User login
Link Found Between Agent Orange Exposure and Multiple Myeloma
There was a 2.4-fold increased risk for monoclonal gammopathy of undetermined significance (MGUS), a precursor to multiple myeloma (MM), for Air Force veterans exposed to Agent Orange, according to a study reported in JAMA Oncology. Already, veterans who develop MM and were exposed to Agent Orange during military service are eligible to receive benefits, but the study further highlights the relationship.
Related: Management of Myeloma and Its Precursor Syndromes
The Agent Orange used during aerial spray missions of herbicides in the Vietnam War contained 2, 4-dichlorophenoxyacetic acid (2,4-D) and 2,4,5-trichlorophenoxyacetic acid (2,4,5-T), as well as human carcinogen 2,3,7,8-tetrachlorodibenzo-p-doxin in variable amounts. After obtaining the laboratory data from 958 serum samples from Air Force personnel, the Air Force Health Studies questionnaire, and results from the physical exam from all participants, researchers were able to compare their findings with control group veterans.
Related: Nephrotic Syndrome Is a Marker for Occult Cancer
The researchers created 2 test groups from Air Force veterans. The first were 777 participants of Operation Ranch Hand, who conducted aerial herbicidal missions from 1962 to 1971, and the second group was made up of 1,174 participants, who had similar duties but did not participate in the missions.
The risk of MGUS was more pronounced in veterans aged > 70 years (odds ratio [OR], 3.4; 95% confidence interval [CI], 1.27-4.44; P = .007). Among veterans aged > 70 years, there was not a significant increase in risk (OR, 1.4%; 95% CI, 0.55-3.63; P = .63). The crude prevalence of overall MGUS was 7.1% (34 of 479) in the exposed veterans, compared with 3.1% (15 of 479) in the comparison group.
Source
Landgren O, Shim YK, Michalek J, et al. JAMA Oncol. [Published online ahead of print September 3, 2015.]
doi: 10.1001/jamaoncol.2015.2938.
There was a 2.4-fold increased risk for monoclonal gammopathy of undetermined significance (MGUS), a precursor to multiple myeloma (MM), for Air Force veterans exposed to Agent Orange, according to a study reported in JAMA Oncology. Already, veterans who develop MM and were exposed to Agent Orange during military service are eligible to receive benefits, but the study further highlights the relationship.
Related: Management of Myeloma and Its Precursor Syndromes
The Agent Orange used during aerial spray missions of herbicides in the Vietnam War contained 2, 4-dichlorophenoxyacetic acid (2,4-D) and 2,4,5-trichlorophenoxyacetic acid (2,4,5-T), as well as human carcinogen 2,3,7,8-tetrachlorodibenzo-p-doxin in variable amounts. After obtaining the laboratory data from 958 serum samples from Air Force personnel, the Air Force Health Studies questionnaire, and results from the physical exam from all participants, researchers were able to compare their findings with control group veterans.
Related: Nephrotic Syndrome Is a Marker for Occult Cancer
The researchers created 2 test groups from Air Force veterans. The first were 777 participants of Operation Ranch Hand, who conducted aerial herbicidal missions from 1962 to 1971, and the second group was made up of 1,174 participants, who had similar duties but did not participate in the missions.
The risk of MGUS was more pronounced in veterans aged > 70 years (odds ratio [OR], 3.4; 95% confidence interval [CI], 1.27-4.44; P = .007). Among veterans aged > 70 years, there was not a significant increase in risk (OR, 1.4%; 95% CI, 0.55-3.63; P = .63). The crude prevalence of overall MGUS was 7.1% (34 of 479) in the exposed veterans, compared with 3.1% (15 of 479) in the comparison group.
Source
Landgren O, Shim YK, Michalek J, et al. JAMA Oncol. [Published online ahead of print September 3, 2015.]
doi: 10.1001/jamaoncol.2015.2938.
There was a 2.4-fold increased risk for monoclonal gammopathy of undetermined significance (MGUS), a precursor to multiple myeloma (MM), for Air Force veterans exposed to Agent Orange, according to a study reported in JAMA Oncology. Already, veterans who develop MM and were exposed to Agent Orange during military service are eligible to receive benefits, but the study further highlights the relationship.
Related: Management of Myeloma and Its Precursor Syndromes
The Agent Orange used during aerial spray missions of herbicides in the Vietnam War contained 2, 4-dichlorophenoxyacetic acid (2,4-D) and 2,4,5-trichlorophenoxyacetic acid (2,4,5-T), as well as human carcinogen 2,3,7,8-tetrachlorodibenzo-p-doxin in variable amounts. After obtaining the laboratory data from 958 serum samples from Air Force personnel, the Air Force Health Studies questionnaire, and results from the physical exam from all participants, researchers were able to compare their findings with control group veterans.
Related: Nephrotic Syndrome Is a Marker for Occult Cancer
The researchers created 2 test groups from Air Force veterans. The first were 777 participants of Operation Ranch Hand, who conducted aerial herbicidal missions from 1962 to 1971, and the second group was made up of 1,174 participants, who had similar duties but did not participate in the missions.
The risk of MGUS was more pronounced in veterans aged > 70 years (odds ratio [OR], 3.4; 95% confidence interval [CI], 1.27-4.44; P = .007). Among veterans aged > 70 years, there was not a significant increase in risk (OR, 1.4%; 95% CI, 0.55-3.63; P = .63). The crude prevalence of overall MGUS was 7.1% (34 of 479) in the exposed veterans, compared with 3.1% (15 of 479) in the comparison group.
Source
Landgren O, Shim YK, Michalek J, et al. JAMA Oncol. [Published online ahead of print September 3, 2015.]
doi: 10.1001/jamaoncol.2015.2938.
Patchouli
Pogostemon cablin, known in the West as patchouli or guang huo-xiang in China, is a long-time staple in traditional Chinese medicine for various indications, particularly gastrointestinal and skin disorders1.
Patchouli oil, which contains several mono- and sesquiterpenoids, alkaloids, and flavonoids, is thought to possess significant anti-inflammatory and antioxidant qualities2.In fact, it is reputed to impart antiviral, antioxidant, anti-inflammatory, and analgesic effects, and is also known to protect intestinal barrier function3. Peng et al. have found that patchouli oil exerts significant antibacterial activity against methicillin-resistant Staphylococcus aureus (MRSA)4.
After a comprehensive 2013 review, Chen et al. deemed P. cablin to have potential clinical benefits as an effective adaptogenic herbal treatment3. It is thought to have some antiacne properties as well1. Further, P. cablin is among the Top 10 most-often-used traditional Chinese medicine prescriptions for skin care and appearance1.
In Brazil, China, Indonesia, and Malaysia, P. cablin is cultivated for its essential oil, which plays an important role in the perfume industry. Patchouli essential oil, featured in perfumes, soaps, cosmetics, and as incense, is used by aromatherapists for its calming and reviving effects. The essential oil has also been shown to impart antioxidant activity5.
In 2014, Lin et al. studied the protective effects of P. cablin essential oil against ultraviolet (UV)-induced skin photoaging in mice. The researchers applied patchouli oil for 2 hours before UV exposure to the dorsal depilated skin of mice. They found that patchouli oil doses of 6 mg/mouse and 9 mg/mouse significantly suppressed skin wrinkle formation, mitigated skin elasticity impairment, and augmented collagen content (21.9% and 26.3%, respectively). The same doses also yielded significant reductions in epidermal thickness and malondialdehyde content, and blocked the disruption of collagen and elastic fibers. Patchouli oil treatment also resulted in the up-regulation of the antioxidant enzymes superoxide dismutase, glutathione peroxidase, and catalase. The investigators concluded that patchouli oil, perhaps due to its antioxidant characteristics, and sesquiterpene constituents in particular, was effective in preventing photoaging in mice, and warrants attention as a potential agent to hinder photoaging in humans1.
Feng et al. also investigated the effects of topically applied patchouli alcohol on UV-induced photoaging in mice that year. For 9 weeks, investigators applied patchouli oil solution or a vehicle to the depilated dorsal skin of 6-week-old mice. They found that patchouli oil significantly hastened the recovery of UV-induced skin lesions, which they ascribed to the antioxidant and anti-inflammatory activity of the agent and its down-regulation of the expression of matrix metalloproteinase (MMP)-1 and MMP-32.
Antimicrobial and mosquito repellent activity
In a 2005 study by Trongtokit et al. of the mosquito-repellent activity of 38 essential oils at three concentrations (10%, 50%, or undiluted) against the mosquito Aedes aegypti under laboratory conditions using human volunteers, undiluted P. cablin oil was one of four [along with Cymbopogon nardus (citronella), Syzygium aromaticum (clove), and Zanthoxylum limonella (Thai name: makaen)] undiluted oils to yield an effect, 2 hours of full repellency. The investigators then tested the same concentrations of these oils for repellency against Culex quinquefasciatus (the Southern house mosquito) and Anopheles dirus (the mosquito considered to be a vector of malaria in Asian forested zones. The undiluted oils provided the greatest protection, with clove oil rendering the most durable repellency6.
Photoaging
Wu et al. determined the acaricidal activity of compounds extracted from patchouli oil against the house dust mite (Dermatophagoides farinae) in 2012. They isolated 2-(1,3-dihydroxy-but-2-enylidene)-6-methyl-3-oxo-heptanoic acid (DHEMH), the hydrolysate of pogostone, and 15 other constituents in patchouli oil, ultimately ascertaining that DHEMH and patchouli oil itself were the most toxic substances to D. farinae. The investigators concluded that patchouli oil and DHEMH warrant consideration and more study for their acaricidal potential as environmentally friendly, effective, and simple fumigant alternatives to chemical agents7.
In 2013, Yang et al. used molecular docking technology to evaluate the antibacterial activity of patchouli oil in vitro. They identified 26 compounds in patchouli oil displaying antibacterial activity, with pogostone and (-)-patchouli alcohol exhibiting the strongest activity8. Later that year, Yang et al. used the same technology to establish that Herba pogostemonis oil exhibited potent antibacterial effects, especially the constituents pogostone and (-)-Herba pogostemonis alcohol9. Raharjo and Fatchiyah also used molecular docking tools and Chimera 1.7s viewer software in a virtual screening of compounds from patchouli oil, concluding that alpha-patchouli alcohol is a potential inhibitor of the cyclo-oxygenase (COX)-1 enzyme. This is notable given the pivotal role of COX-1 in the inflammatory response10.
The next year, Peng et al. isolated one of the primary constituents of patchouli oil, pogostone, and assessed its antibacterial activity in vitro and in vivo. They found that pogostone suppressed both gram-negative and gram-positive bacteria in vitro. The researchers noted that pogostone was active against some drug-resistant bacteria (such as MRSA). Via intraperitoneal injection, pogostone displayed antibacterial activity in male and female Kunming mice against Escherichia coli and MRSA. At concentrations of 50 and 100 mg/kg, 90% of the mice infected with E. coli were protected; 60% of the mice at 25 mg/kg were protected. For mice with MRSA, 60% were protected at a dose of 100 mg/kg and 50% at a dose of 50 mg/kg. The investigators concluded that pogostone is a viable antibacterial agent for clinical use4.
Transdermal delivery
A 2008 study by Luo et al. showed that patchouli oil was among three volatile oils that improved the skin penetration of the flavonoids baicalin11. It was less effective than several compounds, including clove oil, camphor, menthol, and oleic acid, as a transdermal enhancer in a subsequent study by Zheng et al.12.
Conclusion
Patchouli oil continues to be used today in traditional Chinese medicine. In the West, the established literature on Pogostemon cablin is thin, but what has emerged recently, particularly studies on the protection against photoaging in mice, supports the continued investigation of this ancient herb to determine its potential role in dermatologic practice. As it is, much more research is necessary.
References
1. J Ethnopharmacol. 2014;154(2):408-18.
2. Eur J Pharm Sci. 2014;63:113-23.
3. Expert Opin Investig Drugs. 2013;22(2):245-57.
4. Chin Med J. (Engl) 2014;127(23):4001-5.
5. J Agric Food Chem. 2007;55(5):1737-42
6. Phytother Res. 2005;19(4):303-9.
7. Chem Pharm Bull (Tokyo). 2012;60(2):178-82.
8. Iran J Pharm Res. 2013 Summer;12(3):307-16.
9. Pak J Pharm Sci. 2013;26(6):1173-9.
10. Bioinformation 2013;9(6):321-4.
11. Zhong Yao Cai. 2008;31(11):1721-4
12. Zhongguo Zhong Yao Za Zhi. 2009;34(20):2599-603.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in the Design District in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote the textbook, “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and a book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). She has contributed to the Cosmeceutical Critique column in Dermatology News since January 2001. Her latest book, “Cosmeceuticals and Cosmetic Ingredients,” was published in November 2014. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Evolus, Galderma, GlaxoSmithKline, Kythera Biopharmaceuticals, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Topix Pharmaceuticals, and Unilever.
Pogostemon cablin, known in the West as patchouli or guang huo-xiang in China, is a long-time staple in traditional Chinese medicine for various indications, particularly gastrointestinal and skin disorders1.
Patchouli oil, which contains several mono- and sesquiterpenoids, alkaloids, and flavonoids, is thought to possess significant anti-inflammatory and antioxidant qualities2.In fact, it is reputed to impart antiviral, antioxidant, anti-inflammatory, and analgesic effects, and is also known to protect intestinal barrier function3. Peng et al. have found that patchouli oil exerts significant antibacterial activity against methicillin-resistant Staphylococcus aureus (MRSA)4.
After a comprehensive 2013 review, Chen et al. deemed P. cablin to have potential clinical benefits as an effective adaptogenic herbal treatment3. It is thought to have some antiacne properties as well1. Further, P. cablin is among the Top 10 most-often-used traditional Chinese medicine prescriptions for skin care and appearance1.
In Brazil, China, Indonesia, and Malaysia, P. cablin is cultivated for its essential oil, which plays an important role in the perfume industry. Patchouli essential oil, featured in perfumes, soaps, cosmetics, and as incense, is used by aromatherapists for its calming and reviving effects. The essential oil has also been shown to impart antioxidant activity5.
In 2014, Lin et al. studied the protective effects of P. cablin essential oil against ultraviolet (UV)-induced skin photoaging in mice. The researchers applied patchouli oil for 2 hours before UV exposure to the dorsal depilated skin of mice. They found that patchouli oil doses of 6 mg/mouse and 9 mg/mouse significantly suppressed skin wrinkle formation, mitigated skin elasticity impairment, and augmented collagen content (21.9% and 26.3%, respectively). The same doses also yielded significant reductions in epidermal thickness and malondialdehyde content, and blocked the disruption of collagen and elastic fibers. Patchouli oil treatment also resulted in the up-regulation of the antioxidant enzymes superoxide dismutase, glutathione peroxidase, and catalase. The investigators concluded that patchouli oil, perhaps due to its antioxidant characteristics, and sesquiterpene constituents in particular, was effective in preventing photoaging in mice, and warrants attention as a potential agent to hinder photoaging in humans1.
