User login
Troponin elevation after noncardiac surgery: Significance and management
More than 200 million patients undergo noncardiac surgery each year, and the volume is increasing.1 Cardiovascular complications are a major cause of morbidity and mortality in the perioperative period.
Before the advent of modern cardiac biomarkers, an estimated 2% to 3% of all patients undergoing noncardiac surgery had a major adverse cardiac event.2 However, more recent studies suggest that 5% to 25% of patients have troponin elevations after noncardiac surgery, depending on the patient population,3–6 and many are asymptomatic, suggesting that many patients are sustaining undetected myocardial injury. Those who suffer a myocardial infarction or myocardial injury have elevated morbidity and mortality rates, not only perioperatively, but also at 30 days and even at up to 1 year.3–5,7–11
Yet there are almost no data on how best to manage these patients; the available guidelines, therefore, do not provide sufficient recommendations for clinical practice.
To address the lack of guidelines, we examine the incidence and proposed mechanisms of myocardial injury after noncardiac surgery, suggest an approach to identifying patients at risk, recommend treatment strategies, and consider future directions.
CARDIAC BIOMARKERS
When cardiac cellular injury from ischemia, direct trauma, or other cause disrupts the cell membrane, intracellular contents enter the extracellular space, including the blood stream. If the myocyte damage is extensive enough, biochemical assays can detect these substances.
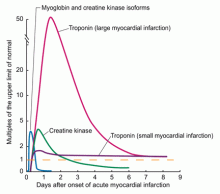
Troponin, creatine kinase, myoglobin, and lactate dehydrogenase are common biomarkers of necrosis that, when detected in the plasma, may indicate cardiac injury. Each can be detected at varying times after cardiac injury (Figure 1).12
Cardiac troponins I and T
Of the biomarkers, cardiac troponin I and cardiac troponin T are now the most widely used and are the most specific for myocyte injury.
Troponins are proteins that regulate the calcium-induced interaction between myosin and actin that results in muscle contraction. Troponin is a complex consisting of three subunits: troponin C, troponin I, and troponin T. The cardiac troponin I and T isoforms are distinct from those found in skeletal muscle, making them specific for myocyte injury, and they are currently the recommended markers for diagnosing acute myocardial infarction.13
The troponin immunoassays currently available are not standardized among laboratories and point-of-care methods, and thus, levels cannot be compared across testing centers.14 Each assay has unique performance characteristics, but guidelines recommend using the 99th percentile value from a normal reference population for a given assay to define whether myocardial injury is present.13
Troponin elevation has prognostic value in patients presenting with acute coronary syndromes,15–18 and the degree of elevation correlates with infarct size.19–21
Controversy exists as to whether troponin and other biomarkers are released only after myocardial necrosis or after reversible injury as well. Using newer, highly sensitive assays, troponin elevations have been detected after short periods of ischemia during stress testing22,23 and in patients with stable angina,24 suggesting that reversible cardiac stress and injury can lead to troponin release. This mechanism may play an important role during the myocardial injury that can occur in patients undergoing noncardiac surgery.
MYOCARDIAL INFARCTION vs MYOCARDIAL INJURY
In 2000, the Joint Task Force of the European Society of Cardiology, American College of Cardiology Foundation, American Heart Association, and World Heart Federation revised the criteria for the diagnosis of myocardial infarction created by the World Health Organization in 1979. The definition was revised again in 2007 and once more in 2012 to create the third universal definition of myocardial infarction.
Acute myocardial infarction
Acute myocardial infarction is defined as evidence of myocardial necrosis in a setting of myocardial ischemia, not related to causes such as trauma or pulmonary embolism, with a rise or a fall (or a rise and a fall) of cardiac biomarkers (at least one value being above the 99th percentile in the reference population) and any of the following:
- Symptoms of ischemia
- New ST-segment changes or new left bundle branch block
- Pathologic Q waves
- Imaging evidence of new loss of viable myocardium or new regional wall-motion abnormality
- Intracoronary thrombus by angiography or autopsy.13
Myocardial injury after noncardiac surgery
Studies10,11 have shown that many patients undergoing noncardiac surgery have evidence of cardiac biomarker release but do not meet the universal definition of myocardial infarction.
The Perioperative Ischemic Evaluation (POISE) trial10 reported that 415 (5%) of its patients met the definition of myocardial infarction, of whom only about 35% had symptoms of ischemia. Another 697 patients (8.3%) had isolated elevations in biomarkers without meeting the definition of myocardial infarction.
The VISION study11 (Vascular Events in Noncardiac Surgery Patients Cohort Evaluation) prospectively screened more than 15,000 patients in several countries for troponin elevation during the first 3 postoperative days and for ischemic symptoms and features. Of the patients screened, approximately 1,200 (8%) had troponin elevations, with fewer than half fulfilling the criteria for myocardial infarction.
In another study, van Waes et al6 prospectively screened 2,232 patients ages 60 and older undergoing intermediate- to high-risk noncardiac surgery. Troponin levels were elevated in 19% of the patients, but only 10 of these patients met the universal definition of myocardial infarction.
In all of these studies, patients with isolated elevation in myocardial biomarkers had worse short-term and long-term outcomes than those without. These observations led to a proposed definition of “myocardial injury after noncardiac surgery” that is broader than that of myocardial infarction and requires only elevation of cardiac biomarkers judged to be due to myocardial ischemia (ie, not from another obvious cause such as pulmonary embolism or myocarditis).3
FIVE TYPES OF MYOCARDIAL INFARCTION
The Joint Task Force13 categorizes myocardial infarction into five distinct types:
- Type 1—due to plaque rupture
- Type 2—due to imbalance between oxygen supply and demand
- Type 3—sudden cardiac death
- Type 4a—associated with percutaneous coronary intervention
- Type 4b—associated with stent thrombosis
- Type 5—associated with coronary artery bypass surgery.
Types 1 and 2 have both been implicated in perioperative myocardial infarction and injury. Patient characteristics and the physiologic response to surgical and anesthetic stressors likely contribute to the development of myocardial infarction and injury after noncardiac surgery.
Plaque rupture as a cause of postoperative myocardial infarction
The mechanism of type 1 myocardial infarction—plaque rupture or erosion leading to thrombosis and infarction—plays a significant role in most cases of acute coronary syndromes. Its role in perioperative and postoperative myocardial infarction or injury, however, is less clear.
In an autopsy study of 26 patients who died of myocardial infarction after noncardiac surgery, plaque rupture was evident in 12 (46%).25 A prospective angiographic study of 120 patients with acute coronary syndromes after noncardiac surgery found that nearly 50% had evidence of plaque rupture.26
Higher levels of catecholamines, cortisol,27,28 platelet reactivity,29 procoagulant factors,30 and coronary artery shear stress31 are all present in the postoperative period and may contribute to an increased propensity for plaque rupture or erosion. Whether plaque rupture is present in patients who have isolated troponin elevation but do not meet the criteria for myocardial infarction has not been investigated.
Oxygen supply-demand imbalance during and after surgery
Oxygen supply-demand imbalance (the mechanism in type 2 myocardial infarction) leading to myocyte stress, ischemia, and subsequent infarction is likely common in the perioperative and postoperative periods. As previously discussed, this imbalance may be present with or without symptoms.
Oxygen demand may increase in this period as a result of tachycardia32 caused by bleeding, pain, and catecholamines or increased wall stress from hypertension due to vasoconstriction or pain.33 Oxygen supply can be decreased secondary to tachycardia, anemia,34 hypotension, hypoxemia, hypercarbia, intravascular fluid shifts (bleeding or volume overload), or coronary vasoconstriction.33,35
These mechanisms of myocardial injury, infarction, or both can occur with or without underlying significant obstructive coronary artery disease. However, severe coronary artery disease is more common in those who have had a perioperative myocardial infarction.36
POSTOPERATIVE TROPONIN ELEVATION CARRIES A WORSE PROGNOSIS
Patients who suffer a myocardial infarction after noncardiac surgery have worse short- and long-term outcomes than their counterparts.4,5,7, 8,10,33 In the POISE trial,10 the 30-day mortality rate was 11.6% in those who had had a perioperative myocardial infarction, compared with 2.2% in those who did not (P < .001). The patients who had had a myocardial infarction were also more likely to have nonfatal cardiac arrest, coronary revascularization, and congestive heart failure.
Myocardial injury not fulfilling the criteria for myocardial infarction after noncardiac surgery is also associated with worse short-term and long-term outcomes.3,6,10,11,37,38 POISE patients with isolated elevations in cardiac biomarkers had a higher 30-day risk of coronary revascularization and nonfatal arrest.10 In the VISION trial, an elevation in troponin was the strongest predictor of death within 30 days after noncardiac surgery. This analysis also showed that the higher the peak troponin value, the greater the risk of death and the shorter the median time until death.11
A meta-analysis of 14 studies in 3,139 patients found that elevated troponin after noncardiac surgery was an independent predictor of death within 1 year (odds ratio [OR] 6.7, 95% confidence interval [CI] 4.1–10.9) and beyond 1 year (OR 1.8, 95% CI 1.4–2.3).37
SHOULD SCREENING BE ROUTINE AFTER NONCARDIAC SURGERY?
Since patients suffering myocardial infarction or injury after noncardiac surgery have a worse prognosis, many experts advocate routinely screening all high-risk patients and those undergoing moderate- to high-risk surgery. Many tools exist to determine which patients undergoing noncardiac surgery are at high risk of cardiac complications.
The revised Goldman Cardiac Risk Index is commonly used and well validated. Variables in this index that predict major cardiac complications are:
- High-risk surgery (vascular surgery, orthopedic surgery, and intraperitoneal or intrathoracic surgery)
- History of ischemic heart disease
- History of congestive heart failure
- History of cerebrovascular disease
- Diabetes requiring insulin therapy
- Chronic kidney disease with a creatinine > 2.0 mg/dL.
The more of these variables that are present, the higher the risk of perioperative cardiac events2,4:
- No risk factors: 0.4% risk (95% CI 0.1–0.8)
- One risk factor: 1.0% risk (95% CI 0.5–1.4)
- Two risk factors: 2.4% risk (95% CI 1.3–3.5)
- Three or more risk factors: 5.4% risk (95% CI 2.7–7.9).
Current guidelines from the American College of Cardiology and the American Heart Association give a class I recommendation (the highest) for measuring troponin levels after noncardiac surgery in patients who have symptoms or signs suggesting myocardial ischemia. They give a class IIb recommendation (usefulness is less well established) for screening those at high risk but without symptoms or signs of ischemia, despite the previously cited evidence that patients with troponin elevation are at increased risk. The IIb recommendation is due to a lack of validated treatment strategies to modify and attenuate the recognized risk with troponin elevation in this setting.39
LITTLE EVIDENCE TO GUIDE TREATMENT
In current practice, internists and cardiologists are often asked to consult on patients with troponin elevations noted after noncardiac surgery. Although published and ongoing studies examine strategies to prevent cardiovascular events during noncardiac surgery, we lack data on managing the cases of myocardial infarction and injury that actually occur after noncardiac surgery.
When managing a patient who has a troponin elevation after surgery, many clinical factors must be weighed, including hemodynamic and clinical stability and risk of bleeding. Confronted with ST-segment elevation myocardial infarction or high-risk non–ST-segment elevation myocardial infarction, most clinicians would favor an early invasive reperfusion strategy in accordance with guidelines on managing acute coronary syndrome. Fibrinolytic drugs for ST-segment elevation myocardial infarction are likely to be contraindicated in the postoperative period because they pose an unacceptable risk of bleeding.
Guideline-directed medical therapies for those suffering perioperative myocardial infarction may lower the risk of future cardiovascular events, as suggested by a retrospective study of 66 patients diagnosed with perioperative myocardial infarction after vascular surgery.40 Those in whom medical therapy for coronary artery disease was not intensified—defined as adding or increasing the dose of antiplatelet agent, statin, beta-blocker, or angiotensin-converting enzyme inhibitor—had higher rates of cardiovascular events at 12 months (hazard ratio [HR] 2.80, 95% CI 1.05–24.2).40
In those with asymptomatic myocardial infarction or isolated elevation in cardiac biomarkers, no treatment strategies have been assessed prospectively or in randomized trials. However, statins and aspirin have been suggested as providing some benefit. In a substudy of the POISE trial, the use of aspirin was associated with a 46% reduction in the 30-day mortality rate in those suffering a perioperative myocardial infarction, and statins were associated with a 76% reduction.10 In a single-center retrospective analysis of 337 patients undergoing moderate- to high-risk vascular surgery, statin therapy was associated with a lower 1-year mortality rate (OR 0.63, 95% CI 0.40–0.98).38
We propose a treatment algorithm for patients identified as having cardiovascular events after noncardiac surgery (Figure 2), based on current evidence and guidelines. Ultimately, treatment decisions should be tailored to the individual patient. Discussion of the risks and benefits of therapeutic options should include the patient and surgeon.
Ongoing and future trials
Ongoing and future trials are aimed at addressing definitive treatment strategies in this patient population.
The MANAGE trial (Management of Myocardial Injury After Non-cardiac Surgery Trial) is randomizing patients suffering myocardial injury after noncardiac surgery to receive either dabigatran and omeprazole or placebo to assess the efficacy of these agents in preventing major adverse cardiac events and the safety of anticoagulation (ClinicalTrials.gov Identifier: NCT01661101).
The INTREPID trial (Study of Ticagrelor Versus Aspirin Treatment in Patients With Myocardial Injury Post Major Non-Cardiac Surgery) will assess the efficacy and safety of ticagrelor treatment compared with aspirin in a similar population (ClinicalTrial.gov Identifier: NCT02291419). The trial will enroll approximately 1,000 patients identified as having a postoperative troponin elevation more than two times the upper limit of normal of the assay during the index hospitalization (Figure 3). Enrollment was to have begun in mid-2015.
- Weiser TG, Regenbogen SE, Thompson KD, et al. An estimation of the global volume of surgery: a modelling strategy based on available data. Lancet 2008; 372:139–144.
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100:1043–1049.
- Botto F, Alonso-Coello P, Chan MT, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology 2014; 120:564–578.
- Devereaux PJ, Goldman L, Cook DJ, Gilbert K, Leslie K, Guyatt GH. Perioperative cardiac events in patients undergoing noncardiac surgery: a review of the magnitude of the problem, the pathophysiology of the events and methods to estimate and communicate risk. CMAJ 2005; 173:627–634.
- McFalls EO, Ward HB, Moritz TE, et al. Predictors and outcomes of a perioperative myocardial infarction following elective vascular surgery in patients with documented coronary artery disease: results of the CARP trial. Eur Heart J 2008; 29:394–401.
- van Waes JA, Nathoe HM, de Graaff JC, et al. Myocardial injury after noncardiac surgery and its association with short-term mortality. Circulation 2013; 127:2264–2271.
- Badner NH, Knill RL, Brown JE, Novick TV, Gelb AW. Myocardial infarction after noncardiac surgery. Anesthesiology 1998; 88:572–578.
- Kim LJ, Martinez EA, Faraday N, et al. Cardiac troponin I predicts short-term mortality in vascular surgery patients. Circulation 2002; 106:2366–2371.
- Landesberg G, Shatz V, Akopnik I, et al. Association of cardiac troponin, CK-MB, and postoperative myocardial ischemia with long-term survival after major vascular surgery. J Am Coll Cardiol 2003; 42:1547–1554.
- Devereaux PJ, Xavier D, Pogue J, et al. Characteristics and short-term prognosis of perioperative myocardial infarction in patients undergoing noncardiac surgery: a cohort study. Ann Intern Med 2011; 154:523–528.
- Devereaux PJ, Chan MT, Alonso-Coello P, et al. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA 2012; 307:2295–2304.
- Kumar A, Cannon CP. Acute coronary syndromes: diagnosis and management, part I. Mayo Clin Proc 2009; 84:917–938.
- Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. Circulation 2012; 126:2020–2035.
- Apple FS, Quist HE, Doyle PJ, Otto AP, Murakami MM. Plasma 99th percentile reference limits for cardiac troponin and creatine kinase MB mass for use with European Society of Cardiology/American College of Cardiology consensus recommendations. Clin Chem 2003; 49:1331–1336.
- Ottani F, Galvani M, Nicolini FA, et al. Elevated cardiac troponin levels predict the risk of adverse outcome in patients with acute coronary syndromes. Am Heart J 2000; 140:917–927.
- Ohman EM, Armstrong PW, White HD, et al. Risk stratification with a point-of-care cardiac troponin T test in acute myocardial infarction. GUSTO III investigators. Global Use of Strategies to Open Occluded Coronary Arteries. Am J Cardiol 1999; 84:1281–1286.
- deFilippi CR, Tocchi M, Parmar RJ, et al. Cardiac troponin T in chest pain unit patients without ischemic electrocardiographic changes: angiographic correlates and long-term clinical outcomes. J Am Coll Cardiol 2000; 35:1827–1834.
- Heidenreich PA, Alloggiamento T, Melsop K, McDonald KM, Go AS, Hlatky MA. The prognostic value of troponin in patients with non-ST elevation acute coronary syndromes: a meta-analysis. J Am Coll Cardiol 2001; 38:478–485.
- Steen H, Giannitsis E, Futterer S, Merten C, Juenger C, Katus HA. Cardiac troponin T at 96 hours after acute myocardial infarction correlates with infarct size and cardiac function. J Am Coll Cardiol 2006; 48:2192–2194.
- Licka M, Zimmermann R, Zehelein J, Dengler TJ, Katus HA, Kubler W. Troponin T concentrations 72 hours after myocardial infarction as a serological estimate of infarct size. Heart 2002; 87:520–524.
- Vasile VC, Babuin L, Giannitsis E, Katus HA, Jaffe AS. Relationship of MRI-determined infarct size and cTnI measurements in patients with ST-elevation myocardial infarction. Clin Chem 2008; 54:617–619.
- Sabatine MS, Morrow DA, de Lemos JA, Jarolim P, Braunwald E. Detection of acute changes in circulating troponin in the setting of transient stress test-induced myocardial ischaemia using an ultrasensitive assay: results from TIMI 35. Eur Heart J 2009; 30:162–169.
- Siriwardena M, Campbell V, Richards AM, Pemberton CJ. Cardiac biomarker responses to dobutamine stress echocardiography in healthy volunteers and patients with coronary artery disease. Clin Chem 2012; 58:1492–1494.
- Turer AT, Addo TA, Martin JL, et al. Myocardial ischemia induced by rapid atrial pacing causes troponin T release detectable by a highly sensitive assay: insights from a coronary sinus sampling study. J Am Coll Cardiol 2011; 57:2398–2405.
- Cohen MC, Aretz TH. Histological analysis of coronary artery lesions in fatal postoperative myocardial infarction. Cardiovasc Pathol 1999; 8:133–139.
- Gualandro DM, Campos CA, Calderaro D, et al. Coronary plaque rupture in patients with myocardial infarction after noncardiac surgery: frequent and dangerous. Atherosclerosis 2012; 222:191–195.
- Sametz W, Metzler H, Gries M, et al. Perioperative catecholamine changes in cardiac risk patients. Eur J Clin Invest 1999; 29:582–587.
- Frank SM, Higgins MS, Breslow MJ, et al. The catecholamine, cortisol, and hemodynamic responses to mild perioperative hypothermia. A randomized clinical trial. Anesthesiology 1995; 82:83–93.
- Rosenfeld BA, Faraday N, Campbell D, et al. Perioperative platelet reactivity and the effects of clonidine. Anesthesiology 1993; 79:255–261.
- Lison S, Weiss G, Spannagl M, Heindl B. Postoperative changes in procoagulant factors after major surgery. Blood Coagul Fibrinolysis 2011; 22:190–196.
- Fukumoto Y, Hiro T, Fujii T, et al. Localized elevation of shear stress is related to coronary plaque rupture: a 3-dimensional intravascular ultrasound study with in-vivo color mapping of shear stress distribution. J Am Coll Cardiol 2008; 51:645–650.
- Feringa HH, Bax JJ, Boersma E, et al. High-dose beta-blockers and tight heart rate control reduce myocardial ischemia and troponin T release in vascular surgery patients. Circulation 2006; 114:I-344–I-349.
- Landesberg G. The pathophysiology of perioperative myocardial infarction: facts and perspectives. J Cardiothorac Vasc Anesth 2003; 17:90–100.
- Nelson AH, Fleisher LA, Rosenbaum SH. Relationship between postoperative anemia and cardiac morbidity in high-risk vascular patients in the intensive care unit. Crit Care Med 1993; 21:860–866.
- Landesberg G, Beattie WS, Mosseri M, Jaffe AS, Alpert JS. Perioperative myocardial infarction. Circulation 2009; 119:2936–2944.
- Ellis SG, Hertzer NR, Young JR, Brener S. Angiographic correlates of cardiac death and myocardial infarction complicating major nonthoracic vascular surgery. Am J Cardiol 1996; 77:1126–1128.
- Levy M, Heels-Ansdell D, Hiralal R, et al. Prognostic value of troponin and creatine kinase muscle and brain isoenzyme measurement after noncardiac surgery: a systematic review and meta-analysis. Anesthesiology 2011; 114:796–806.
- Garcia S, Marston N, Sandoval Y, et al. Prognostic value of 12-lead electrocardiogram and peak troponin I level after vascular surgery. J Vasc Surg 2013; 57:166–172.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. J Am Coll Cardiol 2014; 64:e77–e137.
- Foucrier A, Rodseth R, Aissaoui M, et al. The long-term impact of early cardiovascular therapy intensification for postoperative troponin elevation after major vascular surgery. Anesth Analg 2014; 119:1053–1063.
More than 200 million patients undergo noncardiac surgery each year, and the volume is increasing.1 Cardiovascular complications are a major cause of morbidity and mortality in the perioperative period.
Before the advent of modern cardiac biomarkers, an estimated 2% to 3% of all patients undergoing noncardiac surgery had a major adverse cardiac event.2 However, more recent studies suggest that 5% to 25% of patients have troponin elevations after noncardiac surgery, depending on the patient population,3–6 and many are asymptomatic, suggesting that many patients are sustaining undetected myocardial injury. Those who suffer a myocardial infarction or myocardial injury have elevated morbidity and mortality rates, not only perioperatively, but also at 30 days and even at up to 1 year.3–5,7–11
Yet there are almost no data on how best to manage these patients; the available guidelines, therefore, do not provide sufficient recommendations for clinical practice.
To address the lack of guidelines, we examine the incidence and proposed mechanisms of myocardial injury after noncardiac surgery, suggest an approach to identifying patients at risk, recommend treatment strategies, and consider future directions.
CARDIAC BIOMARKERS
When cardiac cellular injury from ischemia, direct trauma, or other cause disrupts the cell membrane, intracellular contents enter the extracellular space, including the blood stream. If the myocyte damage is extensive enough, biochemical assays can detect these substances.
Troponin, creatine kinase, myoglobin, and lactate dehydrogenase are common biomarkers of necrosis that, when detected in the plasma, may indicate cardiac injury. Each can be detected at varying times after cardiac injury (Figure 1).12
Cardiac troponins I and T
Of the biomarkers, cardiac troponin I and cardiac troponin T are now the most widely used and are the most specific for myocyte injury.
Troponins are proteins that regulate the calcium-induced interaction between myosin and actin that results in muscle contraction. Troponin is a complex consisting of three subunits: troponin C, troponin I, and troponin T. The cardiac troponin I and T isoforms are distinct from those found in skeletal muscle, making them specific for myocyte injury, and they are currently the recommended markers for diagnosing acute myocardial infarction.13
The troponin immunoassays currently available are not standardized among laboratories and point-of-care methods, and thus, levels cannot be compared across testing centers.14 Each assay has unique performance characteristics, but guidelines recommend using the 99th percentile value from a normal reference population for a given assay to define whether myocardial injury is present.13
Troponin elevation has prognostic value in patients presenting with acute coronary syndromes,15–18 and the degree of elevation correlates with infarct size.19–21
Controversy exists as to whether troponin and other biomarkers are released only after myocardial necrosis or after reversible injury as well. Using newer, highly sensitive assays, troponin elevations have been detected after short periods of ischemia during stress testing22,23 and in patients with stable angina,24 suggesting that reversible cardiac stress and injury can lead to troponin release. This mechanism may play an important role during the myocardial injury that can occur in patients undergoing noncardiac surgery.
MYOCARDIAL INFARCTION vs MYOCARDIAL INJURY
In 2000, the Joint Task Force of the European Society of Cardiology, American College of Cardiology Foundation, American Heart Association, and World Heart Federation revised the criteria for the diagnosis of myocardial infarction created by the World Health Organization in 1979. The definition was revised again in 2007 and once more in 2012 to create the third universal definition of myocardial infarction.
Acute myocardial infarction
Acute myocardial infarction is defined as evidence of myocardial necrosis in a setting of myocardial ischemia, not related to causes such as trauma or pulmonary embolism, with a rise or a fall (or a rise and a fall) of cardiac biomarkers (at least one value being above the 99th percentile in the reference population) and any of the following:
- Symptoms of ischemia
- New ST-segment changes or new left bundle branch block
- Pathologic Q waves
- Imaging evidence of new loss of viable myocardium or new regional wall-motion abnormality
- Intracoronary thrombus by angiography or autopsy.13
Myocardial injury after noncardiac surgery
Studies10,11 have shown that many patients undergoing noncardiac surgery have evidence of cardiac biomarker release but do not meet the universal definition of myocardial infarction.
The Perioperative Ischemic Evaluation (POISE) trial10 reported that 415 (5%) of its patients met the definition of myocardial infarction, of whom only about 35% had symptoms of ischemia. Another 697 patients (8.3%) had isolated elevations in biomarkers without meeting the definition of myocardial infarction.
The VISION study11 (Vascular Events in Noncardiac Surgery Patients Cohort Evaluation) prospectively screened more than 15,000 patients in several countries for troponin elevation during the first 3 postoperative days and for ischemic symptoms and features. Of the patients screened, approximately 1,200 (8%) had troponin elevations, with fewer than half fulfilling the criteria for myocardial infarction.
In another study, van Waes et al6 prospectively screened 2,232 patients ages 60 and older undergoing intermediate- to high-risk noncardiac surgery. Troponin levels were elevated in 19% of the patients, but only 10 of these patients met the universal definition of myocardial infarction.
In all of these studies, patients with isolated elevation in myocardial biomarkers had worse short-term and long-term outcomes than those without. These observations led to a proposed definition of “myocardial injury after noncardiac surgery” that is broader than that of myocardial infarction and requires only elevation of cardiac biomarkers judged to be due to myocardial ischemia (ie, not from another obvious cause such as pulmonary embolism or myocarditis).3
FIVE TYPES OF MYOCARDIAL INFARCTION
The Joint Task Force13 categorizes myocardial infarction into five distinct types:
- Type 1—due to plaque rupture
- Type 2—due to imbalance between oxygen supply and demand
- Type 3—sudden cardiac death
- Type 4a—associated with percutaneous coronary intervention
- Type 4b—associated with stent thrombosis
- Type 5—associated with coronary artery bypass surgery.
Types 1 and 2 have both been implicated in perioperative myocardial infarction and injury. Patient characteristics and the physiologic response to surgical and anesthetic stressors likely contribute to the development of myocardial infarction and injury after noncardiac surgery.
Plaque rupture as a cause of postoperative myocardial infarction
The mechanism of type 1 myocardial infarction—plaque rupture or erosion leading to thrombosis and infarction—plays a significant role in most cases of acute coronary syndromes. Its role in perioperative and postoperative myocardial infarction or injury, however, is less clear.
In an autopsy study of 26 patients who died of myocardial infarction after noncardiac surgery, plaque rupture was evident in 12 (46%).25 A prospective angiographic study of 120 patients with acute coronary syndromes after noncardiac surgery found that nearly 50% had evidence of plaque rupture.26
Higher levels of catecholamines, cortisol,27,28 platelet reactivity,29 procoagulant factors,30 and coronary artery shear stress31 are all present in the postoperative period and may contribute to an increased propensity for plaque rupture or erosion. Whether plaque rupture is present in patients who have isolated troponin elevation but do not meet the criteria for myocardial infarction has not been investigated.
Oxygen supply-demand imbalance during and after surgery
Oxygen supply-demand imbalance (the mechanism in type 2 myocardial infarction) leading to myocyte stress, ischemia, and subsequent infarction is likely common in the perioperative and postoperative periods. As previously discussed, this imbalance may be present with or without symptoms.
Oxygen demand may increase in this period as a result of tachycardia32 caused by bleeding, pain, and catecholamines or increased wall stress from hypertension due to vasoconstriction or pain.33 Oxygen supply can be decreased secondary to tachycardia, anemia,34 hypotension, hypoxemia, hypercarbia, intravascular fluid shifts (bleeding or volume overload), or coronary vasoconstriction.33,35
These mechanisms of myocardial injury, infarction, or both can occur with or without underlying significant obstructive coronary artery disease. However, severe coronary artery disease is more common in those who have had a perioperative myocardial infarction.36
POSTOPERATIVE TROPONIN ELEVATION CARRIES A WORSE PROGNOSIS
Patients who suffer a myocardial infarction after noncardiac surgery have worse short- and long-term outcomes than their counterparts.4,5,7, 8,10,33 In the POISE trial,10 the 30-day mortality rate was 11.6% in those who had had a perioperative myocardial infarction, compared with 2.2% in those who did not (P < .001). The patients who had had a myocardial infarction were also more likely to have nonfatal cardiac arrest, coronary revascularization, and congestive heart failure.
Myocardial injury not fulfilling the criteria for myocardial infarction after noncardiac surgery is also associated with worse short-term and long-term outcomes.3,6,10,11,37,38 POISE patients with isolated elevations in cardiac biomarkers had a higher 30-day risk of coronary revascularization and nonfatal arrest.10 In the VISION trial, an elevation in troponin was the strongest predictor of death within 30 days after noncardiac surgery. This analysis also showed that the higher the peak troponin value, the greater the risk of death and the shorter the median time until death.11
A meta-analysis of 14 studies in 3,139 patients found that elevated troponin after noncardiac surgery was an independent predictor of death within 1 year (odds ratio [OR] 6.7, 95% confidence interval [CI] 4.1–10.9) and beyond 1 year (OR 1.8, 95% CI 1.4–2.3).37
SHOULD SCREENING BE ROUTINE AFTER NONCARDIAC SURGERY?
Since patients suffering myocardial infarction or injury after noncardiac surgery have a worse prognosis, many experts advocate routinely screening all high-risk patients and those undergoing moderate- to high-risk surgery. Many tools exist to determine which patients undergoing noncardiac surgery are at high risk of cardiac complications.
The revised Goldman Cardiac Risk Index is commonly used and well validated. Variables in this index that predict major cardiac complications are:
- High-risk surgery (vascular surgery, orthopedic surgery, and intraperitoneal or intrathoracic surgery)
- History of ischemic heart disease
- History of congestive heart failure
- History of cerebrovascular disease
- Diabetes requiring insulin therapy
- Chronic kidney disease with a creatinine > 2.0 mg/dL.
The more of these variables that are present, the higher the risk of perioperative cardiac events2,4:
- No risk factors: 0.4% risk (95% CI 0.1–0.8)
- One risk factor: 1.0% risk (95% CI 0.5–1.4)
- Two risk factors: 2.4% risk (95% CI 1.3–3.5)
- Three or more risk factors: 5.4% risk (95% CI 2.7–7.9).
Current guidelines from the American College of Cardiology and the American Heart Association give a class I recommendation (the highest) for measuring troponin levels after noncardiac surgery in patients who have symptoms or signs suggesting myocardial ischemia. They give a class IIb recommendation (usefulness is less well established) for screening those at high risk but without symptoms or signs of ischemia, despite the previously cited evidence that patients with troponin elevation are at increased risk. The IIb recommendation is due to a lack of validated treatment strategies to modify and attenuate the recognized risk with troponin elevation in this setting.39
LITTLE EVIDENCE TO GUIDE TREATMENT
In current practice, internists and cardiologists are often asked to consult on patients with troponin elevations noted after noncardiac surgery. Although published and ongoing studies examine strategies to prevent cardiovascular events during noncardiac surgery, we lack data on managing the cases of myocardial infarction and injury that actually occur after noncardiac surgery.
When managing a patient who has a troponin elevation after surgery, many clinical factors must be weighed, including hemodynamic and clinical stability and risk of bleeding. Confronted with ST-segment elevation myocardial infarction or high-risk non–ST-segment elevation myocardial infarction, most clinicians would favor an early invasive reperfusion strategy in accordance with guidelines on managing acute coronary syndrome. Fibrinolytic drugs for ST-segment elevation myocardial infarction are likely to be contraindicated in the postoperative period because they pose an unacceptable risk of bleeding.
Guideline-directed medical therapies for those suffering perioperative myocardial infarction may lower the risk of future cardiovascular events, as suggested by a retrospective study of 66 patients diagnosed with perioperative myocardial infarction after vascular surgery.40 Those in whom medical therapy for coronary artery disease was not intensified—defined as adding or increasing the dose of antiplatelet agent, statin, beta-blocker, or angiotensin-converting enzyme inhibitor—had higher rates of cardiovascular events at 12 months (hazard ratio [HR] 2.80, 95% CI 1.05–24.2).40
In those with asymptomatic myocardial infarction or isolated elevation in cardiac biomarkers, no treatment strategies have been assessed prospectively or in randomized trials. However, statins and aspirin have been suggested as providing some benefit. In a substudy of the POISE trial, the use of aspirin was associated with a 46% reduction in the 30-day mortality rate in those suffering a perioperative myocardial infarction, and statins were associated with a 76% reduction.10 In a single-center retrospective analysis of 337 patients undergoing moderate- to high-risk vascular surgery, statin therapy was associated with a lower 1-year mortality rate (OR 0.63, 95% CI 0.40–0.98).38
We propose a treatment algorithm for patients identified as having cardiovascular events after noncardiac surgery (Figure 2), based on current evidence and guidelines. Ultimately, treatment decisions should be tailored to the individual patient. Discussion of the risks and benefits of therapeutic options should include the patient and surgeon.
Ongoing and future trials
Ongoing and future trials are aimed at addressing definitive treatment strategies in this patient population.
The MANAGE trial (Management of Myocardial Injury After Non-cardiac Surgery Trial) is randomizing patients suffering myocardial injury after noncardiac surgery to receive either dabigatran and omeprazole or placebo to assess the efficacy of these agents in preventing major adverse cardiac events and the safety of anticoagulation (ClinicalTrials.gov Identifier: NCT01661101).
The INTREPID trial (Study of Ticagrelor Versus Aspirin Treatment in Patients With Myocardial Injury Post Major Non-Cardiac Surgery) will assess the efficacy and safety of ticagrelor treatment compared with aspirin in a similar population (ClinicalTrial.gov Identifier: NCT02291419). The trial will enroll approximately 1,000 patients identified as having a postoperative troponin elevation more than two times the upper limit of normal of the assay during the index hospitalization (Figure 3). Enrollment was to have begun in mid-2015.
More than 200 million patients undergo noncardiac surgery each year, and the volume is increasing.1 Cardiovascular complications are a major cause of morbidity and mortality in the perioperative period.
Before the advent of modern cardiac biomarkers, an estimated 2% to 3% of all patients undergoing noncardiac surgery had a major adverse cardiac event.2 However, more recent studies suggest that 5% to 25% of patients have troponin elevations after noncardiac surgery, depending on the patient population,3–6 and many are asymptomatic, suggesting that many patients are sustaining undetected myocardial injury. Those who suffer a myocardial infarction or myocardial injury have elevated morbidity and mortality rates, not only perioperatively, but also at 30 days and even at up to 1 year.3–5,7–11
Yet there are almost no data on how best to manage these patients; the available guidelines, therefore, do not provide sufficient recommendations for clinical practice.
To address the lack of guidelines, we examine the incidence and proposed mechanisms of myocardial injury after noncardiac surgery, suggest an approach to identifying patients at risk, recommend treatment strategies, and consider future directions.
CARDIAC BIOMARKERS
When cardiac cellular injury from ischemia, direct trauma, or other cause disrupts the cell membrane, intracellular contents enter the extracellular space, including the blood stream. If the myocyte damage is extensive enough, biochemical assays can detect these substances.
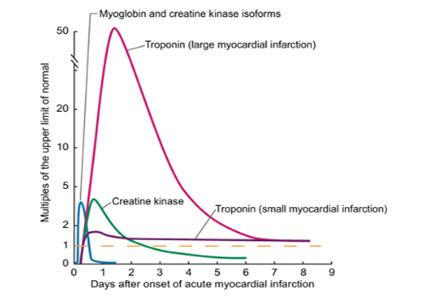
Troponin, creatine kinase, myoglobin, and lactate dehydrogenase are common biomarkers of necrosis that, when detected in the plasma, may indicate cardiac injury. Each can be detected at varying times after cardiac injury (Figure 1).12
Cardiac troponins I and T
Of the biomarkers, cardiac troponin I and cardiac troponin T are now the most widely used and are the most specific for myocyte injury.
Troponins are proteins that regulate the calcium-induced interaction between myosin and actin that results in muscle contraction. Troponin is a complex consisting of three subunits: troponin C, troponin I, and troponin T. The cardiac troponin I and T isoforms are distinct from those found in skeletal muscle, making them specific for myocyte injury, and they are currently the recommended markers for diagnosing acute myocardial infarction.13
The troponin immunoassays currently available are not standardized among laboratories and point-of-care methods, and thus, levels cannot be compared across testing centers.14 Each assay has unique performance characteristics, but guidelines recommend using the 99th percentile value from a normal reference population for a given assay to define whether myocardial injury is present.13
Troponin elevation has prognostic value in patients presenting with acute coronary syndromes,15–18 and the degree of elevation correlates with infarct size.19–21
Controversy exists as to whether troponin and other biomarkers are released only after myocardial necrosis or after reversible injury as well. Using newer, highly sensitive assays, troponin elevations have been detected after short periods of ischemia during stress testing22,23 and in patients with stable angina,24 suggesting that reversible cardiac stress and injury can lead to troponin release. This mechanism may play an important role during the myocardial injury that can occur in patients undergoing noncardiac surgery.
MYOCARDIAL INFARCTION vs MYOCARDIAL INJURY
In 2000, the Joint Task Force of the European Society of Cardiology, American College of Cardiology Foundation, American Heart Association, and World Heart Federation revised the criteria for the diagnosis of myocardial infarction created by the World Health Organization in 1979. The definition was revised again in 2007 and once more in 2012 to create the third universal definition of myocardial infarction.
Acute myocardial infarction
Acute myocardial infarction is defined as evidence of myocardial necrosis in a setting of myocardial ischemia, not related to causes such as trauma or pulmonary embolism, with a rise or a fall (or a rise and a fall) of cardiac biomarkers (at least one value being above the 99th percentile in the reference population) and any of the following:
- Symptoms of ischemia
- New ST-segment changes or new left bundle branch block
- Pathologic Q waves
- Imaging evidence of new loss of viable myocardium or new regional wall-motion abnormality
- Intracoronary thrombus by angiography or autopsy.13
Myocardial injury after noncardiac surgery
Studies10,11 have shown that many patients undergoing noncardiac surgery have evidence of cardiac biomarker release but do not meet the universal definition of myocardial infarction.
The Perioperative Ischemic Evaluation (POISE) trial10 reported that 415 (5%) of its patients met the definition of myocardial infarction, of whom only about 35% had symptoms of ischemia. Another 697 patients (8.3%) had isolated elevations in biomarkers without meeting the definition of myocardial infarction.
The VISION study11 (Vascular Events in Noncardiac Surgery Patients Cohort Evaluation) prospectively screened more than 15,000 patients in several countries for troponin elevation during the first 3 postoperative days and for ischemic symptoms and features. Of the patients screened, approximately 1,200 (8%) had troponin elevations, with fewer than half fulfilling the criteria for myocardial infarction.
In another study, van Waes et al6 prospectively screened 2,232 patients ages 60 and older undergoing intermediate- to high-risk noncardiac surgery. Troponin levels were elevated in 19% of the patients, but only 10 of these patients met the universal definition of myocardial infarction.
In all of these studies, patients with isolated elevation in myocardial biomarkers had worse short-term and long-term outcomes than those without. These observations led to a proposed definition of “myocardial injury after noncardiac surgery” that is broader than that of myocardial infarction and requires only elevation of cardiac biomarkers judged to be due to myocardial ischemia (ie, not from another obvious cause such as pulmonary embolism or myocarditis).3
FIVE TYPES OF MYOCARDIAL INFARCTION
The Joint Task Force13 categorizes myocardial infarction into five distinct types:
- Type 1—due to plaque rupture
- Type 2—due to imbalance between oxygen supply and demand
- Type 3—sudden cardiac death
- Type 4a—associated with percutaneous coronary intervention
- Type 4b—associated with stent thrombosis
- Type 5—associated with coronary artery bypass surgery.
Types 1 and 2 have both been implicated in perioperative myocardial infarction and injury. Patient characteristics and the physiologic response to surgical and anesthetic stressors likely contribute to the development of myocardial infarction and injury after noncardiac surgery.
Plaque rupture as a cause of postoperative myocardial infarction
The mechanism of type 1 myocardial infarction—plaque rupture or erosion leading to thrombosis and infarction—plays a significant role in most cases of acute coronary syndromes. Its role in perioperative and postoperative myocardial infarction or injury, however, is less clear.
In an autopsy study of 26 patients who died of myocardial infarction after noncardiac surgery, plaque rupture was evident in 12 (46%).25 A prospective angiographic study of 120 patients with acute coronary syndromes after noncardiac surgery found that nearly 50% had evidence of plaque rupture.26
Higher levels of catecholamines, cortisol,27,28 platelet reactivity,29 procoagulant factors,30 and coronary artery shear stress31 are all present in the postoperative period and may contribute to an increased propensity for plaque rupture or erosion. Whether plaque rupture is present in patients who have isolated troponin elevation but do not meet the criteria for myocardial infarction has not been investigated.
Oxygen supply-demand imbalance during and after surgery
Oxygen supply-demand imbalance (the mechanism in type 2 myocardial infarction) leading to myocyte stress, ischemia, and subsequent infarction is likely common in the perioperative and postoperative periods. As previously discussed, this imbalance may be present with or without symptoms.
Oxygen demand may increase in this period as a result of tachycardia32 caused by bleeding, pain, and catecholamines or increased wall stress from hypertension due to vasoconstriction or pain.33 Oxygen supply can be decreased secondary to tachycardia, anemia,34 hypotension, hypoxemia, hypercarbia, intravascular fluid shifts (bleeding or volume overload), or coronary vasoconstriction.33,35
These mechanisms of myocardial injury, infarction, or both can occur with or without underlying significant obstructive coronary artery disease. However, severe coronary artery disease is more common in those who have had a perioperative myocardial infarction.36
POSTOPERATIVE TROPONIN ELEVATION CARRIES A WORSE PROGNOSIS
Patients who suffer a myocardial infarction after noncardiac surgery have worse short- and long-term outcomes than their counterparts.4,5,7, 8,10,33 In the POISE trial,10 the 30-day mortality rate was 11.6% in those who had had a perioperative myocardial infarction, compared with 2.2% in those who did not (P < .001). The patients who had had a myocardial infarction were also more likely to have nonfatal cardiac arrest, coronary revascularization, and congestive heart failure.
Myocardial injury not fulfilling the criteria for myocardial infarction after noncardiac surgery is also associated with worse short-term and long-term outcomes.3,6,10,11,37,38 POISE patients with isolated elevations in cardiac biomarkers had a higher 30-day risk of coronary revascularization and nonfatal arrest.10 In the VISION trial, an elevation in troponin was the strongest predictor of death within 30 days after noncardiac surgery. This analysis also showed that the higher the peak troponin value, the greater the risk of death and the shorter the median time until death.11
A meta-analysis of 14 studies in 3,139 patients found that elevated troponin after noncardiac surgery was an independent predictor of death within 1 year (odds ratio [OR] 6.7, 95% confidence interval [CI] 4.1–10.9) and beyond 1 year (OR 1.8, 95% CI 1.4–2.3).37
SHOULD SCREENING BE ROUTINE AFTER NONCARDIAC SURGERY?
Since patients suffering myocardial infarction or injury after noncardiac surgery have a worse prognosis, many experts advocate routinely screening all high-risk patients and those undergoing moderate- to high-risk surgery. Many tools exist to determine which patients undergoing noncardiac surgery are at high risk of cardiac complications.
