User login
Septic Arthritis and Osteomyelitis Caused by Pasteurella multocida
A few days after an incidental cat bite, a patient presented to the emergency department for treatment of poison sumac exposure. He was discharged with oral methylprednisolone for the dermatitis and returned 1 week later with symptoms, examination findings, and laboratory results consistent with sepsis and bilateral upper extremity necrotizing soft-tissue infections. After administering multiple irrigation and débridement procedures, hyperbaric oxygen treatments, and an antibiotic regimen, the patient’s status greatly improved. However, the patient returned 1 month later with a new sternoclavicular joint prominence that was associated with painful crepitus. Additionally, he noted that his wrists were gradually becoming more swollen and painful. Imaging studies showed a lytic destruction of the sternoclavicular joint and erosive changes throughout the carpus and radiocarpal joint bilaterally, consistent with osteomyelitis. The patient was treated with ertapenem for 6 weeks, and his polyarthropathy resolved. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
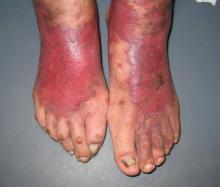
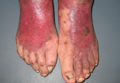
A 73-year-old, right-hand–dominant man with no notable medical history presented to the emergency department for treatment of poison sumac exposure, incidentally, a few days after being bitten by a cat on the bilateral distal upper extremities. He was prescribed a course of oral methylprednisolone for dermatitis. A week later, the patient returned to the emergency department with altered mental status, fevers, diaphoresis, lethargy, and polyarthralgia. At the time of presentation, the patient’s vital signs were labile, and he was found to have extensive bilateral upper extremity erythema, blistering, petechiae, purpuric lesions, and exquisite pain with passive range of motion of his fingers and wrists. His leukocyte count was 25.1 × 109/L, and he had elevated C-reactive protein level and erythrocyte sedimentation rate of 150 mg/L and 120 mm/h, respectively. He was admitted for management of sepsis and presumed bilateral upper extremity necrotizing soft-tissue infection.
Broad-spectrum intravenous (IV) antibiotics (vancomycin, piperacillin, tazobactam) were initiated after blood cultures were obtained, and the patient was taken emergently to the operating theatre for irrigation and débridement of his hands and wrists bilaterally. Arthrotomy of the wrist and débridement of the distal extensor compartment and its tenosynovium were performed on the right forearm, in addition to a decompressive fasciotomy of the left forearm. Postoperatively, the patient’s mental status improved and his vital signs gradually normalized. He received multiple hyperbaric oxygen treatments and underwent several additional operative débridement procedures with eventual closure of his wounds. At initial presentation, the differential diagnosis for the severe soft-tissue infection included necrotizing fasciitis or myositis caused by any of a variety of bacterial pathogens. Most notably, it was important to elicit the history of a cat bite to include and consider Pasteurella multocida as a potential pathogen. Initial cultures supported the diagnosis of acute P multocida necrotizing skin and soft-tissue infection, in addition to septic arthritis. The patient’s blood and intraoperative wound cultures grew P multocida. The antibiotic treatment was tailored initially to ampicillin and sulbactam and then to a final regimen of orally administered ciprofloxacin (750 mg twice a day), once susceptibility testing was performed on the cultures. On hospital day 10, the patient was discharged home, receiving a 6-week course of ciprofloxacin to complete the 8-week course of treatment.
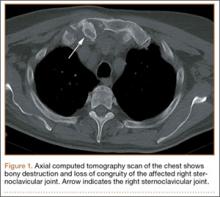
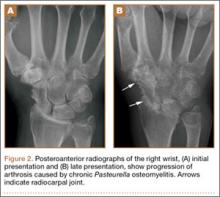
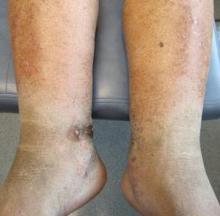
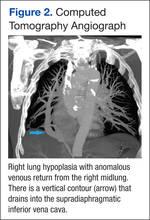
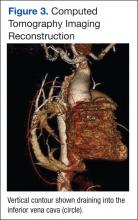
At follow-up, approximately 1 month after discharge, the patient noted that he had developed a new right sternoclavicular joint prominence that was associated with painful crepitus. He also noted that his wrists were gradually becoming more swollen and painful bilaterally. Computed tomography scans of the chest were obtained to evaluate the sternoclavicular joint (Figure 1). Repeat radiographs of the wrists were also obtained (Figure 2). Imaging showed lytic destruction of the sternoclavicular joint and erosive changes throughout the carpus and radiocarpal joint, consistent with osteomyelitis. The C-reactive protein level and erythrocyte sedimentation rate at this time were 34 mg/L and 124 mm/h, respectively.
The patient returned to the operating room for débridement and biopsy of the right sternoclavicular joint and left wrist. This patient’s delayed presentation was characterized by a subacute worsening of isolated musculoskeletal complaints. The differential diagnosis then included infection with the same bacterial pathogen versus reactive or inflammatory arthritis. Several intraoperative cultures failed to grow any bacteria, including P multocida, although P multocida was the presumptive cause of the erosive polyarthropathy, considering that symptoms eventually resolved with a repeated course of IV-administered ertapenem for 6 weeks. The patient experienced complete resolution of his joint pain and swelling. He was able to resume his activities of daily living and had no further recurrence of symptoms at follow-up 3 months later.
Discussion
Cat bites often are the source of Pasteurella species infections because the bacteria are carried by more than 90% of cats.1 These types of infections can cause septic arthritis, osteomyelitis, and deep subcutaneous and myofascial infections because of the sharp and narrow morphology of cat teeth. The infections can progress to necrotizing fasciitis and myositis if not recognized early, as was the case with our patient. Prophylactic antibiotic administration for animal bites is controversial and is not a universal practice.1,2Pasteurella bacteremia is an atypical progression that occurs more often in patients with pneumonia, septic arthritis, or meningitis. Cases of Pasteurella sepsis, necrotizing fasciitis, and septic arthritis have been reported.3-7 However, associated progressive septic arthritis and osteomyelitis, despite initial clinical improvement, have not been reported. Severe infection (ie, sepsis and septic shock) can occur in infants, pregnant women, and other immunocompromised patients.7 Immune suppression of our patient with steroid medication for poison sumac dermatitis likely contributed to the progression and systemic spread of an initially benign cat bite. Before prescribing steroids, it is imperative to ask about exposures and encourage patients to seek prompt medical attention with worsening or new symptoms. Healthy individuals rarely develop bacteremia; however, in these cases, mortality remains high at approximately 25%.4,6
The clinical course of this case emphasizes the need for vigilance and thoroughness in obtaining histories from patients presenting with seemingly benign complaints, especially in vulnerable populations, such as infants, pregnant women, and immunocompromised adults. In this case, the progression of symptoms might have been avoided if the patient’s dermatitis had been treated conservatively or with topical rather than systemic steroids.
1. Esposito S, Picciolli I, Semino M, Principi N. Dog and cat bite-associated infections in children. Eur J Clin Microbiol Infect Dis. 2013;32(8):971-976.
2. Medeiros I, Saconato H. Antibiotic prophylaxis for mammalian bites. Cochrane Database Syst Rev. 2001;(2):CD001738.
3. Haybaeck J, Schindler C, Braza P, Willinger B, Drlicek M. Rapidly progressive and lethal septicemia due to infection with Pasteurella multocida in an infant. Wien Klin Wochenschr. 2009;121(5-6):216-219.
4. Migliore E, Serraino C, Brignone C, et al. Pasteurella multocida infection in a cirrhotic patient: case report, microbiological aspects and a review of the literature. Adv Med Sci. 2009;54(1):109-112.
5. Mugambi SM, Ullian ME. Bacteremia, sepsis, and peritonitis with Pasteurella multocida in a peritoneal dialysis patient. Perit Dial Int. 2010;30(3):381-383.
6. Weber DJ, Wolfson JS, Swartz MN, Hooper DC. Pasteurella multocida infections. Report of 34 cases and review of the literature. Medicine (Baltimore). 1984;63(3):133-154.
7. Oehler RL, Velez AP, Mizrachi M, Lamarche J, Gompf S. Bite-related and septic syndromes caused by cats and dogs. Lancet Infect Dis. 2009;9(7):439-447.
A few days after an incidental cat bite, a patient presented to the emergency department for treatment of poison sumac exposure. He was discharged with oral methylprednisolone for the dermatitis and returned 1 week later with symptoms, examination findings, and laboratory results consistent with sepsis and bilateral upper extremity necrotizing soft-tissue infections. After administering multiple irrigation and débridement procedures, hyperbaric oxygen treatments, and an antibiotic regimen, the patient’s status greatly improved. However, the patient returned 1 month later with a new sternoclavicular joint prominence that was associated with painful crepitus. Additionally, he noted that his wrists were gradually becoming more swollen and painful. Imaging studies showed a lytic destruction of the sternoclavicular joint and erosive changes throughout the carpus and radiocarpal joint bilaterally, consistent with osteomyelitis. The patient was treated with ertapenem for 6 weeks, and his polyarthropathy resolved. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 73-year-old, right-hand–dominant man with no notable medical history presented to the emergency department for treatment of poison sumac exposure, incidentally, a few days after being bitten by a cat on the bilateral distal upper extremities. He was prescribed a course of oral methylprednisolone for dermatitis. A week later, the patient returned to the emergency department with altered mental status, fevers, diaphoresis, lethargy, and polyarthralgia. At the time of presentation, the patient’s vital signs were labile, and he was found to have extensive bilateral upper extremity erythema, blistering, petechiae, purpuric lesions, and exquisite pain with passive range of motion of his fingers and wrists. His leukocyte count was 25.1 × 109/L, and he had elevated C-reactive protein level and erythrocyte sedimentation rate of 150 mg/L and 120 mm/h, respectively. He was admitted for management of sepsis and presumed bilateral upper extremity necrotizing soft-tissue infection.
Broad-spectrum intravenous (IV) antibiotics (vancomycin, piperacillin, tazobactam) were initiated after blood cultures were obtained, and the patient was taken emergently to the operating theatre for irrigation and débridement of his hands and wrists bilaterally. Arthrotomy of the wrist and débridement of the distal extensor compartment and its tenosynovium were performed on the right forearm, in addition to a decompressive fasciotomy of the left forearm. Postoperatively, the patient’s mental status improved and his vital signs gradually normalized. He received multiple hyperbaric oxygen treatments and underwent several additional operative débridement procedures with eventual closure of his wounds. At initial presentation, the differential diagnosis for the severe soft-tissue infection included necrotizing fasciitis or myositis caused by any of a variety of bacterial pathogens. Most notably, it was important to elicit the history of a cat bite to include and consider Pasteurella multocida as a potential pathogen. Initial cultures supported the diagnosis of acute P multocida necrotizing skin and soft-tissue infection, in addition to septic arthritis. The patient’s blood and intraoperative wound cultures grew P multocida. The antibiotic treatment was tailored initially to ampicillin and sulbactam and then to a final regimen of orally administered ciprofloxacin (750 mg twice a day), once susceptibility testing was performed on the cultures. On hospital day 10, the patient was discharged home, receiving a 6-week course of ciprofloxacin to complete the 8-week course of treatment.
At follow-up, approximately 1 month after discharge, the patient noted that he had developed a new right sternoclavicular joint prominence that was associated with painful crepitus. He also noted that his wrists were gradually becoming more swollen and painful bilaterally. Computed tomography scans of the chest were obtained to evaluate the sternoclavicular joint (Figure 1). Repeat radiographs of the wrists were also obtained (Figure 2). Imaging showed lytic destruction of the sternoclavicular joint and erosive changes throughout the carpus and radiocarpal joint, consistent with osteomyelitis. The C-reactive protein level and erythrocyte sedimentation rate at this time were 34 mg/L and 124 mm/h, respectively.
The patient returned to the operating room for débridement and biopsy of the right sternoclavicular joint and left wrist. This patient’s delayed presentation was characterized by a subacute worsening of isolated musculoskeletal complaints. The differential diagnosis then included infection with the same bacterial pathogen versus reactive or inflammatory arthritis. Several intraoperative cultures failed to grow any bacteria, including P multocida, although P multocida was the presumptive cause of the erosive polyarthropathy, considering that symptoms eventually resolved with a repeated course of IV-administered ertapenem for 6 weeks. The patient experienced complete resolution of his joint pain and swelling. He was able to resume his activities of daily living and had no further recurrence of symptoms at follow-up 3 months later.
Discussion
Cat bites often are the source of Pasteurella species infections because the bacteria are carried by more than 90% of cats.1 These types of infections can cause septic arthritis, osteomyelitis, and deep subcutaneous and myofascial infections because of the sharp and narrow morphology of cat teeth. The infections can progress to necrotizing fasciitis and myositis if not recognized early, as was the case with our patient. Prophylactic antibiotic administration for animal bites is controversial and is not a universal practice.1,2Pasteurella bacteremia is an atypical progression that occurs more often in patients with pneumonia, septic arthritis, or meningitis. Cases of Pasteurella sepsis, necrotizing fasciitis, and septic arthritis have been reported.3-7 However, associated progressive septic arthritis and osteomyelitis, despite initial clinical improvement, have not been reported. Severe infection (ie, sepsis and septic shock) can occur in infants, pregnant women, and other immunocompromised patients.7 Immune suppression of our patient with steroid medication for poison sumac dermatitis likely contributed to the progression and systemic spread of an initially benign cat bite. Before prescribing steroids, it is imperative to ask about exposures and encourage patients to seek prompt medical attention with worsening or new symptoms. Healthy individuals rarely develop bacteremia; however, in these cases, mortality remains high at approximately 25%.4,6
The clinical course of this case emphasizes the need for vigilance and thoroughness in obtaining histories from patients presenting with seemingly benign complaints, especially in vulnerable populations, such as infants, pregnant women, and immunocompromised adults. In this case, the progression of symptoms might have been avoided if the patient’s dermatitis had been treated conservatively or with topical rather than systemic steroids.
A few days after an incidental cat bite, a patient presented to the emergency department for treatment of poison sumac exposure. He was discharged with oral methylprednisolone for the dermatitis and returned 1 week later with symptoms, examination findings, and laboratory results consistent with sepsis and bilateral upper extremity necrotizing soft-tissue infections. After administering multiple irrigation and débridement procedures, hyperbaric oxygen treatments, and an antibiotic regimen, the patient’s status greatly improved. However, the patient returned 1 month later with a new sternoclavicular joint prominence that was associated with painful crepitus. Additionally, he noted that his wrists were gradually becoming more swollen and painful. Imaging studies showed a lytic destruction of the sternoclavicular joint and erosive changes throughout the carpus and radiocarpal joint bilaterally, consistent with osteomyelitis. The patient was treated with ertapenem for 6 weeks, and his polyarthropathy resolved. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 73-year-old, right-hand–dominant man with no notable medical history presented to the emergency department for treatment of poison sumac exposure, incidentally, a few days after being bitten by a cat on the bilateral distal upper extremities. He was prescribed a course of oral methylprednisolone for dermatitis. A week later, the patient returned to the emergency department with altered mental status, fevers, diaphoresis, lethargy, and polyarthralgia. At the time of presentation, the patient’s vital signs were labile, and he was found to have extensive bilateral upper extremity erythema, blistering, petechiae, purpuric lesions, and exquisite pain with passive range of motion of his fingers and wrists. His leukocyte count was 25.1 × 109/L, and he had elevated C-reactive protein level and erythrocyte sedimentation rate of 150 mg/L and 120 mm/h, respectively. He was admitted for management of sepsis and presumed bilateral upper extremity necrotizing soft-tissue infection.
Broad-spectrum intravenous (IV) antibiotics (vancomycin, piperacillin, tazobactam) were initiated after blood cultures were obtained, and the patient was taken emergently to the operating theatre for irrigation and débridement of his hands and wrists bilaterally. Arthrotomy of the wrist and débridement of the distal extensor compartment and its tenosynovium were performed on the right forearm, in addition to a decompressive fasciotomy of the left forearm. Postoperatively, the patient’s mental status improved and his vital signs gradually normalized. He received multiple hyperbaric oxygen treatments and underwent several additional operative débridement procedures with eventual closure of his wounds. At initial presentation, the differential diagnosis for the severe soft-tissue infection included necrotizing fasciitis or myositis caused by any of a variety of bacterial pathogens. Most notably, it was important to elicit the history of a cat bite to include and consider Pasteurella multocida as a potential pathogen. Initial cultures supported the diagnosis of acute P multocida necrotizing skin and soft-tissue infection, in addition to septic arthritis. The patient’s blood and intraoperative wound cultures grew P multocida. The antibiotic treatment was tailored initially to ampicillin and sulbactam and then to a final regimen of orally administered ciprofloxacin (750 mg twice a day), once susceptibility testing was performed on the cultures. On hospital day 10, the patient was discharged home, receiving a 6-week course of ciprofloxacin to complete the 8-week course of treatment.
At follow-up, approximately 1 month after discharge, the patient noted that he had developed a new right sternoclavicular joint prominence that was associated with painful crepitus. He also noted that his wrists were gradually becoming more swollen and painful bilaterally. Computed tomography scans of the chest were obtained to evaluate the sternoclavicular joint (Figure 1). Repeat radiographs of the wrists were also obtained (Figure 2). Imaging showed lytic destruction of the sternoclavicular joint and erosive changes throughout the carpus and radiocarpal joint, consistent with osteomyelitis. The C-reactive protein level and erythrocyte sedimentation rate at this time were 34 mg/L and 124 mm/h, respectively.
The patient returned to the operating room for débridement and biopsy of the right sternoclavicular joint and left wrist. This patient’s delayed presentation was characterized by a subacute worsening of isolated musculoskeletal complaints. The differential diagnosis then included infection with the same bacterial pathogen versus reactive or inflammatory arthritis. Several intraoperative cultures failed to grow any bacteria, including P multocida, although P multocida was the presumptive cause of the erosive polyarthropathy, considering that symptoms eventually resolved with a repeated course of IV-administered ertapenem for 6 weeks. The patient experienced complete resolution of his joint pain and swelling. He was able to resume his activities of daily living and had no further recurrence of symptoms at follow-up 3 months later.
Discussion
Cat bites often are the source of Pasteurella species infections because the bacteria are carried by more than 90% of cats.1 These types of infections can cause septic arthritis, osteomyelitis, and deep subcutaneous and myofascial infections because of the sharp and narrow morphology of cat teeth. The infections can progress to necrotizing fasciitis and myositis if not recognized early, as was the case with our patient. Prophylactic antibiotic administration for animal bites is controversial and is not a universal practice.1,2Pasteurella bacteremia is an atypical progression that occurs more often in patients with pneumonia, septic arthritis, or meningitis. Cases of Pasteurella sepsis, necrotizing fasciitis, and septic arthritis have been reported.3-7 However, associated progressive septic arthritis and osteomyelitis, despite initial clinical improvement, have not been reported. Severe infection (ie, sepsis and septic shock) can occur in infants, pregnant women, and other immunocompromised patients.7 Immune suppression of our patient with steroid medication for poison sumac dermatitis likely contributed to the progression and systemic spread of an initially benign cat bite. Before prescribing steroids, it is imperative to ask about exposures and encourage patients to seek prompt medical attention with worsening or new symptoms. Healthy individuals rarely develop bacteremia; however, in these cases, mortality remains high at approximately 25%.4,6
The clinical course of this case emphasizes the need for vigilance and thoroughness in obtaining histories from patients presenting with seemingly benign complaints, especially in vulnerable populations, such as infants, pregnant women, and immunocompromised adults. In this case, the progression of symptoms might have been avoided if the patient’s dermatitis had been treated conservatively or with topical rather than systemic steroids.
1. Esposito S, Picciolli I, Semino M, Principi N. Dog and cat bite-associated infections in children. Eur J Clin Microbiol Infect Dis. 2013;32(8):971-976.
2. Medeiros I, Saconato H. Antibiotic prophylaxis for mammalian bites. Cochrane Database Syst Rev. 2001;(2):CD001738.
3. Haybaeck J, Schindler C, Braza P, Willinger B, Drlicek M. Rapidly progressive and lethal septicemia due to infection with Pasteurella multocida in an infant. Wien Klin Wochenschr. 2009;121(5-6):216-219.
4. Migliore E, Serraino C, Brignone C, et al. Pasteurella multocida infection in a cirrhotic patient: case report, microbiological aspects and a review of the literature. Adv Med Sci. 2009;54(1):109-112.
5. Mugambi SM, Ullian ME. Bacteremia, sepsis, and peritonitis with Pasteurella multocida in a peritoneal dialysis patient. Perit Dial Int. 2010;30(3):381-383.
6. Weber DJ, Wolfson JS, Swartz MN, Hooper DC. Pasteurella multocida infections. Report of 34 cases and review of the literature. Medicine (Baltimore). 1984;63(3):133-154.
7. Oehler RL, Velez AP, Mizrachi M, Lamarche J, Gompf S. Bite-related and septic syndromes caused by cats and dogs. Lancet Infect Dis. 2009;9(7):439-447.
1. Esposito S, Picciolli I, Semino M, Principi N. Dog and cat bite-associated infections in children. Eur J Clin Microbiol Infect Dis. 2013;32(8):971-976.
2. Medeiros I, Saconato H. Antibiotic prophylaxis for mammalian bites. Cochrane Database Syst Rev. 2001;(2):CD001738.
3. Haybaeck J, Schindler C, Braza P, Willinger B, Drlicek M. Rapidly progressive and lethal septicemia due to infection with Pasteurella multocida in an infant. Wien Klin Wochenschr. 2009;121(5-6):216-219.
4. Migliore E, Serraino C, Brignone C, et al. Pasteurella multocida infection in a cirrhotic patient: case report, microbiological aspects and a review of the literature. Adv Med Sci. 2009;54(1):109-112.
5. Mugambi SM, Ullian ME. Bacteremia, sepsis, and peritonitis with Pasteurella multocida in a peritoneal dialysis patient. Perit Dial Int. 2010;30(3):381-383.
6. Weber DJ, Wolfson JS, Swartz MN, Hooper DC. Pasteurella multocida infections. Report of 34 cases and review of the literature. Medicine (Baltimore). 1984;63(3):133-154.
7. Oehler RL, Velez AP, Mizrachi M, Lamarche J, Gompf S. Bite-related and septic syndromes caused by cats and dogs. Lancet Infect Dis. 2009;9(7):439-447.
Supinator Cyst in a Young Female Softball Player Successfully Treated With Aspiration
Ganglion cysts around the elbow joint are unusual, with fewer than 25 citations (most of which are case reports) in the English-language literature. Among the many causes of elbow pain, cysts are chiefly diagnosed by advanced imaging. When an elbow ganglion or perineural cyst is symptomatic, treatment has ranged from nonoperative to surgical intervention. Our case report is the first documented ultrasound-guided aspiration and cortisone injection to successfully alleviate a patient’s symptoms. The procedures and outcomes of minimally invasive ultrasound-guided aspiration and steroid injections have not been described for cysts around the elbow. The patient and patient’s guardian provided written informed consent for print and electronic publication of this case report.
Case Report
A 14-year-old female freshman varsity softball pitcher on multiple teams presented with 6 months of vague right elbow pain. She was unable to pitch and had intermittent sharp pain localized to the lateral proximal forearm. She was, however, able to bat without pain and denied any radiating paresthesias. Despite a reduction in sports activities, the symptoms did not improve.
On physical examination, there was preserved strength that was symmetric with the contralateral side of all major muscles innervated by the radial nerve in the right arm, including full wrist, thumb, and finger extension. Sensation was intact to light touch in all major nervous distributions of the right and left upper extremities. She was tender to palpation at the radiocapitellar joint anteriorly, as well as just distally. The patient was also tender with motion through the proximal radial head. She had pain with resisted finger extension; however, resisted supination elicited no discomfort or pain.
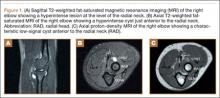
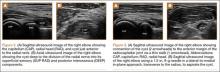
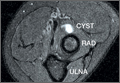
The initial diagnostic workup included radiographs of the right elbow, a magnetic resonance imaging (MRI) scan, and an ultrasound. Elbow radiographs revealed no abnormalities. The MRI scan showed a well-circumscribed ovoid T2-hyperintense structure within the supinator muscle measuring 0.6×0.6×0.4 cm (longitudinal × anteroposterior × transverse), just deep to the split of the superficial and deep radial nerves (Figures 1A-1C). A musculoskeletal ultrasound was performed to further characterize and determine the relationship to neurovascular structures. Longitudinal (Figure 2A) and transverse (Figure 2B) images showed a hypoechoic cystic structure, separate from any local nerve, and without Doppler flow, consistent with what was seen on MRI. Additionally, there was an apparent stalk communicating with the anterior margin of the radiocapitellar articulation, seen on longitudinal images, suggesting an extension of the joint capsule (Figure 3A).
We diagnosed the patient with a radiocapitellar ganglion cyst. Her symptoms continued despite several sessions of physical therapy and cessation from all throwing. Given the ultrasound and MRI findings, and continuation of the symptoms despite conservative treatment, alternative treatment plans were discussed with the patient. These included continued activity modification and nonoperative treatment, open excision of the cyst, or aspiration of the cyst under ultrasound guidance. All appropriate risks and benefits were discussed, including possibility of nerve damage given the proximity of the cyst to the radial nerve branches. After a thorough discussion with both patient and family, a plan was made to undergo aspiration under ultrasound guidance. This was carried out using a lateral-to-medial in-plane approach, transverse to the radius. Using a 19-g, 1.5-inch needle (Figure 3B), 1 mL of serosanguinous fluid was aspirated from the cyst, followed by injection of 40 mg methylprednisolone sodium succinate.
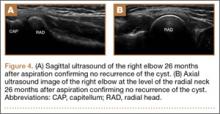
The patient made a dramatic recovery within 8 days after aspiration. On examination, she had full strength to resisted flexion, extension, pronation, and supination; had no tenderness to palpation over the supinator; and no pain with resisted finger extension. She began dedicated physical therapy and a gradual return to throwing. She was able to return to her original level of softball activities 2 months after the aspiration. The patient continued to be symptom-free 26 months after the aspiration/injection. There was no evidence of recurrence of the ganglion on repeat ultrasound at her most recent follow-up (Figures 4A, 4B).
Discussion
Our review of the English-language literature identified 23 reports of cysts in and around the supinator muscle. Ganglion cysts are benign lesions that are uncommonly seen about the elbow. This highlights the rarity of this diagnosis, as well as the need for recognition of its existence. Cysts located in the substance of the nerve1-5 and extraneural ganglia causing symptomatic nerve compression have been described. These extraneural ganglia have been reported to cause compression of the ulnar nerve,1-4,6 posterior interosseous nerve (PIN),5,7-12 and radial nerve,13 and isolated compression of the radial sensory branch.14-17 Ganglion cyst compression in the elbow can result in pain, decreased motor function, and decreased sensation. The PIN syndrome is primarily a motor deficiency, whereas isolated compression of the sensory branches of the radial nerve presents as pain along the radial tunnel and extensor muscle mass.17
Most ganglion cysts are formed when joint fluid extrudes through a defect in the joint capsule; they have also been described originating from a nonunion site.18 When conservative treatment fails, surgical excision has been recommended.5,6,8-10,12-16 We present the first known case of successful ultrasound-guided aspiration and injection of a ganglion cyst from the proximal radiocapitellar joint.
In the earliest described case in 1955, Broomhead19 noted exploration was essential to establish the diagnosis of nerve palsy. In 1966, Bowen and Stone7 were the first to report PIN compression by a ganglion and that compression was likely where nerves pass through confined spaces. In keeping with the known potential for compression of the common peroneal nerve around the fibular head, Bowen and Stone7 posited that the same could be true of the PIN coursing through the supinator and around the radial neck.
Many authors have noted that nerve palsy either improves with rest or worsens with heavy manual work.3,20,21 These observations suggest that dynamic factors in addition to compression of the nerve by the ganglion may influence the occurrence of the nerve palsy.14 This is in line with our patient whose symptoms worsened after pitching.
Ogino and colleagues20 reported on the first use of ultrasonography as a screening examination for a ganglion, particularly when palpation was difficult. Ultrasound allows a detailed assessment of peripheral nerve continuity with a mass, differentiating an intraneural lesion from an adjacent extrinsic ganglion.13 Tonkin10 published the first description of MRI used for the diagnosis of an elbow cyst, and its use has been supported by others.5,8,20 The typical appearance of ganglion cysts on MRI include low signal on T1-weighted images and very high signal on T2-weighted images. Only the periphery of the mass is enhanced by gadolinium, if used.
As recently as 2009, Jou and associates13 suggested that surgical excision should be performed promptly to ensure optimal recovery from a nerve palsy. Many authors agree that early diagnosis and careful surgical excision is associated with a satisfactory outcome without recurrence of the cyst.5,6,8-10,12-15 There are only 4 published case reports14-17 of ganglions causing isolated compression of the superficial radial sensory nerve, as in our case. Their patients had pain with exertional trauma14 as did our patient, a positive Tinel sign,15 and resolution of symptoms after surgical excision without recurrence.14-16 Mileti and colleagues16 state that standard management for resistant radial tunnel syndrome is open decompression of the radial nerve.
In the last decade, a few reports of arthroscopic excision being a viable and safe alternative to open excision have been published.16,22,23 In 2000, Feldman22 described the benefits of an arthroscopic approach as decreased soft-tissue dissection, increased ability to identify intra-articular pathology, and similar recurrence rates to open procedures. He reported 1 transient neurapraxia of the superficial radial nerve from the arthroscopy, highlighting a risk of arthroscopic treatment.
An alternative to open or arthroscopic cyst decompression is aspiration. The only mention of aspiration in the literature comes from Broomhead19 in 1955 when he described 2 patients in whom treatment by aspiration was unsuccessful in relieving their symptoms. Yamazaki and colleagues12 noted that 1 of their 14 patients with PIN palsies caused by ganglions at the elbow underwent puncture of the ganglion with recovery of the paralysis. With the aid of ultrasound guidance, we were able to accurately locate the ganglion cyst, aspirate its contents, and inject methylprednisolone sodium succinate. Our patient continued to be symptom-free and was an active pitcher on a varsity softball team 26 months after aspiration.
Conclusion
This case report describes a rare location for a ganglion cyst in a high-level softball player. To our knowledge, successful treatment with ultrasound-guided aspiration and injection of a supinator cyst has not been reported in the literature. This case report highlights the importance of a careful diagnosis of this condition and an alternative treatment algorithm.
1. Boursinos LA, Dimitriou CG. Ulnar nerve compression in the cubital tunnel by an epineural ganglion: a case report. Hand (N Y). 2007;2(1):12-15.
2. Ferlic DC, Ries MD. Epineural ganglion of the ulnar nerve at the elbow. J Hand Surg Am. 1990;15(6):996-998.
3. Ming Chan K, Thompson S, Amirjani N, Satkunam L, Strohschlein FJ, Lobay GL. Compression of the ulnar nerve at the elbow by an intraneural ganglion. J Clin Neurosci. 2003;10(2):245-248.
4. Sharma RR, Pawar SJ, Delmendo A, Mahapatra AK. Symptomatic epineural ganglion cyst of the ulnar nerve in the cubital tunnel: a case report and brief review of the literature. J Clin Neurosci. 2000;7(6):542-543.
5. Hashizume H, Nishida K, Nanba Y, Inoue H, Konishiike T. Intraneural ganglion of the posterior interosseous nerve with lateral elbow pain. J Hand Surg Br. 1995;20(5):649-651.
6. Kato H, Hirayama T, Minami A, Iwasaki N, Hirachi K. Cubital tunnel syndrome associated with medial elbow Ganglia and osteoarthritis of the elbow. J Bone Joint Surg Am. 2002;84(8):1413-1419.
7. Bowen TL, Stone KH. Posterior interosseous nerve paralysis caused by a ganglion at the elbow. J Bone Joint Surg Br. 1966;48(4):774-776.
8. Ly JQ, Barrett TJ, Beall DP, Bertagnolli R. MRI diagnosis of occult ganglion compression of the posterior interosseous nerve and associated supinator muscle pathology. Clin Imaging. 2005;29(5):362-363.
9. McCollam SM, Corley FG, Green DP. Posterior interosseous nerve palsy caused by ganglions of the proximal radioulnar joint. J Hand Surg Am. 1988;13(5):725-728.
10. Tonkin MA. Posterior interosseous nerve axonotmesis from compression by a ganglion. J Hand Surg Br. 1990;15(4):491-493.
11. Tuygun H, Kose O, Gorgec M. Partial paralysis of the posterior interosseous nerve caused by a ganglion. J Hand Surg Eur. 2008;33(4):540-541.
12. Yamazaki H, Kato H, Hata Y, Murakami N, Saitoh S. The two locations of ganglions causing radial nerve palsy. J Hand Surg Eur. 2007;32(3):341-345.
13. Jou IM, Wang HN, Wang PH, Yong IS, Su WR. Compression of the radial nerve at the elbow by a ganglion: two case reports. J Med Case Rep. 2009;3:7258.
14. Hermansdorfer JD, Greider JL, Dell PC. A case report of a compressive neuropathy of the radial sensory nerve caused by a ganglion cyst at the elbow. Orthopedics. 1986;9(7):1005-1006.
15. McFarlane J, Trehan R, Olivera M, Jones C, Blease S, Davey P. A ganglion cyst at the elbow causing superficial radial nerve compression: a case report. J Med Case Rep. 2008;2:122.
16. Mileti J, Largacha M, O’Driscoll SW. Radial tunnel syndrome caused by ganglion cyst: treatment by arthroscopic cyst decompression. Arthroscopy. 2004;20(5):e39-e44.
17. Plancher KD, Peterson RK, Steichen JB. Compressive neuropathies and tendinopathies in the athletic elbow and wrist. Clin Sports Med. 1996;15(2):331-371.
18. Chim H, Yam AK, Teoh LC. Elbow ganglion arising from medial epicondyle pseudarthrosis. Hand Surg. 2007;12(3):155-158.
19. Broomhead IW. Ganglia associated with elbow and knee joints. Lancet. 1955;269(6885):317-319.
20. Ogino T, Minami A, Kato H. Diagnosis of radial nerve palsy caused by ganglion with use of different imaging techniques. J Hand Surg Am. 1991;16(2):230-235.
21. Spinner M, Spencer PS. Nerve compression lesions of the upper extremity. A clinical and experimental review. Clin Orthop Relat Res. 1974;(104):46-67.
22. Feldman MD. Arthroscopic excision of a ganglion cyst from the elbow. Arthroscopy. 2000;16(6):661-664.
23. Kirpalani PA, Lee HK, Lee YS, Han CW. Transarticular arthroscopic excision of an elbow cyst. Acta Orthop Belg. 2005;71(4):477-480.
Ganglion cysts around the elbow joint are unusual, with fewer than 25 citations (most of which are case reports) in the English-language literature. Among the many causes of elbow pain, cysts are chiefly diagnosed by advanced imaging. When an elbow ganglion or perineural cyst is symptomatic, treatment has ranged from nonoperative to surgical intervention. Our case report is the first documented ultrasound-guided aspiration and cortisone injection to successfully alleviate a patient’s symptoms. The procedures and outcomes of minimally invasive ultrasound-guided aspiration and steroid injections have not been described for cysts around the elbow. The patient and patient’s guardian provided written informed consent for print and electronic publication of this case report.
Case Report
A 14-year-old female freshman varsity softball pitcher on multiple teams presented with 6 months of vague right elbow pain. She was unable to pitch and had intermittent sharp pain localized to the lateral proximal forearm. She was, however, able to bat without pain and denied any radiating paresthesias. Despite a reduction in sports activities, the symptoms did not improve.
On physical examination, there was preserved strength that was symmetric with the contralateral side of all major muscles innervated by the radial nerve in the right arm, including full wrist, thumb, and finger extension. Sensation was intact to light touch in all major nervous distributions of the right and left upper extremities. She was tender to palpation at the radiocapitellar joint anteriorly, as well as just distally. The patient was also tender with motion through the proximal radial head. She had pain with resisted finger extension; however, resisted supination elicited no discomfort or pain.
The initial diagnostic workup included radiographs of the right elbow, a magnetic resonance imaging (MRI) scan, and an ultrasound. Elbow radiographs revealed no abnormalities. The MRI scan showed a well-circumscribed ovoid T2-hyperintense structure within the supinator muscle measuring 0.6×0.6×0.4 cm (longitudinal × anteroposterior × transverse), just deep to the split of the superficial and deep radial nerves (Figures 1A-1C). A musculoskeletal ultrasound was performed to further characterize and determine the relationship to neurovascular structures. Longitudinal (Figure 2A) and transverse (Figure 2B) images showed a hypoechoic cystic structure, separate from any local nerve, and without Doppler flow, consistent with what was seen on MRI. Additionally, there was an apparent stalk communicating with the anterior margin of the radiocapitellar articulation, seen on longitudinal images, suggesting an extension of the joint capsule (Figure 3A).
We diagnosed the patient with a radiocapitellar ganglion cyst. Her symptoms continued despite several sessions of physical therapy and cessation from all throwing. Given the ultrasound and MRI findings, and continuation of the symptoms despite conservative treatment, alternative treatment plans were discussed with the patient. These included continued activity modification and nonoperative treatment, open excision of the cyst, or aspiration of the cyst under ultrasound guidance. All appropriate risks and benefits were discussed, including possibility of nerve damage given the proximity of the cyst to the radial nerve branches. After a thorough discussion with both patient and family, a plan was made to undergo aspiration under ultrasound guidance. This was carried out using a lateral-to-medial in-plane approach, transverse to the radius. Using a 19-g, 1.5-inch needle (Figure 3B), 1 mL of serosanguinous fluid was aspirated from the cyst, followed by injection of 40 mg methylprednisolone sodium succinate.
The patient made a dramatic recovery within 8 days after aspiration. On examination, she had full strength to resisted flexion, extension, pronation, and supination; had no tenderness to palpation over the supinator; and no pain with resisted finger extension. She began dedicated physical therapy and a gradual return to throwing. She was able to return to her original level of softball activities 2 months after the aspiration. The patient continued to be symptom-free 26 months after the aspiration/injection. There was no evidence of recurrence of the ganglion on repeat ultrasound at her most recent follow-up (Figures 4A, 4B).
Discussion
Our review of the English-language literature identified 23 reports of cysts in and around the supinator muscle. Ganglion cysts are benign lesions that are uncommonly seen about the elbow. This highlights the rarity of this diagnosis, as well as the need for recognition of its existence. Cysts located in the substance of the nerve1-5 and extraneural ganglia causing symptomatic nerve compression have been described. These extraneural ganglia have been reported to cause compression of the ulnar nerve,1-4,6 posterior interosseous nerve (PIN),5,7-12 and radial nerve,13 and isolated compression of the radial sensory branch.14-17 Ganglion cyst compression in the elbow can result in pain, decreased motor function, and decreased sensation. The PIN syndrome is primarily a motor deficiency, whereas isolated compression of the sensory branches of the radial nerve presents as pain along the radial tunnel and extensor muscle mass.17
Most ganglion cysts are formed when joint fluid extrudes through a defect in the joint capsule; they have also been described originating from a nonunion site.18 When conservative treatment fails, surgical excision has been recommended.5,6,8-10,12-16 We present the first known case of successful ultrasound-guided aspiration and injection of a ganglion cyst from the proximal radiocapitellar joint.
In the earliest described case in 1955, Broomhead19 noted exploration was essential to establish the diagnosis of nerve palsy. In 1966, Bowen and Stone7 were the first to report PIN compression by a ganglion and that compression was likely where nerves pass through confined spaces. In keeping with the known potential for compression of the common peroneal nerve around the fibular head, Bowen and Stone7 posited that the same could be true of the PIN coursing through the supinator and around the radial neck.
Many authors have noted that nerve palsy either improves with rest or worsens with heavy manual work.3,20,21 These observations suggest that dynamic factors in addition to compression of the nerve by the ganglion may influence the occurrence of the nerve palsy.14 This is in line with our patient whose symptoms worsened after pitching.
Ogino and colleagues20 reported on the first use of ultrasonography as a screening examination for a ganglion, particularly when palpation was difficult. Ultrasound allows a detailed assessment of peripheral nerve continuity with a mass, differentiating an intraneural lesion from an adjacent extrinsic ganglion.13 Tonkin10 published the first description of MRI used for the diagnosis of an elbow cyst, and its use has been supported by others.5,8,20 The typical appearance of ganglion cysts on MRI include low signal on T1-weighted images and very high signal on T2-weighted images. Only the periphery of the mass is enhanced by gadolinium, if used.
As recently as 2009, Jou and associates13 suggested that surgical excision should be performed promptly to ensure optimal recovery from a nerve palsy. Many authors agree that early diagnosis and careful surgical excision is associated with a satisfactory outcome without recurrence of the cyst.5,6,8-10,12-15 There are only 4 published case reports14-17 of ganglions causing isolated compression of the superficial radial sensory nerve, as in our case. Their patients had pain with exertional trauma14 as did our patient, a positive Tinel sign,15 and resolution of symptoms after surgical excision without recurrence.14-16 Mileti and colleagues16 state that standard management for resistant radial tunnel syndrome is open decompression of the radial nerve.
In the last decade, a few reports of arthroscopic excision being a viable and safe alternative to open excision have been published.16,22,23 In 2000, Feldman22 described the benefits of an arthroscopic approach as decreased soft-tissue dissection, increased ability to identify intra-articular pathology, and similar recurrence rates to open procedures. He reported 1 transient neurapraxia of the superficial radial nerve from the arthroscopy, highlighting a risk of arthroscopic treatment.
An alternative to open or arthroscopic cyst decompression is aspiration. The only mention of aspiration in the literature comes from Broomhead19 in 1955 when he described 2 patients in whom treatment by aspiration was unsuccessful in relieving their symptoms. Yamazaki and colleagues12 noted that 1 of their 14 patients with PIN palsies caused by ganglions at the elbow underwent puncture of the ganglion with recovery of the paralysis. With the aid of ultrasound guidance, we were able to accurately locate the ganglion cyst, aspirate its contents, and inject methylprednisolone sodium succinate. Our patient continued to be symptom-free and was an active pitcher on a varsity softball team 26 months after aspiration.
