User login
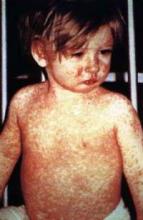
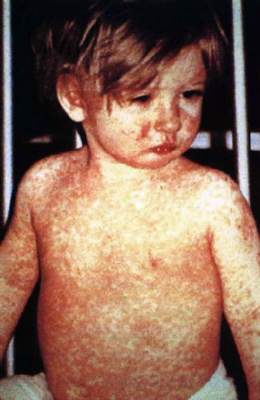
CDC: Suspect measles when seeing fever and rash
Physicians should have a high degree of suspicion for measles when a patient presents with fever, rash, and other measles-related symptoms, CDC officials advised Jan. 29.
“I’m urging all health care professionals to think ‘measles’ when they’re evaluating patients with fever, rash, and other measles-related symptoms,” Dr. Anne Schuchat, the U.S. assistant surgeon general, and the director of the Centers for Disease Control and Prevention’s National Center for Immunization and Respiratory Diseases, said during a press conference. “Health care professionals need to know the guidelines for infection control and reporting measles cases, and they should work to ensure that patients are getting the best protection possible against measles, which is on-time MMR [measles, mumps, and rubella] vaccination, to protect them whether at home or abroad.”
Early symptoms include cough, runny nose and red, light-sensitive eyes. Two to four days later, a fine rash of red spots develops on the face and then gradually spreads down over the entire body. Fever, which can reach 103-105° F, comes with the rash. White spots, called Koplik spots, may appear on the inside of the cheeks.
Measles is more contagious than almost any other disease. The virus that causes measles lives in the nose and throat of infected people and is sprayed into the air when an infected person sneezes, coughs or talks, and can stay in the air for up to 2 hours. People with measles can spread the disease starting 4 days before the rash begins until 4 days after it appears.
At least 84 people across 14 states have been diagnosed recently with measles, including at least 67 who are thought to have been infected in mid- to late December while visiting Disneyland in Anaheim, Calif., Dr. Schuchat said. “We believe someone got infected [with measles] overseas, visited the Disneyland park, and spread the disease to others.” Those people went on to expose others in a variety of settings, including schools, day care centers, outpatient clinics, and airplanes, she added.
“This is not a problem of the measles vaccine not working, this is a problem of the measles vaccine not being used,” Dr. Schuchat said.
The CDC’s Advisory Committee on Immunization Practices recommends that children between 6 months and 12 months of age be vaccinated, particularly if they will be traveling, since many countries still experience measles on a much larger scale than in the United States.
For parents who balk at this, Dr Schuchat said, “The reason that MMR is recommended at 12 months routinely is because babies are exquisitely vulnerable to measles and the complications from measles.”
The CDC does not recommend the vaccine for children younger than 6 months and urged physicians to remind parents to have their children vaccinated with the recommended two additional doses after their children reach 12 months. “Between 6 and 12 months, it will protect, but it won’t last that long.”
The CDC is also urging adults to be sure of their vaccination status. Adults who are unsure whether they are immune to the virus, either through vaccination or from having had the disease, should get vaccinated or at the least discuss it with their physician. “There is no harm in getting another MMR vaccine if you’ve already been vaccinated,” Dr. Schuchat said.
The majority of measles cases over the past few years, she said, have been in persons who were unvaccinated, primarily because of personal beliefs. Others, however, were unvaccinated because of a missed opportunity.
“People were at the doctor’s office and didn’t get their vaccine because they had an illness, but we recommend you get vaccinated when you are there,” she said.
Already in January 2015, the United States has seen more than the median number of measles cases typically recorded annually since the virus was eliminated as a native disease. In 2014, a record number of 644 cases from 27 states reported to CDC’s National Center for Immunization and Respiratory Diseases.
“This is a wake-up call to make sure that we keep measles from gaining a foothold in our country,” Dr. Schuchat said.
The recommended vaccination schedules for children and adults can be found on the CDC website.
On Twitter @whitneymcknight
*Updated on 1/30/15
Physicians should have a high degree of suspicion for measles when a patient presents with fever, rash, and other measles-related symptoms, CDC officials advised Jan. 29.
“I’m urging all health care professionals to think ‘measles’ when they’re evaluating patients with fever, rash, and other measles-related symptoms,” Dr. Anne Schuchat, the U.S. assistant surgeon general, and the director of the Centers for Disease Control and Prevention’s National Center for Immunization and Respiratory Diseases, said during a press conference. “Health care professionals need to know the guidelines for infection control and reporting measles cases, and they should work to ensure that patients are getting the best protection possible against measles, which is on-time MMR [measles, mumps, and rubella] vaccination, to protect them whether at home or abroad.”
Early symptoms include cough, runny nose and red, light-sensitive eyes. Two to four days later, a fine rash of red spots develops on the face and then gradually spreads down over the entire body. Fever, which can reach 103-105° F, comes with the rash. White spots, called Koplik spots, may appear on the inside of the cheeks.
Measles is more contagious than almost any other disease. The virus that causes measles lives in the nose and throat of infected people and is sprayed into the air when an infected person sneezes, coughs or talks, and can stay in the air for up to 2 hours. People with measles can spread the disease starting 4 days before the rash begins until 4 days after it appears.
At least 84 people across 14 states have been diagnosed recently with measles, including at least 67 who are thought to have been infected in mid- to late December while visiting Disneyland in Anaheim, Calif., Dr. Schuchat said. “We believe someone got infected [with measles] overseas, visited the Disneyland park, and spread the disease to others.” Those people went on to expose others in a variety of settings, including schools, day care centers, outpatient clinics, and airplanes, she added.
“This is not a problem of the measles vaccine not working, this is a problem of the measles vaccine not being used,” Dr. Schuchat said.
The CDC’s Advisory Committee on Immunization Practices recommends that children between 6 months and 12 months of age be vaccinated, particularly if they will be traveling, since many countries still experience measles on a much larger scale than in the United States.
For parents who balk at this, Dr Schuchat said, “The reason that MMR is recommended at 12 months routinely is because babies are exquisitely vulnerable to measles and the complications from measles.”
The CDC does not recommend the vaccine for children younger than 6 months and urged physicians to remind parents to have their children vaccinated with the recommended two additional doses after their children reach 12 months. “Between 6 and 12 months, it will protect, but it won’t last that long.”
The CDC is also urging adults to be sure of their vaccination status. Adults who are unsure whether they are immune to the virus, either through vaccination or from having had the disease, should get vaccinated or at the least discuss it with their physician. “There is no harm in getting another MMR vaccine if you’ve already been vaccinated,” Dr. Schuchat said.
The majority of measles cases over the past few years, she said, have been in persons who were unvaccinated, primarily because of personal beliefs. Others, however, were unvaccinated because of a missed opportunity.
“People were at the doctor’s office and didn’t get their vaccine because they had an illness, but we recommend you get vaccinated when you are there,” she said.
Already in January 2015, the United States has seen more than the median number of measles cases typically recorded annually since the virus was eliminated as a native disease. In 2014, a record number of 644 cases from 27 states reported to CDC’s National Center for Immunization and Respiratory Diseases.
“This is a wake-up call to make sure that we keep measles from gaining a foothold in our country,” Dr. Schuchat said.
The recommended vaccination schedules for children and adults can be found on the CDC website.
On Twitter @whitneymcknight
*Updated on 1/30/15
Physicians should have a high degree of suspicion for measles when a patient presents with fever, rash, and other measles-related symptoms, CDC officials advised Jan. 29.
“I’m urging all health care professionals to think ‘measles’ when they’re evaluating patients with fever, rash, and other measles-related symptoms,” Dr. Anne Schuchat, the U.S. assistant surgeon general, and the director of the Centers for Disease Control and Prevention’s National Center for Immunization and Respiratory Diseases, said during a press conference. “Health care professionals need to know the guidelines for infection control and reporting measles cases, and they should work to ensure that patients are getting the best protection possible against measles, which is on-time MMR [measles, mumps, and rubella] vaccination, to protect them whether at home or abroad.”
Early symptoms include cough, runny nose and red, light-sensitive eyes. Two to four days later, a fine rash of red spots develops on the face and then gradually spreads down over the entire body. Fever, which can reach 103-105° F, comes with the rash. White spots, called Koplik spots, may appear on the inside of the cheeks.
Measles is more contagious than almost any other disease. The virus that causes measles lives in the nose and throat of infected people and is sprayed into the air when an infected person sneezes, coughs or talks, and can stay in the air for up to 2 hours. People with measles can spread the disease starting 4 days before the rash begins until 4 days after it appears.
At least 84 people across 14 states have been diagnosed recently with measles, including at least 67 who are thought to have been infected in mid- to late December while visiting Disneyland in Anaheim, Calif., Dr. Schuchat said. “We believe someone got infected [with measles] overseas, visited the Disneyland park, and spread the disease to others.” Those people went on to expose others in a variety of settings, including schools, day care centers, outpatient clinics, and airplanes, she added.
“This is not a problem of the measles vaccine not working, this is a problem of the measles vaccine not being used,” Dr. Schuchat said.
The CDC’s Advisory Committee on Immunization Practices recommends that children between 6 months and 12 months of age be vaccinated, particularly if they will be traveling, since many countries still experience measles on a much larger scale than in the United States.
For parents who balk at this, Dr Schuchat said, “The reason that MMR is recommended at 12 months routinely is because babies are exquisitely vulnerable to measles and the complications from measles.”
The CDC does not recommend the vaccine for children younger than 6 months and urged physicians to remind parents to have their children vaccinated with the recommended two additional doses after their children reach 12 months. “Between 6 and 12 months, it will protect, but it won’t last that long.”
The CDC is also urging adults to be sure of their vaccination status. Adults who are unsure whether they are immune to the virus, either through vaccination or from having had the disease, should get vaccinated or at the least discuss it with their physician. “There is no harm in getting another MMR vaccine if you’ve already been vaccinated,” Dr. Schuchat said.
The majority of measles cases over the past few years, she said, have been in persons who were unvaccinated, primarily because of personal beliefs. Others, however, were unvaccinated because of a missed opportunity.
“People were at the doctor’s office and didn’t get their vaccine because they had an illness, but we recommend you get vaccinated when you are there,” she said.
Already in January 2015, the United States has seen more than the median number of measles cases typically recorded annually since the virus was eliminated as a native disease. In 2014, a record number of 644 cases from 27 states reported to CDC’s National Center for Immunization and Respiratory Diseases.
“This is a wake-up call to make sure that we keep measles from gaining a foothold in our country,” Dr. Schuchat said.
The recommended vaccination schedules for children and adults can be found on the CDC website.
