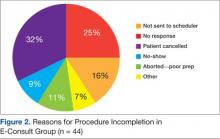
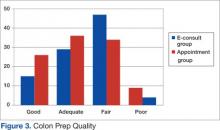
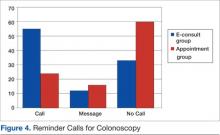
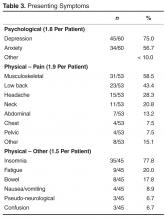
User login
Midfoot Sprains in the National Football League
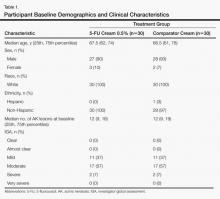
Midfoot (Lisfranc) joint injuries are uncommon in the general population, with a reported incidence ranging from 1 per 50,000 to 1 per 60,000 per year.1,2 The majority of these midfoot injuries result from high-velocity direct trauma involving severe disruption of the tarsometatarsal joint.1-6 Most of the literature on Lisfranc injuries are based on cohorts that include trauma patients. On the other hand, low-velocity indirect injuries of the tarsometatarsal joint have also been associated with midfoot or Lisfranc sprains.7 These injuries are even less extensively studied in athletes, who may sustain them from torsion or the shoe–surface interface.8
Foot and ankle injuries are among the most common injuries in athletes and represent 16% to 22% of all sports injuries.9 Although midfoot sprains are not common in the general population, sporting activities appear to result in a higher rate of midfoot injury, especially in elite athletes. In fact, midfoot sprains comprise the second most common athlete injury to the foot, after metatarsophalangeal joint injuries.10 Football players are especially prone to midfoot sprains; incidence is 4% per year, with offensive linemen sustaining 29.2% of midfoot sprains.10 The most common mechanism of injury is an axial longitudinal force while the foot is plantarflexed and slightly rotated.11,12
There is a paucity of literature detailing the impact of midfoot injuries on football players.8,10,13 A study of 23 collegiate football players found that they may have initially underwent a long period of acute disability but had very minor long-term complaints resulting in residual functional disability.10 However, there are no case series detailing the impact of midfoot sprains on professional football players for whom delayed return to sport can potentially have a devastating impact on a career in terms of both acute- and long-term disability.
We conducted a study to further define the mechanism of injury, diagnosis, treatment, and outcomes among National Football League (NFL) players with midfoot sprains. In addition, we aimed to provide a qualitative analysis of diagnostic and treatment algorithms being used by NFL team physicians in their management of midfoot sprains in these high-level contact athletes.
Materials and Methods
We evaluated midfoot sprains in NFL players in 2 specific phases. In phase 1, we retrospectively reviewed prospectively collected data involving midfoot sprains in professional players from a single NFL team over a 15-year period. In phase 2, we collated diagnostic and treatment algorithms for midfoot sprains among all 32 NFL team physicians by means of a structured questionnaire. Institutional review board approval was obtained for this study at the investigators’ institution.
In phase 1, a NFL team injury database was reviewed for midfoot sprains that had been prospectively entered by a team-certified athletic trainer after consultation with the head orthopedic team physician. All injury and diagnostic modalities and treatments were then analyzed. These included player position, foot and ankle protective gear (none, tape, brace, or unknown), playing surface (grass, AstroTurf, FieldTurf, or unknown), field condition (normal, wet, hard, or unknown), onset of injury (acute, chronic, or unknown), place of injury (game or practice), time of injury in game or practice (first quarter, second quarter, third quarter, fourth quarter, or unknown), type of play (collision, tackled, tackling, blocked, blocking, running/cutting, kicking, or unknown), and mechanism of injury (direct, torsion, shearing, or unknown).
Once the diagnosis was confirmed by physical examination and radiographic findings, midfoot sprain treatment was initiated based on the following algorithm protocols. Nondisplaced sprains were treated with a period of immobilization in a cam walker with progression to weight-bearing as tolerated (grade 1). Once asymptomatic, rehabilitation was initiated, including range of motion, strengthening, and proprioception, and gradual return to play as tolerated. Injuries with subtle diastasis (2-5 mm) were typically treated with nonoperative management in the same manner as the nondisplaced sprain protocol (grade 2); however, signs of gross instability indicated the potential requirement for surgical management. Some of these injuries underwent stress-testing to determine if there was gross instability. If the injury had subtle diastasis with instability or frank (>5 mm) displacement (grade 3), then surgical management was performed with closed versus open reduction and internal fixation (ORIF). The postoperative course included no weight-bearing for 4 to 6 weeks followed by partial weight-bearing for an additional 4 to 6 weeks. After approximately 8 to 12 postoperative weeks, screw removal was performed followed by progression to full weight-bearing and a comprehensive rehabilitation program, including range of motion, strengthening, proprioception, and gradual return to play. Return to play was allowed when the athlete was asymptomatic and had normal range of motion and strength. Time lost from participation was then recorded based on the dates of injury and return to play.
To further elucidate long-term postinjury playing status, we then gathered information from the www.NFL.com historical and current player databases as previously described by Shah and colleagues.14 From this website, we documented the number of regular-season and postseason games as well as the number of seasons before and after the injury. To be included in the series, the athlete had to have been on the active roster for an NFL franchise at the time of injury. Successful return to play was defined as actual return to play in regular season or postseason NFL games after the midfoot sprain.
In phase 2, a structured electronic questionnaire was sent to all 32 NFL team physicians. The questionnaire was compiled to gather information relating to current diagnostic, treatment, and outcome algorithms in the management of midfoot sprains involving professional football players. Each questionnaire was sent by e-mail to all survey participants and included an embedded link to a secure online survey resource (REDCap Survey Software Version 1.3.9; Vanderbilt University, Nashville, Tennessee). Once the electronic questionnaire was completed by each NFL team physician, results were exported in spreadsheet format for descriptive data analysis.
The retrospective case series and NFL team physician survey data were then analyzed. A descriptive analysis was performed for all variables, including means and minimum–maximum range for quantitative variables as well as frequencies and percentages for qualitative variables. Depending on injury severity, an independent-sample t test with corresponding P values was also calculated for time lost from participation.
Results
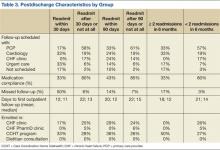
The retrospective review of the prospectively collected NFL injury database revealed there were 15 midfoot sprains during the study period. A statistical and descriptive analysis was performed for all study parameters, including player, field, injury, and outcome-specific data. For player, field, and injury-specific data, the results are summarized in the Table.
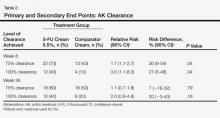
All grade 1 midfoot sprains (7 nondisplaced) and grade 2 midfoot sprains (5 with subtle diastasis and no instability) were treated with nonoperative management. The 12 players were allowed to return to play without the need for subsequent surgery within the same season. In the evaluation of return to play, based on the severity of the midfoot sprain, there was a statistically significant (P = .047) difference in mean (SD) time lost from participation between the grade 1 sprain group, 3.1 (1.9) days, and the grade 2 sprain group, 36 (26.1) days. Overall, nonoperative treatment of either grade 1 or grade 2 midfoot sprains resulted in a mean of 11.7 days of time lost from participation. In 1 patient with a grade 2 midfoot sprain, the injury occurred toward the end of the season, and the patient was not able to return to play during the remaining 42 days of the season. However, this patient returned to play the next season and had no residual problems.
Three grade 3 injuries (midfoot sprains with frank displacement) required surgical management with ORIF. One patient returned to play the same season, in 73 days; however, the other 2 patients had injuries toward the end of the season (29 and 77 days remaining) and were not able to return to play the same season. However, both these patients returned to play the next season and had no persistent problems. In terms of complications within the same season, there were no recurrent injuries reported after successful return to play.
When evaluating long-term postinjury playing status, we found that 11 (92%) of the 12 NFL players who had nonoperative treatment successfully returned to play. The only player who did not return to an NFL regular season or postseason game was an active-roster NFL player who never actually played in an NFL game before or after his midfoot sprain injury. Our series of NFL players played on average 1.9 years (range, 0-7 years) before the midfoot injury and 5.5 years (range, 0-14 years) after the midfoot injury. In terms of NFL regular-season and postseason games played, our cohort of NFL players played on average 24.0 games (range, 0-80 games) before the midfoot injury and 77.7 games (range, 0-226 games) after the midfoot injury. In fact, 10 of the 12 NFL players (83%) who had nonoperative treatment played more games and seasons after their midfoot injury.
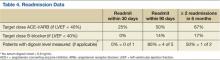
The surveys from phase 2 were completed by all 32 NFL team physicians. When evaluating the severity of midfoot sprains, 63% of the NFL team physicians perform stress-view radiographs. To ascertain NFL team physicians’ management decisions, we evaluated midfoot sprain results according to injury severity, including amount of diastasis.
When managing midfoot sprains with no diastasis, 94% of the team physicians use immobilization, including 27 with a cam walker and 2 with a cast; however, 2 physicians (6%) use only ankle taping or an Ace bandage. Initial weight-bearing status varies among the NFL team physicians, but most (78%) choose to protect the player, including 17 non-weight-bearing, 8 partial weight-bearing, and 7 weight-bearing as tolerated. Most physicians ideally progress players to full weight-bearing by 3 weeks (12% immediately, 12% by week 1, 41% by week 2, 16% by week 3, and 19% from 4-6 weeks).
In the management of midfoot sprains with subtle diastasis, there is variation in treatment modes among the NFL team physicians, with 53% using nonoperative management (34% cam walker, 19% cast) and 47% suggesting operative management. Regardless of treatment, most physicians (97%) maintain initial non-weight-bearing restrictions. In fact, only 1 physician first recommended partial weight-bearing, which corresponded to initial treatment in a cam walker.
In terms of midfoot sprains with frank diastasis, 94% of the NFL team physicians indicated surgical management is warranted, with only 2 physicians (6%) recommending initial nonoperative management with a cam walker. Regardless of treatment, all the physicians (100%) implemented initial non-weight-bearing restrictions. Once surgical treatment was recommended, the preferred fixation method was ORIF using screws (94%) as opposed to closed reduction and internal fixation with percutaneous Kirschner wires (6%). Most of the physicians (59%) do not allow return to play until midfoot hardware is removed; however, 38% allow full participation with contact, and 3% allow partial participation with no contact. Removal of midfoot fixation is an important factor for most of the physicians before considering return to play, and 69% recommend hardware removal after 11 weeks. However, the specific timeline for hardware removal varied among these physicians, with 28% opting for removal at 11 to 12 weeks, 16% at 13 to 14 weeks, 12.5% at 7 to 8 weeks, 12.5% at 15 to 16 weeks, 12.5% at more than 16 weeks, 12.5% never, and 6% at 9 to 10 weeks.
The midfoot sprain treatment protocol (nonoperative vs operative management) based on injury severity was an important factor in considering return-to-play guidelines. When evaluating time lost from participation because of midfoot sprains, most of the NFL team physicians anticipated a period of 5 to 8 weeks when considering nonoperative management (56%) and more than 17 weeks after operative management (53%). In evaluating nonoperative management protocols, return-to-play guidelines were relatively expeditious, with 56% of the physicians estimating from 5 to 8 weeks, 22% from 1 to 4 weeks, 13% from 9 to 12 weeks, 6% from 13 to 16 weeks, and 3% longer than 20 weeks. In comparison to nonoperative management, return-to-play guidelines for operative management were prolonged, with 53% of the physicians estimating more than 20 weeks, 25% from 17 to 20 weeks, 13% from 13 to 16 weeks, and 9% from 9 to 12 weeks.
