User login
Lenalidomide, thalidomide in melphalan regimen yields similar progression-free survival
Swapping lenalidomide for thalidomide in a standard regimen for transplant-ineligible patients with untreated multiple myeloma did not improve efficacy, but the toxicity profile may favor the use of lenalidomide in a maintenance regimen, results of a randomized trial suggest.
Among patients with previously untreated multiple myeloma who were not eligible for autologous stem cell transplant, neither median progression-free survival (PFS) nor overall survival (OS) were significantly different for patients treated with either melphalan, prednisone, and thalidomide (MPT-T) followed by thalidomide maintenance, or with the same regimen with lenalidomide (Revlimid) substituted for thalidomide (MPR-R), reported Dr. Sonja Zweegman of Vrije University Medical Center in Amsterdam.
“MPR-R has no advantage over MPT-T with respect to response rate, PFS, and OS. However, the use of thalidomide as maintenance therapy was associated with a high rate of clinically significant neuropathy and is therefore not preferred for maintenance strategies,” they wrote (Blood 2016;127[9];1109-16).
The investigators randomly assigned 637 transplant-ineligible patients with newly-diagnosed multiple myeloma to receive nine 4-week cycles of either MPT-T (318 patients) or MPR-R (319 patients). At 36 months’ median follow-up, median PFS, the primary endpoint, was 20 months for patients treated with MPT-T, compared with 23 months for those treated with MPR-R. This translated into a hazard ratio (HR) of 0.87, P = .12).
The overall response rates were 81% for MPT-T and 84% for MPR-R. Very good partial responses or better were seen in 47% and 45%, of patients, respectively. The complete response rate with MPT-T was 10%, compared with 13% for MPR-R. Median time to response and time to maximum response were similar between the arms.
OS at 2, 3, and 4 years in the MPT-T and MPR-R arms was 73% vs. 84%, 64% vs 69%, and 52% vs. 56%, respectively. These differences were not statistically significant.
The proportion of patients with one or more grade 3 or 4 adverse events was 81% with thalidomide and 86% with lenalidomide.
The investigators noted, however, that there was a high rate of discontinuation during induction therapy in each arm, with 49% of those starting on MPT-T and 41% of those starting on MPR-R halting therapy. Most of the patients who discontinued were older than age 75. Early treatment deaths (within three cycles) occurred in 13 patients on MPT-T, and 8 on MPR-R.
Among patients who started on maintenance therapy, significantly more patients on thalidomide had to discontinue thalidomide than did patients who started on lenalidomide maintenance (60% vs, 17%, P = .017).
The primary reason for the higher rate of discontinuation of MPT-T maintenance was neuropathy, which occurred in 87% of the discontinuations in this study arm, compared with 3% of those in the lenalidomide arm. Neuropathy of at least grade 3 was 16% in the MPT-T arm vs. 2% in MPR-R, resulting in a significantly shorter duration of maintenance therapy (5 vs. 17 months in MPR-R), irrespective of age.
Hematologic toxicities were higher in the MPR-R group, especially grades 3 and 4 neutropenia (64% vs 27%), but this did not translate into a higher clinical infection rate, and the toxicities were manageable in older patients, the investigators reported.
Swapping lenalidomide for thalidomide in a standard regimen for transplant-ineligible patients with untreated multiple myeloma did not improve efficacy, but the toxicity profile may favor the use of lenalidomide in a maintenance regimen, results of a randomized trial suggest.
Among patients with previously untreated multiple myeloma who were not eligible for autologous stem cell transplant, neither median progression-free survival (PFS) nor overall survival (OS) were significantly different for patients treated with either melphalan, prednisone, and thalidomide (MPT-T) followed by thalidomide maintenance, or with the same regimen with lenalidomide (Revlimid) substituted for thalidomide (MPR-R), reported Dr. Sonja Zweegman of Vrije University Medical Center in Amsterdam.
“MPR-R has no advantage over MPT-T with respect to response rate, PFS, and OS. However, the use of thalidomide as maintenance therapy was associated with a high rate of clinically significant neuropathy and is therefore not preferred for maintenance strategies,” they wrote (Blood 2016;127[9];1109-16).
The investigators randomly assigned 637 transplant-ineligible patients with newly-diagnosed multiple myeloma to receive nine 4-week cycles of either MPT-T (318 patients) or MPR-R (319 patients). At 36 months’ median follow-up, median PFS, the primary endpoint, was 20 months for patients treated with MPT-T, compared with 23 months for those treated with MPR-R. This translated into a hazard ratio (HR) of 0.87, P = .12).
The overall response rates were 81% for MPT-T and 84% for MPR-R. Very good partial responses or better were seen in 47% and 45%, of patients, respectively. The complete response rate with MPT-T was 10%, compared with 13% for MPR-R. Median time to response and time to maximum response were similar between the arms.
OS at 2, 3, and 4 years in the MPT-T and MPR-R arms was 73% vs. 84%, 64% vs 69%, and 52% vs. 56%, respectively. These differences were not statistically significant.
The proportion of patients with one or more grade 3 or 4 adverse events was 81% with thalidomide and 86% with lenalidomide.
The investigators noted, however, that there was a high rate of discontinuation during induction therapy in each arm, with 49% of those starting on MPT-T and 41% of those starting on MPR-R halting therapy. Most of the patients who discontinued were older than age 75. Early treatment deaths (within three cycles) occurred in 13 patients on MPT-T, and 8 on MPR-R.
Among patients who started on maintenance therapy, significantly more patients on thalidomide had to discontinue thalidomide than did patients who started on lenalidomide maintenance (60% vs, 17%, P = .017).
The primary reason for the higher rate of discontinuation of MPT-T maintenance was neuropathy, which occurred in 87% of the discontinuations in this study arm, compared with 3% of those in the lenalidomide arm. Neuropathy of at least grade 3 was 16% in the MPT-T arm vs. 2% in MPR-R, resulting in a significantly shorter duration of maintenance therapy (5 vs. 17 months in MPR-R), irrespective of age.
Hematologic toxicities were higher in the MPR-R group, especially grades 3 and 4 neutropenia (64% vs 27%), but this did not translate into a higher clinical infection rate, and the toxicities were manageable in older patients, the investigators reported.
Swapping lenalidomide for thalidomide in a standard regimen for transplant-ineligible patients with untreated multiple myeloma did not improve efficacy, but the toxicity profile may favor the use of lenalidomide in a maintenance regimen, results of a randomized trial suggest.
Among patients with previously untreated multiple myeloma who were not eligible for autologous stem cell transplant, neither median progression-free survival (PFS) nor overall survival (OS) were significantly different for patients treated with either melphalan, prednisone, and thalidomide (MPT-T) followed by thalidomide maintenance, or with the same regimen with lenalidomide (Revlimid) substituted for thalidomide (MPR-R), reported Dr. Sonja Zweegman of Vrije University Medical Center in Amsterdam.
“MPR-R has no advantage over MPT-T with respect to response rate, PFS, and OS. However, the use of thalidomide as maintenance therapy was associated with a high rate of clinically significant neuropathy and is therefore not preferred for maintenance strategies,” they wrote (Blood 2016;127[9];1109-16).
The investigators randomly assigned 637 transplant-ineligible patients with newly-diagnosed multiple myeloma to receive nine 4-week cycles of either MPT-T (318 patients) or MPR-R (319 patients). At 36 months’ median follow-up, median PFS, the primary endpoint, was 20 months for patients treated with MPT-T, compared with 23 months for those treated with MPR-R. This translated into a hazard ratio (HR) of 0.87, P = .12).
The overall response rates were 81% for MPT-T and 84% for MPR-R. Very good partial responses or better were seen in 47% and 45%, of patients, respectively. The complete response rate with MPT-T was 10%, compared with 13% for MPR-R. Median time to response and time to maximum response were similar between the arms.
OS at 2, 3, and 4 years in the MPT-T and MPR-R arms was 73% vs. 84%, 64% vs 69%, and 52% vs. 56%, respectively. These differences were not statistically significant.
The proportion of patients with one or more grade 3 or 4 adverse events was 81% with thalidomide and 86% with lenalidomide.
The investigators noted, however, that there was a high rate of discontinuation during induction therapy in each arm, with 49% of those starting on MPT-T and 41% of those starting on MPR-R halting therapy. Most of the patients who discontinued were older than age 75. Early treatment deaths (within three cycles) occurred in 13 patients on MPT-T, and 8 on MPR-R.
Among patients who started on maintenance therapy, significantly more patients on thalidomide had to discontinue thalidomide than did patients who started on lenalidomide maintenance (60% vs, 17%, P = .017).
The primary reason for the higher rate of discontinuation of MPT-T maintenance was neuropathy, which occurred in 87% of the discontinuations in this study arm, compared with 3% of those in the lenalidomide arm. Neuropathy of at least grade 3 was 16% in the MPT-T arm vs. 2% in MPR-R, resulting in a significantly shorter duration of maintenance therapy (5 vs. 17 months in MPR-R), irrespective of age.
Hematologic toxicities were higher in the MPR-R group, especially grades 3 and 4 neutropenia (64% vs 27%), but this did not translate into a higher clinical infection rate, and the toxicities were manageable in older patients, the investigators reported.
FROM BLOOD
Key clinical point: Lenalidomide or thalidomide added to a melphalan backbone regimen yielded similar outcomes in transplant-ineligible patients with newly diagnosed multiple myeloma.
Major finding: At 36 months median follow-up, median PFS was 20 months for patients treated with thalidomide, compared with 23 months for those treated with lenalidomide.
Data source: Randomized controlled trial of 637 patients with multiple myeloma who were not candidates for autologous stem cell transplantation.
Disclosures: The Dutch Cancer Society, Norwegian Cancer Society, and Celgene supported the study. Dr. Zweegman and other colleagues reported financial relationships with Celgene.
MACRA Provides New Direction for U.S. Healthcare
Last year, Congress passed legislation to permanently eliminate the Sustainable Growth Rate (SGR) formula, created in 1997 and designed to hold Medicare Part B or outpatient spending under control. Allowing the SGR to go into effect would have severely cut physician reimbursements in recent years, but Congress passed legislation each year to temporarily avert these cuts (also known annually as the “doc fix”). In search of a permanent solution, the passage of bipartisan legislation permanently repealing the SGR in 2015 was hailed as a way to ensure more certainty around the future of Medicare payments for physicians.
This legislation (H.R. 2, 114th Congress), sponsored by Rep. Michael C. Burgess (R-Texas) and entitled “Medicare Access and CHIP Reauthorization Act of 2015,” or MACRA, does much more than simply remove the SGR’s threat of broader Medicare payment cuts. The law changes the ways physicians are reimbursed by Medicare and continues to shift our healthcare system away from volume-based reimbursements and toward a value-based payment system.
What Is MIPS?
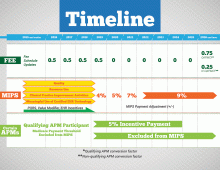
MACRA creates two value-based payment tracks for physicians. The first, the Merit-Based Incentive Payment System (MIPS), is closer to the old fee-for-service model of reimbursement. However, MIPS takes into account both volume and quality (i.e., payment is adjusted based on physician-quality scores). These physician-specific scores broaden the scope of quality measurement by including new measures related to resource utilization, electronic health record (EHR) use, and clinical improvement practices, along with the traditional clinical quality markers.
Under MIPS, the current Physician Quality Reporting System (PQRS), EHR Incentive Program, and Physician Value-Based Modifier all will be integrated into this single-payment adjustment.
The range of potential payment adjustments based on a physician’s MIPS score grows each year through 2022 (in 2022, adjustments can range from +9% to -9%). The program is budget neutral, which means that increases in payments to high-scoring providers will be offset by decreases in payments to low-scoring providers. For 2019 to 2024, there also will be an additional payment adjustment given to the highest MIPS performers for exceptional performance.
A benefit of MIPS is that it will streamline the various quality-reporting programs currently in place into one single program and does not ask physicians to assume any additional financial risk related to outcomes when taking care of patients. However, the particulars of how the MIPS score will be calculated are yet to be determined, and much of the utility and palatability of this score will depend on the chosen metrics. The goal of these metrics should be that they are meaningful, valid, and attributable to specific providers.
What Are APMs?
The other payment option MACRA provides for physicians allows them to opt out of MIPS and participate in the Alternative Payment Models (APMs) track. To incentivize physicians to take part in this riskier track, providers taking part in APMs will receive some extra money for their participation: a 5% annual lump sum bonus on reimbursement payments. To clarify, qualifying APMs are those where providers take on “more than nominal” financial risk, report on their quality measures, and use certified EHR technology.
To qualify as a participant in an APM (for example, the Medicare Shared Savings Program), providers must hit a threshold for percentage of total revenue received or percentage of patients from qualifying APMs. This threshold will increase over time. For example, from 2019 to 2020, providers must obtain at least 25% of their Medicare revenue or patients via APMs, whereas in 2023, 75% of their Medicare revenue or \ patients will need to come from APMs.
Providers will benefit from the increased reimbursement offered if they participate in APMs. There also is funding allocated in MACRA to help develop quality measures, with a call for physician leads to develop quality standards. This payment model, however, does come with increased financial risk for the provider contingent on patient outcomes. In addition, it may be difficult for all providers to hit the thresholds for participation.
Stick with MIPS? Or Take the Plunge with APM?
How MACRA affects you will depend a lot on the practice environment. As described above, MACRA is designed to move physicians into risk-based payment structures if possible. If possible, or otherwise, to simplify the current fee-for-service mechanism of payment by consolidating various Medicare pay-for-performance programs.
Let’s look at a few scenarios:
Hospitalist A works for a physician group that assumes risk for patients in a MACRA-approved APM and sees only those inpatients as opposed to unassigned patients. Therefore, almost all of hospitalist A’s patients are covered by risk-based contracts, and hospitalist A might be well positioned for the new APM structure.
Hospitalist B works for a group, or a university, and sees whatever patients are admitted to the hospital. Hospitalist B’s eligibility to participate in the APM will depend on the percentage of patients in alternative payment models in their market. If hospitalist B’s market has many Medicare accountable care organizations, and Medicaid and the commercial insurers compensate through a risk-sharing model, hospitalist B might reach the threshold. This is more accidental than planned, however, and hospitalist B might not be able to consistently hit this threshold year after year.