Conclusion
This case report describes a rare location for a ganglion cyst in a high-level softball player. To our knowledge, successful treatment with ultrasound-guided aspiration and injection of a supinator cyst has not been reported in the literature. This case report highlights the importance of a careful diagnosis of this condition and an alternative treatment algorithm.
Ganglion cysts around the elbow joint are unusual, with fewer than 25 citations (most of which are case reports) in the English-language literature. Among the many causes of elbow pain, cysts are chiefly diagnosed by advanced imaging. When an elbow ganglion or perineural cyst is symptomatic, treatment has ranged from nonoperative to surgical intervention. Our case report is the first documented ultrasound-guided aspiration and cortisone injection to successfully alleviate a patient’s symptoms. The procedures and outcomes of minimally invasive ultrasound-guided aspiration and steroid injections have not been described for cysts around the elbow. The patient and patient’s guardian provided written informed consent for print and electronic publication of this case report.
Case Report
A 14-year-old female freshman varsity softball pitcher on multiple teams presented with 6 months of vague right elbow pain. She was unable to pitch and had intermittent sharp pain localized to the lateral proximal forearm. She was, however, able to bat without pain and denied any radiating paresthesias. Despite a reduction in sports activities, the symptoms did not improve.
On physical examination, there was preserved strength that was symmetric with the contralateral side of all major muscles innervated by the radial nerve in the right arm, including full wrist, thumb, and finger extension. Sensation was intact to light touch in all major nervous distributions of the right and left upper extremities. She was tender to palpation at the radiocapitellar joint anteriorly, as well as just distally. The patient was also tender with motion through the proximal radial head. She had pain with resisted finger extension; however, resisted supination elicited no discomfort or pain.
The initial diagnostic workup included radiographs of the right elbow, a magnetic resonance imaging (MRI) scan, and an ultrasound. Elbow radiographs revealed no abnormalities. The MRI scan showed a well-circumscribed ovoid T2-hyperintense structure within the supinator muscle measuring 0.6×0.6×0.4 cm (longitudinal × anteroposterior × transverse), just deep to the split of the superficial and deep radial nerves (Figures 1A-1C). A musculoskeletal ultrasound was performed to further characterize and determine the relationship to neurovascular structures. Longitudinal (Figure 2A) and transverse (Figure 2B) images showed a hypoechoic cystic structure, separate from any local nerve, and without Doppler flow, consistent with what was seen on MRI. Additionally, there was an apparent stalk communicating with the anterior margin of the radiocapitellar articulation, seen on longitudinal images, suggesting an extension of the joint capsule (Figure 3A).
We diagnosed the patient with a radiocapitellar ganglion cyst. Her symptoms continued despite several sessions of physical therapy and cessation from all throwing. Given the ultrasound and MRI findings, and continuation of the symptoms despite conservative treatment, alternative treatment plans were discussed with the patient. These included continued activity modification and nonoperative treatment, open excision of the cyst, or aspiration of the cyst under ultrasound guidance. All appropriate risks and benefits were discussed, including possibility of nerve damage given the proximity of the cyst to the radial nerve branches. After a thorough discussion with both patient and family, a plan was made to undergo aspiration under ultrasound guidance. This was carried out using a lateral-to-medial in-plane approach, transverse to the radius. Using a 19-g, 1.5-inch needle (Figure 3B), 1 mL of serosanguinous fluid was aspirated from the cyst, followed by injection of 40 mg methylprednisolone sodium succinate.
The patient made a dramatic recovery within 8 days after aspiration. On examination, she had full strength to resisted flexion, extension, pronation, and supination; had no tenderness to palpation over the supinator; and no pain with resisted finger extension. She began dedicated physical therapy and a gradual return to throwing. She was able to return to her original level of softball activities 2 months after the aspiration. The patient continued to be symptom-free 26 months after the aspiration/injection. There was no evidence of recurrence of the ganglion on repeat ultrasound at her most recent follow-up (Figures 4A, 4B).
Discussion
Our review of the English-language literature identified 23 reports of cysts in and around the supinator muscle. Ganglion cysts are benign lesions that are uncommonly seen about the elbow. This highlights the rarity of this diagnosis, as well as the need for recognition of its existence. Cysts located in the substance of the nerve1-5 and extraneural ganglia causing symptomatic nerve compression have been described. These extraneural ganglia have been reported to cause compression of the ulnar nerve,1-4,6 posterior interosseous nerve (PIN),5,7-12 and radial nerve,13 and isolated compression of the radial sensory branch.14-17 Ganglion cyst compression in the elbow can result in pain, decreased motor function, and decreased sensation. The PIN syndrome is primarily a motor deficiency, whereas isolated compression of the sensory branches of the radial nerve presents as pain along the radial tunnel and extensor muscle mass.17
Most ganglion cysts are formed when joint fluid extrudes through a defect in the joint capsule; they have also been described originating from a nonunion site.18 When conservative treatment fails, surgical excision has been recommended.5,6,8-10,12-16 We present the first known case of successful ultrasound-guided aspiration and injection of a ganglion cyst from the proximal radiocapitellar joint.
In the earliest described case in 1955, Broomhead19 noted exploration was essential to establish the diagnosis of nerve palsy. In 1966, Bowen and Stone7 were the first to report PIN compression by a ganglion and that compression was likely where nerves pass through confined spaces. In keeping with the known potential for compression of the common peroneal nerve around the fibular head, Bowen and Stone7 posited that the same could be true of the PIN coursing through the supinator and around the radial neck.
Many authors have noted that nerve palsy either improves with rest or worsens with heavy manual work.3,20,21 These observations suggest that dynamic factors in addition to compression of the nerve by the ganglion may influence the occurrence of the nerve palsy.14 This is in line with our patient whose symptoms worsened after pitching.
Ogino and colleagues20 reported on the first use of ultrasonography as a screening examination for a ganglion, particularly when palpation was difficult. Ultrasound allows a detailed assessment of peripheral nerve continuity with a mass, differentiating an intraneural lesion from an adjacent extrinsic ganglion.13 Tonkin10 published the first description of MRI used for the diagnosis of an elbow cyst, and its use has been supported by others.5,8,20 The typical appearance of ganglion cysts on MRI include low signal on T1-weighted images and very high signal on T2-weighted images. Only the periphery of the mass is enhanced by gadolinium, if used.
As recently as 2009, Jou and associates13 suggested that surgical excision should be performed promptly to ensure optimal recovery from a nerve palsy. Many authors agree that early diagnosis and careful surgical excision is associated with a satisfactory outcome without recurrence of the cyst.5,6,8-10,12-15 There are only 4 published case reports14-17 of ganglions causing isolated compression of the superficial radial sensory nerve, as in our case. Their patients had pain with exertional trauma14 as did our patient, a positive Tinel sign,15 and resolution of symptoms after surgical excision without recurrence.14-16 Mileti and colleagues16 state that standard management for resistant radial tunnel syndrome is open decompression of the radial nerve.
In the last decade, a few reports of arthroscopic excision being a viable and safe alternative to open excision have been published.16,22,23 In 2000, Feldman22 described the benefits of an arthroscopic approach as decreased soft-tissue dissection, increased ability to identify intra-articular pathology, and similar recurrence rates to open procedures. He reported 1 transient neurapraxia of the superficial radial nerve from the arthroscopy, highlighting a risk of arthroscopic treatment.
An alternative to open or arthroscopic cyst decompression is aspiration. The only mention of aspiration in the literature comes from Broomhead19 in 1955 when he described 2 patients in whom treatment by aspiration was unsuccessful in relieving their symptoms. Yamazaki and colleagues12 noted that 1 of their 14 patients with PIN palsies caused by ganglions at the elbow underwent puncture of the ganglion with recovery of the paralysis. With the aid of ultrasound guidance, we were able to accurately locate the ganglion cyst, aspirate its contents, and inject methylprednisolone sodium succinate. Our patient continued to be symptom-free and was an active pitcher on a varsity softball team 26 months after aspiration.
Conclusion
This case report describes a rare location for a ganglion cyst in a high-level softball player. To our knowledge, successful treatment with ultrasound-guided aspiration and injection of a supinator cyst has not been reported in the literature. This case report highlights the importance of a careful diagnosis of this condition and an alternative treatment algorithm.
1. Boursinos LA, Dimitriou CG. Ulnar nerve compression in the cubital tunnel by an epineural ganglion: a case report. Hand (N Y). 2007;2(1):12-15.
2. Ferlic DC, Ries MD. Epineural ganglion of the ulnar nerve at the elbow. J Hand Surg Am. 1990;15(6):996-998.
3. Ming Chan K, Thompson S, Amirjani N, Satkunam L, Strohschlein FJ, Lobay GL. Compression of the ulnar nerve at the elbow by an intraneural ganglion. J Clin Neurosci. 2003;10(2):245-248.
4. Sharma RR, Pawar SJ, Delmendo A, Mahapatra AK. Symptomatic epineural ganglion cyst of the ulnar nerve in the cubital tunnel: a case report and brief review of the literature. J Clin Neurosci. 2000;7(6):542-543.
5. Hashizume H, Nishida K, Nanba Y, Inoue H, Konishiike T. Intraneural ganglion of the posterior interosseous nerve with lateral elbow pain. J Hand Surg Br. 1995;20(5):649-651.
6. Kato H, Hirayama T, Minami A, Iwasaki N, Hirachi K. Cubital tunnel syndrome associated with medial elbow Ganglia and osteoarthritis of the elbow. J Bone Joint Surg Am. 2002;84(8):1413-1419.
7. Bowen TL, Stone KH. Posterior interosseous nerve paralysis caused by a ganglion at the elbow. J Bone Joint Surg Br. 1966;48(4):774-776.
8. Ly JQ, Barrett TJ, Beall DP, Bertagnolli R. MRI diagnosis of occult ganglion compression of the posterior interosseous nerve and associated supinator muscle pathology. Clin Imaging. 2005;29(5):362-363.
9. McCollam SM, Corley FG, Green DP. Posterior interosseous nerve palsy caused by ganglions of the proximal radioulnar joint. J Hand Surg Am. 1988;13(5):725-728.
10. Tonkin MA. Posterior interosseous nerve axonotmesis from compression by a ganglion. J Hand Surg Br. 1990;15(4):491-493.
11. Tuygun H, Kose O, Gorgec M. Partial paralysis of the posterior interosseous nerve caused by a ganglion. J Hand Surg Eur. 2008;33(4):540-541.
12. Yamazaki H, Kato H, Hata Y, Murakami N, Saitoh S. The two locations of ganglions causing radial nerve palsy. J Hand Surg Eur. 2007;32(3):341-345.
13. Jou IM, Wang HN, Wang PH, Yong IS, Su WR. Compression of the radial nerve at the elbow by a ganglion: two case reports. J Med Case Rep. 2009;3:7258.
14. Hermansdorfer JD, Greider JL, Dell PC. A case report of a compressive neuropathy of the radial sensory nerve caused by a ganglion cyst at the elbow. Orthopedics. 1986;9(7):1005-1006.
15. McFarlane J, Trehan R, Olivera M, Jones C, Blease S, Davey P. A ganglion cyst at the elbow causing superficial radial nerve compression: a case report. J Med Case Rep. 2008;2:122.
16. Mileti J, Largacha M, O’Driscoll SW. Radial tunnel syndrome caused by ganglion cyst: treatment by arthroscopic cyst decompression. Arthroscopy. 2004;20(5):e39-e44.
17. Plancher KD, Peterson RK, Steichen JB. Compressive neuropathies and tendinopathies in the athletic elbow and wrist. Clin Sports Med. 1996;15(2):331-371.
18. Chim H, Yam AK, Teoh LC. Elbow ganglion arising from medial epicondyle pseudarthrosis. Hand Surg. 2007;12(3):155-158.
19. Broomhead IW. Ganglia associated with elbow and knee joints. Lancet. 1955;269(6885):317-319.
20. Ogino T, Minami A, Kato H. Diagnosis of radial nerve palsy caused by ganglion with use of different imaging techniques. J Hand Surg Am. 1991;16(2):230-235.
21. Spinner M, Spencer PS. Nerve compression lesions of the upper extremity. A clinical and experimental review. Clin Orthop Relat Res. 1974;(104):46-67.
22. Feldman MD. Arthroscopic excision of a ganglion cyst from the elbow. Arthroscopy. 2000;16(6):661-664.
23. Kirpalani PA, Lee HK, Lee YS, Han CW. Transarticular arthroscopic excision of an elbow cyst. Acta Orthop Belg. 2005;71(4):477-480.
1. Boursinos LA, Dimitriou CG. Ulnar nerve compression in the cubital tunnel by an epineural ganglion: a case report. Hand (N Y). 2007;2(1):12-15.
2. Ferlic DC, Ries MD. Epineural ganglion of the ulnar nerve at the elbow. J Hand Surg Am. 1990;15(6):996-998.
3. Ming Chan K, Thompson S, Amirjani N, Satkunam L, Strohschlein FJ, Lobay GL. Compression of the ulnar nerve at the elbow by an intraneural ganglion. J Clin Neurosci. 2003;10(2):245-248.
4. Sharma RR, Pawar SJ, Delmendo A, Mahapatra AK. Symptomatic epineural ganglion cyst of the ulnar nerve in the cubital tunnel: a case report and brief review of the literature. J Clin Neurosci. 2000;7(6):542-543.
5. Hashizume H, Nishida K, Nanba Y, Inoue H, Konishiike T. Intraneural ganglion of the posterior interosseous nerve with lateral elbow pain. J Hand Surg Br. 1995;20(5):649-651.
6. Kato H, Hirayama T, Minami A, Iwasaki N, Hirachi K. Cubital tunnel syndrome associated with medial elbow Ganglia and osteoarthritis of the elbow. J Bone Joint Surg Am. 2002;84(8):1413-1419.
7. Bowen TL, Stone KH. Posterior interosseous nerve paralysis caused by a ganglion at the elbow. J Bone Joint Surg Br. 1966;48(4):774-776.
8. Ly JQ, Barrett TJ, Beall DP, Bertagnolli R. MRI diagnosis of occult ganglion compression of the posterior interosseous nerve and associated supinator muscle pathology. Clin Imaging. 2005;29(5):362-363.
9. McCollam SM, Corley FG, Green DP. Posterior interosseous nerve palsy caused by ganglions of the proximal radioulnar joint. J Hand Surg Am. 1988;13(5):725-728.
10. Tonkin MA. Posterior interosseous nerve axonotmesis from compression by a ganglion. J Hand Surg Br. 1990;15(4):491-493.
11. Tuygun H, Kose O, Gorgec M. Partial paralysis of the posterior interosseous nerve caused by a ganglion. J Hand Surg Eur. 2008;33(4):540-541.
12. Yamazaki H, Kato H, Hata Y, Murakami N, Saitoh S. The two locations of ganglions causing radial nerve palsy. J Hand Surg Eur. 2007;32(3):341-345.
13. Jou IM, Wang HN, Wang PH, Yong IS, Su WR. Compression of the radial nerve at the elbow by a ganglion: two case reports. J Med Case Rep. 2009;3:7258.
14. Hermansdorfer JD, Greider JL, Dell PC. A case report of a compressive neuropathy of the radial sensory nerve caused by a ganglion cyst at the elbow. Orthopedics. 1986;9(7):1005-1006.
15. McFarlane J, Trehan R, Olivera M, Jones C, Blease S, Davey P. A ganglion cyst at the elbow causing superficial radial nerve compression: a case report. J Med Case Rep. 2008;2:122.
16. Mileti J, Largacha M, O’Driscoll SW. Radial tunnel syndrome caused by ganglion cyst: treatment by arthroscopic cyst decompression. Arthroscopy. 2004;20(5):e39-e44.
17. Plancher KD, Peterson RK, Steichen JB. Compressive neuropathies and tendinopathies in the athletic elbow and wrist. Clin Sports Med. 1996;15(2):331-371.
18. Chim H, Yam AK, Teoh LC. Elbow ganglion arising from medial epicondyle pseudarthrosis. Hand Surg. 2007;12(3):155-158.
19. Broomhead IW. Ganglia associated with elbow and knee joints. Lancet. 1955;269(6885):317-319.
20. Ogino T, Minami A, Kato H. Diagnosis of radial nerve palsy caused by ganglion with use of different imaging techniques. J Hand Surg Am. 1991;16(2):230-235.
21. Spinner M, Spencer PS. Nerve compression lesions of the upper extremity. A clinical and experimental review. Clin Orthop Relat Res. 1974;(104):46-67.
22. Feldman MD. Arthroscopic excision of a ganglion cyst from the elbow. Arthroscopy. 2000;16(6):661-664.
23. Kirpalani PA, Lee HK, Lee YS, Han CW. Transarticular arthroscopic excision of an elbow cyst. Acta Orthop Belg. 2005;71(4):477-480.
Abdominal distention • loss of appetite • elevated creatinine • Dx?
THE CASE
A 21-year-old male college student sought care at our urology clinic for a 2-year history of progressive abdominal distention and loss of appetite due to abdominal pressure. On physical examination, his abdomen was distended and tense, but without any tenderness on palpation or any costovertebral angle tenderness. He had no abdominal or flank pain, and wasn’t in acute distress. His blood pressure was normal.
Initial lab test results were significant for elevated creatinine at 2.7 mg/dL (normal: 0.7-1.3 mg/dL) and blood urea nitrogen (BUN) at 31.1 mg/dL (normal: 6-20 mg/dL). Results of a complete blood count (CBC) were within normal ranges, including a white blood cell (WBC) count of 7900, hemoglobin level of 15.1 g/dL, and platelet count of 217,000/mcL. A urinalysis showed only a mild increase in the WBC count.
THE DIAGNOSIS
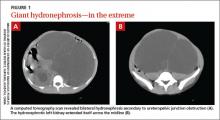
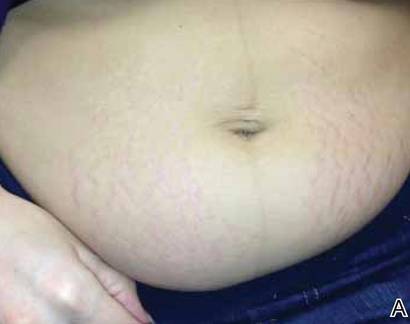
We performed a computed tomography (CT) scan of the patient’s abdomen, which revealed bilateral hydronephrosis secondary to ureteropelvic junction obstruction (UPJO). The patient’s right kidney was mildly to moderately enlarged, but the left kidney was massive (FIGURE 1A). The hydronephrotic left kidney had extended itself across the midline (FIGURE 1B), pushed the ipsilateral diaphragm upward, and displaced the bladder downward.
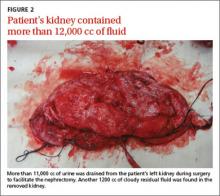
The patient underwent right-sided ureteral stent placement for temporary drainage and a complete left-sided nephrectomy. During the surgery, the left kidney was first aspirated, and more than 11,000 cc of clear urine was drained. (Aspiration reduced the kidney size, allowing the surgeon to make a smaller incision.) The removed kidney contained an additional 1200 cc of cloudy residual fluid (FIGURE 2). UPJO was confirmed by the pathological examination of the excised organ.
DISCUSSION
UPJO is the most common etiology for congenital hydronephrosis.1 Because it can cause little to no pain, hydronephrosis secondary to UPJO can be asymptomatic and may not present until later in life. Frequently, an abdominal mass is the initial clinical presentation.
When the hydronephrotic fluid exceeds 1000 cc, the condition is referred to as giant hydronephrosis.2 Although several cases of giant hydronephrosis secondary to UPJO have been reported in the medical literature,3-5 the volume of the hydronephrotic fluid in these cases rarely exceeded 10,000 cc. We believe our patient may be the most severe case of hydronephrosis secondary to bilateral UPJO, with 12,200 cc of fluid. His condition reached this late stage only because his right kidney retained adequate function.
Diagnosis of hydronephrosis is straightforward with an abdominal ultrasound and/or CT scan. Widespread use of abdominal ultrasound as a screening tool has significantly increased the diagnosis of asymptomatic hydronephrosis, and many cases are secondary to UPJO.6 The true incidence of UPJO is unknown, but it is more prevalent in males than in females, and in 10% to 40% of cases, the condition is bilateral.7 Congenital UPJO typically results from intrinsic pathology of the ureter. The diseased segment is often fibrotic, strictured, and aperistaltic.8
Treatment choice depends on whether renal function can be preserved
Treatment of hydronephrosis is straightforward; when there is little or no salvageable renal function (<10%), a simple nephrectomy is indicated, as was the case for our patient. Nephrectomy can be accomplished by either an open or laparoscopic approach.
When there is salvageable renal function, treatment options include pyeloplasty and pyelotomy. Traditionally, open dismembered pyeloplasty has been the gold standard. However, with advances in endoscopic and laparoscopic techniques, there has been a shift toward minimally invasive procedures. Laparoscopic pyeloplasty—with or without robotic assistance—and endoscopic pyelotomy—with either a percutaneous or retrograde approach—are now typically performed. Ureteral stenting should only be used as a temporary measure.
Our patient. Four weeks after the nephrectomy, our patient underwent a right side pyeloplasty, which was successful. He had an uneventful recovery from both procedures. His renal function stabilized and other than routine follow-up, he required no additional treatment.
THE TAKEAWAY
Most cases of hydronephrosis in young people are due to congenital abnormalities, and UPJO is the leading cause. However, the condition can be asymptomatic and may not present until later in life. Whenever a patient presents with an asymptomatic abdominal mass, hydronephrosis should be part of the differential diagnosis. Treatment options include nephrectomy when there is no salvageable kidney function or pyeloplasty and pyelotomy when some kidney function can be preserved.
1. Brown T, Mandell J, Lebowitz RL. Neonatal hydronephrosis in the era ultrasonography. AJR Am J Roentgenol. 1987;148:959-963.
2. Stirling WC. Massive hydronephrosis complicated by hydroureter: Report of 3 cases. J Urol. 1939;42:520.
3. Chiang PH, Chen MT, Chou YH, et al. Giant hydronephrosis: report of 4 cases with review of the literature. J Formos Med Assoc. 1990;89:811-817.
4. Aguiar MFM, Oliveira APS, Silva SC, et al. Giant hydronephrosis secondary to ureteropelvic junction obstruction. Gazzetta Medica Italiana-Archivio per le Scienze Mediche. 2009;168:207.
5. Sepulveda L, Rodriguesa F. Giant hydronephrosis - a late diagnosis of ureteropelvic junction obstruction. World J Nephrol Urol. 2013;2:33.
6. Bernstein GT, Mandell J, Lebowitz RL, et al. Ureteropelvic junction obstruction in neonate. J Urol. 1988;140:1216-1221.
7. Johnston JH, Evans JP, Glassberg KI, et al. Pelvic hydronephrosis in children: a review of 219 personal cases. J Urol. 1977;117:97-101.
8. Gosling JA, Dixon JS. Functional obstruction of the ureter and renal pelvis. A histological and electron microscopic study. Br J Urol. 1978;50:145-152.
THE CASE
A 21-year-old male college student sought care at our urology clinic for a 2-year history of progressive abdominal distention and loss of appetite due to abdominal pressure. On physical examination, his abdomen was distended and tense, but without any tenderness on palpation or any costovertebral angle tenderness. He had no abdominal or flank pain, and wasn’t in acute distress. His blood pressure was normal.
Initial lab test results were significant for elevated creatinine at 2.7 mg/dL (normal: 0.7-1.3 mg/dL) and blood urea nitrogen (BUN) at 31.1 mg/dL (normal: 6-20 mg/dL). Results of a complete blood count (CBC) were within normal ranges, including a white blood cell (WBC) count of 7900, hemoglobin level of 15.1 g/dL, and platelet count of 217,000/mcL. A urinalysis showed only a mild increase in the WBC count.
THE DIAGNOSIS
We performed a computed tomography (CT) scan of the patient’s abdomen, which revealed bilateral hydronephrosis secondary to ureteropelvic junction obstruction (UPJO). The patient’s right kidney was mildly to moderately enlarged, but the left kidney was massive (FIGURE 1A). The hydronephrotic left kidney had extended itself across the midline (FIGURE 1B), pushed the ipsilateral diaphragm upward, and displaced the bladder downward.
The patient underwent right-sided ureteral stent placement for temporary drainage and a complete left-sided nephrectomy. During the surgery, the left kidney was first aspirated, and more than 11,000 cc of clear urine was drained. (Aspiration reduced the kidney size, allowing the surgeon to make a smaller incision.) The removed kidney contained an additional 1200 cc of cloudy residual fluid (FIGURE 2). UPJO was confirmed by the pathological examination of the excised organ.
DISCUSSION
UPJO is the most common etiology for congenital hydronephrosis.1 Because it can cause little to no pain, hydronephrosis secondary to UPJO can be asymptomatic and may not present until later in life. Frequently, an abdominal mass is the initial clinical presentation.
When the hydronephrotic fluid exceeds 1000 cc, the condition is referred to as giant hydronephrosis.2 Although several cases of giant hydronephrosis secondary to UPJO have been reported in the medical literature,3-5 the volume of the hydronephrotic fluid in these cases rarely exceeded 10,000 cc. We believe our patient may be the most severe case of hydronephrosis secondary to bilateral UPJO, with 12,200 cc of fluid. His condition reached this late stage only because his right kidney retained adequate function.
Diagnosis of hydronephrosis is straightforward with an abdominal ultrasound and/or CT scan. Widespread use of abdominal ultrasound as a screening tool has significantly increased the diagnosis of asymptomatic hydronephrosis, and many cases are secondary to UPJO.6 The true incidence of UPJO is unknown, but it is more prevalent in males than in females, and in 10% to 40% of cases, the condition is bilateral.7 Congenital UPJO typically results from intrinsic pathology of the ureter. The diseased segment is often fibrotic, strictured, and aperistaltic.8
Treatment choice depends on whether renal function can be preserved
Treatment of hydronephrosis is straightforward; when there is little or no salvageable renal function (<10%), a simple nephrectomy is indicated, as was the case for our patient. Nephrectomy can be accomplished by either an open or laparoscopic approach.
When there is salvageable renal function, treatment options include pyeloplasty and pyelotomy. Traditionally, open dismembered pyeloplasty has been the gold standard. However, with advances in endoscopic and laparoscopic techniques, there has been a shift toward minimally invasive procedures. Laparoscopic pyeloplasty—with or without robotic assistance—and endoscopic pyelotomy—with either a percutaneous or retrograde approach—are now typically performed. Ureteral stenting should only be used as a temporary measure.
Our patient. Four weeks after the nephrectomy, our patient underwent a right side pyeloplasty, which was successful. He had an uneventful recovery from both procedures. His renal function stabilized and other than routine follow-up, he required no additional treatment.
THE TAKEAWAY
Most cases of hydronephrosis in young people are due to congenital abnormalities, and UPJO is the leading cause. However, the condition can be asymptomatic and may not present until later in life. Whenever a patient presents with an asymptomatic abdominal mass, hydronephrosis should be part of the differential diagnosis. Treatment options include nephrectomy when there is no salvageable kidney function or pyeloplasty and pyelotomy when some kidney function can be preserved.
THE CASE
A 21-year-old male college student sought care at our urology clinic for a 2-year history of progressive abdominal distention and loss of appetite due to abdominal pressure. On physical examination, his abdomen was distended and tense, but without any tenderness on palpation or any costovertebral angle tenderness. He had no abdominal or flank pain, and wasn’t in acute distress. His blood pressure was normal.
Initial lab test results were significant for elevated creatinine at 2.7 mg/dL (normal: 0.7-1.3 mg/dL) and blood urea nitrogen (BUN) at 31.1 mg/dL (normal: 6-20 mg/dL). Results of a complete blood count (CBC) were within normal ranges, including a white blood cell (WBC) count of 7900, hemoglobin level of 15.1 g/dL, and platelet count of 217,000/mcL. A urinalysis showed only a mild increase in the WBC count.
THE DIAGNOSIS
We performed a computed tomography (CT) scan of the patient’s abdomen, which revealed bilateral hydronephrosis secondary to ureteropelvic junction obstruction (UPJO). The patient’s right kidney was mildly to moderately enlarged, but the left kidney was massive (FIGURE 1A). The hydronephrotic left kidney had extended itself across the midline (FIGURE 1B), pushed the ipsilateral diaphragm upward, and displaced the bladder downward.
The patient underwent right-sided ureteral stent placement for temporary drainage and a complete left-sided nephrectomy. During the surgery, the left kidney was first aspirated, and more than 11,000 cc of clear urine was drained. (Aspiration reduced the kidney size, allowing the surgeon to make a smaller incision.) The removed kidney contained an additional 1200 cc of cloudy residual fluid (FIGURE 2). UPJO was confirmed by the pathological examination of the excised organ.
DISCUSSION
UPJO is the most common etiology for congenital hydronephrosis.1 Because it can cause little to no pain, hydronephrosis secondary to UPJO can be asymptomatic and may not present until later in life. Frequently, an abdominal mass is the initial clinical presentation.
When the hydronephrotic fluid exceeds 1000 cc, the condition is referred to as giant hydronephrosis.2 Although several cases of giant hydronephrosis secondary to UPJO have been reported in the medical literature,3-5 the volume of the hydronephrotic fluid in these cases rarely exceeded 10,000 cc. We believe our patient may be the most severe case of hydronephrosis secondary to bilateral UPJO, with 12,200 cc of fluid. His condition reached this late stage only because his right kidney retained adequate function.
Diagnosis of hydronephrosis is straightforward with an abdominal ultrasound and/or CT scan. Widespread use of abdominal ultrasound as a screening tool has significantly increased the diagnosis of asymptomatic hydronephrosis, and many cases are secondary to UPJO.6 The true incidence of UPJO is unknown, but it is more prevalent in males than in females, and in 10% to 40% of cases, the condition is bilateral.7 Congenital UPJO typically results from intrinsic pathology of the ureter. The diseased segment is often fibrotic, strictured, and aperistaltic.8
Treatment choice depends on whether renal function can be preserved
Treatment of hydronephrosis is straightforward; when there is little or no salvageable renal function (<10%), a simple nephrectomy is indicated, as was the case for our patient. Nephrectomy can be accomplished by either an open or laparoscopic approach.
When there is salvageable renal function, treatment options include pyeloplasty and pyelotomy. Traditionally, open dismembered pyeloplasty has been the gold standard. However, with advances in endoscopic and laparoscopic techniques, there has been a shift toward minimally invasive procedures. Laparoscopic pyeloplasty—with or without robotic assistance—and endoscopic pyelotomy—with either a percutaneous or retrograde approach—are now typically performed. Ureteral stenting should only be used as a temporary measure.
Our patient. Four weeks after the nephrectomy, our patient underwent a right side pyeloplasty, which was successful. He had an uneventful recovery from both procedures. His renal function stabilized and other than routine follow-up, he required no additional treatment.
THE TAKEAWAY
Most cases of hydronephrosis in young people are due to congenital abnormalities, and UPJO is the leading cause. However, the condition can be asymptomatic and may not present until later in life. Whenever a patient presents with an asymptomatic abdominal mass, hydronephrosis should be part of the differential diagnosis. Treatment options include nephrectomy when there is no salvageable kidney function or pyeloplasty and pyelotomy when some kidney function can be preserved.
1. Brown T, Mandell J, Lebowitz RL. Neonatal hydronephrosis in the era ultrasonography. AJR Am J Roentgenol. 1987;148:959-963.
2. Stirling WC. Massive hydronephrosis complicated by hydroureter: Report of 3 cases. J Urol. 1939;42:520.
3. Chiang PH, Chen MT, Chou YH, et al. Giant hydronephrosis: report of 4 cases with review of the literature. J Formos Med Assoc. 1990;89:811-817.
4. Aguiar MFM, Oliveira APS, Silva SC, et al. Giant hydronephrosis secondary to ureteropelvic junction obstruction. Gazzetta Medica Italiana-Archivio per le Scienze Mediche. 2009;168:207.
5. Sepulveda L, Rodriguesa F. Giant hydronephrosis - a late diagnosis of ureteropelvic junction obstruction. World J Nephrol Urol. 2013;2:33.
6. Bernstein GT, Mandell J, Lebowitz RL, et al. Ureteropelvic junction obstruction in neonate. J Urol. 1988;140:1216-1221.
7. Johnston JH, Evans JP, Glassberg KI, et al. Pelvic hydronephrosis in children: a review of 219 personal cases. J Urol. 1977;117:97-101.
8. Gosling JA, Dixon JS. Functional obstruction of the ureter and renal pelvis. A histological and electron microscopic study. Br J Urol. 1978;50:145-152.
1. Brown T, Mandell J, Lebowitz RL. Neonatal hydronephrosis in the era ultrasonography. AJR Am J Roentgenol. 1987;148:959-963.
2. Stirling WC. Massive hydronephrosis complicated by hydroureter: Report of 3 cases. J Urol. 1939;42:520.
3. Chiang PH, Chen MT, Chou YH, et al. Giant hydronephrosis: report of 4 cases with review of the literature. J Formos Med Assoc. 1990;89:811-817.
4. Aguiar MFM, Oliveira APS, Silva SC, et al. Giant hydronephrosis secondary to ureteropelvic junction obstruction. Gazzetta Medica Italiana-Archivio per le Scienze Mediche. 2009;168:207.
5. Sepulveda L, Rodriguesa F. Giant hydronephrosis - a late diagnosis of ureteropelvic junction obstruction. World J Nephrol Urol. 2013;2:33.
6. Bernstein GT, Mandell J, Lebowitz RL, et al. Ureteropelvic junction obstruction in neonate. J Urol. 1988;140:1216-1221.
7. Johnston JH, Evans JP, Glassberg KI, et al. Pelvic hydronephrosis in children: a review of 219 personal cases. J Urol. 1977;117:97-101.
8. Gosling JA, Dixon JS. Functional obstruction of the ureter and renal pelvis. A histological and electron microscopic study. Br J Urol. 1978;50:145-152.
Medical Stabilization and Clearance of the Psychiatric Patient
Dr Mallory is a professor of emergency medicine at the University of Louisville School of Medicine in Louisville, Kentucky. Mr Knight is a senior medical student at the University of Louisville School of Medicine, Kentucky.
Two Questions to Guide the Emergency Physician’s Workup:
Are the psychiatric symptoms caused or exacerbated by an underlying medical condition?
Are chronic medical conditions stable enough to be managed within the scope of a psychiatric facility?
Case Scenarios
Case 1
A 19-year-old college freshman presented to the ED, reporting poor sleep, loss of appetite, and generalized disinterest in school. She stated that she had been unable to concentrate on studying for her midterm exams. She further admitted to having thoughts of loneliness and suicide.
Case 2
Following a relationship break-up, a 28-year-old man with a history of bipolar disorder was brought to the ED after slitting both of his wrists. He was agitated and tremulous, stating that he “wanted to die.” His medications included divalproex and lithium.
Case 3
A 52-year-old woman with a medical history of hypertension, emphysema, and schizophrenia presented to the ED, asking to see the on-call psychiatrist because she did not feel the injection of intramuscular ziprasidone she had received the previous day was controlling her hallucinations. Moreover, she believed that the medication was causing the pain she was experiencing in her left shoulder.
Overview
Already challenged by the increasing medical complexity of patients, overcrowding, limited consultation availability, measurable quality outcomes expectations, and patient safety issues, emergency physicians (EPs) are also treating an increasing number of patients with behavioral health emergencies. At least 6% of all ED visits in 2001 were attributable to a primary psychiatric presentation, with steady annual increases seen over the past decade.1 In 2010, the Agency for Healthcare Research and Quality reported that 12.5% of ED visits were related to mental disorders and/or substance abuse.2
Initial assessments, management, and consultations on patients with behavioral health presentations occur daily in every ED. As the aforementioned brief case vignettes suggest, the EP is faced with a wide variability of their clinical presentations.
Limitations and Liabilities
For centuries, psychiatrists have sought medical causes of psychiatric problems, which alone might mandate a “medical clearance” for behavioral health patients who present with new or routine exacerbations of behavioral issues. Such “psych clearance” requests are routinely expected and performed in most EDs today. Despite efforts to define the term itself (see Box), to standardize patient selection for the process and to identify the appropriate depth of testing, a definition of “routine” screening for underlying medical causes of acute psychiatric presentations remains elusive. The goals of screening may differ between specialists in psychiatry and emergency medicine, and may vary by patient demographics, local expertise, practice patterns, and the variability of medical backup for psychiatrists in stand-alone psychiatric facilities.
The term medical clearance is vague, controversial, and often misinterpreted. Since it is not possible to screen and diagnose all potential concurrent medical illnesses in the ED, some authors prefer the terms “evaluation for medical stability,” or “focused medical assessment.”6 Defining expectations and limits of such an examination, screening for medical mimics, and developing a process for clear communication between providers that includes standard documentation of care are useful in establishing system-specific evaluations of care—and in building a consensus among providers regarding diagnostic testing in the ED.6-8
The literature demonstrates that little is gained from the routine EP screening of patients in need of psychiatric care, particularly screening beyond a focused medical examination.8-15 Unfortunately, firm conclusions are difficult to reach because published studies have neither uniformly documented the same elements of the histories, physical examinations, and diagnostic testing nor have they stratified the indications for laboratory investigations by the pretest probability of underlying disease. They also have not described whether or how disposition decisions would have been altered.
While much of the literature focuses on the controversies surrounding laboratory testing and a lack of standardized guidelines in the medical clearance process, decision-making errors and human bias are also reported. Consequences of prematurely anchoring presenting medical symptoms to a patient’s known psychiatric disorder may be compounded by key assessment procedures less often performed on patients admitted to psychiatric units compared with patients admitted to medical floors.10 The increasing burden on staff time by agitated patients and ”boarders” awaiting limited inpatient psychiatric beds, personal attitudes toward suicide and substance abuse, and the operational challenges of the medical clearance process itself all may adversely affect the care of these patients.16 In fact, the issue of disparate equality of emergency care for patients with behavioral health issues as compared to emergency medicine patients with medical and surgical complaints has been raised.17 While focused emergency care has been developed and implemented for medical presentations such as chest pain and multiple trauma, the lack of similar protocols for behavioral health emergencies may explain why complete assessments of psychiatric patients’ mental status or cognitive abilities are rarely performed and documented.18
Purposeful Medical Stabilization
Beginning with the end in mind, one purpose of the medical clearance process is to determine whether the psychiatric patient’s presentation is caused or exacerbated by a medical illness. Armed with the knowledge to recognize emergency conditions that present with undifferentiated symptoms (ie, medical mimics), along with the capability to treat exacerbations of chronic illness, the medical screening process for patients with acute psychiatric symptoms is not unlike the approach to any other patient in the ED—stabilize (or exclude) emergencies, provide comfort, and arrange a safe disposition. It is important to remember that psychiatric patients have a higher incidence of chronic medical conditions, are at greater risk of injury, and have a shorter lifespan than the general population. Viewing medical clearance in this larger context may help EPs avoid inappropriate diagnostic anchoring, provide a rationale framework for diagnostic testing, and build trust and rapport with both psychiatric and medical colleagues. The identification of medical urgencies prior to psychiatric admission can avert morbidity.7,19
Similar to any patient requiring hospital admission, making a safe disposition decision for a psychiatric patient must take into account the level of nursing care and monitoring needed for the patient. Once an emergency medical condition is excluded, the EP must assess whether the patient’s chronic medical conditions are stable enough to be managed on a psychiatric unit or in a freestanding psychiatric facility. Decisions to order additional tests, initiate or restart treatment for chronic conditions (eg, hypertension, diabetes), and make ongoing medical treatment recommendations can be done on a case-by-case basis and in direct communication with the primary care provider and consultants—in a similar fashion as for any ED patient.
Suggestions for Safe and Focused Medical Stabilization
Stratify Risk
Emergency physicians should pay attention to the patient’s vital signs. Retrospective evidence suggests the likelihood of an underlying medical cause of psychiatric symptoms is low in patients with normal vital signs, as well as those who have a known psychiatric history, who are younger than age 30 years, who have intact orientation, who have no visual hallucinations, and who show no evidence of an acute medical problem. Structured assessment and screening tools to assist in the medical clearance of psychiatric patients are becoming validated.20,21 Conversely, the EP should have a high index of suspicion that a patient's agitation is the result of an underlying medical condition when it is accompanied by abnormal vital signs, immunosuppression, and/or preexisting neurological disease.22
Suspect and Treat Medical Mimics
Suspected medical mimics should always be treated with specific attention to excluding or treating delirium. By definition, delirium is characterized by the acute onset of either a waxing and waning or fluctuating sensorium, and requires reexamination over time. Disorientation and memory difficulties are symptoms of impaired brain functioning and represent a medical emergency requiring acute assessment and treatment.
Many underlying medical and/or organic causes of psychiatric symptoms (eg, trauma, neurology, cardiology, infectious disease, endocrine metabolic/electrolyte function abnormalities, heavy metal poisoning, withdrawal syndromes) can cause delirium. Differentiating between delirium and dementia can prove particularly difficult in elderly patients. When in doubt, or in the absence of prior psychiatric history, the EP should assume an underlying medical cause for psychiatric symptoms and proceed with medical admission. In general, geriatric patients do not fare as well in psychiatric units compared to medical units.23
Search for Collateral Information
The history from a psychotic or agitated patient may be limited. Therefore, collateral history obtained from family, friends, staff, and prehospital providers can be very useful and even essential. A careful review of the patient’s past and current medication lists is important to identify side effects and can indicate subtle withdrawal syndromes.
