User login
Oral antiamyloid shows disease-modifying potential Phase 3 trial underway
BOSTON – , represented by positive changes in plasma and imaging biomarkers of Alzheimer’s disease pathology.
Use of the drug, ALZ-801 (Alzheon), led to a significant reduction of plasma phosphorylated–tau 181 (p-tau181) , a marker of amyloid-induced neuronal injury in Alzheimer’s disease, as well as slowing of hippocampal atrophy and stabilization of cognition.
“The 12-month results of our phase 2 trial support the finding that ALZ-801 blocks misfolding of amyloid monomers and subsequent formation of neurotoxic amyloid oligomers, the key initial step in the amyloid aggregation cascade, which leads to a rapid and sustained reduction of brain neurodegeneration as measured by plasma p-tau181,” John Hey, PhD, Alzheon’s chief scientific officer, said in a statement.
“The severalfold greater reduction on the p-tau181 biomarker in plasma compared to plaque-clearing antiamyloid antibodies, combined with preservation of brain hippocampal volume and their positive correlations with cognitive benefits, further validate the disease-modifying effects of ALZ-801 in Alzheimer’s patients,” Dr. Hey added.
The results were presented at the 2023 annual meeting of the American Academy of Neurology.
ALZ-801 is an optimized prodrug of tramiprosate that has been shown to inhibit amyloid-beta 42 aggregation into toxic oligomers.
The ongoing phase 2 study is evaluating the effects of oral ALZ-801 (265 mg twice daily) on biomarkers of Alzheimer’s disease pathology for 84 adults with early Alzheimer’s disease who have either the APOE4/4 or APOE3/4 genotype. These genotypes represent the majority of patients with Alzheimer’s disease.
The mean age of the cohort was 69 years, and 51% are women; 70% had mild cognitive impairment, and 30% had mild Alzheimer’s disease. The mean Mini-Mental State Examination score for the cohort was 26.0. Roughly half were taking a cholinesterase inhibitor.
Significant plasma p-tau181 reduction was observed at 13 weeks. Levels were reduced by 41% by 52 weeks (P = .016). There was also a significant 5% reduction in plasma amyloid-beta 42 and 40 at 52 weeks (P = .002 and P = .005, respectively), Dr. Hey reported.
After 12 months of treatment, hippocampal atrophy was reduced by about 23%, and expansion of ventricular volume was reduced by about 15%, both in comparison with matched controls from the Alzheimer’s Disease Neuroimaging Initiative.
Composite cognitive z-score improved significantly at 13 and 26 weeks and remained above baseline at 52 weeks in comparison with matched ADNI controls. “These are very promising data,” Dr. Hey told conference attendees.
He noted that the safety profile of ALZ-801 remains favorable and consistent with prior safety data. Common adverse events were mild nausea and SARS-CoV-2 infection. There were no drug-related serious events or amyloid-related imaging abnormalities–edema (ARIA-E).
The phase 3 APOLLOE4 study of ALZ-801 is underway. This double-blind, randomized study is comparing oral ALZ-801 with placebo over 78 weeks for roughly 300 adults with early Alzheimer’s disease who have the APOE4/4 genotype. APOLLOE4 is expected to be completed in mid 2024.
The APOLLOE4 study is supported by a $47 million grant from the National Institute on Aging. The U.S. Food and Drug Administration has granted ALZ-801 fast-track designation.
More accessible option?
Reached for comment, Percy Griffin, PhD, Alzheimer’s Association director of scientific engagement, noted that the “biggest difference between this drug and others is that it is taken orally, rather than delivered through an infusion. This is important and valuable for reducing patient and caregiver burden and increasing ease of use and access.”
It’s also noteworthy that ALZ-801 was not associated with ARIA-E, “which has been reported in other antiamyloid trials and can occasionally be serious,” Dr. Griffin said.
Overall, he said the results are “encouraging, but more work is needed. If studies results continue to be positive, this treatment may provide a more accessible option for people who are at higher risk of ARIA,” Dr. Griffin said.
The study was funded by Alzheon. Dr. Hey is an employee of Alzheon and holds stock in the company. Dr. Griffin has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
BOSTON – , represented by positive changes in plasma and imaging biomarkers of Alzheimer’s disease pathology.
Use of the drug, ALZ-801 (Alzheon), led to a significant reduction of plasma phosphorylated–tau 181 (p-tau181) , a marker of amyloid-induced neuronal injury in Alzheimer’s disease, as well as slowing of hippocampal atrophy and stabilization of cognition.
“The 12-month results of our phase 2 trial support the finding that ALZ-801 blocks misfolding of amyloid monomers and subsequent formation of neurotoxic amyloid oligomers, the key initial step in the amyloid aggregation cascade, which leads to a rapid and sustained reduction of brain neurodegeneration as measured by plasma p-tau181,” John Hey, PhD, Alzheon’s chief scientific officer, said in a statement.
“The severalfold greater reduction on the p-tau181 biomarker in plasma compared to plaque-clearing antiamyloid antibodies, combined with preservation of brain hippocampal volume and their positive correlations with cognitive benefits, further validate the disease-modifying effects of ALZ-801 in Alzheimer’s patients,” Dr. Hey added.
The results were presented at the 2023 annual meeting of the American Academy of Neurology.
ALZ-801 is an optimized prodrug of tramiprosate that has been shown to inhibit amyloid-beta 42 aggregation into toxic oligomers.
The ongoing phase 2 study is evaluating the effects of oral ALZ-801 (265 mg twice daily) on biomarkers of Alzheimer’s disease pathology for 84 adults with early Alzheimer’s disease who have either the APOE4/4 or APOE3/4 genotype. These genotypes represent the majority of patients with Alzheimer’s disease.
The mean age of the cohort was 69 years, and 51% are women; 70% had mild cognitive impairment, and 30% had mild Alzheimer’s disease. The mean Mini-Mental State Examination score for the cohort was 26.0. Roughly half were taking a cholinesterase inhibitor.
Significant plasma p-tau181 reduction was observed at 13 weeks. Levels were reduced by 41% by 52 weeks (P = .016). There was also a significant 5% reduction in plasma amyloid-beta 42 and 40 at 52 weeks (P = .002 and P = .005, respectively), Dr. Hey reported.
After 12 months of treatment, hippocampal atrophy was reduced by about 23%, and expansion of ventricular volume was reduced by about 15%, both in comparison with matched controls from the Alzheimer’s Disease Neuroimaging Initiative.
Composite cognitive z-score improved significantly at 13 and 26 weeks and remained above baseline at 52 weeks in comparison with matched ADNI controls. “These are very promising data,” Dr. Hey told conference attendees.
He noted that the safety profile of ALZ-801 remains favorable and consistent with prior safety data. Common adverse events were mild nausea and SARS-CoV-2 infection. There were no drug-related serious events or amyloid-related imaging abnormalities–edema (ARIA-E).
The phase 3 APOLLOE4 study of ALZ-801 is underway. This double-blind, randomized study is comparing oral ALZ-801 with placebo over 78 weeks for roughly 300 adults with early Alzheimer’s disease who have the APOE4/4 genotype. APOLLOE4 is expected to be completed in mid 2024.
The APOLLOE4 study is supported by a $47 million grant from the National Institute on Aging. The U.S. Food and Drug Administration has granted ALZ-801 fast-track designation.
More accessible option?
Reached for comment, Percy Griffin, PhD, Alzheimer’s Association director of scientific engagement, noted that the “biggest difference between this drug and others is that it is taken orally, rather than delivered through an infusion. This is important and valuable for reducing patient and caregiver burden and increasing ease of use and access.”
It’s also noteworthy that ALZ-801 was not associated with ARIA-E, “which has been reported in other antiamyloid trials and can occasionally be serious,” Dr. Griffin said.
Overall, he said the results are “encouraging, but more work is needed. If studies results continue to be positive, this treatment may provide a more accessible option for people who are at higher risk of ARIA,” Dr. Griffin said.
The study was funded by Alzheon. Dr. Hey is an employee of Alzheon and holds stock in the company. Dr. Griffin has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
BOSTON – , represented by positive changes in plasma and imaging biomarkers of Alzheimer’s disease pathology.
Use of the drug, ALZ-801 (Alzheon), led to a significant reduction of plasma phosphorylated–tau 181 (p-tau181) , a marker of amyloid-induced neuronal injury in Alzheimer’s disease, as well as slowing of hippocampal atrophy and stabilization of cognition.
“The 12-month results of our phase 2 trial support the finding that ALZ-801 blocks misfolding of amyloid monomers and subsequent formation of neurotoxic amyloid oligomers, the key initial step in the amyloid aggregation cascade, which leads to a rapid and sustained reduction of brain neurodegeneration as measured by plasma p-tau181,” John Hey, PhD, Alzheon’s chief scientific officer, said in a statement.
“The severalfold greater reduction on the p-tau181 biomarker in plasma compared to plaque-clearing antiamyloid antibodies, combined with preservation of brain hippocampal volume and their positive correlations with cognitive benefits, further validate the disease-modifying effects of ALZ-801 in Alzheimer’s patients,” Dr. Hey added.
The results were presented at the 2023 annual meeting of the American Academy of Neurology.
ALZ-801 is an optimized prodrug of tramiprosate that has been shown to inhibit amyloid-beta 42 aggregation into toxic oligomers.
The ongoing phase 2 study is evaluating the effects of oral ALZ-801 (265 mg twice daily) on biomarkers of Alzheimer’s disease pathology for 84 adults with early Alzheimer’s disease who have either the APOE4/4 or APOE3/4 genotype. These genotypes represent the majority of patients with Alzheimer’s disease.
The mean age of the cohort was 69 years, and 51% are women; 70% had mild cognitive impairment, and 30% had mild Alzheimer’s disease. The mean Mini-Mental State Examination score for the cohort was 26.0. Roughly half were taking a cholinesterase inhibitor.
Significant plasma p-tau181 reduction was observed at 13 weeks. Levels were reduced by 41% by 52 weeks (P = .016). There was also a significant 5% reduction in plasma amyloid-beta 42 and 40 at 52 weeks (P = .002 and P = .005, respectively), Dr. Hey reported.
After 12 months of treatment, hippocampal atrophy was reduced by about 23%, and expansion of ventricular volume was reduced by about 15%, both in comparison with matched controls from the Alzheimer’s Disease Neuroimaging Initiative.
Composite cognitive z-score improved significantly at 13 and 26 weeks and remained above baseline at 52 weeks in comparison with matched ADNI controls. “These are very promising data,” Dr. Hey told conference attendees.
He noted that the safety profile of ALZ-801 remains favorable and consistent with prior safety data. Common adverse events were mild nausea and SARS-CoV-2 infection. There were no drug-related serious events or amyloid-related imaging abnormalities–edema (ARIA-E).
The phase 3 APOLLOE4 study of ALZ-801 is underway. This double-blind, randomized study is comparing oral ALZ-801 with placebo over 78 weeks for roughly 300 adults with early Alzheimer’s disease who have the APOE4/4 genotype. APOLLOE4 is expected to be completed in mid 2024.