In addition, just working within the model will probably not be enough to qualify. Hospitalist B will need to also take on “more than nominal risk” as a participant in the model. In an employed academic setting, where the hospital is taking on risk as part of an APM, it is unlikely hospitalist B will qualify just by virtue of hospital employment. Hospitalist B must also meet/exceed the patient or payment thresholds under the model.
Bottom line: Given the current situation, we expect many hospitalists will likely be required to participate in MIPS and not qualify for APMs. Understanding the details and expectations now will help them be successful in the future.
Is MACRA Good for Hospitalists?
Most of organized medicine is happy to be free from the annual threat of reimbursement cuts. In addition, the new law might streamline quality reporting. But the specific upside depends on your perspective.
With APMs, a hospitalist might enjoy more upside potential, particularly for high-quality work and EHR use. However, whether it is realistic for most hospitalists to even participate in the model depends on many factors, as described previously, and SHM is advocating for the law to be implemented in ways that will more readily accommodate hospitalist practice and employment structures.
For example, the SHM Public Policy Committee has provided the Centers for Medicare & Medicaid Services (CMS) with realistic options for implementing the APM framework that would allow hospitalist B in the above example to qualify as an APM participant.
With MIPS, the benefit to hospitalists depends a fair amount on the way the law is implemented: how quality reporting happens, what metrics will count as quality improvement efforts, and how utilization of EHRs is measured.
What Issues Should Hospitalists Be Aware Of?
As MACRA is further developed, the main issue for hospitalists will be to ensure fairness in assessing quality and incentive payments. As previously encountered with quality reporting, hospitalists are not differentiated clearly from outpatient providers. As a result, they could suffer from the comparison of their quality outcomes for their sicker hospitalized patients to the patients cared for in a typical primary-care internal medicine practice. This inaccurate comparison poses problems in both models.
A potential solution would be a hospitalist-specific billing code, which would make it easier to identify hospitalists. SHM applied for and advocated for the approval of such a billing code and the request was recently approved by CMS.
In addition, as hospitalists mostly work in groups with shift-based schedules, thus sharing care of patients, individual identifiers may not be as significant as possibly looking at hospital, system, or team-based metrics. Using facility performance measures for both clinical quality and performance improvement—where hospitalists can opt to align with their hospital, which is already reporting quality outcomes—might be one way out of this conundrum. It would take into account the type of facility-level quality improvement work many hospitalists participate in. This also would decrease reporting burden for hospitalist groups.
SHM has advocated for this solution and was able to ensure this concept was included in the law; however, it is unclear when or how CMS will implement it.
To summarize, looking good in quality reporting will continue to be a challenge for hospitalists. It will be critical to keep pressure on CMS to implement solutions that account for the unique situation of our specialty.
Another issue to be aware of is the ability of hospitalists to participate in APMs. As with other facility-based providers, hospitalists have little control over whether their facility participates in an APM. Ways to ensure hospitalists can reach thresholds for participation could include allowing the various APMs that hospitalist patients are aligned with count toward an individual hospitalists’ APM participation total—a solution that SHM is advocating for Medicare to include in the APM framework.
What’s Next?
Much remains to be solidified regarding implementation of MACRA, despite the fact it goes live in a few short years (see Figure 1). CMS has asked for comments and stakeholder input regarding MIPS and APMs, and it will be releasing the first round of rules around MACRA this year.
SHM is actively working with CMS to ensure this legislation will reflect the work we are doing as hospitalists to provide high-quality clinical care for our patients and enhance the performance of our hospitals and health system. TH
Dr. Doctoroff is a hospitalist at Beth Israel Deaconess Medical Center and an instructor of medicine at Harvard Medical School in Boston. Dr. Dutta is a hospitalist at Rush University Medical Center and an assistant professor of medicine at Rush Medical College in Chicago. Both are members of the SHM Public Policy Committee.
Last year, Congress passed legislation to permanently eliminate the Sustainable Growth Rate (SGR) formula, created in 1997 and designed to hold Medicare Part B or outpatient spending under control. Allowing the SGR to go into effect would have severely cut physician reimbursements in recent years, but Congress passed legislation each year to temporarily avert these cuts (also known annually as the “doc fix”). In search of a permanent solution, the passage of bipartisan legislation permanently repealing the SGR in 2015 was hailed as a way to ensure more certainty around the future of Medicare payments for physicians.
This legislation (H.R. 2, 114th Congress), sponsored by Rep. Michael C. Burgess (R-Texas) and entitled “Medicare Access and CHIP Reauthorization Act of 2015,” or MACRA, does much more than simply remove the SGR’s threat of broader Medicare payment cuts. The law changes the ways physicians are reimbursed by Medicare and continues to shift our healthcare system away from volume-based reimbursements and toward a value-based payment system.
What Is MIPS?
MACRA creates two value-based payment tracks for physicians. The first, the Merit-Based Incentive Payment System (MIPS), is closer to the old fee-for-service model of reimbursement. However, MIPS takes into account both volume and quality (i.e., payment is adjusted based on physician-quality scores). These physician-specific scores broaden the scope of quality measurement by including new measures related to resource utilization, electronic health record (EHR) use, and clinical improvement practices, along with the traditional clinical quality markers.
Under MIPS, the current Physician Quality Reporting System (PQRS), EHR Incentive Program, and Physician Value-Based Modifier all will be integrated into this single-payment adjustment.
The range of potential payment adjustments based on a physician’s MIPS score grows each year through 2022 (in 2022, adjustments can range from +9% to -9%). The program is budget neutral, which means that increases in payments to high-scoring providers will be offset by decreases in payments to low-scoring providers. For 2019 to 2024, there also will be an additional payment adjustment given to the highest MIPS performers for exceptional performance.
A benefit of MIPS is that it will streamline the various quality-reporting programs currently in place into one single program and does not ask physicians to assume any additional financial risk related to outcomes when taking care of patients. However, the particulars of how the MIPS score will be calculated are yet to be determined, and much of the utility and palatability of this score will depend on the chosen metrics. The goal of these metrics should be that they are meaningful, valid, and attributable to specific providers.
What Are APMs?
The other payment option MACRA provides for physicians allows them to opt out of MIPS and participate in the Alternative Payment Models (APMs) track. To incentivize physicians to take part in this riskier track, providers taking part in APMs will receive some extra money for their participation: a 5% annual lump sum bonus on reimbursement payments. To clarify, qualifying APMs are those where providers take on “more than nominal” financial risk, report on their quality measures, and use certified EHR technology.
To qualify as a participant in an APM (for example, the Medicare Shared Savings Program), providers must hit a threshold for percentage of total revenue received or percentage of patients from qualifying APMs. This threshold will increase over time. For example, from 2019 to 2020, providers must obtain at least 25% of their Medicare revenue or patients via APMs, whereas in 2023, 75% of their Medicare revenue or \ patients will need to come from APMs.
Providers will benefit from the increased reimbursement offered if they participate in APMs. There also is funding allocated in MACRA to help develop quality measures, with a call for physician leads to develop quality standards. This payment model, however, does come with increased financial risk for the provider contingent on patient outcomes. In addition, it may be difficult for all providers to hit the thresholds for participation.
Stick with MIPS? Or Take the Plunge with APM?
How MACRA affects you will depend a lot on the practice environment. As described above, MACRA is designed to move physicians into risk-based payment structures if possible. If possible, or otherwise, to simplify the current fee-for-service mechanism of payment by consolidating various Medicare pay-for-performance programs.
Let’s look at a few scenarios:
Hospitalist A works for a physician group that assumes risk for patients in a MACRA-approved APM and sees only those inpatients as opposed to unassigned patients. Therefore, almost all of hospitalist A’s patients are covered by risk-based contracts, and hospitalist A might be well positioned for the new APM structure.
Hospitalist B works for a group, or a university, and sees whatever patients are admitted to the hospital. Hospitalist B’s eligibility to participate in the APM will depend on the percentage of patients in alternative payment models in their market. If hospitalist B’s market has many Medicare accountable care organizations, and Medicaid and the commercial insurers compensate through a risk-sharing model, hospitalist B might reach the threshold. This is more accidental than planned, however, and hospitalist B might not be able to consistently hit this threshold year after year.
In addition, just working within the model will probably not be enough to qualify. Hospitalist B will need to also take on “more than nominal risk” as a participant in the model. In an employed academic setting, where the hospital is taking on risk as part of an APM, it is unlikely hospitalist B will qualify just by virtue of hospital employment. Hospitalist B must also meet/exceed the patient or payment thresholds under the model.
Bottom line: Given the current situation, we expect many hospitalists will likely be required to participate in MIPS and not qualify for APMs. Understanding the details and expectations now will help them be successful in the future.
Is MACRA Good for Hospitalists?
Most of organized medicine is happy to be free from the annual threat of reimbursement cuts. In addition, the new law might streamline quality reporting. But the specific upside depends on your perspective.
With APMs, a hospitalist might enjoy more upside potential, particularly for high-quality work and EHR use. However, whether it is realistic for most hospitalists to even participate in the model depends on many factors, as described previously, and SHM is advocating for the law to be implemented in ways that will more readily accommodate hospitalist practice and employment structures.
For example, the SHM Public Policy Committee has provided the Centers for Medicare & Medicaid Services (CMS) with realistic options for implementing the APM framework that would allow hospitalist B in the above example to qualify as an APM participant.
With MIPS, the benefit to hospitalists depends a fair amount on the way the law is implemented: how quality reporting happens, what metrics will count as quality improvement efforts, and how utilization of EHRs is measured.
What Issues Should Hospitalists Be Aware Of?
As MACRA is further developed, the main issue for hospitalists will be to ensure fairness in assessing quality and incentive payments. As previously encountered with quality reporting, hospitalists are not differentiated clearly from outpatient providers. As a result, they could suffer from the comparison of their quality outcomes for their sicker hospitalized patients to the patients cared for in a typical primary-care internal medicine practice. This inaccurate comparison poses problems in both models.
A potential solution would be a hospitalist-specific billing code, which would make it easier to identify hospitalists. SHM applied for and advocated for the approval of such a billing code and the request was recently approved by CMS.
In addition, as hospitalists mostly work in groups with shift-based schedules, thus sharing care of patients, individual identifiers may not be as significant as possibly looking at hospital, system, or team-based metrics. Using facility performance measures for both clinical quality and performance improvement—where hospitalists can opt to align with their hospital, which is already reporting quality outcomes—might be one way out of this conundrum. It would take into account the type of facility-level quality improvement work many hospitalists participate in. This also would decrease reporting burden for hospitalist groups.
SHM has advocated for this solution and was able to ensure this concept was included in the law; however, it is unclear when or how CMS will implement it.
To summarize, looking good in quality reporting will continue to be a challenge for hospitalists. It will be critical to keep pressure on CMS to implement solutions that account for the unique situation of our specialty.
Another issue to be aware of is the ability of hospitalists to participate in APMs. As with other facility-based providers, hospitalists have little control over whether their facility participates in an APM. Ways to ensure hospitalists can reach thresholds for participation could include allowing the various APMs that hospitalist patients are aligned with count toward an individual hospitalists’ APM participation total—a solution that SHM is advocating for Medicare to include in the APM framework.
What’s Next?
Much remains to be solidified regarding implementation of MACRA, despite the fact it goes live in a few short years (see Figure 1). CMS has asked for comments and stakeholder input regarding MIPS and APMs, and it will be releasing the first round of rules around MACRA this year.
SHM is actively working with CMS to ensure this legislation will reflect the work we are doing as hospitalists to provide high-quality clinical care for our patients and enhance the performance of our hospitals and health system. TH
Dr. Doctoroff is a hospitalist at Beth Israel Deaconess Medical Center and an instructor of medicine at Harvard Medical School in Boston. Dr. Dutta is a hospitalist at Rush University Medical Center and an assistant professor of medicine at Rush Medical College in Chicago. Both are members of the SHM Public Policy Committee.
Last year, Congress passed legislation to permanently eliminate the Sustainable Growth Rate (SGR) formula, created in 1997 and designed to hold Medicare Part B or outpatient spending under control. Allowing the SGR to go into effect would have severely cut physician reimbursements in recent years, but Congress passed legislation each year to temporarily avert these cuts (also known annually as the “doc fix”). In search of a permanent solution, the passage of bipartisan legislation permanently repealing the SGR in 2015 was hailed as a way to ensure more certainty around the future of Medicare payments for physicians.
This legislation (H.R. 2, 114th Congress), sponsored by Rep. Michael C. Burgess (R-Texas) and entitled “Medicare Access and CHIP Reauthorization Act of 2015,” or MACRA, does much more than simply remove the SGR’s threat of broader Medicare payment cuts. The law changes the ways physicians are reimbursed by Medicare and continues to shift our healthcare system away from volume-based reimbursements and toward a value-based payment system.
What Is MIPS?
MACRA creates two value-based payment tracks for physicians. The first, the Merit-Based Incentive Payment System (MIPS), is closer to the old fee-for-service model of reimbursement. However, MIPS takes into account both volume and quality (i.e., payment is adjusted based on physician-quality scores). These physician-specific scores broaden the scope of quality measurement by including new measures related to resource utilization, electronic health record (EHR) use, and clinical improvement practices, along with the traditional clinical quality markers.
Under MIPS, the current Physician Quality Reporting System (PQRS), EHR Incentive Program, and Physician Value-Based Modifier all will be integrated into this single-payment adjustment.
The range of potential payment adjustments based on a physician’s MIPS score grows each year through 2022 (in 2022, adjustments can range from +9% to -9%). The program is budget neutral, which means that increases in payments to high-scoring providers will be offset by decreases in payments to low-scoring providers. For 2019 to 2024, there also will be an additional payment adjustment given to the highest MIPS performers for exceptional performance.
