User login
How EHRs went wrong
If, like me, you believe that electronic health records will play an important role in future of medicine but can’t figure out why the federal government is spending $19 billion dollars incentivizing the adoption of systems that aren’t ready for prime time, I have found an answer.
An article in the New York Times ("A Digital Shift on Health Data Swells Profits in an Industry," Feb. 19, 2013) provided a glimpse into the backstage story of some questionable associations that led to bad decisions.
Glen E. Tullman, who until recently was the chief executive of Allscripts, one of the three dominant players in the electronic health information business, was health technology advisor to the 2008 Obama campaign. Since President Obama took office in 2009, he has reportedly visited the White House on at least seven occasions. Mr. Tullman, who was quoted as saying, "We really haven’t done any lobbying," characterized his time in Washington as education. According to the New York Times article, Allscripts annual sales have more than doubled since 2009 to an estimated $1.44 billion dollars in 2012.
It appears that the lobbying efforts by Mr. Tullman and other members of the industry were instrumental in creating a timetable of incentives that had physicians and hospitals rushing to jump on the EHR train before it left the station – and before it was road worthy. The result has been huge profits to the largest digital records companies while smaller companies that may have been less ready to compete have withered, the Times said.
One could argue that this is just another example of survival of the fittest in the best tradition of American free market capitalism. The problem is that the subsidies have tilted the playing field, and the resulting products have not met the promises made by those who lobbied for them. The even bigger problem is that the government also failed to secure from the industry any guarantees that EHR systems would meet a set of minimum standards and be compatible with one another.
As physicians, we also must share some of the blame for this EHR debacle.
We have not been thoughtful consumers. Those of us in small physician-owned groups must understand the relationship between our overhead and the bottom line and carefully weigh whether an incentive makes sense for us financially. If we decide to buy an EHR, we must be good shoppers. We must visit several practices that match our demographic and have been using for several years the system we are considering – even if this means flying to other cities to get a broad sampling. We should drive a hard bargain with incentives for support and severe financial penalties for failure to produce. And we mustn’t be afraid to say to the vendors that either we or they aren’t ready.
Most of us, however, no longer practice in physician-owned practices anymore. For a variety of reasons, we have allowed others to make decisions that dictate how we practice medicine in the real world. Most of these "others" aren’t physicians, and if they were once physicians, they certainly aren’t now in the true sense of the word and they don’t have recent practical experience of seeing real patients in real time. These others are often the folks who choose when and from which vendors medical practices buy their EHRs.
By joining larger and larger provider organizations, practicing physicians have lost their ability to provide critical input into the choice of tools with which they will practice. The result has been large investments in EHR systems that neither save money nor provide better care. We can only hope that from the ashes of this first failed attempt will come a system that does what we and our patients want it to do.
Dr. Wilkoff practices general pediatrics in a multispecialty group practice in Brunswick, Maine. E-mail Dr. Wilkoff at pdnews@frontlinemedcom.com.
If, like me, you believe that electronic health records will play an important role in future of medicine but can’t figure out why the federal government is spending $19 billion dollars incentivizing the adoption of systems that aren’t ready for prime time, I have found an answer.
An article in the New York Times ("A Digital Shift on Health Data Swells Profits in an Industry," Feb. 19, 2013) provided a glimpse into the backstage story of some questionable associations that led to bad decisions.
Glen E. Tullman, who until recently was the chief executive of Allscripts, one of the three dominant players in the electronic health information business, was health technology advisor to the 2008 Obama campaign. Since President Obama took office in 2009, he has reportedly visited the White House on at least seven occasions. Mr. Tullman, who was quoted as saying, "We really haven’t done any lobbying," characterized his time in Washington as education. According to the New York Times article, Allscripts annual sales have more than doubled since 2009 to an estimated $1.44 billion dollars in 2012.
It appears that the lobbying efforts by Mr. Tullman and other members of the industry were instrumental in creating a timetable of incentives that had physicians and hospitals rushing to jump on the EHR train before it left the station – and before it was road worthy. The result has been huge profits to the largest digital records companies while smaller companies that may have been less ready to compete have withered, the Times said.
One could argue that this is just another example of survival of the fittest in the best tradition of American free market capitalism. The problem is that the subsidies have tilted the playing field, and the resulting products have not met the promises made by those who lobbied for them. The even bigger problem is that the government also failed to secure from the industry any guarantees that EHR systems would meet a set of minimum standards and be compatible with one another.
As physicians, we also must share some of the blame for this EHR debacle.
We have not been thoughtful consumers. Those of us in small physician-owned groups must understand the relationship between our overhead and the bottom line and carefully weigh whether an incentive makes sense for us financially. If we decide to buy an EHR, we must be good shoppers. We must visit several practices that match our demographic and have been using for several years the system we are considering – even if this means flying to other cities to get a broad sampling. We should drive a hard bargain with incentives for support and severe financial penalties for failure to produce. And we mustn’t be afraid to say to the vendors that either we or they aren’t ready.
Most of us, however, no longer practice in physician-owned practices anymore. For a variety of reasons, we have allowed others to make decisions that dictate how we practice medicine in the real world. Most of these "others" aren’t physicians, and if they were once physicians, they certainly aren’t now in the true sense of the word and they don’t have recent practical experience of seeing real patients in real time. These others are often the folks who choose when and from which vendors medical practices buy their EHRs.
By joining larger and larger provider organizations, practicing physicians have lost their ability to provide critical input into the choice of tools with which they will practice. The result has been large investments in EHR systems that neither save money nor provide better care. We can only hope that from the ashes of this first failed attempt will come a system that does what we and our patients want it to do.
Dr. Wilkoff practices general pediatrics in a multispecialty group practice in Brunswick, Maine. E-mail Dr. Wilkoff at pdnews@frontlinemedcom.com.
If, like me, you believe that electronic health records will play an important role in future of medicine but can’t figure out why the federal government is spending $19 billion dollars incentivizing the adoption of systems that aren’t ready for prime time, I have found an answer.
An article in the New York Times ("A Digital Shift on Health Data Swells Profits in an Industry," Feb. 19, 2013) provided a glimpse into the backstage story of some questionable associations that led to bad decisions.
Glen E. Tullman, who until recently was the chief executive of Allscripts, one of the three dominant players in the electronic health information business, was health technology advisor to the 2008 Obama campaign. Since President Obama took office in 2009, he has reportedly visited the White House on at least seven occasions. Mr. Tullman, who was quoted as saying, "We really haven’t done any lobbying," characterized his time in Washington as education. According to the New York Times article, Allscripts annual sales have more than doubled since 2009 to an estimated $1.44 billion dollars in 2012.
It appears that the lobbying efforts by Mr. Tullman and other members of the industry were instrumental in creating a timetable of incentives that had physicians and hospitals rushing to jump on the EHR train before it left the station – and before it was road worthy. The result has been huge profits to the largest digital records companies while smaller companies that may have been less ready to compete have withered, the Times said.
One could argue that this is just another example of survival of the fittest in the best tradition of American free market capitalism. The problem is that the subsidies have tilted the playing field, and the resulting products have not met the promises made by those who lobbied for them. The even bigger problem is that the government also failed to secure from the industry any guarantees that EHR systems would meet a set of minimum standards and be compatible with one another.
As physicians, we also must share some of the blame for this EHR debacle.
We have not been thoughtful consumers. Those of us in small physician-owned groups must understand the relationship between our overhead and the bottom line and carefully weigh whether an incentive makes sense for us financially. If we decide to buy an EHR, we must be good shoppers. We must visit several practices that match our demographic and have been using for several years the system we are considering – even if this means flying to other cities to get a broad sampling. We should drive a hard bargain with incentives for support and severe financial penalties for failure to produce. And we mustn’t be afraid to say to the vendors that either we or they aren’t ready.
Most of us, however, no longer practice in physician-owned practices anymore. For a variety of reasons, we have allowed others to make decisions that dictate how we practice medicine in the real world. Most of these "others" aren’t physicians, and if they were once physicians, they certainly aren’t now in the true sense of the word and they don’t have recent practical experience of seeing real patients in real time. These others are often the folks who choose when and from which vendors medical practices buy their EHRs.
By joining larger and larger provider organizations, practicing physicians have lost their ability to provide critical input into the choice of tools with which they will practice. The result has been large investments in EHR systems that neither save money nor provide better care. We can only hope that from the ashes of this first failed attempt will come a system that does what we and our patients want it to do.
Dr. Wilkoff practices general pediatrics in a multispecialty group practice in Brunswick, Maine. E-mail Dr. Wilkoff at pdnews@frontlinemedcom.com.
The Corporation Cardiologist
In bygone days, your community hospital was a place where babies were born and gallbladders were removed. They were often run by city governments or local religious organizations. Of course, a lot has changed since then. Now your hospital advertises on television and extols the viewer about the medical miracles that are performed inside its walls. They also are getting bigger and merging with smaller and occasionally coequal institutions in the name of efficiency and in the effort to expand their patient catchment.
In an even larger sense, hospitals have been gobbled up by insurance companies and by for-profit networks in an attempt to maximize profits and minimize overhead. Some prestigious hospitals like the Mayo Clinic, the Cleveland Clinic, and MD Anderson Cancer Center have even established affiliations with community hospitals thousands of miles away seemingly to improve local care and at the same time to expand their referral network. Where local hospitals are not adequate and the market beckons, some have even built their own facilities not only in the United States, but also in countries around the globe.
The intent of these network affiliations is not only to improve their image but to impart some of their prestige to the local entities as well. As a result of these mergers and consolidations, they are positioning themselves to be more competitive in the new world of health care. Some would profess the altruism of providing better care either locally or at a distance, but in the long run, economics and market share are the driving force. They have not been concerned with delivering babies or taking out gallbladders for a long time.
Few can predict what the new world will look like, but it is quite certain that the Affordable Care Act will re-create or substantially modify American health care as we know it. The potential of attracting thousands of previously uninsured patients, who – with the help of the federal government – can come in the front door for care rather that using the back door of the emergency department for treatment, will be an important target.
In this environment, the practicing physician is caught in the changing tide. Many who are not in the swim will be washed up on the beach. Cardiology, along with oncology and gastroenterology, are the prime targets for the anticipated efficiencies evolving from the hospital system expansions and mergers. Although there is a well-recognized need for primary care health care professionals, much of this need is already being filled by nonphysician professionals. It is possible that cardiology, which has been one of the star profit centers, could become a target for consolidation and economy in the future. We may be seeing some of this, as the opportunities for finishing trainees appear to be diminishing.
It is obvious that there has been a major shift in the setting cardiology practices in the last few years. Since 2007, the proportion of physician-owned practices has decreased substantially. While the number of cardiologists employed by hospitals has grown from 11% to 35%, physician-owned practices have decreased from 59% to 36%. This migration of private practice to hospital-based practice is sure to continue. Cardiology practice will soon be directed by managers representing corporate health care who will be intent on putting in place programs that will establish protocol-driven therapy in the name of "quality" and "cost." Many of the changes will lead to better outcomes. Patient "satisfaction" will be measured by metrics already operational in the corporate environment. As the era of cardiology entrepreneurism faces institutional controls, such profit centers as imaging already are facing significant obstacles. The "down side" of this process will be the death of medical care as we knew it. It was not all bad. That mode of physician-driven, patient-centered care will not be easily transferred into the corporate care environment. The physician will need to ensure that at least that vestige of old-style medical care will not be entirely lost.
Dr. Goldstein, medical editor of Cardiology News, is professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. He is on data safety monitoring committees for the National Institutes of Health and several pharmaceutical companies.
In bygone days, your community hospital was a place where babies were born and gallbladders were removed. They were often run by city governments or local religious organizations. Of course, a lot has changed since then. Now your hospital advertises on television and extols the viewer about the medical miracles that are performed inside its walls. They also are getting bigger and merging with smaller and occasionally coequal institutions in the name of efficiency and in the effort to expand their patient catchment.
In an even larger sense, hospitals have been gobbled up by insurance companies and by for-profit networks in an attempt to maximize profits and minimize overhead. Some prestigious hospitals like the Mayo Clinic, the Cleveland Clinic, and MD Anderson Cancer Center have even established affiliations with community hospitals thousands of miles away seemingly to improve local care and at the same time to expand their referral network. Where local hospitals are not adequate and the market beckons, some have even built their own facilities not only in the United States, but also in countries around the globe.
The intent of these network affiliations is not only to improve their image but to impart some of their prestige to the local entities as well. As a result of these mergers and consolidations, they are positioning themselves to be more competitive in the new world of health care. Some would profess the altruism of providing better care either locally or at a distance, but in the long run, economics and market share are the driving force. They have not been concerned with delivering babies or taking out gallbladders for a long time.