The revised Goldman Cardiac Risk Index is commonly used and well validated. Variables in this index that predict major cardiac complications are:
- High-risk surgery (vascular surgery, orthopedic surgery, and intraperitoneal or intrathoracic surgery)
- History of ischemic heart disease
- History of congestive heart failure
- History of cerebrovascular disease
- Diabetes requiring insulin therapy
- Chronic kidney disease with a creatinine > 2.0 mg/dL.
The more of these variables that are present, the higher the risk of perioperative cardiac events2,4:
- No risk factors: 0.4% risk (95% CI 0.1–0.8)
- One risk factor: 1.0% risk (95% CI 0.5–1.4)
- Two risk factors: 2.4% risk (95% CI 1.3–3.5)
- Three or more risk factors: 5.4% risk (95% CI 2.7–7.9).
Current guidelines from the American College of Cardiology and the American Heart Association give a class I recommendation (the highest) for measuring troponin levels after noncardiac surgery in patients who have symptoms or signs suggesting myocardial ischemia. They give a class IIb recommendation (usefulness is less well established) for screening those at high risk but without symptoms or signs of ischemia, despite the previously cited evidence that patients with troponin elevation are at increased risk. The IIb recommendation is due to a lack of validated treatment strategies to modify and attenuate the recognized risk with troponin elevation in this setting.39
LITTLE EVIDENCE TO GUIDE TREATMENT
In current practice, internists and cardiologists are often asked to consult on patients with troponin elevations noted after noncardiac surgery. Although published and ongoing studies examine strategies to prevent cardiovascular events during noncardiac surgery, we lack data on managing the cases of myocardial infarction and injury that actually occur after noncardiac surgery.
When managing a patient who has a troponin elevation after surgery, many clinical factors must be weighed, including hemodynamic and clinical stability and risk of bleeding. Confronted with ST-segment elevation myocardial infarction or high-risk non–ST-segment elevation myocardial infarction, most clinicians would favor an early invasive reperfusion strategy in accordance with guidelines on managing acute coronary syndrome. Fibrinolytic drugs for ST-segment elevation myocardial infarction are likely to be contraindicated in the postoperative period because they pose an unacceptable risk of bleeding.
Guideline-directed medical therapies for those suffering perioperative myocardial infarction may lower the risk of future cardiovascular events, as suggested by a retrospective study of 66 patients diagnosed with perioperative myocardial infarction after vascular surgery.40 Those in whom medical therapy for coronary artery disease was not intensified—defined as adding or increasing the dose of antiplatelet agent, statin, beta-blocker, or angiotensin-converting enzyme inhibitor—had higher rates of cardiovascular events at 12 months (hazard ratio [HR] 2.80, 95% CI 1.05–24.2).40
In those with asymptomatic myocardial infarction or isolated elevation in cardiac biomarkers, no treatment strategies have been assessed prospectively or in randomized trials. However, statins and aspirin have been suggested as providing some benefit. In a substudy of the POISE trial, the use of aspirin was associated with a 46% reduction in the 30-day mortality rate in those suffering a perioperative myocardial infarction, and statins were associated with a 76% reduction.10 In a single-center retrospective analysis of 337 patients undergoing moderate- to high-risk vascular surgery, statin therapy was associated with a lower 1-year mortality rate (OR 0.63, 95% CI 0.40–0.98).38
We propose a treatment algorithm for patients identified as having cardiovascular events after noncardiac surgery (Figure 2), based on current evidence and guidelines. Ultimately, treatment decisions should be tailored to the individual patient. Discussion of the risks and benefits of therapeutic options should include the patient and surgeon.
Ongoing and future trials
Ongoing and future trials are aimed at addressing definitive treatment strategies in this patient population.
The MANAGE trial (Management of Myocardial Injury After Non-cardiac Surgery Trial) is randomizing patients suffering myocardial injury after noncardiac surgery to receive either dabigatran and omeprazole or placebo to assess the efficacy of these agents in preventing major adverse cardiac events and the safety of anticoagulation (ClinicalTrials.gov Identifier: NCT01661101).
The INTREPID trial (Study of Ticagrelor Versus Aspirin Treatment in Patients With Myocardial Injury Post Major Non-Cardiac Surgery) will assess the efficacy and safety of ticagrelor treatment compared with aspirin in a similar population (ClinicalTrial.gov Identifier: NCT02291419). The trial will enroll approximately 1,000 patients identified as having a postoperative troponin elevation more than two times the upper limit of normal of the assay during the index hospitalization (Figure 3). Enrollment was to have begun in mid-2015.
- Weiser TG, Regenbogen SE, Thompson KD, et al. An estimation of the global volume of surgery: a modelling strategy based on available data. Lancet 2008; 372:139–144.
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100:1043–1049.
- Botto F, Alonso-Coello P, Chan MT, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology 2014; 120:564–578.
- Devereaux PJ, Goldman L, Cook DJ, Gilbert K, Leslie K, Guyatt GH. Perioperative cardiac events in patients undergoing noncardiac surgery: a review of the magnitude of the problem, the pathophysiology of the events and methods to estimate and communicate risk. CMAJ 2005; 173:627–634.
- McFalls EO, Ward HB, Moritz TE, et al. Predictors and outcomes of a perioperative myocardial infarction following elective vascular surgery in patients with documented coronary artery disease: results of the CARP trial. Eur Heart J 2008; 29:394–401.
- van Waes JA, Nathoe HM, de Graaff JC, et al. Myocardial injury after noncardiac surgery and its association with short-term mortality. Circulation 2013; 127:2264–2271.
- Badner NH, Knill RL, Brown JE, Novick TV, Gelb AW. Myocardial infarction after noncardiac surgery. Anesthesiology 1998; 88:572–578.
- Kim LJ, Martinez EA, Faraday N, et al. Cardiac troponin I predicts short-term mortality in vascular surgery patients. Circulation 2002; 106:2366–2371.
- Landesberg G, Shatz V, Akopnik I, et al. Association of cardiac troponin, CK-MB, and postoperative myocardial ischemia with long-term survival after major vascular surgery. J Am Coll Cardiol 2003; 42:1547–1554.
- Devereaux PJ, Xavier D, Pogue J, et al. Characteristics and short-term prognosis of perioperative myocardial infarction in patients undergoing noncardiac surgery: a cohort study. Ann Intern Med 2011; 154:523–528.
- Devereaux PJ, Chan MT, Alonso-Coello P, et al. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA 2012; 307:2295–2304.
- Kumar A, Cannon CP. Acute coronary syndromes: diagnosis and management, part I. Mayo Clin Proc 2009; 84:917–938.
- Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. Circulation 2012; 126:2020–2035.
- Apple FS, Quist HE, Doyle PJ, Otto AP, Murakami MM. Plasma 99th percentile reference limits for cardiac troponin and creatine kinase MB mass for use with European Society of Cardiology/American College of Cardiology consensus recommendations. Clin Chem 2003; 49:1331–1336.
- Ottani F, Galvani M, Nicolini FA, et al. Elevated cardiac troponin levels predict the risk of adverse outcome in patients with acute coronary syndromes. Am Heart J 2000; 140:917–927.
- Ohman EM, Armstrong PW, White HD, et al. Risk stratification with a point-of-care cardiac troponin T test in acute myocardial infarction. GUSTO III investigators. Global Use of Strategies to Open Occluded Coronary Arteries. Am J Cardiol 1999; 84:1281–1286.
- deFilippi CR, Tocchi M, Parmar RJ, et al. Cardiac troponin T in chest pain unit patients without ischemic electrocardiographic changes: angiographic correlates and long-term clinical outcomes. J Am Coll Cardiol 2000; 35:1827–1834.
- Heidenreich PA, Alloggiamento T, Melsop K, McDonald KM, Go AS, Hlatky MA. The prognostic value of troponin in patients with non-ST elevation acute coronary syndromes: a meta-analysis. J Am Coll Cardiol 2001; 38:478–485.
- Steen H, Giannitsis E, Futterer S, Merten C, Juenger C, Katus HA. Cardiac troponin T at 96 hours after acute myocardial infarction correlates with infarct size and cardiac function. J Am Coll Cardiol 2006; 48:2192–2194.
- Licka M, Zimmermann R, Zehelein J, Dengler TJ, Katus HA, Kubler W. Troponin T concentrations 72 hours after myocardial infarction as a serological estimate of infarct size. Heart 2002; 87:520–524.
- Vasile VC, Babuin L, Giannitsis E, Katus HA, Jaffe AS. Relationship of MRI-determined infarct size and cTnI measurements in patients with ST-elevation myocardial infarction. Clin Chem 2008; 54:617–619.
- Sabatine MS, Morrow DA, de Lemos JA, Jarolim P, Braunwald E. Detection of acute changes in circulating troponin in the setting of transient stress test-induced myocardial ischaemia using an ultrasensitive assay: results from TIMI 35. Eur Heart J 2009; 30:162–169.
- Siriwardena M, Campbell V, Richards AM, Pemberton CJ. Cardiac biomarker responses to dobutamine stress echocardiography in healthy volunteers and patients with coronary artery disease. Clin Chem 2012; 58:1492–1494.
- Turer AT, Addo TA, Martin JL, et al. Myocardial ischemia induced by rapid atrial pacing causes troponin T release detectable by a highly sensitive assay: insights from a coronary sinus sampling study. J Am Coll Cardiol 2011; 57:2398–2405.
- Cohen MC, Aretz TH. Histological analysis of coronary artery lesions in fatal postoperative myocardial infarction. Cardiovasc Pathol 1999; 8:133–139.
- Gualandro DM, Campos CA, Calderaro D, et al. Coronary plaque rupture in patients with myocardial infarction after noncardiac surgery: frequent and dangerous. Atherosclerosis 2012; 222:191–195.
- Sametz W, Metzler H, Gries M, et al. Perioperative catecholamine changes in cardiac risk patients. Eur J Clin Invest 1999; 29:582–587.
- Frank SM, Higgins MS, Breslow MJ, et al. The catecholamine, cortisol, and hemodynamic responses to mild perioperative hypothermia. A randomized clinical trial. Anesthesiology 1995; 82:83–93.
- Rosenfeld BA, Faraday N, Campbell D, et al. Perioperative platelet reactivity and the effects of clonidine. Anesthesiology 1993; 79:255–261.
- Lison S, Weiss G, Spannagl M, Heindl B. Postoperative changes in procoagulant factors after major surgery. Blood Coagul Fibrinolysis 2011; 22:190–196.
- Fukumoto Y, Hiro T, Fujii T, et al. Localized elevation of shear stress is related to coronary plaque rupture: a 3-dimensional intravascular ultrasound study with in-vivo color mapping of shear stress distribution. J Am Coll Cardiol 2008; 51:645–650.
- Feringa HH, Bax JJ, Boersma E, et al. High-dose beta-blockers and tight heart rate control reduce myocardial ischemia and troponin T release in vascular surgery patients. Circulation 2006; 114:I-344–I-349.
- Landesberg G. The pathophysiology of perioperative myocardial infarction: facts and perspectives. J Cardiothorac Vasc Anesth 2003; 17:90–100.
- Nelson AH, Fleisher LA, Rosenbaum SH. Relationship between postoperative anemia and cardiac morbidity in high-risk vascular patients in the intensive care unit. Crit Care Med 1993; 21:860–866.
- Landesberg G, Beattie WS, Mosseri M, Jaffe AS, Alpert JS. Perioperative myocardial infarction. Circulation 2009; 119:2936–2944.
- Ellis SG, Hertzer NR, Young JR, Brener S. Angiographic correlates of cardiac death and myocardial infarction complicating major nonthoracic vascular surgery. Am J Cardiol 1996; 77:1126–1128.
- Levy M, Heels-Ansdell D, Hiralal R, et al. Prognostic value of troponin and creatine kinase muscle and brain isoenzyme measurement after noncardiac surgery: a systematic review and meta-analysis. Anesthesiology 2011; 114:796–806.
- Garcia S, Marston N, Sandoval Y, et al. Prognostic value of 12-lead electrocardiogram and peak troponin I level after vascular surgery. J Vasc Surg 2013; 57:166–172.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. J Am Coll Cardiol 2014; 64:e77–e137.
- Foucrier A, Rodseth R, Aissaoui M, et al. The long-term impact of early cardiovascular therapy intensification for postoperative troponin elevation after major vascular surgery. Anesth Analg 2014; 119:1053–1063.
- Weiser TG, Regenbogen SE, Thompson KD, et al. An estimation of the global volume of surgery: a modelling strategy based on available data. Lancet 2008; 372:139–144.
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100:1043–1049.
- Botto F, Alonso-Coello P, Chan MT, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology 2014; 120:564–578.
- Devereaux PJ, Goldman L, Cook DJ, Gilbert K, Leslie K, Guyatt GH. Perioperative cardiac events in patients undergoing noncardiac surgery: a review of the magnitude of the problem, the pathophysiology of the events and methods to estimate and communicate risk. CMAJ 2005; 173:627–634.
- McFalls EO, Ward HB, Moritz TE, et al. Predictors and outcomes of a perioperative myocardial infarction following elective vascular surgery in patients with documented coronary artery disease: results of the CARP trial. Eur Heart J 2008; 29:394–401.
- van Waes JA, Nathoe HM, de Graaff JC, et al. Myocardial injury after noncardiac surgery and its association with short-term mortality. Circulation 2013; 127:2264–2271.
- Badner NH, Knill RL, Brown JE, Novick TV, Gelb AW. Myocardial infarction after noncardiac surgery. Anesthesiology 1998; 88:572–578.
- Kim LJ, Martinez EA, Faraday N, et al. Cardiac troponin I predicts short-term mortality in vascular surgery patients. Circulation 2002; 106:2366–2371.
- Landesberg G, Shatz V, Akopnik I, et al. Association of cardiac troponin, CK-MB, and postoperative myocardial ischemia with long-term survival after major vascular surgery. J Am Coll Cardiol 2003; 42:1547–1554.
- Devereaux PJ, Xavier D, Pogue J, et al. Characteristics and short-term prognosis of perioperative myocardial infarction in patients undergoing noncardiac surgery: a cohort study. Ann Intern Med 2011; 154:523–528.
- Devereaux PJ, Chan MT, Alonso-Coello P, et al. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA 2012; 307:2295–2304.
- Kumar A, Cannon CP. Acute coronary syndromes: diagnosis and management, part I. Mayo Clin Proc 2009; 84:917–938.
- Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. Circulation 2012; 126:2020–2035.
- Apple FS, Quist HE, Doyle PJ, Otto AP, Murakami MM. Plasma 99th percentile reference limits for cardiac troponin and creatine kinase MB mass for use with European Society of Cardiology/American College of Cardiology consensus recommendations. Clin Chem 2003; 49:1331–1336.
- Ottani F, Galvani M, Nicolini FA, et al. Elevated cardiac troponin levels predict the risk of adverse outcome in patients with acute coronary syndromes. Am Heart J 2000; 140:917–927.
- Ohman EM, Armstrong PW, White HD, et al. Risk stratification with a point-of-care cardiac troponin T test in acute myocardial infarction. GUSTO III investigators. Global Use of Strategies to Open Occluded Coronary Arteries. Am J Cardiol 1999; 84:1281–1286.
- deFilippi CR, Tocchi M, Parmar RJ, et al. Cardiac troponin T in chest pain unit patients without ischemic electrocardiographic changes: angiographic correlates and long-term clinical outcomes. J Am Coll Cardiol 2000; 35:1827–1834.
- Heidenreich PA, Alloggiamento T, Melsop K, McDonald KM, Go AS, Hlatky MA. The prognostic value of troponin in patients with non-ST elevation acute coronary syndromes: a meta-analysis. J Am Coll Cardiol 2001; 38:478–485.
- Steen H, Giannitsis E, Futterer S, Merten C, Juenger C, Katus HA. Cardiac troponin T at 96 hours after acute myocardial infarction correlates with infarct size and cardiac function. J Am Coll Cardiol 2006; 48:2192–2194.
- Licka M, Zimmermann R, Zehelein J, Dengler TJ, Katus HA, Kubler W. Troponin T concentrations 72 hours after myocardial infarction as a serological estimate of infarct size. Heart 2002; 87:520–524.
- Vasile VC, Babuin L, Giannitsis E, Katus HA, Jaffe AS. Relationship of MRI-determined infarct size and cTnI measurements in patients with ST-elevation myocardial infarction. Clin Chem 2008; 54:617–619.
- Sabatine MS, Morrow DA, de Lemos JA, Jarolim P, Braunwald E. Detection of acute changes in circulating troponin in the setting of transient stress test-induced myocardial ischaemia using an ultrasensitive assay: results from TIMI 35. Eur Heart J 2009; 30:162–169.
- Siriwardena M, Campbell V, Richards AM, Pemberton CJ. Cardiac biomarker responses to dobutamine stress echocardiography in healthy volunteers and patients with coronary artery disease. Clin Chem 2012; 58:1492–1494.
- Turer AT, Addo TA, Martin JL, et al. Myocardial ischemia induced by rapid atrial pacing causes troponin T release detectable by a highly sensitive assay: insights from a coronary sinus sampling study. J Am Coll Cardiol 2011; 57:2398–2405.
- Cohen MC, Aretz TH. Histological analysis of coronary artery lesions in fatal postoperative myocardial infarction. Cardiovasc Pathol 1999; 8:133–139.
- Gualandro DM, Campos CA, Calderaro D, et al. Coronary plaque rupture in patients with myocardial infarction after noncardiac surgery: frequent and dangerous. Atherosclerosis 2012; 222:191–195.
- Sametz W, Metzler H, Gries M, et al. Perioperative catecholamine changes in cardiac risk patients. Eur J Clin Invest 1999; 29:582–587.
- Frank SM, Higgins MS, Breslow MJ, et al. The catecholamine, cortisol, and hemodynamic responses to mild perioperative hypothermia. A randomized clinical trial. Anesthesiology 1995; 82:83–93.
- Rosenfeld BA, Faraday N, Campbell D, et al. Perioperative platelet reactivity and the effects of clonidine. Anesthesiology 1993; 79:255–261.
- Lison S, Weiss G, Spannagl M, Heindl B. Postoperative changes in procoagulant factors after major surgery. Blood Coagul Fibrinolysis 2011; 22:190–196.
- Fukumoto Y, Hiro T, Fujii T, et al. Localized elevation of shear stress is related to coronary plaque rupture: a 3-dimensional intravascular ultrasound study with in-vivo color mapping of shear stress distribution. J Am Coll Cardiol 2008; 51:645–650.
- Feringa HH, Bax JJ, Boersma E, et al. High-dose beta-blockers and tight heart rate control reduce myocardial ischemia and troponin T release in vascular surgery patients. Circulation 2006; 114:I-344–I-349.
- Landesberg G. The pathophysiology of perioperative myocardial infarction: facts and perspectives. J Cardiothorac Vasc Anesth 2003; 17:90–100.
- Nelson AH, Fleisher LA, Rosenbaum SH. Relationship between postoperative anemia and cardiac morbidity in high-risk vascular patients in the intensive care unit. Crit Care Med 1993; 21:860–866.
- Landesberg G, Beattie WS, Mosseri M, Jaffe AS, Alpert JS. Perioperative myocardial infarction. Circulation 2009; 119:2936–2944.
- Ellis SG, Hertzer NR, Young JR, Brener S. Angiographic correlates of cardiac death and myocardial infarction complicating major nonthoracic vascular surgery. Am J Cardiol 1996; 77:1126–1128.
- Levy M, Heels-Ansdell D, Hiralal R, et al. Prognostic value of troponin and creatine kinase muscle and brain isoenzyme measurement after noncardiac surgery: a systematic review and meta-analysis. Anesthesiology 2011; 114:796–806.
- Garcia S, Marston N, Sandoval Y, et al. Prognostic value of 12-lead electrocardiogram and peak troponin I level after vascular surgery. J Vasc Surg 2013; 57:166–172.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. J Am Coll Cardiol 2014; 64:e77–e137.
- Foucrier A, Rodseth R, Aissaoui M, et al. The long-term impact of early cardiovascular therapy intensification for postoperative troponin elevation after major vascular surgery. Anesth Analg 2014; 119:1053–1063.
KEY POINTS
- Cardiovascular events are a major cause of morbidity and mortality in patients undergoing noncardiac surgery and occur frequently, especially in high-risk patients.
- Myocardial injury or infarction after noncardiac surgery heightens the short- and long-term risk of mortality and major adverse cardiac events.
- The dominant mechanism of myocardial injury after noncardiac surgery remains uncertain.
- In the absence of therapies proven to affect the outcome, the benefit of screening to identify these patients remains uncertain.
- Clinical trials are under way to help clinicians provide optimal care to this at-risk population.
Common Misconceptions of Seizures and Epilepsy
Click here for a partial list of common questions and assumptions that individuals who are newly diagnosed with epilepsy or seizures may have.
Click here for a partial list of common questions and assumptions that individuals who are newly diagnosed with epilepsy or seizures may have.
Click here for a partial list of common questions and assumptions that individuals who are newly diagnosed with epilepsy or seizures may have.
ESC: Aldosterone blockade fails to fly for early MI in ALBATROSS
LONDON – Aldosterone blockade with oral spironolactone showed a disappointing lack of clinical benefit when initiated in the first hours after an acute MI without heart failure in the large, randomized ALBATROSS trial.
ALBATROSS did, however, flash a silver lining under one wing: A whopping 80% reduction in 6-month mortality in a prespecified subgroup analysis restricted to the 1,229 participants with ST-elevation MI, Dr. Gilles Montalescot reported at the annual congress of the European Society of Cardiology.
Although this finding is intriguing, hypothesis-generating, and definitely warrants a confirmatory study, he continued, mortality was nevertheless merely a secondary endpoint in ALBATROSS (Aldosterone Lethal Effects Blockade in Acute Myocardial Infarction Treated With or Without Reperfusion to Improve Outcome and Survival at Six Months Follow-up).
In contrast, the primary composite outcome was negative, so the takeaway message is clear: “The results of the ALBATROSS study do not warrant the extension of aldosterone blockade to MI patients without heart failure,” said Dr. Montalescot, professor of cardiology at the University of Paris.
ALBATROSS was a multicenter French trial that randomly assigned 1,603 acute MI patients to standard therapy alone or with added mineralocorticoid antagonist therapy started within the first 2 days of their coronary event. Often the aldosterone antagonist was begun in the ambulance en route to the hospital.
The primary endpoint was a composite of death, resuscitated cardiac arrest, ventricular fibrillation or tachycardia, heart failure, or an indication for an implantable cardioverter defibrillator. There were 194 such events, and they occurred at a similar rate in the patients who got 25 mg/day of spironolactone and those who did not.
The rationale for ALBATROSS was sound, according to the cardiologist. Aldosterone is a stress hormone released in acute MI. It has deleterious cardiac effects, including arrhythmias, heart failure, and a dose-dependent increase in mortality, so it makes good sense to block it as soon as possible in MI patients. In the EPHESUS trial, the aldosterone antagonist eplerenone, when started 3-14 days post MI in patients with early heart failure, significantly reduced mortality (N Engl J Med. 2003 Apr 3;348[14]:1309-2), with the bulk of the benefit occurring in patients in whom the drug was started 3-7 days post MI.
Last year, Dr. Montalescot and his coinvestigators published the REMINDER study, in which 1,012 ST-elevation MI (STEMI) patients without heart failure were randomized to eplerenone or placebo within the first 24 hours. The study showed a significant reduction in levels of brain natriuretic peptide or N-terminal pro-BNP in the eplerenone arm (Eur Heart J. 2014 Sep 7;35[34]:2295-302), but that’s not a clinical endpoint. ALBATROSS was the first study to look at the clinical impact of commencing mineralocorticoid antagonist therapy prior to day 3 post MI.
Discussant Dr. John McMurray, professor of cardiology at the University of Glasgow, said that ALBATROSS was simply underpowered and thus leaves unanswered the clinically important question of whether early initiation of aldosterone blockade post MI in patients without heart failure confers clinical benefit. The investigators projected a total of 269 events in the composite endpoint but got only 194 because the study participants were so well treated and contemporary medical and interventional therapies are quite effective.
He dismissed the sharp reduction seen in 6-month mortality with spironolactone in the STEMI patients as “just implausible – we don’t know of any treatments in medicine that reduce mortality by 80%.”
Noting that there were only 28 deaths in the study, Dr. McMurray asserted that “a subgroup analysis on such a small number of events is never going to give you a reliable result.” Moreover, he added, “subgroup analysis is even more treacherous when the overall trial is underpowered.”
Dr. Montalescot replied that, while he considers the signal of a mortality benefit for aldosterone blockade in STEMI patients worthy of pursuit in a large randomized trial, the prospects for mounting such a study are poor. The medications are now available as generics, so there is no commercial incentive. The French Ministry of Health, which funded ALBATROSS, isn’t prepared to back a follow-up study. The best hope is that eventually one of the pharmaceutical companies developing third-generation aldosterone antagonists, now in phase II studies, will become interested, he said.
Dr. Montalescot said that, while he receives research grants and consulting fees from numerous pharmaceutical companies, these commercial relationships aren’t relevant to the government-funded ALBATROSS trial.
LONDON – Aldosterone blockade with oral spironolactone showed a disappointing lack of clinical benefit when initiated in the first hours after an acute MI without heart failure in the large, randomized ALBATROSS trial.
ALBATROSS did, however, flash a silver lining under one wing: A whopping 80% reduction in 6-month mortality in a prespecified subgroup analysis restricted to the 1,229 participants with ST-elevation MI, Dr. Gilles Montalescot reported at the annual congress of the European Society of Cardiology.
Although this finding is intriguing, hypothesis-generating, and definitely warrants a confirmatory study, he continued, mortality was nevertheless merely a secondary endpoint in ALBATROSS (Aldosterone Lethal Effects Blockade in Acute Myocardial Infarction Treated With or Without Reperfusion to Improve Outcome and Survival at Six Months Follow-up).
In contrast, the primary composite outcome was negative, so the takeaway message is clear: “The results of the ALBATROSS study do not warrant the extension of aldosterone blockade to MI patients without heart failure,” said Dr. Montalescot, professor of cardiology at the University of Paris.
ALBATROSS was a multicenter French trial that randomly assigned 1,603 acute MI patients to standard therapy alone or with added mineralocorticoid antagonist therapy started within the first 2 days of their coronary event. Often the aldosterone antagonist was begun in the ambulance en route to the hospital.
The primary endpoint was a composite of death, resuscitated cardiac arrest, ventricular fibrillation or tachycardia, heart failure, or an indication for an implantable cardioverter defibrillator. There were 194 such events, and they occurred at a similar rate in the patients who got 25 mg/day of spironolactone and those who did not.
The rationale for ALBATROSS was sound, according to the cardiologist. Aldosterone is a stress hormone released in acute MI. It has deleterious cardiac effects, including arrhythmias, heart failure, and a dose-dependent increase in mortality, so it makes good sense to block it as soon as possible in MI patients. In the EPHESUS trial, the aldosterone antagonist eplerenone, when started 3-14 days post MI in patients with early heart failure, significantly reduced mortality (N Engl J Med. 2003 Apr 3;348[14]:1309-2), with the bulk of the benefit occurring in patients in whom the drug was started 3-7 days post MI.
Last year, Dr. Montalescot and his coinvestigators published the REMINDER study, in which 1,012 ST-elevation MI (STEMI) patients without heart failure were randomized to eplerenone or placebo within the first 24 hours. The study showed a significant reduction in levels of brain natriuretic peptide or N-terminal pro-BNP in the eplerenone arm (Eur Heart J. 2014 Sep 7;35[34]:2295-302), but that’s not a clinical endpoint. ALBATROSS was the first study to look at the clinical impact of commencing mineralocorticoid antagonist therapy prior to day 3 post MI.
Discussant Dr. John McMurray, professor of cardiology at the University of Glasgow, said that ALBATROSS was simply underpowered and thus leaves unanswered the clinically important question of whether early initiation of aldosterone blockade post MI in patients without heart failure confers clinical benefit. The investigators projected a total of 269 events in the composite endpoint but got only 194 because the study participants were so well treated and contemporary medical and interventional therapies are quite effective.
He dismissed the sharp reduction seen in 6-month mortality with spironolactone in the STEMI patients as “just implausible – we don’t know of any treatments in medicine that reduce mortality by 80%.”
Noting that there were only 28 deaths in the study, Dr. McMurray asserted that “a subgroup analysis on such a small number of events is never going to give you a reliable result.” Moreover, he added, “subgroup analysis is even more treacherous when the overall trial is underpowered.”
Dr. Montalescot replied that, while he considers the signal of a mortality benefit for aldosterone blockade in STEMI patients worthy of pursuit in a large randomized trial, the prospects for mounting such a study are poor. The medications are now available as generics, so there is no commercial incentive. The French Ministry of Health, which funded ALBATROSS, isn’t prepared to back a follow-up study. The best hope is that eventually one of the pharmaceutical companies developing third-generation aldosterone antagonists, now in phase II studies, will become interested, he said.
Dr. Montalescot said that, while he receives research grants and consulting fees from numerous pharmaceutical companies, these commercial relationships aren’t relevant to the government-funded ALBATROSS trial.
LONDON – Aldosterone blockade with oral spironolactone showed a disappointing lack of clinical benefit when initiated in the first hours after an acute MI without heart failure in the large, randomized ALBATROSS trial.
ALBATROSS did, however, flash a silver lining under one wing: A whopping 80% reduction in 6-month mortality in a prespecified subgroup analysis restricted to the 1,229 participants with ST-elevation MI, Dr. Gilles Montalescot reported at the annual congress of the European Society of Cardiology.
Although this finding is intriguing, hypothesis-generating, and definitely warrants a confirmatory study, he continued, mortality was nevertheless merely a secondary endpoint in ALBATROSS (Aldosterone Lethal Effects Blockade in Acute Myocardial Infarction Treated With or Without Reperfusion to Improve Outcome and Survival at Six Months Follow-up).
In contrast, the primary composite outcome was negative, so the takeaway message is clear: “The results of the ALBATROSS study do not warrant the extension of aldosterone blockade to MI patients without heart failure,” said Dr. Montalescot, professor of cardiology at the University of Paris.
ALBATROSS was a multicenter French trial that randomly assigned 1,603 acute MI patients to standard therapy alone or with added mineralocorticoid antagonist therapy started within the first 2 days of their coronary event. Often the aldosterone antagonist was begun in the ambulance en route to the hospital.
The primary endpoint was a composite of death, resuscitated cardiac arrest, ventricular fibrillation or tachycardia, heart failure, or an indication for an implantable cardioverter defibrillator. There were 194 such events, and they occurred at a similar rate in the patients who got 25 mg/day of spironolactone and those who did not.
The rationale for ALBATROSS was sound, according to the cardiologist. Aldosterone is a stress hormone released in acute MI. It has deleterious cardiac effects, including arrhythmias, heart failure, and a dose-dependent increase in mortality, so it makes good sense to block it as soon as possible in MI patients. In the EPHESUS trial, the aldosterone antagonist eplerenone, when started 3-14 days post MI in patients with early heart failure, significantly reduced mortality (N Engl J Med. 2003 Apr 3;348[14]:1309-2), with the bulk of the benefit occurring in patients in whom the drug was started 3-7 days post MI.
Last year, Dr. Montalescot and his coinvestigators published the REMINDER study, in which 1,012 ST-elevation MI (STEMI) patients without heart failure were randomized to eplerenone or placebo within the first 24 hours. The study showed a significant reduction in levels of brain natriuretic peptide or N-terminal pro-BNP in the eplerenone arm (Eur Heart J. 2014 Sep 7;35[34]:2295-302), but that’s not a clinical endpoint. ALBATROSS was the first study to look at the clinical impact of commencing mineralocorticoid antagonist therapy prior to day 3 post MI.
Discussant Dr. John McMurray, professor of cardiology at the University of Glasgow, said that ALBATROSS was simply underpowered and thus leaves unanswered the clinically important question of whether early initiation of aldosterone blockade post MI in patients without heart failure confers clinical benefit. The investigators projected a total of 269 events in the composite endpoint but got only 194 because the study participants were so well treated and contemporary medical and interventional therapies are quite effective.
He dismissed the sharp reduction seen in 6-month mortality with spironolactone in the STEMI patients as “just implausible – we don’t know of any treatments in medicine that reduce mortality by 80%.”
Noting that there were only 28 deaths in the study, Dr. McMurray asserted that “a subgroup analysis on such a small number of events is never going to give you a reliable result.” Moreover, he added, “subgroup analysis is even more treacherous when the overall trial is underpowered.”
Dr. Montalescot replied that, while he considers the signal of a mortality benefit for aldosterone blockade in STEMI patients worthy of pursuit in a large randomized trial, the prospects for mounting such a study are poor. The medications are now available as generics, so there is no commercial incentive. The French Ministry of Health, which funded ALBATROSS, isn’t prepared to back a follow-up study. The best hope is that eventually one of the pharmaceutical companies developing third-generation aldosterone antagonists, now in phase II studies, will become interested, he said.
Dr. Montalescot said that, while he receives research grants and consulting fees from numerous pharmaceutical companies, these commercial relationships aren’t relevant to the government-funded ALBATROSS trial.
AT THE ESC CONGRESS 2015
Key clinical point: Giving aldosterone antagonists to acute MI patients without heart failure doesn’t improve clinical outcomes.
Major finding: The 6-month rate of a multipronged composite clinical endpoint was closely similar, regardless of whether patients with acute MI without heart failure were placed on spironolactone within the first couple of days post-MI.
Data source: ALBATROSS was an open-label, multicenter French study in which 1,603 patients were randomized to 6 months of aldosterone blockade or not within the first hours after an acute MI without heart failure.
Disclosures: The investigator-initiated ALBATROSS trial was funded by the French Ministry of Health.
Tips for Seniors With Epilepsy -- Avoiding Falls
Prolonged TV watching linked to fatal PE
LONDON—Results of a large study suggest that watching television for prolonged periods may increase a person’s risk of fatal pulmonary embolism (PE).
The study, which included more than 86,000 subjects, showed that watching an average of 5 or more hours of TV per day was associated with more than twice the risk of fatal PE as watching less than 2.5 hours daily.
And the risk was higher among younger subjects than older ones.
Toru Shirakawa, of Osaka University in Japan, presented this research at the ESC Congress 2015 (abstract P2686*).
“We showed that prolonged television viewing may be a risky behavior for death from pulmonary embolism,” Shirakawa said. “Leg immobility during television viewing may, in part, explain the finding. Public awareness of the risk of pulmonary embolism from lengthy leg immobility is essential.”
For this research, Shirakawa and his colleagues evaluated 86,024 individuals—36,007 men and 50,017 women ages 40 to 79—who were participating in the Japanese Collaborative Cohort Study.
The subjects completed a self-administered questionnaire that included information about average time spent watching TV each day. They were followed for a median of 18.4 years until 2009. Mortality from PE was determined from death certificates.
Subjects were divided into 3 groups according to the amount of TV they watched per day: less than 2.5 hours, 2.5 to 4.9 hours, and 5 or more hours.
The researchers calculated the risk of death from PE according to the amount of TV watched after adjusting for subjects’ age at baseline, gender, history of hypertension, history of diabetes, smoking status, drinking status, body mass index, walking and sports habits, and menopausal status.
During the follow-up period, there were 59 deaths from PE. And the multiavariate analysis revealed a link between extended TV viewing and fatal PE.
Compared to subjects who tended to watch less than 2.5 hours of TV per day, those who watched an average of 2.5 to 4.9 hours had an increased risk of fatal PE (hazard ratio [HR]=1.59). And the risk was greater among subjects whose average TV viewing time was more than 5 hours per day (HR=2.36).
Among subjects ages 40 to 59, the association between prolonged TV watching and fatal PE was more prominent.
Watching 2.5 to 4.9 hours of TV a day more than tripled the risk of fatal PE when compared to watching less than 2.5 hours (HR=3.24). And watching TV for more than 5 hours a day was associated with a more than 6-fold greater risk of fatal PE than watching less than 2.5 hours (HR=6.49).
Because prolonged leg immobility may explain these findings, Shirakawa and his colleagues recommend taking simple steps to prevent PE while watching TV for extended periods.
“[T]ake a break, stand up, and walk around during the television viewing,” he said. “Drinking water for preventing dehydration is also important.”
Shirakawa also noted that other media-based activities involving prolonged sitting may pose a risk of fatal PE.
“In this era of information technology, use of other visual-based media devices, such as personal computers or smartphones, is popular,” he said.
“Prolonged computer gaming has been associated with death from pulmonary embolism, but, to our knowledge, a relationship with prolonged smartphone use has not yet been reported. More research is needed to assess the risks of prolonged use of new technologies on pulmonary embolism morbidity and mortality.” ![]()
*Information in the abstract differs from that presented at the meeting.
LONDON—Results of a large study suggest that watching television for prolonged periods may increase a person’s risk of fatal pulmonary embolism (PE).
The study, which included more than 86,000 subjects, showed that watching an average of 5 or more hours of TV per day was associated with more than twice the risk of fatal PE as watching less than 2.5 hours daily.
And the risk was higher among younger subjects than older ones.
Toru Shirakawa, of Osaka University in Japan, presented this research at the ESC Congress 2015 (abstract P2686*).
“We showed that prolonged television viewing may be a risky behavior for death from pulmonary embolism,” Shirakawa said. “Leg immobility during television viewing may, in part, explain the finding. Public awareness of the risk of pulmonary embolism from lengthy leg immobility is essential.”
For this research, Shirakawa and his colleagues evaluated 86,024 individuals—36,007 men and 50,017 women ages 40 to 79—who were participating in the Japanese Collaborative Cohort Study.
The subjects completed a self-administered questionnaire that included information about average time spent watching TV each day. They were followed for a median of 18.4 years until 2009. Mortality from PE was determined from death certificates.
Subjects were divided into 3 groups according to the amount of TV they watched per day: less than 2.5 hours, 2.5 to 4.9 hours, and 5 or more hours.
The researchers calculated the risk of death from PE according to the amount of TV watched after adjusting for subjects’ age at baseline, gender, history of hypertension, history of diabetes, smoking status, drinking status, body mass index, walking and sports habits, and menopausal status.
During the follow-up period, there were 59 deaths from PE. And the multiavariate analysis revealed a link between extended TV viewing and fatal PE.
Compared to subjects who tended to watch less than 2.5 hours of TV per day, those who watched an average of 2.5 to 4.9 hours had an increased risk of fatal PE (hazard ratio [HR]=1.59). And the risk was greater among subjects whose average TV viewing time was more than 5 hours per day (HR=2.36).
Among subjects ages 40 to 59, the association between prolonged TV watching and fatal PE was more prominent.
Watching 2.5 to 4.9 hours of TV a day more than tripled the risk of fatal PE when compared to watching less than 2.5 hours (HR=3.24). And watching TV for more than 5 hours a day was associated with a more than 6-fold greater risk of fatal PE than watching less than 2.5 hours (HR=6.49).
Because prolonged leg immobility may explain these findings, Shirakawa and his colleagues recommend taking simple steps to prevent PE while watching TV for extended periods.
“[T]ake a break, stand up, and walk around during the television viewing,” he said. “Drinking water for preventing dehydration is also important.”
Shirakawa also noted that other media-based activities involving prolonged sitting may pose a risk of fatal PE.
“In this era of information technology, use of other visual-based media devices, such as personal computers or smartphones, is popular,” he said.
“Prolonged computer gaming has been associated with death from pulmonary embolism, but, to our knowledge, a relationship with prolonged smartphone use has not yet been reported. More research is needed to assess the risks of prolonged use of new technologies on pulmonary embolism morbidity and mortality.” ![]()
*Information in the abstract differs from that presented at the meeting.
LONDON—Results of a large study suggest that watching television for prolonged periods may increase a person’s risk of fatal pulmonary embolism (PE).
The study, which included more than 86,000 subjects, showed that watching an average of 5 or more hours of TV per day was associated with more than twice the risk of fatal PE as watching less than 2.5 hours daily.
And the risk was higher among younger subjects than older ones.
Toru Shirakawa, of Osaka University in Japan, presented this research at the ESC Congress 2015 (abstract P2686*).
“We showed that prolonged television viewing may be a risky behavior for death from pulmonary embolism,” Shirakawa said. “Leg immobility during television viewing may, in part, explain the finding. Public awareness of the risk of pulmonary embolism from lengthy leg immobility is essential.”
For this research, Shirakawa and his colleagues evaluated 86,024 individuals—36,007 men and 50,017 women ages 40 to 79—who were participating in the Japanese Collaborative Cohort Study.
The subjects completed a self-administered questionnaire that included information about average time spent watching TV each day. They were followed for a median of 18.4 years until 2009. Mortality from PE was determined from death certificates.
Subjects were divided into 3 groups according to the amount of TV they watched per day: less than 2.5 hours, 2.5 to 4.9 hours, and 5 or more hours.
The researchers calculated the risk of death from PE according to the amount of TV watched after adjusting for subjects’ age at baseline, gender, history of hypertension, history of diabetes, smoking status, drinking status, body mass index, walking and sports habits, and menopausal status.
During the follow-up period, there were 59 deaths from PE. And the multiavariate analysis revealed a link between extended TV viewing and fatal PE.
Compared to subjects who tended to watch less than 2.5 hours of TV per day, those who watched an average of 2.5 to 4.9 hours had an increased risk of fatal PE (hazard ratio [HR]=1.59). And the risk was greater among subjects whose average TV viewing time was more than 5 hours per day (HR=2.36).
Among subjects ages 40 to 59, the association between prolonged TV watching and fatal PE was more prominent.
Watching 2.5 to 4.9 hours of TV a day more than tripled the risk of fatal PE when compared to watching less than 2.5 hours (HR=3.24). And watching TV for more than 5 hours a day was associated with a more than 6-fold greater risk of fatal PE than watching less than 2.5 hours (HR=6.49).
Because prolonged leg immobility may explain these findings, Shirakawa and his colleagues recommend taking simple steps to prevent PE while watching TV for extended periods.
“[T]ake a break, stand up, and walk around during the television viewing,” he said. “Drinking water for preventing dehydration is also important.”
Shirakawa also noted that other media-based activities involving prolonged sitting may pose a risk of fatal PE.
“In this era of information technology, use of other visual-based media devices, such as personal computers or smartphones, is popular,” he said.
“Prolonged computer gaming has been associated with death from pulmonary embolism, but, to our knowledge, a relationship with prolonged smartphone use has not yet been reported. More research is needed to assess the risks of prolonged use of new technologies on pulmonary embolism morbidity and mortality.” ![]()
*Information in the abstract differs from that presented at the meeting.
Extra education about apixaban may be unnecessary
Photo courtesy of Pfizer
and Bristol-Myers Squibb
LONDON—An educational program designed to improve adherence to the anticoagulant apixaban proved ineffective in a phase 4 trial of patients with atrial fibrillation (AF).
But that’s because adherence was high whether patients completed the program or not.
This suggests current measures used to inform patients about apixaban may be sufficient to ensure treatment adherence, researchers said.
They presented these findings at the ESC Congress 2015 (abstract 2191).
The research, known as the AEGEAN trial, was sponsored by Bristol-Myers Squibb, the company co-developing apixaban (Eliquis) with Pfizer.
“We used the best possible tools for the educational program, including the usual staff and procedures of the anticoagulation clinics, and all of this was useless,” said study investigator Gilles Montalescot, MD, PhD, of Hospitalier Universitaire Pitié-Salpêtrière in Paris, France.
“However, the trial showed very good adherence to apixaban, leaving little room for improvement with an educational program, suggesting one more advantage of prescribing non-vitamin K antagonists (VKAs) over VKAs in that there is apparently no need for additional education and information.”
Dr Montalescot and his colleagues conducted this study in 1162 AF patients receiving apixaban as stroke prophylaxis.
Roughly half of the patients (n=579) completed an educational program promoting treatment adherence, and the other half (n=583) received the usual information about their disease and the treatment.
The educational program included a patient information booklet explaining AF and anticoagulant treatment for stroke prevention, reminder tools (eg, a key ring and mobile phone alerts), and access to a virtual clinic utilizing staff from existing VKA monitoring clinics.
The researchers assessed differences between the 2 patient groups with regard to treatment adherence (defined as continuous, twice-daily dosing, with an occasional missed dose allowed) and treatment persistence (defined as absence of discontinuation for 30 consecutive days) over a 6-month observational period.
Adherence/persistence was measured using an electronic device that holds a blister pack of medication and records each time the pack is removed.
The researchers found no additional value of the educational program for either outcome.
At 24 weeks, the adherence rate was 88.5% in the control group and 88.3% in the education group (P=0.89). Treatment persistence rates were 90.5% and 91.1%, respectively (P=0.76).
For the second part of this study, the researchers are investigating long-term treatment adherence and the value of an educational program beyond 6 months.
“Future studies may want to test more aggressive and more costly educational programs,” Dr Montalescot noted. “But, in the meantime, the adherence and persistence rates we measured are quite reassuring with the common practice and usual mode of prescription of this medication.” ![]()

