User login
Finding Ways to Overcome HIV and Domestic Violence
“Intersecting epidemics.” That is how the Obama Administration sees the health crises of intimate partner violence (IPV) and HIV (human immunodeficiency virus). More than half of HIV-positive women—double the national rate—experience violence from their partners.
In 2012, President Obama published a memorandum outlining plans to address the crises, including establishing the Interagency Federal Working Group on the Intersection of HIV/AIDS. In October 2014 (Domestic Violence [DV] Awareness Month), the Working Group released its report on how the effort is going: Update on Efforts to Address the Intersection of HIV/AIDS, Violence against Women and Girls, and Gender-Related Health Disparities. This update details how various federal agencies have been working to meet the president’s goals. The results have been far-reaching and comprehensive, ranging from programs to train health care professionals in screening and counseling to educate men and boys about their role in preventing violence.
The 5 core objectives for action, released in the September 2013 Working Group report, are (1) Improve health for women by screening for IPV and HIV; (2) Improve outcomes for women in HIV care by addressing violence and trauma; (3) Address contributing factors that increase the risk of violence for women and girls living with HIV; (4) Expand public outreach, education, and prevention efforts regarding violence against women and girls; and (5) Support research to better understand the scope of the intersection of HIV and violence against women and girls and develop effective interventions.
Related: Protecting Our Children
In an October 10, 2014, blog post for the Council on Women and Girls, Working Group member Maggie Czarnogorski, MD, MPH, a senior policy advisor in the Office of National AIDS Policy, on detail from VA, and Tiffany McNair, MD, MPH, a White House Fellow in the Office of the Vice President, pointed to 2 major accomplishments. One is the collaboration between the U.S. Department of Justice and the U.S. Department of Housing and Urban Development’s Housing Opportunities for People with AIDS, which allocates funding and resources to support transitional housing for women who are both living with HIV and experiencing violence in their lives. The second is the Substance Abuse and Mental Health Services Administration’s “trauma-informed approaches,” which aim to help people modify negative behaviors resulting from trauma and ultimately improve health outcomes.
Many of the projects discussed in the 2014 update are educational, focusing on training and disseminating information. For example, NIH created a special Internet portal for information from the HHS 2013 Symposium on the Intersection of HIV and IPV. The Administration for Children and Families (ACF) and the Office of Minority Health have collaborated to support resource centers that develop and disseminate the CORE Linkage to Life Program to both domestic violence and HIV/AIDS care providers, to improve health outcomes for African Americans and Latinos recently discharged form correctional and substance abuse inpatient facilities.
For each objective, the report outlined “concrete, nonexhaustive, recommended actions for specific federal agencies.” On the federal level, the Working Group stated, many goals have been met, while other activities have produced meaningful changes. Some highlights:
A new HRSA-CDC project, Partnerships for Care, is requiring IPV-related training for the HIV workforce. HRSA has also collaborated with ACF to disseminate HIV and IPV screening and counseling tools to a variety of programs, such as rural health programs, maternal and child health programs, and National Health Service Corps providers.
The Office of Population Affairs (OPA) has trained staff from Title X-funded family planning clinics about HIV/AIDS, IPV, and family planning, and the importance of screening. The OPA is also promoting screening for HIV and IPV in reproductive health clinics.
VA has several initiatives in the works, such as developing protocols for coscreening for HIV, IPV, and military sexual trauma. In 2014, VA launched the National DV/IPV Assistance Program in Care Management and Social Work Service, which will implement an IPV screening program and link women who are victims of violence to community organizations that can help them. VA is also promoting HIV screening for all veterans and has national recommendations for IPV screening. It will pilot the use of the Extended Hits Insults Threatens Screams (E-HITS) screening tool and Dangerous Assessment questions to screen for IPV.
Related: Recovering From Military Sexual Trauma
The 2014 update demonstrates “promising trends” and marks the first step toward addressing the needs of women with HIV/AIDS who are victims—or may be victims—of violence, as noted by Dr. Czarnogorski and Dr. McNair. The Working Group is looking forward to building on the momentum by scaling up effective strategies, incorporating trauma-informed approaches, and expanding outreach to high-risk groups. They hope to witness, they conclude, “both individual and broad societal-level impact—and to sustain that impact for the women and girls counting on us.”
“Intersecting epidemics.” That is how the Obama Administration sees the health crises of intimate partner violence (IPV) and HIV (human immunodeficiency virus). More than half of HIV-positive women—double the national rate—experience violence from their partners.
In 2012, President Obama published a memorandum outlining plans to address the crises, including establishing the Interagency Federal Working Group on the Intersection of HIV/AIDS. In October 2014 (Domestic Violence [DV] Awareness Month), the Working Group released its report on how the effort is going: Update on Efforts to Address the Intersection of HIV/AIDS, Violence against Women and Girls, and Gender-Related Health Disparities. This update details how various federal agencies have been working to meet the president’s goals. The results have been far-reaching and comprehensive, ranging from programs to train health care professionals in screening and counseling to educate men and boys about their role in preventing violence.
The 5 core objectives for action, released in the September 2013 Working Group report, are (1) Improve health for women by screening for IPV and HIV; (2) Improve outcomes for women in HIV care by addressing violence and trauma; (3) Address contributing factors that increase the risk of violence for women and girls living with HIV; (4) Expand public outreach, education, and prevention efforts regarding violence against women and girls; and (5) Support research to better understand the scope of the intersection of HIV and violence against women and girls and develop effective interventions.
Related: Protecting Our Children
In an October 10, 2014, blog post for the Council on Women and Girls, Working Group member Maggie Czarnogorski, MD, MPH, a senior policy advisor in the Office of National AIDS Policy, on detail from VA, and Tiffany McNair, MD, MPH, a White House Fellow in the Office of the Vice President, pointed to 2 major accomplishments. One is the collaboration between the U.S. Department of Justice and the U.S. Department of Housing and Urban Development’s Housing Opportunities for People with AIDS, which allocates funding and resources to support transitional housing for women who are both living with HIV and experiencing violence in their lives. The second is the Substance Abuse and Mental Health Services Administration’s “trauma-informed approaches,” which aim to help people modify negative behaviors resulting from trauma and ultimately improve health outcomes.
Many of the projects discussed in the 2014 update are educational, focusing on training and disseminating information. For example, NIH created a special Internet portal for information from the HHS 2013 Symposium on the Intersection of HIV and IPV. The Administration for Children and Families (ACF) and the Office of Minority Health have collaborated to support resource centers that develop and disseminate the CORE Linkage to Life Program to both domestic violence and HIV/AIDS care providers, to improve health outcomes for African Americans and Latinos recently discharged form correctional and substance abuse inpatient facilities.
For each objective, the report outlined “concrete, nonexhaustive, recommended actions for specific federal agencies.” On the federal level, the Working Group stated, many goals have been met, while other activities have produced meaningful changes. Some highlights:
A new HRSA-CDC project, Partnerships for Care, is requiring IPV-related training for the HIV workforce. HRSA has also collaborated with ACF to disseminate HIV and IPV screening and counseling tools to a variety of programs, such as rural health programs, maternal and child health programs, and National Health Service Corps providers.
The Office of Population Affairs (OPA) has trained staff from Title X-funded family planning clinics about HIV/AIDS, IPV, and family planning, and the importance of screening. The OPA is also promoting screening for HIV and IPV in reproductive health clinics.
VA has several initiatives in the works, such as developing protocols for coscreening for HIV, IPV, and military sexual trauma. In 2014, VA launched the National DV/IPV Assistance Program in Care Management and Social Work Service, which will implement an IPV screening program and link women who are victims of violence to community organizations that can help them. VA is also promoting HIV screening for all veterans and has national recommendations for IPV screening. It will pilot the use of the Extended Hits Insults Threatens Screams (E-HITS) screening tool and Dangerous Assessment questions to screen for IPV.
Related: Recovering From Military Sexual Trauma
The 2014 update demonstrates “promising trends” and marks the first step toward addressing the needs of women with HIV/AIDS who are victims—or may be victims—of violence, as noted by Dr. Czarnogorski and Dr. McNair. The Working Group is looking forward to building on the momentum by scaling up effective strategies, incorporating trauma-informed approaches, and expanding outreach to high-risk groups. They hope to witness, they conclude, “both individual and broad societal-level impact—and to sustain that impact for the women and girls counting on us.”
“Intersecting epidemics.” That is how the Obama Administration sees the health crises of intimate partner violence (IPV) and HIV (human immunodeficiency virus). More than half of HIV-positive women—double the national rate—experience violence from their partners.
In 2012, President Obama published a memorandum outlining plans to address the crises, including establishing the Interagency Federal Working Group on the Intersection of HIV/AIDS. In October 2014 (Domestic Violence [DV] Awareness Month), the Working Group released its report on how the effort is going: Update on Efforts to Address the Intersection of HIV/AIDS, Violence against Women and Girls, and Gender-Related Health Disparities. This update details how various federal agencies have been working to meet the president’s goals. The results have been far-reaching and comprehensive, ranging from programs to train health care professionals in screening and counseling to educate men and boys about their role in preventing violence.
The 5 core objectives for action, released in the September 2013 Working Group report, are (1) Improve health for women by screening for IPV and HIV; (2) Improve outcomes for women in HIV care by addressing violence and trauma; (3) Address contributing factors that increase the risk of violence for women and girls living with HIV; (4) Expand public outreach, education, and prevention efforts regarding violence against women and girls; and (5) Support research to better understand the scope of the intersection of HIV and violence against women and girls and develop effective interventions.
Related: Protecting Our Children
In an October 10, 2014, blog post for the Council on Women and Girls, Working Group member Maggie Czarnogorski, MD, MPH, a senior policy advisor in the Office of National AIDS Policy, on detail from VA, and Tiffany McNair, MD, MPH, a White House Fellow in the Office of the Vice President, pointed to 2 major accomplishments. One is the collaboration between the U.S. Department of Justice and the U.S. Department of Housing and Urban Development’s Housing Opportunities for People with AIDS, which allocates funding and resources to support transitional housing for women who are both living with HIV and experiencing violence in their lives. The second is the Substance Abuse and Mental Health Services Administration’s “trauma-informed approaches,” which aim to help people modify negative behaviors resulting from trauma and ultimately improve health outcomes.
Many of the projects discussed in the 2014 update are educational, focusing on training and disseminating information. For example, NIH created a special Internet portal for information from the HHS 2013 Symposium on the Intersection of HIV and IPV. The Administration for Children and Families (ACF) and the Office of Minority Health have collaborated to support resource centers that develop and disseminate the CORE Linkage to Life Program to both domestic violence and HIV/AIDS care providers, to improve health outcomes for African Americans and Latinos recently discharged form correctional and substance abuse inpatient facilities.
For each objective, the report outlined “concrete, nonexhaustive, recommended actions for specific federal agencies.” On the federal level, the Working Group stated, many goals have been met, while other activities have produced meaningful changes. Some highlights:
A new HRSA-CDC project, Partnerships for Care, is requiring IPV-related training for the HIV workforce. HRSA has also collaborated with ACF to disseminate HIV and IPV screening and counseling tools to a variety of programs, such as rural health programs, maternal and child health programs, and National Health Service Corps providers.
The Office of Population Affairs (OPA) has trained staff from Title X-funded family planning clinics about HIV/AIDS, IPV, and family planning, and the importance of screening. The OPA is also promoting screening for HIV and IPV in reproductive health clinics.
VA has several initiatives in the works, such as developing protocols for coscreening for HIV, IPV, and military sexual trauma. In 2014, VA launched the National DV/IPV Assistance Program in Care Management and Social Work Service, which will implement an IPV screening program and link women who are victims of violence to community organizations that can help them. VA is also promoting HIV screening for all veterans and has national recommendations for IPV screening. It will pilot the use of the Extended Hits Insults Threatens Screams (E-HITS) screening tool and Dangerous Assessment questions to screen for IPV.
Related: Recovering From Military Sexual Trauma
The 2014 update demonstrates “promising trends” and marks the first step toward addressing the needs of women with HIV/AIDS who are victims—or may be victims—of violence, as noted by Dr. Czarnogorski and Dr. McNair. The Working Group is looking forward to building on the momentum by scaling up effective strategies, incorporating trauma-informed approaches, and expanding outreach to high-risk groups. They hope to witness, they conclude, “both individual and broad societal-level impact—and to sustain that impact for the women and girls counting on us.”