Few can predict what the new world will look like, but it is quite certain that the Affordable Care Act will re-create or substantially modify American health care as we know it. The potential of attracting thousands of previously uninsured patients, who – with the help of the federal government – can come in the front door for care rather that using the back door of the emergency department for treatment, will be an important target.
In this environment, the practicing physician is caught in the changing tide. Many who are not in the swim will be washed up on the beach. Cardiology, along with oncology and gastroenterology, are the prime targets for the anticipated efficiencies evolving from the hospital system expansions and mergers. Although there is a well-recognized need for primary care health care professionals, much of this need is already being filled by nonphysician professionals. It is possible that cardiology, which has been one of the star profit centers, could become a target for consolidation and economy in the future. We may be seeing some of this, as the opportunities for finishing trainees appear to be diminishing.
It is obvious that there has been a major shift in the setting cardiology practices in the last few years. Since 2007, the proportion of physician-owned practices has decreased substantially. While the number of cardiologists employed by hospitals has grown from 11% to 35%, physician-owned practices have decreased from 59% to 36%. This migration of private practice to hospital-based practice is sure to continue. Cardiology practice will soon be directed by managers representing corporate health care who will be intent on putting in place programs that will establish protocol-driven therapy in the name of "quality" and "cost." Many of the changes will lead to better outcomes. Patient "satisfaction" will be measured by metrics already operational in the corporate environment. As the era of cardiology entrepreneurism faces institutional controls, such profit centers as imaging already are facing significant obstacles. The "down side" of this process will be the death of medical care as we knew it. It was not all bad. That mode of physician-driven, patient-centered care will not be easily transferred into the corporate care environment. The physician will need to ensure that at least that vestige of old-style medical care will not be entirely lost.
Dr. Goldstein, medical editor of Cardiology News, is professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. He is on data safety monitoring committees for the National Institutes of Health and several pharmaceutical companies.
In bygone days, your community hospital was a place where babies were born and gallbladders were removed. They were often run by city governments or local religious organizations. Of course, a lot has changed since then. Now your hospital advertises on television and extols the viewer about the medical miracles that are performed inside its walls. They also are getting bigger and merging with smaller and occasionally coequal institutions in the name of efficiency and in the effort to expand their patient catchment.
In an even larger sense, hospitals have been gobbled up by insurance companies and by for-profit networks in an attempt to maximize profits and minimize overhead. Some prestigious hospitals like the Mayo Clinic, the Cleveland Clinic, and MD Anderson Cancer Center have even established affiliations with community hospitals thousands of miles away seemingly to improve local care and at the same time to expand their referral network. Where local hospitals are not adequate and the market beckons, some have even built their own facilities not only in the United States, but also in countries around the globe.
The intent of these network affiliations is not only to improve their image but to impart some of their prestige to the local entities as well. As a result of these mergers and consolidations, they are positioning themselves to be more competitive in the new world of health care. Some would profess the altruism of providing better care either locally or at a distance, but in the long run, economics and market share are the driving force. They have not been concerned with delivering babies or taking out gallbladders for a long time.
Few can predict what the new world will look like, but it is quite certain that the Affordable Care Act will re-create or substantially modify American health care as we know it. The potential of attracting thousands of previously uninsured patients, who – with the help of the federal government – can come in the front door for care rather that using the back door of the emergency department for treatment, will be an important target.
In this environment, the practicing physician is caught in the changing tide. Many who are not in the swim will be washed up on the beach. Cardiology, along with oncology and gastroenterology, are the prime targets for the anticipated efficiencies evolving from the hospital system expansions and mergers. Although there is a well-recognized need for primary care health care professionals, much of this need is already being filled by nonphysician professionals. It is possible that cardiology, which has been one of the star profit centers, could become a target for consolidation and economy in the future. We may be seeing some of this, as the opportunities for finishing trainees appear to be diminishing.
It is obvious that there has been a major shift in the setting cardiology practices in the last few years. Since 2007, the proportion of physician-owned practices has decreased substantially. While the number of cardiologists employed by hospitals has grown from 11% to 35%, physician-owned practices have decreased from 59% to 36%. This migration of private practice to hospital-based practice is sure to continue. Cardiology practice will soon be directed by managers representing corporate health care who will be intent on putting in place programs that will establish protocol-driven therapy in the name of "quality" and "cost." Many of the changes will lead to better outcomes. Patient "satisfaction" will be measured by metrics already operational in the corporate environment. As the era of cardiology entrepreneurism faces institutional controls, such profit centers as imaging already are facing significant obstacles. The "down side" of this process will be the death of medical care as we knew it. It was not all bad. That mode of physician-driven, patient-centered care will not be easily transferred into the corporate care environment. The physician will need to ensure that at least that vestige of old-style medical care will not be entirely lost.
Dr. Goldstein, medical editor of Cardiology News, is professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. He is on data safety monitoring committees for the National Institutes of Health and several pharmaceutical companies.
Make your practice's Facebook page a success
LAS VEGAS – Hoping to make your practice’s Facebook page a success? Engage with your Facebook followers.
According to Monique Ramsey, founder of Del Mar, Calif.–based Cosmetic Social Media, the best path to social media success involves fostering. "Think about who your consumer is and what she wants to learn about," she advised.
"Provide that information to her and let her share it with her friends, because then your name is attached to that piece of content. And note it may not have anything to do with your cosmetic surgery practice, but this kind of a post will keep people coming to your page and get higher visibility for your posts in the newsfeed," she added.
The goal is to have your posts reach more than the Facebook average, which is about 16% of your Facebook followers. With more than 1 billion people using Facebook, physicians need to be able to "cut through the noise," Ms. Ramsey explained at the annual meeting of the American Academy of Cosmetic Surgery.
It’s okay for 20% of your posts to be about your practice – perhaps advertising special events and promotions – but the remaining 80% should be informative and off the topic yet engaging, meaningful, and fun.
For example, Ms. Ramsey crafted questions for one of her client’s Facebook page intended to trigger engagement in the form of comments. One read "It’s National Wine Day. Are you a red, white or bubbly?" Another post example was crafted to encourage fans to hit the "like" button and featured two cars, sporting false eyelashes which read, "Bet you the lady car uses Latisse – hit your LIKE button if you do too!" Both questions created a spike in traffic, engagement rate, and conversation on her client’s Facebook page, she said. The latter resulted in a reach of over 10,000 people and an engagement rate of over 11% (2% is average).
"You’re trying to create an emotional connection with your Facebook followers," Ms. Ramsey said. "When you’re doing your job right, you will. You will feel like a friend to people. You want people to feel like they’re part of a community. You want to be a resource for them. You want to share your own content as well as other people’s content. Social media is very reciprocal, so give a little and get a lot."
Other tips she shared for optimizing a practice’s Facebook page include the following:
• Be yourself. "Credibility and trust are important," Ms. Ramsey said. "Be authentic. Be humble. Think about influencing conversations, not controlling conversations. You can influence behavior but you don’t have to control it."
• Change your cover image at least monthly. That’s because cover images "get, on average, nine times more engagement than a regular post," she said. "Celebrate your 100th fan, or use this space to advertise a promotion you have going on."
• Make sure your avatar is superb. Ninety percent of Facebook users see your content in their newsfeed, "so it really needs to be good and easy to see," she said. "People prefer to hear from a face of the practice rather than a logo or building because we humans communicate with people, not logos."
• Monitor replies to your posts. If someone is sending abusive messages, "you can hide those messages and block that person from your page. Or it might be better to leave it there and let your community come to your defense on your behalf. I have only had to take down two messages on behalf of clients. Both were from competing physicians."
Ms. Ramsey is the founder of Cosmetic Social Media and had no other financial conflicts to disclose.
LAS VEGAS – Hoping to make your practice’s Facebook page a success? Engage with your Facebook followers.
According to Monique Ramsey, founder of Del Mar, Calif.–based Cosmetic Social Media, the best path to social media success involves fostering. "Think about who your consumer is and what she wants to learn about," she advised.
"Provide that information to her and let her share it with her friends, because then your name is attached to that piece of content. And note it may not have anything to do with your cosmetic surgery practice, but this kind of a post will keep people coming to your page and get higher visibility for your posts in the newsfeed," she added.
The goal is to have your posts reach more than the Facebook average, which is about 16% of your Facebook followers. With more than 1 billion people using Facebook, physicians need to be able to "cut through the noise," Ms. Ramsey explained at the annual meeting of the American Academy of Cosmetic Surgery.
It’s okay for 20% of your posts to be about your practice – perhaps advertising special events and promotions – but the remaining 80% should be informative and off the topic yet engaging, meaningful, and fun.
For example, Ms. Ramsey crafted questions for one of her client’s Facebook page intended to trigger engagement in the form of comments. One read "It’s National Wine Day. Are you a red, white or bubbly?" Another post example was crafted to encourage fans to hit the "like" button and featured two cars, sporting false eyelashes which read, "Bet you the lady car uses Latisse – hit your LIKE button if you do too!" Both questions created a spike in traffic, engagement rate, and conversation on her client’s Facebook page, she said. The latter resulted in a reach of over 10,000 people and an engagement rate of over 11% (2% is average).
"You’re trying to create an emotional connection with your Facebook followers," Ms. Ramsey said. "When you’re doing your job right, you will. You will feel like a friend to people. You want people to feel like they’re part of a community. You want to be a resource for them. You want to share your own content as well as other people’s content. Social media is very reciprocal, so give a little and get a lot."
Other tips she shared for optimizing a practice’s Facebook page include the following:
• Be yourself. "Credibility and trust are important," Ms. Ramsey said. "Be authentic. Be humble. Think about influencing conversations, not controlling conversations. You can influence behavior but you don’t have to control it."
• Change your cover image at least monthly. That’s because cover images "get, on average, nine times more engagement than a regular post," she said. "Celebrate your 100th fan, or use this space to advertise a promotion you have going on."
• Make sure your avatar is superb. Ninety percent of Facebook users see your content in their newsfeed, "so it really needs to be good and easy to see," she said. "People prefer to hear from a face of the practice rather than a logo or building because we humans communicate with people, not logos."
• Monitor replies to your posts. If someone is sending abusive messages, "you can hide those messages and block that person from your page. Or it might be better to leave it there and let your community come to your defense on your behalf. I have only had to take down two messages on behalf of clients. Both were from competing physicians."
Ms. Ramsey is the founder of Cosmetic Social Media and had no other financial conflicts to disclose.
LAS VEGAS – Hoping to make your practice’s Facebook page a success? Engage with your Facebook followers.
According to Monique Ramsey, founder of Del Mar, Calif.–based Cosmetic Social Media, the best path to social media success involves fostering. "Think about who your consumer is and what she wants to learn about," she advised.
"Provide that information to her and let her share it with her friends, because then your name is attached to that piece of content. And note it may not have anything to do with your cosmetic surgery practice, but this kind of a post will keep people coming to your page and get higher visibility for your posts in the newsfeed," she added.
The goal is to have your posts reach more than the Facebook average, which is about 16% of your Facebook followers. With more than 1 billion people using Facebook, physicians need to be able to "cut through the noise," Ms. Ramsey explained at the annual meeting of the American Academy of Cosmetic Surgery.
It’s okay for 20% of your posts to be about your practice – perhaps advertising special events and promotions – but the remaining 80% should be informative and off the topic yet engaging, meaningful, and fun.
For example, Ms. Ramsey crafted questions for one of her client’s Facebook page intended to trigger engagement in the form of comments. One read "It’s National Wine Day. Are you a red, white or bubbly?" Another post example was crafted to encourage fans to hit the "like" button and featured two cars, sporting false eyelashes which read, "Bet you the lady car uses Latisse – hit your LIKE button if you do too!" Both questions created a spike in traffic, engagement rate, and conversation on her client’s Facebook page, she said. The latter resulted in a reach of over 10,000 people and an engagement rate of over 11% (2% is average).
"You’re trying to create an emotional connection with your Facebook followers," Ms. Ramsey said. "When you’re doing your job right, you will. You will feel like a friend to people. You want people to feel like they’re part of a community. You want to be a resource for them. You want to share your own content as well as other people’s content. Social media is very reciprocal, so give a little and get a lot."
Other tips she shared for optimizing a practice’s Facebook page include the following:
• Be yourself. "Credibility and trust are important," Ms. Ramsey said. "Be authentic. Be humble. Think about influencing conversations, not controlling conversations. You can influence behavior but you don’t have to control it."
• Change your cover image at least monthly. That’s because cover images "get, on average, nine times more engagement than a regular post," she said. "Celebrate your 100th fan, or use this space to advertise a promotion you have going on."
• Make sure your avatar is superb. Ninety percent of Facebook users see your content in their newsfeed, "so it really needs to be good and easy to see," she said. "People prefer to hear from a face of the practice rather than a logo or building because we humans communicate with people, not logos."