Photo courtesy of Pfizer
and Bristol-Myers Squibb
LONDON—An educational program designed to improve adherence to the anticoagulant apixaban proved ineffective in a phase 4 trial of patients with atrial fibrillation (AF).
But that’s because adherence was high whether patients completed the program or not.
This suggests current measures used to inform patients about apixaban may be sufficient to ensure treatment adherence, researchers said.
They presented these findings at the ESC Congress 2015 (abstract 2191).
The research, known as the AEGEAN trial, was sponsored by Bristol-Myers Squibb, the company co-developing apixaban (Eliquis) with Pfizer.
“We used the best possible tools for the educational program, including the usual staff and procedures of the anticoagulation clinics, and all of this was useless,” said study investigator Gilles Montalescot, MD, PhD, of Hospitalier Universitaire Pitié-Salpêtrière in Paris, France.
“However, the trial showed very good adherence to apixaban, leaving little room for improvement with an educational program, suggesting one more advantage of prescribing non-vitamin K antagonists (VKAs) over VKAs in that there is apparently no need for additional education and information.”
Dr Montalescot and his colleagues conducted this study in 1162 AF patients receiving apixaban as stroke prophylaxis.
Roughly half of the patients (n=579) completed an educational program promoting treatment adherence, and the other half (n=583) received the usual information about their disease and the treatment.
The educational program included a patient information booklet explaining AF and anticoagulant treatment for stroke prevention, reminder tools (eg, a key ring and mobile phone alerts), and access to a virtual clinic utilizing staff from existing VKA monitoring clinics.
The researchers assessed differences between the 2 patient groups with regard to treatment adherence (defined as continuous, twice-daily dosing, with an occasional missed dose allowed) and treatment persistence (defined as absence of discontinuation for 30 consecutive days) over a 6-month observational period.
Adherence/persistence was measured using an electronic device that holds a blister pack of medication and records each time the pack is removed.
The researchers found no additional value of the educational program for either outcome.
At 24 weeks, the adherence rate was 88.5% in the control group and 88.3% in the education group (P=0.89). Treatment persistence rates were 90.5% and 91.1%, respectively (P=0.76).
For the second part of this study, the researchers are investigating long-term treatment adherence and the value of an educational program beyond 6 months.
“Future studies may want to test more aggressive and more costly educational programs,” Dr Montalescot noted. “But, in the meantime, the adherence and persistence rates we measured are quite reassuring with the common practice and usual mode of prescription of this medication.” ![]()

