User login
No added benefit from revascularization in low-risk CAS
MUNICH – , suggests a planned interim analysis of ECST-2.
Almost 430 patients with symptomatic and asymptomatic atherosclerotic carotid stenosis greater than or equal to 50% and a Carotid Artery Risk (CAR) score less than 20% were randomly assigned to OMT alone or OMT plus revascularization with carotid endarterectomy (CEA) or carotid artery stenting.
The study, which was presented at the annual European Stroke Organisation Conference, was stopped early because of slow recruitment.
Nevertheless, the current results showed that there was no significant difference at 2 years between the treatment groups in the rate of a composite endpoint, as well as the occurrence of any stroke, myocardial infarction, and periprocedural death.
In other words, “there was no evidence of benefit at 2 years from additional carotid revascularization” in patients with carotid stenosis who had a low to intermediate predicted stroke risk, said study presenter Paul Nederkoorn, MD, PhD, department of neurology, Amsterdam UMC, University of Amsterdam.
He added, however, that the complete 2 years will include additional analyses, including an analysis of silent infarcts on MRI, which may affect the results, and that longer clinical follow-up is required.
Future work will include the design and validation of a novel stroke risk prediction tool that will include MRI plaque imaging and will allow individualized patient selection for revascularization, as well as a cost-effectiveness analysis, he noted.
Conclusions ‘difficult’
Session co-chair Peter Kelly, MD, professor of neurology at Mater University Hospital/University College Dublin, and president-elect of the European Stroke Association, described the findings as “interesting” and that it was “great to see them.”
“I’m sure we’ll be discussing these results for a while,” he added.
But co-chair Else Charlotte Sandset, MD, PhD, a consultant neurologist in the Stroke Unit, department of neurology, Oslo University Hospital, said that it’s “difficult to draw firm conclusions from the trial.”
The patients were highly selected, recruitment was “perhaps a bit too slow,” and the study was probably conducted over too many sites, she said in an interview.
Dr. Sandset also noted that the options available for OMT have changed over the course of the study, as well as the overall approach to management.
“We are more aware of how we should treat” these patients, and “we’re probably a bit more aggressive,” which will have shifted the outcomes in the comparator arm as the study progressed.
“That is the challenge of doing these trials that take many years to run – our practice changes.”
‘Old evidence’
In his presentation, Dr. Nederkoorn pointed out that, while the current guidelines for CEA are “robust,” they are based on “old evidence” from trials conducted 20-30 years ago.
During that time, he said, medical treatment has improved significantly, and the risk for stroke has approximately halved. Yet the decision to perform CEA is still largely based on the degree of stenosis and the patient’s symptom status.
Dr. Nederkoorn suggested, however, that factors such as plaque ulceration and patient characteristics and comorbidities might influence the risk-benefit ratio for revascularization.
The current trial was therefore established to test the hypothesis that patients with carotid stenosis greater than or equal to 50% and a low to intermediate risk of stroke will not benefit from additional carotid revascularization on top of optimized medical therapy.
The team conducted a prospective, multicenter, open clinical trial in which patients with both symptomatic and asymptomatic atherosclerotic carotid stenosis were randomly assigned to revascularization plus OMT or OMT alone.
Dr. Nederkoorn explained that a low to intermediate 5-year risk for stroke was established using the CAR score less than 20%.
This is based on a range of parameters, including the sex and age of the patient, degree of stenosis, the type of and time since the event, and the presence of comorbidities, among other factors.
He said that the data was originally derived from the NASCET trial, which was published in 1998, and the first ECST trial, published in the same year.
Since then, the risk of ipsilateral stroke has “strongly declined,” Dr. Nederkoorn said, and so the CAR score was recalibrated to reflect the likely benefit of current OMT.
For the trial, OMT included antihypertensive and cholesterol-lowering medications, and dietary changes, alongside antiplatelet agents and anticoagulation, if indicated, to achieve predefined, guideline-led lipid and blood pressure targets.
Revascularization included CEA and coronary artery stenting in selected patients and was recommended to be performed within 2 weeks of randomization in symptomatic patients and within 4 weeks in asymptomatic patients.
When the trial started in 2012, the intention was to recruit 2,000 patients, with a planned interim analysis after enrollment of 320 patients.
However, recruitment was suspended in 2019, with 429 patients having been enrolled, as it was clear that achieving a cohort of 2,000 patients was “not practical without a change in the trial design” to include MRI plaque imaging and without further funding.
Dr. Nederkoorn showed that the baseline characteristics of the OMT and revascularization plus OMT groups were comparable. The average age of the patients in the groups was 71-72 years, and 31% were female.
Symptomatic disease was present in about 40% of patients, and about 76% had hypertension. Type 2 diabetes was reported in roughly one-quarter of the patients.
There was no difference in the time from randomization to the revascularization procedure between patients with asymptomatic and symptomatic disease.
Moving to the primary outcome, which was a composite of periprocedural death within 90 days of randomization and clinically manifest stroke or myocardial infarction at 2 years, Dr. Nederkoorn showed that there was no significant difference between the treatment groups.
Despite a suggestion that patients undergoing revascularization experienced “more harm” in the initial follow-up period, particularly in patients with a CAR score greater than 10%, the event curves met at around 18 months.
Overall, the hazard ratio between revascularization plus OMT versus OMT alone was 0.96 (95% confidence interval, 0.53-1.76, P = .90).
Breaking down the composite endpoint, there was a numerically lower rate of any stroke with OMT alone, compared with revascularization plus OMT over the study period, but again the difference was not significant at 2 years, at a hazard ratio of 0.68 (95% CI, 0.32-1.42, P = .30).
There was only one case of periprocedural death, in the revascularization arm. Although myocardial infarction was numerically twice as likely with OMT alone, compared with the combined intervention arm, the difference was not significant, at a hazard ratio of 2.00 (95% CI, 0.68-5.84, P = .21).
The study was funded by the National Institute for Health and Care Research, the Swiss National Science Foundation, The Netherlands Organisation of Scientific Research, and the Leeds Neurology Foundation. No relevant financial relationships were declared.
A version of this article first appeared on Medscape.com.
MUNICH – , suggests a planned interim analysis of ECST-2.
Almost 430 patients with symptomatic and asymptomatic atherosclerotic carotid stenosis greater than or equal to 50% and a Carotid Artery Risk (CAR) score less than 20% were randomly assigned to OMT alone or OMT plus revascularization with carotid endarterectomy (CEA) or carotid artery stenting.
The study, which was presented at the annual European Stroke Organisation Conference, was stopped early because of slow recruitment.
Nevertheless, the current results showed that there was no significant difference at 2 years between the treatment groups in the rate of a composite endpoint, as well as the occurrence of any stroke, myocardial infarction, and periprocedural death.
In other words, “there was no evidence of benefit at 2 years from additional carotid revascularization” in patients with carotid stenosis who had a low to intermediate predicted stroke risk, said study presenter Paul Nederkoorn, MD, PhD, department of neurology, Amsterdam UMC, University of Amsterdam.
He added, however, that the complete 2 years will include additional analyses, including an analysis of silent infarcts on MRI, which may affect the results, and that longer clinical follow-up is required.
Future work will include the design and validation of a novel stroke risk prediction tool that will include MRI plaque imaging and will allow individualized patient selection for revascularization, as well as a cost-effectiveness analysis, he noted.
Conclusions ‘difficult’
Session co-chair Peter Kelly, MD, professor of neurology at Mater University Hospital/University College Dublin, and president-elect of the European Stroke Association, described the findings as “interesting” and that it was “great to see them.”
“I’m sure we’ll be discussing these results for a while,” he added.
But co-chair Else Charlotte Sandset, MD, PhD, a consultant neurologist in the Stroke Unit, department of neurology, Oslo University Hospital, said that it’s “difficult to draw firm conclusions from the trial.”
The patients were highly selected, recruitment was “perhaps a bit too slow,” and the study was probably conducted over too many sites, she said in an interview.
Dr. Sandset also noted that the options available for OMT have changed over the course of the study, as well as the overall approach to management.
“We are more aware of how we should treat” these patients, and “we’re probably a bit more aggressive,” which will have shifted the outcomes in the comparator arm as the study progressed.
“That is the challenge of doing these trials that take many years to run – our practice changes.”
‘Old evidence’
In his presentation, Dr. Nederkoorn pointed out that, while the current guidelines for CEA are “robust,” they are based on “old evidence” from trials conducted 20-30 years ago.
During that time, he said, medical treatment has improved significantly, and the risk for stroke has approximately halved. Yet the decision to perform CEA is still largely based on the degree of stenosis and the patient’s symptom status.
Dr. Nederkoorn suggested, however, that factors such as plaque ulceration and patient characteristics and comorbidities might influence the risk-benefit ratio for revascularization.
The current trial was therefore established to test the hypothesis that patients with carotid stenosis greater than or equal to 50% and a low to intermediate risk of stroke will not benefit from additional carotid revascularization on top of optimized medical therapy.
The team conducted a prospective, multicenter, open clinical trial in which patients with both symptomatic and asymptomatic atherosclerotic carotid stenosis were randomly assigned to revascularization plus OMT or OMT alone.
Dr. Nederkoorn explained that a low to intermediate 5-year risk for stroke was established using the CAR score less than 20%.
This is based on a range of parameters, including the sex and age of the patient, degree of stenosis, the type of and time since the event, and the presence of comorbidities, among other factors.
He said that the data was originally derived from the NASCET trial, which was published in 1998, and the first ECST trial, published in the same year.
Since then, the risk of ipsilateral stroke has “strongly declined,” Dr. Nederkoorn said, and so the CAR score was recalibrated to reflect the likely benefit of current OMT.
For the trial, OMT included antihypertensive and cholesterol-lowering medications, and dietary changes, alongside antiplatelet agents and anticoagulation, if indicated, to achieve predefined, guideline-led lipid and blood pressure targets.
Revascularization included CEA and coronary artery stenting in selected patients and was recommended to be performed within 2 weeks of randomization in symptomatic patients and within 4 weeks in asymptomatic patients.
When the trial started in 2012, the intention was to recruit 2,000 patients, with a planned interim analysis after enrollment of 320 patients.
However, recruitment was suspended in 2019, with 429 patients having been enrolled, as it was clear that achieving a cohort of 2,000 patients was “not practical without a change in the trial design” to include MRI plaque imaging and without further funding.
Dr. Nederkoorn showed that the baseline characteristics of the OMT and revascularization plus OMT groups were comparable. The average age of the patients in the groups was 71-72 years, and 31% were female.
Symptomatic disease was present in about 40% of patients, and about 76% had hypertension. Type 2 diabetes was reported in roughly one-quarter of the patients.
There was no difference in the time from randomization to the revascularization procedure between patients with asymptomatic and symptomatic disease.
Moving to the primary outcome, which was a composite of periprocedural death within 90 days of randomization and clinically manifest stroke or myocardial infarction at 2 years, Dr. Nederkoorn showed that there was no significant difference between the treatment groups.
Despite a suggestion that patients undergoing revascularization experienced “more harm” in the initial follow-up period, particularly in patients with a CAR score greater than 10%, the event curves met at around 18 months.
Overall, the hazard ratio between revascularization plus OMT versus OMT alone was 0.96 (95% confidence interval, 0.53-1.76, P = .90).
Breaking down the composite endpoint, there was a numerically lower rate of any stroke with OMT alone, compared with revascularization plus OMT over the study period, but again the difference was not significant at 2 years, at a hazard ratio of 0.68 (95% CI, 0.32-1.42, P = .30).
There was only one case of periprocedural death, in the revascularization arm. Although myocardial infarction was numerically twice as likely with OMT alone, compared with the combined intervention arm, the difference was not significant, at a hazard ratio of 2.00 (95% CI, 0.68-5.84, P = .21).
The study was funded by the National Institute for Health and Care Research, the Swiss National Science Foundation, The Netherlands Organisation of Scientific Research, and the Leeds Neurology Foundation. No relevant financial relationships were declared.
A version of this article first appeared on Medscape.com.
MUNICH – , suggests a planned interim analysis of ECST-2.
Almost 430 patients with symptomatic and asymptomatic atherosclerotic carotid stenosis greater than or equal to 50% and a Carotid Artery Risk (CAR) score less than 20% were randomly assigned to OMT alone or OMT plus revascularization with carotid endarterectomy (CEA) or carotid artery stenting.
The study, which was presented at the annual European Stroke Organisation Conference, was stopped early because of slow recruitment.
Nevertheless, the current results showed that there was no significant difference at 2 years between the treatment groups in the rate of a composite endpoint, as well as the occurrence of any stroke, myocardial infarction, and periprocedural death.
In other words, “there was no evidence of benefit at 2 years from additional carotid revascularization” in patients with carotid stenosis who had a low to intermediate predicted stroke risk, said study presenter Paul Nederkoorn, MD, PhD, department of neurology, Amsterdam UMC, University of Amsterdam.
He added, however, that the complete 2 years will include additional analyses, including an analysis of silent infarcts on MRI, which may affect the results, and that longer clinical follow-up is required.
Future work will include the design and validation of a novel stroke risk prediction tool that will include MRI plaque imaging and will allow individualized patient selection for revascularization, as well as a cost-effectiveness analysis, he noted.
Conclusions ‘difficult’
Session co-chair Peter Kelly, MD, professor of neurology at Mater University Hospital/University College Dublin, and president-elect of the European Stroke Association, described the findings as “interesting” and that it was “great to see them.”
“I’m sure we’ll be discussing these results for a while,” he added.
But co-chair Else Charlotte Sandset, MD, PhD, a consultant neurologist in the Stroke Unit, department of neurology, Oslo University Hospital, said that it’s “difficult to draw firm conclusions from the trial.”
The patients were highly selected, recruitment was “perhaps a bit too slow,” and the study was probably conducted over too many sites, she said in an interview.
Dr. Sandset also noted that the options available for OMT have changed over the course of the study, as well as the overall approach to management.
“We are more aware of how we should treat” these patients, and “we’re probably a bit more aggressive,” which will have shifted the outcomes in the comparator arm as the study progressed.
“That is the challenge of doing these trials that take many years to run – our practice changes.”
‘Old evidence’
In his presentation, Dr. Nederkoorn pointed out that, while the current guidelines for CEA are “robust,” they are based on “old evidence” from trials conducted 20-30 years ago.
During that time, he said, medical treatment has improved significantly, and the risk for stroke has approximately halved. Yet the decision to perform CEA is still largely based on the degree of stenosis and the patient’s symptom status.
Dr. Nederkoorn suggested, however, that factors such as plaque ulceration and patient characteristics and comorbidities might influence the risk-benefit ratio for revascularization.
The current trial was therefore established to test the hypothesis that patients with carotid stenosis greater than or equal to 50% and a low to intermediate risk of stroke will not benefit from additional carotid revascularization on top of optimized medical therapy.
The team conducted a prospective, multicenter, open clinical trial in which patients with both symptomatic and asymptomatic atherosclerotic carotid stenosis were randomly assigned to revascularization plus OMT or OMT alone.
Dr. Nederkoorn explained that a low to intermediate 5-year risk for stroke was established using the CAR score less than 20%.
This is based on a range of parameters, including the sex and age of the patient, degree of stenosis, the type of and time since the event, and the presence of comorbidities, among other factors.
He said that the data was originally derived from the NASCET trial, which was published in 1998, and the first ECST trial, published in the same year.
Since then, the risk of ipsilateral stroke has “strongly declined,” Dr. Nederkoorn said, and so the CAR score was recalibrated to reflect the likely benefit of current OMT.
For the trial, OMT included antihypertensive and cholesterol-lowering medications, and dietary changes, alongside antiplatelet agents and anticoagulation, if indicated, to achieve predefined, guideline-led lipid and blood pressure targets.
Revascularization included CEA and coronary artery stenting in selected patients and was recommended to be performed within 2 weeks of randomization in symptomatic patients and within 4 weeks in asymptomatic patients.
When the trial started in 2012, the intention was to recruit 2,000 patients, with a planned interim analysis after enrollment of 320 patients.
However, recruitment was suspended in 2019, with 429 patients having been enrolled, as it was clear that achieving a cohort of 2,000 patients was “not practical without a change in the trial design” to include MRI plaque imaging and without further funding.
Dr. Nederkoorn showed that the baseline characteristics of the OMT and revascularization plus OMT groups were comparable. The average age of the patients in the groups was 71-72 years, and 31% were female.
Symptomatic disease was present in about 40% of patients, and about 76% had hypertension. Type 2 diabetes was reported in roughly one-quarter of the patients.
There was no difference in the time from randomization to the revascularization procedure between patients with asymptomatic and symptomatic disease.
Moving to the primary outcome, which was a composite of periprocedural death within 90 days of randomization and clinically manifest stroke or myocardial infarction at 2 years, Dr. Nederkoorn showed that there was no significant difference between the treatment groups.
Despite a suggestion that patients undergoing revascularization experienced “more harm” in the initial follow-up period, particularly in patients with a CAR score greater than 10%, the event curves met at around 18 months.
Overall, the hazard ratio between revascularization plus OMT versus OMT alone was 0.96 (95% confidence interval, 0.53-1.76, P = .90).
