User login
Combo targets AML, BL in the same way

Image by Ed Uthman
Combining a cholesterol-lowering drug and a contraceptive steroid could be a safe, effective treatment for leukemias, lymphomas, and other malignancies, according to researchers.
Their work helps explain how this combination treatment, bezafibrate and medroxyprogesterone acetate (BaP), kills cancer cells.
The team discovered that BaP’s mechanism of action is the same in acute myeloid leukemia (AML) and Burkitt lymphoma (BL) cells.
The findings have been published in Cancer Research.
BaP has been shown to prolong survival in early stage trials of elderly AML patients, when compared to standard palliative care. BaP has also been used alongside chemotherapy to successfully treat children with BL.
However, it was unclear whether BaP’s activity against these 2 very different malignancies was mediated by a common mechanism or by different effects in each cancer.
To gain some insight, Andrew Southam, PhD, of the University of Birmingham in the UK, and his colleagues investigated the drugs’ effects on the metabolism and chemical make-up of AML and BL cells.
They found that, in both cell types, BaP blocks stearoyl CoA desaturase, an enzyme crucial to the production of fatty acids, which cancer cells need to grow and multiply. The team also showed that BaP’s ability to deactivate stearoyl CoA desaturase was what prompted the cancer cells to die.
“Developing drugs to target the fatty-acid building blocks of cancer cells has been a promising area of research in recent years,” Dr Southam said. “It is very exciting we have identified these non-toxic drugs already sitting on pharmacy shelves.”
He and his colleagues believe these findings also open up the possibility that BaP could be used to treat other cancers that rely on high levels of stearoyl CoA desaturase to grow, including chronic lymphocytic leukemia and some types of non-Hodgkin lymphoma, as well as prostate, colon, and esophageal cancers.
“This drug combination shows real promise,” said Chris Bunce, PhD, also of the University of Birmingham.
“Affordable, effective, non-toxic treatments that extend survival, while offering a good quality of life, are in demand for almost all types of cancer.” ![]()

Image by Ed Uthman
Combining a cholesterol-lowering drug and a contraceptive steroid could be a safe, effective treatment for leukemias, lymphomas, and other malignancies, according to researchers.
Their work helps explain how this combination treatment, bezafibrate and medroxyprogesterone acetate (BaP), kills cancer cells.
The team discovered that BaP’s mechanism of action is the same in acute myeloid leukemia (AML) and Burkitt lymphoma (BL) cells.
The findings have been published in Cancer Research.
BaP has been shown to prolong survival in early stage trials of elderly AML patients, when compared to standard palliative care. BaP has also been used alongside chemotherapy to successfully treat children with BL.
However, it was unclear whether BaP’s activity against these 2 very different malignancies was mediated by a common mechanism or by different effects in each cancer.
To gain some insight, Andrew Southam, PhD, of the University of Birmingham in the UK, and his colleagues investigated the drugs’ effects on the metabolism and chemical make-up of AML and BL cells.
They found that, in both cell types, BaP blocks stearoyl CoA desaturase, an enzyme crucial to the production of fatty acids, which cancer cells need to grow and multiply. The team also showed that BaP’s ability to deactivate stearoyl CoA desaturase was what prompted the cancer cells to die.
“Developing drugs to target the fatty-acid building blocks of cancer cells has been a promising area of research in recent years,” Dr Southam said. “It is very exciting we have identified these non-toxic drugs already sitting on pharmacy shelves.”
He and his colleagues believe these findings also open up the possibility that BaP could be used to treat other cancers that rely on high levels of stearoyl CoA desaturase to grow, including chronic lymphocytic leukemia and some types of non-Hodgkin lymphoma, as well as prostate, colon, and esophageal cancers.
“This drug combination shows real promise,” said Chris Bunce, PhD, also of the University of Birmingham.
“Affordable, effective, non-toxic treatments that extend survival, while offering a good quality of life, are in demand for almost all types of cancer.” ![]()

Image by Ed Uthman
Combining a cholesterol-lowering drug and a contraceptive steroid could be a safe, effective treatment for leukemias, lymphomas, and other malignancies, according to researchers.
Their work helps explain how this combination treatment, bezafibrate and medroxyprogesterone acetate (BaP), kills cancer cells.
The team discovered that BaP’s mechanism of action is the same in acute myeloid leukemia (AML) and Burkitt lymphoma (BL) cells.
The findings have been published in Cancer Research.
BaP has been shown to prolong survival in early stage trials of elderly AML patients, when compared to standard palliative care. BaP has also been used alongside chemotherapy to successfully treat children with BL.
However, it was unclear whether BaP’s activity against these 2 very different malignancies was mediated by a common mechanism or by different effects in each cancer.
To gain some insight, Andrew Southam, PhD, of the University of Birmingham in the UK, and his colleagues investigated the drugs’ effects on the metabolism and chemical make-up of AML and BL cells.
They found that, in both cell types, BaP blocks stearoyl CoA desaturase, an enzyme crucial to the production of fatty acids, which cancer cells need to grow and multiply. The team also showed that BaP’s ability to deactivate stearoyl CoA desaturase was what prompted the cancer cells to die.
“Developing drugs to target the fatty-acid building blocks of cancer cells has been a promising area of research in recent years,” Dr Southam said. “It is very exciting we have identified these non-toxic drugs already sitting on pharmacy shelves.”
He and his colleagues believe these findings also open up the possibility that BaP could be used to treat other cancers that rely on high levels of stearoyl CoA desaturase to grow, including chronic lymphocytic leukemia and some types of non-Hodgkin lymphoma, as well as prostate, colon, and esophageal cancers.
“This drug combination shows real promise,” said Chris Bunce, PhD, also of the University of Birmingham.
“Affordable, effective, non-toxic treatments that extend survival, while offering a good quality of life, are in demand for almost all types of cancer.” ![]()
Consider laser ablation therapy for treatment of benign thyroid nodules
NASHVILLE – Ultrasound-guided laser ablation therapy was found to be a clinically safe, effective, and well-tolerated option for the treatment of benign thyroid nodules, both solid and mixed, in a retrospective, multicenter study presented at the annual meeting of the American Association of Clinical Endocrinologists.
“We know that image-guided laser ablation of solid thyroid nodules has demonstrated favorable results in several prospective randomized trials,” said Dr. Enrico Papini of Regina Apostolorum Hospital in Rome. “However, these results were obtained in selected patients, with single treatments and fixed modalities of treatment; so the question is, what happens in a real. clinical practice?”
Dr. Papini explained that the aim of the study was to assess clinical efficacy and side effects of laser ablation therapy (LAT) in a large series of unselected benign thyroid nodules of variable structure and size, using data from centers who use LAT as a standard operating technique. Patients with solid or mixed nodules with up to 40% fluid composition, benign cytological findings, and normal thyroid function were included.
Clinical records of 1,534 thyroid nodules in 1,531 patients, all of whom were treated in the last 10 years, was collected from eight Italian thyroid referral centers. A total of 1,837 LAT procedures were performed on these nodules, of which 1,280 (83% of 1,534) were treated in a single session. All nodules were treated in no more than three consecutive sessions, with a fixed output power of 3 watts. According to Dr. Papini, the laser is only fired for up to 10 minutes.
Mean nodule volume significantly decreased following LAT from 27 ± 24 mL at baseline to 8 ± 8 mL at 12 months after treatment (P < .001), and mean nodule volume reduction was 72% ± 11%, with an overall range of 48%-100%. Mixed nodules experienced significantly larger decreases than solid ones. On average, mixed nodule volume decreased 79% ± 7%, versus 72% ± 11% for solid nodules (P < .001) because of fluid components being drained prior to LAT.
Symptoms decreased from 49% at baseline to 10% at 12 months post-treatment. Similarly robust reductions were also seen in cosmetic signs, which decreased 86% to 8% over 12 months. Only 17 patients experienced a complication, including 8 with a “major” complication of dysphonia, which resolved within 2-84 days, and 9 with “minor” complications, such as skin burn and hematoma.
“Laser ablation was performed in outpatient setting, with no hospital admission after treatment,” said Dr. Papini. “It was well-tolerated, and severe pain – requiring more than 3 days of analgesics – occurred in less than 2% of patients.”
Dr. Papini did not report any relevant financial disclosures.