Selectively Test After a Thorough Examination
Inadequate history and physical examinations are cited as leading contributors to missed underlying medical causes of illness in psychiatric presentations.24 While the Mini-Mental Status Exam has been widely used to uncover and characterize altered mental status in the elderly, the Quick Confusion Scale provides comparable results, is quicker to administer, and is thought to be more appropriate in the ED setting.25,26
Recognizing both the limitations and utility of focused laboratory and drug testing, the American College of Emergency Physicians’ clinical policy guidelines state that routine laboratory testing in adult psychiatric patients who are otherwise asymptomatic, alert, and cooperative is unnecessary. The patient’s cognitive abilities, rather than specific toxicological screening results, should guide the timing of psychiatric referral. Additionally, EPs may consider using a period of observation to determine if psychiatric symptoms resolve along with intoxication.5 Please make this a new paragraph. by consultants to obtain routine testing, urine toxicology screening and serum alcohol testing were felt to be more necessary than blood work.27 However, researchers in emergency medicine and emergency psychiatry acknowledge toxicological testing limitations. Routine urine assays do not test for many psychoactive substances and, depending upon the drug of interest, some assays may have poor sensitivities.28 Results of both retrospective and prospective studies show that drug screen results (or their absence entirely) did not change the disposition of emergency psychiatric patients.29,30 Frequent reassessment of the apparently intoxicated psychiatric patient with consultation as soon as he or she is capable of making decisions by demonstrating intact cognitive ability is good medicine and helps with throughput in both the ED and emergency psychiatry unit.
It remains unclear if other factors, such as exclusion of a medical mimic, new onset or a change in psychiatric symptoms, admission/reimbursement requirements for inpatient care, and the need for transfer to a freestanding psychiatric hospital, contribute to either the perceived need or true indication for urine drug screening and blood alcohol testing. There are opportunities for quality improvement in institutions with nonselective, “routine” laboratory testing requirements.31,32
Selectively Treat Agitation and Pain
The use of verbal de-escalation techniques and appropriately directed pharmacotherapy for the acutely agitated patient provides immediate safety, establishes the rapport necessary for effective history taking and physical examination cooperation, and should assist with the timely identification of underlying medical conditions. In some patients with underlying neurological and psychiatric conditions, acute pain may be poorly communicated or present only as agitation.33
In lieu of simply writing “medically cleared” on a patient’s chart and arranging an emergency psychiatric assessment, a direct phone call to the mental health provider, a structured transition of care document for the behavioral health team to reference, or a standard discharge summary of the ED workup that includes testing rationale and future management recommendations34 will facilitate continuity of care, prevent redundancy, and improve the overall care of psychiatric patients.
System Collaborations
Building alliances with hospital-based and community mental health providers based upon best practices would intuitively seem to benefit EDs and patients. The lack of established benchmarks of care remains a major impediment to quality assurance and performance improvement efforts in the provision of psychiatric emergency care, including the medical clearance process. Although currently lacking in application, the creation of a common standard for documenting, abstracting, and reporting the nature and management of psychiatric emergencies has been demonstrated.35
Both academic psychiatric emergency centers and the referral patterns of large community hospital systems can be complex, making not only access to care confusing or unnecessarily difficult for psychiatric patients, but also communication difficulties for caregivers. However, unique collaborative opportunities to streamline medical clearance and psychiatric assessment do exist.36 The Alameda Model, for example, provides guidelines—albeit funding challenges—for interdisciplinary triage and treatment processes that ultimately serve patients and decompresses the burden on EDs of caring for patients with psychiatric illness.37
Case Scenarios Continued
Case 1
[The 19-year-old college freshman with poor sleep, loss of appetite, and generalized disinterest in school]
Normal vital signs were noted at presentation and no past medical or psychiatric history was identified. The patient reported that she had drunk a beer at a party earlier that evening, believing that it would “cheer her up.” She expressed feelings of hopeless and stated that she had recurrent thoughts of jumping off her 5-story dormitory building.
The EP performed a physical examination, including a bedside pregnancy test, which was negative. Toxicological testing was not performed because the patient’s cognitive abilities were intact. Emergent psychiatric consultation was arranged.
Case 2
[The 28-year-old man with a history of bipolar disorder who presented with slit wrists]
On examination, the patient was found to be tachycardic and clinically dehydrated. Old records report cocaine abuse and noncompliance with medication and therapy. His agitation was treated with a benzodiazepine, and he was rehydrated with intravenous fluids. Laboratory studies included evaluation of electrolytes, creatinine, blood urea nitrogen, therapeutic drug levels, and a urine toxicological screen. An electrocardiogram (ECG) also was obtained. The patient’s lithium level was found to be significantly elevated, requiring dialysis. His wounds were sutured, and medical admission with an inpatient psychiatric consultation was arranged.
Case 3
[The 52-year-old woman with a medical history of hypertension, emphysema, and schizophrenia]
An ECG showed no evidence of ischemia. The patient’s caretaker brother reported his sister’s behavior seemed usual to him. He further noted that the patient had a past history of bursitis in her left shoulder. The patient denied any chest pain or shortness of breath. No evidence of substance abuse was identified in the medical record.
Further inquiry into the patient’s medical history revealed she had undergone cardiac catheterization for atypical chest pain 6 months prior, the results of which were normal. The patient’s vital signs remained normal. Except for reproducible pain on range of motion of the left shoulder, the physical examination was also normal. She was able to carry on a conversation with the EP about her favorite television shows, and she gave no indication of intent to harm herself or others. Nonsteroidal anti-inflammatory drugs were recommended to alleviate the shoulder pain, and the patient was discharged to her routine outpatient psychiatric follow-up.
Conclusion
Emergency physicians are trained to assess patients with undifferentiated presentations, including acute psychiatric complaints. Acute delirium should be identified and treated medically. Consideration of other medical mimics should be included through a careful review of vital signs, history, physical examination, and collateral information. As safety-net physicians, EPs should consider evaluating and treating comorbid medical conditions in psychiatric patients referred for medical clearance who may require psychiatric admission. As with any other ED presentation, the EP should maintain a low threshold for testing in high-risk patients.
Routine medical clearance as a prerequisite for psychiatric assessment of patients presenting with acute psychiatric symptoms who are at low-risk for underlying medical causes is costly and unsupported by the literature. Toxicological testing may be indicated in select instances, but rarely alters patient disposition. Through collaboration, education, and the development of assessment protocols, EPs and psychiatry specialists can minimize testing and provide safe, efficient care of patients with acute psychiatric presentations.
Dr Mallory is a professor of emergency medicine at the University of Louisville School of Medicine in Louisville, Kentucky. Mr Knight is a senior medical student at the University of Louisville School of Medicine, Kentucky.
- Larkin GL, Claassen CA, Emond JA, Pelletier AJ, Camargo CA. Trends in U.S. emergency department visits for mental health conditions, 1992 to 2001. Psychiatr Serv. 2005;56(6):671-677.
- Owens, PL, Mutter, R, Stocks, C. Mental Health and Substance Abuse-Related Emergency Department Visits among Adults, 2007. Statistical Brief #92. Rockville, MD: Agency for Healthcare Research and Quality; 2010.
- American College of Emergency Physicians. ACEP Emergency Medicine Practice Committee. Care of the Psychiatric Patient in the Emergency Department—A Review of the Literature. 2014. http://www.acep.org/uploadedFiles/ACEP/Clinical_and_Practice_Management/Resources/Mental_Health_and_Substance_Abuse/Psychiatric%20Patient%20Care%20in%20the%20ED%202014.pdf. Accessed July 13, 2015.
- Emembolu FN, Zun LS. Medical clearance in the emergency department: Is testing indicated? Prim Psych. 2010;17(6):29-34. http://primarypsychiatry.com/wp-content/uploads/import/0610PP_Emembolu.pdf. Accessed July 13, 2015.
- Lukens TW, Wolf SJ, Edlow JA, et al; American College of Emergency Physicians Clinical Policies Subcommittee (Writing Committee) on Critical Issues in the Diagnosis and Management of the Adult Psychiatric Patient in the Emergency Department. Clinical policy: critical issues in the diagnosis and management of the adult psychiatric patient in the emergency department. Ann Emerg Med. 2006;47(1):79-99.
- Zun LS. Evidence-based evaluation of psychiatric patients. J Emerg Med. 2005;28(1):35-39.
- Gregory RJ, Nihalani ND, Rodriguez E. Medical screening in the emergency department for psychiatric admissions: a procedural analysis. Gen Hosp Psychiatry. 2004;26(5):405-410.
- Zun LS, Hernandez R, Thompson R, Downey L. Comparison of EPs’ and psychiatrists’ laboratory assessment of psychiatric patients. Am J Emerg Med. 2004;22(3):175-180.
- Henneman PL, Mendoza R, Lewis RJ. Prospective evaluation of emergency department medical clearance. Ann Emerg Med. 1994;24(4):672-677.
- Reeves RR, Pendarvis EJ, Kimble R. Unrecognized medical emergencies admitted to psychiatric units. Am J Emerg Med. 2000;18(4):390-393.
- Han JH, Zimmerman EE, Cutler N, et al. Delirium in older emergency department patients: recognition, risk factors, and psychomotor subtypes. Acad Emerg Med. 2009;16(3):193-200.
- Janiak BD, Atteberry S. Medical clearance of the psychiatric patient in the emergency department. J Emerg Med. 2012;43(5):866-870.
- Olshaker JS, Browne B, Jerrard DA, Prendergast H, Stair TO. Medical clearance and screening of psychiatric patients in the emergency department. Acad Emerg Med. 1997;4(2):124-128.
- Korn CS, Currier GW, Henderson SO. “Medical clearance” of psychiatric patients without medical complaints in the Emergency Department. J Emerg Med. 2000;18(2):173-176.
- Amin M, Wang J. Routine laboratory testing to evaluate for medical illness in psychiatric patients in the emergency department is largely unrevealing. West J Emerg Med. 2009;10(2):97-100.
- Zun LS. Pitfalls in the care of the psychiatric patient in the emergency department. J Emerg Med. 2012;43(5):829-835.
- Zun LS. An issue of equity of care: psychiatric patients must be treated “on par” with medical patients. Am J Psychiatry. 2014;171(7):716-719.
- Szpakowicz M, Herd A. “Medically cleared”: how well are patients with psychiatric presentations examined by emergency physicians? J Emerg Med. 2008;35(4):369-372.
- Sood TR, Mcstay CM. Evaluation of the psychiatric patient. Emerg Med Clin North Am. 2009;27(4): 669-683, ix.
- Shah SJ, Fiorito M, McNamara RM. A screening tool to medically clear psychiatric patients in the emergency department. J Emerg Med. 2012;43(5):871-875.
- Miller AC, Frei SP, Rupp VA, Joho BS, Miller KM, Bond WF. Validation of a triage algorithm for psychiatric screening (TAPS) for patients with psychiatric chief complaints. J Am Osteopath Assoc. 2012;112(8):502-508.
- Nordstrom K, Zun LS, Wilson MP, et al. Medical evaluation and triage of the agitated patient: consensus statement of the american association for emergency psychiatry project Beta medical evaluation workgroup. West J Emerg Med. 2012;13(1):3-10.
- Reeves RR, Parker JD, Burke RS, Hart RH. Inappropriate psychiatric admission of elderly patients with unrecognized delirium. South Med J. 2010;103(2):111-115.
- Koranyi EK, Potoczny WM. Physical illnesses underlying psychiatric symptoms. Psychother Psychosom. 1992;58(3-4):155-160.
- Dziedzic L, Brady WJ, Lindsay R, Huff JS. The use of the mini-mental status examination in the ED evaluation of the elderly. Am J Emerg Med. 1998;16(7):686-689.
- Huff JS, Farace E, Brady WJ, Kheir J, Shawver G. The quick confusion scale in the ED: comparison with the mini-mental state examination. Am J Emerg Med. 2001;19(6):461-464.
- Broderick KB, Lerner EB, McCourt JD, Fraser E, Salerno K. Emergency physician practices and requirements regarding the medical screening examination of psychiatric patients. Acad Emerg Med. 2002;9(1):88-92.
- 2agøien G, Morken G, Zahlsen K, Aamo T, Spigset O. Evaluation of a urine on-site drugs of abuse screening test in patients admitted to a psychiatric emergency unit. J Clin Psychopharmacol. 2009;29(3):248-254.
- Fortu JM, Kim IK, Cooper A, Condra C, Lorenz DJ, Pierce MC. Psychiatric patients in the pediatric emergency department undergoing routine urine toxicology screens for medical clearance: results and use. Pediatr Emerg Care. 2009;25(6):387-392.
- Eisen JS, Sivilotti ML, Boyd KU, Barton DG, Fortier CJ, Collier CP. Screening urine for drugs of abuse in the emergency department: do test results affect physicians’ patient care decisions? CJEM. 2004;6(2):104-111.
- Donofrio JJ, Santillanes G, McCammack BD, et al. Clinical utility of screening laboratory tests in pediatric psychiatric patients presenting to the emergency department for medical clearance. Ann Emerg Med. 2014;63(6):666-675.
- Donofrio JJ, Horeczko T, Kaji A, Santillanes G, Claudius I. Most routine laboratory testing of pediatric psychiatric patients in the emergency department is not medically necessary. Health Aff (Millwood). 2015;34(5):812-818.
- Zun LS, Downey LV. Level of agitation of psychiatric patients presenting to an emergency department. Prim Care Companion J Clin Psychiatry. 2008;10(2):108-113.
- Tintinalli JE, Peacock FW 4th, Wright MA. Emergency medical evaluation of psychiatric patients. Ann Emerg Med. 1994;23(4):859-862.
- Boudreaux ED, Allen MH, Claassen C, et al. The Psychiatric Emergency Research Collaboration-01: methods and results. Gen Hosp Psychiatry. 2009;31(6):515-522.
- Currier GW, Allen M. Organization and function of academic psychiatric emergency services. Gen Hosp Psychiatry. 2003;25(2):124-129.
- Moulin A, Jones K. The alameda model: an effort worth emulating. West J Emerg Med. 2014;15(1):7-8.
Dr Mallory is a professor of emergency medicine at the University of Louisville School of Medicine in Louisville, Kentucky. Mr Knight is a senior medical student at the University of Louisville School of Medicine, Kentucky.
Two Questions to Guide the Emergency Physician’s Workup:
Are the psychiatric symptoms caused or exacerbated by an underlying medical condition?
Are chronic medical conditions stable enough to be managed within the scope of a psychiatric facility?
Case Scenarios
Case 1
A 19-year-old college freshman presented to the ED, reporting poor sleep, loss of appetite, and generalized disinterest in school. She stated that she had been unable to concentrate on studying for her midterm exams. She further admitted to having thoughts of loneliness and suicide.
Case 2
Following a relationship break-up, a 28-year-old man with a history of bipolar disorder was brought to the ED after slitting both of his wrists. He was agitated and tremulous, stating that he “wanted to die.” His medications included divalproex and lithium.
Case 3
A 52-year-old woman with a medical history of hypertension, emphysema, and schizophrenia presented to the ED, asking to see the on-call psychiatrist because she did not feel the injection of intramuscular ziprasidone she had received the previous day was controlling her hallucinations. Moreover, she believed that the medication was causing the pain she was experiencing in her left shoulder.
Overview
Already challenged by the increasing medical complexity of patients, overcrowding, limited consultation availability, measurable quality outcomes expectations, and patient safety issues, emergency physicians (EPs) are also treating an increasing number of patients with behavioral health emergencies. At least 6% of all ED visits in 2001 were attributable to a primary psychiatric presentation, with steady annual increases seen over the past decade.1 In 2010, the Agency for Healthcare Research and Quality reported that 12.5% of ED visits were related to mental disorders and/or substance abuse.2
Initial assessments, management, and consultations on patients with behavioral health presentations occur daily in every ED. As the aforementioned brief case vignettes suggest, the EP is faced with a wide variability of their clinical presentations.
Limitations and Liabilities
For centuries, psychiatrists have sought medical causes of psychiatric problems, which alone might mandate a “medical clearance” for behavioral health patients who present with new or routine exacerbations of behavioral issues. Such “psych clearance” requests are routinely expected and performed in most EDs today. Despite efforts to define the term itself (see Box), to standardize patient selection for the process and to identify the appropriate depth of testing, a definition of “routine” screening for underlying medical causes of acute psychiatric presentations remains elusive. The goals of screening may differ between specialists in psychiatry and emergency medicine, and may vary by patient demographics, local expertise, practice patterns, and the variability of medical backup for psychiatrists in stand-alone psychiatric facilities.
The term medical clearance is vague, controversial, and often misinterpreted. Since it is not possible to screen and diagnose all potential concurrent medical illnesses in the ED, some authors prefer the terms “evaluation for medical stability,” or “focused medical assessment.”6 Defining expectations and limits of such an examination, screening for medical mimics, and developing a process for clear communication between providers that includes standard documentation of care are useful in establishing system-specific evaluations of care—and in building a consensus among providers regarding diagnostic testing in the ED.6-8
The literature demonstrates that little is gained from the routine EP screening of patients in need of psychiatric care, particularly screening beyond a focused medical examination.8-15 Unfortunately, firm conclusions are difficult to reach because published studies have neither uniformly documented the same elements of the histories, physical examinations, and diagnostic testing nor have they stratified the indications for laboratory investigations by the pretest probability of underlying disease. They also have not described whether or how disposition decisions would have been altered.
While much of the literature focuses on the controversies surrounding laboratory testing and a lack of standardized guidelines in the medical clearance process, decision-making errors and human bias are also reported. Consequences of prematurely anchoring presenting medical symptoms to a patient’s known psychiatric disorder may be compounded by key assessment procedures less often performed on patients admitted to psychiatric units compared with patients admitted to medical floors.10 The increasing burden on staff time by agitated patients and ”boarders” awaiting limited inpatient psychiatric beds, personal attitudes toward suicide and substance abuse, and the operational challenges of the medical clearance process itself all may adversely affect the care of these patients.16 In fact, the issue of disparate equality of emergency care for patients with behavioral health issues as compared to emergency medicine patients with medical and surgical complaints has been raised.17 While focused emergency care has been developed and implemented for medical presentations such as chest pain and multiple trauma, the lack of similar protocols for behavioral health emergencies may explain why complete assessments of psychiatric patients’ mental status or cognitive abilities are rarely performed and documented.18
Purposeful Medical Stabilization
Beginning with the end in mind, one purpose of the medical clearance process is to determine whether the psychiatric patient’s presentation is caused or exacerbated by a medical illness. Armed with the knowledge to recognize emergency conditions that present with undifferentiated symptoms (ie, medical mimics), along with the capability to treat exacerbations of chronic illness, the medical screening process for patients with acute psychiatric symptoms is not unlike the approach to any other patient in the ED—stabilize (or exclude) emergencies, provide comfort, and arrange a safe disposition. It is important to remember that psychiatric patients have a higher incidence of chronic medical conditions, are at greater risk of injury, and have a shorter lifespan than the general population. Viewing medical clearance in this larger context may help EPs avoid inappropriate diagnostic anchoring, provide a rationale framework for diagnostic testing, and build trust and rapport with both psychiatric and medical colleagues. The identification of medical urgencies prior to psychiatric admission can avert morbidity.7,19
Similar to any patient requiring hospital admission, making a safe disposition decision for a psychiatric patient must take into account the level of nursing care and monitoring needed for the patient. Once an emergency medical condition is excluded, the EP must assess whether the patient’s chronic medical conditions are stable enough to be managed on a psychiatric unit or in a freestanding psychiatric facility. Decisions to order additional tests, initiate or restart treatment for chronic conditions (eg, hypertension, diabetes), and make ongoing medical treatment recommendations can be done on a case-by-case basis and in direct communication with the primary care provider and consultants—in a similar fashion as for any ED patient.
Suggestions for Safe and Focused Medical Stabilization
Stratify Risk
Emergency physicians should pay attention to the patient’s vital signs. Retrospective evidence suggests the likelihood of an underlying medical cause of psychiatric symptoms is low in patients with normal vital signs, as well as those who have a known psychiatric history, who are younger than age 30 years, who have intact orientation, who have no visual hallucinations, and who show no evidence of an acute medical problem. Structured assessment and screening tools to assist in the medical clearance of psychiatric patients are becoming validated.20,21 Conversely, the EP should have a high index of suspicion that a patient's agitation is the result of an underlying medical condition when it is accompanied by abnormal vital signs, immunosuppression, and/or preexisting neurological disease.22
Suspect and Treat Medical Mimics
Suspected medical mimics should always be treated with specific attention to excluding or treating delirium. By definition, delirium is characterized by the acute onset of either a waxing and waning or fluctuating sensorium, and requires reexamination over time. Disorientation and memory difficulties are symptoms of impaired brain functioning and represent a medical emergency requiring acute assessment and treatment.
Many underlying medical and/or organic causes of psychiatric symptoms (eg, trauma, neurology, cardiology, infectious disease, endocrine metabolic/electrolyte function abnormalities, heavy metal poisoning, withdrawal syndromes) can cause delirium. Differentiating between delirium and dementia can prove particularly difficult in elderly patients. When in doubt, or in the absence of prior psychiatric history, the EP should assume an underlying medical cause for psychiatric symptoms and proceed with medical admission. In general, geriatric patients do not fare as well in psychiatric units compared to medical units.23
Search for Collateral Information
The history from a psychotic or agitated patient may be limited. Therefore, collateral history obtained from family, friends, staff, and prehospital providers can be very useful and even essential. A careful review of the patient’s past and current medication lists is important to identify side effects and can indicate subtle withdrawal syndromes.
Selectively Test After a Thorough Examination
Inadequate history and physical examinations are cited as leading contributors to missed underlying medical causes of illness in psychiatric presentations.24 While the Mini-Mental Status Exam has been widely used to uncover and characterize altered mental status in the elderly, the Quick Confusion Scale provides comparable results, is quicker to administer, and is thought to be more appropriate in the ED setting.25,26
Recognizing both the limitations and utility of focused laboratory and drug testing, the American College of Emergency Physicians’ clinical policy guidelines state that routine laboratory testing in adult psychiatric patients who are otherwise asymptomatic, alert, and cooperative is unnecessary. The patient’s cognitive abilities, rather than specific toxicological screening results, should guide the timing of psychiatric referral. Additionally, EPs may consider using a period of observation to determine if psychiatric symptoms resolve along with intoxication.5 Please make this a new paragraph. by consultants to obtain routine testing, urine toxicology screening and serum alcohol testing were felt to be more necessary than blood work.27 However, researchers in emergency medicine and emergency psychiatry acknowledge toxicological testing limitations. Routine urine assays do not test for many psychoactive substances and, depending upon the drug of interest, some assays may have poor sensitivities.28 Results of both retrospective and prospective studies show that drug screen results (or their absence entirely) did not change the disposition of emergency psychiatric patients.29,30 Frequent reassessment of the apparently intoxicated psychiatric patient with consultation as soon as he or she is capable of making decisions by demonstrating intact cognitive ability is good medicine and helps with throughput in both the ED and emergency psychiatry unit.
It remains unclear if other factors, such as exclusion of a medical mimic, new onset or a change in psychiatric symptoms, admission/reimbursement requirements for inpatient care, and the need for transfer to a freestanding psychiatric hospital, contribute to either the perceived need or true indication for urine drug screening and blood alcohol testing. There are opportunities for quality improvement in institutions with nonselective, “routine” laboratory testing requirements.31,32
Selectively Treat Agitation and Pain
The use of verbal de-escalation techniques and appropriately directed pharmacotherapy for the acutely agitated patient provides immediate safety, establishes the rapport necessary for effective history taking and physical examination cooperation, and should assist with the timely identification of underlying medical conditions. In some patients with underlying neurological and psychiatric conditions, acute pain may be poorly communicated or present only as agitation.33
In lieu of simply writing “medically cleared” on a patient’s chart and arranging an emergency psychiatric assessment, a direct phone call to the mental health provider, a structured transition of care document for the behavioral health team to reference, or a standard discharge summary of the ED workup that includes testing rationale and future management recommendations34 will facilitate continuity of care, prevent redundancy, and improve the overall care of psychiatric patients.
System Collaborations
Building alliances with hospital-based and community mental health providers based upon best practices would intuitively seem to benefit EDs and patients. The lack of established benchmarks of care remains a major impediment to quality assurance and performance improvement efforts in the provision of psychiatric emergency care, including the medical clearance process. Although currently lacking in application, the creation of a common standard for documenting, abstracting, and reporting the nature and management of psychiatric emergencies has been demonstrated.35
Both academic psychiatric emergency centers and the referral patterns of large community hospital systems can be complex, making not only access to care confusing or unnecessarily difficult for psychiatric patients, but also communication difficulties for caregivers. However, unique collaborative opportunities to streamline medical clearance and psychiatric assessment do exist.36 The Alameda Model, for example, provides guidelines—albeit funding challenges—for interdisciplinary triage and treatment processes that ultimately serve patients and decompresses the burden on EDs of caring for patients with psychiatric illness.37
Case Scenarios Continued
Case 1
[The 19-year-old college freshman with poor sleep, loss of appetite, and generalized disinterest in school]
Normal vital signs were noted at presentation and no past medical or psychiatric history was identified. The patient reported that she had drunk a beer at a party earlier that evening, believing that it would “cheer her up.” She expressed feelings of hopeless and stated that she had recurrent thoughts of jumping off her 5-story dormitory building.
The EP performed a physical examination, including a bedside pregnancy test, which was negative. Toxicological testing was not performed because the patient’s cognitive abilities were intact. Emergent psychiatric consultation was arranged.
Case 2
[The 28-year-old man with a history of bipolar disorder who presented with slit wrists]
On examination, the patient was found to be tachycardic and clinically dehydrated. Old records report cocaine abuse and noncompliance with medication and therapy. His agitation was treated with a benzodiazepine, and he was rehydrated with intravenous fluids. Laboratory studies included evaluation of electrolytes, creatinine, blood urea nitrogen, therapeutic drug levels, and a urine toxicological screen. An electrocardiogram (ECG) also was obtained. The patient’s lithium level was found to be significantly elevated, requiring dialysis. His wounds were sutured, and medical admission with an inpatient psychiatric consultation was arranged.
Case 3
[The 52-year-old woman with a medical history of hypertension, emphysema, and schizophrenia]
An ECG showed no evidence of ischemia. The patient’s caretaker brother reported his sister’s behavior seemed usual to him. He further noted that the patient had a past history of bursitis in her left shoulder. The patient denied any chest pain or shortness of breath. No evidence of substance abuse was identified in the medical record.
Further inquiry into the patient’s medical history revealed she had undergone cardiac catheterization for atypical chest pain 6 months prior, the results of which were normal. The patient’s vital signs remained normal. Except for reproducible pain on range of motion of the left shoulder, the physical examination was also normal. She was able to carry on a conversation with the EP about her favorite television shows, and she gave no indication of intent to harm herself or others. Nonsteroidal anti-inflammatory drugs were recommended to alleviate the shoulder pain, and the patient was discharged to her routine outpatient psychiatric follow-up.
Conclusion
Emergency physicians are trained to assess patients with undifferentiated presentations, including acute psychiatric complaints. Acute delirium should be identified and treated medically. Consideration of other medical mimics should be included through a careful review of vital signs, history, physical examination, and collateral information. As safety-net physicians, EPs should consider evaluating and treating comorbid medical conditions in psychiatric patients referred for medical clearance who may require psychiatric admission. As with any other ED presentation, the EP should maintain a low threshold for testing in high-risk patients.
Routine medical clearance as a prerequisite for psychiatric assessment of patients presenting with acute psychiatric symptoms who are at low-risk for underlying medical causes is costly and unsupported by the literature. Toxicological testing may be indicated in select instances, but rarely alters patient disposition. Through collaboration, education, and the development of assessment protocols, EPs and psychiatry specialists can minimize testing and provide safe, efficient care of patients with acute psychiatric presentations.
Dr Mallory is a professor of emergency medicine at the University of Louisville School of Medicine in Louisville, Kentucky. Mr Knight is a senior medical student at the University of Louisville School of Medicine, Kentucky.
Dr Mallory is a professor of emergency medicine at the University of Louisville School of Medicine in Louisville, Kentucky. Mr Knight is a senior medical student at the University of Louisville School of Medicine, Kentucky.
Two Questions to Guide the Emergency Physician’s Workup:
Are the psychiatric symptoms caused or exacerbated by an underlying medical condition?
Are chronic medical conditions stable enough to be managed within the scope of a psychiatric facility?
Case Scenarios
Case 1
A 19-year-old college freshman presented to the ED, reporting poor sleep, loss of appetite, and generalized disinterest in school. She stated that she had been unable to concentrate on studying for her midterm exams. She further admitted to having thoughts of loneliness and suicide.
Case 2
Following a relationship break-up, a 28-year-old man with a history of bipolar disorder was brought to the ED after slitting both of his wrists. He was agitated and tremulous, stating that he “wanted to die.” His medications included divalproex and lithium.
Case 3
A 52-year-old woman with a medical history of hypertension, emphysema, and schizophrenia presented to the ED, asking to see the on-call psychiatrist because she did not feel the injection of intramuscular ziprasidone she had received the previous day was controlling her hallucinations. Moreover, she believed that the medication was causing the pain she was experiencing in her left shoulder.
Overview
Already challenged by the increasing medical complexity of patients, overcrowding, limited consultation availability, measurable quality outcomes expectations, and patient safety issues, emergency physicians (EPs) are also treating an increasing number of patients with behavioral health emergencies. At least 6% of all ED visits in 2001 were attributable to a primary psychiatric presentation, with steady annual increases seen over the past decade.1 In 2010, the Agency for Healthcare Research and Quality reported that 12.5% of ED visits were related to mental disorders and/or substance abuse.2
Initial assessments, management, and consultations on patients with behavioral health presentations occur daily in every ED. As the aforementioned brief case vignettes suggest, the EP is faced with a wide variability of their clinical presentations.
Limitations and Liabilities
For centuries, psychiatrists have sought medical causes of psychiatric problems, which alone might mandate a “medical clearance” for behavioral health patients who present with new or routine exacerbations of behavioral issues. Such “psych clearance” requests are routinely expected and performed in most EDs today. Despite efforts to define the term itself (see Box), to standardize patient selection for the process and to identify the appropriate depth of testing, a definition of “routine” screening for underlying medical causes of acute psychiatric presentations remains elusive. The goals of screening may differ between specialists in psychiatry and emergency medicine, and may vary by patient demographics, local expertise, practice patterns, and the variability of medical backup for psychiatrists in stand-alone psychiatric facilities.
The term medical clearance is vague, controversial, and often misinterpreted. Since it is not possible to screen and diagnose all potential concurrent medical illnesses in the ED, some authors prefer the terms “evaluation for medical stability,” or “focused medical assessment.”6 Defining expectations and limits of such an examination, screening for medical mimics, and developing a process for clear communication between providers that includes standard documentation of care are useful in establishing system-specific evaluations of care—and in building a consensus among providers regarding diagnostic testing in the ED.6-8
The literature demonstrates that little is gained from the routine EP screening of patients in need of psychiatric care, particularly screening beyond a focused medical examination.8-15 Unfortunately, firm conclusions are difficult to reach because published studies have neither uniformly documented the same elements of the histories, physical examinations, and diagnostic testing nor have they stratified the indications for laboratory investigations by the pretest probability of underlying disease. They also have not described whether or how disposition decisions would have been altered.
While much of the literature focuses on the controversies surrounding laboratory testing and a lack of standardized guidelines in the medical clearance process, decision-making errors and human bias are also reported. Consequences of prematurely anchoring presenting medical symptoms to a patient’s known psychiatric disorder may be compounded by key assessment procedures less often performed on patients admitted to psychiatric units compared with patients admitted to medical floors.10 The increasing burden on staff time by agitated patients and ”boarders” awaiting limited inpatient psychiatric beds, personal attitudes toward suicide and substance abuse, and the operational challenges of the medical clearance process itself all may adversely affect the care of these patients.16 In fact, the issue of disparate equality of emergency care for patients with behavioral health issues as compared to emergency medicine patients with medical and surgical complaints has been raised.17 While focused emergency care has been developed and implemented for medical presentations such as chest pain and multiple trauma, the lack of similar protocols for behavioral health emergencies may explain why complete assessments of psychiatric patients’ mental status or cognitive abilities are rarely performed and documented.18
Purposeful Medical Stabilization
Beginning with the end in mind, one purpose of the medical clearance process is to determine whether the psychiatric patient’s presentation is caused or exacerbated by a medical illness. Armed with the knowledge to recognize emergency conditions that present with undifferentiated symptoms (ie, medical mimics), along with the capability to treat exacerbations of chronic illness, the medical screening process for patients with acute psychiatric symptoms is not unlike the approach to any other patient in the ED—stabilize (or exclude) emergencies, provide comfort, and arrange a safe disposition. It is important to remember that psychiatric patients have a higher incidence of chronic medical conditions, are at greater risk of injury, and have a shorter lifespan than the general population. Viewing medical clearance in this larger context may help EPs avoid inappropriate diagnostic anchoring, provide a rationale framework for diagnostic testing, and build trust and rapport with both psychiatric and medical colleagues. The identification of medical urgencies prior to psychiatric admission can avert morbidity.7,19
Similar to any patient requiring hospital admission, making a safe disposition decision for a psychiatric patient must take into account the level of nursing care and monitoring needed for the patient. Once an emergency medical condition is excluded, the EP must assess whether the patient’s chronic medical conditions are stable enough to be managed on a psychiatric unit or in a freestanding psychiatric facility. Decisions to order additional tests, initiate or restart treatment for chronic conditions (eg, hypertension, diabetes), and make ongoing medical treatment recommendations can be done on a case-by-case basis and in direct communication with the primary care provider and consultants—in a similar fashion as for any ED patient.
Suggestions for Safe and Focused Medical Stabilization
Stratify Risk
Emergency physicians should pay attention to the patient’s vital signs. Retrospective evidence suggests the likelihood of an underlying medical cause of psychiatric symptoms is low in patients with normal vital signs, as well as those who have a known psychiatric history, who are younger than age 30 years, who have intact orientation, who have no visual hallucinations, and who show no evidence of an acute medical problem. Structured assessment and screening tools to assist in the medical clearance of psychiatric patients are becoming validated.20,21 Conversely, the EP should have a high index of suspicion that a patient's agitation is the result of an underlying medical condition when it is accompanied by abnormal vital signs, immunosuppression, and/or preexisting neurological disease.22
Suspect and Treat Medical Mimics
Suspected medical mimics should always be treated with specific attention to excluding or treating delirium. By definition, delirium is characterized by the acute onset of either a waxing and waning or fluctuating sensorium, and requires reexamination over time. Disorientation and memory difficulties are symptoms of impaired brain functioning and represent a medical emergency requiring acute assessment and treatment.
Many underlying medical and/or organic causes of psychiatric symptoms (eg, trauma, neurology, cardiology, infectious disease, endocrine metabolic/electrolyte function abnormalities, heavy metal poisoning, withdrawal syndromes) can cause delirium. Differentiating between delirium and dementia can prove particularly difficult in elderly patients. When in doubt, or in the absence of prior psychiatric history, the EP should assume an underlying medical cause for psychiatric symptoms and proceed with medical admission. In general, geriatric patients do not fare as well in psychiatric units compared to medical units.23
Search for Collateral Information
The history from a psychotic or agitated patient may be limited. Therefore, collateral history obtained from family, friends, staff, and prehospital providers can be very useful and even essential. A careful review of the patient’s past and current medication lists is important to identify side effects and can indicate subtle withdrawal syndromes.
Selectively Test After a Thorough Examination
Inadequate history and physical examinations are cited as leading contributors to missed underlying medical causes of illness in psychiatric presentations.24 While the Mini-Mental Status Exam has been widely used to uncover and characterize altered mental status in the elderly, the Quick Confusion Scale provides comparable results, is quicker to administer, and is thought to be more appropriate in the ED setting.25,26
Recognizing both the limitations and utility of focused laboratory and drug testing, the American College of Emergency Physicians’ clinical policy guidelines state that routine laboratory testing in adult psychiatric patients who are otherwise asymptomatic, alert, and cooperative is unnecessary. The patient’s cognitive abilities, rather than specific toxicological screening results, should guide the timing of psychiatric referral. Additionally, EPs may consider using a period of observation to determine if psychiatric symptoms resolve along with intoxication.5 Please make this a new paragraph. by consultants to obtain routine testing, urine toxicology screening and serum alcohol testing were felt to be more necessary than blood work.27 However, researchers in emergency medicine and emergency psychiatry acknowledge toxicological testing limitations. Routine urine assays do not test for many psychoactive substances and, depending upon the drug of interest, some assays may have poor sensitivities.28 Results of both retrospective and prospective studies show that drug screen results (or their absence entirely) did not change the disposition of emergency psychiatric patients.29,30 Frequent reassessment of the apparently intoxicated psychiatric patient with consultation as soon as he or she is capable of making decisions by demonstrating intact cognitive ability is good medicine and helps with throughput in both the ED and emergency psychiatry unit.
It remains unclear if other factors, such as exclusion of a medical mimic, new onset or a change in psychiatric symptoms, admission/reimbursement requirements for inpatient care, and the need for transfer to a freestanding psychiatric hospital, contribute to either the perceived need or true indication for urine drug screening and blood alcohol testing. There are opportunities for quality improvement in institutions with nonselective, “routine” laboratory testing requirements.31,32
Selectively Treat Agitation and Pain
The use of verbal de-escalation techniques and appropriately directed pharmacotherapy for the acutely agitated patient provides immediate safety, establishes the rapport necessary for effective history taking and physical examination cooperation, and should assist with the timely identification of underlying medical conditions. In some patients with underlying neurological and psychiatric conditions, acute pain may be poorly communicated or present only as agitation.33
In lieu of simply writing “medically cleared” on a patient’s chart and arranging an emergency psychiatric assessment, a direct phone call to the mental health provider, a structured transition of care document for the behavioral health team to reference, or a standard discharge summary of the ED workup that includes testing rationale and future management recommendations34 will facilitate continuity of care, prevent redundancy, and improve the overall care of psychiatric patients.
System Collaborations
Building alliances with hospital-based and community mental health providers based upon best practices would intuitively seem to benefit EDs and patients. The lack of established benchmarks of care remains a major impediment to quality assurance and performance improvement efforts in the provision of psychiatric emergency care, including the medical clearance process. Although currently lacking in application, the creation of a common standard for documenting, abstracting, and reporting the nature and management of psychiatric emergencies has been demonstrated.35
Both academic psychiatric emergency centers and the referral patterns of large community hospital systems can be complex, making not only access to care confusing or unnecessarily difficult for psychiatric patients, but also communication difficulties for caregivers. However, unique collaborative opportunities to streamline medical clearance and psychiatric assessment do exist.36 The Alameda Model, for example, provides guidelines—albeit funding challenges—for interdisciplinary triage and treatment processes that ultimately serve patients and decompresses the burden on EDs of caring for patients with psychiatric illness.37
Case Scenarios Continued
Case 1
[The 19-year-old college freshman with poor sleep, loss of appetite, and generalized disinterest in school]
Normal vital signs were noted at presentation and no past medical or psychiatric history was identified. The patient reported that she had drunk a beer at a party earlier that evening, believing that it would “cheer her up.” She expressed feelings of hopeless and stated that she had recurrent thoughts of jumping off her 5-story dormitory building.
The EP performed a physical examination, including a bedside pregnancy test, which was negative. Toxicological testing was not performed because the patient’s cognitive abilities were intact. Emergent psychiatric consultation was arranged.
Case 2
[The 28-year-old man with a history of bipolar disorder who presented with slit wrists]
On examination, the patient was found to be tachycardic and clinically dehydrated. Old records report cocaine abuse and noncompliance with medication and therapy. His agitation was treated with a benzodiazepine, and he was rehydrated with intravenous fluids. Laboratory studies included evaluation of electrolytes, creatinine, blood urea nitrogen, therapeutic drug levels, and a urine toxicological screen. An electrocardiogram (ECG) also was obtained. The patient’s lithium level was found to be significantly elevated, requiring dialysis. His wounds were sutured, and medical admission with an inpatient psychiatric consultation was arranged.
Case 3
[The 52-year-old woman with a medical history of hypertension, emphysema, and schizophrenia]
An ECG showed no evidence of ischemia. The patient’s caretaker brother reported his sister’s behavior seemed usual to him. He further noted that the patient had a past history of bursitis in her left shoulder. The patient denied any chest pain or shortness of breath. No evidence of substance abuse was identified in the medical record.
Further inquiry into the patient’s medical history revealed she had undergone cardiac catheterization for atypical chest pain 6 months prior, the results of which were normal. The patient’s vital signs remained normal. Except for reproducible pain on range of motion of the left shoulder, the physical examination was also normal. She was able to carry on a conversation with the EP about her favorite television shows, and she gave no indication of intent to harm herself or others. Nonsteroidal anti-inflammatory drugs were recommended to alleviate the shoulder pain, and the patient was discharged to her routine outpatient psychiatric follow-up.
Conclusion
Emergency physicians are trained to assess patients with undifferentiated presentations, including acute psychiatric complaints. Acute delirium should be identified and treated medically. Consideration of other medical mimics should be included through a careful review of vital signs, history, physical examination, and collateral information. As safety-net physicians, EPs should consider evaluating and treating comorbid medical conditions in psychiatric patients referred for medical clearance who may require psychiatric admission. As with any other ED presentation, the EP should maintain a low threshold for testing in high-risk patients.
Routine medical clearance as a prerequisite for psychiatric assessment of patients presenting with acute psychiatric symptoms who are at low-risk for underlying medical causes is costly and unsupported by the literature. Toxicological testing may be indicated in select instances, but rarely alters patient disposition. Through collaboration, education, and the development of assessment protocols, EPs and psychiatry specialists can minimize testing and provide safe, efficient care of patients with acute psychiatric presentations.
Dr Mallory is a professor of emergency medicine at the University of Louisville School of Medicine in Louisville, Kentucky. Mr Knight is a senior medical student at the University of Louisville School of Medicine, Kentucky.
- Larkin GL, Claassen CA, Emond JA, Pelletier AJ, Camargo CA. Trends in U.S. emergency department visits for mental health conditions, 1992 to 2001. Psychiatr Serv. 2005;56(6):671-677.
- Owens, PL, Mutter, R, Stocks, C. Mental Health and Substance Abuse-Related Emergency Department Visits among Adults, 2007. Statistical Brief #92. Rockville, MD: Agency for Healthcare Research and Quality; 2010.
