User login
Apply now for 2016 international scholarships for surgical education
The American College of Surgeons (ACS) Division of Education and the International Relations Committee have announced the availability of two international scholarships focused on surgical education for 2016. These awards will offer faculty members from countries other than the U.S. and Canada the opportunity to participate in a variety of faculty development activities that will result in acquisition of new knowledge and skills in surgical education and training. The intent of the program is to help scholars improve surgical education and training in their home institutions and countries. All application materials and supporting documents are due no later than May 2 for attendance at the ACS Clinical Congress 2016, October 16−20 in Washington, DC.
About the program
The two scholars will participate in the annual Clinical Congress, including the Surgical Education: Principles and Practice course, as well as other plenary sessions and Postgraduate Courses that address surgical education and training needs across the continuum of professional development. This continuum may include the needs of practicing surgeons through their entire careers, as well as the needs of surgery residents, medical students, and other members of the surgical team.
After the Clinical Congress, each scholar will visit two Level I ACS Accredited Education Institutes (ACS-AEIs) selected in advance based on the scholars’ interest areas in surgical education and training. At the conclusion of the Clinical Congress and the scholars’ visits to the ACS-AEIs, each recipient will send to the International Relations Committee and the Division of Education a brief report outlining the outcomes that have been achieved as a result of their scholarship; this report should focus specifically on the objectives outlined in their application for the scholarship. The scholarships will facilitate the scholars’ involvement in subsequent collaborative ventures in education and training under the aegis of the ACS Division of Education.
Each scholarship provides a stipend of $10,000, supporting travel and per diem in North America and the cost of Postgraduate Courses undertaken at the Clinical Congress and at the ACS-AEIs to be visited. Clinical Congress registration and fees for attendance at the Surgical Education: Principles and Practices course will be provided. Assistance will be offered to reserve affordable housing in Washington, DC, during the Clinical Congress.
Application requirements
Applicants must document prior experience in surgical education and training, such as involvement in the development and evaluation of education modules, use of novel teaching and assessment strategies, or curriculum design. In addition, applicants must submit a one-paragraph description of their education philosophies, a list of specific educational goals and objectives for their visits, and evidence of support of these goals and objectives from the leadership at their home institutions. These documents will be reviewed by the Division of Education as part of the selection process. At least 5 years of experience beyond completion of all training and fellowships is required. Scholarships must be used in the year awarded; they may not be postponed.
Full scholarship requirements for this program may be reviewed at facs.org/member-services/scholarships/international/issurged. The application for the scholarship may be accessed at the bottom of the requirements page. Questions should be directed to Kate Early, ACS International Liaison, at kearly@facs.org.
The American College of Surgeons (ACS) Division of Education and the International Relations Committee have announced the availability of two international scholarships focused on surgical education for 2016. These awards will offer faculty members from countries other than the U.S. and Canada the opportunity to participate in a variety of faculty development activities that will result in acquisition of new knowledge and skills in surgical education and training. The intent of the program is to help scholars improve surgical education and training in their home institutions and countries. All application materials and supporting documents are due no later than May 2 for attendance at the ACS Clinical Congress 2016, October 16−20 in Washington, DC.
About the program
The two scholars will participate in the annual Clinical Congress, including the Surgical Education: Principles and Practice course, as well as other plenary sessions and Postgraduate Courses that address surgical education and training needs across the continuum of professional development. This continuum may include the needs of practicing surgeons through their entire careers, as well as the needs of surgery residents, medical students, and other members of the surgical team.
After the Clinical Congress, each scholar will visit two Level I ACS Accredited Education Institutes (ACS-AEIs) selected in advance based on the scholars’ interest areas in surgical education and training. At the conclusion of the Clinical Congress and the scholars’ visits to the ACS-AEIs, each recipient will send to the International Relations Committee and the Division of Education a brief report outlining the outcomes that have been achieved as a result of their scholarship; this report should focus specifically on the objectives outlined in their application for the scholarship. The scholarships will facilitate the scholars’ involvement in subsequent collaborative ventures in education and training under the aegis of the ACS Division of Education.
Each scholarship provides a stipend of $10,000, supporting travel and per diem in North America and the cost of Postgraduate Courses undertaken at the Clinical Congress and at the ACS-AEIs to be visited. Clinical Congress registration and fees for attendance at the Surgical Education: Principles and Practices course will be provided. Assistance will be offered to reserve affordable housing in Washington, DC, during the Clinical Congress.
Application requirements
Applicants must document prior experience in surgical education and training, such as involvement in the development and evaluation of education modules, use of novel teaching and assessment strategies, or curriculum design. In addition, applicants must submit a one-paragraph description of their education philosophies, a list of specific educational goals and objectives for their visits, and evidence of support of these goals and objectives from the leadership at their home institutions. These documents will be reviewed by the Division of Education as part of the selection process. At least 5 years of experience beyond completion of all training and fellowships is required. Scholarships must be used in the year awarded; they may not be postponed.
Full scholarship requirements for this program may be reviewed at facs.org/member-services/scholarships/international/issurged. The application for the scholarship may be accessed at the bottom of the requirements page. Questions should be directed to Kate Early, ACS International Liaison, at kearly@facs.org.
The American College of Surgeons (ACS) Division of Education and the International Relations Committee have announced the availability of two international scholarships focused on surgical education for 2016. These awards will offer faculty members from countries other than the U.S. and Canada the opportunity to participate in a variety of faculty development activities that will result in acquisition of new knowledge and skills in surgical education and training. The intent of the program is to help scholars improve surgical education and training in their home institutions and countries. All application materials and supporting documents are due no later than May 2 for attendance at the ACS Clinical Congress 2016, October 16−20 in Washington, DC.
About the program
The two scholars will participate in the annual Clinical Congress, including the Surgical Education: Principles and Practice course, as well as other plenary sessions and Postgraduate Courses that address surgical education and training needs across the continuum of professional development. This continuum may include the needs of practicing surgeons through their entire careers, as well as the needs of surgery residents, medical students, and other members of the surgical team.
After the Clinical Congress, each scholar will visit two Level I ACS Accredited Education Institutes (ACS-AEIs) selected in advance based on the scholars’ interest areas in surgical education and training. At the conclusion of the Clinical Congress and the scholars’ visits to the ACS-AEIs, each recipient will send to the International Relations Committee and the Division of Education a brief report outlining the outcomes that have been achieved as a result of their scholarship; this report should focus specifically on the objectives outlined in their application for the scholarship. The scholarships will facilitate the scholars’ involvement in subsequent collaborative ventures in education and training under the aegis of the ACS Division of Education.
Each scholarship provides a stipend of $10,000, supporting travel and per diem in North America and the cost of Postgraduate Courses undertaken at the Clinical Congress and at the ACS-AEIs to be visited. Clinical Congress registration and fees for attendance at the Surgical Education: Principles and Practices course will be provided. Assistance will be offered to reserve affordable housing in Washington, DC, during the Clinical Congress.
Application requirements
Applicants must document prior experience in surgical education and training, such as involvement in the development and evaluation of education modules, use of novel teaching and assessment strategies, or curriculum design. In addition, applicants must submit a one-paragraph description of their education philosophies, a list of specific educational goals and objectives for their visits, and evidence of support of these goals and objectives from the leadership at their home institutions. These documents will be reviewed by the Division of Education as part of the selection process. At least 5 years of experience beyond completion of all training and fellowships is required. Scholarships must be used in the year awarded; they may not be postponed.
Full scholarship requirements for this program may be reviewed at facs.org/member-services/scholarships/international/issurged. The application for the scholarship may be accessed at the bottom of the requirements page. Questions should be directed to Kate Early, ACS International Liaison, at kearly@facs.org.
International Guest Scholarships available for 2017
The American College of Surgeons (ACS) offers International Guest Scholarships to young surgeons from countries other than the U.S. or Canada who have demonstrated a strong interest in teaching and research. Twelve scholarships are available for 2017, in the amount of $10,000 each, and will provide the scholars with an opportunity to engage in clinical, teaching, and research activities in the U.S. and Canada and to attend and participate fully in the educational opportunities and activities of the ACS Clinical Congress. For consideration by the Selection Committee, completed applications for the 2017 International Guest Scholarships and all supporting documentation must be received at the office of the International Liaison Section by June 30, 2016.
Paul R. Hawley, MD, FACS(Hon), Past-ACS Executive Director (1950−1961), left a legacy to the College for the scholarship endowment. More recently, gifts to the International Guest Scholarship endowment from the family of Abdol Islami, MD, FACS, and Joan Islami; the Stavros Niarchos Foundation; and others have enabled the College to increase the number of these scholarship awards.
The scholarship requirements are as follows:
• Applicants must be medical school graduates who have completed their surgical training.
• Applicants must be between 35 and 50 years old on the date that the completed application is filed.
• Applicants must submit their applications from their intended permanent location. Applications will be accepted for processing only if the applicants have been in surgical practice, teaching, or research for a minimum of 1 year at their intended permanent location, following completion of all formal training (including fellowships and scholarships).
• Applicants must have demonstrated a commitment to teaching and/or research in accordance with the standards of the applicant’s country.
• Applicants early in their careers are deemed more suitable than those surgeons who are in senior academic positions.
• Applicants must submit a fully completed application form available on the ACS website at facs.org/member-services/scholarships/international/igs. The application and accompanying materials must be submitted in English. Submission of a curriculum vitae without a completed application is unacceptable.
• Applicants must provide a list of all of their publication credits and must submit three complete reprints or manuscripts from that list.
• Preference may be given to applicants who have not already experienced training or surgical fellowships in the U.S. or Canada.
• Applicants must submit independently prepared letters of recommendation from three of their colleagues. One letter must be from the chair of the department in which the applicant holds an academic appointment or an ACS Fellow residing in the applicant’s country. This letter must include a statement regarding the nature and extent of the teaching and other academic involvement of the applicant. Letters of recommendation should be submitted by the person making the recommendation.
• The online application form is structured to assist the Scholarship Selection Subcommittee and assists the applicant in submitting a structured curriculum vitae.
