User login
Crisis Mode and Information Exchange
Using electronic health records to improve the continuity of care between hospital units does not replace the need for interpersonal communication to improve transitions of care. Hospital personnel play a critical role in accurately exchanging patient information during patient transfers, a process requiring accurate communication between hospital units to prevent system failures.[1] Because poor communication contributes to preventable adverse events,[2] and effective communication during handoffs decreases medical errors and readmissions,[3] hospitals need to ensure their work environments are conducive to effective communication.
Individuals working under time constraints and heavy workloads could potentially be at high risk of misinterpreting or delivering inaccurate information,[4] partially due to limited ability to accurately process and communicate information under stressful circumstances. Furthermore, because time‐constrained decision makers tend to use less information and less rigorous decision strategies,[5] work climates characterized by staff members doing too many things too quickly could cause patient health information to be lost during transitions of care across hospital units.
Current studies illustrate scenarios in which demanding or time‐constrained work environments caused information exchange errors. One study found that the increased rate of prescribing errors was partially attributed to a high‐demand work environment characterized by working after hours and multitasking.[6] Other studies found that clinicians' limited time to relay and respond to information and ask clarifying questions during patient handoffs was partially attributed to the fast‐faced and chaotic environment of the emergency room.[7, 8] These studies are consistent with another study that found patient handoffs between emergency departments and inpatient wards were inadequate, partially due to less interactive and more rushed communication.[9] The fact that communication breakdowns are widely cited as barriers to patient handoffs[7, 8, 10] and facilitators of medical errors,[7, 8] further underscores the detrimental effect that crisis mode work climates could have on exchanging patient information during transitions of care.
The objective of this analysis was to evaluate the extent to which a crisis mode work climate impacts the occurrence of patient information exchange problems. Estimating associations between hospital staff members' perceptions of crisis mode work climates and perceptions of information exchange problems provide insights as to whether high‐demand and time‐constrained work climates negatively impact the exchange of patient information. Because hospital staff members working under time constraints and heavy workloads could potentially be at high risk of misinterpreting or delivering inaccurate information, we hypothesized that higher levels of a perceived crisis mode work climate would be associated with higher levels of perceived problems with information exchange across hospital units.
METHODS
Data Source
Data originated from the Agency of Healthcare Research and Quality 2010 Hospital Survey on Patient Safety Culture. This validated survey, designed to assess the safety climate within acute‐care settings, remains an important annual survey deployed each year to track changes and factors impacting patient safety.[11] We included only those respondents who self‐reported their position as a nurse, physician, pharmacist, dietician, therapist, technician, patient care assistant, or hospital unit secretary, all of whom are likely responsible for exchanging patient information across hospital units. For this reason, we excluded respondents who self‐reported their position as administrative or miscellaneous. Applying these exclusion criteria resulted in 247,104 respondents across 884 hospitals.
Conceptual Framework
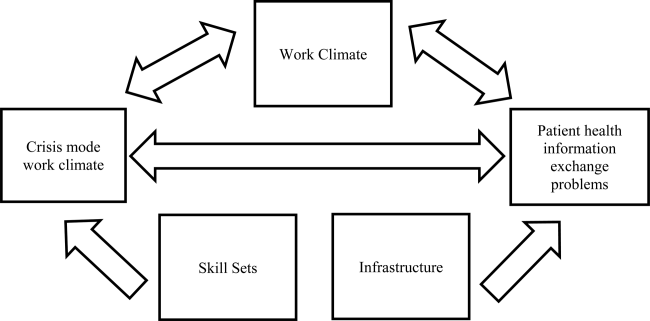
The relationship between perceived crisis mode work climates and patient information exchange problems is likely influenced by staff skill levels, work climate, and infrastructure (Figure 1). With respect to skill levels, hospital staff members with many years of experience compared to those with fewer years may be relatively desensitized to chaotic work environments and consequently have higher thresholds for perceiving crisis modes. Number of hours worked per week likely impacts perceived crisis mode as illustrated in 1 study finding that full‐time nurses reported a significantly lower work pace compared to part‐time nurses.[12] Years of experience likely impacts perception of information exchange problems, particularly if staff members with many years of experience are familiar enough with hospital systems or protocols to easily detect exchange errors or mishaps.
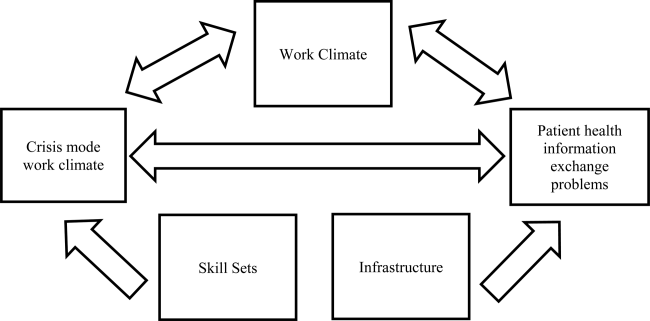
With respect to work climates, workers' perception of cooperation, coordination and patient safety, and specific hospital unit likely impact perceptions of crisis work mode and information exchange problems. For example, hospital staff members reporting high levels of cooperation, coordination, and patient safety likely perceive fewer crisis modes and information exchange problems compared to those in less‐cooperative hospital units. Furthermore, the heterogeneity of work cultures across departments within a hospital results in department‐specific perceptions of crisis mode climates and information exchange problems. Infrastructure factors, such as hospital size, teaching and ownership status, and census region, likely impact the amount of resources available for staffing and infrastructure, which in turn could impact work pace and information exchange accuracy.
Variable Definitions
We defined our predictor as the perceived presence of a crisis mode work climate as captured from the survey questionnaire item: We work in crisis mode trying to do too much, too quickly. This question item had a Likert response scale comprised of the following 5 answer choices: (1) strongly disagree, (2) disagree, (3) neutral, (4) agree, (5) strongly agree. We created a 3‐level response variable by aggregating the agree and disagree responses, respectively, as the first 2 levels, and retaining the neutral response as the third level. Consequently, those responding strongly disagree or disagree were classified as working in lowcrisis mode work climates and those responding strongly agree or agree were classified as working in high crisis mode work climates. We defined our outcome measure as the presence of patient information exchange problems as captured from the survey questionnaire item: Problems often occur in the exchange of information across hospital units. Because this question item had a Likert response scale similar to the crisis mode question predictor, we also created a 3‐level categorical variable in the same fashion. Consequently, those responding strongly disagree or disagree were classified as perceiving no problems exchanging patient information, and those responding strongly agree or agree were classified as perceiving problems exchanging patient information. For the fewer than 10% of the respondents with missing data on either the predictor our outcome variables, the mode measure of central tendency was imputed, a methodology validated in a previous study.[13]
We also included questionnaire items that captured staff skill levels, work climate, and infrastructure as covariates to account for potential confounders (Figure 1). The staff skill levels domain included years of experience working in the hospital, specialty, and unit; current staff position; and extent of patient contact. The work climate domain included respondent perceptions of coordination and cooperation, patient safety, and primary work area or unit in which the provider reported working. The hospital infrastructure domain included bed size, census region, teaching status, and government ownership status. For the fewer than 10% of the respondents with missing data on any of the categorical variables, the mode measure of central tendency was imputed, a methodology validated in a previous study.[13]
Analytic Approach
We used multivariable ordinal regressions to estimate the likelihood of perceived problems in patient information exchange conditional upon perceptions of a crisis mode work climate, controlling for staff skill levels, work climate, and hospital infrastructure. Our estimates therefore reflect the likelihood of hospital staff responding strongly agree or agree to the question Problems often occur in the exchange of information across hospital units conditional upon responding strongly agree or agree to the question We work in crisis mode trying to do too much, too quickly. In addition to controlling for hospital‐specific response rates, we also adjusted our standard errors to account for the clustering of respondents within hospitals. All analyses were conducted in SAS version 9.2 (SAS Institute Inc., Cary, NC).
RESULTS
The hospital sample averaged 279 respondents per hospital with a 56% response rate. Most hospitals were located in the Central region of the United States, and 32% and 19% were teaching and government‐owned hospitals, respectively. Forty‐three percent and 44% of the hospitals in the sample were designated as small and medium hospitals, respectively (Table 1).
| Characteristics | % |
|---|---|
| |
| Hospital characteristics, N=884 | |
| Bed size | |
| Small, 199 | 43.5 |
| Medium, 100399 | 43.8 |
| Large, 400 plus | 12.7 |
| Teaching status | |
| Yes | 32.2 |
| No | 67.8 |
| Government ownership | |
| Yes | 19.5 |
| No | 80.5 |
| Census region | |
| Mid‐Atlantic and New England | 8.7 |
| South Atlantic | 14.8 |
| Central | 57.2 |
| Mountain | 7.7 |
| Pacific | 11.5 |
| Response rate, mean (SD) | 0.56 (0.28) |
| Respondents per hospital, mean (SD) | 279 (358) |
| Respondent characteristics, N=274,140 | |
| How long have you worked in your current specialty or profession? | |
| <1 year | 5.8 |
| 15 years | 32.8 |
| 610 years | 16.2 |
| 1115 years | 12.0 |
| 1620 years | 10.6 |
| 21 years | 22.7 |
| How long have you worked in this hospital? | |
| <1 year | 9.8 |
| 15 years | 42.8 |
| 610 years | 17.8 |
| 1115 years | 9.0 |
| 1620 years | 8.2 |
| 21 years | 12.4 |
| How long have you worked in your current hospital work area/unit? | |
| <1 year | 13.1 |
| 15 years | 48.0 |
| 610 years | 18.1 |
| 1115 years | 8.1 |
| 1620 years | 6.0 |
| 21 years | 6.7 |
| Typically, how many hours per week do you work in this hospital? | |
| <20 hours | 4.8 |
| 2039 hours | 39.9 |
| 4059 hours | 48.8 |
| 6079 hours | 4.2 |
| 8099 hours | 2.1 |
| 100 hours | 0.11 |
| What is your staff position in this hospital? | |
| Registered nurse | 51.2 |
| Technician (eg, ECG, lab, radiology) | 14.1 |
| Unit assistant/clerk/secretary | 8.5 |
| Patient care assistant/hospital aide/care partner | 7.4 |
| Physical, occupational, or speech therapist | 3.7 |
| Attending/staff physician | 3.5 |
| LVN/LPN | 3.0 |
| Respiratory therapist | 2.9 |
| Pharmacist | 2.2 |
| Physician assistant/nurse practitioner | 1.4 |
| Resident physician/physician in training | 1.2 |
| Dietician | 0.83 |
| In your staff position, do you typically have direct interaction or contact with patients? | |
| Yes | 86.6 |
| No | 13.4 |
| What is your primary work area or unit in this hospital? | |
| Other | 27.7 |
| Medicine (nonsurgical) | 11.1 |
| Surgery | 10.0 |
| Intensive care unit (any type) | 8.6 |
| Many different hospital units/no specific unit | 6.8 |
| Radiology | 6.2 |
| Emergency department | 5.8 |
| Obstetrics | 4.9 |
| Laboratory | 4.9 |
| Rehabilitation | 4.2 |
| Pediatrics | 3.8 |
| Pharmacy | 3.2 |
| Psychiatry/mental health | 2.1 |
| Anesthesiology | 0.55 |
Thirty‐seven percent of the respondents have worked in their current specialty or profession for 5 years or less (Table 1). Over half of the respondents have worked in their current hospital for 5 years or less, whereas 61% have worked in their current unit within the hospital for 5 years or less. Forty‐nine percent work at least 40 hours per week. Registered nurses and technicians represented the 2 largest subgroups of staff positions, comprising 51% and 14% of the sample, respectively. Dieticians and resident physicians, on the other hand, represented the 2 smallest subgroups of staff positions, comprising 0.83% and 1.2% of the sample, respectively. Eighty‐seven percent of the respondents have direct interaction or contact with patients. Apart from those responding other as their hospital unit, nonsurgical medicine and surgery represented the largest subgroup primary work areas, comprising 11% and 10% of the sample, respectively. In contrast, psychiatry and anesthesiology represented the 2 smallest subgroups of primary work areas, comprising 2.1% and 0.55% of the sample, respectively (Table 1).
Respondents scored relatively high with regard to teamwork and helping each other out under hurried or busy circumstances. For example, 85% agreed or strongly agreed that their unit worked together as a team to get work done when a lot of work needed to be completed quickly, and 68% agreed or strongly agreed that individuals within their unit helped out when an area in their unit became busy (Table 1). Despite this cooperation, 31% agreed or strongly agreed that hospital units did not coordinate well together. Paradoxically, 57% agreed or strongly agreed that there was good cooperation among hospital units that needed to work together. Seventy‐five percent of the respondents reported excellent or very good patient safety levels within their unit, although 53% agreed or strongly agreed that staff worked longer hours than was best for patient care (Table 1).
With regard to perceived crisis mode work climate, 32% and 47% reported agreeing and disagreeing, respectively, that their work unit worked in crisis mode trying to do too much too quickly (Table 2). With regard to perceived problems with patient information exchange, 27% and 36% reported agreeing and disagreeing, respectively, that information exchange problems occurred across hospital units (Table 2).
| Perceptions | % |
|---|---|
| We work in crisis mode trying to do too much, too quickly | |
| Strongly disagree | 8.1 |
| Disagree | 39.2 |
| Neutral | 21.0 |
| Agree | 24.3 |
| Strongly agree | 7.5 |
| Problems often occur in the exchange of information across hospital units | |
| Strongly disagree | 4.6 |
| Disagree | 31.3 |
| Neutral | 37.3 |
| Agree | 24.0 |
| Strongly agree | 2.7 |
| When a lot of work needs to be done quickly, we work together as a team to get the work done. | |
| Strongly disagree | 1.5 |
| Disagree | 6.1 |
| Neutral | 7.5 |
| Agree | 53.6 |
| Strongly agree | 31.2 |
| When one area in this unit gets really busy, others help out. | |
| Strongly disagree | 3.9 |
| Disagree | 13.9 |
| Neutral | 13.7 |
| Agree | 52.6 |
| Strongly agree | 15.8 |
| Hospital units do not coordinate well with each other. | |
| Strongly disagree | 5.6 |
| Disagree | 38.8 |
| Neutral | 23.7 |
| Agree | 25.3 |
| Strongly agree | 6.6 |
| There is good cooperation among hospital units that need to work together. | |
| Strongly disagree | 2.7 |
| Disagree | 15.1 |
| Neutral | 24.7 |
| Agree | 51.1 |
| Strongly agree | 6.3 |
| Please give your work area/unit in this hospital an overall grade on patient safety. | |
| Excellent | 23.0 |
| Very good | 49.8 |
| Acceptable | 21.8 |
| Poor | 4.6 |
| Failing | 0.76 |
| Staff in this unit work longer hours than is best for patient care. | |
| Strongly disagree | 11.5 |
| Disagree | 42.2 |
| Neutral | 23.6 |
| Agree | 18.4 |
| Strongly agree | 6.3 |
In the unadjusted analyses, crisis mode perceptions and information exchange problem perceptions were significantly associated. Among those who agreed that their work unit worked in crisis mode, a larger proportion of respondents agreed (41%) versus disagreed (24%) that problems often occurred in exchanging patient information across units (Table 3). In contrast, among those who disagreed that their work unit worked in crisis mode, a larger proportion of respondents disagreed (47%) versus agreed (19%) that problems often occurred in exchanging patient information across units (Table 3).
| Problems Often Occur in Exchange of Information Across Hospital Units | |||
|---|---|---|---|
| Agree (N=66,115), Row %* | Neutral (N=92,228), Row % | Disagree (N=88,797), Row % | |
| |||
| Crisis Mode Work Climate | |||
| Agree (N=78,253) | 40.8 | 35.4 | 23.8 |
| Neutral (N=51,836)‖ | 22.9 | 48.9 | 28.2 |
| Disagree (N=116,781) | 19.0 | 33.5 | 47.5 |
In the multivariable ordinal regression, compared to those who disagreed that their unit worked in crisis mode, those who agreed were 1.6 times more likely to report that problems often occurred in exchanging patient information across units (odds ratio [OR]: 1.6, 95% confidence interval [CI]: 1.58‐1.65) (Table 4). Additionally, some key covariates were independently associated with perceptions of information exchange problems. Two of these covariates measured workplace coordination. Those who reported that hospital units did not cooperate well together were more likely to report problematic information exchange compared to those who reported that hospital units did cooperate well (OR: 4.7, 95% CI: 4.35.0). Relatedly, those who reported that hospital units did coordinate well were less likely to report problematic information exchange compared to those who reported that hospital units did not coordinate well (OR: 0.10, 95% CI: 0.10‐0.11). Two other covariates measured patient contact and perceptions about long working hours. Those who reported having direct interaction or contact with patients were less likely to report problematic information exchange compared to those who reported not having direct interaction or contact with patients (OR: 0.85, 95% CI: 0.83‐0.87). Those who reported that staff did not work longer hours than was better for patient care were less likely to report problematic information exchange compared to those who did report working longer hours than was better for patient care (OR: 0.76, 95% CI: 0.73 0.79). One covariate measured hospital size. Those who reported working in smaller hospitals were less likely to report problematic information exchange compared to those reporting working in large hospitals (OR: 0.66, 95% CI 0.59‐0.75) (Table 4).
| Characteristic | Unadjusted OR (95% CI) | Adjusted OR* (95% CI) |
|---|---|---|
| ||
| Primary predictor of interest | ||
| Crisis mode work climate | ||
| Agree | 3.0 (2.9‐3.1) | 1.6 (1.5‐1.6) |
| Neutral | 1.8 (1.7‐1.8) | 1.3 (1.2‐1.3) |
| Disagree | Reference | Reference |
| Hospital characteristics | ||
| Bed Size | ||
| Small, 624 | 0.51 (0.44‐0.59) | 0.66 (0.59‐0.75) |
| Small, 249 | 0.59 (0.53‐0.66) | 0.77 (0.70‐0.84) |
| Small, 5099 | 0.65 (0.58‐0.73) | 0.78 (0.71‐0.84) |
| Medium, 100199 | 0.85 (0.77‐0.95) | 0.92 (0.86‐1.0) |
| Medium, 200299 | 1.0 (0.98‐1.1) | 0.97 (0.90‐1.0) |
| Medium, 300399 | 0.96 (0.85‐1.1) | 1.0 (0.92‐1.1) |
| Large, 400499 | 0.99 (0.86‐1.1) | 0.96 (0.87‐1.0) |
| Large, 500 plus | Reference | Reference |
| Teaching status | ||
| No | 0.81 (0.76‐0.87) | 1.0 (0.95‐1.0) |
| Yes | Reference | Reference |
| Government ownership | ||
| No | 1.1 (1.01.2) | 1.0 (0.98‐1.1) |
| Yes | Reference | Reference |
| Census region | ||
| Mid‐Atlantic and New England | 1.0 (0.88‐1.1) | 0.91 (0.84‐0.99) |
| South Atlantic | 0.95 (0.85‐1.1) | 1.0 (0.95‐1.1) |
| Central 1 | 0.95 (0.85‐1.0) | 0.95 (0.89‐1.0) |
| Central 2 | 0.71 (0.62‐0.81) | 0.91 (0.83‐0.99) |
| Central 3 | 0.80 (0.71‐0.91) | 0.97 (0.90‐1.0) |
| Central 4 | 0.76 (0.68‐0.86) | 0.93 (0.85‐1.0) |
| Mountain | 0.84 (0.73‐0.96) | 0.98 (0.90‐1.1) |
| Pacific | Reference | Reference |
| Average survey response rate within hospital | 0.65 (0.58‐0.72) | 0.93 (0.82‐1.0) |
| Respondent characteristics | ||
| How long have you worked in your current specialty or profession? | ||
| <1 year | 0.75 (0.73‐0.78) | 1.03 (0.99‐1.1) |
| 15 years | 0.99 (0.97‐1.0) | 1.1 (1.1‐1.1) |
| 610 years | 1.0 (1.01.1) | 0.99 (0.96‐1.0) |
| 1115 years | 1.0 (1.01.1) | 1.0 (0.97‐1.0) |
| 1620 years | 1.0 (0.98‐1.0) | 0.97 (0.94‐1.0) |
| 21 years | Reference | Reference |
| How long have you worked in this hospital? | ||
| <1 year | 0.75 (0.73‐0.77) | 0.90 (0.85‐0.90) |
| 15 years | 1.03 (1.001.05) | 0.99 (0.95‐1.0) |
| 610 years | 1.1 (1.1‐1.1) | 0.99 (0.95‐1.0) |
| 1115 years | 1.1 (1. 01.1) | 1.0 (0.96‐1.0) |
| 1620 years | 1.1 (1.01.1) | 0.98 (0.94‐1.0) |
| 21 years | Reference | Reference |
| How long have you worked in your current hospital work area/unit? | ||
| <1 year | 0.79 (0.76‐0.82) | 0.98 (0.93‐1.0) |
| 15 years | 1.0 (1.01.1) | 1.0 (0.99‐1.1) |
| 610 years | 1.1 (1.1‐1.1) | 1.0 (1.01.1) |
| 1115 years | 1.1 (1.01.1) | 1.0 (0.99‐1.1) |
| 1620 years | 1.1 (1.01.1) | 1.1 (1.01.1) |
| 21 years | Reference | Reference |
| Typically, how many hours per week do you work in this hospital? | ||
| <20 | 0.63 (0.50‐0.79) | 0.91 (0.72‐1.2) |
| 2039 | 0.75 (0.59‐0.94) | 0.90 (0.71‐1.1) |
| 4059 | 0.87 (0.69‐1.1) | 1.1 (0.85‐1.4) |
| 6079 | 0.95 (0.75‐1.2) | 1.0 (0.82‐1.3) |
| 8099 | 0.99 (0.78‐1.2) | 1.1 (0.86‐1.4) |
| 100 | Reference | Reference |
| What is your staff position in this hospital? | ||
| Registered nurse | 0.92 (0.90‐0.94) | 1.1 (0.98‐1.0) |
| Technician (eg, ECG, lab, radiology) | Reference | Reference |
| Unit assistant/clerk/secretary | 0.79 (0.76‐0.81) | 0.94 (0.80‐0.96) |
| Patient care assistant/hospital aide/care partner | 0.78 (0.75‐0.81) | 0.96 (0.90‐0.98) |
| Physical, occupational, or speech therapist | 0.88 (0.84‐0.92) | 1.2 (1.1‐1.2) |
| Attending/staff physician | 1.0 (0.97‐1.1) | 1.3 (1.2‐1.3) |
| LVN/LPN | 0.89 (0.85‐0.94) | 1.0 (0.92‐1.0) |
| Respiratory therapist | 0.84 (0.80‐0.88) | 0.97 (0.89‐1.0) |
| Pharmacist | 1.5 (1.4‐1.6) | 1.3 (1.1‐1.3) |
| Physician assistant/nurse practitioner | 0.93 (0.87‐1.0) | 1.2 (1.1‐1.2) |
| Resident physician/physician in training | 0.96 (0.89‐1.0) | 1.3 (1.2‐1.4) |
| Dietician | 0.86 (0.79‐0.94) | 1.2 (1.1‐1.3) |
| In your staff position, do you typically have direct interaction or contact with patients? | ||
| Yes | 0.83 (0.82‐0.85) | 0.85 (0.83‐0.87) |
| No | Reference | Reference |
| What is your primary work area or unit in this hospital? | ||
| Other | Reference | Reference |
| Medicine (nonsurgical) | 1.1 (1.01.1) | 0.84 (0.82‐0.89) |
| Surgery | 1.1 (1.1‐1.2) | 0.88 (0.86‐0.91) |
| Intensive care unit (any type) | 0.93 (0.90‐0.96) | 0.78 (0.76‐0.81) |
| Many different hospital units/no specific unit | 1.2 (1.1‐1.2) | 1.0 (0.98‐ 1.0) |
| Radiology | 1.1 (1.1‐1.1) | 1.0 (1.01.1) |
| Emergency department | 1.0 (0.97‐1.0) | 0.57 (0.55‐0.60) |
| Obstetrics | 0.76 (0.73‐0.79) | 0.66 (0.63‐0.69) |
| Laboratory | 1.2 (1.2‐1.3) | 1.0 (1.01.1) |
| Rehabilitation | 1.0 (0.97‐1.0) | 1.0 (0.98‐1.1) |
| Pediatrics | 0.90 (0.86‐0.94) | 0.83 (0.80‐0.87) |
| Pharmacy | 1.6 (1.5‐1.7) | 1.1 (1.01.2) |
| Psychiatry/mental health | 1.2 (1.1‐1.2) | 0.96 (0.90‐1.0) |
| Anesthesiology | 1.1 (1.01.3) | 0.93 (0.83‐1.0) |
| Respondent perceptions | ||
| When a lot of work needs to be done quickly, we work together as a team to get the work done. | ||
| Strongly disagree | 3.2 (3.03.4) | 1.0 (0.98‐1.1) |
| Disagree | 3.2 (3.13.3) | 1.0 (1.01.1) |
| Neutral | 2.3 (2.2‐2.4) | 0.98 (0.94‐1.0) |
| Agree | 2.3 (2.2‐2.4) | 1.0 (1.002‐1.04) |
| Strongly agree | Reference | Reference |
| Staff in this unit work longer hours than is best for patient care. | ||
| Strongly disagree | 0.51 (0.48‐0.53) | 0.76 (0.73‐0.79) |
| Disagree | 0.68 (0.67‐0.70) | 0.81 (0.78‐0.84) |
| Neutral | 0.94 (0.91‐0.97) | 0.93 (0.90‐0.97) |
| Agree | 1.0 (0.99‐1.1) | 0.94 (0.91‐0.98) |
| Strongly agree | Reference | Reference |
| When 1 area in this unit gets really busy, others help out. | ||
| Strongly disagree | 3.8 (3.7 ‐ 4.0) | 1.0 (0.96‐1.1) |
| Disagree | 3.0 (2.9‐3.1) | 1.0 (0.99‐1.1) |
| Neutral | 2.2 (2.12.3) | 1.0 (0.97‐1.0) |
| Agree | 1.5 (1.5‐1.6) | 0.99 (0.96‐1.0) |
| Strongly agree | Reference | Reference |
| Hospital units do not coordinate well with each other. | ||
| Strongly disagree | 0.03 (0.03‐0.04) | 0.10 (0.10‐0.11) |
| Disagree | 0.08 (0.08‐0.08) | 0.18 (0.17‐0.19) |
| Neutral | 0.21 (0.20‐0.22) | 0.32 (0.30‐0.33) |
| Agree | 0.50 (0.48‐0.52) | 0.61 (0.58‐0.63) |
| Strongly agree | Reference | Reference |
| There is good cooperation among hospital units that need to work together. | ||
| Strongly disagree | 20.1 (18.921.5) | 4.7 (4.35.0) |
| Disagree | 14.2 (13.614.9) | 4.2 (4.14.5) |
| Neutral | 6.7 (6.47.0) | 2.7 (2.6‐2.8) |
| Agree | 2.4 (2.3‐2.5) | 1.6 (1.6‐1.7) |
| Strongly agree | Reference | Reference |
| Please give your work area/unit in this hospital an overall grade on patient safety | ||
| Excellent | 0.13 (0.12‐0.14) | 0.47 (0.42‐0.52) |
| Very good | 0.24 (0.21‐0.26) | 0.63 (0.57‐0.70) |
| Acceptable | 0.49 (0.45‐0.54) | 0.79 (0.72‐0.88) |
| Poor | 0.83 (0.75‐0.92) | 0.92 (0.83‐1.03) |
| Failing | Reference | Reference |
DISCUSSION
Our results illustrate that when hospital staff agree that their hospital works in crisis mode, they are more likely to agree that their hospital unit had frequent problems exchanging patient information across units. Because hospital staff working under time constraints and heavy workloads could potentially be at risk of misinterpreting or delivering inaccurate information, these results imply that crisis mode work climates increase the risk of problematic health information exchange. An equally plausible interpretation could be that problematic patient health information exchange increases the risk of hospital staff perceiving crisis mode work climates. Given that information gaps are associated with patient handoff errors,[14] and that patient handoff errors are associated with adverse events,[2, 3, 6, 8] an urgent need exists to implement information exchange systems that prevent information gaps from harming patients. Consequently, hospitals need to implement workflow strategies that prevent information gaps from undermining patient safety during transitions of care.
Other factors affect information exchange apart from crisis mode work climate, as illustrated by the significant associations of key covariates in the multivariate model. The effect found between perceived coordination and information exchange implies that improving information exchange requires good cooperation and coordination. The effect found between patient contact and information exchange implies that working directly with patients improves either the accuracy or the perception of information exchange. Finally, the effect found between hospital size and information exchange suggests that small hospitals are less likely than large hospitals to have information exchange problem. The geographical dispersion and the complexity of larger institutions could result in information exchange problems due to more confusion and less in‐person communication.
Because problematic patient information exchanges are associated with hospital size, coordination, and patient contact, in addition to crisis mode work climate, multifaceted solutions are necessary to resolve the problem. For example, hospital interventions designed to improve coordination could in turn attenuate perceived crisis modes. Furthermore, tailoring these interventions to hospitals that belong to complex geographically dispersed provider networks would likely decrease errors during transitions of care. Because multiple factors cause information exchange problems, implementing interventions that improve both coordination and crisis mode work climates would likely result in a greater net improvement compared to interventions focused solely on decreasing crisis mode work climates.
Some limitations of our paper are worth noting. First, we did not have information on the volume of data exchanged or the functionality levels of the electronic health record systems, both of which likely impact the accuracy of patient information exchange. For example, hospitals with smaller versus larger amounts of data exchanged could be less prone to error. On the other hand, this risk of error could be reduced even further by implementing robust health information technology (IT) systems that improve the accuracy of information transfer. This is consistent with studies showing that hospitals without computerized provider order entry (CPOE) systems have been shown to have higher medication error rates compared to those hospitals with CPOE systems.[15] Therefore, omitting data volume and health IT capabilities from the multivariate model could introduce unobserved heterogeneity, resulting in biased associations between perceived crisis mode work climate and perceived information exchange problems. Second, the cross‐sectional design limits our ability to infer causality because we are not certain whether the perceived crisis mode occurred before, after, or simultaneously to perceived information exchange problems. Third, the self‐reported nature of the questionnaire items does not provide information on observed levels of crisis mode and exchange problems, which could be inconsistent with perceived levels. Fourth, the relatively low within‐hospital response rate decreases the external validity of our findings. For example, if responders' perceptions of crisis mode or information exchange problems significantly differed from nonresponders, our results would not be generalizable to the larger population of acute‐care hospitals across the United States. Therefore, conclusions should be viewed with caution if applying these results to hospitals with respondents significantly differing from those contained within our sample.
Despite these limitations, the large sample size in conjunction with the use of data from a survey having acceptable psychometric properties[16] strengthens the external and internal validity of our findings. Although questionnaire items measuring perceptions are relatively subjective in nature compared to using metrics that capture observed problems or crisis modes, we argue that staff perception data are equally informative, as they guide organization leaders on how to improve workplace performance. Because a core concept of high reliability organizations (HROs) is to preserve constant awareness by key leaders and staff of the state of the systems and processes that affect patient care,[17] HROs could benefit from knowing the extent to which staff perceptions impact patient care. From a methods perspective, the multivariable ordinal regressions enabled us to control for potential confounders that if omitted could have resulted in biased estimates. Furthermore, low levels of multicollinearity as illustrated by low variation inflation factors enabled us to isolate the independent effect of crisis mode perceptions. Including hospital size and hospital work unit as covariates was an additional methodological strength helping account for the unobserved heterogeneity caused by excluding volume of data exchanged or health IT system capability. For example, because larger compared to smaller hospitals usually have more sophisticated health IT systems,[15] including bed size in the model theoretically captures some of the variation that would have been captured if we were able to include a covariate measuring health IT capability. Last, using ordinal regression facilitates interpretation of the findings because the questionnaire items for the predictor and outcome were originally captured on a Likert scale.
Our findings underscore the significant impact that work climate has on accurate information exchange, and ultimately patient safety. Improving patient safety is imperative for hospitals, especially within the context of recent regulations stemming from the Affordable Care Act that incentivize hospitals to reduce readmissions[18] and improve transitions of care.[19] Because accurate health information exchange is a critical component of patient care, resolving barriers that decrease the accuracy of this exchange is essential. Therefore, future studies need to continue examining these associations within the context of study designs that incorporate longitudinal data and datasets that include objective measures capturing crisis mode work climates and information exchange problems. Because effective communication during handoffs is associated with decreases in medical errors and readmissions, hospitals need to continually ensure that work environments are conducive to effective patient information exchange.
Disclosures
Nothing to report
- , , . Safety and complexity: inter‐departmental relationships as a threat to patient safety in the operating department. J Health Organ Manag. 2006;20(2–3):227–242.
- , , , , . Communication loads on clinical staff in the emergency department. Med J Aust. 2002;176(9):415–418.
- , , , et al. Rates of medical errors and preventable adverse events among hospitalized children following implementation of a resident handoff bundle. JAMA. 2013;310(21):2262–2270.
- , , . The Handbook of Group Communication Theory and Research. Thousand Oaks, CA: Sage Publications; 1999.
- , , . Judgment and decision making under time pressure. In: Svenson O, Maule AJ, eds. Time Pressure and Stress in Human Judgment and Decision Making. New York, NY: Plenum Press; 1993:27–40.
- , , , , . Learning from error: identifying contributory causes of medication errors in an Australian hospital. Med J Aust. 2008;188(5):276–279.
- , , . Communicating in the “gray zone”: perceptions about emergency physician hospitalist handoffs and patient safety. Acad Emerg Med. 2007;14(10):884–894.
- , . Emergency physician intershift handovers: an analysis of our transitional care. Pediatr Emerg Care. 2006;22(10):751–754.
- , , , , , . Dropping the baton: a qualitative analysis of failures during the transition from emergency department to inpatient care. Ann Emerg Med. 2009;53(6):701–710.
- , , , . Lost in translation: challenges and opportunities in physician‐to‐physician communication during patient handoffs. Acad Med. 2005;80(12):1094–1099.
- , , , et al. Hospital Survey on Patient Safety Culture: 2010 user comparative database report. (Prepared by Westat, Rockville, MD, under Contract No. HHSA 290200710024C). Rockville, MD: Agency for Healthcare Research and Quality; February 2010. AHRQ Publication No. 10‐0026.
- , , , , . Physiological and behavioural response patterns at work among hospital nurses. J Nurs Manag. 2011;19(1):57–68.
- , . The treatment of missing values and its effect in the classifier accuracy. In: Banks D, House L, McMorris FR, Arabie P, Gaul W, eds. Classification, Clustering and Data Mining Applications. Berlin, Germany: Springer‐Verlag; 2004.
- , . A systematic review of failures in handoff communication during intrahospital transfers. Jt Comm J Qual Patient Saf. 2011;37(6):274–284.
- , , , et al. Reduction in medication errors in hospitals due to adoption of computerized provider order entry systems. J Am Med Inform Assoc. 2013;20(3):470–476.
- , . Multilevel psychometric properties of the AHRQ hospital survey on patient safety culture. BMC Health Serv Res. 2010;10:199.
- , , , et al. Becoming a High Reliability Organization: Operational Advice for Hospital Leaders. Prepared by the Lewin Group under contract no. 290‐04‐0011. AHRQ publication no. 08–0022. Rockville, MD: Agency for Healthcare Research and Quality; 2008.
- Health policy brief: Medicare hospital readmissions reduction program. Health Affairs. November 12, 2013. Available at: http://www.healthaffairs.org/healthpolicybriefs/brief.php?brief_id=102. Accessed August 11, 2014.
- , , , , . The care span: the importance of transitional care in achieving health reform. Health Aff (Millwood). 2011;30(4):746–754.
Using electronic health records to improve the continuity of care between hospital units does not replace the need for interpersonal communication to improve transitions of care. Hospital personnel play a critical role in accurately exchanging patient information during patient transfers, a process requiring accurate communication between hospital units to prevent system failures.[1] Because poor communication contributes to preventable adverse events,[2] and effective communication during handoffs decreases medical errors and readmissions,[3] hospitals need to ensure their work environments are conducive to effective communication.
Individuals working under time constraints and heavy workloads could potentially be at high risk of misinterpreting or delivering inaccurate information,[4] partially due to limited ability to accurately process and communicate information under stressful circumstances. Furthermore, because time‐constrained decision makers tend to use less information and less rigorous decision strategies,[5] work climates characterized by staff members doing too many things too quickly could cause patient health information to be lost during transitions of care across hospital units.
Current studies illustrate scenarios in which demanding or time‐constrained work environments caused information exchange errors. One study found that the increased rate of prescribing errors was partially attributed to a high‐demand work environment characterized by working after hours and multitasking.[6] Other studies found that clinicians' limited time to relay and respond to information and ask clarifying questions during patient handoffs was partially attributed to the fast‐faced and chaotic environment of the emergency room.[7, 8] These studies are consistent with another study that found patient handoffs between emergency departments and inpatient wards were inadequate, partially due to less interactive and more rushed communication.[9] The fact that communication breakdowns are widely cited as barriers to patient handoffs[7, 8, 10] and facilitators of medical errors,[7, 8] further underscores the detrimental effect that crisis mode work climates could have on exchanging patient information during transitions of care.
The objective of this analysis was to evaluate the extent to which a crisis mode work climate impacts the occurrence of patient information exchange problems. Estimating associations between hospital staff members' perceptions of crisis mode work climates and perceptions of information exchange problems provide insights as to whether high‐demand and time‐constrained work climates negatively impact the exchange of patient information. Because hospital staff members working under time constraints and heavy workloads could potentially be at high risk of misinterpreting or delivering inaccurate information, we hypothesized that higher levels of a perceived crisis mode work climate would be associated with higher levels of perceived problems with information exchange across hospital units.
METHODS
Data Source
Data originated from the Agency of Healthcare Research and Quality 2010 Hospital Survey on Patient Safety Culture. This validated survey, designed to assess the safety climate within acute‐care settings, remains an important annual survey deployed each year to track changes and factors impacting patient safety.[11] We included only those respondents who self‐reported their position as a nurse, physician, pharmacist, dietician, therapist, technician, patient care assistant, or hospital unit secretary, all of whom are likely responsible for exchanging patient information across hospital units. For this reason, we excluded respondents who self‐reported their position as administrative or miscellaneous. Applying these exclusion criteria resulted in 247,104 respondents across 884 hospitals.
Conceptual Framework
The relationship between perceived crisis mode work climates and patient information exchange problems is likely influenced by staff skill levels, work climate, and infrastructure (Figure 1). With respect to skill levels, hospital staff members with many years of experience compared to those with fewer years may be relatively desensitized to chaotic work environments and consequently have higher thresholds for perceiving crisis modes. Number of hours worked per week likely impacts perceived crisis mode as illustrated in 1 study finding that full‐time nurses reported a significantly lower work pace compared to part‐time nurses.[12] Years of experience likely impacts perception of information exchange problems, particularly if staff members with many years of experience are familiar enough with hospital systems or protocols to easily detect exchange errors or mishaps.

With respect to work climates, workers' perception of cooperation, coordination and patient safety, and specific hospital unit likely impact perceptions of crisis work mode and information exchange problems. For example, hospital staff members reporting high levels of cooperation, coordination, and patient safety likely perceive fewer crisis modes and information exchange problems compared to those in less‐cooperative hospital units. Furthermore, the heterogeneity of work cultures across departments within a hospital results in department‐specific perceptions of crisis mode climates and information exchange problems. Infrastructure factors, such as hospital size, teaching and ownership status, and census region, likely impact the amount of resources available for staffing and infrastructure, which in turn could impact work pace and information exchange accuracy.
Variable Definitions
We defined our predictor as the perceived presence of a crisis mode work climate as captured from the survey questionnaire item: We work in crisis mode trying to do too much, too quickly. This question item had a Likert response scale comprised of the following 5 answer choices: (1) strongly disagree, (2) disagree, (3) neutral, (4) agree, (5) strongly agree. We created a 3‐level response variable by aggregating the agree and disagree responses, respectively, as the first 2 levels, and retaining the neutral response as the third level. Consequently, those responding strongly disagree or disagree were classified as working in lowcrisis mode work climates and those responding strongly agree or agree were classified as working in high crisis mode work climates. We defined our outcome measure as the presence of patient information exchange problems as captured from the survey questionnaire item: Problems often occur in the exchange of information across hospital units. Because this question item had a Likert response scale similar to the crisis mode question predictor, we also created a 3‐level categorical variable in the same fashion. Consequently, those responding strongly disagree or disagree were classified as perceiving no problems exchanging patient information, and those responding strongly agree or agree were classified as perceiving problems exchanging patient information. For the fewer than 10% of the respondents with missing data on either the predictor our outcome variables, the mode measure of central tendency was imputed, a methodology validated in a previous study.[13]
We also included questionnaire items that captured staff skill levels, work climate, and infrastructure as covariates to account for potential confounders (Figure 1). The staff skill levels domain included years of experience working in the hospital, specialty, and unit; current staff position; and extent of patient contact. The work climate domain included respondent perceptions of coordination and cooperation, patient safety, and primary work area or unit in which the provider reported working. The hospital infrastructure domain included bed size, census region, teaching status, and government ownership status. For the fewer than 10% of the respondents with missing data on any of the categorical variables, the mode measure of central tendency was imputed, a methodology validated in a previous study.[13]
Analytic Approach
We used multivariable ordinal regressions to estimate the likelihood of perceived problems in patient information exchange conditional upon perceptions of a crisis mode work climate, controlling for staff skill levels, work climate, and hospital infrastructure. Our estimates therefore reflect the likelihood of hospital staff responding strongly agree or agree to the question Problems often occur in the exchange of information across hospital units conditional upon responding strongly agree or agree to the question We work in crisis mode trying to do too much, too quickly. In addition to controlling for hospital‐specific response rates, we also adjusted our standard errors to account for the clustering of respondents within hospitals. All analyses were conducted in SAS version 9.2 (SAS Institute Inc., Cary, NC).
RESULTS
The hospital sample averaged 279 respondents per hospital with a 56% response rate. Most hospitals were located in the Central region of the United States, and 32% and 19% were teaching and government‐owned hospitals, respectively. Forty‐three percent and 44% of the hospitals in the sample were designated as small and medium hospitals, respectively (Table 1).
| Characteristics | % |
|---|---|
| |
| Hospital characteristics, N=884 | |
| Bed size | |
| Small, 199 | 43.5 |
| Medium, 100399 | 43.8 |
| Large, 400 plus | 12.7 |
| Teaching status | |
| Yes | 32.2 |
| No | 67.8 |
| Government ownership | |
| Yes | 19.5 |
| No | 80.5 |
| Census region | |
| Mid‐Atlantic and New England | 8.7 |
| South Atlantic | 14.8 |
| Central | 57.2 |
| Mountain | 7.7 |
| Pacific | 11.5 |
| Response rate, mean (SD) | 0.56 (0.28) |
| Respondents per hospital, mean (SD) | 279 (358) |
| Respondent characteristics, N=274,140 | |
| How long have you worked in your current specialty or profession? | |
| <1 year | 5.8 |
| 15 years | 32.8 |
| 610 years | 16.2 |
| 1115 years | 12.0 |
| 1620 years | 10.6 |
| 21 years | 22.7 |
| How long have you worked in this hospital? | |
| <1 year | 9.8 |
| 15 years | 42.8 |
| 610 years | 17.8 |
| 1115 years | 9.0 |
| 1620 years | 8.2 |
| 21 years | 12.4 |
| How long have you worked in your current hospital work area/unit? | |
| <1 year | 13.1 |
| 15 years | 48.0 |
| 610 years | 18.1 |
| 1115 years | 8.1 |
| 1620 years | 6.0 |
| 21 years | 6.7 |
| Typically, how many hours per week do you work in this hospital? | |
| <20 hours | 4.8 |
| 2039 hours | 39.9 |
| 4059 hours | 48.8 |
| 6079 hours | 4.2 |
| 8099 hours | 2.1 |
| 100 hours | 0.11 |
| What is your staff position in this hospital? | |
| Registered nurse | 51.2 |
| Technician (eg, ECG, lab, radiology) | 14.1 |
| Unit assistant/clerk/secretary | 8.5 |
| Patient care assistant/hospital aide/care partner | 7.4 |
| Physical, occupational, or speech therapist | 3.7 |
| Attending/staff physician | 3.5 |
| LVN/LPN | 3.0 |
| Respiratory therapist | 2.9 |
| Pharmacist | 2.2 |
| Physician assistant/nurse practitioner | 1.4 |
| Resident physician/physician in training | 1.2 |
| Dietician | 0.83 |
| In your staff position, do you typically have direct interaction or contact with patients? | |
| Yes | 86.6 |
| No | 13.4 |
| What is your primary work area or unit in this hospital? | |
| Other | 27.7 |
| Medicine (nonsurgical) | 11.1 |
| Surgery | 10.0 |
| Intensive care unit (any type) | 8.6 |
| Many different hospital units/no specific unit | 6.8 |
| Radiology | 6.2 |
| Emergency department | 5.8 |
| Obstetrics | 4.9 |
| Laboratory | 4.9 |
| Rehabilitation | 4.2 |
| Pediatrics | 3.8 |
| Pharmacy | 3.2 |
| Psychiatry/mental health | 2.1 |
| Anesthesiology | 0.55 |
Thirty‐seven percent of the respondents have worked in their current specialty or profession for 5 years or less (Table 1). Over half of the respondents have worked in their current hospital for 5 years or less, whereas 61% have worked in their current unit within the hospital for 5 years or less. Forty‐nine percent work at least 40 hours per week. Registered nurses and technicians represented the 2 largest subgroups of staff positions, comprising 51% and 14% of the sample, respectively. Dieticians and resident physicians, on the other hand, represented the 2 smallest subgroups of staff positions, comprising 0.83% and 1.2% of the sample, respectively. Eighty‐seven percent of the respondents have direct interaction or contact with patients. Apart from those responding other as their hospital unit, nonsurgical medicine and surgery represented the largest subgroup primary work areas, comprising 11% and 10% of the sample, respectively. In contrast, psychiatry and anesthesiology represented the 2 smallest subgroups of primary work areas, comprising 2.1% and 0.55% of the sample, respectively (Table 1).
Respondents scored relatively high with regard to teamwork and helping each other out under hurried or busy circumstances. For example, 85% agreed or strongly agreed that their unit worked together as a team to get work done when a lot of work needed to be completed quickly, and 68% agreed or strongly agreed that individuals within their unit helped out when an area in their unit became busy (Table 1). Despite this cooperation, 31% agreed or strongly agreed that hospital units did not coordinate well together. Paradoxically, 57% agreed or strongly agreed that there was good cooperation among hospital units that needed to work together. Seventy‐five percent of the respondents reported excellent or very good patient safety levels within their unit, although 53% agreed or strongly agreed that staff worked longer hours than was best for patient care (Table 1).
With regard to perceived crisis mode work climate, 32% and 47% reported agreeing and disagreeing, respectively, that their work unit worked in crisis mode trying to do too much too quickly (Table 2). With regard to perceived problems with patient information exchange, 27% and 36% reported agreeing and disagreeing, respectively, that information exchange problems occurred across hospital units (Table 2).
| Perceptions | % |
|---|---|
| We work in crisis mode trying to do too much, too quickly | |
| Strongly disagree | 8.1 |
| Disagree | 39.2 |
| Neutral | 21.0 |
| Agree | 24.3 |
| Strongly agree | 7.5 |
| Problems often occur in the exchange of information across hospital units | |
| Strongly disagree | 4.6 |
| Disagree | 31.3 |
| Neutral | 37.3 |
| Agree | 24.0 |
| Strongly agree | 2.7 |
| When a lot of work needs to be done quickly, we work together as a team to get the work done. | |
| Strongly disagree | 1.5 |
| Disagree | 6.1 |
| Neutral | 7.5 |
| Agree | 53.6 |
| Strongly agree | 31.2 |
| When one area in this unit gets really busy, others help out. | |
| Strongly disagree | 3.9 |
| Disagree | 13.9 |
| Neutral | 13.7 |
| Agree | 52.6 |
| Strongly agree | 15.8 |
| Hospital units do not coordinate well with each other. | |
| Strongly disagree | 5.6 |
| Disagree | 38.8 |
| Neutral | 23.7 |
| Agree | 25.3 |
| Strongly agree | 6.6 |
| There is good cooperation among hospital units that need to work together. | |
| Strongly disagree | 2.7 |
| Disagree | 15.1 |
| Neutral | 24.7 |
| Agree | 51.1 |
| Strongly agree | 6.3 |
| Please give your work area/unit in this hospital an overall grade on patient safety. | |
| Excellent | 23.0 |
| Very good | 49.8 |
| Acceptable | 21.8 |
| Poor | 4.6 |
| Failing | 0.76 |
| Staff in this unit work longer hours than is best for patient care. | |
| Strongly disagree | 11.5 |
| Disagree | 42.2 |
| Neutral | 23.6 |
| Agree | 18.4 |
| Strongly agree | 6.3 |
In the unadjusted analyses, crisis mode perceptions and information exchange problem perceptions were significantly associated. Among those who agreed that their work unit worked in crisis mode, a larger proportion of respondents agreed (41%) versus disagreed (24%) that problems often occurred in exchanging patient information across units (Table 3). In contrast, among those who disagreed that their work unit worked in crisis mode, a larger proportion of respondents disagreed (47%) versus agreed (19%) that problems often occurred in exchanging patient information across units (Table 3).
| Problems Often Occur in Exchange of Information Across Hospital Units | |||
|---|---|---|---|
| Agree (N=66,115), Row %* | Neutral (N=92,228), Row % | Disagree (N=88,797), Row % | |
| |||
| Crisis Mode Work Climate | |||
| Agree (N=78,253) | 40.8 | 35.4 | 23.8 |
| Neutral (N=51,836)‖ | 22.9 | 48.9 | 28.2 |
| Disagree (N=116,781) | 19.0 | 33.5 | 47.5 |
In the multivariable ordinal regression, compared to those who disagreed that their unit worked in crisis mode, those who agreed were 1.6 times more likely to report that problems often occurred in exchanging patient information across units (odds ratio [OR]: 1.6, 95% confidence interval [CI]: 1.58‐1.65) (Table 4). Additionally, some key covariates were independently associated with perceptions of information exchange problems. Two of these covariates measured workplace coordination. Those who reported that hospital units did not cooperate well together were more likely to report problematic information exchange compared to those who reported that hospital units did cooperate well (OR: 4.7, 95% CI: 4.35.0). Relatedly, those who reported that hospital units did coordinate well were less likely to report problematic information exchange compared to those who reported that hospital units did not coordinate well (OR: 0.10, 95% CI: 0.10‐0.11). Two other covariates measured patient contact and perceptions about long working hours. Those who reported having direct interaction or contact with patients were less likely to report problematic information exchange compared to those who reported not having direct interaction or contact with patients (OR: 0.85, 95% CI: 0.83‐0.87). Those who reported that staff did not work longer hours than was better for patient care were less likely to report problematic information exchange compared to those who did report working longer hours than was better for patient care (OR: 0.76, 95% CI: 0.73 0.79). One covariate measured hospital size. Those who reported working in smaller hospitals were less likely to report problematic information exchange compared to those reporting working in large hospitals (OR: 0.66, 95% CI 0.59‐0.75) (Table 4).
| Characteristic | Unadjusted OR (95% CI) | Adjusted OR* (95% CI) |
|---|---|---|
| ||
| Primary predictor of interest | ||
| Crisis mode work climate | ||
| Agree | 3.0 (2.9‐3.1) | 1.6 (1.5‐1.6) |
| Neutral | 1.8 (1.7‐1.8) | 1.3 (1.2‐1.3) |
| Disagree | Reference | Reference |
| Hospital characteristics | ||
| Bed Size | ||
| Small, 624 | 0.51 (0.44‐0.59) | 0.66 (0.59‐0.75) |
| Small, 249 | 0.59 (0.53‐0.66) | 0.77 (0.70‐0.84) |
| Small, 5099 | 0.65 (0.58‐0.73) | 0.78 (0.71‐0.84) |
| Medium, 100199 | 0.85 (0.77‐0.95) | 0.92 (0.86‐1.0) |
| Medium, 200299 | 1.0 (0.98‐1.1) | 0.97 (0.90‐1.0) |
| Medium, 300399 | 0.96 (0.85‐1.1) | 1.0 (0.92‐1.1) |
| Large, 400499 | 0.99 (0.86‐1.1) | 0.96 (0.87‐1.0) |
| Large, 500 plus | Reference | Reference |
| Teaching status | ||
| No | 0.81 (0.76‐0.87) | 1.0 (0.95‐1.0) |
| Yes | Reference | Reference |
| Government ownership | ||
| No | 1.1 (1.01.2) | 1.0 (0.98‐1.1) |
| Yes | Reference | Reference |
| Census region | ||
| Mid‐Atlantic and New England | 1.0 (0.88‐1.1) | 0.91 (0.84‐0.99) |
| South Atlantic | 0.95 (0.85‐1.1) | 1.0 (0.95‐1.1) |
| Central 1 | 0.95 (0.85‐1.0) | 0.95 (0.89‐1.0) |
| Central 2 | 0.71 (0.62‐0.81) | 0.91 (0.83‐0.99) |
| Central 3 | 0.80 (0.71‐0.91) | 0.97 (0.90‐1.0) |
| Central 4 | 0.76 (0.68‐0.86) | 0.93 (0.85‐1.0) |
| Mountain | 0.84 (0.73‐0.96) | 0.98 (0.90‐1.1) |
| Pacific | Reference | Reference |
| Average survey response rate within hospital | 0.65 (0.58‐0.72) | 0.93 (0.82‐1.0) |
| Respondent characteristics | ||
| How long have you worked in your current specialty or profession? | ||
| <1 year | 0.75 (0.73‐0.78) | 1.03 (0.99‐1.1) |
| 15 years | 0.99 (0.97‐1.0) | 1.1 (1.1‐1.1) |
| 610 years | 1.0 (1.01.1) | 0.99 (0.96‐1.0) |
| 1115 years | 1.0 (1.01.1) | 1.0 (0.97‐1.0) |
| 1620 years | 1.0 (0.98‐1.0) | 0.97 (0.94‐1.0) |
| 21 years | Reference | Reference |
| How long have you worked in this hospital? | ||
| <1 year | 0.75 (0.73‐0.77) | 0.90 (0.85‐0.90) |
| 15 years | 1.03 (1.001.05) | 0.99 (0.95‐1.0) |
| 610 years | 1.1 (1.1‐1.1) | 0.99 (0.95‐1.0) |
| 1115 years | 1.1 (1. 01.1) | 1.0 (0.96‐1.0) |
| 1620 years | 1.1 (1.01.1) | 0.98 (0.94‐1.0) |
| 21 years | Reference | Reference |
| How long have you worked in your current hospital work area/unit? | ||
| <1 year | 0.79 (0.76‐0.82) | 0.98 (0.93‐1.0) |
| 15 years | 1.0 (1.01.1) | 1.0 (0.99‐1.1) |
| 610 years | 1.1 (1.1‐1.1) | 1.0 (1.01.1) |
| 1115 years | 1.1 (1.01.1) | 1.0 (0.99‐1.1) |
| 1620 years | 1.1 (1.01.1) | 1.1 (1.01.1) |
| 21 years | Reference | Reference |
| Typically, how many hours per week do you work in this hospital? | ||
| <20 | 0.63 (0.50‐0.79) | 0.91 (0.72‐1.2) |
| 2039 | 0.75 (0.59‐0.94) | 0.90 (0.71‐1.1) |
| 4059 | 0.87 (0.69‐1.1) | 1.1 (0.85‐1.4) |
| 6079 | 0.95 (0.75‐1.2) | 1.0 (0.82‐1.3) |
| 8099 | 0.99 (0.78‐1.2) | 1.1 (0.86‐1.4) |
| 100 | Reference | Reference |
| What is your staff position in this hospital? | ||
| Registered nurse | 0.92 (0.90‐0.94) | 1.1 (0.98‐1.0) |
| Technician (eg, ECG, lab, radiology) | Reference | Reference |
| Unit assistant/clerk/secretary | 0.79 (0.76‐0.81) | 0.94 (0.80‐0.96) |
| Patient care assistant/hospital aide/care partner | 0.78 (0.75‐0.81) | 0.96 (0.90‐0.98) |
| Physical, occupational, or speech therapist | 0.88 (0.84‐0.92) | 1.2 (1.1‐1.2) |
| Attending/staff physician | 1.0 (0.97‐1.1) | 1.3 (1.2‐1.3) |
| LVN/LPN | 0.89 (0.85‐0.94) | 1.0 (0.92‐1.0) |
| Respiratory therapist | 0.84 (0.80‐0.88) | 0.97 (0.89‐1.0) |
| Pharmacist | 1.5 (1.4‐1.6) | 1.3 (1.1‐1.3) |
| Physician assistant/nurse practitioner | 0.93 (0.87‐1.0) | 1.2 (1.1‐1.2) |
| Resident physician/physician in training | 0.96 (0.89‐1.0) | 1.3 (1.2‐1.4) |
| Dietician | 0.86 (0.79‐0.94) | 1.2 (1.1‐1.3) |
| In your staff position, do you typically have direct interaction or contact with patients? | ||
| Yes | 0.83 (0.82‐0.85) | 0.85 (0.83‐0.87) |
| No | Reference | Reference |
| What is your primary work area or unit in this hospital? | ||
| Other | Reference | Reference |
| Medicine (nonsurgical) | 1.1 (1.01.1) | 0.84 (0.82‐0.89) |
| Surgery | 1.1 (1.1‐1.2) | 0.88 (0.86‐0.91) |
| Intensive care unit (any type) | 0.93 (0.90‐0.96) | 0.78 (0.76‐0.81) |
| Many different hospital units/no specific unit | 1.2 (1.1‐1.2) | 1.0 (0.98‐ 1.0) |
| Radiology | 1.1 (1.1‐1.1) | 1.0 (1.01.1) |
| Emergency department | 1.0 (0.97‐1.0) | 0.57 (0.55‐0.60) |
| Obstetrics | 0.76 (0.73‐0.79) | 0.66 (0.63‐0.69) |
| Laboratory | 1.2 (1.2‐1.3) | 1.0 (1.01.1) |
| Rehabilitation | 1.0 (0.97‐1.0) | 1.0 (0.98‐1.1) |
| Pediatrics | 0.90 (0.86‐0.94) | 0.83 (0.80‐0.87) |
| Pharmacy | 1.6 (1.5‐1.7) | 1.1 (1.01.2) |
| Psychiatry/mental health | 1.2 (1.1‐1.2) | 0.96 (0.90‐1.0) |
| Anesthesiology | 1.1 (1.01.3) | 0.93 (0.83‐1.0) |
| Respondent perceptions | ||
| When a lot of work needs to be done quickly, we work together as a team to get the work done. | ||
| Strongly disagree | 3.2 (3.03.4) | 1.0 (0.98‐1.1) |
| Disagree | 3.2 (3.13.3) | 1.0 (1.01.1) |
| Neutral | 2.3 (2.2‐2.4) | 0.98 (0.94‐1.0) |
| Agree | 2.3 (2.2‐2.4) | 1.0 (1.002‐1.04) |
| Strongly agree | Reference | Reference |
| Staff in this unit work longer hours than is best for patient care. | ||
| Strongly disagree | 0.51 (0.48‐0.53) | 0.76 (0.73‐0.79) |
| Disagree | 0.68 (0.67‐0.70) | 0.81 (0.78‐0.84) |
| Neutral | 0.94 (0.91‐0.97) | 0.93 (0.90‐0.97) |
| Agree | 1.0 (0.99‐1.1) | 0.94 (0.91‐0.98) |
| Strongly agree | Reference | Reference |
| When 1 area in this unit gets really busy, others help out. | ||
| Strongly disagree | 3.8 (3.7 ‐ 4.0) | 1.0 (0.96‐1.1) |
| Disagree | 3.0 (2.9‐3.1) | 1.0 (0.99‐1.1) |
| Neutral | 2.2 (2.12.3) | 1.0 (0.97‐1.0) |
| Agree | 1.5 (1.5‐1.6) | 0.99 (0.96‐1.0) |
| Strongly agree | Reference | Reference |
| Hospital units do not coordinate well with each other. | ||
| Strongly disagree | 0.03 (0.03‐0.04) | 0.10 (0.10‐0.11) |
| Disagree | 0.08 (0.08‐0.08) | 0.18 (0.17‐0.19) |
| Neutral | 0.21 (0.20‐0.22) | 0.32 (0.30‐0.33) |
| Agree | 0.50 (0.48‐0.52) | 0.61 (0.58‐0.63) |
| Strongly agree | Reference | Reference |
| There is good cooperation among hospital units that need to work together. | ||
| Strongly disagree | 20.1 (18.921.5) | 4.7 (4.35.0) |
| Disagree | 14.2 (13.614.9) | 4.2 (4.14.5) |
| Neutral | 6.7 (6.47.0) | 2.7 (2.6‐2.8) |
| Agree | 2.4 (2.3‐2.5) | 1.6 (1.6‐1.7) |
| Strongly agree | Reference | Reference |
| Please give your work area/unit in this hospital an overall grade on patient safety | ||
| Excellent | 0.13 (0.12‐0.14) | 0.47 (0.42‐0.52) |
| Very good | 0.24 (0.21‐0.26) | 0.63 (0.57‐0.70) |
| Acceptable | 0.49 (0.45‐0.54) | 0.79 (0.72‐0.88) |
| Poor | 0.83 (0.75‐0.92) | 0.92 (0.83‐1.03) |
| Failing | Reference | Reference |
DISCUSSION
Our results illustrate that when hospital staff agree that their hospital works in crisis mode, they are more likely to agree that their hospital unit had frequent problems exchanging patient information across units. Because hospital staff working under time constraints and heavy workloads could potentially be at risk of misinterpreting or delivering inaccurate information, these results imply that crisis mode work climates increase the risk of problematic health information exchange. An equally plausible interpretation could be that problematic patient health information exchange increases the risk of hospital staff perceiving crisis mode work climates. Given that information gaps are associated with patient handoff errors,[14] and that patient handoff errors are associated with adverse events,[2, 3, 6, 8] an urgent need exists to implement information exchange systems that prevent information gaps from harming patients. Consequently, hospitals need to implement workflow strategies that prevent information gaps from undermining patient safety during transitions of care.
Other factors affect information exchange apart from crisis mode work climate, as illustrated by the significant associations of key covariates in the multivariate model. The effect found between perceived coordination and information exchange implies that improving information exchange requires good cooperation and coordination. The effect found between patient contact and information exchange implies that working directly with patients improves either the accuracy or the perception of information exchange. Finally, the effect found between hospital size and information exchange suggests that small hospitals are less likely than large hospitals to have information exchange problem. The geographical dispersion and the complexity of larger institutions could result in information exchange problems due to more confusion and less in‐person communication.
Because problematic patient information exchanges are associated with hospital size, coordination, and patient contact, in addition to crisis mode work climate, multifaceted solutions are necessary to resolve the problem. For example, hospital interventions designed to improve coordination could in turn attenuate perceived crisis modes. Furthermore, tailoring these interventions to hospitals that belong to complex geographically dispersed provider networks would likely decrease errors during transitions of care. Because multiple factors cause information exchange problems, implementing interventions that improve both coordination and crisis mode work climates would likely result in a greater net improvement compared to interventions focused solely on decreasing crisis mode work climates.
Some limitations of our paper are worth noting. First, we did not have information on the volume of data exchanged or the functionality levels of the electronic health record systems, both of which likely impact the accuracy of patient information exchange. For example, hospitals with smaller versus larger amounts of data exchanged could be less prone to error. On the other hand, this risk of error could be reduced even further by implementing robust health information technology (IT) systems that improve the accuracy of information transfer. This is consistent with studies showing that hospitals without computerized provider order entry (CPOE) systems have been shown to have higher medication error rates compared to those hospitals with CPOE systems.[15] Therefore, omitting data volume and health IT capabilities from the multivariate model could introduce unobserved heterogeneity, resulting in biased associations between perceived crisis mode work climate and perceived information exchange problems. Second, the cross‐sectional design limits our ability to infer causality because we are not certain whether the perceived crisis mode occurred before, after, or simultaneously to perceived information exchange problems. Third, the self‐reported nature of the questionnaire items does not provide information on observed levels of crisis mode and exchange problems, which could be inconsistent with perceived levels. Fourth, the relatively low within‐hospital response rate decreases the external validity of our findings. For example, if responders' perceptions of crisis mode or information exchange problems significantly differed from nonresponders, our results would not be generalizable to the larger population of acute‐care hospitals across the United States. Therefore, conclusions should be viewed with caution if applying these results to hospitals with respondents significantly differing from those contained within our sample.
Despite these limitations, the large sample size in conjunction with the use of data from a survey having acceptable psychometric properties[16] strengthens the external and internal validity of our findings. Although questionnaire items measuring perceptions are relatively subjective in nature compared to using metrics that capture observed problems or crisis modes, we argue that staff perception data are equally informative, as they guide organization leaders on how to improve workplace performance. Because a core concept of high reliability organizations (HROs) is to preserve constant awareness by key leaders and staff of the state of the systems and processes that affect patient care,[17] HROs could benefit from knowing the extent to which staff perceptions impact patient care. From a methods perspective, the multivariable ordinal regressions enabled us to control for potential confounders that if omitted could have resulted in biased estimates. Furthermore, low levels of multicollinearity as illustrated by low variation inflation factors enabled us to isolate the independent effect of crisis mode perceptions. Including hospital size and hospital work unit as covariates was an additional methodological strength helping account for the unobserved heterogeneity caused by excluding volume of data exchanged or health IT system capability. For example, because larger compared to smaller hospitals usually have more sophisticated health IT systems,[15] including bed size in the model theoretically captures some of the variation that would have been captured if we were able to include a covariate measuring health IT capability. Last, using ordinal regression facilitates interpretation of the findings because the questionnaire items for the predictor and outcome were originally captured on a Likert scale.
Our findings underscore the significant impact that work climate has on accurate information exchange, and ultimately patient safety. Improving patient safety is imperative for hospitals, especially within the context of recent regulations stemming from the Affordable Care Act that incentivize hospitals to reduce readmissions[18] and improve transitions of care.[19] Because accurate health information exchange is a critical component of patient care, resolving barriers that decrease the accuracy of this exchange is essential. Therefore, future studies need to continue examining these associations within the context of study designs that incorporate longitudinal data and datasets that include objective measures capturing crisis mode work climates and information exchange problems. Because effective communication during handoffs is associated with decreases in medical errors and readmissions, hospitals need to continually ensure that work environments are conducive to effective patient information exchange.
Disclosures
Nothing to report
Using electronic health records to improve the continuity of care between hospital units does not replace the need for interpersonal communication to improve transitions of care. Hospital personnel play a critical role in accurately exchanging patient information during patient transfers, a process requiring accurate communication between hospital units to prevent system failures.[1] Because poor communication contributes to preventable adverse events,[2] and effective communication during handoffs decreases medical errors and readmissions,[3] hospitals need to ensure their work environments are conducive to effective communication.
Individuals working under time constraints and heavy workloads could potentially be at high risk of misinterpreting or delivering inaccurate information,[4] partially due to limited ability to accurately process and communicate information under stressful circumstances. Furthermore, because time‐constrained decision makers tend to use less information and less rigorous decision strategies,[5] work climates characterized by staff members doing too many things too quickly could cause patient health information to be lost during transitions of care across hospital units.
Current studies illustrate scenarios in which demanding or time‐constrained work environments caused information exchange errors. One study found that the increased rate of prescribing errors was partially attributed to a high‐demand work environment characterized by working after hours and multitasking.[6] Other studies found that clinicians' limited time to relay and respond to information and ask clarifying questions during patient handoffs was partially attributed to the fast‐faced and chaotic environment of the emergency room.[7, 8] These studies are consistent with another study that found patient handoffs between emergency departments and inpatient wards were inadequate, partially due to less interactive and more rushed communication.[9] The fact that communication breakdowns are widely cited as barriers to patient handoffs[7, 8, 10] and facilitators of medical errors,[7, 8] further underscores the detrimental effect that crisis mode work climates could have on exchanging patient information during transitions of care.
The objective of this analysis was to evaluate the extent to which a crisis mode work climate impacts the occurrence of patient information exchange problems. Estimating associations between hospital staff members' perceptions of crisis mode work climates and perceptions of information exchange problems provide insights as to whether high‐demand and time‐constrained work climates negatively impact the exchange of patient information. Because hospital staff members working under time constraints and heavy workloads could potentially be at high risk of misinterpreting or delivering inaccurate information, we hypothesized that higher levels of a perceived crisis mode work climate would be associated with higher levels of perceived problems with information exchange across hospital units.
METHODS
Data Source
Data originated from the Agency of Healthcare Research and Quality 2010 Hospital Survey on Patient Safety Culture. This validated survey, designed to assess the safety climate within acute‐care settings, remains an important annual survey deployed each year to track changes and factors impacting patient safety.[11] We included only those respondents who self‐reported their position as a nurse, physician, pharmacist, dietician, therapist, technician, patient care assistant, or hospital unit secretary, all of whom are likely responsible for exchanging patient information across hospital units. For this reason, we excluded respondents who self‐reported their position as administrative or miscellaneous. Applying these exclusion criteria resulted in 247,104 respondents across 884 hospitals.
Conceptual Framework
The relationship between perceived crisis mode work climates and patient information exchange problems is likely influenced by staff skill levels, work climate, and infrastructure (Figure 1). With respect to skill levels, hospital staff members with many years of experience compared to those with fewer years may be relatively desensitized to chaotic work environments and consequently have higher thresholds for perceiving crisis modes. Number of hours worked per week likely impacts perceived crisis mode as illustrated in 1 study finding that full‐time nurses reported a significantly lower work pace compared to part‐time nurses.[12] Years of experience likely impacts perception of information exchange problems, particularly if staff members with many years of experience are familiar enough with hospital systems or protocols to easily detect exchange errors or mishaps.

With respect to work climates, workers' perception of cooperation, coordination and patient safety, and specific hospital unit likely impact perceptions of crisis work mode and information exchange problems. For example, hospital staff members reporting high levels of cooperation, coordination, and patient safety likely perceive fewer crisis modes and information exchange problems compared to those in less‐cooperative hospital units. Furthermore, the heterogeneity of work cultures across departments within a hospital results in department‐specific perceptions of crisis mode climates and information exchange problems. Infrastructure factors, such as hospital size, teaching and ownership status, and census region, likely impact the amount of resources available for staffing and infrastructure, which in turn could impact work pace and information exchange accuracy.
Variable Definitions
We defined our predictor as the perceived presence of a crisis mode work climate as captured from the survey questionnaire item: We work in crisis mode trying to do too much, too quickly. This question item had a Likert response scale comprised of the following 5 answer choices: (1) strongly disagree, (2) disagree, (3) neutral, (4) agree, (5) strongly agree. We created a 3‐level response variable by aggregating the agree and disagree responses, respectively, as the first 2 levels, and retaining the neutral response as the third level. Consequently, those responding strongly disagree or disagree were classified as working in lowcrisis mode work climates and those responding strongly agree or agree were classified as working in high crisis mode work climates. We defined our outcome measure as the presence of patient information exchange problems as captured from the survey questionnaire item: Problems often occur in the exchange of information across hospital units. Because this question item had a Likert response scale similar to the crisis mode question predictor, we also created a 3‐level categorical variable in the same fashion. Consequently, those responding strongly disagree or disagree were classified as perceiving no problems exchanging patient information, and those responding strongly agree or agree were classified as perceiving problems exchanging patient information. For the fewer than 10% of the respondents with missing data on either the predictor our outcome variables, the mode measure of central tendency was imputed, a methodology validated in a previous study.[13]
We also included questionnaire items that captured staff skill levels, work climate, and infrastructure as covariates to account for potential confounders (Figure 1). The staff skill levels domain included years of experience working in the hospital, specialty, and unit; current staff position; and extent of patient contact. The work climate domain included respondent perceptions of coordination and cooperation, patient safety, and primary work area or unit in which the provider reported working. The hospital infrastructure domain included bed size, census region, teaching status, and government ownership status. For the fewer than 10% of the respondents with missing data on any of the categorical variables, the mode measure of central tendency was imputed, a methodology validated in a previous study.[13]
Analytic Approach
We used multivariable ordinal regressions to estimate the likelihood of perceived problems in patient information exchange conditional upon perceptions of a crisis mode work climate, controlling for staff skill levels, work climate, and hospital infrastructure. Our estimates therefore reflect the likelihood of hospital staff responding strongly agree or agree to the question Problems often occur in the exchange of information across hospital units conditional upon responding strongly agree or agree to the question We work in crisis mode trying to do too much, too quickly. In addition to controlling for hospital‐specific response rates, we also adjusted our standard errors to account for the clustering of respondents within hospitals. All analyses were conducted in SAS version 9.2 (SAS Institute Inc., Cary, NC).
RESULTS
The hospital sample averaged 279 respondents per hospital with a 56% response rate. Most hospitals were located in the Central region of the United States, and 32% and 19% were teaching and government‐owned hospitals, respectively. Forty‐three percent and 44% of the hospitals in the sample were designated as small and medium hospitals, respectively (Table 1).
| Characteristics | % |
|---|---|
| |
| Hospital characteristics, N=884 | |
| Bed size | |
| Small, 199 | 43.5 |
| Medium, 100399 | 43.8 |
| Large, 400 plus | 12.7 |
| Teaching status | |
| Yes | 32.2 |
| No | 67.8 |
| Government ownership | |
| Yes | 19.5 |
| No | 80.5 |
| Census region | |
| Mid‐Atlantic and New England | 8.7 |
| South Atlantic | 14.8 |
| Central | 57.2 |
| Mountain | 7.7 |
| Pacific | 11.5 |
| Response rate, mean (SD) | 0.56 (0.28) |
| Respondents per hospital, mean (SD) | 279 (358) |
| Respondent characteristics, N=274,140 | |
| How long have you worked in your current specialty or profession? | |
| <1 year | 5.8 |
| 15 years | 32.8 |
| 610 years | 16.2 |
| 1115 years | 12.0 |
| 1620 years | 10.6 |
| 21 years | 22.7 |
| How long have you worked in this hospital? | |
| <1 year | 9.8 |
| 15 years | 42.8 |
| 610 years | 17.8 |
| 1115 years | 9.0 |
| 1620 years | 8.2 |
| 21 years | 12.4 |
| How long have you worked in your current hospital work area/unit? | |
| <1 year | 13.1 |
| 15 years | 48.0 |
| 610 years | 18.1 |
| 1115 years | 8.1 |
| 1620 years | 6.0 |
| 21 years | 6.7 |
| Typically, how many hours per week do you work in this hospital? | |
| <20 hours | 4.8 |
| 2039 hours | 39.9 |
| 4059 hours | 48.8 |
| 6079 hours | 4.2 |
| 8099 hours | 2.1 |
| 100 hours | 0.11 |
| What is your staff position in this hospital? | |
| Registered nurse | 51.2 |
| Technician (eg, ECG, lab, radiology) | 14.1 |
| Unit assistant/clerk/secretary | 8.5 |
| Patient care assistant/hospital aide/care partner | 7.4 |
| Physical, occupational, or speech therapist | 3.7 |
| Attending/staff physician | 3.5 |
| LVN/LPN | 3.0 |
| Respiratory therapist | 2.9 |
| Pharmacist | 2.2 |
| Physician assistant/nurse practitioner | 1.4 |
| Resident physician/physician in training | 1.2 |
| Dietician | 0.83 |
| In your staff position, do you typically have direct interaction or contact with patients? | |
| Yes | 86.6 |
| No | 13.4 |
| What is your primary work area or unit in this hospital? | |
| Other | 27.7 |
| Medicine (nonsurgical) | 11.1 |
| Surgery | 10.0 |
| Intensive care unit (any type) | 8.6 |
| Many different hospital units/no specific unit | 6.8 |
| Radiology | 6.2 |
| Emergency department | 5.8 |
| Obstetrics | 4.9 |
| Laboratory | 4.9 |
| Rehabilitation | 4.2 |
| Pediatrics | 3.8 |
| Pharmacy | 3.2 |
| Psychiatry/mental health | 2.1 |
| Anesthesiology | 0.55 |
Thirty‐seven percent of the respondents have worked in their current specialty or profession for 5 years or less (Table 1). Over half of the respondents have worked in their current hospital for 5 years or less, whereas 61% have worked in their current unit within the hospital for 5 years or less. Forty‐nine percent work at least 40 hours per week. Registered nurses and technicians represented the 2 largest subgroups of staff positions, comprising 51% and 14% of the sample, respectively. Dieticians and resident physicians, on the other hand, represented the 2 smallest subgroups of staff positions, comprising 0.83% and 1.2% of the sample, respectively. Eighty‐seven percent of the respondents have direct interaction or contact with patients. Apart from those responding other as their hospital unit, nonsurgical medicine and surgery represented the largest subgroup primary work areas, comprising 11% and 10% of the sample, respectively. In contrast, psychiatry and anesthesiology represented the 2 smallest subgroups of primary work areas, comprising 2.1% and 0.55% of the sample, respectively (Table 1).
Respondents scored relatively high with regard to teamwork and helping each other out under hurried or busy circumstances. For example, 85% agreed or strongly agreed that their unit worked together as a team to get work done when a lot of work needed to be completed quickly, and 68% agreed or strongly agreed that individuals within their unit helped out when an area in their unit became busy (Table 1). Despite this cooperation, 31% agreed or strongly agreed that hospital units did not coordinate well together. Paradoxically, 57% agreed or strongly agreed that there was good cooperation among hospital units that needed to work together. Seventy‐five percent of the respondents reported excellent or very good patient safety levels within their unit, although 53% agreed or strongly agreed that staff worked longer hours than was best for patient care (Table 1).
