User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
div[contains(@class, 'medstat-accordion-set article-series')]
‘Reform School’ for Pharmacy Benefit Managers: How Might Legislation Help Patients?
The term “reform school” is a bit outdated. It used to refer to institutions where young offenders were sent instead of prison. Some argue that pharmacy benefit managers (PBMs) should bypass reform school and go straight to prison. “PBM reform” has become a ubiquitous term, encompassing any legislative or regulatory efforts aimed at curbing PBMs’ bad behavior. When discussing PBM reform, it’s crucial to understand the various segments of the healthcare system affected by PBMs. This complexity often makes it challenging to determine what these reform packages would actually achieve and who they would benefit.
Pharmacists have long been vocal critics of PBMs, and while their issues are extremely important, it is essential to remember that the ultimate victims of PBM misconduct, in terms of access to care, are patients. At some point, we will all be patients, making this issue universally relevant. It has been quite challenging to follow federal legislation on this topic as these packages attempt to address a number of bad behaviors by PBMs affecting a variety of victims. This discussion will examine those reforms that would directly improve patient’s access to available and affordable medications.
Policy Categories of PBM Reform
There are five policy categories of PBM reform legislation overall, including three that have the greatest potential to directly address patient needs. The first is patient access to medications (utilization management, copay assistance, prior authorization, etc.), followed by delinking drug list prices from PBM income and pass-through of price concessions from the manufacturer. The remaining two categories involve transparency and pharmacy-facing reform, both of which are very important. However, this discussion will revolve around the first three categories. It should be noted that many of the legislation packages addressing the categories of patient access, delinking, and pass-through also include transparency issues, particularly as they relate to pharmacy-facing issues.
Patient Access to Medications — Step Therapy Legislation
One of the major obstacles to patient access to medications is the use of PBM utilization management tools such as step therapy (“fail first”), prior authorizations, nonmedical switching, and formulary exclusions. These tools dictate when patients can obtain necessary medications and for how long patients who are stable on their current treatments can remain on them.
While many states have enacted step therapy reforms to prevent stable patients from being whip-sawed between medications that maximize PBM profits (often labeled as “savings”), these state protections apply only to state-regulated health plans. These include fully insured health plans and those offered through the Affordable Care Act’s Health Insurance Marketplace. It also includes state employees, state corrections, and, in some cases, state labor unions. State legislation does not extend to patients covered by employer self-insured health plans, called ERISA plans for the federal law that governs employee benefit plans, the Employee Retirement Income Security Act. These ERISA plans include nearly 35 million people nationwide.
This is where the Safe Step Act (S.652/H.R.2630) becomes crucial, as it allows employees to request exceptions to harmful fail-first protocols. The bill has gained significant momentum, having been reported out of the Senate HELP Committee and discussed in House markups. The Safe Step Act would mandate that an exception to a step therapy protocol must be granted if:
- The required treatment has been ineffective
- The treatment is expected to be ineffective, and delaying effective treatment would lead to irreversible consequences
- The treatment will cause or is likely to cause an adverse reaction
- The treatment is expected to prevent the individual from performing daily activities or occupational responsibilities
- The individual is stable on their current prescription drugs
- There are other circumstances as determined by the Employee Benefits Security Administration
This legislation is vital for ensuring that patients have timely access to the medications they need without unnecessary delays or disruptions.
Patient Access to Medications — Prior Authorizations
Another significant issue affecting patient access to medications is prior authorizations (PAs). According to an American Medical Association survey, nearly one in four physicians (24%) report that a PA has led to a serious adverse event for a patient in their care. In rheumatology, PAs often result in delays in care (even for those initially approved) and a significant increase in steroid usage. In particular, PAs in Medicare Advantage (MA) plans are harmful to Medicare beneficiaries.
The Improving Seniors’ Timely Access to Care Act (H.R.8702 / S.4532) aims to reform PAs used in MA plans, making the process more efficient and transparent to improve access to care for seniors. Unfortunately, it does not cover Part D drugs and may only cover Part B drugs depending on the MA plan’s benefit package. Here are the key provisions of the act:
- Electronic PA: Implementing real-time decisions for routinely approved items and services.
- Transparency: Requiring annual publication of PA information, such as the percentage of requests approved and the average response time.
- Quality and Timeliness Standards: The Centers for Medicare & Medicaid Services (CMS) will set standards for the quality and timeliness of PA determinations.
- Streamlining Approvals: Simplifying the approval process and reducing the time allowed for health plans to consider PA requests.
This bill passed the House in September 2022 but stalled in the Senate because of an unfavorable Congressional Budget Office score. CMS has since finalized portions of this bill via regulation, zeroing out the CBO score and increasing the chances of its passage.
Delinking Drug Prices from PBM Income and Pass-Through of Price Concessions
Affordability is a crucial aspect of accessibility, especially when it comes to medications. Over the years, we’ve learned that PBMs often favor placing the highest list price drugs on formularies because the rebates and various fees they receive from manufacturers are based on a percentage of the list price. In other words, the higher the medication’s price, the more money the PBM makes.
This practice is evident in both commercial and government formularies, where brand-name drugs are often preferred, while lower-priced generics are either excluded or placed on higher tiers. As a result, while major PBMs benefit from these rebates and fees, patients continue to pay their cost share based on the list price of the medication.
To improve the affordability of medications, a key aspect of PBM reform should be to disincentivize PBMs from selecting higher-priced medications and/or require the pass-through of manufacturer price concessions to patients.
Several major PBM reform bills are currently being considered that address either the delinking of price concessions from the list price of the drug or some form of pass-through of these concessions. These reforms are essential to ensure that patients can access affordable medications without being burdened by inflated costs.
The legislation includes the Pharmacy Benefit Manager Reform Act (S.1339); the Modernizing & Ensuring PBM Accountability Act (S.2973); the Better Mental Health Care, Lower Cost Drugs, and Extenders Act (S.3430); the Protecting Patients Against PBM Abuses Act (H.R. 2880); the DRUG Act (S.2474 / H.R.6283); and the Share the Savings with Seniors Act (S.2474 / H.R.5376).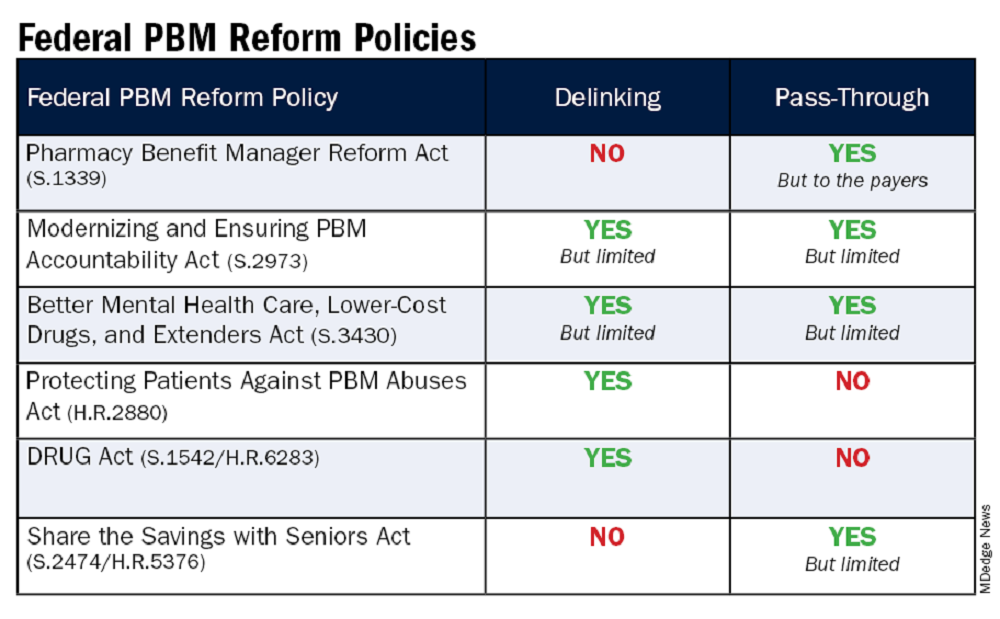
As with all legislation, there are limitations and compromises in each of these. However, these bills are a good first step in addressing PBM remuneration (rebates and fees) based on the list price of the drug and/or passing through to the patient the benefit of manufacturer price concessions. By focusing on key areas like utilization management, delinking drug prices from PBM income, and allowing patients to directly benefit from manufacturer price concessions, we can work toward a more equitable and efficient healthcare system. Reigning in PBM bad behavior is a challenge, but the potential benefits for patient care and access make it a crucial fight worth pursuing.
Please help in efforts to improve patients’ access to available and affordable medications by contacting your representatives in Congress to impart to them the importance of passing legislation. The CSRO’s legislative map tool can help to inform you of the latest information on these and other bills and assist you in engaging with your representatives on them.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s vice president of Advocacy and Government Affairs and its immediate past president, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. She has no relevant conflicts of interest to disclose. You can reach her at rhnews@mdedge.com.
The term “reform school” is a bit outdated. It used to refer to institutions where young offenders were sent instead of prison. Some argue that pharmacy benefit managers (PBMs) should bypass reform school and go straight to prison. “PBM reform” has become a ubiquitous term, encompassing any legislative or regulatory efforts aimed at curbing PBMs’ bad behavior. When discussing PBM reform, it’s crucial to understand the various segments of the healthcare system affected by PBMs. This complexity often makes it challenging to determine what these reform packages would actually achieve and who they would benefit.
Pharmacists have long been vocal critics of PBMs, and while their issues are extremely important, it is essential to remember that the ultimate victims of PBM misconduct, in terms of access to care, are patients. At some point, we will all be patients, making this issue universally relevant. It has been quite challenging to follow federal legislation on this topic as these packages attempt to address a number of bad behaviors by PBMs affecting a variety of victims. This discussion will examine those reforms that would directly improve patient’s access to available and affordable medications.
Policy Categories of PBM Reform
There are five policy categories of PBM reform legislation overall, including three that have the greatest potential to directly address patient needs. The first is patient access to medications (utilization management, copay assistance, prior authorization, etc.), followed by delinking drug list prices from PBM income and pass-through of price concessions from the manufacturer. The remaining two categories involve transparency and pharmacy-facing reform, both of which are very important. However, this discussion will revolve around the first three categories. It should be noted that many of the legislation packages addressing the categories of patient access, delinking, and pass-through also include transparency issues, particularly as they relate to pharmacy-facing issues.
Patient Access to Medications — Step Therapy Legislation
One of the major obstacles to patient access to medications is the use of PBM utilization management tools such as step therapy (“fail first”), prior authorizations, nonmedical switching, and formulary exclusions. These tools dictate when patients can obtain necessary medications and for how long patients who are stable on their current treatments can remain on them.
While many states have enacted step therapy reforms to prevent stable patients from being whip-sawed between medications that maximize PBM profits (often labeled as “savings”), these state protections apply only to state-regulated health plans. These include fully insured health plans and those offered through the Affordable Care Act’s Health Insurance Marketplace. It also includes state employees, state corrections, and, in some cases, state labor unions. State legislation does not extend to patients covered by employer self-insured health plans, called ERISA plans for the federal law that governs employee benefit plans, the Employee Retirement Income Security Act. These ERISA plans include nearly 35 million people nationwide.
This is where the Safe Step Act (S.652/H.R.2630) becomes crucial, as it allows employees to request exceptions to harmful fail-first protocols. The bill has gained significant momentum, having been reported out of the Senate HELP Committee and discussed in House markups. The Safe Step Act would mandate that an exception to a step therapy protocol must be granted if:
- The required treatment has been ineffective
- The treatment is expected to be ineffective, and delaying effective treatment would lead to irreversible consequences
- The treatment will cause or is likely to cause an adverse reaction
- The treatment is expected to prevent the individual from performing daily activities or occupational responsibilities
- The individual is stable on their current prescription drugs
- There are other circumstances as determined by the Employee Benefits Security Administration
This legislation is vital for ensuring that patients have timely access to the medications they need without unnecessary delays or disruptions.
Patient Access to Medications — Prior Authorizations
Another significant issue affecting patient access to medications is prior authorizations (PAs). According to an American Medical Association survey, nearly one in four physicians (24%) report that a PA has led to a serious adverse event for a patient in their care. In rheumatology, PAs often result in delays in care (even for those initially approved) and a significant increase in steroid usage. In particular, PAs in Medicare Advantage (MA) plans are harmful to Medicare beneficiaries.