A benefit of MIPS is that it will streamline the various quality-reporting programs currently in place into one single program and does not ask physicians to assume any additional financial risk related to outcomes when taking care of patients. However, the particulars of how the MIPS score will be calculated are yet to be determined, and much of the utility and palatability of this score will depend on the chosen metrics. The goal of these metrics should be that they are meaningful, valid, and attributable to specific providers.
What Are APMs?
The other payment option MACRA provides for physicians allows them to opt out of MIPS and participate in the Alternative Payment Models (APMs) track. To incentivize physicians to take part in this riskier track, providers taking part in APMs will receive some extra money for their participation: a 5% annual lump sum bonus on reimbursement payments. To clarify, qualifying APMs are those where providers take on “more than nominal” financial risk, report on their quality measures, and use certified EHR technology.
To qualify as a participant in an APM (for example, the Medicare Shared Savings Program), providers must hit a threshold for percentage of total revenue received or percentage of patients from qualifying APMs. This threshold will increase over time. For example, from 2019 to 2020, providers must obtain at least 25% of their Medicare revenue or patients via APMs, whereas in 2023, 75% of their Medicare revenue or \ patients will need to come from APMs.
Providers will benefit from the increased reimbursement offered if they participate in APMs. There also is funding allocated in MACRA to help develop quality measures, with a call for physician leads to develop quality standards. This payment model, however, does come with increased financial risk for the provider contingent on patient outcomes. In addition, it may be difficult for all providers to hit the thresholds for participation.
Stick with MIPS? Or Take the Plunge with APM?
How MACRA affects you will depend a lot on the practice environment. As described above, MACRA is designed to move physicians into risk-based payment structures if possible. If possible, or otherwise, to simplify the current fee-for-service mechanism of payment by consolidating various Medicare pay-for-performance programs.
Let’s look at a few scenarios:
Hospitalist A works for a physician group that assumes risk for patients in a MACRA-approved APM and sees only those inpatients as opposed to unassigned patients. Therefore, almost all of hospitalist A’s patients are covered by risk-based contracts, and hospitalist A might be well positioned for the new APM structure.
Hospitalist B works for a group, or a university, and sees whatever patients are admitted to the hospital. Hospitalist B’s eligibility to participate in the APM will depend on the percentage of patients in alternative payment models in their market. If hospitalist B’s market has many Medicare accountable care organizations, and Medicaid and the commercial insurers compensate through a risk-sharing model, hospitalist B might reach the threshold. This is more accidental than planned, however, and hospitalist B might not be able to consistently hit this threshold year after year.
In addition, just working within the model will probably not be enough to qualify. Hospitalist B will need to also take on “more than nominal risk” as a participant in the model. In an employed academic setting, where the hospital is taking on risk as part of an APM, it is unlikely hospitalist B will qualify just by virtue of hospital employment. Hospitalist B must also meet/exceed the patient or payment thresholds under the model.
Bottom line: Given the current situation, we expect many hospitalists will likely be required to participate in MIPS and not qualify for APMs. Understanding the details and expectations now will help them be successful in the future.
Is MACRA Good for Hospitalists?
Most of organized medicine is happy to be free from the annual threat of reimbursement cuts. In addition, the new law might streamline quality reporting. But the specific upside depends on your perspective.
With APMs, a hospitalist might enjoy more upside potential, particularly for high-quality work and EHR use. However, whether it is realistic for most hospitalists to even participate in the model depends on many factors, as described previously, and SHM is advocating for the law to be implemented in ways that will more readily accommodate hospitalist practice and employment structures.
For example, the SHM Public Policy Committee has provided the Centers for Medicare & Medicaid Services (CMS) with realistic options for implementing the APM framework that would allow hospitalist B in the above example to qualify as an APM participant.
With MIPS, the benefit to hospitalists depends a fair amount on the way the law is implemented: how quality reporting happens, what metrics will count as quality improvement efforts, and how utilization of EHRs is measured.
What Issues Should Hospitalists Be Aware Of?
As MACRA is further developed, the main issue for hospitalists will be to ensure fairness in assessing quality and incentive payments. As previously encountered with quality reporting, hospitalists are not differentiated clearly from outpatient providers. As a result, they could suffer from the comparison of their quality outcomes for their sicker hospitalized patients to the patients cared for in a typical primary-care internal medicine practice. This inaccurate comparison poses problems in both models.
A potential solution would be a hospitalist-specific billing code, which would make it easier to identify hospitalists. SHM applied for and advocated for the approval of such a billing code and the request was recently approved by CMS.
In addition, as hospitalists mostly work in groups with shift-based schedules, thus sharing care of patients, individual identifiers may not be as significant as possibly looking at hospital, system, or team-based metrics. Using facility performance measures for both clinical quality and performance improvement—where hospitalists can opt to align with their hospital, which is already reporting quality outcomes—might be one way out of this conundrum. It would take into account the type of facility-level quality improvement work many hospitalists participate in. This also would decrease reporting burden for hospitalist groups.
SHM has advocated for this solution and was able to ensure this concept was included in the law; however, it is unclear when or how CMS will implement it.
To summarize, looking good in quality reporting will continue to be a challenge for hospitalists. It will be critical to keep pressure on CMS to implement solutions that account for the unique situation of our specialty.
Another issue to be aware of is the ability of hospitalists to participate in APMs. As with other facility-based providers, hospitalists have little control over whether their facility participates in an APM. Ways to ensure hospitalists can reach thresholds for participation could include allowing the various APMs that hospitalist patients are aligned with count toward an individual hospitalists’ APM participation total—a solution that SHM is advocating for Medicare to include in the APM framework.
What’s Next?
Much remains to be solidified regarding implementation of MACRA, despite the fact it goes live in a few short years (see Figure 1). CMS has asked for comments and stakeholder input regarding MIPS and APMs, and it will be releasing the first round of rules around MACRA this year.
SHM is actively working with CMS to ensure this legislation will reflect the work we are doing as hospitalists to provide high-quality clinical care for our patients and enhance the performance of our hospitals and health system. TH
Dr. Doctoroff is a hospitalist at Beth Israel Deaconess Medical Center and an instructor of medicine at Harvard Medical School in Boston. Dr. Dutta is a hospitalist at Rush University Medical Center and an assistant professor of medicine at Rush Medical College in Chicago. Both are members of the SHM Public Policy Committee.
SHM’s Twitter Contest Encourages Appropriate Antibiotic Prescribing
- Identify opportunities to engage with all hospital-based clinicians to improve antibiotic stewardship in your hospital.
- Pay attention to appropriate antibiotic choice and resistance patterns and identify mechanisms to educate providers on overprescribing in your hospital.
- Consider the following:
Adhere to antibiotic treatment guidelines.
Track the day.
Set a stop date.
Reevaluate therapy.
Streamline therapy.
Avoid automatic time courses.
Not only did participants receive recognition for their efforts hanging up the posters and engaging their teams, the posters’ presence in various hospitals and offices around the country created thousands of impressions among hospital-based staff and others directly responsible for proper antibiotic prescribing.
Although the contest is over, you can still help facilitate culture change related to appropriate antibiotic prescribing. Follow SHM on Twitter @SHMLive, and continue to visit FightTheResistance.org for the latest updates on the campaign and new tools to promote antibiotic stewardship. TH
Brett Radler is SHM’s communications coordinator.
- Identify opportunities to engage with all hospital-based clinicians to improve antibiotic stewardship in your hospital.
- Pay attention to appropriate antibiotic choice and resistance patterns and identify mechanisms to educate providers on overprescribing in your hospital.
- Consider the following:
Adhere to antibiotic treatment guidelines.
Track the day.
Set a stop date.
Reevaluate therapy.
Streamline therapy.
Avoid automatic time courses.
Not only did participants receive recognition for their efforts hanging up the posters and engaging their teams, the posters’ presence in various hospitals and offices around the country created thousands of impressions among hospital-based staff and others directly responsible for proper antibiotic prescribing.
Although the contest is over, you can still help facilitate culture change related to appropriate antibiotic prescribing. Follow SHM on Twitter @SHMLive, and continue to visit FightTheResistance.org for the latest updates on the campaign and new tools to promote antibiotic stewardship. TH
Brett Radler is SHM’s communications coordinator.
- Identify opportunities to engage with all hospital-based clinicians to improve antibiotic stewardship in your hospital.
- Pay attention to appropriate antibiotic choice and resistance patterns and identify mechanisms to educate providers on overprescribing in your hospital.
- Consider the following:
Adhere to antibiotic treatment guidelines.
Track the day.
Set a stop date.
Reevaluate therapy.
Streamline therapy.
Avoid automatic time courses.
Not only did participants receive recognition for their efforts hanging up the posters and engaging their teams, the posters’ presence in various hospitals and offices around the country created thousands of impressions among hospital-based staff and others directly responsible for proper antibiotic prescribing.
Although the contest is over, you can still help facilitate culture change related to appropriate antibiotic prescribing. Follow SHM on Twitter @SHMLive, and continue to visit FightTheResistance.org for the latest updates on the campaign and new tools to promote antibiotic stewardship. TH
Brett Radler is SHM’s communications coordinator.
Gut microbes affect platelet function, thrombosis risk

Image by Andre E.X. Brown
New research indicates that gut microbes alter platelet function, which affects the risk of thrombosis and related events like heart attack and stroke.
When the nutrient choline, which is abundant in animal products like meat and egg yolk, is ingested, gut microbes play a role in breaking it down and producing the compound TMAO.
Recent studies have shown that blood TMAO levels are associated with a heightened risk of heart attack and stroke.
The new study, published in Cell, suggests that TMAO encourages hyper-reactive platelet function, thereby increasing the likelihood of thrombosis.
Researchers said this could be the mechanism by which TMAO increases the risk of heart attack and stroke. And these findings reveal a previously unrecognized mechanistic link between specific dietary nutrients, gut microbes, platelet function, and thrombosis risk.
“It is remarkable that gut microbes produce a compound that alters platelet function and thrombotic heart attack and stroke risk,” said study author Stanley Hazen, MD, PhD, of the Cleveland Clinic in Ohio.
“This new link helps explain how diet-induced TMAO generation is mechanistically linked to development of lethal adverse complications of heart disease.”
Dr Hazen and his colleagues first discovered a link between TMAO, gut microbes, and heart disease 5 years ago.
For the current study, the researchers analyzed blood levels of TMAO in more than 4000 patients and saw a significant correlation between higher TMAO and thrombosis potential. This led to the hypothesis that TMAO may directly impact platelet function.
Subsequent studies with both human platelets and animal models confirmed that TMAO makes platelets hyper-reactive, heightening thrombosis potential and accelerating clotting rates.
“We have shown that TMAO fundamentally alters calcium signaling within platelets,” Dr Hazen said. “When TMAO is elevated, platelet responsiveness to known triggers like thrombin, collagen, or ADP is heightened.”
“In general, there’s a broad range for how quickly different people will form clots. However, across the board, when TMAO is elevated, platelet responsiveness jumps to the hyper-reactive side of normal.”
Dr Hazen and his colleagues said these results suggest that lowering TMAO—via dietary manipulation, alteration in microbial community with a probiotic or prebiotic, or direct pharmacological inhibition of microbial enzymes involved in TMA production—may be a way to reduce the risk of thrombotic events.
They noted that, unlike current antiplatelet therapies, targeting TMAO would likely reduce platelet hyper-responsiveness to the normal range and not induce impairment in overall platelet function. So the intervention could attenuate pro-thrombotic tendencies without increasing the risk of bleeding complications. ![]()

Image by Andre E.X. Brown
New research indicates that gut microbes alter platelet function, which affects the risk of thrombosis and related events like heart attack and stroke.
When the nutrient choline, which is abundant in animal products like meat and egg yolk, is ingested, gut microbes play a role in breaking it down and producing the compound TMAO.
Recent studies have shown that blood TMAO levels are associated with a heightened risk of heart attack and stroke.
The new study, published in Cell, suggests that TMAO encourages hyper-reactive platelet function, thereby increasing the likelihood of thrombosis.
Researchers said this could be the mechanism by which TMAO increases the risk of heart attack and stroke. And these findings reveal a previously unrecognized mechanistic link between specific dietary nutrients, gut microbes, platelet function, and thrombosis risk.
“It is remarkable that gut microbes produce a compound that alters platelet function and thrombotic heart attack and stroke risk,” said study author Stanley Hazen, MD, PhD, of the Cleveland Clinic in Ohio.
“This new link helps explain how diet-induced TMAO generation is mechanistically linked to development of lethal adverse complications of heart disease.”
Dr Hazen and his colleagues first discovered a link between TMAO, gut microbes, and heart disease 5 years ago.
For the current study, the researchers analyzed blood levels of TMAO in more than 4000 patients and saw a significant correlation between higher TMAO and thrombosis potential. This led to the hypothesis that TMAO may directly impact platelet function.
Subsequent studies with both human platelets and animal models confirmed that TMAO makes platelets hyper-reactive, heightening thrombosis potential and accelerating clotting rates.
“We have shown that TMAO fundamentally alters calcium signaling within platelets,” Dr Hazen said. “When TMAO is elevated, platelet responsiveness to known triggers like thrombin, collagen, or ADP is heightened.”
“In general, there’s a broad range for how quickly different people will form clots. However, across the board, when TMAO is elevated, platelet responsiveness jumps to the hyper-reactive side of normal.”
Dr Hazen and his colleagues said these results suggest that lowering TMAO—via dietary manipulation, alteration in microbial community with a probiotic or prebiotic, or direct pharmacological inhibition of microbial enzymes involved in TMA production—may be a way to reduce the risk of thrombotic events.
They noted that, unlike current antiplatelet therapies, targeting TMAO would likely reduce platelet hyper-responsiveness to the normal range and not induce impairment in overall platelet function. So the intervention could attenuate pro-thrombotic tendencies without increasing the risk of bleeding complications. ![]()

Image by Andre E.X. Brown
New research indicates that gut microbes alter platelet function, which affects the risk of thrombosis and related events like heart attack and stroke.
When the nutrient choline, which is abundant in animal products like meat and egg yolk, is ingested, gut microbes play a role in breaking it down and producing the compound TMAO.
Recent studies have shown that blood TMAO levels are associated with a heightened risk of heart attack and stroke.