With regard to perceived crisis mode work climate, 32% and 47% reported agreeing and disagreeing, respectively, that their work unit worked in crisis mode trying to do too much too quickly (Table 2). With regard to perceived problems with patient information exchange, 27% and 36% reported agreeing and disagreeing, respectively, that information exchange problems occurred across hospital units (Table 2).
| Perceptions | % |
|---|---|
| We work in crisis mode trying to do too much, too quickly | |
| Strongly disagree | 8.1 |
| Disagree | 39.2 |
| Neutral | 21.0 |
| Agree | 24.3 |
| Strongly agree | 7.5 |
| Problems often occur in the exchange of information across hospital units | |
| Strongly disagree | 4.6 |
| Disagree | 31.3 |
| Neutral | 37.3 |
| Agree | 24.0 |
| Strongly agree | 2.7 |
| When a lot of work needs to be done quickly, we work together as a team to get the work done. | |
| Strongly disagree | 1.5 |
| Disagree | 6.1 |
| Neutral | 7.5 |
| Agree | 53.6 |
| Strongly agree | 31.2 |
| When one area in this unit gets really busy, others help out. | |
| Strongly disagree | 3.9 |
| Disagree | 13.9 |
| Neutral | 13.7 |
| Agree | 52.6 |
| Strongly agree | 15.8 |
| Hospital units do not coordinate well with each other. | |
| Strongly disagree | 5.6 |
| Disagree | 38.8 |
| Neutral | 23.7 |
| Agree | 25.3 |
| Strongly agree | 6.6 |
| There is good cooperation among hospital units that need to work together. | |
| Strongly disagree | 2.7 |
| Disagree | 15.1 |
| Neutral | 24.7 |
| Agree | 51.1 |
| Strongly agree | 6.3 |
| Please give your work area/unit in this hospital an overall grade on patient safety. | |
| Excellent | 23.0 |
| Very good | 49.8 |
| Acceptable | 21.8 |
| Poor | 4.6 |
| Failing | 0.76 |
| Staff in this unit work longer hours than is best for patient care. | |
| Strongly disagree | 11.5 |
| Disagree | 42.2 |
| Neutral | 23.6 |
| Agree | 18.4 |
| Strongly agree | 6.3 |
In the unadjusted analyses, crisis mode perceptions and information exchange problem perceptions were significantly associated. Among those who agreed that their work unit worked in crisis mode, a larger proportion of respondents agreed (41%) versus disagreed (24%) that problems often occurred in exchanging patient information across units (Table 3). In contrast, among those who disagreed that their work unit worked in crisis mode, a larger proportion of respondents disagreed (47%) versus agreed (19%) that problems often occurred in exchanging patient information across units (Table 3).
| Problems Often Occur in Exchange of Information Across Hospital Units | |||
|---|---|---|---|
| Agree (N=66,115), Row %* | Neutral (N=92,228), Row % | Disagree (N=88,797), Row % | |
| |||
| Crisis Mode Work Climate | |||
| Agree (N=78,253) | 40.8 | 35.4 | 23.8 |
| Neutral (N=51,836)‖ | 22.9 | 48.9 | 28.2 |
| Disagree (N=116,781) | 19.0 | 33.5 | 47.5 |
In the multivariable ordinal regression, compared to those who disagreed that their unit worked in crisis mode, those who agreed were 1.6 times more likely to report that problems often occurred in exchanging patient information across units (odds ratio [OR]: 1.6, 95% confidence interval [CI]: 1.58‐1.65) (Table 4). Additionally, some key covariates were independently associated with perceptions of information exchange problems. Two of these covariates measured workplace coordination. Those who reported that hospital units did not cooperate well together were more likely to report problematic information exchange compared to those who reported that hospital units did cooperate well (OR: 4.7, 95% CI: 4.35.0). Relatedly, those who reported that hospital units did coordinate well were less likely to report problematic information exchange compared to those who reported that hospital units did not coordinate well (OR: 0.10, 95% CI: 0.10‐0.11). Two other covariates measured patient contact and perceptions about long working hours. Those who reported having direct interaction or contact with patients were less likely to report problematic information exchange compared to those who reported not having direct interaction or contact with patients (OR: 0.85, 95% CI: 0.83‐0.87). Those who reported that staff did not work longer hours than was better for patient care were less likely to report problematic information exchange compared to those who did report working longer hours than was better for patient care (OR: 0.76, 95% CI: 0.73 0.79). One covariate measured hospital size. Those who reported working in smaller hospitals were less likely to report problematic information exchange compared to those reporting working in large hospitals (OR: 0.66, 95% CI 0.59‐0.75) (Table 4).
| Characteristic | Unadjusted OR (95% CI) | Adjusted OR* (95% CI) |
|---|---|---|
| ||
| Primary predictor of interest | ||
| Crisis mode work climate | ||
| Agree | 3.0 (2.9‐3.1) | 1.6 (1.5‐1.6) |
| Neutral | 1.8 (1.7‐1.8) | 1.3 (1.2‐1.3) |
| Disagree | Reference | Reference |
| Hospital characteristics | ||
| Bed Size | ||
| Small, 624 | 0.51 (0.44‐0.59) | 0.66 (0.59‐0.75) |
| Small, 249 | 0.59 (0.53‐0.66) | 0.77 (0.70‐0.84) |
| Small, 5099 | 0.65 (0.58‐0.73) | 0.78 (0.71‐0.84) |
| Medium, 100199 | 0.85 (0.77‐0.95) | 0.92 (0.86‐1.0) |
| Medium, 200299 | 1.0 (0.98‐1.1) | 0.97 (0.90‐1.0) |
| Medium, 300399 | 0.96 (0.85‐1.1) | 1.0 (0.92‐1.1) |
| Large, 400499 | 0.99 (0.86‐1.1) | 0.96 (0.87‐1.0) |
| Large, 500 plus | Reference | Reference |
| Teaching status | ||
| No | 0.81 (0.76‐0.87) | 1.0 (0.95‐1.0) |
| Yes | Reference | Reference |
| Government ownership | ||
| No | 1.1 (1.01.2) | 1.0 (0.98‐1.1) |
| Yes | Reference | Reference |
| Census region | ||
| Mid‐Atlantic and New England | 1.0 (0.88‐1.1) | 0.91 (0.84‐0.99) |
| South Atlantic | 0.95 (0.85‐1.1) | 1.0 (0.95‐1.1) |
| Central 1 | 0.95 (0.85‐1.0) | 0.95 (0.89‐1.0) |
| Central 2 | 0.71 (0.62‐0.81) | 0.91 (0.83‐0.99) |
| Central 3 | 0.80 (0.71‐0.91) | 0.97 (0.90‐1.0) |
| Central 4 | 0.76 (0.68‐0.86) | 0.93 (0.85‐1.0) |
| Mountain | 0.84 (0.73‐0.96) | 0.98 (0.90‐1.1) |
| Pacific | Reference | Reference |
| Average survey response rate within hospital | 0.65 (0.58‐0.72) | 0.93 (0.82‐1.0) |
| Respondent characteristics | ||
| How long have you worked in your current specialty or profession? | ||
| <1 year | 0.75 (0.73‐0.78) | 1.03 (0.99‐1.1) |
| 15 years | 0.99 (0.97‐1.0) | 1.1 (1.1‐1.1) |
| 610 years | 1.0 (1.01.1) | 0.99 (0.96‐1.0) |
| 1115 years | 1.0 (1.01.1) | 1.0 (0.97‐1.0) |
| 1620 years | 1.0 (0.98‐1.0) | 0.97 (0.94‐1.0) |
| 21 years | Reference | Reference |
| How long have you worked in this hospital? | ||
| <1 year | 0.75 (0.73‐0.77) | 0.90 (0.85‐0.90) |
| 15 years | 1.03 (1.001.05) | 0.99 (0.95‐1.0) |
| 610 years | 1.1 (1.1‐1.1) | 0.99 (0.95‐1.0) |
| 1115 years | 1.1 (1. 01.1) | 1.0 (0.96‐1.0) |
| 1620 years | 1.1 (1.01.1) | 0.98 (0.94‐1.0) |
| 21 years | Reference | Reference |
| How long have you worked in your current hospital work area/unit? | ||
| <1 year | 0.79 (0.76‐0.82) | 0.98 (0.93‐1.0) |
| 15 years | 1.0 (1.01.1) | 1.0 (0.99‐1.1) |
| 610 years | 1.1 (1.1‐1.1) | 1.0 (1.01.1) |
| 1115 years | 1.1 (1.01.1) | 1.0 (0.99‐1.1) |
| 1620 years | 1.1 (1.01.1) | 1.1 (1.01.1) |
| 21 years | Reference | Reference |
| Typically, how many hours per week do you work in this hospital? | ||
| <20 | 0.63 (0.50‐0.79) | 0.91 (0.72‐1.2) |
| 2039 | 0.75 (0.59‐0.94) | 0.90 (0.71‐1.1) |
| 4059 | 0.87 (0.69‐1.1) | 1.1 (0.85‐1.4) |
| 6079 | 0.95 (0.75‐1.2) | 1.0 (0.82‐1.3) |
| 8099 | 0.99 (0.78‐1.2) | 1.1 (0.86‐1.4) |
| 100 | Reference | Reference |
| What is your staff position in this hospital? | ||
| Registered nurse | 0.92 (0.90‐0.94) | 1.1 (0.98‐1.0) |
| Technician (eg, ECG, lab, radiology) | Reference | Reference |
| Unit assistant/clerk/secretary | 0.79 (0.76‐0.81) | 0.94 (0.80‐0.96) |
| Patient care assistant/hospital aide/care partner | 0.78 (0.75‐0.81) | 0.96 (0.90‐0.98) |
| Physical, occupational, or speech therapist | 0.88 (0.84‐0.92) | 1.2 (1.1‐1.2) |
| Attending/staff physician | 1.0 (0.97‐1.1) | 1.3 (1.2‐1.3) |
| LVN/LPN | 0.89 (0.85‐0.94) | 1.0 (0.92‐1.0) |
| Respiratory therapist | 0.84 (0.80‐0.88) | 0.97 (0.89‐1.0) |
| Pharmacist | 1.5 (1.4‐1.6) | 1.3 (1.1‐1.3) |
| Physician assistant/nurse practitioner | 0.93 (0.87‐1.0) | 1.2 (1.1‐1.2) |
| Resident physician/physician in training | 0.96 (0.89‐1.0) | 1.3 (1.2‐1.4) |
| Dietician | 0.86 (0.79‐0.94) | 1.2 (1.1‐1.3) |
| In your staff position, do you typically have direct interaction or contact with patients? | ||
| Yes | 0.83 (0.82‐0.85) | 0.85 (0.83‐0.87) |
| No | Reference | Reference |
| What is your primary work area or unit in this hospital? | ||
| Other | Reference | Reference |
| Medicine (nonsurgical) | 1.1 (1.01.1) | 0.84 (0.82‐0.89) |
| Surgery | 1.1 (1.1‐1.2) | 0.88 (0.86‐0.91) |
| Intensive care unit (any type) | 0.93 (0.90‐0.96) | 0.78 (0.76‐0.81) |
| Many different hospital units/no specific unit | 1.2 (1.1‐1.2) | 1.0 (0.98‐ 1.0) |
| Radiology | 1.1 (1.1‐1.1) | 1.0 (1.01.1) |
| Emergency department | 1.0 (0.97‐1.0) | 0.57 (0.55‐0.60) |
| Obstetrics | 0.76 (0.73‐0.79) | 0.66 (0.63‐0.69) |
| Laboratory | 1.2 (1.2‐1.3) | 1.0 (1.01.1) |
| Rehabilitation | 1.0 (0.97‐1.0) | 1.0 (0.98‐1.1) |
| Pediatrics | 0.90 (0.86‐0.94) | 0.83 (0.80‐0.87) |
| Pharmacy | 1.6 (1.5‐1.7) | 1.1 (1.01.2) |
| Psychiatry/mental health | 1.2 (1.1‐1.2) | 0.96 (0.90‐1.0) |
| Anesthesiology | 1.1 (1.01.3) | 0.93 (0.83‐1.0) |
| Respondent perceptions | ||
| When a lot of work needs to be done quickly, we work together as a team to get the work done. | ||
| Strongly disagree | 3.2 (3.03.4) | 1.0 (0.98‐1.1) |
| Disagree | 3.2 (3.13.3) | 1.0 (1.01.1) |
| Neutral | 2.3 (2.2‐2.4) | 0.98 (0.94‐1.0) |
| Agree | 2.3 (2.2‐2.4) | 1.0 (1.002‐1.04) |
| Strongly agree | Reference | Reference |
| Staff in this unit work longer hours than is best for patient care. | ||
| Strongly disagree | 0.51 (0.48‐0.53) | 0.76 (0.73‐0.79) |
| Disagree | 0.68 (0.67‐0.70) | 0.81 (0.78‐0.84) |
| Neutral | 0.94 (0.91‐0.97) | 0.93 (0.90‐0.97) |
| Agree | 1.0 (0.99‐1.1) | 0.94 (0.91‐0.98) |
| Strongly agree | Reference | Reference |
| When 1 area in this unit gets really busy, others help out. | ||
| Strongly disagree | 3.8 (3.7 ‐ 4.0) | 1.0 (0.96‐1.1) |
| Disagree | 3.0 (2.9‐3.1) | 1.0 (0.99‐1.1) |
| Neutral | 2.2 (2.12.3) | 1.0 (0.97‐1.0) |
| Agree | 1.5 (1.5‐1.6) | 0.99 (0.96‐1.0) |
| Strongly agree | Reference | Reference |
| Hospital units do not coordinate well with each other. | ||
| Strongly disagree | 0.03 (0.03‐0.04) | 0.10 (0.10‐0.11) |
| Disagree | 0.08 (0.08‐0.08) | 0.18 (0.17‐0.19) |
| Neutral | 0.21 (0.20‐0.22) | 0.32 (0.30‐0.33) |
| Agree | 0.50 (0.48‐0.52) | 0.61 (0.58‐0.63) |
| Strongly agree | Reference | Reference |
| There is good cooperation among hospital units that need to work together. | ||
| Strongly disagree | 20.1 (18.921.5) | 4.7 (4.35.0) |
| Disagree | 14.2 (13.614.9) | 4.2 (4.14.5) |
| Neutral | 6.7 (6.47.0) | 2.7 (2.6‐2.8) |
| Agree | 2.4 (2.3‐2.5) | 1.6 (1.6‐1.7) |
| Strongly agree | Reference | Reference |
| Please give your work area/unit in this hospital an overall grade on patient safety | ||
| Excellent | 0.13 (0.12‐0.14) | 0.47 (0.42‐0.52) |
| Very good | 0.24 (0.21‐0.26) | 0.63 (0.57‐0.70) |
| Acceptable | 0.49 (0.45‐0.54) | 0.79 (0.72‐0.88) |
| Poor | 0.83 (0.75‐0.92) | 0.92 (0.83‐1.03) |
| Failing | Reference | Reference |
DISCUSSION
Our results illustrate that when hospital staff agree that their hospital works in crisis mode, they are more likely to agree that their hospital unit had frequent problems exchanging patient information across units. Because hospital staff working under time constraints and heavy workloads could potentially be at risk of misinterpreting or delivering inaccurate information, these results imply that crisis mode work climates increase the risk of problematic health information exchange. An equally plausible interpretation could be that problematic patient health information exchange increases the risk of hospital staff perceiving crisis mode work climates. Given that information gaps are associated with patient handoff errors,[14] and that patient handoff errors are associated with adverse events,[2, 3, 6, 8] an urgent need exists to implement information exchange systems that prevent information gaps from harming patients. Consequently, hospitals need to implement workflow strategies that prevent information gaps from undermining patient safety during transitions of care.
Other factors affect information exchange apart from crisis mode work climate, as illustrated by the significant associations of key covariates in the multivariate model. The effect found between perceived coordination and information exchange implies that improving information exchange requires good cooperation and coordination. The effect found between patient contact and information exchange implies that working directly with patients improves either the accuracy or the perception of information exchange. Finally, the effect found between hospital size and information exchange suggests that small hospitals are less likely than large hospitals to have information exchange problem. The geographical dispersion and the complexity of larger institutions could result in information exchange problems due to more confusion and less in‐person communication.
Because problematic patient information exchanges are associated with hospital size, coordination, and patient contact, in addition to crisis mode work climate, multifaceted solutions are necessary to resolve the problem. For example, hospital interventions designed to improve coordination could in turn attenuate perceived crisis modes. Furthermore, tailoring these interventions to hospitals that belong to complex geographically dispersed provider networks would likely decrease errors during transitions of care. Because multiple factors cause information exchange problems, implementing interventions that improve both coordination and crisis mode work climates would likely result in a greater net improvement compared to interventions focused solely on decreasing crisis mode work climates.
Some limitations of our paper are worth noting. First, we did not have information on the volume of data exchanged or the functionality levels of the electronic health record systems, both of which likely impact the accuracy of patient information exchange. For example, hospitals with smaller versus larger amounts of data exchanged could be less prone to error. On the other hand, this risk of error could be reduced even further by implementing robust health information technology (IT) systems that improve the accuracy of information transfer. This is consistent with studies showing that hospitals without computerized provider order entry (CPOE) systems have been shown to have higher medication error rates compared to those hospitals with CPOE systems.[15] Therefore, omitting data volume and health IT capabilities from the multivariate model could introduce unobserved heterogeneity, resulting in biased associations between perceived crisis mode work climate and perceived information exchange problems. Second, the cross‐sectional design limits our ability to infer causality because we are not certain whether the perceived crisis mode occurred before, after, or simultaneously to perceived information exchange problems. Third, the self‐reported nature of the questionnaire items does not provide information on observed levels of crisis mode and exchange problems, which could be inconsistent with perceived levels. Fourth, the relatively low within‐hospital response rate decreases the external validity of our findings. For example, if responders' perceptions of crisis mode or information exchange problems significantly differed from nonresponders, our results would not be generalizable to the larger population of acute‐care hospitals across the United States. Therefore, conclusions should be viewed with caution if applying these results to hospitals with respondents significantly differing from those contained within our sample.
Despite these limitations, the large sample size in conjunction with the use of data from a survey having acceptable psychometric properties[16] strengthens the external and internal validity of our findings. Although questionnaire items measuring perceptions are relatively subjective in nature compared to using metrics that capture observed problems or crisis modes, we argue that staff perception data are equally informative, as they guide organization leaders on how to improve workplace performance. Because a core concept of high reliability organizations (HROs) is to preserve constant awareness by key leaders and staff of the state of the systems and processes that affect patient care,[17] HROs could benefit from knowing the extent to which staff perceptions impact patient care. From a methods perspective, the multivariable ordinal regressions enabled us to control for potential confounders that if omitted could have resulted in biased estimates. Furthermore, low levels of multicollinearity as illustrated by low variation inflation factors enabled us to isolate the independent effect of crisis mode perceptions. Including hospital size and hospital work unit as covariates was an additional methodological strength helping account for the unobserved heterogeneity caused by excluding volume of data exchanged or health IT system capability. For example, because larger compared to smaller hospitals usually have more sophisticated health IT systems,[15] including bed size in the model theoretically captures some of the variation that would have been captured if we were able to include a covariate measuring health IT capability. Last, using ordinal regression facilitates interpretation of the findings because the questionnaire items for the predictor and outcome were originally captured on a Likert scale.
Our findings underscore the significant impact that work climate has on accurate information exchange, and ultimately patient safety. Improving patient safety is imperative for hospitals, especially within the context of recent regulations stemming from the Affordable Care Act that incentivize hospitals to reduce readmissions[18] and improve transitions of care.[19] Because accurate health information exchange is a critical component of patient care, resolving barriers that decrease the accuracy of this exchange is essential. Therefore, future studies need to continue examining these associations within the context of study designs that incorporate longitudinal data and datasets that include objective measures capturing crisis mode work climates and information exchange problems. Because effective communication during handoffs is associated with decreases in medical errors and readmissions, hospitals need to continually ensure that work environments are conducive to effective patient information exchange.
Disclosures
Nothing to report
- , , . Safety and complexity: inter‐departmental relationships as a threat to patient safety in the operating department. J Health Organ Manag. 2006;20(2–3):227–242.
- , , , , . Communication loads on clinical staff in the emergency department. Med J Aust. 2002;176(9):415–418.
- , , , et al. Rates of medical errors and preventable adverse events among hospitalized children following implementation of a resident handoff bundle. JAMA. 2013;310(21):2262–2270.
- , , . The Handbook of Group Communication Theory and Research. Thousand Oaks, CA: Sage Publications; 1999.
- , , . Judgment and decision making under time pressure. In: Svenson O, Maule AJ, eds. Time Pressure and Stress in Human Judgment and Decision Making. New York, NY: Plenum Press; 1993:27–40.
- , , , , . Learning from error: identifying contributory causes of medication errors in an Australian hospital. Med J Aust. 2008;188(5):276–279.
- , , . Communicating in the “gray zone”: perceptions about emergency physician hospitalist handoffs and patient safety. Acad Emerg Med. 2007;14(10):884–894.
- , . Emergency physician intershift handovers: an analysis of our transitional care. Pediatr Emerg Care. 2006;22(10):751–754.
- , , , , , . Dropping the baton: a qualitative analysis of failures during the transition from emergency department to inpatient care. Ann Emerg Med. 2009;53(6):701–710.
- , , , . Lost in translation: challenges and opportunities in physician‐to‐physician communication during patient handoffs. Acad Med. 2005;80(12):1094–1099.
- , , , et al. Hospital Survey on Patient Safety Culture: 2010 user comparative database report. (Prepared by Westat, Rockville, MD, under Contract No. HHSA 290200710024C). Rockville, MD: Agency for Healthcare Research and Quality; February 2010. AHRQ Publication No. 10‐0026.
- , , , , . Physiological and behavioural response patterns at work among hospital nurses. J Nurs Manag. 2011;19(1):57–68.
- , . The treatment of missing values and its effect in the classifier accuracy. In: Banks D, House L, McMorris FR, Arabie P, Gaul W, eds. Classification, Clustering and Data Mining Applications. Berlin, Germany: Springer‐Verlag; 2004.
- , . A systematic review of failures in handoff communication during intrahospital transfers. Jt Comm J Qual Patient Saf. 2011;37(6):274–284.
- , , , et al. Reduction in medication errors in hospitals due to adoption of computerized provider order entry systems. J Am Med Inform Assoc. 2013;20(3):470–476.
- , . Multilevel psychometric properties of the AHRQ hospital survey on patient safety culture. BMC Health Serv Res. 2010;10:199.
- , , , et al. Becoming a High Reliability Organization: Operational Advice for Hospital Leaders. Prepared by the Lewin Group under contract no. 290‐04‐0011. AHRQ publication no. 08–0022. Rockville, MD: Agency for Healthcare Research and Quality; 2008.
- Health policy brief: Medicare hospital readmissions reduction program. Health Affairs. November 12, 2013. Available at: http://www.healthaffairs.org/healthpolicybriefs/brief.php?brief_id=102. Accessed August 11, 2014.
- , , , , . The care span: the importance of transitional care in achieving health reform. Health Aff (Millwood). 2011;30(4):746–754.
- , , . Safety and complexity: inter‐departmental relationships as a threat to patient safety in the operating department. J Health Organ Manag. 2006;20(2–3):227–242.
- , , , , . Communication loads on clinical staff in the emergency department. Med J Aust. 2002;176(9):415–418.
- , , , et al. Rates of medical errors and preventable adverse events among hospitalized children following implementation of a resident handoff bundle. JAMA. 2013;310(21):2262–2270.
- , , . The Handbook of Group Communication Theory and Research. Thousand Oaks, CA: Sage Publications; 1999.
- , , . Judgment and decision making under time pressure. In: Svenson O, Maule AJ, eds. Time Pressure and Stress in Human Judgment and Decision Making. New York, NY: Plenum Press; 1993:27–40.
- , , , , . Learning from error: identifying contributory causes of medication errors in an Australian hospital. Med J Aust. 2008;188(5):276–279.
- , , . Communicating in the “gray zone”: perceptions about emergency physician hospitalist handoffs and patient safety. Acad Emerg Med. 2007;14(10):884–894.
- , . Emergency physician intershift handovers: an analysis of our transitional care. Pediatr Emerg Care. 2006;22(10):751–754.
- , , , , , . Dropping the baton: a qualitative analysis of failures during the transition from emergency department to inpatient care. Ann Emerg Med. 2009;53(6):701–710.
- , , , . Lost in translation: challenges and opportunities in physician‐to‐physician communication during patient handoffs. Acad Med. 2005;80(12):1094–1099.
- , , , et al. Hospital Survey on Patient Safety Culture: 2010 user comparative database report. (Prepared by Westat, Rockville, MD, under Contract No. HHSA 290200710024C). Rockville, MD: Agency for Healthcare Research and Quality; February 2010. AHRQ Publication No. 10‐0026.
- , , , , . Physiological and behavioural response patterns at work among hospital nurses. J Nurs Manag. 2011;19(1):57–68.
- , . The treatment of missing values and its effect in the classifier accuracy. In: Banks D, House L, McMorris FR, Arabie P, Gaul W, eds. Classification, Clustering and Data Mining Applications. Berlin, Germany: Springer‐Verlag; 2004.
- , . A systematic review of failures in handoff communication during intrahospital transfers. Jt Comm J Qual Patient Saf. 2011;37(6):274–284.
- , , , et al. Reduction in medication errors in hospitals due to adoption of computerized provider order entry systems. J Am Med Inform Assoc. 2013;20(3):470–476.
- , . Multilevel psychometric properties of the AHRQ hospital survey on patient safety culture. BMC Health Serv Res. 2010;10:199.
- , , , et al. Becoming a High Reliability Organization: Operational Advice for Hospital Leaders. Prepared by the Lewin Group under contract no. 290‐04‐0011. AHRQ publication no. 08–0022. Rockville, MD: Agency for Healthcare Research and Quality; 2008.
- Health policy brief: Medicare hospital readmissions reduction program. Health Affairs. November 12, 2013. Available at: http://www.healthaffairs.org/healthpolicybriefs/brief.php?brief_id=102. Accessed August 11, 2014.
- , , , , . The care span: the importance of transitional care in achieving health reform. Health Aff (Millwood). 2011;30(4):746–754.
© 2014 Society of Hospital Medicine
Improved Function and Joint Kinematics After Correction of Tibial Malalignment
The tibia is the most commonly fractured long bone in adults, and tibial malunions occur in up to 60% of these patients.1,2 Persistent tibial malalignment, particularly varus alignment, negatively alters gait and joint kinematics, leading to altered weight-bearing forces across the knee and ankle joints. These altered forces may lead to osteoarthritis.3-8
Several studies have identified a relationship between extent of tibial malalignment and changes in joint reaction forces.3,5-7,9-13 Puno and colleagues14 developed a mathematical model to better define the changes in neighboring joints relative to the pattern of the tibia malalignment. Not surprisingly, their work showed that, with distal tibial malunions, altered stress concentrations were realized at the ankle joint, and more proximal tibial deformities led to larger alterations in the joint stresses at the knee. More recently, van der Schoot and colleagues8 found a high prevalence of ipsilateral ankle osteoarthritis with tibial malalignment of 5° or more, and Greenwood and colleagues15 showed a higher incidence of knee pain, lower limb osteoarthritis, and disability in patients with previous tibia fractures. Given these findings, it would seem that correction of tibial malalignment would lead to normative lower extremity joint kinematic values, joint reaction forces, and overall quality of life (QOL).
The ability to ambulate has been recognized as an important milestone in functional recovery after lower extremity injury.2,16,17 Gait analysis, assessment of joint kinematics, and QOL and health status questionnaires can provide information to evaluate rehabilitation protocols, treatment algorithms, and surgical outcomes. Recently, these measures have been used to assess patients recovering from acetabular fractures, femoral shaft fractures, and calcaneal fractures.4,11,17-24 However, no study has used these measures to assess the benefits of surgical correction of malaligned tibias.
We conducted a study to determine improvement in gait, joint kinematics, and patients’ perceptions of overall well-being after surgical correction of tibial malunions. The null hypothesis was that correction of tibial malunion would have no effect on gait, joint kinematics, or patients’ perceptions of function and QOL.
Materials and Methods
This prospective double-time-point study, which was approved by the Institutional Review Board of Washington University/Barnes-Jewish Hospital, evaluated 11 consecutive patients with a varus tibial malunion treated by a single surgeon between September 2003 and January 2006. All patients were treated using a technique that included oblique osteotomy and open reduction and internal fixation (ORIF) or osteotomy and intramedullary nailing. Study inclusion criteria were age 18 years or older; symptomatic varus malunion of the tibia of 10º or more; absence of a developmental or pathologic process leading to the fracture and subsequent deformity; no neurologic deficit of either lower extremity or contralateral lower extremity deformity; and ability to ambulate 9 meters with or without use of an assistive device.
The 11 patients (6 men, 5 women) who met these criteria enrolled in the study. Mean age was 53 years (range, 43-68 years). Eight malunions involved the left tibia. The mechanisms of injury were motor vehicle crash (6 patients), fall from a great height (3), being struck by a motor vehicle (1), and gunshot (1). Mean time from injury to corrective surgery was 16.9 years (range, 1-34 years). Before surgery, each patient had a thorough physical examination, with plain radiographs, including anteroposterior (AP), lateral, and oblique views, obtained to assess degree of limb malalignment. Patients completed the Short Form-36 (SF-36) and the Musculoskeletal Function Assessment (MFA) and underwent joint kinematics and gait analysis. Five malunions were located in the mid-diaphysis of the tibia, 3 in the proximal third, and 2 in the distal third of the tibial shaft. One patient had posttraumatic deformity involving the proximal and the mid-diaphysis (Table 1). After surgery, each patient was followed at regular intervals in the surgeon’s private office. Minimum follow-up was 7 months (mean, 11 months; range 7-17 months). At follow-up, radiographs were obtained, and each patient completed the SF-36 and the MFA and underwent joint kinematics and gait analysis.
We obtained preoperative AP and lateral radiographs of the malaligned and contralateral normal tibias for each patient. Angular deformity was determined in the sagittal and coronal planes to determine location and magnitude of the deformity. Specifically, on each AP and lateral radiograph, a line was drawn the length of the tibia proximal and distal to the area of the deformity. The angle generated by the intersection of these lines on the AP and lateral radiographs was then plotted on a grid to determine the precise plane and magnitude of the deformity (Table 2).1,12 Clinically, relevant rotational deformity of the involved limb was assessed by physical examination, and the results were compared with those of the contralateral limb. Owing to the lack of considerable rotational deformity in any of these 11 patients, we did not obtain computed tomography scans for further assessment of rotation.
Perioperative intravenous antibiotics were administered: 2 g cefazolin 30 minutes before incision and 1 g every 8 hours for 24 hours after surgery. A pneumatic tourniquet was placed on the proximal thigh, and the entire leg was prepared and draped in a sterile fashion. The limb was elevated and exsanguinated with an Esmark bandage and the tourniquet raised to 250 mm Hg. With fluoroscopy, the site of the tibial deformity was identified. Generally, an incision was made centered over the apex of the deformity and one fingerbreadth lateral to the palpable tibial crest. In most cases, the anterolateral aspect of the tibia was exposed while minimizing soft-tissue and periosteal stripping. The plane of the maximum deformity was identified with both direct visualization and fluoroscopy. The osteotomy was performed with an oscillating saw, and in each case a fibular osteotomy was also performed. Malalignment was corrected using a combination of manual manipulation and femoral distractor.25,26 Intraoperative biplanar radiographs were compared with our preoperative plan and with reversed images of the contralateral tibia to assess correction of the deformity. If lengthening was required, in addition to the tibial osteotomy, a corticotomy was created, and a circular external fixator applied and distraction osteogenesis performed.
We maintained the limbs in a short-leg splint for about 10 days after surgery and then initiated active-assisted range of motion of neighboring joints. Patients were maintained on toe-touch weight-bearing for the initial 6 weeks and were then advanced to partial weight-bearing (23 kg). Physical therapy for lower extremity strengthening and gait training was started 6 weeks after surgery. Three months after surgery, patients were advanced to weight-bearing as tolerated and were allowed to return to their activities of daily living without restrictions if radiographs and clinical examination were consistent with healing of the osteotomy.
Each patient was examined and radiographs obtained at regular intervals (2, 6, and 12 weeks and then about every 3 months) after surgery until healing. Bone union was determined by history and physical examination with pain-free weight-bearing without use of assistive devices and by return of functional use of the extremity. Radiographic union was considered to have occurred when bridging trabeculae were present across the osteotomy and there was no loosening or failure of the implants. Occasionally, if there were questions regarding healing, a musculoskeletal radiologist was consulted. Acceptable tibia alignment was defined as alignment of less than 5° varus or less than 10° valgus in the coronal plane and less than 15° procurvatum or recurvatum in the sagittal plane. Immediate postoperative radiographs and most recent radiographs were used to determine the final amount of angular correction.27
Two patients subsequently required secondary operative procedures. One had varus collapse through the regenerate, and the other developed a nonunion of the osteotomy site and required exchange intramedullary nailing. In each case, the final assessment was done after the patient had healed after the second surgery and had fully recovered.
Perceived Functional Assessment
The MFA is a 100-item self-administered QOL questionnaire designed to assess self-perception of physical, psychological, and social well-being in patients with a musculoskeletal injury or condition. The MFA provides a summary score and separate score for each of 10 functional domains. The lower the score, the better the patient’s perception of function. Validated and published norms are available.20,28-30
Perceived Health Status
The Short Form-36 is a 36-item multipurpose self-administered health survey questionnaire. The SF-36, which assesses overall health status, provides a Physical Component Score (PCS) and a Mental Component Score (MCS). The higher the score, the better the patient’s perception of function. Validated and published norms are available.31
Gait Analysis
Video data from a 6-camera high-resolution system (Motion Analysis, Santa Rosa, California) were used to assess gait. A set of 3 reflective surface markers was placed on each of 4 areas: trunk, thighs, legs, and feet.18,19 The patient walked barefoot along a 9-meter walkway, and video data were collected during the middle 2 meters. For each patient, data from 4 to 7 trials were collected. Computerized software produced data describing the averaged joint angle as a function of the gait cycle for each of the 3 principal planes of the body. Specific points in the gait cycle were analyzed. Variables included maximum knee varus in stance phase; maximum knee valgus in swing; maximum knee flexion in stance and swing; minimum knee flexion in stance; maximum ankle inversion in terminal stance; maximum ankle eversion in stance; maximum ankle dorsiflexion in stance and swing; and maximum ankle plantarflexion at takeoff. In addition to the lower extremity joint kinematics, angular measurements, basic gait measurements of step length, stride length, cadence, and speed were also recorded.
Statistical Analysis
Paired t tests were used to determine if significant changes occurred as a consequence of the surgery for the outcome variables (P < .05). Normative gait data were used to assess the quality of any changes that occurred in the variables, but no statistical analysis was performed to determine significant differences.18
Results
All 11 patients had clinical and radiographic evidence of healing and deformity correction at most recent follow-up. Nine patients (82%) healed after the index procedure. Mean total angular correction in the coronal plane was 21° (range, 14° varus to 7° valgus), and mean total angular correction in the sagittal plane was 9° (range, 21° recurvatum to 15° procurvatum) (Table 2).
For the group, mean preoperative MFA score was 39 (SD, 18; range, 10-69), and mean postoperative MFA score was 28 (SD, 14; range, 8-53). Patients reported the most improvement in 2 domains: In Leisure, mean (SD) preoperative score was 8 (2), and mean postoperative score was 5 (2); in Emotional, mean preoperative score was 5 (2), and mean postoperative score was 4 (1). The other domains were not significantly different between the 2 assessments.
On the SF-36, mean (SD) PCS significantly (P < .05) improved from 32 (8) to 43 (9). Mean (SD) MCS showed little change: preoperative, 46 (16); postoperative, 48 (13). The PCS subcategories that showed the most improvement were Physical Function, mean (SD) preoperative, 26 (20), to postoperative, 52 (26); Role of Physical Health, preoperative, 18 (24), to postoperative, 60 (41); and Bodily Pain, preoperative, 39 (27), to 58 (18).
The results from the preoperative and postoperative gait analysis showed no significant differences for the ankle, knee, and hip variables during swing phase (Table 3). In an analysis of the changes in joint kinematics during stance, maximum hip adduction (increased) and maximum knee varus (decreased) on the operative side were significantly improved toward normative values as a consequence of the surgery (Table 3). The other kinematic stance variables were not significantly different. No significant changes were observed in stance time, step length, stride length, cadence, or speed as a consequence of the surgery (Table 4).
Discussion
Correction of malaligned tibias leads to improved limb alignment and patients’ perceptions of functional abilities and health but had a limited effect on joint kinematics and gait. In a group of like patients, we used common techniques to realign malunited tibias and validated instruments to measure functional outcome, health status, joint kinematics, and gait. The goals of this study were to evaluate changes in perceived function and health status and changes in joint kinematics and gait as a result of correction of a posttraumatic limb deformity.
Other investigators have reported outcomes of treating symptomatic malunions,32 nonunions,24 and leg-length discrepancies.33 In these reports, correction of deformity improved patient satisfaction and function, though objective means of assessment were infrequently used. Good results were reported with use of a dome-shaped supramalleolar osteotomy for the correction of tibial malunion.32 In this study, supramalleolar osteotomy was performed on 8 patients for correction of a malunited tibia. Postoperative assessment included subjective assessment of pain, limp, appearance, instability, and activity. Of these 8 patients, 7 reported overall symptomatic improvement after healing, and the 1 who lost the deformity correction remained symptomatic. Significant improvement in overall health has been reported after successful treatment of tibia nonunions.24 The investigators used the SF-36 to assess patients who underwent treatment for a tibial nonunion. Analysis of these patients’ results showed a significant improvement in physical and mental functioning after healing. In addition, improved gait symmetry was reported in patients successfully treated for leg-length discrepancies.33 Unfortunately, how improvement in gait related to overall patient function was not assessed. In the present study, we used stringent objective and subjective validated instruments to assess changes in joint gait kinematics and functional outcome before and after treatment of a tibial malunion. In general, our results are consistent with published results and indicate that realignment of tibial malunions improves patients’ perceptions of function. Our results also indicate improvements toward normative values in maximal hip adduction and knee varus, thus confirming the efficacy of the surgery from a functional perspective. Unfortunately, we did not show significant improvements in the remaining joint kinematics measurements or temporal gait parameters.
It is not entirely clear whether tibial malalignment leads to degenerative changes of the ipsilateral knee and/or ankle and what role this might play in functioning. In a retrospective analysis of 92 patients, angular deformity within 15° of normal alignment did not lead to ankle arthrosis.9 Milner and colleagues4 found that, though varus malunion of the tibia may lead to arthrosis of the medial compartment of the knee, other factors were more important in causing arthrosis of the ankle.
Wu and colleagues34 used tibial osteotomies in New Zealand white rabbits to investigate cartilage and bone changes of the knee after creation of varus or valgus tibial deformities. Thirty-four weeks after osteotomy, rabbits with up to 30° of deformity had severe cartilage changes with osteophytes, fibrillation, derangement of cell columns, and associated increased subchondral bone density of the knees. Cadaveric studies have also shown increased contact pressures within the knees and ankles with ever increasing amounts of tibial deformity.6,10 In each cadaveric study, malalignment in the distal third of the tibia caused the largest changes in the ankle, and changes in the alignment in the proximal third caused the largest changes in the knee.
Consistent with these animal and cadaveric studies are several retrospective clinical studies that have correlated tibial malalignment (particularly varus) with development of knee and ankle arthrosis.3,5,8 Kettelkamp and colleagues3 found a direct correlation between magnitude of deformity and length of time with development of knee arthrosis. These findings have led many to recommend that surgeons try to restore tibial alignment to as near normal as possible to reduce the likelihood of arthrosis after tibia fracture. We found significant improvement toward normative values for maximum hip adduction (increased) and tibial varus (decreased) after surgery. These improvements would shift the weight-bearing forces back to the central part of the knee and therefore more uniformly distribute weight-bearing forces.
Posttraumatic arthrosis that develops after fracture is thought to result from increased joint pressures and possibly factors related to the injury. Although surgical correction of tibial alignment is unlikely to reverse these cartilage changes, it may restore joint pressure symmetry and “offload” compromised compartments. Offloading of already degenerative compartments may explain our patients’ improved perceptions of function and overall health status.
There were several limitations to our study. First, plain radiographs of malaligned and uninjured tibia and fibula were used, and these do not allow complete assessment of the weight-bearing access of the limb. Our patients, however, had isolated tibia fractures, which involved a normal limb before injury, so any alterations in joint kinematics, gait, or function would likely be the result of the fracture. Another limitation of our study is its nonrandomized design. However, the patients reflect the typical heterogeneous trauma patient population, who typically develop tibial malunions and seek correction. Another limitation was the lack of a treatment protocol regarding exact surgical technique and implants used to stabilize the osteotomies. In general, the patients were treated similarly, and their preoperative and postoperative assessments were exactly the same, as was their state-of-the-art joint kinematics and gait analysis, combined with the use of previously validated outcome measures. In addition, the lack of improvement in gait could have resulted from postoperative physical therapy that focused on joint mobilization and muscle strengthening and not on correction of abnormal gait parameters noted on preoperative gait analysis. Despite the potential limitations of the study, surgical correction of these symptomatic tibial malunions resulted in significant improvement in functional outcome and improved joint kinematics on the operative side.
Conclusion
Significant effort should be made to restore and maintain near-anatomical tibial alignment until a tibia fracture is healed. In patients who develop a symptomatic tibial malunion, surgical correction should be undertaken with the intent to restore normal limb alignment and improve joint kinematics, function, and overall health status.
1. Probe RA. Lower extremity angular malunion: evaluation and surgical correction. J Am Acad Orthop Surg. 2003;11(5):302-311.
2. van der Linden W, Larsson K. Plate fixation versus conservative treatment of tibial shaft fractures. A randomized trial. J Bone Joint Surg Am. 1979;61(6):873-878.
3. Kettelkamp DB, Hillberry BM, Murrish DE, Heck DA. Degenerative arthritis of the knee secondary to fracture malunion. Clin Orthop. 1988;(234):159-169.
4. Milner SA, Davis TR, Muir KR, Greenwood DC, Doherty M. Long-term outcome after tibial shaft fracture: is malunion important? J Bone Joint Surg Am. 2002;84(6):971-980.
5. Puno RM, Vaughan JJ, Stetten ML, Johnson JR. Long-term effects of tibial angular malunion on the knee and ankle joints. J Orthop Trauma. 1991;5(3):247-254.
6. Tarr RR, Resnick CT, Wagner KS, Sarmiento A. Changes in tibiotalar joint contact areas following experimentally induced tibial angular deformities. Clin Orthop. 1985;(199):72-80.
7. Ting AJ, Tarr RR, Sarmiento A, Wagner K, Resnick C. The role of subtalar motion and ankle contact pressure changes from angular deformities of the tibia. Foot Ankle. 1987;7(5):290-299.
8. van der Schoot DK, Den Outer AJ, Bode PJ, Obermann WR, van Vugt AB. Degenerative changes at the knee and ankle related to malunion of tibial fractures. 15-year follow-up of 88 patients. J Bone Joint Surg Br. 1996;78(5):722-725.
9. Kristensen KD, Kiaer T, Blicher J. No arthrosis of the ankle 20 years after malaligned tibial-shaft fracture. Acta Orthop Scand. 1989;60(2):208-209.
10. McKellop HA, Sigholm G, Redfern FC, Doyle B, Sarmiento A, Luck JV Sr. The effect of simulated fracture-angulations of the tibia on cartilage pressures in the knee joint. J Bone Joint Surg Am. 1991;73(9):1382-1391.
11. Merchant TC, Dietz FR. Long-term follow-up after fractures of the tibial and fibular shafts. J Bone Joint Surg Am. 1989;71(4):599-606.
12. Paley D, Herzenberg JE, Tetsworth K, McKie J, Bhave A. Deformity planning for frontal and sagittal plane corrective osteotomies. Orthop Clin North Am. 1994;25(3):425-465.
13. Perry J. Gait Analysis: Normal and Pathological Function. Thorofare, NJ: Slack; 1992.
14. Puno RM, Vaughan JJ, von Fraunhofer JA, Stetten ML, Johnson JR. A method of determining the angular malalignments of the knee and ankle joints resulting from a tibial malunion. Clin Orthop. 1987;(223):213-219.
15. Greenwood DC, Muir KR, Doherty M, Milner SA, Stevens M, Davis TR. Conservatively managed tibial shaft fractures in Nottingham, UK: are pain, osteoarthritis, and disability long-term complications? J Epidemiol Community Health. 1997;51(6):701-704.
16. Dehne E, Deffer PA, Hall RM, Brown PW, Johnson EV. The natural history of the fractured tibia. Surg Clin North Am. 1961;41(6):1495-1513.
17. Kitaoka HB, Schaap EJ, Chao EY, An KN. Displaced intra-articular fractures of the calcaneus treated non-operatively. Clinical results and analysis of motion and ground-reaction and temporal forces. J Bone Joint Surg Am. 1994;76(10):1531-1540.
18. Borrelli J Jr, Goldfarb C, Ricci W, Wagner JM, Engsberg JR. Functional outcome after isolated acetabular fractures. J Orthop Trauma. 2002;16(2):73-81.
19. Borrelli J Jr, Ricci WM, Anglen JO, Gregush R, Engsberg J. Muscle strength recovery and its effects on outcome after open reduction and internal fixation of acetabular fractures. J Orthop Trauma. 2006;20(6):388-395.
20. Jaglal S, Lakhani Z, Schatzker J. Reliability, validity, and responsiveness of the lower extremity measure for patients with a hip fracture. J Bone Joint Surg Am. 2000;82(7):955-962.
21. Madsen MS, Ritter MA, Morris HH, et al. The effect of total hip arthroplasty surgical approach on gait. J Orthop Res. 2004;22(1):44-50.
22. Mittlmeier T, Morlock MM, Hertlein H, et al. Analysis of morphology and gait function after intraarticular calcaneal fracture. J Orthop Trauma. 1993;7(4):303-310.
23. Song KM, Halliday SE, Little DG. The effect of limb-length discrepancy on gait. J Bone Joint Surg Am. 1997;79(11):1690-1698.
24. Zlowodzki M, Obremskey WT, Thomison JB, Kregor PJ. Functional outcome after treatment of lower-extremity nonunions. J Trauma. 2005;58(2):312-317.
25. Sanders R, Anglen JO, Mark JB. Oblique osteotomy for the correction of tibial malunion. J Bone Joint Surg Am. 1995;77(2):240-246.
26. Sangeorzan BJ, Sangeorzan BP, Hansen ST Jr, Judd RP. Mathematically directed single-cut osteotomy for correction of tibial malunion. J Orthop Trauma. 1989;3(4):267-275.
27. Borrelli J Jr, Leduc S, Gregush R, Ricci WM. Tricortical bone grafts for treatment of malaligned tibias and fibulas. Clin Orthop. 2009;467(4):1056-1063.
28. Engelberg R, Martin DP, Agel J, Obremsky W, Coronado G, Swiontkowski MF. Musculoskeletal Function Assessment instrument: criterion and construct validity. J Orthop Res. 1996;14(2):182-192.
29. Engelberg R, Martin DP, Agel J, Swiontkowski MF. Musculoskeletal Function Assessment: reference values for patient and non-patient samples. J Orthop Res. 1999;17(1):101-109.
30. Swiontkowski MF, Engelberg R, Martin DP, Agel J. Short Musculoskeletal Function Assessment questionnaire: validity, reliability, and responsiveness. J Bone Joint Surg Am. 1999;81(9):1245-1260.
31. Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473-483.
32. Graehl PM, Hersh MR, Heckman JD. Supramalleolar osteotomy for the treatment of symptomatic tibial malunion. J Orthop Trauma. 1987;1(4):281-292.
33. Bhave A, Paley D, Herzenberg JE. Improvement in gait parameters after lengthening for the treatment of limb-length discrepancy. J Bone Joint Surg Am. 1999;81(4):529-534.
34. Wu DD, Burr DB, Boyd RD, Radin EL. Bone and cartilage changes following experimental varus or valgus tibial angulation. J Orthop Res. 1990;8(4):572-585.
The tibia is the most commonly fractured long bone in adults, and tibial malunions occur in up to 60% of these patients.1,2 Persistent tibial malalignment, particularly varus alignment, negatively alters gait and joint kinematics, leading to altered weight-bearing forces across the knee and ankle joints. These altered forces may lead to osteoarthritis.3-8
Several studies have identified a relationship between extent of tibial malalignment and changes in joint reaction forces.3,5-7,9-13 Puno and colleagues14 developed a mathematical model to better define the changes in neighboring joints relative to the pattern of the tibia malalignment. Not surprisingly, their work showed that, with distal tibial malunions, altered stress concentrations were realized at the ankle joint, and more proximal tibial deformities led to larger alterations in the joint stresses at the knee. More recently, van der Schoot and colleagues8 found a high prevalence of ipsilateral ankle osteoarthritis with tibial malalignment of 5° or more, and Greenwood and colleagues15 showed a higher incidence of knee pain, lower limb osteoarthritis, and disability in patients with previous tibia fractures. Given these findings, it would seem that correction of tibial malalignment would lead to normative lower extremity joint kinematic values, joint reaction forces, and overall quality of life (QOL).
The ability to ambulate has been recognized as an important milestone in functional recovery after lower extremity injury.2,16,17 Gait analysis, assessment of joint kinematics, and QOL and health status questionnaires can provide information to evaluate rehabilitation protocols, treatment algorithms, and surgical outcomes. Recently, these measures have been used to assess patients recovering from acetabular fractures, femoral shaft fractures, and calcaneal fractures.4,11,17-24 However, no study has used these measures to assess the benefits of surgical correction of malaligned tibias.
We conducted a study to determine improvement in gait, joint kinematics, and patients’ perceptions of overall well-being after surgical correction of tibial malunions. The null hypothesis was that correction of tibial malunion would have no effect on gait, joint kinematics, or patients’ perceptions of function and QOL.
Materials and Methods
This prospective double-time-point study, which was approved by the Institutional Review Board of Washington University/Barnes-Jewish Hospital, evaluated 11 consecutive patients with a varus tibial malunion treated by a single surgeon between September 2003 and January 2006. All patients were treated using a technique that included oblique osteotomy and open reduction and internal fixation (ORIF) or osteotomy and intramedullary nailing. Study inclusion criteria were age 18 years or older; symptomatic varus malunion of the tibia of 10º or more; absence of a developmental or pathologic process leading to the fracture and subsequent deformity; no neurologic deficit of either lower extremity or contralateral lower extremity deformity; and ability to ambulate 9 meters with or without use of an assistive device.
The 11 patients (6 men, 5 women) who met these criteria enrolled in the study. Mean age was 53 years (range, 43-68 years). Eight malunions involved the left tibia. The mechanisms of injury were motor vehicle crash (6 patients), fall from a great height (3), being struck by a motor vehicle (1), and gunshot (1). Mean time from injury to corrective surgery was 16.9 years (range, 1-34 years). Before surgery, each patient had a thorough physical examination, with plain radiographs, including anteroposterior (AP), lateral, and oblique views, obtained to assess degree of limb malalignment. Patients completed the Short Form-36 (SF-36) and the Musculoskeletal Function Assessment (MFA) and underwent joint kinematics and gait analysis. Five malunions were located in the mid-diaphysis of the tibia, 3 in the proximal third, and 2 in the distal third of the tibial shaft. One patient had posttraumatic deformity involving the proximal and the mid-diaphysis (Table 1). After surgery, each patient was followed at regular intervals in the surgeon’s private office. Minimum follow-up was 7 months (mean, 11 months; range 7-17 months). At follow-up, radiographs were obtained, and each patient completed the SF-36 and the MFA and underwent joint kinematics and gait analysis.
We obtained preoperative AP and lateral radiographs of the malaligned and contralateral normal tibias for each patient. Angular deformity was determined in the sagittal and coronal planes to determine location and magnitude of the deformity. Specifically, on each AP and lateral radiograph, a line was drawn the length of the tibia proximal and distal to the area of the deformity. The angle generated by the intersection of these lines on the AP and lateral radiographs was then plotted on a grid to determine the precise plane and magnitude of the deformity (Table 2).1,12 Clinically, relevant rotational deformity of the involved limb was assessed by physical examination, and the results were compared with those of the contralateral limb. Owing to the lack of considerable rotational deformity in any of these 11 patients, we did not obtain computed tomography scans for further assessment of rotation.
Perioperative intravenous antibiotics were administered: 2 g cefazolin 30 minutes before incision and 1 g every 8 hours for 24 hours after surgery. A pneumatic tourniquet was placed on the proximal thigh, and the entire leg was prepared and draped in a sterile fashion. The limb was elevated and exsanguinated with an Esmark bandage and the tourniquet raised to 250 mm Hg. With fluoroscopy, the site of the tibial deformity was identified. Generally, an incision was made centered over the apex of the deformity and one fingerbreadth lateral to the palpable tibial crest. In most cases, the anterolateral aspect of the tibia was exposed while minimizing soft-tissue and periosteal stripping. The plane of the maximum deformity was identified with both direct visualization and fluoroscopy. The osteotomy was performed with an oscillating saw, and in each case a fibular osteotomy was also performed. Malalignment was corrected using a combination of manual manipulation and femoral distractor.25,26 Intraoperative biplanar radiographs were compared with our preoperative plan and with reversed images of the contralateral tibia to assess correction of the deformity. If lengthening was required, in addition to the tibial osteotomy, a corticotomy was created, and a circular external fixator applied and distraction osteogenesis performed.
We maintained the limbs in a short-leg splint for about 10 days after surgery and then initiated active-assisted range of motion of neighboring joints. Patients were maintained on toe-touch weight-bearing for the initial 6 weeks and were then advanced to partial weight-bearing (23 kg). Physical therapy for lower extremity strengthening and gait training was started 6 weeks after surgery. Three months after surgery, patients were advanced to weight-bearing as tolerated and were allowed to return to their activities of daily living without restrictions if radiographs and clinical examination were consistent with healing of the osteotomy.
Each patient was examined and radiographs obtained at regular intervals (2, 6, and 12 weeks and then about every 3 months) after surgery until healing. Bone union was determined by history and physical examination with pain-free weight-bearing without use of assistive devices and by return of functional use of the extremity. Radiographic union was considered to have occurred when bridging trabeculae were present across the osteotomy and there was no loosening or failure of the implants. Occasionally, if there were questions regarding healing, a musculoskeletal radiologist was consulted. Acceptable tibia alignment was defined as alignment of less than 5° varus or less than 10° valgus in the coronal plane and less than 15° procurvatum or recurvatum in the sagittal plane. Immediate postoperative radiographs and most recent radiographs were used to determine the final amount of angular correction.27
Two patients subsequently required secondary operative procedures. One had varus collapse through the regenerate, and the other developed a nonunion of the osteotomy site and required exchange intramedullary nailing. In each case, the final assessment was done after the patient had healed after the second surgery and had fully recovered.
Perceived Functional Assessment
The MFA is a 100-item self-administered QOL questionnaire designed to assess self-perception of physical, psychological, and social well-being in patients with a musculoskeletal injury or condition. The MFA provides a summary score and separate score for each of 10 functional domains. The lower the score, the better the patient’s perception of function. Validated and published norms are available.20,28-30
Perceived Health Status
The Short Form-36 is a 36-item multipurpose self-administered health survey questionnaire. The SF-36, which assesses overall health status, provides a Physical Component Score (PCS) and a Mental Component Score (MCS). The higher the score, the better the patient’s perception of function. Validated and published norms are available.31
Gait Analysis
Video data from a 6-camera high-resolution system (Motion Analysis, Santa Rosa, California) were used to assess gait. A set of 3 reflective surface markers was placed on each of 4 areas: trunk, thighs, legs, and feet.18,19 The patient walked barefoot along a 9-meter walkway, and video data were collected during the middle 2 meters. For each patient, data from 4 to 7 trials were collected. Computerized software produced data describing the averaged joint angle as a function of the gait cycle for each of the 3 principal planes of the body. Specific points in the gait cycle were analyzed. Variables included maximum knee varus in stance phase; maximum knee valgus in swing; maximum knee flexion in stance and swing; minimum knee flexion in stance; maximum ankle inversion in terminal stance; maximum ankle eversion in stance; maximum ankle dorsiflexion in stance and swing; and maximum ankle plantarflexion at takeoff. In addition to the lower extremity joint kinematics, angular measurements, basic gait measurements of step length, stride length, cadence, and speed were also recorded.
Statistical Analysis
Paired t tests were used to determine if significant changes occurred as a consequence of the surgery for the outcome variables (P < .05). Normative gait data were used to assess the quality of any changes that occurred in the variables, but no statistical analysis was performed to determine significant differences.18
Results
All 11 patients had clinical and radiographic evidence of healing and deformity correction at most recent follow-up. Nine patients (82%) healed after the index procedure. Mean total angular correction in the coronal plane was 21° (range, 14° varus to 7° valgus), and mean total angular correction in the sagittal plane was 9° (range, 21° recurvatum to 15° procurvatum) (Table 2).
For the group, mean preoperative MFA score was 39 (SD, 18; range, 10-69), and mean postoperative MFA score was 28 (SD, 14; range, 8-53). Patients reported the most improvement in 2 domains: In Leisure, mean (SD) preoperative score was 8 (2), and mean postoperative score was 5 (2); in Emotional, mean preoperative score was 5 (2), and mean postoperative score was 4 (1). The other domains were not significantly different between the 2 assessments.
On the SF-36, mean (SD) PCS significantly (P < .05) improved from 32 (8) to 43 (9). Mean (SD) MCS showed little change: preoperative, 46 (16); postoperative, 48 (13). The PCS subcategories that showed the most improvement were Physical Function, mean (SD) preoperative, 26 (20), to postoperative, 52 (26); Role of Physical Health, preoperative, 18 (24), to postoperative, 60 (41); and Bodily Pain, preoperative, 39 (27), to 58 (18).
The results from the preoperative and postoperative gait analysis showed no significant differences for the ankle, knee, and hip variables during swing phase (Table 3). In an analysis of the changes in joint kinematics during stance, maximum hip adduction (increased) and maximum knee varus (decreased) on the operative side were significantly improved toward normative values as a consequence of the surgery (Table 3). The other kinematic stance variables were not significantly different. No significant changes were observed in stance time, step length, stride length, cadence, or speed as a consequence of the surgery (Table 4).
Discussion
Correction of malaligned tibias leads to improved limb alignment and patients’ perceptions of functional abilities and health but had a limited effect on joint kinematics and gait. In a group of like patients, we used common techniques to realign malunited tibias and validated instruments to measure functional outcome, health status, joint kinematics, and gait. The goals of this study were to evaluate changes in perceived function and health status and changes in joint kinematics and gait as a result of correction of a posttraumatic limb deformity.
Other investigators have reported outcomes of treating symptomatic malunions,32 nonunions,24 and leg-length discrepancies.33 In these reports, correction of deformity improved patient satisfaction and function, though objective means of assessment were infrequently used. Good results were reported with use of a dome-shaped supramalleolar osteotomy for the correction of tibial malunion.32 In this study, supramalleolar osteotomy was performed on 8 patients for correction of a malunited tibia. Postoperative assessment included subjective assessment of pain, limp, appearance, instability, and activity. Of these 8 patients, 7 reported overall symptomatic improvement after healing, and the 1 who lost the deformity correction remained symptomatic. Significant improvement in overall health has been reported after successful treatment of tibia nonunions.24 The investigators used the SF-36 to assess patients who underwent treatment for a tibial nonunion. Analysis of these patients’ results showed a significant improvement in physical and mental functioning after healing. In addition, improved gait symmetry was reported in patients successfully treated for leg-length discrepancies.33 Unfortunately, how improvement in gait related to overall patient function was not assessed. In the present study, we used stringent objective and subjective validated instruments to assess changes in joint gait kinematics and functional outcome before and after treatment of a tibial malunion. In general, our results are consistent with published results and indicate that realignment of tibial malunions improves patients’ perceptions of function. Our results also indicate improvements toward normative values in maximal hip adduction and knee varus, thus confirming the efficacy of the surgery from a functional perspective. Unfortunately, we did not show significant improvements in the remaining joint kinematics measurements or temporal gait parameters.
It is not entirely clear whether tibial malalignment leads to degenerative changes of the ipsilateral knee and/or ankle and what role this might play in functioning. In a retrospective analysis of 92 patients, angular deformity within 15° of normal alignment did not lead to ankle arthrosis.9 Milner and colleagues4 found that, though varus malunion of the tibia may lead to arthrosis of the medial compartment of the knee, other factors were more important in causing arthrosis of the ankle.
Wu and colleagues34 used tibial osteotomies in New Zealand white rabbits to investigate cartilage and bone changes of the knee after creation of varus or valgus tibial deformities. Thirty-four weeks after osteotomy, rabbits with up to 30° of deformity had severe cartilage changes with osteophytes, fibrillation, derangement of cell columns, and associated increased subchondral bone density of the knees. Cadaveric studies have also shown increased contact pressures within the knees and ankles with ever increasing amounts of tibial deformity.6,10 In each cadaveric study, malalignment in the distal third of the tibia caused the largest changes in the ankle, and changes in the alignment in the proximal third caused the largest changes in the knee.
Consistent with these animal and cadaveric studies are several retrospective clinical studies that have correlated tibial malalignment (particularly varus) with development of knee and ankle arthrosis.3,5,8 Kettelkamp and colleagues3 found a direct correlation between magnitude of deformity and length of time with development of knee arthrosis. These findings have led many to recommend that surgeons try to restore tibial alignment to as near normal as possible to reduce the likelihood of arthrosis after tibia fracture. We found significant improvement toward normative values for maximum hip adduction (increased) and tibial varus (decreased) after surgery. These improvements would shift the weight-bearing forces back to the central part of the knee and therefore more uniformly distribute weight-bearing forces.
Posttraumatic arthrosis that develops after fracture is thought to result from increased joint pressures and possibly factors related to the injury. Although surgical correction of tibial alignment is unlikely to reverse these cartilage changes, it may restore joint pressure symmetry and “offload” compromised compartments. Offloading of already degenerative compartments may explain our patients’ improved perceptions of function and overall health status.
There were several limitations to our study. First, plain radiographs of malaligned and uninjured tibia and fibula were used, and these do not allow complete assessment of the weight-bearing access of the limb. Our patients, however, had isolated tibia fractures, which involved a normal limb before injury, so any alterations in joint kinematics, gait, or function would likely be the result of the fracture. Another limitation of our study is its nonrandomized design. However, the patients reflect the typical heterogeneous trauma patient population, who typically develop tibial malunions and seek correction. Another limitation was the lack of a treatment protocol regarding exact surgical technique and implants used to stabilize the osteotomies. In general, the patients were treated similarly, and their preoperative and postoperative assessments were exactly the same, as was their state-of-the-art joint kinematics and gait analysis, combined with the use of previously validated outcome measures. In addition, the lack of improvement in gait could have resulted from postoperative physical therapy that focused on joint mobilization and muscle strengthening and not on correction of abnormal gait parameters noted on preoperative gait analysis. Despite the potential limitations of the study, surgical correction of these symptomatic tibial malunions resulted in significant improvement in functional outcome and improved joint kinematics on the operative side.
Conclusion
Significant effort should be made to restore and maintain near-anatomical tibial alignment until a tibia fracture is healed. In patients who develop a symptomatic tibial malunion, surgical correction should be undertaken with the intent to restore normal limb alignment and improve joint kinematics, function, and overall health status.
The tibia is the most commonly fractured long bone in adults, and tibial malunions occur in up to 60% of these patients.1,2 Persistent tibial malalignment, particularly varus alignment, negatively alters gait and joint kinematics, leading to altered weight-bearing forces across the knee and ankle joints. These altered forces may lead to osteoarthritis.3-8
Several studies have identified a relationship between extent of tibial malalignment and changes in joint reaction forces.3,5-7,9-13 Puno and colleagues14 developed a mathematical model to better define the changes in neighboring joints relative to the pattern of the tibia malalignment. Not surprisingly, their work showed that, with distal tibial malunions, altered stress concentrations were realized at the ankle joint, and more proximal tibial deformities led to larger alterations in the joint stresses at the knee. More recently, van der Schoot and colleagues8 found a high prevalence of ipsilateral ankle osteoarthritis with tibial malalignment of 5° or more, and Greenwood and colleagues15 showed a higher incidence of knee pain, lower limb osteoarthritis, and disability in patients with previous tibia fractures. Given these findings, it would seem that correction of tibial malalignment would lead to normative lower extremity joint kinematic values, joint reaction forces, and overall quality of life (QOL).
The ability to ambulate has been recognized as an important milestone in functional recovery after lower extremity injury.2,16,17 Gait analysis, assessment of joint kinematics, and QOL and health status questionnaires can provide information to evaluate rehabilitation protocols, treatment algorithms, and surgical outcomes. Recently, these measures have been used to assess patients recovering from acetabular fractures, femoral shaft fractures, and calcaneal fractures.4,11,17-24 However, no study has used these measures to assess the benefits of surgical correction of malaligned tibias.
We conducted a study to determine improvement in gait, joint kinematics, and patients’ perceptions of overall well-being after surgical correction of tibial malunions. The null hypothesis was that correction of tibial malunion would have no effect on gait, joint kinematics, or patients’ perceptions of function and QOL.
Materials and Methods
This prospective double-time-point study, which was approved by the Institutional Review Board of Washington University/Barnes-Jewish Hospital, evaluated 11 consecutive patients with a varus tibial malunion treated by a single surgeon between September 2003 and January 2006. All patients were treated using a technique that included oblique osteotomy and open reduction and internal fixation (ORIF) or osteotomy and intramedullary nailing. Study inclusion criteria were age 18 years or older; symptomatic varus malunion of the tibia of 10º or more; absence of a developmental or pathologic process leading to the fracture and subsequent deformity; no neurologic deficit of either lower extremity or contralateral lower extremity deformity; and ability to ambulate 9 meters with or without use of an assistive device.
The 11 patients (6 men, 5 women) who met these criteria enrolled in the study. Mean age was 53 years (range, 43-68 years). Eight malunions involved the left tibia. The mechanisms of injury were motor vehicle crash (6 patients), fall from a great height (3), being struck by a motor vehicle (1), and gunshot (1). Mean time from injury to corrective surgery was 16.9 years (range, 1-34 years). Before surgery, each patient had a thorough physical examination, with plain radiographs, including anteroposterior (AP), lateral, and oblique views, obtained to assess degree of limb malalignment. Patients completed the Short Form-36 (SF-36) and the Musculoskeletal Function Assessment (MFA) and underwent joint kinematics and gait analysis. Five malunions were located in the mid-diaphysis of the tibia, 3 in the proximal third, and 2 in the distal third of the tibial shaft. One patient had posttraumatic deformity involving the proximal and the mid-diaphysis (Table 1). After surgery, each patient was followed at regular intervals in the surgeon’s private office. Minimum follow-up was 7 months (mean, 11 months; range 7-17 months). At follow-up, radiographs were obtained, and each patient completed the SF-36 and the MFA and underwent joint kinematics and gait analysis.
We obtained preoperative AP and lateral radiographs of the malaligned and contralateral normal tibias for each patient. Angular deformity was determined in the sagittal and coronal planes to determine location and magnitude of the deformity. Specifically, on each AP and lateral radiograph, a line was drawn the length of the tibia proximal and distal to the area of the deformity. The angle generated by the intersection of these lines on the AP and lateral radiographs was then plotted on a grid to determine the precise plane and magnitude of the deformity (Table 2).1,12 Clinically, relevant rotational deformity of the involved limb was assessed by physical examination, and the results were compared with those of the contralateral limb. Owing to the lack of considerable rotational deformity in any of these 11 patients, we did not obtain computed tomography scans for further assessment of rotation.
Perioperative intravenous antibiotics were administered: 2 g cefazolin 30 minutes before incision and 1 g every 8 hours for 24 hours after surgery. A pneumatic tourniquet was placed on the proximal thigh, and the entire leg was prepared and draped in a sterile fashion. The limb was elevated and exsanguinated with an Esmark bandage and the tourniquet raised to 250 mm Hg. With fluoroscopy, the site of the tibial deformity was identified. Generally, an incision was made centered over the apex of the deformity and one fingerbreadth lateral to the palpable tibial crest. In most cases, the anterolateral aspect of the tibia was exposed while minimizing soft-tissue and periosteal stripping. The plane of the maximum deformity was identified with both direct visualization and fluoroscopy. The osteotomy was performed with an oscillating saw, and in each case a fibular osteotomy was also performed. Malalignment was corrected using a combination of manual manipulation and femoral distractor.25,26 Intraoperative biplanar radiographs were compared with our preoperative plan and with reversed images of the contralateral tibia to assess correction of the deformity. If lengthening was required, in addition to the tibial osteotomy, a corticotomy was created, and a circular external fixator applied and distraction osteogenesis performed.
We maintained the limbs in a short-leg splint for about 10 days after surgery and then initiated active-assisted range of motion of neighboring joints. Patients were maintained on toe-touch weight-bearing for the initial 6 weeks and were then advanced to partial weight-bearing (23 kg). Physical therapy for lower extremity strengthening and gait training was started 6 weeks after surgery. Three months after surgery, patients were advanced to weight-bearing as tolerated and were allowed to return to their activities of daily living without restrictions if radiographs and clinical examination were consistent with healing of the osteotomy.
Each patient was examined and radiographs obtained at regular intervals (2, 6, and 12 weeks and then about every 3 months) after surgery until healing. Bone union was determined by history and physical examination with pain-free weight-bearing without use of assistive devices and by return of functional use of the extremity. Radiographic union was considered to have occurred when bridging trabeculae were present across the osteotomy and there was no loosening or failure of the implants. Occasionally, if there were questions regarding healing, a musculoskeletal radiologist was consulted. Acceptable tibia alignment was defined as alignment of less than 5° varus or less than 10° valgus in the coronal plane and less than 15° procurvatum or recurvatum in the sagittal plane. Immediate postoperative radiographs and most recent radiographs were used to determine the final amount of angular correction.27
Two patients subsequently required secondary operative procedures. One had varus collapse through the regenerate, and the other developed a nonunion of the osteotomy site and required exchange intramedullary nailing. In each case, the final assessment was done after the patient had healed after the second surgery and had fully recovered.
Perceived Functional Assessment
The MFA is a 100-item self-administered QOL questionnaire designed to assess self-perception of physical, psychological, and social well-being in patients with a musculoskeletal injury or condition. The MFA provides a summary score and separate score for each of 10 functional domains. The lower the score, the better the patient’s perception of function. Validated and published norms are available.20,28-30
Perceived Health Status
The Short Form-36 is a 36-item multipurpose self-administered health survey questionnaire. The SF-36, which assesses overall health status, provides a Physical Component Score (PCS) and a Mental Component Score (MCS). The higher the score, the better the patient’s perception of function. Validated and published norms are available.31
Gait Analysis
Video data from a 6-camera high-resolution system (Motion Analysis, Santa Rosa, California) were used to assess gait. A set of 3 reflective surface markers was placed on each of 4 areas: trunk, thighs, legs, and feet.18,19 The patient walked barefoot along a 9-meter walkway, and video data were collected during the middle 2 meters. For each patient, data from 4 to 7 trials were collected. Computerized software produced data describing the averaged joint angle as a function of the gait cycle for each of the 3 principal planes of the body. Specific points in the gait cycle were analyzed. Variables included maximum knee varus in stance phase; maximum knee valgus in swing; maximum knee flexion in stance and swing; minimum knee flexion in stance; maximum ankle inversion in terminal stance; maximum ankle eversion in stance; maximum ankle dorsiflexion in stance and swing; and maximum ankle plantarflexion at takeoff. In addition to the lower extremity joint kinematics, angular measurements, basic gait measurements of step length, stride length, cadence, and speed were also recorded.
Statistical Analysis
Paired t tests were used to determine if significant changes occurred as a consequence of the surgery for the outcome variables (P < .05). Normative gait data were used to assess the quality of any changes that occurred in the variables, but no statistical analysis was performed to determine significant differences.18
Results
All 11 patients had clinical and radiographic evidence of healing and deformity correction at most recent follow-up. Nine patients (82%) healed after the index procedure. Mean total angular correction in the coronal plane was 21° (range, 14° varus to 7° valgus), and mean total angular correction in the sagittal plane was 9° (range, 21° recurvatum to 15° procurvatum) (Table 2).
For the group, mean preoperative MFA score was 39 (SD, 18; range, 10-69), and mean postoperative MFA score was 28 (SD, 14; range, 8-53). Patients reported the most improvement in 2 domains: In Leisure, mean (SD) preoperative score was 8 (2), and mean postoperative score was 5 (2); in Emotional, mean preoperative score was 5 (2), and mean postoperative score was 4 (1). The other domains were not significantly different between the 2 assessments.
On the SF-36, mean (SD) PCS significantly (P < .05) improved from 32 (8) to 43 (9). Mean (SD) MCS showed little change: preoperative, 46 (16); postoperative, 48 (13). The PCS subcategories that showed the most improvement were Physical Function, mean (SD) preoperative, 26 (20), to postoperative, 52 (26); Role of Physical Health, preoperative, 18 (24), to postoperative, 60 (41); and Bodily Pain, preoperative, 39 (27), to 58 (18).
The results from the preoperative and postoperative gait analysis showed no significant differences for the ankle, knee, and hip variables during swing phase (Table 3). In an analysis of the changes in joint kinematics during stance, maximum hip adduction (increased) and maximum knee varus (decreased) on the operative side were significantly improved toward normative values as a consequence of the surgery (Table 3). The other kinematic stance variables were not significantly different. No significant changes were observed in stance time, step length, stride length, cadence, or speed as a consequence of the surgery (Table 4).
Discussion
Correction of malaligned tibias leads to improved limb alignment and patients’ perceptions of functional abilities and health but had a limited effect on joint kinematics and gait. In a group of like patients, we used common techniques to realign malunited tibias and validated instruments to measure functional outcome, health status, joint kinematics, and gait. The goals of this study were to evaluate changes in perceived function and health status and changes in joint kinematics and gait as a result of correction of a posttraumatic limb deformity.
Other investigators have reported outcomes of treating symptomatic malunions,32 nonunions,24 and leg-length discrepancies.33 In these reports, correction of deformity improved patient satisfaction and function, though objective means of assessment were infrequently used. Good results were reported with use of a dome-shaped supramalleolar osteotomy for the correction of tibial malunion.32 In this study, supramalleolar osteotomy was performed on 8 patients for correction of a malunited tibia. Postoperative assessment included subjective assessment of pain, limp, appearance, instability, and activity. Of these 8 patients, 7 reported overall symptomatic improvement after healing, and the 1 who lost the deformity correction remained symptomatic. Significant improvement in overall health has been reported after successful treatment of tibia nonunions.24 The investigators used the SF-36 to assess patients who underwent treatment for a tibial nonunion. Analysis of these patients’ results showed a significant improvement in physical and mental functioning after healing. In addition, improved gait symmetry was reported in patients successfully treated for leg-length discrepancies.33 Unfortunately, how improvement in gait related to overall patient function was not assessed. In the present study, we used stringent objective and subjective validated instruments to assess changes in joint gait kinematics and functional outcome before and after treatment of a tibial malunion. In general, our results are consistent with published results and indicate that realignment of tibial malunions improves patients’ perceptions of function. Our results also indicate improvements toward normative values in maximal hip adduction and knee varus, thus confirming the efficacy of the surgery from a functional perspective. Unfortunately, we did not show significant improvements in the remaining joint kinematics measurements or temporal gait parameters.
It is not entirely clear whether tibial malalignment leads to degenerative changes of the ipsilateral knee and/or ankle and what role this might play in functioning. In a retrospective analysis of 92 patients, angular deformity within 15° of normal alignment did not lead to ankle arthrosis.9 Milner and colleagues4 found that, though varus malunion of the tibia may lead to arthrosis of the medial compartment of the knee, other factors were more important in causing arthrosis of the ankle.
Wu and colleagues34 used tibial osteotomies in New Zealand white rabbits to investigate cartilage and bone changes of the knee after creation of varus or valgus tibial deformities. Thirty-four weeks after osteotomy, rabbits with up to 30° of deformity had severe cartilage changes with osteophytes, fibrillation, derangement of cell columns, and associated increased subchondral bone density of the knees. Cadaveric studies have also shown increased contact pressures within the knees and ankles with ever increasing amounts of tibial deformity.6,10 In each cadaveric study, malalignment in the distal third of the tibia caused the largest changes in the ankle, and changes in the alignment in the proximal third caused the largest changes in the knee.
Consistent with these animal and cadaveric studies are several retrospective clinical studies that have correlated tibial malalignment (particularly varus) with development of knee and ankle arthrosis.3,5,8 Kettelkamp and colleagues3 found a direct correlation between magnitude of deformity and length of time with development of knee arthrosis. These findings have led many to recommend that surgeons try to restore tibial alignment to as near normal as possible to reduce the likelihood of arthrosis after tibia fracture. We found significant improvement toward normative values for maximum hip adduction (increased) and tibial varus (decreased) after surgery. These improvements would shift the weight-bearing forces back to the central part of the knee and therefore more uniformly distribute weight-bearing forces.
Posttraumatic arthrosis that develops after fracture is thought to result from increased joint pressures and possibly factors related to the injury. Although surgical correction of tibial alignment is unlikely to reverse these cartilage changes, it may restore joint pressure symmetry and “offload” compromised compartments. Offloading of already degenerative compartments may explain our patients’ improved perceptions of function and overall health status.
There were several limitations to our study. First, plain radiographs of malaligned and uninjured tibia and fibula were used, and these do not allow complete assessment of the weight-bearing access of the limb. Our patients, however, had isolated tibia fractures, which involved a normal limb before injury, so any alterations in joint kinematics, gait, or function would likely be the result of the fracture. Another limitation of our study is its nonrandomized design. However, the patients reflect the typical heterogeneous trauma patient population, who typically develop tibial malunions and seek correction. Another limitation was the lack of a treatment protocol regarding exact surgical technique and implants used to stabilize the osteotomies. In general, the patients were treated similarly, and their preoperative and postoperative assessments were exactly the same, as was their state-of-the-art joint kinematics and gait analysis, combined with the use of previously validated outcome measures. In addition, the lack of improvement in gait could have resulted from postoperative physical therapy that focused on joint mobilization and muscle strengthening and not on correction of abnormal gait parameters noted on preoperative gait analysis. Despite the potential limitations of the study, surgical correction of these symptomatic tibial malunions resulted in significant improvement in functional outcome and improved joint kinematics on the operative side.
Conclusion
Significant effort should be made to restore and maintain near-anatomical tibial alignment until a tibia fracture is healed. In patients who develop a symptomatic tibial malunion, surgical correction should be undertaken with the intent to restore normal limb alignment and improve joint kinematics, function, and overall health status.
1. Probe RA. Lower extremity angular malunion: evaluation and surgical correction. J Am Acad Orthop Surg. 2003;11(5):302-311.
2. van der Linden W, Larsson K. Plate fixation versus conservative treatment of tibial shaft fractures. A randomized trial. J Bone Joint Surg Am. 1979;61(6):873-878.
3. Kettelkamp DB, Hillberry BM, Murrish DE, Heck DA. Degenerative arthritis of the knee secondary to fracture malunion. Clin Orthop. 1988;(234):159-169.
4. Milner SA, Davis TR, Muir KR, Greenwood DC, Doherty M. Long-term outcome after tibial shaft fracture: is malunion important? J Bone Joint Surg Am. 2002;84(6):971-980.
5. Puno RM, Vaughan JJ, Stetten ML, Johnson JR. Long-term effects of tibial angular malunion on the knee and ankle joints. J Orthop Trauma. 1991;5(3):247-254.