On Twitter @whitneymcknight
*Updated on 1/30/15
FROM A CDC PRESS CONFERENCE
VHA Clarifies VISN Restructuring Plan
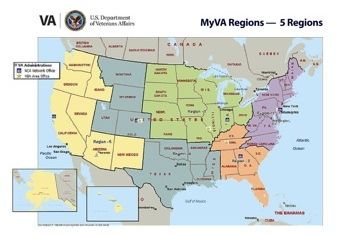
On January 26, 2015, Secretary of Veterans Affairs Robert A. McDonald revealed a plan to restructure the 21 VISNs to better align services for veterans. So what exactly will that entail? For starters, many VISN boundaries will need to be redrawn, but the VISNs are not going away any time soon. Instead, organizations and services within the VA will begin work to ensure their structures are aligned with the 5-region framework by the end of June 2015.
“The regions are going to play an increasingly important role for leadership in VHA and VA in general, but the VISNs will continue to exist,” Acting Director of VHA Health Systems Communications John Goodrich told Federal Practitioner.
Secretary McDonald’s announcement came almost 3 months after he shared preliminary plans to restructure the VA. The initiative, known as MyVA, seeks to ensure that veterans have a clear understanding of VA services and simplifies and streamlines the process of accessing those services. MyVA empowers employees with the authority, knowledge, and tools they need to solve veterans’ problems and take action. The regional structure also will help to ensure that products and services VA delivers to veterans are integrated within the organization.
Related: Maintaining the Public Trust
“We want every veteran to have a seamless, integrated, and responsive VA customer service experience every time. This regional alignment is the first step in empowering veterans to interact with one VA—MyVA,” said Secretary McDonald. “Ultimately, this reform will improve the veteran experience by enabling veterans to more easily navigate VA and access their earned care and benefits.”
Veterans Service Organizations were invited to attend the January 26 briefing. “Paralyzed Veterans of America will remain focused on being the lead voice for veterans with the greatest needs as we monitor the ongoing impact of these MyVA initiatives,” Paralyzed Veterans of America Executive Director Homer Townsend responded.
This isn’t the first time VA has reorganized. The VISN structure was implemented only in 1995 under then Under Secretary for Health Kenneth W. Kizer, MD, MPH. Dr. Kizer’s goal, as stated in his March 17, 1995, Vision for Change, was to “transform [VHA] to a more efficient and patient-centered health care system.” In 2002, VISNs 13 and 14 were joined to form VISN 23.
Related: 2015 Directory of VA and DoD Facilities
The 2015 restructuring is just 1 of 4 goals Secretary McDonald seeks to achieve through the MyVA initiative. Others include establishing a VA-wide customer service organization, working with partners to establish a national network of Community Veteran Advisory Councils, and identifying opportunities for VA to realign its internal business processes into a shared services model.
More information is expected in the coming weeks.
On January 26, 2015, Secretary of Veterans Affairs Robert A. McDonald revealed a plan to restructure the 21 VISNs to better align services for veterans. So what exactly will that entail? For starters, many VISN boundaries will need to be redrawn, but the VISNs are not going away any time soon. Instead, organizations and services within the VA will begin work to ensure their structures are aligned with the 5-region framework by the end of June 2015.
“The regions are going to play an increasingly important role for leadership in VHA and VA in general, but the VISNs will continue to exist,” Acting Director of VHA Health Systems Communications John Goodrich told Federal Practitioner.
Secretary McDonald’s announcement came almost 3 months after he shared preliminary plans to restructure the VA. The initiative, known as MyVA, seeks to ensure that veterans have a clear understanding of VA services and simplifies and streamlines the process of accessing those services. MyVA empowers employees with the authority, knowledge, and tools they need to solve veterans’ problems and take action. The regional structure also will help to ensure that products and services VA delivers to veterans are integrated within the organization.
Related: Maintaining the Public Trust
“We want every veteran to have a seamless, integrated, and responsive VA customer service experience every time. This regional alignment is the first step in empowering veterans to interact with one VA—MyVA,” said Secretary McDonald. “Ultimately, this reform will improve the veteran experience by enabling veterans to more easily navigate VA and access their earned care and benefits.”
Veterans Service Organizations were invited to attend the January 26 briefing. “Paralyzed Veterans of America will remain focused on being the lead voice for veterans with the greatest needs as we monitor the ongoing impact of these MyVA initiatives,” Paralyzed Veterans of America Executive Director Homer Townsend responded.
This isn’t the first time VA has reorganized. The VISN structure was implemented only in 1995 under then Under Secretary for Health Kenneth W. Kizer, MD, MPH. Dr. Kizer’s goal, as stated in his March 17, 1995, Vision for Change, was to “transform [VHA] to a more efficient and patient-centered health care system.” In 2002, VISNs 13 and 14 were joined to form VISN 23.
Related: 2015 Directory of VA and DoD Facilities
The 2015 restructuring is just 1 of 4 goals Secretary McDonald seeks to achieve through the MyVA initiative. Others include establishing a VA-wide customer service organization, working with partners to establish a national network of Community Veteran Advisory Councils, and identifying opportunities for VA to realign its internal business processes into a shared services model.
More information is expected in the coming weeks.
On January 26, 2015, Secretary of Veterans Affairs Robert A. McDonald revealed a plan to restructure the 21 VISNs to better align services for veterans. So what exactly will that entail? For starters, many VISN boundaries will need to be redrawn, but the VISNs are not going away any time soon. Instead, organizations and services within the VA will begin work to ensure their structures are aligned with the 5-region framework by the end of June 2015.
“The regions are going to play an increasingly important role for leadership in VHA and VA in general, but the VISNs will continue to exist,” Acting Director of VHA Health Systems Communications John Goodrich told Federal Practitioner.
Secretary McDonald’s announcement came almost 3 months after he shared preliminary plans to restructure the VA. The initiative, known as MyVA, seeks to ensure that veterans have a clear understanding of VA services and simplifies and streamlines the process of accessing those services. MyVA empowers employees with the authority, knowledge, and tools they need to solve veterans’ problems and take action. The regional structure also will help to ensure that products and services VA delivers to veterans are integrated within the organization.
Related: Maintaining the Public Trust
“We want every veteran to have a seamless, integrated, and responsive VA customer service experience every time. This regional alignment is the first step in empowering veterans to interact with one VA—MyVA,” said Secretary McDonald. “Ultimately, this reform will improve the veteran experience by enabling veterans to more easily navigate VA and access their earned care and benefits.”
Veterans Service Organizations were invited to attend the January 26 briefing. “Paralyzed Veterans of America will remain focused on being the lead voice for veterans with the greatest needs as we monitor the ongoing impact of these MyVA initiatives,” Paralyzed Veterans of America Executive Director Homer Townsend responded.
This isn’t the first time VA has reorganized. The VISN structure was implemented only in 1995 under then Under Secretary for Health Kenneth W. Kizer, MD, MPH. Dr. Kizer’s goal, as stated in his March 17, 1995, Vision for Change, was to “transform [VHA] to a more efficient and patient-centered health care system.” In 2002, VISNs 13 and 14 were joined to form VISN 23.
Related: 2015 Directory of VA and DoD Facilities
The 2015 restructuring is just 1 of 4 goals Secretary McDonald seeks to achieve through the MyVA initiative. Others include establishing a VA-wide customer service organization, working with partners to establish a national network of Community Veteran Advisory Councils, and identifying opportunities for VA to realign its internal business processes into a shared services model.
More information is expected in the coming weeks.
HHS Contracts for Quick and Easy Flu Tests
Being able to quickly and decisively distinguish viral influenza infection from bacterial infection could help reduce the risk of antibiotic overuse. That’s why the HHS Office of the Assistant Secretary for Preparedness and Response (ASPR) is awarding contracts to 2 companies to research and develop simple and inexpensive screening tests.
One company, Alere Inc. (Waltham, Massachusetts), is working on a low-cost molecular test. The iNAT Influenza A&B test could yield results within 15 minutes and show whether the patient has influenza A or B. The second company, InDevR Inc. (Boulder, Colorado), is developing a biochip test, FluChip-8G, to identify seasonal flu viruses and recognize novel flu viruses within 4 hours. Both tests use swabs from a patient’s nasal passage.
Related: Stopping the Spread of Germs
“Administering fast and inexpensive tests at the point of care has tangible benefits to personal and public health, particularly in helping doctors prescribe the right therapy immediately,” said the ASPR Biomedical Advanced Research and Development Authority (BARDA) director, Robin Robinson, PhD. “Prescribing medication or other therapies in a more targeted way is good stewardship and will be critical to reducing the risk of antimicrobial resistance.”
Better tests available in more settings can also alert health care workers and public health authorities to community outbreaks of respiratory illness and signal new viruses, according to HHS.
Related: Global Health Cooperation
The ASPR’s BARDA will oversee the development programs. BARDA is also supporting development of other diagnostic platforms, including a test to identify drug resistance in influenza, new vaccine technology, antiviral drugs, and low-cost, portable ventilators.
Being able to quickly and decisively distinguish viral influenza infection from bacterial infection could help reduce the risk of antibiotic overuse. That’s why the HHS Office of the Assistant Secretary for Preparedness and Response (ASPR) is awarding contracts to 2 companies to research and develop simple and inexpensive screening tests.
One company, Alere Inc. (Waltham, Massachusetts), is working on a low-cost molecular test. The iNAT Influenza A&B test could yield results within 15 minutes and show whether the patient has influenza A or B. The second company, InDevR Inc. (Boulder, Colorado), is developing a biochip test, FluChip-8G, to identify seasonal flu viruses and recognize novel flu viruses within 4 hours. Both tests use swabs from a patient’s nasal passage.
Related: Stopping the Spread of Germs
“Administering fast and inexpensive tests at the point of care has tangible benefits to personal and public health, particularly in helping doctors prescribe the right therapy immediately,” said the ASPR Biomedical Advanced Research and Development Authority (BARDA) director, Robin Robinson, PhD. “Prescribing medication or other therapies in a more targeted way is good stewardship and will be critical to reducing the risk of antimicrobial resistance.”
Better tests available in more settings can also alert health care workers and public health authorities to community outbreaks of respiratory illness and signal new viruses, according to HHS.
Related: Global Health Cooperation
The ASPR’s BARDA will oversee the development programs. BARDA is also supporting development of other diagnostic platforms, including a test to identify drug resistance in influenza, new vaccine technology, antiviral drugs, and low-cost, portable ventilators.
Being able to quickly and decisively distinguish viral influenza infection from bacterial infection could help reduce the risk of antibiotic overuse. That’s why the HHS Office of the Assistant Secretary for Preparedness and Response (ASPR) is awarding contracts to 2 companies to research and develop simple and inexpensive screening tests.
One company, Alere Inc. (Waltham, Massachusetts), is working on a low-cost molecular test. The iNAT Influenza A&B test could yield results within 15 minutes and show whether the patient has influenza A or B. The second company, InDevR Inc. (Boulder, Colorado), is developing a biochip test, FluChip-8G, to identify seasonal flu viruses and recognize novel flu viruses within 4 hours. Both tests use swabs from a patient’s nasal passage.