Discussion
Lisfranc and midfoot injuries remain a controversial topic in sports medicine. Several authors have argued that anatomical reduction of the tarsometatarsal joint in the setting of a Lisfranc injury yields optimal outcomes.15,16 Some studies have also suggested that purely ligamentous Lisfranc injuries may be more of a problem than bony injuries, which may have the benefit of osseous healing.15,17 Anatomical reduction can minimize the potential for arch collapse by maintaining the normal tarsometatarsal relationship. However, there are no long-term data to determine how midfoot arthrosis is affected by ORIF, which typically involves hardware traversing joints. Some have even argued that primary tarsometatarsal arthrodesis should be the treatment of choice, as the midfoot has limited native motion, and successful arthrodesis eliminates the potential for midfoot arthrosis.17,18 However, we are unaware of any studies that have routinely performed arthrodesis in an athletic population.
The majority of studies on midfoot injuries have evaluated individuals involved in traumatic accidents, most commonly motor vehicle collisions. The present study suggests there may be a subset of injuries in athletes that have yet to be adequately studied. Anecdotally, the NFL team physicians surveyed in our study suggested that midfoot sprains with no or subtle displacement may be treated with nonoperative measures while yielding satisfactory clinical outcomes. These results have been quantified in return-to-play status. Our subset of athletes from an NFL team corroborates these findings, even though the series was small (15 patients). Our survey results also suggest there is considerable variation in the “optimal” management plan among the physicians treating these elite athletes. Most would agree that the nondisplaced injuries can be managed conservatively and that the severely displaced injuries should be managed operatively, but the natural history of those injuries with subtle diastasis remains unclear. When operative intervention is implemented, hardware removal versus retention must also be considered when allowing for return to play. Although one would assume that motion-related hardware failure would be possible at the tarsometatarsal joints, this concept has yet to be clearly defined in the literature.
The present study also demonstrates that most athletes with these midfoot injuries can return to play at the elite NFL level, as evidenced by their short- and long-term return to play. However, it was not possible to differentiate the specific return-to-play level related to preinjury performance level. Furthermore, this relatively short-term NFL career follow-up study was not able to elucidate the long-term consequences of these injuries. In fact, arch collapse and acquired flatfoot deformity could eventually result from this injury, and long-term outcomes would be of particular interest in patients who have subtle diastasis and who are treated nonoperatively.
Although previous studies have supported operative management for Lisfranc injuries involving subtle diastasis, more than half of the NFL team physicians surveyed in this study use nonoperative treatment for these injuries.19 Future studies should evaluate stress-imaging to define the effect of stability or latent diastasis on long-term outcomes. Nonetheless, the present study demonstrates that a large cohort of NFL team physicians supports nonoperative management for these Lisfranc injuries with subtle diastasis, even in elite athletes. Additional prospective studies are needed to provide a more rigorous injury evaluation and closer follow-up, including subjective and objective outcomes, to further define the indications for management options for midfoot sprains in this population of contact athletes.
1. Aitken AP, Poulson D. Dislocations of the tarsometatarsal joint. J Bone Joint Surg Am. 1963;45:246-260.
2. Hardcastle PH, Reschauer R, Kutscha-Lissberg E, Schoffmann W. Injuries to the tarsometatarsal joint. Incidence, classification and treatment. J Bone Joint Surg Br. 1982;64(3):349-356.
3. Arntz CT, Veith RG, Hansen ST Jr. Fractures and fracture-dislocations of the tarsometatarsal joint. J Bone Joint Surg Am. 1988(2);70:173-181.
4. Goossens M, De Stoop N. Lisfranc’s fracture-dislocations: etiology, radiology, and results of treatment. A review of 20 cases. Clin Orthop. 1983;(176):154-162.
5. Myerson M. The diagnosis and treatment of injuries to the Lisfranc joint complex. Orthop Clin North Am. 1989;20(4):655-664.
6. Wiley JJ. The mechanism of tarso-metatarsal joint injuries. J Bone Joint Surg Br. 1971;53(3):474-482.
7. Faciszewski T, Burks RT, Manaster BJ. Subtle injuries of the Lisfranc joint. J Bone Joint Surg Am. 1990;72(10):1519-1522.
8. Nunley JA, Vertullo CJ. Classification, investigation, and management of midfoot sprains: Lisfranc injuries in the athlete. Am J Sports Med. 2002;30(6):871-878.
9. Garrick JG, Requa RK. The epidemiology of foot and ankle injuries in sports. Clin Sports Med. 1988;7(1):29-36.
10. Meyer SA, Callaghan JJ, Albright JP, Crowley ET, Powell JW. Midfoot sprains in collegiate football players. Am J Sports Med. 1994;22(3):392-401.
11. Shapiro MS, Wascher DC, Finerman GA. Rupture of Lisfranc’s ligament in athletes. Am J Sports Med. 1994;22(5):687-691.
12. Curtis MJ, Myerson M, Szura B. Tarsometatarsal joint injuries in the athlete. Am J Sports Med. 1993;21(4):497-502.
13. Harwood MI, Raikin SM. A Lisfranc fracture-dislocation in a football player. J Am Board Fam Pract. 2003;16(1):69-72.
14. Shah VM, Andrews JR, Fleisig GS, et al. Return to play after anterior cruciate ligament reconstruction in National Football League athletes. Am J Sports Med. 2010;38(11):2233-2239.
15. Kuo RS, Tejwani NC, Digiovanni CW, et al. Outcome after open reduction and internal fixation of Lisfranc joint injuries. J Bone Joint Surg Am. 2000;82(11):1609-1618.
16. Myerson MS, Cerrato RA. Current management of tarsometatarsal injuries in the athlete. J Bone Joint Surg Am. 2008;90(11):2522-2533.
17. Ly TV, Coetzee JC. Treatment of primarily ligamentous Lisfranc joint injuries: primary arthrodesis compared with open reduction and internal fixation. A prospective, randomized study. J Bone Joint Surg Am. 2006;88(3):514-520.
18. Coetzee JC, Ly TV. Treatment of primarily ligamentous Lisfranc joint injuries: primary arthrodesis compared with open reduction and internal fixation. Surgical technique. J Bone Joint Surg Am. 2007;89(suppl 2 pt1):122-127.
19. Ardoin GT, Anderson RB. Subtle Lisfranc injury. Tech Foot Ankle. 2010;9:100-106.
Midfoot (Lisfranc) joint injuries are uncommon in the general population, with a reported incidence ranging from 1 per 50,000 to 1 per 60,000 per year.1,2 The majority of these midfoot injuries result from high-velocity direct trauma involving severe disruption of the tarsometatarsal joint.1-6 Most of the literature on Lisfranc injuries are based on cohorts that include trauma patients. On the other hand, low-velocity indirect injuries of the tarsometatarsal joint have also been associated with midfoot or Lisfranc sprains.7 These injuries are even less extensively studied in athletes, who may sustain them from torsion or the shoe–surface interface.8
Foot and ankle injuries are among the most common injuries in athletes and represent 16% to 22% of all sports injuries.9 Although midfoot sprains are not common in the general population, sporting activities appear to result in a higher rate of midfoot injury, especially in elite athletes. In fact, midfoot sprains comprise the second most common athlete injury to the foot, after metatarsophalangeal joint injuries.10 Football players are especially prone to midfoot sprains; incidence is 4% per year, with offensive linemen sustaining 29.2% of midfoot sprains.10 The most common mechanism of injury is an axial longitudinal force while the foot is plantarflexed and slightly rotated.11,12
There is a paucity of literature detailing the impact of midfoot injuries on football players.8,10,13 A study of 23 collegiate football players found that they may have initially underwent a long period of acute disability but had very minor long-term complaints resulting in residual functional disability.10 However, there are no case series detailing the impact of midfoot sprains on professional football players for whom delayed return to sport can potentially have a devastating impact on a career in terms of both acute- and long-term disability.
We conducted a study to further define the mechanism of injury, diagnosis, treatment, and outcomes among National Football League (NFL) players with midfoot sprains. In addition, we aimed to provide a qualitative analysis of diagnostic and treatment algorithms being used by NFL team physicians in their management of midfoot sprains in these high-level contact athletes.
Materials and Methods
We evaluated midfoot sprains in NFL players in 2 specific phases. In phase 1, we retrospectively reviewed prospectively collected data involving midfoot sprains in professional players from a single NFL team over a 15-year period. In phase 2, we collated diagnostic and treatment algorithms for midfoot sprains among all 32 NFL team physicians by means of a structured questionnaire. Institutional review board approval was obtained for this study at the investigators’ institution.
In phase 1, a NFL team injury database was reviewed for midfoot sprains that had been prospectively entered by a team-certified athletic trainer after consultation with the head orthopedic team physician. All injury and diagnostic modalities and treatments were then analyzed. These included player position, foot and ankle protective gear (none, tape, brace, or unknown), playing surface (grass, AstroTurf, FieldTurf, or unknown), field condition (normal, wet, hard, or unknown), onset of injury (acute, chronic, or unknown), place of injury (game or practice), time of injury in game or practice (first quarter, second quarter, third quarter, fourth quarter, or unknown), type of play (collision, tackled, tackling, blocked, blocking, running/cutting, kicking, or unknown), and mechanism of injury (direct, torsion, shearing, or unknown).
Once the diagnosis was confirmed by physical examination and radiographic findings, midfoot sprain treatment was initiated based on the following algorithm protocols. Nondisplaced sprains were treated with a period of immobilization in a cam walker with progression to weight-bearing as tolerated (grade 1). Once asymptomatic, rehabilitation was initiated, including range of motion, strengthening, and proprioception, and gradual return to play as tolerated. Injuries with subtle diastasis (2-5 mm) were typically treated with nonoperative management in the same manner as the nondisplaced sprain protocol (grade 2); however, signs of gross instability indicated the potential requirement for surgical management. Some of these injuries underwent stress-testing to determine if there was gross instability. If the injury had subtle diastasis with instability or frank (>5 mm) displacement (grade 3), then surgical management was performed with closed versus open reduction and internal fixation (ORIF). The postoperative course included no weight-bearing for 4 to 6 weeks followed by partial weight-bearing for an additional 4 to 6 weeks. After approximately 8 to 12 postoperative weeks, screw removal was performed followed by progression to full weight-bearing and a comprehensive rehabilitation program, including range of motion, strengthening, proprioception, and gradual return to play. Return to play was allowed when the athlete was asymptomatic and had normal range of motion and strength. Time lost from participation was then recorded based on the dates of injury and return to play.
To further elucidate long-term postinjury playing status, we then gathered information from the www.NFL.com historical and current player databases as previously described by Shah and colleagues.14 From this website, we documented the number of regular-season and postseason games as well as the number of seasons before and after the injury. To be included in the series, the athlete had to have been on the active roster for an NFL franchise at the time of injury. Successful return to play was defined as actual return to play in regular season or postseason NFL games after the midfoot sprain.
In phase 2, a structured electronic questionnaire was sent to all 32 NFL team physicians. The questionnaire was compiled to gather information relating to current diagnostic, treatment, and outcome algorithms in the management of midfoot sprains involving professional football players. Each questionnaire was sent by e-mail to all survey participants and included an embedded link to a secure online survey resource (REDCap Survey Software Version 1.3.9; Vanderbilt University, Nashville, Tennessee). Once the electronic questionnaire was completed by each NFL team physician, results were exported in spreadsheet format for descriptive data analysis.
The retrospective case series and NFL team physician survey data were then analyzed. A descriptive analysis was performed for all variables, including means and minimum–maximum range for quantitative variables as well as frequencies and percentages for qualitative variables. Depending on injury severity, an independent-sample t test with corresponding P values was also calculated for time lost from participation.