Breaking down the composite endpoint, there was a numerically lower rate of any stroke with OMT alone, compared with revascularization plus OMT over the study period, but again the difference was not significant at 2 years, at a hazard ratio of 0.68 (95% CI, 0.32-1.42, P = .30).
There was only one case of periprocedural death, in the revascularization arm. Although myocardial infarction was numerically twice as likely with OMT alone, compared with the combined intervention arm, the difference was not significant, at a hazard ratio of 2.00 (95% CI, 0.68-5.84, P = .21).
The study was funded by the National Institute for Health and Care Research, the Swiss National Science Foundation, The Netherlands Organisation of Scientific Research, and the Leeds Neurology Foundation. No relevant financial relationships were declared.
A version of this article first appeared on Medscape.com.
FROM ESOC 2023
Itchy scaling rash
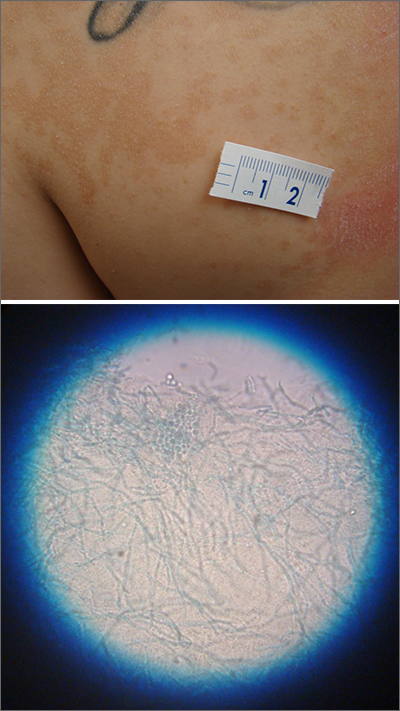
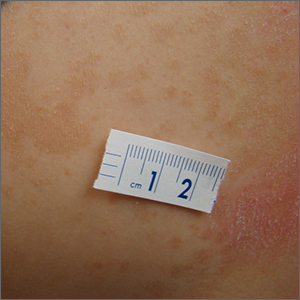
A waxing and waning rash with fine scale is classic for tinea versicolor (TV). A potassium hydroxide (KOH) prep with Swartz-Lamkins stain confirmed the presence of the spaghetti-and-meatballs pattern of Malassezia furfur (MF).
TV is a skin infection caused by M furfur. TF is notorious for the variety of colors that are seen clinically, including hyperpigmentation, as seen in a recent installment in this column.1 It can also appear as hypopigmented lesions or tan macules and patches with fine scale, as was seen in this patient. Hypopigmentation is often more pronounced on sun-exposed areas of the body. The MF produces azelaic acid. The azelaic acid blocks tyrosinase, which hinders melanocyte function and leads to hypopigmentation.2 As a result, areas of skin that are affected by TV do not tan as much as the surrounding skin, making the lesions more pronounced.
First line treatment of TV includes topical antifungal preparations, such as the “azoles” (eg, clotrimazole, ketoconazole, miconazole) twice daily for 2 to 4 weeks. However, the large surface areas involved would require a large amount of these antifungal preparations that come in relatively small tubes. Thus, for many years, clinicians have turned to economical over-the-counter dandruff shampoos with either selenium sulfide or zinc pyrithione that provide excellent results. These shampoos are applied to the entire trunk at full strength, allowed to dry, and then washed off later following various timed protocols. If topical therapy is not successful, or if there is a recurrence, systemic antifungal medications are used. Oral options include fluconazole 200 mg to 300 mg orally once a week for 2 weeks and itraconazole 200 mg orally once a day for 7 days.3 Ketoconazole is avoided as a systemic antifungal (except in life-threatening situations) due to its higher rate of liver dysfunction.
This patient was instructed to apply full-strength selenium sulfide shampoo to his entire trunk in the evening, allow it to dry, then wash it off the next morning and repeat in 1 week. An alternate regimen is to leave it on for 1 hour before washing and repeat daily for 1 week. At the patient’s follow-up appointment a month later, the rash and itching had resolved.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Jasser J, Stulberg D. Teen with hyperpigmented skin lesions. J Fam Pract. 2022;71. Published December 2022. Accessed May 26, 2023. www.mdedge.com/familymedicine/article/260076/dermatology/teen-hyperpigmented-skin-lesions. doi: 10.12788/jfp.0529
2. Leung AK, Barankin B, Lam JM, et al. Tinea versicolor: an updated review. Drugs Context. 2022;11:2022-9-2. doi: 10.7573/dic.2022-9-2
3. Gupta AK, Foley KA. Antifungal treatment for pityriasis versicolor. J Fungi (Basel). 2015;1:13-29. doi: 10.3390/jof1010013

A waxing and waning rash with fine scale is classic for tinea versicolor (TV). A potassium hydroxide (KOH) prep with Swartz-Lamkins stain confirmed the presence of the spaghetti-and-meatballs pattern of Malassezia furfur (MF).
TV is a skin infection caused by M furfur. TF is notorious for the variety of colors that are seen clinically, including hyperpigmentation, as seen in a recent installment in this column.1 It can also appear as hypopigmented lesions or tan macules and patches with fine scale, as was seen in this patient. Hypopigmentation is often more pronounced on sun-exposed areas of the body. The MF produces azelaic acid. The azelaic acid blocks tyrosinase, which hinders melanocyte function and leads to hypopigmentation.2 As a result, areas of skin that are affected by TV do not tan as much as the surrounding skin, making the lesions more pronounced.
First line treatment of TV includes topical antifungal preparations, such as the “azoles” (eg, clotrimazole, ketoconazole, miconazole) twice daily for 2 to 4 weeks. However, the large surface areas involved would require a large amount of these antifungal preparations that come in relatively small tubes. Thus, for many years, clinicians have turned to economical over-the-counter dandruff shampoos with either selenium sulfide or zinc pyrithione that provide excellent results. These shampoos are applied to the entire trunk at full strength, allowed to dry, and then washed off later following various timed protocols. If topical therapy is not successful, or if there is a recurrence, systemic antifungal medications are used. Oral options include fluconazole 200 mg to 300 mg orally once a week for 2 weeks and itraconazole 200 mg orally once a day for 7 days.3 Ketoconazole is avoided as a systemic antifungal (except in life-threatening situations) due to its higher rate of liver dysfunction.
This patient was instructed to apply full-strength selenium sulfide shampoo to his entire trunk in the evening, allow it to dry, then wash it off the next morning and repeat in 1 week. An alternate regimen is to leave it on for 1 hour before washing and repeat daily for 1 week. At the patient’s follow-up appointment a month later, the rash and itching had resolved.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.

A waxing and waning rash with fine scale is classic for tinea versicolor (TV). A potassium hydroxide (KOH) prep with Swartz-Lamkins stain confirmed the presence of the spaghetti-and-meatballs pattern of Malassezia furfur (MF).
TV is a skin infection caused by M furfur. TF is notorious for the variety of colors that are seen clinically, including hyperpigmentation, as seen in a recent installment in this column.1 It can also appear as hypopigmented lesions or tan macules and patches with fine scale, as was seen in this patient. Hypopigmentation is often more pronounced on sun-exposed areas of the body. The MF produces azelaic acid. The azelaic acid blocks tyrosinase, which hinders melanocyte function and leads to hypopigmentation.2 As a result, areas of skin that are affected by TV do not tan as much as the surrounding skin, making the lesions more pronounced.
First line treatment of TV includes topical antifungal preparations, such as the “azoles” (eg, clotrimazole, ketoconazole, miconazole) twice daily for 2 to 4 weeks. However, the large surface areas involved would require a large amount of these antifungal preparations that come in relatively small tubes. Thus, for many years, clinicians have turned to economical over-the-counter dandruff shampoos with either selenium sulfide or zinc pyrithione that provide excellent results. These shampoos are applied to the entire trunk at full strength, allowed to dry, and then washed off later following various timed protocols. If topical therapy is not successful, or if there is a recurrence, systemic antifungal medications are used. Oral options include fluconazole 200 mg to 300 mg orally once a week for 2 weeks and itraconazole 200 mg orally once a day for 7 days.3 Ketoconazole is avoided as a systemic antifungal (except in life-threatening situations) due to its higher rate of liver dysfunction.
This patient was instructed to apply full-strength selenium sulfide shampoo to his entire trunk in the evening, allow it to dry, then wash it off the next morning and repeat in 1 week. An alternate regimen is to leave it on for 1 hour before washing and repeat daily for 1 week. At the patient’s follow-up appointment a month later, the rash and itching had resolved.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Jasser J, Stulberg D. Teen with hyperpigmented skin lesions. J Fam Pract. 2022;71. Published December 2022. Accessed May 26, 2023. www.mdedge.com/familymedicine/article/260076/dermatology/teen-hyperpigmented-skin-lesions. doi: 10.12788/jfp.0529
2. Leung AK, Barankin B, Lam JM, et al. Tinea versicolor: an updated review. Drugs Context. 2022;11:2022-9-2. doi: 10.7573/dic.2022-9-2
3. Gupta AK, Foley KA. Antifungal treatment for pityriasis versicolor. J Fungi (Basel). 2015;1:13-29. doi: 10.3390/jof1010013
1. Jasser J, Stulberg D. Teen with hyperpigmented skin lesions. J Fam Pract. 2022;71. Published December 2022. Accessed May 26, 2023. www.mdedge.com/familymedicine/article/260076/dermatology/teen-hyperpigmented-skin-lesions. doi: 10.12788/jfp.0529
2. Leung AK, Barankin B, Lam JM, et al. Tinea versicolor: an updated review. Drugs Context. 2022;11:2022-9-2. doi: 10.7573/dic.2022-9-2
3. Gupta AK, Foley KA. Antifungal treatment for pityriasis versicolor. J Fungi (Basel). 2015;1:13-29. doi: 10.3390/jof1010013
Advances in Microbiome Therapeutics From DDW 2023
Study results of microbiotic therapeutics for Clostridioides difficile infection (CDI) and a microbial dietary score that points to increased cancer risk are the microbiome advances from Digestive Disease Week 2023, as selected by Dr Purna Kashyap, from the Mayo Clinic in Rochester, Minnesota.
Dr Kashyap starts with four studies examining microbiotic therapeutics for patients with CDI; the first two looked at RBX2660, which was recently approved by the US Food and Drug Administration (FDA).
The first study showed that clonal engraftment of RBX2660 microbiota was associated with clinical response to the treatment, while the second indicated that the therapy is safe and effective in immunocompromised patients.
Next, Dr Kashyap discusses a study of SER-109, also recently approved by the FDA. ESOSPOR IV revealed that the oral microbiome therapeutic achieved durable responses, even in patients with two or more CDI recurrences.
After discussing a final CDI study that may provide a mechanism for the effectiveness of the live biotherapeutic VE303, he moves on to colon cancer.
Dr Kashyap explains that a microbial dietary score was found to be associated not only with low-quality diets but also with an increased risk for colorectal cancer.
--
Purna C. Kashyap, MBBS, Professor of Medicine and Physiology; Consultant, Gastroenterology and Hepatology, Mayo Clinic, Rochester, Minnesota
Purna C. Kashyap, MBBS, has disclosed no relevant financial relationships.
Digestive Disease Week® was sponsored by the American Gastroenterological Association, the American Association for the Study of Liver Diseases, the American Society for Gastrointestinal Endoscopy, and the Society for Surgery of the Alimentary Tract.
Study results of microbiotic therapeutics for Clostridioides difficile infection (CDI) and a microbial dietary score that points to increased cancer risk are the microbiome advances from Digestive Disease Week 2023, as selected by Dr Purna Kashyap, from the Mayo Clinic in Rochester, Minnesota.
Dr Kashyap starts with four studies examining microbiotic therapeutics for patients with CDI; the first two looked at RBX2660, which was recently approved by the US Food and Drug Administration (FDA).
The first study showed that clonal engraftment of RBX2660 microbiota was associated with clinical response to the treatment, while the second indicated that the therapy is safe and effective in immunocompromised patients.
Next, Dr Kashyap discusses a study of SER-109, also recently approved by the FDA. ESOSPOR IV revealed that the oral microbiome therapeutic achieved durable responses, even in patients with two or more CDI recurrences.
After discussing a final CDI study that may provide a mechanism for the effectiveness of the live biotherapeutic VE303, he moves on to colon cancer.
Dr Kashyap explains that a microbial dietary score was found to be associated not only with low-quality diets but also with an increased risk for colorectal cancer.
--
Purna C. Kashyap, MBBS, Professor of Medicine and Physiology; Consultant, Gastroenterology and Hepatology, Mayo Clinic, Rochester, Minnesota
Purna C. Kashyap, MBBS, has disclosed no relevant financial relationships.
Digestive Disease Week® was sponsored by the American Gastroenterological Association, the American Association for the Study of Liver Diseases, the American Society for Gastrointestinal Endoscopy, and the Society for Surgery of the Alimentary Tract.
Study results of microbiotic therapeutics for Clostridioides difficile infection (CDI) and a microbial dietary score that points to increased cancer risk are the microbiome advances from Digestive Disease Week 2023, as selected by Dr Purna Kashyap, from the Mayo Clinic in Rochester, Minnesota.
Dr Kashyap starts with four studies examining microbiotic therapeutics for patients with CDI; the first two looked at RBX2660, which was recently approved by the US Food and Drug Administration (FDA).
The first study showed that clonal engraftment of RBX2660 microbiota was associated with clinical response to the treatment, while the second indicated that the therapy is safe and effective in immunocompromised patients.
Next, Dr Kashyap discusses a study of SER-109, also recently approved by the FDA. ESOSPOR IV revealed that the oral microbiome therapeutic achieved durable responses, even in patients with two or more CDI recurrences.
After discussing a final CDI study that may provide a mechanism for the effectiveness of the live biotherapeutic VE303, he moves on to colon cancer.
Dr Kashyap explains that a microbial dietary score was found to be associated not only with low-quality diets but also with an increased risk for colorectal cancer.
--
Purna C. Kashyap, MBBS, Professor of Medicine and Physiology; Consultant, Gastroenterology and Hepatology, Mayo Clinic, Rochester, Minnesota
Purna C. Kashyap, MBBS, has disclosed no relevant financial relationships.
Digestive Disease Week® was sponsored by the American Gastroenterological Association, the American Association for the Study of Liver Diseases, the American Society for Gastrointestinal Endoscopy, and the Society for Surgery of the Alimentary Tract.

Internists in 2022: Increased earnings can’t stop rising dissatisfaction
Internists experienced many of the usual ups and downs regarding nonclinical matters in 2022: Compensation was up, but satisfaction with compensation was down; the percentage of internists who would choose another specialty was up and time spent on paperwork and administration was down only slightly.
A year that began with the COVID-19 Omicron surge ended with many of the same old issues regaining the attention of physicians, according to those who responded to Medscape’s annual compensation survey, which was conducted from Oct. 2, 2022, to Jan. 17, 2023.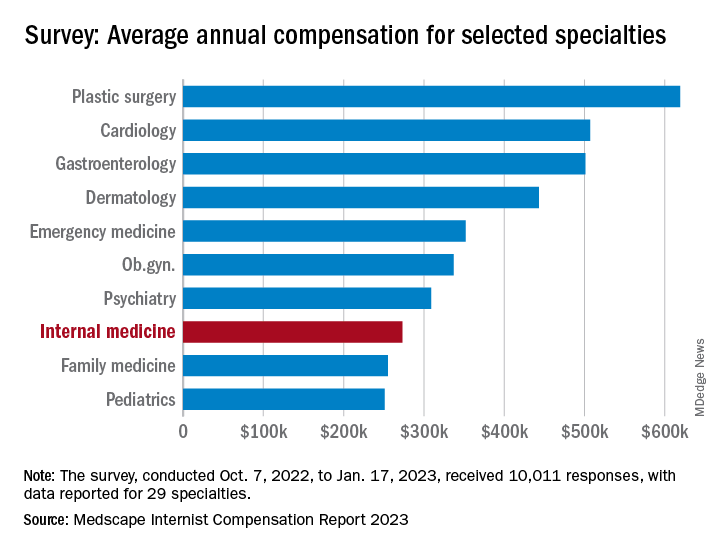
“Decreasing Medicare reimbursement and poor payor mix destroy our income,” one physician wrote, and another said that “patients have become rude and come with poor information from social media.” One respondent described the situation this way: “Overwhelming burnout. I had to reduce my hours to keep myself from quitting medicine completely.”
For internists at least, some of the survey results were positive. For the 13% of the 10,011 respondents who practice internal medicine, average compensation went from $264,000 in 2021 to $273,000 in 2022, an increase of almost 4% that matched the average for all physicians. Among the other primary care specialists, pediatricians did almost as well with a 3% increase, but ob.gyns. and family physicians only managed to keep their 2022 earnings at 2021 levels.
Overall physician compensation for 2022 was $352,000, an increase of almost 18% since 2018. “Supply and demand is the biggest driver,” Mike Belkin, JD, of physician recruitment firm Merritt Hawkins, said in an interview. “Organizations understand it’s not getting any easier to get good candidates, and so for the most part, physicians are getting good offers.”
