User login
UTI, then massive hemorrhage
UTI, THEN MASSIVE HEMORRHAGE
A woman in her 60s was hospitalized with a urinary tract infection (UTI). She was treated with antibiotics and intravenous (IV) fluids but developed deep vein thrombosis (DVT) at the IV site. Enoxaparin sodium was ordered to treat the clot. After 3 days, she suffered a massive abdominal hemorrhage. When she woke from resuscitation, her weight had doubled. She developed a methicillin-resistant Staphylococcus aureus (MRSA) infection, then Clostridium difficile infection due to antibiotics, plus bedsores. Multiple surgeries left her with an abdominal wall defect that cannot be repaired, and a permanent hernia. She was hospitalized for 75 days.
PATIENT’S CLAIM The hemorrhage was caused when enoxaparin was given at 1.5 times the proper dosage because the patient’s weight was overestimated by 50%. Excessive blood, plasma, and fluids caused her weight to double after resuscitation. Her intestines were forced out of her abdominal cavity by the hemorrhage. A permanent hernia, visible underneath her skin, causes pain.
DEFENDANTS’ DEFENSE The patient’s preexisting diabetes, heart condition, high cholesterol levels, and orthopedic issues impacted her condition. She was not compliant in managing her diabetes, causing many of the current problems.
VERDICT A $9.3 million Connecticut verdict was returned.
Related article: Update: Pelvic floor dysfunction Autumn L. Edenfield, MD, and Cindy L. Amundsen, MD (October 2012)
CESAREAN DELAYED UNNECESSARILY
At 37 weeks’ gestation, a mother reported decreased fetal movement. When the biophysical profile test scored 8/8 and the fetal heart rate was reassuring, the attending ObGyn discharged the patient. However, it was the middle of the night, and the nurse kept the mother in the emergency department (ED). At 8:30 am, the fetus began to show signs of fetal distress. Three ObGyns agreed to monitor labor, although one physician wanted delivery to occur that morning.
The next morning, a second biophysical profile scored 2/8, but the on-call ObGyn misunderstood the score as 6/8 and scheduled cesarean delivery for noon. Two hours after the second biophysical profile, the fetal heart rate crashed. A nurse called the ObGyn, who began an emergency cesarean 15 minutes later. The baby, born lifeless, was resuscitated. The child suffered permanent brain damage, and has cerebral palsy, severe cognitive deficits and speech deficits, and walks with an abnormal gait.
PARENTS’ CLAIM A physician did not see the patient for 24 hours, once the decision was made to monitor the mother, even though the fetal heart rate continued to decline. A biophysical profile test score of 2/8 indicates the need for immediate delivery. An earlier cesarean delivery could have reduced the child’s injuries.
DEFENDANTS’ DEFENSE After a settlement was reached with the hospital, the trial continued against the delivering ObGyn. He claimed that decreased fetal movement indicated that the brain injury had occurred 1 to 4 days before the mother came to the ED. The technician had manipulated the mother’s abdomen to wake the fetus before starting the first biophysical profile, which invalidated the score. The nurse miscommunicated the score of the second biophysical profile.
VERDICT A gross $29.8 million Illinois verdict was returned that included a $1.65 million settlement with the hospital.
WAS FACILITY ADEQUATELY STAFFED AFTER HURRICANE IKE?
A mother was admitted to a hospital for induction of labor in September 2008. After birth, the child was found to have cerebral palsy.
PARENTS’ CLAIM The mother should have been sent to another facility before delivery was induced because the hospital was short-staffed and low on resources due to Hurricane Ike. Too much oxytocin was used to induce contractions, which led to a lack of oxygen for the fetus. All prenatal testing had shown a healthy fetus. A cesarean delivery should have occurred when fetal distress was noted.
DEFENDANTS’ DEFENSE The mother had gastric bypass surgery 8 months before she became pregnant, and smoked during pregnancy, which accounted for the infant’s injuries. Treatment during labor and delivery was appropriate. Hospital staffing and resources were adequate.
VERDICT A $6.5 million Texas settlement was reached.
PLACENTA ACCRETA; MOTHER DIES
A 33-year-old woman became pregnant with her second child. A variety of conditions caused this to be high-risk pregnancy, so she saw a maternal-fetal medicine (MFM) specialist 2 months before delivery. The MFM reported that his examination and the ultrasonography (US) results were normal.
The ObGyn who provided prenatal care and delivered her first child scheduled cesarean delivery. During the procedure, the ObGyn noticed a 3- to 4-inch lesion where the placenta had penetrated the uterus. When the placenta was removed, the patient began to hemorrhage and a hysterectomy was performed. The hemorrhage created blood clots that led to gangrene in the patient’s extremities. She died 5 days after giving birth.
ESTATE’S CLAIM Both the MFM and the ObGyn failed to recognize placenta accreta on US prior to delivery. The ObGyn should have performed US prior to beginning cesarean delivery. The hospital’s protocols were not followed: the ObGyn should have stopped the procedure and called for extra surgical assistance and additional blood when he encountered placenta accreta, and again when the patient began to hemorrhage. Placenta accreta does not have to be fatal if detected and managed properly.
DEFENDANTS’ DEFENSE There was no negligence; the patient was treated properly.
VERDICT A $15.5 million Illinois verdict was returned against both physicians and the medical center.
Related article: Is the risk of placenta accreta in a subsequent pregnancy higher after emergent primary cesarean or after elective primary cesarean? Yinka Oyelese, MD (Examining the Evidence, December 2013)
ANTICONVULSANT AND MIGRAINE MEDS TAKEN DURING PREGNANCY
A woman was prescribed topiramate (Topamax) for migraine headaches and hand tremors during the first trimester of her pregnancy in 2007. With a history of seizures, she also took several anticonvulsants throughout her pregnancy. Her child was diagnosed with right unilateral cleft lip (cheiloschisis) in utero. The condition had not been surgically corrected at the time of trial.
PARENTS’ CLAIM The use of topiramate caused the child’s cleft lip. Janssen Pharmaceuticals, the manufacturer of Topamax, knew about the risk of birth defects associated with the drug in 2007, but failed to provide adequate warnings.
DEFENDANTS’ DEFENSE The mother received at least two warnings from her physician regarding the potential risks of anticonvulsant and antiepileptic drugs and the importance of not becoming pregnant while taking the medications. An action against the physician was barred by the applicable statute of limitations. The mother had taken topiramate prescribed to her mother for a time; such actions should release Janssen from liability.
VERDICT A $11 million Pennsylvania verdict was returned.
PID MASKS ECTOPIC PREGNANCY
A woman in her 40s became pregnant. On the first two prenatal diagnostic imaging studies, the ObGyn saw an intrauterine pregnancy. He later realized that the pregnancy was ectopic after beta human chorionic gonadotrophin (beta-hCG) blood levels were abnormal. During surgery to terminate the pregnancy, he found he had to perform a total hysterectomy because the patient had extensive pelvic inflammatory disease (PID) caused by a long history of sexually transmitted disease.
PATIENT’S CLAIM If the ectopic pregnancy had been diagnosed earlier, one of her ovaries could have been preserved, saving her from the symptoms of surgical menopause.
PHYSICIAN’S DEFENSE PID had caused the ovaries, numerous fibroid tumors, and the uterus to fuse into one mass. That was why the first two imaging studies appeared to show an intrauterine pregnancy. It was not possible to diagnose the extent of the problem until surgery. The patient did not have a true ectopic pregnancy.
The patient’s difficulties occurred during a 2-week time period in which she had one visit with him and another visit to an ED where two other physicians examined her and missed the diagnosis.
VERDICT A Michigan defense verdict was returned.
ILIAC ARTERY INJURED DURING LAPAROSCOPIC SURGERY; PATIENT DIES
A 40-year-old woman underwent laparoscopic gynecologic surgery performed by her ObGyn. During the procedure, the patient’s left internal iliac artery was punctured, but the injury was not recognized at the time. She was discharged the same day. The next morning, she went into hypovolemic shock due to internal bleeding. She was taken to the ED, where she died.
ESTATE’S CLAIM The ObGyn, anesthesiologist, and hospital staff were negligent in their postoperative care. The anesthesiologist prescribed pain medication that masked the injury; the patient was discharged from the postanesthesia unit too early and without proper examination. The nursing staff did not react to the patient’s reports of abdominal pain, nor did they properly assess her condition prior to discharge. The ObGyn failed to return a phone call the evening after the procedure.
DEFENDANTS’ DEFENSE The ObGyn settled before trial. The anesthesiologist and hospital denied negligence: care was proper and followed all protocols.
VERDICT A confidential California settlement was reached with the ObGyn. A defense verdict was returned for the anesthesiologist and hospital.
Related article: Anatomy for the laparoscopic surgeon Emad Mikhail, MD; Lauren Scott, MD; Stuart Hart, MD, MS (April 2014)
GENETIC TESTING MISSED A KEY DIAGNOSIS
A 40-year-old woman underwent genetic testing after she became pregnant. She was assured that there were no abnormalities that would impact her child.
The baby was born with Wolf-Hirschhorn syndrome, characterized by facial deformities, intellectual disabilities, delayed growth, and seizures. The child is nonverbal, deaf, and blind. She uses a feeding tube and requires 24-hour care.
PARENTS’ CLAIM The genetic testing was improperly conducted. The mother would have had an abortion if she’d known that the child was so disabled.
DEFENDANTS’ DEFENSE Settlements were mediated.
VERDICT A $6.15 million New Jersey settlement was reached on behalf of the hospital and two laboratory technicians, and a $1 million settlement was reached with the director of the genetic laboratory.
HEAT INJURY TO COLON: ABSCESSES, PERITONITIS
A 43-year-old patient had a history of symptomatic uterine fibroids and infertility. Her ObGyn performed a hysteroscopy because he suspected endometriosis, but found none. He then successfully removed a large uterine fibroid during laparoscopic myomectomy. The patient was discharged the same day.
Two days later, the patient developed abdominal pain, nausea, and fever. She went to the ED and was taken into emergency surgery after a CT scan showed free air and fluid in her abdomen. She suffered multiple abscesses and peritonitis.
PATIENT’S CLAIM The ObGyn was negligent in performing the surgery: the sigmoid colon sustained a thermal injury, which caused the abscesses and peritonitis.
PHYSICIAN’S DEFENSE There was no evidence of thermal injury during the original operation; heat damage can and does occur in the absence of negligence. The patient’s previously unknown diverticulitis contributed to the development of the recurrent abscesses and peritonitis.
VERDICT A Florida defense verdict was returned.
RUPTURED UTERUS IS UNDETECTED
During labor and delivery, a declining fetal heart rate was observed, but there was an hour’s delay before cesarean delivery was started. The child suffered a hypoxic brain injury. He has spastic quadriplegia, cannot speak, and requires a respirator and feeding tube.
PARENTS’ CLAIM The mother suffered a ruptured uterus during labor that was not recognized by the ObGyn or nursing staff.
DEFENDANTS’ DEFENSE A settlement was reached during trial.
VERDICT A $7.5 million New Jersey settlement was reached.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Tell us what you think!
Drop us a line and let us know what you think about this or other current articles, which topics you'd like to see covered in future issues, and what challenges you face in daily practice. Tell us what you think by emailing us at: obg@frontlinemedcom.com Please include your name, city and state.
Stay in touch! Your feedback is important to us!
UTI, THEN MASSIVE HEMORRHAGE
A woman in her 60s was hospitalized with a urinary tract infection (UTI). She was treated with antibiotics and intravenous (IV) fluids but developed deep vein thrombosis (DVT) at the IV site. Enoxaparin sodium was ordered to treat the clot. After 3 days, she suffered a massive abdominal hemorrhage. When she woke from resuscitation, her weight had doubled. She developed a methicillin-resistant Staphylococcus aureus (MRSA) infection, then Clostridium difficile infection due to antibiotics, plus bedsores. Multiple surgeries left her with an abdominal wall defect that cannot be repaired, and a permanent hernia. She was hospitalized for 75 days.
PATIENT’S CLAIM The hemorrhage was caused when enoxaparin was given at 1.5 times the proper dosage because the patient’s weight was overestimated by 50%. Excessive blood, plasma, and fluids caused her weight to double after resuscitation. Her intestines were forced out of her abdominal cavity by the hemorrhage. A permanent hernia, visible underneath her skin, causes pain.
DEFENDANTS’ DEFENSE The patient’s preexisting diabetes, heart condition, high cholesterol levels, and orthopedic issues impacted her condition. She was not compliant in managing her diabetes, causing many of the current problems.
VERDICT A $9.3 million Connecticut verdict was returned.
Related article: Update: Pelvic floor dysfunction Autumn L. Edenfield, MD, and Cindy L. Amundsen, MD (October 2012)
CESAREAN DELAYED UNNECESSARILY
At 37 weeks’ gestation, a mother reported decreased fetal movement. When the biophysical profile test scored 8/8 and the fetal heart rate was reassuring, the attending ObGyn discharged the patient. However, it was the middle of the night, and the nurse kept the mother in the emergency department (ED). At 8:30 am, the fetus began to show signs of fetal distress. Three ObGyns agreed to monitor labor, although one physician wanted delivery to occur that morning.
The next morning, a second biophysical profile scored 2/8, but the on-call ObGyn misunderstood the score as 6/8 and scheduled cesarean delivery for noon. Two hours after the second biophysical profile, the fetal heart rate crashed. A nurse called the ObGyn, who began an emergency cesarean 15 minutes later. The baby, born lifeless, was resuscitated. The child suffered permanent brain damage, and has cerebral palsy, severe cognitive deficits and speech deficits, and walks with an abnormal gait.
PARENTS’ CLAIM A physician did not see the patient for 24 hours, once the decision was made to monitor the mother, even though the fetal heart rate continued to decline. A biophysical profile test score of 2/8 indicates the need for immediate delivery. An earlier cesarean delivery could have reduced the child’s injuries.
DEFENDANTS’ DEFENSE After a settlement was reached with the hospital, the trial continued against the delivering ObGyn. He claimed that decreased fetal movement indicated that the brain injury had occurred 1 to 4 days before the mother came to the ED. The technician had manipulated the mother’s abdomen to wake the fetus before starting the first biophysical profile, which invalidated the score. The nurse miscommunicated the score of the second biophysical profile.
VERDICT A gross $29.8 million Illinois verdict was returned that included a $1.65 million settlement with the hospital.
WAS FACILITY ADEQUATELY STAFFED AFTER HURRICANE IKE?
A mother was admitted to a hospital for induction of labor in September 2008. After birth, the child was found to have cerebral palsy.
PARENTS’ CLAIM The mother should have been sent to another facility before delivery was induced because the hospital was short-staffed and low on resources due to Hurricane Ike. Too much oxytocin was used to induce contractions, which led to a lack of oxygen for the fetus. All prenatal testing had shown a healthy fetus. A cesarean delivery should have occurred when fetal distress was noted.
DEFENDANTS’ DEFENSE The mother had gastric bypass surgery 8 months before she became pregnant, and smoked during pregnancy, which accounted for the infant’s injuries. Treatment during labor and delivery was appropriate. Hospital staffing and resources were adequate.
VERDICT A $6.5 million Texas settlement was reached.
PLACENTA ACCRETA; MOTHER DIES
A 33-year-old woman became pregnant with her second child. A variety of conditions caused this to be high-risk pregnancy, so she saw a maternal-fetal medicine (MFM) specialist 2 months before delivery. The MFM reported that his examination and the ultrasonography (US) results were normal.
The ObGyn who provided prenatal care and delivered her first child scheduled cesarean delivery. During the procedure, the ObGyn noticed a 3- to 4-inch lesion where the placenta had penetrated the uterus. When the placenta was removed, the patient began to hemorrhage and a hysterectomy was performed. The hemorrhage created blood clots that led to gangrene in the patient’s extremities. She died 5 days after giving birth.
ESTATE’S CLAIM Both the MFM and the ObGyn failed to recognize placenta accreta on US prior to delivery. The ObGyn should have performed US prior to beginning cesarean delivery. The hospital’s protocols were not followed: the ObGyn should have stopped the procedure and called for extra surgical assistance and additional blood when he encountered placenta accreta, and again when the patient began to hemorrhage. Placenta accreta does not have to be fatal if detected and managed properly.
DEFENDANTS’ DEFENSE There was no negligence; the patient was treated properly.
VERDICT A $15.5 million Illinois verdict was returned against both physicians and the medical center.
Related article: Is the risk of placenta accreta in a subsequent pregnancy higher after emergent primary cesarean or after elective primary cesarean? Yinka Oyelese, MD (Examining the Evidence, December 2013)
ANTICONVULSANT AND MIGRAINE MEDS TAKEN DURING PREGNANCY
A woman was prescribed topiramate (Topamax) for migraine headaches and hand tremors during the first trimester of her pregnancy in 2007. With a history of seizures, she also took several anticonvulsants throughout her pregnancy. Her child was diagnosed with right unilateral cleft lip (cheiloschisis) in utero. The condition had not been surgically corrected at the time of trial.
PARENTS’ CLAIM The use of topiramate caused the child’s cleft lip. Janssen Pharmaceuticals, the manufacturer of Topamax, knew about the risk of birth defects associated with the drug in 2007, but failed to provide adequate warnings.
DEFENDANTS’ DEFENSE The mother received at least two warnings from her physician regarding the potential risks of anticonvulsant and antiepileptic drugs and the importance of not becoming pregnant while taking the medications. An action against the physician was barred by the applicable statute of limitations. The mother had taken topiramate prescribed to her mother for a time; such actions should release Janssen from liability.
VERDICT A $11 million Pennsylvania verdict was returned.
PID MASKS ECTOPIC PREGNANCY
A woman in her 40s became pregnant. On the first two prenatal diagnostic imaging studies, the ObGyn saw an intrauterine pregnancy. He later realized that the pregnancy was ectopic after beta human chorionic gonadotrophin (beta-hCG) blood levels were abnormal. During surgery to terminate the pregnancy, he found he had to perform a total hysterectomy because the patient had extensive pelvic inflammatory disease (PID) caused by a long history of sexually transmitted disease.
PATIENT’S CLAIM If the ectopic pregnancy had been diagnosed earlier, one of her ovaries could have been preserved, saving her from the symptoms of surgical menopause.
PHYSICIAN’S DEFENSE PID had caused the ovaries, numerous fibroid tumors, and the uterus to fuse into one mass. That was why the first two imaging studies appeared to show an intrauterine pregnancy. It was not possible to diagnose the extent of the problem until surgery. The patient did not have a true ectopic pregnancy.
The patient’s difficulties occurred during a 2-week time period in which she had one visit with him and another visit to an ED where two other physicians examined her and missed the diagnosis.
VERDICT A Michigan defense verdict was returned.
ILIAC ARTERY INJURED DURING LAPAROSCOPIC SURGERY; PATIENT DIES
A 40-year-old woman underwent laparoscopic gynecologic surgery performed by her ObGyn. During the procedure, the patient’s left internal iliac artery was punctured, but the injury was not recognized at the time. She was discharged the same day. The next morning, she went into hypovolemic shock due to internal bleeding. She was taken to the ED, where she died.
ESTATE’S CLAIM The ObGyn, anesthesiologist, and hospital staff were negligent in their postoperative care. The anesthesiologist prescribed pain medication that masked the injury; the patient was discharged from the postanesthesia unit too early and without proper examination. The nursing staff did not react to the patient’s reports of abdominal pain, nor did they properly assess her condition prior to discharge. The ObGyn failed to return a phone call the evening after the procedure.
DEFENDANTS’ DEFENSE The ObGyn settled before trial. The anesthesiologist and hospital denied negligence: care was proper and followed all protocols.
VERDICT A confidential California settlement was reached with the ObGyn. A defense verdict was returned for the anesthesiologist and hospital.
Related article: Anatomy for the laparoscopic surgeon Emad Mikhail, MD; Lauren Scott, MD; Stuart Hart, MD, MS (April 2014)
GENETIC TESTING MISSED A KEY DIAGNOSIS
A 40-year-old woman underwent genetic testing after she became pregnant. She was assured that there were no abnormalities that would impact her child.
The baby was born with Wolf-Hirschhorn syndrome, characterized by facial deformities, intellectual disabilities, delayed growth, and seizures. The child is nonverbal, deaf, and blind. She uses a feeding tube and requires 24-hour care.
PARENTS’ CLAIM The genetic testing was improperly conducted. The mother would have had an abortion if she’d known that the child was so disabled.
DEFENDANTS’ DEFENSE Settlements were mediated.
VERDICT A $6.15 million New Jersey settlement was reached on behalf of the hospital and two laboratory technicians, and a $1 million settlement was reached with the director of the genetic laboratory.
