User login
Panel Calls for More Safety Data for Hepatitis B Vaccine
SILVER SPRING, MD. – Despite evidence of effectiveness and enthusiasm about its potential, more safety data in thousands of people are needed before a two-dose hepatitis B vaccine can be approved for use in adults, according to the majority of a Food and Drug Administration advisory panel.
At a meeting on Nov. 15, the FDA’s Vaccines and Related Biological Products Advisory Committee voted 13 to 1 that the immunogenicity data on the Heplisav vaccine were adequate to support its effectiveness in preventing hepatitis B infection in adults aged 18-70 years. The proposed indication for the vaccine, which combines hepatitis B surface antigen (HBsAg) with a novel adjuvant to enhance the immune response, is for the active immunization against all known subtypes of the hepatitis B virus in adults aged 18-70 years.
But the panel voted 8 to 5, with one abstention, that the available data were not adequate to support the safety of the vaccine, citing the need for more data because the adjuvant, a Toll-like receptor 9 agonist, is not included in any available vaccine. Almost 4,000 people received the vaccine in two phase III studies. The manufacturer, Dynavax Technologies, also has proposed a postmarketing safety study that will enroll up to 30,000 recipients of the vaccine in a managed care organization. Panelists voting no on the safety question said that more data from a more ethnically diverse population than those enrolled in the studies would be needed in as many as 10,000 patients before approval.
While a hepatitis B vaccine that is more immunogenic in populations that do not respond as well to the hepatitis B vaccines would be beneficial, "I don’t think the safety data is sufficiently large to support a recommendation for use in the general adult population given that this vaccine contains a new adjuvant," said one of the panelists, Dr. Melinda Wharton, deputy director of the National Center for Immunization and Respiratory Diseases at the Centers for Disease Control and Prevention.
The two Heplisav doses are administered intramuscularly 1 month apart, compared with the 0, 1, and 6 month schedule for the two currently approved hepatitis B vaccines, Engerix-B and Recombivax HB.
The two phase III noninferiority studies of healthy adults aged 18-70 years compared immune response to vaccination with Heplisav (administered at 0 and 1 months, with a saline placebo administered at 6 months) in 3,778 adults, and with Engerix-B (administered at 0, 1, and 6 months) in 1,089 people. The primary immunogenicity end point was the seroprotection rate (SPR) – an anti-HBsAg level of 10 mIU/mL or greater, recognized as conferring protection against hepatitis B virus infection. In both studies, the SPR results for Heplisav met the noninferiority criteria for the studies.
In the two studies, the SPRs were higher among those who received Heplisav: 95% and 90% at 3 months (8 weeks after the last active dose), compared with 81.1% (4 weeks after the last dose) and 70.5% (8 weeks after the last dose), respectively, of those who received Engerix-B.
The most common adverse event associated with the vaccine was injection-site reaction in both groups. Rates of severe adverse events were lower among those who received Heplisav, and rates of autoimmune events and autoantibody conversions were similar in the two groups, according to Dynavax.
However, thyroid-related adverse events, which could be representative of autoimmune events, were reported in a higher proportion of people who received Heplisav. Cases of serious events, although rare, included one of Wegener’s granulomatosis and one of Guillain-Barré syndrome. Autoimmune diseases are relatively rare in the general population, and a large sample size of patients was necessary to accurately evaluate the associated risk, according to the FDA reviewer.
An increased risk of autoimmune reactions is a theoretical risk with adjuvants.
The FDA’s deadline for making a decision on the approval is Feb. 24, 2013, according to Dynavax. If approved, the company plans to market the vaccine as Heplisav. The vaccine also is under review in Europe.
The FDA usually follows the recommendations of its advisory panels, which are not binding. Panelists have been cleared of potential conflicts of interest related to the topic of the meeting, although a panelist may be given a waiver for conflict of interest, but none were granted at this meeting.
SILVER SPRING, MD. – Despite evidence of effectiveness and enthusiasm about its potential, more safety data in thousands of people are needed before a two-dose hepatitis B vaccine can be approved for use in adults, according to the majority of a Food and Drug Administration advisory panel.
At a meeting on Nov. 15, the FDA’s Vaccines and Related Biological Products Advisory Committee voted 13 to 1 that the immunogenicity data on the Heplisav vaccine were adequate to support its effectiveness in preventing hepatitis B infection in adults aged 18-70 years. The proposed indication for the vaccine, which combines hepatitis B surface antigen (HBsAg) with a novel adjuvant to enhance the immune response, is for the active immunization against all known subtypes of the hepatitis B virus in adults aged 18-70 years.
But the panel voted 8 to 5, with one abstention, that the available data were not adequate to support the safety of the vaccine, citing the need for more data because the adjuvant, a Toll-like receptor 9 agonist, is not included in any available vaccine. Almost 4,000 people received the vaccine in two phase III studies. The manufacturer, Dynavax Technologies, also has proposed a postmarketing safety study that will enroll up to 30,000 recipients of the vaccine in a managed care organization. Panelists voting no on the safety question said that more data from a more ethnically diverse population than those enrolled in the studies would be needed in as many as 10,000 patients before approval.
While a hepatitis B vaccine that is more immunogenic in populations that do not respond as well to the hepatitis B vaccines would be beneficial, "I don’t think the safety data is sufficiently large to support a recommendation for use in the general adult population given that this vaccine contains a new adjuvant," said one of the panelists, Dr. Melinda Wharton, deputy director of the National Center for Immunization and Respiratory Diseases at the Centers for Disease Control and Prevention.
The two Heplisav doses are administered intramuscularly 1 month apart, compared with the 0, 1, and 6 month schedule for the two currently approved hepatitis B vaccines, Engerix-B and Recombivax HB.
The two phase III noninferiority studies of healthy adults aged 18-70 years compared immune response to vaccination with Heplisav (administered at 0 and 1 months, with a saline placebo administered at 6 months) in 3,778 adults, and with Engerix-B (administered at 0, 1, and 6 months) in 1,089 people. The primary immunogenicity end point was the seroprotection rate (SPR) – an anti-HBsAg level of 10 mIU/mL or greater, recognized as conferring protection against hepatitis B virus infection. In both studies, the SPR results for Heplisav met the noninferiority criteria for the studies.
In the two studies, the SPRs were higher among those who received Heplisav: 95% and 90% at 3 months (8 weeks after the last active dose), compared with 81.1% (4 weeks after the last dose) and 70.5% (8 weeks after the last dose), respectively, of those who received Engerix-B.
The most common adverse event associated with the vaccine was injection-site reaction in both groups. Rates of severe adverse events were lower among those who received Heplisav, and rates of autoimmune events and autoantibody conversions were similar in the two groups, according to Dynavax.
However, thyroid-related adverse events, which could be representative of autoimmune events, were reported in a higher proportion of people who received Heplisav. Cases of serious events, although rare, included one of Wegener’s granulomatosis and one of Guillain-Barré syndrome. Autoimmune diseases are relatively rare in the general population, and a large sample size of patients was necessary to accurately evaluate the associated risk, according to the FDA reviewer.
An increased risk of autoimmune reactions is a theoretical risk with adjuvants.
The FDA’s deadline for making a decision on the approval is Feb. 24, 2013, according to Dynavax. If approved, the company plans to market the vaccine as Heplisav. The vaccine also is under review in Europe.
The FDA usually follows the recommendations of its advisory panels, which are not binding. Panelists have been cleared of potential conflicts of interest related to the topic of the meeting, although a panelist may be given a waiver for conflict of interest, but none were granted at this meeting.
SILVER SPRING, MD. – Despite evidence of effectiveness and enthusiasm about its potential, more safety data in thousands of people are needed before a two-dose hepatitis B vaccine can be approved for use in adults, according to the majority of a Food and Drug Administration advisory panel.
At a meeting on Nov. 15, the FDA’s Vaccines and Related Biological Products Advisory Committee voted 13 to 1 that the immunogenicity data on the Heplisav vaccine were adequate to support its effectiveness in preventing hepatitis B infection in adults aged 18-70 years. The proposed indication for the vaccine, which combines hepatitis B surface antigen (HBsAg) with a novel adjuvant to enhance the immune response, is for the active immunization against all known subtypes of the hepatitis B virus in adults aged 18-70 years.
But the panel voted 8 to 5, with one abstention, that the available data were not adequate to support the safety of the vaccine, citing the need for more data because the adjuvant, a Toll-like receptor 9 agonist, is not included in any available vaccine. Almost 4,000 people received the vaccine in two phase III studies. The manufacturer, Dynavax Technologies, also has proposed a postmarketing safety study that will enroll up to 30,000 recipients of the vaccine in a managed care organization. Panelists voting no on the safety question said that more data from a more ethnically diverse population than those enrolled in the studies would be needed in as many as 10,000 patients before approval.
While a hepatitis B vaccine that is more immunogenic in populations that do not respond as well to the hepatitis B vaccines would be beneficial, "I don’t think the safety data is sufficiently large to support a recommendation for use in the general adult population given that this vaccine contains a new adjuvant," said one of the panelists, Dr. Melinda Wharton, deputy director of the National Center for Immunization and Respiratory Diseases at the Centers for Disease Control and Prevention.
The two Heplisav doses are administered intramuscularly 1 month apart, compared with the 0, 1, and 6 month schedule for the two currently approved hepatitis B vaccines, Engerix-B and Recombivax HB.
The two phase III noninferiority studies of healthy adults aged 18-70 years compared immune response to vaccination with Heplisav (administered at 0 and 1 months, with a saline placebo administered at 6 months) in 3,778 adults, and with Engerix-B (administered at 0, 1, and 6 months) in 1,089 people. The primary immunogenicity end point was the seroprotection rate (SPR) – an anti-HBsAg level of 10 mIU/mL or greater, recognized as conferring protection against hepatitis B virus infection. In both studies, the SPR results for Heplisav met the noninferiority criteria for the studies.
In the two studies, the SPRs were higher among those who received Heplisav: 95% and 90% at 3 months (8 weeks after the last active dose), compared with 81.1% (4 weeks after the last dose) and 70.5% (8 weeks after the last dose), respectively, of those who received Engerix-B.