CDC: Flu remains widespread; antivirals underutilized
The approximate midpoint of the 2014-2015 flu season has been reached, disease remains widespread, and antiviral flu medications remain underutilized, according to the Centers for Disease Control and Prevention.
As predicted, this season is proving particularly severe due to the predominance of antigenically drifted H3N2 virus strains, and the CDC is urging clinicians to maintain a high index of suspicion for flu and to prescribe antivirals earlier and more aggressively for patients presenting with flulike illness – especially young children, those over age 65 years, and those with underlying conditions, even if their symptoms are mild, CDC Director Thomas R. Frieden said during a press briefing.
A health advisory on the topic was issued Jan. 9, he noted.
“The CDC has recommended the use of antiviral drugs as an adjunct to vaccination. They are the only medicines that can specifically treat influenza illness, and in the context of an H3N2 predominant season with a less effective vaccine, treatment with antiflu drugs is even more important than usual,” he said, referring to the two neuraminidase inhibitors currently approved for treating influenza: oseltamivir and zanamivir.
About two-thirds of the H3N2 viruses analyzed this season are different from the H3N2 virus included in this year’s vaccine, which is why the vaccine is expected to have reduced effectiveness, he explained.
This makes early antiviral treatment all the more important, he added, noting that treatment within 2 days of symptom onset is optimal, but later treatment can also offer benefit.
“CDC scientists have looked very carefully at the use of influenza drugs in the clinical setting, and the conclusion is clear: They work, but they aren’t being used nearly enough. They can reduce symptoms, shorten duration of illness, and prevent serious complications,” he said.
Early treatment may help patients avoid hospitalization and could be life saving, he added.
Many patients are unaware that effective prescription treatments exist, and many doctors are not using the treatments as recommended. In one study, fewer than one in five eligible high-risk patients received treatment.
“So we’re expanding our efforts to reach clinicians about reminders about the importance of these drugs,” he said, noting that if high-risk patients with underlying conditions such as asthma, sickle cell disease, renal disease, or diabetes were treated, “tens of thousands of hospitalizations, and thousands of deaths could potentially be prevented.”
Dr. Frieden suggested that clinicians consider phone triage lines for high-risk patients to discuss symptoms over the phone and to “facilitate early initiation of treatment.”
An antiviral prescription can be provided without testing and before an office visit, he added.
The typical flu season lasts about 13 weeks on average, and the season began about 7 weeks ago. The continued high rate of H3N2 disease has had the greatest impact on older adults; that is typical of such seasons, which often involve a higher rate of hospitalizations and deaths.
In fact, hospitalization rates in the over 65 age group are rising sharply, Dr. Frieden said.
Last week the rate was 52 per 100,000 population, and this week the rate was 92 per 100,000 population. The cumulative rate 2 years ago when H3N2 viruses last predominated was 183 per 100,000, he noted.
“We wouldn’t be surprised to see something very similar this year,” he said.
Young children are also severely affected; thus far in the season there have been 26 reported pediatric flu-related deaths.
However, there are early signs that infection rates are declining in areas where the season started earlier, such as the Southeast, and the number of patients presenting to a doctor with flulike symptoms has also declined slightly, but it is too soon to tell whether disease activity has peaked, he said, stressing that “we still have several weeks of flu activity ahead.”
It’s not too late to get vaccinated, and despite the reduced efficacy of this year’s vaccine against the circulating H3N2 strains, vaccination may still offer some benefit.
“There are other strains out there as well,” he said, noting that a proportion of cases from other strains, such as influenza B, tend to occur late in the season, and this year’s vaccine offers a good match for influenza B viruses.
Pneumococcal vaccination is also important, particularly for older adults and those at high risk , he said.
Last September the CDC announced a new recommendation that all adults over age 65 years should get two different pneumococcal vaccines. Such vaccination may help prevent flu-related pneumonia.
Patients with the flu or flulike illness should be advised to cover their cough and to stay home from work or school to protect those who are most vulnerable to the flu, he said.
The approximate midpoint of the 2014-2015 flu season has been reached, disease remains widespread, and antiviral flu medications remain underutilized, according to the Centers for Disease Control and Prevention.
As predicted, this season is proving particularly severe due to the predominance of antigenically drifted H3N2 virus strains, and the CDC is urging clinicians to maintain a high index of suspicion for flu and to prescribe antivirals earlier and more aggressively for patients presenting with flulike illness – especially young children, those over age 65 years, and those with underlying conditions, even if their symptoms are mild, CDC Director Thomas R. Frieden said during a press briefing.
A health advisory on the topic was issued Jan. 9, he noted.
“The CDC has recommended the use of antiviral drugs as an adjunct to vaccination. They are the only medicines that can specifically treat influenza illness, and in the context of an H3N2 predominant season with a less effective vaccine, treatment with antiflu drugs is even more important than usual,” he said, referring to the two neuraminidase inhibitors currently approved for treating influenza: oseltamivir and zanamivir.
About two-thirds of the H3N2 viruses analyzed this season are different from the H3N2 virus included in this year’s vaccine, which is why the vaccine is expected to have reduced effectiveness, he explained.
This makes early antiviral treatment all the more important, he added, noting that treatment within 2 days of symptom onset is optimal, but later treatment can also offer benefit.
“CDC scientists have looked very carefully at the use of influenza drugs in the clinical setting, and the conclusion is clear: They work, but they aren’t being used nearly enough. They can reduce symptoms, shorten duration of illness, and prevent serious complications,” he said.
Early treatment may help patients avoid hospitalization and could be life saving, he added.
Many patients are unaware that effective prescription treatments exist, and many doctors are not using the treatments as recommended. In one study, fewer than one in five eligible high-risk patients received treatment.
“So we’re expanding our efforts to reach clinicians about reminders about the importance of these drugs,” he said, noting that if high-risk patients with underlying conditions such as asthma, sickle cell disease, renal disease, or diabetes were treated, “tens of thousands of hospitalizations, and thousands of deaths could potentially be prevented.”
Dr. Frieden suggested that clinicians consider phone triage lines for high-risk patients to discuss symptoms over the phone and to “facilitate early initiation of treatment.”
An antiviral prescription can be provided without testing and before an office visit, he added.
The typical flu season lasts about 13 weeks on average, and the season began about 7 weeks ago. The continued high rate of H3N2 disease has had the greatest impact on older adults; that is typical of such seasons, which often involve a higher rate of hospitalizations and deaths.
In fact, hospitalization rates in the over 65 age group are rising sharply, Dr. Frieden said.
Last week the rate was 52 per 100,000 population, and this week the rate was 92 per 100,000 population. The cumulative rate 2 years ago when H3N2 viruses last predominated was 183 per 100,000, he noted.
“We wouldn’t be surprised to see something very similar this year,” he said.
Young children are also severely affected; thus far in the season there have been 26 reported pediatric flu-related deaths.
However, there are early signs that infection rates are declining in areas where the season started earlier, such as the Southeast, and the number of patients presenting to a doctor with flulike symptoms has also declined slightly, but it is too soon to tell whether disease activity has peaked, he said, stressing that “we still have several weeks of flu activity ahead.”
It’s not too late to get vaccinated, and despite the reduced efficacy of this year’s vaccine against the circulating H3N2 strains, vaccination may still offer some benefit.
“There are other strains out there as well,” he said, noting that a proportion of cases from other strains, such as influenza B, tend to occur late in the season, and this year’s vaccine offers a good match for influenza B viruses.
Pneumococcal vaccination is also important, particularly for older adults and those at high risk , he said.
Last September the CDC announced a new recommendation that all adults over age 65 years should get two different pneumococcal vaccines. Such vaccination may help prevent flu-related pneumonia.
Patients with the flu or flulike illness should be advised to cover their cough and to stay home from work or school to protect those who are most vulnerable to the flu, he said.
The approximate midpoint of the 2014-2015 flu season has been reached, disease remains widespread, and antiviral flu medications remain underutilized, according to the Centers for Disease Control and Prevention.
As predicted, this season is proving particularly severe due to the predominance of antigenically drifted H3N2 virus strains, and the CDC is urging clinicians to maintain a high index of suspicion for flu and to prescribe antivirals earlier and more aggressively for patients presenting with flulike illness – especially young children, those over age 65 years, and those with underlying conditions, even if their symptoms are mild, CDC Director Thomas R. Frieden said during a press briefing.
A health advisory on the topic was issued Jan. 9, he noted.
“The CDC has recommended the use of antiviral drugs as an adjunct to vaccination. They are the only medicines that can specifically treat influenza illness, and in the context of an H3N2 predominant season with a less effective vaccine, treatment with antiflu drugs is even more important than usual,” he said, referring to the two neuraminidase inhibitors currently approved for treating influenza: oseltamivir and zanamivir.
About two-thirds of the H3N2 viruses analyzed this season are different from the H3N2 virus included in this year’s vaccine, which is why the vaccine is expected to have reduced effectiveness, he explained.
This makes early antiviral treatment all the more important, he added, noting that treatment within 2 days of symptom onset is optimal, but later treatment can also offer benefit.
“CDC scientists have looked very carefully at the use of influenza drugs in the clinical setting, and the conclusion is clear: They work, but they aren’t being used nearly enough. They can reduce symptoms, shorten duration of illness, and prevent serious complications,” he said.
Early treatment may help patients avoid hospitalization and could be life saving, he added.
Many patients are unaware that effective prescription treatments exist, and many doctors are not using the treatments as recommended. In one study, fewer than one in five eligible high-risk patients received treatment.
“So we’re expanding our efforts to reach clinicians about reminders about the importance of these drugs,” he said, noting that if high-risk patients with underlying conditions such as asthma, sickle cell disease, renal disease, or diabetes were treated, “tens of thousands of hospitalizations, and thousands of deaths could potentially be prevented.”
Dr. Frieden suggested that clinicians consider phone triage lines for high-risk patients to discuss symptoms over the phone and to “facilitate early initiation of treatment.”
An antiviral prescription can be provided without testing and before an office visit, he added.
The typical flu season lasts about 13 weeks on average, and the season began about 7 weeks ago. The continued high rate of H3N2 disease has had the greatest impact on older adults; that is typical of such seasons, which often involve a higher rate of hospitalizations and deaths.
In fact, hospitalization rates in the over 65 age group are rising sharply, Dr. Frieden said.
Last week the rate was 52 per 100,000 population, and this week the rate was 92 per 100,000 population. The cumulative rate 2 years ago when H3N2 viruses last predominated was 183 per 100,000, he noted.
“We wouldn’t be surprised to see something very similar this year,” he said.
Young children are also severely affected; thus far in the season there have been 26 reported pediatric flu-related deaths.
However, there are early signs that infection rates are declining in areas where the season started earlier, such as the Southeast, and the number of patients presenting to a doctor with flulike symptoms has also declined slightly, but it is too soon to tell whether disease activity has peaked, he said, stressing that “we still have several weeks of flu activity ahead.”
It’s not too late to get vaccinated, and despite the reduced efficacy of this year’s vaccine against the circulating H3N2 strains, vaccination may still offer some benefit.
“There are other strains out there as well,” he said, noting that a proportion of cases from other strains, such as influenza B, tend to occur late in the season, and this year’s vaccine offers a good match for influenza B viruses.
Pneumococcal vaccination is also important, particularly for older adults and those at high risk , he said.
Last September the CDC announced a new recommendation that all adults over age 65 years should get two different pneumococcal vaccines. Such vaccination may help prevent flu-related pneumonia.
Patients with the flu or flulike illness should be advised to cover their cough and to stay home from work or school to protect those who are most vulnerable to the flu, he said.
Edoxaban approved for atrial fib, DVT, and PE indications
Edoxaban, a selective factor Xa-inhibitor, has been approved by the Food and Drug Administration for reducing the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation, with a statement in the boxed warning that it should not be used in patients with normal renal function.
