predicted a positive test for men.
Chest pain was the most common primary symptom reported by nearly three-quarters of women and men and was described as “crushing/pressure/squeezing/tightness” 53% and 46% of the time, respectively (P less than .001). Dyspnea was the second most frequent primary symptom at 15% for both sexes. Women were more likely than men to describe back pain, neck or jaw pain, or palpitations, but only 0.6% to 2.7% of patients ranked these among their main symptoms.
“Further studies are warranted to examine the underlying pathophysiology and implications for clinical care of the sex-based clinical differences observed along the entire diagnostic pathway of suspected CAD, including risk factor burden, presenting symptoms, and testing results,” the researchers concluded.
The PROMISE study was funded by the National Heart, Lung, and Blood Institute. Ms. Hemal had no disclosures. Senior author Dr. Pamela S. Douglas disclosed grant support from HeartFlow and having served on a data and safety monitoring board for General Electric Healthcare. Two of the other 15 coinvestigators also disclosed relationships with industry; the rest had no disclosures.
Women with suspected coronary artery disease had similar symptoms and more heart disease risk factors, compared with men, but were assessed as lower risk by their providers and on all standard risk scores, according to a secondary analysis of the PROMISE trial.
The results “highlight the need for sex-specific approaches to coronary artery disease evaluation and testing,” said Kshipra Hemal at Duke Clinical Research Institute in Durham, N.C., and her associates. The findings will be presented April 3 at the annual meeting of the American College of Cardiology and were published online March 23 in the Journal of the American College of Cardiology: Cardiovascular Imaging.
The PROMISE (Prospective Multicenter Imaging Study for the Evaluation of Chest Pain) trial is one of the largest contemporary trials of symptomatic, nonacute suspected CAD. The study included 10,003 stable outpatients, nearly half of whom were women. The researchers calculated the 2008 Framingham score, 2013 Atherosclerotic Cardiovascular Disease score, 1979 Diamond and Forrester score, modified 2011 Diamond and Forrester score, and 2012 combined Diamond-Forrester and Coronary Artery Surgery Study scores for all patients. Patients also were randomly assigned to either anatomical testing with CT angiography or to functional testing with exercise electrocardiogram, stress nuclear imaging, or stress echocardiogram (J Am Coll Cardiol Img. 2016 Mar 23. doi: 10.1016/j.jcmg.2016.02.001).
Women in the study were an average of 3 years older than the men and were significantly more likely to be hypertensive (67% vs. 63%), dyslipidemic (69% vs. 66%), and to have a family history of premature CAD (35% vs. 29%; P less than .01 for all comparisons), the researchers reported. Nonetheless, all five risk scores characterized women as lower risk than men (P less than .001 for mean differences). Moreover, before testing, providers characterized 41% of women having a low (less than 30%) likelihood of CAD, compared with 34% of men (P less than .001).
Women were more likely than men to be referred for stress echocardiography or nuclear stress test, but only 9.7% had a positive noninvasive test, compared with 15% of men (P less than .001), the researchers also reported. “A number of characteristics predicted positive test results, and many characteristics were similar between the sexes,” they added. “However, in multivariable models, key predictors of test positivity were few and varied by sex.” Body mass index and Framingham risk score predicted a positive test for women, while both the Framingham and modified Diamond-Forrester risk scores predicted a positive test for men.
Chest pain was the most common primary symptom reported by nearly three-quarters of women and men and was described as “crushing/pressure/squeezing/tightness” 53% and 46% of the time, respectively (P less than .001). Dyspnea was the second most frequent primary symptom at 15% for both sexes. Women were more likely than men to describe back pain, neck or jaw pain, or palpitations, but only 0.6% to 2.7% of patients ranked these among their main symptoms.
“Further studies are warranted to examine the underlying pathophysiology and implications for clinical care of the sex-based clinical differences observed along the entire diagnostic pathway of suspected CAD, including risk factor burden, presenting symptoms, and testing results,” the researchers concluded.
The PROMISE study was funded by the National Heart, Lung, and Blood Institute. Ms. Hemal had no disclosures. Senior author Dr. Pamela S. Douglas disclosed grant support from HeartFlow and having served on a data and safety monitoring board for General Electric Healthcare. Two of the other 15 coinvestigators also disclosed relationships with industry; the rest had no disclosures.
FROM ACC 16
Key clinical point: Women with suspected coronary artery disease had similar symptoms and more risk factors for coronary artery disease, compared with men, but were classified as lower risk on risk scores and by providers.
Major finding: All risk scores assessed women as being at lower risk than men. Providers characterized 41% of pretest women and 34% of men as low risk (P less than .001).
Data source: A prospective, multicenter, randomized trial of 10,003 symptomatic outpatients with suspected coronary artery disease.
Disclosures: The PROMISE study was funded by the National Heart, Lung, and Blood Institute. Dr. Hemal had no disclosures. Senior author Dr. Pamela Douglas disclosed grant support from HeartFlow and having served on a data and safety monitoring board for General Electric Healthcare. Two of the other 15 coinvestigators also disclosed relationships with industry; the rest had no disclosures.
Providers need protocols in place to better treat transgender youth
WASHINGTON – Practices and clinics should implement cultural humility training and gender protocols for dealing with transgender youth because many of these youth and their parents find health care experiences to be difficult when procedures for treating and interacting with transgender youth are confusing or nonexistent.
The study is part of an ongoing effort to “provide high-quality, respectful health care for transgender youth [because] these youth are at greatly increased risk of issues including substance abuse, depression, anxiety, homelessness, and suicide,” explained Julia M. Crouch of Seattle Children’s Research Institute.
“A growing body of evidence shows improved health outcomes for transgender youth who received support from family, schools, and providers, and there are now an increasing number of multidisciplinary gender clinics throughout the country [that] have been demonstrated to be a feasible and effective way to provide coordinated care to this population,” Ms. Crouch said at the annual meeting of the Society for Adolescent Health and Medicine.
Ms. Crouch and her coinvestigators recruited transgender youth between the ages of 14 and 22 years, along with parents of transgender youth, all of whom were evaluated and enrolled from the Seattle metropolitan area. Both parents and youth were given the option of participating in either a semistructured interview or a focus group discussion, during which investigators learned about the concerns and experiences of both youth and parents when visiting their health care clinics (J Adolesc Health. 2011 Apr;48[4]:351-7; J Adolesc Health. 2011 Apr;48(4):351-7)..
In total, 13 youth and 16 parents – the latter of whom were not made up of 8 pairs of parents, but rather 16 individual parents of transgender youth who were not necessarily the same 13 youth recruited for the study – were eventually selected for inclusion. The parents split evenly between opting for interviews and focus groups, while four of the youth chose interviews and the remaining nine chose focus groups. Seven youth identified themselves as male, three identified as female, and the remaining three identified as “genderqueer or gender fluid.”
Analysis of interview and focus group conversations identified six key “barriers to care” that Ms. Crouch and her coauthors call necessary to rectify in order to improve the quality of health care provided to transgender youth. These are:
• The dearth of health care providers with sufficient knowledge and interest in working with transgender youth.
• The lack of access to pubertal blockers and cross-sex hormones.
• Doctors and their staff who are unable or unwilling to use the names and pronouns that youths prefer to go by.
• Patients being made to feel uncomfortable or “not normal” by health care providers,
• The lack of a set protocol or treatment methodology for dealing with transgender youth.
• The lack of coordination between health care providers and specialties on treating transgender youth – specifically, a lack of cohesive care between mental and medical health care.
“ ‘It was hard enough to find providers who were accepting new patients, worked with adolescents and my insurance, [and] on top of it, finding someone who was transfriendly made it all but impossible,’ ” Ms. Crouch recalled one transgender youth saying during the study.
Ms. Crouch also recounted that providers’ inability to consistently use the correct pronoun when talking about or to a transgender patient was harmful, and despite most instances being dismissed as unintentional, several were said to be intentional and malicious.
“For example, one parent told us [their] doctor said, ‘her, her, her,’ and [her] son, who was 10 years old, said, ‘him, him, him,’ and the doctor got mad, became dismissive and irritated, and kept saying ‘her,’ ” said Ms. Crouch.
The average age of the youth was 18 years, and of the parents was 49 years. The parents were 88% white and 75% female, with 44% holding at least a college degree. Most of the youth were white (69%), and 77% had either completed high school or some college. Youth and parents were recruited in 2015 from local clinics in the Seattle area, as well as through local and national listservs. Thirty-three percent of the parents were from outside the state of Washington.
The study was funded by the Center for Diversity and Health Equity, and the Clinical and Translational Research Faculty Research Support Fund, at Seattle Children’s Research Institute. Ms. Crouch did not report any relevant financial disclosures.
WASHINGTON – Practices and clinics should implement cultural humility training and gender protocols for dealing with transgender youth because many of these youth and their parents find health care experiences to be difficult when procedures for treating and interacting with transgender youth are confusing or nonexistent.
The study is part of an ongoing effort to “provide high-quality, respectful health care for transgender youth [because] these youth are at greatly increased risk of issues including substance abuse, depression, anxiety, homelessness, and suicide,” explained Julia M. Crouch of Seattle Children’s Research Institute.
“A growing body of evidence shows improved health outcomes for transgender youth who received support from family, schools, and providers, and there are now an increasing number of multidisciplinary gender clinics throughout the country [that] have been demonstrated to be a feasible and effective way to provide coordinated care to this population,” Ms. Crouch said at the annual meeting of the Society for Adolescent Health and Medicine.