NASHVILLE – Ultrasound-guided laser ablation therapy was found to be a clinically safe, effective, and well-tolerated option for the treatment of benign thyroid nodules, both solid and mixed, in a retrospective, multicenter study presented at the annual meeting of the American Association of Clinical Endocrinologists.
“We know that image-guided laser ablation of solid thyroid nodules has demonstrated favorable results in several prospective randomized trials,” said Dr. Enrico Papini of Regina Apostolorum Hospital in Rome. “However, these results were obtained in selected patients, with single treatments and fixed modalities of treatment; so the question is, what happens in a real. clinical practice?”
Dr. Papini explained that the aim of the study was to assess clinical efficacy and side effects of laser ablation therapy (LAT) in a large series of unselected benign thyroid nodules of variable structure and size, using data from centers who use LAT as a standard operating technique. Patients with solid or mixed nodules with up to 40% fluid composition, benign cytological findings, and normal thyroid function were included.
Clinical records of 1,534 thyroid nodules in 1,531 patients, all of whom were treated in the last 10 years, was collected from eight Italian thyroid referral centers. A total of 1,837 LAT procedures were performed on these nodules, of which 1,280 (83% of 1,534) were treated in a single session. All nodules were treated in no more than three consecutive sessions, with a fixed output power of 3 watts. According to Dr. Papini, the laser is only fired for up to 10 minutes.
Mean nodule volume significantly decreased following LAT from 27 ± 24 mL at baseline to 8 ± 8 mL at 12 months after treatment (P < .001), and mean nodule volume reduction was 72% ± 11%, with an overall range of 48%-100%. Mixed nodules experienced significantly larger decreases than solid ones. On average, mixed nodule volume decreased 79% ± 7%, versus 72% ± 11% for solid nodules (P < .001) because of fluid components being drained prior to LAT.
Symptoms decreased from 49% at baseline to 10% at 12 months post-treatment. Similarly robust reductions were also seen in cosmetic signs, which decreased 86% to 8% over 12 months. Only 17 patients experienced a complication, including 8 with a “major” complication of dysphonia, which resolved within 2-84 days, and 9 with “minor” complications, such as skin burn and hematoma.
“Laser ablation was performed in outpatient setting, with no hospital admission after treatment,” said Dr. Papini. “It was well-tolerated, and severe pain – requiring more than 3 days of analgesics – occurred in less than 2% of patients.”
Dr. Papini did not report any relevant financial disclosures.
NASHVILLE – Ultrasound-guided laser ablation therapy was found to be a clinically safe, effective, and well-tolerated option for the treatment of benign thyroid nodules, both solid and mixed, in a retrospective, multicenter study presented at the annual meeting of the American Association of Clinical Endocrinologists.
“We know that image-guided laser ablation of solid thyroid nodules has demonstrated favorable results in several prospective randomized trials,” said Dr. Enrico Papini of Regina Apostolorum Hospital in Rome. “However, these results were obtained in selected patients, with single treatments and fixed modalities of treatment; so the question is, what happens in a real. clinical practice?”
Dr. Papini explained that the aim of the study was to assess clinical efficacy and side effects of laser ablation therapy (LAT) in a large series of unselected benign thyroid nodules of variable structure and size, using data from centers who use LAT as a standard operating technique. Patients with solid or mixed nodules with up to 40% fluid composition, benign cytological findings, and normal thyroid function were included.
Clinical records of 1,534 thyroid nodules in 1,531 patients, all of whom were treated in the last 10 years, was collected from eight Italian thyroid referral centers. A total of 1,837 LAT procedures were performed on these nodules, of which 1,280 (83% of 1,534) were treated in a single session. All nodules were treated in no more than three consecutive sessions, with a fixed output power of 3 watts. According to Dr. Papini, the laser is only fired for up to 10 minutes.
Mean nodule volume significantly decreased following LAT from 27 ± 24 mL at baseline to 8 ± 8 mL at 12 months after treatment (P < .001), and mean nodule volume reduction was 72% ± 11%, with an overall range of 48%-100%. Mixed nodules experienced significantly larger decreases than solid ones. On average, mixed nodule volume decreased 79% ± 7%, versus 72% ± 11% for solid nodules (P < .001) because of fluid components being drained prior to LAT.
Symptoms decreased from 49% at baseline to 10% at 12 months post-treatment. Similarly robust reductions were also seen in cosmetic signs, which decreased 86% to 8% over 12 months. Only 17 patients experienced a complication, including 8 with a “major” complication of dysphonia, which resolved within 2-84 days, and 9 with “minor” complications, such as skin burn and hematoma.
“Laser ablation was performed in outpatient setting, with no hospital admission after treatment,” said Dr. Papini. “It was well-tolerated, and severe pain – requiring more than 3 days of analgesics – occurred in less than 2% of patients.”
Dr. Papini did not report any relevant financial disclosures.
AT AACE 2015
Key clinical point: Ultrasound-guided laser ablation therapy is a clinically effective and well-tolerated tool for treating benign solid and mixed thyroid nodules.
Major finding: In 1,837 treatments for 1,534 nodules, mean nodule volume decreased from 27 ± 24 mL at baseline to 8 ± 8 mL at 12 months after treatment (P < .001), and mean nodule volume reduction was 72% ± 11% (range 48%-100%).
Data source: Retrospective, multicenter study of 1,534 benign solid and mixed thyroid nodules.
Disclosures: Dr. Papini did not report any relevant financial disclosures.
Botox treatments improve urinary incontinence in neurogenic bladder dysfunction
NEW ORLEANS – Regular injections of onabotulinumtoxinA significantly decreased urinary incontinence in patients with neurogenic detrusor bladder overactivity over 4 years of follow-up in 4-year extension study results of a randomized trial.
Incontinence episodes decreased from an average of four per day to one or less after each treatment, Dr. Eric Rovner said at the annual meeting of the American Urological Association.
Each treatment was effective for about 9 months, and the benefit consistent throughout the 4-year study, said Dr. Rovner of the Medical University of South Carolina, Charleston.
About 90% of patients had at least a 50% reduction in incontinence episodes, and more than half experienced a complete cessation of incontinence.
OnabotulinumtoxinA (Botox) was approved in 2011 as a treatment for neurogenic urinary incontinence. Each treatment consists of 20 injections delivered cystoscopically.
Dr. Rovner reported a post hoc analysis of 227 patients who completed 4 years of treatment – a 1-year placebo-controlled trial, and 3 years of open-label extension with a dosage of 200 units of onabotulinumtoxinA.
Patients were relatively young (mean 45 years); about half were male. Most (53%) had multiple sclerosis. The remainder had a spinal cord injury that affected bladder function. Half were taking an anticholinergic medication, but had not responded to it.
Most patients (71%) were already performing intermittent catheterization. Despite that, they had a mean of four incontinence episodes each day.
Over the entire 4 years, onabotulinumtoxinA was associated with significant and consistent improvements in incontinence, with a mean decrease of up to 3.8 incidents per day each year. Each year, about 90% experienced at least a 50% improvement. About half experienced a complete cessation of incontinence over the period.
Urinary tract infections occurred in 20% of patients in years 1 and 2, and 18% in years 3 and 4, which was not significantly different. Urinary retention was highest in year 1 (12%) and dropped to 2% by years 3 and 4.
In the first year, 39% of those who didn’t need intermittent catheterization at baseline had to begin doing so. By year 2, the de novo catheterization rate was 11%. It was 8% in year 3, and in year 4, there were no new catheterizations.
These changes were not only statistically significant, but clinically important, Dr. Rovner said. On a secondary measure, the Incontinence Quality of Life Questionnaire (I-QOL), patients experienced a mean increase of more than 30 points over each study year. An 11-point change is usually considered clinically meaningful, he said.
“This was making a big difference for these patients.”
Dr. Rovner disclosed relationships with Allergan and a number of other pharmaceutical and medical device companies.
On Twitter @alz_gal
NEW ORLEANS – Regular injections of onabotulinumtoxinA significantly decreased urinary incontinence in patients with neurogenic detrusor bladder overactivity over 4 years of follow-up in 4-year extension study results of a randomized trial.
Incontinence episodes decreased from an average of four per day to one or less after each treatment, Dr. Eric Rovner said at the annual meeting of the American Urological Association.
Each treatment was effective for about 9 months, and the benefit consistent throughout the 4-year study, said Dr. Rovner of the Medical University of South Carolina, Charleston.