The new study, published in Cell, suggests that TMAO encourages hyper-reactive platelet function, thereby increasing the likelihood of thrombosis.
Researchers said this could be the mechanism by which TMAO increases the risk of heart attack and stroke. And these findings reveal a previously unrecognized mechanistic link between specific dietary nutrients, gut microbes, platelet function, and thrombosis risk.
“It is remarkable that gut microbes produce a compound that alters platelet function and thrombotic heart attack and stroke risk,” said study author Stanley Hazen, MD, PhD, of the Cleveland Clinic in Ohio.
“This new link helps explain how diet-induced TMAO generation is mechanistically linked to development of lethal adverse complications of heart disease.”
Dr Hazen and his colleagues first discovered a link between TMAO, gut microbes, and heart disease 5 years ago.
For the current study, the researchers analyzed blood levels of TMAO in more than 4000 patients and saw a significant correlation between higher TMAO and thrombosis potential. This led to the hypothesis that TMAO may directly impact platelet function.
Subsequent studies with both human platelets and animal models confirmed that TMAO makes platelets hyper-reactive, heightening thrombosis potential and accelerating clotting rates.
“We have shown that TMAO fundamentally alters calcium signaling within platelets,” Dr Hazen said. “When TMAO is elevated, platelet responsiveness to known triggers like thrombin, collagen, or ADP is heightened.”
“In general, there’s a broad range for how quickly different people will form clots. However, across the board, when TMAO is elevated, platelet responsiveness jumps to the hyper-reactive side of normal.”
Dr Hazen and his colleagues said these results suggest that lowering TMAO—via dietary manipulation, alteration in microbial community with a probiotic or prebiotic, or direct pharmacological inhibition of microbial enzymes involved in TMA production—may be a way to reduce the risk of thrombotic events.
They noted that, unlike current antiplatelet therapies, targeting TMAO would likely reduce platelet hyper-responsiveness to the normal range and not induce impairment in overall platelet function. So the intervention could attenuate pro-thrombotic tendencies without increasing the risk of bleeding complications. ![]()
Negative cancer trials have long-term impact

for a clinical trial
Photo by Esther Dyson
Cancer trials with negative results don’t make an immediate splash in the scientific literature, but they do have a long-term impact on research, according to a study published in JAMA Oncology.
Researchers found that first reports of positive phase 3 cancer trials were twice as likely as first reports of negative phase 3 cancer trials
to be cited in scientific journals.
But over time, when all articles associated with the trials were considered, the scientific impact of negative trials and positive trials was about the same.
“Negative trials aren’t scientific failures,” said study author Joseph Unger, PhD, of the Fred Hutchinson Cancer Research Center in Seattle, Washington.
“We found that they have a positive, lasting impact on cancer research.”
Dr Unger and his colleagues analyzed every randomized, phase 3 cancer trial completed by the cooperative group SWOG from 1984 to 2014. This amounted to 94 studies involving 46,424 patients.
Of those 94 studies, 26 were positive, meaning that the treatment tested performed measurably better than the standard treatment at the time.
Analyses revealed that primary manuscripts first announcing these encouraging results were published in journals with higher impact factors and were cited twice as often as primary manuscripts of negative trials.
The mean 2-year impact factor of the journals was 28 for positive trials and 18 for negative trials (P=0.007). And the mean number of citations per year was 43 for positive trials and 21 for negative trials (P=0.03).
However, when the researchers looked at the number of citations from all primary and secondary manuscripts, they did not see a significant difference between positive and negative trials. The mean number of citations per year was 55 and 45, respectively (P=0.53).
“Negative trials matter because they tell us what doesn’t work, which can be as important as what does,” said study author Dawn Hershman, MD, of Columbia University Medical Center in New York, New York.
“Negative trials are also critical for secondary research, which mines existing trial data to answer new questions in cancer care and prevention. Negative trials are used frequently in secondary research and add great value to the scientific community.” ![]()

for a clinical trial
Photo by Esther Dyson
Cancer trials with negative results don’t make an immediate splash in the scientific literature, but they do have a long-term impact on research, according to a study published in JAMA Oncology.
Researchers found that first reports of positive phase 3 cancer trials were twice as likely as first reports of negative phase 3 cancer trials
to be cited in scientific journals.
But over time, when all articles associated with the trials were considered, the scientific impact of negative trials and positive trials was about the same.
“Negative trials aren’t scientific failures,” said study author Joseph Unger, PhD, of the Fred Hutchinson Cancer Research Center in Seattle, Washington.
“We found that they have a positive, lasting impact on cancer research.”
Dr Unger and his colleagues analyzed every randomized, phase 3 cancer trial completed by the cooperative group SWOG from 1984 to 2014. This amounted to 94 studies involving 46,424 patients.
Of those 94 studies, 26 were positive, meaning that the treatment tested performed measurably better than the standard treatment at the time.
Analyses revealed that primary manuscripts first announcing these encouraging results were published in journals with higher impact factors and were cited twice as often as primary manuscripts of negative trials.
The mean 2-year impact factor of the journals was 28 for positive trials and 18 for negative trials (P=0.007). And the mean number of citations per year was 43 for positive trials and 21 for negative trials (P=0.03).
However, when the researchers looked at the number of citations from all primary and secondary manuscripts, they did not see a significant difference between positive and negative trials. The mean number of citations per year was 55 and 45, respectively (P=0.53).
“Negative trials matter because they tell us what doesn’t work, which can be as important as what does,” said study author Dawn Hershman, MD, of Columbia University Medical Center in New York, New York.
“Negative trials are also critical for secondary research, which mines existing trial data to answer new questions in cancer care and prevention. Negative trials are used frequently in secondary research and add great value to the scientific community.” ![]()

for a clinical trial
Photo by Esther Dyson
Cancer trials with negative results don’t make an immediate splash in the scientific literature, but they do have a long-term impact on research, according to a study published in JAMA Oncology.
Researchers found that first reports of positive phase 3 cancer trials were twice as likely as first reports of negative phase 3 cancer trials
to be cited in scientific journals.
But over time, when all articles associated with the trials were considered, the scientific impact of negative trials and positive trials was about the same.
“Negative trials aren’t scientific failures,” said study author Joseph Unger, PhD, of the Fred Hutchinson Cancer Research Center in Seattle, Washington.
“We found that they have a positive, lasting impact on cancer research.”
Dr Unger and his colleagues analyzed every randomized, phase 3 cancer trial completed by the cooperative group SWOG from 1984 to 2014. This amounted to 94 studies involving 46,424 patients.
Of those 94 studies, 26 were positive, meaning that the treatment tested performed measurably better than the standard treatment at the time.
Analyses revealed that primary manuscripts first announcing these encouraging results were published in journals with higher impact factors and were cited twice as often as primary manuscripts of negative trials.
The mean 2-year impact factor of the journals was 28 for positive trials and 18 for negative trials (P=0.007). And the mean number of citations per year was 43 for positive trials and 21 for negative trials (P=0.03).
However, when the researchers looked at the number of citations from all primary and secondary manuscripts, they did not see a significant difference between positive and negative trials. The mean number of citations per year was 55 and 45, respectively (P=0.53).
“Negative trials matter because they tell us what doesn’t work, which can be as important as what does,” said study author Dawn Hershman, MD, of Columbia University Medical Center in New York, New York.
“Negative trials are also critical for secondary research, which mines existing trial data to answer new questions in cancer care and prevention. Negative trials are used frequently in secondary research and add great value to the scientific community.” ![]()
Nemolizumab improved most common symptoms in moderate, severe atopic dermatitis
WASHINGTON – Nemolizumab, an anti-interleukin-31 receptor A monoclonal antibody, was shown to rapidly and consistently improve pruritus, dermatitis, and sleep disturbance in patients with previously uncontrolled moderate-to-severe atopic dermatitis in a phase II, randomized, double-blind, placebo-controlled trial.
“The patients notice that the itch is gone very quickly, sometimes within days, even before their dermatitis starts to heal,” said Dr. Jon M. Hanifin, one of the treatment’s investigators. “It’s very exciting.”
Dr. Hanifin, a researcher at Oregon Health & Science University, Portland, made his comments while presenting the data during the late-breaking clinical research session at this year’s annual meeting of the American Academy of Dermatology.
The novel treatment, currently known as CIM331 (Chugai) targets elevated levels of interleukin-31, a cytokine that has been implicated in the pathophysiology of atopic dermatitis (AD) and pruritus.
The multicenter, multi-dose study assigned 264 patients, primarily in their mid-30s, with moderate-to-severe AD, uncontrolled by topical treatments, to either placebo or 0.1 mg/kg, 0.5 mg/kg, or 2.0 mg/kg CIM331 every 4 weeks. The study completion rate was 82%.
Using a visual analog scale of 0 to 10, with 10 characterized as “the worst imaginable,” at week 12, patients in the three study groups reported −41.5%, −61.2%, and −60.5% reductions in pruritus vs. −20.1% for placebo (P less than .01 for all).
The “significant reductions” in itch began as early as week 1, particularly in the group treated with the 2-mg/kg dose. Dr. Hanifin said that the patients had a very high mean pruritis score, and some had as much as half of their body surface area affected by AD.
Rescue medications such as topical corticosteroids were not permitted until after at least 1 month of treatment. Patients given rescue therapies were required to have both itch and dermatitis. “This was kind of a tough thing to get through, but [patients] did okay,” Dr. Hanifin said.
Patients treated with the 0.5-mg/kg dose showed the most improvement from baseline in Eczema Area and Severity Index scores at week 12 at −44.6%, compared with −20.9% for placebo. Across the study arms, the proportion of static Investigator’s Global Assessment of 1 or less was 20.9% vs. 4.7% for placebo. Additionally, Dr. Hanifin said sleep latency in the study groups was improved by half and there was an increase in total overall sleep time.
CIM331 was well tolerated, with the most common adverse events being exacerbation of AD and nasopharyngitis. “The risk of immune suppression seemed to be low,” Dr. Hanifin said.
On Twitter @whitneymcknight
WASHINGTON – Nemolizumab, an anti-interleukin-31 receptor A monoclonal antibody, was shown to rapidly and consistently improve pruritus, dermatitis, and sleep disturbance in patients with previously uncontrolled moderate-to-severe atopic dermatitis in a phase II, randomized, double-blind, placebo-controlled trial.
“The patients notice that the itch is gone very quickly, sometimes within days, even before their dermatitis starts to heal,” said Dr. Jon M. Hanifin, one of the treatment’s investigators. “It’s very exciting.”
Dr. Hanifin, a researcher at Oregon Health & Science University, Portland, made his comments while presenting the data during the late-breaking clinical research session at this year’s annual meeting of the American Academy of Dermatology.
The novel treatment, currently known as CIM331 (Chugai) targets elevated levels of interleukin-31, a cytokine that has been implicated in the pathophysiology of atopic dermatitis (AD) and pruritus.
The multicenter, multi-dose study assigned 264 patients, primarily in their mid-30s, with moderate-to-severe AD, uncontrolled by topical treatments, to either placebo or 0.1 mg/kg, 0.5 mg/kg, or 2.0 mg/kg CIM331 every 4 weeks. The study completion rate was 82%.
Using a visual analog scale of 0 to 10, with 10 characterized as “the worst imaginable,” at week 12, patients in the three study groups reported −41.5%, −61.2%, and −60.5% reductions in pruritus vs. −20.1% for placebo (P less than .01 for all).
The “significant reductions” in itch began as early as week 1, particularly in the group treated with the 2-mg/kg dose. Dr. Hanifin said that the patients had a very high mean pruritis score, and some had as much as half of their body surface area affected by AD.
Rescue medications such as topical corticosteroids were not permitted until after at least 1 month of treatment. Patients given rescue therapies were required to have both itch and dermatitis. “This was kind of a tough thing to get through, but [patients] did okay,” Dr. Hanifin said.
Patients treated with the 0.5-mg/kg dose showed the most improvement from baseline in Eczema Area and Severity Index scores at week 12 at −44.6%, compared with −20.9% for placebo. Across the study arms, the proportion of static Investigator’s Global Assessment of 1 or less was 20.9% vs. 4.7% for placebo. Additionally, Dr. Hanifin said sleep latency in the study groups was improved by half and there was an increase in total overall sleep time.
CIM331 was well tolerated, with the most common adverse events being exacerbation of AD and nasopharyngitis. “The risk of immune suppression seemed to be low,” Dr. Hanifin said.
On Twitter @whitneymcknight
WASHINGTON – Nemolizumab, an anti-interleukin-31 receptor A monoclonal antibody, was shown to rapidly and consistently improve pruritus, dermatitis, and sleep disturbance in patients with previously uncontrolled moderate-to-severe atopic dermatitis in a phase II, randomized, double-blind, placebo-controlled trial.
“The patients notice that the itch is gone very quickly, sometimes within days, even before their dermatitis starts to heal,” said Dr. Jon M. Hanifin, one of the treatment’s investigators. “It’s very exciting.”
Dr. Hanifin, a researcher at Oregon Health & Science University, Portland, made his comments while presenting the data during the late-breaking clinical research session at this year’s annual meeting of the American Academy of Dermatology.
The novel treatment, currently known as CIM331 (Chugai) targets elevated levels of interleukin-31, a cytokine that has been implicated in the pathophysiology of atopic dermatitis (AD) and pruritus.
The multicenter, multi-dose study assigned 264 patients, primarily in their mid-30s, with moderate-to-severe AD, uncontrolled by topical treatments, to either placebo or 0.1 mg/kg, 0.5 mg/kg, or 2.0 mg/kg CIM331 every 4 weeks. The study completion rate was 82%.
Using a visual analog scale of 0 to 10, with 10 characterized as “the worst imaginable,” at week 12, patients in the three study groups reported −41.5%, −61.2%, and −60.5% reductions in pruritus vs. −20.1% for placebo (P less than .01 for all).
The “significant reductions” in itch began as early as week 1, particularly in the group treated with the 2-mg/kg dose. Dr. Hanifin said that the patients had a very high mean pruritis score, and some had as much as half of their body surface area affected by AD.