6. Tarr RR, Resnick CT, Wagner KS, Sarmiento A. Changes in tibiotalar joint contact areas following experimentally induced tibial angular deformities. Clin Orthop. 1985;(199):72-80.
7. Ting AJ, Tarr RR, Sarmiento A, Wagner K, Resnick C. The role of subtalar motion and ankle contact pressure changes from angular deformities of the tibia. Foot Ankle. 1987;7(5):290-299.
8. van der Schoot DK, Den Outer AJ, Bode PJ, Obermann WR, van Vugt AB. Degenerative changes at the knee and ankle related to malunion of tibial fractures. 15-year follow-up of 88 patients. J Bone Joint Surg Br. 1996;78(5):722-725.
9. Kristensen KD, Kiaer T, Blicher J. No arthrosis of the ankle 20 years after malaligned tibial-shaft fracture. Acta Orthop Scand. 1989;60(2):208-209.
10. McKellop HA, Sigholm G, Redfern FC, Doyle B, Sarmiento A, Luck JV Sr. The effect of simulated fracture-angulations of the tibia on cartilage pressures in the knee joint. J Bone Joint Surg Am. 1991;73(9):1382-1391.
11. Merchant TC, Dietz FR. Long-term follow-up after fractures of the tibial and fibular shafts. J Bone Joint Surg Am. 1989;71(4):599-606.
12. Paley D, Herzenberg JE, Tetsworth K, McKie J, Bhave A. Deformity planning for frontal and sagittal plane corrective osteotomies. Orthop Clin North Am. 1994;25(3):425-465.
13. Perry J. Gait Analysis: Normal and Pathological Function. Thorofare, NJ: Slack; 1992.
14. Puno RM, Vaughan JJ, von Fraunhofer JA, Stetten ML, Johnson JR. A method of determining the angular malalignments of the knee and ankle joints resulting from a tibial malunion. Clin Orthop. 1987;(223):213-219.
15. Greenwood DC, Muir KR, Doherty M, Milner SA, Stevens M, Davis TR. Conservatively managed tibial shaft fractures in Nottingham, UK: are pain, osteoarthritis, and disability long-term complications? J Epidemiol Community Health. 1997;51(6):701-704.
16. Dehne E, Deffer PA, Hall RM, Brown PW, Johnson EV. The natural history of the fractured tibia. Surg Clin North Am. 1961;41(6):1495-1513.
17. Kitaoka HB, Schaap EJ, Chao EY, An KN. Displaced intra-articular fractures of the calcaneus treated non-operatively. Clinical results and analysis of motion and ground-reaction and temporal forces. J Bone Joint Surg Am. 1994;76(10):1531-1540.
18. Borrelli J Jr, Goldfarb C, Ricci W, Wagner JM, Engsberg JR. Functional outcome after isolated acetabular fractures. J Orthop Trauma. 2002;16(2):73-81.
19. Borrelli J Jr, Ricci WM, Anglen JO, Gregush R, Engsberg J. Muscle strength recovery and its effects on outcome after open reduction and internal fixation of acetabular fractures. J Orthop Trauma. 2006;20(6):388-395.
20. Jaglal S, Lakhani Z, Schatzker J. Reliability, validity, and responsiveness of the lower extremity measure for patients with a hip fracture. J Bone Joint Surg Am. 2000;82(7):955-962.
21. Madsen MS, Ritter MA, Morris HH, et al. The effect of total hip arthroplasty surgical approach on gait. J Orthop Res. 2004;22(1):44-50.
22. Mittlmeier T, Morlock MM, Hertlein H, et al. Analysis of morphology and gait function after intraarticular calcaneal fracture. J Orthop Trauma. 1993;7(4):303-310.
23. Song KM, Halliday SE, Little DG. The effect of limb-length discrepancy on gait. J Bone Joint Surg Am. 1997;79(11):1690-1698.
24. Zlowodzki M, Obremskey WT, Thomison JB, Kregor PJ. Functional outcome after treatment of lower-extremity nonunions. J Trauma. 2005;58(2):312-317.
25. Sanders R, Anglen JO, Mark JB. Oblique osteotomy for the correction of tibial malunion. J Bone Joint Surg Am. 1995;77(2):240-246.
26. Sangeorzan BJ, Sangeorzan BP, Hansen ST Jr, Judd RP. Mathematically directed single-cut osteotomy for correction of tibial malunion. J Orthop Trauma. 1989;3(4):267-275.
27. Borrelli J Jr, Leduc S, Gregush R, Ricci WM. Tricortical bone grafts for treatment of malaligned tibias and fibulas. Clin Orthop. 2009;467(4):1056-1063.
28. Engelberg R, Martin DP, Agel J, Obremsky W, Coronado G, Swiontkowski MF. Musculoskeletal Function Assessment instrument: criterion and construct validity. J Orthop Res. 1996;14(2):182-192.
29. Engelberg R, Martin DP, Agel J, Swiontkowski MF. Musculoskeletal Function Assessment: reference values for patient and non-patient samples. J Orthop Res. 1999;17(1):101-109.
30. Swiontkowski MF, Engelberg R, Martin DP, Agel J. Short Musculoskeletal Function Assessment questionnaire: validity, reliability, and responsiveness. J Bone Joint Surg Am. 1999;81(9):1245-1260.
31. Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473-483.
32. Graehl PM, Hersh MR, Heckman JD. Supramalleolar osteotomy for the treatment of symptomatic tibial malunion. J Orthop Trauma. 1987;1(4):281-292.
33. Bhave A, Paley D, Herzenberg JE. Improvement in gait parameters after lengthening for the treatment of limb-length discrepancy. J Bone Joint Surg Am. 1999;81(4):529-534.
34. Wu DD, Burr DB, Boyd RD, Radin EL. Bone and cartilage changes following experimental varus or valgus tibial angulation. J Orthop Res. 1990;8(4):572-585.
1. Probe RA. Lower extremity angular malunion: evaluation and surgical correction. J Am Acad Orthop Surg. 2003;11(5):302-311.
2. van der Linden W, Larsson K. Plate fixation versus conservative treatment of tibial shaft fractures. A randomized trial. J Bone Joint Surg Am. 1979;61(6):873-878.
3. Kettelkamp DB, Hillberry BM, Murrish DE, Heck DA. Degenerative arthritis of the knee secondary to fracture malunion. Clin Orthop. 1988;(234):159-169.
4. Milner SA, Davis TR, Muir KR, Greenwood DC, Doherty M. Long-term outcome after tibial shaft fracture: is malunion important? J Bone Joint Surg Am. 2002;84(6):971-980.
5. Puno RM, Vaughan JJ, Stetten ML, Johnson JR. Long-term effects of tibial angular malunion on the knee and ankle joints. J Orthop Trauma. 1991;5(3):247-254.
6. Tarr RR, Resnick CT, Wagner KS, Sarmiento A. Changes in tibiotalar joint contact areas following experimentally induced tibial angular deformities. Clin Orthop. 1985;(199):72-80.
7. Ting AJ, Tarr RR, Sarmiento A, Wagner K, Resnick C. The role of subtalar motion and ankle contact pressure changes from angular deformities of the tibia. Foot Ankle. 1987;7(5):290-299.
8. van der Schoot DK, Den Outer AJ, Bode PJ, Obermann WR, van Vugt AB. Degenerative changes at the knee and ankle related to malunion of tibial fractures. 15-year follow-up of 88 patients. J Bone Joint Surg Br. 1996;78(5):722-725.
9. Kristensen KD, Kiaer T, Blicher J. No arthrosis of the ankle 20 years after malaligned tibial-shaft fracture. Acta Orthop Scand. 1989;60(2):208-209.
10. McKellop HA, Sigholm G, Redfern FC, Doyle B, Sarmiento A, Luck JV Sr. The effect of simulated fracture-angulations of the tibia on cartilage pressures in the knee joint. J Bone Joint Surg Am. 1991;73(9):1382-1391.
11. Merchant TC, Dietz FR. Long-term follow-up after fractures of the tibial and fibular shafts. J Bone Joint Surg Am. 1989;71(4):599-606.
12. Paley D, Herzenberg JE, Tetsworth K, McKie J, Bhave A. Deformity planning for frontal and sagittal plane corrective osteotomies. Orthop Clin North Am. 1994;25(3):425-465.
13. Perry J. Gait Analysis: Normal and Pathological Function. Thorofare, NJ: Slack; 1992.
14. Puno RM, Vaughan JJ, von Fraunhofer JA, Stetten ML, Johnson JR. A method of determining the angular malalignments of the knee and ankle joints resulting from a tibial malunion. Clin Orthop. 1987;(223):213-219.
15. Greenwood DC, Muir KR, Doherty M, Milner SA, Stevens M, Davis TR. Conservatively managed tibial shaft fractures in Nottingham, UK: are pain, osteoarthritis, and disability long-term complications? J Epidemiol Community Health. 1997;51(6):701-704.
16. Dehne E, Deffer PA, Hall RM, Brown PW, Johnson EV. The natural history of the fractured tibia. Surg Clin North Am. 1961;41(6):1495-1513.
17. Kitaoka HB, Schaap EJ, Chao EY, An KN. Displaced intra-articular fractures of the calcaneus treated non-operatively. Clinical results and analysis of motion and ground-reaction and temporal forces. J Bone Joint Surg Am. 1994;76(10):1531-1540.
18. Borrelli J Jr, Goldfarb C, Ricci W, Wagner JM, Engsberg JR. Functional outcome after isolated acetabular fractures. J Orthop Trauma. 2002;16(2):73-81.
19. Borrelli J Jr, Ricci WM, Anglen JO, Gregush R, Engsberg J. Muscle strength recovery and its effects on outcome after open reduction and internal fixation of acetabular fractures. J Orthop Trauma. 2006;20(6):388-395.
20. Jaglal S, Lakhani Z, Schatzker J. Reliability, validity, and responsiveness of the lower extremity measure for patients with a hip fracture. J Bone Joint Surg Am. 2000;82(7):955-962.
21. Madsen MS, Ritter MA, Morris HH, et al. The effect of total hip arthroplasty surgical approach on gait. J Orthop Res. 2004;22(1):44-50.
22. Mittlmeier T, Morlock MM, Hertlein H, et al. Analysis of morphology and gait function after intraarticular calcaneal fracture. J Orthop Trauma. 1993;7(4):303-310.
23. Song KM, Halliday SE, Little DG. The effect of limb-length discrepancy on gait. J Bone Joint Surg Am. 1997;79(11):1690-1698.
24. Zlowodzki M, Obremskey WT, Thomison JB, Kregor PJ. Functional outcome after treatment of lower-extremity nonunions. J Trauma. 2005;58(2):312-317.
25. Sanders R, Anglen JO, Mark JB. Oblique osteotomy for the correction of tibial malunion. J Bone Joint Surg Am. 1995;77(2):240-246.
26. Sangeorzan BJ, Sangeorzan BP, Hansen ST Jr, Judd RP. Mathematically directed single-cut osteotomy for correction of tibial malunion. J Orthop Trauma. 1989;3(4):267-275.
27. Borrelli J Jr, Leduc S, Gregush R, Ricci WM. Tricortical bone grafts for treatment of malaligned tibias and fibulas. Clin Orthop. 2009;467(4):1056-1063.
28. Engelberg R, Martin DP, Agel J, Obremsky W, Coronado G, Swiontkowski MF. Musculoskeletal Function Assessment instrument: criterion and construct validity. J Orthop Res. 1996;14(2):182-192.
29. Engelberg R, Martin DP, Agel J, Swiontkowski MF. Musculoskeletal Function Assessment: reference values for patient and non-patient samples. J Orthop Res. 1999;17(1):101-109.
30. Swiontkowski MF, Engelberg R, Martin DP, Agel J. Short Musculoskeletal Function Assessment questionnaire: validity, reliability, and responsiveness. J Bone Joint Surg Am. 1999;81(9):1245-1260.
31. Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473-483.
32. Graehl PM, Hersh MR, Heckman JD. Supramalleolar osteotomy for the treatment of symptomatic tibial malunion. J Orthop Trauma. 1987;1(4):281-292.
33. Bhave A, Paley D, Herzenberg JE. Improvement in gait parameters after lengthening for the treatment of limb-length discrepancy. J Bone Joint Surg Am. 1999;81(4):529-534.
34. Wu DD, Burr DB, Boyd RD, Radin EL. Bone and cartilage changes following experimental varus or valgus tibial angulation. J Orthop Res. 1990;8(4):572-585.
Efficacy of Skin Preparation in Eradicating Organisms Before Total Knee Arthroplasty
Knee arthroplasty continues to be one of the most common and successful methods for treating severe arthritis and other painful arthropathies. Increasing steadily from 1998 to 2008, and with more than 676,000 procedures performed in 2008, knee arthroplasty remains the most common surgical joint replacement procedure.1
Although perioperative and long-term complications are uncommon, infection remains one of the most serious complications of total knee arthroplasty (TKA). Some studies have found a post-TKA infection rate of less than 1%.2 The solution of 2% chlorhexidine gluconate and 70% isopropyl alcohol (Chloraprep; Medi-Flex, Overland Park, Kansas) is commonly used for antiseptic skin preparation before surgery. Studies have shown significant decreases in post-TKA infection rates with preoperative use.3,4 Another study evaluated the efficacy of 3 different skin solutions and found Chloraprep to be the most efficient in eradicating bacteria from the foot and ankle before surgery. The investigators noted that, even with preoperative use of Chloraprep, 23% of patients had residual bacteria on the surface of the skin between the toes.5 Like the foot, the popliteal fossa is an intertriginous area that may harbor normal flora, including gram-positive cocci, in large numbers, mainly because of the contact between 2 skin surfaces. Although postoperative infection rates decrease with use of Chloraprep, its presurgical efficacy in killing bacteria on another intertriginous area, the popliteal fossa, is largely unknown. Also unknown are susceptible organism species and organism population numbers.
Concerned that our skin preparation might be ineffective, we conducted a study to evaluate the efficacy of Chloraprep skin preparation in eradicating organisms before TKA, to isolate the type and number of organisms, and to evaluate several other contributing factors that could lead to infection.
Materials and Methods
This prospective study included 99 patients who were undergoing primary TKA at John Peter Smith Hospital between July 1, 2011 and August 31, 2012. An attempt was made to enroll consecutive TKA patients, and all patients agreed to participate, but a few were not enrolled because the study team had not asked for their consent before they were taken to the operating room. Patients did not receive monetary compensation for participation. Exclusion criteria were pregnancy, imprisonment, and age under 18 years. The study was approved by the institutional review boards at John Peter Smith Hospital and the University of North Texas Health Science Center.
Each lower extremity was prepared with Chloraprep according to the manufacturer’s instructions. Preparation was done by well-trained operating room staff members who were supervised by the surgeon (Dr. Sanchez or Dr. Wagner) but were not involved in the study. With use of the Chloraprep applicator, the solution was applied in a back-and-forth manner to the entire operative leg for at least 30 seconds, and then discarded. This scrub procedure was repeated with a second applicator before standard drapes were placed. The leg was left to air-dry for at least 30 seconds, and the drapes were placed before postsolution swabbing and before the iodine-impregnated adhesive drape was placed around the knee. During drying, the solution was not blotted, wiped away, or touched with instrumentation. Patients were swabbed with an epidermal sterile swab in the popliteal fossa of the knee undergoing surgery, both before solution application (presolution swab) and after (postsolution swab). Only the operating surgeon participated in swabbing the patients. Aerobic and anaerobic swabs were vigorously rubbed over a 2- to 3-in wide area across the entire posterior flexion crease surface.
The collected pre- and postsolution swabs were sent to John Peter Smith Laboratory for identification of organisms. Anaerobic swabs were cultured in thioglycolate broth and on 4 plates: MacConkey agar, Columbia colistin–nalidixic acid agar, chocolate agar, and sheep blood agar. Aerobic swabs were cultured in thioglycolate broth with hemin and vitamin K and on 4 plates: anaerobic blood agar, bile esculin agar, kanamycin and vancomycin agar, and Columbia colistin–nalidixic acid agar. Anaerobic plates were incubated in an anoxic environment. The plates were then read daily, and final reports were issued after 48 hours (for aerobic bacterial isolates) and 72 hours (for anaerobic bacterial isolates), as was the standard at the time.
Additional patient data were collected for possible correlations: American Society of Anesthesiologists (ASA) classification (physical status),6 body mass index (BMI), age, sex, arthroplasty type (unilateral, bilateral), and diabetic status. In addition, patients were asked if they had used Hibiclens antiseptic/antimicrobial skin cleanser daily during the week before surgery—as they had been instructed to do—and the number of times they had used the cleanser.
Study data were analyzed and were used to stratify patients into several groups. Each group had multiple factors evaluated.
Descriptive statistics were used to characterize the patient demographic information. Chi-square analyses were performed to evaluate the difference between presence of organisms before and after solution application, and the data were also layered with reported Hibiclens cleanser use. In addition, binary logistic regression was used to determine if demographic variables could predict presence of organism isolates before and after solution application. Data analyses were conducted using IBM SPSS Statistics Version 20.
Results
No patient had a postoperative infection. Culture isolates grew in 20 (20%) of the 99 patients before solution application and in 5 (5%) of the 99 after application. Of the 20 patients with presolution culture isolates, 16 (80%) had 1 bacterial isolate, and 4 (20%) had 2 or more species. Presolution isolates included normal flora (10, 50%), coagulase-negative Staphylococcus aureus (6, 30%), rare Bacillus (3, 15%), Micrococcus luteus (1, 5%), rare gram-negative (1, 5%), rare gram-positive (1, 5%), and Staphylococcus hominis (1, 5%) (Figure 1). Postsolution isolates included coagulase-negative S aureus (3, 60%), rare Bacillus (1, 20%), and rare Serratia odorifera (1, 20%) (Figure 2). Two postsolution isolates did not have an associated presolution isolate. Presolution organism isolation was an important predictor of postsolution organism isolation (P < .046).
BMI was recorded for all patients. Mean BMI was 35 (range, 20-63). Distribution was as follows: BMI under 20 (3 patients), under 30 (30 patients), under 40 (47 patients), under 50 (14 patients), under 60 (4 patients), and over 60 (1 patient). Mean presolution BMI was significantly (P < .03) higher for patients with bacterial isolates than for patients without isolates (38 and 34, respectively). Mean postsolution BMI was 40 for patients with bacterial isolates and 35 for patients without isolates (Figure 3). Of the 33 patients with BMI under 30, 3 (9%) had presolution isolates and 1 (3%) had postsolution isolates. Of the 66 patients with BMI over 30, 17 (26%) had presolution isolates and 4 (6%) had postsolution isolates (Table).
Of the 99 patients, 30 (30%) had diabetes. Of these 30 patients, 9 (30%) had presolution isolates (45% of all presolution isolates) and 3 (10%) had postsolution isolates (60% of all postsolution isolates.) Although neither pre- nor postsolution results were statistically significant (P = .172) for increasing organism isolation in patients with diabetes, the odds ratio for these patients was 3.6 when the focus was on the likelihood of postsolution organism isolation.
Mean age was 57 years (range, 29-87 years). Results were not statistically significant for age being a likely factor for organism isolate prediction.
There were 81 women and 18 men in the study. Of the 81 women, 16 (20%) had positive presolution cultures and 5 (6%) had positive postsolution cultures. Of the 18 men, 4 (22%) had positive presolution cultures and none had a positive postsolution culture.
Race was recorded. Forty-nine patients were white, 27 black, 18 Hispanic, and 5 unknown. Presolution, 12 whites (24%), 5 blacks (19%), and 3 Hispanics (17%) had positive cultures. Postsolution, 1 white (2%), 1 black (4%), 3 Hispanics (17%), and 1 patient of unknown race (20%) had positive cultures.
ASA classifications were recorded and analyzed. Of the 99 patients, 38 were classified ASA-2, 60 were ASA-3, and 1 was ASA-4. Presolution, 9 (24%) of the 38 ASA-2 patients and 11 (18%) of the 60 ASA-3 patients had positive cultures; postsolution, 2 (5%) of the 38 ASA-2 patients and 3 (5%) of the 60 ASA-3 patients had positive cultures. The 1 ASA-4 patient had neither presolution nor postsolution positive cultures.
Types of TKA (bilateral, unilateral) were recorded. Of the 99 patients, 89 had unilateral TKAs and 10 had bilateral TKAs. Presolution, 19 (21%) of the 89 unilaterals and 1 (10%) of the 10 bilaterals had positive cultures. Postsolution, 5 (6%) of the 89 unilaterals and none of the 10 bilaterals had positive cultures.
Patients were also verbally asked how many cleanser baths they had taken before surgery. Of the 99 patients, 88 reported having taken 1 or more cleanser baths, and 1 reported no baths; 10 patients’ responses were not available. The 88 patients who had taken at least 1 cleanser bath were divided into 3 groups: 1 bath (35 patients), 2 baths (49 patients), and 3 or more baths (4 patients). Presolution, positive cultures were found for 18 (20%) of the 88 patients; for 7 (20%) of the 35 patients with 1 bath; for 10 (20%) of the 49 patients with 2 baths; and for 1 (25%) of the 4 patients with 3 or more baths. Postsolution, positive cultures were found for 5 (6%) of the 88 patients; for 2 (6%) of the 35 patients with 1 bath; for 3 (6%) of the 49 patients with 2 baths; and for 0 (0%) of the 4 patients with 3 or more baths. The 1 patient with no baths did not have a positive culture. Of the 10 patients whose responses were unavailable, 2 patients had positive presolution cultures and no patients had a positive postsolution culture.
Discussion
The efficacy of using Chloraprep before TKA has not been well assessed in orthopedic practice. However, compared with other preoperative solutions, chlorhexidine has been shown to be significantly better in preventing post-TKA infections.4 Other studies have found it far more effective than other commonly used surgical preparations in eliminating microorganisms in hip arthroplasty and foot surgery.5,7 Our study, focused on the efficacy of Chloraprep in killing bacteria, found the solution effective in removing 85% (17/20) of cultured presolution organisms.
Of the bacterial isolates cultured, normal flora were effectively removed from all associated postsolution cultures. Although most of the bacterial isolates were eliminated after solution application, both coagulase-negative S aureus and rare Bacillus species were found both pre- and postsolution, suggesting either inadequate skin preparation or resistant bacteria.
With respect to the secondary variables, our study data showed that BMI was an important predictor for bacterial isolates, significantly so presolution (P < .03). Mean BMI for the overall study was 35, firmly in the obese category. Only when BMI increased to 38 did it become significant as a predictor for postsolution organisms. Mean postsolution BMI was even higher, 40, which is in the morbidly obese category. Interestingly, the percentage of nonobese patients (BMI, <30) with positive presolution cultures was only 9%, versus the 20% with positive presolution cultures overall. In addition, 1 nonobese patient had positive postsolution cultures.
Other studies have linked higher BMI to higher rates of surgical site infection and other complications, but it is unknown if the infections are due to higher bacterial counts in the patients with high BMI or to other factors, such as reduced wound healing or decreased immune response. More research is needed to determine if the number of organisms in patients with high BMI correlates to a higher risk for surgical site infection.8 As expected, along with BMI (>38), presolution organism isolation was an important predictor for postsolution organism isolation. Patients with presolution organism isolation were 24 times more likely to have postsolution isolates.
Even though diabetic status was not significant for predicting bacterial isolation, patients with diabetes were 3.6 times more likely than patients without diabetes to have a positive culture. Other studies have shown that, compared with patients without diabetes, patients with diabetes had a higher chance of postoperative infection.9,10
In this study, 18 of 20 patients with presolution organism isolates reported they had been compliant in taking the recommended preoperative cleanser baths. This finding may indicate that preoperative cleanser baths are ineffective. However, only 20% of our patients had positive presolution cultures, whereas Ostrander and colleagues5 reported 30% positive pre-preparation cultures from the anterior knee. A recent Cochrane Database System Review did not provide clear evidence of benefit for preoperative showering or bathing with chlorhexidine over other wash products.11 Although their benefit may be questionable, we will continue to recommend preoperative cleanser baths.
One limitation of this study is sample size. Although size was sufficient for determining the efficacy of Chloraprep in the intertriginous area of the back of the knee, the lack of statistical significance (eg, effect of diabetes) may not be accurate. In addition, because the nurse who prepared patients’ skin was aware of the study and was supervised in every case, it is possible that the preparation was done more carefully than usual, resulting in more negative cultures than average. Also, compliance in taking preoperative cleanser baths was subjectively determined. Patients may have reported more baths than were actually taken. Still another study limitation is that 2 postsolution isolates did not have an associated presolution isolate. Although we think this may have resulted from laboratory contamination, it is possible the presolution swabs did not accurately determine true bacterial counts in these cases.
Conclusion
A study that showed significant residual bacteria between patients’ toes after chlorhexidine skin preparation5 left us concerned that Chloraprep skin preparation for TKA might not be adequate. The present study showed that this solution was effective in eliminating bacteria from the intertriginous area of the back of the knee in 95% of patients. Skin preparation appears to be less effective in patients with higher BMI.
1. Losina E, Thornhill TS, Rome BN, Wright J, Katz JN. The dramatic increase in total knee replacement utilization rates in the United States cannot be fully explained by growth in population size and the obesity epidemic. J Bone Joint Surg Am. 2012;94(3):201-207.
2. Poultsides LA, Ma Y, Della Valle AG, Chiu YL, Sculco TP, Memtsoudis SG. In-hospital surgical site infections after primary hip and knee arthroplasty—incidence and risk factors. J Arthroplasty. 2013;28(3):385-389.
3. Johnson AJ, Kapadia BH, Daley JA, Molina CB, Mont MA. Chlorhexidine reduces infections in knee arthroplasty. J Knee Surg. 2013;26(3):213-218.
4. Zywiel MG, Daley JA, Delanois RE, Naziri Q, Johnson AJ, Mont MA. Advance pre-operative chlorhexidine reduces the incidence of surgical site infections in knee arthroplasty. Int Orthop. 2011;35(7):1001-1006.
5. Ostrander RV, Botte MJ, Brage ME. Efficacy of surgical preparation solutions in foot and ankle surgery. J Bone Joint Surg Am. 2005;87(5):980-985.
6. Wolters U, Wolf T, Stützer H, Schröder T. ASA classification and perioperative variables as predictors of postoperative outcome. Br J Anaesth. 1996;77(2):217-222.
7. Johnson AJ, Daley JA, Zywiel MG, Delanois RE, Mont MA. Preoperative chlorhexidine preparation and the incidence of surgical site infections after hip arthroplasty. J Arthroplasty. 2010;25(6 suppl):98-102.
8. Samson AJ, Mercer GE, Campbell DG. Total knee replacement in the morbidly obese: a literature review. ANZ J Surg. 2010;80(9):595-599.
9. Iorio R, Williams KM, Marcantonio AJ, Specht LM, Tilzey JF, Healy WL. Diabetes mellitus, hemoglobin A1C, and the incidence of total joint arthroplasty infection. J Arthroplasty. 2012;27(5):726-729.
10. Viens NA, Hug KT, Marchant MH, Cook C, Vail TP, Bolognesi MP. Role of diabetes type in perioperative outcomes after hip and knee arthroplasty in the United States. J Surg Orthop Adv. 2012;21(4):253-260.
11. Webster J, Osborne S. Preoperative bathing or showering with skin antiseptics to prevent surgical site infection. Cochrane Database Syst Rev. 2012;9:CD004985.
Knee arthroplasty continues to be one of the most common and successful methods for treating severe arthritis and other painful arthropathies. Increasing steadily from 1998 to 2008, and with more than 676,000 procedures performed in 2008, knee arthroplasty remains the most common surgical joint replacement procedure.1
Although perioperative and long-term complications are uncommon, infection remains one of the most serious complications of total knee arthroplasty (TKA). Some studies have found a post-TKA infection rate of less than 1%.2 The solution of 2% chlorhexidine gluconate and 70% isopropyl alcohol (Chloraprep; Medi-Flex, Overland Park, Kansas) is commonly used for antiseptic skin preparation before surgery. Studies have shown significant decreases in post-TKA infection rates with preoperative use.3,4 Another study evaluated the efficacy of 3 different skin solutions and found Chloraprep to be the most efficient in eradicating bacteria from the foot and ankle before surgery. The investigators noted that, even with preoperative use of Chloraprep, 23% of patients had residual bacteria on the surface of the skin between the toes.5 Like the foot, the popliteal fossa is an intertriginous area that may harbor normal flora, including gram-positive cocci, in large numbers, mainly because of the contact between 2 skin surfaces. Although postoperative infection rates decrease with use of Chloraprep, its presurgical efficacy in killing bacteria on another intertriginous area, the popliteal fossa, is largely unknown. Also unknown are susceptible organism species and organism population numbers.
Concerned that our skin preparation might be ineffective, we conducted a study to evaluate the efficacy of Chloraprep skin preparation in eradicating organisms before TKA, to isolate the type and number of organisms, and to evaluate several other contributing factors that could lead to infection.
Materials and Methods
This prospective study included 99 patients who were undergoing primary TKA at John Peter Smith Hospital between July 1, 2011 and August 31, 2012. An attempt was made to enroll consecutive TKA patients, and all patients agreed to participate, but a few were not enrolled because the study team had not asked for their consent before they were taken to the operating room. Patients did not receive monetary compensation for participation. Exclusion criteria were pregnancy, imprisonment, and age under 18 years. The study was approved by the institutional review boards at John Peter Smith Hospital and the University of North Texas Health Science Center.
Each lower extremity was prepared with Chloraprep according to the manufacturer’s instructions. Preparation was done by well-trained operating room staff members who were supervised by the surgeon (Dr. Sanchez or Dr. Wagner) but were not involved in the study. With use of the Chloraprep applicator, the solution was applied in a back-and-forth manner to the entire operative leg for at least 30 seconds, and then discarded. This scrub procedure was repeated with a second applicator before standard drapes were placed. The leg was left to air-dry for at least 30 seconds, and the drapes were placed before postsolution swabbing and before the iodine-impregnated adhesive drape was placed around the knee. During drying, the solution was not blotted, wiped away, or touched with instrumentation. Patients were swabbed with an epidermal sterile swab in the popliteal fossa of the knee undergoing surgery, both before solution application (presolution swab) and after (postsolution swab). Only the operating surgeon participated in swabbing the patients. Aerobic and anaerobic swabs were vigorously rubbed over a 2- to 3-in wide area across the entire posterior flexion crease surface.
The collected pre- and postsolution swabs were sent to John Peter Smith Laboratory for identification of organisms. Anaerobic swabs were cultured in thioglycolate broth and on 4 plates: MacConkey agar, Columbia colistin–nalidixic acid agar, chocolate agar, and sheep blood agar. Aerobic swabs were cultured in thioglycolate broth with hemin and vitamin K and on 4 plates: anaerobic blood agar, bile esculin agar, kanamycin and vancomycin agar, and Columbia colistin–nalidixic acid agar. Anaerobic plates were incubated in an anoxic environment. The plates were then read daily, and final reports were issued after 48 hours (for aerobic bacterial isolates) and 72 hours (for anaerobic bacterial isolates), as was the standard at the time.
Additional patient data were collected for possible correlations: American Society of Anesthesiologists (ASA) classification (physical status),6 body mass index (BMI), age, sex, arthroplasty type (unilateral, bilateral), and diabetic status. In addition, patients were asked if they had used Hibiclens antiseptic/antimicrobial skin cleanser daily during the week before surgery—as they had been instructed to do—and the number of times they had used the cleanser.
Study data were analyzed and were used to stratify patients into several groups. Each group had multiple factors evaluated.
Descriptive statistics were used to characterize the patient demographic information. Chi-square analyses were performed to evaluate the difference between presence of organisms before and after solution application, and the data were also layered with reported Hibiclens cleanser use. In addition, binary logistic regression was used to determine if demographic variables could predict presence of organism isolates before and after solution application. Data analyses were conducted using IBM SPSS Statistics Version 20.
Results
No patient had a postoperative infection. Culture isolates grew in 20 (20%) of the 99 patients before solution application and in 5 (5%) of the 99 after application. Of the 20 patients with presolution culture isolates, 16 (80%) had 1 bacterial isolate, and 4 (20%) had 2 or more species. Presolution isolates included normal flora (10, 50%), coagulase-negative Staphylococcus aureus (6, 30%), rare Bacillus (3, 15%), Micrococcus luteus (1, 5%), rare gram-negative (1, 5%), rare gram-positive (1, 5%), and Staphylococcus hominis (1, 5%) (Figure 1). Postsolution isolates included coagulase-negative S aureus (3, 60%), rare Bacillus (1, 20%), and rare Serratia odorifera (1, 20%) (Figure 2). Two postsolution isolates did not have an associated presolution isolate. Presolution organism isolation was an important predictor of postsolution organism isolation (P < .046).
BMI was recorded for all patients. Mean BMI was 35 (range, 20-63). Distribution was as follows: BMI under 20 (3 patients), under 30 (30 patients), under 40 (47 patients), under 50 (14 patients), under 60 (4 patients), and over 60 (1 patient). Mean presolution BMI was significantly (P < .03) higher for patients with bacterial isolates than for patients without isolates (38 and 34, respectively). Mean postsolution BMI was 40 for patients with bacterial isolates and 35 for patients without isolates (Figure 3). Of the 33 patients with BMI under 30, 3 (9%) had presolution isolates and 1 (3%) had postsolution isolates. Of the 66 patients with BMI over 30, 17 (26%) had presolution isolates and 4 (6%) had postsolution isolates (Table).
Of the 99 patients, 30 (30%) had diabetes. Of these 30 patients, 9 (30%) had presolution isolates (45% of all presolution isolates) and 3 (10%) had postsolution isolates (60% of all postsolution isolates.) Although neither pre- nor postsolution results were statistically significant (P = .172) for increasing organism isolation in patients with diabetes, the odds ratio for these patients was 3.6 when the focus was on the likelihood of postsolution organism isolation.
Mean age was 57 years (range, 29-87 years). Results were not statistically significant for age being a likely factor for organism isolate prediction.
There were 81 women and 18 men in the study. Of the 81 women, 16 (20%) had positive presolution cultures and 5 (6%) had positive postsolution cultures. Of the 18 men, 4 (22%) had positive presolution cultures and none had a positive postsolution culture.
Race was recorded. Forty-nine patients were white, 27 black, 18 Hispanic, and 5 unknown. Presolution, 12 whites (24%), 5 blacks (19%), and 3 Hispanics (17%) had positive cultures. Postsolution, 1 white (2%), 1 black (4%), 3 Hispanics (17%), and 1 patient of unknown race (20%) had positive cultures.
ASA classifications were recorded and analyzed. Of the 99 patients, 38 were classified ASA-2, 60 were ASA-3, and 1 was ASA-4. Presolution, 9 (24%) of the 38 ASA-2 patients and 11 (18%) of the 60 ASA-3 patients had positive cultures; postsolution, 2 (5%) of the 38 ASA-2 patients and 3 (5%) of the 60 ASA-3 patients had positive cultures. The 1 ASA-4 patient had neither presolution nor postsolution positive cultures.
Types of TKA (bilateral, unilateral) were recorded. Of the 99 patients, 89 had unilateral TKAs and 10 had bilateral TKAs. Presolution, 19 (21%) of the 89 unilaterals and 1 (10%) of the 10 bilaterals had positive cultures. Postsolution, 5 (6%) of the 89 unilaterals and none of the 10 bilaterals had positive cultures.
Patients were also verbally asked how many cleanser baths they had taken before surgery. Of the 99 patients, 88 reported having taken 1 or more cleanser baths, and 1 reported no baths; 10 patients’ responses were not available. The 88 patients who had taken at least 1 cleanser bath were divided into 3 groups: 1 bath (35 patients), 2 baths (49 patients), and 3 or more baths (4 patients). Presolution, positive cultures were found for 18 (20%) of the 88 patients; for 7 (20%) of the 35 patients with 1 bath; for 10 (20%) of the 49 patients with 2 baths; and for 1 (25%) of the 4 patients with 3 or more baths. Postsolution, positive cultures were found for 5 (6%) of the 88 patients; for 2 (6%) of the 35 patients with 1 bath; for 3 (6%) of the 49 patients with 2 baths; and for 0 (0%) of the 4 patients with 3 or more baths. The 1 patient with no baths did not have a positive culture. Of the 10 patients whose responses were unavailable, 2 patients had positive presolution cultures and no patients had a positive postsolution culture.
Discussion
The efficacy of using Chloraprep before TKA has not been well assessed in orthopedic practice. However, compared with other preoperative solutions, chlorhexidine has been shown to be significantly better in preventing post-TKA infections.4 Other studies have found it far more effective than other commonly used surgical preparations in eliminating microorganisms in hip arthroplasty and foot surgery.5,7 Our study, focused on the efficacy of Chloraprep in killing bacteria, found the solution effective in removing 85% (17/20) of cultured presolution organisms.
Of the bacterial isolates cultured, normal flora were effectively removed from all associated postsolution cultures. Although most of the bacterial isolates were eliminated after solution application, both coagulase-negative S aureus and rare Bacillus species were found both pre- and postsolution, suggesting either inadequate skin preparation or resistant bacteria.
With respect to the secondary variables, our study data showed that BMI was an important predictor for bacterial isolates, significantly so presolution (P < .03). Mean BMI for the overall study was 35, firmly in the obese category. Only when BMI increased to 38 did it become significant as a predictor for postsolution organisms. Mean postsolution BMI was even higher, 40, which is in the morbidly obese category. Interestingly, the percentage of nonobese patients (BMI, <30) with positive presolution cultures was only 9%, versus the 20% with positive presolution cultures overall. In addition, 1 nonobese patient had positive postsolution cultures.
Other studies have linked higher BMI to higher rates of surgical site infection and other complications, but it is unknown if the infections are due to higher bacterial counts in the patients with high BMI or to other factors, such as reduced wound healing or decreased immune response. More research is needed to determine if the number of organisms in patients with high BMI correlates to a higher risk for surgical site infection.8 As expected, along with BMI (>38), presolution organism isolation was an important predictor for postsolution organism isolation. Patients with presolution organism isolation were 24 times more likely to have postsolution isolates.
Even though diabetic status was not significant for predicting bacterial isolation, patients with diabetes were 3.6 times more likely than patients without diabetes to have a positive culture. Other studies have shown that, compared with patients without diabetes, patients with diabetes had a higher chance of postoperative infection.9,10
In this study, 18 of 20 patients with presolution organism isolates reported they had been compliant in taking the recommended preoperative cleanser baths. This finding may indicate that preoperative cleanser baths are ineffective. However, only 20% of our patients had positive presolution cultures, whereas Ostrander and colleagues5 reported 30% positive pre-preparation cultures from the anterior knee. A recent Cochrane Database System Review did not provide clear evidence of benefit for preoperative showering or bathing with chlorhexidine over other wash products.11 Although their benefit may be questionable, we will continue to recommend preoperative cleanser baths.
One limitation of this study is sample size. Although size was sufficient for determining the efficacy of Chloraprep in the intertriginous area of the back of the knee, the lack of statistical significance (eg, effect of diabetes) may not be accurate. In addition, because the nurse who prepared patients’ skin was aware of the study and was supervised in every case, it is possible that the preparation was done more carefully than usual, resulting in more negative cultures than average. Also, compliance in taking preoperative cleanser baths was subjectively determined. Patients may have reported more baths than were actually taken. Still another study limitation is that 2 postsolution isolates did not have an associated presolution isolate. Although we think this may have resulted from laboratory contamination, it is possible the presolution swabs did not accurately determine true bacterial counts in these cases.
Conclusion
A study that showed significant residual bacteria between patients’ toes after chlorhexidine skin preparation5 left us concerned that Chloraprep skin preparation for TKA might not be adequate. The present study showed that this solution was effective in eliminating bacteria from the intertriginous area of the back of the knee in 95% of patients. Skin preparation appears to be less effective in patients with higher BMI.
Knee arthroplasty continues to be one of the most common and successful methods for treating severe arthritis and other painful arthropathies. Increasing steadily from 1998 to 2008, and with more than 676,000 procedures performed in 2008, knee arthroplasty remains the most common surgical joint replacement procedure.1
Although perioperative and long-term complications are uncommon, infection remains one of the most serious complications of total knee arthroplasty (TKA). Some studies have found a post-TKA infection rate of less than 1%.2 The solution of 2% chlorhexidine gluconate and 70% isopropyl alcohol (Chloraprep; Medi-Flex, Overland Park, Kansas) is commonly used for antiseptic skin preparation before surgery. Studies have shown significant decreases in post-TKA infection rates with preoperative use.3,4 Another study evaluated the efficacy of 3 different skin solutions and found Chloraprep to be the most efficient in eradicating bacteria from the foot and ankle before surgery. The investigators noted that, even with preoperative use of Chloraprep, 23% of patients had residual bacteria on the surface of the skin between the toes.5 Like the foot, the popliteal fossa is an intertriginous area that may harbor normal flora, including gram-positive cocci, in large numbers, mainly because of the contact between 2 skin surfaces. Although postoperative infection rates decrease with use of Chloraprep, its presurgical efficacy in killing bacteria on another intertriginous area, the popliteal fossa, is largely unknown. Also unknown are susceptible organism species and organism population numbers.
Concerned that our skin preparation might be ineffective, we conducted a study to evaluate the efficacy of Chloraprep skin preparation in eradicating organisms before TKA, to isolate the type and number of organisms, and to evaluate several other contributing factors that could lead to infection.
Materials and Methods
This prospective study included 99 patients who were undergoing primary TKA at John Peter Smith Hospital between July 1, 2011 and August 31, 2012. An attempt was made to enroll consecutive TKA patients, and all patients agreed to participate, but a few were not enrolled because the study team had not asked for their consent before they were taken to the operating room. Patients did not receive monetary compensation for participation. Exclusion criteria were pregnancy, imprisonment, and age under 18 years. The study was approved by the institutional review boards at John Peter Smith Hospital and the University of North Texas Health Science Center.
Each lower extremity was prepared with Chloraprep according to the manufacturer’s instructions. Preparation was done by well-trained operating room staff members who were supervised by the surgeon (Dr. Sanchez or Dr. Wagner) but were not involved in the study. With use of the Chloraprep applicator, the solution was applied in a back-and-forth manner to the entire operative leg for at least 30 seconds, and then discarded. This scrub procedure was repeated with a second applicator before standard drapes were placed. The leg was left to air-dry for at least 30 seconds, and the drapes were placed before postsolution swabbing and before the iodine-impregnated adhesive drape was placed around the knee. During drying, the solution was not blotted, wiped away, or touched with instrumentation. Patients were swabbed with an epidermal sterile swab in the popliteal fossa of the knee undergoing surgery, both before solution application (presolution swab) and after (postsolution swab). Only the operating surgeon participated in swabbing the patients. Aerobic and anaerobic swabs were vigorously rubbed over a 2- to 3-in wide area across the entire posterior flexion crease surface.
The collected pre- and postsolution swabs were sent to John Peter Smith Laboratory for identification of organisms. Anaerobic swabs were cultured in thioglycolate broth and on 4 plates: MacConkey agar, Columbia colistin–nalidixic acid agar, chocolate agar, and sheep blood agar. Aerobic swabs were cultured in thioglycolate broth with hemin and vitamin K and on 4 plates: anaerobic blood agar, bile esculin agar, kanamycin and vancomycin agar, and Columbia colistin–nalidixic acid agar. Anaerobic plates were incubated in an anoxic environment. The plates were then read daily, and final reports were issued after 48 hours (for aerobic bacterial isolates) and 72 hours (for anaerobic bacterial isolates), as was the standard at the time.
Additional patient data were collected for possible correlations: American Society of Anesthesiologists (ASA) classification (physical status),6 body mass index (BMI), age, sex, arthroplasty type (unilateral, bilateral), and diabetic status. In addition, patients were asked if they had used Hibiclens antiseptic/antimicrobial skin cleanser daily during the week before surgery—as they had been instructed to do—and the number of times they had used the cleanser.
Study data were analyzed and were used to stratify patients into several groups. Each group had multiple factors evaluated.
Descriptive statistics were used to characterize the patient demographic information. Chi-square analyses were performed to evaluate the difference between presence of organisms before and after solution application, and the data were also layered with reported Hibiclens cleanser use. In addition, binary logistic regression was used to determine if demographic variables could predict presence of organism isolates before and after solution application. Data analyses were conducted using IBM SPSS Statistics Version 20.
Results
No patient had a postoperative infection. Culture isolates grew in 20 (20%) of the 99 patients before solution application and in 5 (5%) of the 99 after application. Of the 20 patients with presolution culture isolates, 16 (80%) had 1 bacterial isolate, and 4 (20%) had 2 or more species. Presolution isolates included normal flora (10, 50%), coagulase-negative Staphylococcus aureus (6, 30%), rare Bacillus (3, 15%), Micrococcus luteus (1, 5%), rare gram-negative (1, 5%), rare gram-positive (1, 5%), and Staphylococcus hominis (1, 5%) (Figure 1). Postsolution isolates included coagulase-negative S aureus (3, 60%), rare Bacillus (1, 20%), and rare Serratia odorifera (1, 20%) (Figure 2). Two postsolution isolates did not have an associated presolution isolate. Presolution organism isolation was an important predictor of postsolution organism isolation (P < .046).
BMI was recorded for all patients. Mean BMI was 35 (range, 20-63). Distribution was as follows: BMI under 20 (3 patients), under 30 (30 patients), under 40 (47 patients), under 50 (14 patients), under 60 (4 patients), and over 60 (1 patient). Mean presolution BMI was significantly (P < .03) higher for patients with bacterial isolates than for patients without isolates (38 and 34, respectively). Mean postsolution BMI was 40 for patients with bacterial isolates and 35 for patients without isolates (Figure 3). Of the 33 patients with BMI under 30, 3 (9%) had presolution isolates and 1 (3%) had postsolution isolates. Of the 66 patients with BMI over 30, 17 (26%) had presolution isolates and 4 (6%) had postsolution isolates (Table).
Of the 99 patients, 30 (30%) had diabetes. Of these 30 patients, 9 (30%) had presolution isolates (45% of all presolution isolates) and 3 (10%) had postsolution isolates (60% of all postsolution isolates.) Although neither pre- nor postsolution results were statistically significant (P = .172) for increasing organism isolation in patients with diabetes, the odds ratio for these patients was 3.6 when the focus was on the likelihood of postsolution organism isolation.
Mean age was 57 years (range, 29-87 years). Results were not statistically significant for age being a likely factor for organism isolate prediction.
There were 81 women and 18 men in the study. Of the 81 women, 16 (20%) had positive presolution cultures and 5 (6%) had positive postsolution cultures. Of the 18 men, 4 (22%) had positive presolution cultures and none had a positive postsolution culture.
Race was recorded. Forty-nine patients were white, 27 black, 18 Hispanic, and 5 unknown. Presolution, 12 whites (24%), 5 blacks (19%), and 3 Hispanics (17%) had positive cultures. Postsolution, 1 white (2%), 1 black (4%), 3 Hispanics (17%), and 1 patient of unknown race (20%) had positive cultures.
ASA classifications were recorded and analyzed. Of the 99 patients, 38 were classified ASA-2, 60 were ASA-3, and 1 was ASA-4. Presolution, 9 (24%) of the 38 ASA-2 patients and 11 (18%) of the 60 ASA-3 patients had positive cultures; postsolution, 2 (5%) of the 38 ASA-2 patients and 3 (5%) of the 60 ASA-3 patients had positive cultures. The 1 ASA-4 patient had neither presolution nor postsolution positive cultures.
Types of TKA (bilateral, unilateral) were recorded. Of the 99 patients, 89 had unilateral TKAs and 10 had bilateral TKAs. Presolution, 19 (21%) of the 89 unilaterals and 1 (10%) of the 10 bilaterals had positive cultures. Postsolution, 5 (6%) of the 89 unilaterals and none of the 10 bilaterals had positive cultures.
Patients were also verbally asked how many cleanser baths they had taken before surgery. Of the 99 patients, 88 reported having taken 1 or more cleanser baths, and 1 reported no baths; 10 patients’ responses were not available. The 88 patients who had taken at least 1 cleanser bath were divided into 3 groups: 1 bath (35 patients), 2 baths (49 patients), and 3 or more baths (4 patients). Presolution, positive cultures were found for 18 (20%) of the 88 patients; for 7 (20%) of the 35 patients with 1 bath; for 10 (20%) of the 49 patients with 2 baths; and for 1 (25%) of the 4 patients with 3 or more baths. Postsolution, positive cultures were found for 5 (6%) of the 88 patients; for 2 (6%) of the 35 patients with 1 bath; for 3 (6%) of the 49 patients with 2 baths; and for 0 (0%) of the 4 patients with 3 or more baths. The 1 patient with no baths did not have a positive culture. Of the 10 patients whose responses were unavailable, 2 patients had positive presolution cultures and no patients had a positive postsolution culture.
Discussion
The efficacy of using Chloraprep before TKA has not been well assessed in orthopedic practice. However, compared with other preoperative solutions, chlorhexidine has been shown to be significantly better in preventing post-TKA infections.4 Other studies have found it far more effective than other commonly used surgical preparations in eliminating microorganisms in hip arthroplasty and foot surgery.5,7 Our study, focused on the efficacy of Chloraprep in killing bacteria, found the solution effective in removing 85% (17/20) of cultured presolution organisms.
Of the bacterial isolates cultured, normal flora were effectively removed from all associated postsolution cultures. Although most of the bacterial isolates were eliminated after solution application, both coagulase-negative S aureus and rare Bacillus species were found both pre- and postsolution, suggesting either inadequate skin preparation or resistant bacteria.
With respect to the secondary variables, our study data showed that BMI was an important predictor for bacterial isolates, significantly so presolution (P < .03). Mean BMI for the overall study was 35, firmly in the obese category. Only when BMI increased to 38 did it become significant as a predictor for postsolution organisms. Mean postsolution BMI was even higher, 40, which is in the morbidly obese category. Interestingly, the percentage of nonobese patients (BMI, <30) with positive presolution cultures was only 9%, versus the 20% with positive presolution cultures overall. In addition, 1 nonobese patient had positive postsolution cultures.
Other studies have linked higher BMI to higher rates of surgical site infection and other complications, but it is unknown if the infections are due to higher bacterial counts in the patients with high BMI or to other factors, such as reduced wound healing or decreased immune response. More research is needed to determine if the number of organisms in patients with high BMI correlates to a higher risk for surgical site infection.8 As expected, along with BMI (>38), presolution organism isolation was an important predictor for postsolution organism isolation. Patients with presolution organism isolation were 24 times more likely to have postsolution isolates.
Even though diabetic status was not significant for predicting bacterial isolation, patients with diabetes were 3.6 times more likely than patients without diabetes to have a positive culture. Other studies have shown that, compared with patients without diabetes, patients with diabetes had a higher chance of postoperative infection.9,10
In this study, 18 of 20 patients with presolution organism isolates reported they had been compliant in taking the recommended preoperative cleanser baths. This finding may indicate that preoperative cleanser baths are ineffective. However, only 20% of our patients had positive presolution cultures, whereas Ostrander and colleagues5 reported 30% positive pre-preparation cultures from the anterior knee. A recent Cochrane Database System Review did not provide clear evidence of benefit for preoperative showering or bathing with chlorhexidine over other wash products.11 Although their benefit may be questionable, we will continue to recommend preoperative cleanser baths.
One limitation of this study is sample size. Although size was sufficient for determining the efficacy of Chloraprep in the intertriginous area of the back of the knee, the lack of statistical significance (eg, effect of diabetes) may not be accurate. In addition, because the nurse who prepared patients’ skin was aware of the study and was supervised in every case, it is possible that the preparation was done more carefully than usual, resulting in more negative cultures than average. Also, compliance in taking preoperative cleanser baths was subjectively determined. Patients may have reported more baths than were actually taken. Still another study limitation is that 2 postsolution isolates did not have an associated presolution isolate. Although we think this may have resulted from laboratory contamination, it is possible the presolution swabs did not accurately determine true bacterial counts in these cases.
Conclusion
A study that showed significant residual bacteria between patients’ toes after chlorhexidine skin preparation5 left us concerned that Chloraprep skin preparation for TKA might not be adequate. The present study showed that this solution was effective in eliminating bacteria from the intertriginous area of the back of the knee in 95% of patients. Skin preparation appears to be less effective in patients with higher BMI.
1. Losina E, Thornhill TS, Rome BN, Wright J, Katz JN. The dramatic increase in total knee replacement utilization rates in the United States cannot be fully explained by growth in population size and the obesity epidemic. J Bone Joint Surg Am. 2012;94(3):201-207.
2. Poultsides LA, Ma Y, Della Valle AG, Chiu YL, Sculco TP, Memtsoudis SG. In-hospital surgical site infections after primary hip and knee arthroplasty—incidence and risk factors. J Arthroplasty. 2013;28(3):385-389.
3. Johnson AJ, Kapadia BH, Daley JA, Molina CB, Mont MA. Chlorhexidine reduces infections in knee arthroplasty. J Knee Surg. 2013;26(3):213-218.
4. Zywiel MG, Daley JA, Delanois RE, Naziri Q, Johnson AJ, Mont MA. Advance pre-operative chlorhexidine reduces the incidence of surgical site infections in knee arthroplasty. Int Orthop. 2011;35(7):1001-1006.
5. Ostrander RV, Botte MJ, Brage ME. Efficacy of surgical preparation solutions in foot and ankle surgery. J Bone Joint Surg Am. 2005;87(5):980-985.
6. Wolters U, Wolf T, Stützer H, Schröder T. ASA classification and perioperative variables as predictors of postoperative outcome. Br J Anaesth. 1996;77(2):217-222.
7. Johnson AJ, Daley JA, Zywiel MG, Delanois RE, Mont MA. Preoperative chlorhexidine preparation and the incidence of surgical site infections after hip arthroplasty. J Arthroplasty. 2010;25(6 suppl):98-102.
8. Samson AJ, Mercer GE, Campbell DG. Total knee replacement in the morbidly obese: a literature review. ANZ J Surg. 2010;80(9):595-599.
9. Iorio R, Williams KM, Marcantonio AJ, Specht LM, Tilzey JF, Healy WL. Diabetes mellitus, hemoglobin A1C, and the incidence of total joint arthroplasty infection. J Arthroplasty. 2012;27(5):726-729.
10. Viens NA, Hug KT, Marchant MH, Cook C, Vail TP, Bolognesi MP. Role of diabetes type in perioperative outcomes after hip and knee arthroplasty in the United States. J Surg Orthop Adv. 2012;21(4):253-260.
11. Webster J, Osborne S. Preoperative bathing or showering with skin antiseptics to prevent surgical site infection. Cochrane Database Syst Rev. 2012;9:CD004985.
1. Losina E, Thornhill TS, Rome BN, Wright J, Katz JN. The dramatic increase in total knee replacement utilization rates in the United States cannot be fully explained by growth in population size and the obesity epidemic. J Bone Joint Surg Am. 2012;94(3):201-207.
2. Poultsides LA, Ma Y, Della Valle AG, Chiu YL, Sculco TP, Memtsoudis SG. In-hospital surgical site infections after primary hip and knee arthroplasty—incidence and risk factors. J Arthroplasty. 2013;28(3):385-389.
3. Johnson AJ, Kapadia BH, Daley JA, Molina CB, Mont MA. Chlorhexidine reduces infections in knee arthroplasty. J Knee Surg. 2013;26(3):213-218.
4. Zywiel MG, Daley JA, Delanois RE, Naziri Q, Johnson AJ, Mont MA. Advance pre-operative chlorhexidine reduces the incidence of surgical site infections in knee arthroplasty. Int Orthop. 2011;35(7):1001-1006.
5. Ostrander RV, Botte MJ, Brage ME. Efficacy of surgical preparation solutions in foot and ankle surgery. J Bone Joint Surg Am. 2005;87(5):980-985.
6. Wolters U, Wolf T, Stützer H, Schröder T. ASA classification and perioperative variables as predictors of postoperative outcome. Br J Anaesth. 1996;77(2):217-222.
7. Johnson AJ, Daley JA, Zywiel MG, Delanois RE, Mont MA. Preoperative chlorhexidine preparation and the incidence of surgical site infections after hip arthroplasty. J Arthroplasty. 2010;25(6 suppl):98-102.
8. Samson AJ, Mercer GE, Campbell DG. Total knee replacement in the morbidly obese: a literature review. ANZ J Surg. 2010;80(9):595-599.
9. Iorio R, Williams KM, Marcantonio AJ, Specht LM, Tilzey JF, Healy WL. Diabetes mellitus, hemoglobin A1C, and the incidence of total joint arthroplasty infection. J Arthroplasty. 2012;27(5):726-729.
10. Viens NA, Hug KT, Marchant MH, Cook C, Vail TP, Bolognesi MP. Role of diabetes type in perioperative outcomes after hip and knee arthroplasty in the United States. J Surg Orthop Adv. 2012;21(4):253-260.
11. Webster J, Osborne S. Preoperative bathing or showering with skin antiseptics to prevent surgical site infection. Cochrane Database Syst Rev. 2012;9:CD004985.
Effect of Day of the Week of Primary Total Hip Arthroplasty on Length of Stay at a University-Based Teaching Medical Center
With health care costs increasing and economic resources diminishing, substantial efforts have been directed toward improving the quality of care delivered in a cost-effective manner. For a total hip arthroplasty (THA) performed in the United States between 1997 and 2001, total hospital cost, including direct and indirect costs, was estimated as averaging $13,339.1 In 2012, this cost was estimated to be between $43,000 and $100,000.2 This overall cost estimate, along with the rate at which the procedure is performed, may present an opportunity for cost savings.
Length of hospital stay (LHS) is an important outcome measure that has been assessed for optimal health care delivery. Prolonged LHS implies increased resource expenditure. Therefore, it is crucial to identify factors associated with prolonged LHS in order to reduce costs. Investigations have identified factors shown to affect LHS after THA. These factors include advanced age, medical comorbidities, obesity, intraoperative time, anesthesia technique, surgical site infection, and incision length.3-7
We conducted a study to identify the patient and clinical factors that affect LHS and to determine whether the specific day of the week when primary THA is performed affects LHS at a large tertiary-care university-based medical center. This information may prove valuable to hospital planning committees allotting operating room time and floor staffing for elective surgical cases with the goal of delivering cost-efficient care.
Materials and Methods
After obtaining institutional review board approval for this study, we retrospectively analyzed all primary unilateral THAs (273 patients) performed at our institution, a tertiary-care teaching hospital, between January 2010 and May 2011. The majority of the surgeries were performed through a posterior approach, and a majority of the implants were uncemented. All patients followed the same postoperative clinical pathway; no fast-track pathway was used.
The combined effects of day of surgery, American Society of Anesthesiologists (ASA) grade, anesthesia type, intraoperative time, estimated blood loss (EBL), incision length, presence of complications, age, sex, body mass index (BMI), disposition (skilled nursing facility vs home), transfusion, hematocrit, and hemoglobin on LHS were analyzed using a multiple quasi-Poisson regression model that included a random effect for surgeon. A Poisson regression model (typically used for count data) was deemed appropriate, as LHS was reported in whole days; a quasi-Poisson model relaxes the Poisson model assumption that the variance in the data equals the mean. The random effect for surgeon adjusts for any correlation among data from surgeries conducted by the same surgeon.
All complications were recorded. Complications included excess wound drainage,8 wound hematoma (a case of excess wound drainage necessitated surgical irrigation and débridement), new-onset atrial fibrillation, non-ST-elevation myocardial infarction, atrial flutter, urinary tract infection, pulmonary embolism, disseminated intravascular coagulation, hepatic decompensation as manifested by elevated liver enzymes, pneumonia, gastroesophageal reflux disease, gastric ulcer, sepsis, delirium, hypotension, and dysphagia.
The parameter estimates reported from the quasi-Poisson regression model are incident rate ratios (IRRs). IRR represents the change in expected LHS for a 1-unit change in a continuous variable (eg, age) or between categories of a categorical variable (eg, sex). IRR higher than 1 indicates higher risk as the continuous variable increases or a higher risk relative to the comparator group for a categorical variable. IRR lower than 1 indicates lower risk.
Results
Table 1 summarizes patient characteristics by surgical day. Mean LHS ranged from a minimum of 3.7 days for patients who had surgery on a Monday to a maximum of 4.2 days for patients who had surgery on a Thursday.
Table 2 summarizes results of the multivariate quasi-Poisson regression analysis of LHS by surgical day, ASA grade, anesthesia type, intraoperative time, EBL, incision length, presence of complications, age, sex, and BMI. With all other variables included in the model adjusted for, each additional point in ASA grade was associated with a 12% increase in LHS (P = .019). In addition, with all other variables included in the model adjusted for, LHS was 33% longer for patients with complications than for patients without complications (P < .001) and 12% longer for patients who received transfusions than for patients who did not (P = .046). LHS did not differ significantly by the day of the week when the surgery was performed (P = .496). Disposition status (skilled nursing facility vs home) as a variable to determine LHS did approach statistical significance (P = .061). As the effect size we were interested in detecting was an approximate 1-day increase in LHS for patients who had surgery later in the week relative to patients who had surgery earlier in the week, our sample size was adequate (range of required sample size, 200-300 patients). This study had 99% power to detect a 27% increase in LHS (equivalent to 1 day or more).
Discussion
This retrospective analysis explored how day of the week of primary THA affected LHS. Various confounders, such as surgery and patient factors, were also examined so that the multivariate analysis would be able to isolate the effects of surgical day of the week on LHS.
Effect of day of the week of primary THA on LHS was not investigated in the United States before. In Denmark, in a study similar to ours, Husted and colleagues4 found a 400% increase in the probability of LHS of more than 3 days when patients operated on a Thursday were compared with patients operated on a Friday. The authors reasoned that the Thursday patients most likely had a compromised physical therapy protocol owing to the inclusion of weekend days in the crucial postoperative period. LHS was consequently increased so that these patients would achieve their therapy goals before being discharged. Our investigation showed that LHS did not differ significantly by surgical day of the week. Although patients who had THA on a Thursday had 15% longer LHS than patients who had THA on a Monday, this difference was not statistically significant (P = .496), even though the study was adequately powered to detect a change in LHS of a whole day.
Table 3 summarizes the difference in quantum of workforce on weekdays and weekends at our center. The physiotherapy sessions were reduced to 1 per day. Nurse practitioners and discharge planners were not available on weekends, and some skilled nursing facilities and rehabilitation centers refused to accept patients on weekends. At our center, a teaching institute, the clinical duties of discharge planners and nurse practitioners were assumed by licensed physicians (orthopedic residents covering the arthroplasty team on weekends). This could be one of several possible reasons our study failed to detect statistically significant difference between the 2 groups. This kind of alternative arrangement may not be possible at many other centers. However, our study results provide a reasonably accurate logistical aim with regard to workforce availability on weekends to keep LHS in check.
The importance of giving patients an inpatient physical therapy regimen in timely fashion has been demonstrated in other studies. Munin and colleagues,9 in a randomized controlled trial, evaluated 71 patients who underwent elective hip and knee arthroplasty and received 2 different physical therapy regimens. Patients started their in-treatment physical therapy on postoperative day 3 or 7. Mean total LHS was shorter in the 3-day group (11.7 days) than in the 7-day group (14.5 days) (P < .001). Brusco and colleagues10 also showed that introducing weekend physical therapy services significantly reduced LHS in patients who underwent THA (10.6 vs 12.5 days; P < .05). Rapoport and Judd-Van Eerd11 retrospectively analyzed orthopedic surgery LHS, comparing patients treated in a community hospital during a period of 5-days-a-week physical therapy coverage and patients treated during a period of 7-days-a-week physical therapy coverage. The 7-days-a-week group had significantly statistically shorter mean LHS.
Another rationale for analyzing the impact of surgical day of the week stems from the expectation that patients who undergo THA on Wednesday or Thursday and are scheduled to have physical therapy or be discharged on the weekend may be affected not only by reduced inpatient weekend physical therapy coverage but also by difficulties in being transferred to a skilled nursing facility or rehabilitation center if not discharged home. In our study, the patients who were to be discharged to a rehabilitation center were delayed by 12.5%, and this statistic trended toward significance (P = .061). Our literature search did not turn up any studies, US or European, specifically linking LHS to discharge disposition (whether patient is discharged home or to a skilled nursing facility or rehabilitation center).
Reduced medical staffing on weekends may not only affect the quality of in-hospital patient care but may also result in unnecessary delays in discharge. Chow and Szeto12 retrospectively analyzed the medical records of all acute medical wards in a university hospital and compared weekend discharge rates before and after implementation of a work ordinance, which decreased the physician workforce by half on Saturday and Sunday. Results showed a 2.7% decrease in the weekend discharge rate after the work ordinance was established. The number of weekday discharges between the 2 time periods did not differ. Increasing the workforce availability presents a challenge in academic medical centers where graduate medical education enforces a strict cap on resident duty hours. Under these circumstances, a more feasible approach to decreasing LHS for THA patients is for surgical planning committees to provide the joint replacement services with operative block times early in the workweek.
Even though the organizational structure at our center is strong enough to provide for an adequate weekend workforce to discharge these patients, this study had a few limitations. We could not study readmission rates and whether the transition to home health and home physical therapy for the patients who went home was seamless.
We found that only 3 patient characteristics had a significant effect on LHS: higher ASA grade (a surrogate for medical comorbidities), requirement for blood transfusion, and presence of complications. In Denmark, blood transfusion increased the likelihood of longer LHS by 400%.4 In that study, patients who were ASA grades 1 and 2 had 60% and 20% decreased likelihood of LHS of more than 3 days compared with patients who were ASA grade 3. Similarly, in 2009, Mears and colleagues5 found 4 factors related to increased LHS: female sex (P < .001), older age (P < .001), higher ASA grade (3, P < .01; 4, P < .001), and increased blood loss (P < .001).5
Conclusion
Over the past decade, there has been a significant reduction in LHS after THA, from a mean of 3 weeks to 4 days. Advances in implant technology, delivery of in-home physical therapy, and improved prevention and management of postoperative complications have contributed to this decline. Early identification of patients with transfusion requirements may be helpful in expediting their care. Although guidelines are in place for transfusion, further study in this regard may be needed. It is important to continue to identify surgery and patient factors that affect LHS, but the importance of organizational and planning issues in optimizing hospital health care expenditures cannot be ignored. Further study of providing a specific discharge planning service to identify patients’ discharge needs (home vs extended care facility) may help reduce LHS.
1. Antoniou J, Martineau PA, Filion KB, et al. In-hospital cost of total hip arthroplasty in Canada and the United States. J Bone Joint Surg Am. 2004;86(11):2435-2439.
2. Kumar S, Breuing R, Chahal R. Globalization of health care delivery in the United States through medical tourism. J Health Commun. 2012;17(2):177-198.
3. Foote J, Panchoo K, Blair P, Bannister G. Length of stay following primary total hip replacement. Ann R Coll Surg Engl. 2009;91(6):500-504.
4. Husted H, Holm G, Jacobsen S. Predictors of length of stay and patient satisfaction after hip and knee replacement surgery: fast-track experience in 712 patients. Acta Orthop. 2008;79(2):168-173.
5. Mears DC, Mears SC, Chelly JE, Dai F, Vulakovich KL. THA with a minimally invasive technique, multi-modal anesthesia, and home rehabilitation: factors associated with early discharge? Clin Orthop. 2009;467(6):1412-1417.
6. Peck CN, Foster A, McLauchlan GJ. Reducing incision length or intensifying rehabilitation: what makes the difference to length of stay in total hip replacement in a UK setting? Int Orthop. 2006;30(5):395-398.
7. Weaver F, Hynes D, Hopkinson W, et al. Preoperative risks and outcomes of hip and knee arthroplasty in the Veterans Health Administration. J Arthroplasty. 2003;18(6):693-708.
8. Patel VP, Walsh M, Sehgal B, Preston C, DeWal H, Di Cesare PE. Factors associated with prolonged wound drainage after primary total hip and knee arthroplasty. J Bone Joint Surg Am. 2007;89(1):33-38.
9. Munin MC, Rudy TE, Glynn NW, Crossett LS, Rubash HE. Early inpatient rehabilitation after elective hip and knee arthroplasty. JAMA. 1998;279(11):847-852.
10. Brusco NK, Shields N, Taylor NF, Paratz J. A Saturday physiotherapy service may decrease length of stay in patients undergoing rehabilitation in hospital: a randomised controlled trial. Aust J Physiother. 2007;53(2):75-81.
11. Rapoport J, Judd-Van Eerd M. Impact of physical therapy weekend coverage on length of stay in an acute care community hospital. Phys Ther. 1989;69(1):32-37.
12. Chow KM, Szeto CC. Impact of enforcing the Labour Ordinance, with 1-in-7-day off for hospital doctors, on weekend hospital discharge rate. J Public Health (Oxf). 2005;27(2):189-191.
With health care costs increasing and economic resources diminishing, substantial efforts have been directed toward improving the quality of care delivered in a cost-effective manner. For a total hip arthroplasty (THA) performed in the United States between 1997 and 2001, total hospital cost, including direct and indirect costs, was estimated as averaging $13,339.1 In 2012, this cost was estimated to be between $43,000 and $100,000.2 This overall cost estimate, along with the rate at which the procedure is performed, may present an opportunity for cost savings.
Length of hospital stay (LHS) is an important outcome measure that has been assessed for optimal health care delivery. Prolonged LHS implies increased resource expenditure. Therefore, it is crucial to identify factors associated with prolonged LHS in order to reduce costs. Investigations have identified factors shown to affect LHS after THA. These factors include advanced age, medical comorbidities, obesity, intraoperative time, anesthesia technique, surgical site infection, and incision length.3-7
We conducted a study to identify the patient and clinical factors that affect LHS and to determine whether the specific day of the week when primary THA is performed affects LHS at a large tertiary-care university-based medical center. This information may prove valuable to hospital planning committees allotting operating room time and floor staffing for elective surgical cases with the goal of delivering cost-efficient care.
Materials and Methods
After obtaining institutional review board approval for this study, we retrospectively analyzed all primary unilateral THAs (273 patients) performed at our institution, a tertiary-care teaching hospital, between January 2010 and May 2011. The majority of the surgeries were performed through a posterior approach, and a majority of the implants were uncemented. All patients followed the same postoperative clinical pathway; no fast-track pathway was used.
The combined effects of day of surgery, American Society of Anesthesiologists (ASA) grade, anesthesia type, intraoperative time, estimated blood loss (EBL), incision length, presence of complications, age, sex, body mass index (BMI), disposition (skilled nursing facility vs home), transfusion, hematocrit, and hemoglobin on LHS were analyzed using a multiple quasi-Poisson regression model that included a random effect for surgeon. A Poisson regression model (typically used for count data) was deemed appropriate, as LHS was reported in whole days; a quasi-Poisson model relaxes the Poisson model assumption that the variance in the data equals the mean. The random effect for surgeon adjusts for any correlation among data from surgeries conducted by the same surgeon.
All complications were recorded. Complications included excess wound drainage,8 wound hematoma (a case of excess wound drainage necessitated surgical irrigation and débridement), new-onset atrial fibrillation, non-ST-elevation myocardial infarction, atrial flutter, urinary tract infection, pulmonary embolism, disseminated intravascular coagulation, hepatic decompensation as manifested by elevated liver enzymes, pneumonia, gastroesophageal reflux disease, gastric ulcer, sepsis, delirium, hypotension, and dysphagia.
The parameter estimates reported from the quasi-Poisson regression model are incident rate ratios (IRRs). IRR represents the change in expected LHS for a 1-unit change in a continuous variable (eg, age) or between categories of a categorical variable (eg, sex). IRR higher than 1 indicates higher risk as the continuous variable increases or a higher risk relative to the comparator group for a categorical variable. IRR lower than 1 indicates lower risk.
Results
Table 1 summarizes patient characteristics by surgical day. Mean LHS ranged from a minimum of 3.7 days for patients who had surgery on a Monday to a maximum of 4.2 days for patients who had surgery on a Thursday.
Table 2 summarizes results of the multivariate quasi-Poisson regression analysis of LHS by surgical day, ASA grade, anesthesia type, intraoperative time, EBL, incision length, presence of complications, age, sex, and BMI. With all other variables included in the model adjusted for, each additional point in ASA grade was associated with a 12% increase in LHS (P = .019). In addition, with all other variables included in the model adjusted for, LHS was 33% longer for patients with complications than for patients without complications (P < .001) and 12% longer for patients who received transfusions than for patients who did not (P = .046). LHS did not differ significantly by the day of the week when the surgery was performed (P = .496). Disposition status (skilled nursing facility vs home) as a variable to determine LHS did approach statistical significance (P = .061). As the effect size we were interested in detecting was an approximate 1-day increase in LHS for patients who had surgery later in the week relative to patients who had surgery earlier in the week, our sample size was adequate (range of required sample size, 200-300 patients). This study had 99% power to detect a 27% increase in LHS (equivalent to 1 day or more).
Discussion
This retrospective analysis explored how day of the week of primary THA affected LHS. Various confounders, such as surgery and patient factors, were also examined so that the multivariate analysis would be able to isolate the effects of surgical day of the week on LHS.
Effect of day of the week of primary THA on LHS was not investigated in the United States before. In Denmark, in a study similar to ours, Husted and colleagues4 found a 400% increase in the probability of LHS of more than 3 days when patients operated on a Thursday were compared with patients operated on a Friday. The authors reasoned that the Thursday patients most likely had a compromised physical therapy protocol owing to the inclusion of weekend days in the crucial postoperative period. LHS was consequently increased so that these patients would achieve their therapy goals before being discharged. Our investigation showed that LHS did not differ significantly by surgical day of the week. Although patients who had THA on a Thursday had 15% longer LHS than patients who had THA on a Monday, this difference was not statistically significant (P = .496), even though the study was adequately powered to detect a change in LHS of a whole day.
Table 3 summarizes the difference in quantum of workforce on weekdays and weekends at our center. The physiotherapy sessions were reduced to 1 per day. Nurse practitioners and discharge planners were not available on weekends, and some skilled nursing facilities and rehabilitation centers refused to accept patients on weekends. At our center, a teaching institute, the clinical duties of discharge planners and nurse practitioners were assumed by licensed physicians (orthopedic residents covering the arthroplasty team on weekends). This could be one of several possible reasons our study failed to detect statistically significant difference between the 2 groups. This kind of alternative arrangement may not be possible at many other centers. However, our study results provide a reasonably accurate logistical aim with regard to workforce availability on weekends to keep LHS in check.
The importance of giving patients an inpatient physical therapy regimen in timely fashion has been demonstrated in other studies. Munin and colleagues,9 in a randomized controlled trial, evaluated 71 patients who underwent elective hip and knee arthroplasty and received 2 different physical therapy regimens. Patients started their in-treatment physical therapy on postoperative day 3 or 7. Mean total LHS was shorter in the 3-day group (11.7 days) than in the 7-day group (14.5 days) (P < .001). Brusco and colleagues10 also showed that introducing weekend physical therapy services significantly reduced LHS in patients who underwent THA (10.6 vs 12.5 days; P < .05). Rapoport and Judd-Van Eerd11 retrospectively analyzed orthopedic surgery LHS, comparing patients treated in a community hospital during a period of 5-days-a-week physical therapy coverage and patients treated during a period of 7-days-a-week physical therapy coverage. The 7-days-a-week group had significantly statistically shorter mean LHS.
Another rationale for analyzing the impact of surgical day of the week stems from the expectation that patients who undergo THA on Wednesday or Thursday and are scheduled to have physical therapy or be discharged on the weekend may be affected not only by reduced inpatient weekend physical therapy coverage but also by difficulties in being transferred to a skilled nursing facility or rehabilitation center if not discharged home. In our study, the patients who were to be discharged to a rehabilitation center were delayed by 12.5%, and this statistic trended toward significance (P = .061). Our literature search did not turn up any studies, US or European, specifically linking LHS to discharge disposition (whether patient is discharged home or to a skilled nursing facility or rehabilitation center).
Reduced medical staffing on weekends may not only affect the quality of in-hospital patient care but may also result in unnecessary delays in discharge. Chow and Szeto12 retrospectively analyzed the medical records of all acute medical wards in a university hospital and compared weekend discharge rates before and after implementation of a work ordinance, which decreased the physician workforce by half on Saturday and Sunday. Results showed a 2.7% decrease in the weekend discharge rate after the work ordinance was established. The number of weekday discharges between the 2 time periods did not differ. Increasing the workforce availability presents a challenge in academic medical centers where graduate medical education enforces a strict cap on resident duty hours. Under these circumstances, a more feasible approach to decreasing LHS for THA patients is for surgical planning committees to provide the joint replacement services with operative block times early in the workweek.
Even though the organizational structure at our center is strong enough to provide for an adequate weekend workforce to discharge these patients, this study had a few limitations. We could not study readmission rates and whether the transition to home health and home physical therapy for the patients who went home was seamless.
We found that only 3 patient characteristics had a significant effect on LHS: higher ASA grade (a surrogate for medical comorbidities), requirement for blood transfusion, and presence of complications. In Denmark, blood transfusion increased the likelihood of longer LHS by 400%.4 In that study, patients who were ASA grades 1 and 2 had 60% and 20% decreased likelihood of LHS of more than 3 days compared with patients who were ASA grade 3. Similarly, in 2009, Mears and colleagues5 found 4 factors related to increased LHS: female sex (P < .001), older age (P < .001), higher ASA grade (3, P < .01; 4, P < .001), and increased blood loss (P < .001).5
Conclusion
Over the past decade, there has been a significant reduction in LHS after THA, from a mean of 3 weeks to 4 days. Advances in implant technology, delivery of in-home physical therapy, and improved prevention and management of postoperative complications have contributed to this decline. Early identification of patients with transfusion requirements may be helpful in expediting their care. Although guidelines are in place for transfusion, further study in this regard may be needed. It is important to continue to identify surgery and patient factors that affect LHS, but the importance of organizational and planning issues in optimizing hospital health care expenditures cannot be ignored. Further study of providing a specific discharge planning service to identify patients’ discharge needs (home vs extended care facility) may help reduce LHS.
With health care costs increasing and economic resources diminishing, substantial efforts have been directed toward improving the quality of care delivered in a cost-effective manner. For a total hip arthroplasty (THA) performed in the United States between 1997 and 2001, total hospital cost, including direct and indirect costs, was estimated as averaging $13,339.1 In 2012, this cost was estimated to be between $43,000 and $100,000.2 This overall cost estimate, along with the rate at which the procedure is performed, may present an opportunity for cost savings.
Length of hospital stay (LHS) is an important outcome measure that has been assessed for optimal health care delivery. Prolonged LHS implies increased resource expenditure. Therefore, it is crucial to identify factors associated with prolonged LHS in order to reduce costs. Investigations have identified factors shown to affect LHS after THA. These factors include advanced age, medical comorbidities, obesity, intraoperative time, anesthesia technique, surgical site infection, and incision length.3-7
We conducted a study to identify the patient and clinical factors that affect LHS and to determine whether the specific day of the week when primary THA is performed affects LHS at a large tertiary-care university-based medical center. This information may prove valuable to hospital planning committees allotting operating room time and floor staffing for elective surgical cases with the goal of delivering cost-efficient care.