About 90% of patients had at least a 50% reduction in incontinence episodes, and more than half experienced a complete cessation of incontinence.
OnabotulinumtoxinA (Botox) was approved in 2011 as a treatment for neurogenic urinary incontinence. Each treatment consists of 20 injections delivered cystoscopically.
Dr. Rovner reported a post hoc analysis of 227 patients who completed 4 years of treatment – a 1-year placebo-controlled trial, and 3 years of open-label extension with a dosage of 200 units of onabotulinumtoxinA.
Patients were relatively young (mean 45 years); about half were male. Most (53%) had multiple sclerosis. The remainder had a spinal cord injury that affected bladder function. Half were taking an anticholinergic medication, but had not responded to it.
Most patients (71%) were already performing intermittent catheterization. Despite that, they had a mean of four incontinence episodes each day.
Over the entire 4 years, onabotulinumtoxinA was associated with significant and consistent improvements in incontinence, with a mean decrease of up to 3.8 incidents per day each year. Each year, about 90% experienced at least a 50% improvement. About half experienced a complete cessation of incontinence over the period.
Urinary tract infections occurred in 20% of patients in years 1 and 2, and 18% in years 3 and 4, which was not significantly different. Urinary retention was highest in year 1 (12%) and dropped to 2% by years 3 and 4.
In the first year, 39% of those who didn’t need intermittent catheterization at baseline had to begin doing so. By year 2, the de novo catheterization rate was 11%. It was 8% in year 3, and in year 4, there were no new catheterizations.
These changes were not only statistically significant, but clinically important, Dr. Rovner said. On a secondary measure, the Incontinence Quality of Life Questionnaire (I-QOL), patients experienced a mean increase of more than 30 points over each study year. An 11-point change is usually considered clinically meaningful, he said.
“This was making a big difference for these patients.”
Dr. Rovner disclosed relationships with Allergan and a number of other pharmaceutical and medical device companies.
On Twitter @alz_gal
NEW ORLEANS – Regular injections of onabotulinumtoxinA significantly decreased urinary incontinence in patients with neurogenic detrusor bladder overactivity over 4 years of follow-up in 4-year extension study results of a randomized trial.
Incontinence episodes decreased from an average of four per day to one or less after each treatment, Dr. Eric Rovner said at the annual meeting of the American Urological Association.
Each treatment was effective for about 9 months, and the benefit consistent throughout the 4-year study, said Dr. Rovner of the Medical University of South Carolina, Charleston.
About 90% of patients had at least a 50% reduction in incontinence episodes, and more than half experienced a complete cessation of incontinence.
OnabotulinumtoxinA (Botox) was approved in 2011 as a treatment for neurogenic urinary incontinence. Each treatment consists of 20 injections delivered cystoscopically.
Dr. Rovner reported a post hoc analysis of 227 patients who completed 4 years of treatment – a 1-year placebo-controlled trial, and 3 years of open-label extension with a dosage of 200 units of onabotulinumtoxinA.
Patients were relatively young (mean 45 years); about half were male. Most (53%) had multiple sclerosis. The remainder had a spinal cord injury that affected bladder function. Half were taking an anticholinergic medication, but had not responded to it.
Most patients (71%) were already performing intermittent catheterization. Despite that, they had a mean of four incontinence episodes each day.
Over the entire 4 years, onabotulinumtoxinA was associated with significant and consistent improvements in incontinence, with a mean decrease of up to 3.8 incidents per day each year. Each year, about 90% experienced at least a 50% improvement. About half experienced a complete cessation of incontinence over the period.
Urinary tract infections occurred in 20% of patients in years 1 and 2, and 18% in years 3 and 4, which was not significantly different. Urinary retention was highest in year 1 (12%) and dropped to 2% by years 3 and 4.
In the first year, 39% of those who didn’t need intermittent catheterization at baseline had to begin doing so. By year 2, the de novo catheterization rate was 11%. It was 8% in year 3, and in year 4, there were no new catheterizations.
These changes were not only statistically significant, but clinically important, Dr. Rovner said. On a secondary measure, the Incontinence Quality of Life Questionnaire (I-QOL), patients experienced a mean increase of more than 30 points over each study year. An 11-point change is usually considered clinically meaningful, he said.
“This was making a big difference for these patients.”
Dr. Rovner disclosed relationships with Allergan and a number of other pharmaceutical and medical device companies.
On Twitter @alz_gal
AT THE AUA ANNUAL MEETING
Key clinical point: OnabotulinumtoxinA injections produced a consistent and significant improvement in urinary incontinence in patients with neurogenic detrusor bladder overactivity.
Major finding: Nearly 90% of patients had at least a 50% improvement in incontinence episodes, and almost half experienced a complete cessation.
Data source: A post hoc analysis of 4-year results from a 1-year, randomized, placebo-controlled trial with 3 years of open-label extension in 227 patients.
Disclosures: Dr. Rovner disclosed relationships with Allergan and a number of other pharmaceutical and medical device companies.
How mAbs produce lasting antitumor effects

Photo by Linda Bartlett
Results of preclinical research help explain how antitumor monoclonal antibodies (mAbs) fight lymphoma.
Researchers uncovered a 2-step process that revolves around 2 antibody-binding receptors found on different types of immune cells.
Experiments suggested that these Fc receptors are needed to eradicate lymphoma and ensure it doesn’t return.
The researchers reported these findings in an article published in Cell.
“These findings suggests ways current anticancer antibody treatments might be improved, as well as combined with other immune-system-stimulating therapies to help cancer patients,” said study author Jeffrey Ravetch, MD, PhD, of The Rockefeller University in New York, New York.
Previous research has shown that antitumor mAbs bind to Fc receptors on activated immune cells, prompting those immune cells to kill tumors.
However, it was unclear which Fc receptors are involved or how the tumor killing led the immune system to generate memory T cells against these same antigens, in case the tumor producing them should return.
Dr Ravetch and David DiLillo, PhD, also of The Rockefeller University, investigated this process by injecting CD20-expressing lymphoma cells into mice with immune systems engineered to contain human Fc receptors, treating the mice with anti-CD20 mAbs, and then re-introducing lymphoma.
Wild-type C57BL/6 mice received syngeneic EL4 lymphoma cells expressing human CD20 (EL4-hCD20 cells). When these mice received treatment with an mIgG2a isotype anti-hCD20 mAb, they all survived.
Ninety days later, after the mAb had been cleared from their systems, the mice were re-challenged with EL4-hCD20 tumor cells, at a dose 10-fold greater than the initial challenge, but they did not receive any additional mAb.
All of these mice survived, but tumor/mAb-primed mice that were re-challenged with EL4-wild-type cells, which don’t express hCD20, had poor survival. Results were similar with a different anti-hCD20 mAb, clone 2B8.
The researchers also re-challenged tumor/mAb-primed mice with B6BL lymphoma cells that expressed either hCD20 or an irrelevant antigen, mCD20. All of the mice re-challenged with B6BL-mCD20 cells had died by day 31, but 80% of the mice re-challenged with B6BL-hCD20 cells survived at least 90 days.
Drs Ravetch and DiLillo said these results suggest an anti-hCD20 immune response is generated after the initial FcγR-mediated clearance of tumor cells by antibody-dependent cellular cytotoxicity.
The researchers then took a closer look at the role of Fc receptors, keeping in mind that different types of immune cells can express different receptors.
Based on the cells the researchers thought were involved, they looked to the Fc receptors expressed by cytotoxic immune cells that carried out the initial attack on tumors, as well as the Fc receptors found on dendritic cells, which are crucial to the formation of memory T cells.
To test the involvement of these receptors, the pair altered the mAbs delivered to the lymphoma-infected mice so as to change their affinity for the receptors. Then, they looked for changes in the survival rate of the mice after the first and second challenges with lymphoma cells.
When they dissected this process, the researchers uncovered 2 steps. The Fc receptor FcγRIIIA, which is found on macrophages, responded to mAbs and prompted the macrophages to engulf and destroy the antibody-laden tumor cells.
These same antibodies, still attached to tumor antigens, activated a second receptor, FcγRIIA, on dendritic cells, which used the antigen to prime T cells. The result was the generation of a T-cell memory response that protected the mice against future lymphoma cells expressing CD20.