The most common adverse event associated with the vaccine was injection-site reaction in both groups. Rates of severe adverse events were lower among those who received Heplisav, and rates of autoimmune events and autoantibody conversions were similar in the two groups, according to Dynavax.
However, thyroid-related adverse events, which could be representative of autoimmune events, were reported in a higher proportion of people who received Heplisav. Cases of serious events, although rare, included one of Wegener’s granulomatosis and one of Guillain-Barré syndrome. Autoimmune diseases are relatively rare in the general population, and a large sample size of patients was necessary to accurately evaluate the associated risk, according to the FDA reviewer.
An increased risk of autoimmune reactions is a theoretical risk with adjuvants.
The FDA’s deadline for making a decision on the approval is Feb. 24, 2013, according to Dynavax. If approved, the company plans to market the vaccine as Heplisav. The vaccine also is under review in Europe.
The FDA usually follows the recommendations of its advisory panels, which are not binding. Panelists have been cleared of potential conflicts of interest related to the topic of the meeting, although a panelist may be given a waiver for conflict of interest, but none were granted at this meeting.
AT A MEETING OF THE FDA'S VACCINES AND RELATED BIOLOGICAL PRODUCTS ADVISORY COMMITTEE
Does an Active Childhood Build Strong Knees?
WASHINGTON – Active children may have stronger knees as adults, based on data from a long-term follow-up study of approximately 300 children. The findings were presented at the annual meeting of the American College of Rheumatology.
Although physical activity is recommended for children to improve joint health and function, the correlation between childhood exercise and adult bone structure has not been well studied, said Dr. Graeme Jones, who is professor of rheumatology and epidemiology and head of the musculoskeletal unit at the Menzies Research Institute as well as head of the department of rheumatology at Royal Hobart Hospital, both in Hobart, Australia.
Data from previous studies have shown that children who engaged in vigorous activity in childhood had greater cartilage deposition in their knees compared with less active children, said Dr. Jones.
"The idea would be that if you develop more cartilage in childhood and it lasts until adult life, you can prevent the development of osteoarthritis," he said.
In 1985, data were collected on 8,500 Australian children in the population-based Childhood Determinants of Adult Health study. In that study, researchers measured the children’s fitness based on measures of hand strength, leg strength, run times, sit-ups, and physical work capacity at 170 beats per minute (PWC170).
To measure the long-term impact of exercise on knee structure, Dr. Jones and his colleagues reviewed data from 298 study participants at ages 31-41 years. Approximately half of the participants were female.
The researchers assessed tibial bone area (the size of the knee joint) and the amount of cartilage using T1-weighted fat-suppressed magnetic resonance imaging.
All measures of childhood physical activity levels were significantly associated with increased tibial bone area. These associations included 0.48 cm2 per 100 mW (a measure of work) for PWC170, 1.49 cm2 per 100 g of hand muscle strength, 0.29 cm2 per 100 g of leg muscle strength, and 0.28 cm2 per 10 sit-ups.
In addition, childhood PWC170 was significantly associated with an increased medial tibial cartilage volume in adulthood (0.1 mm3 per 100 mW). This association was approximately 33% weaker, though still significant, after adjusting for tibial bone area, Dr. Jones said. Hand muscle strength and sit-ups were significantly associated with increased medial tibial cartilage volume before adjusting for bone area, but the association became nonsignificant after adjusting for bone area.
"What this suggests to us is that the response to physical activity in childhood is to increase the size of the bone to adjust for this and to spread the load out, and the cartilage will then expand to cover the bone area or the area of contact," said Dr. Jones.
These associations were independent of fitness performance measures and medial tibial cartilage volume in adulthood, he added.
The findings suggest that childhood exposure to physical activity has a long-term protective effect on knee joint health. Therefore, "we need to get children as active as we can," he said.
The study was funded by the National Health and Medical Research Council of Australia. Dr. Jones had no financial conflicts to disclose.
WASHINGTON – Active children may have stronger knees as adults, based on data from a long-term follow-up study of approximately 300 children. The findings were presented at the annual meeting of the American College of Rheumatology.
Although physical activity is recommended for children to improve joint health and function, the correlation between childhood exercise and adult bone structure has not been well studied, said Dr. Graeme Jones, who is professor of rheumatology and epidemiology and head of the musculoskeletal unit at the Menzies Research Institute as well as head of the department of rheumatology at Royal Hobart Hospital, both in Hobart, Australia.
Data from previous studies have shown that children who engaged in vigorous activity in childhood had greater cartilage deposition in their knees compared with less active children, said Dr. Jones.
"The idea would be that if you develop more cartilage in childhood and it lasts until adult life, you can prevent the development of osteoarthritis," he said.
In 1985, data were collected on 8,500 Australian children in the population-based Childhood Determinants of Adult Health study. In that study, researchers measured the children’s fitness based on measures of hand strength, leg strength, run times, sit-ups, and physical work capacity at 170 beats per minute (PWC170).
To measure the long-term impact of exercise on knee structure, Dr. Jones and his colleagues reviewed data from 298 study participants at ages 31-41 years. Approximately half of the participants were female.
The researchers assessed tibial bone area (the size of the knee joint) and the amount of cartilage using T1-weighted fat-suppressed magnetic resonance imaging.
All measures of childhood physical activity levels were significantly associated with increased tibial bone area. These associations included 0.48 cm2 per 100 mW (a measure of work) for PWC170, 1.49 cm2 per 100 g of hand muscle strength, 0.29 cm2 per 100 g of leg muscle strength, and 0.28 cm2 per 10 sit-ups.
In addition, childhood PWC170 was significantly associated with an increased medial tibial cartilage volume in adulthood (0.1 mm3 per 100 mW). This association was approximately 33% weaker, though still significant, after adjusting for tibial bone area, Dr. Jones said. Hand muscle strength and sit-ups were significantly associated with increased medial tibial cartilage volume before adjusting for bone area, but the association became nonsignificant after adjusting for bone area.
"What this suggests to us is that the response to physical activity in childhood is to increase the size of the bone to adjust for this and to spread the load out, and the cartilage will then expand to cover the bone area or the area of contact," said Dr. Jones.
These associations were independent of fitness performance measures and medial tibial cartilage volume in adulthood, he added.
The findings suggest that childhood exposure to physical activity has a long-term protective effect on knee joint health. Therefore, "we need to get children as active as we can," he said.
The study was funded by the National Health and Medical Research Council of Australia. Dr. Jones had no financial conflicts to disclose.
WASHINGTON – Active children may have stronger knees as adults, based on data from a long-term follow-up study of approximately 300 children. The findings were presented at the annual meeting of the American College of Rheumatology.
Although physical activity is recommended for children to improve joint health and function, the correlation between childhood exercise and adult bone structure has not been well studied, said Dr. Graeme Jones, who is professor of rheumatology and epidemiology and head of the musculoskeletal unit at the Menzies Research Institute as well as head of the department of rheumatology at Royal Hobart Hospital, both in Hobart, Australia.
Data from previous studies have shown that children who engaged in vigorous activity in childhood had greater cartilage deposition in their knees compared with less active children, said Dr. Jones.
"The idea would be that if you develop more cartilage in childhood and it lasts until adult life, you can prevent the development of osteoarthritis," he said.
In 1985, data were collected on 8,500 Australian children in the population-based Childhood Determinants of Adult Health study. In that study, researchers measured the children’s fitness based on measures of hand strength, leg strength, run times, sit-ups, and physical work capacity at 170 beats per minute (PWC170).
To measure the long-term impact of exercise on knee structure, Dr. Jones and his colleagues reviewed data from 298 study participants at ages 31-41 years. Approximately half of the participants were female.
The researchers assessed tibial bone area (the size of the knee joint) and the amount of cartilage using T1-weighted fat-suppressed magnetic resonance imaging.
All measures of childhood physical activity levels were significantly associated with increased tibial bone area. These associations included 0.48 cm2 per 100 mW (a measure of work) for PWC170, 1.49 cm2 per 100 g of hand muscle strength, 0.29 cm2 per 100 g of leg muscle strength, and 0.28 cm2 per 10 sit-ups.
In addition, childhood PWC170 was significantly associated with an increased medial tibial cartilage volume in adulthood (0.1 mm3 per 100 mW). This association was approximately 33% weaker, though still significant, after adjusting for tibial bone area, Dr. Jones said. Hand muscle strength and sit-ups were significantly associated with increased medial tibial cartilage volume before adjusting for bone area, but the association became nonsignificant after adjusting for bone area.
"What this suggests to us is that the response to physical activity in childhood is to increase the size of the bone to adjust for this and to spread the load out, and the cartilage will then expand to cover the bone area or the area of contact," said Dr. Jones.
These associations were independent of fitness performance measures and medial tibial cartilage volume in adulthood, he added.
The findings suggest that childhood exposure to physical activity has a long-term protective effect on knee joint health. Therefore, "we need to get children as active as we can," he said.
The study was funded by the National Health and Medical Research Council of Australia. Dr. Jones had no financial conflicts to disclose.
AT THE ANNUAL MEETING OF THE AMERICAN COLLEGE OF RHEUMATOLOGY
Major Finding: Childhood physical activity was significantly associated with increased tibial bone area in early adulthood, including an improvement of 0.29 cm2 per 100 g as a measure of leg muscle strength.
Data Source: The data come from 298 participants in the Childhood Determinants of Adult Health study.
Disclosures: The study was funded by the National Health and Medical Research Council of Australia. Dr. Jones had no financial conflicts to disclose.
Findings could revolutionize malaria drug testing

a red blood cell; Credit: St Jude
Children’s Research Hospital
Scientists have reported that injecting cryopreserved malaria sporozoites in human subjects can result in controlled infection.
This indicates that direct injection of sporozoites can be used in lieu of mosquito bites to test the efficacy of malaria drugs or vaccines in human volunteers.
The findings from this study were reported at the annual meeting of the American Society of Tropical Medicine and Hygiene and published online in the American Journal of Tropical Medicine and Hygiene.