Results
The retrospective review of the prospectively collected NFL injury database revealed there were 15 midfoot sprains during the study period. A statistical and descriptive analysis was performed for all study parameters, including player, field, injury, and outcome-specific data. For player, field, and injury-specific data, the results are summarized in the Table.
All grade 1 midfoot sprains (7 nondisplaced) and grade 2 midfoot sprains (5 with subtle diastasis and no instability) were treated with nonoperative management. The 12 players were allowed to return to play without the need for subsequent surgery within the same season. In the evaluation of return to play, based on the severity of the midfoot sprain, there was a statistically significant (P = .047) difference in mean (SD) time lost from participation between the grade 1 sprain group, 3.1 (1.9) days, and the grade 2 sprain group, 36 (26.1) days. Overall, nonoperative treatment of either grade 1 or grade 2 midfoot sprains resulted in a mean of 11.7 days of time lost from participation. In 1 patient with a grade 2 midfoot sprain, the injury occurred toward the end of the season, and the patient was not able to return to play during the remaining 42 days of the season. However, this patient returned to play the next season and had no residual problems.
Three grade 3 injuries (midfoot sprains with frank displacement) required surgical management with ORIF. One patient returned to play the same season, in 73 days; however, the other 2 patients had injuries toward the end of the season (29 and 77 days remaining) and were not able to return to play the same season. However, both these patients returned to play the next season and had no persistent problems. In terms of complications within the same season, there were no recurrent injuries reported after successful return to play.
When evaluating long-term postinjury playing status, we found that 11 (92%) of the 12 NFL players who had nonoperative treatment successfully returned to play. The only player who did not return to an NFL regular season or postseason game was an active-roster NFL player who never actually played in an NFL game before or after his midfoot sprain injury. Our series of NFL players played on average 1.9 years (range, 0-7 years) before the midfoot injury and 5.5 years (range, 0-14 years) after the midfoot injury. In terms of NFL regular-season and postseason games played, our cohort of NFL players played on average 24.0 games (range, 0-80 games) before the midfoot injury and 77.7 games (range, 0-226 games) after the midfoot injury. In fact, 10 of the 12 NFL players (83%) who had nonoperative treatment played more games and seasons after their midfoot injury.
The surveys from phase 2 were completed by all 32 NFL team physicians. When evaluating the severity of midfoot sprains, 63% of the NFL team physicians perform stress-view radiographs. To ascertain NFL team physicians’ management decisions, we evaluated midfoot sprain results according to injury severity, including amount of diastasis.
When managing midfoot sprains with no diastasis, 94% of the team physicians use immobilization, including 27 with a cam walker and 2 with a cast; however, 2 physicians (6%) use only ankle taping or an Ace bandage. Initial weight-bearing status varies among the NFL team physicians, but most (78%) choose to protect the player, including 17 non-weight-bearing, 8 partial weight-bearing, and 7 weight-bearing as tolerated. Most physicians ideally progress players to full weight-bearing by 3 weeks (12% immediately, 12% by week 1, 41% by week 2, 16% by week 3, and 19% from 4-6 weeks).
In the management of midfoot sprains with subtle diastasis, there is variation in treatment modes among the NFL team physicians, with 53% using nonoperative management (34% cam walker, 19% cast) and 47% suggesting operative management. Regardless of treatment, most physicians (97%) maintain initial non-weight-bearing restrictions. In fact, only 1 physician first recommended partial weight-bearing, which corresponded to initial treatment in a cam walker.
In terms of midfoot sprains with frank diastasis, 94% of the NFL team physicians indicated surgical management is warranted, with only 2 physicians (6%) recommending initial nonoperative management with a cam walker. Regardless of treatment, all the physicians (100%) implemented initial non-weight-bearing restrictions. Once surgical treatment was recommended, the preferred fixation method was ORIF using screws (94%) as opposed to closed reduction and internal fixation with percutaneous Kirschner wires (6%). Most of the physicians (59%) do not allow return to play until midfoot hardware is removed; however, 38% allow full participation with contact, and 3% allow partial participation with no contact. Removal of midfoot fixation is an important factor for most of the physicians before considering return to play, and 69% recommend hardware removal after 11 weeks. However, the specific timeline for hardware removal varied among these physicians, with 28% opting for removal at 11 to 12 weeks, 16% at 13 to 14 weeks, 12.5% at 7 to 8 weeks, 12.5% at 15 to 16 weeks, 12.5% at more than 16 weeks, 12.5% never, and 6% at 9 to 10 weeks.
The midfoot sprain treatment protocol (nonoperative vs operative management) based on injury severity was an important factor in considering return-to-play guidelines. When evaluating time lost from participation because of midfoot sprains, most of the NFL team physicians anticipated a period of 5 to 8 weeks when considering nonoperative management (56%) and more than 17 weeks after operative management (53%). In evaluating nonoperative management protocols, return-to-play guidelines were relatively expeditious, with 56% of the physicians estimating from 5 to 8 weeks, 22% from 1 to 4 weeks, 13% from 9 to 12 weeks, 6% from 13 to 16 weeks, and 3% longer than 20 weeks. In comparison to nonoperative management, return-to-play guidelines for operative management were prolonged, with 53% of the physicians estimating more than 20 weeks, 25% from 17 to 20 weeks, 13% from 13 to 16 weeks, and 9% from 9 to 12 weeks.
Discussion
Lisfranc and midfoot injuries remain a controversial topic in sports medicine. Several authors have argued that anatomical reduction of the tarsometatarsal joint in the setting of a Lisfranc injury yields optimal outcomes.15,16 Some studies have also suggested that purely ligamentous Lisfranc injuries may be more of a problem than bony injuries, which may have the benefit of osseous healing.15,17 Anatomical reduction can minimize the potential for arch collapse by maintaining the normal tarsometatarsal relationship. However, there are no long-term data to determine how midfoot arthrosis is affected by ORIF, which typically involves hardware traversing joints. Some have even argued that primary tarsometatarsal arthrodesis should be the treatment of choice, as the midfoot has limited native motion, and successful arthrodesis eliminates the potential for midfoot arthrosis.17,18 However, we are unaware of any studies that have routinely performed arthrodesis in an athletic population.
The majority of studies on midfoot injuries have evaluated individuals involved in traumatic accidents, most commonly motor vehicle collisions. The present study suggests there may be a subset of injuries in athletes that have yet to be adequately studied. Anecdotally, the NFL team physicians surveyed in our study suggested that midfoot sprains with no or subtle displacement may be treated with nonoperative measures while yielding satisfactory clinical outcomes. These results have been quantified in return-to-play status. Our subset of athletes from an NFL team corroborates these findings, even though the series was small (15 patients). Our survey results also suggest there is considerable variation in the “optimal” management plan among the physicians treating these elite athletes. Most would agree that the nondisplaced injuries can be managed conservatively and that the severely displaced injuries should be managed operatively, but the natural history of those injuries with subtle diastasis remains unclear. When operative intervention is implemented, hardware removal versus retention must also be considered when allowing for return to play. Although one would assume that motion-related hardware failure would be possible at the tarsometatarsal joints, this concept has yet to be clearly defined in the literature.
The present study also demonstrates that most athletes with these midfoot injuries can return to play at the elite NFL level, as evidenced by their short- and long-term return to play. However, it was not possible to differentiate the specific return-to-play level related to preinjury performance level. Furthermore, this relatively short-term NFL career follow-up study was not able to elucidate the long-term consequences of these injuries. In fact, arch collapse and acquired flatfoot deformity could eventually result from this injury, and long-term outcomes would be of particular interest in patients who have subtle diastasis and who are treated nonoperatively.
Although previous studies have supported operative management for Lisfranc injuries involving subtle diastasis, more than half of the NFL team physicians surveyed in this study use nonoperative treatment for these injuries.19 Future studies should evaluate stress-imaging to define the effect of stability or latent diastasis on long-term outcomes. Nonetheless, the present study demonstrates that a large cohort of NFL team physicians supports nonoperative management for these Lisfranc injuries with subtle diastasis, even in elite athletes. Additional prospective studies are needed to provide a more rigorous injury evaluation and closer follow-up, including subjective and objective outcomes, to further define the indications for management options for midfoot sprains in this population of contact athletes.
Midfoot (Lisfranc) joint injuries are uncommon in the general population, with a reported incidence ranging from 1 per 50,000 to 1 per 60,000 per year.1,2 The majority of these midfoot injuries result from high-velocity direct trauma involving severe disruption of the tarsometatarsal joint.1-6 Most of the literature on Lisfranc injuries are based on cohorts that include trauma patients. On the other hand, low-velocity indirect injuries of the tarsometatarsal joint have also been associated with midfoot or Lisfranc sprains.7 These injuries are even less extensively studied in athletes, who may sustain them from torsion or the shoe–surface interface.8
Foot and ankle injuries are among the most common injuries in athletes and represent 16% to 22% of all sports injuries.9 Although midfoot sprains are not common in the general population, sporting activities appear to result in a higher rate of midfoot injury, especially in elite athletes. In fact, midfoot sprains comprise the second most common athlete injury to the foot, after metatarsophalangeal joint injuries.10 Football players are especially prone to midfoot sprains; incidence is 4% per year, with offensive linemen sustaining 29.2% of midfoot sprains.10 The most common mechanism of injury is an axial longitudinal force while the foot is plantarflexed and slightly rotated.11,12
There is a paucity of literature detailing the impact of midfoot injuries on football players.8,10,13 A study of 23 collegiate football players found that they may have initially underwent a long period of acute disability but had very minor long-term complaints resulting in residual functional disability.10 However, there are no case series detailing the impact of midfoot sprains on professional football players for whom delayed return to sport can potentially have a devastating impact on a career in terms of both acute- and long-term disability.
We conducted a study to further define the mechanism of injury, diagnosis, treatment, and outcomes among National Football League (NFL) players with midfoot sprains. In addition, we aimed to provide a qualitative analysis of diagnostic and treatment algorithms being used by NFL team physicians in their management of midfoot sprains in these high-level contact athletes.
Materials and Methods
We evaluated midfoot sprains in NFL players in 2 specific phases. In phase 1, we retrospectively reviewed prospectively collected data involving midfoot sprains in professional players from a single NFL team over a 15-year period. In phase 2, we collated diagnostic and treatment algorithms for midfoot sprains among all 32 NFL team physicians by means of a structured questionnaire. Institutional review board approval was obtained for this study at the investigators’ institution.
In phase 1, a NFL team injury database was reviewed for midfoot sprains that had been prospectively entered by a team-certified athletic trainer after consultation with the head orthopedic team physician. All injury and diagnostic modalities and treatments were then analyzed. These included player position, foot and ankle protective gear (none, tape, brace, or unknown), playing surface (grass, AstroTurf, FieldTurf, or unknown), field condition (normal, wet, hard, or unknown), onset of injury (acute, chronic, or unknown), place of injury (game or practice), time of injury in game or practice (first quarter, second quarter, third quarter, fourth quarter, or unknown), type of play (collision, tackled, tackling, blocked, blocking, running/cutting, kicking, or unknown), and mechanism of injury (direct, torsion, shearing, or unknown).