“By engineering the antibodies so as to increase their affinity for both FcγRIIIA and FcγRIIA, we were able to optimize both steps in this process,” Dr DiLillo said.
“Current antibody therapies are only engineered to improve the immediate killing of tumor cells but not the formation of immunological memory. We are proposing that an ideal antibody therapy would be engineered to take full advantage of both steps.” ![]()

Photo by Linda Bartlett
Results of preclinical research help explain how antitumor monoclonal antibodies (mAbs) fight lymphoma.
Researchers uncovered a 2-step process that revolves around 2 antibody-binding receptors found on different types of immune cells.
Experiments suggested that these Fc receptors are needed to eradicate lymphoma and ensure it doesn’t return.
The researchers reported these findings in an article published in Cell.
“These findings suggests ways current anticancer antibody treatments might be improved, as well as combined with other immune-system-stimulating therapies to help cancer patients,” said study author Jeffrey Ravetch, MD, PhD, of The Rockefeller University in New York, New York.
Previous research has shown that antitumor mAbs bind to Fc receptors on activated immune cells, prompting those immune cells to kill tumors.
However, it was unclear which Fc receptors are involved or how the tumor killing led the immune system to generate memory T cells against these same antigens, in case the tumor producing them should return.
Dr Ravetch and David DiLillo, PhD, also of The Rockefeller University, investigated this process by injecting CD20-expressing lymphoma cells into mice with immune systems engineered to contain human Fc receptors, treating the mice with anti-CD20 mAbs, and then re-introducing lymphoma.
Wild-type C57BL/6 mice received syngeneic EL4 lymphoma cells expressing human CD20 (EL4-hCD20 cells). When these mice received treatment with an mIgG2a isotype anti-hCD20 mAb, they all survived.
Ninety days later, after the mAb had been cleared from their systems, the mice were re-challenged with EL4-hCD20 tumor cells, at a dose 10-fold greater than the initial challenge, but they did not receive any additional mAb.
All of these mice survived, but tumor/mAb-primed mice that were re-challenged with EL4-wild-type cells, which don’t express hCD20, had poor survival. Results were similar with a different anti-hCD20 mAb, clone 2B8.
The researchers also re-challenged tumor/mAb-primed mice with B6BL lymphoma cells that expressed either hCD20 or an irrelevant antigen, mCD20. All of the mice re-challenged with B6BL-mCD20 cells had died by day 31, but 80% of the mice re-challenged with B6BL-hCD20 cells survived at least 90 days.
Drs Ravetch and DiLillo said these results suggest an anti-hCD20 immune response is generated after the initial FcγR-mediated clearance of tumor cells by antibody-dependent cellular cytotoxicity.
The researchers then took a closer look at the role of Fc receptors, keeping in mind that different types of immune cells can express different receptors.
Based on the cells the researchers thought were involved, they looked to the Fc receptors expressed by cytotoxic immune cells that carried out the initial attack on tumors, as well as the Fc receptors found on dendritic cells, which are crucial to the formation of memory T cells.
To test the involvement of these receptors, the pair altered the mAbs delivered to the lymphoma-infected mice so as to change their affinity for the receptors. Then, they looked for changes in the survival rate of the mice after the first and second challenges with lymphoma cells.
When they dissected this process, the researchers uncovered 2 steps. The Fc receptor FcγRIIIA, which is found on macrophages, responded to mAbs and prompted the macrophages to engulf and destroy the antibody-laden tumor cells.
These same antibodies, still attached to tumor antigens, activated a second receptor, FcγRIIA, on dendritic cells, which used the antigen to prime T cells. The result was the generation of a T-cell memory response that protected the mice against future lymphoma cells expressing CD20.
“By engineering the antibodies so as to increase their affinity for both FcγRIIIA and FcγRIIA, we were able to optimize both steps in this process,” Dr DiLillo said.
“Current antibody therapies are only engineered to improve the immediate killing of tumor cells but not the formation of immunological memory. We are proposing that an ideal antibody therapy would be engineered to take full advantage of both steps.” ![]()

Photo by Linda Bartlett
Results of preclinical research help explain how antitumor monoclonal antibodies (mAbs) fight lymphoma.
Researchers uncovered a 2-step process that revolves around 2 antibody-binding receptors found on different types of immune cells.
Experiments suggested that these Fc receptors are needed to eradicate lymphoma and ensure it doesn’t return.
The researchers reported these findings in an article published in Cell.
“These findings suggests ways current anticancer antibody treatments might be improved, as well as combined with other immune-system-stimulating therapies to help cancer patients,” said study author Jeffrey Ravetch, MD, PhD, of The Rockefeller University in New York, New York.
Previous research has shown that antitumor mAbs bind to Fc receptors on activated immune cells, prompting those immune cells to kill tumors.
However, it was unclear which Fc receptors are involved or how the tumor killing led the immune system to generate memory T cells against these same antigens, in case the tumor producing them should return.
Dr Ravetch and David DiLillo, PhD, also of The Rockefeller University, investigated this process by injecting CD20-expressing lymphoma cells into mice with immune systems engineered to contain human Fc receptors, treating the mice with anti-CD20 mAbs, and then re-introducing lymphoma.
Wild-type C57BL/6 mice received syngeneic EL4 lymphoma cells expressing human CD20 (EL4-hCD20 cells). When these mice received treatment with an mIgG2a isotype anti-hCD20 mAb, they all survived.
Ninety days later, after the mAb had been cleared from their systems, the mice were re-challenged with EL4-hCD20 tumor cells, at a dose 10-fold greater than the initial challenge, but they did not receive any additional mAb.
All of these mice survived, but tumor/mAb-primed mice that were re-challenged with EL4-wild-type cells, which don’t express hCD20, had poor survival. Results were similar with a different anti-hCD20 mAb, clone 2B8.
The researchers also re-challenged tumor/mAb-primed mice with B6BL lymphoma cells that expressed either hCD20 or an irrelevant antigen, mCD20. All of the mice re-challenged with B6BL-mCD20 cells had died by day 31, but 80% of the mice re-challenged with B6BL-hCD20 cells survived at least 90 days.
Drs Ravetch and DiLillo said these results suggest an anti-hCD20 immune response is generated after the initial FcγR-mediated clearance of tumor cells by antibody-dependent cellular cytotoxicity.
The researchers then took a closer look at the role of Fc receptors, keeping in mind that different types of immune cells can express different receptors.
Based on the cells the researchers thought were involved, they looked to the Fc receptors expressed by cytotoxic immune cells that carried out the initial attack on tumors, as well as the Fc receptors found on dendritic cells, which are crucial to the formation of memory T cells.
To test the involvement of these receptors, the pair altered the mAbs delivered to the lymphoma-infected mice so as to change their affinity for the receptors. Then, they looked for changes in the survival rate of the mice after the first and second challenges with lymphoma cells.
When they dissected this process, the researchers uncovered 2 steps. The Fc receptor FcγRIIIA, which is found on macrophages, responded to mAbs and prompted the macrophages to engulf and destroy the antibody-laden tumor cells.
These same antibodies, still attached to tumor antigens, activated a second receptor, FcγRIIA, on dendritic cells, which used the antigen to prime T cells. The result was the generation of a T-cell memory response that protected the mice against future lymphoma cells expressing CD20.
“By engineering the antibodies so as to increase their affinity for both FcγRIIIA and FcγRIIA, we were able to optimize both steps in this process,” Dr DiLillo said.
“Current antibody therapies are only engineered to improve the immediate killing of tumor cells but not the formation of immunological memory. We are proposing that an ideal antibody therapy would be engineered to take full advantage of both steps.” ![]()
Corrona Begins
Over the last 15 years the treatment of psoriasis has been transformed with the advent of biologic agents. Now we have a whole new generation of treatments that is emerging. With all of these therapeutic options, the dermatologic community is in need of increasing data to help us further understand both the therapies and the disease state.
A new independent US psoriasis registry has been established. This registry is a joint collaboration with the National Psoriasis Foundation and Corrona, Inc (Consortium of Rheumatology Researchers of North America, Inc). Data will be gathered through comprehensive questionnaires completed by patients and their dermatologists during appointments.
The registry will function to collect and analyze clinical data, and thereby allow investigators to achieve the following: (1) compare the safety and effectiveness of psoriasis treatments, (2) better understand psoriasis comorbidities, and (3) explore the natural history of the disease.