The warning reflects the results of a subgroup analysis in the pivotal trial, which found that the 60-mg dose was superior to warfarin in terms of reducing the stroke risk in mildly renally impaired patients, but was worse in patients with normal renal function. This was the main focus of a meeting of the FDA’s Cardiovascular and Renal Drugs Advisory Panel meeting in October, in which the panel voted 9-1 to recommend approval of edoxaban for this indication, but had mixed opinions on whether approval should be limited to patients with mild to moderate renal impairment.
The approved prescribing information recommends that a patient’s creatinine clearance should be checked before edoxaban is prescribed. “Patients with creatinine clearance greater than 95 mL/min have an increased risk of stroke, compared to similar patients given warfarin,” and should be treated with another anticoagulant, the FDA said in the Jan. 9 statement announcing the approval. The recommended dose for those with a creatinine clearance between 50 mL/min and 95 mL/min is 60 mg once a day; for those with a creatinine clearance of 15-50 mL/min, the recommended dose is 30 mg once a day, according to the prescribing information.
Edoxaban, the fourth novel oral anticoagulant drug approved by the FDA, will be marketed as Savaysa by Daiichi Sankyo. It was also approved to treat deep vein thrombosis and pulmonary embolism following 5-10 days of initial therapy with a parenteral anticoagulant. The recommended dose for this indication is 60 mg once a day. For patients with a creatinine clearance of 15-50 mL/min, or who weigh up to 60 kg (about 132 pounds), or who are taking “certain P-glycoprotein inhibitors,” the 30-mg/day dose is recommended.
Approval for the nonvalvular AF indication was based on ENGAGE AF-TIMI 48 (Effective Anticoagulation with Factor Xa Next Generation in Atrial Fibrillation) study, comparing once-daily edoxaban (60 mg and 30 mg) to warfarin in 21,015 patients with nonvalvular AF, at a moderate to high risk of thromboembolic events (N. Engl. J. Med. 2013;369:2093-104). Over a median of almost 3 years, both doses were noninferior to warfarin in the primary efficacy endpoint, the occurrence of first stroke or of a systemic embolic event. Overall, major bleeding events were significantly lower among those on the 60-mg and 30-mg doses, compared with those on warfarin. However, the rate of ischemic stroke was higher relative to warfarin in patients with a creatinine clearance over 95 mL/min.
About half of the edoxaban dose is eliminated by the kidneys, and patients with a creatinine clearance above 95 mL/min have lower plasma edoxaban levels, according to a statement in the clinical trials section of the prescribing information, which adds: “Given the clear relationship of dose and blood levels to effectiveness in the ENGAGE AF-TIMI 48 study, it could be anticipated that patients with better renal function would show a smaller effect of Savaysa, compared to warfarin than would patients with mildly impaired renal function, and this was in fact observed.”
Approval of the DVT and PE indication was based on the Hokusai-VTE study of about 8,200 people comparing edoxaban to warfarin, which found that the edoxaban 60 mg once a day was noninferior to warfarin in the rate of symptomatic venous thromboembolism (3.2% vs. 3.5% in those on warfarin). The rate of major or clinically relevant nonmajor bleeding events was 8.5% among those on edoxaban vs. 10.3% in those on warfarin (N. Engl. J. Med. 2013;369:1406-15).
Bleeding and anemia were the most common adverse events among patients with nonvalvular atrial fibrillation in clinical trials, and “as with other FDA-approved anticlotting drugs, bleeding, including life-threatening bleeding, is the most serious risk with Savaysa,” the FDA statement said. Among those treated for DVT and PE, the most common adverse events were bleeding, rash, abnormal liver function tests, and anemia.
Savaysa is the fourth novel oral anticoagulant to be cleared by the FDA, after dabigatran (Pradaxa), rivaroxaban (Xarelto), and apixaban (Eliquis).
Serious adverse events associated with edoxaban should be reported to the FDA’s MedWatch program or at 800-332-1088.
A precedent does not come readily to mind where one restricts the use of a drug to patients with mild to moderately impaired renal function to optimize the benefit-risk balance. Typically, one avoids the drug or reduces the dose in such subgroups. For this reason, I think the drug is likely going to be a “nonstarter” for many clinicians.
During the FDA Advisory Committee panel meeting on edoxaban, the emphasis was on excluding patients with a creatinine clearance greater than 80 mL/min (representing about 37% of patients in the pivotal trial). The FDA approval used a cut-off of 95 mL/min (representing about 22% of patients in the pivotal trial). Even though the latter increases the eligible pool of patients for edoxaban, the major challenge is going to be clinical acceptability and marketability, especially given no unique advantage of this drug over other approved novel anticoagulants.
The CrCl cut off of 95mL/min applies to nonvalvular atrial fibrillation indication, and not to the venous thromboembolism (DVT/PE) indication. However, the latter indication requires 5-10 days of parenteral anticoagulant therapy, which puts it at a disadvantage compared with rivaroxaban or apixaban.
It is interesting to note that active pathological bleeding qualifies as a contraindication, but use in patients with normal renal function does not. There is a twofold increased risk of overall stroke or systemic embolism (primary endpoint) and ischemic stroke in patients with normal renal function (CrCl > 95 mL/min) along with an attenuation of the hemorrhagic risk advantage in this subgroup. Even though adjudicated major bleeding is still favorable for edoxaban in this subgroup, in my opinion the ischemic stroke risk outweighs the major bleeding advantage.
Sanjay Kaul, M.D., is professor of medicine at the University of California, Los Angeles. He was a member of the FDA’s Cardiovascular and Renal Drugs Advisory Committee that reviewed edoxaban at a meeting on Oct. 30, 2014.
A precedent does not come readily to mind where one restricts the use of a drug to patients with mild to moderately impaired renal function to optimize the benefit-risk balance. Typically, one avoids the drug or reduces the dose in such subgroups. For this reason, I think the drug is likely going to be a “nonstarter” for many clinicians.
During the FDA Advisory Committee panel meeting on edoxaban, the emphasis was on excluding patients with a creatinine clearance greater than 80 mL/min (representing about 37% of patients in the pivotal trial). The FDA approval used a cut-off of 95 mL/min (representing about 22% of patients in the pivotal trial). Even though the latter increases the eligible pool of patients for edoxaban, the major challenge is going to be clinical acceptability and marketability, especially given no unique advantage of this drug over other approved novel anticoagulants.
The CrCl cut off of 95mL/min applies to nonvalvular atrial fibrillation indication, and not to the venous thromboembolism (DVT/PE) indication. However, the latter indication requires 5-10 days of parenteral anticoagulant therapy, which puts it at a disadvantage compared with rivaroxaban or apixaban.
It is interesting to note that active pathological bleeding qualifies as a contraindication, but use in patients with normal renal function does not. There is a twofold increased risk of overall stroke or systemic embolism (primary endpoint) and ischemic stroke in patients with normal renal function (CrCl > 95 mL/min) along with an attenuation of the hemorrhagic risk advantage in this subgroup. Even though adjudicated major bleeding is still favorable for edoxaban in this subgroup, in my opinion the ischemic stroke risk outweighs the major bleeding advantage.
Sanjay Kaul, M.D., is professor of medicine at the University of California, Los Angeles. He was a member of the FDA’s Cardiovascular and Renal Drugs Advisory Committee that reviewed edoxaban at a meeting on Oct. 30, 2014.
A precedent does not come readily to mind where one restricts the use of a drug to patients with mild to moderately impaired renal function to optimize the benefit-risk balance. Typically, one avoids the drug or reduces the dose in such subgroups. For this reason, I think the drug is likely going to be a “nonstarter” for many clinicians.
During the FDA Advisory Committee panel meeting on edoxaban, the emphasis was on excluding patients with a creatinine clearance greater than 80 mL/min (representing about 37% of patients in the pivotal trial). The FDA approval used a cut-off of 95 mL/min (representing about 22% of patients in the pivotal trial). Even though the latter increases the eligible pool of patients for edoxaban, the major challenge is going to be clinical acceptability and marketability, especially given no unique advantage of this drug over other approved novel anticoagulants.
The CrCl cut off of 95mL/min applies to nonvalvular atrial fibrillation indication, and not to the venous thromboembolism (DVT/PE) indication. However, the latter indication requires 5-10 days of parenteral anticoagulant therapy, which puts it at a disadvantage compared with rivaroxaban or apixaban.
It is interesting to note that active pathological bleeding qualifies as a contraindication, but use in patients with normal renal function does not. There is a twofold increased risk of overall stroke or systemic embolism (primary endpoint) and ischemic stroke in patients with normal renal function (CrCl > 95 mL/min) along with an attenuation of the hemorrhagic risk advantage in this subgroup. Even though adjudicated major bleeding is still favorable for edoxaban in this subgroup, in my opinion the ischemic stroke risk outweighs the major bleeding advantage.
Sanjay Kaul, M.D., is professor of medicine at the University of California, Los Angeles. He was a member of the FDA’s Cardiovascular and Renal Drugs Advisory Committee that reviewed edoxaban at a meeting on Oct. 30, 2014.
Edoxaban, a selective factor Xa-inhibitor, has been approved by the Food and Drug Administration for reducing the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation, with a statement in the boxed warning that it should not be used in patients with normal renal function.
The warning reflects the results of a subgroup analysis in the pivotal trial, which found that the 60-mg dose was superior to warfarin in terms of reducing the stroke risk in mildly renally impaired patients, but was worse in patients with normal renal function. This was the main focus of a meeting of the FDA’s Cardiovascular and Renal Drugs Advisory Panel meeting in October, in which the panel voted 9-1 to recommend approval of edoxaban for this indication, but had mixed opinions on whether approval should be limited to patients with mild to moderate renal impairment.
The approved prescribing information recommends that a patient’s creatinine clearance should be checked before edoxaban is prescribed. “Patients with creatinine clearance greater than 95 mL/min have an increased risk of stroke, compared to similar patients given warfarin,” and should be treated with another anticoagulant, the FDA said in the Jan. 9 statement announcing the approval. The recommended dose for those with a creatinine clearance between 50 mL/min and 95 mL/min is 60 mg once a day; for those with a creatinine clearance of 15-50 mL/min, the recommended dose is 30 mg once a day, according to the prescribing information.
Edoxaban, the fourth novel oral anticoagulant drug approved by the FDA, will be marketed as Savaysa by Daiichi Sankyo. It was also approved to treat deep vein thrombosis and pulmonary embolism following 5-10 days of initial therapy with a parenteral anticoagulant. The recommended dose for this indication is 60 mg once a day. For patients with a creatinine clearance of 15-50 mL/min, or who weigh up to 60 kg (about 132 pounds), or who are taking “certain P-glycoprotein inhibitors,” the 30-mg/day dose is recommended.
Approval for the nonvalvular AF indication was based on ENGAGE AF-TIMI 48 (Effective Anticoagulation with Factor Xa Next Generation in Atrial Fibrillation) study, comparing once-daily edoxaban (60 mg and 30 mg) to warfarin in 21,015 patients with nonvalvular AF, at a moderate to high risk of thromboembolic events (N. Engl. J. Med. 2013;369:2093-104). Over a median of almost 3 years, both doses were noninferior to warfarin in the primary efficacy endpoint, the occurrence of first stroke or of a systemic embolic event. Overall, major bleeding events were significantly lower among those on the 60-mg and 30-mg doses, compared with those on warfarin. However, the rate of ischemic stroke was higher relative to warfarin in patients with a creatinine clearance over 95 mL/min.
About half of the edoxaban dose is eliminated by the kidneys, and patients with a creatinine clearance above 95 mL/min have lower plasma edoxaban levels, according to a statement in the clinical trials section of the prescribing information, which adds: “Given the clear relationship of dose and blood levels to effectiveness in the ENGAGE AF-TIMI 48 study, it could be anticipated that patients with better renal function would show a smaller effect of Savaysa, compared to warfarin than would patients with mildly impaired renal function, and this was in fact observed.”
Approval of the DVT and PE indication was based on the Hokusai-VTE study of about 8,200 people comparing edoxaban to warfarin, which found that the edoxaban 60 mg once a day was noninferior to warfarin in the rate of symptomatic venous thromboembolism (3.2% vs. 3.5% in those on warfarin). The rate of major or clinically relevant nonmajor bleeding events was 8.5% among those on edoxaban vs. 10.3% in those on warfarin (N. Engl. J. Med. 2013;369:1406-15).