The latest increase in earnings among internists also included a decline: The disparity between mens’ and womens’ compensation dropped from 24% in 2021 to 16% in 2022. The gap was slightly larger for all physicians in 2022, with men earning about 19% more than women, and larger again among specialists at 27%, but both of those figures are lower than in recent years, Medscape said.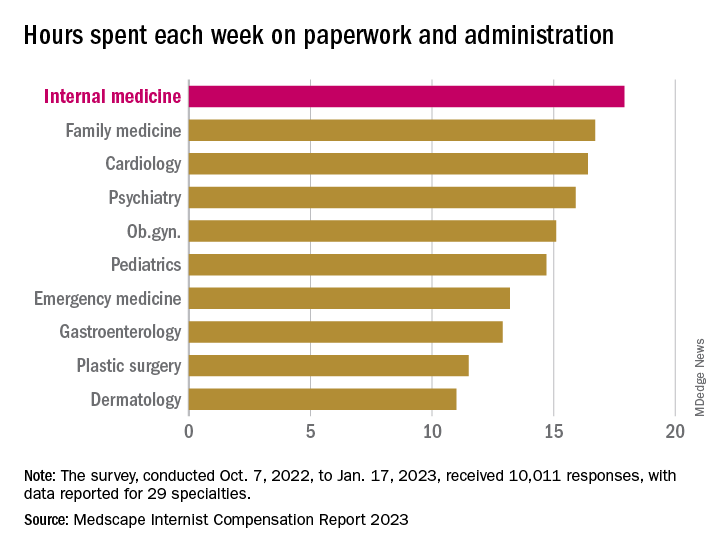
Satisfaction with their compensation, however, was not high for internists: Only 43% feel that they are fairly paid, coming in above only ophthalmology (42%) and infectious diseases (35%) and well below psychiatry (68%) at the top of the list, the Medscape data show. In the 2022 report, 49% of internists said that they had been fairly paid.
In another source of potential dissatisfaction, internist respondents reported spending an average of 17.9 hours each week on paperwork and administration, just below the survey leaders, physical medicine and rehabilitation (18.5 hours) and nephrology (18.1 hours) and well above anesthesiology, which was the lowest of the 29 specialties at 9.0 hours, and the 2022 average of 15.5 hours for all physicians, Medscape said. A small bright spot comes in the form of a decline from the internists’ time of 18.7 hours per week in 2021.
When asked if they would choose medicine again, 72% of internist respondents and 73% of all physicians said yes, with emergency medicine (65%) and dermatology (86%) representing the two extremes. A question about specialty choice showed internists to be the least likely of the 29 included specialties to follow the same path, with 61% (down from 63% in 2022) approving their initial selection, versus 97% for plastic surgeons, Medscape reported.
Commenters among the survey respondents were not identified by specialty, but dissatisfaction on many fronts was a definite theme:
- “Our costs go up, and our reimbursement does not.”
- “Our practice was acquired by venture capital firms; they slashed costs.”
- “My productivity bonus should have come to $45,000. Instead I was paid only $15,000. Yet cardiologists and administrators who were working from home part of the year received their full bonus.”
- “I will no longer practice cookbook mediocrity.”
Internists experienced many of the usual ups and downs regarding nonclinical matters in 2022: Compensation was up, but satisfaction with compensation was down; the percentage of internists who would choose another specialty was up and time spent on paperwork and administration was down only slightly.
A year that began with the COVID-19 Omicron surge ended with many of the same old issues regaining the attention of physicians, according to those who responded to Medscape’s annual compensation survey, which was conducted from Oct. 2, 2022, to Jan. 17, 2023.
“Decreasing Medicare reimbursement and poor payor mix destroy our income,” one physician wrote, and another said that “patients have become rude and come with poor information from social media.” One respondent described the situation this way: “Overwhelming burnout. I had to reduce my hours to keep myself from quitting medicine completely.”
For internists at least, some of the survey results were positive. For the 13% of the 10,011 respondents who practice internal medicine, average compensation went from $264,000 in 2021 to $273,000 in 2022, an increase of almost 4% that matched the average for all physicians. Among the other primary care specialists, pediatricians did almost as well with a 3% increase, but ob.gyns. and family physicians only managed to keep their 2022 earnings at 2021 levels.
Overall physician compensation for 2022 was $352,000, an increase of almost 18% since 2018. “Supply and demand is the biggest driver,” Mike Belkin, JD, of physician recruitment firm Merritt Hawkins, said in an interview. “Organizations understand it’s not getting any easier to get good candidates, and so for the most part, physicians are getting good offers.”
The latest increase in earnings among internists also included a decline: The disparity between mens’ and womens’ compensation dropped from 24% in 2021 to 16% in 2022. The gap was slightly larger for all physicians in 2022, with men earning about 19% more than women, and larger again among specialists at 27%, but both of those figures are lower than in recent years, Medscape said.
Satisfaction with their compensation, however, was not high for internists: Only 43% feel that they are fairly paid, coming in above only ophthalmology (42%) and infectious diseases (35%) and well below psychiatry (68%) at the top of the list, the Medscape data show. In the 2022 report, 49% of internists said that they had been fairly paid.
In another source of potential dissatisfaction, internist respondents reported spending an average of 17.9 hours each week on paperwork and administration, just below the survey leaders, physical medicine and rehabilitation (18.5 hours) and nephrology (18.1 hours) and well above anesthesiology, which was the lowest of the 29 specialties at 9.0 hours, and the 2022 average of 15.5 hours for all physicians, Medscape said. A small bright spot comes in the form of a decline from the internists’ time of 18.7 hours per week in 2021.
When asked if they would choose medicine again, 72% of internist respondents and 73% of all physicians said yes, with emergency medicine (65%) and dermatology (86%) representing the two extremes. A question about specialty choice showed internists to be the least likely of the 29 included specialties to follow the same path, with 61% (down from 63% in 2022) approving their initial selection, versus 97% for plastic surgeons, Medscape reported.
Commenters among the survey respondents were not identified by specialty, but dissatisfaction on many fronts was a definite theme:
- “Our costs go up, and our reimbursement does not.”
- “Our practice was acquired by venture capital firms; they slashed costs.”
- “My productivity bonus should have come to $45,000. Instead I was paid only $15,000. Yet cardiologists and administrators who were working from home part of the year received their full bonus.”
- “I will no longer practice cookbook mediocrity.”
Internists experienced many of the usual ups and downs regarding nonclinical matters in 2022: Compensation was up, but satisfaction with compensation was down; the percentage of internists who would choose another specialty was up and time spent on paperwork and administration was down only slightly.
A year that began with the COVID-19 Omicron surge ended with many of the same old issues regaining the attention of physicians, according to those who responded to Medscape’s annual compensation survey, which was conducted from Oct. 2, 2022, to Jan. 17, 2023.
“Decreasing Medicare reimbursement and poor payor mix destroy our income,” one physician wrote, and another said that “patients have become rude and come with poor information from social media.” One respondent described the situation this way: “Overwhelming burnout. I had to reduce my hours to keep myself from quitting medicine completely.”
For internists at least, some of the survey results were positive. For the 13% of the 10,011 respondents who practice internal medicine, average compensation went from $264,000 in 2021 to $273,000 in 2022, an increase of almost 4% that matched the average for all physicians. Among the other primary care specialists, pediatricians did almost as well with a 3% increase, but ob.gyns. and family physicians only managed to keep their 2022 earnings at 2021 levels.
Overall physician compensation for 2022 was $352,000, an increase of almost 18% since 2018. “Supply and demand is the biggest driver,” Mike Belkin, JD, of physician recruitment firm Merritt Hawkins, said in an interview. “Organizations understand it’s not getting any easier to get good candidates, and so for the most part, physicians are getting good offers.”
The latest increase in earnings among internists also included a decline: The disparity between mens’ and womens’ compensation dropped from 24% in 2021 to 16% in 2022. The gap was slightly larger for all physicians in 2022, with men earning about 19% more than women, and larger again among specialists at 27%, but both of those figures are lower than in recent years, Medscape said.
Satisfaction with their compensation, however, was not high for internists: Only 43% feel that they are fairly paid, coming in above only ophthalmology (42%) and infectious diseases (35%) and well below psychiatry (68%) at the top of the list, the Medscape data show. In the 2022 report, 49% of internists said that they had been fairly paid.
In another source of potential dissatisfaction, internist respondents reported spending an average of 17.9 hours each week on paperwork and administration, just below the survey leaders, physical medicine and rehabilitation (18.5 hours) and nephrology (18.1 hours) and well above anesthesiology, which was the lowest of the 29 specialties at 9.0 hours, and the 2022 average of 15.5 hours for all physicians, Medscape said. A small bright spot comes in the form of a decline from the internists’ time of 18.7 hours per week in 2021.
When asked if they would choose medicine again, 72% of internist respondents and 73% of all physicians said yes, with emergency medicine (65%) and dermatology (86%) representing the two extremes. A question about specialty choice showed internists to be the least likely of the 29 included specialties to follow the same path, with 61% (down from 63% in 2022) approving their initial selection, versus 97% for plastic surgeons, Medscape reported.
Commenters among the survey respondents were not identified by specialty, but dissatisfaction on many fronts was a definite theme:
- “Our costs go up, and our reimbursement does not.”
- “Our practice was acquired by venture capital firms; they slashed costs.”
- “My productivity bonus should have come to $45,000. Instead I was paid only $15,000. Yet cardiologists and administrators who were working from home part of the year received their full bonus.”
- “I will no longer practice cookbook mediocrity.”
Researchers discover brain abnormalities in babies who had SIDS
For decades, researchers have been trying to understand why some otherwise healthy babies under 1 year old mysteriously die during their sleep. SIDS is the leading cause of infant death in the U.S., affecting 103 out of every 100,000 babies.
The new study found that babies who died of SIDS had abnormalities in certain brain receptors responsible for waking and restoring breathing. The scientists decided to look at the babies’ brains at the molecular level because previous research showed that the same kind of brain receptors in rodents are responsible for protective breathing functions during sleep.
The study was published in the Journal of Neuropathology & Experimental Neurology. The researchers compared brain stems from 70 babies, some of whom died of SIDS and some who died of other causes.
Despite discovering the differences in the babies’ brains, the lead author of the paper said more study is needed.
Robin Haynes, PhD, who studies SIDS at Boston Children’s Hospital, said in a statement that “the relationship between the abnormalities and cause of death remains unknown.”
She said there is no way to identify babies with the brain abnormalities, and “thus, adherence to safe-sleep practices remains critical.”
The American Academy of Pediatrics recommends numerous steps for creating a safe sleeping environment for babies, including placing babies on their backs on a firm surface. Education campaigns targeting parents and caregivers in the 1990s are largely considered successful, but SIDS rates have remained steady since the practices became widely used.
A version of this article first appeared on WebMD.com.
For decades, researchers have been trying to understand why some otherwise healthy babies under 1 year old mysteriously die during their sleep. SIDS is the leading cause of infant death in the U.S., affecting 103 out of every 100,000 babies.
The new study found that babies who died of SIDS had abnormalities in certain brain receptors responsible for waking and restoring breathing. The scientists decided to look at the babies’ brains at the molecular level because previous research showed that the same kind of brain receptors in rodents are responsible for protective breathing functions during sleep.
The study was published in the Journal of Neuropathology & Experimental Neurology. The researchers compared brain stems from 70 babies, some of whom died of SIDS and some who died of other causes.
Despite discovering the differences in the babies’ brains, the lead author of the paper said more study is needed.
Robin Haynes, PhD, who studies SIDS at Boston Children’s Hospital, said in a statement that “the relationship between the abnormalities and cause of death remains unknown.”
She said there is no way to identify babies with the brain abnormalities, and “thus, adherence to safe-sleep practices remains critical.”
The American Academy of Pediatrics recommends numerous steps for creating a safe sleeping environment for babies, including placing babies on their backs on a firm surface. Education campaigns targeting parents and caregivers in the 1990s are largely considered successful, but SIDS rates have remained steady since the practices became widely used.
A version of this article first appeared on WebMD.com.
For decades, researchers have been trying to understand why some otherwise healthy babies under 1 year old mysteriously die during their sleep. SIDS is the leading cause of infant death in the U.S., affecting 103 out of every 100,000 babies.
The new study found that babies who died of SIDS had abnormalities in certain brain receptors responsible for waking and restoring breathing. The scientists decided to look at the babies’ brains at the molecular level because previous research showed that the same kind of brain receptors in rodents are responsible for protective breathing functions during sleep.
The study was published in the Journal of Neuropathology & Experimental Neurology. The researchers compared brain stems from 70 babies, some of whom died of SIDS and some who died of other causes.
Despite discovering the differences in the babies’ brains, the lead author of the paper said more study is needed.
Robin Haynes, PhD, who studies SIDS at Boston Children’s Hospital, said in a statement that “the relationship between the abnormalities and cause of death remains unknown.”
She said there is no way to identify babies with the brain abnormalities, and “thus, adherence to safe-sleep practices remains critical.”
The American Academy of Pediatrics recommends numerous steps for creating a safe sleeping environment for babies, including placing babies on their backs on a firm surface. Education campaigns targeting parents and caregivers in the 1990s are largely considered successful, but SIDS rates have remained steady since the practices became widely used.
A version of this article first appeared on WebMD.com.
FROM THE JOURNAL OF NEUROPATHY & EXPERIMENTAL NEUROLOGY
Key Takeaways in Ulcerative Colitis From DDW 2023
Efficacy and long-term safety data of novel drugs, thiopurine withdrawal, and the effect of high-dose opioid use on outcomes are among the key takeaways in ulcerative colitis from Digestive Disease Week (DDW) 2023, as reported by Dr Joseph Feuerstein, from Harvard Medical School, in Boston, Massachusetts.
Dr Feuerstein starts with the QUASAR study of the IL-13 inhibitor guselkumab, which showed that the drug was associated with significantly improved clinical remission over placebo.
Next, an open-label extension of the True North study demonstrated that the oral S1P receptor modulator ozanimod proved to be safe over a 3-year follow-up period. Another trial examining safety found that withdrawal from thiopurine and vedolizumab combination therapy may not be a viable strategy.
Dr Feuerstein then turns to a retrospective analysis of older patients who underwent segmental colectomy in which the procedure was associated with low rates of complications and postoperative flares.
Finally, another retrospective study suggested that, contrary to expectations, high-dose opioid use does not appear to worsen clinical outcomes in acute severe ulcerative colitis.
--
Joseph D. Feuerstein, MD, Associate Professor of Medicine, Department of Gastroenterology, Harvard Medical School; Attending in Gastroenterology, Center for Inflammatory Bowel Disease, Beth Israel Deaconess Medical Center, Boston, Massachusetts
Joseph D. Feuerstein, MD, has disclosed no relevant financial relationships.
Digestive Disease Week® was sponsored by the American Gastroenterological Association, the American Association for the Study of Liver Diseases, the American Society for Gastrointestinal Endoscopy, and the Society for Surgery of the Alimentary Tract.
Efficacy and long-term safety data of novel drugs, thiopurine withdrawal, and the effect of high-dose opioid use on outcomes are among the key takeaways in ulcerative colitis from Digestive Disease Week (DDW) 2023, as reported by Dr Joseph Feuerstein, from Harvard Medical School, in Boston, Massachusetts.
Dr Feuerstein starts with the QUASAR study of the IL-13 inhibitor guselkumab, which showed that the drug was associated with significantly improved clinical remission over placebo.
Next, an open-label extension of the True North study demonstrated that the oral S1P receptor modulator ozanimod proved to be safe over a 3-year follow-up period. Another trial examining safety found that withdrawal from thiopurine and vedolizumab combination therapy may not be a viable strategy.
Dr Feuerstein then turns to a retrospective analysis of older patients who underwent segmental colectomy in which the procedure was associated with low rates of complications and postoperative flares.
Finally, another retrospective study suggested that, contrary to expectations, high-dose opioid use does not appear to worsen clinical outcomes in acute severe ulcerative colitis.
--
Joseph D. Feuerstein, MD, Associate Professor of Medicine, Department of Gastroenterology, Harvard Medical School; Attending in Gastroenterology, Center for Inflammatory Bowel Disease, Beth Israel Deaconess Medical Center, Boston, Massachusetts
Joseph D. Feuerstein, MD, has disclosed no relevant financial relationships.
Digestive Disease Week® was sponsored by the American Gastroenterological Association, the American Association for the Study of Liver Diseases, the American Society for Gastrointestinal Endoscopy, and the Society for Surgery of the Alimentary Tract.
Efficacy and long-term safety data of novel drugs, thiopurine withdrawal, and the effect of high-dose opioid use on outcomes are among the key takeaways in ulcerative colitis from Digestive Disease Week (DDW) 2023, as reported by Dr Joseph Feuerstein, from Harvard Medical School, in Boston, Massachusetts.
Dr Feuerstein starts with the QUASAR study of the IL-13 inhibitor guselkumab, which showed that the drug was associated with significantly improved clinical remission over placebo.
Next, an open-label extension of the True North study demonstrated that the oral S1P receptor modulator ozanimod proved to be safe over a 3-year follow-up period. Another trial examining safety found that withdrawal from thiopurine and vedolizumab combination therapy may not be a viable strategy.