“Our study shows it’s possible to manufacture and then administer controlled doses of malaria parasites using a needle and syringe to deliver a formulation that can meet regulatory standards for purity and dose consistency,” said study author Meta Roestenberg, MD, of the Radboud University Nijmegen Medical Center in The Netherlands.
She and her colleagues noted that the current method of testing malaria drugs and vaccines by exposing subjects to mosquito bites is technically complex and costly. And there are only a few places in the world today where such work is being done.
In addition, when using mosquitoes to deliver malaria parasites, it can be difficult to ensure that all subjects receive the same level of infection. And that can influence the outcome of the treatment.
So Dr Roestenberg and her colleagues set out to find a better way. They tested cryopreserved Plasmodium falciparum sporozoites that were harvested from the salivary glands of infected mosquitoes and had been frozen for more than 2 years. The sporozoites were manufactured by the Maryland-based biotechnology company Sanaria Inc.
The researchers enrolled 18 healthy volunteers and divided them into 3 groups. The first group received 2500 sporozoites, the second received 10,000 sporozoites, and the third received 25,000 sporozoites.
The team found that 84% of participants—5 of the 6 volunteers in each group—were safely and successfully infected with malaria. And there were no differences among the groups in the time it took for the infection to develop or the presentation of symptoms. The volunteers who developed infections subsequently received treatment and quickly recovered without incident.
“We have demonstrated the potential to develop what you might call ‘the human challenge trial in a bottle’ that could be available to scientists anywhere who need to know how a new drug or vaccine would fare against a real but carefully controlled and calibrated malaria infection,” said study author Stephen L. Hoffman, MD, of Sanaria Inc.
The researchers also said these results could aid the development of whole parasite vaccines.
“A major challenge to realizing the potential of whole parasite vaccines is the development of a stable, consistent formulation of sporozoites that can be manufactured, preserved, and used like any other vaccine,” said study author Robert W. Sauerwein, MD, PhD, also of Radboud University Nijmegen Medical Center.
Sanaria is currently pursuing clinical trials to test 2 different approaches to whole parasite vaccination: irradiated sporozoites and inducing controlled infections in tandem with the administration of antimalarial drugs.
Researchers are also planning additional trials to ensure the infection produced with the cryopreserved sporozoites mirrors what one would experience through bites from infected mosquitoes. ![]()

a red blood cell; Credit: St Jude
Children’s Research Hospital
Scientists have reported that injecting cryopreserved malaria sporozoites in human subjects can result in controlled infection.
This indicates that direct injection of sporozoites can be used in lieu of mosquito bites to test the efficacy of malaria drugs or vaccines in human volunteers.
The findings from this study were reported at the annual meeting of the American Society of Tropical Medicine and Hygiene and published online in the American Journal of Tropical Medicine and Hygiene.
“Our study shows it’s possible to manufacture and then administer controlled doses of malaria parasites using a needle and syringe to deliver a formulation that can meet regulatory standards for purity and dose consistency,” said study author Meta Roestenberg, MD, of the Radboud University Nijmegen Medical Center in The Netherlands.
She and her colleagues noted that the current method of testing malaria drugs and vaccines by exposing subjects to mosquito bites is technically complex and costly. And there are only a few places in the world today where such work is being done.
In addition, when using mosquitoes to deliver malaria parasites, it can be difficult to ensure that all subjects receive the same level of infection. And that can influence the outcome of the treatment.
So Dr Roestenberg and her colleagues set out to find a better way. They tested cryopreserved Plasmodium falciparum sporozoites that were harvested from the salivary glands of infected mosquitoes and had been frozen for more than 2 years. The sporozoites were manufactured by the Maryland-based biotechnology company Sanaria Inc.
The researchers enrolled 18 healthy volunteers and divided them into 3 groups. The first group received 2500 sporozoites, the second received 10,000 sporozoites, and the third received 25,000 sporozoites.
The team found that 84% of participants—5 of the 6 volunteers in each group—were safely and successfully infected with malaria. And there were no differences among the groups in the time it took for the infection to develop or the presentation of symptoms. The volunteers who developed infections subsequently received treatment and quickly recovered without incident.
“We have demonstrated the potential to develop what you might call ‘the human challenge trial in a bottle’ that could be available to scientists anywhere who need to know how a new drug or vaccine would fare against a real but carefully controlled and calibrated malaria infection,” said study author Stephen L. Hoffman, MD, of Sanaria Inc.
The researchers also said these results could aid the development of whole parasite vaccines.
“A major challenge to realizing the potential of whole parasite vaccines is the development of a stable, consistent formulation of sporozoites that can be manufactured, preserved, and used like any other vaccine,” said study author Robert W. Sauerwein, MD, PhD, also of Radboud University Nijmegen Medical Center.
Sanaria is currently pursuing clinical trials to test 2 different approaches to whole parasite vaccination: irradiated sporozoites and inducing controlled infections in tandem with the administration of antimalarial drugs.
Researchers are also planning additional trials to ensure the infection produced with the cryopreserved sporozoites mirrors what one would experience through bites from infected mosquitoes. ![]()

a red blood cell; Credit: St Jude
Children’s Research Hospital
Scientists have reported that injecting cryopreserved malaria sporozoites in human subjects can result in controlled infection.
This indicates that direct injection of sporozoites can be used in lieu of mosquito bites to test the efficacy of malaria drugs or vaccines in human volunteers.
The findings from this study were reported at the annual meeting of the American Society of Tropical Medicine and Hygiene and published online in the American Journal of Tropical Medicine and Hygiene.
“Our study shows it’s possible to manufacture and then administer controlled doses of malaria parasites using a needle and syringe to deliver a formulation that can meet regulatory standards for purity and dose consistency,” said study author Meta Roestenberg, MD, of the Radboud University Nijmegen Medical Center in The Netherlands.
She and her colleagues noted that the current method of testing malaria drugs and vaccines by exposing subjects to mosquito bites is technically complex and costly. And there are only a few places in the world today where such work is being done.
In addition, when using mosquitoes to deliver malaria parasites, it can be difficult to ensure that all subjects receive the same level of infection. And that can influence the outcome of the treatment.
So Dr Roestenberg and her colleagues set out to find a better way. They tested cryopreserved Plasmodium falciparum sporozoites that were harvested from the salivary glands of infected mosquitoes and had been frozen for more than 2 years. The sporozoites were manufactured by the Maryland-based biotechnology company Sanaria Inc.
The researchers enrolled 18 healthy volunteers and divided them into 3 groups. The first group received 2500 sporozoites, the second received 10,000 sporozoites, and the third received 25,000 sporozoites.
The team found that 84% of participants—5 of the 6 volunteers in each group—were safely and successfully infected with malaria. And there were no differences among the groups in the time it took for the infection to develop or the presentation of symptoms. The volunteers who developed infections subsequently received treatment and quickly recovered without incident.
“We have demonstrated the potential to develop what you might call ‘the human challenge trial in a bottle’ that could be available to scientists anywhere who need to know how a new drug or vaccine would fare against a real but carefully controlled and calibrated malaria infection,” said study author Stephen L. Hoffman, MD, of Sanaria Inc.
The researchers also said these results could aid the development of whole parasite vaccines.
“A major challenge to realizing the potential of whole parasite vaccines is the development of a stable, consistent formulation of sporozoites that can be manufactured, preserved, and used like any other vaccine,” said study author Robert W. Sauerwein, MD, PhD, also of Radboud University Nijmegen Medical Center.
Sanaria is currently pursuing clinical trials to test 2 different approaches to whole parasite vaccination: irradiated sporozoites and inducing controlled infections in tandem with the administration of antimalarial drugs.
Researchers are also planning additional trials to ensure the infection produced with the cryopreserved sporozoites mirrors what one would experience through bites from infected mosquitoes. ![]()
Accountable-Care Organizations on the Horizon for Hospitalists
Every HM group should look into transitioning from a fee-for-service model to an accountable-care organization (ACO), a leading hospitalist told conference attendees recently at the Third National Accountable Care Organization Congress.
"You need to be tackling it now, but that doesn't mean you need to be aggressively doing it now," Edward Murphy, MD, chairman of Sound Physicians, told eWire days before he spoke at the ACO Congress on Oct. 31 in Los Angeles. "By tackling it, you need to be doing a hard-nosed assessment of what's best for your group and your patients."
Question: Why is the ACO model so difficult in some instances?
Answer: The problem with the healthcare system today is we’ve spent 100 years building up a system that is designed around, and competent at, delivering services for fees. We're really not set up to manage care.
Q: What is the No. 1 thing you want hospitalists to know about ACOs today?
A: Figure out every single day how to improve the care of your patients at a lower cost. And then, how you can demonstrate it in a quantitative and clear way. ACO-style payments are really only a value proposition centered on providing superior outcomes for patients at higher satisfaction for lower cost. That’s a fundamental value that will always be noteworthy.
Q: Is a hospitalist's job to lead the charge toward ACOs, or support those who do?
A: That's the sort of thing that people on the ground don't have to be told. They just know. If I were the leader of a hospitalist group someplace and really thought the smart thing to do was to think about how to move to an accountable-care model … I would know from my discussions with my colleagues, my discussions with hospital executives where everybody was.
Visit our website for more information about ACOs.
Every HM group should look into transitioning from a fee-for-service model to an accountable-care organization (ACO), a leading hospitalist told conference attendees recently at the Third National Accountable Care Organization Congress.
"You need to be tackling it now, but that doesn't mean you need to be aggressively doing it now," Edward Murphy, MD, chairman of Sound Physicians, told eWire days before he spoke at the ACO Congress on Oct. 31 in Los Angeles. "By tackling it, you need to be doing a hard-nosed assessment of what's best for your group and your patients."
Question: Why is the ACO model so difficult in some instances?
Answer: The problem with the healthcare system today is we’ve spent 100 years building up a system that is designed around, and competent at, delivering services for fees. We're really not set up to manage care.
Q: What is the No. 1 thing you want hospitalists to know about ACOs today?