Bleeding and anemia were the most common adverse events among patients with nonvalvular atrial fibrillation in clinical trials, and “as with other FDA-approved anticlotting drugs, bleeding, including life-threatening bleeding, is the most serious risk with Savaysa,” the FDA statement said. Among those treated for DVT and PE, the most common adverse events were bleeding, rash, abnormal liver function tests, and anemia.
Savaysa is the fourth novel oral anticoagulant to be cleared by the FDA, after dabigatran (Pradaxa), rivaroxaban (Xarelto), and apixaban (Eliquis).
Serious adverse events associated with edoxaban should be reported to the FDA’s MedWatch program or at 800-332-1088.
Edoxaban, a selective factor Xa-inhibitor, has been approved by the Food and Drug Administration for reducing the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation, with a statement in the boxed warning that it should not be used in patients with normal renal function.
The warning reflects the results of a subgroup analysis in the pivotal trial, which found that the 60-mg dose was superior to warfarin in terms of reducing the stroke risk in mildly renally impaired patients, but was worse in patients with normal renal function. This was the main focus of a meeting of the FDA’s Cardiovascular and Renal Drugs Advisory Panel meeting in October, in which the panel voted 9-1 to recommend approval of edoxaban for this indication, but had mixed opinions on whether approval should be limited to patients with mild to moderate renal impairment.
The approved prescribing information recommends that a patient’s creatinine clearance should be checked before edoxaban is prescribed. “Patients with creatinine clearance greater than 95 mL/min have an increased risk of stroke, compared to similar patients given warfarin,” and should be treated with another anticoagulant, the FDA said in the Jan. 9 statement announcing the approval. The recommended dose for those with a creatinine clearance between 50 mL/min and 95 mL/min is 60 mg once a day; for those with a creatinine clearance of 15-50 mL/min, the recommended dose is 30 mg once a day, according to the prescribing information.
Edoxaban, the fourth novel oral anticoagulant drug approved by the FDA, will be marketed as Savaysa by Daiichi Sankyo. It was also approved to treat deep vein thrombosis and pulmonary embolism following 5-10 days of initial therapy with a parenteral anticoagulant. The recommended dose for this indication is 60 mg once a day. For patients with a creatinine clearance of 15-50 mL/min, or who weigh up to 60 kg (about 132 pounds), or who are taking “certain P-glycoprotein inhibitors,” the 30-mg/day dose is recommended.
Approval for the nonvalvular AF indication was based on ENGAGE AF-TIMI 48 (Effective Anticoagulation with Factor Xa Next Generation in Atrial Fibrillation) study, comparing once-daily edoxaban (60 mg and 30 mg) to warfarin in 21,015 patients with nonvalvular AF, at a moderate to high risk of thromboembolic events (N. Engl. J. Med. 2013;369:2093-104). Over a median of almost 3 years, both doses were noninferior to warfarin in the primary efficacy endpoint, the occurrence of first stroke or of a systemic embolic event. Overall, major bleeding events were significantly lower among those on the 60-mg and 30-mg doses, compared with those on warfarin. However, the rate of ischemic stroke was higher relative to warfarin in patients with a creatinine clearance over 95 mL/min.
About half of the edoxaban dose is eliminated by the kidneys, and patients with a creatinine clearance above 95 mL/min have lower plasma edoxaban levels, according to a statement in the clinical trials section of the prescribing information, which adds: “Given the clear relationship of dose and blood levels to effectiveness in the ENGAGE AF-TIMI 48 study, it could be anticipated that patients with better renal function would show a smaller effect of Savaysa, compared to warfarin than would patients with mildly impaired renal function, and this was in fact observed.”
Approval of the DVT and PE indication was based on the Hokusai-VTE study of about 8,200 people comparing edoxaban to warfarin, which found that the edoxaban 60 mg once a day was noninferior to warfarin in the rate of symptomatic venous thromboembolism (3.2% vs. 3.5% in those on warfarin). The rate of major or clinically relevant nonmajor bleeding events was 8.5% among those on edoxaban vs. 10.3% in those on warfarin (N. Engl. J. Med. 2013;369:1406-15).
Bleeding and anemia were the most common adverse events among patients with nonvalvular atrial fibrillation in clinical trials, and “as with other FDA-approved anticlotting drugs, bleeding, including life-threatening bleeding, is the most serious risk with Savaysa,” the FDA statement said. Among those treated for DVT and PE, the most common adverse events were bleeding, rash, abnormal liver function tests, and anemia.
Savaysa is the fourth novel oral anticoagulant to be cleared by the FDA, after dabigatran (Pradaxa), rivaroxaban (Xarelto), and apixaban (Eliquis).
Serious adverse events associated with edoxaban should be reported to the FDA’s MedWatch program or at 800-332-1088.
VA Doctor Killed in Shooting at El Paso VA Clinic
In response to the murder of Timothy Fjordbak, PsyD, at the El Paso VA Health Care System (VAHCS) earlier this month, the VA insists it will address staffing shortages and security and surveillance systems nationwide, including motion detectors, cameras, and door alarms. Under current federal law, it is unlawful to be in possession of a firearm on federal grounds unless otherwise authorized.
The murder-suicide in El Paso was one among a long line of violent acts committed on federal health facility grounds. Former chief of police at the Cheyenne VAMC in Wyoming John Glidewell told the Washington Post, “These are the same issues we have been screaming (begging) for help with the entire 10.5 years I have been with the VA Police.”
This comes less than a year after a former VA employee shot and wounded an employee at the Dayton VAMC in Ohio. Craig Larson from the VA’s Chicago regional public affairs office noted that the facility, like El Paso, did not have metal detectors, a policy that could have been implemented at the local level. Even the Washington DC VAMC did not announce their installing of metal detectors until 2013.
The area surrounding the El Paso VAHCS and William Beaumont Army Medical Center was on lockdown after the shooting in accordance with VA’s emergency response plan as the Fort Bliss military police and El Paso and federal law enforcement responded to the incident. The City of El Paso Police Department reported no other patients or staff members were physically harmed. The El Paso VAHCS opened for patient appointments Friday at 1:00 PM under increased security measures, including 100% identification check and random checking of bags and parcels. Security has since increased to 100% bags check. The facility was closed Wednesday and Thursday.
As of January 12, 2015, entrance to the El Paso VAHCS will only be possible through the main floor (3rd floor). Enhanced security procedures will be in place until further notice.
During the emergency closing 2 weeks ago, veterans with existing appointments at the El Paso VAHCS were contacted for rescheduling. Community-based outpatient clinics at Eastside El Paso and La Cruces, New Mexico, were on normal operating hours.
A Mobile Vet Center was deployed January 8, 2015, to provide counseling services to veterans, servicemembers, families, and VA staff members. The Mobile Vet Center operated at the El Paso Community College until 4:30 PM local time Thursday and with the exception of Sunday operated 8:00 AM through 4:30 PM daily through Tuesday, January 13, at the El Paso VAHCS main campus.
The El Paso VAHCS provides primary and specialized ambulatory care services at its main campus, with consultants and fee-basis specialists supplementing the medical staff. The facility is co-located with the 148-bed William Beaumont Army Medical Center at 5005 North Piedras Street near Fort Bliss.
In response to the murder of Timothy Fjordbak, PsyD, at the El Paso VA Health Care System (VAHCS) earlier this month, the VA insists it will address staffing shortages and security and surveillance systems nationwide, including motion detectors, cameras, and door alarms. Under current federal law, it is unlawful to be in possession of a firearm on federal grounds unless otherwise authorized.
The murder-suicide in El Paso was one among a long line of violent acts committed on federal health facility grounds. Former chief of police at the Cheyenne VAMC in Wyoming John Glidewell told the Washington Post, “These are the same issues we have been screaming (begging) for help with the entire 10.5 years I have been with the VA Police.”
This comes less than a year after a former VA employee shot and wounded an employee at the Dayton VAMC in Ohio. Craig Larson from the VA’s Chicago regional public affairs office noted that the facility, like El Paso, did not have metal detectors, a policy that could have been implemented at the local level. Even the Washington DC VAMC did not announce their installing of metal detectors until 2013.
The area surrounding the El Paso VAHCS and William Beaumont Army Medical Center was on lockdown after the shooting in accordance with VA’s emergency response plan as the Fort Bliss military police and El Paso and federal law enforcement responded to the incident. The City of El Paso Police Department reported no other patients or staff members were physically harmed. The El Paso VAHCS opened for patient appointments Friday at 1:00 PM under increased security measures, including 100% identification check and random checking of bags and parcels. Security has since increased to 100% bags check. The facility was closed Wednesday and Thursday.
As of January 12, 2015, entrance to the El Paso VAHCS will only be possible through the main floor (3rd floor). Enhanced security procedures will be in place until further notice.
During the emergency closing 2 weeks ago, veterans with existing appointments at the El Paso VAHCS were contacted for rescheduling. Community-based outpatient clinics at Eastside El Paso and La Cruces, New Mexico, were on normal operating hours.
A Mobile Vet Center was deployed January 8, 2015, to provide counseling services to veterans, servicemembers, families, and VA staff members. The Mobile Vet Center operated at the El Paso Community College until 4:30 PM local time Thursday and with the exception of Sunday operated 8:00 AM through 4:30 PM daily through Tuesday, January 13, at the El Paso VAHCS main campus.
The El Paso VAHCS provides primary and specialized ambulatory care services at its main campus, with consultants and fee-basis specialists supplementing the medical staff. The facility is co-located with the 148-bed William Beaumont Army Medical Center at 5005 North Piedras Street near Fort Bliss.
In response to the murder of Timothy Fjordbak, PsyD, at the El Paso VA Health Care System (VAHCS) earlier this month, the VA insists it will address staffing shortages and security and surveillance systems nationwide, including motion detectors, cameras, and door alarms. Under current federal law, it is unlawful to be in possession of a firearm on federal grounds unless otherwise authorized.
The murder-suicide in El Paso was one among a long line of violent acts committed on federal health facility grounds. Former chief of police at the Cheyenne VAMC in Wyoming John Glidewell told the Washington Post, “These are the same issues we have been screaming (begging) for help with the entire 10.5 years I have been with the VA Police.”
This comes less than a year after a former VA employee shot and wounded an employee at the Dayton VAMC in Ohio. Craig Larson from the VA’s Chicago regional public affairs office noted that the facility, like El Paso, did not have metal detectors, a policy that could have been implemented at the local level. Even the Washington DC VAMC did not announce their installing of metal detectors until 2013.
The area surrounding the El Paso VAHCS and William Beaumont Army Medical Center was on lockdown after the shooting in accordance with VA’s emergency response plan as the Fort Bliss military police and El Paso and federal law enforcement responded to the incident. The City of El Paso Police Department reported no other patients or staff members were physically harmed. The El Paso VAHCS opened for patient appointments Friday at 1:00 PM under increased security measures, including 100% identification check and random checking of bags and parcels. Security has since increased to 100% bags check. The facility was closed Wednesday and Thursday.
As of January 12, 2015, entrance to the El Paso VAHCS will only be possible through the main floor (3rd floor). Enhanced security procedures will be in place until further notice.
During the emergency closing 2 weeks ago, veterans with existing appointments at the El Paso VAHCS were contacted for rescheduling. Community-based outpatient clinics at Eastside El Paso and La Cruces, New Mexico, were on normal operating hours.
A Mobile Vet Center was deployed January 8, 2015, to provide counseling services to veterans, servicemembers, families, and VA staff members. The Mobile Vet Center operated at the El Paso Community College until 4:30 PM local time Thursday and with the exception of Sunday operated 8:00 AM through 4:30 PM daily through Tuesday, January 13, at the El Paso VAHCS main campus.
The El Paso VAHCS provides primary and specialized ambulatory care services at its main campus, with consultants and fee-basis specialists supplementing the medical staff. The facility is co-located with the 148-bed William Beaumont Army Medical Center at 5005 North Piedras Street near Fort Bliss.
Alcohol poisoning kills an average of six people each day
Every day, an average of six people in the United States die from alcohol poisoning—the majority of them middle-aged men, according to a new Vital Signs report from the Centers for Disease Control and Prevention.
“This is likely to be an underestimate,” the CDC’s Deputy Principal Director, Ileana Arias, Ph.D., said during a Jan. 6, 2015 press briefing.