The Improving Seniors’ Timely Access to Care Act (H.R.8702 / S.4532) aims to reform PAs used in MA plans, making the process more efficient and transparent to improve access to care for seniors. Unfortunately, it does not cover Part D drugs and may only cover Part B drugs depending on the MA plan’s benefit package. Here are the key provisions of the act:
- Electronic PA: Implementing real-time decisions for routinely approved items and services.
- Transparency: Requiring annual publication of PA information, such as the percentage of requests approved and the average response time.
- Quality and Timeliness Standards: The Centers for Medicare & Medicaid Services (CMS) will set standards for the quality and timeliness of PA determinations.
- Streamlining Approvals: Simplifying the approval process and reducing the time allowed for health plans to consider PA requests.
This bill passed the House in September 2022 but stalled in the Senate because of an unfavorable Congressional Budget Office score. CMS has since finalized portions of this bill via regulation, zeroing out the CBO score and increasing the chances of its passage.
Delinking Drug Prices from PBM Income and Pass-Through of Price Concessions
Affordability is a crucial aspect of accessibility, especially when it comes to medications. Over the years, we’ve learned that PBMs often favor placing the highest list price drugs on formularies because the rebates and various fees they receive from manufacturers are based on a percentage of the list price. In other words, the higher the medication’s price, the more money the PBM makes.
This practice is evident in both commercial and government formularies, where brand-name drugs are often preferred, while lower-priced generics are either excluded or placed on higher tiers. As a result, while major PBMs benefit from these rebates and fees, patients continue to pay their cost share based on the list price of the medication.
To improve the affordability of medications, a key aspect of PBM reform should be to disincentivize PBMs from selecting higher-priced medications and/or require the pass-through of manufacturer price concessions to patients.
Several major PBM reform bills are currently being considered that address either the delinking of price concessions from the list price of the drug or some form of pass-through of these concessions. These reforms are essential to ensure that patients can access affordable medications without being burdened by inflated costs.
The legislation includes the Pharmacy Benefit Manager Reform Act (S.1339); the Modernizing & Ensuring PBM Accountability Act (S.2973); the Better Mental Health Care, Lower Cost Drugs, and Extenders Act (S.3430); the Protecting Patients Against PBM Abuses Act (H.R. 2880); the DRUG Act (S.2474 / H.R.6283); and the Share the Savings with Seniors Act (S.2474 / H.R.5376).
As with all legislation, there are limitations and compromises in each of these. However, these bills are a good first step in addressing PBM remuneration (rebates and fees) based on the list price of the drug and/or passing through to the patient the benefit of manufacturer price concessions. By focusing on key areas like utilization management, delinking drug prices from PBM income, and allowing patients to directly benefit from manufacturer price concessions, we can work toward a more equitable and efficient healthcare system. Reigning in PBM bad behavior is a challenge, but the potential benefits for patient care and access make it a crucial fight worth pursuing.
Please help in efforts to improve patients’ access to available and affordable medications by contacting your representatives in Congress to impart to them the importance of passing legislation. The CSRO’s legislative map tool can help to inform you of the latest information on these and other bills and assist you in engaging with your representatives on them.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s vice president of Advocacy and Government Affairs and its immediate past president, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. She has no relevant conflicts of interest to disclose. You can reach her at rhnews@mdedge.com.
The term “reform school” is a bit outdated. It used to refer to institutions where young offenders were sent instead of prison. Some argue that pharmacy benefit managers (PBMs) should bypass reform school and go straight to prison. “PBM reform” has become a ubiquitous term, encompassing any legislative or regulatory efforts aimed at curbing PBMs’ bad behavior. When discussing PBM reform, it’s crucial to understand the various segments of the healthcare system affected by PBMs. This complexity often makes it challenging to determine what these reform packages would actually achieve and who they would benefit.
Pharmacists have long been vocal critics of PBMs, and while their issues are extremely important, it is essential to remember that the ultimate victims of PBM misconduct, in terms of access to care, are patients. At some point, we will all be patients, making this issue universally relevant. It has been quite challenging to follow federal legislation on this topic as these packages attempt to address a number of bad behaviors by PBMs affecting a variety of victims. This discussion will examine those reforms that would directly improve patient’s access to available and affordable medications.
Policy Categories of PBM Reform
There are five policy categories of PBM reform legislation overall, including three that have the greatest potential to directly address patient needs. The first is patient access to medications (utilization management, copay assistance, prior authorization, etc.), followed by delinking drug list prices from PBM income and pass-through of price concessions from the manufacturer. The remaining two categories involve transparency and pharmacy-facing reform, both of which are very important. However, this discussion will revolve around the first three categories. It should be noted that many of the legislation packages addressing the categories of patient access, delinking, and pass-through also include transparency issues, particularly as they relate to pharmacy-facing issues.
Patient Access to Medications — Step Therapy Legislation
One of the major obstacles to patient access to medications is the use of PBM utilization management tools such as step therapy (“fail first”), prior authorizations, nonmedical switching, and formulary exclusions. These tools dictate when patients can obtain necessary medications and for how long patients who are stable on their current treatments can remain on them.
While many states have enacted step therapy reforms to prevent stable patients from being whip-sawed between medications that maximize PBM profits (often labeled as “savings”), these state protections apply only to state-regulated health plans. These include fully insured health plans and those offered through the Affordable Care Act’s Health Insurance Marketplace. It also includes state employees, state corrections, and, in some cases, state labor unions. State legislation does not extend to patients covered by employer self-insured health plans, called ERISA plans for the federal law that governs employee benefit plans, the Employee Retirement Income Security Act. These ERISA plans include nearly 35 million people nationwide.
This is where the Safe Step Act (S.652/H.R.2630) becomes crucial, as it allows employees to request exceptions to harmful fail-first protocols. The bill has gained significant momentum, having been reported out of the Senate HELP Committee and discussed in House markups. The Safe Step Act would mandate that an exception to a step therapy protocol must be granted if:
- The required treatment has been ineffective
- The treatment is expected to be ineffective, and delaying effective treatment would lead to irreversible consequences
- The treatment will cause or is likely to cause an adverse reaction
- The treatment is expected to prevent the individual from performing daily activities or occupational responsibilities
- The individual is stable on their current prescription drugs
- There are other circumstances as determined by the Employee Benefits Security Administration
This legislation is vital for ensuring that patients have timely access to the medications they need without unnecessary delays or disruptions.
Patient Access to Medications — Prior Authorizations
Another significant issue affecting patient access to medications is prior authorizations (PAs). According to an American Medical Association survey, nearly one in four physicians (24%) report that a PA has led to a serious adverse event for a patient in their care. In rheumatology, PAs often result in delays in care (even for those initially approved) and a significant increase in steroid usage. In particular, PAs in Medicare Advantage (MA) plans are harmful to Medicare beneficiaries.
The Improving Seniors’ Timely Access to Care Act (H.R.8702 / S.4532) aims to reform PAs used in MA plans, making the process more efficient and transparent to improve access to care for seniors. Unfortunately, it does not cover Part D drugs and may only cover Part B drugs depending on the MA plan’s benefit package. Here are the key provisions of the act:
- Electronic PA: Implementing real-time decisions for routinely approved items and services.
- Transparency: Requiring annual publication of PA information, such as the percentage of requests approved and the average response time.
- Quality and Timeliness Standards: The Centers for Medicare & Medicaid Services (CMS) will set standards for the quality and timeliness of PA determinations.
- Streamlining Approvals: Simplifying the approval process and reducing the time allowed for health plans to consider PA requests.
This bill passed the House in September 2022 but stalled in the Senate because of an unfavorable Congressional Budget Office score. CMS has since finalized portions of this bill via regulation, zeroing out the CBO score and increasing the chances of its passage.
Delinking Drug Prices from PBM Income and Pass-Through of Price Concessions
Affordability is a crucial aspect of accessibility, especially when it comes to medications. Over the years, we’ve learned that PBMs often favor placing the highest list price drugs on formularies because the rebates and various fees they receive from manufacturers are based on a percentage of the list price. In other words, the higher the medication’s price, the more money the PBM makes.
This practice is evident in both commercial and government formularies, where brand-name drugs are often preferred, while lower-priced generics are either excluded or placed on higher tiers. As a result, while major PBMs benefit from these rebates and fees, patients continue to pay their cost share based on the list price of the medication.
To improve the affordability of medications, a key aspect of PBM reform should be to disincentivize PBMs from selecting higher-priced medications and/or require the pass-through of manufacturer price concessions to patients.
Several major PBM reform bills are currently being considered that address either the delinking of price concessions from the list price of the drug or some form of pass-through of these concessions. These reforms are essential to ensure that patients can access affordable medications without being burdened by inflated costs.
The legislation includes the Pharmacy Benefit Manager Reform Act (S.1339); the Modernizing & Ensuring PBM Accountability Act (S.2973); the Better Mental Health Care, Lower Cost Drugs, and Extenders Act (S.3430); the Protecting Patients Against PBM Abuses Act (H.R. 2880); the DRUG Act (S.2474 / H.R.6283); and the Share the Savings with Seniors Act (S.2474 / H.R.5376).
As with all legislation, there are limitations and compromises in each of these. However, these bills are a good first step in addressing PBM remuneration (rebates and fees) based on the list price of the drug and/or passing through to the patient the benefit of manufacturer price concessions. By focusing on key areas like utilization management, delinking drug prices from PBM income, and allowing patients to directly benefit from manufacturer price concessions, we can work toward a more equitable and efficient healthcare system. Reigning in PBM bad behavior is a challenge, but the potential benefits for patient care and access make it a crucial fight worth pursuing.
Please help in efforts to improve patients’ access to available and affordable medications by contacting your representatives in Congress to impart to them the importance of passing legislation. The CSRO’s legislative map tool can help to inform you of the latest information on these and other bills and assist you in engaging with your representatives on them.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s vice president of Advocacy and Government Affairs and its immediate past president, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. She has no relevant conflicts of interest to disclose. You can reach her at rhnews@mdedge.com.
Belimumab Hits Newer Remission, Low Disease Activity Metrics
TOPLINE:
A greater proportion of patients with active systemic lupus erythematosus (SLE) treated with belimumab plus standard therapy achieved the newest definitions for remission and low disease activity compared with those treated with placebo plus standard therapy, with benefits observed as early as week 28 for remission and week 8 for disease activity, according to pooled results from five clinical trials.
METHODOLOGY:
- Researchers conducted an integrated post hoc analysis of five randomized phase 3 clinical trials to evaluate the attainment of remission and low disease activity in adult patients with active, autoantibody-positive SLE.
- A total of 3086 patients (median age, 36 years; 94% women) were randomly assigned to receive standard therapy with intravenous belimumab 10 mg/kg monthly or subcutaneous belimumab 200 mg weekly (n = 1869) or placebo (n = 1217).
- The proportion of patients who achieved definitions of remission in SLE (DORIS) remission and lupus low disease activity state (LLDAS) by visit up to week 52 was assessed.
- The analysis also evaluated the time taken to achieve sustained (at least two consecutive visits) and maintained (up to week 52) DORIS remission and LLDAS.
TAKEAWAY:
- At week 52, a higher proportion of patients receiving belimumab vs placebo achieved DORIS remission (8% vs 6%; risk ratio [RR], 1.51; P = .0055) and LLDAS (17% vs 10%; RR, 1.74; P < .0001).
- The earliest observed significant benefit of belimumab over placebo in patients with a higher baseline disease activity was at week 20 for DORIS remission (RR, 2.09; P = .043) and at week 16 for LLDAS (RR, 1.46; P = .034), with both maintained through week 52.
- The proportion of patients who attained DORIS remission and LLDAS as early as week 28 and week 8, respectively, was higher in the belimumab group than in the placebo group, with both maintained through week 52.
- Patients on belimumab were more likely to have a sustained and maintained DORIS remission (hazard ratio [HR], 1.53; P = .013) and LLDAS (HR, 1.79; P < .0001) at any timepoint.
IN PRACTICE:
“The data clearly support that belimumab is a valuable addition toward accomplishing and maintaining remission or LLDAS,” George Bertsias, MD, PhD, University of Crete Medical School, Heraklion, Greece, and Jinoos Yazdany, MD, University of California San Francisco, wrote in a related comment.
SOURCE:
This study, led by Ioannis Parodis, MD, Karolinska Institutet and Karolinska University Hospital, Stockholm, Sweden, was published online on August 26, 2024, in The Lancet Rheumatology.