A: Figure out every single day how to improve the care of your patients at a lower cost. And then, how you can demonstrate it in a quantitative and clear way. ACO-style payments are really only a value proposition centered on providing superior outcomes for patients at higher satisfaction for lower cost. That’s a fundamental value that will always be noteworthy.
Q: Is a hospitalist's job to lead the charge toward ACOs, or support those who do?
A: That's the sort of thing that people on the ground don't have to be told. They just know. If I were the leader of a hospitalist group someplace and really thought the smart thing to do was to think about how to move to an accountable-care model … I would know from my discussions with my colleagues, my discussions with hospital executives where everybody was.
Visit our website for more information about ACOs.
Every HM group should look into transitioning from a fee-for-service model to an accountable-care organization (ACO), a leading hospitalist told conference attendees recently at the Third National Accountable Care Organization Congress.
"You need to be tackling it now, but that doesn't mean you need to be aggressively doing it now," Edward Murphy, MD, chairman of Sound Physicians, told eWire days before he spoke at the ACO Congress on Oct. 31 in Los Angeles. "By tackling it, you need to be doing a hard-nosed assessment of what's best for your group and your patients."
Question: Why is the ACO model so difficult in some instances?
Answer: The problem with the healthcare system today is we’ve spent 100 years building up a system that is designed around, and competent at, delivering services for fees. We're really not set up to manage care.
Q: What is the No. 1 thing you want hospitalists to know about ACOs today?
A: Figure out every single day how to improve the care of your patients at a lower cost. And then, how you can demonstrate it in a quantitative and clear way. ACO-style payments are really only a value proposition centered on providing superior outcomes for patients at higher satisfaction for lower cost. That’s a fundamental value that will always be noteworthy.
Q: Is a hospitalist's job to lead the charge toward ACOs, or support those who do?
A: That's the sort of thing that people on the ground don't have to be told. They just know. If I were the leader of a hospitalist group someplace and really thought the smart thing to do was to think about how to move to an accountable-care model … I would know from my discussions with my colleagues, my discussions with hospital executives where everybody was.
Visit our website for more information about ACOs.
End-of-Life Care Can Bring on Challenges for Hospitalists
How do you cope with a family that wants you to "do everything" for their seriously ill loved one? This dilemma was one of the topics explored at the Management of the Hospitalized Patient conference held last month at the University of California at San Francisco (UCSF).
"We don't actually know what 'everything' means to the family, not without probing into a range of possible meanings," said presenter Steve Pantilat, MD, FACP, SFHM, hospitalist and director of the palliative-care service at UCSF Medical Center. "The family may not have a clear understanding of what 'everything' entails, including mechanical ventilation or cardio-pulmonary resuscitation. I prefer to ask, 'How were you hoping we could help?' The answer can provide a great deal of insight."
In spite of various tools to aid decisions, prognosis is inherently uncertain, said co-presenter Matthew Gonzales, MD, assistant professor of hospital medicine and palliative care at UCSF Medical Center. "We use the Palliative Performance Scale [PDF]."
The family might not trust the hospitalist’s prognosis, especially when meeting the doctor for the first time in a stressful situation, and there might be disagreements within the family about the course of treatment, Dr. Gonzales said. Cultural differences also come into play.
"I have started to ask, 'How do you decide these questions in your family?' because of the differences within a cultural group," Dr. Pantilat said. "If they talk about hoping for a miracle, I probe the meaning of 'miracle' to them. Physicians can't work on the basis of miracles; they have to practice medicine. And you should resist getting drawn into a religious debate. That's a loser for the physician."
Visit our website for more information about palliative care.
How do you cope with a family that wants you to "do everything" for their seriously ill loved one? This dilemma was one of the topics explored at the Management of the Hospitalized Patient conference held last month at the University of California at San Francisco (UCSF).
"We don't actually know what 'everything' means to the family, not without probing into a range of possible meanings," said presenter Steve Pantilat, MD, FACP, SFHM, hospitalist and director of the palliative-care service at UCSF Medical Center. "The family may not have a clear understanding of what 'everything' entails, including mechanical ventilation or cardio-pulmonary resuscitation. I prefer to ask, 'How were you hoping we could help?' The answer can provide a great deal of insight."
In spite of various tools to aid decisions, prognosis is inherently uncertain, said co-presenter Matthew Gonzales, MD, assistant professor of hospital medicine and palliative care at UCSF Medical Center. "We use the Palliative Performance Scale [PDF]."
The family might not trust the hospitalist’s prognosis, especially when meeting the doctor for the first time in a stressful situation, and there might be disagreements within the family about the course of treatment, Dr. Gonzales said. Cultural differences also come into play.
"I have started to ask, 'How do you decide these questions in your family?' because of the differences within a cultural group," Dr. Pantilat said. "If they talk about hoping for a miracle, I probe the meaning of 'miracle' to them. Physicians can't work on the basis of miracles; they have to practice medicine. And you should resist getting drawn into a religious debate. That's a loser for the physician."
Visit our website for more information about palliative care.
How do you cope with a family that wants you to "do everything" for their seriously ill loved one? This dilemma was one of the topics explored at the Management of the Hospitalized Patient conference held last month at the University of California at San Francisco (UCSF).
"We don't actually know what 'everything' means to the family, not without probing into a range of possible meanings," said presenter Steve Pantilat, MD, FACP, SFHM, hospitalist and director of the palliative-care service at UCSF Medical Center. "The family may not have a clear understanding of what 'everything' entails, including mechanical ventilation or cardio-pulmonary resuscitation. I prefer to ask, 'How were you hoping we could help?' The answer can provide a great deal of insight."
In spite of various tools to aid decisions, prognosis is inherently uncertain, said co-presenter Matthew Gonzales, MD, assistant professor of hospital medicine and palliative care at UCSF Medical Center. "We use the Palliative Performance Scale [PDF]."
The family might not trust the hospitalist’s prognosis, especially when meeting the doctor for the first time in a stressful situation, and there might be disagreements within the family about the course of treatment, Dr. Gonzales said. Cultural differences also come into play.
"I have started to ask, 'How do you decide these questions in your family?' because of the differences within a cultural group," Dr. Pantilat said. "If they talk about hoping for a miracle, I probe the meaning of 'miracle' to them. Physicians can't work on the basis of miracles; they have to practice medicine. And you should resist getting drawn into a religious debate. That's a loser for the physician."
Visit our website for more information about palliative care.
Excessive Acetaminophen Dosing Seen Among Inpatients
Four percent of hospitalized adolescents and adults at two academic tertiary care medical centers received supratherapeutic doses of acetaminophen during their stays, according to a report published online Nov. 12 in Archives of Internal Medicine.
"These were not isolated events but often were successive and overlapping." Nearly half of the exposed patients received 5 g or more of acetaminophen per day, and 40% received excessive dosing for 3 or more days, said Dr. Li Zhou of Partners HealthCare System, Wellesley, Mass., and her associates.
Such overmedicating puts patients at unnecessary risk for hepatotoxicity, acute liver failure, and even death. In this study, even inpatients at specific risk for liver damage didn’t escape excessive dosing with acetaminophen: 22% of the elderly and 18% of patients with existing chronic liver disease were given acetaminophen in amounts exceeding their recommended limit of 3 g/day, said Dr. Zhou, who is also at Brigham and Women’s Hospital and Harvard Medical School, Boston, and her colleagues.
The risk factors most strongly associated with supratherapeutic dosing were receiving recurring scheduled dosing (that is, standing orders) as opposed to as-needed or one-time-only administration, which carried a hazard ratio of 16.6; receiving multiple products containing acetaminophen, which carried a hazard ratio of 2.4 for each additional product; and receiving products containing 500 mg of acetaminophen, which carried an HR of 1.9.
The investigators used the hospitals’ electronic medication administration record systems to assess acetaminophen exposure in the inpatient setting – a topic that has received little attention from researchers before now, they wrote. The study population comprised the 14,411 inpatients aged 12 and older who received any acetaminophen during their stays in a 3-month period. That accounts for about 61% of the entire patient population hospitalized at the two centers during the study period.
The average age of the study subjects was 55 years (range, 12-110 years), and a little more than one-third were older than 65. Approximately 75 had chronic liver disease.
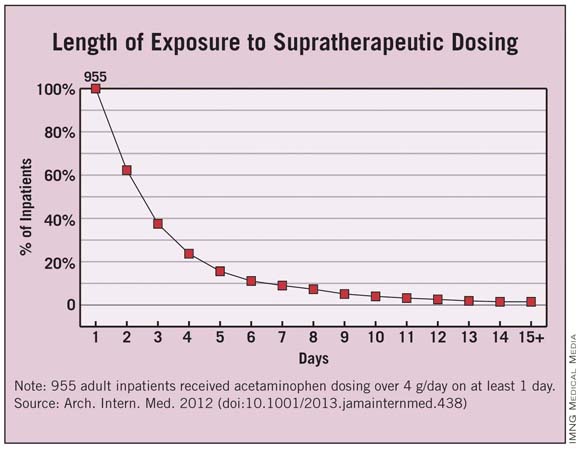
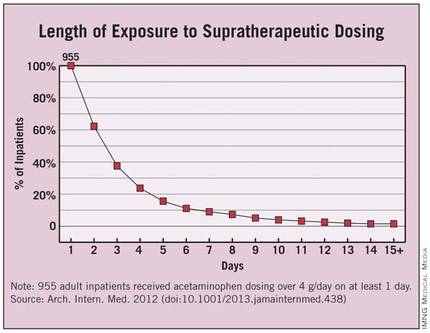
Overall, 955 patients were given acetaminophen in doses exceeding the 4-g/day limit. This represents 4% of the entire hospitalized population and 6.6% of the cohort of patients given any acetaminophen, the researchers said (Arch. Intern. Med. 2012 [doi:10.1001/2013.jamainternmed.438]).
Each patient who received supratherapeutic dosing had a mean of five such incidents during the course of a mean of 3 days. Instances of such overmedicating ranged as high as 48 separate occasions over the course of 30 days.