Dr. Arias highlighted findings from a study of alcohol poisoning among people aged 15 and older that coauthor Dr. Robert D. Brewer and associates conducted using multiple cause-of-death data from the National Vital Statistics for 2010-2012. They found that more than 2,200 Americans died each year of alcohol poisoning, for an average of six deaths every day each year. Three in four alcohol poisoning deaths involved adults 35-54 years old, mostly men.
The researchers determined that binge drinking, defined as consuming four or more drinks for women and five or more drinks for men during a period of 2-3 hours, accounted for most of the deaths. “Despite the risks, more than 38 million U.S. adults report binge drinking about four times per month and consume an average of eight drinks per binge,” Dr. Arias said. “Alcohol poisoning is caused by consuming a very large amount of alcohol in a very short period of time.”
A person’s response to alcohol can vary depending on many factors, including the grade of alcohol consumed, the health of the drinker, and whether the drinker has consumed other drugs. “But the key point is this: The more you drink, the greater you are at risk of poisoning and of death,” she said.
Dr. Arias noted that a 12-ounce can of 5% beer contains the same amount of alcohol as a 5-ounce glass of 12% wine or 1.5 ounces of 80-proof distilled spirits. “It’s also best to avoid drinks with unknown alcohol content and be very cautious when mixing alcohol with energy drinks,” she said. “Caffeine can mask alcohol’s effects, causing you to drink more than you intended [to].”
When assessed by race and ethnicity, the majority of alcohol-poisoning deaths occurred among non-Hispanic whites. However, American Indians and Alaska Natives had the most alcohol-poisoning deaths per million people. Alcohol-poisoning deaths also varied widely across states, ranging from 5.3 deaths per million residents in Alabama to 46.5 deaths per million residents in Alaska. “Alcohol dependence was identified as a factor in 30% of these deaths and other drugs contributed to 3% of the deaths,” she said.
Life-threatening signs of alcohol poisoning include the inability to wake up from sleep, vomiting, seizures, slow or irregular breathing or heart rate, and low body temperature, bluish skin color, or pallor. Dr. Arias said that health professionals can play a role in the prevention of deaths related to alcohol poisoning by screening all adult patients for excessive drinking, counseling those who do so to help them drink less, and referring excessive drinkers who are alcohol dependent for specialized treatment.
“The bottom line is that binge drinking can be lethal,” she said. “Alcohol poisoning is killing people across the lifespan, but in particular men in the prime of their lives.”
None of the researchers reported having relevant financial disclosures.
On Twitter @dougbrunk
Every day, an average of six people in the United States die from alcohol poisoning—the majority of them middle-aged men, according to a new Vital Signs report from the Centers for Disease Control and Prevention.
“This is likely to be an underestimate,” the CDC’s Deputy Principal Director, Ileana Arias, Ph.D., said during a Jan. 6, 2015 press briefing.
Dr. Arias highlighted findings from a study of alcohol poisoning among people aged 15 and older that coauthor Dr. Robert D. Brewer and associates conducted using multiple cause-of-death data from the National Vital Statistics for 2010-2012. They found that more than 2,200 Americans died each year of alcohol poisoning, for an average of six deaths every day each year. Three in four alcohol poisoning deaths involved adults 35-54 years old, mostly men.
The researchers determined that binge drinking, defined as consuming four or more drinks for women and five or more drinks for men during a period of 2-3 hours, accounted for most of the deaths. “Despite the risks, more than 38 million U.S. adults report binge drinking about four times per month and consume an average of eight drinks per binge,” Dr. Arias said. “Alcohol poisoning is caused by consuming a very large amount of alcohol in a very short period of time.”
A person’s response to alcohol can vary depending on many factors, including the grade of alcohol consumed, the health of the drinker, and whether the drinker has consumed other drugs. “But the key point is this: The more you drink, the greater you are at risk of poisoning and of death,” she said.
Dr. Arias noted that a 12-ounce can of 5% beer contains the same amount of alcohol as a 5-ounce glass of 12% wine or 1.5 ounces of 80-proof distilled spirits. “It’s also best to avoid drinks with unknown alcohol content and be very cautious when mixing alcohol with energy drinks,” she said. “Caffeine can mask alcohol’s effects, causing you to drink more than you intended [to].”
When assessed by race and ethnicity, the majority of alcohol-poisoning deaths occurred among non-Hispanic whites. However, American Indians and Alaska Natives had the most alcohol-poisoning deaths per million people. Alcohol-poisoning deaths also varied widely across states, ranging from 5.3 deaths per million residents in Alabama to 46.5 deaths per million residents in Alaska. “Alcohol dependence was identified as a factor in 30% of these deaths and other drugs contributed to 3% of the deaths,” she said.
Life-threatening signs of alcohol poisoning include the inability to wake up from sleep, vomiting, seizures, slow or irregular breathing or heart rate, and low body temperature, bluish skin color, or pallor. Dr. Arias said that health professionals can play a role in the prevention of deaths related to alcohol poisoning by screening all adult patients for excessive drinking, counseling those who do so to help them drink less, and referring excessive drinkers who are alcohol dependent for specialized treatment.
“The bottom line is that binge drinking can be lethal,” she said. “Alcohol poisoning is killing people across the lifespan, but in particular men in the prime of their lives.”
None of the researchers reported having relevant financial disclosures.
On Twitter @dougbrunk
Every day, an average of six people in the United States die from alcohol poisoning—the majority of them middle-aged men, according to a new Vital Signs report from the Centers for Disease Control and Prevention.
“This is likely to be an underestimate,” the CDC’s Deputy Principal Director, Ileana Arias, Ph.D., said during a Jan. 6, 2015 press briefing.
Dr. Arias highlighted findings from a study of alcohol poisoning among people aged 15 and older that coauthor Dr. Robert D. Brewer and associates conducted using multiple cause-of-death data from the National Vital Statistics for 2010-2012. They found that more than 2,200 Americans died each year of alcohol poisoning, for an average of six deaths every day each year. Three in four alcohol poisoning deaths involved adults 35-54 years old, mostly men.
The researchers determined that binge drinking, defined as consuming four or more drinks for women and five or more drinks for men during a period of 2-3 hours, accounted for most of the deaths. “Despite the risks, more than 38 million U.S. adults report binge drinking about four times per month and consume an average of eight drinks per binge,” Dr. Arias said. “Alcohol poisoning is caused by consuming a very large amount of alcohol in a very short period of time.”
A person’s response to alcohol can vary depending on many factors, including the grade of alcohol consumed, the health of the drinker, and whether the drinker has consumed other drugs. “But the key point is this: The more you drink, the greater you are at risk of poisoning and of death,” she said.
Dr. Arias noted that a 12-ounce can of 5% beer contains the same amount of alcohol as a 5-ounce glass of 12% wine or 1.5 ounces of 80-proof distilled spirits. “It’s also best to avoid drinks with unknown alcohol content and be very cautious when mixing alcohol with energy drinks,” she said. “Caffeine can mask alcohol’s effects, causing you to drink more than you intended [to].”
When assessed by race and ethnicity, the majority of alcohol-poisoning deaths occurred among non-Hispanic whites. However, American Indians and Alaska Natives had the most alcohol-poisoning deaths per million people. Alcohol-poisoning deaths also varied widely across states, ranging from 5.3 deaths per million residents in Alabama to 46.5 deaths per million residents in Alaska. “Alcohol dependence was identified as a factor in 30% of these deaths and other drugs contributed to 3% of the deaths,” she said.
Life-threatening signs of alcohol poisoning include the inability to wake up from sleep, vomiting, seizures, slow or irregular breathing or heart rate, and low body temperature, bluish skin color, or pallor. Dr. Arias said that health professionals can play a role in the prevention of deaths related to alcohol poisoning by screening all adult patients for excessive drinking, counseling those who do so to help them drink less, and referring excessive drinkers who are alcohol dependent for specialized treatment.
“The bottom line is that binge drinking can be lethal,” she said. “Alcohol poisoning is killing people across the lifespan, but in particular men in the prime of their lives.”
None of the researchers reported having relevant financial disclosures.
On Twitter @dougbrunk
Key clinical point: An estimated six people in the United States die each day from alcohol poisoning.
Major finding: More than 2,200 Americans die each year of alcohol poisoning, or an average of six deaths every day each year.
Data source: An analysis of multiple cause-of-death data from the National Vital Statistics for 2010-2012.
Disclosures: None of the researchers reported having relevant financial disclosures.
Medicare @ 50: Popular with patients; problematic for physicians
When President Lyndon B. Johnson signed Medicare into law on July 30, 1965, he vowed that the program would give seniors access to the “healing miracle of modern medicine” and save them from exhausting their savings when they became ill.
For the most part, the program has delivered on those bold promises. Americans over age 65 years are living longer, healthier lives, and they are doing so without fear that a trip to the hospital will lead to bankruptcy. That security has only increased over time, as Congress added prescription drug coverage and most recently, free preventive care. In 1972, the Medicare program was extended to the long-term disabled and those with end stage renal disease.
Medicare “fundamentally changed the way we thought about health in our nation for our seniors,” said Dr. Georges C. Benjamin, executive director of the American Public Health Association. “This probably was the most important tool to lift seniors out of poverty. The economic benefit of this, in addition to the health benefit, was enormous.”
Along with coverage of hospital stays and physician services for seniors under Medicare, the Social Security Act Amendments of 1965 also created Medicaid, a voluntary federal-state partnership to provide medical care and long-term care services to low-income Americans.
Changing medical practice
When combined with the Civil Rights Act of 1964, Medicare desegregated all hospitals that participated in the program. Prior to the start of Medicare on July 1, 1966, a large number of hospitals around the country were still racially segregated in some manner. In many areas of the South, a separate hospital system served the black community and were the only places where black physicians could train and practice (Milbank Q. 2005; 83: 247-69). But in a matter of months, hospitals across the country integrated their facilities and staff in a relative peaceful manner, according to a study conducted by the Commonwealth Fund.
“Now, physicians of color could not be kept out of hospitals as a matter of policy,” Dr. Benjamin said. “It dramatically improved practice opportunities for physicians and access to care opportunities for patients.”
Stuart Guterman, Ph.D., vice president for Medicare and cost control at the Commonwealth Fund, said it’s one of the lesser-known elements of Medicare’s history that the law became an impetus to overcoming racial barriers for both patients and physicians.
Changing payment incentives
Medicare payments opened up a significant new stream of revenue for hospitals that made desegregation more palatable. But hospitals weren’t the only ones who benefited financially from the new program.
Despite the American Medical Association’s fight against the creation of Medicare, during which officials famously decried the program as “socialized medicine,” physicians were financial winners under the new law, at least at first.
Much like hospitals, doctors got paid for the first time for treating patients who they had previously seen as charity care. And, in those early years of the Medicare program, the government paid on a charge basis. The result was that Medicare spending was quickly out of control.
Cost controls were added first for hospital care. In 1983, Medicare adopted the inpatient hospital prospective payment system, which replaced hospitals’ cost-based payments with the diagnosis-related group (DRG) system.
“Changing the notion of what hospitals did from each individual service or each individual day to the hospital stay, really changed the way hospital care was looked at,” Dr. Guterman said.
It took somewhat longer for federal health officials to tackle payment on the physician side. Throughout the 1980s, Congress froze physician payment rates in an effort to address rising costs. In 1984, Congress established the Participating Physicians’ Program, which required physicians to accept assignment for all Medicare patients and services rather than on a service-by-service basis. It also barred balance billing of Medicare beneficiaries.
In 1989, Congress made the most significant change yet to physician payment, creating the Resource-Based Relative Value Scale (RBRVS) under the Omnibus Budget Reconciliation Act. Medicare officials used the new RBRVS to establish a standardized physician payment schedule in 1992. Under the new system, payments were determined by calculating the costs of physician work, practice expenses, and professional liability insurance. Payments are then adjusted by a conversion factor and geographic cost differences.
“The impact of the fee schedule all depends on where you sit,” said Paul B. Ginsburg, Ph.D., director of health policy at the Schaeffer Center for Health Policy and Economics at the University of Southern California.