HEAT INJURY TO COLON: ABSCESSES, PERITONITIS
A 43-year-old patient had a history of symptomatic uterine fibroids and infertility. Her ObGyn performed a hysteroscopy because he suspected endometriosis, but found none. He then successfully removed a large uterine fibroid during laparoscopic myomectomy. The patient was discharged the same day.
Two days later, the patient developed abdominal pain, nausea, and fever. She went to the ED and was taken into emergency surgery after a CT scan showed free air and fluid in her abdomen. She suffered multiple abscesses and peritonitis.
PATIENT’S CLAIM The ObGyn was negligent in performing the surgery: the sigmoid colon sustained a thermal injury, which caused the abscesses and peritonitis.
PHYSICIAN’S DEFENSE There was no evidence of thermal injury during the original operation; heat damage can and does occur in the absence of negligence. The patient’s previously unknown diverticulitis contributed to the development of the recurrent abscesses and peritonitis.
VERDICT A Florida defense verdict was returned.
RUPTURED UTERUS IS UNDETECTED
During labor and delivery, a declining fetal heart rate was observed, but there was an hour’s delay before cesarean delivery was started. The child suffered a hypoxic brain injury. He has spastic quadriplegia, cannot speak, and requires a respirator and feeding tube.
PARENTS’ CLAIM The mother suffered a ruptured uterus during labor that was not recognized by the ObGyn or nursing staff.
DEFENDANTS’ DEFENSE A settlement was reached during trial.
VERDICT A $7.5 million New Jersey settlement was reached.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Tell us what you think!
Drop us a line and let us know what you think about this or other current articles, which topics you'd like to see covered in future issues, and what challenges you face in daily practice. Tell us what you think by emailing us at: obg@frontlinemedcom.com Please include your name, city and state.
Stay in touch! Your feedback is important to us!
UTI, THEN MASSIVE HEMORRHAGE
A woman in her 60s was hospitalized with a urinary tract infection (UTI). She was treated with antibiotics and intravenous (IV) fluids but developed deep vein thrombosis (DVT) at the IV site. Enoxaparin sodium was ordered to treat the clot. After 3 days, she suffered a massive abdominal hemorrhage. When she woke from resuscitation, her weight had doubled. She developed a methicillin-resistant Staphylococcus aureus (MRSA) infection, then Clostridium difficile infection due to antibiotics, plus bedsores. Multiple surgeries left her with an abdominal wall defect that cannot be repaired, and a permanent hernia. She was hospitalized for 75 days.
PATIENT’S CLAIM The hemorrhage was caused when enoxaparin was given at 1.5 times the proper dosage because the patient’s weight was overestimated by 50%. Excessive blood, plasma, and fluids caused her weight to double after resuscitation. Her intestines were forced out of her abdominal cavity by the hemorrhage. A permanent hernia, visible underneath her skin, causes pain.
DEFENDANTS’ DEFENSE The patient’s preexisting diabetes, heart condition, high cholesterol levels, and orthopedic issues impacted her condition. She was not compliant in managing her diabetes, causing many of the current problems.
VERDICT A $9.3 million Connecticut verdict was returned.
Related article: Update: Pelvic floor dysfunction Autumn L. Edenfield, MD, and Cindy L. Amundsen, MD (October 2012)
CESAREAN DELAYED UNNECESSARILY
At 37 weeks’ gestation, a mother reported decreased fetal movement. When the biophysical profile test scored 8/8 and the fetal heart rate was reassuring, the attending ObGyn discharged the patient. However, it was the middle of the night, and the nurse kept the mother in the emergency department (ED). At 8:30 am, the fetus began to show signs of fetal distress. Three ObGyns agreed to monitor labor, although one physician wanted delivery to occur that morning.
The next morning, a second biophysical profile scored 2/8, but the on-call ObGyn misunderstood the score as 6/8 and scheduled cesarean delivery for noon. Two hours after the second biophysical profile, the fetal heart rate crashed. A nurse called the ObGyn, who began an emergency cesarean 15 minutes later. The baby, born lifeless, was resuscitated. The child suffered permanent brain damage, and has cerebral palsy, severe cognitive deficits and speech deficits, and walks with an abnormal gait.
PARENTS’ CLAIM A physician did not see the patient for 24 hours, once the decision was made to monitor the mother, even though the fetal heart rate continued to decline. A biophysical profile test score of 2/8 indicates the need for immediate delivery. An earlier cesarean delivery could have reduced the child’s injuries.
DEFENDANTS’ DEFENSE After a settlement was reached with the hospital, the trial continued against the delivering ObGyn. He claimed that decreased fetal movement indicated that the brain injury had occurred 1 to 4 days before the mother came to the ED. The technician had manipulated the mother’s abdomen to wake the fetus before starting the first biophysical profile, which invalidated the score. The nurse miscommunicated the score of the second biophysical profile.
VERDICT A gross $29.8 million Illinois verdict was returned that included a $1.65 million settlement with the hospital.
WAS FACILITY ADEQUATELY STAFFED AFTER HURRICANE IKE?
A mother was admitted to a hospital for induction of labor in September 2008. After birth, the child was found to have cerebral palsy.
PARENTS’ CLAIM The mother should have been sent to another facility before delivery was induced because the hospital was short-staffed and low on resources due to Hurricane Ike. Too much oxytocin was used to induce contractions, which led to a lack of oxygen for the fetus. All prenatal testing had shown a healthy fetus. A cesarean delivery should have occurred when fetal distress was noted.
DEFENDANTS’ DEFENSE The mother had gastric bypass surgery 8 months before she became pregnant, and smoked during pregnancy, which accounted for the infant’s injuries. Treatment during labor and delivery was appropriate. Hospital staffing and resources were adequate.
VERDICT A $6.5 million Texas settlement was reached.
PLACENTA ACCRETA; MOTHER DIES
A 33-year-old woman became pregnant with her second child. A variety of conditions caused this to be high-risk pregnancy, so she saw a maternal-fetal medicine (MFM) specialist 2 months before delivery. The MFM reported that his examination and the ultrasonography (US) results were normal.
The ObGyn who provided prenatal care and delivered her first child scheduled cesarean delivery. During the procedure, the ObGyn noticed a 3- to 4-inch lesion where the placenta had penetrated the uterus. When the placenta was removed, the patient began to hemorrhage and a hysterectomy was performed. The hemorrhage created blood clots that led to gangrene in the patient’s extremities. She died 5 days after giving birth.
ESTATE’S CLAIM Both the MFM and the ObGyn failed to recognize placenta accreta on US prior to delivery. The ObGyn should have performed US prior to beginning cesarean delivery. The hospital’s protocols were not followed: the ObGyn should have stopped the procedure and called for extra surgical assistance and additional blood when he encountered placenta accreta, and again when the patient began to hemorrhage. Placenta accreta does not have to be fatal if detected and managed properly.
DEFENDANTS’ DEFENSE There was no negligence; the patient was treated properly.
VERDICT A $15.5 million Illinois verdict was returned against both physicians and the medical center.
Related article: Is the risk of placenta accreta in a subsequent pregnancy higher after emergent primary cesarean or after elective primary cesarean? Yinka Oyelese, MD (Examining the Evidence, December 2013)
ANTICONVULSANT AND MIGRAINE MEDS TAKEN DURING PREGNANCY
A woman was prescribed topiramate (Topamax) for migraine headaches and hand tremors during the first trimester of her pregnancy in 2007. With a history of seizures, she also took several anticonvulsants throughout her pregnancy. Her child was diagnosed with right unilateral cleft lip (cheiloschisis) in utero. The condition had not been surgically corrected at the time of trial.
PARENTS’ CLAIM The use of topiramate caused the child’s cleft lip. Janssen Pharmaceuticals, the manufacturer of Topamax, knew about the risk of birth defects associated with the drug in 2007, but failed to provide adequate warnings.
DEFENDANTS’ DEFENSE The mother received at least two warnings from her physician regarding the potential risks of anticonvulsant and antiepileptic drugs and the importance of not becoming pregnant while taking the medications. An action against the physician was barred by the applicable statute of limitations. The mother had taken topiramate prescribed to her mother for a time; such actions should release Janssen from liability.
VERDICT A $11 million Pennsylvania verdict was returned.
PID MASKS ECTOPIC PREGNANCY
A woman in her 40s became pregnant. On the first two prenatal diagnostic imaging studies, the ObGyn saw an intrauterine pregnancy. He later realized that the pregnancy was ectopic after beta human chorionic gonadotrophin (beta-hCG) blood levels were abnormal. During surgery to terminate the pregnancy, he found he had to perform a total hysterectomy because the patient had extensive pelvic inflammatory disease (PID) caused by a long history of sexually transmitted disease.
PATIENT’S CLAIM If the ectopic pregnancy had been diagnosed earlier, one of her ovaries could have been preserved, saving her from the symptoms of surgical menopause.
PHYSICIAN’S DEFENSE PID had caused the ovaries, numerous fibroid tumors, and the uterus to fuse into one mass. That was why the first two imaging studies appeared to show an intrauterine pregnancy. It was not possible to diagnose the extent of the problem until surgery. The patient did not have a true ectopic pregnancy.
The patient’s difficulties occurred during a 2-week time period in which she had one visit with him and another visit to an ED where two other physicians examined her and missed the diagnosis.
VERDICT A Michigan defense verdict was returned.
ILIAC ARTERY INJURED DURING LAPAROSCOPIC SURGERY; PATIENT DIES
A 40-year-old woman underwent laparoscopic gynecologic surgery performed by her ObGyn. During the procedure, the patient’s left internal iliac artery was punctured, but the injury was not recognized at the time. She was discharged the same day. The next morning, she went into hypovolemic shock due to internal bleeding. She was taken to the ED, where she died.
ESTATE’S CLAIM The ObGyn, anesthesiologist, and hospital staff were negligent in their postoperative care. The anesthesiologist prescribed pain medication that masked the injury; the patient was discharged from the postanesthesia unit too early and without proper examination. The nursing staff did not react to the patient’s reports of abdominal pain, nor did they properly assess her condition prior to discharge. The ObGyn failed to return a phone call the evening after the procedure.
DEFENDANTS’ DEFENSE The ObGyn settled before trial. The anesthesiologist and hospital denied negligence: care was proper and followed all protocols.
VERDICT A confidential California settlement was reached with the ObGyn. A defense verdict was returned for the anesthesiologist and hospital.
Related article: Anatomy for the laparoscopic surgeon Emad Mikhail, MD; Lauren Scott, MD; Stuart Hart, MD, MS (April 2014)
GENETIC TESTING MISSED A KEY DIAGNOSIS
A 40-year-old woman underwent genetic testing after she became pregnant. She was assured that there were no abnormalities that would impact her child.
The baby was born with Wolf-Hirschhorn syndrome, characterized by facial deformities, intellectual disabilities, delayed growth, and seizures. The child is nonverbal, deaf, and blind. She uses a feeding tube and requires 24-hour care.
PARENTS’ CLAIM The genetic testing was improperly conducted. The mother would have had an abortion if she’d known that the child was so disabled.
DEFENDANTS’ DEFENSE Settlements were mediated.
VERDICT A $6.15 million New Jersey settlement was reached on behalf of the hospital and two laboratory technicians, and a $1 million settlement was reached with the director of the genetic laboratory.
HEAT INJURY TO COLON: ABSCESSES, PERITONITIS
A 43-year-old patient had a history of symptomatic uterine fibroids and infertility. Her ObGyn performed a hysteroscopy because he suspected endometriosis, but found none. He then successfully removed a large uterine fibroid during laparoscopic myomectomy. The patient was discharged the same day.
Two days later, the patient developed abdominal pain, nausea, and fever. She went to the ED and was taken into emergency surgery after a CT scan showed free air and fluid in her abdomen. She suffered multiple abscesses and peritonitis.
PATIENT’S CLAIM The ObGyn was negligent in performing the surgery: the sigmoid colon sustained a thermal injury, which caused the abscesses and peritonitis.
PHYSICIAN’S DEFENSE There was no evidence of thermal injury during the original operation; heat damage can and does occur in the absence of negligence. The patient’s previously unknown diverticulitis contributed to the development of the recurrent abscesses and peritonitis.
VERDICT A Florida defense verdict was returned.
RUPTURED UTERUS IS UNDETECTED
During labor and delivery, a declining fetal heart rate was observed, but there was an hour’s delay before cesarean delivery was started. The child suffered a hypoxic brain injury. He has spastic quadriplegia, cannot speak, and requires a respirator and feeding tube.
PARENTS’ CLAIM The mother suffered a ruptured uterus during labor that was not recognized by the ObGyn or nursing staff.
DEFENDANTS’ DEFENSE A settlement was reached during trial.
VERDICT A $7.5 million New Jersey settlement was reached.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Tell us what you think!
Drop us a line and let us know what you think about this or other current articles, which topics you'd like to see covered in future issues, and what challenges you face in daily practice. Tell us what you think by emailing us at: obg@frontlinemedcom.com Please include your name, city and state.
Stay in touch! Your feedback is important to us!
NHL among top 10 most common cancers in US

Credit: Rhoda Baer
A new report shows the rate of invasive cancer among US men and women dropped slightly from 2009 to 2010, and the most common cancers were solid tumor malignancies.
However, non-Hodgkin lymphoma (NHL) consistently rated among the top 10 most common cancers, regardless of patient gender or race.
The report was prepared by the Centers for Disease Control and Prevention (CDC) and appears in the current Morbidity and Mortality Weekly Report.
Researchers analyzed new cases of invasive cancers diagnosed in 2010 and reported to the CDC’s National Program of Cancer Registries and the National Cancer Institute’s Surveillance, Epidemiology and Results Program.
Data from all states (except Arkansas and Minnesota) and the District of Columbia were included in the analysis, which covered 97% of the US population.
The researchers found the rates of invasive cancer cases dropped from 459 per 100,000 people in 2009 to 446 per 100,000 in 2010.
Cancer rates were higher among men (503 per 100,000) than women (405 per 100,000). In all, there were 745,383 cases reported among men and 711,113 among women in 2010.
The highest rates were for cancers of the prostate (126 per 100,000), female breast (119 per 100,000), lung and bronchus (62 per 100,000), and colon and rectum (40 per 100,000). Together, these accounted for half of all cancer cases in the US.
However, hematologic malignancies were fairly common as well. NHL was the 6th most common cancer for men of all races/ethnicities except Hispanic. For this group, NHL was the 4th most common cancer.
NHL was the 7th most common cancer for black and white women and 6th for the remaining groups, which included American Indian/Alaskan native, Asian/Pacific Islander, and Hispanic women.
Leukemia and myeloma were also among the top 10 most common invasive cancers for certain patients.
Leukemia was the 9th most common cancer for Hispanic and white men and the 10th for American Indian/Alaskan Native women. Myeloma was the 8th most common cancer for black women.
Overall, cancer rates were highest among black patients (455 per 100,000), followed by whites (445 per 100,000), Hispanics (344 per 100,000), Asian/Pacific Islanders (289 per 100,000), and American Indians/Alaskan Natives (270 per 100,000). ![]()

Credit: Rhoda Baer
A new report shows the rate of invasive cancer among US men and women dropped slightly from 2009 to 2010, and the most common cancers were solid tumor malignancies.
However, non-Hodgkin lymphoma (NHL) consistently rated among the top 10 most common cancers, regardless of patient gender or race.
The report was prepared by the Centers for Disease Control and Prevention (CDC) and appears in the current Morbidity and Mortality Weekly Report.
Researchers analyzed new cases of invasive cancers diagnosed in 2010 and reported to the CDC’s National Program of Cancer Registries and the National Cancer Institute’s Surveillance, Epidemiology and Results Program.
Data from all states (except Arkansas and Minnesota) and the District of Columbia were included in the analysis, which covered 97% of the US population.
The researchers found the rates of invasive cancer cases dropped from 459 per 100,000 people in 2009 to 446 per 100,000 in 2010.
Cancer rates were higher among men (503 per 100,000) than women (405 per 100,000). In all, there were 745,383 cases reported among men and 711,113 among women in 2010.
The highest rates were for cancers of the prostate (126 per 100,000), female breast (119 per 100,000), lung and bronchus (62 per 100,000), and colon and rectum (40 per 100,000). Together, these accounted for half of all cancer cases in the US.
However, hematologic malignancies were fairly common as well. NHL was the 6th most common cancer for men of all races/ethnicities except Hispanic. For this group, NHL was the 4th most common cancer.
NHL was the 7th most common cancer for black and white women and 6th for the remaining groups, which included American Indian/Alaskan native, Asian/Pacific Islander, and Hispanic women.
Leukemia and myeloma were also among the top 10 most common invasive cancers for certain patients.
Leukemia was the 9th most common cancer for Hispanic and white men and the 10th for American Indian/Alaskan Native women. Myeloma was the 8th most common cancer for black women.
Overall, cancer rates were highest among black patients (455 per 100,000), followed by whites (445 per 100,000), Hispanics (344 per 100,000), Asian/Pacific Islanders (289 per 100,000), and American Indians/Alaskan Natives (270 per 100,000). ![]()

Credit: Rhoda Baer
A new report shows the rate of invasive cancer among US men and women dropped slightly from 2009 to 2010, and the most common cancers were solid tumor malignancies.
However, non-Hodgkin lymphoma (NHL) consistently rated among the top 10 most common cancers, regardless of patient gender or race.
The report was prepared by the Centers for Disease Control and Prevention (CDC) and appears in the current Morbidity and Mortality Weekly Report.
Researchers analyzed new cases of invasive cancers diagnosed in 2010 and reported to the CDC’s National Program of Cancer Registries and the National Cancer Institute’s Surveillance, Epidemiology and Results Program.
Data from all states (except Arkansas and Minnesota) and the District of Columbia were included in the analysis, which covered 97% of the US population.
The researchers found the rates of invasive cancer cases dropped from 459 per 100,000 people in 2009 to 446 per 100,000 in 2010.
Cancer rates were higher among men (503 per 100,000) than women (405 per 100,000). In all, there were 745,383 cases reported among men and 711,113 among women in 2010.
The highest rates were for cancers of the prostate (126 per 100,000), female breast (119 per 100,000), lung and bronchus (62 per 100,000), and colon and rectum (40 per 100,000). Together, these accounted for half of all cancer cases in the US.
However, hematologic malignancies were fairly common as well. NHL was the 6th most common cancer for men of all races/ethnicities except Hispanic. For this group, NHL was the 4th most common cancer.
NHL was the 7th most common cancer for black and white women and 6th for the remaining groups, which included American Indian/Alaskan native, Asian/Pacific Islander, and Hispanic women.
Leukemia and myeloma were also among the top 10 most common invasive cancers for certain patients.
Leukemia was the 9th most common cancer for Hispanic and white men and the 10th for American Indian/Alaskan Native women. Myeloma was the 8th most common cancer for black women.
Overall, cancer rates were highest among black patients (455 per 100,000), followed by whites (445 per 100,000), Hispanics (344 per 100,000), Asian/Pacific Islanders (289 per 100,000), and American Indians/Alaskan Natives (270 per 100,000). ![]()
Discovery could aid treatment of hemolysis

Credit: NHLBI
Results of preclinical research could aid the development of new treatments for hemolysis, which may have implications for patients with sickle cell anemia and those who receive blood transfusions.
The researchers were investigating the possibility of using haptoglobin to prevent the chemical reactions triggered by hemoglobin after hemolysis.
Haptoglobin is known to bind acellular adult hemoglobin dimers and facilitate their clearance after hemolysis.
But haptoglobin exists in different forms. The 3 main phenotypes—Hp1-1, Hp2-1, and Hp2-2—have diverse structural configurations, and previous research suggested they have different biological activities.
With the current study, however, the researchers showed the different forms of haptoglobin actually exhibit similar activity.
Todd L. Mollan, PhD, of the Center for Biologics Evaluation and Research at the Food and Drug Administration in Bethesda, Maryland, and his colleagues presented these findings in Free Radical Biology and Medicine.
The researchers studied hemoglobin dimers in complex with unfractionated haptoglobin (a mixture of Hp1-1, Hp2-1, and Hp2-2); fractionated, dimeric haptoglobin (Hp1-1); and fractionated, polymeric haptoglobin (predominantly Hp2-2, with minor amounts of Hp2-1).
The team also complexed ferrous and ferric hemoglobins with unfractionated haptoglobin and its fractionated forms.
Experiments revealed no significant differences among the different complexes with regard to hemoglobin-haptoglobin binding kinetics, hydrogen-peroxide-driven oxidative transitions of the heme iron, radical formation, heme loss, or intrinsic redox potential.
The researchers said these results should be taken into account when designing phenotype-specific haptoglobin therapies. ![]()

Credit: NHLBI
Results of preclinical research could aid the development of new treatments for hemolysis, which may have implications for patients with sickle cell anemia and those who receive blood transfusions.