Ms. Crouch and her coinvestigators recruited transgender youth between the ages of 14 and 22 years, along with parents of transgender youth, all of whom were evaluated and enrolled from the Seattle metropolitan area. Both parents and youth were given the option of participating in either a semistructured interview or a focus group discussion, during which investigators learned about the concerns and experiences of both youth and parents when visiting their health care clinics (J Adolesc Health. 2011 Apr;48[4]:351-7; J Adolesc Health. 2011 Apr;48(4):351-7)..
In total, 13 youth and 16 parents – the latter of whom were not made up of 8 pairs of parents, but rather 16 individual parents of transgender youth who were not necessarily the same 13 youth recruited for the study – were eventually selected for inclusion. The parents split evenly between opting for interviews and focus groups, while four of the youth chose interviews and the remaining nine chose focus groups. Seven youth identified themselves as male, three identified as female, and the remaining three identified as “genderqueer or gender fluid.”
Analysis of interview and focus group conversations identified six key “barriers to care” that Ms. Crouch and her coauthors call necessary to rectify in order to improve the quality of health care provided to transgender youth. These are:
• The dearth of health care providers with sufficient knowledge and interest in working with transgender youth.
• The lack of access to pubertal blockers and cross-sex hormones.
• Doctors and their staff who are unable or unwilling to use the names and pronouns that youths prefer to go by.
• Patients being made to feel uncomfortable or “not normal” by health care providers,
• The lack of a set protocol or treatment methodology for dealing with transgender youth.
• The lack of coordination between health care providers and specialties on treating transgender youth – specifically, a lack of cohesive care between mental and medical health care.
“ ‘It was hard enough to find providers who were accepting new patients, worked with adolescents and my insurance, [and] on top of it, finding someone who was transfriendly made it all but impossible,’ ” Ms. Crouch recalled one transgender youth saying during the study.
Ms. Crouch also recounted that providers’ inability to consistently use the correct pronoun when talking about or to a transgender patient was harmful, and despite most instances being dismissed as unintentional, several were said to be intentional and malicious.
“For example, one parent told us [their] doctor said, ‘her, her, her,’ and [her] son, who was 10 years old, said, ‘him, him, him,’ and the doctor got mad, became dismissive and irritated, and kept saying ‘her,’ ” said Ms. Crouch.
The average age of the youth was 18 years, and of the parents was 49 years. The parents were 88% white and 75% female, with 44% holding at least a college degree. Most of the youth were white (69%), and 77% had either completed high school or some college. Youth and parents were recruited in 2015 from local clinics in the Seattle area, as well as through local and national listservs. Thirty-three percent of the parents were from outside the state of Washington.
The study was funded by the Center for Diversity and Health Equity, and the Clinical and Translational Research Faculty Research Support Fund, at Seattle Children’s Research Institute. Ms. Crouch did not report any relevant financial disclosures.
WASHINGTON – Practices and clinics should implement cultural humility training and gender protocols for dealing with transgender youth because many of these youth and their parents find health care experiences to be difficult when procedures for treating and interacting with transgender youth are confusing or nonexistent.
The study is part of an ongoing effort to “provide high-quality, respectful health care for transgender youth [because] these youth are at greatly increased risk of issues including substance abuse, depression, anxiety, homelessness, and suicide,” explained Julia M. Crouch of Seattle Children’s Research Institute.
“A growing body of evidence shows improved health outcomes for transgender youth who received support from family, schools, and providers, and there are now an increasing number of multidisciplinary gender clinics throughout the country [that] have been demonstrated to be a feasible and effective way to provide coordinated care to this population,” Ms. Crouch said at the annual meeting of the Society for Adolescent Health and Medicine.
Ms. Crouch and her coinvestigators recruited transgender youth between the ages of 14 and 22 years, along with parents of transgender youth, all of whom were evaluated and enrolled from the Seattle metropolitan area. Both parents and youth were given the option of participating in either a semistructured interview or a focus group discussion, during which investigators learned about the concerns and experiences of both youth and parents when visiting their health care clinics (J Adolesc Health. 2011 Apr;48[4]:351-7; J Adolesc Health. 2011 Apr;48(4):351-7)..
In total, 13 youth and 16 parents – the latter of whom were not made up of 8 pairs of parents, but rather 16 individual parents of transgender youth who were not necessarily the same 13 youth recruited for the study – were eventually selected for inclusion. The parents split evenly between opting for interviews and focus groups, while four of the youth chose interviews and the remaining nine chose focus groups. Seven youth identified themselves as male, three identified as female, and the remaining three identified as “genderqueer or gender fluid.”
Analysis of interview and focus group conversations identified six key “barriers to care” that Ms. Crouch and her coauthors call necessary to rectify in order to improve the quality of health care provided to transgender youth. These are:
• The dearth of health care providers with sufficient knowledge and interest in working with transgender youth.
• The lack of access to pubertal blockers and cross-sex hormones.
• Doctors and their staff who are unable or unwilling to use the names and pronouns that youths prefer to go by.
• Patients being made to feel uncomfortable or “not normal” by health care providers,
• The lack of a set protocol or treatment methodology for dealing with transgender youth.
• The lack of coordination between health care providers and specialties on treating transgender youth – specifically, a lack of cohesive care between mental and medical health care.
“ ‘It was hard enough to find providers who were accepting new patients, worked with adolescents and my insurance, [and] on top of it, finding someone who was transfriendly made it all but impossible,’ ” Ms. Crouch recalled one transgender youth saying during the study.
Ms. Crouch also recounted that providers’ inability to consistently use the correct pronoun when talking about or to a transgender patient was harmful, and despite most instances being dismissed as unintentional, several were said to be intentional and malicious.
“For example, one parent told us [their] doctor said, ‘her, her, her,’ and [her] son, who was 10 years old, said, ‘him, him, him,’ and the doctor got mad, became dismissive and irritated, and kept saying ‘her,’ ” said Ms. Crouch.
The average age of the youth was 18 years, and of the parents was 49 years. The parents were 88% white and 75% female, with 44% holding at least a college degree. Most of the youth were white (69%), and 77% had either completed high school or some college. Youth and parents were recruited in 2015 from local clinics in the Seattle area, as well as through local and national listservs. Thirty-three percent of the parents were from outside the state of Washington.
The study was funded by the Center for Diversity and Health Equity, and the Clinical and Translational Research Faculty Research Support Fund, at Seattle Children’s Research Institute. Ms. Crouch did not report any relevant financial disclosures.
AT SAHM 16
Key clinical point: Cultural humility training and gender protocols should be adopted for all centers handling transgender youth.
Major finding: A small group of transgender youth and parents of transgender youth outlined six specific protocols that should be adopted by clinicians to improve the treatment of transgender youth.
Data source: A study of 13 transgender youth and 16 parents via interview and focus group discussions.
Disclosures: The study was funded by the Center for Diversity and Health Equity, and the Clinical and Translational Research Faculty Research Support Fund, at Seattle Children’s Research Institute. Ms. Crouch did not report any relevant financial disclosures.
Pediatric mental illness dx, drug prescribing vary widely
A lack of psychiatrists only partially accounted for substantial variations in rates of mental illness diagnosis and prescriptions for psychotropic medications in practices nationwide, a study has shown.
Although a lack of available specialty care was associated with significantly higher odds of a diagnosis or prescription, the colocation of mental health professionals or percentage of children in foster care treated in a practice did not fully explain the differences.
Among 294,748 children aged 4-18 years, seen one or more times in 43 primary care practices nationwide, 15% received a mental health diagnosis between Jan. 1, 2009, and June 30, 2014. Psychotropic medications were prescribed to 14%, reported lead researcher Stephanie L. Mayne of the center for pediatric clinical effectiveness at the the Children’s Hospital of Philadelphia (Pediatrics. 2016 doi: 10.1542/peds.2015-2974).
The most common diagnosis was attention-deficit/hyperactivity disorder at a rate of between 1% and 16%. Differences in other diagnoses “were smaller, but still meaningful” at ranges of 1%-8% for anxiety, 0%-5% for depression, 0.2%-3% for autism, 0%-3% for conduct disorder, and 0%-2% for oppositional-defiant disorder. Bipolar disorder was “uncommon” at less than 1%, Ms. Mayne and her associates reported.
The rate of children receiving any psychotropic medication was between 4% and 26%, while the proportion of patients receiving two or more medication classes ranged between 1% and 12%. Prescription rates for specific medication classes also varied at between 4% and 18% for stimulants, 1% and 12% for antidepressants, 0.1% and 8% for alpha-agonists, and 0.1% and 5% for second-generation antipsychotics.
“Primary care providers’ level of agreement with current guidelines, perceived self-efficacy in diagnosing or treating particular conditions, training, relationships with schools, and reimbursement from insurers might affect prescribing practices,” Ms. Mayne and her associates wrote.
“Even with colocation, barriers such as financial differences in reimbursement for medical and mental health services, difficulties with information sharing, differing expertise, and limited hours may impede integration,” they commented.
Dr. Alexander G. Fiks is an investigator for Pfizer; the other researchers said they had no relevant financial disclosures. This study was funded by the National Institutes of Health and the National Institute of Child Health and Human Development under the Best Pharmaceuticals for Children Act.
On Twitter @whitneymcknight
The integration of mental health services into primary care is an important strategy for increasing access. Future studies that investigate variations in mental health care seen in the primary care setting can help us better understand the quality of this care and consistency with published guidelines.
 |
Dr. Lee Savio Beers |
Increased education and support for primary care physicians is essential, as they are at the front lines of providing care to children with mental and behavioral health concerns. However, working together with specialty mental health providers is also important, as they are important partners in the early identification, diagnosis, and treatment of mental disorders.
Education and consultation models such as Child Psychiatry Access Programs can significantly improve a primary care physician’s capacity to care for children with mental health concerns in the medical home, and arrange for appropriate specialty mental health treatment when indicated.
