User login
How can we effectively treat stress urinary incontinence without drugs or surgery?
Pelvic floor muscle training (PFMT) and intravaginal electrical stimulation seem to be the best bets. PFMT increases urinary continence and improves symptoms of stress urinary incontinence (SUI) (strength of recommendation [SOR]: A, systematic review or randomized, controlled trials [RCTs]). PFMT also improves quality of life (QOL) (activity and psychological impact) (SOR: B, 1 RCT).
Intravaginal electrical stimulation increases urinary continence and improves SUI symptoms; percutaneous electrical stimulation also improves SUI symptoms and likely improves QOL measures (SOR: A, systematic review).
Magnetic stimulation doesn’t increase continence, has mixed effects on SUI symptoms, and produces no clinically meaningful improvement in QOL (SOR: B, heterogeneous RCTs with conflicting results). Vaginal cones don’t increase continence or QOL (SOR: B, 2 RCTs with methodologic flaws).
EVIDENCE SUMMARY
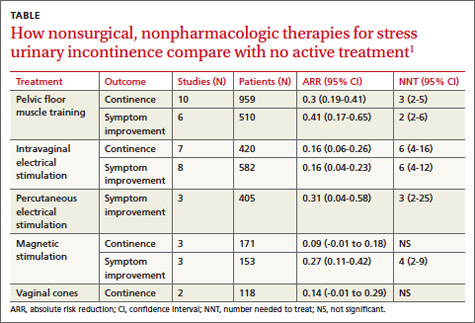
A systematic review by the Agency for Healthcare Research and Quality of adult female outpatients with SUI examined the effectiveness of PFMT, electrical stimulation, magnetic stimulation, and vaginal cones compared with no active treatment or sham treatment to produce continence (90% to 100% symptom reduction) or improve symptoms (at least 50% patient-reported symptom reduction).1 The TABLE summarizes the results.1 Investigators also assessed improvement in patient-reported QOL.
Pelvic floor muscle training improves continence, quality of life
A meta-analysis of 10 RCTs demonstrated that PFMT produced continence more often than placebo, and a meta-analysis of 6 RCTs found that PFMT improved SUI symptoms.1 PFMT regimens ranged in duration from 8 weeks to 6 months, including unsupervised treatment (8 to 12 repetitions, 3 to 10 times a day) and supervised treatment (as long as an hour, as often as 3 times a week).1
Both unsupervised and supervised PFMT produced similar results. One RCT evaluating QOL measures found that PFMT improved activity and reduced psychological impact (number needed to treat [NNT]=1; 95% confidence interval [CI], 1-2).1
Intravaginal electrical stimulation improves continence and symptoms
A meta-analysis of 7 RCTs found that intravaginal electrical stimulation increased continence compared with sham treatment.1 A meta-analysis of 8 RCTs found that intravaginal electrical stimulation also improved SUI symptoms.1 All of the trials used electrical stimulation at frequencies between 4 and 50 Hz for 15 to 20 minutes, 1 to 3 times daily for 4 to 15 weeks.
Percutaneous electrical stimulation improves symptoms
A meta-analysis of 3 RCTs found that percutaneous electrical stimulation improved SUI symptoms compared with no active treatment. Four RCTs found that electrical stimulation improved QOL, although a meta-analysis couldn’t be performed because of clinical heterogeneity.1
Magnetic stimulation produces conflicting results
A meta-analysis of 3 RCTs found that magnetic stimulation at frequencies of 10 to 18.5 Hz given over 1 to 8 weeks didn’t increase continence. A meta-analysis of an additional 3 RCTs concluded that magnetic stimulation improved continence, but the individual studies reported conflicting results and were heterogenous.1
Two RCTs evaluating QOL scores found conflicting results. One study found a mean difference of 3.9 points on the 100-point Incontinence Quality of Life Questionnaire (95% CI, 2.08-5.72; minimal clinically important difference rated 2-5 points).1
Vaginal cones are ineffective and not well-tolerated
Two RCTs found that vaginal cones didn’t improve continence or QOL compared with no treatment. Investigators reported high discontinuation rates and adverse effects with the cones, which weighed 20 to 70 g and were worn for 20 minutes a day for as long as 24 weeks.1
RECOMMENDATIONS
The National Institute for Health and Care Excellence recommends PFMT comprising at least 8 contractions 3 times daily for at least 3 months as first-line therapy for women with SUI.2 They don’t recommend electrical stimulation or intravaginal devices for women who can actively contract their pelvic floor muscles. The American College of Obstetricians and Gynecologists recommends PFMT as first-line therapy for women with SUI and states that PFMT is more effective than electrical stimulation or vaginal cones.3
1. Nonsurgical treatments for urinary incontinence in adult women: Diagnosis and comparative effectiveness. Executive summary. Agency for Healthcare Research and Quality Web site. Available at: http://effectivehealthcare.ahrq.gov/ehc/products/169/1021/CER36_Urinary-Incontinence_execsumm.pdf. Accessed March 19, 2014.
2. Urinary Incontinence: The management of urinary incontinence in women. NICE Clinical Guideline 171. London: NICE; 2006. National Institute for Health and Care Excellence Web site. Available at: www.nice.org.uk/CG171. Accessed March 19, 2014.
3. American College of Obstetricians and Gynecologists. Urinary incontinence in women. Obstet Gynecol. 2005;105:1533-1545.
AHIP; stress urinary incontinence; SUI; pelvic floor muscle training; PFMT; intravaginal electrical stimulation
Pelvic floor muscle training (PFMT) and intravaginal electrical stimulation seem to be the best bets. PFMT increases urinary continence and improves symptoms of stress urinary incontinence (SUI) (strength of recommendation [SOR]: A, systematic review or randomized, controlled trials [RCTs]). PFMT also improves quality of life (QOL) (activity and psychological impact) (SOR: B, 1 RCT).
Intravaginal electrical stimulation increases urinary continence and improves SUI symptoms; percutaneous electrical stimulation also improves SUI symptoms and likely improves QOL measures (SOR: A, systematic review).
Magnetic stimulation doesn’t increase continence, has mixed effects on SUI symptoms, and produces no clinically meaningful improvement in QOL (SOR: B, heterogeneous RCTs with conflicting results). Vaginal cones don’t increase continence or QOL (SOR: B, 2 RCTs with methodologic flaws).
EVIDENCE SUMMARY
A systematic review by the Agency for Healthcare Research and Quality of adult female outpatients with SUI examined the effectiveness of PFMT, electrical stimulation, magnetic stimulation, and vaginal cones compared with no active treatment or sham treatment to produce continence (90% to 100% symptom reduction) or improve symptoms (at least 50% patient-reported symptom reduction).1 The TABLE summarizes the results.1 Investigators also assessed improvement in patient-reported QOL.
Pelvic floor muscle training improves continence, quality of life
A meta-analysis of 10 RCTs demonstrated that PFMT produced continence more often than placebo, and a meta-analysis of 6 RCTs found that PFMT improved SUI symptoms.1 PFMT regimens ranged in duration from 8 weeks to 6 months, including unsupervised treatment (8 to 12 repetitions, 3 to 10 times a day) and supervised treatment (as long as an hour, as often as 3 times a week).1
Both unsupervised and supervised PFMT produced similar results. One RCT evaluating QOL measures found that PFMT improved activity and reduced psychological impact (number needed to treat [NNT]=1; 95% confidence interval [CI], 1-2).1
Intravaginal electrical stimulation improves continence and symptoms
A meta-analysis of 7 RCTs found that intravaginal electrical stimulation increased continence compared with sham treatment.1 A meta-analysis of 8 RCTs found that intravaginal electrical stimulation also improved SUI symptoms.1 All of the trials used electrical stimulation at frequencies between 4 and 50 Hz for 15 to 20 minutes, 1 to 3 times daily for 4 to 15 weeks.
Percutaneous electrical stimulation improves symptoms
A meta-analysis of 3 RCTs found that percutaneous electrical stimulation improved SUI symptoms compared with no active treatment. Four RCTs found that electrical stimulation improved QOL, although a meta-analysis couldn’t be performed because of clinical heterogeneity.1

Magnetic stimulation produces conflicting results
A meta-analysis of 3 RCTs found that magnetic stimulation at frequencies of 10 to 18.5 Hz given over 1 to 8 weeks didn’t increase continence. A meta-analysis of an additional 3 RCTs concluded that magnetic stimulation improved continence, but the individual studies reported conflicting results and were heterogenous.1
Two RCTs evaluating QOL scores found conflicting results. One study found a mean difference of 3.9 points on the 100-point Incontinence Quality of Life Questionnaire (95% CI, 2.08-5.72; minimal clinically important difference rated 2-5 points).1
Vaginal cones are ineffective and not well-tolerated
Two RCTs found that vaginal cones didn’t improve continence or QOL compared with no treatment. Investigators reported high discontinuation rates and adverse effects with the cones, which weighed 20 to 70 g and were worn for 20 minutes a day for as long as 24 weeks.1
RECOMMENDATIONS
The National Institute for Health and Care Excellence recommends PFMT comprising at least 8 contractions 3 times daily for at least 3 months as first-line therapy for women with SUI.2 They don’t recommend electrical stimulation or intravaginal devices for women who can actively contract their pelvic floor muscles. The American College of Obstetricians and Gynecologists recommends PFMT as first-line therapy for women with SUI and states that PFMT is more effective than electrical stimulation or vaginal cones.3
Pelvic floor muscle training (PFMT) and intravaginal electrical stimulation seem to be the best bets. PFMT increases urinary continence and improves symptoms of stress urinary incontinence (SUI) (strength of recommendation [SOR]: A, systematic review or randomized, controlled trials [RCTs]). PFMT also improves quality of life (QOL) (activity and psychological impact) (SOR: B, 1 RCT).
Intravaginal electrical stimulation increases urinary continence and improves SUI symptoms; percutaneous electrical stimulation also improves SUI symptoms and likely improves QOL measures (SOR: A, systematic review).
Magnetic stimulation doesn’t increase continence, has mixed effects on SUI symptoms, and produces no clinically meaningful improvement in QOL (SOR: B, heterogeneous RCTs with conflicting results). Vaginal cones don’t increase continence or QOL (SOR: B, 2 RCTs with methodologic flaws).
EVIDENCE SUMMARY
A systematic review by the Agency for Healthcare Research and Quality of adult female outpatients with SUI examined the effectiveness of PFMT, electrical stimulation, magnetic stimulation, and vaginal cones compared with no active treatment or sham treatment to produce continence (90% to 100% symptom reduction) or improve symptoms (at least 50% patient-reported symptom reduction).1 The TABLE summarizes the results.1 Investigators also assessed improvement in patient-reported QOL.
Pelvic floor muscle training improves continence, quality of life
A meta-analysis of 10 RCTs demonstrated that PFMT produced continence more often than placebo, and a meta-analysis of 6 RCTs found that PFMT improved SUI symptoms.1 PFMT regimens ranged in duration from 8 weeks to 6 months, including unsupervised treatment (8 to 12 repetitions, 3 to 10 times a day) and supervised treatment (as long as an hour, as often as 3 times a week).1
Both unsupervised and supervised PFMT produced similar results. One RCT evaluating QOL measures found that PFMT improved activity and reduced psychological impact (number needed to treat [NNT]=1; 95% confidence interval [CI], 1-2).1
Intravaginal electrical stimulation improves continence and symptoms
A meta-analysis of 7 RCTs found that intravaginal electrical stimulation increased continence compared with sham treatment.1 A meta-analysis of 8 RCTs found that intravaginal electrical stimulation also improved SUI symptoms.1 All of the trials used electrical stimulation at frequencies between 4 and 50 Hz for 15 to 20 minutes, 1 to 3 times daily for 4 to 15 weeks.
Percutaneous electrical stimulation improves symptoms
A meta-analysis of 3 RCTs found that percutaneous electrical stimulation improved SUI symptoms compared with no active treatment. Four RCTs found that electrical stimulation improved QOL, although a meta-analysis couldn’t be performed because of clinical heterogeneity.1

Magnetic stimulation produces conflicting results
A meta-analysis of 3 RCTs found that magnetic stimulation at frequencies of 10 to 18.5 Hz given over 1 to 8 weeks didn’t increase continence. A meta-analysis of an additional 3 RCTs concluded that magnetic stimulation improved continence, but the individual studies reported conflicting results and were heterogenous.1
Two RCTs evaluating QOL scores found conflicting results. One study found a mean difference of 3.9 points on the 100-point Incontinence Quality of Life Questionnaire (95% CI, 2.08-5.72; minimal clinically important difference rated 2-5 points).1
Vaginal cones are ineffective and not well-tolerated
Two RCTs found that vaginal cones didn’t improve continence or QOL compared with no treatment. Investigators reported high discontinuation rates and adverse effects with the cones, which weighed 20 to 70 g and were worn for 20 minutes a day for as long as 24 weeks.1
RECOMMENDATIONS
The National Institute for Health and Care Excellence recommends PFMT comprising at least 8 contractions 3 times daily for at least 3 months as first-line therapy for women with SUI.2 They don’t recommend electrical stimulation or intravaginal devices for women who can actively contract their pelvic floor muscles. The American College of Obstetricians and Gynecologists recommends PFMT as first-line therapy for women with SUI and states that PFMT is more effective than electrical stimulation or vaginal cones.3
1. Nonsurgical treatments for urinary incontinence in adult women: Diagnosis and comparative effectiveness. Executive summary. Agency for Healthcare Research and Quality Web site. Available at: http://effectivehealthcare.ahrq.gov/ehc/products/169/1021/CER36_Urinary-Incontinence_execsumm.pdf. Accessed March 19, 2014.
2. Urinary Incontinence: The management of urinary incontinence in women. NICE Clinical Guideline 171. London: NICE; 2006. National Institute for Health and Care Excellence Web site. Available at: www.nice.org.uk/CG171. Accessed March 19, 2014.
3. American College of Obstetricians and Gynecologists. Urinary incontinence in women. Obstet Gynecol. 2005;105:1533-1545.
1. Nonsurgical treatments for urinary incontinence in adult women: Diagnosis and comparative effectiveness. Executive summary. Agency for Healthcare Research and Quality Web site. Available at: http://effectivehealthcare.ahrq.gov/ehc/products/169/1021/CER36_Urinary-Incontinence_execsumm.pdf. Accessed March 19, 2014.
2. Urinary Incontinence: The management of urinary incontinence in women. NICE Clinical Guideline 171. London: NICE; 2006. National Institute for Health and Care Excellence Web site. Available at: www.nice.org.uk/CG171. Accessed March 19, 2014.
3. American College of Obstetricians and Gynecologists. Urinary incontinence in women. Obstet Gynecol. 2005;105:1533-1545.
AHIP; stress urinary incontinence; SUI; pelvic floor muscle training; PFMT; intravaginal electrical stimulation
AHIP; stress urinary incontinence; SUI; pelvic floor muscle training; PFMT; intravaginal electrical stimulation
Evidence-based answers from the Family Physicians Inquiries Network
Pitfalls & pearls for 8 common lab tests
› When interpreting hemoglobin A1c (HbA1c) levels, assess for anemia and other comorbidities that can significantly affect the lifespan of red blood cells and skew HbA1c test results. B
› Order nonfasting lipid panels for patients for whom fasting laboratory tests are difficult to obtain, as they have good clinical utility in screening and initial treatment. A
› Avoid routine thyroid-stimulating hormone (TSH) testing in asymptomatic adults; when testing is indicated, start with TSH. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Laboratory mistakes are not defined as diagnostic errors, but they contribute significantly to the thousands of medical errors that occur every year.1 Part of the problem: While accurate interpretation of lab tests often depends on the use of statistical concepts we all learned in medical training, it is difficult to find the time to incorporate these principles into a busy practice.
Overuse of lab tests presents problems, as well. Because “normal ranges” for test results are based on statistical analysis, as many as 5% of patients in a standard distribution fall outside of the range.2 It is important to order only the tests you really need, as extra testing automatically means more false positive results.
This article was written with such pitfalls in mind. In the pages that follow, we focus on 8 types of tests family physicians rely on regularly—all cases in which test results are reliable only if comorbidities, pre- and post-test probabilities, and clinical context are carefully considered. To help you put these lab tests into the proper context, we’ve addressed a key question—and highlighted both pitfalls and pearls—about each.
1. Hemoglobin A1c: How does anemia affect it?
Hemoglobin A1c (HbA1c) can be measured in many ways, including high-performance liquid chromatography, boronate affinity, capillary electrophoresis, and immunoassay, all of which can provide equivalent values without significant variability.3,4 In interpreting these tests, however, it is important to understand the effect that anemia has on HbA1c.
It's important to order only the tests you really need, as extra testing automatically means more false positive results.
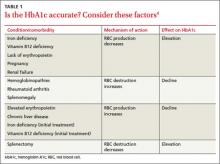
Two primary variables influencing HbA1c are the average glucose level and the average lifespan of red blood cells (RBCs). Normally, there is a direct correlation between average serum glucose and HbA1c.4 In patients with anemia, however, this relationship is less clear, and may be affected by erythropoiesis and RBC destruction.5 In iron deficiency anemia (IDA),6,7 hemoglobin production falls secondary to iron stores, resulting in microcytic cells with a longer lifespan and elevated HbA1c. In at least one study,5,7 HbA1c approached levels associated with diabetes (with increases as high as 1.5%) in nondiabetic patients, but resolved with treatment of IDA.
Increased destruction as well as increased production of RBCs lowers their lifespan, and in turn decreases HbA1c levels (TABLE 1).4 This can be seen in conditions such as splenomegaly and hemoglobinopathies. In patients with hemoglobinopathies, the percentage of hemoglobin A is significantly decreased, often to undetectable levels—thereby making HbA1c tests inaccurate. Hemoglobin electrophoresis and determination of glycation by capillary electrophoresis or high-performance liquid chromatography can be used instead, but neither is practical because of cost and limited availability.4,8,9
THE TAKEAWAY: When you evaluate HbA1c test results, it is crucial to assess the patient for anemia and other conditions or comorbidities that can significantly affect RBC lifespan and skew test results.2,4-6
2. D-dimer: When should you use it?
D-dimer is a fibrin degradation product that is increased when active clotting is present,10 and its assay—which has high sensitivity and low specificity—is widely used to screen for pulmonary embolism (PE) and deep vein thrombosis (DVT). While the minimal number of false negatives makes the D-dimer a good screening test, the higher rate of false positives makes it difficult to arrive at a definitive diagnosis. Appropriate use of the D-dimer assay is crucial to minimize the potential for adverse consequences, such as bleeding in patients who are subjected to unnecessary anticoagulation because of false positive results.
Further testing typically follows. A positive D-dimer test is commonly followed by a computed tomography (CT) scan of the chest or a ventilation/perfusion scan to establish a PE or DVT diagnosis. But this subsequent testing increases both the cost of health care and the patient’s radiation exposure. Use of these subsequent scans can be reduced by first considering the patient’s pretest probability for PE or DVT. The Wells’ Criteria (available at www.mdcalc.com/wells-criteriafor-pulmonary-embolism-pe/) and Geneva Score (Revised) (www.mdcalc.com/genevascore-revised-for-pulmonary-embolism/) can both be used for this purpose.10,11
Patients with high pretest probability should undergo immediate scanning, foregoing the D-dimer—which should be reserved for patients who have a low or moderate pretest probability but sufficient reason to suspect PE or DVT.10-12
The low specificity of the D-dimer assay poses another challenge to its effective use. There are many things that can increase D-dimer levels, such as age, cancer, prolonged immobility, autoimmune disease, inflammation, sickle cell disease, pregnancy, trauma, and surgery.13-15 All these factors must be taken into consideration prior to ordering this test.
In fact, one recent study found that using an age-adjusted D-dimer cutoff (patient’s age in years x 10 mcg/L)—rather than a conventional cutoff of 500 mcg/L—for patients older than 50 years reduces false positives without substantially increasing false negatives.16
Also of note: An anticoagulant can decrease D-dimer levels in plasma, so the test should not be used to rule out PE or DVT in patients who are undergoing anticoagulation.13,15
THE TAKEAWAY: In evaluating patients for PE or DVT, use the Wells’ Criteria or Geneva Score (Revised) to determine a patient’s pretest probability of disease. Use the D-dimer assay to safely rule out these conditions in patients with a low or intermediate pretest probability, but go directly to scans for those with a high pretest probability.
3. Lipid panels: How important is fasting?
Patients are often instructed to report for fasting lab studies, specifically for lipid profiles. Traditionally, this had been defined as an 8- to 12-hour period without food.17 In clinical practice, however, this is often misinterpreted by patients, who may be confused about the duration of the fast or unsure about whether to eat or drink immediately before the test.
Studies investigating the effect of meals on lab values have found that triglycerides are consistently elevated postprandially, to a maximum of 12 hours.18-21 The effect of the fasting state on total cholesterol, low-density lipoprotein (LDL) cholesterol, and high-density lipoprotein (HDL) cholesterol is more controversial; while some postprandial differences have been detected, the clinical relevance is equivocal.18-21
Nonfasting lipid values can offer useful information, particularly in patients who are unwilling or unable to return for fasting labs. The US Preventive Services Task Force (USPSTF) supports this practice.22 Because guidelines for evaluation and treatment are based on fasting lipids, however, fasting lab work should be used, whenever possible, for initiating treatment and monitoring patients with abnormal values. If nonfasting lipids are used, it is crucial to factor in the postprandial effects on triglycerides and the subsequent difficulty of assessing LDL cholesterol levels.
THE TAKEAWAY: The clinical relevance of postprandial vs fasting lipid levels is equivocal. Nonfasting lipid panels have reasonable clinical utility in screening and initial treatment, particularly in cases in which obtaining fasting lab values may be problematic.18,19
4. Mononucleoosis spot test: When should you use it?
The monospot test is a latex assay that causes hemagglutination of horse RBCs in the presence of heterophile antibodies characteristic of infectious mononucleosis.23 The antibodies develop within the first 7 days of onset of symptoms, but do not peak for 2 to 5 weeks.24 As a result, monospot testing yields a high incidence of false negatives during the first 2 weeks of active infection.25 False negatives are also common in patients younger than 14 years. Heterophile antibodies may be present for up to a year after active infection.24
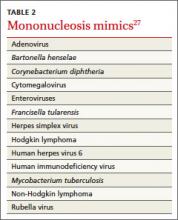
Patients at increased risk for splenic rupture, such as athletes, pose considerable diagnostic difficulty.26 When there is strong clinical suspicion of mononucleosis despite a negative monospot test in such high-risk individuals, follow-up testing is recommended to differentiate it from other mononucleosis-like illnesses (TABLE 2).27 The optimal combination of Epstein-Barr virus (EBV) serologic testing consists of the antibody titration of 4 markers: immunoglobulins M (IgM) and G (IgG) to the viral capsid antigen, IgM to the early antigen, and antibody to Epstein-Barr nuclear antigen (EBNA).28 Acute phase reactants in the setting of an antibody to EBNA could indicate reactivation. A positive test does not exclude other medical causes, however, because up to 20% of patients have acute phase antibodies that persist for years.29
Appropriate diagnosis is important because of the significant morbidity associated with EBV. Risk of splenic injury is greatest between 4 and 21 days after onset of symptoms but persists at 7 weeks,26 so conservative therapy followed by monospot retesting one week later is a reasonable approach.
Mononucleosis or routine tonsillitis? It is important to note that there is no evidence that a positive monospot test will affect the management or outcome of routine tonsillitis, raising questions of the utility of the test in such cases. A better approach: Reserve testing for patients with additional findings—ie, splenomegaly—or whose symptoms have persisted ≥ 2 weeks.
THE TAKEAWAY: Wait at least 2 weeks to conduct monospot testing in patients with routine tonsillitis. If strong clinical suspicion exists, proceed with specific IgM and IgG serologic testing.24,25,27,28
5. Evaluating prescription drug levels: Which factors interfere?
Correct interpretation of lab tests conducted to measure prescription drug levels has major implications with regard to patient safety, particularly for medications with a narrow therapeutic index.
Most drug level tests measure the total concentration, which includes both bound and unbound (free) forms. The unbound forms are the active components of the drug; thus, for an accurate evaluation, it is important to be aware of factors that increase free drug concentration. Chief among them is low protein levels, or hypoalbuminemia.30
Risk factors for hypoalbuminemia include significant burns, advanced age, pregnancy, malnutrition, and human immunodeficiency virus/acquired immune deficiency syndrome (HIV/AIDS).30 HIV/AIDS is a particularly high risk because certain protease inhibitors are highly protein bound.
Drug protein binding is classified as low, moderate, or high. The main proteins involved in the process are albumin, alpha-1-acid glycoprotein, and lipoprotein. Medications that are highly protein bound (>80%) are the most affected by low protein levels: Problems can arise when drugs completely bind to all the available proteins and excess drug availability increases free drug levels.
Medications that are most likely to be affected by a high degree of protein binding include carbamazepine, cyclosporine, mycophenolic acid, phenytoin, protease inhibitors (with the exception of indinavir), tacrolimus, and valproic acid. It is important to consider free levels when you order medication assays for these drugs to avoid misinterpreting the serum levels as being too low-a scenario that raises the risk of drug toxicity and adverse outcomes.30,31
A study of 119 phenytoin samples from 70 patients found significantly higher free phenytoin levels in patients with lower albumin levels.32 Higher free phenytoin levels were also seen in older patients and in those with diminished renal function (creatinine clearance <25 mL/min).32 The degree of protein binding is affected by both the serum drug concentration and the albumin level, with saturable protein binding occurring at higher drug levels.33
Calculate phenytoin levels with this equation. To calculate corrected phenytoin levels in patients with low albumin levels, use the following formula, known as the Sheiner-Tozer equation:34
Concentration adjusted=concentration reported/([adjustment x serum albumin] + 0.1); adjustment=0.2 for creatinine clearance ≥20 or 0.1 for creatinine clearance <20.
Additional causes of misinterpreted drug levels. While hypoalbuminemia plays a major role in the misinterpretation of drug levels, other factors affect serum drug concentration, as well. These include drug-drug interactions, which can significantly increase the concentration of the medications involved, and the timing of the test with regard to medication administration. Digoxin levels, in particular, need to be drawn at least 6 to 8 hours after the last dose is taken to allow for appropriate drug distribution.35
THE TAKEAWAY: It is essential to consider free drug level monitoring in patients who either have hypoalbuminemia or have one or more risk factors for hypoalbuminemia to avoid falsely low estimation of drug levels.36,37
6 Liver function tests: Necessary for patients on statin therapy?
Since statins gained US Food and Drug Administration (FDA) approval, the drugs have been associated with increased liver function tests (LFTs). Indeed, there had been a long-standing belief, based on clinical trials, that by monitoring alanine aminotransferase (ALT) and maintaining it at <3 times the upper limit normal (ULN), hepatotoxicity could be avoided.38 In clinical practice, however, further ALT elevation is frequently allowed based on patient tolerability.
In February 2012, the FDA revised its safety data to reflect this practice.39 The FDA update confirmed that routine LFT monitoring is unnecessary for patients on statins—and that it is not very effective in identifying or preventing liver damage.
Overall, serious hepatotoxicity is very rare, with an incidence ≤2 per 1 million patient-years.39 The National Lipid Association Statin Safety Assessment Task Force recommends repeating LFTs that are 3 to 5 times the ULN within 6 months and continuing with the statin dose if the patient is asymptomatic.38
THE TAKEAWAY: Routine liver function monitoring is not necessary for patients on statins. A better approach: Obtain baseline ALT levels, and repeat the testing only as clinically indicated thereafter.38,39
7. Urine drug screens: Which factors affect their accuracy?
The gold standard for testing for drugs of abuse, urine drug screens (UDS) have good sensitivity and specificity, easy administration, and reasonable cost.40 UDS can detect various narcotics, such as morphine, oxycodone, ,and methadone, and identify other illicit drugs, although which drugs and metabolites are tested for is laboratory- and test-specific.
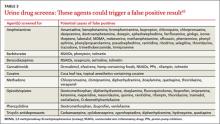
Cross-reactivity. There are 6 currently available immunoassays, all of which use competitive binding between the sample drug and a drug chemically labeled with an enzyme, radioisotope, or fluorophore. The sample drug and labeled drug compete for substrate binding sites on drug-specific antibodies.41,42 Similar to competitive binding for enzymatic reactions in the body, the substrate binding site can experience cross-reactivity—causing substances other than the drug in question to bind to the immunoglobulin, leading to a false positive result (TABLE 3).43 Other factors that can alter the results include the cutoff value of the test and the absorption, distribution, metabolism, and excretion of the drug.42 Thus, a confirmatory test of gas chromatography-mass spectrometry is recommended before making decisions based on the results of UDS.43-45
Routine screens for patients on chronic opioid therapy. Routine use of UDS in emergency departments is no longer recommended, based on evidence that the results are unlikely to have a significant effect on patient management.46 For patients on chronic opioid therapy, however, routine screening has proven helpful in detecting prescription opioid abuse, illicit drug use, and diversion. Up to 34% of patients on prescription opioids have been found to be using illicit drugs, as well.42
THE TAKEAWAY: Use UDS as a tool in managing patients on chronic opioid therapy, but before acting on results, assess for factors, such as the use of oral or topical medications and the cutoff value of the test, that may be associated with false positive or false negative results.43-45
8. Thyroid function testing: When should you test?
Thyroid-stimulating hormone (TSH) is the first-line test when investigating presumed hyper- or hypothyroidism.47,48 Third-generation chemiluminometric assays can reliably measure TSH concentrations <0.01 mU/L by using multiple antibodies to produce a sandwich-type effect on the molecule in question.49
TSH levels exhibit diurnal variation, however, and are affected by other medications, including steroids, opiates, and some antihistamines, among others, as well as comorbidities.47,48 Chronic and acute conditions unrelated to thyroid disease can cause transient changes in TSH concentrations, and have the potential to modify the binding capacity of plasma thyroid hormone binding proteins.48 Thus, TSH should be ordered for hospitalized patients only when clinical suspicion of a thyroid problem exists.48 The USPSTF recommends against routine TSH screening for asymptomatic adults.46
How to respond to abnormal results. For patients found to have abnormal TSH levels, free T4 (fT4) is the next test to order.47,49 An fT4 assay is a superior indicator of thyroid status because it is not affected by changes in iodothyronine-binding proteins, which influence total hormone measurements.49 The results will be elevated in hyperthyroidism and reduced in hypothyroidism.47
Triiodothyronine (T3) measures can be useful in diagnosing Graves’ disease, in which T3 toxicosis may be the initial symptom—or an indication of a relapse. Because T3 is often a peripheral product, however, nonthyroid illnesses and medications can cause artifactually abnormal results.49
Other thyroid-specific labs include thyroid ,antibodies such as antithyroid peroxidase, antithyroglobulin, and TSH receptor, both blocking and stimulating.49 Thyroglobulin is a precursor form of thyroid hormone and should be measured when factitious hyperthyroidism is suspected. Management of hyper- and hypothyroidism often is independent of etiology. Retesting TSH to assess treatment response should be postponed until ≥2 months after any change in medication or dosing.50
Thyroid studies can be very difficult to interpret. TSH should be the first test ordered. However, if TSH values do not match the clinical picture, fT4, T3, and other thyroid tests that are less affected by outside factors can be useful in identifying the cause.
THE TAKEAWAY: Routine TSH testing is not indicated for asymptomatic adults. When evaluating thyroid function is clinically indicated, TSH is the initial test of choice.47,48,51
CORRESPONDENCE
Joshua Tessier, DO, Iowa Lutheran Family Medicine Residency, 840 East University Avenue, Des Moines, IA 50316; joshua.tessier@unitypoint.org
1. Ottomano C. Errors in medicine and errors in laboratory medicine: what is the difference? Blood Transfus. 2010;8;79-81.
2. Wallach JB. Introduction to normal values (reference ranges). Interpretation of Diagnostic Tests. 8th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2007:3-7.
3. Halwachs-Baumann G, Katzensteiner S, Schnedl W, et al. Comparative evaluation of three assay systems for automated determination of hemoglobin A1c. Clin Chem. 1997;43:511-517.
4. Gallagher EJ, Le Roith D, Bloomgarden Z. Review of hemoglobin A(1c) in the management of diabetes. J Diabetes. 2009;1:9-17.
5. Franco RS. The measurement and importance of red cell survival. Am J Hematol. 2009;84:109-114.
6. Tarim O, Küçükerdog˘an A, Gunay U, et al. Effects of iron deficiency anemia on hemoglobin A1c in type 1 diabetes mellitus. Pediatr Int. 1999;41:357-362.
7. Kim C, Bullard KM, Herman WH, et al. Association between iron deficiency and A1C levels among adults without diabetes in the National Health and Nutrition Examination Survey, 1999-2006. Diabetes Care. 2010;33:780-785.
8. Higgins T, Stewart D, Boehr E. Challenges in HbA1c analysis and reporting: an interesting case illustrating the many pitfalls. Clin Biochem. 2008;41:1104-1106.
9. Mongia SK, Little RR, Rohlfing CL, et al. Effects of hemoglobin C and S traits on the results of 14 commercial glycated hemoglobin assays. Am J Clin Pathol. 2008;130:136-140.
10. Brown MD, Rowe BH, Reeves MJ, et al. The accuracy of the enzyme-linked immunosorbent assay D-dimer test in the diagnosis of pulmonary embolism: a meta-analysis. Ann Emerg Med. 2002;40:133-144.
11. Squizzato A, Ageno W. What is the next step in D-dimer research? Education of physicians. Intern Emerg Med. 2006;1:165.
12. Kabrhel C, Mark Courtney D, Camargo CA Jr, et al. Potential impact of adjusting the threshold of the quantitative D-dimer based on pretest probability of acute pulmonary embolism. Acad Emerg Med. 2009;16:325-332.
13. Kabrhel C, Mark Courtney MD, Camargo CA Jr, et al. Factors associated with positive D-dimer results in patients evaluated for pulmonary embolism. Acad Emerg Med. 2010;17:589-597.
14. Berman AR. Pulmonary embolism in the elderly. Clin Geriatr Med. 2001;17:107-130.
15. Bruinstroop E, van de Ree MA, Huisman MV. The use of Ddimer in specific clinical conditions: a narrative review. Eur J Intern Med. 2009;20:441-446.
16. Schouten HJ, Geersing GI, Koek HL, et al. Diagnostic accuracy ,of conventional or age adjusted D-dimer cut-off values in older patients with suspected venous thromboembolism: systematic review and meta-analysis. BMJ. 2013;346:f2492.
17. Turgeon ML. Linne & Ringsrud’s Clinical Laboratory Science. 5th ed. Saint Louis, MO: Mosby; 2007:50.
18. Cohn JS,McNamara JR, Schaefer EJ. Lipoprotein cholesterol concentrations in the plasma of human subjects as measured in the fed and fasted states. Clin Chem. 1988;34:2456-2459.
19. Watts GF, Cohn JS. Whither the lipid profile: feast, famine, or no free lunch? Clin Chem. 2011;57:363-365.
20. Mora S, Rifai N, Buring JE, et al. Fasting compared with nonfasting lipids and apolipoproteins for predicting incident cardiovascular events. Circulation. 2008;118:993-1001.
21. Nordestgaard BG, Langsted A, Freiberg JJ. Nonfasting hyperlipidemia and cardiovascular disease. Curr Drug Targets. 2009;10:328-335.
22. Screening for lipid disorders in adults: US Preventive Services Task Force Recommendation statement. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/uspstf08/lipid/lipidrs.htm. Accessed March 13, 2014.
23. Wolf DM, Friedrichs I, Toma AG. Lymphocyte-white blood cell count ratio: a quickly available screening tool to differentiate acute purulent tonsillitis from glandular fever. Arch Otolaryngol Head Neck Surg. 2007;133:61-64.
24. McCormack R, O’Shea T. The uptake and use of the Monospot test in patients with tonsillitis. Ir Med J. 2009;102:226-228.
25. Ebell MH. Epstein-Barr virus infectious mononucleosis. Am Fam Physician. 2004;70:1279-1287.
26. Waninger KN, Harcke HT. Determination of safe return to play for athletes recovering from infectious mononucleosis: a review of the literature. Clin J Sport Med. 2005;15:410-416.
27. Hurt C, Tammaro D. Diagnostic evaluation of mononucleosislike illnesses. Am J Med. 2007;120:911.e1-911.e8.
28. Vouloumanou EK, Rafailidis PI, Falagas ME. Current diagnosis and management of infectious mononucleosis. Curr Opin Hematol. 2012;19:14-20.
29. Epstein-Barr virus and infectious mononucleosis. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/epstein-barr/laboratory-testing.html. Updated January 7, 2014. Accessed March 12, 2014.
30. Dasgupta A. Clinical utility of free drug monitoring. Clin Chem Lab Med. 2002;40:986-993.
31. Dasgupta A. Usefulness of monitoring free (unbound) concentrations of therapeutic drugs in patient management. Clin Chim Acta. 2007;377:1-13.
32. Iwamoto T, Kagawa Y, Naito Y, et al. Clinical evaluation of plasma free phenytoin measurement and factors influencing its protein binding. Biopharm Drug Dispos. 2006;27:77-84.
33. Hermida J, Tutor JC. A theoretical method for normalizing total serum valproic acid concentration in hypoalbuminemic patients. J Pharmacol Sci. 2005;97:489-493.
34. Wolf GK, McClain CD, Zurakowski D, et al. Total phenytoin concentrations do not accurately predict free phenytoin concentrations in critically ill children. Pediatr Crit Care Med. 2006;7:434-439; quiz 440.
35. Lanoxin (digoxin) [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2011.
36. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143-3421.
37. De Backer G, Ambrosini E, Borch-Johnsen K, et al; Third Joint Force of European and other Societies on Cardiovascular Disease and Prevention in Clinical Practice. European guidelines on cardiovascular disease and prevention in clinical practice. Atherosclerosis. 2003;171:145-155.
38. McKenney JM, Davidson MH, Jacobson TA, et al. Final conclusions and recommendations of the National Lipid Association Statin Safety Assessment Task Force. Am J Cardiol. 2006;97:89C-94C.
39. FDA drug safety communication: important safety label changes to cholesterol-lowering statin drugs. US Food and Drug Administration Web site. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm293101.htm. Updated July 3, 2012. Accessed May 17, 2013.
40. Eskridge KD, Guthrie SK. Clinical issues associated with urine testing of substances of abuse. Pharmacotherapy. 1997;17:497-510.
41. Herring C, Muzyk AJ, Johnston C. Interferences with urine drug screens. J Pharm Pract. 2001;24:102-108.
42. Jaffee WB, Trucco E, Teter C, et al. Focus on alcohol & drug abuse: ensuring validity in urine drug testing. Psychiatr Serv. 2008;59:140-142.
43. Brahm NC, Yeager LL, Fox MD, et al. Commonly prescribed medications and potential false-positive urine drug screens. Am J Health Syst Pharm. 2010;67:1344-1350.
44. Moeller KE, Lee KC, Kissack JC. Urine drug screening: Practical guide for clinicians. Mayo Clin Proc. 2008;83:66-76.
45. Christo PJ, Manchikanti L, Ruan X, et al. Urine drug testing in chronic pain. Pain Physician. 2011;14:123-143.
46. Tenenbein M. Do you really need that emergency drug screen? Clin Toxicol (Phila). 2009;47:286-291.
47. Gaitonde DY, Rowley KD, Sweeney LB. Hypothyroidism: an update. Am Fam Physician. 2012;86:244-251.
48. UK guidelines for the use of thyroid function tests. British Thyroid Association Web site. Available at: http://www.british-thyroid-association.org/info-for-patients/Docs/TFT_guideline_final_version_July_2006.pdf. Accessed March 11, 2014.
49. Volpé, R. Rational use of thyroid function tests. Crit Rev Clin Lab Sci. 1997;34:405-438.
50. Graber JR, Cobin RH, Gharib H, et al; American Association of Clinical Endocrinologists and American Thyroid Association Taskforce on Hypothyroidism in Adults. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr Pract. 2012;18:988-1028.
51. Helfand M; US Preventive Services Task Force. Screening for subclinical thyroid dysfunction in nonpregnant adults: a summary of the evidence for the US Preventive Services Task Force. Ann Intern Med. 2004;140:128-141.
› When interpreting hemoglobin A1c (HbA1c) levels, assess for anemia and other comorbidities that can significantly affect the lifespan of red blood cells and skew HbA1c test results. B
› Order nonfasting lipid panels for patients for whom fasting laboratory tests are difficult to obtain, as they have good clinical utility in screening and initial treatment. A
› Avoid routine thyroid-stimulating hormone (TSH) testing in asymptomatic adults; when testing is indicated, start with TSH. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Laboratory mistakes are not defined as diagnostic errors, but they contribute significantly to the thousands of medical errors that occur every year.1 Part of the problem: While accurate interpretation of lab tests often depends on the use of statistical concepts we all learned in medical training, it is difficult to find the time to incorporate these principles into a busy practice.
Overuse of lab tests presents problems, as well. Because “normal ranges” for test results are based on statistical analysis, as many as 5% of patients in a standard distribution fall outside of the range.2 It is important to order only the tests you really need, as extra testing automatically means more false positive results.
This article was written with such pitfalls in mind. In the pages that follow, we focus on 8 types of tests family physicians rely on regularly—all cases in which test results are reliable only if comorbidities, pre- and post-test probabilities, and clinical context are carefully considered. To help you put these lab tests into the proper context, we’ve addressed a key question—and highlighted both pitfalls and pearls—about each.
1. Hemoglobin A1c: How does anemia affect it?
Hemoglobin A1c (HbA1c) can be measured in many ways, including high-performance liquid chromatography, boronate affinity, capillary electrophoresis, and immunoassay, all of which can provide equivalent values without significant variability.3,4 In interpreting these tests, however, it is important to understand the effect that anemia has on HbA1c.
It's important to order only the tests you really need, as extra testing automatically means more false positive results.
Two primary variables influencing HbA1c are the average glucose level and the average lifespan of red blood cells (RBCs). Normally, there is a direct correlation between average serum glucose and HbA1c.4 In patients with anemia, however, this relationship is less clear, and may be affected by erythropoiesis and RBC destruction.5 In iron deficiency anemia (IDA),6,7 hemoglobin production falls secondary to iron stores, resulting in microcytic cells with a longer lifespan and elevated HbA1c. In at least one study,5,7 HbA1c approached levels associated with diabetes (with increases as high as 1.5%) in nondiabetic patients, but resolved with treatment of IDA.
Increased destruction as well as increased production of RBCs lowers their lifespan, and in turn decreases HbA1c levels (TABLE 1).4 This can be seen in conditions such as splenomegaly and hemoglobinopathies. In patients with hemoglobinopathies, the percentage of hemoglobin A is significantly decreased, often to undetectable levels—thereby making HbA1c tests inaccurate. Hemoglobin electrophoresis and determination of glycation by capillary electrophoresis or high-performance liquid chromatography can be used instead, but neither is practical because of cost and limited availability.4,8,9
THE TAKEAWAY: When you evaluate HbA1c test results, it is crucial to assess the patient for anemia and other conditions or comorbidities that can significantly affect RBC lifespan and skew test results.2,4-6
2. D-dimer: When should you use it?
D-dimer is a fibrin degradation product that is increased when active clotting is present,10 and its assay—which has high sensitivity and low specificity—is widely used to screen for pulmonary embolism (PE) and deep vein thrombosis (DVT). While the minimal number of false negatives makes the D-dimer a good screening test, the higher rate of false positives makes it difficult to arrive at a definitive diagnosis. Appropriate use of the D-dimer assay is crucial to minimize the potential for adverse consequences, such as bleeding in patients who are subjected to unnecessary anticoagulation because of false positive results.
Further testing typically follows. A positive D-dimer test is commonly followed by a computed tomography (CT) scan of the chest or a ventilation/perfusion scan to establish a PE or DVT diagnosis. But this subsequent testing increases both the cost of health care and the patient’s radiation exposure. Use of these subsequent scans can be reduced by first considering the patient’s pretest probability for PE or DVT. The Wells’ Criteria (available at www.mdcalc.com/wells-criteriafor-pulmonary-embolism-pe/) and Geneva Score (Revised) (www.mdcalc.com/genevascore-revised-for-pulmonary-embolism/) can both be used for this purpose.10,11
Patients with high pretest probability should undergo immediate scanning, foregoing the D-dimer—which should be reserved for patients who have a low or moderate pretest probability but sufficient reason to suspect PE or DVT.10-12
The low specificity of the D-dimer assay poses another challenge to its effective use. There are many things that can increase D-dimer levels, such as age, cancer, prolonged immobility, autoimmune disease, inflammation, sickle cell disease, pregnancy, trauma, and surgery.13-15 All these factors must be taken into consideration prior to ordering this test.
In fact, one recent study found that using an age-adjusted D-dimer cutoff (patient’s age in years x 10 mcg/L)—rather than a conventional cutoff of 500 mcg/L—for patients older than 50 years reduces false positives without substantially increasing false negatives.16
Also of note: An anticoagulant can decrease D-dimer levels in plasma, so the test should not be used to rule out PE or DVT in patients who are undergoing anticoagulation.13,15
THE TAKEAWAY: In evaluating patients for PE or DVT, use the Wells’ Criteria or Geneva Score (Revised) to determine a patient’s pretest probability of disease. Use the D-dimer assay to safely rule out these conditions in patients with a low or intermediate pretest probability, but go directly to scans for those with a high pretest probability.
3. Lipid panels: How important is fasting?
Patients are often instructed to report for fasting lab studies, specifically for lipid profiles. Traditionally, this had been defined as an 8- to 12-hour period without food.17 In clinical practice, however, this is often misinterpreted by patients, who may be confused about the duration of the fast or unsure about whether to eat or drink immediately before the test.
Studies investigating the effect of meals on lab values have found that triglycerides are consistently elevated postprandially, to a maximum of 12 hours.18-21 The effect of the fasting state on total cholesterol, low-density lipoprotein (LDL) cholesterol, and high-density lipoprotein (HDL) cholesterol is more controversial; while some postprandial differences have been detected, the clinical relevance is equivocal.18-21
Nonfasting lipid values can offer useful information, particularly in patients who are unwilling or unable to return for fasting labs. The US Preventive Services Task Force (USPSTF) supports this practice.22 Because guidelines for evaluation and treatment are based on fasting lipids, however, fasting lab work should be used, whenever possible, for initiating treatment and monitoring patients with abnormal values. If nonfasting lipids are used, it is crucial to factor in the postprandial effects on triglycerides and the subsequent difficulty of assessing LDL cholesterol levels.
THE TAKEAWAY: The clinical relevance of postprandial vs fasting lipid levels is equivocal. Nonfasting lipid panels have reasonable clinical utility in screening and initial treatment, particularly in cases in which obtaining fasting lab values may be problematic.18,19
4. Mononucleoosis spot test: When should you use it?
The monospot test is a latex assay that causes hemagglutination of horse RBCs in the presence of heterophile antibodies characteristic of infectious mononucleosis.23 The antibodies develop within the first 7 days of onset of symptoms, but do not peak for 2 to 5 weeks.24 As a result, monospot testing yields a high incidence of false negatives during the first 2 weeks of active infection.25 False negatives are also common in patients younger than 14 years. Heterophile antibodies may be present for up to a year after active infection.24
Patients at increased risk for splenic rupture, such as athletes, pose considerable diagnostic difficulty.26 When there is strong clinical suspicion of mononucleosis despite a negative monospot test in such high-risk individuals, follow-up testing is recommended to differentiate it from other mononucleosis-like illnesses (TABLE 2).27 The optimal combination of Epstein-Barr virus (EBV) serologic testing consists of the antibody titration of 4 markers: immunoglobulins M (IgM) and G (IgG) to the viral capsid antigen, IgM to the early antigen, and antibody to Epstein-Barr nuclear antigen (EBNA).28 Acute phase reactants in the setting of an antibody to EBNA could indicate reactivation. A positive test does not exclude other medical causes, however, because up to 20% of patients have acute phase antibodies that persist for years.29
Appropriate diagnosis is important because of the significant morbidity associated with EBV. Risk of splenic injury is greatest between 4 and 21 days after onset of symptoms but persists at 7 weeks,26 so conservative therapy followed by monospot retesting one week later is a reasonable approach.
Mononucleosis or routine tonsillitis? It is important to note that there is no evidence that a positive monospot test will affect the management or outcome of routine tonsillitis, raising questions of the utility of the test in such cases. A better approach: Reserve testing for patients with additional findings—ie, splenomegaly—or whose symptoms have persisted ≥ 2 weeks.
THE TAKEAWAY: Wait at least 2 weeks to conduct monospot testing in patients with routine tonsillitis. If strong clinical suspicion exists, proceed with specific IgM and IgG serologic testing.24,25,27,28
5. Evaluating prescription drug levels: Which factors interfere?
Correct interpretation of lab tests conducted to measure prescription drug levels has major implications with regard to patient safety, particularly for medications with a narrow therapeutic index.
Most drug level tests measure the total concentration, which includes both bound and unbound (free) forms. The unbound forms are the active components of the drug; thus, for an accurate evaluation, it is important to be aware of factors that increase free drug concentration. Chief among them is low protein levels, or hypoalbuminemia.30
Risk factors for hypoalbuminemia include significant burns, advanced age, pregnancy, malnutrition, and human immunodeficiency virus/acquired immune deficiency syndrome (HIV/AIDS).30 HIV/AIDS is a particularly high risk because certain protease inhibitors are highly protein bound.
Drug protein binding is classified as low, moderate, or high. The main proteins involved in the process are albumin, alpha-1-acid glycoprotein, and lipoprotein. Medications that are highly protein bound (>80%) are the most affected by low protein levels: Problems can arise when drugs completely bind to all the available proteins and excess drug availability increases free drug levels.
Medications that are most likely to be affected by a high degree of protein binding include carbamazepine, cyclosporine, mycophenolic acid, phenytoin, protease inhibitors (with the exception of indinavir), tacrolimus, and valproic acid. It is important to consider free levels when you order medication assays for these drugs to avoid misinterpreting the serum levels as being too low-a scenario that raises the risk of drug toxicity and adverse outcomes.30,31
A study of 119 phenytoin samples from 70 patients found significantly higher free phenytoin levels in patients with lower albumin levels.32 Higher free phenytoin levels were also seen in older patients and in those with diminished renal function (creatinine clearance <25 mL/min).32 The degree of protein binding is affected by both the serum drug concentration and the albumin level, with saturable protein binding occurring at higher drug levels.33
Calculate phenytoin levels with this equation. To calculate corrected phenytoin levels in patients with low albumin levels, use the following formula, known as the Sheiner-Tozer equation:34
Concentration adjusted=concentration reported/([adjustment x serum albumin] + 0.1); adjustment=0.2 for creatinine clearance ≥20 or 0.1 for creatinine clearance <20.
Additional causes of misinterpreted drug levels. While hypoalbuminemia plays a major role in the misinterpretation of drug levels, other factors affect serum drug concentration, as well. These include drug-drug interactions, which can significantly increase the concentration of the medications involved, and the timing of the test with regard to medication administration. Digoxin levels, in particular, need to be drawn at least 6 to 8 hours after the last dose is taken to allow for appropriate drug distribution.35
THE TAKEAWAY: It is essential to consider free drug level monitoring in patients who either have hypoalbuminemia or have one or more risk factors for hypoalbuminemia to avoid falsely low estimation of drug levels.36,37
6 Liver function tests: Necessary for patients on statin therapy?
Since statins gained US Food and Drug Administration (FDA) approval, the drugs have been associated with increased liver function tests (LFTs). Indeed, there had been a long-standing belief, based on clinical trials, that by monitoring alanine aminotransferase (ALT) and maintaining it at <3 times the upper limit normal (ULN), hepatotoxicity could be avoided.38 In clinical practice, however, further ALT elevation is frequently allowed based on patient tolerability.
In February 2012, the FDA revised its safety data to reflect this practice.39 The FDA update confirmed that routine LFT monitoring is unnecessary for patients on statins—and that it is not very effective in identifying or preventing liver damage.
Overall, serious hepatotoxicity is very rare, with an incidence ≤2 per 1 million patient-years.39 The National Lipid Association Statin Safety Assessment Task Force recommends repeating LFTs that are 3 to 5 times the ULN within 6 months and continuing with the statin dose if the patient is asymptomatic.38
THE TAKEAWAY: Routine liver function monitoring is not necessary for patients on statins. A better approach: Obtain baseline ALT levels, and repeat the testing only as clinically indicated thereafter.38,39
7. Urine drug screens: Which factors affect their accuracy?
The gold standard for testing for drugs of abuse, urine drug screens (UDS) have good sensitivity and specificity, easy administration, and reasonable cost.40 UDS can detect various narcotics, such as morphine, oxycodone, ,and methadone, and identify other illicit drugs, although which drugs and metabolites are tested for is laboratory- and test-specific.
Cross-reactivity. There are 6 currently available immunoassays, all of which use competitive binding between the sample drug and a drug chemically labeled with an enzyme, radioisotope, or fluorophore. The sample drug and labeled drug compete for substrate binding sites on drug-specific antibodies.41,42 Similar to competitive binding for enzymatic reactions in the body, the substrate binding site can experience cross-reactivity—causing substances other than the drug in question to bind to the immunoglobulin, leading to a false positive result (TABLE 3).43 Other factors that can alter the results include the cutoff value of the test and the absorption, distribution, metabolism, and excretion of the drug.42 Thus, a confirmatory test of gas chromatography-mass spectrometry is recommended before making decisions based on the results of UDS.43-45
Routine screens for patients on chronic opioid therapy. Routine use of UDS in emergency departments is no longer recommended, based on evidence that the results are unlikely to have a significant effect on patient management.46 For patients on chronic opioid therapy, however, routine screening has proven helpful in detecting prescription opioid abuse, illicit drug use, and diversion. Up to 34% of patients on prescription opioids have been found to be using illicit drugs, as well.42
THE TAKEAWAY: Use UDS as a tool in managing patients on chronic opioid therapy, but before acting on results, assess for factors, such as the use of oral or topical medications and the cutoff value of the test, that may be associated with false positive or false negative results.43-45
8. Thyroid function testing: When should you test?
Thyroid-stimulating hormone (TSH) is the first-line test when investigating presumed hyper- or hypothyroidism.47,48 Third-generation chemiluminometric assays can reliably measure TSH concentrations <0.01 mU/L by using multiple antibodies to produce a sandwich-type effect on the molecule in question.49
TSH levels exhibit diurnal variation, however, and are affected by other medications, including steroids, opiates, and some antihistamines, among others, as well as comorbidities.47,48 Chronic and acute conditions unrelated to thyroid disease can cause transient changes in TSH concentrations, and have the potential to modify the binding capacity of plasma thyroid hormone binding proteins.48 Thus, TSH should be ordered for hospitalized patients only when clinical suspicion of a thyroid problem exists.48 The USPSTF recommends against routine TSH screening for asymptomatic adults.46
How to respond to abnormal results. For patients found to have abnormal TSH levels, free T4 (fT4) is the next test to order.47,49 An fT4 assay is a superior indicator of thyroid status because it is not affected by changes in iodothyronine-binding proteins, which influence total hormone measurements.49 The results will be elevated in hyperthyroidism and reduced in hypothyroidism.47
Triiodothyronine (T3) measures can be useful in diagnosing Graves’ disease, in which T3 toxicosis may be the initial symptom—or an indication of a relapse. Because T3 is often a peripheral product, however, nonthyroid illnesses and medications can cause artifactually abnormal results.49
Other thyroid-specific labs include thyroid ,antibodies such as antithyroid peroxidase, antithyroglobulin, and TSH receptor, both blocking and stimulating.49 Thyroglobulin is a precursor form of thyroid hormone and should be measured when factitious hyperthyroidism is suspected. Management of hyper- and hypothyroidism often is independent of etiology. Retesting TSH to assess treatment response should be postponed until ≥2 months after any change in medication or dosing.50
Thyroid studies can be very difficult to interpret. TSH should be the first test ordered. However, if TSH values do not match the clinical picture, fT4, T3, and other thyroid tests that are less affected by outside factors can be useful in identifying the cause.
THE TAKEAWAY: Routine TSH testing is not indicated for asymptomatic adults. When evaluating thyroid function is clinically indicated, TSH is the initial test of choice.47,48,51
CORRESPONDENCE
Joshua Tessier, DO, Iowa Lutheran Family Medicine Residency, 840 East University Avenue, Des Moines, IA 50316; joshua.tessier@unitypoint.org
› When interpreting hemoglobin A1c (HbA1c) levels, assess for anemia and other comorbidities that can significantly affect the lifespan of red blood cells and skew HbA1c test results. B
› Order nonfasting lipid panels for patients for whom fasting laboratory tests are difficult to obtain, as they have good clinical utility in screening and initial treatment. A
› Avoid routine thyroid-stimulating hormone (TSH) testing in asymptomatic adults; when testing is indicated, start with TSH. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Laboratory mistakes are not defined as diagnostic errors, but they contribute significantly to the thousands of medical errors that occur every year.1 Part of the problem: While accurate interpretation of lab tests often depends on the use of statistical concepts we all learned in medical training, it is difficult to find the time to incorporate these principles into a busy practice.
Overuse of lab tests presents problems, as well. Because “normal ranges” for test results are based on statistical analysis, as many as 5% of patients in a standard distribution fall outside of the range.2 It is important to order only the tests you really need, as extra testing automatically means more false positive results.
This article was written with such pitfalls in mind. In the pages that follow, we focus on 8 types of tests family physicians rely on regularly—all cases in which test results are reliable only if comorbidities, pre- and post-test probabilities, and clinical context are carefully considered. To help you put these lab tests into the proper context, we’ve addressed a key question—and highlighted both pitfalls and pearls—about each.
1. Hemoglobin A1c: How does anemia affect it?
Hemoglobin A1c (HbA1c) can be measured in many ways, including high-performance liquid chromatography, boronate affinity, capillary electrophoresis, and immunoassay, all of which can provide equivalent values without significant variability.3,4 In interpreting these tests, however, it is important to understand the effect that anemia has on HbA1c.
It's important to order only the tests you really need, as extra testing automatically means more false positive results.
Two primary variables influencing HbA1c are the average glucose level and the average lifespan of red blood cells (RBCs). Normally, there is a direct correlation between average serum glucose and HbA1c.4 In patients with anemia, however, this relationship is less clear, and may be affected by erythropoiesis and RBC destruction.5 In iron deficiency anemia (IDA),6,7 hemoglobin production falls secondary to iron stores, resulting in microcytic cells with a longer lifespan and elevated HbA1c. In at least one study,5,7 HbA1c approached levels associated with diabetes (with increases as high as 1.5%) in nondiabetic patients, but resolved with treatment of IDA.
Increased destruction as well as increased production of RBCs lowers their lifespan, and in turn decreases HbA1c levels (TABLE 1).4 This can be seen in conditions such as splenomegaly and hemoglobinopathies. In patients with hemoglobinopathies, the percentage of hemoglobin A is significantly decreased, often to undetectable levels—thereby making HbA1c tests inaccurate. Hemoglobin electrophoresis and determination of glycation by capillary electrophoresis or high-performance liquid chromatography can be used instead, but neither is practical because of cost and limited availability.4,8,9
THE TAKEAWAY: When you evaluate HbA1c test results, it is crucial to assess the patient for anemia and other conditions or comorbidities that can significantly affect RBC lifespan and skew test results.2,4-6
2. D-dimer: When should you use it?
D-dimer is a fibrin degradation product that is increased when active clotting is present,10 and its assay—which has high sensitivity and low specificity—is widely used to screen for pulmonary embolism (PE) and deep vein thrombosis (DVT). While the minimal number of false negatives makes the D-dimer a good screening test, the higher rate of false positives makes it difficult to arrive at a definitive diagnosis. Appropriate use of the D-dimer assay is crucial to minimize the potential for adverse consequences, such as bleeding in patients who are subjected to unnecessary anticoagulation because of false positive results.