The registry will begin recruiting patients this year. Initially, the registry will track the drug safety reporting for secukinumab. The goal is for the CORRONA psoriasis registry to enroll at least 3000 patients with psoriasis who are taking secukinumab and then follow their treatment for at least 8 years.
In addition to studying safety and effectiveness of therapeutics, the registry also will help identify potential etiologies of psoriasis, study the relationship between psoriasis and other health conditions, and examine the impact of the condition on quality of life, among other outcomes.
To become an investigator in the registry or learn more about it, visit www.psoriasis.org/corrona-registry.
What’s the issue?
This registry is a welcomed addition to the study of psoriasis. It has the potential to add critical information in the years to come.
Over the last 15 years the treatment of psoriasis has been transformed with the advent of biologic agents. Now we have a whole new generation of treatments that is emerging. With all of these therapeutic options, the dermatologic community is in need of increasing data to help us further understand both the therapies and the disease state.
A new independent US psoriasis registry has been established. This registry is a joint collaboration with the National Psoriasis Foundation and Corrona, Inc (Consortium of Rheumatology Researchers of North America, Inc). Data will be gathered through comprehensive questionnaires completed by patients and their dermatologists during appointments.
The registry will function to collect and analyze clinical data, and thereby allow investigators to achieve the following: (1) compare the safety and effectiveness of psoriasis treatments, (2) better understand psoriasis comorbidities, and (3) explore the natural history of the disease.
The registry will begin recruiting patients this year. Initially, the registry will track the drug safety reporting for secukinumab. The goal is for the CORRONA psoriasis registry to enroll at least 3000 patients with psoriasis who are taking secukinumab and then follow their treatment for at least 8 years.
In addition to studying safety and effectiveness of therapeutics, the registry also will help identify potential etiologies of psoriasis, study the relationship between psoriasis and other health conditions, and examine the impact of the condition on quality of life, among other outcomes.
To become an investigator in the registry or learn more about it, visit www.psoriasis.org/corrona-registry.
What’s the issue?
This registry is a welcomed addition to the study of psoriasis. It has the potential to add critical information in the years to come.
Over the last 15 years the treatment of psoriasis has been transformed with the advent of biologic agents. Now we have a whole new generation of treatments that is emerging. With all of these therapeutic options, the dermatologic community is in need of increasing data to help us further understand both the therapies and the disease state.
A new independent US psoriasis registry has been established. This registry is a joint collaboration with the National Psoriasis Foundation and Corrona, Inc (Consortium of Rheumatology Researchers of North America, Inc). Data will be gathered through comprehensive questionnaires completed by patients and their dermatologists during appointments.
The registry will function to collect and analyze clinical data, and thereby allow investigators to achieve the following: (1) compare the safety and effectiveness of psoriasis treatments, (2) better understand psoriasis comorbidities, and (3) explore the natural history of the disease.
The registry will begin recruiting patients this year. Initially, the registry will track the drug safety reporting for secukinumab. The goal is for the CORRONA psoriasis registry to enroll at least 3000 patients with psoriasis who are taking secukinumab and then follow their treatment for at least 8 years.
In addition to studying safety and effectiveness of therapeutics, the registry also will help identify potential etiologies of psoriasis, study the relationship between psoriasis and other health conditions, and examine the impact of the condition on quality of life, among other outcomes.
To become an investigator in the registry or learn more about it, visit www.psoriasis.org/corrona-registry.
What’s the issue?
This registry is a welcomed addition to the study of psoriasis. It has the potential to add critical information in the years to come.
SAEM: STEMI in the ED: Will lower incidence threaten timely care?
SAN DIEGO – Although cardiovascular disease is on the rise, incidence of ST-elevation myocardial infarction has steadily declined in recent years, with STEMI visits to emergency departments dropping by almost a third between 2006 and 2011, and STEMI-related hospitalizations down as well.
The decline is likely the result of better medical management of known cardiovascular disease, resulting in fewer STEMIs. It may also stem from the bypassing of emergency departments by emergency medical technicians, who can take patients straight to a catheterization lab when they detect STEMI, said Dr. Michael J. Ward, a leading researcher in emergency health care from Vanderbilt University in Nashville, Tenn., at the annual meeting of the Society for Academic Emergency Medicine.
But this trend, while a good thing for most patients, presents potential pitfalls for emergency departments in achieving timely treatment, he said.
In a STEMI incidence study using data on about 1.43 million ED STEMI visits from the Nationwide Emergency Department Sample (NEDS) during 2006-2011, ED STEMI visits per 10,000 U.S. adults declined significantly, from 10.1 in 2006 to 7.3 in 2011. Declines were seen across all age groups and regions during the study period, Dr. Ward and colleagues found in their recently published study (Am. J. Cardiol. 2015;115:167-70).
In a separate analysis of the same data, transfer rates of STEMI patients increased from 15% in 2006 to 20.6% in 2011. Patients without insurance were 60% (adjusted odds ratio, 1.64) more likely to be transferred when presenting to an ED with STEMI than patients with insurance, the investigators found.
Both trends – the decline in presentations to the ED and the increase in transfers – could mean higher risk for patients presenting to EDs with STEMI, Dr. Ward said in an interview.
“You basically have 90 minutes from the time a STEMI patient presents to get the vessel open,” Dr. Ward said. “There’s really very little margin for error. If you’re seeing fewer STEMIs, are you and your staff going to be less practiced? And what if patients present unusually? What if it’s not the older male with chest pain, but a younger female with back pain or just not feeling well?”
The finding of an increase in transfers is problematic as well, he said. “Only about a third of ED facilities have catheterization capabilities. As EDs see fewer and fewer STEMI patients, they may not be able to maintain their ability to recognize and care for them, or develop a lower threshold for transfer.”
Even after adjusting for confounders such as age, presentation at a rural facility, and presentation on a weekend, the likelihood for transfer among self-pay patients, compared with those with any form of insurance, including Medicare and Medicaid, was increased by 64%, Dr. Ward reported.
The findings show that STEMI patients without insurance “are much more likely to be transferred, receiving less timely and therefore lower quality care for the most severe form of heart attack,” Dr. Ward said.
The reasons for this are unknown, he said. “One may be that patients without insurance are presenting to facilities that don’t have the ability to treat them: rural facilities, or facilities without the capability to treat this particular type of emergency. The other possibility is that they’re presenting to one that does have the capability, yet they’re still being transferred.”
Even if a patient with STEMI presents to a facility without the capability to treat a STEMI, and there’s another next door that can, “it still introduces a significant delay,” and with that higher risks, he said.
Dr. Ward’s research was funded by grants from the National Institutes of Health. He disclosed no conflicts of interest.
SAN DIEGO – Although cardiovascular disease is on the rise, incidence of ST-elevation myocardial infarction has steadily declined in recent years, with STEMI visits to emergency departments dropping by almost a third between 2006 and 2011, and STEMI-related hospitalizations down as well.
The decline is likely the result of better medical management of known cardiovascular disease, resulting in fewer STEMIs. It may also stem from the bypassing of emergency departments by emergency medical technicians, who can take patients straight to a catheterization lab when they detect STEMI, said Dr. Michael J. Ward, a leading researcher in emergency health care from Vanderbilt University in Nashville, Tenn., at the annual meeting of the Society for Academic Emergency Medicine.
But this trend, while a good thing for most patients, presents potential pitfalls for emergency departments in achieving timely treatment, he said.
In a STEMI incidence study using data on about 1.43 million ED STEMI visits from the Nationwide Emergency Department Sample (NEDS) during 2006-2011, ED STEMI visits per 10,000 U.S. adults declined significantly, from 10.1 in 2006 to 7.3 in 2011. Declines were seen across all age groups and regions during the study period, Dr. Ward and colleagues found in their recently published study (Am. J. Cardiol. 2015;115:167-70).
In a separate analysis of the same data, transfer rates of STEMI patients increased from 15% in 2006 to 20.6% in 2011. Patients without insurance were 60% (adjusted odds ratio, 1.64) more likely to be transferred when presenting to an ED with STEMI than patients with insurance, the investigators found.
Both trends – the decline in presentations to the ED and the increase in transfers – could mean higher risk for patients presenting to EDs with STEMI, Dr. Ward said in an interview.