• Monitor replies to your posts. If someone is sending abusive messages, "you can hide those messages and block that person from your page. Or it might be better to leave it there and let your community come to your defense on your behalf. I have only had to take down two messages on behalf of clients. Both were from competing physicians."
Ms. Ramsey is the founder of Cosmetic Social Media and had no other financial conflicts to disclose.
EXPERT ANALYSIS FROM THE AACS ANNUAL MEETING
Unresponsive Woman Extricated From Car
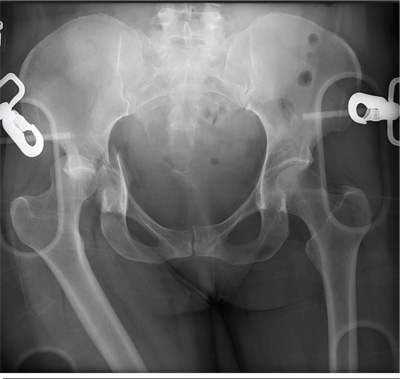
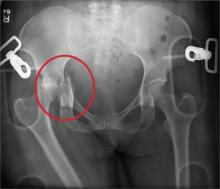
An unidentified female is brought in as a trauma code. She was apparently in a motor vehicle crash, the specific details of which are unclear. Emergency medical personnel describe extensive damage to her vehicle, which resulted in a pro-longed extrication time. Due to unresponsiveness at the scene, she was intubated in the field. The patient is probably in her late 30s to early 40s. She has two large-bore IV lines with normal saline infusing at a wide-open rate. Blood pressure is 90/60 mm Hg and heart rate, 140 beats/min. She has several superficial lacerations on her head, her pupils are fixed and dilated, and there is minimal withdrawal to pain in her extremities. No other trauma is im-mediately evident. Portable radiographs of her chest and pelvis are obtained prior to sending her for CT. Pelvis radiograph is shown. What is your impression?
After 15 Years, Still Losing Hair, Only Faster
ANSWER
This is a classic clinical picture of androgenetic alopecia (choice “a”). See discussion for more details.
Alopecia areata (choice “b”) usually manifests acutely and leads to complete hair loss in a well-defined, annular pattern. It typically resolves on its own, with or without treatment.
Telogen effluvium (choice “c”) involves generalized hair loss without a pattern. The hair is actually “lost,” meaning markedly increased amounts of hair are seen in the comb, brush, sink, or shower. This results in an increasingly visible scalp.
Without a clear clinical picture of alopecia, a biopsy might have been indicated—primarily to rule out conditions such as lupus erythematosus (choice “d”), which can involve hair loss of various kinds. The negative ANA result obtained by the patient’s primary care provider helped rule out this diagnosis.
DISCUSSION
Androgenetic alopecia (AGA) affects both men and women, though the latter begin to develop it about 10 years later, on average, than men do. Among women, 13% develop AGA before menopause, while 75% note its appearance postmenopausally.
In both sexes, AGA results from the gradual conversion of terminal hairs to vellus hairs, with miniaturization of the follicles. Hair loss in men starts in the vertex, followed by bitemporal recession. In women, AGA primarily affects the crown of the scalp, often with partial preservation of the frontal hairline.
Dihydrotestosterone (DHT) appears to be the main culprit; testosterone is converted to DHT by means of the enzyme 5α-reductase. One of the most effective medications for AGA in men has been finasteride, which blocks the effects of 5α-reductase and can at least slow the rate of hair loss. Unfortunately, finasteride does not appear to be effective in treating AGA in women.
Women do, however, appear to respond to minoxidil, a topically applied solution, better than men. The response is moderate at best, and any hair gained is lost if the treatment is discontinued. Interestingly, the stronger 5% solution of minoxidil in women does not produce any demonstrable improvement over that seen with the 2% solution.
From a practical diagnostic standpoint, it is quite common for women with longstanding mild to moderate AGA to present with an acute episode of telogen effluvium (TE), in which hair all over the scalp falls out. Careful history taking is necessary to tease these stories apart, since TE will typically resolve on its own. The most common causes of TE, in my experience, are stress, extreme weight loss, and as a consequence of general anesthesia. For unknown reasons, TE is almost nonexistent in men.
TREATMENT
This patient chose to use 5% OTC minoxidil, an antihypertensive with an unknown mode of action in AGA. She’ll confine its application to the affected areas of the scalp, since unwanted hair growth has been reported on the face with the use of this medication.
ANSWER
This is a classic clinical picture of androgenetic alopecia (choice “a”). See discussion for more details.
Alopecia areata (choice “b”) usually manifests acutely and leads to complete hair loss in a well-defined, annular pattern. It typically resolves on its own, with or without treatment.
Telogen effluvium (choice “c”) involves generalized hair loss without a pattern. The hair is actually “lost,” meaning markedly increased amounts of hair are seen in the comb, brush, sink, or shower. This results in an increasingly visible scalp.
Without a clear clinical picture of alopecia, a biopsy might have been indicated—primarily to rule out conditions such as lupus erythematosus (choice “d”), which can involve hair loss of various kinds. The negative ANA result obtained by the patient’s primary care provider helped rule out this diagnosis.
DISCUSSION
Androgenetic alopecia (AGA) affects both men and women, though the latter begin to develop it about 10 years later, on average, than men do. Among women, 13% develop AGA before menopause, while 75% note its appearance postmenopausally.
In both sexes, AGA results from the gradual conversion of terminal hairs to vellus hairs, with miniaturization of the follicles. Hair loss in men starts in the vertex, followed by bitemporal recession. In women, AGA primarily affects the crown of the scalp, often with partial preservation of the frontal hairline.
Dihydrotestosterone (DHT) appears to be the main culprit; testosterone is converted to DHT by means of the enzyme 5α-reductase. One of the most effective medications for AGA in men has been finasteride, which blocks the effects of 5α-reductase and can at least slow the rate of hair loss. Unfortunately, finasteride does not appear to be effective in treating AGA in women.
Women do, however, appear to respond to minoxidil, a topically applied solution, better than men. The response is moderate at best, and any hair gained is lost if the treatment is discontinued. Interestingly, the stronger 5% solution of minoxidil in women does not produce any demonstrable improvement over that seen with the 2% solution.
From a practical diagnostic standpoint, it is quite common for women with longstanding mild to moderate AGA to present with an acute episode of telogen effluvium (TE), in which hair all over the scalp falls out. Careful history taking is necessary to tease these stories apart, since TE will typically resolve on its own. The most common causes of TE, in my experience, are stress, extreme weight loss, and as a consequence of general anesthesia. For unknown reasons, TE is almost nonexistent in men.
TREATMENT
This patient chose to use 5% OTC minoxidil, an antihypertensive with an unknown mode of action in AGA. She’ll confine its application to the affected areas of the scalp, since unwanted hair growth has been reported on the face with the use of this medication.
ANSWER
This is a classic clinical picture of androgenetic alopecia (choice “a”). See discussion for more details.
Alopecia areata (choice “b”) usually manifests acutely and leads to complete hair loss in a well-defined, annular pattern. It typically resolves on its own, with or without treatment.
Telogen effluvium (choice “c”) involves generalized hair loss without a pattern. The hair is actually “lost,” meaning markedly increased amounts of hair are seen in the comb, brush, sink, or shower. This results in an increasingly visible scalp.
Without a clear clinical picture of alopecia, a biopsy might have been indicated—primarily to rule out conditions such as lupus erythematosus (choice “d”), which can involve hair loss of various kinds. The negative ANA result obtained by the patient’s primary care provider helped rule out this diagnosis.
DISCUSSION
Androgenetic alopecia (AGA) affects both men and women, though the latter begin to develop it about 10 years later, on average, than men do. Among women, 13% develop AGA before menopause, while 75% note its appearance postmenopausally.
In both sexes, AGA results from the gradual conversion of terminal hairs to vellus hairs, with miniaturization of the follicles. Hair loss in men starts in the vertex, followed by bitemporal recession. In women, AGA primarily affects the crown of the scalp, often with partial preservation of the frontal hairline.
Dihydrotestosterone (DHT) appears to be the main culprit; testosterone is converted to DHT by means of the enzyme 5α-reductase. One of the most effective medications for AGA in men has been finasteride, which blocks the effects of 5α-reductase and can at least slow the rate of hair loss. Unfortunately, finasteride does not appear to be effective in treating AGA in women.
Women do, however, appear to respond to minoxidil, a topically applied solution, better than men. The response is moderate at best, and any hair gained is lost if the treatment is discontinued. Interestingly, the stronger 5% solution of minoxidil in women does not produce any demonstrable improvement over that seen with the 2% solution.
From a practical diagnostic standpoint, it is quite common for women with longstanding mild to moderate AGA to present with an acute episode of telogen effluvium (TE), in which hair all over the scalp falls out. Careful history taking is necessary to tease these stories apart, since TE will typically resolve on its own. The most common causes of TE, in my experience, are stress, extreme weight loss, and as a consequence of general anesthesia. For unknown reasons, TE is almost nonexistent in men.
TREATMENT
This patient chose to use 5% OTC minoxidil, an antihypertensive with an unknown mode of action in AGA. She’ll confine its application to the affected areas of the scalp, since unwanted hair growth has been reported on the face with the use of this medication.
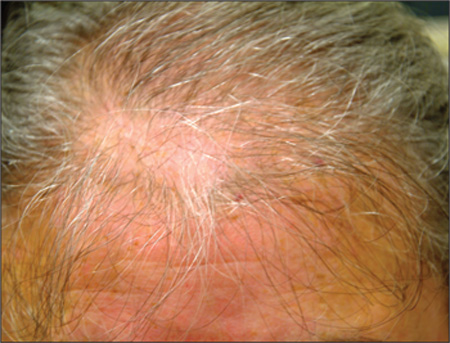
A 43-year-old woman presents to dermatology with the extremely common complaint of hair loss. The problem is not new; she first noticed it 15 years ago. But the loss has now progressed to such an extent that the patient consulted her primary care provider. Blood tests were ordered, including complete blood count, antinuclear antibody (ANA), and thy-roid-stimulating hormone; all results were within normal limits. And so she decided to seek a specialist’s assessment. The patient is going through menopause—without the aid of medication—and claims to be otherwise healthy. She denies finding increased amounts of lost hair in her comb, brush, shower, or sink. She further denies any symptoms in her scalp. Her mother and one sister had similar problems with their scalp hair. Examination reveals extensive thinning of hair, which is almost totally confined to the crown of her scalp, with faint but obvious preservation of a thin band of the frontal hairline. There is no appreciable disruption of the skin surface in the scalp (eg, scaling, redness, edema, or scarring).
Where do people want to die?
Where do you want to die? Strange question, indeed, and one most of us would rather not think about, but one day we all will take our final breath and pass on from life as we know it.
While most people died at home at the turn of the 20th century, by the 1960s, more than two-thirds of deaths occurred in institutions. The birth of the hospice movement in the 1970s did swing the pendulum somewhat back toward death at home, the place preferred by the vast majority of people surveyed, but most people still die in an institution setting, according to an article in April issue of Journal of Hospital Medicine called "Where do you want to spend your last days of life? Low concordance between preferred and actual site of death among hospitalized adults."
"In this observational study of 458 ethnically diverse, mostly male patients of low socioeconomic status, the vast majority (75%) expressed their desire to pass away at home, 10% wanted to spend their last days in a hospital setting, 6% preferred a nursing home, and 4% wanted to die while in an inpatient hospice facility. The remaining 5% either had no preference or refused to answer (J. Hosp. Med. 2013 April;8:178-83).
During the period of this study, 123 participants died. Unfortunately, only 37% died where wanted to.
The dying process is a painful reality that affects not only the patient, but his or her entire family as well. This topic has been discussed in the medical literature for decades and rightly so. A 1984 article in the New England Journal of Medicine, "The physician’s responsibility toward hopelessly ill patients: A second look," addressed issues that are just as relevant today as they were decades ago. For instance, when physicians discuss life-threatening illnesses, are patients capable of truly accepting and processing the information? How much information should we give? What is the optimal timing for telling patients they are terminally ill and how do we give provide this devastating information in a compassionate manner that will not make them give up all hope? (N. Engl. J. Med. 1984; 310:955-9)
These and other questions commonly plague busy physicians. Nevertheless, if the results of the most recent study can be extrapolated to the population at large, and the majority of patients are not able to spend their last days where they choose, perhaps we as hospitalists can help swing the pendulum back in their favor by having the hard conversations with patients and their families earlier. Consulting social workers, case managers, and even hospice coordinators early in the process also can help patients and their families take important steps to plan for the final days and improve patients’ chances of actually passing away in the place where they feel most comfortable and least stressed.
The final days of life are very precious. We owe it to our patients to make them as happy and carefree as possible.
Dr. Hester is a hospitalist with Baltimore-Washington Medical Center, Glen Burnie, Md., who has a passion for empowering patients to partner in their health care. She is the creator of the Patient Whiz, a mobile app for iOS.