LIMITATIONS:
Due to the post hoc nature of the analysis, the trials were not specifically designed to have adequate statistical power to demonstrate the difference between patients who did or did not achieve DORIS remission or LLDAS. The analysis was limited to patients who met the eligibility criteria, and the outcomes are not generalizable to populations outside a clinical trial setting. The study population had high disease activity, which made it challenging to attain the treatment targets.
DISCLOSURES:
The five trials included in this analysis were funded by GSK. The study was supported by the Swedish Rheumatism Association, King Gustaf V’s 80-year Foundation, the Swedish Society of Medicine, Nyckelfonden, Professor Nanna Svartz Foundation, Ulla and Roland Gustafsson Foundation, Region Stockholm, and Karolinska Institutet. Some authors reported receiving grants, speaker honoraria, or consulting fees from various pharmaceutical companies. Some authors reported being employees and owning stocks and shares of GSK.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
A greater proportion of patients with active systemic lupus erythematosus (SLE) treated with belimumab plus standard therapy achieved the newest definitions for remission and low disease activity compared with those treated with placebo plus standard therapy, with benefits observed as early as week 28 for remission and week 8 for disease activity, according to pooled results from five clinical trials.
METHODOLOGY:
- Researchers conducted an integrated post hoc analysis of five randomized phase 3 clinical trials to evaluate the attainment of remission and low disease activity in adult patients with active, autoantibody-positive SLE.
- A total of 3086 patients (median age, 36 years; 94% women) were randomly assigned to receive standard therapy with intravenous belimumab 10 mg/kg monthly or subcutaneous belimumab 200 mg weekly (n = 1869) or placebo (n = 1217).
- The proportion of patients who achieved definitions of remission in SLE (DORIS) remission and lupus low disease activity state (LLDAS) by visit up to week 52 was assessed.
- The analysis also evaluated the time taken to achieve sustained (at least two consecutive visits) and maintained (up to week 52) DORIS remission and LLDAS.
TAKEAWAY:
- At week 52, a higher proportion of patients receiving belimumab vs placebo achieved DORIS remission (8% vs 6%; risk ratio [RR], 1.51; P = .0055) and LLDAS (17% vs 10%; RR, 1.74; P < .0001).
- The earliest observed significant benefit of belimumab over placebo in patients with a higher baseline disease activity was at week 20 for DORIS remission (RR, 2.09; P = .043) and at week 16 for LLDAS (RR, 1.46; P = .034), with both maintained through week 52.
- The proportion of patients who attained DORIS remission and LLDAS as early as week 28 and week 8, respectively, was higher in the belimumab group than in the placebo group, with both maintained through week 52.
- Patients on belimumab were more likely to have a sustained and maintained DORIS remission (hazard ratio [HR], 1.53; P = .013) and LLDAS (HR, 1.79; P < .0001) at any timepoint.
IN PRACTICE:
“The data clearly support that belimumab is a valuable addition toward accomplishing and maintaining remission or LLDAS,” George Bertsias, MD, PhD, University of Crete Medical School, Heraklion, Greece, and Jinoos Yazdany, MD, University of California San Francisco, wrote in a related comment.
SOURCE:
This study, led by Ioannis Parodis, MD, Karolinska Institutet and Karolinska University Hospital, Stockholm, Sweden, was published online on August 26, 2024, in The Lancet Rheumatology.
LIMITATIONS:
Due to the post hoc nature of the analysis, the trials were not specifically designed to have adequate statistical power to demonstrate the difference between patients who did or did not achieve DORIS remission or LLDAS. The analysis was limited to patients who met the eligibility criteria, and the outcomes are not generalizable to populations outside a clinical trial setting. The study population had high disease activity, which made it challenging to attain the treatment targets.
DISCLOSURES:
The five trials included in this analysis were funded by GSK. The study was supported by the Swedish Rheumatism Association, King Gustaf V’s 80-year Foundation, the Swedish Society of Medicine, Nyckelfonden, Professor Nanna Svartz Foundation, Ulla and Roland Gustafsson Foundation, Region Stockholm, and Karolinska Institutet. Some authors reported receiving grants, speaker honoraria, or consulting fees from various pharmaceutical companies. Some authors reported being employees and owning stocks and shares of GSK.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
A greater proportion of patients with active systemic lupus erythematosus (SLE) treated with belimumab plus standard therapy achieved the newest definitions for remission and low disease activity compared with those treated with placebo plus standard therapy, with benefits observed as early as week 28 for remission and week 8 for disease activity, according to pooled results from five clinical trials.
METHODOLOGY:
- Researchers conducted an integrated post hoc analysis of five randomized phase 3 clinical trials to evaluate the attainment of remission and low disease activity in adult patients with active, autoantibody-positive SLE.
- A total of 3086 patients (median age, 36 years; 94% women) were randomly assigned to receive standard therapy with intravenous belimumab 10 mg/kg monthly or subcutaneous belimumab 200 mg weekly (n = 1869) or placebo (n = 1217).
- The proportion of patients who achieved definitions of remission in SLE (DORIS) remission and lupus low disease activity state (LLDAS) by visit up to week 52 was assessed.
- The analysis also evaluated the time taken to achieve sustained (at least two consecutive visits) and maintained (up to week 52) DORIS remission and LLDAS.
TAKEAWAY:
- At week 52, a higher proportion of patients receiving belimumab vs placebo achieved DORIS remission (8% vs 6%; risk ratio [RR], 1.51; P = .0055) and LLDAS (17% vs 10%; RR, 1.74; P < .0001).
- The earliest observed significant benefit of belimumab over placebo in patients with a higher baseline disease activity was at week 20 for DORIS remission (RR, 2.09; P = .043) and at week 16 for LLDAS (RR, 1.46; P = .034), with both maintained through week 52.
- The proportion of patients who attained DORIS remission and LLDAS as early as week 28 and week 8, respectively, was higher in the belimumab group than in the placebo group, with both maintained through week 52.
- Patients on belimumab were more likely to have a sustained and maintained DORIS remission (hazard ratio [HR], 1.53; P = .013) and LLDAS (HR, 1.79; P < .0001) at any timepoint.
IN PRACTICE:
“The data clearly support that belimumab is a valuable addition toward accomplishing and maintaining remission or LLDAS,” George Bertsias, MD, PhD, University of Crete Medical School, Heraklion, Greece, and Jinoos Yazdany, MD, University of California San Francisco, wrote in a related comment.
SOURCE:
This study, led by Ioannis Parodis, MD, Karolinska Institutet and Karolinska University Hospital, Stockholm, Sweden, was published online on August 26, 2024, in The Lancet Rheumatology.
LIMITATIONS:
Due to the post hoc nature of the analysis, the trials were not specifically designed to have adequate statistical power to demonstrate the difference between patients who did or did not achieve DORIS remission or LLDAS. The analysis was limited to patients who met the eligibility criteria, and the outcomes are not generalizable to populations outside a clinical trial setting. The study population had high disease activity, which made it challenging to attain the treatment targets.
DISCLOSURES:
The five trials included in this analysis were funded by GSK. The study was supported by the Swedish Rheumatism Association, King Gustaf V’s 80-year Foundation, the Swedish Society of Medicine, Nyckelfonden, Professor Nanna Svartz Foundation, Ulla and Roland Gustafsson Foundation, Region Stockholm, and Karolinska Institutet. Some authors reported receiving grants, speaker honoraria, or consulting fees from various pharmaceutical companies. Some authors reported being employees and owning stocks and shares of GSK.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
AI-Powered Clinical Documentation Tool Reduces EHR Time for Clinicians
TOPLINE:
An artificial intelligence (AI)-powered clinical documentation tool helped reduce time spent on electronic health records (EHR) at home for almost 48% physicians, and nearly 45% reported less weekly time spent on EHR tasks outside of normal work hours.
METHODOLOGY:
- Researchers recruited 112 clinicians from family medicine, internal medicine, and general pediatrics in North Carolina and Georgia.
- Patients were divided into an intervention group (n = 85) and control group (n = 55), with the intervention group receiving a 1-hour training program on a commercially available AI tool.
- A seven-question survey was administered to participants before and 5 weeks after the intervention to evaluate their experience.
TAKEAWAY:
- The researchers found 47.1% of clinicians in the intervention group reported spending less time on the EHR at home compared with 14.5% in the control group (P < .001); 44.7% reported decreased weekly time on the EHR outside normal work hours compared with 20% in the control group (P = .003).
- The study revealed 43.5% of physicians who used the AI instrument reported spending less time on documentation after visits compared with 18.2% in the control group (P = .002).
- Further, 44.7% reported less frustration when using the EHR compared with 14.5% in the control group (P < .001).
IN PRACTICE:
“Approximately half of clinicians using the AI-powered clinical documentation tool based on interest reported a positive outcome, potentially reducing burnout. However, a significant subset did not find time-saving benefits or improved EHR experience,” the authors of the study wrote.
SOURCE:
The study was led by Tsai-Ling Liu, PhD, Center for Health System Sciences, Atrium Health in Charlotte, North Carolina. It was published online in JAMA Network Open.
LIMITATIONS:
The researchers reported potential selection and recall bias in both groups. Additional research is needed to find areas of improvement and assess the effects on clinician groups and health systems, they said.
DISCLOSURES:
Andrew McWilliams, MD, MPH, reported receiving grants from the Agency for Healthcare Research Quality, the National Institutes of Health, and the Duke Endowment unrelated to this work. Ajay Dharod, MD, reported his role as an electronic health record consultant for the Association of American Medical College CORE program. Jeffrey Cleveland, MD, disclosed his participation on the Executive Client Council, a noncompensated advisory group, for Nuance/Microsoft.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
An artificial intelligence (AI)-powered clinical documentation tool helped reduce time spent on electronic health records (EHR) at home for almost 48% physicians, and nearly 45% reported less weekly time spent on EHR tasks outside of normal work hours.
METHODOLOGY:
- Researchers recruited 112 clinicians from family medicine, internal medicine, and general pediatrics in North Carolina and Georgia.
- Patients were divided into an intervention group (n = 85) and control group (n = 55), with the intervention group receiving a 1-hour training program on a commercially available AI tool.
- A seven-question survey was administered to participants before and 5 weeks after the intervention to evaluate their experience.
TAKEAWAY:
- The researchers found 47.1% of clinicians in the intervention group reported spending less time on the EHR at home compared with 14.5% in the control group (P < .001); 44.7% reported decreased weekly time on the EHR outside normal work hours compared with 20% in the control group (P = .003).
- The study revealed 43.5% of physicians who used the AI instrument reported spending less time on documentation after visits compared with 18.2% in the control group (P = .002).
- Further, 44.7% reported less frustration when using the EHR compared with 14.5% in the control group (P < .001).
IN PRACTICE:
“Approximately half of clinicians using the AI-powered clinical documentation tool based on interest reported a positive outcome, potentially reducing burnout. However, a significant subset did not find time-saving benefits or improved EHR experience,” the authors of the study wrote.
SOURCE:
The study was led by Tsai-Ling Liu, PhD, Center for Health System Sciences, Atrium Health in Charlotte, North Carolina. It was published online in JAMA Network Open.
LIMITATIONS:
The researchers reported potential selection and recall bias in both groups. Additional research is needed to find areas of improvement and assess the effects on clinician groups and health systems, they said.
DISCLOSURES:
Andrew McWilliams, MD, MPH, reported receiving grants from the Agency for Healthcare Research Quality, the National Institutes of Health, and the Duke Endowment unrelated to this work. Ajay Dharod, MD, reported his role as an electronic health record consultant for the Association of American Medical College CORE program. Jeffrey Cleveland, MD, disclosed his participation on the Executive Client Council, a noncompensated advisory group, for Nuance/Microsoft.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
An artificial intelligence (AI)-powered clinical documentation tool helped reduce time spent on electronic health records (EHR) at home for almost 48% physicians, and nearly 45% reported less weekly time spent on EHR tasks outside of normal work hours.
METHODOLOGY:
- Researchers recruited 112 clinicians from family medicine, internal medicine, and general pediatrics in North Carolina and Georgia.
- Patients were divided into an intervention group (n = 85) and control group (n = 55), with the intervention group receiving a 1-hour training program on a commercially available AI tool.
- A seven-question survey was administered to participants before and 5 weeks after the intervention to evaluate their experience.