Approximately 40% of the 955 patients received supratherapeutic dosing for 3 or more days, and 4% received it for 10 or more days.
"Our findings show that despite policies and procedures to monitor and control patients’ acetaminophen exposure, the incidence of supratherapeutic acetaminophen dosing in hospitalized patients remains high," Dr. Zhou and her associates said.
Previous studies have shown that many physicians and nurses aren’t aware of the maximal recommended daily dosing of acetaminophen, and that many have some difficulty identifying which products contain the agent. Therefore, increased training is warranted "to help clinicians identify acetaminophen-containing products and monitor closely the daily dose of acetaminophen," the investigators said.
A reassessment of long-accepted dosing regimens may also be warranted, "especially the scheduled use of products containing 500 mg of acetaminophen," they added.
The study was funded by the Partners-Siemens Research Council. The investigators reported no relevant financial conflicts.
Dr. Li Zhou and her colleagues have identified a serious threat to patient safety and a cause for great concern, said Dr. Walter H. Ettinger.
"On an annualized basis, more than 3,800 patients at these two hospitals alone were put at unnecessary risk. If the estimates in this manuscript are generalizable, hundreds of thousands of patients nationwide may be receiving toxic doses of acetaminophen while in the hospital," he noted.
The study is important, "but it is incomplete and illustrates the obstacles we must overcome in using [health information technology] to drive performance. The authors identified a major flaw in the way in which a common drug is used in the hospital setting, and in a rapid-learning, high-performance health system, the results would be rapidly disseminated, together with a definitive solution for preventing excessive dosing in the future. The authors suggested educating health care providers about the risks of acetaminophen use and changing hospital policies to reduce risk factors for excessive dosing as a means of prevention. But excessive dosing of acetaminophen should be a ‘never event.’ The best way to prevent excessive dosing is to engineer a process and imbed it in the [electronic health record] such that it creates a hard stop that prevents ordering and administering more than 4 g/day of acetaminophen."
Dr. Ettinger is at the University of Massachusetts, Worcester, and at Accretive Health, Chicago, a health care technology and services firm. He reported no other relevant financial conflicts. These remarks were taken from his invited commentary accompanying Dr. Zhou’s report (Arch. Intern. Med. 2012 [doi:10.1001/2013.jamainternmed.607]).
Dr. Li Zhou and her colleagues have identified a serious threat to patient safety and a cause for great concern, said Dr. Walter H. Ettinger.
"On an annualized basis, more than 3,800 patients at these two hospitals alone were put at unnecessary risk. If the estimates in this manuscript are generalizable, hundreds of thousands of patients nationwide may be receiving toxic doses of acetaminophen while in the hospital," he noted.
The study is important, "but it is incomplete and illustrates the obstacles we must overcome in using [health information technology] to drive performance. The authors identified a major flaw in the way in which a common drug is used in the hospital setting, and in a rapid-learning, high-performance health system, the results would be rapidly disseminated, together with a definitive solution for preventing excessive dosing in the future. The authors suggested educating health care providers about the risks of acetaminophen use and changing hospital policies to reduce risk factors for excessive dosing as a means of prevention. But excessive dosing of acetaminophen should be a ‘never event.’ The best way to prevent excessive dosing is to engineer a process and imbed it in the [electronic health record] such that it creates a hard stop that prevents ordering and administering more than 4 g/day of acetaminophen."
Dr. Ettinger is at the University of Massachusetts, Worcester, and at Accretive Health, Chicago, a health care technology and services firm. He reported no other relevant financial conflicts. These remarks were taken from his invited commentary accompanying Dr. Zhou’s report (Arch. Intern. Med. 2012 [doi:10.1001/2013.jamainternmed.607]).
Dr. Li Zhou and her colleagues have identified a serious threat to patient safety and a cause for great concern, said Dr. Walter H. Ettinger.
"On an annualized basis, more than 3,800 patients at these two hospitals alone were put at unnecessary risk. If the estimates in this manuscript are generalizable, hundreds of thousands of patients nationwide may be receiving toxic doses of acetaminophen while in the hospital," he noted.
The study is important, "but it is incomplete and illustrates the obstacles we must overcome in using [health information technology] to drive performance. The authors identified a major flaw in the way in which a common drug is used in the hospital setting, and in a rapid-learning, high-performance health system, the results would be rapidly disseminated, together with a definitive solution for preventing excessive dosing in the future. The authors suggested educating health care providers about the risks of acetaminophen use and changing hospital policies to reduce risk factors for excessive dosing as a means of prevention. But excessive dosing of acetaminophen should be a ‘never event.’ The best way to prevent excessive dosing is to engineer a process and imbed it in the [electronic health record] such that it creates a hard stop that prevents ordering and administering more than 4 g/day of acetaminophen."
Dr. Ettinger is at the University of Massachusetts, Worcester, and at Accretive Health, Chicago, a health care technology and services firm. He reported no other relevant financial conflicts. These remarks were taken from his invited commentary accompanying Dr. Zhou’s report (Arch. Intern. Med. 2012 [doi:10.1001/2013.jamainternmed.607]).
Four percent of hospitalized adolescents and adults at two academic tertiary care medical centers received supratherapeutic doses of acetaminophen during their stays, according to a report published online Nov. 12 in Archives of Internal Medicine.
"These were not isolated events but often were successive and overlapping." Nearly half of the exposed patients received 5 g or more of acetaminophen per day, and 40% received excessive dosing for 3 or more days, said Dr. Li Zhou of Partners HealthCare System, Wellesley, Mass., and her associates.

Such overmedicating puts patients at unnecessary risk for hepatotoxicity, acute liver failure, and even death. In this study, even inpatients at specific risk for liver damage didn’t escape excessive dosing with acetaminophen: 22% of the elderly and 18% of patients with existing chronic liver disease were given acetaminophen in amounts exceeding their recommended limit of 3 g/day, said Dr. Zhou, who is also at Brigham and Women’s Hospital and Harvard Medical School, Boston, and her colleagues.
The risk factors most strongly associated with supratherapeutic dosing were receiving recurring scheduled dosing (that is, standing orders) as opposed to as-needed or one-time-only administration, which carried a hazard ratio of 16.6; receiving multiple products containing acetaminophen, which carried a hazard ratio of 2.4 for each additional product; and receiving products containing 500 mg of acetaminophen, which carried an HR of 1.9.
The investigators used the hospitals’ electronic medication administration record systems to assess acetaminophen exposure in the inpatient setting – a topic that has received little attention from researchers before now, they wrote. The study population comprised the 14,411 inpatients aged 12 and older who received any acetaminophen during their stays in a 3-month period. That accounts for about 61% of the entire patient population hospitalized at the two centers during the study period.
The average age of the study subjects was 55 years (range, 12-110 years), and a little more than one-third were older than 65. Approximately 75 had chronic liver disease.
Overall, 955 patients were given acetaminophen in doses exceeding the 4-g/day limit. This represents 4% of the entire hospitalized population and 6.6% of the cohort of patients given any acetaminophen, the researchers said (Arch. Intern. Med. 2012 [doi:10.1001/2013.jamainternmed.438]).
Each patient who received supratherapeutic dosing had a mean of five such incidents during the course of a mean of 3 days. Instances of such overmedicating ranged as high as 48 separate occasions over the course of 30 days.
Approximately 40% of the 955 patients received supratherapeutic dosing for 3 or more days, and 4% received it for 10 or more days.
"Our findings show that despite policies and procedures to monitor and control patients’ acetaminophen exposure, the incidence of supratherapeutic acetaminophen dosing in hospitalized patients remains high," Dr. Zhou and her associates said.
Previous studies have shown that many physicians and nurses aren’t aware of the maximal recommended daily dosing of acetaminophen, and that many have some difficulty identifying which products contain the agent. Therefore, increased training is warranted "to help clinicians identify acetaminophen-containing products and monitor closely the daily dose of acetaminophen," the investigators said.
A reassessment of long-accepted dosing regimens may also be warranted, "especially the scheduled use of products containing 500 mg of acetaminophen," they added.
The study was funded by the Partners-Siemens Research Council. The investigators reported no relevant financial conflicts.
Four percent of hospitalized adolescents and adults at two academic tertiary care medical centers received supratherapeutic doses of acetaminophen during their stays, according to a report published online Nov. 12 in Archives of Internal Medicine.
"These were not isolated events but often were successive and overlapping." Nearly half of the exposed patients received 5 g or more of acetaminophen per day, and 40% received excessive dosing for 3 or more days, said Dr. Li Zhou of Partners HealthCare System, Wellesley, Mass., and her associates.

Such overmedicating puts patients at unnecessary risk for hepatotoxicity, acute liver failure, and even death. In this study, even inpatients at specific risk for liver damage didn’t escape excessive dosing with acetaminophen: 22% of the elderly and 18% of patients with existing chronic liver disease were given acetaminophen in amounts exceeding their recommended limit of 3 g/day, said Dr. Zhou, who is also at Brigham and Women’s Hospital and Harvard Medical School, Boston, and her colleagues.
The risk factors most strongly associated with supratherapeutic dosing were receiving recurring scheduled dosing (that is, standing orders) as opposed to as-needed or one-time-only administration, which carried a hazard ratio of 16.6; receiving multiple products containing acetaminophen, which carried a hazard ratio of 2.4 for each additional product; and receiving products containing 500 mg of acetaminophen, which carried an HR of 1.9.
The investigators used the hospitals’ electronic medication administration record systems to assess acetaminophen exposure in the inpatient setting – a topic that has received little attention from researchers before now, they wrote. The study population comprised the 14,411 inpatients aged 12 and older who received any acetaminophen during their stays in a 3-month period. That accounts for about 61% of the entire patient population hospitalized at the two centers during the study period.
The average age of the study subjects was 55 years (range, 12-110 years), and a little more than one-third were older than 65. Approximately 75 had chronic liver disease.
