User login
Donanemab bests aducanumab in head-to-head Alzheimer’s trial
BOSTON –
Nearly 40% of patients treated with donanemab had amyloid clearance at 6 months compared with less than 2% of those who received aducanumab, which was approved in 2021 amid a great deal of controversy.
Titration for donanemab progressed more quickly, with participants receiving a maximum dose twice as early as those on aducanumab, without any increase in rates of amyloid-related imaging abnormalities (ARIA) – the most common side effect of amyloid drugs.
Early results from the randomized phase 3 TRAILBLAZER-ALZ 4 trial of donanemab come just 3 months after the Food and Drug Administration denied manufacturer Eli Lilly’s request for accelerated approval for the drug.
“This study shows that the drug with the quicker titration scheme, donanemab, produced more amyloid lowering and did it without having more ARIA,” said lead investigator Stephen P. Salloway, MD, director of the Memory and Aging Program at Butler Hospital in Providence, R.I., and a professor of neurology at Brown University.
The findings were presented at the 2023 annual meeting of the American Academy of Neurology.
Multicenter, head-to-head trial
Donanemab received breakthrough therapy designation in 2021. The drug works similarly to aducanumab and lecanemab, which was approved earlier this year. All three bind to different parts of the amyloid molecule and stimulate an immune response to help clear amyloid plaques, although they each have a distinctive binding component.
TRAILBLAZER-ALZ 4 was conducted at 31 sites across the United States, enrolling 140 patients aged 50-85 years with early and symptomatic Alzheimer’s disease. Study participants received donanemab or aducanumab at escalating doses for 18 months.
Donanemab was titrated more quickly, with participants receiving 700 mg via IV infusion once a month for 3 months before reaching the maximum dose of 1,400 mg in the fourth month of the study.
Aducanumab titration was slower, beginning at 1 mg/kg via IV monthly for 2 months, then 3 mg/kg for another 2 months, and 6 mg/kg for 2 more months before reaching the maximum dose of 10 mg/kg in the seventh month.
After 6 months of treatment, PET scan analysis revealed that 37.9% of donanemab-treated patients achieved amyloid clearance compared with just 1.6% of those who received aducanumab (P < .001).
Among patients with intermediate tau levels (n = 27 for donanemab and n = 28 for aducanumab), 38.5% of those who received donanemab achieved amyloid clearance compared with 3.8% of patients in the aducanumab group (P = .008).
Amyloid levels were 65.2% lower in donanemab patients, while levels in those receiving aducanumab were reduced by 17.0% (P < .001). Among those with intermediate tau, amyloid levels decreased with donanemab by 63.9% and 25.4% with aducanumab (P ≤ .001).
Investigators also noted a greater reduction in plasma ptau217 with donanemab.
Adverse events were similar between groups, with 62.0% of the donanemab group and 66.7% of aducanumab-treated participants reporting an adverse event.
There were no serious adverse events due to ARIA with donanemab, but one participant in the aducanumab group had a serious adverse event linked to ARIA.
“Even though the amyloid lowering was greater with donanemab, the rate of ARIA was similar, which suggests that the speed and depth of amyloid removal is not driving ARIA,” Dr. Salloway said.
There are three other Trailblazer trials of donanemab. Unlike in similar trials, participants in all three of these studies who received the trial drug could discontinue treatment once criteria for amyloid clearance were met.
That’s precisely what happened with Trailblazer 2, the study on which Lilly based its request for accelerated approval. Ironically, that trial design also contributed to the FDA’s decision to reject that request.
The FDA required data from at least 100 patients who had received donanemab for a minimum of 1 year. While the trial included more than 100 patients, many patients discontinued treatment early after achieving the targeted amount of amyloid clearance.
“They had success, and they got punished for it, in my opinion,” Dr. Salloway said.
Final data from Trailblazer 2 is due in the next month, and if results are positive, Lilly is expected to file for full approval.
Questions remain
“This is an interesting study that suggests donanemab may remove amyloid faster in more people than aducanumab,” said Heather Snyder, PhD, Alzheimer’s Association vice president of medical and scientific relations, who commented on the findings.
Howard Fillit, MD, cofounder and chief science officer at the Alzheimer’s Drug Discovery Foundation, also commented on the findings. He noted that faster amyloid clearance “means less time for requiring sometimes burdensome and expensive infusions.”
Both Dr. Snyder and Dr. Fillit noted that longer-term results are needed, along with studies of whether amyloid clearance offers a protective benefit against Alzheimer’s dementia. More results from Trailblazer 4 will be reported after 12 months and again at 18 months.
“There are obviously still a lot of questions about these drugs and whether reducing amyloid plaque will actually preserve cognitive function or at least slow decline,” Dr. Fillit said.
It will also be important to understand the timing of treatment, including when anti-amyloid therapies should be administered and for how long.
“It will be important to understand how these results translate to patient care and treatment plans, should this drug receive FDA approval,” Dr. Snyder said. “Patients should have the opportunity to make a decision, alongside their physician, on a treatment path that is right for them.”
The study was funded by Eli Lilly. Dr. Salloway has been a consultant for Biogen, EISAI, Lilly, Genentech, Novo Nordisk, Prothena, and others. Dr. Snyder and Dr. Fillit have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
BOSTON –
Nearly 40% of patients treated with donanemab had amyloid clearance at 6 months compared with less than 2% of those who received aducanumab, which was approved in 2021 amid a great deal of controversy.
Titration for donanemab progressed more quickly, with participants receiving a maximum dose twice as early as those on aducanumab, without any increase in rates of amyloid-related imaging abnormalities (ARIA) – the most common side effect of amyloid drugs.
Early results from the randomized phase 3 TRAILBLAZER-ALZ 4 trial of donanemab come just 3 months after the Food and Drug Administration denied manufacturer Eli Lilly’s request for accelerated approval for the drug.
“This study shows that the drug with the quicker titration scheme, donanemab, produced more amyloid lowering and did it without having more ARIA,” said lead investigator Stephen P. Salloway, MD, director of the Memory and Aging Program at Butler Hospital in Providence, R.I., and a professor of neurology at Brown University.
The findings were presented at the 2023 annual meeting of the American Academy of Neurology.
Multicenter, head-to-head trial
Donanemab received breakthrough therapy designation in 2021. The drug works similarly to aducanumab and lecanemab, which was approved earlier this year. All three bind to different parts of the amyloid molecule and stimulate an immune response to help clear amyloid plaques, although they each have a distinctive binding component.
TRAILBLAZER-ALZ 4 was conducted at 31 sites across the United States, enrolling 140 patients aged 50-85 years with early and symptomatic Alzheimer’s disease. Study participants received donanemab or aducanumab at escalating doses for 18 months.
Donanemab was titrated more quickly, with participants receiving 700 mg via IV infusion once a month for 3 months before reaching the maximum dose of 1,400 mg in the fourth month of the study.
Aducanumab titration was slower, beginning at 1 mg/kg via IV monthly for 2 months, then 3 mg/kg for another 2 months, and 6 mg/kg for 2 more months before reaching the maximum dose of 10 mg/kg in the seventh month.
After 6 months of treatment, PET scan analysis revealed that 37.9% of donanemab-treated patients achieved amyloid clearance compared with just 1.6% of those who received aducanumab (P < .001).
Among patients with intermediate tau levels (n = 27 for donanemab and n = 28 for aducanumab), 38.5% of those who received donanemab achieved amyloid clearance compared with 3.8% of patients in the aducanumab group (P = .008).
Amyloid levels were 65.2% lower in donanemab patients, while levels in those receiving aducanumab were reduced by 17.0% (P < .001). Among those with intermediate tau, amyloid levels decreased with donanemab by 63.9% and 25.4% with aducanumab (P ≤ .001).
Investigators also noted a greater reduction in plasma ptau217 with donanemab.
Adverse events were similar between groups, with 62.0% of the donanemab group and 66.7% of aducanumab-treated participants reporting an adverse event.
There were no serious adverse events due to ARIA with donanemab, but one participant in the aducanumab group had a serious adverse event linked to ARIA.
“Even though the amyloid lowering was greater with donanemab, the rate of ARIA was similar, which suggests that the speed and depth of amyloid removal is not driving ARIA,” Dr. Salloway said.
There are three other Trailblazer trials of donanemab. Unlike in similar trials, participants in all three of these studies who received the trial drug could discontinue treatment once criteria for amyloid clearance were met.
That’s precisely what happened with Trailblazer 2, the study on which Lilly based its request for accelerated approval. Ironically, that trial design also contributed to the FDA’s decision to reject that request.
The FDA required data from at least 100 patients who had received donanemab for a minimum of 1 year. While the trial included more than 100 patients, many patients discontinued treatment early after achieving the targeted amount of amyloid clearance.
“They had success, and they got punished for it, in my opinion,” Dr. Salloway said.
Final data from Trailblazer 2 is due in the next month, and if results are positive, Lilly is expected to file for full approval.
Questions remain
“This is an interesting study that suggests donanemab may remove amyloid faster in more people than aducanumab,” said Heather Snyder, PhD, Alzheimer’s Association vice president of medical and scientific relations, who commented on the findings.
Howard Fillit, MD, cofounder and chief science officer at the Alzheimer’s Drug Discovery Foundation, also commented on the findings. He noted that faster amyloid clearance “means less time for requiring sometimes burdensome and expensive infusions.”
Both Dr. Snyder and Dr. Fillit noted that longer-term results are needed, along with studies of whether amyloid clearance offers a protective benefit against Alzheimer’s dementia. More results from Trailblazer 4 will be reported after 12 months and again at 18 months.
“There are obviously still a lot of questions about these drugs and whether reducing amyloid plaque will actually preserve cognitive function or at least slow decline,” Dr. Fillit said.
It will also be important to understand the timing of treatment, including when anti-amyloid therapies should be administered and for how long.
“It will be important to understand how these results translate to patient care and treatment plans, should this drug receive FDA approval,” Dr. Snyder said. “Patients should have the opportunity to make a decision, alongside their physician, on a treatment path that is right for them.”
The study was funded by Eli Lilly. Dr. Salloway has been a consultant for Biogen, EISAI, Lilly, Genentech, Novo Nordisk, Prothena, and others. Dr. Snyder and Dr. Fillit have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
BOSTON –
Nearly 40% of patients treated with donanemab had amyloid clearance at 6 months compared with less than 2% of those who received aducanumab, which was approved in 2021 amid a great deal of controversy.
Titration for donanemab progressed more quickly, with participants receiving a maximum dose twice as early as those on aducanumab, without any increase in rates of amyloid-related imaging abnormalities (ARIA) – the most common side effect of amyloid drugs.
Early results from the randomized phase 3 TRAILBLAZER-ALZ 4 trial of donanemab come just 3 months after the Food and Drug Administration denied manufacturer Eli Lilly’s request for accelerated approval for the drug.
“This study shows that the drug with the quicker titration scheme, donanemab, produced more amyloid lowering and did it without having more ARIA,” said lead investigator Stephen P. Salloway, MD, director of the Memory and Aging Program at Butler Hospital in Providence, R.I., and a professor of neurology at Brown University.
The findings were presented at the 2023 annual meeting of the American Academy of Neurology.
Multicenter, head-to-head trial
Donanemab received breakthrough therapy designation in 2021. The drug works similarly to aducanumab and lecanemab, which was approved earlier this year. All three bind to different parts of the amyloid molecule and stimulate an immune response to help clear amyloid plaques, although they each have a distinctive binding component.
TRAILBLAZER-ALZ 4 was conducted at 31 sites across the United States, enrolling 140 patients aged 50-85 years with early and symptomatic Alzheimer’s disease. Study participants received donanemab or aducanumab at escalating doses for 18 months.
Donanemab was titrated more quickly, with participants receiving 700 mg via IV infusion once a month for 3 months before reaching the maximum dose of 1,400 mg in the fourth month of the study.
Aducanumab titration was slower, beginning at 1 mg/kg via IV monthly for 2 months, then 3 mg/kg for another 2 months, and 6 mg/kg for 2 more months before reaching the maximum dose of 10 mg/kg in the seventh month.
After 6 months of treatment, PET scan analysis revealed that 37.9% of donanemab-treated patients achieved amyloid clearance compared with just 1.6% of those who received aducanumab (P < .001).
Among patients with intermediate tau levels (n = 27 for donanemab and n = 28 for aducanumab), 38.5% of those who received donanemab achieved amyloid clearance compared with 3.8% of patients in the aducanumab group (P = .008).
Amyloid levels were 65.2% lower in donanemab patients, while levels in those receiving aducanumab were reduced by 17.0% (P < .001). Among those with intermediate tau, amyloid levels decreased with donanemab by 63.9% and 25.4% with aducanumab (P ≤ .001).
Investigators also noted a greater reduction in plasma ptau217 with donanemab.
Adverse events were similar between groups, with 62.0% of the donanemab group and 66.7% of aducanumab-treated participants reporting an adverse event.
There were no serious adverse events due to ARIA with donanemab, but one participant in the aducanumab group had a serious adverse event linked to ARIA.
“Even though the amyloid lowering was greater with donanemab, the rate of ARIA was similar, which suggests that the speed and depth of amyloid removal is not driving ARIA,” Dr. Salloway said.
There are three other Trailblazer trials of donanemab. Unlike in similar trials, participants in all three of these studies who received the trial drug could discontinue treatment once criteria for amyloid clearance were met.
That’s precisely what happened with Trailblazer 2, the study on which Lilly based its request for accelerated approval. Ironically, that trial design also contributed to the FDA’s decision to reject that request.
The FDA required data from at least 100 patients who had received donanemab for a minimum of 1 year. While the trial included more than 100 patients, many patients discontinued treatment early after achieving the targeted amount of amyloid clearance.
“They had success, and they got punished for it, in my opinion,” Dr. Salloway said.
Final data from Trailblazer 2 is due in the next month, and if results are positive, Lilly is expected to file for full approval.
Questions remain
“This is an interesting study that suggests donanemab may remove amyloid faster in more people than aducanumab,” said Heather Snyder, PhD, Alzheimer’s Association vice president of medical and scientific relations, who commented on the findings.
Howard Fillit, MD, cofounder and chief science officer at the Alzheimer’s Drug Discovery Foundation, also commented on the findings. He noted that faster amyloid clearance “means less time for requiring sometimes burdensome and expensive infusions.”
Both Dr. Snyder and Dr. Fillit noted that longer-term results are needed, along with studies of whether amyloid clearance offers a protective benefit against Alzheimer’s dementia. More results from Trailblazer 4 will be reported after 12 months and again at 18 months.
“There are obviously still a lot of questions about these drugs and whether reducing amyloid plaque will actually preserve cognitive function or at least slow decline,” Dr. Fillit said.
It will also be important to understand the timing of treatment, including when anti-amyloid therapies should be administered and for how long.
“It will be important to understand how these results translate to patient care and treatment plans, should this drug receive FDA approval,” Dr. Snyder said. “Patients should have the opportunity to make a decision, alongside their physician, on a treatment path that is right for them.”
The study was funded by Eli Lilly. Dr. Salloway has been a consultant for Biogen, EISAI, Lilly, Genentech, Novo Nordisk, Prothena, and others. Dr. Snyder and Dr. Fillit have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM AAN 2023
Forget Fibrates for Cardiovascular Risk Reduction: Commentary on the Failure and Implications of the PROMINENT Trial
In this supplement to Family Medicine, Charles P Vega, MD, and Pamela R Kushner, MD, discuss failure of the PROMINENT trial and implications for use of fibrates to reduce cardiovascular risk.
In this supplement to Family Medicine, Charles P Vega, MD, and Pamela R Kushner, MD, discuss failure of the PROMINENT trial and implications for use of fibrates to reduce cardiovascular risk.
In this supplement to Family Medicine, Charles P Vega, MD, and Pamela R Kushner, MD, discuss failure of the PROMINENT trial and implications for use of fibrates to reduce cardiovascular risk.
New hope for adult children with ‘failure to launch’ syndrome
WASHINGTON – , a new pilot study shows.
Known as failure to launch (FTL) syndrome, the criteria for this condition include the absence of a neurodevelopmental, mental, or intellectual condition, difficulty adapting to the challenges of adulthood, and living with or at the expense of parents.
Results suggest that the program benefits families dealing with FTL, said study investigator Uri Berger, PhD, postdoctoral associate, Yale Child Study Center Anxiety and Mood Disorders Program, New Haven, Conn.
“If you encounter parents who are say 50-60 years old who have a child with FTL, you can tell them there’s something they can do; there’s work they can do even if their child is refusing to go to therapy,” he said.
The findings were presented as part of the Anxiety and Depression Association of America Anxiety & Depression conference.
Anxious, isolated
Estimates suggest that there are 3.3 million physically able adults with FTL and that the disorder may be on the rise. These individuals often present with mental health symptoms including anxiety, depression, and suicidality, and tend to be socially isolated.
The investigators noted that intervening is often challenging because individuals with the syndrome are frequently noncompliant with therapy, and currently there is no standard of care.
“The longer you’re isolated, the harder it is getting out of your cocoon, and when these adult children get to the point where they seek help, they’re less likely to comply,” he said. However, he noted, this is not because they are lazy; it’s that they’re “very, very anxious.”
Parents and other family members are also negatively affected. Dr. Berger noted that 15% of parents of a child with FTL equate their caregiver burden with having a family member with a chronic physical illness. “It’s huge; parents go through hell and it’s very hard on them. Many believe it is their fault and they feel a lot of shame.”
Supportive Parenting for Anxious Childhood Emotions (SPACE) is a manualized, parent-based program for childhood anxiety and obsessive-compulsive disorder. It has been tested in clinical trials and found to be noninferior to cognitive behavioral therapy for childhood anxiety.
The research adapted it to treat FTL. SPACE-FTL focuses on reducing parents’ family accommodation (FA), a descriptor for a child’s excessive dependence on their parents to help them avoid anxiety-provoking situations.
The study examined the feasibility, acceptability, and treatment satisfaction and its effect on adult child psychopathology symptoms, parents’ FA, and the paternal burden of caring for adult children.
The study included parents (mean age, 59.46 years; 85% female) of 40 adult children with FTL (mean age, 23.51 years; 20% female) from across the United States.
Parents were randomized to a 13-week wait-list or the SPACE-FTL program, which involves 13-20 therapy sessions, depending on the need. The average number of sessions in the study was 15. The program has five key components:
- Providing information emphasizing FTL as not a character flaw but a problem with anxiety.
- Helping parents identify how they accommodate their child’s behavior, and facilitating an environment that encourages independence.
- Getting parents to show acceptance and confidence in their child who’s trying to overcome anxiety when, for example, they seek employment, instead of being overprotective and demanding.
- Focusing on change nonconfrontationally.
- Involving other family, community members, and professionals who can support the parent, child, or both.
The recruitment, treatment sessions, and assessments were all done online. Most participants rated the intervention as highly satisfactory on the Client Satisfaction Questionnaire (CSQ-8; mean score, 27.7 out of a maximum of 32). About 60% of the offspring no longer met full criteria for FTL (P < .001; Cohen’s D = 1.76).
All children of the wait-listed parents still met criteria for FTL.
FTL symptoms decreased significantly in the offspring of the intervention group, as seen in both in the Adult Entitled Dependence Scale (AED; P < .05; Cohen’s D = 0.84); and the Adaptive Behaviors Scale (ABS; P < .05; Cohen’s D = 0.70).
There was no change in anxiety as assessed by the Adult Behavior Checklist (ABCL). But Dr. Berger noted that child anxiety is difficult to assess through parental report.
“This population is self-isolating and parents sometimes don’t know what’s going on,” and ABCL measures may not be “as sensitive as we would have liked them to be,” Dr. Berger said.
Parental burden was significantly decreased as measured by the Zarit Burden Interview (ZBI; P < .05; Cohen’s D = 0.70). In addition, family accommodation decreased significantly as determined by the Family Accommodation Scale–Anxiety (FASA; P < .05; Cohen’s D = 0.70).
Innovative work
In a comment, Jonathan E. Alpert, MD, PhD, chair, department of psychiatry and behavioral sciences, and professor of psychiatry, neuroscience, and pediatrics, Albert Einstein College of Medicine, New York, described the program as “innovative.”
He noted that the SPACE-FTL approach provides parents with education and skills to reduce behaviors that reinforce their child’s avoidance of independent activities. Such behaviors “may inadvertently contribute to the adult child remaining stuck,” he said.
“Through its involvement of parents and use of a structured approach, SPACE-FTL is a very interesting step toward more evidence-based therapies.”
However, he noted that the number of study participants is still “very low” and further work is needed to better characterize this condition and develop effective therapies.
He noted that parents of adult children with FTL should not be judged or blamed. “They have been living with a worrisome problem for years and are simply doing their best to cope as any of us would do.”
In addition, he noted that some adult children aren’t capable of launching because of a serious mental illness or substance use disorder that needs treatment.
It’s unclear just how many adult children have FTL, as the condition lacks formal, agreed-upon clinical and research criteria and a reliable evidence base for treatment, Dr. Alpert said.
“Whatever the actual numbers of FTL, my anecdotal clinical experience suggests that it is a very common problem which is understudied.”
He added that the definitions of FTL should include cultural context. In some groups, it’s quite normal for adults in their 20s, 30s, or even older to live with their parents, Dr. Alpert said.
Dr. Berger and Dr. Albert report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
WASHINGTON – , a new pilot study shows.
Known as failure to launch (FTL) syndrome, the criteria for this condition include the absence of a neurodevelopmental, mental, or intellectual condition, difficulty adapting to the challenges of adulthood, and living with or at the expense of parents.
Results suggest that the program benefits families dealing with FTL, said study investigator Uri Berger, PhD, postdoctoral associate, Yale Child Study Center Anxiety and Mood Disorders Program, New Haven, Conn.
“If you encounter parents who are say 50-60 years old who have a child with FTL, you can tell them there’s something they can do; there’s work they can do even if their child is refusing to go to therapy,” he said.
The findings were presented as part of the Anxiety and Depression Association of America Anxiety & Depression conference.
Anxious, isolated
Estimates suggest that there are 3.3 million physically able adults with FTL and that the disorder may be on the rise. These individuals often present with mental health symptoms including anxiety, depression, and suicidality, and tend to be socially isolated.
The investigators noted that intervening is often challenging because individuals with the syndrome are frequently noncompliant with therapy, and currently there is no standard of care.
“The longer you’re isolated, the harder it is getting out of your cocoon, and when these adult children get to the point where they seek help, they’re less likely to comply,” he said. However, he noted, this is not because they are lazy; it’s that they’re “very, very anxious.”
Parents and other family members are also negatively affected. Dr. Berger noted that 15% of parents of a child with FTL equate their caregiver burden with having a family member with a chronic physical illness. “It’s huge; parents go through hell and it’s very hard on them. Many believe it is their fault and they feel a lot of shame.”
Supportive Parenting for Anxious Childhood Emotions (SPACE) is a manualized, parent-based program for childhood anxiety and obsessive-compulsive disorder. It has been tested in clinical trials and found to be noninferior to cognitive behavioral therapy for childhood anxiety.
The research adapted it to treat FTL. SPACE-FTL focuses on reducing parents’ family accommodation (FA), a descriptor for a child’s excessive dependence on their parents to help them avoid anxiety-provoking situations.
The study examined the feasibility, acceptability, and treatment satisfaction and its effect on adult child psychopathology symptoms, parents’ FA, and the paternal burden of caring for adult children.
The study included parents (mean age, 59.46 years; 85% female) of 40 adult children with FTL (mean age, 23.51 years; 20% female) from across the United States.
Parents were randomized to a 13-week wait-list or the SPACE-FTL program, which involves 13-20 therapy sessions, depending on the need. The average number of sessions in the study was 15. The program has five key components:
- Providing information emphasizing FTL as not a character flaw but a problem with anxiety.
- Helping parents identify how they accommodate their child’s behavior, and facilitating an environment that encourages independence.
- Getting parents to show acceptance and confidence in their child who’s trying to overcome anxiety when, for example, they seek employment, instead of being overprotective and demanding.
- Focusing on change nonconfrontationally.
- Involving other family, community members, and professionals who can support the parent, child, or both.
The recruitment, treatment sessions, and assessments were all done online. Most participants rated the intervention as highly satisfactory on the Client Satisfaction Questionnaire (CSQ-8; mean score, 27.7 out of a maximum of 32). About 60% of the offspring no longer met full criteria for FTL (P < .001; Cohen’s D = 1.76).
All children of the wait-listed parents still met criteria for FTL.
FTL symptoms decreased significantly in the offspring of the intervention group, as seen in both in the Adult Entitled Dependence Scale (AED; P < .05; Cohen’s D = 0.84); and the Adaptive Behaviors Scale (ABS; P < .05; Cohen’s D = 0.70).
There was no change in anxiety as assessed by the Adult Behavior Checklist (ABCL). But Dr. Berger noted that child anxiety is difficult to assess through parental report.
“This population is self-isolating and parents sometimes don’t know what’s going on,” and ABCL measures may not be “as sensitive as we would have liked them to be,” Dr. Berger said.
Parental burden was significantly decreased as measured by the Zarit Burden Interview (ZBI; P < .05; Cohen’s D = 0.70). In addition, family accommodation decreased significantly as determined by the Family Accommodation Scale–Anxiety (FASA; P < .05; Cohen’s D = 0.70).
Innovative work
In a comment, Jonathan E. Alpert, MD, PhD, chair, department of psychiatry and behavioral sciences, and professor of psychiatry, neuroscience, and pediatrics, Albert Einstein College of Medicine, New York, described the program as “innovative.”
He noted that the SPACE-FTL approach provides parents with education and skills to reduce behaviors that reinforce their child’s avoidance of independent activities. Such behaviors “may inadvertently contribute to the adult child remaining stuck,” he said.
“Through its involvement of parents and use of a structured approach, SPACE-FTL is a very interesting step toward more evidence-based therapies.”
However, he noted that the number of study participants is still “very low” and further work is needed to better characterize this condition and develop effective therapies.
He noted that parents of adult children with FTL should not be judged or blamed. “They have been living with a worrisome problem for years and are simply doing their best to cope as any of us would do.”
In addition, he noted that some adult children aren’t capable of launching because of a serious mental illness or substance use disorder that needs treatment.
It’s unclear just how many adult children have FTL, as the condition lacks formal, agreed-upon clinical and research criteria and a reliable evidence base for treatment, Dr. Alpert said.
“Whatever the actual numbers of FTL, my anecdotal clinical experience suggests that it is a very common problem which is understudied.”
He added that the definitions of FTL should include cultural context. In some groups, it’s quite normal for adults in their 20s, 30s, or even older to live with their parents, Dr. Alpert said.
Dr. Berger and Dr. Albert report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
WASHINGTON – , a new pilot study shows.
Known as failure to launch (FTL) syndrome, the criteria for this condition include the absence of a neurodevelopmental, mental, or intellectual condition, difficulty adapting to the challenges of adulthood, and living with or at the expense of parents.
Results suggest that the program benefits families dealing with FTL, said study investigator Uri Berger, PhD, postdoctoral associate, Yale Child Study Center Anxiety and Mood Disorders Program, New Haven, Conn.
“If you encounter parents who are say 50-60 years old who have a child with FTL, you can tell them there’s something they can do; there’s work they can do even if their child is refusing to go to therapy,” he said.
The findings were presented as part of the Anxiety and Depression Association of America Anxiety & Depression conference.
Anxious, isolated
Estimates suggest that there are 3.3 million physically able adults with FTL and that the disorder may be on the rise. These individuals often present with mental health symptoms including anxiety, depression, and suicidality, and tend to be socially isolated.
The investigators noted that intervening is often challenging because individuals with the syndrome are frequently noncompliant with therapy, and currently there is no standard of care.
“The longer you’re isolated, the harder it is getting out of your cocoon, and when these adult children get to the point where they seek help, they’re less likely to comply,” he said. However, he noted, this is not because they are lazy; it’s that they’re “very, very anxious.”
Parents and other family members are also negatively affected. Dr. Berger noted that 15% of parents of a child with FTL equate their caregiver burden with having a family member with a chronic physical illness. “It’s huge; parents go through hell and it’s very hard on them. Many believe it is their fault and they feel a lot of shame.”
Supportive Parenting for Anxious Childhood Emotions (SPACE) is a manualized, parent-based program for childhood anxiety and obsessive-compulsive disorder. It has been tested in clinical trials and found to be noninferior to cognitive behavioral therapy for childhood anxiety.
The research adapted it to treat FTL. SPACE-FTL focuses on reducing parents’ family accommodation (FA), a descriptor for a child’s excessive dependence on their parents to help them avoid anxiety-provoking situations.
The study examined the feasibility, acceptability, and treatment satisfaction and its effect on adult child psychopathology symptoms, parents’ FA, and the paternal burden of caring for adult children.
The study included parents (mean age, 59.46 years; 85% female) of 40 adult children with FTL (mean age, 23.51 years; 20% female) from across the United States.
Parents were randomized to a 13-week wait-list or the SPACE-FTL program, which involves 13-20 therapy sessions, depending on the need. The average number of sessions in the study was 15. The program has five key components:
- Providing information emphasizing FTL as not a character flaw but a problem with anxiety.
- Helping parents identify how they accommodate their child’s behavior, and facilitating an environment that encourages independence.
- Getting parents to show acceptance and confidence in their child who’s trying to overcome anxiety when, for example, they seek employment, instead of being overprotective and demanding.
- Focusing on change nonconfrontationally.
- Involving other family, community members, and professionals who can support the parent, child, or both.
The recruitment, treatment sessions, and assessments were all done online. Most participants rated the intervention as highly satisfactory on the Client Satisfaction Questionnaire (CSQ-8; mean score, 27.7 out of a maximum of 32). About 60% of the offspring no longer met full criteria for FTL (P < .001; Cohen’s D = 1.76).
All children of the wait-listed parents still met criteria for FTL.
FTL symptoms decreased significantly in the offspring of the intervention group, as seen in both in the Adult Entitled Dependence Scale (AED; P < .05; Cohen’s D = 0.84); and the Adaptive Behaviors Scale (ABS; P < .05; Cohen’s D = 0.70).
There was no change in anxiety as assessed by the Adult Behavior Checklist (ABCL). But Dr. Berger noted that child anxiety is difficult to assess through parental report.
“This population is self-isolating and parents sometimes don’t know what’s going on,” and ABCL measures may not be “as sensitive as we would have liked them to be,” Dr. Berger said.
Parental burden was significantly decreased as measured by the Zarit Burden Interview (ZBI; P < .05; Cohen’s D = 0.70). In addition, family accommodation decreased significantly as determined by the Family Accommodation Scale–Anxiety (FASA; P < .05; Cohen’s D = 0.70).
Innovative work
In a comment, Jonathan E. Alpert, MD, PhD, chair, department of psychiatry and behavioral sciences, and professor of psychiatry, neuroscience, and pediatrics, Albert Einstein College of Medicine, New York, described the program as “innovative.”
He noted that the SPACE-FTL approach provides parents with education and skills to reduce behaviors that reinforce their child’s avoidance of independent activities. Such behaviors “may inadvertently contribute to the adult child remaining stuck,” he said.
“Through its involvement of parents and use of a structured approach, SPACE-FTL is a very interesting step toward more evidence-based therapies.”
However, he noted that the number of study participants is still “very low” and further work is needed to better characterize this condition and develop effective therapies.
He noted that parents of adult children with FTL should not be judged or blamed. “They have been living with a worrisome problem for years and are simply doing their best to cope as any of us would do.”
In addition, he noted that some adult children aren’t capable of launching because of a serious mental illness or substance use disorder that needs treatment.
It’s unclear just how many adult children have FTL, as the condition lacks formal, agreed-upon clinical and research criteria and a reliable evidence base for treatment, Dr. Alpert said.
“Whatever the actual numbers of FTL, my anecdotal clinical experience suggests that it is a very common problem which is understudied.”
He added that the definitions of FTL should include cultural context. In some groups, it’s quite normal for adults in their 20s, 30s, or even older to live with their parents, Dr. Alpert said.
Dr. Berger and Dr. Albert report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ADAA 2023
Commentary: Surgical, Tamoxifen, and Genetic Considerations in Breast Cancer, May 2023
A cohort study by Minami and colleagues assessed the association between surgery type (lumpectomy vs mastectomy) and change in frailty status in older patients with early-stage breast cancer (BC) undergoing locoregional therapy. The study included 31,084 women, age ≥ 65 years, with ductal carcinoma in situ (n = 9962) or stage I hormone receptor–positive (HR+) and ERBB2+ (human epidermal growth factor receptor 2 positive [HER2+]) BC (n = 21,122), of which 22.6% and 77.4% of patients underwent mastectomy and lumpectomy, respectively. The study showed that older patients who underwent mastectomy vs lumpectomy were more likely to experience worse frailty (adjusted odds ratio 1.31; 95% CI 1.23-1.39). Additionally, women who were robust vs having moderate to severe frailty at baseline, ≥ 75 years vs 65-69 years, or African American/Black vs non-Hispanic White, had significantly higher odds of decline. Given that prior data have shown comparable survival between lumpectomy and mastectomy, careful and thoughtful treatment considerations are needed before deciding to intensify surgical management in this population, even in women who do not appear frail at baseline.
Low-dose tamoxifen continues to prevent BC recurrence in breast noninvasive neoplasia
Low-dose tamoxifen is a treatment option for women with noninvasive BC, especially if the patient was not able to tolerate the standard dose of 20 mg daily. The phase 3 TAM-01 trial included 500 women with intraepithelial neoplasia of the breast who were randomly assigned to receive low-dose tamoxifen (5 mg once daily) or placebo. The 10-year follow-up analysis by Lazzeroni and colleagues showed that treatment with low-dose tamoxifen for 3 years continued to prevent a BC recurrence for at least 7 years after treatment cessation. After a median follow-up of 9.7 years, fewer cases of both invasive and in situ BC (hazard ratio 0.58; log-rank P = .03) and contralateral BC (hazard ratio 0.36; P = .025) were reported in the tamoxifen vs placebo group. These results are meaningful, especially in a setting of an optimal safety profile, where patients on low-dose tamoxifen were experiencing similar menopausal symptoms to placebo, and serious adverse events, such as deep vein thrombosis and pulmonary embolism, were not increased during low-dose tamoxifen therapy. This is different from the threefold increased risk reported with standard dosing.
Worse survival in BRCA1/2 germline mutation carriers receiving ET in HR+/HER2− BC
Inconsistent data have been reported on the prognostic impact of BRCA1/2 mutation in HR+ BC. A retrospective study by Frenel and colleagues included 13,776 patients with metastatic BC (MBC) from the Epidemiological Strategy and Medical Economics (ESME) MBC database, of which 676 and 170 patients were germline BRCA wild-type (gBRCAwt) and germline BRCA mutation (gBRCAm) carriers, respectively. They looked at outcomes and first-line endocrine treatment efficacy in patients with HR+/HER2- MBC, treated in a pre–cyclin-dependent kinase (CDK) 4/6 inhibitors era. The results showed that gBRCAm carriers had shorter overall survival (OS; adjusted hazard ratio [aHR] 1.26; P = .024) and progression-free survival (PFS; aHR 1.21; P = .017) compared with gBRCAwt carriers. Furthermore, among those treated with front-line endocrine therapy, gBRCAm patients had lower adjusted OS (aHR [95% CI] 1.54 [1.03-2.32]) and PFS (aHR [95% CI] 1.58 [1.17-2.12]) compared with gBRCAwt patients. Outcomes were similar for gBRCAm patients who received first-line chemotherapy compared with the gBRCAwt group (OS: aHR [95% CI] 1.12 [0.88-1.41]; first-line PFS: aHR [95% CI] 1.09 [0.90-1.31]). A previous retrospective study by Lambertini and colleagues, focusing on young patients with gBRCAm, also showed a tendency for a worse distant recurrence-free interval (aHR 1.39; 95% CI 0.94-2.05) in patients with HR+ BC. Additional studies are needed, especially in the setting of an evolving treatment landscape that includes CDK4/6 inhibitors and poly-ADP ribose polymerase (PARP) inhibitors.
A cohort study by Minami and colleagues assessed the association between surgery type (lumpectomy vs mastectomy) and change in frailty status in older patients with early-stage breast cancer (BC) undergoing locoregional therapy. The study included 31,084 women, age ≥ 65 years, with ductal carcinoma in situ (n = 9962) or stage I hormone receptor–positive (HR+) and ERBB2+ (human epidermal growth factor receptor 2 positive [HER2+]) BC (n = 21,122), of which 22.6% and 77.4% of patients underwent mastectomy and lumpectomy, respectively. The study showed that older patients who underwent mastectomy vs lumpectomy were more likely to experience worse frailty (adjusted odds ratio 1.31; 95% CI 1.23-1.39). Additionally, women who were robust vs having moderate to severe frailty at baseline, ≥ 75 years vs 65-69 years, or African American/Black vs non-Hispanic White, had significantly higher odds of decline. Given that prior data have shown comparable survival between lumpectomy and mastectomy, careful and thoughtful treatment considerations are needed before deciding to intensify surgical management in this population, even in women who do not appear frail at baseline.
Low-dose tamoxifen continues to prevent BC recurrence in breast noninvasive neoplasia
Low-dose tamoxifen is a treatment option for women with noninvasive BC, especially if the patient was not able to tolerate the standard dose of 20 mg daily. The phase 3 TAM-01 trial included 500 women with intraepithelial neoplasia of the breast who were randomly assigned to receive low-dose tamoxifen (5 mg once daily) or placebo. The 10-year follow-up analysis by Lazzeroni and colleagues showed that treatment with low-dose tamoxifen for 3 years continued to prevent a BC recurrence for at least 7 years after treatment cessation. After a median follow-up of 9.7 years, fewer cases of both invasive and in situ BC (hazard ratio 0.58; log-rank P = .03) and contralateral BC (hazard ratio 0.36; P = .025) were reported in the tamoxifen vs placebo group. These results are meaningful, especially in a setting of an optimal safety profile, where patients on low-dose tamoxifen were experiencing similar menopausal symptoms to placebo, and serious adverse events, such as deep vein thrombosis and pulmonary embolism, were not increased during low-dose tamoxifen therapy. This is different from the threefold increased risk reported with standard dosing.