Where do you want to die? Strange question, indeed, and one most of us would rather not think about, but one day we all will take our final breath and pass on from life as we know it.
While most people died at home at the turn of the 20th century, by the 1960s, more than two-thirds of deaths occurred in institutions. The birth of the hospice movement in the 1970s did swing the pendulum somewhat back toward death at home, the place preferred by the vast majority of people surveyed, but most people still die in an institution setting, according to an article in April issue of Journal of Hospital Medicine called "Where do you want to spend your last days of life? Low concordance between preferred and actual site of death among hospitalized adults."
"In this observational study of 458 ethnically diverse, mostly male patients of low socioeconomic status, the vast majority (75%) expressed their desire to pass away at home, 10% wanted to spend their last days in a hospital setting, 6% preferred a nursing home, and 4% wanted to die while in an inpatient hospice facility. The remaining 5% either had no preference or refused to answer (J. Hosp. Med. 2013 April;8:178-83).
During the period of this study, 123 participants died. Unfortunately, only 37% died where wanted to.
The dying process is a painful reality that affects not only the patient, but his or her entire family as well. This topic has been discussed in the medical literature for decades and rightly so. A 1984 article in the New England Journal of Medicine, "The physician’s responsibility toward hopelessly ill patients: A second look," addressed issues that are just as relevant today as they were decades ago. For instance, when physicians discuss life-threatening illnesses, are patients capable of truly accepting and processing the information? How much information should we give? What is the optimal timing for telling patients they are terminally ill and how do we give provide this devastating information in a compassionate manner that will not make them give up all hope? (N. Engl. J. Med. 1984; 310:955-9)
These and other questions commonly plague busy physicians. Nevertheless, if the results of the most recent study can be extrapolated to the population at large, and the majority of patients are not able to spend their last days where they choose, perhaps we as hospitalists can help swing the pendulum back in their favor by having the hard conversations with patients and their families earlier. Consulting social workers, case managers, and even hospice coordinators early in the process also can help patients and their families take important steps to plan for the final days and improve patients’ chances of actually passing away in the place where they feel most comfortable and least stressed.
The final days of life are very precious. We owe it to our patients to make them as happy and carefree as possible.
Dr. Hester is a hospitalist with Baltimore-Washington Medical Center, Glen Burnie, Md., who has a passion for empowering patients to partner in their health care. She is the creator of the Patient Whiz, a mobile app for iOS.
Where do you want to die? Strange question, indeed, and one most of us would rather not think about, but one day we all will take our final breath and pass on from life as we know it.
While most people died at home at the turn of the 20th century, by the 1960s, more than two-thirds of deaths occurred in institutions. The birth of the hospice movement in the 1970s did swing the pendulum somewhat back toward death at home, the place preferred by the vast majority of people surveyed, but most people still die in an institution setting, according to an article in April issue of Journal of Hospital Medicine called "Where do you want to spend your last days of life? Low concordance between preferred and actual site of death among hospitalized adults."
"In this observational study of 458 ethnically diverse, mostly male patients of low socioeconomic status, the vast majority (75%) expressed their desire to pass away at home, 10% wanted to spend their last days in a hospital setting, 6% preferred a nursing home, and 4% wanted to die while in an inpatient hospice facility. The remaining 5% either had no preference or refused to answer (J. Hosp. Med. 2013 April;8:178-83).
During the period of this study, 123 participants died. Unfortunately, only 37% died where wanted to.
The dying process is a painful reality that affects not only the patient, but his or her entire family as well. This topic has been discussed in the medical literature for decades and rightly so. A 1984 article in the New England Journal of Medicine, "The physician’s responsibility toward hopelessly ill patients: A second look," addressed issues that are just as relevant today as they were decades ago. For instance, when physicians discuss life-threatening illnesses, are patients capable of truly accepting and processing the information? How much information should we give? What is the optimal timing for telling patients they are terminally ill and how do we give provide this devastating information in a compassionate manner that will not make them give up all hope? (N. Engl. J. Med. 1984; 310:955-9)
These and other questions commonly plague busy physicians. Nevertheless, if the results of the most recent study can be extrapolated to the population at large, and the majority of patients are not able to spend their last days where they choose, perhaps we as hospitalists can help swing the pendulum back in their favor by having the hard conversations with patients and their families earlier. Consulting social workers, case managers, and even hospice coordinators early in the process also can help patients and their families take important steps to plan for the final days and improve patients’ chances of actually passing away in the place where they feel most comfortable and least stressed.
The final days of life are very precious. We owe it to our patients to make them as happy and carefree as possible.
Dr. Hester is a hospitalist with Baltimore-Washington Medical Center, Glen Burnie, Md., who has a passion for empowering patients to partner in their health care. She is the creator of the Patient Whiz, a mobile app for iOS.
No benefit of endovascular therapy added to TPA for stroke
Functional outcomes in patients treated with intravenous tissue plasminogen activator with or without endovascular therapy after a moderate to severe acute ischemic stroke were not significantly different, and safety outcomes were similar, in a study that was stopped early because of these results.
In the IMS (Interventional Management of Stroke) III study, 40.8% of patients randomized to receive endovascular therapy plus intravenous TPA met the primary endpoint, a measure of functional independence -- a modified Rankin score of 2 or less at 90 days -- compared with 38.7% among those who had intravenous TPA alone, a difference that was not statistically significant, reported Dr. Joseph Broderick of the University of Cincinnati Neuroscience Institute, and the other IMS III investigators.
Mortality and other safety outcomes were also not significantly different between the two groups of patients in the study, which was stopped early because of futility after 656 of the planned 900 patients had been randomized.
The study was published online to coincide with the presentation of the results at the International Stroke Conference (N. Engl. J. Med. 2013 [doi:10.1056/NEJMoa1214300]).
Referring to the lack of randomized clinical trial data, the authors pointed out that it is uncertain whether endovascular therapy (which includes endovascular pharmacologic thrombolysis and, more recently, the use of stent retrievers) alone or combined with intravenous TPA is a more effective treatment of acute stroke than intravenous TPA alone, "the only proven reperfusion therapy for acute ischemic stroke."
In the study, conducted at 58 centers in the United States, Canada, Australia, and Europe, 434 patients were randomized to endovascular therapy plus intravenous TPA and 222 were randomized to standard treatment with intravenous TPA alone (started within 3 hours of stroke onset). The median age of those enrolled was 68-69 years (range, 23-89 years), a little over half were men, about 14% were black or Hispanic, and the median the National Institute of Health Stroke Scale (NIHSS) score was 16-17 (8-19 is a moderately severe stroke and 20 or greater is a severe stroke At the beginning of the study, only one thrombectomy device had been cleared by the Food and Drug Administration and, as the trial continued, other devices were used as they became cleared for use in the different countries.
In addition to the main finding, there were no differences in the primary outcome among those patients with an NIHSS score of 20 or more, and those with a score of 19 or lower, said the authors, who had hypothesized that endovascular therapy would have greater efficacy in patients with more-severe strokes since they "have the highest likelihood of occlusion in a major intracranial artery and the greatest volume of ischemic brain at risk."
They had also hypothesized that receiving endovascular therapy earlier would be associated with a greater benefit, but this was also not a significant factor in outcomes.
Mortality at 90 days was 19.1% in the endovascular therapy group and 21.6% in the intravenous TPA–alone group. Within 30 hours of TPA initiation, 6.2% of those on endovascular therapy and 5.9% of those on TPA alone had a symptomatic intracerebral hemorrhage. The differences in mortality at 7 days and in parenchymal hematoma rates were also not significantly different between the two groups. The rate of asymptomatic intracerebral hemorrhage, however, was significantly higher in the endovascular group.
Outcomes consistently trended better with combined therapy in patients with strokes involving larger artery occlusions and those with the shortest times from stroke onset to initiation of treatment, although because of small patient numbers the differences didn’t achieve statistical significance. These will be the subgroups that ought to be the focus of future clinical trials, Dr. Broderick said in a press briefing at the conference.
The underlying rationale for combined therapy is that intravenous TPA can quickly be started in the emergency department while the endovascular device therapy team is assembling, often at another hospital, which entails time-consuming patient transfer.
Intravenous TPA is the only proven therapy for acute ischemic stroke, but endovascular therapy is more effective at achieving recanalization. The study results bore this out: for example, the rate of partial or complete recanalization at 24 hours for an occlusion in the internal carotid artery was 81% with combined therapy compared to 35% with intravenous TPA alone. Yet this higher recanalization rate bore no clinical benefit, possibly because recanalization occurred too late, after ischemia had turned into infarction, Dr. Broderick explained.
"IMS III is going to be disappointing for a lot of people who are proponents of endovascular therapy. However, there is a light at the end of the tunnel in that there are these subgroups who may benefit," Dr. Brian Silver, who was not involved in the trial, said in an interview.
"The most critical feature is to treat the patients as soon as possible when they arrive in the emergency department, perhaps within 90 minutes. I think that’s the best chance for recovery. We are nowhere near what’s being done in cardiology, where there are door-to-balloon times of an hour. We need to do that in stroke. Since we're dealing with an organ that's more sensitive than the heart to ischemia, we probably need to be even faster than what's being done in cardiology. There is definitely room for improvement in our systems, perhaps by having the endovascular team stay in the hospital. Expense will be the limitation," according to Dr. Silver, director of the stroke center at Brown University, Providence, R.I.
IMS III investigator and interventional neuroradiologist Dr. Thomas A. Tomsick said in an interview that the study results won't change his own clinical practice.
"IMS III is by no means the final word on combined therapy. In Cincinnati tomorrow, if a patient with a large NIH Stroke Severity score shows up and we're treating him with IV TPA at 2 hours from stroke onset, we're not going to do a CT angiogram to evaluate that patient. He's going to the cath lab for angiography to see if there's a clot suitable for endovascular therapy," said Dr. Tomsick, professor of radiology at the University of Cincinnati.
Five different endovascular device therapies were utilized in IMS III. As new devices reached clinical practice, their use was allowed by investigators in order to keep the randomized trial clinically relevant. But recruitment for the study was slow because so many clinicians were already convinced by anecdotal experience that combined therapy is better. So the endovascular therapies used most frequently in IMS III aren't the ones widely used in clinical practice today. Major new randomized trials are now getting underway comparing combined therapy using state-of-the-art, more effective stent clot retriever devices to intravenous TPA alone, he added.
In the New England Journal of Medicine report, the authors noted that "the use of randomization in ongoing and future stroke trials, rather than the treatment of eligible patients with endovascular therapy outside any trial, and minimization of the time to treatment will be essential for assessing the potential benefit of endovascular therapy for acute ischemic stroke."
No matter how future trials of combined therapy turn out, endovascular therapy is not going away, Dr. Broderick observed.
"It's a very good tool. The reason why is there are patients who can't get TPA. For example, roughly 5% of patients who undergo coronary artery bypass surgery have a stroke. If you have somebody with a big stroke 2 days after having their chest cracked, you can't use TPA. In that case, those endovascular devices are the way we can get up in there and get rid of the clot," he explained.
In an editorial accompanying the report in the New England Journal of Medicine (doi: 10.1056/NEJMe1215730), Dr. Marc I. Chimowitz declared that the clinical implication of IMS III is that endovascular therapy remains unproven and intravenous TPA should continue to be the first-line treatment for patients with acute ischemic stroke within 4.5 hours after stroke onset.
While new clinical trials featuring more effective IV clot busters, such as tenecteplase, and next-generation endovascular devices are urgently needed in an effort to improve stroke outcomes, patient recruitment is likely to continue to be a challenge in the current environment. This could be overcome if Medicare were to place a moratorium on reimbursement for endovascular therapy of acute ischemic stroke except as part of a randomized trial, according to Dr. Chimowitz, professor of neurology at the Medical University of South Carolina, Charleston.
The study was supported with grants from NIH and the National Institute of Neurological Disorders and Stroke; and by Genentech (which supplied the TPA); and EKOS, Concentric Medical, and Cordis Neurovascular (which supplied catheters); and Actilyse (alteplase) manufacturer Boehringer Ingelheim (which, along with Genentech and EKOS, provided support for investigator meetings). Dr. Broderick disclosed consulting fees from PhotoThera. Of the 28 other authors, disclosures for 14 were listed and included having received consulting fees, grant support, and/or lecture fees from a variety of device and pharmaceutical companies that include Genentech. Dr. Chimowitz, Dr. Silver, and Dr. Tomsick reported having no financial conflicts.
The key to understanding the results of this study is the difference in the recanalization rates between the two groups and their lack of relationship to outcome. In the IV TPA plus catheter directed TPA group, the recanalization rate was higher than in the IV alone group, but the clinical outcomes were not better. Undoubtedly this is due to the fact that the recanalization was accomplished after brain tissue death had already occurred.