Dr. Lee Savio Beers is the medical director for municipal and regional affairs for the Child Health Advocacy Institute at Children’s National Medical Center, the director of the Washington, D.C., Mental Health Access in Pediatrics (DC MAP) program, and an assistant professor of pediatrics at George Washington University, all in Washington. She had no relevant financial disclosures.
The integration of mental health services into primary care is an important strategy for increasing access. Future studies that investigate variations in mental health care seen in the primary care setting can help us better understand the quality of this care and consistency with published guidelines.
 |
Dr. Lee Savio Beers |
Increased education and support for primary care physicians is essential, as they are at the front lines of providing care to children with mental and behavioral health concerns. However, working together with specialty mental health providers is also important, as they are important partners in the early identification, diagnosis, and treatment of mental disorders.
Education and consultation models such as Child Psychiatry Access Programs can significantly improve a primary care physician’s capacity to care for children with mental health concerns in the medical home, and arrange for appropriate specialty mental health treatment when indicated.
Dr. Lee Savio Beers is the medical director for municipal and regional affairs for the Child Health Advocacy Institute at Children’s National Medical Center, the director of the Washington, D.C., Mental Health Access in Pediatrics (DC MAP) program, and an assistant professor of pediatrics at George Washington University, all in Washington. She had no relevant financial disclosures.
The integration of mental health services into primary care is an important strategy for increasing access. Future studies that investigate variations in mental health care seen in the primary care setting can help us better understand the quality of this care and consistency with published guidelines.
 |
Dr. Lee Savio Beers |
Increased education and support for primary care physicians is essential, as they are at the front lines of providing care to children with mental and behavioral health concerns. However, working together with specialty mental health providers is also important, as they are important partners in the early identification, diagnosis, and treatment of mental disorders.
Education and consultation models such as Child Psychiatry Access Programs can significantly improve a primary care physician’s capacity to care for children with mental health concerns in the medical home, and arrange for appropriate specialty mental health treatment when indicated.
Dr. Lee Savio Beers is the medical director for municipal and regional affairs for the Child Health Advocacy Institute at Children’s National Medical Center, the director of the Washington, D.C., Mental Health Access in Pediatrics (DC MAP) program, and an assistant professor of pediatrics at George Washington University, all in Washington. She had no relevant financial disclosures.
A lack of psychiatrists only partially accounted for substantial variations in rates of mental illness diagnosis and prescriptions for psychotropic medications in practices nationwide, a study has shown.
Although a lack of available specialty care was associated with significantly higher odds of a diagnosis or prescription, the colocation of mental health professionals or percentage of children in foster care treated in a practice did not fully explain the differences.
Among 294,748 children aged 4-18 years, seen one or more times in 43 primary care practices nationwide, 15% received a mental health diagnosis between Jan. 1, 2009, and June 30, 2014. Psychotropic medications were prescribed to 14%, reported lead researcher Stephanie L. Mayne of the center for pediatric clinical effectiveness at the the Children’s Hospital of Philadelphia (Pediatrics. 2016 doi: 10.1542/peds.2015-2974).
The most common diagnosis was attention-deficit/hyperactivity disorder at a rate of between 1% and 16%. Differences in other diagnoses “were smaller, but still meaningful” at ranges of 1%-8% for anxiety, 0%-5% for depression, 0.2%-3% for autism, 0%-3% for conduct disorder, and 0%-2% for oppositional-defiant disorder. Bipolar disorder was “uncommon” at less than 1%, Ms. Mayne and her associates reported.
The rate of children receiving any psychotropic medication was between 4% and 26%, while the proportion of patients receiving two or more medication classes ranged between 1% and 12%. Prescription rates for specific medication classes also varied at between 4% and 18% for stimulants, 1% and 12% for antidepressants, 0.1% and 8% for alpha-agonists, and 0.1% and 5% for second-generation antipsychotics.
“Primary care providers’ level of agreement with current guidelines, perceived self-efficacy in diagnosing or treating particular conditions, training, relationships with schools, and reimbursement from insurers might affect prescribing practices,” Ms. Mayne and her associates wrote.
“Even with colocation, barriers such as financial differences in reimbursement for medical and mental health services, difficulties with information sharing, differing expertise, and limited hours may impede integration,” they commented.
Dr. Alexander G. Fiks is an investigator for Pfizer; the other researchers said they had no relevant financial disclosures. This study was funded by the National Institutes of Health and the National Institute of Child Health and Human Development under the Best Pharmaceuticals for Children Act.
On Twitter @whitneymcknight
A lack of psychiatrists only partially accounted for substantial variations in rates of mental illness diagnosis and prescriptions for psychotropic medications in practices nationwide, a study has shown.
Although a lack of available specialty care was associated with significantly higher odds of a diagnosis or prescription, the colocation of mental health professionals or percentage of children in foster care treated in a practice did not fully explain the differences.
Among 294,748 children aged 4-18 years, seen one or more times in 43 primary care practices nationwide, 15% received a mental health diagnosis between Jan. 1, 2009, and June 30, 2014. Psychotropic medications were prescribed to 14%, reported lead researcher Stephanie L. Mayne of the center for pediatric clinical effectiveness at the the Children’s Hospital of Philadelphia (Pediatrics. 2016 doi: 10.1542/peds.2015-2974).
The most common diagnosis was attention-deficit/hyperactivity disorder at a rate of between 1% and 16%. Differences in other diagnoses “were smaller, but still meaningful” at ranges of 1%-8% for anxiety, 0%-5% for depression, 0.2%-3% for autism, 0%-3% for conduct disorder, and 0%-2% for oppositional-defiant disorder. Bipolar disorder was “uncommon” at less than 1%, Ms. Mayne and her associates reported.
The rate of children receiving any psychotropic medication was between 4% and 26%, while the proportion of patients receiving two or more medication classes ranged between 1% and 12%. Prescription rates for specific medication classes also varied at between 4% and 18% for stimulants, 1% and 12% for antidepressants, 0.1% and 8% for alpha-agonists, and 0.1% and 5% for second-generation antipsychotics.
“Primary care providers’ level of agreement with current guidelines, perceived self-efficacy in diagnosing or treating particular conditions, training, relationships with schools, and reimbursement from insurers might affect prescribing practices,” Ms. Mayne and her associates wrote.
“Even with colocation, barriers such as financial differences in reimbursement for medical and mental health services, difficulties with information sharing, differing expertise, and limited hours may impede integration,” they commented.
Dr. Alexander G. Fiks is an investigator for Pfizer; the other researchers said they had no relevant financial disclosures. This study was funded by the National Institutes of Health and the National Institute of Child Health and Human Development under the Best Pharmaceuticals for Children Act.
On Twitter @whitneymcknight
FROM PEDIATRICS
Key clinical point: A lack of psychiatrists only partially accounted for substantial variations in rates of mental illness diagnosis and prescriptions for psychotropic medications given in practices nationwide, a study has shown.
Major finding: Nationwide, 15% of pediatric patients received a mental health diagnosis, and 14% were prescribed psychotropic medications in primary care, regardless of colocated mental health services.
Data source: A retrospective study of electronic health records for 294,748 patients aged 4-18 years.
Disclosures: Dr. Alexander G. Fiks is an investigator for Pfizer; the other researchers said they had no relevant financial disclosures. This study was funded by the National Institutes of Health and the National Institute of Child Health and Human Development under the Best Pharmaceuticals for Children Act.
Kidney Stones? It’s Time to Rethink Those Meds
PRACTICE CHANGER
Do not prescribe tamsulosin or nifedipine for stone expulsion in patients with ureteral stones that are ≤ 10 mm.1
Strength of recommendation
A: Based on a high-quality randomized controlled trial (RCT).1
Bob Z, age 48, presents to the emergency department (ED) with unspecified groin pain. CT of the kidney, ureter, and bladder (CT KUB) finds evidence of a single ureteral stone measuring 8 mm. He’s prescribed medication for the pain and discharged. The day after his ED visit, he comes to your office to discuss further treatment options. Should you prescribe tamsulosin or nifedipine to help him pass the stone?
The most recent National Health and Nutrition Examination Survey found kidney stones affect 8.8% of the population.2 Outpatient therapy is indicated for patients with ureteric colic secondary to stones ≤ 10 mm who do not have uncontrolled pain, impaired kidney function, or severe infection. Routine outpatient care includes oral hydration, antiemetics, and pain medications.
Medical expulsive therapy (MET) is also used to facilitate stone passage. MET is increasingly becoming part of routine care; use of MET in kidney stone patients in the United States has grown from 14% in 2009 to 64% in 2012.3,4
The joint European Association of Urology/American Urological Association Nephrolithiasis Guideline Panel supports the use of MET.5 Meta-analyses of multiple RCTs suggest that an α-blocker (tamsulosin) or a calcium channel blocker (nifedipine) can reduce pain and lead to quicker stone passage and a higher rate of eventual stone passage when compared to placebo or observation.6,7 However, these reviews included small, heterogeneous studies with a high or unclear risk for bias.
Continue for the study summary >>
STUDY SUMMARY
MET doesn’t increase the rate of stone passage
The SUSPEND (Spontaneous Urinary Stone Passage ENabled by Drugs) trial1 was a multicenter RCT designed to determine the effectiveness of tamsulosin or nifedipine as MET for patients ages 18 to 65 with a single ureteric stone measuring ≤ 10 mm on CT KUB, which has 98% diagnostic accuracy.8 (Stones > 10 mm typically require surgery or lithotripsy.)