Worse survival in BRCA1/2 germline mutation carriers receiving ET in HR+/HER2− BC
Inconsistent data have been reported on the prognostic impact of BRCA1/2 mutation in HR+ BC. A retrospective study by Frenel and colleagues included 13,776 patients with metastatic BC (MBC) from the Epidemiological Strategy and Medical Economics (ESME) MBC database, of which 676 and 170 patients were germline BRCA wild-type (gBRCAwt) and germline BRCA mutation (gBRCAm) carriers, respectively. They looked at outcomes and first-line endocrine treatment efficacy in patients with HR+/HER2- MBC, treated in a pre–cyclin-dependent kinase (CDK) 4/6 inhibitors era. The results showed that gBRCAm carriers had shorter overall survival (OS; adjusted hazard ratio [aHR] 1.26; P = .024) and progression-free survival (PFS; aHR 1.21; P = .017) compared with gBRCAwt carriers. Furthermore, among those treated with front-line endocrine therapy, gBRCAm patients had lower adjusted OS (aHR [95% CI] 1.54 [1.03-2.32]) and PFS (aHR [95% CI] 1.58 [1.17-2.12]) compared with gBRCAwt patients. Outcomes were similar for gBRCAm patients who received first-line chemotherapy compared with the gBRCAwt group (OS: aHR [95% CI] 1.12 [0.88-1.41]; first-line PFS: aHR [95% CI] 1.09 [0.90-1.31]). A previous retrospective study by Lambertini and colleagues, focusing on young patients with gBRCAm, also showed a tendency for a worse distant recurrence-free interval (aHR 1.39; 95% CI 0.94-2.05) in patients with HR+ BC. Additional studies are needed, especially in the setting of an evolving treatment landscape that includes CDK4/6 inhibitors and poly-ADP ribose polymerase (PARP) inhibitors.
A cohort study by Minami and colleagues assessed the association between surgery type (lumpectomy vs mastectomy) and change in frailty status in older patients with early-stage breast cancer (BC) undergoing locoregional therapy. The study included 31,084 women, age ≥ 65 years, with ductal carcinoma in situ (n = 9962) or stage I hormone receptor–positive (HR+) and ERBB2+ (human epidermal growth factor receptor 2 positive [HER2+]) BC (n = 21,122), of which 22.6% and 77.4% of patients underwent mastectomy and lumpectomy, respectively. The study showed that older patients who underwent mastectomy vs lumpectomy were more likely to experience worse frailty (adjusted odds ratio 1.31; 95% CI 1.23-1.39). Additionally, women who were robust vs having moderate to severe frailty at baseline, ≥ 75 years vs 65-69 years, or African American/Black vs non-Hispanic White, had significantly higher odds of decline. Given that prior data have shown comparable survival between lumpectomy and mastectomy, careful and thoughtful treatment considerations are needed before deciding to intensify surgical management in this population, even in women who do not appear frail at baseline.
Low-dose tamoxifen continues to prevent BC recurrence in breast noninvasive neoplasia
Low-dose tamoxifen is a treatment option for women with noninvasive BC, especially if the patient was not able to tolerate the standard dose of 20 mg daily. The phase 3 TAM-01 trial included 500 women with intraepithelial neoplasia of the breast who were randomly assigned to receive low-dose tamoxifen (5 mg once daily) or placebo. The 10-year follow-up analysis by Lazzeroni and colleagues showed that treatment with low-dose tamoxifen for 3 years continued to prevent a BC recurrence for at least 7 years after treatment cessation. After a median follow-up of 9.7 years, fewer cases of both invasive and in situ BC (hazard ratio 0.58; log-rank P = .03) and contralateral BC (hazard ratio 0.36; P = .025) were reported in the tamoxifen vs placebo group. These results are meaningful, especially in a setting of an optimal safety profile, where patients on low-dose tamoxifen were experiencing similar menopausal symptoms to placebo, and serious adverse events, such as deep vein thrombosis and pulmonary embolism, were not increased during low-dose tamoxifen therapy. This is different from the threefold increased risk reported with standard dosing.
Worse survival in BRCA1/2 germline mutation carriers receiving ET in HR+/HER2− BC
Inconsistent data have been reported on the prognostic impact of BRCA1/2 mutation in HR+ BC. A retrospective study by Frenel and colleagues included 13,776 patients with metastatic BC (MBC) from the Epidemiological Strategy and Medical Economics (ESME) MBC database, of which 676 and 170 patients were germline BRCA wild-type (gBRCAwt) and germline BRCA mutation (gBRCAm) carriers, respectively. They looked at outcomes and first-line endocrine treatment efficacy in patients with HR+/HER2- MBC, treated in a pre–cyclin-dependent kinase (CDK) 4/6 inhibitors era. The results showed that gBRCAm carriers had shorter overall survival (OS; adjusted hazard ratio [aHR] 1.26; P = .024) and progression-free survival (PFS; aHR 1.21; P = .017) compared with gBRCAwt carriers. Furthermore, among those treated with front-line endocrine therapy, gBRCAm patients had lower adjusted OS (aHR [95% CI] 1.54 [1.03-2.32]) and PFS (aHR [95% CI] 1.58 [1.17-2.12]) compared with gBRCAwt patients. Outcomes were similar for gBRCAm patients who received first-line chemotherapy compared with the gBRCAwt group (OS: aHR [95% CI] 1.12 [0.88-1.41]; first-line PFS: aHR [95% CI] 1.09 [0.90-1.31]). A previous retrospective study by Lambertini and colleagues, focusing on young patients with gBRCAm, also showed a tendency for a worse distant recurrence-free interval (aHR 1.39; 95% CI 0.94-2.05) in patients with HR+ BC. Additional studies are needed, especially in the setting of an evolving treatment landscape that includes CDK4/6 inhibitors and poly-ADP ribose polymerase (PARP) inhibitors.
Cautious optimism for new Alzheimer’s disease biomarkers and treatments, expert says
SAN DIEGO – said a presenter at the annual meeting of the American College of Physicians.
Dementia prevalence is increasing as the proportion of the U.S. population older than 65 rises, said Zaldy Tan, MD, professor of neurology at Cedars-Sinai Medical Center, Los Angeles. AD deaths more than doubled between 2000 and 2018, he noted, while deaths from HIV infection, stroke, and heart disease decreased.
Most people in the United States who have AD are White, but studies suggest that, compared with Whites, the risk of AD is two times higher in Blacks and 1.5 times higher in Hispanics . “These data suggest that both genes and social determinants of health are at play,” Dr. Tan said.
Diagnosis of Alzheimer’s disease
The different types of dementia make it challenging for primary care physicians to identify the cause of cognitive impairment. “Even though AD is the most common type, clinicians should keep in mind that another type of dementia may be the cause of cognitive impairment,” Dr. Tan cautioned. Other dementia diagnoses include vascular, Lewy body, and frontotemporal.
Diagnostic criteria for AD include evidence of significant cognitive decline in at least one cognitive domain that interferes with independence in everyday activities, as well as the absence of another mental disorder or delirium that would explain the cognitive deficits.
“We see many patients with depressive symptoms and mild cognitive impairment, and it is not always easy to tell which of them have dementia because of the overlap in the symptoms of depression and AD,” said internist Roderick Kim, MD, of Grand Rapids, Mich., who attended the session.
It can be challenging to convince patients to undergo the appropriate diagnostic workup, Dr. Kim said. “This can delay treatment, so it is important to explain to patients that cognitive decline can progress quickly and that there are treatment options to slow it down.”
Why do we need biomarkers for Alzheimer’s disease?
AD is characterized by a long preclinical phase with no specific symptoms other than the typical signs and symptoms of aging, Dr. Tan said. That means cognitive impairment progresses rapidly after diagnosis in most patients with AD.
“In most cases, an accurate history, physical and neurologic examinations, basic labs, and neuroimaging are sufficient for memory loss evaluation. However, as more disease-modifying therapies come to market, biomarkers will rise in importance in primary care,” he said.
This long asymptomatic phase of AD creates the need for diagnostic biomarkers for an earlier diagnosis, he said. Amyloid-beta and tau deposits in PET images and the levels of amyloid-beta seeds, phosphorylated tau, and neurofilament light chain in the cerebrospinal fluid can be used as diagnostic biomarkers in patients with suspected AD. Emerging blood biomarkers for earlier detection include the levels of amyloid-beta1–42, phosphorylated tau, and neurofilament light chain.
With biomarkers and other new tools for the diagnosis of dementia in primary care, Dr. Tan said: “The greatest challenge is cost, as blood-based biomarkers are not currently covered by insurance and still rather costly. In addition, blood-based biomarkers will need to receive [Food and Drug Administration] approval in order to have more widespread availability.”
New and emerging therapies for Alzheimer’s disease
There are two classes of FDA-approved medications to manage cognitive symptoms of dementia: acetylcholinesterase inhibitors and N-methyl-D-aspartate receptor antagonists. The selections may be trial and error for each patient, Dr. Tan said.
“The approved medications can exert subtle benefits that are clinically observable. Thus, barring any contraindications or intolerance, most patients with AD would benefit from a trial of one or both of these medication classes,” said Dr. Tan. He added that it is equally important to wean off and discontinue these medications if there is intolerance or lack of a subjective or objective beneficial response.
Other medications are available for some of the most common behavioral problems associated with dementia, such as agitation, depression, and disorientation. Dr. Tan advised not to prescribe behavioral medications until nonpharmacologic interventions prove to be ineffective or impractical. Behavioral medications have many side effects, some of which are potentially serious, he said, so the risk-benefit ratio should be considered.
In his own practice, when nonpharmacologic strategies do not improve the behavioral symptoms of dementia, Dr. Tan said that, “in cases where a person is at risk of harm to themselves or others, a discussion with the patient and their caregivers about the pros and cons of medications to treat the behavior need to be had. Careful monitoring of the response and dose escalation or deprescribing when appropriate is important to keep in mind.”
Disease-modifying agents have recently provided new hope for AD treatment. Aducanumab and lecanemab, both monoclonal antibodies that target amyloids, are the first two drugs that received accelerated FDA approval for AD.
Although these monoclonal antibodies can help clear deposited amyloid plaques and show some benefit in slowing cognitive impairment in clinical trials, the real-world benefits were unclear enough for Medicare to limit coverage to people enrolled in approved studies to gather more data. Additionally, these agents can cause potentially amyloid-related imaging abnormalities, which may indicate edema, effusion, or microhemorrhage. Therefore, clinicians need to have a clear conversation of risks and benefits with patients and caregivers about these treatments.
Looking ahead
When asked about the most promising emerging technologies or techniques related to dementia diagnosis and management, Dr. Tan noted that multiple technology companies and start-ups are looking for new ways to detect dementia earlier or keep persons with dementia safe at home. Some devices deliver brain waves, computerized brain games or tests, automated pill dispensers, and fall monitors.
“Some of these are potentially helpful, but not every person with dementia will benefit. In addition, most of these technologies are out-of-pocket expenses for the patients and their families. It is important to know what is out there but also be cautious about outrageous claims,” he added.
Dr. Tan reported no relationships with entities whose primary business is producing, marketing, selling, reselling, or distributing health care products used by or on patients.
SAN DIEGO – said a presenter at the annual meeting of the American College of Physicians.
Dementia prevalence is increasing as the proportion of the U.S. population older than 65 rises, said Zaldy Tan, MD, professor of neurology at Cedars-Sinai Medical Center, Los Angeles. AD deaths more than doubled between 2000 and 2018, he noted, while deaths from HIV infection, stroke, and heart disease decreased.
Most people in the United States who have AD are White, but studies suggest that, compared with Whites, the risk of AD is two times higher in Blacks and 1.5 times higher in Hispanics . “These data suggest that both genes and social determinants of health are at play,” Dr. Tan said.
Diagnosis of Alzheimer’s disease
The different types of dementia make it challenging for primary care physicians to identify the cause of cognitive impairment. “Even though AD is the most common type, clinicians should keep in mind that another type of dementia may be the cause of cognitive impairment,” Dr. Tan cautioned. Other dementia diagnoses include vascular, Lewy body, and frontotemporal.
Diagnostic criteria for AD include evidence of significant cognitive decline in at least one cognitive domain that interferes with independence in everyday activities, as well as the absence of another mental disorder or delirium that would explain the cognitive deficits.
“We see many patients with depressive symptoms and mild cognitive impairment, and it is not always easy to tell which of them have dementia because of the overlap in the symptoms of depression and AD,” said internist Roderick Kim, MD, of Grand Rapids, Mich., who attended the session.
It can be challenging to convince patients to undergo the appropriate diagnostic workup, Dr. Kim said. “This can delay treatment, so it is important to explain to patients that cognitive decline can progress quickly and that there are treatment options to slow it down.”
Why do we need biomarkers for Alzheimer’s disease?
AD is characterized by a long preclinical phase with no specific symptoms other than the typical signs and symptoms of aging, Dr. Tan said. That means cognitive impairment progresses rapidly after diagnosis in most patients with AD.
“In most cases, an accurate history, physical and neurologic examinations, basic labs, and neuroimaging are sufficient for memory loss evaluation. However, as more disease-modifying therapies come to market, biomarkers will rise in importance in primary care,” he said.
This long asymptomatic phase of AD creates the need for diagnostic biomarkers for an earlier diagnosis, he said. Amyloid-beta and tau deposits in PET images and the levels of amyloid-beta seeds, phosphorylated tau, and neurofilament light chain in the cerebrospinal fluid can be used as diagnostic biomarkers in patients with suspected AD. Emerging blood biomarkers for earlier detection include the levels of amyloid-beta1–42, phosphorylated tau, and neurofilament light chain.
With biomarkers and other new tools for the diagnosis of dementia in primary care, Dr. Tan said: “The greatest challenge is cost, as blood-based biomarkers are not currently covered by insurance and still rather costly. In addition, blood-based biomarkers will need to receive [Food and Drug Administration] approval in order to have more widespread availability.”
New and emerging therapies for Alzheimer’s disease
There are two classes of FDA-approved medications to manage cognitive symptoms of dementia: acetylcholinesterase inhibitors and N-methyl-D-aspartate receptor antagonists. The selections may be trial and error for each patient, Dr. Tan said.
“The approved medications can exert subtle benefits that are clinically observable. Thus, barring any contraindications or intolerance, most patients with AD would benefit from a trial of one or both of these medication classes,” said Dr. Tan. He added that it is equally important to wean off and discontinue these medications if there is intolerance or lack of a subjective or objective beneficial response.
Other medications are available for some of the most common behavioral problems associated with dementia, such as agitation, depression, and disorientation. Dr. Tan advised not to prescribe behavioral medications until nonpharmacologic interventions prove to be ineffective or impractical. Behavioral medications have many side effects, some of which are potentially serious, he said, so the risk-benefit ratio should be considered.
In his own practice, when nonpharmacologic strategies do not improve the behavioral symptoms of dementia, Dr. Tan said that, “in cases where a person is at risk of harm to themselves or others, a discussion with the patient and their caregivers about the pros and cons of medications to treat the behavior need to be had. Careful monitoring of the response and dose escalation or deprescribing when appropriate is important to keep in mind.”
Disease-modifying agents have recently provided new hope for AD treatment. Aducanumab and lecanemab, both monoclonal antibodies that target amyloids, are the first two drugs that received accelerated FDA approval for AD.
Although these monoclonal antibodies can help clear deposited amyloid plaques and show some benefit in slowing cognitive impairment in clinical trials, the real-world benefits were unclear enough for Medicare to limit coverage to people enrolled in approved studies to gather more data. Additionally, these agents can cause potentially amyloid-related imaging abnormalities, which may indicate edema, effusion, or microhemorrhage. Therefore, clinicians need to have a clear conversation of risks and benefits with patients and caregivers about these treatments.
Looking ahead
When asked about the most promising emerging technologies or techniques related to dementia diagnosis and management, Dr. Tan noted that multiple technology companies and start-ups are looking for new ways to detect dementia earlier or keep persons with dementia safe at home. Some devices deliver brain waves, computerized brain games or tests, automated pill dispensers, and fall monitors.
“Some of these are potentially helpful, but not every person with dementia will benefit. In addition, most of these technologies are out-of-pocket expenses for the patients and their families. It is important to know what is out there but also be cautious about outrageous claims,” he added.
Dr. Tan reported no relationships with entities whose primary business is producing, marketing, selling, reselling, or distributing health care products used by or on patients.
SAN DIEGO – said a presenter at the annual meeting of the American College of Physicians.
Dementia prevalence is increasing as the proportion of the U.S. population older than 65 rises, said Zaldy Tan, MD, professor of neurology at Cedars-Sinai Medical Center, Los Angeles. AD deaths more than doubled between 2000 and 2018, he noted, while deaths from HIV infection, stroke, and heart disease decreased.
Most people in the United States who have AD are White, but studies suggest that, compared with Whites, the risk of AD is two times higher in Blacks and 1.5 times higher in Hispanics . “These data suggest that both genes and social determinants of health are at play,” Dr. Tan said.
Diagnosis of Alzheimer’s disease
The different types of dementia make it challenging for primary care physicians to identify the cause of cognitive impairment. “Even though AD is the most common type, clinicians should keep in mind that another type of dementia may be the cause of cognitive impairment,” Dr. Tan cautioned. Other dementia diagnoses include vascular, Lewy body, and frontotemporal.
Diagnostic criteria for AD include evidence of significant cognitive decline in at least one cognitive domain that interferes with independence in everyday activities, as well as the absence of another mental disorder or delirium that would explain the cognitive deficits.
“We see many patients with depressive symptoms and mild cognitive impairment, and it is not always easy to tell which of them have dementia because of the overlap in the symptoms of depression and AD,” said internist Roderick Kim, MD, of Grand Rapids, Mich., who attended the session.
It can be challenging to convince patients to undergo the appropriate diagnostic workup, Dr. Kim said. “This can delay treatment, so it is important to explain to patients that cognitive decline can progress quickly and that there are treatment options to slow it down.”
Why do we need biomarkers for Alzheimer’s disease?
AD is characterized by a long preclinical phase with no specific symptoms other than the typical signs and symptoms of aging, Dr. Tan said. That means cognitive impairment progresses rapidly after diagnosis in most patients with AD.
“In most cases, an accurate history, physical and neurologic examinations, basic labs, and neuroimaging are sufficient for memory loss evaluation. However, as more disease-modifying therapies come to market, biomarkers will rise in importance in primary care,” he said.
This long asymptomatic phase of AD creates the need for diagnostic biomarkers for an earlier diagnosis, he said. Amyloid-beta and tau deposits in PET images and the levels of amyloid-beta seeds, phosphorylated tau, and neurofilament light chain in the cerebrospinal fluid can be used as diagnostic biomarkers in patients with suspected AD. Emerging blood biomarkers for earlier detection include the levels of amyloid-beta1–42, phosphorylated tau, and neurofilament light chain.
With biomarkers and other new tools for the diagnosis of dementia in primary care, Dr. Tan said: “The greatest challenge is cost, as blood-based biomarkers are not currently covered by insurance and still rather costly. In addition, blood-based biomarkers will need to receive [Food and Drug Administration] approval in order to have more widespread availability.”
New and emerging therapies for Alzheimer’s disease
There are two classes of FDA-approved medications to manage cognitive symptoms of dementia: acetylcholinesterase inhibitors and N-methyl-D-aspartate receptor antagonists. The selections may be trial and error for each patient, Dr. Tan said.
“The approved medications can exert subtle benefits that are clinically observable. Thus, barring any contraindications or intolerance, most patients with AD would benefit from a trial of one or both of these medication classes,” said Dr. Tan. He added that it is equally important to wean off and discontinue these medications if there is intolerance or lack of a subjective or objective beneficial response.
Other medications are available for some of the most common behavioral problems associated with dementia, such as agitation, depression, and disorientation. Dr. Tan advised not to prescribe behavioral medications until nonpharmacologic interventions prove to be ineffective or impractical. Behavioral medications have many side effects, some of which are potentially serious, he said, so the risk-benefit ratio should be considered.
In his own practice, when nonpharmacologic strategies do not improve the behavioral symptoms of dementia, Dr. Tan said that, “in cases where a person is at risk of harm to themselves or others, a discussion with the patient and their caregivers about the pros and cons of medications to treat the behavior need to be had. Careful monitoring of the response and dose escalation or deprescribing when appropriate is important to keep in mind.”
Disease-modifying agents have recently provided new hope for AD treatment. Aducanumab and lecanemab, both monoclonal antibodies that target amyloids, are the first two drugs that received accelerated FDA approval for AD.
Although these monoclonal antibodies can help clear deposited amyloid plaques and show some benefit in slowing cognitive impairment in clinical trials, the real-world benefits were unclear enough for Medicare to limit coverage to people enrolled in approved studies to gather more data. Additionally, these agents can cause potentially amyloid-related imaging abnormalities, which may indicate edema, effusion, or microhemorrhage. Therefore, clinicians need to have a clear conversation of risks and benefits with patients and caregivers about these treatments.
Looking ahead
When asked about the most promising emerging technologies or techniques related to dementia diagnosis and management, Dr. Tan noted that multiple technology companies and start-ups are looking for new ways to detect dementia earlier or keep persons with dementia safe at home. Some devices deliver brain waves, computerized brain games or tests, automated pill dispensers, and fall monitors.
“Some of these are potentially helpful, but not every person with dementia will benefit. In addition, most of these technologies are out-of-pocket expenses for the patients and their families. It is important to know what is out there but also be cautious about outrageous claims,” he added.
Dr. Tan reported no relationships with entities whose primary business is producing, marketing, selling, reselling, or distributing health care products used by or on patients.
AT INTERNAL MEDICINE 2023
Liquid biopsy assay can predict CRC recurrence early
A (CRC).
Patients who were ctDNA methylation positive 1 month after surgery were 17.5 times more likely to relapse, compared with ctDNA-negative patients. And following adjuvant chemotherapy, ctDNA-positive patients had a significantly shorter recurrence-free survival than their ctDNA-negative peers.
Overall, “we found that ctDNA methylation was the most significant prognostic factor for recurrence-free survival among all clinicopathologic risk factors on multivariable analysis,” the authors, led by Shaobo Mo, MD, Fudan University Shanghai (China) Cancer Center, reported in research published in JAMA Oncology.
Van Morris, MD, an oncologist with University of Texas MD Anderson Cancer Center, Houston, who was not involved in the research, noted that other commercially available ctDNA assays have achieved similar findings, but this assay involves the least number of biomarkers.
More notably, the broader message emerging from this research is that “ctDNA is a powerful tool in oncology that is here to stay,” said Dr. Morris.
Dr. Morris added a note of caution, however: Despite the study providing further support for this technology in CRC, “we do not have definitive predictive utility for routinely guiding adjuvant chemotherapy decisions with the use of this [or other] ctDNA assays.”
Recurrence common, predictors important
CRC has a relatively high recurrence rate even after curative-intent therapies, with a 5-year survival rate as low as 60%. Adjuvant chemotherapy in patients with stage III CRC generally lowers the risk of recurrence by about 10%-20%; however, the benefits of adjuvant chemotherapy among patients with stage II CRC remain unclear.
Strategies to identify patients most likely to relapse after adjuvant therapy largely focus on CRC stage and clinical risk factors, though postoperative ctDNA testing has emerged as a tool to help identify patients at risk for recurrence. Often, however, this approach involves ultradeep next-generation sequencing, which limits the strategy’s ease of implementation and cost effectiveness.
As an alternative, the authors used a plasma ctDNA methylation test, ColonAiQ, which identifies the presence of six genomic biomarkers hypermethylated in CRC. This test avoids the complex process of primary tumor profiling among individual patients.
In the multicenter, prospective longitudinal cohort study, conducted from December 2019 to February 2022, Dr. Mo and colleagues evaluated 1,228 blood samples from 299 patients with stage I-III CRC. Samples were collected before and after surgery, during and after adjuvant chemotherapy, and every 3 months for up to 2 years.
Of 296 patients with preoperative samples available, as many as 232 (78.4%) tested positive for at least one of the 6 ctDNA methylation markers. The detection rates were 65.1% for stage I CRC, 82.7% for stage II disease, and 81.5% for stage III disease.
At postoperative month 1, ctDNA methylation–positive patients were 17.5 times more likely to relapse, compared with ctDNA-negative patients (hazard ratio, 17.5; P < .001).
When integrating carcinoembryonic antigen testing alongside ctDNA testing, patients with positive test results had significantly worse prognoses, compared with those who had negative results (HR, 19.0; P < .001).
The association of ctDNA methylation positivity at postoperative month 1 and CRC recurrence was consistent across varying durations and intensities of adjuvant chemotherapy. The researchers found that ctDNA methylation analysis detected CRC recurrence a median of 3.3 months earlier than radiologically confirmed recurrence.
Patients who were ctDNA positive also had significantly shorter periods of recurrence-free survival following adjuvant chemotherapy, compared with ctDNA-negative patients (HR, 13.8; P < .001). That effect was enhanced when positive ctDNA status was maintained longitudinally, compared with those who were persistently ctDNA negative (HR, 68.8; P < .001).
More specifically, 140 patients exhibited sustained ctDNA-positive status over time; 6 of 7 ctDNA-positive patients experienced recurrence within 12 months, whereas 129 of 133 ctDNA-negative patients (97%) remained relapse free. And being ctDNA negative before surgery indicated patients’ relapse risk, with 95.3% of patients who were ctDNA negative presurgery remaining relapse free.
Dr. Mo and colleagues concluded that the simplicity of the assay work flow and convenience of taking blood samples make this approach practical and cost effective in the clinical setting.
In an editorial published alongside the study, Juan Ruiz-Bañobre, MD, PhD, and Ajay Goel, PhD, noted that the field is evolving rapidly but “there is substantial value in prospectively validating the clinical importance of ColonAiQ in randomized clinical trials.”
“If successful, this liquid biopsy assay could represent a simple and cost-effective means for a more accessible and facile decentralized implementation in routine clinical practice,” said Dr. Ruiz-Bañobre, of the University of Santiago de Compostela, A Coruña, Spain, and Dr. Goel, from City of Hope Comprehensive Cancer Center in Duarte, Calif.
Several of the study coauthors are employees of Singlera Genomics, which makes the ColonAiQ test. Dr. Ruiz-Bañobre reported grants from the Spanish Cooperative Group for the Treatment of Digestive Tumors and support from Institute of Health Carlos III. Dr. Morris is the principal investigator on the NRG GI005 trial of the Guardant Reveal liquid biopsy, sponsored by Guardant Health in collaboration with funding support from the National Cancer Institute.
A version of this article first appeared on Medscape.com.
A (CRC).
Patients who were ctDNA methylation positive 1 month after surgery were 17.5 times more likely to relapse, compared with ctDNA-negative patients. And following adjuvant chemotherapy, ctDNA-positive patients had a significantly shorter recurrence-free survival than their ctDNA-negative peers.
Overall, “we found that ctDNA methylation was the most significant prognostic factor for recurrence-free survival among all clinicopathologic risk factors on multivariable analysis,” the authors, led by Shaobo Mo, MD, Fudan University Shanghai (China) Cancer Center, reported in research published in JAMA Oncology.
Van Morris, MD, an oncologist with University of Texas MD Anderson Cancer Center, Houston, who was not involved in the research, noted that other commercially available ctDNA assays have achieved similar findings, but this assay involves the least number of biomarkers.
More notably, the broader message emerging from this research is that “ctDNA is a powerful tool in oncology that is here to stay,” said Dr. Morris.
Dr. Morris added a note of caution, however: Despite the study providing further support for this technology in CRC, “we do not have definitive predictive utility for routinely guiding adjuvant chemotherapy decisions with the use of this [or other] ctDNA assays.”
Recurrence common, predictors important
CRC has a relatively high recurrence rate even after curative-intent therapies, with a 5-year survival rate as low as 60%. Adjuvant chemotherapy in patients with stage III CRC generally lowers the risk of recurrence by about 10%-20%; however, the benefits of adjuvant chemotherapy among patients with stage II CRC remain unclear.
Strategies to identify patients most likely to relapse after adjuvant therapy largely focus on CRC stage and clinical risk factors, though postoperative ctDNA testing has emerged as a tool to help identify patients at risk for recurrence. Often, however, this approach involves ultradeep next-generation sequencing, which limits the strategy’s ease of implementation and cost effectiveness.
As an alternative, the authors used a plasma ctDNA methylation test, ColonAiQ, which identifies the presence of six genomic biomarkers hypermethylated in CRC. This test avoids the complex process of primary tumor profiling among individual patients.
In the multicenter, prospective longitudinal cohort study, conducted from December 2019 to February 2022, Dr. Mo and colleagues evaluated 1,228 blood samples from 299 patients with stage I-III CRC. Samples were collected before and after surgery, during and after adjuvant chemotherapy, and every 3 months for up to 2 years.
Of 296 patients with preoperative samples available, as many as 232 (78.4%) tested positive for at least one of the 6 ctDNA methylation markers. The detection rates were 65.1% for stage I CRC, 82.7% for stage II disease, and 81.5% for stage III disease.
At postoperative month 1, ctDNA methylation–positive patients were 17.5 times more likely to relapse, compared with ctDNA-negative patients (hazard ratio, 17.5; P < .001).
When integrating carcinoembryonic antigen testing alongside ctDNA testing, patients with positive test results had significantly worse prognoses, compared with those who had negative results (HR, 19.0; P < .001).
The association of ctDNA methylation positivity at postoperative month 1 and CRC recurrence was consistent across varying durations and intensities of adjuvant chemotherapy. The researchers found that ctDNA methylation analysis detected CRC recurrence a median of 3.3 months earlier than radiologically confirmed recurrence.
Patients who were ctDNA positive also had significantly shorter periods of recurrence-free survival following adjuvant chemotherapy, compared with ctDNA-negative patients (HR, 13.8; P < .001). That effect was enhanced when positive ctDNA status was maintained longitudinally, compared with those who were persistently ctDNA negative (HR, 68.8; P < .001).
More specifically, 140 patients exhibited sustained ctDNA-positive status over time; 6 of 7 ctDNA-positive patients experienced recurrence within 12 months, whereas 129 of 133 ctDNA-negative patients (97%) remained relapse free. And being ctDNA negative before surgery indicated patients’ relapse risk, with 95.3% of patients who were ctDNA negative presurgery remaining relapse free.
Dr. Mo and colleagues concluded that the simplicity of the assay work flow and convenience of taking blood samples make this approach practical and cost effective in the clinical setting.
In an editorial published alongside the study, Juan Ruiz-Bañobre, MD, PhD, and Ajay Goel, PhD, noted that the field is evolving rapidly but “there is substantial value in prospectively validating the clinical importance of ColonAiQ in randomized clinical trials.”
“If successful, this liquid biopsy assay could represent a simple and cost-effective means for a more accessible and facile decentralized implementation in routine clinical practice,” said Dr. Ruiz-Bañobre, of the University of Santiago de Compostela, A Coruña, Spain, and Dr. Goel, from City of Hope Comprehensive Cancer Center in Duarte, Calif.
Several of the study coauthors are employees of Singlera Genomics, which makes the ColonAiQ test. Dr. Ruiz-Bañobre reported grants from the Spanish Cooperative Group for the Treatment of Digestive Tumors and support from Institute of Health Carlos III. Dr. Morris is the principal investigator on the NRG GI005 trial of the Guardant Reveal liquid biopsy, sponsored by Guardant Health in collaboration with funding support from the National Cancer Institute.
A version of this article first appeared on Medscape.com.
A (CRC).
Patients who were ctDNA methylation positive 1 month after surgery were 17.5 times more likely to relapse, compared with ctDNA-negative patients. And following adjuvant chemotherapy, ctDNA-positive patients had a significantly shorter recurrence-free survival than their ctDNA-negative peers.
Overall, “we found that ctDNA methylation was the most significant prognostic factor for recurrence-free survival among all clinicopathologic risk factors on multivariable analysis,” the authors, led by Shaobo Mo, MD, Fudan University Shanghai (China) Cancer Center, reported in research published in JAMA Oncology.
Van Morris, MD, an oncologist with University of Texas MD Anderson Cancer Center, Houston, who was not involved in the research, noted that other commercially available ctDNA assays have achieved similar findings, but this assay involves the least number of biomarkers.
More notably, the broader message emerging from this research is that “ctDNA is a powerful tool in oncology that is here to stay,” said Dr. Morris.
Dr. Morris added a note of caution, however: Despite the study providing further support for this technology in CRC, “we do not have definitive predictive utility for routinely guiding adjuvant chemotherapy decisions with the use of this [or other] ctDNA assays.”
Recurrence common, predictors important
CRC has a relatively high recurrence rate even after curative-intent therapies, with a 5-year survival rate as low as 60%. Adjuvant chemotherapy in patients with stage III CRC generally lowers the risk of recurrence by about 10%-20%; however, the benefits of adjuvant chemotherapy among patients with stage II CRC remain unclear.
Strategies to identify patients most likely to relapse after adjuvant therapy largely focus on CRC stage and clinical risk factors, though postoperative ctDNA testing has emerged as a tool to help identify patients at risk for recurrence. Often, however, this approach involves ultradeep next-generation sequencing, which limits the strategy’s ease of implementation and cost effectiveness.
As an alternative, the authors used a plasma ctDNA methylation test, ColonAiQ, which identifies the presence of six genomic biomarkers hypermethylated in CRC. This test avoids the complex process of primary tumor profiling among individual patients.
In the multicenter, prospective longitudinal cohort study, conducted from December 2019 to February 2022, Dr. Mo and colleagues evaluated 1,228 blood samples from 299 patients with stage I-III CRC. Samples were collected before and after surgery, during and after adjuvant chemotherapy, and every 3 months for up to 2 years.
Of 296 patients with preoperative samples available, as many as 232 (78.4%) tested positive for at least one of the 6 ctDNA methylation markers. The detection rates were 65.1% for stage I CRC, 82.7% for stage II disease, and 81.5% for stage III disease.
At postoperative month 1, ctDNA methylation–positive patients were 17.5 times more likely to relapse, compared with ctDNA-negative patients (hazard ratio, 17.5; P < .001).
When integrating carcinoembryonic antigen testing alongside ctDNA testing, patients with positive test results had significantly worse prognoses, compared with those who had negative results (HR, 19.0; P < .001).
The association of ctDNA methylation positivity at postoperative month 1 and CRC recurrence was consistent across varying durations and intensities of adjuvant chemotherapy. The researchers found that ctDNA methylation analysis detected CRC recurrence a median of 3.3 months earlier than radiologically confirmed recurrence.
Patients who were ctDNA positive also had significantly shorter periods of recurrence-free survival following adjuvant chemotherapy, compared with ctDNA-negative patients (HR, 13.8; P < .001). That effect was enhanced when positive ctDNA status was maintained longitudinally, compared with those who were persistently ctDNA negative (HR, 68.8; P < .001).
More specifically, 140 patients exhibited sustained ctDNA-positive status over time; 6 of 7 ctDNA-positive patients experienced recurrence within 12 months, whereas 129 of 133 ctDNA-negative patients (97%) remained relapse free. And being ctDNA negative before surgery indicated patients’ relapse risk, with 95.3% of patients who were ctDNA negative presurgery remaining relapse free.
Dr. Mo and colleagues concluded that the simplicity of the assay work flow and convenience of taking blood samples make this approach practical and cost effective in the clinical setting.
In an editorial published alongside the study, Juan Ruiz-Bañobre, MD, PhD, and Ajay Goel, PhD, noted that the field is evolving rapidly but “there is substantial value in prospectively validating the clinical importance of ColonAiQ in randomized clinical trials.”
“If successful, this liquid biopsy assay could represent a simple and cost-effective means for a more accessible and facile decentralized implementation in routine clinical practice,” said Dr. Ruiz-Bañobre, of the University of Santiago de Compostela, A Coruña, Spain, and Dr. Goel, from City of Hope Comprehensive Cancer Center in Duarte, Calif.
Several of the study coauthors are employees of Singlera Genomics, which makes the ColonAiQ test. Dr. Ruiz-Bañobre reported grants from the Spanish Cooperative Group for the Treatment of Digestive Tumors and support from Institute of Health Carlos III. Dr. Morris is the principal investigator on the NRG GI005 trial of the Guardant Reveal liquid biopsy, sponsored by Guardant Health in collaboration with funding support from the National Cancer Institute.
A version of this article first appeared on Medscape.com.
FROM JAMA ONCOLOGY
Experts outline comprehensive preeclampsia prevention strategy
Preeclampsia is a leading cause of maternal mortality and premature births. The report, published in the American Journal of Obstetrics and Gynecology, developed by a working group of clinicians, researchers, patients, advocates, and payers, recommends daily low-dose aspirin, surveillance, behavioral strategies, patient and provider education, long-term follow-up, and addressing social determinants of health.
Titled “Care plan for individuals at risk for preeclampsia: Shared approach to education, strategies for prevention, surveillance and follow up,” the report includes recommendations for providers and for patients at moderate to high risk of preeclampsia.
Top recommendations for providers include performing a risk assessment, including social determinants of health, medication recommendations (including daily aspirin and antihypertensive therapy), and behavioral recommendations (including specific information about diet, exercise, and sleep.)
The recommendations for patients include asking providers about aspirin use, checking blood pressure at home, and reporting any readings greater than 140/90. For those with BPs measuring 140/90 mm Hg or higher, the plan recommends antihypertensive therapy. The recommendations include making changes to diet, exercise, and sleep in consultation with providers.
Home blood pressure checks controversial
James Roberts, MD, a maternal-fetal medicine researcher at the Magee-Women’s Research Institute at University of Pittsburgh Medical Center and lead author on the paper, told this publication the home blood pressure checks may be the most controversial item in the report as not all insurers cover the at-home equipment.
In this report, the authors write that the working group “strongly advocates that payers of health care services cover the modest expense of home blood pressure determination including equipment and training.”
Dr. Roberts is the founding principal investigator of the Global Pregnancy Collaboration (CoLab), a consortium of 40 centers and one of the groups leading the creation of this report.
He said that while most of the recommendations are already recommended in guidelines, the report puts the preeclampsia plan into easy-to-read steps and downloadable checklists and compiles the evidence all in one place.
Dr. Roberts said the working group hopes this report will be adapted into guidelines developed by the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine, and made part of electronic health records.
So far, the authors say, a comprehensive, integrated preeclampsia care plan has not been widely adopted.
Fewer than half of patients at risk receive aspirin
The coauthors note that “today, most pregnant individuals at increased risk do not receive even one of the interventions to prevent preeclampsia. For example, less than half of high-risk patients receive low-dose aspirin.”