• The International Guest Scholarships must be used in the year for which they are designated. They may not be postponed.
• Applicants who are awarded scholarships will provide a full written report of the experiences provided through the scholarships upon completion of their tours.
• An unsuccessful applicant may reapply only twice and only by completing and submitting a new application and new supporting documentation.
The scholarships will provide successful applicants with public recognition of their presence. Assistance will be provided in arranging visits, following the Clinical Congress, to various clinics and universities of their choice.
For consideration by the Selection Committee, applicants must fulfill all of the requirements. Applicants are urged to submit their completed applications and supporting documents as early as possible to provide sufficient time for processing.
Send supporting materials to International Liaison Section, American College of Surgeons, 633 N. Saint Clair St., Chicago, IL, 60611-3211; or via fax to 312-202-5021. Questions should be directed to Kate Early, International Liaison, at kearly@facs.org.
The American College of Surgeons (ACS) offers International Guest Scholarships to young surgeons from countries other than the U.S. or Canada who have demonstrated a strong interest in teaching and research. Twelve scholarships are available for 2017, in the amount of $10,000 each, and will provide the scholars with an opportunity to engage in clinical, teaching, and research activities in the U.S. and Canada and to attend and participate fully in the educational opportunities and activities of the ACS Clinical Congress. For consideration by the Selection Committee, completed applications for the 2017 International Guest Scholarships and all supporting documentation must be received at the office of the International Liaison Section by June 30, 2016.
Paul R. Hawley, MD, FACS(Hon), Past-ACS Executive Director (1950−1961), left a legacy to the College for the scholarship endowment. More recently, gifts to the International Guest Scholarship endowment from the family of Abdol Islami, MD, FACS, and Joan Islami; the Stavros Niarchos Foundation; and others have enabled the College to increase the number of these scholarship awards.
The scholarship requirements are as follows:
• Applicants must be medical school graduates who have completed their surgical training.
• Applicants must be between 35 and 50 years old on the date that the completed application is filed.
• Applicants must submit their applications from their intended permanent location. Applications will be accepted for processing only if the applicants have been in surgical practice, teaching, or research for a minimum of 1 year at their intended permanent location, following completion of all formal training (including fellowships and scholarships).
• Applicants must have demonstrated a commitment to teaching and/or research in accordance with the standards of the applicant’s country.
• Applicants early in their careers are deemed more suitable than those surgeons who are in senior academic positions.
• Applicants must submit a fully completed application form available on the ACS website at facs.org/member-services/scholarships/international/igs. The application and accompanying materials must be submitted in English. Submission of a curriculum vitae without a completed application is unacceptable.
• Applicants must provide a list of all of their publication credits and must submit three complete reprints or manuscripts from that list.
• Preference may be given to applicants who have not already experienced training or surgical fellowships in the U.S. or Canada.
• Applicants must submit independently prepared letters of recommendation from three of their colleagues. One letter must be from the chair of the department in which the applicant holds an academic appointment or an ACS Fellow residing in the applicant’s country. This letter must include a statement regarding the nature and extent of the teaching and other academic involvement of the applicant. Letters of recommendation should be submitted by the person making the recommendation.
• The online application form is structured to assist the Scholarship Selection Subcommittee and assists the applicant in submitting a structured curriculum vitae.
• The International Guest Scholarships must be used in the year for which they are designated. They may not be postponed.
• Applicants who are awarded scholarships will provide a full written report of the experiences provided through the scholarships upon completion of their tours.
• An unsuccessful applicant may reapply only twice and only by completing and submitting a new application and new supporting documentation.
The scholarships will provide successful applicants with public recognition of their presence. Assistance will be provided in arranging visits, following the Clinical Congress, to various clinics and universities of their choice.
For consideration by the Selection Committee, applicants must fulfill all of the requirements. Applicants are urged to submit their completed applications and supporting documents as early as possible to provide sufficient time for processing.
Send supporting materials to International Liaison Section, American College of Surgeons, 633 N. Saint Clair St., Chicago, IL, 60611-3211; or via fax to 312-202-5021. Questions should be directed to Kate Early, International Liaison, at kearly@facs.org.
The American College of Surgeons (ACS) offers International Guest Scholarships to young surgeons from countries other than the U.S. or Canada who have demonstrated a strong interest in teaching and research. Twelve scholarships are available for 2017, in the amount of $10,000 each, and will provide the scholars with an opportunity to engage in clinical, teaching, and research activities in the U.S. and Canada and to attend and participate fully in the educational opportunities and activities of the ACS Clinical Congress. For consideration by the Selection Committee, completed applications for the 2017 International Guest Scholarships and all supporting documentation must be received at the office of the International Liaison Section by June 30, 2016.
Paul R. Hawley, MD, FACS(Hon), Past-ACS Executive Director (1950−1961), left a legacy to the College for the scholarship endowment. More recently, gifts to the International Guest Scholarship endowment from the family of Abdol Islami, MD, FACS, and Joan Islami; the Stavros Niarchos Foundation; and others have enabled the College to increase the number of these scholarship awards.
The scholarship requirements are as follows:
• Applicants must be medical school graduates who have completed their surgical training.
• Applicants must be between 35 and 50 years old on the date that the completed application is filed.
• Applicants must submit their applications from their intended permanent location. Applications will be accepted for processing only if the applicants have been in surgical practice, teaching, or research for a minimum of 1 year at their intended permanent location, following completion of all formal training (including fellowships and scholarships).
• Applicants must have demonstrated a commitment to teaching and/or research in accordance with the standards of the applicant’s country.
• Applicants early in their careers are deemed more suitable than those surgeons who are in senior academic positions.
• Applicants must submit a fully completed application form available on the ACS website at facs.org/member-services/scholarships/international/igs. The application and accompanying materials must be submitted in English. Submission of a curriculum vitae without a completed application is unacceptable.
• Applicants must provide a list of all of their publication credits and must submit three complete reprints or manuscripts from that list.
• Preference may be given to applicants who have not already experienced training or surgical fellowships in the U.S. or Canada.
• Applicants must submit independently prepared letters of recommendation from three of their colleagues. One letter must be from the chair of the department in which the applicant holds an academic appointment or an ACS Fellow residing in the applicant’s country. This letter must include a statement regarding the nature and extent of the teaching and other academic involvement of the applicant. Letters of recommendation should be submitted by the person making the recommendation.
• The online application form is structured to assist the Scholarship Selection Subcommittee and assists the applicant in submitting a structured curriculum vitae.
• The International Guest Scholarships must be used in the year for which they are designated. They may not be postponed.
• Applicants who are awarded scholarships will provide a full written report of the experiences provided through the scholarships upon completion of their tours.
• An unsuccessful applicant may reapply only twice and only by completing and submitting a new application and new supporting documentation.
The scholarships will provide successful applicants with public recognition of their presence. Assistance will be provided in arranging visits, following the Clinical Congress, to various clinics and universities of their choice.
For consideration by the Selection Committee, applicants must fulfill all of the requirements. Applicants are urged to submit their completed applications and supporting documents as early as possible to provide sufficient time for processing.
Send supporting materials to International Liaison Section, American College of Surgeons, 633 N. Saint Clair St., Chicago, IL, 60611-3211; or via fax to 312-202-5021. Questions should be directed to Kate Early, International Liaison, at kearly@facs.org.
Community Surgeon Travel Awards available for 2017
Applications and supporting documentation for two 2017 Community Surgeon Travel Awards, sponsored by the International Relations Committee of the American College of Surgeons (ACS), are due July 1, 2016. The travel awards, $4,000 each and available to surgeons ages 30–50 years, allow international surgeons to attend and participate in the educational activities of the annual ACS Clinical Congress. The awards are intended specifically to assist surgeons who work in community or regional hospitals or clinics in countries other than the United States and Canada, or who are from under-resourced academic departments of surgery in under-resourced countries.
The College will cover each awardee’s registration fees for Clinical Congress 2017, October 22−26, in San Diego, CA, as well as the cost of one Postgraduate Course at the meeting. The ACS also will assist the recipients in finding preferential housing in an economical hotel. All applicants will be notified of the Selection Committee’s decision in November 2016.
Application requirements are as follows:
• Applicants must be medical school graduates who have completed their surgical training.
• Applicants must be between 30 and 50 years old on the date that the application is filed.
• Applicants must submit their applications from their intended permanent location. Applications will be accepted for processing only when the applicants have been in surgical practice, teaching, or research for at least 1 year at their intended permanent location and following completion of all formal training (including fellowships and scholarships).
• Applicants must show evidence of commitment to quality care, surgical teaching, and improving access to surgical care in their community.
• Applicants must submit a fully completed application form provided on the ACS website at facs.org/member-services/scholarships/international/communitytravel. The application and accompanying materials must be submitted in English. Submission of a curriculum vitae without a completed application is unacceptable.
• Applicants who have not already experienced training or surgical fellowships in the U.S. or Canada will receive preference for the awards.
• Applicants must submit independently prepared letters of recommendation from three colleagues. One letter must be from the chair of the department in which the applicant holds a clinical or academic appointment or from an ACS Fellow residing in their country. The recommendation letter must directly address the applicant’s commitment to quality care, surgical teaching, and improving access to surgical care locally. Letters of recommendation should be submitted by the individuals making the recommendations.
• The Community Surgeon Travel Awards must be used in the year for which they are designated. They may not be postponed.
• Awardees are expected to provide a written report upon their return home, specifically focusing on the value of the visit to the awardee and the potentially beneficial effect for patients in the country of origin.
• Unsuccessful applicants may reapply only twice and only by completing and submitting a new application together with new supporting documentation.
To qualify for consideration by the Selection Committee, all of the requirements must be fulfilled.
Supporting materials and questions should be directed to Kate Early, International Liaison, at kearly@facs.org or faxed to 312-202-5021.