The researchers were investigating the possibility of using haptoglobin to prevent the chemical reactions triggered by hemoglobin after hemolysis.
Haptoglobin is known to bind acellular adult hemoglobin dimers and facilitate their clearance after hemolysis.
But haptoglobin exists in different forms. The 3 main phenotypes—Hp1-1, Hp2-1, and Hp2-2—have diverse structural configurations, and previous research suggested they have different biological activities.
With the current study, however, the researchers showed the different forms of haptoglobin actually exhibit similar activity.
Todd L. Mollan, PhD, of the Center for Biologics Evaluation and Research at the Food and Drug Administration in Bethesda, Maryland, and his colleagues presented these findings in Free Radical Biology and Medicine.
The researchers studied hemoglobin dimers in complex with unfractionated haptoglobin (a mixture of Hp1-1, Hp2-1, and Hp2-2); fractionated, dimeric haptoglobin (Hp1-1); and fractionated, polymeric haptoglobin (predominantly Hp2-2, with minor amounts of Hp2-1).
The team also complexed ferrous and ferric hemoglobins with unfractionated haptoglobin and its fractionated forms.
Experiments revealed no significant differences among the different complexes with regard to hemoglobin-haptoglobin binding kinetics, hydrogen-peroxide-driven oxidative transitions of the heme iron, radical formation, heme loss, or intrinsic redox potential.
The researchers said these results should be taken into account when designing phenotype-specific haptoglobin therapies. ![]()

Credit: NHLBI
Results of preclinical research could aid the development of new treatments for hemolysis, which may have implications for patients with sickle cell anemia and those who receive blood transfusions.
The researchers were investigating the possibility of using haptoglobin to prevent the chemical reactions triggered by hemoglobin after hemolysis.
Haptoglobin is known to bind acellular adult hemoglobin dimers and facilitate their clearance after hemolysis.
But haptoglobin exists in different forms. The 3 main phenotypes—Hp1-1, Hp2-1, and Hp2-2—have diverse structural configurations, and previous research suggested they have different biological activities.
With the current study, however, the researchers showed the different forms of haptoglobin actually exhibit similar activity.
Todd L. Mollan, PhD, of the Center for Biologics Evaluation and Research at the Food and Drug Administration in Bethesda, Maryland, and his colleagues presented these findings in Free Radical Biology and Medicine.
The researchers studied hemoglobin dimers in complex with unfractionated haptoglobin (a mixture of Hp1-1, Hp2-1, and Hp2-2); fractionated, dimeric haptoglobin (Hp1-1); and fractionated, polymeric haptoglobin (predominantly Hp2-2, with minor amounts of Hp2-1).
The team also complexed ferrous and ferric hemoglobins with unfractionated haptoglobin and its fractionated forms.
Experiments revealed no significant differences among the different complexes with regard to hemoglobin-haptoglobin binding kinetics, hydrogen-peroxide-driven oxidative transitions of the heme iron, radical formation, heme loss, or intrinsic redox potential.
The researchers said these results should be taken into account when designing phenotype-specific haptoglobin therapies. ![]()
NICE OKs rituximab for ANCA-associated vasculitis

Credit: Linda Bartlett
The UK’s National Institute for Health and Care Excellence (NICE) has published a final guidance recommending rituximab (MabThera) as a treatment option for adults with anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis.
The guidance applies to adults with severe microscopic polyangiitis or granulomatosis with polyangiitis.
The guidance recommends rituximab in combination with glucocorticoids for certain patients in this population.
According to NICE, the treatment is suitable only if:
- The patient is in danger of exceeding the maximum amount of cyclophosphamide
- The patient cannot or should not receive cyclophosphamide
- Loss of fertility (due to cyclophosphamide) is a concern
- The patient’s disease did not respond to a course of cyclophosphamide lasting 3 to 6 months
- The patient has had uroepithelial cancer.
About ANCA-associated vasculitis
ANCA-associated vasculitis is an inflammatory autoimmune disease affecting the blood vessel walls. It can affect many organs and leads to tissue breakdown and damage. Granulomatosis with polyangiitis and microscopic polyangiitis are types of ANCA-associated vasculitis that affect small blood vessels.
ANCA-associated vasculitis usually affects the lungs, kidneys, ears, nose or sinuses. Depending on the organs involved, it can cause bleeding, rash, or deafness.
The aim of treatment is initially to induce remission, then to maintain remission and treat relapse when necessary. With adequate ongoing care, most patients with ANCA-associated vasculitis will have a good quality of life and normal life expectancy.
“The effects of vasculitis, as well as the stress of the fear of relapse, can often have a significant detrimental impact on patients’ quality of life,” said Carole Longson, PhD, Health Technology Evaluation Centre Director at NICE.
“The introduction of immunosuppressant therapy with cyclophosphamide and corticosteroids has dramatically improved the prognosis from a condition with high mortality to being a chronic disease with a relapsing and remitting course. However, these treatments are associated with substantial side effects that can further impair patients’ quality of life.”
Rituximab for ANCA-associated vasculitis
An independent advisory committee informed NICE that rituximab is a clinically effective and cost-effective option for some patients with severe microscopic polyangiitis or granulomatosis with polyangiitis.
“The committee heard that rituximab is the first effective treatment since the introduction of cyclophosphamide in the 1970s,” Dr Longson said. “In addition, they heard from the clinical specialists and patient experts that induction treatment with rituximab was 4 weeks instead of up to 6 months with cyclophosphamide, which was more convenient for patients.”
“The committee concluded that rituximab is an innovative treatment for vasculitis and that this benefit is important to patients. The committee also concluded that rituximab is a cost-effective use of NHS resources for those groups specified in the guidance.”
Rituximab is priced at £174.63 per 10 mL vial and £873.15 per 50 mL vial (excluding tax). The manufacturer’s estimate of the average cost of a course of treatment is £4889.64 (based on 1.79 m2 body surface area and no vial sharing). Costs may vary in different settings because of negotiated procurement discounts. ![]()

Credit: Linda Bartlett
The UK’s National Institute for Health and Care Excellence (NICE) has published a final guidance recommending rituximab (MabThera) as a treatment option for adults with anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis.
The guidance applies to adults with severe microscopic polyangiitis or granulomatosis with polyangiitis.
The guidance recommends rituximab in combination with glucocorticoids for certain patients in this population.
According to NICE, the treatment is suitable only if:
- The patient is in danger of exceeding the maximum amount of cyclophosphamide
- The patient cannot or should not receive cyclophosphamide
- Loss of fertility (due to cyclophosphamide) is a concern
- The patient’s disease did not respond to a course of cyclophosphamide lasting 3 to 6 months
- The patient has had uroepithelial cancer.
About ANCA-associated vasculitis
ANCA-associated vasculitis is an inflammatory autoimmune disease affecting the blood vessel walls. It can affect many organs and leads to tissue breakdown and damage. Granulomatosis with polyangiitis and microscopic polyangiitis are types of ANCA-associated vasculitis that affect small blood vessels.
ANCA-associated vasculitis usually affects the lungs, kidneys, ears, nose or sinuses. Depending on the organs involved, it can cause bleeding, rash, or deafness.
The aim of treatment is initially to induce remission, then to maintain remission and treat relapse when necessary. With adequate ongoing care, most patients with ANCA-associated vasculitis will have a good quality of life and normal life expectancy.
“The effects of vasculitis, as well as the stress of the fear of relapse, can often have a significant detrimental impact on patients’ quality of life,” said Carole Longson, PhD, Health Technology Evaluation Centre Director at NICE.
“The introduction of immunosuppressant therapy with cyclophosphamide and corticosteroids has dramatically improved the prognosis from a condition with high mortality to being a chronic disease with a relapsing and remitting course. However, these treatments are associated with substantial side effects that can further impair patients’ quality of life.”
Rituximab for ANCA-associated vasculitis
An independent advisory committee informed NICE that rituximab is a clinically effective and cost-effective option for some patients with severe microscopic polyangiitis or granulomatosis with polyangiitis.
“The committee heard that rituximab is the first effective treatment since the introduction of cyclophosphamide in the 1970s,” Dr Longson said. “In addition, they heard from the clinical specialists and patient experts that induction treatment with rituximab was 4 weeks instead of up to 6 months with cyclophosphamide, which was more convenient for patients.”
“The committee concluded that rituximab is an innovative treatment for vasculitis and that this benefit is important to patients. The committee also concluded that rituximab is a cost-effective use of NHS resources for those groups specified in the guidance.”
Rituximab is priced at £174.63 per 10 mL vial and £873.15 per 50 mL vial (excluding tax). The manufacturer’s estimate of the average cost of a course of treatment is £4889.64 (based on 1.79 m2 body surface area and no vial sharing). Costs may vary in different settings because of negotiated procurement discounts. ![]()

Credit: Linda Bartlett
The UK’s National Institute for Health and Care Excellence (NICE) has published a final guidance recommending rituximab (MabThera) as a treatment option for adults with anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis.
The guidance applies to adults with severe microscopic polyangiitis or granulomatosis with polyangiitis.
The guidance recommends rituximab in combination with glucocorticoids for certain patients in this population.
According to NICE, the treatment is suitable only if:
- The patient is in danger of exceeding the maximum amount of cyclophosphamide
- The patient cannot or should not receive cyclophosphamide
- Loss of fertility (due to cyclophosphamide) is a concern
- The patient’s disease did not respond to a course of cyclophosphamide lasting 3 to 6 months
- The patient has had uroepithelial cancer.
About ANCA-associated vasculitis
ANCA-associated vasculitis is an inflammatory autoimmune disease affecting the blood vessel walls. It can affect many organs and leads to tissue breakdown and damage. Granulomatosis with polyangiitis and microscopic polyangiitis are types of ANCA-associated vasculitis that affect small blood vessels.
ANCA-associated vasculitis usually affects the lungs, kidneys, ears, nose or sinuses. Depending on the organs involved, it can cause bleeding, rash, or deafness.
The aim of treatment is initially to induce remission, then to maintain remission and treat relapse when necessary. With adequate ongoing care, most patients with ANCA-associated vasculitis will have a good quality of life and normal life expectancy.
“The effects of vasculitis, as well as the stress of the fear of relapse, can often have a significant detrimental impact on patients’ quality of life,” said Carole Longson, PhD, Health Technology Evaluation Centre Director at NICE.
“The introduction of immunosuppressant therapy with cyclophosphamide and corticosteroids has dramatically improved the prognosis from a condition with high mortality to being a chronic disease with a relapsing and remitting course. However, these treatments are associated with substantial side effects that can further impair patients’ quality of life.”
Rituximab for ANCA-associated vasculitis
An independent advisory committee informed NICE that rituximab is a clinically effective and cost-effective option for some patients with severe microscopic polyangiitis or granulomatosis with polyangiitis.
“The committee heard that rituximab is the first effective treatment since the introduction of cyclophosphamide in the 1970s,” Dr Longson said. “In addition, they heard from the clinical specialists and patient experts that induction treatment with rituximab was 4 weeks instead of up to 6 months with cyclophosphamide, which was more convenient for patients.”
“The committee concluded that rituximab is an innovative treatment for vasculitis and that this benefit is important to patients. The committee also concluded that rituximab is a cost-effective use of NHS resources for those groups specified in the guidance.”
Rituximab is priced at £174.63 per 10 mL vial and £873.15 per 50 mL vial (excluding tax). The manufacturer’s estimate of the average cost of a course of treatment is £4889.64 (based on 1.79 m2 body surface area and no vial sharing). Costs may vary in different settings because of negotiated procurement discounts. ![]()
Study provides insights on gene linked to lymphoma
Research in zebrafish embryos may help explain the link between Max’s giant-associated protein (MGA) and Richter’s syndrome.
Previous studies showed that mutations in MGA are associated with a high risk of Richter’s syndrome, a rare lymphoma that can occur in patients with chronic lymphocytic leukemia.
Now, a team of biologists has discovered how MGA controls developmental processes. They described their discoveries in Developmental Cell.
“The same genes that are involved in building a person during embryonic development can mutate and cause cancer later in life,” said study author Scott Dougan, PhD, of the University of Georgia in Athens.
“No one has done a systematic study of MGA, but now that some studies connect it to cancer, there is tremendous interest.”
Preliminary tests have suggested that Richter’s syndrome might develop when MGA does not successfully control the activities of the MYC oncogene.
Dr Dougan and his colleagues decided to alter the levels of MGA in zebrafish embryos to see if they could discover any other roles for MGA.
They found that MGA also helps control the expression of bone morphogenetic proteins (BMPs). Specifically, a transcription factor complex consisting of MGA, Max, and Smad4 controls the expression of bmp2b/swirl in the zebrafish yolk syncytial layer. And this controls BMP signaling throughout the embryo.
BMPs are responsible for bone development in the embryo, but, in adults, changes in BMP activity can result in tumor development. This research suggests MGA may be part of this transformation.
“Scientists are only beginning to understand the roles this MGA protein plays, but our tests show that MGA may control many more processes than first imagined,” Dr Dougan said. “MGA may be involved in a number of other cancers, but we need to do more research before we’re sure.”
In the coming months, Dr Dougan and his colleagues plan to further examine the roles of MGA to determine when it controls MYC, when it controls BMP, and how it is involved in tumor formation.
“[W]e need investigations like these to understand the fundamentals of our biology,” Dr Dougan said. “Once we have this understanding, we can begin to develop new therapies to treat diseases in new, more effective ways.” ![]()

Research in zebrafish embryos may help explain the link between Max’s giant-associated protein (MGA) and Richter’s syndrome.
Previous studies showed that mutations in MGA are associated with a high risk of Richter’s syndrome, a rare lymphoma that can occur in patients with chronic lymphocytic leukemia.
Now, a team of biologists has discovered how MGA controls developmental processes. They described their discoveries in Developmental Cell.
“The same genes that are involved in building a person during embryonic development can mutate and cause cancer later in life,” said study author Scott Dougan, PhD, of the University of Georgia in Athens.
“No one has done a systematic study of MGA, but now that some studies connect it to cancer, there is tremendous interest.”
Preliminary tests have suggested that Richter’s syndrome might develop when MGA does not successfully control the activities of the MYC oncogene.
Dr Dougan and his colleagues decided to alter the levels of MGA in zebrafish embryos to see if they could discover any other roles for MGA.
They found that MGA also helps control the expression of bone morphogenetic proteins (BMPs). Specifically, a transcription factor complex consisting of MGA, Max, and Smad4 controls the expression of bmp2b/swirl in the zebrafish yolk syncytial layer. And this controls BMP signaling throughout the embryo.
BMPs are responsible for bone development in the embryo, but, in adults, changes in BMP activity can result in tumor development. This research suggests MGA may be part of this transformation.
“Scientists are only beginning to understand the roles this MGA protein plays, but our tests show that MGA may control many more processes than first imagined,” Dr Dougan said. “MGA may be involved in a number of other cancers, but we need to do more research before we’re sure.”
In the coming months, Dr Dougan and his colleagues plan to further examine the roles of MGA to determine when it controls MYC, when it controls BMP, and how it is involved in tumor formation.
“[W]e need investigations like these to understand the fundamentals of our biology,” Dr Dougan said. “Once we have this understanding, we can begin to develop new therapies to treat diseases in new, more effective ways.” ![]()

Research in zebrafish embryos may help explain the link between Max’s giant-associated protein (MGA) and Richter’s syndrome.
Previous studies showed that mutations in MGA are associated with a high risk of Richter’s syndrome, a rare lymphoma that can occur in patients with chronic lymphocytic leukemia.
Now, a team of biologists has discovered how MGA controls developmental processes. They described their discoveries in Developmental Cell.
“The same genes that are involved in building a person during embryonic development can mutate and cause cancer later in life,” said study author Scott Dougan, PhD, of the University of Georgia in Athens.
“No one has done a systematic study of MGA, but now that some studies connect it to cancer, there is tremendous interest.”
Preliminary tests have suggested that Richter’s syndrome might develop when MGA does not successfully control the activities of the MYC oncogene.
Dr Dougan and his colleagues decided to alter the levels of MGA in zebrafish embryos to see if they could discover any other roles for MGA.
They found that MGA also helps control the expression of bone morphogenetic proteins (BMPs). Specifically, a transcription factor complex consisting of MGA, Max, and Smad4 controls the expression of bmp2b/swirl in the zebrafish yolk syncytial layer. And this controls BMP signaling throughout the embryo.
BMPs are responsible for bone development in the embryo, but, in adults, changes in BMP activity can result in tumor development. This research suggests MGA may be part of this transformation.
“Scientists are only beginning to understand the roles this MGA protein plays, but our tests show that MGA may control many more processes than first imagined,” Dr Dougan said. “MGA may be involved in a number of other cancers, but we need to do more research before we’re sure.”
In the coming months, Dr Dougan and his colleagues plan to further examine the roles of MGA to determine when it controls MYC, when it controls BMP, and how it is involved in tumor formation.
“[W]e need investigations like these to understand the fundamentals of our biology,” Dr Dougan said. “Once we have this understanding, we can begin to develop new therapies to treat diseases in new, more effective ways.” ![]()
Pediatric Inpatient Guidelines Quality
Researchers from the Pediatric Research in Inpatient Settings (PRIS) network, an open pediatric hospitalist research network,[1] have identified inpatient pediatric medical and surgical conditions considered high priority for quality improvement (QI) initiatives and/or comparative effectiveness research based on prevalence, cost, and interhospital variation in resource utilization.[2] One approach for improving the quality of care within hospitals is to operationalize evidence‐based guidelines into practice.[3] Although guidelines may be used by individual clinicians, systematic adoption by hospitals into clinical workflow has the potential to influence providers to adhere to evidence‐based care, reduce unwarranted variation, and ultimately improve patient outcomes.[3, 4, 5, 6]
There are critical appraisal tools to measure the methodological quality, as defined by the Institute of Medicine (IOM) and others in their guidelines.[7, 8, 9, 10, 11, 12] One such validated tool is the AGREE II instrument, created by the AGREE (Appraisal of Guidelines for REsearch and Evaluation) collaboration.[13, 14] It defines methodological quality as the confidence that the biases linked to the rigor of development, presentation, and applicability of a clinical practice guideline have been minimized and that each step of the development process is clearly reported.[13]
The objective of our study was to rate the methodological quality of national guidelines for 20 of the PRIS priority pediatric inpatient conditions.[2] Our intent in pursuing this project was 2‐fold: first, to inform pediatric inpatient QI initiatives, and second, to call out priority pediatric inpatient conditions for which high methodological‐quality guidelines are currently lacking.
METHODS
The study methods involved (1) prioritizing pediatric inpatient conditions, (2) identifying national guidelines for the priority conditions, and (3) rating the methodological quality of available guidelines. This study was considered nonhuman‐subject research (A. Johnson, personal e‐mail communication, November 14, 2012), and the original prioritization study was deemed exempt from review by the institutional review board of the Children's Hospital of Philadelphia under 45 CFR 46.102(f).[2]
Prioritizing Pediatric Inpatient Conditions
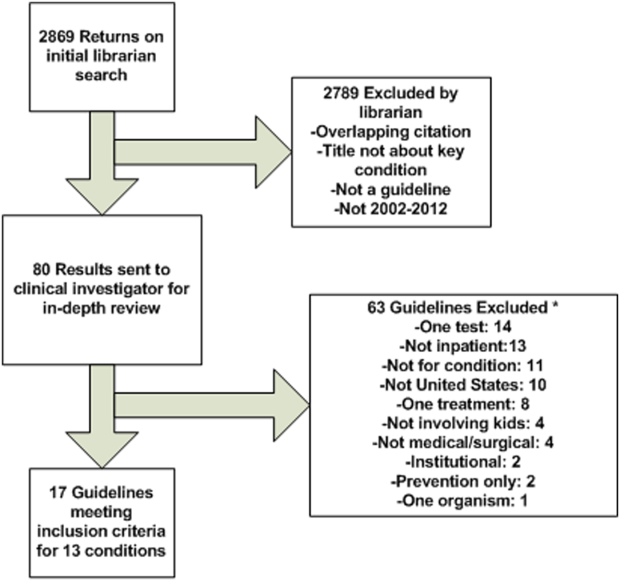
Methods for developing the prioritization list are published elsewhere in detail and briefly described here.[1] An International Classification of Diseases, 9th Revision, Clinical Modification‐based clinical condition grouper was created for primary discharge diagnosis codes for inpatient, ambulatory surgery, and observation unit encounters accounting for either 80% of all encounters or 80% of all charges for over 3.4 million discharges from 2004 to 2009 for 38 children's hospitals in the Pediatric Health Information Systems (PHIS) database, which includes administrative and billing data.[15] A standardized cost master index was created to assign the same unit cost for each billable item (calculated as the median of median hospital unit costs) to allow for comparisons of resource utilization across hospitals (eg, the cost of a chest x‐ray was set to be the same across all hospitals in 2009 dollars). Total hospital costs were then recalculated for every admission by multiplying the standardized cost master index by the number of units for each item in the hospital bill, and then summing the standardized costs of each line item in every bill. Conditions were ranked based on prevalence and total cost across all hospitals in the study period. The variation in standardized costs across hospitals for each condition was determined.