A big part of this plan, Dr. Roberts said, calls for further educating both providers and patients.
Vesna Garovic, MD, PhD, a preeclampsia specialist at the Mayo Clinic in Rochester, Minn., who was not part of the working group, said, “This is the first comprehensive plan that provides a safe, cost-effective approach to reduce the risk of preeclampsia in individuals at moderate to high risk for this condition who qualify to receive aspirin for prevention.”
Dr. Garovic said the plan is novel in several ways, including the multispecialty input that also includes patients and advocates. Also, she says, it can be easily included in electronic health records and routine care of patients.
“The recommendations that were made, other than self-monitoring of blood pressure, are already standard of care. It will be important to understand as to which extent this comprehensive program, compared to the standard approach, would reduce further the risk of preeclampsia,” Dr. Garovic said. “A prospective, adequately powered comparative study would not only address this question, but will investigate compliance of providers and pregnant women with this shared approach, as well as patient satisfaction.”
The authors note the approach presented is for care in developed countries and that low- and middle-income countries would need to tailor the plan. The Care Plan is also meant only for prevention and is not meant to guide care for women who have developed preeclampsia.
Funding was provided to The Precia Group and the Global Pregnancy Collaboration to assemble this care plan by Mirvie, which is developing a biochemical predictor for preeclampsia. Precia and CoLab used a portion of these funds to support the time of some of the authors. Mirvie had no part in selecting authors or in the content of the manuscript.
Several authors received an honorarium for participation in the Working Group that developed the Care Plan. Two coauthors are site principal investigators overseeing sample collection on a Mirvie project. The remaining authors and Dr. Garovic report no conflicts of interest.
Preeclampsia is a leading cause of maternal mortality and premature births. The report, published in the American Journal of Obstetrics and Gynecology, developed by a working group of clinicians, researchers, patients, advocates, and payers, recommends daily low-dose aspirin, surveillance, behavioral strategies, patient and provider education, long-term follow-up, and addressing social determinants of health.
Titled “Care plan for individuals at risk for preeclampsia: Shared approach to education, strategies for prevention, surveillance and follow up,” the report includes recommendations for providers and for patients at moderate to high risk of preeclampsia.
Top recommendations for providers include performing a risk assessment, including social determinants of health, medication recommendations (including daily aspirin and antihypertensive therapy), and behavioral recommendations (including specific information about diet, exercise, and sleep.)
The recommendations for patients include asking providers about aspirin use, checking blood pressure at home, and reporting any readings greater than 140/90. For those with BPs measuring 140/90 mm Hg or higher, the plan recommends antihypertensive therapy. The recommendations include making changes to diet, exercise, and sleep in consultation with providers.
Home blood pressure checks controversial
James Roberts, MD, a maternal-fetal medicine researcher at the Magee-Women’s Research Institute at University of Pittsburgh Medical Center and lead author on the paper, told this publication the home blood pressure checks may be the most controversial item in the report as not all insurers cover the at-home equipment.
In this report, the authors write that the working group “strongly advocates that payers of health care services cover the modest expense of home blood pressure determination including equipment and training.”
Dr. Roberts is the founding principal investigator of the Global Pregnancy Collaboration (CoLab), a consortium of 40 centers and one of the groups leading the creation of this report.
He said that while most of the recommendations are already recommended in guidelines, the report puts the preeclampsia plan into easy-to-read steps and downloadable checklists and compiles the evidence all in one place.
Dr. Roberts said the working group hopes this report will be adapted into guidelines developed by the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine, and made part of electronic health records.
So far, the authors say, a comprehensive, integrated preeclampsia care plan has not been widely adopted.
Fewer than half of patients at risk receive aspirin
The coauthors note that “today, most pregnant individuals at increased risk do not receive even one of the interventions to prevent preeclampsia. For example, less than half of high-risk patients receive low-dose aspirin.”
A big part of this plan, Dr. Roberts said, calls for further educating both providers and patients.
Vesna Garovic, MD, PhD, a preeclampsia specialist at the Mayo Clinic in Rochester, Minn., who was not part of the working group, said, “This is the first comprehensive plan that provides a safe, cost-effective approach to reduce the risk of preeclampsia in individuals at moderate to high risk for this condition who qualify to receive aspirin for prevention.”
Dr. Garovic said the plan is novel in several ways, including the multispecialty input that also includes patients and advocates. Also, she says, it can be easily included in electronic health records and routine care of patients.
“The recommendations that were made, other than self-monitoring of blood pressure, are already standard of care. It will be important to understand as to which extent this comprehensive program, compared to the standard approach, would reduce further the risk of preeclampsia,” Dr. Garovic said. “A prospective, adequately powered comparative study would not only address this question, but will investigate compliance of providers and pregnant women with this shared approach, as well as patient satisfaction.”
The authors note the approach presented is for care in developed countries and that low- and middle-income countries would need to tailor the plan. The Care Plan is also meant only for prevention and is not meant to guide care for women who have developed preeclampsia.
Funding was provided to The Precia Group and the Global Pregnancy Collaboration to assemble this care plan by Mirvie, which is developing a biochemical predictor for preeclampsia. Precia and CoLab used a portion of these funds to support the time of some of the authors. Mirvie had no part in selecting authors or in the content of the manuscript.
Several authors received an honorarium for participation in the Working Group that developed the Care Plan. Two coauthors are site principal investigators overseeing sample collection on a Mirvie project. The remaining authors and Dr. Garovic report no conflicts of interest.
Preeclampsia is a leading cause of maternal mortality and premature births. The report, published in the American Journal of Obstetrics and Gynecology, developed by a working group of clinicians, researchers, patients, advocates, and payers, recommends daily low-dose aspirin, surveillance, behavioral strategies, patient and provider education, long-term follow-up, and addressing social determinants of health.
Titled “Care plan for individuals at risk for preeclampsia: Shared approach to education, strategies for prevention, surveillance and follow up,” the report includes recommendations for providers and for patients at moderate to high risk of preeclampsia.
Top recommendations for providers include performing a risk assessment, including social determinants of health, medication recommendations (including daily aspirin and antihypertensive therapy), and behavioral recommendations (including specific information about diet, exercise, and sleep.)
The recommendations for patients include asking providers about aspirin use, checking blood pressure at home, and reporting any readings greater than 140/90. For those with BPs measuring 140/90 mm Hg or higher, the plan recommends antihypertensive therapy. The recommendations include making changes to diet, exercise, and sleep in consultation with providers.
Home blood pressure checks controversial
James Roberts, MD, a maternal-fetal medicine researcher at the Magee-Women’s Research Institute at University of Pittsburgh Medical Center and lead author on the paper, told this publication the home blood pressure checks may be the most controversial item in the report as not all insurers cover the at-home equipment.
In this report, the authors write that the working group “strongly advocates that payers of health care services cover the modest expense of home blood pressure determination including equipment and training.”
Dr. Roberts is the founding principal investigator of the Global Pregnancy Collaboration (CoLab), a consortium of 40 centers and one of the groups leading the creation of this report.
He said that while most of the recommendations are already recommended in guidelines, the report puts the preeclampsia plan into easy-to-read steps and downloadable checklists and compiles the evidence all in one place.
Dr. Roberts said the working group hopes this report will be adapted into guidelines developed by the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine, and made part of electronic health records.
So far, the authors say, a comprehensive, integrated preeclampsia care plan has not been widely adopted.
Fewer than half of patients at risk receive aspirin
The coauthors note that “today, most pregnant individuals at increased risk do not receive even one of the interventions to prevent preeclampsia. For example, less than half of high-risk patients receive low-dose aspirin.”
A big part of this plan, Dr. Roberts said, calls for further educating both providers and patients.
Vesna Garovic, MD, PhD, a preeclampsia specialist at the Mayo Clinic in Rochester, Minn., who was not part of the working group, said, “This is the first comprehensive plan that provides a safe, cost-effective approach to reduce the risk of preeclampsia in individuals at moderate to high risk for this condition who qualify to receive aspirin for prevention.”
Dr. Garovic said the plan is novel in several ways, including the multispecialty input that also includes patients and advocates. Also, she says, it can be easily included in electronic health records and routine care of patients.
“The recommendations that were made, other than self-monitoring of blood pressure, are already standard of care. It will be important to understand as to which extent this comprehensive program, compared to the standard approach, would reduce further the risk of preeclampsia,” Dr. Garovic said. “A prospective, adequately powered comparative study would not only address this question, but will investigate compliance of providers and pregnant women with this shared approach, as well as patient satisfaction.”
The authors note the approach presented is for care in developed countries and that low- and middle-income countries would need to tailor the plan. The Care Plan is also meant only for prevention and is not meant to guide care for women who have developed preeclampsia.
Funding was provided to The Precia Group and the Global Pregnancy Collaboration to assemble this care plan by Mirvie, which is developing a biochemical predictor for preeclampsia. Precia and CoLab used a portion of these funds to support the time of some of the authors. Mirvie had no part in selecting authors or in the content of the manuscript.
Several authors received an honorarium for participation in the Working Group that developed the Care Plan. Two coauthors are site principal investigators overseeing sample collection on a Mirvie project. The remaining authors and Dr. Garovic report no conflicts of interest.
FROM AMERICAN JOURNAL OF OBSTETRICS AND GYNECOLOGY
High-dose vitamin D and MS relapse: New phase 3 data
, results from a randomized control trial show. However, at least one expert believes the study’s exclusion criteria may have been too broad.
The investigation of vitamin D to prevent relapse of MS is based on older observational studies of people who already had higher blood levels of vitamin D and were less likely to develop MS, said study investigator Ellen Mowry, MD, Richard T. and Frances W. Johnson professor of neurology, Johns Hopkins University, Baltimore.
Later research where participants were given vitamin D as a therapeutic option for MS “were disappointing as the vitamin D had minimal effect,” she said.
“While we were excited by early data suggesting that vitamin D may have an important impact on MS, it’s essential to follow those linkage studies with the gold standard clinical evidence, which we have here,” Dr. Mowry added.
The findings were published online in eClinicalMedicine.
No difference in relapse risk
The multisite, phase 3 Vitamin D to Ameliorate MS (VIDAMS) clinical trial included 172 participants aged 18-50 years with RRMS from 16 neurology clinics between 2012 and 2019.
Inclusion criteria were having one or more clinical episodes of MS in the past year and at least one brain lesion on MRI in the past year or having two or more clinical episodes in the past year. Eligible participants also had to have a score of 4 or less on the Kurtzke Expanded Disability Status Scale.
A total of 83 participants were randomly assigned to receive low-dose vitamin D3 (600 IU/day) and 89 to receive high-dose vitamin D3 (5,000 IU/day). Each participant took the vitamin tablet with glatiramer acetate, a synthetic protein that simulates myelin.
Participants were assessed every 12 weeks to measure serum 25(OH)D levels and every 24 weeks for a number of movement and coordination tests, as well as two 3T clinical brain MRIs to check for lesions.
By the trial’s end at 96 weeks, the researchers found no differences in relapse risk between the high- and low-dose groups (P = .57). In addition, there were no differences in MRI outcomes between the two groups.
Dr. Mowry said that more than a few people have asked her if she is disappointed by the results of the VIDAMS trial. “I tell them that no, I’m not – that we are scientists and clinicians, and it is our job to understand what they can do to fight their disease. And if the answer is not vitamin D, that’s OK – we have many other ideas.”
These include helping patients minimize cardiometabolic comorbidities, such as heart disease and blood pressure, she said.
Exclusion criteria too broad?
Commenting on the findings, Alberto Ascherio, MD, professor of epidemiology and nutrition at Harvard School of Public Health, Boston, said a key principle of recommending vitamin supplements is that they are, generally speaking, only beneficial for individuals with vitamin deficiencies.
He noted that “patients with vitamin D deficiency (25(OH)D < 15 ng/mL, which corresponds to 37.5 nmol/L) were excluded from this study. Most importantly, the baseline mean 25(OH)D levels were about 30 ng/mL (75 nmol/L), which is considered a sufficient level (the IOM considers 20 ng/mL = 50 nmol/L as an adequate level),” with the level further increasing during the trial due to the supplementation.
“It would be a serious mistake to conclude from this trial (or any of the previous trials) that vitamin D supplementation is not important in MS patients,” Dr. Ascherio said.
He added that many individuals with MS have serum vitamin D levels below 20 ng/mL (50 nmol/L) and that this was the median serum value in studies among individuals with MS in Europe.
“These patients would almost certainly benefit from moderate doses of vitamin D supplements or judicious UV light exposure. Most likely even patients with sufficient but suboptimal 25(OH)D levels (between 20 and 30 ng/mL, or 50 and 75 nmol/L) would benefit from an increase,” he said.
The study was funded by the National Multiple Sclerosis Society, Teva Neuroscience, and the National Institute of Health. Dr. Mowry reported grant support from the National MS Society, Biogen, Genentech, and Teva Neuroscience; honoraria from UpToDate; and consulting fees from BeCare Link.
A version of this article first appeared on Medscape.com.
, results from a randomized control trial show. However, at least one expert believes the study’s exclusion criteria may have been too broad.
The investigation of vitamin D to prevent relapse of MS is based on older observational studies of people who already had higher blood levels of vitamin D and were less likely to develop MS, said study investigator Ellen Mowry, MD, Richard T. and Frances W. Johnson professor of neurology, Johns Hopkins University, Baltimore.
Later research where participants were given vitamin D as a therapeutic option for MS “were disappointing as the vitamin D had minimal effect,” she said.
“While we were excited by early data suggesting that vitamin D may have an important impact on MS, it’s essential to follow those linkage studies with the gold standard clinical evidence, which we have here,” Dr. Mowry added.
The findings were published online in eClinicalMedicine.
No difference in relapse risk
The multisite, phase 3 Vitamin D to Ameliorate MS (VIDAMS) clinical trial included 172 participants aged 18-50 years with RRMS from 16 neurology clinics between 2012 and 2019.
Inclusion criteria were having one or more clinical episodes of MS in the past year and at least one brain lesion on MRI in the past year or having two or more clinical episodes in the past year. Eligible participants also had to have a score of 4 or less on the Kurtzke Expanded Disability Status Scale.
A total of 83 participants were randomly assigned to receive low-dose vitamin D3 (600 IU/day) and 89 to receive high-dose vitamin D3 (5,000 IU/day). Each participant took the vitamin tablet with glatiramer acetate, a synthetic protein that simulates myelin.
Participants were assessed every 12 weeks to measure serum 25(OH)D levels and every 24 weeks for a number of movement and coordination tests, as well as two 3T clinical brain MRIs to check for lesions.
By the trial’s end at 96 weeks, the researchers found no differences in relapse risk between the high- and low-dose groups (P = .57). In addition, there were no differences in MRI outcomes between the two groups.
Dr. Mowry said that more than a few people have asked her if she is disappointed by the results of the VIDAMS trial. “I tell them that no, I’m not – that we are scientists and clinicians, and it is our job to understand what they can do to fight their disease. And if the answer is not vitamin D, that’s OK – we have many other ideas.”
These include helping patients minimize cardiometabolic comorbidities, such as heart disease and blood pressure, she said.
Exclusion criteria too broad?
Commenting on the findings, Alberto Ascherio, MD, professor of epidemiology and nutrition at Harvard School of Public Health, Boston, said a key principle of recommending vitamin supplements is that they are, generally speaking, only beneficial for individuals with vitamin deficiencies.
He noted that “patients with vitamin D deficiency (25(OH)D < 15 ng/mL, which corresponds to 37.5 nmol/L) were excluded from this study. Most importantly, the baseline mean 25(OH)D levels were about 30 ng/mL (75 nmol/L), which is considered a sufficient level (the IOM considers 20 ng/mL = 50 nmol/L as an adequate level),” with the level further increasing during the trial due to the supplementation.
“It would be a serious mistake to conclude from this trial (or any of the previous trials) that vitamin D supplementation is not important in MS patients,” Dr. Ascherio said.
He added that many individuals with MS have serum vitamin D levels below 20 ng/mL (50 nmol/L) and that this was the median serum value in studies among individuals with MS in Europe.
“These patients would almost certainly benefit from moderate doses of vitamin D supplements or judicious UV light exposure. Most likely even patients with sufficient but suboptimal 25(OH)D levels (between 20 and 30 ng/mL, or 50 and 75 nmol/L) would benefit from an increase,” he said.
The study was funded by the National Multiple Sclerosis Society, Teva Neuroscience, and the National Institute of Health. Dr. Mowry reported grant support from the National MS Society, Biogen, Genentech, and Teva Neuroscience; honoraria from UpToDate; and consulting fees from BeCare Link.
A version of this article first appeared on Medscape.com.
, results from a randomized control trial show. However, at least one expert believes the study’s exclusion criteria may have been too broad.
The investigation of vitamin D to prevent relapse of MS is based on older observational studies of people who already had higher blood levels of vitamin D and were less likely to develop MS, said study investigator Ellen Mowry, MD, Richard T. and Frances W. Johnson professor of neurology, Johns Hopkins University, Baltimore.
Later research where participants were given vitamin D as a therapeutic option for MS “were disappointing as the vitamin D had minimal effect,” she said.
“While we were excited by early data suggesting that vitamin D may have an important impact on MS, it’s essential to follow those linkage studies with the gold standard clinical evidence, which we have here,” Dr. Mowry added.
The findings were published online in eClinicalMedicine.
No difference in relapse risk
The multisite, phase 3 Vitamin D to Ameliorate MS (VIDAMS) clinical trial included 172 participants aged 18-50 years with RRMS from 16 neurology clinics between 2012 and 2019.
Inclusion criteria were having one or more clinical episodes of MS in the past year and at least one brain lesion on MRI in the past year or having two or more clinical episodes in the past year. Eligible participants also had to have a score of 4 or less on the Kurtzke Expanded Disability Status Scale.
A total of 83 participants were randomly assigned to receive low-dose vitamin D3 (600 IU/day) and 89 to receive high-dose vitamin D3 (5,000 IU/day). Each participant took the vitamin tablet with glatiramer acetate, a synthetic protein that simulates myelin.
Participants were assessed every 12 weeks to measure serum 25(OH)D levels and every 24 weeks for a number of movement and coordination tests, as well as two 3T clinical brain MRIs to check for lesions.
By the trial’s end at 96 weeks, the researchers found no differences in relapse risk between the high- and low-dose groups (P = .57). In addition, there were no differences in MRI outcomes between the two groups.
Dr. Mowry said that more than a few people have asked her if she is disappointed by the results of the VIDAMS trial. “I tell them that no, I’m not – that we are scientists and clinicians, and it is our job to understand what they can do to fight their disease. And if the answer is not vitamin D, that’s OK – we have many other ideas.”
These include helping patients minimize cardiometabolic comorbidities, such as heart disease and blood pressure, she said.
Exclusion criteria too broad?
Commenting on the findings, Alberto Ascherio, MD, professor of epidemiology and nutrition at Harvard School of Public Health, Boston, said a key principle of recommending vitamin supplements is that they are, generally speaking, only beneficial for individuals with vitamin deficiencies.
He noted that “patients with vitamin D deficiency (25(OH)D < 15 ng/mL, which corresponds to 37.5 nmol/L) were excluded from this study. Most importantly, the baseline mean 25(OH)D levels were about 30 ng/mL (75 nmol/L), which is considered a sufficient level (the IOM considers 20 ng/mL = 50 nmol/L as an adequate level),” with the level further increasing during the trial due to the supplementation.
“It would be a serious mistake to conclude from this trial (or any of the previous trials) that vitamin D supplementation is not important in MS patients,” Dr. Ascherio said.
He added that many individuals with MS have serum vitamin D levels below 20 ng/mL (50 nmol/L) and that this was the median serum value in studies among individuals with MS in Europe.
“These patients would almost certainly benefit from moderate doses of vitamin D supplements or judicious UV light exposure. Most likely even patients with sufficient but suboptimal 25(OH)D levels (between 20 and 30 ng/mL, or 50 and 75 nmol/L) would benefit from an increase,” he said.
The study was funded by the National Multiple Sclerosis Society, Teva Neuroscience, and the National Institute of Health. Dr. Mowry reported grant support from the National MS Society, Biogen, Genentech, and Teva Neuroscience; honoraria from UpToDate; and consulting fees from BeCare Link.
A version of this article first appeared on Medscape.com.
FROM ECLINICALMEDICINE
Interventional psychiatry (Part 1)
Advances in the understanding of neurobiological and neuropsychiatric pathophysiology have opened new avenues of treatment for psychiatric patients. Historically, with a few exceptions, most psychiatric medications have been administered orally. However, many of the newer treatments require some form of specialized administration because they cannot be taken orally due to their chemical property (such as aducanumab); because there is the need to produce stable blood levels of the medication (such as brexanolone); because oral administration greatly diminished efficacy (such as oral vs IV magnesium or scopolamine), or because the treatment is focused on specific brain structures. This need for specialized administration has created a subspecialty called interventional psychiatry.
Part 1 of this 2-part article provides an overview of 1 type of interventional psychiatry: parenterally administered medications, including those administered via IV. We also describe 3 other interventional approaches to treatment: stellate ganglion blocks, glabellar botulinum toxin (BT) injections, and trigger point injections. In Part 2 we will review interventional approaches that involve neuromodulation.
Parenteral medications in psychiatry
In general, IV and IM medications can be more potent that oral medications due to their overall faster onset of action and higher blood concentrations. These more invasive forms of administration can have significant limitations, such as a risk of infection at the injection site, the need to be administered in a medical setting, additional time, and patient discomfort.
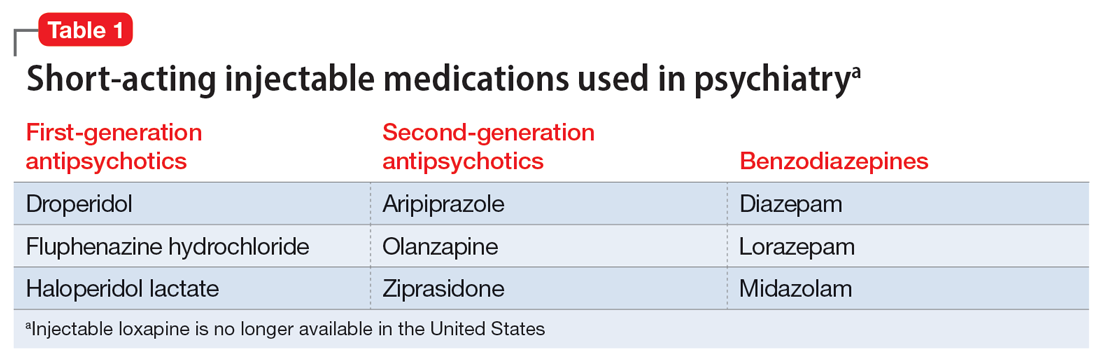
Table 1 lists short-acting injectable medications used in psychiatry, and Table 2 lists long-acting injectable medications. Parenteral administration of antipsychotics is performed to alleviate acute agitation or for chronic symptom control. These medications generally are not considered interventional treatments, but could be classified as such due to their invasive nature.1 Furthermore, inhalable loxapine—which is indicated for managing acute agitation—requires a Risk Evaluation and Mitigation Strategy program consisting of 2 hours observation and monitoring of respiratory status.2,3 Other indications for parenteral treatments include IM naltrexone extended release4 and subcutaneous injections of buprenorphine extended release5 and risperidone.6
IV administration
Most IV treatments described in this article are not FDA-approved for psychiatric treatment. Despite this, many interventional psychiatric treatments are part of clinical practice. IV infusion of ketamine is the most widely known and most researched of these. Table 3 lists other IV treatments that could be used as psychiatric treatment.
Ketamine
Since the early 1960s, ketamine has been used as a surgical anesthetic for animals. In the United States, it was approved for human surgical anesthesia in 1970. It was widely used during the Vietnam War due to its lack of inhibition of respiratory drive; medical staff first noticed an improvement in depressive symptoms and the resolution of suicidal ideation in patients who received ketamine. This led to further research on ketamine, particularly to determine its application in treatment-resistant depression (TRD) and other conditions.7 IV ketamine administration is most widely researched, but IM injections, intranasal sprays, and lozenges are also available. The dissociative properties of ketamine have led to its recreational use.8
Hypotheses for the mechanism of action of ketamine as an antidepressant include direct synaptic or extrasynaptic (GluN2B-selective), N-methyl-
Continue to: Ketamine is a schedule...
Ketamine is a schedule III medication with addictive properties. Delirium, panic attacks, hallucinations, nightmares, dysphoria, and paranoia may occur during and after use.13 Premedication with benzodiazepines, most notably lorazepam, is occasionally used to minimize ketamine’s adverse effects, but this generally is not recommended because doing so reduces ketamine’s antidepressant effects.14 Driving and operating heavy machinery is contraindicated after IV infusion. The usual protocol involves an IV infusion of ketamine 0.4 mg/kg to 1 mg/kg dosing over 1 hour. Doses between 0.4 mg/kg and 0.6 mg/kg are most common. Ketamine has a therapeutic window; doses >0.5 mg/kg are progressively less effective.15 Unlike the recommendation after esketamine administration, after receiving ketamine, patients remain in the care of their treatment team for <2 hours.
Esketamine, the S enantiomer of ketamine, was FDA-approved for TRD as an intranasal formulation. Esketamine is more commonly used than IV ketamine because it is FDA-approved and typically covered by insurance, but it may not be as effective.16 An economic analysis by Brendle et al17 suggested insurance companies would lower costs if they covered ketamine infusions vs intranasal esketamine.
Aducanumab and lecanemab
The most recent FDA-approved interventional agents are aducanumab and lecanemab, which are indicated for treating Alzheimer disease.18,19 Both are human monoclonal antibodies that bind selectively and with high affinity to amyloid beta plaque aggregates and promote their removal by Fc receptor–mediated phagocytosis.20
FDA approval of aducanumab and lecanemab was controversial. Initially, aducanumab’s safety monitoring board performed a futility analysis that suggested aducanumab was unlikely to separate from placebo, and the research was stopped.21 The manufacturer petitioned the FDA to consider the medication for accelerated approval on the basis of biomarker data showing that amyloid beta plaque aggregates become smaller. Current FDA approval is temporary to allow patients access to this potentially beneficial agent, but the manufacturer must supply clinical evidence that the reduction of amyloid beta plaques is associated with desirable changes in the course of Alzheimer disease, or risk losing the approval.
Lecanemab is also a human monoclonal antibody intended to remove amyloid beta plaques that was FDA-approved under the accelerated approval pathway.22 Unlike aducanumab, lecanemab demonstrated a statistically significant (although clinically imperceptible) reduction in the rate of cognitive decline; it did not show cognitive improvement.23 Lecanemab also significantly reduced amyloid beta plaques.23
Continue to: Aducanumab and lecanemab are generally...
Aducanumab and lecanemab are generally not covered by insurance and typically cost >$26,000 annually. Both are administered by IV infusion once a month. More monoclonal antibody medications for treating early Alzheimer disease are in the late stages of development, most notably donanebab.24 Observations during clinical trials found that in the later stages of Alzheimer disease, forceful removal of plaques by the autoimmune process damages neurons, while in less dense deposits of early dementia such removal is not harmful to the cells and prevents amyloid buildup.
Brexanolone is an aqueous formulation of allopregnanolone, a major metabolite of progesterone and a positive allosteric modulator of GABA-A receptors.25 Its levels are maximal at the end of the third trimester of pregnancy and fall rapidly following delivery. Research showed a 3-day infusion was rapidly and significantly effective for treating postpartum depression26 and brexanolone received FDA approval for this indication in March 2019.27 However, various administrative, economic, insurance, and other hurdles make it difficult for patients to access this treatment. Despite its rapid onset of action (usually 48 hours), brexanolone takes an average of 15 days to go through the prior authorization process.28 In addition to the need for prior authorization, the main impediment to the use of brexanolone is the 3-day infusion schedule, which greatly magnifies the cost but is partially circumvented by the availability of dedicated outpatient centers.
Magnesium
Magnesium is on the World Health Organization’s Model List of Essential Medicines.29 There has been extensive research on the use of magnesium sulfate for psychiatric indications, especially for depression.30 Magnesium functions similarly to calcium channel blockers by competitively blocking intracellular calcium channels, decreasing calcium availability, and inhibiting smooth muscle contractility.31 It also competes with calcium at the motor end plate, reducing excitation by inhibiting the release of acetylcholine.32 This property is used for high-dose IV magnesium treatment of impending preterm labor in obstetrics. Magnesium sulfate is the drug of choice in treating eclamptic seizures and preventing seizures in severe preeclampsia or gestational hypertension with severe features.33 It is also used to treat torsade de pointes, severe asthma exacerbations, constipation, and barium poisoning.34 Beneficial use in asthma treatment35 and the treatment of migraine36 have also been reported.
IV magnesium in myocardial infarction may be harmful,37 though outside of acute cardiac events, magnesium is found to be safe.38
Oral magnesium sulfate is a common over-the-counter anxiety remedy. As a general cell stabilizer (mediated by the reduction of intracellular calcium), magnesium is potentially beneficial outside of its muscle-relaxing properties, although muscle relaxing can benefit anxious patients. It is used to treat anxiety,39 alcohol withdrawal,40 and fear.41 Low magnesium blood levels are found in patients with depression, schizophrenia,42 and attention-deficit/hyperactivity disorder.43 However, it is important to note that the therapeutic effect of magnesium when treating anxiety and headache is independent of preinfusion magnesium blood levels.43
Continue to: The efficacy of oral magnesium...
The efficacy of oral magnesium is not robust. However, IV administration has a pronounced beneficial effect as an abortive and preventative treatment in many patients with anxiety.44
IV administration of magnesium can produce adverse effects, including flushing, sweating, hypotension, depressed reflexes, flaccid paralysis, hypothermia, circulatory collapse, and cardiac and CNS depression. These complications are very rare and dose-dependent.45 Magnesium is excreted by the kidneys, and dosing must be decreased in patients with kidney failure. The most common adverse effect is local burning along the vein upon infusion; small doses of IV lidocaine can remedy this. Hot flashes are also common.45
Various dosing strategies are available. In patients with anxiety, application dosing is based on the recommended preeclampsia IV dose of 4 g diluted in 250 mL of 5% dextrose. Much higher doses may be used in obstetrics. Unlike in obstetrics, for psychiatric indications, magnesium is administered over 60 to 90 minutes. Heart monitoring is recommended.
Scopolamine
Scopolamine is primarily used to relieve nausea, vomiting, and dizziness associated with motion sickness and recovery from anesthesia. It is also used in ophthalmology and in patients with excessive sweating. In off-label and experimental applications, scopolamine has been used in patients with TRD.46
Scopolamine is an anticholinergic medication. It is a nonselective antagonist at muscarinic receptors.47 Tricyclic antidepressants (TCAs) possess strong anticholinergic function. Newer generations of antidepressants were designed specifically not to have this function because it was believed to be an unwanted and potentially dangerous adverse effect. However, data suggest that anticholinergic action is important in decreasing depressive symptoms. Several hypotheses of anticholinergic effects on depression have been published since the 1970s. They include the cholinergic-adrenergic hypothesis,48 acetylcholine predominance relative to adrenergic action hypothesis,49 and insecticide poisoning observations.50 Centrally acting physostigmine (but not peripherally acting neostigmine) was reported to control mania.48,51 A genetic connection between the M2acetylcholine receptor in patients with major depressive disorder (MDD) and alcohol use disorder is also suggestive.52
Continue to: Multiple animal studies show...
Multiple animal studies show direct improvement in mobility and a decrease in despair upon introducing anticholinergic substances.53-55 The cholinergic theory of depression has been studied in several controlled clinical human studies.56,57 Use of a short-acting anticholinergic glycopyrrolate during electroconvulsive therapy (ECT) may contribute to the procedure’s efficacy.
Human research shows scopolamine has a higher efficacy as an antidepressant and anti-anxiety medication in women than in men,58 possibly because estrogen increases the activity of choline acetyltransferase and release of acetylcholine.59,60 M2receptors mediate estrogen influence on the NMDAR, which may explain the anticholinergic effects of depression treatments in women.61
Another proposed mechanism of action of scopolamine is a potent inhibition of the NMDAR.62 Rapid treatments of depression may be based on this mechanism. Examples of such treatments include IV ketamine and sleep deprivation.63 IV scopolamine shows potency in treating MDD and bipolar depression. This treatment should be reserved for patients who do not respond to or are not candidates for other usual treatment modalities of MDD and for the most severe cases. Scopolamine is 30 times more potent than amitriptyline in anticholinergic effect and reportedly produces sustained improvement in MDD.64
Scopolamine has no black-box warnings. It has not been studied in pregnant women and is not recommended for use during pregnancy. Due to possible negative cardiovascular effects, a normal electrocardiogram is required before the start of treatment. Exercise caution in patients with glaucoma, benign prostatic enlargement, gastroparesis, unstable cardiovascular status, or severe renal impairment.
Treatment with scopolamine is not indicated for patients with myasthenia gravis, psychosis, or seizures. Patients must be off potassium for 3 days before beginning scopolamine treatment. Patients should consult with their cardiologist before having a scopolamine infusion. Adverse reactions may include psychosis, tachycardia, seizures, paralytic ileus, and glaucoma exacerbation. The most common adverse effects of scopolamine infusion treatment include drowsiness, dry mouth, blurred vision, lightheadedness, and dizziness. Due to possible drowsiness, operating motor vehicles or heavy machinery must be avoided on the day of treatment.65 Overall, the adverse effects of scopolamine are preventable and manageable, and its antidepressant efficacy is noteworthy.66
Continue to: Treatment typically consists of 3 consecutive infusions...
Treatment typically consists of 3 consecutive infusions of 4 mcg/kg separated by 3 to 5 days.56 It is possible to have a longer treatment course if the patient experiences only partial improvement. Repeated courses or maintenance treatment (similar to ECT maintenance) are utilized in some patients if indicated. Cardiac monitoring is mandatory.
Clomipramine
Clomipramine, a TCA, acts as a preferential inhibitor of 5-hydroxytryptamine uptake and has proven effective in managing depression, TRD, and obsessive-compulsive disorder (OCD).67 Although this medication has reported treatment benefits for patients with phobia, panic disorder,15 chronic pain,68 Tourette syndrome,69 premature ejaculation, anorexia nervosa,70 cataplexy,49 and enuresis,71 it is FDA-approved only for the treatment of OCD.72 Clomipramine may also be beneficial for pain and headache, possibly because of its anti-inflammatory action.73 The anticholinergic effects of clomipramine may add to its efficacy in depression.
The pathophysiology of MDD is connected to hyperactivity of the HPA axis and elevated cortisol levels. Higher clomipramine plasma levels show a linear correlation with lower cortisol secretion and levels, possibly aiding in the treatment of MDD and anxiety.74 The higher the blood level, the more pronounced clomipramine’s therapeutic effect across multiple domains.75
IV infusion of clomipramine produces the highest concentration in the shortest time, but overall, research does not necessarily support increased efficacy of IV over oral administration. There is evidence suggesting that subgroups of patients with severe, treatment-refractory OCD may benefit from IV agents and research suggests a faster onset of action.76 Faster onset of symptom relief is the basis for IV clomipramine use. In patients with OCD, it can take several months for oral medications to produce therapeutic benefits; not all patients can tolerate this. In such scenarios, IV administration may be considered, though it is not appropriate for routine use until more research is available. Patients with treatment-resistant OCD who have exhausted other means of symptom relief may also be candidates for IV treatment.
The adverse effects of IV clomipramine are no different from oral use, though they may be more pronounced. A pretreatment cardiac exam is desirable because clomipramine, like other TCAs, may be cardiotoxic. The anticholinergic adverse effects of TCAs are well known to clinicians77 and partially explained in the scopolamine section of this article. It is not advisable to combine clomipramine with other TCAs or serotonin reuptake inhibitors. Clomipramine also should not be combined with monoamine oxidase inhibitors, though such a combination was reported in medical literature.78 Combination with antiarrhythmics such as lidocaine or opioids such as fentanyl or and tramadol is highly discouraged (fentanyl and tramadol also have serotonergic effects).79
Continue to: Clomipramine for IV use is not commercially available...
Clomipramine for IV use is not commercially available and must be sterilely compounded. The usual course of treatment is a series of 3 infusions: 150 mg on Day 1, 200 mg on Day 2 or Day 3, and 250 mg on Day 3, Day 4, or Day 5, depending on tolerability. A protocol with a 50 mg/d starting dose and titration up to a maximum dose of 225 mg/d over 5 to 7 days has been suggested for inpatient settings.67 Titration to 250 mg is more common.80
A longer series may be performed, but this increases the likelihood of adverse effects. Booster and maintenance treatments are also completed when required. Cardiac monitoring is mandatory.
Vortioxetine and citalopram
IV treatment of depression with
Injections and blocks
Three interventional approaches to treatment that possess psychotherapeutic potential include stellate ganglion blocks (SGBs), glabellar BT injections, and trigger point injections (TPIs). None of these are FDA-approved for psychiatric treatment.
Stellate ganglion blocks
The sympathetic nervous system is involved in autonomic hyperarousal and is at the core of posttraumatic symptomatology.83 Insomnia, anxiety, irritability, hypervigilance, and other excitatory CNS events are connected to the sympathetic nervous system and amygdala activation is commonly observed in those exposed to extreme stress or traumatic events.84
Continue to: SGBs were first performed 100 years ago...