Applications and supporting documentation for two 2017 Community Surgeon Travel Awards, sponsored by the International Relations Committee of the American College of Surgeons (ACS), are due July 1, 2016. The travel awards, $4,000 each and available to surgeons ages 30–50 years, allow international surgeons to attend and participate in the educational activities of the annual ACS Clinical Congress. The awards are intended specifically to assist surgeons who work in community or regional hospitals or clinics in countries other than the United States and Canada, or who are from under-resourced academic departments of surgery in under-resourced countries.
The College will cover each awardee’s registration fees for Clinical Congress 2017, October 22−26, in San Diego, CA, as well as the cost of one Postgraduate Course at the meeting. The ACS also will assist the recipients in finding preferential housing in an economical hotel. All applicants will be notified of the Selection Committee’s decision in November 2016.
Application requirements are as follows:
• Applicants must be medical school graduates who have completed their surgical training.
• Applicants must be between 30 and 50 years old on the date that the application is filed.
• Applicants must submit their applications from their intended permanent location. Applications will be accepted for processing only when the applicants have been in surgical practice, teaching, or research for at least 1 year at their intended permanent location and following completion of all formal training (including fellowships and scholarships).
• Applicants must show evidence of commitment to quality care, surgical teaching, and improving access to surgical care in their community.
• Applicants must submit a fully completed application form provided on the ACS website at facs.org/member-services/scholarships/international/communitytravel. The application and accompanying materials must be submitted in English. Submission of a curriculum vitae without a completed application is unacceptable.
• Applicants who have not already experienced training or surgical fellowships in the U.S. or Canada will receive preference for the awards.
• Applicants must submit independently prepared letters of recommendation from three colleagues. One letter must be from the chair of the department in which the applicant holds a clinical or academic appointment or from an ACS Fellow residing in their country. The recommendation letter must directly address the applicant’s commitment to quality care, surgical teaching, and improving access to surgical care locally. Letters of recommendation should be submitted by the individuals making the recommendations.
• The Community Surgeon Travel Awards must be used in the year for which they are designated. They may not be postponed.
• Awardees are expected to provide a written report upon their return home, specifically focusing on the value of the visit to the awardee and the potentially beneficial effect for patients in the country of origin.
• Unsuccessful applicants may reapply only twice and only by completing and submitting a new application together with new supporting documentation.
To qualify for consideration by the Selection Committee, all of the requirements must be fulfilled.
Supporting materials and questions should be directed to Kate Early, International Liaison, at kearly@facs.org or faxed to 312-202-5021.
Applications and supporting documentation for two 2017 Community Surgeon Travel Awards, sponsored by the International Relations Committee of the American College of Surgeons (ACS), are due July 1, 2016. The travel awards, $4,000 each and available to surgeons ages 30–50 years, allow international surgeons to attend and participate in the educational activities of the annual ACS Clinical Congress. The awards are intended specifically to assist surgeons who work in community or regional hospitals or clinics in countries other than the United States and Canada, or who are from under-resourced academic departments of surgery in under-resourced countries.
The College will cover each awardee’s registration fees for Clinical Congress 2017, October 22−26, in San Diego, CA, as well as the cost of one Postgraduate Course at the meeting. The ACS also will assist the recipients in finding preferential housing in an economical hotel. All applicants will be notified of the Selection Committee’s decision in November 2016.
Application requirements are as follows:
• Applicants must be medical school graduates who have completed their surgical training.
• Applicants must be between 30 and 50 years old on the date that the application is filed.
• Applicants must submit their applications from their intended permanent location. Applications will be accepted for processing only when the applicants have been in surgical practice, teaching, or research for at least 1 year at their intended permanent location and following completion of all formal training (including fellowships and scholarships).
• Applicants must show evidence of commitment to quality care, surgical teaching, and improving access to surgical care in their community.
• Applicants must submit a fully completed application form provided on the ACS website at facs.org/member-services/scholarships/international/communitytravel. The application and accompanying materials must be submitted in English. Submission of a curriculum vitae without a completed application is unacceptable.
• Applicants who have not already experienced training or surgical fellowships in the U.S. or Canada will receive preference for the awards.
• Applicants must submit independently prepared letters of recommendation from three colleagues. One letter must be from the chair of the department in which the applicant holds a clinical or academic appointment or from an ACS Fellow residing in their country. The recommendation letter must directly address the applicant’s commitment to quality care, surgical teaching, and improving access to surgical care locally. Letters of recommendation should be submitted by the individuals making the recommendations.
• The Community Surgeon Travel Awards must be used in the year for which they are designated. They may not be postponed.
• Awardees are expected to provide a written report upon their return home, specifically focusing on the value of the visit to the awardee and the potentially beneficial effect for patients in the country of origin.
• Unsuccessful applicants may reapply only twice and only by completing and submitting a new application together with new supporting documentation.
To qualify for consideration by the Selection Committee, all of the requirements must be fulfilled.
Supporting materials and questions should be directed to Kate Early, International Liaison, at kearly@facs.org or faxed to 312-202-5021.
Save the date for the ACS Surgeons as Leaders Course in June
Save the date for the American College of Surgeons (ACS) Surgeons as Leaders: From Operating Room to Boardroom course, June 5–8 in Durham, NC. Surgeons who aspire to meet the challenges of exemplary leadership across all settings are encouraged to join senior surgical leaders in the three-day course.
Faculty will include the following:
• Course Chair Andrew L. Warshaw, MD, FACS, FRCSEd(Hon), senior consultant, international and regional clinical relations, Massachusetts General Hospital and Partners HealthCare, Boston, MA, and Immediate Past-President of the ACS.
• Julie A. Freischlag, MD, FACS, vice-chancellor, human health sciences, and dean, school of medicine; University of California-Davis Health System, and Past-Chair of the ACS Board of Regents.
• Matthew M. Hutter, MD, MPH, FACS, director, Codman Center for Clinical Effectiveness in Surgery, Massachusetts General Hospital, and associate professor of surgery, Harvard Medical School, Boston.
• Larry R. Kaiser, MD, FACS, president and chief executive officer, Temple University Health System, and dean, Lewis Katz School of Medicine, Temple University, Philadelphia, PA.
• Fabrizio Michelassi, MD, FACS, Lewis Atterbury Stimson Professor and chairman, department of surgery, Weill Cornell Medical College; surgeon in chief, New York-Presbyterian/Weill Cornell Medical Center, New York, NY; and Chair, ACS Board of Governors.
• Carlos A. Pellegrini, MD, FACS, FRCSI(Hon), FRCS(Hon), FRCSEd(Hon), chief medical officer, UW Medicine; vice-president for medical affairs, University of Washington, Seattle; and ACS Past-President.
• Nathaniel J. Soper, MD, FACS, Loyal and Edith Davis Professor and chair, department of surgery, and surgeon in chief, Northwestern Medicine, Chicago, IL, and a Past-Governor of the ACS.
• Beth H. Sutton, MD, FACS, general surgeon, Wichita Falls, TX; clinical professor of surgery, University of Texas Southwestern Medical School, Dallas; and ACS Regent.
• Michael Useem, PhD, William and Jacalyn Egan Professor of Management and director, Center for Leadership and Change Management, Wharton School of University of Pennsylvania, Philadelphia.
• The keynote speaker will be David F. Torchiana, MD, FACS, president and chief executive officer, Partners HealthCare System, Boston
Organized by the ACS Division of Education, the course will help surgeons exhibit leadership attributes; use consensus development and vision to set, align, and achieve goals; build and maintain effective teams; identify factors that hamper the ability to lead; change culture, resolve conflict, and balance demands within the larger environment; and translate the principles of leadership into action. For additional information, e-mail ulangenscheidt@facs.org, or call 312-202-5018. ♦
Save the date for the American College of Surgeons (ACS) Surgeons as Leaders: From Operating Room to Boardroom course, June 5–8 in Durham, NC. Surgeons who aspire to meet the challenges of exemplary leadership across all settings are encouraged to join senior surgical leaders in the three-day course.
Faculty will include the following:
• Course Chair Andrew L. Warshaw, MD, FACS, FRCSEd(Hon), senior consultant, international and regional clinical relations, Massachusetts General Hospital and Partners HealthCare, Boston, MA, and Immediate Past-President of the ACS.
• Julie A. Freischlag, MD, FACS, vice-chancellor, human health sciences, and dean, school of medicine; University of California-Davis Health System, and Past-Chair of the ACS Board of Regents.
• Matthew M. Hutter, MD, MPH, FACS, director, Codman Center for Clinical Effectiveness in Surgery, Massachusetts General Hospital, and associate professor of surgery, Harvard Medical School, Boston.
• Larry R. Kaiser, MD, FACS, president and chief executive officer, Temple University Health System, and dean, Lewis Katz School of Medicine, Temple University, Philadelphia, PA.
• Fabrizio Michelassi, MD, FACS, Lewis Atterbury Stimson Professor and chairman, department of surgery, Weill Cornell Medical College; surgeon in chief, New York-Presbyterian/Weill Cornell Medical Center, New York, NY; and Chair, ACS Board of Governors.
• Carlos A. Pellegrini, MD, FACS, FRCSI(Hon), FRCS(Hon), FRCSEd(Hon), chief medical officer, UW Medicine; vice-president for medical affairs, University of Washington, Seattle; and ACS Past-President.
• Nathaniel J. Soper, MD, FACS, Loyal and Edith Davis Professor and chair, department of surgery, and surgeon in chief, Northwestern Medicine, Chicago, IL, and a Past-Governor of the ACS.
• Beth H. Sutton, MD, FACS, general surgeon, Wichita Falls, TX; clinical professor of surgery, University of Texas Southwestern Medical School, Dallas; and ACS Regent.
• Michael Useem, PhD, William and Jacalyn Egan Professor of Management and director, Center for Leadership and Change Management, Wharton School of University of Pennsylvania, Philadelphia.