Materials and Methods
After obtaining institutional review board approval for this study, we retrospectively analyzed all primary unilateral THAs (273 patients) performed at our institution, a tertiary-care teaching hospital, between January 2010 and May 2011. The majority of the surgeries were performed through a posterior approach, and a majority of the implants were uncemented. All patients followed the same postoperative clinical pathway; no fast-track pathway was used.
The combined effects of day of surgery, American Society of Anesthesiologists (ASA) grade, anesthesia type, intraoperative time, estimated blood loss (EBL), incision length, presence of complications, age, sex, body mass index (BMI), disposition (skilled nursing facility vs home), transfusion, hematocrit, and hemoglobin on LHS were analyzed using a multiple quasi-Poisson regression model that included a random effect for surgeon. A Poisson regression model (typically used for count data) was deemed appropriate, as LHS was reported in whole days; a quasi-Poisson model relaxes the Poisson model assumption that the variance in the data equals the mean. The random effect for surgeon adjusts for any correlation among data from surgeries conducted by the same surgeon.
All complications were recorded. Complications included excess wound drainage,8 wound hematoma (a case of excess wound drainage necessitated surgical irrigation and débridement), new-onset atrial fibrillation, non-ST-elevation myocardial infarction, atrial flutter, urinary tract infection, pulmonary embolism, disseminated intravascular coagulation, hepatic decompensation as manifested by elevated liver enzymes, pneumonia, gastroesophageal reflux disease, gastric ulcer, sepsis, delirium, hypotension, and dysphagia.
The parameter estimates reported from the quasi-Poisson regression model are incident rate ratios (IRRs). IRR represents the change in expected LHS for a 1-unit change in a continuous variable (eg, age) or between categories of a categorical variable (eg, sex). IRR higher than 1 indicates higher risk as the continuous variable increases or a higher risk relative to the comparator group for a categorical variable. IRR lower than 1 indicates lower risk.
Results
Table 1 summarizes patient characteristics by surgical day. Mean LHS ranged from a minimum of 3.7 days for patients who had surgery on a Monday to a maximum of 4.2 days for patients who had surgery on a Thursday.
Table 2 summarizes results of the multivariate quasi-Poisson regression analysis of LHS by surgical day, ASA grade, anesthesia type, intraoperative time, EBL, incision length, presence of complications, age, sex, and BMI. With all other variables included in the model adjusted for, each additional point in ASA grade was associated with a 12% increase in LHS (P = .019). In addition, with all other variables included in the model adjusted for, LHS was 33% longer for patients with complications than for patients without complications (P < .001) and 12% longer for patients who received transfusions than for patients who did not (P = .046). LHS did not differ significantly by the day of the week when the surgery was performed (P = .496). Disposition status (skilled nursing facility vs home) as a variable to determine LHS did approach statistical significance (P = .061). As the effect size we were interested in detecting was an approximate 1-day increase in LHS for patients who had surgery later in the week relative to patients who had surgery earlier in the week, our sample size was adequate (range of required sample size, 200-300 patients). This study had 99% power to detect a 27% increase in LHS (equivalent to 1 day or more).
Discussion
This retrospective analysis explored how day of the week of primary THA affected LHS. Various confounders, such as surgery and patient factors, were also examined so that the multivariate analysis would be able to isolate the effects of surgical day of the week on LHS.
Effect of day of the week of primary THA on LHS was not investigated in the United States before. In Denmark, in a study similar to ours, Husted and colleagues4 found a 400% increase in the probability of LHS of more than 3 days when patients operated on a Thursday were compared with patients operated on a Friday. The authors reasoned that the Thursday patients most likely had a compromised physical therapy protocol owing to the inclusion of weekend days in the crucial postoperative period. LHS was consequently increased so that these patients would achieve their therapy goals before being discharged. Our investigation showed that LHS did not differ significantly by surgical day of the week. Although patients who had THA on a Thursday had 15% longer LHS than patients who had THA on a Monday, this difference was not statistically significant (P = .496), even though the study was adequately powered to detect a change in LHS of a whole day.
Table 3 summarizes the difference in quantum of workforce on weekdays and weekends at our center. The physiotherapy sessions were reduced to 1 per day. Nurse practitioners and discharge planners were not available on weekends, and some skilled nursing facilities and rehabilitation centers refused to accept patients on weekends. At our center, a teaching institute, the clinical duties of discharge planners and nurse practitioners were assumed by licensed physicians (orthopedic residents covering the arthroplasty team on weekends). This could be one of several possible reasons our study failed to detect statistically significant difference between the 2 groups. This kind of alternative arrangement may not be possible at many other centers. However, our study results provide a reasonably accurate logistical aim with regard to workforce availability on weekends to keep LHS in check.
The importance of giving patients an inpatient physical therapy regimen in timely fashion has been demonstrated in other studies. Munin and colleagues,9 in a randomized controlled trial, evaluated 71 patients who underwent elective hip and knee arthroplasty and received 2 different physical therapy regimens. Patients started their in-treatment physical therapy on postoperative day 3 or 7. Mean total LHS was shorter in the 3-day group (11.7 days) than in the 7-day group (14.5 days) (P < .001). Brusco and colleagues10 also showed that introducing weekend physical therapy services significantly reduced LHS in patients who underwent THA (10.6 vs 12.5 days; P < .05). Rapoport and Judd-Van Eerd11 retrospectively analyzed orthopedic surgery LHS, comparing patients treated in a community hospital during a period of 5-days-a-week physical therapy coverage and patients treated during a period of 7-days-a-week physical therapy coverage. The 7-days-a-week group had significantly statistically shorter mean LHS.
Another rationale for analyzing the impact of surgical day of the week stems from the expectation that patients who undergo THA on Wednesday or Thursday and are scheduled to have physical therapy or be discharged on the weekend may be affected not only by reduced inpatient weekend physical therapy coverage but also by difficulties in being transferred to a skilled nursing facility or rehabilitation center if not discharged home. In our study, the patients who were to be discharged to a rehabilitation center were delayed by 12.5%, and this statistic trended toward significance (P = .061). Our literature search did not turn up any studies, US or European, specifically linking LHS to discharge disposition (whether patient is discharged home or to a skilled nursing facility or rehabilitation center).
Reduced medical staffing on weekends may not only affect the quality of in-hospital patient care but may also result in unnecessary delays in discharge. Chow and Szeto12 retrospectively analyzed the medical records of all acute medical wards in a university hospital and compared weekend discharge rates before and after implementation of a work ordinance, which decreased the physician workforce by half on Saturday and Sunday. Results showed a 2.7% decrease in the weekend discharge rate after the work ordinance was established. The number of weekday discharges between the 2 time periods did not differ. Increasing the workforce availability presents a challenge in academic medical centers where graduate medical education enforces a strict cap on resident duty hours. Under these circumstances, a more feasible approach to decreasing LHS for THA patients is for surgical planning committees to provide the joint replacement services with operative block times early in the workweek.
Even though the organizational structure at our center is strong enough to provide for an adequate weekend workforce to discharge these patients, this study had a few limitations. We could not study readmission rates and whether the transition to home health and home physical therapy for the patients who went home was seamless.
We found that only 3 patient characteristics had a significant effect on LHS: higher ASA grade (a surrogate for medical comorbidities), requirement for blood transfusion, and presence of complications. In Denmark, blood transfusion increased the likelihood of longer LHS by 400%.4 In that study, patients who were ASA grades 1 and 2 had 60% and 20% decreased likelihood of LHS of more than 3 days compared with patients who were ASA grade 3. Similarly, in 2009, Mears and colleagues5 found 4 factors related to increased LHS: female sex (P < .001), older age (P < .001), higher ASA grade (3, P < .01; 4, P < .001), and increased blood loss (P < .001).5
Conclusion
Over the past decade, there has been a significant reduction in LHS after THA, from a mean of 3 weeks to 4 days. Advances in implant technology, delivery of in-home physical therapy, and improved prevention and management of postoperative complications have contributed to this decline. Early identification of patients with transfusion requirements may be helpful in expediting their care. Although guidelines are in place for transfusion, further study in this regard may be needed. It is important to continue to identify surgery and patient factors that affect LHS, but the importance of organizational and planning issues in optimizing hospital health care expenditures cannot be ignored. Further study of providing a specific discharge planning service to identify patients’ discharge needs (home vs extended care facility) may help reduce LHS.
1. Antoniou J, Martineau PA, Filion KB, et al. In-hospital cost of total hip arthroplasty in Canada and the United States. J Bone Joint Surg Am. 2004;86(11):2435-2439.
2. Kumar S, Breuing R, Chahal R. Globalization of health care delivery in the United States through medical tourism. J Health Commun. 2012;17(2):177-198.
3. Foote J, Panchoo K, Blair P, Bannister G. Length of stay following primary total hip replacement. Ann R Coll Surg Engl. 2009;91(6):500-504.
4. Husted H, Holm G, Jacobsen S. Predictors of length of stay and patient satisfaction after hip and knee replacement surgery: fast-track experience in 712 patients. Acta Orthop. 2008;79(2):168-173.
5. Mears DC, Mears SC, Chelly JE, Dai F, Vulakovich KL. THA with a minimally invasive technique, multi-modal anesthesia, and home rehabilitation: factors associated with early discharge? Clin Orthop. 2009;467(6):1412-1417.
6. Peck CN, Foster A, McLauchlan GJ. Reducing incision length or intensifying rehabilitation: what makes the difference to length of stay in total hip replacement in a UK setting? Int Orthop. 2006;30(5):395-398.
7. Weaver F, Hynes D, Hopkinson W, et al. Preoperative risks and outcomes of hip and knee arthroplasty in the Veterans Health Administration. J Arthroplasty. 2003;18(6):693-708.
8. Patel VP, Walsh M, Sehgal B, Preston C, DeWal H, Di Cesare PE. Factors associated with prolonged wound drainage after primary total hip and knee arthroplasty. J Bone Joint Surg Am. 2007;89(1):33-38.
9. Munin MC, Rudy TE, Glynn NW, Crossett LS, Rubash HE. Early inpatient rehabilitation after elective hip and knee arthroplasty. JAMA. 1998;279(11):847-852.
10. Brusco NK, Shields N, Taylor NF, Paratz J. A Saturday physiotherapy service may decrease length of stay in patients undergoing rehabilitation in hospital: a randomised controlled trial. Aust J Physiother. 2007;53(2):75-81.
11. Rapoport J, Judd-Van Eerd M. Impact of physical therapy weekend coverage on length of stay in an acute care community hospital. Phys Ther. 1989;69(1):32-37.
12. Chow KM, Szeto CC. Impact of enforcing the Labour Ordinance, with 1-in-7-day off for hospital doctors, on weekend hospital discharge rate. J Public Health (Oxf). 2005;27(2):189-191.
1. Antoniou J, Martineau PA, Filion KB, et al. In-hospital cost of total hip arthroplasty in Canada and the United States. J Bone Joint Surg Am. 2004;86(11):2435-2439.
2. Kumar S, Breuing R, Chahal R. Globalization of health care delivery in the United States through medical tourism. J Health Commun. 2012;17(2):177-198.
3. Foote J, Panchoo K, Blair P, Bannister G. Length of stay following primary total hip replacement. Ann R Coll Surg Engl. 2009;91(6):500-504.
4. Husted H, Holm G, Jacobsen S. Predictors of length of stay and patient satisfaction after hip and knee replacement surgery: fast-track experience in 712 patients. Acta Orthop. 2008;79(2):168-173.
5. Mears DC, Mears SC, Chelly JE, Dai F, Vulakovich KL. THA with a minimally invasive technique, multi-modal anesthesia, and home rehabilitation: factors associated with early discharge? Clin Orthop. 2009;467(6):1412-1417.
6. Peck CN, Foster A, McLauchlan GJ. Reducing incision length or intensifying rehabilitation: what makes the difference to length of stay in total hip replacement in a UK setting? Int Orthop. 2006;30(5):395-398.
7. Weaver F, Hynes D, Hopkinson W, et al. Preoperative risks and outcomes of hip and knee arthroplasty in the Veterans Health Administration. J Arthroplasty. 2003;18(6):693-708.
8. Patel VP, Walsh M, Sehgal B, Preston C, DeWal H, Di Cesare PE. Factors associated with prolonged wound drainage after primary total hip and knee arthroplasty. J Bone Joint Surg Am. 2007;89(1):33-38.
9. Munin MC, Rudy TE, Glynn NW, Crossett LS, Rubash HE. Early inpatient rehabilitation after elective hip and knee arthroplasty. JAMA. 1998;279(11):847-852.
10. Brusco NK, Shields N, Taylor NF, Paratz J. A Saturday physiotherapy service may decrease length of stay in patients undergoing rehabilitation in hospital: a randomised controlled trial. Aust J Physiother. 2007;53(2):75-81.
11. Rapoport J, Judd-Van Eerd M. Impact of physical therapy weekend coverage on length of stay in an acute care community hospital. Phys Ther. 1989;69(1):32-37.
12. Chow KM, Szeto CC. Impact of enforcing the Labour Ordinance, with 1-in-7-day off for hospital doctors, on weekend hospital discharge rate. J Public Health (Oxf). 2005;27(2):189-191.
Metal Ion Levels in Maternal and Placental Blood After Metal-on-Metal Total Hip Arthroplasty
Serum metal ion levels are increased after primary total hip arthroplasty (THA) with all types of head-liner bearing surfaces.1-4 In recent years, there has been increasing concern regarding elevated metal ion levels and adverse local and systemic effects, in particular, from metal-on-metal (MOM) implants.5 There have been reports of adverse local tissue reactions (ALTR) and systemic effects associated with elevated metal ion levels from MOM THA.6-10 MOM hip resurfacings have been advocated in the literature for use in select patient populations, such as young, active individuals.11,12 Purported benefits of MOM-bearing surfaces in this patient population include reduced wear and the prevention of osteolysis.13 While the incidence of ALTR has been reported to be approximately 1% within 5 years after MOM hip resurfacing, the prevalence of ALTR at long-term follow-up is unknown.14 Adverse local tissue reactions in hip resurfacing are related in part to femoral head coverage by the acetabular cup, as well as component design and orientation. The risk for ALTR with MOM THA has been reported to correlate with the level of serum metal ion levels because of the bearing surface, along with taper corrosion and corrosion secondary to the large surface area of the femoral head.13-15 The overall clinical and prognostic value of metal ion levels in cases of MOM bearings remains controversial and without clear cut-off values.15
The long-term physiologic response to elevated serum metal ion levels from THA implants remains unknown and is of particular concern in pregnant women because of potential transplacental transfer. Several studies have shown that DNA (deoxyribonucleic acid) and chromosomal changes can occur in patients with both MOM and non-MOM hip implants.16-18 Techniques to accurately measure the levels of metal ions, including cobalt, chromium, and titanium in the serum, have improved substantially in recent years and have been used successfully in clinical applications with low detection limits.2,4,19,20 Evidence shows that pregnancy-related complications in women with well-functioning cemented, hybrid, or uncemented metal-on-polyethylene THA are not different from those in healthy women.21 However, it is unclear if metal ions can cross the placenta and negatively affect the development of a fetus in mothers with MOM-bearing THAs.22 We conducted this study to determine if metal ions can traverse the placenta by measuring serum metal ion concentrations in mothers with and without MOM THA and the corresponding levels in umbilical cord blood samples.
Materials and Methods
Ten patients were prospectively enrolled in this study, which was approved by the institutional review boards at all 3 participating hospitals prior to initiation. All patients provided informed consent and agreed to maternal and umbilical cord blood sampling. Seven of the 10 patients did not have any metallic implants and served as controls. The remaining 3 patients had either a MOM unilateral primary THA (1 patient) or unilateral MOM hip-resurfacing arthroplasty (2 patients) with no other metal implants in the body. For all subjects, maternal and umbilical cord blood was obtained at the time of delivery.
Group Demographics
The 3 women in the implant group had a mean age of 32.3 years (range, 23-39 years) at time of delivery. The first patient had posttraumatic osteoarthritis and underwent right THA using a modular MOM 60-mm acetabular component, a 36-mm cobalt chrome head, and a cementless, titanium proximally porous-coated femoral component (Pinnacle Total Hip System and Summit Total Hip System; DePuy Synthes, Warsaw, Indiana). Her infant was born 2 years after index THA. The second patient had a diagnosis of degenerative osteoarthritis secondary to developmental dysplasia of the hip and underwent a hybrid MOM hip resurfacing with a monoblock 50-mm cup and 40-mm cobalt chrome head (Conserve Plus; Wright Medical Technology, Arlington, Tennessee). She gave birth to her infant 6 years after her hip resurfacing. The third patient also had a diagnosis of degenerative osteoarthritis secondary to developmental dysplasia of the hip and underwent hip resurfacing with a 54-mm monoblock cup and 44-mm cobalt chrome head (Conserve Plus). Her infant was born 4 years after her resurfacing arthroplasty. All of the infants were born healthy, and the deliveries were uneventful and without complications. Seven women with a mean age of 32.1 years (range, 24-37 years) and their infants served as controls at the time of delivery. None of the women in the control group had a history of renal impairment, inherited genetic disorders, or metal implants.
Serum Metal Ion Analysis
Blood samples were collected using S-Monovette polypropylene syringes (Sarstedt, Princeton, New Jersey), a multi-adapter, and infusion set following an established technique.1 All vessels and utensils used for specimen collection were verified to be free of metal contamination. Three 10-mL syringes were drawn, and each syringe was labeled to indicate the sequence of collection. The first 10 mL were drawn to rinse the needle and adapter. Blood was then allowed to clot naturally and centrifuged at 1850 rpm for 30 minutes, separating samples into cell and serum fractions that were stored in labeled vials at -80ºC. All specimen manipulations after collection were carried out in a class-100 environment using a SterilGARD Biological Safety Cabinet (Baker, Sanford, Maine) and class-100 gloves (Oak Technical, Ravenna, Ohio) to minimize atmospheric and manual contamination. Maternal and umbilical cord blood metal ion levels were tested using high-resolution sector-field inductively-coupled plasma-mass spectrometry (HR-SF-ICPMS) (Element 2; Thermo Fisher Scientific, Bremen, Germany) following the method of additions as described previously.23 The HR-SF-ICPMS machine was equipped with an SC-E2 autosampler, Teflon nebulizer and spray chamber, sapphire injector (Elemental Scientific, Omaha, Nebraska) and platinum cones. All calibration and internal standard solutions were prepared by gradual dilutions of single-element standard solutions (1000 μg/mL from High Purity Standards, Charleston, South Carolina). The certified reference material, Seronorm Trace Elements Serum (SERO, Billingstad, Norway), were routinely analyzed with samples. The serum concentrations of cobalt, chromium, titanium, and nickel were measured with detection limits in ng/mL (parts per billion) of 0.04 for cobalt, 0.015 for chromium, 0.2 for titanium, and 0.17 for nickel.4 Concentrations below the detection limit were approximated as one-half of the detection limit by convention to calculate means.
Statistical Analysis
The data reported are the means for each group for each of the metal ion levels analyzed. Intergroup comparisons were made with the Mann-Whitney-Wilcoxon test using SPSS statistics software (SPSS Science Inc, Chicago, Illinois) to compare implant and control groups in regards to serum metal ion levels. Intragroup comparisons were made using the Friedman test with significance set at P < .05. Spearman rank-order correlation tests were used to investigate relationships between maternal and infant serum metal ion levels.
Results
The Table shows the mean serum metal ion levels of chromium, cobalt, titanium, and nickel for both groups. The implant-group mothers had significantly higher chromium and cobalt levels than the control-group mothers, with mean chromium levels of 1.87 ng/mL vs 0.16 ng/mL (P = .01) and mean cobalt levels of 0.97 ng/mL vs 0.20 ng/mL (P = .01), respectively. All control-group maternal chromium and cobalt levels were lower than the implant group. There were no significant differences detected between the implant-group and control-group mothers with respect to serum titanium or nickel levels.
The implant-group cord blood samples also had significantly higher chromium and cobalt levels than did the control-group infants with mean chromium levels of 0.29 ng/mL vs 0.10 ng/mL (P = .03) and mean cobalt levels of 0.49 ng/mL vs 0.16 ng/mL (P = .01), respectively. All but 1 of the control-group infants had chromium levels that were lower than the implant-group infant chromium levels. All of the control-group infant cobalt levels were lower than the implant-group infant cobalt levels. In the mother (I-1) who had a titanium-containing implant, her titanium level was 1.77 ng/mL and her cord blood level was 0.78 ng/mL. In contrast, the other 2 patients did not have titanium-containing implants and had corresponding metal levels of 0.10 ng/mL and cord blood levels either below the detection limit or just slightly above it. No statistically significant differences were found between the implant- and control-group infants with respect to serum titanium or nickel levels.
Considering the implant and control groups separately, we found no statistically significant differences between the maternal and infant titanium levels or the maternal and infant nickel levels. In the implant group, the mother’s chromium level was always higher than her infant’s chromium level, and the mother’s cobalt level was always higher than her infant’s cobalt level (P= .08). In the control group, there was no correlation between the maternal and infant chromium levels, or between the maternal and infant cobalt levels. In the implant group, the maternal and infant chromium levels were highly correlated (r = 1), as were the maternal and infant cobalt levels (r = 1).
When infants’ chromium levels were expressed as a percentage of their mothers’ chromium levels, the mean was 15.4% (range, 12.3%-18%) for the implant group and 58.9% (range, 13.5%-165.7%) for the control group (P = .05). The mean infant cobalt level, expressed as percentage of maternal cobalt level, was 50% (range, 45.8%-53.1%) for the implant group and 76.6% (range, 59.1%-150.8%) for the control group (P = .01).
Discussion
Cobalt and chromium serum metal ion levels obtained from mothers with MOM implants and umbilical cord samples were significantly elevated in comparison with controls. There was also a strong correlation between implant-group maternal cobalt and chromium levels and implant-group infant cobalt and chromium levels; however, no such correlation existed in the control group. Our results suggest that cobalt and chromium cross the placental barrier. Implant mothers had higher chromium and cobalt levels compared with implant babies, and these babies had approximately 15% of the level of chromium and 50% of the level of cobalt when compared to implant maternal levels. This finding suggests that the placenta modulates the transfer of chromium and cobalt to the fetus.
Two studies have reported chromium and cobalt levels in maternal and umbilical cord sera after MOM THA.5,24 Brodner and colleagues5 determined the maternal serum levels of cobalt and chromium in 3 women 3.8 years after MOM THA and compared those to cobalt and chromium levels obtained from umbilical cord blood. At the time of delivery, the maternal chromium concentrations in the 3 patients were 1.6 ng/mL, 0.5 ng/mL, and 0.9 ng/mL, and the cobalt concentrations were 1 ng/mL in 1 patient and below the detection limit in the other 2 patients. Cobalt and chromium concentrations of the 3 umbilical cord sera were below the detection limit. The authors concluded that cobalt and chromium did not cross the placenta based on their laboratory detection limits. Metal ion levels were measured using atomic absorption spectrometry with relatively high detection limits of 0.3 ng/mL, which is not as sensitive as the HR-SF-ICPMS technique used in the present study that has detection limits of 0.04 ng/mL for cobalt and 0.015 ng/mL for chromium. The relatively high detection limits of atomic absorption spectrometry were likely responsible for the authors’ inability to detect elevated chromium and cobalt levels in umbilical cord sera.
Ziaee and colleagues24 used HR-SF-ICPMS, as we did in this study, to measure the mean concentrations of cobalt and chromium ion levels in 10 maternal and umbilical cord blood samples in women with Birmingham MOM hip-resurfacing prostheses (Smith & Nephew, Warwick, United Kingdom). Nine of those patients had a unilateral resurfacing and 1 patient had bilateral-resurfacing prostheses. The mean maternal age was 31 years, and mean duration between hip resurfacing and delivery was 53 months. Ten normal controls were also tested with a mean maternal age of 30.9 years. The authors found that the mean cord blood level of cobalt in the study patients was 0.83 ng/mL, significantly higher (P < .01) than cobalt levels in the control group, which measured 0.33 ng/mL. The mean cord blood levels of chromium in the study and control groups were 0.37 ng/mL and 0.19 ng/mL, respectively. No children were reported to have evidence of congenital anomalies. Similar to our findings, they noted a modulatory effect on the transfer of metal ions across the placenta in patients with MOM prostheses.24 They reported the relative levels of cobalt and chromium in offspring to be 60.4% and 29.4% of the maternal ion levels, respectively. Control-group infants had mean cobalt and chromium levels that were 98.5% and 97.2% of mean maternal levels, respectively.
The transfer of metal ions across the placenta in control subjects is an expected finding because cobalt and chromium are essential trace elements required by the developing fetus. Rudge and colleagues25 estimated a transplacental transfer rate of 45% for cobalt in a series of 62 paired samples of maternal and cord blood. DeSouza and coauthors26 reported a series of 3 patients with MOM hip resurfacings who had the prosthesis in situ during pregnancy and found no teratogenic effects of metal ion transfer across the placenta. Umbilical cord blood chromium levels were less than 25% of the maternal serum levels and cord blood cobalt levels were approximately 50% that of maternal blood.26 In an animal experiment, Wallach and Verch18 also reported that maternal chromium levels can be decreased because of placental uptake.
Ziaee and colleagues24 tested metal ion levels using whole blood in contrast to serum, as we did in the present study. Daniel and coauthors16, who reported on the validity of serum levels as a surrogate measure of systemic exposure to metal ions in hip replacement, suggested that serum and whole blood metal ion levels cannot be interconverted because metal within cells are not in dynamic equilibrium with extracellular levels. They concluded that serum metal ion concentrations are not a useful surrogate measure of systemic metal ion exposure based on the wide variability seen in normalized and Bland-Altman scatterplots.16 However, it is important to note that Bland-Altman plots are user-dependent in determining significance, and results can vary based on the parameters tested. A high correlation does not automatically imply that there is a good agreement between 2 methods because a widespread sample could influence results. Whole blood analysis requires more processing steps, thus providing an increased chance of contamination and variability compared with serum metal level analysis. In our experiences, serum metal ion analysis has been shown to have accurate and reproducible results in clinical situations.2,4
While there is insufficient literature that specifically studies the effects of elevated metal ion levels on maternal and fetal subjects, there have been no reported negative effects in human babies even when maternal ion levels are elevated enough to be associated with ALTR. A case report by Fritzsche and colleagues27 reported a mother with bilateral MOM THA, a recurrent pseudotumor, and high blood levels of chromium (39 ng/mL) and cobalt (138 ng/mL) at 12 weeks gestation. The child was born at 38 weeks gestation with cord blood chromium and cobalt levels of 2.1 ng/mL and 75 ng/mL, respectively. The infant’s metal ion levels remained elevated at age 8 weeks with a chromium level of 2.5 ng/mL and cobalt level of 13 ng/mL and no signs of toxicity by age 14 weeks. In an animal model, Saxena and colleagues28 found that chromium in the hexavalent form passed through the placenta in mice and rats that were fed high doses of potassium dichromate. Trivalent chromium was not found to cross the placenta. In a follow-up study, Junaid and coauthors29 investigated the effects of elevated chromium levels in female mice given potassium dichromate in drinking water on days 14 to 19 of pregnancy. Animals receiving high-dose chromium had significantly higher incidences of postimplantation loss along with subdermal hemorrhagic patches and reduced ossification.29 Cobalt has not been shown to be teratogenic or cause fetotoxicity in a rat animal model given daily doses of as much as 100 mg/kg cobalt (II) chloride on days 6 to 15 of gestation.30
It is important to recognize that rodent data are limited and may not provide accurate translational insight into the effects of metal ions in human maternal and fetal subjects. Mammalian species have significant heterogeneity in the structure and function of their placentas. Rurak31 has shown that rodents have an additional persisting yolk sac placenta that allows the transfer of maternal immunoglobulins to the fetus. Humans, on the other hand, have a yolk sac placenta that regresses early in pregnancy. Differing placental biologic function makes it difficult to extrapolate the effects of metal ions in rodents to human subjects.
It is also important to note that serum levels of cobalt, chromium, and titanium can remain persistently elevated in well-functioning metal-on-polyethylene THA for several years and that elevated metal ion levels are not confined to MOM bearings.2 Levine and colleagues4 reported that serum levels of cobalt, chromium, and titanium remain persistently elevated after 10 years in a cohort of 27 well-functioning primary metal-on-polyethylene THA (hybrid, cobalt-chrome, titanium). Cobalt concentrations were elevated in all implants compared with controls at all follow-up periods through 10 years with absolute values less than 1 ng/mL. The authors noted that metal release at the modular femoral head-neck junctions was likely the dominant source of serum cobalt and chromium rather than passive dissolution. Hsu and colleagues32 have also shown that patients undergoing a second metal-on-polyethylene THA after primary THA have elevated serum metal ion levels (cobalt, chromium, titanium) up to 6 years after second surgery. Reported cobalt concentrations in patients with unilateral THA reached a maximum of 0.5 ng/mL during the follow-up course compared with 1.5 ng/mL for patients with bilateral THA. It is unknown what the potential metal ion transfer load would be in mothers with metal-on-polyethylene THA and associated taper corrosion to their infants.
Conclusion
Mothers with MOM-bearing implants and their children have higher cobalt and chromium levels than control subjects, demonstrating that the placenta is not a complete barrier to metal ion transport, although it seems to have a modulating effect. Physicians and women of child-bearing age should be aware of these findings when considering the use of MOM-bearing couples for THA. The effects of metal ions on long-term maternal and fetal health require research through serial clinical exams and metal ion level testing in prospective studies of different THA-bearing surfaces.
1. Jacobs JJ, Skipor AK, Black J, Urban R, Galante JO. Release and excretion of metal in patients who have a total hip-replacement component made of titanium-base alloy. J Bone Joint Surg Am. 1991;73(10):1475-1486.
2. Jacobs JJ, Skipor AK, Patterson LM, et al. Metal release in patients who have had a primary total hip arthroplasty. A prospective, controlled, longitudinal study. J Bone Joint Surg Am. 1998;80(10):1447-1458.
3. Jacobs JJ, Skipor AK, Urban RM, Black J, Manion LM, Galante JO. Transport of metal degradation products of titanium alloy total hip replacements to reticuloendothelial organs. An autopsy study. Trans Soc Biomater. 1994;18:318-325.
4. Levine BR, Hsu AR, Skipor AK, et al. Ten-year outcome of serum metal ion levels after primary total hip arthroplasty: a concise follow-up of a previous report. J Bone Joint Surg Am. 2013;95(6):512-518.
5. Brodner W, Grohs JG, Bancher-Todesca D, et al. Does the placenta inhibit the passage of chromium and cobalt after metal-on-metal total hip arthroplasty? J Arthroplasty. 2004;19(8 suppl 3):102-106.
6. Hsu AR, Gross CE, Levine BR. Pseudotumor from modular neck corrosion after ceramic-on-polyethylene total hip arthroplasty. Am J Orthop. 2012;41(9):422-426.
7. Tower SS. Arthroprosthetic cobaltism: neurological and cardiac manifestations in two patients with metal-on-metal arthroplasty: a case report. J Bone Joint Surg Am. 2010;92(17):2847-2851.
8. Urban RM, Jacobs JJ, Tomlinson MJ, Gavrilovic J, Black J, Peoc’h M. Dissemination of wear particles to the liver, spleen, and abdominal lymph nodes of patients with hip or knee replacement. J Bone Joint Surg Am. 2000;82(4):457-476.
9. Watters TS, Eward WC, Hallows RK, Dodd LG, Wellman SS, Bolognesi MP. Pseudotumor with superimposed periprosthetic infection following metal-on-metal total hip arthroplasty: a case report. J Bone Joint Surg Am. 2010;92(7):1666-1669.
10. Willert HG, Buchhorn GH, Fayyazi A, et al. Metal-on-metal bearings and hypersensitivity in patients with artificial hip joints. A clinical and histomorphological study. J Bone Joint Surg Am. 2005;87(1):28-36.
11. Nunley RM, Della Valle CJ, Barrack RL. Is patient selection important for hip resurfacing? Clin Orthop. 2009;467(1):56-65.
12. Treacy RB, McBryde CW, Pynsent PB. Birmingham hip resurfacing arthroplasty. A minimum follow-up of five years. J Bone Joint Surg Br. 2005;87(2):167-170.
13. Dorr LD, Wan Z, Longjohn DB, Dubois B, Murken R. Total hip arthroplasty with use of the Metasul metal-on-metal articulation. Four to seven-year results. J Bone Joint Surg Am. 2000;82(6):789-798.
14. Pandit H, Glyn-Jones S, McLardy-Smith P, et al. Pseudotumours associated with metal-on-metal hip resurfacings. J Bone Joint Surg Br. 2008;90(7):847-851.
15. Hartmann A, Hannemann F, Lutzner J, et al. Metal ion concentrations in body fluids after implantation of hip replacements with metal-on-metal bearing--systematic review of clinical and epidemiological studies. PLoS One. 2013;8(8):e70359.
16. Daniel J, Ziaee H, Pynsent PB, McMinn DJ. The validity of serum levels as a surrogate measure of systemic exposure to metal ions in hip replacement. J Bone Joint Surg Br. 2007;89(6):736-741.
17. Merritt K, Brown SA. Release of hexavalent chromium from corrosion of stainless steel and cobalt-chromium alloys. J Biomed Mater Res. 1995;29(5):627-633.
18. Wallach S, Verch RL. Placental transport of chromium. J Am Coll Nutr. 1984;3(1):69-74.
19. Jacobs JJ, Gilbert JL, Urban RM. Corrosion of metal orthopaedic implants. J Bone Joint Surg Am. 1998;80(2):268-282.
20. Van Der Straeten C, Grammatopoulos G, Gill HS, Calistri A, Campbell P, De Smet KA. The 2012 Otto Aufranc Award: The interpretation of metal ion levels in unilateral and bilateral hip resurfacing. Clin Orthop. 2013;471(2):377-385.
21. Sierra RJ, Trousdale RT, Cabanela ME. Pregnancy and childbirth after total hip arthroplasty. J Bone Joint Surg Br. 2005;87(1):21-24.
22. Antoniou J, Zukor DJ, Mwale F, Minarik W, Petit A, Huk OL. Metal ion levels in the blood of patients after hip resurfacing: a comparison between twenty-eight and thirty-six-millimeter-head metal-on-metal prostheses. J Bone Joint Surg Am. 2008;90(Suppl 3):142-148.
23. Iavicoli I, Falcone G, Alessandrelli M, et al. The release of metals from metal-on-metal surface arthroplasty of the hip. J Trace Elem Med Biol. 2006;20(1):25-31.
24. Ziaee H, Daniel J, Datta AK, Blunt S, McMinn DJ. Transplacental transfer of cobalt and chromium in patients with metal-on-metal hip arthroplasty: a controlled study. J Bone Joint Surg Br. 2007;89(3):301-305.
25. Rudge CV, Rollin HB, Nogueira CM, Thomassen Y, Rudge MC, Odland JO. The placenta as a barrier for toxic and essential elements in paired maternal and cord blood samples of South African delivering women. J Environ Monit. 2009;11(7):1322-1330.
26. deSouza RM, Wallace D, Costa ML, Krikler SJ. Transplacental passage of metal ions in women with hip resurfacing: no teratogenic effects observed. Hip Int. 2012;22(1):96-99.
27. Fritzsche J, Borisch C, Schaefer C. Case report: High chromium and cobalt levels in a pregnant patient with bilateral metal-on-metal hip arthroplasties. Clin Orthop. 2012;470(8):2325-2331.
28. Saxena DK, Murthy RC, Jain VK, Chandra SV. Fetoplacental-maternal uptake of hexavalent chromium administered orally in rats and mice. Bull Environ Contam Toxicol. 1990;45(3):430-435.
29. Junaid M, Murthy RC, Saxena DK. Chromium fetotoxicity in mice during late pregnancy. Vet Hum Toxicol. 1995;37(4):320-323.
30. Paternain JL, Domingo JL, Corbella J. Developmental toxicity of cobalt in the rat. J Toxicol Environ Health. 1988;24(2):193-200.
31. Rurak DW. Development and function of the placenta. In: Harding R, Bocking AD, eds. Fetal Growth and Development. Cambridge, UK: Cambridge University Press; 2001.
32. Hsu AR, Levine BR, Skipor AK, Hallab NJ, Paprosky WG, Jacobs JJ. Effect of a second joint arthroplasty on metal ion levels after primary total hip arthroplasty. Am J Orthop. 2013;42(10):E84-E87.
Serum metal ion levels are increased after primary total hip arthroplasty (THA) with all types of head-liner bearing surfaces.1-4 In recent years, there has been increasing concern regarding elevated metal ion levels and adverse local and systemic effects, in particular, from metal-on-metal (MOM) implants.5 There have been reports of adverse local tissue reactions (ALTR) and systemic effects associated with elevated metal ion levels from MOM THA.6-10 MOM hip resurfacings have been advocated in the literature for use in select patient populations, such as young, active individuals.11,12 Purported benefits of MOM-bearing surfaces in this patient population include reduced wear and the prevention of osteolysis.13 While the incidence of ALTR has been reported to be approximately 1% within 5 years after MOM hip resurfacing, the prevalence of ALTR at long-term follow-up is unknown.14 Adverse local tissue reactions in hip resurfacing are related in part to femoral head coverage by the acetabular cup, as well as component design and orientation. The risk for ALTR with MOM THA has been reported to correlate with the level of serum metal ion levels because of the bearing surface, along with taper corrosion and corrosion secondary to the large surface area of the femoral head.13-15 The overall clinical and prognostic value of metal ion levels in cases of MOM bearings remains controversial and without clear cut-off values.15
The long-term physiologic response to elevated serum metal ion levels from THA implants remains unknown and is of particular concern in pregnant women because of potential transplacental transfer. Several studies have shown that DNA (deoxyribonucleic acid) and chromosomal changes can occur in patients with both MOM and non-MOM hip implants.16-18 Techniques to accurately measure the levels of metal ions, including cobalt, chromium, and titanium in the serum, have improved substantially in recent years and have been used successfully in clinical applications with low detection limits.2,4,19,20 Evidence shows that pregnancy-related complications in women with well-functioning cemented, hybrid, or uncemented metal-on-polyethylene THA are not different from those in healthy women.21 However, it is unclear if metal ions can cross the placenta and negatively affect the development of a fetus in mothers with MOM-bearing THAs.22 We conducted this study to determine if metal ions can traverse the placenta by measuring serum metal ion concentrations in mothers with and without MOM THA and the corresponding levels in umbilical cord blood samples.
Materials and Methods
Ten patients were prospectively enrolled in this study, which was approved by the institutional review boards at all 3 participating hospitals prior to initiation. All patients provided informed consent and agreed to maternal and umbilical cord blood sampling. Seven of the 10 patients did not have any metallic implants and served as controls. The remaining 3 patients had either a MOM unilateral primary THA (1 patient) or unilateral MOM hip-resurfacing arthroplasty (2 patients) with no other metal implants in the body. For all subjects, maternal and umbilical cord blood was obtained at the time of delivery.
Group Demographics
The 3 women in the implant group had a mean age of 32.3 years (range, 23-39 years) at time of delivery. The first patient had posttraumatic osteoarthritis and underwent right THA using a modular MOM 60-mm acetabular component, a 36-mm cobalt chrome head, and a cementless, titanium proximally porous-coated femoral component (Pinnacle Total Hip System and Summit Total Hip System; DePuy Synthes, Warsaw, Indiana). Her infant was born 2 years after index THA. The second patient had a diagnosis of degenerative osteoarthritis secondary to developmental dysplasia of the hip and underwent a hybrid MOM hip resurfacing with a monoblock 50-mm cup and 40-mm cobalt chrome head (Conserve Plus; Wright Medical Technology, Arlington, Tennessee). She gave birth to her infant 6 years after her hip resurfacing. The third patient also had a diagnosis of degenerative osteoarthritis secondary to developmental dysplasia of the hip and underwent hip resurfacing with a 54-mm monoblock cup and 44-mm cobalt chrome head (Conserve Plus). Her infant was born 4 years after her resurfacing arthroplasty. All of the infants were born healthy, and the deliveries were uneventful and without complications. Seven women with a mean age of 32.1 years (range, 24-37 years) and their infants served as controls at the time of delivery. None of the women in the control group had a history of renal impairment, inherited genetic disorders, or metal implants.
Serum Metal Ion Analysis
Blood samples were collected using S-Monovette polypropylene syringes (Sarstedt, Princeton, New Jersey), a multi-adapter, and infusion set following an established technique.1 All vessels and utensils used for specimen collection were verified to be free of metal contamination. Three 10-mL syringes were drawn, and each syringe was labeled to indicate the sequence of collection. The first 10 mL were drawn to rinse the needle and adapter. Blood was then allowed to clot naturally and centrifuged at 1850 rpm for 30 minutes, separating samples into cell and serum fractions that were stored in labeled vials at -80ºC. All specimen manipulations after collection were carried out in a class-100 environment using a SterilGARD Biological Safety Cabinet (Baker, Sanford, Maine) and class-100 gloves (Oak Technical, Ravenna, Ohio) to minimize atmospheric and manual contamination. Maternal and umbilical cord blood metal ion levels were tested using high-resolution sector-field inductively-coupled plasma-mass spectrometry (HR-SF-ICPMS) (Element 2; Thermo Fisher Scientific, Bremen, Germany) following the method of additions as described previously.23 The HR-SF-ICPMS machine was equipped with an SC-E2 autosampler, Teflon nebulizer and spray chamber, sapphire injector (Elemental Scientific, Omaha, Nebraska) and platinum cones. All calibration and internal standard solutions were prepared by gradual dilutions of single-element standard solutions (1000 μg/mL from High Purity Standards, Charleston, South Carolina). The certified reference material, Seronorm Trace Elements Serum (SERO, Billingstad, Norway), were routinely analyzed with samples. The serum concentrations of cobalt, chromium, titanium, and nickel were measured with detection limits in ng/mL (parts per billion) of 0.04 for cobalt, 0.015 for chromium, 0.2 for titanium, and 0.17 for nickel.4 Concentrations below the detection limit were approximated as one-half of the detection limit by convention to calculate means.
Statistical Analysis
The data reported are the means for each group for each of the metal ion levels analyzed. Intergroup comparisons were made with the Mann-Whitney-Wilcoxon test using SPSS statistics software (SPSS Science Inc, Chicago, Illinois) to compare implant and control groups in regards to serum metal ion levels. Intragroup comparisons were made using the Friedman test with significance set at P < .05. Spearman rank-order correlation tests were used to investigate relationships between maternal and infant serum metal ion levels.
Results
The Table shows the mean serum metal ion levels of chromium, cobalt, titanium, and nickel for both groups. The implant-group mothers had significantly higher chromium and cobalt levels than the control-group mothers, with mean chromium levels of 1.87 ng/mL vs 0.16 ng/mL (P = .01) and mean cobalt levels of 0.97 ng/mL vs 0.20 ng/mL (P = .01), respectively. All control-group maternal chromium and cobalt levels were lower than the implant group. There were no significant differences detected between the implant-group and control-group mothers with respect to serum titanium or nickel levels.
The implant-group cord blood samples also had significantly higher chromium and cobalt levels than did the control-group infants with mean chromium levels of 0.29 ng/mL vs 0.10 ng/mL (P = .03) and mean cobalt levels of 0.49 ng/mL vs 0.16 ng/mL (P = .01), respectively. All but 1 of the control-group infants had chromium levels that were lower than the implant-group infant chromium levels. All of the control-group infant cobalt levels were lower than the implant-group infant cobalt levels. In the mother (I-1) who had a titanium-containing implant, her titanium level was 1.77 ng/mL and her cord blood level was 0.78 ng/mL. In contrast, the other 2 patients did not have titanium-containing implants and had corresponding metal levels of 0.10 ng/mL and cord blood levels either below the detection limit or just slightly above it. No statistically significant differences were found between the implant- and control-group infants with respect to serum titanium or nickel levels.
Considering the implant and control groups separately, we found no statistically significant differences between the maternal and infant titanium levels or the maternal and infant nickel levels. In the implant group, the mother’s chromium level was always higher than her infant’s chromium level, and the mother’s cobalt level was always higher than her infant’s cobalt level (P= .08). In the control group, there was no correlation between the maternal and infant chromium levels, or between the maternal and infant cobalt levels. In the implant group, the maternal and infant chromium levels were highly correlated (r = 1), as were the maternal and infant cobalt levels (r = 1).
When infants’ chromium levels were expressed as a percentage of their mothers’ chromium levels, the mean was 15.4% (range, 12.3%-18%) for the implant group and 58.9% (range, 13.5%-165.7%) for the control group (P = .05). The mean infant cobalt level, expressed as percentage of maternal cobalt level, was 50% (range, 45.8%-53.1%) for the implant group and 76.6% (range, 59.1%-150.8%) for the control group (P = .01).
Discussion
Cobalt and chromium serum metal ion levels obtained from mothers with MOM implants and umbilical cord samples were significantly elevated in comparison with controls. There was also a strong correlation between implant-group maternal cobalt and chromium levels and implant-group infant cobalt and chromium levels; however, no such correlation existed in the control group. Our results suggest that cobalt and chromium cross the placental barrier. Implant mothers had higher chromium and cobalt levels compared with implant babies, and these babies had approximately 15% of the level of chromium and 50% of the level of cobalt when compared to implant maternal levels. This finding suggests that the placenta modulates the transfer of chromium and cobalt to the fetus.
Two studies have reported chromium and cobalt levels in maternal and umbilical cord sera after MOM THA.5,24 Brodner and colleagues5 determined the maternal serum levels of cobalt and chromium in 3 women 3.8 years after MOM THA and compared those to cobalt and chromium levels obtained from umbilical cord blood. At the time of delivery, the maternal chromium concentrations in the 3 patients were 1.6 ng/mL, 0.5 ng/mL, and 0.9 ng/mL, and the cobalt concentrations were 1 ng/mL in 1 patient and below the detection limit in the other 2 patients. Cobalt and chromium concentrations of the 3 umbilical cord sera were below the detection limit. The authors concluded that cobalt and chromium did not cross the placenta based on their laboratory detection limits. Metal ion levels were measured using atomic absorption spectrometry with relatively high detection limits of 0.3 ng/mL, which is not as sensitive as the HR-SF-ICPMS technique used in the present study that has detection limits of 0.04 ng/mL for cobalt and 0.015 ng/mL for chromium. The relatively high detection limits of atomic absorption spectrometry were likely responsible for the authors’ inability to detect elevated chromium and cobalt levels in umbilical cord sera.
Ziaee and colleagues24 used HR-SF-ICPMS, as we did in this study, to measure the mean concentrations of cobalt and chromium ion levels in 10 maternal and umbilical cord blood samples in women with Birmingham MOM hip-resurfacing prostheses (Smith & Nephew, Warwick, United Kingdom). Nine of those patients had a unilateral resurfacing and 1 patient had bilateral-resurfacing prostheses. The mean maternal age was 31 years, and mean duration between hip resurfacing and delivery was 53 months. Ten normal controls were also tested with a mean maternal age of 30.9 years. The authors found that the mean cord blood level of cobalt in the study patients was 0.83 ng/mL, significantly higher (P < .01) than cobalt levels in the control group, which measured 0.33 ng/mL. The mean cord blood levels of chromium in the study and control groups were 0.37 ng/mL and 0.19 ng/mL, respectively. No children were reported to have evidence of congenital anomalies. Similar to our findings, they noted a modulatory effect on the transfer of metal ions across the placenta in patients with MOM prostheses.24 They reported the relative levels of cobalt and chromium in offspring to be 60.4% and 29.4% of the maternal ion levels, respectively. Control-group infants had mean cobalt and chromium levels that were 98.5% and 97.2% of mean maternal levels, respectively.
The transfer of metal ions across the placenta in control subjects is an expected finding because cobalt and chromium are essential trace elements required by the developing fetus. Rudge and colleagues25 estimated a transplacental transfer rate of 45% for cobalt in a series of 62 paired samples of maternal and cord blood. DeSouza and coauthors26 reported a series of 3 patients with MOM hip resurfacings who had the prosthesis in situ during pregnancy and found no teratogenic effects of metal ion transfer across the placenta. Umbilical cord blood chromium levels were less than 25% of the maternal serum levels and cord blood cobalt levels were approximately 50% that of maternal blood.26 In an animal experiment, Wallach and Verch18 also reported that maternal chromium levels can be decreased because of placental uptake.
Ziaee and colleagues24 tested metal ion levels using whole blood in contrast to serum, as we did in the present study. Daniel and coauthors16, who reported on the validity of serum levels as a surrogate measure of systemic exposure to metal ions in hip replacement, suggested that serum and whole blood metal ion levels cannot be interconverted because metal within cells are not in dynamic equilibrium with extracellular levels. They concluded that serum metal ion concentrations are not a useful surrogate measure of systemic metal ion exposure based on the wide variability seen in normalized and Bland-Altman scatterplots.16 However, it is important to note that Bland-Altman plots are user-dependent in determining significance, and results can vary based on the parameters tested. A high correlation does not automatically imply that there is a good agreement between 2 methods because a widespread sample could influence results. Whole blood analysis requires more processing steps, thus providing an increased chance of contamination and variability compared with serum metal level analysis. In our experiences, serum metal ion analysis has been shown to have accurate and reproducible results in clinical situations.2,4
While there is insufficient literature that specifically studies the effects of elevated metal ion levels on maternal and fetal subjects, there have been no reported negative effects in human babies even when maternal ion levels are elevated enough to be associated with ALTR. A case report by Fritzsche and colleagues27 reported a mother with bilateral MOM THA, a recurrent pseudotumor, and high blood levels of chromium (39 ng/mL) and cobalt (138 ng/mL) at 12 weeks gestation. The child was born at 38 weeks gestation with cord blood chromium and cobalt levels of 2.1 ng/mL and 75 ng/mL, respectively. The infant’s metal ion levels remained elevated at age 8 weeks with a chromium level of 2.5 ng/mL and cobalt level of 13 ng/mL and no signs of toxicity by age 14 weeks. In an animal model, Saxena and colleagues28 found that chromium in the hexavalent form passed through the placenta in mice and rats that were fed high doses of potassium dichromate. Trivalent chromium was not found to cross the placenta. In a follow-up study, Junaid and coauthors29 investigated the effects of elevated chromium levels in female mice given potassium dichromate in drinking water on days 14 to 19 of pregnancy. Animals receiving high-dose chromium had significantly higher incidences of postimplantation loss along with subdermal hemorrhagic patches and reduced ossification.29 Cobalt has not been shown to be teratogenic or cause fetotoxicity in a rat animal model given daily doses of as much as 100 mg/kg cobalt (II) chloride on days 6 to 15 of gestation.30
It is important to recognize that rodent data are limited and may not provide accurate translational insight into the effects of metal ions in human maternal and fetal subjects. Mammalian species have significant heterogeneity in the structure and function of their placentas. Rurak31 has shown that rodents have an additional persisting yolk sac placenta that allows the transfer of maternal immunoglobulins to the fetus. Humans, on the other hand, have a yolk sac placenta that regresses early in pregnancy. Differing placental biologic function makes it difficult to extrapolate the effects of metal ions in rodents to human subjects.
It is also important to note that serum levels of cobalt, chromium, and titanium can remain persistently elevated in well-functioning metal-on-polyethylene THA for several years and that elevated metal ion levels are not confined to MOM bearings.2 Levine and colleagues4 reported that serum levels of cobalt, chromium, and titanium remain persistently elevated after 10 years in a cohort of 27 well-functioning primary metal-on-polyethylene THA (hybrid, cobalt-chrome, titanium). Cobalt concentrations were elevated in all implants compared with controls at all follow-up periods through 10 years with absolute values less than 1 ng/mL. The authors noted that metal release at the modular femoral head-neck junctions was likely the dominant source of serum cobalt and chromium rather than passive dissolution. Hsu and colleagues32 have also shown that patients undergoing a second metal-on-polyethylene THA after primary THA have elevated serum metal ion levels (cobalt, chromium, titanium) up to 6 years after second surgery. Reported cobalt concentrations in patients with unilateral THA reached a maximum of 0.5 ng/mL during the follow-up course compared with 1.5 ng/mL for patients with bilateral THA. It is unknown what the potential metal ion transfer load would be in mothers with metal-on-polyethylene THA and associated taper corrosion to their infants.
Conclusion
Mothers with MOM-bearing implants and their children have higher cobalt and chromium levels than control subjects, demonstrating that the placenta is not a complete barrier to metal ion transport, although it seems to have a modulating effect. Physicians and women of child-bearing age should be aware of these findings when considering the use of MOM-bearing couples for THA. The effects of metal ions on long-term maternal and fetal health require research through serial clinical exams and metal ion level testing in prospective studies of different THA-bearing surfaces.
Serum metal ion levels are increased after primary total hip arthroplasty (THA) with all types of head-liner bearing surfaces.1-4 In recent years, there has been increasing concern regarding elevated metal ion levels and adverse local and systemic effects, in particular, from metal-on-metal (MOM) implants.5 There have been reports of adverse local tissue reactions (ALTR) and systemic effects associated with elevated metal ion levels from MOM THA.6-10 MOM hip resurfacings have been advocated in the literature for use in select patient populations, such as young, active individuals.11,12 Purported benefits of MOM-bearing surfaces in this patient population include reduced wear and the prevention of osteolysis.13 While the incidence of ALTR has been reported to be approximately 1% within 5 years after MOM hip resurfacing, the prevalence of ALTR at long-term follow-up is unknown.14 Adverse local tissue reactions in hip resurfacing are related in part to femoral head coverage by the acetabular cup, as well as component design and orientation. The risk for ALTR with MOM THA has been reported to correlate with the level of serum metal ion levels because of the bearing surface, along with taper corrosion and corrosion secondary to the large surface area of the femoral head.13-15 The overall clinical and prognostic value of metal ion levels in cases of MOM bearings remains controversial and without clear cut-off values.15
The long-term physiologic response to elevated serum metal ion levels from THA implants remains unknown and is of particular concern in pregnant women because of potential transplacental transfer. Several studies have shown that DNA (deoxyribonucleic acid) and chromosomal changes can occur in patients with both MOM and non-MOM hip implants.16-18 Techniques to accurately measure the levels of metal ions, including cobalt, chromium, and titanium in the serum, have improved substantially in recent years and have been used successfully in clinical applications with low detection limits.2,4,19,20 Evidence shows that pregnancy-related complications in women with well-functioning cemented, hybrid, or uncemented metal-on-polyethylene THA are not different from those in healthy women.21 However, it is unclear if metal ions can cross the placenta and negatively affect the development of a fetus in mothers with MOM-bearing THAs.22 We conducted this study to determine if metal ions can traverse the placenta by measuring serum metal ion concentrations in mothers with and without MOM THA and the corresponding levels in umbilical cord blood samples.
Materials and Methods
Ten patients were prospectively enrolled in this study, which was approved by the institutional review boards at all 3 participating hospitals prior to initiation. All patients provided informed consent and agreed to maternal and umbilical cord blood sampling. Seven of the 10 patients did not have any metallic implants and served as controls. The remaining 3 patients had either a MOM unilateral primary THA (1 patient) or unilateral MOM hip-resurfacing arthroplasty (2 patients) with no other metal implants in the body. For all subjects, maternal and umbilical cord blood was obtained at the time of delivery.
Group Demographics
The 3 women in the implant group had a mean age of 32.3 years (range, 23-39 years) at time of delivery. The first patient had posttraumatic osteoarthritis and underwent right THA using a modular MOM 60-mm acetabular component, a 36-mm cobalt chrome head, and a cementless, titanium proximally porous-coated femoral component (Pinnacle Total Hip System and Summit Total Hip System; DePuy Synthes, Warsaw, Indiana). Her infant was born 2 years after index THA. The second patient had a diagnosis of degenerative osteoarthritis secondary to developmental dysplasia of the hip and underwent a hybrid MOM hip resurfacing with a monoblock 50-mm cup and 40-mm cobalt chrome head (Conserve Plus; Wright Medical Technology, Arlington, Tennessee). She gave birth to her infant 6 years after her hip resurfacing. The third patient also had a diagnosis of degenerative osteoarthritis secondary to developmental dysplasia of the hip and underwent hip resurfacing with a 54-mm monoblock cup and 44-mm cobalt chrome head (Conserve Plus). Her infant was born 4 years after her resurfacing arthroplasty. All of the infants were born healthy, and the deliveries were uneventful and without complications. Seven women with a mean age of 32.1 years (range, 24-37 years) and their infants served as controls at the time of delivery. None of the women in the control group had a history of renal impairment, inherited genetic disorders, or metal implants.
Serum Metal Ion Analysis
Blood samples were collected using S-Monovette polypropylene syringes (Sarstedt, Princeton, New Jersey), a multi-adapter, and infusion set following an established technique.1 All vessels and utensils used for specimen collection were verified to be free of metal contamination. Three 10-mL syringes were drawn, and each syringe was labeled to indicate the sequence of collection. The first 10 mL were drawn to rinse the needle and adapter. Blood was then allowed to clot naturally and centrifuged at 1850 rpm for 30 minutes, separating samples into cell and serum fractions that were stored in labeled vials at -80ºC. All specimen manipulations after collection were carried out in a class-100 environment using a SterilGARD Biological Safety Cabinet (Baker, Sanford, Maine) and class-100 gloves (Oak Technical, Ravenna, Ohio) to minimize atmospheric and manual contamination. Maternal and umbilical cord blood metal ion levels were tested using high-resolution sector-field inductively-coupled plasma-mass spectrometry (HR-SF-ICPMS) (Element 2; Thermo Fisher Scientific, Bremen, Germany) following the method of additions as described previously.23 The HR-SF-ICPMS machine was equipped with an SC-E2 autosampler, Teflon nebulizer and spray chamber, sapphire injector (Elemental Scientific, Omaha, Nebraska) and platinum cones. All calibration and internal standard solutions were prepared by gradual dilutions of single-element standard solutions (1000 μg/mL from High Purity Standards, Charleston, South Carolina). The certified reference material, Seronorm Trace Elements Serum (SERO, Billingstad, Norway), were routinely analyzed with samples. The serum concentrations of cobalt, chromium, titanium, and nickel were measured with detection limits in ng/mL (parts per billion) of 0.04 for cobalt, 0.015 for chromium, 0.2 for titanium, and 0.17 for nickel.4 Concentrations below the detection limit were approximated as one-half of the detection limit by convention to calculate means.
Statistical Analysis
The data reported are the means for each group for each of the metal ion levels analyzed. Intergroup comparisons were made with the Mann-Whitney-Wilcoxon test using SPSS statistics software (SPSS Science Inc, Chicago, Illinois) to compare implant and control groups in regards to serum metal ion levels. Intragroup comparisons were made using the Friedman test with significance set at P < .05. Spearman rank-order correlation tests were used to investigate relationships between maternal and infant serum metal ion levels.
Results
The Table shows the mean serum metal ion levels of chromium, cobalt, titanium, and nickel for both groups. The implant-group mothers had significantly higher chromium and cobalt levels than the control-group mothers, with mean chromium levels of 1.87 ng/mL vs 0.16 ng/mL (P = .01) and mean cobalt levels of 0.97 ng/mL vs 0.20 ng/mL (P = .01), respectively. All control-group maternal chromium and cobalt levels were lower than the implant group. There were no significant differences detected between the implant-group and control-group mothers with respect to serum titanium or nickel levels.
The implant-group cord blood samples also had significantly higher chromium and cobalt levels than did the control-group infants with mean chromium levels of 0.29 ng/mL vs 0.10 ng/mL (P = .03) and mean cobalt levels of 0.49 ng/mL vs 0.16 ng/mL (P = .01), respectively. All but 1 of the control-group infants had chromium levels that were lower than the implant-group infant chromium levels. All of the control-group infant cobalt levels were lower than the implant-group infant cobalt levels. In the mother (I-1) who had a titanium-containing implant, her titanium level was 1.77 ng/mL and her cord blood level was 0.78 ng/mL. In contrast, the other 2 patients did not have titanium-containing implants and had corresponding metal levels of 0.10 ng/mL and cord blood levels either below the detection limit or just slightly above it. No statistically significant differences were found between the implant- and control-group infants with respect to serum titanium or nickel levels.
Considering the implant and control groups separately, we found no statistically significant differences between the maternal and infant titanium levels or the maternal and infant nickel levels. In the implant group, the mother’s chromium level was always higher than her infant’s chromium level, and the mother’s cobalt level was always higher than her infant’s cobalt level (P= .08). In the control group, there was no correlation between the maternal and infant chromium levels, or between the maternal and infant cobalt levels. In the implant group, the maternal and infant chromium levels were highly correlated (r = 1), as were the maternal and infant cobalt levels (r = 1).
When infants’ chromium levels were expressed as a percentage of their mothers’ chromium levels, the mean was 15.4% (range, 12.3%-18%) for the implant group and 58.9% (range, 13.5%-165.7%) for the control group (P = .05). The mean infant cobalt level, expressed as percentage of maternal cobalt level, was 50% (range, 45.8%-53.1%) for the implant group and 76.6% (range, 59.1%-150.8%) for the control group (P = .01).
Discussion
Cobalt and chromium serum metal ion levels obtained from mothers with MOM implants and umbilical cord samples were significantly elevated in comparison with controls. There was also a strong correlation between implant-group maternal cobalt and chromium levels and implant-group infant cobalt and chromium levels; however, no such correlation existed in the control group. Our results suggest that cobalt and chromium cross the placental barrier. Implant mothers had higher chromium and cobalt levels compared with implant babies, and these babies had approximately 15% of the level of chromium and 50% of the level of cobalt when compared to implant maternal levels. This finding suggests that the placenta modulates the transfer of chromium and cobalt to the fetus.
Two studies have reported chromium and cobalt levels in maternal and umbilical cord sera after MOM THA.5,24 Brodner and colleagues5 determined the maternal serum levels of cobalt and chromium in 3 women 3.8 years after MOM THA and compared those to cobalt and chromium levels obtained from umbilical cord blood. At the time of delivery, the maternal chromium concentrations in the 3 patients were 1.6 ng/mL, 0.5 ng/mL, and 0.9 ng/mL, and the cobalt concentrations were 1 ng/mL in 1 patient and below the detection limit in the other 2 patients. Cobalt and chromium concentrations of the 3 umbilical cord sera were below the detection limit. The authors concluded that cobalt and chromium did not cross the placenta based on their laboratory detection limits. Metal ion levels were measured using atomic absorption spectrometry with relatively high detection limits of 0.3 ng/mL, which is not as sensitive as the HR-SF-ICPMS technique used in the present study that has detection limits of 0.04 ng/mL for cobalt and 0.015 ng/mL for chromium. The relatively high detection limits of atomic absorption spectrometry were likely responsible for the authors’ inability to detect elevated chromium and cobalt levels in umbilical cord sera.
Ziaee and colleagues24 used HR-SF-ICPMS, as we did in this study, to measure the mean concentrations of cobalt and chromium ion levels in 10 maternal and umbilical cord blood samples in women with Birmingham MOM hip-resurfacing prostheses (Smith & Nephew, Warwick, United Kingdom). Nine of those patients had a unilateral resurfacing and 1 patient had bilateral-resurfacing prostheses. The mean maternal age was 31 years, and mean duration between hip resurfacing and delivery was 53 months. Ten normal controls were also tested with a mean maternal age of 30.9 years. The authors found that the mean cord blood level of cobalt in the study patients was 0.83 ng/mL, significantly higher (P < .01) than cobalt levels in the control group, which measured 0.33 ng/mL. The mean cord blood levels of chromium in the study and control groups were 0.37 ng/mL and 0.19 ng/mL, respectively. No children were reported to have evidence of congenital anomalies. Similar to our findings, they noted a modulatory effect on the transfer of metal ions across the placenta in patients with MOM prostheses.24 They reported the relative levels of cobalt and chromium in offspring to be 60.4% and 29.4% of the maternal ion levels, respectively. Control-group infants had mean cobalt and chromium levels that were 98.5% and 97.2% of mean maternal levels, respectively.
The transfer of metal ions across the placenta in control subjects is an expected finding because cobalt and chromium are essential trace elements required by the developing fetus. Rudge and colleagues25 estimated a transplacental transfer rate of 45% for cobalt in a series of 62 paired samples of maternal and cord blood. DeSouza and coauthors26 reported a series of 3 patients with MOM hip resurfacings who had the prosthesis in situ during pregnancy and found no teratogenic effects of metal ion transfer across the placenta. Umbilical cord blood chromium levels were less than 25% of the maternal serum levels and cord blood cobalt levels were approximately 50% that of maternal blood.26 In an animal experiment, Wallach and Verch18 also reported that maternal chromium levels can be decreased because of placental uptake.
Ziaee and colleagues24 tested metal ion levels using whole blood in contrast to serum, as we did in the present study. Daniel and coauthors16, who reported on the validity of serum levels as a surrogate measure of systemic exposure to metal ions in hip replacement, suggested that serum and whole blood metal ion levels cannot be interconverted because metal within cells are not in dynamic equilibrium with extracellular levels. They concluded that serum metal ion concentrations are not a useful surrogate measure of systemic metal ion exposure based on the wide variability seen in normalized and Bland-Altman scatterplots.16 However, it is important to note that Bland-Altman plots are user-dependent in determining significance, and results can vary based on the parameters tested. A high correlation does not automatically imply that there is a good agreement between 2 methods because a widespread sample could influence results. Whole blood analysis requires more processing steps, thus providing an increased chance of contamination and variability compared with serum metal level analysis. In our experiences, serum metal ion analysis has been shown to have accurate and reproducible results in clinical situations.2,4
While there is insufficient literature that specifically studies the effects of elevated metal ion levels on maternal and fetal subjects, there have been no reported negative effects in human babies even when maternal ion levels are elevated enough to be associated with ALTR. A case report by Fritzsche and colleagues27 reported a mother with bilateral MOM THA, a recurrent pseudotumor, and high blood levels of chromium (39 ng/mL) and cobalt (138 ng/mL) at 12 weeks gestation. The child was born at 38 weeks gestation with cord blood chromium and cobalt levels of 2.1 ng/mL and 75 ng/mL, respectively. The infant’s metal ion levels remained elevated at age 8 weeks with a chromium level of 2.5 ng/mL and cobalt level of 13 ng/mL and no signs of toxicity by age 14 weeks. In an animal model, Saxena and colleagues28 found that chromium in the hexavalent form passed through the placenta in mice and rats that were fed high doses of potassium dichromate. Trivalent chromium was not found to cross the placenta. In a follow-up study, Junaid and coauthors29 investigated the effects of elevated chromium levels in female mice given potassium dichromate in drinking water on days 14 to 19 of pregnancy. Animals receiving high-dose chromium had significantly higher incidences of postimplantation loss along with subdermal hemorrhagic patches and reduced ossification.29 Cobalt has not been shown to be teratogenic or cause fetotoxicity in a rat animal model given daily doses of as much as 100 mg/kg cobalt (II) chloride on days 6 to 15 of gestation.30
It is important to recognize that rodent data are limited and may not provide accurate translational insight into the effects of metal ions in human maternal and fetal subjects. Mammalian species have significant heterogeneity in the structure and function of their placentas. Rurak31 has shown that rodents have an additional persisting yolk sac placenta that allows the transfer of maternal immunoglobulins to the fetus. Humans, on the other hand, have a yolk sac placenta that regresses early in pregnancy. Differing placental biologic function makes it difficult to extrapolate the effects of metal ions in rodents to human subjects.
It is also important to note that serum levels of cobalt, chromium, and titanium can remain persistently elevated in well-functioning metal-on-polyethylene THA for several years and that elevated metal ion levels are not confined to MOM bearings.2 Levine and colleagues4 reported that serum levels of cobalt, chromium, and titanium remain persistently elevated after 10 years in a cohort of 27 well-functioning primary metal-on-polyethylene THA (hybrid, cobalt-chrome, titanium). Cobalt concentrations were elevated in all implants compared with controls at all follow-up periods through 10 years with absolute values less than 1 ng/mL. The authors noted that metal release at the modular femoral head-neck junctions was likely the dominant source of serum cobalt and chromium rather than passive dissolution. Hsu and colleagues32 have also shown that patients undergoing a second metal-on-polyethylene THA after primary THA have elevated serum metal ion levels (cobalt, chromium, titanium) up to 6 years after second surgery. Reported cobalt concentrations in patients with unilateral THA reached a maximum of 0.5 ng/mL during the follow-up course compared with 1.5 ng/mL for patients with bilateral THA. It is unknown what the potential metal ion transfer load would be in mothers with metal-on-polyethylene THA and associated taper corrosion to their infants.
Conclusion
Mothers with MOM-bearing implants and their children have higher cobalt and chromium levels than control subjects, demonstrating that the placenta is not a complete barrier to metal ion transport, although it seems to have a modulating effect. Physicians and women of child-bearing age should be aware of these findings when considering the use of MOM-bearing couples for THA. The effects of metal ions on long-term maternal and fetal health require research through serial clinical exams and metal ion level testing in prospective studies of different THA-bearing surfaces.
1. Jacobs JJ, Skipor AK, Black J, Urban R, Galante JO. Release and excretion of metal in patients who have a total hip-replacement component made of titanium-base alloy. J Bone Joint Surg Am. 1991;73(10):1475-1486.
2. Jacobs JJ, Skipor AK, Patterson LM, et al. Metal release in patients who have had a primary total hip arthroplasty. A prospective, controlled, longitudinal study. J Bone Joint Surg Am. 1998;80(10):1447-1458.
3. Jacobs JJ, Skipor AK, Urban RM, Black J, Manion LM, Galante JO. Transport of metal degradation products of titanium alloy total hip replacements to reticuloendothelial organs. An autopsy study. Trans Soc Biomater. 1994;18:318-325.
4. Levine BR, Hsu AR, Skipor AK, et al. Ten-year outcome of serum metal ion levels after primary total hip arthroplasty: a concise follow-up of a previous report. J Bone Joint Surg Am. 2013;95(6):512-518.
5. Brodner W, Grohs JG, Bancher-Todesca D, et al. Does the placenta inhibit the passage of chromium and cobalt after metal-on-metal total hip arthroplasty? J Arthroplasty. 2004;19(8 suppl 3):102-106.
6. Hsu AR, Gross CE, Levine BR. Pseudotumor from modular neck corrosion after ceramic-on-polyethylene total hip arthroplasty. Am J Orthop. 2012;41(9):422-426.
7. Tower SS. Arthroprosthetic cobaltism: neurological and cardiac manifestations in two patients with metal-on-metal arthroplasty: a case report. J Bone Joint Surg Am. 2010;92(17):2847-2851.
8. Urban RM, Jacobs JJ, Tomlinson MJ, Gavrilovic J, Black J, Peoc’h M. Dissemination of wear particles to the liver, spleen, and abdominal lymph nodes of patients with hip or knee replacement. J Bone Joint Surg Am. 2000;82(4):457-476.
9. Watters TS, Eward WC, Hallows RK, Dodd LG, Wellman SS, Bolognesi MP. Pseudotumor with superimposed periprosthetic infection following metal-on-metal total hip arthroplasty: a case report. J Bone Joint Surg Am. 2010;92(7):1666-1669.
10. Willert HG, Buchhorn GH, Fayyazi A, et al. Metal-on-metal bearings and hypersensitivity in patients with artificial hip joints. A clinical and histomorphological study. J Bone Joint Surg Am. 2005;87(1):28-36.
11. Nunley RM, Della Valle CJ, Barrack RL. Is patient selection important for hip resurfacing? Clin Orthop. 2009;467(1):56-65.
12. Treacy RB, McBryde CW, Pynsent PB. Birmingham hip resurfacing arthroplasty. A minimum follow-up of five years. J Bone Joint Surg Br. 2005;87(2):167-170.
13. Dorr LD, Wan Z, Longjohn DB, Dubois B, Murken R. Total hip arthroplasty with use of the Metasul metal-on-metal articulation. Four to seven-year results. J Bone Joint Surg Am. 2000;82(6):789-798.
14. Pandit H, Glyn-Jones S, McLardy-Smith P, et al. Pseudotumours associated with metal-on-metal hip resurfacings. J Bone Joint Surg Br. 2008;90(7):847-851.
15. Hartmann A, Hannemann F, Lutzner J, et al. Metal ion concentrations in body fluids after implantation of hip replacements with metal-on-metal bearing--systematic review of clinical and epidemiological studies. PLoS One. 2013;8(8):e70359.
16. Daniel J, Ziaee H, Pynsent PB, McMinn DJ. The validity of serum levels as a surrogate measure of systemic exposure to metal ions in hip replacement. J Bone Joint Surg Br. 2007;89(6):736-741.
17. Merritt K, Brown SA. Release of hexavalent chromium from corrosion of stainless steel and cobalt-chromium alloys. J Biomed Mater Res. 1995;29(5):627-633.
18. Wallach S, Verch RL. Placental transport of chromium. J Am Coll Nutr. 1984;3(1):69-74.
19. Jacobs JJ, Gilbert JL, Urban RM. Corrosion of metal orthopaedic implants. J Bone Joint Surg Am. 1998;80(2):268-282.
20. Van Der Straeten C, Grammatopoulos G, Gill HS, Calistri A, Campbell P, De Smet KA. The 2012 Otto Aufranc Award: The interpretation of metal ion levels in unilateral and bilateral hip resurfacing. Clin Orthop. 2013;471(2):377-385.
21. Sierra RJ, Trousdale RT, Cabanela ME. Pregnancy and childbirth after total hip arthroplasty. J Bone Joint Surg Br. 2005;87(1):21-24.
22. Antoniou J, Zukor DJ, Mwale F, Minarik W, Petit A, Huk OL. Metal ion levels in the blood of patients after hip resurfacing: a comparison between twenty-eight and thirty-six-millimeter-head metal-on-metal prostheses. J Bone Joint Surg Am. 2008;90(Suppl 3):142-148.
23. Iavicoli I, Falcone G, Alessandrelli M, et al. The release of metals from metal-on-metal surface arthroplasty of the hip. J Trace Elem Med Biol. 2006;20(1):25-31.
24. Ziaee H, Daniel J, Datta AK, Blunt S, McMinn DJ. Transplacental transfer of cobalt and chromium in patients with metal-on-metal hip arthroplasty: a controlled study. J Bone Joint Surg Br. 2007;89(3):301-305.
25. Rudge CV, Rollin HB, Nogueira CM, Thomassen Y, Rudge MC, Odland JO. The placenta as a barrier for toxic and essential elements in paired maternal and cord blood samples of South African delivering women. J Environ Monit. 2009;11(7):1322-1330.
26. deSouza RM, Wallace D, Costa ML, Krikler SJ. Transplacental passage of metal ions in women with hip resurfacing: no teratogenic effects observed. Hip Int. 2012;22(1):96-99.
27. Fritzsche J, Borisch C, Schaefer C. Case report: High chromium and cobalt levels in a pregnant patient with bilateral metal-on-metal hip arthroplasties. Clin Orthop. 2012;470(8):2325-2331.
28. Saxena DK, Murthy RC, Jain VK, Chandra SV. Fetoplacental-maternal uptake of hexavalent chromium administered orally in rats and mice. Bull Environ Contam Toxicol. 1990;45(3):430-435.
29. Junaid M, Murthy RC, Saxena DK. Chromium fetotoxicity in mice during late pregnancy. Vet Hum Toxicol. 1995;37(4):320-323.
30. Paternain JL, Domingo JL, Corbella J. Developmental toxicity of cobalt in the rat. J Toxicol Environ Health. 1988;24(2):193-200.
31. Rurak DW. Development and function of the placenta. In: Harding R, Bocking AD, eds. Fetal Growth and Development. Cambridge, UK: Cambridge University Press; 2001.
32. Hsu AR, Levine BR, Skipor AK, Hallab NJ, Paprosky WG, Jacobs JJ. Effect of a second joint arthroplasty on metal ion levels after primary total hip arthroplasty. Am J Orthop. 2013;42(10):E84-E87.
1. Jacobs JJ, Skipor AK, Black J, Urban R, Galante JO. Release and excretion of metal in patients who have a total hip-replacement component made of titanium-base alloy. J Bone Joint Surg Am. 1991;73(10):1475-1486.
2. Jacobs JJ, Skipor AK, Patterson LM, et al. Metal release in patients who have had a primary total hip arthroplasty. A prospective, controlled, longitudinal study. J Bone Joint Surg Am. 1998;80(10):1447-1458.
3. Jacobs JJ, Skipor AK, Urban RM, Black J, Manion LM, Galante JO. Transport of metal degradation products of titanium alloy total hip replacements to reticuloendothelial organs. An autopsy study. Trans Soc Biomater. 1994;18:318-325.
4. Levine BR, Hsu AR, Skipor AK, et al. Ten-year outcome of serum metal ion levels after primary total hip arthroplasty: a concise follow-up of a previous report. J Bone Joint Surg Am. 2013;95(6):512-518.
5. Brodner W, Grohs JG, Bancher-Todesca D, et al. Does the placenta inhibit the passage of chromium and cobalt after metal-on-metal total hip arthroplasty? J Arthroplasty. 2004;19(8 suppl 3):102-106.
6. Hsu AR, Gross CE, Levine BR. Pseudotumor from modular neck corrosion after ceramic-on-polyethylene total hip arthroplasty. Am J Orthop. 2012;41(9):422-426.
7. Tower SS. Arthroprosthetic cobaltism: neurological and cardiac manifestations in two patients with metal-on-metal arthroplasty: a case report. J Bone Joint Surg Am. 2010;92(17):2847-2851.
8. Urban RM, Jacobs JJ, Tomlinson MJ, Gavrilovic J, Black J, Peoc’h M. Dissemination of wear particles to the liver, spleen, and abdominal lymph nodes of patients with hip or knee replacement. J Bone Joint Surg Am. 2000;82(4):457-476.
9. Watters TS, Eward WC, Hallows RK, Dodd LG, Wellman SS, Bolognesi MP. Pseudotumor with superimposed periprosthetic infection following metal-on-metal total hip arthroplasty: a case report. J Bone Joint Surg Am. 2010;92(7):1666-1669.
10. Willert HG, Buchhorn GH, Fayyazi A, et al. Metal-on-metal bearings and hypersensitivity in patients with artificial hip joints. A clinical and histomorphological study. J Bone Joint Surg Am. 2005;87(1):28-36.
11. Nunley RM, Della Valle CJ, Barrack RL. Is patient selection important for hip resurfacing? Clin Orthop. 2009;467(1):56-65.
12. Treacy RB, McBryde CW, Pynsent PB. Birmingham hip resurfacing arthroplasty. A minimum follow-up of five years. J Bone Joint Surg Br. 2005;87(2):167-170.