SGBs were first performed 100 years ago and reported to have beneficial psychiatric effects at the end of the 1940s. In 1998 in Finland, improvement of posttraumatic stress disorder (PTSD) symptoms was observed accidentally via thoracic level spine blocks.85 In 2006, cervical level sympathetic blocks were shown to be effective for PTSD symptom control.86 By the end of 2010, Veterans Administration hospitals adopted SGBs to treat veterans with PTSD.87,88 The first multisite, randomized clinical trial of
Since the stellate ganglion is connected to the amygdala, SGB has also been assessed for treating anxiety and depression.89,90 Outside of PTSD, SGBs are used to treat complex regional pain syndrome,91 phantom limb pain, trigeminal neuralgia,92 intractable angina,93 and postherpetic neuralgia in the head, neck, upper chest, or arms.94 The procedure consists of an injection of a local anesthetic through a 25-gauge needle into the stellate sympathetic ganglion at the C6 or C7 vertebral levels. An injection into C6 is considered safer because of specific cervical spine anatomy. Ideally, fluoroscopic guidance or ultrasound is used to guide needle insertion.95
A severe drop in blood pressure may be associated with SGBs and is mitigated by IV hydration. Other adverse effects include red eyes, drooping of the eyelids, nasal congestion, hoarseness, difficulty swallowing, a sensation of a “lump” in the throat, and a sensation of warmth or tingling in the arm or hand. Bilateral SGB is contraindicated due to the danger of respiratory arrest.96
Glabellar BT injections
OnabotulinumtoxinA (BT) injection was first approved for therapeutic use in 1989 for eye muscle disorders such as strabismus97 and blepharospasm.98 It was later approved for several other indications, including cosmetic use, hyperhidrosis, migraine prevention, neurogenic bladder disorder, overactive bladder, urinary incontinence, and spasticity.99-104 BT is used off-label for achalasia and sialorrhea.105,106 Its mechanism of action is primarily attributed to muscle paralysis by blocking presynaptic acetylcholine release into neuromuscular junctions.107
Facial BT injections are usually administered for cosmetic purposes, but smoothing forehead wrinkles and frown lines (the glabellar region of the face) both have antidepressant effects.108 BT injections into the glabellar region also demonstrate antidepressant effects, particularly in patients with comorbid migraines and MDD.109 Early case observations supported the independent benefit of the toxin on MDD when the toxin was injected into the glabellar region.110,111 The most frequent protocol involves injections in the procerus and corrugator muscles.
Continue to: The facial feedback/emotional proprioception hypothesis...
The facial feedback/emotional proprioception hypothesis has dominated thinking about the mood-improving effects of BT. The theory is that blocking muscular expression of sadness (especially in the face) interrupts the experience of sadness; therefore, depression subsides.112,113 However, BT injections in the muscles involved in the smile and an expression of positive emotions (lateral part of the musculus orbicularis oculi) have been associated with increased MDD scores.114 Thus, the mechanism clearly involves more than the cosmetic effect, since facial muscle injections in rats also have antidepressant effects.115
The use of progressive muscle relaxation is well-established in psychiatric treatment. The investigated conditions of increased muscle tone, especially torticollis and blepharospasm, are associated with MDD, and it may be speculated that proprioceptive feedback from the affected muscles may be causally involved in this association.116-118 Activity of the corrugator muscle has been positively associated with increased amygdala activity.119 This suggests a potential similar mechanism to that hypothesized for SGB.
Alternatively, BT is commonly used to treat chronic conditions that may contribute to depression; its success in relieving the underlying problem may indirectly relieve MDD. Thus, in a postmarketing safety evaluation of BT, MDD was demonstrated 40% to 88% less often by patients treated with BT for 6 of the 8 conditions and injection sites, such as in spasms and spasticity of arms and legs, torticollis and neck pain, and axilla and palm injections for hyperhidrosis. In a parotid and submandibular glands BT injection subcohort, no patients experienced depressive symptoms.120
Medicinal BT is generally considered safe. The most common adverse effects are hypersensitivity, injection site reactions, and other adverse effects specific to the injection site.121 Additionally, the cosmetic effects are transient, given the nature of the medication.
Trigger point injections
TPIs in the neck and shoulders are frequently used to treat tension headaches and various referred pain locations in the face and arms. Tension and depression frequently overlap in clinical practice.122 Relieving muscle tension (with resulting trigger points) improves muscle function and mood.
Continue to: The higher the number of active...
The higher the number of active trigger points (TPs), the greater the physical burden of headache and the higher the anxiety level. Gender differences in TP prevalence and TPI efficacy have been described in the literature. For example, the number of active TPs seems directly associated with anxiety levels in women but not in men.123 Although TPs appear to be more closely associated with anxiety than depression,124 depression associated with muscle tension does improve with TPIs. European studies have demonstrated a decrease in scores on the Hamilton Depression Rating Scale and Hamilton Anxiety Rating Scale following TPI treatment.125 The effect may be indirect, as when a patient’s pain is relieved, sleep and other psychiatric symptoms improve.126
A randomized controlled trial by Castro Sánchez et al127 demonstrated that dry needling therapy in patients with fibromyalgia syndrome (FMS) showed improvements in pain pressure thresholds, body pain, vitality, and social function, as well as total FMS symptoms, quality of sleep, anxiety, hospital anxiety and depression, general pain intensity, and fatigue.
Myofascial pain syndrome, catastrophizing, and muscle tension are common in patients with depression, anxiety, and somatization. Local TPI therapy aimed at inactivating pain generators is supported by moderate quality evidence. All manner of therapies have been described, including injection of saline, corticosteroids, local anesthetic agents, and dry needling. BT injections in chronic TPs are also practiced, though no specific injection therapy has been reliably shown to be more advantageous than another. The benefits of TPIs may be derived from the needle itself rather than from any specific substance injected. Stimulation of a local twitch response with direct needling of the TP appears of importance. There is no established consensus regarding the number of injection points, frequency of administration, and volume or type of injectate.128
Adverse effects of TPIs relate to the nature of the invasive therapy, with the risk of tissue damage and bleeding. Pneumothorax risk is present with needle insertion at the neck and thorax.129 Patients with diabetes may experience variations in blood sugar control if steroids are used.
Bottom Line
Interventional treatment modalities that may have a role in psychiatric treatment include IV administration of ketamine, aducanumab, lecanemab, brexanolone, magnesium, scopolamine, and clomipramine. Other interventional approaches include stellate ganglion blocks, glabellar botulinum toxin injections, and trigger point injections.
Related Resources
- Dokucu ME, Janicak PG. Nontraditional therapies for treatment-resistant depression. Current Psychiatry. 2021; 20(9):38-43,49. doi:10.12788/cp.0166
- Kim J, Khoury R, Grossberg GT. Botulinum toxin: emerging psychiatric indications. Current Psychiatry. 2018;17(12):8-18.
Drug Brand Names
Aducanumab • Aduhelm
Aripiprazole • Abilify
Aripiprazole lauroxil • Aristada
Brexanolone • Zulresso
Buprenorphine • Sublocade
Citalopram • Celexa
Clomipramine • Anafranil
Diazepam • Valium
Droperidol • Inapsine
Esketamine • Spravato
Fentanyl • Actiq
Fluphenazine decanoate • Modecate
Fluphenazine hydrochloride • Prolixin
Haloperidol decanoate • Haldol decanoate
Haloperidol lactate • Haldol
Ketamine • Ketalar
Lecanemab • Leqembi
Lidocaine • Xylocaine
Lorazepam • Ativan
Loxapine inhaled • Adasuve
Naltrexone • Vivitrol
Magnesium sulfate • Sulfamag
Midazolam • Versed
Olanzapine • Zyprexa
OnabotulinumtoxinA injection • Botox
Paliperidone • Invega Hafyera, Invega Sustenna, Invega Trinza
Rapamycin • Rapamune, Sirolimus
Risperidone • Perseris
Risperidone microspheres • Risperdal Consta, Rykindo
Scopolamine • Hyoscine
Tramadol • Conzip
Vortioxetine • Trintellix
Ziprasidone • Geodon
1. Vincent KM, Ryan M, Palmer E, et al. Interventional psychiatry. Postgrad Med. 2020;132(7):573-574.
2. Allen MH, Feifel D, Lesem MD, et al. Efficacy and safety of loxapine for inhalation in the treatment of agitation in patients with schizophrenia: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2011;72(10):1313-1321.
3. Kwentus J, Riesenberg RA, Marandi M, et al. Rapid acute treatment of agitation in patients with bipolar I disorder: a multicenter, randomized, placebo-controlled clinical trial with inhaled loxapine. Bipolar Disord. 2012;14(1):31-40.
4. Lee JD, Nunes EV Jr, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391(10118):309-318.
5. Haight BR, Learned SM, Laffont CM, et al. Efficacy and safety of a monthly buprenorphine depot injection for opioid use disorder: a multicentre, randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet. 2019;393(10173):778-790.
6. Andorn A, Graham J, Csernansky J, et al. Monthly extended-release risperidone (RBP-7000) in the treatment of schizophrenia: results from the phase 3 program. J Clin Psychopharmacol. 2019;39(5):428-433.
7. Dundee TW. Twenty-five years of ketamine. A report of an international meeting. Anaesthesia. 1990;45(2):159. doi:10.1111/j.1365-2044.1990.tb14287.x
8. White PF, Way WL, Trevor AJ. Ketamine--its pharmacology and therapeutic uses. Anesthesiology. 1982;56(2):119-136. doi:10.1097/00000542-198202000-00007
9. Zanos P, Gould TD. Mechanisms of ketamine action as an antidepressant. Mol Psychiatry. 2018;23(4):801-811.
10. Molero P, Ramos-Quiroga JA, Martin-Santos R, et al. Antidepressant efficacy and tolerability of ketamine and esketamine: a critical review. CNS Drugs. 2018;32(5):411-420. doi:10.1007/s40263-018-0519-3
11. Williams NR, Heifets BD, Blasey C, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018;175(12):1205-1215.
12. Witkin JM, Martin AE, Golani LK, et al. Rapid-acting antidepressants. Adv Pharmacol. 2019;86:47-96.
13. Strayer RJ, Nelson LS. Adverse events associated with ketamine for procedural sedation in adults. Am J Emerg Med. 2008;26(9):985-1028. doi:10.1016/j.ajem.2007.12.005
14. Frye MA, Blier P, Tye SJ. Concomitant benzodiazepine use attenuates ketamine response: implications for large scale study design and clinical development. J Clin Psychopharmacol. 2015;35(3):334-336.
15. Fava M, Freeman MP, Flynn M, et al. Double-blind, placebo-controlled, dose-ranging trial of intravenous ketamine as adjunctive therapy in treatment-resistant depression (TRD). Mol Psychiatry. 2020;25(7):1592-1603.
16. Bahji A, Vazquez GH, Zarate CA Jr. Comparative efficacy of racemic ketamine and esketamine for depression: a systematic review and meta-analysis. J Affect Disord. 2021;278:542-555. Erratum in: J Affect Disord. 2021;281:1001.
17. Brendle M, Robison R, Malone DC. Cost-effectiveness of esketamine nasal spray compared to intravenous ketamine for patients with treatment-resistant depression in the US utilizing clinical trial efficacy and real-world effectiveness estimates. J Affect Disord. 2022;319:388-396.
18. Dhillon S. Aducanumab: first approval. Drugs. 2021;81(12):1437-1443. Erratum in: Drugs. 2021;81(14):1701.
19. van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023;388(1):9-21. doi:10.1056/NEJMoa2212948
20. Sevigny J, Chiao P, Bussière T, et al. The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature. 2016;537(7618):50-56. Update in: Nature. 2017;546(7659):564.
21. Fillit H, Green A. Aducanumab and the FDA – where are we now? Nat Rev Neurol. 2021;17(3):129-130.
22. Reardon S. FDA approves Alzheimer’s drug lecanemab amid safety concerns. Nature. 2023;613(7943):227-228. doi:10.1038/d41586-023-00030-3
23. McDade E, Cummings JL, Dhadda S, et al. Lecanemab in patients with early Alzheimer’s disease: detailed results on biomarker, cognitive, and clinical effects from the randomized and open-label extension of the phase 2 proof-of-concept study. Alzheimers Res Ther. 2022;14(1):191. doi:10.1186/s13195-022-01124-2
24. Mintun MA, Lo AC, Evans CD, et al. Donanemab in early Alzheimer’s disease. N Engl J Med. 2021;384(18):1691-1704.
25. Luisi S, Petraglia F, Benedetto C, et al. Serum allopregnanolone levels in pregnant women: changes during pregnancy, at delivery, and in hypertensive patients. J Clin Endocrinol Metab. 2000;85(7):2429-2433.
26. Meltzer-Brody S, Colquhoun H, Riesenberg R, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392(10152):1058-1070.
27. Powell JG, Garland S, Preston K, et al. Brexanolone (Zulresso): finally, an FDA-approved treatment for postpartum depression. Ann Pharmacother. 2020;54(2):157-163.
28. Patterson R, Krohn H, Richardson E, et al. A brexanolone treatment program at an academic medical center: patient selection, 90-day posttreatment outcomes, and lessons learned. J Acad Consult Liaison Psychiatry. 2022;63(1):14-22.
29. World Health Organization. WHO model list of essential medicines - 22nd list (2021). World Health Organization. September 30, 2021. Accessed April 7, 2023. https://www.who.int/publications/i/item/WHO-MHP-HPS-EML-2021.02
30. Eby GA, Eby KL, Mruk H. Magnesium and major depression. In: Vink R, Nechifor M, eds. Magnesium in the Central Nervous System. University of Adelaide Press; 2011.
31. Plant TM, Zeleznik AJ. Knobil and Neill’s Physiology of Reproduction. 4th ed. Elsevier Inc.; 2015:2503-2550.
32. Sidebotham D, Le Grice IJ. Physiology and pathophysiology. In: Sidebotham D, McKee A, Gillham M, Levy J. Cardiothoracic Critical Care. Elsevier, Inc.; 2007:3-27.
33. Duley L, Gülmezoglu AM, Henderson-Smart DJ, et al. Magnesium sulphate and other anticonvulsants for women with pre-eclampsia. Cochrane Database Syst Rev. 2010;2010(11):CD000025.
34. Emergency supply of medicines. In: British National Formulary. British Medical Association, Royal Pharmaceutical Society; 2015:6. Accessed April 7, 2023. https://www.academia.edu/35076015/british_national_formulary_2015_pdf
35. Kwofie K, Wolfson AB. Intravenous magnesium sulfate for acute asthma exacerbation in children and adults. Am Fam Physician. 2021;103(4):245-246.
36. Patniyot IR, Gelfand AA. Acute treatment therapies for pediatric migraine: a qualitative systematic review. Headache. 2016;56(1):49-70.
37. Wang X, Du X, Yang H, et al. Use of intravenous magnesium sulfate among patients with acute myocardial infarction in China from 2001 to 2015: China PEACE-Retrospective AMI Study. BMJ Open. 2020;10(3):e033269.
38. Karhu E, Atlas SE, Jinrun G, et al. Intravenous infusion of magnesium sulfate is not associated with cardiovascular, liver, kidney, and metabolic toxicity in adults. J Clin Transl Res. 2018;4(1):47-55.
39. Noah L, Pickering G, Mazur A, et al. Impact of magnesium supplementation, in combination with vitamin B6, on stress and magnesium status: secondary data from a randomized controlled trial. Magnes Res. 2020;33(3):45-57.
40. Erstad BL, Cotugno CL. Management of alcohol withdrawal. Am J Health Syst Pharm. 1995;52(7):697-709.
41. Abumaria N, Luo L, Ahn M, et al. Magnesium supplement enhances spatial-context pattern separation and prevents fear overgeneralization. Behav Pharmacol. 2013;24(4):255-263.
42. Kirov GK, Tsachev KN. Magnesium, schizophrenia and manic-depressive disease. Neuropsychobiology. 1990;23(2):79-81.
43. Botturi A, Ciappolino V, Delvecchio G, et al. The role and the effect of magnesium in mental disorders: a systematic review. Nutrients. 2020;12(6):1661.
44. Kirkland AE, Sarlo GL, Holton KF. The role of magnesium in neurological disorders. Nutrients. 2018;10(6):730.
45. Magnesium sulfate intravenous side effects by likelihood and severity. WebMD. Accessed April 9, 2023. https://www.webmd.com/drugs/2/drug-149570/magnesium-sulfate-intravenous/details/list-sideeffects
46. Scopolamine base transdermal system – uses, side effects, and more. WebMD. Accessed April 9, 2023. https://www.webmd.com/drugs/2/drug-14032/scopolamine-transdermal/details
47. Bolden C, Cusack B, Richelson E. Antagonism by antimuscarinic and neuroleptic compounds at the five cloned human muscarinic cholinergic receptors expressed in Chinese hamster ovary cells. J Pharmacol Exp Ther. 1992;260(2):576-580.
48. Janowsky DS, el-Yousef MK, Davis JM, et al. A cholinergic-adrenergic hypothesis of mania and depression. Lancet. 1972;2(7778):632-635.
49. Janowsky DS, Risch SC, Gillin JC. Adrenergic-cholinergic balance and the treatment of affective disorders. Prog Neuropsychopharmacol Biol Psychiatry. 1983;7(2-3):297-307.
50. Gershon S, Shaw FH. Psychiatric sequelae of chronic exposure to organophosphorous insecticides. Lancet. 1972;1(7191):1371-1374.
51. Davis KL, Berger PA, Hollister LE, et al. Physostigmine in mania. Arch Gen Psychiatry. 1978;35(1):119-122.
52. Wang JC, Hinrichs AL, Stock H, et al. Evidence of common and specific genetic effects: association of the muscarinic acetylcholine receptor M2 (CHRM2) gene with alcohol dependence and major depressive syndrome. Hum Mol Genet. 2004;13(17):1903-1911.
53. Brown RG. Effects of antidepressants and anticholinergics in a mouse “behavioral despair” test. Eur J Pharmacol. 1979;58(3):331-334.
54. Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266(5604):730-732.
55. Ji CX, Zhang JJ. Effect of scopolamine on depression in mice. Abstract in English. Yao Xue Xue Bao. 2011;46(4):400-405.
56. Furey ML, Drevets WC. Antidepressant efficacy of the antimuscarinic drug scopolamine: a randomized, placebo-controlled clinical trial. Arch Gen Psychiatry. 2006;63(10):1121-1129.
57. Drevets WC, Furey ML. Replication of scopolamine’s antidepressant efficacy in major depressive disorder: a randomized, placebo-controlled clinical trial. Biol Psychiatry. 2010;67(5):432-438.
58. Furey ML, Khanna A, Hoffman EM, et al. Scopolamine produces larger antidepressant and antianxiety effects in women than in men. Neuropsychopharmacology. 2010;35(12):2479-2488.
59. Gibbs RB, Gabor R, Cox T, et al. Effects of raloxifene and estradiol on hippocampal acetylcholine release and spatial learning in the rat. Psychoneuroendocrinology. 2004;29(6):741-748.
60. Pongrac JL, Gibbs RB, Defranco DB. Estrogen-mediated regulation of cholinergic expression in basal forebrain neurons requires extracellular-signal-regulated kinase activity. Neuroscience. 2004;124(4):809-816.
61. Daniel JM, Dohanich GP. Acetylcholine mediates the estrogen-induced increase in NMDA receptor binding in CA1 of the hippocampus and the associated improvement in working memory. J Neurosci. 2001;21(17):6949-6956.
62. Gerhard DM, Wohleb ES, Duman RS. Emerging treatment mechanisms for depression: focus on glutamate and synaptic plasticity. Drug Discov Today. 2016;21(3):454-464.
63. Voderholzer U. Sleep deprivation and antidepressant treatment. Dialogues Clin Neurosci. 2003;5(4):366-369.
64. Hasselmann H. Scopolamine and depression: a role for muscarinic antagonism? CNS Neurol Disord Drug Targets. 2014;13(4):673-683.
65. Transderm scopolamine [prescribing information]. Warren, NJ: GSK Consumer Healthcare; 2019.
66. Jaffe RJ, Novakovic V, Peselow ED. Scopolamine as an antidepressant: a systematic review. Clin Neuropharmacol. 2013;36(1):24-26.
67. Karameh WK, Khani M. Intravenous clomipramine for treatment-resistant obsessive-compulsive disorder. Int J Neuropsychopharmacol. 2015;19(2):pyv084.
68. Andrews ET, Beattie RM, Tighe MP. Functional abdominal pain: what clinicians need to know. Arch Dis Child. 2020;105(10):938-944. doi:10.1136/archdischild-2020-318825
69. Aliane V, Pérez S, Bohren Y, et al. Key role of striatal cholinergic interneurons in processes leading to arrest of motor stereotypies. Brain. 2011;134(Pt 1):110-118. doi:10.1093/brain/awq285
70. Tzavara ET, Bymaster FP, Davis RJ, et al. M4 muscarinic receptors regulate the dynamics of cholinergic and dopaminergic neurotransmission: relevance to the pathophysiology and treatment of related CNS pathologies. FASEB J. 2004;18(12):1410-1412. doi:10.1096/fj.04-1575fje
71. Korczyn AD, Kish I. The mechanism of imipramine in enuresis nocturna. Clin Exp Pharmacol Physiol. 1979;6(1):31-35. doi:10.1111/j.1440-1681.1979.tb00004.x
72. Trimble MR. Worldwide use of clomipramine. J Clin Psychiatry. 1990;51(Suppl):51-54; discussion 55-58.
73. Gong W, Zhang S, Zong Y, et al. Involvement of the microglial NLRP3 inflammasome in the anti-inflammatory effect of the antidepressant clomipramine. J Affect Disord. 2019;254:15-25.
74. Piwowarska J, Wrzosek M, Radziwon’-Zaleska M. Serum cortisol concentration in patients with major depression after treatment with clomipramine. Pharmacol Rep. 2009;61(4):604-611.
75. Danish University Antidepressant Group (DUAG). Clomipramine dose-effect study in patients with depression: clinical end points and pharmacokinetics. Clin Pharmacol Ther. 1999;66(2):152-165.
76. Moukaddam NJ, Hirschfeld RMA. Intravenous antidepressants: a review. Depress Anxiety. 2004;19(1):1-9.
77. Gerretsen P, Pollock BG. Rediscovering adverse anticholinergic effects. J Clin Psychiatry. 2011;72(6):869-870. doi:10.4088/JCP.11ac07093
78. Thomas SJ, Shin M, McInnis MG, et al. Combination therapy with monoamine oxidase inhibitors and other antidepressants or stimulants: strategies for the management of treatment-resistant depression. Pharmacotherapy. 2015;35(4):433-449. doi:10.1002/phar.1576
79. Robles LA. Serotonin syndrome induced by fentanyl in a child: case report. Clin Neuropharmacol. 2015;38(5):206-208. doi:10.1097/WNF.0000000000000100
80. Fallon BA, Liebowitz MR, Campeas R, et al. Intravenous clomipramine for obsessive-compulsive disorder refractory to oral clomipramine: a placebo-controlled study. Arch Gen Psychiatry. 1998;55(10):918-924.
81. Vieta E, Florea I, Schmidt SN, et al. Intravenous vortioxetine to accelerate onset of effect in major depressive disorder: a 2-week, randomized, double-blind, placebo-controlled study. Int Clin Psychopharmacol. 2019;34(4):153-160.
82. Kasper S, Müller-Spahn F. Intravenous antidepressant treatment: focus on citalopram. Eur Arch Psychiatry Clin Neurosci. 2002;252(3):105-109.
83. Togay B, El-Mallakh RS. Posttraumatic stress disorder: from pathophysiology to pharmacology. Current Psychiatry. 2020;19(5):33-39.
84. Adhikari A, Lerner TN, Finkelstein J, et al. Basomedial amygdala mediates top-down control of anxiety and fear. Nature. 2015;527(7577):179-185. doi:10.1038/nature15698
85. Lipov E. In search of an effective treatment for combat-related post-traumatic stress disorder (PTSD): can the stellate ganglion block be the answer? Pain Pract. 2010;10(4):265-266.
86. Lipov E, Ritchie EC. A review of the use of stellate ganglion block in the treatment of PTSD. Curr Psychiatry Rep. 2015;17(8):599.
87. Olmsted KLR, Bartoszek M, McLean B, et al. Effect of stellate ganglion block treatment on posttraumatic stress disorder symptoms: a randomized clinical trial. JAMA Psychiatry. 2020;77(2):130-138.
88. Lipov E, Candido K. The successful use of left-sided stellate ganglion block in patients that fail to respond to right-sided stellate ganglion block for the treatment of post-traumatic stress disorder symptoms: a retrospective analysis of 205 patients. Mil Med. 2021;186(11-12):319-320.
89. Li Y, Loshak H. Stellate ganglion block for the treatment of post-traumatic stress disorder, depression, and anxiety. Canadian J Health Technol. 2021;1(3):1-30.
90. Kerzner J, Liu H, Demchenko I, et al. Stellate ganglion block for psychiatric disorders: a systematic review of the clinical research landscape. Chronic Stress (Thousand Oaks). 2021;5:24705470211055176.
91. Wie C, Gupta R, Maloney J, et al. Interventional modalities to treat complex regional pain syndrome. Curr Pain Headache Rep. 2021;25(2):10. doi:10.1007/s11916-020-00904-5
92. Chaturvedi A, Dash HH. Sympathetic blockade for the relief of chronic pain. J Indian Med Assoc. 2001;99(12):698-703.
93. Chester M, Hammond C. Leach A. Long-term benefits of stellate ganglion block in severe chronic refractory angina. Pain. 2000;87(1):103-105. doi:10.1016/S0304-3959(00)00270-0
94. Jeon Y. Therapeutic potential of stellate ganglion block in orofacial pain: a mini review. J Dent Anesth Pain Med. 2016;16(3):159-163. doi:10.17245/jdapm.2016.16.3.159
95. Shan HH, Chen HF, Ni Y, et al. Effects of stellate ganglion block through different approaches under guidance of ultrasound. Front Surg. 2022;8:797793. doi:10.3389/fsurg.2021.797793
96. Goel V, Patwardhan AM, Ibrahim M, et al. Complications associated with stellate ganglion nerve block: a systematic review. Reg Anesth Pain Med. 2019;rapm-2018-100127. doi:10.1136/rapm-2018-100127
97. Rowe FJ, Noonan CP. Botulinum toxin for the treatment of strabismus. Cochrane Database Syst Rev. 2017;3(3):CD006499.
98. Roggenkämper P, Jost WH, Bihari K, et al. Efficacy and safety of a new botulinum toxin type A free of complexing proteins in the treatment of blepharospasm. J Neural Transm (Vienna). 2006;113(3):303-312.
99. Heckmann M, Ceballos-Baumann AO, Plewig G; Hyperhidrosis Study Group. Botulinum toxin A for axillary hyperhidrosis (excessive sweating). N Engl J Med. 2001;344(7):488-493.
100. Carruthers JA, Lowe NJ, Menter MA, et al. A multicenter, double-blind, randomized, placebo-controlled study of the efficacy and safety of botulinum toxin type A in the treatment of glabellar lines. J Am Acad Dermatol. 2002;46(6):840-849.
101. Schurch B, de Sèze M, Denys P, et al. Botulinum toxin type A is a safe and effective treatment for neurogenic urinary incontinence: results of a single treatment, randomized, placebo controlled 6-month study. J Urol. 2005;174:196–200.
102. Aurora SK, Winner P, Freeman MC, et al. OnabotulinumtoxinA for treatment of chronic migraine: Pooled analyses of the 56-week PREEMPT clinical program. Headache. 2011;51(9):1358-1373.
103. Dashtipour K, Chen JJ, Walker HW, et al. Systematic literature review of abobotulinumtoxinA in clinical trials for adult upper limb spasticity. Am J Phys Med Rehabil. 2015;94(3):229-238.
104. Nitti VW, Dmochowski R, Herschorn S, et al. OnabotulinumtoxinA for the treatment of patients with overactive bladder and urinary incontinence: results of a phase 3, randomized, placebo-controlled trial. J Urol. 2017;197(2S):S216-S223.
105. Jongerius PH, van den Hoogen FJA, van Limbeek J, et al. Effect of botulinum toxin in the treatment of drooling: a controlled clinical trial. Pediatrics. 2004;114(3):620-627.
106. Zaninotto, G. Annese V, Costantini M, et al. Randomized controlled trial of botulinum toxin versus laparoscopic heller myotomy for esophageal achalasia. Ann Surg. 2004;239(3):364-370.
107. Dressler D, Adib Saberi F. Botulinum toxin: mechanisms of action. Eur Neurol. 2005;53:3-9.
108. Lewis MB, Bowler PJ. Botulinum toxin cosmetic therapy correlates with a more positive mood. J Cosmet Dermatol. 2009;8(1):24-26.
109. Affatato O, Moulin TC, Pisanu C, et al. High efficacy of onabotulinumtoxinA treatment in patients with comorbid migraine and depression: a meta-analysis. J Transl Med. 2021;19(1):133.
110. Finzi E, Wasserman E. Treatment of depression with botulinum toxin A: a case series. Dermatol Surg. 2006;32(5):645-649; discussion 649-650.
111. Schulze J, Neumann I, Magid M, et al. Botulinum toxin for the management of depression: an updated review of the evidence and meta-analysis. J Psychiatr Res. 2021;135:332-340.
112. Finzi E, Rosenthal NE. Emotional proprioception: treatment of depression with afferent facial feedback. J Psychiatr Res. 2016;80:93-96.
113. Söderkvist S, Ohlén K, Dimberg U. How the experience of emotion is modulated by facial feedback. J Nonverbal Behav. 2018;42(1):129-151.
114. Lewis, MB. The interactions between botulinum-toxin-based facial treatments and embodied emotions. Sci Rep. 2018;8(1):14720.
115. Li Y, Liu J, Liu X, et al. Antidepressant-like action of single facial injection of botulinum neurotoxin A is associated with augmented 5-HT levels and BDNF/ERK/CREB pathways in mouse brain. Neurosci Bull. 2019;35(4):661-672. Erratum in: Neurosci Bull. 2019;35(4):779-780.
116. Gündel H, Wolf A, Xidara V, et al. High psychiatric comorbidity in spasmodic torticollis: a controlled study. J Nerv Ment Dis. 2003;191(7):465-473.
117. Hall TA, McGwin G Jr, Searcey K, et al. Health-related quality of life and psychosocial characteristics of patients with benign essential blepharospasm. Arch Ophthalmol. 2006;124(1):116-119.
118. Ceylan D, Erer S, Zarifog˘lu M, et al. Evaluation of anxiety and depression scales and quality of life in cervical dystonia patients on botulinum toxin therapy and their relatives. Neurol Sci. 2019;40(4):725-731.
119. Heller AS, Lapate RC, Mayer KE, et al. The face of negative affect: trial-by-trial corrugator responses to negative pictures are positively associated with amygdala and negatively associated with ventromedial prefrontal cortex activity. J Cogn Neurosci. 2014;26(9):2102-2110.
120. Makunts T, Wollmer MA, Abagyan R. Postmarketing safety surveillance data reveals antidepressant effects of botulinum toxin across various indications and injection sites. Sci Rep. 2020;10(1):12851.
121. Ahsanuddin S, Roy S, Nasser W, et al. Adverse events associated with botox as reported in a Food and Drug Administration database. Aesthetic Plast Surg. 2021;45(3):1201-1209. doi:10.1007/s00266-020-02027-z
122. Kashif M, Tahir S, Ashfaq F, et al. Association of myofascial trigger points in neck and shoulder region with depression, anxiety, and stress among university students. J Pak Med Assoc. 2021;71(9):2139-2142.
123. Cigarán-Méndez M, Jiménez-Antona C, Parás-Bravo P, et al. Active trigger points are associated with anxiety and widespread pressure pain sensitivity in women, but not men, with tension type headache. Pain Pract. 2019;19(5):522-529.
124. Palacios-Ceña M, Castaldo M, Wang K, et al. Relationship of active trigger points with related disability and anxiety in people with tension-type headache. Medicine (Baltimore). 2017;96(13):e6548.
125. Karadas Ö, Inan LE, Ulas Ü, et al. Efficacy of local lidocaine application on anxiety and depression and its curative effect on patients with chronic tension-type headache. Eur Neurol. 2013;70(1-2):95-101.
126. Gerwin RD. Classification, epidemiology and natural history of myofascial pain syndrome. Curr Pain Headache Rep. 2001;5(5):412-420.
127. Castro Sánchez AM, García López H, Fernández Sánchez M, et al. Improvement in clinical outcomes after dry needling versus myofascial release on pain pressure thresholds, quality of life, fatigue, pain intensity, quality of sleep, anxiety, and depression in patients with fibromyalgia syndrome. Disabil Rehabil. 2019;41(19):2235-2246.
128. Healy GM, Finn DP, O’Gorman DA, et al. Pretreatment anxiety and pain acceptance are associated with response to trigger point injection therapy for chronic myofascial pain. Pain Med. 2015;16(10):1955-1966.
129. Morjaria JB, Lakshminarayana UB, Liu-Shiu-Cheong P, et al. Pneumothorax: a tale of pain or spontaneity. Ther Adv Chronic Dis. 2014;5(6):269-273.
Advances in the understanding of neurobiological and neuropsychiatric pathophysiology have opened new avenues of treatment for psychiatric patients. Historically, with a few exceptions, most psychiatric medications have been administered orally. However, many of the newer treatments require some form of specialized administration because they cannot be taken orally due to their chemical property (such as aducanumab); because there is the need to produce stable blood levels of the medication (such as brexanolone); because oral administration greatly diminished efficacy (such as oral vs IV magnesium or scopolamine), or because the treatment is focused on specific brain structures. This need for specialized administration has created a subspecialty called interventional psychiatry.
Part 1 of this 2-part article provides an overview of 1 type of interventional psychiatry: parenterally administered medications, including those administered via IV. We also describe 3 other interventional approaches to treatment: stellate ganglion blocks, glabellar botulinum toxin (BT) injections, and trigger point injections. In Part 2 we will review interventional approaches that involve neuromodulation.
Parenteral medications in psychiatry
In general, IV and IM medications can be more potent that oral medications due to their overall faster onset of action and higher blood concentrations. These more invasive forms of administration can have significant limitations, such as a risk of infection at the injection site, the need to be administered in a medical setting, additional time, and patient discomfort.

Table 1 lists short-acting injectable medications used in psychiatry, and Table 2 lists long-acting injectable medications. Parenteral administration of antipsychotics is performed to alleviate acute agitation or for chronic symptom control. These medications generally are not considered interventional treatments, but could be classified as such due to their invasive nature.1 Furthermore, inhalable loxapine—which is indicated for managing acute agitation—requires a Risk Evaluation and Mitigation Strategy program consisting of 2 hours observation and monitoring of respiratory status.2,3 Other indications for parenteral treatments include IM naltrexone extended release4 and subcutaneous injections of buprenorphine extended release5 and risperidone.6
IV administration
Most IV treatments described in this article are not FDA-approved for psychiatric treatment. Despite this, many interventional psychiatric treatments are part of clinical practice. IV infusion of ketamine is the most widely known and most researched of these. Table 3 lists other IV treatments that could be used as psychiatric treatment.
Ketamine
Since the early 1960s, ketamine has been used as a surgical anesthetic for animals. In the United States, it was approved for human surgical anesthesia in 1970. It was widely used during the Vietnam War due to its lack of inhibition of respiratory drive; medical staff first noticed an improvement in depressive symptoms and the resolution of suicidal ideation in patients who received ketamine. This led to further research on ketamine, particularly to determine its application in treatment-resistant depression (TRD) and other conditions.7 IV ketamine administration is most widely researched, but IM injections, intranasal sprays, and lozenges are also available. The dissociative properties of ketamine have led to its recreational use.8
Hypotheses for the mechanism of action of ketamine as an antidepressant include direct synaptic or extrasynaptic (GluN2B-selective), N-methyl-
Continue to: Ketamine is a schedule...
Ketamine is a schedule III medication with addictive properties. Delirium, panic attacks, hallucinations, nightmares, dysphoria, and paranoia may occur during and after use.13 Premedication with benzodiazepines, most notably lorazepam, is occasionally used to minimize ketamine’s adverse effects, but this generally is not recommended because doing so reduces ketamine’s antidepressant effects.14 Driving and operating heavy machinery is contraindicated after IV infusion. The usual protocol involves an IV infusion of ketamine 0.4 mg/kg to 1 mg/kg dosing over 1 hour. Doses between 0.4 mg/kg and 0.6 mg/kg are most common. Ketamine has a therapeutic window; doses >0.5 mg/kg are progressively less effective.15 Unlike the recommendation after esketamine administration, after receiving ketamine, patients remain in the care of their treatment team for <2 hours.
Esketamine, the S enantiomer of ketamine, was FDA-approved for TRD as an intranasal formulation. Esketamine is more commonly used than IV ketamine because it is FDA-approved and typically covered by insurance, but it may not be as effective.16 An economic analysis by Brendle et al17 suggested insurance companies would lower costs if they covered ketamine infusions vs intranasal esketamine.
Aducanumab and lecanemab
The most recent FDA-approved interventional agents are aducanumab and lecanemab, which are indicated for treating Alzheimer disease.18,19 Both are human monoclonal antibodies that bind selectively and with high affinity to amyloid beta plaque aggregates and promote their removal by Fc receptor–mediated phagocytosis.20
FDA approval of aducanumab and lecanemab was controversial. Initially, aducanumab’s safety monitoring board performed a futility analysis that suggested aducanumab was unlikely to separate from placebo, and the research was stopped.21 The manufacturer petitioned the FDA to consider the medication for accelerated approval on the basis of biomarker data showing that amyloid beta plaque aggregates become smaller. Current FDA approval is temporary to allow patients access to this potentially beneficial agent, but the manufacturer must supply clinical evidence that the reduction of amyloid beta plaques is associated with desirable changes in the course of Alzheimer disease, or risk losing the approval.
Lecanemab is also a human monoclonal antibody intended to remove amyloid beta plaques that was FDA-approved under the accelerated approval pathway.22 Unlike aducanumab, lecanemab demonstrated a statistically significant (although clinically imperceptible) reduction in the rate of cognitive decline; it did not show cognitive improvement.23 Lecanemab also significantly reduced amyloid beta plaques.23
Continue to: Aducanumab and lecanemab are generally...
Aducanumab and lecanemab are generally not covered by insurance and typically cost >$26,000 annually. Both are administered by IV infusion once a month. More monoclonal antibody medications for treating early Alzheimer disease are in the late stages of development, most notably donanebab.24 Observations during clinical trials found that in the later stages of Alzheimer disease, forceful removal of plaques by the autoimmune process damages neurons, while in less dense deposits of early dementia such removal is not harmful to the cells and prevents amyloid buildup.
Brexanolone is an aqueous formulation of allopregnanolone, a major metabolite of progesterone and a positive allosteric modulator of GABA-A receptors.25 Its levels are maximal at the end of the third trimester of pregnancy and fall rapidly following delivery. Research showed a 3-day infusion was rapidly and significantly effective for treating postpartum depression26 and brexanolone received FDA approval for this indication in March 2019.27 However, various administrative, economic, insurance, and other hurdles make it difficult for patients to access this treatment. Despite its rapid onset of action (usually 48 hours), brexanolone takes an average of 15 days to go through the prior authorization process.28 In addition to the need for prior authorization, the main impediment to the use of brexanolone is the 3-day infusion schedule, which greatly magnifies the cost but is partially circumvented by the availability of dedicated outpatient centers.