Feng et al. also investigated the effects of topically applied patchouli alcohol on UV-induced photoaging in mice that year. For 9 weeks, investigators applied patchouli oil solution or a vehicle to the depilated dorsal skin of 6-week-old mice. They found that patchouli oil significantly hastened the recovery of UV-induced skin lesions, which they ascribed to the antioxidant and anti-inflammatory activity of the agent and its down-regulation of the expression of matrix metalloproteinase (MMP)-1 and MMP-32.
Antimicrobial and mosquito repellent activity
In a 2005 study by Trongtokit et al. of the mosquito-repellent activity of 38 essential oils at three concentrations (10%, 50%, or undiluted) against the mosquito Aedes aegypti under laboratory conditions using human volunteers, undiluted P. cablin oil was one of four [along with Cymbopogon nardus (citronella), Syzygium aromaticum (clove), and Zanthoxylum limonella (Thai name: makaen)] undiluted oils to yield an effect, 2 hours of full repellency. The investigators then tested the same concentrations of these oils for repellency against Culex quinquefasciatus (the Southern house mosquito) and Anopheles dirus (the mosquito considered to be a vector of malaria in Asian forested zones. The undiluted oils provided the greatest protection, with clove oil rendering the most durable repellency6.
Photoaging
Wu et al. determined the acaricidal activity of compounds extracted from patchouli oil against the house dust mite (Dermatophagoides farinae) in 2012. They isolated 2-(1,3-dihydroxy-but-2-enylidene)-6-methyl-3-oxo-heptanoic acid (DHEMH), the hydrolysate of pogostone, and 15 other constituents in patchouli oil, ultimately ascertaining that DHEMH and patchouli oil itself were the most toxic substances to D. farinae. The investigators concluded that patchouli oil and DHEMH warrant consideration and more study for their acaricidal potential as environmentally friendly, effective, and simple fumigant alternatives to chemical agents7.
In 2013, Yang et al. used molecular docking technology to evaluate the antibacterial activity of patchouli oil in vitro. They identified 26 compounds in patchouli oil displaying antibacterial activity, with pogostone and (-)-patchouli alcohol exhibiting the strongest activity8. Later that year, Yang et al. used the same technology to establish that Herba pogostemonis oil exhibited potent antibacterial effects, especially the constituents pogostone and (-)-Herba pogostemonis alcohol9. Raharjo and Fatchiyah also used molecular docking tools and Chimera 1.7s viewer software in a virtual screening of compounds from patchouli oil, concluding that alpha-patchouli alcohol is a potential inhibitor of the cyclo-oxygenase (COX)-1 enzyme. This is notable given the pivotal role of COX-1 in the inflammatory response10.
The next year, Peng et al. isolated one of the primary constituents of patchouli oil, pogostone, and assessed its antibacterial activity in vitro and in vivo. They found that pogostone suppressed both gram-negative and gram-positive bacteria in vitro. The researchers noted that pogostone was active against some drug-resistant bacteria (such as MRSA). Via intraperitoneal injection, pogostone displayed antibacterial activity in male and female Kunming mice against Escherichia coli and MRSA. At concentrations of 50 and 100 mg/kg, 90% of the mice infected with E. coli were protected; 60% of the mice at 25 mg/kg were protected. For mice with MRSA, 60% were protected at a dose of 100 mg/kg and 50% at a dose of 50 mg/kg. The investigators concluded that pogostone is a viable antibacterial agent for clinical use4.
Transdermal delivery
A 2008 study by Luo et al. showed that patchouli oil was among three volatile oils that improved the skin penetration of the flavonoids baicalin11. It was less effective than several compounds, including clove oil, camphor, menthol, and oleic acid, as a transdermal enhancer in a subsequent study by Zheng et al.12.
Conclusion
Patchouli oil continues to be used today in traditional Chinese medicine. In the West, the established literature on Pogostemon cablin is thin, but what has emerged recently, particularly studies on the protection against photoaging in mice, supports the continued investigation of this ancient herb to determine its potential role in dermatologic practice. As it is, much more research is necessary.
References
1. J Ethnopharmacol. 2014;154(2):408-18.
2. Eur J Pharm Sci. 2014;63:113-23.
3. Expert Opin Investig Drugs. 2013;22(2):245-57.
4. Chin Med J. (Engl) 2014;127(23):4001-5.
5. J Agric Food Chem. 2007;55(5):1737-42
6. Phytother Res. 2005;19(4):303-9.
7. Chem Pharm Bull (Tokyo). 2012;60(2):178-82.
8. Iran J Pharm Res. 2013 Summer;12(3):307-16.
9. Pak J Pharm Sci. 2013;26(6):1173-9.
10. Bioinformation 2013;9(6):321-4.
11. Zhong Yao Cai. 2008;31(11):1721-4
12. Zhongguo Zhong Yao Za Zhi. 2009;34(20):2599-603.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in the Design District in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote the textbook, “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and a book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). She has contributed to the Cosmeceutical Critique column in Dermatology News since January 2001. Her latest book, “Cosmeceuticals and Cosmetic Ingredients,” was published in November 2014. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Evolus, Galderma, GlaxoSmithKline, Kythera Biopharmaceuticals, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Topix Pharmaceuticals, and Unilever.
Pogostemon cablin, known in the West as patchouli or guang huo-xiang in China, is a long-time staple in traditional Chinese medicine for various indications, particularly gastrointestinal and skin disorders1.
Patchouli oil, which contains several mono- and sesquiterpenoids, alkaloids, and flavonoids, is thought to possess significant anti-inflammatory and antioxidant qualities2.In fact, it is reputed to impart antiviral, antioxidant, anti-inflammatory, and analgesic effects, and is also known to protect intestinal barrier function3. Peng et al. have found that patchouli oil exerts significant antibacterial activity against methicillin-resistant Staphylococcus aureus (MRSA)4.
After a comprehensive 2013 review, Chen et al. deemed P. cablin to have potential clinical benefits as an effective adaptogenic herbal treatment3. It is thought to have some antiacne properties as well1. Further, P. cablin is among the Top 10 most-often-used traditional Chinese medicine prescriptions for skin care and appearance1.
In Brazil, China, Indonesia, and Malaysia, P. cablin is cultivated for its essential oil, which plays an important role in the perfume industry. Patchouli essential oil, featured in perfumes, soaps, cosmetics, and as incense, is used by aromatherapists for its calming and reviving effects. The essential oil has also been shown to impart antioxidant activity5.
In 2014, Lin et al. studied the protective effects of P. cablin essential oil against ultraviolet (UV)-induced skin photoaging in mice. The researchers applied patchouli oil for 2 hours before UV exposure to the dorsal depilated skin of mice. They found that patchouli oil doses of 6 mg/mouse and 9 mg/mouse significantly suppressed skin wrinkle formation, mitigated skin elasticity impairment, and augmented collagen content (21.9% and 26.3%, respectively). The same doses also yielded significant reductions in epidermal thickness and malondialdehyde content, and blocked the disruption of collagen and elastic fibers. Patchouli oil treatment also resulted in the up-regulation of the antioxidant enzymes superoxide dismutase, glutathione peroxidase, and catalase. The investigators concluded that patchouli oil, perhaps due to its antioxidant characteristics, and sesquiterpene constituents in particular, was effective in preventing photoaging in mice, and warrants attention as a potential agent to hinder photoaging in humans1.
Feng et al. also investigated the effects of topically applied patchouli alcohol on UV-induced photoaging in mice that year. For 9 weeks, investigators applied patchouli oil solution or a vehicle to the depilated dorsal skin of 6-week-old mice. They found that patchouli oil significantly hastened the recovery of UV-induced skin lesions, which they ascribed to the antioxidant and anti-inflammatory activity of the agent and its down-regulation of the expression of matrix metalloproteinase (MMP)-1 and MMP-32.
Antimicrobial and mosquito repellent activity
In a 2005 study by Trongtokit et al. of the mosquito-repellent activity of 38 essential oils at three concentrations (10%, 50%, or undiluted) against the mosquito Aedes aegypti under laboratory conditions using human volunteers, undiluted P. cablin oil was one of four [along with Cymbopogon nardus (citronella), Syzygium aromaticum (clove), and Zanthoxylum limonella (Thai name: makaen)] undiluted oils to yield an effect, 2 hours of full repellency. The investigators then tested the same concentrations of these oils for repellency against Culex quinquefasciatus (the Southern house mosquito) and Anopheles dirus (the mosquito considered to be a vector of malaria in Asian forested zones. The undiluted oils provided the greatest protection, with clove oil rendering the most durable repellency6.
Photoaging
Wu et al. determined the acaricidal activity of compounds extracted from patchouli oil against the house dust mite (Dermatophagoides farinae) in 2012. They isolated 2-(1,3-dihydroxy-but-2-enylidene)-6-methyl-3-oxo-heptanoic acid (DHEMH), the hydrolysate of pogostone, and 15 other constituents in patchouli oil, ultimately ascertaining that DHEMH and patchouli oil itself were the most toxic substances to D. farinae. The investigators concluded that patchouli oil and DHEMH warrant consideration and more study for their acaricidal potential as environmentally friendly, effective, and simple fumigant alternatives to chemical agents7.
In 2013, Yang et al. used molecular docking technology to evaluate the antibacterial activity of patchouli oil in vitro. They identified 26 compounds in patchouli oil displaying antibacterial activity, with pogostone and (-)-patchouli alcohol exhibiting the strongest activity8. Later that year, Yang et al. used the same technology to establish that Herba pogostemonis oil exhibited potent antibacterial effects, especially the constituents pogostone and (-)-Herba pogostemonis alcohol9. Raharjo and Fatchiyah also used molecular docking tools and Chimera 1.7s viewer software in a virtual screening of compounds from patchouli oil, concluding that alpha-patchouli alcohol is a potential inhibitor of the cyclo-oxygenase (COX)-1 enzyme. This is notable given the pivotal role of COX-1 in the inflammatory response10.
The next year, Peng et al. isolated one of the primary constituents of patchouli oil, pogostone, and assessed its antibacterial activity in vitro and in vivo. They found that pogostone suppressed both gram-negative and gram-positive bacteria in vitro. The researchers noted that pogostone was active against some drug-resistant bacteria (such as MRSA). Via intraperitoneal injection, pogostone displayed antibacterial activity in male and female Kunming mice against Escherichia coli and MRSA. At concentrations of 50 and 100 mg/kg, 90% of the mice infected with E. coli were protected; 60% of the mice at 25 mg/kg were protected. For mice with MRSA, 60% were protected at a dose of 100 mg/kg and 50% at a dose of 50 mg/kg. The investigators concluded that pogostone is a viable antibacterial agent for clinical use4.
Transdermal delivery
A 2008 study by Luo et al. showed that patchouli oil was among three volatile oils that improved the skin penetration of the flavonoids baicalin11. It was less effective than several compounds, including clove oil, camphor, menthol, and oleic acid, as a transdermal enhancer in a subsequent study by Zheng et al.12.
Conclusion
Patchouli oil continues to be used today in traditional Chinese medicine. In the West, the established literature on Pogostemon cablin is thin, but what has emerged recently, particularly studies on the protection against photoaging in mice, supports the continued investigation of this ancient herb to determine its potential role in dermatologic practice. As it is, much more research is necessary.
References
1. J Ethnopharmacol. 2014;154(2):408-18.
2. Eur J Pharm Sci. 2014;63:113-23.
3. Expert Opin Investig Drugs. 2013;22(2):245-57.
4. Chin Med J. (Engl) 2014;127(23):4001-5.
5. J Agric Food Chem. 2007;55(5):1737-42
6. Phytother Res. 2005;19(4):303-9.
7. Chem Pharm Bull (Tokyo). 2012;60(2):178-82.
8. Iran J Pharm Res. 2013 Summer;12(3):307-16.
9. Pak J Pharm Sci. 2013;26(6):1173-9.
10. Bioinformation 2013;9(6):321-4.
11. Zhong Yao Cai. 2008;31(11):1721-4
12. Zhongguo Zhong Yao Za Zhi. 2009;34(20):2599-603.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in the Design District in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote the textbook, “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and a book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). She has contributed to the Cosmeceutical Critique column in Dermatology News since January 2001. Her latest book, “Cosmeceuticals and Cosmetic Ingredients,” was published in November 2014. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Evolus, Galderma, GlaxoSmithKline, Kythera Biopharmaceuticals, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Topix Pharmaceuticals, and Unilever.
Targeting STAT3 to prevent relapse in AML

Compounds that target a novel binding site on STAT3 may one day be used to prevent relapse in acute myeloid leukemia (AML), according to researchers.
STAT3 is known to interfere with chemotherapy and is thought to play a role in many cases of AML relapse.
But preclinical research suggests a compound known as MM-206 can disrupt STAT3’s disease-promoting effects by targeting a previously unknown ligand-binding site on the protein.
Zachary Ball, PhD, of Rice University in Houston, Texas, and his colleagues described this research in Angewandte Chemie.
The team first discovered that the coiled–coil domain (CCD) is a novel ligand-binding site on STAT3. Then, they identified a naphthalene sulfonamide compound, known as C188, that can target CCD and inhibit STAT3.
The researchers tested C188 and compounds synthesized from it—C188-9, C188-9-Rh2, and MM-206—in vitro and in vivo. Only MM-206 proved effective in vivo.
“Our main advance, from a medicinal perspective, is that this compound also works in a mouse model,” Dr Ball said. “All the other compounds worked in cells, but, in mice, they weren’t potent enough or stable enough.”
MM-206 inhibited STAT3 phosphorylation and induced apoptosis in 3 different AML cell lines. The compound also prompted apoptosis in primary tumor cells from pediatric AML patients.
Among mice engrafted with luciferase-expressing MV4-11 AML cells, those that received MM-206 exhibited slower disease progression than untreated mice.
When the mice received 4 weeks of treatment with MM-206, they had significantly fewer tumor cells in their bone marrow and lived significantly longer than control mice (P=0.019 at 10 weeks).
Follow-up studies should lead to improved versions of MM-206, according to Dr Ball.
“The discovery raises new questions about STAT3 biology and points the way to future anticancer approaches,” he said, “including combination therapies of coiled-coil STAT3 inhibitors in tandem with other agents.” ![]()

Compounds that target a novel binding site on STAT3 may one day be used to prevent relapse in acute myeloid leukemia (AML), according to researchers.
STAT3 is known to interfere with chemotherapy and is thought to play a role in many cases of AML relapse.
But preclinical research suggests a compound known as MM-206 can disrupt STAT3’s disease-promoting effects by targeting a previously unknown ligand-binding site on the protein.
Zachary Ball, PhD, of Rice University in Houston, Texas, and his colleagues described this research in Angewandte Chemie.