The APOLLOE4 study is supported by a $47 million grant from the National Institute on Aging. The U.S. Food and Drug Administration has granted ALZ-801 fast-track designation.
More accessible option?
Reached for comment, Percy Griffin, PhD, Alzheimer’s Association director of scientific engagement, noted that the “biggest difference between this drug and others is that it is taken orally, rather than delivered through an infusion. This is important and valuable for reducing patient and caregiver burden and increasing ease of use and access.”
It’s also noteworthy that ALZ-801 was not associated with ARIA-E, “which has been reported in other antiamyloid trials and can occasionally be serious,” Dr. Griffin said.
Overall, he said the results are “encouraging, but more work is needed. If studies results continue to be positive, this treatment may provide a more accessible option for people who are at higher risk of ARIA,” Dr. Griffin said.
The study was funded by Alzheon. Dr. Hey is an employee of Alzheon and holds stock in the company. Dr. Griffin has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM AAN 2023
Patients With Newly Diagnosed Mantle Cell Lymphoma and the Relevance of Clinical Trials
What is the significance of the recent TRIANGLE study on mantle cell lymphoma (MCL)?
Dr. LaCasce: The TRIANGLE study is extremely important in previously untreated, transplant-eligible patients with MCL. The cutoff age for transplants varies by center and is between 60 and 75 years. In the absence of a TP53 mutation, we have typically used induction chemotherapy followed by autologous stem-cell transplant (ASCT), followed by 3 years of maintenance rituximab. Obviously, this is a lot of therapy.
The TRIANGLE study was a 3-arm study in which ibrutinib-containing therapy was compared with standard RCHOP/RDHAP followed by ASCT. Maintenance rituximab became standard of care midway through the trial and was added. In the first experimental arm, ibrutinib was combined with RCHOP and then given as maintenance for 2 years following ASCT. The second experimental arm included the same schedule of ibrutinib and omitted the ASCT.
The results are early, but what has been presented thus far, ibrutinib induction and maintenance with ASCT is clearly superior to the standard arm with ASCT. Although the data are not statistically mature, the failure-free survival of the 2 ibrutinib arms was similar, suggesting that transplant may not be necessary. Longer follow-up is necessary to confirm this conclusion and assess overall survival in all 3 arms.
If the results hold, ASCT could become a thing of the past or perhaps used in the second line. With the favorable activity of chimeric antigen receptor (CAR) T-cell therapy, however, it is unclear whether ASCT would be used in second line. Avoiding the sequential use of ASCT and CAR T-cell therapy is appealing given the stem-cell damage that can result. It is appealing to think about not using ASCT upfront, because ASCT increases the risk of myelodysplastic syndrome.
The TRIANGLE data are likely to change the frontline management of MCL. Although ibrutinib was the first Bruton tyrosine kinase (BTK) inhibitor approved in MCL and has obviously changed the field dramatically, it is significantly less well-tolerated than the next generation of drugs—acalabrutinib and zanubrutinib. I suspect these will be substituted for ibrutinib and we will see even more tolerable upfront regimens for patients with newly diagnosed MCL.
Have there been any disparities that you found in patients newly diagnosed with MCL regarding age, sex, or ethnicity?
Dr. LaCasce: MCL typically affects patients in their 60s. It is rare in young patients, and approximately 75% of the cases are male. If you look at the demographics, it is more common in White patients and less common in Hispanic and African American patients. In addition, there is an association with farming, which likely contribute to the demographics of patients with MCL.
What is your recommended approach to managing patients newly diagnosed with MCL in your day-to-day practice?
Dr. LaCasce: Management is a bit tricky right now because the TRIANGLE study is not part of any guidelines thus far. Therefore, most would argue the standard treatment continues to include ASCT upfront. There is an important, large randomized study (NCT03267433) going on in the United States that is assessing the role of ASCT in patients who are in MRD-negative complete remission at the end of induction therapy. These patients are randomized to ASCT plus maintenance rituximab versus maintenance rituximab alone.
We are still enrolling patients to participate in this study, which is addressing a different question than TRIANGLE. I think we will learn a lot from this study. For patients who are not interested in participating in this study, we talk about the risks and benefits of ASCT.
One or 2 years ago, I would have strongly encouraged patients who were appropriate candidates to consider transplant in first remission. With the TRIANGLE data, however, and now that we have CAR T-cell therapy, I think it is more important to tailor the recommendation to the individual patient. If a patient is reluctant about ASCT and the associated risks, I do not push it.
If patients want the most aggressive approach associated with the longest remissions, at this moment, before TRIANGLE findings have been adopted into guidelines, I continue to recommend ASCT. For patients who have TP53 mutation, however, we treat with typically less aggressive therapy, as this patient population does not benefit from ASCT. We look forward to more data incorporating BTK inhibitors upfront, particularly for this group of patients, who tend to have a more adverse prognosis.
Do you feel MCL data and clinical trials are important areas of focus for your colleagues?
Dr. LaCasce: Yes. I think it is a rapidly evolving field, which is really exciting. We are seeing data now from the bispecific antibodies in the relapsed/refractory setting. We also need more data using pirtobrutinib for patients who have had BTK inhibitors and compare pirtobrutinib (a non-covalent BTK inhibitor) with the covalent BTK inhibitors.
I would strongly encourage patients to participate in clinical trials so that we can better answer these important questions. When patients go online and read about MCL, they often see a median survival of 3 to 4 years, which is completely outdated. The overall prognosis of MCL has changed dramatically since I have been in the field. Hopefully, survival will continue to improve, and therapies will become more tolerable, as well.
What is the significance of the recent TRIANGLE study on mantle cell lymphoma (MCL)?
Dr. LaCasce: The TRIANGLE study is extremely important in previously untreated, transplant-eligible patients with MCL. The cutoff age for transplants varies by center and is between 60 and 75 years. In the absence of a TP53 mutation, we have typically used induction chemotherapy followed by autologous stem-cell transplant (ASCT), followed by 3 years of maintenance rituximab. Obviously, this is a lot of therapy.
The TRIANGLE study was a 3-arm study in which ibrutinib-containing therapy was compared with standard RCHOP/RDHAP followed by ASCT. Maintenance rituximab became standard of care midway through the trial and was added. In the first experimental arm, ibrutinib was combined with RCHOP and then given as maintenance for 2 years following ASCT. The second experimental arm included the same schedule of ibrutinib and omitted the ASCT.
The results are early, but what has been presented thus far, ibrutinib induction and maintenance with ASCT is clearly superior to the standard arm with ASCT. Although the data are not statistically mature, the failure-free survival of the 2 ibrutinib arms was similar, suggesting that transplant may not be necessary. Longer follow-up is necessary to confirm this conclusion and assess overall survival in all 3 arms.
If the results hold, ASCT could become a thing of the past or perhaps used in the second line. With the favorable activity of chimeric antigen receptor (CAR) T-cell therapy, however, it is unclear whether ASCT would be used in second line. Avoiding the sequential use of ASCT and CAR T-cell therapy is appealing given the stem-cell damage that can result. It is appealing to think about not using ASCT upfront, because ASCT increases the risk of myelodysplastic syndrome.
The TRIANGLE data are likely to change the frontline management of MCL. Although ibrutinib was the first Bruton tyrosine kinase (BTK) inhibitor approved in MCL and has obviously changed the field dramatically, it is significantly less well-tolerated than the next generation of drugs—acalabrutinib and zanubrutinib. I suspect these will be substituted for ibrutinib and we will see even more tolerable upfront regimens for patients with newly diagnosed MCL.
Have there been any disparities that you found in patients newly diagnosed with MCL regarding age, sex, or ethnicity?
Dr. LaCasce: MCL typically affects patients in their 60s. It is rare in young patients, and approximately 75% of the cases are male. If you look at the demographics, it is more common in White patients and less common in Hispanic and African American patients. In addition, there is an association with farming, which likely contribute to the demographics of patients with MCL.
What is your recommended approach to managing patients newly diagnosed with MCL in your day-to-day practice?
Dr. LaCasce: Management is a bit tricky right now because the TRIANGLE study is not part of any guidelines thus far. Therefore, most would argue the standard treatment continues to include ASCT upfront. There is an important, large randomized study (NCT03267433) going on in the United States that is assessing the role of ASCT in patients who are in MRD-negative complete remission at the end of induction therapy. These patients are randomized to ASCT plus maintenance rituximab versus maintenance rituximab alone.
We are still enrolling patients to participate in this study, which is addressing a different question than TRIANGLE. I think we will learn a lot from this study. For patients who are not interested in participating in this study, we talk about the risks and benefits of ASCT.
One or 2 years ago, I would have strongly encouraged patients who were appropriate candidates to consider transplant in first remission. With the TRIANGLE data, however, and now that we have CAR T-cell therapy, I think it is more important to tailor the recommendation to the individual patient. If a patient is reluctant about ASCT and the associated risks, I do not push it.
If patients want the most aggressive approach associated with the longest remissions, at this moment, before TRIANGLE findings have been adopted into guidelines, I continue to recommend ASCT. For patients who have TP53 mutation, however, we treat with typically less aggressive therapy, as this patient population does not benefit from ASCT. We look forward to more data incorporating BTK inhibitors upfront, particularly for this group of patients, who tend to have a more adverse prognosis.
Do you feel MCL data and clinical trials are important areas of focus for your colleagues?
Dr. LaCasce: Yes. I think it is a rapidly evolving field, which is really exciting. We are seeing data now from the bispecific antibodies in the relapsed/refractory setting. We also need more data using pirtobrutinib for patients who have had BTK inhibitors and compare pirtobrutinib (a non-covalent BTK inhibitor) with the covalent BTK inhibitors.
I would strongly encourage patients to participate in clinical trials so that we can better answer these important questions. When patients go online and read about MCL, they often see a median survival of 3 to 4 years, which is completely outdated. The overall prognosis of MCL has changed dramatically since I have been in the field. Hopefully, survival will continue to improve, and therapies will become more tolerable, as well.
What is the significance of the recent TRIANGLE study on mantle cell lymphoma (MCL)?
Dr. LaCasce: The TRIANGLE study is extremely important in previously untreated, transplant-eligible patients with MCL. The cutoff age for transplants varies by center and is between 60 and 75 years. In the absence of a TP53 mutation, we have typically used induction chemotherapy followed by autologous stem-cell transplant (ASCT), followed by 3 years of maintenance rituximab. Obviously, this is a lot of therapy.
The TRIANGLE study was a 3-arm study in which ibrutinib-containing therapy was compared with standard RCHOP/RDHAP followed by ASCT. Maintenance rituximab became standard of care midway through the trial and was added. In the first experimental arm, ibrutinib was combined with RCHOP and then given as maintenance for 2 years following ASCT. The second experimental arm included the same schedule of ibrutinib and omitted the ASCT.