Overall, 955 patients were given acetaminophen in doses exceeding the 4-g/day limit. This represents 4% of the entire hospitalized population and 6.6% of the cohort of patients given any acetaminophen, the researchers said (Arch. Intern. Med. 2012 [doi:10.1001/2013.jamainternmed.438]).
Each patient who received supratherapeutic dosing had a mean of five such incidents during the course of a mean of 3 days. Instances of such overmedicating ranged as high as 48 separate occasions over the course of 30 days.
Approximately 40% of the 955 patients received supratherapeutic dosing for 3 or more days, and 4% received it for 10 or more days.
"Our findings show that despite policies and procedures to monitor and control patients’ acetaminophen exposure, the incidence of supratherapeutic acetaminophen dosing in hospitalized patients remains high," Dr. Zhou and her associates said.
Previous studies have shown that many physicians and nurses aren’t aware of the maximal recommended daily dosing of acetaminophen, and that many have some difficulty identifying which products contain the agent. Therefore, increased training is warranted "to help clinicians identify acetaminophen-containing products and monitor closely the daily dose of acetaminophen," the investigators said.
A reassessment of long-accepted dosing regimens may also be warranted, "especially the scheduled use of products containing 500 mg of acetaminophen," they added.
The study was funded by the Partners-Siemens Research Council. The investigators reported no relevant financial conflicts.
FROM ARCHIVES OF INTERNAL MEDICINE
Major Finding: Four percent of all adolescent and adult inpatients at two hospitals received supratherapeutic doses of acetaminophen during their stays, including 22% of the elderly and 18% of patients with chronic liver disease.
Data Source: Data are based on acetaminophen exposure determined from electronic medication administration records for 14,411 inpatients aged 12 and older at two urban academic tertiary care hospitals during a 3-month period.
Disclosures: The study was funded by the Partners-Siemens Research Council. The authors reported no relevant financial conflicts.
Informed Consent: Information Transfer or Communication?
Obtaining informed consent for surgery is one of the most common tasks surgeons perform. Ensuring that a patient has the capacity to make a decision and then explaining the indications, risks, and alternatives to the surgery is something that surgeons do hundreds of times a year. Although the process is routine, it reflects the importance of respecting patients’ autonomy in making informed decisions about their health care. Informed consent has been an accepted practice in American surgery for decades, but questions about the process can arise and elicit varied responses among surgeons.
Recently, an experienced colleague (Dr. S) called me to discuss a case that had been troubling him. A 68-year-old man had been referred to him for a surgical opinion after experiencing recurrent episodes of diverticulitis. He was a former insurance executive, now retired. He was married, but came without his wife to the appointment with Dr. S.
After completing the history and physical examination and reviewing the imaging, Dr. S recommended an elective sigmoid colon resection. He explained to the patient what was involved in the surgery and the expected recovery. According to Dr. S, the patient then stated, "I understand. When can we schedule it?" At this point, Dr. S stated that a few more things needed to be reviewed and began to discuss the potential risks of the surgery. The patient stopped him abruptly, saying that he knew that there were risks, but he preferred not to hear about them.
When Dr. S responded that it is always good to know the risks when deciding to have surgery, the patient stated, "Look, I trust my primary care doctor who sent me to see you. You seem like a good surgeon, and I have confidence in you. I don’t want to hear about the risks, because my hearing them won’t make them not happen and will only make me worry. I’ll sign anything that you want, but I don’t want you to tell me the risks."
Dr. S felt torn. He had always felt that the consent process was primarily about informing the patient of what could happen. Reviewing the potential risks would help the patient to become better informed in making the decision to have surgery. Dr. S felt that his patient’s desire not to be informed had short-circuited the process, and it now seemed incomplete.
Dr. S offered to call the patient’s wife to discuss the risks with her, but the patient said that would not be necessary since he was making his own decisions. Although there was no hostility in the patient’s remarks, Dr. S felt that he had pushed hard enough, so he obtained a signature on the consent form and documented in his note that he had offered to inform the patient of the risks but that the patient had refused to hear them.
In the 3 weeks between this office visit and the surgery, Dr. S related the case to a number of his surgical colleagues and received diametrically opposing views. One group of surgeons stated that as the surgeon, Dr. S had an ethical responsibility to ensure that his patient knew of the risks of the procedure. They felt strongly that unless Dr. S had reviewed these risks with the patient, the consent was not valid because "it was not truly informed." Another group of surgeons felt that the ethical responsibility was not to inform the patient, but rather to provide the opportunity for the patient to be informed. This second group felt that pushing the patient to hear information that he did not want to hear would actually be violating his autonomous choice not to be informed.
These opposing views of what to do in this case help to clarify what I believe is one of the central points of confusion about the informed consent process as it is currently used. Surgeons and patients alike often believe that the information transfer – that is, describing the risks of the surgery – is the central goal of the process. However, many studies have shown that patients do not actually remember much of what they are told by their surgeons. Specifically, few risks are remembered even on the same day that the surgeon has reviewed them and the consent form is signed. These data clearly suggest to me that the more important aspect of the informed consent process is the communication between the surgeon and the patient.
Communication suggests something more than providing a lecture to a patient. It suggests the importance of listening and responding to requests for more or less information. Given that the requirement for informed consent prior to surgery is grounded on the principle of respecting a patient’s autonomy, it seems clear that when a patient does not want to hear the information that a surgeon wants to convey, the surgeon must respect the patient’s choice not to be informed.
Although Dr. S had felt that something was missing in the consent process since it did not include "the usual discussion of risks," the patient’s choices had been respected and Dr. S had fully discharged his responsibilities in obtaining the patient’s informed consent.
Dr. Angelos is an ACS Fellow, the Linda Kohler Anderson Professor of Surgery and Surgical Ethics, chief, endocrine surgery, and associate director of the MacLean Center for Clinical Medical Ethics at the University of Chicago.
Obtaining informed consent for surgery is one of the most common tasks surgeons perform. Ensuring that a patient has the capacity to make a decision and then explaining the indications, risks, and alternatives to the surgery is something that surgeons do hundreds of times a year. Although the process is routine, it reflects the importance of respecting patients’ autonomy in making informed decisions about their health care. Informed consent has been an accepted practice in American surgery for decades, but questions about the process can arise and elicit varied responses among surgeons.
Recently, an experienced colleague (Dr. S) called me to discuss a case that had been troubling him. A 68-year-old man had been referred to him for a surgical opinion after experiencing recurrent episodes of diverticulitis. He was a former insurance executive, now retired. He was married, but came without his wife to the appointment with Dr. S.
After completing the history and physical examination and reviewing the imaging, Dr. S recommended an elective sigmoid colon resection. He explained to the patient what was involved in the surgery and the expected recovery. According to Dr. S, the patient then stated, "I understand. When can we schedule it?" At this point, Dr. S stated that a few more things needed to be reviewed and began to discuss the potential risks of the surgery. The patient stopped him abruptly, saying that he knew that there were risks, but he preferred not to hear about them.
When Dr. S responded that it is always good to know the risks when deciding to have surgery, the patient stated, "Look, I trust my primary care doctor who sent me to see you. You seem like a good surgeon, and I have confidence in you. I don’t want to hear about the risks, because my hearing them won’t make them not happen and will only make me worry. I’ll sign anything that you want, but I don’t want you to tell me the risks."
Dr. S felt torn. He had always felt that the consent process was primarily about informing the patient of what could happen. Reviewing the potential risks would help the patient to become better informed in making the decision to have surgery. Dr. S felt that his patient’s desire not to be informed had short-circuited the process, and it now seemed incomplete.
Dr. S offered to call the patient’s wife to discuss the risks with her, but the patient said that would not be necessary since he was making his own decisions. Although there was no hostility in the patient’s remarks, Dr. S felt that he had pushed hard enough, so he obtained a signature on the consent form and documented in his note that he had offered to inform the patient of the risks but that the patient had refused to hear them.
In the 3 weeks between this office visit and the surgery, Dr. S related the case to a number of his surgical colleagues and received diametrically opposing views. One group of surgeons stated that as the surgeon, Dr. S had an ethical responsibility to ensure that his patient knew of the risks of the procedure. They felt strongly that unless Dr. S had reviewed these risks with the patient, the consent was not valid because "it was not truly informed." Another group of surgeons felt that the ethical responsibility was not to inform the patient, but rather to provide the opportunity for the patient to be informed. This second group felt that pushing the patient to hear information that he did not want to hear would actually be violating his autonomous choice not to be informed.
These opposing views of what to do in this case help to clarify what I believe is one of the central points of confusion about the informed consent process as it is currently used. Surgeons and patients alike often believe that the information transfer – that is, describing the risks of the surgery – is the central goal of the process. However, many studies have shown that patients do not actually remember much of what they are told by their surgeons. Specifically, few risks are remembered even on the same day that the surgeon has reviewed them and the consent form is signed. These data clearly suggest to me that the more important aspect of the informed consent process is the communication between the surgeon and the patient.
Communication suggests something more than providing a lecture to a patient. It suggests the importance of listening and responding to requests for more or less information. Given that the requirement for informed consent prior to surgery is grounded on the principle of respecting a patient’s autonomy, it seems clear that when a patient does not want to hear the information that a surgeon wants to convey, the surgeon must respect the patient’s choice not to be informed.
Although Dr. S had felt that something was missing in the consent process since it did not include "the usual discussion of risks," the patient’s choices had been respected and Dr. S had fully discharged his responsibilities in obtaining the patient’s informed consent.
Dr. Angelos is an ACS Fellow, the Linda Kohler Anderson Professor of Surgery and Surgical Ethics, chief, endocrine surgery, and associate director of the MacLean Center for Clinical Medical Ethics at the University of Chicago.
Obtaining informed consent for surgery is one of the most common tasks surgeons perform. Ensuring that a patient has the capacity to make a decision and then explaining the indications, risks, and alternatives to the surgery is something that surgeons do hundreds of times a year. Although the process is routine, it reflects the importance of respecting patients’ autonomy in making informed decisions about their health care. Informed consent has been an accepted practice in American surgery for decades, but questions about the process can arise and elicit varied responses among surgeons.