Further testing typically follows. A positive D-dimer test is commonly followed by a computed tomography (CT) scan of the chest or a ventilation/perfusion scan to establish a PE or DVT diagnosis. But this subsequent testing increases both the cost of health care and the patient’s radiation exposure. Use of these subsequent scans can be reduced by first considering the patient’s pretest probability for PE or DVT. The Wells’ Criteria (available at www.mdcalc.com/wells-criteriafor-pulmonary-embolism-pe/) and Geneva Score (Revised) (www.mdcalc.com/genevascore-revised-for-pulmonary-embolism/) can both be used for this purpose.10,11
Patients with high pretest probability should undergo immediate scanning, foregoing the D-dimer—which should be reserved for patients who have a low or moderate pretest probability but sufficient reason to suspect PE or DVT.10-12
The low specificity of the D-dimer assay poses another challenge to its effective use. There are many things that can increase D-dimer levels, such as age, cancer, prolonged immobility, autoimmune disease, inflammation, sickle cell disease, pregnancy, trauma, and surgery.13-15 All these factors must be taken into consideration prior to ordering this test.
In fact, one recent study found that using an age-adjusted D-dimer cutoff (patient’s age in years x 10 mcg/L)—rather than a conventional cutoff of 500 mcg/L—for patients older than 50 years reduces false positives without substantially increasing false negatives.16
Also of note: An anticoagulant can decrease D-dimer levels in plasma, so the test should not be used to rule out PE or DVT in patients who are undergoing anticoagulation.13,15
THE TAKEAWAY: In evaluating patients for PE or DVT, use the Wells’ Criteria or Geneva Score (Revised) to determine a patient’s pretest probability of disease. Use the D-dimer assay to safely rule out these conditions in patients with a low or intermediate pretest probability, but go directly to scans for those with a high pretest probability.
3. Lipid panels: How important is fasting?
Patients are often instructed to report for fasting lab studies, specifically for lipid profiles. Traditionally, this had been defined as an 8- to 12-hour period without food.17 In clinical practice, however, this is often misinterpreted by patients, who may be confused about the duration of the fast or unsure about whether to eat or drink immediately before the test.
Studies investigating the effect of meals on lab values have found that triglycerides are consistently elevated postprandially, to a maximum of 12 hours.18-21 The effect of the fasting state on total cholesterol, low-density lipoprotein (LDL) cholesterol, and high-density lipoprotein (HDL) cholesterol is more controversial; while some postprandial differences have been detected, the clinical relevance is equivocal.18-21
Nonfasting lipid values can offer useful information, particularly in patients who are unwilling or unable to return for fasting labs. The US Preventive Services Task Force (USPSTF) supports this practice.22 Because guidelines for evaluation and treatment are based on fasting lipids, however, fasting lab work should be used, whenever possible, for initiating treatment and monitoring patients with abnormal values. If nonfasting lipids are used, it is crucial to factor in the postprandial effects on triglycerides and the subsequent difficulty of assessing LDL cholesterol levels.
THE TAKEAWAY: The clinical relevance of postprandial vs fasting lipid levels is equivocal. Nonfasting lipid panels have reasonable clinical utility in screening and initial treatment, particularly in cases in which obtaining fasting lab values may be problematic.18,19
4. Mononucleoosis spot test: When should you use it?
The monospot test is a latex assay that causes hemagglutination of horse RBCs in the presence of heterophile antibodies characteristic of infectious mononucleosis.23 The antibodies develop within the first 7 days of onset of symptoms, but do not peak for 2 to 5 weeks.24 As a result, monospot testing yields a high incidence of false negatives during the first 2 weeks of active infection.25 False negatives are also common in patients younger than 14 years. Heterophile antibodies may be present for up to a year after active infection.24
Patients at increased risk for splenic rupture, such as athletes, pose considerable diagnostic difficulty.26 When there is strong clinical suspicion of mononucleosis despite a negative monospot test in such high-risk individuals, follow-up testing is recommended to differentiate it from other mononucleosis-like illnesses (TABLE 2).27 The optimal combination of Epstein-Barr virus (EBV) serologic testing consists of the antibody titration of 4 markers: immunoglobulins M (IgM) and G (IgG) to the viral capsid antigen, IgM to the early antigen, and antibody to Epstein-Barr nuclear antigen (EBNA).28 Acute phase reactants in the setting of an antibody to EBNA could indicate reactivation. A positive test does not exclude other medical causes, however, because up to 20% of patients have acute phase antibodies that persist for years.29
Appropriate diagnosis is important because of the significant morbidity associated with EBV. Risk of splenic injury is greatest between 4 and 21 days after onset of symptoms but persists at 7 weeks,26 so conservative therapy followed by monospot retesting one week later is a reasonable approach.
Mononucleosis or routine tonsillitis? It is important to note that there is no evidence that a positive monospot test will affect the management or outcome of routine tonsillitis, raising questions of the utility of the test in such cases. A better approach: Reserve testing for patients with additional findings—ie, splenomegaly—or whose symptoms have persisted ≥ 2 weeks.
THE TAKEAWAY: Wait at least 2 weeks to conduct monospot testing in patients with routine tonsillitis. If strong clinical suspicion exists, proceed with specific IgM and IgG serologic testing.24,25,27,28
5. Evaluating prescription drug levels: Which factors interfere?
Correct interpretation of lab tests conducted to measure prescription drug levels has major implications with regard to patient safety, particularly for medications with a narrow therapeutic index.
Most drug level tests measure the total concentration, which includes both bound and unbound (free) forms. The unbound forms are the active components of the drug; thus, for an accurate evaluation, it is important to be aware of factors that increase free drug concentration. Chief among them is low protein levels, or hypoalbuminemia.30
Risk factors for hypoalbuminemia include significant burns, advanced age, pregnancy, malnutrition, and human immunodeficiency virus/acquired immune deficiency syndrome (HIV/AIDS).30 HIV/AIDS is a particularly high risk because certain protease inhibitors are highly protein bound.
Drug protein binding is classified as low, moderate, or high. The main proteins involved in the process are albumin, alpha-1-acid glycoprotein, and lipoprotein. Medications that are highly protein bound (>80%) are the most affected by low protein levels: Problems can arise when drugs completely bind to all the available proteins and excess drug availability increases free drug levels.
Medications that are most likely to be affected by a high degree of protein binding include carbamazepine, cyclosporine, mycophenolic acid, phenytoin, protease inhibitors (with the exception of indinavir), tacrolimus, and valproic acid. It is important to consider free levels when you order medication assays for these drugs to avoid misinterpreting the serum levels as being too low-a scenario that raises the risk of drug toxicity and adverse outcomes.30,31
A study of 119 phenytoin samples from 70 patients found significantly higher free phenytoin levels in patients with lower albumin levels.32 Higher free phenytoin levels were also seen in older patients and in those with diminished renal function (creatinine clearance <25 mL/min).32 The degree of protein binding is affected by both the serum drug concentration and the albumin level, with saturable protein binding occurring at higher drug levels.33
Calculate phenytoin levels with this equation. To calculate corrected phenytoin levels in patients with low albumin levels, use the following formula, known as the Sheiner-Tozer equation:34
Concentration adjusted=concentration reported/([adjustment x serum albumin] + 0.1); adjustment=0.2 for creatinine clearance ≥20 or 0.1 for creatinine clearance <20.
Additional causes of misinterpreted drug levels. While hypoalbuminemia plays a major role in the misinterpretation of drug levels, other factors affect serum drug concentration, as well. These include drug-drug interactions, which can significantly increase the concentration of the medications involved, and the timing of the test with regard to medication administration. Digoxin levels, in particular, need to be drawn at least 6 to 8 hours after the last dose is taken to allow for appropriate drug distribution.35
THE TAKEAWAY: It is essential to consider free drug level monitoring in patients who either have hypoalbuminemia or have one or more risk factors for hypoalbuminemia to avoid falsely low estimation of drug levels.36,37
6 Liver function tests: Necessary for patients on statin therapy?
Since statins gained US Food and Drug Administration (FDA) approval, the drugs have been associated with increased liver function tests (LFTs). Indeed, there had been a long-standing belief, based on clinical trials, that by monitoring alanine aminotransferase (ALT) and maintaining it at <3 times the upper limit normal (ULN), hepatotoxicity could be avoided.38 In clinical practice, however, further ALT elevation is frequently allowed based on patient tolerability.
In February 2012, the FDA revised its safety data to reflect this practice.39 The FDA update confirmed that routine LFT monitoring is unnecessary for patients on statins—and that it is not very effective in identifying or preventing liver damage.
Overall, serious hepatotoxicity is very rare, with an incidence ≤2 per 1 million patient-years.39 The National Lipid Association Statin Safety Assessment Task Force recommends repeating LFTs that are 3 to 5 times the ULN within 6 months and continuing with the statin dose if the patient is asymptomatic.38
THE TAKEAWAY: Routine liver function monitoring is not necessary for patients on statins. A better approach: Obtain baseline ALT levels, and repeat the testing only as clinically indicated thereafter.38,39
7. Urine drug screens: Which factors affect their accuracy?
The gold standard for testing for drugs of abuse, urine drug screens (UDS) have good sensitivity and specificity, easy administration, and reasonable cost.40 UDS can detect various narcotics, such as morphine, oxycodone, ,and methadone, and identify other illicit drugs, although which drugs and metabolites are tested for is laboratory- and test-specific.
Cross-reactivity. There are 6 currently available immunoassays, all of which use competitive binding between the sample drug and a drug chemically labeled with an enzyme, radioisotope, or fluorophore. The sample drug and labeled drug compete for substrate binding sites on drug-specific antibodies.41,42 Similar to competitive binding for enzymatic reactions in the body, the substrate binding site can experience cross-reactivity—causing substances other than the drug in question to bind to the immunoglobulin, leading to a false positive result (TABLE 3).43 Other factors that can alter the results include the cutoff value of the test and the absorption, distribution, metabolism, and excretion of the drug.42 Thus, a confirmatory test of gas chromatography-mass spectrometry is recommended before making decisions based on the results of UDS.43-45
Routine screens for patients on chronic opioid therapy. Routine use of UDS in emergency departments is no longer recommended, based on evidence that the results are unlikely to have a significant effect on patient management.46 For patients on chronic opioid therapy, however, routine screening has proven helpful in detecting prescription opioid abuse, illicit drug use, and diversion. Up to 34% of patients on prescription opioids have been found to be using illicit drugs, as well.42
THE TAKEAWAY: Use UDS as a tool in managing patients on chronic opioid therapy, but before acting on results, assess for factors, such as the use of oral or topical medications and the cutoff value of the test, that may be associated with false positive or false negative results.43-45
8. Thyroid function testing: When should you test?
Thyroid-stimulating hormone (TSH) is the first-line test when investigating presumed hyper- or hypothyroidism.47,48 Third-generation chemiluminometric assays can reliably measure TSH concentrations <0.01 mU/L by using multiple antibodies to produce a sandwich-type effect on the molecule in question.49
TSH levels exhibit diurnal variation, however, and are affected by other medications, including steroids, opiates, and some antihistamines, among others, as well as comorbidities.47,48 Chronic and acute conditions unrelated to thyroid disease can cause transient changes in TSH concentrations, and have the potential to modify the binding capacity of plasma thyroid hormone binding proteins.48 Thus, TSH should be ordered for hospitalized patients only when clinical suspicion of a thyroid problem exists.48 The USPSTF recommends against routine TSH screening for asymptomatic adults.46
How to respond to abnormal results. For patients found to have abnormal TSH levels, free T4 (fT4) is the next test to order.47,49 An fT4 assay is a superior indicator of thyroid status because it is not affected by changes in iodothyronine-binding proteins, which influence total hormone measurements.49 The results will be elevated in hyperthyroidism and reduced in hypothyroidism.47
Triiodothyronine (T3) measures can be useful in diagnosing Graves’ disease, in which T3 toxicosis may be the initial symptom—or an indication of a relapse. Because T3 is often a peripheral product, however, nonthyroid illnesses and medications can cause artifactually abnormal results.49
Other thyroid-specific labs include thyroid ,antibodies such as antithyroid peroxidase, antithyroglobulin, and TSH receptor, both blocking and stimulating.49 Thyroglobulin is a precursor form of thyroid hormone and should be measured when factitious hyperthyroidism is suspected. Management of hyper- and hypothyroidism often is independent of etiology. Retesting TSH to assess treatment response should be postponed until ≥2 months after any change in medication or dosing.50
Thyroid studies can be very difficult to interpret. TSH should be the first test ordered. However, if TSH values do not match the clinical picture, fT4, T3, and other thyroid tests that are less affected by outside factors can be useful in identifying the cause.
THE TAKEAWAY: Routine TSH testing is not indicated for asymptomatic adults. When evaluating thyroid function is clinically indicated, TSH is the initial test of choice.47,48,51
CORRESPONDENCE
Joshua Tessier, DO, Iowa Lutheran Family Medicine Residency, 840 East University Avenue, Des Moines, IA 50316; joshua.tessier@unitypoint.org
1. Ottomano C. Errors in medicine and errors in laboratory medicine: what is the difference? Blood Transfus. 2010;8;79-81.
2. Wallach JB. Introduction to normal values (reference ranges). Interpretation of Diagnostic Tests. 8th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2007:3-7.
3. Halwachs-Baumann G, Katzensteiner S, Schnedl W, et al. Comparative evaluation of three assay systems for automated determination of hemoglobin A1c. Clin Chem. 1997;43:511-517.
4. Gallagher EJ, Le Roith D, Bloomgarden Z. Review of hemoglobin A(1c) in the management of diabetes. J Diabetes. 2009;1:9-17.
5. Franco RS. The measurement and importance of red cell survival. Am J Hematol. 2009;84:109-114.
6. Tarim O, Küçükerdog˘an A, Gunay U, et al. Effects of iron deficiency anemia on hemoglobin A1c in type 1 diabetes mellitus. Pediatr Int. 1999;41:357-362.
7. Kim C, Bullard KM, Herman WH, et al. Association between iron deficiency and A1C levels among adults without diabetes in the National Health and Nutrition Examination Survey, 1999-2006. Diabetes Care. 2010;33:780-785.
8. Higgins T, Stewart D, Boehr E. Challenges in HbA1c analysis and reporting: an interesting case illustrating the many pitfalls. Clin Biochem. 2008;41:1104-1106.
9. Mongia SK, Little RR, Rohlfing CL, et al. Effects of hemoglobin C and S traits on the results of 14 commercial glycated hemoglobin assays. Am J Clin Pathol. 2008;130:136-140.
10. Brown MD, Rowe BH, Reeves MJ, et al. The accuracy of the enzyme-linked immunosorbent assay D-dimer test in the diagnosis of pulmonary embolism: a meta-analysis. Ann Emerg Med. 2002;40:133-144.
11. Squizzato A, Ageno W. What is the next step in D-dimer research? Education of physicians. Intern Emerg Med. 2006;1:165.
12. Kabrhel C, Mark Courtney D, Camargo CA Jr, et al. Potential impact of adjusting the threshold of the quantitative D-dimer based on pretest probability of acute pulmonary embolism. Acad Emerg Med. 2009;16:325-332.
13. Kabrhel C, Mark Courtney MD, Camargo CA Jr, et al. Factors associated with positive D-dimer results in patients evaluated for pulmonary embolism. Acad Emerg Med. 2010;17:589-597.
14. Berman AR. Pulmonary embolism in the elderly. Clin Geriatr Med. 2001;17:107-130.
15. Bruinstroop E, van de Ree MA, Huisman MV. The use of Ddimer in specific clinical conditions: a narrative review. Eur J Intern Med. 2009;20:441-446.
16. Schouten HJ, Geersing GI, Koek HL, et al. Diagnostic accuracy ,of conventional or age adjusted D-dimer cut-off values in older patients with suspected venous thromboembolism: systematic review and meta-analysis. BMJ. 2013;346:f2492.
17. Turgeon ML. Linne & Ringsrud’s Clinical Laboratory Science. 5th ed. Saint Louis, MO: Mosby; 2007:50.
18. Cohn JS,McNamara JR, Schaefer EJ. Lipoprotein cholesterol concentrations in the plasma of human subjects as measured in the fed and fasted states. Clin Chem. 1988;34:2456-2459.
19. Watts GF, Cohn JS. Whither the lipid profile: feast, famine, or no free lunch? Clin Chem. 2011;57:363-365.
20. Mora S, Rifai N, Buring JE, et al. Fasting compared with nonfasting lipids and apolipoproteins for predicting incident cardiovascular events. Circulation. 2008;118:993-1001.
21. Nordestgaard BG, Langsted A, Freiberg JJ. Nonfasting hyperlipidemia and cardiovascular disease. Curr Drug Targets. 2009;10:328-335.
22. Screening for lipid disorders in adults: US Preventive Services Task Force Recommendation statement. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/uspstf08/lipid/lipidrs.htm. Accessed March 13, 2014.
23. Wolf DM, Friedrichs I, Toma AG. Lymphocyte-white blood cell count ratio: a quickly available screening tool to differentiate acute purulent tonsillitis from glandular fever. Arch Otolaryngol Head Neck Surg. 2007;133:61-64.
24. McCormack R, O’Shea T. The uptake and use of the Monospot test in patients with tonsillitis. Ir Med J. 2009;102:226-228.
25. Ebell MH. Epstein-Barr virus infectious mononucleosis. Am Fam Physician. 2004;70:1279-1287.
26. Waninger KN, Harcke HT. Determination of safe return to play for athletes recovering from infectious mononucleosis: a review of the literature. Clin J Sport Med. 2005;15:410-416.
27. Hurt C, Tammaro D. Diagnostic evaluation of mononucleosislike illnesses. Am J Med. 2007;120:911.e1-911.e8.
28. Vouloumanou EK, Rafailidis PI, Falagas ME. Current diagnosis and management of infectious mononucleosis. Curr Opin Hematol. 2012;19:14-20.
29. Epstein-Barr virus and infectious mononucleosis. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/epstein-barr/laboratory-testing.html. Updated January 7, 2014. Accessed March 12, 2014.
30. Dasgupta A. Clinical utility of free drug monitoring. Clin Chem Lab Med. 2002;40:986-993.
31. Dasgupta A. Usefulness of monitoring free (unbound) concentrations of therapeutic drugs in patient management. Clin Chim Acta. 2007;377:1-13.
32. Iwamoto T, Kagawa Y, Naito Y, et al. Clinical evaluation of plasma free phenytoin measurement and factors influencing its protein binding. Biopharm Drug Dispos. 2006;27:77-84.
33. Hermida J, Tutor JC. A theoretical method for normalizing total serum valproic acid concentration in hypoalbuminemic patients. J Pharmacol Sci. 2005;97:489-493.
34. Wolf GK, McClain CD, Zurakowski D, et al. Total phenytoin concentrations do not accurately predict free phenytoin concentrations in critically ill children. Pediatr Crit Care Med. 2006;7:434-439; quiz 440.
35. Lanoxin (digoxin) [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2011.
36. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143-3421.
37. De Backer G, Ambrosini E, Borch-Johnsen K, et al; Third Joint Force of European and other Societies on Cardiovascular Disease and Prevention in Clinical Practice. European guidelines on cardiovascular disease and prevention in clinical practice. Atherosclerosis. 2003;171:145-155.
38. McKenney JM, Davidson MH, Jacobson TA, et al. Final conclusions and recommendations of the National Lipid Association Statin Safety Assessment Task Force. Am J Cardiol. 2006;97:89C-94C.
39. FDA drug safety communication: important safety label changes to cholesterol-lowering statin drugs. US Food and Drug Administration Web site. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm293101.htm. Updated July 3, 2012. Accessed May 17, 2013.
40. Eskridge KD, Guthrie SK. Clinical issues associated with urine testing of substances of abuse. Pharmacotherapy. 1997;17:497-510.
41. Herring C, Muzyk AJ, Johnston C. Interferences with urine drug screens. J Pharm Pract. 2001;24:102-108.
42. Jaffee WB, Trucco E, Teter C, et al. Focus on alcohol & drug abuse: ensuring validity in urine drug testing. Psychiatr Serv. 2008;59:140-142.
43. Brahm NC, Yeager LL, Fox MD, et al. Commonly prescribed medications and potential false-positive urine drug screens. Am J Health Syst Pharm. 2010;67:1344-1350.
44. Moeller KE, Lee KC, Kissack JC. Urine drug screening: Practical guide for clinicians. Mayo Clin Proc. 2008;83:66-76.
45. Christo PJ, Manchikanti L, Ruan X, et al. Urine drug testing in chronic pain. Pain Physician. 2011;14:123-143.
46. Tenenbein M. Do you really need that emergency drug screen? Clin Toxicol (Phila). 2009;47:286-291.
47. Gaitonde DY, Rowley KD, Sweeney LB. Hypothyroidism: an update. Am Fam Physician. 2012;86:244-251.
48. UK guidelines for the use of thyroid function tests. British Thyroid Association Web site. Available at: http://www.british-thyroid-association.org/info-for-patients/Docs/TFT_guideline_final_version_July_2006.pdf. Accessed March 11, 2014.
49. Volpé, R. Rational use of thyroid function tests. Crit Rev Clin Lab Sci. 1997;34:405-438.
50. Graber JR, Cobin RH, Gharib H, et al; American Association of Clinical Endocrinologists and American Thyroid Association Taskforce on Hypothyroidism in Adults. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr Pract. 2012;18:988-1028.
51. Helfand M; US Preventive Services Task Force. Screening for subclinical thyroid dysfunction in nonpregnant adults: a summary of the evidence for the US Preventive Services Task Force. Ann Intern Med. 2004;140:128-141.
1. Ottomano C. Errors in medicine and errors in laboratory medicine: what is the difference? Blood Transfus. 2010;8;79-81.
2. Wallach JB. Introduction to normal values (reference ranges). Interpretation of Diagnostic Tests. 8th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2007:3-7.
3. Halwachs-Baumann G, Katzensteiner S, Schnedl W, et al. Comparative evaluation of three assay systems for automated determination of hemoglobin A1c. Clin Chem. 1997;43:511-517.
4. Gallagher EJ, Le Roith D, Bloomgarden Z. Review of hemoglobin A(1c) in the management of diabetes. J Diabetes. 2009;1:9-17.
5. Franco RS. The measurement and importance of red cell survival. Am J Hematol. 2009;84:109-114.
6. Tarim O, Küçükerdog˘an A, Gunay U, et al. Effects of iron deficiency anemia on hemoglobin A1c in type 1 diabetes mellitus. Pediatr Int. 1999;41:357-362.
7. Kim C, Bullard KM, Herman WH, et al. Association between iron deficiency and A1C levels among adults without diabetes in the National Health and Nutrition Examination Survey, 1999-2006. Diabetes Care. 2010;33:780-785.
8. Higgins T, Stewart D, Boehr E. Challenges in HbA1c analysis and reporting: an interesting case illustrating the many pitfalls. Clin Biochem. 2008;41:1104-1106.
9. Mongia SK, Little RR, Rohlfing CL, et al. Effects of hemoglobin C and S traits on the results of 14 commercial glycated hemoglobin assays. Am J Clin Pathol. 2008;130:136-140.
10. Brown MD, Rowe BH, Reeves MJ, et al. The accuracy of the enzyme-linked immunosorbent assay D-dimer test in the diagnosis of pulmonary embolism: a meta-analysis. Ann Emerg Med. 2002;40:133-144.
11. Squizzato A, Ageno W. What is the next step in D-dimer research? Education of physicians. Intern Emerg Med. 2006;1:165.
12. Kabrhel C, Mark Courtney D, Camargo CA Jr, et al. Potential impact of adjusting the threshold of the quantitative D-dimer based on pretest probability of acute pulmonary embolism. Acad Emerg Med. 2009;16:325-332.
13. Kabrhel C, Mark Courtney MD, Camargo CA Jr, et al. Factors associated with positive D-dimer results in patients evaluated for pulmonary embolism. Acad Emerg Med. 2010;17:589-597.
14. Berman AR. Pulmonary embolism in the elderly. Clin Geriatr Med. 2001;17:107-130.
15. Bruinstroop E, van de Ree MA, Huisman MV. The use of Ddimer in specific clinical conditions: a narrative review. Eur J Intern Med. 2009;20:441-446.
16. Schouten HJ, Geersing GI, Koek HL, et al. Diagnostic accuracy ,of conventional or age adjusted D-dimer cut-off values in older patients with suspected venous thromboembolism: systematic review and meta-analysis. BMJ. 2013;346:f2492.
17. Turgeon ML. Linne & Ringsrud’s Clinical Laboratory Science. 5th ed. Saint Louis, MO: Mosby; 2007:50.
18. Cohn JS,McNamara JR, Schaefer EJ. Lipoprotein cholesterol concentrations in the plasma of human subjects as measured in the fed and fasted states. Clin Chem. 1988;34:2456-2459.
19. Watts GF, Cohn JS. Whither the lipid profile: feast, famine, or no free lunch? Clin Chem. 2011;57:363-365.
20. Mora S, Rifai N, Buring JE, et al. Fasting compared with nonfasting lipids and apolipoproteins for predicting incident cardiovascular events. Circulation. 2008;118:993-1001.
21. Nordestgaard BG, Langsted A, Freiberg JJ. Nonfasting hyperlipidemia and cardiovascular disease. Curr Drug Targets. 2009;10:328-335.
22. Screening for lipid disorders in adults: US Preventive Services Task Force Recommendation statement. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/uspstf08/lipid/lipidrs.htm. Accessed March 13, 2014.
23. Wolf DM, Friedrichs I, Toma AG. Lymphocyte-white blood cell count ratio: a quickly available screening tool to differentiate acute purulent tonsillitis from glandular fever. Arch Otolaryngol Head Neck Surg. 2007;133:61-64.
24. McCormack R, O’Shea T. The uptake and use of the Monospot test in patients with tonsillitis. Ir Med J. 2009;102:226-228.
25. Ebell MH. Epstein-Barr virus infectious mononucleosis. Am Fam Physician. 2004;70:1279-1287.
26. Waninger KN, Harcke HT. Determination of safe return to play for athletes recovering from infectious mononucleosis: a review of the literature. Clin J Sport Med. 2005;15:410-416.
27. Hurt C, Tammaro D. Diagnostic evaluation of mononucleosislike illnesses. Am J Med. 2007;120:911.e1-911.e8.
28. Vouloumanou EK, Rafailidis PI, Falagas ME. Current diagnosis and management of infectious mononucleosis. Curr Opin Hematol. 2012;19:14-20.
29. Epstein-Barr virus and infectious mononucleosis. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/epstein-barr/laboratory-testing.html. Updated January 7, 2014. Accessed March 12, 2014.
30. Dasgupta A. Clinical utility of free drug monitoring. Clin Chem Lab Med. 2002;40:986-993.
31. Dasgupta A. Usefulness of monitoring free (unbound) concentrations of therapeutic drugs in patient management. Clin Chim Acta. 2007;377:1-13.
32. Iwamoto T, Kagawa Y, Naito Y, et al. Clinical evaluation of plasma free phenytoin measurement and factors influencing its protein binding. Biopharm Drug Dispos. 2006;27:77-84.
33. Hermida J, Tutor JC. A theoretical method for normalizing total serum valproic acid concentration in hypoalbuminemic patients. J Pharmacol Sci. 2005;97:489-493.
34. Wolf GK, McClain CD, Zurakowski D, et al. Total phenytoin concentrations do not accurately predict free phenytoin concentrations in critically ill children. Pediatr Crit Care Med. 2006;7:434-439; quiz 440.
35. Lanoxin (digoxin) [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2011.
36. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143-3421.
37. De Backer G, Ambrosini E, Borch-Johnsen K, et al; Third Joint Force of European and other Societies on Cardiovascular Disease and Prevention in Clinical Practice. European guidelines on cardiovascular disease and prevention in clinical practice. Atherosclerosis. 2003;171:145-155.
38. McKenney JM, Davidson MH, Jacobson TA, et al. Final conclusions and recommendations of the National Lipid Association Statin Safety Assessment Task Force. Am J Cardiol. 2006;97:89C-94C.
39. FDA drug safety communication: important safety label changes to cholesterol-lowering statin drugs. US Food and Drug Administration Web site. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm293101.htm. Updated July 3, 2012. Accessed May 17, 2013.
40. Eskridge KD, Guthrie SK. Clinical issues associated with urine testing of substances of abuse. Pharmacotherapy. 1997;17:497-510.
41. Herring C, Muzyk AJ, Johnston C. Interferences with urine drug screens. J Pharm Pract. 2001;24:102-108.
42. Jaffee WB, Trucco E, Teter C, et al. Focus on alcohol & drug abuse: ensuring validity in urine drug testing. Psychiatr Serv. 2008;59:140-142.
43. Brahm NC, Yeager LL, Fox MD, et al. Commonly prescribed medications and potential false-positive urine drug screens. Am J Health Syst Pharm. 2010;67:1344-1350.
44. Moeller KE, Lee KC, Kissack JC. Urine drug screening: Practical guide for clinicians. Mayo Clin Proc. 2008;83:66-76.
45. Christo PJ, Manchikanti L, Ruan X, et al. Urine drug testing in chronic pain. Pain Physician. 2011;14:123-143.
46. Tenenbein M. Do you really need that emergency drug screen? Clin Toxicol (Phila). 2009;47:286-291.
47. Gaitonde DY, Rowley KD, Sweeney LB. Hypothyroidism: an update. Am Fam Physician. 2012;86:244-251.
48. UK guidelines for the use of thyroid function tests. British Thyroid Association Web site. Available at: http://www.british-thyroid-association.org/info-for-patients/Docs/TFT_guideline_final_version_July_2006.pdf. Accessed March 11, 2014.
49. Volpé, R. Rational use of thyroid function tests. Crit Rev Clin Lab Sci. 1997;34:405-438.
50. Graber JR, Cobin RH, Gharib H, et al; American Association of Clinical Endocrinologists and American Thyroid Association Taskforce on Hypothyroidism in Adults. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr Pract. 2012;18:988-1028.
51. Helfand M; US Preventive Services Task Force. Screening for subclinical thyroid dysfunction in nonpregnant adults: a summary of the evidence for the US Preventive Services Task Force. Ann Intern Med. 2004;140:128-141.
How STAT3 blocks an antitumor mechanism
Credit: Ed Uthman
Researchers say they’ve discovered how the protein STAT3 interferes with an antitumor mechanism in cells, thereby promoting the growth of lymphoma and other cancers.
The group made their discovery using the Epstein-Barr virus (EBV) as a tool to investigate cancer development.
“Our findings add to the short list of known mechanisms by which a key cellular antitumor barrier is breached by STAT3 prior to cancer development,” said Sumita Bhaduri-McIntosh, MD, PhD, of Stony Brook University School of Medicine in New York.
“Because STAT3 interferes with this innate antitumor mechanism in cells, the opposite occurs when blood cells are infected in the lab with the cancer-causing virus EBV, and the cells continue to divide—a necessary step in cancer development.”
Dr Bhaduri-McIntosh and her colleagues described their research in PNAS.
The team explained that STAT3 inhibits a cancer-suppressing cellular activity called the DNA damage response (DDR). Normally, this response pauses cell division, allowing for the repair of damaged DNA.
But this study showed that EBV not only causes DNA damage when it infects and replicates in cells; it also activates and increases STAT3 expression. This starts a chain reaction that leads to an “un-pause” in cell division, thereby promoting cell proliferation. This, in combination with other pro-proliferative effects of the virus, can lead to cancers.
The researchers found that DDR does detect replication stress-associated DNA damage resulting from EBV infection. But signaling downstream of ATR proteins is impaired by STAT3. And this leads to relaxation of the intra-S phase checkpoint of the cell cycle.
STAT3 interrupts signaling from ATR to the protein Chk1 by promoting the loss of Claspin, a protein that assists ATR to phosphorylate Chk1. The loss of Claspin, which facilitates cell proliferation, is mediated by caspase 7.
Previous research suggested that STAT3 and Chk1 are potential targets for cancer therapies. Dr Bhaduri-McIntosh’s team said their results provide new insight into anticancer drug development because they reveal a mechanistic link between these 2 proteins.
Dr Bhaduri-McIntosh emphasized that, because STAT3 is involved in most cancers, her group’s findings could potentially impact the prevention or treatment of several types of cancer—something her lab is investigating. ![]()

Credit: Ed Uthman
Researchers say they’ve discovered how the protein STAT3 interferes with an antitumor mechanism in cells, thereby promoting the growth of lymphoma and other cancers.
The group made their discovery using the Epstein-Barr virus (EBV) as a tool to investigate cancer development.
“Our findings add to the short list of known mechanisms by which a key cellular antitumor barrier is breached by STAT3 prior to cancer development,” said Sumita Bhaduri-McIntosh, MD, PhD, of Stony Brook University School of Medicine in New York.
“Because STAT3 interferes with this innate antitumor mechanism in cells, the opposite occurs when blood cells are infected in the lab with the cancer-causing virus EBV, and the cells continue to divide—a necessary step in cancer development.”
Dr Bhaduri-McIntosh and her colleagues described their research in PNAS.
The team explained that STAT3 inhibits a cancer-suppressing cellular activity called the DNA damage response (DDR). Normally, this response pauses cell division, allowing for the repair of damaged DNA.
But this study showed that EBV not only causes DNA damage when it infects and replicates in cells; it also activates and increases STAT3 expression. This starts a chain reaction that leads to an “un-pause” in cell division, thereby promoting cell proliferation. This, in combination with other pro-proliferative effects of the virus, can lead to cancers.
The researchers found that DDR does detect replication stress-associated DNA damage resulting from EBV infection. But signaling downstream of ATR proteins is impaired by STAT3. And this leads to relaxation of the intra-S phase checkpoint of the cell cycle.
STAT3 interrupts signaling from ATR to the protein Chk1 by promoting the loss of Claspin, a protein that assists ATR to phosphorylate Chk1. The loss of Claspin, which facilitates cell proliferation, is mediated by caspase 7.
Previous research suggested that STAT3 and Chk1 are potential targets for cancer therapies. Dr Bhaduri-McIntosh’s team said their results provide new insight into anticancer drug development because they reveal a mechanistic link between these 2 proteins.
Dr Bhaduri-McIntosh emphasized that, because STAT3 is involved in most cancers, her group’s findings could potentially impact the prevention or treatment of several types of cancer—something her lab is investigating. ![]()

Credit: Ed Uthman
Researchers say they’ve discovered how the protein STAT3 interferes with an antitumor mechanism in cells, thereby promoting the growth of lymphoma and other cancers.
The group made their discovery using the Epstein-Barr virus (EBV) as a tool to investigate cancer development.
“Our findings add to the short list of known mechanisms by which a key cellular antitumor barrier is breached by STAT3 prior to cancer development,” said Sumita Bhaduri-McIntosh, MD, PhD, of Stony Brook University School of Medicine in New York.
“Because STAT3 interferes with this innate antitumor mechanism in cells, the opposite occurs when blood cells are infected in the lab with the cancer-causing virus EBV, and the cells continue to divide—a necessary step in cancer development.”
Dr Bhaduri-McIntosh and her colleagues described their research in PNAS.
The team explained that STAT3 inhibits a cancer-suppressing cellular activity called the DNA damage response (DDR). Normally, this response pauses cell division, allowing for the repair of damaged DNA.
But this study showed that EBV not only causes DNA damage when it infects and replicates in cells; it also activates and increases STAT3 expression. This starts a chain reaction that leads to an “un-pause” in cell division, thereby promoting cell proliferation. This, in combination with other pro-proliferative effects of the virus, can lead to cancers.
The researchers found that DDR does detect replication stress-associated DNA damage resulting from EBV infection. But signaling downstream of ATR proteins is impaired by STAT3. And this leads to relaxation of the intra-S phase checkpoint of the cell cycle.
STAT3 interrupts signaling from ATR to the protein Chk1 by promoting the loss of Claspin, a protein that assists ATR to phosphorylate Chk1. The loss of Claspin, which facilitates cell proliferation, is mediated by caspase 7.
Previous research suggested that STAT3 and Chk1 are potential targets for cancer therapies. Dr Bhaduri-McIntosh’s team said their results provide new insight into anticancer drug development because they reveal a mechanistic link between these 2 proteins.
Dr Bhaduri-McIntosh emphasized that, because STAT3 is involved in most cancers, her group’s findings could potentially impact the prevention or treatment of several types of cancer—something her lab is investigating. ![]()
Mediterranean diet tied to decreased platelets, WBCs

part of a Mediterranean diet
In a large study, individuals who strictly followed a Mediterranean diet had lower levels of platelets and white blood cells (WBCs) than those who deviated from the diet.
And the lower cell counts were associated with lower levels of inflammation.
The research also suggested the diet as a whole, and not just certain components, was responsible for these markers of improved health.
Marialaura Bonaccio, PhD, of the IRCCS Istituto Neurologico Mediterraneo NEUROMED in Italy, and her colleagues reported these findings in Blood.
The team noted that the Mediterranean diet—which is characterized by a wide consumption of plant foods, cereals, legumes, fish, and olive oil, as well as moderate wine consumption—has long been hailed as a heart-healthy eating plan. And previous research suggested the diet can reduce inflammation.
But the connection between the diet and levels of platelets and WBCs, 2 specific inflammatory markers in the body, has remained unclear.
“We undertook this study to understand the correlation between consuming a Mediterranean diet and specific health markers, including platelet levels and white blood cell counts, which can more specifically explain the diet’s benefits in reducing the long-term risk of cerebral and heart disease or other chronic conditions,” Dr Bonaccio said.
To do this, she and her colleagues analyzed the eating habits of 14,586 healthy Italian men and women aged 35 and older. At baseline, all subjects were healthy.
The researchers measured total platelet and WBC counts and grouped participants according to their levels (low, normal, or high), based on age- and gender-specific cut-offs.
Participants with high platelet levels were younger and had a greater incidence of high cholesterol and increased levels of common inflammation marker C-reactive protein when compared to subjects in the normal or low-platelet categories.
Individuals in the high-WBC category were mainly younger, male, and smokers. They had a higher body-mass index and higher levels of C-reactive protein and blood glucose than subjects in the other groups. They also showed higher prevalence of high blood pressure and high cholesterol.
The researchers determined participants’ adherence to a Mediterranean using 2 dietary scoring systems, the Mediterranean diet score or the Italian Mediterranean Index, which helped to accurately determine intake levels and portion sizes.
Results of these analyses revealed that adherence to the Mediterranean diet was directly related to lower levels of platelets and WBCs (P<0.0001 and P=0.008, respectively), which was correlated with lower levels of inflammation.
When compared with participants who did not follow the eating plan as closely, subjects who strictly followed the diet were less likely to belong to the group with the highest platelet counts (odds ratio=0.50) and more likely to belong to the group with the lowest WBC counts (odds ratio=1.41).
“Because the study included healthy participants, the lower levels of platelets and white blood cells in those who were more strictly consuming a Mediterranean diet indicate that this eating plan could account for substantial changes within normal ranges of variability,” Dr Bonaccio said.
“This is an important finding that has implications for how these anti-inflammatory markers are tracked among the general population.”
The researchers also evaluated the role of specific components of the diet to help clarify the observed correlation, including food antioxidant content and fiber intake, both of which have previously been connected to cardiovascular benefits.
These components only partially accounted for the link between the diet and WBC count. And they did not fully explain the correlation to platelet levels.
“An important finding of this study is that it indicates that the Mediterranean diet as a whole, and not just a few specific ingredients, is likely responsible for the beneficial health outcomes among the healthy population and should be encouraged as part of healthy eating habits,” Dr Bonaccio said.
“Building on these important findings, we continue to study this population to determine if the dietary habits may have an influence on cardiovascular disease-related mortality.” ![]()

part of a Mediterranean diet
In a large study, individuals who strictly followed a Mediterranean diet had lower levels of platelets and white blood cells (WBCs) than those who deviated from the diet.
And the lower cell counts were associated with lower levels of inflammation.
The research also suggested the diet as a whole, and not just certain components, was responsible for these markers of improved health.
Marialaura Bonaccio, PhD, of the IRCCS Istituto Neurologico Mediterraneo NEUROMED in Italy, and her colleagues reported these findings in Blood.
The team noted that the Mediterranean diet—which is characterized by a wide consumption of plant foods, cereals, legumes, fish, and olive oil, as well as moderate wine consumption—has long been hailed as a heart-healthy eating plan. And previous research suggested the diet can reduce inflammation.
But the connection between the diet and levels of platelets and WBCs, 2 specific inflammatory markers in the body, has remained unclear.
“We undertook this study to understand the correlation between consuming a Mediterranean diet and specific health markers, including platelet levels and white blood cell counts, which can more specifically explain the diet’s benefits in reducing the long-term risk of cerebral and heart disease or other chronic conditions,” Dr Bonaccio said.
To do this, she and her colleagues analyzed the eating habits of 14,586 healthy Italian men and women aged 35 and older. At baseline, all subjects were healthy.
The researchers measured total platelet and WBC counts and grouped participants according to their levels (low, normal, or high), based on age- and gender-specific cut-offs.
Participants with high platelet levels were younger and had a greater incidence of high cholesterol and increased levels of common inflammation marker C-reactive protein when compared to subjects in the normal or low-platelet categories.
Individuals in the high-WBC category were mainly younger, male, and smokers. They had a higher body-mass index and higher levels of C-reactive protein and blood glucose than subjects in the other groups. They also showed higher prevalence of high blood pressure and high cholesterol.
The researchers determined participants’ adherence to a Mediterranean using 2 dietary scoring systems, the Mediterranean diet score or the Italian Mediterranean Index, which helped to accurately determine intake levels and portion sizes.
Results of these analyses revealed that adherence to the Mediterranean diet was directly related to lower levels of platelets and WBCs (P<0.0001 and P=0.008, respectively), which was correlated with lower levels of inflammation.
When compared with participants who did not follow the eating plan as closely, subjects who strictly followed the diet were less likely to belong to the group with the highest platelet counts (odds ratio=0.50) and more likely to belong to the group with the lowest WBC counts (odds ratio=1.41).
“Because the study included healthy participants, the lower levels of platelets and white blood cells in those who were more strictly consuming a Mediterranean diet indicate that this eating plan could account for substantial changes within normal ranges of variability,” Dr Bonaccio said.
“This is an important finding that has implications for how these anti-inflammatory markers are tracked among the general population.”
The researchers also evaluated the role of specific components of the diet to help clarify the observed correlation, including food antioxidant content and fiber intake, both of which have previously been connected to cardiovascular benefits.
These components only partially accounted for the link between the diet and WBC count. And they did not fully explain the correlation to platelet levels.
“An important finding of this study is that it indicates that the Mediterranean diet as a whole, and not just a few specific ingredients, is likely responsible for the beneficial health outcomes among the healthy population and should be encouraged as part of healthy eating habits,” Dr Bonaccio said.
“Building on these important findings, we continue to study this population to determine if the dietary habits may have an influence on cardiovascular disease-related mortality.” ![]()

part of a Mediterranean diet
In a large study, individuals who strictly followed a Mediterranean diet had lower levels of platelets and white blood cells (WBCs) than those who deviated from the diet.
And the lower cell counts were associated with lower levels of inflammation.
The research also suggested the diet as a whole, and not just certain components, was responsible for these markers of improved health.
Marialaura Bonaccio, PhD, of the IRCCS Istituto Neurologico Mediterraneo NEUROMED in Italy, and her colleagues reported these findings in Blood.
The team noted that the Mediterranean diet—which is characterized by a wide consumption of plant foods, cereals, legumes, fish, and olive oil, as well as moderate wine consumption—has long been hailed as a heart-healthy eating plan. And previous research suggested the diet can reduce inflammation.
But the connection between the diet and levels of platelets and WBCs, 2 specific inflammatory markers in the body, has remained unclear.
“We undertook this study to understand the correlation between consuming a Mediterranean diet and specific health markers, including platelet levels and white blood cell counts, which can more specifically explain the diet’s benefits in reducing the long-term risk of cerebral and heart disease or other chronic conditions,” Dr Bonaccio said.
To do this, she and her colleagues analyzed the eating habits of 14,586 healthy Italian men and women aged 35 and older. At baseline, all subjects were healthy.
The researchers measured total platelet and WBC counts and grouped participants according to their levels (low, normal, or high), based on age- and gender-specific cut-offs.
Participants with high platelet levels were younger and had a greater incidence of high cholesterol and increased levels of common inflammation marker C-reactive protein when compared to subjects in the normal or low-platelet categories.
Individuals in the high-WBC category were mainly younger, male, and smokers. They had a higher body-mass index and higher levels of C-reactive protein and blood glucose than subjects in the other groups. They also showed higher prevalence of high blood pressure and high cholesterol.
The researchers determined participants’ adherence to a Mediterranean using 2 dietary scoring systems, the Mediterranean diet score or the Italian Mediterranean Index, which helped to accurately determine intake levels and portion sizes.
Results of these analyses revealed that adherence to the Mediterranean diet was directly related to lower levels of platelets and WBCs (P<0.0001 and P=0.008, respectively), which was correlated with lower levels of inflammation.
When compared with participants who did not follow the eating plan as closely, subjects who strictly followed the diet were less likely to belong to the group with the highest platelet counts (odds ratio=0.50) and more likely to belong to the group with the lowest WBC counts (odds ratio=1.41).
“Because the study included healthy participants, the lower levels of platelets and white blood cells in those who were more strictly consuming a Mediterranean diet indicate that this eating plan could account for substantial changes within normal ranges of variability,” Dr Bonaccio said.
“This is an important finding that has implications for how these anti-inflammatory markers are tracked among the general population.”
The researchers also evaluated the role of specific components of the diet to help clarify the observed correlation, including food antioxidant content and fiber intake, both of which have previously been connected to cardiovascular benefits.
These components only partially accounted for the link between the diet and WBC count. And they did not fully explain the correlation to platelet levels.
“An important finding of this study is that it indicates that the Mediterranean diet as a whole, and not just a few specific ingredients, is likely responsible for the beneficial health outcomes among the healthy population and should be encouraged as part of healthy eating habits,” Dr Bonaccio said.
“Building on these important findings, we continue to study this population to determine if the dietary habits may have an influence on cardiovascular disease-related mortality.” ![]()
FDA working to alleviate saline shortage
In an attempt to alleviate the shortage of saline (0.9% sodium chloride injection) in the US, the Food and Drug Administration (FDA) is allowing saline products to be imported from Norway.
The company importing the products is Fresenius Kabi USA. The FDA inspected the company’s Norway manufacturing facility and found the site meets FDA standards.
So Fresenius Kabi has begun importing Sodium Chloride 0.9% Freeflex Injection Solution for Intravenous Infusion.
The European product contains the same active ingredient in the same concentration as the 0.9% sodium chloride injection products approved in the US.
For a complete list of all the Fresenius Kabi saline products, as well as a list of differences between the European and US prescribing information, see the “Dear Healthcare Professional” letter posted on the FDA website.
The FDA is asking that healthcare professionals contact Fresenius Kabi USA directly to obtain saline products. The company’s customer service number is 1-888-386-1300.
The FDA concedes that, while the shipments from Norway will help, they will not resolve the saline shortage. However, the agency says it is working closely with manufacturers to meet the needs for saline across the US. ![]()

In an attempt to alleviate the shortage of saline (0.9% sodium chloride injection) in the US, the Food and Drug Administration (FDA) is allowing saline products to be imported from Norway.
The company importing the products is Fresenius Kabi USA. The FDA inspected the company’s Norway manufacturing facility and found the site meets FDA standards.
So Fresenius Kabi has begun importing Sodium Chloride 0.9% Freeflex Injection Solution for Intravenous Infusion.
The European product contains the same active ingredient in the same concentration as the 0.9% sodium chloride injection products approved in the US.
For a complete list of all the Fresenius Kabi saline products, as well as a list of differences between the European and US prescribing information, see the “Dear Healthcare Professional” letter posted on the FDA website.
The FDA is asking that healthcare professionals contact Fresenius Kabi USA directly to obtain saline products. The company’s customer service number is 1-888-386-1300.
The FDA concedes that, while the shipments from Norway will help, they will not resolve the saline shortage. However, the agency says it is working closely with manufacturers to meet the needs for saline across the US. ![]()

In an attempt to alleviate the shortage of saline (0.9% sodium chloride injection) in the US, the Food and Drug Administration (FDA) is allowing saline products to be imported from Norway.
The company importing the products is Fresenius Kabi USA. The FDA inspected the company’s Norway manufacturing facility and found the site meets FDA standards.
So Fresenius Kabi has begun importing Sodium Chloride 0.9% Freeflex Injection Solution for Intravenous Infusion.
The European product contains the same active ingredient in the same concentration as the 0.9% sodium chloride injection products approved in the US.
For a complete list of all the Fresenius Kabi saline products, as well as a list of differences between the European and US prescribing information, see the “Dear Healthcare Professional” letter posted on the FDA website.
The FDA is asking that healthcare professionals contact Fresenius Kabi USA directly to obtain saline products. The company’s customer service number is 1-888-386-1300.
The FDA concedes that, while the shipments from Norway will help, they will not resolve the saline shortage. However, the agency says it is working closely with manufacturers to meet the needs for saline across the US. ![]()
Cangrelor bests clopidogrel for stent thrombosis

Credit: Andre E.X. Brown
WASHINGTON, DC—A new analysis suggests cangrelor can reduce the risk of stent thrombosis in patients undergoing percutaneous coronary intervention (PCI), when compared to clopidogrel.
In fact, cangrelor was an independent predictor of freedom from stent thrombosis at 30 days after PCI.
These results, obtained by analyzing patients from the CHAMPION PHOENIX trial, were presented at the American College of Cardiology’s 63rd Annual Scientific Session & Expo (presentation 2105-290).
CHAMPION PHOENIX was a prospective, double-blind trial that included 11,145 patients. They were randomized to receive intravenous cangrelor or oral clopidogrel at the time of PCI.
In a previous analysis of the trial data, researchers found that cangrelor reduced the overall odds of complications from stenting procedures—including death, myocardial infarction, ischemia-driven revascularization, and stent thrombosis—compared to clopidogrel. However, cangrelor also increased the risk of major and minor bleeding.
In the new analysis, an independent core laboratory blinded to the treatment performed angiographic analysis in 10,939 of the patients. The researchers defined stent thrombosis as the occurrence of intraprocedural stent thrombosis (IPST) or ARC-defined stent thrombosis (definite or probable).
Stent thrombosis occurred in 120 patients (1.1%) at 48 hours after PCI and in 175 patients (1.6%) at 30 days. The occurrence of stent thrombosis at 48 hours and 30 days was associated with a marked increase in 30-day mortality, with odds ratios (ORs) of 15.3 (P<0.001) and 55.2 (P<0.001), respectively.
IPST, ARC acute stent thrombosis (≤ 24 hrs), and ARC subacute stent thrombosis (1-30 days) occurred in 89 (0.8%), 32 (0.3%), and 60 (0.5%) patients, respectively.
Each type of stent thrombosis was associated with an increase in 30-day mortality. The ORs were 17.4 for IPST (P<0.001), 43.3 for ARC acute stent thrombosis (P<0.001), and 189.1 for ARC subacute stent thrombosis (P<0.001).
“Regardless of the exact type of stent thrombosis, it remains associated with a high rate of death,” said investigator Deepak L. Bhatt, MD, MPH, a professor at Harvard Medical School and co-chair of the CHAMPION program.
However, patients who received cangrelor were less likely than those treated with clopidogrel to develop stent thrombosis, both at 48 hours and at 30 days.
At 48 hours, stent thrombosis had occurred in 0.8% and 1.4% of patients, respectively (P=0.01). And at 30 days, stent thrombosis had occurred in 1.3% and 1.9%, respectively (P=0.01).
Cangrelor appeared to be more effective than clopidogrel at preventing all types of stent thrombosis, although the difference was only statistically significant for IPST.
IPST occurred in 0.6% and 1.0% of patients, respectively (P=0.04). Acute ARC stent thrombosis occurred in 0.2% and 0.4%, respectively (P=0.8). And subacute ARC stent thrombosis occurred in 0.5% and 0.6%, respectively (P=0.60).
Multivariable analysis suggested the use of cangrelor was as an independent predictor of freedom from stent thrombosis at 30 days.
The CHAMPION PHOENIX trial was funded by The Medicines Company, makers of cangrelor. ![]()

Credit: Andre E.X. Brown
WASHINGTON, DC—A new analysis suggests cangrelor can reduce the risk of stent thrombosis in patients undergoing percutaneous coronary intervention (PCI), when compared to clopidogrel.
In fact, cangrelor was an independent predictor of freedom from stent thrombosis at 30 days after PCI.
These results, obtained by analyzing patients from the CHAMPION PHOENIX trial, were presented at the American College of Cardiology’s 63rd Annual Scientific Session & Expo (presentation 2105-290).
CHAMPION PHOENIX was a prospective, double-blind trial that included 11,145 patients. They were randomized to receive intravenous cangrelor or oral clopidogrel at the time of PCI.
In a previous analysis of the trial data, researchers found that cangrelor reduced the overall odds of complications from stenting procedures—including death, myocardial infarction, ischemia-driven revascularization, and stent thrombosis—compared to clopidogrel. However, cangrelor also increased the risk of major and minor bleeding.
In the new analysis, an independent core laboratory blinded to the treatment performed angiographic analysis in 10,939 of the patients. The researchers defined stent thrombosis as the occurrence of intraprocedural stent thrombosis (IPST) or ARC-defined stent thrombosis (definite or probable).
Stent thrombosis occurred in 120 patients (1.1%) at 48 hours after PCI and in 175 patients (1.6%) at 30 days. The occurrence of stent thrombosis at 48 hours and 30 days was associated with a marked increase in 30-day mortality, with odds ratios (ORs) of 15.3 (P<0.001) and 55.2 (P<0.001), respectively.
IPST, ARC acute stent thrombosis (≤ 24 hrs), and ARC subacute stent thrombosis (1-30 days) occurred in 89 (0.8%), 32 (0.3%), and 60 (0.5%) patients, respectively.
Each type of stent thrombosis was associated with an increase in 30-day mortality. The ORs were 17.4 for IPST (P<0.001), 43.3 for ARC acute stent thrombosis (P<0.001), and 189.1 for ARC subacute stent thrombosis (P<0.001).
“Regardless of the exact type of stent thrombosis, it remains associated with a high rate of death,” said investigator Deepak L. Bhatt, MD, MPH, a professor at Harvard Medical School and co-chair of the CHAMPION program.
However, patients who received cangrelor were less likely than those treated with clopidogrel to develop stent thrombosis, both at 48 hours and at 30 days.
At 48 hours, stent thrombosis had occurred in 0.8% and 1.4% of patients, respectively (P=0.01). And at 30 days, stent thrombosis had occurred in 1.3% and 1.9%, respectively (P=0.01).
Cangrelor appeared to be more effective than clopidogrel at preventing all types of stent thrombosis, although the difference was only statistically significant for IPST.
IPST occurred in 0.6% and 1.0% of patients, respectively (P=0.04). Acute ARC stent thrombosis occurred in 0.2% and 0.4%, respectively (P=0.8). And subacute ARC stent thrombosis occurred in 0.5% and 0.6%, respectively (P=0.60).
Multivariable analysis suggested the use of cangrelor was as an independent predictor of freedom from stent thrombosis at 30 days.
The CHAMPION PHOENIX trial was funded by The Medicines Company, makers of cangrelor. ![]()

Credit: Andre E.X. Brown
WASHINGTON, DC—A new analysis suggests cangrelor can reduce the risk of stent thrombosis in patients undergoing percutaneous coronary intervention (PCI), when compared to clopidogrel.
In fact, cangrelor was an independent predictor of freedom from stent thrombosis at 30 days after PCI.
These results, obtained by analyzing patients from the CHAMPION PHOENIX trial, were presented at the American College of Cardiology’s 63rd Annual Scientific Session & Expo (presentation 2105-290).
CHAMPION PHOENIX was a prospective, double-blind trial that included 11,145 patients. They were randomized to receive intravenous cangrelor or oral clopidogrel at the time of PCI.
In a previous analysis of the trial data, researchers found that cangrelor reduced the overall odds of complications from stenting procedures—including death, myocardial infarction, ischemia-driven revascularization, and stent thrombosis—compared to clopidogrel. However, cangrelor also increased the risk of major and minor bleeding.
In the new analysis, an independent core laboratory blinded to the treatment performed angiographic analysis in 10,939 of the patients. The researchers defined stent thrombosis as the occurrence of intraprocedural stent thrombosis (IPST) or ARC-defined stent thrombosis (definite or probable).
Stent thrombosis occurred in 120 patients (1.1%) at 48 hours after PCI and in 175 patients (1.6%) at 30 days. The occurrence of stent thrombosis at 48 hours and 30 days was associated with a marked increase in 30-day mortality, with odds ratios (ORs) of 15.3 (P<0.001) and 55.2 (P<0.001), respectively.
IPST, ARC acute stent thrombosis (≤ 24 hrs), and ARC subacute stent thrombosis (1-30 days) occurred in 89 (0.8%), 32 (0.3%), and 60 (0.5%) patients, respectively.
Each type of stent thrombosis was associated with an increase in 30-day mortality. The ORs were 17.4 for IPST (P<0.001), 43.3 for ARC acute stent thrombosis (P<0.001), and 189.1 for ARC subacute stent thrombosis (P<0.001).
“Regardless of the exact type of stent thrombosis, it remains associated with a high rate of death,” said investigator Deepak L. Bhatt, MD, MPH, a professor at Harvard Medical School and co-chair of the CHAMPION program.
However, patients who received cangrelor were less likely than those treated with clopidogrel to develop stent thrombosis, both at 48 hours and at 30 days.
At 48 hours, stent thrombosis had occurred in 0.8% and 1.4% of patients, respectively (P=0.01). And at 30 days, stent thrombosis had occurred in 1.3% and 1.9%, respectively (P=0.01).
Cangrelor appeared to be more effective than clopidogrel at preventing all types of stent thrombosis, although the difference was only statistically significant for IPST.
IPST occurred in 0.6% and 1.0% of patients, respectively (P=0.04). Acute ARC stent thrombosis occurred in 0.2% and 0.4%, respectively (P=0.8). And subacute ARC stent thrombosis occurred in 0.5% and 0.6%, respectively (P=0.60).
Multivariable analysis suggested the use of cangrelor was as an independent predictor of freedom from stent thrombosis at 30 days.