Once the diagnosis was confirmed by physical examination and radiographic findings, midfoot sprain treatment was initiated based on the following algorithm protocols. Nondisplaced sprains were treated with a period of immobilization in a cam walker with progression to weight-bearing as tolerated (grade 1). Once asymptomatic, rehabilitation was initiated, including range of motion, strengthening, and proprioception, and gradual return to play as tolerated. Injuries with subtle diastasis (2-5 mm) were typically treated with nonoperative management in the same manner as the nondisplaced sprain protocol (grade 2); however, signs of gross instability indicated the potential requirement for surgical management. Some of these injuries underwent stress-testing to determine if there was gross instability. If the injury had subtle diastasis with instability or frank (>5 mm) displacement (grade 3), then surgical management was performed with closed versus open reduction and internal fixation (ORIF). The postoperative course included no weight-bearing for 4 to 6 weeks followed by partial weight-bearing for an additional 4 to 6 weeks. After approximately 8 to 12 postoperative weeks, screw removal was performed followed by progression to full weight-bearing and a comprehensive rehabilitation program, including range of motion, strengthening, proprioception, and gradual return to play. Return to play was allowed when the athlete was asymptomatic and had normal range of motion and strength. Time lost from participation was then recorded based on the dates of injury and return to play.
To further elucidate long-term postinjury playing status, we then gathered information from the www.NFL.com historical and current player databases as previously described by Shah and colleagues.14 From this website, we documented the number of regular-season and postseason games as well as the number of seasons before and after the injury. To be included in the series, the athlete had to have been on the active roster for an NFL franchise at the time of injury. Successful return to play was defined as actual return to play in regular season or postseason NFL games after the midfoot sprain.
In phase 2, a structured electronic questionnaire was sent to all 32 NFL team physicians. The questionnaire was compiled to gather information relating to current diagnostic, treatment, and outcome algorithms in the management of midfoot sprains involving professional football players. Each questionnaire was sent by e-mail to all survey participants and included an embedded link to a secure online survey resource (REDCap Survey Software Version 1.3.9; Vanderbilt University, Nashville, Tennessee). Once the electronic questionnaire was completed by each NFL team physician, results were exported in spreadsheet format for descriptive data analysis.
The retrospective case series and NFL team physician survey data were then analyzed. A descriptive analysis was performed for all variables, including means and minimum–maximum range for quantitative variables as well as frequencies and percentages for qualitative variables. Depending on injury severity, an independent-sample t test with corresponding P values was also calculated for time lost from participation.
Results
The retrospective review of the prospectively collected NFL injury database revealed there were 15 midfoot sprains during the study period. A statistical and descriptive analysis was performed for all study parameters, including player, field, injury, and outcome-specific data. For player, field, and injury-specific data, the results are summarized in the Table.
All grade 1 midfoot sprains (7 nondisplaced) and grade 2 midfoot sprains (5 with subtle diastasis and no instability) were treated with nonoperative management. The 12 players were allowed to return to play without the need for subsequent surgery within the same season. In the evaluation of return to play, based on the severity of the midfoot sprain, there was a statistically significant (P = .047) difference in mean (SD) time lost from participation between the grade 1 sprain group, 3.1 (1.9) days, and the grade 2 sprain group, 36 (26.1) days. Overall, nonoperative treatment of either grade 1 or grade 2 midfoot sprains resulted in a mean of 11.7 days of time lost from participation. In 1 patient with a grade 2 midfoot sprain, the injury occurred toward the end of the season, and the patient was not able to return to play during the remaining 42 days of the season. However, this patient returned to play the next season and had no residual problems.
Three grade 3 injuries (midfoot sprains with frank displacement) required surgical management with ORIF. One patient returned to play the same season, in 73 days; however, the other 2 patients had injuries toward the end of the season (29 and 77 days remaining) and were not able to return to play the same season. However, both these patients returned to play the next season and had no persistent problems. In terms of complications within the same season, there were no recurrent injuries reported after successful return to play.
When evaluating long-term postinjury playing status, we found that 11 (92%) of the 12 NFL players who had nonoperative treatment successfully returned to play. The only player who did not return to an NFL regular season or postseason game was an active-roster NFL player who never actually played in an NFL game before or after his midfoot sprain injury. Our series of NFL players played on average 1.9 years (range, 0-7 years) before the midfoot injury and 5.5 years (range, 0-14 years) after the midfoot injury. In terms of NFL regular-season and postseason games played, our cohort of NFL players played on average 24.0 games (range, 0-80 games) before the midfoot injury and 77.7 games (range, 0-226 games) after the midfoot injury. In fact, 10 of the 12 NFL players (83%) who had nonoperative treatment played more games and seasons after their midfoot injury.
The surveys from phase 2 were completed by all 32 NFL team physicians. When evaluating the severity of midfoot sprains, 63% of the NFL team physicians perform stress-view radiographs. To ascertain NFL team physicians’ management decisions, we evaluated midfoot sprain results according to injury severity, including amount of diastasis.
When managing midfoot sprains with no diastasis, 94% of the team physicians use immobilization, including 27 with a cam walker and 2 with a cast; however, 2 physicians (6%) use only ankle taping or an Ace bandage. Initial weight-bearing status varies among the NFL team physicians, but most (78%) choose to protect the player, including 17 non-weight-bearing, 8 partial weight-bearing, and 7 weight-bearing as tolerated. Most physicians ideally progress players to full weight-bearing by 3 weeks (12% immediately, 12% by week 1, 41% by week 2, 16% by week 3, and 19% from 4-6 weeks).
In the management of midfoot sprains with subtle diastasis, there is variation in treatment modes among the NFL team physicians, with 53% using nonoperative management (34% cam walker, 19% cast) and 47% suggesting operative management. Regardless of treatment, most physicians (97%) maintain initial non-weight-bearing restrictions. In fact, only 1 physician first recommended partial weight-bearing, which corresponded to initial treatment in a cam walker.
In terms of midfoot sprains with frank diastasis, 94% of the NFL team physicians indicated surgical management is warranted, with only 2 physicians (6%) recommending initial nonoperative management with a cam walker. Regardless of treatment, all the physicians (100%) implemented initial non-weight-bearing restrictions. Once surgical treatment was recommended, the preferred fixation method was ORIF using screws (94%) as opposed to closed reduction and internal fixation with percutaneous Kirschner wires (6%). Most of the physicians (59%) do not allow return to play until midfoot hardware is removed; however, 38% allow full participation with contact, and 3% allow partial participation with no contact. Removal of midfoot fixation is an important factor for most of the physicians before considering return to play, and 69% recommend hardware removal after 11 weeks. However, the specific timeline for hardware removal varied among these physicians, with 28% opting for removal at 11 to 12 weeks, 16% at 13 to 14 weeks, 12.5% at 7 to 8 weeks, 12.5% at 15 to 16 weeks, 12.5% at more than 16 weeks, 12.5% never, and 6% at 9 to 10 weeks.
The midfoot sprain treatment protocol (nonoperative vs operative management) based on injury severity was an important factor in considering return-to-play guidelines. When evaluating time lost from participation because of midfoot sprains, most of the NFL team physicians anticipated a period of 5 to 8 weeks when considering nonoperative management (56%) and more than 17 weeks after operative management (53%). In evaluating nonoperative management protocols, return-to-play guidelines were relatively expeditious, with 56% of the physicians estimating from 5 to 8 weeks, 22% from 1 to 4 weeks, 13% from 9 to 12 weeks, 6% from 13 to 16 weeks, and 3% longer than 20 weeks. In comparison to nonoperative management, return-to-play guidelines for operative management were prolonged, with 53% of the physicians estimating more than 20 weeks, 25% from 17 to 20 weeks, 13% from 13 to 16 weeks, and 9% from 9 to 12 weeks.
Discussion
Lisfranc and midfoot injuries remain a controversial topic in sports medicine. Several authors have argued that anatomical reduction of the tarsometatarsal joint in the setting of a Lisfranc injury yields optimal outcomes.15,16 Some studies have also suggested that purely ligamentous Lisfranc injuries may be more of a problem than bony injuries, which may have the benefit of osseous healing.15,17 Anatomical reduction can minimize the potential for arch collapse by maintaining the normal tarsometatarsal relationship. However, there are no long-term data to determine how midfoot arthrosis is affected by ORIF, which typically involves hardware traversing joints. Some have even argued that primary tarsometatarsal arthrodesis should be the treatment of choice, as the midfoot has limited native motion, and successful arthrodesis eliminates the potential for midfoot arthrosis.17,18 However, we are unaware of any studies that have routinely performed arthrodesis in an athletic population.
The majority of studies on midfoot injuries have evaluated individuals involved in traumatic accidents, most commonly motor vehicle collisions. The present study suggests there may be a subset of injuries in athletes that have yet to be adequately studied. Anecdotally, the NFL team physicians surveyed in our study suggested that midfoot sprains with no or subtle displacement may be treated with nonoperative measures while yielding satisfactory clinical outcomes. These results have been quantified in return-to-play status. Our subset of athletes from an NFL team corroborates these findings, even though the series was small (15 patients). Our survey results also suggest there is considerable variation in the “optimal” management plan among the physicians treating these elite athletes. Most would agree that the nondisplaced injuries can be managed conservatively and that the severely displaced injuries should be managed operatively, but the natural history of those injuries with subtle diastasis remains unclear. When operative intervention is implemented, hardware removal versus retention must also be considered when allowing for return to play. Although one would assume that motion-related hardware failure would be possible at the tarsometatarsal joints, this concept has yet to be clearly defined in the literature.
The present study also demonstrates that most athletes with these midfoot injuries can return to play at the elite NFL level, as evidenced by their short- and long-term return to play. However, it was not possible to differentiate the specific return-to-play level related to preinjury performance level. Furthermore, this relatively short-term NFL career follow-up study was not able to elucidate the long-term consequences of these injuries. In fact, arch collapse and acquired flatfoot deformity could eventually result from this injury, and long-term outcomes would be of particular interest in patients who have subtle diastasis and who are treated nonoperatively.
Although previous studies have supported operative management for Lisfranc injuries involving subtle diastasis, more than half of the NFL team physicians surveyed in this study use nonoperative treatment for these injuries.19 Future studies should evaluate stress-imaging to define the effect of stability or latent diastasis on long-term outcomes. Nonetheless, the present study demonstrates that a large cohort of NFL team physicians supports nonoperative management for these Lisfranc injuries with subtle diastasis, even in elite athletes. Additional prospective studies are needed to provide a more rigorous injury evaluation and closer follow-up, including subjective and objective outcomes, to further define the indications for management options for midfoot sprains in this population of contact athletes.
1. Aitken AP, Poulson D. Dislocations of the tarsometatarsal joint. J Bone Joint Surg Am. 1963;45:246-260.
2. Hardcastle PH, Reschauer R, Kutscha-Lissberg E, Schoffmann W. Injuries to the tarsometatarsal joint. Incidence, classification and treatment. J Bone Joint Surg Br. 1982;64(3):349-356.
3. Arntz CT, Veith RG, Hansen ST Jr. Fractures and fracture-dislocations of the tarsometatarsal joint. J Bone Joint Surg Am. 1988(2);70:173-181.
4. Goossens M, De Stoop N. Lisfranc’s fracture-dislocations: etiology, radiology, and results of treatment. A review of 20 cases. Clin Orthop. 1983;(176):154-162.
5. Myerson M. The diagnosis and treatment of injuries to the Lisfranc joint complex. Orthop Clin North Am. 1989;20(4):655-664.
6. Wiley JJ. The mechanism of tarso-metatarsal joint injuries. J Bone Joint Surg Br. 1971;53(3):474-482.
7. Faciszewski T, Burks RT, Manaster BJ. Subtle injuries of the Lisfranc joint. J Bone Joint Surg Am. 1990;72(10):1519-1522.
8. Nunley JA, Vertullo CJ. Classification, investigation, and management of midfoot sprains: Lisfranc injuries in the athlete. Am J Sports Med. 2002;30(6):871-878.
9. Garrick JG, Requa RK. The epidemiology of foot and ankle injuries in sports. Clin Sports Med. 1988;7(1):29-36.
10. Meyer SA, Callaghan JJ, Albright JP, Crowley ET, Powell JW. Midfoot sprains in collegiate football players. Am J Sports Med. 1994;22(3):392-401.