• The keynote speaker will be David F. Torchiana, MD, FACS, president and chief executive officer, Partners HealthCare System, Boston
Organized by the ACS Division of Education, the course will help surgeons exhibit leadership attributes; use consensus development and vision to set, align, and achieve goals; build and maintain effective teams; identify factors that hamper the ability to lead; change culture, resolve conflict, and balance demands within the larger environment; and translate the principles of leadership into action. For additional information, e-mail ulangenscheidt@facs.org, or call 312-202-5018. ♦
Save the date for the American College of Surgeons (ACS) Surgeons as Leaders: From Operating Room to Boardroom course, June 5–8 in Durham, NC. Surgeons who aspire to meet the challenges of exemplary leadership across all settings are encouraged to join senior surgical leaders in the three-day course.
Faculty will include the following:
• Course Chair Andrew L. Warshaw, MD, FACS, FRCSEd(Hon), senior consultant, international and regional clinical relations, Massachusetts General Hospital and Partners HealthCare, Boston, MA, and Immediate Past-President of the ACS.
• Julie A. Freischlag, MD, FACS, vice-chancellor, human health sciences, and dean, school of medicine; University of California-Davis Health System, and Past-Chair of the ACS Board of Regents.
• Matthew M. Hutter, MD, MPH, FACS, director, Codman Center for Clinical Effectiveness in Surgery, Massachusetts General Hospital, and associate professor of surgery, Harvard Medical School, Boston.
• Larry R. Kaiser, MD, FACS, president and chief executive officer, Temple University Health System, and dean, Lewis Katz School of Medicine, Temple University, Philadelphia, PA.
• Fabrizio Michelassi, MD, FACS, Lewis Atterbury Stimson Professor and chairman, department of surgery, Weill Cornell Medical College; surgeon in chief, New York-Presbyterian/Weill Cornell Medical Center, New York, NY; and Chair, ACS Board of Governors.
• Carlos A. Pellegrini, MD, FACS, FRCSI(Hon), FRCS(Hon), FRCSEd(Hon), chief medical officer, UW Medicine; vice-president for medical affairs, University of Washington, Seattle; and ACS Past-President.
• Nathaniel J. Soper, MD, FACS, Loyal and Edith Davis Professor and chair, department of surgery, and surgeon in chief, Northwestern Medicine, Chicago, IL, and a Past-Governor of the ACS.
• Beth H. Sutton, MD, FACS, general surgeon, Wichita Falls, TX; clinical professor of surgery, University of Texas Southwestern Medical School, Dallas; and ACS Regent.
• Michael Useem, PhD, William and Jacalyn Egan Professor of Management and director, Center for Leadership and Change Management, Wharton School of University of Pennsylvania, Philadelphia.
• The keynote speaker will be David F. Torchiana, MD, FACS, president and chief executive officer, Partners HealthCare System, Boston
Organized by the ACS Division of Education, the course will help surgeons exhibit leadership attributes; use consensus development and vision to set, align, and achieve goals; build and maintain effective teams; identify factors that hamper the ability to lead; change culture, resolve conflict, and balance demands within the larger environment; and translate the principles of leadership into action. For additional information, e-mail ulangenscheidt@facs.org, or call 312-202-5018. ♦
Register Now for 2016 Leadership & Advocacy Summit
Register today for the American College of Surgeons (ACS) 2016 Leadership & Advocacy Summit, April 9-12, at the J.W. Marriott, Washington, DC. This dual meeting offers volunteer leaders and advocates educational sessions focused on effective surgeon leadership, as well as interactive advocacy training with coordinated visits to congressional offices.
The 2016 Leadership Summit will commence Saturday, April 9, with an evening Welcome Reception, followed the next day by presentations on strategic thinking, the latest on social media for surgeons, building better team communication, improving emotional intelligence, and leading through team conflict, among other topics. Find more information about the Leadership Summit at https://www.facs.org/advocacy/participate/summit-2016. Summit attendees will also meet over lunch in small groups organized by state/region to identify areas for unified efforts in the upcoming year. The Leadership Summit preliminary agenda is available at https://www.facs.org/advocacy/participate/summit-2016/leadership-agenda.
The Advocacy Summit will kick off the evening of April 10 with a dinner featuring political pundit and MSNBC Hardball host and Today Show commentator Chris Matthews. The next day, a number of speakers will discuss the political environment in Washington, DC, and provide updates on important health care issues. Monday’s program will include a luncheon sponsored by the ACS Professional Association’s political action committee (ACSPA-SurgeonsPAC), featuring Larry J. Sabato, PhD, election analyst and author; professor of politics, University of Virginia Center for Politics, Charlottesville. Participants will use the lessons learned at the Advocacy Summit in meetings with their senators and representatives and/or congressional staff on Tuesday. Find more information on the Advocacy Summit at https://www.facs.org/advocacy/participate/summit-2016/advocacy-agenda.
Register today for the American College of Surgeons (ACS) 2016 Leadership & Advocacy Summit, April 9-12, at the J.W. Marriott, Washington, DC. This dual meeting offers volunteer leaders and advocates educational sessions focused on effective surgeon leadership, as well as interactive advocacy training with coordinated visits to congressional offices.
The 2016 Leadership Summit will commence Saturday, April 9, with an evening Welcome Reception, followed the next day by presentations on strategic thinking, the latest on social media for surgeons, building better team communication, improving emotional intelligence, and leading through team conflict, among other topics. Find more information about the Leadership Summit at https://www.facs.org/advocacy/participate/summit-2016. Summit attendees will also meet over lunch in small groups organized by state/region to identify areas for unified efforts in the upcoming year. The Leadership Summit preliminary agenda is available at https://www.facs.org/advocacy/participate/summit-2016/leadership-agenda.
The Advocacy Summit will kick off the evening of April 10 with a dinner featuring political pundit and MSNBC Hardball host and Today Show commentator Chris Matthews. The next day, a number of speakers will discuss the political environment in Washington, DC, and provide updates on important health care issues. Monday’s program will include a luncheon sponsored by the ACS Professional Association’s political action committee (ACSPA-SurgeonsPAC), featuring Larry J. Sabato, PhD, election analyst and author; professor of politics, University of Virginia Center for Politics, Charlottesville. Participants will use the lessons learned at the Advocacy Summit in meetings with their senators and representatives and/or congressional staff on Tuesday. Find more information on the Advocacy Summit at https://www.facs.org/advocacy/participate/summit-2016/advocacy-agenda.
Register today for the American College of Surgeons (ACS) 2016 Leadership & Advocacy Summit, April 9-12, at the J.W. Marriott, Washington, DC. This dual meeting offers volunteer leaders and advocates educational sessions focused on effective surgeon leadership, as well as interactive advocacy training with coordinated visits to congressional offices.
The 2016 Leadership Summit will commence Saturday, April 9, with an evening Welcome Reception, followed the next day by presentations on strategic thinking, the latest on social media for surgeons, building better team communication, improving emotional intelligence, and leading through team conflict, among other topics. Find more information about the Leadership Summit at https://www.facs.org/advocacy/participate/summit-2016. Summit attendees will also meet over lunch in small groups organized by state/region to identify areas for unified efforts in the upcoming year. The Leadership Summit preliminary agenda is available at https://www.facs.org/advocacy/participate/summit-2016/leadership-agenda.
The Advocacy Summit will kick off the evening of April 10 with a dinner featuring political pundit and MSNBC Hardball host and Today Show commentator Chris Matthews. The next day, a number of speakers will discuss the political environment in Washington, DC, and provide updates on important health care issues. Monday’s program will include a luncheon sponsored by the ACS Professional Association’s political action committee (ACSPA-SurgeonsPAC), featuring Larry J. Sabato, PhD, election analyst and author; professor of politics, University of Virginia Center for Politics, Charlottesville. Participants will use the lessons learned at the Advocacy Summit in meetings with their senators and representatives and/or congressional staff on Tuesday. Find more information on the Advocacy Summit at https://www.facs.org/advocacy/participate/summit-2016/advocacy-agenda.
ACS cosponsors fellowships in ethics and leadership
The American College of Surgeons (ACS) Division of Education is offering two new fellowships – one in conjunction with the MacLean Center for Clinical Medical Ethics, University of Chicago, IL, and the other with the department of surgery at the University of Wisconsin (UW), Madison.
The MacLean Center will prepare two surgeons for careers that combine clinical surgery with scholarly studies in surgical ethics, beginning with a 5-week, full-time course in Chicago in July and August. From September 2016 to June 2017, fellowship recipients will meet weekly for a structured ethics curriculum. In addition, fellows will participate in an ethics consultation service and complete a research project. For additional information, contact Patrice Gabler Blair, MPH, Associate Director, ACS Division of Education, at pblair@facs.org. Application materials are due April 30, 2016.
In addition, the ACS Division of Education and the UW department of surgery have developed a fellowship program that will allow surgery residents who have completed 2 or 3 years of postgraduate training to attain leadership skills in surgical education. This 2-year fellowship also allows fellows to participate in the UW School of Education master’s degree program. Faculty from the ACS Division of Education, UW department of surgery, and UW School of Education will guide the participants in a mentored surgical education research project. Two years of funding will become available in July 2016. Additional information can be found online at www.surgery.wisc.edu/uw-acs or by contacting Maria Branca-Afrazi, department of surgery, UW School of Medicine and Public Health, at afrazi@surgery.wisc.edu. Applications will be accepted on a rolling basis until the positions are filled.
The American College of Surgeons (ACS) Division of Education is offering two new fellowships – one in conjunction with the MacLean Center for Clinical Medical Ethics, University of Chicago, IL, and the other with the department of surgery at the University of Wisconsin (UW), Madison.
The MacLean Center will prepare two surgeons for careers that combine clinical surgery with scholarly studies in surgical ethics, beginning with a 5-week, full-time course in Chicago in July and August. From September 2016 to June 2017, fellowship recipients will meet weekly for a structured ethics curriculum. In addition, fellows will participate in an ethics consultation service and complete a research project. For additional information, contact Patrice Gabler Blair, MPH, Associate Director, ACS Division of Education, at pblair@facs.org. Application materials are due April 30, 2016.