The fee schedule was positive for primary care physicians at first, but a flawed updating process over the decades led to differences in Medicare payments by specialty, largely favoring procedural specialists over those who billed for evaluation and management services.
But Dr. Ginsburg said Medicare is now on the cusp of an even more important payment change as federal officials begin to move away from fee-for-service and toward payment approaches that emphasize quality and value.
While some new payment model experiments have been promising, the attempts by the Centers for Medicare & Medicaid Services have been “fumbling,” Dr. Ginsburg said.
Changing physician satisfaction
Medicare beneficiaries are typically happier with their health coverage than individuals enrolled in private, employer-sponsored insurance and are less worried about financial barriers to care, Dr. Guterman said.
“I think it’s fair to say that it’s one of the more popular federal programs ever,” he said. “You hear people frequently talking about how touchy it is to make any changes to the program because people are very wary of having that benefit diluted.”
That’s not the case for physicians.
“The physicians I’ve talked to over the last 2 years feel like they’re drowning in red tape,” said Dr. Austin King, president of the Texas Medical Association and a head and neck surgeon in Abilene.
The result is that physicians are becoming employees in large physician practices or hospitals, or they are moving to set up boutique practices, Dr. King said. “Right now, the physicians are not real happy with the program.”
Dr. King predicted that physicians will continue to move out of the program, creating patient access issues, unless Congress gives them a way to recoup their costs, such as through the practice of balance billing.
Resolving issues like the looming annual cuts from the Sustainable Growth Rate formula and eliminating the Affordable Care Act’s Independent Payment Advisory Board would put some predictability back into the system, Dr. King said, adding that it’s not enough because it would still leave the numerous regulatory requirements, which many physicians “agonize” over.
It’s that kind of agony that keeps Dr. Jane Orient, a general internist in Tucson, Ariz., from participating in Medicare. Dr. Orient opted out of the program in 1990, around the time that Medicare began requiring that physicians file claims for payment. Previously, patients could pay her directly for services and be reimbursed by Medicare.
“I decided I just could not do that,” said Dr. Orient, executive director of the Association of American Physicians and Surgeons. “For me to file the claim and figure out what all those E&M guidelines meant, was just impossible. I certainly couldn’t do it with the staff I had.”
The fundamental problem, said Rep. Michael C. Burgess (R-Texas), cochair of the Congressional Health Caucus and an ob.gyn., is that patients get their care covered at a price they can afford, while Medicare’s payment to physicians doesn’t cover the cost of care.
“What has always been a bad situation is getting worse and worse with every passing year,” Rep. Burgess said.
Rep. Phil Roe (R-Tenn.), cochair of the GOP Doctors Caucus, said the problem for lawmakers is that they must fix the physician payment system before patient access is significantly affected. How much time do they have?
Rep. Roe, an ob.gyn., said physicians will hang on for as long as they can. But if Medicare continues to cut payments, an access crisis is coming.
If Medicare’s solution to their financing problem is to cut providers, he said, physicians will look for a way out of the program, and that will start with large-scale retirements of older physicians.
“They are all looking for the exit signs now,” Rep. Roe said. 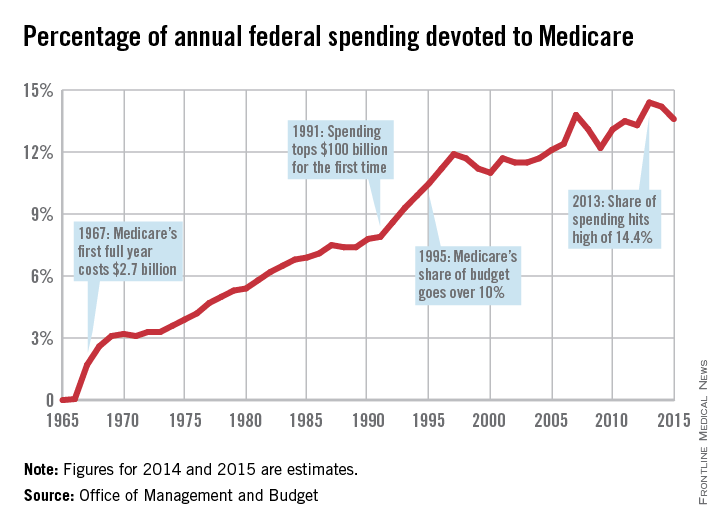
|
This is the first in a series of articles tracing the 50-year history of Medicare and its impact on physicians and patients. Future articles will focus on the public health impact of Medicare, the origins of the Sustainable Growth Rate formula, and the long-term financial viability of the program.
mschneider@frontlinemedcom.com
On Twitter @maryellenny
When President Lyndon B. Johnson signed Medicare into law on July 30, 1965, he vowed that the program would give seniors access to the “healing miracle of modern medicine” and save them from exhausting their savings when they became ill.
For the most part, the program has delivered on those bold promises. Americans over age 65 years are living longer, healthier lives, and they are doing so without fear that a trip to the hospital will lead to bankruptcy. That security has only increased over time, as Congress added prescription drug coverage and most recently, free preventive care. In 1972, the Medicare program was extended to the long-term disabled and those with end stage renal disease.
Medicare “fundamentally changed the way we thought about health in our nation for our seniors,” said Dr. Georges C. Benjamin, executive director of the American Public Health Association. “This probably was the most important tool to lift seniors out of poverty. The economic benefit of this, in addition to the health benefit, was enormous.”
Along with coverage of hospital stays and physician services for seniors under Medicare, the Social Security Act Amendments of 1965 also created Medicaid, a voluntary federal-state partnership to provide medical care and long-term care services to low-income Americans.
Changing medical practice
When combined with the Civil Rights Act of 1964, Medicare desegregated all hospitals that participated in the program. Prior to the start of Medicare on July 1, 1966, a large number of hospitals around the country were still racially segregated in some manner. In many areas of the South, a separate hospital system served the black community and were the only places where black physicians could train and practice (Milbank Q. 2005; 83: 247-69). But in a matter of months, hospitals across the country integrated their facilities and staff in a relative peaceful manner, according to a study conducted by the Commonwealth Fund.
“Now, physicians of color could not be kept out of hospitals as a matter of policy,” Dr. Benjamin said. “It dramatically improved practice opportunities for physicians and access to care opportunities for patients.”
Stuart Guterman, Ph.D., vice president for Medicare and cost control at the Commonwealth Fund, said it’s one of the lesser-known elements of Medicare’s history that the law became an impetus to overcoming racial barriers for both patients and physicians.
Changing payment incentives
Medicare payments opened up a significant new stream of revenue for hospitals that made desegregation more palatable. But hospitals weren’t the only ones who benefited financially from the new program.
Despite the American Medical Association’s fight against the creation of Medicare, during which officials famously decried the program as “socialized medicine,” physicians were financial winners under the new law, at least at first.
Much like hospitals, doctors got paid for the first time for treating patients who they had previously seen as charity care. And, in those early years of the Medicare program, the government paid on a charge basis. The result was that Medicare spending was quickly out of control.
Cost controls were added first for hospital care. In 1983, Medicare adopted the inpatient hospital prospective payment system, which replaced hospitals’ cost-based payments with the diagnosis-related group (DRG) system.
“Changing the notion of what hospitals did from each individual service or each individual day to the hospital stay, really changed the way hospital care was looked at,” Dr. Guterman said.
It took somewhat longer for federal health officials to tackle payment on the physician side. Throughout the 1980s, Congress froze physician payment rates in an effort to address rising costs. In 1984, Congress established the Participating Physicians’ Program, which required physicians to accept assignment for all Medicare patients and services rather than on a service-by-service basis. It also barred balance billing of Medicare beneficiaries.
In 1989, Congress made the most significant change yet to physician payment, creating the Resource-Based Relative Value Scale (RBRVS) under the Omnibus Budget Reconciliation Act. Medicare officials used the new RBRVS to establish a standardized physician payment schedule in 1992. Under the new system, payments were determined by calculating the costs of physician work, practice expenses, and professional liability insurance. Payments are then adjusted by a conversion factor and geographic cost differences.
“The impact of the fee schedule all depends on where you sit,” said Paul B. Ginsburg, Ph.D., director of health policy at the Schaeffer Center for Health Policy and Economics at the University of Southern California.
The fee schedule was positive for primary care physicians at first, but a flawed updating process over the decades led to differences in Medicare payments by specialty, largely favoring procedural specialists over those who billed for evaluation and management services.
But Dr. Ginsburg said Medicare is now on the cusp of an even more important payment change as federal officials begin to move away from fee-for-service and toward payment approaches that emphasize quality and value.
While some new payment model experiments have been promising, the attempts by the Centers for Medicare & Medicaid Services have been “fumbling,” Dr. Ginsburg said.
Changing physician satisfaction
Medicare beneficiaries are typically happier with their health coverage than individuals enrolled in private, employer-sponsored insurance and are less worried about financial barriers to care, Dr. Guterman said.
“I think it’s fair to say that it’s one of the more popular federal programs ever,” he said. “You hear people frequently talking about how touchy it is to make any changes to the program because people are very wary of having that benefit diluted.”
That’s not the case for physicians.
“The physicians I’ve talked to over the last 2 years feel like they’re drowning in red tape,” said Dr. Austin King, president of the Texas Medical Association and a head and neck surgeon in Abilene.
The result is that physicians are becoming employees in large physician practices or hospitals, or they are moving to set up boutique practices, Dr. King said. “Right now, the physicians are not real happy with the program.”
Dr. King predicted that physicians will continue to move out of the program, creating patient access issues, unless Congress gives them a way to recoup their costs, such as through the practice of balance billing.
Resolving issues like the looming annual cuts from the Sustainable Growth Rate formula and eliminating the Affordable Care Act’s Independent Payment Advisory Board would put some predictability back into the system, Dr. King said, adding that it’s not enough because it would still leave the numerous regulatory requirements, which many physicians “agonize” over.
It’s that kind of agony that keeps Dr. Jane Orient, a general internist in Tucson, Ariz., from participating in Medicare. Dr. Orient opted out of the program in 1990, around the time that Medicare began requiring that physicians file claims for payment. Previously, patients could pay her directly for services and be reimbursed by Medicare.
“I decided I just could not do that,” said Dr. Orient, executive director of the Association of American Physicians and Surgeons. “For me to file the claim and figure out what all those E&M guidelines meant, was just impossible. I certainly couldn’t do it with the staff I had.”
The fundamental problem, said Rep. Michael C. Burgess (R-Texas), cochair of the Congressional Health Caucus and an ob.gyn., is that patients get their care covered at a price they can afford, while Medicare’s payment to physicians doesn’t cover the cost of care.
“What has always been a bad situation is getting worse and worse with every passing year,” Rep. Burgess said.
Rep. Phil Roe (R-Tenn.), cochair of the GOP Doctors Caucus, said the problem for lawmakers is that they must fix the physician payment system before patient access is significantly affected. How much time do they have?
Rep. Roe, an ob.gyn., said physicians will hang on for as long as they can. But if Medicare continues to cut payments, an access crisis is coming.
If Medicare’s solution to their financing problem is to cut providers, he said, physicians will look for a way out of the program, and that will start with large-scale retirements of older physicians.
“They are all looking for the exit signs now,” Rep. Roe said. 
|
This is the first in a series of articles tracing the 50-year history of Medicare and its impact on physicians and patients. Future articles will focus on the public health impact of Medicare, the origins of the Sustainable Growth Rate formula, and the long-term financial viability of the program.
mschneider@frontlinemedcom.com
On Twitter @maryellenny
When President Lyndon B. Johnson signed Medicare into law on July 30, 1965, he vowed that the program would give seniors access to the “healing miracle of modern medicine” and save them from exhausting their savings when they became ill.
For the most part, the program has delivered on those bold promises. Americans over age 65 years are living longer, healthier lives, and they are doing so without fear that a trip to the hospital will lead to bankruptcy. That security has only increased over time, as Congress added prescription drug coverage and most recently, free preventive care. In 1972, the Medicare program was extended to the long-term disabled and those with end stage renal disease.
Medicare “fundamentally changed the way we thought about health in our nation for our seniors,” said Dr. Georges C. Benjamin, executive director of the American Public Health Association. “This probably was the most important tool to lift seniors out of poverty. The economic benefit of this, in addition to the health benefit, was enormous.”