For the current study, conditions were considered if they had a top 20 prevalence rank, a top 20 cost rank, high variation (intraclass correlation coefficient >0.1) in standardized costs across hospitals, a minimum number of PHIS hospitals with annualized overexpenditures (using the standardized cost master) of at least $50,000 when compared to the mean, or a minimum median of 200 cases per hospital over the 6‐year study period to assure sufficient hospital volume for future interventions. This resulted in 29 conditions; the selected 20 conditions matched the top 20 prevalence rank (see Supporting Information, Table 1, in the online version of this article).[2]
| Condition by PRIS Priority Rank | Guidelines Meeting Inclusion Criteriaa | Guidelines Citation | Mean Overall Reviewer Methodological Quality Rating (Rater 1, Rater 2)b | Recommended for Use in the Pediatric Inpatient Setting, Mean (Rater 1, Rater 2)c | Weighted Kappa(95% Confidence Interval) |
|---|---|---|---|---|---|
| |||||
| Otitis media, unspecified, s | 1 | American Academy of Family Physicians; American Academy of Otolaryngology‐Head and Neck Surgery; American Academy of Pediatrics Subcommittee on Otitis Media With Effusion. Clinical Practice Guidelines: Otitis media with effusion. Pediatrics. 2004 May;113(5):1412‐29. | 6 (6, 6) | 3 (3, 3) | 0.76 (0.490.93) |
| Hypertrophy of tonsils and adenoids, s | 1 | Baugh RF et al. Clinical practice guideline: tonsillectomy in children. Otolaryngol Head Neck Surg. 2011;144(1 suppl):S1S30. | 6.5 (7, 6) | 3 (3, 3) | 0.49 (0.050.81) |
| Asthma, m | 1 | National Heart, Lung, and Blood Institute; National Asthma Education and Prevention Program. Expert panel report 3 (EPR‐3): guidelines for the diagnosis and management of asthma‐full report 2007. Pages 1‐440. Available at: | 7 (7, 7) | 3 (3, 3) | 0.62 (0.210.87) |
| Bronchiolitis, m | 1 | American Academy of Pediatrics Subcommittee on Diagnosis and Management of Bronchiolitis. Diagnosis and management of bronchiolitis. Pediatrics. 2006;118:17741793. | 6.5 (6, 7) | 3 (3, 3) | 0.95 (0.871.00) |
| Pneumonia, m | 1 | Bradley JS et al.The management of community‐acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53(7):e25e76. | 6 (6, 6) | 3 (3, 3) | 0.82 (0.640.96) |
| Dental caries, s | 1 | American Academy on Pediatric Dentistry Clinical Affairs CommitteePulp Therapy Subcommittee; American Academy on Pediatric Dentistry Council on Clinical Affairs. Guideline on pulp therapy for primary and young permanent teeth. Pediatr Dent. 2008;30:170174. | 3 (3, 3) | 1.5 (1, 2) | 0.51 (0.140.83) |
| Chemotherapy, m | 0 | ||||
| Cellulitis, m | 1 | Stevens DL et al. Practice guidelines for the diagnosis and management of skin and softtissue infections. Clin Infect Dis. 2005;41:13731406. | 4.5 (4, 5) | 2.5 (2, 3) | 0.52 (0.150.79) |
| Inguinal hernia, s | 0 | ||||
| Gastroesophageal reflux and esophagitis, m, s | 2 | Vandenplas Y et al. Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of NASPGHAN and ESPGHAN. J Pediatr Gastroenterol Nutr. 2009;49(4):498547. | 5 (5, 5) | 3 (3, 3) | 0.69 (0.450.87) |
| Furuta GT et al. Eosinophilic esophagitis in children and adults: a systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology. 2007;133:13421363. | 5 (5, 5) | 2.5 (2, 3) | 0.93 (0.850.98) | ||
| Dehydration, m | 0 | ||||
| Redundant prepuce and phimosis, s | 1 | American Academy of Pediatrics Task Force on Circumcision. Male circumcision. Pediatrics. 2012;130(3):e756e785. | 6 (6, 6) | 3 (3, 3) | 0.66 (0.250.89) |
| Abdominal pain, m | 0 | ||||
| Other convulsions, m | 0 | ||||
| Urinary tract infection, m | 1 | Roberts KB et al. Urinary tract infection: clinical practice guideline for the diagnosis and management of the initial UTI in febrile infants and children 2 to 24 months. Pediatrics. 2011;128:595610. | 5.5 (5, 6) | 2.5 (2, 3) | 0.62 (0.230.84) |
| Acute appendicitis without peritonitis, s | 1 | Solomkin JS et al. Diagnosis and management of complicated intra‐abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis. 2010;50:133164. | 4.5 (5, 4) | 2.5 (3, 2) | 0.37 (0.110.81) |
| Eso‐ exo‐ hetero‐, and hypertropia, s | 0 | ||||
| Fever, m | 0 | ||||
| Seizures with and without intractable epilepsy, m | 3 | Brophy GM et al; Neurocritical Care Society Status Epilepticus Guideline Writing Committee. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care. 2012;17:323. | 5 (5, 5) | 3 (3, 3) | 0.95 (0.870.99) |
| Hirtz D et al. Practice parameter: treatment of the child with a first unprovoked seizure: report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 2003;60:166175. | 5 (5, 5) | 2.5 (2, 3) | 0.73 (0.410.94) | ||
| Riviello JJ Jr et al. Practice parameter: diagnostic assessment of the child with status epilepticus (an evidence‐based review): report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 2006;67:15421550. | 5 (4, 6) | 2.5 (2, 3) | 0.80 (0.630.94) | ||
| Sickle cell disease with crisis, m | 2 | Section on Hematology/Oncology Committee on Genetics; American Academy of Pediatrics. Health supervision for children with sickle cell disease. Pediatrics. 2002;109:526535. | 3.5 (3, 4) | 1.5 (1, 2) | 0.92 (0.800.98) |
| National Heart, Lung, and Blood Institute, National Institutes of Health. The management of sickle cell disease. National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD. Available at: | 4 (4, 4) | 2.5 (2, 3) | 0.91 (0.800.97) | ||
Identifying National Guidelines
We developed a search protocol (see Supporting Information, Table 2, in the online version of this article) using condition‐specific keywords and the following criteria: guideline, pediatric, 2002 to 2012. A medical librarian (E.E.) used the protocol to search PubMed, National Guidelines Clearing House, and the American Academy of Pediatrics website for guidelines for the 20 selected conditions.
We limited our study to US national guidelines published or updated from 2002 to 2012 to be most relevant to the 38 US children's hospitals in the original study. Guidelines had to address either medical or surgical or both types of inpatient management for the condition, depending on how the condition was categorized on the PRIS prioritization list. For example, to target inpatient issues, otitis media was treated as a surgical condition when the prioritization list was created, therefore guidelines included in our study needed to address surgical management (ie, myringotomy or tympanostomy tubes).[2] Guidelines specific to 1 organism, test, or treatment were a priori excluded, as they would not map well to the prioritization list, and would be difficult to interpret. Guidelines focusing exclusively on condition prevention were also excluded. Guidelines with a broad subject matter (eg, abdominal infection) or unclear age were included if they contained a significant focus on the condition of interest (eg, appendicitis without peritonitis), such that the course of pediatric inpatient care was described for that condition. Retracted or outdated (superseded by a more current version) guidelines were excluded.
An investigator (G.H.) reviewed potentially relevant results from the librarian's search. For example, the search for tonsillectomy guidelines retrieved a guideline on the use of polysomnography prior to tonsillectomy in children but did not cover the inpatient management or tonsillectomy procedure.[16] This guideline was excluded from our study, as it focused on a specific test and did not discuss surgical management of the condition.
Rating Methodological Quality of Guidelines
Methodological quality of guidelines was rated with the AGREE II tool by 2 investigators (G.H. and K.N.).[13, 17] This tool has 2 overall guideline assessments and 23 subcomponents within 6 domains, reflecting many of the IOM's recommendations for methodological quality in guidelines: scope and purpose, stakeholder involvement, rigor of development, clarity of presentation, applicability, and editorial independence.[8, 17]
The AGREE II tool rates each of the 23 subcomponent questions using a 7‐point scale (1=strongly disagree7=strongly agree). We followed the AGREE II user's manual suggestion in rating subcomponents as 1, indicating an absence of information for that question if the question was not addressed within the guideline.[13] The AGREE II user's manual describes the option of creating standardized domain scores; however, as the objective of our study was to assess the overall methodological quality of the guideline and not to highlight particular areas of strengths/weaknesses in the domains, we elected to present raw scores only.[13]
For the overall guideline rating item 1 (Rate the overall quality of this guideline.) the AGREE II tool instructs that a score of 1 indicates lowest possible quality and 7 indicates highest possible quality.[13] As these score anchors are far apart with no guide for interpretation of intermediate results, we modified the descriptive terms on the tool to define scores <3 as low quality, scores 3 to 5 as moderate quality, and scores >5 as high quality to allow for easier interpretation of our results. We also modified the final overall recommendation score (on a 3‐point scale) from I would recommend this guideline for use to I would recommend this guideline for use in the pediatric inpatient setting.[13, 17] A score of 1 indicated to not recommend, 2 indicated to recommend with modifications, and 3 indicated to recommend without modification.
Significant discrepancies (>2‐point difference on overall rating) between the 2 raters were to be settled by consensus scoring by 3 senior investigators blinded to previous reviews, using a modified Delphi technique.[18]
Inter‐rater reliability was measured using a weighted kappa coefficient and reported using a bootstrapped method with 95% confidence intervals. Interpretation of kappa is such that 0 is the amount of agreement that would be expected by chance, and 1 is perfect agreement, with previous researchers stating scores >0.81 indicate almost perfect agreement.[19]
RESULTS
The librarian's search retrieved 2869 potential results (Figure 1). Seventeen guidelines met inclusion criteria for 13 of the 20 priority conditions. Seven conditions did not have national guidelines meeting inclusion criteria. Table 1 displays the 20 medical and surgical conditions on the modified PRIS prioritization list, including overall guideline scoring, recommendation scores, and kappa results for each guideline. The highest methodological‐quality guidelines were for asthma,[20] tonsillectomy,[21] and bronchiolitis[22] (mean overall rating 7, 6.5, and 6.5, respectively). The lowest methodological‐quality guidelines were for 2 sickle cell disease guidelines[23, 24] and 1 dental caries guideline[25] (mean overall rating 4, 3.5, and 3, respectively). Seven guidelines were rated as high overall quality, and 10 guidelines were rated as moderate overall quality. Eight of the 17 guidelines[20, 21, 22, 26, 27, 28, 29, 30] were recommended for use in the pediatric inpatient setting without modification by both reviewers. Two guidelines (for dental caries[25] and sickle cell[23]) were not recommended for use by 1 reviewer.
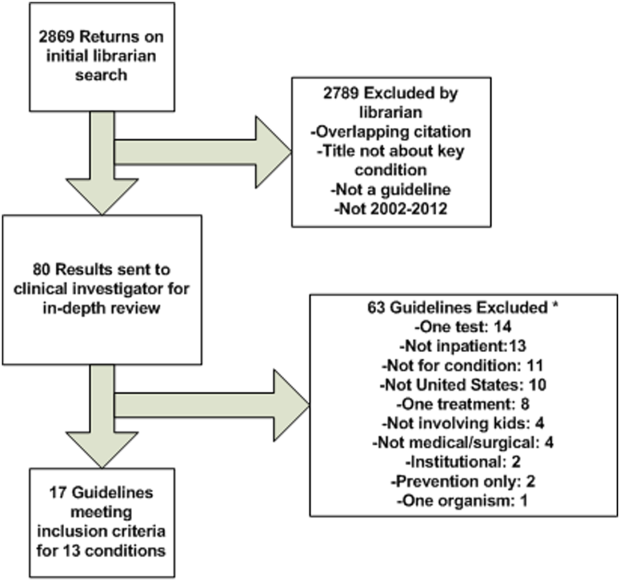
As an example of scoring, a national guideline for asthma had high overall scores (7 from each reviewer) and high scores across most AGREE II subcomponents. The guideline was found by both reviewers to be systematic in describing guideline development with clearly stated recommendations linked to the available evidence (including strengths and limitations) and implementation considerations.[20] Conversely, a national guideline for sickle cell disease had moderate overall scores (scores of 3 and 4) and low‐moderate scores across the majority of the subcomponent items.[23] The reviewers believe that this guideline would have been strengthened by increased transparency in guideline development, discussion of the evidence surrounding recommendations, and discussion of implementation factors. A table with detailed scoring of each guideline is available (see Supporting Information, Table 3, in the online version of this article).
Agreement between the 2 raters was almost perfect,[19] with an overall boot‐strapped weighted kappa of 0.83 (95% confidence interval 0.780.87) across 850 scores. There were no discrepancies between reviewers in overall scoring requiring consensus scoring.
DISCUSSION
Using a modified version of a published prioritization list for inpatient pediatric conditions, we found national guidelines for 13 of 20 conditions with high prevalence, cost, and interhospital variation in resource utilization. Seven conditions had no national guidelines published within the past 10 years applicable for use in the pediatric inpatient setting. Of 17 guidelines for 13 conditions, 10 had moderate and 7 had high methodological quality.
Our findings add to the literature describing methodological quality of guidelines. Many publications focus on the methodological quality of guidelines as a group and use a standardized instrument (eg, the AGREE II tool) to rate within domains (eg, domain 1: scope and purpose) across guidelines in an effort to encourage improvement in developing and reporting in guidelines.[31, 32] Our study differs in that we chose to focus on the overall quality rating of individual guidelines for specific prioritized conditions to allow hospitals to guide QI initiatives. One study that had a similar aim to ours surveyed Dutch pediatricians to select priority conditions and used the AGREE II tool to rate 17 guidelines, recommending 14 for use in the Netherlands.[33]
Identifying high methodological‐quality guidelines is only 1 in a series of steps prior to successful guideline implementation in hospitals. Other aspects of guidelines, including the strength of the evidence (eg, from randomized controlled trials) and subsequent force and clarity (eg, use of must instead of consider) of recommendations, may affect clinician or patient adherence, work processes, and ultimately patient outcomes. Strong evidence should translate into forceful and clear recommendations. Authors with the Yale Guideline Recommendation Corpus describe significant variation in reporting of guideline recommendations, and further studies have shown that the force and clarity of a recommendation is associated with adherence rates.[34, 35, 36, 37] Unfortunately, current guideline appraisal tools lack the means to score the strength of evidence, and force and clarity of recommendations.[10]
Implementation science demonstrates that there are many important factors in translating best practice into improvements in clinical care. In addition to implementation considerations such as adherence, patient preferences, and work processes, variability in methodological quality, strength of evidence, and force and clarity of recommendations may be additional reasons why evidence for the impact of guidelines on patient outcomes remains mixed in the literature.[38] One recent study found that adherence to antibiotics recommended within a national pediatric community‐acquired pneumonia guideline, which had a high methodological‐quality score in our study, did not change hospital length of stay or readmissions.[29, 39] There are several possible interpretations for this. Recommendations may not have been based upon strong evidence, research methodology assessing how adherence to recommendations impacts patient outcomes may have been limited, or the outcomes measured in current studies (such as readmission) are not the outcomes that may be improved by adherence to these recommendations (such as decreasing antimicrobial resistance). These are important considerations when hospitals are incorporating recommendations from guidelines into practice. Hospitals should assess the multiple aspects of guidelines, including methodological quality, which our study helps to identify, strength of evidence, and force and clarity of recommendations, as well as adherence, patient preferences, work processes, and key outcome measures when implementing guidelines into clinical practice. A study utilizing a robust QI methodology demonstrated that clinician adherence to several elements in an asthma guideline, which also had a high methodological‐quality score in our study, led to a significant decrease in 6‐month hospital and emergency department readmission for asthma.[6, 20]
Our study also highlights that several pediatric conditions with high prevalence, cost, and interhospital resource utilization variation lack recent national pediatric guidelines applicable to the inpatient setting. If strong evidence exists for these priority conditions, professional societies should create high methodological‐quality guidelines with strong and clear recommendations. If evidence is lacking for these priority conditions, then investigators should focus on generating research in these areas.
There are several limitations to this study. The AGREE II tool does not have a mechanism to measure the strength of evidence used in a guideline. Methodological quality of a guideline alone may not translate into improved outcomes. Conditions may have national guidelines published before 2002, institution‐specific or international guidelines, or adult guidelines that might be amenable to use in the pediatric inpatient setting but were not included in this study. Several conditions on the prioritization list are broad in nature (eg, dehydration) and may not be amenable to the creation of guidelines. Other conditions on the prioritization list (eg, chemotherapy or cellulitis) may have useful guidelines within the context of specific conditions (eg, acute lymphoblastic leukemia) or for specific organisms (eg, methicillin‐resistant Staphylococcus aureus). We elected to exclude these narrower guidelines to focus on broad and comprehensive guidelines applicable to a wider range of clinical situations. Additionally, although use of a validated tool attempts to objectively guide ratings, the rating of quality is to some degree subjective. Finally, our study used a previously published prioritization list using data from children's hospitals, and the list likely under‐represents conditions commonly managed in community hospitals (eg, hyperbilirubinemia).[2] Exclusion of these conditions was not reflective of importance or quality of available national guidelines.
CONCLUSIONS
Our study adds to recent publications on the need to prioritize conditions for QI in children's hospitals. We identified a group of moderate to high methodological‐quality national guidelines for pediatric inpatient conditions with high prevalence, cost, and variation in interhospital resource utilization. Not all prioritized conditions have national high methodological‐quality guidelines available. Hospitals should prioritize conditions with high methodological‐quality guidelines to allocate resources for QI initiatives. Professional societies should focus their efforts on producing methodologically sound guidelines for prioritized conditions currently lacking high‐quality guidelines if sufficient evidence exists.
Acknowledgements
The authors thank Christopher G. Maloney, MD, PhD, for critical review of the manuscript, and Gregory J. Stoddard, MS, for statistical support. Mr. Stoddard's work is supported by the University of Utah Study Design and Biostatistics Center, with funding in part from the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through grant 8UL1TR000105 (formerly UL1RR025764).
Disclosures
Sanjay Mahant, Ron Keren, and Raj Srivastava are all Executive Council members of the Pediatric Research in Inpatient Settings (PRIS) Network. PRIS, Sanjay Mahant, Ron Keren, and Raj Srivastava are all supported by grants from the Children's Hospital Association. Sanjay Mahant is also supported by research grants from the Canadian Institute of Health Research and Physician Services Incorporated. Ron Keren and Raj Srivastava also serve as medical legal consultants. The remaining authors have no financial relationships relevant to this article to disclose.
- , . Development of the Pediatric Research in Inpatient Settings (PRIS) Network: lessons learned. J Hosp Med. 2012;7(8):661–664.
- , , , et al. Prioritization of comparative effectiveness research topics in hospital pediatrics. Arch Pediatr Adolesc Med. 2012;166(12):1–10.
- , . How Intermountain trimmed health care costs through robust quality improvement efforts. Health Aff (Millwood). 2011;30(6):1185–1191.
- . Making it easy to do it right. N Engl J Med. 2001;345(13):991–993.
- , , , , . Clinical guidelines: potential benefits, limitations, and harms of clinical guidelines. BMJ. 1999;318(7182):527–530.
- , , , et al. The Joint Commission Children's Asthma Care quality measures and asthma readmissions. Pediatrics. 2012;130(3):482–491.
- , , , et al. Toward transparent clinical policies. Pediatrics. 2008;121(3):643–646.
- Field MJ, Lohr KN, eds.; Committee to Advise the Public Health Service on Clinical Practice Guidelines, Institute of Medicine. Clinical Practice Guidelines: Directions for a New Program. Washington, DC: National Academies Press; 1990.
- , , , , . Development and application of a generic methodology to assess the quality of clinical guidelines. Int J Qual Health Care. 1999;11(1):21–28.
- , , , , . A systematic review of appraisal tools for clinical practice guidelines: multiple similarities and one common deficit. Int J Qual Health Care. 2005;17(3):235–242.
- , , . How to decide whether a clinical practice guideline is trustworthy. JAMA. 2013;309(2):139–140.