“You basically have 90 minutes from the time a STEMI patient presents to get the vessel open,” Dr. Ward said. “There’s really very little margin for error. If you’re seeing fewer STEMIs, are you and your staff going to be less practiced? And what if patients present unusually? What if it’s not the older male with chest pain, but a younger female with back pain or just not feeling well?”
The finding of an increase in transfers is problematic as well, he said. “Only about a third of ED facilities have catheterization capabilities. As EDs see fewer and fewer STEMI patients, they may not be able to maintain their ability to recognize and care for them, or develop a lower threshold for transfer.”
Even after adjusting for confounders such as age, presentation at a rural facility, and presentation on a weekend, the likelihood for transfer among self-pay patients, compared with those with any form of insurance, including Medicare and Medicaid, was increased by 64%, Dr. Ward reported.
The findings show that STEMI patients without insurance “are much more likely to be transferred, receiving less timely and therefore lower quality care for the most severe form of heart attack,” Dr. Ward said.
The reasons for this are unknown, he said. “One may be that patients without insurance are presenting to facilities that don’t have the ability to treat them: rural facilities, or facilities without the capability to treat this particular type of emergency. The other possibility is that they’re presenting to one that does have the capability, yet they’re still being transferred.”
Even if a patient with STEMI presents to a facility without the capability to treat a STEMI, and there’s another next door that can, “it still introduces a significant delay,” and with that higher risks, he said.
Dr. Ward’s research was funded by grants from the National Institutes of Health. He disclosed no conflicts of interest.
SAN DIEGO – Although cardiovascular disease is on the rise, incidence of ST-elevation myocardial infarction has steadily declined in recent years, with STEMI visits to emergency departments dropping by almost a third between 2006 and 2011, and STEMI-related hospitalizations down as well.
The decline is likely the result of better medical management of known cardiovascular disease, resulting in fewer STEMIs. It may also stem from the bypassing of emergency departments by emergency medical technicians, who can take patients straight to a catheterization lab when they detect STEMI, said Dr. Michael J. Ward, a leading researcher in emergency health care from Vanderbilt University in Nashville, Tenn., at the annual meeting of the Society for Academic Emergency Medicine.
But this trend, while a good thing for most patients, presents potential pitfalls for emergency departments in achieving timely treatment, he said.
In a STEMI incidence study using data on about 1.43 million ED STEMI visits from the Nationwide Emergency Department Sample (NEDS) during 2006-2011, ED STEMI visits per 10,000 U.S. adults declined significantly, from 10.1 in 2006 to 7.3 in 2011. Declines were seen across all age groups and regions during the study period, Dr. Ward and colleagues found in their recently published study (Am. J. Cardiol. 2015;115:167-70).
In a separate analysis of the same data, transfer rates of STEMI patients increased from 15% in 2006 to 20.6% in 2011. Patients without insurance were 60% (adjusted odds ratio, 1.64) more likely to be transferred when presenting to an ED with STEMI than patients with insurance, the investigators found.
Both trends – the decline in presentations to the ED and the increase in transfers – could mean higher risk for patients presenting to EDs with STEMI, Dr. Ward said in an interview.
“You basically have 90 minutes from the time a STEMI patient presents to get the vessel open,” Dr. Ward said. “There’s really very little margin for error. If you’re seeing fewer STEMIs, are you and your staff going to be less practiced? And what if patients present unusually? What if it’s not the older male with chest pain, but a younger female with back pain or just not feeling well?”
The finding of an increase in transfers is problematic as well, he said. “Only about a third of ED facilities have catheterization capabilities. As EDs see fewer and fewer STEMI patients, they may not be able to maintain their ability to recognize and care for them, or develop a lower threshold for transfer.”
Even after adjusting for confounders such as age, presentation at a rural facility, and presentation on a weekend, the likelihood for transfer among self-pay patients, compared with those with any form of insurance, including Medicare and Medicaid, was increased by 64%, Dr. Ward reported.
The findings show that STEMI patients without insurance “are much more likely to be transferred, receiving less timely and therefore lower quality care for the most severe form of heart attack,” Dr. Ward said.
The reasons for this are unknown, he said. “One may be that patients without insurance are presenting to facilities that don’t have the ability to treat them: rural facilities, or facilities without the capability to treat this particular type of emergency. The other possibility is that they’re presenting to one that does have the capability, yet they’re still being transferred.”
Even if a patient with STEMI presents to a facility without the capability to treat a STEMI, and there’s another next door that can, “it still introduces a significant delay,” and with that higher risks, he said.
Dr. Ward’s research was funded by grants from the National Institutes of Health. He disclosed no conflicts of interest.
AT SAEM 2015
Key clinical point: A decline in STEMI visits to the ED and a rise in transfers of STEMI patients from the ED present challenges to timely catheterization in ED settings.
Major finding: ED visits to emergency departments for STEMI dropped about 30% from 2006 to 2011, a period in which STEMI-related transfers in the ED rose from 15% to more than 20.6%. Risk of transfer was markedly higher (adjusted OR, 1.64) for uninsured patients.
Data source: Records from 1,428,653 ED STEMI visits, including 259,376 (18.2%) transfers, from the Nationwide Emergency Department Sample.
Disclosures: None
Grip strength predicts cardiovascular mortality
Grip strength measurement is a quick and inexpensive way to stratify an individual’s risk of all-cause mortality, and can be a strong predictor of cardiovascular mortality and a moderately strong predictor of incident cardiovascular disease, according to new research in The Lancet.
In a large, longitudinal population study, nearly 140,000 participants identified from the Prospective Urban-Rural Epidemiology (PURE) study were examined during 2003-2009. Grip strength was measured via handgrip dynamometer.
After adjustment, grip strength was inversely associated with all-cause mortality (hazard ratio per 5-kg reduction in grip strength 1.16), cardiovascular mortality (1.17), noncardiovascular mortality (1.17), myocardial infarction (1.07), and stroke (1.09). The results were highly statistically significant and largely similar across various socioeconomic groups, although risk of cancer and grip strength were positively associated more often among patients in higher-income countries than in middle- and lower-income countries, reported Dr. Darryl P. Leong of McMaster University, Hamilton, Ont., and his colleagues.
The researchers also attempted to assess the prognostic value of grip strength on other medical conditions, but “found no significant association” between grip strength and incident diabetes and no association between grip strength and risk of hospital admission for pneumonia or COPD, injury from fall, or fracture.
“Our study suggests that measurement of grip strength is a simple, inexpensive risk-stratifying method to assess risk of death, particularly in individuals who develop a major illness, and that muscle strength is a risk marker for incident cardiovascular disease in a number of countries and populations,” the investigators wrote.
Read the entire article here.
Grip strength measurement is a quick and inexpensive way to stratify an individual’s risk of all-cause mortality, and can be a strong predictor of cardiovascular mortality and a moderately strong predictor of incident cardiovascular disease, according to new research in The Lancet.
In a large, longitudinal population study, nearly 140,000 participants identified from the Prospective Urban-Rural Epidemiology (PURE) study were examined during 2003-2009. Grip strength was measured via handgrip dynamometer.
After adjustment, grip strength was inversely associated with all-cause mortality (hazard ratio per 5-kg reduction in grip strength 1.16), cardiovascular mortality (1.17), noncardiovascular mortality (1.17), myocardial infarction (1.07), and stroke (1.09). The results were highly statistically significant and largely similar across various socioeconomic groups, although risk of cancer and grip strength were positively associated more often among patients in higher-income countries than in middle- and lower-income countries, reported Dr. Darryl P. Leong of McMaster University, Hamilton, Ont., and his colleagues.
The researchers also attempted to assess the prognostic value of grip strength on other medical conditions, but “found no significant association” between grip strength and incident diabetes and no association between grip strength and risk of hospital admission for pneumonia or COPD, injury from fall, or fracture.
“Our study suggests that measurement of grip strength is a simple, inexpensive risk-stratifying method to assess risk of death, particularly in individuals who develop a major illness, and that muscle strength is a risk marker for incident cardiovascular disease in a number of countries and populations,” the investigators wrote.
Read the entire article here.
Grip strength measurement is a quick and inexpensive way to stratify an individual’s risk of all-cause mortality, and can be a strong predictor of cardiovascular mortality and a moderately strong predictor of incident cardiovascular disease, according to new research in The Lancet.