Related: Stopping the Spread of Germs
“Administering fast and inexpensive tests at the point of care has tangible benefits to personal and public health, particularly in helping doctors prescribe the right therapy immediately,” said the ASPR Biomedical Advanced Research and Development Authority (BARDA) director, Robin Robinson, PhD. “Prescribing medication or other therapies in a more targeted way is good stewardship and will be critical to reducing the risk of antimicrobial resistance.”
Better tests available in more settings can also alert health care workers and public health authorities to community outbreaks of respiratory illness and signal new viruses, according to HHS.
Related: Global Health Cooperation
The ASPR’s BARDA will oversee the development programs. BARDA is also supporting development of other diagnostic platforms, including a test to identify drug resistance in influenza, new vaccine technology, antiviral drugs, and low-cost, portable ventilators.
Second group B meningitis vaccine wins FDA nod of approval
A second serogroup B meningococcal vaccine has been approved by the Food and Drug Administration, based on an international study of over 2,500 adolescents and young adults, the agency announced on Jan. 23.
The vaccine is approved to prevent invasive meningococcal disease caused by Neisseria meningitidis serogroup B in people aged 10-25 years. The vaccine will be marketed as Bexsero, by Novartis Vaccines and Diagnostics, according to the FDA statement. The first serogroup B meningococcal vaccine was approved in October 2014; that vaccine is Trumenba, manufactured by Wyeth Pharmaceuticals. “The approval of these vaccines represents a major public health accomplishment toward preventing this life-threatening disease,” Dr. Karen Midthun, director of the FDA’s Center for Biologics Evaluation and Research, said in the statement.
Before the two vaccines were approved, commercially available FDA-approved meningococcal vaccines covered four of the five main serogroups of N. meningitidis bacteria that cause meningococcal disease (A, C, Y, and W). In 2012, about 160 of the approximately 500 cases of meningococcal disease reported in the United States were caused by serogroup B, according to Centers for Disease Control and Prevention data cited in the FDA statement.
Approval of Bexsero was based on three studies of about 2,600 adolescents and young adults in Canada, Australia, Chile, and the United Kingdom. After two doses of the vaccine, 62%-88% of recipients “had antibodies in their blood that killed three different N. meningitidis serogroup B strains in tests carried out in a laboratory,” compared with 0-23% before they were vaccinated, according to the FDA statement. The most common adverse effects associated with the vaccine include pain and swelling at the injection site, headache, diarrhea, muscle pain, joint pain, fatigue, and chills. Safety information is from an evaluation of 5,000 people who received the vaccine in the United States and other countries, and in over 15,000 people who received the vaccine during two outbreaks of meningococcal disease at universities in the United States.
Almost 30,000 doses of the vaccine were provided to students and staff members during outbreaks of meningitis B at Princeton (New Jersey) University and the University of California, Santa Barbara, according to Novartis.
Because this was an accelerated approval, Novartis will conduct studies to determine if the vaccine is effective against other strains of N. meningitidis serogroup B. Bexsero was approved in people aged 2 months and older in Europe in January 2013.
More information on serogroup B meningococcal vaccine and the outbreaks is available on the CDC website at http://www.cdc.gov/meningococcal/outbreaks/vaccine-serogroupB.html
A second serogroup B meningococcal vaccine has been approved by the Food and Drug Administration, based on an international study of over 2,500 adolescents and young adults, the agency announced on Jan. 23.
The vaccine is approved to prevent invasive meningococcal disease caused by Neisseria meningitidis serogroup B in people aged 10-25 years. The vaccine will be marketed as Bexsero, by Novartis Vaccines and Diagnostics, according to the FDA statement. The first serogroup B meningococcal vaccine was approved in October 2014; that vaccine is Trumenba, manufactured by Wyeth Pharmaceuticals. “The approval of these vaccines represents a major public health accomplishment toward preventing this life-threatening disease,” Dr. Karen Midthun, director of the FDA’s Center for Biologics Evaluation and Research, said in the statement.
Before the two vaccines were approved, commercially available FDA-approved meningococcal vaccines covered four of the five main serogroups of N. meningitidis bacteria that cause meningococcal disease (A, C, Y, and W). In 2012, about 160 of the approximately 500 cases of meningococcal disease reported in the United States were caused by serogroup B, according to Centers for Disease Control and Prevention data cited in the FDA statement.
Approval of Bexsero was based on three studies of about 2,600 adolescents and young adults in Canada, Australia, Chile, and the United Kingdom. After two doses of the vaccine, 62%-88% of recipients “had antibodies in their blood that killed three different N. meningitidis serogroup B strains in tests carried out in a laboratory,” compared with 0-23% before they were vaccinated, according to the FDA statement. The most common adverse effects associated with the vaccine include pain and swelling at the injection site, headache, diarrhea, muscle pain, joint pain, fatigue, and chills. Safety information is from an evaluation of 5,000 people who received the vaccine in the United States and other countries, and in over 15,000 people who received the vaccine during two outbreaks of meningococcal disease at universities in the United States.
Almost 30,000 doses of the vaccine were provided to students and staff members during outbreaks of meningitis B at Princeton (New Jersey) University and the University of California, Santa Barbara, according to Novartis.
Because this was an accelerated approval, Novartis will conduct studies to determine if the vaccine is effective against other strains of N. meningitidis serogroup B. Bexsero was approved in people aged 2 months and older in Europe in January 2013.
More information on serogroup B meningococcal vaccine and the outbreaks is available on the CDC website at http://www.cdc.gov/meningococcal/outbreaks/vaccine-serogroupB.html
A second serogroup B meningococcal vaccine has been approved by the Food and Drug Administration, based on an international study of over 2,500 adolescents and young adults, the agency announced on Jan. 23.
The vaccine is approved to prevent invasive meningococcal disease caused by Neisseria meningitidis serogroup B in people aged 10-25 years. The vaccine will be marketed as Bexsero, by Novartis Vaccines and Diagnostics, according to the FDA statement. The first serogroup B meningococcal vaccine was approved in October 2014; that vaccine is Trumenba, manufactured by Wyeth Pharmaceuticals. “The approval of these vaccines represents a major public health accomplishment toward preventing this life-threatening disease,” Dr. Karen Midthun, director of the FDA’s Center for Biologics Evaluation and Research, said in the statement.
Before the two vaccines were approved, commercially available FDA-approved meningococcal vaccines covered four of the five main serogroups of N. meningitidis bacteria that cause meningococcal disease (A, C, Y, and W). In 2012, about 160 of the approximately 500 cases of meningococcal disease reported in the United States were caused by serogroup B, according to Centers for Disease Control and Prevention data cited in the FDA statement.
Approval of Bexsero was based on three studies of about 2,600 adolescents and young adults in Canada, Australia, Chile, and the United Kingdom. After two doses of the vaccine, 62%-88% of recipients “had antibodies in their blood that killed three different N. meningitidis serogroup B strains in tests carried out in a laboratory,” compared with 0-23% before they were vaccinated, according to the FDA statement. The most common adverse effects associated with the vaccine include pain and swelling at the injection site, headache, diarrhea, muscle pain, joint pain, fatigue, and chills. Safety information is from an evaluation of 5,000 people who received the vaccine in the United States and other countries, and in over 15,000 people who received the vaccine during two outbreaks of meningococcal disease at universities in the United States.
Almost 30,000 doses of the vaccine were provided to students and staff members during outbreaks of meningitis B at Princeton (New Jersey) University and the University of California, Santa Barbara, according to Novartis.
Because this was an accelerated approval, Novartis will conduct studies to determine if the vaccine is effective against other strains of N. meningitidis serogroup B. Bexsero was approved in people aged 2 months and older in Europe in January 2013.
More information on serogroup B meningococcal vaccine and the outbreaks is available on the CDC website at http://www.cdc.gov/meningococcal/outbreaks/vaccine-serogroupB.html
FROM THE FDA
FDA panel backs antifungal for invasive aspergillosis, mucormycosis
SILVER SPRING, MD. – A novel treatment for invasive aspergillosis and invasive mucormycosis gained the support of a Food and Drug Administration advisory panel, although members were more ambivalent about the mucormycosis indication, based on the small amount of data available in those patients.
At a meeting on Jan. 22, the FDA’s Anti-Infective Drugs Advisory Committee voted 11-0 that there was “substantial evidence” that isavuconazonium, an antifungal prodrug, was safe and effective for the treatment of invasive aspergillosis, a life-threatening condition most commonly seen in immunocompromised patients. The drug was studied in a phase III study comparing isavuconazonium to voriconazole, the standard of care, in more than 500 patients.
The panel voted 8-2, with one abstention, that there was substantial evidence it was safe and effective for treating patients with invasive mucormycosis, with panelists voting on both sides of the question citing the study of only 37 patients that used historical controls as an issue. Those supporting approval for mucormycosis said that, if approved, the manufacturer, Astellas, should be required to conduct a phase IV trial further evaluating this treatment for patients with this infection.
Isavuconazonium is a prodrug of isavuconazole, a triazole antifungal, and would be available in an oral capsule formulation and a powder formulation that is reconstituted for intravenous administration (and needs to be administered through an in-line filter in case of particulate formation).
For aspergillosis, “I do believe that this drug provides a reasonable alternative to the current therapies that are available without additional toxicities,” said panelist Dr. Paige Waterman of the Global Emerging Infections Surveillance and Response System at the Walter Reed Army Medical Center, Silver Spring, Md. Other panelists agreed with her recommendations that labeling should make clear that it should not be used in people under age 18 years or in pregnant women, that a filter should be used when administered intravenously, and that labeling should include a statement about the increased risk of hepatotoxicity that also appears in the labeling of other drugs in the same class.
Because it has been associated with a shortened QT interval, she said that screening ECGs should be recommended for patients and that there should be some extra caution when it is prescribed to people who are Asian or of Asian descent, since drug concentrations were slightly higher among Asian patients who received the drug. Other recommendations from panelists included the need to conduct studies of the drug to provide information on therapeutic drug monitoring and in people under 18 years.
For treatment of invasive mucormycosis, an even rarer fungal infection which, in hospitals, is associated with the use of contaminated materials or organ transplantation, the panelists were more hesitant, but those voting in favor of approval cited the significance of the condition and the reasonable efficacy results in the study, stressing that postmarketing studies were critical. Panelists also noted that more clinical data are clearly needed in patients with this infection and the lack of data directly comparing it to amphotericin B – the only FDA-approved drug for this indication – was problematic.
“This drug really does fill an unmet need; I have high hopes that it is at least as good as amphotericin. But I do think we need more data to confirm that,” said Dr. Michael Neely, chair of antimicrobial stewardship at Children’s Hospital, Los Angeles, who was among those voting in favor of this indication. It appears to have a better safety profile and it would be available in both oral and intravenous formulations, he added. Amphotericin B is available only in an intravenous (IV) formulation.
If approved, isavuconazonium would provide an alternative to voriconazole for treating aspergillosis, and the IV formulation does not contain cyclodextrin, unlike the IV formulation of voriconazole, which limits its use in patients with moderate to severe renal dysfunction, according to Astellas. Safety concerns specific to isavuconazonium include QT-segment shortening and particulate formation in the IV formulation, according to the FDA.