The CHAMPION PHOENIX trial was funded by The Medicines Company, makers of cangrelor. ![]()
Inpatient Trainee Clinical Exposures
The clinical learning model in medical education, specifically in the third and fourth years of medical school and in residency and fellowship training, is driven by direct patient‐care experiences and complemented by mentorship and supervision provided by experienced physicians.[1] Despite the emphasis on experiential learning in medical school and graduate training, the ability of educators to quantify the clinical experiences of learners has been limited. Case logs, often self‐reported, are frequently required during educational rotations to attempt to measure clinical experience.[2] Logs have been utilized to document diagnoses, demographics, disease severity, procedures, and chief complaints.[3, 4, 5, 6] Unfortunately, self‐reported logs are vulnerable to delayed updates, misreported data, and unreliable data validation.[7, 8] Automated data collection has been shown to be more reliable than self‐reported logs.[8, 9]
The enhanced data mining methods now available allow educators to appraise learners' exposures during patient‐care interactions beyond just the diagnosis or chief complaint (eg, how many electrocardiograms do our learners evaluate during a cardiology rotation, how often do our learners gain experience prescribing a specific class of antibiotics, how many of the patients seen by our learners are diabetic). For example, a learner's interaction with a patient during an inpatient admission for community‐acquired pneumonia, at minimum, would include assessing of past medical history, reviewing outpatient medications and allergies, evaluating tests completed (chest x‐ray, complete blood count, blood cultures), prescribing antibiotics, and monitoring comorbidities. The lack of knowledge regarding the frequency and context of these exposures is a key gap in our understanding of the clinical experience of inpatient trainees. Additionally, there are no data on clinical exposures specific to team‐based inpatient learning. When a rotation is team‐based, the educational experience is not limited to the learner's assigned patients, and this arrangement allows for educational exposures from patients who are not the learner's primary assignments through experiences gained during team rounds, cross‐coverage assessments, and informal discussions of patient care.
In this study, we quantify the clinical exposures of learners on an acting internship (AI) rotation in internal medicine by utilizing the Veterans Affairs (VA) electronic medical records (EMR) as collected through the VA Veterans Integrated Service Network 10 Clinical Data Warehouse (CDW). The AI or subinternship is a medical school clinical rotation typically completed in the fourth year, where the learning experience is expected to mirror a 1‐month rotation of a first‐year resident.[10] The AI has historically been defined as an experiential curriculum, during which students assume many of the responsibilities and activities that they will manage as graduate medical trainees.[10, 11] The exposures of AI learners include primary diagnoses encountered, problem lists evaluated at the time of admission, medications prescribed, laboratory tests ordered, and radiologic imaging evaluated. We additionally explored the exposures of the AI learner's team to assess the experiences available through team‐based care.
METHODS
This study was completed at the Louis Stokes Veterans Affairs Medical Center (LSVAMC) in Cleveland, Ohio, which is an academic affiliate of the Case Western Reserve University School of Medicine. The study was approved by the LSVAMC institutional review board.
At the LSVAMC, the AI rotation in internal medicine is a 4‐week inpatient rotation for fourth‐year medical students, in which the student is assigned to an inpatient medical team consisting of an attending physician, a senior resident, and a combination of first‐year residents and acting interns. Compared to a first‐year resident, the acting intern is assigned approximately half of the number of admissions. The teams rounds as a group at least once per day. Acting interns are permitted to place orders and write notes in the EMR; all orders require a cosignature by a resident or attending physician to be released.
We identified students who rotated through the LSVAMC for an AI in internal medicine rotation from July 2008 to November 2011 from rotation records. Using the CDW, we queried student names and their rotation dates and analyzed the results using a Structured Query Language Query Analyzer. Each student's patient encounters during the rotation were identified. A patient encounter was defined as a patient for whom the student wrote at least 1 note titled either Medicine Admission Note or Medicine Inpatient Progress Note, on any of the dates during their AI rotation. We then counted the total number of notes written by each student during their rotation. A patient identifier is associated with each note. The number of distinct patient identifiers was also tallied to establish the total number of patients seen during the rotation by the individual student as the primary caregiver.
We associated each patient encounter with an inpatient admission profile that included patient admission and discharge dates, International Classification of Diseases, 9th Revision (ICD‐9) diagnosis codes, and admitting specialty. Primary diagnosis codes were queried for each admission and were counted for individual students and in aggregate. We tallied both the individual student and aggregate patient medications prescribed during the dates of admission and ordered to a patient location consistent with an acute medical ward (therefore excluding orders placed if a patient was transferred to an intensive care unit). Similar queries were completed for laboratory and radiological testing.
The VA EMR keeps an active problem list on each patient, and items are associated with an ICD‐9 code. To assemble the active problems available for evaluation by the student on the day of a patient's admission, we queried all problem list items added prior to, but not discontinued before, the day of admission. We then tallied the results for every patient seen by each individual student and in aggregate.
To assess the team exposures for each AI student, we queried all discharge summaries cosigned by the student's attending during the dates of the student's rotation. We assumed the student's team members wrote these discharge summaries. After excluding the student's patients, the resultant list represented the team patient exposures for each student. This list was also queried for the number of patients seen, primary diagnoses, medications, problems, labs, and radiology. The number of team admissions counted included all patients who spent at least 1 day on the team while the student was rotating. All other team exposure counts completed included only patients who were both admitted and discharged within the dates of the student's rotation.
RESULTS
An AI rotation is 4 weeks in duration. Students competed a total of 128 rotations from July 30, 2008 through November 21, 2011. We included all rotations during this time period in the analysis. Tables 1, 2, 3, 4, 5 report results in 4 categories. The Student category tallies the total number of specific exposures (diagnoses, problems, medications, lab values, or radiology tests) for all patients primarily assigned to a student. The Team category tallies the total number of exposures for all patients assigned to other members of the student's inpatient team. The Primary % category identifies the percentage of students who had at least 1 assigned patient with the evaluated clinical exposure. The All Patients % category identifies the percentage of students who had at least 1 student‐assigned patient or at least 1 team‐assigned patient with the evaluated clinical exposure.
| Diagnosis | Student | Team | Primary% | All Patients % |
|---|---|---|---|---|
| Obstructive chronic bronchitis, with acute exacerbation | 102 | 241 | 57% | 91% |
| Pneumonia, organism unspecified | 91 | 228 | 49% | 91% |
| Acute renal failure, unspecified | 73 | 170 | 46% | 83% |
| Urinary tract infection, site not specified | 69 | 149 | 43% | 87% |
| Congestive heart failure, unspecified | 65 | 114 | 41% | 68% |
| Alcohol withdrawal | 46 | 101 | 26% | 61% |
| Alcoholic cirrhosis of liver | 28 | 98 | 16% | 57% |
| Cellulitis and abscess of leg, except foot | 26 | 61 | 18% | 45% |
| Acute pancreatitis | 23 | 51 | 16% | 43% |
| Intestinal infection due to Clostridium difficile | 22 | 30 | 17% | 33% |
| Malignant neoplasm of bronchus and lung, unspecified | 22 | 38 | 16% | 35% |
| Acute on chronic diastolic heart failure | 22 | 45 | 16% | 39% |
| Encounter for antineoplastic chemotherapy | 21 | 96 | 15% | 48% |
| Dehydration | 19 | 78 | 13% | 46% |
| Anemia, unspecified | 19 | 36 | 13% | 30% |
| Pneumonitis due to inhalation of food or vomitus | 19 | 25 | 13% | 24% |
| Syncope and collapse | 16 | 38 | 13% | 39% |
| Other pulmonary embolism and infarction | 15 | 41 | 12% | 26% |
| Unspecified pleural effusion | 15 | 37 | 10% | 34% |
| Acute respiratory failure | 15 | 42 | 11% | 35% |
| Problem | Student | Team | Primary% | All Patients % |
|---|---|---|---|---|
| Hypertension | 1,665 | 3,280 | 100% | 100% |
| Tobacco use disorder | 1,350 | 2,759 | 100% | 100% |
| Unknown cause morbidity/mortality | 1,154 | 2,370 | 100% | 100% |
| Hyperlipidemia | 1,036 | 2,044 | 99% | 100% |
| Diabetes mellitus 2 without complication | 865 | 1,709 | 100% | 100% |
| Chronic airway obstruction | 600 | 1,132 | 100% | 100% |
| Esophageal reflux | 583 | 1,131 | 99% | 100% |
| Depressive disorder | 510 | 1,005 | 100% | 100% |
| Dermatophytosis of nail | 498 | 939 | 98% | 100% |
| Alcohol dependence | 441 | 966 | 97% | 100% |
| Chronic ischemic heart disease | 385 | 758 | 95% | 100% |
| Osteoarthritis | 383 | 791 | 96% | 100% |
| Lumbago | 357 | 692 | 97% | 100% |
| Current useanticoagulation | 342 | 629 | 94% | 100% |
| Anemia | 337 | 674 | 97% | 100% |
| Inhibited sex excitement | 317 | 610 | 91% | 100% |
| Congestive heart failure | 294 | 551 | 91% | 100% |
| Peripheral vascular disease | 288 | 529 | 88% | 99% |
| Sensorineural hearing loss | 280 | 535 | 88% | 99% |
| Post‐traumatic stress disorder | 274 | 528 | 91% | 100% |
| Pure hypercholesterolemia | 262 | 521 | 88% | 100% |
| Coronary atherosclerosis | 259 | 396 | 87% | 95% |
| Obesity | 246 | 509 | 89% | 99% |
| Atrial fibrillation | 236 | 469 | 85% | 100% |
| Gout | 216 | 389 | 85% | 100% |
| Medication | Student | Team | Primary% | All Patients % |
|---|---|---|---|---|
| Omeprazole | 1,372 | 2,981 | 99% | 100% |
| Heparin | 1,067 | 2,271 | 95% | 96% |
| Sodium chloride 0.9% | 925 | 2,036 | 99% | 100% |
| Aspirin | 844 | 1,782 | 98% | 100% |
| Potassium chloride | 707 | 1,387 | 99% | 100% |
| Metoprolol tartrate | 693 | 1,318 | 98% | 100% |
| Insulin regular | 692 | 1,518 | 99% | 100% |
| Acetaminophen | 669 | 1,351 | 98% | 100% |
| Simvastatin | 648 | 1,408 | 99% | 100% |
| Lisinopril | 582 | 1,309 | 98% | 100% |
| Furosemide | 577 | 1,186 | 98% | 100% |
| Docusate sodium | 541 | 1,127 | 98% | 100% |
| Vancomycin | 531 | 977 | 98% | 100% |
| Multivitamin | 478 | 1,074 | 96% | 100% |
| Piperacillin/tazobactam | 470 | 781 | 98% | 100% |
| Selected examples | ||||
| Prednisone | 305 | 613 | 93% | 100% |
| Insulin glargine | 244 | 492 | 81% | 98% |
| Spironolactone | 167 | 380 | 73% | 98% |
| Digoxin | 68 | 125 | 40% | 77% |
| Meropenem | 16 | 21 | 11% | 24% |
| Lab Test | Student | Team | Primary% | All Patients % |
|---|---|---|---|---|
| ||||
| Fingerstick glucose | 12,869 | 24,946 | 100% | 100% |
| Renal panel (serum sodium) | 7,728 | 14,504 | 100% | 100% |
| Complete blood count (blood hematocrit) | 7,372 | 14,188 | 100% | 100% |
| International normalized ratio | 3,725 | 6,259 | 100% | 100% |
| Liver function tests (serum SGOT) | 1,570 | 3,180 | 99% | 100% |
| Urinalysis (urine nitrite) | 789 | 1,537 | 100% | 100% |
| Arterial blood gas (arterial blood pH) | 767 | 704 | 78% | 99% |
| Hemoglobin A1C | 485 | 1,177 | 96% | 100% |
| Fractional excretion of sodium (urine creatinine) | 336 | 677 | 85% | 99% |
| Lactic acid | 195 | 314 | 65% | 96% |
| Ferritin | 193 | 413 | 74% | 99% |
| Thyroid‐stimulating hormone | 184 | 391 | 55% | 64% |
| Lipase | 157 | 317 | 58% | 91% |
| Hepatitis C antibody | 139 | 327 | 70% | 98% |
| Haptoglobin | 101 | 208 | 46% | 83% |
| B‐type natriuretic peptide | 98 | 212 | 48% | 87% |
| Cortisol | 70 | 119 | 34% | 60% |
| Rapid plasma reagin | 70 | 173 | 44% | 82% |
| Urine legionella antigen | 70 | 126 | 38% | 64% |
| D‐dimer | 59 | 111 | 34% | 72% |
| Digoxin | 45 | 69 | 18% | 39% |
| Paracentesis labs (peritoneal fluid total protein) | 34 | 47 | 16% | 34% |
| Thoracentesis labs (pleural fluid WBC count) | 33 | 42 | 20% | 38% |
| C‐reactive protein | 30 | 65 | 17% | 34% |
| Lumbar puncture labs (cerebrospinal fluid WBC count) | 22 | 57 | 11% | 27% |
| Arthrocentesis (synovial fluid WBC count) | 14 | 23 | 9% | 23% |
| Radiology Test | Student | Team | Primary% | All Patients % |
|---|---|---|---|---|
| ||||
| Chest,2 views,PA and lateral | 938 | 1,955 | 100% | 100% |
| Chest portable | 414 | 751 | 96% | 100% |
| CT head without contrast | 235 | 499 | 82% | 100% |
| CT abdomen with contrast | 218 | 365 | 59% | 71% |
| CT pelvis with contrast | 213 | 364 | 59% | 70% |
| CT chest with contrast | 163 | 351 | 75% | 99% |
| Ultrasound kidney, bilateral | 119 | 208 | 61% | 92% |
| Abdomen 1 view | 107 | 220 | 59% | 93% |
| Ultrasound liver | 100 | 183 | 48% | 82% |
| Modified barium swallow | 93 | 130 | 53% | 82% |
| PET scan | 93 | 181 | 49% | 79% |
| Selected examples | ||||
| Acute abdomen series | 85 | 177 | 48% | 81% |
| CT chest, PE protocol | 67 | 126 | 37% | 73% |
| MRI brain with andwithout contrast | 56 | 109 | 34% | 66% |
| Chest decubitus | 51 | 76 | 34% | 60% |
| Portable KUBfor Dobhoff placement | 42 | 62 | 30% | 48% |
| Ventilation/perfusion lung scan | 15 | 25 | 12% | 27% |
| Ultrasound thyroid | 8 | 16 | 5% | 17% |
Distinct Patients and Progress Notes
The mean number of progress notes written by a student was 67.2 (standard deviation [SD] 16.3). The mean number of distinct patients evaluated by a student during a rotation was 18.4 (SD 4.2). The mean number of team admissions per student rotation was 46.7 (SD 9.6) distinct patients.
Primary Diagnoses
A total of 2213 primary diagnoses were documented on patients assigned to students on AI rotations. A total of 5323 primary diagnoses were documented on patients assigned to other members of the team during the students' rotations. Therefore, the mean number of primary diagnoses seen by a student during a rotation was 58.9 (17.3 primary diagnoses for student‐assigned patients and 41.6 primary diagnoses for team patients). The students and teams encountered similar diagnoses (Table 1).
Problem List
Students and teams evaluated a total of 40,015 and 78,643 past medical problems, respectively. The mean number of problems seen by a student during a rotation was 927 (313 student, 614 team). Table 2 reports the most frequent problems assigned to primary student admissions. Students and teams evaluated similar problems. Hepatitis C (196 student, 410 team) was the only team problem that was in the team top 25 but not in the student top 25.
Medications
A total of 38,149 medications were prescribed to the students' primary patients. A total of 77,738 medications were prescribed to patients assigned to the rest of the team. The mean number of medication exposures for a student during a rotation was 905 (298 student, 607 team). The most frequently prescribed medications were similar between student and the team (Table 3). Team medications that were in the top 25 but not in the student top 25 included: hydralazine (300 student, 629 team), prednisone (305 student, 613 team), and oxycodone/acetaminophen (286 student, 608 team).
Labs
All laboratory tests with reported results were tallied. For common laboratory panels, single lab values (eg, serum hematocrit for a complete blood count) were selected as proxies to count the number of studies completed and evaluated. Table 4 shows a cross‐section of laboratory tests evaluated during AI rotations.
Radiology
A total of 6197 radiology tests were completed on patients assigned to students, whereas 11,761 radiology tests were completed on patients assigned to other team members. The mean number of radiology exposures for a student was 140 (48 student, 92 team). The most frequently seen radiology tests were similar between student and the team (Table 5).
DISCUSSION
As medical educators, we assume that the clinical training years allow learners to develop essential skills through their varied clinical experiences. Through exposure to direct patient care, to medical decision‐making scenarios, and to senior physician management practices, trainees build the knowledge base for independent practice. To ensure there is sufficient clinical exposure, data on what trainees are encountering may prove beneficial.
In this novel study, we quantified what learners encounter during a 1‐month team‐based inpatient rotation at a large teaching hospital. We effectively measured a number of aspects of internal medicine inpatient training that have been difficult to quantify in the past. The ability to extract learner‐specific data is becoming increasingly available in academic teaching hospitals. For example, VA medical centers have available a daily updated national data warehouse. The other steps necessary for using learner‐specific data include an understanding of the local inpatient processhow tests are ordered, what note titles are used by traineesas well as someone able to build the queries necessary for data extraction. Once built, data extraction should be able to continue as an automated process and used in real time by medical educators.
Our method of data collection has limitations. The orders placed on a learner's primary patients may not have been placed by the learner. For example, orders may have been placed by an overnight resident cross‐covering the learner's patients. We assumed that learners evaluated the results of all tests (or medication changes) that occurred at any time during their rotation, including cross‐cover periods or days off. In addition, our method for evaluating team exposure underestimates the number of team patients calculated for each learner by limiting the query only to patients whose hospital stay was completed before the student left the inpatient service. It is also difficult to know the how many of the exposures are realized by the learner. Differences in learner attention, contrasts in rounding styles, and varying presentation methods will affect the number of exposures truly attained by the learner. Finally, not all clinical exposures can be evaluated through review of an EMR. Clinical experiences, such as care coordination, patient education, and family counseling, cannot be easily extracted.
Data mining EMRs can enhance clinical medical education. Although our data collection was completed retrospectively, we could easily provide learner‐specific data in real time to ward attendings, chief residents, and program directors. This information could direct the development of teaching tools and individualization of curricula. Perhaps, even more importantly, it would also allow educators to define curricular gaps. Whether these gaps are due to the particular patient demographics of a medical center, the practice patterns and strengths of a particular institution, or career interests of a trainee, these gaps may skew the patient‐care experiences encountered by individual trainees. We can use these data to identify differences in clinical experience and then develop opportunities for learnersclinical, didactic, or simulatedto address deficiencies and provide well‐rounded clinical experiences.
Further investigation to better understand the relationship between direct patient‐care experience and clinical skill acquisition is needed. This information could help guide the development of standards on the number of exposures we expect our learners to have with different diagnostic or treatment modalities prior to independent practice. Using learner data to better understand the clinical experiences of our medical trainees, we can hopefully develop more precise and focused curricula to ensure we produce competent graduates.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the Louis Stokes Cleveland VA Medical Center. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs.
- Accreditation Council for Graduate Medical Education. Program requirements for graduate medical education in internal medicine. Available at: http://www.acgme.org/acgmeweb/Portals/0/PFAssets/2013-PR-FAQ-PIF/140_internal_medicine_07012013.pdf. Originally accessed December 18, 2012.
- , , . Residents make their lists and program directors check them twice: reviewing case logs. J Grad Med Educ. 2012;34:257–260.
- , , , et al. Quantifying internal medicine resident clinical experience using resident‐selected primary diagnosis codes. J Hosp Med. 2011;6(7):395–400.
- , , , et al. Documenting and comparing medical students' clinical experiences. JAMA. 2001;286:1035–1040.
- , , , , . Use of an electronic medical record to profile the continuity clinic experiences of primary care residents. Acad Med. 2005;80:390–394.
- , , . Using a Web‐based system to monitor practice profiles in primary care residency training. Can Fam Physician. 2011;57:1030–1037.
- , , . An automated electronic case log: using electronic information systems to assess training in emergency medicine. Acad Emergency Med. 2006;13:733–739.
- , , , , , . The design and implementation of an automated system for logging clinical experiences using an anesthesia information management system. Anesth Analg. 2011;112(2):422–429.
- , , , . Validation of an electronic system for recording medical student patient encounters. AMIA Annu Symp Proc. 2008;2008:510–514.
- . The structure and content of the medical subinternship: a national survey. J Gen Intern Med. 2001;16:550–553.
- , . Education for practice: the role of practical experience in undergraduate and general clinical training. Med Educ. 1989;23:189–195.
The clinical learning model in medical education, specifically in the third and fourth years of medical school and in residency and fellowship training, is driven by direct patient‐care experiences and complemented by mentorship and supervision provided by experienced physicians.[1] Despite the emphasis on experiential learning in medical school and graduate training, the ability of educators to quantify the clinical experiences of learners has been limited. Case logs, often self‐reported, are frequently required during educational rotations to attempt to measure clinical experience.[2] Logs have been utilized to document diagnoses, demographics, disease severity, procedures, and chief complaints.[3, 4, 5, 6] Unfortunately, self‐reported logs are vulnerable to delayed updates, misreported data, and unreliable data validation.[7, 8] Automated data collection has been shown to be more reliable than self‐reported logs.[8, 9]
The enhanced data mining methods now available allow educators to appraise learners' exposures during patient‐care interactions beyond just the diagnosis or chief complaint (eg, how many electrocardiograms do our learners evaluate during a cardiology rotation, how often do our learners gain experience prescribing a specific class of antibiotics, how many of the patients seen by our learners are diabetic). For example, a learner's interaction with a patient during an inpatient admission for community‐acquired pneumonia, at minimum, would include assessing of past medical history, reviewing outpatient medications and allergies, evaluating tests completed (chest x‐ray, complete blood count, blood cultures), prescribing antibiotics, and monitoring comorbidities. The lack of knowledge regarding the frequency and context of these exposures is a key gap in our understanding of the clinical experience of inpatient trainees. Additionally, there are no data on clinical exposures specific to team‐based inpatient learning. When a rotation is team‐based, the educational experience is not limited to the learner's assigned patients, and this arrangement allows for educational exposures from patients who are not the learner's primary assignments through experiences gained during team rounds, cross‐coverage assessments, and informal discussions of patient care.
In this study, we quantify the clinical exposures of learners on an acting internship (AI) rotation in internal medicine by utilizing the Veterans Affairs (VA) electronic medical records (EMR) as collected through the VA Veterans Integrated Service Network 10 Clinical Data Warehouse (CDW). The AI or subinternship is a medical school clinical rotation typically completed in the fourth year, where the learning experience is expected to mirror a 1‐month rotation of a first‐year resident.[10] The AI has historically been defined as an experiential curriculum, during which students assume many of the responsibilities and activities that they will manage as graduate medical trainees.[10, 11] The exposures of AI learners include primary diagnoses encountered, problem lists evaluated at the time of admission, medications prescribed, laboratory tests ordered, and radiologic imaging evaluated. We additionally explored the exposures of the AI learner's team to assess the experiences available through team‐based care.
METHODS
This study was completed at the Louis Stokes Veterans Affairs Medical Center (LSVAMC) in Cleveland, Ohio, which is an academic affiliate of the Case Western Reserve University School of Medicine. The study was approved by the LSVAMC institutional review board.
At the LSVAMC, the AI rotation in internal medicine is a 4‐week inpatient rotation for fourth‐year medical students, in which the student is assigned to an inpatient medical team consisting of an attending physician, a senior resident, and a combination of first‐year residents and acting interns. Compared to a first‐year resident, the acting intern is assigned approximately half of the number of admissions. The teams rounds as a group at least once per day. Acting interns are permitted to place orders and write notes in the EMR; all orders require a cosignature by a resident or attending physician to be released.
We identified students who rotated through the LSVAMC for an AI in internal medicine rotation from July 2008 to November 2011 from rotation records. Using the CDW, we queried student names and their rotation dates and analyzed the results using a Structured Query Language Query Analyzer. Each student's patient encounters during the rotation were identified. A patient encounter was defined as a patient for whom the student wrote at least 1 note titled either Medicine Admission Note or Medicine Inpatient Progress Note, on any of the dates during their AI rotation. We then counted the total number of notes written by each student during their rotation. A patient identifier is associated with each note. The number of distinct patient identifiers was also tallied to establish the total number of patients seen during the rotation by the individual student as the primary caregiver.
We associated each patient encounter with an inpatient admission profile that included patient admission and discharge dates, International Classification of Diseases, 9th Revision (ICD‐9) diagnosis codes, and admitting specialty. Primary diagnosis codes were queried for each admission and were counted for individual students and in aggregate. We tallied both the individual student and aggregate patient medications prescribed during the dates of admission and ordered to a patient location consistent with an acute medical ward (therefore excluding orders placed if a patient was transferred to an intensive care unit). Similar queries were completed for laboratory and radiological testing.
The VA EMR keeps an active problem list on each patient, and items are associated with an ICD‐9 code. To assemble the active problems available for evaluation by the student on the day of a patient's admission, we queried all problem list items added prior to, but not discontinued before, the day of admission. We then tallied the results for every patient seen by each individual student and in aggregate.
To assess the team exposures for each AI student, we queried all discharge summaries cosigned by the student's attending during the dates of the student's rotation. We assumed the student's team members wrote these discharge summaries. After excluding the student's patients, the resultant list represented the team patient exposures for each student. This list was also queried for the number of patients seen, primary diagnoses, medications, problems, labs, and radiology. The number of team admissions counted included all patients who spent at least 1 day on the team while the student was rotating. All other team exposure counts completed included only patients who were both admitted and discharged within the dates of the student's rotation.
RESULTS
An AI rotation is 4 weeks in duration. Students competed a total of 128 rotations from July 30, 2008 through November 21, 2011. We included all rotations during this time period in the analysis. Tables 1, 2, 3, 4, 5 report results in 4 categories. The Student category tallies the total number of specific exposures (diagnoses, problems, medications, lab values, or radiology tests) for all patients primarily assigned to a student. The Team category tallies the total number of exposures for all patients assigned to other members of the student's inpatient team. The Primary % category identifies the percentage of students who had at least 1 assigned patient with the evaluated clinical exposure. The All Patients % category identifies the percentage of students who had at least 1 student‐assigned patient or at least 1 team‐assigned patient with the evaluated clinical exposure.
| Diagnosis | Student | Team | Primary% | All Patients % |
|---|---|---|---|---|
| Obstructive chronic bronchitis, with acute exacerbation | 102 | 241 | 57% | 91% |
| Pneumonia, organism unspecified | 91 | 228 | 49% | 91% |
| Acute renal failure, unspecified | 73 | 170 | 46% | 83% |
| Urinary tract infection, site not specified | 69 | 149 | 43% | 87% |
| Congestive heart failure, unspecified | 65 | 114 | 41% | 68% |
| Alcohol withdrawal | 46 | 101 | 26% | 61% |
| Alcoholic cirrhosis of liver | 28 | 98 | 16% | 57% |
| Cellulitis and abscess of leg, except foot | 26 | 61 | 18% | 45% |
| Acute pancreatitis | 23 | 51 | 16% | 43% |
| Intestinal infection due to Clostridium difficile | 22 | 30 | 17% | 33% |
| Malignant neoplasm of bronchus and lung, unspecified | 22 | 38 | 16% | 35% |
| Acute on chronic diastolic heart failure | 22 | 45 | 16% | 39% |
| Encounter for antineoplastic chemotherapy | 21 | 96 | 15% | 48% |
| Dehydration | 19 | 78 | 13% | 46% |
| Anemia, unspecified | 19 | 36 | 13% | 30% |
| Pneumonitis due to inhalation of food or vomitus | 19 | 25 | 13% | 24% |
| Syncope and collapse | 16 | 38 | 13% | 39% |
| Other pulmonary embolism and infarction | 15 | 41 | 12% | 26% |
| Unspecified pleural effusion | 15 | 37 | 10% | 34% |
| Acute respiratory failure | 15 | 42 | 11% | 35% |
| Problem | Student | Team | Primary% | All Patients % |
|---|---|---|---|---|
| Hypertension | 1,665 | 3,280 | 100% | 100% |
| Tobacco use disorder | 1,350 | 2,759 | 100% | 100% |
| Unknown cause morbidity/mortality | 1,154 | 2,370 | 100% | 100% |
| Hyperlipidemia | 1,036 | 2,044 | 99% | 100% |
| Diabetes mellitus 2 without complication | 865 | 1,709 | 100% | 100% |
| Chronic airway obstruction | 600 | 1,132 | 100% | 100% |
| Esophageal reflux | 583 | 1,131 | 99% | 100% |
| Depressive disorder | 510 | 1,005 | 100% | 100% |
| Dermatophytosis of nail | 498 | 939 | 98% | 100% |
| Alcohol dependence | 441 | 966 | 97% | 100% |
| Chronic ischemic heart disease | 385 | 758 | 95% | 100% |
| Osteoarthritis | 383 | 791 | 96% | 100% |
| Lumbago | 357 | 692 | 97% | 100% |
| Current useanticoagulation | 342 | 629 | 94% | 100% |
| Anemia | 337 | 674 | 97% | 100% |
| Inhibited sex excitement | 317 | 610 | 91% | 100% |
| Congestive heart failure | 294 | 551 | 91% | 100% |
| Peripheral vascular disease | 288 | 529 | 88% | 99% |
| Sensorineural hearing loss | 280 | 535 | 88% | 99% |
| Post‐traumatic stress disorder | 274 | 528 | 91% | 100% |
| Pure hypercholesterolemia | 262 | 521 | 88% | 100% |
| Coronary atherosclerosis | 259 | 396 | 87% | 95% |
| Obesity | 246 | 509 | 89% | 99% |
| Atrial fibrillation | 236 | 469 | 85% | 100% |
| Gout | 216 | 389 | 85% | 100% |
| Medication | Student | Team | Primary% | All Patients % |
|---|---|---|---|---|
| Omeprazole | 1,372 | 2,981 | 99% | 100% |
| Heparin | 1,067 | 2,271 | 95% | 96% |
| Sodium chloride 0.9% | 925 | 2,036 | 99% | 100% |
| Aspirin | 844 | 1,782 | 98% | 100% |
| Potassium chloride | 707 | 1,387 | 99% | 100% |
| Metoprolol tartrate | 693 | 1,318 | 98% | 100% |
| Insulin regular | 692 | 1,518 | 99% | 100% |
| Acetaminophen | 669 | 1,351 | 98% | 100% |
| Simvastatin | 648 | 1,408 | 99% | 100% |
| Lisinopril | 582 | 1,309 | 98% | 100% |
| Furosemide | 577 | 1,186 | 98% | 100% |
| Docusate sodium | 541 | 1,127 | 98% | 100% |
| Vancomycin | 531 | 977 | 98% | 100% |
| Multivitamin | 478 | 1,074 | 96% | 100% |
| Piperacillin/tazobactam | 470 | 781 | 98% | 100% |
| Selected examples | ||||
| Prednisone | 305 | 613 | 93% | 100% |
| Insulin glargine | 244 | 492 | 81% | 98% |
| Spironolactone | 167 | 380 | 73% | 98% |
| Digoxin | 68 | 125 | 40% | 77% |
| Meropenem | 16 | 21 | 11% | 24% |
| Lab Test | Student | Team | Primary% | All Patients % |
|---|---|---|---|---|
| ||||
| Fingerstick glucose | 12,869 | 24,946 | 100% | 100% |
| Renal panel (serum sodium) | 7,728 | 14,504 | 100% | 100% |
| Complete blood count (blood hematocrit) | 7,372 | 14,188 | 100% | 100% |
| International normalized ratio | 3,725 | 6,259 | 100% | 100% |
| Liver function tests (serum SGOT) | 1,570 | 3,180 | 99% | 100% |
| Urinalysis (urine nitrite) | 789 | 1,537 | 100% | 100% |
| Arterial blood gas (arterial blood pH) | 767 | 704 | 78% | 99% |
| Hemoglobin A1C | 485 | 1,177 | 96% | 100% |
| Fractional excretion of sodium (urine creatinine) | 336 | 677 | 85% | 99% |
| Lactic acid | 195 | 314 | 65% | 96% |
| Ferritin | 193 | 413 | 74% | 99% |
| Thyroid‐stimulating hormone | 184 | 391 | 55% | 64% |
| Lipase | 157 | 317 | 58% | 91% |
| Hepatitis C antibody | 139 | 327 | 70% | 98% |
| Haptoglobin | 101 | 208 | 46% | 83% |
| B‐type natriuretic peptide | 98 | 212 | 48% | 87% |
| Cortisol | 70 | 119 | 34% | 60% |
| Rapid plasma reagin | 70 | 173 | 44% | 82% |
| Urine legionella antigen | 70 | 126 | 38% | 64% |
| D‐dimer | 59 | 111 | 34% | 72% |
| Digoxin | 45 | 69 | 18% | 39% |
| Paracentesis labs (peritoneal fluid total protein) | 34 | 47 | 16% | 34% |
| Thoracentesis labs (pleural fluid WBC count) | 33 | 42 | 20% | 38% |
| C‐reactive protein | 30 | 65 | 17% | 34% |
| Lumbar puncture labs (cerebrospinal fluid WBC count) | 22 | 57 | 11% | 27% |
| Arthrocentesis (synovial fluid WBC count) | 14 | 23 | 9% | 23% |
| Radiology Test | Student | Team | Primary% | All Patients % |
|---|---|---|---|---|
| ||||
| Chest,2 views,PA and lateral | 938 | 1,955 | 100% | 100% |
| Chest portable | 414 | 751 | 96% | 100% |
| CT head without contrast | 235 | 499 | 82% | 100% |
| CT abdomen with contrast | 218 | 365 | 59% | 71% |
| CT pelvis with contrast | 213 | 364 | 59% | 70% |
| CT chest with contrast | 163 | 351 | 75% | 99% |
| Ultrasound kidney, bilateral | 119 | 208 | 61% | 92% |
| Abdomen 1 view | 107 | 220 | 59% | 93% |
| Ultrasound liver | 100 | 183 | 48% | 82% |
| Modified barium swallow | 93 | 130 | 53% | 82% |
| PET scan | 93 | 181 | 49% | 79% |
| Selected examples | ||||
| Acute abdomen series | 85 | 177 | 48% | 81% |
| CT chest, PE protocol | 67 | 126 | 37% | 73% |
| MRI brain with andwithout contrast | 56 | 109 | 34% | 66% |
| Chest decubitus | 51 | 76 | 34% | 60% |
| Portable KUBfor Dobhoff placement | 42 | 62 | 30% | 48% |
| Ventilation/perfusion lung scan | 15 | 25 | 12% | 27% |
| Ultrasound thyroid | 8 | 16 | 5% | 17% |
Distinct Patients and Progress Notes
The mean number of progress notes written by a student was 67.2 (standard deviation [SD] 16.3). The mean number of distinct patients evaluated by a student during a rotation was 18.4 (SD 4.2). The mean number of team admissions per student rotation was 46.7 (SD 9.6) distinct patients.
Primary Diagnoses
A total of 2213 primary diagnoses were documented on patients assigned to students on AI rotations. A total of 5323 primary diagnoses were documented on patients assigned to other members of the team during the students' rotations. Therefore, the mean number of primary diagnoses seen by a student during a rotation was 58.9 (17.3 primary diagnoses for student‐assigned patients and 41.6 primary diagnoses for team patients). The students and teams encountered similar diagnoses (Table 1).
Problem List
Students and teams evaluated a total of 40,015 and 78,643 past medical problems, respectively. The mean number of problems seen by a student during a rotation was 927 (313 student, 614 team). Table 2 reports the most frequent problems assigned to primary student admissions. Students and teams evaluated similar problems. Hepatitis C (196 student, 410 team) was the only team problem that was in the team top 25 but not in the student top 25.
Medications
A total of 38,149 medications were prescribed to the students' primary patients. A total of 77,738 medications were prescribed to patients assigned to the rest of the team. The mean number of medication exposures for a student during a rotation was 905 (298 student, 607 team). The most frequently prescribed medications were similar between student and the team (Table 3). Team medications that were in the top 25 but not in the student top 25 included: hydralazine (300 student, 629 team), prednisone (305 student, 613 team), and oxycodone/acetaminophen (286 student, 608 team).
Labs
All laboratory tests with reported results were tallied. For common laboratory panels, single lab values (eg, serum hematocrit for a complete blood count) were selected as proxies to count the number of studies completed and evaluated. Table 4 shows a cross‐section of laboratory tests evaluated during AI rotations.
Radiology
A total of 6197 radiology tests were completed on patients assigned to students, whereas 11,761 radiology tests were completed on patients assigned to other team members. The mean number of radiology exposures for a student was 140 (48 student, 92 team). The most frequently seen radiology tests were similar between student and the team (Table 5).
DISCUSSION
As medical educators, we assume that the clinical training years allow learners to develop essential skills through their varied clinical experiences. Through exposure to direct patient care, to medical decision‐making scenarios, and to senior physician management practices, trainees build the knowledge base for independent practice. To ensure there is sufficient clinical exposure, data on what trainees are encountering may prove beneficial.
In this novel study, we quantified what learners encounter during a 1‐month team‐based inpatient rotation at a large teaching hospital. We effectively measured a number of aspects of internal medicine inpatient training that have been difficult to quantify in the past. The ability to extract learner‐specific data is becoming increasingly available in academic teaching hospitals. For example, VA medical centers have available a daily updated national data warehouse. The other steps necessary for using learner‐specific data include an understanding of the local inpatient processhow tests are ordered, what note titles are used by traineesas well as someone able to build the queries necessary for data extraction. Once built, data extraction should be able to continue as an automated process and used in real time by medical educators.
Our method of data collection has limitations. The orders placed on a learner's primary patients may not have been placed by the learner. For example, orders may have been placed by an overnight resident cross‐covering the learner's patients. We assumed that learners evaluated the results of all tests (or medication changes) that occurred at any time during their rotation, including cross‐cover periods or days off. In addition, our method for evaluating team exposure underestimates the number of team patients calculated for each learner by limiting the query only to patients whose hospital stay was completed before the student left the inpatient service. It is also difficult to know the how many of the exposures are realized by the learner. Differences in learner attention, contrasts in rounding styles, and varying presentation methods will affect the number of exposures truly attained by the learner. Finally, not all clinical exposures can be evaluated through review of an EMR. Clinical experiences, such as care coordination, patient education, and family counseling, cannot be easily extracted.
Data mining EMRs can enhance clinical medical education. Although our data collection was completed retrospectively, we could easily provide learner‐specific data in real time to ward attendings, chief residents, and program directors. This information could direct the development of teaching tools and individualization of curricula. Perhaps, even more importantly, it would also allow educators to define curricular gaps. Whether these gaps are due to the particular patient demographics of a medical center, the practice patterns and strengths of a particular institution, or career interests of a trainee, these gaps may skew the patient‐care experiences encountered by individual trainees. We can use these data to identify differences in clinical experience and then develop opportunities for learnersclinical, didactic, or simulatedto address deficiencies and provide well‐rounded clinical experiences.
Further investigation to better understand the relationship between direct patient‐care experience and clinical skill acquisition is needed. This information could help guide the development of standards on the number of exposures we expect our learners to have with different diagnostic or treatment modalities prior to independent practice. Using learner data to better understand the clinical experiences of our medical trainees, we can hopefully develop more precise and focused curricula to ensure we produce competent graduates.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the Louis Stokes Cleveland VA Medical Center. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs.
The clinical learning model in medical education, specifically in the third and fourth years of medical school and in residency and fellowship training, is driven by direct patient‐care experiences and complemented by mentorship and supervision provided by experienced physicians.[1] Despite the emphasis on experiential learning in medical school and graduate training, the ability of educators to quantify the clinical experiences of learners has been limited. Case logs, often self‐reported, are frequently required during educational rotations to attempt to measure clinical experience.[2] Logs have been utilized to document diagnoses, demographics, disease severity, procedures, and chief complaints.[3, 4, 5, 6] Unfortunately, self‐reported logs are vulnerable to delayed updates, misreported data, and unreliable data validation.[7, 8] Automated data collection has been shown to be more reliable than self‐reported logs.[8, 9]
The enhanced data mining methods now available allow educators to appraise learners' exposures during patient‐care interactions beyond just the diagnosis or chief complaint (eg, how many electrocardiograms do our learners evaluate during a cardiology rotation, how often do our learners gain experience prescribing a specific class of antibiotics, how many of the patients seen by our learners are diabetic). For example, a learner's interaction with a patient during an inpatient admission for community‐acquired pneumonia, at minimum, would include assessing of past medical history, reviewing outpatient medications and allergies, evaluating tests completed (chest x‐ray, complete blood count, blood cultures), prescribing antibiotics, and monitoring comorbidities. The lack of knowledge regarding the frequency and context of these exposures is a key gap in our understanding of the clinical experience of inpatient trainees. Additionally, there are no data on clinical exposures specific to team‐based inpatient learning. When a rotation is team‐based, the educational experience is not limited to the learner's assigned patients, and this arrangement allows for educational exposures from patients who are not the learner's primary assignments through experiences gained during team rounds, cross‐coverage assessments, and informal discussions of patient care.
In this study, we quantify the clinical exposures of learners on an acting internship (AI) rotation in internal medicine by utilizing the Veterans Affairs (VA) electronic medical records (EMR) as collected through the VA Veterans Integrated Service Network 10 Clinical Data Warehouse (CDW). The AI or subinternship is a medical school clinical rotation typically completed in the fourth year, where the learning experience is expected to mirror a 1‐month rotation of a first‐year resident.[10] The AI has historically been defined as an experiential curriculum, during which students assume many of the responsibilities and activities that they will manage as graduate medical trainees.[10, 11] The exposures of AI learners include primary diagnoses encountered, problem lists evaluated at the time of admission, medications prescribed, laboratory tests ordered, and radiologic imaging evaluated. We additionally explored the exposures of the AI learner's team to assess the experiences available through team‐based care.
METHODS
This study was completed at the Louis Stokes Veterans Affairs Medical Center (LSVAMC) in Cleveland, Ohio, which is an academic affiliate of the Case Western Reserve University School of Medicine. The study was approved by the LSVAMC institutional review board.
At the LSVAMC, the AI rotation in internal medicine is a 4‐week inpatient rotation for fourth‐year medical students, in which the student is assigned to an inpatient medical team consisting of an attending physician, a senior resident, and a combination of first‐year residents and acting interns. Compared to a first‐year resident, the acting intern is assigned approximately half of the number of admissions. The teams rounds as a group at least once per day. Acting interns are permitted to place orders and write notes in the EMR; all orders require a cosignature by a resident or attending physician to be released.
We identified students who rotated through the LSVAMC for an AI in internal medicine rotation from July 2008 to November 2011 from rotation records. Using the CDW, we queried student names and their rotation dates and analyzed the results using a Structured Query Language Query Analyzer. Each student's patient encounters during the rotation were identified. A patient encounter was defined as a patient for whom the student wrote at least 1 note titled either Medicine Admission Note or Medicine Inpatient Progress Note, on any of the dates during their AI rotation. We then counted the total number of notes written by each student during their rotation. A patient identifier is associated with each note. The number of distinct patient identifiers was also tallied to establish the total number of patients seen during the rotation by the individual student as the primary caregiver.
We associated each patient encounter with an inpatient admission profile that included patient admission and discharge dates, International Classification of Diseases, 9th Revision (ICD‐9) diagnosis codes, and admitting specialty. Primary diagnosis codes were queried for each admission and were counted for individual students and in aggregate. We tallied both the individual student and aggregate patient medications prescribed during the dates of admission and ordered to a patient location consistent with an acute medical ward (therefore excluding orders placed if a patient was transferred to an intensive care unit). Similar queries were completed for laboratory and radiological testing.
The VA EMR keeps an active problem list on each patient, and items are associated with an ICD‐9 code. To assemble the active problems available for evaluation by the student on the day of a patient's admission, we queried all problem list items added prior to, but not discontinued before, the day of admission. We then tallied the results for every patient seen by each individual student and in aggregate.
To assess the team exposures for each AI student, we queried all discharge summaries cosigned by the student's attending during the dates of the student's rotation. We assumed the student's team members wrote these discharge summaries. After excluding the student's patients, the resultant list represented the team patient exposures for each student. This list was also queried for the number of patients seen, primary diagnoses, medications, problems, labs, and radiology. The number of team admissions counted included all patients who spent at least 1 day on the team while the student was rotating. All other team exposure counts completed included only patients who were both admitted and discharged within the dates of the student's rotation.
RESULTS
An AI rotation is 4 weeks in duration. Students competed a total of 128 rotations from July 30, 2008 through November 21, 2011. We included all rotations during this time period in the analysis. Tables 1, 2, 3, 4, 5 report results in 4 categories. The Student category tallies the total number of specific exposures (diagnoses, problems, medications, lab values, or radiology tests) for all patients primarily assigned to a student. The Team category tallies the total number of exposures for all patients assigned to other members of the student's inpatient team. The Primary % category identifies the percentage of students who had at least 1 assigned patient with the evaluated clinical exposure. The All Patients % category identifies the percentage of students who had at least 1 student‐assigned patient or at least 1 team‐assigned patient with the evaluated clinical exposure.
| Diagnosis | Student | Team | Primary% | All Patients % |
|---|---|---|---|---|
| Obstructive chronic bronchitis, with acute exacerbation | 102 | 241 | 57% | 91% |
| Pneumonia, organism unspecified | 91 | 228 | 49% | 91% |
| Acute renal failure, unspecified | 73 | 170 | 46% | 83% |
| Urinary tract infection, site not specified | 69 | 149 | 43% | 87% |
| Congestive heart failure, unspecified | 65 | 114 | 41% | 68% |
| Alcohol withdrawal | 46 | 101 | 26% | 61% |
| Alcoholic cirrhosis of liver | 28 | 98 | 16% | 57% |
| Cellulitis and abscess of leg, except foot | 26 | 61 | 18% | 45% |
| Acute pancreatitis | 23 | 51 | 16% | 43% |
| Intestinal infection due to Clostridium difficile | 22 | 30 | 17% | 33% |
| Malignant neoplasm of bronchus and lung, unspecified | 22 | 38 | 16% | 35% |
| Acute on chronic diastolic heart failure | 22 | 45 | 16% | 39% |
| Encounter for antineoplastic chemotherapy | 21 | 96 | 15% | 48% |
| Dehydration | 19 | 78 | 13% | 46% |
| Anemia, unspecified | 19 | 36 | 13% | 30% |
| Pneumonitis due to inhalation of food or vomitus | 19 | 25 | 13% | 24% |
| Syncope and collapse | 16 | 38 | 13% | 39% |
| Other pulmonary embolism and infarction | 15 | 41 | 12% | 26% |
| Unspecified pleural effusion | 15 | 37 | 10% | 34% |
| Acute respiratory failure | 15 | 42 | 11% | 35% |
| Problem | Student | Team | Primary% | All Patients % |
|---|---|---|---|---|
| Hypertension | 1,665 | 3,280 | 100% | 100% |
| Tobacco use disorder | 1,350 | 2,759 | 100% | 100% |
| Unknown cause morbidity/mortality | 1,154 | 2,370 | 100% | 100% |
| Hyperlipidemia | 1,036 | 2,044 | 99% | 100% |
| Diabetes mellitus 2 without complication | 865 | 1,709 | 100% | 100% |
| Chronic airway obstruction | 600 | 1,132 | 100% | 100% |
| Esophageal reflux | 583 | 1,131 | 99% | 100% |
| Depressive disorder | 510 | 1,005 | 100% | 100% |
| Dermatophytosis of nail | 498 | 939 | 98% | 100% |
| Alcohol dependence | 441 | 966 | 97% | 100% |
| Chronic ischemic heart disease | 385 | 758 | 95% | 100% |
| Osteoarthritis | 383 | 791 | 96% | 100% |
| Lumbago | 357 | 692 | 97% | 100% |
| Current useanticoagulation | 342 | 629 | 94% | 100% |
| Anemia | 337 | 674 | 97% | 100% |
| Inhibited sex excitement | 317 | 610 | 91% | 100% |
| Congestive heart failure | 294 | 551 | 91% | 100% |
| Peripheral vascular disease | 288 | 529 | 88% | 99% |
| Sensorineural hearing loss | 280 | 535 | 88% | 99% |
| Post‐traumatic stress disorder | 274 | 528 | 91% | 100% |
| Pure hypercholesterolemia | 262 | 521 | 88% | 100% |
| Coronary atherosclerosis | 259 | 396 | 87% | 95% |
| Obesity | 246 | 509 | 89% | 99% |
| Atrial fibrillation | 236 | 469 | 85% | 100% |
| Gout | 216 | 389 | 85% | 100% |
| Medication | Student | Team | Primary% | All Patients % |
|---|---|---|---|---|
| Omeprazole | 1,372 | 2,981 | 99% | 100% |
| Heparin | 1,067 | 2,271 | 95% | 96% |
| Sodium chloride 0.9% | 925 | 2,036 | 99% | 100% |
| Aspirin | 844 | 1,782 | 98% | 100% |
| Potassium chloride | 707 | 1,387 | 99% | 100% |
| Metoprolol tartrate | 693 | 1,318 | 98% | 100% |
| Insulin regular | 692 | 1,518 | 99% | 100% |
| Acetaminophen | 669 | 1,351 | 98% | 100% |
| Simvastatin | 648 | 1,408 | 99% | 100% |
| Lisinopril | 582 | 1,309 | 98% | 100% |
| Furosemide | 577 | 1,186 | 98% | 100% |
| Docusate sodium | 541 | 1,127 | 98% | 100% |
| Vancomycin | 531 | 977 | 98% | 100% |
| Multivitamin | 478 | 1,074 | 96% | 100% |
| Piperacillin/tazobactam | 470 | 781 | 98% | 100% |
| Selected examples | ||||
| Prednisone | 305 | 613 | 93% | 100% |
| Insulin glargine | 244 | 492 | 81% | 98% |
| Spironolactone | 167 | 380 | 73% | 98% |
| Digoxin | 68 | 125 | 40% | 77% |
| Meropenem | 16 | 21 | 11% | 24% |
| Lab Test | Student | Team | Primary% | All Patients % |
|---|---|---|---|---|
| ||||
| Fingerstick glucose | 12,869 | 24,946 | 100% | 100% |
| Renal panel (serum sodium) | 7,728 | 14,504 | 100% | 100% |
| Complete blood count (blood hematocrit) | 7,372 | 14,188 | 100% | 100% |
| International normalized ratio | 3,725 | 6,259 | 100% | 100% |
| Liver function tests (serum SGOT) | 1,570 | 3,180 | 99% | 100% |
| Urinalysis (urine nitrite) | 789 | 1,537 | 100% | 100% |
| Arterial blood gas (arterial blood pH) | 767 | 704 | 78% | 99% |
| Hemoglobin A1C | 485 | 1,177 | 96% | 100% |
| Fractional excretion of sodium (urine creatinine) | 336 | 677 | 85% | 99% |
| Lactic acid | 195 | 314 | 65% | 96% |
| Ferritin | 193 | 413 | 74% | 99% |
| Thyroid‐stimulating hormone | 184 | 391 | 55% | 64% |
| Lipase | 157 | 317 | 58% | 91% |
| Hepatitis C antibody | 139 | 327 | 70% | 98% |
| Haptoglobin | 101 | 208 | 46% | 83% |
| B‐type natriuretic peptide | 98 | 212 | 48% | 87% |
| Cortisol | 70 | 119 | 34% | 60% |
| Rapid plasma reagin | 70 | 173 | 44% | 82% |
| Urine legionella antigen | 70 | 126 | 38% | 64% |
| D‐dimer | 59 | 111 | 34% | 72% |
| Digoxin | 45 | 69 | 18% | 39% |
| Paracentesis labs (peritoneal fluid total protein) | 34 | 47 | 16% | 34% |
| Thoracentesis labs (pleural fluid WBC count) | 33 | 42 | 20% | 38% |
| C‐reactive protein | 30 | 65 | 17% | 34% |
| Lumbar puncture labs (cerebrospinal fluid WBC count) | 22 | 57 | 11% | 27% |
| Arthrocentesis (synovial fluid WBC count) | 14 | 23 | 9% | 23% |
| Radiology Test | Student | Team | Primary% | All Patients % |
|---|---|---|---|---|
| ||||
| Chest,2 views,PA and lateral | 938 | 1,955 | 100% | 100% |
| Chest portable | 414 | 751 | 96% | 100% |
| CT head without contrast | 235 | 499 | 82% | 100% |
| CT abdomen with contrast | 218 | 365 | 59% | 71% |
| CT pelvis with contrast | 213 | 364 | 59% | 70% |
| CT chest with contrast | 163 | 351 | 75% | 99% |
| Ultrasound kidney, bilateral | 119 | 208 | 61% | 92% |
| Abdomen 1 view | 107 | 220 | 59% | 93% |
| Ultrasound liver | 100 | 183 | 48% | 82% |
| Modified barium swallow | 93 | 130 | 53% | 82% |
| PET scan | 93 | 181 | 49% | 79% |
| Selected examples | ||||
| Acute abdomen series | 85 | 177 | 48% | 81% |
| CT chest, PE protocol | 67 | 126 | 37% | 73% |
| MRI brain with andwithout contrast | 56 | 109 | 34% | 66% |
| Chest decubitus | 51 | 76 | 34% | 60% |
| Portable KUBfor Dobhoff placement | 42 | 62 | 30% | 48% |
| Ventilation/perfusion lung scan | 15 | 25 | 12% | 27% |
| Ultrasound thyroid | 8 | 16 | 5% | 17% |
Distinct Patients and Progress Notes
The mean number of progress notes written by a student was 67.2 (standard deviation [SD] 16.3). The mean number of distinct patients evaluated by a student during a rotation was 18.4 (SD 4.2). The mean number of team admissions per student rotation was 46.7 (SD 9.6) distinct patients.
Primary Diagnoses
A total of 2213 primary diagnoses were documented on patients assigned to students on AI rotations. A total of 5323 primary diagnoses were documented on patients assigned to other members of the team during the students' rotations. Therefore, the mean number of primary diagnoses seen by a student during a rotation was 58.9 (17.3 primary diagnoses for student‐assigned patients and 41.6 primary diagnoses for team patients). The students and teams encountered similar diagnoses (Table 1).
Problem List
Students and teams evaluated a total of 40,015 and 78,643 past medical problems, respectively. The mean number of problems seen by a student during a rotation was 927 (313 student, 614 team). Table 2 reports the most frequent problems assigned to primary student admissions. Students and teams evaluated similar problems. Hepatitis C (196 student, 410 team) was the only team problem that was in the team top 25 but not in the student top 25.
Medications
A total of 38,149 medications were prescribed to the students' primary patients. A total of 77,738 medications were prescribed to patients assigned to the rest of the team. The mean number of medication exposures for a student during a rotation was 905 (298 student, 607 team). The most frequently prescribed medications were similar between student and the team (Table 3). Team medications that were in the top 25 but not in the student top 25 included: hydralazine (300 student, 629 team), prednisone (305 student, 613 team), and oxycodone/acetaminophen (286 student, 608 team).
Labs
All laboratory tests with reported results were tallied. For common laboratory panels, single lab values (eg, serum hematocrit for a complete blood count) were selected as proxies to count the number of studies completed and evaluated. Table 4 shows a cross‐section of laboratory tests evaluated during AI rotations.
Radiology
A total of 6197 radiology tests were completed on patients assigned to students, whereas 11,761 radiology tests were completed on patients assigned to other team members. The mean number of radiology exposures for a student was 140 (48 student, 92 team). The most frequently seen radiology tests were similar between student and the team (Table 5).
DISCUSSION
As medical educators, we assume that the clinical training years allow learners to develop essential skills through their varied clinical experiences. Through exposure to direct patient care, to medical decision‐making scenarios, and to senior physician management practices, trainees build the knowledge base for independent practice. To ensure there is sufficient clinical exposure, data on what trainees are encountering may prove beneficial.
In this novel study, we quantified what learners encounter during a 1‐month team‐based inpatient rotation at a large teaching hospital. We effectively measured a number of aspects of internal medicine inpatient training that have been difficult to quantify in the past. The ability to extract learner‐specific data is becoming increasingly available in academic teaching hospitals. For example, VA medical centers have available a daily updated national data warehouse. The other steps necessary for using learner‐specific data include an understanding of the local inpatient processhow tests are ordered, what note titles are used by traineesas well as someone able to build the queries necessary for data extraction. Once built, data extraction should be able to continue as an automated process and used in real time by medical educators.
Our method of data collection has limitations. The orders placed on a learner's primary patients may not have been placed by the learner. For example, orders may have been placed by an overnight resident cross‐covering the learner's patients. We assumed that learners evaluated the results of all tests (or medication changes) that occurred at any time during their rotation, including cross‐cover periods or days off. In addition, our method for evaluating team exposure underestimates the number of team patients calculated for each learner by limiting the query only to patients whose hospital stay was completed before the student left the inpatient service. It is also difficult to know the how many of the exposures are realized by the learner. Differences in learner attention, contrasts in rounding styles, and varying presentation methods will affect the number of exposures truly attained by the learner. Finally, not all clinical exposures can be evaluated through review of an EMR. Clinical experiences, such as care coordination, patient education, and family counseling, cannot be easily extracted.
Data mining EMRs can enhance clinical medical education. Although our data collection was completed retrospectively, we could easily provide learner‐specific data in real time to ward attendings, chief residents, and program directors. This information could direct the development of teaching tools and individualization of curricula. Perhaps, even more importantly, it would also allow educators to define curricular gaps. Whether these gaps are due to the particular patient demographics of a medical center, the practice patterns and strengths of a particular institution, or career interests of a trainee, these gaps may skew the patient‐care experiences encountered by individual trainees. We can use these data to identify differences in clinical experience and then develop opportunities for learnersclinical, didactic, or simulatedto address deficiencies and provide well‐rounded clinical experiences.
Further investigation to better understand the relationship between direct patient‐care experience and clinical skill acquisition is needed. This information could help guide the development of standards on the number of exposures we expect our learners to have with different diagnostic or treatment modalities prior to independent practice. Using learner data to better understand the clinical experiences of our medical trainees, we can hopefully develop more precise and focused curricula to ensure we produce competent graduates.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the Louis Stokes Cleveland VA Medical Center. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs.
- Accreditation Council for Graduate Medical Education. Program requirements for graduate medical education in internal medicine. Available at: http://www.acgme.org/acgmeweb/Portals/0/PFAssets/2013-PR-FAQ-PIF/140_internal_medicine_07012013.pdf. Originally accessed December 18, 2012.
- , , . Residents make their lists and program directors check them twice: reviewing case logs. J Grad Med Educ. 2012;34:257–260.
- , , , et al. Quantifying internal medicine resident clinical experience using resident‐selected primary diagnosis codes. J Hosp Med. 2011;6(7):395–400.
- , , , et al. Documenting and comparing medical students' clinical experiences. JAMA. 2001;286:1035–1040.
- , , , , . Use of an electronic medical record to profile the continuity clinic experiences of primary care residents. Acad Med. 2005;80:390–394.
- , , . Using a Web‐based system to monitor practice profiles in primary care residency training. Can Fam Physician. 2011;57:1030–1037.
- , , . An automated electronic case log: using electronic information systems to assess training in emergency medicine. Acad Emergency Med. 2006;13:733–739.
- , , , , , . The design and implementation of an automated system for logging clinical experiences using an anesthesia information management system. Anesth Analg. 2011;112(2):422–429.
- , , , . Validation of an electronic system for recording medical student patient encounters. AMIA Annu Symp Proc. 2008;2008:510–514.
- . The structure and content of the medical subinternship: a national survey. J Gen Intern Med. 2001;16:550–553.
- , . Education for practice: the role of practical experience in undergraduate and general clinical training. Med Educ. 1989;23:189–195.