In a large, longitudinal population study, nearly 140,000 participants identified from the Prospective Urban-Rural Epidemiology (PURE) study were examined during 2003-2009. Grip strength was measured via handgrip dynamometer.
After adjustment, grip strength was inversely associated with all-cause mortality (hazard ratio per 5-kg reduction in grip strength 1.16), cardiovascular mortality (1.17), noncardiovascular mortality (1.17), myocardial infarction (1.07), and stroke (1.09). The results were highly statistically significant and largely similar across various socioeconomic groups, although risk of cancer and grip strength were positively associated more often among patients in higher-income countries than in middle- and lower-income countries, reported Dr. Darryl P. Leong of McMaster University, Hamilton, Ont., and his colleagues.
The researchers also attempted to assess the prognostic value of grip strength on other medical conditions, but “found no significant association” between grip strength and incident diabetes and no association between grip strength and risk of hospital admission for pneumonia or COPD, injury from fall, or fracture.
“Our study suggests that measurement of grip strength is a simple, inexpensive risk-stratifying method to assess risk of death, particularly in individuals who develop a major illness, and that muscle strength is a risk marker for incident cardiovascular disease in a number of countries and populations,” the investigators wrote.
Read the entire article here.
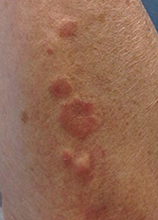
Make the Diagnosis - May 2015
Diagnosis: Granuloma annulare
Granuloma annulare (GA) is a self-limited cutaneous disorder predominantly seen in women that affects children and adults. The cause is unknown. Inciting factors can include herpes zoster infection, sun exposure, medications, and trauma.
Several clinical variants exist. Localized GA is the most common form, often presenting in the first three decades of life as an asymptomatic, erythematous, annular plaque with a firm border and central clearing localized to the wrists, ankles, and dorsal hands or feet. Generalized GA accounts for 15% of reported cases and presents in the fourth to seventh decades of life as multiple asymptomatic or pruritic skin-colored or erythematous papules and plaques on the trunk and extremities. Subcutaneous GA is more common in children and presents as multiple painless nodules on the scalp or extremities. Patch GA can be localized or generalized. Perforating GA presents as asymptomatic erythematous papules that evolve into yellow, umbilicated papules with a clear-to-white discharge.
Histopathologically, an interstitial or palisading pattern is seen with a dermal lymphohistiocytic infiltrate, degenerated collagen, and mucin deposition (visualized with alcian blue or colloidal iron stains). The interstitial pattern presents in the majority of cases.
Diagnosis of GA is predominantly clinically based. When the diagnosis is questionable or the presentation is atypical, biopsy is useful. Granuloma annulare is often self-limiting and resolves within 2 years, although recurrence is possible. First-line therapy for localized GA includes high-potency topical corticosteroids or intralesional corticosteroids. Other treatments include cryotherapy, phototherapy, and topical tacrolimus. For generalized GA, topical or intralesional corticosteroids may be used for select lesions. Topical calcineurin inhibitors, light therapy, hydroxychloroquine, isotretinion, and dapsone also have been reported as treatments in the literature.
Diagnosis: Granuloma annulare
Granuloma annulare (GA) is a self-limited cutaneous disorder predominantly seen in women that affects children and adults. The cause is unknown. Inciting factors can include herpes zoster infection, sun exposure, medications, and trauma.
Several clinical variants exist. Localized GA is the most common form, often presenting in the first three decades of life as an asymptomatic, erythematous, annular plaque with a firm border and central clearing localized to the wrists, ankles, and dorsal hands or feet. Generalized GA accounts for 15% of reported cases and presents in the fourth to seventh decades of life as multiple asymptomatic or pruritic skin-colored or erythematous papules and plaques on the trunk and extremities. Subcutaneous GA is more common in children and presents as multiple painless nodules on the scalp or extremities. Patch GA can be localized or generalized. Perforating GA presents as asymptomatic erythematous papules that evolve into yellow, umbilicated papules with a clear-to-white discharge.
Histopathologically, an interstitial or palisading pattern is seen with a dermal lymphohistiocytic infiltrate, degenerated collagen, and mucin deposition (visualized with alcian blue or colloidal iron stains). The interstitial pattern presents in the majority of cases.
Diagnosis of GA is predominantly clinically based. When the diagnosis is questionable or the presentation is atypical, biopsy is useful. Granuloma annulare is often self-limiting and resolves within 2 years, although recurrence is possible. First-line therapy for localized GA includes high-potency topical corticosteroids or intralesional corticosteroids. Other treatments include cryotherapy, phototherapy, and topical tacrolimus. For generalized GA, topical or intralesional corticosteroids may be used for select lesions. Topical calcineurin inhibitors, light therapy, hydroxychloroquine, isotretinion, and dapsone also have been reported as treatments in the literature.
Diagnosis: Granuloma annulare
Granuloma annulare (GA) is a self-limited cutaneous disorder predominantly seen in women that affects children and adults. The cause is unknown. Inciting factors can include herpes zoster infection, sun exposure, medications, and trauma.
Several clinical variants exist. Localized GA is the most common form, often presenting in the first three decades of life as an asymptomatic, erythematous, annular plaque with a firm border and central clearing localized to the wrists, ankles, and dorsal hands or feet. Generalized GA accounts for 15% of reported cases and presents in the fourth to seventh decades of life as multiple asymptomatic or pruritic skin-colored or erythematous papules and plaques on the trunk and extremities. Subcutaneous GA is more common in children and presents as multiple painless nodules on the scalp or extremities. Patch GA can be localized or generalized. Perforating GA presents as asymptomatic erythematous papules that evolve into yellow, umbilicated papules with a clear-to-white discharge.
Histopathologically, an interstitial or palisading pattern is seen with a dermal lymphohistiocytic infiltrate, degenerated collagen, and mucin deposition (visualized with alcian blue or colloidal iron stains). The interstitial pattern presents in the majority of cases.
Diagnosis of GA is predominantly clinically based. When the diagnosis is questionable or the presentation is atypical, biopsy is useful. Granuloma annulare is often self-limiting and resolves within 2 years, although recurrence is possible. First-line therapy for localized GA includes high-potency topical corticosteroids or intralesional corticosteroids. Other treatments include cryotherapy, phototherapy, and topical tacrolimus. For generalized GA, topical or intralesional corticosteroids may be used for select lesions. Topical calcineurin inhibitors, light therapy, hydroxychloroquine, isotretinion, and dapsone also have been reported as treatments in the literature.
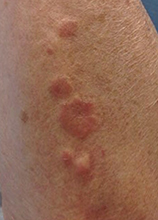
This case and photo were submitted by Orli Stern of Ross University and Dr. Donna Bilu Martin of Premier Dermatology, MD. A 60-year-old female with no significant past medical history presented with a 3-month history of asymptomatic, erythematous, firm, annular plaques on her bilateral proximal upper extremities and dorsal hands. The lesions have not been treated in the past.
Tips for Minimizing Recurrence of Seizures
Combo can fight infection, GVHD

Image courtesy of NIAID
NEW ORLEANS—Results of a phase 1 trial suggest that modified T cells can fight infection in patients who have undergone haploidentical hematopoietic
stem cell transplant (haplo-HSCT), and subsequent administration of a bio-inert drug can ameliorate graft-vs-host disease (GVHD) in these patients.
Researchers introduced the suicide gene inducible caspase 9 (iC9) into T cells and infused them into transplant recipients to promote immune reconstitution.
For patients who went on to develop GVHD, the researchers activated the suicide gene by administering a dose of the drug rimiducid (AP1903).
This cleared the patients of GVHD symptoms without jeopardizing the remaining T cells’ ability to fight infection.
The researchers presented these results at the American Society of Gene and Cell Therapy Annual Meeting and reported them in Blood.
The trial was sponsored by Baylor College of Medicine, but Bellicum Pharmaceuticals is the company developing rimiducid and the so-called iC9 “safety switch,” also known as CaspaCIDe.
“We’ve shown that the therapy works, fighting viruses that threaten immune-compromised patients,” said Xiaoou Zhou, PhD, of Baylor College of Medicine in Houston, Texas.
“We have also shown that the switch can turn off the T cells that reproduce out of control, attacking the patient’s graft-vs-host disease. This study was the first to look at any potential effect on the ability of the T cells to fight infection. We found there was no compromise.”