The randomized, double-blind, international, noninferiority study compared treatment with isavuconazonium to voriconazole in 516 adults with invasive aspergillosis. In the randomized study, the mean age of patients was 51 years, 60% were men, most were white, 11% were in the United States and Canada, 20% had had an allogeneic bone marrow transplant, and 70% had an uncontrolled malignancy at baseline (Infect. Drug Resist. 2013;6:16374). The primary effectiveness endpoint, all-cause mortality through day 42, was 19% in those on isavuconazonium, compared with 20% in those on voriconazole. The study met the prespecified noninferiority margin. The rates of deaths and serious adverse events were similar to voriconazole, and there were fewer adverse events in those on isavuconazonium requiring discontinuation of the drug (14% vs. 23%).
Differences in adverse events included lower rates of hepatobiliary disorders, including hyperbilirubinemia, abnormal hepatic function, and jaundice (9% vs. 16%); skin and subcutaneous tissue disorders, including rash, erythema, and drug eruption (34% vs 43%) associated with isavuconazonium, compared with voriconazole. Common adverse events included nausea (28%), vomiting (25%), and diarrhea (24%). Decreases in the QT segment occurred in 7.5% of those on isavuconazonium, compared with 4.5% of those on voriconazole, but were not associated with clinical events.
The prospective, open-label, single-arm study evaluated isavuconazonium in 37 patients with proven or probable mucormycosis infections, whose mean age was 49 years; 59% had a hematologic malignancy, and about 40% were neutropenic at baseline. All-cause mortality at day 42 was almost 38%, which was similar to the mortality rate for amphotericin in the literature, according to Astellas.
There are about 12,000 cases of aspergillosis and about 500 cases of mucormycosis in the United States every year, the company said. In addition to voriconazole, other drugs approved for invasive aspergillosis include amphotericin formulations, itraconazole, and caspofungin. The FDA usually follows the recommendations of its advisory panels. The FDA is expected to make a decision by March 8, according to Astellas, which plans to market the drug as Cresemba if approved. It is also under review in Europe for the same indications.
Panelists were cleared of potential conflicts of interest related to the topic of the meeting. In some cases, a panelist may be given a waiver but not at this meeting.
Dr. Daniel Ouellette, FCCP, comments: Serious fungal infections are being increasingly recognized in patients who are immunocompromised or critically ill, but effective treatments with an acceptable side-effect profile have been sparse. Just a few years ago, the mainstay of treatment was amphotericin, an agent whose use has been associated with a variety of complications. However, novel agents have been developed that hold promise. The recent development of isavuconazonium, a pro-drug of isavuconazole, may lead to further augmentation of the clinician's armamentarium in fighting these illnesses.
Dr. Daniel Ouellette, FCCP, comments: Serious fungal infections are being increasingly recognized in patients who are immunocompromised or critically ill, but effective treatments with an acceptable side-effect profile have been sparse. Just a few years ago, the mainstay of treatment was amphotericin, an agent whose use has been associated with a variety of complications. However, novel agents have been developed that hold promise. The recent development of isavuconazonium, a pro-drug of isavuconazole, may lead to further augmentation of the clinician's armamentarium in fighting these illnesses.
Dr. Daniel Ouellette, FCCP, comments: Serious fungal infections are being increasingly recognized in patients who are immunocompromised or critically ill, but effective treatments with an acceptable side-effect profile have been sparse. Just a few years ago, the mainstay of treatment was amphotericin, an agent whose use has been associated with a variety of complications. However, novel agents have been developed that hold promise. The recent development of isavuconazonium, a pro-drug of isavuconazole, may lead to further augmentation of the clinician's armamentarium in fighting these illnesses.
SILVER SPRING, MD. – A novel treatment for invasive aspergillosis and invasive mucormycosis gained the support of a Food and Drug Administration advisory panel, although members were more ambivalent about the mucormycosis indication, based on the small amount of data available in those patients.
At a meeting on Jan. 22, the FDA’s Anti-Infective Drugs Advisory Committee voted 11-0 that there was “substantial evidence” that isavuconazonium, an antifungal prodrug, was safe and effective for the treatment of invasive aspergillosis, a life-threatening condition most commonly seen in immunocompromised patients. The drug was studied in a phase III study comparing isavuconazonium to voriconazole, the standard of care, in more than 500 patients.
The panel voted 8-2, with one abstention, that there was substantial evidence it was safe and effective for treating patients with invasive mucormycosis, with panelists voting on both sides of the question citing the study of only 37 patients that used historical controls as an issue. Those supporting approval for mucormycosis said that, if approved, the manufacturer, Astellas, should be required to conduct a phase IV trial further evaluating this treatment for patients with this infection.
Isavuconazonium is a prodrug of isavuconazole, a triazole antifungal, and would be available in an oral capsule formulation and a powder formulation that is reconstituted for intravenous administration (and needs to be administered through an in-line filter in case of particulate formation).
For aspergillosis, “I do believe that this drug provides a reasonable alternative to the current therapies that are available without additional toxicities,” said panelist Dr. Paige Waterman of the Global Emerging Infections Surveillance and Response System at the Walter Reed Army Medical Center, Silver Spring, Md. Other panelists agreed with her recommendations that labeling should make clear that it should not be used in people under age 18 years or in pregnant women, that a filter should be used when administered intravenously, and that labeling should include a statement about the increased risk of hepatotoxicity that also appears in the labeling of other drugs in the same class.
Because it has been associated with a shortened QT interval, she said that screening ECGs should be recommended for patients and that there should be some extra caution when it is prescribed to people who are Asian or of Asian descent, since drug concentrations were slightly higher among Asian patients who received the drug. Other recommendations from panelists included the need to conduct studies of the drug to provide information on therapeutic drug monitoring and in people under 18 years.
For treatment of invasive mucormycosis, an even rarer fungal infection which, in hospitals, is associated with the use of contaminated materials or organ transplantation, the panelists were more hesitant, but those voting in favor of approval cited the significance of the condition and the reasonable efficacy results in the study, stressing that postmarketing studies were critical. Panelists also noted that more clinical data are clearly needed in patients with this infection and the lack of data directly comparing it to amphotericin B – the only FDA-approved drug for this indication – was problematic.
“This drug really does fill an unmet need; I have high hopes that it is at least as good as amphotericin. But I do think we need more data to confirm that,” said Dr. Michael Neely, chair of antimicrobial stewardship at Children’s Hospital, Los Angeles, who was among those voting in favor of this indication. It appears to have a better safety profile and it would be available in both oral and intravenous formulations, he added. Amphotericin B is available only in an intravenous (IV) formulation.
If approved, isavuconazonium would provide an alternative to voriconazole for treating aspergillosis, and the IV formulation does not contain cyclodextrin, unlike the IV formulation of voriconazole, which limits its use in patients with moderate to severe renal dysfunction, according to Astellas. Safety concerns specific to isavuconazonium include QT-segment shortening and particulate formation in the IV formulation, according to the FDA.
The randomized, double-blind, international, noninferiority study compared treatment with isavuconazonium to voriconazole in 516 adults with invasive aspergillosis. In the randomized study, the mean age of patients was 51 years, 60% were men, most were white, 11% were in the United States and Canada, 20% had had an allogeneic bone marrow transplant, and 70% had an uncontrolled malignancy at baseline (Infect. Drug Resist. 2013;6:16374). The primary effectiveness endpoint, all-cause mortality through day 42, was 19% in those on isavuconazonium, compared with 20% in those on voriconazole. The study met the prespecified noninferiority margin. The rates of deaths and serious adverse events were similar to voriconazole, and there were fewer adverse events in those on isavuconazonium requiring discontinuation of the drug (14% vs. 23%).
Differences in adverse events included lower rates of hepatobiliary disorders, including hyperbilirubinemia, abnormal hepatic function, and jaundice (9% vs. 16%); skin and subcutaneous tissue disorders, including rash, erythema, and drug eruption (34% vs 43%) associated with isavuconazonium, compared with voriconazole. Common adverse events included nausea (28%), vomiting (25%), and diarrhea (24%). Decreases in the QT segment occurred in 7.5% of those on isavuconazonium, compared with 4.5% of those on voriconazole, but were not associated with clinical events.
The prospective, open-label, single-arm study evaluated isavuconazonium in 37 patients with proven or probable mucormycosis infections, whose mean age was 49 years; 59% had a hematologic malignancy, and about 40% were neutropenic at baseline. All-cause mortality at day 42 was almost 38%, which was similar to the mortality rate for amphotericin in the literature, according to Astellas.
There are about 12,000 cases of aspergillosis and about 500 cases of mucormycosis in the United States every year, the company said. In addition to voriconazole, other drugs approved for invasive aspergillosis include amphotericin formulations, itraconazole, and caspofungin. The FDA usually follows the recommendations of its advisory panels. The FDA is expected to make a decision by March 8, according to Astellas, which plans to market the drug as Cresemba if approved. It is also under review in Europe for the same indications.
Panelists were cleared of potential conflicts of interest related to the topic of the meeting. In some cases, a panelist may be given a waiver but not at this meeting.
SILVER SPRING, MD. – A novel treatment for invasive aspergillosis and invasive mucormycosis gained the support of a Food and Drug Administration advisory panel, although members were more ambivalent about the mucormycosis indication, based on the small amount of data available in those patients.
At a meeting on Jan. 22, the FDA’s Anti-Infective Drugs Advisory Committee voted 11-0 that there was “substantial evidence” that isavuconazonium, an antifungal prodrug, was safe and effective for the treatment of invasive aspergillosis, a life-threatening condition most commonly seen in immunocompromised patients. The drug was studied in a phase III study comparing isavuconazonium to voriconazole, the standard of care, in more than 500 patients.
The panel voted 8-2, with one abstention, that there was substantial evidence it was safe and effective for treating patients with invasive mucormycosis, with panelists voting on both sides of the question citing the study of only 37 patients that used historical controls as an issue. Those supporting approval for mucormycosis said that, if approved, the manufacturer, Astellas, should be required to conduct a phase IV trial further evaluating this treatment for patients with this infection.
Isavuconazonium is a prodrug of isavuconazole, a triazole antifungal, and would be available in an oral capsule formulation and a powder formulation that is reconstituted for intravenous administration (and needs to be administered through an in-line filter in case of particulate formation).
For aspergillosis, “I do believe that this drug provides a reasonable alternative to the current therapies that are available without additional toxicities,” said panelist Dr. Paige Waterman of the Global Emerging Infections Surveillance and Response System at the Walter Reed Army Medical Center, Silver Spring, Md. Other panelists agreed with her recommendations that labeling should make clear that it should not be used in people under age 18 years or in pregnant women, that a filter should be used when administered intravenously, and that labeling should include a statement about the increased risk of hepatotoxicity that also appears in the labeling of other drugs in the same class.