Recently, an experienced colleague (Dr. S) called me to discuss a case that had been troubling him. A 68-year-old man had been referred to him for a surgical opinion after experiencing recurrent episodes of diverticulitis. He was a former insurance executive, now retired. He was married, but came without his wife to the appointment with Dr. S.
After completing the history and physical examination and reviewing the imaging, Dr. S recommended an elective sigmoid colon resection. He explained to the patient what was involved in the surgery and the expected recovery. According to Dr. S, the patient then stated, "I understand. When can we schedule it?" At this point, Dr. S stated that a few more things needed to be reviewed and began to discuss the potential risks of the surgery. The patient stopped him abruptly, saying that he knew that there were risks, but he preferred not to hear about them.
When Dr. S responded that it is always good to know the risks when deciding to have surgery, the patient stated, "Look, I trust my primary care doctor who sent me to see you. You seem like a good surgeon, and I have confidence in you. I don’t want to hear about the risks, because my hearing them won’t make them not happen and will only make me worry. I’ll sign anything that you want, but I don’t want you to tell me the risks."
Dr. S felt torn. He had always felt that the consent process was primarily about informing the patient of what could happen. Reviewing the potential risks would help the patient to become better informed in making the decision to have surgery. Dr. S felt that his patient’s desire not to be informed had short-circuited the process, and it now seemed incomplete.
Dr. S offered to call the patient’s wife to discuss the risks with her, but the patient said that would not be necessary since he was making his own decisions. Although there was no hostility in the patient’s remarks, Dr. S felt that he had pushed hard enough, so he obtained a signature on the consent form and documented in his note that he had offered to inform the patient of the risks but that the patient had refused to hear them.
In the 3 weeks between this office visit and the surgery, Dr. S related the case to a number of his surgical colleagues and received diametrically opposing views. One group of surgeons stated that as the surgeon, Dr. S had an ethical responsibility to ensure that his patient knew of the risks of the procedure. They felt strongly that unless Dr. S had reviewed these risks with the patient, the consent was not valid because "it was not truly informed." Another group of surgeons felt that the ethical responsibility was not to inform the patient, but rather to provide the opportunity for the patient to be informed. This second group felt that pushing the patient to hear information that he did not want to hear would actually be violating his autonomous choice not to be informed.
These opposing views of what to do in this case help to clarify what I believe is one of the central points of confusion about the informed consent process as it is currently used. Surgeons and patients alike often believe that the information transfer – that is, describing the risks of the surgery – is the central goal of the process. However, many studies have shown that patients do not actually remember much of what they are told by their surgeons. Specifically, few risks are remembered even on the same day that the surgeon has reviewed them and the consent form is signed. These data clearly suggest to me that the more important aspect of the informed consent process is the communication between the surgeon and the patient.
Communication suggests something more than providing a lecture to a patient. It suggests the importance of listening and responding to requests for more or less information. Given that the requirement for informed consent prior to surgery is grounded on the principle of respecting a patient’s autonomy, it seems clear that when a patient does not want to hear the information that a surgeon wants to convey, the surgeon must respect the patient’s choice not to be informed.
Although Dr. S had felt that something was missing in the consent process since it did not include "the usual discussion of risks," the patient’s choices had been respected and Dr. S had fully discharged his responsibilities in obtaining the patient’s informed consent.
Dr. Angelos is an ACS Fellow, the Linda Kohler Anderson Professor of Surgery and Surgical Ethics, chief, endocrine surgery, and associate director of the MacLean Center for Clinical Medical Ethics at the University of Chicago.
Twelve Reasons for Considering Buprenorphine as a Frontline Analgesic in the Management of Pain
Mellar P. Davis, MD, FCCP, FAAHPM
ABSTRACT: Buprenorphine is an opioid that has a complex and unique pharmacology which provides some advantages over other potent mu agonists. We review 12 reasons for considering buprenorphine as a frontline analgesic for moderate to severe pain: (1) Buprenorphine is effective in cancer pain; (2) buprenorphine is effective in treating neuropathic pain; (3) buprenorphine treats a broader array of pain phenotypes than do certain potent mu agonists, is associated with less analgesic tolerance, and can be combined with other mu agonists; (4) buprenorphine produces less constipation than do certain other potent mu agonists, and does not adversely affect the sphincter of Oddi; (5) buprenorphine has a ceiling effect on respiratory depression but not analgesia; (6) buprenorphine causes less cognitive impairment than do certain other opioids; (7) buprenorphine is not immunosuppressive like morphine and fentanyl; (8) buprenorphine does not adversely affect the hypothalamic-pituitary-adrenal axis or cause hypogonadism; (9) buprenorphine does not significantly prolong the QTc interval, and is associated with less sudden death than is methadone; (10) buprenorphine is a safe and effective analgesic for the elderly; (11) buprenorphine is one of the safest opioids to use in patients in renal failure and those on dialysis; and (12) withdrawal symptoms are milder and drug dependence is less with buprenorphine. In light of evidence for efficacy, safety, versatility, and cost, buprenorphine should be considered as a first-line analgesic.
*For a PDF of the full article and a Commentary by Paul Sloan, MD, click on the links to the left of this introduction.
Mellar P. Davis, MD, FCCP, FAAHPM
ABSTRACT: Buprenorphine is an opioid that has a complex and unique pharmacology which provides some advantages over other potent mu agonists. We review 12 reasons for considering buprenorphine as a frontline analgesic for moderate to severe pain: (1) Buprenorphine is effective in cancer pain; (2) buprenorphine is effective in treating neuropathic pain; (3) buprenorphine treats a broader array of pain phenotypes than do certain potent mu agonists, is associated with less analgesic tolerance, and can be combined with other mu agonists; (4) buprenorphine produces less constipation than do certain other potent mu agonists, and does not adversely affect the sphincter of Oddi; (5) buprenorphine has a ceiling effect on respiratory depression but not analgesia; (6) buprenorphine causes less cognitive impairment than do certain other opioids; (7) buprenorphine is not immunosuppressive like morphine and fentanyl; (8) buprenorphine does not adversely affect the hypothalamic-pituitary-adrenal axis or cause hypogonadism; (9) buprenorphine does not significantly prolong the QTc interval, and is associated with less sudden death than is methadone; (10) buprenorphine is a safe and effective analgesic for the elderly; (11) buprenorphine is one of the safest opioids to use in patients in renal failure and those on dialysis; and (12) withdrawal symptoms are milder and drug dependence is less with buprenorphine. In light of evidence for efficacy, safety, versatility, and cost, buprenorphine should be considered as a first-line analgesic.
*For a PDF of the full article and a Commentary by Paul Sloan, MD, click on the links to the left of this introduction.
Mellar P. Davis, MD, FCCP, FAAHPM
ABSTRACT: Buprenorphine is an opioid that has a complex and unique pharmacology which provides some advantages over other potent mu agonists. We review 12 reasons for considering buprenorphine as a frontline analgesic for moderate to severe pain: (1) Buprenorphine is effective in cancer pain; (2) buprenorphine is effective in treating neuropathic pain; (3) buprenorphine treats a broader array of pain phenotypes than do certain potent mu agonists, is associated with less analgesic tolerance, and can be combined with other mu agonists; (4) buprenorphine produces less constipation than do certain other potent mu agonists, and does not adversely affect the sphincter of Oddi; (5) buprenorphine has a ceiling effect on respiratory depression but not analgesia; (6) buprenorphine causes less cognitive impairment than do certain other opioids; (7) buprenorphine is not immunosuppressive like morphine and fentanyl; (8) buprenorphine does not adversely affect the hypothalamic-pituitary-adrenal axis or cause hypogonadism; (9) buprenorphine does not significantly prolong the QTc interval, and is associated with less sudden death than is methadone; (10) buprenorphine is a safe and effective analgesic for the elderly; (11) buprenorphine is one of the safest opioids to use in patients in renal failure and those on dialysis; and (12) withdrawal symptoms are milder and drug dependence is less with buprenorphine. In light of evidence for efficacy, safety, versatility, and cost, buprenorphine should be considered as a first-line analgesic.
*For a PDF of the full article and a Commentary by Paul Sloan, MD, click on the links to the left of this introduction.
Highlights from the 2012 Annual Meeting of the American Society of Clinical Oncology
Highlights from the 2012 Annual Meeting of the American Society of Clinical Oncology 48th Annual Meeting
*For a PDF of the full article, click on the link to the left of this introduction.
Highlights from the 2012 Annual Meeting of the American Society of Clinical Oncology 48th Annual Meeting
*For a PDF of the full article, click on the link to the left of this introduction.
Highlights from the 2012 Annual Meeting of the American Society of Clinical Oncology 48th Annual Meeting
*For a PDF of the full article, click on the link to the left of this introduction.
Benefit of Young Liver Donors Scrutinized in Study
BOSTON – Younger age of deceased liver donors appeared to improve patient and graft survival when donors and recipients were both of younger age, but not when younger donor livers went to older recipients, according to a secondary analysis of the Organ Procurement and Transplantation Network national database from 2002 to 2011.
"Matching younger recipients with younger donors appears to be the best strategy in order to maximize life years after liver transplantation. However, before this can be considered for allocation policy, much more work needs to be done," including analyses specific to United Network for Organ Sharing regions, Model for End-Stage Liver Disease groups, and modeling of the potential life-years saved with a change in allocation policy, said lead investigator Dr. Neehar D. Parikh of the Comprehensive Transplant Center at Northwestern University, Chicago.
The risk of recipient death is known to increase with advancing donor age, beginning at ages older than 40 years and especially with donors older than 60 years. Also, advancing recipient age has been shown, with some controversy, to affect outcomes negatively, Dr. Parikh said at the annual meeting of the American Association for the Study of Liver Diseases.
Dr. Parikh and his colleagues’ study of the Organ Procurement and Transplantation Network database analyzed 26,849 cases of liver transplantation in which the donor was younger than the recipient, 782 cases in which they were the same age, and 12,107 cases in which the donor was older than the recipient. They also looked at outcomes of patients aged 18-40 years and those 60 years or older, based on the donor age.