In this RCT, 1,167 adults were randomized to take tamsulosin (0.4 mg/d), nifedipine (30 mg/d), or placebo for four weeks or until the stone spontaneously passed, whichever came first. The participants, clinicians, and research staff were blinded to treatment assignment. The primary outcome was the proportion of participants who spontaneously passed their stone, as indicated in patient self-reported questionnaires and case-report forms completed by researchers. Secondary outcomes were time to stone passage and pain as assessed by analgesic use and a visual analogue scale (VAS).
At four weeks, 1,136 (97%) of the randomized participants had data available for analysis. The proportion of participants who passed their stone did not differ between MET and placebo; 80% of the placebo group (303 of 379 participants) passed the stone, compared with 81% (307 of 378) of the tamsulosin group and 80% (304 of 379) of the nifedipine group. The odds ratio (OR) for MET vs placebo was 1.04 (95% confidence interval [CI], 0.77 to 1.43) and the OR for tamsulosin vs nifedipine was 1.07 (95% CI, 0.74 to 1.53). These findings did not change with further subgroup analysis, including by sex, stone size (≤ 5 mm vs > 5 mm), or stone location.
There were no differences between groups in time to stone passage as measured by clinical report and confirmed by imaging. Time to passage of stone was available for 237 (21% of) participants. The mean days to stone passage was 15.9 (n = 84) for placebo, 16.5 (n = 79) for tamsulosin, and 16.2 (n = 74) for nifedipine, with a MET vs placebo difference of 0.5 days (95% CI, –2.9 to 3.9; P = .78). Sensitivity analysis accounting for bias from missing data did not change this outcome.
No differences in analgesic use or pain. Self-reported use of pain medication during the first four weeks was similar between groups: 59% (placebo patients), 56% (tamsulosin), and 56% (nifedipine). The mean days of pain medication use was 10.5 for placebo, 11.6 for tamsulosin, and 10.7 for nifedipine, with a MET vs placebo difference of 0.6 days (95% CI, –1.6 to 2.8; P = .45).
There was no difference between groups in the VAS pain score at four weeks. The MET vs placebo difference was 0.0 (95% CI, –0.4 to 0.4; P = .96) and the mean VAS pain score was 1.2 for placebo, 1.0 for tamsulosin, and 1.3 for nifedipine.
WHAT’S NEW
This large RCT contradicts results from previous meta-analyses
The SUSPEND study is the first large, multicenter RCT of MET with tamsulosin or nifedipine for kidney stones that used patient-oriented outcomes to find no benefit for stone expulsion, analgesic use, or reported pain compared to placebo. The discrepancy with prior meta-analyses is not unusual. Up to one-third of meta-analyses that show positive outcomes of a therapy are subsequently altered by the inclusion of results from a single, large, well-designed, multicenter RCT.9
Continue for caveats >>
CAVEATS
This trial included fewer women than previous studies
The SUSPEND study included a smaller proportion of women than previously published case series due to a need for a diagnostic CT KUB, which excluded more women than men due to radiation concerns. However, the proportion of women was balanced across all groups in this trial, and there was no evidence that sex impacted the efficacy of treatment for the primary outcome.1
CHALLENGES TO IMPLEMENTATION
We see no challenges to the implementation of this recommendation.
References
1. Pickard R, Starr K, MacLennan G, et al. Medical expulsive therapy in adults with ureteric colic: a multicentre, randomised, placebo-controlled trial. Lancet. 2015;386:341-349.
2. Scales CD Jr, Smith AC, Hanley JM, et al. Prevalence of kidney stones in the United States. Eur Urol. 2012;62:160-165.
3. Fwu CW, Eggers PW, Kimmel PL, et al. Emergency department visits, use of imaging, and drugs for urolithiasis have increased in the United States. Kidney Int. 2013;89:479-486.
4. Bagga H, Appa A, Wang R, et al. 2257 medical expulsion therapy is underutilized in women presenting to an emergency department with acute urinary stone disease. J Urol. 2013; 189:e925-e926.
5. Preminger GM, Tiselius HG, Assimos DG, et al; American Urological Association Education and Research, Inc; European Association of Urology. 2007 Guideline for the management of ureteral calculi. Eur Urol. 2007;52:1610-1631.
6. Campschroer T, Zhu Y, Duijvesz D, et al. Alpha-blockers as medical expulsive therapy for ureteral stones. Cochrane Database Syst Rev. 2014;4:CD008509.
7. Seitz C, Liatsikos E, Porpiglia F, et al. Medical therapy to facilitate the passage of stones: what is the evidence? Eur Urol. 2009;56:455-471.
8. Worster A, Preyra I, Weaver B, et al. The accuracy of noncontrast helical computed tomography versus intravenous pyelography in the diagnosis of suspected acute urolithiasis: a meta-analysis. Ann Emerg Med. 2002;40: 280-286.
9. LeLorier J, Gregoire G, Benhaddad A, et al. Discrepancies between meta-analyses and subsequent large randomized, controlled trials. N Engl J Med. 1997;337:536-542.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2016. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2016;65(2):118-120.
PRACTICE CHANGER
Do not prescribe tamsulosin or nifedipine for stone expulsion in patients with ureteral stones that are ≤ 10 mm.1
Strength of recommendation
A: Based on a high-quality randomized controlled trial (RCT).1
Bob Z, age 48, presents to the emergency department (ED) with unspecified groin pain. CT of the kidney, ureter, and bladder (CT KUB) finds evidence of a single ureteral stone measuring 8 mm. He’s prescribed medication for the pain and discharged. The day after his ED visit, he comes to your office to discuss further treatment options. Should you prescribe tamsulosin or nifedipine to help him pass the stone?
The most recent National Health and Nutrition Examination Survey found kidney stones affect 8.8% of the population.2 Outpatient therapy is indicated for patients with ureteric colic secondary to stones ≤ 10 mm who do not have uncontrolled pain, impaired kidney function, or severe infection. Routine outpatient care includes oral hydration, antiemetics, and pain medications.
Medical expulsive therapy (MET) is also used to facilitate stone passage. MET is increasingly becoming part of routine care; use of MET in kidney stone patients in the United States has grown from 14% in 2009 to 64% in 2012.3,4
The joint European Association of Urology/American Urological Association Nephrolithiasis Guideline Panel supports the use of MET.5 Meta-analyses of multiple RCTs suggest that an α-blocker (tamsulosin) or a calcium channel blocker (nifedipine) can reduce pain and lead to quicker stone passage and a higher rate of eventual stone passage when compared to placebo or observation.6,7 However, these reviews included small, heterogeneous studies with a high or unclear risk for bias.
Continue for the study summary >>
STUDY SUMMARY
MET doesn’t increase the rate of stone passage
The SUSPEND (Spontaneous Urinary Stone Passage ENabled by Drugs) trial1 was a multicenter RCT designed to determine the effectiveness of tamsulosin or nifedipine as MET for patients ages 18 to 65 with a single ureteric stone measuring ≤ 10 mm on CT KUB, which has 98% diagnostic accuracy.8 (Stones > 10 mm typically require surgery or lithotripsy.)
In this RCT, 1,167 adults were randomized to take tamsulosin (0.4 mg/d), nifedipine (30 mg/d), or placebo for four weeks or until the stone spontaneously passed, whichever came first. The participants, clinicians, and research staff were blinded to treatment assignment. The primary outcome was the proportion of participants who spontaneously passed their stone, as indicated in patient self-reported questionnaires and case-report forms completed by researchers. Secondary outcomes were time to stone passage and pain as assessed by analgesic use and a visual analogue scale (VAS).
At four weeks, 1,136 (97%) of the randomized participants had data available for analysis. The proportion of participants who passed their stone did not differ between MET and placebo; 80% of the placebo group (303 of 379 participants) passed the stone, compared with 81% (307 of 378) of the tamsulosin group and 80% (304 of 379) of the nifedipine group. The odds ratio (OR) for MET vs placebo was 1.04 (95% confidence interval [CI], 0.77 to 1.43) and the OR for tamsulosin vs nifedipine was 1.07 (95% CI, 0.74 to 1.53). These findings did not change with further subgroup analysis, including by sex, stone size (≤ 5 mm vs > 5 mm), or stone location.
There were no differences between groups in time to stone passage as measured by clinical report and confirmed by imaging. Time to passage of stone was available for 237 (21% of) participants. The mean days to stone passage was 15.9 (n = 84) for placebo, 16.5 (n = 79) for tamsulosin, and 16.2 (n = 74) for nifedipine, with a MET vs placebo difference of 0.5 days (95% CI, –2.9 to 3.9; P = .78). Sensitivity analysis accounting for bias from missing data did not change this outcome.
No differences in analgesic use or pain. Self-reported use of pain medication during the first four weeks was similar between groups: 59% (placebo patients), 56% (tamsulosin), and 56% (nifedipine). The mean days of pain medication use was 10.5 for placebo, 11.6 for tamsulosin, and 10.7 for nifedipine, with a MET vs placebo difference of 0.6 days (95% CI, –1.6 to 2.8; P = .45).
There was no difference between groups in the VAS pain score at four weeks. The MET vs placebo difference was 0.0 (95% CI, –0.4 to 0.4; P = .96) and the mean VAS pain score was 1.2 for placebo, 1.0 for tamsulosin, and 1.3 for nifedipine.