In addition, the ACS Division of Education and the UW department of surgery have developed a fellowship program that will allow surgery residents who have completed 2 or 3 years of postgraduate training to attain leadership skills in surgical education. This 2-year fellowship also allows fellows to participate in the UW School of Education master’s degree program. Faculty from the ACS Division of Education, UW department of surgery, and UW School of Education will guide the participants in a mentored surgical education research project. Two years of funding will become available in July 2016. Additional information can be found online at www.surgery.wisc.edu/uw-acs or by contacting Maria Branca-Afrazi, department of surgery, UW School of Medicine and Public Health, at afrazi@surgery.wisc.edu. Applications will be accepted on a rolling basis until the positions are filled.
The American College of Surgeons (ACS) Division of Education is offering two new fellowships – one in conjunction with the MacLean Center for Clinical Medical Ethics, University of Chicago, IL, and the other with the department of surgery at the University of Wisconsin (UW), Madison.
The MacLean Center will prepare two surgeons for careers that combine clinical surgery with scholarly studies in surgical ethics, beginning with a 5-week, full-time course in Chicago in July and August. From September 2016 to June 2017, fellowship recipients will meet weekly for a structured ethics curriculum. In addition, fellows will participate in an ethics consultation service and complete a research project. For additional information, contact Patrice Gabler Blair, MPH, Associate Director, ACS Division of Education, at pblair@facs.org. Application materials are due April 30, 2016.
In addition, the ACS Division of Education and the UW department of surgery have developed a fellowship program that will allow surgery residents who have completed 2 or 3 years of postgraduate training to attain leadership skills in surgical education. This 2-year fellowship also allows fellows to participate in the UW School of Education master’s degree program. Faculty from the ACS Division of Education, UW department of surgery, and UW School of Education will guide the participants in a mentored surgical education research project. Two years of funding will become available in July 2016. Additional information can be found online at www.surgery.wisc.edu/uw-acs or by contacting Maria Branca-Afrazi, department of surgery, UW School of Medicine and Public Health, at afrazi@surgery.wisc.edu. Applications will be accepted on a rolling basis until the positions are filled.
New standards for children’s surgery verification released
The American College of Surgeons (ACS) Children’s Surgery Verification Quality Improvement Program recently released its latest standards document, Optimal Resources for Children’s Surgical Care. These standards, developed by the ACS in collaboration with the Task Force for Children’s Surgical Care from 2012 through 2014, are the nation’s first and only multispecialty standards that seek to improve surgical care for pediatric surgical patients.
“This is the first time that there has been a formal delineation of resource standards that relate specifically to children’s surgical care across all relevant disciplines,” said Keith T. Oldham, MD, FACS, chair, Children’s Surgery Verification Quality Improvement Program, and surgeon in chief, Children’s Hospital of Wisconsin, Milwaukee.
The pilot phase of the program launched in April 2015. Within 1 month, six pilot site visits were completed at diverse institutions nationwide. The final document includes revisions to the 2014 draft standards and updates from lessons learned during the pilot phase of the program, such as the need for alternative training pathways for anesthesiology, emergency medicine, and radiology. The new standards also clearly define the safety data elements required for all level designations.
The new standards document comes in advance of the online application – a prereview questionnaire for centers seeking designation through the Children’s Surgery Verification Quality Improvement Program – expected to launch later this year.
“The standards presented in this document are the basis for the Children’s Surgery Verification Quality Improvement Program, for which the ACS will visit centers periodically and verify that relevant standards are met and related quality improvement mechanisms are in place,” Dr. Oldham said.
To access the standards, visit facs.org/quality-programs/childrens-surgery.
The American College of Surgeons (ACS) Children’s Surgery Verification Quality Improvement Program recently released its latest standards document, Optimal Resources for Children’s Surgical Care. These standards, developed by the ACS in collaboration with the Task Force for Children’s Surgical Care from 2012 through 2014, are the nation’s first and only multispecialty standards that seek to improve surgical care for pediatric surgical patients.
“This is the first time that there has been a formal delineation of resource standards that relate specifically to children’s surgical care across all relevant disciplines,” said Keith T. Oldham, MD, FACS, chair, Children’s Surgery Verification Quality Improvement Program, and surgeon in chief, Children’s Hospital of Wisconsin, Milwaukee.
The pilot phase of the program launched in April 2015. Within 1 month, six pilot site visits were completed at diverse institutions nationwide. The final document includes revisions to the 2014 draft standards and updates from lessons learned during the pilot phase of the program, such as the need for alternative training pathways for anesthesiology, emergency medicine, and radiology. The new standards also clearly define the safety data elements required for all level designations.
The new standards document comes in advance of the online application – a prereview questionnaire for centers seeking designation through the Children’s Surgery Verification Quality Improvement Program – expected to launch later this year.
“The standards presented in this document are the basis for the Children’s Surgery Verification Quality Improvement Program, for which the ACS will visit centers periodically and verify that relevant standards are met and related quality improvement mechanisms are in place,” Dr. Oldham said.
To access the standards, visit facs.org/quality-programs/childrens-surgery.
The American College of Surgeons (ACS) Children’s Surgery Verification Quality Improvement Program recently released its latest standards document, Optimal Resources for Children’s Surgical Care. These standards, developed by the ACS in collaboration with the Task Force for Children’s Surgical Care from 2012 through 2014, are the nation’s first and only multispecialty standards that seek to improve surgical care for pediatric surgical patients.
“This is the first time that there has been a formal delineation of resource standards that relate specifically to children’s surgical care across all relevant disciplines,” said Keith T. Oldham, MD, FACS, chair, Children’s Surgery Verification Quality Improvement Program, and surgeon in chief, Children’s Hospital of Wisconsin, Milwaukee.
The pilot phase of the program launched in April 2015. Within 1 month, six pilot site visits were completed at diverse institutions nationwide. The final document includes revisions to the 2014 draft standards and updates from lessons learned during the pilot phase of the program, such as the need for alternative training pathways for anesthesiology, emergency medicine, and radiology. The new standards also clearly define the safety data elements required for all level designations.
The new standards document comes in advance of the online application – a prereview questionnaire for centers seeking designation through the Children’s Surgery Verification Quality Improvement Program – expected to launch later this year.
“The standards presented in this document are the basis for the Children’s Surgery Verification Quality Improvement Program, for which the ACS will visit centers periodically and verify that relevant standards are met and related quality improvement mechanisms are in place,” Dr. Oldham said.
To access the standards, visit facs.org/quality-programs/childrens-surgery.
Lower the CT to check the heart for embolic sources in acute stroke
LOS ANGELES – Enlarge the field of CT angiography to include the heart in acute ischemic stroke patients; you’ll quickly identify sources of cardiogenic emboli and other problems that will otherwise be missed, according to investigators from the National University Hospital, Singapore.
It adds only a few seconds to the scan, with no extra contrast or meaningful increase in radiation. There’s no need to gate the heart with beta-blockers.
Among 20 acute ischemic stroke patients presenting within 4.5 hours of symptom onset, Dr. Leonard Yeo and his coinvestigators found one with a localized dissection in the ascending aorta, another with a ventricular thrombus, and a third with an atrial appendage blood clot. Both thrombus cases were confirmed by transesophageal echocardiography and started on anticoagulation the next day. The 2-phase, 64-slice nongated cardiac CT angiographies (CTA) were done in the same sitting as the brain CTA.
“Scans with 1-mm thick slices are best for screening for thrombus and structural abnormalities that cause embolism. Remarkably, [even without gating], the detail is excellent. There’s very little downside [to this, and] it maximizes your return on scans that are already a part of most acute stroke protocols,” said Dr. Yeo, a neurologist at the hospital.
“Since most of our patients get a CTA during acute stroke, it made sense to check the heart for embolic sources.” There isn’t any time to give a beta-blocker, so “these were nongated” scans, Dr. Yeo said during his presentation at the International Stroke Conference, sponsored by the American Heart Association.
If it’s confirmed that nongated heart CTAs provide useful information, “we will probably all be doing this in the future. Everybody does CTs for the head in acute stroke, so all you do is go down a little lower” without any more contrast. “Within an hour of somebody presenting, you know what they have,” said Dr. Robert Hart, a neurology professor at McMaster University in Hamilton, Ont., and co-moderator of Dr. Yeo’s presentation.
In most places, acute ischemic stroke patients only get an ECG. Transesophageal echocardiography (TEE) is also good for checking the heart, but it usually comes later. It “excels at detecting abnormalities with medium embolic risk,” such as patent foramen ovale and septal aneurysm. “However, for these medium-risk cardiac sources of embolism, the optimal choice of therapy is not clear. Unlike high-risk sources which require anticoagulation, TEE does not provide therapeutic gains in terms of clinical decision making,” Dr. Yeo said.
Nongated cardiac CTAs during acute stroke, he added, also check chamber, valve, pericardial, and great vessel morphology, as well as abnormal chambers-vessel communications and “left ventricular aneurysms that can rupture with [tissue plasminogen activator], with catastrophic consequences.”
The mean age in the study was 64 years old, and about 60% of the subjects were men. None of the patients were dead at 3 months, and by then eight (40%) had modified Rankin Scale scores of 0-1. Patients were excluded if they had contraindications to IV contrast, or were unable to provide informed consent. CTA images were read by the treating neurologist and radiologist.
The work was funded by the Singapore Ministry of Health’s National Medical Research Council. The investigators have no relevant disclosures.
LOS ANGELES – Enlarge the field of CT angiography to include the heart in acute ischemic stroke patients; you’ll quickly identify sources of cardiogenic emboli and other problems that will otherwise be missed, according to investigators from the National University Hospital, Singapore.
It adds only a few seconds to the scan, with no extra contrast or meaningful increase in radiation. There’s no need to gate the heart with beta-blockers.
Among 20 acute ischemic stroke patients presenting within 4.5 hours of symptom onset, Dr. Leonard Yeo and his coinvestigators found one with a localized dissection in the ascending aorta, another with a ventricular thrombus, and a third with an atrial appendage blood clot. Both thrombus cases were confirmed by transesophageal echocardiography and started on anticoagulation the next day. The 2-phase, 64-slice nongated cardiac CT angiographies (CTA) were done in the same sitting as the brain CTA.