Photo courtesy of Pfizer
and Bristol-Myers Squibb
LONDON—An educational program designed to improve adherence to the anticoagulant apixaban proved ineffective in a phase 4 trial of patients with atrial fibrillation (AF).
But that’s because adherence was high whether patients completed the program or not.
This suggests current measures used to inform patients about apixaban may be sufficient to ensure treatment adherence, researchers said.
They presented these findings at the ESC Congress 2015 (abstract 2191).
The research, known as the AEGEAN trial, was sponsored by Bristol-Myers Squibb, the company co-developing apixaban (Eliquis) with Pfizer.
“We used the best possible tools for the educational program, including the usual staff and procedures of the anticoagulation clinics, and all of this was useless,” said study investigator Gilles Montalescot, MD, PhD, of Hospitalier Universitaire Pitié-Salpêtrière in Paris, France.
“However, the trial showed very good adherence to apixaban, leaving little room for improvement with an educational program, suggesting one more advantage of prescribing non-vitamin K antagonists (VKAs) over VKAs in that there is apparently no need for additional education and information.”
Dr Montalescot and his colleagues conducted this study in 1162 AF patients receiving apixaban as stroke prophylaxis.
Roughly half of the patients (n=579) completed an educational program promoting treatment adherence, and the other half (n=583) received the usual information about their disease and the treatment.
The educational program included a patient information booklet explaining AF and anticoagulant treatment for stroke prevention, reminder tools (eg, a key ring and mobile phone alerts), and access to a virtual clinic utilizing staff from existing VKA monitoring clinics.
The researchers assessed differences between the 2 patient groups with regard to treatment adherence (defined as continuous, twice-daily dosing, with an occasional missed dose allowed) and treatment persistence (defined as absence of discontinuation for 30 consecutive days) over a 6-month observational period.
Adherence/persistence was measured using an electronic device that holds a blister pack of medication and records each time the pack is removed.
The researchers found no additional value of the educational program for either outcome.
At 24 weeks, the adherence rate was 88.5% in the control group and 88.3% in the education group (P=0.89). Treatment persistence rates were 90.5% and 91.1%, respectively (P=0.76).
For the second part of this study, the researchers are investigating long-term treatment adherence and the value of an educational program beyond 6 months.
“Future studies may want to test more aggressive and more costly educational programs,” Dr Montalescot noted. “But, in the meantime, the adherence and persistence rates we measured are quite reassuring with the common practice and usual mode of prescription of this medication.” ![]()
Early intervention may forestall menopause-related skin aging
NEW YORK – Evidence is mounting that early intervention in the menopausal transition could help forestall some of the skin aging associated with estrogen decline.
Estrogen supplementation and collagen stimulation both seem effective in preserving the integrity of a woman’s skin as levels of the hormone decrease, Dr. Diane Madfes said at the American Academy of Dermatology summer meeting.
Type 3 collagen decreases by up to 50% within a few years of menopause, said Dr. Madfes, a dermatologist in New York. This is directly related to a loss of estrogen receptor beta in the dermal matrix, which promotes collagen formation.
“There is a theory – the timing hypothesis – that we have a window of opportunity to intervene. If we can stimulate the collagen before the receptors go down, maybe we can have a beneficial effect on skin.”
Any method of collagen stimulation should work, she said: laser resurfacing, microneedling, or radiofrequency. “We are very good about being able to stimulate collagen. The method doesn’t matter as much as the timing. The important thing is to intervene early. If you see your patients starting to sag, see a loss of elasticity, that is the time to intervene. Get at the collagen while it’s still receptive.”
Estrogen exerts a plethora of antiaging, skin-preserving effects. “We know that a decrease in estrogen is related to telomere shortening. Estrogen protects against oxidative damage. It signals keratinocytes through IGF-1,” she said.
The hormone also protects skin’s water-binding qualities by promoting mucopolysaccharides, sebum production, barrier function, and hyaluronic acid. It may even play a role in protecting against ultraviolet light. Estrogen downregulation affects healing by inhibiting the proliferation of keratinocytes and the proliferation and migration of fibroblasts.
All these add up to rapid skin aging after estrogen levels drop.
“The visible effects of aging on women’s skin are not so much related to her chronological age as to the years after menopause,” Dr. Madfes said – a finding that is particularly illustrated in young women with surgical menopause and those with breast cancer who take tamoxifen. The observation seems to suggest that early intervention with estrogen might help prevent at least some of the signs of aging.
The ongoing KEEPS trial (Kronos Early Estrogen Prevention Study) may shed some light on the issue. KEEPS has randomized 729 women aged 42-58 years to oral or transdermal estrogen; the primary endpoint is rate of atherosclerosis. But an ancillary study is looking at the effect of estrogen on skin wrinkles and skin rigidity.
The substudy is based on positive findings of a 1996 study, which found evidence for facial application of topical estrogen designed for vulvar use. After 6 months, elasticity and firmness significantly improved. Skin moisture increased, as did type 3 collagen and collagen fibers.
Some women do use topical estrogens on their faces. “It seems to promote skin thickening and tightening,“ Dr. Madfes said, although a recent editorial suggested that using the product anywhere but on the genitals can cause estrogen-mediated side effects in both children and pets.
But recommending estrogen is fraught with controversy. Large studies have come to conflicting conclusions about its benefit and safety. And prescribing estrogen is not really within a dermatologist’s purview.
“It’s not for us to suggest that women go on hormone therapy. But we can explain these things and ask if she is taking it, or if she’s talked to her gynecologist about it.”
Dr. Madfes has no financial disclosures to report.
On Twitter @Alz_Gal
NEW YORK – Evidence is mounting that early intervention in the menopausal transition could help forestall some of the skin aging associated with estrogen decline.
Estrogen supplementation and collagen stimulation both seem effective in preserving the integrity of a woman’s skin as levels of the hormone decrease, Dr. Diane Madfes said at the American Academy of Dermatology summer meeting.
Type 3 collagen decreases by up to 50% within a few years of menopause, said Dr. Madfes, a dermatologist in New York. This is directly related to a loss of estrogen receptor beta in the dermal matrix, which promotes collagen formation.
“There is a theory – the timing hypothesis – that we have a window of opportunity to intervene. If we can stimulate the collagen before the receptors go down, maybe we can have a beneficial effect on skin.”
Any method of collagen stimulation should work, she said: laser resurfacing, microneedling, or radiofrequency. “We are very good about being able to stimulate collagen. The method doesn’t matter as much as the timing. The important thing is to intervene early. If you see your patients starting to sag, see a loss of elasticity, that is the time to intervene. Get at the collagen while it’s still receptive.”
Estrogen exerts a plethora of antiaging, skin-preserving effects. “We know that a decrease in estrogen is related to telomere shortening. Estrogen protects against oxidative damage. It signals keratinocytes through IGF-1,” she said.
The hormone also protects skin’s water-binding qualities by promoting mucopolysaccharides, sebum production, barrier function, and hyaluronic acid. It may even play a role in protecting against ultraviolet light. Estrogen downregulation affects healing by inhibiting the proliferation of keratinocytes and the proliferation and migration of fibroblasts.
All these add up to rapid skin aging after estrogen levels drop.
“The visible effects of aging on women’s skin are not so much related to her chronological age as to the years after menopause,” Dr. Madfes said – a finding that is particularly illustrated in young women with surgical menopause and those with breast cancer who take tamoxifen. The observation seems to suggest that early intervention with estrogen might help prevent at least some of the signs of aging.
The ongoing KEEPS trial (Kronos Early Estrogen Prevention Study) may shed some light on the issue. KEEPS has randomized 729 women aged 42-58 years to oral or transdermal estrogen; the primary endpoint is rate of atherosclerosis. But an ancillary study is looking at the effect of estrogen on skin wrinkles and skin rigidity.
The substudy is based on positive findings of a 1996 study, which found evidence for facial application of topical estrogen designed for vulvar use. After 6 months, elasticity and firmness significantly improved. Skin moisture increased, as did type 3 collagen and collagen fibers.
Some women do use topical estrogens on their faces. “It seems to promote skin thickening and tightening,“ Dr. Madfes said, although a recent editorial suggested that using the product anywhere but on the genitals can cause estrogen-mediated side effects in both children and pets.
But recommending estrogen is fraught with controversy. Large studies have come to conflicting conclusions about its benefit and safety. And prescribing estrogen is not really within a dermatologist’s purview.
“It’s not for us to suggest that women go on hormone therapy. But we can explain these things and ask if she is taking it, or if she’s talked to her gynecologist about it.”
Dr. Madfes has no financial disclosures to report.
On Twitter @Alz_Gal
NEW YORK – Evidence is mounting that early intervention in the menopausal transition could help forestall some of the skin aging associated with estrogen decline.
Estrogen supplementation and collagen stimulation both seem effective in preserving the integrity of a woman’s skin as levels of the hormone decrease, Dr. Diane Madfes said at the American Academy of Dermatology summer meeting.
Type 3 collagen decreases by up to 50% within a few years of menopause, said Dr. Madfes, a dermatologist in New York. This is directly related to a loss of estrogen receptor beta in the dermal matrix, which promotes collagen formation.
“There is a theory – the timing hypothesis – that we have a window of opportunity to intervene. If we can stimulate the collagen before the receptors go down, maybe we can have a beneficial effect on skin.”
Any method of collagen stimulation should work, she said: laser resurfacing, microneedling, or radiofrequency. “We are very good about being able to stimulate collagen. The method doesn’t matter as much as the timing. The important thing is to intervene early. If you see your patients starting to sag, see a loss of elasticity, that is the time to intervene. Get at the collagen while it’s still receptive.”
Estrogen exerts a plethora of antiaging, skin-preserving effects. “We know that a decrease in estrogen is related to telomere shortening. Estrogen protects against oxidative damage. It signals keratinocytes through IGF-1,” she said.
The hormone also protects skin’s water-binding qualities by promoting mucopolysaccharides, sebum production, barrier function, and hyaluronic acid. It may even play a role in protecting against ultraviolet light. Estrogen downregulation affects healing by inhibiting the proliferation of keratinocytes and the proliferation and migration of fibroblasts.
All these add up to rapid skin aging after estrogen levels drop.
“The visible effects of aging on women’s skin are not so much related to her chronological age as to the years after menopause,” Dr. Madfes said – a finding that is particularly illustrated in young women with surgical menopause and those with breast cancer who take tamoxifen. The observation seems to suggest that early intervention with estrogen might help prevent at least some of the signs of aging.
The ongoing KEEPS trial (Kronos Early Estrogen Prevention Study) may shed some light on the issue. KEEPS has randomized 729 women aged 42-58 years to oral or transdermal estrogen; the primary endpoint is rate of atherosclerosis. But an ancillary study is looking at the effect of estrogen on skin wrinkles and skin rigidity.
The substudy is based on positive findings of a 1996 study, which found evidence for facial application of topical estrogen designed for vulvar use. After 6 months, elasticity and firmness significantly improved. Skin moisture increased, as did type 3 collagen and collagen fibers.
Some women do use topical estrogens on their faces. “It seems to promote skin thickening and tightening,“ Dr. Madfes said, although a recent editorial suggested that using the product anywhere but on the genitals can cause estrogen-mediated side effects in both children and pets.
But recommending estrogen is fraught with controversy. Large studies have come to conflicting conclusions about its benefit and safety. And prescribing estrogen is not really within a dermatologist’s purview.
“It’s not for us to suggest that women go on hormone therapy. But we can explain these things and ask if she is taking it, or if she’s talked to her gynecologist about it.”
Dr. Madfes has no financial disclosures to report.
On Twitter @Alz_Gal
EXPERT ANALYSIS FROM THE AAD SUMMER ACADEMY 2015
Public uninformed about cancer therapies, survey suggests