- American College of Emergency Physicians. ACEP Emergency Medicine Practice Committee. Care of the Psychiatric Patient in the Emergency Department—A Review of the Literature. 2014. http://www.acep.org/uploadedFiles/ACEP/Clinical_and_Practice_Management/Resources/Mental_Health_and_Substance_Abuse/Psychiatric%20Patient%20Care%20in%20the%20ED%202014.pdf. Accessed July 13, 2015.
- Emembolu FN, Zun LS. Medical clearance in the emergency department: Is testing indicated? Prim Psych. 2010;17(6):29-34. http://primarypsychiatry.com/wp-content/uploads/import/0610PP_Emembolu.pdf. Accessed July 13, 2015.
- Lukens TW, Wolf SJ, Edlow JA, et al; American College of Emergency Physicians Clinical Policies Subcommittee (Writing Committee) on Critical Issues in the Diagnosis and Management of the Adult Psychiatric Patient in the Emergency Department. Clinical policy: critical issues in the diagnosis and management of the adult psychiatric patient in the emergency department. Ann Emerg Med. 2006;47(1):79-99.
- Zun LS. Evidence-based evaluation of psychiatric patients. J Emerg Med. 2005;28(1):35-39.
- Gregory RJ, Nihalani ND, Rodriguez E. Medical screening in the emergency department for psychiatric admissions: a procedural analysis. Gen Hosp Psychiatry. 2004;26(5):405-410.
- Zun LS, Hernandez R, Thompson R, Downey L. Comparison of EPs’ and psychiatrists’ laboratory assessment of psychiatric patients. Am J Emerg Med. 2004;22(3):175-180.
- Henneman PL, Mendoza R, Lewis RJ. Prospective evaluation of emergency department medical clearance. Ann Emerg Med. 1994;24(4):672-677.
- Reeves RR, Pendarvis EJ, Kimble R. Unrecognized medical emergencies admitted to psychiatric units. Am J Emerg Med. 2000;18(4):390-393.
- Han JH, Zimmerman EE, Cutler N, et al. Delirium in older emergency department patients: recognition, risk factors, and psychomotor subtypes. Acad Emerg Med. 2009;16(3):193-200.
- Janiak BD, Atteberry S. Medical clearance of the psychiatric patient in the emergency department. J Emerg Med. 2012;43(5):866-870.
- Olshaker JS, Browne B, Jerrard DA, Prendergast H, Stair TO. Medical clearance and screening of psychiatric patients in the emergency department. Acad Emerg Med. 1997;4(2):124-128.
- Korn CS, Currier GW, Henderson SO. “Medical clearance” of psychiatric patients without medical complaints in the Emergency Department. J Emerg Med. 2000;18(2):173-176.
- Amin M, Wang J. Routine laboratory testing to evaluate for medical illness in psychiatric patients in the emergency department is largely unrevealing. West J Emerg Med. 2009;10(2):97-100.
- Zun LS. Pitfalls in the care of the psychiatric patient in the emergency department. J Emerg Med. 2012;43(5):829-835.
- Zun LS. An issue of equity of care: psychiatric patients must be treated “on par” with medical patients. Am J Psychiatry. 2014;171(7):716-719.
- Szpakowicz M, Herd A. “Medically cleared”: how well are patients with psychiatric presentations examined by emergency physicians? J Emerg Med. 2008;35(4):369-372.
- Sood TR, Mcstay CM. Evaluation of the psychiatric patient. Emerg Med Clin North Am. 2009;27(4): 669-683, ix.
- Shah SJ, Fiorito M, McNamara RM. A screening tool to medically clear psychiatric patients in the emergency department. J Emerg Med. 2012;43(5):871-875.
- Miller AC, Frei SP, Rupp VA, Joho BS, Miller KM, Bond WF. Validation of a triage algorithm for psychiatric screening (TAPS) for patients with psychiatric chief complaints. J Am Osteopath Assoc. 2012;112(8):502-508.
- Nordstrom K, Zun LS, Wilson MP, et al. Medical evaluation and triage of the agitated patient: consensus statement of the american association for emergency psychiatry project Beta medical evaluation workgroup. West J Emerg Med. 2012;13(1):3-10.
- Reeves RR, Parker JD, Burke RS, Hart RH. Inappropriate psychiatric admission of elderly patients with unrecognized delirium. South Med J. 2010;103(2):111-115.
- Koranyi EK, Potoczny WM. Physical illnesses underlying psychiatric symptoms. Psychother Psychosom. 1992;58(3-4):155-160.
- Dziedzic L, Brady WJ, Lindsay R, Huff JS. The use of the mini-mental status examination in the ED evaluation of the elderly. Am J Emerg Med. 1998;16(7):686-689.
- Huff JS, Farace E, Brady WJ, Kheir J, Shawver G. The quick confusion scale in the ED: comparison with the mini-mental state examination. Am J Emerg Med. 2001;19(6):461-464.
- Broderick KB, Lerner EB, McCourt JD, Fraser E, Salerno K. Emergency physician practices and requirements regarding the medical screening examination of psychiatric patients. Acad Emerg Med. 2002;9(1):88-92.
- 2agøien G, Morken G, Zahlsen K, Aamo T, Spigset O. Evaluation of a urine on-site drugs of abuse screening test in patients admitted to a psychiatric emergency unit. J Clin Psychopharmacol. 2009;29(3):248-254.
- Fortu JM, Kim IK, Cooper A, Condra C, Lorenz DJ, Pierce MC. Psychiatric patients in the pediatric emergency department undergoing routine urine toxicology screens for medical clearance: results and use. Pediatr Emerg Care. 2009;25(6):387-392.
- Eisen JS, Sivilotti ML, Boyd KU, Barton DG, Fortier CJ, Collier CP. Screening urine for drugs of abuse in the emergency department: do test results affect physicians’ patient care decisions? CJEM. 2004;6(2):104-111.
- Donofrio JJ, Santillanes G, McCammack BD, et al. Clinical utility of screening laboratory tests in pediatric psychiatric patients presenting to the emergency department for medical clearance. Ann Emerg Med. 2014;63(6):666-675.
- Donofrio JJ, Horeczko T, Kaji A, Santillanes G, Claudius I. Most routine laboratory testing of pediatric psychiatric patients in the emergency department is not medically necessary. Health Aff (Millwood). 2015;34(5):812-818.
- Zun LS, Downey LV. Level of agitation of psychiatric patients presenting to an emergency department. Prim Care Companion J Clin Psychiatry. 2008;10(2):108-113.
- Tintinalli JE, Peacock FW 4th, Wright MA. Emergency medical evaluation of psychiatric patients. Ann Emerg Med. 1994;23(4):859-862.
- Boudreaux ED, Allen MH, Claassen C, et al. The Psychiatric Emergency Research Collaboration-01: methods and results. Gen Hosp Psychiatry. 2009;31(6):515-522.
- Currier GW, Allen M. Organization and function of academic psychiatric emergency services. Gen Hosp Psychiatry. 2003;25(2):124-129.
- Moulin A, Jones K. The alameda model: an effort worth emulating. West J Emerg Med. 2014;15(1):7-8.
- Larkin GL, Claassen CA, Emond JA, Pelletier AJ, Camargo CA. Trends in U.S. emergency department visits for mental health conditions, 1992 to 2001. Psychiatr Serv. 2005;56(6):671-677.
- Owens, PL, Mutter, R, Stocks, C. Mental Health and Substance Abuse-Related Emergency Department Visits among Adults, 2007. Statistical Brief #92. Rockville, MD: Agency for Healthcare Research and Quality; 2010.
- American College of Emergency Physicians. ACEP Emergency Medicine Practice Committee. Care of the Psychiatric Patient in the Emergency Department—A Review of the Literature. 2014. http://www.acep.org/uploadedFiles/ACEP/Clinical_and_Practice_Management/Resources/Mental_Health_and_Substance_Abuse/Psychiatric%20Patient%20Care%20in%20the%20ED%202014.pdf. Accessed July 13, 2015.
- Emembolu FN, Zun LS. Medical clearance in the emergency department: Is testing indicated? Prim Psych. 2010;17(6):29-34. http://primarypsychiatry.com/wp-content/uploads/import/0610PP_Emembolu.pdf. Accessed July 13, 2015.
- Lukens TW, Wolf SJ, Edlow JA, et al; American College of Emergency Physicians Clinical Policies Subcommittee (Writing Committee) on Critical Issues in the Diagnosis and Management of the Adult Psychiatric Patient in the Emergency Department. Clinical policy: critical issues in the diagnosis and management of the adult psychiatric patient in the emergency department. Ann Emerg Med. 2006;47(1):79-99.
- Zun LS. Evidence-based evaluation of psychiatric patients. J Emerg Med. 2005;28(1):35-39.
- Gregory RJ, Nihalani ND, Rodriguez E. Medical screening in the emergency department for psychiatric admissions: a procedural analysis. Gen Hosp Psychiatry. 2004;26(5):405-410.
- Zun LS, Hernandez R, Thompson R, Downey L. Comparison of EPs’ and psychiatrists’ laboratory assessment of psychiatric patients. Am J Emerg Med. 2004;22(3):175-180.
- Henneman PL, Mendoza R, Lewis RJ. Prospective evaluation of emergency department medical clearance. Ann Emerg Med. 1994;24(4):672-677.
- Reeves RR, Pendarvis EJ, Kimble R. Unrecognized medical emergencies admitted to psychiatric units. Am J Emerg Med. 2000;18(4):390-393.
- Han JH, Zimmerman EE, Cutler N, et al. Delirium in older emergency department patients: recognition, risk factors, and psychomotor subtypes. Acad Emerg Med. 2009;16(3):193-200.
- Janiak BD, Atteberry S. Medical clearance of the psychiatric patient in the emergency department. J Emerg Med. 2012;43(5):866-870.
- Olshaker JS, Browne B, Jerrard DA, Prendergast H, Stair TO. Medical clearance and screening of psychiatric patients in the emergency department. Acad Emerg Med. 1997;4(2):124-128.
- Korn CS, Currier GW, Henderson SO. “Medical clearance” of psychiatric patients without medical complaints in the Emergency Department. J Emerg Med. 2000;18(2):173-176.
- Amin M, Wang J. Routine laboratory testing to evaluate for medical illness in psychiatric patients in the emergency department is largely unrevealing. West J Emerg Med. 2009;10(2):97-100.
- Zun LS. Pitfalls in the care of the psychiatric patient in the emergency department. J Emerg Med. 2012;43(5):829-835.
- Zun LS. An issue of equity of care: psychiatric patients must be treated “on par” with medical patients. Am J Psychiatry. 2014;171(7):716-719.
- Szpakowicz M, Herd A. “Medically cleared”: how well are patients with psychiatric presentations examined by emergency physicians? J Emerg Med. 2008;35(4):369-372.
- Sood TR, Mcstay CM. Evaluation of the psychiatric patient. Emerg Med Clin North Am. 2009;27(4): 669-683, ix.
- Shah SJ, Fiorito M, McNamara RM. A screening tool to medically clear psychiatric patients in the emergency department. J Emerg Med. 2012;43(5):871-875.
- Miller AC, Frei SP, Rupp VA, Joho BS, Miller KM, Bond WF. Validation of a triage algorithm for psychiatric screening (TAPS) for patients with psychiatric chief complaints. J Am Osteopath Assoc. 2012;112(8):502-508.
- Nordstrom K, Zun LS, Wilson MP, et al. Medical evaluation and triage of the agitated patient: consensus statement of the american association for emergency psychiatry project Beta medical evaluation workgroup. West J Emerg Med. 2012;13(1):3-10.
- Reeves RR, Parker JD, Burke RS, Hart RH. Inappropriate psychiatric admission of elderly patients with unrecognized delirium. South Med J. 2010;103(2):111-115.
- Koranyi EK, Potoczny WM. Physical illnesses underlying psychiatric symptoms. Psychother Psychosom. 1992;58(3-4):155-160.
- Dziedzic L, Brady WJ, Lindsay R, Huff JS. The use of the mini-mental status examination in the ED evaluation of the elderly. Am J Emerg Med. 1998;16(7):686-689.
- Huff JS, Farace E, Brady WJ, Kheir J, Shawver G. The quick confusion scale in the ED: comparison with the mini-mental state examination. Am J Emerg Med. 2001;19(6):461-464.
- Broderick KB, Lerner EB, McCourt JD, Fraser E, Salerno K. Emergency physician practices and requirements regarding the medical screening examination of psychiatric patients. Acad Emerg Med. 2002;9(1):88-92.
- 2agøien G, Morken G, Zahlsen K, Aamo T, Spigset O. Evaluation of a urine on-site drugs of abuse screening test in patients admitted to a psychiatric emergency unit. J Clin Psychopharmacol. 2009;29(3):248-254.
- Fortu JM, Kim IK, Cooper A, Condra C, Lorenz DJ, Pierce MC. Psychiatric patients in the pediatric emergency department undergoing routine urine toxicology screens for medical clearance: results and use. Pediatr Emerg Care. 2009;25(6):387-392.
- Eisen JS, Sivilotti ML, Boyd KU, Barton DG, Fortier CJ, Collier CP. Screening urine for drugs of abuse in the emergency department: do test results affect physicians’ patient care decisions? CJEM. 2004;6(2):104-111.
- Donofrio JJ, Santillanes G, McCammack BD, et al. Clinical utility of screening laboratory tests in pediatric psychiatric patients presenting to the emergency department for medical clearance. Ann Emerg Med. 2014;63(6):666-675.
- Donofrio JJ, Horeczko T, Kaji A, Santillanes G, Claudius I. Most routine laboratory testing of pediatric psychiatric patients in the emergency department is not medically necessary. Health Aff (Millwood). 2015;34(5):812-818.
- Zun LS, Downey LV. Level of agitation of psychiatric patients presenting to an emergency department. Prim Care Companion J Clin Psychiatry. 2008;10(2):108-113.
- Tintinalli JE, Peacock FW 4th, Wright MA. Emergency medical evaluation of psychiatric patients. Ann Emerg Med. 1994;23(4):859-862.
- Boudreaux ED, Allen MH, Claassen C, et al. The Psychiatric Emergency Research Collaboration-01: methods and results. Gen Hosp Psychiatry. 2009;31(6):515-522.
- Currier GW, Allen M. Organization and function of academic psychiatric emergency services. Gen Hosp Psychiatry. 2003;25(2):124-129.
- Moulin A, Jones K. The alameda model: an effort worth emulating. West J Emerg Med. 2014;15(1):7-8.
Case Studies in Toxicology: When Doing More for the Sake of Better Health Goes Wrong
Case
A 62-year-old man with a history of hypercholesterolemia and HIV infection presented to the ED for evaluation of diffuse myalgia and tea-colored urine. His medication history included lopinavir/ritonavir (Kaletra) and simvastatin. A week prior to presentation, the patient’s primary care physician had instructed him to increase his daily dose of simvastatin from 40 mg to 80 mg. The patient stated that he had taken simvastatin 80 mg daily for approximately 5 days and then, 2 days prior to presentation, had independently further increased the dose to 160 mg daily.
In the ED, the patient reported feeling fatigued. His initial vital signs were: blood pressure, 129/86 mm Hg; heart rate, 93 beats/minute; respiratory rate, 17 breaths/minute; and temperature, 98.5˚F. Oxygen saturation was 98% on room air. His physical examination was unremarkable. Initial laboratory testing revealed the following: creatine kinase (CK) 350,000 U/L; blood urea nitrogen, 27 mg/dL; creatinine, 0.7 mg/dL; aspartate aminotransferase (AST), 2,950 U/L; and alanine aminotransferase (ALT), 1,305 U/L.
What can cause tea-colored/cola-colored urine and myalgia?
Numerous medications can result in dark-colored urine. These include antimalarial drugs such as chloroquine and primaquine; antibiotics such as metronidazole or nitrofurantoin; and the muscle relaxant methocarbamol. Myalgia and tea-colored urine are the hallmarks of rhabdomyolysis. Rhabdomyolysis involves the destruction of myocytes, which can occur as a result of a long list of processes, including crush injuries, poor oxygenation or perfusion, hypermetabolic states, and direct (or indirect) toxin-mediated myocyte damage.1 The list of toxic substances that can cause rhabdomyolysis is extensive, and statins are one of the most common drug-induced causes (Table).
Simvastatin is one of seven currently available 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors (ie, statins) that are commonly used to treat hypercholesterolemia. Because simvastatin is lipophilic, it can more readily cross cell membranes than nonlipophilic statins such as pravastatin. Simvastatin, therefore, has a propensity to disrupt the cellular integrity of myocytes and hepatocytes.What is the likely cause of this patient’s rhabdomyolysis?
At doses greater than 40 mg daily, simvastatin is associated with myalgia, myositis, and rhabdomyolysis. In December 2011, the US Food and Drug Administration (FDA) released a drug safety announcement recommending the originally approved maximum daily dose of simvastatin 80 mg be limited to patients who have already tolerated that dose for at least 12 months without evidence of muscular injury. The FDA further recommended no new patients be escalated to this dose. According to the FDA, patients taking 80 mg of simvastatin daily are also at increased risk of myopathy.
The metabolism of simvastatin, in addition to increased dosage of the drug, contributes to its potential for adverse effects. Of the seven available statins, only atorvastatin, lovastatin, and simvastatin are metabolized by the cytochrome P450 3A4 (CYP3A4). Lovastatin and simvastatin appear to have the highest potential for drug-drug interactions when coadministered with drugs that inhibit this enzyme (eg, ritonavir).2 The resulting elevation in blood concentration of simvastatin increases the risk of rhabdomyolysis. Other nonlipophilic statins, such as pravastatin, which are mostly eliminated unchanged in the urine and bile, would be preferable for patients taking CYP3A4 inhibitors.
How should patients with rhabdomyolysis be monitored?
Statins interfere with the myocyte’s ability to produce adenosine triphosphate, most likely by depleting coenzyme Q—one of the complexes found in the electron transport chain of the mitochondria. Under conditions of a high-energy requirement, myocytes incapable of producing sufficient energy ultimately fail and lyse, releasing cellular contents such as CK and myoglobin.1 The serum CK activity serves as a marker of muscle injury and should be monitored closely in patients with rhabdomyolysis. Although values above 5,000 U/L has been associated with renal injury,4 in healthy patients with access to hydration, renal injury is relatively uncommon with CK activities less than 50,000 U/L. Even though the prediction of renal failure is difficult, a validated nephrotoxicity prediction instrument using the patient’s age, gender, and initial laboratory data (serum creatinine, calcium, CK, phosphate, and bicarbonate) is available.5
Although the association between rhabdomyolysis and acute renal injury is well established, the mechanism remains unclear. Myoglobin from skeletal myocytes passes through the glomerulus without causing damage and is reabsorbed in the proximal renal tubular cell. Iron is subsequently released from the porphyrin ring and, in large concentrations, exceeds the binding capacity of the tissue ferritin. Because it is a transition metal, the free iron ion participates in oxidant stress reactions causing direct injury to the renal tubular cells.6 Furthermore, myoglobin also combines with renal tubular proteins, a process enhanced by an environment with lower pH, to form casts and cause renal tubular obstruction.
Patients with rhabdomyolysis may also be at risk for aminotransferase elevation, as occurred in the patient presented here. This elevation is most likely due to myocyte injury. In addition, potassium release due to myocyte destruction may cause life-threatening hyperkalemia, and phosphate liberation from these myocytes may cause hypocalcemia. Laboratory monitoring along with an electrocardiogram should be performed as required.
What is the treatment for rhabdomyolysis?
No adequate randomized controlled trials exist to guide the treatment of patients with rhabdomyolysis. As a result, recommendations for management come from retrospective observational studies, animal studies, case reports, and expert opinion.7
Once airway, breathing, and circulation have been addressed, patients with statin-induced rhabdomyolysis should be immediately treated with intravenous (IV) fluids to maintain renal perfusion, which helps to limit acute renal injury. Normal saline appears to be the most recommended fluid type, with a goal of maintaining a urine output of approximately 3 to 5 mL/kg/h.4,7
Some recommendations include the use of a sodium bicarbonate infusion to raise the urine pH, which may help limit the formation of renal casts from myoglobin. The data to support the benefit of sodium bicarbonate, however, is weak.3 A 2013 systematic review indicated that sodium bicarbonate should only be used to treat severe metabolic acidosis in patients with rhabdomyolysis.4
In addition to sodium bicarbonate, the use of diuretics is also discouraged by current recommendations. In patients with refractory electrolyte abnormalities or renal failure, hemodialysis may be required. Before disposition of a patient, his or her medication list should be reconciled to reflect statin discontinuation.
Case Conclusion
The patient received IV normal saline to maintain his urine output at 2 to 3 cc/kg/h. His repeat creatinine was 0.8 mg/dL and remained stable on repeat testing. His CK and AST concentrations trended down during his hospitalization. On hospital day 4, laboratory values were CK, less than 10,000 U/L; AST, 56 U/L; and ALT, 23 U/L. He had normal serum potassium levels and no dysrhythmia on electrocardiogram. His symptoms resolved on hospital day 2, and he was discharged on hospital day 4 with instructions to discontinue simvastatin.
Dr Fernandez is a senior toxicology fellow, department of emergency medicine, New York University School of Medicine. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
- Bench-to-bedside review: Rhabdomyolysis—an overview for clinicians. Crit Care. 2005;9(2):158-169.
- Chauvin B, Drouot S, Barrail-Tran A, Taburet AM. Drug-drug interactions between HMG-CoA reductase inhibitors (statins) and antiviral protease inhibitors. Clin Pharmacokinet. 2013;52(10):815-831.
- Brown CV, Rhee P, Chan L, Evans K, Demetriades D, Velmahos GC. Preventing renal failure in patients with rhabdomyolysis: do bicarbonate and mannitol make a difference? J Trauma. 2004;56(6):1191-1196.
- Scharman EJ, Troutman WG. Prevention of kidney injury following rhabdomyolysis: a systematic review. Ann Pharmacother. 2013;47(1):90-105.
- McMahon GM, Zeng X, Waikar SS. A risk prediction score for kidney failure or mortality in rhabdomyolysis. JAMA Intern Med. 2013;173(19):1821-1828.
- Visweswaran P, Guntupalli J. Rhabdomyolysis. Crit Care Clin. 1999;15(2):415-428, ix-x.
- Zimmerman JL, Shen MC. Rhabdomyolysis. Chest. 2013;144(3):1058-1065.
Case
A 62-year-old man with a history of hypercholesterolemia and HIV infection presented to the ED for evaluation of diffuse myalgia and tea-colored urine. His medication history included lopinavir/ritonavir (Kaletra) and simvastatin. A week prior to presentation, the patient’s primary care physician had instructed him to increase his daily dose of simvastatin from 40 mg to 80 mg. The patient stated that he had taken simvastatin 80 mg daily for approximately 5 days and then, 2 days prior to presentation, had independently further increased the dose to 160 mg daily.
In the ED, the patient reported feeling fatigued. His initial vital signs were: blood pressure, 129/86 mm Hg; heart rate, 93 beats/minute; respiratory rate, 17 breaths/minute; and temperature, 98.5˚F. Oxygen saturation was 98% on room air. His physical examination was unremarkable. Initial laboratory testing revealed the following: creatine kinase (CK) 350,000 U/L; blood urea nitrogen, 27 mg/dL; creatinine, 0.7 mg/dL; aspartate aminotransferase (AST), 2,950 U/L; and alanine aminotransferase (ALT), 1,305 U/L.
What can cause tea-colored/cola-colored urine and myalgia?
Numerous medications can result in dark-colored urine. These include antimalarial drugs such as chloroquine and primaquine; antibiotics such as metronidazole or nitrofurantoin; and the muscle relaxant methocarbamol. Myalgia and tea-colored urine are the hallmarks of rhabdomyolysis. Rhabdomyolysis involves the destruction of myocytes, which can occur as a result of a long list of processes, including crush injuries, poor oxygenation or perfusion, hypermetabolic states, and direct (or indirect) toxin-mediated myocyte damage.1 The list of toxic substances that can cause rhabdomyolysis is extensive, and statins are one of the most common drug-induced causes (Table).
Simvastatin is one of seven currently available 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors (ie, statins) that are commonly used to treat hypercholesterolemia. Because simvastatin is lipophilic, it can more readily cross cell membranes than nonlipophilic statins such as pravastatin. Simvastatin, therefore, has a propensity to disrupt the cellular integrity of myocytes and hepatocytes.What is the likely cause of this patient’s rhabdomyolysis?
At doses greater than 40 mg daily, simvastatin is associated with myalgia, myositis, and rhabdomyolysis. In December 2011, the US Food and Drug Administration (FDA) released a drug safety announcement recommending the originally approved maximum daily dose of simvastatin 80 mg be limited to patients who have already tolerated that dose for at least 12 months without evidence of muscular injury. The FDA further recommended no new patients be escalated to this dose. According to the FDA, patients taking 80 mg of simvastatin daily are also at increased risk of myopathy.
The metabolism of simvastatin, in addition to increased dosage of the drug, contributes to its potential for adverse effects. Of the seven available statins, only atorvastatin, lovastatin, and simvastatin are metabolized by the cytochrome P450 3A4 (CYP3A4). Lovastatin and simvastatin appear to have the highest potential for drug-drug interactions when coadministered with drugs that inhibit this enzyme (eg, ritonavir).2 The resulting elevation in blood concentration of simvastatin increases the risk of rhabdomyolysis. Other nonlipophilic statins, such as pravastatin, which are mostly eliminated unchanged in the urine and bile, would be preferable for patients taking CYP3A4 inhibitors.
How should patients with rhabdomyolysis be monitored?
Statins interfere with the myocyte’s ability to produce adenosine triphosphate, most likely by depleting coenzyme Q—one of the complexes found in the electron transport chain of the mitochondria. Under conditions of a high-energy requirement, myocytes incapable of producing sufficient energy ultimately fail and lyse, releasing cellular contents such as CK and myoglobin.1 The serum CK activity serves as a marker of muscle injury and should be monitored closely in patients with rhabdomyolysis. Although values above 5,000 U/L has been associated with renal injury,4 in healthy patients with access to hydration, renal injury is relatively uncommon with CK activities less than 50,000 U/L. Even though the prediction of renal failure is difficult, a validated nephrotoxicity prediction instrument using the patient’s age, gender, and initial laboratory data (serum creatinine, calcium, CK, phosphate, and bicarbonate) is available.5
Although the association between rhabdomyolysis and acute renal injury is well established, the mechanism remains unclear. Myoglobin from skeletal myocytes passes through the glomerulus without causing damage and is reabsorbed in the proximal renal tubular cell. Iron is subsequently released from the porphyrin ring and, in large concentrations, exceeds the binding capacity of the tissue ferritin. Because it is a transition metal, the free iron ion participates in oxidant stress reactions causing direct injury to the renal tubular cells.6 Furthermore, myoglobin also combines with renal tubular proteins, a process enhanced by an environment with lower pH, to form casts and cause renal tubular obstruction.
Patients with rhabdomyolysis may also be at risk for aminotransferase elevation, as occurred in the patient presented here. This elevation is most likely due to myocyte injury. In addition, potassium release due to myocyte destruction may cause life-threatening hyperkalemia, and phosphate liberation from these myocytes may cause hypocalcemia. Laboratory monitoring along with an electrocardiogram should be performed as required.
What is the treatment for rhabdomyolysis?
No adequate randomized controlled trials exist to guide the treatment of patients with rhabdomyolysis. As a result, recommendations for management come from retrospective observational studies, animal studies, case reports, and expert opinion.7
Once airway, breathing, and circulation have been addressed, patients with statin-induced rhabdomyolysis should be immediately treated with intravenous (IV) fluids to maintain renal perfusion, which helps to limit acute renal injury. Normal saline appears to be the most recommended fluid type, with a goal of maintaining a urine output of approximately 3 to 5 mL/kg/h.4,7
Some recommendations include the use of a sodium bicarbonate infusion to raise the urine pH, which may help limit the formation of renal casts from myoglobin. The data to support the benefit of sodium bicarbonate, however, is weak.3 A 2013 systematic review indicated that sodium bicarbonate should only be used to treat severe metabolic acidosis in patients with rhabdomyolysis.4
In addition to sodium bicarbonate, the use of diuretics is also discouraged by current recommendations. In patients with refractory electrolyte abnormalities or renal failure, hemodialysis may be required. Before disposition of a patient, his or her medication list should be reconciled to reflect statin discontinuation.
Case Conclusion
The patient received IV normal saline to maintain his urine output at 2 to 3 cc/kg/h. His repeat creatinine was 0.8 mg/dL and remained stable on repeat testing. His CK and AST concentrations trended down during his hospitalization. On hospital day 4, laboratory values were CK, less than 10,000 U/L; AST, 56 U/L; and ALT, 23 U/L. He had normal serum potassium levels and no dysrhythmia on electrocardiogram. His symptoms resolved on hospital day 2, and he was discharged on hospital day 4 with instructions to discontinue simvastatin.
Dr Fernandez is a senior toxicology fellow, department of emergency medicine, New York University School of Medicine. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
Case
A 62-year-old man with a history of hypercholesterolemia and HIV infection presented to the ED for evaluation of diffuse myalgia and tea-colored urine. His medication history included lopinavir/ritonavir (Kaletra) and simvastatin. A week prior to presentation, the patient’s primary care physician had instructed him to increase his daily dose of simvastatin from 40 mg to 80 mg. The patient stated that he had taken simvastatin 80 mg daily for approximately 5 days and then, 2 days prior to presentation, had independently further increased the dose to 160 mg daily.
In the ED, the patient reported feeling fatigued. His initial vital signs were: blood pressure, 129/86 mm Hg; heart rate, 93 beats/minute; respiratory rate, 17 breaths/minute; and temperature, 98.5˚F. Oxygen saturation was 98% on room air. His physical examination was unremarkable. Initial laboratory testing revealed the following: creatine kinase (CK) 350,000 U/L; blood urea nitrogen, 27 mg/dL; creatinine, 0.7 mg/dL; aspartate aminotransferase (AST), 2,950 U/L; and alanine aminotransferase (ALT), 1,305 U/L.
What can cause tea-colored/cola-colored urine and myalgia?
Numerous medications can result in dark-colored urine. These include antimalarial drugs such as chloroquine and primaquine; antibiotics such as metronidazole or nitrofurantoin; and the muscle relaxant methocarbamol. Myalgia and tea-colored urine are the hallmarks of rhabdomyolysis. Rhabdomyolysis involves the destruction of myocytes, which can occur as a result of a long list of processes, including crush injuries, poor oxygenation or perfusion, hypermetabolic states, and direct (or indirect) toxin-mediated myocyte damage.1 The list of toxic substances that can cause rhabdomyolysis is extensive, and statins are one of the most common drug-induced causes (Table).
Simvastatin is one of seven currently available 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors (ie, statins) that are commonly used to treat hypercholesterolemia. Because simvastatin is lipophilic, it can more readily cross cell membranes than nonlipophilic statins such as pravastatin. Simvastatin, therefore, has a propensity to disrupt the cellular integrity of myocytes and hepatocytes.What is the likely cause of this patient’s rhabdomyolysis?
At doses greater than 40 mg daily, simvastatin is associated with myalgia, myositis, and rhabdomyolysis. In December 2011, the US Food and Drug Administration (FDA) released a drug safety announcement recommending the originally approved maximum daily dose of simvastatin 80 mg be limited to patients who have already tolerated that dose for at least 12 months without evidence of muscular injury. The FDA further recommended no new patients be escalated to this dose. According to the FDA, patients taking 80 mg of simvastatin daily are also at increased risk of myopathy.
The metabolism of simvastatin, in addition to increased dosage of the drug, contributes to its potential for adverse effects. Of the seven available statins, only atorvastatin, lovastatin, and simvastatin are metabolized by the cytochrome P450 3A4 (CYP3A4). Lovastatin and simvastatin appear to have the highest potential for drug-drug interactions when coadministered with drugs that inhibit this enzyme (eg, ritonavir).2 The resulting elevation in blood concentration of simvastatin increases the risk of rhabdomyolysis. Other nonlipophilic statins, such as pravastatin, which are mostly eliminated unchanged in the urine and bile, would be preferable for patients taking CYP3A4 inhibitors.
How should patients with rhabdomyolysis be monitored?
Statins interfere with the myocyte’s ability to produce adenosine triphosphate, most likely by depleting coenzyme Q—one of the complexes found in the electron transport chain of the mitochondria. Under conditions of a high-energy requirement, myocytes incapable of producing sufficient energy ultimately fail and lyse, releasing cellular contents such as CK and myoglobin.1 The serum CK activity serves as a marker of muscle injury and should be monitored closely in patients with rhabdomyolysis. Although values above 5,000 U/L has been associated with renal injury,4 in healthy patients with access to hydration, renal injury is relatively uncommon with CK activities less than 50,000 U/L. Even though the prediction of renal failure is difficult, a validated nephrotoxicity prediction instrument using the patient’s age, gender, and initial laboratory data (serum creatinine, calcium, CK, phosphate, and bicarbonate) is available.5
Although the association between rhabdomyolysis and acute renal injury is well established, the mechanism remains unclear. Myoglobin from skeletal myocytes passes through the glomerulus without causing damage and is reabsorbed in the proximal renal tubular cell. Iron is subsequently released from the porphyrin ring and, in large concentrations, exceeds the binding capacity of the tissue ferritin. Because it is a transition metal, the free iron ion participates in oxidant stress reactions causing direct injury to the renal tubular cells.6 Furthermore, myoglobin also combines with renal tubular proteins, a process enhanced by an environment with lower pH, to form casts and cause renal tubular obstruction.
Patients with rhabdomyolysis may also be at risk for aminotransferase elevation, as occurred in the patient presented here. This elevation is most likely due to myocyte injury. In addition, potassium release due to myocyte destruction may cause life-threatening hyperkalemia, and phosphate liberation from these myocytes may cause hypocalcemia. Laboratory monitoring along with an electrocardiogram should be performed as required.
What is the treatment for rhabdomyolysis?
No adequate randomized controlled trials exist to guide the treatment of patients with rhabdomyolysis. As a result, recommendations for management come from retrospective observational studies, animal studies, case reports, and expert opinion.7
Once airway, breathing, and circulation have been addressed, patients with statin-induced rhabdomyolysis should be immediately treated with intravenous (IV) fluids to maintain renal perfusion, which helps to limit acute renal injury. Normal saline appears to be the most recommended fluid type, with a goal of maintaining a urine output of approximately 3 to 5 mL/kg/h.4,7
Some recommendations include the use of a sodium bicarbonate infusion to raise the urine pH, which may help limit the formation of renal casts from myoglobin. The data to support the benefit of sodium bicarbonate, however, is weak.3 A 2013 systematic review indicated that sodium bicarbonate should only be used to treat severe metabolic acidosis in patients with rhabdomyolysis.4
In addition to sodium bicarbonate, the use of diuretics is also discouraged by current recommendations. In patients with refractory electrolyte abnormalities or renal failure, hemodialysis may be required. Before disposition of a patient, his or her medication list should be reconciled to reflect statin discontinuation.
Case Conclusion
The patient received IV normal saline to maintain his urine output at 2 to 3 cc/kg/h. His repeat creatinine was 0.8 mg/dL and remained stable on repeat testing. His CK and AST concentrations trended down during his hospitalization. On hospital day 4, laboratory values were CK, less than 10,000 U/L; AST, 56 U/L; and ALT, 23 U/L. He had normal serum potassium levels and no dysrhythmia on electrocardiogram. His symptoms resolved on hospital day 2, and he was discharged on hospital day 4 with instructions to discontinue simvastatin.
Dr Fernandez is a senior toxicology fellow, department of emergency medicine, New York University School of Medicine. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
- Bench-to-bedside review: Rhabdomyolysis—an overview for clinicians. Crit Care. 2005;9(2):158-169.
- Chauvin B, Drouot S, Barrail-Tran A, Taburet AM. Drug-drug interactions between HMG-CoA reductase inhibitors (statins) and antiviral protease inhibitors. Clin Pharmacokinet. 2013;52(10):815-831.
- Brown CV, Rhee P, Chan L, Evans K, Demetriades D, Velmahos GC. Preventing renal failure in patients with rhabdomyolysis: do bicarbonate and mannitol make a difference? J Trauma. 2004;56(6):1191-1196.
- Scharman EJ, Troutman WG. Prevention of kidney injury following rhabdomyolysis: a systematic review. Ann Pharmacother. 2013;47(1):90-105.
- McMahon GM, Zeng X, Waikar SS. A risk prediction score for kidney failure or mortality in rhabdomyolysis. JAMA Intern Med. 2013;173(19):1821-1828.
- Visweswaran P, Guntupalli J. Rhabdomyolysis. Crit Care Clin. 1999;15(2):415-428, ix-x.
- Zimmerman JL, Shen MC. Rhabdomyolysis. Chest. 2013;144(3):1058-1065.
- Bench-to-bedside review: Rhabdomyolysis—an overview for clinicians. Crit Care. 2005;9(2):158-169.
- Chauvin B, Drouot S, Barrail-Tran A, Taburet AM. Drug-drug interactions between HMG-CoA reductase inhibitors (statins) and antiviral protease inhibitors. Clin Pharmacokinet. 2013;52(10):815-831.
- Brown CV, Rhee P, Chan L, Evans K, Demetriades D, Velmahos GC. Preventing renal failure in patients with rhabdomyolysis: do bicarbonate and mannitol make a difference? J Trauma. 2004;56(6):1191-1196.
- Scharman EJ, Troutman WG. Prevention of kidney injury following rhabdomyolysis: a systematic review. Ann Pharmacother. 2013;47(1):90-105.
- McMahon GM, Zeng X, Waikar SS. A risk prediction score for kidney failure or mortality in rhabdomyolysis. JAMA Intern Med. 2013;173(19):1821-1828.
- Visweswaran P, Guntupalli J. Rhabdomyolysis. Crit Care Clin. 1999;15(2):415-428, ix-x.
- Zimmerman JL, Shen MC. Rhabdomyolysis. Chest. 2013;144(3):1058-1065.
Isolated splenic metastasis in a patient with two distinct genitourinary malignancies
Click on the PDF icon at the top of this introduction to read the full article.
Click on the PDF icon at the top of this introduction to read the full article.
Click on the PDF icon at the top of this introduction to read the full article.
Non–HIV-Related Kaposi Sarcoma in 2 Hispanic Patients Arising in the Setting of Chronic Venous Insufficiency
Kaposi sarcoma (KS) is a vascular neoplasm associated with human herpesvirus 8 (HHV-8) infection that is conventionally divided into 4 clinical variants: classic, African, transplant associated, and AIDS related. In the United States, classic KS predominantly is observed in elderly white men of Ashkenazi Jewish or Mediterranean descent.1
The clinical and histological changes seen in KS can be confused with those from chronic venous insufficiency. Both conditions tend to present with purple-colored patches, plaques, or nodules on the lower extremities. Histologically, both KS and chronic venous insufficiency are characterized by blood vessel and endothelial cell proliferation in the papillary dermis, red blood cell extravasation, and hemosiderin deposition.2 Nevertheless, KS can be diagnosed based on the presence of neoplastic spindle-shaped cells and positive immunostaining for HHV-8 antigen.3
We describe the cases of 2 elderly Hispanic patients in the United States with no history of human immunodeficiency virus (HIV), immunosuppression, or travel to the Mediterranean region who presented with erythematous to violaceous papules, plaques, and nodules on the distal lower extremities in the setting of chronic venous insufficiency. We review the relationship between KS and chronic venous insufficiency and suggest that these presentations may represent a distinct clinical variant of KS.
Case Reports
Patient 1
An 83-year-old Hispanic woman with a history of hypertension, atrial fibrillation, and chronic venous insufficiency presented with a chronic painful violaceous eruption on the lower legs of 3 years’ duration. The patient reported that the erythematous patches, which she described as bruiselike, originally developed 3 years prior after starting warfarin therapy for atrial fibrillation. At that time, a biopsy indicated findings of pigmented purpuric dermatosis and negative immunostaining for HHV-8. Her condition had worsened over the last 6 months with the development of tender eroded plaques on the dorsal aspects of the feet (Figure 1) and purple-brown patches and plaques on the legs. Prior treatment with topical corticosteroids and a short course of prednisone was unsuccessful. Of note, the patient had no history of immunosuppression or HIV and was not of Mediterranean or African descent.
Punch biopsies from the left dorsal foot and left lower leg revealed dermal fibrosis, a proliferation of small blood vessels with plump endothelial cells, and foci of spindled endothelial cells with narrow slitlike spaces containing erythrocytes (Figure 2). There also were extravasated erythrocytes and a sparse inflammatory cell infiltrate comprised of lymphocytes, eosinophils, neutrophils, and plasma cells. Perls Prussian blue stain highlighted numerous siderophages scattered in the dermis. Based on these findings, immunostaining for HHV-8 was performed and highlighted reactivity of the spindled cells (Figure 3). The patient was diagnosed with KS in the setting of chronic venous insufficiency.
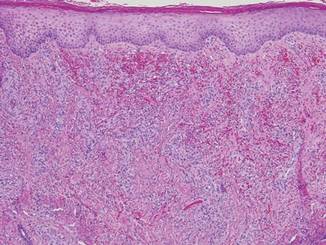
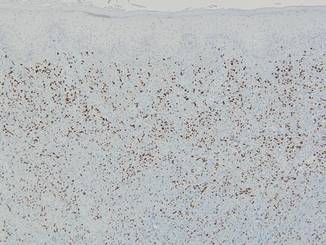 |
Patient 2
A 67-year-old Hispanic man with a history of diabetes mellitus presented with 10 asymptomatic purple papules and hyperkeratotic and hyperpigmented plaques on the distal aspect of the legs of 1 year’s duration in the setting of chronic venous insufficiency (Figure 4). The patient had no history of immunosuppression or HIV and was not of Mediterranean or African descent. An excisional biopsy from the left fourth toe revealed a cellular dermal vascular proliferation associated with numerous small slitlike vessels and scattered dilated blood vessels with prominent hemorrhage and surrounding dermal fibrosis (Figure 5). The lesion was markedly cellular with nuclear atypia. Many cells showed enlarged oval- to spindle-shaped hyperchromatic nuclei, some with prominent nucleoli and scant amounts of eosinophilic cytoplasm. Although the differential diagnosis included an unusual cellular pyogenic granuloma with atypia in the setting of chronic venous insufficiency, the degree of cellularity and presence of spindled cells associated with slitlike vessels were more consistent with a pyogenic granulomalike variant of KS. This variant is rare but has previously been reported.4 Many of the tumor nucleoli stained strongly for HHV-8, which is a diagnostic finding of KS (Figure 6). The patient subsequently was diagnosed with KS.