The results are early, but what has been presented thus far, ibrutinib induction and maintenance with ASCT is clearly superior to the standard arm with ASCT. Although the data are not statistically mature, the failure-free survival of the 2 ibrutinib arms was similar, suggesting that transplant may not be necessary. Longer follow-up is necessary to confirm this conclusion and assess overall survival in all 3 arms.
If the results hold, ASCT could become a thing of the past or perhaps used in the second line. With the favorable activity of chimeric antigen receptor (CAR) T-cell therapy, however, it is unclear whether ASCT would be used in second line. Avoiding the sequential use of ASCT and CAR T-cell therapy is appealing given the stem-cell damage that can result. It is appealing to think about not using ASCT upfront, because ASCT increases the risk of myelodysplastic syndrome.
The TRIANGLE data are likely to change the frontline management of MCL. Although ibrutinib was the first Bruton tyrosine kinase (BTK) inhibitor approved in MCL and has obviously changed the field dramatically, it is significantly less well-tolerated than the next generation of drugs—acalabrutinib and zanubrutinib. I suspect these will be substituted for ibrutinib and we will see even more tolerable upfront regimens for patients with newly diagnosed MCL.
Have there been any disparities that you found in patients newly diagnosed with MCL regarding age, sex, or ethnicity?
Dr. LaCasce: MCL typically affects patients in their 60s. It is rare in young patients, and approximately 75% of the cases are male. If you look at the demographics, it is more common in White patients and less common in Hispanic and African American patients. In addition, there is an association with farming, which likely contribute to the demographics of patients with MCL.
What is your recommended approach to managing patients newly diagnosed with MCL in your day-to-day practice?
Dr. LaCasce: Management is a bit tricky right now because the TRIANGLE study is not part of any guidelines thus far. Therefore, most would argue the standard treatment continues to include ASCT upfront. There is an important, large randomized study (NCT03267433) going on in the United States that is assessing the role of ASCT in patients who are in MRD-negative complete remission at the end of induction therapy. These patients are randomized to ASCT plus maintenance rituximab versus maintenance rituximab alone.
We are still enrolling patients to participate in this study, which is addressing a different question than TRIANGLE. I think we will learn a lot from this study. For patients who are not interested in participating in this study, we talk about the risks and benefits of ASCT.
One or 2 years ago, I would have strongly encouraged patients who were appropriate candidates to consider transplant in first remission. With the TRIANGLE data, however, and now that we have CAR T-cell therapy, I think it is more important to tailor the recommendation to the individual patient. If a patient is reluctant about ASCT and the associated risks, I do not push it.
If patients want the most aggressive approach associated with the longest remissions, at this moment, before TRIANGLE findings have been adopted into guidelines, I continue to recommend ASCT. For patients who have TP53 mutation, however, we treat with typically less aggressive therapy, as this patient population does not benefit from ASCT. We look forward to more data incorporating BTK inhibitors upfront, particularly for this group of patients, who tend to have a more adverse prognosis.
Do you feel MCL data and clinical trials are important areas of focus for your colleagues?
Dr. LaCasce: Yes. I think it is a rapidly evolving field, which is really exciting. We are seeing data now from the bispecific antibodies in the relapsed/refractory setting. We also need more data using pirtobrutinib for patients who have had BTK inhibitors and compare pirtobrutinib (a non-covalent BTK inhibitor) with the covalent BTK inhibitors.
I would strongly encourage patients to participate in clinical trials so that we can better answer these important questions. When patients go online and read about MCL, they often see a median survival of 3 to 4 years, which is completely outdated. The overall prognosis of MCL has changed dramatically since I have been in the field. Hopefully, survival will continue to improve, and therapies will become more tolerable, as well.
Long-COVID rate may be higher with rheumatic diseases
MANCHESTER, England – Data from the COVAD-2 e-survey suggest that people with a rheumatic disease are twice as likely as are those without to experience long-term effects after contracting COVID-19.
The prevalence of post–COVID-19 condition (PCC), the term the World Health Organization advocates for describing the widely popularized term long COVID, was 10.8% among people with autoimmune rheumatic diseases (AIRDs) vs. 5.3% among those with no autoimmune condition (designated as “healthy controls”). The odds ratio was 2.1, with a 95% confidence interval of 1.4-3.2 and a P-value of .002.
The prevalence in people with nonrheumatic autoimmune diseases was also higher than it was in the control participants but still lower, at 7.3%, than in those with AIRDs.
“Our findings highlight the importance of close monitoring for PCC,” Arvind Nune, MBBCh, MSc, said in a virtual poster presentation at the annual meeting of the British Society for Rheumatology.
They also show the need for “appropriate referral for optimized multidisciplinary care for patients with autoimmune rheumatic diseases during the recovery period following COVID-19,” added Dr. Nune, who works for Southport (England) and Ormskirk Hospital NHS Trust.
In an interview, he noted that it was patients who had a severe COVID-19 course or had other coexisting conditions that appeared to experience more long-term effects than did their less-affected counterparts.
Commenting on the study, Jeffrey A. Sparks, MD, MMSc, told this news organization: “This is one of the first studies to find that the prevalence of long COVID is higher among people with systemic rheumatic diseases than those without.”
Dr. Sparks, who is based at Brigham and Women’s Hospital and Harvard Medical School in Boston, added: “Since the symptoms of long COVID and rheumatic diseases can overlap substantially, more work will need to be done to determine whether COVID may have induced flares, new symptoms, or whether the finding is due to the presence of the chronic rheumatic disease.”
The COVAD study
Using an electronic survey platform, the COVAD study has been set up to look at the long-term efficacy and safety of COVID-19 vaccinations in patients with AIRDs. It’s now a large international effort involving more than 150 collaborating clinics in 106 countries.
A huge amount of data has been collected. “We collected demographics, details of autoimmune disease, including treatment, comorbidity, COVID infection, vaccination history and outcomes, date on flares, and validated patient-reported outcomes, including pain, fatigue, physical function, and quality of life,” Dr. Nune said in his presentation.
A total of 12,358 people who were invited to participate responded to the e-survey. Of them, 2,640 were confirmed to have COVID-19. Because the analysis aimed to look at PCC, anyone who had completed the survey less than 3 months after infection was excluded. This left 1,677 eligible respondents, of whom, an overall 8.7% (n = 136) were identified as having PCC.
“The [WHO] definition for PCC was employed, which is persistent signs or symptoms beyond 3 months of COVID-19 infection lasting at least 2 months,” Dr. Nune told this news organization.
“Symptoms could be anything from fatigue to breathlessness to arthralgias,” he added. However, the focus of the present analysis was to look at how many people were experiencing the condition rather than specific symptoms.
A higher risk for PCC was seen in women than in men (OR, 2.9; 95% CI, 1.1-7.7; P = .037) in the entire cohort.
In addition, those with comorbidities were found to have a greater chance of long-term sequelae from COVID-19 than were those without comorbid disease (OR, 2.8; 95% CI, 1.4-5.7; P = .005).
Patients who experienced more severe acute COVID-19, such as those who needed intensive care treatment, oxygen therapy, or advanced treatment for COVID-19 with monoclonal antibodies, were significantly more likely to later have PCC than were those who did not (OR, 3.8; 95% CI, 1.1-13.6; P = .039).
Having PCC was also associated with poorer patient-reported outcomes for physical function, compared with not having PCC. “However, no association with disease flares of underlying rheumatic diseases or immunosuppressive drugs used were noted,” Dr. Nune said.
These new findings from the COVAD study should be published soon. Dr. Nune suggested that the findings might be used to help identify patients early so that they can be referred to the appropriate services in good time.
The COVAD study was independently supported. Dr. Nune reports no relevant financial relationships. Dr. Sparks is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the R. Bruce and Joan M. Mickey Research Scholar Fund, and the Llura Gund Award for Rheumatoid Arthritis Research and Care. Dr. Sparks has received research support from Bristol-Myers Squibb and performed consultancy for AbbVie, Amgen, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead, Inova Diagnostics, Janssen, Optum, and Pfizer.
MANCHESTER, England – Data from the COVAD-2 e-survey suggest that people with a rheumatic disease are twice as likely as are those without to experience long-term effects after contracting COVID-19.
The prevalence of post–COVID-19 condition (PCC), the term the World Health Organization advocates for describing the widely popularized term long COVID, was 10.8% among people with autoimmune rheumatic diseases (AIRDs) vs. 5.3% among those with no autoimmune condition (designated as “healthy controls”). The odds ratio was 2.1, with a 95% confidence interval of 1.4-3.2 and a P-value of .002.
The prevalence in people with nonrheumatic autoimmune diseases was also higher than it was in the control participants but still lower, at 7.3%, than in those with AIRDs.
“Our findings highlight the importance of close monitoring for PCC,” Arvind Nune, MBBCh, MSc, said in a virtual poster presentation at the annual meeting of the British Society for Rheumatology.
They also show the need for “appropriate referral for optimized multidisciplinary care for patients with autoimmune rheumatic diseases during the recovery period following COVID-19,” added Dr. Nune, who works for Southport (England) and Ormskirk Hospital NHS Trust.
In an interview, he noted that it was patients who had a severe COVID-19 course or had other coexisting conditions that appeared to experience more long-term effects than did their less-affected counterparts.
Commenting on the study, Jeffrey A. Sparks, MD, MMSc, told this news organization: “This is one of the first studies to find that the prevalence of long COVID is higher among people with systemic rheumatic diseases than those without.”
Dr. Sparks, who is based at Brigham and Women’s Hospital and Harvard Medical School in Boston, added: “Since the symptoms of long COVID and rheumatic diseases can overlap substantially, more work will need to be done to determine whether COVID may have induced flares, new symptoms, or whether the finding is due to the presence of the chronic rheumatic disease.”
The COVAD study
Using an electronic survey platform, the COVAD study has been set up to look at the long-term efficacy and safety of COVID-19 vaccinations in patients with AIRDs. It’s now a large international effort involving more than 150 collaborating clinics in 106 countries.
A huge amount of data has been collected. “We collected demographics, details of autoimmune disease, including treatment, comorbidity, COVID infection, vaccination history and outcomes, date on flares, and validated patient-reported outcomes, including pain, fatigue, physical function, and quality of life,” Dr. Nune said in his presentation.
A total of 12,358 people who were invited to participate responded to the e-survey. Of them, 2,640 were confirmed to have COVID-19. Because the analysis aimed to look at PCC, anyone who had completed the survey less than 3 months after infection was excluded. This left 1,677 eligible respondents, of whom, an overall 8.7% (n = 136) were identified as having PCC.
“The [WHO] definition for PCC was employed, which is persistent signs or symptoms beyond 3 months of COVID-19 infection lasting at least 2 months,” Dr. Nune told this news organization.
“Symptoms could be anything from fatigue to breathlessness to arthralgias,” he added. However, the focus of the present analysis was to look at how many people were experiencing the condition rather than specific symptoms.
A higher risk for PCC was seen in women than in men (OR, 2.9; 95% CI, 1.1-7.7; P = .037) in the entire cohort.