receiving chemotherapy
Photo by Rhoda Baer
Results of a new survey suggest many adults in the UK may be uninformed about cancer treatment options, despite broad media coverage of these therapies.
Personalized drug treatment, immunotherapy, and proton beam therapy have all been covered by the lay media and featured in news stories across the globe.
But a survey of more than 2000 UK adults showed that most respondents were not aware of these treatment types.
Only 19% of respondents said they had heard about immunotherapy, 29% had heard of personalized drug treatment, and 30% had heard of proton beam therapy.
The survey, which included 2081 adults, was conducted online by YouGov in June. It was commissioned by Cancer Research UK and other members of the Radiotherapy Awareness Programme.
The primary goal of the survey was to examine public awareness of radiotherapy. And the results showed that many respondents were unaware of newer, more targeted radiotherapy options.
Respondents were largely uninformed about other types of cancer treatment as well. However, of the respondents who elected to give their opinion (n=1877), most said the National Health Service (NHS) should fund chemotherapy and other drug treatments over radiotherapy.
Survey questions and responses were as follows.
Radiotherapy
Before taking this survey, which, if any, of the following types of radiotherapy had you heard of?
| Intensity-modulated radiotherapy | 4% |
| Stereotactic radiotherapy/
stereotactic ablative radiotherapy |
3% |
| Image-guided radiotherapy | 9% |
| Proton beam therapy | 30% |
| Brachytherapy | 5% |
| Radiofrequency ablation | 7% |
| Cyberknife | 4% |
| Gammaknife | 6% |
| Higgs-boson radiotherapy
(red herring option) |
6% |
| Carbon ion radiotherapy
(red herring option) |
3% |
| None of these | 50% |
| Prefer not to say | 11% |
Other cancer treatments
Which, if any, of the following specific types of cancer treatments/tests had you heard of before taking this survey?
| Immunotherapy | 19% |
| Personalized drugs | 29% |
| Monoclonal antibodies | 5% |
| High-dose chemotherapy
with stem cell transplant |
26% |
| Tablet chemotherapy | 28% |
| Molecular diagnostic tests | 6% |
| Robotically assisted surgery/Da Vinci robot | 12% |
| Laparoscopic (keyhole) surgery | 39% |
| None of these | 32% |
| Prefer not to say | 11% |
NHS funding
What level of priority do you think the NHS should give to funding each of the following 4 types of cancer treatments?
| Treatment | 1st priority | 2nd priority | 3rd priority | Lowest priority |
| Chemotherapy &
other drug treatments |
57% | 29% | 10% | 4% |
| Surgery | 29% | 35% | 31% | 5% |
| Radiotherapy | 9% | 32% | 53% | 5% |
| Alternative treatments | 5% | 4% | 6% | 86% |
![]()