11. Shapiro MS, Wascher DC, Finerman GA. Rupture of Lisfranc’s ligament in athletes. Am J Sports Med. 1994;22(5):687-691.
12. Curtis MJ, Myerson M, Szura B. Tarsometatarsal joint injuries in the athlete. Am J Sports Med. 1993;21(4):497-502.
13. Harwood MI, Raikin SM. A Lisfranc fracture-dislocation in a football player. J Am Board Fam Pract. 2003;16(1):69-72.
14. Shah VM, Andrews JR, Fleisig GS, et al. Return to play after anterior cruciate ligament reconstruction in National Football League athletes. Am J Sports Med. 2010;38(11):2233-2239.
15. Kuo RS, Tejwani NC, Digiovanni CW, et al. Outcome after open reduction and internal fixation of Lisfranc joint injuries. J Bone Joint Surg Am. 2000;82(11):1609-1618.
16. Myerson MS, Cerrato RA. Current management of tarsometatarsal injuries in the athlete. J Bone Joint Surg Am. 2008;90(11):2522-2533.
17. Ly TV, Coetzee JC. Treatment of primarily ligamentous Lisfranc joint injuries: primary arthrodesis compared with open reduction and internal fixation. A prospective, randomized study. J Bone Joint Surg Am. 2006;88(3):514-520.
18. Coetzee JC, Ly TV. Treatment of primarily ligamentous Lisfranc joint injuries: primary arthrodesis compared with open reduction and internal fixation. Surgical technique. J Bone Joint Surg Am. 2007;89(suppl 2 pt1):122-127.
19. Ardoin GT, Anderson RB. Subtle Lisfranc injury. Tech Foot Ankle. 2010;9:100-106.
1. Aitken AP, Poulson D. Dislocations of the tarsometatarsal joint. J Bone Joint Surg Am. 1963;45:246-260.
2. Hardcastle PH, Reschauer R, Kutscha-Lissberg E, Schoffmann W. Injuries to the tarsometatarsal joint. Incidence, classification and treatment. J Bone Joint Surg Br. 1982;64(3):349-356.
3. Arntz CT, Veith RG, Hansen ST Jr. Fractures and fracture-dislocations of the tarsometatarsal joint. J Bone Joint Surg Am. 1988(2);70:173-181.
4. Goossens M, De Stoop N. Lisfranc’s fracture-dislocations: etiology, radiology, and results of treatment. A review of 20 cases. Clin Orthop. 1983;(176):154-162.
5. Myerson M. The diagnosis and treatment of injuries to the Lisfranc joint complex. Orthop Clin North Am. 1989;20(4):655-664.
6. Wiley JJ. The mechanism of tarso-metatarsal joint injuries. J Bone Joint Surg Br. 1971;53(3):474-482.
7. Faciszewski T, Burks RT, Manaster BJ. Subtle injuries of the Lisfranc joint. J Bone Joint Surg Am. 1990;72(10):1519-1522.
8. Nunley JA, Vertullo CJ. Classification, investigation, and management of midfoot sprains: Lisfranc injuries in the athlete. Am J Sports Med. 2002;30(6):871-878.
9. Garrick JG, Requa RK. The epidemiology of foot and ankle injuries in sports. Clin Sports Med. 1988;7(1):29-36.
10. Meyer SA, Callaghan JJ, Albright JP, Crowley ET, Powell JW. Midfoot sprains in collegiate football players. Am J Sports Med. 1994;22(3):392-401.
11. Shapiro MS, Wascher DC, Finerman GA. Rupture of Lisfranc’s ligament in athletes. Am J Sports Med. 1994;22(5):687-691.
12. Curtis MJ, Myerson M, Szura B. Tarsometatarsal joint injuries in the athlete. Am J Sports Med. 1993;21(4):497-502.
13. Harwood MI, Raikin SM. A Lisfranc fracture-dislocation in a football player. J Am Board Fam Pract. 2003;16(1):69-72.
14. Shah VM, Andrews JR, Fleisig GS, et al. Return to play after anterior cruciate ligament reconstruction in National Football League athletes. Am J Sports Med. 2010;38(11):2233-2239.
15. Kuo RS, Tejwani NC, Digiovanni CW, et al. Outcome after open reduction and internal fixation of Lisfranc joint injuries. J Bone Joint Surg Am. 2000;82(11):1609-1618.
16. Myerson MS, Cerrato RA. Current management of tarsometatarsal injuries in the athlete. J Bone Joint Surg Am. 2008;90(11):2522-2533.
17. Ly TV, Coetzee JC. Treatment of primarily ligamentous Lisfranc joint injuries: primary arthrodesis compared with open reduction and internal fixation. A prospective, randomized study. J Bone Joint Surg Am. 2006;88(3):514-520.
18. Coetzee JC, Ly TV. Treatment of primarily ligamentous Lisfranc joint injuries: primary arthrodesis compared with open reduction and internal fixation. Surgical technique. J Bone Joint Surg Am. 2007;89(suppl 2 pt1):122-127.
19. Ardoin GT, Anderson RB. Subtle Lisfranc injury. Tech Foot Ankle. 2010;9:100-106.
Osteoid Osteomas of the Foot and Ankle: A Study of Patients Over a 20-Year Period
Because of the complex anatomy of the ankle joint and foot, the wide array of possible bone and soft-tissue injuries, and the uncommon occurrence of tumors at these sites, osteoid osteomas (OOs) are often not included in the differential diagnosis of foot and ankle pain.1,2 Patients with OO usually complain of severe pain that is worse at night and is relieved with use of nonsteroidal anti-inflammatory drugs (NSAIDs).1-4 This classic clinical presentation, combined with the characteristic imaging features, facilitates making an accurate diagnosis.
OOs were first described in 1935 by Jaffe,5 who characterized them as benign, solitary, osteoblastic tumors consisting of atypical bone and osteoid. On radiographs and thin-slice computed tomography (CT), these tumors are small osteolytic lesions surrounded by a larger region of cortical thickening, medullary sclerosis, and benign periosteal new bone formation.4,6,7 They often contain a central focus of calcification—the nidus. OOs typically occur in children and young adults; the majority of patients are younger than 25 years. OOs show a predilection for the appendicular skeleton, with the majority of the lesions in the femur and tibia.4,6,7 OOs infrequently occur in the bones of the hands and feet.8-12 Previous studies of foot and ankle OOs have been predominantly limited to case reports; the largest study, conducted almost 20 years ago, included only 10 patients.1
We conducted a study to evaluate the epidemiology and radiographic features of foot and ankle OOs, to evaluate surgical treatment options and outcomes in patients with foot and ankle OOs, and to evaluate the disease course of patients with foot and ankle OOs treated surgically or with radiofrequency ablation (RFA).
Materials and Methods
After obtaining approval from our institutional review board, we retrospectively reviewed all cases of patients who underwent a surgical or an interventional radiologic procedure and had a preoperative diagnosis of a lower extremity OO between 1990 and 2010. Only patients with a histologically confirmed diagnosis of OO were included in the review of foot and ankle cases.
The medical records of patients with a diagnosis of foot or ankle OO were reviewed for patient sex, age, OO site, clinical presentation, radiographic studies, pain characteristics, treatment modality, histologic diagnosis, and clinical outcome of the surgical or RFA procedure. Preoperative and postoperative clinical outcome scores were calculated using American Orthopedic Foot and Ankle Society (AOFAS) scores.
Whether to perform surgical excision or RFA was discussed between the treating surgeon and the radiologist before treatment. The goal was to treat each lesion while minimizing damage to normal, surrounding structures. If there was any question whether a lesion could be something other than OO based on radiographic features, the lesion was treated with surgical excision. Surgical excision consisted of curettage and bone grafting or en bloc removal. Surgical hardware was placed only when an osteotomy was needed to access the lesion. RFA was performed by consultant musculoskeletal radiologists. Before ablation, a CT-guided needle biopsy of the lesion was performed to obtain tissue for pathologic diagnosis. Recurrence was defined as return of preoperative symptoms after treatment, along with radiographic features of recurrence.
Statistical analysis was done with SPSS software (IBM, New York, New York) using unpaired Student t tests and Fisher exact tests. Statistical significance was set at P < .05.
Results
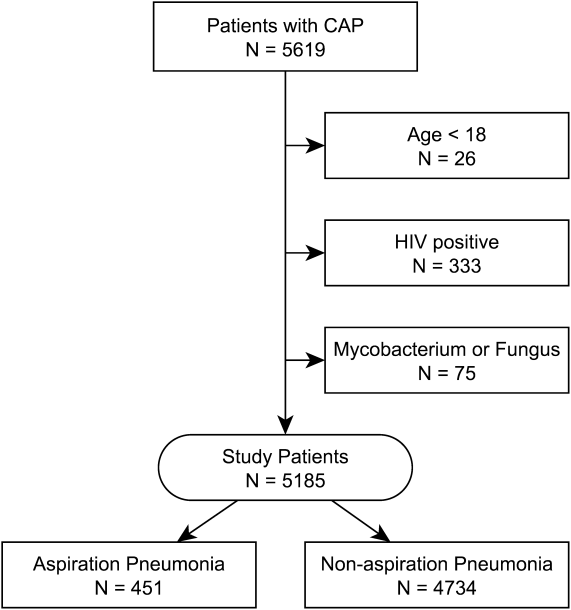
Of the 117 patients with a lower extremity OO, 13 (11%) had it in the bones of the foot or ankle (Table). Mean age at presentation was 20.1 years (range, 9-38 years). There was no statistically significant difference in age between patients with foot or ankle OO and patients with OO of the long bones of the lower extremity (P = .27). Of the 13 patients, 12 were male and 1 was female (Table). The foot and ankle OO sites were the talus (n = 5), the distal tibia/plafond (n = 3), the calcaneus (n = 2), the tarsal bones (n = 2), and the phalanx (n = 1). All 13 foot and ankle lesions were histologically confirmed as OO.
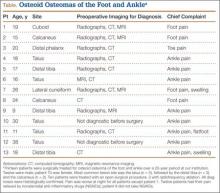
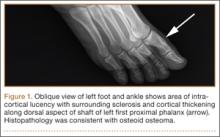
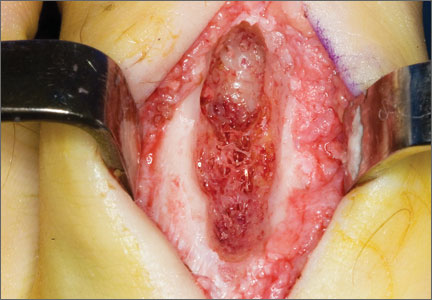
The 13 patients’ primary complaint was foot or ankle pain. Ten of the 13 were referred to our institution for clinical workup and management of foot or ankle pain and for assessment of radiographic features of OO (Figure 1). For all patients in the study, preoperative plain film radiographs of the affected extremity were obtained. Nine patients (69%) had a CT scan (Figure 2), 6 (46%) had a magnetic resonance imaging (MRI) scan, and 2 (15%) had a bone scan. Despite undergoing advanced imaging (1 CT, 1 MRI), 2 patients (15%) did not get a differential diagnosis of OO before being treated. The same 2 patients did not have radiographic images available for review to determine why a differential diagnosis of OO was not included based on imaging features prior to surgery. For the patients who did not have a diagnosis of OO before being evaluated at our institution, preliminary diagnoses included osteomyelitis and painful osteophytes. Twelve of the 13 patients complained of pain that was worse at night and was not relieved with use of NSAIDs. Mean time from symptom onset to presentation at our institution was 14.4 months (range, 3-42 months). All patients reported pain relief after the procedure. There was a significant (P = .0001) increase in AOFAS scores after surgery. Mean AOFAS score was 65.42 (range, 54-80) before surgery and 97.91 (range, 90-100) after surgery.