13. Dorr LD, Wan Z, Longjohn DB, Dubois B, Murken R. Total hip arthroplasty with use of the Metasul metal-on-metal articulation. Four to seven-year results. J Bone Joint Surg Am. 2000;82(6):789-798.
14. Pandit H, Glyn-Jones S, McLardy-Smith P, et al. Pseudotumours associated with metal-on-metal hip resurfacings. J Bone Joint Surg Br. 2008;90(7):847-851.
15. Hartmann A, Hannemann F, Lutzner J, et al. Metal ion concentrations in body fluids after implantation of hip replacements with metal-on-metal bearing--systematic review of clinical and epidemiological studies. PLoS One. 2013;8(8):e70359.
16. Daniel J, Ziaee H, Pynsent PB, McMinn DJ. The validity of serum levels as a surrogate measure of systemic exposure to metal ions in hip replacement. J Bone Joint Surg Br. 2007;89(6):736-741.
17. Merritt K, Brown SA. Release of hexavalent chromium from corrosion of stainless steel and cobalt-chromium alloys. J Biomed Mater Res. 1995;29(5):627-633.
18. Wallach S, Verch RL. Placental transport of chromium. J Am Coll Nutr. 1984;3(1):69-74.
19. Jacobs JJ, Gilbert JL, Urban RM. Corrosion of metal orthopaedic implants. J Bone Joint Surg Am. 1998;80(2):268-282.
20. Van Der Straeten C, Grammatopoulos G, Gill HS, Calistri A, Campbell P, De Smet KA. The 2012 Otto Aufranc Award: The interpretation of metal ion levels in unilateral and bilateral hip resurfacing. Clin Orthop. 2013;471(2):377-385.
21. Sierra RJ, Trousdale RT, Cabanela ME. Pregnancy and childbirth after total hip arthroplasty. J Bone Joint Surg Br. 2005;87(1):21-24.
22. Antoniou J, Zukor DJ, Mwale F, Minarik W, Petit A, Huk OL. Metal ion levels in the blood of patients after hip resurfacing: a comparison between twenty-eight and thirty-six-millimeter-head metal-on-metal prostheses. J Bone Joint Surg Am. 2008;90(Suppl 3):142-148.
23. Iavicoli I, Falcone G, Alessandrelli M, et al. The release of metals from metal-on-metal surface arthroplasty of the hip. J Trace Elem Med Biol. 2006;20(1):25-31.
24. Ziaee H, Daniel J, Datta AK, Blunt S, McMinn DJ. Transplacental transfer of cobalt and chromium in patients with metal-on-metal hip arthroplasty: a controlled study. J Bone Joint Surg Br. 2007;89(3):301-305.
25. Rudge CV, Rollin HB, Nogueira CM, Thomassen Y, Rudge MC, Odland JO. The placenta as a barrier for toxic and essential elements in paired maternal and cord blood samples of South African delivering women. J Environ Monit. 2009;11(7):1322-1330.
26. deSouza RM, Wallace D, Costa ML, Krikler SJ. Transplacental passage of metal ions in women with hip resurfacing: no teratogenic effects observed. Hip Int. 2012;22(1):96-99.
27. Fritzsche J, Borisch C, Schaefer C. Case report: High chromium and cobalt levels in a pregnant patient with bilateral metal-on-metal hip arthroplasties. Clin Orthop. 2012;470(8):2325-2331.
28. Saxena DK, Murthy RC, Jain VK, Chandra SV. Fetoplacental-maternal uptake of hexavalent chromium administered orally in rats and mice. Bull Environ Contam Toxicol. 1990;45(3):430-435.
29. Junaid M, Murthy RC, Saxena DK. Chromium fetotoxicity in mice during late pregnancy. Vet Hum Toxicol. 1995;37(4):320-323.
30. Paternain JL, Domingo JL, Corbella J. Developmental toxicity of cobalt in the rat. J Toxicol Environ Health. 1988;24(2):193-200.
31. Rurak DW. Development and function of the placenta. In: Harding R, Bocking AD, eds. Fetal Growth and Development. Cambridge, UK: Cambridge University Press; 2001.
32. Hsu AR, Levine BR, Skipor AK, Hallab NJ, Paprosky WG, Jacobs JJ. Effect of a second joint arthroplasty on metal ion levels after primary total hip arthroplasty. Am J Orthop. 2013;42(10):E84-E87.
Radiofrequency Stimulation for Potential Healing of Meniscal Injuries in the Avascular Zone
Partial meniscectomy of tears in the avascular zone remains one of the most common orthopedic procedures. While results of partial meniscectomy in younger patients have excellent short- to medium-term results, the long-term clinical outcomes are often less favorable.1-3 Repair in the avascular “white-white” zone has resulted in lower patient satisfaction scores and higher revision surgery rates.4-7 Consequently, most tears in this region have been treated with partial meniscectomy.
The inability to repair rather than resect menisci with avascular tears has led to extensive research. Techniques such as trephination and rasping to initiate an angiogenic response have had inconsistent and unreliable results when applied to the white-white zone.8-13 In contrast, Tasto and colleagues14 have shown that radiofrequency (RF) applied to hypovascular tissue can not only stimulate tissue vascularity, but also increase organization of fibroblastic cells. In addition, Tasto and colleagues15,16 have shown that RF application can significantly improve histologic healing and clinical outcomes in refractory cases of Achilles tendinopathy and lateral epicondylitis. In Japanese white rabbit menisci, Higuchi and coauthors17 applied monopolar RF at 60°C and 40W to avascular zone tears to fuse the tissue. They found a significant increase in fibroblast proliferation and fusion of collagen fibers at 2, 4, and 12 weeks after surgery. They also found significant acellular zones of meniscus tissue and attributed these findings to fibrochondrocyte death because of thermal treatment.
This body of research led to the present study, which evaluates the effect of low-temperature, bipolar RF stimulation, in conjunction with suture repair, on the healing of tears in the avascular white-white zone of the meniscus both in vivo and ex vivo. We performed gross and histologic analyses of the treatment groups for the in vivo aspect of the study and biochemical analyses to study the ex vivo effects of RF treatment. 3H-thymidine incorporation has been shown to be a reliable indicator of cellular proliferation in several studies, and this was measured in our treatment groups.18-21 In addition, the response of mitogenic growth factors (IGF-1, bFGF) and angiogenic markers (VEGF, αV) to RF treatment was studied.22 We hypothesized that bipolar RF application would show increased gross, histologic, and biochemical healing when combined with suture repair of longitudinal avascular zone meniscus tears.
Materials and Methods
Creation of Meniscal Tears
Fifty-four healthy, skeletally mature male and female adult New Zealand white rabbits aged 7 to 18 months were used for the study. All procedures conformed to the guidelines of our university’s institutional animal care and use committee and the American Association for Accreditation of Laboratory Animal Care. All rabbits underwent a surgical procedure in which a longitudinal tear was created in the avascular white-white zone of the medial meniscus. Using sterile technique and instrumentation, a medial parapatellar incision was made on the left knee of each rabbit. The patella was retracted laterally, exposing the medial meniscus. The tibia was then externally rotated to sublux the anterior horn of the medial meniscus anteriorly. A longitudinal full-thickness meniscal tear (3-4 mm in length) was created in the avascular zone (inner third) of the anterior horn of the medial meniscus using an 11-blade scalpel (Figures 1A, 1B). The location of the tear was grossly performed in the same location in each meniscus. The rabbits were randomly divided into 3 treatment groups: 1, 2, and 3 (Table 1). Group 1 (n = 6) served as a control with no repair or RF treatment applied. Group 2 (n = 15) underwent suture repair only of the meniscal tear using 5-0 nylon suture in a horizontal mattress pattern (Figure 2A). Group 3 (n = 33) underwent suture repair after RF stimulation was applied to both sides of the meniscus tear (Figures 2B, 2C). RF was applied using a 0.8-mm TOPAZ MicroDebrider (ArthroCare, Sunnyvale, California) set at level 4 (175 V-RMS) for 500 milliseconds. Lactated ringer’s solution was continuously infused through the probe via sterile tubing to prevent overheating.
After meniscal treatment, hemostasis of the surrounding surgical dissection was achieved to prevent hematoma formation, and the wounds were irrigated. The patellae were relocated and the arthrotomies were closed with a running 2-0 vicryl suture. Fascial and subcutaneous layers were closed with a running 3-0 vicryl suture, and skin was closed with subcuticular 4‑0 vicryl sutures. The rabbit limbs were allowed weight-bearing with unrestricted range of motion within 2x2x2-ft cages.
For all groups, menisci were explanted at 28 and 84 days for gross and histologic analysis. For biochemical assessments, menisci were explanted at 9, 28, and 84 days (Table 1).
Gross Analysis
Immediately after specimen removal, all medial menisci were evaluated for gross morphology. A grading system was used for organization and classification of data (Table 2). Three blinded orthopedic surgeon-observers performed all grading. Grade A was considered complete healing of the meniscus. Grade B involved complete healing with a trace of injury remaining on the surface of the meniscus. Grade C represented incomplete healing with a full-thickness injury that was stable to stress of the repair site with an arthroscopic probe. Grade D had no healing with the injured region unstable to stress of the repair site with an arthroscopic probe.
Histologic Analysis and Microscopic Grading of Meniscal Healing
After gross evaluation by the 3 blinded observers, each meniscus was fixed for 24 hours in 10% buffered formalin. Each specimen was then embedded in paraffin and cut into 6-µm slices along the radial plane. The tissue samples were stained with hematoxylin-eosin, and microscopic grading was assigned. The grading system was the same as that used for gross morphologic analysis.
Biochemical Analysis
To determine whether RF treatment stimulated a healing response in the avascular zone of the meniscus, measurements of specific biochemical markers were analyzed at 9, 28, and 84 days after treatment. As a control, unrepaired meniscal tissue from the contralateral knee was also analyzed. 3H-thymidine incorporation into the meniscus was measured to assess cell proliferation.23 At sacrifice, control and treated menisci were dissected and immediately placed into sterile culture media (Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum, antibiotic, and fungicide). 3H-thymidine was added at a concentration of 5µCi/mL of media to each tube. After incubation for 48 hours at 37°C under 5% CO2, the menisci were removed and dialyzed against water for 24 hours to remove unincorporated thymidine. After washing, the menisci were lyophilized, aliquots weighed, and radioactivity determined by liquid scintillation spectrometry. Results are expressed as counts per minute per mg dry tissue weight.
Semiquantitative reverse transcription polymerase chain reaction (RT-PCR) was used to determine mRNA expression of mitogenic growth factors, IGF-1 and bFGF, and angiogenic markers, αV and VEFG.24 National Institutes of Health (NIH) image-analysis software (version 1.61; NIH, Bethesda, Maryland) was used to quantitatively scan RT-PCR profiles after agarose gel electrophoresis and ethidium bromide visualization. Values were normalized to the housekeeping gene, GAPDH.
Statistical Analysis
Data are expressed as mean (SD) and evaluated using an unpaired Student t test between groups. Statistical significance was established at P < .05.
Results
Gross Morphology
Analysis of gross morphology showed signs of healing only in the group treated with suture repair combined with RF treatment (Table 3). In group 1 (meniscal injury only) and group 2 (suture repair only), no healing occurred at 28 and 84 days (Figure 3A). A meniscal grading system was developed to better describe the varying levels of healing shown in the suture-plus-RF-treatment group (Table 2). Of the specimens that showed healing in group 3, 1 had complete healing (grade A) within the avascular zone of the meniscus at 84 days (Figure 3B). In addition, 4 specimens subjected to suture repair and RF treatment had complete healing with only a trace of injured tissue remaining (grade B). Fourteen specimens in group 3 had incomplete healing with lesions stable to stress suggesting early signs of healing (grade C). In total, 58% of menisci treated with RF showed signs of healing while the remaining 14 specimens in group 3 showed none (grade D).
Histologic Examination
The histology correlated well with gross analysis. No microscopic evidence of healing was seen in groups 1 and 2 (Figure 3C). Of the specimens treated with suture repair combined with RF, 19 (58%) showed varying degrees of histologic healing. While gross morphologic examination showed that only 1 specimen had complete healing, microscopic analysis showed that 1 specimen from group 3 had grade B healing on gross analysis but grade A healing on histologic analysis. Thus, upon histologic examination, 2 specimens showed complete healing of injuries in the avascular zone of the meniscus when treated with suture repair combined with RF treatment rather than the 1 specimen seen on gross morphology (Figure 3D).
Biochemical Analysis
Biochemical assessments were performed at 9, 28, and 84 days after surgery. 3H-thymidine incorporation was studied as a marker for cellular proliferation, and its levels were significantly higher in meniscus explants treated with RF (Figure 4). The mean (SD) rate of incorporation for meniscal tears treated with suture repair plus RF was 590 (80) cpm/mg dry tissue at 9 days. This value was approximately 40% greater than the menisci treated with suture repair only, which had a mean (SD) value of 380 (30) cpm/mg (P < .05). Normal, unrepaired meniscal tissue had a mean (SD) 3H-thymidine incorporation rate of 250 (35) cpm/mg. By 84 days, thymidine levels returned to uninjured levels in both suture-only and RF-treated menisci. Semiquantitative RT-PCR analysis showed that, 9 days after repair, the RF-treated menisci had increased mRNA expression of IGF-1, bFGF, VEGF, and αV relative to untreated repairs (Figure 5). There was a statistically significant acute phase response in IGF-1, bFGF, VEGF and αV in groups treated with RF at 9 days (P > .05).
Adverse Outcomes
There were no surgical complications. During the histologic evaluation, there were no incidences of fibrochondrocyte cell death or damage from the application of RF treatment.
Discussion
RF treatments have been used for many years in various medical and surgical applications. Presently, the most common implementation of RF is for cutting and coagulating tissue during surgery. More recently, however, several publications have shown that when used properly and safely, RF can be an effective surgical adjunct for tendinosis recalcitrant to conservative therapy.15-17,25-32
Many have suggested that RF coblation is successful in these clinical scenarios because of its ability to promote an increased angiogenic and fibroblastic response in hypovascular tissue.29,33,34
This body of literature led to the evaluation of RF coblation in treating meniscal tears in the avascular zone. Studies have shown poor success of meniscus repairs done in the avascular zone; however, our data demonstrate that supplementing suture repair with RF treatment may improve the acute-phase healing response. Although the control and suture-repair groups showed no signs of healing, the suture-repair-combined-with-RF-treatment group had 2 specimens in which complete gross and histologic healing occurred. In addition, 19 (58%) specimens in the RF group showed gross or histologic signs of healing.
Biochemically, 3H-thymidine incorporation was examined to assess cellular proliferation. Mitogenic (IGF, bFGF) and angiogenic (VEGF, αV) growth factors were measured as markers of an increased healing response. Compared with noninjured meniscal tissue, 3H-thymidine incorporation was significantly increased in both the suture and suture-combined-with-RF-treatment groups at 9 and 28 days after surgery. Between the suture and suture-RF groups, RF treatment led to a 40% greater increase in 3H-thymidine incorporation suggesting greater cellular proliferation in the immediate postoperative period. With respect to mitogenic and angiogenic factors, IGF, bFGF, VEGF, and αV were only significantly increased when RF was combined with suture repair. All 4 factors are important regulators of vasculogenesis, angiogenesis, wound healing, bone remodeling, and neurogenesis. The suture repair–only group showed no upregulation of these factors compared with uninjured controls.
Our study has several strengths. Using an animal model with menisci grossly similar to that of humans, we performed a controlled study comparing 2 treatment options, suture repair only and suture repair combined with RF treatment.35,36 The animal model also enabled second-look examinations at designated intervals. We analyzed the effect of RF treatment on concrete measures, such as gross, histologic, and biochemical healing. In particular, the biochemical analysis may indicate that RF treatment can increase the proliferative, mitogenic, and angiogenic capabilities of surrounding progenitor cells. This was evidenced by the statistically significant increase we saw in IGF-1, bFGF, VEGF, and αV at 9 and 28 days compared with controls.
Meniscal tears in the avascular zone represent a significant treatment dilemma for the physiologically young patient population. While partial meniscectomy provides excellent short-term relief, the long-term outcome of this intervention is degenerative joint disease. Meniscal repair in the central two-thirds of the meniscus has shown poor results. Our study presents data that show supplementing suture repair of avascular meniscal tears with RF can lead to increased gross, histologic, and biochemical healing in the New Zealand white rabbit. While these results are encouraging, studies with longer follow-up and specimens that represent the human menisci are necessary to determine whether these preliminary results would translate to human meniscal tears in the avascular zone.
Weaknesses of our study include the use of an animal model and the location of the tear created in the menisci. While using an animal model had many strengths, the results of our study are probably not strong enough to immediately extrapolate the use of RF in human meniscal repairs. However, the data we obtained are very encouraging and perhaps suggest that RF warrants human trials. Our open surgical technique made it difficult to create and repair a tear on the posterior horn of the medical meniscus without completely dislocating the knee anteriorly. As a result, the knees were subluxed anteriorly, and the meniscal tears and repairs were performed more anteriorly. The more anterior aspects of the menisci do not undergo the same rotational and axial loads as the posterior horn, and it is unclear whether this difference would contribute to the results we obtained from RF treatment. In addition, the tears were surgically created and the repair was done during the same procedure. Patients do not present in this manner, and this further underscores the need for a clinical trial to determine the effectiveness of this treatment option in humans.
Conclusion
RF-based supplementation of meniscal repairs in the avascular zone showed acute signs of biochemical healing in 58% of New Zealand white rabbit specimens. In addition, gross and histologic evaluations showed an increase in healing compared with controls. Two specimens treated with RF in combination with suture repair had complete healing. These results illustrate the effectiveness of RF in stimulating a healing response in hypovascular tissue. Clinical trials are necessary to determine the effectiveness of this treatment in humans.
1. Fauno P, Nielsen AB. Arthroscopic partial meniscectomy: a long-term follow-up. Arthroscopy. 1992;8(3):345-349.
2. Lynch MA, Henning CE, Glick KR, Jr. Knee joint surface changes. Long-term follow-up meniscus tear treatment in stable anterior cruciate ligament reconstructions. Clin Orthop. 1983;172:148-153.
3. Roos H, Lauren M, Adalberth T, Roos EM, Jonsson K, Lohmander LS. Knee osteoarthritis after meniscectomy: prevalence of radiographic changes after twenty-one years, compared with matched controls. Arthritis Rheum. 1998;41(4):687-693.
4. Hennerbichler A, Moutos FT, Hennerbichler D, Weinberg JB, Guilak F. Repair response of the inner and outer regions of the porcine meniscus in vitro. Am J Sports Med. 2007;35(5):754‑762.
5. Gershuni DH, Hargens AR, Danzig LA. Regional nutrition and cellularity of the meniscus. Implications for tear and repair. Sports Med. 1988;5(5):322-327.
6. Gershuni DH, Skyhar MJ, Danzig LA, Camp J, Hargens AR, Akeson WH. Experimental models to promote healing of tears in the avascular segment of canine knee menisci. J Bone Joint Surg Am. 1989;71(9):1363-1370.
7. Papachristou G, Efstathopoulos N, Plessas S, Levidiotis C, Chronopoulos E, Sourlas J. Isolated meniscal repair in the avascular area. Acta Orthop Belg. 2003;69(4):341-345.
8. Fox JM, Rintz KG, Ferkel RD. Trephination of incomplete meniscal tears. Arthroscopy. 1993;9(4):451-455.
9. Zhang Z, Arnold JA, Williams T, McCann B. Repairs by trephination and suturing of longitudinal injuries in the avascular area of the meniscus in goats. Am J Sports Med. 1995;23(1):35-41.
10. Zhang ZN, Tu KY, Xu YK, Zhang WM, Liu ZT, Ou SH. Treatment of longitudinal injuries in avascular area of meniscus in dogs by trephination. Arthroscopy. 1988;4(3):151-159.
11. Ochi M, Uchio Y, Okuda K, Shu N, Yamaguchi H, Sakai Y. Expression of cytokines after meniscal rasping to promote meniscal healing. Arthroscopy. 2001;17(7):724-731.
12. Okuda K, Ochi M, Shu N, Uchio Y. Meniscal rasping for repair of meniscal tear in the avascular zone. Arthroscopy. 1999;15(3):281-286.
13. Uchio Y, Ochi M, Adachi N, Kawasaki K, Iwasa J. Results of rasping of meniscal tears with and without anterior cruciate ligament injury as evaluated by second-look arthroscopy. Arthroscopy. 2003;19(5):463-469.
14. Tasto JP, Cummings J, Medlock V, Harwood F, Hardesty R, Amiel D. The tendon treatment center: new horizons in the treatment of tendinosis. Arthroscopy. 2003;19(suppl 1):213-223.
15. Tasto JP. The role of radiofrequency-based devices in shaping the future of orthopedic surgery. Orthopedics. 2006;29(10):874-875.
16. Tasto JP, Cummings J, Medlock V, Hardesty R, Amiel D. Microtenotomy using a radiofrequency probe to treat lateral epicondylitis. Arthroscopy. 2005;21(7):851-860.
17. Higuchi H, Kimura M, Kobayashi A, Hatayama K, Takagishi K. A novel treatment of hypermobile lateral meniscus with monopolar radiofrequency energy. Arthroscopy 2004;20 (suppl 2):1-5.
18. Tonna EA, Cronkite EP. The periosteum. Autoradiographic studies on cellular proliferation and transformation utilizing tritiated thymidine. Clin Orthop. 1963;30:218-233.
19. Madewell BR. Serum thymidine kinase activity: an alternative to histologic markers of cellular proliferation in canine lymphoma. J Vet Intern Med. 2004;18(5):595-596.
20. Mujoomdar M, Bennett A, Hoskin D, Blay J. Adenosine stimulation of proliferation of breast carcinoma cell lines: evaluation of the [3H]thymidine assay system and modulatory effects of the cellular microenvironment in vitro. J Cell Physiol. 2004;201(3):429-438.
21. Vander Borght T, Labar D, Pauwels S, Lambotte L. Production of [2-11C]thymidine for quantification of cellular proliferation with PET. Int J Rad Appl Instrum A. 1991;42(1):103-104.
22. Spindler KP, Mayes CE, Miller RR, Imro AK, Davidson JM. Regional mitogenic response of the meniscus to platelet-derived growth factor (PDGF-AB). J Orthop Res. 1995;13(2):201-207.
23. Thomopoulos S, Zaegel M, Das R, et al. PDGF-BB released in tendon repair using a novel delivery system promotes cell proliferation and collagen remodeling. J Orthop Res. 2007;25(10):1358-1368.
24. Pennock AT, Robertson CM, Emmerson BC, Harwood FL, Amiel D. Role of apoptotic and matrix-degrading genes in articular cartilage and meniscus of mature and aged rabbits during development of osteoarthritis. Arthritis Rheum. 2007;56(5):1529-1536.
25. Allen RT, Tasto JP, Cummings J, Robertson CM, Amiel D. Meniscal debridement with an arthroscopic radiofrequency wand versus an arthroscopic shaver: comparative effects on menisci and underlying articular cartilage. Arthroscopy. 2006;22(4):385-393.
26. Figueroa D, Calvo R, Vaisman A, et al. Bipolar radiofrequency in the human meniscus. Comparative study between patients younger and older than 40 years of age. Knee. 2007;14(5):357-360.
27. Friedman M, LoSavio P, Ibrahim H, Ramakrishnan V. Radiofrequency tonsil reduction: safety, morbidity, and efficacy. Laryngoscope. 2003;113(5):882-887.
28. Hall DJ, Littlefield PD, Birkmire-Peters DP, Holtel MR. Radiofrequency ablation versus electrocautery in tonsillectomy. Otolaryngol Head Neck Surg. 2004;130(3):300-305.
29. Kaplan H, Gat A. Clinical and histopathological results following TriPollar radiofrequency skin treatments. J Cosmet Laser Ther. 2009;11(2):78-84.
30. Mancini PF. Coblation: a new technology and technique for skin resurfacing and other aesthetic surgical procedures. Aesthetic Plast Surg. 2001;2595):372-377.
31. Penka I, Kaplan Z, Sefr R, Sirotek L, Eber Z, Ondrák M. Use of radiofrequency ablation in the treatment of malignant liver lesions. Hepatogastroenterology. 2008;55(82-83):562-567.
32. Tasto JP, Ash SA. Current uses of radiofrequency in arthroscopic knee surgery. Am J Knee Surg. 1999;12(3):186-191.
33. Amiel D, Ball ST, Tasto JP. Chondrocyte viability and metabolic activity after treatment of bovine articular cartilage with bipolar radiofrequency: an in vitro study. Arthroscopy. 2004;20(5):503-510.
34. Barry KJ, Kaplan J, Connolly RJ, et al. The effect of radiofrequency-generated thermal energy on the mechanical and histologic characteristics of the arterial wall in vivo: implications for radiofrequency angioplasty. Am Heart J. 1989;117(2):332-341.
35. Hoch DH, Grodzinsky AJ, Koob TJ, Albert ML, Eyre DR. Early changes in material properties of rabbit articular cartilage after meniscectomy. J Orthop Res. 1983;1(1):4-12.
36. Thompson AM, Stockwell RA. An ultrastructural study of the marginal transitional zone in the rabbit knee joint. J Anat. 1983;136(Pt 4):701-713.
Partial meniscectomy of tears in the avascular zone remains one of the most common orthopedic procedures. While results of partial meniscectomy in younger patients have excellent short- to medium-term results, the long-term clinical outcomes are often less favorable.1-3 Repair in the avascular “white-white” zone has resulted in lower patient satisfaction scores and higher revision surgery rates.4-7 Consequently, most tears in this region have been treated with partial meniscectomy.
The inability to repair rather than resect menisci with avascular tears has led to extensive research. Techniques such as trephination and rasping to initiate an angiogenic response have had inconsistent and unreliable results when applied to the white-white zone.8-13 In contrast, Tasto and colleagues14 have shown that radiofrequency (RF) applied to hypovascular tissue can not only stimulate tissue vascularity, but also increase organization of fibroblastic cells. In addition, Tasto and colleagues15,16 have shown that RF application can significantly improve histologic healing and clinical outcomes in refractory cases of Achilles tendinopathy and lateral epicondylitis. In Japanese white rabbit menisci, Higuchi and coauthors17 applied monopolar RF at 60°C and 40W to avascular zone tears to fuse the tissue. They found a significant increase in fibroblast proliferation and fusion of collagen fibers at 2, 4, and 12 weeks after surgery. They also found significant acellular zones of meniscus tissue and attributed these findings to fibrochondrocyte death because of thermal treatment.
This body of research led to the present study, which evaluates the effect of low-temperature, bipolar RF stimulation, in conjunction with suture repair, on the healing of tears in the avascular white-white zone of the meniscus both in vivo and ex vivo. We performed gross and histologic analyses of the treatment groups for the in vivo aspect of the study and biochemical analyses to study the ex vivo effects of RF treatment. 3H-thymidine incorporation has been shown to be a reliable indicator of cellular proliferation in several studies, and this was measured in our treatment groups.18-21 In addition, the response of mitogenic growth factors (IGF-1, bFGF) and angiogenic markers (VEGF, αV) to RF treatment was studied.22 We hypothesized that bipolar RF application would show increased gross, histologic, and biochemical healing when combined with suture repair of longitudinal avascular zone meniscus tears.
Materials and Methods
Creation of Meniscal Tears
Fifty-four healthy, skeletally mature male and female adult New Zealand white rabbits aged 7 to 18 months were used for the study. All procedures conformed to the guidelines of our university’s institutional animal care and use committee and the American Association for Accreditation of Laboratory Animal Care. All rabbits underwent a surgical procedure in which a longitudinal tear was created in the avascular white-white zone of the medial meniscus. Using sterile technique and instrumentation, a medial parapatellar incision was made on the left knee of each rabbit. The patella was retracted laterally, exposing the medial meniscus. The tibia was then externally rotated to sublux the anterior horn of the medial meniscus anteriorly. A longitudinal full-thickness meniscal tear (3-4 mm in length) was created in the avascular zone (inner third) of the anterior horn of the medial meniscus using an 11-blade scalpel (Figures 1A, 1B). The location of the tear was grossly performed in the same location in each meniscus. The rabbits were randomly divided into 3 treatment groups: 1, 2, and 3 (Table 1). Group 1 (n = 6) served as a control with no repair or RF treatment applied. Group 2 (n = 15) underwent suture repair only of the meniscal tear using 5-0 nylon suture in a horizontal mattress pattern (Figure 2A). Group 3 (n = 33) underwent suture repair after RF stimulation was applied to both sides of the meniscus tear (Figures 2B, 2C). RF was applied using a 0.8-mm TOPAZ MicroDebrider (ArthroCare, Sunnyvale, California) set at level 4 (175 V-RMS) for 500 milliseconds. Lactated ringer’s solution was continuously infused through the probe via sterile tubing to prevent overheating.
After meniscal treatment, hemostasis of the surrounding surgical dissection was achieved to prevent hematoma formation, and the wounds were irrigated. The patellae were relocated and the arthrotomies were closed with a running 2-0 vicryl suture. Fascial and subcutaneous layers were closed with a running 3-0 vicryl suture, and skin was closed with subcuticular 4‑0 vicryl sutures. The rabbit limbs were allowed weight-bearing with unrestricted range of motion within 2x2x2-ft cages.
For all groups, menisci were explanted at 28 and 84 days for gross and histologic analysis. For biochemical assessments, menisci were explanted at 9, 28, and 84 days (Table 1).
Gross Analysis
Immediately after specimen removal, all medial menisci were evaluated for gross morphology. A grading system was used for organization and classification of data (Table 2). Three blinded orthopedic surgeon-observers performed all grading. Grade A was considered complete healing of the meniscus. Grade B involved complete healing with a trace of injury remaining on the surface of the meniscus. Grade C represented incomplete healing with a full-thickness injury that was stable to stress of the repair site with an arthroscopic probe. Grade D had no healing with the injured region unstable to stress of the repair site with an arthroscopic probe.
Histologic Analysis and Microscopic Grading of Meniscal Healing
After gross evaluation by the 3 blinded observers, each meniscus was fixed for 24 hours in 10% buffered formalin. Each specimen was then embedded in paraffin and cut into 6-µm slices along the radial plane. The tissue samples were stained with hematoxylin-eosin, and microscopic grading was assigned. The grading system was the same as that used for gross morphologic analysis.
Biochemical Analysis
To determine whether RF treatment stimulated a healing response in the avascular zone of the meniscus, measurements of specific biochemical markers were analyzed at 9, 28, and 84 days after treatment. As a control, unrepaired meniscal tissue from the contralateral knee was also analyzed. 3H-thymidine incorporation into the meniscus was measured to assess cell proliferation.23 At sacrifice, control and treated menisci were dissected and immediately placed into sterile culture media (Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum, antibiotic, and fungicide). 3H-thymidine was added at a concentration of 5µCi/mL of media to each tube. After incubation for 48 hours at 37°C under 5% CO2, the menisci were removed and dialyzed against water for 24 hours to remove unincorporated thymidine. After washing, the menisci were lyophilized, aliquots weighed, and radioactivity determined by liquid scintillation spectrometry. Results are expressed as counts per minute per mg dry tissue weight.
Semiquantitative reverse transcription polymerase chain reaction (RT-PCR) was used to determine mRNA expression of mitogenic growth factors, IGF-1 and bFGF, and angiogenic markers, αV and VEFG.24 National Institutes of Health (NIH) image-analysis software (version 1.61; NIH, Bethesda, Maryland) was used to quantitatively scan RT-PCR profiles after agarose gel electrophoresis and ethidium bromide visualization. Values were normalized to the housekeeping gene, GAPDH.
Statistical Analysis
Data are expressed as mean (SD) and evaluated using an unpaired Student t test between groups. Statistical significance was established at P < .05.
Results
Gross Morphology
Analysis of gross morphology showed signs of healing only in the group treated with suture repair combined with RF treatment (Table 3). In group 1 (meniscal injury only) and group 2 (suture repair only), no healing occurred at 28 and 84 days (Figure 3A). A meniscal grading system was developed to better describe the varying levels of healing shown in the suture-plus-RF-treatment group (Table 2). Of the specimens that showed healing in group 3, 1 had complete healing (grade A) within the avascular zone of the meniscus at 84 days (Figure 3B). In addition, 4 specimens subjected to suture repair and RF treatment had complete healing with only a trace of injured tissue remaining (grade B). Fourteen specimens in group 3 had incomplete healing with lesions stable to stress suggesting early signs of healing (grade C). In total, 58% of menisci treated with RF showed signs of healing while the remaining 14 specimens in group 3 showed none (grade D).
Histologic Examination
The histology correlated well with gross analysis. No microscopic evidence of healing was seen in groups 1 and 2 (Figure 3C). Of the specimens treated with suture repair combined with RF, 19 (58%) showed varying degrees of histologic healing. While gross morphologic examination showed that only 1 specimen had complete healing, microscopic analysis showed that 1 specimen from group 3 had grade B healing on gross analysis but grade A healing on histologic analysis. Thus, upon histologic examination, 2 specimens showed complete healing of injuries in the avascular zone of the meniscus when treated with suture repair combined with RF treatment rather than the 1 specimen seen on gross morphology (Figure 3D).
Biochemical Analysis
Biochemical assessments were performed at 9, 28, and 84 days after surgery. 3H-thymidine incorporation was studied as a marker for cellular proliferation, and its levels were significantly higher in meniscus explants treated with RF (Figure 4). The mean (SD) rate of incorporation for meniscal tears treated with suture repair plus RF was 590 (80) cpm/mg dry tissue at 9 days. This value was approximately 40% greater than the menisci treated with suture repair only, which had a mean (SD) value of 380 (30) cpm/mg (P < .05). Normal, unrepaired meniscal tissue had a mean (SD) 3H-thymidine incorporation rate of 250 (35) cpm/mg. By 84 days, thymidine levels returned to uninjured levels in both suture-only and RF-treated menisci. Semiquantitative RT-PCR analysis showed that, 9 days after repair, the RF-treated menisci had increased mRNA expression of IGF-1, bFGF, VEGF, and αV relative to untreated repairs (Figure 5). There was a statistically significant acute phase response in IGF-1, bFGF, VEGF and αV in groups treated with RF at 9 days (P > .05).
Adverse Outcomes
There were no surgical complications. During the histologic evaluation, there were no incidences of fibrochondrocyte cell death or damage from the application of RF treatment.
Discussion
RF treatments have been used for many years in various medical and surgical applications. Presently, the most common implementation of RF is for cutting and coagulating tissue during surgery. More recently, however, several publications have shown that when used properly and safely, RF can be an effective surgical adjunct for tendinosis recalcitrant to conservative therapy.15-17,25-32
Many have suggested that RF coblation is successful in these clinical scenarios because of its ability to promote an increased angiogenic and fibroblastic response in hypovascular tissue.29,33,34
This body of literature led to the evaluation of RF coblation in treating meniscal tears in the avascular zone. Studies have shown poor success of meniscus repairs done in the avascular zone; however, our data demonstrate that supplementing suture repair with RF treatment may improve the acute-phase healing response. Although the control and suture-repair groups showed no signs of healing, the suture-repair-combined-with-RF-treatment group had 2 specimens in which complete gross and histologic healing occurred. In addition, 19 (58%) specimens in the RF group showed gross or histologic signs of healing.
Biochemically, 3H-thymidine incorporation was examined to assess cellular proliferation. Mitogenic (IGF, bFGF) and angiogenic (VEGF, αV) growth factors were measured as markers of an increased healing response. Compared with noninjured meniscal tissue, 3H-thymidine incorporation was significantly increased in both the suture and suture-combined-with-RF-treatment groups at 9 and 28 days after surgery. Between the suture and suture-RF groups, RF treatment led to a 40% greater increase in 3H-thymidine incorporation suggesting greater cellular proliferation in the immediate postoperative period. With respect to mitogenic and angiogenic factors, IGF, bFGF, VEGF, and αV were only significantly increased when RF was combined with suture repair. All 4 factors are important regulators of vasculogenesis, angiogenesis, wound healing, bone remodeling, and neurogenesis. The suture repair–only group showed no upregulation of these factors compared with uninjured controls.
Our study has several strengths. Using an animal model with menisci grossly similar to that of humans, we performed a controlled study comparing 2 treatment options, suture repair only and suture repair combined with RF treatment.35,36 The animal model also enabled second-look examinations at designated intervals. We analyzed the effect of RF treatment on concrete measures, such as gross, histologic, and biochemical healing. In particular, the biochemical analysis may indicate that RF treatment can increase the proliferative, mitogenic, and angiogenic capabilities of surrounding progenitor cells. This was evidenced by the statistically significant increase we saw in IGF-1, bFGF, VEGF, and αV at 9 and 28 days compared with controls.
Meniscal tears in the avascular zone represent a significant treatment dilemma for the physiologically young patient population. While partial meniscectomy provides excellent short-term relief, the long-term outcome of this intervention is degenerative joint disease. Meniscal repair in the central two-thirds of the meniscus has shown poor results. Our study presents data that show supplementing suture repair of avascular meniscal tears with RF can lead to increased gross, histologic, and biochemical healing in the New Zealand white rabbit. While these results are encouraging, studies with longer follow-up and specimens that represent the human menisci are necessary to determine whether these preliminary results would translate to human meniscal tears in the avascular zone.
Weaknesses of our study include the use of an animal model and the location of the tear created in the menisci. While using an animal model had many strengths, the results of our study are probably not strong enough to immediately extrapolate the use of RF in human meniscal repairs. However, the data we obtained are very encouraging and perhaps suggest that RF warrants human trials. Our open surgical technique made it difficult to create and repair a tear on the posterior horn of the medical meniscus without completely dislocating the knee anteriorly. As a result, the knees were subluxed anteriorly, and the meniscal tears and repairs were performed more anteriorly. The more anterior aspects of the menisci do not undergo the same rotational and axial loads as the posterior horn, and it is unclear whether this difference would contribute to the results we obtained from RF treatment. In addition, the tears were surgically created and the repair was done during the same procedure. Patients do not present in this manner, and this further underscores the need for a clinical trial to determine the effectiveness of this treatment option in humans.
Conclusion
RF-based supplementation of meniscal repairs in the avascular zone showed acute signs of biochemical healing in 58% of New Zealand white rabbit specimens. In addition, gross and histologic evaluations showed an increase in healing compared with controls. Two specimens treated with RF in combination with suture repair had complete healing. These results illustrate the effectiveness of RF in stimulating a healing response in hypovascular tissue. Clinical trials are necessary to determine the effectiveness of this treatment in humans.
Partial meniscectomy of tears in the avascular zone remains one of the most common orthopedic procedures. While results of partial meniscectomy in younger patients have excellent short- to medium-term results, the long-term clinical outcomes are often less favorable.1-3 Repair in the avascular “white-white” zone has resulted in lower patient satisfaction scores and higher revision surgery rates.4-7 Consequently, most tears in this region have been treated with partial meniscectomy.
The inability to repair rather than resect menisci with avascular tears has led to extensive research. Techniques such as trephination and rasping to initiate an angiogenic response have had inconsistent and unreliable results when applied to the white-white zone.8-13 In contrast, Tasto and colleagues14 have shown that radiofrequency (RF) applied to hypovascular tissue can not only stimulate tissue vascularity, but also increase organization of fibroblastic cells. In addition, Tasto and colleagues15,16 have shown that RF application can significantly improve histologic healing and clinical outcomes in refractory cases of Achilles tendinopathy and lateral epicondylitis. In Japanese white rabbit menisci, Higuchi and coauthors17 applied monopolar RF at 60°C and 40W to avascular zone tears to fuse the tissue. They found a significant increase in fibroblast proliferation and fusion of collagen fibers at 2, 4, and 12 weeks after surgery. They also found significant acellular zones of meniscus tissue and attributed these findings to fibrochondrocyte death because of thermal treatment.
This body of research led to the present study, which evaluates the effect of low-temperature, bipolar RF stimulation, in conjunction with suture repair, on the healing of tears in the avascular white-white zone of the meniscus both in vivo and ex vivo. We performed gross and histologic analyses of the treatment groups for the in vivo aspect of the study and biochemical analyses to study the ex vivo effects of RF treatment. 3H-thymidine incorporation has been shown to be a reliable indicator of cellular proliferation in several studies, and this was measured in our treatment groups.18-21 In addition, the response of mitogenic growth factors (IGF-1, bFGF) and angiogenic markers (VEGF, αV) to RF treatment was studied.22 We hypothesized that bipolar RF application would show increased gross, histologic, and biochemical healing when combined with suture repair of longitudinal avascular zone meniscus tears.
Materials and Methods
Creation of Meniscal Tears
Fifty-four healthy, skeletally mature male and female adult New Zealand white rabbits aged 7 to 18 months were used for the study. All procedures conformed to the guidelines of our university’s institutional animal care and use committee and the American Association for Accreditation of Laboratory Animal Care. All rabbits underwent a surgical procedure in which a longitudinal tear was created in the avascular white-white zone of the medial meniscus. Using sterile technique and instrumentation, a medial parapatellar incision was made on the left knee of each rabbit. The patella was retracted laterally, exposing the medial meniscus. The tibia was then externally rotated to sublux the anterior horn of the medial meniscus anteriorly. A longitudinal full-thickness meniscal tear (3-4 mm in length) was created in the avascular zone (inner third) of the anterior horn of the medial meniscus using an 11-blade scalpel (Figures 1A, 1B). The location of the tear was grossly performed in the same location in each meniscus. The rabbits were randomly divided into 3 treatment groups: 1, 2, and 3 (Table 1). Group 1 (n = 6) served as a control with no repair or RF treatment applied. Group 2 (n = 15) underwent suture repair only of the meniscal tear using 5-0 nylon suture in a horizontal mattress pattern (Figure 2A). Group 3 (n = 33) underwent suture repair after RF stimulation was applied to both sides of the meniscus tear (Figures 2B, 2C). RF was applied using a 0.8-mm TOPAZ MicroDebrider (ArthroCare, Sunnyvale, California) set at level 4 (175 V-RMS) for 500 milliseconds. Lactated ringer’s solution was continuously infused through the probe via sterile tubing to prevent overheating.
After meniscal treatment, hemostasis of the surrounding surgical dissection was achieved to prevent hematoma formation, and the wounds were irrigated. The patellae were relocated and the arthrotomies were closed with a running 2-0 vicryl suture. Fascial and subcutaneous layers were closed with a running 3-0 vicryl suture, and skin was closed with subcuticular 4‑0 vicryl sutures. The rabbit limbs were allowed weight-bearing with unrestricted range of motion within 2x2x2-ft cages.
For all groups, menisci were explanted at 28 and 84 days for gross and histologic analysis. For biochemical assessments, menisci were explanted at 9, 28, and 84 days (Table 1).
Gross Analysis
Immediately after specimen removal, all medial menisci were evaluated for gross morphology. A grading system was used for organization and classification of data (Table 2). Three blinded orthopedic surgeon-observers performed all grading. Grade A was considered complete healing of the meniscus. Grade B involved complete healing with a trace of injury remaining on the surface of the meniscus. Grade C represented incomplete healing with a full-thickness injury that was stable to stress of the repair site with an arthroscopic probe. Grade D had no healing with the injured region unstable to stress of the repair site with an arthroscopic probe.
Histologic Analysis and Microscopic Grading of Meniscal Healing
After gross evaluation by the 3 blinded observers, each meniscus was fixed for 24 hours in 10% buffered formalin. Each specimen was then embedded in paraffin and cut into 6-µm slices along the radial plane. The tissue samples were stained with hematoxylin-eosin, and microscopic grading was assigned. The grading system was the same as that used for gross morphologic analysis.
Biochemical Analysis
To determine whether RF treatment stimulated a healing response in the avascular zone of the meniscus, measurements of specific biochemical markers were analyzed at 9, 28, and 84 days after treatment. As a control, unrepaired meniscal tissue from the contralateral knee was also analyzed. 3H-thymidine incorporation into the meniscus was measured to assess cell proliferation.23 At sacrifice, control and treated menisci were dissected and immediately placed into sterile culture media (Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum, antibiotic, and fungicide). 3H-thymidine was added at a concentration of 5µCi/mL of media to each tube. After incubation for 48 hours at 37°C under 5% CO2, the menisci were removed and dialyzed against water for 24 hours to remove unincorporated thymidine. After washing, the menisci were lyophilized, aliquots weighed, and radioactivity determined by liquid scintillation spectrometry. Results are expressed as counts per minute per mg dry tissue weight.
Semiquantitative reverse transcription polymerase chain reaction (RT-PCR) was used to determine mRNA expression of mitogenic growth factors, IGF-1 and bFGF, and angiogenic markers, αV and VEFG.24 National Institutes of Health (NIH) image-analysis software (version 1.61; NIH, Bethesda, Maryland) was used to quantitatively scan RT-PCR profiles after agarose gel electrophoresis and ethidium bromide visualization. Values were normalized to the housekeeping gene, GAPDH.
Statistical Analysis
Data are expressed as mean (SD) and evaluated using an unpaired Student t test between groups. Statistical significance was established at P < .05.
Results
Gross Morphology
Analysis of gross morphology showed signs of healing only in the group treated with suture repair combined with RF treatment (Table 3). In group 1 (meniscal injury only) and group 2 (suture repair only), no healing occurred at 28 and 84 days (Figure 3A). A meniscal grading system was developed to better describe the varying levels of healing shown in the suture-plus-RF-treatment group (Table 2). Of the specimens that showed healing in group 3, 1 had complete healing (grade A) within the avascular zone of the meniscus at 84 days (Figure 3B). In addition, 4 specimens subjected to suture repair and RF treatment had complete healing with only a trace of injured tissue remaining (grade B). Fourteen specimens in group 3 had incomplete healing with lesions stable to stress suggesting early signs of healing (grade C). In total, 58% of menisci treated with RF showed signs of healing while the remaining 14 specimens in group 3 showed none (grade D).
Histologic Examination
The histology correlated well with gross analysis. No microscopic evidence of healing was seen in groups 1 and 2 (Figure 3C). Of the specimens treated with suture repair combined with RF, 19 (58%) showed varying degrees of histologic healing. While gross morphologic examination showed that only 1 specimen had complete healing, microscopic analysis showed that 1 specimen from group 3 had grade B healing on gross analysis but grade A healing on histologic analysis. Thus, upon histologic examination, 2 specimens showed complete healing of injuries in the avascular zone of the meniscus when treated with suture repair combined with RF treatment rather than the 1 specimen seen on gross morphology (Figure 3D).
Biochemical Analysis
Biochemical assessments were performed at 9, 28, and 84 days after surgery. 3H-thymidine incorporation was studied as a marker for cellular proliferation, and its levels were significantly higher in meniscus explants treated with RF (Figure 4). The mean (SD) rate of incorporation for meniscal tears treated with suture repair plus RF was 590 (80) cpm/mg dry tissue at 9 days. This value was approximately 40% greater than the menisci treated with suture repair only, which had a mean (SD) value of 380 (30) cpm/mg (P < .05). Normal, unrepaired meniscal tissue had a mean (SD) 3H-thymidine incorporation rate of 250 (35) cpm/mg. By 84 days, thymidine levels returned to uninjured levels in both suture-only and RF-treated menisci. Semiquantitative RT-PCR analysis showed that, 9 days after repair, the RF-treated menisci had increased mRNA expression of IGF-1, bFGF, VEGF, and αV relative to untreated repairs (Figure 5). There was a statistically significant acute phase response in IGF-1, bFGF, VEGF and αV in groups treated with RF at 9 days (P > .05).
Adverse Outcomes
There were no surgical complications. During the histologic evaluation, there were no incidences of fibrochondrocyte cell death or damage from the application of RF treatment.
Discussion
RF treatments have been used for many years in various medical and surgical applications. Presently, the most common implementation of RF is for cutting and coagulating tissue during surgery. More recently, however, several publications have shown that when used properly and safely, RF can be an effective surgical adjunct for tendinosis recalcitrant to conservative therapy.15-17,25-32
Many have suggested that RF coblation is successful in these clinical scenarios because of its ability to promote an increased angiogenic and fibroblastic response in hypovascular tissue.29,33,34
This body of literature led to the evaluation of RF coblation in treating meniscal tears in the avascular zone. Studies have shown poor success of meniscus repairs done in the avascular zone; however, our data demonstrate that supplementing suture repair with RF treatment may improve the acute-phase healing response. Although the control and suture-repair groups showed no signs of healing, the suture-repair-combined-with-RF-treatment group had 2 specimens in which complete gross and histologic healing occurred. In addition, 19 (58%) specimens in the RF group showed gross or histologic signs of healing.
Biochemically, 3H-thymidine incorporation was examined to assess cellular proliferation. Mitogenic (IGF, bFGF) and angiogenic (VEGF, αV) growth factors were measured as markers of an increased healing response. Compared with noninjured meniscal tissue, 3H-thymidine incorporation was significantly increased in both the suture and suture-combined-with-RF-treatment groups at 9 and 28 days after surgery. Between the suture and suture-RF groups, RF treatment led to a 40% greater increase in 3H-thymidine incorporation suggesting greater cellular proliferation in the immediate postoperative period. With respect to mitogenic and angiogenic factors, IGF, bFGF, VEGF, and αV were only significantly increased when RF was combined with suture repair. All 4 factors are important regulators of vasculogenesis, angiogenesis, wound healing, bone remodeling, and neurogenesis. The suture repair–only group showed no upregulation of these factors compared with uninjured controls.
Our study has several strengths. Using an animal model with menisci grossly similar to that of humans, we performed a controlled study comparing 2 treatment options, suture repair only and suture repair combined with RF treatment.35,36 The animal model also enabled second-look examinations at designated intervals. We analyzed the effect of RF treatment on concrete measures, such as gross, histologic, and biochemical healing. In particular, the biochemical analysis may indicate that RF treatment can increase the proliferative, mitogenic, and angiogenic capabilities of surrounding progenitor cells. This was evidenced by the statistically significant increase we saw in IGF-1, bFGF, VEGF, and αV at 9 and 28 days compared with controls.
Meniscal tears in the avascular zone represent a significant treatment dilemma for the physiologically young patient population. While partial meniscectomy provides excellent short-term relief, the long-term outcome of this intervention is degenerative joint disease. Meniscal repair in the central two-thirds of the meniscus has shown poor results. Our study presents data that show supplementing suture repair of avascular meniscal tears with RF can lead to increased gross, histologic, and biochemical healing in the New Zealand white rabbit. While these results are encouraging, studies with longer follow-up and specimens that represent the human menisci are necessary to determine whether these preliminary results would translate to human meniscal tears in the avascular zone.
Weaknesses of our study include the use of an animal model and the location of the tear created in the menisci. While using an animal model had many strengths, the results of our study are probably not strong enough to immediately extrapolate the use of RF in human meniscal repairs. However, the data we obtained are very encouraging and perhaps suggest that RF warrants human trials. Our open surgical technique made it difficult to create and repair a tear on the posterior horn of the medical meniscus without completely dislocating the knee anteriorly. As a result, the knees were subluxed anteriorly, and the meniscal tears and repairs were performed more anteriorly. The more anterior aspects of the menisci do not undergo the same rotational and axial loads as the posterior horn, and it is unclear whether this difference would contribute to the results we obtained from RF treatment. In addition, the tears were surgically created and the repair was done during the same procedure. Patients do not present in this manner, and this further underscores the need for a clinical trial to determine the effectiveness of this treatment option in humans.
Conclusion
RF-based supplementation of meniscal repairs in the avascular zone showed acute signs of biochemical healing in 58% of New Zealand white rabbit specimens. In addition, gross and histologic evaluations showed an increase in healing compared with controls. Two specimens treated with RF in combination with suture repair had complete healing. These results illustrate the effectiveness of RF in stimulating a healing response in hypovascular tissue. Clinical trials are necessary to determine the effectiveness of this treatment in humans.
1. Fauno P, Nielsen AB. Arthroscopic partial meniscectomy: a long-term follow-up. Arthroscopy. 1992;8(3):345-349.
2. Lynch MA, Henning CE, Glick KR, Jr. Knee joint surface changes. Long-term follow-up meniscus tear treatment in stable anterior cruciate ligament reconstructions. Clin Orthop. 1983;172:148-153.
3. Roos H, Lauren M, Adalberth T, Roos EM, Jonsson K, Lohmander LS. Knee osteoarthritis after meniscectomy: prevalence of radiographic changes after twenty-one years, compared with matched controls. Arthritis Rheum. 1998;41(4):687-693.
4. Hennerbichler A, Moutos FT, Hennerbichler D, Weinberg JB, Guilak F. Repair response of the inner and outer regions of the porcine meniscus in vitro. Am J Sports Med. 2007;35(5):754‑762.
5. Gershuni DH, Hargens AR, Danzig LA. Regional nutrition and cellularity of the meniscus. Implications for tear and repair. Sports Med. 1988;5(5):322-327.
6. Gershuni DH, Skyhar MJ, Danzig LA, Camp J, Hargens AR, Akeson WH. Experimental models to promote healing of tears in the avascular segment of canine knee menisci. J Bone Joint Surg Am. 1989;71(9):1363-1370.
7. Papachristou G, Efstathopoulos N, Plessas S, Levidiotis C, Chronopoulos E, Sourlas J. Isolated meniscal repair in the avascular area. Acta Orthop Belg. 2003;69(4):341-345.
8. Fox JM, Rintz KG, Ferkel RD. Trephination of incomplete meniscal tears. Arthroscopy. 1993;9(4):451-455.
9. Zhang Z, Arnold JA, Williams T, McCann B. Repairs by trephination and suturing of longitudinal injuries in the avascular area of the meniscus in goats. Am J Sports Med. 1995;23(1):35-41.
10. Zhang ZN, Tu KY, Xu YK, Zhang WM, Liu ZT, Ou SH. Treatment of longitudinal injuries in avascular area of meniscus in dogs by trephination. Arthroscopy. 1988;4(3):151-159.
11. Ochi M, Uchio Y, Okuda K, Shu N, Yamaguchi H, Sakai Y. Expression of cytokines after meniscal rasping to promote meniscal healing. Arthroscopy. 2001;17(7):724-731.
12. Okuda K, Ochi M, Shu N, Uchio Y. Meniscal rasping for repair of meniscal tear in the avascular zone. Arthroscopy. 1999;15(3):281-286.
13. Uchio Y, Ochi M, Adachi N, Kawasaki K, Iwasa J. Results of rasping of meniscal tears with and without anterior cruciate ligament injury as evaluated by second-look arthroscopy. Arthroscopy. 2003;19(5):463-469.
14. Tasto JP, Cummings J, Medlock V, Harwood F, Hardesty R, Amiel D. The tendon treatment center: new horizons in the treatment of tendinosis. Arthroscopy. 2003;19(suppl 1):213-223.
15. Tasto JP. The role of radiofrequency-based devices in shaping the future of orthopedic surgery. Orthopedics. 2006;29(10):874-875.
16. Tasto JP, Cummings J, Medlock V, Hardesty R, Amiel D. Microtenotomy using a radiofrequency probe to treat lateral epicondylitis. Arthroscopy. 2005;21(7):851-860.
17. Higuchi H, Kimura M, Kobayashi A, Hatayama K, Takagishi K. A novel treatment of hypermobile lateral meniscus with monopolar radiofrequency energy. Arthroscopy 2004;20 (suppl 2):1-5.
18. Tonna EA, Cronkite EP. The periosteum. Autoradiographic studies on cellular proliferation and transformation utilizing tritiated thymidine. Clin Orthop. 1963;30:218-233.
19. Madewell BR. Serum thymidine kinase activity: an alternative to histologic markers of cellular proliferation in canine lymphoma. J Vet Intern Med. 2004;18(5):595-596.
20. Mujoomdar M, Bennett A, Hoskin D, Blay J. Adenosine stimulation of proliferation of breast carcinoma cell lines: evaluation of the [3H]thymidine assay system and modulatory effects of the cellular microenvironment in vitro. J Cell Physiol. 2004;201(3):429-438.
21. Vander Borght T, Labar D, Pauwels S, Lambotte L. Production of [2-11C]thymidine for quantification of cellular proliferation with PET. Int J Rad Appl Instrum A. 1991;42(1):103-104.
22. Spindler KP, Mayes CE, Miller RR, Imro AK, Davidson JM. Regional mitogenic response of the meniscus to platelet-derived growth factor (PDGF-AB). J Orthop Res. 1995;13(2):201-207.
23. Thomopoulos S, Zaegel M, Das R, et al. PDGF-BB released in tendon repair using a novel delivery system promotes cell proliferation and collagen remodeling. J Orthop Res. 2007;25(10):1358-1368.
24. Pennock AT, Robertson CM, Emmerson BC, Harwood FL, Amiel D. Role of apoptotic and matrix-degrading genes in articular cartilage and meniscus of mature and aged rabbits during development of osteoarthritis. Arthritis Rheum. 2007;56(5):1529-1536.
25. Allen RT, Tasto JP, Cummings J, Robertson CM, Amiel D. Meniscal debridement with an arthroscopic radiofrequency wand versus an arthroscopic shaver: comparative effects on menisci and underlying articular cartilage. Arthroscopy. 2006;22(4):385-393.
26. Figueroa D, Calvo R, Vaisman A, et al. Bipolar radiofrequency in the human meniscus. Comparative study between patients younger and older than 40 years of age. Knee. 2007;14(5):357-360.
27. Friedman M, LoSavio P, Ibrahim H, Ramakrishnan V. Radiofrequency tonsil reduction: safety, morbidity, and efficacy. Laryngoscope. 2003;113(5):882-887.
28. Hall DJ, Littlefield PD, Birkmire-Peters DP, Holtel MR. Radiofrequency ablation versus electrocautery in tonsillectomy. Otolaryngol Head Neck Surg. 2004;130(3):300-305.
29. Kaplan H, Gat A. Clinical and histopathological results following TriPollar radiofrequency skin treatments. J Cosmet Laser Ther. 2009;11(2):78-84.
30. Mancini PF. Coblation: a new technology and technique for skin resurfacing and other aesthetic surgical procedures. Aesthetic Plast Surg. 2001;2595):372-377.
31. Penka I, Kaplan Z, Sefr R, Sirotek L, Eber Z, Ondrák M. Use of radiofrequency ablation in the treatment of malignant liver lesions. Hepatogastroenterology. 2008;55(82-83):562-567.
32. Tasto JP, Ash SA. Current uses of radiofrequency in arthroscopic knee surgery. Am J Knee Surg. 1999;12(3):186-191.
33. Amiel D, Ball ST, Tasto JP. Chondrocyte viability and metabolic activity after treatment of bovine articular cartilage with bipolar radiofrequency: an in vitro study. Arthroscopy. 2004;20(5):503-510.
34. Barry KJ, Kaplan J, Connolly RJ, et al. The effect of radiofrequency-generated thermal energy on the mechanical and histologic characteristics of the arterial wall in vivo: implications for radiofrequency angioplasty. Am Heart J. 1989;117(2):332-341.
35. Hoch DH, Grodzinsky AJ, Koob TJ, Albert ML, Eyre DR. Early changes in material properties of rabbit articular cartilage after meniscectomy. J Orthop Res. 1983;1(1):4-12.
36. Thompson AM, Stockwell RA. An ultrastructural study of the marginal transitional zone in the rabbit knee joint. J Anat. 1983;136(Pt 4):701-713.
1. Fauno P, Nielsen AB. Arthroscopic partial meniscectomy: a long-term follow-up. Arthroscopy. 1992;8(3):345-349.
2. Lynch MA, Henning CE, Glick KR, Jr. Knee joint surface changes. Long-term follow-up meniscus tear treatment in stable anterior cruciate ligament reconstructions. Clin Orthop. 1983;172:148-153.
3. Roos H, Lauren M, Adalberth T, Roos EM, Jonsson K, Lohmander LS. Knee osteoarthritis after meniscectomy: prevalence of radiographic changes after twenty-one years, compared with matched controls. Arthritis Rheum. 1998;41(4):687-693.
4. Hennerbichler A, Moutos FT, Hennerbichler D, Weinberg JB, Guilak F. Repair response of the inner and outer regions of the porcine meniscus in vitro. Am J Sports Med. 2007;35(5):754‑762.
5. Gershuni DH, Hargens AR, Danzig LA. Regional nutrition and cellularity of the meniscus. Implications for tear and repair. Sports Med. 1988;5(5):322-327.
6. Gershuni DH, Skyhar MJ, Danzig LA, Camp J, Hargens AR, Akeson WH. Experimental models to promote healing of tears in the avascular segment of canine knee menisci. J Bone Joint Surg Am. 1989;71(9):1363-1370.
7. Papachristou G, Efstathopoulos N, Plessas S, Levidiotis C, Chronopoulos E, Sourlas J. Isolated meniscal repair in the avascular area. Acta Orthop Belg. 2003;69(4):341-345.
8. Fox JM, Rintz KG, Ferkel RD. Trephination of incomplete meniscal tears. Arthroscopy. 1993;9(4):451-455.
9. Zhang Z, Arnold JA, Williams T, McCann B. Repairs by trephination and suturing of longitudinal injuries in the avascular area of the meniscus in goats. Am J Sports Med. 1995;23(1):35-41.
10. Zhang ZN, Tu KY, Xu YK, Zhang WM, Liu ZT, Ou SH. Treatment of longitudinal injuries in avascular area of meniscus in dogs by trephination. Arthroscopy. 1988;4(3):151-159.
11. Ochi M, Uchio Y, Okuda K, Shu N, Yamaguchi H, Sakai Y. Expression of cytokines after meniscal rasping to promote meniscal healing. Arthroscopy. 2001;17(7):724-731.
12. Okuda K, Ochi M, Shu N, Uchio Y. Meniscal rasping for repair of meniscal tear in the avascular zone. Arthroscopy. 1999;15(3):281-286.
13. Uchio Y, Ochi M, Adachi N, Kawasaki K, Iwasa J. Results of rasping of meniscal tears with and without anterior cruciate ligament injury as evaluated by second-look arthroscopy. Arthroscopy. 2003;19(5):463-469.
14. Tasto JP, Cummings J, Medlock V, Harwood F, Hardesty R, Amiel D. The tendon treatment center: new horizons in the treatment of tendinosis. Arthroscopy. 2003;19(suppl 1):213-223.
15. Tasto JP. The role of radiofrequency-based devices in shaping the future of orthopedic surgery. Orthopedics. 2006;29(10):874-875.
16. Tasto JP, Cummings J, Medlock V, Hardesty R, Amiel D. Microtenotomy using a radiofrequency probe to treat lateral epicondylitis. Arthroscopy. 2005;21(7):851-860.
17. Higuchi H, Kimura M, Kobayashi A, Hatayama K, Takagishi K. A novel treatment of hypermobile lateral meniscus with monopolar radiofrequency energy. Arthroscopy 2004;20 (suppl 2):1-5.
18. Tonna EA, Cronkite EP. The periosteum. Autoradiographic studies on cellular proliferation and transformation utilizing tritiated thymidine. Clin Orthop. 1963;30:218-233.
19. Madewell BR. Serum thymidine kinase activity: an alternative to histologic markers of cellular proliferation in canine lymphoma. J Vet Intern Med. 2004;18(5):595-596.
20. Mujoomdar M, Bennett A, Hoskin D, Blay J. Adenosine stimulation of proliferation of breast carcinoma cell lines: evaluation of the [3H]thymidine assay system and modulatory effects of the cellular microenvironment in vitro. J Cell Physiol. 2004;201(3):429-438.
21. Vander Borght T, Labar D, Pauwels S, Lambotte L. Production of [2-11C]thymidine for quantification of cellular proliferation with PET. Int J Rad Appl Instrum A. 1991;42(1):103-104.
22. Spindler KP, Mayes CE, Miller RR, Imro AK, Davidson JM. Regional mitogenic response of the meniscus to platelet-derived growth factor (PDGF-AB). J Orthop Res. 1995;13(2):201-207.
23. Thomopoulos S, Zaegel M, Das R, et al. PDGF-BB released in tendon repair using a novel delivery system promotes cell proliferation and collagen remodeling. J Orthop Res. 2007;25(10):1358-1368.
24. Pennock AT, Robertson CM, Emmerson BC, Harwood FL, Amiel D. Role of apoptotic and matrix-degrading genes in articular cartilage and meniscus of mature and aged rabbits during development of osteoarthritis. Arthritis Rheum. 2007;56(5):1529-1536.
25. Allen RT, Tasto JP, Cummings J, Robertson CM, Amiel D. Meniscal debridement with an arthroscopic radiofrequency wand versus an arthroscopic shaver: comparative effects on menisci and underlying articular cartilage. Arthroscopy. 2006;22(4):385-393.
26. Figueroa D, Calvo R, Vaisman A, et al. Bipolar radiofrequency in the human meniscus. Comparative study between patients younger and older than 40 years of age. Knee. 2007;14(5):357-360.
27. Friedman M, LoSavio P, Ibrahim H, Ramakrishnan V. Radiofrequency tonsil reduction: safety, morbidity, and efficacy. Laryngoscope. 2003;113(5):882-887.
28. Hall DJ, Littlefield PD, Birkmire-Peters DP, Holtel MR. Radiofrequency ablation versus electrocautery in tonsillectomy. Otolaryngol Head Neck Surg. 2004;130(3):300-305.
29. Kaplan H, Gat A. Clinical and histopathological results following TriPollar radiofrequency skin treatments. J Cosmet Laser Ther. 2009;11(2):78-84.
30. Mancini PF. Coblation: a new technology and technique for skin resurfacing and other aesthetic surgical procedures. Aesthetic Plast Surg. 2001;2595):372-377.
31. Penka I, Kaplan Z, Sefr R, Sirotek L, Eber Z, Ondrák M. Use of radiofrequency ablation in the treatment of malignant liver lesions. Hepatogastroenterology. 2008;55(82-83):562-567.
32. Tasto JP, Ash SA. Current uses of radiofrequency in arthroscopic knee surgery. Am J Knee Surg. 1999;12(3):186-191.
33. Amiel D, Ball ST, Tasto JP. Chondrocyte viability and metabolic activity after treatment of bovine articular cartilage with bipolar radiofrequency: an in vitro study. Arthroscopy. 2004;20(5):503-510.
34. Barry KJ, Kaplan J, Connolly RJ, et al. The effect of radiofrequency-generated thermal energy on the mechanical and histologic characteristics of the arterial wall in vivo: implications for radiofrequency angioplasty. Am Heart J. 1989;117(2):332-341.
35. Hoch DH, Grodzinsky AJ, Koob TJ, Albert ML, Eyre DR. Early changes in material properties of rabbit articular cartilage after meniscectomy. J Orthop Res. 1983;1(1):4-12.
36. Thompson AM, Stockwell RA. An ultrastructural study of the marginal transitional zone in the rabbit knee joint. J Anat. 1983;136(Pt 4):701-713.
Inpatient Pediatric Service Redesign
Given its positive effects on improving effectiveness and efficiency, Lean Six Sigma (LSS) is a business approach that is receiving a great deal of attention in the healthcare industry.[1, 2, 3, 4, 5, 6, 7] Although there are differences between Lean and Six Sigma, at their core they are both customer‐centered, quality methodologies designed to improve process efficiency and product quality through waste elimination, creating standardized work and reducing variation.[8]
Six Sigma is a rigorous problem‐focused process improvement method that focuses on defect removal, variation reduction, and customer satisfaction that relies heavily on statistical analysis. It includes 5 steps: define, measure, analyze, improvement, and control.[7, 8] Six Sigma assumes through variation reduction, defect removal, and meeting customer specifications, the performance of the organization can be improved and also meet the requirements of the customer.[8]
Lean is more process‐focused. It places emphasis on creating flow by removing waste and getting the steps of any given process in the right sequence.[8] In Lean terms, waste is defined as anything that the customer does not value and anything that is not done right the first time.[9] This category of waste is termed nonvalue adding and unnecessary. It is estimated that 30% to 50% of all steps of hospital processes are nonvalue adding and unnecessary, and therefore can be defined as waste.[10] Lean identifies 8 different types of nonvalue adding and unnecessary wastes. They are defects and rework, overproduction, waiting, nonutilization of resources, transport, inventory, motion, and extra processing. Waste creates delays that negatively impact patient care and reduce healthcare productivity.[10] Therefore, it makes sense to apply Lean concepts of waste identification and elimination to improve process efficiency. For example, when a facility is at or exceeds its bed capacity, any delay in discharge creates throughput delays throughout the hospital.[5] Discharge delays often result in emergency department (ED) overcrowding, and also affects a hospital's ability to accommodate internal downgrades and outside referrals in a timely fashion.[11, 12] However, because the sequence of steps of the discharge process is variable and not standardized, the goal to achieve early discharges remains elusive.[13]
There are emerging data to support that current rounding censuses exceed most hospitalist's abilities to deliver safe and efficient care.[12, 14, 15, 16] It is unclear what that threshold should be, but the current industry standard has nonacademic hospitalists seeing 15 patients per day. Therefore, high patient censuses could be contributing to delays in patient discharge times that effect hospital throughput. We speculated that by implementing a lean, quick‐strike approach[17] designed to improve the sequencing of housestaff, attending, and nursing work by eliminating the wastes of rework, waiting, extra processing, and nonutilization of physician resources by restaffing, we could improve patient discharge times. We augmented the intervention by creating standardized workflow expectations, a discharge checklist, and implemented daily interdisciplinary discharge planning huddles.
We hypothesized these interventions would improve the median time of discharge order entry and time of patient discharge. Primary outcome measures were: (1) the change in time of discharge order and discharge time and (2) the proportion of patients discharged before noon and 2 pm. Secondary outcomes that were used as balance measures were length of stay (LOS) and 7‐day, 14‐day, and 30‐day readmission rates.
METHODS
Study Design
This was a prospective quality improvement intervention with concurrent controls aimed to determine if discharge efficiency could be improved by load‐balancing our service line with existing faculty and residents, creating daily standard work using a discharge checklist and interdisciplinary huddles (see Supporting Figure 1 through Supporting Figure 3 in the online version of this article). All discharge data were collected as part of our medical center's Department of Logistics standard data collection procedures using solutions from TeleTracking Technologies, Inc. (Pittsburgh, PA). All patients discharged Monday through Friday from the pediatric hospitalist service prior to the 6‐month high‐census period (before intervention) and the 6‐month high‐census period (intervention period) were included in the study. To serve as our control, we collected the same discharge data during the same time periods for the remaining services of the children's hospital. This study was approved by Penn State Hershey Medical Center's institutional review board.
Study Setting
The study was conducted at the Penn State Hershey Children's Hospital (PSHCH), which is a physically free‐standing 133‐bed university‐based tertiary care hospital located in central Pennsylvania. The hospital has 36 pediatric medical/surgical beds located in 2 units (1 general and 1 intermediate care). PSHCH performs approximately 4100 admissions per year, of which approximately 1100 are performed by the Division of Pediatric Hospital Medicine. Our division is composed of 8 academic hospitalists with 1 to 20+ years' experience. Historically, the months of October through April are months when our service‐line has average daily censuses (ADC) that routinely exceed 12 patients per hospitalist. During these months, the median times patient discharge orders are placed and patient discharges occur historically approach 2 pm and 4 pm, respectively, and exceed our internal benchmark by 2 hours. Discharges from the remaining medical and surgical service lines at PSHCH that occurred Monday through Friday during the concurrent pre‐ and postintervention time periods served as the control group.
Needs Assessment and LSS
Traditionally, morning patient rounds are allotted approximately 180 minutes. Therefore, a rounding team can only be expected to spend 13 minutes or less per patient when the census exceeds 12 patients. The cycle time to perform 1 discharge using our electronic medical record is approximately 20 minutes, which is almost 10 minutes longer than the allotted time per patient. During high‐census months, our service averages 4 to 5 discharges per day. To accommodate performing discharges during rounds would require spending 80 to 100 minutes of the 180 available minutes. This would leave only 80 to 100 minutes to see the remaining 8 to 10 patients. As a result of these constraints, discharges are typically completed by the residents in unsupervised batches each afternoon following the noon conference (Figure 1A).
Because LSS focuses on eliminating nonvalue adding and unnecessary waste by load balancing processes and minimizing batching tasks,[8] this approach should lead one to question whether the current rounding model that requires 1 attending to see >12 high‐acuity patients with a maximum of 13 minutes per patient is system design flaw that leads to errors and inefficiency,[16] Theoretically, having an additional attending present would allow teams to resequence the work on smaller batches of patients and double the time to spend on each patient. This could create the opportunity to do value‐added work at the bedside in the presence of the family and nurse and eliminate the amount of nursing rework and time spent as work in progress on dischargeable patients (Figure 1).
Additionally, improving discharge efficiency creates virtual beds. Virtual beds permit hospitals to accommodate additional admissions despite operating with a fixed‐bed capacity. A way to calculate virtual beds is to calculate the reduction in LOS, and multiply that by the number of admissions per year divided by 36518 (see Supporting Figure 4 in the online version of this article). Our study was intended to determine the impact of discharge efficiency on this metric.
Intervention
We re‐structured our service line in a way that would balance both physician workload needs and patient expectations. To accomplish this, off service attendings were reallocated to round with a smaller resident team on fewer patients for the duration of the 6‐month study. Each member of the division agreed to work an average of 3 more weeks per year. One work day was estimated to be approximately 10 hours and 1 work week equaled 5 days, which asked for 150 hours of additional work per year. Because there increases in functional FTEs, the 2 teams consolidated into 1 team each weekend, to meet the group requirement that this model not result in additional weekend coverage. A balanced workload also theoretically allows the physician to spend more time at the bedside in direct patient care and resident education activities/observations.
In addition to reallocating physician and resident resources, our model created standard work expectations to reduce the variations in physician work sequences that can account for delayed discharge orders and delayed discharges, which is also an LSS principle. The intervention consisted of 3 changes: (1) fundamentally altering the composition of the rounding teams to optimize the provider: patient ratio; (2) defining rounding standards to expedite discharges; and (3) establishing a daily predischarge planning process.
The preintervention team typically had 1 attending, 1 to 2 senior residents, and 2 to 3 interns. The intervention period required creating 2 independently functioning teams, each composed of 1 hospitalist attending, and a minimum of 1 senior and 1 intern. The intervention occurred November through April, when the censuses predictably exceed 12 patients for the rounding attending. Because both teams functioned independently, all of the patients were divided equally between the 2 teams. Each team carried a panel of patients that included new, established, and dischargeable patients (Figure 1). We did not compare the number of provider handoffs before and during the intervention or time spent per patient.
Because the intervention required increasing the number of weeks on‐service by 2 to 3weeks per physician to reduce clinical work time, it meant redeploying previously off‐service attendings to coincide with peak demands. This aspect of the intervention made group buy‐in mandatory. The group agreed to distribute the predictably heavy workload that usually falls on 1 attending by adding a second attending for the busiest 6 months of the year. Our division voted unanimously to adopt this model despite the increase in service time, as long as weekend coverage was not increased.
As part of the intervention, we created standard work expectations within our division to (1) start rounds on dischargeable patients who were identified the prior evening during the (2) interdisciplinary huddle, and (3) have the entire departure process completed at the bedside using a discharge checklist (see Supporting Figure 1 through Figure 3 in the online version of this article). The expectations included a standard script for beginning rounds, selecting patients who could be discharged first, and completing all necessary discharge computer work at the bedside, before proceeding to the next patient. The daily predischarge huddle was instituted each afternoon to prepare discharges that were expected to occur the following day. The huddles were attended by care coordinators, social workers, and both medical teams. During the huddle, the team discussed anticipated discharges, scheduled follow‐up appointments and testing, faxed necessary prescriptions, and arranged any needed home services.
Inclusion and Exclusion Criteria for Patients
All patients discharged from the pediatric hospitalist inpatient service between Monday and Friday from April 8, 2013 to October 25, 2013 (preimplementation cohort) and October 28, 2013 to April 18, 2014 (postimplementation cohort) were eligible for inclusion. This included admitted patients and observation status patients. Patients discharged from the remaining PSHCH medical and surgical service lines were included in the control group analysis using the above criteria.
OUTCOME MEASURES
Throughput and Patient‐Level Outcomes
Primary outcomes included (1) time of electronic discharge order placement, (2) actual patient discharge time, (3) proportion of patients discharged before noon and 2 pm, (4) 7‐day, 14‐day, and 30‐day readmission rates, (5) length of stay (LOS), and (6) average daily census (ADC).
Statistical Analysis
The null hypothesis was that there would be no difference in discharge order time, discharge time, LOS, readmission rates, and daily discharges in the preintervention group compared to the intervention group. For time of order placement and actual patient discharge, the significance was assessed using Wilcoxon rank sum test and expressed as median time among the groups. Patient discharge before noon/2 pm was assessed by a logistic regression model. The predictor being the intervention group with the results expressed as odds ratios of discharge before noon/2 pm comparing the intervention group to the preintervention group. Readmission rates were assessed using a [2] test to see if there was a significant difference from what would be expected. Last, LOS and ADC were assessed by a Student t test and expressed as the means. The data were analyzed using SAS version 9.3 (SAS Institute, Cary, NC).
RESULTS
For our division's service line, both the ADC and number of patients discharged per day were significantly higher during the intervention months (Table 1). By comparison, the control group had a significantly lower ADC and lower average of discharges per day in the intervention time period. The new model permitted the teams to enter discharge orders earlier in the day, which ultimately lead to earlier patient discharges. The additive effect of the 3 interventions had a statistically significant effect on process efficiency metrics (Table 1). The median discharge order entry time decreased by 200 minutes from 14:05 to 10:45, and the median time of patient discharge decreased by 93 minutes from 15:48 to 14:15. By comparison, the median time of discharge order entry decreased 13:13 to 12:56 pm, but the median time of discharge increased 5 minutes 14:45 versus 14:50 in the control group. A significantly higher proportion of patients were discharged by noon (27% vs 14%; P<0.0001; odds ratio [OR]:2.2; 95% confidence interval [CI]: 1.6‐3.1) and by 2 pm during the intervention period (47% vs 30%; P<0.0001; OR: 2.1; 95% CI: 1.6‐2.7). There was no observed difference in the proportion of patients who were discharged by noon or 2 pm in the control group. Finally, in the intervention group, approximately 50% of patients had discharge orders entered before noon compared to 23% in the control group (Figure 2). The intervention demonstrated statistical significance in shifting the time of discharge order entry and the time of patient discharge when compared to the relatively less burdened PSHCH control group (Figures 2 and 3). As seen in Figure 4, the results were sustained for the duration of the study and appeared to improve throughout intervention. Finally, readmission rates at 7, 14, and 30 days postdischarge and LOS were not negatively affected (Table 1) in either the intervention or control group.
| Outcomes | Experimental Model | Control Group | ||||
|---|---|---|---|---|---|---|
| Preintervention, n=421 | Intervention, n=552 | P Value | Preintervention, n=1,390 | Intervention, n=1,146 | P Value | |
| Average daily census | 9.7 | 12.4 | <0.0001 | 45.9 | 43.4 | 0.002 |
| Discharges per day | 3.1 | 4.5 | <0.00001 | 9.5 | 9.2 | 0.419 |
| Average length of stay | 3.1 | 3.0 | 0.864 | 6.3 | 5.9 | 0.714 |
| Discharge order time, median | 14:05 | 10:45 | <0.0001 | 13:13 | 12:56 | 0.053 |
| Discharge from hospital, median | 15:48 | 14:15 | <0.0001 | 14:45 | 14:50 | 0.113 |
| Patients discharged before noon | 59 (14%) | 147 (27%) | <0.0001 | 176 (13%) | 170 (15%) | 0.138 |
| Patients discharged before 2 pm | 128 (30%) | 261 (47%) | <0.0001 | 519 (38%) | 447 (39%) | 0.512 |
| 7‐day readmission rates | 3.1% | 3.5% | 0.965 | 6.7% | 6.8% | 0.970 |
| 14‐day readmission rates | 5.8% | 5.8% | 0.981 | 12.0% | 13.5% | 0.301 |
| 30‐day readmission rates | 9.4% | 9.1% | 0.703 | 20.0% | 20.6% | 0.705 |
DISCUSSION
We demonstrated a statistically significant and what appears to be a sustainable improvement in median discharge order times, discharge times, and proportion of discharges by noon and 2 pm. Ours was the only service line in our medical center to achieve a median time discharge before our institution's internal metric of 2 pm and maintain it for 3 consecutive months. Additionally, the process demonstrated consistent performance independent of the varying styles and experience of the rounding attending during the busiest months of the year without incurring a negative impact on LOS or readmission rates.