receiving chemotherapy
Photo by Rhoda Baer
Results of a new survey suggest many adults in the UK may be uninformed about cancer treatment options, despite broad media coverage of these therapies.
Personalized drug treatment, immunotherapy, and proton beam therapy have all been covered by the lay media and featured in news stories across the globe.
But a survey of more than 2000 UK adults showed that most respondents were not aware of these treatment types.
Only 19% of respondents said they had heard about immunotherapy, 29% had heard of personalized drug treatment, and 30% had heard of proton beam therapy.
The survey, which included 2081 adults, was conducted online by YouGov in June. It was commissioned by Cancer Research UK and other members of the Radiotherapy Awareness Programme.
The primary goal of the survey was to examine public awareness of radiotherapy. And the results showed that many respondents were unaware of newer, more targeted radiotherapy options.
Respondents were largely uninformed about other types of cancer treatment as well. However, of the respondents who elected to give their opinion (n=1877), most said the National Health Service (NHS) should fund chemotherapy and other drug treatments over radiotherapy.
Survey questions and responses were as follows.
Radiotherapy
Before taking this survey, which, if any, of the following types of radiotherapy had you heard of?
| Intensity-modulated radiotherapy | 4% |
| Stereotactic radiotherapy/
stereotactic ablative radiotherapy |
3% |
| Image-guided radiotherapy | 9% |
| Proton beam therapy | 30% |
| Brachytherapy | 5% |
| Radiofrequency ablation | 7% |
| Cyberknife | 4% |
| Gammaknife | 6% |
| Higgs-boson radiotherapy
(red herring option) |
6% |
| Carbon ion radiotherapy
(red herring option) |
3% |
| None of these | 50% |
| Prefer not to say | 11% |
Other cancer treatments
Which, if any, of the following specific types of cancer treatments/tests had you heard of before taking this survey?
| Immunotherapy | 19% |
| Personalized drugs | 29% |
| Monoclonal antibodies | 5% |
| High-dose chemotherapy
with stem cell transplant |
26% |
| Tablet chemotherapy | 28% |
| Molecular diagnostic tests | 6% |
| Robotically assisted surgery/Da Vinci robot | 12% |
| Laparoscopic (keyhole) surgery | 39% |
| None of these | 32% |
| Prefer not to say | 11% |
NHS funding
What level of priority do you think the NHS should give to funding each of the following 4 types of cancer treatments?
| Treatment | 1st priority | 2nd priority | 3rd priority | Lowest priority |
| Chemotherapy &
other drug treatments |
57% | 29% | 10% | 4% |
| Surgery | 29% | 35% | 31% | 5% |
| Radiotherapy | 9% | 32% | 53% | 5% |
| Alternative treatments | 5% | 4% | 6% | 86% |
![]()