Hence the next and critical question is....what would the result be if we could administer the catheter directed TPA in a timely fashion, as is done with acute myocardial infarction? If the study had been performed in centers where this therapy was available without delay, would the results have been different? I think almost certainly. While it is true that this therapy is not available as widely as coronary interventions, it is critical for us to know whether patients who are fortunate enough to be treated in an institution where the therapy is available should receive it. Another disappointing aspect of the study design was the inability to perform subgroup analysis. While it appeared that patients with larger stroke distribution might benefit, the study was apparently not powered to detect this difference. Lastly, it is puzzling that the investigators themselves do not appear to believe the results of their own study, with two of them indicating the results would not change their own practice. If that's the case, why spend all this time and money designing a trial that doesn't answer the questions?
Dr. Cynthia K. Shortell is Professor and Chief, Division of Vascular Surgery, Duke University Medical Center, and an associate medical editor for Vascular Specialist.
The key to understanding the results of this study is the difference in the recanalization rates between the two groups and their lack of relationship to outcome. In the IV TPA plus catheter directed TPA group, the recanalization rate was higher than in the IV alone group, but the clinical outcomes were not better. Undoubtedly this is due to the fact that the recanalization was accomplished after brain tissue death had already occurred.
Hence the next and critical question is....what would the result be if we could administer the catheter directed TPA in a timely fashion, as is done with acute myocardial infarction? If the study had been performed in centers where this therapy was available without delay, would the results have been different? I think almost certainly. While it is true that this therapy is not available as widely as coronary interventions, it is critical for us to know whether patients who are fortunate enough to be treated in an institution where the therapy is available should receive it. Another disappointing aspect of the study design was the inability to perform subgroup analysis. While it appeared that patients with larger stroke distribution might benefit, the study was apparently not powered to detect this difference. Lastly, it is puzzling that the investigators themselves do not appear to believe the results of their own study, with two of them indicating the results would not change their own practice. If that's the case, why spend all this time and money designing a trial that doesn't answer the questions?
Dr. Cynthia K. Shortell is Professor and Chief, Division of Vascular Surgery, Duke University Medical Center, and an associate medical editor for Vascular Specialist.
The key to understanding the results of this study is the difference in the recanalization rates between the two groups and their lack of relationship to outcome. In the IV TPA plus catheter directed TPA group, the recanalization rate was higher than in the IV alone group, but the clinical outcomes were not better. Undoubtedly this is due to the fact that the recanalization was accomplished after brain tissue death had already occurred.
Hence the next and critical question is....what would the result be if we could administer the catheter directed TPA in a timely fashion, as is done with acute myocardial infarction? If the study had been performed in centers where this therapy was available without delay, would the results have been different? I think almost certainly. While it is true that this therapy is not available as widely as coronary interventions, it is critical for us to know whether patients who are fortunate enough to be treated in an institution where the therapy is available should receive it. Another disappointing aspect of the study design was the inability to perform subgroup analysis. While it appeared that patients with larger stroke distribution might benefit, the study was apparently not powered to detect this difference. Lastly, it is puzzling that the investigators themselves do not appear to believe the results of their own study, with two of them indicating the results would not change their own practice. If that's the case, why spend all this time and money designing a trial that doesn't answer the questions?
Dr. Cynthia K. Shortell is Professor and Chief, Division of Vascular Surgery, Duke University Medical Center, and an associate medical editor for Vascular Specialist.
Functional outcomes in patients treated with intravenous tissue plasminogen activator with or without endovascular therapy after a moderate to severe acute ischemic stroke were not significantly different, and safety outcomes were similar, in a study that was stopped early because of these results.
In the IMS (Interventional Management of Stroke) III study, 40.8% of patients randomized to receive endovascular therapy plus intravenous TPA met the primary endpoint, a measure of functional independence -- a modified Rankin score of 2 or less at 90 days -- compared with 38.7% among those who had intravenous TPA alone, a difference that was not statistically significant, reported Dr. Joseph Broderick of the University of Cincinnati Neuroscience Institute, and the other IMS III investigators.
Mortality and other safety outcomes were also not significantly different between the two groups of patients in the study, which was stopped early because of futility after 656 of the planned 900 patients had been randomized.
The study was published online to coincide with the presentation of the results at the International Stroke Conference (N. Engl. J. Med. 2013 [doi:10.1056/NEJMoa1214300]).
Referring to the lack of randomized clinical trial data, the authors pointed out that it is uncertain whether endovascular therapy (which includes endovascular pharmacologic thrombolysis and, more recently, the use of stent retrievers) alone or combined with intravenous TPA is a more effective treatment of acute stroke than intravenous TPA alone, "the only proven reperfusion therapy for acute ischemic stroke."
In the study, conducted at 58 centers in the United States, Canada, Australia, and Europe, 434 patients were randomized to endovascular therapy plus intravenous TPA and 222 were randomized to standard treatment with intravenous TPA alone (started within 3 hours of stroke onset). The median age of those enrolled was 68-69 years (range, 23-89 years), a little over half were men, about 14% were black or Hispanic, and the median the National Institute of Health Stroke Scale (NIHSS) score was 16-17 (8-19 is a moderately severe stroke and 20 or greater is a severe stroke At the beginning of the study, only one thrombectomy device had been cleared by the Food and Drug Administration and, as the trial continued, other devices were used as they became cleared for use in the different countries.
In addition to the main finding, there were no differences in the primary outcome among those patients with an NIHSS score of 20 or more, and those with a score of 19 or lower, said the authors, who had hypothesized that endovascular therapy would have greater efficacy in patients with more-severe strokes since they "have the highest likelihood of occlusion in a major intracranial artery and the greatest volume of ischemic brain at risk."
They had also hypothesized that receiving endovascular therapy earlier would be associated with a greater benefit, but this was also not a significant factor in outcomes.
Mortality at 90 days was 19.1% in the endovascular therapy group and 21.6% in the intravenous TPA–alone group. Within 30 hours of TPA initiation, 6.2% of those on endovascular therapy and 5.9% of those on TPA alone had a symptomatic intracerebral hemorrhage. The differences in mortality at 7 days and in parenchymal hematoma rates were also not significantly different between the two groups. The rate of asymptomatic intracerebral hemorrhage, however, was significantly higher in the endovascular group.
Outcomes consistently trended better with combined therapy in patients with strokes involving larger artery occlusions and those with the shortest times from stroke onset to initiation of treatment, although because of small patient numbers the differences didn’t achieve statistical significance. These will be the subgroups that ought to be the focus of future clinical trials, Dr. Broderick said in a press briefing at the conference.
The underlying rationale for combined therapy is that intravenous TPA can quickly be started in the emergency department while the endovascular device therapy team is assembling, often at another hospital, which entails time-consuming patient transfer.
Intravenous TPA is the only proven therapy for acute ischemic stroke, but endovascular therapy is more effective at achieving recanalization. The study results bore this out: for example, the rate of partial or complete recanalization at 24 hours for an occlusion in the internal carotid artery was 81% with combined therapy compared to 35% with intravenous TPA alone. Yet this higher recanalization rate bore no clinical benefit, possibly because recanalization occurred too late, after ischemia had turned into infarction, Dr. Broderick explained.
"IMS III is going to be disappointing for a lot of people who are proponents of endovascular therapy. However, there is a light at the end of the tunnel in that there are these subgroups who may benefit," Dr. Brian Silver, who was not involved in the trial, said in an interview.
"The most critical feature is to treat the patients as soon as possible when they arrive in the emergency department, perhaps within 90 minutes. I think that’s the best chance for recovery. We are nowhere near what’s being done in cardiology, where there are door-to-balloon times of an hour. We need to do that in stroke. Since we're dealing with an organ that's more sensitive than the heart to ischemia, we probably need to be even faster than what's being done in cardiology. There is definitely room for improvement in our systems, perhaps by having the endovascular team stay in the hospital. Expense will be the limitation," according to Dr. Silver, director of the stroke center at Brown University, Providence, R.I.
IMS III investigator and interventional neuroradiologist Dr. Thomas A. Tomsick said in an interview that the study results won't change his own clinical practice.
"IMS III is by no means the final word on combined therapy. In Cincinnati tomorrow, if a patient with a large NIH Stroke Severity score shows up and we're treating him with IV TPA at 2 hours from stroke onset, we're not going to do a CT angiogram to evaluate that patient. He's going to the cath lab for angiography to see if there's a clot suitable for endovascular therapy," said Dr. Tomsick, professor of radiology at the University of Cincinnati.
Five different endovascular device therapies were utilized in IMS III. As new devices reached clinical practice, their use was allowed by investigators in order to keep the randomized trial clinically relevant. But recruitment for the study was slow because so many clinicians were already convinced by anecdotal experience that combined therapy is better. So the endovascular therapies used most frequently in IMS III aren't the ones widely used in clinical practice today. Major new randomized trials are now getting underway comparing combined therapy using state-of-the-art, more effective stent clot retriever devices to intravenous TPA alone, he added.
In the New England Journal of Medicine report, the authors noted that "the use of randomization in ongoing and future stroke trials, rather than the treatment of eligible patients with endovascular therapy outside any trial, and minimization of the time to treatment will be essential for assessing the potential benefit of endovascular therapy for acute ischemic stroke."
No matter how future trials of combined therapy turn out, endovascular therapy is not going away, Dr. Broderick observed.
"It's a very good tool. The reason why is there are patients who can't get TPA. For example, roughly 5% of patients who undergo coronary artery bypass surgery have a stroke. If you have somebody with a big stroke 2 days after having their chest cracked, you can't use TPA. In that case, those endovascular devices are the way we can get up in there and get rid of the clot," he explained.
In an editorial accompanying the report in the New England Journal of Medicine (doi: 10.1056/NEJMe1215730), Dr. Marc I. Chimowitz declared that the clinical implication of IMS III is that endovascular therapy remains unproven and intravenous TPA should continue to be the first-line treatment for patients with acute ischemic stroke within 4.5 hours after stroke onset.
While new clinical trials featuring more effective IV clot busters, such as tenecteplase, and next-generation endovascular devices are urgently needed in an effort to improve stroke outcomes, patient recruitment is likely to continue to be a challenge in the current environment. This could be overcome if Medicare were to place a moratorium on reimbursement for endovascular therapy of acute ischemic stroke except as part of a randomized trial, according to Dr. Chimowitz, professor of neurology at the Medical University of South Carolina, Charleston.
The study was supported with grants from NIH and the National Institute of Neurological Disorders and Stroke; and by Genentech (which supplied the TPA); and EKOS, Concentric Medical, and Cordis Neurovascular (which supplied catheters); and Actilyse (alteplase) manufacturer Boehringer Ingelheim (which, along with Genentech and EKOS, provided support for investigator meetings). Dr. Broderick disclosed consulting fees from PhotoThera. Of the 28 other authors, disclosures for 14 were listed and included having received consulting fees, grant support, and/or lecture fees from a variety of device and pharmaceutical companies that include Genentech. Dr. Chimowitz, Dr. Silver, and Dr. Tomsick reported having no financial conflicts.
Functional outcomes in patients treated with intravenous tissue plasminogen activator with or without endovascular therapy after a moderate to severe acute ischemic stroke were not significantly different, and safety outcomes were similar, in a study that was stopped early because of these results.
In the IMS (Interventional Management of Stroke) III study, 40.8% of patients randomized to receive endovascular therapy plus intravenous TPA met the primary endpoint, a measure of functional independence -- a modified Rankin score of 2 or less at 90 days -- compared with 38.7% among those who had intravenous TPA alone, a difference that was not statistically significant, reported Dr. Joseph Broderick of the University of Cincinnati Neuroscience Institute, and the other IMS III investigators.
Mortality and other safety outcomes were also not significantly different between the two groups of patients in the study, which was stopped early because of futility after 656 of the planned 900 patients had been randomized.
The study was published online to coincide with the presentation of the results at the International Stroke Conference (N. Engl. J. Med. 2013 [doi:10.1056/NEJMoa1214300]).
Referring to the lack of randomized clinical trial data, the authors pointed out that it is uncertain whether endovascular therapy (which includes endovascular pharmacologic thrombolysis and, more recently, the use of stent retrievers) alone or combined with intravenous TPA is a more effective treatment of acute stroke than intravenous TPA alone, "the only proven reperfusion therapy for acute ischemic stroke."
In the study, conducted at 58 centers in the United States, Canada, Australia, and Europe, 434 patients were randomized to endovascular therapy plus intravenous TPA and 222 were randomized to standard treatment with intravenous TPA alone (started within 3 hours of stroke onset). The median age of those enrolled was 68-69 years (range, 23-89 years), a little over half were men, about 14% were black or Hispanic, and the median the National Institute of Health Stroke Scale (NIHSS) score was 16-17 (8-19 is a moderately severe stroke and 20 or greater is a severe stroke At the beginning of the study, only one thrombectomy device had been cleared by the Food and Drug Administration and, as the trial continued, other devices were used as they became cleared for use in the different countries.