Although our intervention demonstrated statistical significance in shifting the discharge distribution curves by almost 2 hours, more relevant is its potential clinical and financial impact. First, it puts our hospital in compliance with the Joint Commission's recommendations standard LD.04.03.1, stipulating that hospitals measure and set goals for mitigating and managing the flow of patients though the hospital. Second, our findings confirm the results of earlier studies suggesting that shifting discharge times could likely be achieved without the additional staff, but with alterations in staff shift scheduling.[11] Third, by doing required discharge work at the bedside and making it available earlier in the day, every day, we consistently reduced patient waiting along the entire supply chain.
Advancing the discharge time creates virtual beds that allow our facility to theoretically accommodate new patients. Using the calculation in the Methods section (see Supporting Figure 4 in the online version of this article) on how to calculate virtual beds, we determined that our intervention created between 0.30 and 0.38 virtual beds in a hospital with only 72 beds. We calculated that this would create 6.8 more open bed hours per day, 74 additional patient days per year, and assuming patients were waiting for the beds and rapid bed turnover, our intervention theoretically created the capacity to accommodate approximately 25 additional admissions per year (see Supporting Figure 4 in the online version of this article). As the only children's hospital in the region, this intervention will enable our organization to provide timelier access and possibly reduce time sensitive medical errors.
Timelier evaluations also have revenue potential by eliminating lost referrals, thus turning waste into value. When comparing the previous year's high‐census monthsOctober through Marchthere were 20 lost referrals due to lack of bed capacity, as compared to zero lost referrals during our intervention period. By accommodating these 20 additional admissions, we estimated this generated between $275,000 and $412,000 dollars in additional revenue without additional resources but simply staffing to demand.
Finally, when we looked at patient satisfaction metrics obtained through Press Ganey (PG), comparing the time periods we observed that overall satisfaction increased from the 91 percentile to the 94 percentile, trust in doctor increased from the 20 percentile to the 70 percentile, and would recommend this hospital to others increased from the 53 percentile to the 75 percentile. Interestingly, despite being a study that improved discharge efficiency, none of the discharge metrics gathered by PG improved. It is possible that this is a limitation of the PG survey, or could reflect the possibility that our new process exposed that our discharge order entry and discharge processes are misaligned.
When we surveyed the nursing staff and members of the division regarding whether or not to continue the intervention rounding model, 75% and 100%, respectively, voted in favor of continuing with the intervention model. Unfortunately, housestaff satisfaction was not measured for this study.
Despite more weeks in the hospital, but because there was better process sequencing, our providers indicated that because the workload of the primary attending was reduced and the workload for the additional attending was light, there was ample time to engage in afternoon nonclinical activities (Figure 1B). In fact, several division members assumed departmental and educational leadership positions, and others volunteered to facilitate highly valued, but unsubsidized, afternoon medical student and resident teaching sessions that occurred solely as a result of the resequenced and redistributed clinical load.
There are limitations to this study. First, because 3 interventions were implemented simultaneously, it is difficult to identify which component of the intervention was the primary driver for the measured differences. It is conceivable the proactive discharge planning that occurred during the afternoon predischarge planning huddles allowed the teams to complete discharge requirements the night before anticipated discharge therefore expediting the next morning's discharge. A second limitation was not simultaneously comparing the traditional rounding structure with the experimental model. One could argue that the improved efficiency we observed was not due to any of the interventions and represents secular trends that all residents' teams experience through the course of the year as they get more adept at performing patient discharges. However, when we compare our performance to the control group performance, this efficiency trend was not present. Additionally, it was possible that attendings were so result focused that they delayed discharges if the 2 pm discharge goal was missed for that day and planned for early discharges the following morning. If this behavior occurred, this would likely have been reflected as increases in our LOS data, however this was not observed. Third, because our preintervention data reflected discharge behaviors during a low‐census period, it is possible that there was less urgency to discharge patients when bed capacity issues did not exist. Comparing the intervention period to a period when censuses are similar would better address this issue. Finally, although we assert that attending workload is a fundamental waste‐producing constraint in the discharge process, this study did not determine what the optimal patient census should be.
Most hospitalists struggle with finding a balance of meeting patient quality and administrative productivity demands.[16] Hospitalists at academic medical centers have the added demand of maintaining their educational mission. Since 2001, the Institute of Medicine[19] has advocated process re‐engineering using more patient‐centered approaches. A recent study found that when hospitals reach capacity, the excess workload placed on internal medicine hospitalists reduces efficiency and increases costs.[16] Interestingly, in a study conducted by McMahon et al., they found that reducing team censuses by 50%, resident educational outcomes can be improved.[20] Similar to this study, our study reduced attending workload by 50% with the goal to assess the impact on discharge efficiency rather than educational outcomes. Also similar to that study, we radically altered the operational model in which physicians historically had functioned.[14, 20] Because the rounding structures in both studies reduced patient:provider ratios we believe that our model will successfully balance education, patient quality, and productivity.
LSS, when thoughtfully applied to the problems we face, could be part of the solution. It delivers quick results without large capital investments, by identifying and implementing high‐leverage changes that value a creative solution before a capital investment. One of the strengths of this model is that it does not require substantial financial investment to produce these outcomes. Because the morning clinical loads were more evenly distributed during the busiest months of the year, our division members were able to engage in nonclinical duties and teaching sessions, both of which often required afternoon commitments, but permitted us to balance work and professional achievement (Figure 1B). Finally, as part of any new process, one must consider the factors that influence its sustainability: provider level satisfaction, impact of the process change, and remuneration. Because the intervention reduced lost referrals, the departmental and institutional leadership agreed to financially incentivize the value‐generating potential this intervention had on increasing patient access by facilitating organizational throughput. Therefore, having met the three aforementioned elements, we believe this model is sustainable.
Although many studies remain results focused with aims at documenting how hospital processes fail when overburdened, our study takes a novel process‐focused approach to look at how processes can excel during periods of high demands, simply by reallocating existing resources.
Medicine is in the midst of multiple paradigm shifts involving resident work hour reduction, public safety reporting, reimbursement constraints, and value‐driven care, to name a few. Whether we take a resident or patient‐centered approach, it seems highly unlikely that the current approaches will meet these demands without making significant changes in how we deliver care. Next steps should include construction of a value stream map (VSM), with the input of all of the process stakeholders, that diagrams the entire discharge process. The VSM should highlight all nonvalue adding steps and eliminate them. They are likely a contributing cause to the disproportionate reduction in time of discharge order entry (200 minutes) versus actual discharge (93 minutes) seen in our study. Future work needs to establish the generalizability and sustainability of this model across other hospital service lines. Future studies should establish if this model has sustained impact on patient, provider, and resident satisfaction and overall system efficiency (ED boarding), with aims to quantify the revenue generating potential that occurs through waste elimination.
We close with the following thought: [T]o ask people to make different decisions without fundamentally changing the equation presented to them is wrong. If we wish to change the types of decisions our people make, we owe it to them to design and build processes that will put them in a position to succeed.[21]
Acknowledgements
The authors recognize the contributions made by members of the Division of Pediatric Hospital Medicine at PSHCH, without which this project would not have been possible. They are: Drs. Marta Biderman, Anika Kumar, Margaret Mikula, Chris O'Hara, Brandon Smith, and Ron Williams, and Heidi Wolf, and Lyndsay Gardener, CRNP. This project would not have been possible without the help from Brenda Ruhl, Manjula Narasimhan, and Heather Boyle from the Department of Logistics. Finally, the authors would like to thank the following for their thoughtful reviews of this manuscript: Drs. Lisa Scalzi, Barbara Ostrov, Jed Gonzalo, Chris Sciamanna, and Matt Wain.
Disclosures: Nothing to report.
- . Theory of constraints—a review of the philosophy and its applications. Int J Oper Prod Man. 1998;18(4):336–355.
- , , , et al. Use of lean and six sigma methodology to improve operating room efficiency in a high‐volume tertiary‐care academic medical center. J Am Coll Surg. 2011;213(1):83–92; discussion 93–84.
- , . The promise of Lean in health care. Mayo Clin Proc. 2013;88(1):74–82.
- , , . Assessing the evidence of Six Sigma and Lean in the health care industry. Qual Manag Health Care. 2010;19(3):211–225.
- , , , et al. Redesigning care at the Flinders Medical Centre: clinical process redesign using “lean thinking”. Med J Aust. 2008;188(6 suppl):S27–S31.
- , , , , . Impact of 5 years of lean six sigma in a University Medical Center. Qual Manag Health Care. 2012;21(4):262–268.
- , , , et al. A Lean Six Sigma quality improvement project to increase discharge paperwork completeness for admission to a comprehensive integrated inpatient rehabilitation program. Am J Med Qual. 2013;28(4):301–307.
- . How to compare Six Sigma, lean and the theory of constraints—a framework for choosing what's best for your organization. Qual Prog. 2002;35(3):73–78.
- , , , , . Application of lean manufacturing techniques in the Emergency Department. J Emerg Med. 2009;37(2):177–182.
- . Lean Hospitals: Improving Quality, Patient Safety, and Employee Engagement. 2nd ed. New York, NY: Productivity Press/Taylor 2012.
- , , , , , . The relationship between inpatient discharge timing and emergency department boarding. J Emerg Med. 2012;42(2):186–196.
- , , , . Unravelling relationships: Hospital occupancy levels, discharge timing and emergency department access block. Emerg Med Aust. 2012;24(5):510–517.
- . Need to speed up discharges? The pros and cons of putting discharges on the clock. Today's Hospitalist. 2013. Accessed November 2014. Available at: http://www.todayshospitalist.com/index.php?b=articles_read3(2):144–150.
- , . Hospitalist staffing requirements. Eff Clin Pract. 1999;2(3):126–130.
- , , , , . Effect of hospitalist workload on the quality and efficiency of care. JAMA Intern Med. 2014;174(5):786–793.
- . Accelerating Lean Six Sigma Results: How to Achieve Improvement Excellence in the New Economy. Plantation, FL: J. Ross Publishing; 2011.
- . “Virtual bed capacity” may offer revenue boost for hospitals. Healthcare Finance. Accessed October 2014. Available at: http://www.healthcarefinancenews.com/blog/virtual‐bed‐capacity‐may‐offer‐revenue‐boost‐hospitals. Published November 30, 2011.
- Institute of Medicine. Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy Press; 2001.
- , , , , . Evaluation of a redesign initiative in an internal‐medicine residency. N Engl J Med. 2010;362(14):1304–1311.
- John Kim and Associates website. Available at: http://www.johnkimconsulting.com. Accessed June 2014.
Given its positive effects on improving effectiveness and efficiency, Lean Six Sigma (LSS) is a business approach that is receiving a great deal of attention in the healthcare industry.[1, 2, 3, 4, 5, 6, 7] Although there are differences between Lean and Six Sigma, at their core they are both customer‐centered, quality methodologies designed to improve process efficiency and product quality through waste elimination, creating standardized work and reducing variation.[8]
Six Sigma is a rigorous problem‐focused process improvement method that focuses on defect removal, variation reduction, and customer satisfaction that relies heavily on statistical analysis. It includes 5 steps: define, measure, analyze, improvement, and control.[7, 8] Six Sigma assumes through variation reduction, defect removal, and meeting customer specifications, the performance of the organization can be improved and also meet the requirements of the customer.[8]
Lean is more process‐focused. It places emphasis on creating flow by removing waste and getting the steps of any given process in the right sequence.[8] In Lean terms, waste is defined as anything that the customer does not value and anything that is not done right the first time.[9] This category of waste is termed nonvalue adding and unnecessary. It is estimated that 30% to 50% of all steps of hospital processes are nonvalue adding and unnecessary, and therefore can be defined as waste.[10] Lean identifies 8 different types of nonvalue adding and unnecessary wastes. They are defects and rework, overproduction, waiting, nonutilization of resources, transport, inventory, motion, and extra processing. Waste creates delays that negatively impact patient care and reduce healthcare productivity.[10] Therefore, it makes sense to apply Lean concepts of waste identification and elimination to improve process efficiency. For example, when a facility is at or exceeds its bed capacity, any delay in discharge creates throughput delays throughout the hospital.[5] Discharge delays often result in emergency department (ED) overcrowding, and also affects a hospital's ability to accommodate internal downgrades and outside referrals in a timely fashion.[11, 12] However, because the sequence of steps of the discharge process is variable and not standardized, the goal to achieve early discharges remains elusive.[13]
There are emerging data to support that current rounding censuses exceed most hospitalist's abilities to deliver safe and efficient care.[12, 14, 15, 16] It is unclear what that threshold should be, but the current industry standard has nonacademic hospitalists seeing 15 patients per day. Therefore, high patient censuses could be contributing to delays in patient discharge times that effect hospital throughput. We speculated that by implementing a lean, quick‐strike approach[17] designed to improve the sequencing of housestaff, attending, and nursing work by eliminating the wastes of rework, waiting, extra processing, and nonutilization of physician resources by restaffing, we could improve patient discharge times. We augmented the intervention by creating standardized workflow expectations, a discharge checklist, and implemented daily interdisciplinary discharge planning huddles.
We hypothesized these interventions would improve the median time of discharge order entry and time of patient discharge. Primary outcome measures were: (1) the change in time of discharge order and discharge time and (2) the proportion of patients discharged before noon and 2 pm. Secondary outcomes that were used as balance measures were length of stay (LOS) and 7‐day, 14‐day, and 30‐day readmission rates.
METHODS
Study Design
This was a prospective quality improvement intervention with concurrent controls aimed to determine if discharge efficiency could be improved by load‐balancing our service line with existing faculty and residents, creating daily standard work using a discharge checklist and interdisciplinary huddles (see Supporting Figure 1 through Supporting Figure 3 in the online version of this article). All discharge data were collected as part of our medical center's Department of Logistics standard data collection procedures using solutions from TeleTracking Technologies, Inc. (Pittsburgh, PA). All patients discharged Monday through Friday from the pediatric hospitalist service prior to the 6‐month high‐census period (before intervention) and the 6‐month high‐census period (intervention period) were included in the study. To serve as our control, we collected the same discharge data during the same time periods for the remaining services of the children's hospital. This study was approved by Penn State Hershey Medical Center's institutional review board.
Study Setting
The study was conducted at the Penn State Hershey Children's Hospital (PSHCH), which is a physically free‐standing 133‐bed university‐based tertiary care hospital located in central Pennsylvania. The hospital has 36 pediatric medical/surgical beds located in 2 units (1 general and 1 intermediate care). PSHCH performs approximately 4100 admissions per year, of which approximately 1100 are performed by the Division of Pediatric Hospital Medicine. Our division is composed of 8 academic hospitalists with 1 to 20+ years' experience. Historically, the months of October through April are months when our service‐line has average daily censuses (ADC) that routinely exceed 12 patients per hospitalist. During these months, the median times patient discharge orders are placed and patient discharges occur historically approach 2 pm and 4 pm, respectively, and exceed our internal benchmark by 2 hours. Discharges from the remaining medical and surgical service lines at PSHCH that occurred Monday through Friday during the concurrent pre‐ and postintervention time periods served as the control group.
Needs Assessment and LSS
Traditionally, morning patient rounds are allotted approximately 180 minutes. Therefore, a rounding team can only be expected to spend 13 minutes or less per patient when the census exceeds 12 patients. The cycle time to perform 1 discharge using our electronic medical record is approximately 20 minutes, which is almost 10 minutes longer than the allotted time per patient. During high‐census months, our service averages 4 to 5 discharges per day. To accommodate performing discharges during rounds would require spending 80 to 100 minutes of the 180 available minutes. This would leave only 80 to 100 minutes to see the remaining 8 to 10 patients. As a result of these constraints, discharges are typically completed by the residents in unsupervised batches each afternoon following the noon conference (Figure 1A).

Because LSS focuses on eliminating nonvalue adding and unnecessary waste by load balancing processes and minimizing batching tasks,[8] this approach should lead one to question whether the current rounding model that requires 1 attending to see >12 high‐acuity patients with a maximum of 13 minutes per patient is system design flaw that leads to errors and inefficiency,[16] Theoretically, having an additional attending present would allow teams to resequence the work on smaller batches of patients and double the time to spend on each patient. This could create the opportunity to do value‐added work at the bedside in the presence of the family and nurse and eliminate the amount of nursing rework and time spent as work in progress on dischargeable patients (Figure 1).
Additionally, improving discharge efficiency creates virtual beds. Virtual beds permit hospitals to accommodate additional admissions despite operating with a fixed‐bed capacity. A way to calculate virtual beds is to calculate the reduction in LOS, and multiply that by the number of admissions per year divided by 36518 (see Supporting Figure 4 in the online version of this article). Our study was intended to determine the impact of discharge efficiency on this metric.
Intervention
We re‐structured our service line in a way that would balance both physician workload needs and patient expectations. To accomplish this, off service attendings were reallocated to round with a smaller resident team on fewer patients for the duration of the 6‐month study. Each member of the division agreed to work an average of 3 more weeks per year. One work day was estimated to be approximately 10 hours and 1 work week equaled 5 days, which asked for 150 hours of additional work per year. Because there increases in functional FTEs, the 2 teams consolidated into 1 team each weekend, to meet the group requirement that this model not result in additional weekend coverage. A balanced workload also theoretically allows the physician to spend more time at the bedside in direct patient care and resident education activities/observations.
In addition to reallocating physician and resident resources, our model created standard work expectations to reduce the variations in physician work sequences that can account for delayed discharge orders and delayed discharges, which is also an LSS principle. The intervention consisted of 3 changes: (1) fundamentally altering the composition of the rounding teams to optimize the provider: patient ratio; (2) defining rounding standards to expedite discharges; and (3) establishing a daily predischarge planning process.
The preintervention team typically had 1 attending, 1 to 2 senior residents, and 2 to 3 interns. The intervention period required creating 2 independently functioning teams, each composed of 1 hospitalist attending, and a minimum of 1 senior and 1 intern. The intervention occurred November through April, when the censuses predictably exceed 12 patients for the rounding attending. Because both teams functioned independently, all of the patients were divided equally between the 2 teams. Each team carried a panel of patients that included new, established, and dischargeable patients (Figure 1). We did not compare the number of provider handoffs before and during the intervention or time spent per patient.
Because the intervention required increasing the number of weeks on‐service by 2 to 3weeks per physician to reduce clinical work time, it meant redeploying previously off‐service attendings to coincide with peak demands. This aspect of the intervention made group buy‐in mandatory. The group agreed to distribute the predictably heavy workload that usually falls on 1 attending by adding a second attending for the busiest 6 months of the year. Our division voted unanimously to adopt this model despite the increase in service time, as long as weekend coverage was not increased.
As part of the intervention, we created standard work expectations within our division to (1) start rounds on dischargeable patients who were identified the prior evening during the (2) interdisciplinary huddle, and (3) have the entire departure process completed at the bedside using a discharge checklist (see Supporting Figure 1 through Figure 3 in the online version of this article). The expectations included a standard script for beginning rounds, selecting patients who could be discharged first, and completing all necessary discharge computer work at the bedside, before proceeding to the next patient. The daily predischarge huddle was instituted each afternoon to prepare discharges that were expected to occur the following day. The huddles were attended by care coordinators, social workers, and both medical teams. During the huddle, the team discussed anticipated discharges, scheduled follow‐up appointments and testing, faxed necessary prescriptions, and arranged any needed home services.
Inclusion and Exclusion Criteria for Patients
All patients discharged from the pediatric hospitalist inpatient service between Monday and Friday from April 8, 2013 to October 25, 2013 (preimplementation cohort) and October 28, 2013 to April 18, 2014 (postimplementation cohort) were eligible for inclusion. This included admitted patients and observation status patients. Patients discharged from the remaining PSHCH medical and surgical service lines were included in the control group analysis using the above criteria.
OUTCOME MEASURES
Throughput and Patient‐Level Outcomes
Primary outcomes included (1) time of electronic discharge order placement, (2) actual patient discharge time, (3) proportion of patients discharged before noon and 2 pm, (4) 7‐day, 14‐day, and 30‐day readmission rates, (5) length of stay (LOS), and (6) average daily census (ADC).
Statistical Analysis
The null hypothesis was that there would be no difference in discharge order time, discharge time, LOS, readmission rates, and daily discharges in the preintervention group compared to the intervention group. For time of order placement and actual patient discharge, the significance was assessed using Wilcoxon rank sum test and expressed as median time among the groups. Patient discharge before noon/2 pm was assessed by a logistic regression model. The predictor being the intervention group with the results expressed as odds ratios of discharge before noon/2 pm comparing the intervention group to the preintervention group. Readmission rates were assessed using a [2] test to see if there was a significant difference from what would be expected. Last, LOS and ADC were assessed by a Student t test and expressed as the means. The data were analyzed using SAS version 9.3 (SAS Institute, Cary, NC).
RESULTS
For our division's service line, both the ADC and number of patients discharged per day were significantly higher during the intervention months (Table 1). By comparison, the control group had a significantly lower ADC and lower average of discharges per day in the intervention time period. The new model permitted the teams to enter discharge orders earlier in the day, which ultimately lead to earlier patient discharges. The additive effect of the 3 interventions had a statistically significant effect on process efficiency metrics (Table 1). The median discharge order entry time decreased by 200 minutes from 14:05 to 10:45, and the median time of patient discharge decreased by 93 minutes from 15:48 to 14:15. By comparison, the median time of discharge order entry decreased 13:13 to 12:56 pm, but the median time of discharge increased 5 minutes 14:45 versus 14:50 in the control group. A significantly higher proportion of patients were discharged by noon (27% vs 14%; P<0.0001; odds ratio [OR]:2.2; 95% confidence interval [CI]: 1.6‐3.1) and by 2 pm during the intervention period (47% vs 30%; P<0.0001; OR: 2.1; 95% CI: 1.6‐2.7). There was no observed difference in the proportion of patients who were discharged by noon or 2 pm in the control group. Finally, in the intervention group, approximately 50% of patients had discharge orders entered before noon compared to 23% in the control group (Figure 2). The intervention demonstrated statistical significance in shifting the time of discharge order entry and the time of patient discharge when compared to the relatively less burdened PSHCH control group (Figures 2 and 3). As seen in Figure 4, the results were sustained for the duration of the study and appeared to improve throughout intervention. Finally, readmission rates at 7, 14, and 30 days postdischarge and LOS were not negatively affected (Table 1) in either the intervention or control group.
| Outcomes | Experimental Model | Control Group | ||||
|---|---|---|---|---|---|---|
| Preintervention, n=421 | Intervention, n=552 | P Value | Preintervention, n=1,390 | Intervention, n=1,146 | P Value | |
| Average daily census | 9.7 | 12.4 | <0.0001 | 45.9 | 43.4 | 0.002 |
| Discharges per day | 3.1 | 4.5 | <0.00001 | 9.5 | 9.2 | 0.419 |
| Average length of stay | 3.1 | 3.0 | 0.864 | 6.3 | 5.9 | 0.714 |
| Discharge order time, median | 14:05 | 10:45 | <0.0001 | 13:13 | 12:56 | 0.053 |
| Discharge from hospital, median | 15:48 | 14:15 | <0.0001 | 14:45 | 14:50 | 0.113 |
| Patients discharged before noon | 59 (14%) | 147 (27%) | <0.0001 | 176 (13%) | 170 (15%) | 0.138 |
| Patients discharged before 2 pm | 128 (30%) | 261 (47%) | <0.0001 | 519 (38%) | 447 (39%) | 0.512 |
| 7‐day readmission rates | 3.1% | 3.5% | 0.965 | 6.7% | 6.8% | 0.970 |
| 14‐day readmission rates | 5.8% | 5.8% | 0.981 | 12.0% | 13.5% | 0.301 |
| 30‐day readmission rates | 9.4% | 9.1% | 0.703 | 20.0% | 20.6% | 0.705 |



DISCUSSION
We demonstrated a statistically significant and what appears to be a sustainable improvement in median discharge order times, discharge times, and proportion of discharges by noon and 2 pm. Ours was the only service line in our medical center to achieve a median time discharge before our institution's internal metric of 2 pm and maintain it for 3 consecutive months. Additionally, the process demonstrated consistent performance independent of the varying styles and experience of the rounding attending during the busiest months of the year without incurring a negative impact on LOS or readmission rates.
Although our intervention demonstrated statistical significance in shifting the discharge distribution curves by almost 2 hours, more relevant is its potential clinical and financial impact. First, it puts our hospital in compliance with the Joint Commission's recommendations standard LD.04.03.1, stipulating that hospitals measure and set goals for mitigating and managing the flow of patients though the hospital. Second, our findings confirm the results of earlier studies suggesting that shifting discharge times could likely be achieved without the additional staff, but with alterations in staff shift scheduling.[11] Third, by doing required discharge work at the bedside and making it available earlier in the day, every day, we consistently reduced patient waiting along the entire supply chain.
Advancing the discharge time creates virtual beds that allow our facility to theoretically accommodate new patients. Using the calculation in the Methods section (see Supporting Figure 4 in the online version of this article) on how to calculate virtual beds, we determined that our intervention created between 0.30 and 0.38 virtual beds in a hospital with only 72 beds. We calculated that this would create 6.8 more open bed hours per day, 74 additional patient days per year, and assuming patients were waiting for the beds and rapid bed turnover, our intervention theoretically created the capacity to accommodate approximately 25 additional admissions per year (see Supporting Figure 4 in the online version of this article). As the only children's hospital in the region, this intervention will enable our organization to provide timelier access and possibly reduce time sensitive medical errors.
Timelier evaluations also have revenue potential by eliminating lost referrals, thus turning waste into value. When comparing the previous year's high‐census monthsOctober through Marchthere were 20 lost referrals due to lack of bed capacity, as compared to zero lost referrals during our intervention period. By accommodating these 20 additional admissions, we estimated this generated between $275,000 and $412,000 dollars in additional revenue without additional resources but simply staffing to demand.
Finally, when we looked at patient satisfaction metrics obtained through Press Ganey (PG), comparing the time periods we observed that overall satisfaction increased from the 91 percentile to the 94 percentile, trust in doctor increased from the 20 percentile to the 70 percentile, and would recommend this hospital to others increased from the 53 percentile to the 75 percentile. Interestingly, despite being a study that improved discharge efficiency, none of the discharge metrics gathered by PG improved. It is possible that this is a limitation of the PG survey, or could reflect the possibility that our new process exposed that our discharge order entry and discharge processes are misaligned.
When we surveyed the nursing staff and members of the division regarding whether or not to continue the intervention rounding model, 75% and 100%, respectively, voted in favor of continuing with the intervention model. Unfortunately, housestaff satisfaction was not measured for this study.
Despite more weeks in the hospital, but because there was better process sequencing, our providers indicated that because the workload of the primary attending was reduced and the workload for the additional attending was light, there was ample time to engage in afternoon nonclinical activities (Figure 1B). In fact, several division members assumed departmental and educational leadership positions, and others volunteered to facilitate highly valued, but unsubsidized, afternoon medical student and resident teaching sessions that occurred solely as a result of the resequenced and redistributed clinical load.
There are limitations to this study. First, because 3 interventions were implemented simultaneously, it is difficult to identify which component of the intervention was the primary driver for the measured differences. It is conceivable the proactive discharge planning that occurred during the afternoon predischarge planning huddles allowed the teams to complete discharge requirements the night before anticipated discharge therefore expediting the next morning's discharge. A second limitation was not simultaneously comparing the traditional rounding structure with the experimental model. One could argue that the improved efficiency we observed was not due to any of the interventions and represents secular trends that all residents' teams experience through the course of the year as they get more adept at performing patient discharges. However, when we compare our performance to the control group performance, this efficiency trend was not present. Additionally, it was possible that attendings were so result focused that they delayed discharges if the 2 pm discharge goal was missed for that day and planned for early discharges the following morning. If this behavior occurred, this would likely have been reflected as increases in our LOS data, however this was not observed. Third, because our preintervention data reflected discharge behaviors during a low‐census period, it is possible that there was less urgency to discharge patients when bed capacity issues did not exist. Comparing the intervention period to a period when censuses are similar would better address this issue. Finally, although we assert that attending workload is a fundamental waste‐producing constraint in the discharge process, this study did not determine what the optimal patient census should be.
Most hospitalists struggle with finding a balance of meeting patient quality and administrative productivity demands.[16] Hospitalists at academic medical centers have the added demand of maintaining their educational mission. Since 2001, the Institute of Medicine[19] has advocated process re‐engineering using more patient‐centered approaches. A recent study found that when hospitals reach capacity, the excess workload placed on internal medicine hospitalists reduces efficiency and increases costs.[16] Interestingly, in a study conducted by McMahon et al., they found that reducing team censuses by 50%, resident educational outcomes can be improved.[20] Similar to this study, our study reduced attending workload by 50% with the goal to assess the impact on discharge efficiency rather than educational outcomes. Also similar to that study, we radically altered the operational model in which physicians historically had functioned.[14, 20] Because the rounding structures in both studies reduced patient:provider ratios we believe that our model will successfully balance education, patient quality, and productivity.
LSS, when thoughtfully applied to the problems we face, could be part of the solution. It delivers quick results without large capital investments, by identifying and implementing high‐leverage changes that value a creative solution before a capital investment. One of the strengths of this model is that it does not require substantial financial investment to produce these outcomes. Because the morning clinical loads were more evenly distributed during the busiest months of the year, our division members were able to engage in nonclinical duties and teaching sessions, both of which often required afternoon commitments, but permitted us to balance work and professional achievement (Figure 1B). Finally, as part of any new process, one must consider the factors that influence its sustainability: provider level satisfaction, impact of the process change, and remuneration. Because the intervention reduced lost referrals, the departmental and institutional leadership agreed to financially incentivize the value‐generating potential this intervention had on increasing patient access by facilitating organizational throughput. Therefore, having met the three aforementioned elements, we believe this model is sustainable.
Although many studies remain results focused with aims at documenting how hospital processes fail when overburdened, our study takes a novel process‐focused approach to look at how processes can excel during periods of high demands, simply by reallocating existing resources.
Medicine is in the midst of multiple paradigm shifts involving resident work hour reduction, public safety reporting, reimbursement constraints, and value‐driven care, to name a few. Whether we take a resident or patient‐centered approach, it seems highly unlikely that the current approaches will meet these demands without making significant changes in how we deliver care. Next steps should include construction of a value stream map (VSM), with the input of all of the process stakeholders, that diagrams the entire discharge process. The VSM should highlight all nonvalue adding steps and eliminate them. They are likely a contributing cause to the disproportionate reduction in time of discharge order entry (200 minutes) versus actual discharge (93 minutes) seen in our study. Future work needs to establish the generalizability and sustainability of this model across other hospital service lines. Future studies should establish if this model has sustained impact on patient, provider, and resident satisfaction and overall system efficiency (ED boarding), with aims to quantify the revenue generating potential that occurs through waste elimination.
We close with the following thought: [T]o ask people to make different decisions without fundamentally changing the equation presented to them is wrong. If we wish to change the types of decisions our people make, we owe it to them to design and build processes that will put them in a position to succeed.[21]
Acknowledgements
The authors recognize the contributions made by members of the Division of Pediatric Hospital Medicine at PSHCH, without which this project would not have been possible. They are: Drs. Marta Biderman, Anika Kumar, Margaret Mikula, Chris O'Hara, Brandon Smith, and Ron Williams, and Heidi Wolf, and Lyndsay Gardener, CRNP. This project would not have been possible without the help from Brenda Ruhl, Manjula Narasimhan, and Heather Boyle from the Department of Logistics. Finally, the authors would like to thank the following for their thoughtful reviews of this manuscript: Drs. Lisa Scalzi, Barbara Ostrov, Jed Gonzalo, Chris Sciamanna, and Matt Wain.
Disclosures: Nothing to report.
Given its positive effects on improving effectiveness and efficiency, Lean Six Sigma (LSS) is a business approach that is receiving a great deal of attention in the healthcare industry.[1, 2, 3, 4, 5, 6, 7] Although there are differences between Lean and Six Sigma, at their core they are both customer‐centered, quality methodologies designed to improve process efficiency and product quality through waste elimination, creating standardized work and reducing variation.[8]
Six Sigma is a rigorous problem‐focused process improvement method that focuses on defect removal, variation reduction, and customer satisfaction that relies heavily on statistical analysis. It includes 5 steps: define, measure, analyze, improvement, and control.[7, 8] Six Sigma assumes through variation reduction, defect removal, and meeting customer specifications, the performance of the organization can be improved and also meet the requirements of the customer.[8]
Lean is more process‐focused. It places emphasis on creating flow by removing waste and getting the steps of any given process in the right sequence.[8] In Lean terms, waste is defined as anything that the customer does not value and anything that is not done right the first time.[9] This category of waste is termed nonvalue adding and unnecessary. It is estimated that 30% to 50% of all steps of hospital processes are nonvalue adding and unnecessary, and therefore can be defined as waste.[10] Lean identifies 8 different types of nonvalue adding and unnecessary wastes. They are defects and rework, overproduction, waiting, nonutilization of resources, transport, inventory, motion, and extra processing. Waste creates delays that negatively impact patient care and reduce healthcare productivity.[10] Therefore, it makes sense to apply Lean concepts of waste identification and elimination to improve process efficiency. For example, when a facility is at or exceeds its bed capacity, any delay in discharge creates throughput delays throughout the hospital.[5] Discharge delays often result in emergency department (ED) overcrowding, and also affects a hospital's ability to accommodate internal downgrades and outside referrals in a timely fashion.[11, 12] However, because the sequence of steps of the discharge process is variable and not standardized, the goal to achieve early discharges remains elusive.[13]
There are emerging data to support that current rounding censuses exceed most hospitalist's abilities to deliver safe and efficient care.[12, 14, 15, 16] It is unclear what that threshold should be, but the current industry standard has nonacademic hospitalists seeing 15 patients per day. Therefore, high patient censuses could be contributing to delays in patient discharge times that effect hospital throughput. We speculated that by implementing a lean, quick‐strike approach[17] designed to improve the sequencing of housestaff, attending, and nursing work by eliminating the wastes of rework, waiting, extra processing, and nonutilization of physician resources by restaffing, we could improve patient discharge times. We augmented the intervention by creating standardized workflow expectations, a discharge checklist, and implemented daily interdisciplinary discharge planning huddles.
We hypothesized these interventions would improve the median time of discharge order entry and time of patient discharge. Primary outcome measures were: (1) the change in time of discharge order and discharge time and (2) the proportion of patients discharged before noon and 2 pm. Secondary outcomes that were used as balance measures were length of stay (LOS) and 7‐day, 14‐day, and 30‐day readmission rates.
METHODS
Study Design
This was a prospective quality improvement intervention with concurrent controls aimed to determine if discharge efficiency could be improved by load‐balancing our service line with existing faculty and residents, creating daily standard work using a discharge checklist and interdisciplinary huddles (see Supporting Figure 1 through Supporting Figure 3 in the online version of this article). All discharge data were collected as part of our medical center's Department of Logistics standard data collection procedures using solutions from TeleTracking Technologies, Inc. (Pittsburgh, PA). All patients discharged Monday through Friday from the pediatric hospitalist service prior to the 6‐month high‐census period (before intervention) and the 6‐month high‐census period (intervention period) were included in the study. To serve as our control, we collected the same discharge data during the same time periods for the remaining services of the children's hospital. This study was approved by Penn State Hershey Medical Center's institutional review board.
Study Setting
The study was conducted at the Penn State Hershey Children's Hospital (PSHCH), which is a physically free‐standing 133‐bed university‐based tertiary care hospital located in central Pennsylvania. The hospital has 36 pediatric medical/surgical beds located in 2 units (1 general and 1 intermediate care). PSHCH performs approximately 4100 admissions per year, of which approximately 1100 are performed by the Division of Pediatric Hospital Medicine. Our division is composed of 8 academic hospitalists with 1 to 20+ years' experience. Historically, the months of October through April are months when our service‐line has average daily censuses (ADC) that routinely exceed 12 patients per hospitalist. During these months, the median times patient discharge orders are placed and patient discharges occur historically approach 2 pm and 4 pm, respectively, and exceed our internal benchmark by 2 hours. Discharges from the remaining medical and surgical service lines at PSHCH that occurred Monday through Friday during the concurrent pre‐ and postintervention time periods served as the control group.
Needs Assessment and LSS
Traditionally, morning patient rounds are allotted approximately 180 minutes. Therefore, a rounding team can only be expected to spend 13 minutes or less per patient when the census exceeds 12 patients. The cycle time to perform 1 discharge using our electronic medical record is approximately 20 minutes, which is almost 10 minutes longer than the allotted time per patient. During high‐census months, our service averages 4 to 5 discharges per day. To accommodate performing discharges during rounds would require spending 80 to 100 minutes of the 180 available minutes. This would leave only 80 to 100 minutes to see the remaining 8 to 10 patients. As a result of these constraints, discharges are typically completed by the residents in unsupervised batches each afternoon following the noon conference (Figure 1A).

Because LSS focuses on eliminating nonvalue adding and unnecessary waste by load balancing processes and minimizing batching tasks,[8] this approach should lead one to question whether the current rounding model that requires 1 attending to see >12 high‐acuity patients with a maximum of 13 minutes per patient is system design flaw that leads to errors and inefficiency,[16] Theoretically, having an additional attending present would allow teams to resequence the work on smaller batches of patients and double the time to spend on each patient. This could create the opportunity to do value‐added work at the bedside in the presence of the family and nurse and eliminate the amount of nursing rework and time spent as work in progress on dischargeable patients (Figure 1).
Additionally, improving discharge efficiency creates virtual beds. Virtual beds permit hospitals to accommodate additional admissions despite operating with a fixed‐bed capacity. A way to calculate virtual beds is to calculate the reduction in LOS, and multiply that by the number of admissions per year divided by 36518 (see Supporting Figure 4 in the online version of this article). Our study was intended to determine the impact of discharge efficiency on this metric.
Intervention
We re‐structured our service line in a way that would balance both physician workload needs and patient expectations. To accomplish this, off service attendings were reallocated to round with a smaller resident team on fewer patients for the duration of the 6‐month study. Each member of the division agreed to work an average of 3 more weeks per year. One work day was estimated to be approximately 10 hours and 1 work week equaled 5 days, which asked for 150 hours of additional work per year. Because there increases in functional FTEs, the 2 teams consolidated into 1 team each weekend, to meet the group requirement that this model not result in additional weekend coverage. A balanced workload also theoretically allows the physician to spend more time at the bedside in direct patient care and resident education activities/observations.
In addition to reallocating physician and resident resources, our model created standard work expectations to reduce the variations in physician work sequences that can account for delayed discharge orders and delayed discharges, which is also an LSS principle. The intervention consisted of 3 changes: (1) fundamentally altering the composition of the rounding teams to optimize the provider: patient ratio; (2) defining rounding standards to expedite discharges; and (3) establishing a daily predischarge planning process.
The preintervention team typically had 1 attending, 1 to 2 senior residents, and 2 to 3 interns. The intervention period required creating 2 independently functioning teams, each composed of 1 hospitalist attending, and a minimum of 1 senior and 1 intern. The intervention occurred November through April, when the censuses predictably exceed 12 patients for the rounding attending. Because both teams functioned independently, all of the patients were divided equally between the 2 teams. Each team carried a panel of patients that included new, established, and dischargeable patients (Figure 1). We did not compare the number of provider handoffs before and during the intervention or time spent per patient.
Because the intervention required increasing the number of weeks on‐service by 2 to 3weeks per physician to reduce clinical work time, it meant redeploying previously off‐service attendings to coincide with peak demands. This aspect of the intervention made group buy‐in mandatory. The group agreed to distribute the predictably heavy workload that usually falls on 1 attending by adding a second attending for the busiest 6 months of the year. Our division voted unanimously to adopt this model despite the increase in service time, as long as weekend coverage was not increased.
As part of the intervention, we created standard work expectations within our division to (1) start rounds on dischargeable patients who were identified the prior evening during the (2) interdisciplinary huddle, and (3) have the entire departure process completed at the bedside using a discharge checklist (see Supporting Figure 1 through Figure 3 in the online version of this article). The expectations included a standard script for beginning rounds, selecting patients who could be discharged first, and completing all necessary discharge computer work at the bedside, before proceeding to the next patient. The daily predischarge huddle was instituted each afternoon to prepare discharges that were expected to occur the following day. The huddles were attended by care coordinators, social workers, and both medical teams. During the huddle, the team discussed anticipated discharges, scheduled follow‐up appointments and testing, faxed necessary prescriptions, and arranged any needed home services.
Inclusion and Exclusion Criteria for Patients
All patients discharged from the pediatric hospitalist inpatient service between Monday and Friday from April 8, 2013 to October 25, 2013 (preimplementation cohort) and October 28, 2013 to April 18, 2014 (postimplementation cohort) were eligible for inclusion. This included admitted patients and observation status patients. Patients discharged from the remaining PSHCH medical and surgical service lines were included in the control group analysis using the above criteria.
OUTCOME MEASURES
Throughput and Patient‐Level Outcomes
Primary outcomes included (1) time of electronic discharge order placement, (2) actual patient discharge time, (3) proportion of patients discharged before noon and 2 pm, (4) 7‐day, 14‐day, and 30‐day readmission rates, (5) length of stay (LOS), and (6) average daily census (ADC).
Statistical Analysis
The null hypothesis was that there would be no difference in discharge order time, discharge time, LOS, readmission rates, and daily discharges in the preintervention group compared to the intervention group. For time of order placement and actual patient discharge, the significance was assessed using Wilcoxon rank sum test and expressed as median time among the groups. Patient discharge before noon/2 pm was assessed by a logistic regression model. The predictor being the intervention group with the results expressed as odds ratios of discharge before noon/2 pm comparing the intervention group to the preintervention group. Readmission rates were assessed using a [2] test to see if there was a significant difference from what would be expected. Last, LOS and ADC were assessed by a Student t test and expressed as the means. The data were analyzed using SAS version 9.3 (SAS Institute, Cary, NC).
RESULTS
For our division's service line, both the ADC and number of patients discharged per day were significantly higher during the intervention months (Table 1). By comparison, the control group had a significantly lower ADC and lower average of discharges per day in the intervention time period. The new model permitted the teams to enter discharge orders earlier in the day, which ultimately lead to earlier patient discharges. The additive effect of the 3 interventions had a statistically significant effect on process efficiency metrics (Table 1). The median discharge order entry time decreased by 200 minutes from 14:05 to 10:45, and the median time of patient discharge decreased by 93 minutes from 15:48 to 14:15. By comparison, the median time of discharge order entry decreased 13:13 to 12:56 pm, but the median time of discharge increased 5 minutes 14:45 versus 14:50 in the control group. A significantly higher proportion of patients were discharged by noon (27% vs 14%; P<0.0001; odds ratio [OR]:2.2; 95% confidence interval [CI]: 1.6‐3.1) and by 2 pm during the intervention period (47% vs 30%; P<0.0001; OR: 2.1; 95% CI: 1.6‐2.7). There was no observed difference in the proportion of patients who were discharged by noon or 2 pm in the control group. Finally, in the intervention group, approximately 50% of patients had discharge orders entered before noon compared to 23% in the control group (Figure 2). The intervention demonstrated statistical significance in shifting the time of discharge order entry and the time of patient discharge when compared to the relatively less burdened PSHCH control group (Figures 2 and 3). As seen in Figure 4, the results were sustained for the duration of the study and appeared to improve throughout intervention. Finally, readmission rates at 7, 14, and 30 days postdischarge and LOS were not negatively affected (Table 1) in either the intervention or control group.
| Outcomes | Experimental Model | Control Group | ||||
|---|---|---|---|---|---|---|
| Preintervention, n=421 | Intervention, n=552 | P Value | Preintervention, n=1,390 | Intervention, n=1,146 | P Value | |
| Average daily census | 9.7 | 12.4 | <0.0001 | 45.9 | 43.4 | 0.002 |
| Discharges per day | 3.1 | 4.5 | <0.00001 | 9.5 | 9.2 | 0.419 |
| Average length of stay | 3.1 | 3.0 | 0.864 | 6.3 | 5.9 | 0.714 |
| Discharge order time, median | 14:05 | 10:45 | <0.0001 | 13:13 | 12:56 | 0.053 |
| Discharge from hospital, median | 15:48 | 14:15 | <0.0001 | 14:45 | 14:50 | 0.113 |
| Patients discharged before noon | 59 (14%) | 147 (27%) | <0.0001 | 176 (13%) | 170 (15%) | 0.138 |
| Patients discharged before 2 pm | 128 (30%) | 261 (47%) | <0.0001 | 519 (38%) | 447 (39%) | 0.512 |
| 7‐day readmission rates | 3.1% | 3.5% | 0.965 | 6.7% | 6.8% | 0.970 |
| 14‐day readmission rates | 5.8% | 5.8% | 0.981 | 12.0% | 13.5% | 0.301 |
| 30‐day readmission rates | 9.4% | 9.1% | 0.703 | 20.0% | 20.6% | 0.705 |



DISCUSSION
We demonstrated a statistically significant and what appears to be a sustainable improvement in median discharge order times, discharge times, and proportion of discharges by noon and 2 pm. Ours was the only service line in our medical center to achieve a median time discharge before our institution's internal metric of 2 pm and maintain it for 3 consecutive months. Additionally, the process demonstrated consistent performance independent of the varying styles and experience of the rounding attending during the busiest months of the year without incurring a negative impact on LOS or readmission rates.
Although our intervention demonstrated statistical significance in shifting the discharge distribution curves by almost 2 hours, more relevant is its potential clinical and financial impact. First, it puts our hospital in compliance with the Joint Commission's recommendations standard LD.04.03.1, stipulating that hospitals measure and set goals for mitigating and managing the flow of patients though the hospital. Second, our findings confirm the results of earlier studies suggesting that shifting discharge times could likely be achieved without the additional staff, but with alterations in staff shift scheduling.[11] Third, by doing required discharge work at the bedside and making it available earlier in the day, every day, we consistently reduced patient waiting along the entire supply chain.
Advancing the discharge time creates virtual beds that allow our facility to theoretically accommodate new patients. Using the calculation in the Methods section (see Supporting Figure 4 in the online version of this article) on how to calculate virtual beds, we determined that our intervention created between 0.30 and 0.38 virtual beds in a hospital with only 72 beds. We calculated that this would create 6.8 more open bed hours per day, 74 additional patient days per year, and assuming patients were waiting for the beds and rapid bed turnover, our intervention theoretically created the capacity to accommodate approximately 25 additional admissions per year (see Supporting Figure 4 in the online version of this article). As the only children's hospital in the region, this intervention will enable our organization to provide timelier access and possibly reduce time sensitive medical errors.
Timelier evaluations also have revenue potential by eliminating lost referrals, thus turning waste into value. When comparing the previous year's high‐census monthsOctober through Marchthere were 20 lost referrals due to lack of bed capacity, as compared to zero lost referrals during our intervention period. By accommodating these 20 additional admissions, we estimated this generated between $275,000 and $412,000 dollars in additional revenue without additional resources but simply staffing to demand.
Finally, when we looked at patient satisfaction metrics obtained through Press Ganey (PG), comparing the time periods we observed that overall satisfaction increased from the 91 percentile to the 94 percentile, trust in doctor increased from the 20 percentile to the 70 percentile, and would recommend this hospital to others increased from the 53 percentile to the 75 percentile. Interestingly, despite being a study that improved discharge efficiency, none of the discharge metrics gathered by PG improved. It is possible that this is a limitation of the PG survey, or could reflect the possibility that our new process exposed that our discharge order entry and discharge processes are misaligned.
When we surveyed the nursing staff and members of the division regarding whether or not to continue the intervention rounding model, 75% and 100%, respectively, voted in favor of continuing with the intervention model. Unfortunately, housestaff satisfaction was not measured for this study.
Despite more weeks in the hospital, but because there was better process sequencing, our providers indicated that because the workload of the primary attending was reduced and the workload for the additional attending was light, there was ample time to engage in afternoon nonclinical activities (Figure 1B). In fact, several division members assumed departmental and educational leadership positions, and others volunteered to facilitate highly valued, but unsubsidized, afternoon medical student and resident teaching sessions that occurred solely as a result of the resequenced and redistributed clinical load.
There are limitations to this study. First, because 3 interventions were implemented simultaneously, it is difficult to identify which component of the intervention was the primary driver for the measured differences. It is conceivable the proactive discharge planning that occurred during the afternoon predischarge planning huddles allowed the teams to complete discharge requirements the night before anticipated discharge therefore expediting the next morning's discharge. A second limitation was not simultaneously comparing the traditional rounding structure with the experimental model. One could argue that the improved efficiency we observed was not due to any of the interventions and represents secular trends that all residents' teams experience through the course of the year as they get more adept at performing patient discharges. However, when we compare our performance to the control group performance, this efficiency trend was not present. Additionally, it was possible that attendings were so result focused that they delayed discharges if the 2 pm discharge goal was missed for that day and planned for early discharges the following morning. If this behavior occurred, this would likely have been reflected as increases in our LOS data, however this was not observed. Third, because our preintervention data reflected discharge behaviors during a low‐census period, it is possible that there was less urgency to discharge patients when bed capacity issues did not exist. Comparing the intervention period to a period when censuses are similar would better address this issue. Finally, although we assert that attending workload is a fundamental waste‐producing constraint in the discharge process, this study did not determine what the optimal patient census should be.
Most hospitalists struggle with finding a balance of meeting patient quality and administrative productivity demands.[16] Hospitalists at academic medical centers have the added demand of maintaining their educational mission. Since 2001, the Institute of Medicine[19] has advocated process re‐engineering using more patient‐centered approaches. A recent study found that when hospitals reach capacity, the excess workload placed on internal medicine hospitalists reduces efficiency and increases costs.[16] Interestingly, in a study conducted by McMahon et al., they found that reducing team censuses by 50%, resident educational outcomes can be improved.[20] Similar to this study, our study reduced attending workload by 50% with the goal to assess the impact on discharge efficiency rather than educational outcomes. Also similar to that study, we radically altered the operational model in which physicians historically had functioned.[14, 20] Because the rounding structures in both studies reduced patient:provider ratios we believe that our model will successfully balance education, patient quality, and productivity.
LSS, when thoughtfully applied to the problems we face, could be part of the solution. It delivers quick results without large capital investments, by identifying and implementing high‐leverage changes that value a creative solution before a capital investment. One of the strengths of this model is that it does not require substantial financial investment to produce these outcomes. Because the morning clinical loads were more evenly distributed during the busiest months of the year, our division members were able to engage in nonclinical duties and teaching sessions, both of which often required afternoon commitments, but permitted us to balance work and professional achievement (Figure 1B). Finally, as part of any new process, one must consider the factors that influence its sustainability: provider level satisfaction, impact of the process change, and remuneration. Because the intervention reduced lost referrals, the departmental and institutional leadership agreed to financially incentivize the value‐generating potential this intervention had on increasing patient access by facilitating organizational throughput. Therefore, having met the three aforementioned elements, we believe this model is sustainable.
Although many studies remain results focused with aims at documenting how hospital processes fail when overburdened, our study takes a novel process‐focused approach to look at how processes can excel during periods of high demands, simply by reallocating existing resources.
Medicine is in the midst of multiple paradigm shifts involving resident work hour reduction, public safety reporting, reimbursement constraints, and value‐driven care, to name a few. Whether we take a resident or patient‐centered approach, it seems highly unlikely that the current approaches will meet these demands without making significant changes in how we deliver care. Next steps should include construction of a value stream map (VSM), with the input of all of the process stakeholders, that diagrams the entire discharge process. The VSM should highlight all nonvalue adding steps and eliminate them. They are likely a contributing cause to the disproportionate reduction in time of discharge order entry (200 minutes) versus actual discharge (93 minutes) seen in our study. Future work needs to establish the generalizability and sustainability of this model across other hospital service lines. Future studies should establish if this model has sustained impact on patient, provider, and resident satisfaction and overall system efficiency (ED boarding), with aims to quantify the revenue generating potential that occurs through waste elimination.
We close with the following thought: [T]o ask people to make different decisions without fundamentally changing the equation presented to them is wrong. If we wish to change the types of decisions our people make, we owe it to them to design and build processes that will put them in a position to succeed.[21]
Acknowledgements
The authors recognize the contributions made by members of the Division of Pediatric Hospital Medicine at PSHCH, without which this project would not have been possible. They are: Drs. Marta Biderman, Anika Kumar, Margaret Mikula, Chris O'Hara, Brandon Smith, and Ron Williams, and Heidi Wolf, and Lyndsay Gardener, CRNP. This project would not have been possible without the help from Brenda Ruhl, Manjula Narasimhan, and Heather Boyle from the Department of Logistics. Finally, the authors would like to thank the following for their thoughtful reviews of this manuscript: Drs. Lisa Scalzi, Barbara Ostrov, Jed Gonzalo, Chris Sciamanna, and Matt Wain.
Disclosures: Nothing to report.
- . Theory of constraints—a review of the philosophy and its applications. Int J Oper Prod Man. 1998;18(4):336–355.
- , , , et al. Use of lean and six sigma methodology to improve operating room efficiency in a high‐volume tertiary‐care academic medical center. J Am Coll Surg. 2011;213(1):83–92; discussion 93–84.
- , . The promise of Lean in health care. Mayo Clin Proc. 2013;88(1):74–82.
- , , . Assessing the evidence of Six Sigma and Lean in the health care industry. Qual Manag Health Care. 2010;19(3):211–225.
- , , , et al. Redesigning care at the Flinders Medical Centre: clinical process redesign using “lean thinking”. Med J Aust. 2008;188(6 suppl):S27–S31.
- , , , , . Impact of 5 years of lean six sigma in a University Medical Center. Qual Manag Health Care. 2012;21(4):262–268.
- , , , et al. A Lean Six Sigma quality improvement project to increase discharge paperwork completeness for admission to a comprehensive integrated inpatient rehabilitation program. Am J Med Qual. 2013;28(4):301–307.
- . How to compare Six Sigma, lean and the theory of constraints—a framework for choosing what's best for your organization. Qual Prog. 2002;35(3):73–78.
- , , , , . Application of lean manufacturing techniques in the Emergency Department. J Emerg Med. 2009;37(2):177–182.
- . Lean Hospitals: Improving Quality, Patient Safety, and Employee Engagement. 2nd ed. New York, NY: Productivity Press/Taylor 2012.
- , , , , , . The relationship between inpatient discharge timing and emergency department boarding. J Emerg Med. 2012;42(2):186–196.
- , , , . Unravelling relationships: Hospital occupancy levels, discharge timing and emergency department access block. Emerg Med Aust. 2012;24(5):510–517.
- . Need to speed up discharges? The pros and cons of putting discharges on the clock. Today's Hospitalist. 2013. Accessed November 2014. Available at: http://www.todayshospitalist.com/index.php?b=articles_read3(2):144–150.
- , . Hospitalist staffing requirements. Eff Clin Pract. 1999;2(3):126–130.
- , , , , . Effect of hospitalist workload on the quality and efficiency of care. JAMA Intern Med. 2014;174(5):786–793.
- . Accelerating Lean Six Sigma Results: How to Achieve Improvement Excellence in the New Economy. Plantation, FL: J. Ross Publishing; 2011.
- . “Virtual bed capacity” may offer revenue boost for hospitals. Healthcare Finance. Accessed October 2014. Available at: http://www.healthcarefinancenews.com/blog/virtual‐bed‐capacity‐may‐offer‐revenue‐boost‐hospitals. Published November 30, 2011.
- Institute of Medicine. Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy Press; 2001.
- , , , , . Evaluation of a redesign initiative in an internal‐medicine residency. N Engl J Med. 2010;362(14):1304–1311.
- John Kim and Associates website. Available at: http://www.johnkimconsulting.com. Accessed June 2014.
- . Theory of constraints—a review of the philosophy and its applications. Int J Oper Prod Man. 1998;18(4):336–355.
- , , , et al. Use of lean and six sigma methodology to improve operating room efficiency in a high‐volume tertiary‐care academic medical center. J Am Coll Surg. 2011;213(1):83–92; discussion 93–84.
- , . The promise of Lean in health care. Mayo Clin Proc. 2013;88(1):74–82.
- , , . Assessing the evidence of Six Sigma and Lean in the health care industry. Qual Manag Health Care. 2010;19(3):211–225.
- , , , et al. Redesigning care at the Flinders Medical Centre: clinical process redesign using “lean thinking”. Med J Aust. 2008;188(6 suppl):S27–S31.
- , , , , . Impact of 5 years of lean six sigma in a University Medical Center. Qual Manag Health Care. 2012;21(4):262–268.
- , , , et al. A Lean Six Sigma quality improvement project to increase discharge paperwork completeness for admission to a comprehensive integrated inpatient rehabilitation program. Am J Med Qual. 2013;28(4):301–307.
- . How to compare Six Sigma, lean and the theory of constraints—a framework for choosing what's best for your organization. Qual Prog. 2002;35(3):73–78.
- , , , , . Application of lean manufacturing techniques in the Emergency Department. J Emerg Med. 2009;37(2):177–182.
- . Lean Hospitals: Improving Quality, Patient Safety, and Employee Engagement. 2nd ed. New York, NY: Productivity Press/Taylor 2012.
- , , , , , . The relationship between inpatient discharge timing and emergency department boarding. J Emerg Med. 2012;42(2):186–196.
- , , , . Unravelling relationships: Hospital occupancy levels, discharge timing and emergency department access block. Emerg Med Aust. 2012;24(5):510–517.
- . Need to speed up discharges? The pros and cons of putting discharges on the clock. Today's Hospitalist. 2013. Accessed November 2014. Available at: http://www.todayshospitalist.com/index.php?b=articles_read3(2):144–150.
- , . Hospitalist staffing requirements. Eff Clin Pract. 1999;2(3):126–130.
- , , , , . Effect of hospitalist workload on the quality and efficiency of care. JAMA Intern Med. 2014;174(5):786–793.
- . Accelerating Lean Six Sigma Results: How to Achieve Improvement Excellence in the New Economy. Plantation, FL: J. Ross Publishing; 2011.
- . “Virtual bed capacity” may offer revenue boost for hospitals. Healthcare Finance. Accessed October 2014. Available at: http://www.healthcarefinancenews.com/blog/virtual‐bed‐capacity‐may‐offer‐revenue‐boost‐hospitals. Published November 30, 2011.
- Institute of Medicine. Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy Press; 2001.
- , , , , . Evaluation of a redesign initiative in an internal‐medicine residency. N Engl J Med. 2010;362(14):1304–1311.
- John Kim and Associates website. Available at: http://www.johnkimconsulting.com. Accessed June 2014.
© 2014 Society of Hospital Medicine
In‐Hospital Asthma Resource Utilization
Pediatric hospitalizations for obesity‐related conditions have doubled in the last decade, mirroring the trend of higher levels of childhood obesity in the United States.[1, 2, 3] Recent studies have demonstrated worsened pediatric in‐hospital outcomes, including mortality and increased resource utilization, for children with obesity across a range of diagnoses.[4, 5, 6, 7, 8, 9, 10] Although the mechanisms driving the association between obesity and in‐hospital outcomes are not fully known, for asthma it is believed that adipocytes expressing inflammatory markers create a low level of systemic inflammation, thereby increasing the severity of allergic‐type illnesses and decreasing the response to anti‐inflammatory medications, such as steroids.[11, 12, 13, 14, 15, 16, 17, 18] The relationship of obesity and in‐hospital asthma outcomes is of particular interest because status asthmaticus is the most common reason for admission in children aged 3 to 12 years, accounting for approximately 150,000 admissions (7.4% of all hospitalizations for children and adolescents) and $835 million in hospital costs annually.[19]
Few prior studies have examined the association of obesity and asthma outcomes in the in‐hospital setting. The studies examining this association have found patients with obesity to have a longer hospital length of stay (LOS) and increased hospital costs.[8, 9, 20] Obesity has also been associated with increased respiratory treatments and supplemental oxygen requirements.[20] Associations between obesity and admission rates from the emergency department (ED) for pediatric asthma have been inconsistent.[21, 22] Most of these prior studies had several limitations in identifying patients with obesity, including using weight‐for‐age percentiles or International Classification of Diseases, Ninth Revision (ICD‐9) codes, rather than body mass index (BMI) percentile for age, the currently recommended method.[23, 24, 25] Methods other than BMI have the potential to either underestimate obesity (ie, ICD‐9 codes)[26] or to confound weight with adiposity (ie, weight‐for‐age percentiles),[27] thereby skewing the primary exposure of interest.
In the present study, we sought to examine associations between obesity and in‐hospital outcomes for pediatric status asthmaticus using the currently endorsed method for identifying obesity in children, BMI percentile for age.[23, 24, 25] The outcomes of interest included a broad range of in‐hospital measures, including resource utilization (medication and radiology use), readmission rates, billed charges, and LOS. We hypothesize that obesity, due to its proinflammatory state, would result in increased LOS, increased resource utilization, and an increased readmission rate for children admitted with status asthmaticus.
METHODS
Data Sources
Data for this retrospective cross‐sectional study were obtained from 2 sources. First, we queried the Pediatric Health Information System (PHIS) administrative database, which draws information from multiple children's hospitals to identify patients at our 2 institutions of interest who met study criteria. The PHIS database also was used to collect patient demographic data. PHIS is an administrative database operated by Children's Hospital Association (Overland Park, KS) containing clinical and billing data from 43 tertiary care, freestanding children's hospitals, including data on 41 ICD‐9 diagnoses, billed charges, and LOS. Based on the primary diagnosis, PHIS assigns each discharge to an All Patient Refined‐Diagnosis Related Group (APR‐DRG v.24) (3M Health information Systems, St. Paul, MN). APR‐DRGs allow similar diagnoses to be grouped together.[28, 29] PHIS also uses ICD‐9 codes to identify patients with a complex chronic condition (CCC).[30, 31] CCCs are those conditions that can be reasonably expected to last at least 12 months (unless death intervenes) and to involve either several different organ systems or one system severely enough to require specialty pediatric care and probably some period of hospitalization in a tertiary care center.[30, 31] PHIS data quality is ensured through a collaborative effort of the participating hospitals, the Children's Hospital Association, and Truven Healthcare.
Second, standardized chart reviews were then performed to collect clinical data not found in PHIS: BMI, LOS in hours, and medications administered, including total number of albuterol treatments administered during both the admission and the associated preceding ED visit.
Study Setting and Participants
All admissions examined in this study were at Children's Mercy Hospitals. Children's Mercy Hospitals includes 2 separate hospitals: 1 hospital is a 354‐bed academic, tertiary care freestanding children's hospital located in Kansas City, Missouri; a second, smaller, 50‐bed freestanding hospital is located in Overland Park, Kansas. Both hospitals have pediatric emergency departments. Inclusion criteria included patients aged 5 to 17 years discharged for status asthmaticus (APR‐DRG 141) at Children's Mercy Hospital from October 1, 2011 to September 30, 2012, with a recorded BMI during the admission or within 30 days of the admission. Patients between the ages of 2 and 5 years old were not included because of the incidence of viral‐induced wheezing in this age group and therefore possible miscoding of the asthma diagnosis. Exclusion criteria included a concurrent diagnosis of a CCC or bacterial pneumonia because these conditions could alter LOS, resource utilization, and readmission rates independent of the subject's status asthmaticus. In addition, to account for differences in the amount of treatment given in the pre‐inpatient setting, patients not initially treated through the hospital's ED were excluded. For patients with multiple admissions during the study period for the same diagnosis, only the index admission was examined. The institutional review board at Children's Mercy Hospital approved this study with waiver of informed consent.
Study Definitions
BMI percentile for age was used as both a continuous and categorical predictor variable. As a categorical variable it was divided into 4 categories: underweight (BMI <5%), normal weight (BMI 5%84%), overweight (BMI 85%94%), and obese (BMI 95%).[23] Race was categorized non‐Hispanic white, non‐Hispanic black, and other. Other included Asian, Pacific Islander, American Indian, and other. Ethnicity was categorized as Hispanic and non‐Hispanic. Insurance categories included private (commercial or TRICARE), public (Medicaid and Title V), and other (uninsured, self‐pay, and other). Adjusted billed charges were calculated for each hospitalization. Adjusted billed charges are the billed charges adjusted by the US Centers of Medicare and Medicaid Services' price/wage index for the study site's location.[32, 33]
To compare albuterol of different delivery methods, albuterol equivalents were calculated. Based upon prior research demonstrating equal efficacy between albuterol administered by nebulizer and metered‐dose inhaler (MDI),[34] every 2.5 mg of albuterol administered by nebulizer was treated as equivalent to 2 sprays of albuterol (90 g/spray) administered by MDI. Therefore, albuterol 2.5 mg nebulized and 2 sprays of albuterol (90 g/spray) were each defined as 1 albuterol equivalent. To compare continuous administration of nebulized albuterol with intermittent administration of albuterol, the total milligrams of continuously nebulized albuterol were examined. Per protocol at the study site, 10 mg per hour of continuous albuterol are administered for patients 5 years and younger and, for children 6 years and older, 15 mg per hour of continuous albuterol are administered. Based upon milligrams of albuterol nebulized, 5‐year‐old subjects receiving an hour of continuous albuterol would equal 4 albuterol equivalents (or 4 treatments of nebulized albuterol 2.5 mg/treatment or 4 treatments of albuterol 90 g/spray 2 sprays/treatment); for patients 6 years and older, an hour of continuous albuterol would equal 6 albuterol equivalents (or 6 treatments of nebulized albuterol 2.5 mg/treatment or 6 treatments of albuterol 90 g/spray 2 sprays/treatment). The variable total albuterol was then created to include albuterol equivalents delivered by metered dose inhaler and as both single and continuous nebulized treatments.
Main Exposure
The main exposure of interest was BMI percentile for age.
Outcome Measures
The main outcome measure was inpatient LOS measured in hours. Secondary outcome measures included the total albuterol (in the inpatient setting as well as combined inpatient and ED settings) and the administration of intravenous IV fluids and intramuscular (IM) or IV systemic steroids. Other secondary measures included readmission for status asthmaticus during the study period, adjusted billed charges, and inpatient chest radiograph utilization.
Statistical Analyses
We summarized categorical variables with frequencies and percentages, and used [2] test across BMI categories. The non‐normal distribution of continuous dependent variables (LOS, number of albuterol treatments, billed charges) were summarized with medians and interquartile ranges (IQRs). Kruskal‐Wallis test was used to examine outcomes across BMI categories. For regression models, BMI percentile for age was divided into deciles and treated as a continuous predictor. Factors used in the regression models included age, gender, race, ethnicity, and insurance. Total albuterol received in the ED was also included in the model to adjust for differences in the amount of treatment received prior to admission. Incidence rate ratios were created using Poisson regression for continuous outcomes (LOS, billed charges, and number of albuterol equivalent treatments administered), and odds ratios were created using logistic regression for dichotomous outcomes. All statistical analyses were performed using IBM SPSS Statistics version 20 (IBM, Armonk, NY), and P values <0.05 were considered statistically significant.
RESULTS
Patient Characteristics
Of 788 patients admitted for status asthmaticus during the study period, 518 (65.7%) met inclusion criteria; 42 (5.3%) did not meet inclusion criteria due to lack of a documented BMI (Table 1). Most patients were normal weight (59.7%). Approximately one‐third (36.7%) were either overweight or obese. The median age was 8 years, with patients with obesity being significantly older than underweight patients (9 vs 7.5 years, P<0.001). The majority of patients were black/African American (56.9%) and non‐Hispanic (88.6%). The percentage of patients who were obese was higher in patients of other race (29.3%) than whites (20.2%) or blacks (16.3%) (P<0.05). Patients of Hispanic ethnicity had a higher rate of obesity compared to non‐Hispanic patients (37.3% vs 17.4%, P<0.01). There were no differences in BMI categories for insurance.
| Patient Characteristics | Total | Category of Body Mass Index Percentile for Age | ||||
|---|---|---|---|---|---|---|
| Underweight | Normal | Overweight | Obese | P* | ||
| ||||||
| Total patients, n (%) | 518 | 18 (3.5) | 310 (59.8) | 88 (17.0) | 102 (19.7) | |
| Age, y, median (IQR) | 8 (611) | 7.5 (5.89) | 8 (610) | 8 (610) | 9 (712) | <0.001 |
| Gender, n (%) | ||||||
| Male | 309 | 12 (3.9) | 184 (59.5) | 46 (14.9) | 67 (21.7) | 0.27 |
| Female | 209 | 6 (2.9) | 126 (60.3) | 42 (20.1) | 35 (16.7) | |
| Race, n (%) | ||||||
| Non‐Hispanic white | 124 | 8 (6.5) | 76 (61.3) | 15 (12.1) | 25 (20.2) | 0.021 |
| Non‐Hispanic black | 295 | 7 (2.4) | 182 (61.7) | 58 (19.7) | 48 (16.3) | |
| Other | 99 | 3 (3.0) | 52 (52.5) | 15 (15.2) | 29 (29.3) | |
| Ethnicity, n (%) | ||||||
| Hispanic | 59 | 1 (1.7) | 25 (42.4) | 11 (18.6) | 22 (37.3) | 0.002 |
| Non‐Hispanic | 459 | 17 (3.7) | 285 (62.1) | 77 (16.8) | 80 (17.4) | |
| Insurance, n (%) | ||||||
| Private | 163 | 10 (6.1) | 97 (59.5) | 28 (17.2) | 28 (17.2) | 0.48 |
| Public | 313 | 7 (2.2) | 190 (60.7) | 51 (16.3) | 65 (20.8) | |
| Other | 42 | 1 (2.4) | 23 (54.8) | 9 (21.4) | 9 (21.4) | |
LOS and Resource Utilization
The median LOS for all patients was approximately 1 day (Table 2). The median number of albuterol treatments in the inpatient setting was 14 (IQR, 824). When albuterol treatments given in the ED were included, the median number of treatments increased to 38 (IQR, 2848). Approximately one‐half of patients required supplemental oxygen, one‐third received IV fluids, and one‐fifth received either IV or IM steroids (with all but 1.6% of the remaining patients receiving oral steroids). Less than 5% of the study population received magnesium sulfate, epinephrine, required intensive care unit (ICU) admission, or were readmitted for status asthmaticus within 30 days. Approximately 15% of patients received a chest radiograph. The median adjusted billed charge was approximately $7,000. There were no differences in any of these outcomes by BMI category (P>0.05).
| Total | Body Mass Index Category | ||||
|---|---|---|---|---|---|
| Underweight | Normal | Overweight | Obese | ||
| |||||
| Total Patients, n (%) | 518 | 18 (3.5) | 310 (59.8) | 88 (17.0) | 102 (19.7) |
| LOS, h, median (IQR) | 26 (1841) | 41 (19.560.5) | 26 (1841) | 26 (19.2540) | 31 (1942) |
| Inpatient albuterol equivalents, median (IQR) | 14(824) | 19 (9.528) | 14 (824) | 14 (8.522) | 16 (824) |
| Total albuterol equivalents, median (IQR) | 38 (2848) | 34 (2734) | 36 (2848) | 37 (2849.5) | 40 (3052) |
| Adjusted billed charges, $, median (IQR) | 6,999.5 (52929258) | 7,457 (56048536) | 6876 (52379390) | 7056 (54099061) | 7198 (53319306) |
| All readmits, n (%) | 44 (8.5) | 2 (11.1) | 29 (9.4) | 7 (8.0) | 6 (5.9) |
| Readmits within 30 days, n (%) | 11 (2.1) | 1 (5.6) | 7 (2.3) | 1 (1.1) | 2 (2.0) |
| ICU admissions, n (%) | 24 (4.6) | 0 (0) | 13 (4.2) | 7 (8.0) | 4 (3.9) |
| Chest radiograph, n (%) | 64 (12.4) | 5 (27.8) | 34 (11.0) | 12 (13.6) | 13 (12.7) |
| Oxygen, n (%) | 255 (49.2) | 11 (61.1) | 157 (50.6) | 42 (47.7) | 45 (44.1) |
| IV/IM steroid, n (%) | 93 (18.0) | 2 (11.1) | 53 (17.1) | 18 (20.5) | 20 (19.6) |
| Epinephrine, n (%) | 2 (0.4) | 0 (0) | 2 (0.6) | 0 (0) | 0 (0) |
| Magnesium, n (%) | 15 (2.9) | 0 (0) | 8 (2.6) | 3 (3.4) | 4 (3.9) |
| IV fluids, n (%) | 152 (29.3) | 4 (22.2) | 85 (27.4) | 31 (35.2) | 32 (31.4) |
Multivariable Results
After adjusting for age, gender, race, ethnicity, and insurance, the decile of BMI percentile for age showed a small negative association with LOS. Specifically, for each decile increase for BMI percentile for age, LOS decreased by approximately 2%. BMI percentile for age was not associated with other measures of resource utilization including total albuterol use, adjusted billed charges, readmission, ICU care, receipt of supplemental oxygen or a chest radiograph, IV fluids, or other medications (IV/IM steroids, epinephrine, or magnesium sulfate).
DISCUSSION
Our study suggests that the decile of BMI percentile for age is inversely associated with LOS but did not have a clinically meaningful effect. Secondary measures, such as total albuterol needs and adjusted billed charges, did not show an association with BMI percentile for age. There were also no associations between BMI percentile for age and other resource utilization outcomes.
Our findings differ from previous studies examining in‐hospital status asthmaticus in children who are overweight or obese. In addition, the present study was able to adjust for therapies received prior to admission. Carroll et al. demonstrated an increased LOS of approximately 3 days for overweight or obese asthmatics admitted to the ICU with status asthmaticus as well as increased duration of supplemental oxygen, continuous albuterol, and intravenous steroids.[20] It is possible that differences in methodology (ie, weight‐for‐age percentile vs BMI percentile for age, inclusion of ED treatments), different thresholds for treatment of status asthmaticus outside the ICU, or differences in patient populations studied (ie, only ICU patients vs all in‐hospital patients) explain the difference between their findings and the present study. The present study's use of BMI percentile for age follows current recommendations for classifying a patient as obese or overweight.[23, 24, 25] However, the use of classifications other than BMI percentile for age would tend to bias toward the null hypothesis, whereas in Carroll's study children who were overweight or obese had increased resource utilization. Additionally, in the time frame between this publication and the current study, many hospitals worked to standardize asthma hospitalizations by creating weaning protocols for albuterol, thereby decreasing LOS for all asthmatics, which may also affect the differences in LOS between groups of obese and nonobese patients.[35]
Woolford et al. found approximately a one‐half‐day increase in LOS and $2,000 higher mean charges for patients admitted with status asthmaticus and a secondary diagnosis of obesity.[8, 9] Study location and differing methods for defining obesity may account for the discrepancy between Woolford's findings and our study. We examined children admitted to the inpatient floor of a tertiary care children's hospital compared to Woolford et al.'s examination of pediatric patients admitted to all hospitals via the Kids' Inpatient Database (Healthcare Cost and Utilization Project, Agency for Healthcare Research and Quality). That study also relied on the coding of obesity as an ICD‐9 diagnosis, rather than examining the BMI of all admitted patients. Previous research has demonstrated that relying on a coded diagnosis of obesity is not as sensitive as measurement.[26] By relying on ICD‐9 diagnosis coding, only patients with very high BMIs may be diagnosed with obesity during the admission and therefore only associations between very high BMI and status asthmaticus will be examined.
There are several limitations to our findings. First, our study was limited to a single, tertiary care children's hospital and may not be generalizable to other hospitals. Our hospital standardizes the treatment of inpatient status asthmatics by formation of a respiratory care plan, involving interval scoring of respiratory symptoms and automatic spacing of albuterol treatments. This likely minimizes physician‐to‐physician variation. Second, we included only those patients who were initially treated within the ED associated with the admitting hospital to minimize the effects of timing for treatments prior to admittance. This excluded those patients first cared for by their primary care physician or by an outlying ED. Therefore, our sample may be biased toward a study population less connected to a medical home and therefore possibly poorer asthma control. Third, to utilize the most accurate method to define obesity, we excluded approximately 5% of eligible patients because BMI was unavailable. This may have included children with more severe asthma symptoms, as a height measurement may have been deferred due to their higher acuity. Asthma severity or chronicity would be associated with our outcomes of interest. However, we were unable to collect reliable data on severity or chronicity. Finally, to measure the amount of total albuterol needed by a patient during the ED and inpatient admissions, albuterol treatments delivered by MDI, nebulizer, or continuously were converted into total albuterol. Although based upon total milligram dosing and studies comparing routes of albuterol administration,[34] the validity of this conversion is unknown.
CONCLUSION
Although BMI percentile for age is inversely associated with LOS for in‐hospital pediatric status asthmaticus, the impact of BMI on this outcome likely is not clinically meaningful. Future investigations should examine other elements of BMI and in‐hospital status asthmaticus, such as any associations between BMI and admission rates.
Acknowledgements
The authors offer their appreciation to their research assistant, Amy Lee, for her support and dedication to this project.
Disclosures
Internal funds from Children's Mercy Hospital and Clinics supported the conduct of this work. The authors report no conflicts of interest.
- , , . Incidence of childhood obesity in the United States. N Engl J Med. 2014;370(5):403–411.
- , . Prevalence and trends in obesity and severe obesity among children in the United States, 1999–2012. JAMA Pediatr. 2014;168(6):561–566.
- , , , . Effects of childhood obesity on hospital care and costs, 1999–2005. Health Affairs. 2009;28(4):w751–w760.
- , , , , . Influence of obesity on clinical outcomes in hospitalized children: a systematic review. JAMA Pediatr. 2013;167(5):476–482.