The team first discovered that the coiled–coil domain (CCD) is a novel ligand-binding site on STAT3. Then, they identified a naphthalene sulfonamide compound, known as C188, that can target CCD and inhibit STAT3.
The researchers tested C188 and compounds synthesized from it—C188-9, C188-9-Rh2, and MM-206—in vitro and in vivo. Only MM-206 proved effective in vivo.
“Our main advance, from a medicinal perspective, is that this compound also works in a mouse model,” Dr Ball said. “All the other compounds worked in cells, but, in mice, they weren’t potent enough or stable enough.”
MM-206 inhibited STAT3 phosphorylation and induced apoptosis in 3 different AML cell lines. The compound also prompted apoptosis in primary tumor cells from pediatric AML patients.
Among mice engrafted with luciferase-expressing MV4-11 AML cells, those that received MM-206 exhibited slower disease progression than untreated mice.
When the mice received 4 weeks of treatment with MM-206, they had significantly fewer tumor cells in their bone marrow and lived significantly longer than control mice (P=0.019 at 10 weeks).
Follow-up studies should lead to improved versions of MM-206, according to Dr Ball.
“The discovery raises new questions about STAT3 biology and points the way to future anticancer approaches,” he said, “including combination therapies of coiled-coil STAT3 inhibitors in tandem with other agents.” ![]()

Compounds that target a novel binding site on STAT3 may one day be used to prevent relapse in acute myeloid leukemia (AML), according to researchers.
STAT3 is known to interfere with chemotherapy and is thought to play a role in many cases of AML relapse.
But preclinical research suggests a compound known as MM-206 can disrupt STAT3’s disease-promoting effects by targeting a previously unknown ligand-binding site on the protein.
Zachary Ball, PhD, of Rice University in Houston, Texas, and his colleagues described this research in Angewandte Chemie.
The team first discovered that the coiled–coil domain (CCD) is a novel ligand-binding site on STAT3. Then, they identified a naphthalene sulfonamide compound, known as C188, that can target CCD and inhibit STAT3.
The researchers tested C188 and compounds synthesized from it—C188-9, C188-9-Rh2, and MM-206—in vitro and in vivo. Only MM-206 proved effective in vivo.
“Our main advance, from a medicinal perspective, is that this compound also works in a mouse model,” Dr Ball said. “All the other compounds worked in cells, but, in mice, they weren’t potent enough or stable enough.”
MM-206 inhibited STAT3 phosphorylation and induced apoptosis in 3 different AML cell lines. The compound also prompted apoptosis in primary tumor cells from pediatric AML patients.
Among mice engrafted with luciferase-expressing MV4-11 AML cells, those that received MM-206 exhibited slower disease progression than untreated mice.
When the mice received 4 weeks of treatment with MM-206, they had significantly fewer tumor cells in their bone marrow and lived significantly longer than control mice (P=0.019 at 10 weeks).
Follow-up studies should lead to improved versions of MM-206, according to Dr Ball.
“The discovery raises new questions about STAT3 biology and points the way to future anticancer approaches,” he said, “including combination therapies of coiled-coil STAT3 inhibitors in tandem with other agents.” ![]()
Combination induces remission in advanced MM

Photo from Penn Medicine
Investigators have described a 3-pronged treatment approach that induced sustained remission in a patient with advanced multiple myeloma (MM).
The treatment combined chemotherapy, autologous stem cell transplant, and the chimeric antigen receptor T-cell therapy CTL019.
The patient, who had received 9 prior lines of therapy, responded to this combination despite the fact that 99.95% of her neoplastic plasma cells did not express CD19.
“There was some skepticism about whether a CD19-directed therapy would work in this disease, since nearly all of these patients’ cancerous plasma cells do not express CD19,” said study author Edward Stadtmauer, MD, of the University of Pennsylvania in Philadelphia.
“Since there was data showing that the possible stem cells can be CD19-positive, our hypothesis was that we may be able to devise a therapy targeted at early precursors of those cells.”
Dr Stadtmauer and his colleagues described this case, which is part of a larger trial, in NEJM.
Prior to receiving the combination therapy, the MM patient had received 9 different treatment regimens in the 5 years since her diagnosis (at age 43).
The patient had received regimens containing lenalidomide, bortezomib, dexamethasone, carfilzomib, pomalidomide, vorinostat, clarithromycin, and elotuzumab. She also received a previous autologous stem cell transplant, which had only controlled her disease for a few months.
For this study, the patient received high-dose melphalan (140 mg/m2), reinfusion of autologous stem cells (≥2.1×106 cells per kg of body weight), and a CTL019 infusion 12 days later.
Response and adverse effects
The patient experienced transplant-related side effects prior to receiving CTL019, including grade 4 neutropenia and thrombocytopenia, grade 2 nausea and anorexia, neutropenic fever, and Staphylococcus aureus bacteremia. By day 100 after transplant, all transplant-related adverse effects had resolved.
The patient had hypogammaglobulinemia before transplant that persisted at day 100 after transplant and was attributed to the effects of CTL019 on normal B cells and plasma cells. There were no other adverse events attributable to CTL019.
On day 130 after transplant, the patient began to receive lenalidomide maintenance at 5 mg daily (an optional part of the protocol). Her dose was later reduced to 5 mg twice weekly because of gastrointestinal side effects.
At day 130 after CTL019 infusion, tests revealed no evidence of disease. The patient—who was the first to be treated as part of this trial—remains in remission more than 12 months after receiving this therapy.
“We couldn’t be more pleased with this patient’s response,” said study author Alfred Garfall, MD, of the University of Pennsylvania.
“We believe her CTL019 cells made the difference, since we would not have expected such a durable remission with a transplant alone, considering the very transient response this patient had to her first transplant several years ago.”
Dr Garfall and his colleagues also reported on the trial’s overall progress. Of the 10 patients who have received this combination therapy to date, 6 remain progression-free, although 2 patients have been treated very recently.
The additional CTL019-attributable side effects have been grade 1 cytokine release syndrome (n=1) and grade 3 enterocolitis due to autologous graft-vs-host disease (n=1).
This trial is sponsored by Novartis, the company developing CTL019, and others. CTL019 was originally developed at the University of Pennsylvania, but the university licensed the technology to Novartis. ![]()

Photo from Penn Medicine
Investigators have described a 3-pronged treatment approach that induced sustained remission in a patient with advanced multiple myeloma (MM).
The treatment combined chemotherapy, autologous stem cell transplant, and the chimeric antigen receptor T-cell therapy CTL019.
The patient, who had received 9 prior lines of therapy, responded to this combination despite the fact that 99.95% of her neoplastic plasma cells did not express CD19.
“There was some skepticism about whether a CD19-directed therapy would work in this disease, since nearly all of these patients’ cancerous plasma cells do not express CD19,” said study author Edward Stadtmauer, MD, of the University of Pennsylvania in Philadelphia.
“Since there was data showing that the possible stem cells can be CD19-positive, our hypothesis was that we may be able to devise a therapy targeted at early precursors of those cells.”
Dr Stadtmauer and his colleagues described this case, which is part of a larger trial, in NEJM.
Prior to receiving the combination therapy, the MM patient had received 9 different treatment regimens in the 5 years since her diagnosis (at age 43).
The patient had received regimens containing lenalidomide, bortezomib, dexamethasone, carfilzomib, pomalidomide, vorinostat, clarithromycin, and elotuzumab. She also received a previous autologous stem cell transplant, which had only controlled her disease for a few months.
For this study, the patient received high-dose melphalan (140 mg/m2), reinfusion of autologous stem cells (≥2.1×106 cells per kg of body weight), and a CTL019 infusion 12 days later.
Response and adverse effects
The patient experienced transplant-related side effects prior to receiving CTL019, including grade 4 neutropenia and thrombocytopenia, grade 2 nausea and anorexia, neutropenic fever, and Staphylococcus aureus bacteremia. By day 100 after transplant, all transplant-related adverse effects had resolved.
The patient had hypogammaglobulinemia before transplant that persisted at day 100 after transplant and was attributed to the effects of CTL019 on normal B cells and plasma cells. There were no other adverse events attributable to CTL019.
On day 130 after transplant, the patient began to receive lenalidomide maintenance at 5 mg daily (an optional part of the protocol). Her dose was later reduced to 5 mg twice weekly because of gastrointestinal side effects.
At day 130 after CTL019 infusion, tests revealed no evidence of disease. The patient—who was the first to be treated as part of this trial—remains in remission more than 12 months after receiving this therapy.
“We couldn’t be more pleased with this patient’s response,” said study author Alfred Garfall, MD, of the University of Pennsylvania.
“We believe her CTL019 cells made the difference, since we would not have expected such a durable remission with a transplant alone, considering the very transient response this patient had to her first transplant several years ago.”
Dr Garfall and his colleagues also reported on the trial’s overall progress. Of the 10 patients who have received this combination therapy to date, 6 remain progression-free, although 2 patients have been treated very recently.
The additional CTL019-attributable side effects have been grade 1 cytokine release syndrome (n=1) and grade 3 enterocolitis due to autologous graft-vs-host disease (n=1).
This trial is sponsored by Novartis, the company developing CTL019, and others. CTL019 was originally developed at the University of Pennsylvania, but the university licensed the technology to Novartis. ![]()

Photo from Penn Medicine
Investigators have described a 3-pronged treatment approach that induced sustained remission in a patient with advanced multiple myeloma (MM).
The treatment combined chemotherapy, autologous stem cell transplant, and the chimeric antigen receptor T-cell therapy CTL019.
The patient, who had received 9 prior lines of therapy, responded to this combination despite the fact that 99.95% of her neoplastic plasma cells did not express CD19.
“There was some skepticism about whether a CD19-directed therapy would work in this disease, since nearly all of these patients’ cancerous plasma cells do not express CD19,” said study author Edward Stadtmauer, MD, of the University of Pennsylvania in Philadelphia.
“Since there was data showing that the possible stem cells can be CD19-positive, our hypothesis was that we may be able to devise a therapy targeted at early precursors of those cells.”
Dr Stadtmauer and his colleagues described this case, which is part of a larger trial, in NEJM.
Prior to receiving the combination therapy, the MM patient had received 9 different treatment regimens in the 5 years since her diagnosis (at age 43).
The patient had received regimens containing lenalidomide, bortezomib, dexamethasone, carfilzomib, pomalidomide, vorinostat, clarithromycin, and elotuzumab. She also received a previous autologous stem cell transplant, which had only controlled her disease for a few months.
For this study, the patient received high-dose melphalan (140 mg/m2), reinfusion of autologous stem cells (≥2.1×106 cells per kg of body weight), and a CTL019 infusion 12 days later.
Response and adverse effects
The patient experienced transplant-related side effects prior to receiving CTL019, including grade 4 neutropenia and thrombocytopenia, grade 2 nausea and anorexia, neutropenic fever, and Staphylococcus aureus bacteremia. By day 100 after transplant, all transplant-related adverse effects had resolved.
The patient had hypogammaglobulinemia before transplant that persisted at day 100 after transplant and was attributed to the effects of CTL019 on normal B cells and plasma cells. There were no other adverse events attributable to CTL019.
On day 130 after transplant, the patient began to receive lenalidomide maintenance at 5 mg daily (an optional part of the protocol). Her dose was later reduced to 5 mg twice weekly because of gastrointestinal side effects.
At day 130 after CTL019 infusion, tests revealed no evidence of disease. The patient—who was the first to be treated as part of this trial—remains in remission more than 12 months after receiving this therapy.
“We couldn’t be more pleased with this patient’s response,” said study author Alfred Garfall, MD, of the University of Pennsylvania.
“We believe her CTL019 cells made the difference, since we would not have expected such a durable remission with a transplant alone, considering the very transient response this patient had to her first transplant several years ago.”
Dr Garfall and his colleagues also reported on the trial’s overall progress. Of the 10 patients who have received this combination therapy to date, 6 remain progression-free, although 2 patients have been treated very recently.
The additional CTL019-attributable side effects have been grade 1 cytokine release syndrome (n=1) and grade 3 enterocolitis due to autologous graft-vs-host disease (n=1).
This trial is sponsored by Novartis, the company developing CTL019, and others. CTL019 was originally developed at the University of Pennsylvania, but the university licensed the technology to Novartis. ![]()
Groups crowd-source cancer research

Photo by Rhoda Baer
In an attempt to crowd-source cancer research, a pair of Canadian organizations made a small molecule they developed freely available to researchers.
The goal was to fast-track the testing of new treatment strategies and facilitate sharing of the results.
Researchers have already completed preclinical studies of this molecule, a WDR5 inhibitor called OICR-9429, showing that it is active against breast cancer and acute myeloid leukemia (AML).
The organizations that developed OICR-9429 are the Ontario Institute for Cancer Research (OICR) and the Structural Genomics Consortium (SGC).
“In the time that it would normally take to negotiate a legal agreement to provide OICR-9429 to other research teams, we have received results back from our collaborators showing that it can kill 2 different types of cancer cells,” said Cheryl Arrowsmith, PhD, of SGC Toronto in Ontario, Canada.
“Opening our chemistry capabilities to the world’s scientists allowed us to crowd-source and accelerate the research.”
A study published in Nature Chemical Biology suggested that WDR5 is a therapeutic target for a subtype of AML, and OICR-9429 could therefore be used to treat the disease.
The researchers noted that mutations in C/EBPα occur in about 9% of AML cases and lead to the expression of a 30-kDa dominant negative isoform (C/EBPα-p30).
Their experiments revealed that C/EBPα p30 preferentially interacts with WDR5. So it was no surprise that OICR-9429 inhibited proliferation and induced differentiation in p30-expressing AML cells.
In a study published in Nature, OICR-9429 inhibited cancer cell growth in a panel of breast cancer cell lines driven by mutated forms of p53.
Researchers said this work has implications beyond breast cancer because p53 is mutated in at least half of all cancers and is dysregulated in others.
“It is remarkable how quickly our research results were translated into discoveries by the groups around the world,” said Rima Al-awar, PhD, of OICR in Toronto.
“We are looking forward to seeing more research conducted with OICR-9429 and showing that this new approach to early stage drug discovery has significant advantages.”
The SGC and OICR teams said they are continuing their collaboration to identify additional molecules to advance cancer drug discovery. ![]()

Photo by Rhoda Baer
In an attempt to crowd-source cancer research, a pair of Canadian organizations made a small molecule they developed freely available to researchers.
The goal was to fast-track the testing of new treatment strategies and facilitate sharing of the results.
Researchers have already completed preclinical studies of this molecule, a WDR5 inhibitor called OICR-9429, showing that it is active against breast cancer and acute myeloid leukemia (AML).
The organizations that developed OICR-9429 are the Ontario Institute for Cancer Research (OICR) and the Structural Genomics Consortium (SGC).
“In the time that it would normally take to negotiate a legal agreement to provide OICR-9429 to other research teams, we have received results back from our collaborators showing that it can kill 2 different types of cancer cells,” said Cheryl Arrowsmith, PhD, of SGC Toronto in Ontario, Canada.