- Field MJ, Lohr KN, eds.; Committee to Advise the Public Health Service on Clinical Practice Guidelines, Institute of Medicine. Guidelines for Clinical Practice: From Development to Use. Washington, DC: National Academies Press; 1992.
- The AGREE Collaboration. Development and validation of an international appraisal instrument for assessing the quality of clinical practice guidelines: the AGREE project. Qual Saf Health Care. 2003;12:18–23.
- . AGREE II‐improving the quality of clinical care. Lancet. 2010;376(9747):1128–1129.
- , , , . Corticosteroids and mortality in children with bacterial meningitis. JAMA. 2008;299(17):2048–2055.
- , , , et al. Clinical practice guideline: polysomnography for sleep‐disordered breathing prior to tonsillectomy in children. Otolaryngol Head Neck Surg. 2011;145(1 suppl):S1–S15.
- , , , et al. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010;182(18):E839–E842.
- , . The Delphi method as a research tool: an example, design considerations and applications. Inform Manag. 2004;42(1):15–29.
- , . The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174.
- National Heart, Lung, and Blood Institute; National Asthma Education and Prevention Program. Expert panel report 3 (EPR‐3): guidelines for the diagnosis and management of asthma‐full report 2007. Pages 1–440. Available at: http://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.pdf. Accessed on 24 August 2012.
- , , , et al. Clinical practice guideline: tonsillectomy in children. Otolaryngol Head Neck Surg. 2011;144(1 suppl):S1–S30.
- American Academy of Pediatrics Subcommittee on Diagnosis and Management of Bronchiolitis. Diagnosis and management of bronchiolitis. Pediatrics. 2006;118:1774–1793.
- Health supervision for children with sickle cell disease. Pediatrics. 2002;109(3):526–535.
- National Heart, Lung, and Blood Institute, National Institutes of Health. The management of sickle cell disease. National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD. Available at: http://www.nhlbi.nih.gov/health/prof/blood/sickle/sc_mngt.pdf. Revised June 2002. Accessed on 13 October 2012.
- American Academy on Pediatric Dentistry Clinical Affairs Committee–Pulp Therapy Subcommittee; American Academy on Pediatric Dentistry Council on Clinical Affairs. Guideline on pulp therapy for primary and young permanent teeth. Pediatr Dent. 2008;30:170–174.
- , , , et al.; Neurocritical Care Society Status Epilepticus Guideline Writing Committee. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care. 2012;17:3–23.
- American Academy of Pediatrics Task Force on Circumcision. Male circumcision. Pediatrics. 2012;130(3):e756–e785.
- , , , et al. Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN). J Pediatr Gastroenterol Nutr. 2009;49(4):498–547.
- , , , et al. The management of community‐acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53(7):e25–e76.
- American Academy of Family Physicians; American Academy of Otolaryngology‐Head and Neck Surgery; American Academy of Pediatrics Subcommittee on Otitis Media With Effusion. Clinical Practice Guidelines: Otitis media with effusion. Pediatrics. 2004 May;113(5):1412–29.
- , , . Are guidelines following guidelines? The methodological quality of clinical practice guidelines in the peer‐reviewed medical literature. JAMA. 1999;281(20):1900–1905.
- , , , . Quality of reporting and evidence in American Academy of Pediatrics guidelines. Pediatrics. 2013;131(4):732–738.
- , , . Quality of evidence‐based pediatric guidelines. Pediatrics. 2005;115(5):1378–1391.
- , , . The Yale Guideline Recommendation Corpus: a representative sample of the knowledge content of guidelines. Int J Med Inform. 2009;78(5):354–363.
- , , . How often is strength of recommendation indicated in guidelines? Analysis of the Yale Guideline Recommendation Corpus. AMIA Annu Symp Proc. 2008:984.
- , , . Clinical practice guideline development manual, third edition: a quality‐driven approach for translating evidence into action. Otolaryngol Head Neck Surg. 2013;148(1 suppl):S1–S55.
- , , , , , . Attributes of clinical guidelines that influence use of guidelines in general practice: observational study. BMJ. 1998;317(7162):858–861.
- , , , et al. Toward evidence‐based quality improvement. Evidence (and its limitations) of the effectiveness of guideline dissemination and implementation strategies 1966–1998. J Gen Intern Med. 2006;21(suppl 2):S14–S20.
- , , , . Effectiveness of antimicrobial guidelines for community‐acquired pneumonia in children. Pediatrics. 2012;129(5):e1326–e1333.
Researchers from the Pediatric Research in Inpatient Settings (PRIS) network, an open pediatric hospitalist research network,[1] have identified inpatient pediatric medical and surgical conditions considered high priority for quality improvement (QI) initiatives and/or comparative effectiveness research based on prevalence, cost, and interhospital variation in resource utilization.[2] One approach for improving the quality of care within hospitals is to operationalize evidence‐based guidelines into practice.[3] Although guidelines may be used by individual clinicians, systematic adoption by hospitals into clinical workflow has the potential to influence providers to adhere to evidence‐based care, reduce unwarranted variation, and ultimately improve patient outcomes.[3, 4, 5, 6]
There are critical appraisal tools to measure the methodological quality, as defined by the Institute of Medicine (IOM) and others in their guidelines.[7, 8, 9, 10, 11, 12] One such validated tool is the AGREE II instrument, created by the AGREE (Appraisal of Guidelines for REsearch and Evaluation) collaboration.[13, 14] It defines methodological quality as the confidence that the biases linked to the rigor of development, presentation, and applicability of a clinical practice guideline have been minimized and that each step of the development process is clearly reported.[13]
The objective of our study was to rate the methodological quality of national guidelines for 20 of the PRIS priority pediatric inpatient conditions.[2] Our intent in pursuing this project was 2‐fold: first, to inform pediatric inpatient QI initiatives, and second, to call out priority pediatric inpatient conditions for which high methodological‐quality guidelines are currently lacking.
METHODS
The study methods involved (1) prioritizing pediatric inpatient conditions, (2) identifying national guidelines for the priority conditions, and (3) rating the methodological quality of available guidelines. This study was considered nonhuman‐subject research (A. Johnson, personal e‐mail communication, November 14, 2012), and the original prioritization study was deemed exempt from review by the institutional review board of the Children's Hospital of Philadelphia under 45 CFR 46.102(f).[2]
Prioritizing Pediatric Inpatient Conditions
Methods for developing the prioritization list are published elsewhere in detail and briefly described here.[1] An International Classification of Diseases, 9th Revision, Clinical Modification‐based clinical condition grouper was created for primary discharge diagnosis codes for inpatient, ambulatory surgery, and observation unit encounters accounting for either 80% of all encounters or 80% of all charges for over 3.4 million discharges from 2004 to 2009 for 38 children's hospitals in the Pediatric Health Information Systems (PHIS) database, which includes administrative and billing data.[15] A standardized cost master index was created to assign the same unit cost for each billable item (calculated as the median of median hospital unit costs) to allow for comparisons of resource utilization across hospitals (eg, the cost of a chest x‐ray was set to be the same across all hospitals in 2009 dollars). Total hospital costs were then recalculated for every admission by multiplying the standardized cost master index by the number of units for each item in the hospital bill, and then summing the standardized costs of each line item in every bill. Conditions were ranked based on prevalence and total cost across all hospitals in the study period. The variation in standardized costs across hospitals for each condition was determined.
For the current study, conditions were considered if they had a top 20 prevalence rank, a top 20 cost rank, high variation (intraclass correlation coefficient >0.1) in standardized costs across hospitals, a minimum number of PHIS hospitals with annualized overexpenditures (using the standardized cost master) of at least $50,000 when compared to the mean, or a minimum median of 200 cases per hospital over the 6‐year study period to assure sufficient hospital volume for future interventions. This resulted in 29 conditions; the selected 20 conditions matched the top 20 prevalence rank (see Supporting Information, Table 1, in the online version of this article).[2]
| Condition by PRIS Priority Rank | Guidelines Meeting Inclusion Criteriaa | Guidelines Citation | Mean Overall Reviewer Methodological Quality Rating (Rater 1, Rater 2)b | Recommended for Use in the Pediatric Inpatient Setting, Mean (Rater 1, Rater 2)c | Weighted Kappa(95% Confidence Interval) |
|---|---|---|---|---|---|
| |||||
| Otitis media, unspecified, s | 1 | American Academy of Family Physicians; American Academy of Otolaryngology‐Head and Neck Surgery; American Academy of Pediatrics Subcommittee on Otitis Media With Effusion. Clinical Practice Guidelines: Otitis media with effusion. Pediatrics. 2004 May;113(5):1412‐29. | 6 (6, 6) | 3 (3, 3) | 0.76 (0.490.93) |
| Hypertrophy of tonsils and adenoids, s | 1 | Baugh RF et al. Clinical practice guideline: tonsillectomy in children. Otolaryngol Head Neck Surg. 2011;144(1 suppl):S1S30. | 6.5 (7, 6) | 3 (3, 3) | 0.49 (0.050.81) |
| Asthma, m | 1 | National Heart, Lung, and Blood Institute; National Asthma Education and Prevention Program. Expert panel report 3 (EPR‐3): guidelines for the diagnosis and management of asthma‐full report 2007. Pages 1‐440. Available at: | 7 (7, 7) | 3 (3, 3) | 0.62 (0.210.87) |
| Bronchiolitis, m | 1 | American Academy of Pediatrics Subcommittee on Diagnosis and Management of Bronchiolitis. Diagnosis and management of bronchiolitis. Pediatrics. 2006;118:17741793. | 6.5 (6, 7) | 3 (3, 3) | 0.95 (0.871.00) |
| Pneumonia, m | 1 | Bradley JS et al.The management of community‐acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53(7):e25e76. | 6 (6, 6) | 3 (3, 3) | 0.82 (0.640.96) |
| Dental caries, s | 1 | American Academy on Pediatric Dentistry Clinical Affairs CommitteePulp Therapy Subcommittee; American Academy on Pediatric Dentistry Council on Clinical Affairs. Guideline on pulp therapy for primary and young permanent teeth. Pediatr Dent. 2008;30:170174. | 3 (3, 3) | 1.5 (1, 2) | 0.51 (0.140.83) |
| Chemotherapy, m | 0 | ||||
| Cellulitis, m | 1 | Stevens DL et al. Practice guidelines for the diagnosis and management of skin and softtissue infections. Clin Infect Dis. 2005;41:13731406. | 4.5 (4, 5) | 2.5 (2, 3) | 0.52 (0.150.79) |
| Inguinal hernia, s | 0 | ||||
| Gastroesophageal reflux and esophagitis, m, s | 2 | Vandenplas Y et al. Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of NASPGHAN and ESPGHAN. J Pediatr Gastroenterol Nutr. 2009;49(4):498547. | 5 (5, 5) | 3 (3, 3) | 0.69 (0.450.87) |
| Furuta GT et al. Eosinophilic esophagitis in children and adults: a systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology. 2007;133:13421363. | 5 (5, 5) | 2.5 (2, 3) | 0.93 (0.850.98) | ||
| Dehydration, m | 0 | ||||
| Redundant prepuce and phimosis, s | 1 | American Academy of Pediatrics Task Force on Circumcision. Male circumcision. Pediatrics. 2012;130(3):e756e785. | 6 (6, 6) | 3 (3, 3) | 0.66 (0.250.89) |
| Abdominal pain, m | 0 | ||||
| Other convulsions, m | 0 | ||||
| Urinary tract infection, m | 1 | Roberts KB et al. Urinary tract infection: clinical practice guideline for the diagnosis and management of the initial UTI in febrile infants and children 2 to 24 months. Pediatrics. 2011;128:595610. | 5.5 (5, 6) | 2.5 (2, 3) | 0.62 (0.230.84) |
| Acute appendicitis without peritonitis, s | 1 | Solomkin JS et al. Diagnosis and management of complicated intra‐abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis. 2010;50:133164. | 4.5 (5, 4) | 2.5 (3, 2) | 0.37 (0.110.81) |
| Eso‐ exo‐ hetero‐, and hypertropia, s | 0 | ||||
| Fever, m | 0 | ||||
| Seizures with and without intractable epilepsy, m | 3 | Brophy GM et al; Neurocritical Care Society Status Epilepticus Guideline Writing Committee. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care. 2012;17:323. | 5 (5, 5) | 3 (3, 3) | 0.95 (0.870.99) |
| Hirtz D et al. Practice parameter: treatment of the child with a first unprovoked seizure: report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 2003;60:166175. | 5 (5, 5) | 2.5 (2, 3) | 0.73 (0.410.94) | ||
| Riviello JJ Jr et al. Practice parameter: diagnostic assessment of the child with status epilepticus (an evidence‐based review): report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 2006;67:15421550. | 5 (4, 6) | 2.5 (2, 3) | 0.80 (0.630.94) | ||
| Sickle cell disease with crisis, m | 2 | Section on Hematology/Oncology Committee on Genetics; American Academy of Pediatrics. Health supervision for children with sickle cell disease. Pediatrics. 2002;109:526535. | 3.5 (3, 4) | 1.5 (1, 2) | 0.92 (0.800.98) |
| National Heart, Lung, and Blood Institute, National Institutes of Health. The management of sickle cell disease. National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD. Available at: | 4 (4, 4) | 2.5 (2, 3) | 0.91 (0.800.97) | ||
Identifying National Guidelines
We developed a search protocol (see Supporting Information, Table 2, in the online version of this article) using condition‐specific keywords and the following criteria: guideline, pediatric, 2002 to 2012. A medical librarian (E.E.) used the protocol to search PubMed, National Guidelines Clearing House, and the American Academy of Pediatrics website for guidelines for the 20 selected conditions.
We limited our study to US national guidelines published or updated from 2002 to 2012 to be most relevant to the 38 US children's hospitals in the original study. Guidelines had to address either medical or surgical or both types of inpatient management for the condition, depending on how the condition was categorized on the PRIS prioritization list. For example, to target inpatient issues, otitis media was treated as a surgical condition when the prioritization list was created, therefore guidelines included in our study needed to address surgical management (ie, myringotomy or tympanostomy tubes).[2] Guidelines specific to 1 organism, test, or treatment were a priori excluded, as they would not map well to the prioritization list, and would be difficult to interpret. Guidelines focusing exclusively on condition prevention were also excluded. Guidelines with a broad subject matter (eg, abdominal infection) or unclear age were included if they contained a significant focus on the condition of interest (eg, appendicitis without peritonitis), such that the course of pediatric inpatient care was described for that condition. Retracted or outdated (superseded by a more current version) guidelines were excluded.
An investigator (G.H.) reviewed potentially relevant results from the librarian's search. For example, the search for tonsillectomy guidelines retrieved a guideline on the use of polysomnography prior to tonsillectomy in children but did not cover the inpatient management or tonsillectomy procedure.[16] This guideline was excluded from our study, as it focused on a specific test and did not discuss surgical management of the condition.
Rating Methodological Quality of Guidelines
Methodological quality of guidelines was rated with the AGREE II tool by 2 investigators (G.H. and K.N.).[13, 17] This tool has 2 overall guideline assessments and 23 subcomponents within 6 domains, reflecting many of the IOM's recommendations for methodological quality in guidelines: scope and purpose, stakeholder involvement, rigor of development, clarity of presentation, applicability, and editorial independence.[8, 17]
The AGREE II tool rates each of the 23 subcomponent questions using a 7‐point scale (1=strongly disagree7=strongly agree). We followed the AGREE II user's manual suggestion in rating subcomponents as 1, indicating an absence of information for that question if the question was not addressed within the guideline.[13] The AGREE II user's manual describes the option of creating standardized domain scores; however, as the objective of our study was to assess the overall methodological quality of the guideline and not to highlight particular areas of strengths/weaknesses in the domains, we elected to present raw scores only.[13]
For the overall guideline rating item 1 (Rate the overall quality of this guideline.) the AGREE II tool instructs that a score of 1 indicates lowest possible quality and 7 indicates highest possible quality.[13] As these score anchors are far apart with no guide for interpretation of intermediate results, we modified the descriptive terms on the tool to define scores <3 as low quality, scores 3 to 5 as moderate quality, and scores >5 as high quality to allow for easier interpretation of our results. We also modified the final overall recommendation score (on a 3‐point scale) from I would recommend this guideline for use to I would recommend this guideline for use in the pediatric inpatient setting.[13, 17] A score of 1 indicated to not recommend, 2 indicated to recommend with modifications, and 3 indicated to recommend without modification.
Significant discrepancies (>2‐point difference on overall rating) between the 2 raters were to be settled by consensus scoring by 3 senior investigators blinded to previous reviews, using a modified Delphi technique.[18]
Inter‐rater reliability was measured using a weighted kappa coefficient and reported using a bootstrapped method with 95% confidence intervals. Interpretation of kappa is such that 0 is the amount of agreement that would be expected by chance, and 1 is perfect agreement, with previous researchers stating scores >0.81 indicate almost perfect agreement.[19]
RESULTS
The librarian's search retrieved 2869 potential results (Figure 1). Seventeen guidelines met inclusion criteria for 13 of the 20 priority conditions. Seven conditions did not have national guidelines meeting inclusion criteria. Table 1 displays the 20 medical and surgical conditions on the modified PRIS prioritization list, including overall guideline scoring, recommendation scores, and kappa results for each guideline. The highest methodological‐quality guidelines were for asthma,[20] tonsillectomy,[21] and bronchiolitis[22] (mean overall rating 7, 6.5, and 6.5, respectively). The lowest methodological‐quality guidelines were for 2 sickle cell disease guidelines[23, 24] and 1 dental caries guideline[25] (mean overall rating 4, 3.5, and 3, respectively). Seven guidelines were rated as high overall quality, and 10 guidelines were rated as moderate overall quality. Eight of the 17 guidelines[20, 21, 22, 26, 27, 28, 29, 30] were recommended for use in the pediatric inpatient setting without modification by both reviewers. Two guidelines (for dental caries[25] and sickle cell[23]) were not recommended for use by 1 reviewer.

As an example of scoring, a national guideline for asthma had high overall scores (7 from each reviewer) and high scores across most AGREE II subcomponents. The guideline was found by both reviewers to be systematic in describing guideline development with clearly stated recommendations linked to the available evidence (including strengths and limitations) and implementation considerations.[20] Conversely, a national guideline for sickle cell disease had moderate overall scores (scores of 3 and 4) and low‐moderate scores across the majority of the subcomponent items.[23] The reviewers believe that this guideline would have been strengthened by increased transparency in guideline development, discussion of the evidence surrounding recommendations, and discussion of implementation factors. A table with detailed scoring of each guideline is available (see Supporting Information, Table 3, in the online version of this article).
Agreement between the 2 raters was almost perfect,[19] with an overall boot‐strapped weighted kappa of 0.83 (95% confidence interval 0.780.87) across 850 scores. There were no discrepancies between reviewers in overall scoring requiring consensus scoring.
DISCUSSION
Using a modified version of a published prioritization list for inpatient pediatric conditions, we found national guidelines for 13 of 20 conditions with high prevalence, cost, and interhospital variation in resource utilization. Seven conditions had no national guidelines published within the past 10 years applicable for use in the pediatric inpatient setting. Of 17 guidelines for 13 conditions, 10 had moderate and 7 had high methodological quality.
Our findings add to the literature describing methodological quality of guidelines. Many publications focus on the methodological quality of guidelines as a group and use a standardized instrument (eg, the AGREE II tool) to rate within domains (eg, domain 1: scope and purpose) across guidelines in an effort to encourage improvement in developing and reporting in guidelines.[31, 32] Our study differs in that we chose to focus on the overall quality rating of individual guidelines for specific prioritized conditions to allow hospitals to guide QI initiatives. One study that had a similar aim to ours surveyed Dutch pediatricians to select priority conditions and used the AGREE II tool to rate 17 guidelines, recommending 14 for use in the Netherlands.[33]
Identifying high methodological‐quality guidelines is only 1 in a series of steps prior to successful guideline implementation in hospitals. Other aspects of guidelines, including the strength of the evidence (eg, from randomized controlled trials) and subsequent force and clarity (eg, use of must instead of consider) of recommendations, may affect clinician or patient adherence, work processes, and ultimately patient outcomes. Strong evidence should translate into forceful and clear recommendations. Authors with the Yale Guideline Recommendation Corpus describe significant variation in reporting of guideline recommendations, and further studies have shown that the force and clarity of a recommendation is associated with adherence rates.[34, 35, 36, 37] Unfortunately, current guideline appraisal tools lack the means to score the strength of evidence, and force and clarity of recommendations.[10]
Implementation science demonstrates that there are many important factors in translating best practice into improvements in clinical care. In addition to implementation considerations such as adherence, patient preferences, and work processes, variability in methodological quality, strength of evidence, and force and clarity of recommendations may be additional reasons why evidence for the impact of guidelines on patient outcomes remains mixed in the literature.[38] One recent study found that adherence to antibiotics recommended within a national pediatric community‐acquired pneumonia guideline, which had a high methodological‐quality score in our study, did not change hospital length of stay or readmissions.[29, 39] There are several possible interpretations for this. Recommendations may not have been based upon strong evidence, research methodology assessing how adherence to recommendations impacts patient outcomes may have been limited, or the outcomes measured in current studies (such as readmission) are not the outcomes that may be improved by adherence to these recommendations (such as decreasing antimicrobial resistance). These are important considerations when hospitals are incorporating recommendations from guidelines into practice. Hospitals should assess the multiple aspects of guidelines, including methodological quality, which our study helps to identify, strength of evidence, and force and clarity of recommendations, as well as adherence, patient preferences, work processes, and key outcome measures when implementing guidelines into clinical practice. A study utilizing a robust QI methodology demonstrated that clinician adherence to several elements in an asthma guideline, which also had a high methodological‐quality score in our study, led to a significant decrease in 6‐month hospital and emergency department readmission for asthma.[6, 20]
Our study also highlights that several pediatric conditions with high prevalence, cost, and interhospital resource utilization variation lack recent national pediatric guidelines applicable to the inpatient setting. If strong evidence exists for these priority conditions, professional societies should create high methodological‐quality guidelines with strong and clear recommendations. If evidence is lacking for these priority conditions, then investigators should focus on generating research in these areas.