Rescue medications such as topical corticosteroids were not permitted until after at least 1 month of treatment. Patients given rescue therapies were required to have both itch and dermatitis. “This was kind of a tough thing to get through, but [patients] did okay,” Dr. Hanifin said.
Patients treated with the 0.5-mg/kg dose showed the most improvement from baseline in Eczema Area and Severity Index scores at week 12 at −44.6%, compared with −20.9% for placebo. Across the study arms, the proportion of static Investigator’s Global Assessment of 1 or less was 20.9% vs. 4.7% for placebo. Additionally, Dr. Hanifin said sleep latency in the study groups was improved by half and there was an increase in total overall sleep time.
CIM331 was well tolerated, with the most common adverse events being exacerbation of AD and nasopharyngitis. “The risk of immune suppression seemed to be low,” Dr. Hanifin said.
On Twitter @whitneymcknight
AT AAD 2016
Key clinical point: Nemolizumab rapidly and consistently improved pruritus, dermatitis and sleep disturbance in patients with previously uncontrolled moderate-to-severe atopic dermatitis.
Major finding: At week 12, patients treated with nemolizumab reported reductions in pruritus of −41.5% for 0.1 mg/kg, −61.2% for 0.5 mg/kg, and −60.5% 2.0 mg/kg vs. −20.1% for placebo (P less than .01 for all).
Data source: Randomized, double-blind, placebo-controlled, multi-center, multi-dose phase II study of 264 patients with moderate-to-severe atopic dermatitis.
Disclosures: This trial was sponsored by Chugai Pharmaceuticals.
Outcomes in major surgery unchanged by continuing clopidogrel
MONTREAL – Patients who stayed on antiplatelet therapy close to – or even up until – major surgery fared just as well as those who stopped their medication earlier in a retrospective, single-center study.
The study found no difference in blood product administration, adverse perioperative events, or all-cause 30-day mortality regardless of whether patients stopped clopidogrel (Plavix) the recommended 5 days before surgery.
“We believe that continuing clopidogrel in elective and emergent surgical situations appears to be safe, and may challenge the current recommendations,” said presenter Dr. David Strosberg.
The study addressed a thorny question for surgeons, Dr. Strosberg said. “As surgeons, we face a dilemma: Do we take the risk of thrombotic complications in stopping the antiplatelet drugs, or do we take the risk of increased surgical bleeding with continuing therapy?”
The package insert for clopidogrel advises discontinuation 5 days prior to surgery. However, manufacturer labeling also states that discontinuation of clopidogrel can lead to adverse cardiac events, said Dr. Strosberg, a general surgery resident at Ohio State University in Columbus.
The aim of the study, presented at the annual meeting of the Central Surgical Association, was to ascertain whether continuing antiplatelet therapy increased the rate of adverse surgical outcomes in those undergoing major emergent or elective surgery.
Dr. Strosberg and his colleagues retrospectively reviewed the record of patients over a 4-year period at a single institution and included those undergoing major general, thoracic, or vascular surgery who were taking clopidogrel at the time of presentation.
Data collected included patient characteristics, including demographic data and comorbidities, as well as transfusion requirements and perioperative events.
A total of 200 patients who had 205 qualifying procedures and were taking clopidogrel were included in the study. Of these, 116 patients (Group A) had their clopidogrel held for at least 5 days preoperatively. The remaining 89 patients (Group B) had their clopidogrel held for less than 5 days, or not at all.
Patient demographics were similar between the two groups. Patients in Group A were more likely to have emergency surgery, to have peripheral stents placed, to have COPD or peripheral vascular disease, to have a malignancy, and to have received aspirin within five days of surgery (P less than .01 for all).
Blood product administration rates and volumes did not differ significantly between the two groups, and there was no difference between the groups in the incidence of myocardial infarctions, cerebrovascular events, or acute visceral or lower extremity ischemia.
Three patients in each group died within 30 days of the procedure, a nonsignificant difference. However, in the group that had clopidogrel held, three patients had perioperative myocardial infarctions, and two of these patients died. In discussing the study, Dr. Michael Dalsing said, “I think a lot of us would accept bleeding more over myocardial infarction.”
A subgroup analysis of the group who had clopidogrel held for fewer than 5 days compared outcomes for emergent vs. non-emergent surgery. The emergent surgery subgroup had a significantly higher rate of preoperative platelet transfusions, although numbers overall were small (2/17, 11.8%, vs. 0/72; P = .03).
Dr. Strosberg noted study limitations that included the retrospective, single-center nature of the study, and the fact that one variable, estimated blood loss, is notoriously subjective and inaccurate.
Dr. Dalsing, chief of vascular surgery at Indiana University, Indianapolis, said that he “was surprised that not even one patient went back for postoperative bleeding in this high-risk group of patients,” and raised the question of potential selection bias. Dr. Strosberg replied that comorbidities were ascertained by the physician at the time of surgery planning; since no differences were seen between study groups, investigators didn’t go back and parse out details about comorbid conditions.
In discussion following the presentation, surgeons spoke to the real-world challenges of performing surgery on a patient with antiplatelet therapy on board.
“Overall, I think your data support kind of a bias I have. Since I’m a vascular surgeon, we almost always operate on clopidogrel, and I don’t know if our bleeding risk is worse or better. But it’s something we almost have to do to keep our grafts going,” Dr. Dalsing said.
Dr. Peter Henke, professor of vascular surgery at the University of Michigan, Ann Arbor, said, “I’d be a little bit cautious with this. If you’ve ever done a big aortic procedure on someone on Plavix, I’ve seen them lose up to a couple of liters of blood just with oozing.”
“Those of us who do open aortic surgery know that very few things bleed like the back wall of an aortic anastomosis of a patient on Plavix,” echoed Dr. Peter Rossi, associate professor of vascular surgery at the Medical College of Wisconsin, Milwaukee.
The study authors reported no relevant disclosures.
On Twitter @karioakes
MONTREAL – Patients who stayed on antiplatelet therapy close to – or even up until – major surgery fared just as well as those who stopped their medication earlier in a retrospective, single-center study.
The study found no difference in blood product administration, adverse perioperative events, or all-cause 30-day mortality regardless of whether patients stopped clopidogrel (Plavix) the recommended 5 days before surgery.
“We believe that continuing clopidogrel in elective and emergent surgical situations appears to be safe, and may challenge the current recommendations,” said presenter Dr. David Strosberg.
The study addressed a thorny question for surgeons, Dr. Strosberg said. “As surgeons, we face a dilemma: Do we take the risk of thrombotic complications in stopping the antiplatelet drugs, or do we take the risk of increased surgical bleeding with continuing therapy?”
The package insert for clopidogrel advises discontinuation 5 days prior to surgery. However, manufacturer labeling also states that discontinuation of clopidogrel can lead to adverse cardiac events, said Dr. Strosberg, a general surgery resident at Ohio State University in Columbus.
The aim of the study, presented at the annual meeting of the Central Surgical Association, was to ascertain whether continuing antiplatelet therapy increased the rate of adverse surgical outcomes in those undergoing major emergent or elective surgery.
Dr. Strosberg and his colleagues retrospectively reviewed the record of patients over a 4-year period at a single institution and included those undergoing major general, thoracic, or vascular surgery who were taking clopidogrel at the time of presentation.
Data collected included patient characteristics, including demographic data and comorbidities, as well as transfusion requirements and perioperative events.
A total of 200 patients who had 205 qualifying procedures and were taking clopidogrel were included in the study. Of these, 116 patients (Group A) had their clopidogrel held for at least 5 days preoperatively. The remaining 89 patients (Group B) had their clopidogrel held for less than 5 days, or not at all.
Patient demographics were similar between the two groups. Patients in Group A were more likely to have emergency surgery, to have peripheral stents placed, to have COPD or peripheral vascular disease, to have a malignancy, and to have received aspirin within five days of surgery (P less than .01 for all).
Blood product administration rates and volumes did not differ significantly between the two groups, and there was no difference between the groups in the incidence of myocardial infarctions, cerebrovascular events, or acute visceral or lower extremity ischemia.
Three patients in each group died within 30 days of the procedure, a nonsignificant difference. However, in the group that had clopidogrel held, three patients had perioperative myocardial infarctions, and two of these patients died. In discussing the study, Dr. Michael Dalsing said, “I think a lot of us would accept bleeding more over myocardial infarction.”
A subgroup analysis of the group who had clopidogrel held for fewer than 5 days compared outcomes for emergent vs. non-emergent surgery. The emergent surgery subgroup had a significantly higher rate of preoperative platelet transfusions, although numbers overall were small (2/17, 11.8%, vs. 0/72; P = .03).
Dr. Strosberg noted study limitations that included the retrospective, single-center nature of the study, and the fact that one variable, estimated blood loss, is notoriously subjective and inaccurate.
Dr. Dalsing, chief of vascular surgery at Indiana University, Indianapolis, said that he “was surprised that not even one patient went back for postoperative bleeding in this high-risk group of patients,” and raised the question of potential selection bias. Dr. Strosberg replied that comorbidities were ascertained by the physician at the time of surgery planning; since no differences were seen between study groups, investigators didn’t go back and parse out details about comorbid conditions.
In discussion following the presentation, surgeons spoke to the real-world challenges of performing surgery on a patient with antiplatelet therapy on board.
“Overall, I think your data support kind of a bias I have. Since I’m a vascular surgeon, we almost always operate on clopidogrel, and I don’t know if our bleeding risk is worse or better. But it’s something we almost have to do to keep our grafts going,” Dr. Dalsing said.
Dr. Peter Henke, professor of vascular surgery at the University of Michigan, Ann Arbor, said, “I’d be a little bit cautious with this. If you’ve ever done a big aortic procedure on someone on Plavix, I’ve seen them lose up to a couple of liters of blood just with oozing.”
“Those of us who do open aortic surgery know that very few things bleed like the back wall of an aortic anastomosis of a patient on Plavix,” echoed Dr. Peter Rossi, associate professor of vascular surgery at the Medical College of Wisconsin, Milwaukee.
The study authors reported no relevant disclosures.
On Twitter @karioakes
MONTREAL – Patients who stayed on antiplatelet therapy close to – or even up until – major surgery fared just as well as those who stopped their medication earlier in a retrospective, single-center study.
The study found no difference in blood product administration, adverse perioperative events, or all-cause 30-day mortality regardless of whether patients stopped clopidogrel (Plavix) the recommended 5 days before surgery.
“We believe that continuing clopidogrel in elective and emergent surgical situations appears to be safe, and may challenge the current recommendations,” said presenter Dr. David Strosberg.
The study addressed a thorny question for surgeons, Dr. Strosberg said. “As surgeons, we face a dilemma: Do we take the risk of thrombotic complications in stopping the antiplatelet drugs, or do we take the risk of increased surgical bleeding with continuing therapy?”
The package insert for clopidogrel advises discontinuation 5 days prior to surgery. However, manufacturer labeling also states that discontinuation of clopidogrel can lead to adverse cardiac events, said Dr. Strosberg, a general surgery resident at Ohio State University in Columbus.
The aim of the study, presented at the annual meeting of the Central Surgical Association, was to ascertain whether continuing antiplatelet therapy increased the rate of adverse surgical outcomes in those undergoing major emergent or elective surgery.
Dr. Strosberg and his colleagues retrospectively reviewed the record of patients over a 4-year period at a single institution and included those undergoing major general, thoracic, or vascular surgery who were taking clopidogrel at the time of presentation.
Data collected included patient characteristics, including demographic data and comorbidities, as well as transfusion requirements and perioperative events.
A total of 200 patients who had 205 qualifying procedures and were taking clopidogrel were included in the study. Of these, 116 patients (Group A) had their clopidogrel held for at least 5 days preoperatively. The remaining 89 patients (Group B) had their clopidogrel held for less than 5 days, or not at all.
Patient demographics were similar between the two groups. Patients in Group A were more likely to have emergency surgery, to have peripheral stents placed, to have COPD or peripheral vascular disease, to have a malignancy, and to have received aspirin within five days of surgery (P less than .01 for all).
Blood product administration rates and volumes did not differ significantly between the two groups, and there was no difference between the groups in the incidence of myocardial infarctions, cerebrovascular events, or acute visceral or lower extremity ischemia.
Three patients in each group died within 30 days of the procedure, a nonsignificant difference. However, in the group that had clopidogrel held, three patients had perioperative myocardial infarctions, and two of these patients died. In discussing the study, Dr. Michael Dalsing said, “I think a lot of us would accept bleeding more over myocardial infarction.”
A subgroup analysis of the group who had clopidogrel held for fewer than 5 days compared outcomes for emergent vs. non-emergent surgery. The emergent surgery subgroup had a significantly higher rate of preoperative platelet transfusions, although numbers overall were small (2/17, 11.8%, vs. 0/72; P = .03).
Dr. Strosberg noted study limitations that included the retrospective, single-center nature of the study, and the fact that one variable, estimated blood loss, is notoriously subjective and inaccurate.
Dr. Dalsing, chief of vascular surgery at Indiana University, Indianapolis, said that he “was surprised that not even one patient went back for postoperative bleeding in this high-risk group of patients,” and raised the question of potential selection bias. Dr. Strosberg replied that comorbidities were ascertained by the physician at the time of surgery planning; since no differences were seen between study groups, investigators didn’t go back and parse out details about comorbid conditions.
In discussion following the presentation, surgeons spoke to the real-world challenges of performing surgery on a patient with antiplatelet therapy on board.
“Overall, I think your data support kind of a bias I have. Since I’m a vascular surgeon, we almost always operate on clopidogrel, and I don’t know if our bleeding risk is worse or better. But it’s something we almost have to do to keep our grafts going,” Dr. Dalsing said.
Dr. Peter Henke, professor of vascular surgery at the University of Michigan, Ann Arbor, said, “I’d be a little bit cautious with this. If you’ve ever done a big aortic procedure on someone on Plavix, I’ve seen them lose up to a couple of liters of blood just with oozing.”
“Those of us who do open aortic surgery know that very few things bleed like the back wall of an aortic anastomosis of a patient on Plavix,” echoed Dr. Peter Rossi, associate professor of vascular surgery at the Medical College of Wisconsin, Milwaukee.