TAKEAWAY:
- The researchers found 47.1% of clinicians in the intervention group reported spending less time on the EHR at home compared with 14.5% in the control group (P < .001); 44.7% reported decreased weekly time on the EHR outside normal work hours compared with 20% in the control group (P = .003).
- The study revealed 43.5% of physicians who used the AI instrument reported spending less time on documentation after visits compared with 18.2% in the control group (P = .002).
- Further, 44.7% reported less frustration when using the EHR compared with 14.5% in the control group (P < .001).
IN PRACTICE:
“Approximately half of clinicians using the AI-powered clinical documentation tool based on interest reported a positive outcome, potentially reducing burnout. However, a significant subset did not find time-saving benefits or improved EHR experience,” the authors of the study wrote.
SOURCE:
The study was led by Tsai-Ling Liu, PhD, Center for Health System Sciences, Atrium Health in Charlotte, North Carolina. It was published online in JAMA Network Open.
LIMITATIONS:
The researchers reported potential selection and recall bias in both groups. Additional research is needed to find areas of improvement and assess the effects on clinician groups and health systems, they said.
DISCLOSURES:
Andrew McWilliams, MD, MPH, reported receiving grants from the Agency for Healthcare Research Quality, the National Institutes of Health, and the Duke Endowment unrelated to this work. Ajay Dharod, MD, reported his role as an electronic health record consultant for the Association of American Medical College CORE program. Jeffrey Cleveland, MD, disclosed his participation on the Executive Client Council, a noncompensated advisory group, for Nuance/Microsoft.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Are Pharmacy Deserts Worsening Health Disparities?
TOPLINE:
Pharmacy closures in the United States are creating “pharmacy deserts,” disproportionately affecting socially vulnerable communities. High social vulnerability and low primary care practitioner (PCP) density are linked to increased pharmacy desert density.
METHODOLOGY:
- Data through 2020 on communities located 10 or more miles from the nearest retail pharmacy were sourced from TelePharm Map.
- Counties were stratified as having a high pharmacy desert density if the number of pharmacy deserts per 1000 inhabitants was in the 80th percentile or higher.
- Social vulnerability index and healthcare practitioner data were obtained from the Agency for Toxic Substances and Disease Registry and the Area Health Resources Files.
- PCP density was calculated as the number of PCPs per 10,000 inhabitants.
- A total of 3143 counties were analyzed, with 1447 (46%) having at least one pharmacy desert.
TAKEAWAY:
- Counties with a high pharmacy desert density had a higher social vulnerability index than those with a low pharmacy desert density (P = .006).
- Areas with a high pharmacy desert density had lower median PCP density than those with low or no pharmacy desert density (P < .001).
- High social vulnerability index (odds ratio [OR], 1.35; 95% CI, 1.07-1.70; P = .01) and low PCP density (OR, 2.27; 95% CI, 1.80-2.86; P < .001) were associated with a higher likelihood for a county to have a high pharmacy desert density.
- Pharmacy closures are leaving more individuals without easy access to medications, with disproportionate consequences for certain communities.
IN PRACTICE:
“As high pharmacy desert density counties also have a lower PCP density, patients residing in these regions face increased barriers to accessing primary healthcare needs,” wrote the authors of the study.
SOURCE:
The study was led by Giovanni Catalano, MD, Muhammad Muntazir Mehdi Khan, MBBS, and Timothy M. Pawlik, MD, PhD, MPH, MTS, MBA, Department of Surgery, The Ohio State University Wexner Medical Center in Columbus, Ohio. It was published online in JAMA Network Open.
LIMITATIONS:
The cross-sectional design of the study limited the ability to draw causal inferences. The study relied on public county-level data, which may not have captured all relevant variables. The use of the social vulnerability index and PCP density as proxies did not fully represent the complexity of pharmacy access issues. The study’s findings were not generalizable to regions outside the United States.
DISCLOSURES:
No relevant conflicts of interest were disclosed by the authors. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Pharmacy closures in the United States are creating “pharmacy deserts,” disproportionately affecting socially vulnerable communities. High social vulnerability and low primary care practitioner (PCP) density are linked to increased pharmacy desert density.
METHODOLOGY:
- Data through 2020 on communities located 10 or more miles from the nearest retail pharmacy were sourced from TelePharm Map.
- Counties were stratified as having a high pharmacy desert density if the number of pharmacy deserts per 1000 inhabitants was in the 80th percentile or higher.
- Social vulnerability index and healthcare practitioner data were obtained from the Agency for Toxic Substances and Disease Registry and the Area Health Resources Files.
- PCP density was calculated as the number of PCPs per 10,000 inhabitants.
- A total of 3143 counties were analyzed, with 1447 (46%) having at least one pharmacy desert.
TAKEAWAY:
- Counties with a high pharmacy desert density had a higher social vulnerability index than those with a low pharmacy desert density (P = .006).
- Areas with a high pharmacy desert density had lower median PCP density than those with low or no pharmacy desert density (P < .001).
- High social vulnerability index (odds ratio [OR], 1.35; 95% CI, 1.07-1.70; P = .01) and low PCP density (OR, 2.27; 95% CI, 1.80-2.86; P < .001) were associated with a higher likelihood for a county to have a high pharmacy desert density.
- Pharmacy closures are leaving more individuals without easy access to medications, with disproportionate consequences for certain communities.
IN PRACTICE:
“As high pharmacy desert density counties also have a lower PCP density, patients residing in these regions face increased barriers to accessing primary healthcare needs,” wrote the authors of the study.
SOURCE:
The study was led by Giovanni Catalano, MD, Muhammad Muntazir Mehdi Khan, MBBS, and Timothy M. Pawlik, MD, PhD, MPH, MTS, MBA, Department of Surgery, The Ohio State University Wexner Medical Center in Columbus, Ohio. It was published online in JAMA Network Open.
LIMITATIONS:
The cross-sectional design of the study limited the ability to draw causal inferences. The study relied on public county-level data, which may not have captured all relevant variables. The use of the social vulnerability index and PCP density as proxies did not fully represent the complexity of pharmacy access issues. The study’s findings were not generalizable to regions outside the United States.
DISCLOSURES:
No relevant conflicts of interest were disclosed by the authors. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Pharmacy closures in the United States are creating “pharmacy deserts,” disproportionately affecting socially vulnerable communities. High social vulnerability and low primary care practitioner (PCP) density are linked to increased pharmacy desert density.
METHODOLOGY:
- Data through 2020 on communities located 10 or more miles from the nearest retail pharmacy were sourced from TelePharm Map.
- Counties were stratified as having a high pharmacy desert density if the number of pharmacy deserts per 1000 inhabitants was in the 80th percentile or higher.
- Social vulnerability index and healthcare practitioner data were obtained from the Agency for Toxic Substances and Disease Registry and the Area Health Resources Files.
- PCP density was calculated as the number of PCPs per 10,000 inhabitants.
- A total of 3143 counties were analyzed, with 1447 (46%) having at least one pharmacy desert.
TAKEAWAY:
- Counties with a high pharmacy desert density had a higher social vulnerability index than those with a low pharmacy desert density (P = .006).
- Areas with a high pharmacy desert density had lower median PCP density than those with low or no pharmacy desert density (P < .001).
- High social vulnerability index (odds ratio [OR], 1.35; 95% CI, 1.07-1.70; P = .01) and low PCP density (OR, 2.27; 95% CI, 1.80-2.86; P < .001) were associated with a higher likelihood for a county to have a high pharmacy desert density.
- Pharmacy closures are leaving more individuals without easy access to medications, with disproportionate consequences for certain communities.
IN PRACTICE:
“As high pharmacy desert density counties also have a lower PCP density, patients residing in these regions face increased barriers to accessing primary healthcare needs,” wrote the authors of the study.
SOURCE:
The study was led by Giovanni Catalano, MD, Muhammad Muntazir Mehdi Khan, MBBS, and Timothy M. Pawlik, MD, PhD, MPH, MTS, MBA, Department of Surgery, The Ohio State University Wexner Medical Center in Columbus, Ohio. It was published online in JAMA Network Open.
LIMITATIONS:
The cross-sectional design of the study limited the ability to draw causal inferences. The study relied on public county-level data, which may not have captured all relevant variables. The use of the social vulnerability index and PCP density as proxies did not fully represent the complexity of pharmacy access issues. The study’s findings were not generalizable to regions outside the United States.
DISCLOSURES:
No relevant conflicts of interest were disclosed by the authors. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
UVA Defends Medical School Dean, Hospital CEO After Docs Call for Their Removal
The University of Virginia (UVA) is defending the CEO of its health system and its medical school dean in the wake of a very public call for their removal.
At least 128 members of the University of Virginia faculty who are employed by both the medical school and the UVA Physicians Group wrote to the UVA Board of Visitors and its peer-elected faculty leaders, expressing no confidence in K. Craig Kent, MD, CEO of UVA Health and executive vice president for health affairs, and Melina Kibbe, MD, dean of the medical school and chief health affairs officer.
Dr. Kibbe, a vascular surgeon and researcher, is also the editor in chief of JAMA Surgery.
“We call for the immediate removal of Craig Kent and Melina Kibbe,” wrote the physicians.
The letter alleged that patient safety was compromised because doctors, nurses, and other staff were pressured to abstain from reporting safety concerns and that physicians had been hired “despite concerns regarding integrity and quality.” Those who raised safety concerns faced “explicit and implicit threats and retaliation,” including delays and denials of promotion and tenure, said the letter.
The September 5 letter did not include signatures. The authors said that names were being protected, but that they would share the names with a limited audience.
UVA President Jim Ryan took issue with the notion that the signees were anonymous. He said in his own letter to medical school faculty that some of the accusations were about matters that had already been addressed or that were being worked on. As far as allegations that he was not previously aware of, “we will do our best to investigate,” he said.
The faculty who signed the letter “have besmirched the reputations of not just Melina and Craig,” wrote Mr. Ryan. “They have unfairly — and I trust unwittingly — cast a shadow over the great work of the entire health system and medical school.”
The authors claimed that reports about bullying and harassment of trainees had been “suppressed, minimized, and subsequently altered.”
And they said that spending on leadership was prioritized over addressing clinical and technical staff shortages. Whistleblowers who reported fraud were not protected, and clinicians were pressured to modify patient records to “obfuscate adverse outcomes and boost productivity metrics,” they wrote.
The 128 members of the UVA Physicians Group who signed the letter represent about 10% of the 1400 medical school faculty members.
It is not the first time that Dr. Kent has been given a vote of no confidence. In 2017, when he was the dean of the College of Medicine at the Ohio State University, Dr. Kent was accused in a “no confidence” letter from 25 physicians and faculty of helping to undermine the school’s mission and taking actions that led to resignations and early retirements of many staff, the Columbus Dispatch reported.
William G. Crutchfield Jr., a member of the UVA Health System Board, defended Dr. Kent and Dr. Kibbe in a lengthy statement shared with this news organization. He said that UVA Health’s four hospitals had received “A” ratings for safety, and that the system has a 5.1% turnover rate compared with a national average of 8.3%.
Dr. Kent and Dr. Kibbe have recruited faculty from top academic medical centers, Mr. Crutchfield wrote.
“If our work environment were so toxic, these people would not have joined our faculty,” he wrote.
Mr. Crutchfield credited Dr. Kent and Dr. Kibbe with crafting a new 10-year strategic plan and for hiring a chief strategy officer to lead the plan — a move that replaced “expensive outside consultants.”
Mr. Ryan said in his letter that his inbox “is overflowing with testimonials from some of the 1200-plus faculty who did not sign the letter, who attest that the health system today — under Melina and Craig’s leadership — is in the best shape it has ever been in, and that they have addressed changes that have needed to be made for more than two decades.”
A request to see some of these positive testimonials was not answered by press time.
Mr. Crutchfield, like Mr. Ryan, said that the letter writers were doing more harm than good.
“If a small cabal of people hiding behind anonymity can force outstanding leaders out of UVA, it will make it extremely difficult to recruit outstanding new physicians, nurses, technicians, and administrators,” he wrote.
A version of this article first appeared on Medscape.com.
The University of Virginia (UVA) is defending the CEO of its health system and its medical school dean in the wake of a very public call for their removal.
At least 128 members of the University of Virginia faculty who are employed by both the medical school and the UVA Physicians Group wrote to the UVA Board of Visitors and its peer-elected faculty leaders, expressing no confidence in K. Craig Kent, MD, CEO of UVA Health and executive vice president for health affairs, and Melina Kibbe, MD, dean of the medical school and chief health affairs officer.