In addition to the main finding, there were no differences in the primary outcome among those patients with an NIHSS score of 20 or more, and those with a score of 19 or lower, said the authors, who had hypothesized that endovascular therapy would have greater efficacy in patients with more-severe strokes since they "have the highest likelihood of occlusion in a major intracranial artery and the greatest volume of ischemic brain at risk."
They had also hypothesized that receiving endovascular therapy earlier would be associated with a greater benefit, but this was also not a significant factor in outcomes.
Mortality at 90 days was 19.1% in the endovascular therapy group and 21.6% in the intravenous TPA–alone group. Within 30 hours of TPA initiation, 6.2% of those on endovascular therapy and 5.9% of those on TPA alone had a symptomatic intracerebral hemorrhage. The differences in mortality at 7 days and in parenchymal hematoma rates were also not significantly different between the two groups. The rate of asymptomatic intracerebral hemorrhage, however, was significantly higher in the endovascular group.
Outcomes consistently trended better with combined therapy in patients with strokes involving larger artery occlusions and those with the shortest times from stroke onset to initiation of treatment, although because of small patient numbers the differences didn’t achieve statistical significance. These will be the subgroups that ought to be the focus of future clinical trials, Dr. Broderick said in a press briefing at the conference.
The underlying rationale for combined therapy is that intravenous TPA can quickly be started in the emergency department while the endovascular device therapy team is assembling, often at another hospital, which entails time-consuming patient transfer.
Intravenous TPA is the only proven therapy for acute ischemic stroke, but endovascular therapy is more effective at achieving recanalization. The study results bore this out: for example, the rate of partial or complete recanalization at 24 hours for an occlusion in the internal carotid artery was 81% with combined therapy compared to 35% with intravenous TPA alone. Yet this higher recanalization rate bore no clinical benefit, possibly because recanalization occurred too late, after ischemia had turned into infarction, Dr. Broderick explained.
"IMS III is going to be disappointing for a lot of people who are proponents of endovascular therapy. However, there is a light at the end of the tunnel in that there are these subgroups who may benefit," Dr. Brian Silver, who was not involved in the trial, said in an interview.
"The most critical feature is to treat the patients as soon as possible when they arrive in the emergency department, perhaps within 90 minutes. I think that’s the best chance for recovery. We are nowhere near what’s being done in cardiology, where there are door-to-balloon times of an hour. We need to do that in stroke. Since we're dealing with an organ that's more sensitive than the heart to ischemia, we probably need to be even faster than what's being done in cardiology. There is definitely room for improvement in our systems, perhaps by having the endovascular team stay in the hospital. Expense will be the limitation," according to Dr. Silver, director of the stroke center at Brown University, Providence, R.I.
IMS III investigator and interventional neuroradiologist Dr. Thomas A. Tomsick said in an interview that the study results won't change his own clinical practice.
"IMS III is by no means the final word on combined therapy. In Cincinnati tomorrow, if a patient with a large NIH Stroke Severity score shows up and we're treating him with IV TPA at 2 hours from stroke onset, we're not going to do a CT angiogram to evaluate that patient. He's going to the cath lab for angiography to see if there's a clot suitable for endovascular therapy," said Dr. Tomsick, professor of radiology at the University of Cincinnati.
Five different endovascular device therapies were utilized in IMS III. As new devices reached clinical practice, their use was allowed by investigators in order to keep the randomized trial clinically relevant. But recruitment for the study was slow because so many clinicians were already convinced by anecdotal experience that combined therapy is better. So the endovascular therapies used most frequently in IMS III aren't the ones widely used in clinical practice today. Major new randomized trials are now getting underway comparing combined therapy using state-of-the-art, more effective stent clot retriever devices to intravenous TPA alone, he added.
In the New England Journal of Medicine report, the authors noted that "the use of randomization in ongoing and future stroke trials, rather than the treatment of eligible patients with endovascular therapy outside any trial, and minimization of the time to treatment will be essential for assessing the potential benefit of endovascular therapy for acute ischemic stroke."
No matter how future trials of combined therapy turn out, endovascular therapy is not going away, Dr. Broderick observed.
"It's a very good tool. The reason why is there are patients who can't get TPA. For example, roughly 5% of patients who undergo coronary artery bypass surgery have a stroke. If you have somebody with a big stroke 2 days after having their chest cracked, you can't use TPA. In that case, those endovascular devices are the way we can get up in there and get rid of the clot," he explained.
In an editorial accompanying the report in the New England Journal of Medicine (doi: 10.1056/NEJMe1215730), Dr. Marc I. Chimowitz declared that the clinical implication of IMS III is that endovascular therapy remains unproven and intravenous TPA should continue to be the first-line treatment for patients with acute ischemic stroke within 4.5 hours after stroke onset.
While new clinical trials featuring more effective IV clot busters, such as tenecteplase, and next-generation endovascular devices are urgently needed in an effort to improve stroke outcomes, patient recruitment is likely to continue to be a challenge in the current environment. This could be overcome if Medicare were to place a moratorium on reimbursement for endovascular therapy of acute ischemic stroke except as part of a randomized trial, according to Dr. Chimowitz, professor of neurology at the Medical University of South Carolina, Charleston.
The study was supported with grants from NIH and the National Institute of Neurological Disorders and Stroke; and by Genentech (which supplied the TPA); and EKOS, Concentric Medical, and Cordis Neurovascular (which supplied catheters); and Actilyse (alteplase) manufacturer Boehringer Ingelheim (which, along with Genentech and EKOS, provided support for investigator meetings). Dr. Broderick disclosed consulting fees from PhotoThera. Of the 28 other authors, disclosures for 14 were listed and included having received consulting fees, grant support, and/or lecture fees from a variety of device and pharmaceutical companies that include Genentech. Dr. Chimowitz, Dr. Silver, and Dr. Tomsick reported having no financial conflicts.
FROM THE INTERNATIONAL STROKE CONFERENCE
Major Finding: Endovascular therapy plus intravenous TPA showed no added outcome benefits, compared with TPA alone, with 40.8% and 38.7% of patients, respectively, reaching functional independence at 90 days.
Data Source: An international, phase III study of 656 patients with an acute moderate to severe ischemic stroke randomized 2:1 to IV TPA with endovascular therapy or IV TPA alone.
Disclosures: The study was supported with grants from NIH and the National Institute of Neurological Disorders and Stroke, and by Genentech (supplier of TPA), and EKOS, Concentric Medical, and Cordis Neurovascular (which supplied catheters). Dr. Broderick disclosed consulting fees from PhotoThera. Of the 28 other authors, disclosures for 14 were listed and included having received consulting fees, grant support, and/or lecture fees from a variety of device and pharmaceutical companies that included Genentech. Dr. Chimowitz, Dr. Silver, and Dr. Tomsick reported having no financial conflicts.
Risk of HCV transmission very low in monogamous heterosexual couples
The risk of sexually transmitting a chronic hepatitis C infection to a long-term monogamous heterosexual partner is very low, averaging just about 1% per year.
That risk level works out to a transmission rate of about one in every 190,000 sexual contacts, Dr. Norah Terrault and her colleagues reported in the April issue of Hepatology (2013;57:881-9).
The cross-sectional study also found that no one sexual practice – including anal intercourse or intercourse during menses – significantly increased the risk of transmission, wrote Dr. Terrault of the University of California, San Francisco. The findings can be used to provide "unambiguous and reassuring counseling messages," she and her coinvestigators noted.
The study included 500 subjects with chronic HCV infections, and their sexual partners. All couples reported longtime, monogamous relationships (median duration, 15 years); however, the relationship duration varied widely, spanning 2-52 years.
Each of the partners was interviewed separately about their sexual contacts and practices. At the time of interview, the index subjects were a median of 49 years old and the partners, a median of 48 years.
The HCV-positive subjects reported the highest incidence of past risk factors, including blood transfusions before 1992 (32%), injected illegal drugs (54%), and being stuck by a bloody sharp item in a hospital (4%). Nearly half (46%) reported having had at least 20 lifetime sexual partners, with 21% having had 50 or more.
However, partners also reported some risk factors: 11% had an early transfusion, 2% used illegal drugs, and 2% had a hospital sharps incident. Many (27%) also reported having had at least 20 sexual partners.
Among the 500 couples, 20 partners (4%) were coinfected with HCV. Of these, nine were concordantly infected, eight discordantly, and three were indeterminate.
Six of the concordant couples underwent phylogenetic typing. Three were infected with the same HCV isolate and three with different strains. The investigators estimated the time of transmission and any additional risk factor among the three couples with concordant strains.
For the first couple, with an 18-year relationship, transmission probably occurred after about 6.5 years. The female partner had a history of injected drug use, while the male had no identifiable risk factors.
The second couple had a 28-year relationship; transmission probably occurred at around 15 years, the investigators said. "The female partner had a history of injectable drug use and both partners reported more than 20 prior sexual partners, a history of sexual transmitted diseases, and a history of snorting of drugs."
For the third couple, who had been together for 10 years, transmission probably occurred at around year 6. "The male partner had a history of injectable drug use, of being stuck by a sharp bloody object while working in a hospital, and more than 20 prior sexual partners; both partners reported snorting drugs and sharing snorting equipment."
The investigators determined that these infections were probably sexually transmitted between the partners – a prevalence of about 1%. "The estimated risk per sexual contact ranged from 1/380,000 to 1/190, 000," they said.
However, they were unable to identify any behaviors that significantly increased the risk of transmission. Compared with couples without coinfection, coinfected couples were more likely to have vaginal intercourse during menses (100% vs. 66%), more likely to have anal intercourse (67% vs. 30%), and less likely to use condoms (0% vs. 30%), but none of these differences was statistically significant.
"HCV transmission by sex from chronically infected persons to their heterosexual partners in a long-term monogamous relationship likely occurs, but is a rare event," the authors concluded. "Our results provide a basis for specific counseling messages that clinicians can use with their patients... [that] support the current national recommendations that couples not change their sexual practices if they are in a monogamous heterosexual relationship."
None of the study authors reported any financial conflicts.
The risk of sexually transmitting a chronic hepatitis C infection to a long-term monogamous heterosexual partner is very low, averaging just about 1% per year.
That risk level works out to a transmission rate of about one in every 190,000 sexual contacts, Dr. Norah Terrault and her colleagues reported in the April issue of Hepatology (2013;57:881-9).
The cross-sectional study also found that no one sexual practice – including anal intercourse or intercourse during menses – significantly increased the risk of transmission, wrote Dr. Terrault of the University of California, San Francisco. The findings can be used to provide "unambiguous and reassuring counseling messages," she and her coinvestigators noted.
The study included 500 subjects with chronic HCV infections, and their sexual partners. All couples reported longtime, monogamous relationships (median duration, 15 years); however, the relationship duration varied widely, spanning 2-52 years.
Each of the partners was interviewed separately about their sexual contacts and practices. At the time of interview, the index subjects were a median of 49 years old and the partners, a median of 48 years.
The HCV-positive subjects reported the highest incidence of past risk factors, including blood transfusions before 1992 (32%), injected illegal drugs (54%), and being stuck by a bloody sharp item in a hospital (4%). Nearly half (46%) reported having had at least 20 lifetime sexual partners, with 21% having had 50 or more.
However, partners also reported some risk factors: 11% had an early transfusion, 2% used illegal drugs, and 2% had a hospital sharps incident. Many (27%) also reported having had at least 20 sexual partners.
Among the 500 couples, 20 partners (4%) were coinfected with HCV. Of these, nine were concordantly infected, eight discordantly, and three were indeterminate.
Six of the concordant couples underwent phylogenetic typing. Three were infected with the same HCV isolate and three with different strains. The investigators estimated the time of transmission and any additional risk factor among the three couples with concordant strains.
For the first couple, with an 18-year relationship, transmission probably occurred after about 6.5 years. The female partner had a history of injected drug use, while the male had no identifiable risk factors.
The second couple had a 28-year relationship; transmission probably occurred at around 15 years, the investigators said. "The female partner had a history of injectable drug use and both partners reported more than 20 prior sexual partners, a history of sexual transmitted diseases, and a history of snorting of drugs."
For the third couple, who had been together for 10 years, transmission probably occurred at around year 6. "The male partner had a history of injectable drug use, of being stuck by a sharp bloody object while working in a hospital, and more than 20 prior sexual partners; both partners reported snorting drugs and sharing snorting equipment."
The investigators determined that these infections were probably sexually transmitted between the partners – a prevalence of about 1%. "The estimated risk per sexual contact ranged from 1/380,000 to 1/190, 000," they said.