Because it has been associated with a shortened QT interval, she said that screening ECGs should be recommended for patients and that there should be some extra caution when it is prescribed to people who are Asian or of Asian descent, since drug concentrations were slightly higher among Asian patients who received the drug. Other recommendations from panelists included the need to conduct studies of the drug to provide information on therapeutic drug monitoring and in people under 18 years.
For treatment of invasive mucormycosis, an even rarer fungal infection which, in hospitals, is associated with the use of contaminated materials or organ transplantation, the panelists were more hesitant, but those voting in favor of approval cited the significance of the condition and the reasonable efficacy results in the study, stressing that postmarketing studies were critical. Panelists also noted that more clinical data are clearly needed in patients with this infection and the lack of data directly comparing it to amphotericin B – the only FDA-approved drug for this indication – was problematic.
“This drug really does fill an unmet need; I have high hopes that it is at least as good as amphotericin. But I do think we need more data to confirm that,” said Dr. Michael Neely, chair of antimicrobial stewardship at Children’s Hospital, Los Angeles, who was among those voting in favor of this indication. It appears to have a better safety profile and it would be available in both oral and intravenous formulations, he added. Amphotericin B is available only in an intravenous (IV) formulation.
If approved, isavuconazonium would provide an alternative to voriconazole for treating aspergillosis, and the IV formulation does not contain cyclodextrin, unlike the IV formulation of voriconazole, which limits its use in patients with moderate to severe renal dysfunction, according to Astellas. Safety concerns specific to isavuconazonium include QT-segment shortening and particulate formation in the IV formulation, according to the FDA.
The randomized, double-blind, international, noninferiority study compared treatment with isavuconazonium to voriconazole in 516 adults with invasive aspergillosis. In the randomized study, the mean age of patients was 51 years, 60% were men, most were white, 11% were in the United States and Canada, 20% had had an allogeneic bone marrow transplant, and 70% had an uncontrolled malignancy at baseline (Infect. Drug Resist. 2013;6:16374). The primary effectiveness endpoint, all-cause mortality through day 42, was 19% in those on isavuconazonium, compared with 20% in those on voriconazole. The study met the prespecified noninferiority margin. The rates of deaths and serious adverse events were similar to voriconazole, and there were fewer adverse events in those on isavuconazonium requiring discontinuation of the drug (14% vs. 23%).
Differences in adverse events included lower rates of hepatobiliary disorders, including hyperbilirubinemia, abnormal hepatic function, and jaundice (9% vs. 16%); skin and subcutaneous tissue disorders, including rash, erythema, and drug eruption (34% vs 43%) associated with isavuconazonium, compared with voriconazole. Common adverse events included nausea (28%), vomiting (25%), and diarrhea (24%). Decreases in the QT segment occurred in 7.5% of those on isavuconazonium, compared with 4.5% of those on voriconazole, but were not associated with clinical events.
The prospective, open-label, single-arm study evaluated isavuconazonium in 37 patients with proven or probable mucormycosis infections, whose mean age was 49 years; 59% had a hematologic malignancy, and about 40% were neutropenic at baseline. All-cause mortality at day 42 was almost 38%, which was similar to the mortality rate for amphotericin in the literature, according to Astellas.
There are about 12,000 cases of aspergillosis and about 500 cases of mucormycosis in the United States every year, the company said. In addition to voriconazole, other drugs approved for invasive aspergillosis include amphotericin formulations, itraconazole, and caspofungin. The FDA usually follows the recommendations of its advisory panels. The FDA is expected to make a decision by March 8, according to Astellas, which plans to market the drug as Cresemba if approved. It is also under review in Europe for the same indications.
Panelists were cleared of potential conflicts of interest related to the topic of the meeting. In some cases, a panelist may be given a waiver but not at this meeting.
AT AN FDA ADVISORY COMMITTEE MEETING
Congress to doctors: How can we pay for SGR fix?
WASHINGTON – Legislators on the Health Subcommittee looked to physicians and health care providers for ways to pay for a repeal of the Medicare Sustainable Growth Rate formula at a hearing Jan. 22.
The subcommittee is looking “at opportunities for pay-fors” to fund the already-agreed-upon bipartisan legislation to permanently repeal and replace the SGR, according to Rep. Joseph Pitts (R-Pa.), Health Subcommittee chairman. The panel’s ranking member, Rep. Gene Green, (D-Tex.), noted that he didn’t mind looking outside of health care to cover the estimated $140 billion cost of repeal.
Dr. Barbara L. McAneny, chair of the American Medical Association Board of Trustees, said that her organization needed more guidance from the subcommittee before leaders could recommend specific offsets.
The issue “is a very difficult one because, within the health care sector, so many people are struggling now just to keep their doors open to their patients, that for us from within the health care sector to really come up with a specific pay-for may not be as useful until there are some guidelines set up by Congress,” Dr. McAneny testified. “What are the rules of this particular budgetary process? How do we fit those things within that? I think the AMA stands ready to assist and help by weighing in on any given suggestions, but I think we are very uneasy and feel that we don’t really have the ability to give you specific pay-fors.”
Her testimony drew sharp criticism from Rep. Larry Bucshon, (R-Ind.).
“I would just implore you to really reconsider that and the AMA reconsider and maybe help us rather than waiting for other options and coming out and saying up or down, we disagree or we agree,” Rep. Bucshon said. “If you are going to offer an opinion at the end, then you should be part of the offering solutions on the front side. … If you are just going to wait and be a critic and not offer solutions yourself, to me that’s not very helpful.”
Others testifying before the subcommittee noted that despite the committee being open to all avenues to finance the SGR bill, Medicare would likely bear some of those costs.
“The [American Hospital Association] cannot support any proposal to fix the physician payment problem at the expense of funding for services provided by other caregivers,” AHA President and CEO Richard Umbdenstock testified, adding that the organization “cannot simply oppose payment cuts without supporting other solutions.”
Mr. Umbdenstock highlighted four solutions that the AHA supports: combining Medicare Part A and Part B with a unified deductible and coinsurance; higher premiums for beneficiaries coming into Medicare as well as means-testing for premiums; altering incentives to first-dollar coverage for Medigap so that beneficiaries will be more aware of how they are choosing the health care they need; and medical liability reform.
Mr. Umbdenstock added that these suggestions have general bipartisan support and “would not only generate savings, but also put the Medicare program on firmer financial footing for years to come.”
Eric Schneidewind, president-elect of AARP, also offered a number of solutions to the committee and suggested that maybe Congress does not need to fully fund the SGR bill.
“In light of current and future savings in the Medicare program, Congress would be justified in not fully offsetting the cost of a permanent repeal at this time,” Mr. Schneidewind said. He also added that legislators could consider expansion of competitive bidding for durable medical equipment, equalize payments based on physician site of services, be more aggressive in collecting overpayments to Medicare Advantage plans, increasing transitional care and chronic care management, and encourage the use of all highly skilled clinicians.
But what likely would be considered more controversial were Mr. Schneidewind’s suggestions related to drugs.
“AARP believes that any discussion of budget offsets for Medicare reimbursement reform should include savings from prescription drugs,” he said. “We urge you to give strong consideration to the following prescription drug proposals that could save at least $150 billion.”
Those proposals included offering Medicaid-level drug rebates to beneficiaries who are eligible for both Medicare and Medicaid, giving the secretary of Health and Human Services the power to negotiate drug prices, reduce the exclusivity period for biologics, prohibit pay-for-delay agreements (when a brand-name drug manufacturer pays to delay the launch of a generic equivalent), and prohibit the use of Risk Evaluation and Mitigation Strategies (REMS) to block generic and biosimilar drug development.
WASHINGTON – Legislators on the Health Subcommittee looked to physicians and health care providers for ways to pay for a repeal of the Medicare Sustainable Growth Rate formula at a hearing Jan. 22.
The subcommittee is looking “at opportunities for pay-fors” to fund the already-agreed-upon bipartisan legislation to permanently repeal and replace the SGR, according to Rep. Joseph Pitts (R-Pa.), Health Subcommittee chairman. The panel’s ranking member, Rep. Gene Green, (D-Tex.), noted that he didn’t mind looking outside of health care to cover the estimated $140 billion cost of repeal.
Dr. Barbara L. McAneny, chair of the American Medical Association Board of Trustees, said that her organization needed more guidance from the subcommittee before leaders could recommend specific offsets.
The issue “is a very difficult one because, within the health care sector, so many people are struggling now just to keep their doors open to their patients, that for us from within the health care sector to really come up with a specific pay-for may not be as useful until there are some guidelines set up by Congress,” Dr. McAneny testified. “What are the rules of this particular budgetary process? How do we fit those things within that? I think the AMA stands ready to assist and help by weighing in on any given suggestions, but I think we are very uneasy and feel that we don’t really have the ability to give you specific pay-fors.”
Her testimony drew sharp criticism from Rep. Larry Bucshon, (R-Ind.).
“I would just implore you to really reconsider that and the AMA reconsider and maybe help us rather than waiting for other options and coming out and saying up or down, we disagree or we agree,” Rep. Bucshon said. “If you are going to offer an opinion at the end, then you should be part of the offering solutions on the front side. … If you are just going to wait and be a critic and not offer solutions yourself, to me that’s not very helpful.”
Others testifying before the subcommittee noted that despite the committee being open to all avenues to finance the SGR bill, Medicare would likely bear some of those costs.
“The [American Hospital Association] cannot support any proposal to fix the physician payment problem at the expense of funding for services provided by other caregivers,” AHA President and CEO Richard Umbdenstock testified, adding that the organization “cannot simply oppose payment cuts without supporting other solutions.”
Mr. Umbdenstock highlighted four solutions that the AHA supports: combining Medicare Part A and Part B with a unified deductible and coinsurance; higher premiums for beneficiaries coming into Medicare as well as means-testing for premiums; altering incentives to first-dollar coverage for Medigap so that beneficiaries will be more aware of how they are choosing the health care they need; and medical liability reform.
Mr. Umbdenstock added that these suggestions have general bipartisan support and “would not only generate savings, but also put the Medicare program on firmer financial footing for years to come.”
Eric Schneidewind, president-elect of AARP, also offered a number of solutions to the committee and suggested that maybe Congress does not need to fully fund the SGR bill.
“In light of current and future savings in the Medicare program, Congress would be justified in not fully offsetting the cost of a permanent repeal at this time,” Mr. Schneidewind said. He also added that legislators could consider expansion of competitive bidding for durable medical equipment, equalize payments based on physician site of services, be more aggressive in collecting overpayments to Medicare Advantage plans, increasing transitional care and chronic care management, and encourage the use of all highly skilled clinicians.
But what likely would be considered more controversial were Mr. Schneidewind’s suggestions related to drugs.
“AARP believes that any discussion of budget offsets for Medicare reimbursement reform should include savings from prescription drugs,” he said. “We urge you to give strong consideration to the following prescription drug proposals that could save at least $150 billion.”