In addition, those with comorbidities were found to have a greater chance of long-term sequelae from COVID-19 than were those without comorbid disease (OR, 2.8; 95% CI, 1.4-5.7; P = .005).
Patients who experienced more severe acute COVID-19, such as those who needed intensive care treatment, oxygen therapy, or advanced treatment for COVID-19 with monoclonal antibodies, were significantly more likely to later have PCC than were those who did not (OR, 3.8; 95% CI, 1.1-13.6; P = .039).
Having PCC was also associated with poorer patient-reported outcomes for physical function, compared with not having PCC. “However, no association with disease flares of underlying rheumatic diseases or immunosuppressive drugs used were noted,” Dr. Nune said.
These new findings from the COVAD study should be published soon. Dr. Nune suggested that the findings might be used to help identify patients early so that they can be referred to the appropriate services in good time.
The COVAD study was independently supported. Dr. Nune reports no relevant financial relationships. Dr. Sparks is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the R. Bruce and Joan M. Mickey Research Scholar Fund, and the Llura Gund Award for Rheumatoid Arthritis Research and Care. Dr. Sparks has received research support from Bristol-Myers Squibb and performed consultancy for AbbVie, Amgen, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead, Inova Diagnostics, Janssen, Optum, and Pfizer.
MANCHESTER, England – Data from the COVAD-2 e-survey suggest that people with a rheumatic disease are twice as likely as are those without to experience long-term effects after contracting COVID-19.
The prevalence of post–COVID-19 condition (PCC), the term the World Health Organization advocates for describing the widely popularized term long COVID, was 10.8% among people with autoimmune rheumatic diseases (AIRDs) vs. 5.3% among those with no autoimmune condition (designated as “healthy controls”). The odds ratio was 2.1, with a 95% confidence interval of 1.4-3.2 and a P-value of .002.
The prevalence in people with nonrheumatic autoimmune diseases was also higher than it was in the control participants but still lower, at 7.3%, than in those with AIRDs.
“Our findings highlight the importance of close monitoring for PCC,” Arvind Nune, MBBCh, MSc, said in a virtual poster presentation at the annual meeting of the British Society for Rheumatology.
They also show the need for “appropriate referral for optimized multidisciplinary care for patients with autoimmune rheumatic diseases during the recovery period following COVID-19,” added Dr. Nune, who works for Southport (England) and Ormskirk Hospital NHS Trust.
In an interview, he noted that it was patients who had a severe COVID-19 course or had other coexisting conditions that appeared to experience more long-term effects than did their less-affected counterparts.
Commenting on the study, Jeffrey A. Sparks, MD, MMSc, told this news organization: “This is one of the first studies to find that the prevalence of long COVID is higher among people with systemic rheumatic diseases than those without.”
Dr. Sparks, who is based at Brigham and Women’s Hospital and Harvard Medical School in Boston, added: “Since the symptoms of long COVID and rheumatic diseases can overlap substantially, more work will need to be done to determine whether COVID may have induced flares, new symptoms, or whether the finding is due to the presence of the chronic rheumatic disease.”
The COVAD study
Using an electronic survey platform, the COVAD study has been set up to look at the long-term efficacy and safety of COVID-19 vaccinations in patients with AIRDs. It’s now a large international effort involving more than 150 collaborating clinics in 106 countries.
A huge amount of data has been collected. “We collected demographics, details of autoimmune disease, including treatment, comorbidity, COVID infection, vaccination history and outcomes, date on flares, and validated patient-reported outcomes, including pain, fatigue, physical function, and quality of life,” Dr. Nune said in his presentation.
A total of 12,358 people who were invited to participate responded to the e-survey. Of them, 2,640 were confirmed to have COVID-19. Because the analysis aimed to look at PCC, anyone who had completed the survey less than 3 months after infection was excluded. This left 1,677 eligible respondents, of whom, an overall 8.7% (n = 136) were identified as having PCC.
“The [WHO] definition for PCC was employed, which is persistent signs or symptoms beyond 3 months of COVID-19 infection lasting at least 2 months,” Dr. Nune told this news organization.
“Symptoms could be anything from fatigue to breathlessness to arthralgias,” he added. However, the focus of the present analysis was to look at how many people were experiencing the condition rather than specific symptoms.
A higher risk for PCC was seen in women than in men (OR, 2.9; 95% CI, 1.1-7.7; P = .037) in the entire cohort.
In addition, those with comorbidities were found to have a greater chance of long-term sequelae from COVID-19 than were those without comorbid disease (OR, 2.8; 95% CI, 1.4-5.7; P = .005).
Patients who experienced more severe acute COVID-19, such as those who needed intensive care treatment, oxygen therapy, or advanced treatment for COVID-19 with monoclonal antibodies, were significantly more likely to later have PCC than were those who did not (OR, 3.8; 95% CI, 1.1-13.6; P = .039).
Having PCC was also associated with poorer patient-reported outcomes for physical function, compared with not having PCC. “However, no association with disease flares of underlying rheumatic diseases or immunosuppressive drugs used were noted,” Dr. Nune said.
These new findings from the COVAD study should be published soon. Dr. Nune suggested that the findings might be used to help identify patients early so that they can be referred to the appropriate services in good time.
The COVAD study was independently supported. Dr. Nune reports no relevant financial relationships. Dr. Sparks is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the R. Bruce and Joan M. Mickey Research Scholar Fund, and the Llura Gund Award for Rheumatoid Arthritis Research and Care. Dr. Sparks has received research support from Bristol-Myers Squibb and performed consultancy for AbbVie, Amgen, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead, Inova Diagnostics, Janssen, Optum, and Pfizer.
AT BSR 2023
The nation’s health secretary has this obstetrician on call
She’s seen progress, albeit slow, over three decades, yet the number of maternal deaths each year continues to rise.
Luckily, she’s got the ear of President Joe Biden’s health secretary.
Dr. Reyes, 64, is married to Health and Human Services Secretary Xavier Becerra, who is championing the administration’s initiative to require all states to provide Medicaid coverage to mothers for a year after giving birth. In March, the Centers for Disease Control and Prevention released data showing a 40% increase in U.S. maternal deaths from 2020 to 2021. The mortality rate among Black women was 2.6 times that of white women, no matter their economic status.
Over the years, Mr. Becerra has spoken highly of his wife’s expertise, but she downplays her influence, saying her husband of nearly 35 years “had it in him to begin with” to improve health care for women and to demand fewer pregnancy-related deaths. She, too, describes the nation’s high maternal mortality rate as unacceptable and preventable.
Dr. Reyes, a Latina who grew up as one of eight children in California’s agricultural heartland, now practices perinatology at the University of California, Davis. She is a member of a California Department of Public Health panel that reviews cases of maternal deaths and recommends improvements. And she chairs the board of the California Health Care Foundation, a nonprofit that works to increase health care access. (California Healthline is an editorially independent service of the California Health Care Foundation.)
Her work has been a blend of medicine and advocacy, and she worries recent federal court rulings will erode hard-fought victories regarding the safety of pregnant women and their babies. She discussed the nation’s maternal health crisis and health care disparities in an interview, which has been edited for length and clarity.
Question: When did you first realize there are disparities in the health care system?
Answer: When I was in high school in the Fresno Unified School District, we were under a consent decree to desegregate. And I was, at the time, student body president at Roosevelt High School. I was asked to be on this unified school district desegregation task force, where the district had to come up with a plan.
It was a time when I really had incredible exposure to how policies are made at a larger level, societal level, that really determine where people live, where they can seek health care, where they go to school. That experience had a tremendous impact on my life in terms of what I wanted to do in a career and how to give back.
Q: The U.S. has one of the best health care systems in the world, yet the maternal mortality rate is high compared with other developed countries. Why do think that is?
A. What we know by the CDC and maternal mortality review committees is that about 60% of maternal deaths are considered preventable. And that’s really been a lot of what I’ve tried to focus on: What can we do to reduce the severity of disease? Or what can we do within the role that we play in maternal health that can reduce that?
We know that there are societal issues absolutely that increase women’s risks and there are public health issues. But there’s a role that hospitals play in helping reduce that risk. Ten years ago, I was on the maternal mortality review committee for the state of California when we started reviewing cases of women who died within hospital systems to see, “Is there a role that we can play in a hospital system to reduce that risk?”
We recognized that sometimes there were conditions that were not recognized early enough so that there was a delay in the care. Sometimes there was a misdiagnosis. Or in some hospital systems, especially rural systems where there aren’t as many resources, sometimes there was the lack of specialists available. So, we’ve identified these risks and said, “We can do something about them.”
Q: You served on a federal panel 20 years ago that published a groundbreaking report identifying racism in health care. It seems as if we could be much further along.
A. The purpose of that committee was to really answer the question: Do patients receive a different level of care based on race? Looking back, we knew there was something there, but we really didn’t know. And it took months for the committee to come to that agreement, that there was a difference. I mean, that was honestly monumental, because we just didn’t have that level of consensus before. And so just to say “That treatment is unequal and it’s unacceptable” was really profound.
We thought that the 700-page report was going to be a time period where there was going to be tremendous movement, and I think I’ve learned over 20 years that change doesn’t happen quickly, especially when providers and health systems don’t see that they play a role. It’s like … “OK, so maybe it exists, but not for me.”
We all saw George Floyd and how he was treated. And during COVID we saw a tremendous difference in who was dying, right? Underrepresented minorities – certainly much higher. It was that culmination that made us realize the elephant in the room. We can’t ignore that this does exist, that there is a difference in how people are treated, even in our health care system.
Q: When addressing racism in health care, you talk about diversifying not just the health care workforce, but also the boardrooms of hospitals and health systems. Why is that important?
A. At the board level, change is hard. But we all play a role because leadership really helps determine much of what’s carried out. So, to have a leadership that is understanding and representative of the communities they serve, I think it has been demonstrated that we do make a difference.
Q: As a health care provider, do you have a wish list of policies you’d like the government to take up?
A. There was tremendous effort around offering preventive health services as a part of what was covered under the Affordable Care Act. And individuals exhaled, finally thinking this is a tremendous win, especially for women in pregnancy. Because we fought for preventive health services to help them have access so they can prepare for their pregnancy. So, for women, this was huge. But now with the Texas federal court ruling that the U.S. Preventive Services Task Force didn’t have any authority, it is a tremendous step backward.
We have culturally, linguistically appropriate standards in place, but it’s a matter in terms of how they’re carried out by state and by individual hospital systems. My wish list is that we really do listen to our patients, speak to them in a language of their choice, and provide them written materials in the language of their choice. We don’t fully do that.
Q: You mentioned one Texas ruling on the ACA. What’s your take on the ruling by another Texas judge suspending the abortion pill? And the U.S. Supreme Court’s overturning of Roe v. Wade?A. As a maternal-fetal medicine specialist who tries to help women plan for pregnancies, those rulings are a tremendous setback.
Q: And what about women of color? Will they find access to abortion services more difficult?