Magnesium
Magnesium is on the World Health Organization’s Model List of Essential Medicines.29 There has been extensive research on the use of magnesium sulfate for psychiatric indications, especially for depression.30 Magnesium functions similarly to calcium channel blockers by competitively blocking intracellular calcium channels, decreasing calcium availability, and inhibiting smooth muscle contractility.31 It also competes with calcium at the motor end plate, reducing excitation by inhibiting the release of acetylcholine.32 This property is used for high-dose IV magnesium treatment of impending preterm labor in obstetrics. Magnesium sulfate is the drug of choice in treating eclamptic seizures and preventing seizures in severe preeclampsia or gestational hypertension with severe features.33 It is also used to treat torsade de pointes, severe asthma exacerbations, constipation, and barium poisoning.34 Beneficial use in asthma treatment35 and the treatment of migraine36 have also been reported.
IV magnesium in myocardial infarction may be harmful,37 though outside of acute cardiac events, magnesium is found to be safe.38
Oral magnesium sulfate is a common over-the-counter anxiety remedy. As a general cell stabilizer (mediated by the reduction of intracellular calcium), magnesium is potentially beneficial outside of its muscle-relaxing properties, although muscle relaxing can benefit anxious patients. It is used to treat anxiety,39 alcohol withdrawal,40 and fear.41 Low magnesium blood levels are found in patients with depression, schizophrenia,42 and attention-deficit/hyperactivity disorder.43 However, it is important to note that the therapeutic effect of magnesium when treating anxiety and headache is independent of preinfusion magnesium blood levels.43
Continue to: The efficacy of oral magnesium...
The efficacy of oral magnesium is not robust. However, IV administration has a pronounced beneficial effect as an abortive and preventative treatment in many patients with anxiety.44
IV administration of magnesium can produce adverse effects, including flushing, sweating, hypotension, depressed reflexes, flaccid paralysis, hypothermia, circulatory collapse, and cardiac and CNS depression. These complications are very rare and dose-dependent.45 Magnesium is excreted by the kidneys, and dosing must be decreased in patients with kidney failure. The most common adverse effect is local burning along the vein upon infusion; small doses of IV lidocaine can remedy this. Hot flashes are also common.45
Various dosing strategies are available. In patients with anxiety, application dosing is based on the recommended preeclampsia IV dose of 4 g diluted in 250 mL of 5% dextrose. Much higher doses may be used in obstetrics. Unlike in obstetrics, for psychiatric indications, magnesium is administered over 60 to 90 minutes. Heart monitoring is recommended.
Scopolamine
Scopolamine is primarily used to relieve nausea, vomiting, and dizziness associated with motion sickness and recovery from anesthesia. It is also used in ophthalmology and in patients with excessive sweating. In off-label and experimental applications, scopolamine has been used in patients with TRD.46
Scopolamine is an anticholinergic medication. It is a nonselective antagonist at muscarinic receptors.47 Tricyclic antidepressants (TCAs) possess strong anticholinergic function. Newer generations of antidepressants were designed specifically not to have this function because it was believed to be an unwanted and potentially dangerous adverse effect. However, data suggest that anticholinergic action is important in decreasing depressive symptoms. Several hypotheses of anticholinergic effects on depression have been published since the 1970s. They include the cholinergic-adrenergic hypothesis,48 acetylcholine predominance relative to adrenergic action hypothesis,49 and insecticide poisoning observations.50 Centrally acting physostigmine (but not peripherally acting neostigmine) was reported to control mania.48,51 A genetic connection between the M2acetylcholine receptor in patients with major depressive disorder (MDD) and alcohol use disorder is also suggestive.52
Continue to: Multiple animal studies show...
Multiple animal studies show direct improvement in mobility and a decrease in despair upon introducing anticholinergic substances.53-55 The cholinergic theory of depression has been studied in several controlled clinical human studies.56,57 Use of a short-acting anticholinergic glycopyrrolate during electroconvulsive therapy (ECT) may contribute to the procedure’s efficacy.
Human research shows scopolamine has a higher efficacy as an antidepressant and anti-anxiety medication in women than in men,58 possibly because estrogen increases the activity of choline acetyltransferase and release of acetylcholine.59,60 M2receptors mediate estrogen influence on the NMDAR, which may explain the anticholinergic effects of depression treatments in women.61
Another proposed mechanism of action of scopolamine is a potent inhibition of the NMDAR.62 Rapid treatments of depression may be based on this mechanism. Examples of such treatments include IV ketamine and sleep deprivation.63 IV scopolamine shows potency in treating MDD and bipolar depression. This treatment should be reserved for patients who do not respond to or are not candidates for other usual treatment modalities of MDD and for the most severe cases. Scopolamine is 30 times more potent than amitriptyline in anticholinergic effect and reportedly produces sustained improvement in MDD.64
Scopolamine has no black-box warnings. It has not been studied in pregnant women and is not recommended for use during pregnancy. Due to possible negative cardiovascular effects, a normal electrocardiogram is required before the start of treatment. Exercise caution in patients with glaucoma, benign prostatic enlargement, gastroparesis, unstable cardiovascular status, or severe renal impairment.
Treatment with scopolamine is not indicated for patients with myasthenia gravis, psychosis, or seizures. Patients must be off potassium for 3 days before beginning scopolamine treatment. Patients should consult with their cardiologist before having a scopolamine infusion. Adverse reactions may include psychosis, tachycardia, seizures, paralytic ileus, and glaucoma exacerbation. The most common adverse effects of scopolamine infusion treatment include drowsiness, dry mouth, blurred vision, lightheadedness, and dizziness. Due to possible drowsiness, operating motor vehicles or heavy machinery must be avoided on the day of treatment.65 Overall, the adverse effects of scopolamine are preventable and manageable, and its antidepressant efficacy is noteworthy.66
Continue to: Treatment typically consists of 3 consecutive infusions...
Treatment typically consists of 3 consecutive infusions of 4 mcg/kg separated by 3 to 5 days.56 It is possible to have a longer treatment course if the patient experiences only partial improvement. Repeated courses or maintenance treatment (similar to ECT maintenance) are utilized in some patients if indicated. Cardiac monitoring is mandatory.
Clomipramine
Clomipramine, a TCA, acts as a preferential inhibitor of 5-hydroxytryptamine uptake and has proven effective in managing depression, TRD, and obsessive-compulsive disorder (OCD).67 Although this medication has reported treatment benefits for patients with phobia, panic disorder,15 chronic pain,68 Tourette syndrome,69 premature ejaculation, anorexia nervosa,70 cataplexy,49 and enuresis,71 it is FDA-approved only for the treatment of OCD.72 Clomipramine may also be beneficial for pain and headache, possibly because of its anti-inflammatory action.73 The anticholinergic effects of clomipramine may add to its efficacy in depression.
The pathophysiology of MDD is connected to hyperactivity of the HPA axis and elevated cortisol levels. Higher clomipramine plasma levels show a linear correlation with lower cortisol secretion and levels, possibly aiding in the treatment of MDD and anxiety.74 The higher the blood level, the more pronounced clomipramine’s therapeutic effect across multiple domains.75
IV infusion of clomipramine produces the highest concentration in the shortest time, but overall, research does not necessarily support increased efficacy of IV over oral administration. There is evidence suggesting that subgroups of patients with severe, treatment-refractory OCD may benefit from IV agents and research suggests a faster onset of action.76 Faster onset of symptom relief is the basis for IV clomipramine use. In patients with OCD, it can take several months for oral medications to produce therapeutic benefits; not all patients can tolerate this. In such scenarios, IV administration may be considered, though it is not appropriate for routine use until more research is available. Patients with treatment-resistant OCD who have exhausted other means of symptom relief may also be candidates for IV treatment.
The adverse effects of IV clomipramine are no different from oral use, though they may be more pronounced. A pretreatment cardiac exam is desirable because clomipramine, like other TCAs, may be cardiotoxic. The anticholinergic adverse effects of TCAs are well known to clinicians77 and partially explained in the scopolamine section of this article. It is not advisable to combine clomipramine with other TCAs or serotonin reuptake inhibitors. Clomipramine also should not be combined with monoamine oxidase inhibitors, though such a combination was reported in medical literature.78 Combination with antiarrhythmics such as lidocaine or opioids such as fentanyl or and tramadol is highly discouraged (fentanyl and tramadol also have serotonergic effects).79
Continue to: Clomipramine for IV use is not commercially available...
Clomipramine for IV use is not commercially available and must be sterilely compounded. The usual course of treatment is a series of 3 infusions: 150 mg on Day 1, 200 mg on Day 2 or Day 3, and 250 mg on Day 3, Day 4, or Day 5, depending on tolerability. A protocol with a 50 mg/d starting dose and titration up to a maximum dose of 225 mg/d over 5 to 7 days has been suggested for inpatient settings.67 Titration to 250 mg is more common.80
A longer series may be performed, but this increases the likelihood of adverse effects. Booster and maintenance treatments are also completed when required. Cardiac monitoring is mandatory.
Vortioxetine and citalopram
IV treatment of depression with
Injections and blocks
Three interventional approaches to treatment that possess psychotherapeutic potential include stellate ganglion blocks (SGBs), glabellar BT injections, and trigger point injections (TPIs). None of these are FDA-approved for psychiatric treatment.
Stellate ganglion blocks
The sympathetic nervous system is involved in autonomic hyperarousal and is at the core of posttraumatic symptomatology.83 Insomnia, anxiety, irritability, hypervigilance, and other excitatory CNS events are connected to the sympathetic nervous system and amygdala activation is commonly observed in those exposed to extreme stress or traumatic events.84
Continue to: SGBs were first performed 100 years ago...
SGBs were first performed 100 years ago and reported to have beneficial psychiatric effects at the end of the 1940s. In 1998 in Finland, improvement of posttraumatic stress disorder (PTSD) symptoms was observed accidentally via thoracic level spine blocks.85 In 2006, cervical level sympathetic blocks were shown to be effective for PTSD symptom control.86 By the end of 2010, Veterans Administration hospitals adopted SGBs to treat veterans with PTSD.87,88 The first multisite, randomized clinical trial of
Since the stellate ganglion is connected to the amygdala, SGB has also been assessed for treating anxiety and depression.89,90 Outside of PTSD, SGBs are used to treat complex regional pain syndrome,91 phantom limb pain, trigeminal neuralgia,92 intractable angina,93 and postherpetic neuralgia in the head, neck, upper chest, or arms.94 The procedure consists of an injection of a local anesthetic through a 25-gauge needle into the stellate sympathetic ganglion at the C6 or C7 vertebral levels. An injection into C6 is considered safer because of specific cervical spine anatomy. Ideally, fluoroscopic guidance or ultrasound is used to guide needle insertion.95
A severe drop in blood pressure may be associated with SGBs and is mitigated by IV hydration. Other adverse effects include red eyes, drooping of the eyelids, nasal congestion, hoarseness, difficulty swallowing, a sensation of a “lump” in the throat, and a sensation of warmth or tingling in the arm or hand. Bilateral SGB is contraindicated due to the danger of respiratory arrest.96
Glabellar BT injections
OnabotulinumtoxinA (BT) injection was first approved for therapeutic use in 1989 for eye muscle disorders such as strabismus97 and blepharospasm.98 It was later approved for several other indications, including cosmetic use, hyperhidrosis, migraine prevention, neurogenic bladder disorder, overactive bladder, urinary incontinence, and spasticity.99-104 BT is used off-label for achalasia and sialorrhea.105,106 Its mechanism of action is primarily attributed to muscle paralysis by blocking presynaptic acetylcholine release into neuromuscular junctions.107
Facial BT injections are usually administered for cosmetic purposes, but smoothing forehead wrinkles and frown lines (the glabellar region of the face) both have antidepressant effects.108 BT injections into the glabellar region also demonstrate antidepressant effects, particularly in patients with comorbid migraines and MDD.109 Early case observations supported the independent benefit of the toxin on MDD when the toxin was injected into the glabellar region.110,111 The most frequent protocol involves injections in the procerus and corrugator muscles.
Continue to: The facial feedback/emotional proprioception hypothesis...
The facial feedback/emotional proprioception hypothesis has dominated thinking about the mood-improving effects of BT. The theory is that blocking muscular expression of sadness (especially in the face) interrupts the experience of sadness; therefore, depression subsides.112,113 However, BT injections in the muscles involved in the smile and an expression of positive emotions (lateral part of the musculus orbicularis oculi) have been associated with increased MDD scores.114 Thus, the mechanism clearly involves more than the cosmetic effect, since facial muscle injections in rats also have antidepressant effects.115
The use of progressive muscle relaxation is well-established in psychiatric treatment. The investigated conditions of increased muscle tone, especially torticollis and blepharospasm, are associated with MDD, and it may be speculated that proprioceptive feedback from the affected muscles may be causally involved in this association.116-118 Activity of the corrugator muscle has been positively associated with increased amygdala activity.119 This suggests a potential similar mechanism to that hypothesized for SGB.
Alternatively, BT is commonly used to treat chronic conditions that may contribute to depression; its success in relieving the underlying problem may indirectly relieve MDD. Thus, in a postmarketing safety evaluation of BT, MDD was demonstrated 40% to 88% less often by patients treated with BT for 6 of the 8 conditions and injection sites, such as in spasms and spasticity of arms and legs, torticollis and neck pain, and axilla and palm injections for hyperhidrosis. In a parotid and submandibular glands BT injection subcohort, no patients experienced depressive symptoms.120
Medicinal BT is generally considered safe. The most common adverse effects are hypersensitivity, injection site reactions, and other adverse effects specific to the injection site.121 Additionally, the cosmetic effects are transient, given the nature of the medication.
Trigger point injections
TPIs in the neck and shoulders are frequently used to treat tension headaches and various referred pain locations in the face and arms. Tension and depression frequently overlap in clinical practice.122 Relieving muscle tension (with resulting trigger points) improves muscle function and mood.
Continue to: The higher the number of active...
The higher the number of active trigger points (TPs), the greater the physical burden of headache and the higher the anxiety level. Gender differences in TP prevalence and TPI efficacy have been described in the literature. For example, the number of active TPs seems directly associated with anxiety levels in women but not in men.123 Although TPs appear to be more closely associated with anxiety than depression,124 depression associated with muscle tension does improve with TPIs. European studies have demonstrated a decrease in scores on the Hamilton Depression Rating Scale and Hamilton Anxiety Rating Scale following TPI treatment.125 The effect may be indirect, as when a patient’s pain is relieved, sleep and other psychiatric symptoms improve.126
A randomized controlled trial by Castro Sánchez et al127 demonstrated that dry needling therapy in patients with fibromyalgia syndrome (FMS) showed improvements in pain pressure thresholds, body pain, vitality, and social function, as well as total FMS symptoms, quality of sleep, anxiety, hospital anxiety and depression, general pain intensity, and fatigue.
Myofascial pain syndrome, catastrophizing, and muscle tension are common in patients with depression, anxiety, and somatization. Local TPI therapy aimed at inactivating pain generators is supported by moderate quality evidence. All manner of therapies have been described, including injection of saline, corticosteroids, local anesthetic agents, and dry needling. BT injections in chronic TPs are also practiced, though no specific injection therapy has been reliably shown to be more advantageous than another. The benefits of TPIs may be derived from the needle itself rather than from any specific substance injected. Stimulation of a local twitch response with direct needling of the TP appears of importance. There is no established consensus regarding the number of injection points, frequency of administration, and volume or type of injectate.128
Adverse effects of TPIs relate to the nature of the invasive therapy, with the risk of tissue damage and bleeding. Pneumothorax risk is present with needle insertion at the neck and thorax.129 Patients with diabetes may experience variations in blood sugar control if steroids are used.
Bottom Line
Interventional treatment modalities that may have a role in psychiatric treatment include IV administration of ketamine, aducanumab, lecanemab, brexanolone, magnesium, scopolamine, and clomipramine. Other interventional approaches include stellate ganglion blocks, glabellar botulinum toxin injections, and trigger point injections.
Related Resources
- Dokucu ME, Janicak PG. Nontraditional therapies for treatment-resistant depression. Current Psychiatry. 2021; 20(9):38-43,49. doi:10.12788/cp.0166
- Kim J, Khoury R, Grossberg GT. Botulinum toxin: emerging psychiatric indications. Current Psychiatry. 2018;17(12):8-18.
Drug Brand Names
Aducanumab • Aduhelm
Aripiprazole • Abilify
Aripiprazole lauroxil • Aristada
Brexanolone • Zulresso
Buprenorphine • Sublocade
Citalopram • Celexa
Clomipramine • Anafranil
Diazepam • Valium
Droperidol • Inapsine
Esketamine • Spravato
Fentanyl • Actiq
Fluphenazine decanoate • Modecate
Fluphenazine hydrochloride • Prolixin
Haloperidol decanoate • Haldol decanoate
Haloperidol lactate • Haldol
Ketamine • Ketalar
Lecanemab • Leqembi
Lidocaine • Xylocaine
Lorazepam • Ativan
Loxapine inhaled • Adasuve
Naltrexone • Vivitrol
Magnesium sulfate • Sulfamag
Midazolam • Versed
Olanzapine • Zyprexa
OnabotulinumtoxinA injection • Botox
Paliperidone • Invega Hafyera, Invega Sustenna, Invega Trinza
Rapamycin • Rapamune, Sirolimus
Risperidone • Perseris
Risperidone microspheres • Risperdal Consta, Rykindo
Scopolamine • Hyoscine
Tramadol • Conzip
Vortioxetine • Trintellix
Ziprasidone • Geodon
Advances in the understanding of neurobiological and neuropsychiatric pathophysiology have opened new avenues of treatment for psychiatric patients. Historically, with a few exceptions, most psychiatric medications have been administered orally. However, many of the newer treatments require some form of specialized administration because they cannot be taken orally due to their chemical property (such as aducanumab); because there is the need to produce stable blood levels of the medication (such as brexanolone); because oral administration greatly diminished efficacy (such as oral vs IV magnesium or scopolamine), or because the treatment is focused on specific brain structures. This need for specialized administration has created a subspecialty called interventional psychiatry.
Part 1 of this 2-part article provides an overview of 1 type of interventional psychiatry: parenterally administered medications, including those administered via IV. We also describe 3 other interventional approaches to treatment: stellate ganglion blocks, glabellar botulinum toxin (BT) injections, and trigger point injections. In Part 2 we will review interventional approaches that involve neuromodulation.
Parenteral medications in psychiatry
In general, IV and IM medications can be more potent that oral medications due to their overall faster onset of action and higher blood concentrations. These more invasive forms of administration can have significant limitations, such as a risk of infection at the injection site, the need to be administered in a medical setting, additional time, and patient discomfort.

Table 1 lists short-acting injectable medications used in psychiatry, and Table 2 lists long-acting injectable medications. Parenteral administration of antipsychotics is performed to alleviate acute agitation or for chronic symptom control. These medications generally are not considered interventional treatments, but could be classified as such due to their invasive nature.1 Furthermore, inhalable loxapine—which is indicated for managing acute agitation—requires a Risk Evaluation and Mitigation Strategy program consisting of 2 hours observation and monitoring of respiratory status.2,3 Other indications for parenteral treatments include IM naltrexone extended release4 and subcutaneous injections of buprenorphine extended release5 and risperidone.6
IV administration
Most IV treatments described in this article are not FDA-approved for psychiatric treatment. Despite this, many interventional psychiatric treatments are part of clinical practice. IV infusion of ketamine is the most widely known and most researched of these. Table 3 lists other IV treatments that could be used as psychiatric treatment.
Ketamine
Since the early 1960s, ketamine has been used as a surgical anesthetic for animals. In the United States, it was approved for human surgical anesthesia in 1970. It was widely used during the Vietnam War due to its lack of inhibition of respiratory drive; medical staff first noticed an improvement in depressive symptoms and the resolution of suicidal ideation in patients who received ketamine. This led to further research on ketamine, particularly to determine its application in treatment-resistant depression (TRD) and other conditions.7 IV ketamine administration is most widely researched, but IM injections, intranasal sprays, and lozenges are also available. The dissociative properties of ketamine have led to its recreational use.8
Hypotheses for the mechanism of action of ketamine as an antidepressant include direct synaptic or extrasynaptic (GluN2B-selective), N-methyl-
Continue to: Ketamine is a schedule...
Ketamine is a schedule III medication with addictive properties. Delirium, panic attacks, hallucinations, nightmares, dysphoria, and paranoia may occur during and after use.13 Premedication with benzodiazepines, most notably lorazepam, is occasionally used to minimize ketamine’s adverse effects, but this generally is not recommended because doing so reduces ketamine’s antidepressant effects.14 Driving and operating heavy machinery is contraindicated after IV infusion. The usual protocol involves an IV infusion of ketamine 0.4 mg/kg to 1 mg/kg dosing over 1 hour. Doses between 0.4 mg/kg and 0.6 mg/kg are most common. Ketamine has a therapeutic window; doses >0.5 mg/kg are progressively less effective.15 Unlike the recommendation after esketamine administration, after receiving ketamine, patients remain in the care of their treatment team for <2 hours.
Esketamine, the S enantiomer of ketamine, was FDA-approved for TRD as an intranasal formulation. Esketamine is more commonly used than IV ketamine because it is FDA-approved and typically covered by insurance, but it may not be as effective.16 An economic analysis by Brendle et al17 suggested insurance companies would lower costs if they covered ketamine infusions vs intranasal esketamine.
Aducanumab and lecanemab
The most recent FDA-approved interventional agents are aducanumab and lecanemab, which are indicated for treating Alzheimer disease.18,19 Both are human monoclonal antibodies that bind selectively and with high affinity to amyloid beta plaque aggregates and promote their removal by Fc receptor–mediated phagocytosis.20
FDA approval of aducanumab and lecanemab was controversial. Initially, aducanumab’s safety monitoring board performed a futility analysis that suggested aducanumab was unlikely to separate from placebo, and the research was stopped.21 The manufacturer petitioned the FDA to consider the medication for accelerated approval on the basis of biomarker data showing that amyloid beta plaque aggregates become smaller. Current FDA approval is temporary to allow patients access to this potentially beneficial agent, but the manufacturer must supply clinical evidence that the reduction of amyloid beta plaques is associated with desirable changes in the course of Alzheimer disease, or risk losing the approval.
Lecanemab is also a human monoclonal antibody intended to remove amyloid beta plaques that was FDA-approved under the accelerated approval pathway.22 Unlike aducanumab, lecanemab demonstrated a statistically significant (although clinically imperceptible) reduction in the rate of cognitive decline; it did not show cognitive improvement.23 Lecanemab also significantly reduced amyloid beta plaques.23
Continue to: Aducanumab and lecanemab are generally...
Aducanumab and lecanemab are generally not covered by insurance and typically cost >$26,000 annually. Both are administered by IV infusion once a month. More monoclonal antibody medications for treating early Alzheimer disease are in the late stages of development, most notably donanebab.24 Observations during clinical trials found that in the later stages of Alzheimer disease, forceful removal of plaques by the autoimmune process damages neurons, while in less dense deposits of early dementia such removal is not harmful to the cells and prevents amyloid buildup.
Brexanolone is an aqueous formulation of allopregnanolone, a major metabolite of progesterone and a positive allosteric modulator of GABA-A receptors.25 Its levels are maximal at the end of the third trimester of pregnancy and fall rapidly following delivery. Research showed a 3-day infusion was rapidly and significantly effective for treating postpartum depression26 and brexanolone received FDA approval for this indication in March 2019.27 However, various administrative, economic, insurance, and other hurdles make it difficult for patients to access this treatment. Despite its rapid onset of action (usually 48 hours), brexanolone takes an average of 15 days to go through the prior authorization process.28 In addition to the need for prior authorization, the main impediment to the use of brexanolone is the 3-day infusion schedule, which greatly magnifies the cost but is partially circumvented by the availability of dedicated outpatient centers.
Magnesium
Magnesium is on the World Health Organization’s Model List of Essential Medicines.29 There has been extensive research on the use of magnesium sulfate for psychiatric indications, especially for depression.30 Magnesium functions similarly to calcium channel blockers by competitively blocking intracellular calcium channels, decreasing calcium availability, and inhibiting smooth muscle contractility.31 It also competes with calcium at the motor end plate, reducing excitation by inhibiting the release of acetylcholine.32 This property is used for high-dose IV magnesium treatment of impending preterm labor in obstetrics. Magnesium sulfate is the drug of choice in treating eclamptic seizures and preventing seizures in severe preeclampsia or gestational hypertension with severe features.33 It is also used to treat torsade de pointes, severe asthma exacerbations, constipation, and barium poisoning.34 Beneficial use in asthma treatment35 and the treatment of migraine36 have also been reported.
IV magnesium in myocardial infarction may be harmful,37 though outside of acute cardiac events, magnesium is found to be safe.38
Oral magnesium sulfate is a common over-the-counter anxiety remedy. As a general cell stabilizer (mediated by the reduction of intracellular calcium), magnesium is potentially beneficial outside of its muscle-relaxing properties, although muscle relaxing can benefit anxious patients. It is used to treat anxiety,39 alcohol withdrawal,40 and fear.41 Low magnesium blood levels are found in patients with depression, schizophrenia,42 and attention-deficit/hyperactivity disorder.43 However, it is important to note that the therapeutic effect of magnesium when treating anxiety and headache is independent of preinfusion magnesium blood levels.43
Continue to: The efficacy of oral magnesium...
The efficacy of oral magnesium is not robust. However, IV administration has a pronounced beneficial effect as an abortive and preventative treatment in many patients with anxiety.44
IV administration of magnesium can produce adverse effects, including flushing, sweating, hypotension, depressed reflexes, flaccid paralysis, hypothermia, circulatory collapse, and cardiac and CNS depression. These complications are very rare and dose-dependent.45 Magnesium is excreted by the kidneys, and dosing must be decreased in patients with kidney failure. The most common adverse effect is local burning along the vein upon infusion; small doses of IV lidocaine can remedy this. Hot flashes are also common.45
Various dosing strategies are available. In patients with anxiety, application dosing is based on the recommended preeclampsia IV dose of 4 g diluted in 250 mL of 5% dextrose. Much higher doses may be used in obstetrics. Unlike in obstetrics, for psychiatric indications, magnesium is administered over 60 to 90 minutes. Heart monitoring is recommended.
Scopolamine
Scopolamine is primarily used to relieve nausea, vomiting, and dizziness associated with motion sickness and recovery from anesthesia. It is also used in ophthalmology and in patients with excessive sweating. In off-label and experimental applications, scopolamine has been used in patients with TRD.46
Scopolamine is an anticholinergic medication. It is a nonselective antagonist at muscarinic receptors.47 Tricyclic antidepressants (TCAs) possess strong anticholinergic function. Newer generations of antidepressants were designed specifically not to have this function because it was believed to be an unwanted and potentially dangerous adverse effect. However, data suggest that anticholinergic action is important in decreasing depressive symptoms. Several hypotheses of anticholinergic effects on depression have been published since the 1970s. They include the cholinergic-adrenergic hypothesis,48 acetylcholine predominance relative to adrenergic action hypothesis,49 and insecticide poisoning observations.50 Centrally acting physostigmine (but not peripherally acting neostigmine) was reported to control mania.48,51 A genetic connection between the M2acetylcholine receptor in patients with major depressive disorder (MDD) and alcohol use disorder is also suggestive.52
Continue to: Multiple animal studies show...
Multiple animal studies show direct improvement in mobility and a decrease in despair upon introducing anticholinergic substances.53-55 The cholinergic theory of depression has been studied in several controlled clinical human studies.56,57 Use of a short-acting anticholinergic glycopyrrolate during electroconvulsive therapy (ECT) may contribute to the procedure’s efficacy.
Human research shows scopolamine has a higher efficacy as an antidepressant and anti-anxiety medication in women than in men,58 possibly because estrogen increases the activity of choline acetyltransferase and release of acetylcholine.59,60 M2receptors mediate estrogen influence on the NMDAR, which may explain the anticholinergic effects of depression treatments in women.61
Another proposed mechanism of action of scopolamine is a potent inhibition of the NMDAR.62 Rapid treatments of depression may be based on this mechanism. Examples of such treatments include IV ketamine and sleep deprivation.63 IV scopolamine shows potency in treating MDD and bipolar depression. This treatment should be reserved for patients who do not respond to or are not candidates for other usual treatment modalities of MDD and for the most severe cases. Scopolamine is 30 times more potent than amitriptyline in anticholinergic effect and reportedly produces sustained improvement in MDD.64
Scopolamine has no black-box warnings. It has not been studied in pregnant women and is not recommended for use during pregnancy. Due to possible negative cardiovascular effects, a normal electrocardiogram is required before the start of treatment. Exercise caution in patients with glaucoma, benign prostatic enlargement, gastroparesis, unstable cardiovascular status, or severe renal impairment.
Treatment with scopolamine is not indicated for patients with myasthenia gravis, psychosis, or seizures. Patients must be off potassium for 3 days before beginning scopolamine treatment. Patients should consult with their cardiologist before having a scopolamine infusion. Adverse reactions may include psychosis, tachycardia, seizures, paralytic ileus, and glaucoma exacerbation. The most common adverse effects of scopolamine infusion treatment include drowsiness, dry mouth, blurred vision, lightheadedness, and dizziness. Due to possible drowsiness, operating motor vehicles or heavy machinery must be avoided on the day of treatment.65 Overall, the adverse effects of scopolamine are preventable and manageable, and its antidepressant efficacy is noteworthy.66
Continue to: Treatment typically consists of 3 consecutive infusions...
Treatment typically consists of 3 consecutive infusions of 4 mcg/kg separated by 3 to 5 days.56 It is possible to have a longer treatment course if the patient experiences only partial improvement. Repeated courses or maintenance treatment (similar to ECT maintenance) are utilized in some patients if indicated. Cardiac monitoring is mandatory.
Clomipramine
Clomipramine, a TCA, acts as a preferential inhibitor of 5-hydroxytryptamine uptake and has proven effective in managing depression, TRD, and obsessive-compulsive disorder (OCD).67 Although this medication has reported treatment benefits for patients with phobia, panic disorder,15 chronic pain,68 Tourette syndrome,69 premature ejaculation, anorexia nervosa,70 cataplexy,49 and enuresis,71 it is FDA-approved only for the treatment of OCD.72 Clomipramine may also be beneficial for pain and headache, possibly because of its anti-inflammatory action.73 The anticholinergic effects of clomipramine may add to its efficacy in depression.
The pathophysiology of MDD is connected to hyperactivity of the HPA axis and elevated cortisol levels. Higher clomipramine plasma levels show a linear correlation with lower cortisol secretion and levels, possibly aiding in the treatment of MDD and anxiety.74 The higher the blood level, the more pronounced clomipramine’s therapeutic effect across multiple domains.75
IV infusion of clomipramine produces the highest concentration in the shortest time, but overall, research does not necessarily support increased efficacy of IV over oral administration. There is evidence suggesting that subgroups of patients with severe, treatment-refractory OCD may benefit from IV agents and research suggests a faster onset of action.76 Faster onset of symptom relief is the basis for IV clomipramine use. In patients with OCD, it can take several months for oral medications to produce therapeutic benefits; not all patients can tolerate this. In such scenarios, IV administration may be considered, though it is not appropriate for routine use until more research is available. Patients with treatment-resistant OCD who have exhausted other means of symptom relief may also be candidates for IV treatment.
The adverse effects of IV clomipramine are no different from oral use, though they may be more pronounced. A pretreatment cardiac exam is desirable because clomipramine, like other TCAs, may be cardiotoxic. The anticholinergic adverse effects of TCAs are well known to clinicians77 and partially explained in the scopolamine section of this article. It is not advisable to combine clomipramine with other TCAs or serotonin reuptake inhibitors. Clomipramine also should not be combined with monoamine oxidase inhibitors, though such a combination was reported in medical literature.78 Combination with antiarrhythmics such as lidocaine or opioids such as fentanyl or and tramadol is highly discouraged (fentanyl and tramadol also have serotonergic effects).79
Continue to: Clomipramine for IV use is not commercially available...
Clomipramine for IV use is not commercially available and must be sterilely compounded. The usual course of treatment is a series of 3 infusions: 150 mg on Day 1, 200 mg on Day 2 or Day 3, and 250 mg on Day 3, Day 4, or Day 5, depending on tolerability. A protocol with a 50 mg/d starting dose and titration up to a maximum dose of 225 mg/d over 5 to 7 days has been suggested for inpatient settings.67 Titration to 250 mg is more common.80
A longer series may be performed, but this increases the likelihood of adverse effects. Booster and maintenance treatments are also completed when required. Cardiac monitoring is mandatory.
Vortioxetine and citalopram
IV treatment of depression with
Injections and blocks
Three interventional approaches to treatment that possess psychotherapeutic potential include stellate ganglion blocks (SGBs), glabellar BT injections, and trigger point injections (TPIs). None of these are FDA-approved for psychiatric treatment.
Stellate ganglion blocks
The sympathetic nervous system is involved in autonomic hyperarousal and is at the core of posttraumatic symptomatology.83 Insomnia, anxiety, irritability, hypervigilance, and other excitatory CNS events are connected to the sympathetic nervous system and amygdala activation is commonly observed in those exposed to extreme stress or traumatic events.84
Continue to: SGBs were first performed 100 years ago...
SGBs were first performed 100 years ago and reported to have beneficial psychiatric effects at the end of the 1940s. In 1998 in Finland, improvement of posttraumatic stress disorder (PTSD) symptoms was observed accidentally via thoracic level spine blocks.85 In 2006, cervical level sympathetic blocks were shown to be effective for PTSD symptom control.86 By the end of 2010, Veterans Administration hospitals adopted SGBs to treat veterans with PTSD.87,88 The first multisite, randomized clinical trial of
Since the stellate ganglion is connected to the amygdala, SGB has also been assessed for treating anxiety and depression.89,90 Outside of PTSD, SGBs are used to treat complex regional pain syndrome,91 phantom limb pain, trigeminal neuralgia,92 intractable angina,93 and postherpetic neuralgia in the head, neck, upper chest, or arms.94 The procedure consists of an injection of a local anesthetic through a 25-gauge needle into the stellate sympathetic ganglion at the C6 or C7 vertebral levels. An injection into C6 is considered safer because of specific cervical spine anatomy. Ideally, fluoroscopic guidance or ultrasound is used to guide needle insertion.95
A severe drop in blood pressure may be associated with SGBs and is mitigated by IV hydration. Other adverse effects include red eyes, drooping of the eyelids, nasal congestion, hoarseness, difficulty swallowing, a sensation of a “lump” in the throat, and a sensation of warmth or tingling in the arm or hand. Bilateral SGB is contraindicated due to the danger of respiratory arrest.96
Glabellar BT injections
OnabotulinumtoxinA (BT) injection was first approved for therapeutic use in 1989 for eye muscle disorders such as strabismus97 and blepharospasm.98 It was later approved for several other indications, including cosmetic use, hyperhidrosis, migraine prevention, neurogenic bladder disorder, overactive bladder, urinary incontinence, and spasticity.99-104 BT is used off-label for achalasia and sialorrhea.105,106 Its mechanism of action is primarily attributed to muscle paralysis by blocking presynaptic acetylcholine release into neuromuscular junctions.107
Facial BT injections are usually administered for cosmetic purposes, but smoothing forehead wrinkles and frown lines (the glabellar region of the face) both have antidepressant effects.108 BT injections into the glabellar region also demonstrate antidepressant effects, particularly in patients with comorbid migraines and MDD.109 Early case observations supported the independent benefit of the toxin on MDD when the toxin was injected into the glabellar region.110,111 The most frequent protocol involves injections in the procerus and corrugator muscles.
Continue to: The facial feedback/emotional proprioception hypothesis...
The facial feedback/emotional proprioception hypothesis has dominated thinking about the mood-improving effects of BT. The theory is that blocking muscular expression of sadness (especially in the face) interrupts the experience of sadness; therefore, depression subsides.112,113 However, BT injections in the muscles involved in the smile and an expression of positive emotions (lateral part of the musculus orbicularis oculi) have been associated with increased MDD scores.114 Thus, the mechanism clearly involves more than the cosmetic effect, since facial muscle injections in rats also have antidepressant effects.115
The use of progressive muscle relaxation is well-established in psychiatric treatment. The investigated conditions of increased muscle tone, especially torticollis and blepharospasm, are associated with MDD, and it may be speculated that proprioceptive feedback from the affected muscles may be causally involved in this association.116-118 Activity of the corrugator muscle has been positively associated with increased amygdala activity.119 This suggests a potential similar mechanism to that hypothesized for SGB.
Alternatively, BT is commonly used to treat chronic conditions that may contribute to depression; its success in relieving the underlying problem may indirectly relieve MDD. Thus, in a postmarketing safety evaluation of BT, MDD was demonstrated 40% to 88% less often by patients treated with BT for 6 of the 8 conditions and injection sites, such as in spasms and spasticity of arms and legs, torticollis and neck pain, and axilla and palm injections for hyperhidrosis. In a parotid and submandibular glands BT injection subcohort, no patients experienced depressive symptoms.120
Medicinal BT is generally considered safe. The most common adverse effects are hypersensitivity, injection site reactions, and other adverse effects specific to the injection site.121 Additionally, the cosmetic effects are transient, given the nature of the medication.
Trigger point injections
TPIs in the neck and shoulders are frequently used to treat tension headaches and various referred pain locations in the face and arms. Tension and depression frequently overlap in clinical practice.122 Relieving muscle tension (with resulting trigger points) improves muscle function and mood.
Continue to: The higher the number of active...
The higher the number of active trigger points (TPs), the greater the physical burden of headache and the higher the anxiety level. Gender differences in TP prevalence and TPI efficacy have been described in the literature. For example, the number of active TPs seems directly associated with anxiety levels in women but not in men.123 Although TPs appear to be more closely associated with anxiety than depression,124 depression associated with muscle tension does improve with TPIs. European studies have demonstrated a decrease in scores on the Hamilton Depression Rating Scale and Hamilton Anxiety Rating Scale following TPI treatment.125 The effect may be indirect, as when a patient’s pain is relieved, sleep and other psychiatric symptoms improve.126
A randomized controlled trial by Castro Sánchez et al127 demonstrated that dry needling therapy in patients with fibromyalgia syndrome (FMS) showed improvements in pain pressure thresholds, body pain, vitality, and social function, as well as total FMS symptoms, quality of sleep, anxiety, hospital anxiety and depression, general pain intensity, and fatigue.