Comment
These 2 cases represent unusual clinical presentations of KS in Hispanic patients with no known risk factors for KS. In patient 1, an initial skin biopsy prompted HHV-8 testing due to suspicion for KS. At that time, HHV-8 was negative, perhaps because of technical deficiencies in the staining protocol; alternatively, the patient may have subsequently developed KS. Both patients had known chronic venous insufficiency. However, biopsies revealed spindle-shaped cells forming clefts and positivity for HHV-8. We propose that these cases may represent an additional clinical variant of KS, chronic venous insufficiency–associated KS.
If similar presentations of KS are identified, studies will need to be done to uncover the specific risk factors involved. Human herpesvirus 8 is not sufficient for the development of KS on its own, as oncogenesis of KS requires immunodeficiency or an additional environmental factor such as diabetes mellitus.5 Through impaired microvascular circulation and the release of hypoxia-inducible factor 1a, diabetes can promote KS-herpesvirus replication. Therefore, the risk for KS is increased in individuals with diabetes regardless of a negative history of immunodeficiency, which may have been the case in patient 2.
Our cases suggest that chronic venous insufficiency may be another factor that predisposes immunocompetent individuals to KS. Chronic venous insufficiency can cause hypoxia, promoting the release of cytokines and angiogenic factors responsible for the formation of vascular tumors such as KS.6 Once present, KS can worsen preexisting stasis dermatitis by compressing the external lymphatics and exacerbating lymphedema.7 Stasis dermatitis and KS may be part of a self-perpetuating cycle that involves obstruction due to secondary lymphadenopathy, the development of lymphedema, and the release of cytokines and growth factors that lead to further vascular proliferation.8
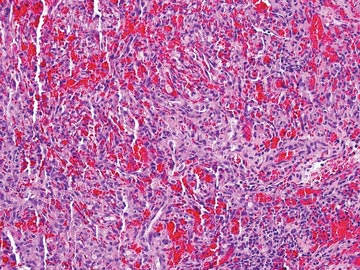 | 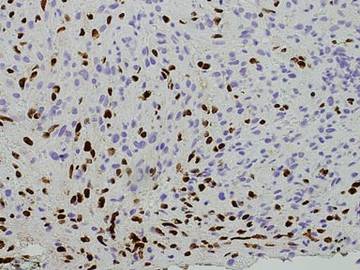 |
In summary, we present 2 cases of non–HIV-related KS that may represent an additional clinical variant of KS that mimics and/or arises in chronic venous insufficiency and appears as papules and plaques in elderly patients who are Hispanic, immunocompetent, and HIV negative. We suggest including KS in the differential diagnosis for chronic venous insufficiency, especially in cases with an unusual clinical appearance or course. In these cases, skin biopsy with HHV-8 testing may be warranted.
Acknowledgment
The authors would like to acknowledge William Putnam, BFA, New York, New York, for his assistance with the figures.
1. Hiatt KM, Nelson AM, Lichy JH, et al. Classic Kaposi sarcoma in the United States over the last two decades: a clinicopathologic and molecular study of 438 non-HIV-related Kaposi Sarcoma patients with comparison to HIV-related Kaposi Sarcoma [published online ahead of print March 28, 2008]. Mod Pathol. 2008;21:572-582.
2. Weaver J, Billings SD. Initial presentation of stasis dermatitis mimicking solitary lesions: a previously unrecognized clinical scenario. J Am Acad Dermatol. 2009;61:1028-1032.
3. Bulat V, Lugovic´ L, Šitum M, et al. Acroangiodermatitis (pseudo-Kaposi sarcoma) as part of chronic venous insufficiency. Acta Clin Croat. 2007;46:273-277.
4. Urquhart JL, Uzieblo A, Kohler S. Detection of HHV-8 in pyogenic granuloma-like Kaposi sarcoma. Am J Dermatopathol. 2006;28:317-321.
5. Anderson LA, Lauria C, Romano N, et al. Risk factors for classical Kaposi sarcoma in a population-based case-control study in Sicily. Cancer Epidemiol Biomarkers Prev. 2008;17:3435-3443.
6. Lunardi-Iskandar Y, Bryant JL, Zeman RA, et al. Tumorigenesis and metastasis of neoplastic Kaposi’s sarcoma cell line in immunodeficient mice blocked by a human pregnancy hormone. Nature. 1995;375:64-68.
7. Allen PJ, Gillespie DL, Redfield RR, et al. Lower extremity lymphedema caused by acquired immune deficiency syndrome-related Kaposi’s sarcoma: case report and review of the literature. J Vasc Surg. 1995;22:178-181.
8. Ramdial PK, Chetty R, Singh B, et al. Lymphedematous HIV-associated Kaposi’s sarcoma. J Cutan Pathol. 2006;33:474-481.
Kaposi sarcoma (KS) is a vascular neoplasm associated with human herpesvirus 8 (HHV-8) infection that is conventionally divided into 4 clinical variants: classic, African, transplant associated, and AIDS related. In the United States, classic KS predominantly is observed in elderly white men of Ashkenazi Jewish or Mediterranean descent.1
The clinical and histological changes seen in KS can be confused with those from chronic venous insufficiency. Both conditions tend to present with purple-colored patches, plaques, or nodules on the lower extremities. Histologically, both KS and chronic venous insufficiency are characterized by blood vessel and endothelial cell proliferation in the papillary dermis, red blood cell extravasation, and hemosiderin deposition.2 Nevertheless, KS can be diagnosed based on the presence of neoplastic spindle-shaped cells and positive immunostaining for HHV-8 antigen.3
We describe the cases of 2 elderly Hispanic patients in the United States with no history of human immunodeficiency virus (HIV), immunosuppression, or travel to the Mediterranean region who presented with erythematous to violaceous papules, plaques, and nodules on the distal lower extremities in the setting of chronic venous insufficiency. We review the relationship between KS and chronic venous insufficiency and suggest that these presentations may represent a distinct clinical variant of KS.
Case Reports
Patient 1
An 83-year-old Hispanic woman with a history of hypertension, atrial fibrillation, and chronic venous insufficiency presented with a chronic painful violaceous eruption on the lower legs of 3 years’ duration. The patient reported that the erythematous patches, which she described as bruiselike, originally developed 3 years prior after starting warfarin therapy for atrial fibrillation. At that time, a biopsy indicated findings of pigmented purpuric dermatosis and negative immunostaining for HHV-8. Her condition had worsened over the last 6 months with the development of tender eroded plaques on the dorsal aspects of the feet (Figure 1) and purple-brown patches and plaques on the legs. Prior treatment with topical corticosteroids and a short course of prednisone was unsuccessful. Of note, the patient had no history of immunosuppression or HIV and was not of Mediterranean or African descent.
Punch biopsies from the left dorsal foot and left lower leg revealed dermal fibrosis, a proliferation of small blood vessels with plump endothelial cells, and foci of spindled endothelial cells with narrow slitlike spaces containing erythrocytes (Figure 2). There also were extravasated erythrocytes and a sparse inflammatory cell infiltrate comprised of lymphocytes, eosinophils, neutrophils, and plasma cells. Perls Prussian blue stain highlighted numerous siderophages scattered in the dermis. Based on these findings, immunostaining for HHV-8 was performed and highlighted reactivity of the spindled cells (Figure 3). The patient was diagnosed with KS in the setting of chronic venous insufficiency.

 |
Patient 2
A 67-year-old Hispanic man with a history of diabetes mellitus presented with 10 asymptomatic purple papules and hyperkeratotic and hyperpigmented plaques on the distal aspect of the legs of 1 year’s duration in the setting of chronic venous insufficiency (Figure 4). The patient had no history of immunosuppression or HIV and was not of Mediterranean or African descent. An excisional biopsy from the left fourth toe revealed a cellular dermal vascular proliferation associated with numerous small slitlike vessels and scattered dilated blood vessels with prominent hemorrhage and surrounding dermal fibrosis (Figure 5). The lesion was markedly cellular with nuclear atypia. Many cells showed enlarged oval- to spindle-shaped hyperchromatic nuclei, some with prominent nucleoli and scant amounts of eosinophilic cytoplasm. Although the differential diagnosis included an unusual cellular pyogenic granuloma with atypia in the setting of chronic venous insufficiency, the degree of cellularity and presence of spindled cells associated with slitlike vessels were more consistent with a pyogenic granulomalike variant of KS. This variant is rare but has previously been reported.4 Many of the tumor nucleoli stained strongly for HHV-8, which is a diagnostic finding of KS (Figure 6). The patient subsequently was diagnosed with KS.
Comment
These 2 cases represent unusual clinical presentations of KS in Hispanic patients with no known risk factors for KS. In patient 1, an initial skin biopsy prompted HHV-8 testing due to suspicion for KS. At that time, HHV-8 was negative, perhaps because of technical deficiencies in the staining protocol; alternatively, the patient may have subsequently developed KS. Both patients had known chronic venous insufficiency. However, biopsies revealed spindle-shaped cells forming clefts and positivity for HHV-8. We propose that these cases may represent an additional clinical variant of KS, chronic venous insufficiency–associated KS.
If similar presentations of KS are identified, studies will need to be done to uncover the specific risk factors involved. Human herpesvirus 8 is not sufficient for the development of KS on its own, as oncogenesis of KS requires immunodeficiency or an additional environmental factor such as diabetes mellitus.5 Through impaired microvascular circulation and the release of hypoxia-inducible factor 1a, diabetes can promote KS-herpesvirus replication. Therefore, the risk for KS is increased in individuals with diabetes regardless of a negative history of immunodeficiency, which may have been the case in patient 2.
Our cases suggest that chronic venous insufficiency may be another factor that predisposes immunocompetent individuals to KS. Chronic venous insufficiency can cause hypoxia, promoting the release of cytokines and angiogenic factors responsible for the formation of vascular tumors such as KS.6 Once present, KS can worsen preexisting stasis dermatitis by compressing the external lymphatics and exacerbating lymphedema.7 Stasis dermatitis and KS may be part of a self-perpetuating cycle that involves obstruction due to secondary lymphadenopathy, the development of lymphedema, and the release of cytokines and growth factors that lead to further vascular proliferation.8
 |  |
In summary, we present 2 cases of non–HIV-related KS that may represent an additional clinical variant of KS that mimics and/or arises in chronic venous insufficiency and appears as papules and plaques in elderly patients who are Hispanic, immunocompetent, and HIV negative. We suggest including KS in the differential diagnosis for chronic venous insufficiency, especially in cases with an unusual clinical appearance or course. In these cases, skin biopsy with HHV-8 testing may be warranted.
Acknowledgment
The authors would like to acknowledge William Putnam, BFA, New York, New York, for his assistance with the figures.
Kaposi sarcoma (KS) is a vascular neoplasm associated with human herpesvirus 8 (HHV-8) infection that is conventionally divided into 4 clinical variants: classic, African, transplant associated, and AIDS related. In the United States, classic KS predominantly is observed in elderly white men of Ashkenazi Jewish or Mediterranean descent.1
The clinical and histological changes seen in KS can be confused with those from chronic venous insufficiency. Both conditions tend to present with purple-colored patches, plaques, or nodules on the lower extremities. Histologically, both KS and chronic venous insufficiency are characterized by blood vessel and endothelial cell proliferation in the papillary dermis, red blood cell extravasation, and hemosiderin deposition.2 Nevertheless, KS can be diagnosed based on the presence of neoplastic spindle-shaped cells and positive immunostaining for HHV-8 antigen.3
We describe the cases of 2 elderly Hispanic patients in the United States with no history of human immunodeficiency virus (HIV), immunosuppression, or travel to the Mediterranean region who presented with erythematous to violaceous papules, plaques, and nodules on the distal lower extremities in the setting of chronic venous insufficiency. We review the relationship between KS and chronic venous insufficiency and suggest that these presentations may represent a distinct clinical variant of KS.
Case Reports
Patient 1
An 83-year-old Hispanic woman with a history of hypertension, atrial fibrillation, and chronic venous insufficiency presented with a chronic painful violaceous eruption on the lower legs of 3 years’ duration. The patient reported that the erythematous patches, which she described as bruiselike, originally developed 3 years prior after starting warfarin therapy for atrial fibrillation. At that time, a biopsy indicated findings of pigmented purpuric dermatosis and negative immunostaining for HHV-8. Her condition had worsened over the last 6 months with the development of tender eroded plaques on the dorsal aspects of the feet (Figure 1) and purple-brown patches and plaques on the legs. Prior treatment with topical corticosteroids and a short course of prednisone was unsuccessful. Of note, the patient had no history of immunosuppression or HIV and was not of Mediterranean or African descent.
Punch biopsies from the left dorsal foot and left lower leg revealed dermal fibrosis, a proliferation of small blood vessels with plump endothelial cells, and foci of spindled endothelial cells with narrow slitlike spaces containing erythrocytes (Figure 2). There also were extravasated erythrocytes and a sparse inflammatory cell infiltrate comprised of lymphocytes, eosinophils, neutrophils, and plasma cells. Perls Prussian blue stain highlighted numerous siderophages scattered in the dermis. Based on these findings, immunostaining for HHV-8 was performed and highlighted reactivity of the spindled cells (Figure 3). The patient was diagnosed with KS in the setting of chronic venous insufficiency.

 |
Patient 2
A 67-year-old Hispanic man with a history of diabetes mellitus presented with 10 asymptomatic purple papules and hyperkeratotic and hyperpigmented plaques on the distal aspect of the legs of 1 year’s duration in the setting of chronic venous insufficiency (Figure 4). The patient had no history of immunosuppression or HIV and was not of Mediterranean or African descent. An excisional biopsy from the left fourth toe revealed a cellular dermal vascular proliferation associated with numerous small slitlike vessels and scattered dilated blood vessels with prominent hemorrhage and surrounding dermal fibrosis (Figure 5). The lesion was markedly cellular with nuclear atypia. Many cells showed enlarged oval- to spindle-shaped hyperchromatic nuclei, some with prominent nucleoli and scant amounts of eosinophilic cytoplasm. Although the differential diagnosis included an unusual cellular pyogenic granuloma with atypia in the setting of chronic venous insufficiency, the degree of cellularity and presence of spindled cells associated with slitlike vessels were more consistent with a pyogenic granulomalike variant of KS. This variant is rare but has previously been reported.4 Many of the tumor nucleoli stained strongly for HHV-8, which is a diagnostic finding of KS (Figure 6). The patient subsequently was diagnosed with KS.
Comment
These 2 cases represent unusual clinical presentations of KS in Hispanic patients with no known risk factors for KS. In patient 1, an initial skin biopsy prompted HHV-8 testing due to suspicion for KS. At that time, HHV-8 was negative, perhaps because of technical deficiencies in the staining protocol; alternatively, the patient may have subsequently developed KS. Both patients had known chronic venous insufficiency. However, biopsies revealed spindle-shaped cells forming clefts and positivity for HHV-8. We propose that these cases may represent an additional clinical variant of KS, chronic venous insufficiency–associated KS.
If similar presentations of KS are identified, studies will need to be done to uncover the specific risk factors involved. Human herpesvirus 8 is not sufficient for the development of KS on its own, as oncogenesis of KS requires immunodeficiency or an additional environmental factor such as diabetes mellitus.5 Through impaired microvascular circulation and the release of hypoxia-inducible factor 1a, diabetes can promote KS-herpesvirus replication. Therefore, the risk for KS is increased in individuals with diabetes regardless of a negative history of immunodeficiency, which may have been the case in patient 2.
Our cases suggest that chronic venous insufficiency may be another factor that predisposes immunocompetent individuals to KS. Chronic venous insufficiency can cause hypoxia, promoting the release of cytokines and angiogenic factors responsible for the formation of vascular tumors such as KS.6 Once present, KS can worsen preexisting stasis dermatitis by compressing the external lymphatics and exacerbating lymphedema.7 Stasis dermatitis and KS may be part of a self-perpetuating cycle that involves obstruction due to secondary lymphadenopathy, the development of lymphedema, and the release of cytokines and growth factors that lead to further vascular proliferation.8
 |  |
In summary, we present 2 cases of non–HIV-related KS that may represent an additional clinical variant of KS that mimics and/or arises in chronic venous insufficiency and appears as papules and plaques in elderly patients who are Hispanic, immunocompetent, and HIV negative. We suggest including KS in the differential diagnosis for chronic venous insufficiency, especially in cases with an unusual clinical appearance or course. In these cases, skin biopsy with HHV-8 testing may be warranted.
Acknowledgment
The authors would like to acknowledge William Putnam, BFA, New York, New York, for his assistance with the figures.
1. Hiatt KM, Nelson AM, Lichy JH, et al. Classic Kaposi sarcoma in the United States over the last two decades: a clinicopathologic and molecular study of 438 non-HIV-related Kaposi Sarcoma patients with comparison to HIV-related Kaposi Sarcoma [published online ahead of print March 28, 2008]. Mod Pathol. 2008;21:572-582.
2. Weaver J, Billings SD. Initial presentation of stasis dermatitis mimicking solitary lesions: a previously unrecognized clinical scenario. J Am Acad Dermatol. 2009;61:1028-1032.
3. Bulat V, Lugovic´ L, Šitum M, et al. Acroangiodermatitis (pseudo-Kaposi sarcoma) as part of chronic venous insufficiency. Acta Clin Croat. 2007;46:273-277.
4. Urquhart JL, Uzieblo A, Kohler S. Detection of HHV-8 in pyogenic granuloma-like Kaposi sarcoma. Am J Dermatopathol. 2006;28:317-321.
5. Anderson LA, Lauria C, Romano N, et al. Risk factors for classical Kaposi sarcoma in a population-based case-control study in Sicily. Cancer Epidemiol Biomarkers Prev. 2008;17:3435-3443.
6. Lunardi-Iskandar Y, Bryant JL, Zeman RA, et al. Tumorigenesis and metastasis of neoplastic Kaposi’s sarcoma cell line in immunodeficient mice blocked by a human pregnancy hormone. Nature. 1995;375:64-68.
7. Allen PJ, Gillespie DL, Redfield RR, et al. Lower extremity lymphedema caused by acquired immune deficiency syndrome-related Kaposi’s sarcoma: case report and review of the literature. J Vasc Surg. 1995;22:178-181.
8. Ramdial PK, Chetty R, Singh B, et al. Lymphedematous HIV-associated Kaposi’s sarcoma. J Cutan Pathol. 2006;33:474-481.
1. Hiatt KM, Nelson AM, Lichy JH, et al. Classic Kaposi sarcoma in the United States over the last two decades: a clinicopathologic and molecular study of 438 non-HIV-related Kaposi Sarcoma patients with comparison to HIV-related Kaposi Sarcoma [published online ahead of print March 28, 2008]. Mod Pathol. 2008;21:572-582.
2. Weaver J, Billings SD. Initial presentation of stasis dermatitis mimicking solitary lesions: a previously unrecognized clinical scenario. J Am Acad Dermatol. 2009;61:1028-1032.
3. Bulat V, Lugovic´ L, Šitum M, et al. Acroangiodermatitis (pseudo-Kaposi sarcoma) as part of chronic venous insufficiency. Acta Clin Croat. 2007;46:273-277.
4. Urquhart JL, Uzieblo A, Kohler S. Detection of HHV-8 in pyogenic granuloma-like Kaposi sarcoma. Am J Dermatopathol. 2006;28:317-321.
5. Anderson LA, Lauria C, Romano N, et al. Risk factors for classical Kaposi sarcoma in a population-based case-control study in Sicily. Cancer Epidemiol Biomarkers Prev. 2008;17:3435-3443.
6. Lunardi-Iskandar Y, Bryant JL, Zeman RA, et al. Tumorigenesis and metastasis of neoplastic Kaposi’s sarcoma cell line in immunodeficient mice blocked by a human pregnancy hormone. Nature. 1995;375:64-68.
7. Allen PJ, Gillespie DL, Redfield RR, et al. Lower extremity lymphedema caused by acquired immune deficiency syndrome-related Kaposi’s sarcoma: case report and review of the literature. J Vasc Surg. 1995;22:178-181.
8. Ramdial PK, Chetty R, Singh B, et al. Lymphedematous HIV-associated Kaposi’s sarcoma. J Cutan Pathol. 2006;33:474-481.
Practice Points
- The 4 clinical variants of Kaposi sarcoma (KS) are classic, African, transplant associated, and AIDS related.
- Human herpesvirus 8 (HHV-8) staining can be used to confirm the presence of KS.
- A subset of patients with chronic venous insufficiency with no other risk factors for KS have been found to have violaceous plaques that test positive for HHV-8.
- Chronic venous insufficiency may be a predisposing factor to KS in immunocompetent individuals and may constitute an additional clinical variant of KS.
Unusual Congenital Pulmonary Anomaly in an Adult Patient With Dyspnea
Anatomic variations may result in abnormal return from the pulmonary veins to the right side of the heart. This group of congenital anomalies, also known as partial anomalous pulmonary venous return (PAPVR), may connect oxygenated blood from the pulmonary vein to a systemic vein before reaching the right atrium. The most common PAPVR is derived from the left upper pulmonary vein, which then connects to the left innominate vein and drains into the superior vena cava (SVC).
Scimitar syndrome is a rare PAPVR variant in which part of or the entire right lung is drained by the pulmonary vein into the inferior vena cava (IVC), giving the curvilinear dimension the appearance of a Middle Eastern sword (scimitar). The syndrome is frequently associated with other abnormalities, such as right lung hypoplasia and abnormal right lung lobation, dextroposition of the heart, right pulmonary artery hypoplasia, systemic arterial blood supply to the right lower lung from the infradiaphragmatic aorta, atrial septal defects of the secundum type, right-sided diaphragmatic hernia, and horseshoe lung.1,2 The syndrome was first described in 1836 by Cooper during an autopsy of an infant, and Dotter diagnosed the first symptomatic patient in 1949.3,4
Case Report
A 62-year-old man, former smoker (40 pack-year), with a past medical history of arterial hypertension and asthma visited the clinic, reporting exertional dyspnea. He also reported oppressive, retrosternally located exertional chest pain, 6/10 in intensity, of 3 minutes’ duration that radiated to the right chest and ameliorated with rest. Symptoms had occurred every other day for the past year. His physical exam was remarkable for central obesity. Lung auscultation was essentially clear. There was no jugular vein distention. The patient’s heart showed a regular rate and rhythm without evidence of murmurs or gallops. There was no evidence of leg edema or cyanosis. The patient’s resting oxygen saturation of 98% remained unchanged after exercise.
Related: Venous Thromboembolism Prophylaxis in Acutely Ill Veterans With Respiratory Disease
An electrocardiogram showed normal sinus rhythm with no ischemic changes. A pulmonary function test showed a forced expiratory volume (FEV1) of 1.44 L (61% of predicted), forced vital capacity (FVC) of 1.99 L (68% of predicted), and slow vital capacity (SVC) of 2.09 L (60% of predicted), with an FEV1/SVC ratio of 68% of predicted. These results suggested moderate-to-severe obstructive ventilatory impairment.
There was no response to bronchodilator therapy. Lung volumes were measured by plethysmography. The residual volume (RV), total lung capacity (TLC), and RV/TLC ratio were 2.57 L (147% of predicted), 4.66 L (88% of predicted), and 55%, respectively, suggesting severe air trapping. Diffusion lung capacity (DLCO) testing revealed 16.95 mL/min/mm Hg (73% of predicted) when corrected by hemoglobin and DLCO/alveolar volume of 4.97 mL/min/mm Hg/L (114% of predicted). This result was consistent with a mild reduction of gas transfer, which normalized when corrected by alveolar volume.
A posteroanterior chest radiograph image was remarkable for mediastinal shifting toward the right side, volume loss of the right lung, and evidence of a previous gunshot on the right chest wall (Figure 1). Previous chest imaging done in October 2009 showed an opacification of the right lower lung with indistinctness of the right cardiac border and partial obliteration of the right hemidiaphragm. The patient was treated with inhaled steroids and long- acting bronchodilators with partial improvement in dyspnea symptoms.
Myocardial perfusion imaging revealed scintigraphic evidence of heart rate-induced ischemia on the inferior and apical wall segments of the left ventricular myocardium. A transthoracic echocardiogram showed a very poor echocardiographic window. Left ventricular function seemed preserved. Transesophageal echocardiography was scheduled, but the patient missed the appointment.
Cardiac catheterization was only remarkable for 40% to 50% obstruction of the mid-left anterior descending artery, which did not explain the patient’s dyspnea or chest pain. Right side pressures were described as follows: right atrial mean, 10 mm Hg; right ventricle, 36/8 mm Hg; pulmonary artery, 33/16 mm Hg; pulmonary artery mean, 23 mm Hg; pulmonary capillary wedge pressure, 12 mm Hg; and a mean arterial pressure of 100 mm Hg. He had a left ventricle ejection fraction of 60%.
Because images suggested dextroposition of the heart and right lung hypoplasia, a chest computed tomography (CT) and angiography were done (Figure 2). The images showed hypoplasia of the right lung field with an anomalous venous return from the right midlung, having a vertical contour that drained into the supradiaphragmatic IVC. In addition, CT reconstruction demarcated the last mentioned contour draining into the IVC, consistent with scimitar syndrome (Figure 3). The patient was treated conservatively due to age, optimizing therapy for obstructive lung and cardiovascular disease.
Discussion
Partial anomalous pulmonary venous return is a relatively uncommon congenital anomaly, accounting for 0.5% to 1% of congenital heart disease.4,5 The characteristic abnormality is PAPVR of part of or the entire right lung to the IVC, either below the diaphragm or at the junction of the IVC and the right atrium. The rare combination (3%-5%) of an association of PAPVR, right lung hypoplasia, and dextroposition of the heart is designated scimitar syndrome. The scimitar vein sign is a characteristic chest roentgenographic finding of a crescentlike shadow in the right lower lung field where the curvilinear dimension gives the appearance of a scimitar sword.
Related: Another Reason Not to Smoke: Acute Eosinophilic Pneunomia
Normally, the pulmonary veins from the right and left lung carry oxygenated blood into the left atrium, then to the left ventricle, and then flowing out systemically. The SCV and IVC return the deoxygenated blood from the body system to the right atrium. From the right atrium, blood flows into the right ventricle, and then through pulmonary arteries, reaching the lungs where oxygenation occurs. In this syndrome, a left-to-right shunt is established when the anomalous pulmonary vein drains blood from the right lung into the IVC, resulting in an increased risk of developing right ventricular failure due to long-standing right ventricular volume overload.
Presentation and Diagnosis
There are two clinical presentations of scimitar syndrome: infantile and pediatric/adult. Infantile scimitar syndrome has a clinical presentation of tachypnea and heart failure within the first 2 months of life, with a high mortality rate. The pediatric/adult type is milder and frequently asymptomatic, and the diagnosis is usually incidental after performing an imaging study. Scimitar vein sign appears in 70% of the noninfantile cases, and lung hypoplasia is less severe. A spirometry may reveal mild deficits in vital capacity and FEV1. An electrocardiogram may show right ventricular hypertrophy.
Cardiac catheterization is required to confirm the diagnosis. Additionally, this procedure can help in the assessment of the pulmonary venous drainage course, pulmonary artery anatomy and pressure, scimitar vein stenosis, and presence of left-to-right shunt or other cardiac anomalies, if present. Other modalities have been suggested as alternative methods for diagnosing this condition, including the use of coronary CT and 3D echocardiography.6,7 However, these diagnostic tests are not available in all facilities and are very costly.
Treatment and Prognosis
Vida and colleagues conducted a multicentric study for the European Congenital Heart Surgeons Association on scimitar syndrome.8 Data were collected from 1997 to 2007 for 68 patients who underwent a surgical procedure. A total of 11 patients were categorized as late onset, and when compared with the infantile category, they had fewer postoperatory complications, hospital mortality, late mortality, and were less likely to develop pulmonary hypertension. Both pulmonary stenosis and pulmonary hypertension were linked with poor outcomes. It seems the younger the patient (infantile), the higher the possibility of complications and mortality. Adults who are incidentally diagnosed have a better outcome if asymptomatic. Findings such as hypoplastic lungs may predispose these patients to developing recurrent pneumonias.8,9
Related: Prevention of Venous Thromboembolism After Total Joint Replacement: A Rivaroxaban Update
Dusenbery and colleagues documented in a cohort study the relationship between poor survival and other variables. Significant variables included age at presentation, nonatrial septal defect (non-ASD) congenital heart disease, left pulmonary vein stenosis, and pulmonary artery pressure (PAP) at the time of presentation. Predictors of survival for nonsurgical patients were directly related to PAP at presentation and absence of non-ASD congenital heart disease. If the patient’s PAP is less than half of the systemic pressure, the survival is near 100% at 5 years from initial presentation.9
Surgery is the definitive treatment for PAPVR. However, asymptomatic patients with PAPVR with small left-to-right shunt do not require intervention, as the defect has no significant clinical impact, and patients have a normal life expectancy without correction.10
Surgical treatment may be considered in the following circumstances:
- A hemodynamically significant left-to-right shunt (a ratio of pulmonary to systemic blood flow is greater than 2:1), often manifested as right ventricular volume overload
- Recurrent pulmonary infections
- Compression or obstruction of surrounding structures caused by the anomalous vein
- During surgical repair of other major cardiac lesions, depending on the surgical risk of a repair and level and degree of shunting
Surgical options include redirecting the venous drainage to the left atria, ligation/embolization of vascular supply to the sequestered lobe, and pneumonectomy. The procedure complications may include thrombosis of the scimitar vein, lung infarct, hemoptysis, and pulmonary hypertension, which may lead to resection of the lung.11,12 Surgical procedures are recommended in cases where the patient has had recurrent lung infections or a significant degree of shunting. Studies have compared both approaches, demonstrating a better outcome after 10 years for those patients who were medically treated considering the aforementioned surgical indications.
Conclusion
Scimitar syndrome is a rare but welldescribed constellation of cardiopulmonary anomalies, accounting for 0.5% to 1% of congenital heart disease. It is a variant of PAPVR, in which part of or even the entire right lung is drained by right pulmonary veins that connect anomalously to the IVC. Although a diagnosis can be made by chest radiograph, further imaging is needed to corroborate the diagnosis and demonstrate other associated abnormalities.
Additional tests have been described in the literature, but these procedures are not available in all facilities and may incur a higher cost. Therefore, CT angiographic reconstruction is an alternative, noninvasive procedure. Surgery is the definitive treatment; however, asymptomatic patients with PAPVR and small left-to-right shunt do not require intervention.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Cooper G. Case of malformation of the thoracic viscera: consisting of imperfect development of the right lung, and transposition of the heart. London Med Gaz. 1836;18:600-602.
2. Spentzouris G, Zandian A, Cesmebasi A, et al. The clinical anatomy of the inferior vena cava: a review of common congenital anomalies and the considerations for clinicians. Clin Anat. 2014;27(8):1234-1243.
3. Neill CA, Ferencz C, Sabiston DC, Sheldon H. The familial occurrence of hypoplastic right lung with systemic arterial supply and venous drainage “scimitar syndrome.” Bull Johns Hopkins Hosp. 1960;107:1-21.
4. Ward KE, Mullins CE. Anomalous pulmonary venous connections, pulmonary vein stenosis, and atresia of the common pulmonary vein. In: Garson A, Bricker JT, Fisher DJ, Neish SR, eds. The Science and Practice of Pediatric Cardiology. 2nd ed. Baltimore, MD: Williams and Wilkins; 1998:1431-1461.
5. Garcia-Barreto L, Vega W, Deliz R, Rodriguez W. Right hilar abnormality in a young man. Respiration. 1996;63(4):246-250.
6. Simmons DB, Menon RS, Pomeroy WL, Batts TC, Slim AM. An unusual presentation of scimitar syndrome in a military service member. Case Rep Vasc Med. 2013;2013:632402.
7. Palios J, Pernetz MA, Clements S Jr, Lerakis S. Three-dimensional echocardiography images showing anomalous pulmonary venous return in an adult with scimitar syndrome. Echocardiography. 2014;31(3):E103.
8. Vida VL, Padalino MA, Boccuzzo G, et al. Scimitar syndrome: a European Congenital Heart Surgeons Association (ECHSA) multicentric study. Circulation. 2010;122(12):1159-1166.
9. Dusenbery SM, Geva T, Seale A, et al. Outcome predictors and implications for management of scimitar syndrome. Am Heart J. 2013;165(5):770-777.
10. Sehgal A, Loughran-Fowlds A. Scimitar syndrome. Indian J Pediatr. 2005;72(3):249-251.
11. Najm HK, Williams WG, Coles JG, Rebeyka IM, Freedom RM. Scimitar syndrome: twenty years’ experience and results of repair. J Thorac Cardiovasc Surg. 1996;112(5):1161-1169.
12. Dupuis C, Charaf LA, Brevière GM, Abou P, Rémy-Jardin M, Helmius G. The “adult” form of the scimitar syndrome. Am J Cardiol. 1992;70(4):502-507.
Anatomic variations may result in abnormal return from the pulmonary veins to the right side of the heart. This group of congenital anomalies, also known as partial anomalous pulmonary venous return (PAPVR), may connect oxygenated blood from the pulmonary vein to a systemic vein before reaching the right atrium. The most common PAPVR is derived from the left upper pulmonary vein, which then connects to the left innominate vein and drains into the superior vena cava (SVC).
Scimitar syndrome is a rare PAPVR variant in which part of or the entire right lung is drained by the pulmonary vein into the inferior vena cava (IVC), giving the curvilinear dimension the appearance of a Middle Eastern sword (scimitar). The syndrome is frequently associated with other abnormalities, such as right lung hypoplasia and abnormal right lung lobation, dextroposition of the heart, right pulmonary artery hypoplasia, systemic arterial blood supply to the right lower lung from the infradiaphragmatic aorta, atrial septal defects of the secundum type, right-sided diaphragmatic hernia, and horseshoe lung.1,2 The syndrome was first described in 1836 by Cooper during an autopsy of an infant, and Dotter diagnosed the first symptomatic patient in 1949.3,4
Case Report
A 62-year-old man, former smoker (40 pack-year), with a past medical history of arterial hypertension and asthma visited the clinic, reporting exertional dyspnea. He also reported oppressive, retrosternally located exertional chest pain, 6/10 in intensity, of 3 minutes’ duration that radiated to the right chest and ameliorated with rest. Symptoms had occurred every other day for the past year. His physical exam was remarkable for central obesity. Lung auscultation was essentially clear. There was no jugular vein distention. The patient’s heart showed a regular rate and rhythm without evidence of murmurs or gallops. There was no evidence of leg edema or cyanosis. The patient’s resting oxygen saturation of 98% remained unchanged after exercise.
Related: Venous Thromboembolism Prophylaxis in Acutely Ill Veterans With Respiratory Disease
An electrocardiogram showed normal sinus rhythm with no ischemic changes. A pulmonary function test showed a forced expiratory volume (FEV1) of 1.44 L (61% of predicted), forced vital capacity (FVC) of 1.99 L (68% of predicted), and slow vital capacity (SVC) of 2.09 L (60% of predicted), with an FEV1/SVC ratio of 68% of predicted. These results suggested moderate-to-severe obstructive ventilatory impairment.
There was no response to bronchodilator therapy. Lung volumes were measured by plethysmography. The residual volume (RV), total lung capacity (TLC), and RV/TLC ratio were 2.57 L (147% of predicted), 4.66 L (88% of predicted), and 55%, respectively, suggesting severe air trapping. Diffusion lung capacity (DLCO) testing revealed 16.95 mL/min/mm Hg (73% of predicted) when corrected by hemoglobin and DLCO/alveolar volume of 4.97 mL/min/mm Hg/L (114% of predicted). This result was consistent with a mild reduction of gas transfer, which normalized when corrected by alveolar volume.
A posteroanterior chest radiograph image was remarkable for mediastinal shifting toward the right side, volume loss of the right lung, and evidence of a previous gunshot on the right chest wall (Figure 1). Previous chest imaging done in October 2009 showed an opacification of the right lower lung with indistinctness of the right cardiac border and partial obliteration of the right hemidiaphragm. The patient was treated with inhaled steroids and long- acting bronchodilators with partial improvement in dyspnea symptoms.
Myocardial perfusion imaging revealed scintigraphic evidence of heart rate-induced ischemia on the inferior and apical wall segments of the left ventricular myocardium. A transthoracic echocardiogram showed a very poor echocardiographic window. Left ventricular function seemed preserved. Transesophageal echocardiography was scheduled, but the patient missed the appointment.
Cardiac catheterization was only remarkable for 40% to 50% obstruction of the mid-left anterior descending artery, which did not explain the patient’s dyspnea or chest pain. Right side pressures were described as follows: right atrial mean, 10 mm Hg; right ventricle, 36/8 mm Hg; pulmonary artery, 33/16 mm Hg; pulmonary artery mean, 23 mm Hg; pulmonary capillary wedge pressure, 12 mm Hg; and a mean arterial pressure of 100 mm Hg. He had a left ventricle ejection fraction of 60%.
Because images suggested dextroposition of the heart and right lung hypoplasia, a chest computed tomography (CT) and angiography were done (Figure 2). The images showed hypoplasia of the right lung field with an anomalous venous return from the right midlung, having a vertical contour that drained into the supradiaphragmatic IVC. In addition, CT reconstruction demarcated the last mentioned contour draining into the IVC, consistent with scimitar syndrome (Figure 3). The patient was treated conservatively due to age, optimizing therapy for obstructive lung and cardiovascular disease.
Discussion
Partial anomalous pulmonary venous return is a relatively uncommon congenital anomaly, accounting for 0.5% to 1% of congenital heart disease.4,5 The characteristic abnormality is PAPVR of part of or the entire right lung to the IVC, either below the diaphragm or at the junction of the IVC and the right atrium. The rare combination (3%-5%) of an association of PAPVR, right lung hypoplasia, and dextroposition of the heart is designated scimitar syndrome. The scimitar vein sign is a characteristic chest roentgenographic finding of a crescentlike shadow in the right lower lung field where the curvilinear dimension gives the appearance of a scimitar sword.
Related: Another Reason Not to Smoke: Acute Eosinophilic Pneunomia
Normally, the pulmonary veins from the right and left lung carry oxygenated blood into the left atrium, then to the left ventricle, and then flowing out systemically. The SCV and IVC return the deoxygenated blood from the body system to the right atrium. From the right atrium, blood flows into the right ventricle, and then through pulmonary arteries, reaching the lungs where oxygenation occurs. In this syndrome, a left-to-right shunt is established when the anomalous pulmonary vein drains blood from the right lung into the IVC, resulting in an increased risk of developing right ventricular failure due to long-standing right ventricular volume overload.
Presentation and Diagnosis
There are two clinical presentations of scimitar syndrome: infantile and pediatric/adult. Infantile scimitar syndrome has a clinical presentation of tachypnea and heart failure within the first 2 months of life, with a high mortality rate. The pediatric/adult type is milder and frequently asymptomatic, and the diagnosis is usually incidental after performing an imaging study. Scimitar vein sign appears in 70% of the noninfantile cases, and lung hypoplasia is less severe. A spirometry may reveal mild deficits in vital capacity and FEV1. An electrocardiogram may show right ventricular hypertrophy.
Cardiac catheterization is required to confirm the diagnosis. Additionally, this procedure can help in the assessment of the pulmonary venous drainage course, pulmonary artery anatomy and pressure, scimitar vein stenosis, and presence of left-to-right shunt or other cardiac anomalies, if present. Other modalities have been suggested as alternative methods for diagnosing this condition, including the use of coronary CT and 3D echocardiography.6,7 However, these diagnostic tests are not available in all facilities and are very costly.
Treatment and Prognosis
Vida and colleagues conducted a multicentric study for the European Congenital Heart Surgeons Association on scimitar syndrome.8 Data were collected from 1997 to 2007 for 68 patients who underwent a surgical procedure. A total of 11 patients were categorized as late onset, and when compared with the infantile category, they had fewer postoperatory complications, hospital mortality, late mortality, and were less likely to develop pulmonary hypertension. Both pulmonary stenosis and pulmonary hypertension were linked with poor outcomes. It seems the younger the patient (infantile), the higher the possibility of complications and mortality. Adults who are incidentally diagnosed have a better outcome if asymptomatic. Findings such as hypoplastic lungs may predispose these patients to developing recurrent pneumonias.8,9
Related: Prevention of Venous Thromboembolism After Total Joint Replacement: A Rivaroxaban Update
Dusenbery and colleagues documented in a cohort study the relationship between poor survival and other variables. Significant variables included age at presentation, nonatrial septal defect (non-ASD) congenital heart disease, left pulmonary vein stenosis, and pulmonary artery pressure (PAP) at the time of presentation. Predictors of survival for nonsurgical patients were directly related to PAP at presentation and absence of non-ASD congenital heart disease. If the patient’s PAP is less than half of the systemic pressure, the survival is near 100% at 5 years from initial presentation.9
Surgery is the definitive treatment for PAPVR. However, asymptomatic patients with PAPVR with small left-to-right shunt do not require intervention, as the defect has no significant clinical impact, and patients have a normal life expectancy without correction.10
Surgical treatment may be considered in the following circumstances:
- A hemodynamically significant left-to-right shunt (a ratio of pulmonary to systemic blood flow is greater than 2:1), often manifested as right ventricular volume overload
- Recurrent pulmonary infections
- Compression or obstruction of surrounding structures caused by the anomalous vein
- During surgical repair of other major cardiac lesions, depending on the surgical risk of a repair and level and degree of shunting
Surgical options include redirecting the venous drainage to the left atria, ligation/embolization of vascular supply to the sequestered lobe, and pneumonectomy. The procedure complications may include thrombosis of the scimitar vein, lung infarct, hemoptysis, and pulmonary hypertension, which may lead to resection of the lung.11,12 Surgical procedures are recommended in cases where the patient has had recurrent lung infections or a significant degree of shunting. Studies have compared both approaches, demonstrating a better outcome after 10 years for those patients who were medically treated considering the aforementioned surgical indications.