Dr Feuerstein then turns to a retrospective analysis of older patients who underwent segmental colectomy in which the procedure was associated with low rates of complications and postoperative flares.
Finally, another retrospective study suggested that, contrary to expectations, high-dose opioid use does not appear to worsen clinical outcomes in acute severe ulcerative colitis.
--
Joseph D. Feuerstein, MD, Associate Professor of Medicine, Department of Gastroenterology, Harvard Medical School; Attending in Gastroenterology, Center for Inflammatory Bowel Disease, Beth Israel Deaconess Medical Center, Boston, Massachusetts
Joseph D. Feuerstein, MD, has disclosed no relevant financial relationships.
Digestive Disease Week® was sponsored by the American Gastroenterological Association, the American Association for the Study of Liver Diseases, the American Society for Gastrointestinal Endoscopy, and the Society for Surgery of the Alimentary Tract.

Noncardiac mortality is not increased by revascularization in a meta-analysis: New data refute recent study
In response to a randomized trial that associated elective revascularization for ischemia with an increase in noncardiac mortality versus medical therapy alone, a meta-analysis with a far larger dataset challenges this assertion, suggesting the initial conclusion is due to a type 1 error.
, reports William Wijns, MD, PhD, professor of interventional cardiology, National University of Ireland, Galway.
The larger pool of data from the meta-analysis was considered compelling by several experts at the annual meeting of the European Association of Percutaneous Cardiovascular Interventions, where it was presented.
“I think these data will close once and forever this controversy,” said Davide Capodanno, MD, PhD, a professor of cardiology and interventional cardiologist at the University of Catania (Italy).
Evidence for an unexpected increased risk of noncardiac mortality was drawn from the ISCHEMIA-EXTEND study, which was published earlier this year. Numerous prior studies comparing percutaneous intervention (PCI) to medical therapy for relief of ischemia had shown no such safety signal.
The ISCHEMIA-EXTEND study provided long-term follow up of patients enrolled in ISCHEMIA, a study that randomized patients with stable coronary disease and moderate or severe ischemia to PCI or a conservative approach. After 3.2 years of follow up, there was no reduction in risk of cardiovascular events or all-cause death. While this lack of benefit was a disappointing result from the perspective of interventional cardiology, there was also no increase in these risks.
In ISCHEMIA-EXTEND, the more than 5,000 patients originally randomized were followed for an additional 2.5 years (total 5.7 years). During this extended period, the estimated 7-year risk of cardiovascular mortality was 22% lower in the group randomized to PCI (hazard ratio, 0.78; 95% confidence interval, 0.63-0.96) but the noncardiac mortality was increased by 44% (HR, 1.44; 95% CI, 1.08-1.91). Because of the counterbalancing effects on survival, all-cause mortality was similar in the two groups.
The newly completed meta-analysis was undertaken to address this surprising result not least because the increased rates of noncardiac death did not have a plausible explanation, according to Dr. Wijns.
When the patients from the 18 randomized trials were compared, noncardiac death occurred in 4.68% of the 8,665 patients assigned to elective revascularization and in 4.17% of the 8,243 patients assigned to medical therapy alone at an average follow up of 5.7 years.
This difference was not significant overall (HR, 1.09; 95% CI, 0.94-1.26; P = .26) or after sensitivity analyses. For example, there was no difference (P = .52) between an invasive or conservative approach after controlling for length of follow up.
There was also no heterogeneity (I2 = 0%) among the studies when ISCHEMIA-EXTEND was excluded.
Absence of negative effect ‘is confirmed’
On the basis of a Bayesian meta-analysis designed to account for residual uncertainty (relative risk, 1.08, 95% CI, 0.90-1.30) and the consistency of results among all studies with the exception of ISCHEMIA-EXTEND (RR, 1.0; 95% CI, 0.84-1;18; P = .7), “the absence of a negative effect of revascularization on noncardiac death was confirmed,” Dr. Wijns reported.
Based on the preponderance of evidence assembled in this meta-analysis, the “noncardiac mortality excess risk observed following revascularization relative to medical therapy was confined to a single large trial and is likely due to a type 1 error,” Dr. Wijns reported. He noted that this study is “the first large-scale meta-analysis study designed to systematically evaluate potential differences in noncardiac mortality between treatment strategies for chronic coronary syndromes.”
Eliano P. Navarese, MD, PhD, an associate professor of interventional cardiology at Nicolaus Copernicus University, Bydgoszcz, Poland, was the lead author of this study and Dr. Wijns was a coinvestigator. The study was published simultaneously in the Journal of the American College of Cardiology at the time of the EuroPCR meeting.
In the late-breaking session where these data were presented, there was a general consensus among invited panelists that the data are convincing. For example, Michael Joner, MD, PhD, director of early clinical trials, German Heart Centre, Munich, agreed that these data “resolve the issue.”
Bernard de Bruyne, MD, PhD, an interventional cardiologist associated with the Cardiovascular Center Aalst, Kraainem, Belgium, also agreed that these data argue convincingly against the concern raised by publication of ISCHEMIA-EXTEND, but he added that this controversy has raised an important issue.
“We should always be reporting all-cause mortality, not just cardiovascular mortality, in our clinical trials,” he said, emphasizing that extending all-cause survival, not just preventing cardiovascular-related events, should be recognized as the goal of invasive strategies.
In an editorial accompanying the publication, Dr. Harvey D. White, MD, Te Whatu Ora-Health New Zealand, Auckland, writes similarly that the current findings, “alert us to the importance of adjudicating causes of death in clinical trials.
“The current trial-level meta-analysis may seem to dispel concerns about increases in noncardiac and cardiovascular deaths seen in some revascularization trials, but paradoxically, it has raised the need for more and careful analysis of causes of death,” Dr. White notes. He feels the signal of increased noncardiac or noncardiovascular death in ISCHEMIA EXTEND and the REVIVED trials is something “that we should pay attention to and explore the possibility that increased radiation doses with PCI may cause increased rates of cancer.”
Further study, including longer follow-up, other datasets, and quality of life data including cognitive function and “patient-focused outcomes such as day alive out of hospital,” is needed, he concludes.
Dr. Navarese has received research grants from Abbott and Amgen and lecture fees/honoraria from Amgen, AstraZeneca, Bayer, Pfizer, and Sanofi-Regeneron. Dr. Wijns reports financial relationships with Argonauts, Corrib Core Laboratory, and Rede Optimus Research. Dr. Capodanno reports financial relationships with Amgen, Daiichi Sankyo, and Sanofi. Dr. de Bruyne and Dr. Joner report financial relationships with multiple pharmaceutical and device manufacturers. Prof. White, as the John Neutze scholar, is supported by the Green Lane Research and Educational Fund. Prof. White has received grant support paid to the institution and fees for serving on steering committees of multiple trials sponsored by various companies.
In response to a randomized trial that associated elective revascularization for ischemia with an increase in noncardiac mortality versus medical therapy alone, a meta-analysis with a far larger dataset challenges this assertion, suggesting the initial conclusion is due to a type 1 error.
, reports William Wijns, MD, PhD, professor of interventional cardiology, National University of Ireland, Galway.
The larger pool of data from the meta-analysis was considered compelling by several experts at the annual meeting of the European Association of Percutaneous Cardiovascular Interventions, where it was presented.
“I think these data will close once and forever this controversy,” said Davide Capodanno, MD, PhD, a professor of cardiology and interventional cardiologist at the University of Catania (Italy).
Evidence for an unexpected increased risk of noncardiac mortality was drawn from the ISCHEMIA-EXTEND study, which was published earlier this year. Numerous prior studies comparing percutaneous intervention (PCI) to medical therapy for relief of ischemia had shown no such safety signal.
The ISCHEMIA-EXTEND study provided long-term follow up of patients enrolled in ISCHEMIA, a study that randomized patients with stable coronary disease and moderate or severe ischemia to PCI or a conservative approach. After 3.2 years of follow up, there was no reduction in risk of cardiovascular events or all-cause death. While this lack of benefit was a disappointing result from the perspective of interventional cardiology, there was also no increase in these risks.
In ISCHEMIA-EXTEND, the more than 5,000 patients originally randomized were followed for an additional 2.5 years (total 5.7 years). During this extended period, the estimated 7-year risk of cardiovascular mortality was 22% lower in the group randomized to PCI (hazard ratio, 0.78; 95% confidence interval, 0.63-0.96) but the noncardiac mortality was increased by 44% (HR, 1.44; 95% CI, 1.08-1.91). Because of the counterbalancing effects on survival, all-cause mortality was similar in the two groups.
The newly completed meta-analysis was undertaken to address this surprising result not least because the increased rates of noncardiac death did not have a plausible explanation, according to Dr. Wijns.
When the patients from the 18 randomized trials were compared, noncardiac death occurred in 4.68% of the 8,665 patients assigned to elective revascularization and in 4.17% of the 8,243 patients assigned to medical therapy alone at an average follow up of 5.7 years.
This difference was not significant overall (HR, 1.09; 95% CI, 0.94-1.26; P = .26) or after sensitivity analyses. For example, there was no difference (P = .52) between an invasive or conservative approach after controlling for length of follow up.
There was also no heterogeneity (I2 = 0%) among the studies when ISCHEMIA-EXTEND was excluded.
Absence of negative effect ‘is confirmed’
On the basis of a Bayesian meta-analysis designed to account for residual uncertainty (relative risk, 1.08, 95% CI, 0.90-1.30) and the consistency of results among all studies with the exception of ISCHEMIA-EXTEND (RR, 1.0; 95% CI, 0.84-1;18; P = .7), “the absence of a negative effect of revascularization on noncardiac death was confirmed,” Dr. Wijns reported.
Based on the preponderance of evidence assembled in this meta-analysis, the “noncardiac mortality excess risk observed following revascularization relative to medical therapy was confined to a single large trial and is likely due to a type 1 error,” Dr. Wijns reported. He noted that this study is “the first large-scale meta-analysis study designed to systematically evaluate potential differences in noncardiac mortality between treatment strategies for chronic coronary syndromes.”
Eliano P. Navarese, MD, PhD, an associate professor of interventional cardiology at Nicolaus Copernicus University, Bydgoszcz, Poland, was the lead author of this study and Dr. Wijns was a coinvestigator. The study was published simultaneously in the Journal of the American College of Cardiology at the time of the EuroPCR meeting.
In the late-breaking session where these data were presented, there was a general consensus among invited panelists that the data are convincing. For example, Michael Joner, MD, PhD, director of early clinical trials, German Heart Centre, Munich, agreed that these data “resolve the issue.”
Bernard de Bruyne, MD, PhD, an interventional cardiologist associated with the Cardiovascular Center Aalst, Kraainem, Belgium, also agreed that these data argue convincingly against the concern raised by publication of ISCHEMIA-EXTEND, but he added that this controversy has raised an important issue.
“We should always be reporting all-cause mortality, not just cardiovascular mortality, in our clinical trials,” he said, emphasizing that extending all-cause survival, not just preventing cardiovascular-related events, should be recognized as the goal of invasive strategies.
In an editorial accompanying the publication, Dr. Harvey D. White, MD, Te Whatu Ora-Health New Zealand, Auckland, writes similarly that the current findings, “alert us to the importance of adjudicating causes of death in clinical trials.
“The current trial-level meta-analysis may seem to dispel concerns about increases in noncardiac and cardiovascular deaths seen in some revascularization trials, but paradoxically, it has raised the need for more and careful analysis of causes of death,” Dr. White notes. He feels the signal of increased noncardiac or noncardiovascular death in ISCHEMIA EXTEND and the REVIVED trials is something “that we should pay attention to and explore the possibility that increased radiation doses with PCI may cause increased rates of cancer.”
Further study, including longer follow-up, other datasets, and quality of life data including cognitive function and “patient-focused outcomes such as day alive out of hospital,” is needed, he concludes.
Dr. Navarese has received research grants from Abbott and Amgen and lecture fees/honoraria from Amgen, AstraZeneca, Bayer, Pfizer, and Sanofi-Regeneron. Dr. Wijns reports financial relationships with Argonauts, Corrib Core Laboratory, and Rede Optimus Research. Dr. Capodanno reports financial relationships with Amgen, Daiichi Sankyo, and Sanofi. Dr. de Bruyne and Dr. Joner report financial relationships with multiple pharmaceutical and device manufacturers. Prof. White, as the John Neutze scholar, is supported by the Green Lane Research and Educational Fund. Prof. White has received grant support paid to the institution and fees for serving on steering committees of multiple trials sponsored by various companies.
In response to a randomized trial that associated elective revascularization for ischemia with an increase in noncardiac mortality versus medical therapy alone, a meta-analysis with a far larger dataset challenges this assertion, suggesting the initial conclusion is due to a type 1 error.
, reports William Wijns, MD, PhD, professor of interventional cardiology, National University of Ireland, Galway.
The larger pool of data from the meta-analysis was considered compelling by several experts at the annual meeting of the European Association of Percutaneous Cardiovascular Interventions, where it was presented.
“I think these data will close once and forever this controversy,” said Davide Capodanno, MD, PhD, a professor of cardiology and interventional cardiologist at the University of Catania (Italy).
Evidence for an unexpected increased risk of noncardiac mortality was drawn from the ISCHEMIA-EXTEND study, which was published earlier this year. Numerous prior studies comparing percutaneous intervention (PCI) to medical therapy for relief of ischemia had shown no such safety signal.
The ISCHEMIA-EXTEND study provided long-term follow up of patients enrolled in ISCHEMIA, a study that randomized patients with stable coronary disease and moderate or severe ischemia to PCI or a conservative approach. After 3.2 years of follow up, there was no reduction in risk of cardiovascular events or all-cause death. While this lack of benefit was a disappointing result from the perspective of interventional cardiology, there was also no increase in these risks.
In ISCHEMIA-EXTEND, the more than 5,000 patients originally randomized were followed for an additional 2.5 years (total 5.7 years). During this extended period, the estimated 7-year risk of cardiovascular mortality was 22% lower in the group randomized to PCI (hazard ratio, 0.78; 95% confidence interval, 0.63-0.96) but the noncardiac mortality was increased by 44% (HR, 1.44; 95% CI, 1.08-1.91). Because of the counterbalancing effects on survival, all-cause mortality was similar in the two groups.
The newly completed meta-analysis was undertaken to address this surprising result not least because the increased rates of noncardiac death did not have a plausible explanation, according to Dr. Wijns.
When the patients from the 18 randomized trials were compared, noncardiac death occurred in 4.68% of the 8,665 patients assigned to elective revascularization and in 4.17% of the 8,243 patients assigned to medical therapy alone at an average follow up of 5.7 years.
This difference was not significant overall (HR, 1.09; 95% CI, 0.94-1.26; P = .26) or after sensitivity analyses. For example, there was no difference (P = .52) between an invasive or conservative approach after controlling for length of follow up.
There was also no heterogeneity (I2 = 0%) among the studies when ISCHEMIA-EXTEND was excluded.
Absence of negative effect ‘is confirmed’
On the basis of a Bayesian meta-analysis designed to account for residual uncertainty (relative risk, 1.08, 95% CI, 0.90-1.30) and the consistency of results among all studies with the exception of ISCHEMIA-EXTEND (RR, 1.0; 95% CI, 0.84-1;18; P = .7), “the absence of a negative effect of revascularization on noncardiac death was confirmed,” Dr. Wijns reported.
Based on the preponderance of evidence assembled in this meta-analysis, the “noncardiac mortality excess risk observed following revascularization relative to medical therapy was confined to a single large trial and is likely due to a type 1 error,” Dr. Wijns reported. He noted that this study is “the first large-scale meta-analysis study designed to systematically evaluate potential differences in noncardiac mortality between treatment strategies for chronic coronary syndromes.”
Eliano P. Navarese, MD, PhD, an associate professor of interventional cardiology at Nicolaus Copernicus University, Bydgoszcz, Poland, was the lead author of this study and Dr. Wijns was a coinvestigator. The study was published simultaneously in the Journal of the American College of Cardiology at the time of the EuroPCR meeting.
In the late-breaking session where these data were presented, there was a general consensus among invited panelists that the data are convincing. For example, Michael Joner, MD, PhD, director of early clinical trials, German Heart Centre, Munich, agreed that these data “resolve the issue.”
Bernard de Bruyne, MD, PhD, an interventional cardiologist associated with the Cardiovascular Center Aalst, Kraainem, Belgium, also agreed that these data argue convincingly against the concern raised by publication of ISCHEMIA-EXTEND, but he added that this controversy has raised an important issue.
“We should always be reporting all-cause mortality, not just cardiovascular mortality, in our clinical trials,” he said, emphasizing that extending all-cause survival, not just preventing cardiovascular-related events, should be recognized as the goal of invasive strategies.
In an editorial accompanying the publication, Dr. Harvey D. White, MD, Te Whatu Ora-Health New Zealand, Auckland, writes similarly that the current findings, “alert us to the importance of adjudicating causes of death in clinical trials.