Those proposals included offering Medicaid-level drug rebates to beneficiaries who are eligible for both Medicare and Medicaid, giving the secretary of Health and Human Services the power to negotiate drug prices, reduce the exclusivity period for biologics, prohibit pay-for-delay agreements (when a brand-name drug manufacturer pays to delay the launch of a generic equivalent), and prohibit the use of Risk Evaluation and Mitigation Strategies (REMS) to block generic and biosimilar drug development.
WASHINGTON – Legislators on the Health Subcommittee looked to physicians and health care providers for ways to pay for a repeal of the Medicare Sustainable Growth Rate formula at a hearing Jan. 22.
The subcommittee is looking “at opportunities for pay-fors” to fund the already-agreed-upon bipartisan legislation to permanently repeal and replace the SGR, according to Rep. Joseph Pitts (R-Pa.), Health Subcommittee chairman. The panel’s ranking member, Rep. Gene Green, (D-Tex.), noted that he didn’t mind looking outside of health care to cover the estimated $140 billion cost of repeal.
Dr. Barbara L. McAneny, chair of the American Medical Association Board of Trustees, said that her organization needed more guidance from the subcommittee before leaders could recommend specific offsets.
The issue “is a very difficult one because, within the health care sector, so many people are struggling now just to keep their doors open to their patients, that for us from within the health care sector to really come up with a specific pay-for may not be as useful until there are some guidelines set up by Congress,” Dr. McAneny testified. “What are the rules of this particular budgetary process? How do we fit those things within that? I think the AMA stands ready to assist and help by weighing in on any given suggestions, but I think we are very uneasy and feel that we don’t really have the ability to give you specific pay-fors.”
Her testimony drew sharp criticism from Rep. Larry Bucshon, (R-Ind.).
“I would just implore you to really reconsider that and the AMA reconsider and maybe help us rather than waiting for other options and coming out and saying up or down, we disagree or we agree,” Rep. Bucshon said. “If you are going to offer an opinion at the end, then you should be part of the offering solutions on the front side. … If you are just going to wait and be a critic and not offer solutions yourself, to me that’s not very helpful.”
Others testifying before the subcommittee noted that despite the committee being open to all avenues to finance the SGR bill, Medicare would likely bear some of those costs.
“The [American Hospital Association] cannot support any proposal to fix the physician payment problem at the expense of funding for services provided by other caregivers,” AHA President and CEO Richard Umbdenstock testified, adding that the organization “cannot simply oppose payment cuts without supporting other solutions.”
Mr. Umbdenstock highlighted four solutions that the AHA supports: combining Medicare Part A and Part B with a unified deductible and coinsurance; higher premiums for beneficiaries coming into Medicare as well as means-testing for premiums; altering incentives to first-dollar coverage for Medigap so that beneficiaries will be more aware of how they are choosing the health care they need; and medical liability reform.
Mr. Umbdenstock added that these suggestions have general bipartisan support and “would not only generate savings, but also put the Medicare program on firmer financial footing for years to come.”
Eric Schneidewind, president-elect of AARP, also offered a number of solutions to the committee and suggested that maybe Congress does not need to fully fund the SGR bill.
“In light of current and future savings in the Medicare program, Congress would be justified in not fully offsetting the cost of a permanent repeal at this time,” Mr. Schneidewind said. He also added that legislators could consider expansion of competitive bidding for durable medical equipment, equalize payments based on physician site of services, be more aggressive in collecting overpayments to Medicare Advantage plans, increasing transitional care and chronic care management, and encourage the use of all highly skilled clinicians.
But what likely would be considered more controversial were Mr. Schneidewind’s suggestions related to drugs.
“AARP believes that any discussion of budget offsets for Medicare reimbursement reform should include savings from prescription drugs,” he said. “We urge you to give strong consideration to the following prescription drug proposals that could save at least $150 billion.”
Those proposals included offering Medicaid-level drug rebates to beneficiaries who are eligible for both Medicare and Medicaid, giving the secretary of Health and Human Services the power to negotiate drug prices, reduce the exclusivity period for biologics, prohibit pay-for-delay agreements (when a brand-name drug manufacturer pays to delay the launch of a generic equivalent), and prohibit the use of Risk Evaluation and Mitigation Strategies (REMS) to block generic and biosimilar drug development.
AT A HOUSE ENERGY AND COMMERCE HEALTH SUBCOMMITTEE HEARING
SAMHSA Awards Funds for Tribal Youth Programs
The Substance Abuse and Mental Health Services Administration (SAMHSA) has awarded up to $294 million for programs that help promote prevention and treatment of mental health and substance use disorders among tribal children, adolescents, and young adults.
The grants are going to tribal communities across the country to expand and enhance behavioral health care services. The programs receiving grant money include:
- Circles of Care VI focuses on developing infrastructure to improve the mental health and wellness of children, youth, and families in American Indian/Alaska Native (AI/AN) communities. The program provides tribal and urban AI/AN communities with tools and resources to plan and design a holistic, community-based, coordinated system of care. In the system of care approach, families and youth work in partnership with public and private organizations to design mental health services and supports that are effective, build on the strengths of individuals, and address each person’s cultural and linguistic needs, according to SAMHSA. The program also aims to address “historically traumatic events” that have resulted in a significant loss of culture, language, and traditional ways of life;
Related: Banning Smoking in Tribal Casinos
- State/Tribal Youth Suicide Prevention Cooperative Agreements support states and tribes (including Alaska villages and urban AI organizations) in developing and implementing statewide or tribal youth suicide prevention and early intervention strategies via public/private collaboration among schools, juvenile justice systems, foster care systems, substance abuse and mental health programs, and other organizations that support children and youth. Among other goals, funding will boost the number of people trained to identify and refer youth at risk for suicide as well as improve continuity of care and follow-up for at-risk youth discharged from emergency departments and inpatient psychiatric units;
Related: Taking a New Approach to Tribal Child Welfare
- SAMHSA Treatment Drug Courts enhance substance abuse treatment services in existing adult Tribal Healing to Wellness Courts (the tribal version of adult drug courts) and in Juvenile Treatment Drug Courts (tribal or nontribal). Drug courts are specially designed court calendars intended to reduce recidivism and substance abuse and increase successful habilitation through early, continuous, and intense judicially supervised treatment. Although nearly 2,500 drug courts were created in 2012, many lack the funding they need for substance abuse treatment, SAMHSA said. The administration’s goal is to meet the clinical needs of clients and ensure that they are treated using evidence-based practices consistent with the disease model and the problem-solving model, rather than with the traditional court case-processing model.
Related: Controlling Substance Abuse
“SAMHSA has a deep commitment to helping tribes advance the behavioral health services of their communities, particularly with regard to tribal youth,” said SAMHSA Administrator Pamela S. Hyde. “It is essential that these young people have access to all the prevention, treatment, and recovery services they may need, so that they—and the tribes—can flourish.”
The Substance Abuse and Mental Health Services Administration (SAMHSA) has awarded up to $294 million for programs that help promote prevention and treatment of mental health and substance use disorders among tribal children, adolescents, and young adults.
The grants are going to tribal communities across the country to expand and enhance behavioral health care services. The programs receiving grant money include:
- Circles of Care VI focuses on developing infrastructure to improve the mental health and wellness of children, youth, and families in American Indian/Alaska Native (AI/AN) communities. The program provides tribal and urban AI/AN communities with tools and resources to plan and design a holistic, community-based, coordinated system of care. In the system of care approach, families and youth work in partnership with public and private organizations to design mental health services and supports that are effective, build on the strengths of individuals, and address each person’s cultural and linguistic needs, according to SAMHSA. The program also aims to address “historically traumatic events” that have resulted in a significant loss of culture, language, and traditional ways of life;
Related: Banning Smoking in Tribal Casinos
- State/Tribal Youth Suicide Prevention Cooperative Agreements support states and tribes (including Alaska villages and urban AI organizations) in developing and implementing statewide or tribal youth suicide prevention and early intervention strategies via public/private collaboration among schools, juvenile justice systems, foster care systems, substance abuse and mental health programs, and other organizations that support children and youth. Among other goals, funding will boost the number of people trained to identify and refer youth at risk for suicide as well as improve continuity of care and follow-up for at-risk youth discharged from emergency departments and inpatient psychiatric units;
Related: Taking a New Approach to Tribal Child Welfare
- SAMHSA Treatment Drug Courts enhance substance abuse treatment services in existing adult Tribal Healing to Wellness Courts (the tribal version of adult drug courts) and in Juvenile Treatment Drug Courts (tribal or nontribal). Drug courts are specially designed court calendars intended to reduce recidivism and substance abuse and increase successful habilitation through early, continuous, and intense judicially supervised treatment. Although nearly 2,500 drug courts were created in 2012, many lack the funding they need for substance abuse treatment, SAMHSA said. The administration’s goal is to meet the clinical needs of clients and ensure that they are treated using evidence-based practices consistent with the disease model and the problem-solving model, rather than with the traditional court case-processing model.
Related: Controlling Substance Abuse
“SAMHSA has a deep commitment to helping tribes advance the behavioral health services of their communities, particularly with regard to tribal youth,” said SAMHSA Administrator Pamela S. Hyde. “It is essential that these young people have access to all the prevention, treatment, and recovery services they may need, so that they—and the tribes—can flourish.”
The Substance Abuse and Mental Health Services Administration (SAMHSA) has awarded up to $294 million for programs that help promote prevention and treatment of mental health and substance use disorders among tribal children, adolescents, and young adults.
The grants are going to tribal communities across the country to expand and enhance behavioral health care services. The programs receiving grant money include:
- Circles of Care VI focuses on developing infrastructure to improve the mental health and wellness of children, youth, and families in American Indian/Alaska Native (AI/AN) communities. The program provides tribal and urban AI/AN communities with tools and resources to plan and design a holistic, community-based, coordinated system of care. In the system of care approach, families and youth work in partnership with public and private organizations to design mental health services and supports that are effective, build on the strengths of individuals, and address each person’s cultural and linguistic needs, according to SAMHSA. The program also aims to address “historically traumatic events” that have resulted in a significant loss of culture, language, and traditional ways of life;
Related: Banning Smoking in Tribal Casinos
- State/Tribal Youth Suicide Prevention Cooperative Agreements support states and tribes (including Alaska villages and urban AI organizations) in developing and implementing statewide or tribal youth suicide prevention and early intervention strategies via public/private collaboration among schools, juvenile justice systems, foster care systems, substance abuse and mental health programs, and other organizations that support children and youth. Among other goals, funding will boost the number of people trained to identify and refer youth at risk for suicide as well as improve continuity of care and follow-up for at-risk youth discharged from emergency departments and inpatient psychiatric units;
Related: Taking a New Approach to Tribal Child Welfare
- SAMHSA Treatment Drug Courts enhance substance abuse treatment services in existing adult Tribal Healing to Wellness Courts (the tribal version of adult drug courts) and in Juvenile Treatment Drug Courts (tribal or nontribal). Drug courts are specially designed court calendars intended to reduce recidivism and substance abuse and increase successful habilitation through early, continuous, and intense judicially supervised treatment. Although nearly 2,500 drug courts were created in 2012, many lack the funding they need for substance abuse treatment, SAMHSA said. The administration’s goal is to meet the clinical needs of clients and ensure that they are treated using evidence-based practices consistent with the disease model and the problem-solving model, rather than with the traditional court case-processing model.