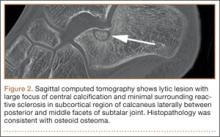
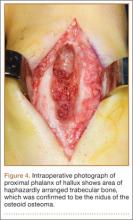
Before 1998, all foot and ankle OOs (n = 6) were treated with surgical excision. After RFA was introduced at our institution, 3 foot and ankle OOs (43%) were treated with RFA (Figures 3A, 3B), and 4 (57%) were treated with surgical curettage (Figure 4). The 4 surgical patients’ OOs were not amenable to RFA primarily because of anatomical considerations: In 2 patients, the OO was too near the articular surface; in another patient, the lesion was in intimate contact with a neurovascular bundle; in the fourth patient, the lesion was amenable to RFA, but the patient’s family selected surgical curettage instead.
Mean tumor nidus size was 7.5 mm (range, 3-12 mm). Bone graft was placed in 3 patients (30%), and surgical hardware was placed to repair a medial malleolar osteotomy in 1 (10%) of the patients treated surgically. The majority of the lesions (8) were in cancellous bone in a subcortical location. Three lesions were intracortical. Seven lesions were intra-articular, and 4 were extra-articular. Two patients did not have radiographic images available for review.
One patient had a recurrence of OO and underwent a repeat procedure 4 months after the initial one. At final follow-up, on average 1 year after the initial procedure (range, 2 weeks–3 years), there were no reported recurrences. One patient underwent a procedure to remove painful hardware that had been implanted, during the primary procedure, to repair the medial malleolar osteotomy used to access the lesion. Recurrence rates for RFA (n = 1) and surgical excision (n = 0) were similar.
Discussion
OOs are relatively common bone tumors that account for about 13% of all benign bone tumors.4,13 OOs typically occur in children or young adults—the majority of patients are younger than 25 years—and are 3 times more common in males than females.4,13 Our findings for all patients with a lower extremity OO are consistent with those previously reported: male predominance (75 males, 42 females) and mean age under 25 years (mean age, 18.7 years). In patients with foot or ankle OO, male predominance was substantially greater (12 males, 1 female), though mean age at presentation (20.1 years) was similar.
Local pain is the most common complaint in patients who present with OO.4,13 Pain is thought to be generated by a combination of multiple nerve endings in the tumor14 and prostaglandin production by the tumor nidus (prostaglandins E2 and I2)3 causing an inflammatory reaction.6 In accord with previous studies,4 localized foot or ankle pain was the most common complaint at time of presentation in our study; 100% of our patients had it. All but 1 patient (92%) in our study described pain that was worse at night and relieved by aspirin or other nonsteroidal anti-inflammatory medications. Pain reduction after NSAID use was observed in 92% (12/13) of our patients as well; the 1 patient who did not report pain relief had not used NSAIDs before being evaluated at our institution. Our patient population reported night pain and pain relief with NSAID use more frequently than patients in other studies did.15,16
The bone most commonly involved in our patients’ foot and ankle OOs was the talus (5/13, 38%). This is in accord with 1 study1 but contradicts another, in which the most common foot and ankle site was the calcaneus.17 The site of the lesion in the bone can be subclassified as cortical, cancellous, or subperiosteal.11,12 Cortical OOs were the most common in our study, but in previous reports the most common were subperiosteal and cancellous.1,11 As all our OOs were cortical, we classified them (on the basis of the relationship of the nidus to the cortex) as intracortical, periosteal, or subcortical (endosteal) instead of subperiosteal or cancellous. Three of our patients’ lesions were intracortical, 8 were subcortical, and 2 patients did not have radiographs available for review at the time of the study.
Although the classic clinical presentation of OO is often sufficient to raise suspicion for the diagnosis, imaging studies play a crucial role in accurate diagnosis. An accurate diagnosis of OO in the long bones can be made if the lesion presents with characteristic imaging features, as a small round lytic lesion with associated cortical thickening, medullary sclerosis, and chronic benign periosteal new bone formation.15 In some cases, however, the nidus may be obscured by the extensive associated reactive changes on the radiographs, and therefore the differential diagnosis may also include stress fracture, Brodie abscess, or even osteosarcoma. High-resolution CT is the imaging modality of choice for accurate diagnosis of OO, and it often plays an instrumental role in making the diagnosis and excluding other diagnostic possibilities.15-17
As foot OOs often occur near the joint (7 intra-articular lesions in our study), they often lack the exuberant periosteal reaction, cortical thickening, and reactive medullary sclerosis that characterize these lesions in the appendicular skeleton.17 In addition, the anatomical complexity of the small bones of the foot and ankle, particularly the hindfoot, where the bones are flat and irregular, makes identifying the lesions difficult.17 Conventional radiographs are the initial imaging modality of choice for evaluating patients with a clinical suspicion of OO, and they may identify the tumor. However, if radiographs are nondiagnostic, and the diagnosis of OO is suspected, high-resolution CT should be performed.
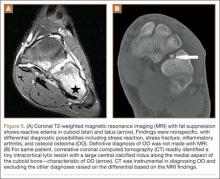
MRI is commonly used to assess for ligamentous, tendinous, and articular cartilage injuries in patients with ankle and hindfoot pain. However, as already discussed, and as reported in previous studies,17 accurate diagnosis of OO can be challenging with MRI (Figure 5A), and often the patients who had MRI scans then underwent CT (Table) for the definitive diagnosis (Figure 5B). In only 1 patient in our study was MRI used to make the preoperative diagnosis of OO (Table). In 2 patients (15%), even advanced imaging did not result in OO being included in the differential diagnosis. This is consistent with other reports, which found that a diagnosis was not made in 11% of patients.16 Although almost a quarter of patients did not have radiographic features diagnostic of OO, CT is the modality of choice for all patients who have clinical features suggestive of a diagnosis of OO.
Surgical treatment of OO is effective when the entire nidus is removed, with excision providing rapid pain relief.4,6,7,11,12 Historically, the tumor was often treated with wide, en bloc resection, but this is a large operation involving removal of a substantial amount of surrounding normal bone, as the lesion is often difficult to identify intraoperatively without preoperative localization.4,6,13 Curettage was performed on the lesion to reduce the amount of bone removed.4 Both techniques are reportedly very successful in treating OOs, with recurrence rates ranging from 0% to 15%.18,19 In our study, none of the surgically treated lesions recurred, and their AOFAS score improved from 67.11 (range, 54-80) before surgery to 98.33 (range, 93-100) after surgery. However, all surgically treated patients required a mean of 3 weeks (0-2.5 months) of either partial weight-bearing or non-weight-bearing of the affected extremity. A variety of treatment techniques have been used as alternatives to surgical resection in an attempt to treat OOs effectively and minimize damage to the surrounding normal bone.4,6,13 These techniques have included percutaneous CT-guided tumor excision with a trephine; percutaneous or surgical ablation using laser, cryotherapy, or ethanol; CT-guided localization followed by operative excision; and CT-guided percutaneous RFA.4,6,13,20 Over the past 2 decades, CT-guided percutaneous RFA has evolved to become the treatment of choice for painful OOs of the appendicular skeleton.15,21,22 The success of this procedure depends on accurate preprocedure diagnosis and precise anatomical localization with CT. Our results correlate with those in series reported in the literature, showing no significant difference in tumor recurrence rates between this technique and surgical excision.22
In our study, 3 patients were treated with CT-guided RFA. Because of recurrent pain, 1 of these patients had a repeat RFA 4 months after the initial procedure. After the second procedure, the patient was asymptomatic. Pain recurrence rates have ranged from 2% to 11% in large series of treated nonspinal OOs.21-23 Our RFA patients’ mean AOFAS score notably improved from 60.33 (range, 60-61) before surgery to 96.66 (range, 90-100) after surgery.
One of the distinct advantages of CT-guided RFA of OO is that it provides a minimally invasive technique for curative treatment with minimal damage to the adjacent normal bone by providing selective and controlled ablation of the tumor nidus.15 Additional advantages are that it can be performed as an outpatient procedure, and patients convalesce quickly with unrestricted weight-bearing and immediate return to activities of daily living.21-23 In addition, when RFA and surgical excision were compared on their average costs of hospitalization and treatment for OO, RFA was found to be less expensive.24
There were no RFA-related complications in our study population, but complications have been reported (albeit rarely) in other large studies of using RFA throughout the appendicular skeleton.21,25 Reported complications include skin burns, nerve damage, reflex sympathetic dystrophy, cellulitis, and thrombophlebitis.21,25 To reduce the risk for these complications, the investigators emphasized the importance of avoiding use of RFA for lesions near a neurovascular bundle (<1.5 cm away) or in a superficial location near the surface of the skin (<1.0 cm away).21,25
We believe that surgical resection and RFA provide equally effective treatment outcomes for patients with foot and ankle OOs. The major contraindication to RFA is anatomical proximity (<1.5 cm) to a major neurovascular bundle. Theoretically, articular cartilage can be damaged during RFA.21,25 To our knowledge, there have been no reported complications involving articular cartilage damage. However, surgeons should carefully measure the distance from lesion to articular cartilage and select the treatment option that will cause the least amount of damage to the cartilage.
Two limitations of this study are its retrospective nature and relatively small number of patients. As all the lesions in the study were treated surgically or with RFA, we are unable to comment on the natural history of untreated foot and ankle OOs. Although there were no recurrences, late recurrence is possible with longer follow-up. However, we think this study will not only increase familiarity with the imaging features of OOs involving the bones of the foot and ankle, but it will help clinicians formulate optimal treatment plans.
Overall, OOs are relatively common benign bone tumors, with limited reports of their occurrence in the foot and ankle. There should be a high index of suspicion for the diagnosis if a patient presents with the symptoms classically associated with the tumor, but in some cases the diagnosis can be challenging. Proper imaging is essential for prompt and accurate diagnosis.
1. Shereff MJ, Cullivan WT, Johnson KA. Osteoid-osteoma of the foot. J Bone Joint Surg Am. 1983;65(5):638-641.
2. Snow SW, Sobel M, DiCarlo EF, Thompson FM, Deland JT. Chronic ankle pain caused by osteoid osteoma of the neck of the talus. Foot Ankle Int. 1997;18(2):98-101.
3. Greco F, Tamburrelli F, Ciabattoni G. Prostaglandins in osteoid osteoma. Int Orthop. 1991;15(1):35-37.
4. Lee EH, Shafi M, Hui JH. Osteoid osteoma: a current review. J Ped Orthop. 2006;26(5):695-700.
5. Jaffe HL. Osteoid-osteoma: a benign osteoblastic tumour composed of osteoid and atypical bone. Arch Surg. 1935;31:19.
6. Ghanem I. The management of osteoid osteoma: updates and controversies. Curr Opin Pediatr. 2006;18(1):36-41.
7. Klein MH, Shankman S. Osteoid osteoma: radiologic and pathologic correlation. Skeletal Radiol. 1992;21(1):23-31.
8. Casadei R, Ferraro A, Ferruzzi A, Biagini R, Ruggieri P. Bone tumors of the foot: epidemiology and diagnosis. Chir Organi Mov. 1991;76(1):47-62.
9. Ebrahimzadeh MH, Ahmadzadeh-Chabock H, Ebrahimzadeh AR. Osteoid osteoma: a diagnosis for radicular pain of extremities. Orthopedics. 2009;32(11):821.