Dr. Kibbe, a vascular surgeon and researcher, is also the editor in chief of JAMA Surgery.
“We call for the immediate removal of Craig Kent and Melina Kibbe,” wrote the physicians.
The letter alleged that patient safety was compromised because doctors, nurses, and other staff were pressured to abstain from reporting safety concerns and that physicians had been hired “despite concerns regarding integrity and quality.” Those who raised safety concerns faced “explicit and implicit threats and retaliation,” including delays and denials of promotion and tenure, said the letter.
The September 5 letter did not include signatures. The authors said that names were being protected, but that they would share the names with a limited audience.
UVA President Jim Ryan took issue with the notion that the signees were anonymous. He said in his own letter to medical school faculty that some of the accusations were about matters that had already been addressed or that were being worked on. As far as allegations that he was not previously aware of, “we will do our best to investigate,” he said.
The faculty who signed the letter “have besmirched the reputations of not just Melina and Craig,” wrote Mr. Ryan. “They have unfairly — and I trust unwittingly — cast a shadow over the great work of the entire health system and medical school.”
The authors claimed that reports about bullying and harassment of trainees had been “suppressed, minimized, and subsequently altered.”
And they said that spending on leadership was prioritized over addressing clinical and technical staff shortages. Whistleblowers who reported fraud were not protected, and clinicians were pressured to modify patient records to “obfuscate adverse outcomes and boost productivity metrics,” they wrote.
The 128 members of the UVA Physicians Group who signed the letter represent about 10% of the 1400 medical school faculty members.
It is not the first time that Dr. Kent has been given a vote of no confidence. In 2017, when he was the dean of the College of Medicine at the Ohio State University, Dr. Kent was accused in a “no confidence” letter from 25 physicians and faculty of helping to undermine the school’s mission and taking actions that led to resignations and early retirements of many staff, the Columbus Dispatch reported.
William G. Crutchfield Jr., a member of the UVA Health System Board, defended Dr. Kent and Dr. Kibbe in a lengthy statement shared with this news organization. He said that UVA Health’s four hospitals had received “A” ratings for safety, and that the system has a 5.1% turnover rate compared with a national average of 8.3%.
Dr. Kent and Dr. Kibbe have recruited faculty from top academic medical centers, Mr. Crutchfield wrote.
“If our work environment were so toxic, these people would not have joined our faculty,” he wrote.
Mr. Crutchfield credited Dr. Kent and Dr. Kibbe with crafting a new 10-year strategic plan and for hiring a chief strategy officer to lead the plan — a move that replaced “expensive outside consultants.”
Mr. Ryan said in his letter that his inbox “is overflowing with testimonials from some of the 1200-plus faculty who did not sign the letter, who attest that the health system today — under Melina and Craig’s leadership — is in the best shape it has ever been in, and that they have addressed changes that have needed to be made for more than two decades.”
A request to see some of these positive testimonials was not answered by press time.
Mr. Crutchfield, like Mr. Ryan, said that the letter writers were doing more harm than good.
“If a small cabal of people hiding behind anonymity can force outstanding leaders out of UVA, it will make it extremely difficult to recruit outstanding new physicians, nurses, technicians, and administrators,” he wrote.
A version of this article first appeared on Medscape.com.
The University of Virginia (UVA) is defending the CEO of its health system and its medical school dean in the wake of a very public call for their removal.
At least 128 members of the University of Virginia faculty who are employed by both the medical school and the UVA Physicians Group wrote to the UVA Board of Visitors and its peer-elected faculty leaders, expressing no confidence in K. Craig Kent, MD, CEO of UVA Health and executive vice president for health affairs, and Melina Kibbe, MD, dean of the medical school and chief health affairs officer.
Dr. Kibbe, a vascular surgeon and researcher, is also the editor in chief of JAMA Surgery.
“We call for the immediate removal of Craig Kent and Melina Kibbe,” wrote the physicians.
The letter alleged that patient safety was compromised because doctors, nurses, and other staff were pressured to abstain from reporting safety concerns and that physicians had been hired “despite concerns regarding integrity and quality.” Those who raised safety concerns faced “explicit and implicit threats and retaliation,” including delays and denials of promotion and tenure, said the letter.
The September 5 letter did not include signatures. The authors said that names were being protected, but that they would share the names with a limited audience.
UVA President Jim Ryan took issue with the notion that the signees were anonymous. He said in his own letter to medical school faculty that some of the accusations were about matters that had already been addressed or that were being worked on. As far as allegations that he was not previously aware of, “we will do our best to investigate,” he said.
The faculty who signed the letter “have besmirched the reputations of not just Melina and Craig,” wrote Mr. Ryan. “They have unfairly — and I trust unwittingly — cast a shadow over the great work of the entire health system and medical school.”
The authors claimed that reports about bullying and harassment of trainees had been “suppressed, minimized, and subsequently altered.”
And they said that spending on leadership was prioritized over addressing clinical and technical staff shortages. Whistleblowers who reported fraud were not protected, and clinicians were pressured to modify patient records to “obfuscate adverse outcomes and boost productivity metrics,” they wrote.
The 128 members of the UVA Physicians Group who signed the letter represent about 10% of the 1400 medical school faculty members.
It is not the first time that Dr. Kent has been given a vote of no confidence. In 2017, when he was the dean of the College of Medicine at the Ohio State University, Dr. Kent was accused in a “no confidence” letter from 25 physicians and faculty of helping to undermine the school’s mission and taking actions that led to resignations and early retirements of many staff, the Columbus Dispatch reported.
William G. Crutchfield Jr., a member of the UVA Health System Board, defended Dr. Kent and Dr. Kibbe in a lengthy statement shared with this news organization. He said that UVA Health’s four hospitals had received “A” ratings for safety, and that the system has a 5.1% turnover rate compared with a national average of 8.3%.
Dr. Kent and Dr. Kibbe have recruited faculty from top academic medical centers, Mr. Crutchfield wrote.
“If our work environment were so toxic, these people would not have joined our faculty,” he wrote.
Mr. Crutchfield credited Dr. Kent and Dr. Kibbe with crafting a new 10-year strategic plan and for hiring a chief strategy officer to lead the plan — a move that replaced “expensive outside consultants.”
Mr. Ryan said in his letter that his inbox “is overflowing with testimonials from some of the 1200-plus faculty who did not sign the letter, who attest that the health system today — under Melina and Craig’s leadership — is in the best shape it has ever been in, and that they have addressed changes that have needed to be made for more than two decades.”
A request to see some of these positive testimonials was not answered by press time.
Mr. Crutchfield, like Mr. Ryan, said that the letter writers were doing more harm than good.
“If a small cabal of people hiding behind anonymity can force outstanding leaders out of UVA, it will make it extremely difficult to recruit outstanding new physicians, nurses, technicians, and administrators,” he wrote.
A version of this article first appeared on Medscape.com.
Early Use of Steroids Linked to Prolonged Treatment in Early Rheumatoid Arthritis
TOPLINE:
A substantial proportion of older adults with early rheumatoid arthritis (RA) initiate glucocorticoids before receiving care from a rheumatologist. The early initiation of glucocorticoids in this group is associated with prolonged use.
METHODOLOGY:
- Researchers analyzed data from Medicare claims and Rheumatology Informatics System for Effectiveness registry of the American College of Rheumatology from 2016 to 2018 to assess the relationship between the timing of glucocorticoid initiation and the subsequent duration of glucocorticoid use in older adults with early RA in the United States.
- They included 1733 patients aged ≥ 65 years (mean age, 76 years; 67% women) with early RA.
- Glucocorticoid initiation was defined as the first use between 3 months before and 6 months after entrance into rheumatology care.
- The continuous administration of glucocorticoid therapy was monitored for all individuals who initiated glucocorticoid treatment for up to 12 months after entering rheumatology care.
- The primary outcome was the duration of continuous glucocorticoid use after entering rheumatology care.
TAKEAWAY:
- Glucocorticoids were initiated in 41% of patients, with 65% starting them before the initial RA diagnosis by a rheumatologist. The median duration of glucocorticoid use was 157 days.
- Patients with early RA who initiated glucocorticoids before entering rheumatology care showed a significantly longer duration of glucocorticoid use than those who initiated it later (median, 186 vs 97 days; P < .0001).
- Patients who initiated glucocorticoids before entering rheumatology care were 39% less likely to stop its use within 1 year (hazard ratio, 0.61; 95% CI, 0.51-0.74).
- The mean daily dose of glucocorticoids was < 5 mg/d for patients who received them for at least 3 months, indicating a trend toward low-dose, long-term use.
IN PRACTICE:
“Initiatives to reduce GC [glucocorticoid] exposure among patients with eRA [early RA] will likely require attention to rheumatology workforce shortages and close collaboration between rheumatologists and primary care clinicians to expedite referrals to rheumatology care,” the authors wrote.
SOURCE:
This study was led by Andriko Palmowski, MD, Department of Rheumatology and Clinical Immunology, Charité – Universitätsmedizin Berlin in Germany, and was published online in Seminars in Arthritis and Rheumatism.
LIMITATIONS:
The observational nature of this study limited the ability to establish causality between early glucocorticoid initiation and the prolonged use of glucocorticoids. Moreover, the study population was limited to older adults, which affected the generalizability of the findings to younger populations. It also did not account for the fact that patients with more severe early RA were more likely to start glucocorticoid therapy early and continue it for longer durations.
DISCLOSURES:
The study was supported by research grants from the Deutsche Autoimmun-Stiftung and other sources. Some authors declared receiving grant support, consultancy fees, honoraria, and travel expenses and had other ties with various pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
A substantial proportion of older adults with early rheumatoid arthritis (RA) initiate glucocorticoids before receiving care from a rheumatologist. The early initiation of glucocorticoids in this group is associated with prolonged use.
METHODOLOGY:
- Researchers analyzed data from Medicare claims and Rheumatology Informatics System for Effectiveness registry of the American College of Rheumatology from 2016 to 2018 to assess the relationship between the timing of glucocorticoid initiation and the subsequent duration of glucocorticoid use in older adults with early RA in the United States.
- They included 1733 patients aged ≥ 65 years (mean age, 76 years; 67% women) with early RA.
- Glucocorticoid initiation was defined as the first use between 3 months before and 6 months after entrance into rheumatology care.
- The continuous administration of glucocorticoid therapy was monitored for all individuals who initiated glucocorticoid treatment for up to 12 months after entering rheumatology care.
- The primary outcome was the duration of continuous glucocorticoid use after entering rheumatology care.
TAKEAWAY:
- Glucocorticoids were initiated in 41% of patients, with 65% starting them before the initial RA diagnosis by a rheumatologist. The median duration of glucocorticoid use was 157 days.
- Patients with early RA who initiated glucocorticoids before entering rheumatology care showed a significantly longer duration of glucocorticoid use than those who initiated it later (median, 186 vs 97 days; P < .0001).
- Patients who initiated glucocorticoids before entering rheumatology care were 39% less likely to stop its use within 1 year (hazard ratio, 0.61; 95% CI, 0.51-0.74).
- The mean daily dose of glucocorticoids was < 5 mg/d for patients who received them for at least 3 months, indicating a trend toward low-dose, long-term use.
IN PRACTICE:
“Initiatives to reduce GC [glucocorticoid] exposure among patients with eRA [early RA] will likely require attention to rheumatology workforce shortages and close collaboration between rheumatologists and primary care clinicians to expedite referrals to rheumatology care,” the authors wrote.
SOURCE:
This study was led by Andriko Palmowski, MD, Department of Rheumatology and Clinical Immunology, Charité – Universitätsmedizin Berlin in Germany, and was published online in Seminars in Arthritis and Rheumatism.
LIMITATIONS:
The observational nature of this study limited the ability to establish causality between early glucocorticoid initiation and the prolonged use of glucocorticoids. Moreover, the study population was limited to older adults, which affected the generalizability of the findings to younger populations. It also did not account for the fact that patients with more severe early RA were more likely to start glucocorticoid therapy early and continue it for longer durations.
DISCLOSURES:
The study was supported by research grants from the Deutsche Autoimmun-Stiftung and other sources. Some authors declared receiving grant support, consultancy fees, honoraria, and travel expenses and had other ties with various pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
A substantial proportion of older adults with early rheumatoid arthritis (RA) initiate glucocorticoids before receiving care from a rheumatologist. The early initiation of glucocorticoids in this group is associated with prolonged use.