“The current trial-level meta-analysis may seem to dispel concerns about increases in noncardiac and cardiovascular deaths seen in some revascularization trials, but paradoxically, it has raised the need for more and careful analysis of causes of death,” Dr. White notes. He feels the signal of increased noncardiac or noncardiovascular death in ISCHEMIA EXTEND and the REVIVED trials is something “that we should pay attention to and explore the possibility that increased radiation doses with PCI may cause increased rates of cancer.”
Further study, including longer follow-up, other datasets, and quality of life data including cognitive function and “patient-focused outcomes such as day alive out of hospital,” is needed, he concludes.
Dr. Navarese has received research grants from Abbott and Amgen and lecture fees/honoraria from Amgen, AstraZeneca, Bayer, Pfizer, and Sanofi-Regeneron. Dr. Wijns reports financial relationships with Argonauts, Corrib Core Laboratory, and Rede Optimus Research. Dr. Capodanno reports financial relationships with Amgen, Daiichi Sankyo, and Sanofi. Dr. de Bruyne and Dr. Joner report financial relationships with multiple pharmaceutical and device manufacturers. Prof. White, as the John Neutze scholar, is supported by the Green Lane Research and Educational Fund. Prof. White has received grant support paid to the institution and fees for serving on steering committees of multiple trials sponsored by various companies.
FROM EUROPCR 2023
PTSD, anxiety linked to out-of-hospital cardiac arrest
Investigators compared more than 35,000 OHCA case patients with a similar number of matched control persons and found an almost 1.5 times higher hazard of long-term stress conditions among OHCA case patients, compared with control persons, with a similar hazard for anxiety. Posttraumatic stress disorder was associated with an almost twofold higher risk of OHCA.
The findings applied equally to men and women and were independent of the presence of cardiovascular disease (CVD).
“This study raises awareness of the higher risks of OHCA and early risk monitoring to prevent OHCA in patients with stress-related disorders and anxiety,” write Talip Eroglu, of the department of cardiology, Copenhagen University Hospital, and colleagues.
The study was published online in BMJ Open Heart.
Stress disorders and anxiety overrepresented
OHCA “predominantly arises from lethal cardiac arrhythmias ... that occur most frequently in the setting of coronary heart disease,” the authors write. However, increasing evidence suggests that rates of OHCA may also be increased in association with noncardiac diseases.
Individuals with stress-related disorders and anxiety are “overrepresented” among victims of cardiac arrest as well as those with multiple CVDs. But previous studies of OHCA have been limited by small numbers of cardiac arrests. In addition, those studies involved only data from selected populations or used in-hospital diagnosis to identify cardiac arrest, thereby potentially omitting OHCA patients who died prior to hospital admission.
The researchers therefore turned to data from Danish health registries that include a large, unselected cohort of patients with OHCA to investigate whether long-term stress conditions (that is, PTSD and adjustment disorder) or anxiety disorder were associated with OHCA.
They stratified the cohort according to sex, age, and CVD to identify which risk factor confers the highest risk of OHCA in patients with long-term stress conditions or anxiety, and they conducted sensitivity analyses of potential confounders, such as depression.
The design was a nested-case control model in which records at an individual patient level across registries were cross-linked to data from other national registries and were compared to matched control persons from the general population (35,195 OHCAs and 351,950 matched control persons; median IQR age, 72 [62-81] years; 66.82% men).
The prevalence of comorbidities and use of cardiovascular drugs were higher among OHCA case patients than among non-OHCA control persons.
Keep aware of stress and anxiety as risk factors
Among OHCA and non-OHCA participants, long-term stress conditions were diagnosed in 0.92% and 0.45%, respectively. Anxiety was diagnosed in 0.85% of OHCA case patients and in 0.37% of non-OHCA control persons.
These conditions were associated with a higher rate of OHCA after adjustment for common OHCA risk factors.
There were no significant differences in results when the researchers adjusted for the use of anxiolytics and antidepressants.
When they examined the prevalence of concomitant medication use or comorbidities, they found that depression was more frequent among patients with long-term stress and anxiety, compared with individuals with neither of those diagnoses. Additionally, patients with long-term stress and anxiety more often used anxiolytics, antidepressants, and QT-prolonging drugs.
Stratification of the analyses according to sex revealed that the OHCA rate was increased in both women and men with long-term stress and anxiety. There were no significant differences between the sexes. There were also no significant differences between the association among different age groups, nor between patients with and those without CVD, ischemic heart disease, or heart failure.
Previous research has shown associations of stress-related disorders or anxiety with cardiovascular outcomes, including myocardial infarction, heart failure, and cerebrovascular disease. These disorders might be “biological mediators in the causal pathway of OHCA” and contribute to the increased OHCA rate associated with stress-related disorders and anxiety, the authors suggest.
Nevertheless, they note, stress-related disorders and anxiety remained significantly associated with OHCA after controlling for these variables, “suggesting that it is unlikely that traditional risk factors of OHCA alone explain this relationship.”
They suggest several potential mechanisms. One is that the relationship is likely mediated by the activity of the sympathetic autonomic nervous system, which “leads to an increase in heart rate, release of neurotransmitters into the circulation, and local release of neurotransmitters in the heart.”
Each of these factors “may potentially influence cardiac electrophysiology and facilitate ventricular arrhythmias and OHCA.”
In addition to a biological mechanism, behavioral and psychosocial factors may also contribute to OHCA risk, since stress-related disorders and anxiety “often lead to unhealthy lifestyle, such as smoking and lower physical activity, which in turn may increase the risk of OHCA.” Given the absence of data on these features in the registries the investigators used, they were unable to account for them.
However, “it is unlikely that knowledge of these factors would have altered our conclusions considering that we have adjusted for all the relevant cardiovascular comorbidities.”
Similarly, other psychiatric disorders, such as depression, can contribute to OHCA risk, but they adjusted for depression in their multivariable analyses.
“Awareness of the higher risks of OHCA in patients with stress-related disorders and anxiety is important when treating these patients,” they conclude.
Detrimental to the heart, not just the psyche
Glenn Levine, MD, master clinician and professor of medicine, Baylor College of Medicine, Houston, called it an “important study in that it is a large, nationwide cohort study and thus provides important information to complement much smaller, focused studies.”
Like those other studies, “it finds that negative psychological health, specifically, long-term stress (as well as anxiety), is associated with a significantly increased risk of out-of-hospital cardiac arrest,” continued Dr. Levine, who is the chief of the cardiology section at Michael E. DeBakey VA Medical Center, Houston, and was not involved with the study.
Dr. Levine thinks the study “does a good job, as best one can for such a study, in trying to control for other factors, and zeroing in specifically on stress (and anxiety), trying to assess their independent contributions to the risk of developing cardiac arrest.”
The take-home message for clinicians and patients “is that negative psychological stress factors, such as stress and anxiety, are not only detrimental to one’s psychological health but likely increase one’s risk for adverse cardiac events, such as cardiac arrest,” he stated.
No specific funding for the study was disclosed. Mr. Eroglu has disclosed no relevant financial relationships. The other authors’ disclosures are listed in the original article. Dr. Levine reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators compared more than 35,000 OHCA case patients with a similar number of matched control persons and found an almost 1.5 times higher hazard of long-term stress conditions among OHCA case patients, compared with control persons, with a similar hazard for anxiety. Posttraumatic stress disorder was associated with an almost twofold higher risk of OHCA.
The findings applied equally to men and women and were independent of the presence of cardiovascular disease (CVD).
“This study raises awareness of the higher risks of OHCA and early risk monitoring to prevent OHCA in patients with stress-related disorders and anxiety,” write Talip Eroglu, of the department of cardiology, Copenhagen University Hospital, and colleagues.
The study was published online in BMJ Open Heart.
Stress disorders and anxiety overrepresented
OHCA “predominantly arises from lethal cardiac arrhythmias ... that occur most frequently in the setting of coronary heart disease,” the authors write. However, increasing evidence suggests that rates of OHCA may also be increased in association with noncardiac diseases.
Individuals with stress-related disorders and anxiety are “overrepresented” among victims of cardiac arrest as well as those with multiple CVDs. But previous studies of OHCA have been limited by small numbers of cardiac arrests. In addition, those studies involved only data from selected populations or used in-hospital diagnosis to identify cardiac arrest, thereby potentially omitting OHCA patients who died prior to hospital admission.
The researchers therefore turned to data from Danish health registries that include a large, unselected cohort of patients with OHCA to investigate whether long-term stress conditions (that is, PTSD and adjustment disorder) or anxiety disorder were associated with OHCA.
They stratified the cohort according to sex, age, and CVD to identify which risk factor confers the highest risk of OHCA in patients with long-term stress conditions or anxiety, and they conducted sensitivity analyses of potential confounders, such as depression.
The design was a nested-case control model in which records at an individual patient level across registries were cross-linked to data from other national registries and were compared to matched control persons from the general population (35,195 OHCAs and 351,950 matched control persons; median IQR age, 72 [62-81] years; 66.82% men).
The prevalence of comorbidities and use of cardiovascular drugs were higher among OHCA case patients than among non-OHCA control persons.
Keep aware of stress and anxiety as risk factors
Among OHCA and non-OHCA participants, long-term stress conditions were diagnosed in 0.92% and 0.45%, respectively. Anxiety was diagnosed in 0.85% of OHCA case patients and in 0.37% of non-OHCA control persons.
These conditions were associated with a higher rate of OHCA after adjustment for common OHCA risk factors.
There were no significant differences in results when the researchers adjusted for the use of anxiolytics and antidepressants.
When they examined the prevalence of concomitant medication use or comorbidities, they found that depression was more frequent among patients with long-term stress and anxiety, compared with individuals with neither of those diagnoses. Additionally, patients with long-term stress and anxiety more often used anxiolytics, antidepressants, and QT-prolonging drugs.
Stratification of the analyses according to sex revealed that the OHCA rate was increased in both women and men with long-term stress and anxiety. There were no significant differences between the sexes. There were also no significant differences between the association among different age groups, nor between patients with and those without CVD, ischemic heart disease, or heart failure.
Previous research has shown associations of stress-related disorders or anxiety with cardiovascular outcomes, including myocardial infarction, heart failure, and cerebrovascular disease. These disorders might be “biological mediators in the causal pathway of OHCA” and contribute to the increased OHCA rate associated with stress-related disorders and anxiety, the authors suggest.
Nevertheless, they note, stress-related disorders and anxiety remained significantly associated with OHCA after controlling for these variables, “suggesting that it is unlikely that traditional risk factors of OHCA alone explain this relationship.”
They suggest several potential mechanisms. One is that the relationship is likely mediated by the activity of the sympathetic autonomic nervous system, which “leads to an increase in heart rate, release of neurotransmitters into the circulation, and local release of neurotransmitters in the heart.”
Each of these factors “may potentially influence cardiac electrophysiology and facilitate ventricular arrhythmias and OHCA.”
In addition to a biological mechanism, behavioral and psychosocial factors may also contribute to OHCA risk, since stress-related disorders and anxiety “often lead to unhealthy lifestyle, such as smoking and lower physical activity, which in turn may increase the risk of OHCA.” Given the absence of data on these features in the registries the investigators used, they were unable to account for them.
However, “it is unlikely that knowledge of these factors would have altered our conclusions considering that we have adjusted for all the relevant cardiovascular comorbidities.”
Similarly, other psychiatric disorders, such as depression, can contribute to OHCA risk, but they adjusted for depression in their multivariable analyses.
“Awareness of the higher risks of OHCA in patients with stress-related disorders and anxiety is important when treating these patients,” they conclude.
Detrimental to the heart, not just the psyche
Glenn Levine, MD, master clinician and professor of medicine, Baylor College of Medicine, Houston, called it an “important study in that it is a large, nationwide cohort study and thus provides important information to complement much smaller, focused studies.”
Like those other studies, “it finds that negative psychological health, specifically, long-term stress (as well as anxiety), is associated with a significantly increased risk of out-of-hospital cardiac arrest,” continued Dr. Levine, who is the chief of the cardiology section at Michael E. DeBakey VA Medical Center, Houston, and was not involved with the study.
Dr. Levine thinks the study “does a good job, as best one can for such a study, in trying to control for other factors, and zeroing in specifically on stress (and anxiety), trying to assess their independent contributions to the risk of developing cardiac arrest.”
The take-home message for clinicians and patients “is that negative psychological stress factors, such as stress and anxiety, are not only detrimental to one’s psychological health but likely increase one’s risk for adverse cardiac events, such as cardiac arrest,” he stated.
No specific funding for the study was disclosed. Mr. Eroglu has disclosed no relevant financial relationships. The other authors’ disclosures are listed in the original article. Dr. Levine reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators compared more than 35,000 OHCA case patients with a similar number of matched control persons and found an almost 1.5 times higher hazard of long-term stress conditions among OHCA case patients, compared with control persons, with a similar hazard for anxiety. Posttraumatic stress disorder was associated with an almost twofold higher risk of OHCA.
The findings applied equally to men and women and were independent of the presence of cardiovascular disease (CVD).
“This study raises awareness of the higher risks of OHCA and early risk monitoring to prevent OHCA in patients with stress-related disorders and anxiety,” write Talip Eroglu, of the department of cardiology, Copenhagen University Hospital, and colleagues.
The study was published online in BMJ Open Heart.
Stress disorders and anxiety overrepresented
OHCA “predominantly arises from lethal cardiac arrhythmias ... that occur most frequently in the setting of coronary heart disease,” the authors write. However, increasing evidence suggests that rates of OHCA may also be increased in association with noncardiac diseases.
Individuals with stress-related disorders and anxiety are “overrepresented” among victims of cardiac arrest as well as those with multiple CVDs. But previous studies of OHCA have been limited by small numbers of cardiac arrests. In addition, those studies involved only data from selected populations or used in-hospital diagnosis to identify cardiac arrest, thereby potentially omitting OHCA patients who died prior to hospital admission.
The researchers therefore turned to data from Danish health registries that include a large, unselected cohort of patients with OHCA to investigate whether long-term stress conditions (that is, PTSD and adjustment disorder) or anxiety disorder were associated with OHCA.
They stratified the cohort according to sex, age, and CVD to identify which risk factor confers the highest risk of OHCA in patients with long-term stress conditions or anxiety, and they conducted sensitivity analyses of potential confounders, such as depression.
The design was a nested-case control model in which records at an individual patient level across registries were cross-linked to data from other national registries and were compared to matched control persons from the general population (35,195 OHCAs and 351,950 matched control persons; median IQR age, 72 [62-81] years; 66.82% men).
The prevalence of comorbidities and use of cardiovascular drugs were higher among OHCA case patients than among non-OHCA control persons.
Keep aware of stress and anxiety as risk factors
Among OHCA and non-OHCA participants, long-term stress conditions were diagnosed in 0.92% and 0.45%, respectively. Anxiety was diagnosed in 0.85% of OHCA case patients and in 0.37% of non-OHCA control persons.
These conditions were associated with a higher rate of OHCA after adjustment for common OHCA risk factors.
There were no significant differences in results when the researchers adjusted for the use of anxiolytics and antidepressants.
When they examined the prevalence of concomitant medication use or comorbidities, they found that depression was more frequent among patients with long-term stress and anxiety, compared with individuals with neither of those diagnoses. Additionally, patients with long-term stress and anxiety more often used anxiolytics, antidepressants, and QT-prolonging drugs.
Stratification of the analyses according to sex revealed that the OHCA rate was increased in both women and men with long-term stress and anxiety. There were no significant differences between the sexes. There were also no significant differences between the association among different age groups, nor between patients with and those without CVD, ischemic heart disease, or heart failure.
Previous research has shown associations of stress-related disorders or anxiety with cardiovascular outcomes, including myocardial infarction, heart failure, and cerebrovascular disease. These disorders might be “biological mediators in the causal pathway of OHCA” and contribute to the increased OHCA rate associated with stress-related disorders and anxiety, the authors suggest.
Nevertheless, they note, stress-related disorders and anxiety remained significantly associated with OHCA after controlling for these variables, “suggesting that it is unlikely that traditional risk factors of OHCA alone explain this relationship.”
They suggest several potential mechanisms. One is that the relationship is likely mediated by the activity of the sympathetic autonomic nervous system, which “leads to an increase in heart rate, release of neurotransmitters into the circulation, and local release of neurotransmitters in the heart.”
Each of these factors “may potentially influence cardiac electrophysiology and facilitate ventricular arrhythmias and OHCA.”
In addition to a biological mechanism, behavioral and psychosocial factors may also contribute to OHCA risk, since stress-related disorders and anxiety “often lead to unhealthy lifestyle, such as smoking and lower physical activity, which in turn may increase the risk of OHCA.” Given the absence of data on these features in the registries the investigators used, they were unable to account for them.
However, “it is unlikely that knowledge of these factors would have altered our conclusions considering that we have adjusted for all the relevant cardiovascular comorbidities.”
Similarly, other psychiatric disorders, such as depression, can contribute to OHCA risk, but they adjusted for depression in their multivariable analyses.
“Awareness of the higher risks of OHCA in patients with stress-related disorders and anxiety is important when treating these patients,” they conclude.
Detrimental to the heart, not just the psyche
Glenn Levine, MD, master clinician and professor of medicine, Baylor College of Medicine, Houston, called it an “important study in that it is a large, nationwide cohort study and thus provides important information to complement much smaller, focused studies.”