- Accreditation Council for Graduate Medical Education. Program requirements for graduate medical education in internal medicine. Available at: http://www.acgme.org/acgmeweb/Portals/0/PFAssets/2013-PR-FAQ-PIF/140_internal_medicine_07012013.pdf. Originally accessed December 18, 2012.
- , , . Residents make their lists and program directors check them twice: reviewing case logs. J Grad Med Educ. 2012;34:257–260.
- , , , et al. Quantifying internal medicine resident clinical experience using resident‐selected primary diagnosis codes. J Hosp Med. 2011;6(7):395–400.
- , , , et al. Documenting and comparing medical students' clinical experiences. JAMA. 2001;286:1035–1040.
- , , , , . Use of an electronic medical record to profile the continuity clinic experiences of primary care residents. Acad Med. 2005;80:390–394.
- , , . Using a Web‐based system to monitor practice profiles in primary care residency training. Can Fam Physician. 2011;57:1030–1037.
- , , . An automated electronic case log: using electronic information systems to assess training in emergency medicine. Acad Emergency Med. 2006;13:733–739.
- , , , , , . The design and implementation of an automated system for logging clinical experiences using an anesthesia information management system. Anesth Analg. 2011;112(2):422–429.
- , , , . Validation of an electronic system for recording medical student patient encounters. AMIA Annu Symp Proc. 2008;2008:510–514.
- . The structure and content of the medical subinternship: a national survey. J Gen Intern Med. 2001;16:550–553.
- , . Education for practice: the role of practical experience in undergraduate and general clinical training. Med Educ. 1989;23:189–195.
© 2014 Society of Hospital Medicine
Don't forget non-Alzheimer dementias
Dementia is not always due to Alzheimer disease. An accurate diagnosis is important, as the various causative conditions can differ in their course and treatment.
Dementia refers to cognitive impairment severe enough to interfere with the ability to independently perform activities of daily living. It can occur at any age but is most common after age 60. Some studies estimate that 13.9% of people age 71 and older have some form of dementia.1 The prevalence increases with age, ranging from 5% at age 70 to 79 to 37% at age 90 and older.1
Alzheimer disease accounts for about 60% to 80% of cases,2 or an estimated 4.7 million people age 65 and older in the United States, a number anticipated to climb to 13.8 million by 2050.3
Other types of dementia are less often considered and are challenging to recognize, although many have distinct characteristics. This article summarizes the features and management of the more common non-Alzheimer dementias:
- Vascular dementia
- Dementia with Lewy bodies
- Progressive supranuclear palsy
- Corticobasal degeneration
- Multiple system atrophy
- Parkinson disease dementia
- Frontotemporal dementia
- Primary progressive aphasia
- Normal-pressure hydrocephalus
- Rapidly progressive dementia (ie, Creutzfeld-Jakob disease, autoimmune disease).
VASCULAR DEMENTIA
After Alzheimer disease, vascular dementia is the most common dementia, accounting for about 20% to 30% of cases. Clinical criteria have not been widely accepted, although several have been published, including those in the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) and the National Institute of Neurological and Communicative Diseases and Stroke-Association Internationale pour la Recherche et l’Enseignement en Neurosciences.
Risk factors for vascular dementia include cerebrovascular disease (hypertension, diabetes, hyperlipidemia) and coexisting conditions related to atherosclerosis (coronary artery disease, peripheral artery disease).
The Hachinski Ischemic Score is a good bedside tool to help differentiate Alzheimer dementia from vascular dementia.5
Sudden onset and stepwise decline
Vascular dementia often presents as a sudden and stepwise progression of cognitive deficits that stabilize and that are caused by vascular insults (Table 1).6–10 Some patients have continuous decline after a vascular event, indicating that Alzheimer dementia may also be present. Dementia is then defined as a mixed type.
Behavioral problems such as physical aggression, hallucinations, paranoia, and mood fluctuations are common.11
Deficits depend on vascular areas affected
Cognitive deficits are heterogeneous and are often related to the location of the vascular insult. Involvement of subcortical areas may result in executive dysfunction, slowed processing speed, and behavioral changes.12
Executive dysfunction may be identified using the Trail Making Test (Part B) or the Executive Interview (EXIT25). Office-based tools such as the Folstein Mini-Mental State Examination, the Montreal Cognitive Assessment, or the St. Louis University Mental Status Examination may also uncover these deficits.
Focal neurologic deficits may be found on clinical examination.
Structural neuroimaging may identify small strokes in areas of the brain affecting cognitive function or occlusion of a larger vessel associated with more profound neurologic deficits. Neuroimaging findings may not correlate with any significant decline noted by the patient, suggesting “silent” strokes.
Treat symptoms and manage risk factors
Although the US Food and Drug Administration (FDA) has not approved any pharmacotherapy for vascular dementia, commonly prescribed cognitive enhancers have demonstrated some benefit.13
Behavioral problems such as aggression can be disturbing to the patient and the caregiver. Nonpharmacologic methods (eg, redirection, rescheduling care activities to avoid conflict, avoiding issues that lead to agitation) should be tried first to address these problems.
Drug therapy may be used off-label for neuropsychiatric symptoms such as hallucinations, delusions, and combativeness, but clinical trials of these agents for this purpose have shown mixed results,14 and their use is often associated with significant risk.15 Antipsychotic drugs are associated with a risk of death and pneumonia when prescribed for dementia. Many also carry a risk of QT prolongation, which is particularly concerning for patients with coronary artery disease or rhythm disturbances.
The key to reducing further decline is to optimize management of vascular risk factors to reduce stroke risk.
DEMENTIA WITH LEWY BODIES
Dementia with Lewy bodies, the next most common neurodegenerative dementia in the elderly, is characterized by progressive loss of cognitive function, prominent visual hallucinations, and parkinsonism (Table 1).6 Disease progression usually occurs over years but can be more rapid than in Alzheimer disease.
Alpha-synucleinopathy results in dysfunction of synaptic vesicles in presynaptic terminals. Lewy bodies may be diffusely spread in cortical and subcortical areas (appearing as spherical masses).
Visual hallucinations are typical
The McKeith criteria16 are the gold standard for diagnosing probable Lewy body dementia, based on clinical and imaging features (Table 2).
Visual hallucinations are usually well formed and detailed. They may initially be pleasant (eg, seeing children and little people) but may evolve to be accompanied by persecutory delusions.
Parkinsonism develops with or after dementia with Lewy bodies
Dementia with Lewy bodies and Parkinson disease dementia share many clinical and pathologic features; Parkinson dementia also is associated with cortical Lewy bodies.
Parkinsonian features include bradykinesia, masked facies, and rigidity. Resting tremor is less common.
The third report of the Dementia With Lewy Bodies Consortium recommends that the condition be diagnosed if dementia occurs before or concurrently with parkinsonism, and dementia with Parkinson disease should be diagnosed if dementia occurs in the context of well-established Parkinson disease.16 The development of dementia within 12 months of extrapyramidal signs suggests dementia with Lewy bodies.
Cognitive deficits fluctuate
Cognitive impairment in Lewy body dementia is characterized by progressive dementia with fluctuations in cognitive performance. Family members or caregivers may report that the patient can carry on a conversation one day and the next day be confused and inattentive. Compared with those with Alzheimer dementia, patients with Lewy body dementia have better delayed recall but more problems with executive functioning (planning) and visuospatial skills (following an unfamiliar route, copying a figure).
Specialized imaging provides clues
Dementia with Lewy bodies is associated with diffuse brain atrophy, with no established characteristic pattern on structural neuroimaging with computed tomography (CT) or magnetic resonance imaging (MRI).17 The contrast agent ioflupane iodine-123 injection (DaTscan) used with single-photon emission CT (SPECT) detects dopamine transporters, which are reduced in parkinsonian syndromes. The scan can also help differentiate between Alzheimer dementia and Lewy body dementia by detecting the loss of functional dopaminergic terminals in the striatum in Lewy body dementia. Alpha-synuclein imaging may become another useful diagnostic tool in the future.
Alzheimer medications may help in dementia with Lewy bodies
Medications with anticholinergic effects and dopamine agonists should be discontinued because of possible effects on cognitive function and parkinsonism. In one clinical trial,18 rivastigmine (Exelon) was found to help cognitive functioning as well as reduce psychotic symptoms in dementia with Lewy bodies, although a recent Cochrane review could not support the evidence for use of all cholinesterase inhibitors in Lewy body dementia.19 In another trial,20 memantine (Namenda) was found to improve global clinical status and behavioral symptoms of Lewy body dementia.
Treating hallucinations of dementia with Lewy bodies
Patients with dementia with Lewy bodies are extremely sensitive to the extrapyramidal side effects of neuroleptic drugs. Some evidence indicates that the atypical antipsychotic drug quetiapine (Seroquel) helps with prominent and disturbing psychotic features and is less likely to worsen parkinsonism than other antipsychotics.21 The best evidence is for clozapine (Clozaril) as a treatment for hallucinations in Parkinson dementia, but the possible side effect of agranulocytosis limits its clinical use. Other atypical antipsychotics such as risperidone (Risperdal) and olanzapine (Zyprexa) are not recommended.22
PROGRESSIVE SUPRANUCLEAR PALSY
Progressive supranuclear palsy is a sporadic atypical parkinsonian disorder with onset between age 50 and 70. Familial cases are infrequent.
Progressive supranuclear palsy presents as early postural instability, vertical supranuclear gaze palsy, and axial muscle rigidity in the first few years. Disease progression is gradual: one study of 50 patients found that the median time from onset to the first key motor impairment (unintelligible speech, no independent walking, inability to stand unassisted, wheelchair-bound, or recommendation for feeding tube placement) was 4 years.23
Histologically, progressive supranuclear palsy is characterized by accumulation of tau protein aggregates in the basal ganglia, brainstem, and cerebral cortex. The degenerative process involves dopaminergic, cholinergic, and gamma-aminobutyric acid (GABA)-ergic neurons.24
Gait and balance problems predominate early in progressive supranuclear palsy
The most commonly used diagnostic criteria are from the National Institute of Neurological Disorders and Stroke. The diagnosis of probable progressive supranuclear palsy requires vertical gaze palsy and falls or the tendency to fall within the first year of disease onset and exclusion of other causes.
The earliest symptom is usually gait and balance impairment.25 Falls (usually backward) and postural instability occur during the first year in 58% of patients.26 Instead of turning en bloc as in Parkinson disease, patients with progressive supranuclear palsy tend to pivot quickly. Patients may also have a coarse groaning voice and moaning. Insomnia has been reported, but rapid-eye-movement sleep behavior disorders are infrequent (unlike in Parkinson disease, multiple system atrophy, and Lewy body dementia).27
Apathy and extreme mood swings
Cognitive impairment is seen in 50% of patients in the early stage of progressive supranuclear palsy. It mostly involves the frontal lobe, including frontal behavioral disturbances (eg, apathy in 91% of patients26 or pseudobulbar affect and extreme emotional lability) and deficits in abstract thoughts or verbal fluency (to test this, patients are asked to say as many words as possible from a category in a given time). Ideomotor apraxia (inability to correctly imitate hand gestures and voluntarily pantomime tool use, such as pretending to brush hair) is rare, despite corticobasal degeneration.28
Vertical gaze palsy
The hallmark of progressive supranuclear palsy is vertical gaze palsy. Initially, this involves slowing of vertical saccades, followed by diminished vertical gaze and more characteristic downward gaze palsy. These findings may develop over 3 to 4 years. Vertical gaze palsy leads to spilling food and tripping while walking.
The gaze abnormality combined with rare blinking and facial dystonia form the classic facial expression of astonishment called “leonine facies.” The face is stiff and deeply furrowed, with a look of surprise.
Axial (especially neck) rigidity is more prominent than limb rigidity. Retrocollis (the head is drawn back) occurs in less than 25% of patients. Parkinsonian features such as bradykinesia affect nearly half of patients by the time of diagnosis.
Instead of the classic symptoms of progressive supranuclear palsy, about one-third of patients present with progressive supranuclear palsy-parkinsonism, which involves asymmetric parkinsonism that initially responds to levodopa.29
MRI shows ‘hummingbird sign’
Brain MRI shows atrophy of the brainstem, particularly the midbrain. Thinning of the superior part of the midbrain and dilation of the third ventricle (“hummingbird sign” on sagittal sections or “morning glory flower” on axial sections) support a diagnosis of progressive supranuclear palsy and differentiate it from Parkinson disease and other atypical parkinsonian disorders.30,31
Levodopa ineffective for supranuclear palsy
There is no treatment to slow progressive supranuclear palsy. Even in high doses, levodopa rarely alleviates parkinsonian features in a clinically meaningful way.26 Successful experimental biologic therapies have been studied in animal models.32 Davunetide is thought to help with neuronal integrity and cell survival through the stabilization of microtubules in preclinical studies, but it has not been used in clinical practice.33
CORTICOBASAL DEGENERATION
Corticobasal degeneration is a progressive, asymmetric movement disorder often manifesting initially with cognitive or behavioral impairment. It is associated with abnormality of the cytoskeleton protein tau. Onset is usually after age 60.
Asymmetric movement disorder with cognitive dysfunction
This diagnosis is clinical. Diagnostic criteria proposed in 2003 include the following core features34:
- Insidious onset and progressive course
- No identifiable cause
- Cortical dysfunction with at least one of the following: apraxia, alien limb phenomenon (one limb moves involuntarily with complex movements, eg, grabbing the other hand), cortical sensory loss, visual hemineglect, nonfluent aphasia
- Extrapyramidal dysfunction: focal rigidity unresponsive to levodopa, asymmetric dystonia.
An international consortium has developed more specific clinical research criteria for probable and possible corticobasal degeneration.35 In a series of 147 patients, the following clinical features were found: parkinsonism (100%), higher cortical dysfunction (93%), dyspraxia (82%), gait disorder (80%), unilateral limb dystonia (71%), tremor (55%), and dementia (25%).36
Behavioral problems commonly include depression; apathy, irritability, and agitation are also reported.37
Cognitive testing may reveal deficits in frontal-parietal cognitive domains including attention and concentration, executive function, verbal fluency, and visuospatial skills.38 Learning disabilities may be improved with verbal cueing (in contrast to Alzheimer disease). Patients may also have impaired graphesthesia (the ability to recognize writing on the skin only by the sensation of touch).39,40
Motor examination may reveal marked asymmetry. Hand, limb, speech, and gait apraxias are common. Gait is typically slow, with short steps and shuffling, and a wide-based or freezing gait. Arm swing may be absent on one side.
Asymmetric cortical atrophy
Early on, MRI may be normal. As the disease progresses, asymmetric cortical atrophy may be seen, especially in the posterior frontal and parietal lobes.
Levodopa ineffective in corticobasal degeneration
Corticobasal degeneration responds poorly to levodopa. Botulinum toxin has been used to help with dystonia and limb pain.
MULTIPLE SYSTEM ATROPHY
Multiple system atrophy is another atypical parkinsonian disorder, most often diagnosed in men over age 60. It is characterized by sporadic parkinsonism, cerebellar signs (involving balance and coordination), pyramidal tract dysfunction, and autonomic insufficiency in varying combinations. Two major subtypes are recognized, depending on whether the predominating presenting features are cerebellar signs or parkinsonism. In contrast to dementia with Lewy bodies, psychiatric symptoms are not a major feature, except possibly depression.41
Diagnosis requires a sporadic progressive disorder that has features of autonomic failure and poor response of parkinsonism or cerebellar ataxia to levodopa.42
Multiple system atrophy is usually not associated with dementia in the early stages, but patients develop deficits in learning, recognition, memory, and verbal fluency as the disease progresses.43 Rapid-eye-movement sleep behavior disorder has been reported in more than half of patients.44
A neurologic examination provides clues
Parkinsonian features are usually symmetric, in contrast to idiopathic Parkinson disease. These signs may include akinesia with rigidity, postural instability, hypokinetic speech, and tremor.
Cerebellar signs include nystagmus and dysarthria (speech disturbance), and gait and limb ataxia.
Pyramidal features include extensor plantar responses and hyperreflexia.
Autonomic dysfunction includes orthostatic hypotension, bladder and rectal atony, loss of sweating, urinary or fecal incontinence, and erectile dysfunction.
Electromyography may demonstrate decreased anal sphincter tone.
MRI shows atrophy of putamen and pons
Brain MRI may show atrophy of the putamen (hypointensity of the putamen with a hyperintense rim). Pons atrophy may also be present, revealing a “hot cross bun” sign in axial images. These combined findings have specificity above 90% but limited sensitivity. These signs are useful to distinguish multiple system atrophy from Parkinson dementia, but their absence does not exclude the diagnosis of multiple system atrophy.45,46
Multiple system atrophy typically responds poorly to levodopa
Levodopa may improve movement and rigidity, but many respond poorly to treatment or lose response after a few years. Fludrocortisone (Florinef) or vasoconstrictors such as midodrine (Orvaten, Proamatine) may help with orthostatic hypotension.47,48
PARKINSON DISEASE DEMENTIA
Dementia eventually develops in most patients with Parkinson disease. Older age and the akinetic rigid form of the disease are associated with higher risk. Diagnosis of idiopathic Parkinson disease before the development of dementia is essential for the diagnosis.
The Movement Disorder Society Task Force has developed new diagnostic criteria.49 Deficits must be present in at least two of the four core cognitive domains (attention, memory, executive, and visuospatial functions) and must be severe enough to affect daily functioning.
Behavioral symptoms such as affective changes, hallucinations, and apathy are common.
MRI shows characteristic brain atrophy in Parkinson disease dementia
MRI shows reduced gray matter volume in the frontal lobe in patients with Parkinson disease without dementia compared with controls. In Parkinson disease dementia, reduced volume extends to temporal, occipital, and subcortical areas. No significant volumetric differences have been observed in Parkinson dementia compared with dementia with Lewy bodies.50 A greater decrease of glucose metabolism has been found in the inferior parietal and occipital lobes in Parkinson disease dementia than in Parkinson disease without dementia.51
Rivastigmine effective for dementia
A Cochrane review supports the use of acetylcholinesterase inhibitors in patients with Parkinson disease dementia, with a positive impact on global assessment, cognitive function, behavioral disturbance, and activities of daily living rating scales.19 At this time, rivastigmine is the only FDA-approved cholinesterase inhibitor for treating Parkinson disease dementia. In clinical trials, memantine did not improve global clinical status or behavioral symptoms of dementia of Parkinson disease.51
FRONTOTEMPORAL DEMENTIA
Frontotemporal dementia frequently starts before age 65 and accounts for 20% to 50% of dementias in this age group.52 Recognition of the condition in older patients is also growing.53 Frontotemporal dementia encompasses a spectrum of dementias, including behavioral variant frontotemporal dementia, semantic dementia, and progressive nonfluent aphasia.54
Gradual onset of uncharacteristic behaviors
Accepted diagnostic criteria include core features of gradual onset, early decline in social and interpersonal conduct, early impairment of self-regulation, emotional blunting, and loss of insight. Many patients are diagnosed with psychiatric conditions. Changes reported by family and caregivers typically deviate substantially from the person’s usual behavior, such as impulsive and inappropriate behaviors or complete withdrawal and apathy.
Language sometimes affected in frontotemporal dementia
Language impairment may be present in some variants. Behavioral and language changes often accompany other forms of dementia (Alzheimer disease, vascular dementia, primary progressive aphasia), making diagnosis more challenging. Office-based testing often does not reveal any deficits, although the Frontal Behavioral Inventory may help.55 A referral to a clinical neuropsychologist may help identify and quantify cognitive impairments.
MRI shows frontotemporal lobes affected
Structural neuroimaging may not reveal abnormalities initially, but with progression, atrophy may be seen in the frontal and temporal lobes. Functional neuroimaging (positron emission tomography, brain SPECT, functional MRI) show hypometabolism in the same areas.
Treat symptoms
There are no specific FDA-approved therapies for frontotemporal dementia. Acetylcholinesterase inhibitors can help progressive nonfluent aphasia in some cases. Selective serotonin reuptake inhibitors may alleviate depressive symptoms, and low doses of atypical antipsychotic medications may help with impulsivity, disinhibition, and aggressive or disruptive behaviors.56
PRIMARY PROGRESSIVE APHASIA
Language impairment predominates
Primary progressive aphasia is a rare form of dementia in which symptoms typically develop around age 60. Pathology is varied. In a study of 60 patients with initial clinical symptoms of primary progressive aphasia, postmortem histology of brain tissue revealed various findings, including those consistent with Alzheimer pathology and motor neuron diseasetype inclusions.57
Patients typically present with expressive language problems as the primary deficit for the first 2 years of the disease, with preservation in other cognitive areas such as memory, visuospatial skills, and executive function.58 Office-based testing may overstate the severity of the dementia, given the dependence of performance on intact language.
It is important to distinguish primary progressive aphasia from other dementias that also affect language. In the frontal variant of frontotemporal dementia, the primary language problem is anomia (inability to name objects) or diminished speech output, which may be accompanied by behavioral problems. Semantic dementia affects word recognition as well as comprehension. In Alzheimer disease, language may be affected along with memory and other areas of cognitive function.
Imaging shows focal degeneration in the left hemisphere
Structural neuroimaging does not initially reveal any deficits, but later it may reveal atrophy in the frontal, perisylvian complex, and temporal areas of the left hemisphere, reflecting the focal nature of the degeneration.59 Functional neuroimaging (positron emission tomography, SPECT) may reveal hypometabolism or diminished blood flow in these areas prior to changes in structural neuroimaging.60
Other communication methods may help
There are no FDA-approved therapies for primary progressive aphasia. Off-label use of some agents (eg, selective serotonin reuptake inhibitors and small doses of antipsychotic medications) has been found useful in small trials.56 Patients may benefit from learning other forms of communication, such as using sign language, laminated cards with printed words or pictures, or artificial voice synthesizers, to express their needs.
NORMAL-PRESSURE HYDROCEPHALUS
Classic triad: Gait, cognition, incontinence
With the onset of symptoms in the sixth or seventh decade, normal-pressure hydrocephalus affects less than 1% of people age 65 and older. It represents up to 5% of dementias, although estimates are influenced by the varied criteria for diagnosis.61 It is characterized by the classic triad of gait impairment, cognitive impairment, and urinary frequency or incontinence.62
Symptoms progress over a period of years, with gait impairment often predominating. As this triad is common in the geriatric population, identifying other explanations is important. Gait impairment caused by spinal stenosis, peripheral neuropathy, or parkinsonism should be explored. Cognitive impairment could be due to depression, Alzheimer disease, or other forms of dementia. Urinary symptoms may be related to detrusor instability or an enlarged prostate.
Gait impairment initially manifests as slowing of gait, but progresses to difficulty with gait initiation. Gait tends to be wide-based (stance more than 1 foot wide).
Cognitive impairment is typically subcortical, manifested as slowed processing speed and impaired executive function. Recall and working memory may be impaired.
Enlarged ventricles seen on imaging in normal-pressure hydrocephalus
Structural neuroimaging reveals enlarged ventricles (Evan’s ratio > 0.358). This can be difficult to distinguish from ventriculomegaly due to cerebral atrophy; assessing the callosal angle on MRI may distinguish the two.63,64 Diagnosis of normal-pressure hydrocephalus can be confirmed using a cerebrospinal fluid infusion test to assess resistance of fluid to resorption.65
Treat with cerebrospinal fluid drainage
Specific tests should be performed to determine candidacy for surgery. These include a high-volume lumbar puncture (40 to 50 mL) or a trial of external lumbar drainage (10 mL per hour for 48 to 72 hours).65 Definitive treatment is surgical placement of a shunt to allow cerebrospinal fluid to drain into the atria or peritoneal cavity.
Surgery may improve gait, but cognitive symptoms often remain,66 and clinical decline may occur after the shunt is placed. Once gait dysfunction is resolved, other explanations for cognitive impairment or residual gait impairment should be considered. An underlying reason for progression of normal-pressure hydrocephalus symptoms after surgical intervention should be identified.67
RAPIDLY PROGRESSIVE DEMENTIAS
Rapidly progressive dementias are among the most challenging of dementing illnesses. They are characterized by a subacute course and an accelerated rate of decline, developing in less than 2 years. Evaluation should typically be more comprehensive than for other types of dementia. The main goal is to diagnose potentially treatable conditions, such as Hashimoto encephalopathy or paraneoplastic limbic encephalitis, and to distinguish these conditions from diseases with a very poor prognosis, such as Creutzfeldt-Jakob disease.
Creutzfeldt-Jakob disease
Creutzfeldt-Jakob disease is a fatal prion-related neurodegenerative illness. Sporadic disease is most common, but variant, familial, and iatrogenic types have been reported. The most common initial symptoms in sporadic disease are cognitive (39%), cerebellar (21%), behavioral (20%), constitutional (20%), sensory (11%), motor (9%), and visual (7%).68
Chronic neurodegenerative diseases can be misdiagnosed as Creutzfeldt-Jakob disease because of an atypical time course and multi-system neurologic findings.
The US Centers for Disease Control and Prevention has adopted criteria for diagnosing probable Creutzfeldt-Jakob disease (Table 3). Routine investigations should also not suggest an alternative diagnosis.69
Autoimmune diseases
Autoimmune conditions may present as a rapidly progressive dementia, including Hashimoto encephalopathy and antibody-mediated limbic encephalitis, either associated with cancer (paraneoplastic) or without cancer (nonparaneoplastic).
Paraneoplastic limbic encephalitis is a group of inflammatory conditions involving antibodies produced within the cerebrospinal fluid and serum resulting in neurologic symptoms. These antibodies react against proteins expressed mostly by a tumor somewhere else in the body.70
Hashimoto encephalitis is a subacute to chronic encephalopathy that may present as dementia with abnormally high levels of thyroid antibodies. The symptoms can vary from confusion to psychosis. There are two main presentations: one involves a relapsing-remitting course with stroke-like episodes (27% of patients) and the second consists of insidious onset of seizures (66% of patients).
Diagnosis involves testing for elevated anti-thyroid peroxidase and thyroglobulin antibodies. MRI findings are nonspecific. Hashimoto encephalitis responds to treatment with corticosteroids, plasmapheresis, or immunosuppressive therapy.71
- Plassman BL, Langa KM, Fisher GG, et al. Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology 2007; 29:125–132.
- Alzheimer’s Association. 2013 Alzheimer’s disease facts and figures. http://www.alz.org/downloads/facts_figures_2013.pdf. Accessed February 3, 2014.
- Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010-2050) estimated using the 2010 census. Neurology 2013; 80:1778–1783.
- Hou CE, Carlin D, Miller BL. Non-Alzheimer’s disease dementias: anatomic, clinical, and molecular correlates. Can J Psychiatry 2004; 49:164–171.
- Hachinski VC, Iliff LD, Zilhka E, et al. Cerebral blood flow in dementia. Arch Neurol 1975; 32:632–637.
- Fereshtehnejad SM, Religa D, Westman E, Aarsland D, Lökk J, Eriksdotter M. Demography, diagnostics, and medication in dementia with Lewy bodies and Parkinson’s disease with dementia: data from the Swedish Dementia Quality Registry (SveDem). Neuropsychiatr Dis Treat 2013; 9:927–935.
- Nomura T, Inoue Y, Takigawa H, Nakashima K. Comparison of REM sleep behaviour disorder variables between patients with progressive supranuclear palsy and those with Parkinson’s disease. Parkinsonism Relat Disord 2012; 18:394–396.
- Davis PH, Golbe LI, Duvoisin RC, Schoenberg BS. Risk factors for progrssive supranuclear palsy. Neurology 1988; 38:1546–1552.
- Tousi B, Schuele SU, Subramanian T. A 46-year-old woman with rigidity and frequent falls. Cleve Clin J Med 2005; 72:57–63.
- Cooper AD, Josephs KA. Photophobia, visual hallucinations, and REM sleep behavior disorder in progressive supranuclear palsy and corticobasal degeneration: a prospective study. Parkinsonism Relat Disord 2009; 15:59–61.
- Lyketsos CG, Lopez O, Jones B, Fitzpatrick AL, Breitner J, DeKosky S. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the cardiovascular health study0. JAMA 2002; 288:1475–1483.
- Knopman DS. Dementia and cerebrovascular disease. Mayo Clin Proc 2006; 81:223–230.
- Erkinjuntti T, Kurz A, Gauthier S, Bullock R, Lilienfeld S, Damaraju CV. Efficacy of galantamine in probable vascular dementia and Alzheimer’s disease combined with cerebrovascular disease: a randomised trial. Lancet 2002; 359:1283–1290.
- Sink KM, Holden KF, Yaffe K. Pharmacological treatment of neuropsychiatric symptoms of dementia: a review of the evidence. JAMA 2005; 293:596–608.
- Schneider LS, Dagerman K, Insel PS. Efficacy and adverse effects of atypical antipsychotics for dementia: meta-analysis of randomized, placebo-controlled trials. Am J Geriatr Psychiatry 2006; 14:191–210.
- McKeith IG, Dickson DW, Lowe J, et al; Consortium on DLB. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 2005; 65:1863–1872.
- Tartaglia MC, Rosen HJ, Miller BL. Neuroimaging in dementia. Neurotherapeutics 2011; 8:82–92.
- McKeith I, Del Ser T, Spano P, et al. Efficacy of rivastigmine in dementia with Lewy bodies: a randomised, double-blind, placebo-controlled international study. Lancet 2000; 356:2031–2036.
- Rolinski M, Fox C, Maidment I, McShane R. Cholinesterase inhibitors for dementia with Lewy bodies, Parkinson’s disease dementia and cognitive impairment in Parkinson’s disease. Cochrane Database Syst Rev 2012; 3:CD006504.
- Emre M, Tsolaki M, Bonucelli U, et al; on behalf of the 11018 Study Investigators. Memantine for patients with Parkinson’s disease dementia or dementia with Lewy bodies: a randomised, double-blind, placebo-controlled trial. Lancet Neurol 2010; 9:969–977.
- Kurlan R, Cummings J, Raman R, Thal L; Alzheimer’s Disease Cooperative Study Group. Quetiapine for agitation or psychosis in patients with dementia and parkinsonism. Neurology 2007; 68:1356–1363.
- Ballard C, Aarsland D, Francis P, Corbett A. Neuropsychiatric symptoms in patients with dementias associated with cortical Lewy bodies: pathophysiology, clinical features, and pharmacological management. Drugs Aging 2013; 30:603–611.
- Goetz CG, Leurgans S, Lang AE, Litvan I. Progression of gait, speech and swallowing deficits in progressive supranuclear palsy. Neurology 2003; 60:917–922.
- Kasashima S, Oda Y. Cholinergic neuronal loss in the basal forebrain and mesopontine tegmentum of progressive supranuclear palsy and corticobasal degeneration. Acta Neuropathol 2003; 105:117–124.
- Fahn S, Jankovic J, Hallett M, editors. Principles and Practice of Movement Disorders. 2nd ed. New York, NY: Elsevier/Saunders; 2011.
- Litvan I, Mangone CA, McKee A, et al. Natural history of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome) and clinical predictors of survival: a clinicopathological study. J Neurol Neurosurg Psychiatry 1996; 60:615–620.
- Boeve BF, Silber MH, Parisi JE, et al. Synucleinopathy pathology and REM sleep behavior disorder plus dementia or parkinsonism. Neurology 2003; 61:40–45.
- Pharr V, Uttl B, Stark M, Litvan I, Fantie B, Grafman J. Comparison of apraxia in corticobasal degeneration and progressive supranuclear palsy. Neurology 2001; 56:957–963.
- Williams DR, Lees AJ. What features improve the accuracy of the clinical diagnosis of progressive supranuclear palsy-parkinsonism (PSP-P)? Mov Disord 2010; 25:357–362.
- Wenning GK, Litvan I, Tolosa E. Milestones in atypical and secondary parkinsonisms. Mov Disord 2011; 26:1083–1095.
- Gallucci M, Limbucci N, Catalucci A, Caulo M. Neurodegenerative diseases. Radiol Clin North Am 2008; 46:799–817.
- Stamelou M, de Silva R, Arias-Carrión O, et al. Rational therapeutic approaches to progressive supranuclear palsy. Brain 2010; 133:1578–1590.
- Gold M, Lorenzl S, Stewart AJ, Morimoto BH, Williams DR, Gozes I. Critical appraisal of the role of davunetide in the treatment of progressive supranuclear palsy. Neuropsychiatr Dis Treat 2012; 8:85–93.
- Boeve BF, Lang AE, Litvan I. Corticobasal degeneration and its relationship to progressive supranuclear palsy and frontotemporal dementia. Ann Neurol 2003; 54(suppl 5):S15–S19.
- Armstrong MJ, Litvan I, Lang AE, et al. Criteria for the diagnosis of corticobasal degeneration. Neurology 2013; 80:496–503.
- Kompoliti K, Goetz CG, Boeve BF, et al. Clinical presentation and pharmacological therapy in corticobasal degeneration. Arch Neurol 1998; 55:957–961.
- Litvan I, Cummings JL, Mega M. Neuropsychiatric features of corticobasal degeneration. J Neurol Neurosurg Psychiatry 1998; 65:717–721.
- Pillon B, Blin J, Vidailhet M, et al. The neuropsychological pattern of corticobasal degeneration: comparison with progressive supranuclear palsy and Alzheimer’s disease. Neurology 1995; 45:1477–1483.
- Tang-Wai DF, Josephs KA, Boeve BF, Petersen RC, Parisi JE, Dickson DW. Coexistent Lewy body disease in a case of “visual variant of Alzheimer’s disease.” J Neurol Neurosurg Psychiatry 2003; 74:389.
- Tang-Wai DF, Josephs KA, Boeve BF, Dickson DW, Parisi JE, Petersen RC. Pathologically confirmed corticobasal degeneration presenting with visuospatial dysfunction. Neurology 2003; 61:1134–1135.
- Goto K, Ueki A, Shimode H, Shinjo H, Miwa C, Morita Y. Depression in multiple system atrophy: a case report. Psychiatry Clin Neurosci 2000; 54:507–511.
- Gilman S, Wenning GK, Low PA, et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology 2008; 71:670–676.
- Berent S, Giordani B, Gilman S, et al. Patterns of neuropsychological performance in multiple system atrophy compared to sporadic and hereditary olivopontocerebellar atrophy. Brain Cogn 2002; 50:194–206.
- Ghorayeb I, Yekhlef F, Chrysostome V, Balestre E, Bioulac B, Tison F. Sleep disorders and their determinants in multiple system atrophy. J Neurol Neurosurg Psychiatry 2002; 72:798–800.
- Schrag A, Kingsley D, Phatouros C, et al. Clinical usefulness of magnetic resonance imaging in multiple system atrophy. J Neurol Neurosurg Psychiatry 1998; 65:65–71.
- Massey LA, Micallef C, Paviour DC, et al. Conventional magnetic resonance imaging in confirmed progressive supranuclear palsy and multiple system atrophy. Mov Disord 2012; 27:1754–1762.
- Low PA, Gilden JL, Freeman R, Sheng KN, McElligott MA. Efficacy of midodrine vs placebo in neurogenic orthostatic hypotension. A randomized, double-blind multicenter study. Midodrine Study Group. JAMA 1997; 277:1046–1051.
- Mathias CJ, Kimber JR. Postural hypotension: causes, clinical features, investigation, and management. Annu Rev Med 1999; 50:317–336.
- Emre M, Aarsland D, Brown R, et al. Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov Disord 2007; 22:1689–1707.
- Burton EJ, McKeith IG, Burn DJ, Williams ED, O’Brien JT. Cerebral atrophy in Parkinson’s disease with and without dementia: a comparison with Alzheimer’s disease, dementia with Lewy bodies and controls. Brain 2004; 127:791–800.
- Piert M, Koeppe RA, Giordani B, Minoshima S, Kuhl DE. Determination of regional rate constants from dynamic FDG-PET studies in Parkinson’s disease. J Nucl Med 1996; 37:1115–1122.
- Ratnavalli E, Brayne C, Dawson K, Hodges JR. The prevalence of frontotemporal dementia. Neurology 2002; 58:1615–1621.
- Baborie A, Griffiths TD, Jaros E, et al. Frontotemporal dementia in elderly individuals. Arch Neurol 2012; 69:1052–1960.
- Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 1998; 51:1546–1554.
- Kertesz A, Nadkarni N, Davidson W, Thomas AW. The Frontal Behavioral Inventory in the differential diagnosis of frontotemporal dementia. J Int Neuropsychol Soc 2000; 6:460–468.
- Mendez MF, Lauterbach EC, Sampson SM; ANPA Committee on Research. An evidence-based review of the psychopathology of frontotemporal dementia: a report of the ANPA Committee on Research. J Neuropsychiatry Clin Neurosci 2008; 20:130–149.
- Kertesz A, McMonagle P, Blair M, Davidson W, Munoz DG. The evolution and pathology of frontotemporal dementia. Brain 2005; 128:1996–2005.
- Mesulam MM. Primary progressive aphasia—a language-based dementia. N Engl J Med 2003; 349:1535–1542.
- Turner RS, Kenyon LC, Trojanowski JQ, Gonatas N, Grossman M. Clinical, neuroimaging, and pathologic features of progressive nonfluent aphasia. Ann Neurol 1996; 39:166–173.
- Abe K, Ukita H, Yanagihara T. Imaging in primary progressive aphasia. Neuroradiology 1997; 39:556–559.
- Trenkwalder C, Schwarz J, Gebhard J, et al. Starnberg trial on epidemiology of parkinsonism and hypertension in the elderly. Prevalence of Parkinson’s disease and related disorders assessed by a door-to-door survey of inhabitants older than 65 years. Arch Neurol 1995; 52:1017–1022.
- Hakim S, Adams RD. The special clinical problem of symptomatic hydrocephalus with normal cerebrospinal fluid pressure. Observations on cerebrospinal fluid hydrodynamics. J Neurol Sci 1965; 2:307–327.
- Vanneste JA. Diagnosis and management of normal-pressure hydrocephalus. J Neurol 2000; 247:5–14.
- Ishii K, Kanda T, Harada A, et al. Clinical impact of the callosal angle in the diagnosis of idiopathic normal pressure hydrocephalus. Eur Radiol 2008; 18:2678–2683.
- Marmarou A, Bergsneider M, Klinge P, Relkin N, Black PM. The value of supplemental prognostic tests for the preoperative assessment of idiopathic normal-pressure hydrocephalus. Neurosurgery 2005; 57(suppl 3):S17–S28.
- Bergsneider M, Miller C, Vespa PM, Hu X. Surgical management of adult hydrocephalus. Neurosurgery 2008; 62(suppl 2):643–659.
- Malm J, Graff-Radford NR, Ishikawa M, et al. Influence of comorbidities in idiopathic normal pressure hydrocephalus—research and clinical care. A report of the ISHCSF task force on comorbidities in INPH. Fluids Barriers CNS 2013; 10:22.
- Rabinovici GD, Wang PN, Levin J, et al. First symptom in sporadic Creutzfeldt-Jakob disease. Neurology 2006; 66:286–287.
- Zerr I, Kallenberg K, Summers DM, et al. Updated clinical diagnostic criteria for sporadic Creutzfeldt-Jakob disease. Brain 2009; 132:2659–2668.
- Dalmau J, Rosenfeld MR. Paraneoplastic syndromes of the CNS. Lancet Neurol 2008; 7:327–340.
- Chong JY, Rowland LP, Utiger RD. Hashimoto encephalopathy: syndrome or myth? Arch Neurol 2003; 60:164–171.
Dementia is not always due to Alzheimer disease. An accurate diagnosis is important, as the various causative conditions can differ in their course and treatment.
Dementia refers to cognitive impairment severe enough to interfere with the ability to independently perform activities of daily living. It can occur at any age but is most common after age 60. Some studies estimate that 13.9% of people age 71 and older have some form of dementia.1 The prevalence increases with age, ranging from 5% at age 70 to 79 to 37% at age 90 and older.1
Alzheimer disease accounts for about 60% to 80% of cases,2 or an estimated 4.7 million people age 65 and older in the United States, a number anticipated to climb to 13.8 million by 2050.3
Other types of dementia are less often considered and are challenging to recognize, although many have distinct characteristics. This article summarizes the features and management of the more common non-Alzheimer dementias:
- Vascular dementia
- Dementia with Lewy bodies
- Progressive supranuclear palsy
- Corticobasal degeneration
- Multiple system atrophy
- Parkinson disease dementia
- Frontotemporal dementia
- Primary progressive aphasia
- Normal-pressure hydrocephalus
- Rapidly progressive dementia (ie, Creutzfeld-Jakob disease, autoimmune disease).
VASCULAR DEMENTIA
After Alzheimer disease, vascular dementia is the most common dementia, accounting for about 20% to 30% of cases. Clinical criteria have not been widely accepted, although several have been published, including those in the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) and the National Institute of Neurological and Communicative Diseases and Stroke-Association Internationale pour la Recherche et l’Enseignement en Neurosciences.
Risk factors for vascular dementia include cerebrovascular disease (hypertension, diabetes, hyperlipidemia) and coexisting conditions related to atherosclerosis (coronary artery disease, peripheral artery disease).
The Hachinski Ischemic Score is a good bedside tool to help differentiate Alzheimer dementia from vascular dementia.5
Sudden onset and stepwise decline
Vascular dementia often presents as a sudden and stepwise progression of cognitive deficits that stabilize and that are caused by vascular insults (Table 1).6–10 Some patients have continuous decline after a vascular event, indicating that Alzheimer dementia may also be present. Dementia is then defined as a mixed type.
Behavioral problems such as physical aggression, hallucinations, paranoia, and mood fluctuations are common.11
Deficits depend on vascular areas affected
Cognitive deficits are heterogeneous and are often related to the location of the vascular insult. Involvement of subcortical areas may result in executive dysfunction, slowed processing speed, and behavioral changes.12
Executive dysfunction may be identified using the Trail Making Test (Part B) or the Executive Interview (EXIT25). Office-based tools such as the Folstein Mini-Mental State Examination, the Montreal Cognitive Assessment, or the St. Louis University Mental Status Examination may also uncover these deficits.
Focal neurologic deficits may be found on clinical examination.
Structural neuroimaging may identify small strokes in areas of the brain affecting cognitive function or occlusion of a larger vessel associated with more profound neurologic deficits. Neuroimaging findings may not correlate with any significant decline noted by the patient, suggesting “silent” strokes.
Treat symptoms and manage risk factors
Although the US Food and Drug Administration (FDA) has not approved any pharmacotherapy for vascular dementia, commonly prescribed cognitive enhancers have demonstrated some benefit.13
Behavioral problems such as aggression can be disturbing to the patient and the caregiver. Nonpharmacologic methods (eg, redirection, rescheduling care activities to avoid conflict, avoiding issues that lead to agitation) should be tried first to address these problems.
Drug therapy may be used off-label for neuropsychiatric symptoms such as hallucinations, delusions, and combativeness, but clinical trials of these agents for this purpose have shown mixed results,14 and their use is often associated with significant risk.15 Antipsychotic drugs are associated with a risk of death and pneumonia when prescribed for dementia. Many also carry a risk of QT prolongation, which is particularly concerning for patients with coronary artery disease or rhythm disturbances.
The key to reducing further decline is to optimize management of vascular risk factors to reduce stroke risk.
DEMENTIA WITH LEWY BODIES
Dementia with Lewy bodies, the next most common neurodegenerative dementia in the elderly, is characterized by progressive loss of cognitive function, prominent visual hallucinations, and parkinsonism (Table 1).6 Disease progression usually occurs over years but can be more rapid than in Alzheimer disease.
Alpha-synucleinopathy results in dysfunction of synaptic vesicles in presynaptic terminals. Lewy bodies may be diffusely spread in cortical and subcortical areas (appearing as spherical masses).
Visual hallucinations are typical
The McKeith criteria16 are the gold standard for diagnosing probable Lewy body dementia, based on clinical and imaging features (Table 2).
Visual hallucinations are usually well formed and detailed. They may initially be pleasant (eg, seeing children and little people) but may evolve to be accompanied by persecutory delusions.
Parkinsonism develops with or after dementia with Lewy bodies
Dementia with Lewy bodies and Parkinson disease dementia share many clinical and pathologic features; Parkinson dementia also is associated with cortical Lewy bodies.
Parkinsonian features include bradykinesia, masked facies, and rigidity. Resting tremor is less common.
The third report of the Dementia With Lewy Bodies Consortium recommends that the condition be diagnosed if dementia occurs before or concurrently with parkinsonism, and dementia with Parkinson disease should be diagnosed if dementia occurs in the context of well-established Parkinson disease.16 The development of dementia within 12 months of extrapyramidal signs suggests dementia with Lewy bodies.
Cognitive deficits fluctuate
Cognitive impairment in Lewy body dementia is characterized by progressive dementia with fluctuations in cognitive performance. Family members or caregivers may report that the patient can carry on a conversation one day and the next day be confused and inattentive. Compared with those with Alzheimer dementia, patients with Lewy body dementia have better delayed recall but more problems with executive functioning (planning) and visuospatial skills (following an unfamiliar route, copying a figure).
Specialized imaging provides clues
Dementia with Lewy bodies is associated with diffuse brain atrophy, with no established characteristic pattern on structural neuroimaging with computed tomography (CT) or magnetic resonance imaging (MRI).17 The contrast agent ioflupane iodine-123 injection (DaTscan) used with single-photon emission CT (SPECT) detects dopamine transporters, which are reduced in parkinsonian syndromes. The scan can also help differentiate between Alzheimer dementia and Lewy body dementia by detecting the loss of functional dopaminergic terminals in the striatum in Lewy body dementia. Alpha-synuclein imaging may become another useful diagnostic tool in the future.
Alzheimer medications may help in dementia with Lewy bodies
Medications with anticholinergic effects and dopamine agonists should be discontinued because of possible effects on cognitive function and parkinsonism. In one clinical trial,18 rivastigmine (Exelon) was found to help cognitive functioning as well as reduce psychotic symptoms in dementia with Lewy bodies, although a recent Cochrane review could not support the evidence for use of all cholinesterase inhibitors in Lewy body dementia.19 In another trial,20 memantine (Namenda) was found to improve global clinical status and behavioral symptoms of Lewy body dementia.
Treating hallucinations of dementia with Lewy bodies
Patients with dementia with Lewy bodies are extremely sensitive to the extrapyramidal side effects of neuroleptic drugs. Some evidence indicates that the atypical antipsychotic drug quetiapine (Seroquel) helps with prominent and disturbing psychotic features and is less likely to worsen parkinsonism than other antipsychotics.21 The best evidence is for clozapine (Clozaril) as a treatment for hallucinations in Parkinson dementia, but the possible side effect of agranulocytosis limits its clinical use. Other atypical antipsychotics such as risperidone (Risperdal) and olanzapine (Zyprexa) are not recommended.22
PROGRESSIVE SUPRANUCLEAR PALSY
Progressive supranuclear palsy is a sporadic atypical parkinsonian disorder with onset between age 50 and 70. Familial cases are infrequent.
Progressive supranuclear palsy presents as early postural instability, vertical supranuclear gaze palsy, and axial muscle rigidity in the first few years. Disease progression is gradual: one study of 50 patients found that the median time from onset to the first key motor impairment (unintelligible speech, no independent walking, inability to stand unassisted, wheelchair-bound, or recommendation for feeding tube placement) was 4 years.23
Histologically, progressive supranuclear palsy is characterized by accumulation of tau protein aggregates in the basal ganglia, brainstem, and cerebral cortex. The degenerative process involves dopaminergic, cholinergic, and gamma-aminobutyric acid (GABA)-ergic neurons.24
Gait and balance problems predominate early in progressive supranuclear palsy
The most commonly used diagnostic criteria are from the National Institute of Neurological Disorders and Stroke. The diagnosis of probable progressive supranuclear palsy requires vertical gaze palsy and falls or the tendency to fall within the first year of disease onset and exclusion of other causes.
The earliest symptom is usually gait and balance impairment.25 Falls (usually backward) and postural instability occur during the first year in 58% of patients.26 Instead of turning en bloc as in Parkinson disease, patients with progressive supranuclear palsy tend to pivot quickly. Patients may also have a coarse groaning voice and moaning. Insomnia has been reported, but rapid-eye-movement sleep behavior disorders are infrequent (unlike in Parkinson disease, multiple system atrophy, and Lewy body dementia).27
Apathy and extreme mood swings
Cognitive impairment is seen in 50% of patients in the early stage of progressive supranuclear palsy. It mostly involves the frontal lobe, including frontal behavioral disturbances (eg, apathy in 91% of patients26 or pseudobulbar affect and extreme emotional lability) and deficits in abstract thoughts or verbal fluency (to test this, patients are asked to say as many words as possible from a category in a given time). Ideomotor apraxia (inability to correctly imitate hand gestures and voluntarily pantomime tool use, such as pretending to brush hair) is rare, despite corticobasal degeneration.28
Vertical gaze palsy
The hallmark of progressive supranuclear palsy is vertical gaze palsy. Initially, this involves slowing of vertical saccades, followed by diminished vertical gaze and more characteristic downward gaze palsy. These findings may develop over 3 to 4 years. Vertical gaze palsy leads to spilling food and tripping while walking.
The gaze abnormality combined with rare blinking and facial dystonia form the classic facial expression of astonishment called “leonine facies.” The face is stiff and deeply furrowed, with a look of surprise.
Axial (especially neck) rigidity is more prominent than limb rigidity. Retrocollis (the head is drawn back) occurs in less than 25% of patients. Parkinsonian features such as bradykinesia affect nearly half of patients by the time of diagnosis.
Instead of the classic symptoms of progressive supranuclear palsy, about one-third of patients present with progressive supranuclear palsy-parkinsonism, which involves asymmetric parkinsonism that initially responds to levodopa.29
MRI shows ‘hummingbird sign’
Brain MRI shows atrophy of the brainstem, particularly the midbrain. Thinning of the superior part of the midbrain and dilation of the third ventricle (“hummingbird sign” on sagittal sections or “morning glory flower” on axial sections) support a diagnosis of progressive supranuclear palsy and differentiate it from Parkinson disease and other atypical parkinsonian disorders.30,31
Levodopa ineffective for supranuclear palsy
There is no treatment to slow progressive supranuclear palsy. Even in high doses, levodopa rarely alleviates parkinsonian features in a clinically meaningful way.26 Successful experimental biologic therapies have been studied in animal models.32 Davunetide is thought to help with neuronal integrity and cell survival through the stabilization of microtubules in preclinical studies, but it has not been used in clinical practice.33
CORTICOBASAL DEGENERATION
Corticobasal degeneration is a progressive, asymmetric movement disorder often manifesting initially with cognitive or behavioral impairment. It is associated with abnormality of the cytoskeleton protein tau. Onset is usually after age 60.
Asymmetric movement disorder with cognitive dysfunction
This diagnosis is clinical. Diagnostic criteria proposed in 2003 include the following core features34:
- Insidious onset and progressive course
- No identifiable cause
- Cortical dysfunction with at least one of the following: apraxia, alien limb phenomenon (one limb moves involuntarily with complex movements, eg, grabbing the other hand), cortical sensory loss, visual hemineglect, nonfluent aphasia
- Extrapyramidal dysfunction: focal rigidity unresponsive to levodopa, asymmetric dystonia.
An international consortium has developed more specific clinical research criteria for probable and possible corticobasal degeneration.35 In a series of 147 patients, the following clinical features were found: parkinsonism (100%), higher cortical dysfunction (93%), dyspraxia (82%), gait disorder (80%), unilateral limb dystonia (71%), tremor (55%), and dementia (25%).36
Behavioral problems commonly include depression; apathy, irritability, and agitation are also reported.37
Cognitive testing may reveal deficits in frontal-parietal cognitive domains including attention and concentration, executive function, verbal fluency, and visuospatial skills.38 Learning disabilities may be improved with verbal cueing (in contrast to Alzheimer disease). Patients may also have impaired graphesthesia (the ability to recognize writing on the skin only by the sensation of touch).39,40
Motor examination may reveal marked asymmetry. Hand, limb, speech, and gait apraxias are common. Gait is typically slow, with short steps and shuffling, and a wide-based or freezing gait. Arm swing may be absent on one side.
Asymmetric cortical atrophy
Early on, MRI may be normal. As the disease progresses, asymmetric cortical atrophy may be seen, especially in the posterior frontal and parietal lobes.
Levodopa ineffective in corticobasal degeneration
Corticobasal degeneration responds poorly to levodopa. Botulinum toxin has been used to help with dystonia and limb pain.
MULTIPLE SYSTEM ATROPHY
Multiple system atrophy is another atypical parkinsonian disorder, most often diagnosed in men over age 60. It is characterized by sporadic parkinsonism, cerebellar signs (involving balance and coordination), pyramidal tract dysfunction, and autonomic insufficiency in varying combinations. Two major subtypes are recognized, depending on whether the predominating presenting features are cerebellar signs or parkinsonism. In contrast to dementia with Lewy bodies, psychiatric symptoms are not a major feature, except possibly depression.41
Diagnosis requires a sporadic progressive disorder that has features of autonomic failure and poor response of parkinsonism or cerebellar ataxia to levodopa.42
Multiple system atrophy is usually not associated with dementia in the early stages, but patients develop deficits in learning, recognition, memory, and verbal fluency as the disease progresses.43 Rapid-eye-movement sleep behavior disorder has been reported in more than half of patients.44
A neurologic examination provides clues
Parkinsonian features are usually symmetric, in contrast to idiopathic Parkinson disease. These signs may include akinesia with rigidity, postural instability, hypokinetic speech, and tremor.
Cerebellar signs include nystagmus and dysarthria (speech disturbance), and gait and limb ataxia.
Pyramidal features include extensor plantar responses and hyperreflexia.
Autonomic dysfunction includes orthostatic hypotension, bladder and rectal atony, loss of sweating, urinary or fecal incontinence, and erectile dysfunction.
Electromyography may demonstrate decreased anal sphincter tone.
MRI shows atrophy of putamen and pons
Brain MRI may show atrophy of the putamen (hypointensity of the putamen with a hyperintense rim). Pons atrophy may also be present, revealing a “hot cross bun” sign in axial images. These combined findings have specificity above 90% but limited sensitivity. These signs are useful to distinguish multiple system atrophy from Parkinson dementia, but their absence does not exclude the diagnosis of multiple system atrophy.45,46
Multiple system atrophy typically responds poorly to levodopa
Levodopa may improve movement and rigidity, but many respond poorly to treatment or lose response after a few years. Fludrocortisone (Florinef) or vasoconstrictors such as midodrine (Orvaten, Proamatine) may help with orthostatic hypotension.47,48
PARKINSON DISEASE DEMENTIA
Dementia eventually develops in most patients with Parkinson disease. Older age and the akinetic rigid form of the disease are associated with higher risk. Diagnosis of idiopathic Parkinson disease before the development of dementia is essential for the diagnosis.
The Movement Disorder Society Task Force has developed new diagnostic criteria.49 Deficits must be present in at least two of the four core cognitive domains (attention, memory, executive, and visuospatial functions) and must be severe enough to affect daily functioning.
Behavioral symptoms such as affective changes, hallucinations, and apathy are common.
MRI shows characteristic brain atrophy in Parkinson disease dementia
MRI shows reduced gray matter volume in the frontal lobe in patients with Parkinson disease without dementia compared with controls. In Parkinson disease dementia, reduced volume extends to temporal, occipital, and subcortical areas. No significant volumetric differences have been observed in Parkinson dementia compared with dementia with Lewy bodies.50 A greater decrease of glucose metabolism has been found in the inferior parietal and occipital lobes in Parkinson disease dementia than in Parkinson disease without dementia.51
Rivastigmine effective for dementia
A Cochrane review supports the use of acetylcholinesterase inhibitors in patients with Parkinson disease dementia, with a positive impact on global assessment, cognitive function, behavioral disturbance, and activities of daily living rating scales.19 At this time, rivastigmine is the only FDA-approved cholinesterase inhibitor for treating Parkinson disease dementia. In clinical trials, memantine did not improve global clinical status or behavioral symptoms of dementia of Parkinson disease.51
FRONTOTEMPORAL DEMENTIA
Frontotemporal dementia frequently starts before age 65 and accounts for 20% to 50% of dementias in this age group.52 Recognition of the condition in older patients is also growing.53 Frontotemporal dementia encompasses a spectrum of dementias, including behavioral variant frontotemporal dementia, semantic dementia, and progressive nonfluent aphasia.54
Gradual onset of uncharacteristic behaviors
Accepted diagnostic criteria include core features of gradual onset, early decline in social and interpersonal conduct, early impairment of self-regulation, emotional blunting, and loss of insight. Many patients are diagnosed with psychiatric conditions. Changes reported by family and caregivers typically deviate substantially from the person’s usual behavior, such as impulsive and inappropriate behaviors or complete withdrawal and apathy.
Language sometimes affected in frontotemporal dementia
Language impairment may be present in some variants. Behavioral and language changes often accompany other forms of dementia (Alzheimer disease, vascular dementia, primary progressive aphasia), making diagnosis more challenging. Office-based testing often does not reveal any deficits, although the Frontal Behavioral Inventory may help.55 A referral to a clinical neuropsychologist may help identify and quantify cognitive impairments.
MRI shows frontotemporal lobes affected
Structural neuroimaging may not reveal abnormalities initially, but with progression, atrophy may be seen in the frontal and temporal lobes. Functional neuroimaging (positron emission tomography, brain SPECT, functional MRI) show hypometabolism in the same areas.
Treat symptoms
There are no specific FDA-approved therapies for frontotemporal dementia. Acetylcholinesterase inhibitors can help progressive nonfluent aphasia in some cases. Selective serotonin reuptake inhibitors may alleviate depressive symptoms, and low doses of atypical antipsychotic medications may help with impulsivity, disinhibition, and aggressive or disruptive behaviors.56
PRIMARY PROGRESSIVE APHASIA
Language impairment predominates
Primary progressive aphasia is a rare form of dementia in which symptoms typically develop around age 60. Pathology is varied. In a study of 60 patients with initial clinical symptoms of primary progressive aphasia, postmortem histology of brain tissue revealed various findings, including those consistent with Alzheimer pathology and motor neuron diseasetype inclusions.57
Patients typically present with expressive language problems as the primary deficit for the first 2 years of the disease, with preservation in other cognitive areas such as memory, visuospatial skills, and executive function.58 Office-based testing may overstate the severity of the dementia, given the dependence of performance on intact language.
It is important to distinguish primary progressive aphasia from other dementias that also affect language. In the frontal variant of frontotemporal dementia, the primary language problem is anomia (inability to name objects) or diminished speech output, which may be accompanied by behavioral problems. Semantic dementia affects word recognition as well as comprehension. In Alzheimer disease, language may be affected along with memory and other areas of cognitive function.
Imaging shows focal degeneration in the left hemisphere
Structural neuroimaging does not initially reveal any deficits, but later it may reveal atrophy in the frontal, perisylvian complex, and temporal areas of the left hemisphere, reflecting the focal nature of the degeneration.59 Functional neuroimaging (positron emission tomography, SPECT) may reveal hypometabolism or diminished blood flow in these areas prior to changes in structural neuroimaging.60
Other communication methods may help
There are no FDA-approved therapies for primary progressive aphasia. Off-label use of some agents (eg, selective serotonin reuptake inhibitors and small doses of antipsychotic medications) has been found useful in small trials.56 Patients may benefit from learning other forms of communication, such as using sign language, laminated cards with printed words or pictures, or artificial voice synthesizers, to express their needs.