The study authors reported no relevant disclosures.
On Twitter @karioakes
AT THE ANNUAL MEETING OF THE CENTRAL SURGICAL ASSOCIATION
Key clinical point: Outcomes were similar whether patients did or didn’t stop clopidogrel before major surgery.
Major finding: No significant differences in blood product use, adverse events, or death were seen with continuing clopidogrel.
Data source: Retrospective, single-center review of 200 patients undergoing major elective or emergent surgery and taking clopidogrel.
Disclosures: The study authors reported no relevant disclosures.
SHM Announces 2016 Awards of Excellence Winners
The Society of Hospital Medicine (SHM) created the Awards of Excellence Program to honor its members whose exemplary contributions to the hospital medicine movement merit acknowledgment and celebration. In honor of their achievements, recipients of each Award of Excellence receive an all-expense paid trip to SHM’s annual meeting.
Award recipients also receive recognition on stage in front of friends, family, and colleagues at SHM’s annual meeting, in The Hospitalist, and on www.hospitalmedicine.org.
Congratulations to this year’s winners:
Clinical Excellence
Mark Thoelke, MD, SFHM
Dr. Thoelke became the first hospitalist at Barnes-Jewish Hospital in 1998 and helped form the Hospital Medicine Division of the Washington University School of Medicine in St. Louis in 2000, one of the first divisions in the U.S. The division is now composed of 70 physicians and eight nurse practitioners and consistently turns in superior performances on clinical outcomes as measured by UnitedHealthcare. The division has led the way with innovations in care models and teaching models and was one of the first to offer a sub-internship experience on the non-teaching service and one of the first to offer co-management with their oncology service in 2002. Dr. Thoelke still spends two-thirds of his time on clinical services and states that his job satisfaction comes largely from patient care and teaching.
Humanitarian Services
Bijay Acharya, MBBS, MD
Dr. Acharya works as a hospitalist at Massachusetts General Hospital in Boston and is currently completing the Harvard Medical School/CRICO Fellowship in Patient Safety and Quality. His humanitarian work started when he was in medical school, where he led many health camps in extremely poor villages, ran blood-donation drives, and established the poor-patient fund. After graduation, Dr. Acharya, with his friends, worked to establish a nonprofit clinic named NyayaHealth (now Possible) to serve the healthcare needs of a very remote district in rural Nepal. Prior to the clinic, there was no physician for more than a quarter million people. Recently, after the massive earthquake in Nepal, Dr. Acharya led the relief efforts for the earthquake victims. Dr. Acharya strongly believes in the capacity of hospitalists to be strong advocates for their patients, peers, and communities, both locally and globally.
Non-Physician
Tiffani M. Panek, MA, SFHM, CLHM
Panek is the hospitalist administrator for the Division of Hospital Medicine at the Johns Hopkins Bayview Medical Center in Baltimore. She is a Senior Fellow in Hospital Medicine and has also received her Certificate of Leadership in Hospital Medicine (CLHM) from SHM. She has been at Johns Hopkins for more than 12 years and has been instrumental in the significant growth and success of the Division of Hospital Medicine. Within SHM, she has been a member of the Practice Administrators Committee for three years and was recently elected to a two-year term as vice president of SHM’s Maryland Chapter. She is the first administrator to be elected to chapter leadership, to receive the CLHM, and to have an abstract accepted at an SHM annual meeting.
Outstanding Service
Thomas McIlraith, MD, SFHM, CLHM
Dr. McIlraith is the chairman of the Hospital Medicine Department at Mercy Medical Group in Sacramento, Calif. He improved patient flow between admissions and rounding with a novel operational system called Central Coordination, and it is now the standard for the Dignity Health facilities in Sacramento. The system markedly improved ED response, on-call hospitalist stress, and patient continuity. He has led many other quality and operational improvements, including unit-based rounding, rapid-response team development, and staff restructuring to improve physician coverage. Most recently, he became a leader in the Patient Experience Movement by developing the “Cognitive/Emotional Disconnect” model for understanding patient experience in hospital medicine. He is a member of the SHM Practice Management Committee.
Research
Vineet Chopra, MD, MSc, FHM
Dr. Chopra is an assistant professor of medicine and research scientist in the Patient Safety Enhancement Program at the University of Michigan and Ann Arbor VA Medical Center. Dr. Chopra’s research efforts are centered on improving the safety of hospitalized patients by preventing hospital-acquired complications. Using peripherally inserted central catheters (PICCs) as a model for this inquiry, his work has focused on quantifying current use of PICCs in hospitalized patients, estimating the risk of complications, and defining innovative ways to improve decision making for these devices. His research has been cited 1,962 times (1,580 times since 2010). He is an associate editor of The American Journal of Medicine and the Journal of Hospital Medicine and will serve as chair of SHM’s Research Committee in 2016.
Teaching
Alberto Puig, MD, PhD, SFHM
Dr. Puig has spent his career fully devoted to medical and clinical education. He is an associate professor of medicine at Harvard Medical School in Boston and director of the core educator faculty in the Department of Medicine at Massachusetts General Hospital, where he leads a unique group of physician-teachers fully devoted to clinical education. He is a regular discussant on educational programs for the academy at Harvard Medical School, and his contributions to medical education and clinical hospital teaching have made him a celebrated teacher and educator. Dr. Puig has played an important role at SHM and in the field of hospital medicine through his efforts as a medical educator; he is an avid student of the history of medicine and has been a frequent presenter at SHM’s annual meeting on this topic.
Teamwork
WellSpan Health, Active Bed Management
With the launch of ABM, Dr. Pfeiffer and Dr. Landis hoped to decrease ED length of stay by standardizing the hospitalist processes surrounding admission orders in computerized physician order entry. Ultimately, ABM at WellSpan has maintained the fastest time-to-admission order entry for any service at York Hospital—a decrease to 10 minutes from 80—with less variation for two years. ABM has also sustained national benchmark ED length of stay when the hospital is functioning at general capacity.
ABM also became instrumental in process and outcome objectives from a number of other hospital-wide initiatives. With ABM, more than 90% of a physician’s patient load is on one medical unit (up from 40%), which allowed the hospitalists to implement structured interdisciplinary bedside rounds (SIBR) on all medical units in York and Gettysburg hospitals. The success of ABM and SIBR allowed a transition-of-care project to focus on efficient discharges. Furthermore, Dr. Pfeiffer led a direct admission task force to improve direct admission referrals, safety, and acceptance, the number of which has since doubled. Without hospitalists’ ongoing leadership and effective teamwork, these significant improvements would not have been possible or sustained. TH
The Society of Hospital Medicine (SHM) created the Awards of Excellence Program to honor its members whose exemplary contributions to the hospital medicine movement merit acknowledgment and celebration. In honor of their achievements, recipients of each Award of Excellence receive an all-expense paid trip to SHM’s annual meeting.
Award recipients also receive recognition on stage in front of friends, family, and colleagues at SHM’s annual meeting, in The Hospitalist, and on www.hospitalmedicine.org.
Congratulations to this year’s winners:
Clinical Excellence
Mark Thoelke, MD, SFHM
Dr. Thoelke became the first hospitalist at Barnes-Jewish Hospital in 1998 and helped form the Hospital Medicine Division of the Washington University School of Medicine in St. Louis in 2000, one of the first divisions in the U.S. The division is now composed of 70 physicians and eight nurse practitioners and consistently turns in superior performances on clinical outcomes as measured by UnitedHealthcare. The division has led the way with innovations in care models and teaching models and was one of the first to offer a sub-internship experience on the non-teaching service and one of the first to offer co-management with their oncology service in 2002. Dr. Thoelke still spends two-thirds of his time on clinical services and states that his job satisfaction comes largely from patient care and teaching.
Humanitarian Services
Bijay Acharya, MBBS, MD
Dr. Acharya works as a hospitalist at Massachusetts General Hospital in Boston and is currently completing the Harvard Medical School/CRICO Fellowship in Patient Safety and Quality. His humanitarian work started when he was in medical school, where he led many health camps in extremely poor villages, ran blood-donation drives, and established the poor-patient fund. After graduation, Dr. Acharya, with his friends, worked to establish a nonprofit clinic named NyayaHealth (now Possible) to serve the healthcare needs of a very remote district in rural Nepal. Prior to the clinic, there was no physician for more than a quarter million people. Recently, after the massive earthquake in Nepal, Dr. Acharya led the relief efforts for the earthquake victims. Dr. Acharya strongly believes in the capacity of hospitalists to be strong advocates for their patients, peers, and communities, both locally and globally.
Non-Physician
Tiffani M. Panek, MA, SFHM, CLHM
Panek is the hospitalist administrator for the Division of Hospital Medicine at the Johns Hopkins Bayview Medical Center in Baltimore. She is a Senior Fellow in Hospital Medicine and has also received her Certificate of Leadership in Hospital Medicine (CLHM) from SHM. She has been at Johns Hopkins for more than 12 years and has been instrumental in the significant growth and success of the Division of Hospital Medicine. Within SHM, she has been a member of the Practice Administrators Committee for three years and was recently elected to a two-year term as vice president of SHM’s Maryland Chapter. She is the first administrator to be elected to chapter leadership, to receive the CLHM, and to have an abstract accepted at an SHM annual meeting.
Outstanding Service
Thomas McIlraith, MD, SFHM, CLHM
Dr. McIlraith is the chairman of the Hospital Medicine Department at Mercy Medical Group in Sacramento, Calif. He improved patient flow between admissions and rounding with a novel operational system called Central Coordination, and it is now the standard for the Dignity Health facilities in Sacramento. The system markedly improved ED response, on-call hospitalist stress, and patient continuity. He has led many other quality and operational improvements, including unit-based rounding, rapid-response team development, and staff restructuring to improve physician coverage. Most recently, he became a leader in the Patient Experience Movement by developing the “Cognitive/Emotional Disconnect” model for understanding patient experience in hospital medicine. He is a member of the SHM Practice Management Committee.
Research
Vineet Chopra, MD, MSc, FHM
Dr. Chopra is an assistant professor of medicine and research scientist in the Patient Safety Enhancement Program at the University of Michigan and Ann Arbor VA Medical Center. Dr. Chopra’s research efforts are centered on improving the safety of hospitalized patients by preventing hospital-acquired complications. Using peripherally inserted central catheters (PICCs) as a model for this inquiry, his work has focused on quantifying current use of PICCs in hospitalized patients, estimating the risk of complications, and defining innovative ways to improve decision making for these devices. His research has been cited 1,962 times (1,580 times since 2010). He is an associate editor of The American Journal of Medicine and the Journal of Hospital Medicine and will serve as chair of SHM’s Research Committee in 2016.
Teaching
Alberto Puig, MD, PhD, SFHM
Dr. Puig has spent his career fully devoted to medical and clinical education. He is an associate professor of medicine at Harvard Medical School in Boston and director of the core educator faculty in the Department of Medicine at Massachusetts General Hospital, where he leads a unique group of physician-teachers fully devoted to clinical education. He is a regular discussant on educational programs for the academy at Harvard Medical School, and his contributions to medical education and clinical hospital teaching have made him a celebrated teacher and educator. Dr. Puig has played an important role at SHM and in the field of hospital medicine through his efforts as a medical educator; he is an avid student of the history of medicine and has been a frequent presenter at SHM’s annual meeting on this topic.
Teamwork
WellSpan Health, Active Bed Management
With the launch of ABM, Dr. Pfeiffer and Dr. Landis hoped to decrease ED length of stay by standardizing the hospitalist processes surrounding admission orders in computerized physician order entry. Ultimately, ABM at WellSpan has maintained the fastest time-to-admission order entry for any service at York Hospital—a decrease to 10 minutes from 80—with less variation for two years. ABM has also sustained national benchmark ED length of stay when the hospital is functioning at general capacity.
ABM also became instrumental in process and outcome objectives from a number of other hospital-wide initiatives. With ABM, more than 90% of a physician’s patient load is on one medical unit (up from 40%), which allowed the hospitalists to implement structured interdisciplinary bedside rounds (SIBR) on all medical units in York and Gettysburg hospitals. The success of ABM and SIBR allowed a transition-of-care project to focus on efficient discharges. Furthermore, Dr. Pfeiffer led a direct admission task force to improve direct admission referrals, safety, and acceptance, the number of which has since doubled. Without hospitalists’ ongoing leadership and effective teamwork, these significant improvements would not have been possible or sustained. TH
The Society of Hospital Medicine (SHM) created the Awards of Excellence Program to honor its members whose exemplary contributions to the hospital medicine movement merit acknowledgment and celebration. In honor of their achievements, recipients of each Award of Excellence receive an all-expense paid trip to SHM’s annual meeting.
Award recipients also receive recognition on stage in front of friends, family, and colleagues at SHM’s annual meeting, in The Hospitalist, and on www.hospitalmedicine.org.
Congratulations to this year’s winners:
Clinical Excellence
Mark Thoelke, MD, SFHM
Dr. Thoelke became the first hospitalist at Barnes-Jewish Hospital in 1998 and helped form the Hospital Medicine Division of the Washington University School of Medicine in St. Louis in 2000, one of the first divisions in the U.S. The division is now composed of 70 physicians and eight nurse practitioners and consistently turns in superior performances on clinical outcomes as measured by UnitedHealthcare. The division has led the way with innovations in care models and teaching models and was one of the first to offer a sub-internship experience on the non-teaching service and one of the first to offer co-management with their oncology service in 2002. Dr. Thoelke still spends two-thirds of his time on clinical services and states that his job satisfaction comes largely from patient care and teaching.
Humanitarian Services
Bijay Acharya, MBBS, MD
Dr. Acharya works as a hospitalist at Massachusetts General Hospital in Boston and is currently completing the Harvard Medical School/CRICO Fellowship in Patient Safety and Quality. His humanitarian work started when he was in medical school, where he led many health camps in extremely poor villages, ran blood-donation drives, and established the poor-patient fund. After graduation, Dr. Acharya, with his friends, worked to establish a nonprofit clinic named NyayaHealth (now Possible) to serve the healthcare needs of a very remote district in rural Nepal. Prior to the clinic, there was no physician for more than a quarter million people. Recently, after the massive earthquake in Nepal, Dr. Acharya led the relief efforts for the earthquake victims. Dr. Acharya strongly believes in the capacity of hospitalists to be strong advocates for their patients, peers, and communities, both locally and globally.