Related: Controlling Substance Abuse
“SAMHSA has a deep commitment to helping tribes advance the behavioral health services of their communities, particularly with regard to tribal youth,” said SAMHSA Administrator Pamela S. Hyde. “It is essential that these young people have access to all the prevention, treatment, and recovery services they may need, so that they—and the tribes—can flourish.”
VA Drastically Cuts Rates of MRSA Infection
An environment that promotes infection prevention and control as “everyone’s responsibility” is at the heart of a “striking” drop in rates of infection with methicillin-resistant Staphylococcus aureus (MRSA), according to VA. Between 2007 and 2012, health care-related MRSA infections dropped 72% among VA patients in intensive care units, from 1.64 to 0.46 per 1,000 patient days. Infection rates for patients in nonintensive care hospital units fell 66%, from 0.47 to 0.16 per 1,000 patient days.
Keeping MRSA rates down matters: A surveillance report from the CDC found 75,309 cases of invasive MRSA infections and 9,670 deaths due to MRSA in 2012. “Health care-associated infections are a major challenge throughout the health care industry,” said VA Interim Under Secretary for Health Dr. Carolyn Clancy, “but we have found in VA that consistently applying some simple preventive strategies can make a very big difference, and that difference is being recognized.”
Related: The Downside of MRSA Patient Isolation
VA’s multifaceted prevention plan includes patient-screening programs for MRSA, contact precautions for hospitalized patients with MRSA, and hand hygiene reminders with readily available hand sanitizer stations placed strategically in common areas, patient wards, and specialty clinics throughout medical centers.
Having a dedicated employee at each medical center to monitor compliance with MRSA protection procedures, train staff, and work with patients and families helps reinforce the commitment to infection control and prevention; as do computerized reminders, online training, frequent measurement, and continual feedback to medical staff, according to VA.
Related: Linezolid Contributes to Clinical Success in MRSA Pneumonia
“VA has a well-earned reputation in successful prevention of MRSA,” said Secretary of Veterans Affairs Robert A. McDonald. “The drop in MRSA rates shows that we are pursuing the right course for prevention and treatment.”
An environment that promotes infection prevention and control as “everyone’s responsibility” is at the heart of a “striking” drop in rates of infection with methicillin-resistant Staphylococcus aureus (MRSA), according to VA. Between 2007 and 2012, health care-related MRSA infections dropped 72% among VA patients in intensive care units, from 1.64 to 0.46 per 1,000 patient days. Infection rates for patients in nonintensive care hospital units fell 66%, from 0.47 to 0.16 per 1,000 patient days.
Keeping MRSA rates down matters: A surveillance report from the CDC found 75,309 cases of invasive MRSA infections and 9,670 deaths due to MRSA in 2012. “Health care-associated infections are a major challenge throughout the health care industry,” said VA Interim Under Secretary for Health Dr. Carolyn Clancy, “but we have found in VA that consistently applying some simple preventive strategies can make a very big difference, and that difference is being recognized.”
Related: The Downside of MRSA Patient Isolation
VA’s multifaceted prevention plan includes patient-screening programs for MRSA, contact precautions for hospitalized patients with MRSA, and hand hygiene reminders with readily available hand sanitizer stations placed strategically in common areas, patient wards, and specialty clinics throughout medical centers.
Having a dedicated employee at each medical center to monitor compliance with MRSA protection procedures, train staff, and work with patients and families helps reinforce the commitment to infection control and prevention; as do computerized reminders, online training, frequent measurement, and continual feedback to medical staff, according to VA.
Related: Linezolid Contributes to Clinical Success in MRSA Pneumonia
“VA has a well-earned reputation in successful prevention of MRSA,” said Secretary of Veterans Affairs Robert A. McDonald. “The drop in MRSA rates shows that we are pursuing the right course for prevention and treatment.”
An environment that promotes infection prevention and control as “everyone’s responsibility” is at the heart of a “striking” drop in rates of infection with methicillin-resistant Staphylococcus aureus (MRSA), according to VA. Between 2007 and 2012, health care-related MRSA infections dropped 72% among VA patients in intensive care units, from 1.64 to 0.46 per 1,000 patient days. Infection rates for patients in nonintensive care hospital units fell 66%, from 0.47 to 0.16 per 1,000 patient days.
Keeping MRSA rates down matters: A surveillance report from the CDC found 75,309 cases of invasive MRSA infections and 9,670 deaths due to MRSA in 2012. “Health care-associated infections are a major challenge throughout the health care industry,” said VA Interim Under Secretary for Health Dr. Carolyn Clancy, “but we have found in VA that consistently applying some simple preventive strategies can make a very big difference, and that difference is being recognized.”
Related: The Downside of MRSA Patient Isolation
VA’s multifaceted prevention plan includes patient-screening programs for MRSA, contact precautions for hospitalized patients with MRSA, and hand hygiene reminders with readily available hand sanitizer stations placed strategically in common areas, patient wards, and specialty clinics throughout medical centers.
Having a dedicated employee at each medical center to monitor compliance with MRSA protection procedures, train staff, and work with patients and families helps reinforce the commitment to infection control and prevention; as do computerized reminders, online training, frequent measurement, and continual feedback to medical staff, according to VA.
Related: Linezolid Contributes to Clinical Success in MRSA Pneumonia
“VA has a well-earned reputation in successful prevention of MRSA,” said Secretary of Veterans Affairs Robert A. McDonald. “The drop in MRSA rates shows that we are pursuing the right course for prevention and treatment.”
So far, flu vaccine only 23% effective
So far this flu season, more than two-thirds of influenza A (H3N2) viruses differ from the components of the 2014-2015 influenza vaccine. In addition, the overall estimated effectiveness of the 2014-2015 influenza vaccine for preventing laboratory-confirmed influenza infection is only 23%.
Those are key findings from an analysis of 2,321 children and adults who presented to one of five study sites in the United States with acute respiratory illness between Nov. 10, 2014, and Jan. 2, 2015.
“Although influenza vaccines are the best tool for prevention of influenza currently available, more effective vaccines are needed,” wrote Brendan Flannery, Ph.D., of the influenza division at the CDC’s National Center for Immunization and Respiratory Disease, and his associates. The report is in the Jan. 16, 2015, issue of the CDC’s Morbidity and Mortality Weekly Report (MMWR 2015 Jan. 16;64:10-15). “Antiviral medications are an important adjunct in the treatment and control of influenza for the 2014-2015 season and should be used as recommended, regardless of patient vaccination status.”
Targeted groups for antiviral treatment include any patient with suspected or confirmed influenza who is hospitalized, has severe or progressive illness, or is at high risk for complications from influenza, even if the illness seems mild. “Persons at high risk include young children (especially those younger than age 2), pregnant women, persons with chronic medical conditions like asthma, diabetes, or heart disease, and adults aged 65 years and older,” the researchers wrote. “Ideally, antiviral treatment should be initiated within 48 hours of symptom onset, when treatment is most effective.”
Though spot shortages of Tamiflu and other antiviral drugs have been reported in the United States, the CDC investigators noted that it may be necessary for physicians and patients to contact more than one pharmacy to fill a prescription. Updates on the supply of antiviral drugs can be found here.
Dr. Flannery and his associates acknowledged certain limitations of their analysis, including the fact that future estimates could differ as more data become available and that the current estimates “are limited to the prevention of outpatient medical visits, rather than more severe illness outcomes, such as hospitalization or death.”
The researchers reported having no relevant financial conflicts.
On Twitter @dougbrunk
So far this flu season, more than two-thirds of influenza A (H3N2) viruses differ from the components of the 2014-2015 influenza vaccine. In addition, the overall estimated effectiveness of the 2014-2015 influenza vaccine for preventing laboratory-confirmed influenza infection is only 23%.
Those are key findings from an analysis of 2,321 children and adults who presented to one of five study sites in the United States with acute respiratory illness between Nov. 10, 2014, and Jan. 2, 2015.
“Although influenza vaccines are the best tool for prevention of influenza currently available, more effective vaccines are needed,” wrote Brendan Flannery, Ph.D., of the influenza division at the CDC’s National Center for Immunization and Respiratory Disease, and his associates. The report is in the Jan. 16, 2015, issue of the CDC’s Morbidity and Mortality Weekly Report (MMWR 2015 Jan. 16;64:10-15). “Antiviral medications are an important adjunct in the treatment and control of influenza for the 2014-2015 season and should be used as recommended, regardless of patient vaccination status.”
Targeted groups for antiviral treatment include any patient with suspected or confirmed influenza who is hospitalized, has severe or progressive illness, or is at high risk for complications from influenza, even if the illness seems mild. “Persons at high risk include young children (especially those younger than age 2), pregnant women, persons with chronic medical conditions like asthma, diabetes, or heart disease, and adults aged 65 years and older,” the researchers wrote. “Ideally, antiviral treatment should be initiated within 48 hours of symptom onset, when treatment is most effective.”
Though spot shortages of Tamiflu and other antiviral drugs have been reported in the United States, the CDC investigators noted that it may be necessary for physicians and patients to contact more than one pharmacy to fill a prescription. Updates on the supply of antiviral drugs can be found here.
Dr. Flannery and his associates acknowledged certain limitations of their analysis, including the fact that future estimates could differ as more data become available and that the current estimates “are limited to the prevention of outpatient medical visits, rather than more severe illness outcomes, such as hospitalization or death.”
The researchers reported having no relevant financial conflicts.
On Twitter @dougbrunk
So far this flu season, more than two-thirds of influenza A (H3N2) viruses differ from the components of the 2014-2015 influenza vaccine. In addition, the overall estimated effectiveness of the 2014-2015 influenza vaccine for preventing laboratory-confirmed influenza infection is only 23%.
Those are key findings from an analysis of 2,321 children and adults who presented to one of five study sites in the United States with acute respiratory illness between Nov. 10, 2014, and Jan. 2, 2015.
“Although influenza vaccines are the best tool for prevention of influenza currently available, more effective vaccines are needed,” wrote Brendan Flannery, Ph.D., of the influenza division at the CDC’s National Center for Immunization and Respiratory Disease, and his associates. The report is in the Jan. 16, 2015, issue of the CDC’s Morbidity and Mortality Weekly Report (MMWR 2015 Jan. 16;64:10-15). “Antiviral medications are an important adjunct in the treatment and control of influenza for the 2014-2015 season and should be used as recommended, regardless of patient vaccination status.”
Targeted groups for antiviral treatment include any patient with suspected or confirmed influenza who is hospitalized, has severe or progressive illness, or is at high risk for complications from influenza, even if the illness seems mild. “Persons at high risk include young children (especially those younger than age 2), pregnant women, persons with chronic medical conditions like asthma, diabetes, or heart disease, and adults aged 65 years and older,” the researchers wrote. “Ideally, antiviral treatment should be initiated within 48 hours of symptom onset, when treatment is most effective.”