Conclusion
Scimitar syndrome is a rare but welldescribed constellation of cardiopulmonary anomalies, accounting for 0.5% to 1% of congenital heart disease. It is a variant of PAPVR, in which part of or even the entire right lung is drained by right pulmonary veins that connect anomalously to the IVC. Although a diagnosis can be made by chest radiograph, further imaging is needed to corroborate the diagnosis and demonstrate other associated abnormalities.
Additional tests have been described in the literature, but these procedures are not available in all facilities and may incur a higher cost. Therefore, CT angiographic reconstruction is an alternative, noninvasive procedure. Surgery is the definitive treatment; however, asymptomatic patients with PAPVR and small left-to-right shunt do not require intervention.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Anatomic variations may result in abnormal return from the pulmonary veins to the right side of the heart. This group of congenital anomalies, also known as partial anomalous pulmonary venous return (PAPVR), may connect oxygenated blood from the pulmonary vein to a systemic vein before reaching the right atrium. The most common PAPVR is derived from the left upper pulmonary vein, which then connects to the left innominate vein and drains into the superior vena cava (SVC).
Scimitar syndrome is a rare PAPVR variant in which part of or the entire right lung is drained by the pulmonary vein into the inferior vena cava (IVC), giving the curvilinear dimension the appearance of a Middle Eastern sword (scimitar). The syndrome is frequently associated with other abnormalities, such as right lung hypoplasia and abnormal right lung lobation, dextroposition of the heart, right pulmonary artery hypoplasia, systemic arterial blood supply to the right lower lung from the infradiaphragmatic aorta, atrial septal defects of the secundum type, right-sided diaphragmatic hernia, and horseshoe lung.1,2 The syndrome was first described in 1836 by Cooper during an autopsy of an infant, and Dotter diagnosed the first symptomatic patient in 1949.3,4
Case Report
A 62-year-old man, former smoker (40 pack-year), with a past medical history of arterial hypertension and asthma visited the clinic, reporting exertional dyspnea. He also reported oppressive, retrosternally located exertional chest pain, 6/10 in intensity, of 3 minutes’ duration that radiated to the right chest and ameliorated with rest. Symptoms had occurred every other day for the past year. His physical exam was remarkable for central obesity. Lung auscultation was essentially clear. There was no jugular vein distention. The patient’s heart showed a regular rate and rhythm without evidence of murmurs or gallops. There was no evidence of leg edema or cyanosis. The patient’s resting oxygen saturation of 98% remained unchanged after exercise.
Related: Venous Thromboembolism Prophylaxis in Acutely Ill Veterans With Respiratory Disease
An electrocardiogram showed normal sinus rhythm with no ischemic changes. A pulmonary function test showed a forced expiratory volume (FEV1) of 1.44 L (61% of predicted), forced vital capacity (FVC) of 1.99 L (68% of predicted), and slow vital capacity (SVC) of 2.09 L (60% of predicted), with an FEV1/SVC ratio of 68% of predicted. These results suggested moderate-to-severe obstructive ventilatory impairment.
There was no response to bronchodilator therapy. Lung volumes were measured by plethysmography. The residual volume (RV), total lung capacity (TLC), and RV/TLC ratio were 2.57 L (147% of predicted), 4.66 L (88% of predicted), and 55%, respectively, suggesting severe air trapping. Diffusion lung capacity (DLCO) testing revealed 16.95 mL/min/mm Hg (73% of predicted) when corrected by hemoglobin and DLCO/alveolar volume of 4.97 mL/min/mm Hg/L (114% of predicted). This result was consistent with a mild reduction of gas transfer, which normalized when corrected by alveolar volume.
A posteroanterior chest radiograph image was remarkable for mediastinal shifting toward the right side, volume loss of the right lung, and evidence of a previous gunshot on the right chest wall (Figure 1). Previous chest imaging done in October 2009 showed an opacification of the right lower lung with indistinctness of the right cardiac border and partial obliteration of the right hemidiaphragm. The patient was treated with inhaled steroids and long- acting bronchodilators with partial improvement in dyspnea symptoms.
Myocardial perfusion imaging revealed scintigraphic evidence of heart rate-induced ischemia on the inferior and apical wall segments of the left ventricular myocardium. A transthoracic echocardiogram showed a very poor echocardiographic window. Left ventricular function seemed preserved. Transesophageal echocardiography was scheduled, but the patient missed the appointment.
Cardiac catheterization was only remarkable for 40% to 50% obstruction of the mid-left anterior descending artery, which did not explain the patient’s dyspnea or chest pain. Right side pressures were described as follows: right atrial mean, 10 mm Hg; right ventricle, 36/8 mm Hg; pulmonary artery, 33/16 mm Hg; pulmonary artery mean, 23 mm Hg; pulmonary capillary wedge pressure, 12 mm Hg; and a mean arterial pressure of 100 mm Hg. He had a left ventricle ejection fraction of 60%.
Because images suggested dextroposition of the heart and right lung hypoplasia, a chest computed tomography (CT) and angiography were done (Figure 2). The images showed hypoplasia of the right lung field with an anomalous venous return from the right midlung, having a vertical contour that drained into the supradiaphragmatic IVC. In addition, CT reconstruction demarcated the last mentioned contour draining into the IVC, consistent with scimitar syndrome (Figure 3). The patient was treated conservatively due to age, optimizing therapy for obstructive lung and cardiovascular disease.
Discussion
Partial anomalous pulmonary venous return is a relatively uncommon congenital anomaly, accounting for 0.5% to 1% of congenital heart disease.4,5 The characteristic abnormality is PAPVR of part of or the entire right lung to the IVC, either below the diaphragm or at the junction of the IVC and the right atrium. The rare combination (3%-5%) of an association of PAPVR, right lung hypoplasia, and dextroposition of the heart is designated scimitar syndrome. The scimitar vein sign is a characteristic chest roentgenographic finding of a crescentlike shadow in the right lower lung field where the curvilinear dimension gives the appearance of a scimitar sword.
Related: Another Reason Not to Smoke: Acute Eosinophilic Pneunomia
Normally, the pulmonary veins from the right and left lung carry oxygenated blood into the left atrium, then to the left ventricle, and then flowing out systemically. The SCV and IVC return the deoxygenated blood from the body system to the right atrium. From the right atrium, blood flows into the right ventricle, and then through pulmonary arteries, reaching the lungs where oxygenation occurs. In this syndrome, a left-to-right shunt is established when the anomalous pulmonary vein drains blood from the right lung into the IVC, resulting in an increased risk of developing right ventricular failure due to long-standing right ventricular volume overload.
Presentation and Diagnosis
There are two clinical presentations of scimitar syndrome: infantile and pediatric/adult. Infantile scimitar syndrome has a clinical presentation of tachypnea and heart failure within the first 2 months of life, with a high mortality rate. The pediatric/adult type is milder and frequently asymptomatic, and the diagnosis is usually incidental after performing an imaging study. Scimitar vein sign appears in 70% of the noninfantile cases, and lung hypoplasia is less severe. A spirometry may reveal mild deficits in vital capacity and FEV1. An electrocardiogram may show right ventricular hypertrophy.
Cardiac catheterization is required to confirm the diagnosis. Additionally, this procedure can help in the assessment of the pulmonary venous drainage course, pulmonary artery anatomy and pressure, scimitar vein stenosis, and presence of left-to-right shunt or other cardiac anomalies, if present. Other modalities have been suggested as alternative methods for diagnosing this condition, including the use of coronary CT and 3D echocardiography.6,7 However, these diagnostic tests are not available in all facilities and are very costly.
Treatment and Prognosis
Vida and colleagues conducted a multicentric study for the European Congenital Heart Surgeons Association on scimitar syndrome.8 Data were collected from 1997 to 2007 for 68 patients who underwent a surgical procedure. A total of 11 patients were categorized as late onset, and when compared with the infantile category, they had fewer postoperatory complications, hospital mortality, late mortality, and were less likely to develop pulmonary hypertension. Both pulmonary stenosis and pulmonary hypertension were linked with poor outcomes. It seems the younger the patient (infantile), the higher the possibility of complications and mortality. Adults who are incidentally diagnosed have a better outcome if asymptomatic. Findings such as hypoplastic lungs may predispose these patients to developing recurrent pneumonias.8,9
Related: Prevention of Venous Thromboembolism After Total Joint Replacement: A Rivaroxaban Update
Dusenbery and colleagues documented in a cohort study the relationship between poor survival and other variables. Significant variables included age at presentation, nonatrial septal defect (non-ASD) congenital heart disease, left pulmonary vein stenosis, and pulmonary artery pressure (PAP) at the time of presentation. Predictors of survival for nonsurgical patients were directly related to PAP at presentation and absence of non-ASD congenital heart disease. If the patient’s PAP is less than half of the systemic pressure, the survival is near 100% at 5 years from initial presentation.9
Surgery is the definitive treatment for PAPVR. However, asymptomatic patients with PAPVR with small left-to-right shunt do not require intervention, as the defect has no significant clinical impact, and patients have a normal life expectancy without correction.10
Surgical treatment may be considered in the following circumstances:
- A hemodynamically significant left-to-right shunt (a ratio of pulmonary to systemic blood flow is greater than 2:1), often manifested as right ventricular volume overload
- Recurrent pulmonary infections
- Compression or obstruction of surrounding structures caused by the anomalous vein
- During surgical repair of other major cardiac lesions, depending on the surgical risk of a repair and level and degree of shunting
Surgical options include redirecting the venous drainage to the left atria, ligation/embolization of vascular supply to the sequestered lobe, and pneumonectomy. The procedure complications may include thrombosis of the scimitar vein, lung infarct, hemoptysis, and pulmonary hypertension, which may lead to resection of the lung.11,12 Surgical procedures are recommended in cases where the patient has had recurrent lung infections or a significant degree of shunting. Studies have compared both approaches, demonstrating a better outcome after 10 years for those patients who were medically treated considering the aforementioned surgical indications.
Conclusion
Scimitar syndrome is a rare but welldescribed constellation of cardiopulmonary anomalies, accounting for 0.5% to 1% of congenital heart disease. It is a variant of PAPVR, in which part of or even the entire right lung is drained by right pulmonary veins that connect anomalously to the IVC. Although a diagnosis can be made by chest radiograph, further imaging is needed to corroborate the diagnosis and demonstrate other associated abnormalities.
Additional tests have been described in the literature, but these procedures are not available in all facilities and may incur a higher cost. Therefore, CT angiographic reconstruction is an alternative, noninvasive procedure. Surgery is the definitive treatment; however, asymptomatic patients with PAPVR and small left-to-right shunt do not require intervention.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Cooper G. Case of malformation of the thoracic viscera: consisting of imperfect development of the right lung, and transposition of the heart. London Med Gaz. 1836;18:600-602.
2. Spentzouris G, Zandian A, Cesmebasi A, et al. The clinical anatomy of the inferior vena cava: a review of common congenital anomalies and the considerations for clinicians. Clin Anat. 2014;27(8):1234-1243.
3. Neill CA, Ferencz C, Sabiston DC, Sheldon H. The familial occurrence of hypoplastic right lung with systemic arterial supply and venous drainage “scimitar syndrome.” Bull Johns Hopkins Hosp. 1960;107:1-21.
4. Ward KE, Mullins CE. Anomalous pulmonary venous connections, pulmonary vein stenosis, and atresia of the common pulmonary vein. In: Garson A, Bricker JT, Fisher DJ, Neish SR, eds. The Science and Practice of Pediatric Cardiology. 2nd ed. Baltimore, MD: Williams and Wilkins; 1998:1431-1461.
5. Garcia-Barreto L, Vega W, Deliz R, Rodriguez W. Right hilar abnormality in a young man. Respiration. 1996;63(4):246-250.
6. Simmons DB, Menon RS, Pomeroy WL, Batts TC, Slim AM. An unusual presentation of scimitar syndrome in a military service member. Case Rep Vasc Med. 2013;2013:632402.
7. Palios J, Pernetz MA, Clements S Jr, Lerakis S. Three-dimensional echocardiography images showing anomalous pulmonary venous return in an adult with scimitar syndrome. Echocardiography. 2014;31(3):E103.
8. Vida VL, Padalino MA, Boccuzzo G, et al. Scimitar syndrome: a European Congenital Heart Surgeons Association (ECHSA) multicentric study. Circulation. 2010;122(12):1159-1166.
9. Dusenbery SM, Geva T, Seale A, et al. Outcome predictors and implications for management of scimitar syndrome. Am Heart J. 2013;165(5):770-777.
10. Sehgal A, Loughran-Fowlds A. Scimitar syndrome. Indian J Pediatr. 2005;72(3):249-251.
11. Najm HK, Williams WG, Coles JG, Rebeyka IM, Freedom RM. Scimitar syndrome: twenty years’ experience and results of repair. J Thorac Cardiovasc Surg. 1996;112(5):1161-1169.
12. Dupuis C, Charaf LA, Brevière GM, Abou P, Rémy-Jardin M, Helmius G. The “adult” form of the scimitar syndrome. Am J Cardiol. 1992;70(4):502-507.
1. Cooper G. Case of malformation of the thoracic viscera: consisting of imperfect development of the right lung, and transposition of the heart. London Med Gaz. 1836;18:600-602.
2. Spentzouris G, Zandian A, Cesmebasi A, et al. The clinical anatomy of the inferior vena cava: a review of common congenital anomalies and the considerations for clinicians. Clin Anat. 2014;27(8):1234-1243.
3. Neill CA, Ferencz C, Sabiston DC, Sheldon H. The familial occurrence of hypoplastic right lung with systemic arterial supply and venous drainage “scimitar syndrome.” Bull Johns Hopkins Hosp. 1960;107:1-21.
4. Ward KE, Mullins CE. Anomalous pulmonary venous connections, pulmonary vein stenosis, and atresia of the common pulmonary vein. In: Garson A, Bricker JT, Fisher DJ, Neish SR, eds. The Science and Practice of Pediatric Cardiology. 2nd ed. Baltimore, MD: Williams and Wilkins; 1998:1431-1461.
5. Garcia-Barreto L, Vega W, Deliz R, Rodriguez W. Right hilar abnormality in a young man. Respiration. 1996;63(4):246-250.
6. Simmons DB, Menon RS, Pomeroy WL, Batts TC, Slim AM. An unusual presentation of scimitar syndrome in a military service member. Case Rep Vasc Med. 2013;2013:632402.
7. Palios J, Pernetz MA, Clements S Jr, Lerakis S. Three-dimensional echocardiography images showing anomalous pulmonary venous return in an adult with scimitar syndrome. Echocardiography. 2014;31(3):E103.
8. Vida VL, Padalino MA, Boccuzzo G, et al. Scimitar syndrome: a European Congenital Heart Surgeons Association (ECHSA) multicentric study. Circulation. 2010;122(12):1159-1166.
9. Dusenbery SM, Geva T, Seale A, et al. Outcome predictors and implications for management of scimitar syndrome. Am Heart J. 2013;165(5):770-777.
10. Sehgal A, Loughran-Fowlds A. Scimitar syndrome. Indian J Pediatr. 2005;72(3):249-251.
11. Najm HK, Williams WG, Coles JG, Rebeyka IM, Freedom RM. Scimitar syndrome: twenty years’ experience and results of repair. J Thorac Cardiovasc Surg. 1996;112(5):1161-1169.
12. Dupuis C, Charaf LA, Brevière GM, Abou P, Rémy-Jardin M, Helmius G. The “adult” form of the scimitar syndrome. Am J Cardiol. 1992;70(4):502-507.
Testosterone Replacement Therapy: Playing Catch-up With Patients
The objective of this article is to help primary care providers (PCPs) council patients regarding testosterone replacement therapy (TRT). This case will present a patient who initiated TRT at a community-based alternative medicine clinic. The case will be followed by a discussion regarding the standard diagnosis of hypogonadism, the potential benefits and risks of TRT, and a review of the current clinical guideline recommendations. Examples of information being disseminated to the general public by the complementary and alternative medicine (CAM) providers will be briefly reviewed for an increased awareness of the questions patients may pose regarding TRT.
Background
From 2000 to 2011, total testosterone sales increased 12-fold globally.1 Possible causes for the increase involved the aging population, newer options for TRT administration, and increased direct-to-consumer advertising. A low testosterone level (sometimes referred to as low T in consumer marketing materials) is associated with a variety of medical conditions (ie, low mood, increased body fat, declining athletic performance, and decreased sexual performance) that have become increasingly prevalent among middle aged and older men.2 It has also received attention as an intervention to reverse frailty and sarcopenia.3
Testosterone replacement therapy options include injectable solutions, transdermal gels and patches, pellet implants, or buccal tablets. The ease of administration of transdermal testosterone comes at a relatively high cost. Injectable testosterone preparations are generally the least expensive option, and many patients choose injections for this reason.
Related: Keeping an Open Mind on HRT
Testosterone prescriptions were most frequently written by PCPs with 36% coming from family practitioners and 20.1% from internal medicine practices, according to a Kaiser Permanente study.4 Endocrinologists (13.5%) and urologists (6.6%) were less likely to have written the prescriptions for patients.
Due, in part, to direct-to-consumer advertising and to the availability of online medical information, many men now present to their PCP questioning whether they might have low T. Others may have already started therapy at a CAM, integrative medicine, or anti-aging clinic.
Confusing the issue further, some CAM providers promote a variety of off-label medications and nutritional supplements for the treatment of low T, which seems to have struck a chord in the baby boomer generation. No other age group in history has tried to work so intensely on its physical condition and appearance.5 Much of the information marketed to consumers emphasizes that many traditionally trained physicians are not educated in the treatment of low T.
Case Report
Mr. C. is a 65-year-old man who was seen in the primary care clinic for the first time. He was accompanied by his much younger fiancée. She reported that Mr. C.’s energy and sexual interest were declining, and the patient reported his “get up and go had gotten up and left.” They sought medical advice from a CAM provider who ordered blood work and then explained that the symptoms were due to low testosterone. For the past 6 months he had been visiting the clinic weekly for testosterone injections.
Mr. C. reported feeling as good as a “40 year old.” He also reported that he started working with a personal trainer and had given up most junk food and alcohol. He had no symptoms of chest pain, erectile dysfunction, or significant urinary urgency, frequency, or nocturia.
Related: Will Testosterone Therapy Kill Your Patient?
The visits to a CAM provider had been an out-of-pocket expense, and he was hoping to transfer his treatment to the VA so the costs could be covered. Mr. C. failed to bring medical records from the other provider but remembered being told that all his tests were “fine” except for the low testosterone level.
His past history was notable for controlled type 2 diabetes mellitus for 8 years, hypertension, hyperlipidemia, and spinal stenosis. He had no history of benign prostatic hyperplasia or prostate cancer.
In addition to the testosterone (100 mg intramuscular injection weekly), his medication regimen included metoprolol 25 mg twice daily, atorvastatin 20 mg daily, acetaminophen 650 mg 3 times daily as needed, aspirin 81 mg daily, metformin 500 mg twice daily, vitamin D 2,000 IU daily, vitamin B12 1,000 mg daily, and Co-Q10 200 mg daily.
On physical examination, Mr. C.’s vitals were stable and his body mass index was in the overweight range at 29.8 kg/m2. His cardiopulmonary examination was normal. There was increased central obesity without palpable organomegaly. There was no gynecomastia, and he had normal amounts of axillary and pubic hair. There was no peripheral edema; his genitourinary examination included normal-sized testicles, and the prostate was smooth without nodules.
The PCP informed Mr. C. that he was familiar with the evaluation and management of testosterone therapy. He was advised that additional evaluation would be needed before determining whether the clinical benefit of TRT outweighed the potential risks.
Andropause
Testosterone levels in men are known to decline at a rate of 1% per year after aged 30 years.6 About 20% of men aged ≥ 60 years and 50% of men aged ≥ 80 years have low (hypogonadal) total testosterone levels.7 The clinical diagnosis of hypogonadism, however, is made on the basis of signs and symptoms consistent with androgen deficiency and a low serum morning testosterone level measured on serum on multiple occasions.8
Specific clinical signs and symptoms (“A” list) consistent with androgen deficiency include low libido and sexual activity; diminished spontaneous erections; gynecomastia; reduced facial, axillary, or pubic hair; small (≤ 5 mL) testes; inability to father children; loss of height, fractures, or other signs of bone loss; and hot flashes and night sweats.9
Less specific signs and symptoms (“B” list) of androgen deficiency include a decrease in energy or motivation, feelings of sadness or depression, poor concentration or memory, trouble sleeping, increased sleepiness, mild anemia, reduced muscle bulk or strength, increased body fat, and diminished physical performance.9
Making the clinical diagnosis of hypogonadism is challenging, because the clinical symptoms have a high prevalence in the older male population and overlap with many nonendocrine diseases. Testosterone replacement therapy has been associated weakly, but consistently, with improved sexual function,10-12 bone mineral density,13,14 fat free mass,13,14 strength,15,16 lipid profiles,17,18 insulin resistance,17,18 and with an increased time to ST segment depression during stress testing.19,20
Laboratory Evaluation
Serum total testosterone circulates in 3 forms: free testosterone, sex hormone-binding globulin (SHBG)-bound testosterone, and albumin-bound testosterone. Free testosterone is the most bio-available testosterone but represents only 2% to 3% of total testosterone.21 Whether total testosterone or free testosterone measurements most closely correlate with symptomatic androgen deficiency is a matter of debate.21 A total testosterone level is an appropriate screening test in young, healthy, and lean men for whom SHBG levels are presumably normal. However, a free or bioavailable testosterone level should be considered for men when there is a high likelihood of conditions that can affect SHBG levels.
Conditions that can decrease SHBG (and may result in a low total testosterone reading even when the free fraction may be normal) include obesity, metabolic syndrome, type 2 diabetes mellitus, hypothyroidism, nephrotic syndrome, chronic glucocorticoid use, and the use of progestins and anabolic steroids.21 Conditions that can increase SHBG (and may result in a normal total testosterone level in patients with hypogonadism, as they have low levels of free testosterone) include aging, cirrhosis, anticonvulsant use, hyperthyroidism, catabolic conditions, and HIV.21
Related: Effect of Statins on Total Testosterone Levels in Male Veterans
Serum testosterone levels generally peak in the early morning, followed by a progressive decline over the course of the day until they reach a nadir in the evening.21 Although it has been debated that morning testosterone levels are not necessary in older men due to a blunting of the circadian rhythm, many men aged 65 to 80 years who have low T in the afternoon will have normal testosterone levels when retested in the morning.22,23 Readings below a reference range of 280 ng/dL to 300 ng/dL on at least 2 different occasions support a diagnosis of hypogonadism.9
Follicle stimulating hormone (FSH) and luteinizing hormone (LH) laboratory tests may be ordered following confirmation of a low testosterone level. Prolactin levels and iron saturation can help evaluate for the presence of hyperprolactinemia and hemochromatosis, respectively. Primary hypogonadism due to testicular failure is diagnosed with high FSH, high LH, and low testosterone levels. Secondary hypogonadism due to hypothalamic or pituitary failure is diagnosed with low FSH, low LH, and low testosterone levels.
Hypothalamic or pituitary suppression from a nonendocrine condition may result in functional hypogonadotropic hypogonadism (FHH), which can be identified with low (or normal) FSH; low (or normal) LH; and low testosterone levels. Hypogonadotropic hypogonadism has been associated with depression, obesity, stress, and physical exertion; and FHH may also be associated with the use of multiple drugs and drug classes (spironolactone, anabolic and corticosteroids, ketoconazole, ethanol, anticonvulsants, immunosuppressants, tricyclic antidepressants, selective serotonin reuptake inhibitors, antipsychotics, and opioids).24,25 Even statin therapy has been associated with FHH.26,27 Testosterone levels will often recover if or when modifiable factors for FHH are corrected.28
Although there is no consensus on an absolute number that defines a low testosterone level, concern exists that there are economic incentives to raise the bar for normal and thereby increase the potential market for testosterone-raising products.29 Many commercial avenues for the treatment of low T do not follow the standards of the established medical community. Some websites suggest screening for low T with total and free testosterone levels for all men aged > 40 years. Others advise men to consider TRT if they have a total testosterone level of < 500 ng/dL or a free testosterone level that is not in the upper one-third range for men aged 21 to 49 years.30 Of even greater concern, Baillargeon and colleagues reported that 25% of all new androgen users had not had their testosterone levels measured in the 12 months before starting treatment.31 In another study, 40% of men who initiated TRT did not have a baseline measurement.32
Treatments
Before considering TRT, physicians need to emphasize lifestyle modifications as first-line treatment for hypogonadism. The most important modifications include weight loss, tobacco cessation, and moderation in alcohol use.
Patients need to be advised of possible adverse events (AEs) of TRT, which may include gynecomastia, polycythemia, sleep apnea, decreased high-density lipoprotein cholesterol, benign prostatic hypertrophy, infertility, testicular atrophy, and abnormal liver function tests. More recently, several studies have shown an association between TRT and an increase in cardiovascular complications, such as stroke, heart attacks, and death.
Prior to considering TRT, a careful history and physical examination, including a clinical prostate examination, should be performed. Minimum additional tests should include hematocrit, fasting lipid profile (FLP), complete metabolic profile (CMP), and prostate-specific antigen (PSA). Initiation of TRT is not recommended for patients with metastatic prostate cancer; breast cancer; an unevaluated prostate nodule; a PSA > 4 ng/mL (or > 3 ng/mL in African Americans or men with a first-degree relative with prostate cancer); hematocrit > 50%; untreated severe obstructive sleep apnea; uncontrolled or poorly controlled congestive heart failure; or an International Prostate Symptoms Score (IPSS) > 19.9
A past history of prostate cancer had previously been a contraindication for the use of TRT. However, more recent studies have shown that TRT can be used in those who have no evidence of active or metastatic disease and who are under the close supervision of a physician.33-35
Widespread screening is not recommended, and population-based surveys can be unreliable. Fifteen percent of healthy young men, for example, will have a low serum testosterone level in a given 24-hour period.9 Thirty percent of men with an initial testosterone level in the mildly hypogonadal range will have a normal testosterone level when retested; moreover the threshold below which AEs occur remains unknown.9
The goal of TRT is to achieve a total testosterone level in the 400 ng/mL to 700 ng/mL range with improved clinical signs and symptoms.9 Laboratory tests should be conducted at 3 months, 6 months, and then annually. These tests include hematocrit, PSA, and a testosterone level.32 Testing for CMP and FLP should also be considered. If, during therapy, the hematocrit is > 54%, the patient should be assessed for hypoxia and sleep apnea, and treatment should resume at a lower dose only when the hematocrit returns to baseline.9 A digital examination of the prostate is recommended for men with a PSA of > 0.6 ng/mL. A urologic consultation should be obtained for an increase in the PSA of > 1.4 ng/mL over 12 months, a PSA velocity of > 0.4 ng/mL per year (using the PSA after 6 months as a reference), or for an IPPS of > 19.9
Emerging Cardiovascular Concerns
The Testosterone for Older Men study, a randomized, placebo- controlled clinical trial of testosterone therapy in men with a high prevalence of cardiovascular disease, showed significantly greater improvements in leg-press, chest-press, and stair-climbing exercises while carrying a load compared with that in the placebo group.36 However, the study was stopped early due to an increased risk of cardiovascular AEs in those who received testosterone gel.
Vigen and colleagues examined a cohort of veterans who underwent coronary angiography and had a low serum testosterone level.37 The use of TRT in this cohort was also associated with an increased risk of adverse cardiovascular outcomes. This study generated several letters and a recent article in response that vigorously questioned the validity of the methods used and the conclusions reached.38-44 Prior clinical studies of TRT had not detected cardiac AEs, but these trials were generally of short duration and not powered for clinical endpoints.37
A FDA Safety Announcement as well as a VA National Pharmacy Benefits Management bulletin were based on the results of these studies.45 The FDA did not conclude that TRT increased the risk of stroke, heart attack, or death, but health care providers were asked to consider whether the benefits of TRT are likely to exceed the potential risk of treatment.
Direct-to-Consumer Marketing
Some direct-to-consumer marketing promotes the use of aromatase inhibitors, such as anastrozole. This class of medications prevents the conversion of endogenous and exogenous testosterone to estrogen by the aromatase enzyme, which is found predominately in abdominal adipose tissue. There is no evidence that naturally occurring elevations in estrogen cause low testosterone or that treatment of elevated estrogen with an aromatase inhibitor during TRT has any significant clinical benefit in terms of male sexuality.46 Nevertheless, some CAM providers now hypothesize that the increase in cardiovascular AEs with TRT noted in the recent studies may have been due to the increase in estrogen that is associated with TRT.46
The off-label use of clomiphene citrate to block the negative feedback of estrogen on the production of LH has been promoted as another potential treatment to increase testosterone levels. Luteinizing hormone is the pituitary analog of human chorionic gonadotropin (HCG). Many CAM providers also prescribe HCG to increase the testicles’ testosterone production.
Some consumer-focused media insist that the use of either clomiphene citrate or HCG will increase testosterone production and does not cause testicular atrophy, a known TRT- associated AE. This seems to increase the motivation of many men to try these off-label medications.
Some sources even posit a “conspiracy theory” that the FDA and pharmaceutical companies conspire to keep the price of transdermal TRT options high. Men are told that testosterone creams made at compounding pharmacies are much less expensive than are the transdermal pharmaceuticals, and they are urged to see a CAM provider to obtain a prescription for the compounded testosterone. In some cases, a sample prescription is included.47
Many supplements are available that claim to boost testosterone or suppress estrogen. Chrysin, for example, is a bioflavonoid that is marketed as having the potential to act as a natural aromatase inhibitor. Although studies have suggested the potential for chrysin to work in such a manner, the effectiveness may be attenuated by its low bioavailability in supplements.48 Long-term studies have not been conducted.49 Nettle root is a plant-derived compound that is stated to increase free testosterone levels by binding to SHBG, in place of testosterone, and by inhibiting the enzyme that converts testosterone to dihydrotestosterone. The clinical evidence of effectiveness is based on many open studies, and the significance and magnitude of the effect still needs more rigorous evaluation.50
Conclusions
Patients today are barraged with medical information through television, print advertising, radio, and the Internet. A recent study of online sources of herbal product information found that only 10.5% recommended a consultation with a health care professional and < 3% cited scientific literature to accompany their claims.51 Many patients present to their PCP with questions about TRT or have already started an intervention for low T. Complementary and alternative medicine providers of TRT have been able to capture a segment of the population that often has the motivation and disposable income to pursue nontraditional therapies.
All nutritional supplements contain a standard warning from the FDA: “The above statements have not been evaluated by the FDA. This product is not intended to diagnose, treat, cure or prevent any disease.” Providers should remind patients of the statement and point out the contradictions between the statement and the benefits touted by the supplement marketing literature.
Finally, despite the well- established role of testosterone in enhancing libido, its definitive role in erectile function had been controversial until evidence substantiated a key function for this hormone.52 Testosterone may facilitate erection by acting as a vasodilator of the penile arterioles and cavernous sinusoids and may ameliorate the response to the phosphodiesterase-5 inhibitors in hypogonadal men.53 Testosterone replacement alone in hypogonadal men can restore erectile dysfunction.51 However, hypogonadism is not a common finding in those with erectile dysfunction, only occurring in about 5% of cases.53
Allopathic providers are concerned about the vitality and sexual health of their aging male patients, but their enthusiasm for anti-aging treatments is often tempered by evidence-based studies that have shown a lack of efficacy or potentially serious health care risks. Unfortunately, many patients remain unaware of the controversies regarding TRT. For those patients who receive treatment through CAM providers and are convinced of the efficacy of their low-T treatment regimen, it is important to keep lines of communication open.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Handelsman D. Global trends in testosterone prescribing, 2000-2011: expanding the spectrum of prescription drug misuse. Med J Aust. 2013;199(8):548-551.
2. Hackett G. Testosterone and the heart. Int J Clin Pract. 2012;66(7):648-655.
3. Morley JE. Hypogonadism, testosterone, and nursing home residents. J Am Med Dir Assoc. 2013;14(6):381-383.
4. An J, Cheetham TC, Van Den Eeden S. PS3-36: testosterone replacement therapy patterns for aging males in a managed care setting. Clin Med Res. 2013;11(3):141.
5. Moschis G, Lee E, Marthur A, Strautman J. The Maturing Marketplace: Buying Habits of Baby Boomers and Their Parents. Westport, CT: Quorum Books; 2000.
6. Morley JE, Kaiser FE, Perry HM 3rd, et al. Longitudinal changes in testosterone, luteinizing hormone, and follicle stimulating hormone in healthy older men. Metabolism. 1997;46(4):410-413.
7. Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR; Baltimore Longitudinal Study of Aging. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab. 2001;86(2):724-731.
8. Basaria S. Male hypogonadism. Lancet. 2014;383 (9924):1250-1263.
9. Bhasin S, Cunningham GR, Hayes FJ, et al; Task Force, Endocrine Society. Testosterone therapy in men with androgen deficiency syndromes. J Clin Endocrinol Metab. 2010;95(6):2536-2559.
10. Wang C, Swerdloff RS, Iranmanesh A, et al. Transdermal testosterone gel improves sexual function, mood, muscle strength, and body composition parameters in hypogonadal men. J Clin Endocrinol Metab. 2000;85(8):2839-2853.
11. Bolona ER, Uranga MV, Haddad RM, et al. Testosterone use in men with sexual dysfunction: a systematic review and meta-analysis of randomized placebo-controlled trials. Mayo Clin Proc. 2007;82(1):20-28.
12. Isidori AM, Giannetta E, Gianfrilli D, et al. Effects of testosterone on sexual function in men: results of a meta-analysis. Clin Endocrinol (Oxf). 2005;63(4):381-394.
13. Isidori AM, Giannetta E, Greco EA, et al. Effects of testosterone on body composition, bone metabolism and serum lipid profile in middle-aged men: a meta-analysis. Clin Endocrinol (Oxf). 2005;63(3):280-293.
14. Snyder PJ, Peachey H, Berlin JA, et al. Effects of testosterone replacement in hypogonadal men. J Clin Endocrinol Metab. 2000;85(8):2670-2677.
15. Sih R, Morley JE, Kaiser FE, Perry HM 3rd, Patrick P, Ross C. Testosterone replacement in older hypogonadal men: a 12-month randomized controlled trial. J Clin Endocrinol Metab. 1997;82(6):1661-1667.
16. Travison TG, Basaria S, Storer TW, et al. Clinical meaningfulness of the changes in muscle performance and physical function associated with testosterone administration in older men with mobility limitation. J Gerontol A Biol Sci Med Sci. 2011;66(10):1090-1099.
17. Jones TH, Arver S, Behre HM, et al; TIMES2 Investigators. Testosterone replacement in hypogonadal men with type 2 diabetes and/or metabolic syndrome. Diabetes Care. 2011;34(4):828-837.
18. Jones TH, Saad F. The effects of testosterone on risk factors for, and the mediators of, the atherosclerotic process. Atherosclerosis. 2009;207(2):318-327.
19. Mathur A, Malkin C, Saeed B, Muthusamy R, Jones TH, Channer K. Long-term benefits of testosterone replacement therapy on angina threshold and atheroma in men. Eur J Endocrinol. 2009;161(3):443-449.
20. Malkin CJ, Pugh PJ, Morris PD, et al. Testosterone replacement in hypogonadal men with angina improves ischaemic threshold and quality of life. Heart. 2004;90(8):871-876.
21. Paduch DA, Brannigan RE, Fuchs EF, Kim ED, Marmar JL, Sandlow JI. White Paper: The Laboratory Diagnosis of Testosterone Deficiency. http://www.auanet.org/common/pdf/education/clinical -guidance/Testosterone-Deficiency-WhitePaper.pdf. Published 2013. Accessed April 9, 2015.
22. Crawford ED, Barqawi AB, O’Donnell C, Morgentaler A. The association of time of day and serum testosterone concentration in a large screening population. BJU Int. 2007;100(3):509-513.
23. Brambilla DJ, O’Donnell AB, Matsumoto AM, McKinlay JB. Intraindividual variation in levels of serum testosterone and other reproductive and adrenal hormones in men. Clin Endocrinol (Oxf). 2007;67(6):853-862.
24. Kumar P, Kumar N, Thakur DS, Patidar A. Male hypogonadism: symptoms and treatment. J Adv Pharm Technol Res. 2010;1(3):297-301.
25. Montgomery K. Sexual desire disorders. Psychiatry (Edgmont). 2008;5(6):50-55.
26. Corona G, Boddi V, Balercia G, et al. The effect of statin therapy on testosterone levels in subjects consulting for erectile dysfunction. J Sex Med. 2010; 7(4 pt 1):1547-1556.
27. Schooling CM, Yeung SLA, Freeman G, Cowling BJ. The effect of statins on testosterone in men and women, a systematic review and meta-analysis of randomized controlled trials. BMC Med. 2013;11:57.
28. Wu FC, Tajar A, Pye SR, et al; European Male Aging Study Group. Hypothalamic-pituitary-testicular axis disruptions in older men are differentially linked to age and modifiable risk factors: the European Male Aging Study. J Clin Endocrinol Metab. 2008;93(7):2737-2745.
29. Schwartz LM, Woloshin S. Low “T” as in “template”: how to sell disease. JAMA Intern Med. 2013;173(15):1460-1462.
30. Male Hormone Modulation Therapy, Part 2. Life Extension Vitamins Website. http://www.lifeextension vitamins.com/mahomothpa2.html. Accessed April 9, 2015.
31. Baillargeon J, Urban RJ, Ottenbacher KJ, Pierson KS, Goodwin JS. Trends in androgen prescribing in the United States, 2001 to 2011. JAMA Intern Med. 2013;173(15):1465-1466.
32. Layton JB, Li D, Meier CR, et al. Testosterone lab testing and initiation in the United Kingdom and the United States, 2000 to 2011. J Clin Endocrinol Metab. 2014;99(3):835-842.
33. Ramasamy R, Fisher ES, Schlegel PN. Testosterone replacement and prostate cancer. Indian J Urol. 2012;28(2):123-128.
34. Marks, LS, Mazer NA, Mostaghel E, et al. Effect of testosterone replacement therapy on prostate tissue in men with late-onset hypogonadism: a randomized controlled trial. JAMA. 2006;296(19):2351-2361.
35. Coward RM, Simhan J, Carson CC 3rd. Prostate-specific antigen changes and prostate cancer in hypogonadal men treated with testosterone replacement therapy. BJU Int. 2009;103(9):1179-1183.
36. Basaria S, Coviello AD, Travison TG, et al. Adverse events associated with testosterone administration. N Engl J Med. 2010;363(2):109-122.
37. Vigen R, O’Donnell Cl, Barón AE, et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310(17):1829-1836.
38. Morgentaler A, Traish A, Kacker R. Deaths and cardiovascular events in men receiving testosterone. JAMA. 2014;311(9):961-962.
39. Jones TH, Channer K. Deaths and cardiovascular events in men receiving testosterone. JAMA. 2014;311(9):962-963.
40. Katz J, Nadelberg R. Deaths and cardiovascular events in men receiving testosterone. JAMA. 2014;311(9):963.
41. Riche D, Baker WL, Koch CA. Deaths and cardiovascular events in men receiving testosterone. JAMA. 2014;311(9):963-964.
42. Dhindsa S, Batra M, Dandona P. Deaths and cardiovascular events in men receiving testosterone. JAMA. 2014;311(9):964.
43. Ho PM, Barón AE, Wierman M. Deaths and cardiovascular events in men receiving testosterone—reply. JAMA. 2014;311(9):964-965.
44. Traish AM, Guay AT, Morgentaler A. Death by testosterone? We think not! J Sex Med. 2014;11(3):624-629.
45. U.S. Department of Veterans Affairs, Veterans Health Administration (VHA), Pharmacy Benefit Management Services (PBM), Medical Advisory Panel (MAP), and Center for Medication Safety (VA Medsafe. National PBM Bulletin. Testosterone products and cardiovascular safety. http://www.pbm.va.gov/PBM/vacenterformedicationsafety/nationalpbmbulletin/Testosterone_Products_and_Cardiovascular_Safety_NATIONAL_PBM_BULLETIN_02.pdf. Published February 7, 2014. Accessed April 9, 2015.
46. Kacker R, Traish AM, Morgentaler A. Estrogens in men: clinical implications for sexual function and treatment of testosterone deficiency. J Sex Med. 2012;9(6):1681-1696.
47. Faloon W. Vindication. Life Extension Magazine Website. http://www.lef.org/magazine /mag2008/dec2008_Harvard-Experts-Recommend -Testosterone-Replacement_02.htm. Published December 2008. Accessed April 9,2015.
48. Walle T, Otake Y, Brubaker JA, Walle UK, Halushka PV. Disposition and metabolism of the flavonoid chrysin in normal volunteers. Br J Clin Pharmacol. 2001;51(2):143-146.
49. Jana K, Yin X, Schiffer, et al. Chrysin, a natural flavonoid enhances steroidogenesis and steroidogenic acute regulatory protein gene expression in mouse Leydig cells. J Endocrinol. 2008;197(2):315-323.
50. Chrubasik JE, Roufogalis BD, Wagner H, Chrubasik S. A comprehensive review on the stinging nettle effect and efficacy profiles. Part II: urticae radix. Phytomedicine. 2007;14(7-8):568-579.
51. Owens C, Baergen R, Puckett D. Online sources of herbal product information. Am J Med. 2014;127(2):109-115.
52. Blute W, Hakimian P, Kashanian J, Shteynshluyger A, Lee M, Shabsigh R. Erectile dysfunction and testosterone deficiency. Front Horm Res. 2009;37:108-122.