“Scans with 1-mm thick slices are best for screening for thrombus and structural abnormalities that cause embolism. Remarkably, [even without gating], the detail is excellent. There’s very little downside [to this, and] it maximizes your return on scans that are already a part of most acute stroke protocols,” said Dr. Yeo, a neurologist at the hospital.
“Since most of our patients get a CTA during acute stroke, it made sense to check the heart for embolic sources.” There isn’t any time to give a beta-blocker, so “these were nongated” scans, Dr. Yeo said during his presentation at the International Stroke Conference, sponsored by the American Heart Association.
If it’s confirmed that nongated heart CTAs provide useful information, “we will probably all be doing this in the future. Everybody does CTs for the head in acute stroke, so all you do is go down a little lower” without any more contrast. “Within an hour of somebody presenting, you know what they have,” said Dr. Robert Hart, a neurology professor at McMaster University in Hamilton, Ont., and co-moderator of Dr. Yeo’s presentation.
In most places, acute ischemic stroke patients only get an ECG. Transesophageal echocardiography (TEE) is also good for checking the heart, but it usually comes later. It “excels at detecting abnormalities with medium embolic risk,” such as patent foramen ovale and septal aneurysm. “However, for these medium-risk cardiac sources of embolism, the optimal choice of therapy is not clear. Unlike high-risk sources which require anticoagulation, TEE does not provide therapeutic gains in terms of clinical decision making,” Dr. Yeo said.
Nongated cardiac CTAs during acute stroke, he added, also check chamber, valve, pericardial, and great vessel morphology, as well as abnormal chambers-vessel communications and “left ventricular aneurysms that can rupture with [tissue plasminogen activator], with catastrophic consequences.”
The mean age in the study was 64 years old, and about 60% of the subjects were men. None of the patients were dead at 3 months, and by then eight (40%) had modified Rankin Scale scores of 0-1. Patients were excluded if they had contraindications to IV contrast, or were unable to provide informed consent. CTA images were read by the treating neurologist and radiologist.
The work was funded by the Singapore Ministry of Health’s National Medical Research Council. The investigators have no relevant disclosures.
LOS ANGELES – Enlarge the field of CT angiography to include the heart in acute ischemic stroke patients; you’ll quickly identify sources of cardiogenic emboli and other problems that will otherwise be missed, according to investigators from the National University Hospital, Singapore.
It adds only a few seconds to the scan, with no extra contrast or meaningful increase in radiation. There’s no need to gate the heart with beta-blockers.
Among 20 acute ischemic stroke patients presenting within 4.5 hours of symptom onset, Dr. Leonard Yeo and his coinvestigators found one with a localized dissection in the ascending aorta, another with a ventricular thrombus, and a third with an atrial appendage blood clot. Both thrombus cases were confirmed by transesophageal echocardiography and started on anticoagulation the next day. The 2-phase, 64-slice nongated cardiac CT angiographies (CTA) were done in the same sitting as the brain CTA.
“Scans with 1-mm thick slices are best for screening for thrombus and structural abnormalities that cause embolism. Remarkably, [even without gating], the detail is excellent. There’s very little downside [to this, and] it maximizes your return on scans that are already a part of most acute stroke protocols,” said Dr. Yeo, a neurologist at the hospital.
“Since most of our patients get a CTA during acute stroke, it made sense to check the heart for embolic sources.” There isn’t any time to give a beta-blocker, so “these were nongated” scans, Dr. Yeo said during his presentation at the International Stroke Conference, sponsored by the American Heart Association.
If it’s confirmed that nongated heart CTAs provide useful information, “we will probably all be doing this in the future. Everybody does CTs for the head in acute stroke, so all you do is go down a little lower” without any more contrast. “Within an hour of somebody presenting, you know what they have,” said Dr. Robert Hart, a neurology professor at McMaster University in Hamilton, Ont., and co-moderator of Dr. Yeo’s presentation.
In most places, acute ischemic stroke patients only get an ECG. Transesophageal echocardiography (TEE) is also good for checking the heart, but it usually comes later. It “excels at detecting abnormalities with medium embolic risk,” such as patent foramen ovale and septal aneurysm. “However, for these medium-risk cardiac sources of embolism, the optimal choice of therapy is not clear. Unlike high-risk sources which require anticoagulation, TEE does not provide therapeutic gains in terms of clinical decision making,” Dr. Yeo said.
Nongated cardiac CTAs during acute stroke, he added, also check chamber, valve, pericardial, and great vessel morphology, as well as abnormal chambers-vessel communications and “left ventricular aneurysms that can rupture with [tissue plasminogen activator], with catastrophic consequences.”
The mean age in the study was 64 years old, and about 60% of the subjects were men. None of the patients were dead at 3 months, and by then eight (40%) had modified Rankin Scale scores of 0-1. Patients were excluded if they had contraindications to IV contrast, or were unable to provide informed consent. CTA images were read by the treating neurologist and radiologist.
The work was funded by the Singapore Ministry of Health’s National Medical Research Council. The investigators have no relevant disclosures.
AT THE INTERNATIONAL STROKE CONFERENCE
Key clinical point: Nongated heart CTAs may provide useful information in acute ischemic stroke.
Major finding: Among 20 acute ischemic stroke patients presenting within 4.5 hours of symptom onset, one had a localized dissection in the ascending aorta, another had a ventricular thrombus, and a third had an atrial appendage blood clot.
Data source: Prospective investigation of 20 patients.
Disclosures: The work was funded by the Singapore Ministry of Health’s National Medical Research Council. The investigators have no relevant disclosures.
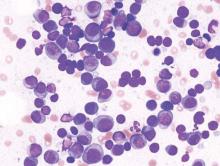
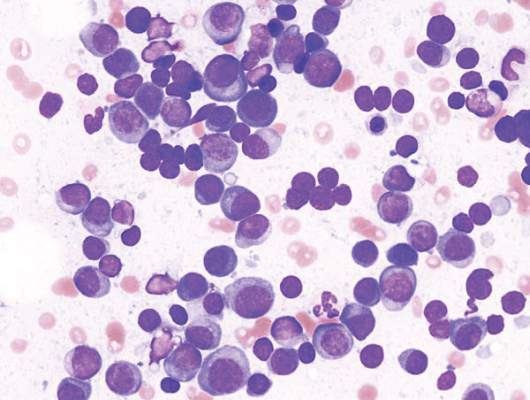
Lenalidomide-dexamethasone yields similar PFS as triplet regimens in elderly multiple myeloma patients
A comparison of lenalidomide-based treatments for multiple myeloma patients who were ineligible for stem cell transplantation showed similar progression-free survival (PFS) for two alkylator-containing triplet regimens and an alkylator-free doublet regimen but a higher risk of hematologic toxicity with a melphalan-prednisone-lenalidomide regimen.
For the triplet regimens, melphalan-prednisone-lenalidomide (MPR) and cyclophosphamide-prednisone-lenalidomide (CPR), the median PFS was 22 months, compared with 21 months for the doublet regimen lenalidomide plus low-dose dexamethasone (Rd). The hazard ratio (HR) was 0.906 (95% CI, 0.739-1.11; P = .344). The 4-year overall survival (OS) was 67% with triplet and 58% with doublet regimens (HR, 0.945; 95% CI, 0.700-1.274; P = .709) (Blood. 2016;127[9]:1102-8).
The major safety concern, according to the researchers, was the higher toxicity with MPR compared with CPR and Rd. The most frequent toxicities of grade 3 or more were hematologic, with at least one reported event in 68% of the MPR arm, 32% of CPR, and 29% or Rd patients (P less than .0001). In a post hoc analysis of safety according to patient fitness, the incidence of at least one hematologic adverse event ocurred in 75% of fit patients in the MPR arm occurred, 34% in CPR, and 29% in Rd; the incidence of at least one hematologic adverse event in intermediate fitness patients in the MPR arm was 61%, 33% in CPR, and 25% in Rd; in frail patients, 75% in MPR, 28% in CPR, and 3% in Rd (P = .001 for MPR vs. Rd and MPR vs. CPR). Nonhematologic adverse events were similar for the three groups and less than 10%.
Previous studies, such as the FIRST trial, showed the superiority of lenalidomide-containing regimens over standard treatments, but a question remained over the best drug to combine with lenalidomide – an alkylating agent or steroid. Separate analysis of the three arms further illustrated that the addition of an alkylating agent did not lead to better response or outcome. Median PFS for MPR, CPR, and Rd arms were 24, 20, and 21 months, respectively; 4-year OS rates were 65%, 68%, and 58%, respectively; overall response rates were 71%, 68%, and 74%, respectively.
Compared with the previous FIRST study, the less intense regimen in this study (Rd administered for only 9 months as induction treatment, followed by maintenance with lenalidomide at a lower dose) resulted in less hematological toxicity.
“This suggests that continuous treatment with Rd can be a valuable option for prolonging PFS and achieving a deeper response, and reducing the dose during maintenance can be a valuable strategy for improving tolerability,” wrote Dr. Valeria Magarotto of the myeloma unit in the division of hematology at the University of Torino (Italy), and colleagues. They added, “A more intensive induction treatment with Rd administered for a limited duration (9 months) followed by a less intensive continuous treatment with lenalidomide alone seems to be a sensible and effective choice.”
The phase III trial included 654 patients with newly diagnosed multiple myeloma who were ineligible for stem cell transplantation due to advanced age (65 years and older) or comorbidities. Patients were randomized to receive MPR (n = 217), CPR (n = 220), or Rd (n = 217).
A comparison of lenalidomide-based treatments for multiple myeloma patients who were ineligible for stem cell transplantation showed similar progression-free survival (PFS) for two alkylator-containing triplet regimens and an alkylator-free doublet regimen but a higher risk of hematologic toxicity with a melphalan-prednisone-lenalidomide regimen.