Like those other studies, “it finds that negative psychological health, specifically, long-term stress (as well as anxiety), is associated with a significantly increased risk of out-of-hospital cardiac arrest,” continued Dr. Levine, who is the chief of the cardiology section at Michael E. DeBakey VA Medical Center, Houston, and was not involved with the study.
Dr. Levine thinks the study “does a good job, as best one can for such a study, in trying to control for other factors, and zeroing in specifically on stress (and anxiety), trying to assess their independent contributions to the risk of developing cardiac arrest.”
The take-home message for clinicians and patients “is that negative psychological stress factors, such as stress and anxiety, are not only detrimental to one’s psychological health but likely increase one’s risk for adverse cardiac events, such as cardiac arrest,” he stated.
No specific funding for the study was disclosed. Mr. Eroglu has disclosed no relevant financial relationships. The other authors’ disclosures are listed in the original article. Dr. Levine reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM BMJ OPEN HEART
Can a saliva test predict the best way to manage obesity?
It sounds like a simple solution to a complicated problem: Find out what kind of obesity someone has based on a one-time genetic saliva test. Then patients and their doctor can get a better idea if antiobesity drugs or other treatments are more likely to work for them.
It’s what Mayo Clinic researchers had in mind when they created four phenotypes of obesity.
Obesity experts not affiliated with the research have some concerns and say independent studies are needed to verify the potential of this strategy.
This research could help predict who will respond best to popular antiobesity medications, said Andres Acosta, MD, PhD, cofounder of Phenomix Sciences, the company behind the tests. These medications include glucagonlike peptide–1 (GLP-1) receptor agonists like liraglutide (Saxenda, Victoza) and semaglutide (Ozempic, Wegovy).
“We know that not everyone on a GLP-1 will respond. In reality, about a third of the patients don’t do well with GLP-1s,” said Dr. Acosta, an assistant professor of medicine and researcher in the division of gastroenterology and hepatology at the Mayo Clinic in Rochester, Minn.
Furthest along in development is the “My Phenome Hungry Gut” test for predicting GLP-1 response. People in this Hungry Gut group tend to empty their stomach after a meal faster and are more likely to feel hungry again a short time later, as explained on the company’s website.
A pilot study to test how well it works started in April at three primary care practices. Plans are to expand real-world testing for this and other obesity types later in 2023.
The other obesity categories are:
- “Hungry brain,” where the brain does not recognize signals that the stomach is full
- “Emotional hunger,” where cravings to eat are driven by emotions, anxiety, and negative feelings
- “Slow burn,” where people have a slow metabolism and low energy level
People in these categories might be more likely to benefit from other obesity management strategies, like changes to their diet or placement of an intragastric balloon.
Some things to consider
While applauding their efforts to be more precise in treating people with obesity, not all experts are convinced this saliva test will be the answer. The company’s research might look promising, but verification of results is warranted.
“Can we get better outcomes with things like this? Well, that’s the hope,” said Jaime Almandoz, MD, medical director of weight wellness at the University of Texas Southwestern Medical Center, Dallas.
“We still don’t have randomized trials where we’re looking at obesity phenotyping yet,” said Dr. Almandoz, who is also a spokesperson for the Obesity Society, a professional group of clinicians, researchers, educators, and others focused on obesity science, treatment, and prevention.
There is always concern when a diagnostic test is being developed for commercial use, said Daniel Bessesen, MD, a professor of medicine–endocrinology, metabolism, and diabetes at the University of Colorado at Denver, Aurora. “What they’re talking about doing is super important. But this is a company. This is a company that is, I think, selling a product.”
In an online search, Dr. Bessesen did not find any external studies that showed how well the saliva testing worked. But referring to work by Dr. Acosta and Michael Camilleri, MD, the other cofounder of Phenomix, he said, “I found some papers that they did that I hadn’t read before that are good.”
“These guys are smart guys. And they’ve done a lot of work on [the movement of food through the gut] and how that correlates with obesity and response to some therapies,” said Dr. Bessesen, who is also a spokesperson for the Obesity Society. “So their scientific work does line up with this area.”
Validation of any research is important because the obesity industry has been known for a lot of lose-weight-quick strategies, some with little or no science behind them, he said.
It is also essential, he said, because “anytime you do something commercial in the area of obesity, you have to acknowledge that people with obesity are a vulnerable population. These people face stigma and bias all the time.”
Removing the stigma
If knowing your obesity type ends up making a difference, it could change the conversation people have with their medical provider, Dr. Acosta said. It could also help remove some of the stigma around obesity.
“We’re going to change the conversation because now we can say: ‘Hey, you have obesity because you have ‘Hungry Gut’ phenotype. And because of that, you’re going to respond to this medication,” Dr. Acosta said. The phenotyping suggests a strong genetic tendency – a biologic basis for obesity.
“So it’s not only a way of taking the blame out, but it’s also way of explaining that there’s a reason why you have obesity,” Dr. Acosta said. It tells people: “You’re not a failure.”
More cost-effective treatment?
Targeting obesity treatment could also save on overall health care costs, Dr. Almandoz said. He estimated a cost of $1,400 per month “for forever and ever semaglutide” or at least $1,400 a month for a 3-month trial to see if this medication works in a particular person with obesity.
“That’s a lot of money when you extrapolate that out over the number of people who probably meet the criteria for treatment,” he said. A total 42% of Americans meet the Centers for Disease Control and Prevention definition for obesity.
“You can imagine the potential cost if we were to provide antiobesity therapies to everybody and we were to use what is the most effective class of medication, which is more than a thousand dollars per month, indefinitely,” Dr. Almandoz said. “Not that we should not treat everybody. That’s not the message I’m saying. But if we’re looking at yield or value in terms of treating obesity in a setting with limited resources, it may be best to start with who is most likely to benefit.”
How they created four obesity types
Starting in 2015, Dr. Acosta and colleagues started comparing tests in people with normal weight versus obesity. They used artificial intelligence and machine learning to classify obesity into 11 types at first. They realized this many obesity types were not practical for doctors and people with obesity, so they combined them into four phenotypes.
“The AI machine learning was followed by, as I like to call, HI, or human intelligence,” he said.
The saliva test checks for about 6,000 relevant genetic single-nucleotide polymorphisms. Six thousand genetic changes may sound like a large number to check; however, the average individual carries 5 million and 6 million SNPs in their DNA.
The results are translated to a score that yields a low risk or high risk for Hungry Gut or other types of obesity. “You can have all six thousand genetic mutations, or you can have zero,” Dr. Acosta said.
Moving forward
After the soft launch of Hungry Gut testing in April, Phenomix plans to continue studying their saliva test on other obesity types.
Dr. Acosta is not aware of any direct competitors to Phenomix, although that could change. “I think we’re the only diagnostic company in the space right now. But if it’s really a $14.8 billion market, we’re going to see a lot of diagnostic companies trying to do what we’re doing – if we’re successful,” he said.
An October 2022 report from Polaris Market Research estimates that the global market for obesity treatment – medications, surgery, and all others – was about $14 billion in 2021. The same report predicts the market will grow to $32 billion by 2030.
A version of this article first appeared on WebMD.com.
It sounds like a simple solution to a complicated problem: Find out what kind of obesity someone has based on a one-time genetic saliva test. Then patients and their doctor can get a better idea if antiobesity drugs or other treatments are more likely to work for them.
It’s what Mayo Clinic researchers had in mind when they created four phenotypes of obesity.
Obesity experts not affiliated with the research have some concerns and say independent studies are needed to verify the potential of this strategy.
This research could help predict who will respond best to popular antiobesity medications, said Andres Acosta, MD, PhD, cofounder of Phenomix Sciences, the company behind the tests. These medications include glucagonlike peptide–1 (GLP-1) receptor agonists like liraglutide (Saxenda, Victoza) and semaglutide (Ozempic, Wegovy).
“We know that not everyone on a GLP-1 will respond. In reality, about a third of the patients don’t do well with GLP-1s,” said Dr. Acosta, an assistant professor of medicine and researcher in the division of gastroenterology and hepatology at the Mayo Clinic in Rochester, Minn.
Furthest along in development is the “My Phenome Hungry Gut” test for predicting GLP-1 response. People in this Hungry Gut group tend to empty their stomach after a meal faster and are more likely to feel hungry again a short time later, as explained on the company’s website.
A pilot study to test how well it works started in April at three primary care practices. Plans are to expand real-world testing for this and other obesity types later in 2023.
The other obesity categories are:
- “Hungry brain,” where the brain does not recognize signals that the stomach is full
- “Emotional hunger,” where cravings to eat are driven by emotions, anxiety, and negative feelings
- “Slow burn,” where people have a slow metabolism and low energy level
People in these categories might be more likely to benefit from other obesity management strategies, like changes to their diet or placement of an intragastric balloon.
Some things to consider
While applauding their efforts to be more precise in treating people with obesity, not all experts are convinced this saliva test will be the answer. The company’s research might look promising, but verification of results is warranted.
“Can we get better outcomes with things like this? Well, that’s the hope,” said Jaime Almandoz, MD, medical director of weight wellness at the University of Texas Southwestern Medical Center, Dallas.
“We still don’t have randomized trials where we’re looking at obesity phenotyping yet,” said Dr. Almandoz, who is also a spokesperson for the Obesity Society, a professional group of clinicians, researchers, educators, and others focused on obesity science, treatment, and prevention.
There is always concern when a diagnostic test is being developed for commercial use, said Daniel Bessesen, MD, a professor of medicine–endocrinology, metabolism, and diabetes at the University of Colorado at Denver, Aurora. “What they’re talking about doing is super important. But this is a company. This is a company that is, I think, selling a product.”
In an online search, Dr. Bessesen did not find any external studies that showed how well the saliva testing worked. But referring to work by Dr. Acosta and Michael Camilleri, MD, the other cofounder of Phenomix, he said, “I found some papers that they did that I hadn’t read before that are good.”
“These guys are smart guys. And they’ve done a lot of work on [the movement of food through the gut] and how that correlates with obesity and response to some therapies,” said Dr. Bessesen, who is also a spokesperson for the Obesity Society. “So their scientific work does line up with this area.”
Validation of any research is important because the obesity industry has been known for a lot of lose-weight-quick strategies, some with little or no science behind them, he said.
It is also essential, he said, because “anytime you do something commercial in the area of obesity, you have to acknowledge that people with obesity are a vulnerable population. These people face stigma and bias all the time.”
Removing the stigma
If knowing your obesity type ends up making a difference, it could change the conversation people have with their medical provider, Dr. Acosta said. It could also help remove some of the stigma around obesity.
“We’re going to change the conversation because now we can say: ‘Hey, you have obesity because you have ‘Hungry Gut’ phenotype. And because of that, you’re going to respond to this medication,” Dr. Acosta said. The phenotyping suggests a strong genetic tendency – a biologic basis for obesity.
“So it’s not only a way of taking the blame out, but it’s also way of explaining that there’s a reason why you have obesity,” Dr. Acosta said. It tells people: “You’re not a failure.”
More cost-effective treatment?
Targeting obesity treatment could also save on overall health care costs, Dr. Almandoz said. He estimated a cost of $1,400 per month “for forever and ever semaglutide” or at least $1,400 a month for a 3-month trial to see if this medication works in a particular person with obesity.
“That’s a lot of money when you extrapolate that out over the number of people who probably meet the criteria for treatment,” he said. A total 42% of Americans meet the Centers for Disease Control and Prevention definition for obesity.
“You can imagine the potential cost if we were to provide antiobesity therapies to everybody and we were to use what is the most effective class of medication, which is more than a thousand dollars per month, indefinitely,” Dr. Almandoz said. “Not that we should not treat everybody. That’s not the message I’m saying. But if we’re looking at yield or value in terms of treating obesity in a setting with limited resources, it may be best to start with who is most likely to benefit.”
How they created four obesity types
Starting in 2015, Dr. Acosta and colleagues started comparing tests in people with normal weight versus obesity. They used artificial intelligence and machine learning to classify obesity into 11 types at first. They realized this many obesity types were not practical for doctors and people with obesity, so they combined them into four phenotypes.
“The AI machine learning was followed by, as I like to call, HI, or human intelligence,” he said.
The saliva test checks for about 6,000 relevant genetic single-nucleotide polymorphisms. Six thousand genetic changes may sound like a large number to check; however, the average individual carries 5 million and 6 million SNPs in their DNA.
The results are translated to a score that yields a low risk or high risk for Hungry Gut or other types of obesity. “You can have all six thousand genetic mutations, or you can have zero,” Dr. Acosta said.
Moving forward
After the soft launch of Hungry Gut testing in April, Phenomix plans to continue studying their saliva test on other obesity types.
Dr. Acosta is not aware of any direct competitors to Phenomix, although that could change. “I think we’re the only diagnostic company in the space right now. But if it’s really a $14.8 billion market, we’re going to see a lot of diagnostic companies trying to do what we’re doing – if we’re successful,” he said.
An October 2022 report from Polaris Market Research estimates that the global market for obesity treatment – medications, surgery, and all others – was about $14 billion in 2021. The same report predicts the market will grow to $32 billion by 2030.
A version of this article first appeared on WebMD.com.
It sounds like a simple solution to a complicated problem: Find out what kind of obesity someone has based on a one-time genetic saliva test. Then patients and their doctor can get a better idea if antiobesity drugs or other treatments are more likely to work for them.
It’s what Mayo Clinic researchers had in mind when they created four phenotypes of obesity.
Obesity experts not affiliated with the research have some concerns and say independent studies are needed to verify the potential of this strategy.
This research could help predict who will respond best to popular antiobesity medications, said Andres Acosta, MD, PhD, cofounder of Phenomix Sciences, the company behind the tests. These medications include glucagonlike peptide–1 (GLP-1) receptor agonists like liraglutide (Saxenda, Victoza) and semaglutide (Ozempic, Wegovy).
“We know that not everyone on a GLP-1 will respond. In reality, about a third of the patients don’t do well with GLP-1s,” said Dr. Acosta, an assistant professor of medicine and researcher in the division of gastroenterology and hepatology at the Mayo Clinic in Rochester, Minn.
Furthest along in development is the “My Phenome Hungry Gut” test for predicting GLP-1 response. People in this Hungry Gut group tend to empty their stomach after a meal faster and are more likely to feel hungry again a short time later, as explained on the company’s website.
A pilot study to test how well it works started in April at three primary care practices. Plans are to expand real-world testing for this and other obesity types later in 2023.
The other obesity categories are:
- “Hungry brain,” where the brain does not recognize signals that the stomach is full
- “Emotional hunger,” where cravings to eat are driven by emotions, anxiety, and negative feelings
- “Slow burn,” where people have a slow metabolism and low energy level
People in these categories might be more likely to benefit from other obesity management strategies, like changes to their diet or placement of an intragastric balloon.
Some things to consider
While applauding their efforts to be more precise in treating people with obesity, not all experts are convinced this saliva test will be the answer. The company’s research might look promising, but verification of results is warranted.
“Can we get better outcomes with things like this? Well, that’s the hope,” said Jaime Almandoz, MD, medical director of weight wellness at the University of Texas Southwestern Medical Center, Dallas.
“We still don’t have randomized trials where we’re looking at obesity phenotyping yet,” said Dr. Almandoz, who is also a spokesperson for the Obesity Society, a professional group of clinicians, researchers, educators, and others focused on obesity science, treatment, and prevention.
There is always concern when a diagnostic test is being developed for commercial use, said Daniel Bessesen, MD, a professor of medicine–endocrinology, metabolism, and diabetes at the University of Colorado at Denver, Aurora. “What they’re talking about doing is super important. But this is a company. This is a company that is, I think, selling a product.”
In an online search, Dr. Bessesen did not find any external studies that showed how well the saliva testing worked. But referring to work by Dr. Acosta and Michael Camilleri, MD, the other cofounder of Phenomix, he said, “I found some papers that they did that I hadn’t read before that are good.”
“These guys are smart guys. And they’ve done a lot of work on [the movement of food through the gut] and how that correlates with obesity and response to some therapies,” said Dr. Bessesen, who is also a spokesperson for the Obesity Society. “So their scientific work does line up with this area.”
Validation of any research is important because the obesity industry has been known for a lot of lose-weight-quick strategies, some with little or no science behind them, he said.
It is also essential, he said, because “anytime you do something commercial in the area of obesity, you have to acknowledge that people with obesity are a vulnerable population. These people face stigma and bias all the time.”
Removing the stigma
If knowing your obesity type ends up making a difference, it could change the conversation people have with their medical provider, Dr. Acosta said. It could also help remove some of the stigma around obesity.
“We’re going to change the conversation because now we can say: ‘Hey, you have obesity because you have ‘Hungry Gut’ phenotype. And because of that, you’re going to respond to this medication,” Dr. Acosta said. The phenotyping suggests a strong genetic tendency – a biologic basis for obesity.
“So it’s not only a way of taking the blame out, but it’s also way of explaining that there’s a reason why you have obesity,” Dr. Acosta said. It tells people: “You’re not a failure.”
More cost-effective treatment?