METHODOLOGY:
- Researchers analyzed data from Medicare claims and Rheumatology Informatics System for Effectiveness registry of the American College of Rheumatology from 2016 to 2018 to assess the relationship between the timing of glucocorticoid initiation and the subsequent duration of glucocorticoid use in older adults with early RA in the United States.
- They included 1733 patients aged ≥ 65 years (mean age, 76 years; 67% women) with early RA.
- Glucocorticoid initiation was defined as the first use between 3 months before and 6 months after entrance into rheumatology care.
- The continuous administration of glucocorticoid therapy was monitored for all individuals who initiated glucocorticoid treatment for up to 12 months after entering rheumatology care.
- The primary outcome was the duration of continuous glucocorticoid use after entering rheumatology care.
TAKEAWAY:
- Glucocorticoids were initiated in 41% of patients, with 65% starting them before the initial RA diagnosis by a rheumatologist. The median duration of glucocorticoid use was 157 days.
- Patients with early RA who initiated glucocorticoids before entering rheumatology care showed a significantly longer duration of glucocorticoid use than those who initiated it later (median, 186 vs 97 days; P < .0001).
- Patients who initiated glucocorticoids before entering rheumatology care were 39% less likely to stop its use within 1 year (hazard ratio, 0.61; 95% CI, 0.51-0.74).
- The mean daily dose of glucocorticoids was < 5 mg/d for patients who received them for at least 3 months, indicating a trend toward low-dose, long-term use.
IN PRACTICE:
“Initiatives to reduce GC [glucocorticoid] exposure among patients with eRA [early RA] will likely require attention to rheumatology workforce shortages and close collaboration between rheumatologists and primary care clinicians to expedite referrals to rheumatology care,” the authors wrote.
SOURCE:
This study was led by Andriko Palmowski, MD, Department of Rheumatology and Clinical Immunology, Charité – Universitätsmedizin Berlin in Germany, and was published online in Seminars in Arthritis and Rheumatism.
LIMITATIONS:
The observational nature of this study limited the ability to establish causality between early glucocorticoid initiation and the prolonged use of glucocorticoids. Moreover, the study population was limited to older adults, which affected the generalizability of the findings to younger populations. It also did not account for the fact that patients with more severe early RA were more likely to start glucocorticoid therapy early and continue it for longer durations.
DISCLOSURES:
The study was supported by research grants from the Deutsche Autoimmun-Stiftung and other sources. Some authors declared receiving grant support, consultancy fees, honoraria, and travel expenses and had other ties with various pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
ANA Testing: When to Tap the Brakes
This transcript has been edited for clarity.
There are five reasons you do not want to order that notorious antinuclear antibody (ANA) test — when a patient comes into your office and you say, “Let’s just run a wellness check” and you order the ANA test, or the patient comes in and says, “Hey doc, order everything, okay?” — without really thinking these things through.
1. I’m sure you know that the ANA test, if positive, does not exclude other conditions. For instance, older women could have a positive ANA test; it’s very common in this group.
2. There’s a high false-positive rate for an ANA test. For instance, cancers and viral infections can cause an ANA test to be positive, and certain medications can cause a false-positive ANA test.
3. Context matters. If you have a patient that has particular symptoms, joint swelling, a strong family history of autoimmune disease, a luminal rash that you can’t understand, hair loss, those kind of things, then yes, when you order that ANA test, it’s going to be valuable. If the patient does not have those symptoms, you are just running down this rabbit hole that causes worry for you and your patient.
4. The ANA test on its own is not helpful until you order the subtypes. Double-stranded DNA and anti-SSA or anti-SSB antibodies are just a few examples of the subtypes of the ANA test that really help you understand what you ordered.
5. The elephant in the room: What is the pretest probability of your diagnostic test — all the symptoms, the hair loss, the malar rash, the sores in the mouth, the joint swelling, the blood in the urine? Fluid around the heart, pericarditis, pleurisy, those kinds of symptoms, right? When you have those symptoms and you order an ANA test, then you have basically put directions into your GPS. So now you know that if the test is positive, these are the things you’re going to do with the test going forward.
I hope that these five things have told you: Hey, before you order that ANA test, let’s make sure that we’re not causing unnecessary stress for our patients and also minimizing unnecessary testing.
Dr. Dada, CEO, Overlake Arthritis and Osteoporosis Center, Bellevue, Washington, disclosed ties with Horizon Pharmaceuticals.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
There are five reasons you do not want to order that notorious antinuclear antibody (ANA) test — when a patient comes into your office and you say, “Let’s just run a wellness check” and you order the ANA test, or the patient comes in and says, “Hey doc, order everything, okay?” — without really thinking these things through.
1. I’m sure you know that the ANA test, if positive, does not exclude other conditions. For instance, older women could have a positive ANA test; it’s very common in this group.
2. There’s a high false-positive rate for an ANA test. For instance, cancers and viral infections can cause an ANA test to be positive, and certain medications can cause a false-positive ANA test.
3. Context matters. If you have a patient that has particular symptoms, joint swelling, a strong family history of autoimmune disease, a luminal rash that you can’t understand, hair loss, those kind of things, then yes, when you order that ANA test, it’s going to be valuable. If the patient does not have those symptoms, you are just running down this rabbit hole that causes worry for you and your patient.
4. The ANA test on its own is not helpful until you order the subtypes. Double-stranded DNA and anti-SSA or anti-SSB antibodies are just a few examples of the subtypes of the ANA test that really help you understand what you ordered.
5. The elephant in the room: What is the pretest probability of your diagnostic test — all the symptoms, the hair loss, the malar rash, the sores in the mouth, the joint swelling, the blood in the urine? Fluid around the heart, pericarditis, pleurisy, those kinds of symptoms, right? When you have those symptoms and you order an ANA test, then you have basically put directions into your GPS. So now you know that if the test is positive, these are the things you’re going to do with the test going forward.
I hope that these five things have told you: Hey, before you order that ANA test, let’s make sure that we’re not causing unnecessary stress for our patients and also minimizing unnecessary testing.
Dr. Dada, CEO, Overlake Arthritis and Osteoporosis Center, Bellevue, Washington, disclosed ties with Horizon Pharmaceuticals.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
There are five reasons you do not want to order that notorious antinuclear antibody (ANA) test — when a patient comes into your office and you say, “Let’s just run a wellness check” and you order the ANA test, or the patient comes in and says, “Hey doc, order everything, okay?” — without really thinking these things through.
1. I’m sure you know that the ANA test, if positive, does not exclude other conditions. For instance, older women could have a positive ANA test; it’s very common in this group.
2. There’s a high false-positive rate for an ANA test. For instance, cancers and viral infections can cause an ANA test to be positive, and certain medications can cause a false-positive ANA test.
3. Context matters. If you have a patient that has particular symptoms, joint swelling, a strong family history of autoimmune disease, a luminal rash that you can’t understand, hair loss, those kind of things, then yes, when you order that ANA test, it’s going to be valuable. If the patient does not have those symptoms, you are just running down this rabbit hole that causes worry for you and your patient.
4. The ANA test on its own is not helpful until you order the subtypes. Double-stranded DNA and anti-SSA or anti-SSB antibodies are just a few examples of the subtypes of the ANA test that really help you understand what you ordered.
5. The elephant in the room: What is the pretest probability of your diagnostic test — all the symptoms, the hair loss, the malar rash, the sores in the mouth, the joint swelling, the blood in the urine? Fluid around the heart, pericarditis, pleurisy, those kinds of symptoms, right? When you have those symptoms and you order an ANA test, then you have basically put directions into your GPS. So now you know that if the test is positive, these are the things you’re going to do with the test going forward.
I hope that these five things have told you: Hey, before you order that ANA test, let’s make sure that we’re not causing unnecessary stress for our patients and also minimizing unnecessary testing.
Dr. Dada, CEO, Overlake Arthritis and Osteoporosis Center, Bellevue, Washington, disclosed ties with Horizon Pharmaceuticals.
A version of this article first appeared on Medscape.com.
The Silent Exodus: Are Nurse Practitioners and Physician Assistants Quiet Quitting?
While she cared deeply about her work, Melissa Adams*, a family nurse practitioner (NP) in Madison, Alabama, was being frequently triple-booked, didn’t feel respected by her office manager, and started to worry about becoming burned out. When she sought help, “the administration was tone-deaf,” she said. “When I asked about what I could do to prevent burnout, they sent me an article about it. It was clear to me that asking for respite from triple-booking and asking to be respected by my office manager wasn’t being heard ... so I thought, ‘how do I fly under the radar and get by with what I can?’ ” That meant focusing on patient care and refusing to take on additional responsibilities, like training new hires or working with students.
“You’re overworked and underpaid, and you start giving less and less of yourself,” Ms. Adams said in an interview.
Quiet quitting, defined as performing only the assigned tasks of the job without making any extra effort or going the proverbial extra mile, has gained attention in the press in recent years. A Gallup poll found that about 50% of the workforce were “quiet quitters” or disengaged.
It may be even more prevalent in healthcare, where a recent survey found that 57% of frontline medical staff, including NPs and physician assistants (PAs), report being disengaged at work.
The Causes of Quiet Quitting
Potential causes of quiet quitting among PAs and NPs include:
- Unrealistic care expectations. Ms. Adams said.
- Lack of trust or respect. Physicians don’t always respect the role that PAs and NPs play in a practice.
- Dissatisfaction with leadership or administration. There’s often a feeling that the PA or NP isn’t “heard” or appreciated.
- Dissatisfaction with pay or working conditions.
- Moral injury. “There’s no way to escape being morally injured when you work with an at-risk population,” said Ms. Adams. “You may see someone who has 20-24 determinants of health, and you’re expected to schlep them through in 8 minutes — you know you’re not able to do what they need.”
What Quiet Quitting Looks Like
Terri Smith*, an NP at an academic medical center outpatient clinic in rural Vermont, said that, while she feels appreciated by her patients and her team, there’s poor communication from the administration, which has caused her to quietly quit.
“I stopped saying ‘yes’ to all the normal committee work and the extra stuff that used to add a lot to my professional enjoyment,” she said. “The last couple of years, my whole motto is to nod and smile when administration says to do something — to put your head down and take care of your patients.”
While the term “quiet quitting” may be new, the issue is not, said Bridget Roberts, PhD, a healthcare executive who ran a large physician’s group of 100 healthcare providers in Jacksonville, Florida, for a decade. “Quiet quitting is a fancy title for employees who are completely disengaged,” said Dr. Roberts. “When they’re on the way out, they ‘check the box’. That’s not a new thing.”
“Typically, the first thing you see is a lot of frustration in that they aren’t able to complete the tasks they have at hand,” said Rebecca Day, PMNHP, a doctoral-educated NP and director of nursing practice at a Federally Qualified Health Center in Corbin, Kentucky. “Staff may be overworked and not have enough time to do what’s required of them with patient care as well as the paperwork required behind the scenes. It [quiet quitting] is doing just enough to get by, but shortcutting as much as they can to try to save some time.”
Addressing Quiet Quitting
Those kinds of shortcuts may affect patients, admits Ms. Smith. “I do think it starts to seep into patient care,” she said. “And that really doesn’t feel good ... at our institution, I’m not just an NP — I’m the nurse, the doctor, the secretary — I’m everybody, and for the last year, almost every single day in clinic, I’m apologizing [to a patient] because we can’t do something.”
Watching for this frustration can help alert administrators to NPs and PAs who may be “checking out” at work. Open lines of communication can help you address the issue. “Ask questions like ‘What could we do differently to make your day easier?’” said Dr. Roberts. Understanding the day-to-day issues NPs and PAs face at work can help in developing a plan to address disengagement.
When Dr. Day sees quiet quitting at her practice, she talks with the advance practice provider about what’s causing the issue. “’Are you overworked? Are you understaffed? Are there problems at home? Do you feel you’re receiving inadequate pay?’ ” she said. “The first thing to do is address that and find mutual ground on the issues…deal with the person as a person and then go back and deal with the person as an employee. If your staff isn’t happy, your clinic isn’t going to be productive.”
Finally, while reasons for quiet quitting may vary, cultivating a collaborative atmosphere where NPs and PAs feel appreciated and valued can help reduce the risk for quiet quitting. “Get to know your advanced practice providers,” said Ms. Adams. “Understand their strengths and what they’re about. It’s not an ‘us vs them’ ... there is a lot more commonality when we approach it that way.” Respect for the integral role that NPs and PAs play in your practice can help reduce the risk for quiet quitting — and help provide better patient care.