- , . Appendicitis in the obese child. J Pediatr Surg. 2007;42(5):857–861.
- , , , , . Obesity: influence on length of hospital stay for the pediatric burn patient. J Burn Care Res. 2010;31(2):251–256.
- , , , , , . The impact of obesity on severely injured children and adolescents. J Pediatr Surg. 2006;41(1):88–91.
- , , , . Incremental hospital charges associated with obesity as a secondary diagnosis in children. Obesity (Silver Spring). 2007;15(7):1895–1901.
- , , , . Persistent gap of incremental charges for obesity as a secondary diagnosis in common pediatric hospitalizations. J Hosp Med. 2009;4(3):149–156.
- , , , . Resource utilization and expenditures for overweight and obese children. Arch Pediatr Adolesc Med. 2007;161(1):11–14.
- , , , , , . Decreased response to inhaled steroids in overweight and obese asthmatic children. J Allergy Clin Immunol. 2011;127(3):741–749.
- , , , , . Body mass and glucocorticoid response in asthma. Am J Respir Crit Care Med. 2008;178(7):682–687.
- , , , ; National Heart, Lung, and Blood Institute's Asthma Clinical Research Network. Body mass index and phenotype in subjects with mild‐to‐moderate persistent asthma. J Allergy Clin Immunol. 2009;123(6):1328–1334.e1.
- , . Obesity and asthma disease phenotypes. Curr Opin Allergy Clin Immunol. 2012;12(1):76–81.
- , . Obesity and the lung: 4. Obesity and asthma. Thorax. 2008;63(11):1018–1023.
- , , , et al. Body mass index and response to asthma therapy: fluticasone propionate/salmeterol versus montelukast. J Asthma. 2010;47(1):76–82.
- , , , et al. Effect of obesity on clinical presentation and response to treatment in asthma. J Asthma. 2006;43(7):553–558.
- , , , . Asthma and obesity in three‐year‐old urban children: role of sex and home environment. J Pediatr. 2011;159(1):14–20.
- , , , . Care of Children and Adolescents in U.S. Hospitals. Rockville, MD: Agency for Healthcare Research and Quality; 2003. Available at: http://archive.ahrq.gov/data/hcup/factbk4/factbk4.pdf. Accessed February 12, 2014.
- , , , . Childhood obesity increases duration of therapy during severe asthma exacerbations. Pediatr Crit Care Med. 2006;7(6):527–531.
- , , , , . Childhood overweight increases hospital admission rates for asthma. Pediatr. 2007;120(4):734–740.
- , , , . Body mass index and acute asthma severity among children presenting to the emergency department. Pediatr Allergy Immunol. 2009;21(3):480–488.
- Centers for Disease Control and Prevention. Basics about childhood obesity. Available at: http://www.cdc.gov/obesity/childhood/basics.html. Accessed February 12, 2014.
- . Screening and interventions for childhood overweight: a summary of evidence for the US Preventive Services Task Force. Pediatrics. 2005;116(1):e125–e144.
- ; Expert Committee. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120(suppl 4):S164–S192.
- , , , . Comparison of ICD code‐based diagnosis of obesity with measured obesity in children and the implications for health care cost estimates. BMC Med Res Methodol. 2011;11(1):173.
- , ; American Academy of Pediatrics Committee on Nutrition. Prevention of pediatric overweight and obesity. Pediatrics. 2003;112(2):424–430.
- . Development of the 3M all patient‐refined diagnosis‐related groups (APR DRGs). Available at: http//www.ahrq.gov/legacy/qual/mortality/Hughes3.htm. Accessed March 1, 2014.
- . 3M Health Information Systems (HIS) APR‐DRG classification software: overview. Available at: http://www.ahrq.gov/legacy/qual/mortality/Hughessumm.htm. Accessed March 1, 2014.
- , , , , . Shifting place of death among children with complex chronic conditions in the United States, 1989–2003. JAMA. 2007;297(24):2725–2732.
- , , . Pediatric deaths attributable to complex chronic conditions: a population‐based study of Washington State, 1980–1997. Pediatrics. 2000;106(1 pt 2):205–209.
- , , , et al. Comparative effectiveness of pleural drainage procedures for the treatment of complicated pneumonia in childhood. J Hosp Med. 2011;6(5):256–263.
- , , , , , . Adjunct corticosteroids in children hospitalized with community‐acquired pneumonia. Pediatrics. 2011;127(2):e255–e263.
- , , . Holding chambers (spacers) versus nebulisers for beta‐agonist treatment of acute asthma. Cochrane Database Syst Rev. 2013;9:CD000052.
- , , , . Effectiveness of a clinical pathway for inpatient asthma management. Pediatrics. 2000;106(5):1006–1012.
Pediatric hospitalizations for obesity‐related conditions have doubled in the last decade, mirroring the trend of higher levels of childhood obesity in the United States.[1, 2, 3] Recent studies have demonstrated worsened pediatric in‐hospital outcomes, including mortality and increased resource utilization, for children with obesity across a range of diagnoses.[4, 5, 6, 7, 8, 9, 10] Although the mechanisms driving the association between obesity and in‐hospital outcomes are not fully known, for asthma it is believed that adipocytes expressing inflammatory markers create a low level of systemic inflammation, thereby increasing the severity of allergic‐type illnesses and decreasing the response to anti‐inflammatory medications, such as steroids.[11, 12, 13, 14, 15, 16, 17, 18] The relationship of obesity and in‐hospital asthma outcomes is of particular interest because status asthmaticus is the most common reason for admission in children aged 3 to 12 years, accounting for approximately 150,000 admissions (7.4% of all hospitalizations for children and adolescents) and $835 million in hospital costs annually.[19]
Few prior studies have examined the association of obesity and asthma outcomes in the in‐hospital setting. The studies examining this association have found patients with obesity to have a longer hospital length of stay (LOS) and increased hospital costs.[8, 9, 20] Obesity has also been associated with increased respiratory treatments and supplemental oxygen requirements.[20] Associations between obesity and admission rates from the emergency department (ED) for pediatric asthma have been inconsistent.[21, 22] Most of these prior studies had several limitations in identifying patients with obesity, including using weight‐for‐age percentiles or International Classification of Diseases, Ninth Revision (ICD‐9) codes, rather than body mass index (BMI) percentile for age, the currently recommended method.[23, 24, 25] Methods other than BMI have the potential to either underestimate obesity (ie, ICD‐9 codes)[26] or to confound weight with adiposity (ie, weight‐for‐age percentiles),[27] thereby skewing the primary exposure of interest.
In the present study, we sought to examine associations between obesity and in‐hospital outcomes for pediatric status asthmaticus using the currently endorsed method for identifying obesity in children, BMI percentile for age.[23, 24, 25] The outcomes of interest included a broad range of in‐hospital measures, including resource utilization (medication and radiology use), readmission rates, billed charges, and LOS. We hypothesize that obesity, due to its proinflammatory state, would result in increased LOS, increased resource utilization, and an increased readmission rate for children admitted with status asthmaticus.
METHODS
Data Sources
Data for this retrospective cross‐sectional study were obtained from 2 sources. First, we queried the Pediatric Health Information System (PHIS) administrative database, which draws information from multiple children's hospitals to identify patients at our 2 institutions of interest who met study criteria. The PHIS database also was used to collect patient demographic data. PHIS is an administrative database operated by Children's Hospital Association (Overland Park, KS) containing clinical and billing data from 43 tertiary care, freestanding children's hospitals, including data on 41 ICD‐9 diagnoses, billed charges, and LOS. Based on the primary diagnosis, PHIS assigns each discharge to an All Patient Refined‐Diagnosis Related Group (APR‐DRG v.24) (3M Health information Systems, St. Paul, MN). APR‐DRGs allow similar diagnoses to be grouped together.[28, 29] PHIS also uses ICD‐9 codes to identify patients with a complex chronic condition (CCC).[30, 31] CCCs are those conditions that can be reasonably expected to last at least 12 months (unless death intervenes) and to involve either several different organ systems or one system severely enough to require specialty pediatric care and probably some period of hospitalization in a tertiary care center.[30, 31] PHIS data quality is ensured through a collaborative effort of the participating hospitals, the Children's Hospital Association, and Truven Healthcare.
Second, standardized chart reviews were then performed to collect clinical data not found in PHIS: BMI, LOS in hours, and medications administered, including total number of albuterol treatments administered during both the admission and the associated preceding ED visit.
Study Setting and Participants
All admissions examined in this study were at Children's Mercy Hospitals. Children's Mercy Hospitals includes 2 separate hospitals: 1 hospital is a 354‐bed academic, tertiary care freestanding children's hospital located in Kansas City, Missouri; a second, smaller, 50‐bed freestanding hospital is located in Overland Park, Kansas. Both hospitals have pediatric emergency departments. Inclusion criteria included patients aged 5 to 17 years discharged for status asthmaticus (APR‐DRG 141) at Children's Mercy Hospital from October 1, 2011 to September 30, 2012, with a recorded BMI during the admission or within 30 days of the admission. Patients between the ages of 2 and 5 years old were not included because of the incidence of viral‐induced wheezing in this age group and therefore possible miscoding of the asthma diagnosis. Exclusion criteria included a concurrent diagnosis of a CCC or bacterial pneumonia because these conditions could alter LOS, resource utilization, and readmission rates independent of the subject's status asthmaticus. In addition, to account for differences in the amount of treatment given in the pre‐inpatient setting, patients not initially treated through the hospital's ED were excluded. For patients with multiple admissions during the study period for the same diagnosis, only the index admission was examined. The institutional review board at Children's Mercy Hospital approved this study with waiver of informed consent.
Study Definitions
BMI percentile for age was used as both a continuous and categorical predictor variable. As a categorical variable it was divided into 4 categories: underweight (BMI <5%), normal weight (BMI 5%84%), overweight (BMI 85%94%), and obese (BMI 95%).[23] Race was categorized non‐Hispanic white, non‐Hispanic black, and other. Other included Asian, Pacific Islander, American Indian, and other. Ethnicity was categorized as Hispanic and non‐Hispanic. Insurance categories included private (commercial or TRICARE), public (Medicaid and Title V), and other (uninsured, self‐pay, and other). Adjusted billed charges were calculated for each hospitalization. Adjusted billed charges are the billed charges adjusted by the US Centers of Medicare and Medicaid Services' price/wage index for the study site's location.[32, 33]
To compare albuterol of different delivery methods, albuterol equivalents were calculated. Based upon prior research demonstrating equal efficacy between albuterol administered by nebulizer and metered‐dose inhaler (MDI),[34] every 2.5 mg of albuterol administered by nebulizer was treated as equivalent to 2 sprays of albuterol (90 g/spray) administered by MDI. Therefore, albuterol 2.5 mg nebulized and 2 sprays of albuterol (90 g/spray) were each defined as 1 albuterol equivalent. To compare continuous administration of nebulized albuterol with intermittent administration of albuterol, the total milligrams of continuously nebulized albuterol were examined. Per protocol at the study site, 10 mg per hour of continuous albuterol are administered for patients 5 years and younger and, for children 6 years and older, 15 mg per hour of continuous albuterol are administered. Based upon milligrams of albuterol nebulized, 5‐year‐old subjects receiving an hour of continuous albuterol would equal 4 albuterol equivalents (or 4 treatments of nebulized albuterol 2.5 mg/treatment or 4 treatments of albuterol 90 g/spray 2 sprays/treatment); for patients 6 years and older, an hour of continuous albuterol would equal 6 albuterol equivalents (or 6 treatments of nebulized albuterol 2.5 mg/treatment or 6 treatments of albuterol 90 g/spray 2 sprays/treatment). The variable total albuterol was then created to include albuterol equivalents delivered by metered dose inhaler and as both single and continuous nebulized treatments.
Main Exposure
The main exposure of interest was BMI percentile for age.
Outcome Measures
The main outcome measure was inpatient LOS measured in hours. Secondary outcome measures included the total albuterol (in the inpatient setting as well as combined inpatient and ED settings) and the administration of intravenous IV fluids and intramuscular (IM) or IV systemic steroids. Other secondary measures included readmission for status asthmaticus during the study period, adjusted billed charges, and inpatient chest radiograph utilization.
Statistical Analyses
We summarized categorical variables with frequencies and percentages, and used [2] test across BMI categories. The non‐normal distribution of continuous dependent variables (LOS, number of albuterol treatments, billed charges) were summarized with medians and interquartile ranges (IQRs). Kruskal‐Wallis test was used to examine outcomes across BMI categories. For regression models, BMI percentile for age was divided into deciles and treated as a continuous predictor. Factors used in the regression models included age, gender, race, ethnicity, and insurance. Total albuterol received in the ED was also included in the model to adjust for differences in the amount of treatment received prior to admission. Incidence rate ratios were created using Poisson regression for continuous outcomes (LOS, billed charges, and number of albuterol equivalent treatments administered), and odds ratios were created using logistic regression for dichotomous outcomes. All statistical analyses were performed using IBM SPSS Statistics version 20 (IBM, Armonk, NY), and P values <0.05 were considered statistically significant.
RESULTS
Patient Characteristics
Of 788 patients admitted for status asthmaticus during the study period, 518 (65.7%) met inclusion criteria; 42 (5.3%) did not meet inclusion criteria due to lack of a documented BMI (Table 1). Most patients were normal weight (59.7%). Approximately one‐third (36.7%) were either overweight or obese. The median age was 8 years, with patients with obesity being significantly older than underweight patients (9 vs 7.5 years, P<0.001). The majority of patients were black/African American (56.9%) and non‐Hispanic (88.6%). The percentage of patients who were obese was higher in patients of other race (29.3%) than whites (20.2%) or blacks (16.3%) (P<0.05). Patients of Hispanic ethnicity had a higher rate of obesity compared to non‐Hispanic patients (37.3% vs 17.4%, P<0.01). There were no differences in BMI categories for insurance.
| Patient Characteristics | Total | Category of Body Mass Index Percentile for Age | ||||
|---|---|---|---|---|---|---|
| Underweight | Normal | Overweight | Obese | P* | ||
| ||||||
| Total patients, n (%) | 518 | 18 (3.5) | 310 (59.8) | 88 (17.0) | 102 (19.7) | |
| Age, y, median (IQR) | 8 (611) | 7.5 (5.89) | 8 (610) | 8 (610) | 9 (712) | <0.001 |
| Gender, n (%) | ||||||
| Male | 309 | 12 (3.9) | 184 (59.5) | 46 (14.9) | 67 (21.7) | 0.27 |
| Female | 209 | 6 (2.9) | 126 (60.3) | 42 (20.1) | 35 (16.7) | |
| Race, n (%) | ||||||
| Non‐Hispanic white | 124 | 8 (6.5) | 76 (61.3) | 15 (12.1) | 25 (20.2) | 0.021 |
| Non‐Hispanic black | 295 | 7 (2.4) | 182 (61.7) | 58 (19.7) | 48 (16.3) | |
| Other | 99 | 3 (3.0) | 52 (52.5) | 15 (15.2) | 29 (29.3) | |
| Ethnicity, n (%) | ||||||
| Hispanic | 59 | 1 (1.7) | 25 (42.4) | 11 (18.6) | 22 (37.3) | 0.002 |
| Non‐Hispanic | 459 | 17 (3.7) | 285 (62.1) | 77 (16.8) | 80 (17.4) | |
| Insurance, n (%) | ||||||
| Private | 163 | 10 (6.1) | 97 (59.5) | 28 (17.2) | 28 (17.2) | 0.48 |
| Public | 313 | 7 (2.2) | 190 (60.7) | 51 (16.3) | 65 (20.8) | |
| Other | 42 | 1 (2.4) | 23 (54.8) | 9 (21.4) | 9 (21.4) | |
LOS and Resource Utilization
The median LOS for all patients was approximately 1 day (Table 2). The median number of albuterol treatments in the inpatient setting was 14 (IQR, 824). When albuterol treatments given in the ED were included, the median number of treatments increased to 38 (IQR, 2848). Approximately one‐half of patients required supplemental oxygen, one‐third received IV fluids, and one‐fifth received either IV or IM steroids (with all but 1.6% of the remaining patients receiving oral steroids). Less than 5% of the study population received magnesium sulfate, epinephrine, required intensive care unit (ICU) admission, or were readmitted for status asthmaticus within 30 days. Approximately 15% of patients received a chest radiograph. The median adjusted billed charge was approximately $7,000. There were no differences in any of these outcomes by BMI category (P>0.05).
| Total | Body Mass Index Category | ||||
|---|---|---|---|---|---|
| Underweight | Normal | Overweight | Obese | ||
| |||||
| Total Patients, n (%) | 518 | 18 (3.5) | 310 (59.8) | 88 (17.0) | 102 (19.7) |
| LOS, h, median (IQR) | 26 (1841) | 41 (19.560.5) | 26 (1841) | 26 (19.2540) | 31 (1942) |
| Inpatient albuterol equivalents, median (IQR) | 14(824) | 19 (9.528) | 14 (824) | 14 (8.522) | 16 (824) |
| Total albuterol equivalents, median (IQR) | 38 (2848) | 34 (2734) | 36 (2848) | 37 (2849.5) | 40 (3052) |
| Adjusted billed charges, $, median (IQR) | 6,999.5 (52929258) | 7,457 (56048536) | 6876 (52379390) | 7056 (54099061) | 7198 (53319306) |
| All readmits, n (%) | 44 (8.5) | 2 (11.1) | 29 (9.4) | 7 (8.0) | 6 (5.9) |
| Readmits within 30 days, n (%) | 11 (2.1) | 1 (5.6) | 7 (2.3) | 1 (1.1) | 2 (2.0) |
| ICU admissions, n (%) | 24 (4.6) | 0 (0) | 13 (4.2) | 7 (8.0) | 4 (3.9) |
| Chest radiograph, n (%) | 64 (12.4) | 5 (27.8) | 34 (11.0) | 12 (13.6) | 13 (12.7) |
| Oxygen, n (%) | 255 (49.2) | 11 (61.1) | 157 (50.6) | 42 (47.7) | 45 (44.1) |
| IV/IM steroid, n (%) | 93 (18.0) | 2 (11.1) | 53 (17.1) | 18 (20.5) | 20 (19.6) |
| Epinephrine, n (%) | 2 (0.4) | 0 (0) | 2 (0.6) | 0 (0) | 0 (0) |
| Magnesium, n (%) | 15 (2.9) | 0 (0) | 8 (2.6) | 3 (3.4) | 4 (3.9) |
| IV fluids, n (%) | 152 (29.3) | 4 (22.2) | 85 (27.4) | 31 (35.2) | 32 (31.4) |
Multivariable Results
After adjusting for age, gender, race, ethnicity, and insurance, the decile of BMI percentile for age showed a small negative association with LOS. Specifically, for each decile increase for BMI percentile for age, LOS decreased by approximately 2%. BMI percentile for age was not associated with other measures of resource utilization including total albuterol use, adjusted billed charges, readmission, ICU care, receipt of supplemental oxygen or a chest radiograph, IV fluids, or other medications (IV/IM steroids, epinephrine, or magnesium sulfate).
DISCUSSION
Our study suggests that the decile of BMI percentile for age is inversely associated with LOS but did not have a clinically meaningful effect. Secondary measures, such as total albuterol needs and adjusted billed charges, did not show an association with BMI percentile for age. There were also no associations between BMI percentile for age and other resource utilization outcomes.
Our findings differ from previous studies examining in‐hospital status asthmaticus in children who are overweight or obese. In addition, the present study was able to adjust for therapies received prior to admission. Carroll et al. demonstrated an increased LOS of approximately 3 days for overweight or obese asthmatics admitted to the ICU with status asthmaticus as well as increased duration of supplemental oxygen, continuous albuterol, and intravenous steroids.[20] It is possible that differences in methodology (ie, weight‐for‐age percentile vs BMI percentile for age, inclusion of ED treatments), different thresholds for treatment of status asthmaticus outside the ICU, or differences in patient populations studied (ie, only ICU patients vs all in‐hospital patients) explain the difference between their findings and the present study. The present study's use of BMI percentile for age follows current recommendations for classifying a patient as obese or overweight.[23, 24, 25] However, the use of classifications other than BMI percentile for age would tend to bias toward the null hypothesis, whereas in Carroll's study children who were overweight or obese had increased resource utilization. Additionally, in the time frame between this publication and the current study, many hospitals worked to standardize asthma hospitalizations by creating weaning protocols for albuterol, thereby decreasing LOS for all asthmatics, which may also affect the differences in LOS between groups of obese and nonobese patients.[35]
Woolford et al. found approximately a one‐half‐day increase in LOS and $2,000 higher mean charges for patients admitted with status asthmaticus and a secondary diagnosis of obesity.[8, 9] Study location and differing methods for defining obesity may account for the discrepancy between Woolford's findings and our study. We examined children admitted to the inpatient floor of a tertiary care children's hospital compared to Woolford et al.'s examination of pediatric patients admitted to all hospitals via the Kids' Inpatient Database (Healthcare Cost and Utilization Project, Agency for Healthcare Research and Quality). That study also relied on the coding of obesity as an ICD‐9 diagnosis, rather than examining the BMI of all admitted patients. Previous research has demonstrated that relying on a coded diagnosis of obesity is not as sensitive as measurement.[26] By relying on ICD‐9 diagnosis coding, only patients with very high BMIs may be diagnosed with obesity during the admission and therefore only associations between very high BMI and status asthmaticus will be examined.
There are several limitations to our findings. First, our study was limited to a single, tertiary care children's hospital and may not be generalizable to other hospitals. Our hospital standardizes the treatment of inpatient status asthmatics by formation of a respiratory care plan, involving interval scoring of respiratory symptoms and automatic spacing of albuterol treatments. This likely minimizes physician‐to‐physician variation. Second, we included only those patients who were initially treated within the ED associated with the admitting hospital to minimize the effects of timing for treatments prior to admittance. This excluded those patients first cared for by their primary care physician or by an outlying ED. Therefore, our sample may be biased toward a study population less connected to a medical home and therefore possibly poorer asthma control. Third, to utilize the most accurate method to define obesity, we excluded approximately 5% of eligible patients because BMI was unavailable. This may have included children with more severe asthma symptoms, as a height measurement may have been deferred due to their higher acuity. Asthma severity or chronicity would be associated with our outcomes of interest. However, we were unable to collect reliable data on severity or chronicity. Finally, to measure the amount of total albuterol needed by a patient during the ED and inpatient admissions, albuterol treatments delivered by MDI, nebulizer, or continuously were converted into total albuterol. Although based upon total milligram dosing and studies comparing routes of albuterol administration,[34] the validity of this conversion is unknown.
CONCLUSION
Although BMI percentile for age is inversely associated with LOS for in‐hospital pediatric status asthmaticus, the impact of BMI on this outcome likely is not clinically meaningful. Future investigations should examine other elements of BMI and in‐hospital status asthmaticus, such as any associations between BMI and admission rates.
Acknowledgements
The authors offer their appreciation to their research assistant, Amy Lee, for her support and dedication to this project.
Disclosures
Internal funds from Children's Mercy Hospital and Clinics supported the conduct of this work. The authors report no conflicts of interest.
Pediatric hospitalizations for obesity‐related conditions have doubled in the last decade, mirroring the trend of higher levels of childhood obesity in the United States.[1, 2, 3] Recent studies have demonstrated worsened pediatric in‐hospital outcomes, including mortality and increased resource utilization, for children with obesity across a range of diagnoses.[4, 5, 6, 7, 8, 9, 10] Although the mechanisms driving the association between obesity and in‐hospital outcomes are not fully known, for asthma it is believed that adipocytes expressing inflammatory markers create a low level of systemic inflammation, thereby increasing the severity of allergic‐type illnesses and decreasing the response to anti‐inflammatory medications, such as steroids.[11, 12, 13, 14, 15, 16, 17, 18] The relationship of obesity and in‐hospital asthma outcomes is of particular interest because status asthmaticus is the most common reason for admission in children aged 3 to 12 years, accounting for approximately 150,000 admissions (7.4% of all hospitalizations for children and adolescents) and $835 million in hospital costs annually.[19]
Few prior studies have examined the association of obesity and asthma outcomes in the in‐hospital setting. The studies examining this association have found patients with obesity to have a longer hospital length of stay (LOS) and increased hospital costs.[8, 9, 20] Obesity has also been associated with increased respiratory treatments and supplemental oxygen requirements.[20] Associations between obesity and admission rates from the emergency department (ED) for pediatric asthma have been inconsistent.[21, 22] Most of these prior studies had several limitations in identifying patients with obesity, including using weight‐for‐age percentiles or International Classification of Diseases, Ninth Revision (ICD‐9) codes, rather than body mass index (BMI) percentile for age, the currently recommended method.[23, 24, 25] Methods other than BMI have the potential to either underestimate obesity (ie, ICD‐9 codes)[26] or to confound weight with adiposity (ie, weight‐for‐age percentiles),[27] thereby skewing the primary exposure of interest.
In the present study, we sought to examine associations between obesity and in‐hospital outcomes for pediatric status asthmaticus using the currently endorsed method for identifying obesity in children, BMI percentile for age.[23, 24, 25] The outcomes of interest included a broad range of in‐hospital measures, including resource utilization (medication and radiology use), readmission rates, billed charges, and LOS. We hypothesize that obesity, due to its proinflammatory state, would result in increased LOS, increased resource utilization, and an increased readmission rate for children admitted with status asthmaticus.
METHODS
Data Sources
Data for this retrospective cross‐sectional study were obtained from 2 sources. First, we queried the Pediatric Health Information System (PHIS) administrative database, which draws information from multiple children's hospitals to identify patients at our 2 institutions of interest who met study criteria. The PHIS database also was used to collect patient demographic data. PHIS is an administrative database operated by Children's Hospital Association (Overland Park, KS) containing clinical and billing data from 43 tertiary care, freestanding children's hospitals, including data on 41 ICD‐9 diagnoses, billed charges, and LOS. Based on the primary diagnosis, PHIS assigns each discharge to an All Patient Refined‐Diagnosis Related Group (APR‐DRG v.24) (3M Health information Systems, St. Paul, MN). APR‐DRGs allow similar diagnoses to be grouped together.[28, 29] PHIS also uses ICD‐9 codes to identify patients with a complex chronic condition (CCC).[30, 31] CCCs are those conditions that can be reasonably expected to last at least 12 months (unless death intervenes) and to involve either several different organ systems or one system severely enough to require specialty pediatric care and probably some period of hospitalization in a tertiary care center.[30, 31] PHIS data quality is ensured through a collaborative effort of the participating hospitals, the Children's Hospital Association, and Truven Healthcare.
Second, standardized chart reviews were then performed to collect clinical data not found in PHIS: BMI, LOS in hours, and medications administered, including total number of albuterol treatments administered during both the admission and the associated preceding ED visit.
Study Setting and Participants
All admissions examined in this study were at Children's Mercy Hospitals. Children's Mercy Hospitals includes 2 separate hospitals: 1 hospital is a 354‐bed academic, tertiary care freestanding children's hospital located in Kansas City, Missouri; a second, smaller, 50‐bed freestanding hospital is located in Overland Park, Kansas. Both hospitals have pediatric emergency departments. Inclusion criteria included patients aged 5 to 17 years discharged for status asthmaticus (APR‐DRG 141) at Children's Mercy Hospital from October 1, 2011 to September 30, 2012, with a recorded BMI during the admission or within 30 days of the admission. Patients between the ages of 2 and 5 years old were not included because of the incidence of viral‐induced wheezing in this age group and therefore possible miscoding of the asthma diagnosis. Exclusion criteria included a concurrent diagnosis of a CCC or bacterial pneumonia because these conditions could alter LOS, resource utilization, and readmission rates independent of the subject's status asthmaticus. In addition, to account for differences in the amount of treatment given in the pre‐inpatient setting, patients not initially treated through the hospital's ED were excluded. For patients with multiple admissions during the study period for the same diagnosis, only the index admission was examined. The institutional review board at Children's Mercy Hospital approved this study with waiver of informed consent.
Study Definitions
BMI percentile for age was used as both a continuous and categorical predictor variable. As a categorical variable it was divided into 4 categories: underweight (BMI <5%), normal weight (BMI 5%84%), overweight (BMI 85%94%), and obese (BMI 95%).[23] Race was categorized non‐Hispanic white, non‐Hispanic black, and other. Other included Asian, Pacific Islander, American Indian, and other. Ethnicity was categorized as Hispanic and non‐Hispanic. Insurance categories included private (commercial or TRICARE), public (Medicaid and Title V), and other (uninsured, self‐pay, and other). Adjusted billed charges were calculated for each hospitalization. Adjusted billed charges are the billed charges adjusted by the US Centers of Medicare and Medicaid Services' price/wage index for the study site's location.[32, 33]
To compare albuterol of different delivery methods, albuterol equivalents were calculated. Based upon prior research demonstrating equal efficacy between albuterol administered by nebulizer and metered‐dose inhaler (MDI),[34] every 2.5 mg of albuterol administered by nebulizer was treated as equivalent to 2 sprays of albuterol (90 g/spray) administered by MDI. Therefore, albuterol 2.5 mg nebulized and 2 sprays of albuterol (90 g/spray) were each defined as 1 albuterol equivalent. To compare continuous administration of nebulized albuterol with intermittent administration of albuterol, the total milligrams of continuously nebulized albuterol were examined. Per protocol at the study site, 10 mg per hour of continuous albuterol are administered for patients 5 years and younger and, for children 6 years and older, 15 mg per hour of continuous albuterol are administered. Based upon milligrams of albuterol nebulized, 5‐year‐old subjects receiving an hour of continuous albuterol would equal 4 albuterol equivalents (or 4 treatments of nebulized albuterol 2.5 mg/treatment or 4 treatments of albuterol 90 g/spray 2 sprays/treatment); for patients 6 years and older, an hour of continuous albuterol would equal 6 albuterol equivalents (or 6 treatments of nebulized albuterol 2.5 mg/treatment or 6 treatments of albuterol 90 g/spray 2 sprays/treatment). The variable total albuterol was then created to include albuterol equivalents delivered by metered dose inhaler and as both single and continuous nebulized treatments.
Main Exposure
The main exposure of interest was BMI percentile for age.
Outcome Measures
The main outcome measure was inpatient LOS measured in hours. Secondary outcome measures included the total albuterol (in the inpatient setting as well as combined inpatient and ED settings) and the administration of intravenous IV fluids and intramuscular (IM) or IV systemic steroids. Other secondary measures included readmission for status asthmaticus during the study period, adjusted billed charges, and inpatient chest radiograph utilization.
Statistical Analyses
We summarized categorical variables with frequencies and percentages, and used [2] test across BMI categories. The non‐normal distribution of continuous dependent variables (LOS, number of albuterol treatments, billed charges) were summarized with medians and interquartile ranges (IQRs). Kruskal‐Wallis test was used to examine outcomes across BMI categories. For regression models, BMI percentile for age was divided into deciles and treated as a continuous predictor. Factors used in the regression models included age, gender, race, ethnicity, and insurance. Total albuterol received in the ED was also included in the model to adjust for differences in the amount of treatment received prior to admission. Incidence rate ratios were created using Poisson regression for continuous outcomes (LOS, billed charges, and number of albuterol equivalent treatments administered), and odds ratios were created using logistic regression for dichotomous outcomes. All statistical analyses were performed using IBM SPSS Statistics version 20 (IBM, Armonk, NY), and P values <0.05 were considered statistically significant.
RESULTS
Patient Characteristics
Of 788 patients admitted for status asthmaticus during the study period, 518 (65.7%) met inclusion criteria; 42 (5.3%) did not meet inclusion criteria due to lack of a documented BMI (Table 1). Most patients were normal weight (59.7%). Approximately one‐third (36.7%) were either overweight or obese. The median age was 8 years, with patients with obesity being significantly older than underweight patients (9 vs 7.5 years, P<0.001). The majority of patients were black/African American (56.9%) and non‐Hispanic (88.6%). The percentage of patients who were obese was higher in patients of other race (29.3%) than whites (20.2%) or blacks (16.3%) (P<0.05). Patients of Hispanic ethnicity had a higher rate of obesity compared to non‐Hispanic patients (37.3% vs 17.4%, P<0.01). There were no differences in BMI categories for insurance.
| Patient Characteristics | Total | Category of Body Mass Index Percentile for Age | ||||
|---|---|---|---|---|---|---|
| Underweight | Normal | Overweight | Obese | P* | ||
| ||||||
| Total patients, n (%) | 518 | 18 (3.5) | 310 (59.8) | 88 (17.0) | 102 (19.7) | |
| Age, y, median (IQR) | 8 (611) | 7.5 (5.89) | 8 (610) | 8 (610) | 9 (712) | <0.001 |
| Gender, n (%) | ||||||
| Male | 309 | 12 (3.9) | 184 (59.5) | 46 (14.9) | 67 (21.7) | 0.27 |
| Female | 209 | 6 (2.9) | 126 (60.3) | 42 (20.1) | 35 (16.7) | |
| Race, n (%) | ||||||
| Non‐Hispanic white | 124 | 8 (6.5) | 76 (61.3) | 15 (12.1) | 25 (20.2) | 0.021 |
| Non‐Hispanic black | 295 | 7 (2.4) | 182 (61.7) | 58 (19.7) | 48 (16.3) | |
| Other | 99 | 3 (3.0) | 52 (52.5) | 15 (15.2) | 29 (29.3) | |
| Ethnicity, n (%) | ||||||
| Hispanic | 59 | 1 (1.7) | 25 (42.4) | 11 (18.6) | 22 (37.3) | 0.002 |
| Non‐Hispanic | 459 | 17 (3.7) | 285 (62.1) | 77 (16.8) | 80 (17.4) | |
| Insurance, n (%) | ||||||
| Private | 163 | 10 (6.1) | 97 (59.5) | 28 (17.2) | 28 (17.2) | 0.48 |
| Public | 313 | 7 (2.2) | 190 (60.7) | 51 (16.3) | 65 (20.8) | |
| Other | 42 | 1 (2.4) | 23 (54.8) | 9 (21.4) | 9 (21.4) | |
LOS and Resource Utilization
The median LOS for all patients was approximately 1 day (Table 2). The median number of albuterol treatments in the inpatient setting was 14 (IQR, 824). When albuterol treatments given in the ED were included, the median number of treatments increased to 38 (IQR, 2848). Approximately one‐half of patients required supplemental oxygen, one‐third received IV fluids, and one‐fifth received either IV or IM steroids (with all but 1.6% of the remaining patients receiving oral steroids). Less than 5% of the study population received magnesium sulfate, epinephrine, required intensive care unit (ICU) admission, or were readmitted for status asthmaticus within 30 days. Approximately 15% of patients received a chest radiograph. The median adjusted billed charge was approximately $7,000. There were no differences in any of these outcomes by BMI category (P>0.05).
| Total | Body Mass Index Category | ||||
|---|---|---|---|---|---|
| Underweight | Normal | Overweight | Obese | ||
| |||||
| Total Patients, n (%) | 518 | 18 (3.5) | 310 (59.8) | 88 (17.0) | 102 (19.7) |
| LOS, h, median (IQR) | 26 (1841) | 41 (19.560.5) | 26 (1841) | 26 (19.2540) | 31 (1942) |
| Inpatient albuterol equivalents, median (IQR) | 14(824) | 19 (9.528) | 14 (824) | 14 (8.522) | 16 (824) |
| Total albuterol equivalents, median (IQR) | 38 (2848) | 34 (2734) | 36 (2848) | 37 (2849.5) | 40 (3052) |
| Adjusted billed charges, $, median (IQR) | 6,999.5 (52929258) | 7,457 (56048536) | 6876 (52379390) | 7056 (54099061) | 7198 (53319306) |
| All readmits, n (%) | 44 (8.5) | 2 (11.1) | 29 (9.4) | 7 (8.0) | 6 (5.9) |
| Readmits within 30 days, n (%) | 11 (2.1) | 1 (5.6) | 7 (2.3) | 1 (1.1) | 2 (2.0) |
| ICU admissions, n (%) | 24 (4.6) | 0 (0) | 13 (4.2) | 7 (8.0) | 4 (3.9) |
| Chest radiograph, n (%) | 64 (12.4) | 5 (27.8) | 34 (11.0) | 12 (13.6) | 13 (12.7) |
| Oxygen, n (%) | 255 (49.2) | 11 (61.1) | 157 (50.6) | 42 (47.7) | 45 (44.1) |
| IV/IM steroid, n (%) | 93 (18.0) | 2 (11.1) | 53 (17.1) | 18 (20.5) | 20 (19.6) |
| Epinephrine, n (%) | 2 (0.4) | 0 (0) | 2 (0.6) | 0 (0) | 0 (0) |
| Magnesium, n (%) | 15 (2.9) | 0 (0) | 8 (2.6) | 3 (3.4) | 4 (3.9) |
| IV fluids, n (%) | 152 (29.3) | 4 (22.2) | 85 (27.4) | 31 (35.2) | 32 (31.4) |
Multivariable Results
After adjusting for age, gender, race, ethnicity, and insurance, the decile of BMI percentile for age showed a small negative association with LOS. Specifically, for each decile increase for BMI percentile for age, LOS decreased by approximately 2%. BMI percentile for age was not associated with other measures of resource utilization including total albuterol use, adjusted billed charges, readmission, ICU care, receipt of supplemental oxygen or a chest radiograph, IV fluids, or other medications (IV/IM steroids, epinephrine, or magnesium sulfate).
DISCUSSION
Our study suggests that the decile of BMI percentile for age is inversely associated with LOS but did not have a clinically meaningful effect. Secondary measures, such as total albuterol needs and adjusted billed charges, did not show an association with BMI percentile for age. There were also no associations between BMI percentile for age and other resource utilization outcomes.
Our findings differ from previous studies examining in‐hospital status asthmaticus in children who are overweight or obese. In addition, the present study was able to adjust for therapies received prior to admission. Carroll et al. demonstrated an increased LOS of approximately 3 days for overweight or obese asthmatics admitted to the ICU with status asthmaticus as well as increased duration of supplemental oxygen, continuous albuterol, and intravenous steroids.[20] It is possible that differences in methodology (ie, weight‐for‐age percentile vs BMI percentile for age, inclusion of ED treatments), different thresholds for treatment of status asthmaticus outside the ICU, or differences in patient populations studied (ie, only ICU patients vs all in‐hospital patients) explain the difference between their findings and the present study. The present study's use of BMI percentile for age follows current recommendations for classifying a patient as obese or overweight.[23, 24, 25] However, the use of classifications other than BMI percentile for age would tend to bias toward the null hypothesis, whereas in Carroll's study children who were overweight or obese had increased resource utilization. Additionally, in the time frame between this publication and the current study, many hospitals worked to standardize asthma hospitalizations by creating weaning protocols for albuterol, thereby decreasing LOS for all asthmatics, which may also affect the differences in LOS between groups of obese and nonobese patients.[35]
Woolford et al. found approximately a one‐half‐day increase in LOS and $2,000 higher mean charges for patients admitted with status asthmaticus and a secondary diagnosis of obesity.[8, 9] Study location and differing methods for defining obesity may account for the discrepancy between Woolford's findings and our study. We examined children admitted to the inpatient floor of a tertiary care children's hospital compared to Woolford et al.'s examination of pediatric patients admitted to all hospitals via the Kids' Inpatient Database (Healthcare Cost and Utilization Project, Agency for Healthcare Research and Quality). That study also relied on the coding of obesity as an ICD‐9 diagnosis, rather than examining the BMI of all admitted patients. Previous research has demonstrated that relying on a coded diagnosis of obesity is not as sensitive as measurement.[26] By relying on ICD‐9 diagnosis coding, only patients with very high BMIs may be diagnosed with obesity during the admission and therefore only associations between very high BMI and status asthmaticus will be examined.
There are several limitations to our findings. First, our study was limited to a single, tertiary care children's hospital and may not be generalizable to other hospitals. Our hospital standardizes the treatment of inpatient status asthmatics by formation of a respiratory care plan, involving interval scoring of respiratory symptoms and automatic spacing of albuterol treatments. This likely minimizes physician‐to‐physician variation. Second, we included only those patients who were initially treated within the ED associated with the admitting hospital to minimize the effects of timing for treatments prior to admittance. This excluded those patients first cared for by their primary care physician or by an outlying ED. Therefore, our sample may be biased toward a study population less connected to a medical home and therefore possibly poorer asthma control. Third, to utilize the most accurate method to define obesity, we excluded approximately 5% of eligible patients because BMI was unavailable. This may have included children with more severe asthma symptoms, as a height measurement may have been deferred due to their higher acuity. Asthma severity or chronicity would be associated with our outcomes of interest. However, we were unable to collect reliable data on severity or chronicity. Finally, to measure the amount of total albuterol needed by a patient during the ED and inpatient admissions, albuterol treatments delivered by MDI, nebulizer, or continuously were converted into total albuterol. Although based upon total milligram dosing and studies comparing routes of albuterol administration,[34] the validity of this conversion is unknown.
CONCLUSION
Although BMI percentile for age is inversely associated with LOS for in‐hospital pediatric status asthmaticus, the impact of BMI on this outcome likely is not clinically meaningful. Future investigations should examine other elements of BMI and in‐hospital status asthmaticus, such as any associations between BMI and admission rates.
Acknowledgements
The authors offer their appreciation to their research assistant, Amy Lee, for her support and dedication to this project.
Disclosures
Internal funds from Children's Mercy Hospital and Clinics supported the conduct of this work. The authors report no conflicts of interest.
- , , . Incidence of childhood obesity in the United States. N Engl J Med. 2014;370(5):403–411.
- , . Prevalence and trends in obesity and severe obesity among children in the United States, 1999–2012. JAMA Pediatr. 2014;168(6):561–566.
- , , , . Effects of childhood obesity on hospital care and costs, 1999–2005. Health Affairs. 2009;28(4):w751–w760.
- , , , , . Influence of obesity on clinical outcomes in hospitalized children: a systematic review. JAMA Pediatr. 2013;167(5):476–482.
- , . Appendicitis in the obese child. J Pediatr Surg. 2007;42(5):857–861.
- , , , , . Obesity: influence on length of hospital stay for the pediatric burn patient. J Burn Care Res. 2010;31(2):251–256.
- , , , , , . The impact of obesity on severely injured children and adolescents. J Pediatr Surg. 2006;41(1):88–91.
- , , , . Incremental hospital charges associated with obesity as a secondary diagnosis in children. Obesity (Silver Spring). 2007;15(7):1895–1901.
- , , , . Persistent gap of incremental charges for obesity as a secondary diagnosis in common pediatric hospitalizations. J Hosp Med. 2009;4(3):149–156.
- , , , . Resource utilization and expenditures for overweight and obese children. Arch Pediatr Adolesc Med. 2007;161(1):11–14.
- , , , , , . Decreased response to inhaled steroids in overweight and obese asthmatic children. J Allergy Clin Immunol. 2011;127(3):741–749.
- , , , , . Body mass and glucocorticoid response in asthma. Am J Respir Crit Care Med. 2008;178(7):682–687.
- , , , ; National Heart, Lung, and Blood Institute's Asthma Clinical Research Network. Body mass index and phenotype in subjects with mild‐to‐moderate persistent asthma. J Allergy Clin Immunol. 2009;123(6):1328–1334.e1.
- , . Obesity and asthma disease phenotypes. Curr Opin Allergy Clin Immunol. 2012;12(1):76–81.
- , . Obesity and the lung: 4. Obesity and asthma. Thorax. 2008;63(11):1018–1023.
- , , , et al. Body mass index and response to asthma therapy: fluticasone propionate/salmeterol versus montelukast. J Asthma. 2010;47(1):76–82.
- , , , et al. Effect of obesity on clinical presentation and response to treatment in asthma. J Asthma. 2006;43(7):553–558.
- , , , . Asthma and obesity in three‐year‐old urban children: role of sex and home environment. J Pediatr. 2011;159(1):14–20.
- , , , . Care of Children and Adolescents in U.S. Hospitals. Rockville, MD: Agency for Healthcare Research and Quality; 2003. Available at: http://archive.ahrq.gov/data/hcup/factbk4/factbk4.pdf. Accessed February 12, 2014.
- , , , . Childhood obesity increases duration of therapy during severe asthma exacerbations. Pediatr Crit Care Med. 2006;7(6):527–531.
- , , , , . Childhood overweight increases hospital admission rates for asthma. Pediatr. 2007;120(4):734–740.
- , , , . Body mass index and acute asthma severity among children presenting to the emergency department. Pediatr Allergy Immunol. 2009;21(3):480–488.
- Centers for Disease Control and Prevention. Basics about childhood obesity. Available at: http://www.cdc.gov/obesity/childhood/basics.html. Accessed February 12, 2014.
- . Screening and interventions for childhood overweight: a summary of evidence for the US Preventive Services Task Force. Pediatrics. 2005;116(1):e125–e144.
- ; Expert Committee. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120(suppl 4):S164–S192.
- , , , . Comparison of ICD code‐based diagnosis of obesity with measured obesity in children and the implications for health care cost estimates. BMC Med Res Methodol. 2011;11(1):173.
- , ; American Academy of Pediatrics Committee on Nutrition. Prevention of pediatric overweight and obesity. Pediatrics. 2003;112(2):424–430.
- . Development of the 3M all patient‐refined diagnosis‐related groups (APR DRGs). Available at: http//www.ahrq.gov/legacy/qual/mortality/Hughes3.htm. Accessed March 1, 2014.
- . 3M Health Information Systems (HIS) APR‐DRG classification software: overview. Available at: http://www.ahrq.gov/legacy/qual/mortality/Hughessumm.htm. Accessed March 1, 2014.
- , , , , . Shifting place of death among children with complex chronic conditions in the United States, 1989–2003. JAMA. 2007;297(24):2725–2732.
- , , . Pediatric deaths attributable to complex chronic conditions: a population‐based study of Washington State, 1980–1997. Pediatrics. 2000;106(1 pt 2):205–209.
- , , , et al. Comparative effectiveness of pleural drainage procedures for the treatment of complicated pneumonia in childhood. J Hosp Med. 2011;6(5):256–263.
- , , , , , . Adjunct corticosteroids in children hospitalized with community‐acquired pneumonia. Pediatrics. 2011;127(2):e255–e263.
- , , . Holding chambers (spacers) versus nebulisers for beta‐agonist treatment of acute asthma. Cochrane Database Syst Rev. 2013;9:CD000052.
- , , , . Effectiveness of a clinical pathway for inpatient asthma management. Pediatrics. 2000;106(5):1006–1012.
- , , . Incidence of childhood obesity in the United States. N Engl J Med. 2014;370(5):403–411.
- , . Prevalence and trends in obesity and severe obesity among children in the United States, 1999–2012. JAMA Pediatr. 2014;168(6):561–566.
- , , , . Effects of childhood obesity on hospital care and costs, 1999–2005. Health Affairs. 2009;28(4):w751–w760.
- , , , , . Influence of obesity on clinical outcomes in hospitalized children: a systematic review. JAMA Pediatr. 2013;167(5):476–482.
- , . Appendicitis in the obese child. J Pediatr Surg. 2007;42(5):857–861.
- , , , , . Obesity: influence on length of hospital stay for the pediatric burn patient. J Burn Care Res. 2010;31(2):251–256.
- , , , , , . The impact of obesity on severely injured children and adolescents. J Pediatr Surg. 2006;41(1):88–91.
- , , , . Incremental hospital charges associated with obesity as a secondary diagnosis in children. Obesity (Silver Spring). 2007;15(7):1895–1901.
- , , , . Persistent gap of incremental charges for obesity as a secondary diagnosis in common pediatric hospitalizations. J Hosp Med. 2009;4(3):149–156.
- , , , . Resource utilization and expenditures for overweight and obese children. Arch Pediatr Adolesc Med. 2007;161(1):11–14.
- , , , , , . Decreased response to inhaled steroids in overweight and obese asthmatic children. J Allergy Clin Immunol. 2011;127(3):741–749.
- , , , , . Body mass and glucocorticoid response in asthma. Am J Respir Crit Care Med. 2008;178(7):682–687.
- , , , ; National Heart, Lung, and Blood Institute's Asthma Clinical Research Network. Body mass index and phenotype in subjects with mild‐to‐moderate persistent asthma. J Allergy Clin Immunol. 2009;123(6):1328–1334.e1.
- , . Obesity and asthma disease phenotypes. Curr Opin Allergy Clin Immunol. 2012;12(1):76–81.
- , . Obesity and the lung: 4. Obesity and asthma. Thorax. 2008;63(11):1018–1023.
- , , , et al. Body mass index and response to asthma therapy: fluticasone propionate/salmeterol versus montelukast. J Asthma. 2010;47(1):76–82.
- , , , et al. Effect of obesity on clinical presentation and response to treatment in asthma. J Asthma. 2006;43(7):553–558.
- , , , . Asthma and obesity in three‐year‐old urban children: role of sex and home environment. J Pediatr. 2011;159(1):14–20.
- , , , . Care of Children and Adolescents in U.S. Hospitals. Rockville, MD: Agency for Healthcare Research and Quality; 2003. Available at: http://archive.ahrq.gov/data/hcup/factbk4/factbk4.pdf. Accessed February 12, 2014.
- , , , . Childhood obesity increases duration of therapy during severe asthma exacerbations. Pediatr Crit Care Med. 2006;7(6):527–531.
- , , , , . Childhood overweight increases hospital admission rates for asthma. Pediatr. 2007;120(4):734–740.
- , , , . Body mass index and acute asthma severity among children presenting to the emergency department. Pediatr Allergy Immunol. 2009;21(3):480–488.
- Centers for Disease Control and Prevention. Basics about childhood obesity. Available at: http://www.cdc.gov/obesity/childhood/basics.html. Accessed February 12, 2014.
- . Screening and interventions for childhood overweight: a summary of evidence for the US Preventive Services Task Force. Pediatrics. 2005;116(1):e125–e144.
- ; Expert Committee. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120(suppl 4):S164–S192.
- , , , . Comparison of ICD code‐based diagnosis of obesity with measured obesity in children and the implications for health care cost estimates. BMC Med Res Methodol. 2011;11(1):173.
- , ; American Academy of Pediatrics Committee on Nutrition. Prevention of pediatric overweight and obesity. Pediatrics. 2003;112(2):424–430.
- . Development of the 3M all patient‐refined diagnosis‐related groups (APR DRGs). Available at: http//www.ahrq.gov/legacy/qual/mortality/Hughes3.htm. Accessed March 1, 2014.
- . 3M Health Information Systems (HIS) APR‐DRG classification software: overview. Available at: http://www.ahrq.gov/legacy/qual/mortality/Hughessumm.htm. Accessed March 1, 2014.
- , , , , . Shifting place of death among children with complex chronic conditions in the United States, 1989–2003. JAMA. 2007;297(24):2725–2732.
- , , . Pediatric deaths attributable to complex chronic conditions: a population‐based study of Washington State, 1980–1997. Pediatrics. 2000;106(1 pt 2):205–209.
- , , , et al. Comparative effectiveness of pleural drainage procedures for the treatment of complicated pneumonia in childhood. J Hosp Med. 2011;6(5):256–263.
- , , , , , . Adjunct corticosteroids in children hospitalized with community‐acquired pneumonia. Pediatrics. 2011;127(2):e255–e263.
- , , . Holding chambers (spacers) versus nebulisers for beta‐agonist treatment of acute asthma. Cochrane Database Syst Rev. 2013;9:CD000052.
- , , , . Effectiveness of a clinical pathway for inpatient asthma management. Pediatrics. 2000;106(5):1006–1012.
© 2014 Society of Hospital Medicine
Severe‐Sepsis Screening Tool
Sepsis remains a significant healthcare burden and is the sixth most common reason for hospitalization in the United States. For patients presenting with severe sepsis, mortality rates are approximately 30%,[1, 2] and sepsis remains the most expensive reason for hospitalization. In 2009, septicemia accounted for nearly $15.4 billion in aggregate hospital costs.[2]
Early identification of sepsis and the timely implementation of goal‐directed therapy significantly decrease sepsis‐related mortality and are cost‐effective,[3, 4, 5] highlighting the need for new clinical strategies to aid in early diagnosis. To date, most studies have focused on the screening and management of sepsis in the emergency department and intensive care unit (ICU),[6, 7] and less is known about the benefits of screening in non‐ICU settings. In the non‐ICU setting, conditions may go unrecognized and treatments delayed. Evidence suggests that patients diagnosed with severe sepsis in the non‐ICU setting are almost twice as likely to die as those diagnosed in an emergency department.[8, 9]
Application of a sepsis screening tool to both medical and surgical patients poses an additional challenge that may impact the screen's performance. The specificity may be compromised by noninfectious causes of systemic inflammatory response syndrome (SIRS) commonly seen in the postsurgical patient. For example, the tachycardia and fever often seen in the postoperative patient are sufficient to qualify for SIRS, making the diagnosis of sepsis more challenging. The purpose of this study was to examine the performance of a nurse‐driven, simple sepsis screening tool in a mixed medical and surgical non‐ICU setting.
METHODS
Setting
This was an observational pilot study of prospectively screened patients admitted to a 26‐bed medical/surgical intermediate care unit with telemetry monitoring in a 613‐bed university tertiary referral hospital over a 1‐month time period. The surgical patient population of this floor consisted of cardiothoracic (50%), general (24%), and vascular surgery (17%) patients as well as a small number of trauma (7%) patients. The medical patient population admitted to this unit included pretransplant and complex medical patients requiring telemetry monitoring. Though the incidence of sepsis specific to this unit was unknown prior to the study, after an analysis of discharges the study team surmised there would be sufficient volume for testing of a nurse‐based screening tool.
Nurse Education
Registered nurses (RNs) working on the study unit had an average of 5 to 7 years of experience. The all‐RN unit was staffed predominantly at a 1:3 RN to patient ratio. RNs were supported by a clinical nurse specialist (CNS) and clinical educator (CE) RN who provided regular ongoing education about infection prevention and identification of common conditions that are seen on the unit.
In the 6 months prior to our sepsis screening initiative, nursing staff had been given more than 8 hours of education on infection‐ and sepsis‐related topics in 15‐ to 20‐minute blocks of time. This dedicated education took place during the nurses' shift in groups of 2 to 3, and was run by the CNS, assistant nurse manager, and CE RN. Nurses were also encouraged to attend an optional 8‐hour sepsis continuing medical education (CME) program. Approximately 20% of the nurses on the study unit attended.
Just prior to the pilot study, nursing staff completed a 1‐hour refresher self‐study module on severe sepsis stressing the importance of early identification. There was also a training month prior to the actual data collection time frame, where unit core trainers (RNs) or champions who had attended the optional 8‐hour sepsis CME conducted 1:1 follow‐up with each RN, reviewing at least 1 of their screens to validate understanding of screening concepts. Each RN was checked off after correctly completing a screen. During the study, unit educators and the CNS provided additional on‐unit in‐service training with screening tool completion instructions and advice on how to incorporate the tool into the RN's current assessment workflow. In addition, the charge nurses were asked to review the screens collected each shift and validate any that may have seemed inconsistent with the RN's verbal report of the patient's status.
The university's institutional review board notice of determination waived review for this study because it was classified as quality improvement.
Screening Tool
A sepsis screening tool was developed as part of a broader initiative to improve sepsis‐related morbidity and mortality at our hospital. The screening tool was adapted from the severe sepsis screening tool created by the Surviving Sepsis Campaign and Institute for Healthcare Improvement,[10] and consisted of a simple 3‐tiered paper‐based screening assessment that was to be completed by the bedside RN (Figure 1). RNs on the pilot medical/surgical intermediate care unit performed the screening assessment with their regular patient assessment at the beginning of each shift.
The first tier of the tool screened for the presence of SIRS. Positive parameters included heart rate >90, temperature >38C or <36C, white blood cell count >12,000 or<4000 or >10% bands, and/or respiratory rate >20 or partial pressure of carbon dioxide (PaCO2) <32 mm Hg. To decrease the number of false‐positive screens in patients whose abnormal vitals could already be attributed to a condition other than sepsis, these symptoms were only scored if they had emerged within the previous 8 hours.
If patients met 2 SIRS criteria, the nurse would move to the second tier of the tool, which involved consideration of possible infection as a contributor to a patient's clinical condition as well as a source of infection. If infection was not suspected, further screening was terminated. If infection was suspected, the patient then met criteria for a positive sepsis screen, and a third tier of screening involving assessment of organ dysfunction was initiated.
If the patient screened positive for sepsis (2 SIRS and suspicion for new infection) or severe sepsis (sepsis with end‐organ dysfunction), nurses were instructed to document this in the patient's electronic medical record (EMR) and call the primary team to initiate actions following the hospital‐wide sepsis guidelines. Any subsequent actions were recorded in the patient's EMR.
Data Collection
Completed sepsis screening forms during the month of October 2010 were reviewed by the authors (E.G., L.S., and P.M.). Data including age, gender, International Classification of Diseases, Ninth Revision (ICD‐9) admission and discharge diagnoses, vital signs, lab results, clinical interventions, and documented clinical decision processes by healthcare staff were collected on patients with a positive screen or those who did not screen positive but had an ICD‐9 code for sepsis, severe sepsis, or septic shock during their hospitalization or at discharge. We also collected demographic and clinical data for a random sample of patients who consistently screened negative for sepsis.
Performance Measurement
The sensitivity and specificity of the screening tool was determined by identifying true‐positive, false‐positive, true‐negative, and false‐negative results and calculating accordingly using a 2 2 contingency table. True positives were defined as cases where patients screened positive for sepsis and had a documented diagnosis of sepsis in their EMR within 24 hours of the positive screening or had an ICD‐9 billing code for sepsis. False‐positive cases were those in which patients screened positive for sepsis but did not have a diagnosis of sepsis by manual chart review nor was there an ICD‐9 code for sepsis for their hospital stay. True‐negative cases were those where patients screened negative and did not have an ICD‐9 code for sepsis. False negatives were cases where patients consistently screened negative for sepsis but had an ICD‐9 code for sepsis.
Clinical Activities
To examine the impact of a positive sepsis screen on subsequent clinical action, we assessed the frequency with which a treatment or diagnostic workup was initiated after a positive screen and compared this to clinical activity initiated after a negative screen. Specifically, the patient's EMR was reviewed for actions including measurement of lactate, blood cultures, administration of broad spectrum antibiotics, administration of fluid boluses, or consultation with or transfer to the ICU. These actions were chosen because they are part of the Surviving Sepsis Bundle, which has been demonstrated to improve mortality rates after diagnosis of severe sepsis or septic shock,[11, 12] and can be done outside of an ICU setting. Because screening was done every 8 hours, clinical activity was only attributed to a positive or negative sepsis screen if it occurred within 8 hours of the screening result. Patients were excluded if there were missing data points that precluded full analysis of their clinical course.
Statistical Analysis
To compare the performance of the screening tool between surgical and medical patients, we calculated 95% confidence intervals of screening test sensitivity and specificity. To test if performance was significantly different between these groups, we performed a nonparametric, 2‐sided, 2‐sample test of proportions. Though similar to a [2] test, the 2‐sided test of proportions allowed us to determine if there was a directional difference in test performance (ie, Does the screening tool perform better or worse in a certain patient group?). We also used the test of proportions to compare differences in the proportion of patients receiving sepsis‐related interventions before and after a positive or negative screening result. For comparisons of demographic variables we used nonparametric tests including the [2] test for categorical variables and the Kruskal‐Wallis test for continuous variables. We used SAS 9.3 (SAS Institute Inc., Cary, NC) to perform our analyses.
RESULTS
Over a 1‐month time period, 2143 screens were completed on 245 patients (169 surgical, 76 medical). The overall incidence of sepsis on the treatment unit during this time period was 9%. Surgical patients had an 8.9% incidence of sepsis, and medical patients had an incidence of 9.2%.
Screening tool performance is presented in Table 1. The screening tool had 95.5% sensitivity and 91.9% specificity, with no significant differences in performance between surgical and medical patients. The overall negative predictive value was 99.5%, also with comparable performance in both surgical and medical patients (P = 0.89). The overall positive predictive value (PPV) was 70% in medical patients and 48% in surgical patients (P = 0.12). Screening tool accuracy for medical and surgical patients was 92%.
| Overall, N = 245 (95% CI) | Surgery, N = 169 (95% CI) | Medicine, N = 76 (95% CI) | P Value* | |
|---|---|---|---|---|
| ||||
| Sensitivity | 95.5% (75%‐99.7%) | 93% (66%‐99.6%) | 100% (56%‐100%) | 0.17 |
| Specificity | 91.9% (87%‐95%) | 90% (84%‐94%) | 95% (87%‐99%) | 0.48 |
| NPV | 99.5% (81%‐100%) | 99.3% (71%‐100%) | 100% (67%‐100%) | 0.89 |
| PPV | 53.8% (39%‐70%) | 48% (23%‐73%) | 70% (30%‐100%) | 0.12 |
| LR+ | 11.8 | 9.3 | 20 | |
| LR | 0.05 | 0.08 | 0 | |
| Confirmed patient diagnosis, overall | ||||
| Sepsis | No sepsis | |||
| Screen positive | 21 (TP) | 18 (FP) | ||
| Screen negative | 1 (FN) | 205 (TN) | ||
| Confirmed patient diagnosis, medicine | ||||
| Sepsis | No sepsis | |||
| Screen positive | 7 (TP) | 3 (FP) | ||
| Screen negative | 0 (FN) | 66 (TN) | ||
| Confirmed patient diagnosis, surgery | ||||
| Sepsis | No sepsis | |||
| Screen positive | 14 (TP) | 15 (FP) | ||
| Screen negative | 1 (FN) | 139 (TN) | ||
Clinical Activities
Of the 39 patients who screened positive for sepsis, nurses classified 20 with sepsis and 19 with severe sepsis. Of these 39 patients, 33 were included in our descriptive analysis of the effect of positive screening results on clinical activity (3 were excluded for admission for sepsis and 3 for missing data). As a comparison, we randomly selected 30 patients of the 206 patients who screened negative for sepsis to evaluate clinical activity before and after a negative screen.
Characteristics of patients screening positive and negative for sepsis are reported in Table 2. We found no statistically significant differences in age, sex, length of hospital stay, or mortality amongst all groups.
| Patient Characteristics | Surgery (Positive) | Medicine (Positive) | Surgery (Negative) | Medicine (Negative) | P Value |
|---|---|---|---|---|---|
| |||||
| No. | 26 | 7 | 20 | 10 | |
| Age, y, mean | 57.8 ( 16.5) | 72.4 ( 16.8) | 64.6 ( 19.4) | 63.6 ( 16.8) | 0.25 |
| % Male (no.) | 50% (13) | 57% (4) | 60% (12) | 60% (6) | 0.27 |
| Length of stay, d, median (IQR) | 9 (716.7) | 7 (5.511.5) | 11 (7.722) | 8 (421) | 0.38 |
| No. of PODs until first positive screen, d, median (IQR) | 2 (13) | N/A | N/A | N/A | |
| % Mortality (no.) | 0% | 14% (1) | 5% (1) | 10% (1) | 0.19 |
Figure 2 illustrates differences in the proportion of patients receiving a clinical action before and after a negative or positive screening test result. In the cohort of 33 patients screening positive for sepsis, clinical action after a positive screen was taken in 4 of the 7 (50%) medical patients and 11 of 26 (42%) surgical patients. In patients screening negative for sepsis we found only 1 incident in which a sepsis‐related action was taken after a negative screen. In this case the patient was admitted to the ICU within 8 hours of a negative screen, though there was no explicit documentation that sepsis was the reason for this admission.
We compared the proportion of patients receiving sepsis‐related treatment before either a negative or positive screen and found no significant difference (Table 3). We then compared the proportion of patients receiving sepsis‐related actions after a positive or negative screening test result and found that the proportion of patients receiving antibiotics, blood cultures, and lactate measurement was significantly higher for patients with a positive sepsis screening result compared to those with a negative screening result (Table 3).
| Intervention and Group | Proportion | P Value |
|---|---|---|
| ||
| Before screening test | ||
| Antibiotics | 0.066 | |
| Positive screen | 45% | |
| Negative screen | 23% | |
| Lactate | 0.837 | |
| Positive screen | 15% | |
| Negative screen | 13% | |
| Blood culture | 0.181 | |
| Positive screen | 18% | |
| Negative screen | 17% | |
| Fluid administration | 0.564 | |
| Positive screen | 6% | |
| Negative screen | 10% | |
| ICU transfer/consult | 0.337 | |
| Positive screen | 3% | |
| Negative screen | 0% | |
| After screening test | ||
| Antibiotics | 0.006 | |
| Positive screen | 58% | |
| Negative screen | 23% | |
| Lactate | 0.018 | |
| Positive screen | 36% | |
| Negative screen | 13% | |
| Blood Culture | 0.002 | |
| Positive screen | 24% | |
| Negative screen | 17% | |
| Fluid administration | 0.112 | |
| Positive screen | 24% | |
| Negative screen | 10% | |
| ICU transfer/consult | 0.175 | |
| Positive screen | 9% | |
| Negative screen | 3% | |
DISCUSSION
Improving recognition and time to treatment of sepsis in a non‐ICU setting is an important step toward decreasing sepsis‐related mortality. Lundberg and colleagues found that mortality rates for patients diagnosed with septic shock on a general ward were higher than for patients diagnosed in the ICU, even though ward patients were younger and healthier at baseline.[8] For ward patients, treatment delays were most profound in initiating vasoactive therapies, and minor delays were encountered in initiating fluid resuscitation. In their international study on the impact of early goal‐directed therapy guidelines, Levy and colleagues found that patients diagnosed with severe sepsis on the wards were almost twice as likely to die as patients diagnosed with sepsis in the emergency department.[9]
We are the first to report about an accurate nurse‐driven SIRS‐based sepsis screening protocol that is effective in the early identification of sepsis in both medical and surgical patients in an intermediate care setting. We found no significant difference in the screening tool performance between the medical and surgical cohorts. This is an important comparison given that SIRS criteria alone can be nonspecific in the postoperative population, where it is common to have hemodynamic changes, elevation of inflammatory markers, and fevers from noninfectious sources.
Our sepsis screening tool was designed in 3 tiers to improve its specificity. The first tier was based strictly on SIRS criteria (eg, tachycardia or fever), whereas the second and third tiers served to increase the specificity of the screening tool by instructing the evaluator to assess possible sources of infection and assess for objective signs of organ dysfunction. We relied heavily on the nursing staff to assess for the presence or absence of infection and believe that the educational component prior to initiating the screening protocol was vital.
EMR‐based screening tools that rely purely on physiologic data have been considered for the early detection and management of sepsis, although they lack the specificity gained through the incorporation of clinical judgment.[13] Sawyer and colleagues report using a real‐time EMR‐based method for early sepsis detection in non‐ICU patients that is based solely on objective measures; however, their PPV was only 19.5%. The model we describe in this study is one that incorporates real‐time physiologic data available from an EMR coupled with the clinical judgment of a bedside registered nurse. As our data suggest, this provides a screen that is both sensitive and specific.
It is interesting to note that in our assessment of clinical action taken 8 hours after a positive screening test (the interval after which a new screening test was performed), the rate of diagnostic workup and/or treatment for sepsis was relatively low. One reason for this could have been that the treating team had suspicion for sepsis prior to a positive screen and had already initiated clinical action. Of the 51 recorded clinical actions taken around the time of a positive screen, the majority (67%) occurred before the screening result. It is also possible that clinical action was not pursued because the treatment team disagreed with a diagnosis of sepsis. Of all the false positive screening cases, manual chart review confirmed that these patients did not have sepsis, nor did they develop sepsis during their index hospital stay. Last, we only recorded clinical actions taken within 8 hours of the first positive screen for sepsis and measured 5 very specific actions. Thus, our analysis may have missed actions taken after 8 hours or actions that differed from the 5 we chose to assess.
Even with the apparently low levels of new clinical activity after a positive screen, when compared to patients who screened negative for sepsis, a significantly higher number of patients who had a positive screen received antibiotics, had lactate measured, and had blood cultures drawn. We did not find a significant difference in the proportion of patients receiving a sepsis‐related clinical action before a screening result (positive or negative), which suggests that a positive screening test may have led to increased clinical action.
A limitation of our study is its small size and that it was conducted in 1 pilot unit. Additionally, our retrospective analysis of clinical care inhibited our ability to fully understand a patient's clinical course or retrieve missing data points. A related limitation is that we could not ascertain how often the screening tool did not identify a case of sepsis before it was clinically diagnosed. Assessing the temporal performance of our screening tool is of great interest and may be more easily performed using an electronic version of the screening tool, which is currently in development.
Using ICD‐9 codes to determine the true‐negative cohort is another limitation of our study. It is well documented that use of administrative data can lead to inaccurate classification of patients.[14] To address this, we performed random audits of 30 test‐negative patients. In doing so we did not find any errors in classification.
Although we did not find a significant difference in screening tool performance between surgical and medical patients, the PPV of the tool was lower in the surgical population (48%) compared to the medical population (70%). The lower PPV observed in surgical patients could be attributable to an overall lower incidence of sepsis in this cohort as well as possible errors in initial assessment of infection, which can be difficult in postsurgical patients. Our retrospective analysis included data from the early months of the screening protocol, a time in which nursing staff was still developing clinical acumen in identifying sepsis. However, this could have led nurses to either overestimate or underestimate the presence of infection in either patient group.
Suspicion for infection is the cornerstone definition of sepsis, and in our screening protocol nurses were charged with making this decision based on their knowledge of the patient's clinical course and current status. Issues concerning nurses' recognition of infection symptoms are an area of opportunity for further research and education and could aid in improving PPV. Clinical judgment could be further bolstered by adding promising laboratory tests such as C‐reactive protein or procalcitonin as objective adjuncts to an initial assessment for sepsis,[15] which could potentially increase screening test PPV.
CONCLUSIONS
A simple screening tool for sepsis performed by the bedside nurse can provide a means to successfully identify sepsis early and lead to more timely diagnostics and treatment in both medical and surgical patients in an intermediate care setting.
ACKNOWLEDGEMENTS
The authors thank Eileen Pummer, quality manager for the sepsis team; Pauline Regner, patient care manager of the pilot study unit; and the nurses who contributed to the screening tool design team and data collection. The authors acknowledge Pooja Loftus for her statistical expertise, and Isabella Chu for her review of the manuscript. Disclosures: Presented as a poster at the 31st Annual Meeting of the Surgical Infection Society, Palm Beach, Florida, May 2011. The authors report no conflicts of interest.
- , , , , , Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303–1310.
- , , Septicemia in U.S. hospitals, 2009. HCUP statistical brief #122. Agency for Healthcare Research and Quality. Available at: http://www.hcup-us.ahrq.gov/reports/statbriefs/sb122.pdf. Published October 2011. Accessed on September 4, 2012.
- , , , Economic implications of an evidence‐based sepsis protocol: can we improve outcomes and lower costs? Crit Care Med. 2007;35(5):1257–1262.
- , , , , , Late compliance with the sepsis resuscitation bundle: impact on mortality. Shock. 2011;36(6):542–547.
- , , , , , The costs and cost‐effectiveness of an integrated sepsis treatment protocol. Crit Care Med. 2008;36(4):1168–1174.
- , , A simple prediction algorithm for bacteraemia in patients with acute febrile illness. QJM. 2005;98(11):813–820.
- , , , et al. Validation of a screening tool for the early identification of sepsis. J Trauma. 2009;66(6):1539–1546; discussion 1546–1547.
- , , , et al. Septic shock: an analysis of outcomes for patients with onset on hospital wards versus intensive care units. Crit Care Med. 1998;26(6):1020–1024.
- , , , et al. The Surviving Sepsis Campaign: results of an international guideline‐based performance improvement program targeting severe sepsis. Crit Care Med. 2010;38(2):367–374.
- Institute of Healthcare Improvement. Evaluation for severe sepsis screening tool. Surviving Sepsis Campaign. Available at: http://www.survivingsepsis.org/About_the_Campaign/Documents/evaluationforseveresepsisscreeningtool.pdf. Accessed on September 30, 2012.
- , , , et al. Impact of the Surviving Sepsis Campaign protocols on hospital length of stay and mortality in septic shock patients: results of a three‐year follow‐up quasi‐experimental study. Crit Care Med. 2010;38(4):1036–1043.
- , , , et al. Reduction of the severe sepsis or septic shock associated mortality by reinforcement of the recommendations bundle: a multicenter study. Ann Fr Anesth Reanim. 2010;29(9):621–628.
- , , , et al. Implementation of a real‐time computerized sepsis alert in nonintensive care unit patients. Crit Care Med. 2011;39(3):469–473.
- , , , Accuracy of administrative data for identifying patients with pneumonia. Am J Med Qual. 2005;20(6):319–328.
- , , , , , Comparison of procalcitonin and C‐reactive protein as markers of sepsis. Crit Care Med. 2003;31(6):1737–1741.
Sepsis remains a significant healthcare burden and is the sixth most common reason for hospitalization in the United States. For patients presenting with severe sepsis, mortality rates are approximately 30%,[1, 2] and sepsis remains the most expensive reason for hospitalization. In 2009, septicemia accounted for nearly $15.4 billion in aggregate hospital costs.[2]
Early identification of sepsis and the timely implementation of goal‐directed therapy significantly decrease sepsis‐related mortality and are cost‐effective,[3, 4, 5] highlighting the need for new clinical strategies to aid in early diagnosis. To date, most studies have focused on the screening and management of sepsis in the emergency department and intensive care unit (ICU),[6, 7] and less is known about the benefits of screening in non‐ICU settings. In the non‐ICU setting, conditions may go unrecognized and treatments delayed. Evidence suggests that patients diagnosed with severe sepsis in the non‐ICU setting are almost twice as likely to die as those diagnosed in an emergency department.[8, 9]
Application of a sepsis screening tool to both medical and surgical patients poses an additional challenge that may impact the screen's performance. The specificity may be compromised by noninfectious causes of systemic inflammatory response syndrome (SIRS) commonly seen in the postsurgical patient. For example, the tachycardia and fever often seen in the postoperative patient are sufficient to qualify for SIRS, making the diagnosis of sepsis more challenging. The purpose of this study was to examine the performance of a nurse‐driven, simple sepsis screening tool in a mixed medical and surgical non‐ICU setting.
METHODS
Setting
This was an observational pilot study of prospectively screened patients admitted to a 26‐bed medical/surgical intermediate care unit with telemetry monitoring in a 613‐bed university tertiary referral hospital over a 1‐month time period. The surgical patient population of this floor consisted of cardiothoracic (50%), general (24%), and vascular surgery (17%) patients as well as a small number of trauma (7%) patients. The medical patient population admitted to this unit included pretransplant and complex medical patients requiring telemetry monitoring. Though the incidence of sepsis specific to this unit was unknown prior to the study, after an analysis of discharges the study team surmised there would be sufficient volume for testing of a nurse‐based screening tool.
Nurse Education
Registered nurses (RNs) working on the study unit had an average of 5 to 7 years of experience. The all‐RN unit was staffed predominantly at a 1:3 RN to patient ratio. RNs were supported by a clinical nurse specialist (CNS) and clinical educator (CE) RN who provided regular ongoing education about infection prevention and identification of common conditions that are seen on the unit.
In the 6 months prior to our sepsis screening initiative, nursing staff had been given more than 8 hours of education on infection‐ and sepsis‐related topics in 15‐ to 20‐minute blocks of time. This dedicated education took place during the nurses' shift in groups of 2 to 3, and was run by the CNS, assistant nurse manager, and CE RN. Nurses were also encouraged to attend an optional 8‐hour sepsis continuing medical education (CME) program. Approximately 20% of the nurses on the study unit attended.
Just prior to the pilot study, nursing staff completed a 1‐hour refresher self‐study module on severe sepsis stressing the importance of early identification. There was also a training month prior to the actual data collection time frame, where unit core trainers (RNs) or champions who had attended the optional 8‐hour sepsis CME conducted 1:1 follow‐up with each RN, reviewing at least 1 of their screens to validate understanding of screening concepts. Each RN was checked off after correctly completing a screen. During the study, unit educators and the CNS provided additional on‐unit in‐service training with screening tool completion instructions and advice on how to incorporate the tool into the RN's current assessment workflow. In addition, the charge nurses were asked to review the screens collected each shift and validate any that may have seemed inconsistent with the RN's verbal report of the patient's status.
The university's institutional review board notice of determination waived review for this study because it was classified as quality improvement.
Screening Tool
A sepsis screening tool was developed as part of a broader initiative to improve sepsis‐related morbidity and mortality at our hospital. The screening tool was adapted from the severe sepsis screening tool created by the Surviving Sepsis Campaign and Institute for Healthcare Improvement,[10] and consisted of a simple 3‐tiered paper‐based screening assessment that was to be completed by the bedside RN (Figure 1). RNs on the pilot medical/surgical intermediate care unit performed the screening assessment with their regular patient assessment at the beginning of each shift.

The first tier of the tool screened for the presence of SIRS. Positive parameters included heart rate >90, temperature >38C or <36C, white blood cell count >12,000 or<4000 or >10% bands, and/or respiratory rate >20 or partial pressure of carbon dioxide (PaCO2) <32 mm Hg. To decrease the number of false‐positive screens in patients whose abnormal vitals could already be attributed to a condition other than sepsis, these symptoms were only scored if they had emerged within the previous 8 hours.
If patients met 2 SIRS criteria, the nurse would move to the second tier of the tool, which involved consideration of possible infection as a contributor to a patient's clinical condition as well as a source of infection. If infection was not suspected, further screening was terminated. If infection was suspected, the patient then met criteria for a positive sepsis screen, and a third tier of screening involving assessment of organ dysfunction was initiated.
If the patient screened positive for sepsis (2 SIRS and suspicion for new infection) or severe sepsis (sepsis with end‐organ dysfunction), nurses were instructed to document this in the patient's electronic medical record (EMR) and call the primary team to initiate actions following the hospital‐wide sepsis guidelines. Any subsequent actions were recorded in the patient's EMR.
Data Collection
Completed sepsis screening forms during the month of October 2010 were reviewed by the authors (E.G., L.S., and P.M.). Data including age, gender, International Classification of Diseases, Ninth Revision (ICD‐9) admission and discharge diagnoses, vital signs, lab results, clinical interventions, and documented clinical decision processes by healthcare staff were collected on patients with a positive screen or those who did not screen positive but had an ICD‐9 code for sepsis, severe sepsis, or septic shock during their hospitalization or at discharge. We also collected demographic and clinical data for a random sample of patients who consistently screened negative for sepsis.
Performance Measurement
The sensitivity and specificity of the screening tool was determined by identifying true‐positive, false‐positive, true‐negative, and false‐negative results and calculating accordingly using a 2 2 contingency table. True positives were defined as cases where patients screened positive for sepsis and had a documented diagnosis of sepsis in their EMR within 24 hours of the positive screening or had an ICD‐9 billing code for sepsis. False‐positive cases were those in which patients screened positive for sepsis but did not have a diagnosis of sepsis by manual chart review nor was there an ICD‐9 code for sepsis for their hospital stay. True‐negative cases were those where patients screened negative and did not have an ICD‐9 code for sepsis. False negatives were cases where patients consistently screened negative for sepsis but had an ICD‐9 code for sepsis.