“Opening our chemistry capabilities to the world’s scientists allowed us to crowd-source and accelerate the research.”
A study published in Nature Chemical Biology suggested that WDR5 is a therapeutic target for a subtype of AML, and OICR-9429 could therefore be used to treat the disease.
The researchers noted that mutations in C/EBPα occur in about 9% of AML cases and lead to the expression of a 30-kDa dominant negative isoform (C/EBPα-p30).
Their experiments revealed that C/EBPα p30 preferentially interacts with WDR5. So it was no surprise that OICR-9429 inhibited proliferation and induced differentiation in p30-expressing AML cells.
In a study published in Nature, OICR-9429 inhibited cancer cell growth in a panel of breast cancer cell lines driven by mutated forms of p53.
Researchers said this work has implications beyond breast cancer because p53 is mutated in at least half of all cancers and is dysregulated in others.
“It is remarkable how quickly our research results were translated into discoveries by the groups around the world,” said Rima Al-awar, PhD, of OICR in Toronto.
“We are looking forward to seeing more research conducted with OICR-9429 and showing that this new approach to early stage drug discovery has significant advantages.”
The SGC and OICR teams said they are continuing their collaboration to identify additional molecules to advance cancer drug discovery. ![]()

Photo by Rhoda Baer
In an attempt to crowd-source cancer research, a pair of Canadian organizations made a small molecule they developed freely available to researchers.
The goal was to fast-track the testing of new treatment strategies and facilitate sharing of the results.
Researchers have already completed preclinical studies of this molecule, a WDR5 inhibitor called OICR-9429, showing that it is active against breast cancer and acute myeloid leukemia (AML).
The organizations that developed OICR-9429 are the Ontario Institute for Cancer Research (OICR) and the Structural Genomics Consortium (SGC).
“In the time that it would normally take to negotiate a legal agreement to provide OICR-9429 to other research teams, we have received results back from our collaborators showing that it can kill 2 different types of cancer cells,” said Cheryl Arrowsmith, PhD, of SGC Toronto in Ontario, Canada.
“Opening our chemistry capabilities to the world’s scientists allowed us to crowd-source and accelerate the research.”
A study published in Nature Chemical Biology suggested that WDR5 is a therapeutic target for a subtype of AML, and OICR-9429 could therefore be used to treat the disease.
The researchers noted that mutations in C/EBPα occur in about 9% of AML cases and lead to the expression of a 30-kDa dominant negative isoform (C/EBPα-p30).
Their experiments revealed that C/EBPα p30 preferentially interacts with WDR5. So it was no surprise that OICR-9429 inhibited proliferation and induced differentiation in p30-expressing AML cells.
In a study published in Nature, OICR-9429 inhibited cancer cell growth in a panel of breast cancer cell lines driven by mutated forms of p53.
Researchers said this work has implications beyond breast cancer because p53 is mutated in at least half of all cancers and is dysregulated in others.
“It is remarkable how quickly our research results were translated into discoveries by the groups around the world,” said Rima Al-awar, PhD, of OICR in Toronto.
“We are looking forward to seeing more research conducted with OICR-9429 and showing that this new approach to early stage drug discovery has significant advantages.”
The SGC and OICR teams said they are continuing their collaboration to identify additional molecules to advance cancer drug discovery. ![]()
Drug gets orphan designation for chronic ITP

The US Food and Drug Administration (FDA) has granted orphan drug designation to the SYK inhibitor fostamatinib as a treatment for patients
with chronic immune thrombocytopenia (ITP).
Fostamatinib (also known as R935788 or R788) has been shown to increase platelet counts in patients with chronic ITP.
The drug, which comes in tablet form, is thought to prevent macrophages from destroying platelets by inhibiting SYK activation.
Fostamatinib previously produced promising results in a phase 2 trial of ITP patients and is now under investigation in a pair of phase 3 trials (NCT02076412 and NCT02076399).
Results from these trials are expected in mid-2016, according to Rigel Pharmaceuticals, Inc., the company developing fostamatinib.
Phase 2 trial
The trial included 16 adults with chronic ITP. Fostamatinib doses began at 75 mg and were increased until a patient experienced a persistent response, toxicity occurred, or the patient reached the maximum dose—175 mg twice daily.
Eight patients achieved persistent responses. They maintained platelet counts above 50,000/mm3 on a median of 95% of study visits and were able to avoid receiving other treatments.
Four other patients experienced transient responses. They had an increase in platelet count from a median minimum of 17,000/mm3 at baseline to a median maximum of 177,000/mm3.
In all 12 responders, the median platelet count increased from 16,000/mm3 at baseline to a median peak of 105,000/mm3 while on fostamatinib.
Adverse events considered probably related to fostamatinib were diarrhea (n=6), an increase in systolic blood pressure of more than 10 mm Hg (n=5), nausea (n=4), headache (n=4), weight gain of more than 5 kg (n=3), vomiting (n=3), abdominal pain (n=3), constipation (n=2), and alanine aminotransferase levels greater than 2 times the upper limit of normal (n=2).
Three patients stopped treatment due to adverse events. One patient developed a urinary tract infection and deep vein thrombosis (both considered unrelated to treatment).
The second patient withdrew consent due to gastrointestinal toxicity. And the last patient withdrew consent due to preexisting elevated transaminase levels that worsened on fostamatinib and prevented increases in the dose.
About orphan designation
The FDA grants orphan designation to drugs that are intended to treat diseases or conditions affecting fewer than 200,000 patients in the US.
Orphan designation provides the sponsor of a drug with various development incentives, including opportunities to apply for research-related tax credits and
grant funding, assistance in designing clinical trials, and 7 years of US marketing exclusivity if the drug is approved. ![]()

The US Food and Drug Administration (FDA) has granted orphan drug designation to the SYK inhibitor fostamatinib as a treatment for patients
with chronic immune thrombocytopenia (ITP).
Fostamatinib (also known as R935788 or R788) has been shown to increase platelet counts in patients with chronic ITP.
The drug, which comes in tablet form, is thought to prevent macrophages from destroying platelets by inhibiting SYK activation.
Fostamatinib previously produced promising results in a phase 2 trial of ITP patients and is now under investigation in a pair of phase 3 trials (NCT02076412 and NCT02076399).
Results from these trials are expected in mid-2016, according to Rigel Pharmaceuticals, Inc., the company developing fostamatinib.
Phase 2 trial
The trial included 16 adults with chronic ITP. Fostamatinib doses began at 75 mg and were increased until a patient experienced a persistent response, toxicity occurred, or the patient reached the maximum dose—175 mg twice daily.
Eight patients achieved persistent responses. They maintained platelet counts above 50,000/mm3 on a median of 95% of study visits and were able to avoid receiving other treatments.
Four other patients experienced transient responses. They had an increase in platelet count from a median minimum of 17,000/mm3 at baseline to a median maximum of 177,000/mm3.
In all 12 responders, the median platelet count increased from 16,000/mm3 at baseline to a median peak of 105,000/mm3 while on fostamatinib.
Adverse events considered probably related to fostamatinib were diarrhea (n=6), an increase in systolic blood pressure of more than 10 mm Hg (n=5), nausea (n=4), headache (n=4), weight gain of more than 5 kg (n=3), vomiting (n=3), abdominal pain (n=3), constipation (n=2), and alanine aminotransferase levels greater than 2 times the upper limit of normal (n=2).
Three patients stopped treatment due to adverse events. One patient developed a urinary tract infection and deep vein thrombosis (both considered unrelated to treatment).
The second patient withdrew consent due to gastrointestinal toxicity. And the last patient withdrew consent due to preexisting elevated transaminase levels that worsened on fostamatinib and prevented increases in the dose.
About orphan designation
The FDA grants orphan designation to drugs that are intended to treat diseases or conditions affecting fewer than 200,000 patients in the US.
Orphan designation provides the sponsor of a drug with various development incentives, including opportunities to apply for research-related tax credits and
grant funding, assistance in designing clinical trials, and 7 years of US marketing exclusivity if the drug is approved. ![]()

The US Food and Drug Administration (FDA) has granted orphan drug designation to the SYK inhibitor fostamatinib as a treatment for patients
with chronic immune thrombocytopenia (ITP).
Fostamatinib (also known as R935788 or R788) has been shown to increase platelet counts in patients with chronic ITP.
The drug, which comes in tablet form, is thought to prevent macrophages from destroying platelets by inhibiting SYK activation.
Fostamatinib previously produced promising results in a phase 2 trial of ITP patients and is now under investigation in a pair of phase 3 trials (NCT02076412 and NCT02076399).
Results from these trials are expected in mid-2016, according to Rigel Pharmaceuticals, Inc., the company developing fostamatinib.
Phase 2 trial
The trial included 16 adults with chronic ITP. Fostamatinib doses began at 75 mg and were increased until a patient experienced a persistent response, toxicity occurred, or the patient reached the maximum dose—175 mg twice daily.
Eight patients achieved persistent responses. They maintained platelet counts above 50,000/mm3 on a median of 95% of study visits and were able to avoid receiving other treatments.
Four other patients experienced transient responses. They had an increase in platelet count from a median minimum of 17,000/mm3 at baseline to a median maximum of 177,000/mm3.
In all 12 responders, the median platelet count increased from 16,000/mm3 at baseline to a median peak of 105,000/mm3 while on fostamatinib.
Adverse events considered probably related to fostamatinib were diarrhea (n=6), an increase in systolic blood pressure of more than 10 mm Hg (n=5), nausea (n=4), headache (n=4), weight gain of more than 5 kg (n=3), vomiting (n=3), abdominal pain (n=3), constipation (n=2), and alanine aminotransferase levels greater than 2 times the upper limit of normal (n=2).
Three patients stopped treatment due to adverse events. One patient developed a urinary tract infection and deep vein thrombosis (both considered unrelated to treatment).
The second patient withdrew consent due to gastrointestinal toxicity. And the last patient withdrew consent due to preexisting elevated transaminase levels that worsened on fostamatinib and prevented increases in the dose.
About orphan designation
The FDA grants orphan designation to drugs that are intended to treat diseases or conditions affecting fewer than 200,000 patients in the US.
Orphan designation provides the sponsor of a drug with various development incentives, including opportunities to apply for research-related tax credits and
grant funding, assistance in designing clinical trials, and 7 years of US marketing exclusivity if the drug is approved. ![]()
Extensive Skin Necrosis From Suspected Levamisole-Contaminated Cocaine
To the Editor:
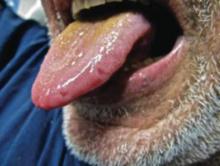
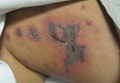
A 52-year-old man presented to the emergency department with skin pain. Although he felt well overall, he reported that he had developed skin sores 3 weeks prior to presentation that were progressively causing skin pain and sleep loss. He acknowledged smoking cigarettes and snorting cocaine but denied intravenous use of cocaine or using any other drugs. His usual medications were lisinopril and tramadol, and he had no known drug allergies. His history was remarkable for methicillin-resistant Staphylococcus aureus (MRSA) septic arthritis of the shoulder and MRSA prepatellar bursitis within the last 2 years. During examination in the emergency department he was alert, afebrile, nontoxic, generally healthy, and in no acute distress. Extensive necrotic skin lesions were present on the trunk, extremities, and both ears. The lesions were large necrotic patches with irregular, sharply angulated borders with thin or ulcerated epidermis surrounded by a bright halo of erythema (Figure 1). Ulcers were noted on the tongue (Figure 2).
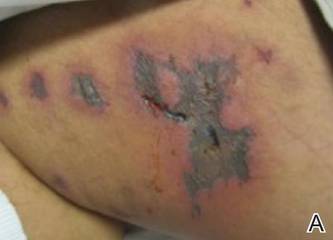 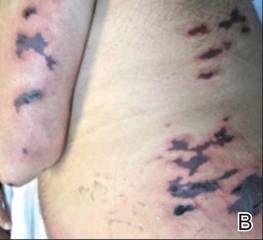  |
| Figure 1. Extensive skin necrosis on the leg from levamisole-contaminated cocaine (A). Necrotic skin lesions also were present on the trunk, arm (B), and ear (C). |
The clinical diagnosis was probable thrombosis of skin vessels with skin necrosis due to cocaine that was likely contaminated with levamisole. Pertinent laboratory results included the following: mild anemia and mild leukopenia; values within reference range for liver function, serum protein electrophoresis, hepatitis profile, human immunodeficiency virus 1 and 2, rapid plasma reagin, and antinuclear antibody; normal thrombotic studies for antithrombin III, protein C, protein S, factor V Leiden, prothrombin mutation G20210A, anticardiolipin IgG, IgM, and IgA; erythrocyte sedimentation rate of 26 mm/h (reference range, 0–15 mm/h); perinuclear antineutrophil cytoplasmic antibody greater than 1:320 (reference range, <1:20) with normal proteinase 3 and myeloperoxidase antibodies; urine positive for cocaine but blood negative for cocaine; normal chest radiograph; and normal electrocardiogram.
The patient was stable with good family support and was discharged from the emergency department to be followed in our dermatology office. The following day his skin biopsies were interpreted as neutrophilic vasculitis with extensive intravascular early and organizing thrombi involving all small- and medium-sized blood vessels consistent with levamisole-induced necrosis or septic vasculitis (Figure 3). With his history of MRSA septic arthritis and bursitis, he was hospitalized for treatment with intravenous vancomycin pending further studies. Skin biopsy for direct immunofluorescence revealed granular deposits of IgM and linear deposits of C3 at the dermoepidermal junction and in blood vessel walls. Two tissue cultures for bacteria and fungi were negative and 2 blood cultures were negative. An echocardiogram was normal and without evidence of emboli. The patient remained stable and antibiotics were discontinued. He was released from the hospital and his skin lesions healed satisfactorily with showering and mupirocin ointment.