However, they were unable to identify any behaviors that significantly increased the risk of transmission. Compared with couples without coinfection, coinfected couples were more likely to have vaginal intercourse during menses (100% vs. 66%), more likely to have anal intercourse (67% vs. 30%), and less likely to use condoms (0% vs. 30%), but none of these differences was statistically significant.
"HCV transmission by sex from chronically infected persons to their heterosexual partners in a long-term monogamous relationship likely occurs, but is a rare event," the authors concluded. "Our results provide a basis for specific counseling messages that clinicians can use with their patients... [that] support the current national recommendations that couples not change their sexual practices if they are in a monogamous heterosexual relationship."
None of the study authors reported any financial conflicts.
The risk of sexually transmitting a chronic hepatitis C infection to a long-term monogamous heterosexual partner is very low, averaging just about 1% per year.
That risk level works out to a transmission rate of about one in every 190,000 sexual contacts, Dr. Norah Terrault and her colleagues reported in the April issue of Hepatology (2013;57:881-9).
The cross-sectional study also found that no one sexual practice – including anal intercourse or intercourse during menses – significantly increased the risk of transmission, wrote Dr. Terrault of the University of California, San Francisco. The findings can be used to provide "unambiguous and reassuring counseling messages," she and her coinvestigators noted.
The study included 500 subjects with chronic HCV infections, and their sexual partners. All couples reported longtime, monogamous relationships (median duration, 15 years); however, the relationship duration varied widely, spanning 2-52 years.
Each of the partners was interviewed separately about their sexual contacts and practices. At the time of interview, the index subjects were a median of 49 years old and the partners, a median of 48 years.
The HCV-positive subjects reported the highest incidence of past risk factors, including blood transfusions before 1992 (32%), injected illegal drugs (54%), and being stuck by a bloody sharp item in a hospital (4%). Nearly half (46%) reported having had at least 20 lifetime sexual partners, with 21% having had 50 or more.
However, partners also reported some risk factors: 11% had an early transfusion, 2% used illegal drugs, and 2% had a hospital sharps incident. Many (27%) also reported having had at least 20 sexual partners.
Among the 500 couples, 20 partners (4%) were coinfected with HCV. Of these, nine were concordantly infected, eight discordantly, and three were indeterminate.
Six of the concordant couples underwent phylogenetic typing. Three were infected with the same HCV isolate and three with different strains. The investigators estimated the time of transmission and any additional risk factor among the three couples with concordant strains.
For the first couple, with an 18-year relationship, transmission probably occurred after about 6.5 years. The female partner had a history of injected drug use, while the male had no identifiable risk factors.
The second couple had a 28-year relationship; transmission probably occurred at around 15 years, the investigators said. "The female partner had a history of injectable drug use and both partners reported more than 20 prior sexual partners, a history of sexual transmitted diseases, and a history of snorting of drugs."
For the third couple, who had been together for 10 years, transmission probably occurred at around year 6. "The male partner had a history of injectable drug use, of being stuck by a sharp bloody object while working in a hospital, and more than 20 prior sexual partners; both partners reported snorting drugs and sharing snorting equipment."
The investigators determined that these infections were probably sexually transmitted between the partners – a prevalence of about 1%. "The estimated risk per sexual contact ranged from 1/380,000 to 1/190, 000," they said.
However, they were unable to identify any behaviors that significantly increased the risk of transmission. Compared with couples without coinfection, coinfected couples were more likely to have vaginal intercourse during menses (100% vs. 66%), more likely to have anal intercourse (67% vs. 30%), and less likely to use condoms (0% vs. 30%), but none of these differences was statistically significant.
"HCV transmission by sex from chronically infected persons to their heterosexual partners in a long-term monogamous relationship likely occurs, but is a rare event," the authors concluded. "Our results provide a basis for specific counseling messages that clinicians can use with their patients... [that] support the current national recommendations that couples not change their sexual practices if they are in a monogamous heterosexual relationship."
None of the study authors reported any financial conflicts.
FROM HEPATOLOGY
Major finding: Sexual transmission of chronic hepatitis C infection averaged about 1% per year in monogamous heterosexual couples.
Data source: A cross-sectional study of 500 couples in long-term monogamous relationships.
Disclosures: None of the study authors reported any financial disclosures.
Sofosbuvir shows sustained virologic response in HCV
Sofosbuvir resulted in a sustained virologic response in 90% of patients infected with hepatitis C virus genotype 1 or 4 at 12 weeks in a phase III trial.
Moreover, in a separate noninferiority analysis, patients with HCV genotypes 2 and 3 taking sofosbuvir had the same rates of sustained virologic response (SVR) as patients taking peginterferon, with fewer side effects, wrote Dr. Eric Lawitz in a report published online April 23 in the New England Journal of Medicine (doi: 10.1056/NEJMoa1214853).
In a report that is also to be presented at the International Liver Congress in Amsterdam, Dr. Lawitz analyzed more than 1,000 previously untreated HCV patients who received treatment with sofosbuvir in two multicenter studies. Sofosbuvir is an investigational nucleotide analogue HCV NS5B polymerase inhibitor.
The first study, the NEUTRINO trial, looked at a single group of 327 patients with genotypes 1, 4, 5, and 6 who received 12 weeks of open-label treatment with sofosbuvir (400 mg/day) plus peginterferon alfa-2a (180 mcg once per week) and ribavirin (given as a divided dose according to body weight, at 1,000 mg/day for patients less than 75 kg and 1,200 mg/day for heavier patients).
Most (89%) of these patients were genotype 1, with 9% having genotype 4, and the remainder having types 5 or 6, according to Dr. Lawitz of the Texas Liver Institute at the University of Texas Health Science Center in San Antonio.
At 12 weeks, SVR, defined by the lower limit of quantification of 25 IU/mL, occurred in 295 of these 327 patients (90%), with little difference in response rate according to genotype.
Having cirrhosis reduced the regimen’s SVR rate to 80%.
The second study assessed by Dr. Lawitz, the FISSION trial, involved 499 patients with HCV genotypes 2 or 3, who were randomized to open-label treatment with either sofosbuvir plus ribavirin for 12 weeks (n = 256) or peginterferon alfa-2a plus ribavirin for 24 weeks (n = 243).
Dosages in the sofosbuvir group were the same as in the NEUTRINO trial; in the peginterferon group, the ribavirin was given as 800 mg/day in two divided doses.
In this analysis, the researchers found that at 12 weeks, both treatment groups had an SVR of 67%.
Once again, the presence of cirrhosis reduced the likelihood of SVR. Genotype 3 also lowered the response, giving SVR rates of 56% in the sofosbuvir group and 63% in the peginterferon group.
In both NEUTRINO and FISSION, deep sequencing analysis of patients taking sofosbuvir who relapsed after having a virologic response showed no evidence of resistance-associated variants.
Discontinuation of sofosbuvir because of adverse events was uncommon in both trials: 2% in the NEUTRINO and 1% in the FISSION.
Indeed, "the influenza-like symptoms and fever that are characteristic of interferon treatment were reported in 16% and 18% of patients receiving peginterferon, respectively, but in only 3% of patients receiving sofosbuvir," Dr. Lawitz and his colleagues wrote.
"Depression, another common side effect of interferon therapy, occurred in 14% of patients receiving peginterferon, as compared with 5% of patients receiving sofosbuvir."
Dr. Lawitz was an investigator in a second, unrelated study to be presented at the International Liver Congress and also reported in the New England Journal of Medicine. In that study, sofosbuvir was again proven to be effective in HCV genotype 2 and 3 patients for whom the traditional peginterferon-ribavirin regimen was not an option. Only the abstract of the study was available at press time (N. Engl. J. Med. 2013 April 23 [doi: 10.1056/NEJMoa1214854]).
The NEUTRINO and FISSION trials were sponsored by Gilead Sciences, maker of sofosbuvir. Dr. Lawitz disclosed financial relationships to Gilead and multiple other pharmaceutical companies. Several authors were employees of Gilead.
The rapid change in the landscape for treating hepatitis C virus infection and the speed of drug development in the field – in the case of sofosbuvir, only a 3-year interval separates the publications of the chemical discovery of the protease inhibitor and its clinical data – may have negatively affected the design of clinical trials in the field.
For example, only one of the two studies conducted by Dr. Lawitz was a randomized controlled trial, and while the Food and Drug Administration has approved an endpoint of an SVR at 12 weeks (rather than the previously approved 24 weeks) for use in HCV trials, there is a small percentage (4%-6%) of patients with relapse of disease 12-24 weeks after treatment with sofosbuvir despite having an SVR. Although viral breakthrough does not appear to happen with sofosbuvir and did not occur in any of these patients, they somehow still relapsed despite meeting the trial’s definition for SVR; the reasons for relapse remain unknown.
However, these concerns may be outweighed by the sofosbuvir regimen’s low incidence of side effects, the relatively short duration of treatment, and the pangenotypic properties of the drug .
Dr. Joost P.H. Drenth is in the department of gastroenterology and hepatology at Radboud University Nijmegen (the Netherlands) Medical Center. He disclosed previous grants from Gilead Sciences, maker of sofosbuvir, and other pharmaceutical companies. His comments are derived from his editorial accompanying the sofosbuvir studies (N. Engl. J. Med. 2013 April 23 [doi: 10.1056/NEJMe1303818]).
The rapid change in the landscape for treating hepatitis C virus infection and the speed of drug development in the field – in the case of sofosbuvir, only a 3-year interval separates the publications of the chemical discovery of the protease inhibitor and its clinical data – may have negatively affected the design of clinical trials in the field.
For example, only one of the two studies conducted by Dr. Lawitz was a randomized controlled trial, and while the Food and Drug Administration has approved an endpoint of an SVR at 12 weeks (rather than the previously approved 24 weeks) for use in HCV trials, there is a small percentage (4%-6%) of patients with relapse of disease 12-24 weeks after treatment with sofosbuvir despite having an SVR. Although viral breakthrough does not appear to happen with sofosbuvir and did not occur in any of these patients, they somehow still relapsed despite meeting the trial’s definition for SVR; the reasons for relapse remain unknown.
However, these concerns may be outweighed by the sofosbuvir regimen’s low incidence of side effects, the relatively short duration of treatment, and the pangenotypic properties of the drug .
Dr. Joost P.H. Drenth is in the department of gastroenterology and hepatology at Radboud University Nijmegen (the Netherlands) Medical Center. He disclosed previous grants from Gilead Sciences, maker of sofosbuvir, and other pharmaceutical companies. His comments are derived from his editorial accompanying the sofosbuvir studies (N. Engl. J. Med. 2013 April 23 [doi: 10.1056/NEJMe1303818]).
The rapid change in the landscape for treating hepatitis C virus infection and the speed of drug development in the field – in the case of sofosbuvir, only a 3-year interval separates the publications of the chemical discovery of the protease inhibitor and its clinical data – may have negatively affected the design of clinical trials in the field.
For example, only one of the two studies conducted by Dr. Lawitz was a randomized controlled trial, and while the Food and Drug Administration has approved an endpoint of an SVR at 12 weeks (rather than the previously approved 24 weeks) for use in HCV trials, there is a small percentage (4%-6%) of patients with relapse of disease 12-24 weeks after treatment with sofosbuvir despite having an SVR. Although viral breakthrough does not appear to happen with sofosbuvir and did not occur in any of these patients, they somehow still relapsed despite meeting the trial’s definition for SVR; the reasons for relapse remain unknown.
However, these concerns may be outweighed by the sofosbuvir regimen’s low incidence of side effects, the relatively short duration of treatment, and the pangenotypic properties of the drug .
Dr. Joost P.H. Drenth is in the department of gastroenterology and hepatology at Radboud University Nijmegen (the Netherlands) Medical Center. He disclosed previous grants from Gilead Sciences, maker of sofosbuvir, and other pharmaceutical companies. His comments are derived from his editorial accompanying the sofosbuvir studies (N. Engl. J. Med. 2013 April 23 [doi: 10.1056/NEJMe1303818]).
Sofosbuvir resulted in a sustained virologic response in 90% of patients infected with hepatitis C virus genotype 1 or 4 at 12 weeks in a phase III trial.
Moreover, in a separate noninferiority analysis, patients with HCV genotypes 2 and 3 taking sofosbuvir had the same rates of sustained virologic response (SVR) as patients taking peginterferon, with fewer side effects, wrote Dr. Eric Lawitz in a report published online April 23 in the New England Journal of Medicine (doi: 10.1056/NEJMoa1214853).
In a report that is also to be presented at the International Liver Congress in Amsterdam, Dr. Lawitz analyzed more than 1,000 previously untreated HCV patients who received treatment with sofosbuvir in two multicenter studies. Sofosbuvir is an investigational nucleotide analogue HCV NS5B polymerase inhibitor.