For the triplet regimens, melphalan-prednisone-lenalidomide (MPR) and cyclophosphamide-prednisone-lenalidomide (CPR), the median PFS was 22 months, compared with 21 months for the doublet regimen lenalidomide plus low-dose dexamethasone (Rd). The hazard ratio (HR) was 0.906 (95% CI, 0.739-1.11; P = .344). The 4-year overall survival (OS) was 67% with triplet and 58% with doublet regimens (HR, 0.945; 95% CI, 0.700-1.274; P = .709) (Blood. 2016;127[9]:1102-8).
The major safety concern, according to the researchers, was the higher toxicity with MPR compared with CPR and Rd. The most frequent toxicities of grade 3 or more were hematologic, with at least one reported event in 68% of the MPR arm, 32% of CPR, and 29% or Rd patients (P less than .0001). In a post hoc analysis of safety according to patient fitness, the incidence of at least one hematologic adverse event ocurred in 75% of fit patients in the MPR arm occurred, 34% in CPR, and 29% in Rd; the incidence of at least one hematologic adverse event in intermediate fitness patients in the MPR arm was 61%, 33% in CPR, and 25% in Rd; in frail patients, 75% in MPR, 28% in CPR, and 3% in Rd (P = .001 for MPR vs. Rd and MPR vs. CPR). Nonhematologic adverse events were similar for the three groups and less than 10%.
Previous studies, such as the FIRST trial, showed the superiority of lenalidomide-containing regimens over standard treatments, but a question remained over the best drug to combine with lenalidomide – an alkylating agent or steroid. Separate analysis of the three arms further illustrated that the addition of an alkylating agent did not lead to better response or outcome. Median PFS for MPR, CPR, and Rd arms were 24, 20, and 21 months, respectively; 4-year OS rates were 65%, 68%, and 58%, respectively; overall response rates were 71%, 68%, and 74%, respectively.
Compared with the previous FIRST study, the less intense regimen in this study (Rd administered for only 9 months as induction treatment, followed by maintenance with lenalidomide at a lower dose) resulted in less hematological toxicity.
“This suggests that continuous treatment with Rd can be a valuable option for prolonging PFS and achieving a deeper response, and reducing the dose during maintenance can be a valuable strategy for improving tolerability,” wrote Dr. Valeria Magarotto of the myeloma unit in the division of hematology at the University of Torino (Italy), and colleagues. They added, “A more intensive induction treatment with Rd administered for a limited duration (9 months) followed by a less intensive continuous treatment with lenalidomide alone seems to be a sensible and effective choice.”
The phase III trial included 654 patients with newly diagnosed multiple myeloma who were ineligible for stem cell transplantation due to advanced age (65 years and older) or comorbidities. Patients were randomized to receive MPR (n = 217), CPR (n = 220), or Rd (n = 217).
A comparison of lenalidomide-based treatments for multiple myeloma patients who were ineligible for stem cell transplantation showed similar progression-free survival (PFS) for two alkylator-containing triplet regimens and an alkylator-free doublet regimen but a higher risk of hematologic toxicity with a melphalan-prednisone-lenalidomide regimen.
For the triplet regimens, melphalan-prednisone-lenalidomide (MPR) and cyclophosphamide-prednisone-lenalidomide (CPR), the median PFS was 22 months, compared with 21 months for the doublet regimen lenalidomide plus low-dose dexamethasone (Rd). The hazard ratio (HR) was 0.906 (95% CI, 0.739-1.11; P = .344). The 4-year overall survival (OS) was 67% with triplet and 58% with doublet regimens (HR, 0.945; 95% CI, 0.700-1.274; P = .709) (Blood. 2016;127[9]:1102-8).
The major safety concern, according to the researchers, was the higher toxicity with MPR compared with CPR and Rd. The most frequent toxicities of grade 3 or more were hematologic, with at least one reported event in 68% of the MPR arm, 32% of CPR, and 29% or Rd patients (P less than .0001). In a post hoc analysis of safety according to patient fitness, the incidence of at least one hematologic adverse event ocurred in 75% of fit patients in the MPR arm occurred, 34% in CPR, and 29% in Rd; the incidence of at least one hematologic adverse event in intermediate fitness patients in the MPR arm was 61%, 33% in CPR, and 25% in Rd; in frail patients, 75% in MPR, 28% in CPR, and 3% in Rd (P = .001 for MPR vs. Rd and MPR vs. CPR). Nonhematologic adverse events were similar for the three groups and less than 10%.
Previous studies, such as the FIRST trial, showed the superiority of lenalidomide-containing regimens over standard treatments, but a question remained over the best drug to combine with lenalidomide – an alkylating agent or steroid. Separate analysis of the three arms further illustrated that the addition of an alkylating agent did not lead to better response or outcome. Median PFS for MPR, CPR, and Rd arms were 24, 20, and 21 months, respectively; 4-year OS rates were 65%, 68%, and 58%, respectively; overall response rates were 71%, 68%, and 74%, respectively.
Compared with the previous FIRST study, the less intense regimen in this study (Rd administered for only 9 months as induction treatment, followed by maintenance with lenalidomide at a lower dose) resulted in less hematological toxicity.
“This suggests that continuous treatment with Rd can be a valuable option for prolonging PFS and achieving a deeper response, and reducing the dose during maintenance can be a valuable strategy for improving tolerability,” wrote Dr. Valeria Magarotto of the myeloma unit in the division of hematology at the University of Torino (Italy), and colleagues. They added, “A more intensive induction treatment with Rd administered for a limited duration (9 months) followed by a less intensive continuous treatment with lenalidomide alone seems to be a sensible and effective choice.”
The phase III trial included 654 patients with newly diagnosed multiple myeloma who were ineligible for stem cell transplantation due to advanced age (65 years and older) or comorbidities. Patients were randomized to receive MPR (n = 217), CPR (n = 220), or Rd (n = 217).
FROM BLOOD
Key clinical point: In elderly patients with newly diagnosed multiple myeloma, progression-free survival was similar for alkylator-containing triplet regimens and an alkylator-free doublet regimen, but the doublet resulted in less hematologic toxicity.
Major finding: Median PFS for MPR, CPR and Rd arms were 24, 20, and 21 months, respectively; 4-year OS rates were 65%, 68%, and 58%, respectively.
Data sources: Phase III trial of 654 patients randomized to receive melphalan-prednisone-lenalidomide (n = 217), cyclophosphamide-prednisone-lenalidomide (n = 220), or lenalidomide plus low-dose dexamethasone (n = 217).
Disclosures: Dr. Magarotto reported having no disclosures. Several of her coauthors reported financial ties to industry sources.
Expert examines secukinumab’s role in ankylosing spondylitis treatment strategies
MAUI, HAWAII – The most important development within the past year in the treatment of ankylosing spondylitis was the Food and Drug Administration approval of secukinumab (Cosentyx) as the first non-tumor necrosis factor inhibitor biologic for this condition – but the interleukin-17A inhibitor is not going to immediately step into a role as a first-line therapy, Dr. Eric M. Ruderman predicted at the 2016 Rheumatology Winter Clinical Symposium.
“In all likelihood nobody’s going to use this as a first-line drug right out of the gate. It’s a drug you’re going to potentially go to in people who haven’t responded to the things that you’ve been comfortable using for the last 10 or 15 years. So the big practical issue becomes, ‘How does secukinumab perform in TNF inhibitor-naive patients versus prior TNF inhibitor inadequate responders?’ ” according to the rheumatologist, who is professor of medicine at Northwestern University in Chicago.
This question has been addressed in secondary analyses of the pivotal phase III MEASURE 1 and MEASURE 2 trials which have been presented at the annual European League Against Rheumatism and American College of Rheumatology meetings. The bottom line was that the therapeutic response rate in both trials was markedly lower in TNF inhibitor inadequate responders than in TNF inhibitor-naive subjects.
“But there still is a significant response rate in the inadequate responders. It’s clearly better than placebo. So this is a drug that may have a role in your practice at the point where patients have failed on one or two anti-TNF biologics,” according to Dr. Ruderman.
The difference between MEASURE 1 and MEASURE 2 is that MEASURE 1 entailed three intravenous loading doses of the biologic at 2-week intervals before switching to monthly subcutaneous dosing, while MEASURE 2 featured subcutaneous loading doses given weekly for 4 weeks before moving to monthly administration. Interestingly, the FDA approval of secukinumab at 150 mg doesn’t call for a loading dose, even though both pivotal trials relied on them, the rheumatologist observed.
At 16 weeks in MEASURE 1, 66% of TNF inhibitor-naive subjects on secukinumab 150 mg had at least a 20% improvement from baseline in ankylosing spondylitis signs and symptoms, or Assessment of Spondyloarthritis International Society (ASAS) 20, compared with 46% of TNF inhibitor inadequate responders. The week 16 ASAS 20 rate in MEASURE 2 was 68% in TNF inhibitor-naive patients and 50% in those with a prior inadequate response to TNF inhibitor therapy.
How should rheumatologists expect secukinumab to perform in daily clinical practice? In the 181 ankylosing spoindylitis patients who completed 52 weeks in the MEASURE 2 extension study, 74% of those on secukinumab at 150 mg had an ASAS 20 response. In both trials, the secukinumab side effect profile was “reasonably clean,” in Dr. Ruderman’s view, with serious adverse events that were similar to placebo.
Serial MRI scans showed rapid resolution of bone marrow edema and inflammation by 16 weeks, an effect sustained through 52 weeks.
The big unanswered question is whether secukinumab prevents radiographic progression of the disease. Serial cervical and spinal X-rays rated using the modified Stoke Ankylosing Spondylitis Spinal Score showed a mean increase of just 0.30 points at 2 years from a baseline of 10.22, with 80% of patients demonstrating no change over time. But there were no untreated controls for comparison in this analysis, so it’s not possible to say whether the drug actually slowed disease progression or that’s the natural history of disease in those subjects, Dr. Ruderman noted.