A. Oh, absolutely. When we speak of underrepresented minorities or those with less resources, they have less resources to then seek the appropriate care. Some women may have the opportunity to go to a different state or seek care elsewhere if their state doesn’t provide it. Many women just don’t have those resources to devote to them and don’t have a choice. So, we will see that disparity widen.
This article was produced by KFF Health News, which publishes California Healthline, an editorially independent service of the California Health Care Foundation.
She’s seen progress, albeit slow, over three decades, yet the number of maternal deaths each year continues to rise.
Luckily, she’s got the ear of President Joe Biden’s health secretary.
Dr. Reyes, 64, is married to Health and Human Services Secretary Xavier Becerra, who is championing the administration’s initiative to require all states to provide Medicaid coverage to mothers for a year after giving birth. In March, the Centers for Disease Control and Prevention released data showing a 40% increase in U.S. maternal deaths from 2020 to 2021. The mortality rate among Black women was 2.6 times that of white women, no matter their economic status.
Over the years, Mr. Becerra has spoken highly of his wife’s expertise, but she downplays her influence, saying her husband of nearly 35 years “had it in him to begin with” to improve health care for women and to demand fewer pregnancy-related deaths. She, too, describes the nation’s high maternal mortality rate as unacceptable and preventable.
Dr. Reyes, a Latina who grew up as one of eight children in California’s agricultural heartland, now practices perinatology at the University of California, Davis. She is a member of a California Department of Public Health panel that reviews cases of maternal deaths and recommends improvements. And she chairs the board of the California Health Care Foundation, a nonprofit that works to increase health care access. (California Healthline is an editorially independent service of the California Health Care Foundation.)
Her work has been a blend of medicine and advocacy, and she worries recent federal court rulings will erode hard-fought victories regarding the safety of pregnant women and their babies. She discussed the nation’s maternal health crisis and health care disparities in an interview, which has been edited for length and clarity.
Question: When did you first realize there are disparities in the health care system?
Answer: When I was in high school in the Fresno Unified School District, we were under a consent decree to desegregate. And I was, at the time, student body president at Roosevelt High School. I was asked to be on this unified school district desegregation task force, where the district had to come up with a plan.
It was a time when I really had incredible exposure to how policies are made at a larger level, societal level, that really determine where people live, where they can seek health care, where they go to school. That experience had a tremendous impact on my life in terms of what I wanted to do in a career and how to give back.
Q: The U.S. has one of the best health care systems in the world, yet the maternal mortality rate is high compared with other developed countries. Why do think that is?
A. What we know by the CDC and maternal mortality review committees is that about 60% of maternal deaths are considered preventable. And that’s really been a lot of what I’ve tried to focus on: What can we do to reduce the severity of disease? Or what can we do within the role that we play in maternal health that can reduce that?
We know that there are societal issues absolutely that increase women’s risks and there are public health issues. But there’s a role that hospitals play in helping reduce that risk. Ten years ago, I was on the maternal mortality review committee for the state of California when we started reviewing cases of women who died within hospital systems to see, “Is there a role that we can play in a hospital system to reduce that risk?”
We recognized that sometimes there were conditions that were not recognized early enough so that there was a delay in the care. Sometimes there was a misdiagnosis. Or in some hospital systems, especially rural systems where there aren’t as many resources, sometimes there was the lack of specialists available. So, we’ve identified these risks and said, “We can do something about them.”
Q: You served on a federal panel 20 years ago that published a groundbreaking report identifying racism in health care. It seems as if we could be much further along.
A. The purpose of that committee was to really answer the question: Do patients receive a different level of care based on race? Looking back, we knew there was something there, but we really didn’t know. And it took months for the committee to come to that agreement, that there was a difference. I mean, that was honestly monumental, because we just didn’t have that level of consensus before. And so just to say “That treatment is unequal and it’s unacceptable” was really profound.
We thought that the 700-page report was going to be a time period where there was going to be tremendous movement, and I think I’ve learned over 20 years that change doesn’t happen quickly, especially when providers and health systems don’t see that they play a role. It’s like … “OK, so maybe it exists, but not for me.”
We all saw George Floyd and how he was treated. And during COVID we saw a tremendous difference in who was dying, right? Underrepresented minorities – certainly much higher. It was that culmination that made us realize the elephant in the room. We can’t ignore that this does exist, that there is a difference in how people are treated, even in our health care system.
Q: When addressing racism in health care, you talk about diversifying not just the health care workforce, but also the boardrooms of hospitals and health systems. Why is that important?
A. At the board level, change is hard. But we all play a role because leadership really helps determine much of what’s carried out. So, to have a leadership that is understanding and representative of the communities they serve, I think it has been demonstrated that we do make a difference.
Q: As a health care provider, do you have a wish list of policies you’d like the government to take up?
A. There was tremendous effort around offering preventive health services as a part of what was covered under the Affordable Care Act. And individuals exhaled, finally thinking this is a tremendous win, especially for women in pregnancy. Because we fought for preventive health services to help them have access so they can prepare for their pregnancy. So, for women, this was huge. But now with the Texas federal court ruling that the U.S. Preventive Services Task Force didn’t have any authority, it is a tremendous step backward.
We have culturally, linguistically appropriate standards in place, but it’s a matter in terms of how they’re carried out by state and by individual hospital systems. My wish list is that we really do listen to our patients, speak to them in a language of their choice, and provide them written materials in the language of their choice. We don’t fully do that.
Q: You mentioned one Texas ruling on the ACA. What’s your take on the ruling by another Texas judge suspending the abortion pill? And the U.S. Supreme Court’s overturning of Roe v. Wade?A. As a maternal-fetal medicine specialist who tries to help women plan for pregnancies, those rulings are a tremendous setback.
Q: And what about women of color? Will they find access to abortion services more difficult?
A. Oh, absolutely. When we speak of underrepresented minorities or those with less resources, they have less resources to then seek the appropriate care. Some women may have the opportunity to go to a different state or seek care elsewhere if their state doesn’t provide it. Many women just don’t have those resources to devote to them and don’t have a choice. So, we will see that disparity widen.
This article was produced by KFF Health News, which publishes California Healthline, an editorially independent service of the California Health Care Foundation.
She’s seen progress, albeit slow, over three decades, yet the number of maternal deaths each year continues to rise.
Luckily, she’s got the ear of President Joe Biden’s health secretary.
Dr. Reyes, 64, is married to Health and Human Services Secretary Xavier Becerra, who is championing the administration’s initiative to require all states to provide Medicaid coverage to mothers for a year after giving birth. In March, the Centers for Disease Control and Prevention released data showing a 40% increase in U.S. maternal deaths from 2020 to 2021. The mortality rate among Black women was 2.6 times that of white women, no matter their economic status.
Over the years, Mr. Becerra has spoken highly of his wife’s expertise, but she downplays her influence, saying her husband of nearly 35 years “had it in him to begin with” to improve health care for women and to demand fewer pregnancy-related deaths. She, too, describes the nation’s high maternal mortality rate as unacceptable and preventable.
Dr. Reyes, a Latina who grew up as one of eight children in California’s agricultural heartland, now practices perinatology at the University of California, Davis. She is a member of a California Department of Public Health panel that reviews cases of maternal deaths and recommends improvements. And she chairs the board of the California Health Care Foundation, a nonprofit that works to increase health care access. (California Healthline is an editorially independent service of the California Health Care Foundation.)
Her work has been a blend of medicine and advocacy, and she worries recent federal court rulings will erode hard-fought victories regarding the safety of pregnant women and their babies. She discussed the nation’s maternal health crisis and health care disparities in an interview, which has been edited for length and clarity.
Question: When did you first realize there are disparities in the health care system?
Answer: When I was in high school in the Fresno Unified School District, we were under a consent decree to desegregate. And I was, at the time, student body president at Roosevelt High School. I was asked to be on this unified school district desegregation task force, where the district had to come up with a plan.
It was a time when I really had incredible exposure to how policies are made at a larger level, societal level, that really determine where people live, where they can seek health care, where they go to school. That experience had a tremendous impact on my life in terms of what I wanted to do in a career and how to give back.
Q: The U.S. has one of the best health care systems in the world, yet the maternal mortality rate is high compared with other developed countries. Why do think that is?
A. What we know by the CDC and maternal mortality review committees is that about 60% of maternal deaths are considered preventable. And that’s really been a lot of what I’ve tried to focus on: What can we do to reduce the severity of disease? Or what can we do within the role that we play in maternal health that can reduce that?
We know that there are societal issues absolutely that increase women’s risks and there are public health issues. But there’s a role that hospitals play in helping reduce that risk. Ten years ago, I was on the maternal mortality review committee for the state of California when we started reviewing cases of women who died within hospital systems to see, “Is there a role that we can play in a hospital system to reduce that risk?”
We recognized that sometimes there were conditions that were not recognized early enough so that there was a delay in the care. Sometimes there was a misdiagnosis. Or in some hospital systems, especially rural systems where there aren’t as many resources, sometimes there was the lack of specialists available. So, we’ve identified these risks and said, “We can do something about them.”
Q: You served on a federal panel 20 years ago that published a groundbreaking report identifying racism in health care. It seems as if we could be much further along.
A. The purpose of that committee was to really answer the question: Do patients receive a different level of care based on race? Looking back, we knew there was something there, but we really didn’t know. And it took months for the committee to come to that agreement, that there was a difference. I mean, that was honestly monumental, because we just didn’t have that level of consensus before. And so just to say “That treatment is unequal and it’s unacceptable” was really profound.
We thought that the 700-page report was going to be a time period where there was going to be tremendous movement, and I think I’ve learned over 20 years that change doesn’t happen quickly, especially when providers and health systems don’t see that they play a role. It’s like … “OK, so maybe it exists, but not for me.”
We all saw George Floyd and how he was treated. And during COVID we saw a tremendous difference in who was dying, right? Underrepresented minorities – certainly much higher. It was that culmination that made us realize the elephant in the room. We can’t ignore that this does exist, that there is a difference in how people are treated, even in our health care system.
Q: When addressing racism in health care, you talk about diversifying not just the health care workforce, but also the boardrooms of hospitals and health systems. Why is that important?
A. At the board level, change is hard. But we all play a role because leadership really helps determine much of what’s carried out. So, to have a leadership that is understanding and representative of the communities they serve, I think it has been demonstrated that we do make a difference.
Q: As a health care provider, do you have a wish list of policies you’d like the government to take up?
A. There was tremendous effort around offering preventive health services as a part of what was covered under the Affordable Care Act. And individuals exhaled, finally thinking this is a tremendous win, especially for women in pregnancy. Because we fought for preventive health services to help them have access so they can prepare for their pregnancy. So, for women, this was huge. But now with the Texas federal court ruling that the U.S. Preventive Services Task Force didn’t have any authority, it is a tremendous step backward.
We have culturally, linguistically appropriate standards in place, but it’s a matter in terms of how they’re carried out by state and by individual hospital systems. My wish list is that we really do listen to our patients, speak to them in a language of their choice, and provide them written materials in the language of their choice. We don’t fully do that.