NORMAL-PRESSURE HYDROCEPHALUS
Classic triad: Gait, cognition, incontinence
With the onset of symptoms in the sixth or seventh decade, normal-pressure hydrocephalus affects less than 1% of people age 65 and older. It represents up to 5% of dementias, although estimates are influenced by the varied criteria for diagnosis.61 It is characterized by the classic triad of gait impairment, cognitive impairment, and urinary frequency or incontinence.62
Symptoms progress over a period of years, with gait impairment often predominating. As this triad is common in the geriatric population, identifying other explanations is important. Gait impairment caused by spinal stenosis, peripheral neuropathy, or parkinsonism should be explored. Cognitive impairment could be due to depression, Alzheimer disease, or other forms of dementia. Urinary symptoms may be related to detrusor instability or an enlarged prostate.
Gait impairment initially manifests as slowing of gait, but progresses to difficulty with gait initiation. Gait tends to be wide-based (stance more than 1 foot wide).
Cognitive impairment is typically subcortical, manifested as slowed processing speed and impaired executive function. Recall and working memory may be impaired.
Enlarged ventricles seen on imaging in normal-pressure hydrocephalus
Structural neuroimaging reveals enlarged ventricles (Evan’s ratio > 0.358). This can be difficult to distinguish from ventriculomegaly due to cerebral atrophy; assessing the callosal angle on MRI may distinguish the two.63,64 Diagnosis of normal-pressure hydrocephalus can be confirmed using a cerebrospinal fluid infusion test to assess resistance of fluid to resorption.65
Treat with cerebrospinal fluid drainage
Specific tests should be performed to determine candidacy for surgery. These include a high-volume lumbar puncture (40 to 50 mL) or a trial of external lumbar drainage (10 mL per hour for 48 to 72 hours).65 Definitive treatment is surgical placement of a shunt to allow cerebrospinal fluid to drain into the atria or peritoneal cavity.
Surgery may improve gait, but cognitive symptoms often remain,66 and clinical decline may occur after the shunt is placed. Once gait dysfunction is resolved, other explanations for cognitive impairment or residual gait impairment should be considered. An underlying reason for progression of normal-pressure hydrocephalus symptoms after surgical intervention should be identified.67
RAPIDLY PROGRESSIVE DEMENTIAS
Rapidly progressive dementias are among the most challenging of dementing illnesses. They are characterized by a subacute course and an accelerated rate of decline, developing in less than 2 years. Evaluation should typically be more comprehensive than for other types of dementia. The main goal is to diagnose potentially treatable conditions, such as Hashimoto encephalopathy or paraneoplastic limbic encephalitis, and to distinguish these conditions from diseases with a very poor prognosis, such as Creutzfeldt-Jakob disease.
Creutzfeldt-Jakob disease
Creutzfeldt-Jakob disease is a fatal prion-related neurodegenerative illness. Sporadic disease is most common, but variant, familial, and iatrogenic types have been reported. The most common initial symptoms in sporadic disease are cognitive (39%), cerebellar (21%), behavioral (20%), constitutional (20%), sensory (11%), motor (9%), and visual (7%).68
Chronic neurodegenerative diseases can be misdiagnosed as Creutzfeldt-Jakob disease because of an atypical time course and multi-system neurologic findings.
The US Centers for Disease Control and Prevention has adopted criteria for diagnosing probable Creutzfeldt-Jakob disease (Table 3). Routine investigations should also not suggest an alternative diagnosis.69
Autoimmune diseases
Autoimmune conditions may present as a rapidly progressive dementia, including Hashimoto encephalopathy and antibody-mediated limbic encephalitis, either associated with cancer (paraneoplastic) or without cancer (nonparaneoplastic).
Paraneoplastic limbic encephalitis is a group of inflammatory conditions involving antibodies produced within the cerebrospinal fluid and serum resulting in neurologic symptoms. These antibodies react against proteins expressed mostly by a tumor somewhere else in the body.70
Hashimoto encephalitis is a subacute to chronic encephalopathy that may present as dementia with abnormally high levels of thyroid antibodies. The symptoms can vary from confusion to psychosis. There are two main presentations: one involves a relapsing-remitting course with stroke-like episodes (27% of patients) and the second consists of insidious onset of seizures (66% of patients).
Diagnosis involves testing for elevated anti-thyroid peroxidase and thyroglobulin antibodies. MRI findings are nonspecific. Hashimoto encephalitis responds to treatment with corticosteroids, plasmapheresis, or immunosuppressive therapy.71
Dementia is not always due to Alzheimer disease. An accurate diagnosis is important, as the various causative conditions can differ in their course and treatment.
Dementia refers to cognitive impairment severe enough to interfere with the ability to independently perform activities of daily living. It can occur at any age but is most common after age 60. Some studies estimate that 13.9% of people age 71 and older have some form of dementia.1 The prevalence increases with age, ranging from 5% at age 70 to 79 to 37% at age 90 and older.1
Alzheimer disease accounts for about 60% to 80% of cases,2 or an estimated 4.7 million people age 65 and older in the United States, a number anticipated to climb to 13.8 million by 2050.3
Other types of dementia are less often considered and are challenging to recognize, although many have distinct characteristics. This article summarizes the features and management of the more common non-Alzheimer dementias:
- Vascular dementia
- Dementia with Lewy bodies
- Progressive supranuclear palsy
- Corticobasal degeneration
- Multiple system atrophy
- Parkinson disease dementia
- Frontotemporal dementia
- Primary progressive aphasia
- Normal-pressure hydrocephalus
- Rapidly progressive dementia (ie, Creutzfeld-Jakob disease, autoimmune disease).
VASCULAR DEMENTIA
After Alzheimer disease, vascular dementia is the most common dementia, accounting for about 20% to 30% of cases. Clinical criteria have not been widely accepted, although several have been published, including those in the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) and the National Institute of Neurological and Communicative Diseases and Stroke-Association Internationale pour la Recherche et l’Enseignement en Neurosciences.
Risk factors for vascular dementia include cerebrovascular disease (hypertension, diabetes, hyperlipidemia) and coexisting conditions related to atherosclerosis (coronary artery disease, peripheral artery disease).
The Hachinski Ischemic Score is a good bedside tool to help differentiate Alzheimer dementia from vascular dementia.5
Sudden onset and stepwise decline
Vascular dementia often presents as a sudden and stepwise progression of cognitive deficits that stabilize and that are caused by vascular insults (Table 1).6–10 Some patients have continuous decline after a vascular event, indicating that Alzheimer dementia may also be present. Dementia is then defined as a mixed type.
Behavioral problems such as physical aggression, hallucinations, paranoia, and mood fluctuations are common.11
Deficits depend on vascular areas affected
Cognitive deficits are heterogeneous and are often related to the location of the vascular insult. Involvement of subcortical areas may result in executive dysfunction, slowed processing speed, and behavioral changes.12
Executive dysfunction may be identified using the Trail Making Test (Part B) or the Executive Interview (EXIT25). Office-based tools such as the Folstein Mini-Mental State Examination, the Montreal Cognitive Assessment, or the St. Louis University Mental Status Examination may also uncover these deficits.
Focal neurologic deficits may be found on clinical examination.
Structural neuroimaging may identify small strokes in areas of the brain affecting cognitive function or occlusion of a larger vessel associated with more profound neurologic deficits. Neuroimaging findings may not correlate with any significant decline noted by the patient, suggesting “silent” strokes.
Treat symptoms and manage risk factors
Although the US Food and Drug Administration (FDA) has not approved any pharmacotherapy for vascular dementia, commonly prescribed cognitive enhancers have demonstrated some benefit.13
Behavioral problems such as aggression can be disturbing to the patient and the caregiver. Nonpharmacologic methods (eg, redirection, rescheduling care activities to avoid conflict, avoiding issues that lead to agitation) should be tried first to address these problems.
Drug therapy may be used off-label for neuropsychiatric symptoms such as hallucinations, delusions, and combativeness, but clinical trials of these agents for this purpose have shown mixed results,14 and their use is often associated with significant risk.15 Antipsychotic drugs are associated with a risk of death and pneumonia when prescribed for dementia. Many also carry a risk of QT prolongation, which is particularly concerning for patients with coronary artery disease or rhythm disturbances.
The key to reducing further decline is to optimize management of vascular risk factors to reduce stroke risk.
DEMENTIA WITH LEWY BODIES
Dementia with Lewy bodies, the next most common neurodegenerative dementia in the elderly, is characterized by progressive loss of cognitive function, prominent visual hallucinations, and parkinsonism (Table 1).6 Disease progression usually occurs over years but can be more rapid than in Alzheimer disease.
Alpha-synucleinopathy results in dysfunction of synaptic vesicles in presynaptic terminals. Lewy bodies may be diffusely spread in cortical and subcortical areas (appearing as spherical masses).
Visual hallucinations are typical
The McKeith criteria16 are the gold standard for diagnosing probable Lewy body dementia, based on clinical and imaging features (Table 2).
Visual hallucinations are usually well formed and detailed. They may initially be pleasant (eg, seeing children and little people) but may evolve to be accompanied by persecutory delusions.
Parkinsonism develops with or after dementia with Lewy bodies
Dementia with Lewy bodies and Parkinson disease dementia share many clinical and pathologic features; Parkinson dementia also is associated with cortical Lewy bodies.
Parkinsonian features include bradykinesia, masked facies, and rigidity. Resting tremor is less common.
The third report of the Dementia With Lewy Bodies Consortium recommends that the condition be diagnosed if dementia occurs before or concurrently with parkinsonism, and dementia with Parkinson disease should be diagnosed if dementia occurs in the context of well-established Parkinson disease.16 The development of dementia within 12 months of extrapyramidal signs suggests dementia with Lewy bodies.
Cognitive deficits fluctuate
Cognitive impairment in Lewy body dementia is characterized by progressive dementia with fluctuations in cognitive performance. Family members or caregivers may report that the patient can carry on a conversation one day and the next day be confused and inattentive. Compared with those with Alzheimer dementia, patients with Lewy body dementia have better delayed recall but more problems with executive functioning (planning) and visuospatial skills (following an unfamiliar route, copying a figure).
Specialized imaging provides clues
Dementia with Lewy bodies is associated with diffuse brain atrophy, with no established characteristic pattern on structural neuroimaging with computed tomography (CT) or magnetic resonance imaging (MRI).17 The contrast agent ioflupane iodine-123 injection (DaTscan) used with single-photon emission CT (SPECT) detects dopamine transporters, which are reduced in parkinsonian syndromes. The scan can also help differentiate between Alzheimer dementia and Lewy body dementia by detecting the loss of functional dopaminergic terminals in the striatum in Lewy body dementia. Alpha-synuclein imaging may become another useful diagnostic tool in the future.
Alzheimer medications may help in dementia with Lewy bodies
Medications with anticholinergic effects and dopamine agonists should be discontinued because of possible effects on cognitive function and parkinsonism. In one clinical trial,18 rivastigmine (Exelon) was found to help cognitive functioning as well as reduce psychotic symptoms in dementia with Lewy bodies, although a recent Cochrane review could not support the evidence for use of all cholinesterase inhibitors in Lewy body dementia.19 In another trial,20 memantine (Namenda) was found to improve global clinical status and behavioral symptoms of Lewy body dementia.
Treating hallucinations of dementia with Lewy bodies
Patients with dementia with Lewy bodies are extremely sensitive to the extrapyramidal side effects of neuroleptic drugs. Some evidence indicates that the atypical antipsychotic drug quetiapine (Seroquel) helps with prominent and disturbing psychotic features and is less likely to worsen parkinsonism than other antipsychotics.21 The best evidence is for clozapine (Clozaril) as a treatment for hallucinations in Parkinson dementia, but the possible side effect of agranulocytosis limits its clinical use. Other atypical antipsychotics such as risperidone (Risperdal) and olanzapine (Zyprexa) are not recommended.22
PROGRESSIVE SUPRANUCLEAR PALSY
Progressive supranuclear palsy is a sporadic atypical parkinsonian disorder with onset between age 50 and 70. Familial cases are infrequent.
Progressive supranuclear palsy presents as early postural instability, vertical supranuclear gaze palsy, and axial muscle rigidity in the first few years. Disease progression is gradual: one study of 50 patients found that the median time from onset to the first key motor impairment (unintelligible speech, no independent walking, inability to stand unassisted, wheelchair-bound, or recommendation for feeding tube placement) was 4 years.23
Histologically, progressive supranuclear palsy is characterized by accumulation of tau protein aggregates in the basal ganglia, brainstem, and cerebral cortex. The degenerative process involves dopaminergic, cholinergic, and gamma-aminobutyric acid (GABA)-ergic neurons.24
Gait and balance problems predominate early in progressive supranuclear palsy
The most commonly used diagnostic criteria are from the National Institute of Neurological Disorders and Stroke. The diagnosis of probable progressive supranuclear palsy requires vertical gaze palsy and falls or the tendency to fall within the first year of disease onset and exclusion of other causes.
The earliest symptom is usually gait and balance impairment.25 Falls (usually backward) and postural instability occur during the first year in 58% of patients.26 Instead of turning en bloc as in Parkinson disease, patients with progressive supranuclear palsy tend to pivot quickly. Patients may also have a coarse groaning voice and moaning. Insomnia has been reported, but rapid-eye-movement sleep behavior disorders are infrequent (unlike in Parkinson disease, multiple system atrophy, and Lewy body dementia).27
Apathy and extreme mood swings
Cognitive impairment is seen in 50% of patients in the early stage of progressive supranuclear palsy. It mostly involves the frontal lobe, including frontal behavioral disturbances (eg, apathy in 91% of patients26 or pseudobulbar affect and extreme emotional lability) and deficits in abstract thoughts or verbal fluency (to test this, patients are asked to say as many words as possible from a category in a given time). Ideomotor apraxia (inability to correctly imitate hand gestures and voluntarily pantomime tool use, such as pretending to brush hair) is rare, despite corticobasal degeneration.28
Vertical gaze palsy
The hallmark of progressive supranuclear palsy is vertical gaze palsy. Initially, this involves slowing of vertical saccades, followed by diminished vertical gaze and more characteristic downward gaze palsy. These findings may develop over 3 to 4 years. Vertical gaze palsy leads to spilling food and tripping while walking.
The gaze abnormality combined with rare blinking and facial dystonia form the classic facial expression of astonishment called “leonine facies.” The face is stiff and deeply furrowed, with a look of surprise.
Axial (especially neck) rigidity is more prominent than limb rigidity. Retrocollis (the head is drawn back) occurs in less than 25% of patients. Parkinsonian features such as bradykinesia affect nearly half of patients by the time of diagnosis.
Instead of the classic symptoms of progressive supranuclear palsy, about one-third of patients present with progressive supranuclear palsy-parkinsonism, which involves asymmetric parkinsonism that initially responds to levodopa.29
MRI shows ‘hummingbird sign’
Brain MRI shows atrophy of the brainstem, particularly the midbrain. Thinning of the superior part of the midbrain and dilation of the third ventricle (“hummingbird sign” on sagittal sections or “morning glory flower” on axial sections) support a diagnosis of progressive supranuclear palsy and differentiate it from Parkinson disease and other atypical parkinsonian disorders.30,31
Levodopa ineffective for supranuclear palsy
There is no treatment to slow progressive supranuclear palsy. Even in high doses, levodopa rarely alleviates parkinsonian features in a clinically meaningful way.26 Successful experimental biologic therapies have been studied in animal models.32 Davunetide is thought to help with neuronal integrity and cell survival through the stabilization of microtubules in preclinical studies, but it has not been used in clinical practice.33
CORTICOBASAL DEGENERATION
Corticobasal degeneration is a progressive, asymmetric movement disorder often manifesting initially with cognitive or behavioral impairment. It is associated with abnormality of the cytoskeleton protein tau. Onset is usually after age 60.
Asymmetric movement disorder with cognitive dysfunction
This diagnosis is clinical. Diagnostic criteria proposed in 2003 include the following core features34:
- Insidious onset and progressive course
- No identifiable cause
- Cortical dysfunction with at least one of the following: apraxia, alien limb phenomenon (one limb moves involuntarily with complex movements, eg, grabbing the other hand), cortical sensory loss, visual hemineglect, nonfluent aphasia
- Extrapyramidal dysfunction: focal rigidity unresponsive to levodopa, asymmetric dystonia.
An international consortium has developed more specific clinical research criteria for probable and possible corticobasal degeneration.35 In a series of 147 patients, the following clinical features were found: parkinsonism (100%), higher cortical dysfunction (93%), dyspraxia (82%), gait disorder (80%), unilateral limb dystonia (71%), tremor (55%), and dementia (25%).36
Behavioral problems commonly include depression; apathy, irritability, and agitation are also reported.37
Cognitive testing may reveal deficits in frontal-parietal cognitive domains including attention and concentration, executive function, verbal fluency, and visuospatial skills.38 Learning disabilities may be improved with verbal cueing (in contrast to Alzheimer disease). Patients may also have impaired graphesthesia (the ability to recognize writing on the skin only by the sensation of touch).39,40
Motor examination may reveal marked asymmetry. Hand, limb, speech, and gait apraxias are common. Gait is typically slow, with short steps and shuffling, and a wide-based or freezing gait. Arm swing may be absent on one side.
Asymmetric cortical atrophy
Early on, MRI may be normal. As the disease progresses, asymmetric cortical atrophy may be seen, especially in the posterior frontal and parietal lobes.
Levodopa ineffective in corticobasal degeneration
Corticobasal degeneration responds poorly to levodopa. Botulinum toxin has been used to help with dystonia and limb pain.
MULTIPLE SYSTEM ATROPHY
Multiple system atrophy is another atypical parkinsonian disorder, most often diagnosed in men over age 60. It is characterized by sporadic parkinsonism, cerebellar signs (involving balance and coordination), pyramidal tract dysfunction, and autonomic insufficiency in varying combinations. Two major subtypes are recognized, depending on whether the predominating presenting features are cerebellar signs or parkinsonism. In contrast to dementia with Lewy bodies, psychiatric symptoms are not a major feature, except possibly depression.41
Diagnosis requires a sporadic progressive disorder that has features of autonomic failure and poor response of parkinsonism or cerebellar ataxia to levodopa.42
Multiple system atrophy is usually not associated with dementia in the early stages, but patients develop deficits in learning, recognition, memory, and verbal fluency as the disease progresses.43 Rapid-eye-movement sleep behavior disorder has been reported in more than half of patients.44
A neurologic examination provides clues
Parkinsonian features are usually symmetric, in contrast to idiopathic Parkinson disease. These signs may include akinesia with rigidity, postural instability, hypokinetic speech, and tremor.
Cerebellar signs include nystagmus and dysarthria (speech disturbance), and gait and limb ataxia.
Pyramidal features include extensor plantar responses and hyperreflexia.
Autonomic dysfunction includes orthostatic hypotension, bladder and rectal atony, loss of sweating, urinary or fecal incontinence, and erectile dysfunction.
Electromyography may demonstrate decreased anal sphincter tone.
MRI shows atrophy of putamen and pons
Brain MRI may show atrophy of the putamen (hypointensity of the putamen with a hyperintense rim). Pons atrophy may also be present, revealing a “hot cross bun” sign in axial images. These combined findings have specificity above 90% but limited sensitivity. These signs are useful to distinguish multiple system atrophy from Parkinson dementia, but their absence does not exclude the diagnosis of multiple system atrophy.45,46
Multiple system atrophy typically responds poorly to levodopa
Levodopa may improve movement and rigidity, but many respond poorly to treatment or lose response after a few years. Fludrocortisone (Florinef) or vasoconstrictors such as midodrine (Orvaten, Proamatine) may help with orthostatic hypotension.47,48
PARKINSON DISEASE DEMENTIA
Dementia eventually develops in most patients with Parkinson disease. Older age and the akinetic rigid form of the disease are associated with higher risk. Diagnosis of idiopathic Parkinson disease before the development of dementia is essential for the diagnosis.
The Movement Disorder Society Task Force has developed new diagnostic criteria.49 Deficits must be present in at least two of the four core cognitive domains (attention, memory, executive, and visuospatial functions) and must be severe enough to affect daily functioning.
Behavioral symptoms such as affective changes, hallucinations, and apathy are common.
MRI shows characteristic brain atrophy in Parkinson disease dementia
MRI shows reduced gray matter volume in the frontal lobe in patients with Parkinson disease without dementia compared with controls. In Parkinson disease dementia, reduced volume extends to temporal, occipital, and subcortical areas. No significant volumetric differences have been observed in Parkinson dementia compared with dementia with Lewy bodies.50 A greater decrease of glucose metabolism has been found in the inferior parietal and occipital lobes in Parkinson disease dementia than in Parkinson disease without dementia.51
Rivastigmine effective for dementia
A Cochrane review supports the use of acetylcholinesterase inhibitors in patients with Parkinson disease dementia, with a positive impact on global assessment, cognitive function, behavioral disturbance, and activities of daily living rating scales.19 At this time, rivastigmine is the only FDA-approved cholinesterase inhibitor for treating Parkinson disease dementia. In clinical trials, memantine did not improve global clinical status or behavioral symptoms of dementia of Parkinson disease.51
FRONTOTEMPORAL DEMENTIA
Frontotemporal dementia frequently starts before age 65 and accounts for 20% to 50% of dementias in this age group.52 Recognition of the condition in older patients is also growing.53 Frontotemporal dementia encompasses a spectrum of dementias, including behavioral variant frontotemporal dementia, semantic dementia, and progressive nonfluent aphasia.54
Gradual onset of uncharacteristic behaviors
Accepted diagnostic criteria include core features of gradual onset, early decline in social and interpersonal conduct, early impairment of self-regulation, emotional blunting, and loss of insight. Many patients are diagnosed with psychiatric conditions. Changes reported by family and caregivers typically deviate substantially from the person’s usual behavior, such as impulsive and inappropriate behaviors or complete withdrawal and apathy.
Language sometimes affected in frontotemporal dementia
Language impairment may be present in some variants. Behavioral and language changes often accompany other forms of dementia (Alzheimer disease, vascular dementia, primary progressive aphasia), making diagnosis more challenging. Office-based testing often does not reveal any deficits, although the Frontal Behavioral Inventory may help.55 A referral to a clinical neuropsychologist may help identify and quantify cognitive impairments.
MRI shows frontotemporal lobes affected
Structural neuroimaging may not reveal abnormalities initially, but with progression, atrophy may be seen in the frontal and temporal lobes. Functional neuroimaging (positron emission tomography, brain SPECT, functional MRI) show hypometabolism in the same areas.
Treat symptoms
There are no specific FDA-approved therapies for frontotemporal dementia. Acetylcholinesterase inhibitors can help progressive nonfluent aphasia in some cases. Selective serotonin reuptake inhibitors may alleviate depressive symptoms, and low doses of atypical antipsychotic medications may help with impulsivity, disinhibition, and aggressive or disruptive behaviors.56
PRIMARY PROGRESSIVE APHASIA
Language impairment predominates
Primary progressive aphasia is a rare form of dementia in which symptoms typically develop around age 60. Pathology is varied. In a study of 60 patients with initial clinical symptoms of primary progressive aphasia, postmortem histology of brain tissue revealed various findings, including those consistent with Alzheimer pathology and motor neuron diseasetype inclusions.57
Patients typically present with expressive language problems as the primary deficit for the first 2 years of the disease, with preservation in other cognitive areas such as memory, visuospatial skills, and executive function.58 Office-based testing may overstate the severity of the dementia, given the dependence of performance on intact language.
It is important to distinguish primary progressive aphasia from other dementias that also affect language. In the frontal variant of frontotemporal dementia, the primary language problem is anomia (inability to name objects) or diminished speech output, which may be accompanied by behavioral problems. Semantic dementia affects word recognition as well as comprehension. In Alzheimer disease, language may be affected along with memory and other areas of cognitive function.
Imaging shows focal degeneration in the left hemisphere
Structural neuroimaging does not initially reveal any deficits, but later it may reveal atrophy in the frontal, perisylvian complex, and temporal areas of the left hemisphere, reflecting the focal nature of the degeneration.59 Functional neuroimaging (positron emission tomography, SPECT) may reveal hypometabolism or diminished blood flow in these areas prior to changes in structural neuroimaging.60
Other communication methods may help
There are no FDA-approved therapies for primary progressive aphasia. Off-label use of some agents (eg, selective serotonin reuptake inhibitors and small doses of antipsychotic medications) has been found useful in small trials.56 Patients may benefit from learning other forms of communication, such as using sign language, laminated cards with printed words or pictures, or artificial voice synthesizers, to express their needs.
NORMAL-PRESSURE HYDROCEPHALUS
Classic triad: Gait, cognition, incontinence
With the onset of symptoms in the sixth or seventh decade, normal-pressure hydrocephalus affects less than 1% of people age 65 and older. It represents up to 5% of dementias, although estimates are influenced by the varied criteria for diagnosis.61 It is characterized by the classic triad of gait impairment, cognitive impairment, and urinary frequency or incontinence.62
Symptoms progress over a period of years, with gait impairment often predominating. As this triad is common in the geriatric population, identifying other explanations is important. Gait impairment caused by spinal stenosis, peripheral neuropathy, or parkinsonism should be explored. Cognitive impairment could be due to depression, Alzheimer disease, or other forms of dementia. Urinary symptoms may be related to detrusor instability or an enlarged prostate.
Gait impairment initially manifests as slowing of gait, but progresses to difficulty with gait initiation. Gait tends to be wide-based (stance more than 1 foot wide).
Cognitive impairment is typically subcortical, manifested as slowed processing speed and impaired executive function. Recall and working memory may be impaired.
Enlarged ventricles seen on imaging in normal-pressure hydrocephalus
Structural neuroimaging reveals enlarged ventricles (Evan’s ratio > 0.358). This can be difficult to distinguish from ventriculomegaly due to cerebral atrophy; assessing the callosal angle on MRI may distinguish the two.63,64 Diagnosis of normal-pressure hydrocephalus can be confirmed using a cerebrospinal fluid infusion test to assess resistance of fluid to resorption.65
Treat with cerebrospinal fluid drainage
Specific tests should be performed to determine candidacy for surgery. These include a high-volume lumbar puncture (40 to 50 mL) or a trial of external lumbar drainage (10 mL per hour for 48 to 72 hours).65 Definitive treatment is surgical placement of a shunt to allow cerebrospinal fluid to drain into the atria or peritoneal cavity.
Surgery may improve gait, but cognitive symptoms often remain,66 and clinical decline may occur after the shunt is placed. Once gait dysfunction is resolved, other explanations for cognitive impairment or residual gait impairment should be considered. An underlying reason for progression of normal-pressure hydrocephalus symptoms after surgical intervention should be identified.67
RAPIDLY PROGRESSIVE DEMENTIAS
Rapidly progressive dementias are among the most challenging of dementing illnesses. They are characterized by a subacute course and an accelerated rate of decline, developing in less than 2 years. Evaluation should typically be more comprehensive than for other types of dementia. The main goal is to diagnose potentially treatable conditions, such as Hashimoto encephalopathy or paraneoplastic limbic encephalitis, and to distinguish these conditions from diseases with a very poor prognosis, such as Creutzfeldt-Jakob disease.
Creutzfeldt-Jakob disease
Creutzfeldt-Jakob disease is a fatal prion-related neurodegenerative illness. Sporadic disease is most common, but variant, familial, and iatrogenic types have been reported. The most common initial symptoms in sporadic disease are cognitive (39%), cerebellar (21%), behavioral (20%), constitutional (20%), sensory (11%), motor (9%), and visual (7%).68
Chronic neurodegenerative diseases can be misdiagnosed as Creutzfeldt-Jakob disease because of an atypical time course and multi-system neurologic findings.
The US Centers for Disease Control and Prevention has adopted criteria for diagnosing probable Creutzfeldt-Jakob disease (Table 3). Routine investigations should also not suggest an alternative diagnosis.69
Autoimmune diseases
Autoimmune conditions may present as a rapidly progressive dementia, including Hashimoto encephalopathy and antibody-mediated limbic encephalitis, either associated with cancer (paraneoplastic) or without cancer (nonparaneoplastic).
Paraneoplastic limbic encephalitis is a group of inflammatory conditions involving antibodies produced within the cerebrospinal fluid and serum resulting in neurologic symptoms. These antibodies react against proteins expressed mostly by a tumor somewhere else in the body.70
Hashimoto encephalitis is a subacute to chronic encephalopathy that may present as dementia with abnormally high levels of thyroid antibodies. The symptoms can vary from confusion to psychosis. There are two main presentations: one involves a relapsing-remitting course with stroke-like episodes (27% of patients) and the second consists of insidious onset of seizures (66% of patients).
Diagnosis involves testing for elevated anti-thyroid peroxidase and thyroglobulin antibodies. MRI findings are nonspecific. Hashimoto encephalitis responds to treatment with corticosteroids, plasmapheresis, or immunosuppressive therapy.71
- Plassman BL, Langa KM, Fisher GG, et al. Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology 2007; 29:125–132.
- Alzheimer’s Association. 2013 Alzheimer’s disease facts and figures. http://www.alz.org/downloads/facts_figures_2013.pdf. Accessed February 3, 2014.
- Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010-2050) estimated using the 2010 census. Neurology 2013; 80:1778–1783.
- Hou CE, Carlin D, Miller BL. Non-Alzheimer’s disease dementias: anatomic, clinical, and molecular correlates. Can J Psychiatry 2004; 49:164–171.
- Hachinski VC, Iliff LD, Zilhka E, et al. Cerebral blood flow in dementia. Arch Neurol 1975; 32:632–637.
- Fereshtehnejad SM, Religa D, Westman E, Aarsland D, Lökk J, Eriksdotter M. Demography, diagnostics, and medication in dementia with Lewy bodies and Parkinson’s disease with dementia: data from the Swedish Dementia Quality Registry (SveDem). Neuropsychiatr Dis Treat 2013; 9:927–935.
- Nomura T, Inoue Y, Takigawa H, Nakashima K. Comparison of REM sleep behaviour disorder variables between patients with progressive supranuclear palsy and those with Parkinson’s disease. Parkinsonism Relat Disord 2012; 18:394–396.
- Davis PH, Golbe LI, Duvoisin RC, Schoenberg BS. Risk factors for progrssive supranuclear palsy. Neurology 1988; 38:1546–1552.
- Tousi B, Schuele SU, Subramanian T. A 46-year-old woman with rigidity and frequent falls. Cleve Clin J Med 2005; 72:57–63.
- Cooper AD, Josephs KA. Photophobia, visual hallucinations, and REM sleep behavior disorder in progressive supranuclear palsy and corticobasal degeneration: a prospective study. Parkinsonism Relat Disord 2009; 15:59–61.
- Lyketsos CG, Lopez O, Jones B, Fitzpatrick AL, Breitner J, DeKosky S. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the cardiovascular health study0. JAMA 2002; 288:1475–1483.
- Knopman DS. Dementia and cerebrovascular disease. Mayo Clin Proc 2006; 81:223–230.
- Erkinjuntti T, Kurz A, Gauthier S, Bullock R, Lilienfeld S, Damaraju CV. Efficacy of galantamine in probable vascular dementia and Alzheimer’s disease combined with cerebrovascular disease: a randomised trial. Lancet 2002; 359:1283–1290.
- Sink KM, Holden KF, Yaffe K. Pharmacological treatment of neuropsychiatric symptoms of dementia: a review of the evidence. JAMA 2005; 293:596–608.
- Schneider LS, Dagerman K, Insel PS. Efficacy and adverse effects of atypical antipsychotics for dementia: meta-analysis of randomized, placebo-controlled trials. Am J Geriatr Psychiatry 2006; 14:191–210.
- McKeith IG, Dickson DW, Lowe J, et al; Consortium on DLB. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 2005; 65:1863–1872.
- Tartaglia MC, Rosen HJ, Miller BL. Neuroimaging in dementia. Neurotherapeutics 2011; 8:82–92.
- McKeith I, Del Ser T, Spano P, et al. Efficacy of rivastigmine in dementia with Lewy bodies: a randomised, double-blind, placebo-controlled international study. Lancet 2000; 356:2031–2036.
- Rolinski M, Fox C, Maidment I, McShane R. Cholinesterase inhibitors for dementia with Lewy bodies, Parkinson’s disease dementia and cognitive impairment in Parkinson’s disease. Cochrane Database Syst Rev 2012; 3:CD006504.
- Emre M, Tsolaki M, Bonucelli U, et al; on behalf of the 11018 Study Investigators. Memantine for patients with Parkinson’s disease dementia or dementia with Lewy bodies: a randomised, double-blind, placebo-controlled trial. Lancet Neurol 2010; 9:969–977.
- Kurlan R, Cummings J, Raman R, Thal L; Alzheimer’s Disease Cooperative Study Group. Quetiapine for agitation or psychosis in patients with dementia and parkinsonism. Neurology 2007; 68:1356–1363.
- Ballard C, Aarsland D, Francis P, Corbett A. Neuropsychiatric symptoms in patients with dementias associated with cortical Lewy bodies: pathophysiology, clinical features, and pharmacological management. Drugs Aging 2013; 30:603–611.
- Goetz CG, Leurgans S, Lang AE, Litvan I. Progression of gait, speech and swallowing deficits in progressive supranuclear palsy. Neurology 2003; 60:917–922.
- Kasashima S, Oda Y. Cholinergic neuronal loss in the basal forebrain and mesopontine tegmentum of progressive supranuclear palsy and corticobasal degeneration. Acta Neuropathol 2003; 105:117–124.
- Fahn S, Jankovic J, Hallett M, editors. Principles and Practice of Movement Disorders. 2nd ed. New York, NY: Elsevier/Saunders; 2011.
- Litvan I, Mangone CA, McKee A, et al. Natural history of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome) and clinical predictors of survival: a clinicopathological study. J Neurol Neurosurg Psychiatry 1996; 60:615–620.
- Boeve BF, Silber MH, Parisi JE, et al. Synucleinopathy pathology and REM sleep behavior disorder plus dementia or parkinsonism. Neurology 2003; 61:40–45.
- Pharr V, Uttl B, Stark M, Litvan I, Fantie B, Grafman J. Comparison of apraxia in corticobasal degeneration and progressive supranuclear palsy. Neurology 2001; 56:957–963.
- Williams DR, Lees AJ. What features improve the accuracy of the clinical diagnosis of progressive supranuclear palsy-parkinsonism (PSP-P)? Mov Disord 2010; 25:357–362.
- Wenning GK, Litvan I, Tolosa E. Milestones in atypical and secondary parkinsonisms. Mov Disord 2011; 26:1083–1095.
- Gallucci M, Limbucci N, Catalucci A, Caulo M. Neurodegenerative diseases. Radiol Clin North Am 2008; 46:799–817.
- Stamelou M, de Silva R, Arias-Carrión O, et al. Rational therapeutic approaches to progressive supranuclear palsy. Brain 2010; 133:1578–1590.
- Gold M, Lorenzl S, Stewart AJ, Morimoto BH, Williams DR, Gozes I. Critical appraisal of the role of davunetide in the treatment of progressive supranuclear palsy. Neuropsychiatr Dis Treat 2012; 8:85–93.
- Boeve BF, Lang AE, Litvan I. Corticobasal degeneration and its relationship to progressive supranuclear palsy and frontotemporal dementia. Ann Neurol 2003; 54(suppl 5):S15–S19.
- Armstrong MJ, Litvan I, Lang AE, et al. Criteria for the diagnosis of corticobasal degeneration. Neurology 2013; 80:496–503.
- Kompoliti K, Goetz CG, Boeve BF, et al. Clinical presentation and pharmacological therapy in corticobasal degeneration. Arch Neurol 1998; 55:957–961.
- Litvan I, Cummings JL, Mega M. Neuropsychiatric features of corticobasal degeneration. J Neurol Neurosurg Psychiatry 1998; 65:717–721.
- Pillon B, Blin J, Vidailhet M, et al. The neuropsychological pattern of corticobasal degeneration: comparison with progressive supranuclear palsy and Alzheimer’s disease. Neurology 1995; 45:1477–1483.
- Tang-Wai DF, Josephs KA, Boeve BF, Petersen RC, Parisi JE, Dickson DW. Coexistent Lewy body disease in a case of “visual variant of Alzheimer’s disease.” J Neurol Neurosurg Psychiatry 2003; 74:389.
- Tang-Wai DF, Josephs KA, Boeve BF, Dickson DW, Parisi JE, Petersen RC. Pathologically confirmed corticobasal degeneration presenting with visuospatial dysfunction. Neurology 2003; 61:1134–1135.
- Goto K, Ueki A, Shimode H, Shinjo H, Miwa C, Morita Y. Depression in multiple system atrophy: a case report. Psychiatry Clin Neurosci 2000; 54:507–511.
- Gilman S, Wenning GK, Low PA, et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology 2008; 71:670–676.
- Berent S, Giordani B, Gilman S, et al. Patterns of neuropsychological performance in multiple system atrophy compared to sporadic and hereditary olivopontocerebellar atrophy. Brain Cogn 2002; 50:194–206.
- Ghorayeb I, Yekhlef F, Chrysostome V, Balestre E, Bioulac B, Tison F. Sleep disorders and their determinants in multiple system atrophy. J Neurol Neurosurg Psychiatry 2002; 72:798–800.
- Schrag A, Kingsley D, Phatouros C, et al. Clinical usefulness of magnetic resonance imaging in multiple system atrophy. J Neurol Neurosurg Psychiatry 1998; 65:65–71.
- Massey LA, Micallef C, Paviour DC, et al. Conventional magnetic resonance imaging in confirmed progressive supranuclear palsy and multiple system atrophy. Mov Disord 2012; 27:1754–1762.
- Low PA, Gilden JL, Freeman R, Sheng KN, McElligott MA. Efficacy of midodrine vs placebo in neurogenic orthostatic hypotension. A randomized, double-blind multicenter study. Midodrine Study Group. JAMA 1997; 277:1046–1051.
- Mathias CJ, Kimber JR. Postural hypotension: causes, clinical features, investigation, and management. Annu Rev Med 1999; 50:317–336.
- Emre M, Aarsland D, Brown R, et al. Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov Disord 2007; 22:1689–1707.
- Burton EJ, McKeith IG, Burn DJ, Williams ED, O’Brien JT. Cerebral atrophy in Parkinson’s disease with and without dementia: a comparison with Alzheimer’s disease, dementia with Lewy bodies and controls. Brain 2004; 127:791–800.
- Piert M, Koeppe RA, Giordani B, Minoshima S, Kuhl DE. Determination of regional rate constants from dynamic FDG-PET studies in Parkinson’s disease. J Nucl Med 1996; 37:1115–1122.
- Ratnavalli E, Brayne C, Dawson K, Hodges JR. The prevalence of frontotemporal dementia. Neurology 2002; 58:1615–1621.
- Baborie A, Griffiths TD, Jaros E, et al. Frontotemporal dementia in elderly individuals. Arch Neurol 2012; 69:1052–1960.
- Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 1998; 51:1546–1554.
- Kertesz A, Nadkarni N, Davidson W, Thomas AW. The Frontal Behavioral Inventory in the differential diagnosis of frontotemporal dementia. J Int Neuropsychol Soc 2000; 6:460–468.
- Mendez MF, Lauterbach EC, Sampson SM; ANPA Committee on Research. An evidence-based review of the psychopathology of frontotemporal dementia: a report of the ANPA Committee on Research. J Neuropsychiatry Clin Neurosci 2008; 20:130–149.
- Kertesz A, McMonagle P, Blair M, Davidson W, Munoz DG. The evolution and pathology of frontotemporal dementia. Brain 2005; 128:1996–2005.
- Mesulam MM. Primary progressive aphasia—a language-based dementia. N Engl J Med 2003; 349:1535–1542.
- Turner RS, Kenyon LC, Trojanowski JQ, Gonatas N, Grossman M. Clinical, neuroimaging, and pathologic features of progressive nonfluent aphasia. Ann Neurol 1996; 39:166–173.
- Abe K, Ukita H, Yanagihara T. Imaging in primary progressive aphasia. Neuroradiology 1997; 39:556–559.
- Trenkwalder C, Schwarz J, Gebhard J, et al. Starnberg trial on epidemiology of parkinsonism and hypertension in the elderly. Prevalence of Parkinson’s disease and related disorders assessed by a door-to-door survey of inhabitants older than 65 years. Arch Neurol 1995; 52:1017–1022.
- Hakim S, Adams RD. The special clinical problem of symptomatic hydrocephalus with normal cerebrospinal fluid pressure. Observations on cerebrospinal fluid hydrodynamics. J Neurol Sci 1965; 2:307–327.
- Vanneste JA. Diagnosis and management of normal-pressure hydrocephalus. J Neurol 2000; 247:5–14.
- Ishii K, Kanda T, Harada A, et al. Clinical impact of the callosal angle in the diagnosis of idiopathic normal pressure hydrocephalus. Eur Radiol 2008; 18:2678–2683.
- Marmarou A, Bergsneider M, Klinge P, Relkin N, Black PM. The value of supplemental prognostic tests for the preoperative assessment of idiopathic normal-pressure hydrocephalus. Neurosurgery 2005; 57(suppl 3):S17–S28.
- Bergsneider M, Miller C, Vespa PM, Hu X. Surgical management of adult hydrocephalus. Neurosurgery 2008; 62(suppl 2):643–659.
- Malm J, Graff-Radford NR, Ishikawa M, et al. Influence of comorbidities in idiopathic normal pressure hydrocephalus—research and clinical care. A report of the ISHCSF task force on comorbidities in INPH. Fluids Barriers CNS 2013; 10:22.
- Rabinovici GD, Wang PN, Levin J, et al. First symptom in sporadic Creutzfeldt-Jakob disease. Neurology 2006; 66:286–287.
- Zerr I, Kallenberg K, Summers DM, et al. Updated clinical diagnostic criteria for sporadic Creutzfeldt-Jakob disease. Brain 2009; 132:2659–2668.
- Dalmau J, Rosenfeld MR. Paraneoplastic syndromes of the CNS. Lancet Neurol 2008; 7:327–340.
- Chong JY, Rowland LP, Utiger RD. Hashimoto encephalopathy: syndrome or myth? Arch Neurol 2003; 60:164–171.
- Plassman BL, Langa KM, Fisher GG, et al. Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology 2007; 29:125–132.
- Alzheimer’s Association. 2013 Alzheimer’s disease facts and figures. http://www.alz.org/downloads/facts_figures_2013.pdf. Accessed February 3, 2014.
- Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010-2050) estimated using the 2010 census. Neurology 2013; 80:1778–1783.
- Hou CE, Carlin D, Miller BL. Non-Alzheimer’s disease dementias: anatomic, clinical, and molecular correlates. Can J Psychiatry 2004; 49:164–171.
- Hachinski VC, Iliff LD, Zilhka E, et al. Cerebral blood flow in dementia. Arch Neurol 1975; 32:632–637.
- Fereshtehnejad SM, Religa D, Westman E, Aarsland D, Lökk J, Eriksdotter M. Demography, diagnostics, and medication in dementia with Lewy bodies and Parkinson’s disease with dementia: data from the Swedish Dementia Quality Registry (SveDem). Neuropsychiatr Dis Treat 2013; 9:927–935.
- Nomura T, Inoue Y, Takigawa H, Nakashima K. Comparison of REM sleep behaviour disorder variables between patients with progressive supranuclear palsy and those with Parkinson’s disease. Parkinsonism Relat Disord 2012; 18:394–396.
- Davis PH, Golbe LI, Duvoisin RC, Schoenberg BS. Risk factors for progrssive supranuclear palsy. Neurology 1988; 38:1546–1552.
- Tousi B, Schuele SU, Subramanian T. A 46-year-old woman with rigidity and frequent falls. Cleve Clin J Med 2005; 72:57–63.
- Cooper AD, Josephs KA. Photophobia, visual hallucinations, and REM sleep behavior disorder in progressive supranuclear palsy and corticobasal degeneration: a prospective study. Parkinsonism Relat Disord 2009; 15:59–61.
- Lyketsos CG, Lopez O, Jones B, Fitzpatrick AL, Breitner J, DeKosky S. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the cardiovascular health study0. JAMA 2002; 288:1475–1483.
- Knopman DS. Dementia and cerebrovascular disease. Mayo Clin Proc 2006; 81:223–230.
- Erkinjuntti T, Kurz A, Gauthier S, Bullock R, Lilienfeld S, Damaraju CV. Efficacy of galantamine in probable vascular dementia and Alzheimer’s disease combined with cerebrovascular disease: a randomised trial. Lancet 2002; 359:1283–1290.
- Sink KM, Holden KF, Yaffe K. Pharmacological treatment of neuropsychiatric symptoms of dementia: a review of the evidence. JAMA 2005; 293:596–608.
- Schneider LS, Dagerman K, Insel PS. Efficacy and adverse effects of atypical antipsychotics for dementia: meta-analysis of randomized, placebo-controlled trials. Am J Geriatr Psychiatry 2006; 14:191–210.
- McKeith IG, Dickson DW, Lowe J, et al; Consortium on DLB. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 2005; 65:1863–1872.
- Tartaglia MC, Rosen HJ, Miller BL. Neuroimaging in dementia. Neurotherapeutics 2011; 8:82–92.
- McKeith I, Del Ser T, Spano P, et al. Efficacy of rivastigmine in dementia with Lewy bodies: a randomised, double-blind, placebo-controlled international study. Lancet 2000; 356:2031–2036.
- Rolinski M, Fox C, Maidment I, McShane R. Cholinesterase inhibitors for dementia with Lewy bodies, Parkinson’s disease dementia and cognitive impairment in Parkinson’s disease. Cochrane Database Syst Rev 2012; 3:CD006504.
- Emre M, Tsolaki M, Bonucelli U, et al; on behalf of the 11018 Study Investigators. Memantine for patients with Parkinson’s disease dementia or dementia with Lewy bodies: a randomised, double-blind, placebo-controlled trial. Lancet Neurol 2010; 9:969–977.
- Kurlan R, Cummings J, Raman R, Thal L; Alzheimer’s Disease Cooperative Study Group. Quetiapine for agitation or psychosis in patients with dementia and parkinsonism. Neurology 2007; 68:1356–1363.
- Ballard C, Aarsland D, Francis P, Corbett A. Neuropsychiatric symptoms in patients with dementias associated with cortical Lewy bodies: pathophysiology, clinical features, and pharmacological management. Drugs Aging 2013; 30:603–611.
- Goetz CG, Leurgans S, Lang AE, Litvan I. Progression of gait, speech and swallowing deficits in progressive supranuclear palsy. Neurology 2003; 60:917–922.
- Kasashima S, Oda Y. Cholinergic neuronal loss in the basal forebrain and mesopontine tegmentum of progressive supranuclear palsy and corticobasal degeneration. Acta Neuropathol 2003; 105:117–124.
- Fahn S, Jankovic J, Hallett M, editors. Principles and Practice of Movement Disorders. 2nd ed. New York, NY: Elsevier/Saunders; 2011.
- Litvan I, Mangone CA, McKee A, et al. Natural history of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome) and clinical predictors of survival: a clinicopathological study. J Neurol Neurosurg Psychiatry 1996; 60:615–620.
- Boeve BF, Silber MH, Parisi JE, et al. Synucleinopathy pathology and REM sleep behavior disorder plus dementia or parkinsonism. Neurology 2003; 61:40–45.
- Pharr V, Uttl B, Stark M, Litvan I, Fantie B, Grafman J. Comparison of apraxia in corticobasal degeneration and progressive supranuclear palsy. Neurology 2001; 56:957–963.
- Williams DR, Lees AJ. What features improve the accuracy of the clinical diagnosis of progressive supranuclear palsy-parkinsonism (PSP-P)? Mov Disord 2010; 25:357–362.
- Wenning GK, Litvan I, Tolosa E. Milestones in atypical and secondary parkinsonisms. Mov Disord 2011; 26:1083–1095.
- Gallucci M, Limbucci N, Catalucci A, Caulo M. Neurodegenerative diseases. Radiol Clin North Am 2008; 46:799–817.
- Stamelou M, de Silva R, Arias-Carrión O, et al. Rational therapeutic approaches to progressive supranuclear palsy. Brain 2010; 133:1578–1590.
- Gold M, Lorenzl S, Stewart AJ, Morimoto BH, Williams DR, Gozes I. Critical appraisal of the role of davunetide in the treatment of progressive supranuclear palsy. Neuropsychiatr Dis Treat 2012; 8:85–93.
- Boeve BF, Lang AE, Litvan I. Corticobasal degeneration and its relationship to progressive supranuclear palsy and frontotemporal dementia. Ann Neurol 2003; 54(suppl 5):S15–S19.
- Armstrong MJ, Litvan I, Lang AE, et al. Criteria for the diagnosis of corticobasal degeneration. Neurology 2013; 80:496–503.
- Kompoliti K, Goetz CG, Boeve BF, et al. Clinical presentation and pharmacological therapy in corticobasal degeneration. Arch Neurol 1998; 55:957–961.
- Litvan I, Cummings JL, Mega M. Neuropsychiatric features of corticobasal degeneration. J Neurol Neurosurg Psychiatry 1998; 65:717–721.
- Pillon B, Blin J, Vidailhet M, et al. The neuropsychological pattern of corticobasal degeneration: comparison with progressive supranuclear palsy and Alzheimer’s disease. Neurology 1995; 45:1477–1483.
- Tang-Wai DF, Josephs KA, Boeve BF, Petersen RC, Parisi JE, Dickson DW. Coexistent Lewy body disease in a case of “visual variant of Alzheimer’s disease.” J Neurol Neurosurg Psychiatry 2003; 74:389.
- Tang-Wai DF, Josephs KA, Boeve BF, Dickson DW, Parisi JE, Petersen RC. Pathologically confirmed corticobasal degeneration presenting with visuospatial dysfunction. Neurology 2003; 61:1134–1135.
- Goto K, Ueki A, Shimode H, Shinjo H, Miwa C, Morita Y. Depression in multiple system atrophy: a case report. Psychiatry Clin Neurosci 2000; 54:507–511.
- Gilman S, Wenning GK, Low PA, et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology 2008; 71:670–676.
- Berent S, Giordani B, Gilman S, et al. Patterns of neuropsychological performance in multiple system atrophy compared to sporadic and hereditary olivopontocerebellar atrophy. Brain Cogn 2002; 50:194–206.
- Ghorayeb I, Yekhlef F, Chrysostome V, Balestre E, Bioulac B, Tison F. Sleep disorders and their determinants in multiple system atrophy. J Neurol Neurosurg Psychiatry 2002; 72:798–800.
- Schrag A, Kingsley D, Phatouros C, et al. Clinical usefulness of magnetic resonance imaging in multiple system atrophy. J Neurol Neurosurg Psychiatry 1998; 65:65–71.
- Massey LA, Micallef C, Paviour DC, et al. Conventional magnetic resonance imaging in confirmed progressive supranuclear palsy and multiple system atrophy. Mov Disord 2012; 27:1754–1762.
- Low PA, Gilden JL, Freeman R, Sheng KN, McElligott MA. Efficacy of midodrine vs placebo in neurogenic orthostatic hypotension. A randomized, double-blind multicenter study. Midodrine Study Group. JAMA 1997; 277:1046–1051.
- Mathias CJ, Kimber JR. Postural hypotension: causes, clinical features, investigation, and management. Annu Rev Med 1999; 50:317–336.
- Emre M, Aarsland D, Brown R, et al. Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov Disord 2007; 22:1689–1707.
- Burton EJ, McKeith IG, Burn DJ, Williams ED, O’Brien JT. Cerebral atrophy in Parkinson’s disease with and without dementia: a comparison with Alzheimer’s disease, dementia with Lewy bodies and controls. Brain 2004; 127:791–800.
- Piert M, Koeppe RA, Giordani B, Minoshima S, Kuhl DE. Determination of regional rate constants from dynamic FDG-PET studies in Parkinson’s disease. J Nucl Med 1996; 37:1115–1122.
- Ratnavalli E, Brayne C, Dawson K, Hodges JR. The prevalence of frontotemporal dementia. Neurology 2002; 58:1615–1621.
- Baborie A, Griffiths TD, Jaros E, et al. Frontotemporal dementia in elderly individuals. Arch Neurol 2012; 69:1052–1960.
- Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 1998; 51:1546–1554.
- Kertesz A, Nadkarni N, Davidson W, Thomas AW. The Frontal Behavioral Inventory in the differential diagnosis of frontotemporal dementia. J Int Neuropsychol Soc 2000; 6:460–468.
- Mendez MF, Lauterbach EC, Sampson SM; ANPA Committee on Research. An evidence-based review of the psychopathology of frontotemporal dementia: a report of the ANPA Committee on Research. J Neuropsychiatry Clin Neurosci 2008; 20:130–149.
- Kertesz A, McMonagle P, Blair M, Davidson W, Munoz DG. The evolution and pathology of frontotemporal dementia. Brain 2005; 128:1996–2005.
- Mesulam MM. Primary progressive aphasia—a language-based dementia. N Engl J Med 2003; 349:1535–1542.
- Turner RS, Kenyon LC, Trojanowski JQ, Gonatas N, Grossman M. Clinical, neuroimaging, and pathologic features of progressive nonfluent aphasia. Ann Neurol 1996; 39:166–173.
- Abe K, Ukita H, Yanagihara T. Imaging in primary progressive aphasia. Neuroradiology 1997; 39:556–559.
- Trenkwalder C, Schwarz J, Gebhard J, et al. Starnberg trial on epidemiology of parkinsonism and hypertension in the elderly. Prevalence of Parkinson’s disease and related disorders assessed by a door-to-door survey of inhabitants older than 65 years. Arch Neurol 1995; 52:1017–1022.
- Hakim S, Adams RD. The special clinical problem of symptomatic hydrocephalus with normal cerebrospinal fluid pressure. Observations on cerebrospinal fluid hydrodynamics. J Neurol Sci 1965; 2:307–327.
- Vanneste JA. Diagnosis and management of normal-pressure hydrocephalus. J Neurol 2000; 247:5–14.
- Ishii K, Kanda T, Harada A, et al. Clinical impact of the callosal angle in the diagnosis of idiopathic normal pressure hydrocephalus. Eur Radiol 2008; 18:2678–2683.
- Marmarou A, Bergsneider M, Klinge P, Relkin N, Black PM. The value of supplemental prognostic tests for the preoperative assessment of idiopathic normal-pressure hydrocephalus. Neurosurgery 2005; 57(suppl 3):S17–S28.
- Bergsneider M, Miller C, Vespa PM, Hu X. Surgical management of adult hydrocephalus. Neurosurgery 2008; 62(suppl 2):643–659.
- Malm J, Graff-Radford NR, Ishikawa M, et al. Influence of comorbidities in idiopathic normal pressure hydrocephalus—research and clinical care. A report of the ISHCSF task force on comorbidities in INPH. Fluids Barriers CNS 2013; 10:22.
- Rabinovici GD, Wang PN, Levin J, et al. First symptom in sporadic Creutzfeldt-Jakob disease. Neurology 2006; 66:286–287.
- Zerr I, Kallenberg K, Summers DM, et al. Updated clinical diagnostic criteria for sporadic Creutzfeldt-Jakob disease. Brain 2009; 132:2659–2668.
- Dalmau J, Rosenfeld MR. Paraneoplastic syndromes of the CNS. Lancet Neurol 2008; 7:327–340.
- Chong JY, Rowland LP, Utiger RD. Hashimoto encephalopathy: syndrome or myth? Arch Neurol 2003; 60:164–171.
KEY POINTS
- Vascular dementia presents as a sudden, stepwise progression of cognitive deficits.
- Lewy body dementia often involves prominent visual hallucinations.
- Progressive supranuclear palsy starts with gait and balance problems caused by downward-gaze palsy.
- Many neurodegenerative conditions involve parkinsonism, but unlike Parkinson disease, they do not tend to respond well to levodopa, and dementia develops early.
- Corticobasal degeneration involves markedly asymmetric parkinsonism.
- Frontotemporal dementia involves dramatic behavior changes, including inappropriate impulsivity and complete apathy.
- Patients with rapidly progressive dementia should be evaluated for a treatable condition such as antibody-mediated encephalitis.
Managing acute coronary syndromes: Decades of progress
Most decisions for managing acute coronary syndromes can be based on ample data from large randomized trials with hard clinical end points, so there is little reason to provide care that is not evidence-based.
This article reviews some of the trials that provide guidance on diagnosing and managing acute coronary syndromes, including the timing of reperfusion and adjunctive therapies in different situations.
MOST ACUTE CORONARY SYNDROMES ARE NON-ST-ELEVATION CONDITIONS
Acute coronary syndromes range from unstable angina and non-ST-elevation myocardial infarction (NSTEMI) to ST-elevation MI (STEMI), reflecting a continuum of severity of coronary stenosis. The degree of coronary occlusion may ultimately determine whether a patient has unstable angina or MI with or without ST elevation.1
The substrate for all of these is vulnerable plaque. Angiographic studies have indicated that in many cases medium-size plaques (30%–40% stenosis) are more likely to rupture than larger, more obstructive ones. Moderate plaques may be vulnerable because they are less mature, with a large lipid core and a thin cap prone to rupture or erode, exposing the thrombogenic subendothelial components.2
Because the vulnerability of a coronary plaque may not correlate with the severity of stenosis before the plaque ruptures, stress tests and symptoms may not predict the risk of MI. The key role of thrombosis in the pathogenesis also highlights the importance of antithrombotic therapy in the acute phases of acute coronary syndromes, which can significantly reduce mortality and morbidity rates.
Perhaps because of the widespread use of aspirin and statins, most patients who currently present with an acute coronary syndrome have either unstable angina or NSTEMI: of about 1.57 million hospital admissions in 2004 for acute coronary syndromes, for example, only 330,000 (21%) were for STEMI.3
DIAGNOSING ACUTE CORONARY SYNDROME
Symptoms may not be classic
The classic symptoms of acute coronary syndromes are intense, oppressive chest pressure radiating to the left arm, but nearly any discomfort “between the nose and navel” (eg, including the jaw, arm, and epigastric and abdominal areas) may be an acute coronary syndrome. Associated symptoms may include chest heaviness or burning, radiation to the jaw, neck, shoulder, back, or arms, and dyspnea.
Particularly in older, female, postoperative, or diabetic patients, the presentation may be atypical or “silent,” including nausea or vomiting; breathlessness; sweating; arrhythmias; or light-headedness. Especially in these groups, symptoms may be mild or subtle, and acute coronary syndrome may manifest only as “not feeling well.”
The differential diagnosis of acute coronary syndromes is broad. Most important to immediately consider are pulmonary embolism and aortic dissection, as they are life-threatening and are treated differently from acute coronary syndromes. Otherwise, it is best to err on the side of caution and treat for an acute coronary syndrome until it is proven otherwise.
Electrocardiography is critical
Electrocardiography (ECG) gives valuable information about the location, extent, and prognosis of infarction, and it is critically important for distinguishing STEMI from NSTEMI, with ST elevation classically diagnostic of complete coronary occlusion. Q waves can occur early and do not necessarily signify completed infarction, as traditionally thought. ST depression or T inversion indicates that total coronary occlusion is unlikely unless they are in a pattern of circumflex infarct associated with an enlarging R wave in lead V1. An ST elevation in RV4 indicates right ventricular infarction.
The appearance on ECG may evolve over time, so a patient with atypical symptoms and a nonspecific electrocardiogram should be observed for 24 hours or until more specific criteria develop.