Though spot shortages of Tamiflu and other antiviral drugs have been reported in the United States, the CDC investigators noted that it may be necessary for physicians and patients to contact more than one pharmacy to fill a prescription. Updates on the supply of antiviral drugs can be found here.
Dr. Flannery and his associates acknowledged certain limitations of their analysis, including the fact that future estimates could differ as more data become available and that the current estimates “are limited to the prevention of outpatient medical visits, rather than more severe illness outcomes, such as hospitalization or death.”
The researchers reported having no relevant financial conflicts.
On Twitter @dougbrunk
FROM MORBIDITY AND MORTALITY WEEKLY REPORT
FDA approves vagal blocking device for obesity
A novel implantable device that delivers electrical pulses to the intra-abdominal vagus nerve has been approved for treatment of obesity in adults, providing a less invasive alternative to bariatric surgery.
The Maestro Rechargeable System is “the first weight loss treatment device that targets the nerve pathway between the brain and the stomach that controls feelings of hunger and fullness,” according to the Food and Drug Administration statement released Jan. 14. The last device approved by the FDA for treatment of obesity was the Realize gastric band, in September 2007.
The device is approved for adults aged 18 and older with a body mass index of at least 40-45 kg/m2, or at least 35-39.9 kg/m2 with a related health condition such as high blood pressure or high cholesterol levels, who have tried to lose weight in a supervised weight management program within the past 5 years.
The system includes a rechargeable electrical pulse generator implanted into the lateral chest wall, connected to two electrical leads placed around the abdominal vagus nerve via a laparoscopic procedure. “It works by sending intermittent electrical pulses to the trunks in the abdominal vagus nerve, which is involved in regulating stomach emptying and signaling to the brain that the stomach feels empty or full,” the FDA statement said, adding: “Although it is known that the electric stimulation blocks nerve activity between the brain and the stomach, the specific mechanisms for weight loss due to use of the device are unknown.”
The manufacturer, EnteroMedics, refers to the treatment as “VBLOC therapy,” delivered by the Maestro System. The company expects that the device will be available this year “on a limited basis” at select Bariatric Centers of Excellence in the United States, according to a statement issued by EnteroMedics on Jan. 14.
FDA approval was based on the results of the ReCharge study of 233 patients with a body mass index of at least 35 kg/m2; the device was activated in 157 patients, and the remaining patients had the device implanted but it was not activated and they served as controls.
After 12 months, those with the activated device lost 8.5% more excess weight than did the controls. Among those who had the device activated, almost 53% lost at least 20% of their excess weight and 38% lost at least 35% of their excess weight, according to the FDA.
The study did not meet the primary effectiveness endpoint, which was that those on active treatment would lose at least 10% more excess weight than would the controls. However, the majority of an FDA advisory panel that reviewed the data at a meeting in June 2014 supported approval, agreeing that the benefits outweighed the risks for the proposed indication. Panelists cited the fact that the study safety endpoint was met and that the device was effective in helping some people lose weight.
The FDA statement said the decision to approve the device was based on the panel’s recommendation, the study results, and an FDA survey of patient preferences for obesity devices, which found that “a group of patients would accept risks associated with this surgically implanted device for the amounts of weight loss expected to be provided by the device.”
As a condition for approval, EnteroMedics is required to conduct a 5-year postmarketing study that will collect safety and effectiveness data in at least 100 patients, including weight loss, adverse events, surgical revisions and explants and changes in obesity-related comorbidities, according to the FDA.
Serious adverse events in the ReCharge study were nausea, pain at the neuroregulator site, vomiting, and surgical complications; other adverse events were heartburn, problems swallowing, belching, mild nausea, and chest pain, the FDA noted.
The EnteroMedics statement says that contraindications for VBLOC therapy include liver cirrhosis, portal hypertension, esophageal varices or an uncorrectable, clinically significant hiatal hernia; patients for whom magnetic resonance imaging or diathermy use is planned; patients at high risk for surgical complications; and patients who have permanently implanted, electrically-powered medical devices or gastrointestinal devices or prostheses, such as pacemakers, implanted defibrillators, or neurostimulators.
A novel implantable device that delivers electrical pulses to the intra-abdominal vagus nerve has been approved for treatment of obesity in adults, providing a less invasive alternative to bariatric surgery.
The Maestro Rechargeable System is “the first weight loss treatment device that targets the nerve pathway between the brain and the stomach that controls feelings of hunger and fullness,” according to the Food and Drug Administration statement released Jan. 14. The last device approved by the FDA for treatment of obesity was the Realize gastric band, in September 2007.
The device is approved for adults aged 18 and older with a body mass index of at least 40-45 kg/m2, or at least 35-39.9 kg/m2 with a related health condition such as high blood pressure or high cholesterol levels, who have tried to lose weight in a supervised weight management program within the past 5 years.
The system includes a rechargeable electrical pulse generator implanted into the lateral chest wall, connected to two electrical leads placed around the abdominal vagus nerve via a laparoscopic procedure. “It works by sending intermittent electrical pulses to the trunks in the abdominal vagus nerve, which is involved in regulating stomach emptying and signaling to the brain that the stomach feels empty or full,” the FDA statement said, adding: “Although it is known that the electric stimulation blocks nerve activity between the brain and the stomach, the specific mechanisms for weight loss due to use of the device are unknown.”
The manufacturer, EnteroMedics, refers to the treatment as “VBLOC therapy,” delivered by the Maestro System. The company expects that the device will be available this year “on a limited basis” at select Bariatric Centers of Excellence in the United States, according to a statement issued by EnteroMedics on Jan. 14.
FDA approval was based on the results of the ReCharge study of 233 patients with a body mass index of at least 35 kg/m2; the device was activated in 157 patients, and the remaining patients had the device implanted but it was not activated and they served as controls.
After 12 months, those with the activated device lost 8.5% more excess weight than did the controls. Among those who had the device activated, almost 53% lost at least 20% of their excess weight and 38% lost at least 35% of their excess weight, according to the FDA.
The study did not meet the primary effectiveness endpoint, which was that those on active treatment would lose at least 10% more excess weight than would the controls. However, the majority of an FDA advisory panel that reviewed the data at a meeting in June 2014 supported approval, agreeing that the benefits outweighed the risks for the proposed indication. Panelists cited the fact that the study safety endpoint was met and that the device was effective in helping some people lose weight.
The FDA statement said the decision to approve the device was based on the panel’s recommendation, the study results, and an FDA survey of patient preferences for obesity devices, which found that “a group of patients would accept risks associated with this surgically implanted device for the amounts of weight loss expected to be provided by the device.”
As a condition for approval, EnteroMedics is required to conduct a 5-year postmarketing study that will collect safety and effectiveness data in at least 100 patients, including weight loss, adverse events, surgical revisions and explants and changes in obesity-related comorbidities, according to the FDA.
Serious adverse events in the ReCharge study were nausea, pain at the neuroregulator site, vomiting, and surgical complications; other adverse events were heartburn, problems swallowing, belching, mild nausea, and chest pain, the FDA noted.
The EnteroMedics statement says that contraindications for VBLOC therapy include liver cirrhosis, portal hypertension, esophageal varices or an uncorrectable, clinically significant hiatal hernia; patients for whom magnetic resonance imaging or diathermy use is planned; patients at high risk for surgical complications; and patients who have permanently implanted, electrically-powered medical devices or gastrointestinal devices or prostheses, such as pacemakers, implanted defibrillators, or neurostimulators.
A novel implantable device that delivers electrical pulses to the intra-abdominal vagus nerve has been approved for treatment of obesity in adults, providing a less invasive alternative to bariatric surgery.
The Maestro Rechargeable System is “the first weight loss treatment device that targets the nerve pathway between the brain and the stomach that controls feelings of hunger and fullness,” according to the Food and Drug Administration statement released Jan. 14. The last device approved by the FDA for treatment of obesity was the Realize gastric band, in September 2007.
The device is approved for adults aged 18 and older with a body mass index of at least 40-45 kg/m2, or at least 35-39.9 kg/m2 with a related health condition such as high blood pressure or high cholesterol levels, who have tried to lose weight in a supervised weight management program within the past 5 years.
The system includes a rechargeable electrical pulse generator implanted into the lateral chest wall, connected to two electrical leads placed around the abdominal vagus nerve via a laparoscopic procedure. “It works by sending intermittent electrical pulses to the trunks in the abdominal vagus nerve, which is involved in regulating stomach emptying and signaling to the brain that the stomach feels empty or full,” the FDA statement said, adding: “Although it is known that the electric stimulation blocks nerve activity between the brain and the stomach, the specific mechanisms for weight loss due to use of the device are unknown.”
The manufacturer, EnteroMedics, refers to the treatment as “VBLOC therapy,” delivered by the Maestro System. The company expects that the device will be available this year “on a limited basis” at select Bariatric Centers of Excellence in the United States, according to a statement issued by EnteroMedics on Jan. 14.
FDA approval was based on the results of the ReCharge study of 233 patients with a body mass index of at least 35 kg/m2; the device was activated in 157 patients, and the remaining patients had the device implanted but it was not activated and they served as controls.
After 12 months, those with the activated device lost 8.5% more excess weight than did the controls. Among those who had the device activated, almost 53% lost at least 20% of their excess weight and 38% lost at least 35% of their excess weight, according to the FDA.
The study did not meet the primary effectiveness endpoint, which was that those on active treatment would lose at least 10% more excess weight than would the controls. However, the majority of an FDA advisory panel that reviewed the data at a meeting in June 2014 supported approval, agreeing that the benefits outweighed the risks for the proposed indication. Panelists cited the fact that the study safety endpoint was met and that the device was effective in helping some people lose weight.
The FDA statement said the decision to approve the device was based on the panel’s recommendation, the study results, and an FDA survey of patient preferences for obesity devices, which found that “a group of patients would accept risks associated with this surgically implanted device for the amounts of weight loss expected to be provided by the device.”
As a condition for approval, EnteroMedics is required to conduct a 5-year postmarketing study that will collect safety and effectiveness data in at least 100 patients, including weight loss, adverse events, surgical revisions and explants and changes in obesity-related comorbidities, according to the FDA.
Serious adverse events in the ReCharge study were nausea, pain at the neuroregulator site, vomiting, and surgical complications; other adverse events were heartburn, problems swallowing, belching, mild nausea, and chest pain, the FDA noted.
The EnteroMedics statement says that contraindications for VBLOC therapy include liver cirrhosis, portal hypertension, esophageal varices or an uncorrectable, clinically significant hiatal hernia; patients for whom magnetic resonance imaging or diathermy use is planned; patients at high risk for surgical complications; and patients who have permanently implanted, electrically-powered medical devices or gastrointestinal devices or prostheses, such as pacemakers, implanted defibrillators, or neurostimulators.