Q: You mentioned one Texas ruling on the ACA. What’s your take on the ruling by another Texas judge suspending the abortion pill? And the U.S. Supreme Court’s overturning of Roe v. Wade?A. As a maternal-fetal medicine specialist who tries to help women plan for pregnancies, those rulings are a tremendous setback.
Q: And what about women of color? Will they find access to abortion services more difficult?
A. Oh, absolutely. When we speak of underrepresented minorities or those with less resources, they have less resources to then seek the appropriate care. Some women may have the opportunity to go to a different state or seek care elsewhere if their state doesn’t provide it. Many women just don’t have those resources to devote to them and don’t have a choice. So, we will see that disparity widen.
This article was produced by KFF Health News, which publishes California Healthline, an editorially independent service of the California Health Care Foundation.
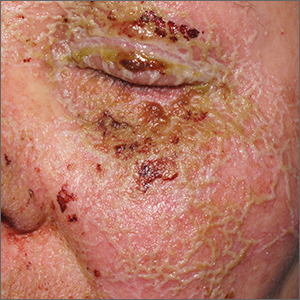
Pustules on face
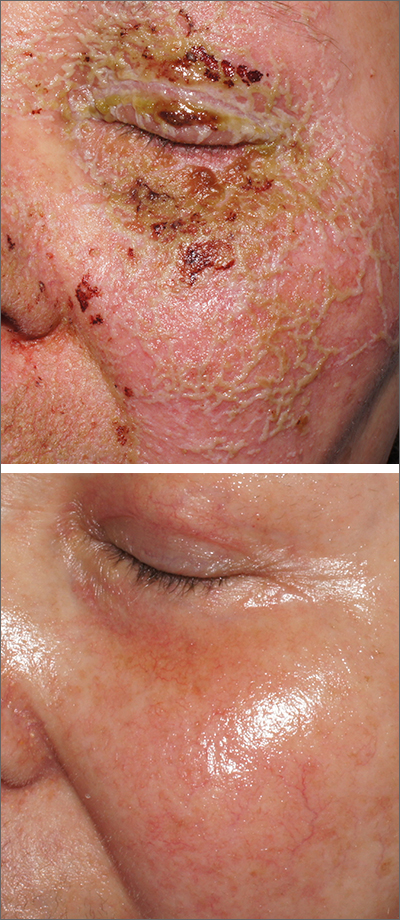
A review of the patient’s chemotherapy medications revealed that 4 weeks earlier, panitumumab had been added to her folinic acid, fluorouracil, and irinotecan (FOLFIRI) regimen. The physician diagnosed this acneiform eruption as an adverse effect of the panitumumab.
Panitumumab is a monoclonal antibody that works to inhibit epidermal growth factor receptor (EGFR) proteins that are overexpressed on some solid tumors and responsible for cancer cell proliferation. EGFR inhibitor–induced acneiform eruptions are common in patients receiving panitumumab.
EGFR proteins have been a target of chemotherapy since the approval of the small molecule erlotinib in 2004. Panitumumab and cetuximab are monoclonal antibodies targeting EGFR and improve long-term survival in patients with metastatic colorectal cancer when added to other standard chemotherapy regimens. EGFR is found throughout the epidermis and all EGFR inhibitors may cause unique skin toxicity not seen with other chemotherapy agents. In 1 study of 229 patients, 59% of patients exhibited skin toxicity at Day 15; the most common examples included widespread acne-like papules and pustules or an eczema-like manifestation.1 Eruptions may be worsened by significant sun exposure while on panitumumab. In this case, the acneiform eruption occurred more intensely along visible facial telangiectasias.
When EGFR inhibitor–induced acneiform eruption occurs, patients commonly develop skin toxicity within the first 2 to 4 weeks of therapy. Pre-therapy doxycycline or minocycline and/or topical steroids may help prevent toxicities from occurring. These same therapies may be used to treat symptoms after they have occurred. More severe cases with systemic symptoms or failure to improve with the above measures may need prednisone or cessation of therapy.
This patient was started on topical hydrocortisone 2.5% ointment twice daily and oral doxycycline 100 mg bid for 6 weeks. She had dramatic improvement within 3 weeks. Doxycycline was subsequently continued at a dose of 100 mg/d and the patient was able to continue with her chemotherapy combination for several more months. Unfortunately, her colon cancer progressed despite therapy and she ultimately died from cancer-related complications.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Bouché O, Ben Abdelghani M, Labourey JL, et al. Management of skin toxicities during panitumumab treatment in metastatic colorectal cancer. World J Gastroenterol. 2019;25:4007-4018. doi: 10.3748/wjg.v25.i29.4007

A review of the patient’s chemotherapy medications revealed that 4 weeks earlier, panitumumab had been added to her folinic acid, fluorouracil, and irinotecan (FOLFIRI) regimen. The physician diagnosed this acneiform eruption as an adverse effect of the panitumumab.
Panitumumab is a monoclonal antibody that works to inhibit epidermal growth factor receptor (EGFR) proteins that are overexpressed on some solid tumors and responsible for cancer cell proliferation. EGFR inhibitor–induced acneiform eruptions are common in patients receiving panitumumab.
EGFR proteins have been a target of chemotherapy since the approval of the small molecule erlotinib in 2004. Panitumumab and cetuximab are monoclonal antibodies targeting EGFR and improve long-term survival in patients with metastatic colorectal cancer when added to other standard chemotherapy regimens. EGFR is found throughout the epidermis and all EGFR inhibitors may cause unique skin toxicity not seen with other chemotherapy agents. In 1 study of 229 patients, 59% of patients exhibited skin toxicity at Day 15; the most common examples included widespread acne-like papules and pustules or an eczema-like manifestation.1 Eruptions may be worsened by significant sun exposure while on panitumumab. In this case, the acneiform eruption occurred more intensely along visible facial telangiectasias.
When EGFR inhibitor–induced acneiform eruption occurs, patients commonly develop skin toxicity within the first 2 to 4 weeks of therapy. Pre-therapy doxycycline or minocycline and/or topical steroids may help prevent toxicities from occurring. These same therapies may be used to treat symptoms after they have occurred. More severe cases with systemic symptoms or failure to improve with the above measures may need prednisone or cessation of therapy.
This patient was started on topical hydrocortisone 2.5% ointment twice daily and oral doxycycline 100 mg bid for 6 weeks. She had dramatic improvement within 3 weeks. Doxycycline was subsequently continued at a dose of 100 mg/d and the patient was able to continue with her chemotherapy combination for several more months. Unfortunately, her colon cancer progressed despite therapy and she ultimately died from cancer-related complications.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.

A review of the patient’s chemotherapy medications revealed that 4 weeks earlier, panitumumab had been added to her folinic acid, fluorouracil, and irinotecan (FOLFIRI) regimen. The physician diagnosed this acneiform eruption as an adverse effect of the panitumumab.
Panitumumab is a monoclonal antibody that works to inhibit epidermal growth factor receptor (EGFR) proteins that are overexpressed on some solid tumors and responsible for cancer cell proliferation. EGFR inhibitor–induced acneiform eruptions are common in patients receiving panitumumab.
EGFR proteins have been a target of chemotherapy since the approval of the small molecule erlotinib in 2004. Panitumumab and cetuximab are monoclonal antibodies targeting EGFR and improve long-term survival in patients with metastatic colorectal cancer when added to other standard chemotherapy regimens. EGFR is found throughout the epidermis and all EGFR inhibitors may cause unique skin toxicity not seen with other chemotherapy agents. In 1 study of 229 patients, 59% of patients exhibited skin toxicity at Day 15; the most common examples included widespread acne-like papules and pustules or an eczema-like manifestation.1 Eruptions may be worsened by significant sun exposure while on panitumumab. In this case, the acneiform eruption occurred more intensely along visible facial telangiectasias.
When EGFR inhibitor–induced acneiform eruption occurs, patients commonly develop skin toxicity within the first 2 to 4 weeks of therapy. Pre-therapy doxycycline or minocycline and/or topical steroids may help prevent toxicities from occurring. These same therapies may be used to treat symptoms after they have occurred. More severe cases with systemic symptoms or failure to improve with the above measures may need prednisone or cessation of therapy.
This patient was started on topical hydrocortisone 2.5% ointment twice daily and oral doxycycline 100 mg bid for 6 weeks. She had dramatic improvement within 3 weeks. Doxycycline was subsequently continued at a dose of 100 mg/d and the patient was able to continue with her chemotherapy combination for several more months. Unfortunately, her colon cancer progressed despite therapy and she ultimately died from cancer-related complications.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Bouché O, Ben Abdelghani M, Labourey JL, et al. Management of skin toxicities during panitumumab treatment in metastatic colorectal cancer. World J Gastroenterol. 2019;25:4007-4018. doi: 10.3748/wjg.v25.i29.4007
1. Bouché O, Ben Abdelghani M, Labourey JL, et al. Management of skin toxicities during panitumumab treatment in metastatic colorectal cancer. World J Gastroenterol. 2019;25:4007-4018. doi: 10.3748/wjg.v25.i29.4007
The next big thing in cancer research
Cancer research has made big strides over the past few decades, leading to better prevention efforts, improved treatment options, and longer survival. Despite the significant progress, there is still a lot of work to do.
More sex-specific research
Sherene Loi, MBBS, PhD, head of the Translational Breast Cancer Genomics and Therapeutics Laboratory at the MacCallum Cancer Centre in Melbourne, said there needs to be more research on the differences in immune-related adverse events and immune responses between the sexes.
Dr. Loi’s recent research in mouse models has revealed that immune checkpoint inhibitors can lead to reduced oocyte reserves, and if those insights are validated in humans, it could have big implications for women of childbearing age who may face premature menopause and infertility.
“It is astonishing to realize that very little research has been done to investigate the long-term reproductive or fertility consequences of new agents we investigate in the phase 3 setting and then prescribe routinely in the curative setting,” Dr. Loi said.
The global cancer community
C. S. Pramesh, MMBS, MS, FRCS, director of Tata Memorial Hospital in Mumbai, India, said that cancer research should prioritize global experiences, instead of focusing so heavily on high-income countries such as the United States.
“With much of the cancer burden likely to fall on low- and middle-income countries, it seems incongruous that almost 90% of cancer research currently takes place in high-income countries,” Dr. Pramesh said. “Neither the discordance between the cancer burden and research funding in high-income countries nor the types of problems or solutions addressed in these countries are relevant to the majority of patients with cancer in the world.”
Bishal Gyawali, MD, PhD, has discussed a similar need to prioritize cancer care in low- and middle-income countries, what he has dubbed “cancer groundshot.”
Dr. Pramesh described a brainstorming session among colleagues with global cancer expertise in which they identified five broad themes especially relevant to a global community. These themes include reducing the burden of patients presenting with advanced disease as well as improving access, affordability, and outcomes through solution-oriented research – goals that are critical but often not prioritized by high-income countries or industry, he said.