receiving chemotherapy
Photo by Rhoda Baer
Results of a new survey suggest many adults in the UK may be uninformed about cancer treatment options, despite broad media coverage of these therapies.
Personalized drug treatment, immunotherapy, and proton beam therapy have all been covered by the lay media and featured in news stories across the globe.
But a survey of more than 2000 UK adults showed that most respondents were not aware of these treatment types.
Only 19% of respondents said they had heard about immunotherapy, 29% had heard of personalized drug treatment, and 30% had heard of proton beam therapy.
The survey, which included 2081 adults, was conducted online by YouGov in June. It was commissioned by Cancer Research UK and other members of the Radiotherapy Awareness Programme.
The primary goal of the survey was to examine public awareness of radiotherapy. And the results showed that many respondents were unaware of newer, more targeted radiotherapy options.
Respondents were largely uninformed about other types of cancer treatment as well. However, of the respondents who elected to give their opinion (n=1877), most said the National Health Service (NHS) should fund chemotherapy and other drug treatments over radiotherapy.
Survey questions and responses were as follows.
Radiotherapy
Before taking this survey, which, if any, of the following types of radiotherapy had you heard of?
| Intensity-modulated radiotherapy | 4% |
| Stereotactic radiotherapy/
stereotactic ablative radiotherapy |
3% |
| Image-guided radiotherapy | 9% |
| Proton beam therapy | 30% |
| Brachytherapy | 5% |
| Radiofrequency ablation | 7% |
| Cyberknife | 4% |
| Gammaknife | 6% |
| Higgs-boson radiotherapy
(red herring option) |
6% |
| Carbon ion radiotherapy
(red herring option) |
3% |
| None of these | 50% |
| Prefer not to say | 11% |
Other cancer treatments
Which, if any, of the following specific types of cancer treatments/tests had you heard of before taking this survey?
| Immunotherapy | 19% |
| Personalized drugs | 29% |
| Monoclonal antibodies | 5% |
| High-dose chemotherapy
with stem cell transplant |
26% |
| Tablet chemotherapy | 28% |
| Molecular diagnostic tests | 6% |
| Robotically assisted surgery/Da Vinci robot | 12% |
| Laparoscopic (keyhole) surgery | 39% |
| None of these | 32% |
| Prefer not to say | 11% |
NHS funding
What level of priority do you think the NHS should give to funding each of the following 4 types of cancer treatments?
| Treatment | 1st priority | 2nd priority | 3rd priority | Lowest priority |
| Chemotherapy &
other drug treatments |
57% | 29% | 10% | 4% |
| Surgery | 29% | 35% | 31% | 5% |
| Radiotherapy | 9% | 32% | 53% | 5% |
| Alternative treatments | 5% | 4% | 6% | 86% |
![]()
Less posttransplant primary biliary cirrhosis with preventive UDCA
Only 21% of liver transplantation patients who received ursodeoxycholic acid (UDCA) after surgery developed recurrent primary biliary cirrhosis, compared with 62% of patients who did not receive the bile acid, researchers reported in the Journal of Hepatology.
The results provide strong evidence that routinely giving liver transplant patients UDCA can prevent or delay recurrent primary biliary cirrhosis, said Alexie Bosch at Hôpital Edouard Herriot in Lyon, France, and his associates.
Primary biliary cirrhosis can recur after liver transplantation and increases the chances of graft dysfunction, the researchers noted. UDCA is the only approved medical treatment for primary biliary cirrhosis in the United States or Europe, but no research team has studied its potential to prevent recurrent primary biliary cirrhosis after liver transplantation, they added. Therefore, they retrospectively studied 90 patients with primary biliary cirrhosis who underwent liver transplantation at five centers in France and Switzerland between 1988 and 2010. In all, 21% of patients received oral UDCA (10-15 mg/kg per day in two divided doses) within 2 weeks after their operation, while the rest received it only if they developed biopsy-confirmed recurrent primary biliary cirrhosis. Biopsies were taken at posttransplant year 1 and every 5 years after that, or when clinically indicated, the investigators noted (J Hepatol. 2015 Aug. 14. doi: 10.1016/j.jhep.2015.07.038).
Patients who received preventive UDCA had a lower cumulative rate of recurrence throughout 15 years of postsurgical follow-up (P = .014), the researchers reported. The chances of recurrent primary biliary cirrhosis at 5, 10, and 15 years after transplantation were 11%, 21%, and 40% in the UDCA group, compared with 32%, 53%, and 70% for patients who did not receive prophylactic UDCA, they added. A multivariable analysis showed that recurrent primary biliary cirrhosis was associated with not receiving prophylactic UDCA (hazard ratio, 0.32; 95% confidence interval, 0.11, 0.91), but was not linked to donor age, Model For End-Stage Liver Disease (MELD) score, or sex mismatch between donor and recipient, the investigators said. Preventive UDCA also was tied to a 1.6-year longer median time to recurrence, although the trend did not reach statistical significance.
Although the study was retrospective and most patients who received UDCA were treated at one transplant center, all centers had similar histologic findings for recurrent primary biliary cirrhosis, said the researchers. Biopsies also were histologically similar regardless of whether they were event driven or obtained based on the study protocol, and time to recurrence did not vary based on biopsy type, they added. “In our multivariate analysis, we took care to account for all risk factors and confounders, as well as to test multilevel models in order to exclude potential misleading results and center effects,” they emphasized. “Given the extremely limited feasibility of prospective studies and the good tolerance and acceptability of long-term UDCA therapy, these results support the extended use of UDCA as prophylaxis for primary biliary cirrhosis recurrence after liver transplantation.”
The researchers declared no funding sources and reported having no conflicts of interest.
Only 21% of liver transplantation patients who received ursodeoxycholic acid (UDCA) after surgery developed recurrent primary biliary cirrhosis, compared with 62% of patients who did not receive the bile acid, researchers reported in the Journal of Hepatology.
The results provide strong evidence that routinely giving liver transplant patients UDCA can prevent or delay recurrent primary biliary cirrhosis, said Alexie Bosch at Hôpital Edouard Herriot in Lyon, France, and his associates.
Primary biliary cirrhosis can recur after liver transplantation and increases the chances of graft dysfunction, the researchers noted. UDCA is the only approved medical treatment for primary biliary cirrhosis in the United States or Europe, but no research team has studied its potential to prevent recurrent primary biliary cirrhosis after liver transplantation, they added. Therefore, they retrospectively studied 90 patients with primary biliary cirrhosis who underwent liver transplantation at five centers in France and Switzerland between 1988 and 2010. In all, 21% of patients received oral UDCA (10-15 mg/kg per day in two divided doses) within 2 weeks after their operation, while the rest received it only if they developed biopsy-confirmed recurrent primary biliary cirrhosis. Biopsies were taken at posttransplant year 1 and every 5 years after that, or when clinically indicated, the investigators noted (J Hepatol. 2015 Aug. 14. doi: 10.1016/j.jhep.2015.07.038).
Patients who received preventive UDCA had a lower cumulative rate of recurrence throughout 15 years of postsurgical follow-up (P = .014), the researchers reported. The chances of recurrent primary biliary cirrhosis at 5, 10, and 15 years after transplantation were 11%, 21%, and 40% in the UDCA group, compared with 32%, 53%, and 70% for patients who did not receive prophylactic UDCA, they added. A multivariable analysis showed that recurrent primary biliary cirrhosis was associated with not receiving prophylactic UDCA (hazard ratio, 0.32; 95% confidence interval, 0.11, 0.91), but was not linked to donor age, Model For End-Stage Liver Disease (MELD) score, or sex mismatch between donor and recipient, the investigators said. Preventive UDCA also was tied to a 1.6-year longer median time to recurrence, although the trend did not reach statistical significance.
Although the study was retrospective and most patients who received UDCA were treated at one transplant center, all centers had similar histologic findings for recurrent primary biliary cirrhosis, said the researchers. Biopsies also were histologically similar regardless of whether they were event driven or obtained based on the study protocol, and time to recurrence did not vary based on biopsy type, they added. “In our multivariate analysis, we took care to account for all risk factors and confounders, as well as to test multilevel models in order to exclude potential misleading results and center effects,” they emphasized. “Given the extremely limited feasibility of prospective studies and the good tolerance and acceptability of long-term UDCA therapy, these results support the extended use of UDCA as prophylaxis for primary biliary cirrhosis recurrence after liver transplantation.”
The researchers declared no funding sources and reported having no conflicts of interest.
Only 21% of liver transplantation patients who received ursodeoxycholic acid (UDCA) after surgery developed recurrent primary biliary cirrhosis, compared with 62% of patients who did not receive the bile acid, researchers reported in the Journal of Hepatology.
The results provide strong evidence that routinely giving liver transplant patients UDCA can prevent or delay recurrent primary biliary cirrhosis, said Alexie Bosch at Hôpital Edouard Herriot in Lyon, France, and his associates.
Primary biliary cirrhosis can recur after liver transplantation and increases the chances of graft dysfunction, the researchers noted. UDCA is the only approved medical treatment for primary biliary cirrhosis in the United States or Europe, but no research team has studied its potential to prevent recurrent primary biliary cirrhosis after liver transplantation, they added. Therefore, they retrospectively studied 90 patients with primary biliary cirrhosis who underwent liver transplantation at five centers in France and Switzerland between 1988 and 2010. In all, 21% of patients received oral UDCA (10-15 mg/kg per day in two divided doses) within 2 weeks after their operation, while the rest received it only if they developed biopsy-confirmed recurrent primary biliary cirrhosis. Biopsies were taken at posttransplant year 1 and every 5 years after that, or when clinically indicated, the investigators noted (J Hepatol. 2015 Aug. 14. doi: 10.1016/j.jhep.2015.07.038).
Patients who received preventive UDCA had a lower cumulative rate of recurrence throughout 15 years of postsurgical follow-up (P = .014), the researchers reported. The chances of recurrent primary biliary cirrhosis at 5, 10, and 15 years after transplantation were 11%, 21%, and 40% in the UDCA group, compared with 32%, 53%, and 70% for patients who did not receive prophylactic UDCA, they added. A multivariable analysis showed that recurrent primary biliary cirrhosis was associated with not receiving prophylactic UDCA (hazard ratio, 0.32; 95% confidence interval, 0.11, 0.91), but was not linked to donor age, Model For End-Stage Liver Disease (MELD) score, or sex mismatch between donor and recipient, the investigators said. Preventive UDCA also was tied to a 1.6-year longer median time to recurrence, although the trend did not reach statistical significance.
Although the study was retrospective and most patients who received UDCA were treated at one transplant center, all centers had similar histologic findings for recurrent primary biliary cirrhosis, said the researchers. Biopsies also were histologically similar regardless of whether they were event driven or obtained based on the study protocol, and time to recurrence did not vary based on biopsy type, they added. “In our multivariate analysis, we took care to account for all risk factors and confounders, as well as to test multilevel models in order to exclude potential misleading results and center effects,” they emphasized. “Given the extremely limited feasibility of prospective studies and the good tolerance and acceptability of long-term UDCA therapy, these results support the extended use of UDCA as prophylaxis for primary biliary cirrhosis recurrence after liver transplantation.”
The researchers declared no funding sources and reported having no conflicts of interest.
FROM THE JOURNAL OF HEPATOLOGY
Key clinical point: Treatment with prophylactic ursodeoxycholic acid might help prevent recurrent primary biliary cirrhosis after liver transplantation.
Major finding: Patients who received preventive UDCA had a lower cumulative rate of recurrence throughout 15 years of follow-up (P = .014).
Data source: Multicenter retrospective study of 90 patients who underwent liver transplantation for primary biliary cirrhosis.
Disclosures: The researchers declared no funding sources and reported having no conflicts of interest.
Imaging the suspected ovarian malignancy: 14 cases
Pelvic ultrasonography remains the preferred imaging method to evaluate most adnexal cysts given its ability to characterize such cysts with high resolution and accuracy. Most cystic adnexal masses have characteristic findings that can guide counseling and management decisions. For instance, mature cystic teratomas have hyperechoic lines/dots and acoustic shadowing; hydrosalpinx are tubular or s shaped and show a “waist sign.”
In parts 1 through 3 of this 4-part series on adnexal pathology, we presented images detailing common benign adnexal cysts, including:
- simple and hemorrhagic cysts (Part 1:Telltale sonographic features of simple and hemorrhagic cysts)
- mature cystic teratomas (dermoid cysts) and endometriomas (Part 2: Imaging the endometrioma and mature cystic teratoma)
- hydrosalpinx and pelvic inclusion cysts (Part 3: “Cogwheel” and other signs of hydrosalpinx and pelvic inclusion cysts)
In this conclusion to the series, we detail imaging for ovarian neoplasias (including cystadenoma and cystadenocarcinoma).
OVARIAN NEOPLASIA
A woman’s lifetime risk of undergoing surgery for suspected ovarian malignancy is 5% to 10% in the United States, and only about 13% to 21% of those undergoing surgery will actually be diagnosed with ovarian cancer.1 Therefore, the goal of diagnostic evaluation is to exclude malignancy.
Diagnostic evaluation includes:
- imaging
- lab work
- history
- physical findings.
The preferred imaging modality for a pelvic mass in asymptomatic premenopausal and postmenopausal women is transvaginal ultrasonography according to the American College of Obstetricians and Gynecologists (ACOG) practice bulletin, which was reaffirmed in 2013.1 “No alternative imaging modality has demonstrated sufficient superiority to transvaginal ultrasonography to justify its routine use.”1
Transvaginal ultrasonography with color Doppler interrogation has demonstrated a sensitivity of 0.86% and a specificity of 0.91% for discriminating between malignant and benign ovarian masses.
Sonographic features that are worrisome for malignancy include:
- Multiple thin septations (if indeterminate, the mass may possibly be benign)
- Thick (> 3 mm), irregular septations
- Focal areas of wall thickening (> 3 mm)
- Mural nodules or papillary projections
- Levine and colleagues note that a cyst with a mural nodule with internal blood flow on color Doppler has the highest likelihood of being malignant2
- Moderate or large amount of ascitic fluid in pelvis (in conjunction with ovarian mass showing the above characteristics)
Various morphology indices have been developed that combine these criteria with ovarian mass volume to determine the preoperative predictive value for malignancy.
In the images that follow, we present 14 cases that demonstrate cystadenoma, low malignant potential tumors, and ovarian neoplasia.
CASE 1. Right ovarian mucinous cystadenoma in 68-year-old woman with uterine prolapse and history of ovarian cyst
CASE 2. Borderline serous cystadenoma in 56-year-old woman with right lower quadrant pain, nausea, and loss of appetite