The first study, the NEUTRINO trial, looked at a single group of 327 patients with genotypes 1, 4, 5, and 6 who received 12 weeks of open-label treatment with sofosbuvir (400 mg/day) plus peginterferon alfa-2a (180 mcg once per week) and ribavirin (given as a divided dose according to body weight, at 1,000 mg/day for patients less than 75 kg and 1,200 mg/day for heavier patients).
Most (89%) of these patients were genotype 1, with 9% having genotype 4, and the remainder having types 5 or 6, according to Dr. Lawitz of the Texas Liver Institute at the University of Texas Health Science Center in San Antonio.
At 12 weeks, SVR, defined by the lower limit of quantification of 25 IU/mL, occurred in 295 of these 327 patients (90%), with little difference in response rate according to genotype.
Having cirrhosis reduced the regimen’s SVR rate to 80%.
The second study assessed by Dr. Lawitz, the FISSION trial, involved 499 patients with HCV genotypes 2 or 3, who were randomized to open-label treatment with either sofosbuvir plus ribavirin for 12 weeks (n = 256) or peginterferon alfa-2a plus ribavirin for 24 weeks (n = 243).
Dosages in the sofosbuvir group were the same as in the NEUTRINO trial; in the peginterferon group, the ribavirin was given as 800 mg/day in two divided doses.
In this analysis, the researchers found that at 12 weeks, both treatment groups had an SVR of 67%.
Once again, the presence of cirrhosis reduced the likelihood of SVR. Genotype 3 also lowered the response, giving SVR rates of 56% in the sofosbuvir group and 63% in the peginterferon group.
In both NEUTRINO and FISSION, deep sequencing analysis of patients taking sofosbuvir who relapsed after having a virologic response showed no evidence of resistance-associated variants.
Discontinuation of sofosbuvir because of adverse events was uncommon in both trials: 2% in the NEUTRINO and 1% in the FISSION.
Indeed, "the influenza-like symptoms and fever that are characteristic of interferon treatment were reported in 16% and 18% of patients receiving peginterferon, respectively, but in only 3% of patients receiving sofosbuvir," Dr. Lawitz and his colleagues wrote.
"Depression, another common side effect of interferon therapy, occurred in 14% of patients receiving peginterferon, as compared with 5% of patients receiving sofosbuvir."
Dr. Lawitz was an investigator in a second, unrelated study to be presented at the International Liver Congress and also reported in the New England Journal of Medicine. In that study, sofosbuvir was again proven to be effective in HCV genotype 2 and 3 patients for whom the traditional peginterferon-ribavirin regimen was not an option. Only the abstract of the study was available at press time (N. Engl. J. Med. 2013 April 23 [doi: 10.1056/NEJMoa1214854]).
The NEUTRINO and FISSION trials were sponsored by Gilead Sciences, maker of sofosbuvir. Dr. Lawitz disclosed financial relationships to Gilead and multiple other pharmaceutical companies. Several authors were employees of Gilead.
Sofosbuvir resulted in a sustained virologic response in 90% of patients infected with hepatitis C virus genotype 1 or 4 at 12 weeks in a phase III trial.
Moreover, in a separate noninferiority analysis, patients with HCV genotypes 2 and 3 taking sofosbuvir had the same rates of sustained virologic response (SVR) as patients taking peginterferon, with fewer side effects, wrote Dr. Eric Lawitz in a report published online April 23 in the New England Journal of Medicine (doi: 10.1056/NEJMoa1214853).
In a report that is also to be presented at the International Liver Congress in Amsterdam, Dr. Lawitz analyzed more than 1,000 previously untreated HCV patients who received treatment with sofosbuvir in two multicenter studies. Sofosbuvir is an investigational nucleotide analogue HCV NS5B polymerase inhibitor.
The first study, the NEUTRINO trial, looked at a single group of 327 patients with genotypes 1, 4, 5, and 6 who received 12 weeks of open-label treatment with sofosbuvir (400 mg/day) plus peginterferon alfa-2a (180 mcg once per week) and ribavirin (given as a divided dose according to body weight, at 1,000 mg/day for patients less than 75 kg and 1,200 mg/day for heavier patients).
Most (89%) of these patients were genotype 1, with 9% having genotype 4, and the remainder having types 5 or 6, according to Dr. Lawitz of the Texas Liver Institute at the University of Texas Health Science Center in San Antonio.
At 12 weeks, SVR, defined by the lower limit of quantification of 25 IU/mL, occurred in 295 of these 327 patients (90%), with little difference in response rate according to genotype.
Having cirrhosis reduced the regimen’s SVR rate to 80%.
The second study assessed by Dr. Lawitz, the FISSION trial, involved 499 patients with HCV genotypes 2 or 3, who were randomized to open-label treatment with either sofosbuvir plus ribavirin for 12 weeks (n = 256) or peginterferon alfa-2a plus ribavirin for 24 weeks (n = 243).
Dosages in the sofosbuvir group were the same as in the NEUTRINO trial; in the peginterferon group, the ribavirin was given as 800 mg/day in two divided doses.
In this analysis, the researchers found that at 12 weeks, both treatment groups had an SVR of 67%.
Once again, the presence of cirrhosis reduced the likelihood of SVR. Genotype 3 also lowered the response, giving SVR rates of 56% in the sofosbuvir group and 63% in the peginterferon group.
In both NEUTRINO and FISSION, deep sequencing analysis of patients taking sofosbuvir who relapsed after having a virologic response showed no evidence of resistance-associated variants.
Discontinuation of sofosbuvir because of adverse events was uncommon in both trials: 2% in the NEUTRINO and 1% in the FISSION.
Indeed, "the influenza-like symptoms and fever that are characteristic of interferon treatment were reported in 16% and 18% of patients receiving peginterferon, respectively, but in only 3% of patients receiving sofosbuvir," Dr. Lawitz and his colleagues wrote.
"Depression, another common side effect of interferon therapy, occurred in 14% of patients receiving peginterferon, as compared with 5% of patients receiving sofosbuvir."
Dr. Lawitz was an investigator in a second, unrelated study to be presented at the International Liver Congress and also reported in the New England Journal of Medicine. In that study, sofosbuvir was again proven to be effective in HCV genotype 2 and 3 patients for whom the traditional peginterferon-ribavirin regimen was not an option. Only the abstract of the study was available at press time (N. Engl. J. Med. 2013 April 23 [doi: 10.1056/NEJMoa1214854]).
The NEUTRINO and FISSION trials were sponsored by Gilead Sciences, maker of sofosbuvir. Dr. Lawitz disclosed financial relationships to Gilead and multiple other pharmaceutical companies. Several authors were employees of Gilead.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Major finding: A regimen containing sofosbuvir gave 90% of patients with genotype 1 or 4 HCV infection an SVR at 12 weeks, whereas genotype 2 and 3 patients had a 12-week SVR rate of 67%.
Data source: Two phase III studies (one single-group, open-label study and one randomized, open-label, active-control trial) of more than 1,000 patients
Disclosures: The NEUTRINO and FISSION trials were sponsored by Gilead Sciences, maker of sofosbuvir. Dr. Lawitz disclosed financial relationships to Gilead and multiple other pharmaceutical companies. Several authors were employees of Gilead.
Stricter Duty-Hour Regulations Tied to Diminished Patient Care
A new report has linked recent changes made to hospital residents’ duty-hour regulations with a reduction in some aspects of patient care.
The study compared the work model for duty-hour regulations implemented in 2011 by the Accreditation Council for Graduate Medical Education (ACGME), which mostly limits first-year residents to a maximum 16-hour shift and older residents to 24 hours, with less restrictive guidelines adopted in 2003. Previously, 30-hour shifts were permitted for all residents.
Researchers at Johns Hopkins University in Baltimore measured residents’ sleep duration, hospital admission volumes, residents’ educational opportunities, the number of handoffs, and patient satisfaction surveys during shifts worked by internal-medicine house staff trainees under both models. The researchers used a three-month crossover design.
Residents slept longer, as expected, but the data showed more handoffs, fewer chances to attend teaching conferences, and reduced intern presence during daytime shifts when trainees followed the more recent work model. The study authors associated the model adopted in 2011 with deterioration in continuity of patient care and perceived quality of care. One of the four house staff teams perceived such a reduced quality of patient care that it terminated the project early.
However, one resident program director says much more research needs to be done to determine the efficacy of the new work-hour rules, particularly on patient and resident satisfaction. “There are things that go along with duty-hours, such as access to information and really well-designed handoff systems, that I think would bring out the safety advantages of duty-hours,” says Ethan Fried, MD, MS, FACP, associate professor of clinical medicine, Columbia College of Physicians and Surgeons and vice chair for education, department of medicine, St. Luke’s-Roosevelt Hospital, both in New York, and a former president of the Association of Program Directors in Internal Medicine.
“One of the reasons you’re not seeing an inflection in safety is because you have duty-hours, but you haven’t got the other system that you need to make duty-hours work. What people have been focused on is pure safety, and that we haven’t been able to demonstrate actual improvement in morbidity, mortality or complications,” he adds. “It’s one of those cases where I don’t know if we’re necessarily asking the right questions.”
Visit our website for more information on duty-hours.
A new report has linked recent changes made to hospital residents’ duty-hour regulations with a reduction in some aspects of patient care.
The study compared the work model for duty-hour regulations implemented in 2011 by the Accreditation Council for Graduate Medical Education (ACGME), which mostly limits first-year residents to a maximum 16-hour shift and older residents to 24 hours, with less restrictive guidelines adopted in 2003. Previously, 30-hour shifts were permitted for all residents.
Researchers at Johns Hopkins University in Baltimore measured residents’ sleep duration, hospital admission volumes, residents’ educational opportunities, the number of handoffs, and patient satisfaction surveys during shifts worked by internal-medicine house staff trainees under both models. The researchers used a three-month crossover design.
Residents slept longer, as expected, but the data showed more handoffs, fewer chances to attend teaching conferences, and reduced intern presence during daytime shifts when trainees followed the more recent work model. The study authors associated the model adopted in 2011 with deterioration in continuity of patient care and perceived quality of care. One of the four house staff teams perceived such a reduced quality of patient care that it terminated the project early.
However, one resident program director says much more research needs to be done to determine the efficacy of the new work-hour rules, particularly on patient and resident satisfaction. “There are things that go along with duty-hours, such as access to information and really well-designed handoff systems, that I think would bring out the safety advantages of duty-hours,” says Ethan Fried, MD, MS, FACP, associate professor of clinical medicine, Columbia College of Physicians and Surgeons and vice chair for education, department of medicine, St. Luke’s-Roosevelt Hospital, both in New York, and a former president of the Association of Program Directors in Internal Medicine.
“One of the reasons you’re not seeing an inflection in safety is because you have duty-hours, but you haven’t got the other system that you need to make duty-hours work. What people have been focused on is pure safety, and that we haven’t been able to demonstrate actual improvement in morbidity, mortality or complications,” he adds. “It’s one of those cases where I don’t know if we’re necessarily asking the right questions.”
Visit our website for more information on duty-hours.
A new report has linked recent changes made to hospital residents’ duty-hour regulations with a reduction in some aspects of patient care.
The study compared the work model for duty-hour regulations implemented in 2011 by the Accreditation Council for Graduate Medical Education (ACGME), which mostly limits first-year residents to a maximum 16-hour shift and older residents to 24 hours, with less restrictive guidelines adopted in 2003. Previously, 30-hour shifts were permitted for all residents.
Researchers at Johns Hopkins University in Baltimore measured residents’ sleep duration, hospital admission volumes, residents’ educational opportunities, the number of handoffs, and patient satisfaction surveys during shifts worked by internal-medicine house staff trainees under both models. The researchers used a three-month crossover design.
Residents slept longer, as expected, but the data showed more handoffs, fewer chances to attend teaching conferences, and reduced intern presence during daytime shifts when trainees followed the more recent work model. The study authors associated the model adopted in 2011 with deterioration in continuity of patient care and perceived quality of care. One of the four house staff teams perceived such a reduced quality of patient care that it terminated the project early.
However, one resident program director says much more research needs to be done to determine the efficacy of the new work-hour rules, particularly on patient and resident satisfaction. “There are things that go along with duty-hours, such as access to information and really well-designed handoff systems, that I think would bring out the safety advantages of duty-hours,” says Ethan Fried, MD, MS, FACP, associate professor of clinical medicine, Columbia College of Physicians and Surgeons and vice chair for education, department of medicine, St. Luke’s-Roosevelt Hospital, both in New York, and a former president of the Association of Program Directors in Internal Medicine.
“One of the reasons you’re not seeing an inflection in safety is because you have duty-hours, but you haven’t got the other system that you need to make duty-hours work. What people have been focused on is pure safety, and that we haven’t been able to demonstrate actual improvement in morbidity, mortality or complications,” he adds. “It’s one of those cases where I don’t know if we’re necessarily asking the right questions.”
Visit our website for more information on duty-hours.