10. Lander PH, Azouz EM, Marton D. Subperiosteal osteoid osteoma of the talus. Clin Radiol. 1986;37(5):491-493.
11. Oztürk A, Yalçinkaya U, Ozkan Y, Yalçin N. Subperiosteal osteoid osteoma in the hallux of a 9-year-old female. J Foot Ankle Surg. 2008;47(6):579-582.
12. Sproule JA, Khan F, Fogarty EE. Osteoid osteoma: painful enlargement of the second toe. Arch Orthop Trauma Surg. 2004;124(5):354-356.
13. Atesok KI, Alman BA, Schemitsch EH, Peyser A, Mankin H. Osteoid osteoma and osteoblastoma. J Am Acad Orthop Surg. 2011;19(11):678-689.
14. Schulman L, Dorfman HD. Nerve fibers in osteoid osteoma. J Bone Joint Surg Am. 1970;52(7):1351-1356.
15. Rosenthal DI, Alexander A, Rosenberg AE, Springfield D. Ablation of osteoid osteomas with a percutaneously placed electrode: a new procedure. Radiology. 1992;183(1):29-33.
16. Gamba JL, Martinez S, Apple J, Harrelson JM, Nunley JA. Computed tomography of axial skeletal osteoid osteomas. AJR Am J Roentgenol. 1984;142(4):769-772.
17. Shukla S, Clarke AW, Saifuddin A. Imaging features of foot osteoid osteoma. Skeletal Radiol. 2010;39(7):683-689.
18. Sluga M, Windhager R, Pfeiffer M, Dominkus M, Kotz R. Peripheral osteoid osteoma. Is there still a place for traditional surgery? J Bone Joint Surg Br. 2002;84(2):249-251.
19. Ward WG, Eckardt JJ, Shayestehfar S, et al. Osteoid osteoma diagnosis and management with low morbidity. Clin Orthop. 1993;(291):229-235.
20. Donahue F, Ahmad A, Mnaymneh W, Pevsner NH. Osteoid osteoma. Computed tomography guided percutaneous excision. Clin Orthop. 1999;(366):191-196.
21. Rosenthal DI, Hornicek FJ, Torriani M, Gebhardt MC, Mankin HJ. Osteoid osteoma: percutaneous treatment with radiofrequency energy. Radiology. 2003;229(1):171-175.
22. Rosenthal DI, Hornicek FJ, Wolfe MW, et al. Percutaneous radiofrequency coagulation of osteoid osteoma compared with operative treatment. J Bone Joint Surg Am. 1998;80(6):815-821.
23. Rosenthal DI, Hornicek FJ, Wolfe MW, Jennings LC, Gebhardt MC, Mankin HJ. Decreasing length of hospital stay in treatment of osteoid osteoma. Clin Orthop. 1999;(361):186-191.
24. Lindner NJ, Scarborough M, Ciccarelli JM, Enneking WF. CT-controlled thermocoagulation of osteoid osteoma in comparison with traditional methods [in German]. Z Orthop Ihre Grenzgeb. 1997;135(6):522-527.
25. Rimondi E, Mavrogenis AF, Rossi G, et al. Radiofrequency ablation for non-spinal osteoid osteomas in 557 patients. Eur Radiol. 2012;22(1):181-188.
Because of the complex anatomy of the ankle joint and foot, the wide array of possible bone and soft-tissue injuries, and the uncommon occurrence of tumors at these sites, osteoid osteomas (OOs) are often not included in the differential diagnosis of foot and ankle pain.1,2 Patients with OO usually complain of severe pain that is worse at night and is relieved with use of nonsteroidal anti-inflammatory drugs (NSAIDs).1-4 This classic clinical presentation, combined with the characteristic imaging features, facilitates making an accurate diagnosis.
OOs were first described in 1935 by Jaffe,5 who characterized them as benign, solitary, osteoblastic tumors consisting of atypical bone and osteoid. On radiographs and thin-slice computed tomography (CT), these tumors are small osteolytic lesions surrounded by a larger region of cortical thickening, medullary sclerosis, and benign periosteal new bone formation.4,6,7 They often contain a central focus of calcification—the nidus. OOs typically occur in children and young adults; the majority of patients are younger than 25 years. OOs show a predilection for the appendicular skeleton, with the majority of the lesions in the femur and tibia.4,6,7 OOs infrequently occur in the bones of the hands and feet.8-12 Previous studies of foot and ankle OOs have been predominantly limited to case reports; the largest study, conducted almost 20 years ago, included only 10 patients.1
We conducted a study to evaluate the epidemiology and radiographic features of foot and ankle OOs, to evaluate surgical treatment options and outcomes in patients with foot and ankle OOs, and to evaluate the disease course of patients with foot and ankle OOs treated surgically or with radiofrequency ablation (RFA).
Materials and Methods
After obtaining approval from our institutional review board, we retrospectively reviewed all cases of patients who underwent a surgical or an interventional radiologic procedure and had a preoperative diagnosis of a lower extremity OO between 1990 and 2010. Only patients with a histologically confirmed diagnosis of OO were included in the review of foot and ankle cases.
The medical records of patients with a diagnosis of foot or ankle OO were reviewed for patient sex, age, OO site, clinical presentation, radiographic studies, pain characteristics, treatment modality, histologic diagnosis, and clinical outcome of the surgical or RFA procedure. Preoperative and postoperative clinical outcome scores were calculated using American Orthopedic Foot and Ankle Society (AOFAS) scores.
Whether to perform surgical excision or RFA was discussed between the treating surgeon and the radiologist before treatment. The goal was to treat each lesion while minimizing damage to normal, surrounding structures. If there was any question whether a lesion could be something other than OO based on radiographic features, the lesion was treated with surgical excision. Surgical excision consisted of curettage and bone grafting or en bloc removal. Surgical hardware was placed only when an osteotomy was needed to access the lesion. RFA was performed by consultant musculoskeletal radiologists. Before ablation, a CT-guided needle biopsy of the lesion was performed to obtain tissue for pathologic diagnosis. Recurrence was defined as return of preoperative symptoms after treatment, along with radiographic features of recurrence.
Statistical analysis was done with SPSS software (IBM, New York, New York) using unpaired Student t tests and Fisher exact tests. Statistical significance was set at P < .05.
Results
Of the 117 patients with a lower extremity OO, 13 (11%) had it in the bones of the foot or ankle (Table). Mean age at presentation was 20.1 years (range, 9-38 years). There was no statistically significant difference in age between patients with foot or ankle OO and patients with OO of the long bones of the lower extremity (P = .27). Of the 13 patients, 12 were male and 1 was female (Table). The foot and ankle OO sites were the talus (n = 5), the distal tibia/plafond (n = 3), the calcaneus (n = 2), the tarsal bones (n = 2), and the phalanx (n = 1). All 13 foot and ankle lesions were histologically confirmed as OO.
The 13 patients’ primary complaint was foot or ankle pain. Ten of the 13 were referred to our institution for clinical workup and management of foot or ankle pain and for assessment of radiographic features of OO (Figure 1). For all patients in the study, preoperative plain film radiographs of the affected extremity were obtained. Nine patients (69%) had a CT scan (Figure 2), 6 (46%) had a magnetic resonance imaging (MRI) scan, and 2 (15%) had a bone scan. Despite undergoing advanced imaging (1 CT, 1 MRI), 2 patients (15%) did not get a differential diagnosis of OO before being treated. The same 2 patients did not have radiographic images available for review to determine why a differential diagnosis of OO was not included based on imaging features prior to surgery. For the patients who did not have a diagnosis of OO before being evaluated at our institution, preliminary diagnoses included osteomyelitis and painful osteophytes. Twelve of the 13 patients complained of pain that was worse at night and was not relieved with use of NSAIDs. Mean time from symptom onset to presentation at our institution was 14.4 months (range, 3-42 months). All patients reported pain relief after the procedure. There was a significant (P = .0001) increase in AOFAS scores after surgery. Mean AOFAS score was 65.42 (range, 54-80) before surgery and 97.91 (range, 90-100) after surgery.
Before 1998, all foot and ankle OOs (n = 6) were treated with surgical excision. After RFA was introduced at our institution, 3 foot and ankle OOs (43%) were treated with RFA (Figures 3A, 3B), and 4 (57%) were treated with surgical curettage (Figure 4). The 4 surgical patients’ OOs were not amenable to RFA primarily because of anatomical considerations: In 2 patients, the OO was too near the articular surface; in another patient, the lesion was in intimate contact with a neurovascular bundle; in the fourth patient, the lesion was amenable to RFA, but the patient’s family selected surgical curettage instead.
Mean tumor nidus size was 7.5 mm (range, 3-12 mm). Bone graft was placed in 3 patients (30%), and surgical hardware was placed to repair a medial malleolar osteotomy in 1 (10%) of the patients treated surgically. The majority of the lesions (8) were in cancellous bone in a subcortical location. Three lesions were intracortical. Seven lesions were intra-articular, and 4 were extra-articular. Two patients did not have radiographic images available for review.
One patient had a recurrence of OO and underwent a repeat procedure 4 months after the initial one. At final follow-up, on average 1 year after the initial procedure (range, 2 weeks–3 years), there were no reported recurrences. One patient underwent a procedure to remove painful hardware that had been implanted, during the primary procedure, to repair the medial malleolar osteotomy used to access the lesion. Recurrence rates for RFA (n = 1) and surgical excision (n = 0) were similar.
Discussion
OOs are relatively common bone tumors that account for about 13% of all benign bone tumors.4,13 OOs typically occur in children or young adults—the majority of patients are younger than 25 years—and are 3 times more common in males than females.4,13 Our findings for all patients with a lower extremity OO are consistent with those previously reported: male predominance (75 males, 42 females) and mean age under 25 years (mean age, 18.7 years). In patients with foot or ankle OO, male predominance was substantially greater (12 males, 1 female), though mean age at presentation (20.1 years) was similar.
Local pain is the most common complaint in patients who present with OO.4,13 Pain is thought to be generated by a combination of multiple nerve endings in the tumor14 and prostaglandin production by the tumor nidus (prostaglandins E2 and I2)3 causing an inflammatory reaction.6 In accord with previous studies,4 localized foot or ankle pain was the most common complaint at time of presentation in our study; 100% of our patients had it. All but 1 patient (92%) in our study described pain that was worse at night and relieved by aspirin or other nonsteroidal anti-inflammatory medications. Pain reduction after NSAID use was observed in 92% (12/13) of our patients as well; the 1 patient who did not report pain relief had not used NSAIDs before being evaluated at our institution. Our patient population reported night pain and pain relief with NSAID use more frequently than patients in other studies did.15,16
The bone most commonly involved in our patients’ foot and ankle OOs was the talus (5/13, 38%). This is in accord with 1 study1 but contradicts another, in which the most common foot and ankle site was the calcaneus.17 The site of the lesion in the bone can be subclassified as cortical, cancellous, or subperiosteal.11,12 Cortical OOs were the most common in our study, but in previous reports the most common were subperiosteal and cancellous.1,11 As all our OOs were cortical, we classified them (on the basis of the relationship of the nidus to the cortex) as intracortical, periosteal, or subcortical (endosteal) instead of subperiosteal or cancellous. Three of our patients’ lesions were intracortical, 8 were subcortical, and 2 patients did not have radiographs available for review at the time of the study.