“Now is the time for the global community to wake up, take notice, and change the direction of cancer research for the larger public good,” Dr. Pramesh said.
Prioritizing combination therapies
The next big focus in cancer research should be to develop effective combination therapies, according to René Bernards, PhD, of The Netherlands Cancer Institute.
“Resistance to therapy remains a major obstacle in the treatment of cancer,” Dr. Bernards said. But, as the AIDS pandemic has taught us, the use of multiple drugs with “nonoverlapping resistance mechanisms can make a deadly disease with a high mutation rate chronic.”
A growing body of evidence highlights the relevance of this strategy to oncology. A recent study, for instance, highlighted the effectiveness of dual immune checkpoint inhibitors to treat advanced melanoma.
“I believe that academic researchers can deliver more clinical benefit to patients by focusing on finding highly effective combinations of existing drugs than by searching for more drug targets,” he said. “Over time, this would also contribute to affordable health care through use of more generic drugs.”
Cancer drugs and the heart
Cardiologist Javid Moslehi, MD, who specializes in the cardiovascular health of patients with cancer, believes cardio-oncology should be the next frontier. During his research fellowship, Dr. Moslehi discovered that “many novel cancer therapies were leading to cardiovascular adverse effects, both during treatment and survivorship.”
But, Dr. Moslehi explained, “we are entering [uncharted] waters.”
Patients who receive immune checkpoint inhibitors may, for instance, develop fulminant myocarditis. Dr. Moslehi and colleagues have also found in preclinical models that abatacept (CTLA4-Ig) may be an effective treatment for myocarditis.
“Because of the targeted nature of new cancer therapies, cardiovascular sequelae may provide insights into cardiac biology, making cardio-oncology a novel platform for cardiovascular investigation,” Dr. Moslehi explained.
Inside rare cancers
William Sellers, MD, director of the Broad Institute of MIT’s Cancer Program, Cambridge, Mass., said rare cancers should be the next focus.
After all, “rare cancers are only rare in isolation,” Dr. Sellers said, noting that these cancers make up 20%-24% of all cancer diagnoses.
Although funding for rare cancer research remains limited, investing more could benefit patients in the long run. In early 2023, Pfizer announced plans to explore more options for early stage treatments for rare diseases and cancers.
“New initiatives supporting direct-to-patient cohort enrollment bridging geographic fragmentation and rare cancer model development, enabling preclinical research to accelerate, are the first steps along a path toward curing these diseases,” he said.
The researchers reported numerous relationships with pharmaceutical companies.
A version of this article first appeared on Medscape.com.
Cancer research has made big strides over the past few decades, leading to better prevention efforts, improved treatment options, and longer survival. Despite the significant progress, there is still a lot of work to do.
More sex-specific research
Sherene Loi, MBBS, PhD, head of the Translational Breast Cancer Genomics and Therapeutics Laboratory at the MacCallum Cancer Centre in Melbourne, said there needs to be more research on the differences in immune-related adverse events and immune responses between the sexes.
Dr. Loi’s recent research in mouse models has revealed that immune checkpoint inhibitors can lead to reduced oocyte reserves, and if those insights are validated in humans, it could have big implications for women of childbearing age who may face premature menopause and infertility.
“It is astonishing to realize that very little research has been done to investigate the long-term reproductive or fertility consequences of new agents we investigate in the phase 3 setting and then prescribe routinely in the curative setting,” Dr. Loi said.
The global cancer community
C. S. Pramesh, MMBS, MS, FRCS, director of Tata Memorial Hospital in Mumbai, India, said that cancer research should prioritize global experiences, instead of focusing so heavily on high-income countries such as the United States.
“With much of the cancer burden likely to fall on low- and middle-income countries, it seems incongruous that almost 90% of cancer research currently takes place in high-income countries,” Dr. Pramesh said. “Neither the discordance between the cancer burden and research funding in high-income countries nor the types of problems or solutions addressed in these countries are relevant to the majority of patients with cancer in the world.”
Bishal Gyawali, MD, PhD, has discussed a similar need to prioritize cancer care in low- and middle-income countries, what he has dubbed “cancer groundshot.”
Dr. Pramesh described a brainstorming session among colleagues with global cancer expertise in which they identified five broad themes especially relevant to a global community. These themes include reducing the burden of patients presenting with advanced disease as well as improving access, affordability, and outcomes through solution-oriented research – goals that are critical but often not prioritized by high-income countries or industry, he said.
“Now is the time for the global community to wake up, take notice, and change the direction of cancer research for the larger public good,” Dr. Pramesh said.
Prioritizing combination therapies
The next big focus in cancer research should be to develop effective combination therapies, according to René Bernards, PhD, of The Netherlands Cancer Institute.
“Resistance to therapy remains a major obstacle in the treatment of cancer,” Dr. Bernards said. But, as the AIDS pandemic has taught us, the use of multiple drugs with “nonoverlapping resistance mechanisms can make a deadly disease with a high mutation rate chronic.”
A growing body of evidence highlights the relevance of this strategy to oncology. A recent study, for instance, highlighted the effectiveness of dual immune checkpoint inhibitors to treat advanced melanoma.
“I believe that academic researchers can deliver more clinical benefit to patients by focusing on finding highly effective combinations of existing drugs than by searching for more drug targets,” he said. “Over time, this would also contribute to affordable health care through use of more generic drugs.”
Cancer drugs and the heart
Cardiologist Javid Moslehi, MD, who specializes in the cardiovascular health of patients with cancer, believes cardio-oncology should be the next frontier. During his research fellowship, Dr. Moslehi discovered that “many novel cancer therapies were leading to cardiovascular adverse effects, both during treatment and survivorship.”
But, Dr. Moslehi explained, “we are entering [uncharted] waters.”
Patients who receive immune checkpoint inhibitors may, for instance, develop fulminant myocarditis. Dr. Moslehi and colleagues have also found in preclinical models that abatacept (CTLA4-Ig) may be an effective treatment for myocarditis.
“Because of the targeted nature of new cancer therapies, cardiovascular sequelae may provide insights into cardiac biology, making cardio-oncology a novel platform for cardiovascular investigation,” Dr. Moslehi explained.
Inside rare cancers
William Sellers, MD, director of the Broad Institute of MIT’s Cancer Program, Cambridge, Mass., said rare cancers should be the next focus.
After all, “rare cancers are only rare in isolation,” Dr. Sellers said, noting that these cancers make up 20%-24% of all cancer diagnoses.
Although funding for rare cancer research remains limited, investing more could benefit patients in the long run. In early 2023, Pfizer announced plans to explore more options for early stage treatments for rare diseases and cancers.
“New initiatives supporting direct-to-patient cohort enrollment bridging geographic fragmentation and rare cancer model development, enabling preclinical research to accelerate, are the first steps along a path toward curing these diseases,” he said.
The researchers reported numerous relationships with pharmaceutical companies.
A version of this article first appeared on Medscape.com.
Cancer research has made big strides over the past few decades, leading to better prevention efforts, improved treatment options, and longer survival. Despite the significant progress, there is still a lot of work to do.
More sex-specific research
Sherene Loi, MBBS, PhD, head of the Translational Breast Cancer Genomics and Therapeutics Laboratory at the MacCallum Cancer Centre in Melbourne, said there needs to be more research on the differences in immune-related adverse events and immune responses between the sexes.
Dr. Loi’s recent research in mouse models has revealed that immune checkpoint inhibitors can lead to reduced oocyte reserves, and if those insights are validated in humans, it could have big implications for women of childbearing age who may face premature menopause and infertility.
“It is astonishing to realize that very little research has been done to investigate the long-term reproductive or fertility consequences of new agents we investigate in the phase 3 setting and then prescribe routinely in the curative setting,” Dr. Loi said.
The global cancer community
C. S. Pramesh, MMBS, MS, FRCS, director of Tata Memorial Hospital in Mumbai, India, said that cancer research should prioritize global experiences, instead of focusing so heavily on high-income countries such as the United States.
“With much of the cancer burden likely to fall on low- and middle-income countries, it seems incongruous that almost 90% of cancer research currently takes place in high-income countries,” Dr. Pramesh said. “Neither the discordance between the cancer burden and research funding in high-income countries nor the types of problems or solutions addressed in these countries are relevant to the majority of patients with cancer in the world.”
Bishal Gyawali, MD, PhD, has discussed a similar need to prioritize cancer care in low- and middle-income countries, what he has dubbed “cancer groundshot.”
Dr. Pramesh described a brainstorming session among colleagues with global cancer expertise in which they identified five broad themes especially relevant to a global community. These themes include reducing the burden of patients presenting with advanced disease as well as improving access, affordability, and outcomes through solution-oriented research – goals that are critical but often not prioritized by high-income countries or industry, he said.
“Now is the time for the global community to wake up, take notice, and change the direction of cancer research for the larger public good,” Dr. Pramesh said.
Prioritizing combination therapies
The next big focus in cancer research should be to develop effective combination therapies, according to René Bernards, PhD, of The Netherlands Cancer Institute.
“Resistance to therapy remains a major obstacle in the treatment of cancer,” Dr. Bernards said. But, as the AIDS pandemic has taught us, the use of multiple drugs with “nonoverlapping resistance mechanisms can make a deadly disease with a high mutation rate chronic.”
A growing body of evidence highlights the relevance of this strategy to oncology. A recent study, for instance, highlighted the effectiveness of dual immune checkpoint inhibitors to treat advanced melanoma.
“I believe that academic researchers can deliver more clinical benefit to patients by focusing on finding highly effective combinations of existing drugs than by searching for more drug targets,” he said. “Over time, this would also contribute to affordable health care through use of more generic drugs.”
Cancer drugs and the heart
Cardiologist Javid Moslehi, MD, who specializes in the cardiovascular health of patients with cancer, believes cardio-oncology should be the next frontier. During his research fellowship, Dr. Moslehi discovered that “many novel cancer therapies were leading to cardiovascular adverse effects, both during treatment and survivorship.”
But, Dr. Moslehi explained, “we are entering [uncharted] waters.”
Patients who receive immune checkpoint inhibitors may, for instance, develop fulminant myocarditis. Dr. Moslehi and colleagues have also found in preclinical models that abatacept (CTLA4-Ig) may be an effective treatment for myocarditis.
“Because of the targeted nature of new cancer therapies, cardiovascular sequelae may provide insights into cardiac biology, making cardio-oncology a novel platform for cardiovascular investigation,” Dr. Moslehi explained.
Inside rare cancers
William Sellers, MD, director of the Broad Institute of MIT’s Cancer Program, Cambridge, Mass., said rare cancers should be the next focus.
After all, “rare cancers are only rare in isolation,” Dr. Sellers said, noting that these cancers make up 20%-24% of all cancer diagnoses.
Although funding for rare cancer research remains limited, investing more could benefit patients in the long run. In early 2023, Pfizer announced plans to explore more options for early stage treatments for rare diseases and cancers.