*Names have been changed.
A version of this article first appeared on Medscape.com.
While she cared deeply about her work, Melissa Adams*, a family nurse practitioner (NP) in Madison, Alabama, was being frequently triple-booked, didn’t feel respected by her office manager, and started to worry about becoming burned out. When she sought help, “the administration was tone-deaf,” she said. “When I asked about what I could do to prevent burnout, they sent me an article about it. It was clear to me that asking for respite from triple-booking and asking to be respected by my office manager wasn’t being heard ... so I thought, ‘how do I fly under the radar and get by with what I can?’ ” That meant focusing on patient care and refusing to take on additional responsibilities, like training new hires or working with students.
“You’re overworked and underpaid, and you start giving less and less of yourself,” Ms. Adams said in an interview.
Quiet quitting, defined as performing only the assigned tasks of the job without making any extra effort or going the proverbial extra mile, has gained attention in the press in recent years. A Gallup poll found that about 50% of the workforce were “quiet quitters” or disengaged.
It may be even more prevalent in healthcare, where a recent survey found that 57% of frontline medical staff, including NPs and physician assistants (PAs), report being disengaged at work.
The Causes of Quiet Quitting
Potential causes of quiet quitting among PAs and NPs include:
- Unrealistic care expectations. Ms. Adams said.
- Lack of trust or respect. Physicians don’t always respect the role that PAs and NPs play in a practice.
- Dissatisfaction with leadership or administration. There’s often a feeling that the PA or NP isn’t “heard” or appreciated.
- Dissatisfaction with pay or working conditions.
- Moral injury. “There’s no way to escape being morally injured when you work with an at-risk population,” said Ms. Adams. “You may see someone who has 20-24 determinants of health, and you’re expected to schlep them through in 8 minutes — you know you’re not able to do what they need.”
What Quiet Quitting Looks Like
Terri Smith*, an NP at an academic medical center outpatient clinic in rural Vermont, said that, while she feels appreciated by her patients and her team, there’s poor communication from the administration, which has caused her to quietly quit.
“I stopped saying ‘yes’ to all the normal committee work and the extra stuff that used to add a lot to my professional enjoyment,” she said. “The last couple of years, my whole motto is to nod and smile when administration says to do something — to put your head down and take care of your patients.”
While the term “quiet quitting” may be new, the issue is not, said Bridget Roberts, PhD, a healthcare executive who ran a large physician’s group of 100 healthcare providers in Jacksonville, Florida, for a decade. “Quiet quitting is a fancy title for employees who are completely disengaged,” said Dr. Roberts. “When they’re on the way out, they ‘check the box’. That’s not a new thing.”
“Typically, the first thing you see is a lot of frustration in that they aren’t able to complete the tasks they have at hand,” said Rebecca Day, PMNHP, a doctoral-educated NP and director of nursing practice at a Federally Qualified Health Center in Corbin, Kentucky. “Staff may be overworked and not have enough time to do what’s required of them with patient care as well as the paperwork required behind the scenes. It [quiet quitting] is doing just enough to get by, but shortcutting as much as they can to try to save some time.”
Addressing Quiet Quitting
Those kinds of shortcuts may affect patients, admits Ms. Smith. “I do think it starts to seep into patient care,” she said. “And that really doesn’t feel good ... at our institution, I’m not just an NP — I’m the nurse, the doctor, the secretary — I’m everybody, and for the last year, almost every single day in clinic, I’m apologizing [to a patient] because we can’t do something.”
Watching for this frustration can help alert administrators to NPs and PAs who may be “checking out” at work. Open lines of communication can help you address the issue. “Ask questions like ‘What could we do differently to make your day easier?’” said Dr. Roberts. Understanding the day-to-day issues NPs and PAs face at work can help in developing a plan to address disengagement.
When Dr. Day sees quiet quitting at her practice, she talks with the advance practice provider about what’s causing the issue. “’Are you overworked? Are you understaffed? Are there problems at home? Do you feel you’re receiving inadequate pay?’ ” she said. “The first thing to do is address that and find mutual ground on the issues…deal with the person as a person and then go back and deal with the person as an employee. If your staff isn’t happy, your clinic isn’t going to be productive.”
Finally, while reasons for quiet quitting may vary, cultivating a collaborative atmosphere where NPs and PAs feel appreciated and valued can help reduce the risk for quiet quitting. “Get to know your advanced practice providers,” said Ms. Adams. “Understand their strengths and what they’re about. It’s not an ‘us vs them’ ... there is a lot more commonality when we approach it that way.” Respect for the integral role that NPs and PAs play in your practice can help reduce the risk for quiet quitting — and help provide better patient care.
*Names have been changed.
A version of this article first appeared on Medscape.com.
While she cared deeply about her work, Melissa Adams*, a family nurse practitioner (NP) in Madison, Alabama, was being frequently triple-booked, didn’t feel respected by her office manager, and started to worry about becoming burned out. When she sought help, “the administration was tone-deaf,” she said. “When I asked about what I could do to prevent burnout, they sent me an article about it. It was clear to me that asking for respite from triple-booking and asking to be respected by my office manager wasn’t being heard ... so I thought, ‘how do I fly under the radar and get by with what I can?’ ” That meant focusing on patient care and refusing to take on additional responsibilities, like training new hires or working with students.
“You’re overworked and underpaid, and you start giving less and less of yourself,” Ms. Adams said in an interview.
Quiet quitting, defined as performing only the assigned tasks of the job without making any extra effort or going the proverbial extra mile, has gained attention in the press in recent years. A Gallup poll found that about 50% of the workforce were “quiet quitters” or disengaged.
It may be even more prevalent in healthcare, where a recent survey found that 57% of frontline medical staff, including NPs and physician assistants (PAs), report being disengaged at work.
The Causes of Quiet Quitting
Potential causes of quiet quitting among PAs and NPs include:
- Unrealistic care expectations. Ms. Adams said.
- Lack of trust or respect. Physicians don’t always respect the role that PAs and NPs play in a practice.
- Dissatisfaction with leadership or administration. There’s often a feeling that the PA or NP isn’t “heard” or appreciated.
- Dissatisfaction with pay or working conditions.
- Moral injury. “There’s no way to escape being morally injured when you work with an at-risk population,” said Ms. Adams. “You may see someone who has 20-24 determinants of health, and you’re expected to schlep them through in 8 minutes — you know you’re not able to do what they need.”
What Quiet Quitting Looks Like
Terri Smith*, an NP at an academic medical center outpatient clinic in rural Vermont, said that, while she feels appreciated by her patients and her team, there’s poor communication from the administration, which has caused her to quietly quit.
“I stopped saying ‘yes’ to all the normal committee work and the extra stuff that used to add a lot to my professional enjoyment,” she said. “The last couple of years, my whole motto is to nod and smile when administration says to do something — to put your head down and take care of your patients.”
While the term “quiet quitting” may be new, the issue is not, said Bridget Roberts, PhD, a healthcare executive who ran a large physician’s group of 100 healthcare providers in Jacksonville, Florida, for a decade. “Quiet quitting is a fancy title for employees who are completely disengaged,” said Dr. Roberts. “When they’re on the way out, they ‘check the box’. That’s not a new thing.”
“Typically, the first thing you see is a lot of frustration in that they aren’t able to complete the tasks they have at hand,” said Rebecca Day, PMNHP, a doctoral-educated NP and director of nursing practice at a Federally Qualified Health Center in Corbin, Kentucky. “Staff may be overworked and not have enough time to do what’s required of them with patient care as well as the paperwork required behind the scenes. It [quiet quitting] is doing just enough to get by, but shortcutting as much as they can to try to save some time.”
Addressing Quiet Quitting
Those kinds of shortcuts may affect patients, admits Ms. Smith. “I do think it starts to seep into patient care,” she said. “And that really doesn’t feel good ... at our institution, I’m not just an NP — I’m the nurse, the doctor, the secretary — I’m everybody, and for the last year, almost every single day in clinic, I’m apologizing [to a patient] because we can’t do something.”
Watching for this frustration can help alert administrators to NPs and PAs who may be “checking out” at work. Open lines of communication can help you address the issue. “Ask questions like ‘What could we do differently to make your day easier?’” said Dr. Roberts. Understanding the day-to-day issues NPs and PAs face at work can help in developing a plan to address disengagement.
When Dr. Day sees quiet quitting at her practice, she talks with the advance practice provider about what’s causing the issue. “’Are you overworked? Are you understaffed? Are there problems at home? Do you feel you’re receiving inadequate pay?’ ” she said. “The first thing to do is address that and find mutual ground on the issues…deal with the person as a person and then go back and deal with the person as an employee. If your staff isn’t happy, your clinic isn’t going to be productive.”
Finally, while reasons for quiet quitting may vary, cultivating a collaborative atmosphere where NPs and PAs feel appreciated and valued can help reduce the risk for quiet quitting. “Get to know your advanced practice providers,” said Ms. Adams. “Understand their strengths and what they’re about. It’s not an ‘us vs them’ ... there is a lot more commonality when we approach it that way.” Respect for the integral role that NPs and PAs play in your practice can help reduce the risk for quiet quitting — and help provide better patient care.
*Names have been changed.
A version of this article first appeared on Medscape.com.
Current Hydroxychloroquine Use in Lupus May Provide Protection Against Cardiovascular Events
TOPLINE:
Current use of hydroxychloroquine is associated with a lower risk for myocardial infarction (MI), stroke, and other thromboembolic events in patients with systemic lupus erythematosus (SLE). This protective effect diminishes after discontinuation of hydroxychloroquine treatment.
METHODOLOGY:
- Researchers used a nested case-control design to evaluate the association between exposure to hydroxychloroquine and the risk for cardiovascular events in patients with SLE.
- They included 52,883 adults with SLE (mean age, 44.23 years; 86.6% women) identified from the National System of Health Databases, which includes 99% of the French population.
- Among these, 1981 individuals with composite cardiovascular conditions were matched with 16,892 control individuals without cardiovascular conditions.
- Patients were categorized on the basis of hydroxychloroquine exposure into current users (last exposure within 90 days before a cardiovascular event), remote users (91-365 days before), and nonusers (no exposure within 365 days).
- The study outcomes included a composite of cardiovascular events, including MI, stroke (including transient ischemic attack), and other thromboembolic events such as phlebitis, thrombophlebitis, venous thrombosis, venous thromboembolism, and pulmonary embolism.
TAKEAWAY:
- Current hydroxychloroquine users had lower odds of experiencing a composite cardiovascular outcome than nonusers (adjusted odds ratio [aOR], 0.63; 95% CI, 0.57-0.70).
- The odds of MI (aOR, 0.72; 95% CI, 0.60-0.87), stroke (aOR, 0.71; 95% CI, 0.61-0.83), and other thromboembolic events (aOR, 0.58; 95% CI, 0.48-0.69) were also lower among current users than among nonusers.
- No significant association was found for remote hydroxychloroquine exposure and the risk for composite cardiovascular events, MI, stroke, and other thromboembolic events.
IN PRACTICE:
“These findings support the protective association of hydroxychloroquine against CV [cardiovascular] events and underscore the importance of continuous hydroxychloroquine therapy for patients diagnosed with SLE,” the authors wrote.
SOURCE:
The study was led by Lamiae Grimaldi-Bensouda, PharmD, PhD, Department of Pharmacology, Hospital Group Paris-Saclay, Assistance Publique-Hôpitaux de Paris, France. It was published online on August 30, 2024, in JAMA Network Open.
LIMITATIONS:
The observational nature of the study may have introduced confounding. Current hydroxychloroquine users were younger than nonusers, with an average age difference of almost 5 years. Current hydroxychloroquine users had a twofold longer duration of onset of SLE and had a higher prevalence of chronic kidney disease compared with nonusers.
DISCLOSURES:
This study was funded by the Banque pour l’Investissement, Deeptech. Some authors declared having financial ties with various institutions and companies outside of the current study.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
Current use of hydroxychloroquine is associated with a lower risk for myocardial infarction (MI), stroke, and other thromboembolic events in patients with systemic lupus erythematosus (SLE). This protective effect diminishes after discontinuation of hydroxychloroquine treatment.