There are several limitations to this study. The AGREE II tool does not have a mechanism to measure the strength of evidence used in a guideline. Methodological quality of a guideline alone may not translate into improved outcomes. Conditions may have national guidelines published before 2002, institution‐specific or international guidelines, or adult guidelines that might be amenable to use in the pediatric inpatient setting but were not included in this study. Several conditions on the prioritization list are broad in nature (eg, dehydration) and may not be amenable to the creation of guidelines. Other conditions on the prioritization list (eg, chemotherapy or cellulitis) may have useful guidelines within the context of specific conditions (eg, acute lymphoblastic leukemia) or for specific organisms (eg, methicillin‐resistant Staphylococcus aureus). We elected to exclude these narrower guidelines to focus on broad and comprehensive guidelines applicable to a wider range of clinical situations. Additionally, although use of a validated tool attempts to objectively guide ratings, the rating of quality is to some degree subjective. Finally, our study used a previously published prioritization list using data from children's hospitals, and the list likely under‐represents conditions commonly managed in community hospitals (eg, hyperbilirubinemia).[2] Exclusion of these conditions was not reflective of importance or quality of available national guidelines.
CONCLUSIONS
Our study adds to recent publications on the need to prioritize conditions for QI in children's hospitals. We identified a group of moderate to high methodological‐quality national guidelines for pediatric inpatient conditions with high prevalence, cost, and variation in interhospital resource utilization. Not all prioritized conditions have national high methodological‐quality guidelines available. Hospitals should prioritize conditions with high methodological‐quality guidelines to allocate resources for QI initiatives. Professional societies should focus their efforts on producing methodologically sound guidelines for prioritized conditions currently lacking high‐quality guidelines if sufficient evidence exists.
Acknowledgements
The authors thank Christopher G. Maloney, MD, PhD, for critical review of the manuscript, and Gregory J. Stoddard, MS, for statistical support. Mr. Stoddard's work is supported by the University of Utah Study Design and Biostatistics Center, with funding in part from the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through grant 8UL1TR000105 (formerly UL1RR025764).
Disclosures
Sanjay Mahant, Ron Keren, and Raj Srivastava are all Executive Council members of the Pediatric Research in Inpatient Settings (PRIS) Network. PRIS, Sanjay Mahant, Ron Keren, and Raj Srivastava are all supported by grants from the Children's Hospital Association. Sanjay Mahant is also supported by research grants from the Canadian Institute of Health Research and Physician Services Incorporated. Ron Keren and Raj Srivastava also serve as medical legal consultants. The remaining authors have no financial relationships relevant to this article to disclose.
Researchers from the Pediatric Research in Inpatient Settings (PRIS) network, an open pediatric hospitalist research network,[1] have identified inpatient pediatric medical and surgical conditions considered high priority for quality improvement (QI) initiatives and/or comparative effectiveness research based on prevalence, cost, and interhospital variation in resource utilization.[2] One approach for improving the quality of care within hospitals is to operationalize evidence‐based guidelines into practice.[3] Although guidelines may be used by individual clinicians, systematic adoption by hospitals into clinical workflow has the potential to influence providers to adhere to evidence‐based care, reduce unwarranted variation, and ultimately improve patient outcomes.[3, 4, 5, 6]
There are critical appraisal tools to measure the methodological quality, as defined by the Institute of Medicine (IOM) and others in their guidelines.[7, 8, 9, 10, 11, 12] One such validated tool is the AGREE II instrument, created by the AGREE (Appraisal of Guidelines for REsearch and Evaluation) collaboration.[13, 14] It defines methodological quality as the confidence that the biases linked to the rigor of development, presentation, and applicability of a clinical practice guideline have been minimized and that each step of the development process is clearly reported.[13]
The objective of our study was to rate the methodological quality of national guidelines for 20 of the PRIS priority pediatric inpatient conditions.[2] Our intent in pursuing this project was 2‐fold: first, to inform pediatric inpatient QI initiatives, and second, to call out priority pediatric inpatient conditions for which high methodological‐quality guidelines are currently lacking.
METHODS
The study methods involved (1) prioritizing pediatric inpatient conditions, (2) identifying national guidelines for the priority conditions, and (3) rating the methodological quality of available guidelines. This study was considered nonhuman‐subject research (A. Johnson, personal e‐mail communication, November 14, 2012), and the original prioritization study was deemed exempt from review by the institutional review board of the Children's Hospital of Philadelphia under 45 CFR 46.102(f).[2]
Prioritizing Pediatric Inpatient Conditions
Methods for developing the prioritization list are published elsewhere in detail and briefly described here.[1] An International Classification of Diseases, 9th Revision, Clinical Modification‐based clinical condition grouper was created for primary discharge diagnosis codes for inpatient, ambulatory surgery, and observation unit encounters accounting for either 80% of all encounters or 80% of all charges for over 3.4 million discharges from 2004 to 2009 for 38 children's hospitals in the Pediatric Health Information Systems (PHIS) database, which includes administrative and billing data.[15] A standardized cost master index was created to assign the same unit cost for each billable item (calculated as the median of median hospital unit costs) to allow for comparisons of resource utilization across hospitals (eg, the cost of a chest x‐ray was set to be the same across all hospitals in 2009 dollars). Total hospital costs were then recalculated for every admission by multiplying the standardized cost master index by the number of units for each item in the hospital bill, and then summing the standardized costs of each line item in every bill. Conditions were ranked based on prevalence and total cost across all hospitals in the study period. The variation in standardized costs across hospitals for each condition was determined.
For the current study, conditions were considered if they had a top 20 prevalence rank, a top 20 cost rank, high variation (intraclass correlation coefficient >0.1) in standardized costs across hospitals, a minimum number of PHIS hospitals with annualized overexpenditures (using the standardized cost master) of at least $50,000 when compared to the mean, or a minimum median of 200 cases per hospital over the 6‐year study period to assure sufficient hospital volume for future interventions. This resulted in 29 conditions; the selected 20 conditions matched the top 20 prevalence rank (see Supporting Information, Table 1, in the online version of this article).[2]
| Condition by PRIS Priority Rank | Guidelines Meeting Inclusion Criteriaa | Guidelines Citation | Mean Overall Reviewer Methodological Quality Rating (Rater 1, Rater 2)b | Recommended for Use in the Pediatric Inpatient Setting, Mean (Rater 1, Rater 2)c | Weighted Kappa(95% Confidence Interval) |
|---|---|---|---|---|---|
| |||||
| Otitis media, unspecified, s | 1 | American Academy of Family Physicians; American Academy of Otolaryngology‐Head and Neck Surgery; American Academy of Pediatrics Subcommittee on Otitis Media With Effusion. Clinical Practice Guidelines: Otitis media with effusion. Pediatrics. 2004 May;113(5):1412‐29. | 6 (6, 6) | 3 (3, 3) | 0.76 (0.490.93) |
| Hypertrophy of tonsils and adenoids, s | 1 | Baugh RF et al. Clinical practice guideline: tonsillectomy in children. Otolaryngol Head Neck Surg. 2011;144(1 suppl):S1S30. | 6.5 (7, 6) | 3 (3, 3) | 0.49 (0.050.81) |
| Asthma, m | 1 | National Heart, Lung, and Blood Institute; National Asthma Education and Prevention Program. Expert panel report 3 (EPR‐3): guidelines for the diagnosis and management of asthma‐full report 2007. Pages 1‐440. Available at: | 7 (7, 7) | 3 (3, 3) | 0.62 (0.210.87) |
| Bronchiolitis, m | 1 | American Academy of Pediatrics Subcommittee on Diagnosis and Management of Bronchiolitis. Diagnosis and management of bronchiolitis. Pediatrics. 2006;118:17741793. | 6.5 (6, 7) | 3 (3, 3) | 0.95 (0.871.00) |
| Pneumonia, m | 1 | Bradley JS et al.The management of community‐acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53(7):e25e76. | 6 (6, 6) | 3 (3, 3) | 0.82 (0.640.96) |
| Dental caries, s | 1 | American Academy on Pediatric Dentistry Clinical Affairs CommitteePulp Therapy Subcommittee; American Academy on Pediatric Dentistry Council on Clinical Affairs. Guideline on pulp therapy for primary and young permanent teeth. Pediatr Dent. 2008;30:170174. | 3 (3, 3) | 1.5 (1, 2) | 0.51 (0.140.83) |
| Chemotherapy, m | 0 | ||||
| Cellulitis, m | 1 | Stevens DL et al. Practice guidelines for the diagnosis and management of skin and softtissue infections. Clin Infect Dis. 2005;41:13731406. | 4.5 (4, 5) | 2.5 (2, 3) | 0.52 (0.150.79) |
| Inguinal hernia, s | 0 | ||||
| Gastroesophageal reflux and esophagitis, m, s | 2 | Vandenplas Y et al. Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of NASPGHAN and ESPGHAN. J Pediatr Gastroenterol Nutr. 2009;49(4):498547. | 5 (5, 5) | 3 (3, 3) | 0.69 (0.450.87) |
| Furuta GT et al. Eosinophilic esophagitis in children and adults: a systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology. 2007;133:13421363. | 5 (5, 5) | 2.5 (2, 3) | 0.93 (0.850.98) | ||
| Dehydration, m | 0 | ||||
| Redundant prepuce and phimosis, s | 1 | American Academy of Pediatrics Task Force on Circumcision. Male circumcision. Pediatrics. 2012;130(3):e756e785. | 6 (6, 6) | 3 (3, 3) | 0.66 (0.250.89) |
| Abdominal pain, m | 0 | ||||
| Other convulsions, m | 0 | ||||
| Urinary tract infection, m | 1 | Roberts KB et al. Urinary tract infection: clinical practice guideline for the diagnosis and management of the initial UTI in febrile infants and children 2 to 24 months. Pediatrics. 2011;128:595610. | 5.5 (5, 6) | 2.5 (2, 3) | 0.62 (0.230.84) |
| Acute appendicitis without peritonitis, s | 1 | Solomkin JS et al. Diagnosis and management of complicated intra‐abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis. 2010;50:133164. | 4.5 (5, 4) | 2.5 (3, 2) | 0.37 (0.110.81) |
| Eso‐ exo‐ hetero‐, and hypertropia, s | 0 | ||||
| Fever, m | 0 | ||||
| Seizures with and without intractable epilepsy, m | 3 | Brophy GM et al; Neurocritical Care Society Status Epilepticus Guideline Writing Committee. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care. 2012;17:323. | 5 (5, 5) | 3 (3, 3) | 0.95 (0.870.99) |
| Hirtz D et al. Practice parameter: treatment of the child with a first unprovoked seizure: report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 2003;60:166175. | 5 (5, 5) | 2.5 (2, 3) | 0.73 (0.410.94) | ||
| Riviello JJ Jr et al. Practice parameter: diagnostic assessment of the child with status epilepticus (an evidence‐based review): report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 2006;67:15421550. | 5 (4, 6) | 2.5 (2, 3) | 0.80 (0.630.94) | ||
| Sickle cell disease with crisis, m | 2 | Section on Hematology/Oncology Committee on Genetics; American Academy of Pediatrics. Health supervision for children with sickle cell disease. Pediatrics. 2002;109:526535. | 3.5 (3, 4) | 1.5 (1, 2) | 0.92 (0.800.98) |
| National Heart, Lung, and Blood Institute, National Institutes of Health. The management of sickle cell disease. National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD. Available at: | 4 (4, 4) | 2.5 (2, 3) | 0.91 (0.800.97) | ||
Identifying National Guidelines
We developed a search protocol (see Supporting Information, Table 2, in the online version of this article) using condition‐specific keywords and the following criteria: guideline, pediatric, 2002 to 2012. A medical librarian (E.E.) used the protocol to search PubMed, National Guidelines Clearing House, and the American Academy of Pediatrics website for guidelines for the 20 selected conditions.
We limited our study to US national guidelines published or updated from 2002 to 2012 to be most relevant to the 38 US children's hospitals in the original study. Guidelines had to address either medical or surgical or both types of inpatient management for the condition, depending on how the condition was categorized on the PRIS prioritization list. For example, to target inpatient issues, otitis media was treated as a surgical condition when the prioritization list was created, therefore guidelines included in our study needed to address surgical management (ie, myringotomy or tympanostomy tubes).[2] Guidelines specific to 1 organism, test, or treatment were a priori excluded, as they would not map well to the prioritization list, and would be difficult to interpret. Guidelines focusing exclusively on condition prevention were also excluded. Guidelines with a broad subject matter (eg, abdominal infection) or unclear age were included if they contained a significant focus on the condition of interest (eg, appendicitis without peritonitis), such that the course of pediatric inpatient care was described for that condition. Retracted or outdated (superseded by a more current version) guidelines were excluded.
An investigator (G.H.) reviewed potentially relevant results from the librarian's search. For example, the search for tonsillectomy guidelines retrieved a guideline on the use of polysomnography prior to tonsillectomy in children but did not cover the inpatient management or tonsillectomy procedure.[16] This guideline was excluded from our study, as it focused on a specific test and did not discuss surgical management of the condition.
Rating Methodological Quality of Guidelines
Methodological quality of guidelines was rated with the AGREE II tool by 2 investigators (G.H. and K.N.).[13, 17] This tool has 2 overall guideline assessments and 23 subcomponents within 6 domains, reflecting many of the IOM's recommendations for methodological quality in guidelines: scope and purpose, stakeholder involvement, rigor of development, clarity of presentation, applicability, and editorial independence.[8, 17]
The AGREE II tool rates each of the 23 subcomponent questions using a 7‐point scale (1=strongly disagree7=strongly agree). We followed the AGREE II user's manual suggestion in rating subcomponents as 1, indicating an absence of information for that question if the question was not addressed within the guideline.[13] The AGREE II user's manual describes the option of creating standardized domain scores; however, as the objective of our study was to assess the overall methodological quality of the guideline and not to highlight particular areas of strengths/weaknesses in the domains, we elected to present raw scores only.[13]
For the overall guideline rating item 1 (Rate the overall quality of this guideline.) the AGREE II tool instructs that a score of 1 indicates lowest possible quality and 7 indicates highest possible quality.[13] As these score anchors are far apart with no guide for interpretation of intermediate results, we modified the descriptive terms on the tool to define scores <3 as low quality, scores 3 to 5 as moderate quality, and scores >5 as high quality to allow for easier interpretation of our results. We also modified the final overall recommendation score (on a 3‐point scale) from I would recommend this guideline for use to I would recommend this guideline for use in the pediatric inpatient setting.[13, 17] A score of 1 indicated to not recommend, 2 indicated to recommend with modifications, and 3 indicated to recommend without modification.
Significant discrepancies (>2‐point difference on overall rating) between the 2 raters were to be settled by consensus scoring by 3 senior investigators blinded to previous reviews, using a modified Delphi technique.[18]
Inter‐rater reliability was measured using a weighted kappa coefficient and reported using a bootstrapped method with 95% confidence intervals. Interpretation of kappa is such that 0 is the amount of agreement that would be expected by chance, and 1 is perfect agreement, with previous researchers stating scores >0.81 indicate almost perfect agreement.[19]
RESULTS
The librarian's search retrieved 2869 potential results (Figure 1). Seventeen guidelines met inclusion criteria for 13 of the 20 priority conditions. Seven conditions did not have national guidelines meeting inclusion criteria. Table 1 displays the 20 medical and surgical conditions on the modified PRIS prioritization list, including overall guideline scoring, recommendation scores, and kappa results for each guideline. The highest methodological‐quality guidelines were for asthma,[20] tonsillectomy,[21] and bronchiolitis[22] (mean overall rating 7, 6.5, and 6.5, respectively). The lowest methodological‐quality guidelines were for 2 sickle cell disease guidelines[23, 24] and 1 dental caries guideline[25] (mean overall rating 4, 3.5, and 3, respectively). Seven guidelines were rated as high overall quality, and 10 guidelines were rated as moderate overall quality. Eight of the 17 guidelines[20, 21, 22, 26, 27, 28, 29, 30] were recommended for use in the pediatric inpatient setting without modification by both reviewers. Two guidelines (for dental caries[25] and sickle cell[23]) were not recommended for use by 1 reviewer.

As an example of scoring, a national guideline for asthma had high overall scores (7 from each reviewer) and high scores across most AGREE II subcomponents. The guideline was found by both reviewers to be systematic in describing guideline development with clearly stated recommendations linked to the available evidence (including strengths and limitations) and implementation considerations.[20] Conversely, a national guideline for sickle cell disease had moderate overall scores (scores of 3 and 4) and low‐moderate scores across the majority of the subcomponent items.[23] The reviewers believe that this guideline would have been strengthened by increased transparency in guideline development, discussion of the evidence surrounding recommendations, and discussion of implementation factors. A table with detailed scoring of each guideline is available (see Supporting Information, Table 3, in the online version of this article).
Agreement between the 2 raters was almost perfect,[19] with an overall boot‐strapped weighted kappa of 0.83 (95% confidence interval 0.780.87) across 850 scores. There were no discrepancies between reviewers in overall scoring requiring consensus scoring.
DISCUSSION
Using a modified version of a published prioritization list for inpatient pediatric conditions, we found national guidelines for 13 of 20 conditions with high prevalence, cost, and interhospital variation in resource utilization. Seven conditions had no national guidelines published within the past 10 years applicable for use in the pediatric inpatient setting. Of 17 guidelines for 13 conditions, 10 had moderate and 7 had high methodological quality.
Our findings add to the literature describing methodological quality of guidelines. Many publications focus on the methodological quality of guidelines as a group and use a standardized instrument (eg, the AGREE II tool) to rate within domains (eg, domain 1: scope and purpose) across guidelines in an effort to encourage improvement in developing and reporting in guidelines.[31, 32] Our study differs in that we chose to focus on the overall quality rating of individual guidelines for specific prioritized conditions to allow hospitals to guide QI initiatives. One study that had a similar aim to ours surveyed Dutch pediatricians to select priority conditions and used the AGREE II tool to rate 17 guidelines, recommending 14 for use in the Netherlands.[33]
Identifying high methodological‐quality guidelines is only 1 in a series of steps prior to successful guideline implementation in hospitals. Other aspects of guidelines, including the strength of the evidence (eg, from randomized controlled trials) and subsequent force and clarity (eg, use of must instead of consider) of recommendations, may affect clinician or patient adherence, work processes, and ultimately patient outcomes. Strong evidence should translate into forceful and clear recommendations. Authors with the Yale Guideline Recommendation Corpus describe significant variation in reporting of guideline recommendations, and further studies have shown that the force and clarity of a recommendation is associated with adherence rates.[34, 35, 36, 37] Unfortunately, current guideline appraisal tools lack the means to score the strength of evidence, and force and clarity of recommendations.[10]
Implementation science demonstrates that there are many important factors in translating best practice into improvements in clinical care. In addition to implementation considerations such as adherence, patient preferences, and work processes, variability in methodological quality, strength of evidence, and force and clarity of recommendations may be additional reasons why evidence for the impact of guidelines on patient outcomes remains mixed in the literature.[38] One recent study found that adherence to antibiotics recommended within a national pediatric community‐acquired pneumonia guideline, which had a high methodological‐quality score in our study, did not change hospital length of stay or readmissions.[29, 39] There are several possible interpretations for this. Recommendations may not have been based upon strong evidence, research methodology assessing how adherence to recommendations impacts patient outcomes may have been limited, or the outcomes measured in current studies (such as readmission) are not the outcomes that may be improved by adherence to these recommendations (such as decreasing antimicrobial resistance). These are important considerations when hospitals are incorporating recommendations from guidelines into practice. Hospitals should assess the multiple aspects of guidelines, including methodological quality, which our study helps to identify, strength of evidence, and force and clarity of recommendations, as well as adherence, patient preferences, work processes, and key outcome measures when implementing guidelines into clinical practice. A study utilizing a robust QI methodology demonstrated that clinician adherence to several elements in an asthma guideline, which also had a high methodological‐quality score in our study, led to a significant decrease in 6‐month hospital and emergency department readmission for asthma.[6, 20]
Our study also highlights that several pediatric conditions with high prevalence, cost, and interhospital resource utilization variation lack recent national pediatric guidelines applicable to the inpatient setting. If strong evidence exists for these priority conditions, professional societies should create high methodological‐quality guidelines with strong and clear recommendations. If evidence is lacking for these priority conditions, then investigators should focus on generating research in these areas.