“New initiatives supporting direct-to-patient cohort enrollment bridging geographic fragmentation and rare cancer model development, enabling preclinical research to accelerate, are the first steps along a path toward curing these diseases,” he said.
The researchers reported numerous relationships with pharmaceutical companies.
A version of this article first appeared on Medscape.com.
FROM CELL
Prostate biopsies a laughing (gas) matter?
An old dog – nitrous oxide – can learn new tricks, managing pain in men undergoing transrectal biopsies, researchers reported at the annual meeting of the American Urological Association.
said Heidi Rayala, MD, PhD, assistant professor of surgery at Harvard Medical School, Boston, who helped conduct the study.
Nitrous oxide is best known as a pain medication and anesthetic during dental procedures and childbirth, after trauma, and in end-of-life care.
In the new study, Dr. Rayala and her colleagues at Harvard and Beth Israel-Deaconess Medical Center, Boston, randomly assigned 128 men to self-administered nitrous oxide (SANO) or oxygen as a placebo. Patients in the SANO group had a smaller change in post-biopsy pain score (Visual Analog Scale for pain, 0.43 vs. 1.03; P = .03) and lower odds of experiencing pain during the procedure (odds ratio, 0.45; confidence interval, 0.21-0.97; P = .04).
A comparison of anxiety scores in the two groups failed to find a statistically significant difference between SANO and placebo. However, more men who received nitrous oxide said they tolerated the procedure “better than expected” (61% vs. 41%; P = 0.02), according to the researchers.
Dr. Rayala said that the researchers used the Nitrouseal system (Sedation Systems), in which the patient holds a mask to their face and works with staff to adjust the gas levels to the desired amount. The system is governed to max out at 50% nitrous oxide, ensuring “minimal sedation concentrations, so anesthesia personnel are not required,” she said.
“At levels of less than 50%, patients respond normally to verbal commands and maintain normal airway reflexes,” Dr. Rayala added. “This provides an advantage in that patients do not require the presence of anesthesia personnel.” And because the body eliminates the gas within about 5 minutes, patients do not require an escort home, she said.
This system is also self-scavenging to protect the operating urologist and other personnel from environmental exposure to nitrous oxide.
Dr. Rayala said that three patients (2.3%) found the mask uncomfortable, but in follow-up studies the clinicians have done a better job of preparing patients for the feeling of the mask, making a marked difference. Headaches and nausea are the most commonly reported complaints at concentrations above 50%.
“We did not have patients report headaches or nausea in new study (by the BIDMC group),” she said. This study has been submitted for publication.
Clinicians outside the United States have been quicker to embrace nitrous oxide for prostate procedures.
In a randomized controlled trial, researchers in Australia found no significant improvement in pain scores at 15 minutes from the use of nitrous oxide during transrectal biopsies; however, improvements were seen in patient-reported discomfort, overall experience, and willingness to undergo repeat biopsies.
Stephen McCombie, MD, a consultant at Perth Urology Clinic, Australia, who has been adapting the nitrous oxide protocol for transrectal biopsies to transperineal procedures, said that the Beth Israel study “adds to the evidence to support adjunct use of mild inhalational anesthetics and analgesics during prostate biopsies to improve the patient experience of the procedure.”
He said that the role for these agents may grow with the global trend away from transrectal prostate biopsies and toward transperineal biopsies, largely driven by increasing rates of sepsis after transrectal biopsies.
“While transperineal biopsies can be more painful then transrectal biopsies when performed under local anesthesia, perhaps due to biopsies being taken through the highly sensate perineum as opposed to above the dentate line, optimization of the technique can significantly reduce the discomfort associated with the procedure, which may be further reduced with these agents,” Dr. McCombie said.
“Studies indicate that transperineal biopsies can be more painful than the traditional transrectal biopsies,” Dr. Rayala said. “We do offer transperineal biopsies at BIDMC, and we are gearing up to repeat the SANO study” for those patients.
Dr. Rayala and Dr. McCombie have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
An old dog – nitrous oxide – can learn new tricks, managing pain in men undergoing transrectal biopsies, researchers reported at the annual meeting of the American Urological Association.
said Heidi Rayala, MD, PhD, assistant professor of surgery at Harvard Medical School, Boston, who helped conduct the study.
Nitrous oxide is best known as a pain medication and anesthetic during dental procedures and childbirth, after trauma, and in end-of-life care.
In the new study, Dr. Rayala and her colleagues at Harvard and Beth Israel-Deaconess Medical Center, Boston, randomly assigned 128 men to self-administered nitrous oxide (SANO) or oxygen as a placebo. Patients in the SANO group had a smaller change in post-biopsy pain score (Visual Analog Scale for pain, 0.43 vs. 1.03; P = .03) and lower odds of experiencing pain during the procedure (odds ratio, 0.45; confidence interval, 0.21-0.97; P = .04).
A comparison of anxiety scores in the two groups failed to find a statistically significant difference between SANO and placebo. However, more men who received nitrous oxide said they tolerated the procedure “better than expected” (61% vs. 41%; P = 0.02), according to the researchers.
Dr. Rayala said that the researchers used the Nitrouseal system (Sedation Systems), in which the patient holds a mask to their face and works with staff to adjust the gas levels to the desired amount. The system is governed to max out at 50% nitrous oxide, ensuring “minimal sedation concentrations, so anesthesia personnel are not required,” she said.
“At levels of less than 50%, patients respond normally to verbal commands and maintain normal airway reflexes,” Dr. Rayala added. “This provides an advantage in that patients do not require the presence of anesthesia personnel.” And because the body eliminates the gas within about 5 minutes, patients do not require an escort home, she said.
This system is also self-scavenging to protect the operating urologist and other personnel from environmental exposure to nitrous oxide.
Dr. Rayala said that three patients (2.3%) found the mask uncomfortable, but in follow-up studies the clinicians have done a better job of preparing patients for the feeling of the mask, making a marked difference. Headaches and nausea are the most commonly reported complaints at concentrations above 50%.
“We did not have patients report headaches or nausea in new study (by the BIDMC group),” she said. This study has been submitted for publication.
Clinicians outside the United States have been quicker to embrace nitrous oxide for prostate procedures.
In a randomized controlled trial, researchers in Australia found no significant improvement in pain scores at 15 minutes from the use of nitrous oxide during transrectal biopsies; however, improvements were seen in patient-reported discomfort, overall experience, and willingness to undergo repeat biopsies.
Stephen McCombie, MD, a consultant at Perth Urology Clinic, Australia, who has been adapting the nitrous oxide protocol for transrectal biopsies to transperineal procedures, said that the Beth Israel study “adds to the evidence to support adjunct use of mild inhalational anesthetics and analgesics during prostate biopsies to improve the patient experience of the procedure.”
He said that the role for these agents may grow with the global trend away from transrectal prostate biopsies and toward transperineal biopsies, largely driven by increasing rates of sepsis after transrectal biopsies.
“While transperineal biopsies can be more painful then transrectal biopsies when performed under local anesthesia, perhaps due to biopsies being taken through the highly sensate perineum as opposed to above the dentate line, optimization of the technique can significantly reduce the discomfort associated with the procedure, which may be further reduced with these agents,” Dr. McCombie said.
“Studies indicate that transperineal biopsies can be more painful than the traditional transrectal biopsies,” Dr. Rayala said. “We do offer transperineal biopsies at BIDMC, and we are gearing up to repeat the SANO study” for those patients.
Dr. Rayala and Dr. McCombie have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
An old dog – nitrous oxide – can learn new tricks, managing pain in men undergoing transrectal biopsies, researchers reported at the annual meeting of the American Urological Association.
said Heidi Rayala, MD, PhD, assistant professor of surgery at Harvard Medical School, Boston, who helped conduct the study.
Nitrous oxide is best known as a pain medication and anesthetic during dental procedures and childbirth, after trauma, and in end-of-life care.
In the new study, Dr. Rayala and her colleagues at Harvard and Beth Israel-Deaconess Medical Center, Boston, randomly assigned 128 men to self-administered nitrous oxide (SANO) or oxygen as a placebo. Patients in the SANO group had a smaller change in post-biopsy pain score (Visual Analog Scale for pain, 0.43 vs. 1.03; P = .03) and lower odds of experiencing pain during the procedure (odds ratio, 0.45; confidence interval, 0.21-0.97; P = .04).
A comparison of anxiety scores in the two groups failed to find a statistically significant difference between SANO and placebo. However, more men who received nitrous oxide said they tolerated the procedure “better than expected” (61% vs. 41%; P = 0.02), according to the researchers.
Dr. Rayala said that the researchers used the Nitrouseal system (Sedation Systems), in which the patient holds a mask to their face and works with staff to adjust the gas levels to the desired amount. The system is governed to max out at 50% nitrous oxide, ensuring “minimal sedation concentrations, so anesthesia personnel are not required,” she said.
“At levels of less than 50%, patients respond normally to verbal commands and maintain normal airway reflexes,” Dr. Rayala added. “This provides an advantage in that patients do not require the presence of anesthesia personnel.” And because the body eliminates the gas within about 5 minutes, patients do not require an escort home, she said.
This system is also self-scavenging to protect the operating urologist and other personnel from environmental exposure to nitrous oxide.
Dr. Rayala said that three patients (2.3%) found the mask uncomfortable, but in follow-up studies the clinicians have done a better job of preparing patients for the feeling of the mask, making a marked difference. Headaches and nausea are the most commonly reported complaints at concentrations above 50%.
“We did not have patients report headaches or nausea in new study (by the BIDMC group),” she said. This study has been submitted for publication.
Clinicians outside the United States have been quicker to embrace nitrous oxide for prostate procedures.
In a randomized controlled trial, researchers in Australia found no significant improvement in pain scores at 15 minutes from the use of nitrous oxide during transrectal biopsies; however, improvements were seen in patient-reported discomfort, overall experience, and willingness to undergo repeat biopsies.
Stephen McCombie, MD, a consultant at Perth Urology Clinic, Australia, who has been adapting the nitrous oxide protocol for transrectal biopsies to transperineal procedures, said that the Beth Israel study “adds to the evidence to support adjunct use of mild inhalational anesthetics and analgesics during prostate biopsies to improve the patient experience of the procedure.”
He said that the role for these agents may grow with the global trend away from transrectal prostate biopsies and toward transperineal biopsies, largely driven by increasing rates of sepsis after transrectal biopsies.
“While transperineal biopsies can be more painful then transrectal biopsies when performed under local anesthesia, perhaps due to biopsies being taken through the highly sensate perineum as opposed to above the dentate line, optimization of the technique can significantly reduce the discomfort associated with the procedure, which may be further reduced with these agents,” Dr. McCombie said.
“Studies indicate that transperineal biopsies can be more painful than the traditional transrectal biopsies,” Dr. Rayala said. “We do offer transperineal biopsies at BIDMC, and we are gearing up to repeat the SANO study” for those patients.
Dr. Rayala and Dr. McCombie have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT AUA 2023