WHAT’S NEW
This large RCT contradicts results from previous meta-analyses
The SUSPEND study is the first large, multicenter RCT of MET with tamsulosin or nifedipine for kidney stones that used patient-oriented outcomes to find no benefit for stone expulsion, analgesic use, or reported pain compared to placebo. The discrepancy with prior meta-analyses is not unusual. Up to one-third of meta-analyses that show positive outcomes of a therapy are subsequently altered by the inclusion of results from a single, large, well-designed, multicenter RCT.9
Continue for caveats >>
CAVEATS
This trial included fewer women than previous studies
The SUSPEND study included a smaller proportion of women than previously published case series due to a need for a diagnostic CT KUB, which excluded more women than men due to radiation concerns. However, the proportion of women was balanced across all groups in this trial, and there was no evidence that sex impacted the efficacy of treatment for the primary outcome.1
CHALLENGES TO IMPLEMENTATION
We see no challenges to the implementation of this recommendation.
References
1. Pickard R, Starr K, MacLennan G, et al. Medical expulsive therapy in adults with ureteric colic: a multicentre, randomised, placebo-controlled trial. Lancet. 2015;386:341-349.
2. Scales CD Jr, Smith AC, Hanley JM, et al. Prevalence of kidney stones in the United States. Eur Urol. 2012;62:160-165.
3. Fwu CW, Eggers PW, Kimmel PL, et al. Emergency department visits, use of imaging, and drugs for urolithiasis have increased in the United States. Kidney Int. 2013;89:479-486.
4. Bagga H, Appa A, Wang R, et al. 2257 medical expulsion therapy is underutilized in women presenting to an emergency department with acute urinary stone disease. J Urol. 2013; 189:e925-e926.
5. Preminger GM, Tiselius HG, Assimos DG, et al; American Urological Association Education and Research, Inc; European Association of Urology. 2007 Guideline for the management of ureteral calculi. Eur Urol. 2007;52:1610-1631.
6. Campschroer T, Zhu Y, Duijvesz D, et al. Alpha-blockers as medical expulsive therapy for ureteral stones. Cochrane Database Syst Rev. 2014;4:CD008509.
7. Seitz C, Liatsikos E, Porpiglia F, et al. Medical therapy to facilitate the passage of stones: what is the evidence? Eur Urol. 2009;56:455-471.
8. Worster A, Preyra I, Weaver B, et al. The accuracy of noncontrast helical computed tomography versus intravenous pyelography in the diagnosis of suspected acute urolithiasis: a meta-analysis. Ann Emerg Med. 2002;40: 280-286.
9. LeLorier J, Gregoire G, Benhaddad A, et al. Discrepancies between meta-analyses and subsequent large randomized, controlled trials. N Engl J Med. 1997;337:536-542.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2016. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2016;65(2):118-120.
PRACTICE CHANGER
Do not prescribe tamsulosin or nifedipine for stone expulsion in patients with ureteral stones that are ≤ 10 mm.1
Strength of recommendation
A: Based on a high-quality randomized controlled trial (RCT).1
Bob Z, age 48, presents to the emergency department (ED) with unspecified groin pain. CT of the kidney, ureter, and bladder (CT KUB) finds evidence of a single ureteral stone measuring 8 mm. He’s prescribed medication for the pain and discharged. The day after his ED visit, he comes to your office to discuss further treatment options. Should you prescribe tamsulosin or nifedipine to help him pass the stone?
The most recent National Health and Nutrition Examination Survey found kidney stones affect 8.8% of the population.2 Outpatient therapy is indicated for patients with ureteric colic secondary to stones ≤ 10 mm who do not have uncontrolled pain, impaired kidney function, or severe infection. Routine outpatient care includes oral hydration, antiemetics, and pain medications.
Medical expulsive therapy (MET) is also used to facilitate stone passage. MET is increasingly becoming part of routine care; use of MET in kidney stone patients in the United States has grown from 14% in 2009 to 64% in 2012.3,4
The joint European Association of Urology/American Urological Association Nephrolithiasis Guideline Panel supports the use of MET.5 Meta-analyses of multiple RCTs suggest that an α-blocker (tamsulosin) or a calcium channel blocker (nifedipine) can reduce pain and lead to quicker stone passage and a higher rate of eventual stone passage when compared to placebo or observation.6,7 However, these reviews included small, heterogeneous studies with a high or unclear risk for bias.
Continue for the study summary >>
STUDY SUMMARY
MET doesn’t increase the rate of stone passage
The SUSPEND (Spontaneous Urinary Stone Passage ENabled by Drugs) trial1 was a multicenter RCT designed to determine the effectiveness of tamsulosin or nifedipine as MET for patients ages 18 to 65 with a single ureteric stone measuring ≤ 10 mm on CT KUB, which has 98% diagnostic accuracy.8 (Stones > 10 mm typically require surgery or lithotripsy.)
In this RCT, 1,167 adults were randomized to take tamsulosin (0.4 mg/d), nifedipine (30 mg/d), or placebo for four weeks or until the stone spontaneously passed, whichever came first. The participants, clinicians, and research staff were blinded to treatment assignment. The primary outcome was the proportion of participants who spontaneously passed their stone, as indicated in patient self-reported questionnaires and case-report forms completed by researchers. Secondary outcomes were time to stone passage and pain as assessed by analgesic use and a visual analogue scale (VAS).
At four weeks, 1,136 (97%) of the randomized participants had data available for analysis. The proportion of participants who passed their stone did not differ between MET and placebo; 80% of the placebo group (303 of 379 participants) passed the stone, compared with 81% (307 of 378) of the tamsulosin group and 80% (304 of 379) of the nifedipine group. The odds ratio (OR) for MET vs placebo was 1.04 (95% confidence interval [CI], 0.77 to 1.43) and the OR for tamsulosin vs nifedipine was 1.07 (95% CI, 0.74 to 1.53). These findings did not change with further subgroup analysis, including by sex, stone size (≤ 5 mm vs > 5 mm), or stone location.
There were no differences between groups in time to stone passage as measured by clinical report and confirmed by imaging. Time to passage of stone was available for 237 (21% of) participants. The mean days to stone passage was 15.9 (n = 84) for placebo, 16.5 (n = 79) for tamsulosin, and 16.2 (n = 74) for nifedipine, with a MET vs placebo difference of 0.5 days (95% CI, –2.9 to 3.9; P = .78). Sensitivity analysis accounting for bias from missing data did not change this outcome.
No differences in analgesic use or pain. Self-reported use of pain medication during the first four weeks was similar between groups: 59% (placebo patients), 56% (tamsulosin), and 56% (nifedipine). The mean days of pain medication use was 10.5 for placebo, 11.6 for tamsulosin, and 10.7 for nifedipine, with a MET vs placebo difference of 0.6 days (95% CI, –1.6 to 2.8; P = .45).
There was no difference between groups in the VAS pain score at four weeks. The MET vs placebo difference was 0.0 (95% CI, –0.4 to 0.4; P = .96) and the mean VAS pain score was 1.2 for placebo, 1.0 for tamsulosin, and 1.3 for nifedipine.
WHAT’S NEW
This large RCT contradicts results from previous meta-analyses
The SUSPEND study is the first large, multicenter RCT of MET with tamsulosin or nifedipine for kidney stones that used patient-oriented outcomes to find no benefit for stone expulsion, analgesic use, or reported pain compared to placebo. The discrepancy with prior meta-analyses is not unusual. Up to one-third of meta-analyses that show positive outcomes of a therapy are subsequently altered by the inclusion of results from a single, large, well-designed, multicenter RCT.9
Continue for caveats >>
CAVEATS
This trial included fewer women than previous studies
The SUSPEND study included a smaller proportion of women than previously published case series due to a need for a diagnostic CT KUB, which excluded more women than men due to radiation concerns. However, the proportion of women was balanced across all groups in this trial, and there was no evidence that sex impacted the efficacy of treatment for the primary outcome.1
CHALLENGES TO IMPLEMENTATION
We see no challenges to the implementation of this recommendation.
References
1. Pickard R, Starr K, MacLennan G, et al. Medical expulsive therapy in adults with ureteric colic: a multicentre, randomised, placebo-controlled trial. Lancet. 2015;386:341-349.
2. Scales CD Jr, Smith AC, Hanley JM, et al. Prevalence of kidney stones in the United States. Eur Urol. 2012;62:160-165.
3. Fwu CW, Eggers PW, Kimmel PL, et al. Emergency department visits, use of imaging, and drugs for urolithiasis have increased in the United States. Kidney Int. 2013;89:479-486.
4. Bagga H, Appa A, Wang R, et al. 2257 medical expulsion therapy is underutilized in women presenting to an emergency department with acute urinary stone disease. J Urol. 2013; 189:e925-e926.
5. Preminger GM, Tiselius HG, Assimos DG, et al; American Urological Association Education and Research, Inc; European Association of Urology. 2007 Guideline for the management of ureteral calculi. Eur Urol. 2007;52:1610-1631.
6. Campschroer T, Zhu Y, Duijvesz D, et al. Alpha-blockers as medical expulsive therapy for ureteral stones. Cochrane Database Syst Rev. 2014;4:CD008509.
7. Seitz C, Liatsikos E, Porpiglia F, et al. Medical therapy to facilitate the passage of stones: what is the evidence? Eur Urol. 2009;56:455-471.
8. Worster A, Preyra I, Weaver B, et al. The accuracy of noncontrast helical computed tomography versus intravenous pyelography in the diagnosis of suspected acute urolithiasis: a meta-analysis. Ann Emerg Med. 2002;40: 280-286.
9. LeLorier J, Gregoire G, Benhaddad A, et al. Discrepancies between meta-analyses and subsequent large randomized, controlled trials. N Engl J Med. 1997;337:536-542.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2016. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2016;65(2):118-120.
Managing endometriosis to prevent ovarian cancer
Endometriosis is a common condition, occurring in this country in 1 of 10 women of reproductive age. An association between endometriosis and subsequent ovarian carcinoma has been reported for decades, yet it is only recently that our knowledge has deepened enough to support more rational methods for preventing the malignancy.