There are several limitations to this study. The AGREE II tool does not have a mechanism to measure the strength of evidence used in a guideline. Methodological quality of a guideline alone may not translate into improved outcomes. Conditions may have national guidelines published before 2002, institution‐specific or international guidelines, or adult guidelines that might be amenable to use in the pediatric inpatient setting but were not included in this study. Several conditions on the prioritization list are broad in nature (eg, dehydration) and may not be amenable to the creation of guidelines. Other conditions on the prioritization list (eg, chemotherapy or cellulitis) may have useful guidelines within the context of specific conditions (eg, acute lymphoblastic leukemia) or for specific organisms (eg, methicillin‐resistant Staphylococcus aureus). We elected to exclude these narrower guidelines to focus on broad and comprehensive guidelines applicable to a wider range of clinical situations. Additionally, although use of a validated tool attempts to objectively guide ratings, the rating of quality is to some degree subjective. Finally, our study used a previously published prioritization list using data from children's hospitals, and the list likely under‐represents conditions commonly managed in community hospitals (eg, hyperbilirubinemia).[2] Exclusion of these conditions was not reflective of importance or quality of available national guidelines.
CONCLUSIONS
Our study adds to recent publications on the need to prioritize conditions for QI in children's hospitals. We identified a group of moderate to high methodological‐quality national guidelines for pediatric inpatient conditions with high prevalence, cost, and variation in interhospital resource utilization. Not all prioritized conditions have national high methodological‐quality guidelines available. Hospitals should prioritize conditions with high methodological‐quality guidelines to allocate resources for QI initiatives. Professional societies should focus their efforts on producing methodologically sound guidelines for prioritized conditions currently lacking high‐quality guidelines if sufficient evidence exists.
Acknowledgements
The authors thank Christopher G. Maloney, MD, PhD, for critical review of the manuscript, and Gregory J. Stoddard, MS, for statistical support. Mr. Stoddard's work is supported by the University of Utah Study Design and Biostatistics Center, with funding in part from the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through grant 8UL1TR000105 (formerly UL1RR025764).
Disclosures
Sanjay Mahant, Ron Keren, and Raj Srivastava are all Executive Council members of the Pediatric Research in Inpatient Settings (PRIS) Network. PRIS, Sanjay Mahant, Ron Keren, and Raj Srivastava are all supported by grants from the Children's Hospital Association. Sanjay Mahant is also supported by research grants from the Canadian Institute of Health Research and Physician Services Incorporated. Ron Keren and Raj Srivastava also serve as medical legal consultants. The remaining authors have no financial relationships relevant to this article to disclose.
- , . Development of the Pediatric Research in Inpatient Settings (PRIS) Network: lessons learned. J Hosp Med. 2012;7(8):661–664.
- , , , et al. Prioritization of comparative effectiveness research topics in hospital pediatrics. Arch Pediatr Adolesc Med. 2012;166(12):1–10.
- , . How Intermountain trimmed health care costs through robust quality improvement efforts. Health Aff (Millwood). 2011;30(6):1185–1191.
- . Making it easy to do it right. N Engl J Med. 2001;345(13):991–993.
- , , , , . Clinical guidelines: potential benefits, limitations, and harms of clinical guidelines. BMJ. 1999;318(7182):527–530.
- , , , et al. The Joint Commission Children's Asthma Care quality measures and asthma readmissions. Pediatrics. 2012;130(3):482–491.
- , , , et al. Toward transparent clinical policies. Pediatrics. 2008;121(3):643–646.
- Field MJ, Lohr KN, eds.; Committee to Advise the Public Health Service on Clinical Practice Guidelines, Institute of Medicine. Clinical Practice Guidelines: Directions for a New Program. Washington, DC: National Academies Press; 1990.
- , , , , . Development and application of a generic methodology to assess the quality of clinical guidelines. Int J Qual Health Care. 1999;11(1):21–28.
- , , , , . A systematic review of appraisal tools for clinical practice guidelines: multiple similarities and one common deficit. Int J Qual Health Care. 2005;17(3):235–242.
- , , . How to decide whether a clinical practice guideline is trustworthy. JAMA. 2013;309(2):139–140.
- Field MJ, Lohr KN, eds.; Committee to Advise the Public Health Service on Clinical Practice Guidelines, Institute of Medicine. Guidelines for Clinical Practice: From Development to Use. Washington, DC: National Academies Press; 1992.
- The AGREE Collaboration. Development and validation of an international appraisal instrument for assessing the quality of clinical practice guidelines: the AGREE project. Qual Saf Health Care. 2003;12:18–23.
- . AGREE II‐improving the quality of clinical care. Lancet. 2010;376(9747):1128–1129.
- , , , . Corticosteroids and mortality in children with bacterial meningitis. JAMA. 2008;299(17):2048–2055.
- , , , et al. Clinical practice guideline: polysomnography for sleep‐disordered breathing prior to tonsillectomy in children. Otolaryngol Head Neck Surg. 2011;145(1 suppl):S1–S15.
- , , , et al. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010;182(18):E839–E842.
- , . The Delphi method as a research tool: an example, design considerations and applications. Inform Manag. 2004;42(1):15–29.
- , . The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174.
- National Heart, Lung, and Blood Institute; National Asthma Education and Prevention Program. Expert panel report 3 (EPR‐3): guidelines for the diagnosis and management of asthma‐full report 2007. Pages 1–440. Available at: http://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.pdf. Accessed on 24 August 2012.
- , , , et al. Clinical practice guideline: tonsillectomy in children. Otolaryngol Head Neck Surg. 2011;144(1 suppl):S1–S30.
- American Academy of Pediatrics Subcommittee on Diagnosis and Management of Bronchiolitis. Diagnosis and management of bronchiolitis. Pediatrics. 2006;118:1774–1793.
- Health supervision for children with sickle cell disease. Pediatrics. 2002;109(3):526–535.
- National Heart, Lung, and Blood Institute, National Institutes of Health. The management of sickle cell disease. National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD. Available at: http://www.nhlbi.nih.gov/health/prof/blood/sickle/sc_mngt.pdf. Revised June 2002. Accessed on 13 October 2012.
- American Academy on Pediatric Dentistry Clinical Affairs Committee–Pulp Therapy Subcommittee; American Academy on Pediatric Dentistry Council on Clinical Affairs. Guideline on pulp therapy for primary and young permanent teeth. Pediatr Dent. 2008;30:170–174.
- , , , et al.; Neurocritical Care Society Status Epilepticus Guideline Writing Committee. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care. 2012;17:3–23.
- American Academy of Pediatrics Task Force on Circumcision. Male circumcision. Pediatrics. 2012;130(3):e756–e785.
- , , , et al. Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN). J Pediatr Gastroenterol Nutr. 2009;49(4):498–547.
- , , , et al. The management of community‐acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53(7):e25–e76.
- American Academy of Family Physicians; American Academy of Otolaryngology‐Head and Neck Surgery; American Academy of Pediatrics Subcommittee on Otitis Media With Effusion. Clinical Practice Guidelines: Otitis media with effusion. Pediatrics. 2004 May;113(5):1412–29.
- , , . Are guidelines following guidelines? The methodological quality of clinical practice guidelines in the peer‐reviewed medical literature. JAMA. 1999;281(20):1900–1905.
- , , , . Quality of reporting and evidence in American Academy of Pediatrics guidelines. Pediatrics. 2013;131(4):732–738.
- , , . Quality of evidence‐based pediatric guidelines. Pediatrics. 2005;115(5):1378–1391.
- , , . The Yale Guideline Recommendation Corpus: a representative sample of the knowledge content of guidelines. Int J Med Inform. 2009;78(5):354–363.
- , , . How often is strength of recommendation indicated in guidelines? Analysis of the Yale Guideline Recommendation Corpus. AMIA Annu Symp Proc. 2008:984.
- , , . Clinical practice guideline development manual, third edition: a quality‐driven approach for translating evidence into action. Otolaryngol Head Neck Surg. 2013;148(1 suppl):S1–S55.
- , , , , , . Attributes of clinical guidelines that influence use of guidelines in general practice: observational study. BMJ. 1998;317(7162):858–861.
- , , , et al. Toward evidence‐based quality improvement. Evidence (and its limitations) of the effectiveness of guideline dissemination and implementation strategies 1966–1998. J Gen Intern Med. 2006;21(suppl 2):S14–S20.
- , , , . Effectiveness of antimicrobial guidelines for community‐acquired pneumonia in children. Pediatrics. 2012;129(5):e1326–e1333.
- , . Development of the Pediatric Research in Inpatient Settings (PRIS) Network: lessons learned. J Hosp Med. 2012;7(8):661–664.
- , , , et al. Prioritization of comparative effectiveness research topics in hospital pediatrics. Arch Pediatr Adolesc Med. 2012;166(12):1–10.
- , . How Intermountain trimmed health care costs through robust quality improvement efforts. Health Aff (Millwood). 2011;30(6):1185–1191.
- . Making it easy to do it right. N Engl J Med. 2001;345(13):991–993.
- , , , , . Clinical guidelines: potential benefits, limitations, and harms of clinical guidelines. BMJ. 1999;318(7182):527–530.
- , , , et al. The Joint Commission Children's Asthma Care quality measures and asthma readmissions. Pediatrics. 2012;130(3):482–491.
- , , , et al. Toward transparent clinical policies. Pediatrics. 2008;121(3):643–646.
- Field MJ, Lohr KN, eds.; Committee to Advise the Public Health Service on Clinical Practice Guidelines, Institute of Medicine. Clinical Practice Guidelines: Directions for a New Program. Washington, DC: National Academies Press; 1990.
- , , , , . Development and application of a generic methodology to assess the quality of clinical guidelines. Int J Qual Health Care. 1999;11(1):21–28.
- , , , , . A systematic review of appraisal tools for clinical practice guidelines: multiple similarities and one common deficit. Int J Qual Health Care. 2005;17(3):235–242.
- , , . How to decide whether a clinical practice guideline is trustworthy. JAMA. 2013;309(2):139–140.
- Field MJ, Lohr KN, eds.; Committee to Advise the Public Health Service on Clinical Practice Guidelines, Institute of Medicine. Guidelines for Clinical Practice: From Development to Use. Washington, DC: National Academies Press; 1992.
- The AGREE Collaboration. Development and validation of an international appraisal instrument for assessing the quality of clinical practice guidelines: the AGREE project. Qual Saf Health Care. 2003;12:18–23.
- . AGREE II‐improving the quality of clinical care. Lancet. 2010;376(9747):1128–1129.
- , , , . Corticosteroids and mortality in children with bacterial meningitis. JAMA. 2008;299(17):2048–2055.
- , , , et al. Clinical practice guideline: polysomnography for sleep‐disordered breathing prior to tonsillectomy in children. Otolaryngol Head Neck Surg. 2011;145(1 suppl):S1–S15.
- , , , et al. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010;182(18):E839–E842.
- , . The Delphi method as a research tool: an example, design considerations and applications. Inform Manag. 2004;42(1):15–29.
- , . The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174.
- National Heart, Lung, and Blood Institute; National Asthma Education and Prevention Program. Expert panel report 3 (EPR‐3): guidelines for the diagnosis and management of asthma‐full report 2007. Pages 1–440. Available at: http://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.pdf. Accessed on 24 August 2012.
- , , , et al. Clinical practice guideline: tonsillectomy in children. Otolaryngol Head Neck Surg. 2011;144(1 suppl):S1–S30.
- American Academy of Pediatrics Subcommittee on Diagnosis and Management of Bronchiolitis. Diagnosis and management of bronchiolitis. Pediatrics. 2006;118:1774–1793.
- Health supervision for children with sickle cell disease. Pediatrics. 2002;109(3):526–535.
- National Heart, Lung, and Blood Institute, National Institutes of Health. The management of sickle cell disease. National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD. Available at: http://www.nhlbi.nih.gov/health/prof/blood/sickle/sc_mngt.pdf. Revised June 2002. Accessed on 13 October 2012.
- American Academy on Pediatric Dentistry Clinical Affairs Committee–Pulp Therapy Subcommittee; American Academy on Pediatric Dentistry Council on Clinical Affairs. Guideline on pulp therapy for primary and young permanent teeth. Pediatr Dent. 2008;30:170–174.
- , , , et al.; Neurocritical Care Society Status Epilepticus Guideline Writing Committee. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care. 2012;17:3–23.
- American Academy of Pediatrics Task Force on Circumcision. Male circumcision. Pediatrics. 2012;130(3):e756–e785.
- , , , et al. Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN). J Pediatr Gastroenterol Nutr. 2009;49(4):498–547.
- , , , et al. The management of community‐acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53(7):e25–e76.
- American Academy of Family Physicians; American Academy of Otolaryngology‐Head and Neck Surgery; American Academy of Pediatrics Subcommittee on Otitis Media With Effusion. Clinical Practice Guidelines: Otitis media with effusion. Pediatrics. 2004 May;113(5):1412–29.
- , , . Are guidelines following guidelines? The methodological quality of clinical practice guidelines in the peer‐reviewed medical literature. JAMA. 1999;281(20):1900–1905.
- , , , . Quality of reporting and evidence in American Academy of Pediatrics guidelines. Pediatrics. 2013;131(4):732–738.
- , , . Quality of evidence‐based pediatric guidelines. Pediatrics. 2005;115(5):1378–1391.
- , , . The Yale Guideline Recommendation Corpus: a representative sample of the knowledge content of guidelines. Int J Med Inform. 2009;78(5):354–363.
- , , . How often is strength of recommendation indicated in guidelines? Analysis of the Yale Guideline Recommendation Corpus. AMIA Annu Symp Proc. 2008:984.
- , , . Clinical practice guideline development manual, third edition: a quality‐driven approach for translating evidence into action. Otolaryngol Head Neck Surg. 2013;148(1 suppl):S1–S55.
- , , , , , . Attributes of clinical guidelines that influence use of guidelines in general practice: observational study. BMJ. 1998;317(7162):858–861.
- , , , et al. Toward evidence‐based quality improvement. Evidence (and its limitations) of the effectiveness of guideline dissemination and implementation strategies 1966–1998. J Gen Intern Med. 2006;21(suppl 2):S14–S20.
- , , , . Effectiveness of antimicrobial guidelines for community‐acquired pneumonia in children. Pediatrics. 2012;129(5):e1326–e1333.
© 2014 Society of Hospital Medicine
New SHM President Outlines “Next Evolution” of Hospitalist Practice
LAS VEGAS—Newly minted SHM President Burke Kealey, MD, SFHM, wants hospitalists to change how they look at health-care affordability, patient health, and the patient experience: He wants you to view them as one thing, not three.
“We put the energy and the effort of the moment behind the squeaky wheel,” Dr. Kealey said in his inaugural address Wednesday at the Mandalay Bay Resort and Casino. “What I would like us to do is all start thinking about all three at the same time, and with equal weight at all times. To me, this is the next evolution of the hospitalist.”
Dr. Kealey, medical director of hospital specialties at HealthPartners Medical Group in St. Paul, Minn., borrowed the approach from the Institute of Healthcare Improvement, whose “Triple Aim” initiative has the same goals. In his address, he told HM14 attendees that to focus on any of the three areas while giving short shrift to the others misses the point of bettering the overall health-care system.
“To improve health, but then people can’t afford that health care, is a nonstarter,” he said. “To make things finally affordable, but then people stay away because it’s a bad experience, makes no sense, either. We must do it all together.”
Dr. Kealey, who replaces Eric Howell, MD, SFHM, as president for the next year, says that as value-based purchasing further connects cost of care to the quality of delivery, hospitalists who link health, experience, and affordability will have success.
“This movement goes beyond just improving scores,” he says. “It improves the health of our patients.”
LAS VEGAS—Newly minted SHM President Burke Kealey, MD, SFHM, wants hospitalists to change how they look at health-care affordability, patient health, and the patient experience: He wants you to view them as one thing, not three.
“We put the energy and the effort of the moment behind the squeaky wheel,” Dr. Kealey said in his inaugural address Wednesday at the Mandalay Bay Resort and Casino. “What I would like us to do is all start thinking about all three at the same time, and with equal weight at all times. To me, this is the next evolution of the hospitalist.”
Dr. Kealey, medical director of hospital specialties at HealthPartners Medical Group in St. Paul, Minn., borrowed the approach from the Institute of Healthcare Improvement, whose “Triple Aim” initiative has the same goals. In his address, he told HM14 attendees that to focus on any of the three areas while giving short shrift to the others misses the point of bettering the overall health-care system.
“To improve health, but then people can’t afford that health care, is a nonstarter,” he said. “To make things finally affordable, but then people stay away because it’s a bad experience, makes no sense, either. We must do it all together.”
Dr. Kealey, who replaces Eric Howell, MD, SFHM, as president for the next year, says that as value-based purchasing further connects cost of care to the quality of delivery, hospitalists who link health, experience, and affordability will have success.
“This movement goes beyond just improving scores,” he says. “It improves the health of our patients.”
LAS VEGAS—Newly minted SHM President Burke Kealey, MD, SFHM, wants hospitalists to change how they look at health-care affordability, patient health, and the patient experience: He wants you to view them as one thing, not three.
“We put the energy and the effort of the moment behind the squeaky wheel,” Dr. Kealey said in his inaugural address Wednesday at the Mandalay Bay Resort and Casino. “What I would like us to do is all start thinking about all three at the same time, and with equal weight at all times. To me, this is the next evolution of the hospitalist.”
Dr. Kealey, medical director of hospital specialties at HealthPartners Medical Group in St. Paul, Minn., borrowed the approach from the Institute of Healthcare Improvement, whose “Triple Aim” initiative has the same goals. In his address, he told HM14 attendees that to focus on any of the three areas while giving short shrift to the others misses the point of bettering the overall health-care system.
“To improve health, but then people can’t afford that health care, is a nonstarter,” he said. “To make things finally affordable, but then people stay away because it’s a bad experience, makes no sense, either. We must do it all together.”
Dr. Kealey, who replaces Eric Howell, MD, SFHM, as president for the next year, says that as value-based purchasing further connects cost of care to the quality of delivery, hospitalists who link health, experience, and affordability will have success.
“This movement goes beyond just improving scores,” he says. “It improves the health of our patients.”
Grassroots Hospitalists Anxious for Bob Wachter’s Keynote
LAS VEGAS—Hospitalist Jennifer Johnson, MD, knows exactly where she’ll be at noon today: sitting in a banquet chair listening to “the father of hospital medicine,” Bob Wachter, MD, MHM, give his annual meeting address.
When you work in technology, “you don’t get to listen to Bill Gates talk,” says Dr. Johnson, who practices at Aurora Medical Center in Grafton, Wis. “It’s one of the few times you get to be in the same room with and hear how the real people at the forefront are thinking. I think that’s very helpful.”
Dr. Johnson, who last heard Dr. Wachter speak at HM12 in San Diego, says she relishes his vision, which goes beyond what most hospitalists’ day-to-day duties encompass.
“No matter what your vocation is, it’s always good to be reminded what more you could do,” she says. “How better you could improve yourself. Instead of stagnating…there’s always a next level, a next step. He’s the guy who thinks that way.”
She’s not alone in that view. Dr. Wachter’s presentation has become a rite of SHM’s annual meeting and an unofficial wrap-up of the four-day convention. This year’s edition is titled “Ten Years of Wachter Keynote: And Now For Something Completely Different.”
Sunil Kartham, MD, a hospitalist at Altru Health System in Grand Forks, N.D., spoke with Dr. Wachter earlier this week, as he wasn’t sure he could stay long enough for the formal address. But whether it’s Dr. Wachter or any of the keynote speakers, Dr. Kartham enjoys hearing HM’s leaders give advice.