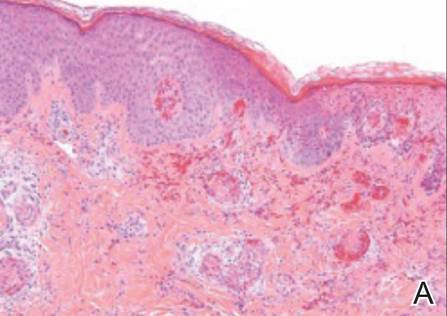 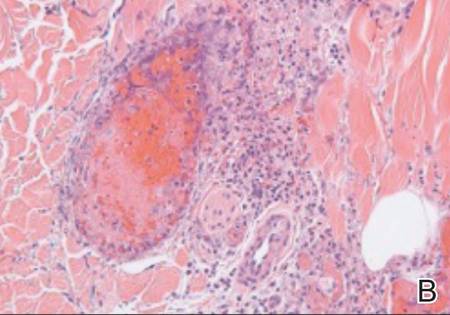 |
| Figure 3. Thrombotic occlusion of blood vessels was seen on histopathology (A and B)(H&E, original magnifications ×100 and ×400).
|
Cocaine is a white powder that is primarily derived from the leaves of the coca plant in South America. It is ingested orally; injected intravenously; snorted intranasally; chewed; eaten; used as a suppository; or dissolved in water and baking soda then heated to crystallization for smoking, which is the most addictive method and known as freebasing. When smoked, crack cocaine produces a crackling sound. Cocaine stimulates the central nervous system similar to amphetamine but may harm any body organ through vasoconstriction/vasospasm and cause skin necrosis without any additive. Perhaps less known is its ability to produce smooth muscle hyperplasia of small vessels and premature atherosclerosis.1
Levamisole has been used to treat worms, cancer, and stimulation of the immune system but currently is used only by veterinarians because of agranulocytosis and vasculitis in humans. As of July 2009, the Drug Enforcement Agency reported that 69% of seized cocaine lots coming into the United States contained levamisole.2 By January 2010, 73.2% of seized cocaine exhibits contained levamisole according to the California Poison Control System, with reports of contamination rates from across the country ranging from 40% to 90%.3 Levamisole is an inexpensive additive to cocaine and may increase the release of brain dopamine.4 It is difficult to detect levamisole in urine due to its short half-life of 5.6 hours and only 2% to 5% of the parent compound being found in the urine.5
Skin necrosis due to cocaine-contaminated levamisole usually occurs in younger individuals who have characteristic skin lesions and a history of cocaine use. Skin lesions usually are multiple, purpuric or necrotic with irregular angulated edges and a halo of erythema. Ear involvement is common but not invariable.6 Descriptive adjectives include branched, netlike, retiform, and stellate, all revealing the compromised underlying dermal and subcutaneous vascular anatomy. Supportive evidence includes a decreased white blood cell count (neutropenia in up to 50%),5 positive antineutrophilic cytoplasmic antibodies,5,7 and/or positive drug screen. Skin biopsy may reveal thrombosis,4 fibrin thrombi without vasculitis,8 or leukocytoclastic vasculitis,4,5 or may suggest septic vasculitis.9 Direct immunofluorescence may suggest an immune complex-mediated vasculitis.5
The differential diagnosis for a patient with purpuric/necrotic skin lesions should be broad and include vasculitis (eg, inflammatory, antineutrophil cytoplasmic antibody positive, septic), hypercoagulopathy (eg, antiphospholipid syndrome, antithrombin III, prothrombin mutation G20210A, factor V Leiden, protein C, protein S), drugs (eg, heparin, warfarin, cocaine with or without levamisole, intravenous drug use, hydroxyurea, ergotamine, propylthiouracil10), calciphylaxis, cold-induced thrombosis, emboli (eg, atheroma, cholesterol, endocarditis, myxoma, aortic angiosarcoma, marantic), febrile ulceronecrotic Mucha-Habermann disease, infection especially if immunosuppressed (eg, disseminated Acanthamoeba/Candida/histoplasmosis/strongyloides/varicella-zoster virus, S aureus, streptococcus, ecthyma gangrenosum, gas gangrene, hemorrhagic smallpox, lues maligna with human immunodeficiency virus, Meleney ulcer, Rocky Mountain spotted fever, Vibrio vulnificus), idiopathic thrombocytopenic purpura, thrombotic thrombocytopenic purpura, thrombocythemia, Waldenström hyperglobulinemic purpura, pyoderma gangrenosum, cancer (eg, paraneoplastic arterial thrombi), oxalosis, paraproteinemia (eg, multiple myeloma), and lupus with generalized coagulopathy. Less likely diagnoses might include Degos disease, factitial dermatitis, foreign bodies, multiple spider bites, paroxysmal nocturnal hemoglobinuria, sickle cell anemia, Buruli ulcer, or thromboangiitis obliterans. Branched, angulated, retiform lesions are an important finding, and some of these diagnostic possibilities are not classically retiform. However, clinical findings are not always classical, and astute physicians want to be circumspect. Had more ominous findings been present in our patient (eg, fever, hemodynamic instability, progressive skin lesions, systemic organ involvement), prompt hospitalization and additional considerations would have been necessary, such as septicemia (eg, meningococcemia, bubonic plague [Black Death], necrotizing fasciitis, purpura fulminans), catastrophic antiphospholipid syndrome, or disseminated intra- vascular coagulation.
The prognosis for skin necrosis caused by levamisole-contaminated cocaine generally is good without long-term sequelae.5 Autoantibody serologies normalize within weeks to months after stopping levamisole.5,8 Our patient recovered with conservative measures.
1. Dhawan SS, Wang BW. Four-extremity gangrene associated with crack cocaine abuse [published online ahead of print October 23, 2006]. Ann Emerg Med. 2007;49:186-189.
2. Centers for Disease Control and Prevention. Agranulocytosis associated with cocaine use—four states, March 2008–November 2009. MMWR Morb Mortal Wkly Rep. 2009;58:1381-1385.
3. Buchanan J; California Poison Control System. Levamisole-contaminated cocaine. Call Us… December 3, 2014. http://www.calpoison.org/hcp/2014/ callusvol12no3.htm. Accessed September 1, 2015.
4. Mouzakis J, Somboonwit C, Lakshmi S, et al. Levamisole induced necrosis of the skin and neutropenia following intranasal cocaine use: a newly recognized syndrome. J Drugs Dermatology. 2011;10:1204-1207.
5. Chung C, Tumeh PC, Birnbaum R, et al. Characteristic purpura of the ears, vasculitis, and neutropenia—a potential public health epidemic associated with levamisole-adulterated cocaine [published online ahead of print June 11, 2011]. J Am Acad Dermatol. 2011;65:722-725.
6. Farhat EK, Muirhead TT, Chaffins ML, et al. Levamisole-induced cutaneous necrosis mimicking coagulopathy. Arch Dermatol. 2010;46:1320-1321.
7. Geller L, Whang TB, Mercer SE. Retiform purpura: a new stigmata of illicit drug use? Dermatol Online J. 2011;17:7.
8. Waller JM, Feramisco JD, Alberta-Wszolek L, et al. Cocaine-associated retiform purpura and neutropenia: is levamisole the culprit [published online ahead of print March 20, 2010]? J Am Acad Dermatol. 2010;63:530-535.
9. Reutemann P, Grenier N, Telang GH. Occlusive vasculopathy with vascular and skin necrosis secondary to smoking crack cocaine. J Am Acad Dermatol. 2011;64:1004-1006.
10. Mahmood T, Delacerda A, Fiala K. Painful purpura on bilateral helices. JAMA Dermatol. 2015;151:551-552.
To the Editor:
A 52-year-old man presented to the emergency department with skin pain. Although he felt well overall, he reported that he had developed skin sores 3 weeks prior to presentation that were progressively causing skin pain and sleep loss. He acknowledged smoking cigarettes and snorting cocaine but denied intravenous use of cocaine or using any other drugs. His usual medications were lisinopril and tramadol, and he had no known drug allergies. His history was remarkable for methicillin-resistant Staphylococcus aureus (MRSA) septic arthritis of the shoulder and MRSA prepatellar bursitis within the last 2 years. During examination in the emergency department he was alert, afebrile, nontoxic, generally healthy, and in no acute distress. Extensive necrotic skin lesions were present on the trunk, extremities, and both ears. The lesions were large necrotic patches with irregular, sharply angulated borders with thin or ulcerated epidermis surrounded by a bright halo of erythema (Figure 1). Ulcers were noted on the tongue (Figure 2).
   |
| Figure 1. Extensive skin necrosis on the leg from levamisole-contaminated cocaine (A). Necrotic skin lesions also were present on the trunk, arm (B), and ear (C). |
The clinical diagnosis was probable thrombosis of skin vessels with skin necrosis due to cocaine that was likely contaminated with levamisole. Pertinent laboratory results included the following: mild anemia and mild leukopenia; values within reference range for liver function, serum protein electrophoresis, hepatitis profile, human immunodeficiency virus 1 and 2, rapid plasma reagin, and antinuclear antibody; normal thrombotic studies for antithrombin III, protein C, protein S, factor V Leiden, prothrombin mutation G20210A, anticardiolipin IgG, IgM, and IgA; erythrocyte sedimentation rate of 26 mm/h (reference range, 0–15 mm/h); perinuclear antineutrophil cytoplasmic antibody greater than 1:320 (reference range, <1:20) with normal proteinase 3 and myeloperoxidase antibodies; urine positive for cocaine but blood negative for cocaine; normal chest radiograph; and normal electrocardiogram.
The patient was stable with good family support and was discharged from the emergency department to be followed in our dermatology office. The following day his skin biopsies were interpreted as neutrophilic vasculitis with extensive intravascular early and organizing thrombi involving all small- and medium-sized blood vessels consistent with levamisole-induced necrosis or septic vasculitis (Figure 3). With his history of MRSA septic arthritis and bursitis, he was hospitalized for treatment with intravenous vancomycin pending further studies. Skin biopsy for direct immunofluorescence revealed granular deposits of IgM and linear deposits of C3 at the dermoepidermal junction and in blood vessel walls. Two tissue cultures for bacteria and fungi were negative and 2 blood cultures were negative. An echocardiogram was normal and without evidence of emboli. The patient remained stable and antibiotics were discontinued. He was released from the hospital and his skin lesions healed satisfactorily with showering and mupirocin ointment.
  |
| Figure 3. Thrombotic occlusion of blood vessels was seen on histopathology (A and B)(H&E, original magnifications ×100 and ×400).
|
Cocaine is a white powder that is primarily derived from the leaves of the coca plant in South America. It is ingested orally; injected intravenously; snorted intranasally; chewed; eaten; used as a suppository; or dissolved in water and baking soda then heated to crystallization for smoking, which is the most addictive method and known as freebasing. When smoked, crack cocaine produces a crackling sound. Cocaine stimulates the central nervous system similar to amphetamine but may harm any body organ through vasoconstriction/vasospasm and cause skin necrosis without any additive. Perhaps less known is its ability to produce smooth muscle hyperplasia of small vessels and premature atherosclerosis.1
Levamisole has been used to treat worms, cancer, and stimulation of the immune system but currently is used only by veterinarians because of agranulocytosis and vasculitis in humans. As of July 2009, the Drug Enforcement Agency reported that 69% of seized cocaine lots coming into the United States contained levamisole.2 By January 2010, 73.2% of seized cocaine exhibits contained levamisole according to the California Poison Control System, with reports of contamination rates from across the country ranging from 40% to 90%.3 Levamisole is an inexpensive additive to cocaine and may increase the release of brain dopamine.4 It is difficult to detect levamisole in urine due to its short half-life of 5.6 hours and only 2% to 5% of the parent compound being found in the urine.5
Skin necrosis due to cocaine-contaminated levamisole usually occurs in younger individuals who have characteristic skin lesions and a history of cocaine use. Skin lesions usually are multiple, purpuric or necrotic with irregular angulated edges and a halo of erythema. Ear involvement is common but not invariable.6 Descriptive adjectives include branched, netlike, retiform, and stellate, all revealing the compromised underlying dermal and subcutaneous vascular anatomy. Supportive evidence includes a decreased white blood cell count (neutropenia in up to 50%),5 positive antineutrophilic cytoplasmic antibodies,5,7 and/or positive drug screen. Skin biopsy may reveal thrombosis,4 fibrin thrombi without vasculitis,8 or leukocytoclastic vasculitis,4,5 or may suggest septic vasculitis.9 Direct immunofluorescence may suggest an immune complex-mediated vasculitis.5
The differential diagnosis for a patient with purpuric/necrotic skin lesions should be broad and include vasculitis (eg, inflammatory, antineutrophil cytoplasmic antibody positive, septic), hypercoagulopathy (eg, antiphospholipid syndrome, antithrombin III, prothrombin mutation G20210A, factor V Leiden, protein C, protein S), drugs (eg, heparin, warfarin, cocaine with or without levamisole, intravenous drug use, hydroxyurea, ergotamine, propylthiouracil10), calciphylaxis, cold-induced thrombosis, emboli (eg, atheroma, cholesterol, endocarditis, myxoma, aortic angiosarcoma, marantic), febrile ulceronecrotic Mucha-Habermann disease, infection especially if immunosuppressed (eg, disseminated Acanthamoeba/Candida/histoplasmosis/strongyloides/varicella-zoster virus, S aureus, streptococcus, ecthyma gangrenosum, gas gangrene, hemorrhagic smallpox, lues maligna with human immunodeficiency virus, Meleney ulcer, Rocky Mountain spotted fever, Vibrio vulnificus), idiopathic thrombocytopenic purpura, thrombotic thrombocytopenic purpura, thrombocythemia, Waldenström hyperglobulinemic purpura, pyoderma gangrenosum, cancer (eg, paraneoplastic arterial thrombi), oxalosis, paraproteinemia (eg, multiple myeloma), and lupus with generalized coagulopathy. Less likely diagnoses might include Degos disease, factitial dermatitis, foreign bodies, multiple spider bites, paroxysmal nocturnal hemoglobinuria, sickle cell anemia, Buruli ulcer, or thromboangiitis obliterans. Branched, angulated, retiform lesions are an important finding, and some of these diagnostic possibilities are not classically retiform. However, clinical findings are not always classical, and astute physicians want to be circumspect. Had more ominous findings been present in our patient (eg, fever, hemodynamic instability, progressive skin lesions, systemic organ involvement), prompt hospitalization and additional considerations would have been necessary, such as septicemia (eg, meningococcemia, bubonic plague [Black Death], necrotizing fasciitis, purpura fulminans), catastrophic antiphospholipid syndrome, or disseminated intra- vascular coagulation.
The prognosis for skin necrosis caused by levamisole-contaminated cocaine generally is good without long-term sequelae.5 Autoantibody serologies normalize within weeks to months after stopping levamisole.5,8 Our patient recovered with conservative measures.
To the Editor:
A 52-year-old man presented to the emergency department with skin pain. Although he felt well overall, he reported that he had developed skin sores 3 weeks prior to presentation that were progressively causing skin pain and sleep loss. He acknowledged smoking cigarettes and snorting cocaine but denied intravenous use of cocaine or using any other drugs. His usual medications were lisinopril and tramadol, and he had no known drug allergies. His history was remarkable for methicillin-resistant Staphylococcus aureus (MRSA) septic arthritis of the shoulder and MRSA prepatellar bursitis within the last 2 years. During examination in the emergency department he was alert, afebrile, nontoxic, generally healthy, and in no acute distress. Extensive necrotic skin lesions were present on the trunk, extremities, and both ears. The lesions were large necrotic patches with irregular, sharply angulated borders with thin or ulcerated epidermis surrounded by a bright halo of erythema (Figure 1). Ulcers were noted on the tongue (Figure 2).
   |
| Figure 1. Extensive skin necrosis on the leg from levamisole-contaminated cocaine (A). Necrotic skin lesions also were present on the trunk, arm (B), and ear (C). |
The clinical diagnosis was probable thrombosis of skin vessels with skin necrosis due to cocaine that was likely contaminated with levamisole. Pertinent laboratory results included the following: mild anemia and mild leukopenia; values within reference range for liver function, serum protein electrophoresis, hepatitis profile, human immunodeficiency virus 1 and 2, rapid plasma reagin, and antinuclear antibody; normal thrombotic studies for antithrombin III, protein C, protein S, factor V Leiden, prothrombin mutation G20210A, anticardiolipin IgG, IgM, and IgA; erythrocyte sedimentation rate of 26 mm/h (reference range, 0–15 mm/h); perinuclear antineutrophil cytoplasmic antibody greater than 1:320 (reference range, <1:20) with normal proteinase 3 and myeloperoxidase antibodies; urine positive for cocaine but blood negative for cocaine; normal chest radiograph; and normal electrocardiogram.