Myofascial pain syndrome, catastrophizing, and muscle tension are common in patients with depression, anxiety, and somatization. Local TPI therapy aimed at inactivating pain generators is supported by moderate quality evidence. All manner of therapies have been described, including injection of saline, corticosteroids, local anesthetic agents, and dry needling. BT injections in chronic TPs are also practiced, though no specific injection therapy has been reliably shown to be more advantageous than another. The benefits of TPIs may be derived from the needle itself rather than from any specific substance injected. Stimulation of a local twitch response with direct needling of the TP appears of importance. There is no established consensus regarding the number of injection points, frequency of administration, and volume or type of injectate.128
Adverse effects of TPIs relate to the nature of the invasive therapy, with the risk of tissue damage and bleeding. Pneumothorax risk is present with needle insertion at the neck and thorax.129 Patients with diabetes may experience variations in blood sugar control if steroids are used.
Bottom Line
Interventional treatment modalities that may have a role in psychiatric treatment include IV administration of ketamine, aducanumab, lecanemab, brexanolone, magnesium, scopolamine, and clomipramine. Other interventional approaches include stellate ganglion blocks, glabellar botulinum toxin injections, and trigger point injections.
Related Resources
- Dokucu ME, Janicak PG. Nontraditional therapies for treatment-resistant depression. Current Psychiatry. 2021; 20(9):38-43,49. doi:10.12788/cp.0166
- Kim J, Khoury R, Grossberg GT. Botulinum toxin: emerging psychiatric indications. Current Psychiatry. 2018;17(12):8-18.
Drug Brand Names
Aducanumab • Aduhelm
Aripiprazole • Abilify
Aripiprazole lauroxil • Aristada
Brexanolone • Zulresso
Buprenorphine • Sublocade
Citalopram • Celexa
Clomipramine • Anafranil
Diazepam • Valium
Droperidol • Inapsine
Esketamine • Spravato
Fentanyl • Actiq
Fluphenazine decanoate • Modecate
Fluphenazine hydrochloride • Prolixin
Haloperidol decanoate • Haldol decanoate
Haloperidol lactate • Haldol
Ketamine • Ketalar
Lecanemab • Leqembi
Lidocaine • Xylocaine
Lorazepam • Ativan
Loxapine inhaled • Adasuve
Naltrexone • Vivitrol
Magnesium sulfate • Sulfamag
Midazolam • Versed
Olanzapine • Zyprexa
OnabotulinumtoxinA injection • Botox
Paliperidone • Invega Hafyera, Invega Sustenna, Invega Trinza
Rapamycin • Rapamune, Sirolimus
Risperidone • Perseris
Risperidone microspheres • Risperdal Consta, Rykindo
Scopolamine • Hyoscine
Tramadol • Conzip
Vortioxetine • Trintellix
Ziprasidone • Geodon
1. Vincent KM, Ryan M, Palmer E, et al. Interventional psychiatry. Postgrad Med. 2020;132(7):573-574.
2. Allen MH, Feifel D, Lesem MD, et al. Efficacy and safety of loxapine for inhalation in the treatment of agitation in patients with schizophrenia: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2011;72(10):1313-1321.
3. Kwentus J, Riesenberg RA, Marandi M, et al. Rapid acute treatment of agitation in patients with bipolar I disorder: a multicenter, randomized, placebo-controlled clinical trial with inhaled loxapine. Bipolar Disord. 2012;14(1):31-40.
4. Lee JD, Nunes EV Jr, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391(10118):309-318.
5. Haight BR, Learned SM, Laffont CM, et al. Efficacy and safety of a monthly buprenorphine depot injection for opioid use disorder: a multicentre, randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet. 2019;393(10173):778-790.
6. Andorn A, Graham J, Csernansky J, et al. Monthly extended-release risperidone (RBP-7000) in the treatment of schizophrenia: results from the phase 3 program. J Clin Psychopharmacol. 2019;39(5):428-433.
7. Dundee TW. Twenty-five years of ketamine. A report of an international meeting. Anaesthesia. 1990;45(2):159. doi:10.1111/j.1365-2044.1990.tb14287.x
8. White PF, Way WL, Trevor AJ. Ketamine--its pharmacology and therapeutic uses. Anesthesiology. 1982;56(2):119-136. doi:10.1097/00000542-198202000-00007
9. Zanos P, Gould TD. Mechanisms of ketamine action as an antidepressant. Mol Psychiatry. 2018;23(4):801-811.
10. Molero P, Ramos-Quiroga JA, Martin-Santos R, et al. Antidepressant efficacy and tolerability of ketamine and esketamine: a critical review. CNS Drugs. 2018;32(5):411-420. doi:10.1007/s40263-018-0519-3
11. Williams NR, Heifets BD, Blasey C, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018;175(12):1205-1215.
12. Witkin JM, Martin AE, Golani LK, et al. Rapid-acting antidepressants. Adv Pharmacol. 2019;86:47-96.
13. Strayer RJ, Nelson LS. Adverse events associated with ketamine for procedural sedation in adults. Am J Emerg Med. 2008;26(9):985-1028. doi:10.1016/j.ajem.2007.12.005
14. Frye MA, Blier P, Tye SJ. Concomitant benzodiazepine use attenuates ketamine response: implications for large scale study design and clinical development. J Clin Psychopharmacol. 2015;35(3):334-336.
15. Fava M, Freeman MP, Flynn M, et al. Double-blind, placebo-controlled, dose-ranging trial of intravenous ketamine as adjunctive therapy in treatment-resistant depression (TRD). Mol Psychiatry. 2020;25(7):1592-1603.
16. Bahji A, Vazquez GH, Zarate CA Jr. Comparative efficacy of racemic ketamine and esketamine for depression: a systematic review and meta-analysis. J Affect Disord. 2021;278:542-555. Erratum in: J Affect Disord. 2021;281:1001.
17. Brendle M, Robison R, Malone DC. Cost-effectiveness of esketamine nasal spray compared to intravenous ketamine for patients with treatment-resistant depression in the US utilizing clinical trial efficacy and real-world effectiveness estimates. J Affect Disord. 2022;319:388-396.
18. Dhillon S. Aducanumab: first approval. Drugs. 2021;81(12):1437-1443. Erratum in: Drugs. 2021;81(14):1701.
19. van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023;388(1):9-21. doi:10.1056/NEJMoa2212948
20. Sevigny J, Chiao P, Bussière T, et al. The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature. 2016;537(7618):50-56. Update in: Nature. 2017;546(7659):564.
21. Fillit H, Green A. Aducanumab and the FDA – where are we now? Nat Rev Neurol. 2021;17(3):129-130.
22. Reardon S. FDA approves Alzheimer’s drug lecanemab amid safety concerns. Nature. 2023;613(7943):227-228. doi:10.1038/d41586-023-00030-3
23. McDade E, Cummings JL, Dhadda S, et al. Lecanemab in patients with early Alzheimer’s disease: detailed results on biomarker, cognitive, and clinical effects from the randomized and open-label extension of the phase 2 proof-of-concept study. Alzheimers Res Ther. 2022;14(1):191. doi:10.1186/s13195-022-01124-2
24. Mintun MA, Lo AC, Evans CD, et al. Donanemab in early Alzheimer’s disease. N Engl J Med. 2021;384(18):1691-1704.
25. Luisi S, Petraglia F, Benedetto C, et al. Serum allopregnanolone levels in pregnant women: changes during pregnancy, at delivery, and in hypertensive patients. J Clin Endocrinol Metab. 2000;85(7):2429-2433.
26. Meltzer-Brody S, Colquhoun H, Riesenberg R, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392(10152):1058-1070.
27. Powell JG, Garland S, Preston K, et al. Brexanolone (Zulresso): finally, an FDA-approved treatment for postpartum depression. Ann Pharmacother. 2020;54(2):157-163.
28. Patterson R, Krohn H, Richardson E, et al. A brexanolone treatment program at an academic medical center: patient selection, 90-day posttreatment outcomes, and lessons learned. J Acad Consult Liaison Psychiatry. 2022;63(1):14-22.
29. World Health Organization. WHO model list of essential medicines - 22nd list (2021). World Health Organization. September 30, 2021. Accessed April 7, 2023. https://www.who.int/publications/i/item/WHO-MHP-HPS-EML-2021.02
30. Eby GA, Eby KL, Mruk H. Magnesium and major depression. In: Vink R, Nechifor M, eds. Magnesium in the Central Nervous System. University of Adelaide Press; 2011.
31. Plant TM, Zeleznik AJ. Knobil and Neill’s Physiology of Reproduction. 4th ed. Elsevier Inc.; 2015:2503-2550.
32. Sidebotham D, Le Grice IJ. Physiology and pathophysiology. In: Sidebotham D, McKee A, Gillham M, Levy J. Cardiothoracic Critical Care. Elsevier, Inc.; 2007:3-27.
33. Duley L, Gülmezoglu AM, Henderson-Smart DJ, et al. Magnesium sulphate and other anticonvulsants for women with pre-eclampsia. Cochrane Database Syst Rev. 2010;2010(11):CD000025.
34. Emergency supply of medicines. In: British National Formulary. British Medical Association, Royal Pharmaceutical Society; 2015:6. Accessed April 7, 2023. https://www.academia.edu/35076015/british_national_formulary_2015_pdf
35. Kwofie K, Wolfson AB. Intravenous magnesium sulfate for acute asthma exacerbation in children and adults. Am Fam Physician. 2021;103(4):245-246.
36. Patniyot IR, Gelfand AA. Acute treatment therapies for pediatric migraine: a qualitative systematic review. Headache. 2016;56(1):49-70.
37. Wang X, Du X, Yang H, et al. Use of intravenous magnesium sulfate among patients with acute myocardial infarction in China from 2001 to 2015: China PEACE-Retrospective AMI Study. BMJ Open. 2020;10(3):e033269.
38. Karhu E, Atlas SE, Jinrun G, et al. Intravenous infusion of magnesium sulfate is not associated with cardiovascular, liver, kidney, and metabolic toxicity in adults. J Clin Transl Res. 2018;4(1):47-55.
39. Noah L, Pickering G, Mazur A, et al. Impact of magnesium supplementation, in combination with vitamin B6, on stress and magnesium status: secondary data from a randomized controlled trial. Magnes Res. 2020;33(3):45-57.
40. Erstad BL, Cotugno CL. Management of alcohol withdrawal. Am J Health Syst Pharm. 1995;52(7):697-709.
41. Abumaria N, Luo L, Ahn M, et al. Magnesium supplement enhances spatial-context pattern separation and prevents fear overgeneralization. Behav Pharmacol. 2013;24(4):255-263.
42. Kirov GK, Tsachev KN. Magnesium, schizophrenia and manic-depressive disease. Neuropsychobiology. 1990;23(2):79-81.
43. Botturi A, Ciappolino V, Delvecchio G, et al. The role and the effect of magnesium in mental disorders: a systematic review. Nutrients. 2020;12(6):1661.
44. Kirkland AE, Sarlo GL, Holton KF. The role of magnesium in neurological disorders. Nutrients. 2018;10(6):730.
45. Magnesium sulfate intravenous side effects by likelihood and severity. WebMD. Accessed April 9, 2023. https://www.webmd.com/drugs/2/drug-149570/magnesium-sulfate-intravenous/details/list-sideeffects
46. Scopolamine base transdermal system – uses, side effects, and more. WebMD. Accessed April 9, 2023. https://www.webmd.com/drugs/2/drug-14032/scopolamine-transdermal/details
47. Bolden C, Cusack B, Richelson E. Antagonism by antimuscarinic and neuroleptic compounds at the five cloned human muscarinic cholinergic receptors expressed in Chinese hamster ovary cells. J Pharmacol Exp Ther. 1992;260(2):576-580.
48. Janowsky DS, el-Yousef MK, Davis JM, et al. A cholinergic-adrenergic hypothesis of mania and depression. Lancet. 1972;2(7778):632-635.
49. Janowsky DS, Risch SC, Gillin JC. Adrenergic-cholinergic balance and the treatment of affective disorders. Prog Neuropsychopharmacol Biol Psychiatry. 1983;7(2-3):297-307.
50. Gershon S, Shaw FH. Psychiatric sequelae of chronic exposure to organophosphorous insecticides. Lancet. 1972;1(7191):1371-1374.
51. Davis KL, Berger PA, Hollister LE, et al. Physostigmine in mania. Arch Gen Psychiatry. 1978;35(1):119-122.
52. Wang JC, Hinrichs AL, Stock H, et al. Evidence of common and specific genetic effects: association of the muscarinic acetylcholine receptor M2 (CHRM2) gene with alcohol dependence and major depressive syndrome. Hum Mol Genet. 2004;13(17):1903-1911.
53. Brown RG. Effects of antidepressants and anticholinergics in a mouse “behavioral despair” test. Eur J Pharmacol. 1979;58(3):331-334.
54. Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266(5604):730-732.
55. Ji CX, Zhang JJ. Effect of scopolamine on depression in mice. Abstract in English. Yao Xue Xue Bao. 2011;46(4):400-405.
56. Furey ML, Drevets WC. Antidepressant efficacy of the antimuscarinic drug scopolamine: a randomized, placebo-controlled clinical trial. Arch Gen Psychiatry. 2006;63(10):1121-1129.
57. Drevets WC, Furey ML. Replication of scopolamine’s antidepressant efficacy in major depressive disorder: a randomized, placebo-controlled clinical trial. Biol Psychiatry. 2010;67(5):432-438.
58. Furey ML, Khanna A, Hoffman EM, et al. Scopolamine produces larger antidepressant and antianxiety effects in women than in men. Neuropsychopharmacology. 2010;35(12):2479-2488.
59. Gibbs RB, Gabor R, Cox T, et al. Effects of raloxifene and estradiol on hippocampal acetylcholine release and spatial learning in the rat. Psychoneuroendocrinology. 2004;29(6):741-748.
60. Pongrac JL, Gibbs RB, Defranco DB. Estrogen-mediated regulation of cholinergic expression in basal forebrain neurons requires extracellular-signal-regulated kinase activity. Neuroscience. 2004;124(4):809-816.
61. Daniel JM, Dohanich GP. Acetylcholine mediates the estrogen-induced increase in NMDA receptor binding in CA1 of the hippocampus and the associated improvement in working memory. J Neurosci. 2001;21(17):6949-6956.
62. Gerhard DM, Wohleb ES, Duman RS. Emerging treatment mechanisms for depression: focus on glutamate and synaptic plasticity. Drug Discov Today. 2016;21(3):454-464.
63. Voderholzer U. Sleep deprivation and antidepressant treatment. Dialogues Clin Neurosci. 2003;5(4):366-369.
64. Hasselmann H. Scopolamine and depression: a role for muscarinic antagonism? CNS Neurol Disord Drug Targets. 2014;13(4):673-683.
65. Transderm scopolamine [prescribing information]. Warren, NJ: GSK Consumer Healthcare; 2019.
66. Jaffe RJ, Novakovic V, Peselow ED. Scopolamine as an antidepressant: a systematic review. Clin Neuropharmacol. 2013;36(1):24-26.
67. Karameh WK, Khani M. Intravenous clomipramine for treatment-resistant obsessive-compulsive disorder. Int J Neuropsychopharmacol. 2015;19(2):pyv084.
68. Andrews ET, Beattie RM, Tighe MP. Functional abdominal pain: what clinicians need to know. Arch Dis Child. 2020;105(10):938-944. doi:10.1136/archdischild-2020-318825
69. Aliane V, Pérez S, Bohren Y, et al. Key role of striatal cholinergic interneurons in processes leading to arrest of motor stereotypies. Brain. 2011;134(Pt 1):110-118. doi:10.1093/brain/awq285
70. Tzavara ET, Bymaster FP, Davis RJ, et al. M4 muscarinic receptors regulate the dynamics of cholinergic and dopaminergic neurotransmission: relevance to the pathophysiology and treatment of related CNS pathologies. FASEB J. 2004;18(12):1410-1412. doi:10.1096/fj.04-1575fje
71. Korczyn AD, Kish I. The mechanism of imipramine in enuresis nocturna. Clin Exp Pharmacol Physiol. 1979;6(1):31-35. doi:10.1111/j.1440-1681.1979.tb00004.x
72. Trimble MR. Worldwide use of clomipramine. J Clin Psychiatry. 1990;51(Suppl):51-54; discussion 55-58.
73. Gong W, Zhang S, Zong Y, et al. Involvement of the microglial NLRP3 inflammasome in the anti-inflammatory effect of the antidepressant clomipramine. J Affect Disord. 2019;254:15-25.
74. Piwowarska J, Wrzosek M, Radziwon’-Zaleska M. Serum cortisol concentration in patients with major depression after treatment with clomipramine. Pharmacol Rep. 2009;61(4):604-611.
75. Danish University Antidepressant Group (DUAG). Clomipramine dose-effect study in patients with depression: clinical end points and pharmacokinetics. Clin Pharmacol Ther. 1999;66(2):152-165.
76. Moukaddam NJ, Hirschfeld RMA. Intravenous antidepressants: a review. Depress Anxiety. 2004;19(1):1-9.
77. Gerretsen P, Pollock BG. Rediscovering adverse anticholinergic effects. J Clin Psychiatry. 2011;72(6):869-870. doi:10.4088/JCP.11ac07093
78. Thomas SJ, Shin M, McInnis MG, et al. Combination therapy with monoamine oxidase inhibitors and other antidepressants or stimulants: strategies for the management of treatment-resistant depression. Pharmacotherapy. 2015;35(4):433-449. doi:10.1002/phar.1576
79. Robles LA. Serotonin syndrome induced by fentanyl in a child: case report. Clin Neuropharmacol. 2015;38(5):206-208. doi:10.1097/WNF.0000000000000100
80. Fallon BA, Liebowitz MR, Campeas R, et al. Intravenous clomipramine for obsessive-compulsive disorder refractory to oral clomipramine: a placebo-controlled study. Arch Gen Psychiatry. 1998;55(10):918-924.
81. Vieta E, Florea I, Schmidt SN, et al. Intravenous vortioxetine to accelerate onset of effect in major depressive disorder: a 2-week, randomized, double-blind, placebo-controlled study. Int Clin Psychopharmacol. 2019;34(4):153-160.
82. Kasper S, Müller-Spahn F. Intravenous antidepressant treatment: focus on citalopram. Eur Arch Psychiatry Clin Neurosci. 2002;252(3):105-109.
83. Togay B, El-Mallakh RS. Posttraumatic stress disorder: from pathophysiology to pharmacology. Current Psychiatry. 2020;19(5):33-39.
84. Adhikari A, Lerner TN, Finkelstein J, et al. Basomedial amygdala mediates top-down control of anxiety and fear. Nature. 2015;527(7577):179-185. doi:10.1038/nature15698
85. Lipov E. In search of an effective treatment for combat-related post-traumatic stress disorder (PTSD): can the stellate ganglion block be the answer? Pain Pract. 2010;10(4):265-266.
86. Lipov E, Ritchie EC. A review of the use of stellate ganglion block in the treatment of PTSD. Curr Psychiatry Rep. 2015;17(8):599.
87. Olmsted KLR, Bartoszek M, McLean B, et al. Effect of stellate ganglion block treatment on posttraumatic stress disorder symptoms: a randomized clinical trial. JAMA Psychiatry. 2020;77(2):130-138.
88. Lipov E, Candido K. The successful use of left-sided stellate ganglion block in patients that fail to respond to right-sided stellate ganglion block for the treatment of post-traumatic stress disorder symptoms: a retrospective analysis of 205 patients. Mil Med. 2021;186(11-12):319-320.
89. Li Y, Loshak H. Stellate ganglion block for the treatment of post-traumatic stress disorder, depression, and anxiety. Canadian J Health Technol. 2021;1(3):1-30.
90. Kerzner J, Liu H, Demchenko I, et al. Stellate ganglion block for psychiatric disorders: a systematic review of the clinical research landscape. Chronic Stress (Thousand Oaks). 2021;5:24705470211055176.
91. Wie C, Gupta R, Maloney J, et al. Interventional modalities to treat complex regional pain syndrome. Curr Pain Headache Rep. 2021;25(2):10. doi:10.1007/s11916-020-00904-5
92. Chaturvedi A, Dash HH. Sympathetic blockade for the relief of chronic pain. J Indian Med Assoc. 2001;99(12):698-703.
93. Chester M, Hammond C. Leach A. Long-term benefits of stellate ganglion block in severe chronic refractory angina. Pain. 2000;87(1):103-105. doi:10.1016/S0304-3959(00)00270-0
94. Jeon Y. Therapeutic potential of stellate ganglion block in orofacial pain: a mini review. J Dent Anesth Pain Med. 2016;16(3):159-163. doi:10.17245/jdapm.2016.16.3.159
95. Shan HH, Chen HF, Ni Y, et al. Effects of stellate ganglion block through different approaches under guidance of ultrasound. Front Surg. 2022;8:797793. doi:10.3389/fsurg.2021.797793
96. Goel V, Patwardhan AM, Ibrahim M, et al. Complications associated with stellate ganglion nerve block: a systematic review. Reg Anesth Pain Med. 2019;rapm-2018-100127. doi:10.1136/rapm-2018-100127
97. Rowe FJ, Noonan CP. Botulinum toxin for the treatment of strabismus. Cochrane Database Syst Rev. 2017;3(3):CD006499.
98. Roggenkämper P, Jost WH, Bihari K, et al. Efficacy and safety of a new botulinum toxin type A free of complexing proteins in the treatment of blepharospasm. J Neural Transm (Vienna). 2006;113(3):303-312.
99. Heckmann M, Ceballos-Baumann AO, Plewig G; Hyperhidrosis Study Group. Botulinum toxin A for axillary hyperhidrosis (excessive sweating). N Engl J Med. 2001;344(7):488-493.
100. Carruthers JA, Lowe NJ, Menter MA, et al. A multicenter, double-blind, randomized, placebo-controlled study of the efficacy and safety of botulinum toxin type A in the treatment of glabellar lines. J Am Acad Dermatol. 2002;46(6):840-849.
101. Schurch B, de Sèze M, Denys P, et al. Botulinum toxin type A is a safe and effective treatment for neurogenic urinary incontinence: results of a single treatment, randomized, placebo controlled 6-month study. J Urol. 2005;174:196–200.
102. Aurora SK, Winner P, Freeman MC, et al. OnabotulinumtoxinA for treatment of chronic migraine: Pooled analyses of the 56-week PREEMPT clinical program. Headache. 2011;51(9):1358-1373.
103. Dashtipour K, Chen JJ, Walker HW, et al. Systematic literature review of abobotulinumtoxinA in clinical trials for adult upper limb spasticity. Am J Phys Med Rehabil. 2015;94(3):229-238.
104. Nitti VW, Dmochowski R, Herschorn S, et al. OnabotulinumtoxinA for the treatment of patients with overactive bladder and urinary incontinence: results of a phase 3, randomized, placebo-controlled trial. J Urol. 2017;197(2S):S216-S223.
105. Jongerius PH, van den Hoogen FJA, van Limbeek J, et al. Effect of botulinum toxin in the treatment of drooling: a controlled clinical trial. Pediatrics. 2004;114(3):620-627.
106. Zaninotto, G. Annese V, Costantini M, et al. Randomized controlled trial of botulinum toxin versus laparoscopic heller myotomy for esophageal achalasia. Ann Surg. 2004;239(3):364-370.
107. Dressler D, Adib Saberi F. Botulinum toxin: mechanisms of action. Eur Neurol. 2005;53:3-9.
108. Lewis MB, Bowler PJ. Botulinum toxin cosmetic therapy correlates with a more positive mood. J Cosmet Dermatol. 2009;8(1):24-26.
109. Affatato O, Moulin TC, Pisanu C, et al. High efficacy of onabotulinumtoxinA treatment in patients with comorbid migraine and depression: a meta-analysis. J Transl Med. 2021;19(1):133.
110. Finzi E, Wasserman E. Treatment of depression with botulinum toxin A: a case series. Dermatol Surg. 2006;32(5):645-649; discussion 649-650.
111. Schulze J, Neumann I, Magid M, et al. Botulinum toxin for the management of depression: an updated review of the evidence and meta-analysis. J Psychiatr Res. 2021;135:332-340.
112. Finzi E, Rosenthal NE. Emotional proprioception: treatment of depression with afferent facial feedback. J Psychiatr Res. 2016;80:93-96.
113. Söderkvist S, Ohlén K, Dimberg U. How the experience of emotion is modulated by facial feedback. J Nonverbal Behav. 2018;42(1):129-151.
114. Lewis, MB. The interactions between botulinum-toxin-based facial treatments and embodied emotions. Sci Rep. 2018;8(1):14720.
115. Li Y, Liu J, Liu X, et al. Antidepressant-like action of single facial injection of botulinum neurotoxin A is associated with augmented 5-HT levels and BDNF/ERK/CREB pathways in mouse brain. Neurosci Bull. 2019;35(4):661-672. Erratum in: Neurosci Bull. 2019;35(4):779-780.
116. Gündel H, Wolf A, Xidara V, et al. High psychiatric comorbidity in spasmodic torticollis: a controlled study. J Nerv Ment Dis. 2003;191(7):465-473.
117. Hall TA, McGwin G Jr, Searcey K, et al. Health-related quality of life and psychosocial characteristics of patients with benign essential blepharospasm. Arch Ophthalmol. 2006;124(1):116-119.
118. Ceylan D, Erer S, Zarifog˘lu M, et al. Evaluation of anxiety and depression scales and quality of life in cervical dystonia patients on botulinum toxin therapy and their relatives. Neurol Sci. 2019;40(4):725-731.
119. Heller AS, Lapate RC, Mayer KE, et al. The face of negative affect: trial-by-trial corrugator responses to negative pictures are positively associated with amygdala and negatively associated with ventromedial prefrontal cortex activity. J Cogn Neurosci. 2014;26(9):2102-2110.
120. Makunts T, Wollmer MA, Abagyan R. Postmarketing safety surveillance data reveals antidepressant effects of botulinum toxin across various indications and injection sites. Sci Rep. 2020;10(1):12851.
121. Ahsanuddin S, Roy S, Nasser W, et al. Adverse events associated with botox as reported in a Food and Drug Administration database. Aesthetic Plast Surg. 2021;45(3):1201-1209. doi:10.1007/s00266-020-02027-z
122. Kashif M, Tahir S, Ashfaq F, et al. Association of myofascial trigger points in neck and shoulder region with depression, anxiety, and stress among university students. J Pak Med Assoc. 2021;71(9):2139-2142.
123. Cigarán-Méndez M, Jiménez-Antona C, Parás-Bravo P, et al. Active trigger points are associated with anxiety and widespread pressure pain sensitivity in women, but not men, with tension type headache. Pain Pract. 2019;19(5):522-529.
124. Palacios-Ceña M, Castaldo M, Wang K, et al. Relationship of active trigger points with related disability and anxiety in people with tension-type headache. Medicine (Baltimore). 2017;96(13):e6548.
125. Karadas Ö, Inan LE, Ulas Ü, et al. Efficacy of local lidocaine application on anxiety and depression and its curative effect on patients with chronic tension-type headache. Eur Neurol. 2013;70(1-2):95-101.
126. Gerwin RD. Classification, epidemiology and natural history of myofascial pain syndrome. Curr Pain Headache Rep. 2001;5(5):412-420.
127. Castro Sánchez AM, García López H, Fernández Sánchez M, et al. Improvement in clinical outcomes after dry needling versus myofascial release on pain pressure thresholds, quality of life, fatigue, pain intensity, quality of sleep, anxiety, and depression in patients with fibromyalgia syndrome. Disabil Rehabil. 2019;41(19):2235-2246.
128. Healy GM, Finn DP, O’Gorman DA, et al. Pretreatment anxiety and pain acceptance are associated with response to trigger point injection therapy for chronic myofascial pain. Pain Med. 2015;16(10):1955-1966.
129. Morjaria JB, Lakshminarayana UB, Liu-Shiu-Cheong P, et al. Pneumothorax: a tale of pain or spontaneity. Ther Adv Chronic Dis. 2014;5(6):269-273.
1. Vincent KM, Ryan M, Palmer E, et al. Interventional psychiatry. Postgrad Med. 2020;132(7):573-574.
2. Allen MH, Feifel D, Lesem MD, et al. Efficacy and safety of loxapine for inhalation in the treatment of agitation in patients with schizophrenia: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2011;72(10):1313-1321.
3. Kwentus J, Riesenberg RA, Marandi M, et al. Rapid acute treatment of agitation in patients with bipolar I disorder: a multicenter, randomized, placebo-controlled clinical trial with inhaled loxapine. Bipolar Disord. 2012;14(1):31-40.
4. Lee JD, Nunes EV Jr, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391(10118):309-318.
5. Haight BR, Learned SM, Laffont CM, et al. Efficacy and safety of a monthly buprenorphine depot injection for opioid use disorder: a multicentre, randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet. 2019;393(10173):778-790.
6. Andorn A, Graham J, Csernansky J, et al. Monthly extended-release risperidone (RBP-7000) in the treatment of schizophrenia: results from the phase 3 program. J Clin Psychopharmacol. 2019;39(5):428-433.
7. Dundee TW. Twenty-five years of ketamine. A report of an international meeting. Anaesthesia. 1990;45(2):159. doi:10.1111/j.1365-2044.1990.tb14287.x
8. White PF, Way WL, Trevor AJ. Ketamine--its pharmacology and therapeutic uses. Anesthesiology. 1982;56(2):119-136. doi:10.1097/00000542-198202000-00007
9. Zanos P, Gould TD. Mechanisms of ketamine action as an antidepressant. Mol Psychiatry. 2018;23(4):801-811.
10. Molero P, Ramos-Quiroga JA, Martin-Santos R, et al. Antidepressant efficacy and tolerability of ketamine and esketamine: a critical review. CNS Drugs. 2018;32(5):411-420. doi:10.1007/s40263-018-0519-3
11. Williams NR, Heifets BD, Blasey C, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018;175(12):1205-1215.
12. Witkin JM, Martin AE, Golani LK, et al. Rapid-acting antidepressants. Adv Pharmacol. 2019;86:47-96.
13. Strayer RJ, Nelson LS. Adverse events associated with ketamine for procedural sedation in adults. Am J Emerg Med. 2008;26(9):985-1028. doi:10.1016/j.ajem.2007.12.005
14. Frye MA, Blier P, Tye SJ. Concomitant benzodiazepine use attenuates ketamine response: implications for large scale study design and clinical development. J Clin Psychopharmacol. 2015;35(3):334-336.
15. Fava M, Freeman MP, Flynn M, et al. Double-blind, placebo-controlled, dose-ranging trial of intravenous ketamine as adjunctive therapy in treatment-resistant depression (TRD). Mol Psychiatry. 2020;25(7):1592-1603.
16. Bahji A, Vazquez GH, Zarate CA Jr. Comparative efficacy of racemic ketamine and esketamine for depression: a systematic review and meta-analysis. J Affect Disord. 2021;278:542-555. Erratum in: J Affect Disord. 2021;281:1001.
17. Brendle M, Robison R, Malone DC. Cost-effectiveness of esketamine nasal spray compared to intravenous ketamine for patients with treatment-resistant depression in the US utilizing clinical trial efficacy and real-world effectiveness estimates. J Affect Disord. 2022;319:388-396.
18. Dhillon S. Aducanumab: first approval. Drugs. 2021;81(12):1437-1443. Erratum in: Drugs. 2021;81(14):1701.
19. van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023;388(1):9-21. doi:10.1056/NEJMoa2212948
20. Sevigny J, Chiao P, Bussière T, et al. The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature. 2016;537(7618):50-56. Update in: Nature. 2017;546(7659):564.
21. Fillit H, Green A. Aducanumab and the FDA – where are we now? Nat Rev Neurol. 2021;17(3):129-130.
22. Reardon S. FDA approves Alzheimer’s drug lecanemab amid safety concerns. Nature. 2023;613(7943):227-228. doi:10.1038/d41586-023-00030-3
23. McDade E, Cummings JL, Dhadda S, et al. Lecanemab in patients with early Alzheimer’s disease: detailed results on biomarker, cognitive, and clinical effects from the randomized and open-label extension of the phase 2 proof-of-concept study. Alzheimers Res Ther. 2022;14(1):191. doi:10.1186/s13195-022-01124-2
24. Mintun MA, Lo AC, Evans CD, et al. Donanemab in early Alzheimer’s disease. N Engl J Med. 2021;384(18):1691-1704.
25. Luisi S, Petraglia F, Benedetto C, et al. Serum allopregnanolone levels in pregnant women: changes during pregnancy, at delivery, and in hypertensive patients. J Clin Endocrinol Metab. 2000;85(7):2429-2433.
26. Meltzer-Brody S, Colquhoun H, Riesenberg R, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392(10152):1058-1070.
27. Powell JG, Garland S, Preston K, et al. Brexanolone (Zulresso): finally, an FDA-approved treatment for postpartum depression. Ann Pharmacother. 2020;54(2):157-163.
28. Patterson R, Krohn H, Richardson E, et al. A brexanolone treatment program at an academic medical center: patient selection, 90-day posttreatment outcomes, and lessons learned. J Acad Consult Liaison Psychiatry. 2022;63(1):14-22.
29. World Health Organization. WHO model list of essential medicines - 22nd list (2021). World Health Organization. September 30, 2021. Accessed April 7, 2023. https://www.who.int/publications/i/item/WHO-MHP-HPS-EML-2021.02
30. Eby GA, Eby KL, Mruk H. Magnesium and major depression. In: Vink R, Nechifor M, eds. Magnesium in the Central Nervous System. University of Adelaide Press; 2011.
31. Plant TM, Zeleznik AJ. Knobil and Neill’s Physiology of Reproduction. 4th ed. Elsevier Inc.; 2015:2503-2550.
32. Sidebotham D, Le Grice IJ. Physiology and pathophysiology. In: Sidebotham D, McKee A, Gillham M, Levy J. Cardiothoracic Critical Care. Elsevier, Inc.; 2007:3-27.
33. Duley L, Gülmezoglu AM, Henderson-Smart DJ, et al. Magnesium sulphate and other anticonvulsants for women with pre-eclampsia. Cochrane Database Syst Rev. 2010;2010(11):CD000025.
34. Emergency supply of medicines. In: British National Formulary. British Medical Association, Royal Pharmaceutical Society; 2015:6. Accessed April 7, 2023. https://www.academia.edu/35076015/british_national_formulary_2015_pdf
35. Kwofie K, Wolfson AB. Intravenous magnesium sulfate for acute asthma exacerbation in children and adults. Am Fam Physician. 2021;103(4):245-246.
36. Patniyot IR, Gelfand AA. Acute treatment therapies for pediatric migraine: a qualitative systematic review. Headache. 2016;56(1):49-70.
37. Wang X, Du X, Yang H, et al. Use of intravenous magnesium sulfate among patients with acute myocardial infarction in China from 2001 to 2015: China PEACE-Retrospective AMI Study. BMJ Open. 2020;10(3):e033269.
38. Karhu E, Atlas SE, Jinrun G, et al. Intravenous infusion of magnesium sulfate is not associated with cardiovascular, liver, kidney, and metabolic toxicity in adults. J Clin Transl Res. 2018;4(1):47-55.
39. Noah L, Pickering G, Mazur A, et al. Impact of magnesium supplementation, in combination with vitamin B6, on stress and magnesium status: secondary data from a randomized controlled trial. Magnes Res. 2020;33(3):45-57.
40. Erstad BL, Cotugno CL. Management of alcohol withdrawal. Am J Health Syst Pharm. 1995;52(7):697-709.
41. Abumaria N, Luo L, Ahn M, et al. Magnesium supplement enhances spatial-context pattern separation and prevents fear overgeneralization. Behav Pharmacol. 2013;24(4):255-263.
42. Kirov GK, Tsachev KN. Magnesium, schizophrenia and manic-depressive disease. Neuropsychobiology. 1990;23(2):79-81.
43. Botturi A, Ciappolino V, Delvecchio G, et al. The role and the effect of magnesium in mental disorders: a systematic review. Nutrients. 2020;12(6):1661.
44. Kirkland AE, Sarlo GL, Holton KF. The role of magnesium in neurological disorders. Nutrients. 2018;10(6):730.
45. Magnesium sulfate intravenous side effects by likelihood and severity. WebMD. Accessed April 9, 2023. https://www.webmd.com/drugs/2/drug-149570/magnesium-sulfate-intravenous/details/list-sideeffects
46. Scopolamine base transdermal system – uses, side effects, and more. WebMD. Accessed April 9, 2023. https://www.webmd.com/drugs/2/drug-14032/scopolamine-transdermal/details
47. Bolden C, Cusack B, Richelson E. Antagonism by antimuscarinic and neuroleptic compounds at the five cloned human muscarinic cholinergic receptors expressed in Chinese hamster ovary cells. J Pharmacol Exp Ther. 1992;260(2):576-580.
48. Janowsky DS, el-Yousef MK, Davis JM, et al. A cholinergic-adrenergic hypothesis of mania and depression. Lancet. 1972;2(7778):632-635.
49. Janowsky DS, Risch SC, Gillin JC. Adrenergic-cholinergic balance and the treatment of affective disorders. Prog Neuropsychopharmacol Biol Psychiatry. 1983;7(2-3):297-307.
50. Gershon S, Shaw FH. Psychiatric sequelae of chronic exposure to organophosphorous insecticides. Lancet. 1972;1(7191):1371-1374.
51. Davis KL, Berger PA, Hollister LE, et al. Physostigmine in mania. Arch Gen Psychiatry. 1978;35(1):119-122.
52. Wang JC, Hinrichs AL, Stock H, et al. Evidence of common and specific genetic effects: association of the muscarinic acetylcholine receptor M2 (CHRM2) gene with alcohol dependence and major depressive syndrome. Hum Mol Genet. 2004;13(17):1903-1911.
53. Brown RG. Effects of antidepressants and anticholinergics in a mouse “behavioral despair” test. Eur J Pharmacol. 1979;58(3):331-334.
54. Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266(5604):730-732.
55. Ji CX, Zhang JJ. Effect of scopolamine on depression in mice. Abstract in English. Yao Xue Xue Bao. 2011;46(4):400-405.