Biomarkers in NSTEMI
In MI, cardiac troponin levels begin to rise about 3 hours after the onset of chest pain, and elevations can last for up to 14 days. Levels can also be mildly elevated chronically in patients with renal dysfunction, so positive biomarker tests in that population should be interpreted cautiously.
For STEMI, the opportunity to reperfuse is lost if one waits for cardiac biomarkers to become elevated. But for NSTEMI, they are highly sensitive and specific for identifying patients at high risk and determining who should be treated aggressively. Patients who are biomarker-negative have a better prognosis than patients with identical symptoms and electrocardiograms who are biomarker-positive.
MI is currently defined as a rise in any biomarker (usually troponin) above the 99th percentile for a reference population, with at least one of the following:
- Ischemic symptoms
- New ST/T changes or left bundle branch block
- Pathologic Q waves
- Loss of myocardium or abnormal wall motion seen by imaging
- Intracoronary thrombus.
REPERFUSION FOR ACUTE STEMI
Because acute coronary syndromes have a common pathophysiology, for the most part, lessons from clinical trials in one syndrome are relevant to the others. However, important differences exist regarding the need for immediate reperfusion in STEMI, since in most cases these patients have total rather than partial occlusion.
Fibrinolysis has limitations
The standard of management for STEMI is immediate reperfusion. The goal is to interrupt the wave front of myocardial necrosis, salvage threatened myocardium, and ultimately improve survival.
Five placebo-controlled trials showed a 30% reduction in the death rate in patients who received fibrinolytic therapy within 6 to 12 hours of presentation.4
Patients with ST elevation or with new bundle branch block benefit most from fibrinolytic therapy. Those with ST depression, T inversion, or nonspecific changes on ECG do not benefit; they probably do not have complete coronary occlusion, so the prothrombotic or platelet-activating effects of fibrinolytic therapy may make them worse.5 Further, fibrinolytic therapy poses the risk of intracranial hemorrhage, which, although rare (occurring in up to 1% of cases depending on the drug regimen), is a devastating complication.
In general, absolute contraindications to fibrinolysis include intracranial abnormalities, hemorrhage, and head trauma. An important relative contraindication is uncontrolled blood pressure (> 180/110 mm Hg at any point during hospitalization, including during the immediate presentation). Studies show that even if blood pressure can be controlled, the risk of intracranial hemorrhage is substantially higher, although the risk may not outweigh the benefit of reperfusion, particularly for large infarctions when percutaneous coronary intervention (PCI) is not available as an alternative to fibrinolysis.
Prompt PCI is preferable to fibrinolysis
If PCI is available on site, there is nearly no role for fibrinolytic therapy. PCI is better than fibrinolytic therapy in terms of the degree of reperfusion, reocclusion, MI recurrence, and mortality rate, and it poses little or no risk of intracranial hemorrhage.6
For either fibrinolytic therapy or percutaneous therapy, “time is muscle”: the longer the ischemic time, the higher the mortality rate (relative risk = 1.075 for every 30 minutes of delay, P = .041).7
At centers that do not have PCI on site, studies (mainly from Europe) have shown that it is better to transport the patient for PCI than to give immediate fibrinolytic therapy.7,8 But because the centers studied tended to have short transport times (usually 40 minutes or less), it is uncertain whether the results are applicable throughout the United States.
The delay between symptom onset and presentation is also relevant. Reperfusion within the first 1 to 2 hours after the onset of symptoms provides the greatest degree of myocardial salvage and of reduction in the risk of death; the extent of benefit thereafter is substantially less. As a result, patients who present very early after symptom onset have the most to lose if their reperfusion is delayed by even a few more hours, whereas patients who have already experienced several hours of pain are affected less by additional delay.9 Thus, patients presenting within the “golden” 1 or 2 hours after symptoms begin should be considered for fibrinolytic therapy if transfer for PCI cannot be done expeditiously. It is important for hospitals without PCI available on site to have a system in place for rapid transport of patients when needed.
Guidelines advise that patients with STEMI should undergo PCI rather than receive fibrinolytic therapy as long as PCI is available within 90 minutes of first medical contact. Otherwise, fibrinolysis should be started within 30 minutes.10 For patients who present several hours after symptom onset, PCI may still be preferable even if the transport time is somewhat longer.
PCI after fibrinolytic therapy
In prior decades, PCI immediately after fibrinolytic therapy was associated with an increased risk of bleeding complications and reinfarction. That has changed with improvements in equipment and antithrombotic therapy.
Two large trials conclusively found that routinely transferring high-risk patients for PCI immediately after receiving fibrinolytic therapy (combined half-dose reteplase [Retavase] and abciximab [ReoPro]11 or full-dose tenecteplase [TNKase]12) resulted in much lower rates of ischemic end points without an increase in bleeding complications compared with transferring patients only for rescue PCI after fibrinolytic therapy.
Routine transfer is now the standard of care for high-risk patients after fibrinolytic therapy and probably is best for all patients after an MI.
MANAGING NSTEMI AND UNSTABLE ANGINA
For patients with NSTEMI, immediate reperfusion is usually not required, although initial triage for “early invasive” vs “initial conservative” management must be done early in the hospital course. Randomized trials have evaluated these two approaches, with most studies in the contemporary era reporting improved outcomes with an early invasive approach.
The TACTICS trial,13 the most important of these, enrolled more than 2,200 patients with unstable angina or NSTEMI and randomized them to an early invasive strategy or a conservative strategy. Overall, results were better with the early invasive strategy.
The ICTUS trial.14 Although several studies showed that an early invasive approach was better, the most recent study using the most modern practices—the ICTUS trial—did not find that it reduced death rates. Most patients eventually underwent angiography and revascularization, but not early on. However, all studies showed that rates of recurrent unstable angina and hospitalization were reduced by an early invasive approach, so revascularization does have a role in stabilizing the patient. But in situations of aggressive medical management with antithrombotic and other therapies, an early conservative approach may be an appropriate alternative for many patients.15
The selection of an invasive vs a conservative approach should include a consideration of risk, which can be estimated using a number of criteria, including the Thrombolysis in Myocardial Infarction (TIMI) or the GRACE risk score. When risk was stratified using the TIMI risk score,16 in the TACTICS trial, the higher the risk score, the more likely patients were to benefit from early revascularization.
When an invasive approach is chosen, it does not appear necessary to take patients to catheterization immediately (within 2–24 hours) compared with later during the hospital course.
The TIMACS trial,17 with more than 3,000 patients, tested the benefits of very early vs later revascularization for patients with NSTEMI and unstable angina. Early intervention did not significantly improve outcomes for the primary composite end point of death, MI, and stroke in the overall population enrolled in the trial, but when the secondary end point of refractory ischemia was added in, early intervention was found to be beneficial overall. Moreover, when stratified by risk, high-risk patients significantly benefited from early intervention for the primary end point.
Guidelines for NSTEMI and unstable angina continue to prefer an early invasive strategy, particularly for high-risk patients, although a conservative strategy is considered acceptable if patients receive intensive evidence-based medical therapy and remain clinically stable.18
ANTITHROMBOTIC THERAPIES
Once a revascularization strategy has been chosen, adjunctive therapies should be considered. The most important are the antithrombotic therapies.
Many drugs target platelet activity. Most important are the thromboxane inhibitor aspirin, the adenosine diphosphate (ADP) receptor antagonists clopidogrel (Plavix), prasugrel (Effient), and ticagrelor (Brilinta), and the glycoprotein (GP) IIb/IIIa antagonists abciximab and eptifibatide (Integrilin). Others, such as thrombin receptor antagonists, are under investigation.19
Aspirin for secondary prevention
Evidence is unequivocal for the benefit of aspirin therapy in patients with established or suspected vascular disease.
The ISIS-2 trial20 compared 35-day mortality rates in 16,000 patients with STEMI who were given aspirin, streptokinase, combined streptokinase and aspirin, or placebo. Mortality rates were reduced by aspirin compared with placebo by an extent similar to that achieved with streptokinase, with a further reduction when aspirin and streptokinase were given together.
Therefore, patients with STEMI should be given aspirin daily indefinitely unless they have true aspirin allergy. The dose is 165 to 325 mg initially and 75 to 162 mg daily thereafter.
For NSTEMI and even for secondary prevention in less-acute situations, a number of smaller trials also provide clear evidence of benefit from aspirin therapy.
The CURRENT-OASIS 7 trial21 showed that low maintenance dosages of aspirin (75–100 mg per day) resulted in the same incidence of ischemic end points (cardiovascular death, MI, or stroke) as higher dosages. Although rates of major bleeding events did not differ, a higher rate of gastrointestinal bleeding was evident at just 30 days in patients taking the higher doses. This large trial clearly established that there is no advantage to daily aspirin doses of more than 100 mg.
DUAL ANTIPLATELET THERAPY IS STANDARD
Standard practice now is to use aspirin plus another antiplatelet agent that acts by inhibiting either the ADP receptor (for which there is the most evidence) or the GP IIb/IIIa receptor (which is becoming less used). Dual therapy should begin early in patients with acute coronary syndrome.
Clopidogrel: Well studied with aspirin
The most commonly used ADP antagonist is clopidogrel, a thienopyridine. Much evidence exists for its benefit.
The CURE trial22 randomized more than 12,000 patients with NSTEMI or unstable angina to aspirin plus either clopidogrel or placebo. The incidence of the combined end point of MI, stroke, and cardiovascular death was 20% lower in the clopidogrel group than in the placebo group over 12 months of follow-up. The benefit of clopidogrel began to occur within the first 24 hours after randomization, with a 33% relative risk reduction in the combined end point of cardiovascular death, MI, stroke, and severe ischemia, demonstrating the importance of starting this agent early in the hospital course.
COMMIT23 found a benefit in adding clopidogrel to aspirin in patients with acute STEMI. Although it was only a 30-day trial, significant risk reduction was found in the dual-therapy group for combined death, stroke, or reinfarction. The results of this brief trial were less definitive, but the pathophysiology was similar to non-ST-elevation acute coronary syndromes, so it is reasonable to extrapolate the long-term findings to this setting.
The CURRENT-OASIS 7 trial21 randomized more than 25,000 patients to either clopidogrel in a double dosage (600 mg load, 150 mg/day for 6 days, then 75 mg/day) or standard dosage (300 mg load, 75 mg/day thereafter). Although no overall benefit was found for the higher dosage, a subgroup of more than 17,000 patients who underwent PCI after randomization had a lower risk of developing stent thrombosis. On the other hand, higher doses of clopidogrel caused more major bleeding events.
Ticagrelor and prasugrel: New alternatives to clopidogrel
The principal limitation of clopidogrel is its metabolism. It is a prodrug, ie, it is not active as taken and must be converted to its active state by cytochrome P450 enzymes in the liver. Patients who bear certain polymorphisms in the genes for these enzymes or who are taking other medications that affect this enzymatic pathway may derive less platelet inhibition from the drug, leading to considerable patient-to-patient variability in the degree of antiplatelet effect.
Alternatives to clopidogrel have been developed that inhibit platelets more intensely, are activated more rapidly, and have less interpatient variability. Available now are ticagrelor and prasugrel.24 Like clopidogrel, prasugrel is absorbed as an inactive prodrug, but it is efficiently metabolized by esterases to an active form, and then by a simpler step within the liver to its fully active metabolite.25 Ticagrelor is active as absorbed.26
Pharmacodynamically, the two drugs perform almost identically and much faster than clopidogrel, with equilibrium platelet inhibition reached in less than 1 hour. The degree of platelet inhibition is also more—sometimes twice as much—with the new drugs compared with clopidogrel, and the effect is much more consistent between patients.
Both clopidogrel and prasugrel permanently inhibit the platelet ADP receptor, and 3 to 7 days are therefore required for their antiplatelet effects to completely wear off. In contrast, ticagrelor is a reversible inhibitor and its effects wear off more rapidly. Despite achieving a much higher level of platelet inhibition than clopidogrel, ticagrelor’s activity falls below that of clopidogrel’s by 48 hours of discontinuing the drugs.
Trial of prasugrel vs clopidogrel
The TRITON-TIMI 38 trial27 enrolled more than 13,000 patients with acute coronary syndromes, randomized to receive, either prasugrel or clopidogrel, in addition to aspirin. The patients were all undergoing PCI, so the findings do not apply to patients treated medically with an early conservative approach. The study drug was given only after the decision was made to perform PCI in patients with non-ST-elevation acute coronary syndrome (but given immediately for patients with STEMI, because nearly all those patients undergo PCI).
Prasugrel was clearly beneficial, with a significant 20% lower rate of the combined end point of cardiovascular death, MI, and stroke at 15 months. However, bleeding risk was higher with prasugrel (2.4% vs 1.8%, hazard ratio 1.32, 95% confidence interval 1.02–1.68, P = .03). Looking at individual end points, the advantages of prasugrel were primarily in reducing rates of stent thrombosis and nonfatal MI. Death rates with the two drugs were equivalent, possibly because of the higher risk of bleeding with prasugrel. Bleeding in the prasugrel group was particularly increased in patients who underwent bypass surgery; more patients also needed transfusion.
Subgroup analysis showed that patients with a history of stroke or transient ischemic attack had higher rates of ischemic and bleeding events with prasugrel than with clopidogrel, leading to these being labeled as absolute contraindications to prasugrel. Patients over age 75 or who weighed less than 60 kg experienced excess bleeding risk that closely matched the reduction in ischemic event rates and thus did not have a net benefit with prasugrel.
Trial of ticagrelor vs clopidogrel
The PLATO trial28 included 18,000 patients, of whom 65% underwent revascularization and 35% were treated medically. The drug—clopidogrel or ticagrelor—was given in addition to aspirin at randomization (within 24 hours of symptom onset); this more closely follows clinical practice, in which dual antiplatelet therapy is started as soon as possible. This difference makes the PLATO study more relevant to practice for patients with non-ST-elevation acute coronary syndrome. Also, because they gave the drugs to all patients regardless of whether they were to undergo PCI, this study likely had a higher-risk population, which may be refected in the higher mortality rate at 30 days (5.9% in the clopidogrel group in the PLATO study vs 3.2% in the clopidogrel group in the TRITON study).
Another important difference between the trials testing prasugrel and ticagrelor is that patients who had already received a thienopyridine were excluded from the prasugrel trial but not from the ticagrelor trial. Nearly half the patients in the ticagrelor group were already taking clopidogrel. The clinical implication is that for patients who arrive from another facility and already have been given clopidogrel, it is safe to give ticagrelor. There is limited information about whether that is also true for prasugrel, although there is no known reason why the safety of adding prasugrel to clopidogrel should be different from that of ticagrelor.
The rate of ischemic events was 20% lower in the ticagrelor group than in the clopidogrel group, importantly including reductions in the incidence of death, MI, and stent thrombosis. There was no increase with ticagrelor compared with clopidogrel in bleeding associated with coronary artery bypass graft surgery, likely because of the more rapid washout of the ticagrelor effect, or in the need for blood transfusions. However, the rate of bleeding unrelated to coronary artery bypass was about 20% higher with ticagrelor.
In summary, more intense platelet inhibition reduces the risk of ischemic events, but, particularly for the irreversible inhibitor prasugrel, at the cost of a higher risk of bleeding. In general, the net benefit of these agents in preventing the irreversible complications of MI and (in the case of ticagrelor) death favor the use of the more intense ADP inhibitors in appropriate patients. Ticagrelor is indicated in patients with acute coronary syndromes undergoing invasive or conservative management; prasugrel is indicated in patients undergoing PCI, but contraindicated in patients with a previous stroke or transient ischemic event. Neither drug is indicated in patients undergoing elective PCI outside the setting of acute coronary syndromes, although these agents may be appropriate in patients with intolerance or allergy to clopidogrel.
Glycoprotein IIb/IIIa antagonists for select cases only
GP IIb/IIIa antagonists such as abciximab were previously used more commonly than they are today. Now, with routine pretreatment using thienopyridines, their role in acute coronary syndromes is less clear. They still play a role when routine dual antiplatelet therapy is not used, when prasugrel or ticagrelor is not used, and when heparin rather than an alternative antithrombin agent is used.
A meta-analysis29 of 3,755 patients showed a clear reduction in ischemic complications with abciximab as an adjunct to primary PCI for STEMI in patients treated with heparin.
Kastrati et al30 found that patients with non-ST-elevation acute coronary syndromes benefited from abciximab at the time of PCI with heparin, even though they had been routinely pretreated with clopidogrel. However, benefits were seen only in high-risk patients who had presented with elevated troponins.
On the other hand, the role of GP IIb/IIIa blockade for “upstream” medical management in patients with acute coronary syndromes has been eroded by several studies.
The ACUITY trial31 randomized more than 9,000 patients to receive either routine treatment with a GP IIb/IIIa inhibitor before angiography or deferred selective use in the catheterization laboratory only for patients undergoing PCI. No significant differences were found in rates of MI and death.
The Early ACS trial32 compared early routine eptifibatide vs delayed, provisional eptifibatide in 9,492 patients with acute coronary syndromes without ST elevation and who were assigned to an invasive strategy. The early-eptifibatide group received two boluses and an infusion of eptifibatide before angiography; the others received a placebo infusion, with provisional eptifibatide after angiography if the patient underwent PCI and was deemed at high risk. No significant difference in rates of death or MI were noted, and the early-eptifibatide group had significantly higher rates of bleeding and need for transfusion.
The FINESSE trial33 also discredited “facilitating” PCI by giving GP IIb/IIIa antagonists in patients with STEMI before arrival in the catheterization laboratory, with no benefit to giving abciximab ahead of time vs in the catheterization laboratory, and with an increased risk of bleeding complications.
These studies have helped narrow the use of GP IIb/IIIa inhibitors to the catheterization laboratory in conjunction with heparin anticoagulation (as compared with bivalirudin [Angiomax]; see below) and only in select or high-risk cases. These drugs are indicated in the medical phase of management only if patients cannot be stabilized by aspirin or ADP inhibition.
NEWER ANTITHROMBOTICS: ADVANTAGES UNCLEAR
The complex coagulation cascade has a number of components, but only a few are targeted by drugs that are approved and recommended: fondaparinux (Arixtra) and oral factor Xa inhibitors affect the prothrombinase complex (including factor X); bivalirudin and oral factor IIa inhibitors affect thrombin; and heparin and the low-molecular-weight heparins inhibit both targets.
Low-molecular-weight heparins
The SYNERGY trial34 randomized nearly 10,000 patients with non-ST-elevation acute coronary syndromes at high risk for ischemic cardiac complications managed with an invasive approach to either the low-molecular-weight heparin enoxaparin (Lovenox) or intravenous unfractionated heparin immediately after enrollment. Most patients underwent catheterization and revascularization. No clinical advantage was found for enoxaparin, and bleeding complications were increased.
The EXTRACT-TIMI 25 trial35 randomized more than 20,000 patients with STEMI who were about to undergo fibrinolysis to receive either enoxaparin throughout hospitalization (average of 8 days) or unfractionated heparin for at least 48 hours. The enoxaparin group had a lower rate of recurrent MI, but it was unclear if the difference was in part attributable to the longer therapy time. The enoxaparin group also had more bleeding.
Fondaparinux
The OASIS-5 trial36,37 compared enoxaparin and fondaparinux, an exclusive factor Xa inhibitor, in more than 20,000 patients with unstable angina or NSTEMI. Fondaparinux was associated with a lower risk of death and reinfarction as well as fewer bleeding events. However, the benefits were almost exclusively in patients treated medically. In those undergoing PCI within the first 8 days, no benefit was found, although there was still a significant reduction in major bleeding events. Catheter thrombosis was also increased in patients taking fondaparinux, but only in those who did not receive adequate unfractionated heparin treatment before PCI.
Bivalirudin superior at time of catheterization
The most significant advance in antithrombotic therapy for patients with acute coronary syndromes is bivalirudin. This drug has a clear role only in the catheterization laboratory, where patients can be switched to it from heparin, low-molecular-weight heparin, or fondaparinux.
Three trials38–40 evaluated the drug in a total of more than 20,000 patients receiving invasive management of coronary artery disease undergoing PCI for elective indications, NSTEMI, or STEMI.
Results were remarkably similar across the three trials. Patients who were treated with bivalirudin alone had the same rate of ischemic end points at 30 days as those receiving heparin plus a GP IIb/IIIa inhibitor, but bivalirudin was associated with a consistent and significant 40% to 50% lower bleeding risk. For the highest-risk patients, those with STEMI, the bivalirudin group also had a significantly lower risk of death at 1 year.41
OTHER DRUGS: EARLY TREATMENT NO LONGER ROUTINE
Most data for the use of therapies aside from antithrombotics are from studies of patients with STEMI, but findings can logically be extrapolated to those with non-ST-elevation acute coronary syndromes.
Beta-blockers: Cardiogenic shock a risk
For beta-blockers, many historical trials were done in stable coronary disease, but there are no large trials in the setting of NSTEMI or unstable angina, and only recently have there been large trials for STEMI. Before the availability of recent evidence, standard practice was to treat STEMI routinely with intravenous metoprolol (Lopressor) and then oral metoprolol.
When large studies were finally conducted, the results were sobering.
COMMIT.42 Nearly 46,000 patients with suspected acute MI were randomized to receive either metoprolol (up to 15 mg intravenously, then 200 mg by mouth daily until discharge or for up to 4 weeks in the hospital) or placebo. Surprisingly, although rates of reinfarction and ventricular fibrillation were lower with metoprolol, a higher risk of cardiogenic shock with early beta-blockade offset these benefits and the net mortality rate was not reduced. This study led to a reduction in the early use of beta-blockers in patients with STEMI.
The standard of care has now shifted from beta-blockers in everyone as early as possible after MI to being more cautious in patients with contraindications, including signs of heart failure or a low-output state, or even in those of advanced age or with borderline low blood pressure or a high heart rate. Patients who present late and therefore may have a larger infarct are also at higher risk.
Although the goal should be to ultimately discharge patients on beta-blocker therapy after an MI, there should be no rush to start one early.
Carvedilol now preferred after STEMI
The CAPRICORN trial43 randomized nearly 2,000 patients following MI with left ventricular dysfunction (an ejection fraction of 40% or below) to either placebo or the beta-blocker carvedilol (Coreg). Patients taking the drug had a clear reduction in rates of death and reinfarction, leading to this drug becoming the beta-blocker of choice in patients with ventricular dysfunction after STEMI.
Angiotensin-converting enzyme inhibitors: Early risk of cardiogenic shock
The use of angiotensin-converting enzyme (ACE) inhibitors after MI is also supported by several studies.44 Two very large studies, one of nearly 60,000 patients and one of nearly 20,000, showed a clear reduction in the mortality rate in those who received an ACE inhibitor. Most of the benefit was in patients with an ejection fraction of less than 40%. On the basis of these trials, ACE inhibitors are indicated for all patients for the first 30 days after MI and then indefinitely for those with left ventricular dysfunction. However, the trial in which an ACE inhibitor was given intravenously early on had to be stopped prematurely because of worse outcomes owing to cardiogenic shock.
These studies highlight again that for patients who are unstable in the first few days of an acute coronary syndrome, it is best to wait until their condition stabilizes and to start these therapies before hospital discharge.
Intensive statin therapy
In the last 20 years, unequivocal evidence has emerged to support the beneficial role of statins for secondary prevention in patients with established coronary artery disease. More-recent trials have also shown that intensive statin therapy (a high dose of a potent statin) improves outcomes better than lower doses.
The PROVE-IT TIMI 22 trial45 randomized patients after an acute coronary syndrome to receive either standard therapy (pravastatin [Pravachol] 40 mg) or intensive therapy (atorvastatin [Lipitor] 80 mg). The intensive-therapy group had a significantly lower rate of major cardiovascular events, and the difference persisted and grew over 30 months of follow-up.
A number of studies confirmed this and broadened the patient population to those with unstable or stable coronary disease. Regardless of the risk profile, the effects were consistent and showed that high-dose statins were better in preventing coronary death and MI.46
Guidelines are evolving toward recommendation of highest doses of statins independently of the target level of low-density lipoprotein cholesterol.
- Antman EM, Anbe DT, Armstrong PW, et al; American College of Cardiology; American Heart Association Task Force on Practice Guidelines; Canadian Cardiovascular Society. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction. Circulation 2004; 110:e82–e292. Erratum in: Circulation 2005; 111:2013–2014.
- Davies MJ. The pathophysiology of acute coronary syndromes. Heart 2000; 83:361–366.
- Rosamond W, Flegal K, Friday G, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics–2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2007; 115:e69–e171.
- Granger CB, Califf RM, Topol EJ. Thrombolytic therapy for acute myocardial infarction. A review. Drugs 1992; 44:293–325.
- Fibrinolytic Therapy Trialists’ (FTT) Collaborative Group. Indications for fibrinolytic therapy in suspected acute myocardial infarction: collaborative overview of early mortality and major morbidity results from all randomised trials of more than 1000 patients. Lancet 1994; 343:311–322.
- Keely EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet 2003; 361:13–20
- De Luca G, Suryapranata H, Ottervanger JP, Antman EM. Time delay to treatment and mortality in primary angioplasty for acute myocardial infarction: every minute of delay counts. Circulation 2004; 109:1223–1225.
- Dalby M, Bouzamondo A, Lechat P, Montalescot G. Transfer for primary angioplasty versus immediate thrombolysis in acute myocardial infarction: a meta-analysis. Circulation 2003; 108:1809–1814.
- Gersh BJ, Stone GW, White HD, Holmes DR Jr. Pharmacological facilitation of primary percutaneous coronary intervention for acute myocardial infarction: is the slope of the curve the shape of the future? JAMA 2005; 293:979–986.
- Antman EM, Hand M, Armstron PW, et al; Canadian Cardiovascular Society; American Academy of Family Physicians; American College of Cardiology; American Heart Association. 2007 focused update of the ACC/AHA 2004 guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2008; 51:210–247.
- Di Mario C, Dudek D, Piscione F, et al; CARESS-in-AMI (Combined Abciximab Reteplase Stent Study in Acute Myocardial Infarction) Investigators. Immediate angioplasty versus standard therapy with rescue angioplasty after thrombolysis in the Combined Abciximab REteplase Stent Study in Acute Myocardial Infarction (CARESS-in-AMI): an open, prospective, randomised, multicentre trial. Lancet 2008; 371:559–568.
- Cantor WJ, Fitchett D, Borgundvaag B, et al; TRANSFER-AMI Trial Investigators. Routine early angioplasty after fibrinolysis for acute myocardial infarction. N Engl J Med 2009; 360:2705–2718.
- Cannon CP, Weintraub WS, Demopoulos LA, et al; TACTICS (Treat Angina With Aggrastat and Determine Cost of Therapy With an Invasive or Conservative Strategy)–Thrombolysis in Myocardial Infarction 18 Investigators. Comparison of early invasive and conservative strategies in patients with unstable coronary syndromes treated with the glycoprotein IIb/IIIa inhibitor tirofiban. N Engl J Med 2001; 344:1879–1887.
- Damman P, Hirsch A, Windhausen F, Tijssen JG, de Winter RJ; ICTUS Investigators. 5-year clinical outcomes in the ICTUS (Invasive versus Conservative Treatment in Unstable coronary Syndromes) trial a randomized comparison of an early invasive versus selective invasive management in patients with non-ST-segment elevation acute coronary syndrome. J Am Coll Cardiol 2010; 55:858–864.
- Bavry AA, Kumbhani DJ, Rassi AN, Bhatt DL, Askari AT. Benefit of early invasive therapy in acute coronary syndromes: a meta-analysis of contemporary randomized clinical trials. J Am Coll Cardiol 2006; 48:1319–1325.
- Antman EM, Cohen M, Bernink PJ, et al. The TIMI risk score for unstable angina/non-ST elevation MI: a method for prognostication and therapeutic decision making. JAMA 2000; 284:835–842.
- Mehta SR, Granger CB, Boden WE, et al; TIMACS Investigators. Early versus delayed invasive intervention in acute coronary syndromes. N Engl J Med 2009; 360:2165–2175.
- Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-Elevation myocardial infarction. J Am Coll Cardiol 2007; 50:e1–e157.
- Yousef O, Bhatt DL. The evolution of antiplatelet therapy in cardiovascular disease. Nat Rev Cardiol 2011; 8:547–559.
- ISIS-2 (Second International Study of Infarct Survival) Collaborative Group. Randomised trial of intravenous streptokinase, oral aspirin, both, or neither among 17,187 cases of suspected acute myocardial infarction: ISIS-2. Lancet 1988; 2:349–360.
- CURRENT-OASIS 7 Investigators; Mehta SR, Bassand JP, Chrolavicius S, et al. Dose comparisons of clopidogrel and aspirin in acute coronary syndromes. N Engl J Med 2010; 363:930–942.
- Yusuf S, Mehta SR, Zhao F, et al; Clopidogrel in Unstable angina to prevent Recurrent Events Trial Investigators. Early and late effects of clopidogrel in patients with acute coronary syndromes. Circulation 2003; 107:966–972.
- Chen ZM, Jiang LX, Chen YP, et al; COMMIT (Clopidogrel and Metoprolol in Myocardial Infarction Trial) collaborative group. Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet 2005; 366:1607–1621.
- Schömig A. Ticagrelor—is there need for a new player in the antiplatelet-therapy field? N Engl J Med 2009; 361:1108–1111.
- Wiviott SD, Antman EM, Braunwald E. Prasugrel. Circulation 2010; 122:394–403.
- Gurbel PA, Bliden KP, Butler K, et al. Randomized double-blind assessment of the ONSET and OFFSET of the antiplatelet effects of ticagrelor versus clopidogrel in patients with stable coronary artery disease: the ONSET/OFFSET study. Circulation 2009; 120:2577–2585.
- Wiviott SD, Braunwald E, McCabe CH, et al; TRITON-TIMI 38 Investigators. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007; 357:2001–2015.
- Wallentin L, Becker RC, Budaj A, et al; PLATO Investigators. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2009; 361:1045–1057.
- de Queiroz Fernandes Araujo JO, Veloso HH, Braga De Paiva JM, Fiho MW, Vincenzo De Paola AA. Efficacy and safety of abciximab on acute myocardial infarction treated with percutaneous coronary interventions: a meta-analysis of randomized, controlled trials. Am Heart J 2004; 148:937–943.
- Kastrati A, Mehilli J, Neuman FJ, et al; Intracoronary Stenting and Antithrombotic: Regimen Rapid Early Action for Coronary Treatment 2 (ISAR-REACT 2) Trial Investigators. Abciximab in patients with acute coronary syndromes undergoing percutaneous coronary intervention after clopidogrel pretreatment: the ISAR-REACT 2 randomized trial. JAMA 2006; 295:1531–1538.
- Stone GW, Bertrand ME, Moses JW, et al; ACUITY Investigators. Routine upstream initiation vs deferred selective use of glycoprotein IIb/IIIa inhibitors in acute coronary syndromes: the ACUITY Timing trial. JAMA 2007; 297:591–602.
- Giugliano RP, White JA, Bode C, et al; Early ACS Investigators. Early vs delayed, provisional eptifibatide in acute coronary syndromes. N Engl J Med 2009; 360:2176–2190.
- Ellis SG, Tendera M, de Belder MA, et al; FINESSE Investigators. Facilitated PCI in patients with ST-elevation myocardial infarction. N Engl J Med 2008; 358:2205–2217.
- Fergusson JJ, Califf RM, Antman EM, et al; SYNERGY Trial Investigators. Enoxaparin vs unfractionated heparin in high-risk patients with non-ST-segment elevation acute coronary syndromes managed with an intended early invasive strategy: primary results of the SYNERGY randomized trial. JAMA 2004; 292:45–54.
- Antman EM, Morrow DA, McCabe CH; EXTRACT-TIMI 25 Investigators. Enoxaparin versus unfractionated heparin with fibrinolysis for ST-elevation myocardial infarction. N Engl J Med 2006; 354:1477–1488.
- The Fifth Organization to Assess Strategies in Acute Ischemic Syndromes Investigators. Comparison of fondaparinux and enoxaparin in acute coronary syndromes. N Engl J Med 2006; 354:1464–1476.
- Mehta SR, Granger CB, Eikelboom JW, et al. Efficacy and safety of fondaparinux versus enoxaparin in patients with acute coronary syndromes undergoing percutaneous coronary intervention: results from the OASIS-5 trial. J Am Coll Cardiol 2007; 50:1742–1751.
- Lincoff AM, Bittl JA, Harrington RA, et al; REPLACE-2 Investigators. Bivalirudin and provisional glycoprotein IIb/IIIa blockade compared with heparin and planned glycoprotein IIb/IIIa blockade during percutaneous coronary intervention: REPLACE-2 randomized trial. JAMA 2003; 289:853–863.
- Stone GW, McLaurin BT, Cox DA, et al; ACUITY Investigators. Bivalirudin for patients with acute coronary syndromes. N Engl J Med 2006; 355:2203–2216.
- Stone GW, Witzenbichler B, Guagliumi G, et al; HORIZONS-AMI Trial Investigators. Bivalirudin during primary PCI in acute myocardial infarction. N Engl J Med 2007; 358:2218–2230.
- Mehran R, Lansky AJ, Witzenbichler B, et al; HORIZONS-AMI Trial Investigators. Bivalirudin in patients undergoing primary angioplasty for acute myocardial infarction (HORIZONS-AMI): 1-year results of a randomised controlled trial. Lancet 2009; 374:1149–1159.
- Chen ZM, Pan HC, Chen YP, et al; COMMIT (Clopidogrel and Metoprolol in Myocardial Infarction Trial) Collaborative Group. Early intravenous then oral metoprolol in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet 2005; 366:1622–1632.
- Dargie JH. Effect of carvedilol on outcome after myocardial infarction in patients with left-ventricular dysfunction: the CAPRICORN randomised trial. Lancet 2001; 357:1385–1390.
- Hennekens CH, Albert CM, Godfried SL, Gaziano JM, Buring JE. Adjunctive drug therapy of acute myocardial infarction—evidence from clinical trials. N Engl J Med 1996; 335:1660–1667.
- Cannon CP, Braunwald E, McCabe CH, et al; Pravastatin or Atorvastatin Evaluation and Infection Therapy–Thrombolysis in Myocardial Infarction 22 Investigators. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004; 350:1495–1504.
- Cannon CP, Steinberg BA, Murphy SA, Mega JL, Braunwald E. Meta-analysis of cardiovascular outcomes trials comparing intensive versus moderate statin therapy. J Am Coll Cardiol 2006; 48:438–445.
Most decisions for managing acute coronary syndromes can be based on ample data from large randomized trials with hard clinical end points, so there is little reason to provide care that is not evidence-based.
This article reviews some of the trials that provide guidance on diagnosing and managing acute coronary syndromes, including the timing of reperfusion and adjunctive therapies in different situations.
MOST ACUTE CORONARY SYNDROMES ARE NON-ST-ELEVATION CONDITIONS
Acute coronary syndromes range from unstable angina and non-ST-elevation myocardial infarction (NSTEMI) to ST-elevation MI (STEMI), reflecting a continuum of severity of coronary stenosis. The degree of coronary occlusion may ultimately determine whether a patient has unstable angina or MI with or without ST elevation.1
The substrate for all of these is vulnerable plaque. Angiographic studies have indicated that in many cases medium-size plaques (30%–40% stenosis) are more likely to rupture than larger, more obstructive ones. Moderate plaques may be vulnerable because they are less mature, with a large lipid core and a thin cap prone to rupture or erode, exposing the thrombogenic subendothelial components.2
Because the vulnerability of a coronary plaque may not correlate with the severity of stenosis before the plaque ruptures, stress tests and symptoms may not predict the risk of MI. The key role of thrombosis in the pathogenesis also highlights the importance of antithrombotic therapy in the acute phases of acute coronary syndromes, which can significantly reduce mortality and morbidity rates.
Perhaps because of the widespread use of aspirin and statins, most patients who currently present with an acute coronary syndrome have either unstable angina or NSTEMI: of about 1.57 million hospital admissions in 2004 for acute coronary syndromes, for example, only 330,000 (21%) were for STEMI.3
DIAGNOSING ACUTE CORONARY SYNDROME
Symptoms may not be classic
The classic symptoms of acute coronary syndromes are intense, oppressive chest pressure radiating to the left arm, but nearly any discomfort “between the nose and navel” (eg, including the jaw, arm, and epigastric and abdominal areas) may be an acute coronary syndrome. Associated symptoms may include chest heaviness or burning, radiation to the jaw, neck, shoulder, back, or arms, and dyspnea.
Particularly in older, female, postoperative, or diabetic patients, the presentation may be atypical or “silent,” including nausea or vomiting; breathlessness; sweating; arrhythmias; or light-headedness. Especially in these groups, symptoms may be mild or subtle, and acute coronary syndrome may manifest only as “not feeling well.”
The differential diagnosis of acute coronary syndromes is broad. Most important to immediately consider are pulmonary embolism and aortic dissection, as they are life-threatening and are treated differently from acute coronary syndromes. Otherwise, it is best to err on the side of caution and treat for an acute coronary syndrome until it is proven otherwise.
Electrocardiography is critical
Electrocardiography (ECG) gives valuable information about the location, extent, and prognosis of infarction, and it is critically important for distinguishing STEMI from NSTEMI, with ST elevation classically diagnostic of complete coronary occlusion. Q waves can occur early and do not necessarily signify completed infarction, as traditionally thought. ST depression or T inversion indicates that total coronary occlusion is unlikely unless they are in a pattern of circumflex infarct associated with an enlarging R wave in lead V1. An ST elevation in RV4 indicates right ventricular infarction.
The appearance on ECG may evolve over time, so a patient with atypical symptoms and a nonspecific electrocardiogram should be observed for 24 hours or until more specific criteria develop.
Biomarkers in NSTEMI
In MI, cardiac troponin levels begin to rise about 3 hours after the onset of chest pain, and elevations can last for up to 14 days. Levels can also be mildly elevated chronically in patients with renal dysfunction, so positive biomarker tests in that population should be interpreted cautiously.
For STEMI, the opportunity to reperfuse is lost if one waits for cardiac biomarkers to become elevated. But for NSTEMI, they are highly sensitive and specific for identifying patients at high risk and determining who should be treated aggressively. Patients who are biomarker-negative have a better prognosis than patients with identical symptoms and electrocardiograms who are biomarker-positive.
MI is currently defined as a rise in any biomarker (usually troponin) above the 99th percentile for a reference population, with at least one of the following:
- Ischemic symptoms
- New ST/T changes or left bundle branch block
- Pathologic Q waves
- Loss of myocardium or abnormal wall motion seen by imaging
- Intracoronary thrombus.
REPERFUSION FOR ACUTE STEMI
Because acute coronary syndromes have a common pathophysiology, for the most part, lessons from clinical trials in one syndrome are relevant to the others. However, important differences exist regarding the need for immediate reperfusion in STEMI, since in most cases these patients have total rather than partial occlusion.
Fibrinolysis has limitations
The standard of management for STEMI is immediate reperfusion. The goal is to interrupt the wave front of myocardial necrosis, salvage threatened myocardium, and ultimately improve survival.
Five placebo-controlled trials showed a 30% reduction in the death rate in patients who received fibrinolytic therapy within 6 to 12 hours of presentation.4
Patients with ST elevation or with new bundle branch block benefit most from fibrinolytic therapy. Those with ST depression, T inversion, or nonspecific changes on ECG do not benefit; they probably do not have complete coronary occlusion, so the prothrombotic or platelet-activating effects of fibrinolytic therapy may make them worse.5 Further, fibrinolytic therapy poses the risk of intracranial hemorrhage, which, although rare (occurring in up to 1% of cases depending on the drug regimen), is a devastating complication.
In general, absolute contraindications to fibrinolysis include intracranial abnormalities, hemorrhage, and head trauma. An important relative contraindication is uncontrolled blood pressure (> 180/110 mm Hg at any point during hospitalization, including during the immediate presentation). Studies show that even if blood pressure can be controlled, the risk of intracranial hemorrhage is substantially higher, although the risk may not outweigh the benefit of reperfusion, particularly for large infarctions when percutaneous coronary intervention (PCI) is not available as an alternative to fibrinolysis.
Prompt PCI is preferable to fibrinolysis
If PCI is available on site, there is nearly no role for fibrinolytic therapy. PCI is better than fibrinolytic therapy in terms of the degree of reperfusion, reocclusion, MI recurrence, and mortality rate, and it poses little or no risk of intracranial hemorrhage.6
For either fibrinolytic therapy or percutaneous therapy, “time is muscle”: the longer the ischemic time, the higher the mortality rate (relative risk = 1.075 for every 30 minutes of delay, P = .041).7
At centers that do not have PCI on site, studies (mainly from Europe) have shown that it is better to transport the patient for PCI than to give immediate fibrinolytic therapy.7,8 But because the centers studied tended to have short transport times (usually 40 minutes or less), it is uncertain whether the results are applicable throughout the United States.
The delay between symptom onset and presentation is also relevant. Reperfusion within the first 1 to 2 hours after the onset of symptoms provides the greatest degree of myocardial salvage and of reduction in the risk of death; the extent of benefit thereafter is substantially less. As a result, patients who present very early after symptom onset have the most to lose if their reperfusion is delayed by even a few more hours, whereas patients who have already experienced several hours of pain are affected less by additional delay.9 Thus, patients presenting within the “golden” 1 or 2 hours after symptoms begin should be considered for fibrinolytic therapy if transfer for PCI cannot be done expeditiously. It is important for hospitals without PCI available on site to have a system in place for rapid transport of patients when needed.
Guidelines advise that patients with STEMI should undergo PCI rather than receive fibrinolytic therapy as long as PCI is available within 90 minutes of first medical contact. Otherwise, fibrinolysis should be started within 30 minutes.10 For patients who present several hours after symptom onset, PCI may still be preferable even if the transport time is somewhat longer.
PCI after fibrinolytic therapy
In prior decades, PCI immediately after fibrinolytic therapy was associated with an increased risk of bleeding complications and reinfarction. That has changed with improvements in equipment and antithrombotic therapy.
Two large trials conclusively found that routinely transferring high-risk patients for PCI immediately after receiving fibrinolytic therapy (combined half-dose reteplase [Retavase] and abciximab [ReoPro]11 or full-dose tenecteplase [TNKase]12) resulted in much lower rates of ischemic end points without an increase in bleeding complications compared with transferring patients only for rescue PCI after fibrinolytic therapy.
Routine transfer is now the standard of care for high-risk patients after fibrinolytic therapy and probably is best for all patients after an MI.
MANAGING NSTEMI AND UNSTABLE ANGINA
For patients with NSTEMI, immediate reperfusion is usually not required, although initial triage for “early invasive” vs “initial conservative” management must be done early in the hospital course. Randomized trials have evaluated these two approaches, with most studies in the contemporary era reporting improved outcomes with an early invasive approach.
The TACTICS trial,13 the most important of these, enrolled more than 2,200 patients with unstable angina or NSTEMI and randomized them to an early invasive strategy or a conservative strategy. Overall, results were better with the early invasive strategy.
The ICTUS trial.14 Although several studies showed that an early invasive approach was better, the most recent study using the most modern practices—the ICTUS trial—did not find that it reduced death rates. Most patients eventually underwent angiography and revascularization, but not early on. However, all studies showed that rates of recurrent unstable angina and hospitalization were reduced by an early invasive approach, so revascularization does have a role in stabilizing the patient. But in situations of aggressive medical management with antithrombotic and other therapies, an early conservative approach may be an appropriate alternative for many patients.15
The selection of an invasive vs a conservative approach should include a consideration of risk, which can be estimated using a number of criteria, including the Thrombolysis in Myocardial Infarction (TIMI) or the GRACE risk score. When risk was stratified using the TIMI risk score,16 in the TACTICS trial, the higher the risk score, the more likely patients were to benefit from early revascularization.
When an invasive approach is chosen, it does not appear necessary to take patients to catheterization immediately (within 2–24 hours) compared with later during the hospital course.
The TIMACS trial,17 with more than 3,000 patients, tested the benefits of very early vs later revascularization for patients with NSTEMI and unstable angina. Early intervention did not significantly improve outcomes for the primary composite end point of death, MI, and stroke in the overall population enrolled in the trial, but when the secondary end point of refractory ischemia was added in, early intervention was found to be beneficial overall. Moreover, when stratified by risk, high-risk patients significantly benefited from early intervention for the primary end point.
Guidelines for NSTEMI and unstable angina continue to prefer an early invasive strategy, particularly for high-risk patients, although a conservative strategy is considered acceptable if patients receive intensive evidence-based medical therapy and remain clinically stable.18
ANTITHROMBOTIC THERAPIES
Once a revascularization strategy has been chosen, adjunctive therapies should be considered. The most important are the antithrombotic therapies.
Many drugs target platelet activity. Most important are the thromboxane inhibitor aspirin, the adenosine diphosphate (ADP) receptor antagonists clopidogrel (Plavix), prasugrel (Effient), and ticagrelor (Brilinta), and the glycoprotein (GP) IIb/IIIa antagonists abciximab and eptifibatide (Integrilin). Others, such as thrombin receptor antagonists, are under investigation.19
Aspirin for secondary prevention
Evidence is unequivocal for the benefit of aspirin therapy in patients with established or suspected vascular disease.
The ISIS-2 trial20 compared 35-day mortality rates in 16,000 patients with STEMI who were given aspirin, streptokinase, combined streptokinase and aspirin, or placebo. Mortality rates were reduced by aspirin compared with placebo by an extent similar to that achieved with streptokinase, with a further reduction when aspirin and streptokinase were given together.
Therefore, patients with STEMI should be given aspirin daily indefinitely unless they have true aspirin allergy. The dose is 165 to 325 mg initially and 75 to 162 mg daily thereafter.
For NSTEMI and even for secondary prevention in less-acute situations, a number of smaller trials also provide clear evidence of benefit from aspirin therapy.
The CURRENT-OASIS 7 trial21 showed that low maintenance dosages of aspirin (75–100 mg per day) resulted in the same incidence of ischemic end points (cardiovascular death, MI, or stroke) as higher dosages. Although rates of major bleeding events did not differ, a higher rate of gastrointestinal bleeding was evident at just 30 days in patients taking the higher doses. This large trial clearly established that there is no advantage to daily aspirin doses of more than 100 mg.
DUAL ANTIPLATELET THERAPY IS STANDARD
Standard practice now is to use aspirin plus another antiplatelet agent that acts by inhibiting either the ADP receptor (for which there is the most evidence) or the GP IIb/IIIa receptor (which is becoming less used). Dual therapy should begin early in patients with acute coronary syndrome.
Clopidogrel: Well studied with aspirin
The most commonly used ADP antagonist is clopidogrel, a thienopyridine. Much evidence exists for its benefit.
The CURE trial22 randomized more than 12,000 patients with NSTEMI or unstable angina to aspirin plus either clopidogrel or placebo. The incidence of the combined end point of MI, stroke, and cardiovascular death was 20% lower in the clopidogrel group than in the placebo group over 12 months of follow-up. The benefit of clopidogrel began to occur within the first 24 hours after randomization, with a 33% relative risk reduction in the combined end point of cardiovascular death, MI, stroke, and severe ischemia, demonstrating the importance of starting this agent early in the hospital course.
COMMIT23 found a benefit in adding clopidogrel to aspirin in patients with acute STEMI. Although it was only a 30-day trial, significant risk reduction was found in the dual-therapy group for combined death, stroke, or reinfarction. The results of this brief trial were less definitive, but the pathophysiology was similar to non-ST-elevation acute coronary syndromes, so it is reasonable to extrapolate the long-term findings to this setting.
The CURRENT-OASIS 7 trial21 randomized more than 25,000 patients to either clopidogrel in a double dosage (600 mg load, 150 mg/day for 6 days, then 75 mg/day) or standard dosage (300 mg load, 75 mg/day thereafter). Although no overall benefit was found for the higher dosage, a subgroup of more than 17,000 patients who underwent PCI after randomization had a lower risk of developing stent thrombosis. On the other hand, higher doses of clopidogrel caused more major bleeding events.
Ticagrelor and prasugrel: New alternatives to clopidogrel
The principal limitation of clopidogrel is its metabolism. It is a prodrug, ie, it is not active as taken and must be converted to its active state by cytochrome P450 enzymes in the liver. Patients who bear certain polymorphisms in the genes for these enzymes or who are taking other medications that affect this enzymatic pathway may derive less platelet inhibition from the drug, leading to considerable patient-to-patient variability in the degree of antiplatelet effect.
Alternatives to clopidogrel have been developed that inhibit platelets more intensely, are activated more rapidly, and have less interpatient variability. Available now are ticagrelor and prasugrel.24 Like clopidogrel, prasugrel is absorbed as an inactive prodrug, but it is efficiently metabolized by esterases to an active form, and then by a simpler step within the liver to its fully active metabolite.25 Ticagrelor is active as absorbed.26
Pharmacodynamically, the two drugs perform almost identically and much faster than clopidogrel, with equilibrium platelet inhibition reached in less than 1 hour. The degree of platelet inhibition is also more—sometimes twice as much—with the new drugs compared with clopidogrel, and the effect is much more consistent between patients.
Both clopidogrel and prasugrel permanently inhibit the platelet ADP receptor, and 3 to 7 days are therefore required for their antiplatelet effects to completely wear off. In contrast, ticagrelor is a reversible inhibitor and its effects wear off more rapidly. Despite achieving a much higher level of platelet inhibition than clopidogrel, ticagrelor’s activity falls below that of clopidogrel’s by 48 hours of discontinuing the drugs.
Trial of prasugrel vs clopidogrel
The TRITON-TIMI 38 trial27 enrolled more than 13,000 patients with acute coronary syndromes, randomized to receive, either prasugrel or clopidogrel, in addition to aspirin. The patients were all undergoing PCI, so the findings do not apply to patients treated medically with an early conservative approach. The study drug was given only after the decision was made to perform PCI in patients with non-ST-elevation acute coronary syndrome (but given immediately for patients with STEMI, because nearly all those patients undergo PCI).
Prasugrel was clearly beneficial, with a significant 20% lower rate of the combined end point of cardiovascular death, MI, and stroke at 15 months. However, bleeding risk was higher with prasugrel (2.4% vs 1.8%, hazard ratio 1.32, 95% confidence interval 1.02–1.68, P = .03). Looking at individual end points, the advantages of prasugrel were primarily in reducing rates of stent thrombosis and nonfatal MI. Death rates with the two drugs were equivalent, possibly because of the higher risk of bleeding with prasugrel. Bleeding in the prasugrel group was particularly increased in patients who underwent bypass surgery; more patients also needed transfusion.
Subgroup analysis showed that patients with a history of stroke or transient ischemic attack had higher rates of ischemic and bleeding events with prasugrel than with clopidogrel, leading to these being labeled as absolute contraindications to prasugrel. Patients over age 75 or who weighed less than 60 kg experienced excess bleeding risk that closely matched the reduction in ischemic event rates and thus did not have a net benefit with prasugrel.
Trial of ticagrelor vs clopidogrel
The PLATO trial28 included 18,000 patients, of whom 65% underwent revascularization and 35% were treated medically. The drug—clopidogrel or ticagrelor—was given in addition to aspirin at randomization (within 24 hours of symptom onset); this more closely follows clinical practice, in which dual antiplatelet therapy is started as soon as possible. This difference makes the PLATO study more relevant to practice for patients with non-ST-elevation acute coronary syndrome. Also, because they gave the drugs to all patients regardless of whether they were to undergo PCI, this study likely had a higher-risk population, which may be refected in the higher mortality rate at 30 days (5.9% in the clopidogrel group in the PLATO study vs 3.2% in the clopidogrel group in the TRITON study).
Another important difference between the trials testing prasugrel and ticagrelor is that patients who had already received a thienopyridine were excluded from the prasugrel trial but not from the ticagrelor trial. Nearly half the patients in the ticagrelor group were already taking clopidogrel. The clinical implication is that for patients who arrive from another facility and already have been given clopidogrel, it is safe to give ticagrelor. There is limited information about whether that is also true for prasugrel, although there is no known reason why the safety of adding prasugrel to clopidogrel should be different from that of ticagrelor.
The rate of ischemic events was 20% lower in the ticagrelor group than in the clopidogrel group, importantly including reductions in the incidence of death, MI, and stent thrombosis. There was no increase with ticagrelor compared with clopidogrel in bleeding associated with coronary artery bypass graft surgery, likely because of the more rapid washout of the ticagrelor effect, or in the need for blood transfusions. However, the rate of bleeding unrelated to coronary artery bypass was about 20% higher with ticagrelor.
In summary, more intense platelet inhibition reduces the risk of ischemic events, but, particularly for the irreversible inhibitor prasugrel, at the cost of a higher risk of bleeding. In general, the net benefit of these agents in preventing the irreversible complications of MI and (in the case of ticagrelor) death favor the use of the more intense ADP inhibitors in appropriate patients. Ticagrelor is indicated in patients with acute coronary syndromes undergoing invasive or conservative management; prasugrel is indicated in patients undergoing PCI, but contraindicated in patients with a previous stroke or transient ischemic event. Neither drug is indicated in patients undergoing elective PCI outside the setting of acute coronary syndromes, although these agents may be appropriate in patients with intolerance or allergy to clopidogrel.
Glycoprotein IIb/IIIa antagonists for select cases only
GP IIb/IIIa antagonists such as abciximab were previously used more commonly than they are today. Now, with routine pretreatment using thienopyridines, their role in acute coronary syndromes is less clear. They still play a role when routine dual antiplatelet therapy is not used, when prasugrel or ticagrelor is not used, and when heparin rather than an alternative antithrombin agent is used.
A meta-analysis29 of 3,755 patients showed a clear reduction in ischemic complications with abciximab as an adjunct to primary PCI for STEMI in patients treated with heparin.
Kastrati et al30 found that patients with non-ST-elevation acute coronary syndromes benefited from abciximab at the time of PCI with heparin, even though they had been routinely pretreated with clopidogrel. However, benefits were seen only in high-risk patients who had presented with elevated troponins.
On the other hand, the role of GP IIb/IIIa blockade for “upstream” medical management in patients with acute coronary syndromes has been eroded by several studies.
The ACUITY trial31 randomized more than 9,000 patients to receive either routine treatment with a GP IIb/IIIa inhibitor before angiography or deferred selective use in the catheterization laboratory only for patients undergoing PCI. No significant differences were found in rates of MI and death.
The Early ACS trial32 compared early routine eptifibatide vs delayed, provisional eptifibatide in 9,492 patients with acute coronary syndromes without ST elevation and who were assigned to an invasive strategy. The early-eptifibatide group received two boluses and an infusion of eptifibatide before angiography; the others received a placebo infusion, with provisional eptifibatide after angiography if the patient underwent PCI and was deemed at high risk. No significant difference in rates of death or MI were noted, and the early-eptifibatide group had significantly higher rates of bleeding and need for transfusion.
The FINESSE trial33 also discredited “facilitating” PCI by giving GP IIb/IIIa antagonists in patients with STEMI before arrival in the catheterization laboratory, with no benefit to giving abciximab ahead of time vs in the catheterization laboratory, and with an increased risk of bleeding complications.
These studies have helped narrow the use of GP IIb/IIIa inhibitors to the catheterization laboratory in conjunction with heparin anticoagulation (as compared with bivalirudin [Angiomax]; see below) and only in select or high-risk cases. These drugs are indicated in the medical phase of management only if patients cannot be stabilized by aspirin or ADP inhibition.
NEWER ANTITHROMBOTICS: ADVANTAGES UNCLEAR
The complex coagulation cascade has a number of components, but only a few are targeted by drugs that are approved and recommended: fondaparinux (Arixtra) and oral factor Xa inhibitors affect the prothrombinase complex (including factor X); bivalirudin and oral factor IIa inhibitors affect thrombin; and heparin and the low-molecular-weight heparins inhibit both targets.
Low-molecular-weight heparins
The SYNERGY trial34 randomized nearly 10,000 patients with non-ST-elevation acute coronary syndromes at high risk for ischemic cardiac complications managed with an invasive approach to either the low-molecular-weight heparin enoxaparin (Lovenox) or intravenous unfractionated heparin immediately after enrollment. Most patients underwent catheterization and revascularization. No clinical advantage was found for enoxaparin, and bleeding complications were increased.
The EXTRACT-TIMI 25 trial35 randomized more than 20,000 patients with STEMI who were about to undergo fibrinolysis to receive either enoxaparin throughout hospitalization (average of 8 days) or unfractionated heparin for at least 48 hours. The enoxaparin group had a lower rate of recurrent MI, but it was unclear if the difference was in part attributable to the longer therapy time. The enoxaparin group also had more bleeding.
Fondaparinux
The OASIS-5 trial36,37 compared enoxaparin and fondaparinux, an exclusive factor Xa inhibitor, in more than 20,000 patients with unstable angina or NSTEMI. Fondaparinux was associated with a lower risk of death and reinfarction as well as fewer bleeding events. However, the benefits were almost exclusively in patients treated medically. In those undergoing PCI within the first 8 days, no benefit was found, although there was still a significant reduction in major bleeding events. Catheter thrombosis was also increased in patients taking fondaparinux, but only in those who did not receive adequate unfractionated heparin treatment before PCI.
Bivalirudin superior at time of catheterization
The most significant advance in antithrombotic therapy for patients with acute coronary syndromes is bivalirudin. This drug has a clear role only in the catheterization laboratory, where patients can be switched to it from heparin, low-molecular-weight heparin, or fondaparinux.
Three trials38–40 evaluated the drug in a total of more than 20,000 patients receiving invasive management of coronary artery disease undergoing PCI for elective indications, NSTEMI, or STEMI.
Results were remarkably similar across the three trials. Patients who were treated with bivalirudin alone had the same rate of ischemic end points at 30 days as those receiving heparin plus a GP IIb/IIIa inhibitor, but bivalirudin was associated with a consistent and significant 40% to 50% lower bleeding risk. For the highest-risk patients, those with STEMI, the bivalirudin group also had a significantly lower risk of death at 1 year.41
OTHER DRUGS: EARLY TREATMENT NO LONGER ROUTINE
Most data for the use of therapies aside from antithrombotics are from studies of patients with STEMI, but findings can logically be extrapolated to those with non-ST-elevation acute coronary syndromes.
Beta-blockers: Cardiogenic shock a risk
For beta-blockers, many historical trials were done in stable coronary disease, but there are no large trials in the setting of NSTEMI or unstable angina, and only recently have there been large trials for STEMI. Before the availability of recent evidence, standard practice was to treat STEMI routinely with intravenous metoprolol (Lopressor) and then oral metoprolol.
When large studies were finally conducted, the results were sobering.
COMMIT.42 Nearly 46,000 patients with suspected acute MI were randomized to receive either metoprolol (up to 15 mg intravenously, then 200 mg by mouth daily until discharge or for up to 4 weeks in the hospital) or placebo. Surprisingly, although rates of reinfarction and ventricular fibrillation were lower with metoprolol, a higher risk of cardiogenic shock with early beta-blockade offset these benefits and the net mortality rate was not reduced. This study led to a reduction in the early use of beta-blockers in patients with STEMI.
The standard of care has now shifted from beta-blockers in everyone as early as possible after MI to being more cautious in patients with contraindications, including signs of heart failure or a low-output state, or even in those of advanced age or with borderline low blood pressure or a high heart rate. Patients who present late and therefore may have a larger infarct are also at higher risk.
Although the goal should be to ultimately discharge patients on beta-blocker therapy after an MI, there should be no rush to start one early.