Along with coverage of hospital stays and physician services for seniors under Medicare, the Social Security Act Amendments of 1965 also created Medicaid, a voluntary federal-state partnership to provide medical care and long-term care services to low-income Americans.
Changing medical practice
When combined with the Civil Rights Act of 1964, Medicare desegregated all hospitals that participated in the program. Prior to the start of Medicare on July 1, 1966, a large number of hospitals around the country were still racially segregated in some manner. In many areas of the South, a separate hospital system served the black community and were the only places where black physicians could train and practice (Milbank Q. 2005; 83: 247-69). But in a matter of months, hospitals across the country integrated their facilities and staff in a relative peaceful manner, according to a study conducted by the Commonwealth Fund.
“Now, physicians of color could not be kept out of hospitals as a matter of policy,” Dr. Benjamin said. “It dramatically improved practice opportunities for physicians and access to care opportunities for patients.”
Stuart Guterman, Ph.D., vice president for Medicare and cost control at the Commonwealth Fund, said it’s one of the lesser-known elements of Medicare’s history that the law became an impetus to overcoming racial barriers for both patients and physicians.
Changing payment incentives
Medicare payments opened up a significant new stream of revenue for hospitals that made desegregation more palatable. But hospitals weren’t the only ones who benefited financially from the new program.
Despite the American Medical Association’s fight against the creation of Medicare, during which officials famously decried the program as “socialized medicine,” physicians were financial winners under the new law, at least at first.
Much like hospitals, doctors got paid for the first time for treating patients who they had previously seen as charity care. And, in those early years of the Medicare program, the government paid on a charge basis. The result was that Medicare spending was quickly out of control.
Cost controls were added first for hospital care. In 1983, Medicare adopted the inpatient hospital prospective payment system, which replaced hospitals’ cost-based payments with the diagnosis-related group (DRG) system.
“Changing the notion of what hospitals did from each individual service or each individual day to the hospital stay, really changed the way hospital care was looked at,” Dr. Guterman said.
It took somewhat longer for federal health officials to tackle payment on the physician side. Throughout the 1980s, Congress froze physician payment rates in an effort to address rising costs. In 1984, Congress established the Participating Physicians’ Program, which required physicians to accept assignment for all Medicare patients and services rather than on a service-by-service basis. It also barred balance billing of Medicare beneficiaries.
In 1989, Congress made the most significant change yet to physician payment, creating the Resource-Based Relative Value Scale (RBRVS) under the Omnibus Budget Reconciliation Act. Medicare officials used the new RBRVS to establish a standardized physician payment schedule in 1992. Under the new system, payments were determined by calculating the costs of physician work, practice expenses, and professional liability insurance. Payments are then adjusted by a conversion factor and geographic cost differences.
“The impact of the fee schedule all depends on where you sit,” said Paul B. Ginsburg, Ph.D., director of health policy at the Schaeffer Center for Health Policy and Economics at the University of Southern California.
The fee schedule was positive for primary care physicians at first, but a flawed updating process over the decades led to differences in Medicare payments by specialty, largely favoring procedural specialists over those who billed for evaluation and management services.
But Dr. Ginsburg said Medicare is now on the cusp of an even more important payment change as federal officials begin to move away from fee-for-service and toward payment approaches that emphasize quality and value.
While some new payment model experiments have been promising, the attempts by the Centers for Medicare & Medicaid Services have been “fumbling,” Dr. Ginsburg said.
Changing physician satisfaction
Medicare beneficiaries are typically happier with their health coverage than individuals enrolled in private, employer-sponsored insurance and are less worried about financial barriers to care, Dr. Guterman said.
“I think it’s fair to say that it’s one of the more popular federal programs ever,” he said. “You hear people frequently talking about how touchy it is to make any changes to the program because people are very wary of having that benefit diluted.”
That’s not the case for physicians.
“The physicians I’ve talked to over the last 2 years feel like they’re drowning in red tape,” said Dr. Austin King, president of the Texas Medical Association and a head and neck surgeon in Abilene.
The result is that physicians are becoming employees in large physician practices or hospitals, or they are moving to set up boutique practices, Dr. King said. “Right now, the physicians are not real happy with the program.”
Dr. King predicted that physicians will continue to move out of the program, creating patient access issues, unless Congress gives them a way to recoup their costs, such as through the practice of balance billing.
Resolving issues like the looming annual cuts from the Sustainable Growth Rate formula and eliminating the Affordable Care Act’s Independent Payment Advisory Board would put some predictability back into the system, Dr. King said, adding that it’s not enough because it would still leave the numerous regulatory requirements, which many physicians “agonize” over.
It’s that kind of agony that keeps Dr. Jane Orient, a general internist in Tucson, Ariz., from participating in Medicare. Dr. Orient opted out of the program in 1990, around the time that Medicare began requiring that physicians file claims for payment. Previously, patients could pay her directly for services and be reimbursed by Medicare.
“I decided I just could not do that,” said Dr. Orient, executive director of the Association of American Physicians and Surgeons. “For me to file the claim and figure out what all those E&M guidelines meant, was just impossible. I certainly couldn’t do it with the staff I had.”
The fundamental problem, said Rep. Michael C. Burgess (R-Texas), cochair of the Congressional Health Caucus and an ob.gyn., is that patients get their care covered at a price they can afford, while Medicare’s payment to physicians doesn’t cover the cost of care.
“What has always been a bad situation is getting worse and worse with every passing year,” Rep. Burgess said.
Rep. Phil Roe (R-Tenn.), cochair of the GOP Doctors Caucus, said the problem for lawmakers is that they must fix the physician payment system before patient access is significantly affected. How much time do they have?
Rep. Roe, an ob.gyn., said physicians will hang on for as long as they can. But if Medicare continues to cut payments, an access crisis is coming.
If Medicare’s solution to their financing problem is to cut providers, he said, physicians will look for a way out of the program, and that will start with large-scale retirements of older physicians.
“They are all looking for the exit signs now,” Rep. Roe said. 
|
This is the first in a series of articles tracing the 50-year history of Medicare and its impact on physicians and patients. Future articles will focus on the public health impact of Medicare, the origins of the Sustainable Growth Rate formula, and the long-term financial viability of the program.
mschneider@frontlinemedcom.com
On Twitter @maryellenny
FDA approves IV antibacterial for complicated UTIs, abdominal infections
A combination of a cephalosporin and a beta-lactamase inhibitor in an intravenous formulation has been approved for treating complicated intra-abdominal infections and complicated urinary tract infections in adults, the Food and Drug Administration announced on Dec. 19.
The cephalosporin is ceftolozane and the beta-lactamase inhibitor is tazobactam; it will be marketed as Zerbaxa by Cubist Pharmaceuticals.
This is the fourth antibacterial drug product approved by the FDA in 2014 and, like the other three, it was designated as a Qualified Infectious Disease Product (QIDP) and was given priority review, according to the FDA statement. Zerbaxa was granted a QIDP designation under the Generating Antibiotic Incentives Now (GAIN) Act of the FDA Safety and Innovation Act, “because it is an antibacterial or antifungal human drug intended to treat a serious or life-threatening infection,” the statement said.
As part of the QIDP program, the manufacturer has also been granted an extra 5 years of “exclusivity” – exclusive marketing rights – by the FDA.
Ceftolozane-tazobactam was approved for treating complicated intra-abdominal infections in combination with metronidazole; approval for this indication was based on a study of 979 adults, randomized to the combination or to meropenem.
Approval for complicated urinary tract infections, including pyelonephritis, was based on a study of 1,068 adults, randomized to treatment with ceftolozane-tazobactam or levofloxacin.
The prescribing information includes a warning about decreased efficacy in patients with renal impairment (a baseline creatinine clearance of 30-50 mL/min or less, and the recommendation to monitor creatinine clearance “at least daily in patients with changing renal function,” and to adjust dose accordingly. Nausea, diarrhea, headache, and fever were the most common adverse events in studies, according to the FDA statement.
The other antibacterials approved by the FDA in 2014 were approved for treating acute bacterial skin and skin structure infections caused by certain susceptible bacteria. They were dalbavancin (Dalvance), approved in May; tedizolid (Sivextro), approved in June; and oritavancin (Orbactiv), approved in August.
Serious adverse events associated with Zerbaxa should be reported to the FDA’s MedWatch program at 800-332-1088 or http://www.fda.gov/Safety/MedWatch/HowToReport/default.htm.
A combination of a cephalosporin and a beta-lactamase inhibitor in an intravenous formulation has been approved for treating complicated intra-abdominal infections and complicated urinary tract infections in adults, the Food and Drug Administration announced on Dec. 19.
The cephalosporin is ceftolozane and the beta-lactamase inhibitor is tazobactam; it will be marketed as Zerbaxa by Cubist Pharmaceuticals.
This is the fourth antibacterial drug product approved by the FDA in 2014 and, like the other three, it was designated as a Qualified Infectious Disease Product (QIDP) and was given priority review, according to the FDA statement. Zerbaxa was granted a QIDP designation under the Generating Antibiotic Incentives Now (GAIN) Act of the FDA Safety and Innovation Act, “because it is an antibacterial or antifungal human drug intended to treat a serious or life-threatening infection,” the statement said.
As part of the QIDP program, the manufacturer has also been granted an extra 5 years of “exclusivity” – exclusive marketing rights – by the FDA.
Ceftolozane-tazobactam was approved for treating complicated intra-abdominal infections in combination with metronidazole; approval for this indication was based on a study of 979 adults, randomized to the combination or to meropenem.
Approval for complicated urinary tract infections, including pyelonephritis, was based on a study of 1,068 adults, randomized to treatment with ceftolozane-tazobactam or levofloxacin.
The prescribing information includes a warning about decreased efficacy in patients with renal impairment (a baseline creatinine clearance of 30-50 mL/min or less, and the recommendation to monitor creatinine clearance “at least daily in patients with changing renal function,” and to adjust dose accordingly. Nausea, diarrhea, headache, and fever were the most common adverse events in studies, according to the FDA statement.
The other antibacterials approved by the FDA in 2014 were approved for treating acute bacterial skin and skin structure infections caused by certain susceptible bacteria. They were dalbavancin (Dalvance), approved in May; tedizolid (Sivextro), approved in June; and oritavancin (Orbactiv), approved in August.
Serious adverse events associated with Zerbaxa should be reported to the FDA’s MedWatch program at 800-332-1088 or http://www.fda.gov/Safety/MedWatch/HowToReport/default.htm.
A combination of a cephalosporin and a beta-lactamase inhibitor in an intravenous formulation has been approved for treating complicated intra-abdominal infections and complicated urinary tract infections in adults, the Food and Drug Administration announced on Dec. 19.
The cephalosporin is ceftolozane and the beta-lactamase inhibitor is tazobactam; it will be marketed as Zerbaxa by Cubist Pharmaceuticals.
This is the fourth antibacterial drug product approved by the FDA in 2014 and, like the other three, it was designated as a Qualified Infectious Disease Product (QIDP) and was given priority review, according to the FDA statement. Zerbaxa was granted a QIDP designation under the Generating Antibiotic Incentives Now (GAIN) Act of the FDA Safety and Innovation Act, “because it is an antibacterial or antifungal human drug intended to treat a serious or life-threatening infection,” the statement said.
As part of the QIDP program, the manufacturer has also been granted an extra 5 years of “exclusivity” – exclusive marketing rights – by the FDA.
Ceftolozane-tazobactam was approved for treating complicated intra-abdominal infections in combination with metronidazole; approval for this indication was based on a study of 979 adults, randomized to the combination or to meropenem.
Approval for complicated urinary tract infections, including pyelonephritis, was based on a study of 1,068 adults, randomized to treatment with ceftolozane-tazobactam or levofloxacin.
The prescribing information includes a warning about decreased efficacy in patients with renal impairment (a baseline creatinine clearance of 30-50 mL/min or less, and the recommendation to monitor creatinine clearance “at least daily in patients with changing renal function,” and to adjust dose accordingly. Nausea, diarrhea, headache, and fever were the most common adverse events in studies, according to the FDA statement.
The other antibacterials approved by the FDA in 2014 were approved for treating acute bacterial skin and skin structure infections caused by certain susceptible bacteria. They were dalbavancin (Dalvance), approved in May; tedizolid (Sivextro), approved in June; and oritavancin (Orbactiv), approved in August.