56. Furey ML, Drevets WC. Antidepressant efficacy of the antimuscarinic drug scopolamine: a randomized, placebo-controlled clinical trial. Arch Gen Psychiatry. 2006;63(10):1121-1129.
57. Drevets WC, Furey ML. Replication of scopolamine’s antidepressant efficacy in major depressive disorder: a randomized, placebo-controlled clinical trial. Biol Psychiatry. 2010;67(5):432-438.
58. Furey ML, Khanna A, Hoffman EM, et al. Scopolamine produces larger antidepressant and antianxiety effects in women than in men. Neuropsychopharmacology. 2010;35(12):2479-2488.
59. Gibbs RB, Gabor R, Cox T, et al. Effects of raloxifene and estradiol on hippocampal acetylcholine release and spatial learning in the rat. Psychoneuroendocrinology. 2004;29(6):741-748.
60. Pongrac JL, Gibbs RB, Defranco DB. Estrogen-mediated regulation of cholinergic expression in basal forebrain neurons requires extracellular-signal-regulated kinase activity. Neuroscience. 2004;124(4):809-816.
61. Daniel JM, Dohanich GP. Acetylcholine mediates the estrogen-induced increase in NMDA receptor binding in CA1 of the hippocampus and the associated improvement in working memory. J Neurosci. 2001;21(17):6949-6956.
62. Gerhard DM, Wohleb ES, Duman RS. Emerging treatment mechanisms for depression: focus on glutamate and synaptic plasticity. Drug Discov Today. 2016;21(3):454-464.
63. Voderholzer U. Sleep deprivation and antidepressant treatment. Dialogues Clin Neurosci. 2003;5(4):366-369.
64. Hasselmann H. Scopolamine and depression: a role for muscarinic antagonism? CNS Neurol Disord Drug Targets. 2014;13(4):673-683.
65. Transderm scopolamine [prescribing information]. Warren, NJ: GSK Consumer Healthcare; 2019.
66. Jaffe RJ, Novakovic V, Peselow ED. Scopolamine as an antidepressant: a systematic review. Clin Neuropharmacol. 2013;36(1):24-26.
67. Karameh WK, Khani M. Intravenous clomipramine for treatment-resistant obsessive-compulsive disorder. Int J Neuropsychopharmacol. 2015;19(2):pyv084.
68. Andrews ET, Beattie RM, Tighe MP. Functional abdominal pain: what clinicians need to know. Arch Dis Child. 2020;105(10):938-944. doi:10.1136/archdischild-2020-318825
69. Aliane V, Pérez S, Bohren Y, et al. Key role of striatal cholinergic interneurons in processes leading to arrest of motor stereotypies. Brain. 2011;134(Pt 1):110-118. doi:10.1093/brain/awq285
70. Tzavara ET, Bymaster FP, Davis RJ, et al. M4 muscarinic receptors regulate the dynamics of cholinergic and dopaminergic neurotransmission: relevance to the pathophysiology and treatment of related CNS pathologies. FASEB J. 2004;18(12):1410-1412. doi:10.1096/fj.04-1575fje
71. Korczyn AD, Kish I. The mechanism of imipramine in enuresis nocturna. Clin Exp Pharmacol Physiol. 1979;6(1):31-35. doi:10.1111/j.1440-1681.1979.tb00004.x
72. Trimble MR. Worldwide use of clomipramine. J Clin Psychiatry. 1990;51(Suppl):51-54; discussion 55-58.
73. Gong W, Zhang S, Zong Y, et al. Involvement of the microglial NLRP3 inflammasome in the anti-inflammatory effect of the antidepressant clomipramine. J Affect Disord. 2019;254:15-25.
74. Piwowarska J, Wrzosek M, Radziwon’-Zaleska M. Serum cortisol concentration in patients with major depression after treatment with clomipramine. Pharmacol Rep. 2009;61(4):604-611.
75. Danish University Antidepressant Group (DUAG). Clomipramine dose-effect study in patients with depression: clinical end points and pharmacokinetics. Clin Pharmacol Ther. 1999;66(2):152-165.
76. Moukaddam NJ, Hirschfeld RMA. Intravenous antidepressants: a review. Depress Anxiety. 2004;19(1):1-9.
77. Gerretsen P, Pollock BG. Rediscovering adverse anticholinergic effects. J Clin Psychiatry. 2011;72(6):869-870. doi:10.4088/JCP.11ac07093
78. Thomas SJ, Shin M, McInnis MG, et al. Combination therapy with monoamine oxidase inhibitors and other antidepressants or stimulants: strategies for the management of treatment-resistant depression. Pharmacotherapy. 2015;35(4):433-449. doi:10.1002/phar.1576
79. Robles LA. Serotonin syndrome induced by fentanyl in a child: case report. Clin Neuropharmacol. 2015;38(5):206-208. doi:10.1097/WNF.0000000000000100
80. Fallon BA, Liebowitz MR, Campeas R, et al. Intravenous clomipramine for obsessive-compulsive disorder refractory to oral clomipramine: a placebo-controlled study. Arch Gen Psychiatry. 1998;55(10):918-924.
81. Vieta E, Florea I, Schmidt SN, et al. Intravenous vortioxetine to accelerate onset of effect in major depressive disorder: a 2-week, randomized, double-blind, placebo-controlled study. Int Clin Psychopharmacol. 2019;34(4):153-160.
82. Kasper S, Müller-Spahn F. Intravenous antidepressant treatment: focus on citalopram. Eur Arch Psychiatry Clin Neurosci. 2002;252(3):105-109.
83. Togay B, El-Mallakh RS. Posttraumatic stress disorder: from pathophysiology to pharmacology. Current Psychiatry. 2020;19(5):33-39.
84. Adhikari A, Lerner TN, Finkelstein J, et al. Basomedial amygdala mediates top-down control of anxiety and fear. Nature. 2015;527(7577):179-185. doi:10.1038/nature15698
85. Lipov E. In search of an effective treatment for combat-related post-traumatic stress disorder (PTSD): can the stellate ganglion block be the answer? Pain Pract. 2010;10(4):265-266.
86. Lipov E, Ritchie EC. A review of the use of stellate ganglion block in the treatment of PTSD. Curr Psychiatry Rep. 2015;17(8):599.
87. Olmsted KLR, Bartoszek M, McLean B, et al. Effect of stellate ganglion block treatment on posttraumatic stress disorder symptoms: a randomized clinical trial. JAMA Psychiatry. 2020;77(2):130-138.
88. Lipov E, Candido K. The successful use of left-sided stellate ganglion block in patients that fail to respond to right-sided stellate ganglion block for the treatment of post-traumatic stress disorder symptoms: a retrospective analysis of 205 patients. Mil Med. 2021;186(11-12):319-320.
89. Li Y, Loshak H. Stellate ganglion block for the treatment of post-traumatic stress disorder, depression, and anxiety. Canadian J Health Technol. 2021;1(3):1-30.
90. Kerzner J, Liu H, Demchenko I, et al. Stellate ganglion block for psychiatric disorders: a systematic review of the clinical research landscape. Chronic Stress (Thousand Oaks). 2021;5:24705470211055176.
91. Wie C, Gupta R, Maloney J, et al. Interventional modalities to treat complex regional pain syndrome. Curr Pain Headache Rep. 2021;25(2):10. doi:10.1007/s11916-020-00904-5
92. Chaturvedi A, Dash HH. Sympathetic blockade for the relief of chronic pain. J Indian Med Assoc. 2001;99(12):698-703.
93. Chester M, Hammond C. Leach A. Long-term benefits of stellate ganglion block in severe chronic refractory angina. Pain. 2000;87(1):103-105. doi:10.1016/S0304-3959(00)00270-0
94. Jeon Y. Therapeutic potential of stellate ganglion block in orofacial pain: a mini review. J Dent Anesth Pain Med. 2016;16(3):159-163. doi:10.17245/jdapm.2016.16.3.159
95. Shan HH, Chen HF, Ni Y, et al. Effects of stellate ganglion block through different approaches under guidance of ultrasound. Front Surg. 2022;8:797793. doi:10.3389/fsurg.2021.797793
96. Goel V, Patwardhan AM, Ibrahim M, et al. Complications associated with stellate ganglion nerve block: a systematic review. Reg Anesth Pain Med. 2019;rapm-2018-100127. doi:10.1136/rapm-2018-100127
97. Rowe FJ, Noonan CP. Botulinum toxin for the treatment of strabismus. Cochrane Database Syst Rev. 2017;3(3):CD006499.
98. Roggenkämper P, Jost WH, Bihari K, et al. Efficacy and safety of a new botulinum toxin type A free of complexing proteins in the treatment of blepharospasm. J Neural Transm (Vienna). 2006;113(3):303-312.
99. Heckmann M, Ceballos-Baumann AO, Plewig G; Hyperhidrosis Study Group. Botulinum toxin A for axillary hyperhidrosis (excessive sweating). N Engl J Med. 2001;344(7):488-493.
100. Carruthers JA, Lowe NJ, Menter MA, et al. A multicenter, double-blind, randomized, placebo-controlled study of the efficacy and safety of botulinum toxin type A in the treatment of glabellar lines. J Am Acad Dermatol. 2002;46(6):840-849.
101. Schurch B, de Sèze M, Denys P, et al. Botulinum toxin type A is a safe and effective treatment for neurogenic urinary incontinence: results of a single treatment, randomized, placebo controlled 6-month study. J Urol. 2005;174:196–200.
102. Aurora SK, Winner P, Freeman MC, et al. OnabotulinumtoxinA for treatment of chronic migraine: Pooled analyses of the 56-week PREEMPT clinical program. Headache. 2011;51(9):1358-1373.
103. Dashtipour K, Chen JJ, Walker HW, et al. Systematic literature review of abobotulinumtoxinA in clinical trials for adult upper limb spasticity. Am J Phys Med Rehabil. 2015;94(3):229-238.
104. Nitti VW, Dmochowski R, Herschorn S, et al. OnabotulinumtoxinA for the treatment of patients with overactive bladder and urinary incontinence: results of a phase 3, randomized, placebo-controlled trial. J Urol. 2017;197(2S):S216-S223.
105. Jongerius PH, van den Hoogen FJA, van Limbeek J, et al. Effect of botulinum toxin in the treatment of drooling: a controlled clinical trial. Pediatrics. 2004;114(3):620-627.
106. Zaninotto, G. Annese V, Costantini M, et al. Randomized controlled trial of botulinum toxin versus laparoscopic heller myotomy for esophageal achalasia. Ann Surg. 2004;239(3):364-370.
107. Dressler D, Adib Saberi F. Botulinum toxin: mechanisms of action. Eur Neurol. 2005;53:3-9.
108. Lewis MB, Bowler PJ. Botulinum toxin cosmetic therapy correlates with a more positive mood. J Cosmet Dermatol. 2009;8(1):24-26.
109. Affatato O, Moulin TC, Pisanu C, et al. High efficacy of onabotulinumtoxinA treatment in patients with comorbid migraine and depression: a meta-analysis. J Transl Med. 2021;19(1):133.
110. Finzi E, Wasserman E. Treatment of depression with botulinum toxin A: a case series. Dermatol Surg. 2006;32(5):645-649; discussion 649-650.
111. Schulze J, Neumann I, Magid M, et al. Botulinum toxin for the management of depression: an updated review of the evidence and meta-analysis. J Psychiatr Res. 2021;135:332-340.
112. Finzi E, Rosenthal NE. Emotional proprioception: treatment of depression with afferent facial feedback. J Psychiatr Res. 2016;80:93-96.
113. Söderkvist S, Ohlén K, Dimberg U. How the experience of emotion is modulated by facial feedback. J Nonverbal Behav. 2018;42(1):129-151.
114. Lewis, MB. The interactions between botulinum-toxin-based facial treatments and embodied emotions. Sci Rep. 2018;8(1):14720.
115. Li Y, Liu J, Liu X, et al. Antidepressant-like action of single facial injection of botulinum neurotoxin A is associated with augmented 5-HT levels and BDNF/ERK/CREB pathways in mouse brain. Neurosci Bull. 2019;35(4):661-672. Erratum in: Neurosci Bull. 2019;35(4):779-780.
116. Gündel H, Wolf A, Xidara V, et al. High psychiatric comorbidity in spasmodic torticollis: a controlled study. J Nerv Ment Dis. 2003;191(7):465-473.
117. Hall TA, McGwin G Jr, Searcey K, et al. Health-related quality of life and psychosocial characteristics of patients with benign essential blepharospasm. Arch Ophthalmol. 2006;124(1):116-119.
118. Ceylan D, Erer S, Zarifog˘lu M, et al. Evaluation of anxiety and depression scales and quality of life in cervical dystonia patients on botulinum toxin therapy and their relatives. Neurol Sci. 2019;40(4):725-731.
119. Heller AS, Lapate RC, Mayer KE, et al. The face of negative affect: trial-by-trial corrugator responses to negative pictures are positively associated with amygdala and negatively associated with ventromedial prefrontal cortex activity. J Cogn Neurosci. 2014;26(9):2102-2110.
120. Makunts T, Wollmer MA, Abagyan R. Postmarketing safety surveillance data reveals antidepressant effects of botulinum toxin across various indications and injection sites. Sci Rep. 2020;10(1):12851.
121. Ahsanuddin S, Roy S, Nasser W, et al. Adverse events associated with botox as reported in a Food and Drug Administration database. Aesthetic Plast Surg. 2021;45(3):1201-1209. doi:10.1007/s00266-020-02027-z
122. Kashif M, Tahir S, Ashfaq F, et al. Association of myofascial trigger points in neck and shoulder region with depression, anxiety, and stress among university students. J Pak Med Assoc. 2021;71(9):2139-2142.
123. Cigarán-Méndez M, Jiménez-Antona C, Parás-Bravo P, et al. Active trigger points are associated with anxiety and widespread pressure pain sensitivity in women, but not men, with tension type headache. Pain Pract. 2019;19(5):522-529.
124. Palacios-Ceña M, Castaldo M, Wang K, et al. Relationship of active trigger points with related disability and anxiety in people with tension-type headache. Medicine (Baltimore). 2017;96(13):e6548.
125. Karadas Ö, Inan LE, Ulas Ü, et al. Efficacy of local lidocaine application on anxiety and depression and its curative effect on patients with chronic tension-type headache. Eur Neurol. 2013;70(1-2):95-101.
126. Gerwin RD. Classification, epidemiology and natural history of myofascial pain syndrome. Curr Pain Headache Rep. 2001;5(5):412-420.
127. Castro Sánchez AM, García López H, Fernández Sánchez M, et al. Improvement in clinical outcomes after dry needling versus myofascial release on pain pressure thresholds, quality of life, fatigue, pain intensity, quality of sleep, anxiety, and depression in patients with fibromyalgia syndrome. Disabil Rehabil. 2019;41(19):2235-2246.
128. Healy GM, Finn DP, O’Gorman DA, et al. Pretreatment anxiety and pain acceptance are associated with response to trigger point injection therapy for chronic myofascial pain. Pain Med. 2015;16(10):1955-1966.
129. Morjaria JB, Lakshminarayana UB, Liu-Shiu-Cheong P, et al. Pneumothorax: a tale of pain or spontaneity. Ther Adv Chronic Dis. 2014;5(6):269-273.
De-pathologizing gender identity: Psychiatry’s role
Treating patients who are transgender or gender diverse (TGGD) requires an understanding of the social and psychological factors that have a unique impact on this population. As clinicians, it is our responsibility to understand the social, cultural, and political issues our patients face, both historically and currently. In this article, we provide information about the nature of gender and gender identity as separate from biological sex and informed by a person’s perception of self as male, female, nonbinary, or other variation.
Psychiatrists must be aware of how individuals who are TGGD have been perceived, classified, and treated by the medical profession, as this history is often a source of mistrust and a barrier to treatment for patients who need psychiatric care. This includes awareness of the “gatekeeping” role that persists in medical institutions today: applying strict eligibility criteria to determine the “fitness” of individuals who are transgender to pursue medical transition, as compared to the informed-consent model that is widely applied to other medical interventions. Our review of minority stress theory, as applicable to this patient population, provides a context and framework for empathic approaches to care for patients who are TGGD. Recognizing barriers to care and ways in which we can create a supportive environment for treatment will allow for tailored approaches that better fit the unique needs of this patient population.
The gender binary
In Western societies, gender has often been viewed as “binary,” oppositional, and directly correlated with physical sex or presumed anatomy.1 The theory of gender essentialism insists that sex and gender are indistinguishable from one another and provide 2 “natural” and distinct categories: women and men. The “gender/sex” binary refers to the belief that individuals born with 2 X chromosomes will inherently develop into and fulfill the social roles of women, and those born with an X and a Y chromosome will develop into and fulfill the social roles of men.1 In this context, “sex” refers to biological characteristics of individuals, including combinations of sex chromosomes, anatomy, and the development of sex characteristics during puberty. The term “gender” refers to the social, cultural, and behavioral aspects of being a man, woman, both, or neither, and “gender identity” refers to one’s internal, individual sense of self and experience of gender (Figure 12). Many Western cultures are now facing destabilization of the gender/sex binary in social, political, and interpersonal contexts.1 This is perhaps most clearly seen in the battle for self-determination and protection by laws affecting individuals who are transgender as well as the determination of other groups to maintain traditional sex and gender roles, often through political action. Historically, individuals who are TGGD have been present in a variety of cultures. For example, most Native American cultures have revered other-gendered individuals, more recently referred to as “two-spirited.” Similarly, the Bugis people of South Sulawesi, Indonesia, recognize 5 genders that exist on a nonbinary spectrum.3
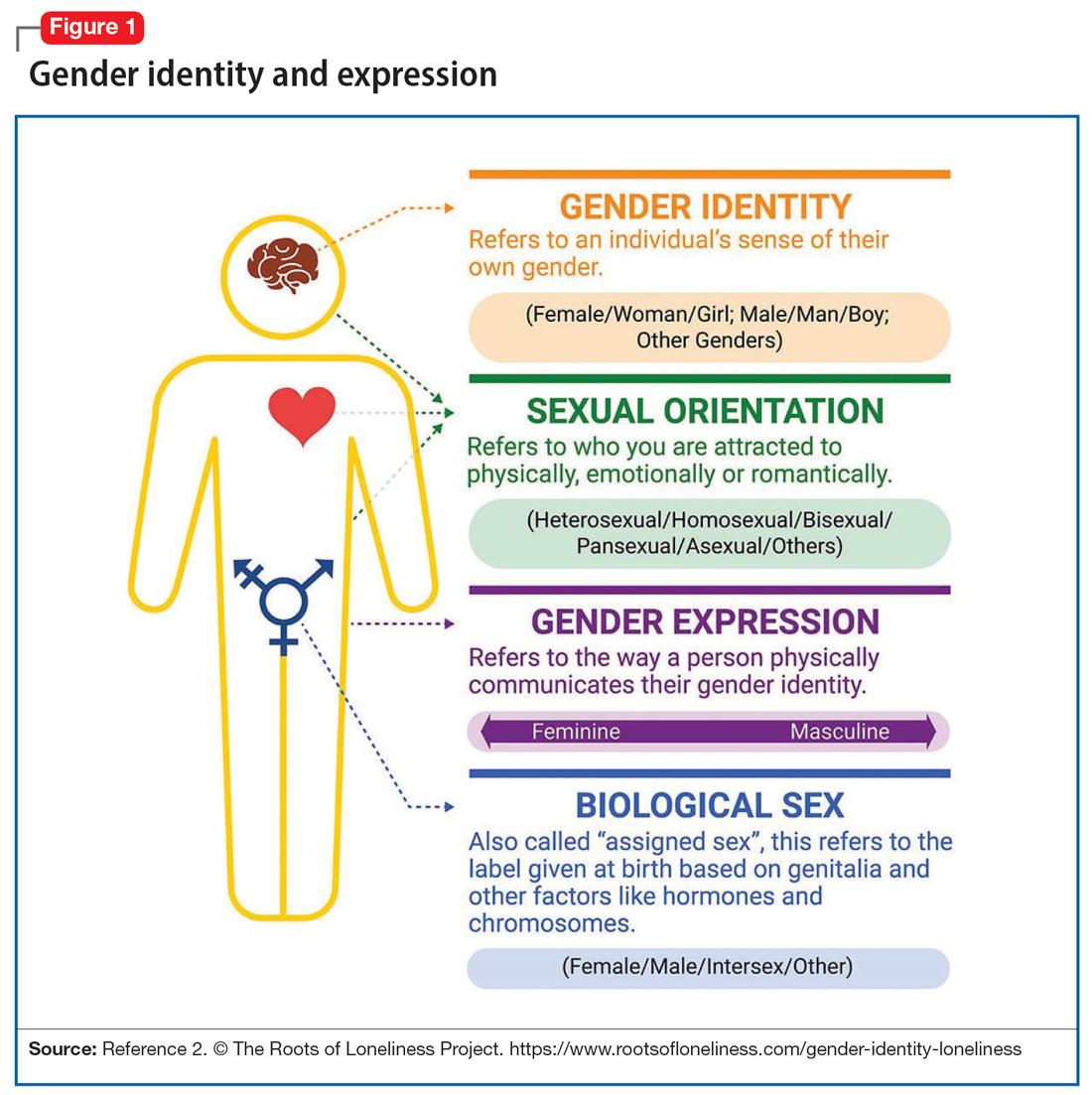
Despite its prevalence in Western society, scientific evidence for the gender/sex binary is lacking. The gender similarities hypothesis states that males and females are similar in most, but not all, psychological variables and is supported by multiple meta-analyses examining psychological gender differences.4 In a 2005 review of 46 meta-analyses of gender-differences, studied through behavior analysis, effect sizes for gender differences were trivial or small in almost 75% of examined variables.5 Analyzing for internal consistency among studies showing large gender/sex differences, Joel et al6 found that, on measures of personality traits, attitudes, interests, and behaviors were rarely homogenous in the brains of males or females. In fact, <1% of study participants showed only masculine or feminine traits, whereas 55% showed a combination, or mosaic, of these traits.6 These findings were supported by further research in behavioral neuroendocrinology that demonstrated a lack of hormonal evidence for 2 distinct sexes. Both estrogen (the “female” hormone) and testosterone (the “male” hormone) are produced by both biological males and females. Further, levels of estradiol do not significantly differ between males and females, and, in fact, in nonpregnant females, estradiol levels are more similar to those of males than to those of pregnant females.1 In the last decade, imaging studies of the human brain have shown that brain structure and connectivity in individuals who are transgender are more similar to those of their experienced gender than of their natal sex.7 In social analyses of intersex individuals (individuals born with ambiguous physical sex characteristics), surgical assignment into the binary gender system did not improve—and often worsened—feelings of isolation and shame.1
The National Institutes of Health defines gender as “socially constructed and enacted roles and behaviors which occur in a historical and cultural context and vary across societies and time.”8 The World Health Organization (WHO) provides a similar definition, and the evidence to support this exists in social-role theory, social-identity theory, and the stereotype-content model. However, despite evidence disputing a gender/sex binary, this method of classifying individuals into a dyad persists in many areas of modern culture, from gender-specific physical spaces (bathrooms, classrooms, store brands), language (pronouns), and laws. This desire for categorization helps fulfill social and psychological needs of groups and individuals by providing group identities and giving structure to the complexity of modern-day life. Identity and group membership provide a sense of belonging, source of self-esteem, and avoidance of ambiguity. Binary gender stereotypes provide expectations that allow anticipation and prediction of our social environments.9 However, the harm of perpetuating the false gender/sex binary is well documented and includes social and economic penalties, extreme violence, and even death. The field of medicine has not been immune from practices that implicitly endorse the gender/sex connection, as seen in the erroneous use of gender in biomedical writings at the highest levels and evidenced in research examining “gender” differences in disease incidence.
Gender diversity as a pathology
The American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders (DSM) has been a source of pathologizing gender diversity since the 1960s, with the introduction of “transsexualism” in DSM-II10 and “gender identity disorder of childhood” in DSM-III.11 These diagnoses were listed under the headings of “sexual deviations” and “psychosexual disorders” in the respective DSM editions. This illustrates how gender diversity was viewed as a mental illness/defect. As the DSM developed through various revisions, so have these diagnoses. DSM-IV used the diagnosis “gender identity disorder.”12 Psychiatry has evolved away from this line of thinking by focusing on the distress from biological sex characteristics that are “incongruent” with an individual’s gender identity, leading to the development of the gender dysphoria diagnosis.13 While this has been a positive step in psychiatry’s efforts to de-pathologize individuals who are gender-diverse, it raises the question: should such diagnoses be included in the DSM at all?
The gender dysphoria diagnosis continues to be needed by many individuals who are TGGD in order to access gender-affirming health care services. Mental health professionals are placed in a gatekeeping role by the expectation that they provide letters of “support” to indicate an individual is of sound mind and consistent gender identity to have services covered by insurance providers. In this way, the insurance industry and the field of medicine continue to believe that individuals who are TGGD need psychiatric permission and/or counsel regarding their gender identity. This can place psychiatry in a role of controlling access to necessary care while also creating a possible distrust in our ability to provide care to patients who are gender-diverse. This is particularly problematic given the high rates of depression, anxiety, trauma, and substance use within these communities.14 In the WHO’s ICD-11, gender dysphoria was changed to gender incongruence and is contained in the category of “Conditions related to sexual health.”15 This indicates continued evolution of how medicine views individuals who are TGGD, and offers hope that psychiatry and the DSM will follow suit.
Continue to: Minority stress theory
Minority stress theory
Ilan Meyer’s minority stress theory explores how cultural and social factors impact mental health functioning (Figure 216). Minority stress theory, which was originally developed for what at the time was described as the lesbian, gay, and bisexual communities, purports that the higher prevalence of mental health disorders among such individuals is likely due to social stigma, discrimination, and stressors associated with minority status. More recently, minority stress theory has been expanded to provide framework for individuals who are TGGD. Hendricks et al17 explain how distal, proximal, and resilience factors contribute to mental health outcomes among these individuals. Distal factors, such as gender-related discrimination, harassment, violence, and rejection, explain how systemic, cultural, and environmental events lead to overt stress. Proximal factors consist of an individual’s expectation and anticipation of negative and stressful events and the internalization of negative attitudes and prejudice (ie, internalized transphobia). Resilience factors consist of community connectedness and within-group identification and can help mediate the negative effects of distal and proximal factors.
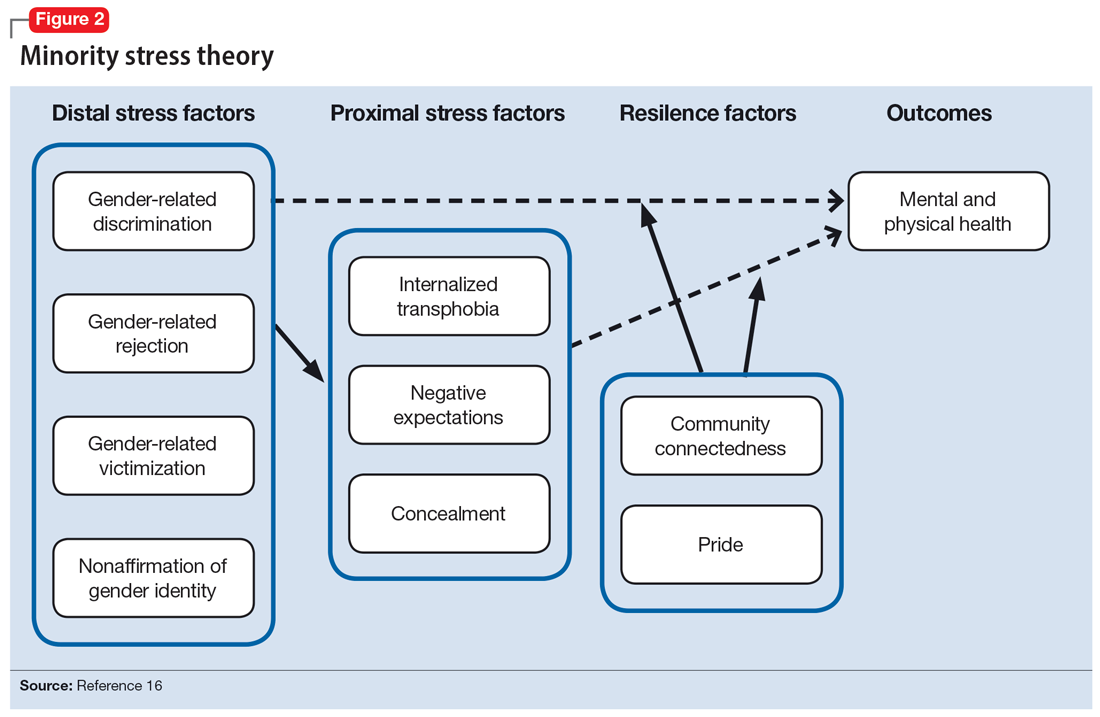
As clinicians, understanding our patients’ experiences and expectations can help us better engage with them and create an environment of safety and healing. Minority stress theory framework suggests that patients may start treatment with distrust or suspicion in light of previous negative experiences. They may also be likely to expect clinicians to be judgmental or to lack understanding of them. The 2015 US Transgender Survey found that 33% of individuals who are TGGD who sought medical treatment in the past year had at least 1 negative experience related to their gender identity (Table 118). Twenty-four percent reported having to educate their clinician about people who are TGGD, while 15% reported the health care professional asked invasive or unnecessary questions about their gender status that were unrelated to their visit. While psychiatry is often distinct from the larger medical field, it is important to understand the negative encounters individuals who are TGGD have likely experienced in medicine, and how those events may skew their feelings about psychiatric treatment. This is especially salient given the higher prevalence of various psychiatric disorders among individuals who are TGGD.18
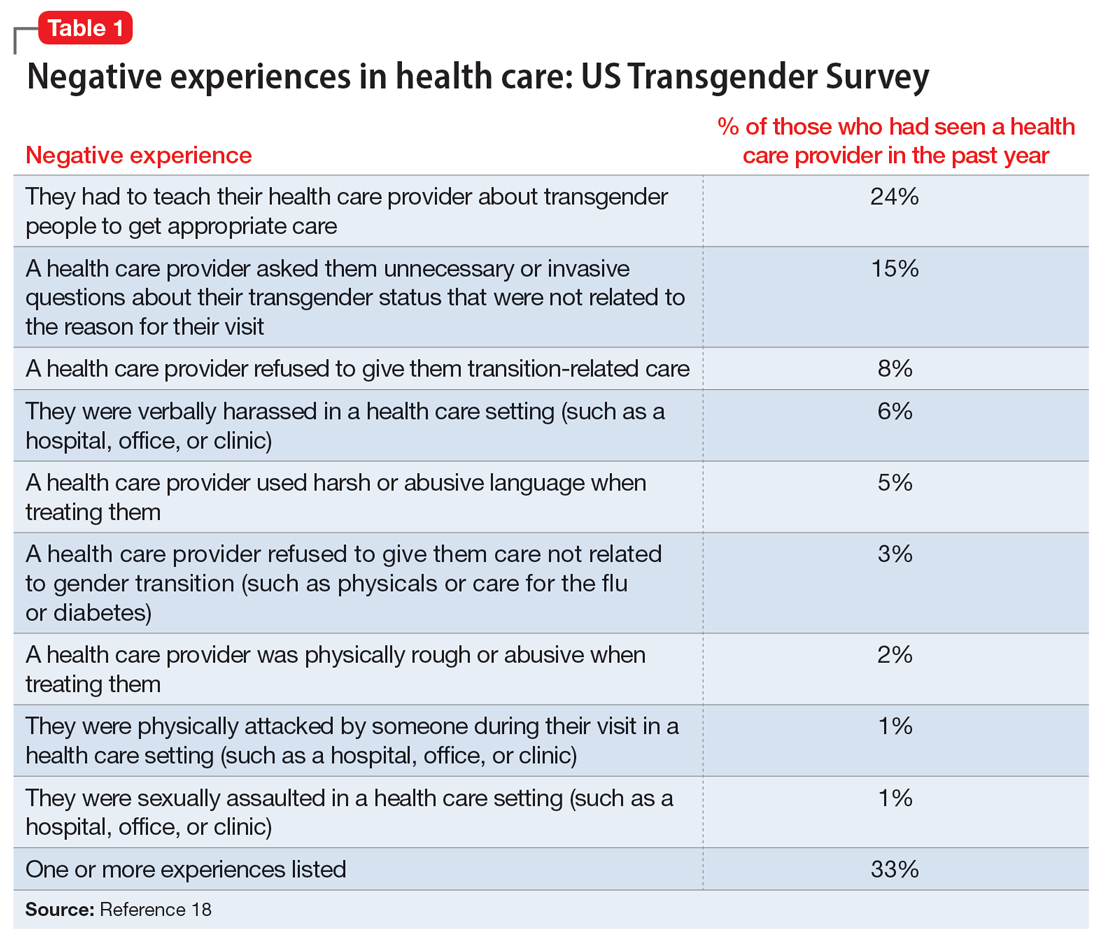
According to the US Transgender Survey, 39% of participants were currently experiencing serious psychological distress, which is nearly 8 times the rate in the US population (5%).18 When extrapolated, this data indicates that we in psychiatry are likely to work with individuals who identify as TGGD, regardless of our expertise. Additionally, research indicates that having access to gender-affirming care—such as hormone replacement therapy, gender-affirming surgery, voice therapy, and other treatments—greatly improves mental health issues such as anxiety, depression, and suicidality among individuals who are TGGD.19,20 It is in this way we in psychiatry must do more than just care for our patients by becoming advocates for them to receive the care they need and deserve. While at times we may want to stay out of politics and other public discourse, it is becoming increasingly necessary as health care is entrenched in politics.
Clinical applicability
Because individuals who are TGGD experience higher rates of depression, anxiety, substance use, and other psychiatric disorders,14 it is increasingly likely that many clinicians will be presented with opportunities to treat such individuals. Despite high rates of psychiatric disorders, individuals who are TGGD often avoid treatment due to concerns about being pathologized, stereotyped, and/or encountering professionals who lack the knowledge to treat them as they are.21 Several studies recommend clinicians better equip themselves to appropriately provide services to individuals who are TGGD.21 Some advise seeking education to understand the unique needs of these patients and to help stay current with appropriate terminology and language (Table 222). This also implies not relying on patients to educate clinicians in understanding their specific needs and experiences.
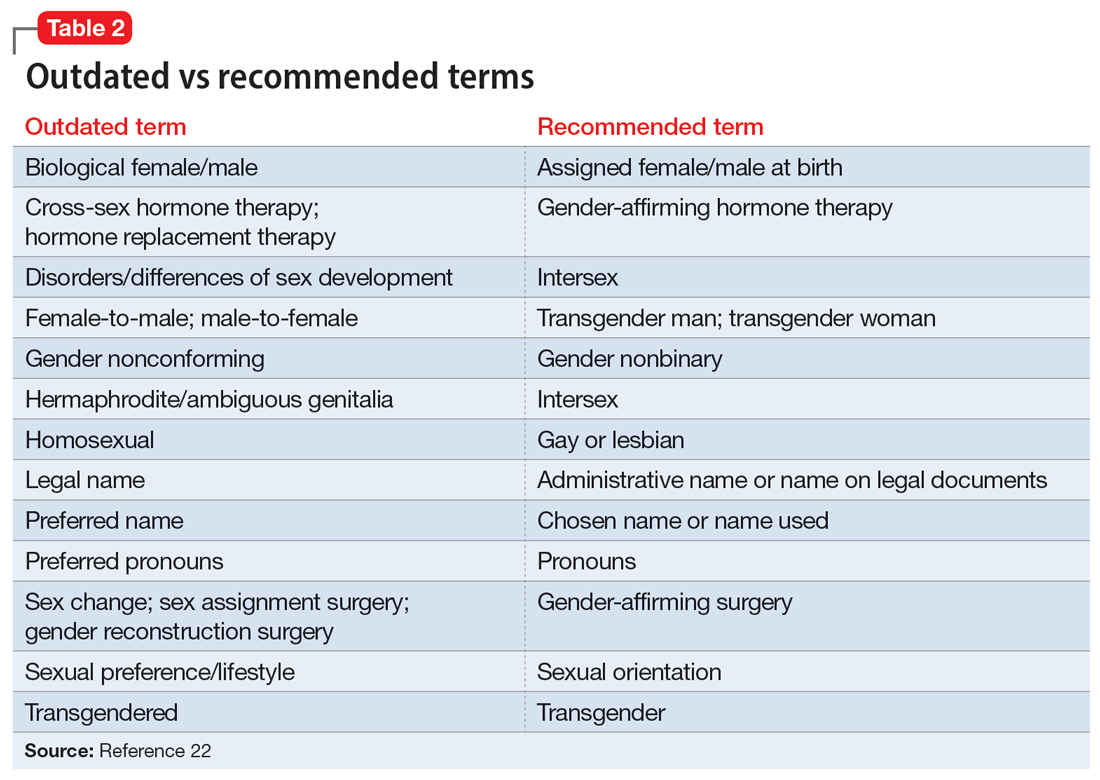
Making assumptions about a patient’s identity is one of the most commonly reported issues by individuals who are TGGD. Therefore, it is critical to avoid making assumptions about patients based on binary stereotypes.23,24 We can circumvent these mistakes by asking every patient for their name and pronouns, and introducing ourselves with our pronouns. This illustrates an openness and understanding of the importance of identity and language, and makes it common practice from the outset. Integrating the use of gender-neutral language into paperwork, intake forms, charting, and conversation will also help avoid the pitfalls of misgendering and making false assumptions. This will also allow for support staff, medical assistants, and others to use correct language with patients. Having a patient’s used name and pronouns visible for everyone who works with the patient is necessary to effectively meet the patient’s needs. Additionally, understanding that the range of experiences and needs for individuals who are TGGD is heterogeneous can help reduce assumptions and ensure we are asking for needed information. It is also important to ask for only relevant information needed to provide treatment.
Continue to: Resources are widely available...
Resources are widely available to aid in the care of individuals who are TGGD. In 2022, the World Professional Association for Transgender Health released new guidelines—Standards of Care 8—for working with individuals who are TGGD.25 While these standards include a section dedicated to mental health, they also provide guidelines on education, assessments, specific demographic groups, hormone therapy, primary care, and sexual health. Additionally, while we may not want the role of gatekeeping for individuals to receive gender-affirming care, we work within a health care and insurance system that continues to require psychiatric assessment for such surgeries. In this role, we must do our part to educate ourselves in how to best provide these assessments and letters of support to help patients receive appropriate and life-saving care.