Each year, approximately 22,000 new cases of ovarian cancer are diagnosed. The lifetime risk of developing this malignancy is low, but it is the deadliest of the gynecologic malignancies, with diagnosis usually made in advanced stages when prognosis is poor.
Endometriosis shows some characteristics of malignancy, such as the development of local and distant foci, and attachment to and invasion of other tissues with subsequent damage to these tissues. Endometriosis also is characterized by recurrent, unregulated cell proliferation and estrogen-dependent growth.
Our attempts during the past 2 decades to detect ovarian carcinoma at the early stages through a combined screening modality involving transvaginal ultrasound and a test for the serum level of cancer antigen 125 have failed to provide any survival benefit or even any measurable reduction in morbidity. Today, early-stage ovarian carcinoma, which has a 5-year survival rate of more than 90%, is diagnosed in only a minority of women.
There is good news, however. In recent years our insight into the pathophysiology of ovarian cancer has deepened, providing us with a new paradigm for ovarian cancer pathogenesis that divides ovarian epithelial carcinoma into two distinct types with distinct molecular profiles – one which originates largely in the distal portion of the fallopian tube and the other which traces back to endometriosis.
This new paradigm strengthens and helps to explain the reported association between endometriosis and ovarian cancer. It also has important clinical implications for current practice. While we have much more to learn about the etiology of endometriosis and the causes of malignant transformation, our current knowledge provides a strong rationale for identification and close monitoring of some patients with endometriosis deemed at risk for ovarian cancer, risk-reducing medical management, earlier and more meticulous surgical treatment, and close monitoring.
By combining this new approach to endometriosis with consideration of salpingectomy after completion of childbearing, we have an unprecedented opportunity to reduce the incidence of epithelial ovarian cancer.
Dual pathogenesis
The majority of ovarian cancers are of epithelial origin and fall into four histologic categories: serous, endometrioid, clear cell, and mucinous. In recent years, we have gained a deeper understanding of the pathogenesis of ovarian carcinoma, with an array of epidemiologic, histologic, and molecular data showing us that epithelial ovarian cancers are also of two distinct types (Am J Obstet Gynecol. 2015 Sep;213[3]:262-7).
One of these types, a high-grade serous carcinoma, appears to arise in many cases in the epithelium of the fallopian tube. The other type of tumor is a low-grade carcinoma – particularly of the endometrioid and clear cell histologic subtypes – that originates largely from ovarian endometriotic lesions or from borderline serous tumors in the case of serous histology.
The majority of diagnosed stage 1 ovarian cancers are carcinomas of this low-grade type and not high-grade serous carcinomas. In a study of 76 consecutive stage 1 carcinomas, investigators found that ovarian endometriosis was present in 40 of the 76 cases. More than two-thirds of the 76 cases (71%) were nonserous cancers, and almost all of these cases were associated with endometriosis based on histologic examination (Fertil Steril. 2007 Oct;88[4]:906-10).
This study was among the first to show that the majority of stage 1 ovarian carcinomas are not high-grade serous carcinomas, but rather nonserous, primarily endometrioid and clear cell, cancers. The research demonstrated that endometriosis should be viewed as a potential precursor lesion to specific subtypes of ovarian cancer.
The malignant transformation of endometriosis was first suggested by Dr. J. A. Sampson in 1925, and a number of studies – in addition to the 2007 landmark study – have since described ovarian cancer arising from endometriosis, based on the frequent co-occurrence in surgical specimens.
Most recently, a study from the Ovarian Cancer Association Consortium (OCAC) found that women who reported a history of endometriosis had a significantly higher risk of developing ovarian cancer than the general population (odds ratio, 1.46).
Investigators of this critical study pooled data from 13 ovarian cancer case-control studies involving more than 13,226 controls and 7,911 women with invasive epithelial ovarian cancer – 818 (6.2%) and 738 (9.3%) of whom, respectively, reported a history of endometriosis. Specifically, they determined that self-reported endometriosis was associated with a 3.05-fold increased risk for clear cell invasive ovarian cancer and a 2.04-fold increased risk of endometrioid ovarian cancer.
Moreover, a significant association between preexisting endometriosis and low-grade serous invasive ovarian cancer (OR, 2.11) was demonstrated, while no association was found between endometriosis and the risk of high-grade serous invasive ovarian cancer (Lancet Oncol. 2012 Apr;13[4]:385-94).
A second recently published report – a meta-analysis of 20 case-control and 15 cohort studies published between 1990 and 2012 and involving more than 444,000 patients – found that endometriosis increased cancer risk in case-control or two-arm cohort studies by 27% (relative risk, 1.265) and by approximately 80% in single-arm cohort studies (standard incidence ratio, 1.797). Endometrioid and clear cell carcinomas were more common in endometriosis-associated ovarian cancer, while serous carcinoma was less frequent (Br J Cancer. 2014 Apr 2;110[7]:1878-90).
Findings of both of these large studies have served to clarify the association between endometriosis and specific histologic subtypes and suggested that there are important differences in the pathogenesis of low-grade and high-grade serous ovarian carcinomas.
Clinical implications
It is not clear what causes malignant transformation or what predisposes some patients with endometriosis to develop ovarian cancer, but the risk likely involves genetic and epigenetic influences as well as immunologic, inflammatory, and hormonal factors.
The molecular profiles of the main two types of ovarian cancer are different: While the majority of high-grade serous ovarian tumors are characterized by TP53 mutations, the low-grade carcinomas are characterized by a variety of mutations, including KRAS, BRAF, ERBB2, CTNNB1, and BCL2 mutations.
There currently are not enough data to recommend genetic screening tests in patients with endometriosis, but our hope is that we eventually will be able to screen for “high-risk” endometriotic lesions by testing for genes specific to various histologic subtypes of low-grade ovarian cancer, or by finding and utilizing other biomarkers.
In the meantime, we believe it is important to more thoroughly treat endometriosis and to identify and follow women with a history of the condition, especially those with a long-standing history, those with a history of endometriosis associated with infertility, and those with ovarian endometrioma. Each of these factors predisposes patients to a higher risk of malignant transformation.
Complete surgical resection of all visible endometriosis is the most effective treatment and will afford the best cancer prevention, even in women who are asymptomatic. In a recent Swedish national registry case-control study, women who underwent radical surgical excision of all visible endometriosis were significantly less likely (OR, 0.30) to develop ovarian cancer (Acta Obstet Gynecol Scand. 2013 May;92[5]:546-54).
Suppressive hormonal therapy is another treatment option for patients with no interest in conceiving. Most large endometriomas are functional ovarian cysts that have been invaded by cortical ovarian endometriosis or by small primary endometriomas (J Reprod Med. 1992 Sep;37[9]:771-6).
While hormonal therapy will not always result in complete regression of endometriotic lesions, it will decrease the recurrence rate of endometriomas and can be considered for long-term prevention of potentially premalignant lesions. It is most effective when it follows surgical excision of endometriomas and associated endometriosis.
A patient who has completed childbearing at the time of surgical resection may be offered bilateral salpingectomy, regardless of menopausal status. Salpingectomy in both average and high-risk populations (e.g., BRCA 1/2 carriers) not only prevents high-grade serous carcinoma by eliminating the site of origin, but also may decrease the risk of endometrioid and clear cell carcinoma by blocking the passageway that enables the flow of endometrium and factors that induce inflammation. It is estimated that the procedure reduces the risk of ovarian cancer by 40%.
Interestingly, tubal ligation has historically been shown to decrease the risk of ovarian cancer, and recent data have shown that the risk of endometrioid and clear cell carcinoma is cut even more than the risk of high-grade serous carcinoma (Int J Epidemiol. 2013 Apr;42[2]:579-89).
The Society of Gynecologic Oncology recommends that risk-reducing salpingectomy be considered at the time of hysterectomy or other abdominal or pelvic surgery, and in lieu of tubal ligation. The American College of Obstetricians and Gynecologists similarly has stated that prophylactic salpingectomy may offer clinicians the opportunity to prevent ovarian cancer in their patients. Salpingectomy is an important option for all patients, but is especially important when the fallopian tubes are found to be damaged by endometriosis and/or pelvic inflammatory disease. When imaging studies show that endometriomas are present and resection is not performed, pelvic ultrasound should become part of the patient’s routine examination.
Most endometriomas have a homogeneous appearance; any significant increase in size or a change in the homogeneous cystic characteristics to a more heterogeneous appearance with mural components should raise suspicion about malignant change.
It can be difficult to detect relatively small endocystic components with ultrasound, so if there is any doubt about whether there is some heterogeneous consistency, an MRI should be performed. MRI is showing more promise in detecting malignant change. Hyperdense mural nodules within the ovary and rapid growth of an endometrioma have both been associated with malignant transformation and can be seen on these images.
In a cohort study comparing MRI findings of 10 patients with ovarian adenocarcinoma to 10 patients with benign endometriomas, investigators found mural nodules in all 10 malignancies but in only three of the benign cases (AJR Am J Roentgenol. 2000 Nov;175[5]:1423-30).
Long-term follow-up is necessary to understand the timeline of transformation in patients with mural nodules. This together with increasing knowledge of molecular events underpinning evolution of endometriosis will lead to better screening and preventive strategies.
Dr. Nezhat is the director of minimally invasive gynecologic surgery and robotics at Winthrop University Hospital in Mineola, N.Y., and an adjunct professor of obstetrics, gynecology, and reproductive medicine at the State University of New York at Stony Brook. He reported having no financial disclosures.
Endometriosis is a common condition, occurring in this country in 1 of 10 women of reproductive age. An association between endometriosis and subsequent ovarian carcinoma has been reported for decades, yet it is only recently that our knowledge has deepened enough to support more rational methods for preventing the malignancy.