Effect of NSAID dosing frequency on progression
On the topic of preventing radiographic progression in ankylosing spondylitis, the rheumatologist highlighted a prospective study presented at last year’s EULAR meeting and published online last summer (Ann Rheum Dis. 2015 Aug 4. doi: 10.1136/annrheumdis-2015-207897) that demonstrated that continuous use of diclofenac didn’t do any better at preventing radiographic spinal disease progression than on-demand use of the nonsteroidal anti-inflammatory drug (NSAID) over the course of 2 years.
“There’s been a lot of noise in the ankylosing spondylitis community about the potential benefit of NSAIDs in preventing structural progression. Previous information suggested that staying on them continuously actually reduced radiographic progression. This diclofenac study has shaken things up a little. It raises the question of whether there is any added benefit for NSAIDs in terms of structural progression,” he commented.
Current ACR/SAA/SPARTAN guidelines, which predate the study, feature a conditional recommendation that patients with active ankylosing spondylitis stay on continuous NSAID therapy.
Secukinumab is also approved for treatment of psoriasis and psoriatic arthritis.
Dr. Ruderman reported serving as a consultant to and/or receiving research grants from numerous pharmaceutical companies, including Novartis, which markets secukinumab.
MAUI, HAWAII – The most important development within the past year in the treatment of ankylosing spondylitis was the Food and Drug Administration approval of secukinumab (Cosentyx) as the first non-tumor necrosis factor inhibitor biologic for this condition – but the interleukin-17A inhibitor is not going to immediately step into a role as a first-line therapy, Dr. Eric M. Ruderman predicted at the 2016 Rheumatology Winter Clinical Symposium.
“In all likelihood nobody’s going to use this as a first-line drug right out of the gate. It’s a drug you’re going to potentially go to in people who haven’t responded to the things that you’ve been comfortable using for the last 10 or 15 years. So the big practical issue becomes, ‘How does secukinumab perform in TNF inhibitor-naive patients versus prior TNF inhibitor inadequate responders?’ ” according to the rheumatologist, who is professor of medicine at Northwestern University in Chicago.
This question has been addressed in secondary analyses of the pivotal phase III MEASURE 1 and MEASURE 2 trials which have been presented at the annual European League Against Rheumatism and American College of Rheumatology meetings. The bottom line was that the therapeutic response rate in both trials was markedly lower in TNF inhibitor inadequate responders than in TNF inhibitor-naive subjects.
“But there still is a significant response rate in the inadequate responders. It’s clearly better than placebo. So this is a drug that may have a role in your practice at the point where patients have failed on one or two anti-TNF biologics,” according to Dr. Ruderman.
The difference between MEASURE 1 and MEASURE 2 is that MEASURE 1 entailed three intravenous loading doses of the biologic at 2-week intervals before switching to monthly subcutaneous dosing, while MEASURE 2 featured subcutaneous loading doses given weekly for 4 weeks before moving to monthly administration. Interestingly, the FDA approval of secukinumab at 150 mg doesn’t call for a loading dose, even though both pivotal trials relied on them, the rheumatologist observed.
At 16 weeks in MEASURE 1, 66% of TNF inhibitor-naive subjects on secukinumab 150 mg had at least a 20% improvement from baseline in ankylosing spondylitis signs and symptoms, or Assessment of Spondyloarthritis International Society (ASAS) 20, compared with 46% of TNF inhibitor inadequate responders. The week 16 ASAS 20 rate in MEASURE 2 was 68% in TNF inhibitor-naive patients and 50% in those with a prior inadequate response to TNF inhibitor therapy.
How should rheumatologists expect secukinumab to perform in daily clinical practice? In the 181 ankylosing spoindylitis patients who completed 52 weeks in the MEASURE 2 extension study, 74% of those on secukinumab at 150 mg had an ASAS 20 response. In both trials, the secukinumab side effect profile was “reasonably clean,” in Dr. Ruderman’s view, with serious adverse events that were similar to placebo.
Serial MRI scans showed rapid resolution of bone marrow edema and inflammation by 16 weeks, an effect sustained through 52 weeks.
The big unanswered question is whether secukinumab prevents radiographic progression of the disease. Serial cervical and spinal X-rays rated using the modified Stoke Ankylosing Spondylitis Spinal Score showed a mean increase of just 0.30 points at 2 years from a baseline of 10.22, with 80% of patients demonstrating no change over time. But there were no untreated controls for comparison in this analysis, so it’s not possible to say whether the drug actually slowed disease progression or that’s the natural history of disease in those subjects, Dr. Ruderman noted.
Effect of NSAID dosing frequency on progression
On the topic of preventing radiographic progression in ankylosing spondylitis, the rheumatologist highlighted a prospective study presented at last year’s EULAR meeting and published online last summer (Ann Rheum Dis. 2015 Aug 4. doi: 10.1136/annrheumdis-2015-207897) that demonstrated that continuous use of diclofenac didn’t do any better at preventing radiographic spinal disease progression than on-demand use of the nonsteroidal anti-inflammatory drug (NSAID) over the course of 2 years.
“There’s been a lot of noise in the ankylosing spondylitis community about the potential benefit of NSAIDs in preventing structural progression. Previous information suggested that staying on them continuously actually reduced radiographic progression. This diclofenac study has shaken things up a little. It raises the question of whether there is any added benefit for NSAIDs in terms of structural progression,” he commented.
Current ACR/SAA/SPARTAN guidelines, which predate the study, feature a conditional recommendation that patients with active ankylosing spondylitis stay on continuous NSAID therapy.
Secukinumab is also approved for treatment of psoriasis and psoriatic arthritis.
Dr. Ruderman reported serving as a consultant to and/or receiving research grants from numerous pharmaceutical companies, including Novartis, which markets secukinumab.
MAUI, HAWAII – The most important development within the past year in the treatment of ankylosing spondylitis was the Food and Drug Administration approval of secukinumab (Cosentyx) as the first non-tumor necrosis factor inhibitor biologic for this condition – but the interleukin-17A inhibitor is not going to immediately step into a role as a first-line therapy, Dr. Eric M. Ruderman predicted at the 2016 Rheumatology Winter Clinical Symposium.
“In all likelihood nobody’s going to use this as a first-line drug right out of the gate. It’s a drug you’re going to potentially go to in people who haven’t responded to the things that you’ve been comfortable using for the last 10 or 15 years. So the big practical issue becomes, ‘How does secukinumab perform in TNF inhibitor-naive patients versus prior TNF inhibitor inadequate responders?’ ” according to the rheumatologist, who is professor of medicine at Northwestern University in Chicago.
This question has been addressed in secondary analyses of the pivotal phase III MEASURE 1 and MEASURE 2 trials which have been presented at the annual European League Against Rheumatism and American College of Rheumatology meetings. The bottom line was that the therapeutic response rate in both trials was markedly lower in TNF inhibitor inadequate responders than in TNF inhibitor-naive subjects.
“But there still is a significant response rate in the inadequate responders. It’s clearly better than placebo. So this is a drug that may have a role in your practice at the point where patients have failed on one or two anti-TNF biologics,” according to Dr. Ruderman.
The difference between MEASURE 1 and MEASURE 2 is that MEASURE 1 entailed three intravenous loading doses of the biologic at 2-week intervals before switching to monthly subcutaneous dosing, while MEASURE 2 featured subcutaneous loading doses given weekly for 4 weeks before moving to monthly administration. Interestingly, the FDA approval of secukinumab at 150 mg doesn’t call for a loading dose, even though both pivotal trials relied on them, the rheumatologist observed.
At 16 weeks in MEASURE 1, 66% of TNF inhibitor-naive subjects on secukinumab 150 mg had at least a 20% improvement from baseline in ankylosing spondylitis signs and symptoms, or Assessment of Spondyloarthritis International Society (ASAS) 20, compared with 46% of TNF inhibitor inadequate responders. The week 16 ASAS 20 rate in MEASURE 2 was 68% in TNF inhibitor-naive patients and 50% in those with a prior inadequate response to TNF inhibitor therapy.
How should rheumatologists expect secukinumab to perform in daily clinical practice? In the 181 ankylosing spoindylitis patients who completed 52 weeks in the MEASURE 2 extension study, 74% of those on secukinumab at 150 mg had an ASAS 20 response. In both trials, the secukinumab side effect profile was “reasonably clean,” in Dr. Ruderman’s view, with serious adverse events that were similar to placebo.
Serial MRI scans showed rapid resolution of bone marrow edema and inflammation by 16 weeks, an effect sustained through 52 weeks.
The big unanswered question is whether secukinumab prevents radiographic progression of the disease. Serial cervical and spinal X-rays rated using the modified Stoke Ankylosing Spondylitis Spinal Score showed a mean increase of just 0.30 points at 2 years from a baseline of 10.22, with 80% of patients demonstrating no change over time. But there were no untreated controls for comparison in this analysis, so it’s not possible to say whether the drug actually slowed disease progression or that’s the natural history of disease in those subjects, Dr. Ruderman noted.
Effect of NSAID dosing frequency on progression
On the topic of preventing radiographic progression in ankylosing spondylitis, the rheumatologist highlighted a prospective study presented at last year’s EULAR meeting and published online last summer (Ann Rheum Dis. 2015 Aug 4. doi: 10.1136/annrheumdis-2015-207897) that demonstrated that continuous use of diclofenac didn’t do any better at preventing radiographic spinal disease progression than on-demand use of the nonsteroidal anti-inflammatory drug (NSAID) over the course of 2 years.
“There’s been a lot of noise in the ankylosing spondylitis community about the potential benefit of NSAIDs in preventing structural progression. Previous information suggested that staying on them continuously actually reduced radiographic progression. This diclofenac study has shaken things up a little. It raises the question of whether there is any added benefit for NSAIDs in terms of structural progression,” he commented.
Current ACR/SAA/SPARTAN guidelines, which predate the study, feature a conditional recommendation that patients with active ankylosing spondylitis stay on continuous NSAID therapy.
Secukinumab is also approved for treatment of psoriasis and psoriatic arthritis.
Dr. Ruderman reported serving as a consultant to and/or receiving research grants from numerous pharmaceutical companies, including Novartis, which markets secukinumab.
EXPERT ANALYSIS FROM RWCS 2016