The study included 12 patients with a median age of 10 (range, 2-50) who had undergone haplo-HSCT. They received donor-derived T cells engineered with CaspaCIDe using a dose escalation schedule from 1×104 to 5×106 cells/kg, at a median of 42 days after transplant (range, 31-82 days).
All 12 patients had more rapid immune reconstitution and fewer infections after the infusions, when compared with previously reported results in T-cell-depleted, haplo-HSCT procedures. The CaspaCIDe T cells successfully provided protection from EBV, CMV, VZV, HHV6, and BKV viruses.
The researchers said there were no immediate toxicities related to the T-cell infusions, but 4 patients went on to develop GVHD.
Treatment with rimiducid resolved the patients’ GVHD symptoms within 6 to 48 hours. The researchers found that rimiducid could eliminate the uncontrolled T cells in the central nervous system as well as the peripheral blood.
Even after the problematic T cells were killed, the remaining T cells were able to fight infection without causing further GVHD.
One patient experienced a decrease in cell counts after receiving rimiducid, but counts had normalized by 48 hours. The researchers said there were no other immediate or delayed adverse effects associated with the drug.
One patient developed cytokine release syndrome, but this was resolved in 2 hours with a single dose of rimiducid.
“This study shows that infusing larger numbers of haploidentical donor T cells engineered with CaspaCIDe leads to better infection control,” Dr Zhou said. “We also showed that, if GVHD occurs, it can be rapidly controlled and eliminated by removing alloreactive cells with rimiducid in vivo, and that the productive, antiviral and anticancer cells remain, repopulate, and maintain immunity.”
“This is a significant finding that can lead to broader adoption of curative haploidentical transplants for cancers and genetic blood disorders. It also suggests that CaspaCIDe has great potential with CAR T and TCR therapies, where rapid control of dangerous T-cell-mitigated toxicities, such as cytokine release syndrome, is needed to achieve wide adoption.” ![]()

Image courtesy of NIAID
NEW ORLEANS—Results of a phase 1 trial suggest that modified T cells can fight infection in patients who have undergone haploidentical hematopoietic
stem cell transplant (haplo-HSCT), and subsequent administration of a bio-inert drug can ameliorate graft-vs-host disease (GVHD) in these patients.
Researchers introduced the suicide gene inducible caspase 9 (iC9) into T cells and infused them into transplant recipients to promote immune reconstitution.
For patients who went on to develop GVHD, the researchers activated the suicide gene by administering a dose of the drug rimiducid (AP1903).
This cleared the patients of GVHD symptoms without jeopardizing the remaining T cells’ ability to fight infection.
The researchers presented these results at the American Society of Gene and Cell Therapy Annual Meeting and reported them in Blood.
The trial was sponsored by Baylor College of Medicine, but Bellicum Pharmaceuticals is the company developing rimiducid and the so-called iC9 “safety switch,” also known as CaspaCIDe.
“We’ve shown that the therapy works, fighting viruses that threaten immune-compromised patients,” said Xiaoou Zhou, PhD, of Baylor College of Medicine in Houston, Texas.
“We have also shown that the switch can turn off the T cells that reproduce out of control, attacking the patient’s graft-vs-host disease. This study was the first to look at any potential effect on the ability of the T cells to fight infection. We found there was no compromise.”
The study included 12 patients with a median age of 10 (range, 2-50) who had undergone haplo-HSCT. They received donor-derived T cells engineered with CaspaCIDe using a dose escalation schedule from 1×104 to 5×106 cells/kg, at a median of 42 days after transplant (range, 31-82 days).
All 12 patients had more rapid immune reconstitution and fewer infections after the infusions, when compared with previously reported results in T-cell-depleted, haplo-HSCT procedures. The CaspaCIDe T cells successfully provided protection from EBV, CMV, VZV, HHV6, and BKV viruses.
The researchers said there were no immediate toxicities related to the T-cell infusions, but 4 patients went on to develop GVHD.
Treatment with rimiducid resolved the patients’ GVHD symptoms within 6 to 48 hours. The researchers found that rimiducid could eliminate the uncontrolled T cells in the central nervous system as well as the peripheral blood.
Even after the problematic T cells were killed, the remaining T cells were able to fight infection without causing further GVHD.
One patient experienced a decrease in cell counts after receiving rimiducid, but counts had normalized by 48 hours. The researchers said there were no other immediate or delayed adverse effects associated with the drug.
One patient developed cytokine release syndrome, but this was resolved in 2 hours with a single dose of rimiducid.
“This study shows that infusing larger numbers of haploidentical donor T cells engineered with CaspaCIDe leads to better infection control,” Dr Zhou said. “We also showed that, if GVHD occurs, it can be rapidly controlled and eliminated by removing alloreactive cells with rimiducid in vivo, and that the productive, antiviral and anticancer cells remain, repopulate, and maintain immunity.”
“This is a significant finding that can lead to broader adoption of curative haploidentical transplants for cancers and genetic blood disorders. It also suggests that CaspaCIDe has great potential with CAR T and TCR therapies, where rapid control of dangerous T-cell-mitigated toxicities, such as cytokine release syndrome, is needed to achieve wide adoption.” ![]()

Image courtesy of NIAID
NEW ORLEANS—Results of a phase 1 trial suggest that modified T cells can fight infection in patients who have undergone haploidentical hematopoietic
stem cell transplant (haplo-HSCT), and subsequent administration of a bio-inert drug can ameliorate graft-vs-host disease (GVHD) in these patients.
Researchers introduced the suicide gene inducible caspase 9 (iC9) into T cells and infused them into transplant recipients to promote immune reconstitution.
For patients who went on to develop GVHD, the researchers activated the suicide gene by administering a dose of the drug rimiducid (AP1903).
This cleared the patients of GVHD symptoms without jeopardizing the remaining T cells’ ability to fight infection.
The researchers presented these results at the American Society of Gene and Cell Therapy Annual Meeting and reported them in Blood.
The trial was sponsored by Baylor College of Medicine, but Bellicum Pharmaceuticals is the company developing rimiducid and the so-called iC9 “safety switch,” also known as CaspaCIDe.
“We’ve shown that the therapy works, fighting viruses that threaten immune-compromised patients,” said Xiaoou Zhou, PhD, of Baylor College of Medicine in Houston, Texas.
“We have also shown that the switch can turn off the T cells that reproduce out of control, attacking the patient’s graft-vs-host disease. This study was the first to look at any potential effect on the ability of the T cells to fight infection. We found there was no compromise.”
The study included 12 patients with a median age of 10 (range, 2-50) who had undergone haplo-HSCT. They received donor-derived T cells engineered with CaspaCIDe using a dose escalation schedule from 1×104 to 5×106 cells/kg, at a median of 42 days after transplant (range, 31-82 days).
All 12 patients had more rapid immune reconstitution and fewer infections after the infusions, when compared with previously reported results in T-cell-depleted, haplo-HSCT procedures. The CaspaCIDe T cells successfully provided protection from EBV, CMV, VZV, HHV6, and BKV viruses.
The researchers said there were no immediate toxicities related to the T-cell infusions, but 4 patients went on to develop GVHD.
Treatment with rimiducid resolved the patients’ GVHD symptoms within 6 to 48 hours. The researchers found that rimiducid could eliminate the uncontrolled T cells in the central nervous system as well as the peripheral blood.
Even after the problematic T cells were killed, the remaining T cells were able to fight infection without causing further GVHD.
One patient experienced a decrease in cell counts after receiving rimiducid, but counts had normalized by 48 hours. The researchers said there were no other immediate or delayed adverse effects associated with the drug.
One patient developed cytokine release syndrome, but this was resolved in 2 hours with a single dose of rimiducid.
“This study shows that infusing larger numbers of haploidentical donor T cells engineered with CaspaCIDe leads to better infection control,” Dr Zhou said. “We also showed that, if GVHD occurs, it can be rapidly controlled and eliminated by removing alloreactive cells with rimiducid in vivo, and that the productive, antiviral and anticancer cells remain, repopulate, and maintain immunity.”
“This is a significant finding that can lead to broader adoption of curative haploidentical transplants for cancers and genetic blood disorders. It also suggests that CaspaCIDe has great potential with CAR T and TCR therapies, where rapid control of dangerous T-cell-mitigated toxicities, such as cytokine release syndrome, is needed to achieve wide adoption.” ![]()