Each year, approximately 22,000 new cases of ovarian cancer are diagnosed. The lifetime risk of developing this malignancy is low, but it is the deadliest of the gynecologic malignancies, with diagnosis usually made in advanced stages when prognosis is poor.
Endometriosis shows some characteristics of malignancy, such as the development of local and distant foci, and attachment to and invasion of other tissues with subsequent damage to these tissues. Endometriosis also is characterized by recurrent, unregulated cell proliferation and estrogen-dependent growth.
Our attempts during the past 2 decades to detect ovarian carcinoma at the early stages through a combined screening modality involving transvaginal ultrasound and a test for the serum level of cancer antigen 125 have failed to provide any survival benefit or even any measurable reduction in morbidity. Today, early-stage ovarian carcinoma, which has a 5-year survival rate of more than 90%, is diagnosed in only a minority of women.
There is good news, however. In recent years our insight into the pathophysiology of ovarian cancer has deepened, providing us with a new paradigm for ovarian cancer pathogenesis that divides ovarian epithelial carcinoma into two distinct types with distinct molecular profiles – one which originates largely in the distal portion of the fallopian tube and the other which traces back to endometriosis.
This new paradigm strengthens and helps to explain the reported association between endometriosis and ovarian cancer. It also has important clinical implications for current practice. While we have much more to learn about the etiology of endometriosis and the causes of malignant transformation, our current knowledge provides a strong rationale for identification and close monitoring of some patients with endometriosis deemed at risk for ovarian cancer, risk-reducing medical management, earlier and more meticulous surgical treatment, and close monitoring.
By combining this new approach to endometriosis with consideration of salpingectomy after completion of childbearing, we have an unprecedented opportunity to reduce the incidence of epithelial ovarian cancer.
Dual pathogenesis
The majority of ovarian cancers are of epithelial origin and fall into four histologic categories: serous, endometrioid, clear cell, and mucinous. In recent years, we have gained a deeper understanding of the pathogenesis of ovarian carcinoma, with an array of epidemiologic, histologic, and molecular data showing us that epithelial ovarian cancers are also of two distinct types (Am J Obstet Gynecol. 2015 Sep;213[3]:262-7).
One of these types, a high-grade serous carcinoma, appears to arise in many cases in the epithelium of the fallopian tube. The other type of tumor is a low-grade carcinoma – particularly of the endometrioid and clear cell histologic subtypes – that originates largely from ovarian endometriotic lesions or from borderline serous tumors in the case of serous histology.
The majority of diagnosed stage 1 ovarian cancers are carcinomas of this low-grade type and not high-grade serous carcinomas. In a study of 76 consecutive stage 1 carcinomas, investigators found that ovarian endometriosis was present in 40 of the 76 cases. More than two-thirds of the 76 cases (71%) were nonserous cancers, and almost all of these cases were associated with endometriosis based on histologic examination (Fertil Steril. 2007 Oct;88[4]:906-10).
This study was among the first to show that the majority of stage 1 ovarian carcinomas are not high-grade serous carcinomas, but rather nonserous, primarily endometrioid and clear cell, cancers. The research demonstrated that endometriosis should be viewed as a potential precursor lesion to specific subtypes of ovarian cancer.
The malignant transformation of endometriosis was first suggested by Dr. J. A. Sampson in 1925, and a number of studies – in addition to the 2007 landmark study – have since described ovarian cancer arising from endometriosis, based on the frequent co-occurrence in surgical specimens.
Most recently, a study from the Ovarian Cancer Association Consortium (OCAC) found that women who reported a history of endometriosis had a significantly higher risk of developing ovarian cancer than the general population (odds ratio, 1.46).
Investigators of this critical study pooled data from 13 ovarian cancer case-control studies involving more than 13,226 controls and 7,911 women with invasive epithelial ovarian cancer – 818 (6.2%) and 738 (9.3%) of whom, respectively, reported a history of endometriosis. Specifically, they determined that self-reported endometriosis was associated with a 3.05-fold increased risk for clear cell invasive ovarian cancer and a 2.04-fold increased risk of endometrioid ovarian cancer.
Moreover, a significant association between preexisting endometriosis and low-grade serous invasive ovarian cancer (OR, 2.11) was demonstrated, while no association was found between endometriosis and the risk of high-grade serous invasive ovarian cancer (Lancet Oncol. 2012 Apr;13[4]:385-94).
A second recently published report – a meta-analysis of 20 case-control and 15 cohort studies published between 1990 and 2012 and involving more than 444,000 patients – found that endometriosis increased cancer risk in case-control or two-arm cohort studies by 27% (relative risk, 1.265) and by approximately 80% in single-arm cohort studies (standard incidence ratio, 1.797). Endometrioid and clear cell carcinomas were more common in endometriosis-associated ovarian cancer, while serous carcinoma was less frequent (Br J Cancer. 2014 Apr 2;110[7]:1878-90).
Findings of both of these large studies have served to clarify the association between endometriosis and specific histologic subtypes and suggested that there are important differences in the pathogenesis of low-grade and high-grade serous ovarian carcinomas.
Clinical implications
It is not clear what causes malignant transformation or what predisposes some patients with endometriosis to develop ovarian cancer, but the risk likely involves genetic and epigenetic influences as well as immunologic, inflammatory, and hormonal factors.
The molecular profiles of the main two types of ovarian cancer are different: While the majority of high-grade serous ovarian tumors are characterized by TP53 mutations, the low-grade carcinomas are characterized by a variety of mutations, including KRAS, BRAF, ERBB2, CTNNB1, and BCL2 mutations.
There currently are not enough data to recommend genetic screening tests in patients with endometriosis, but our hope is that we eventually will be able to screen for “high-risk” endometriotic lesions by testing for genes specific to various histologic subtypes of low-grade ovarian cancer, or by finding and utilizing other biomarkers.
In the meantime, we believe it is important to more thoroughly treat endometriosis and to identify and follow women with a history of the condition, especially those with a long-standing history, those with a history of endometriosis associated with infertility, and those with ovarian endometrioma. Each of these factors predisposes patients to a higher risk of malignant transformation.
Complete surgical resection of all visible endometriosis is the most effective treatment and will afford the best cancer prevention, even in women who are asymptomatic. In a recent Swedish national registry case-control study, women who underwent radical surgical excision of all visible endometriosis were significantly less likely (OR, 0.30) to develop ovarian cancer (Acta Obstet Gynecol Scand. 2013 May;92[5]:546-54).
Suppressive hormonal therapy is another treatment option for patients with no interest in conceiving. Most large endometriomas are functional ovarian cysts that have been invaded by cortical ovarian endometriosis or by small primary endometriomas (J Reprod Med. 1992 Sep;37[9]:771-6).
While hormonal therapy will not always result in complete regression of endometriotic lesions, it will decrease the recurrence rate of endometriomas and can be considered for long-term prevention of potentially premalignant lesions. It is most effective when it follows surgical excision of endometriomas and associated endometriosis.
A patient who has completed childbearing at the time of surgical resection may be offered bilateral salpingectomy, regardless of menopausal status. Salpingectomy in both average and high-risk populations (e.g., BRCA 1/2 carriers) not only prevents high-grade serous carcinoma by eliminating the site of origin, but also may decrease the risk of endometrioid and clear cell carcinoma by blocking the passageway that enables the flow of endometrium and factors that induce inflammation. It is estimated that the procedure reduces the risk of ovarian cancer by 40%.
Interestingly, tubal ligation has historically been shown to decrease the risk of ovarian cancer, and recent data have shown that the risk of endometrioid and clear cell carcinoma is cut even more than the risk of high-grade serous carcinoma (Int J Epidemiol. 2013 Apr;42[2]:579-89).
The Society of Gynecologic Oncology recommends that risk-reducing salpingectomy be considered at the time of hysterectomy or other abdominal or pelvic surgery, and in lieu of tubal ligation. The American College of Obstetricians and Gynecologists similarly has stated that prophylactic salpingectomy may offer clinicians the opportunity to prevent ovarian cancer in their patients. Salpingectomy is an important option for all patients, but is especially important when the fallopian tubes are found to be damaged by endometriosis and/or pelvic inflammatory disease. When imaging studies show that endometriomas are present and resection is not performed, pelvic ultrasound should become part of the patient’s routine examination.
Most endometriomas have a homogeneous appearance; any significant increase in size or a change in the homogeneous cystic characteristics to a more heterogeneous appearance with mural components should raise suspicion about malignant change.
It can be difficult to detect relatively small endocystic components with ultrasound, so if there is any doubt about whether there is some heterogeneous consistency, an MRI should be performed. MRI is showing more promise in detecting malignant change. Hyperdense mural nodules within the ovary and rapid growth of an endometrioma have both been associated with malignant transformation and can be seen on these images.
In a cohort study comparing MRI findings of 10 patients with ovarian adenocarcinoma to 10 patients with benign endometriomas, investigators found mural nodules in all 10 malignancies but in only three of the benign cases (AJR Am J Roentgenol. 2000 Nov;175[5]:1423-30).
Long-term follow-up is necessary to understand the timeline of transformation in patients with mural nodules. This together with increasing knowledge of molecular events underpinning evolution of endometriosis will lead to better screening and preventive strategies.
Dr. Nezhat is the director of minimally invasive gynecologic surgery and robotics at Winthrop University Hospital in Mineola, N.Y., and an adjunct professor of obstetrics, gynecology, and reproductive medicine at the State University of New York at Stony Brook. He reported having no financial disclosures.