The patient was stable with good family support and was discharged from the emergency department to be followed in our dermatology office. The following day his skin biopsies were interpreted as neutrophilic vasculitis with extensive intravascular early and organizing thrombi involving all small- and medium-sized blood vessels consistent with levamisole-induced necrosis or septic vasculitis (Figure 3). With his history of MRSA septic arthritis and bursitis, he was hospitalized for treatment with intravenous vancomycin pending further studies. Skin biopsy for direct immunofluorescence revealed granular deposits of IgM and linear deposits of C3 at the dermoepidermal junction and in blood vessel walls. Two tissue cultures for bacteria and fungi were negative and 2 blood cultures were negative. An echocardiogram was normal and without evidence of emboli. The patient remained stable and antibiotics were discontinued. He was released from the hospital and his skin lesions healed satisfactorily with showering and mupirocin ointment.
  |
| Figure 3. Thrombotic occlusion of blood vessels was seen on histopathology (A and B)(H&E, original magnifications ×100 and ×400).
|
Cocaine is a white powder that is primarily derived from the leaves of the coca plant in South America. It is ingested orally; injected intravenously; snorted intranasally; chewed; eaten; used as a suppository; or dissolved in water and baking soda then heated to crystallization for smoking, which is the most addictive method and known as freebasing. When smoked, crack cocaine produces a crackling sound. Cocaine stimulates the central nervous system similar to amphetamine but may harm any body organ through vasoconstriction/vasospasm and cause skin necrosis without any additive. Perhaps less known is its ability to produce smooth muscle hyperplasia of small vessels and premature atherosclerosis.1
Levamisole has been used to treat worms, cancer, and stimulation of the immune system but currently is used only by veterinarians because of agranulocytosis and vasculitis in humans. As of July 2009, the Drug Enforcement Agency reported that 69% of seized cocaine lots coming into the United States contained levamisole.2 By January 2010, 73.2% of seized cocaine exhibits contained levamisole according to the California Poison Control System, with reports of contamination rates from across the country ranging from 40% to 90%.3 Levamisole is an inexpensive additive to cocaine and may increase the release of brain dopamine.4 It is difficult to detect levamisole in urine due to its short half-life of 5.6 hours and only 2% to 5% of the parent compound being found in the urine.5
Skin necrosis due to cocaine-contaminated levamisole usually occurs in younger individuals who have characteristic skin lesions and a history of cocaine use. Skin lesions usually are multiple, purpuric or necrotic with irregular angulated edges and a halo of erythema. Ear involvement is common but not invariable.6 Descriptive adjectives include branched, netlike, retiform, and stellate, all revealing the compromised underlying dermal and subcutaneous vascular anatomy. Supportive evidence includes a decreased white blood cell count (neutropenia in up to 50%),5 positive antineutrophilic cytoplasmic antibodies,5,7 and/or positive drug screen. Skin biopsy may reveal thrombosis,4 fibrin thrombi without vasculitis,8 or leukocytoclastic vasculitis,4,5 or may suggest septic vasculitis.9 Direct immunofluorescence may suggest an immune complex-mediated vasculitis.5
The differential diagnosis for a patient with purpuric/necrotic skin lesions should be broad and include vasculitis (eg, inflammatory, antineutrophil cytoplasmic antibody positive, septic), hypercoagulopathy (eg, antiphospholipid syndrome, antithrombin III, prothrombin mutation G20210A, factor V Leiden, protein C, protein S), drugs (eg, heparin, warfarin, cocaine with or without levamisole, intravenous drug use, hydroxyurea, ergotamine, propylthiouracil10), calciphylaxis, cold-induced thrombosis, emboli (eg, atheroma, cholesterol, endocarditis, myxoma, aortic angiosarcoma, marantic), febrile ulceronecrotic Mucha-Habermann disease, infection especially if immunosuppressed (eg, disseminated Acanthamoeba/Candida/histoplasmosis/strongyloides/varicella-zoster virus, S aureus, streptococcus, ecthyma gangrenosum, gas gangrene, hemorrhagic smallpox, lues maligna with human immunodeficiency virus, Meleney ulcer, Rocky Mountain spotted fever, Vibrio vulnificus), idiopathic thrombocytopenic purpura, thrombotic thrombocytopenic purpura, thrombocythemia, Waldenström hyperglobulinemic purpura, pyoderma gangrenosum, cancer (eg, paraneoplastic arterial thrombi), oxalosis, paraproteinemia (eg, multiple myeloma), and lupus with generalized coagulopathy. Less likely diagnoses might include Degos disease, factitial dermatitis, foreign bodies, multiple spider bites, paroxysmal nocturnal hemoglobinuria, sickle cell anemia, Buruli ulcer, or thromboangiitis obliterans. Branched, angulated, retiform lesions are an important finding, and some of these diagnostic possibilities are not classically retiform. However, clinical findings are not always classical, and astute physicians want to be circumspect. Had more ominous findings been present in our patient (eg, fever, hemodynamic instability, progressive skin lesions, systemic organ involvement), prompt hospitalization and additional considerations would have been necessary, such as septicemia (eg, meningococcemia, bubonic plague [Black Death], necrotizing fasciitis, purpura fulminans), catastrophic antiphospholipid syndrome, or disseminated intra- vascular coagulation.
The prognosis for skin necrosis caused by levamisole-contaminated cocaine generally is good without long-term sequelae.5 Autoantibody serologies normalize within weeks to months after stopping levamisole.5,8 Our patient recovered with conservative measures.
1. Dhawan SS, Wang BW. Four-extremity gangrene associated with crack cocaine abuse [published online ahead of print October 23, 2006]. Ann Emerg Med. 2007;49:186-189.
2. Centers for Disease Control and Prevention. Agranulocytosis associated with cocaine use—four states, March 2008–November 2009. MMWR Morb Mortal Wkly Rep. 2009;58:1381-1385.
3. Buchanan J; California Poison Control System. Levamisole-contaminated cocaine. Call Us… December 3, 2014. http://www.calpoison.org/hcp/2014/ callusvol12no3.htm. Accessed September 1, 2015.
4. Mouzakis J, Somboonwit C, Lakshmi S, et al. Levamisole induced necrosis of the skin and neutropenia following intranasal cocaine use: a newly recognized syndrome. J Drugs Dermatology. 2011;10:1204-1207.
5. Chung C, Tumeh PC, Birnbaum R, et al. Characteristic purpura of the ears, vasculitis, and neutropenia—a potential public health epidemic associated with levamisole-adulterated cocaine [published online ahead of print June 11, 2011]. J Am Acad Dermatol. 2011;65:722-725.
6. Farhat EK, Muirhead TT, Chaffins ML, et al. Levamisole-induced cutaneous necrosis mimicking coagulopathy. Arch Dermatol. 2010;46:1320-1321.
7. Geller L, Whang TB, Mercer SE. Retiform purpura: a new stigmata of illicit drug use? Dermatol Online J. 2011;17:7.
8. Waller JM, Feramisco JD, Alberta-Wszolek L, et al. Cocaine-associated retiform purpura and neutropenia: is levamisole the culprit [published online ahead of print March 20, 2010]? J Am Acad Dermatol. 2010;63:530-535.
9. Reutemann P, Grenier N, Telang GH. Occlusive vasculopathy with vascular and skin necrosis secondary to smoking crack cocaine. J Am Acad Dermatol. 2011;64:1004-1006.
10. Mahmood T, Delacerda A, Fiala K. Painful purpura on bilateral helices. JAMA Dermatol. 2015;151:551-552.
1. Dhawan SS, Wang BW. Four-extremity gangrene associated with crack cocaine abuse [published online ahead of print October 23, 2006]. Ann Emerg Med. 2007;49:186-189.
2. Centers for Disease Control and Prevention. Agranulocytosis associated with cocaine use—four states, March 2008–November 2009. MMWR Morb Mortal Wkly Rep. 2009;58:1381-1385.
3. Buchanan J; California Poison Control System. Levamisole-contaminated cocaine. Call Us… December 3, 2014. http://www.calpoison.org/hcp/2014/ callusvol12no3.htm. Accessed September 1, 2015.
4. Mouzakis J, Somboonwit C, Lakshmi S, et al. Levamisole induced necrosis of the skin and neutropenia following intranasal cocaine use: a newly recognized syndrome. J Drugs Dermatology. 2011;10:1204-1207.
5. Chung C, Tumeh PC, Birnbaum R, et al. Characteristic purpura of the ears, vasculitis, and neutropenia—a potential public health epidemic associated with levamisole-adulterated cocaine [published online ahead of print June 11, 2011]. J Am Acad Dermatol. 2011;65:722-725.
6. Farhat EK, Muirhead TT, Chaffins ML, et al. Levamisole-induced cutaneous necrosis mimicking coagulopathy. Arch Dermatol. 2010;46:1320-1321.
7. Geller L, Whang TB, Mercer SE. Retiform purpura: a new stigmata of illicit drug use? Dermatol Online J. 2011;17:7.
8. Waller JM, Feramisco JD, Alberta-Wszolek L, et al. Cocaine-associated retiform purpura and neutropenia: is levamisole the culprit [published online ahead of print March 20, 2010]? J Am Acad Dermatol. 2010;63:530-535.
9. Reutemann P, Grenier N, Telang GH. Occlusive vasculopathy with vascular and skin necrosis secondary to smoking crack cocaine. J Am Acad Dermatol. 2011;64:1004-1006.
10. Mahmood T, Delacerda A, Fiala K. Painful purpura on bilateral helices. JAMA Dermatol. 2015;151:551-552.
Agent blocks STAT3 protein and might prevent AML relapse
The discovery of a new therapeutic target site on the STAT3 oncoprotein – a protein that interferes with chemotherapy by halting deaths of cancerous cells – could cut down on acute myeloid leukemia (AML) relapse in chemoresistant patients, according to research published in Angewandte Chemie.
The STAT3 protein – which stands for “signal transducer and activator of transcription 3” – is a suspected factor in the relapse of nearly 40% of children with AML. A team of researchers from Rice University, working with colleagues at Baylor College of Medicine and the University of Texas MD Anderson Cancer Center, all in Houston, developed a proximity-driven rhodium (II) catalyst known as MM-206, which blocks STAT3 function by identifying the STAT3 coiled-coil domain as a novel ligand-binding site and delivering a naphthalene sulfonamide inhibitor, thus halting the disease promoting effects of STAT3.
The effects were replicated in tumor growth models and testing in a leukemia mouse model suggest this approach can be used to shut down STAT3 activity in AML patients, according to coauthor Dr. Zachary Ball of the department of chemistry at Rice.
Read the full article here: Angew Chem Int Ed Engl. 2015 Sep 7. doi: 10.1002/anie.201506889.
The discovery of a new therapeutic target site on the STAT3 oncoprotein – a protein that interferes with chemotherapy by halting deaths of cancerous cells – could cut down on acute myeloid leukemia (AML) relapse in chemoresistant patients, according to research published in Angewandte Chemie.
The STAT3 protein – which stands for “signal transducer and activator of transcription 3” – is a suspected factor in the relapse of nearly 40% of children with AML. A team of researchers from Rice University, working with colleagues at Baylor College of Medicine and the University of Texas MD Anderson Cancer Center, all in Houston, developed a proximity-driven rhodium (II) catalyst known as MM-206, which blocks STAT3 function by identifying the STAT3 coiled-coil domain as a novel ligand-binding site and delivering a naphthalene sulfonamide inhibitor, thus halting the disease promoting effects of STAT3.
The effects were replicated in tumor growth models and testing in a leukemia mouse model suggest this approach can be used to shut down STAT3 activity in AML patients, according to coauthor Dr. Zachary Ball of the department of chemistry at Rice.
Read the full article here: Angew Chem Int Ed Engl. 2015 Sep 7. doi: 10.1002/anie.201506889.
The discovery of a new therapeutic target site on the STAT3 oncoprotein – a protein that interferes with chemotherapy by halting deaths of cancerous cells – could cut down on acute myeloid leukemia (AML) relapse in chemoresistant patients, according to research published in Angewandte Chemie.
The STAT3 protein – which stands for “signal transducer and activator of transcription 3” – is a suspected factor in the relapse of nearly 40% of children with AML. A team of researchers from Rice University, working with colleagues at Baylor College of Medicine and the University of Texas MD Anderson Cancer Center, all in Houston, developed a proximity-driven rhodium (II) catalyst known as MM-206, which blocks STAT3 function by identifying the STAT3 coiled-coil domain as a novel ligand-binding site and delivering a naphthalene sulfonamide inhibitor, thus halting the disease promoting effects of STAT3.
The effects were replicated in tumor growth models and testing in a leukemia mouse model suggest this approach can be used to shut down STAT3 activity in AML patients, according to coauthor Dr. Zachary Ball of the department of chemistry at Rice.
Read the full article here: Angew Chem Int Ed Engl. 2015 Sep 7. doi: 10.1002/anie.201506889.
FROM ANGEWANDTE CHEMIE
ESC: Too much TV boosts PE risk
LONDON – Middle-aged adults who watch TV for an average of 5 or more hours per night face an adjusted 6.5-fold increased risk of fatal pulmonary embolism, compared with those who watch less than 2.5 hours per night, Toru Shirakawa reported at the annual congress of the European Society of Cardiology.
This was the key lesson gleaned from an analysis from the Japan Collaborative Cohort Study, the first large-scale prospective investigation of the relationship between prolonged television watching and pulmonary embolism (PE). The study included 86,024 Japanese participants aged 40-79 years prospectively followed for a median of 18.4 years, explained Mr. Shirakawa, a medical student at Osaka (Japan) University.
And while many busy medical professionals might presume 5 hours–plus of TV watching per night constitutes extreme behavior, that’s hardly the case. Indeed, according to the Nielsen survey, American adults watch an average of 4.85 hours of TV nightly.
During the study period there were 59 confirmed deaths from PE. In a multivariate analysis adjusted for sex and baseline age, cardiovascular risk factors, and physical activity level, a strong dose-response relationship was evident between hours of TV viewing and fatal PE.