53. Mikhail N. Does testosterone have a role in erectile dysfunction? Am J Med. 2006;119(5):373-382.
The objective of this article is to help primary care providers (PCPs) council patients regarding testosterone replacement therapy (TRT). This case will present a patient who initiated TRT at a community-based alternative medicine clinic. The case will be followed by a discussion regarding the standard diagnosis of hypogonadism, the potential benefits and risks of TRT, and a review of the current clinical guideline recommendations. Examples of information being disseminated to the general public by the complementary and alternative medicine (CAM) providers will be briefly reviewed for an increased awareness of the questions patients may pose regarding TRT.
Background
From 2000 to 2011, total testosterone sales increased 12-fold globally.1 Possible causes for the increase involved the aging population, newer options for TRT administration, and increased direct-to-consumer advertising. A low testosterone level (sometimes referred to as low T in consumer marketing materials) is associated with a variety of medical conditions (ie, low mood, increased body fat, declining athletic performance, and decreased sexual performance) that have become increasingly prevalent among middle aged and older men.2 It has also received attention as an intervention to reverse frailty and sarcopenia.3
Testosterone replacement therapy options include injectable solutions, transdermal gels and patches, pellet implants, or buccal tablets. The ease of administration of transdermal testosterone comes at a relatively high cost. Injectable testosterone preparations are generally the least expensive option, and many patients choose injections for this reason.
Related: Keeping an Open Mind on HRT
Testosterone prescriptions were most frequently written by PCPs with 36% coming from family practitioners and 20.1% from internal medicine practices, according to a Kaiser Permanente study.4 Endocrinologists (13.5%) and urologists (6.6%) were less likely to have written the prescriptions for patients.
Due, in part, to direct-to-consumer advertising and to the availability of online medical information, many men now present to their PCP questioning whether they might have low T. Others may have already started therapy at a CAM, integrative medicine, or anti-aging clinic.
Confusing the issue further, some CAM providers promote a variety of off-label medications and nutritional supplements for the treatment of low T, which seems to have struck a chord in the baby boomer generation. No other age group in history has tried to work so intensely on its physical condition and appearance.5 Much of the information marketed to consumers emphasizes that many traditionally trained physicians are not educated in the treatment of low T.
Case Report
Mr. C. is a 65-year-old man who was seen in the primary care clinic for the first time. He was accompanied by his much younger fiancée. She reported that Mr. C.’s energy and sexual interest were declining, and the patient reported his “get up and go had gotten up and left.” They sought medical advice from a CAM provider who ordered blood work and then explained that the symptoms were due to low testosterone. For the past 6 months he had been visiting the clinic weekly for testosterone injections.
Mr. C. reported feeling as good as a “40 year old.” He also reported that he started working with a personal trainer and had given up most junk food and alcohol. He had no symptoms of chest pain, erectile dysfunction, or significant urinary urgency, frequency, or nocturia.
Related: Will Testosterone Therapy Kill Your Patient?
The visits to a CAM provider had been an out-of-pocket expense, and he was hoping to transfer his treatment to the VA so the costs could be covered. Mr. C. failed to bring medical records from the other provider but remembered being told that all his tests were “fine” except for the low testosterone level.
His past history was notable for controlled type 2 diabetes mellitus for 8 years, hypertension, hyperlipidemia, and spinal stenosis. He had no history of benign prostatic hyperplasia or prostate cancer.
In addition to the testosterone (100 mg intramuscular injection weekly), his medication regimen included metoprolol 25 mg twice daily, atorvastatin 20 mg daily, acetaminophen 650 mg 3 times daily as needed, aspirin 81 mg daily, metformin 500 mg twice daily, vitamin D 2,000 IU daily, vitamin B12 1,000 mg daily, and Co-Q10 200 mg daily.
On physical examination, Mr. C.’s vitals were stable and his body mass index was in the overweight range at 29.8 kg/m2. His cardiopulmonary examination was normal. There was increased central obesity without palpable organomegaly. There was no gynecomastia, and he had normal amounts of axillary and pubic hair. There was no peripheral edema; his genitourinary examination included normal-sized testicles, and the prostate was smooth without nodules.
The PCP informed Mr. C. that he was familiar with the evaluation and management of testosterone therapy. He was advised that additional evaluation would be needed before determining whether the clinical benefit of TRT outweighed the potential risks.
Andropause
Testosterone levels in men are known to decline at a rate of 1% per year after aged 30 years.6 About 20% of men aged ≥ 60 years and 50% of men aged ≥ 80 years have low (hypogonadal) total testosterone levels.7 The clinical diagnosis of hypogonadism, however, is made on the basis of signs and symptoms consistent with androgen deficiency and a low serum morning testosterone level measured on serum on multiple occasions.8
Specific clinical signs and symptoms (“A” list) consistent with androgen deficiency include low libido and sexual activity; diminished spontaneous erections; gynecomastia; reduced facial, axillary, or pubic hair; small (≤ 5 mL) testes; inability to father children; loss of height, fractures, or other signs of bone loss; and hot flashes and night sweats.9
Less specific signs and symptoms (“B” list) of androgen deficiency include a decrease in energy or motivation, feelings of sadness or depression, poor concentration or memory, trouble sleeping, increased sleepiness, mild anemia, reduced muscle bulk or strength, increased body fat, and diminished physical performance.9
Making the clinical diagnosis of hypogonadism is challenging, because the clinical symptoms have a high prevalence in the older male population and overlap with many nonendocrine diseases. Testosterone replacement therapy has been associated weakly, but consistently, with improved sexual function,10-12 bone mineral density,13,14 fat free mass,13,14 strength,15,16 lipid profiles,17,18 insulin resistance,17,18 and with an increased time to ST segment depression during stress testing.19,20
Laboratory Evaluation
Serum total testosterone circulates in 3 forms: free testosterone, sex hormone-binding globulin (SHBG)-bound testosterone, and albumin-bound testosterone. Free testosterone is the most bio-available testosterone but represents only 2% to 3% of total testosterone.21 Whether total testosterone or free testosterone measurements most closely correlate with symptomatic androgen deficiency is a matter of debate.21 A total testosterone level is an appropriate screening test in young, healthy, and lean men for whom SHBG levels are presumably normal. However, a free or bioavailable testosterone level should be considered for men when there is a high likelihood of conditions that can affect SHBG levels.
Conditions that can decrease SHBG (and may result in a low total testosterone reading even when the free fraction may be normal) include obesity, metabolic syndrome, type 2 diabetes mellitus, hypothyroidism, nephrotic syndrome, chronic glucocorticoid use, and the use of progestins and anabolic steroids.21 Conditions that can increase SHBG (and may result in a normal total testosterone level in patients with hypogonadism, as they have low levels of free testosterone) include aging, cirrhosis, anticonvulsant use, hyperthyroidism, catabolic conditions, and HIV.21
Related: Effect of Statins on Total Testosterone Levels in Male Veterans
Serum testosterone levels generally peak in the early morning, followed by a progressive decline over the course of the day until they reach a nadir in the evening.21 Although it has been debated that morning testosterone levels are not necessary in older men due to a blunting of the circadian rhythm, many men aged 65 to 80 years who have low T in the afternoon will have normal testosterone levels when retested in the morning.22,23 Readings below a reference range of 280 ng/dL to 300 ng/dL on at least 2 different occasions support a diagnosis of hypogonadism.9
Follicle stimulating hormone (FSH) and luteinizing hormone (LH) laboratory tests may be ordered following confirmation of a low testosterone level. Prolactin levels and iron saturation can help evaluate for the presence of hyperprolactinemia and hemochromatosis, respectively. Primary hypogonadism due to testicular failure is diagnosed with high FSH, high LH, and low testosterone levels. Secondary hypogonadism due to hypothalamic or pituitary failure is diagnosed with low FSH, low LH, and low testosterone levels.
Hypothalamic or pituitary suppression from a nonendocrine condition may result in functional hypogonadotropic hypogonadism (FHH), which can be identified with low (or normal) FSH; low (or normal) LH; and low testosterone levels. Hypogonadotropic hypogonadism has been associated with depression, obesity, stress, and physical exertion; and FHH may also be associated with the use of multiple drugs and drug classes (spironolactone, anabolic and corticosteroids, ketoconazole, ethanol, anticonvulsants, immunosuppressants, tricyclic antidepressants, selective serotonin reuptake inhibitors, antipsychotics, and opioids).24,25 Even statin therapy has been associated with FHH.26,27 Testosterone levels will often recover if or when modifiable factors for FHH are corrected.28
Although there is no consensus on an absolute number that defines a low testosterone level, concern exists that there are economic incentives to raise the bar for normal and thereby increase the potential market for testosterone-raising products.29 Many commercial avenues for the treatment of low T do not follow the standards of the established medical community. Some websites suggest screening for low T with total and free testosterone levels for all men aged > 40 years. Others advise men to consider TRT if they have a total testosterone level of < 500 ng/dL or a free testosterone level that is not in the upper one-third range for men aged 21 to 49 years.30 Of even greater concern, Baillargeon and colleagues reported that 25% of all new androgen users had not had their testosterone levels measured in the 12 months before starting treatment.31 In another study, 40% of men who initiated TRT did not have a baseline measurement.32
Treatments
Before considering TRT, physicians need to emphasize lifestyle modifications as first-line treatment for hypogonadism. The most important modifications include weight loss, tobacco cessation, and moderation in alcohol use.
Patients need to be advised of possible adverse events (AEs) of TRT, which may include gynecomastia, polycythemia, sleep apnea, decreased high-density lipoprotein cholesterol, benign prostatic hypertrophy, infertility, testicular atrophy, and abnormal liver function tests. More recently, several studies have shown an association between TRT and an increase in cardiovascular complications, such as stroke, heart attacks, and death.
Prior to considering TRT, a careful history and physical examination, including a clinical prostate examination, should be performed. Minimum additional tests should include hematocrit, fasting lipid profile (FLP), complete metabolic profile (CMP), and prostate-specific antigen (PSA). Initiation of TRT is not recommended for patients with metastatic prostate cancer; breast cancer; an unevaluated prostate nodule; a PSA > 4 ng/mL (or > 3 ng/mL in African Americans or men with a first-degree relative with prostate cancer); hematocrit > 50%; untreated severe obstructive sleep apnea; uncontrolled or poorly controlled congestive heart failure; or an International Prostate Symptoms Score (IPSS) > 19.9
A past history of prostate cancer had previously been a contraindication for the use of TRT. However, more recent studies have shown that TRT can be used in those who have no evidence of active or metastatic disease and who are under the close supervision of a physician.33-35
Widespread screening is not recommended, and population-based surveys can be unreliable. Fifteen percent of healthy young men, for example, will have a low serum testosterone level in a given 24-hour period.9 Thirty percent of men with an initial testosterone level in the mildly hypogonadal range will have a normal testosterone level when retested; moreover the threshold below which AEs occur remains unknown.9
The goal of TRT is to achieve a total testosterone level in the 400 ng/mL to 700 ng/mL range with improved clinical signs and symptoms.9 Laboratory tests should be conducted at 3 months, 6 months, and then annually. These tests include hematocrit, PSA, and a testosterone level.32 Testing for CMP and FLP should also be considered. If, during therapy, the hematocrit is > 54%, the patient should be assessed for hypoxia and sleep apnea, and treatment should resume at a lower dose only when the hematocrit returns to baseline.9 A digital examination of the prostate is recommended for men with a PSA of > 0.6 ng/mL. A urologic consultation should be obtained for an increase in the PSA of > 1.4 ng/mL over 12 months, a PSA velocity of > 0.4 ng/mL per year (using the PSA after 6 months as a reference), or for an IPPS of > 19.9
Emerging Cardiovascular Concerns
The Testosterone for Older Men study, a randomized, placebo- controlled clinical trial of testosterone therapy in men with a high prevalence of cardiovascular disease, showed significantly greater improvements in leg-press, chest-press, and stair-climbing exercises while carrying a load compared with that in the placebo group.36 However, the study was stopped early due to an increased risk of cardiovascular AEs in those who received testosterone gel.
Vigen and colleagues examined a cohort of veterans who underwent coronary angiography and had a low serum testosterone level.37 The use of TRT in this cohort was also associated with an increased risk of adverse cardiovascular outcomes. This study generated several letters and a recent article in response that vigorously questioned the validity of the methods used and the conclusions reached.38-44 Prior clinical studies of TRT had not detected cardiac AEs, but these trials were generally of short duration and not powered for clinical endpoints.37
A FDA Safety Announcement as well as a VA National Pharmacy Benefits Management bulletin were based on the results of these studies.45 The FDA did not conclude that TRT increased the risk of stroke, heart attack, or death, but health care providers were asked to consider whether the benefits of TRT are likely to exceed the potential risk of treatment.
Direct-to-Consumer Marketing
Some direct-to-consumer marketing promotes the use of aromatase inhibitors, such as anastrozole. This class of medications prevents the conversion of endogenous and exogenous testosterone to estrogen by the aromatase enzyme, which is found predominately in abdominal adipose tissue. There is no evidence that naturally occurring elevations in estrogen cause low testosterone or that treatment of elevated estrogen with an aromatase inhibitor during TRT has any significant clinical benefit in terms of male sexuality.46 Nevertheless, some CAM providers now hypothesize that the increase in cardiovascular AEs with TRT noted in the recent studies may have been due to the increase in estrogen that is associated with TRT.46
The off-label use of clomiphene citrate to block the negative feedback of estrogen on the production of LH has been promoted as another potential treatment to increase testosterone levels. Luteinizing hormone is the pituitary analog of human chorionic gonadotropin (HCG). Many CAM providers also prescribe HCG to increase the testicles’ testosterone production.
Some consumer-focused media insist that the use of either clomiphene citrate or HCG will increase testosterone production and does not cause testicular atrophy, a known TRT- associated AE. This seems to increase the motivation of many men to try these off-label medications.
Some sources even posit a “conspiracy theory” that the FDA and pharmaceutical companies conspire to keep the price of transdermal TRT options high. Men are told that testosterone creams made at compounding pharmacies are much less expensive than are the transdermal pharmaceuticals, and they are urged to see a CAM provider to obtain a prescription for the compounded testosterone. In some cases, a sample prescription is included.47
Many supplements are available that claim to boost testosterone or suppress estrogen. Chrysin, for example, is a bioflavonoid that is marketed as having the potential to act as a natural aromatase inhibitor. Although studies have suggested the potential for chrysin to work in such a manner, the effectiveness may be attenuated by its low bioavailability in supplements.48 Long-term studies have not been conducted.49 Nettle root is a plant-derived compound that is stated to increase free testosterone levels by binding to SHBG, in place of testosterone, and by inhibiting the enzyme that converts testosterone to dihydrotestosterone. The clinical evidence of effectiveness is based on many open studies, and the significance and magnitude of the effect still needs more rigorous evaluation.50
Conclusions
Patients today are barraged with medical information through television, print advertising, radio, and the Internet. A recent study of online sources of herbal product information found that only 10.5% recommended a consultation with a health care professional and < 3% cited scientific literature to accompany their claims.51 Many patients present to their PCP with questions about TRT or have already started an intervention for low T. Complementary and alternative medicine providers of TRT have been able to capture a segment of the population that often has the motivation and disposable income to pursue nontraditional therapies.
All nutritional supplements contain a standard warning from the FDA: “The above statements have not been evaluated by the FDA. This product is not intended to diagnose, treat, cure or prevent any disease.” Providers should remind patients of the statement and point out the contradictions between the statement and the benefits touted by the supplement marketing literature.
Finally, despite the well- established role of testosterone in enhancing libido, its definitive role in erectile function had been controversial until evidence substantiated a key function for this hormone.52 Testosterone may facilitate erection by acting as a vasodilator of the penile arterioles and cavernous sinusoids and may ameliorate the response to the phosphodiesterase-5 inhibitors in hypogonadal men.53 Testosterone replacement alone in hypogonadal men can restore erectile dysfunction.51 However, hypogonadism is not a common finding in those with erectile dysfunction, only occurring in about 5% of cases.53
Allopathic providers are concerned about the vitality and sexual health of their aging male patients, but their enthusiasm for anti-aging treatments is often tempered by evidence-based studies that have shown a lack of efficacy or potentially serious health care risks. Unfortunately, many patients remain unaware of the controversies regarding TRT. For those patients who receive treatment through CAM providers and are convinced of the efficacy of their low-T treatment regimen, it is important to keep lines of communication open.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
The objective of this article is to help primary care providers (PCPs) council patients regarding testosterone replacement therapy (TRT). This case will present a patient who initiated TRT at a community-based alternative medicine clinic. The case will be followed by a discussion regarding the standard diagnosis of hypogonadism, the potential benefits and risks of TRT, and a review of the current clinical guideline recommendations. Examples of information being disseminated to the general public by the complementary and alternative medicine (CAM) providers will be briefly reviewed for an increased awareness of the questions patients may pose regarding TRT.
Background
From 2000 to 2011, total testosterone sales increased 12-fold globally.1 Possible causes for the increase involved the aging population, newer options for TRT administration, and increased direct-to-consumer advertising. A low testosterone level (sometimes referred to as low T in consumer marketing materials) is associated with a variety of medical conditions (ie, low mood, increased body fat, declining athletic performance, and decreased sexual performance) that have become increasingly prevalent among middle aged and older men.2 It has also received attention as an intervention to reverse frailty and sarcopenia.3
Testosterone replacement therapy options include injectable solutions, transdermal gels and patches, pellet implants, or buccal tablets. The ease of administration of transdermal testosterone comes at a relatively high cost. Injectable testosterone preparations are generally the least expensive option, and many patients choose injections for this reason.
Related: Keeping an Open Mind on HRT
Testosterone prescriptions were most frequently written by PCPs with 36% coming from family practitioners and 20.1% from internal medicine practices, according to a Kaiser Permanente study.4 Endocrinologists (13.5%) and urologists (6.6%) were less likely to have written the prescriptions for patients.
Due, in part, to direct-to-consumer advertising and to the availability of online medical information, many men now present to their PCP questioning whether they might have low T. Others may have already started therapy at a CAM, integrative medicine, or anti-aging clinic.
Confusing the issue further, some CAM providers promote a variety of off-label medications and nutritional supplements for the treatment of low T, which seems to have struck a chord in the baby boomer generation. No other age group in history has tried to work so intensely on its physical condition and appearance.5 Much of the information marketed to consumers emphasizes that many traditionally trained physicians are not educated in the treatment of low T.
Case Report
Mr. C. is a 65-year-old man who was seen in the primary care clinic for the first time. He was accompanied by his much younger fiancée. She reported that Mr. C.’s energy and sexual interest were declining, and the patient reported his “get up and go had gotten up and left.” They sought medical advice from a CAM provider who ordered blood work and then explained that the symptoms were due to low testosterone. For the past 6 months he had been visiting the clinic weekly for testosterone injections.
Mr. C. reported feeling as good as a “40 year old.” He also reported that he started working with a personal trainer and had given up most junk food and alcohol. He had no symptoms of chest pain, erectile dysfunction, or significant urinary urgency, frequency, or nocturia.
Related: Will Testosterone Therapy Kill Your Patient?
The visits to a CAM provider had been an out-of-pocket expense, and he was hoping to transfer his treatment to the VA so the costs could be covered. Mr. C. failed to bring medical records from the other provider but remembered being told that all his tests were “fine” except for the low testosterone level.
His past history was notable for controlled type 2 diabetes mellitus for 8 years, hypertension, hyperlipidemia, and spinal stenosis. He had no history of benign prostatic hyperplasia or prostate cancer.
In addition to the testosterone (100 mg intramuscular injection weekly), his medication regimen included metoprolol 25 mg twice daily, atorvastatin 20 mg daily, acetaminophen 650 mg 3 times daily as needed, aspirin 81 mg daily, metformin 500 mg twice daily, vitamin D 2,000 IU daily, vitamin B12 1,000 mg daily, and Co-Q10 200 mg daily.
On physical examination, Mr. C.’s vitals were stable and his body mass index was in the overweight range at 29.8 kg/m2. His cardiopulmonary examination was normal. There was increased central obesity without palpable organomegaly. There was no gynecomastia, and he had normal amounts of axillary and pubic hair. There was no peripheral edema; his genitourinary examination included normal-sized testicles, and the prostate was smooth without nodules.
The PCP informed Mr. C. that he was familiar with the evaluation and management of testosterone therapy. He was advised that additional evaluation would be needed before determining whether the clinical benefit of TRT outweighed the potential risks.
Andropause
Testosterone levels in men are known to decline at a rate of 1% per year after aged 30 years.6 About 20% of men aged ≥ 60 years and 50% of men aged ≥ 80 years have low (hypogonadal) total testosterone levels.7 The clinical diagnosis of hypogonadism, however, is made on the basis of signs and symptoms consistent with androgen deficiency and a low serum morning testosterone level measured on serum on multiple occasions.8
Specific clinical signs and symptoms (“A” list) consistent with androgen deficiency include low libido and sexual activity; diminished spontaneous erections; gynecomastia; reduced facial, axillary, or pubic hair; small (≤ 5 mL) testes; inability to father children; loss of height, fractures, or other signs of bone loss; and hot flashes and night sweats.9
Less specific signs and symptoms (“B” list) of androgen deficiency include a decrease in energy or motivation, feelings of sadness or depression, poor concentration or memory, trouble sleeping, increased sleepiness, mild anemia, reduced muscle bulk or strength, increased body fat, and diminished physical performance.9
Making the clinical diagnosis of hypogonadism is challenging, because the clinical symptoms have a high prevalence in the older male population and overlap with many nonendocrine diseases. Testosterone replacement therapy has been associated weakly, but consistently, with improved sexual function,10-12 bone mineral density,13,14 fat free mass,13,14 strength,15,16 lipid profiles,17,18 insulin resistance,17,18 and with an increased time to ST segment depression during stress testing.19,20
Laboratory Evaluation
Serum total testosterone circulates in 3 forms: free testosterone, sex hormone-binding globulin (SHBG)-bound testosterone, and albumin-bound testosterone. Free testosterone is the most bio-available testosterone but represents only 2% to 3% of total testosterone.21 Whether total testosterone or free testosterone measurements most closely correlate with symptomatic androgen deficiency is a matter of debate.21 A total testosterone level is an appropriate screening test in young, healthy, and lean men for whom SHBG levels are presumably normal. However, a free or bioavailable testosterone level should be considered for men when there is a high likelihood of conditions that can affect SHBG levels.
Conditions that can decrease SHBG (and may result in a low total testosterone reading even when the free fraction may be normal) include obesity, metabolic syndrome, type 2 diabetes mellitus, hypothyroidism, nephrotic syndrome, chronic glucocorticoid use, and the use of progestins and anabolic steroids.21 Conditions that can increase SHBG (and may result in a normal total testosterone level in patients with hypogonadism, as they have low levels of free testosterone) include aging, cirrhosis, anticonvulsant use, hyperthyroidism, catabolic conditions, and HIV.21
Related: Effect of Statins on Total Testosterone Levels in Male Veterans
Serum testosterone levels generally peak in the early morning, followed by a progressive decline over the course of the day until they reach a nadir in the evening.21 Although it has been debated that morning testosterone levels are not necessary in older men due to a blunting of the circadian rhythm, many men aged 65 to 80 years who have low T in the afternoon will have normal testosterone levels when retested in the morning.22,23 Readings below a reference range of 280 ng/dL to 300 ng/dL on at least 2 different occasions support a diagnosis of hypogonadism.9
Follicle stimulating hormone (FSH) and luteinizing hormone (LH) laboratory tests may be ordered following confirmation of a low testosterone level. Prolactin levels and iron saturation can help evaluate for the presence of hyperprolactinemia and hemochromatosis, respectively. Primary hypogonadism due to testicular failure is diagnosed with high FSH, high LH, and low testosterone levels. Secondary hypogonadism due to hypothalamic or pituitary failure is diagnosed with low FSH, low LH, and low testosterone levels.
Hypothalamic or pituitary suppression from a nonendocrine condition may result in functional hypogonadotropic hypogonadism (FHH), which can be identified with low (or normal) FSH; low (or normal) LH; and low testosterone levels. Hypogonadotropic hypogonadism has been associated with depression, obesity, stress, and physical exertion; and FHH may also be associated with the use of multiple drugs and drug classes (spironolactone, anabolic and corticosteroids, ketoconazole, ethanol, anticonvulsants, immunosuppressants, tricyclic antidepressants, selective serotonin reuptake inhibitors, antipsychotics, and opioids).24,25 Even statin therapy has been associated with FHH.26,27 Testosterone levels will often recover if or when modifiable factors for FHH are corrected.28
Although there is no consensus on an absolute number that defines a low testosterone level, concern exists that there are economic incentives to raise the bar for normal and thereby increase the potential market for testosterone-raising products.29 Many commercial avenues for the treatment of low T do not follow the standards of the established medical community. Some websites suggest screening for low T with total and free testosterone levels for all men aged > 40 years. Others advise men to consider TRT if they have a total testosterone level of < 500 ng/dL or a free testosterone level that is not in the upper one-third range for men aged 21 to 49 years.30 Of even greater concern, Baillargeon and colleagues reported that 25% of all new androgen users had not had their testosterone levels measured in the 12 months before starting treatment.31 In another study, 40% of men who initiated TRT did not have a baseline measurement.32
Treatments
Before considering TRT, physicians need to emphasize lifestyle modifications as first-line treatment for hypogonadism. The most important modifications include weight loss, tobacco cessation, and moderation in alcohol use.
Patients need to be advised of possible adverse events (AEs) of TRT, which may include gynecomastia, polycythemia, sleep apnea, decreased high-density lipoprotein cholesterol, benign prostatic hypertrophy, infertility, testicular atrophy, and abnormal liver function tests. More recently, several studies have shown an association between TRT and an increase in cardiovascular complications, such as stroke, heart attacks, and death.
Prior to considering TRT, a careful history and physical examination, including a clinical prostate examination, should be performed. Minimum additional tests should include hematocrit, fasting lipid profile (FLP), complete metabolic profile (CMP), and prostate-specific antigen (PSA). Initiation of TRT is not recommended for patients with metastatic prostate cancer; breast cancer; an unevaluated prostate nodule; a PSA > 4 ng/mL (or > 3 ng/mL in African Americans or men with a first-degree relative with prostate cancer); hematocrit > 50%; untreated severe obstructive sleep apnea; uncontrolled or poorly controlled congestive heart failure; or an International Prostate Symptoms Score (IPSS) > 19.9
A past history of prostate cancer had previously been a contraindication for the use of TRT. However, more recent studies have shown that TRT can be used in those who have no evidence of active or metastatic disease and who are under the close supervision of a physician.33-35
Widespread screening is not recommended, and population-based surveys can be unreliable. Fifteen percent of healthy young men, for example, will have a low serum testosterone level in a given 24-hour period.9 Thirty percent of men with an initial testosterone level in the mildly hypogonadal range will have a normal testosterone level when retested; moreover the threshold below which AEs occur remains unknown.9
The goal of TRT is to achieve a total testosterone level in the 400 ng/mL to 700 ng/mL range with improved clinical signs and symptoms.9 Laboratory tests should be conducted at 3 months, 6 months, and then annually. These tests include hematocrit, PSA, and a testosterone level.32 Testing for CMP and FLP should also be considered. If, during therapy, the hematocrit is > 54%, the patient should be assessed for hypoxia and sleep apnea, and treatment should resume at a lower dose only when the hematocrit returns to baseline.9 A digital examination of the prostate is recommended for men with a PSA of > 0.6 ng/mL. A urologic consultation should be obtained for an increase in the PSA of > 1.4 ng/mL over 12 months, a PSA velocity of > 0.4 ng/mL per year (using the PSA after 6 months as a reference), or for an IPPS of > 19.9
Emerging Cardiovascular Concerns
The Testosterone for Older Men study, a randomized, placebo- controlled clinical trial of testosterone therapy in men with a high prevalence of cardiovascular disease, showed significantly greater improvements in leg-press, chest-press, and stair-climbing exercises while carrying a load compared with that in the placebo group.36 However, the study was stopped early due to an increased risk of cardiovascular AEs in those who received testosterone gel.
Vigen and colleagues examined a cohort of veterans who underwent coronary angiography and had a low serum testosterone level.37 The use of TRT in this cohort was also associated with an increased risk of adverse cardiovascular outcomes. This study generated several letters and a recent article in response that vigorously questioned the validity of the methods used and the conclusions reached.38-44 Prior clinical studies of TRT had not detected cardiac AEs, but these trials were generally of short duration and not powered for clinical endpoints.37
A FDA Safety Announcement as well as a VA National Pharmacy Benefits Management bulletin were based on the results of these studies.45 The FDA did not conclude that TRT increased the risk of stroke, heart attack, or death, but health care providers were asked to consider whether the benefits of TRT are likely to exceed the potential risk of treatment.
Direct-to-Consumer Marketing
Some direct-to-consumer marketing promotes the use of aromatase inhibitors, such as anastrozole. This class of medications prevents the conversion of endogenous and exogenous testosterone to estrogen by the aromatase enzyme, which is found predominately in abdominal adipose tissue. There is no evidence that naturally occurring elevations in estrogen cause low testosterone or that treatment of elevated estrogen with an aromatase inhibitor during TRT has any significant clinical benefit in terms of male sexuality.46 Nevertheless, some CAM providers now hypothesize that the increase in cardiovascular AEs with TRT noted in the recent studies may have been due to the increase in estrogen that is associated with TRT.46
The off-label use of clomiphene citrate to block the negative feedback of estrogen on the production of LH has been promoted as another potential treatment to increase testosterone levels. Luteinizing hormone is the pituitary analog of human chorionic gonadotropin (HCG). Many CAM providers also prescribe HCG to increase the testicles’ testosterone production.
Some consumer-focused media insist that the use of either clomiphene citrate or HCG will increase testosterone production and does not cause testicular atrophy, a known TRT- associated AE. This seems to increase the motivation of many men to try these off-label medications.
Some sources even posit a “conspiracy theory” that the FDA and pharmaceutical companies conspire to keep the price of transdermal TRT options high. Men are told that testosterone creams made at compounding pharmacies are much less expensive than are the transdermal pharmaceuticals, and they are urged to see a CAM provider to obtain a prescription for the compounded testosterone. In some cases, a sample prescription is included.47
Many supplements are available that claim to boost testosterone or suppress estrogen. Chrysin, for example, is a bioflavonoid that is marketed as having the potential to act as a natural aromatase inhibitor. Although studies have suggested the potential for chrysin to work in such a manner, the effectiveness may be attenuated by its low bioavailability in supplements.48 Long-term studies have not been conducted.49 Nettle root is a plant-derived compound that is stated to increase free testosterone levels by binding to SHBG, in place of testosterone, and by inhibiting the enzyme that converts testosterone to dihydrotestosterone. The clinical evidence of effectiveness is based on many open studies, and the significance and magnitude of the effect still needs more rigorous evaluation.50
Conclusions
Patients today are barraged with medical information through television, print advertising, radio, and the Internet. A recent study of online sources of herbal product information found that only 10.5% recommended a consultation with a health care professional and < 3% cited scientific literature to accompany their claims.51 Many patients present to their PCP with questions about TRT or have already started an intervention for low T. Complementary and alternative medicine providers of TRT have been able to capture a segment of the population that often has the motivation and disposable income to pursue nontraditional therapies.
All nutritional supplements contain a standard warning from the FDA: “The above statements have not been evaluated by the FDA. This product is not intended to diagnose, treat, cure or prevent any disease.” Providers should remind patients of the statement and point out the contradictions between the statement and the benefits touted by the supplement marketing literature.
Finally, despite the well- established role of testosterone in enhancing libido, its definitive role in erectile function had been controversial until evidence substantiated a key function for this hormone.52 Testosterone may facilitate erection by acting as a vasodilator of the penile arterioles and cavernous sinusoids and may ameliorate the response to the phosphodiesterase-5 inhibitors in hypogonadal men.53 Testosterone replacement alone in hypogonadal men can restore erectile dysfunction.51 However, hypogonadism is not a common finding in those with erectile dysfunction, only occurring in about 5% of cases.53
Allopathic providers are concerned about the vitality and sexual health of their aging male patients, but their enthusiasm for anti-aging treatments is often tempered by evidence-based studies that have shown a lack of efficacy or potentially serious health care risks. Unfortunately, many patients remain unaware of the controversies regarding TRT. For those patients who receive treatment through CAM providers and are convinced of the efficacy of their low-T treatment regimen, it is important to keep lines of communication open.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Handelsman D. Global trends in testosterone prescribing, 2000-2011: expanding the spectrum of prescription drug misuse. Med J Aust. 2013;199(8):548-551.
2. Hackett G. Testosterone and the heart. Int J Clin Pract. 2012;66(7):648-655.
3. Morley JE. Hypogonadism, testosterone, and nursing home residents. J Am Med Dir Assoc. 2013;14(6):381-383.
4. An J, Cheetham TC, Van Den Eeden S. PS3-36: testosterone replacement therapy patterns for aging males in a managed care setting. Clin Med Res. 2013;11(3):141.
5. Moschis G, Lee E, Marthur A, Strautman J. The Maturing Marketplace: Buying Habits of Baby Boomers and Their Parents. Westport, CT: Quorum Books; 2000.
6. Morley JE, Kaiser FE, Perry HM 3rd, et al. Longitudinal changes in testosterone, luteinizing hormone, and follicle stimulating hormone in healthy older men. Metabolism. 1997;46(4):410-413.
7. Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR; Baltimore Longitudinal Study of Aging. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab. 2001;86(2):724-731.
8. Basaria S. Male hypogonadism. Lancet. 2014;383 (9924):1250-1263.
9. Bhasin S, Cunningham GR, Hayes FJ, et al; Task Force, Endocrine Society. Testosterone therapy in men with androgen deficiency syndromes. J Clin Endocrinol Metab. 2010;95(6):2536-2559.
10. Wang C, Swerdloff RS, Iranmanesh A, et al. Transdermal testosterone gel improves sexual function, mood, muscle strength, and body composition parameters in hypogonadal men. J Clin Endocrinol Metab. 2000;85(8):2839-2853.
11. Bolona ER, Uranga MV, Haddad RM, et al. Testosterone use in men with sexual dysfunction: a systematic review and meta-analysis of randomized placebo-controlled trials. Mayo Clin Proc. 2007;82(1):20-28.
12. Isidori AM, Giannetta E, Gianfrilli D, et al. Effects of testosterone on sexual function in men: results of a meta-analysis. Clin Endocrinol (Oxf). 2005;63(4):381-394.
13. Isidori AM, Giannetta E, Greco EA, et al. Effects of testosterone on body composition, bone metabolism and serum lipid profile in middle-aged men: a meta-analysis. Clin Endocrinol (Oxf). 2005;63(3):280-293.
14. Snyder PJ, Peachey H, Berlin JA, et al. Effects of testosterone replacement in hypogonadal men. J Clin Endocrinol Metab. 2000;85(8):2670-2677.
15. Sih R, Morley JE, Kaiser FE, Perry HM 3rd, Patrick P, Ross C. Testosterone replacement in older hypogonadal men: a 12-month randomized controlled trial. J Clin Endocrinol Metab. 1997;82(6):1661-1667.
16. Travison TG, Basaria S, Storer TW, et al. Clinical meaningfulness of the changes in muscle performance and physical function associated with testosterone administration in older men with mobility limitation. J Gerontol A Biol Sci Med Sci. 2011;66(10):1090-1099.
17. Jones TH, Arver S, Behre HM, et al; TIMES2 Investigators. Testosterone replacement in hypogonadal men with type 2 diabetes and/or metabolic syndrome. Diabetes Care. 2011;34(4):828-837.
18. Jones TH, Saad F. The effects of testosterone on risk factors for, and the mediators of, the atherosclerotic process. Atherosclerosis. 2009;207(2):318-327.
19. Mathur A, Malkin C, Saeed B, Muthusamy R, Jones TH, Channer K. Long-term benefits of testosterone replacement therapy on angina threshold and atheroma in men. Eur J Endocrinol. 2009;161(3):443-449.
20. Malkin CJ, Pugh PJ, Morris PD, et al. Testosterone replacement in hypogonadal men with angina improves ischaemic threshold and quality of life. Heart. 2004;90(8):871-876.
21. Paduch DA, Brannigan RE, Fuchs EF, Kim ED, Marmar JL, Sandlow JI. White Paper: The Laboratory Diagnosis of Testosterone Deficiency. http://www.auanet.org/common/pdf/education/clinical -guidance/Testosterone-Deficiency-WhitePaper.pdf. Published 2013. Accessed April 9, 2015.
22. Crawford ED, Barqawi AB, O’Donnell C, Morgentaler A. The association of time of day and serum testosterone concentration in a large screening population. BJU Int. 2007;100(3):509-513.
23. Brambilla DJ, O’Donnell AB, Matsumoto AM, McKinlay JB. Intraindividual variation in levels of serum testosterone and other reproductive and adrenal hormones in men. Clin Endocrinol (Oxf). 2007;67(6):853-862.
24. Kumar P, Kumar N, Thakur DS, Patidar A. Male hypogonadism: symptoms and treatment. J Adv Pharm Technol Res. 2010;1(3):297-301.
25. Montgomery K. Sexual desire disorders. Psychiatry (Edgmont). 2008;5(6):50-55.
26. Corona G, Boddi V, Balercia G, et al. The effect of statin therapy on testosterone levels in subjects consulting for erectile dysfunction. J Sex Med. 2010; 7(4 pt 1):1547-1556.
27. Schooling CM, Yeung SLA, Freeman G, Cowling BJ. The effect of statins on testosterone in men and women, a systematic review and meta-analysis of randomized controlled trials. BMC Med. 2013;11:57.
28. Wu FC, Tajar A, Pye SR, et al; European Male Aging Study Group. Hypothalamic-pituitary-testicular axis disruptions in older men are differentially linked to age and modifiable risk factors: the European Male Aging Study. J Clin Endocrinol Metab. 2008;93(7):2737-2745.
29. Schwartz LM, Woloshin S. Low “T” as in “template”: how to sell disease. JAMA Intern Med. 2013;173(15):1460-1462.
30. Male Hormone Modulation Therapy, Part 2. Life Extension Vitamins Website. http://www.lifeextension vitamins.com/mahomothpa2.html. Accessed April 9, 2015.
31. Baillargeon J, Urban RJ, Ottenbacher KJ, Pierson KS, Goodwin JS. Trends in androgen prescribing in the United States, 2001 to 2011. JAMA Intern Med. 2013;173(15):1465-1466.
32. Layton JB, Li D, Meier CR, et al. Testosterone lab testing and initiation in the United Kingdom and the United States, 2000 to 2011. J Clin Endocrinol Metab. 2014;99(3):835-842.
33. Ramasamy R, Fisher ES, Schlegel PN. Testosterone replacement and prostate cancer. Indian J Urol. 2012;28(2):123-128.
34. Marks, LS, Mazer NA, Mostaghel E, et al. Effect of testosterone replacement therapy on prostate tissue in men with late-onset hypogonadism: a randomized controlled trial. JAMA. 2006;296(19):2351-2361.
35. Coward RM, Simhan J, Carson CC 3rd. Prostate-specific antigen changes and prostate cancer in hypogonadal men treated with testosterone replacement therapy. BJU Int. 2009;103(9):1179-1183.
36. Basaria S, Coviello AD, Travison TG, et al. Adverse events associated with testosterone administration. N Engl J Med. 2010;363(2):109-122.
37. Vigen R, O’Donnell Cl, Barón AE, et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310(17):1829-1836.
38. Morgentaler A, Traish A, Kacker R. Deaths and cardiovascular events in men receiving testosterone. JAMA. 2014;311(9):961-962.
39. Jones TH, Channer K. Deaths and cardiovascular events in men receiving testosterone. JAMA. 2014;311(9):962-963.
40. Katz J, Nadelberg R. Deaths and cardiovascular events in men receiving testosterone. JAMA. 2014;311(9):963.
41. Riche D, Baker WL, Koch CA. Deaths and cardiovascular events in men receiving testosterone. JAMA. 2014;311(9):963-964.
42. Dhindsa S, Batra M, Dandona P. Deaths and cardiovascular events in men receiving testosterone. JAMA. 2014;311(9):964.
43. Ho PM, Barón AE, Wierman M. Deaths and cardiovascular events in men receiving testosterone—reply. JAMA. 2014;311(9):964-965.
44. Traish AM, Guay AT, Morgentaler A. Death by testosterone? We think not! J Sex Med. 2014;11(3):624-629.
45. U.S. Department of Veterans Affairs, Veterans Health Administration (VHA), Pharmacy Benefit Management Services (PBM), Medical Advisory Panel (MAP), and Center for Medication Safety (VA Medsafe. National PBM Bulletin. Testosterone products and cardiovascular safety. http://www.pbm.va.gov/PBM/vacenterformedicationsafety/nationalpbmbulletin/Testosterone_Products_and_Cardiovascular_Safety_NATIONAL_PBM_BULLETIN_02.pdf. Published February 7, 2014. Accessed April 9, 2015.
46. Kacker R, Traish AM, Morgentaler A. Estrogens in men: clinical implications for sexual function and treatment of testosterone deficiency. J Sex Med. 2012;9(6):1681-1696.
47. Faloon W. Vindication. Life Extension Magazine Website. http://www.lef.org/magazine /mag2008/dec2008_Harvard-Experts-Recommend -Testosterone-Replacement_02.htm. Published December 2008. Accessed April 9,2015.
48. Walle T, Otake Y, Brubaker JA, Walle UK, Halushka PV. Disposition and metabolism of the flavonoid chrysin in normal volunteers. Br J Clin Pharmacol. 2001;51(2):143-146.
49. Jana K, Yin X, Schiffer, et al. Chrysin, a natural flavonoid enhances steroidogenesis and steroidogenic acute regulatory protein gene expression in mouse Leydig cells. J Endocrinol. 2008;197(2):315-323.
50. Chrubasik JE, Roufogalis BD, Wagner H, Chrubasik S. A comprehensive review on the stinging nettle effect and efficacy profiles. Part II: urticae radix. Phytomedicine. 2007;14(7-8):568-579.