Finally, in order to provide a more comfortable and affirming space for individuals who are TGGD, develop ways to self-assess and monitor the policies, procedures, and language used within your practice, clinic, or institution. Monitoring the language used in charting to ensure consistency with the individual’s gender identity is important for our own understanding of the patient, and for patients to feel seen. This is especially true given patients’ access to medical records under the Cures Act. Moreover, it is essential to be cognizant of how you present clients to others in consultation or care coordination to ensure the patient is identified correctly and consistently by clinicians and staff.
Bottom Line
Understanding the social, cultural, and medical discrimination faced by patients who are transgender or gender diverse can make us better suited to engage and treat these individuals in an affirming and supportive way.
Related Resources
- World Professional Association of Transgender Health (WPATH) Standards of Care—8th edition. https://www.tandfonline.com/doi/pdf/10.1080/26895269.2022.2100644
- The Fenway Institute: National LGBTQIA+ Health Education Center. https://fenwayhealth.org/the-fenway-institute/education/the-national-lgbtia-health-education-center/
1. Morgenroth T, Ryan MK. The effects of gender trouble: an integrative theoretical framework of the perpetuation and disruption of the gender/sex binary. Perspect Psychol Sci. 2021;16(6):1113-1142. doi:10.1177/1745691620902442
2. The Roots of Loneliness Project. Accessed April 8, 2023. https://www.rootsofloneliness.com/gender-identity-loneliness
3. Davies SG. Challenging Gender Norms: Five Genders Among Bugis in Indonesia. Thomson Wadsworth; 2007.
4. Hyde JS. The gender similarities hypothesis. Am Psychol. 2005;60(6):581-592. doi:10.1037/0003-066X.60.6.581
5. Joel D. Beyond the binary: rethinking sex and the brain. Neurosci Biobehav Rev. 2021;122:165-175. doi:10.1016/j.neubiorev.2020.22.018
6. Joel D, Berman Z, Tavor I, et al. Sex beyond the genitalia: the human brain mosaic. Proc Natl Acad Sci U S A. 2015;112(50):15468-15473. doi:10.1073/pnas.1509654112
7. Palmer BF, Clegg DJ. A universally accepted definition of gender will positively impact societal understanding, acceptance, and appropriateness of health care. Mayo Clin Proc. 2020;95(10):2235-2243. doi:10.1016/j.mayocp.2020.01.031
8. Office of Research on Women’s Health. Sex & Gender. National Institutes of Health. Accessed April 6, 2023. https://orwh.od.nih.gov/sex-gender
9. Morgenroth T, Sendén MG, Lindqvist A, et al. Defending the sex/gender binary: the role of gender identification and need for closure. Soc Psychol Pers Sci. 2021;12(5):731-740.
10. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 2nd ed. American Psychiatric Association; 1968.
11. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3rd ed. American Psychiatric Association; 1980.
12. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. American Psychiatric Association; 1994.
13. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Publishing; 2013.
14. Wanta JW, Niforatos JD, Durbak E, et al. Mental health diagnoses among transgender patients in the clinical setting: an all-payer electronic health record study. Transgend Health. 2019;4(1):313-315.
15. World Health Organization. International Statistical Classification of Diseases. 11th ed. World Health Organization; 2019.
16. Meyer IH. Prejudice, social stress, and mental health in lesbian, gay, and bisexual populations: conceptual issues and research evidence. Psychol Bull. 2003;129(5):674-697. doi:10.1037/0033-2909.129.5.674
17. Hendricks ML, Testa RJ. A conceptual framework for clinical work with transgender and gender nonconforming clients: an adaptation of the Minority Stress Model. Profess Psychol: Res Pract. 2012;43(5):460-467. doi:10.1037/a0029597
18. James SE, Herman J, Keisling M, et al. The Report of the 2015 U.S. Transgender Survey. National Center for Transgender Equality; 2016. Accessed April 6, 2023. https://transequality.org/sites/default/files/docs/usts/USTS-Full-Report-Dec17.pdf
19. Almazan AN, Keuroghlian AS. Association between gender-affirming surgeries and mental health outcomes. JAMA Surg. 2021;156(7):611-618. doi:10.1001/jamasurg.2021.0952
20. Tordoff DM, Wanta JW, Collin A, et al. Mental health outcomes in transgender and nonbinary youths receiving gender-affirming care. JAMA Netw Open. 2022;5(2):e220978. doi:10.1001/jamanetworkopen.2022.0978
21. Snow A, Cerel J, Loeffler DN, et al. Barriers to mental health care for transgender and gender-nonconforming adults: a systematic literature review. Health Soc Work. 2019;44(3):149-155. doi:10.1093/hsw/hlz016
22. National LGBTQIA+ Health Education Center. Accessed April 8, 2023. https://www.lgbtqiahealtheducation.org
23. Baldwin A, Dodge B, Schick VR, et al. Transgender and genderqueer individuals’ experiences with health care providers: what’s working, what’s not, and where do we go from here? J Health Care Poor Underserved. 2018;29(4):1300-1318. doi:10.1353/hpu.2018.0097
24. Kcomt L, Gorey KM, Barrett BJ, et al. Healthcare avoidance due to anticipated discrimination among transgender people: a call to create trans-affirmative environments. SSM-Popul Health. 2020;11:100608. doi:10.1016/j.ssmph.2020.100608
25. Coleman E, Radix AE, Bouman WP, et al. Standards of care for the health of transgender and gender diverse people, version 8. Int J Transgender Health. 2022;23(Suppl 1):S1-S259.
Treating patients who are transgender or gender diverse (TGGD) requires an understanding of the social and psychological factors that have a unique impact on this population. As clinicians, it is our responsibility to understand the social, cultural, and political issues our patients face, both historically and currently. In this article, we provide information about the nature of gender and gender identity as separate from biological sex and informed by a person’s perception of self as male, female, nonbinary, or other variation.
Psychiatrists must be aware of how individuals who are TGGD have been perceived, classified, and treated by the medical profession, as this history is often a source of mistrust and a barrier to treatment for patients who need psychiatric care. This includes awareness of the “gatekeeping” role that persists in medical institutions today: applying strict eligibility criteria to determine the “fitness” of individuals who are transgender to pursue medical transition, as compared to the informed-consent model that is widely applied to other medical interventions. Our review of minority stress theory, as applicable to this patient population, provides a context and framework for empathic approaches to care for patients who are TGGD. Recognizing barriers to care and ways in which we can create a supportive environment for treatment will allow for tailored approaches that better fit the unique needs of this patient population.
The gender binary
In Western societies, gender has often been viewed as “binary,” oppositional, and directly correlated with physical sex or presumed anatomy.1 The theory of gender essentialism insists that sex and gender are indistinguishable from one another and provide 2 “natural” and distinct categories: women and men. The “gender/sex” binary refers to the belief that individuals born with 2 X chromosomes will inherently develop into and fulfill the social roles of women, and those born with an X and a Y chromosome will develop into and fulfill the social roles of men.1 In this context, “sex” refers to biological characteristics of individuals, including combinations of sex chromosomes, anatomy, and the development of sex characteristics during puberty. The term “gender” refers to the social, cultural, and behavioral aspects of being a man, woman, both, or neither, and “gender identity” refers to one’s internal, individual sense of self and experience of gender (Figure 12). Many Western cultures are now facing destabilization of the gender/sex binary in social, political, and interpersonal contexts.1 This is perhaps most clearly seen in the battle for self-determination and protection by laws affecting individuals who are transgender as well as the determination of other groups to maintain traditional sex and gender roles, often through political action. Historically, individuals who are TGGD have been present in a variety of cultures. For example, most Native American cultures have revered other-gendered individuals, more recently referred to as “two-spirited.” Similarly, the Bugis people of South Sulawesi, Indonesia, recognize 5 genders that exist on a nonbinary spectrum.3

Despite its prevalence in Western society, scientific evidence for the gender/sex binary is lacking. The gender similarities hypothesis states that males and females are similar in most, but not all, psychological variables and is supported by multiple meta-analyses examining psychological gender differences.4 In a 2005 review of 46 meta-analyses of gender-differences, studied through behavior analysis, effect sizes for gender differences were trivial or small in almost 75% of examined variables.5 Analyzing for internal consistency among studies showing large gender/sex differences, Joel et al6 found that, on measures of personality traits, attitudes, interests, and behaviors were rarely homogenous in the brains of males or females. In fact, <1% of study participants showed only masculine or feminine traits, whereas 55% showed a combination, or mosaic, of these traits.6 These findings were supported by further research in behavioral neuroendocrinology that demonstrated a lack of hormonal evidence for 2 distinct sexes. Both estrogen (the “female” hormone) and testosterone (the “male” hormone) are produced by both biological males and females. Further, levels of estradiol do not significantly differ between males and females, and, in fact, in nonpregnant females, estradiol levels are more similar to those of males than to those of pregnant females.1 In the last decade, imaging studies of the human brain have shown that brain structure and connectivity in individuals who are transgender are more similar to those of their experienced gender than of their natal sex.7 In social analyses of intersex individuals (individuals born with ambiguous physical sex characteristics), surgical assignment into the binary gender system did not improve—and often worsened—feelings of isolation and shame.1
The National Institutes of Health defines gender as “socially constructed and enacted roles and behaviors which occur in a historical and cultural context and vary across societies and time.”8 The World Health Organization (WHO) provides a similar definition, and the evidence to support this exists in social-role theory, social-identity theory, and the stereotype-content model. However, despite evidence disputing a gender/sex binary, this method of classifying individuals into a dyad persists in many areas of modern culture, from gender-specific physical spaces (bathrooms, classrooms, store brands), language (pronouns), and laws. This desire for categorization helps fulfill social and psychological needs of groups and individuals by providing group identities and giving structure to the complexity of modern-day life. Identity and group membership provide a sense of belonging, source of self-esteem, and avoidance of ambiguity. Binary gender stereotypes provide expectations that allow anticipation and prediction of our social environments.9 However, the harm of perpetuating the false gender/sex binary is well documented and includes social and economic penalties, extreme violence, and even death. The field of medicine has not been immune from practices that implicitly endorse the gender/sex connection, as seen in the erroneous use of gender in biomedical writings at the highest levels and evidenced in research examining “gender” differences in disease incidence.
Gender diversity as a pathology
The American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders (DSM) has been a source of pathologizing gender diversity since the 1960s, with the introduction of “transsexualism” in DSM-II10 and “gender identity disorder of childhood” in DSM-III.11 These diagnoses were listed under the headings of “sexual deviations” and “psychosexual disorders” in the respective DSM editions. This illustrates how gender diversity was viewed as a mental illness/defect. As the DSM developed through various revisions, so have these diagnoses. DSM-IV used the diagnosis “gender identity disorder.”12 Psychiatry has evolved away from this line of thinking by focusing on the distress from biological sex characteristics that are “incongruent” with an individual’s gender identity, leading to the development of the gender dysphoria diagnosis.13 While this has been a positive step in psychiatry’s efforts to de-pathologize individuals who are gender-diverse, it raises the question: should such diagnoses be included in the DSM at all?
The gender dysphoria diagnosis continues to be needed by many individuals who are TGGD in order to access gender-affirming health care services. Mental health professionals are placed in a gatekeeping role by the expectation that they provide letters of “support” to indicate an individual is of sound mind and consistent gender identity to have services covered by insurance providers. In this way, the insurance industry and the field of medicine continue to believe that individuals who are TGGD need psychiatric permission and/or counsel regarding their gender identity. This can place psychiatry in a role of controlling access to necessary care while also creating a possible distrust in our ability to provide care to patients who are gender-diverse. This is particularly problematic given the high rates of depression, anxiety, trauma, and substance use within these communities.14 In the WHO’s ICD-11, gender dysphoria was changed to gender incongruence and is contained in the category of “Conditions related to sexual health.”15 This indicates continued evolution of how medicine views individuals who are TGGD, and offers hope that psychiatry and the DSM will follow suit.
Continue to: Minority stress theory
Minority stress theory
Ilan Meyer’s minority stress theory explores how cultural and social factors impact mental health functioning (Figure 216). Minority stress theory, which was originally developed for what at the time was described as the lesbian, gay, and bisexual communities, purports that the higher prevalence of mental health disorders among such individuals is likely due to social stigma, discrimination, and stressors associated with minority status. More recently, minority stress theory has been expanded to provide framework for individuals who are TGGD. Hendricks et al17 explain how distal, proximal, and resilience factors contribute to mental health outcomes among these individuals. Distal factors, such as gender-related discrimination, harassment, violence, and rejection, explain how systemic, cultural, and environmental events lead to overt stress. Proximal factors consist of an individual’s expectation and anticipation of negative and stressful events and the internalization of negative attitudes and prejudice (ie, internalized transphobia). Resilience factors consist of community connectedness and within-group identification and can help mediate the negative effects of distal and proximal factors.

As clinicians, understanding our patients’ experiences and expectations can help us better engage with them and create an environment of safety and healing. Minority stress theory framework suggests that patients may start treatment with distrust or suspicion in light of previous negative experiences. They may also be likely to expect clinicians to be judgmental or to lack understanding of them. The 2015 US Transgender Survey found that 33% of individuals who are TGGD who sought medical treatment in the past year had at least 1 negative experience related to their gender identity (Table 118). Twenty-four percent reported having to educate their clinician about people who are TGGD, while 15% reported the health care professional asked invasive or unnecessary questions about their gender status that were unrelated to their visit. While psychiatry is often distinct from the larger medical field, it is important to understand the negative encounters individuals who are TGGD have likely experienced in medicine, and how those events may skew their feelings about psychiatric treatment. This is especially salient given the higher prevalence of various psychiatric disorders among individuals who are TGGD.18

According to the US Transgender Survey, 39% of participants were currently experiencing serious psychological distress, which is nearly 8 times the rate in the US population (5%).18 When extrapolated, this data indicates that we in psychiatry are likely to work with individuals who identify as TGGD, regardless of our expertise. Additionally, research indicates that having access to gender-affirming care—such as hormone replacement therapy, gender-affirming surgery, voice therapy, and other treatments—greatly improves mental health issues such as anxiety, depression, and suicidality among individuals who are TGGD.19,20 It is in this way we in psychiatry must do more than just care for our patients by becoming advocates for them to receive the care they need and deserve. While at times we may want to stay out of politics and other public discourse, it is becoming increasingly necessary as health care is entrenched in politics.
Clinical applicability
Because individuals who are TGGD experience higher rates of depression, anxiety, substance use, and other psychiatric disorders,14 it is increasingly likely that many clinicians will be presented with opportunities to treat such individuals. Despite high rates of psychiatric disorders, individuals who are TGGD often avoid treatment due to concerns about being pathologized, stereotyped, and/or encountering professionals who lack the knowledge to treat them as they are.21 Several studies recommend clinicians better equip themselves to appropriately provide services to individuals who are TGGD.21 Some advise seeking education to understand the unique needs of these patients and to help stay current with appropriate terminology and language (Table 222). This also implies not relying on patients to educate clinicians in understanding their specific needs and experiences.

Making assumptions about a patient’s identity is one of the most commonly reported issues by individuals who are TGGD. Therefore, it is critical to avoid making assumptions about patients based on binary stereotypes.23,24 We can circumvent these mistakes by asking every patient for their name and pronouns, and introducing ourselves with our pronouns. This illustrates an openness and understanding of the importance of identity and language, and makes it common practice from the outset. Integrating the use of gender-neutral language into paperwork, intake forms, charting, and conversation will also help avoid the pitfalls of misgendering and making false assumptions. This will also allow for support staff, medical assistants, and others to use correct language with patients. Having a patient’s used name and pronouns visible for everyone who works with the patient is necessary to effectively meet the patient’s needs. Additionally, understanding that the range of experiences and needs for individuals who are TGGD is heterogeneous can help reduce assumptions and ensure we are asking for needed information. It is also important to ask for only relevant information needed to provide treatment.
Continue to: Resources are widely available...
Resources are widely available to aid in the care of individuals who are TGGD. In 2022, the World Professional Association for Transgender Health released new guidelines—Standards of Care 8—for working with individuals who are TGGD.25 While these standards include a section dedicated to mental health, they also provide guidelines on education, assessments, specific demographic groups, hormone therapy, primary care, and sexual health. Additionally, while we may not want the role of gatekeeping for individuals to receive gender-affirming care, we work within a health care and insurance system that continues to require psychiatric assessment for such surgeries. In this role, we must do our part to educate ourselves in how to best provide these assessments and letters of support to help patients receive appropriate and life-saving care.
Finally, in order to provide a more comfortable and affirming space for individuals who are TGGD, develop ways to self-assess and monitor the policies, procedures, and language used within your practice, clinic, or institution. Monitoring the language used in charting to ensure consistency with the individual’s gender identity is important for our own understanding of the patient, and for patients to feel seen. This is especially true given patients’ access to medical records under the Cures Act. Moreover, it is essential to be cognizant of how you present clients to others in consultation or care coordination to ensure the patient is identified correctly and consistently by clinicians and staff.
Bottom Line
Understanding the social, cultural, and medical discrimination faced by patients who are transgender or gender diverse can make us better suited to engage and treat these individuals in an affirming and supportive way.
Related Resources
- World Professional Association of Transgender Health (WPATH) Standards of Care—8th edition. https://www.tandfonline.com/doi/pdf/10.1080/26895269.2022.2100644
- The Fenway Institute: National LGBTQIA+ Health Education Center. https://fenwayhealth.org/the-fenway-institute/education/the-national-lgbtia-health-education-center/
Treating patients who are transgender or gender diverse (TGGD) requires an understanding of the social and psychological factors that have a unique impact on this population. As clinicians, it is our responsibility to understand the social, cultural, and political issues our patients face, both historically and currently. In this article, we provide information about the nature of gender and gender identity as separate from biological sex and informed by a person’s perception of self as male, female, nonbinary, or other variation.
Psychiatrists must be aware of how individuals who are TGGD have been perceived, classified, and treated by the medical profession, as this history is often a source of mistrust and a barrier to treatment for patients who need psychiatric care. This includes awareness of the “gatekeeping” role that persists in medical institutions today: applying strict eligibility criteria to determine the “fitness” of individuals who are transgender to pursue medical transition, as compared to the informed-consent model that is widely applied to other medical interventions. Our review of minority stress theory, as applicable to this patient population, provides a context and framework for empathic approaches to care for patients who are TGGD. Recognizing barriers to care and ways in which we can create a supportive environment for treatment will allow for tailored approaches that better fit the unique needs of this patient population.
The gender binary
In Western societies, gender has often been viewed as “binary,” oppositional, and directly correlated with physical sex or presumed anatomy.1 The theory of gender essentialism insists that sex and gender are indistinguishable from one another and provide 2 “natural” and distinct categories: women and men. The “gender/sex” binary refers to the belief that individuals born with 2 X chromosomes will inherently develop into and fulfill the social roles of women, and those born with an X and a Y chromosome will develop into and fulfill the social roles of men.1 In this context, “sex” refers to biological characteristics of individuals, including combinations of sex chromosomes, anatomy, and the development of sex characteristics during puberty. The term “gender” refers to the social, cultural, and behavioral aspects of being a man, woman, both, or neither, and “gender identity” refers to one’s internal, individual sense of self and experience of gender (Figure 12). Many Western cultures are now facing destabilization of the gender/sex binary in social, political, and interpersonal contexts.1 This is perhaps most clearly seen in the battle for self-determination and protection by laws affecting individuals who are transgender as well as the determination of other groups to maintain traditional sex and gender roles, often through political action. Historically, individuals who are TGGD have been present in a variety of cultures. For example, most Native American cultures have revered other-gendered individuals, more recently referred to as “two-spirited.” Similarly, the Bugis people of South Sulawesi, Indonesia, recognize 5 genders that exist on a nonbinary spectrum.3

Despite its prevalence in Western society, scientific evidence for the gender/sex binary is lacking. The gender similarities hypothesis states that males and females are similar in most, but not all, psychological variables and is supported by multiple meta-analyses examining psychological gender differences.4 In a 2005 review of 46 meta-analyses of gender-differences, studied through behavior analysis, effect sizes for gender differences were trivial or small in almost 75% of examined variables.5 Analyzing for internal consistency among studies showing large gender/sex differences, Joel et al6 found that, on measures of personality traits, attitudes, interests, and behaviors were rarely homogenous in the brains of males or females. In fact, <1% of study participants showed only masculine or feminine traits, whereas 55% showed a combination, or mosaic, of these traits.6 These findings were supported by further research in behavioral neuroendocrinology that demonstrated a lack of hormonal evidence for 2 distinct sexes. Both estrogen (the “female” hormone) and testosterone (the “male” hormone) are produced by both biological males and females. Further, levels of estradiol do not significantly differ between males and females, and, in fact, in nonpregnant females, estradiol levels are more similar to those of males than to those of pregnant females.1 In the last decade, imaging studies of the human brain have shown that brain structure and connectivity in individuals who are transgender are more similar to those of their experienced gender than of their natal sex.7 In social analyses of intersex individuals (individuals born with ambiguous physical sex characteristics), surgical assignment into the binary gender system did not improve—and often worsened—feelings of isolation and shame.1
The National Institutes of Health defines gender as “socially constructed and enacted roles and behaviors which occur in a historical and cultural context and vary across societies and time.”8 The World Health Organization (WHO) provides a similar definition, and the evidence to support this exists in social-role theory, social-identity theory, and the stereotype-content model. However, despite evidence disputing a gender/sex binary, this method of classifying individuals into a dyad persists in many areas of modern culture, from gender-specific physical spaces (bathrooms, classrooms, store brands), language (pronouns), and laws. This desire for categorization helps fulfill social and psychological needs of groups and individuals by providing group identities and giving structure to the complexity of modern-day life. Identity and group membership provide a sense of belonging, source of self-esteem, and avoidance of ambiguity. Binary gender stereotypes provide expectations that allow anticipation and prediction of our social environments.9 However, the harm of perpetuating the false gender/sex binary is well documented and includes social and economic penalties, extreme violence, and even death. The field of medicine has not been immune from practices that implicitly endorse the gender/sex connection, as seen in the erroneous use of gender in biomedical writings at the highest levels and evidenced in research examining “gender” differences in disease incidence.
Gender diversity as a pathology
The American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders (DSM) has been a source of pathologizing gender diversity since the 1960s, with the introduction of “transsexualism” in DSM-II10 and “gender identity disorder of childhood” in DSM-III.11 These diagnoses were listed under the headings of “sexual deviations” and “psychosexual disorders” in the respective DSM editions. This illustrates how gender diversity was viewed as a mental illness/defect. As the DSM developed through various revisions, so have these diagnoses. DSM-IV used the diagnosis “gender identity disorder.”12 Psychiatry has evolved away from this line of thinking by focusing on the distress from biological sex characteristics that are “incongruent” with an individual’s gender identity, leading to the development of the gender dysphoria diagnosis.13 While this has been a positive step in psychiatry’s efforts to de-pathologize individuals who are gender-diverse, it raises the question: should such diagnoses be included in the DSM at all?
The gender dysphoria diagnosis continues to be needed by many individuals who are TGGD in order to access gender-affirming health care services. Mental health professionals are placed in a gatekeeping role by the expectation that they provide letters of “support” to indicate an individual is of sound mind and consistent gender identity to have services covered by insurance providers. In this way, the insurance industry and the field of medicine continue to believe that individuals who are TGGD need psychiatric permission and/or counsel regarding their gender identity. This can place psychiatry in a role of controlling access to necessary care while also creating a possible distrust in our ability to provide care to patients who are gender-diverse. This is particularly problematic given the high rates of depression, anxiety, trauma, and substance use within these communities.14 In the WHO’s ICD-11, gender dysphoria was changed to gender incongruence and is contained in the category of “Conditions related to sexual health.”15 This indicates continued evolution of how medicine views individuals who are TGGD, and offers hope that psychiatry and the DSM will follow suit.
Continue to: Minority stress theory
Minority stress theory
Ilan Meyer’s minority stress theory explores how cultural and social factors impact mental health functioning (Figure 216). Minority stress theory, which was originally developed for what at the time was described as the lesbian, gay, and bisexual communities, purports that the higher prevalence of mental health disorders among such individuals is likely due to social stigma, discrimination, and stressors associated with minority status. More recently, minority stress theory has been expanded to provide framework for individuals who are TGGD. Hendricks et al17 explain how distal, proximal, and resilience factors contribute to mental health outcomes among these individuals. Distal factors, such as gender-related discrimination, harassment, violence, and rejection, explain how systemic, cultural, and environmental events lead to overt stress. Proximal factors consist of an individual’s expectation and anticipation of negative and stressful events and the internalization of negative attitudes and prejudice (ie, internalized transphobia). Resilience factors consist of community connectedness and within-group identification and can help mediate the negative effects of distal and proximal factors.

As clinicians, understanding our patients’ experiences and expectations can help us better engage with them and create an environment of safety and healing. Minority stress theory framework suggests that patients may start treatment with distrust or suspicion in light of previous negative experiences. They may also be likely to expect clinicians to be judgmental or to lack understanding of them. The 2015 US Transgender Survey found that 33% of individuals who are TGGD who sought medical treatment in the past year had at least 1 negative experience related to their gender identity (Table 118). Twenty-four percent reported having to educate their clinician about people who are TGGD, while 15% reported the health care professional asked invasive or unnecessary questions about their gender status that were unrelated to their visit. While psychiatry is often distinct from the larger medical field, it is important to understand the negative encounters individuals who are TGGD have likely experienced in medicine, and how those events may skew their feelings about psychiatric treatment. This is especially salient given the higher prevalence of various psychiatric disorders among individuals who are TGGD.18

According to the US Transgender Survey, 39% of participants were currently experiencing serious psychological distress, which is nearly 8 times the rate in the US population (5%).18 When extrapolated, this data indicates that we in psychiatry are likely to work with individuals who identify as TGGD, regardless of our expertise. Additionally, research indicates that having access to gender-affirming care—such as hormone replacement therapy, gender-affirming surgery, voice therapy, and other treatments—greatly improves mental health issues such as anxiety, depression, and suicidality among individuals who are TGGD.19,20 It is in this way we in psychiatry must do more than just care for our patients by becoming advocates for them to receive the care they need and deserve. While at times we may want to stay out of politics and other public discourse, it is becoming increasingly necessary as health care is entrenched in politics.
Clinical applicability
Because individuals who are TGGD experience higher rates of depression, anxiety, substance use, and other psychiatric disorders,14 it is increasingly likely that many clinicians will be presented with opportunities to treat such individuals. Despite high rates of psychiatric disorders, individuals who are TGGD often avoid treatment due to concerns about being pathologized, stereotyped, and/or encountering professionals who lack the knowledge to treat them as they are.21 Several studies recommend clinicians better equip themselves to appropriately provide services to individuals who are TGGD.21 Some advise seeking education to understand the unique needs of these patients and to help stay current with appropriate terminology and language (Table 222). This also implies not relying on patients to educate clinicians in understanding their specific needs and experiences.

Making assumptions about a patient’s identity is one of the most commonly reported issues by individuals who are TGGD. Therefore, it is critical to avoid making assumptions about patients based on binary stereotypes.23,24 We can circumvent these mistakes by asking every patient for their name and pronouns, and introducing ourselves with our pronouns. This illustrates an openness and understanding of the importance of identity and language, and makes it common practice from the outset. Integrating the use of gender-neutral language into paperwork, intake forms, charting, and conversation will also help avoid the pitfalls of misgendering and making false assumptions. This will also allow for support staff, medical assistants, and others to use correct language with patients. Having a patient’s used name and pronouns visible for everyone who works with the patient is necessary to effectively meet the patient’s needs. Additionally, understanding that the range of experiences and needs for individuals who are TGGD is heterogeneous can help reduce assumptions and ensure we are asking for needed information. It is also important to ask for only relevant information needed to provide treatment.
Continue to: Resources are widely available...
Resources are widely available to aid in the care of individuals who are TGGD. In 2022, the World Professional Association for Transgender Health released new guidelines—Standards of Care 8—for working with individuals who are TGGD.25 While these standards include a section dedicated to mental health, they also provide guidelines on education, assessments, specific demographic groups, hormone therapy, primary care, and sexual health. Additionally, while we may not want the role of gatekeeping for individuals to receive gender-affirming care, we work within a health care and insurance system that continues to require psychiatric assessment for such surgeries. In this role, we must do our part to educate ourselves in how to best provide these assessments and letters of support to help patients receive appropriate and life-saving care.
Finally, in order to provide a more comfortable and affirming space for individuals who are TGGD, develop ways to self-assess and monitor the policies, procedures, and language used within your practice, clinic, or institution. Monitoring the language used in charting to ensure consistency with the individual’s gender identity is important for our own understanding of the patient, and for patients to feel seen. This is especially true given patients’ access to medical records under the Cures Act. Moreover, it is essential to be cognizant of how you present clients to others in consultation or care coordination to ensure the patient is identified correctly and consistently by clinicians and staff.
Bottom Line
Understanding the social, cultural, and medical discrimination faced by patients who are transgender or gender diverse can make us better suited to engage and treat these individuals in an affirming and supportive way.
Related Resources
- World Professional Association of Transgender Health (WPATH) Standards of Care—8th edition. https://www.tandfonline.com/doi/pdf/10.1080/26895269.2022.2100644
- The Fenway Institute: National LGBTQIA+ Health Education Center. https://fenwayhealth.org/the-fenway-institute/education/the-national-lgbtia-health-education-center/
1. Morgenroth T, Ryan MK. The effects of gender trouble: an integrative theoretical framework of the perpetuation and disruption of the gender/sex binary. Perspect Psychol Sci. 2021;16(6):1113-1142. doi:10.1177/1745691620902442
2. The Roots of Loneliness Project. Accessed April 8, 2023. https://www.rootsofloneliness.com/gender-identity-loneliness
3. Davies SG. Challenging Gender Norms: Five Genders Among Bugis in Indonesia. Thomson Wadsworth; 2007.
4. Hyde JS. The gender similarities hypothesis. Am Psychol. 2005;60(6):581-592. doi:10.1037/0003-066X.60.6.581
5. Joel D. Beyond the binary: rethinking sex and the brain. Neurosci Biobehav Rev. 2021;122:165-175. doi:10.1016/j.neubiorev.2020.22.018
6. Joel D, Berman Z, Tavor I, et al. Sex beyond the genitalia: the human brain mosaic. Proc Natl Acad Sci U S A. 2015;112(50):15468-15473. doi:10.1073/pnas.1509654112
7. Palmer BF, Clegg DJ. A universally accepted definition of gender will positively impact societal understanding, acceptance, and appropriateness of health care. Mayo Clin Proc. 2020;95(10):2235-2243. doi:10.1016/j.mayocp.2020.01.031
8. Office of Research on Women’s Health. Sex & Gender. National Institutes of Health. Accessed April 6, 2023. https://orwh.od.nih.gov/sex-gender
9. Morgenroth T, Sendén MG, Lindqvist A, et al. Defending the sex/gender binary: the role of gender identification and need for closure. Soc Psychol Pers Sci. 2021;12(5):731-740.
10. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 2nd ed. American Psychiatric Association; 1968.
11. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3rd ed. American Psychiatric Association; 1980.
12. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. American Psychiatric Association; 1994.
13. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Publishing; 2013.
14. Wanta JW, Niforatos JD, Durbak E, et al. Mental health diagnoses among transgender patients in the clinical setting: an all-payer electronic health record study. Transgend Health. 2019;4(1):313-315.
15. World Health Organization. International Statistical Classification of Diseases. 11th ed. World Health Organization; 2019.
16. Meyer IH. Prejudice, social stress, and mental health in lesbian, gay, and bisexual populations: conceptual issues and research evidence. Psychol Bull. 2003;129(5):674-697. doi:10.1037/0033-2909.129.5.674
17. Hendricks ML, Testa RJ. A conceptual framework for clinical work with transgender and gender nonconforming clients: an adaptation of the Minority Stress Model. Profess Psychol: Res Pract. 2012;43(5):460-467. doi:10.1037/a0029597
18. James SE, Herman J, Keisling M, et al. The Report of the 2015 U.S. Transgender Survey. National Center for Transgender Equality; 2016. Accessed April 6, 2023. https://transequality.org/sites/default/files/docs/usts/USTS-Full-Report-Dec17.pdf
19. Almazan AN, Keuroghlian AS. Association between gender-affirming surgeries and mental health outcomes. JAMA Surg. 2021;156(7):611-618. doi:10.1001/jamasurg.2021.0952
20. Tordoff DM, Wanta JW, Collin A, et al. Mental health outcomes in transgender and nonbinary youths receiving gender-affirming care. JAMA Netw Open. 2022;5(2):e220978. doi:10.1001/jamanetworkopen.2022.0978
21. Snow A, Cerel J, Loeffler DN, et al. Barriers to mental health care for transgender and gender-nonconforming adults: a systematic literature review. Health Soc Work. 2019;44(3):149-155. doi:10.1093/hsw/hlz016
22. National LGBTQIA+ Health Education Center. Accessed April 8, 2023. https://www.lgbtqiahealtheducation.org
23. Baldwin A, Dodge B, Schick VR, et al. Transgender and genderqueer individuals’ experiences with health care providers: what’s working, what’s not, and where do we go from here? J Health Care Poor Underserved. 2018;29(4):1300-1318. doi:10.1353/hpu.2018.0097
24. Kcomt L, Gorey KM, Barrett BJ, et al. Healthcare avoidance due to anticipated discrimination among transgender people: a call to create trans-affirmative environments. SSM-Popul Health. 2020;11:100608. doi:10.1016/j.ssmph.2020.100608
25. Coleman E, Radix AE, Bouman WP, et al. Standards of care for the health of transgender and gender diverse people, version 8. Int J Transgender Health. 2022;23(Suppl 1):S1-S259.
1. Morgenroth T, Ryan MK. The effects of gender trouble: an integrative theoretical framework of the perpetuation and disruption of the gender/sex binary. Perspect Psychol Sci. 2021;16(6):1113-1142. doi:10.1177/1745691620902442
2. The Roots of Loneliness Project. Accessed April 8, 2023. https://www.rootsofloneliness.com/gender-identity-loneliness
3. Davies SG. Challenging Gender Norms: Five Genders Among Bugis in Indonesia. Thomson Wadsworth; 2007.
4. Hyde JS. The gender similarities hypothesis. Am Psychol. 2005;60(6):581-592. doi:10.1037/0003-066X.60.6.581
5. Joel D. Beyond the binary: rethinking sex and the brain. Neurosci Biobehav Rev. 2021;122:165-175. doi:10.1016/j.neubiorev.2020.22.018
6. Joel D, Berman Z, Tavor I, et al. Sex beyond the genitalia: the human brain mosaic. Proc Natl Acad Sci U S A. 2015;112(50):15468-15473. doi:10.1073/pnas.1509654112
7. Palmer BF, Clegg DJ. A universally accepted definition of gender will positively impact societal understanding, acceptance, and appropriateness of health care. Mayo Clin Proc. 2020;95(10):2235-2243. doi:10.1016/j.mayocp.2020.01.031
8. Office of Research on Women’s Health. Sex & Gender. National Institutes of Health. Accessed April 6, 2023. https://orwh.od.nih.gov/sex-gender
9. Morgenroth T, Sendén MG, Lindqvist A, et al. Defending the sex/gender binary: the role of gender identification and need for closure. Soc Psychol Pers Sci. 2021;12(5):731-740.
10. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 2nd ed. American Psychiatric Association; 1968.
11. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3rd ed. American Psychiatric Association; 1980.
12. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. American Psychiatric Association; 1994.
13. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Publishing; 2013.
14. Wanta JW, Niforatos JD, Durbak E, et al. Mental health diagnoses among transgender patients in the clinical setting: an all-payer electronic health record study. Transgend Health. 2019;4(1):313-315.
15. World Health Organization. International Statistical Classification of Diseases. 11th ed. World Health Organization; 2019.
16. Meyer IH. Prejudice, social stress, and mental health in lesbian, gay, and bisexual populations: conceptual issues and research evidence. Psychol Bull. 2003;129(5):674-697. doi:10.1037/0033-2909.129.5.674
17. Hendricks ML, Testa RJ. A conceptual framework for clinical work with transgender and gender nonconforming clients: an adaptation of the Minority Stress Model. Profess Psychol: Res Pract. 2012;43(5):460-467. doi:10.1037/a0029597
18. James SE, Herman J, Keisling M, et al. The Report of the 2015 U.S. Transgender Survey. National Center for Transgender Equality; 2016. Accessed April 6, 2023. https://transequality.org/sites/default/files/docs/usts/USTS-Full-Report-Dec17.pdf
19. Almazan AN, Keuroghlian AS. Association between gender-affirming surgeries and mental health outcomes. JAMA Surg. 2021;156(7):611-618. doi:10.1001/jamasurg.2021.0952
20. Tordoff DM, Wanta JW, Collin A, et al. Mental health outcomes in transgender and nonbinary youths receiving gender-affirming care. JAMA Netw Open. 2022;5(2):e220978. doi:10.1001/jamanetworkopen.2022.0978
21. Snow A, Cerel J, Loeffler DN, et al. Barriers to mental health care for transgender and gender-nonconforming adults: a systematic literature review. Health Soc Work. 2019;44(3):149-155. doi:10.1093/hsw/hlz016
22. National LGBTQIA+ Health Education Center. Accessed April 8, 2023. https://www.lgbtqiahealtheducation.org
23. Baldwin A, Dodge B, Schick VR, et al. Transgender and genderqueer individuals’ experiences with health care providers: what’s working, what’s not, and where do we go from here? J Health Care Poor Underserved. 2018;29(4):1300-1318. doi:10.1353/hpu.2018.0097
24. Kcomt L, Gorey KM, Barrett BJ, et al. Healthcare avoidance due to anticipated discrimination among transgender people: a call to create trans-affirmative environments. SSM-Popul Health. 2020;11:100608. doi:10.1016/j.ssmph.2020.100608
25. Coleman E, Radix AE, Bouman WP, et al. Standards of care for the health of transgender and gender diverse people, version 8. Int J Transgender Health. 2022;23(Suppl 1):S1-S259.