CASE 3. Mucinous cystadenoma in 38-year-old woman undergoing sonography for spontaneous abortion

CASE 4. Mucinous cystadenoma in 54-year-old woman undergoing follow-up ultrasound for persistent ovarian cyst
CASE 7. Mature cystic teratoma in 31-year-old woman with progressively heavier bleeding and pelvic pain

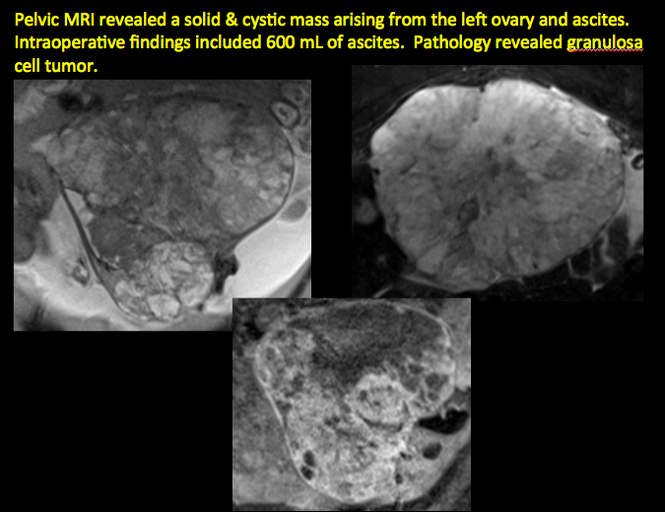
CASE 11. Sex-cord stromal tumor in 61-year-old woman with postmenopausal bleeding
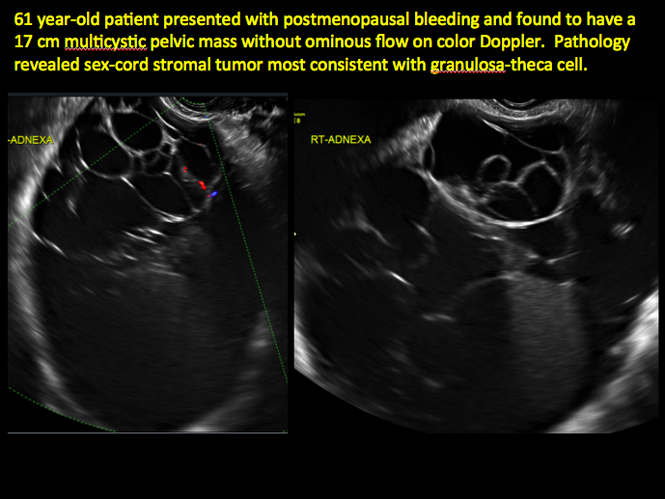
CASE 12. Juvenile granulosa cell tumor in 19-year-old patient with secondary amenorrhea
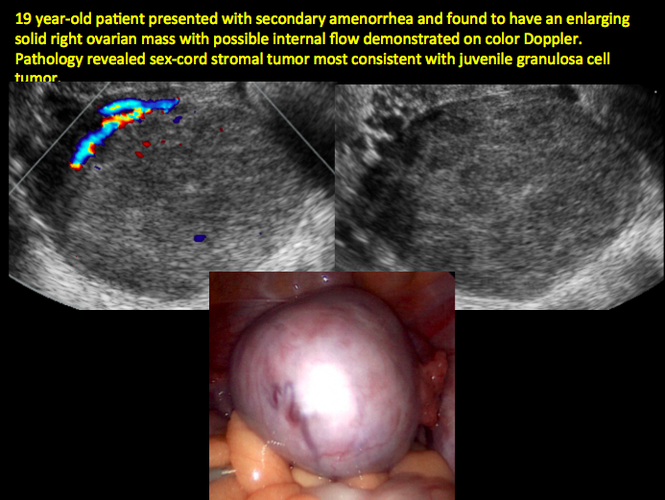
CASE 14. Mucinous borderline tumor in 55-year-old woman with pelvic discomfort
Pelvic ultrasonography remains the preferred imaging method to evaluate most adnexal cysts given its ability to characterize such cysts with high resolution and accuracy. Most cystic adnexal masses have characteristic findings that can guide counseling and management decisions. For instance, mature cystic teratomas have hyperechoic lines/dots and acoustic shadowing; hydrosalpinx are tubular or s shaped and show a “waist sign.”
In parts 1 through 3 of this 4-part series on adnexal pathology, we presented images detailing common benign adnexal cysts, including:
- simple and hemorrhagic cysts (Part 1:Telltale sonographic features of simple and hemorrhagic cysts)
- mature cystic teratomas (dermoid cysts) and endometriomas (Part 2: Imaging the endometrioma and mature cystic teratoma)
- hydrosalpinx and pelvic inclusion cysts (Part 3: “Cogwheel” and other signs of hydrosalpinx and pelvic inclusion cysts)
In this conclusion to the series, we detail imaging for ovarian neoplasias (including cystadenoma and cystadenocarcinoma).
OVARIAN NEOPLASIA
A woman’s lifetime risk of undergoing surgery for suspected ovarian malignancy is 5% to 10% in the United States, and only about 13% to 21% of those undergoing surgery will actually be diagnosed with ovarian cancer.1 Therefore, the goal of diagnostic evaluation is to exclude malignancy.
Diagnostic evaluation includes:
- imaging
- lab work
- history
- physical findings.
The preferred imaging modality for a pelvic mass in asymptomatic premenopausal and postmenopausal women is transvaginal ultrasonography according to the American College of Obstetricians and Gynecologists (ACOG) practice bulletin, which was reaffirmed in 2013.1 “No alternative imaging modality has demonstrated sufficient superiority to transvaginal ultrasonography to justify its routine use.”1
Transvaginal ultrasonography with color Doppler interrogation has demonstrated a sensitivity of 0.86% and a specificity of 0.91% for discriminating between malignant and benign ovarian masses.
Sonographic features that are worrisome for malignancy include:
- Multiple thin septations (if indeterminate, the mass may possibly be benign)
- Thick (> 3 mm), irregular septations
- Focal areas of wall thickening (> 3 mm)
- Mural nodules or papillary projections
- Levine and colleagues note that a cyst with a mural nodule with internal blood flow on color Doppler has the highest likelihood of being malignant2
- Moderate or large amount of ascitic fluid in pelvis (in conjunction with ovarian mass showing the above characteristics)
Various morphology indices have been developed that combine these criteria with ovarian mass volume to determine the preoperative predictive value for malignancy.
In the images that follow, we present 14 cases that demonstrate cystadenoma, low malignant potential tumors, and ovarian neoplasia.
CASE 1. Right ovarian mucinous cystadenoma in 68-year-old woman with uterine prolapse and history of ovarian cyst


CASE 2. Borderline serous cystadenoma in 56-year-old woman with right lower quadrant pain, nausea, and loss of appetite


CASE 3. Mucinous cystadenoma in 38-year-old woman undergoing sonography for spontaneous abortion


CASE 4. Mucinous cystadenoma in 54-year-old woman undergoing follow-up ultrasound for persistent ovarian cyst



CASE 7. Mature cystic teratoma in 31-year-old woman with progressively heavier bleeding and pelvic pain





CASE 11. Sex-cord stromal tumor in 61-year-old woman with postmenopausal bleeding


CASE 12. Juvenile granulosa cell tumor in 19-year-old patient with secondary amenorrhea



CASE 14. Mucinous borderline tumor in 55-year-old woman with pelvic discomfort

Pelvic ultrasonography remains the preferred imaging method to evaluate most adnexal cysts given its ability to characterize such cysts with high resolution and accuracy. Most cystic adnexal masses have characteristic findings that can guide counseling and management decisions. For instance, mature cystic teratomas have hyperechoic lines/dots and acoustic shadowing; hydrosalpinx are tubular or s shaped and show a “waist sign.”
In parts 1 through 3 of this 4-part series on adnexal pathology, we presented images detailing common benign adnexal cysts, including:
- simple and hemorrhagic cysts (Part 1:Telltale sonographic features of simple and hemorrhagic cysts)
- mature cystic teratomas (dermoid cysts) and endometriomas (Part 2: Imaging the endometrioma and mature cystic teratoma)
- hydrosalpinx and pelvic inclusion cysts (Part 3: “Cogwheel” and other signs of hydrosalpinx and pelvic inclusion cysts)
In this conclusion to the series, we detail imaging for ovarian neoplasias (including cystadenoma and cystadenocarcinoma).
OVARIAN NEOPLASIA
A woman’s lifetime risk of undergoing surgery for suspected ovarian malignancy is 5% to 10% in the United States, and only about 13% to 21% of those undergoing surgery will actually be diagnosed with ovarian cancer.1 Therefore, the goal of diagnostic evaluation is to exclude malignancy.
Diagnostic evaluation includes:
- imaging
- lab work
- history
- physical findings.
The preferred imaging modality for a pelvic mass in asymptomatic premenopausal and postmenopausal women is transvaginal ultrasonography according to the American College of Obstetricians and Gynecologists (ACOG) practice bulletin, which was reaffirmed in 2013.1 “No alternative imaging modality has demonstrated sufficient superiority to transvaginal ultrasonography to justify its routine use.”1
Transvaginal ultrasonography with color Doppler interrogation has demonstrated a sensitivity of 0.86% and a specificity of 0.91% for discriminating between malignant and benign ovarian masses.
Sonographic features that are worrisome for malignancy include:
- Multiple thin septations (if indeterminate, the mass may possibly be benign)
- Thick (> 3 mm), irregular septations
- Focal areas of wall thickening (> 3 mm)
- Mural nodules or papillary projections
- Levine and colleagues note that a cyst with a mural nodule with internal blood flow on color Doppler has the highest likelihood of being malignant2
- Moderate or large amount of ascitic fluid in pelvis (in conjunction with ovarian mass showing the above characteristics)
Various morphology indices have been developed that combine these criteria with ovarian mass volume to determine the preoperative predictive value for malignancy.
In the images that follow, we present 14 cases that demonstrate cystadenoma, low malignant potential tumors, and ovarian neoplasia.
CASE 1. Right ovarian mucinous cystadenoma in 68-year-old woman with uterine prolapse and history of ovarian cyst


CASE 2. Borderline serous cystadenoma in 56-year-old woman with right lower quadrant pain, nausea, and loss of appetite


CASE 3. Mucinous cystadenoma in 38-year-old woman undergoing sonography for spontaneous abortion


CASE 4. Mucinous cystadenoma in 54-year-old woman undergoing follow-up ultrasound for persistent ovarian cyst



CASE 7. Mature cystic teratoma in 31-year-old woman with progressively heavier bleeding and pelvic pain





CASE 11. Sex-cord stromal tumor in 61-year-old woman with postmenopausal bleeding


CASE 12. Juvenile granulosa cell tumor in 19-year-old patient with secondary amenorrhea



CASE 14. Mucinous borderline tumor in 55-year-old woman with pelvic discomfort