51. Owens C, Baergen R, Puckett D. Online sources of herbal product information. Am J Med. 2014;127(2):109-115.
52. Blute W, Hakimian P, Kashanian J, Shteynshluyger A, Lee M, Shabsigh R. Erectile dysfunction and testosterone deficiency. Front Horm Res. 2009;37:108-122.
53. Mikhail N. Does testosterone have a role in erectile dysfunction? Am J Med. 2006;119(5):373-382.
1. Handelsman D. Global trends in testosterone prescribing, 2000-2011: expanding the spectrum of prescription drug misuse. Med J Aust. 2013;199(8):548-551.
2. Hackett G. Testosterone and the heart. Int J Clin Pract. 2012;66(7):648-655.
3. Morley JE. Hypogonadism, testosterone, and nursing home residents. J Am Med Dir Assoc. 2013;14(6):381-383.
4. An J, Cheetham TC, Van Den Eeden S. PS3-36: testosterone replacement therapy patterns for aging males in a managed care setting. Clin Med Res. 2013;11(3):141.
5. Moschis G, Lee E, Marthur A, Strautman J. The Maturing Marketplace: Buying Habits of Baby Boomers and Their Parents. Westport, CT: Quorum Books; 2000.
6. Morley JE, Kaiser FE, Perry HM 3rd, et al. Longitudinal changes in testosterone, luteinizing hormone, and follicle stimulating hormone in healthy older men. Metabolism. 1997;46(4):410-413.
7. Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR; Baltimore Longitudinal Study of Aging. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab. 2001;86(2):724-731.
8. Basaria S. Male hypogonadism. Lancet. 2014;383 (9924):1250-1263.
9. Bhasin S, Cunningham GR, Hayes FJ, et al; Task Force, Endocrine Society. Testosterone therapy in men with androgen deficiency syndromes. J Clin Endocrinol Metab. 2010;95(6):2536-2559.
10. Wang C, Swerdloff RS, Iranmanesh A, et al. Transdermal testosterone gel improves sexual function, mood, muscle strength, and body composition parameters in hypogonadal men. J Clin Endocrinol Metab. 2000;85(8):2839-2853.
11. Bolona ER, Uranga MV, Haddad RM, et al. Testosterone use in men with sexual dysfunction: a systematic review and meta-analysis of randomized placebo-controlled trials. Mayo Clin Proc. 2007;82(1):20-28.
12. Isidori AM, Giannetta E, Gianfrilli D, et al. Effects of testosterone on sexual function in men: results of a meta-analysis. Clin Endocrinol (Oxf). 2005;63(4):381-394.
13. Isidori AM, Giannetta E, Greco EA, et al. Effects of testosterone on body composition, bone metabolism and serum lipid profile in middle-aged men: a meta-analysis. Clin Endocrinol (Oxf). 2005;63(3):280-293.
14. Snyder PJ, Peachey H, Berlin JA, et al. Effects of testosterone replacement in hypogonadal men. J Clin Endocrinol Metab. 2000;85(8):2670-2677.
15. Sih R, Morley JE, Kaiser FE, Perry HM 3rd, Patrick P, Ross C. Testosterone replacement in older hypogonadal men: a 12-month randomized controlled trial. J Clin Endocrinol Metab. 1997;82(6):1661-1667.
16. Travison TG, Basaria S, Storer TW, et al. Clinical meaningfulness of the changes in muscle performance and physical function associated with testosterone administration in older men with mobility limitation. J Gerontol A Biol Sci Med Sci. 2011;66(10):1090-1099.
17. Jones TH, Arver S, Behre HM, et al; TIMES2 Investigators. Testosterone replacement in hypogonadal men with type 2 diabetes and/or metabolic syndrome. Diabetes Care. 2011;34(4):828-837.
18. Jones TH, Saad F. The effects of testosterone on risk factors for, and the mediators of, the atherosclerotic process. Atherosclerosis. 2009;207(2):318-327.
19. Mathur A, Malkin C, Saeed B, Muthusamy R, Jones TH, Channer K. Long-term benefits of testosterone replacement therapy on angina threshold and atheroma in men. Eur J Endocrinol. 2009;161(3):443-449.
20. Malkin CJ, Pugh PJ, Morris PD, et al. Testosterone replacement in hypogonadal men with angina improves ischaemic threshold and quality of life. Heart. 2004;90(8):871-876.
21. Paduch DA, Brannigan RE, Fuchs EF, Kim ED, Marmar JL, Sandlow JI. White Paper: The Laboratory Diagnosis of Testosterone Deficiency. http://www.auanet.org/common/pdf/education/clinical -guidance/Testosterone-Deficiency-WhitePaper.pdf. Published 2013. Accessed April 9, 2015.
22. Crawford ED, Barqawi AB, O’Donnell C, Morgentaler A. The association of time of day and serum testosterone concentration in a large screening population. BJU Int. 2007;100(3):509-513.
23. Brambilla DJ, O’Donnell AB, Matsumoto AM, McKinlay JB. Intraindividual variation in levels of serum testosterone and other reproductive and adrenal hormones in men. Clin Endocrinol (Oxf). 2007;67(6):853-862.
24. Kumar P, Kumar N, Thakur DS, Patidar A. Male hypogonadism: symptoms and treatment. J Adv Pharm Technol Res. 2010;1(3):297-301.
25. Montgomery K. Sexual desire disorders. Psychiatry (Edgmont). 2008;5(6):50-55.
26. Corona G, Boddi V, Balercia G, et al. The effect of statin therapy on testosterone levels in subjects consulting for erectile dysfunction. J Sex Med. 2010; 7(4 pt 1):1547-1556.
27. Schooling CM, Yeung SLA, Freeman G, Cowling BJ. The effect of statins on testosterone in men and women, a systematic review and meta-analysis of randomized controlled trials. BMC Med. 2013;11:57.
28. Wu FC, Tajar A, Pye SR, et al; European Male Aging Study Group. Hypothalamic-pituitary-testicular axis disruptions in older men are differentially linked to age and modifiable risk factors: the European Male Aging Study. J Clin Endocrinol Metab. 2008;93(7):2737-2745.
29. Schwartz LM, Woloshin S. Low “T” as in “template”: how to sell disease. JAMA Intern Med. 2013;173(15):1460-1462.
30. Male Hormone Modulation Therapy, Part 2. Life Extension Vitamins Website. http://www.lifeextension vitamins.com/mahomothpa2.html. Accessed April 9, 2015.
31. Baillargeon J, Urban RJ, Ottenbacher KJ, Pierson KS, Goodwin JS. Trends in androgen prescribing in the United States, 2001 to 2011. JAMA Intern Med. 2013;173(15):1465-1466.
32. Layton JB, Li D, Meier CR, et al. Testosterone lab testing and initiation in the United Kingdom and the United States, 2000 to 2011. J Clin Endocrinol Metab. 2014;99(3):835-842.
33. Ramasamy R, Fisher ES, Schlegel PN. Testosterone replacement and prostate cancer. Indian J Urol. 2012;28(2):123-128.
34. Marks, LS, Mazer NA, Mostaghel E, et al. Effect of testosterone replacement therapy on prostate tissue in men with late-onset hypogonadism: a randomized controlled trial. JAMA. 2006;296(19):2351-2361.
35. Coward RM, Simhan J, Carson CC 3rd. Prostate-specific antigen changes and prostate cancer in hypogonadal men treated with testosterone replacement therapy. BJU Int. 2009;103(9):1179-1183.
36. Basaria S, Coviello AD, Travison TG, et al. Adverse events associated with testosterone administration. N Engl J Med. 2010;363(2):109-122.
37. Vigen R, O’Donnell Cl, Barón AE, et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310(17):1829-1836.
38. Morgentaler A, Traish A, Kacker R. Deaths and cardiovascular events in men receiving testosterone. JAMA. 2014;311(9):961-962.
39. Jones TH, Channer K. Deaths and cardiovascular events in men receiving testosterone. JAMA. 2014;311(9):962-963.
40. Katz J, Nadelberg R. Deaths and cardiovascular events in men receiving testosterone. JAMA. 2014;311(9):963.
41. Riche D, Baker WL, Koch CA. Deaths and cardiovascular events in men receiving testosterone. JAMA. 2014;311(9):963-964.
42. Dhindsa S, Batra M, Dandona P. Deaths and cardiovascular events in men receiving testosterone. JAMA. 2014;311(9):964.
43. Ho PM, Barón AE, Wierman M. Deaths and cardiovascular events in men receiving testosterone—reply. JAMA. 2014;311(9):964-965.
44. Traish AM, Guay AT, Morgentaler A. Death by testosterone? We think not! J Sex Med. 2014;11(3):624-629.
45. U.S. Department of Veterans Affairs, Veterans Health Administration (VHA), Pharmacy Benefit Management Services (PBM), Medical Advisory Panel (MAP), and Center for Medication Safety (VA Medsafe. National PBM Bulletin. Testosterone products and cardiovascular safety. http://www.pbm.va.gov/PBM/vacenterformedicationsafety/nationalpbmbulletin/Testosterone_Products_and_Cardiovascular_Safety_NATIONAL_PBM_BULLETIN_02.pdf. Published February 7, 2014. Accessed April 9, 2015.
46. Kacker R, Traish AM, Morgentaler A. Estrogens in men: clinical implications for sexual function and treatment of testosterone deficiency. J Sex Med. 2012;9(6):1681-1696.
47. Faloon W. Vindication. Life Extension Magazine Website. http://www.lef.org/magazine /mag2008/dec2008_Harvard-Experts-Recommend -Testosterone-Replacement_02.htm. Published December 2008. Accessed April 9,2015.
48. Walle T, Otake Y, Brubaker JA, Walle UK, Halushka PV. Disposition and metabolism of the flavonoid chrysin in normal volunteers. Br J Clin Pharmacol. 2001;51(2):143-146.
49. Jana K, Yin X, Schiffer, et al. Chrysin, a natural flavonoid enhances steroidogenesis and steroidogenic acute regulatory protein gene expression in mouse Leydig cells. J Endocrinol. 2008;197(2):315-323.
50. Chrubasik JE, Roufogalis BD, Wagner H, Chrubasik S. A comprehensive review on the stinging nettle effect and efficacy profiles. Part II: urticae radix. Phytomedicine. 2007;14(7-8):568-579.
51. Owens C, Baergen R, Puckett D. Online sources of herbal product information. Am J Med. 2014;127(2):109-115.
52. Blute W, Hakimian P, Kashanian J, Shteynshluyger A, Lee M, Shabsigh R. Erectile dysfunction and testosterone deficiency. Front Horm Res. 2009;37:108-122.
53. Mikhail N. Does testosterone have a role in erectile dysfunction? Am J Med. 2006;119(5):373-382.
Pruritic Urticarial Papules and Plaques of Pregnancy Occurring Postpartum
The cutaneous effects of pregnancy are variable and numerous. We all have likely seen the pigmentary changes induced by pregnancy as well as both exacerbation and complete resolution of preexisting skin conditions. The dermatoses of pregnancy are classified as a group of inflammatory skin conditions exclusively seen in pregnant women, the most common being pruritic urticarial papules and plaques of pregnancy (PUPPP).1 Also known as polymorphic eruption of pregnancy in Europe, PUPPP was first recognized in 1979 as a distinct entity that manifested as an intense pruritic eruption unique to women in the third trimester of pregnancy.2 The condition usually is self-limited, with the majority of cases spontaneously resolving within 4 to 6 weeks after delivery.3,4 Presentation of PUPPP in the postpartum period is rare.1-4 We report a biopsy-proven case of PUPPP in a 30-year-old woman who presented 2 weeks postpartum with an intensely pruritic generalized eruption. A PubMed search of articles indexed for MEDLINE using the search terms pruritic urticarial papules and plaques of pregnancy or polymorphic eruption of pregnancy and postpartum revealed only 5 reports of PUPPP or polymorphic eruption of pregnancy occurring in the postpartum period, 2 occurring in the United States.5-9
Case Report
A 30-year-old woman who was 2 weeks postpartum presented to our dermatology clinic with an intensely pruritic generalized rash. Within 24 hours of delivery of her first child, the patient developed an itchy rash on the abdomen and was started on oral corticosteroids and antihistamines in the hospital. On discharge, she was instructed to follow up with the dermatology department if the rash did not resolve. After leaving the hospital, she reported that the eruption had progressively spread to the buttocks, legs, and arms, and the itching seemed to be worse despite finishing the course of oral corticosteroids and antihistamines.
The patient’s prenatal course was uneventful. She gained 16 kg during pregnancy, with a prepregnancy weight of 50 kg. A healthy male neonate was delivered at 38 weeks’ gestation without complication. The patient’s medical history was unremarkable. Her current medications included prenatal vitamins, oral prednisone, and loratadine, and she reported no known drug allergies.
On physical examination, the patient was afebrile and her blood pressure was normal. Examination of the skin revealed erythematous papules and urticarial plaques involving the abdominal striae with periumbilical sparing (Figure 1A). Similar lesions were noted on the legs, buttocks, and arms (Figure 1B). The face, palms, and soles were uninvolved. No vesicles or pustules were noted. The oral mucosa was pink, moist, and unremarkable.
 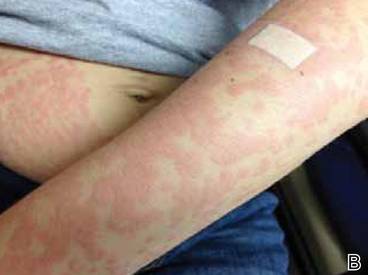 Figure 1. Initial presentation of urticarial plaques involving the abdominal striae with periumbilical sparing (A) and the left arm (B). |
Based on the patient’s clinical presentation, the differential diagnosis included pemphigoid gestationis, a hypersensitivity reaction, cutaneous lupus, cholestasis of pregnancy, and PUPPP. Pruritic urticarial papules and plaques of pregnancy was considered to be unlikely because of the uncharacteristic postpartum presentation of the eruption.
Two 4-mm punch biopsies were performed on the left upper arm and were sent for histopathologic examination and direct immunofluorescence. Laboratory studies including complete blood cell count with differential, complete metabolic panel, antinuclear antibodies, and IgE levels were conducted. The patient was started on triamcinolone cream 0.1% twice daily and her antihistamine was switched from loratadine to cetirizine.
Histopathologic examination revealed a mixed perivascular infiltrate in the superficial dermis consisting of lymphocytes, mast cells, and eosinophils (Figures 2 and 3), which was consistent with a diagnosis of PUPPP. Direct immunofluorescence was negative. Laboratory studies were within reference range and antinuclear antibodies and IgE levels were negative. A diagnosis of postpartum PUPPP was made. Complete resolution of the eruption was experienced by 2-week follow-up (Figures 4A and 4B). The patient noted that her symptoms improved within 2 days of starting topical therapy.
|
|
Comment
Pruritic urticarial papules and plaques of pregnancy complicates 1 of 160 to 1 of 300 pregnancies.1 As seen in our case, the majority of cases of PUPPP are diagnosed in women who are nulliparous or primigravida.10 A study by Aronson et al10 reported that of 57 cases of PUPPP, 24 (42%) patients were primigravida, 16 (28%) were gravida 2, 9 (16%) were gravida 3, 4 (7%) were gravida 4, 3 (5%) were gravida 6, and 1 (2%) was gravida 7. Thirty-nine (68%) patients were nulliparous.10 The average onset of symptoms is approximately 35 weeks’ gestation.9
Classical presentation of PUPPP starts with erythematous papules within the abdominal striae, sparing the periumbilical skin.1 The abdominal striae are most commonly affected, and in some women, it may be the only site affected.10 The lesions then may pro-gress to urticarial plaques involving the extremities, while the face, palms, and soles usually are spared.11 However, clinical manifestations of PUPPP can vary, with reports of targetlike lesions with a surrounding halo resembling erythema multiforme as well as involvement of the face and palmoplantar skin.10-13 Histologic findings are not diagnostic but can help distinguish PUPPP from other pregnancy-associated dermatoses.14 Histologically, PUPPP demonstrates variable epidermal spongiosis and a nonspecific superficial perivascular infiltrate in the dermis composed of lymphocytes with eosinophils or neutrophils, and there may be dermal edema.10,15 Direct immunofluorescence usually is negative in PUPPP; however, 31% of cases have demonstrated deposition of C3 and IgM or IgA, either perivascularly or at the dermoepidermal junction.1,10,15
There are no systemic alterations seen in PUPPP; however, all patients report severe pruritus.12 Pruritic urticarial papules and plaques of pregnancy typically affects women in the third trimester, and delivery is curative in most patients.13 Recurrence of PUPPP usually is not seen with subsequent pregnancies, and the long-term prognosis is excellent.15
The pathogenesis of PUPPP is not well understood and likely is multifactorial. Ohel et al12 found PUPPP to be strongly associated with hypertensive disorders, multiple gestation pregnancies, excessive maternal weight gain, excessive stretching of the abdominal skin, and nulliparity.13 One theory suggests that abdominal skin stretching, if drastic, can damage underlying connective tissue, resulting in the release of antigens that can trigger a reactive inflammatory response.16 The majority of maternal weight gain occurs during the third trimester, which may explain why most cases of PUPPP present in the third trimester.17 Alternative theories have suggested that PUPPP may represent an immunologic response to circulating fetal antigens.18 It is possible, as in our case, that certain nulliparous women who have a healthy weight prior to pregnancy (as determined by a body mass index of 18.5 to 24.9) in combination with excessive weight gain during the third trimester and drastic hormone fluctuations associated with labor and delivery may be at greater risk for developing PUPPP. Another theory may be related to the degree of skin stretching during the third trimester and the abrupt decrease in the stretching of the skin that occurs with delivery.16
Conclusion
Pruritic urticarial papules and plaques of pregnancy can present in a variety of ways, most commonly in the third trimester but also in the postpartum period. When a patient presents in the postpartum period with a pruritic eruption, PUPPP should be included in the differential diagnosis. The pathogenesis of PUPPP is multifactorial and not well understood, and additional research in the field may lead to improved prediction of who may be at risk and what we can do to prevent it.
1. Pomeranz MK. Dermatoses of pregnancy. UpToDate Web site. http://www.uptodate.com/contents/dermatoses-of-pregnancy. Updated December 22, 2014. Accessed May 5, 2015.
2. Lawley TJ, Hertz KC, Wade TR, et al. Pruritic urticarial papules and plaques of pregnancy. JAMA. 1979;241:1696-1699.
3. Kroumpouzos G, Cohen LM. Specific dermatoses of pregnancy: an evidence-based systematic review. Am J Obstet Gynecol. 2003;188:1083-1092.
4. Callen JP, Hanno R. Pruritic urticarial papules and plaques of pregnancy (PUPPP): a clinicopathologic study. J Am Acad Dermatol.1981;5:401-405.
5. Ozcan D, Ozcakmak B, Aydogan FC. J Obstet Gynaecol Res. 2011;37:1158-1161.
6. Journet-Tollhupp J, Tchen T, Remy-Leroux V, et al. Polymorphic eruption of pregnancy and acquired hemophilia A [in French]. Ann Dermatol Venereol. 2010;137:713-717.
7. Buccolo LS, Viera AJ. Pruritic urticarial papules and plaques of pregnancy presenting in the postpartum period: a case report. J Reprod Med. 2005;50:61-63.
8. Kirkup ME, Dunnill MG. Polymorphic eruption of pregnancy developing in the puerrperium. Clin Exp Dermatol. 2002;27:657-660.
9. Yancy KB, Hall RP, Lawley TJ. Pruritic urticarial papules and plaques of pregnancy: clinical experience in twenty-five patients. J Am Acad Dermatol. 1984;10:473-480.
10. Aronson IK, Bond S, Fiedler VC, et al. Pruritic urticarial papules and plaques of pregnancy: clinical and immunopathologic observations in 57 patients. J Am Acad Dermatol. 1998;39:933-939.
11. Roger D, Vaillant L, Fignon A, et al. Specific pruritic dermatoses of pregnancy: a prospective study of 3129 women. Arch Dermatol. 1994;130:734-739.
12. Ohel I, Levy A, Silberstein T, et al. Pregnancy outcome of patients with pruritic urticarial papules and plaques of pregnancy. J Matern Fetal Neonatal Med. 2006;19:305-308.
13. Elling SV, McKenna P, Pawell FC. Pruritic urticarial papules and plaques of pregnancy in twin and triplet pregnancies. J Eur Acad Dermatol Venereol. 2000;14:378-381.
14. Scheinfeld N. Pruritic urticarial papules and plaques of pregnancy wholly abated with one week twice daily application of fluticasone propionate lotion: a case report and review of the literature. Dermatol Online J. 2008;14:4.
15. Shornick JK. Dermatoses of pregnancy. Semin Cutan Med Surg. 1998;17:172-181.
16. Cohen LM, Capeless EL, Krusinski PA, et al. Pruritic urticarial papules and plaques of pregnancy and its relationship to maternal-fetal weight gain and twin pregnancy. Arch Dermatol. 1989;125:1534-1536.
17. Drehmer M, Duncan BB, Kac G, et al. Association of second and third trimester weight gain in pregnancy with maternal and fetal outcomes. PLoS One. 2013;8:e54704.
18. Aractingi S, Berkane N, Bertheau P, et al. Fetal DNA in skin of polymorphic eruptions of pregnancy. Lancet. 1998;352:1898-1901.
The cutaneous effects of pregnancy are variable and numerous. We all have likely seen the pigmentary changes induced by pregnancy as well as both exacerbation and complete resolution of preexisting skin conditions. The dermatoses of pregnancy are classified as a group of inflammatory skin conditions exclusively seen in pregnant women, the most common being pruritic urticarial papules and plaques of pregnancy (PUPPP).1 Also known as polymorphic eruption of pregnancy in Europe, PUPPP was first recognized in 1979 as a distinct entity that manifested as an intense pruritic eruption unique to women in the third trimester of pregnancy.2 The condition usually is self-limited, with the majority of cases spontaneously resolving within 4 to 6 weeks after delivery.3,4 Presentation of PUPPP in the postpartum period is rare.1-4 We report a biopsy-proven case of PUPPP in a 30-year-old woman who presented 2 weeks postpartum with an intensely pruritic generalized eruption. A PubMed search of articles indexed for MEDLINE using the search terms pruritic urticarial papules and plaques of pregnancy or polymorphic eruption of pregnancy and postpartum revealed only 5 reports of PUPPP or polymorphic eruption of pregnancy occurring in the postpartum period, 2 occurring in the United States.5-9
Case Report
A 30-year-old woman who was 2 weeks postpartum presented to our dermatology clinic with an intensely pruritic generalized rash. Within 24 hours of delivery of her first child, the patient developed an itchy rash on the abdomen and was started on oral corticosteroids and antihistamines in the hospital. On discharge, she was instructed to follow up with the dermatology department if the rash did not resolve. After leaving the hospital, she reported that the eruption had progressively spread to the buttocks, legs, and arms, and the itching seemed to be worse despite finishing the course of oral corticosteroids and antihistamines.
The patient’s prenatal course was uneventful. She gained 16 kg during pregnancy, with a prepregnancy weight of 50 kg. A healthy male neonate was delivered at 38 weeks’ gestation without complication. The patient’s medical history was unremarkable. Her current medications included prenatal vitamins, oral prednisone, and loratadine, and she reported no known drug allergies.
On physical examination, the patient was afebrile and her blood pressure was normal. Examination of the skin revealed erythematous papules and urticarial plaques involving the abdominal striae with periumbilical sparing (Figure 1A). Similar lesions were noted on the legs, buttocks, and arms (Figure 1B). The face, palms, and soles were uninvolved. No vesicles or pustules were noted. The oral mucosa was pink, moist, and unremarkable.
  Figure 1. Initial presentation of urticarial plaques involving the abdominal striae with periumbilical sparing (A) and the left arm (B). |
Based on the patient’s clinical presentation, the differential diagnosis included pemphigoid gestationis, a hypersensitivity reaction, cutaneous lupus, cholestasis of pregnancy, and PUPPP. Pruritic urticarial papules and plaques of pregnancy was considered to be unlikely because of the uncharacteristic postpartum presentation of the eruption.
Two 4-mm punch biopsies were performed on the left upper arm and were sent for histopathologic examination and direct immunofluorescence. Laboratory studies including complete blood cell count with differential, complete metabolic panel, antinuclear antibodies, and IgE levels were conducted. The patient was started on triamcinolone cream 0.1% twice daily and her antihistamine was switched from loratadine to cetirizine.
Histopathologic examination revealed a mixed perivascular infiltrate in the superficial dermis consisting of lymphocytes, mast cells, and eosinophils (Figures 2 and 3), which was consistent with a diagnosis of PUPPP. Direct immunofluorescence was negative. Laboratory studies were within reference range and antinuclear antibodies and IgE levels were negative. A diagnosis of postpartum PUPPP was made. Complete resolution of the eruption was experienced by 2-week follow-up (Figures 4A and 4B). The patient noted that her symptoms improved within 2 days of starting topical therapy.
|
|
Comment
Pruritic urticarial papules and plaques of pregnancy complicates 1 of 160 to 1 of 300 pregnancies.1 As seen in our case, the majority of cases of PUPPP are diagnosed in women who are nulliparous or primigravida.10 A study by Aronson et al10 reported that of 57 cases of PUPPP, 24 (42%) patients were primigravida, 16 (28%) were gravida 2, 9 (16%) were gravida 3, 4 (7%) were gravida 4, 3 (5%) were gravida 6, and 1 (2%) was gravida 7. Thirty-nine (68%) patients were nulliparous.10 The average onset of symptoms is approximately 35 weeks’ gestation.9
Classical presentation of PUPPP starts with erythematous papules within the abdominal striae, sparing the periumbilical skin.1 The abdominal striae are most commonly affected, and in some women, it may be the only site affected.10 The lesions then may pro-gress to urticarial plaques involving the extremities, while the face, palms, and soles usually are spared.11 However, clinical manifestations of PUPPP can vary, with reports of targetlike lesions with a surrounding halo resembling erythema multiforme as well as involvement of the face and palmoplantar skin.10-13 Histologic findings are not diagnostic but can help distinguish PUPPP from other pregnancy-associated dermatoses.14 Histologically, PUPPP demonstrates variable epidermal spongiosis and a nonspecific superficial perivascular infiltrate in the dermis composed of lymphocytes with eosinophils or neutrophils, and there may be dermal edema.10,15 Direct immunofluorescence usually is negative in PUPPP; however, 31% of cases have demonstrated deposition of C3 and IgM or IgA, either perivascularly or at the dermoepidermal junction.1,10,15
There are no systemic alterations seen in PUPPP; however, all patients report severe pruritus.12 Pruritic urticarial papules and plaques of pregnancy typically affects women in the third trimester, and delivery is curative in most patients.13 Recurrence of PUPPP usually is not seen with subsequent pregnancies, and the long-term prognosis is excellent.15
The pathogenesis of PUPPP is not well understood and likely is multifactorial. Ohel et al12 found PUPPP to be strongly associated with hypertensive disorders, multiple gestation pregnancies, excessive maternal weight gain, excessive stretching of the abdominal skin, and nulliparity.13 One theory suggests that abdominal skin stretching, if drastic, can damage underlying connective tissue, resulting in the release of antigens that can trigger a reactive inflammatory response.16 The majority of maternal weight gain occurs during the third trimester, which may explain why most cases of PUPPP present in the third trimester.17 Alternative theories have suggested that PUPPP may represent an immunologic response to circulating fetal antigens.18 It is possible, as in our case, that certain nulliparous women who have a healthy weight prior to pregnancy (as determined by a body mass index of 18.5 to 24.9) in combination with excessive weight gain during the third trimester and drastic hormone fluctuations associated with labor and delivery may be at greater risk for developing PUPPP. Another theory may be related to the degree of skin stretching during the third trimester and the abrupt decrease in the stretching of the skin that occurs with delivery.16
Conclusion
Pruritic urticarial papules and plaques of pregnancy can present in a variety of ways, most commonly in the third trimester but also in the postpartum period. When a patient presents in the postpartum period with a pruritic eruption, PUPPP should be included in the differential diagnosis. The pathogenesis of PUPPP is multifactorial and not well understood, and additional research in the field may lead to improved prediction of who may be at risk and what we can do to prevent it.
The cutaneous effects of pregnancy are variable and numerous. We all have likely seen the pigmentary changes induced by pregnancy as well as both exacerbation and complete resolution of preexisting skin conditions. The dermatoses of pregnancy are classified as a group of inflammatory skin conditions exclusively seen in pregnant women, the most common being pruritic urticarial papules and plaques of pregnancy (PUPPP).1 Also known as polymorphic eruption of pregnancy in Europe, PUPPP was first recognized in 1979 as a distinct entity that manifested as an intense pruritic eruption unique to women in the third trimester of pregnancy.2 The condition usually is self-limited, with the majority of cases spontaneously resolving within 4 to 6 weeks after delivery.3,4 Presentation of PUPPP in the postpartum period is rare.1-4 We report a biopsy-proven case of PUPPP in a 30-year-old woman who presented 2 weeks postpartum with an intensely pruritic generalized eruption. A PubMed search of articles indexed for MEDLINE using the search terms pruritic urticarial papules and plaques of pregnancy or polymorphic eruption of pregnancy and postpartum revealed only 5 reports of PUPPP or polymorphic eruption of pregnancy occurring in the postpartum period, 2 occurring in the United States.5-9
Case Report
A 30-year-old woman who was 2 weeks postpartum presented to our dermatology clinic with an intensely pruritic generalized rash. Within 24 hours of delivery of her first child, the patient developed an itchy rash on the abdomen and was started on oral corticosteroids and antihistamines in the hospital. On discharge, she was instructed to follow up with the dermatology department if the rash did not resolve. After leaving the hospital, she reported that the eruption had progressively spread to the buttocks, legs, and arms, and the itching seemed to be worse despite finishing the course of oral corticosteroids and antihistamines.
The patient’s prenatal course was uneventful. She gained 16 kg during pregnancy, with a prepregnancy weight of 50 kg. A healthy male neonate was delivered at 38 weeks’ gestation without complication. The patient’s medical history was unremarkable. Her current medications included prenatal vitamins, oral prednisone, and loratadine, and she reported no known drug allergies.
On physical examination, the patient was afebrile and her blood pressure was normal. Examination of the skin revealed erythematous papules and urticarial plaques involving the abdominal striae with periumbilical sparing (Figure 1A). Similar lesions were noted on the legs, buttocks, and arms (Figure 1B). The face, palms, and soles were uninvolved. No vesicles or pustules were noted. The oral mucosa was pink, moist, and unremarkable.
  Figure 1. Initial presentation of urticarial plaques involving the abdominal striae with periumbilical sparing (A) and the left arm (B). |
Based on the patient’s clinical presentation, the differential diagnosis included pemphigoid gestationis, a hypersensitivity reaction, cutaneous lupus, cholestasis of pregnancy, and PUPPP. Pruritic urticarial papules and plaques of pregnancy was considered to be unlikely because of the uncharacteristic postpartum presentation of the eruption.
Two 4-mm punch biopsies were performed on the left upper arm and were sent for histopathologic examination and direct immunofluorescence. Laboratory studies including complete blood cell count with differential, complete metabolic panel, antinuclear antibodies, and IgE levels were conducted. The patient was started on triamcinolone cream 0.1% twice daily and her antihistamine was switched from loratadine to cetirizine.
Histopathologic examination revealed a mixed perivascular infiltrate in the superficial dermis consisting of lymphocytes, mast cells, and eosinophils (Figures 2 and 3), which was consistent with a diagnosis of PUPPP. Direct immunofluorescence was negative. Laboratory studies were within reference range and antinuclear antibodies and IgE levels were negative. A diagnosis of postpartum PUPPP was made. Complete resolution of the eruption was experienced by 2-week follow-up (Figures 4A and 4B). The patient noted that her symptoms improved within 2 days of starting topical therapy.
|
|
Comment
Pruritic urticarial papules and plaques of pregnancy complicates 1 of 160 to 1 of 300 pregnancies.1 As seen in our case, the majority of cases of PUPPP are diagnosed in women who are nulliparous or primigravida.10 A study by Aronson et al10 reported that of 57 cases of PUPPP, 24 (42%) patients were primigravida, 16 (28%) were gravida 2, 9 (16%) were gravida 3, 4 (7%) were gravida 4, 3 (5%) were gravida 6, and 1 (2%) was gravida 7. Thirty-nine (68%) patients were nulliparous.10 The average onset of symptoms is approximately 35 weeks’ gestation.9
Classical presentation of PUPPP starts with erythematous papules within the abdominal striae, sparing the periumbilical skin.1 The abdominal striae are most commonly affected, and in some women, it may be the only site affected.10 The lesions then may pro-gress to urticarial plaques involving the extremities, while the face, palms, and soles usually are spared.11 However, clinical manifestations of PUPPP can vary, with reports of targetlike lesions with a surrounding halo resembling erythema multiforme as well as involvement of the face and palmoplantar skin.10-13 Histologic findings are not diagnostic but can help distinguish PUPPP from other pregnancy-associated dermatoses.14 Histologically, PUPPP demonstrates variable epidermal spongiosis and a nonspecific superficial perivascular infiltrate in the dermis composed of lymphocytes with eosinophils or neutrophils, and there may be dermal edema.10,15 Direct immunofluorescence usually is negative in PUPPP; however, 31% of cases have demonstrated deposition of C3 and IgM or IgA, either perivascularly or at the dermoepidermal junction.1,10,15
There are no systemic alterations seen in PUPPP; however, all patients report severe pruritus.12 Pruritic urticarial papules and plaques of pregnancy typically affects women in the third trimester, and delivery is curative in most patients.13 Recurrence of PUPPP usually is not seen with subsequent pregnancies, and the long-term prognosis is excellent.15
The pathogenesis of PUPPP is not well understood and likely is multifactorial. Ohel et al12 found PUPPP to be strongly associated with hypertensive disorders, multiple gestation pregnancies, excessive maternal weight gain, excessive stretching of the abdominal skin, and nulliparity.13 One theory suggests that abdominal skin stretching, if drastic, can damage underlying connective tissue, resulting in the release of antigens that can trigger a reactive inflammatory response.16 The majority of maternal weight gain occurs during the third trimester, which may explain why most cases of PUPPP present in the third trimester.17 Alternative theories have suggested that PUPPP may represent an immunologic response to circulating fetal antigens.18 It is possible, as in our case, that certain nulliparous women who have a healthy weight prior to pregnancy (as determined by a body mass index of 18.5 to 24.9) in combination with excessive weight gain during the third trimester and drastic hormone fluctuations associated with labor and delivery may be at greater risk for developing PUPPP. Another theory may be related to the degree of skin stretching during the third trimester and the abrupt decrease in the stretching of the skin that occurs with delivery.16
Conclusion
Pruritic urticarial papules and plaques of pregnancy can present in a variety of ways, most commonly in the third trimester but also in the postpartum period. When a patient presents in the postpartum period with a pruritic eruption, PUPPP should be included in the differential diagnosis. The pathogenesis of PUPPP is multifactorial and not well understood, and additional research in the field may lead to improved prediction of who may be at risk and what we can do to prevent it.
1. Pomeranz MK. Dermatoses of pregnancy. UpToDate Web site. http://www.uptodate.com/contents/dermatoses-of-pregnancy. Updated December 22, 2014. Accessed May 5, 2015.
2. Lawley TJ, Hertz KC, Wade TR, et al. Pruritic urticarial papules and plaques of pregnancy. JAMA. 1979;241:1696-1699.
3. Kroumpouzos G, Cohen LM. Specific dermatoses of pregnancy: an evidence-based systematic review. Am J Obstet Gynecol. 2003;188:1083-1092.
4. Callen JP, Hanno R. Pruritic urticarial papules and plaques of pregnancy (PUPPP): a clinicopathologic study. J Am Acad Dermatol.1981;5:401-405.
5. Ozcan D, Ozcakmak B, Aydogan FC. J Obstet Gynaecol Res. 2011;37:1158-1161.
6. Journet-Tollhupp J, Tchen T, Remy-Leroux V, et al. Polymorphic eruption of pregnancy and acquired hemophilia A [in French]. Ann Dermatol Venereol. 2010;137:713-717.
7. Buccolo LS, Viera AJ. Pruritic urticarial papules and plaques of pregnancy presenting in the postpartum period: a case report. J Reprod Med. 2005;50:61-63.
8. Kirkup ME, Dunnill MG. Polymorphic eruption of pregnancy developing in the puerrperium. Clin Exp Dermatol. 2002;27:657-660.
9. Yancy KB, Hall RP, Lawley TJ. Pruritic urticarial papules and plaques of pregnancy: clinical experience in twenty-five patients. J Am Acad Dermatol. 1984;10:473-480.
10. Aronson IK, Bond S, Fiedler VC, et al. Pruritic urticarial papules and plaques of pregnancy: clinical and immunopathologic observations in 57 patients. J Am Acad Dermatol. 1998;39:933-939.
11. Roger D, Vaillant L, Fignon A, et al. Specific pruritic dermatoses of pregnancy: a prospective study of 3129 women. Arch Dermatol. 1994;130:734-739.
12. Ohel I, Levy A, Silberstein T, et al. Pregnancy outcome of patients with pruritic urticarial papules and plaques of pregnancy. J Matern Fetal Neonatal Med. 2006;19:305-308.
13. Elling SV, McKenna P, Pawell FC. Pruritic urticarial papules and plaques of pregnancy in twin and triplet pregnancies. J Eur Acad Dermatol Venereol. 2000;14:378-381.
14. Scheinfeld N. Pruritic urticarial papules and plaques of pregnancy wholly abated with one week twice daily application of fluticasone propionate lotion: a case report and review of the literature. Dermatol Online J. 2008;14:4.
15. Shornick JK. Dermatoses of pregnancy. Semin Cutan Med Surg. 1998;17:172-181.
16. Cohen LM, Capeless EL, Krusinski PA, et al. Pruritic urticarial papules and plaques of pregnancy and its relationship to maternal-fetal weight gain and twin pregnancy. Arch Dermatol. 1989;125:1534-1536.
17. Drehmer M, Duncan BB, Kac G, et al. Association of second and third trimester weight gain in pregnancy with maternal and fetal outcomes. PLoS One. 2013;8:e54704.
18. Aractingi S, Berkane N, Bertheau P, et al. Fetal DNA in skin of polymorphic eruptions of pregnancy. Lancet. 1998;352:1898-1901.
1. Pomeranz MK. Dermatoses of pregnancy. UpToDate Web site. http://www.uptodate.com/contents/dermatoses-of-pregnancy. Updated December 22, 2014. Accessed May 5, 2015.
2. Lawley TJ, Hertz KC, Wade TR, et al. Pruritic urticarial papules and plaques of pregnancy. JAMA. 1979;241:1696-1699.
3. Kroumpouzos G, Cohen LM. Specific dermatoses of pregnancy: an evidence-based systematic review. Am J Obstet Gynecol. 2003;188:1083-1092.
4. Callen JP, Hanno R. Pruritic urticarial papules and plaques of pregnancy (PUPPP): a clinicopathologic study. J Am Acad Dermatol.1981;5:401-405.
5. Ozcan D, Ozcakmak B, Aydogan FC. J Obstet Gynaecol Res. 2011;37:1158-1161.
6. Journet-Tollhupp J, Tchen T, Remy-Leroux V, et al. Polymorphic eruption of pregnancy and acquired hemophilia A [in French]. Ann Dermatol Venereol. 2010;137:713-717.
7. Buccolo LS, Viera AJ. Pruritic urticarial papules and plaques of pregnancy presenting in the postpartum period: a case report. J Reprod Med. 2005;50:61-63.
8. Kirkup ME, Dunnill MG. Polymorphic eruption of pregnancy developing in the puerrperium. Clin Exp Dermatol. 2002;27:657-660.
9. Yancy KB, Hall RP, Lawley TJ. Pruritic urticarial papules and plaques of pregnancy: clinical experience in twenty-five patients. J Am Acad Dermatol. 1984;10:473-480.
10. Aronson IK, Bond S, Fiedler VC, et al. Pruritic urticarial papules and plaques of pregnancy: clinical and immunopathologic observations in 57 patients. J Am Acad Dermatol. 1998;39:933-939.
11. Roger D, Vaillant L, Fignon A, et al. Specific pruritic dermatoses of pregnancy: a prospective study of 3129 women. Arch Dermatol. 1994;130:734-739.
12. Ohel I, Levy A, Silberstein T, et al. Pregnancy outcome of patients with pruritic urticarial papules and plaques of pregnancy. J Matern Fetal Neonatal Med. 2006;19:305-308.
13. Elling SV, McKenna P, Pawell FC. Pruritic urticarial papules and plaques of pregnancy in twin and triplet pregnancies. J Eur Acad Dermatol Venereol. 2000;14:378-381.
14. Scheinfeld N. Pruritic urticarial papules and plaques of pregnancy wholly abated with one week twice daily application of fluticasone propionate lotion: a case report and review of the literature. Dermatol Online J. 2008;14:4.
15. Shornick JK. Dermatoses of pregnancy. Semin Cutan Med Surg. 1998;17:172-181.
16. Cohen LM, Capeless EL, Krusinski PA, et al. Pruritic urticarial papules and plaques of pregnancy and its relationship to maternal-fetal weight gain and twin pregnancy. Arch Dermatol. 1989;125:1534-1536.
17. Drehmer M, Duncan BB, Kac G, et al. Association of second and third trimester weight gain in pregnancy with maternal and fetal outcomes. PLoS One. 2013;8:e54704.
18. Aractingi S, Berkane N, Bertheau P, et al. Fetal DNA in skin of polymorphic eruptions of pregnancy. Lancet. 1998;352:1898-1901.
Practice Points
- Pruritic urticarial papules and plaques of pregnancy (PUPPP) is an intensely pruritic eruption that typically affects women during the third trimester of pregnancy.
- Because clinical manifestations can vary, PUPPP should be considered in the differential diagnosis when patients present in the postpartum period with a pruritic eruption.
- Histologic findings are not diagnostic but can help distinguish PUPPP from other pregnancy-associated dermatoses.