Targeting obesity treatment could also save on overall health care costs, Dr. Almandoz said. He estimated a cost of $1,400 per month “for forever and ever semaglutide” or at least $1,400 a month for a 3-month trial to see if this medication works in a particular person with obesity.
“That’s a lot of money when you extrapolate that out over the number of people who probably meet the criteria for treatment,” he said. A total 42% of Americans meet the Centers for Disease Control and Prevention definition for obesity.
“You can imagine the potential cost if we were to provide antiobesity therapies to everybody and we were to use what is the most effective class of medication, which is more than a thousand dollars per month, indefinitely,” Dr. Almandoz said. “Not that we should not treat everybody. That’s not the message I’m saying. But if we’re looking at yield or value in terms of treating obesity in a setting with limited resources, it may be best to start with who is most likely to benefit.”
How they created four obesity types
Starting in 2015, Dr. Acosta and colleagues started comparing tests in people with normal weight versus obesity. They used artificial intelligence and machine learning to classify obesity into 11 types at first. They realized this many obesity types were not practical for doctors and people with obesity, so they combined them into four phenotypes.
“The AI machine learning was followed by, as I like to call, HI, or human intelligence,” he said.
The saliva test checks for about 6,000 relevant genetic single-nucleotide polymorphisms. Six thousand genetic changes may sound like a large number to check; however, the average individual carries 5 million and 6 million SNPs in their DNA.
The results are translated to a score that yields a low risk or high risk for Hungry Gut or other types of obesity. “You can have all six thousand genetic mutations, or you can have zero,” Dr. Acosta said.
Moving forward
After the soft launch of Hungry Gut testing in April, Phenomix plans to continue studying their saliva test on other obesity types.
Dr. Acosta is not aware of any direct competitors to Phenomix, although that could change. “I think we’re the only diagnostic company in the space right now. But if it’s really a $14.8 billion market, we’re going to see a lot of diagnostic companies trying to do what we’re doing – if we’re successful,” he said.
An October 2022 report from Polaris Market Research estimates that the global market for obesity treatment – medications, surgery, and all others – was about $14 billion in 2021. The same report predicts the market will grow to $32 billion by 2030.
A version of this article first appeared on WebMD.com.
FDA approves new drug, sotagliflozin, for heart failure
Sotagliflozin, a novel agent that inhibits sodium-glucose cotransporter 1 as well as SGLT2, has received marketing approval from the Food and Drug Administration for reducing the risk for cardiovascular death, hospitalization for heart failure, and urgent heart failure visits in patients with heart failure, and also for preventing these same events in patients with type 2 diabetes, chronic kidney disease (CKD), and other cardiovascular disease risk factors.
This puts sotagliflozin in direct competition with two SGLT2 inhibitors, dapagliflozin (Farxiga) and empagliflozin (Jardiance), that already have indications for preventing heart failure hospitalizations in patients with heart failure as well as approvals for type 2 diabetes and preservation of renal function.
Officials at Lexicon Pharmaceuticals, the company that developed and will market sotagliflozin under the trade name Inpefa, said in a press release that they expect U.S. sales of the agent to begin before the end of June 2023. The release also highlighted that the approval broadly covered use in patients with heart failure across the full range of both reduced and preserved left ventricular ejection fractions.
They base this niche target for sotagliflozin on results from the SOLOIST-WHF trial, which randomized 1,222 patients with type 2 diabetes recently hospitalized for worsening heart failure and showed a significant 33% reduction in the rate of deaths from cardiovascular causes and hospitalizations and urgent visits for heart failure, compared with control patients during a median 9 months of follow-up. Nearly half of the enrolled patients received their first dose while still hospitalized, while the other half received their first dose a median of 2 days after hospital discharge. The drug appeared safe.
Cutting heart failure rehospitalizations in half
An exploratory post hoc analysis of SOLOIST-WHF showed that treatment with sotagliflozin cut the rate of rehospitalizations roughly in half after both 30 and 90 days compared with control patients, according to an abstract presented at the 2022 annual scientific sessions of the AHA that has not yet been published in a peer-reviewed journal.
The only SGLT2 inhibitor tested so far when initiated in patients during hospitalization for heart failure is empagliflozin, in the EMPULSE trial, which randomized 530 patients. EMPULSE also showed that starting an SGLT2 inhibitor in this setting was safe and resulted in significant clinical benefit, the study’s primary endpoint, defined as a composite of death from any cause, number of heart failure events, and time to first heart failure event, or a 5-point or greater difference in change from baseline in the Kansas City Cardiomyopathy Questionnaire Total Symptom Score at 90 days.
In the DELIVER trial, which tested dapagliflozin in patients with heart failure with preserved ejection fraction, roughly 10% of patients started study treatment during or within 30 days of heart failure hospitalization, and in this subgroup, dapagliflozin appeared as effective as it was in the other 90% of patients who did not start the drug during an acute or subacute phase.
Despite the SOLOIST-WHF evidence for sotagliflozin’s safety and efficacy in this economically important clinical setting, some experts say the drug faces an uphill path as it contends for market share against two solidly established, albeit dramatically underused, SGLT2 inhibitors. (Recent data document that 20% or fewer of U.S. patients eligible for treatment with an SGLT2 inhibitor receive it, such as a review of 49,000 patients hospitalized during 2021-2022 with heart failure with reduced ejection fraction.)
Others foresee a clear role for sotagliflozin, particularly because of additional facets of the drug’s performance in trials that they perceive give it an edge over dapagliflozin and empagliflozin. This includes evidence that sotagliflozin treatment uniquely (within the SGLT2 inhibitor class) cuts the rate of strokes and myocardial infarctions, as well as evidence of its apparent ability to lower hemoglobin A1c levels in patients with type 2 diabetes and with an estimated glomerular filtration rate below 30 mL/min per 1.73 m2, a property likely linked to inhibition of SGLT1 in the gut that dampens intestinal glucose absorption.
Sotagliflozin uptake ‘will be a challenge’
“It will be a challenge” for sotagliflozin uptake, given the head start that both dapagliflozin and empagliflozin have had as well-documented agents for patients with heart failure, commented Javed Butler, MD, a heart failure clinician and trialist who is president of the Baylor Scott & White Research Institute in Dallas.
Given the position dapagliflozin and empagliflozin currently have in U.S. heart failure management – with the SGLT2 inhibitor class called out in guidelines as foundational for treating patients with heart failure with reduced ejection fraction and likely soon for heart failure with preserved ejection fraction as well – “I can’t imagine [sotagliflozin] will be considered a preferred option,” Dr. Butler said in an interview.
Another expert was even more dismissive of sotagliflozin’s role.
“There is no persuasive evidence that sotagliflozin has any advantages, compared with the SGLT2 inhibitors, for the treatment of heart failure,” said Milton Packer, MD, a heart failure specialist and trialist at Baylor University Medical Center, Dallas. “I do not see why U.S. physicians might pivot from established SGLT2 inhibitors to sotagliflozin,” unless it was priced “at a very meaningful discount to available SGLT2 inhibitors.”
At the time it announced the FDA’s approval, Lexicon did not provide details on how it would price sotagliflozin. Existing retail prices for dapagliflozin and empagliflozin run about $550-$600/month, a price point that has contributed to slow U.S. uptake of the drug class. But experts anticipate a dramatic shake-up of the U.S. market for SGLT2 inhibitors with expected introduction of a generic SGLT2 inhibitor formulation by 2025, a development that could further dampen sotagliflozin’s prospects.
Other experts are more optimistic about the new agent’s uptake, perhaps none more than Deepak L. Bhatt, MD, MPH, who led both pivotal trials that provide the bulk of sotagliflozin’s evidence package.
In addition to SOLOIST-WHF, Dr. Bhatt also headed the SCORED trial, with 10,584 patients with type 2 diabetes, CKD, and risks for cardiovascular disease randomized to sotagliflozin or placebo and followed for a median of 16 months. The primary result showed that sotagliflozin treatment cut the combined rate of deaths from cardiovascular causes, hospitalizations for heart failure, and urgent visits for heart failure by a significant 26% relative to control patients.
A clear MACE benefit
“The data from SOLOIST-WHF and SCORED look at least as good as the data for the SGLT2 inhibitors for heart failure, and what appears to be different are the rates for MI and stroke in SCORED,” said Dr. Bhatt, director of Mount Sinai Heart, New York.
“I believe the rate of major adverse cardiovascular events [MACE] were reduced [in SCORED], and this is different from the SGLT2 inhibitors,” he said in an interview.
In 2022, Dr. Bhatt reported results from a prespecified secondary analysis of SCORED that showed that treatment with sotagliflozin cut the rate of MACE by a significant 21%-26%, compared with placebo. This finding was, in part, driven by the first data to show a substantial benefit from an SGLT inhibitor on stroke rates.
And while SCORED did not report a significant benefit for slowing progression of CKD, subsequent post hoc analyses have suggested this advantage also in as-yet-unpublished findings, Dr. Bhatt added.
But he said he doubted nephrologists will see it as a first-line agent for slowing CKD progression – an indication already held by dapagliflozin, pending for empagliflozin, and also in place for a third SGLT2 inhibitor, canagliflozin (Invokana) – because sotagliflozin lacks clear significant and prespecified evidence for this effect.
Dr. Bhatt also acknowledged the limitation of sotagliflozin compared with the SGLT2 inhibitors as an agent for glucose control, again because of no evidence for this effect from a prospective analysis and no pending indication for type 2 diabetes treatment. But the SCORED data showed a clear A1c benefit, even in patients with severely reduced renal function.
Mostly for cardiologists? ‘Compelling’ reductions in MIs and strokes
That may mean sotagliflozin “won’t get much use by endocrinologists nor by primary care physicians,” commented Carol L. Wysham, MD, an endocrinologist with MultiCare in Spokane, Wash.
Sotagliflozin “will be a cardiology drug,” and will “have a hard time” competing with the SGLT2 inhibitors, she predicted.
Dr. Bhatt agreed that sotagliflozin “will be perceived as a drug for cardiologists to prescribe. I don’t see endocrinologists, nephrologists, and primary care physicians reaching for this drug if it has a heart failure label.” But, he added, “my hope is that the company files for additional indications. It deserves an indication for glycemic control.”
The evidence for a heart failure benefit from sotagliflozin is “valid and compelling,” and “having this option is great,” commented Mikhail N. Kosiborod, MD, a cardiologist, vice president of research at Saint Luke’s Health System, and codirector of the Haverty Cardiometabolic Center of Excellence at Saint Luke’s Mid America Heart Institute in Kansas City, Mo. But, he added, “it will be a reasonably tall task for sotagliflozin to come from behind and be disruptive in a space where there are already two well-established SGLT2 inhibitors” approved for preventing heart failure hospitalizations, “with a lot of data to back them up,”
The feature that sets sotagliflozin apart from the approved SGLT2 inhibitors is the “really compelling decrease” it produced in rates of MIs and strokes “that we simply do not see with SGLT2 inhibitors,” Dr. Kosiborod said in an interview.
He also cited results from SCORED that suggest “a meaningful reduction in A1c” when indirectly compared with SGLT2 inhibitors, especially in patients with more severe CKD. The lack of a dedicated A1c-lowering trial or an approved type 2 diabetes indication “will not be a problem for cardiologists,” he predicted, but also agreed that it is less likely to be used by primary care physicians in low-risk patients.
“I can see myself prescribing sotagliflozin,” said Dr. Kosiborod, a SCORED coinvestigator, especially for patients with coexisting type 2 diabetes, heart failure, CKD, and atherosclerotic cardiovascular disease. These patients may get “more bang for the buck” because of a reduced risk for MI and stroke, making sotagliflozin “a solid consideration in these patients if the economic factors align.”
Like others, Dr. Kosiborod cited the big impact pricing will have, especially if, as expected, a generic SGLT2 inhibitor soon comes onto the U.S. market. “Access and affordability are very important.”
SOLOIST-WHF and SCORED were sponsored initially by Sanofi and later by Lexicon after Sanofi pulled out of sotagliflozin development. Dr. Butler has been a consultant for Lexicon as well as for AstraZeneca (which markets Farxiga), Boehringer Ingelheim and Lilly (which jointly market Jardiance), and Janssen (which markets Invokana), as well as for numerous other companies. Dr. Packer has been a consultant for AstraZeneca, Boehringer Ingelheim, Lilly, and numerous other companies. Dr. Bhatt was lead investigator for SOLOIST-WHF and SCORED and has been an adviser for Boehringer Ingelheim and Janssen and numerous other companies. Dr. Wysham has been an adviser, speaker, and consultant for AstraZeneca, Boehringer Ingelheim, Lilly, Janssen, Novo Nordisk, and Sanofi, an adviser for Abbott, and a speaker for Insulet. Dr. Kosiborod was a member of the SCORED Steering Committee and has been a consultant for Lexicon, AstraZeneca, Boehringer Ingelheim, Janssen, Lilly, Novo Nordisk, and numerous other companies.
A version of this article first appeared on Medscape.com.
Sotagliflozin, a novel agent that inhibits sodium-glucose cotransporter 1 as well as SGLT2, has received marketing approval from the Food and Drug Administration for reducing the risk for cardiovascular death, hospitalization for heart failure, and urgent heart failure visits in patients with heart failure, and also for preventing these same events in patients with type 2 diabetes, chronic kidney disease (CKD), and other cardiovascular disease risk factors.
This puts sotagliflozin in direct competition with two SGLT2 inhibitors, dapagliflozin (Farxiga) and empagliflozin (Jardiance), that already have indications for preventing heart failure hospitalizations in patients with heart failure as well as approvals for type 2 diabetes and preservation of renal function.
Officials at Lexicon Pharmaceuticals, the company that developed and will market sotagliflozin under the trade name Inpefa, said in a press release that they expect U.S. sales of the agent to begin before the end of June 2023. The release also highlighted that the approval broadly covered use in patients with heart failure across the full range of both reduced and preserved left ventricular ejection fractions.
They base this niche target for sotagliflozin on results from the SOLOIST-WHF trial, which randomized 1,222 patients with type 2 diabetes recently hospitalized for worsening heart failure and showed a significant 33% reduction in the rate of deaths from cardiovascular causes and hospitalizations and urgent visits for heart failure, compared with control patients during a median 9 months of follow-up. Nearly half of the enrolled patients received their first dose while still hospitalized, while the other half received their first dose a median of 2 days after hospital discharge. The drug appeared safe.
Cutting heart failure rehospitalizations in half
An exploratory post hoc analysis of SOLOIST-WHF showed that treatment with sotagliflozin cut the rate of rehospitalizations roughly in half after both 30 and 90 days compared with control patients, according to an abstract presented at the 2022 annual scientific sessions of the AHA that has not yet been published in a peer-reviewed journal.
The only SGLT2 inhibitor tested so far when initiated in patients during hospitalization for heart failure is empagliflozin, in the EMPULSE trial, which randomized 530 patients. EMPULSE also showed that starting an SGLT2 inhibitor in this setting was safe and resulted in significant clinical benefit, the study’s primary endpoint, defined as a composite of death from any cause, number of heart failure events, and time to first heart failure event, or a 5-point or greater difference in change from baseline in the Kansas City Cardiomyopathy Questionnaire Total Symptom Score at 90 days.
In the DELIVER trial, which tested dapagliflozin in patients with heart failure with preserved ejection fraction, roughly 10% of patients started study treatment during or within 30 days of heart failure hospitalization, and in this subgroup, dapagliflozin appeared as effective as it was in the other 90% of patients who did not start the drug during an acute or subacute phase.
Despite the SOLOIST-WHF evidence for sotagliflozin’s safety and efficacy in this economically important clinical setting, some experts say the drug faces an uphill path as it contends for market share against two solidly established, albeit dramatically underused, SGLT2 inhibitors. (Recent data document that 20% or fewer of U.S. patients eligible for treatment with an SGLT2 inhibitor receive it, such as a review of 49,000 patients hospitalized during 2021-2022 with heart failure with reduced ejection fraction.)
Others foresee a clear role for sotagliflozin, particularly because of additional facets of the drug’s performance in trials that they perceive give it an edge over dapagliflozin and empagliflozin. This includes evidence that sotagliflozin treatment uniquely (within the SGLT2 inhibitor class) cuts the rate of strokes and myocardial infarctions, as well as evidence of its apparent ability to lower hemoglobin A1c levels in patients with type 2 diabetes and with an estimated glomerular filtration rate below 30 mL/min per 1.73 m2, a property likely linked to inhibition of SGLT1 in the gut that dampens intestinal glucose absorption.
Sotagliflozin uptake ‘will be a challenge’
“It will be a challenge” for sotagliflozin uptake, given the head start that both dapagliflozin and empagliflozin have had as well-documented agents for patients with heart failure, commented Javed Butler, MD, a heart failure clinician and trialist who is president of the Baylor Scott & White Research Institute in Dallas.
Given the position dapagliflozin and empagliflozin currently have in U.S. heart failure management – with the SGLT2 inhibitor class called out in guidelines as foundational for treating patients with heart failure with reduced ejection fraction and likely soon for heart failure with preserved ejection fraction as well – “I can’t imagine [sotagliflozin] will be considered a preferred option,” Dr. Butler said in an interview.
Another expert was even more dismissive of sotagliflozin’s role.