This association was most pronounced in the 40- to 59-year-olds. Using as a reference group subjects who watched less than 2.5 hours per day, those who watched 2.5-4.9 hours had an adjusted 3.14-fold increased risk of fatal PE. Individuals who watched 5 hours or more – less than the average length of two American football games – were at 6.49-fold increased risk.
The same dose-response association was evident in the full study population spanning ages 40 through 79 years at entry. However, the magnitude of risk attributable to prolonged television watching in the overall group wasn’t as great, since 60- to 79-year-olds face multiple age-related competing mortality risks. Still, 40- to 79-year-olds who watched at least 5 hours of TV daily had an adjusted 2.36-fold greater risk of fatal PE, compared with those who watched for less than 2.5 hours.
Mr. Shirakawa observed that the mechanism of injury is presumably the same as previously reported in studies of “shelter death” during the bombing of London during World War II, as well as “economy-class syndrome,” first described in conjunction with long-distance airplane flights in 1954. Basically, prolonged leg immobility leads to inadequate circulation and resultant venous clot formation. But prolonged TV watching is a much more common risk factor than is economy-class syndrome, he noted.
“The take-home message is this: Public awareness of the risk of pulmonary embolism from lengthy leg immobility is essential. To prevent the occurrence of pulmonary embolism, we recommend the same preventive behavior used against economy-class syndrome. That is, take a break, stand up, and walk around during the television viewing. And drink water to prevent dehydration; that is also important,” Mr. Shirakawa said.
Session cochair Dr. José Ramón González Juanatey observed that the true burden of PE triggered by prolonged TV watching is far greater than documented in the Japanese study because the analysis focused exclusively on fatal cases.
“Only about 10% of cases of pulmonary embolism are immediately fatal events,” commented Dr. González Juanatey of the University of Santiago de Compostela and president of the Spanish Society of Cardiology.
“The absolute risk may be fairly small, but it’s a devastating thing to have happen to you,” added cochair Dr. Ian Graham, professor of cardiovascular medicine at Trinity College, Dublin.
The Japan Collaborative Cohort Study has been funded by scientific grants from various Japanese health and education ministries. Mr. Shirakawa reported having no financial conflicts.
LONDON – Middle-aged adults who watch TV for an average of 5 or more hours per night face an adjusted 6.5-fold increased risk of fatal pulmonary embolism, compared with those who watch less than 2.5 hours per night, Toru Shirakawa reported at the annual congress of the European Society of Cardiology.
This was the key lesson gleaned from an analysis from the Japan Collaborative Cohort Study, the first large-scale prospective investigation of the relationship between prolonged television watching and pulmonary embolism (PE). The study included 86,024 Japanese participants aged 40-79 years prospectively followed for a median of 18.4 years, explained Mr. Shirakawa, a medical student at Osaka (Japan) University.
And while many busy medical professionals might presume 5 hours–plus of TV watching per night constitutes extreme behavior, that’s hardly the case. Indeed, according to the Nielsen survey, American adults watch an average of 4.85 hours of TV nightly.
During the study period there were 59 confirmed deaths from PE. In a multivariate analysis adjusted for sex and baseline age, cardiovascular risk factors, and physical activity level, a strong dose-response relationship was evident between hours of TV viewing and fatal PE.
This association was most pronounced in the 40- to 59-year-olds. Using as a reference group subjects who watched less than 2.5 hours per day, those who watched 2.5-4.9 hours had an adjusted 3.14-fold increased risk of fatal PE. Individuals who watched 5 hours or more – less than the average length of two American football games – were at 6.49-fold increased risk.
The same dose-response association was evident in the full study population spanning ages 40 through 79 years at entry. However, the magnitude of risk attributable to prolonged television watching in the overall group wasn’t as great, since 60- to 79-year-olds face multiple age-related competing mortality risks. Still, 40- to 79-year-olds who watched at least 5 hours of TV daily had an adjusted 2.36-fold greater risk of fatal PE, compared with those who watched for less than 2.5 hours.
Mr. Shirakawa observed that the mechanism of injury is presumably the same as previously reported in studies of “shelter death” during the bombing of London during World War II, as well as “economy-class syndrome,” first described in conjunction with long-distance airplane flights in 1954. Basically, prolonged leg immobility leads to inadequate circulation and resultant venous clot formation. But prolonged TV watching is a much more common risk factor than is economy-class syndrome, he noted.
“The take-home message is this: Public awareness of the risk of pulmonary embolism from lengthy leg immobility is essential. To prevent the occurrence of pulmonary embolism, we recommend the same preventive behavior used against economy-class syndrome. That is, take a break, stand up, and walk around during the television viewing. And drink water to prevent dehydration; that is also important,” Mr. Shirakawa said.
Session cochair Dr. José Ramón González Juanatey observed that the true burden of PE triggered by prolonged TV watching is far greater than documented in the Japanese study because the analysis focused exclusively on fatal cases.
“Only about 10% of cases of pulmonary embolism are immediately fatal events,” commented Dr. González Juanatey of the University of Santiago de Compostela and president of the Spanish Society of Cardiology.
“The absolute risk may be fairly small, but it’s a devastating thing to have happen to you,” added cochair Dr. Ian Graham, professor of cardiovascular medicine at Trinity College, Dublin.
The Japan Collaborative Cohort Study has been funded by scientific grants from various Japanese health and education ministries. Mr. Shirakawa reported having no financial conflicts.
LONDON – Middle-aged adults who watch TV for an average of 5 or more hours per night face an adjusted 6.5-fold increased risk of fatal pulmonary embolism, compared with those who watch less than 2.5 hours per night, Toru Shirakawa reported at the annual congress of the European Society of Cardiology.
This was the key lesson gleaned from an analysis from the Japan Collaborative Cohort Study, the first large-scale prospective investigation of the relationship between prolonged television watching and pulmonary embolism (PE). The study included 86,024 Japanese participants aged 40-79 years prospectively followed for a median of 18.4 years, explained Mr. Shirakawa, a medical student at Osaka (Japan) University.
And while many busy medical professionals might presume 5 hours–plus of TV watching per night constitutes extreme behavior, that’s hardly the case. Indeed, according to the Nielsen survey, American adults watch an average of 4.85 hours of TV nightly.
During the study period there were 59 confirmed deaths from PE. In a multivariate analysis adjusted for sex and baseline age, cardiovascular risk factors, and physical activity level, a strong dose-response relationship was evident between hours of TV viewing and fatal PE.
This association was most pronounced in the 40- to 59-year-olds. Using as a reference group subjects who watched less than 2.5 hours per day, those who watched 2.5-4.9 hours had an adjusted 3.14-fold increased risk of fatal PE. Individuals who watched 5 hours or more – less than the average length of two American football games – were at 6.49-fold increased risk.
The same dose-response association was evident in the full study population spanning ages 40 through 79 years at entry. However, the magnitude of risk attributable to prolonged television watching in the overall group wasn’t as great, since 60- to 79-year-olds face multiple age-related competing mortality risks. Still, 40- to 79-year-olds who watched at least 5 hours of TV daily had an adjusted 2.36-fold greater risk of fatal PE, compared with those who watched for less than 2.5 hours.
Mr. Shirakawa observed that the mechanism of injury is presumably the same as previously reported in studies of “shelter death” during the bombing of London during World War II, as well as “economy-class syndrome,” first described in conjunction with long-distance airplane flights in 1954. Basically, prolonged leg immobility leads to inadequate circulation and resultant venous clot formation. But prolonged TV watching is a much more common risk factor than is economy-class syndrome, he noted.
“The take-home message is this: Public awareness of the risk of pulmonary embolism from lengthy leg immobility is essential. To prevent the occurrence of pulmonary embolism, we recommend the same preventive behavior used against economy-class syndrome. That is, take a break, stand up, and walk around during the television viewing. And drink water to prevent dehydration; that is also important,” Mr. Shirakawa said.
Session cochair Dr. José Ramón González Juanatey observed that the true burden of PE triggered by prolonged TV watching is far greater than documented in the Japanese study because the analysis focused exclusively on fatal cases.
“Only about 10% of cases of pulmonary embolism are immediately fatal events,” commented Dr. González Juanatey of the University of Santiago de Compostela and president of the Spanish Society of Cardiology.
“The absolute risk may be fairly small, but it’s a devastating thing to have happen to you,” added cochair Dr. Ian Graham, professor of cardiovascular medicine at Trinity College, Dublin.
The Japan Collaborative Cohort Study has been funded by scientific grants from various Japanese health and education ministries. Mr. Shirakawa reported having no financial conflicts.
AT THE ESC CONGRESS 2015
Key clinical point: Tuning in to the television for an average of 5 hours or more per day is independently associated with a sharply increased pulmonary embolism risk.
Major finding: Middle-aged Japanese adults who spent 5 or more hours per day watching television were at an adjusted 6.5-fold greater risk of fatal pulmonary embolism than were those who averaged less than 2.5 hours of viewing.
Data source: This prospective Japanese national study included 86,024 participants aged 40-79 years who were followed for a median of 18.4 years.
Disclosures: The Japan Collaborative Cohort Study has been funded by scientific grants from various Japanese health and education ministries. The presenter reported having no financial conflicts.
ESC: Rivaroxaban safety highlighted in real-world setting
LONDON – The factor Xa inhibitor rivaroxaban was associated with low rates of bleeding and stroke in two observational studies that included more than 45,000 people with nonvalvular atrial fibrillation.
The XANTUS (Xarelto for Prevention of Stroke in Patients With Atrial Fibrillation) study involved 6,784 individuals treated at centers in Europe, Canada, and Israel. The incidence of major bleeding was 2.1% per year and the risk of stroke was 0.7% per year. Rates of fatal, critical organ, and intracranial bleeding were also low at 0.2%, 0.7%, and 0.4% per year, respectively.
“The rates of stroke and systemic embolism, all strokes and gastrointestinal bleeding were markedly lower in XANTUS in comparison to ROCKET-AF,” noted Dr. John Camm who presented the XANTUS study findings at the annual congress of the European Society of Cardiology. “Major bleeding was also largely reduced in XANTUS, however the death rate and intracranial hemorrhage rate was similar,” he added.
Dr. Camm, who is professor of clinical cardiology at St George’s Hospital in London, noted that the patient populations and the design of the XANTUS study and phase III ROCKET-AF trial (N Engl J Med. 2011;365:883-91) were slightly different. Patients in the single-arm, prospective, observational XANTUS study were recruited from routine primary care practices and had an overall lower risk of stroke than those enrolled in the randomized, double-blind, controlled clinical trial who were at more moderate to high risk respective CHADS2 scores of 2.0 and 3.5. The incidence of major bleeding was also slightly higher in the ROCKET-AF, at 3.6% per year, which was similar to that seen with warfarin (3.4%; P = .58), the active comparator used.
Nevertheless, the findings of the XANTUS study, which were published online simultaneously with their presentation at the conference (Eur Heart J. Sep 1. doi: 10.1093/eurheartj/ehv466), highlight the “real-world” safety of rivaroxaban, Dr. Camm said.
Results from the separate PMSS (Post-Marketing Safety Surveillance) study reported in a poster at the meeting were similar. The PMSS study is being conducted in the United States and is an ongoing, retrospective, 5-year, observational study of more than 39,000 patients with nonvalvular atrial fibrillation. At 2 years follow-up, the incidence of major bleeding was 2.89% per year and the incidence of fatal bleeding was 0.1% per year (Eur Heart J. 2015;36:687.P4066).
“Real-world research is an essential complement to clinical trials and helps inform treatment decisions,” PMSS study investigator Dr. Frank Peacock said in a press release issued by Janssen. Dr. Peacock, who is professor of emergency medicine at Baylor College of Medicine in Houston, added, “These studies confirm the safety profile of rivaroxaban in real-world settings around the globe.”
The XANTUS and PMSS studies are part of a large postlicensing program and were respectively designed to meet European Medicines Agency and U.S. Food and Drug Administration requirements on the long-term monitoring of medicines. There are also similar programs running in other world regions, such as XANTUS-EL and XANAP.
Other real-world data gleaned from electronic medical records (EMRs) comparing the potential bleeding risks of the factor Xa inhibitor apixaban (Eliquis) versus other available non–vitamin K antagonists (NOACs) including rivaroxaban and the direct thrombin inhibitor dabigatran (Pradaxa) were reported in several posters supported by Bristol-Myers Squibb and Pfizer and in an oral presentation given by Dr. Gregory Lip of the University of Birmingham, England.
Two of the posters reported data from retrospective analyses of different United States EMRs of 29,338 and 35, 757 patients, respectively, with nonvalvular atrial fibrillation newly started on a NOAC or warfarin in 2013 or 2014. Most were started on warfarin (43.3%/69.6%), followed by rivaroxaban (34.3%/17.9%), dabigatran (14.2%/6.8%), and apixaban (8.2%/5.7%).
Results of the first study (Eur Heart J. 2015;36:1085.P6217) showed that patients newly starting treatment with a NOAC had significantly lower rates of major bleeding than those starting treatment with warfarin, which was 4.6% per year versus 2.35% per year for apixiban, 3.38% per year for dabigatran, and 4.57% per year for rivaroxaban in the first study.
In the second study (Eur Heart J. 2015;36:1085.P6215) the respective adjusted hazard ratios for bleeding risk were 1.094, 0.747 and 0.679, comparing rivaroxaban, apixaban, and dabigatran against warfarin.
Other data gleaned from separate U.S. EMRs suggested that apixaban was associated with fewer bleeding-related hospital readmissions than either rivaroxaban or dabigatran in hospitalized patients with nonvalvular atrial fibrillation (Eur Heart J. 2015;36: 1085.P6211).
Dr. Lip presented 6-month follow-up data on more than 60,000 patients with nonvalvular atrial fibrillation who were treated with one of the three NOACs that was recorded in a U.S. medical claims database (Eur Heart J. 2015;36:339.1975). Most of the patients were treated with rivaroxaban (50.6%), with 34.8% treated with dabigatran and 14.6% with apixaban. Unadjusted data showed that the rates of major bleeding were 20.2% per year, 13.2% per year, and 14.5% per year, respectively.
Dr. Lip observed that, after adjusting the data, patients taking dabigatran had higher rates of clinically relevant nonmajor gastrointestinal bleeding (HR =1.24), and that those taking rivaroxaban were more likely to have major (HR = 3.6), clinically relevant nonmajor (HR = 1.43), or any bleeding (HR = 1.41) when compared with apixaban users.
“Larger cohort studies and longer follow-up data of general nonvalvular atrial fibrillation populations will be needed to confirm these early observations,” Dr. Lip concluded.
While real-world research of course has its limitations and cannot replace clinical trial findings as a means to accurately compare the clinical efficacy or safety profiles of different medicines, such studies do provide information that can help inform clinical practice.