Non-Physician
Tiffani M. Panek, MA, SFHM, CLHM
Panek is the hospitalist administrator for the Division of Hospital Medicine at the Johns Hopkins Bayview Medical Center in Baltimore. She is a Senior Fellow in Hospital Medicine and has also received her Certificate of Leadership in Hospital Medicine (CLHM) from SHM. She has been at Johns Hopkins for more than 12 years and has been instrumental in the significant growth and success of the Division of Hospital Medicine. Within SHM, she has been a member of the Practice Administrators Committee for three years and was recently elected to a two-year term as vice president of SHM’s Maryland Chapter. She is the first administrator to be elected to chapter leadership, to receive the CLHM, and to have an abstract accepted at an SHM annual meeting.
Outstanding Service
Thomas McIlraith, MD, SFHM, CLHM
Dr. McIlraith is the chairman of the Hospital Medicine Department at Mercy Medical Group in Sacramento, Calif. He improved patient flow between admissions and rounding with a novel operational system called Central Coordination, and it is now the standard for the Dignity Health facilities in Sacramento. The system markedly improved ED response, on-call hospitalist stress, and patient continuity. He has led many other quality and operational improvements, including unit-based rounding, rapid-response team development, and staff restructuring to improve physician coverage. Most recently, he became a leader in the Patient Experience Movement by developing the “Cognitive/Emotional Disconnect” model for understanding patient experience in hospital medicine. He is a member of the SHM Practice Management Committee.
Research
Vineet Chopra, MD, MSc, FHM
Dr. Chopra is an assistant professor of medicine and research scientist in the Patient Safety Enhancement Program at the University of Michigan and Ann Arbor VA Medical Center. Dr. Chopra’s research efforts are centered on improving the safety of hospitalized patients by preventing hospital-acquired complications. Using peripherally inserted central catheters (PICCs) as a model for this inquiry, his work has focused on quantifying current use of PICCs in hospitalized patients, estimating the risk of complications, and defining innovative ways to improve decision making for these devices. His research has been cited 1,962 times (1,580 times since 2010). He is an associate editor of The American Journal of Medicine and the Journal of Hospital Medicine and will serve as chair of SHM’s Research Committee in 2016.
Teaching
Alberto Puig, MD, PhD, SFHM
Dr. Puig has spent his career fully devoted to medical and clinical education. He is an associate professor of medicine at Harvard Medical School in Boston and director of the core educator faculty in the Department of Medicine at Massachusetts General Hospital, where he leads a unique group of physician-teachers fully devoted to clinical education. He is a regular discussant on educational programs for the academy at Harvard Medical School, and his contributions to medical education and clinical hospital teaching have made him a celebrated teacher and educator. Dr. Puig has played an important role at SHM and in the field of hospital medicine through his efforts as a medical educator; he is an avid student of the history of medicine and has been a frequent presenter at SHM’s annual meeting on this topic.
Teamwork
WellSpan Health, Active Bed Management
With the launch of ABM, Dr. Pfeiffer and Dr. Landis hoped to decrease ED length of stay by standardizing the hospitalist processes surrounding admission orders in computerized physician order entry. Ultimately, ABM at WellSpan has maintained the fastest time-to-admission order entry for any service at York Hospital—a decrease to 10 minutes from 80—with less variation for two years. ABM has also sustained national benchmark ED length of stay when the hospital is functioning at general capacity.
ABM also became instrumental in process and outcome objectives from a number of other hospital-wide initiatives. With ABM, more than 90% of a physician’s patient load is on one medical unit (up from 40%), which allowed the hospitalists to implement structured interdisciplinary bedside rounds (SIBR) on all medical units in York and Gettysburg hospitals. The success of ABM and SIBR allowed a transition-of-care project to focus on efficient discharges. Furthermore, Dr. Pfeiffer led a direct admission task force to improve direct admission referrals, safety, and acceptance, the number of which has since doubled. Without hospitalists’ ongoing leadership and effective teamwork, these significant improvements would not have been possible or sustained. TH
AEs prompt EMA review of idelalisib

Photo courtesy of
Gilead Sciences, Inc.
The European Medicines Agency (EMA) is reviewing the safety of idelalisib (Zydelig), a drug approved to treat chronic lymphocytic leukemia (CLL) and follicular lymphoma in the European Union (EU).
The European Commission (EC) requested the review because of serious adverse events (AEs), including deaths, reported in 3 clinical trials investigating idelalisib in combination with other drugs.
The AEs were mostly infection-related.
The EMA is reviewing data from these studies to assess whether the findings have any consequences for the authorized uses of idelalisib.
In the meantime, the EMA advises that patients starting or already on treatment with idelalisib be carefully monitored for signs of infections. If the drug is well tolerated, treatment should not be stopped.
The EMA is considering whether any other immediate measures are necessary during the review period. The agency said it will communicate further and keep doctors and patients informed as appropriate.
About idelalisib
In the EU, idelalisib is approved for use in combination with rituximab to treat adults with CLL who have received at least 1 prior therapy or as first-line treatment in the presence of 17p deletion or TP53 mutation in CLL patients unsuitable for chemo-immunotherapy.
Idelalisib is also approved as monotherapy for adults with follicular lymphoma that is refractory to 2 prior lines of treatment.
About the trials
The trials in which patients have experienced serious AEs involve patients with CLL and indolent non-Hodgkin lymphoma (NHL).
In one trial (NCT01732926), researchers are evaluating idelalisib in combination with bendamustine and rituximab for previously treated indolent NHL.
In another (NCT01732913), researchers are testing idelalisib in combination with rituximab for previously treated indolent NHL.
And in the third (NCT01980888), researchers are evaluating idelalisib in combination with bendamustine and rituximab in patients with previously untreated CLL.
The EMA noted that these studies are investigating combinations of drugs that are currently not approved in the EU and include patients with disease characteristics different from those covered by the approved indications for idelalisib.
About the review
The EMA has begun the review of idelalisib at the request of the EC, under Article 20 of Directive 2001/83/EC.
The review is being carried out by the EMA’s Pharmacovigilance Risk Assessment Committee, the committee responsible for the evaluation of safety issues for human medicines, which will make a set of recommendations.
Those recommendations will then be forwarded to the Committee for Medicinal Products for Human Use, which is responsible for questions concerning medicines for human use and will adopt a final opinion on the safety of idelalisib.
The final stage of the review procedure is the EC’s adoption of a legally binding decision that is applicable in all EU member states. ![]()

Photo courtesy of
Gilead Sciences, Inc.
The European Medicines Agency (EMA) is reviewing the safety of idelalisib (Zydelig), a drug approved to treat chronic lymphocytic leukemia (CLL) and follicular lymphoma in the European Union (EU).
The European Commission (EC) requested the review because of serious adverse events (AEs), including deaths, reported in 3 clinical trials investigating idelalisib in combination with other drugs.
The AEs were mostly infection-related.
The EMA is reviewing data from these studies to assess whether the findings have any consequences for the authorized uses of idelalisib.
In the meantime, the EMA advises that patients starting or already on treatment with idelalisib be carefully monitored for signs of infections. If the drug is well tolerated, treatment should not be stopped.
The EMA is considering whether any other immediate measures are necessary during the review period. The agency said it will communicate further and keep doctors and patients informed as appropriate.
About idelalisib
In the EU, idelalisib is approved for use in combination with rituximab to treat adults with CLL who have received at least 1 prior therapy or as first-line treatment in the presence of 17p deletion or TP53 mutation in CLL patients unsuitable for chemo-immunotherapy.
Idelalisib is also approved as monotherapy for adults with follicular lymphoma that is refractory to 2 prior lines of treatment.
About the trials
The trials in which patients have experienced serious AEs involve patients with CLL and indolent non-Hodgkin lymphoma (NHL).
In one trial (NCT01732926), researchers are evaluating idelalisib in combination with bendamustine and rituximab for previously treated indolent NHL.
In another (NCT01732913), researchers are testing idelalisib in combination with rituximab for previously treated indolent NHL.
And in the third (NCT01980888), researchers are evaluating idelalisib in combination with bendamustine and rituximab in patients with previously untreated CLL.
The EMA noted that these studies are investigating combinations of drugs that are currently not approved in the EU and include patients with disease characteristics different from those covered by the approved indications for idelalisib.
About the review
The EMA has begun the review of idelalisib at the request of the EC, under Article 20 of Directive 2001/83/EC.
The review is being carried out by the EMA’s Pharmacovigilance Risk Assessment Committee, the committee responsible for the evaluation of safety issues for human medicines, which will make a set of recommendations.
Those recommendations will then be forwarded to the Committee for Medicinal Products for Human Use, which is responsible for questions concerning medicines for human use and will adopt a final opinion on the safety of idelalisib.
The final stage of the review procedure is the EC’s adoption of a legally binding decision that is applicable in all EU member states. ![]()

Photo courtesy of
Gilead Sciences, Inc.
The European Medicines Agency (EMA) is reviewing the safety of idelalisib (Zydelig), a drug approved to treat chronic lymphocytic leukemia (CLL) and follicular lymphoma in the European Union (EU).
The European Commission (EC) requested the review because of serious adverse events (AEs), including deaths, reported in 3 clinical trials investigating idelalisib in combination with other drugs.
The AEs were mostly infection-related.
The EMA is reviewing data from these studies to assess whether the findings have any consequences for the authorized uses of idelalisib.
In the meantime, the EMA advises that patients starting or already on treatment with idelalisib be carefully monitored for signs of infections. If the drug is well tolerated, treatment should not be stopped.
The EMA is considering whether any other immediate measures are necessary during the review period. The agency said it will communicate further and keep doctors and patients informed as appropriate.
About idelalisib
In the EU, idelalisib is approved for use in combination with rituximab to treat adults with CLL who have received at least 1 prior therapy or as first-line treatment in the presence of 17p deletion or TP53 mutation in CLL patients unsuitable for chemo-immunotherapy.
Idelalisib is also approved as monotherapy for adults with follicular lymphoma that is refractory to 2 prior lines of treatment.
About the trials
The trials in which patients have experienced serious AEs involve patients with CLL and indolent non-Hodgkin lymphoma (NHL).
In one trial (NCT01732926), researchers are evaluating idelalisib in combination with bendamustine and rituximab for previously treated indolent NHL.
In another (NCT01732913), researchers are testing idelalisib in combination with rituximab for previously treated indolent NHL.
And in the third (NCT01980888), researchers are evaluating idelalisib in combination with bendamustine and rituximab in patients with previously untreated CLL.
The EMA noted that these studies are investigating combinations of drugs that are currently not approved in the EU and include patients with disease characteristics different from those covered by the approved indications for idelalisib.
About the review
The EMA has begun the review of idelalisib at the request of the EC, under Article 20 of Directive 2001/83/EC.
The review is being carried out by the EMA’s Pharmacovigilance Risk Assessment Committee, the committee responsible for the evaluation of safety issues for human medicines, which will make a set of recommendations.
Those recommendations will then be forwarded to the Committee for Medicinal Products for Human Use, which is responsible for questions concerning medicines for human use and will adopt a final opinion on the safety of idelalisib.
The final stage of the review procedure is the EC’s adoption of a legally binding decision that is applicable in all EU member states. ![]()
Global Surgery: ‘Partnership Among Friends’
Surgery volunteerism has been on the rise for several decades. The American College of Surgeons is increasing its role in organizing and facilitating these programs via Operation Giving Back (OGB). And many ACS members are prominent participants in this endeavor.
A leader in global surgery is Michael L. Bentz, M.D., FAAP, FACS, professor of surgery, pediatrics, and neurosurgery, and chairman of the Division of Plastic and Reconstructive Surgery at the University of Wisconsin School of Medicine and Public Health. Dr. Bentz has led international missions in many countries of the world over nearly 20 years and has helped a team develop a long-term program of clinical care and training in Nicaragua. We talked with him about his experiences.
Q: You have been involved in international surgical missions for many years. Can you tell us something about your early projects?
I was first exposed to international work at the University of Pittsburgh. My mentor J. William Futrell, M.D., FACS, was a veteran of over 30 international surgical trips. I went on the first trip with him to Vietnam in the 1997 and have been going ever since. For that initial trip, we worked with a nonprofit organization called Interplast. I went with a large group of 20 people from the University that included plastic surgery attendings, plastic surgery residents, pediatric attendings, pediatric residents, and nursing and anesthesia staff.
In those days, many trips were based predominantly on clinical care – adult care and pediatric care. Teams would do a certain number of operations and then go home. We did cleft lip repairs, cleft palate repairs, burn reconstruction, congenital hand deformity surgery, and tumor management.
That would result in good outcomes for those who actually had a procedure done. But in any place I have ever worked overseas – Vietnam, China, Russia, Nicaragua – the need is overwhelming. The need far outstripped what surgical missions can provide in isolated, single trips back and forth.
Q: The years have brought changes to these missions. What are the most significant changes over the years in how these missions are conducted?
The scope and direction of global health is moving toward sustainable, long-term, and longitudinal education. In those earlier trips where there was an emphasis on doing as many operations as possible, people meant well – we meant well! But the real impact comes with the longitudinal education investment.
I have never been anywhere around the world where there weren’t interested, very capable, excellent surgeons committed to taking care of their patients who only need some support and facilitation.
If you compare the cases we are able to do on a trip with our partners with the cases they are able to do independently, it’s a logarithmic curve – they are far more productive than we could ever be on any number of trips. There is a multiplier effect that allows many more patients to be taken care of.
Q: Your institution has a long-term relationship with a hospital in Nicaragua. How does this work and what is the role of your team in the program?
The University of Wisconsin Division of Plastic Surgery and the Eduplast Foundation has a team of about 10 that goes to Nicaragua twice a year. Most importantly, we support a residency program in there. We move residents through a 3-year modular program much like programs in the U.S. and then examine them. We facilitate this educational process with trips there and we bring them to our institution in the U.S.