“There is no persuasive evidence that sotagliflozin has any advantages, compared with the SGLT2 inhibitors, for the treatment of heart failure,” said Milton Packer, MD, a heart failure specialist and trialist at Baylor University Medical Center, Dallas. “I do not see why U.S. physicians might pivot from established SGLT2 inhibitors to sotagliflozin,” unless it was priced “at a very meaningful discount to available SGLT2 inhibitors.”
At the time it announced the FDA’s approval, Lexicon did not provide details on how it would price sotagliflozin. Existing retail prices for dapagliflozin and empagliflozin run about $550-$600/month, a price point that has contributed to slow U.S. uptake of the drug class. But experts anticipate a dramatic shake-up of the U.S. market for SGLT2 inhibitors with expected introduction of a generic SGLT2 inhibitor formulation by 2025, a development that could further dampen sotagliflozin’s prospects.
Other experts are more optimistic about the new agent’s uptake, perhaps none more than Deepak L. Bhatt, MD, MPH, who led both pivotal trials that provide the bulk of sotagliflozin’s evidence package.
In addition to SOLOIST-WHF, Dr. Bhatt also headed the SCORED trial, with 10,584 patients with type 2 diabetes, CKD, and risks for cardiovascular disease randomized to sotagliflozin or placebo and followed for a median of 16 months. The primary result showed that sotagliflozin treatment cut the combined rate of deaths from cardiovascular causes, hospitalizations for heart failure, and urgent visits for heart failure by a significant 26% relative to control patients.
A clear MACE benefit
“The data from SOLOIST-WHF and SCORED look at least as good as the data for the SGLT2 inhibitors for heart failure, and what appears to be different are the rates for MI and stroke in SCORED,” said Dr. Bhatt, director of Mount Sinai Heart, New York.
“I believe the rate of major adverse cardiovascular events [MACE] were reduced [in SCORED], and this is different from the SGLT2 inhibitors,” he said in an interview.
In 2022, Dr. Bhatt reported results from a prespecified secondary analysis of SCORED that showed that treatment with sotagliflozin cut the rate of MACE by a significant 21%-26%, compared with placebo. This finding was, in part, driven by the first data to show a substantial benefit from an SGLT inhibitor on stroke rates.
And while SCORED did not report a significant benefit for slowing progression of CKD, subsequent post hoc analyses have suggested this advantage also in as-yet-unpublished findings, Dr. Bhatt added.
But he said he doubted nephrologists will see it as a first-line agent for slowing CKD progression – an indication already held by dapagliflozin, pending for empagliflozin, and also in place for a third SGLT2 inhibitor, canagliflozin (Invokana) – because sotagliflozin lacks clear significant and prespecified evidence for this effect.
Dr. Bhatt also acknowledged the limitation of sotagliflozin compared with the SGLT2 inhibitors as an agent for glucose control, again because of no evidence for this effect from a prospective analysis and no pending indication for type 2 diabetes treatment. But the SCORED data showed a clear A1c benefit, even in patients with severely reduced renal function.
Mostly for cardiologists? ‘Compelling’ reductions in MIs and strokes
That may mean sotagliflozin “won’t get much use by endocrinologists nor by primary care physicians,” commented Carol L. Wysham, MD, an endocrinologist with MultiCare in Spokane, Wash.
Sotagliflozin “will be a cardiology drug,” and will “have a hard time” competing with the SGLT2 inhibitors, she predicted.
Dr. Bhatt agreed that sotagliflozin “will be perceived as a drug for cardiologists to prescribe. I don’t see endocrinologists, nephrologists, and primary care physicians reaching for this drug if it has a heart failure label.” But, he added, “my hope is that the company files for additional indications. It deserves an indication for glycemic control.”
The evidence for a heart failure benefit from sotagliflozin is “valid and compelling,” and “having this option is great,” commented Mikhail N. Kosiborod, MD, a cardiologist, vice president of research at Saint Luke’s Health System, and codirector of the Haverty Cardiometabolic Center of Excellence at Saint Luke’s Mid America Heart Institute in Kansas City, Mo. But, he added, “it will be a reasonably tall task for sotagliflozin to come from behind and be disruptive in a space where there are already two well-established SGLT2 inhibitors” approved for preventing heart failure hospitalizations, “with a lot of data to back them up,”
The feature that sets sotagliflozin apart from the approved SGLT2 inhibitors is the “really compelling decrease” it produced in rates of MIs and strokes “that we simply do not see with SGLT2 inhibitors,” Dr. Kosiborod said in an interview.
He also cited results from SCORED that suggest “a meaningful reduction in A1c” when indirectly compared with SGLT2 inhibitors, especially in patients with more severe CKD. The lack of a dedicated A1c-lowering trial or an approved type 2 diabetes indication “will not be a problem for cardiologists,” he predicted, but also agreed that it is less likely to be used by primary care physicians in low-risk patients.
“I can see myself prescribing sotagliflozin,” said Dr. Kosiborod, a SCORED coinvestigator, especially for patients with coexisting type 2 diabetes, heart failure, CKD, and atherosclerotic cardiovascular disease. These patients may get “more bang for the buck” because of a reduced risk for MI and stroke, making sotagliflozin “a solid consideration in these patients if the economic factors align.”
Like others, Dr. Kosiborod cited the big impact pricing will have, especially if, as expected, a generic SGLT2 inhibitor soon comes onto the U.S. market. “Access and affordability are very important.”
SOLOIST-WHF and SCORED were sponsored initially by Sanofi and later by Lexicon after Sanofi pulled out of sotagliflozin development. Dr. Butler has been a consultant for Lexicon as well as for AstraZeneca (which markets Farxiga), Boehringer Ingelheim and Lilly (which jointly market Jardiance), and Janssen (which markets Invokana), as well as for numerous other companies. Dr. Packer has been a consultant for AstraZeneca, Boehringer Ingelheim, Lilly, and numerous other companies. Dr. Bhatt was lead investigator for SOLOIST-WHF and SCORED and has been an adviser for Boehringer Ingelheim and Janssen and numerous other companies. Dr. Wysham has been an adviser, speaker, and consultant for AstraZeneca, Boehringer Ingelheim, Lilly, Janssen, Novo Nordisk, and Sanofi, an adviser for Abbott, and a speaker for Insulet. Dr. Kosiborod was a member of the SCORED Steering Committee and has been a consultant for Lexicon, AstraZeneca, Boehringer Ingelheim, Janssen, Lilly, Novo Nordisk, and numerous other companies.
A version of this article first appeared on Medscape.com.
Sotagliflozin, a novel agent that inhibits sodium-glucose cotransporter 1 as well as SGLT2, has received marketing approval from the Food and Drug Administration for reducing the risk for cardiovascular death, hospitalization for heart failure, and urgent heart failure visits in patients with heart failure, and also for preventing these same events in patients with type 2 diabetes, chronic kidney disease (CKD), and other cardiovascular disease risk factors.
This puts sotagliflozin in direct competition with two SGLT2 inhibitors, dapagliflozin (Farxiga) and empagliflozin (Jardiance), that already have indications for preventing heart failure hospitalizations in patients with heart failure as well as approvals for type 2 diabetes and preservation of renal function.
Officials at Lexicon Pharmaceuticals, the company that developed and will market sotagliflozin under the trade name Inpefa, said in a press release that they expect U.S. sales of the agent to begin before the end of June 2023. The release also highlighted that the approval broadly covered use in patients with heart failure across the full range of both reduced and preserved left ventricular ejection fractions.
They base this niche target for sotagliflozin on results from the SOLOIST-WHF trial, which randomized 1,222 patients with type 2 diabetes recently hospitalized for worsening heart failure and showed a significant 33% reduction in the rate of deaths from cardiovascular causes and hospitalizations and urgent visits for heart failure, compared with control patients during a median 9 months of follow-up. Nearly half of the enrolled patients received their first dose while still hospitalized, while the other half received their first dose a median of 2 days after hospital discharge. The drug appeared safe.
Cutting heart failure rehospitalizations in half
An exploratory post hoc analysis of SOLOIST-WHF showed that treatment with sotagliflozin cut the rate of rehospitalizations roughly in half after both 30 and 90 days compared with control patients, according to an abstract presented at the 2022 annual scientific sessions of the AHA that has not yet been published in a peer-reviewed journal.
The only SGLT2 inhibitor tested so far when initiated in patients during hospitalization for heart failure is empagliflozin, in the EMPULSE trial, which randomized 530 patients. EMPULSE also showed that starting an SGLT2 inhibitor in this setting was safe and resulted in significant clinical benefit, the study’s primary endpoint, defined as a composite of death from any cause, number of heart failure events, and time to first heart failure event, or a 5-point or greater difference in change from baseline in the Kansas City Cardiomyopathy Questionnaire Total Symptom Score at 90 days.
In the DELIVER trial, which tested dapagliflozin in patients with heart failure with preserved ejection fraction, roughly 10% of patients started study treatment during or within 30 days of heart failure hospitalization, and in this subgroup, dapagliflozin appeared as effective as it was in the other 90% of patients who did not start the drug during an acute or subacute phase.
Despite the SOLOIST-WHF evidence for sotagliflozin’s safety and efficacy in this economically important clinical setting, some experts say the drug faces an uphill path as it contends for market share against two solidly established, albeit dramatically underused, SGLT2 inhibitors. (Recent data document that 20% or fewer of U.S. patients eligible for treatment with an SGLT2 inhibitor receive it, such as a review of 49,000 patients hospitalized during 2021-2022 with heart failure with reduced ejection fraction.)
Others foresee a clear role for sotagliflozin, particularly because of additional facets of the drug’s performance in trials that they perceive give it an edge over dapagliflozin and empagliflozin. This includes evidence that sotagliflozin treatment uniquely (within the SGLT2 inhibitor class) cuts the rate of strokes and myocardial infarctions, as well as evidence of its apparent ability to lower hemoglobin A1c levels in patients with type 2 diabetes and with an estimated glomerular filtration rate below 30 mL/min per 1.73 m2, a property likely linked to inhibition of SGLT1 in the gut that dampens intestinal glucose absorption.
Sotagliflozin uptake ‘will be a challenge’
“It will be a challenge” for sotagliflozin uptake, given the head start that both dapagliflozin and empagliflozin have had as well-documented agents for patients with heart failure, commented Javed Butler, MD, a heart failure clinician and trialist who is president of the Baylor Scott & White Research Institute in Dallas.
Given the position dapagliflozin and empagliflozin currently have in U.S. heart failure management – with the SGLT2 inhibitor class called out in guidelines as foundational for treating patients with heart failure with reduced ejection fraction and likely soon for heart failure with preserved ejection fraction as well – “I can’t imagine [sotagliflozin] will be considered a preferred option,” Dr. Butler said in an interview.
Another expert was even more dismissive of sotagliflozin’s role.
“There is no persuasive evidence that sotagliflozin has any advantages, compared with the SGLT2 inhibitors, for the treatment of heart failure,” said Milton Packer, MD, a heart failure specialist and trialist at Baylor University Medical Center, Dallas. “I do not see why U.S. physicians might pivot from established SGLT2 inhibitors to sotagliflozin,” unless it was priced “at a very meaningful discount to available SGLT2 inhibitors.”
At the time it announced the FDA’s approval, Lexicon did not provide details on how it would price sotagliflozin. Existing retail prices for dapagliflozin and empagliflozin run about $550-$600/month, a price point that has contributed to slow U.S. uptake of the drug class. But experts anticipate a dramatic shake-up of the U.S. market for SGLT2 inhibitors with expected introduction of a generic SGLT2 inhibitor formulation by 2025, a development that could further dampen sotagliflozin’s prospects.
Other experts are more optimistic about the new agent’s uptake, perhaps none more than Deepak L. Bhatt, MD, MPH, who led both pivotal trials that provide the bulk of sotagliflozin’s evidence package.
In addition to SOLOIST-WHF, Dr. Bhatt also headed the SCORED trial, with 10,584 patients with type 2 diabetes, CKD, and risks for cardiovascular disease randomized to sotagliflozin or placebo and followed for a median of 16 months. The primary result showed that sotagliflozin treatment cut the combined rate of deaths from cardiovascular causes, hospitalizations for heart failure, and urgent visits for heart failure by a significant 26% relative to control patients.
A clear MACE benefit
“The data from SOLOIST-WHF and SCORED look at least as good as the data for the SGLT2 inhibitors for heart failure, and what appears to be different are the rates for MI and stroke in SCORED,” said Dr. Bhatt, director of Mount Sinai Heart, New York.
“I believe the rate of major adverse cardiovascular events [MACE] were reduced [in SCORED], and this is different from the SGLT2 inhibitors,” he said in an interview.
In 2022, Dr. Bhatt reported results from a prespecified secondary analysis of SCORED that showed that treatment with sotagliflozin cut the rate of MACE by a significant 21%-26%, compared with placebo. This finding was, in part, driven by the first data to show a substantial benefit from an SGLT inhibitor on stroke rates.
And while SCORED did not report a significant benefit for slowing progression of CKD, subsequent post hoc analyses have suggested this advantage also in as-yet-unpublished findings, Dr. Bhatt added.
But he said he doubted nephrologists will see it as a first-line agent for slowing CKD progression – an indication already held by dapagliflozin, pending for empagliflozin, and also in place for a third SGLT2 inhibitor, canagliflozin (Invokana) – because sotagliflozin lacks clear significant and prespecified evidence for this effect.
Dr. Bhatt also acknowledged the limitation of sotagliflozin compared with the SGLT2 inhibitors as an agent for glucose control, again because of no evidence for this effect from a prospective analysis and no pending indication for type 2 diabetes treatment. But the SCORED data showed a clear A1c benefit, even in patients with severely reduced renal function.
Mostly for cardiologists? ‘Compelling’ reductions in MIs and strokes
That may mean sotagliflozin “won’t get much use by endocrinologists nor by primary care physicians,” commented Carol L. Wysham, MD, an endocrinologist with MultiCare in Spokane, Wash.
Sotagliflozin “will be a cardiology drug,” and will “have a hard time” competing with the SGLT2 inhibitors, she predicted.
Dr. Bhatt agreed that sotagliflozin “will be perceived as a drug for cardiologists to prescribe. I don’t see endocrinologists, nephrologists, and primary care physicians reaching for this drug if it has a heart failure label.” But, he added, “my hope is that the company files for additional indications. It deserves an indication for glycemic control.”
The evidence for a heart failure benefit from sotagliflozin is “valid and compelling,” and “having this option is great,” commented Mikhail N. Kosiborod, MD, a cardiologist, vice president of research at Saint Luke’s Health System, and codirector of the Haverty Cardiometabolic Center of Excellence at Saint Luke’s Mid America Heart Institute in Kansas City, Mo. But, he added, “it will be a reasonably tall task for sotagliflozin to come from behind and be disruptive in a space where there are already two well-established SGLT2 inhibitors” approved for preventing heart failure hospitalizations, “with a lot of data to back them up,”
The feature that sets sotagliflozin apart from the approved SGLT2 inhibitors is the “really compelling decrease” it produced in rates of MIs and strokes “that we simply do not see with SGLT2 inhibitors,” Dr. Kosiborod said in an interview.
He also cited results from SCORED that suggest “a meaningful reduction in A1c” when indirectly compared with SGLT2 inhibitors, especially in patients with more severe CKD. The lack of a dedicated A1c-lowering trial or an approved type 2 diabetes indication “will not be a problem for cardiologists,” he predicted, but also agreed that it is less likely to be used by primary care physicians in low-risk patients.
“I can see myself prescribing sotagliflozin,” said Dr. Kosiborod, a SCORED coinvestigator, especially for patients with coexisting type 2 diabetes, heart failure, CKD, and atherosclerotic cardiovascular disease. These patients may get “more bang for the buck” because of a reduced risk for MI and stroke, making sotagliflozin “a solid consideration in these patients if the economic factors align.”
Like others, Dr. Kosiborod cited the big impact pricing will have, especially if, as expected, a generic SGLT2 inhibitor soon comes onto the U.S. market. “Access and affordability are very important.”
SOLOIST-WHF and SCORED were sponsored initially by Sanofi and later by Lexicon after Sanofi pulled out of sotagliflozin development. Dr. Butler has been a consultant for Lexicon as well as for AstraZeneca (which markets Farxiga), Boehringer Ingelheim and Lilly (which jointly market Jardiance), and Janssen (which markets Invokana), as well as for numerous other companies. Dr. Packer has been a consultant for AstraZeneca, Boehringer Ingelheim, Lilly, and numerous other companies. Dr. Bhatt was lead investigator for SOLOIST-WHF and SCORED and has been an adviser for Boehringer Ingelheim and Janssen and numerous other companies. Dr. Wysham has been an adviser, speaker, and consultant for AstraZeneca, Boehringer Ingelheim, Lilly, Janssen, Novo Nordisk, and Sanofi, an adviser for Abbott, and a speaker for Insulet. Dr. Kosiborod was a member of the SCORED Steering Committee and has been a consultant for Lexicon, AstraZeneca, Boehringer Ingelheim, Janssen, Lilly, Novo Nordisk, and numerous other companies.
A version of this article first appeared on Medscape.com.