Carvedilol now preferred after STEMI
The CAPRICORN trial43 randomized nearly 2,000 patients following MI with left ventricular dysfunction (an ejection fraction of 40% or below) to either placebo or the beta-blocker carvedilol (Coreg). Patients taking the drug had a clear reduction in rates of death and reinfarction, leading to this drug becoming the beta-blocker of choice in patients with ventricular dysfunction after STEMI.
Angiotensin-converting enzyme inhibitors: Early risk of cardiogenic shock
The use of angiotensin-converting enzyme (ACE) inhibitors after MI is also supported by several studies.44 Two very large studies, one of nearly 60,000 patients and one of nearly 20,000, showed a clear reduction in the mortality rate in those who received an ACE inhibitor. Most of the benefit was in patients with an ejection fraction of less than 40%. On the basis of these trials, ACE inhibitors are indicated for all patients for the first 30 days after MI and then indefinitely for those with left ventricular dysfunction. However, the trial in which an ACE inhibitor was given intravenously early on had to be stopped prematurely because of worse outcomes owing to cardiogenic shock.
These studies highlight again that for patients who are unstable in the first few days of an acute coronary syndrome, it is best to wait until their condition stabilizes and to start these therapies before hospital discharge.
Intensive statin therapy
In the last 20 years, unequivocal evidence has emerged to support the beneficial role of statins for secondary prevention in patients with established coronary artery disease. More-recent trials have also shown that intensive statin therapy (a high dose of a potent statin) improves outcomes better than lower doses.
The PROVE-IT TIMI 22 trial45 randomized patients after an acute coronary syndrome to receive either standard therapy (pravastatin [Pravachol] 40 mg) or intensive therapy (atorvastatin [Lipitor] 80 mg). The intensive-therapy group had a significantly lower rate of major cardiovascular events, and the difference persisted and grew over 30 months of follow-up.
A number of studies confirmed this and broadened the patient population to those with unstable or stable coronary disease. Regardless of the risk profile, the effects were consistent and showed that high-dose statins were better in preventing coronary death and MI.46
Guidelines are evolving toward recommendation of highest doses of statins independently of the target level of low-density lipoprotein cholesterol.
Most decisions for managing acute coronary syndromes can be based on ample data from large randomized trials with hard clinical end points, so there is little reason to provide care that is not evidence-based.
This article reviews some of the trials that provide guidance on diagnosing and managing acute coronary syndromes, including the timing of reperfusion and adjunctive therapies in different situations.
MOST ACUTE CORONARY SYNDROMES ARE NON-ST-ELEVATION CONDITIONS
Acute coronary syndromes range from unstable angina and non-ST-elevation myocardial infarction (NSTEMI) to ST-elevation MI (STEMI), reflecting a continuum of severity of coronary stenosis. The degree of coronary occlusion may ultimately determine whether a patient has unstable angina or MI with or without ST elevation.1
The substrate for all of these is vulnerable plaque. Angiographic studies have indicated that in many cases medium-size plaques (30%–40% stenosis) are more likely to rupture than larger, more obstructive ones. Moderate plaques may be vulnerable because they are less mature, with a large lipid core and a thin cap prone to rupture or erode, exposing the thrombogenic subendothelial components.2
Because the vulnerability of a coronary plaque may not correlate with the severity of stenosis before the plaque ruptures, stress tests and symptoms may not predict the risk of MI. The key role of thrombosis in the pathogenesis also highlights the importance of antithrombotic therapy in the acute phases of acute coronary syndromes, which can significantly reduce mortality and morbidity rates.
Perhaps because of the widespread use of aspirin and statins, most patients who currently present with an acute coronary syndrome have either unstable angina or NSTEMI: of about 1.57 million hospital admissions in 2004 for acute coronary syndromes, for example, only 330,000 (21%) were for STEMI.3
DIAGNOSING ACUTE CORONARY SYNDROME
Symptoms may not be classic
The classic symptoms of acute coronary syndromes are intense, oppressive chest pressure radiating to the left arm, but nearly any discomfort “between the nose and navel” (eg, including the jaw, arm, and epigastric and abdominal areas) may be an acute coronary syndrome. Associated symptoms may include chest heaviness or burning, radiation to the jaw, neck, shoulder, back, or arms, and dyspnea.
Particularly in older, female, postoperative, or diabetic patients, the presentation may be atypical or “silent,” including nausea or vomiting; breathlessness; sweating; arrhythmias; or light-headedness. Especially in these groups, symptoms may be mild or subtle, and acute coronary syndrome may manifest only as “not feeling well.”
The differential diagnosis of acute coronary syndromes is broad. Most important to immediately consider are pulmonary embolism and aortic dissection, as they are life-threatening and are treated differently from acute coronary syndromes. Otherwise, it is best to err on the side of caution and treat for an acute coronary syndrome until it is proven otherwise.
Electrocardiography is critical
Electrocardiography (ECG) gives valuable information about the location, extent, and prognosis of infarction, and it is critically important for distinguishing STEMI from NSTEMI, with ST elevation classically diagnostic of complete coronary occlusion. Q waves can occur early and do not necessarily signify completed infarction, as traditionally thought. ST depression or T inversion indicates that total coronary occlusion is unlikely unless they are in a pattern of circumflex infarct associated with an enlarging R wave in lead V1. An ST elevation in RV4 indicates right ventricular infarction.
The appearance on ECG may evolve over time, so a patient with atypical symptoms and a nonspecific electrocardiogram should be observed for 24 hours or until more specific criteria develop.
Biomarkers in NSTEMI
In MI, cardiac troponin levels begin to rise about 3 hours after the onset of chest pain, and elevations can last for up to 14 days. Levels can also be mildly elevated chronically in patients with renal dysfunction, so positive biomarker tests in that population should be interpreted cautiously.
For STEMI, the opportunity to reperfuse is lost if one waits for cardiac biomarkers to become elevated. But for NSTEMI, they are highly sensitive and specific for identifying patients at high risk and determining who should be treated aggressively. Patients who are biomarker-negative have a better prognosis than patients with identical symptoms and electrocardiograms who are biomarker-positive.
MI is currently defined as a rise in any biomarker (usually troponin) above the 99th percentile for a reference population, with at least one of the following:
- Ischemic symptoms
- New ST/T changes or left bundle branch block
- Pathologic Q waves
- Loss of myocardium or abnormal wall motion seen by imaging
- Intracoronary thrombus.
REPERFUSION FOR ACUTE STEMI
Because acute coronary syndromes have a common pathophysiology, for the most part, lessons from clinical trials in one syndrome are relevant to the others. However, important differences exist regarding the need for immediate reperfusion in STEMI, since in most cases these patients have total rather than partial occlusion.
Fibrinolysis has limitations
The standard of management for STEMI is immediate reperfusion. The goal is to interrupt the wave front of myocardial necrosis, salvage threatened myocardium, and ultimately improve survival.
Five placebo-controlled trials showed a 30% reduction in the death rate in patients who received fibrinolytic therapy within 6 to 12 hours of presentation.4
Patients with ST elevation or with new bundle branch block benefit most from fibrinolytic therapy. Those with ST depression, T inversion, or nonspecific changes on ECG do not benefit; they probably do not have complete coronary occlusion, so the prothrombotic or platelet-activating effects of fibrinolytic therapy may make them worse.5 Further, fibrinolytic therapy poses the risk of intracranial hemorrhage, which, although rare (occurring in up to 1% of cases depending on the drug regimen), is a devastating complication.
In general, absolute contraindications to fibrinolysis include intracranial abnormalities, hemorrhage, and head trauma. An important relative contraindication is uncontrolled blood pressure (> 180/110 mm Hg at any point during hospitalization, including during the immediate presentation). Studies show that even if blood pressure can be controlled, the risk of intracranial hemorrhage is substantially higher, although the risk may not outweigh the benefit of reperfusion, particularly for large infarctions when percutaneous coronary intervention (PCI) is not available as an alternative to fibrinolysis.
Prompt PCI is preferable to fibrinolysis
If PCI is available on site, there is nearly no role for fibrinolytic therapy. PCI is better than fibrinolytic therapy in terms of the degree of reperfusion, reocclusion, MI recurrence, and mortality rate, and it poses little or no risk of intracranial hemorrhage.6
For either fibrinolytic therapy or percutaneous therapy, “time is muscle”: the longer the ischemic time, the higher the mortality rate (relative risk = 1.075 for every 30 minutes of delay, P = .041).7
At centers that do not have PCI on site, studies (mainly from Europe) have shown that it is better to transport the patient for PCI than to give immediate fibrinolytic therapy.7,8 But because the centers studied tended to have short transport times (usually 40 minutes or less), it is uncertain whether the results are applicable throughout the United States.
The delay between symptom onset and presentation is also relevant. Reperfusion within the first 1 to 2 hours after the onset of symptoms provides the greatest degree of myocardial salvage and of reduction in the risk of death; the extent of benefit thereafter is substantially less. As a result, patients who present very early after symptom onset have the most to lose if their reperfusion is delayed by even a few more hours, whereas patients who have already experienced several hours of pain are affected less by additional delay.9 Thus, patients presenting within the “golden” 1 or 2 hours after symptoms begin should be considered for fibrinolytic therapy if transfer for PCI cannot be done expeditiously. It is important for hospitals without PCI available on site to have a system in place for rapid transport of patients when needed.
Guidelines advise that patients with STEMI should undergo PCI rather than receive fibrinolytic therapy as long as PCI is available within 90 minutes of first medical contact. Otherwise, fibrinolysis should be started within 30 minutes.10 For patients who present several hours after symptom onset, PCI may still be preferable even if the transport time is somewhat longer.
PCI after fibrinolytic therapy
In prior decades, PCI immediately after fibrinolytic therapy was associated with an increased risk of bleeding complications and reinfarction. That has changed with improvements in equipment and antithrombotic therapy.
Two large trials conclusively found that routinely transferring high-risk patients for PCI immediately after receiving fibrinolytic therapy (combined half-dose reteplase [Retavase] and abciximab [ReoPro]11 or full-dose tenecteplase [TNKase]12) resulted in much lower rates of ischemic end points without an increase in bleeding complications compared with transferring patients only for rescue PCI after fibrinolytic therapy.
Routine transfer is now the standard of care for high-risk patients after fibrinolytic therapy and probably is best for all patients after an MI.
MANAGING NSTEMI AND UNSTABLE ANGINA
For patients with NSTEMI, immediate reperfusion is usually not required, although initial triage for “early invasive” vs “initial conservative” management must be done early in the hospital course. Randomized trials have evaluated these two approaches, with most studies in the contemporary era reporting improved outcomes with an early invasive approach.
The TACTICS trial,13 the most important of these, enrolled more than 2,200 patients with unstable angina or NSTEMI and randomized them to an early invasive strategy or a conservative strategy. Overall, results were better with the early invasive strategy.
The ICTUS trial.14 Although several studies showed that an early invasive approach was better, the most recent study using the most modern practices—the ICTUS trial—did not find that it reduced death rates. Most patients eventually underwent angiography and revascularization, but not early on. However, all studies showed that rates of recurrent unstable angina and hospitalization were reduced by an early invasive approach, so revascularization does have a role in stabilizing the patient. But in situations of aggressive medical management with antithrombotic and other therapies, an early conservative approach may be an appropriate alternative for many patients.15
The selection of an invasive vs a conservative approach should include a consideration of risk, which can be estimated using a number of criteria, including the Thrombolysis in Myocardial Infarction (TIMI) or the GRACE risk score. When risk was stratified using the TIMI risk score,16 in the TACTICS trial, the higher the risk score, the more likely patients were to benefit from early revascularization.
When an invasive approach is chosen, it does not appear necessary to take patients to catheterization immediately (within 2–24 hours) compared with later during the hospital course.
The TIMACS trial,17 with more than 3,000 patients, tested the benefits of very early vs later revascularization for patients with NSTEMI and unstable angina. Early intervention did not significantly improve outcomes for the primary composite end point of death, MI, and stroke in the overall population enrolled in the trial, but when the secondary end point of refractory ischemia was added in, early intervention was found to be beneficial overall. Moreover, when stratified by risk, high-risk patients significantly benefited from early intervention for the primary end point.
Guidelines for NSTEMI and unstable angina continue to prefer an early invasive strategy, particularly for high-risk patients, although a conservative strategy is considered acceptable if patients receive intensive evidence-based medical therapy and remain clinically stable.18
ANTITHROMBOTIC THERAPIES
Once a revascularization strategy has been chosen, adjunctive therapies should be considered. The most important are the antithrombotic therapies.
Many drugs target platelet activity. Most important are the thromboxane inhibitor aspirin, the adenosine diphosphate (ADP) receptor antagonists clopidogrel (Plavix), prasugrel (Effient), and ticagrelor (Brilinta), and the glycoprotein (GP) IIb/IIIa antagonists abciximab and eptifibatide (Integrilin). Others, such as thrombin receptor antagonists, are under investigation.19
Aspirin for secondary prevention
Evidence is unequivocal for the benefit of aspirin therapy in patients with established or suspected vascular disease.
The ISIS-2 trial20 compared 35-day mortality rates in 16,000 patients with STEMI who were given aspirin, streptokinase, combined streptokinase and aspirin, or placebo. Mortality rates were reduced by aspirin compared with placebo by an extent similar to that achieved with streptokinase, with a further reduction when aspirin and streptokinase were given together.
Therefore, patients with STEMI should be given aspirin daily indefinitely unless they have true aspirin allergy. The dose is 165 to 325 mg initially and 75 to 162 mg daily thereafter.
For NSTEMI and even for secondary prevention in less-acute situations, a number of smaller trials also provide clear evidence of benefit from aspirin therapy.
The CURRENT-OASIS 7 trial21 showed that low maintenance dosages of aspirin (75–100 mg per day) resulted in the same incidence of ischemic end points (cardiovascular death, MI, or stroke) as higher dosages. Although rates of major bleeding events did not differ, a higher rate of gastrointestinal bleeding was evident at just 30 days in patients taking the higher doses. This large trial clearly established that there is no advantage to daily aspirin doses of more than 100 mg.
DUAL ANTIPLATELET THERAPY IS STANDARD
Standard practice now is to use aspirin plus another antiplatelet agent that acts by inhibiting either the ADP receptor (for which there is the most evidence) or the GP IIb/IIIa receptor (which is becoming less used). Dual therapy should begin early in patients with acute coronary syndrome.
Clopidogrel: Well studied with aspirin
The most commonly used ADP antagonist is clopidogrel, a thienopyridine. Much evidence exists for its benefit.
The CURE trial22 randomized more than 12,000 patients with NSTEMI or unstable angina to aspirin plus either clopidogrel or placebo. The incidence of the combined end point of MI, stroke, and cardiovascular death was 20% lower in the clopidogrel group than in the placebo group over 12 months of follow-up. The benefit of clopidogrel began to occur within the first 24 hours after randomization, with a 33% relative risk reduction in the combined end point of cardiovascular death, MI, stroke, and severe ischemia, demonstrating the importance of starting this agent early in the hospital course.
COMMIT23 found a benefit in adding clopidogrel to aspirin in patients with acute STEMI. Although it was only a 30-day trial, significant risk reduction was found in the dual-therapy group for combined death, stroke, or reinfarction. The results of this brief trial were less definitive, but the pathophysiology was similar to non-ST-elevation acute coronary syndromes, so it is reasonable to extrapolate the long-term findings to this setting.
The CURRENT-OASIS 7 trial21 randomized more than 25,000 patients to either clopidogrel in a double dosage (600 mg load, 150 mg/day for 6 days, then 75 mg/day) or standard dosage (300 mg load, 75 mg/day thereafter). Although no overall benefit was found for the higher dosage, a subgroup of more than 17,000 patients who underwent PCI after randomization had a lower risk of developing stent thrombosis. On the other hand, higher doses of clopidogrel caused more major bleeding events.
Ticagrelor and prasugrel: New alternatives to clopidogrel
The principal limitation of clopidogrel is its metabolism. It is a prodrug, ie, it is not active as taken and must be converted to its active state by cytochrome P450 enzymes in the liver. Patients who bear certain polymorphisms in the genes for these enzymes or who are taking other medications that affect this enzymatic pathway may derive less platelet inhibition from the drug, leading to considerable patient-to-patient variability in the degree of antiplatelet effect.
Alternatives to clopidogrel have been developed that inhibit platelets more intensely, are activated more rapidly, and have less interpatient variability. Available now are ticagrelor and prasugrel.24 Like clopidogrel, prasugrel is absorbed as an inactive prodrug, but it is efficiently metabolized by esterases to an active form, and then by a simpler step within the liver to its fully active metabolite.25 Ticagrelor is active as absorbed.26
Pharmacodynamically, the two drugs perform almost identically and much faster than clopidogrel, with equilibrium platelet inhibition reached in less than 1 hour. The degree of platelet inhibition is also more—sometimes twice as much—with the new drugs compared with clopidogrel, and the effect is much more consistent between patients.
Both clopidogrel and prasugrel permanently inhibit the platelet ADP receptor, and 3 to 7 days are therefore required for their antiplatelet effects to completely wear off. In contrast, ticagrelor is a reversible inhibitor and its effects wear off more rapidly. Despite achieving a much higher level of platelet inhibition than clopidogrel, ticagrelor’s activity falls below that of clopidogrel’s by 48 hours of discontinuing the drugs.
Trial of prasugrel vs clopidogrel
The TRITON-TIMI 38 trial27 enrolled more than 13,000 patients with acute coronary syndromes, randomized to receive, either prasugrel or clopidogrel, in addition to aspirin. The patients were all undergoing PCI, so the findings do not apply to patients treated medically with an early conservative approach. The study drug was given only after the decision was made to perform PCI in patients with non-ST-elevation acute coronary syndrome (but given immediately for patients with STEMI, because nearly all those patients undergo PCI).
Prasugrel was clearly beneficial, with a significant 20% lower rate of the combined end point of cardiovascular death, MI, and stroke at 15 months. However, bleeding risk was higher with prasugrel (2.4% vs 1.8%, hazard ratio 1.32, 95% confidence interval 1.02–1.68, P = .03). Looking at individual end points, the advantages of prasugrel were primarily in reducing rates of stent thrombosis and nonfatal MI. Death rates with the two drugs were equivalent, possibly because of the higher risk of bleeding with prasugrel. Bleeding in the prasugrel group was particularly increased in patients who underwent bypass surgery; more patients also needed transfusion.
Subgroup analysis showed that patients with a history of stroke or transient ischemic attack had higher rates of ischemic and bleeding events with prasugrel than with clopidogrel, leading to these being labeled as absolute contraindications to prasugrel. Patients over age 75 or who weighed less than 60 kg experienced excess bleeding risk that closely matched the reduction in ischemic event rates and thus did not have a net benefit with prasugrel.
Trial of ticagrelor vs clopidogrel
The PLATO trial28 included 18,000 patients, of whom 65% underwent revascularization and 35% were treated medically. The drug—clopidogrel or ticagrelor—was given in addition to aspirin at randomization (within 24 hours of symptom onset); this more closely follows clinical practice, in which dual antiplatelet therapy is started as soon as possible. This difference makes the PLATO study more relevant to practice for patients with non-ST-elevation acute coronary syndrome. Also, because they gave the drugs to all patients regardless of whether they were to undergo PCI, this study likely had a higher-risk population, which may be refected in the higher mortality rate at 30 days (5.9% in the clopidogrel group in the PLATO study vs 3.2% in the clopidogrel group in the TRITON study).
Another important difference between the trials testing prasugrel and ticagrelor is that patients who had already received a thienopyridine were excluded from the prasugrel trial but not from the ticagrelor trial. Nearly half the patients in the ticagrelor group were already taking clopidogrel. The clinical implication is that for patients who arrive from another facility and already have been given clopidogrel, it is safe to give ticagrelor. There is limited information about whether that is also true for prasugrel, although there is no known reason why the safety of adding prasugrel to clopidogrel should be different from that of ticagrelor.
The rate of ischemic events was 20% lower in the ticagrelor group than in the clopidogrel group, importantly including reductions in the incidence of death, MI, and stent thrombosis. There was no increase with ticagrelor compared with clopidogrel in bleeding associated with coronary artery bypass graft surgery, likely because of the more rapid washout of the ticagrelor effect, or in the need for blood transfusions. However, the rate of bleeding unrelated to coronary artery bypass was about 20% higher with ticagrelor.
In summary, more intense platelet inhibition reduces the risk of ischemic events, but, particularly for the irreversible inhibitor prasugrel, at the cost of a higher risk of bleeding. In general, the net benefit of these agents in preventing the irreversible complications of MI and (in the case of ticagrelor) death favor the use of the more intense ADP inhibitors in appropriate patients. Ticagrelor is indicated in patients with acute coronary syndromes undergoing invasive or conservative management; prasugrel is indicated in patients undergoing PCI, but contraindicated in patients with a previous stroke or transient ischemic event. Neither drug is indicated in patients undergoing elective PCI outside the setting of acute coronary syndromes, although these agents may be appropriate in patients with intolerance or allergy to clopidogrel.
Glycoprotein IIb/IIIa antagonists for select cases only
GP IIb/IIIa antagonists such as abciximab were previously used more commonly than they are today. Now, with routine pretreatment using thienopyridines, their role in acute coronary syndromes is less clear. They still play a role when routine dual antiplatelet therapy is not used, when prasugrel or ticagrelor is not used, and when heparin rather than an alternative antithrombin agent is used.
A meta-analysis29 of 3,755 patients showed a clear reduction in ischemic complications with abciximab as an adjunct to primary PCI for STEMI in patients treated with heparin.
Kastrati et al30 found that patients with non-ST-elevation acute coronary syndromes benefited from abciximab at the time of PCI with heparin, even though they had been routinely pretreated with clopidogrel. However, benefits were seen only in high-risk patients who had presented with elevated troponins.
On the other hand, the role of GP IIb/IIIa blockade for “upstream” medical management in patients with acute coronary syndromes has been eroded by several studies.
The ACUITY trial31 randomized more than 9,000 patients to receive either routine treatment with a GP IIb/IIIa inhibitor before angiography or deferred selective use in the catheterization laboratory only for patients undergoing PCI. No significant differences were found in rates of MI and death.
The Early ACS trial32 compared early routine eptifibatide vs delayed, provisional eptifibatide in 9,492 patients with acute coronary syndromes without ST elevation and who were assigned to an invasive strategy. The early-eptifibatide group received two boluses and an infusion of eptifibatide before angiography; the others received a placebo infusion, with provisional eptifibatide after angiography if the patient underwent PCI and was deemed at high risk. No significant difference in rates of death or MI were noted, and the early-eptifibatide group had significantly higher rates of bleeding and need for transfusion.
The FINESSE trial33 also discredited “facilitating” PCI by giving GP IIb/IIIa antagonists in patients with STEMI before arrival in the catheterization laboratory, with no benefit to giving abciximab ahead of time vs in the catheterization laboratory, and with an increased risk of bleeding complications.
These studies have helped narrow the use of GP IIb/IIIa inhibitors to the catheterization laboratory in conjunction with heparin anticoagulation (as compared with bivalirudin [Angiomax]; see below) and only in select or high-risk cases. These drugs are indicated in the medical phase of management only if patients cannot be stabilized by aspirin or ADP inhibition.
NEWER ANTITHROMBOTICS: ADVANTAGES UNCLEAR
The complex coagulation cascade has a number of components, but only a few are targeted by drugs that are approved and recommended: fondaparinux (Arixtra) and oral factor Xa inhibitors affect the prothrombinase complex (including factor X); bivalirudin and oral factor IIa inhibitors affect thrombin; and heparin and the low-molecular-weight heparins inhibit both targets.
Low-molecular-weight heparins
The SYNERGY trial34 randomized nearly 10,000 patients with non-ST-elevation acute coronary syndromes at high risk for ischemic cardiac complications managed with an invasive approach to either the low-molecular-weight heparin enoxaparin (Lovenox) or intravenous unfractionated heparin immediately after enrollment. Most patients underwent catheterization and revascularization. No clinical advantage was found for enoxaparin, and bleeding complications were increased.
The EXTRACT-TIMI 25 trial35 randomized more than 20,000 patients with STEMI who were about to undergo fibrinolysis to receive either enoxaparin throughout hospitalization (average of 8 days) or unfractionated heparin for at least 48 hours. The enoxaparin group had a lower rate of recurrent MI, but it was unclear if the difference was in part attributable to the longer therapy time. The enoxaparin group also had more bleeding.
Fondaparinux
The OASIS-5 trial36,37 compared enoxaparin and fondaparinux, an exclusive factor Xa inhibitor, in more than 20,000 patients with unstable angina or NSTEMI. Fondaparinux was associated with a lower risk of death and reinfarction as well as fewer bleeding events. However, the benefits were almost exclusively in patients treated medically. In those undergoing PCI within the first 8 days, no benefit was found, although there was still a significant reduction in major bleeding events. Catheter thrombosis was also increased in patients taking fondaparinux, but only in those who did not receive adequate unfractionated heparin treatment before PCI.
Bivalirudin superior at time of catheterization
The most significant advance in antithrombotic therapy for patients with acute coronary syndromes is bivalirudin. This drug has a clear role only in the catheterization laboratory, where patients can be switched to it from heparin, low-molecular-weight heparin, or fondaparinux.
Three trials38–40 evaluated the drug in a total of more than 20,000 patients receiving invasive management of coronary artery disease undergoing PCI for elective indications, NSTEMI, or STEMI.
Results were remarkably similar across the three trials. Patients who were treated with bivalirudin alone had the same rate of ischemic end points at 30 days as those receiving heparin plus a GP IIb/IIIa inhibitor, but bivalirudin was associated with a consistent and significant 40% to 50% lower bleeding risk. For the highest-risk patients, those with STEMI, the bivalirudin group also had a significantly lower risk of death at 1 year.41
OTHER DRUGS: EARLY TREATMENT NO LONGER ROUTINE
Most data for the use of therapies aside from antithrombotics are from studies of patients with STEMI, but findings can logically be extrapolated to those with non-ST-elevation acute coronary syndromes.
Beta-blockers: Cardiogenic shock a risk
For beta-blockers, many historical trials were done in stable coronary disease, but there are no large trials in the setting of NSTEMI or unstable angina, and only recently have there been large trials for STEMI. Before the availability of recent evidence, standard practice was to treat STEMI routinely with intravenous metoprolol (Lopressor) and then oral metoprolol.
When large studies were finally conducted, the results were sobering.
COMMIT.42 Nearly 46,000 patients with suspected acute MI were randomized to receive either metoprolol (up to 15 mg intravenously, then 200 mg by mouth daily until discharge or for up to 4 weeks in the hospital) or placebo. Surprisingly, although rates of reinfarction and ventricular fibrillation were lower with metoprolol, a higher risk of cardiogenic shock with early beta-blockade offset these benefits and the net mortality rate was not reduced. This study led to a reduction in the early use of beta-blockers in patients with STEMI.
The standard of care has now shifted from beta-blockers in everyone as early as possible after MI to being more cautious in patients with contraindications, including signs of heart failure or a low-output state, or even in those of advanced age or with borderline low blood pressure or a high heart rate. Patients who present late and therefore may have a larger infarct are also at higher risk.
Although the goal should be to ultimately discharge patients on beta-blocker therapy after an MI, there should be no rush to start one early.
Carvedilol now preferred after STEMI
The CAPRICORN trial43 randomized nearly 2,000 patients following MI with left ventricular dysfunction (an ejection fraction of 40% or below) to either placebo or the beta-blocker carvedilol (Coreg). Patients taking the drug had a clear reduction in rates of death and reinfarction, leading to this drug becoming the beta-blocker of choice in patients with ventricular dysfunction after STEMI.
Angiotensin-converting enzyme inhibitors: Early risk of cardiogenic shock
The use of angiotensin-converting enzyme (ACE) inhibitors after MI is also supported by several studies.44 Two very large studies, one of nearly 60,000 patients and one of nearly 20,000, showed a clear reduction in the mortality rate in those who received an ACE inhibitor. Most of the benefit was in patients with an ejection fraction of less than 40%. On the basis of these trials, ACE inhibitors are indicated for all patients for the first 30 days after MI and then indefinitely for those with left ventricular dysfunction. However, the trial in which an ACE inhibitor was given intravenously early on had to be stopped prematurely because of worse outcomes owing to cardiogenic shock.
These studies highlight again that for patients who are unstable in the first few days of an acute coronary syndrome, it is best to wait until their condition stabilizes and to start these therapies before hospital discharge.
Intensive statin therapy
In the last 20 years, unequivocal evidence has emerged to support the beneficial role of statins for secondary prevention in patients with established coronary artery disease. More-recent trials have also shown that intensive statin therapy (a high dose of a potent statin) improves outcomes better than lower doses.
The PROVE-IT TIMI 22 trial45 randomized patients after an acute coronary syndrome to receive either standard therapy (pravastatin [Pravachol] 40 mg) or intensive therapy (atorvastatin [Lipitor] 80 mg). The intensive-therapy group had a significantly lower rate of major cardiovascular events, and the difference persisted and grew over 30 months of follow-up.
A number of studies confirmed this and broadened the patient population to those with unstable or stable coronary disease. Regardless of the risk profile, the effects were consistent and showed that high-dose statins were better in preventing coronary death and MI.46
Guidelines are evolving toward recommendation of highest doses of statins independently of the target level of low-density lipoprotein cholesterol.
- Antman EM, Anbe DT, Armstrong PW, et al; American College of Cardiology; American Heart Association Task Force on Practice Guidelines; Canadian Cardiovascular Society. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction. Circulation 2004; 110:e82–e292. Erratum in: Circulation 2005; 111:2013–2014.
- Davies MJ. The pathophysiology of acute coronary syndromes. Heart 2000; 83:361–366.
- Rosamond W, Flegal K, Friday G, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics–2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2007; 115:e69–e171.
- Granger CB, Califf RM, Topol EJ. Thrombolytic therapy for acute myocardial infarction. A review. Drugs 1992; 44:293–325.
- Fibrinolytic Therapy Trialists’ (FTT) Collaborative Group. Indications for fibrinolytic therapy in suspected acute myocardial infarction: collaborative overview of early mortality and major morbidity results from all randomised trials of more than 1000 patients. Lancet 1994; 343:311–322.
- Keely EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet 2003; 361:13–20
- De Luca G, Suryapranata H, Ottervanger JP, Antman EM. Time delay to treatment and mortality in primary angioplasty for acute myocardial infarction: every minute of delay counts. Circulation 2004; 109:1223–1225.
- Dalby M, Bouzamondo A, Lechat P, Montalescot G. Transfer for primary angioplasty versus immediate thrombolysis in acute myocardial infarction: a meta-analysis. Circulation 2003; 108:1809–1814.
- Gersh BJ, Stone GW, White HD, Holmes DR Jr. Pharmacological facilitation of primary percutaneous coronary intervention for acute myocardial infarction: is the slope of the curve the shape of the future? JAMA 2005; 293:979–986.
- Antman EM, Hand M, Armstron PW, et al; Canadian Cardiovascular Society; American Academy of Family Physicians; American College of Cardiology; American Heart Association. 2007 focused update of the ACC/AHA 2004 guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2008; 51:210–247.
- Di Mario C, Dudek D, Piscione F, et al; CARESS-in-AMI (Combined Abciximab Reteplase Stent Study in Acute Myocardial Infarction) Investigators. Immediate angioplasty versus standard therapy with rescue angioplasty after thrombolysis in the Combined Abciximab REteplase Stent Study in Acute Myocardial Infarction (CARESS-in-AMI): an open, prospective, randomised, multicentre trial. Lancet 2008; 371:559–568.
- Cantor WJ, Fitchett D, Borgundvaag B, et al; TRANSFER-AMI Trial Investigators. Routine early angioplasty after fibrinolysis for acute myocardial infarction. N Engl J Med 2009; 360:2705–2718.
- Cannon CP, Weintraub WS, Demopoulos LA, et al; TACTICS (Treat Angina With Aggrastat and Determine Cost of Therapy With an Invasive or Conservative Strategy)–Thrombolysis in Myocardial Infarction 18 Investigators. Comparison of early invasive and conservative strategies in patients with unstable coronary syndromes treated with the glycoprotein IIb/IIIa inhibitor tirofiban. N Engl J Med 2001; 344:1879–1887.
- Damman P, Hirsch A, Windhausen F, Tijssen JG, de Winter RJ; ICTUS Investigators. 5-year clinical outcomes in the ICTUS (Invasive versus Conservative Treatment in Unstable coronary Syndromes) trial a randomized comparison of an early invasive versus selective invasive management in patients with non-ST-segment elevation acute coronary syndrome. J Am Coll Cardiol 2010; 55:858–864.
- Bavry AA, Kumbhani DJ, Rassi AN, Bhatt DL, Askari AT. Benefit of early invasive therapy in acute coronary syndromes: a meta-analysis of contemporary randomized clinical trials. J Am Coll Cardiol 2006; 48:1319–1325.
- Antman EM, Cohen M, Bernink PJ, et al. The TIMI risk score for unstable angina/non-ST elevation MI: a method for prognostication and therapeutic decision making. JAMA 2000; 284:835–842.
- Mehta SR, Granger CB, Boden WE, et al; TIMACS Investigators. Early versus delayed invasive intervention in acute coronary syndromes. N Engl J Med 2009; 360:2165–2175.
- Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-Elevation myocardial infarction. J Am Coll Cardiol 2007; 50:e1–e157.
- Yousef O, Bhatt DL. The evolution of antiplatelet therapy in cardiovascular disease. Nat Rev Cardiol 2011; 8:547–559.
- ISIS-2 (Second International Study of Infarct Survival) Collaborative Group. Randomised trial of intravenous streptokinase, oral aspirin, both, or neither among 17,187 cases of suspected acute myocardial infarction: ISIS-2. Lancet 1988; 2:349–360.
- CURRENT-OASIS 7 Investigators; Mehta SR, Bassand JP, Chrolavicius S, et al. Dose comparisons of clopidogrel and aspirin in acute coronary syndromes. N Engl J Med 2010; 363:930–942.
- Yusuf S, Mehta SR, Zhao F, et al; Clopidogrel in Unstable angina to prevent Recurrent Events Trial Investigators. Early and late effects of clopidogrel in patients with acute coronary syndromes. Circulation 2003; 107:966–972.
- Chen ZM, Jiang LX, Chen YP, et al; COMMIT (Clopidogrel and Metoprolol in Myocardial Infarction Trial) collaborative group. Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet 2005; 366:1607–1621.
- Schömig A. Ticagrelor—is there need for a new player in the antiplatelet-therapy field? N Engl J Med 2009; 361:1108–1111.
- Wiviott SD, Antman EM, Braunwald E. Prasugrel. Circulation 2010; 122:394–403.
- Gurbel PA, Bliden KP, Butler K, et al. Randomized double-blind assessment of the ONSET and OFFSET of the antiplatelet effects of ticagrelor versus clopidogrel in patients with stable coronary artery disease: the ONSET/OFFSET study. Circulation 2009; 120:2577–2585.
- Wiviott SD, Braunwald E, McCabe CH, et al; TRITON-TIMI 38 Investigators. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007; 357:2001–2015.
- Wallentin L, Becker RC, Budaj A, et al; PLATO Investigators. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2009; 361:1045–1057.
- de Queiroz Fernandes Araujo JO, Veloso HH, Braga De Paiva JM, Fiho MW, Vincenzo De Paola AA. Efficacy and safety of abciximab on acute myocardial infarction treated with percutaneous coronary interventions: a meta-analysis of randomized, controlled trials. Am Heart J 2004; 148:937–943.
- Kastrati A, Mehilli J, Neuman FJ, et al; Intracoronary Stenting and Antithrombotic: Regimen Rapid Early Action for Coronary Treatment 2 (ISAR-REACT 2) Trial Investigators. Abciximab in patients with acute coronary syndromes undergoing percutaneous coronary intervention after clopidogrel pretreatment: the ISAR-REACT 2 randomized trial. JAMA 2006; 295:1531–1538.
- Stone GW, Bertrand ME, Moses JW, et al; ACUITY Investigators. Routine upstream initiation vs deferred selective use of glycoprotein IIb/IIIa inhibitors in acute coronary syndromes: the ACUITY Timing trial. JAMA 2007; 297:591–602.
- Giugliano RP, White JA, Bode C, et al; Early ACS Investigators. Early vs delayed, provisional eptifibatide in acute coronary syndromes. N Engl J Med 2009; 360:2176–2190.
- Ellis SG, Tendera M, de Belder MA, et al; FINESSE Investigators. Facilitated PCI in patients with ST-elevation myocardial infarction. N Engl J Med 2008; 358:2205–2217.
- Fergusson JJ, Califf RM, Antman EM, et al; SYNERGY Trial Investigators. Enoxaparin vs unfractionated heparin in high-risk patients with non-ST-segment elevation acute coronary syndromes managed with an intended early invasive strategy: primary results of the SYNERGY randomized trial. JAMA 2004; 292:45–54.
- Antman EM, Morrow DA, McCabe CH; EXTRACT-TIMI 25 Investigators. Enoxaparin versus unfractionated heparin with fibrinolysis for ST-elevation myocardial infarction. N Engl J Med 2006; 354:1477–1488.
- The Fifth Organization to Assess Strategies in Acute Ischemic Syndromes Investigators. Comparison of fondaparinux and enoxaparin in acute coronary syndromes. N Engl J Med 2006; 354:1464–1476.
- Mehta SR, Granger CB, Eikelboom JW, et al. Efficacy and safety of fondaparinux versus enoxaparin in patients with acute coronary syndromes undergoing percutaneous coronary intervention: results from the OASIS-5 trial. J Am Coll Cardiol 2007; 50:1742–1751.
- Lincoff AM, Bittl JA, Harrington RA, et al; REPLACE-2 Investigators. Bivalirudin and provisional glycoprotein IIb/IIIa blockade compared with heparin and planned glycoprotein IIb/IIIa blockade during percutaneous coronary intervention: REPLACE-2 randomized trial. JAMA 2003; 289:853–863.
- Stone GW, McLaurin BT, Cox DA, et al; ACUITY Investigators. Bivalirudin for patients with acute coronary syndromes. N Engl J Med 2006; 355:2203–2216.
- Stone GW, Witzenbichler B, Guagliumi G, et al; HORIZONS-AMI Trial Investigators. Bivalirudin during primary PCI in acute myocardial infarction. N Engl J Med 2007; 358:2218–2230.
- Mehran R, Lansky AJ, Witzenbichler B, et al; HORIZONS-AMI Trial Investigators. Bivalirudin in patients undergoing primary angioplasty for acute myocardial infarction (HORIZONS-AMI): 1-year results of a randomised controlled trial. Lancet 2009; 374:1149–1159.
- Chen ZM, Pan HC, Chen YP, et al; COMMIT (Clopidogrel and Metoprolol in Myocardial Infarction Trial) Collaborative Group. Early intravenous then oral metoprolol in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet 2005; 366:1622–1632.
- Dargie JH. Effect of carvedilol on outcome after myocardial infarction in patients with left-ventricular dysfunction: the CAPRICORN randomised trial. Lancet 2001; 357:1385–1390.
- Hennekens CH, Albert CM, Godfried SL, Gaziano JM, Buring JE. Adjunctive drug therapy of acute myocardial infarction—evidence from clinical trials. N Engl J Med 1996; 335:1660–1667.
- Cannon CP, Braunwald E, McCabe CH, et al; Pravastatin or Atorvastatin Evaluation and Infection Therapy–Thrombolysis in Myocardial Infarction 22 Investigators. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004; 350:1495–1504.
- Cannon CP, Steinberg BA, Murphy SA, Mega JL, Braunwald E. Meta-analysis of cardiovascular outcomes trials comparing intensive versus moderate statin therapy. J Am Coll Cardiol 2006; 48:438–445.
- Antman EM, Anbe DT, Armstrong PW, et al; American College of Cardiology; American Heart Association Task Force on Practice Guidelines; Canadian Cardiovascular Society. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction. Circulation 2004; 110:e82–e292. Erratum in: Circulation 2005; 111:2013–2014.
- Davies MJ. The pathophysiology of acute coronary syndromes. Heart 2000; 83:361–366.
- Rosamond W, Flegal K, Friday G, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics–2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2007; 115:e69–e171.
- Granger CB, Califf RM, Topol EJ. Thrombolytic therapy for acute myocardial infarction. A review. Drugs 1992; 44:293–325.
- Fibrinolytic Therapy Trialists’ (FTT) Collaborative Group. Indications for fibrinolytic therapy in suspected acute myocardial infarction: collaborative overview of early mortality and major morbidity results from all randomised trials of more than 1000 patients. Lancet 1994; 343:311–322.
- Keely EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet 2003; 361:13–20
- De Luca G, Suryapranata H, Ottervanger JP, Antman EM. Time delay to treatment and mortality in primary angioplasty for acute myocardial infarction: every minute of delay counts. Circulation 2004; 109:1223–1225.
- Dalby M, Bouzamondo A, Lechat P, Montalescot G. Transfer for primary angioplasty versus immediate thrombolysis in acute myocardial infarction: a meta-analysis. Circulation 2003; 108:1809–1814.
- Gersh BJ, Stone GW, White HD, Holmes DR Jr. Pharmacological facilitation of primary percutaneous coronary intervention for acute myocardial infarction: is the slope of the curve the shape of the future? JAMA 2005; 293:979–986.
- Antman EM, Hand M, Armstron PW, et al; Canadian Cardiovascular Society; American Academy of Family Physicians; American College of Cardiology; American Heart Association. 2007 focused update of the ACC/AHA 2004 guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2008; 51:210–247.
- Di Mario C, Dudek D, Piscione F, et al; CARESS-in-AMI (Combined Abciximab Reteplase Stent Study in Acute Myocardial Infarction) Investigators. Immediate angioplasty versus standard therapy with rescue angioplasty after thrombolysis in the Combined Abciximab REteplase Stent Study in Acute Myocardial Infarction (CARESS-in-AMI): an open, prospective, randomised, multicentre trial. Lancet 2008; 371:559–568.
- Cantor WJ, Fitchett D, Borgundvaag B, et al; TRANSFER-AMI Trial Investigators. Routine early angioplasty after fibrinolysis for acute myocardial infarction. N Engl J Med 2009; 360:2705–2718.
- Cannon CP, Weintraub WS, Demopoulos LA, et al; TACTICS (Treat Angina With Aggrastat and Determine Cost of Therapy With an Invasive or Conservative Strategy)–Thrombolysis in Myocardial Infarction 18 Investigators. Comparison of early invasive and conservative strategies in patients with unstable coronary syndromes treated with the glycoprotein IIb/IIIa inhibitor tirofiban. N Engl J Med 2001; 344:1879–1887.
- Damman P, Hirsch A, Windhausen F, Tijssen JG, de Winter RJ; ICTUS Investigators. 5-year clinical outcomes in the ICTUS (Invasive versus Conservative Treatment in Unstable coronary Syndromes) trial a randomized comparison of an early invasive versus selective invasive management in patients with non-ST-segment elevation acute coronary syndrome. J Am Coll Cardiol 2010; 55:858–864.
- Bavry AA, Kumbhani DJ, Rassi AN, Bhatt DL, Askari AT. Benefit of early invasive therapy in acute coronary syndromes: a meta-analysis of contemporary randomized clinical trials. J Am Coll Cardiol 2006; 48:1319–1325.
- Antman EM, Cohen M, Bernink PJ, et al. The TIMI risk score for unstable angina/non-ST elevation MI: a method for prognostication and therapeutic decision making. JAMA 2000; 284:835–842.
- Mehta SR, Granger CB, Boden WE, et al; TIMACS Investigators. Early versus delayed invasive intervention in acute coronary syndromes. N Engl J Med 2009; 360:2165–2175.
- Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-Elevation myocardial infarction. J Am Coll Cardiol 2007; 50:e1–e157.
- Yousef O, Bhatt DL. The evolution of antiplatelet therapy in cardiovascular disease. Nat Rev Cardiol 2011; 8:547–559.
- ISIS-2 (Second International Study of Infarct Survival) Collaborative Group. Randomised trial of intravenous streptokinase, oral aspirin, both, or neither among 17,187 cases of suspected acute myocardial infarction: ISIS-2. Lancet 1988; 2:349–360.
- CURRENT-OASIS 7 Investigators; Mehta SR, Bassand JP, Chrolavicius S, et al. Dose comparisons of clopidogrel and aspirin in acute coronary syndromes. N Engl J Med 2010; 363:930–942.
- Yusuf S, Mehta SR, Zhao F, et al; Clopidogrel in Unstable angina to prevent Recurrent Events Trial Investigators. Early and late effects of clopidogrel in patients with acute coronary syndromes. Circulation 2003; 107:966–972.
- Chen ZM, Jiang LX, Chen YP, et al; COMMIT (Clopidogrel and Metoprolol in Myocardial Infarction Trial) collaborative group. Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet 2005; 366:1607–1621.
- Schömig A. Ticagrelor—is there need for a new player in the antiplatelet-therapy field? N Engl J Med 2009; 361:1108–1111.
- Wiviott SD, Antman EM, Braunwald E. Prasugrel. Circulation 2010; 122:394–403.
- Gurbel PA, Bliden KP, Butler K, et al. Randomized double-blind assessment of the ONSET and OFFSET of the antiplatelet effects of ticagrelor versus clopidogrel in patients with stable coronary artery disease: the ONSET/OFFSET study. Circulation 2009; 120:2577–2585.
- Wiviott SD, Braunwald E, McCabe CH, et al; TRITON-TIMI 38 Investigators. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007; 357:2001–2015.
- Wallentin L, Becker RC, Budaj A, et al; PLATO Investigators. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2009; 361:1045–1057.
- de Queiroz Fernandes Araujo JO, Veloso HH, Braga De Paiva JM, Fiho MW, Vincenzo De Paola AA. Efficacy and safety of abciximab on acute myocardial infarction treated with percutaneous coronary interventions: a meta-analysis of randomized, controlled trials. Am Heart J 2004; 148:937–943.
- Kastrati A, Mehilli J, Neuman FJ, et al; Intracoronary Stenting and Antithrombotic: Regimen Rapid Early Action for Coronary Treatment 2 (ISAR-REACT 2) Trial Investigators. Abciximab in patients with acute coronary syndromes undergoing percutaneous coronary intervention after clopidogrel pretreatment: the ISAR-REACT 2 randomized trial. JAMA 2006; 295:1531–1538.
- Stone GW, Bertrand ME, Moses JW, et al; ACUITY Investigators. Routine upstream initiation vs deferred selective use of glycoprotein IIb/IIIa inhibitors in acute coronary syndromes: the ACUITY Timing trial. JAMA 2007; 297:591–602.
- Giugliano RP, White JA, Bode C, et al; Early ACS Investigators. Early vs delayed, provisional eptifibatide in acute coronary syndromes. N Engl J Med 2009; 360:2176–2190.
- Ellis SG, Tendera M, de Belder MA, et al; FINESSE Investigators. Facilitated PCI in patients with ST-elevation myocardial infarction. N Engl J Med 2008; 358:2205–2217.
- Fergusson JJ, Califf RM, Antman EM, et al; SYNERGY Trial Investigators. Enoxaparin vs unfractionated heparin in high-risk patients with non-ST-segment elevation acute coronary syndromes managed with an intended early invasive strategy: primary results of the SYNERGY randomized trial. JAMA 2004; 292:45–54.
- Antman EM, Morrow DA, McCabe CH; EXTRACT-TIMI 25 Investigators. Enoxaparin versus unfractionated heparin with fibrinolysis for ST-elevation myocardial infarction. N Engl J Med 2006; 354:1477–1488.
- The Fifth Organization to Assess Strategies in Acute Ischemic Syndromes Investigators. Comparison of fondaparinux and enoxaparin in acute coronary syndromes. N Engl J Med 2006; 354:1464–1476.
- Mehta SR, Granger CB, Eikelboom JW, et al. Efficacy and safety of fondaparinux versus enoxaparin in patients with acute coronary syndromes undergoing percutaneous coronary intervention: results from the OASIS-5 trial. J Am Coll Cardiol 2007; 50:1742–1751.
- Lincoff AM, Bittl JA, Harrington RA, et al; REPLACE-2 Investigators. Bivalirudin and provisional glycoprotein IIb/IIIa blockade compared with heparin and planned glycoprotein IIb/IIIa blockade during percutaneous coronary intervention: REPLACE-2 randomized trial. JAMA 2003; 289:853–863.
- Stone GW, McLaurin BT, Cox DA, et al; ACUITY Investigators. Bivalirudin for patients with acute coronary syndromes. N Engl J Med 2006; 355:2203–2216.
- Stone GW, Witzenbichler B, Guagliumi G, et al; HORIZONS-AMI Trial Investigators. Bivalirudin during primary PCI in acute myocardial infarction. N Engl J Med 2007; 358:2218–2230.
- Mehran R, Lansky AJ, Witzenbichler B, et al; HORIZONS-AMI Trial Investigators. Bivalirudin in patients undergoing primary angioplasty for acute myocardial infarction (HORIZONS-AMI): 1-year results of a randomised controlled trial. Lancet 2009; 374:1149–1159.
- Chen ZM, Pan HC, Chen YP, et al; COMMIT (Clopidogrel and Metoprolol in Myocardial Infarction Trial) Collaborative Group. Early intravenous then oral metoprolol in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet 2005; 366:1622–1632.
- Dargie JH. Effect of carvedilol on outcome after myocardial infarction in patients with left-ventricular dysfunction: the CAPRICORN randomised trial. Lancet 2001; 357:1385–1390.
- Hennekens CH, Albert CM, Godfried SL, Gaziano JM, Buring JE. Adjunctive drug therapy of acute myocardial infarction—evidence from clinical trials. N Engl J Med 1996; 335:1660–1667.
- Cannon CP, Braunwald E, McCabe CH, et al; Pravastatin or Atorvastatin Evaluation and Infection Therapy–Thrombolysis in Myocardial Infarction 22 Investigators. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004; 350:1495–1504.
- Cannon CP, Steinberg BA, Murphy SA, Mega JL, Braunwald E. Meta-analysis of cardiovascular outcomes trials comparing intensive versus moderate statin therapy. J Am Coll Cardiol 2006; 48:438–445.
KEY POINTS
- For acute ST-elevation myocardial infarction, primary percutaneous coronary intervention is preferred over fibrinolytic therapy if it is available within 90 minutes of first medical contact.
- For non-ST-elevation acute coronary syndromes, either an early invasive or conservative strategy is recommended depending on patient risk and whether intensive medical therapy is available and appropriate.
- Daily aspirin therapy is indicated for all patients with acute coronary syndromes unless they have a true aspirin allergy.
- Adenosine diphosphate receptor inhibitors—clopidogrel, prasugrel, and ticagrelor—reduce ischemic events but increase bleeding risk and should be used only for patients with no history of stroke or transient ischemic attack.
The METEOR trial: No rush to repair a torn meniscus
Many patients who have osteoarthritis of the knee and a torn meniscus can defer having the meniscus repaired and undergo physical therapy instead. If a trial of physical therapy does not help, they can opt for surgery later.
This seems to be the take-home message from the recent Meniscal Tear in Osteoarthritis Research (METEOR) trial,1 which compared the efficacy of arthroscopic partial meniscectomy plus physical therapy vs physical therapy alone for patients with knee symptoms, a meniscal tear, and mild to moderate osteoarthritis of the knee.1
In brief, patients improved to a roughly similar degree with either approach, and although many patients assigned to physical therapy eventually underwent surgery anyway by 6 months, the delay did not adversely affect outcomes.
In this article, we review the background, design, and findings of the METEOR trial, and their implications for clinical practice.
SURGERY: HIGH VOLUME, BUT LITTLE EVIDENCE
Magnetic resonance imaging (MRI) often incidentally reveals meniscal lesions in middle-aged and older patients who have osteoarthritis and knee pain.2 Should these patients undergo arthroscopic meniscal repair? The decision is difficult, since it is hard to distinguish the symptoms of a meniscal tear from those of osteoarthritis.3
Current evidence suggests that, for symptomatic knee osteoarthritis by itself, arthroscopic surgery is no more effective than conservative management.4,5 But what about surgery for a torn meniscus in addition to osteoarthritis?
Osteoarthritis is the most common joint disease, accounting for many physician visits.6 More than 26 million Americans over age 25 have some form of it, and the prevalence of symptomatic, radiographically confirmed osteoarthritis of the knee was 12.1% in the third National Health and Nutrition Examination Survey.7
We used to consider osteoarthritis a “wear-and-tear” disease—thus the term “degenerative joint disease.” But today, we know that it is an active response to injury, involving inflammatory and metabolic pathways.8 Moreover, the risk of osteoarthritis and its progression seems to be higher in those who have had meniscal injury and total or arthroscopic partial meniscectomy.9,10
MRI is not commonly used in managing knee osteoarthritis, but it has been used diagnostically in patients with symptoms of a meniscal tear, such as clicking, locking, popping, giving way, and pain with pivoting or twisting. Traumatic meniscal tears (a longitudinal or radial tear pattern) most often occur in active younger people and often lead to meniscal surgery.11,12 In contrast, degenerative meniscal tears (horizontal, oblique, or complex tear pattern or meniscal maceration) tend to occur in older people,11,12 but how to manage them is not widely agreed upon.
Of note, most patients with osteoarthritis of the knee have torn, macerated, or heavily damaged menisci.13,14 Meniscal lesions are also common in middle-aged people in the general population, with a higher prevalence in people who are older, heavier, or female, or who have a family history of osteoarthritis.15
These abnormalities are only weakly associated with symptoms.2 However, when a patient has knee symptoms and a torn meniscus is detected on MRI, the tear is often assumed to be the source of the symptoms, and meniscal tears are the most common reason for arthroscopy.16
Since we have no way to prevent the progression of joint damage from osteoarthritis with drugs or by any other means, the goal is to alleviate the symptoms. Many patients report pain relief or functional improvement after arthroscopic surgery. But arthroscopic lavage or debridement for osteoarthritis has not been found to be better than conservative treatment or placebo in randomized controlled trials.4,5
In contrast, the current standard treatment for a symptomatic degenerative meniscal tear is arthroscopic partial meniscectomy. Nearly 500,000 of these procedures are performed annually in the United States.16 But based on the best evidence, arthroscopic partial meniscectomy does not result in better pain relief and functional improvement than does physical therapy alone in patients who have a torn meniscus and knee osteoarthritis.17,18
OVERVIEW OF THE METEOR TRIAL
The METEOR trial was a randomized controlled trial conducted at seven US tertiary referral centers. Its aim was to compare the short-term (6-month) and long-term (12-month) efficacy of arthroscopic partial meniscectomy and physical therapy in patients with symptomatic meniscal tear and osteoarthritis of the knee.19 It was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases.1
Patients were age 45 and older
METEOR patients had to be at least 45 years old and have symptomatic meniscal tears and knee osteoarthritis detected on MRI or radiography.1
Osteoarthritis was defined broadly, given that it begins well before the appearance of radiographic evidence such as an osteophyte or joint-space narrowing.19 Patients with cartilage defects on MRI were also enrolled, as were patients with radiographically documented osteoarthritis.19
Patients were considered to have a symptomatic meniscal tear if they had had at least 4 weeks of symptoms (such as episodic pain and pain that was acute and localized to one spot on the knee, as well as typical mechanical pain suggesting a meniscal tear, such as clicking, catching, popping, giving way, or pain with pivoting or twisting) in addition to evidence of a meniscal tear on MRI.19
Patients were excluded if they had a chronically locked knee (a clear-cut indication for arthroscopic partial meniscectomy), advanced osteoarthritis (Kellgren-Lawrence grade 4), inflammatory arthritis, clinically symptomatic chondrocalcinosis, or bilateral symptomatic meniscal tears.19 Patients who had undergone surgery or injection of a viscosupplement in the index knee during the past 4 weeks were also excluded.19
Of 1,330 eligible patients, 351 (26.4%) were enrolled and randomly assigned in a 1:1 ratio to a treatment group by means of a secure program on the trial website.1,19 Of those who were eligible but did not enroll, 195 (14.6%) were not referred and 784 (58.9%) declined to participate. Of those who declined, more preferred surgery than physical therapy (36.1% vs 21%). No information is available on any differences in baseline characteristics between the enrolled patients and the eligible patients who declined.
Randomization was done in blocks of varying size within each site, stratified according to sex and the extent of osteoarthritis on baseline radiography. The extent of osteoarthritis was categorized either as Kellgren-Lawrence grade 0 (normal, no features of osteoarthritis) to grade 2 (definite osteoarthritis, a definite osteophyte without joint-space narrowing) or as Kellgren-Lawrence grade 3 (moderate osteoarthritis, < 50% joint-space narrowing).1,19 The two treatment groups were similar with respect to age, sex, race or ethnicity, baseline Kellgren-Lawrence grade, and baseline Western Ontario and McMaster Universities Arthritis Index (WOMAC) physical function score.1
The mean age of the participants was 58, and 85% were white. Sixty-three percent had Kellgren-Lawrence grade 0 to 2 osteoarthritis, and 27% had grade 3.1
Surgery plus physical therapy vs physical therapy alone
The surgery group underwent arthroscopic partial meniscectomy, which involved trimming the damaged meniscus back to a stable rim1,19 and trimming loose fragments of cartilage and bone.
After the procedure, patients were scheduled for physical therapy. Although there is no consensus on the need for or the effectiveness of postoperative physical therapy in this setting, the investigators believed that including it in both study groups would help to isolate the independent effects of surgery. The physical therapy regimen after surgery was similar to that provided in the nonoperative group.1,19
Physical therapy was designed to address inflammation, range of motion, muscle strength, muscle-length restriction, functional mobility, and proprioception and balance.1,19 There were three stages; criteria for advancing from one phase to the next included the level of self-reported pain, observed strength, range of knee motion, knee effusion, and functional mobility.1,18
The duration of participation varied depending on the pace of improvement. Generally, the program lasted about 6 weeks.1,19
Crossover and other therapies were allowed
Crossover from physical therapy alone to surgery was allowed during the trial if the patient and surgeon thought it was clinically indicated.
Participants in both groups were permitted to take acetaminophen and nonsteroidal anti-inflammatory drugs as needed. Intra-articular injections of glucocorticoids were also allowed during the trial.
OUTCOMES MEASURED
WOMAC physical function score
The primary outcome of the METEOR trial was the difference between the study groups in the change in WOMAC physical function score from baseline to 6 months, at which point participants were expected to have achieved maximum improvement.1,19 Questionnaires were also administered at 3 months to assess the early response to surgery or physical therapy and again at 12 months.
The complete WOMAC also measures pain and stiffness in addition to physical function, with separate subscales for each. The change in WOMAC score is one of the most widely endorsed outcome measures in assessing interventions in osteoarthritis or other conditions of the lower extremities.20 The METEOR trial authors considered the WOMAC scale to be highly valid and reliable, with a Cronbach alpha value of 0.97 (maximum value = 1; the higher the better).