Endometriosis is a common condition, occurring in this country in 1 of 10 women of reproductive age. An association between endometriosis and subsequent ovarian carcinoma has been reported for decades, yet it is only recently that our knowledge has deepened enough to support more rational methods for preventing the malignancy.
Each year, approximately 22,000 new cases of ovarian cancer are diagnosed. The lifetime risk of developing this malignancy is low, but it is the deadliest of the gynecologic malignancies, with diagnosis usually made in advanced stages when prognosis is poor.
Endometriosis shows some characteristics of malignancy, such as the development of local and distant foci, and attachment to and invasion of other tissues with subsequent damage to these tissues. Endometriosis also is characterized by recurrent, unregulated cell proliferation and estrogen-dependent growth.
Our attempts during the past 2 decades to detect ovarian carcinoma at the early stages through a combined screening modality involving transvaginal ultrasound and a test for the serum level of cancer antigen 125 have failed to provide any survival benefit or even any measurable reduction in morbidity. Today, early-stage ovarian carcinoma, which has a 5-year survival rate of more than 90%, is diagnosed in only a minority of women.
There is good news, however. In recent years our insight into the pathophysiology of ovarian cancer has deepened, providing us with a new paradigm for ovarian cancer pathogenesis that divides ovarian epithelial carcinoma into two distinct types with distinct molecular profiles – one which originates largely in the distal portion of the fallopian tube and the other which traces back to endometriosis.
This new paradigm strengthens and helps to explain the reported association between endometriosis and ovarian cancer. It also has important clinical implications for current practice. While we have much more to learn about the etiology of endometriosis and the causes of malignant transformation, our current knowledge provides a strong rationale for identification and close monitoring of some patients with endometriosis deemed at risk for ovarian cancer, risk-reducing medical management, earlier and more meticulous surgical treatment, and close monitoring.
By combining this new approach to endometriosis with consideration of salpingectomy after completion of childbearing, we have an unprecedented opportunity to reduce the incidence of epithelial ovarian cancer.
Dual pathogenesis
The majority of ovarian cancers are of epithelial origin and fall into four histologic categories: serous, endometrioid, clear cell, and mucinous. In recent years, we have gained a deeper understanding of the pathogenesis of ovarian carcinoma, with an array of epidemiologic, histologic, and molecular data showing us that epithelial ovarian cancers are also of two distinct types (Am J Obstet Gynecol. 2015 Sep;213[3]:262-7).
One of these types, a high-grade serous carcinoma, appears to arise in many cases in the epithelium of the fallopian tube. The other type of tumor is a low-grade carcinoma – particularly of the endometrioid and clear cell histologic subtypes – that originates largely from ovarian endometriotic lesions or from borderline serous tumors in the case of serous histology.
The majority of diagnosed stage 1 ovarian cancers are carcinomas of this low-grade type and not high-grade serous carcinomas. In a study of 76 consecutive stage 1 carcinomas, investigators found that ovarian endometriosis was present in 40 of the 76 cases. More than two-thirds of the 76 cases (71%) were nonserous cancers, and almost all of these cases were associated with endometriosis based on histologic examination (Fertil Steril. 2007 Oct;88[4]:906-10).
This study was among the first to show that the majority of stage 1 ovarian carcinomas are not high-grade serous carcinomas, but rather nonserous, primarily endometrioid and clear cell, cancers. The research demonstrated that endometriosis should be viewed as a potential precursor lesion to specific subtypes of ovarian cancer.
The malignant transformation of endometriosis was first suggested by Dr. J. A. Sampson in 1925, and a number of studies – in addition to the 2007 landmark study – have since described ovarian cancer arising from endometriosis, based on the frequent co-occurrence in surgical specimens.
Most recently, a study from the Ovarian Cancer Association Consortium (OCAC) found that women who reported a history of endometriosis had a significantly higher risk of developing ovarian cancer than the general population (odds ratio, 1.46).
Investigators of this critical study pooled data from 13 ovarian cancer case-control studies involving more than 13,226 controls and 7,911 women with invasive epithelial ovarian cancer – 818 (6.2%) and 738 (9.3%) of whom, respectively, reported a history of endometriosis. Specifically, they determined that self-reported endometriosis was associated with a 3.05-fold increased risk for clear cell invasive ovarian cancer and a 2.04-fold increased risk of endometrioid ovarian cancer.
Moreover, a significant association between preexisting endometriosis and low-grade serous invasive ovarian cancer (OR, 2.11) was demonstrated, while no association was found between endometriosis and the risk of high-grade serous invasive ovarian cancer (Lancet Oncol. 2012 Apr;13[4]:385-94).
A second recently published report – a meta-analysis of 20 case-control and 15 cohort studies published between 1990 and 2012 and involving more than 444,000 patients – found that endometriosis increased cancer risk in case-control or two-arm cohort studies by 27% (relative risk, 1.265) and by approximately 80% in single-arm cohort studies (standard incidence ratio, 1.797). Endometrioid and clear cell carcinomas were more common in endometriosis-associated ovarian cancer, while serous carcinoma was less frequent (Br J Cancer. 2014 Apr 2;110[7]:1878-90).
Findings of both of these large studies have served to clarify the association between endometriosis and specific histologic subtypes and suggested that there are important differences in the pathogenesis of low-grade and high-grade serous ovarian carcinomas.
Clinical implications
It is not clear what causes malignant transformation or what predisposes some patients with endometriosis to develop ovarian cancer, but the risk likely involves genetic and epigenetic influences as well as immunologic, inflammatory, and hormonal factors.
The molecular profiles of the main two types of ovarian cancer are different: While the majority of high-grade serous ovarian tumors are characterized by TP53 mutations, the low-grade carcinomas are characterized by a variety of mutations, including KRAS, BRAF, ERBB2, CTNNB1, and BCL2 mutations.
There currently are not enough data to recommend genetic screening tests in patients with endometriosis, but our hope is that we eventually will be able to screen for “high-risk” endometriotic lesions by testing for genes specific to various histologic subtypes of low-grade ovarian cancer, or by finding and utilizing other biomarkers.
In the meantime, we believe it is important to more thoroughly treat endometriosis and to identify and follow women with a history of the condition, especially those with a long-standing history, those with a history of endometriosis associated with infertility, and those with ovarian endometrioma. Each of these factors predisposes patients to a higher risk of malignant transformation.
Complete surgical resection of all visible endometriosis is the most effective treatment and will afford the best cancer prevention, even in women who are asymptomatic. In a recent Swedish national registry case-control study, women who underwent radical surgical excision of all visible endometriosis were significantly less likely (OR, 0.30) to develop ovarian cancer (Acta Obstet Gynecol Scand. 2013 May;92[5]:546-54).
Suppressive hormonal therapy is another treatment option for patients with no interest in conceiving. Most large endometriomas are functional ovarian cysts that have been invaded by cortical ovarian endometriosis or by small primary endometriomas (J Reprod Med. 1992 Sep;37[9]:771-6).
While hormonal therapy will not always result in complete regression of endometriotic lesions, it will decrease the recurrence rate of endometriomas and can be considered for long-term prevention of potentially premalignant lesions. It is most effective when it follows surgical excision of endometriomas and associated endometriosis.
A patient who has completed childbearing at the time of surgical resection may be offered bilateral salpingectomy, regardless of menopausal status. Salpingectomy in both average and high-risk populations (e.g., BRCA 1/2 carriers) not only prevents high-grade serous carcinoma by eliminating the site of origin, but also may decrease the risk of endometrioid and clear cell carcinoma by blocking the passageway that enables the flow of endometrium and factors that induce inflammation. It is estimated that the procedure reduces the risk of ovarian cancer by 40%.
Interestingly, tubal ligation has historically been shown to decrease the risk of ovarian cancer, and recent data have shown that the risk of endometrioid and clear cell carcinoma is cut even more than the risk of high-grade serous carcinoma (Int J Epidemiol. 2013 Apr;42[2]:579-89).
The Society of Gynecologic Oncology recommends that risk-reducing salpingectomy be considered at the time of hysterectomy or other abdominal or pelvic surgery, and in lieu of tubal ligation. The American College of Obstetricians and Gynecologists similarly has stated that prophylactic salpingectomy may offer clinicians the opportunity to prevent ovarian cancer in their patients. Salpingectomy is an important option for all patients, but is especially important when the fallopian tubes are found to be damaged by endometriosis and/or pelvic inflammatory disease. When imaging studies show that endometriomas are present and resection is not performed, pelvic ultrasound should become part of the patient’s routine examination.
Most endometriomas have a homogeneous appearance; any significant increase in size or a change in the homogeneous cystic characteristics to a more heterogeneous appearance with mural components should raise suspicion about malignant change.
It can be difficult to detect relatively small endocystic components with ultrasound, so if there is any doubt about whether there is some heterogeneous consistency, an MRI should be performed. MRI is showing more promise in detecting malignant change. Hyperdense mural nodules within the ovary and rapid growth of an endometrioma have both been associated with malignant transformation and can be seen on these images.
In a cohort study comparing MRI findings of 10 patients with ovarian adenocarcinoma to 10 patients with benign endometriomas, investigators found mural nodules in all 10 malignancies but in only three of the benign cases (AJR Am J Roentgenol. 2000 Nov;175[5]:1423-30).
Long-term follow-up is necessary to understand the timeline of transformation in patients with mural nodules. This together with increasing knowledge of molecular events underpinning evolution of endometriosis will lead to better screening and preventive strategies.
Dr. Nezhat is the director of minimally invasive gynecologic surgery and robotics at Winthrop University Hospital in Mineola, N.Y., and an adjunct professor of obstetrics, gynecology, and reproductive medicine at the State University of New York at Stony Brook. He reported having no financial disclosures.