Clinical Activities
To examine the impact of a positive sepsis screen on subsequent clinical action, we assessed the frequency with which a treatment or diagnostic workup was initiated after a positive screen and compared this to clinical activity initiated after a negative screen. Specifically, the patient's EMR was reviewed for actions including measurement of lactate, blood cultures, administration of broad spectrum antibiotics, administration of fluid boluses, or consultation with or transfer to the ICU. These actions were chosen because they are part of the Surviving Sepsis Bundle, which has been demonstrated to improve mortality rates after diagnosis of severe sepsis or septic shock,[11, 12] and can be done outside of an ICU setting. Because screening was done every 8 hours, clinical activity was only attributed to a positive or negative sepsis screen if it occurred within 8 hours of the screening result. Patients were excluded if there were missing data points that precluded full analysis of their clinical course.
Statistical Analysis
To compare the performance of the screening tool between surgical and medical patients, we calculated 95% confidence intervals of screening test sensitivity and specificity. To test if performance was significantly different between these groups, we performed a nonparametric, 2‐sided, 2‐sample test of proportions. Though similar to a [2] test, the 2‐sided test of proportions allowed us to determine if there was a directional difference in test performance (ie, Does the screening tool perform better or worse in a certain patient group?). We also used the test of proportions to compare differences in the proportion of patients receiving sepsis‐related interventions before and after a positive or negative screening result. For comparisons of demographic variables we used nonparametric tests including the [2] test for categorical variables and the Kruskal‐Wallis test for continuous variables. We used SAS 9.3 (SAS Institute Inc., Cary, NC) to perform our analyses.
RESULTS
Over a 1‐month time period, 2143 screens were completed on 245 patients (169 surgical, 76 medical). The overall incidence of sepsis on the treatment unit during this time period was 9%. Surgical patients had an 8.9% incidence of sepsis, and medical patients had an incidence of 9.2%.
Screening tool performance is presented in Table 1. The screening tool had 95.5% sensitivity and 91.9% specificity, with no significant differences in performance between surgical and medical patients. The overall negative predictive value was 99.5%, also with comparable performance in both surgical and medical patients (P = 0.89). The overall positive predictive value (PPV) was 70% in medical patients and 48% in surgical patients (P = 0.12). Screening tool accuracy for medical and surgical patients was 92%.
| Overall, N = 245 (95% CI) | Surgery, N = 169 (95% CI) | Medicine, N = 76 (95% CI) | P Value* | |
|---|---|---|---|---|
| ||||
| Sensitivity | 95.5% (75%‐99.7%) | 93% (66%‐99.6%) | 100% (56%‐100%) | 0.17 |
| Specificity | 91.9% (87%‐95%) | 90% (84%‐94%) | 95% (87%‐99%) | 0.48 |
| NPV | 99.5% (81%‐100%) | 99.3% (71%‐100%) | 100% (67%‐100%) | 0.89 |
| PPV | 53.8% (39%‐70%) | 48% (23%‐73%) | 70% (30%‐100%) | 0.12 |
| LR+ | 11.8 | 9.3 | 20 | |
| LR | 0.05 | 0.08 | 0 | |
| Confirmed patient diagnosis, overall | ||||
| Sepsis | No sepsis | |||
| Screen positive | 21 (TP) | 18 (FP) | ||
| Screen negative | 1 (FN) | 205 (TN) | ||
| Confirmed patient diagnosis, medicine | ||||
| Sepsis | No sepsis | |||
| Screen positive | 7 (TP) | 3 (FP) | ||
| Screen negative | 0 (FN) | 66 (TN) | ||
| Confirmed patient diagnosis, surgery | ||||
| Sepsis | No sepsis | |||
| Screen positive | 14 (TP) | 15 (FP) | ||
| Screen negative | 1 (FN) | 139 (TN) | ||
Clinical Activities
Of the 39 patients who screened positive for sepsis, nurses classified 20 with sepsis and 19 with severe sepsis. Of these 39 patients, 33 were included in our descriptive analysis of the effect of positive screening results on clinical activity (3 were excluded for admission for sepsis and 3 for missing data). As a comparison, we randomly selected 30 patients of the 206 patients who screened negative for sepsis to evaluate clinical activity before and after a negative screen.
Characteristics of patients screening positive and negative for sepsis are reported in Table 2. We found no statistically significant differences in age, sex, length of hospital stay, or mortality amongst all groups.
| Patient Characteristics | Surgery (Positive) | Medicine (Positive) | Surgery (Negative) | Medicine (Negative) | P Value |
|---|---|---|---|---|---|
| |||||
| No. | 26 | 7 | 20 | 10 | |
| Age, y, mean | 57.8 ( 16.5) | 72.4 ( 16.8) | 64.6 ( 19.4) | 63.6 ( 16.8) | 0.25 |
| % Male (no.) | 50% (13) | 57% (4) | 60% (12) | 60% (6) | 0.27 |
| Length of stay, d, median (IQR) | 9 (716.7) | 7 (5.511.5) | 11 (7.722) | 8 (421) | 0.38 |
| No. of PODs until first positive screen, d, median (IQR) | 2 (13) | N/A | N/A | N/A | |
| % Mortality (no.) | 0% | 14% (1) | 5% (1) | 10% (1) | 0.19 |
Figure 2 illustrates differences in the proportion of patients receiving a clinical action before and after a negative or positive screening test result. In the cohort of 33 patients screening positive for sepsis, clinical action after a positive screen was taken in 4 of the 7 (50%) medical patients and 11 of 26 (42%) surgical patients. In patients screening negative for sepsis we found only 1 incident in which a sepsis‐related action was taken after a negative screen. In this case the patient was admitted to the ICU within 8 hours of a negative screen, though there was no explicit documentation that sepsis was the reason for this admission.

We compared the proportion of patients receiving sepsis‐related treatment before either a negative or positive screen and found no significant difference (Table 3). We then compared the proportion of patients receiving sepsis‐related actions after a positive or negative screening test result and found that the proportion of patients receiving antibiotics, blood cultures, and lactate measurement was significantly higher for patients with a positive sepsis screening result compared to those with a negative screening result (Table 3).
| Intervention and Group | Proportion | P Value |
|---|---|---|
| ||
| Before screening test | ||
| Antibiotics | 0.066 | |
| Positive screen | 45% | |
| Negative screen | 23% | |
| Lactate | 0.837 | |
| Positive screen | 15% | |
| Negative screen | 13% | |
| Blood culture | 0.181 | |
| Positive screen | 18% | |
| Negative screen | 17% | |
| Fluid administration | 0.564 | |
| Positive screen | 6% | |
| Negative screen | 10% | |
| ICU transfer/consult | 0.337 | |
| Positive screen | 3% | |
| Negative screen | 0% | |
| After screening test | ||
| Antibiotics | 0.006 | |
| Positive screen | 58% | |
| Negative screen | 23% | |
| Lactate | 0.018 | |
| Positive screen | 36% | |
| Negative screen | 13% | |
| Blood Culture | 0.002 | |
| Positive screen | 24% | |
| Negative screen | 17% | |
| Fluid administration | 0.112 | |
| Positive screen | 24% | |
| Negative screen | 10% | |
| ICU transfer/consult | 0.175 | |
| Positive screen | 9% | |
| Negative screen | 3% | |
DISCUSSION
Improving recognition and time to treatment of sepsis in a non‐ICU setting is an important step toward decreasing sepsis‐related mortality. Lundberg and colleagues found that mortality rates for patients diagnosed with septic shock on a general ward were higher than for patients diagnosed in the ICU, even though ward patients were younger and healthier at baseline.[8] For ward patients, treatment delays were most profound in initiating vasoactive therapies, and minor delays were encountered in initiating fluid resuscitation. In their international study on the impact of early goal‐directed therapy guidelines, Levy and colleagues found that patients diagnosed with severe sepsis on the wards were almost twice as likely to die as patients diagnosed with sepsis in the emergency department.[9]
We are the first to report about an accurate nurse‐driven SIRS‐based sepsis screening protocol that is effective in the early identification of sepsis in both medical and surgical patients in an intermediate care setting. We found no significant difference in the screening tool performance between the medical and surgical cohorts. This is an important comparison given that SIRS criteria alone can be nonspecific in the postoperative population, where it is common to have hemodynamic changes, elevation of inflammatory markers, and fevers from noninfectious sources.
Our sepsis screening tool was designed in 3 tiers to improve its specificity. The first tier was based strictly on SIRS criteria (eg, tachycardia or fever), whereas the second and third tiers served to increase the specificity of the screening tool by instructing the evaluator to assess possible sources of infection and assess for objective signs of organ dysfunction. We relied heavily on the nursing staff to assess for the presence or absence of infection and believe that the educational component prior to initiating the screening protocol was vital.
EMR‐based screening tools that rely purely on physiologic data have been considered for the early detection and management of sepsis, although they lack the specificity gained through the incorporation of clinical judgment.[13] Sawyer and colleagues report using a real‐time EMR‐based method for early sepsis detection in non‐ICU patients that is based solely on objective measures; however, their PPV was only 19.5%. The model we describe in this study is one that incorporates real‐time physiologic data available from an EMR coupled with the clinical judgment of a bedside registered nurse. As our data suggest, this provides a screen that is both sensitive and specific.
It is interesting to note that in our assessment of clinical action taken 8 hours after a positive screening test (the interval after which a new screening test was performed), the rate of diagnostic workup and/or treatment for sepsis was relatively low. One reason for this could have been that the treating team had suspicion for sepsis prior to a positive screen and had already initiated clinical action. Of the 51 recorded clinical actions taken around the time of a positive screen, the majority (67%) occurred before the screening result. It is also possible that clinical action was not pursued because the treatment team disagreed with a diagnosis of sepsis. Of all the false positive screening cases, manual chart review confirmed that these patients did not have sepsis, nor did they develop sepsis during their index hospital stay. Last, we only recorded clinical actions taken within 8 hours of the first positive screen for sepsis and measured 5 very specific actions. Thus, our analysis may have missed actions taken after 8 hours or actions that differed from the 5 we chose to assess.
Even with the apparently low levels of new clinical activity after a positive screen, when compared to patients who screened negative for sepsis, a significantly higher number of patients who had a positive screen received antibiotics, had lactate measured, and had blood cultures drawn. We did not find a significant difference in the proportion of patients receiving a sepsis‐related clinical action before a screening result (positive or negative), which suggests that a positive screening test may have led to increased clinical action.
A limitation of our study is its small size and that it was conducted in 1 pilot unit. Additionally, our retrospective analysis of clinical care inhibited our ability to fully understand a patient's clinical course or retrieve missing data points. A related limitation is that we could not ascertain how often the screening tool did not identify a case of sepsis before it was clinically diagnosed. Assessing the temporal performance of our screening tool is of great interest and may be more easily performed using an electronic version of the screening tool, which is currently in development.
Using ICD‐9 codes to determine the true‐negative cohort is another limitation of our study. It is well documented that use of administrative data can lead to inaccurate classification of patients.[14] To address this, we performed random audits of 30 test‐negative patients. In doing so we did not find any errors in classification.
Although we did not find a significant difference in screening tool performance between surgical and medical patients, the PPV of the tool was lower in the surgical population (48%) compared to the medical population (70%). The lower PPV observed in surgical patients could be attributable to an overall lower incidence of sepsis in this cohort as well as possible errors in initial assessment of infection, which can be difficult in postsurgical patients. Our retrospective analysis included data from the early months of the screening protocol, a time in which nursing staff was still developing clinical acumen in identifying sepsis. However, this could have led nurses to either overestimate or underestimate the presence of infection in either patient group.
Suspicion for infection is the cornerstone definition of sepsis, and in our screening protocol nurses were charged with making this decision based on their knowledge of the patient's clinical course and current status. Issues concerning nurses' recognition of infection symptoms are an area of opportunity for further research and education and could aid in improving PPV. Clinical judgment could be further bolstered by adding promising laboratory tests such as C‐reactive protein or procalcitonin as objective adjuncts to an initial assessment for sepsis,[15] which could potentially increase screening test PPV.
CONCLUSIONS
A simple screening tool for sepsis performed by the bedside nurse can provide a means to successfully identify sepsis early and lead to more timely diagnostics and treatment in both medical and surgical patients in an intermediate care setting.
ACKNOWLEDGEMENTS
The authors thank Eileen Pummer, quality manager for the sepsis team; Pauline Regner, patient care manager of the pilot study unit; and the nurses who contributed to the screening tool design team and data collection. The authors acknowledge Pooja Loftus for her statistical expertise, and Isabella Chu for her review of the manuscript. Disclosures: Presented as a poster at the 31st Annual Meeting of the Surgical Infection Society, Palm Beach, Florida, May 2011. The authors report no conflicts of interest.
Sepsis remains a significant healthcare burden and is the sixth most common reason for hospitalization in the United States. For patients presenting with severe sepsis, mortality rates are approximately 30%,[1, 2] and sepsis remains the most expensive reason for hospitalization. In 2009, septicemia accounted for nearly $15.4 billion in aggregate hospital costs.[2]
Early identification of sepsis and the timely implementation of goal‐directed therapy significantly decrease sepsis‐related mortality and are cost‐effective,[3, 4, 5] highlighting the need for new clinical strategies to aid in early diagnosis. To date, most studies have focused on the screening and management of sepsis in the emergency department and intensive care unit (ICU),[6, 7] and less is known about the benefits of screening in non‐ICU settings. In the non‐ICU setting, conditions may go unrecognized and treatments delayed. Evidence suggests that patients diagnosed with severe sepsis in the non‐ICU setting are almost twice as likely to die as those diagnosed in an emergency department.[8, 9]
Application of a sepsis screening tool to both medical and surgical patients poses an additional challenge that may impact the screen's performance. The specificity may be compromised by noninfectious causes of systemic inflammatory response syndrome (SIRS) commonly seen in the postsurgical patient. For example, the tachycardia and fever often seen in the postoperative patient are sufficient to qualify for SIRS, making the diagnosis of sepsis more challenging. The purpose of this study was to examine the performance of a nurse‐driven, simple sepsis screening tool in a mixed medical and surgical non‐ICU setting.
METHODS
Setting
This was an observational pilot study of prospectively screened patients admitted to a 26‐bed medical/surgical intermediate care unit with telemetry monitoring in a 613‐bed university tertiary referral hospital over a 1‐month time period. The surgical patient population of this floor consisted of cardiothoracic (50%), general (24%), and vascular surgery (17%) patients as well as a small number of trauma (7%) patients. The medical patient population admitted to this unit included pretransplant and complex medical patients requiring telemetry monitoring. Though the incidence of sepsis specific to this unit was unknown prior to the study, after an analysis of discharges the study team surmised there would be sufficient volume for testing of a nurse‐based screening tool.
Nurse Education
Registered nurses (RNs) working on the study unit had an average of 5 to 7 years of experience. The all‐RN unit was staffed predominantly at a 1:3 RN to patient ratio. RNs were supported by a clinical nurse specialist (CNS) and clinical educator (CE) RN who provided regular ongoing education about infection prevention and identification of common conditions that are seen on the unit.
In the 6 months prior to our sepsis screening initiative, nursing staff had been given more than 8 hours of education on infection‐ and sepsis‐related topics in 15‐ to 20‐minute blocks of time. This dedicated education took place during the nurses' shift in groups of 2 to 3, and was run by the CNS, assistant nurse manager, and CE RN. Nurses were also encouraged to attend an optional 8‐hour sepsis continuing medical education (CME) program. Approximately 20% of the nurses on the study unit attended.
Just prior to the pilot study, nursing staff completed a 1‐hour refresher self‐study module on severe sepsis stressing the importance of early identification. There was also a training month prior to the actual data collection time frame, where unit core trainers (RNs) or champions who had attended the optional 8‐hour sepsis CME conducted 1:1 follow‐up with each RN, reviewing at least 1 of their screens to validate understanding of screening concepts. Each RN was checked off after correctly completing a screen. During the study, unit educators and the CNS provided additional on‐unit in‐service training with screening tool completion instructions and advice on how to incorporate the tool into the RN's current assessment workflow. In addition, the charge nurses were asked to review the screens collected each shift and validate any that may have seemed inconsistent with the RN's verbal report of the patient's status.
The university's institutional review board notice of determination waived review for this study because it was classified as quality improvement.
Screening Tool
A sepsis screening tool was developed as part of a broader initiative to improve sepsis‐related morbidity and mortality at our hospital. The screening tool was adapted from the severe sepsis screening tool created by the Surviving Sepsis Campaign and Institute for Healthcare Improvement,[10] and consisted of a simple 3‐tiered paper‐based screening assessment that was to be completed by the bedside RN (Figure 1). RNs on the pilot medical/surgical intermediate care unit performed the screening assessment with their regular patient assessment at the beginning of each shift.

The first tier of the tool screened for the presence of SIRS. Positive parameters included heart rate >90, temperature >38C or <36C, white blood cell count >12,000 or<4000 or >10% bands, and/or respiratory rate >20 or partial pressure of carbon dioxide (PaCO2) <32 mm Hg. To decrease the number of false‐positive screens in patients whose abnormal vitals could already be attributed to a condition other than sepsis, these symptoms were only scored if they had emerged within the previous 8 hours.
If patients met 2 SIRS criteria, the nurse would move to the second tier of the tool, which involved consideration of possible infection as a contributor to a patient's clinical condition as well as a source of infection. If infection was not suspected, further screening was terminated. If infection was suspected, the patient then met criteria for a positive sepsis screen, and a third tier of screening involving assessment of organ dysfunction was initiated.
If the patient screened positive for sepsis (2 SIRS and suspicion for new infection) or severe sepsis (sepsis with end‐organ dysfunction), nurses were instructed to document this in the patient's electronic medical record (EMR) and call the primary team to initiate actions following the hospital‐wide sepsis guidelines. Any subsequent actions were recorded in the patient's EMR.
Data Collection
Completed sepsis screening forms during the month of October 2010 were reviewed by the authors (E.G., L.S., and P.M.). Data including age, gender, International Classification of Diseases, Ninth Revision (ICD‐9) admission and discharge diagnoses, vital signs, lab results, clinical interventions, and documented clinical decision processes by healthcare staff were collected on patients with a positive screen or those who did not screen positive but had an ICD‐9 code for sepsis, severe sepsis, or septic shock during their hospitalization or at discharge. We also collected demographic and clinical data for a random sample of patients who consistently screened negative for sepsis.
Performance Measurement
The sensitivity and specificity of the screening tool was determined by identifying true‐positive, false‐positive, true‐negative, and false‐negative results and calculating accordingly using a 2 2 contingency table. True positives were defined as cases where patients screened positive for sepsis and had a documented diagnosis of sepsis in their EMR within 24 hours of the positive screening or had an ICD‐9 billing code for sepsis. False‐positive cases were those in which patients screened positive for sepsis but did not have a diagnosis of sepsis by manual chart review nor was there an ICD‐9 code for sepsis for their hospital stay. True‐negative cases were those where patients screened negative and did not have an ICD‐9 code for sepsis. False negatives were cases where patients consistently screened negative for sepsis but had an ICD‐9 code for sepsis.
Clinical Activities
To examine the impact of a positive sepsis screen on subsequent clinical action, we assessed the frequency with which a treatment or diagnostic workup was initiated after a positive screen and compared this to clinical activity initiated after a negative screen. Specifically, the patient's EMR was reviewed for actions including measurement of lactate, blood cultures, administration of broad spectrum antibiotics, administration of fluid boluses, or consultation with or transfer to the ICU. These actions were chosen because they are part of the Surviving Sepsis Bundle, which has been demonstrated to improve mortality rates after diagnosis of severe sepsis or septic shock,[11, 12] and can be done outside of an ICU setting. Because screening was done every 8 hours, clinical activity was only attributed to a positive or negative sepsis screen if it occurred within 8 hours of the screening result. Patients were excluded if there were missing data points that precluded full analysis of their clinical course.
Statistical Analysis
To compare the performance of the screening tool between surgical and medical patients, we calculated 95% confidence intervals of screening test sensitivity and specificity. To test if performance was significantly different between these groups, we performed a nonparametric, 2‐sided, 2‐sample test of proportions. Though similar to a [2] test, the 2‐sided test of proportions allowed us to determine if there was a directional difference in test performance (ie, Does the screening tool perform better or worse in a certain patient group?). We also used the test of proportions to compare differences in the proportion of patients receiving sepsis‐related interventions before and after a positive or negative screening result. For comparisons of demographic variables we used nonparametric tests including the [2] test for categorical variables and the Kruskal‐Wallis test for continuous variables. We used SAS 9.3 (SAS Institute Inc., Cary, NC) to perform our analyses.
RESULTS
Over a 1‐month time period, 2143 screens were completed on 245 patients (169 surgical, 76 medical). The overall incidence of sepsis on the treatment unit during this time period was 9%. Surgical patients had an 8.9% incidence of sepsis, and medical patients had an incidence of 9.2%.
Screening tool performance is presented in Table 1. The screening tool had 95.5% sensitivity and 91.9% specificity, with no significant differences in performance between surgical and medical patients. The overall negative predictive value was 99.5%, also with comparable performance in both surgical and medical patients (P = 0.89). The overall positive predictive value (PPV) was 70% in medical patients and 48% in surgical patients (P = 0.12). Screening tool accuracy for medical and surgical patients was 92%.
| Overall, N = 245 (95% CI) | Surgery, N = 169 (95% CI) | Medicine, N = 76 (95% CI) | P Value* | |
|---|---|---|---|---|
| ||||
| Sensitivity | 95.5% (75%‐99.7%) | 93% (66%‐99.6%) | 100% (56%‐100%) | 0.17 |
| Specificity | 91.9% (87%‐95%) | 90% (84%‐94%) | 95% (87%‐99%) | 0.48 |
| NPV | 99.5% (81%‐100%) | 99.3% (71%‐100%) | 100% (67%‐100%) | 0.89 |
| PPV | 53.8% (39%‐70%) | 48% (23%‐73%) | 70% (30%‐100%) | 0.12 |
| LR+ | 11.8 | 9.3 | 20 | |
| LR | 0.05 | 0.08 | 0 | |
| Confirmed patient diagnosis, overall | ||||
| Sepsis | No sepsis | |||
| Screen positive | 21 (TP) | 18 (FP) | ||
| Screen negative | 1 (FN) | 205 (TN) | ||
| Confirmed patient diagnosis, medicine | ||||
| Sepsis | No sepsis | |||
| Screen positive | 7 (TP) | 3 (FP) | ||
| Screen negative | 0 (FN) | 66 (TN) | ||
| Confirmed patient diagnosis, surgery | ||||
| Sepsis | No sepsis | |||
| Screen positive | 14 (TP) | 15 (FP) | ||
| Screen negative | 1 (FN) | 139 (TN) | ||
Clinical Activities
Of the 39 patients who screened positive for sepsis, nurses classified 20 with sepsis and 19 with severe sepsis. Of these 39 patients, 33 were included in our descriptive analysis of the effect of positive screening results on clinical activity (3 were excluded for admission for sepsis and 3 for missing data). As a comparison, we randomly selected 30 patients of the 206 patients who screened negative for sepsis to evaluate clinical activity before and after a negative screen.
Characteristics of patients screening positive and negative for sepsis are reported in Table 2. We found no statistically significant differences in age, sex, length of hospital stay, or mortality amongst all groups.
| Patient Characteristics | Surgery (Positive) | Medicine (Positive) | Surgery (Negative) | Medicine (Negative) | P Value |
|---|---|---|---|---|---|
| |||||
| No. | 26 | 7 | 20 | 10 | |
| Age, y, mean | 57.8 ( 16.5) | 72.4 ( 16.8) | 64.6 ( 19.4) | 63.6 ( 16.8) | 0.25 |
| % Male (no.) | 50% (13) | 57% (4) | 60% (12) | 60% (6) | 0.27 |
| Length of stay, d, median (IQR) | 9 (716.7) | 7 (5.511.5) | 11 (7.722) | 8 (421) | 0.38 |
| No. of PODs until first positive screen, d, median (IQR) | 2 (13) | N/A | N/A | N/A | |
| % Mortality (no.) | 0% | 14% (1) | 5% (1) | 10% (1) | 0.19 |
Figure 2 illustrates differences in the proportion of patients receiving a clinical action before and after a negative or positive screening test result. In the cohort of 33 patients screening positive for sepsis, clinical action after a positive screen was taken in 4 of the 7 (50%) medical patients and 11 of 26 (42%) surgical patients. In patients screening negative for sepsis we found only 1 incident in which a sepsis‐related action was taken after a negative screen. In this case the patient was admitted to the ICU within 8 hours of a negative screen, though there was no explicit documentation that sepsis was the reason for this admission.

We compared the proportion of patients receiving sepsis‐related treatment before either a negative or positive screen and found no significant difference (Table 3). We then compared the proportion of patients receiving sepsis‐related actions after a positive or negative screening test result and found that the proportion of patients receiving antibiotics, blood cultures, and lactate measurement was significantly higher for patients with a positive sepsis screening result compared to those with a negative screening result (Table 3).
| Intervention and Group | Proportion | P Value |
|---|---|---|
| ||
| Before screening test | ||
| Antibiotics | 0.066 | |
| Positive screen | 45% | |
| Negative screen | 23% | |
| Lactate | 0.837 | |
| Positive screen | 15% | |
| Negative screen | 13% | |
| Blood culture | 0.181 | |
| Positive screen | 18% | |
| Negative screen | 17% | |
| Fluid administration | 0.564 | |
| Positive screen | 6% | |
| Negative screen | 10% | |
| ICU transfer/consult | 0.337 | |
| Positive screen | 3% | |
| Negative screen | 0% | |
| After screening test | ||
| Antibiotics | 0.006 | |
| Positive screen | 58% | |
| Negative screen | 23% | |
| Lactate | 0.018 | |
| Positive screen | 36% | |
| Negative screen | 13% | |
| Blood Culture | 0.002 | |
| Positive screen | 24% | |
| Negative screen | 17% | |
| Fluid administration | 0.112 | |
| Positive screen | 24% | |
| Negative screen | 10% | |
| ICU transfer/consult | 0.175 | |
| Positive screen | 9% | |
| Negative screen | 3% | |
DISCUSSION
Improving recognition and time to treatment of sepsis in a non‐ICU setting is an important step toward decreasing sepsis‐related mortality. Lundberg and colleagues found that mortality rates for patients diagnosed with septic shock on a general ward were higher than for patients diagnosed in the ICU, even though ward patients were younger and healthier at baseline.[8] For ward patients, treatment delays were most profound in initiating vasoactive therapies, and minor delays were encountered in initiating fluid resuscitation. In their international study on the impact of early goal‐directed therapy guidelines, Levy and colleagues found that patients diagnosed with severe sepsis on the wards were almost twice as likely to die as patients diagnosed with sepsis in the emergency department.[9]
We are the first to report about an accurate nurse‐driven SIRS‐based sepsis screening protocol that is effective in the early identification of sepsis in both medical and surgical patients in an intermediate care setting. We found no significant difference in the screening tool performance between the medical and surgical cohorts. This is an important comparison given that SIRS criteria alone can be nonspecific in the postoperative population, where it is common to have hemodynamic changes, elevation of inflammatory markers, and fevers from noninfectious sources.
Our sepsis screening tool was designed in 3 tiers to improve its specificity. The first tier was based strictly on SIRS criteria (eg, tachycardia or fever), whereas the second and third tiers served to increase the specificity of the screening tool by instructing the evaluator to assess possible sources of infection and assess for objective signs of organ dysfunction. We relied heavily on the nursing staff to assess for the presence or absence of infection and believe that the educational component prior to initiating the screening protocol was vital.
EMR‐based screening tools that rely purely on physiologic data have been considered for the early detection and management of sepsis, although they lack the specificity gained through the incorporation of clinical judgment.[13] Sawyer and colleagues report using a real‐time EMR‐based method for early sepsis detection in non‐ICU patients that is based solely on objective measures; however, their PPV was only 19.5%. The model we describe in this study is one that incorporates real‐time physiologic data available from an EMR coupled with the clinical judgment of a bedside registered nurse. As our data suggest, this provides a screen that is both sensitive and specific.
It is interesting to note that in our assessment of clinical action taken 8 hours after a positive screening test (the interval after which a new screening test was performed), the rate of diagnostic workup and/or treatment for sepsis was relatively low. One reason for this could have been that the treating team had suspicion for sepsis prior to a positive screen and had already initiated clinical action. Of the 51 recorded clinical actions taken around the time of a positive screen, the majority (67%) occurred before the screening result. It is also possible that clinical action was not pursued because the treatment team disagreed with a diagnosis of sepsis. Of all the false positive screening cases, manual chart review confirmed that these patients did not have sepsis, nor did they develop sepsis during their index hospital stay. Last, we only recorded clinical actions taken within 8 hours of the first positive screen for sepsis and measured 5 very specific actions. Thus, our analysis may have missed actions taken after 8 hours or actions that differed from the 5 we chose to assess.
Even with the apparently low levels of new clinical activity after a positive screen, when compared to patients who screened negative for sepsis, a significantly higher number of patients who had a positive screen received antibiotics, had lactate measured, and had blood cultures drawn. We did not find a significant difference in the proportion of patients receiving a sepsis‐related clinical action before a screening result (positive or negative), which suggests that a positive screening test may have led to increased clinical action.
A limitation of our study is its small size and that it was conducted in 1 pilot unit. Additionally, our retrospective analysis of clinical care inhibited our ability to fully understand a patient's clinical course or retrieve missing data points. A related limitation is that we could not ascertain how often the screening tool did not identify a case of sepsis before it was clinically diagnosed. Assessing the temporal performance of our screening tool is of great interest and may be more easily performed using an electronic version of the screening tool, which is currently in development.
Using ICD‐9 codes to determine the true‐negative cohort is another limitation of our study. It is well documented that use of administrative data can lead to inaccurate classification of patients.[14] To address this, we performed random audits of 30 test‐negative patients. In doing so we did not find any errors in classification.
Although we did not find a significant difference in screening tool performance between surgical and medical patients, the PPV of the tool was lower in the surgical population (48%) compared to the medical population (70%). The lower PPV observed in surgical patients could be attributable to an overall lower incidence of sepsis in this cohort as well as possible errors in initial assessment of infection, which can be difficult in postsurgical patients. Our retrospective analysis included data from the early months of the screening protocol, a time in which nursing staff was still developing clinical acumen in identifying sepsis. However, this could have led nurses to either overestimate or underestimate the presence of infection in either patient group.
Suspicion for infection is the cornerstone definition of sepsis, and in our screening protocol nurses were charged with making this decision based on their knowledge of the patient's clinical course and current status. Issues concerning nurses' recognition of infection symptoms are an area of opportunity for further research and education and could aid in improving PPV. Clinical judgment could be further bolstered by adding promising laboratory tests such as C‐reactive protein or procalcitonin as objective adjuncts to an initial assessment for sepsis,[15] which could potentially increase screening test PPV.
CONCLUSIONS
A simple screening tool for sepsis performed by the bedside nurse can provide a means to successfully identify sepsis early and lead to more timely diagnostics and treatment in both medical and surgical patients in an intermediate care setting.
ACKNOWLEDGEMENTS
The authors thank Eileen Pummer, quality manager for the sepsis team; Pauline Regner, patient care manager of the pilot study unit; and the nurses who contributed to the screening tool design team and data collection. The authors acknowledge Pooja Loftus for her statistical expertise, and Isabella Chu for her review of the manuscript. Disclosures: Presented as a poster at the 31st Annual Meeting of the Surgical Infection Society, Palm Beach, Florida, May 2011. The authors report no conflicts of interest.
- , , , , , Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303–1310.
- , , Septicemia in U.S. hospitals, 2009. HCUP statistical brief #122. Agency for Healthcare Research and Quality. Available at: http://www.hcup-us.ahrq.gov/reports/statbriefs/sb122.pdf. Published October 2011. Accessed on September 4, 2012.
- , , , Economic implications of an evidence‐based sepsis protocol: can we improve outcomes and lower costs? Crit Care Med. 2007;35(5):1257–1262.
- , , , , , Late compliance with the sepsis resuscitation bundle: impact on mortality. Shock. 2011;36(6):542–547.
- , , , , , The costs and cost‐effectiveness of an integrated sepsis treatment protocol. Crit Care Med. 2008;36(4):1168–1174.
- , , A simple prediction algorithm for bacteraemia in patients with acute febrile illness. QJM. 2005;98(11):813–820.
- , , , et al. Validation of a screening tool for the early identification of sepsis. J Trauma. 2009;66(6):1539–1546; discussion 1546–1547.
- , , , et al. Septic shock: an analysis of outcomes for patients with onset on hospital wards versus intensive care units. Crit Care Med. 1998;26(6):1020–1024.
- , , , et al. The Surviving Sepsis Campaign: results of an international guideline‐based performance improvement program targeting severe sepsis. Crit Care Med. 2010;38(2):367–374.
- Institute of Healthcare Improvement. Evaluation for severe sepsis screening tool. Surviving Sepsis Campaign. Available at: http://www.survivingsepsis.org/About_the_Campaign/Documents/evaluationforseveresepsisscreeningtool.pdf. Accessed on September 30, 2012.
- , , , et al. Impact of the Surviving Sepsis Campaign protocols on hospital length of stay and mortality in septic shock patients: results of a three‐year follow‐up quasi‐experimental study. Crit Care Med. 2010;38(4):1036–1043.
- , , , et al. Reduction of the severe sepsis or septic shock associated mortality by reinforcement of the recommendations bundle: a multicenter study. Ann Fr Anesth Reanim. 2010;29(9):621–628.
- , , , et al. Implementation of a real‐time computerized sepsis alert in nonintensive care unit patients. Crit Care Med. 2011;39(3):469–473.
- , , , Accuracy of administrative data for identifying patients with pneumonia. Am J Med Qual. 2005;20(6):319–328.
- , , , , , Comparison of procalcitonin and C‐reactive protein as markers of sepsis. Crit Care Med. 2003;31(6):1737–1741.
- , , , , , Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303–1310.
- , , Septicemia in U.S. hospitals, 2009. HCUP statistical brief #122. Agency for Healthcare Research and Quality. Available at: http://www.hcup-us.ahrq.gov/reports/statbriefs/sb122.pdf. Published October 2011. Accessed on September 4, 2012.
- , , , Economic implications of an evidence‐based sepsis protocol: can we improve outcomes and lower costs? Crit Care Med. 2007;35(5):1257–1262.
- , , , , , Late compliance with the sepsis resuscitation bundle: impact on mortality. Shock. 2011;36(6):542–547.
- , , , , , The costs and cost‐effectiveness of an integrated sepsis treatment protocol. Crit Care Med. 2008;36(4):1168–1174.
- , , A simple prediction algorithm for bacteraemia in patients with acute febrile illness. QJM. 2005;98(11):813–820.
- , , , et al. Validation of a screening tool for the early identification of sepsis. J Trauma. 2009;66(6):1539–1546; discussion 1546–1547.
- , , , et al. Septic shock: an analysis of outcomes for patients with onset on hospital wards versus intensive care units. Crit Care Med. 1998;26(6):1020–1024.
- , , , et al. The Surviving Sepsis Campaign: results of an international guideline‐based performance improvement program targeting severe sepsis. Crit Care Med. 2010;38(2):367–374.
- Institute of Healthcare Improvement. Evaluation for severe sepsis screening tool. Surviving Sepsis Campaign. Available at: http://www.survivingsepsis.org/About_the_Campaign/Documents/evaluationforseveresepsisscreeningtool.pdf. Accessed on September 30, 2012.
- , , , et al. Impact of the Surviving Sepsis Campaign protocols on hospital length of stay and mortality in septic shock patients: results of a three‐year follow‐up quasi‐experimental study. Crit Care Med. 2010;38(4):1036–1043.
- , , , et al. Reduction of the severe sepsis or septic shock associated mortality by reinforcement of the recommendations bundle: a multicenter study. Ann Fr Anesth Reanim. 2010;29(9):621–628.
- , , , et al. Implementation of a real‐time computerized sepsis alert in nonintensive care unit patients. Crit Care Med. 2011;39(3):469–473.
- , , , Accuracy of administrative data for identifying patients with pneumonia. Am J Med Qual. 2005;20(6):319–328.
- , , , , , Comparison of procalcitonin and C‐reactive protein as markers of sepsis. Crit Care Med. 2003;31(6):1737–1741.
© 2014 Society of Hospital Medicine
Increased Incidence of Patella Baja After Total Knee Arthroplasty Revision for Infection
Patellar height may be important in determining function after total knee arthroplasty (TKA). By altering patellofemoral joint mechanics, patella baja may cause several functional issues after TKA.1-8 Patella baja leads to decreased range of motion (ROM) affecting both extension and flexion.5,8,9 Deep flexion can be restricted in TKA patients with patella baja because of tracking limitations associated with an inferiorly displaced patella. As the knee is brought into flexion, the patella can impinge on the anterior aspect of the tibial polyethylene or the tibial tray—presenting a true block to flexion and potentially altering wear.1,10
Another functional issue with patella baja is loss of strength in the extensor mechanism. The patella serves as a fulcrum for the extensor muscles of the knee. When positioned properly and functioning properly, the patella increases the extensor forces generated. When the patella is positioned in baja, the knee generates decreased extensor mechanism force.6,7 This can result in a lag, with the patient being unable to fully extend the knee. Extension-dependent activities are impaired. Patients with weak extensor function can experience poor function with stair climbing, rising from a chair, and exiting an automobile. The improper function and scarring of the patella can result in increased anterior knee pain and worse functional outcome scores after TKAs.3,9
An abnormally positioned patella can either result from or lead to increased scarring in the knee.9,11 Patellar height is often measured with the Insall-Salvati ratio (ISR), which is the patella tendon length (measurement of the tendon from the tibial tubercle to the inferior pole of the patella) divided by the patellar length (longest measured dimension of the patella) (Figure 1).12 Patella baja is defined as an ISR of less than 0.8. Other indices that reference off the tibial plateau (Blackburne-Peel ratio, Canton-Deschamps ratio) reflect an elevation of the joint line, or pseudobaja, and are unreliable for analysis of patella baja after TKA.13
Postoperative patella baja has been reported in 10% to 34% of primary TKAs.4,7 Inferior positioning of the patella and scarring can cause intraoperative difficulty with exposure and may complicate outcomes.9,13 The exposure scar is often larger in TKA revisions for infection compared with primary TKAs.
We conducted a study to compare the incidence of patella baja in noninfected and infected TKA revisions. We hypothesized that, compared with noninfected knees, infected knees treated with nonarticulating spacers would have a higher incidence of patella baja both before and after surgery secondary to more inflammation, immobilization, and related scarring.
Materials and Methods
We conducted a retrospective case–cohort study of 148 consecutive TKA revisions. All TKA revisions were performed between 2003 and 2009 using a mobile-bearing revision system from a single manufacturer. All surgeries were done at a single institution by the 2 senior surgeons. The surgical approach was a standard medial parapatellar approach without patellar eversion. Our institutional review board approved the study and waived the requirement for informed consent, as this was a retrospective study of existing medical records that posed no more than minimal risk to patients.
To properly evaluate patellar height, orthopedic specialty–trained radiologic technicians obtained preoperative and postoperative weight-bearing radiographs using a standardized lateral radiograph in clinic. Two blinded investigators measured ISR radiographically both before surgery (preexplant for septic revisions) and at latest follow-up (postreplant for septic revisions). Patients with inadequate films and/or patellectomies were excluded, along with patients who had less than 6 months of postoperative follow-up.
Ninety-one patients (101 TKAs) met the study inclusion criteria. Two groups of cases were compared: aseptic revisions (n = 67) and septic revisions (n = 34). Reasons for aseptic revisions included implant loosening (24/67, 35.8%), instability (12/67, 17.9%), pain (12/67, 17.9%), lysis (5/67, 7.5%), stiffness (3/67, 4.5%), and malrotation (2/67, 3.0%). Infection was determined by Musculoskeletal Infection Society criteria, as documented by positive aspirations and/or intraoperative tissue cultures taken at prosthesis explantation, elevated white blood cell count in the aspirate, elevated percentage of polymorphonuclear (PMN) cells in the aspirate, gross purulence, presence of chronic draining sinus, or histologic analysis revealing acute inflammation with more than 5 PMN cells per high power field.14,15
All infected TKAs were treated with 2-stage revisions. The standard of care at our institution through this series was to use a nonarticulating spacer for the treatment of infection. Weight-bearing status varied by extent of bone damage. Six weeks of culture-specific intravenous antibiotics were administered with assistance from an infectious disease consultant. Reimplantation was performed when clinical and laboratory criteria for resolution of infection were met—specifically, when erythrocyte sedimentation rate was less than 30 mm/h, C-reactive protein level was less than 10 mg/L, and aspirates were culture-negative. Mean (range) follow-up was 33.9 (6.2-75.7) months for aseptic revisions and 32.3 (7.5-94.2) months for septic revisions. Radiographic follow-up was performed at each visit, with weight-bearing anteroposterior and posteroanterior views, along with a lateral knee radiograph. At final follow-up, ROM was recorded by the senior attending evaluating the patient.
Categorical variables were statistically analyzed with χ2 tests, and continuous variables were analyzed with Student t test, analysis of variance, and univariate analysis of covariance (ANCOVA). Statistical significance was set at P < .05. Intrarater reliability was measured with the intraclass correlation coefficient (ICC). All statistical analysis was performed with Predictive Analytics SoftWare Statistics Version 20.0 (SPSS, Chicago, Illinois).
Results
Ninety-one consecutive patients (43 men, 48 women) were included in this study. Mean (SD) age was 66.4 (10.1) years. Mean (SD) preoperative ISR in septic and aseptic cases was 0.94 (0.25) for men and 1.02 (0.23) for women (P = .10). Mean postoperative ISR in septic and aseptic cases was 0.84 (0.27) for men and 0.99 (0.23) for women (P = .004). There was a sex difference between septic and aseptic revisions. There were 22 men and 36 women in the aseptic group and 21 men and 12 women in the septic group (P = .01). Men were more likely than women to have septic revisions and patella baja. Table 1 compares the patient demographics of the 2 patient populations. Mean (SD) number of surgeries, including irrigation and débridement procedures before reimplantation, was larger for septic revisions, 2.9 (0.9), than for aseptic revisions, 1.4 (0.8) (P < .001).
Infection was the most common reason for revision and accounted for 33.7% (34/101) of all revisions. Noninfectious indications, in declining order of frequency, included loosening (23.8%, 24/101), instability (11.9%, 12/101), pain (11.9%, 12/101), osteolysis (5.0%, 5/101), polyethylene wear (5.0%, 5/101), failed unicompartmental knee (4.0%, 4/101), stiffness (3.0%, 3/101), and patellar problems (2.0%, 2/101) (Table 2). ISR decreased significantly only in infected revisions. It is important to note that there was not a high incidence of stiffness or patellofemoral failure in revision patients before surgery.
Mean (SD) ISR did not differ between groups before surgery, 1.00 (0.25) for aseptic and 0.96 (0.22) for septic (P = .49), but differed significantly after surgery, 0.99 (0.23) for aseptic and 0.77 (0.24) for septic (P < .001) (Figure 2). The univariate ANCOVA also demonstrated a postoperative difference between groups when taking the preoperative ratio into account: 0.99 (0.23) for aseptic and 0.78 (0.24) for septic (P = .005) (Table 3). Before surgery, 22.4% and 23.9% of the aseptic and septic groups, respectively, had patella baja (P = .58). After surgery, 17.6% and 58.8% of the aseptic and septic groups had patella baja (P = .001) (Table 4). The ICC for preoperative ISR was 0.94, and the ICC for postoperative ISR was 0.96, which indicates excellent agreement of measurements between the 2 blinded investigators.
ROM differed between septic and aseptic groups owing to the difference in postoperative flexion. Mean (SD) postoperative extension was 2.2° (5.4°) for the aseptic group and 5.1° (9.8°) for the septic group—not significantly different (P = .13). Mean (SD) postoperative flexion was 110.2° (18.8°) for the aseptic group and 97.2° (29.4°) for the septic group—significantly different (P = .02). The groups differed significantly (P = .02) in mean (SD) ROM: 108.0° (20.7°) for aseptic and 92.2° (34.6°) for septic (Table 1). ROM was also significantly associated with patella baja (P = .04), as patients with ISR of less than 0.8 had mean (SD) postoperative ROM of 95.1° (31.6°), and patients without patella baja had mean (SD) postoperative ROM of 106.8° (23.6°).
For the septic group, mean (SD) time between first and second stages was 13.0 (8.3) weeks (range, 1-44.3 weeks). Mean (SD) timing of spacer placement was not statistically significantly different (P = .90) between patients who had patella baja, 12.9 (8.8) weeks, and patients who did not have patella baja, 13.2 (7.8) weeks.
Discussion
This study demonstrated that TKAs done for septic reasons resulted in a higher incidence of patella baja and decreased ROM. Incidence of patella baja was higher both before and after revision in septic TKAs than in aseptic TKAs, proving the hypothesis under study. Prerevision incidence was not significantly different, but there was a trend that could not be ignored. This may suggest that there is already an ongoing process in the infected knee that contributes to patella baja; the precise etiology remains unclear and is likely multifactorial. For example, scar formation may be increased in patients with chronic infection, predisposing to patella baja. This assertion is indirectly supported by a recent study from our institution revealing longer average surgical time in septic versus aseptic knee revisions; the difference was thought to reflect increased scar-tissue formation.16 That study also found that patients who underwent septic revisions had significantly more surgical procedures than patients who underwent aseptic revisions. Repetitive surgeries—specifically, repetitive arthrotomies during irrigation and débridement before reimplantation—lead to increased scar formation, which may contribute to preoperative and postoperative patella baja. This may be reflected in the findings that ROM was decreased in patients in the septic group versus patients in the aseptic group and that ROM was decreased in patients with patella baja. In addition, our study found that male patients were more likely to undergo TKA revision for septic reasons and to develop postoperative patella baja. This finding contrasts with that of a study5 that compared preoperative and postoperative ISR in primary TKA and found that women were more likely than men to have patella baja. Although women are more likely to undergo TKA revision,17 men may be more susceptible to infection and subsequent patella baja.
The higher postoperative rate of patella baja in the septic group became statistically significant even when preoperative incidence was considered. This may have been caused by infection-related scarring and by prolonged immobilization of septic knees with use of nonarticulating antibiotic spacers. By keeping these knees immobile with a nonarticulating spacer for a prolonged period in the healing phase of the infection, scar tissue may mature and form over the time between stages. A comparable example may be high tibial osteotomies, in which a high incidence of patella baja has been partly attributed to prolonged casting.11 Future work comparing the results of articulating and nonarticulating spacers will help to determine if immobilization contributes to patella baja in infected TKAs.
There are several limitations to our study. Patient outcome questionnaires were not used, and they would have allowed for the assessment of physical outcomes and emotional satisfaction by comparing outcomes between patients with and without patella baja and comparing septic and aseptic TKAs. In addition, there was no standard method for quantifying difficulty of revision, which would have enabled us to compare difficulty of revision in patients with patella baja.
Conclusion
This study identified a high rate of patella baja and decreased ROM in TKA revisions, particularly infected revisions treated with a nonarticulating spacer. It is important to determine if there are functional consequences. Further investigation is needed regarding the cause, prevention, and management of this potentially debilitating outcome after revision TKA.
1. Aglietti P, Buzzi R, Gaudenzi A. Patello-femoral functional results and complications with the posterior stabilised total condylar knee prosthesis. J Arthroplasty. 1988;3(1):17-25.
2. Fern ED, Winson IG, Getty CJM. Anterior knee pain in rheumatoid patients after total knee replacement: possible selection criteria for patellar resurfacing. J Bone Joint Surg Br. 1992;74(5):745-748.
3. Figgie HE 3rd, Goldberg VM, Heiple KG, Moller HS 3rd, Gordon NH. The influence of tibial-patellofemoral location on function of the knee in patients with the posterior stabilized condylar knee prosthesis. J Bone Surg Surg Am. 1986;68(7):1035-1040.
4. Floren M, Davis J, Peterson MG, Laskin RS. A mini-midvastus capsular approach with patellar displacement decreases the prevalence of patellar baja. J Arthroplasty. 2007;22(6 Suppl 2):51-57.
5. Meneghini RM, Ritter MA, Pierson JL, Meding JB, Berend ME, Faris PM. The effect of the Insall-Salvati ratio on outcome after total knee arthroplasty. J Arthroplasty. 2006;21(6 Suppl 2):116-120.
6. Singerman R, Davy DT, Goldberg VM. Effects of patella alta and patella infera on patellofemoral contact forces. J Biomech. 1994;27(8):1059-1065.
7. Van Eijden TM, Kouwenhoven E, Weijs WA. Mechanics of the patellar articulation: effects of patellar ligament length studied with a mathematical model. Acta Orthop Scand. 1987;58(5):560-566.
8. Weale AE, Murray DW, Newman JH, Ackroyd CE. The length of the patellar tendon after unicompartmental and total knee replacement. J Bone Joint Surg Br. 1999;81(5):790-795.
9. Chonko DJ, Lombardi AV Jr, Berend KR. Patella baja and total knee arthroplasty (TKA): etiology, diagnosis, and management. Surg Technol Int. 2004;12:231-238.
10. Cameron HU, Jung YB. Patella baja complicating total knee arthroplasty. A report of two cases. J Arthroplasty. 1988;3(2):177-180.
11. Scuderi GR, Windsor RE, Insall JN. Observations on patellar height after proximal tibial osteotomy. J Bone Joint Surg Am. 1989;71(2):245-248.
12. Insall JN, Salvati E. Patella position in the normal knee joint. Radiology. 1971;101(1):101-104.
13. Grelsamer RP. Patella baja after total knee arthroplasty: is it really patella baja? J Arthroplasty. 2002;17(1):66-69.
14. Parvizi J, Zmistowski B, Berbari EF, et al. New definition for periprosthetic joint infection: from the Workgroup of the Musculoskeletal Infection Society. Clin Orthop. 2011;469(11):2992-2994.
15. Workgroup Convened by the Musculoskeletal Infection Society. New definition for periprosthetic joint infection. J Arthroplasty. 2011;26(8):1136-1138.
16. Laudermilch DJ, Fedorka CJ, Heyl A, Rao N, McGough RL. Outcomes of revision total knee arthroplasty after methicillin-resistant Staphylococcus aureus infection. Clin Orthop. 2010;468(8):2067-2073.
17. Bozic KJ, Kurtz SM, Lau E, et al. The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop. 2010;468(1):45-51.
Patellar height may be important in determining function after total knee arthroplasty (TKA). By altering patellofemoral joint mechanics, patella baja may cause several functional issues after TKA.1-8 Patella baja leads to decreased range of motion (ROM) affecting both extension and flexion.5,8,9 Deep flexion can be restricted in TKA patients with patella baja because of tracking limitations associated with an inferiorly displaced patella. As the knee is brought into flexion, the patella can impinge on the anterior aspect of the tibial polyethylene or the tibial tray—presenting a true block to flexion and potentially altering wear.1,10
Another functional issue with patella baja is loss of strength in the extensor mechanism. The patella serves as a fulcrum for the extensor muscles of the knee. When positioned properly and functioning properly, the patella increases the extensor forces generated. When the patella is positioned in baja, the knee generates decreased extensor mechanism force.6,7 This can result in a lag, with the patient being unable to fully extend the knee. Extension-dependent activities are impaired. Patients with weak extensor function can experience poor function with stair climbing, rising from a chair, and exiting an automobile. The improper function and scarring of the patella can result in increased anterior knee pain and worse functional outcome scores after TKAs.3,9
An abnormally positioned patella can either result from or lead to increased scarring in the knee.9,11 Patellar height is often measured with the Insall-Salvati ratio (ISR), which is the patella tendon length (measurement of the tendon from the tibial tubercle to the inferior pole of the patella) divided by the patellar length (longest measured dimension of the patella) (Figure 1).12 Patella baja is defined as an ISR of less than 0.8. Other indices that reference off the tibial plateau (Blackburne-Peel ratio, Canton-Deschamps ratio) reflect an elevation of the joint line, or pseudobaja, and are unreliable for analysis of patella baja after TKA.13
Postoperative patella baja has been reported in 10% to 34% of primary TKAs.4,7 Inferior positioning of the patella and scarring can cause intraoperative difficulty with exposure and may complicate outcomes.9,13 The exposure scar is often larger in TKA revisions for infection compared with primary TKAs.
We conducted a study to compare the incidence of patella baja in noninfected and infected TKA revisions. We hypothesized that, compared with noninfected knees, infected knees treated with nonarticulating spacers would have a higher incidence of patella baja both before and after surgery secondary to more inflammation, immobilization, and related scarring.
Materials and Methods
We conducted a retrospective case–cohort study of 148 consecutive TKA revisions. All TKA revisions were performed between 2003 and 2009 using a mobile-bearing revision system from a single manufacturer. All surgeries were done at a single institution by the 2 senior surgeons. The surgical approach was a standard medial parapatellar approach without patellar eversion. Our institutional review board approved the study and waived the requirement for informed consent, as this was a retrospective study of existing medical records that posed no more than minimal risk to patients.
To properly evaluate patellar height, orthopedic specialty–trained radiologic technicians obtained preoperative and postoperative weight-bearing radiographs using a standardized lateral radiograph in clinic. Two blinded investigators measured ISR radiographically both before surgery (preexplant for septic revisions) and at latest follow-up (postreplant for septic revisions). Patients with inadequate films and/or patellectomies were excluded, along with patients who had less than 6 months of postoperative follow-up.
Ninety-one patients (101 TKAs) met the study inclusion criteria. Two groups of cases were compared: aseptic revisions (n = 67) and septic revisions (n = 34). Reasons for aseptic revisions included implant loosening (24/67, 35.8%), instability (12/67, 17.9%), pain (12/67, 17.9%), lysis (5/67, 7.5%), stiffness (3/67, 4.5%), and malrotation (2/67, 3.0%). Infection was determined by Musculoskeletal Infection Society criteria, as documented by positive aspirations and/or intraoperative tissue cultures taken at prosthesis explantation, elevated white blood cell count in the aspirate, elevated percentage of polymorphonuclear (PMN) cells in the aspirate, gross purulence, presence of chronic draining sinus, or histologic analysis revealing acute inflammation with more than 5 PMN cells per high power field.14,15
All infected TKAs were treated with 2-stage revisions. The standard of care at our institution through this series was to use a nonarticulating spacer for the treatment of infection. Weight-bearing status varied by extent of bone damage. Six weeks of culture-specific intravenous antibiotics were administered with assistance from an infectious disease consultant. Reimplantation was performed when clinical and laboratory criteria for resolution of infection were met—specifically, when erythrocyte sedimentation rate was less than 30 mm/h, C-reactive protein level was less than 10 mg/L, and aspirates were culture-negative. Mean (range) follow-up was 33.9 (6.2-75.7) months for aseptic revisions and 32.3 (7.5-94.2) months for septic revisions. Radiographic follow-up was performed at each visit, with weight-bearing anteroposterior and posteroanterior views, along with a lateral knee radiograph. At final follow-up, ROM was recorded by the senior attending evaluating the patient.
Categorical variables were statistically analyzed with χ2 tests, and continuous variables were analyzed with Student t test, analysis of variance, and univariate analysis of covariance (ANCOVA). Statistical significance was set at P < .05. Intrarater reliability was measured with the intraclass correlation coefficient (ICC). All statistical analysis was performed with Predictive Analytics SoftWare Statistics Version 20.0 (SPSS, Chicago, Illinois).
Results
Ninety-one consecutive patients (43 men, 48 women) were included in this study. Mean (SD) age was 66.4 (10.1) years. Mean (SD) preoperative ISR in septic and aseptic cases was 0.94 (0.25) for men and 1.02 (0.23) for women (P = .10). Mean postoperative ISR in septic and aseptic cases was 0.84 (0.27) for men and 0.99 (0.23) for women (P = .004). There was a sex difference between septic and aseptic revisions. There were 22 men and 36 women in the aseptic group and 21 men and 12 women in the septic group (P = .01). Men were more likely than women to have septic revisions and patella baja. Table 1 compares the patient demographics of the 2 patient populations. Mean (SD) number of surgeries, including irrigation and débridement procedures before reimplantation, was larger for septic revisions, 2.9 (0.9), than for aseptic revisions, 1.4 (0.8) (P < .001).
Infection was the most common reason for revision and accounted for 33.7% (34/101) of all revisions. Noninfectious indications, in declining order of frequency, included loosening (23.8%, 24/101), instability (11.9%, 12/101), pain (11.9%, 12/101), osteolysis (5.0%, 5/101), polyethylene wear (5.0%, 5/101), failed unicompartmental knee (4.0%, 4/101), stiffness (3.0%, 3/101), and patellar problems (2.0%, 2/101) (Table 2). ISR decreased significantly only in infected revisions. It is important to note that there was not a high incidence of stiffness or patellofemoral failure in revision patients before surgery.
Mean (SD) ISR did not differ between groups before surgery, 1.00 (0.25) for aseptic and 0.96 (0.22) for septic (P = .49), but differed significantly after surgery, 0.99 (0.23) for aseptic and 0.77 (0.24) for septic (P < .001) (Figure 2). The univariate ANCOVA also demonstrated a postoperative difference between groups when taking the preoperative ratio into account: 0.99 (0.23) for aseptic and 0.78 (0.24) for septic (P = .005) (Table 3). Before surgery, 22.4% and 23.9% of the aseptic and septic groups, respectively, had patella baja (P = .58). After surgery, 17.6% and 58.8% of the aseptic and septic groups had patella baja (P = .001) (Table 4). The ICC for preoperative ISR was 0.94, and the ICC for postoperative ISR was 0.96, which indicates excellent agreement of measurements between the 2 blinded investigators.
ROM differed between septic and aseptic groups owing to the difference in postoperative flexion. Mean (SD) postoperative extension was 2.2° (5.4°) for the aseptic group and 5.1° (9.8°) for the septic group—not significantly different (P = .13). Mean (SD) postoperative flexion was 110.2° (18.8°) for the aseptic group and 97.2° (29.4°) for the septic group—significantly different (P = .02). The groups differed significantly (P = .02) in mean (SD) ROM: 108.0° (20.7°) for aseptic and 92.2° (34.6°) for septic (Table 1). ROM was also significantly associated with patella baja (P = .04), as patients with ISR of less than 0.8 had mean (SD) postoperative ROM of 95.1° (31.6°), and patients without patella baja had mean (SD) postoperative ROM of 106.8° (23.6°).
For the septic group, mean (SD) time between first and second stages was 13.0 (8.3) weeks (range, 1-44.3 weeks). Mean (SD) timing of spacer placement was not statistically significantly different (P = .90) between patients who had patella baja, 12.9 (8.8) weeks, and patients who did not have patella baja, 13.2 (7.8) weeks.
Discussion
This study demonstrated that TKAs done for septic reasons resulted in a higher incidence of patella baja and decreased ROM. Incidence of patella baja was higher both before and after revision in septic TKAs than in aseptic TKAs, proving the hypothesis under study. Prerevision incidence was not significantly different, but there was a trend that could not be ignored. This may suggest that there is already an ongoing process in the infected knee that contributes to patella baja; the precise etiology remains unclear and is likely multifactorial. For example, scar formation may be increased in patients with chronic infection, predisposing to patella baja. This assertion is indirectly supported by a recent study from our institution revealing longer average surgical time in septic versus aseptic knee revisions; the difference was thought to reflect increased scar-tissue formation.16 That study also found that patients who underwent septic revisions had significantly more surgical procedures than patients who underwent aseptic revisions. Repetitive surgeries—specifically, repetitive arthrotomies during irrigation and débridement before reimplantation—lead to increased scar formation, which may contribute to preoperative and postoperative patella baja. This may be reflected in the findings that ROM was decreased in patients in the septic group versus patients in the aseptic group and that ROM was decreased in patients with patella baja. In addition, our study found that male patients were more likely to undergo TKA revision for septic reasons and to develop postoperative patella baja. This finding contrasts with that of a study5 that compared preoperative and postoperative ISR in primary TKA and found that women were more likely than men to have patella baja. Although women are more likely to undergo TKA revision,17 men may be more susceptible to infection and subsequent patella baja.
The higher postoperative rate of patella baja in the septic group became statistically significant even when preoperative incidence was considered. This may have been caused by infection-related scarring and by prolonged immobilization of septic knees with use of nonarticulating antibiotic spacers. By keeping these knees immobile with a nonarticulating spacer for a prolonged period in the healing phase of the infection, scar tissue may mature and form over the time between stages. A comparable example may be high tibial osteotomies, in which a high incidence of patella baja has been partly attributed to prolonged casting.11 Future work comparing the results of articulating and nonarticulating spacers will help to determine if immobilization contributes to patella baja in infected TKAs.
There are several limitations to our study. Patient outcome questionnaires were not used, and they would have allowed for the assessment of physical outcomes and emotional satisfaction by comparing outcomes between patients with and without patella baja and comparing septic and aseptic TKAs. In addition, there was no standard method for quantifying difficulty of revision, which would have enabled us to compare difficulty of revision in patients with patella baja.
Conclusion
This study identified a high rate of patella baja and decreased ROM in TKA revisions, particularly infected revisions treated with a nonarticulating spacer. It is important to determine if there are functional consequences. Further investigation is needed regarding the cause, prevention, and management of this potentially debilitating outcome after revision TKA.
Patellar height may be important in determining function after total knee arthroplasty (TKA). By altering patellofemoral joint mechanics, patella baja may cause several functional issues after TKA.1-8 Patella baja leads to decreased range of motion (ROM) affecting both extension and flexion.5,8,9 Deep flexion can be restricted in TKA patients with patella baja because of tracking limitations associated with an inferiorly displaced patella. As the knee is brought into flexion, the patella can impinge on the anterior aspect of the tibial polyethylene or the tibial tray—presenting a true block to flexion and potentially altering wear.1,10
Another functional issue with patella baja is loss of strength in the extensor mechanism. The patella serves as a fulcrum for the extensor muscles of the knee. When positioned properly and functioning properly, the patella increases the extensor forces generated. When the patella is positioned in baja, the knee generates decreased extensor mechanism force.6,7 This can result in a lag, with the patient being unable to fully extend the knee. Extension-dependent activities are impaired. Patients with weak extensor function can experience poor function with stair climbing, rising from a chair, and exiting an automobile. The improper function and scarring of the patella can result in increased anterior knee pain and worse functional outcome scores after TKAs.3,9
An abnormally positioned patella can either result from or lead to increased scarring in the knee.9,11 Patellar height is often measured with the Insall-Salvati ratio (ISR), which is the patella tendon length (measurement of the tendon from the tibial tubercle to the inferior pole of the patella) divided by the patellar length (longest measured dimension of the patella) (Figure 1).12 Patella baja is defined as an ISR of less than 0.8. Other indices that reference off the tibial plateau (Blackburne-Peel ratio, Canton-Deschamps ratio) reflect an elevation of the joint line, or pseudobaja, and are unreliable for analysis of patella baja after TKA.13
Postoperative patella baja has been reported in 10% to 34% of primary TKAs.4,7 Inferior positioning of the patella and scarring can cause intraoperative difficulty with exposure and may complicate outcomes.9,13 The exposure scar is often larger in TKA revisions for infection compared with primary TKAs.
We conducted a study to compare the incidence of patella baja in noninfected and infected TKA revisions. We hypothesized that, compared with noninfected knees, infected knees treated with nonarticulating spacers would have a higher incidence of patella baja both before and after surgery secondary to more inflammation, immobilization, and related scarring.
Materials and Methods
We conducted a retrospective case–cohort study of 148 consecutive TKA revisions. All TKA revisions were performed between 2003 and 2009 using a mobile-bearing revision system from a single manufacturer. All surgeries were done at a single institution by the 2 senior surgeons. The surgical approach was a standard medial parapatellar approach without patellar eversion. Our institutional review board approved the study and waived the requirement for informed consent, as this was a retrospective study of existing medical records that posed no more than minimal risk to patients.
To properly evaluate patellar height, orthopedic specialty–trained radiologic technicians obtained preoperative and postoperative weight-bearing radiographs using a standardized lateral radiograph in clinic. Two blinded investigators measured ISR radiographically both before surgery (preexplant for septic revisions) and at latest follow-up (postreplant for septic revisions). Patients with inadequate films and/or patellectomies were excluded, along with patients who had less than 6 months of postoperative follow-up.
Ninety-one patients (101 TKAs) met the study inclusion criteria. Two groups of cases were compared: aseptic revisions (n = 67) and septic revisions (n = 34). Reasons for aseptic revisions included implant loosening (24/67, 35.8%), instability (12/67, 17.9%), pain (12/67, 17.9%), lysis (5/67, 7.5%), stiffness (3/67, 4.5%), and malrotation (2/67, 3.0%). Infection was determined by Musculoskeletal Infection Society criteria, as documented by positive aspirations and/or intraoperative tissue cultures taken at prosthesis explantation, elevated white blood cell count in the aspirate, elevated percentage of polymorphonuclear (PMN) cells in the aspirate, gross purulence, presence of chronic draining sinus, or histologic analysis revealing acute inflammation with more than 5 PMN cells per high power field.14,15
All infected TKAs were treated with 2-stage revisions. The standard of care at our institution through this series was to use a nonarticulating spacer for the treatment of infection. Weight-bearing status varied by extent of bone damage. Six weeks of culture-specific intravenous antibiotics were administered with assistance from an infectious disease consultant. Reimplantation was performed when clinical and laboratory criteria for resolution of infection were met—specifically, when erythrocyte sedimentation rate was less than 30 mm/h, C-reactive protein level was less than 10 mg/L, and aspirates were culture-negative. Mean (range) follow-up was 33.9 (6.2-75.7) months for aseptic revisions and 32.3 (7.5-94.2) months for septic revisions. Radiographic follow-up was performed at each visit, with weight-bearing anteroposterior and posteroanterior views, along with a lateral knee radiograph. At final follow-up, ROM was recorded by the senior attending evaluating the patient.
Categorical variables were statistically analyzed with χ2 tests, and continuous variables were analyzed with Student t test, analysis of variance, and univariate analysis of covariance (ANCOVA). Statistical significance was set at P < .05. Intrarater reliability was measured with the intraclass correlation coefficient (ICC). All statistical analysis was performed with Predictive Analytics SoftWare Statistics Version 20.0 (SPSS, Chicago, Illinois).
Results
Ninety-one consecutive patients (43 men, 48 women) were included in this study. Mean (SD) age was 66.4 (10.1) years. Mean (SD) preoperative ISR in septic and aseptic cases was 0.94 (0.25) for men and 1.02 (0.23) for women (P = .10). Mean postoperative ISR in septic and aseptic cases was 0.84 (0.27) for men and 0.99 (0.23) for women (P = .004). There was a sex difference between septic and aseptic revisions. There were 22 men and 36 women in the aseptic group and 21 men and 12 women in the septic group (P = .01). Men were more likely than women to have septic revisions and patella baja. Table 1 compares the patient demographics of the 2 patient populations. Mean (SD) number of surgeries, including irrigation and débridement procedures before reimplantation, was larger for septic revisions, 2.9 (0.9), than for aseptic revisions, 1.4 (0.8) (P < .001).
Infection was the most common reason for revision and accounted for 33.7% (34/101) of all revisions. Noninfectious indications, in declining order of frequency, included loosening (23.8%, 24/101), instability (11.9%, 12/101), pain (11.9%, 12/101), osteolysis (5.0%, 5/101), polyethylene wear (5.0%, 5/101), failed unicompartmental knee (4.0%, 4/101), stiffness (3.0%, 3/101), and patellar problems (2.0%, 2/101) (Table 2). ISR decreased significantly only in infected revisions. It is important to note that there was not a high incidence of stiffness or patellofemoral failure in revision patients before surgery.
Mean (SD) ISR did not differ between groups before surgery, 1.00 (0.25) for aseptic and 0.96 (0.22) for septic (P = .49), but differed significantly after surgery, 0.99 (0.23) for aseptic and 0.77 (0.24) for septic (P < .001) (Figure 2). The univariate ANCOVA also demonstrated a postoperative difference between groups when taking the preoperative ratio into account: 0.99 (0.23) for aseptic and 0.78 (0.24) for septic (P = .005) (Table 3). Before surgery, 22.4% and 23.9% of the aseptic and septic groups, respectively, had patella baja (P = .58). After surgery, 17.6% and 58.8% of the aseptic and septic groups had patella baja (P = .001) (Table 4). The ICC for preoperative ISR was 0.94, and the ICC for postoperative ISR was 0.96, which indicates excellent agreement of measurements between the 2 blinded investigators.
ROM differed between septic and aseptic groups owing to the difference in postoperative flexion. Mean (SD) postoperative extension was 2.2° (5.4°) for the aseptic group and 5.1° (9.8°) for the septic group—not significantly different (P = .13). Mean (SD) postoperative flexion was 110.2° (18.8°) for the aseptic group and 97.2° (29.4°) for the septic group—significantly different (P = .02). The groups differed significantly (P = .02) in mean (SD) ROM: 108.0° (20.7°) for aseptic and 92.2° (34.6°) for septic (Table 1). ROM was also significantly associated with patella baja (P = .04), as patients with ISR of less than 0.8 had mean (SD) postoperative ROM of 95.1° (31.6°), and patients without patella baja had mean (SD) postoperative ROM of 106.8° (23.6°).
For the septic group, mean (SD) time between first and second stages was 13.0 (8.3) weeks (range, 1-44.3 weeks). Mean (SD) timing of spacer placement was not statistically significantly different (P = .90) between patients who had patella baja, 12.9 (8.8) weeks, and patients who did not have patella baja, 13.2 (7.8) weeks.
Discussion
This study demonstrated that TKAs done for septic reasons resulted in a higher incidence of patella baja and decreased ROM. Incidence of patella baja was higher both before and after revision in septic TKAs than in aseptic TKAs, proving the hypothesis under study. Prerevision incidence was not significantly different, but there was a trend that could not be ignored. This may suggest that there is already an ongoing process in the infected knee that contributes to patella baja; the precise etiology remains unclear and is likely multifactorial. For example, scar formation may be increased in patients with chronic infection, predisposing to patella baja. This assertion is indirectly supported by a recent study from our institution revealing longer average surgical time in septic versus aseptic knee revisions; the difference was thought to reflect increased scar-tissue formation.16 That study also found that patients who underwent septic revisions had significantly more surgical procedures than patients who underwent aseptic revisions. Repetitive surgeries—specifically, repetitive arthrotomies during irrigation and débridement before reimplantation—lead to increased scar formation, which may contribute to preoperative and postoperative patella baja. This may be reflected in the findings that ROM was decreased in patients in the septic group versus patients in the aseptic group and that ROM was decreased in patients with patella baja. In addition, our study found that male patients were more likely to undergo TKA revision for septic reasons and to develop postoperative patella baja. This finding contrasts with that of a study5 that compared preoperative and postoperative ISR in primary TKA and found that women were more likely than men to have patella baja. Although women are more likely to undergo TKA revision,17 men may be more susceptible to infection and subsequent patella baja.
The higher postoperative rate of patella baja in the septic group became statistically significant even when preoperative incidence was considered. This may have been caused by infection-related scarring and by prolonged immobilization of septic knees with use of nonarticulating antibiotic spacers. By keeping these knees immobile with a nonarticulating spacer for a prolonged period in the healing phase of the infection, scar tissue may mature and form over the time between stages. A comparable example may be high tibial osteotomies, in which a high incidence of patella baja has been partly attributed to prolonged casting.11 Future work comparing the results of articulating and nonarticulating spacers will help to determine if immobilization contributes to patella baja in infected TKAs.
There are several limitations to our study. Patient outcome questionnaires were not used, and they would have allowed for the assessment of physical outcomes and emotional satisfaction by comparing outcomes between patients with and without patella baja and comparing septic and aseptic TKAs. In addition, there was no standard method for quantifying difficulty of revision, which would have enabled us to compare difficulty of revision in patients with patella baja.
Conclusion
This study identified a high rate of patella baja and decreased ROM in TKA revisions, particularly infected revisions treated with a nonarticulating spacer. It is important to determine if there are functional consequences. Further investigation is needed regarding the cause, prevention, and management of this potentially debilitating outcome after revision TKA.
1. Aglietti P, Buzzi R, Gaudenzi A. Patello-femoral functional results and complications with the posterior stabilised total condylar knee prosthesis. J Arthroplasty. 1988;3(1):17-25.
2. Fern ED, Winson IG, Getty CJM. Anterior knee pain in rheumatoid patients after total knee replacement: possible selection criteria for patellar resurfacing. J Bone Joint Surg Br. 1992;74(5):745-748.
3. Figgie HE 3rd, Goldberg VM, Heiple KG, Moller HS 3rd, Gordon NH. The influence of tibial-patellofemoral location on function of the knee in patients with the posterior stabilized condylar knee prosthesis. J Bone Surg Surg Am. 1986;68(7):1035-1040.
4. Floren M, Davis J, Peterson MG, Laskin RS. A mini-midvastus capsular approach with patellar displacement decreases the prevalence of patellar baja. J Arthroplasty. 2007;22(6 Suppl 2):51-57.
5. Meneghini RM, Ritter MA, Pierson JL, Meding JB, Berend ME, Faris PM. The effect of the Insall-Salvati ratio on outcome after total knee arthroplasty. J Arthroplasty. 2006;21(6 Suppl 2):116-120.
6. Singerman R, Davy DT, Goldberg VM. Effects of patella alta and patella infera on patellofemoral contact forces. J Biomech. 1994;27(8):1059-1065.
7. Van Eijden TM, Kouwenhoven E, Weijs WA. Mechanics of the patellar articulation: effects of patellar ligament length studied with a mathematical model. Acta Orthop Scand. 1987;58(5):560-566.
8. Weale AE, Murray DW, Newman JH, Ackroyd CE. The length of the patellar tendon after unicompartmental and total knee replacement. J Bone Joint Surg Br. 1999;81(5):790-795.
9. Chonko DJ, Lombardi AV Jr, Berend KR. Patella baja and total knee arthroplasty (TKA): etiology, diagnosis, and management. Surg Technol Int. 2004;12:231-238.
10. Cameron HU, Jung YB. Patella baja complicating total knee arthroplasty. A report of two cases. J Arthroplasty. 1988;3(2):177-180.
11. Scuderi GR, Windsor RE, Insall JN. Observations on patellar height after proximal tibial osteotomy. J Bone Joint Surg Am. 1989;71(2):245-248.
12. Insall JN, Salvati E. Patella position in the normal knee joint. Radiology. 1971;101(1):101-104.
13. Grelsamer RP. Patella baja after total knee arthroplasty: is it really patella baja? J Arthroplasty. 2002;17(1):66-69.
14. Parvizi J, Zmistowski B, Berbari EF, et al. New definition for periprosthetic joint infection: from the Workgroup of the Musculoskeletal Infection Society. Clin Orthop. 2011;469(11):2992-2994.
15. Workgroup Convened by the Musculoskeletal Infection Society. New definition for periprosthetic joint infection. J Arthroplasty. 2011;26(8):1136-1138.
16. Laudermilch DJ, Fedorka CJ, Heyl A, Rao N, McGough RL. Outcomes of revision total knee arthroplasty after methicillin-resistant Staphylococcus aureus infection. Clin Orthop. 2010;468(8):2067-2073.
17. Bozic KJ, Kurtz SM, Lau E, et al. The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop. 2010;468(1):45-51.
1. Aglietti P, Buzzi R, Gaudenzi A. Patello-femoral functional results and complications with the posterior stabilised total condylar knee prosthesis. J Arthroplasty. 1988;3(1):17-25.
2. Fern ED, Winson IG, Getty CJM. Anterior knee pain in rheumatoid patients after total knee replacement: possible selection criteria for patellar resurfacing. J Bone Joint Surg Br. 1992;74(5):745-748.
3. Figgie HE 3rd, Goldberg VM, Heiple KG, Moller HS 3rd, Gordon NH. The influence of tibial-patellofemoral location on function of the knee in patients with the posterior stabilized condylar knee prosthesis. J Bone Surg Surg Am. 1986;68(7):1035-1040.
4. Floren M, Davis J, Peterson MG, Laskin RS. A mini-midvastus capsular approach with patellar displacement decreases the prevalence of patellar baja. J Arthroplasty. 2007;22(6 Suppl 2):51-57.
5. Meneghini RM, Ritter MA, Pierson JL, Meding JB, Berend ME, Faris PM. The effect of the Insall-Salvati ratio on outcome after total knee arthroplasty. J Arthroplasty. 2006;21(6 Suppl 2):116-120.
6. Singerman R, Davy DT, Goldberg VM. Effects of patella alta and patella infera on patellofemoral contact forces. J Biomech. 1994;27(8):1059-1065.
7. Van Eijden TM, Kouwenhoven E, Weijs WA. Mechanics of the patellar articulation: effects of patellar ligament length studied with a mathematical model. Acta Orthop Scand. 1987;58(5):560-566.
8. Weale AE, Murray DW, Newman JH, Ackroyd CE. The length of the patellar tendon after unicompartmental and total knee replacement. J Bone Joint Surg Br. 1999;81(5):790-795.
9. Chonko DJ, Lombardi AV Jr, Berend KR. Patella baja and total knee arthroplasty (TKA): etiology, diagnosis, and management. Surg Technol Int. 2004;12:231-238.
10. Cameron HU, Jung YB. Patella baja complicating total knee arthroplasty. A report of two cases. J Arthroplasty. 1988;3(2):177-180.
11. Scuderi GR, Windsor RE, Insall JN. Observations on patellar height after proximal tibial osteotomy. J Bone Joint Surg Am. 1989;71(2):245-248.
12. Insall JN, Salvati E. Patella position in the normal knee joint. Radiology. 1971;101(1):101-104.
13. Grelsamer RP. Patella baja after total knee arthroplasty: is it really patella baja? J Arthroplasty. 2002;17(1):66-69.
14. Parvizi J, Zmistowski B, Berbari EF, et al. New definition for periprosthetic joint infection: from the Workgroup of the Musculoskeletal Infection Society. Clin Orthop. 2011;469(11):2992-2994.
15. Workgroup Convened by the Musculoskeletal Infection Society. New definition for periprosthetic joint infection. J Arthroplasty. 2011;26(8):1136-1138.
16. Laudermilch DJ, Fedorka CJ, Heyl A, Rao N, McGough RL. Outcomes of revision total knee arthroplasty after methicillin-resistant Staphylococcus aureus infection. Clin Orthop. 2010;468(8):2067-2073.
17. Bozic KJ, Kurtz SM, Lau E, et al. The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop. 2010;468(1):45-51.