Over the past 10 years, we have been doing a weekly live webcast of our Plastic Surgery Grand Rounds which is received on several continents. This creates a very valuable bidirectional, and even tridirectional conversation. This webcast is simple, incredibly inexpensive, and has provided hundreds of hours of education over the years in addition to the on-site work we do.
There can be a language barrier in some cases, but we broadcast in English, with occasional translation support. In addition to Nicaragua, our webcast has been received in institutions in Thailand, China, Ecuador and across the United States. We keep records of cases performed. Our plastic surgery residents can get credit for the cases they do under faculty supervision at our international sites if we meet specific criteria set by our Resident Review Committee.
It is important to note that we take care of the patients in our partner institutions in Nicaragua exactly as we would care for patients in our institution in Wisconsin. There is no “practicing” as all operations are done by surgeons appropriately credentialed and trained for the task.
Q: Do you find that there is a cultural gap that you must bridge in working with colleagues and patients in Nicaragua?
Our program has an orientation session for team participants in advance of each trip, where we talk about the mechanics of the trip – safety, medical issues. We also talk about cultural considerations of each site. It is very important that the residents embed in the culture in which we are working. They also need to know the cultural norms of how to communicate with patients, parents, and children. Some of it is simply good manners – acting like your mother taught you!
The team can reside in a local hotel, but often stays in the homes of local hosts, and this can be a beautiful opportunity to learn about local norms and communication.
Q: What is your favorite part of these missions?
I have so many favorite parts! I like caring for people who otherwise might not receive medical care. This is “giving back” and I think all of the participants would agree that we come home feeling like we received much more than we gave. These experiences remind you of why you went to medical school. It is an opportunity to provide something in return for all the investment that has been made in us for our education. In working with colleagues from other countries, I learn as much as I teach. I come back a better surgeon.
The benefits to residents from our institution are many. They learn how to operate in a resource-limited setting, and they return with a greater appreciation for the equipment and supplies we have available at our institution in Madison. The cultural competence and awareness they also learn is an invaluable life skill.
I want to stress that the friendships with our fellow surgeons are what makes this work. We achieve a degree of continuity and even watch our pediatric patients grow up over the years because of our long-term relationship with the hospital in León and our dedicated colleagues there. This is a truly a partnership among friends.
Q: Do you have some advice for a surgeon interested in participating in an international program?
For those surgeons who were not exposed to these programs during residency, finding a mentor or mentoring organization is the way to begin. A beginner should consider making the first couple of trips with someone who knows the ropes in terms of understanding cultural competency, practical issues of safety, and relevant clinical issues. Almost every surgery discipline has an organization with the capability of identifying volunteer surgery groups in their specialty. ACS’ Operation Giving Back is a particularly important resource for helping Fellows find the right international program.
If you would like to learn more about global surgery programs, contact Operation Giving Back at gtefera@facs.org. Or if you would like to share your experiences as an international surgical volunteer, please email this publication at acssurgerynews@frontlinemedcom.com.
Surgery volunteerism has been on the rise for several decades. The American College of Surgeons is increasing its role in organizing and facilitating these programs via Operation Giving Back (OGB). And many ACS members are prominent participants in this endeavor.
A leader in global surgery is Michael L. Bentz, M.D., FAAP, FACS, professor of surgery, pediatrics, and neurosurgery, and chairman of the Division of Plastic and Reconstructive Surgery at the University of Wisconsin School of Medicine and Public Health. Dr. Bentz has led international missions in many countries of the world over nearly 20 years and has helped a team develop a long-term program of clinical care and training in Nicaragua. We talked with him about his experiences.
Q: You have been involved in international surgical missions for many years. Can you tell us something about your early projects?
I was first exposed to international work at the University of Pittsburgh. My mentor J. William Futrell, M.D., FACS, was a veteran of over 30 international surgical trips. I went on the first trip with him to Vietnam in the 1997 and have been going ever since. For that initial trip, we worked with a nonprofit organization called Interplast. I went with a large group of 20 people from the University that included plastic surgery attendings, plastic surgery residents, pediatric attendings, pediatric residents, and nursing and anesthesia staff.
In those days, many trips were based predominantly on clinical care – adult care and pediatric care. Teams would do a certain number of operations and then go home. We did cleft lip repairs, cleft palate repairs, burn reconstruction, congenital hand deformity surgery, and tumor management.
That would result in good outcomes for those who actually had a procedure done. But in any place I have ever worked overseas – Vietnam, China, Russia, Nicaragua – the need is overwhelming. The need far outstripped what surgical missions can provide in isolated, single trips back and forth.
Q: The years have brought changes to these missions. What are the most significant changes over the years in how these missions are conducted?
The scope and direction of global health is moving toward sustainable, long-term, and longitudinal education. In those earlier trips where there was an emphasis on doing as many operations as possible, people meant well – we meant well! But the real impact comes with the longitudinal education investment.
I have never been anywhere around the world where there weren’t interested, very capable, excellent surgeons committed to taking care of their patients who only need some support and facilitation.
If you compare the cases we are able to do on a trip with our partners with the cases they are able to do independently, it’s a logarithmic curve – they are far more productive than we could ever be on any number of trips. There is a multiplier effect that allows many more patients to be taken care of.
Q: Your institution has a long-term relationship with a hospital in Nicaragua. How does this work and what is the role of your team in the program?
The University of Wisconsin Division of Plastic Surgery and the Eduplast Foundation has a team of about 10 that goes to Nicaragua twice a year. Most importantly, we support a residency program in there. We move residents through a 3-year modular program much like programs in the U.S. and then examine them. We facilitate this educational process with trips there and we bring them to our institution in the U.S.
Over the past 10 years, we have been doing a weekly live webcast of our Plastic Surgery Grand Rounds which is received on several continents. This creates a very valuable bidirectional, and even tridirectional conversation. This webcast is simple, incredibly inexpensive, and has provided hundreds of hours of education over the years in addition to the on-site work we do.
There can be a language barrier in some cases, but we broadcast in English, with occasional translation support. In addition to Nicaragua, our webcast has been received in institutions in Thailand, China, Ecuador and across the United States. We keep records of cases performed. Our plastic surgery residents can get credit for the cases they do under faculty supervision at our international sites if we meet specific criteria set by our Resident Review Committee.
It is important to note that we take care of the patients in our partner institutions in Nicaragua exactly as we would care for patients in our institution in Wisconsin. There is no “practicing” as all operations are done by surgeons appropriately credentialed and trained for the task.
Q: Do you find that there is a cultural gap that you must bridge in working with colleagues and patients in Nicaragua?
Our program has an orientation session for team participants in advance of each trip, where we talk about the mechanics of the trip – safety, medical issues. We also talk about cultural considerations of each site. It is very important that the residents embed in the culture in which we are working. They also need to know the cultural norms of how to communicate with patients, parents, and children. Some of it is simply good manners – acting like your mother taught you!
The team can reside in a local hotel, but often stays in the homes of local hosts, and this can be a beautiful opportunity to learn about local norms and communication.
Q: What is your favorite part of these missions?
I have so many favorite parts! I like caring for people who otherwise might not receive medical care. This is “giving back” and I think all of the participants would agree that we come home feeling like we received much more than we gave. These experiences remind you of why you went to medical school. It is an opportunity to provide something in return for all the investment that has been made in us for our education. In working with colleagues from other countries, I learn as much as I teach. I come back a better surgeon.
The benefits to residents from our institution are many. They learn how to operate in a resource-limited setting, and they return with a greater appreciation for the equipment and supplies we have available at our institution in Madison. The cultural competence and awareness they also learn is an invaluable life skill.
I want to stress that the friendships with our fellow surgeons are what makes this work. We achieve a degree of continuity and even watch our pediatric patients grow up over the years because of our long-term relationship with the hospital in León and our dedicated colleagues there. This is a truly a partnership among friends.
Q: Do you have some advice for a surgeon interested in participating in an international program?
For those surgeons who were not exposed to these programs during residency, finding a mentor or mentoring organization is the way to begin. A beginner should consider making the first couple of trips with someone who knows the ropes in terms of understanding cultural competency, practical issues of safety, and relevant clinical issues. Almost every surgery discipline has an organization with the capability of identifying volunteer surgery groups in their specialty. ACS’ Operation Giving Back is a particularly important resource for helping Fellows find the right international program.
If you would like to learn more about global surgery programs, contact Operation Giving Back at gtefera@facs.org. Or if you would like to share your experiences as an international surgical volunteer, please email this publication at acssurgerynews@frontlinemedcom.com.
Surgery volunteerism has been on the rise for several decades. The American College of Surgeons is increasing its role in organizing and facilitating these programs via Operation Giving Back (OGB). And many ACS members are prominent participants in this endeavor.
A leader in global surgery is Michael L. Bentz, M.D., FAAP, FACS, professor of surgery, pediatrics, and neurosurgery, and chairman of the Division of Plastic and Reconstructive Surgery at the University of Wisconsin School of Medicine and Public Health. Dr. Bentz has led international missions in many countries of the world over nearly 20 years and has helped a team develop a long-term program of clinical care and training in Nicaragua. We talked with him about his experiences.
Q: You have been involved in international surgical missions for many years. Can you tell us something about your early projects?
I was first exposed to international work at the University of Pittsburgh. My mentor J. William Futrell, M.D., FACS, was a veteran of over 30 international surgical trips. I went on the first trip with him to Vietnam in the 1997 and have been going ever since. For that initial trip, we worked with a nonprofit organization called Interplast. I went with a large group of 20 people from the University that included plastic surgery attendings, plastic surgery residents, pediatric attendings, pediatric residents, and nursing and anesthesia staff.
In those days, many trips were based predominantly on clinical care – adult care and pediatric care. Teams would do a certain number of operations and then go home. We did cleft lip repairs, cleft palate repairs, burn reconstruction, congenital hand deformity surgery, and tumor management.
That would result in good outcomes for those who actually had a procedure done. But in any place I have ever worked overseas – Vietnam, China, Russia, Nicaragua – the need is overwhelming. The need far outstripped what surgical missions can provide in isolated, single trips back and forth.
Q: The years have brought changes to these missions. What are the most significant changes over the years in how these missions are conducted?
The scope and direction of global health is moving toward sustainable, long-term, and longitudinal education. In those earlier trips where there was an emphasis on doing as many operations as possible, people meant well – we meant well! But the real impact comes with the longitudinal education investment.
I have never been anywhere around the world where there weren’t interested, very capable, excellent surgeons committed to taking care of their patients who only need some support and facilitation.
If you compare the cases we are able to do on a trip with our partners with the cases they are able to do independently, it’s a logarithmic curve – they are far more productive than we could ever be on any number of trips. There is a multiplier effect that allows many more patients to be taken care of.
Q: Your institution has a long-term relationship with a hospital in Nicaragua. How does this work and what is the role of your team in the program?
The University of Wisconsin Division of Plastic Surgery and the Eduplast Foundation has a team of about 10 that goes to Nicaragua twice a year. Most importantly, we support a residency program in there. We move residents through a 3-year modular program much like programs in the U.S. and then examine them. We facilitate this educational process with trips there and we bring them to our institution in the U.S.
Over the past 10 years, we have been doing a weekly live webcast of our Plastic Surgery Grand Rounds which is received on several continents. This creates a very valuable bidirectional, and even tridirectional conversation. This webcast is simple, incredibly inexpensive, and has provided hundreds of hours of education over the years in addition to the on-site work we do.
There can be a language barrier in some cases, but we broadcast in English, with occasional translation support. In addition to Nicaragua, our webcast has been received in institutions in Thailand, China, Ecuador and across the United States. We keep records of cases performed. Our plastic surgery residents can get credit for the cases they do under faculty supervision at our international sites if we meet specific criteria set by our Resident Review Committee.
It is important to note that we take care of the patients in our partner institutions in Nicaragua exactly as we would care for patients in our institution in Wisconsin. There is no “practicing” as all operations are done by surgeons appropriately credentialed and trained for the task.
Q: Do you find that there is a cultural gap that you must bridge in working with colleagues and patients in Nicaragua?
Our program has an orientation session for team participants in advance of each trip, where we talk about the mechanics of the trip – safety, medical issues. We also talk about cultural considerations of each site. It is very important that the residents embed in the culture in which we are working. They also need to know the cultural norms of how to communicate with patients, parents, and children. Some of it is simply good manners – acting like your mother taught you!
The team can reside in a local hotel, but often stays in the homes of local hosts, and this can be a beautiful opportunity to learn about local norms and communication.
Q: What is your favorite part of these missions?
I have so many favorite parts! I like caring for people who otherwise might not receive medical care. This is “giving back” and I think all of the participants would agree that we come home feeling like we received much more than we gave. These experiences remind you of why you went to medical school. It is an opportunity to provide something in return for all the investment that has been made in us for our education. In working with colleagues from other countries, I learn as much as I teach. I come back a better surgeon.
The benefits to residents from our institution are many. They learn how to operate in a resource-limited setting, and they return with a greater appreciation for the equipment and supplies we have available at our institution in Madison. The cultural competence and awareness they also learn is an invaluable life skill.
I want to stress that the friendships with our fellow surgeons are what makes this work. We achieve a degree of continuity and even watch our pediatric patients grow up over the years because of our long-term relationship with the hospital in León and our dedicated colleagues there. This is a truly a partnership among friends.
Q: Do you have some advice for a surgeon interested in participating in an international program?
For those surgeons who were not exposed to these programs during residency, finding a mentor or mentoring organization is the way to begin. A beginner should consider making the first couple of trips with someone who knows the ropes in terms of understanding cultural competency, practical issues of safety, and relevant clinical issues. Almost every surgery discipline has an organization with the capability of identifying volunteer surgery groups in their specialty. ACS’ Operation Giving Back is a particularly important resource for helping Fellows find the right international program.
If you would like to learn more about global surgery programs, contact Operation Giving Back at gtefera@facs.org. Or if you would like to share your experiences as an international surgical volunteer, please email this publication at acssurgerynews@frontlinemedcom.com.