“When you’re individual physicians,” he says, “you don’t know what to expect in the future. When the leaders come and speak, they lay out a map for you…so you can prepare yourself.”
For his part, Dr. Wachter has been priming the crowd for his talk. Earlier this week, he tweeted about his talk: “last 6 min wil b highlight (or lowlite) of conf, posibly of my life.”
LAS VEGAS—Hospitalist Jennifer Johnson, MD, knows exactly where she’ll be at noon today: sitting in a banquet chair listening to “the father of hospital medicine,” Bob Wachter, MD, MHM, give his annual meeting address.
When you work in technology, “you don’t get to listen to Bill Gates talk,” says Dr. Johnson, who practices at Aurora Medical Center in Grafton, Wis. “It’s one of the few times you get to be in the same room with and hear how the real people at the forefront are thinking. I think that’s very helpful.”
Dr. Johnson, who last heard Dr. Wachter speak at HM12 in San Diego, says she relishes his vision, which goes beyond what most hospitalists’ day-to-day duties encompass.
“No matter what your vocation is, it’s always good to be reminded what more you could do,” she says. “How better you could improve yourself. Instead of stagnating…there’s always a next level, a next step. He’s the guy who thinks that way.”
She’s not alone in that view. Dr. Wachter’s presentation has become a rite of SHM’s annual meeting and an unofficial wrap-up of the four-day convention. This year’s edition is titled “Ten Years of Wachter Keynote: And Now For Something Completely Different.”
Sunil Kartham, MD, a hospitalist at Altru Health System in Grand Forks, N.D., spoke with Dr. Wachter earlier this week, as he wasn’t sure he could stay long enough for the formal address. But whether it’s Dr. Wachter or any of the keynote speakers, Dr. Kartham enjoys hearing HM’s leaders give advice.
“When you’re individual physicians,” he says, “you don’t know what to expect in the future. When the leaders come and speak, they lay out a map for you…so you can prepare yourself.”
For his part, Dr. Wachter has been priming the crowd for his talk. Earlier this week, he tweeted about his talk: “last 6 min wil b highlight (or lowlite) of conf, posibly of my life.”
LAS VEGAS—Hospitalist Jennifer Johnson, MD, knows exactly where she’ll be at noon today: sitting in a banquet chair listening to “the father of hospital medicine,” Bob Wachter, MD, MHM, give his annual meeting address.
When you work in technology, “you don’t get to listen to Bill Gates talk,” says Dr. Johnson, who practices at Aurora Medical Center in Grafton, Wis. “It’s one of the few times you get to be in the same room with and hear how the real people at the forefront are thinking. I think that’s very helpful.”
Dr. Johnson, who last heard Dr. Wachter speak at HM12 in San Diego, says she relishes his vision, which goes beyond what most hospitalists’ day-to-day duties encompass.
“No matter what your vocation is, it’s always good to be reminded what more you could do,” she says. “How better you could improve yourself. Instead of stagnating…there’s always a next level, a next step. He’s the guy who thinks that way.”
She’s not alone in that view. Dr. Wachter’s presentation has become a rite of SHM’s annual meeting and an unofficial wrap-up of the four-day convention. This year’s edition is titled “Ten Years of Wachter Keynote: And Now For Something Completely Different.”
Sunil Kartham, MD, a hospitalist at Altru Health System in Grand Forks, N.D., spoke with Dr. Wachter earlier this week, as he wasn’t sure he could stay long enough for the formal address. But whether it’s Dr. Wachter or any of the keynote speakers, Dr. Kartham enjoys hearing HM’s leaders give advice.
“When you’re individual physicians,” he says, “you don’t know what to expect in the future. When the leaders come and speak, they lay out a map for you…so you can prepare yourself.”
For his part, Dr. Wachter has been priming the crowd for his talk. Earlier this week, he tweeted about his talk: “last 6 min wil b highlight (or lowlite) of conf, posibly of my life.”
HM 14 Special Report: Optimizing Patient Flow: The New Challenge for Hospitalists
Presenters:
Jeffrey Frank, MD, MBA; Joseph Guarisco, MD; David Yu, MD
Hospital medicine groups and emergency physicians are overdue for a paradigm shift in the ways we work together. Going forward, HM and ED groups must be tightly linked and integrated to provide the best care for patients and to be aligned with outcomes for which our hospital systems are being reimbursed under value-based purchasing.
Key Takeaways:
- Improving patient flow meets the triple aim: improves outcomes, improves patient experience, and reduces costs.
- The CMS Hospital Compare website publicly reports numerous measures directly related to ED flow time, evaluation by physician, time to pain med for long bone fracture, and LWBS (left without being seen rate). ED TAT (turn-around time) is a surrogate measure for hospital efficiency.
- CMS including ED throughput as a VBP measure. Healthcare payments projected to fall by about 25% in the near future. With margins shrinking, we must all “step up our game.”
- ED and HCAHPS Survey scores trend together; decreasing ED wait times improves overall patient satisfaction. Nationwide ED accounts for 70% of hospital admissions, this is trending upward; addressing the ED wait times in partnership with our ED colleagues is critical to our patient satisfaction scores.
- Decreasing ED wait times requires innovative thinking: the same thinking that got us here, will not solve our problem.
• Increase capacity during peak times by adding lower-cost alternatives to support physicians and keep them practicing at the highest level of our licenses (bring in scribes, personal medical assistants, physician extenders, etc.) Simple addition of more physicians does little toward solving problem of wait times;
• Active bed management and early hospital discharge;
• ED redesign: don’t just add more “beds;” and
• Keep ambulatory patients upright; only the sickest patients require stretcher space.
- ED and HM groups should meet monthly, not only to task issues as a team but to build and strengthen relationships.
- The days of ED/HM group culture of “us” vs. “them” are over—it doesn’t work. We’re all playing on the same team and all for the same goal—providing the best care for the patient. When ED and HM groups truly align and integrate they become a powerful force.
Julianna Lindsey, MD, MBA, FHM, is a Principal, COO & Strategist for Synergy Surgicalists, a practicing hospitalist, and a member of Team Hospitalist. Dr. Lindsey worked a number of years as a full-time ED physician.
Presenters:
Jeffrey Frank, MD, MBA; Joseph Guarisco, MD; David Yu, MD
Hospital medicine groups and emergency physicians are overdue for a paradigm shift in the ways we work together. Going forward, HM and ED groups must be tightly linked and integrated to provide the best care for patients and to be aligned with outcomes for which our hospital systems are being reimbursed under value-based purchasing.
Key Takeaways:
- Improving patient flow meets the triple aim: improves outcomes, improves patient experience, and reduces costs.
- The CMS Hospital Compare website publicly reports numerous measures directly related to ED flow time, evaluation by physician, time to pain med for long bone fracture, and LWBS (left without being seen rate). ED TAT (turn-around time) is a surrogate measure for hospital efficiency.
- CMS including ED throughput as a VBP measure. Healthcare payments projected to fall by about 25% in the near future. With margins shrinking, we must all “step up our game.”
- ED and HCAHPS Survey scores trend together; decreasing ED wait times improves overall patient satisfaction. Nationwide ED accounts for 70% of hospital admissions, this is trending upward; addressing the ED wait times in partnership with our ED colleagues is critical to our patient satisfaction scores.
- Decreasing ED wait times requires innovative thinking: the same thinking that got us here, will not solve our problem.
• Increase capacity during peak times by adding lower-cost alternatives to support physicians and keep them practicing at the highest level of our licenses (bring in scribes, personal medical assistants, physician extenders, etc.) Simple addition of more physicians does little toward solving problem of wait times;
• Active bed management and early hospital discharge;
• ED redesign: don’t just add more “beds;” and
• Keep ambulatory patients upright; only the sickest patients require stretcher space.
- ED and HM groups should meet monthly, not only to task issues as a team but to build and strengthen relationships.
- The days of ED/HM group culture of “us” vs. “them” are over—it doesn’t work. We’re all playing on the same team and all for the same goal—providing the best care for the patient. When ED and HM groups truly align and integrate they become a powerful force.
Julianna Lindsey, MD, MBA, FHM, is a Principal, COO & Strategist for Synergy Surgicalists, a practicing hospitalist, and a member of Team Hospitalist. Dr. Lindsey worked a number of years as a full-time ED physician.
Presenters:
Jeffrey Frank, MD, MBA; Joseph Guarisco, MD; David Yu, MD
Hospital medicine groups and emergency physicians are overdue for a paradigm shift in the ways we work together. Going forward, HM and ED groups must be tightly linked and integrated to provide the best care for patients and to be aligned with outcomes for which our hospital systems are being reimbursed under value-based purchasing.
Key Takeaways:
- Improving patient flow meets the triple aim: improves outcomes, improves patient experience, and reduces costs.
- The CMS Hospital Compare website publicly reports numerous measures directly related to ED flow time, evaluation by physician, time to pain med for long bone fracture, and LWBS (left without being seen rate). ED TAT (turn-around time) is a surrogate measure for hospital efficiency.
- CMS including ED throughput as a VBP measure. Healthcare payments projected to fall by about 25% in the near future. With margins shrinking, we must all “step up our game.”
- ED and HCAHPS Survey scores trend together; decreasing ED wait times improves overall patient satisfaction. Nationwide ED accounts for 70% of hospital admissions, this is trending upward; addressing the ED wait times in partnership with our ED colleagues is critical to our patient satisfaction scores.
- Decreasing ED wait times requires innovative thinking: the same thinking that got us here, will not solve our problem.
• Increase capacity during peak times by adding lower-cost alternatives to support physicians and keep them practicing at the highest level of our licenses (bring in scribes, personal medical assistants, physician extenders, etc.) Simple addition of more physicians does little toward solving problem of wait times;
• Active bed management and early hospital discharge;
• ED redesign: don’t just add more “beds;” and
• Keep ambulatory patients upright; only the sickest patients require stretcher space.
- ED and HM groups should meet monthly, not only to task issues as a team but to build and strengthen relationships.
- The days of ED/HM group culture of “us” vs. “them” are over—it doesn’t work. We’re all playing on the same team and all for the same goal—providing the best care for the patient. When ED and HM groups truly align and integrate they become a powerful force.
Julianna Lindsey, MD, MBA, FHM, is a Principal, COO & Strategist for Synergy Surgicalists, a practicing hospitalist, and a member of Team Hospitalist. Dr. Lindsey worked a number of years as a full-time ED physician.
HM 14 Special Report: Pre-Course Perioperative Medicine: Clinical Facts and Deep Cuts with Case-Based Applications
Presenters:
Steven Cohn, MD, FACP, SFHM; Leonard Feldman, MD, FAAP, FACP, SFHM; Amir Jaffer, MD, MBA, SFHM; Franklin Michota, MD, FHM; Kurt Pfeifer, MD, FACP; Barbara Slawski, MD, MS, FACP
In a session at HM14, a panel of physicians led a discussion about hospitalists' role in perioperative care. Hospitalists are increasingly asked to take on co-management roles for surgical patients. Controversies remain around a number of topics in perioperative care; it is important to separate evidence-based care from “best practice” and develop standardized approaches to perioperative care in all facilities.
Key Takeaways:
- Invoke the “platinum rule” as a medical consultant: treat others as they wish to be treated. Surgeons’ preferences differ from the traditional teaching regarding “rules of medical consulting.” Examples of surgeon preferences as opposed to traditional teaching: consultants should not limit themselves to specific question(s), just take care of the patient’s non-surgical issues; DO write orders in the chart [which] facilitates care; co-management relationship is desired; literature references placed in chart are NOT helpful; daily progress notes and follow-up is desired regardless of patient acuity; verbal discussion of recommendations not always necessary.
- Do not always defer to the surgeon, speak up for the best interest of the patient. If it is not in the patient’s best interest to go to surgery “@ 0700 in a.m.” then say so. Surgeons want us to speak up.
- Principles of perioperative medication management: most mediations can be safely be continued, many need not be continued. Certain medications are essential (cardiac, pulmonary, steroids) but not necessarily on the morning of surgery; some medications require discontinuation or dose adjustment (hypoglycemics, anticoagulants/antiplatelets).
- Perioperative lab testing: cataract surgery is low-risk surgery; laboratory testing NOT required. Base preoperative lab testing on patient risk factors (targeted H&P) and surgical risk factors (low risk/ambulatory surgeries generally do not require testing; consider testing in surgeries with anticipated blood loss). Develop local guidelines to standardize approach to preoperative lab evaluations at each facility/system.
- Sources for two cardiac risk calculators: www.surgicalriskcalculator.com/miorcardiacarrest; riskcalculator.facs.org.
- Periop pulmonary risk assessment: NSQIP Respiratory Failure Index calculator can be found @ www.surgicalriskcalculator.com. Never tell the anesthesiologist what mode of anesthesia to deliver (GETA, regional, neuraxial). ALL surgical patients should use incentive spirometry.
- VTE prophylaxis: VTE prevention is a consequential safety and quality measure, ALL facilities should have a standardized approach to VTE prophylaxis. Surgical patients should be risk-stratified and prophylaxed, weighing risks (VTE and major bleeding). Extended prophylaxis should be used for high-risk cancer and major orthopedic surgery patients.
- Perioperative management of warfarin: INR < 1.2 is achieved by holding warfarin for four doses. Discussion of perioperative anticoagulation strategy with patient, anesthesiologist, and surgeon is critical. Patients on antithrombotic therapy require an individualized custom-tailored approach.
- Perioperative anemia: reserve transfusion for symptoms attributable to anemia, Hgb <7-8 g/dl for hospitalized patients or <8 g/dl in patients with CV disease.
- Post-op fever: atelectasis does NOT cause fever, anesthetics and tissue trauma causing release of pyrogenic cytokines DO cause fever (first 48 hours post-op).
- Visit SHMconsult.com
Julianna Lindsey, MD, MBA, FHM, is Principal COO & Strategist for Synergy Surgicalists and a member of Team Hospitalist.
Presenters:
Steven Cohn, MD, FACP, SFHM; Leonard Feldman, MD, FAAP, FACP, SFHM; Amir Jaffer, MD, MBA, SFHM; Franklin Michota, MD, FHM; Kurt Pfeifer, MD, FACP; Barbara Slawski, MD, MS, FACP
In a session at HM14, a panel of physicians led a discussion about hospitalists' role in perioperative care. Hospitalists are increasingly asked to take on co-management roles for surgical patients. Controversies remain around a number of topics in perioperative care; it is important to separate evidence-based care from “best practice” and develop standardized approaches to perioperative care in all facilities.
Key Takeaways:
- Invoke the “platinum rule” as a medical consultant: treat others as they wish to be treated. Surgeons’ preferences differ from the traditional teaching regarding “rules of medical consulting.” Examples of surgeon preferences as opposed to traditional teaching: consultants should not limit themselves to specific question(s), just take care of the patient’s non-surgical issues; DO write orders in the chart [which] facilitates care; co-management relationship is desired; literature references placed in chart are NOT helpful; daily progress notes and follow-up is desired regardless of patient acuity; verbal discussion of recommendations not always necessary.
- Do not always defer to the surgeon, speak up for the best interest of the patient. If it is not in the patient’s best interest to go to surgery “@ 0700 in a.m.” then say so. Surgeons want us to speak up.
- Principles of perioperative medication management: most mediations can be safely be continued, many need not be continued. Certain medications are essential (cardiac, pulmonary, steroids) but not necessarily on the morning of surgery; some medications require discontinuation or dose adjustment (hypoglycemics, anticoagulants/antiplatelets).
- Perioperative lab testing: cataract surgery is low-risk surgery; laboratory testing NOT required. Base preoperative lab testing on patient risk factors (targeted H&P) and surgical risk factors (low risk/ambulatory surgeries generally do not require testing; consider testing in surgeries with anticipated blood loss). Develop local guidelines to standardize approach to preoperative lab evaluations at each facility/system.
- Sources for two cardiac risk calculators: www.surgicalriskcalculator.com/miorcardiacarrest; riskcalculator.facs.org.
- Periop pulmonary risk assessment: NSQIP Respiratory Failure Index calculator can be found @ www.surgicalriskcalculator.com. Never tell the anesthesiologist what mode of anesthesia to deliver (GETA, regional, neuraxial). ALL surgical patients should use incentive spirometry.
- VTE prophylaxis: VTE prevention is a consequential safety and quality measure, ALL facilities should have a standardized approach to VTE prophylaxis. Surgical patients should be risk-stratified and prophylaxed, weighing risks (VTE and major bleeding). Extended prophylaxis should be used for high-risk cancer and major orthopedic surgery patients.
- Perioperative management of warfarin: INR < 1.2 is achieved by holding warfarin for four doses. Discussion of perioperative anticoagulation strategy with patient, anesthesiologist, and surgeon is critical. Patients on antithrombotic therapy require an individualized custom-tailored approach.
- Perioperative anemia: reserve transfusion for symptoms attributable to anemia, Hgb <7-8 g/dl for hospitalized patients or <8 g/dl in patients with CV disease.
- Post-op fever: atelectasis does NOT cause fever, anesthetics and tissue trauma causing release of pyrogenic cytokines DO cause fever (first 48 hours post-op).
- Visit SHMconsult.com
Julianna Lindsey, MD, MBA, FHM, is Principal COO & Strategist for Synergy Surgicalists and a member of Team Hospitalist.
Presenters:
Steven Cohn, MD, FACP, SFHM; Leonard Feldman, MD, FAAP, FACP, SFHM; Amir Jaffer, MD, MBA, SFHM; Franklin Michota, MD, FHM; Kurt Pfeifer, MD, FACP; Barbara Slawski, MD, MS, FACP
In a session at HM14, a panel of physicians led a discussion about hospitalists' role in perioperative care. Hospitalists are increasingly asked to take on co-management roles for surgical patients. Controversies remain around a number of topics in perioperative care; it is important to separate evidence-based care from “best practice” and develop standardized approaches to perioperative care in all facilities.
Key Takeaways:
- Invoke the “platinum rule” as a medical consultant: treat others as they wish to be treated. Surgeons’ preferences differ from the traditional teaching regarding “rules of medical consulting.” Examples of surgeon preferences as opposed to traditional teaching: consultants should not limit themselves to specific question(s), just take care of the patient’s non-surgical issues; DO write orders in the chart [which] facilitates care; co-management relationship is desired; literature references placed in chart are NOT helpful; daily progress notes and follow-up is desired regardless of patient acuity; verbal discussion of recommendations not always necessary.
- Do not always defer to the surgeon, speak up for the best interest of the patient. If it is not in the patient’s best interest to go to surgery “@ 0700 in a.m.” then say so. Surgeons want us to speak up.
- Principles of perioperative medication management: most mediations can be safely be continued, many need not be continued. Certain medications are essential (cardiac, pulmonary, steroids) but not necessarily on the morning of surgery; some medications require discontinuation or dose adjustment (hypoglycemics, anticoagulants/antiplatelets).
- Perioperative lab testing: cataract surgery is low-risk surgery; laboratory testing NOT required. Base preoperative lab testing on patient risk factors (targeted H&P) and surgical risk factors (low risk/ambulatory surgeries generally do not require testing; consider testing in surgeries with anticipated blood loss). Develop local guidelines to standardize approach to preoperative lab evaluations at each facility/system.
- Sources for two cardiac risk calculators: www.surgicalriskcalculator.com/miorcardiacarrest; riskcalculator.facs.org.
- Periop pulmonary risk assessment: NSQIP Respiratory Failure Index calculator can be found @ www.surgicalriskcalculator.com. Never tell the anesthesiologist what mode of anesthesia to deliver (GETA, regional, neuraxial). ALL surgical patients should use incentive spirometry.
- VTE prophylaxis: VTE prevention is a consequential safety and quality measure, ALL facilities should have a standardized approach to VTE prophylaxis. Surgical patients should be risk-stratified and prophylaxed, weighing risks (VTE and major bleeding). Extended prophylaxis should be used for high-risk cancer and major orthopedic surgery patients.
- Perioperative management of warfarin: INR < 1.2 is achieved by holding warfarin for four doses. Discussion of perioperative anticoagulation strategy with patient, anesthesiologist, and surgeon is critical. Patients on antithrombotic therapy require an individualized custom-tailored approach.
- Perioperative anemia: reserve transfusion for symptoms attributable to anemia, Hgb <7-8 g/dl for hospitalized patients or <8 g/dl in patients with CV disease.
- Post-op fever: atelectasis does NOT cause fever, anesthetics and tissue trauma causing release of pyrogenic cytokines DO cause fever (first 48 hours post-op).
- Visit SHMconsult.com
Julianna Lindsey, MD, MBA, FHM, is Principal COO & Strategist for Synergy Surgicalists and a member of Team Hospitalist.