Serious adverse events associated with Zerbaxa should be reported to the FDA’s MedWatch program at 800-332-1088 or http://www.fda.gov/Safety/MedWatch/HowToReport/default.htm.
Feds release quality data on diabetes, heart disease, infections
Medicare officials have released a slew of new data demonstrating physicians’ and hospitals’ performance on quality measures related to diabetes, cardiovascular care, and hospital-acquired infections and injuries.
The quality data were posted on Medicare’s Physician Compare and Hospital Compare websites, which aim to provide consumers with the information they need to help them select physicians and other health care providers. The information is also available on data.medicare.gov.
“The Compare sites empower consumers with information to help with health care decisions, encourage providers to strive for higher levels of quality, drive overall health system improvement,” Dr. Patrick Conway, chief medical officer and deputy administrator for innovation and quality at the Center for Medicare & Medicaid Services, wrote in a blog post.
For physicians, CMS is posting four measures related to diabetes and heart disease: controlling hemoglobin A1c (less than 8%); controlling blood pressure; prescribing aspirin to patients with diabetes and ischemic vascular disease; and prescribing ACE inhibitors or angiotensin receptor blockers for patients with coronary artery disease and diabetes or left ventricular systolic dysfunction.
The data come from 139 physician group practices, 214 shared savings program accountable care organizations, and 23 Pioneer ACOs.
To make the data more user-friendly, CMS uses stars, followed by a percentage score, to show group performance on individual measures. For instance, if a practice scored 80% on a measure, four stars would be shown along with the 80% score.
This is the second time that CMS officials have added quality data to Physician Compare – the first posting was in February 2014. CMS plans to expand the number of quality measures and the number of providers who are listed on the website. By late 2015, CMS plans to post quality data for group practices of all sizes and some data on individual physicians.
For hospitals, CMS posted performance on reducing hospital-acquired conditions, including central line–associated bloodstream infections, catheter-associated urinary tract infections, pressure ulcers, and accidental punctures and lacerations.
The agency also added data from its Hospital Readmissions Reduction Program, including 30-day readmission rates following elective and primary total hip and/or total knee replacement and the 30-day rate of unplanned readmission for chronic obstructive pulmonary disease.
CMS also released data on payment adjustments made as part of the 2015 Hospital Value-Based Purchasing Program, which ties payment to performance on certain quality measures. In fiscal year 2015 – the third year of the program – 1,714 hospitals will see their payments go up as a result of bonus payments from the program, compared with 1,375 that will see their payments decline, according to CMS.
mschneider@frontlinemedcom.com
On Twitter @maryellenny
Medicare officials have released a slew of new data demonstrating physicians’ and hospitals’ performance on quality measures related to diabetes, cardiovascular care, and hospital-acquired infections and injuries.
The quality data were posted on Medicare’s Physician Compare and Hospital Compare websites, which aim to provide consumers with the information they need to help them select physicians and other health care providers. The information is also available on data.medicare.gov.
“The Compare sites empower consumers with information to help with health care decisions, encourage providers to strive for higher levels of quality, drive overall health system improvement,” Dr. Patrick Conway, chief medical officer and deputy administrator for innovation and quality at the Center for Medicare & Medicaid Services, wrote in a blog post.
For physicians, CMS is posting four measures related to diabetes and heart disease: controlling hemoglobin A1c (less than 8%); controlling blood pressure; prescribing aspirin to patients with diabetes and ischemic vascular disease; and prescribing ACE inhibitors or angiotensin receptor blockers for patients with coronary artery disease and diabetes or left ventricular systolic dysfunction.
The data come from 139 physician group practices, 214 shared savings program accountable care organizations, and 23 Pioneer ACOs.
To make the data more user-friendly, CMS uses stars, followed by a percentage score, to show group performance on individual measures. For instance, if a practice scored 80% on a measure, four stars would be shown along with the 80% score.
This is the second time that CMS officials have added quality data to Physician Compare – the first posting was in February 2014. CMS plans to expand the number of quality measures and the number of providers who are listed on the website. By late 2015, CMS plans to post quality data for group practices of all sizes and some data on individual physicians.
For hospitals, CMS posted performance on reducing hospital-acquired conditions, including central line–associated bloodstream infections, catheter-associated urinary tract infections, pressure ulcers, and accidental punctures and lacerations.
The agency also added data from its Hospital Readmissions Reduction Program, including 30-day readmission rates following elective and primary total hip and/or total knee replacement and the 30-day rate of unplanned readmission for chronic obstructive pulmonary disease.
CMS also released data on payment adjustments made as part of the 2015 Hospital Value-Based Purchasing Program, which ties payment to performance on certain quality measures. In fiscal year 2015 – the third year of the program – 1,714 hospitals will see their payments go up as a result of bonus payments from the program, compared with 1,375 that will see their payments decline, according to CMS.
mschneider@frontlinemedcom.com
On Twitter @maryellenny
Medicare officials have released a slew of new data demonstrating physicians’ and hospitals’ performance on quality measures related to diabetes, cardiovascular care, and hospital-acquired infections and injuries.
The quality data were posted on Medicare’s Physician Compare and Hospital Compare websites, which aim to provide consumers with the information they need to help them select physicians and other health care providers. The information is also available on data.medicare.gov.
“The Compare sites empower consumers with information to help with health care decisions, encourage providers to strive for higher levels of quality, drive overall health system improvement,” Dr. Patrick Conway, chief medical officer and deputy administrator for innovation and quality at the Center for Medicare & Medicaid Services, wrote in a blog post.
For physicians, CMS is posting four measures related to diabetes and heart disease: controlling hemoglobin A1c (less than 8%); controlling blood pressure; prescribing aspirin to patients with diabetes and ischemic vascular disease; and prescribing ACE inhibitors or angiotensin receptor blockers for patients with coronary artery disease and diabetes or left ventricular systolic dysfunction.
The data come from 139 physician group practices, 214 shared savings program accountable care organizations, and 23 Pioneer ACOs.
To make the data more user-friendly, CMS uses stars, followed by a percentage score, to show group performance on individual measures. For instance, if a practice scored 80% on a measure, four stars would be shown along with the 80% score.
This is the second time that CMS officials have added quality data to Physician Compare – the first posting was in February 2014. CMS plans to expand the number of quality measures and the number of providers who are listed on the website. By late 2015, CMS plans to post quality data for group practices of all sizes and some data on individual physicians.
For hospitals, CMS posted performance on reducing hospital-acquired conditions, including central line–associated bloodstream infections, catheter-associated urinary tract infections, pressure ulcers, and accidental punctures and lacerations.
The agency also added data from its Hospital Readmissions Reduction Program, including 30-day readmission rates following elective and primary total hip and/or total knee replacement and the 30-day rate of unplanned readmission for chronic obstructive pulmonary disease.
CMS also released data on payment adjustments made as part of the 2015 Hospital Value-Based Purchasing Program, which ties payment to performance on certain quality measures. In fiscal year 2015 – the third year of the program – 1,714 hospitals will see their payments go up as a result of bonus payments from the program, compared with 1,375 that will see their payments decline, according to CMS.
mschneider@frontlinemedcom.com
On Twitter @maryellenny
FDA: Ease ban on blood donation by MSM
In 2015, the Food and Drug Administration will issue a draft guidance recommending that men who have sex with men can donate blood 1 year after their last sexual contact with another man, replacing the longstanding policy that this group be deferred from donating blood indefinitely, the agency announced on Dec. 23.
The recommendation, under consideration for several years, is based on available scientific evidence, including epidemiologic data and statistical modeling of the impact of this policy change would have on blood supply safety; as well as recommendations of FDA and Department of Health and Human Services advisory committees, Dr. Peter Marks, deputy director of the FDA Center for Biologics Evaluation and Research, said during a press conference.
“Some of the most compelling data in favor of policy change come from Australia,” where HIV epidemiology is similar to that in the United States, Dr. Marks said.
Studies using a national blood surveillance system during the decade after the policy was changed in Australia from indefinite deferral of men who have sex with men (MSM) to 1 year deferral, as proposed in the United States, “documented no adverse effects” on the safety of the country’s blood supply, Dr. Marks said.
The FDA has also been working with the National Heart, Lung, and Blood Institute to implement a surveillance system to monitor the effect of the policy change on the safety of the blood supply. As the data are collected, the policy will be revisited, Dr. Marks said. The proposed policy change will also better align with the blood donor deferral period recommended for other men and women at increased risk of HIV infection, he noted.
Dr. Marks said that the currently available scientific data do not support shortening the deferral period to less than 1 year.
At a meeting in November, the federal Advisory Committee on Blood and Tissue Safety and Availability voted 16-2 to support the 1 year deferral policy for MSM.
The draft guidance will be published in the Federal Register in 2015 and will allow the public to submit comments. Dr. Marks said he could not predict whether the actual policy change would go into effect in 2015.
In 2015, the Food and Drug Administration will issue a draft guidance recommending that men who have sex with men can donate blood 1 year after their last sexual contact with another man, replacing the longstanding policy that this group be deferred from donating blood indefinitely, the agency announced on Dec. 23.
The recommendation, under consideration for several years, is based on available scientific evidence, including epidemiologic data and statistical modeling of the impact of this policy change would have on blood supply safety; as well as recommendations of FDA and Department of Health and Human Services advisory committees, Dr. Peter Marks, deputy director of the FDA Center for Biologics Evaluation and Research, said during a press conference.
“Some of the most compelling data in favor of policy change come from Australia,” where HIV epidemiology is similar to that in the United States, Dr. Marks said.
Studies using a national blood surveillance system during the decade after the policy was changed in Australia from indefinite deferral of men who have sex with men (MSM) to 1 year deferral, as proposed in the United States, “documented no adverse effects” on the safety of the country’s blood supply, Dr. Marks said.
The FDA has also been working with the National Heart, Lung, and Blood Institute to implement a surveillance system to monitor the effect of the policy change on the safety of the blood supply. As the data are collected, the policy will be revisited, Dr. Marks said. The proposed policy change will also better align with the blood donor deferral period recommended for other men and women at increased risk of HIV infection, he noted.
Dr. Marks said that the currently available scientific data do not support shortening the deferral period to less than 1 year.
At a meeting in November, the federal Advisory Committee on Blood and Tissue Safety and Availability voted 16-2 to support the 1 year deferral policy for MSM.
The draft guidance will be published in the Federal Register in 2015 and will allow the public to submit comments. Dr. Marks said he could not predict whether the actual policy change would go into effect in 2015.
In 2015, the Food and Drug Administration will issue a draft guidance recommending that men who have sex with men can donate blood 1 year after their last sexual contact with another man, replacing the longstanding policy that this group be deferred from donating blood indefinitely, the agency announced on Dec. 23.
The recommendation, under consideration for several years, is based on available scientific evidence, including epidemiologic data and statistical modeling of the impact of this policy change would have on blood supply safety; as well as recommendations of FDA and Department of Health and Human Services advisory committees, Dr. Peter Marks, deputy director of the FDA Center for Biologics Evaluation and Research, said during a press conference.
“Some of the most compelling data in favor of policy change come from Australia,” where HIV epidemiology is similar to that in the United States, Dr. Marks said.
Studies using a national blood surveillance system during the decade after the policy was changed in Australia from indefinite deferral of men who have sex with men (MSM) to 1 year deferral, as proposed in the United States, “documented no adverse effects” on the safety of the country’s blood supply, Dr. Marks said.
The FDA has also been working with the National Heart, Lung, and Blood Institute to implement a surveillance system to monitor the effect of the policy change on the safety of the blood supply. As the data are collected, the policy will be revisited, Dr. Marks said. The proposed policy change will also better align with the blood donor deferral period recommended for other men and women at increased risk of HIV infection, he noted.
Dr. Marks said that the currently available scientific data do not support shortening the deferral period to less than 1 year.
At a meeting in November, the federal Advisory Committee on Blood and Tissue Safety and Availability voted 16-2 to support the 1 year deferral policy for MSM.
The draft guidance will be published in the Federal Register in 2015 and will allow the public to submit comments. Dr. Marks said he could not predict whether the actual policy change would go into effect in 2015.