METHODOLOGY:
- Researchers used a nested case-control design to evaluate the association between exposure to hydroxychloroquine and the risk for cardiovascular events in patients with SLE.
- They included 52,883 adults with SLE (mean age, 44.23 years; 86.6% women) identified from the National System of Health Databases, which includes 99% of the French population.
- Among these, 1981 individuals with composite cardiovascular conditions were matched with 16,892 control individuals without cardiovascular conditions.
- Patients were categorized on the basis of hydroxychloroquine exposure into current users (last exposure within 90 days before a cardiovascular event), remote users (91-365 days before), and nonusers (no exposure within 365 days).
- The study outcomes included a composite of cardiovascular events, including MI, stroke (including transient ischemic attack), and other thromboembolic events such as phlebitis, thrombophlebitis, venous thrombosis, venous thromboembolism, and pulmonary embolism.
TAKEAWAY:
- Current hydroxychloroquine users had lower odds of experiencing a composite cardiovascular outcome than nonusers (adjusted odds ratio [aOR], 0.63; 95% CI, 0.57-0.70).
- The odds of MI (aOR, 0.72; 95% CI, 0.60-0.87), stroke (aOR, 0.71; 95% CI, 0.61-0.83), and other thromboembolic events (aOR, 0.58; 95% CI, 0.48-0.69) were also lower among current users than among nonusers.
- No significant association was found for remote hydroxychloroquine exposure and the risk for composite cardiovascular events, MI, stroke, and other thromboembolic events.
IN PRACTICE:
“These findings support the protective association of hydroxychloroquine against CV [cardiovascular] events and underscore the importance of continuous hydroxychloroquine therapy for patients diagnosed with SLE,” the authors wrote.
SOURCE:
The study was led by Lamiae Grimaldi-Bensouda, PharmD, PhD, Department of Pharmacology, Hospital Group Paris-Saclay, Assistance Publique-Hôpitaux de Paris, France. It was published online on August 30, 2024, in JAMA Network Open.
LIMITATIONS:
The observational nature of the study may have introduced confounding. Current hydroxychloroquine users were younger than nonusers, with an average age difference of almost 5 years. Current hydroxychloroquine users had a twofold longer duration of onset of SLE and had a higher prevalence of chronic kidney disease compared with nonusers.
DISCLOSURES:
This study was funded by the Banque pour l’Investissement, Deeptech. Some authors declared having financial ties with various institutions and companies outside of the current study.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
Current use of hydroxychloroquine is associated with a lower risk for myocardial infarction (MI), stroke, and other thromboembolic events in patients with systemic lupus erythematosus (SLE). This protective effect diminishes after discontinuation of hydroxychloroquine treatment.
METHODOLOGY:
- Researchers used a nested case-control design to evaluate the association between exposure to hydroxychloroquine and the risk for cardiovascular events in patients with SLE.
- They included 52,883 adults with SLE (mean age, 44.23 years; 86.6% women) identified from the National System of Health Databases, which includes 99% of the French population.
- Among these, 1981 individuals with composite cardiovascular conditions were matched with 16,892 control individuals without cardiovascular conditions.
- Patients were categorized on the basis of hydroxychloroquine exposure into current users (last exposure within 90 days before a cardiovascular event), remote users (91-365 days before), and nonusers (no exposure within 365 days).
- The study outcomes included a composite of cardiovascular events, including MI, stroke (including transient ischemic attack), and other thromboembolic events such as phlebitis, thrombophlebitis, venous thrombosis, venous thromboembolism, and pulmonary embolism.
TAKEAWAY:
- Current hydroxychloroquine users had lower odds of experiencing a composite cardiovascular outcome than nonusers (adjusted odds ratio [aOR], 0.63; 95% CI, 0.57-0.70).
- The odds of MI (aOR, 0.72; 95% CI, 0.60-0.87), stroke (aOR, 0.71; 95% CI, 0.61-0.83), and other thromboembolic events (aOR, 0.58; 95% CI, 0.48-0.69) were also lower among current users than among nonusers.
- No significant association was found for remote hydroxychloroquine exposure and the risk for composite cardiovascular events, MI, stroke, and other thromboembolic events.
IN PRACTICE:
“These findings support the protective association of hydroxychloroquine against CV [cardiovascular] events and underscore the importance of continuous hydroxychloroquine therapy for patients diagnosed with SLE,” the authors wrote.
SOURCE:
The study was led by Lamiae Grimaldi-Bensouda, PharmD, PhD, Department of Pharmacology, Hospital Group Paris-Saclay, Assistance Publique-Hôpitaux de Paris, France. It was published online on August 30, 2024, in JAMA Network Open.
LIMITATIONS:
The observational nature of the study may have introduced confounding. Current hydroxychloroquine users were younger than nonusers, with an average age difference of almost 5 years. Current hydroxychloroquine users had a twofold longer duration of onset of SLE and had a higher prevalence of chronic kidney disease compared with nonusers.
DISCLOSURES:
This study was funded by the Banque pour l’Investissement, Deeptech. Some authors declared having financial ties with various institutions and companies outside of the current study.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
MRI-Derived Abdominal Adipose Tissue Linked to Chronic Musculoskeletal Pain
TOPLINE:
MRI-derived abdominal adipose tissue is linked to chronic musculoskeletal pain in multiple sites. The association is stronger in women, suggesting sex differences in fat distribution and hormones.
METHODOLOGY:
- Researchers used data from the UK Biobank, a large population-based cohort study, to investigate the associations between MRI-measured abdominal adipose tissue and chronic musculoskeletal pain.
- A total of 32,409 participants (50.8% women; mean age, 55.0 ± 7.4 years) were included in the analysis, with abdominal MRI scans performed at two imaging visits.
- Pain in the neck/shoulder, back, hip, knee, or “all over the body” was assessed, and participants were categorized based on the number of chronic pain sites.
- Mixed-effects ordinal, multinomial, and logistic regression models were used to analyze the associations between visceral adipose tissue (VAT), subcutaneous adipose tissue (SAT), and their ratio with chronic pain.
TAKEAWAY:
- According to the authors, there was a dose-response association between VAT, SAT, and their ratio with the number of chronic pain sites in both women and men.
- Higher levels of abdominal adipose tissue were associated with greater odds of reporting chronic pain in both sexes, with effect estimates being relatively larger in women.
- The researchers found that the VAT/SAT ratio was associated with the number of chronic pain sites and chronic pain in both sexes, reflecting differences in fat distribution and hormones.
- The study suggested that excessive abdominal adipose tissue may be involved in the pathogenesis of multisite and widespread chronic musculoskeletal pain.
IN PRACTICE:
“Abdominal adipose tissue was associated with chronic musculoskeletal pain, suggesting that excessive and ectopic fat depositions may be involved in the pathogenesis of multisite and widespread chronic musculoskeletal pain,” wrote the authors of the study.
SOURCE:
This study was led by Zemene Demelash Kifle, University of Tasmania Menzies Institute for Medical Research in Hobart, Australia. It was published online in Regional Anesthesia & Pain Medicine.
LIMITATIONS:
The study’s limitations included the use of a pain questionnaire that did not assess pain severity, which limited the ability to examine the relationship between fat measures and pain severity. Additionally, MRI was conducted on only two occasions, which may have not captured patterns and fluctuations in chronic pain sites. The relatively small size of the imaging sample, compared with the original baseline sample limited the generalizability of the findings. The predominant White ethnicity of participants also limited the generalizability to diverse populations.
DISCLOSURES:
The study was supported by grants from the Australian National Health and Medical Research Council (NHMRC). Mr. Kifle disclosed receiving grants from the Australian NHMRC. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
MRI-derived abdominal adipose tissue is linked to chronic musculoskeletal pain in multiple sites. The association is stronger in women, suggesting sex differences in fat distribution and hormones.
METHODOLOGY:
- Researchers used data from the UK Biobank, a large population-based cohort study, to investigate the associations between MRI-measured abdominal adipose tissue and chronic musculoskeletal pain.
- A total of 32,409 participants (50.8% women; mean age, 55.0 ± 7.4 years) were included in the analysis, with abdominal MRI scans performed at two imaging visits.
- Pain in the neck/shoulder, back, hip, knee, or “all over the body” was assessed, and participants were categorized based on the number of chronic pain sites.
- Mixed-effects ordinal, multinomial, and logistic regression models were used to analyze the associations between visceral adipose tissue (VAT), subcutaneous adipose tissue (SAT), and their ratio with chronic pain.
TAKEAWAY:
- According to the authors, there was a dose-response association between VAT, SAT, and their ratio with the number of chronic pain sites in both women and men.
- Higher levels of abdominal adipose tissue were associated with greater odds of reporting chronic pain in both sexes, with effect estimates being relatively larger in women.
- The researchers found that the VAT/SAT ratio was associated with the number of chronic pain sites and chronic pain in both sexes, reflecting differences in fat distribution and hormones.
- The study suggested that excessive abdominal adipose tissue may be involved in the pathogenesis of multisite and widespread chronic musculoskeletal pain.
IN PRACTICE:
“Abdominal adipose tissue was associated with chronic musculoskeletal pain, suggesting that excessive and ectopic fat depositions may be involved in the pathogenesis of multisite and widespread chronic musculoskeletal pain,” wrote the authors of the study.
SOURCE:
This study was led by Zemene Demelash Kifle, University of Tasmania Menzies Institute for Medical Research in Hobart, Australia. It was published online in Regional Anesthesia & Pain Medicine.
LIMITATIONS:
The study’s limitations included the use of a pain questionnaire that did not assess pain severity, which limited the ability to examine the relationship between fat measures and pain severity. Additionally, MRI was conducted on only two occasions, which may have not captured patterns and fluctuations in chronic pain sites. The relatively small size of the imaging sample, compared with the original baseline sample limited the generalizability of the findings. The predominant White ethnicity of participants also limited the generalizability to diverse populations.
DISCLOSURES:
The study was supported by grants from the Australian National Health and Medical Research Council (NHMRC). Mr. Kifle disclosed receiving grants from the Australian NHMRC. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
MRI-derived abdominal adipose tissue is linked to chronic musculoskeletal pain in multiple sites. The association is stronger in women, suggesting sex differences in fat distribution and hormones.
METHODOLOGY:
- Researchers used data from the UK Biobank, a large population-based cohort study, to investigate the associations between MRI-measured abdominal adipose tissue and chronic musculoskeletal pain.
- A total of 32,409 participants (50.8% women; mean age, 55.0 ± 7.4 years) were included in the analysis, with abdominal MRI scans performed at two imaging visits.
- Pain in the neck/shoulder, back, hip, knee, or “all over the body” was assessed, and participants were categorized based on the number of chronic pain sites.
- Mixed-effects ordinal, multinomial, and logistic regression models were used to analyze the associations between visceral adipose tissue (VAT), subcutaneous adipose tissue (SAT), and their ratio with chronic pain.
TAKEAWAY:
- According to the authors, there was a dose-response association between VAT, SAT, and their ratio with the number of chronic pain sites in both women and men.
- Higher levels of abdominal adipose tissue were associated with greater odds of reporting chronic pain in both sexes, with effect estimates being relatively larger in women.
- The researchers found that the VAT/SAT ratio was associated with the number of chronic pain sites and chronic pain in both sexes, reflecting differences in fat distribution and hormones.
- The study suggested that excessive abdominal adipose tissue may be involved in the pathogenesis of multisite and widespread chronic musculoskeletal pain.
IN PRACTICE:
“Abdominal adipose tissue was associated with chronic musculoskeletal pain, suggesting that excessive and ectopic fat depositions may be involved in the pathogenesis of multisite and widespread chronic musculoskeletal pain,” wrote the authors of the study.
SOURCE:
This study was led by Zemene Demelash Kifle, University of Tasmania Menzies Institute for Medical Research in Hobart, Australia. It was published online in Regional Anesthesia & Pain Medicine.
LIMITATIONS:
The study’s limitations included the use of a pain questionnaire that did not assess pain severity, which limited the ability to examine the relationship between fat measures and pain severity. Additionally, MRI was conducted on only two occasions, which may have not captured patterns and fluctuations in chronic pain sites. The relatively small size of the imaging sample, compared with the original baseline sample limited the generalizability of the findings. The predominant White ethnicity of participants also limited the generalizability to diverse populations.
DISCLOSURES:
The study was supported by grants from the Australian National Health and Medical Research Council (NHMRC). Mr. Kifle disclosed receiving grants from the Australian NHMRC. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.