As would be expected, recipients had progressively younger mean ages as the donor age increased: 56 years for recipients with younger donors, 52 years for same-age donors, and 48 years for older donors. Hepatitis C virus seropositivity was lowest in recipients who had an older donor (41%), followed by same-age recipient-donor pairs (51%), and recipients who had a younger donor (50%).
In Kaplan-Meier analyses, patient survival at 1 year was significantly worse for those who received a liver from a donor with an older age (86%) or the same age (86%) than from a younger donor (87.4%). Similar results were seen at 3 years between recipients of livers from older (74.5%), same age (73.5%), and younger donors (77.9%).
Older donor age increased the risk for patient death by 14% and graft failure by 13%, in comparison with younger donor age in an adjusted analysis.
For every 10-year increase in patient age for younger donors, the risk of patient mortality increased by 24% and graft failure by 17%. For every 10 years the donor was younger than the recipient, however, there was a 27% decline in the risk of patient mortality and 30% decrease in the risk of graft failure. A small but significance beneficial interaction was found between young donor age and young recipient age on both outcomes.
In contrast, for every 10 years the donor was older than the recipient, the risk of patient mortality increased 9% and the risk of graft failure increased by 22%, and there was no significant interaction between recipient and donor age.
When compared against donor and recipient pairs who were both aged 18-40 years, the risk of patient mortality and graft failure increased significantly for all other combinations of donor and recipient pairs: recipients aged 18-40 years and donors aged 60 years or older (hazard ratios of 1.76 and 1.49, respectively), recipients aged 60 years older and donors aged 18-40 years (HRs of 1.67 and 1.83), and recipients and donors both aged 60 years or older (HRs of 2.12 and 1.87). These results were consistent at both 1 and 3 years.
The overall results "suggest that there is something intrinsic about being older or having an older graft that cannot be remediated by graft or patient age factors," Dr. Parikh said.
The results might be explained in livers from older donors by impaired liver regeneration and increased susceptibility to ischemic reperfusion injury, while older recipients might have decreased circulating stem cells and more competing risks that are not adequately risk adjusted in the available data in older recipients, he said.
One audience member noted that there is still a "huge survival advantage" in all patient populations that receive younger donor livers and that allocating organs based on age is a slippery slope.
Dr. Parikh and his colleagues had no relevant financial disclosures.
BOSTON – Younger age of deceased liver donors appeared to improve patient and graft survival when donors and recipients were both of younger age, but not when younger donor livers went to older recipients, according to a secondary analysis of the Organ Procurement and Transplantation Network national database from 2002 to 2011.
"Matching younger recipients with younger donors appears to be the best strategy in order to maximize life years after liver transplantation. However, before this can be considered for allocation policy, much more work needs to be done," including analyses specific to United Network for Organ Sharing regions, Model for End-Stage Liver Disease groups, and modeling of the potential life-years saved with a change in allocation policy, said lead investigator Dr. Neehar D. Parikh of the Comprehensive Transplant Center at Northwestern University, Chicago.
The risk of recipient death is known to increase with advancing donor age, beginning at ages older than 40 years and especially with donors older than 60 years. Also, advancing recipient age has been shown, with some controversy, to affect outcomes negatively, Dr. Parikh said at the annual meeting of the American Association for the Study of Liver Diseases.
Dr. Parikh and his colleagues’ study of the Organ Procurement and Transplantation Network database analyzed 26,849 cases of liver transplantation in which the donor was younger than the recipient, 782 cases in which they were the same age, and 12,107 cases in which the donor was older than the recipient. They also looked at outcomes of patients aged 18-40 years and those 60 years or older, based on the donor age.
As would be expected, recipients had progressively younger mean ages as the donor age increased: 56 years for recipients with younger donors, 52 years for same-age donors, and 48 years for older donors. Hepatitis C virus seropositivity was lowest in recipients who had an older donor (41%), followed by same-age recipient-donor pairs (51%), and recipients who had a younger donor (50%).
In Kaplan-Meier analyses, patient survival at 1 year was significantly worse for those who received a liver from a donor with an older age (86%) or the same age (86%) than from a younger donor (87.4%). Similar results were seen at 3 years between recipients of livers from older (74.5%), same age (73.5%), and younger donors (77.9%).
Older donor age increased the risk for patient death by 14% and graft failure by 13%, in comparison with younger donor age in an adjusted analysis.
For every 10-year increase in patient age for younger donors, the risk of patient mortality increased by 24% and graft failure by 17%. For every 10 years the donor was younger than the recipient, however, there was a 27% decline in the risk of patient mortality and 30% decrease in the risk of graft failure. A small but significance beneficial interaction was found between young donor age and young recipient age on both outcomes.
In contrast, for every 10 years the donor was older than the recipient, the risk of patient mortality increased 9% and the risk of graft failure increased by 22%, and there was no significant interaction between recipient and donor age.
When compared against donor and recipient pairs who were both aged 18-40 years, the risk of patient mortality and graft failure increased significantly for all other combinations of donor and recipient pairs: recipients aged 18-40 years and donors aged 60 years or older (hazard ratios of 1.76 and 1.49, respectively), recipients aged 60 years older and donors aged 18-40 years (HRs of 1.67 and 1.83), and recipients and donors both aged 60 years or older (HRs of 2.12 and 1.87). These results were consistent at both 1 and 3 years.
The overall results "suggest that there is something intrinsic about being older or having an older graft that cannot be remediated by graft or patient age factors," Dr. Parikh said.
The results might be explained in livers from older donors by impaired liver regeneration and increased susceptibility to ischemic reperfusion injury, while older recipients might have decreased circulating stem cells and more competing risks that are not adequately risk adjusted in the available data in older recipients, he said.
One audience member noted that there is still a "huge survival advantage" in all patient populations that receive younger donor livers and that allocating organs based on age is a slippery slope.
Dr. Parikh and his colleagues had no relevant financial disclosures.
BOSTON – Younger age of deceased liver donors appeared to improve patient and graft survival when donors and recipients were both of younger age, but not when younger donor livers went to older recipients, according to a secondary analysis of the Organ Procurement and Transplantation Network national database from 2002 to 2011.
"Matching younger recipients with younger donors appears to be the best strategy in order to maximize life years after liver transplantation. However, before this can be considered for allocation policy, much more work needs to be done," including analyses specific to United Network for Organ Sharing regions, Model for End-Stage Liver Disease groups, and modeling of the potential life-years saved with a change in allocation policy, said lead investigator Dr. Neehar D. Parikh of the Comprehensive Transplant Center at Northwestern University, Chicago.
The risk of recipient death is known to increase with advancing donor age, beginning at ages older than 40 years and especially with donors older than 60 years. Also, advancing recipient age has been shown, with some controversy, to affect outcomes negatively, Dr. Parikh said at the annual meeting of the American Association for the Study of Liver Diseases.
Dr. Parikh and his colleagues’ study of the Organ Procurement and Transplantation Network database analyzed 26,849 cases of liver transplantation in which the donor was younger than the recipient, 782 cases in which they were the same age, and 12,107 cases in which the donor was older than the recipient. They also looked at outcomes of patients aged 18-40 years and those 60 years or older, based on the donor age.
As would be expected, recipients had progressively younger mean ages as the donor age increased: 56 years for recipients with younger donors, 52 years for same-age donors, and 48 years for older donors. Hepatitis C virus seropositivity was lowest in recipients who had an older donor (41%), followed by same-age recipient-donor pairs (51%), and recipients who had a younger donor (50%).
In Kaplan-Meier analyses, patient survival at 1 year was significantly worse for those who received a liver from a donor with an older age (86%) or the same age (86%) than from a younger donor (87.4%). Similar results were seen at 3 years between recipients of livers from older (74.5%), same age (73.5%), and younger donors (77.9%).
Older donor age increased the risk for patient death by 14% and graft failure by 13%, in comparison with younger donor age in an adjusted analysis.
For every 10-year increase in patient age for younger donors, the risk of patient mortality increased by 24% and graft failure by 17%. For every 10 years the donor was younger than the recipient, however, there was a 27% decline in the risk of patient mortality and 30% decrease in the risk of graft failure. A small but significance beneficial interaction was found between young donor age and young recipient age on both outcomes.
In contrast, for every 10 years the donor was older than the recipient, the risk of patient mortality increased 9% and the risk of graft failure increased by 22%, and there was no significant interaction between recipient and donor age.
When compared against donor and recipient pairs who were both aged 18-40 years, the risk of patient mortality and graft failure increased significantly for all other combinations of donor and recipient pairs: recipients aged 18-40 years and donors aged 60 years or older (hazard ratios of 1.76 and 1.49, respectively), recipients aged 60 years older and donors aged 18-40 years (HRs of 1.67 and 1.83), and recipients and donors both aged 60 years or older (HRs of 2.12 and 1.87). These results were consistent at both 1 and 3 years.
The overall results "suggest that there is something intrinsic about being older or having an older graft that cannot be remediated by graft or patient age factors," Dr. Parikh said.
The results might be explained in livers from older donors by impaired liver regeneration and increased susceptibility to ischemic reperfusion injury, while older recipients might have decreased circulating stem cells and more competing risks that are not adequately risk adjusted in the available data in older recipients, he said.
One audience member noted that there is still a "huge survival advantage" in all patient populations that receive younger donor livers and that allocating organs based on age is a slippery slope.
Dr. Parikh and his colleagues had no relevant financial disclosures.
AT THE ANNUAL MEETING OF THE AMERICAN ASSOCIATION FOR THE STUDY OF LIVER DISEASES
Major Finding: For every 10-year increase in patient age for younger donors, the risk of patient mortality increased by 24% and graft failure by 17%; for every 10 years the donor was younger than the recipient, there was a 27% decline in the risk of patient mortality and a 30% decrease in the risk of graft failure.
Data Source: This was a secondary analysis of the Organ Procurement and Transplantation Network national database from 2002 to 2011 for adult cadaveric liver transplantation.
Disclosures: Dr. Parikh and his colleagues had no relevant financial disclosures.