“With 10 million people in Europe alone affected by atrial fibrillation, a number that is only expected to increase, real-world insights on routine anticoagulation management in everyday clinical practice is increasingly important for physicians and patients,” Dr. Camm noted in a media release on the XANTUS trial issued by the European Society of Cardiology.
Dr. Camm added: “These real-world insights from XANTAS complement and expand on what we already know from clinical trials, and provide physicians with reassurance to prescribe rivaroxaban as an effective and well-tolerated treatment option for the broad range of patients with atrial fibrillation seen in their everyday practice.”
The XANTUS and PMSS studies were supported by Bayer HealthCare and Janssen. The other studies mentioned were supported by Bristol-Myers Squibb and Pfizer. Dr. Camm disclosed acting as a consultant for Bayer Healthcare and other health care companies. Dr. Lip disclosed acting as a consultant for Bayer Healthcare, Bristol-Myers Squibb, and Pfizer as well as other health care companies.
LONDON – The factor Xa inhibitor rivaroxaban was associated with low rates of bleeding and stroke in two observational studies that included more than 45,000 people with nonvalvular atrial fibrillation.
The XANTUS (Xarelto for Prevention of Stroke in Patients With Atrial Fibrillation) study involved 6,784 individuals treated at centers in Europe, Canada, and Israel. The incidence of major bleeding was 2.1% per year and the risk of stroke was 0.7% per year. Rates of fatal, critical organ, and intracranial bleeding were also low at 0.2%, 0.7%, and 0.4% per year, respectively.
“The rates of stroke and systemic embolism, all strokes and gastrointestinal bleeding were markedly lower in XANTUS in comparison to ROCKET-AF,” noted Dr. John Camm who presented the XANTUS study findings at the annual congress of the European Society of Cardiology. “Major bleeding was also largely reduced in XANTUS, however the death rate and intracranial hemorrhage rate was similar,” he added.
Dr. Camm, who is professor of clinical cardiology at St George’s Hospital in London, noted that the patient populations and the design of the XANTUS study and phase III ROCKET-AF trial (N Engl J Med. 2011;365:883-91) were slightly different. Patients in the single-arm, prospective, observational XANTUS study were recruited from routine primary care practices and had an overall lower risk of stroke than those enrolled in the randomized, double-blind, controlled clinical trial who were at more moderate to high risk respective CHADS2 scores of 2.0 and 3.5. The incidence of major bleeding was also slightly higher in the ROCKET-AF, at 3.6% per year, which was similar to that seen with warfarin (3.4%; P = .58), the active comparator used.
Nevertheless, the findings of the XANTUS study, which were published online simultaneously with their presentation at the conference (Eur Heart J. Sep 1. doi: 10.1093/eurheartj/ehv466), highlight the “real-world” safety of rivaroxaban, Dr. Camm said.
Results from the separate PMSS (Post-Marketing Safety Surveillance) study reported in a poster at the meeting were similar. The PMSS study is being conducted in the United States and is an ongoing, retrospective, 5-year, observational study of more than 39,000 patients with nonvalvular atrial fibrillation. At 2 years follow-up, the incidence of major bleeding was 2.89% per year and the incidence of fatal bleeding was 0.1% per year (Eur Heart J. 2015;36:687.P4066).
“Real-world research is an essential complement to clinical trials and helps inform treatment decisions,” PMSS study investigator Dr. Frank Peacock said in a press release issued by Janssen. Dr. Peacock, who is professor of emergency medicine at Baylor College of Medicine in Houston, added, “These studies confirm the safety profile of rivaroxaban in real-world settings around the globe.”
The XANTUS and PMSS studies are part of a large postlicensing program and were respectively designed to meet European Medicines Agency and U.S. Food and Drug Administration requirements on the long-term monitoring of medicines. There are also similar programs running in other world regions, such as XANTUS-EL and XANAP.
Other real-world data gleaned from electronic medical records (EMRs) comparing the potential bleeding risks of the factor Xa inhibitor apixaban (Eliquis) versus other available non–vitamin K antagonists (NOACs) including rivaroxaban and the direct thrombin inhibitor dabigatran (Pradaxa) were reported in several posters supported by Bristol-Myers Squibb and Pfizer and in an oral presentation given by Dr. Gregory Lip of the University of Birmingham, England.
Two of the posters reported data from retrospective analyses of different United States EMRs of 29,338 and 35, 757 patients, respectively, with nonvalvular atrial fibrillation newly started on a NOAC or warfarin in 2013 or 2014. Most were started on warfarin (43.3%/69.6%), followed by rivaroxaban (34.3%/17.9%), dabigatran (14.2%/6.8%), and apixaban (8.2%/5.7%).
Results of the first study (Eur Heart J. 2015;36:1085.P6217) showed that patients newly starting treatment with a NOAC had significantly lower rates of major bleeding than those starting treatment with warfarin, which was 4.6% per year versus 2.35% per year for apixiban, 3.38% per year for dabigatran, and 4.57% per year for rivaroxaban in the first study.
In the second study (Eur Heart J. 2015;36:1085.P6215) the respective adjusted hazard ratios for bleeding risk were 1.094, 0.747 and 0.679, comparing rivaroxaban, apixaban, and dabigatran against warfarin.
Other data gleaned from separate U.S. EMRs suggested that apixaban was associated with fewer bleeding-related hospital readmissions than either rivaroxaban or dabigatran in hospitalized patients with nonvalvular atrial fibrillation (Eur Heart J. 2015;36: 1085.P6211).
Dr. Lip presented 6-month follow-up data on more than 60,000 patients with nonvalvular atrial fibrillation who were treated with one of the three NOACs that was recorded in a U.S. medical claims database (Eur Heart J. 2015;36:339.1975). Most of the patients were treated with rivaroxaban (50.6%), with 34.8% treated with dabigatran and 14.6% with apixaban. Unadjusted data showed that the rates of major bleeding were 20.2% per year, 13.2% per year, and 14.5% per year, respectively.
Dr. Lip observed that, after adjusting the data, patients taking dabigatran had higher rates of clinically relevant nonmajor gastrointestinal bleeding (HR =1.24), and that those taking rivaroxaban were more likely to have major (HR = 3.6), clinically relevant nonmajor (HR = 1.43), or any bleeding (HR = 1.41) when compared with apixaban users.
“Larger cohort studies and longer follow-up data of general nonvalvular atrial fibrillation populations will be needed to confirm these early observations,” Dr. Lip concluded.
While real-world research of course has its limitations and cannot replace clinical trial findings as a means to accurately compare the clinical efficacy or safety profiles of different medicines, such studies do provide information that can help inform clinical practice.
“With 10 million people in Europe alone affected by atrial fibrillation, a number that is only expected to increase, real-world insights on routine anticoagulation management in everyday clinical practice is increasingly important for physicians and patients,” Dr. Camm noted in a media release on the XANTUS trial issued by the European Society of Cardiology.
Dr. Camm added: “These real-world insights from XANTAS complement and expand on what we already know from clinical trials, and provide physicians with reassurance to prescribe rivaroxaban as an effective and well-tolerated treatment option for the broad range of patients with atrial fibrillation seen in their everyday practice.”
The XANTUS and PMSS studies were supported by Bayer HealthCare and Janssen. The other studies mentioned were supported by Bristol-Myers Squibb and Pfizer. Dr. Camm disclosed acting as a consultant for Bayer Healthcare and other health care companies. Dr. Lip disclosed acting as a consultant for Bayer Healthcare, Bristol-Myers Squibb, and Pfizer as well as other health care companies.
LONDON – The factor Xa inhibitor rivaroxaban was associated with low rates of bleeding and stroke in two observational studies that included more than 45,000 people with nonvalvular atrial fibrillation.
The XANTUS (Xarelto for Prevention of Stroke in Patients With Atrial Fibrillation) study involved 6,784 individuals treated at centers in Europe, Canada, and Israel. The incidence of major bleeding was 2.1% per year and the risk of stroke was 0.7% per year. Rates of fatal, critical organ, and intracranial bleeding were also low at 0.2%, 0.7%, and 0.4% per year, respectively.
“The rates of stroke and systemic embolism, all strokes and gastrointestinal bleeding were markedly lower in XANTUS in comparison to ROCKET-AF,” noted Dr. John Camm who presented the XANTUS study findings at the annual congress of the European Society of Cardiology. “Major bleeding was also largely reduced in XANTUS, however the death rate and intracranial hemorrhage rate was similar,” he added.
Dr. Camm, who is professor of clinical cardiology at St George’s Hospital in London, noted that the patient populations and the design of the XANTUS study and phase III ROCKET-AF trial (N Engl J Med. 2011;365:883-91) were slightly different. Patients in the single-arm, prospective, observational XANTUS study were recruited from routine primary care practices and had an overall lower risk of stroke than those enrolled in the randomized, double-blind, controlled clinical trial who were at more moderate to high risk respective CHADS2 scores of 2.0 and 3.5. The incidence of major bleeding was also slightly higher in the ROCKET-AF, at 3.6% per year, which was similar to that seen with warfarin (3.4%; P = .58), the active comparator used.
Nevertheless, the findings of the XANTUS study, which were published online simultaneously with their presentation at the conference (Eur Heart J. Sep 1. doi: 10.1093/eurheartj/ehv466), highlight the “real-world” safety of rivaroxaban, Dr. Camm said.
Results from the separate PMSS (Post-Marketing Safety Surveillance) study reported in a poster at the meeting were similar. The PMSS study is being conducted in the United States and is an ongoing, retrospective, 5-year, observational study of more than 39,000 patients with nonvalvular atrial fibrillation. At 2 years follow-up, the incidence of major bleeding was 2.89% per year and the incidence of fatal bleeding was 0.1% per year (Eur Heart J. 2015;36:687.P4066).
“Real-world research is an essential complement to clinical trials and helps inform treatment decisions,” PMSS study investigator Dr. Frank Peacock said in a press release issued by Janssen. Dr. Peacock, who is professor of emergency medicine at Baylor College of Medicine in Houston, added, “These studies confirm the safety profile of rivaroxaban in real-world settings around the globe.”
The XANTUS and PMSS studies are part of a large postlicensing program and were respectively designed to meet European Medicines Agency and U.S. Food and Drug Administration requirements on the long-term monitoring of medicines. There are also similar programs running in other world regions, such as XANTUS-EL and XANAP.
Other real-world data gleaned from electronic medical records (EMRs) comparing the potential bleeding risks of the factor Xa inhibitor apixaban (Eliquis) versus other available non–vitamin K antagonists (NOACs) including rivaroxaban and the direct thrombin inhibitor dabigatran (Pradaxa) were reported in several posters supported by Bristol-Myers Squibb and Pfizer and in an oral presentation given by Dr. Gregory Lip of the University of Birmingham, England.
Two of the posters reported data from retrospective analyses of different United States EMRs of 29,338 and 35, 757 patients, respectively, with nonvalvular atrial fibrillation newly started on a NOAC or warfarin in 2013 or 2014. Most were started on warfarin (43.3%/69.6%), followed by rivaroxaban (34.3%/17.9%), dabigatran (14.2%/6.8%), and apixaban (8.2%/5.7%).
Results of the first study (Eur Heart J. 2015;36:1085.P6217) showed that patients newly starting treatment with a NOAC had significantly lower rates of major bleeding than those starting treatment with warfarin, which was 4.6% per year versus 2.35% per year for apixiban, 3.38% per year for dabigatran, and 4.57% per year for rivaroxaban in the first study.
In the second study (Eur Heart J. 2015;36:1085.P6215) the respective adjusted hazard ratios for bleeding risk were 1.094, 0.747 and 0.679, comparing rivaroxaban, apixaban, and dabigatran against warfarin.
Other data gleaned from separate U.S. EMRs suggested that apixaban was associated with fewer bleeding-related hospital readmissions than either rivaroxaban or dabigatran in hospitalized patients with nonvalvular atrial fibrillation (Eur Heart J. 2015;36: 1085.P6211).
Dr. Lip presented 6-month follow-up data on more than 60,000 patients with nonvalvular atrial fibrillation who were treated with one of the three NOACs that was recorded in a U.S. medical claims database (Eur Heart J. 2015;36:339.1975). Most of the patients were treated with rivaroxaban (50.6%), with 34.8% treated with dabigatran and 14.6% with apixaban. Unadjusted data showed that the rates of major bleeding were 20.2% per year, 13.2% per year, and 14.5% per year, respectively.
Dr. Lip observed that, after adjusting the data, patients taking dabigatran had higher rates of clinically relevant nonmajor gastrointestinal bleeding (HR =1.24), and that those taking rivaroxaban were more likely to have major (HR = 3.6), clinically relevant nonmajor (HR = 1.43), or any bleeding (HR = 1.41) when compared with apixaban users.
“Larger cohort studies and longer follow-up data of general nonvalvular atrial fibrillation populations will be needed to confirm these early observations,” Dr. Lip concluded.
While real-world research of course has its limitations and cannot replace clinical trial findings as a means to accurately compare the clinical efficacy or safety profiles of different medicines, such studies do provide information that can help inform clinical practice.
“With 10 million people in Europe alone affected by atrial fibrillation, a number that is only expected to increase, real-world insights on routine anticoagulation management in everyday clinical practice is increasingly important for physicians and patients,” Dr. Camm noted in a media release on the XANTUS trial issued by the European Society of Cardiology.
Dr. Camm added: “These real-world insights from XANTAS complement and expand on what we already know from clinical trials, and provide physicians with reassurance to prescribe rivaroxaban as an effective and well-tolerated treatment option for the broad range of patients with atrial fibrillation seen in their everyday practice.”
The XANTUS and PMSS studies were supported by Bayer HealthCare and Janssen. The other studies mentioned were supported by Bristol-Myers Squibb and Pfizer. Dr. Camm disclosed acting as a consultant for Bayer Healthcare and other health care companies. Dr. Lip disclosed acting as a consultant for Bayer Healthcare, Bristol-Myers Squibb, and Pfizer as well as other health care companies.
AT THE ESC CONGRESS 2015
Key clinical point: Data from routine clinical practice studies suggest a low risk of bleeding and stroke with rivaroxaban and other non–vitamin K antagonists.
Major finding: The incidence of major bleeding with rivaroxaban was 2.1% per year and the risk of stroke was 0.7% per year in the XANTUS study.
Data source: More than 45,000 patients with nonvalvular atrial fibrillation treated with rivaroxaban for stroke prevention in two, real-world observational studies and separate electronic medical record analyses of patients treated with apixaban, rivaroxaban, and dabigatran.
Disclosures: The XANTUS and PMSS studies were supported by Bayer HealthCare and Janssen. The other studies mentioned were supported by Bristol-Myers Squibb and Pfizer. Dr. Camm disclosed acting as a consultant for Bayer Healthcare and other health care companies. Dr. Lip disclosed acting as a consultant for Bayer Healthcare, Bristol-Myers Squibb, and Pfizer as well as other health care companies.