No ceiling or floor effects were observed in the WOMAC physical function score in patients with osteoarthritis and a meniscal tear in a pilot study for METEOR.19
In the main METEOR study, WOMAC physical function was scored on a scale of 0 to 100, with a higher score indicating worse physical function.1 Changes in the score were also measured as a yes-or-no question, defined a priori as whether the score declined by at least 8 points, which is considered the minimal clinically important difference in osteoarthritis patients.1,19
KOOS and MOS SF-36 scores
Secondary outcomes were measured in several domains, including pain, generic functional status, quality of life, and health care utilization.1,19
The KOOS (Knee Injury and OA Outcome Scale) is specific for knee pain, being designed to evaluate short-term and long-term symptoms and function in patients with knee injury and associated problems.21 It has five subscales, which are scored separately: pain, other symptoms, activities of daily living, sport and recreation, and knee-related quality of life.21 Since the WOMAC pain scale showed a ceiling effect in the pilot study in patients undergoing surgery, the authors chose the KOOS pain scale as a pain measure.19 Scores were transformed to a 0–100 scale, with a higher score indicating more pain.1
The MOS SF-36 (Medical Outcomes Study 36-item short form) was used to measure general health status and function.1,19
STATISTICAL ANALYSIS: INTENTION-TO-TREAT AND AS-TREATED
The study was powered to detect a 10-point difference in WOMAC physical function scores at 6 months of follow-up between the operative and nonoperative groups, anticipating losses to follow-up and crossover, with preplanned subgroup (Kellgren–Lawrence grade 0–2 vs grade 3) analysis.1,19
The primary analysis used a modified intention-to-treat approach and was implemented with an analysis of covariance with changes in the WOMAC score from baseline to 6 months as the dependent variable, treatment as the independent variable of interest, and study site as a covariate. Other covariates, such as age, sex, and baseline Kellgren-Lawrence grade, were balanced across groups and were therefore not included in the analysis.1,19
Secondary analyses used an “as-treated” approach, ie, according to the treatment actually received.1,19 Secondary intention-to-treat analysis—using binary outcome measures in which treatment failure was defined as improvement in the WOMAC score of less than 8 points or crossing over to the other treatment—was also performed to estimate efficacy at the level of the patient rather than at the group level.1,19
BOTH GROUPS IMPROVED
In the intention-to-treat analyses at 6 months and 12 months after randomization, both groups improved, with no clinically important or statistically significant differences between the groups in functional status (WOMAC score, MOS SF-36 score) or pain (KOOS score).1 The mean improvement (decline) in the WOMAC score from baseline to 6 months was 20.9 points in the surgery group vs 18.5 points in the physical therapy group, a difference of 2.4 points (95% confidence interval [CI], −1.8 to 6.5).1
35% of physical therapy patients underwent surgery by 12 months
Of the 177 patients randomized to physical therapy alone, by 6 months 1 had died, 1 had undergone total knee replacement, 4 had withdrawn, and 2 were lost to follow-up. Of the 169 remaining, 51 (30%) had undergone arthroscopic partial meniscectomy. An additional 8 patients who were assigned to physical therapy crossed over to surgery between 6 and 12 months.1,19
Of the 174 patients randomized to surgery, by 6 months 1 had died, 3 had undergone total knee replacement, 7 had withdrawn, and 2 were ineligible. Of the 161 remaining, 9 (6%) had not undergone the procedure.
Other outcomes
Subgroup analysis based on the baseline radiographic grade (Kellgren-Lawrence grade 0 to 2 vs grade 3) did not show a difference between groups in functional improvement at 6 months (P = .13 for interaction).1
No statistically significant difference was noted in rates of overall or specific adverse events between the two groups over the first 12 months.1 Adverse events rated as mild or moderate in severity occurred in 15 participants in the surgery group and 13 participants in the physical therapy group.1 Long-term risks associated with these interventions are being assessed, and longitudinal assessment of imaging studies is planned to address this question but is not yet available.1,18
In the physical therapy group, 21 patients (12%) received intra-articular glucocorticoid injections, as did 9 patients (6%) in the surgery group.1,19
TRANSLATING THE METEOR RESULTS TO EVERYDAY PRACTICE
There are many challenges in designing surgical trials. Indeed, by one estimate,22 only about 40% of treatment questions involving surgical procedures can be evaluated by a randomized controlled trial.
Although the METEOR trial was not blinded, it was the first large, multicenter, randomized controlled trial to compare arthroscopic partial meniscectomy vs standardized physical therapy by using high-quality methodology such as careful sample-size calculation, balancing the groups according to known prognostic factors with block randomization, and intention-to-treat analysis. Moreover, the outcome measures were obtained from validated self-reporting questionnaires (WOMAC for function and KOOS for pain), reducing the possibility of observer bias.19 In addition, analyses were performed with the analysts blinded to the randomization assignment.
Limitations of the trial
A few limitations of the study are worth noting.
Patients age 45 or older with both symptomatic meniscal tear and osteoarthritis were the target population of this study. However, it is important to distinguish between the study population and the target population in a physician’s practice.
The investigators adopted broad definitions of osteoarthritis and symptoms of meniscal tear. Twenty-one percent of participants had normal findings on plain radiography, with cartilage defects visible only on MRI. Further, episodic pain or acute pain localized to a joint line was regarded as a symptom consistent with a torn meniscus.
In practice, arthroscopic partial meniscectomy is usually considered when a patient with a long history of tolerable osteoarthritis presents with a sudden onset of intolerable pain after a squatting or twisting injury.
In addition, the study population was predominantly white (85%), and the study was performed in tertiary referral academic medical centers. Therefore, the outcomes achieved with surgery or physical therapy may not translate to the community setting. Clinicians must be careful to account for these types of differences in extrapolating to patients in their own practice.
Potential enrollment bias
Although randomization is a rigorous method that eliminates selection bias in assigning individuals to study and control groups, selective enrollment could have created bias.1 As the authors mentioned, only 26% of eligible patients were enrolled, possibly reflecting patients’ or surgeons’ strong preferences for one treatment or the other. Because the study and control groups were hardly random samples of eligible populations, we must be careful in generalizing the efficacy of physical therapy.1
Crossover may have obscured the benefit of surgery
During the first 6 months, 30% of patients crossed over from physical therapy to surgery. High crossover rates in surgical trials are common, especially when comparing surgery with medical therapy.23 Given that most of the patients assigned to only physical therapy who crossed over to surgery did not have substantial improvement in functional status, it seems that crossover occurred by nonrandom factors, potentially biasing the study results. With the high degree of crossover from the nonoperative group to the surgical group, intention-to-treat analysis may have given an inflated estimate of the effect of physical therapy.
To account for crossovers, researchers defined a binary outcome a priori: patients were considered to have had a successful treatment response if they improved by at least 8 points on the WOMAC scale (a clinically important difference) and did not cross over from their assigned treatment. At 6 months, 67.1% of patients assigned to surgery showed a successful treatment response, compared with 43.8% of patients assigned to physical therapy alone (P = .001).1
In patients who crossed over, the last scores before crossover were carried over, and primary analysis of the WOMAC score at 6 months was repeated to estimate the effect of crossovers from the nonoperative to the surgery group. This exploratory analysis showed a 13.0-point improvement in WOMAC score at 6 months with physical therapy alone vs a 20.9-point improvement with surgery, suggesting that the similarity in outcomes between the two groups may be explained in part by additional improvements from surgery for those who crossed over from physical therapy alone.1
Implications for functional improvement
Lacking a comparison group that underwent a sham surgical procedure, one cannot conclude that surgery after crossover improved functional status in those patients. However, there was no significant difference in WOMAC physical function scores at 12 months between the 30% of patients in the physical therapy group who crossed over and underwent surgery during the first 6 months and patients initially assigned to surgery. This finding suggests that physical therapy can be recommended as a first-line therapy, although we must be cautious, given that the physical therapy group required more background therapy (eg, intra-articular glucocorticoid injections), and that this study was not powered to detect such differences at 12 months.
Also, a patient may need to get better quickly, to get back to work, for example. Although the data were not definitive, at 3 months the patients in the surgery group seemed to have better pain control and function than those in the physical therapy group. A cost-benefit analysis of physical therapy compared with surgery for short-term outcomes may be helpful before generalizing these findings.
SURGERY VS SHAM PROCEDURE: THE FIDELITY GROUP RESULTS
In a later publication from the Finnish Degenerative Meniscal Lesion Study (FIDELITY) Group,24 146 patients with symptoms consistent with degenerative meniscal tear but no knee osteoarthritis were randomized to undergo arthroscopic partial meniscectomy or a sham procedure. At 12 months, no differences were noted between the groups in terms of change of symptoms from baseline to 12 months.
The authors concluded that the outcomes with meniscectomy were no better than with a sham procedure.24
SURGERY FIRST, OR PHYSICAL THERAPY FIRST?
The use of knee arthroscopy has increased sharply in middle-aged patients in recent years. Indeed, this demographic group accounts for nearly half of the knee arthroscopic procedures performed for meniscal tears, although the increase may be due in part to issues with surgeons’ coding and insurance authorization.16
The METEOR trial showed that a structured physical therapy program can be as effective as surgery as a first-line therapy in many patients with symptomatic meniscal tears and mild to moderate osteoarthritis. These results should inform clinical practice in that most such patients need not be immediately referred for surgical intervention.
However, a subset of these patients may benefit from surgery rather than nonoperative therapy. Given the potential risks and public health implications of arthroscopic surgery for meniscal tears, further study is needed to better characterize these patients. A randomized sham-controlled trial is under way25 with the goal of assessing the efficacy of arthroscopic partial meniscectomy for medial meniscus tears in patients with or without knee osteoarthritis, and it is hoped this study will shed further light on this issue.
Based on the results of the METEOR trial, the physical therapy regimen that was used may be reasonable before referring patients with knee osteoarthritis and symptomatic meniscal tears for surgery.
- Katz JN, Brophy RH, Chaisson CE, et al. Surgery versus physical therapy for a meniscal tear and osteoarthritis. N Engl J Med 2013; 368:1675–1684.
- Englund M, Guermazi A, Gale D, et al. Incidental meniscal findings on knee MRI in middle-aged and elderly persons. N Engl J Med 2008; 359:1108–115.
- Englund M, Guermazi A, Lohmander SL. The role of the meniscus in knee osteoarthritis: a cause or consequence? Radiol Clin North Am 2009; 47:703–712.
- Moseley JB, O’Malley K, Petersen NJ, et al. A controlled trial of arthroscopic surgery for osteoarthritis of the knee. N Engl J Med 2002; 347:81–68.
- Kirkley A, Birmingham TB, Litchfield RB, et al. A randomized trial of arthroscopic surgery for osteoarthritis of the knee. N Engl J Med 2008; 359:1097–1107.
- Quintana JM, Escobar A, Arostegui I, et al. Prevalence of symptoms of knee or hip joints in older adults from the general population. Aging Clin Exp Res 2008; 20:329–336.
- Lawrence RC, Felson DT, Helmick CG, et al; National Arthritis Data Workgroup. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum 2008; 58:26–35.
- Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum 2012; 64:1697–1707.
- Englund M, Lohmander LS. Risk factors for symptomatic knee osteoarthritis fifteen to twenty-two years after meniscectomy. Arthritis Rheum 2004; 50:2811–2819.
- Papalia R, Del Buono A, Osti L, Denaro V, Maffulli N. Meniscectomy as a risk factor for knee osteoarthritis: a systematic review. Br Med Bull 2011; 99:89–106.
- Englund M, Guermazi A, Roemer FW, et al. Meniscal tear in knees without surgery and the development of radiographic osteoarthritis among middle-aged and elderly persons: The Multicenter Osteoarthritis Study. Arthritis Rheum 2009; 60:831–839.
- Poehling GG, Ruch DS, Chabon SJ. The landscape of meniscal injuries. Clin Sports Med 1990; 9:539–549.
- Bhattacharyya T, Gale D, Dewire P, et al. The clinical importance of meniscal tears demonstrated by magnetic resonance imaging in osteoarthritis of the knee. J Bone Joint Surg Am 2003; 85-A:4–9.
- Sowers M, Karvonen-Gutierrez CA, Jacobson JA, Jiang Y, Yosef M. Associations of anatomical measures from MRI with radiographically defined knee osteoarthritis score, pain, and physical functioning. J Bone Joint Surg Am 2011; 93:241–251.
- Ding C, Martel-Pelletier J, Pelletier JP, et al. Meniscal tear as an osteoarthritis risk factor in a largely non-osteoarthritic cohort: a cross-sectional study. J Rheumatol 2007; 34:776–784.
- Kim S, Bosque J, Meehan JP, Jamali A, Marder R. Increase in outpatient knee arthroscopy in the United States: a comparison of National Surveys of Ambulatory Surgery, 1996 and 2006. J Bone Joint Surg Am 2011; 93:994–1000.
- Herrlin S, Hållander M, Wange P, Weidenhielm L, Werner S. Arthroscopic or conservative treatment of degenerative medial meniscal tears: a prospective randomised trial. Knee Surg Sports Traumatol Arthrosc 2007; 15:393–401.
- Herrlin SV, Wange PO, Lapidus G, Hållander M, Werner S, Weidenhielm L. Is arthroscopic surgery beneficial in treating non-traumatic, degenerative medial meniscal tears? A five year follow-up. Knee Surg Sports Traumatol Arthrosc 2013; 21:358–364.
- Katz JN, Chaisson CE, Cole B, et al. The MeTeOR trial (Meniscal Tear in Osteoarthritis Research): rationale and design features. Contemp Clin Trials 2012; 33:1189–1196.
- Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol 1988; 15:1833–1840.
- Roos EM, Lohmander LS. The Knee injury and Osteoarthritis Outcome Score (KOOS): from joint injury to osteoarthritis. Health Qual Life Outcomes 2003; 1:64.
- Solomon MJ, McLeod RS. Should we be performing more randomized controlled trials evaluating surgical operations? Surgery 1995; 118:459–467.
- Farrokhyar F, Karanicolas PJ, Thoma A, et al. Randomized controlled trials of surgical interventions. Ann Surg 2010; 251:409–416.
- Sihvonen R, Paavola M, Malmivaara A. Arthroscopic partial meniscectomy versus sham surgery for a degenerative meniscal tear. N Engl J Med 2013; 369:2515–2524.
- Hare KB, Lohmander LS, Christensen R, Roos EM. Arthroscopic partial meniscectomy in middle-aged patients with mild or no knee osteoarthritis: a protocol for a double-blind, randomized sham-controlled multicentre trial. BMC Musculoskelet Disord 2013; 14:71.
Many patients who have osteoarthritis of the knee and a torn meniscus can defer having the meniscus repaired and undergo physical therapy instead. If a trial of physical therapy does not help, they can opt for surgery later.
This seems to be the take-home message from the recent Meniscal Tear in Osteoarthritis Research (METEOR) trial,1 which compared the efficacy of arthroscopic partial meniscectomy plus physical therapy vs physical therapy alone for patients with knee symptoms, a meniscal tear, and mild to moderate osteoarthritis of the knee.1
In brief, patients improved to a roughly similar degree with either approach, and although many patients assigned to physical therapy eventually underwent surgery anyway by 6 months, the delay did not adversely affect outcomes.
In this article, we review the background, design, and findings of the METEOR trial, and their implications for clinical practice.
SURGERY: HIGH VOLUME, BUT LITTLE EVIDENCE
Magnetic resonance imaging (MRI) often incidentally reveals meniscal lesions in middle-aged and older patients who have osteoarthritis and knee pain.2 Should these patients undergo arthroscopic meniscal repair? The decision is difficult, since it is hard to distinguish the symptoms of a meniscal tear from those of osteoarthritis.3
Current evidence suggests that, for symptomatic knee osteoarthritis by itself, arthroscopic surgery is no more effective than conservative management.4,5 But what about surgery for a torn meniscus in addition to osteoarthritis?
Osteoarthritis is the most common joint disease, accounting for many physician visits.6 More than 26 million Americans over age 25 have some form of it, and the prevalence of symptomatic, radiographically confirmed osteoarthritis of the knee was 12.1% in the third National Health and Nutrition Examination Survey.7
We used to consider osteoarthritis a “wear-and-tear” disease—thus the term “degenerative joint disease.” But today, we know that it is an active response to injury, involving inflammatory and metabolic pathways.8 Moreover, the risk of osteoarthritis and its progression seems to be higher in those who have had meniscal injury and total or arthroscopic partial meniscectomy.9,10
MRI is not commonly used in managing knee osteoarthritis, but it has been used diagnostically in patients with symptoms of a meniscal tear, such as clicking, locking, popping, giving way, and pain with pivoting or twisting. Traumatic meniscal tears (a longitudinal or radial tear pattern) most often occur in active younger people and often lead to meniscal surgery.11,12 In contrast, degenerative meniscal tears (horizontal, oblique, or complex tear pattern or meniscal maceration) tend to occur in older people,11,12 but how to manage them is not widely agreed upon.
Of note, most patients with osteoarthritis of the knee have torn, macerated, or heavily damaged menisci.13,14 Meniscal lesions are also common in middle-aged people in the general population, with a higher prevalence in people who are older, heavier, or female, or who have a family history of osteoarthritis.15
These abnormalities are only weakly associated with symptoms.2 However, when a patient has knee symptoms and a torn meniscus is detected on MRI, the tear is often assumed to be the source of the symptoms, and meniscal tears are the most common reason for arthroscopy.16
Since we have no way to prevent the progression of joint damage from osteoarthritis with drugs or by any other means, the goal is to alleviate the symptoms. Many patients report pain relief or functional improvement after arthroscopic surgery. But arthroscopic lavage or debridement for osteoarthritis has not been found to be better than conservative treatment or placebo in randomized controlled trials.4,5
In contrast, the current standard treatment for a symptomatic degenerative meniscal tear is arthroscopic partial meniscectomy. Nearly 500,000 of these procedures are performed annually in the United States.16 But based on the best evidence, arthroscopic partial meniscectomy does not result in better pain relief and functional improvement than does physical therapy alone in patients who have a torn meniscus and knee osteoarthritis.17,18
OVERVIEW OF THE METEOR TRIAL
The METEOR trial was a randomized controlled trial conducted at seven US tertiary referral centers. Its aim was to compare the short-term (6-month) and long-term (12-month) efficacy of arthroscopic partial meniscectomy and physical therapy in patients with symptomatic meniscal tear and osteoarthritis of the knee.19 It was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases.1
Patients were age 45 and older
METEOR patients had to be at least 45 years old and have symptomatic meniscal tears and knee osteoarthritis detected on MRI or radiography.1
Osteoarthritis was defined broadly, given that it begins well before the appearance of radiographic evidence such as an osteophyte or joint-space narrowing.19 Patients with cartilage defects on MRI were also enrolled, as were patients with radiographically documented osteoarthritis.19
Patients were considered to have a symptomatic meniscal tear if they had had at least 4 weeks of symptoms (such as episodic pain and pain that was acute and localized to one spot on the knee, as well as typical mechanical pain suggesting a meniscal tear, such as clicking, catching, popping, giving way, or pain with pivoting or twisting) in addition to evidence of a meniscal tear on MRI.19
Patients were excluded if they had a chronically locked knee (a clear-cut indication for arthroscopic partial meniscectomy), advanced osteoarthritis (Kellgren-Lawrence grade 4), inflammatory arthritis, clinically symptomatic chondrocalcinosis, or bilateral symptomatic meniscal tears.19 Patients who had undergone surgery or injection of a viscosupplement in the index knee during the past 4 weeks were also excluded.19
Of 1,330 eligible patients, 351 (26.4%) were enrolled and randomly assigned in a 1:1 ratio to a treatment group by means of a secure program on the trial website.1,19 Of those who were eligible but did not enroll, 195 (14.6%) were not referred and 784 (58.9%) declined to participate. Of those who declined, more preferred surgery than physical therapy (36.1% vs 21%). No information is available on any differences in baseline characteristics between the enrolled patients and the eligible patients who declined.
Randomization was done in blocks of varying size within each site, stratified according to sex and the extent of osteoarthritis on baseline radiography. The extent of osteoarthritis was categorized either as Kellgren-Lawrence grade 0 (normal, no features of osteoarthritis) to grade 2 (definite osteoarthritis, a definite osteophyte without joint-space narrowing) or as Kellgren-Lawrence grade 3 (moderate osteoarthritis, < 50% joint-space narrowing).1,19 The two treatment groups were similar with respect to age, sex, race or ethnicity, baseline Kellgren-Lawrence grade, and baseline Western Ontario and McMaster Universities Arthritis Index (WOMAC) physical function score.1
The mean age of the participants was 58, and 85% were white. Sixty-three percent had Kellgren-Lawrence grade 0 to 2 osteoarthritis, and 27% had grade 3.1
Surgery plus physical therapy vs physical therapy alone
The surgery group underwent arthroscopic partial meniscectomy, which involved trimming the damaged meniscus back to a stable rim1,19 and trimming loose fragments of cartilage and bone.
After the procedure, patients were scheduled for physical therapy. Although there is no consensus on the need for or the effectiveness of postoperative physical therapy in this setting, the investigators believed that including it in both study groups would help to isolate the independent effects of surgery. The physical therapy regimen after surgery was similar to that provided in the nonoperative group.1,19
Physical therapy was designed to address inflammation, range of motion, muscle strength, muscle-length restriction, functional mobility, and proprioception and balance.1,19 There were three stages; criteria for advancing from one phase to the next included the level of self-reported pain, observed strength, range of knee motion, knee effusion, and functional mobility.1,18
The duration of participation varied depending on the pace of improvement. Generally, the program lasted about 6 weeks.1,19
Crossover and other therapies were allowed
Crossover from physical therapy alone to surgery was allowed during the trial if the patient and surgeon thought it was clinically indicated.
Participants in both groups were permitted to take acetaminophen and nonsteroidal anti-inflammatory drugs as needed. Intra-articular injections of glucocorticoids were also allowed during the trial.
OUTCOMES MEASURED
WOMAC physical function score
The primary outcome of the METEOR trial was the difference between the study groups in the change in WOMAC physical function score from baseline to 6 months, at which point participants were expected to have achieved maximum improvement.1,19 Questionnaires were also administered at 3 months to assess the early response to surgery or physical therapy and again at 12 months.
The complete WOMAC also measures pain and stiffness in addition to physical function, with separate subscales for each. The change in WOMAC score is one of the most widely endorsed outcome measures in assessing interventions in osteoarthritis or other conditions of the lower extremities.20 The METEOR trial authors considered the WOMAC scale to be highly valid and reliable, with a Cronbach alpha value of 0.97 (maximum value = 1; the higher the better).
No ceiling or floor effects were observed in the WOMAC physical function score in patients with osteoarthritis and a meniscal tear in a pilot study for METEOR.19
In the main METEOR study, WOMAC physical function was scored on a scale of 0 to 100, with a higher score indicating worse physical function.1 Changes in the score were also measured as a yes-or-no question, defined a priori as whether the score declined by at least 8 points, which is considered the minimal clinically important difference in osteoarthritis patients.1,19
KOOS and MOS SF-36 scores
Secondary outcomes were measured in several domains, including pain, generic functional status, quality of life, and health care utilization.1,19
The KOOS (Knee Injury and OA Outcome Scale) is specific for knee pain, being designed to evaluate short-term and long-term symptoms and function in patients with knee injury and associated problems.21 It has five subscales, which are scored separately: pain, other symptoms, activities of daily living, sport and recreation, and knee-related quality of life.21 Since the WOMAC pain scale showed a ceiling effect in the pilot study in patients undergoing surgery, the authors chose the KOOS pain scale as a pain measure.19 Scores were transformed to a 0–100 scale, with a higher score indicating more pain.1
The MOS SF-36 (Medical Outcomes Study 36-item short form) was used to measure general health status and function.1,19
STATISTICAL ANALYSIS: INTENTION-TO-TREAT AND AS-TREATED
The study was powered to detect a 10-point difference in WOMAC physical function scores at 6 months of follow-up between the operative and nonoperative groups, anticipating losses to follow-up and crossover, with preplanned subgroup (Kellgren–Lawrence grade 0–2 vs grade 3) analysis.1,19
The primary analysis used a modified intention-to-treat approach and was implemented with an analysis of covariance with changes in the WOMAC score from baseline to 6 months as the dependent variable, treatment as the independent variable of interest, and study site as a covariate. Other covariates, such as age, sex, and baseline Kellgren-Lawrence grade, were balanced across groups and were therefore not included in the analysis.1,19
Secondary analyses used an “as-treated” approach, ie, according to the treatment actually received.1,19 Secondary intention-to-treat analysis—using binary outcome measures in which treatment failure was defined as improvement in the WOMAC score of less than 8 points or crossing over to the other treatment—was also performed to estimate efficacy at the level of the patient rather than at the group level.1,19
BOTH GROUPS IMPROVED
In the intention-to-treat analyses at 6 months and 12 months after randomization, both groups improved, with no clinically important or statistically significant differences between the groups in functional status (WOMAC score, MOS SF-36 score) or pain (KOOS score).1 The mean improvement (decline) in the WOMAC score from baseline to 6 months was 20.9 points in the surgery group vs 18.5 points in the physical therapy group, a difference of 2.4 points (95% confidence interval [CI], −1.8 to 6.5).1
35% of physical therapy patients underwent surgery by 12 months
Of the 177 patients randomized to physical therapy alone, by 6 months 1 had died, 1 had undergone total knee replacement, 4 had withdrawn, and 2 were lost to follow-up. Of the 169 remaining, 51 (30%) had undergone arthroscopic partial meniscectomy. An additional 8 patients who were assigned to physical therapy crossed over to surgery between 6 and 12 months.1,19
Of the 174 patients randomized to surgery, by 6 months 1 had died, 3 had undergone total knee replacement, 7 had withdrawn, and 2 were ineligible. Of the 161 remaining, 9 (6%) had not undergone the procedure.
Other outcomes
Subgroup analysis based on the baseline radiographic grade (Kellgren-Lawrence grade 0 to 2 vs grade 3) did not show a difference between groups in functional improvement at 6 months (P = .13 for interaction).1
No statistically significant difference was noted in rates of overall or specific adverse events between the two groups over the first 12 months.1 Adverse events rated as mild or moderate in severity occurred in 15 participants in the surgery group and 13 participants in the physical therapy group.1 Long-term risks associated with these interventions are being assessed, and longitudinal assessment of imaging studies is planned to address this question but is not yet available.1,18
In the physical therapy group, 21 patients (12%) received intra-articular glucocorticoid injections, as did 9 patients (6%) in the surgery group.1,19
TRANSLATING THE METEOR RESULTS TO EVERYDAY PRACTICE
There are many challenges in designing surgical trials. Indeed, by one estimate,22 only about 40% of treatment questions involving surgical procedures can be evaluated by a randomized controlled trial.
Although the METEOR trial was not blinded, it was the first large, multicenter, randomized controlled trial to compare arthroscopic partial meniscectomy vs standardized physical therapy by using high-quality methodology such as careful sample-size calculation, balancing the groups according to known prognostic factors with block randomization, and intention-to-treat analysis. Moreover, the outcome measures were obtained from validated self-reporting questionnaires (WOMAC for function and KOOS for pain), reducing the possibility of observer bias.19 In addition, analyses were performed with the analysts blinded to the randomization assignment.
Limitations of the trial
A few limitations of the study are worth noting.
Patients age 45 or older with both symptomatic meniscal tear and osteoarthritis were the target population of this study. However, it is important to distinguish between the study population and the target population in a physician’s practice.
The investigators adopted broad definitions of osteoarthritis and symptoms of meniscal tear. Twenty-one percent of participants had normal findings on plain radiography, with cartilage defects visible only on MRI. Further, episodic pain or acute pain localized to a joint line was regarded as a symptom consistent with a torn meniscus.
In practice, arthroscopic partial meniscectomy is usually considered when a patient with a long history of tolerable osteoarthritis presents with a sudden onset of intolerable pain after a squatting or twisting injury.
In addition, the study population was predominantly white (85%), and the study was performed in tertiary referral academic medical centers. Therefore, the outcomes achieved with surgery or physical therapy may not translate to the community setting. Clinicians must be careful to account for these types of differences in extrapolating to patients in their own practice.
Potential enrollment bias
Although randomization is a rigorous method that eliminates selection bias in assigning individuals to study and control groups, selective enrollment could have created bias.1 As the authors mentioned, only 26% of eligible patients were enrolled, possibly reflecting patients’ or surgeons’ strong preferences for one treatment or the other. Because the study and control groups were hardly random samples of eligible populations, we must be careful in generalizing the efficacy of physical therapy.1
Crossover may have obscured the benefit of surgery
During the first 6 months, 30% of patients crossed over from physical therapy to surgery. High crossover rates in surgical trials are common, especially when comparing surgery with medical therapy.23 Given that most of the patients assigned to only physical therapy who crossed over to surgery did not have substantial improvement in functional status, it seems that crossover occurred by nonrandom factors, potentially biasing the study results. With the high degree of crossover from the nonoperative group to the surgical group, intention-to-treat analysis may have given an inflated estimate of the effect of physical therapy.
To account for crossovers, researchers defined a binary outcome a priori: patients were considered to have had a successful treatment response if they improved by at least 8 points on the WOMAC scale (a clinically important difference) and did not cross over from their assigned treatment. At 6 months, 67.1% of patients assigned to surgery showed a successful treatment response, compared with 43.8% of patients assigned to physical therapy alone (P = .001).1
In patients who crossed over, the last scores before crossover were carried over, and primary analysis of the WOMAC score at 6 months was repeated to estimate the effect of crossovers from the nonoperative to the surgery group. This exploratory analysis showed a 13.0-point improvement in WOMAC score at 6 months with physical therapy alone vs a 20.9-point improvement with surgery, suggesting that the similarity in outcomes between the two groups may be explained in part by additional improvements from surgery for those who crossed over from physical therapy alone.1
Implications for functional improvement
Lacking a comparison group that underwent a sham surgical procedure, one cannot conclude that surgery after crossover improved functional status in those patients. However, there was no significant difference in WOMAC physical function scores at 12 months between the 30% of patients in the physical therapy group who crossed over and underwent surgery during the first 6 months and patients initially assigned to surgery. This finding suggests that physical therapy can be recommended as a first-line therapy, although we must be cautious, given that the physical therapy group required more background therapy (eg, intra-articular glucocorticoid injections), and that this study was not powered to detect such differences at 12 months.
Also, a patient may need to get better quickly, to get back to work, for example. Although the data were not definitive, at 3 months the patients in the surgery group seemed to have better pain control and function than those in the physical therapy group. A cost-benefit analysis of physical therapy compared with surgery for short-term outcomes may be helpful before generalizing these findings.
SURGERY VS SHAM PROCEDURE: THE FIDELITY GROUP RESULTS
In a later publication from the Finnish Degenerative Meniscal Lesion Study (FIDELITY) Group,24 146 patients with symptoms consistent with degenerative meniscal tear but no knee osteoarthritis were randomized to undergo arthroscopic partial meniscectomy or a sham procedure. At 12 months, no differences were noted between the groups in terms of change of symptoms from baseline to 12 months.
The authors concluded that the outcomes with meniscectomy were no better than with a sham procedure.24
SURGERY FIRST, OR PHYSICAL THERAPY FIRST?
The use of knee arthroscopy has increased sharply in middle-aged patients in recent years. Indeed, this demographic group accounts for nearly half of the knee arthroscopic procedures performed for meniscal tears, although the increase may be due in part to issues with surgeons’ coding and insurance authorization.16
The METEOR trial showed that a structured physical therapy program can be as effective as surgery as a first-line therapy in many patients with symptomatic meniscal tears and mild to moderate osteoarthritis. These results should inform clinical practice in that most such patients need not be immediately referred for surgical intervention.
However, a subset of these patients may benefit from surgery rather than nonoperative therapy. Given the potential risks and public health implications of arthroscopic surgery for meniscal tears, further study is needed to better characterize these patients. A randomized sham-controlled trial is under way25 with the goal of assessing the efficacy of arthroscopic partial meniscectomy for medial meniscus tears in patients with or without knee osteoarthritis, and it is hoped this study will shed further light on this issue.
Based on the results of the METEOR trial, the physical therapy regimen that was used may be reasonable before referring patients with knee osteoarthritis and symptomatic meniscal tears for surgery.
Many patients who have osteoarthritis of the knee and a torn meniscus can defer having the meniscus repaired and undergo physical therapy instead. If a trial of physical therapy does not help, they can opt for surgery later.
This seems to be the take-home message from the recent Meniscal Tear in Osteoarthritis Research (METEOR) trial,1 which compared the efficacy of arthroscopic partial meniscectomy plus physical therapy vs physical therapy alone for patients with knee symptoms, a meniscal tear, and mild to moderate osteoarthritis of the knee.1
In brief, patients improved to a roughly similar degree with either approach, and although many patients assigned to physical therapy eventually underwent surgery anyway by 6 months, the delay did not adversely affect outcomes.
In this article, we review the background, design, and findings of the METEOR trial, and their implications for clinical practice.
SURGERY: HIGH VOLUME, BUT LITTLE EVIDENCE
Magnetic resonance imaging (MRI) often incidentally reveals meniscal lesions in middle-aged and older patients who have osteoarthritis and knee pain.2 Should these patients undergo arthroscopic meniscal repair? The decision is difficult, since it is hard to distinguish the symptoms of a meniscal tear from those of osteoarthritis.3
Current evidence suggests that, for symptomatic knee osteoarthritis by itself, arthroscopic surgery is no more effective than conservative management.4,5 But what about surgery for a torn meniscus in addition to osteoarthritis?
Osteoarthritis is the most common joint disease, accounting for many physician visits.6 More than 26 million Americans over age 25 have some form of it, and the prevalence of symptomatic, radiographically confirmed osteoarthritis of the knee was 12.1% in the third National Health and Nutrition Examination Survey.7
We used to consider osteoarthritis a “wear-and-tear” disease—thus the term “degenerative joint disease.” But today, we know that it is an active response to injury, involving inflammatory and metabolic pathways.8 Moreover, the risk of osteoarthritis and its progression seems to be higher in those who have had meniscal injury and total or arthroscopic partial meniscectomy.9,10
MRI is not commonly used in managing knee osteoarthritis, but it has been used diagnostically in patients with symptoms of a meniscal tear, such as clicking, locking, popping, giving way, and pain with pivoting or twisting. Traumatic meniscal tears (a longitudinal or radial tear pattern) most often occur in active younger people and often lead to meniscal surgery.11,12 In contrast, degenerative meniscal tears (horizontal, oblique, or complex tear pattern or meniscal maceration) tend to occur in older people,11,12 but how to manage them is not widely agreed upon.
Of note, most patients with osteoarthritis of the knee have torn, macerated, or heavily damaged menisci.13,14 Meniscal lesions are also common in middle-aged people in the general population, with a higher prevalence in people who are older, heavier, or female, or who have a family history of osteoarthritis.15
These abnormalities are only weakly associated with symptoms.2 However, when a patient has knee symptoms and a torn meniscus is detected on MRI, the tear is often assumed to be the source of the symptoms, and meniscal tears are the most common reason for arthroscopy.16
Since we have no way to prevent the progression of joint damage from osteoarthritis with drugs or by any other means, the goal is to alleviate the symptoms. Many patients report pain relief or functional improvement after arthroscopic surgery. But arthroscopic lavage or debridement for osteoarthritis has not been found to be better than conservative treatment or placebo in randomized controlled trials.4,5
In contrast, the current standard treatment for a symptomatic degenerative meniscal tear is arthroscopic partial meniscectomy. Nearly 500,000 of these procedures are performed annually in the United States.16 But based on the best evidence, arthroscopic partial meniscectomy does not result in better pain relief and functional improvement than does physical therapy alone in patients who have a torn meniscus and knee osteoarthritis.17,18
OVERVIEW OF THE METEOR TRIAL
The METEOR trial was a randomized controlled trial conducted at seven US tertiary referral centers. Its aim was to compare the short-term (6-month) and long-term (12-month) efficacy of arthroscopic partial meniscectomy and physical therapy in patients with symptomatic meniscal tear and osteoarthritis of the knee.19 It was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases.1
Patients were age 45 and older
METEOR patients had to be at least 45 years old and have symptomatic meniscal tears and knee osteoarthritis detected on MRI or radiography.1
Osteoarthritis was defined broadly, given that it begins well before the appearance of radiographic evidence such as an osteophyte or joint-space narrowing.19 Patients with cartilage defects on MRI were also enrolled, as were patients with radiographically documented osteoarthritis.19
Patients were considered to have a symptomatic meniscal tear if they had had at least 4 weeks of symptoms (such as episodic pain and pain that was acute and localized to one spot on the knee, as well as typical mechanical pain suggesting a meniscal tear, such as clicking, catching, popping, giving way, or pain with pivoting or twisting) in addition to evidence of a meniscal tear on MRI.19
Patients were excluded if they had a chronically locked knee (a clear-cut indication for arthroscopic partial meniscectomy), advanced osteoarthritis (Kellgren-Lawrence grade 4), inflammatory arthritis, clinically symptomatic chondrocalcinosis, or bilateral symptomatic meniscal tears.19 Patients who had undergone surgery or injection of a viscosupplement in the index knee during the past 4 weeks were also excluded.19
Of 1,330 eligible patients, 351 (26.4%) were enrolled and randomly assigned in a 1:1 ratio to a treatment group by means of a secure program on the trial website.1,19 Of those who were eligible but did not enroll, 195 (14.6%) were not referred and 784 (58.9%) declined to participate. Of those who declined, more preferred surgery than physical therapy (36.1% vs 21%). No information is available on any differences in baseline characteristics between the enrolled patients and the eligible patients who declined.
Randomization was done in blocks of varying size within each site, stratified according to sex and the extent of osteoarthritis on baseline radiography. The extent of osteoarthritis was categorized either as Kellgren-Lawrence grade 0 (normal, no features of osteoarthritis) to grade 2 (definite osteoarthritis, a definite osteophyte without joint-space narrowing) or as Kellgren-Lawrence grade 3 (moderate osteoarthritis, < 50% joint-space narrowing).1,19 The two treatment groups were similar with respect to age, sex, race or ethnicity, baseline Kellgren-Lawrence grade, and baseline Western Ontario and McMaster Universities Arthritis Index (WOMAC) physical function score.1
The mean age of the participants was 58, and 85% were white. Sixty-three percent had Kellgren-Lawrence grade 0 to 2 osteoarthritis, and 27% had grade 3.1
Surgery plus physical therapy vs physical therapy alone
The surgery group underwent arthroscopic partial meniscectomy, which involved trimming the damaged meniscus back to a stable rim1,19 and trimming loose fragments of cartilage and bone.
After the procedure, patients were scheduled for physical therapy. Although there is no consensus on the need for or the effectiveness of postoperative physical therapy in this setting, the investigators believed that including it in both study groups would help to isolate the independent effects of surgery. The physical therapy regimen after surgery was similar to that provided in the nonoperative group.1,19
Physical therapy was designed to address inflammation, range of motion, muscle strength, muscle-length restriction, functional mobility, and proprioception and balance.1,19 There were three stages; criteria for advancing from one phase to the next included the level of self-reported pain, observed strength, range of knee motion, knee effusion, and functional mobility.1,18
The duration of participation varied depending on the pace of improvement. Generally, the program lasted about 6 weeks.1,19
Crossover and other therapies were allowed
Crossover from physical therapy alone to surgery was allowed during the trial if the patient and surgeon thought it was clinically indicated.
Participants in both groups were permitted to take acetaminophen and nonsteroidal anti-inflammatory drugs as needed. Intra-articular injections of glucocorticoids were also allowed during the trial.
OUTCOMES MEASURED
WOMAC physical function score
The primary outcome of the METEOR trial was the difference between the study groups in the change in WOMAC physical function score from baseline to 6 months, at which point participants were expected to have achieved maximum improvement.1,19 Questionnaires were also administered at 3 months to assess the early response to surgery or physical therapy and again at 12 months.
The complete WOMAC also measures pain and stiffness in addition to physical function, with separate subscales for each. The change in WOMAC score is one of the most widely endorsed outcome measures in assessing interventions in osteoarthritis or other conditions of the lower extremities.20 The METEOR trial authors considered the WOMAC scale to be highly valid and reliable, with a Cronbach alpha value of 0.97 (maximum value = 1; the higher the better).
No ceiling or floor effects were observed in the WOMAC physical function score in patients with osteoarthritis and a meniscal tear in a pilot study for METEOR.19
In the main METEOR study, WOMAC physical function was scored on a scale of 0 to 100, with a higher score indicating worse physical function.1 Changes in the score were also measured as a yes-or-no question, defined a priori as whether the score declined by at least 8 points, which is considered the minimal clinically important difference in osteoarthritis patients.1,19
KOOS and MOS SF-36 scores
Secondary outcomes were measured in several domains, including pain, generic functional status, quality of life, and health care utilization.1,19
The KOOS (Knee Injury and OA Outcome Scale) is specific for knee pain, being designed to evaluate short-term and long-term symptoms and function in patients with knee injury and associated problems.21 It has five subscales, which are scored separately: pain, other symptoms, activities of daily living, sport and recreation, and knee-related quality of life.21 Since the WOMAC pain scale showed a ceiling effect in the pilot study in patients undergoing surgery, the authors chose the KOOS pain scale as a pain measure.19 Scores were transformed to a 0–100 scale, with a higher score indicating more pain.1
The MOS SF-36 (Medical Outcomes Study 36-item short form) was used to measure general health status and function.1,19
STATISTICAL ANALYSIS: INTENTION-TO-TREAT AND AS-TREATED
The study was powered to detect a 10-point difference in WOMAC physical function scores at 6 months of follow-up between the operative and nonoperative groups, anticipating losses to follow-up and crossover, with preplanned subgroup (Kellgren–Lawrence grade 0–2 vs grade 3) analysis.1,19
The primary analysis used a modified intention-to-treat approach and was implemented with an analysis of covariance with changes in the WOMAC score from baseline to 6 months as the dependent variable, treatment as the independent variable of interest, and study site as a covariate. Other covariates, such as age, sex, and baseline Kellgren-Lawrence grade, were balanced across groups and were therefore not included in the analysis.1,19
Secondary analyses used an “as-treated” approach, ie, according to the treatment actually received.1,19 Secondary intention-to-treat analysis—using binary outcome measures in which treatment failure was defined as improvement in the WOMAC score of less than 8 points or crossing over to the other treatment—was also performed to estimate efficacy at the level of the patient rather than at the group level.1,19
BOTH GROUPS IMPROVED
In the intention-to-treat analyses at 6 months and 12 months after randomization, both groups improved, with no clinically important or statistically significant differences between the groups in functional status (WOMAC score, MOS SF-36 score) or pain (KOOS score).1 The mean improvement (decline) in the WOMAC score from baseline to 6 months was 20.9 points in the surgery group vs 18.5 points in the physical therapy group, a difference of 2.4 points (95% confidence interval [CI], −1.8 to 6.5).1
35% of physical therapy patients underwent surgery by 12 months
Of the 177 patients randomized to physical therapy alone, by 6 months 1 had died, 1 had undergone total knee replacement, 4 had withdrawn, and 2 were lost to follow-up. Of the 169 remaining, 51 (30%) had undergone arthroscopic partial meniscectomy. An additional 8 patients who were assigned to physical therapy crossed over to surgery between 6 and 12 months.1,19
Of the 174 patients randomized to surgery, by 6 months 1 had died, 3 had undergone total knee replacement, 7 had withdrawn, and 2 were ineligible. Of the 161 remaining, 9 (6%) had not undergone the procedure.
Other outcomes
Subgroup analysis based on the baseline radiographic grade (Kellgren-Lawrence grade 0 to 2 vs grade 3) did not show a difference between groups in functional improvement at 6 months (P = .13 for interaction).1
No statistically significant difference was noted in rates of overall or specific adverse events between the two groups over the first 12 months.1 Adverse events rated as mild or moderate in severity occurred in 15 participants in the surgery group and 13 participants in the physical therapy group.1 Long-term risks associated with these interventions are being assessed, and longitudinal assessment of imaging studies is planned to address this question but is not yet available.1,18
In the physical therapy group, 21 patients (12%) received intra-articular glucocorticoid injections, as did 9 patients (6%) in the surgery group.1,19
TRANSLATING THE METEOR RESULTS TO EVERYDAY PRACTICE
There are many challenges in designing surgical trials. Indeed, by one estimate,22 only about 40% of treatment questions involving surgical procedures can be evaluated by a randomized controlled trial.
Although the METEOR trial was not blinded, it was the first large, multicenter, randomized controlled trial to compare arthroscopic partial meniscectomy vs standardized physical therapy by using high-quality methodology such as careful sample-size calculation, balancing the groups according to known prognostic factors with block randomization, and intention-to-treat analysis. Moreover, the outcome measures were obtained from validated self-reporting questionnaires (WOMAC for function and KOOS for pain), reducing the possibility of observer bias.19 In addition, analyses were performed with the analysts blinded to the randomization assignment.
Limitations of the trial
A few limitations of the study are worth noting.
Patients age 45 or older with both symptomatic meniscal tear and osteoarthritis were the target population of this study. However, it is important to distinguish between the study population and the target population in a physician’s practice.
The investigators adopted broad definitions of osteoarthritis and symptoms of meniscal tear. Twenty-one percent of participants had normal findings on plain radiography, with cartilage defects visible only on MRI. Further, episodic pain or acute pain localized to a joint line was regarded as a symptom consistent with a torn meniscus.
In practice, arthroscopic partial meniscectomy is usually considered when a patient with a long history of tolerable osteoarthritis presents with a sudden onset of intolerable pain after a squatting or twisting injury.
In addition, the study population was predominantly white (85%), and the study was performed in tertiary referral academic medical centers. Therefore, the outcomes achieved with surgery or physical therapy may not translate to the community setting. Clinicians must be careful to account for these types of differences in extrapolating to patients in their own practice.
Potential enrollment bias
Although randomization is a rigorous method that eliminates selection bias in assigning individuals to study and control groups, selective enrollment could have created bias.1 As the authors mentioned, only 26% of eligible patients were enrolled, possibly reflecting patients’ or surgeons’ strong preferences for one treatment or the other. Because the study and control groups were hardly random samples of eligible populations, we must be careful in generalizing the efficacy of physical therapy.1
Crossover may have obscured the benefit of surgery
During the first 6 months, 30% of patients crossed over from physical therapy to surgery. High crossover rates in surgical trials are common, especially when comparing surgery with medical therapy.23 Given that most of the patients assigned to only physical therapy who crossed over to surgery did not have substantial improvement in functional status, it seems that crossover occurred by nonrandom factors, potentially biasing the study results. With the high degree of crossover from the nonoperative group to the surgical group, intention-to-treat analysis may have given an inflated estimate of the effect of physical therapy.
To account for crossovers, researchers defined a binary outcome a priori: patients were considered to have had a successful treatment response if they improved by at least 8 points on the WOMAC scale (a clinically important difference) and did not cross over from their assigned treatment. At 6 months, 67.1% of patients assigned to surgery showed a successful treatment response, compared with 43.8% of patients assigned to physical therapy alone (P = .001).1
In patients who crossed over, the last scores before crossover were carried over, and primary analysis of the WOMAC score at 6 months was repeated to estimate the effect of crossovers from the nonoperative to the surgery group. This exploratory analysis showed a 13.0-point improvement in WOMAC score at 6 months with physical therapy alone vs a 20.9-point improvement with surgery, suggesting that the similarity in outcomes between the two groups may be explained in part by additional improvements from surgery for those who crossed over from physical therapy alone.1
Implications for functional improvement
Lacking a comparison group that underwent a sham surgical procedure, one cannot conclude that surgery after crossover improved functional status in those patients. However, there was no significant difference in WOMAC physical function scores at 12 months between the 30% of patients in the physical therapy group who crossed over and underwent surgery during the first 6 months and patients initially assigned to surgery. This finding suggests that physical therapy can be recommended as a first-line therapy, although we must be cautious, given that the physical therapy group required more background therapy (eg, intra-articular glucocorticoid injections), and that this study was not powered to detect such differences at 12 months.
Also, a patient may need to get better quickly, to get back to work, for example. Although the data were not definitive, at 3 months the patients in the surgery group seemed to have better pain control and function than those in the physical therapy group. A cost-benefit analysis of physical therapy compared with surgery for short-term outcomes may be helpful before generalizing these findings.
SURGERY VS SHAM PROCEDURE: THE FIDELITY GROUP RESULTS
In a later publication from the Finnish Degenerative Meniscal Lesion Study (FIDELITY) Group,24 146 patients with symptoms consistent with degenerative meniscal tear but no knee osteoarthritis were randomized to undergo arthroscopic partial meniscectomy or a sham procedure. At 12 months, no differences were noted between the groups in terms of change of symptoms from baseline to 12 months.
The authors concluded that the outcomes with meniscectomy were no better than with a sham procedure.24
SURGERY FIRST, OR PHYSICAL THERAPY FIRST?
The use of knee arthroscopy has increased sharply in middle-aged patients in recent years. Indeed, this demographic group accounts for nearly half of the knee arthroscopic procedures performed for meniscal tears, although the increase may be due in part to issues with surgeons’ coding and insurance authorization.16
The METEOR trial showed that a structured physical therapy program can be as effective as surgery as a first-line therapy in many patients with symptomatic meniscal tears and mild to moderate osteoarthritis. These results should inform clinical practice in that most such patients need not be immediately referred for surgical intervention.
However, a subset of these patients may benefit from surgery rather than nonoperative therapy. Given the potential risks and public health implications of arthroscopic surgery for meniscal tears, further study is needed to better characterize these patients. A randomized sham-controlled trial is under way25 with the goal of assessing the efficacy of arthroscopic partial meniscectomy for medial meniscus tears in patients with or without knee osteoarthritis, and it is hoped this study will shed further light on this issue.
Based on the results of the METEOR trial, the physical therapy regimen that was used may be reasonable before referring patients with knee osteoarthritis and symptomatic meniscal tears for surgery.
- Katz JN, Brophy RH, Chaisson CE, et al. Surgery versus physical therapy for a meniscal tear and osteoarthritis. N Engl J Med 2013; 368:1675–1684.
- Englund M, Guermazi A, Gale D, et al. Incidental meniscal findings on knee MRI in middle-aged and elderly persons. N Engl J Med 2008; 359:1108–115.
- Englund M, Guermazi A, Lohmander SL. The role of the meniscus in knee osteoarthritis: a cause or consequence? Radiol Clin North Am 2009; 47:703–712.
- Moseley JB, O’Malley K, Petersen NJ, et al. A controlled trial of arthroscopic surgery for osteoarthritis of the knee. N Engl J Med 2002; 347:81–68.
- Kirkley A, Birmingham TB, Litchfield RB, et al. A randomized trial of arthroscopic surgery for osteoarthritis of the knee. N Engl J Med 2008; 359:1097–1107.
- Quintana JM, Escobar A, Arostegui I, et al. Prevalence of symptoms of knee or hip joints in older adults from the general population. Aging Clin Exp Res 2008; 20:329–336.
- Lawrence RC, Felson DT, Helmick CG, et al; National Arthritis Data Workgroup. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum 2008; 58:26–35.
- Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum 2012; 64:1697–1707.
- Englund M, Lohmander LS. Risk factors for symptomatic knee osteoarthritis fifteen to twenty-two years after meniscectomy. Arthritis Rheum 2004; 50:2811–2819.
- Papalia R, Del Buono A, Osti L, Denaro V, Maffulli N. Meniscectomy as a risk factor for knee osteoarthritis: a systematic review. Br Med Bull 2011; 99:89–106.
- Englund M, Guermazi A, Roemer FW, et al. Meniscal tear in knees without surgery and the development of radiographic osteoarthritis among middle-aged and elderly persons: The Multicenter Osteoarthritis Study. Arthritis Rheum 2009; 60:831–839.
- Poehling GG, Ruch DS, Chabon SJ. The landscape of meniscal injuries. Clin Sports Med 1990; 9:539–549.
- Bhattacharyya T, Gale D, Dewire P, et al. The clinical importance of meniscal tears demonstrated by magnetic resonance imaging in osteoarthritis of the knee. J Bone Joint Surg Am 2003; 85-A:4–9.
- Sowers M, Karvonen-Gutierrez CA, Jacobson JA, Jiang Y, Yosef M. Associations of anatomical measures from MRI with radiographically defined knee osteoarthritis score, pain, and physical functioning. J Bone Joint Surg Am 2011; 93:241–251.
- Ding C, Martel-Pelletier J, Pelletier JP, et al. Meniscal tear as an osteoarthritis risk factor in a largely non-osteoarthritic cohort: a cross-sectional study. J Rheumatol 2007; 34:776–784.
- Kim S, Bosque J, Meehan JP, Jamali A, Marder R. Increase in outpatient knee arthroscopy in the United States: a comparison of National Surveys of Ambulatory Surgery, 1996 and 2006. J Bone Joint Surg Am 2011; 93:994–1000.
- Herrlin S, Hållander M, Wange P, Weidenhielm L, Werner S. Arthroscopic or conservative treatment of degenerative medial meniscal tears: a prospective randomised trial. Knee Surg Sports Traumatol Arthrosc 2007; 15:393–401.
- Herrlin SV, Wange PO, Lapidus G, Hållander M, Werner S, Weidenhielm L. Is arthroscopic surgery beneficial in treating non-traumatic, degenerative medial meniscal tears? A five year follow-up. Knee Surg Sports Traumatol Arthrosc 2013; 21:358–364.
- Katz JN, Chaisson CE, Cole B, et al. The MeTeOR trial (Meniscal Tear in Osteoarthritis Research): rationale and design features. Contemp Clin Trials 2012; 33:1189–1196.
- Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol 1988; 15:1833–1840.
- Roos EM, Lohmander LS. The Knee injury and Osteoarthritis Outcome Score (KOOS): from joint injury to osteoarthritis. Health Qual Life Outcomes 2003; 1:64.
- Solomon MJ, McLeod RS. Should we be performing more randomized controlled trials evaluating surgical operations? Surgery 1995; 118:459–467.
- Farrokhyar F, Karanicolas PJ, Thoma A, et al. Randomized controlled trials of surgical interventions. Ann Surg 2010; 251:409–416.
- Sihvonen R, Paavola M, Malmivaara A. Arthroscopic partial meniscectomy versus sham surgery for a degenerative meniscal tear. N Engl J Med 2013; 369:2515–2524.
- Hare KB, Lohmander LS, Christensen R, Roos EM. Arthroscopic partial meniscectomy in middle-aged patients with mild or no knee osteoarthritis: a protocol for a double-blind, randomized sham-controlled multicentre trial. BMC Musculoskelet Disord 2013; 14:71.
- Katz JN, Brophy RH, Chaisson CE, et al. Surgery versus physical therapy for a meniscal tear and osteoarthritis. N Engl J Med 2013; 368:1675–1684.
- Englund M, Guermazi A, Gale D, et al. Incidental meniscal findings on knee MRI in middle-aged and elderly persons. N Engl J Med 2008; 359:1108–115.
- Englund M, Guermazi A, Lohmander SL. The role of the meniscus in knee osteoarthritis: a cause or consequence? Radiol Clin North Am 2009; 47:703–712.
- Moseley JB, O’Malley K, Petersen NJ, et al. A controlled trial of arthroscopic surgery for osteoarthritis of the knee. N Engl J Med 2002; 347:81–68.
- Kirkley A, Birmingham TB, Litchfield RB, et al. A randomized trial of arthroscopic surgery for osteoarthritis of the knee. N Engl J Med 2008; 359:1097–1107.
- Quintana JM, Escobar A, Arostegui I, et al. Prevalence of symptoms of knee or hip joints in older adults from the general population. Aging Clin Exp Res 2008; 20:329–336.
- Lawrence RC, Felson DT, Helmick CG, et al; National Arthritis Data Workgroup. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum 2008; 58:26–35.
- Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum 2012; 64:1697–1707.
- Englund M, Lohmander LS. Risk factors for symptomatic knee osteoarthritis fifteen to twenty-two years after meniscectomy. Arthritis Rheum 2004; 50:2811–2819.
- Papalia R, Del Buono A, Osti L, Denaro V, Maffulli N. Meniscectomy as a risk factor for knee osteoarthritis: a systematic review. Br Med Bull 2011; 99:89–106.
- Englund M, Guermazi A, Roemer FW, et al. Meniscal tear in knees without surgery and the development of radiographic osteoarthritis among middle-aged and elderly persons: The Multicenter Osteoarthritis Study. Arthritis Rheum 2009; 60:831–839.
- Poehling GG, Ruch DS, Chabon SJ. The landscape of meniscal injuries. Clin Sports Med 1990; 9:539–549.
- Bhattacharyya T, Gale D, Dewire P, et al. The clinical importance of meniscal tears demonstrated by magnetic resonance imaging in osteoarthritis of the knee. J Bone Joint Surg Am 2003; 85-A:4–9.
- Sowers M, Karvonen-Gutierrez CA, Jacobson JA, Jiang Y, Yosef M. Associations of anatomical measures from MRI with radiographically defined knee osteoarthritis score, pain, and physical functioning. J Bone Joint Surg Am 2011; 93:241–251.
- Ding C, Martel-Pelletier J, Pelletier JP, et al. Meniscal tear as an osteoarthritis risk factor in a largely non-osteoarthritic cohort: a cross-sectional study. J Rheumatol 2007; 34:776–784.
- Kim S, Bosque J, Meehan JP, Jamali A, Marder R. Increase in outpatient knee arthroscopy in the United States: a comparison of National Surveys of Ambulatory Surgery, 1996 and 2006. J Bone Joint Surg Am 2011; 93:994–1000.
- Herrlin S, Hållander M, Wange P, Weidenhielm L, Werner S. Arthroscopic or conservative treatment of degenerative medial meniscal tears: a prospective randomised trial. Knee Surg Sports Traumatol Arthrosc 2007; 15:393–401.
- Herrlin SV, Wange PO, Lapidus G, Hållander M, Werner S, Weidenhielm L. Is arthroscopic surgery beneficial in treating non-traumatic, degenerative medial meniscal tears? A five year follow-up. Knee Surg Sports Traumatol Arthrosc 2013; 21:358–364.
- Katz JN, Chaisson CE, Cole B, et al. The MeTeOR trial (Meniscal Tear in Osteoarthritis Research): rationale and design features. Contemp Clin Trials 2012; 33:1189–1196.
- Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol 1988; 15:1833–1840.
- Roos EM, Lohmander LS. The Knee injury and Osteoarthritis Outcome Score (KOOS): from joint injury to osteoarthritis. Health Qual Life Outcomes 2003; 1:64.
- Solomon MJ, McLeod RS. Should we be performing more randomized controlled trials evaluating surgical operations? Surgery 1995; 118:459–467.
- Farrokhyar F, Karanicolas PJ, Thoma A, et al. Randomized controlled trials of surgical interventions. Ann Surg 2010; 251:409–416.
- Sihvonen R, Paavola M, Malmivaara A. Arthroscopic partial meniscectomy versus sham surgery for a degenerative meniscal tear. N Engl J Med 2013; 369:2515–2524.
- Hare KB, Lohmander LS, Christensen R, Roos EM. Arthroscopic partial meniscectomy in middle-aged patients with mild or no knee osteoarthritis: a protocol for a double-blind, randomized sham-controlled multicentre trial. BMC Musculoskelet Disord 2013; 14:71.
KEY POINTS
- METEOR trial was a randomized controlled trial comparing the short-term and long-term efficacy of arthroscopic partial meniscectomy vs physical therapy in patients with a symptomatic meniscal tear and knee osteoarthritis.
- Both treatment groups in the METEOR trial received physical therapy in order to determine the incremental benefit of surgery and physical therapy compared with physical therapy alone.
- The trial investigators used specific definitions of osteoarthritis and symptoms of meniscal tear.
- Meniscectomy is often performed for patients with symptoms consistent with a meniscal tear and evidence of a meniscal tear on magnetic resonance imaging, but the benefits of this procedure are unclear.