Although the classic clinical presentation of OO is often sufficient to raise suspicion for the diagnosis, imaging studies play a crucial role in accurate diagnosis. An accurate diagnosis of OO in the long bones can be made if the lesion presents with characteristic imaging features, as a small round lytic lesion with associated cortical thickening, medullary sclerosis, and chronic benign periosteal new bone formation.15 In some cases, however, the nidus may be obscured by the extensive associated reactive changes on the radiographs, and therefore the differential diagnosis may also include stress fracture, Brodie abscess, or even osteosarcoma. High-resolution CT is the imaging modality of choice for accurate diagnosis of OO, and it often plays an instrumental role in making the diagnosis and excluding other diagnostic possibilities.15-17
As foot OOs often occur near the joint (7 intra-articular lesions in our study), they often lack the exuberant periosteal reaction, cortical thickening, and reactive medullary sclerosis that characterize these lesions in the appendicular skeleton.17 In addition, the anatomical complexity of the small bones of the foot and ankle, particularly the hindfoot, where the bones are flat and irregular, makes identifying the lesions difficult.17 Conventional radiographs are the initial imaging modality of choice for evaluating patients with a clinical suspicion of OO, and they may identify the tumor. However, if radiographs are nondiagnostic, and the diagnosis of OO is suspected, high-resolution CT should be performed.
MRI is commonly used to assess for ligamentous, tendinous, and articular cartilage injuries in patients with ankle and hindfoot pain. However, as already discussed, and as reported in previous studies,17 accurate diagnosis of OO can be challenging with MRI (Figure 5A), and often the patients who had MRI scans then underwent CT (Table) for the definitive diagnosis (Figure 5B). In only 1 patient in our study was MRI used to make the preoperative diagnosis of OO (Table). In 2 patients (15%), even advanced imaging did not result in OO being included in the differential diagnosis. This is consistent with other reports, which found that a diagnosis was not made in 11% of patients.16 Although almost a quarter of patients did not have radiographic features diagnostic of OO, CT is the modality of choice for all patients who have clinical features suggestive of a diagnosis of OO.
Surgical treatment of OO is effective when the entire nidus is removed, with excision providing rapid pain relief.4,6,7,11,12 Historically, the tumor was often treated with wide, en bloc resection, but this is a large operation involving removal of a substantial amount of surrounding normal bone, as the lesion is often difficult to identify intraoperatively without preoperative localization.4,6,13 Curettage was performed on the lesion to reduce the amount of bone removed.4 Both techniques are reportedly very successful in treating OOs, with recurrence rates ranging from 0% to 15%.18,19 In our study, none of the surgically treated lesions recurred, and their AOFAS score improved from 67.11 (range, 54-80) before surgery to 98.33 (range, 93-100) after surgery. However, all surgically treated patients required a mean of 3 weeks (0-2.5 months) of either partial weight-bearing or non-weight-bearing of the affected extremity. A variety of treatment techniques have been used as alternatives to surgical resection in an attempt to treat OOs effectively and minimize damage to the surrounding normal bone.4,6,13 These techniques have included percutaneous CT-guided tumor excision with a trephine; percutaneous or surgical ablation using laser, cryotherapy, or ethanol; CT-guided localization followed by operative excision; and CT-guided percutaneous RFA.4,6,13,20 Over the past 2 decades, CT-guided percutaneous RFA has evolved to become the treatment of choice for painful OOs of the appendicular skeleton.15,21,22 The success of this procedure depends on accurate preprocedure diagnosis and precise anatomical localization with CT. Our results correlate with those in series reported in the literature, showing no significant difference in tumor recurrence rates between this technique and surgical excision.22
In our study, 3 patients were treated with CT-guided RFA. Because of recurrent pain, 1 of these patients had a repeat RFA 4 months after the initial procedure. After the second procedure, the patient was asymptomatic. Pain recurrence rates have ranged from 2% to 11% in large series of treated nonspinal OOs.21-23 Our RFA patients’ mean AOFAS score notably improved from 60.33 (range, 60-61) before surgery to 96.66 (range, 90-100) after surgery.
One of the distinct advantages of CT-guided RFA of OO is that it provides a minimally invasive technique for curative treatment with minimal damage to the adjacent normal bone by providing selective and controlled ablation of the tumor nidus.15 Additional advantages are that it can be performed as an outpatient procedure, and patients convalesce quickly with unrestricted weight-bearing and immediate return to activities of daily living.21-23 In addition, when RFA and surgical excision were compared on their average costs of hospitalization and treatment for OO, RFA was found to be less expensive.24
There were no RFA-related complications in our study population, but complications have been reported (albeit rarely) in other large studies of using RFA throughout the appendicular skeleton.21,25 Reported complications include skin burns, nerve damage, reflex sympathetic dystrophy, cellulitis, and thrombophlebitis.21,25 To reduce the risk for these complications, the investigators emphasized the importance of avoiding use of RFA for lesions near a neurovascular bundle (<1.5 cm away) or in a superficial location near the surface of the skin (<1.0 cm away).21,25
We believe that surgical resection and RFA provide equally effective treatment outcomes for patients with foot and ankle OOs. The major contraindication to RFA is anatomical proximity (<1.5 cm) to a major neurovascular bundle. Theoretically, articular cartilage can be damaged during RFA.21,25 To our knowledge, there have been no reported complications involving articular cartilage damage. However, surgeons should carefully measure the distance from lesion to articular cartilage and select the treatment option that will cause the least amount of damage to the cartilage.
Two limitations of this study are its retrospective nature and relatively small number of patients. As all the lesions in the study were treated surgically or with RFA, we are unable to comment on the natural history of untreated foot and ankle OOs. Although there were no recurrences, late recurrence is possible with longer follow-up. However, we think this study will not only increase familiarity with the imaging features of OOs involving the bones of the foot and ankle, but it will help clinicians formulate optimal treatment plans.
Overall, OOs are relatively common benign bone tumors, with limited reports of their occurrence in the foot and ankle. There should be a high index of suspicion for the diagnosis if a patient presents with the symptoms classically associated with the tumor, but in some cases the diagnosis can be challenging. Proper imaging is essential for prompt and accurate diagnosis.
Because of the complex anatomy of the ankle joint and foot, the wide array of possible bone and soft-tissue injuries, and the uncommon occurrence of tumors at these sites, osteoid osteomas (OOs) are often not included in the differential diagnosis of foot and ankle pain.1,2 Patients with OO usually complain of severe pain that is worse at night and is relieved with use of nonsteroidal anti-inflammatory drugs (NSAIDs).1-4 This classic clinical presentation, combined with the characteristic imaging features, facilitates making an accurate diagnosis.
OOs were first described in 1935 by Jaffe,5 who characterized them as benign, solitary, osteoblastic tumors consisting of atypical bone and osteoid. On radiographs and thin-slice computed tomography (CT), these tumors are small osteolytic lesions surrounded by a larger region of cortical thickening, medullary sclerosis, and benign periosteal new bone formation.4,6,7 They often contain a central focus of calcification—the nidus. OOs typically occur in children and young adults; the majority of patients are younger than 25 years. OOs show a predilection for the appendicular skeleton, with the majority of the lesions in the femur and tibia.4,6,7 OOs infrequently occur in the bones of the hands and feet.8-12 Previous studies of foot and ankle OOs have been predominantly limited to case reports; the largest study, conducted almost 20 years ago, included only 10 patients.1
We conducted a study to evaluate the epidemiology and radiographic features of foot and ankle OOs, to evaluate surgical treatment options and outcomes in patients with foot and ankle OOs, and to evaluate the disease course of patients with foot and ankle OOs treated surgically or with radiofrequency ablation (RFA).
Materials and Methods
After obtaining approval from our institutional review board, we retrospectively reviewed all cases of patients who underwent a surgical or an interventional radiologic procedure and had a preoperative diagnosis of a lower extremity OO between 1990 and 2010. Only patients with a histologically confirmed diagnosis of OO were included in the review of foot and ankle cases.
The medical records of patients with a diagnosis of foot or ankle OO were reviewed for patient sex, age, OO site, clinical presentation, radiographic studies, pain characteristics, treatment modality, histologic diagnosis, and clinical outcome of the surgical or RFA procedure. Preoperative and postoperative clinical outcome scores were calculated using American Orthopedic Foot and Ankle Society (AOFAS) scores.
Whether to perform surgical excision or RFA was discussed between the treating surgeon and the radiologist before treatment. The goal was to treat each lesion while minimizing damage to normal, surrounding structures. If there was any question whether a lesion could be something other than OO based on radiographic features, the lesion was treated with surgical excision. Surgical excision consisted of curettage and bone grafting or en bloc removal. Surgical hardware was placed only when an osteotomy was needed to access the lesion. RFA was performed by consultant musculoskeletal radiologists. Before ablation, a CT-guided needle biopsy of the lesion was performed to obtain tissue for pathologic diagnosis. Recurrence was defined as return of preoperative symptoms after treatment, along with radiographic features of recurrence.
Statistical analysis was done with SPSS software (IBM, New York, New York) using unpaired Student t tests and Fisher exact tests. Statistical significance was set at P < .05.
Results
Of the 117 patients with a lower extremity OO, 13 (11%) had it in the bones of the foot or ankle (Table). Mean age at presentation was 20.1 years (range, 9-38 years). There was no statistically significant difference in age between patients with foot or ankle OO and patients with OO of the long bones of the lower extremity (P = .27). Of the 13 patients, 12 were male and 1 was female (Table). The foot and ankle OO sites were the talus (n = 5), the distal tibia/plafond (n = 3), the calcaneus (n = 2), the tarsal bones (n = 2), and the phalanx (n = 1). All 13 foot and ankle lesions were histologically confirmed as OO.
The 13 patients’ primary complaint was foot or ankle pain. Ten of the 13 were referred to our institution for clinical workup and management of foot or ankle pain and for assessment of radiographic features of OO (Figure 1). For all patients in the study, preoperative plain film radiographs of the affected extremity were obtained. Nine patients (69%) had a CT scan (Figure 2), 6 (46%) had a magnetic resonance imaging (MRI) scan, and 2 (15%) had a bone scan. Despite undergoing advanced imaging (1 CT, 1 MRI), 2 patients (15%) did not get a differential diagnosis of OO before being treated. The same 2 patients did not have radiographic images available for review to determine why a differential diagnosis of OO was not included based on imaging features prior to surgery. For the patients who did not have a diagnosis of OO before being evaluated at our institution, preliminary diagnoses included osteomyelitis and painful osteophytes. Twelve of the 13 patients complained of pain that was worse at night and was not relieved with use of NSAIDs. Mean time from symptom onset to presentation at our institution was 14.4 months (range, 3-42 months). All patients reported pain relief after the procedure. There was a significant (P = .0001) increase in AOFAS scores after surgery. Mean AOFAS score was 65.42 (range, 54-80) before surgery and 97.91 (range, 90-100) after surgery.
Before 1998, all foot and ankle OOs (n = 6) were treated with surgical excision. After RFA was introduced at our institution, 3 foot and ankle OOs (43%) were treated with RFA (Figures 3A, 3B), and 4 (57%) were treated with surgical curettage (Figure 4). The 4 surgical patients’ OOs were not amenable to RFA primarily because of anatomical considerations: In 2 patients, the OO was too near the articular surface; in another patient, the lesion was in intimate contact with a neurovascular bundle; in the fourth patient, the lesion was amenable to RFA, but the patient’s family selected surgical curettage instead.
Mean tumor nidus size was 7.5 mm (range, 3-12 mm). Bone graft was placed in 3 patients (30%), and surgical hardware was placed to repair a medial malleolar osteotomy in 1 (10%) of the patients treated surgically. The majority of the lesions (8) were in cancellous bone in a subcortical location. Three lesions were intracortical. Seven lesions were intra-articular, and 4 were extra-articular. Two patients did not have radiographic images available for review.
One patient had a recurrence of OO and underwent a repeat procedure 4 months after the initial one. At final follow-up, on average 1 year after the initial procedure (range, 2 weeks–3 years), there were no reported recurrences. One patient underwent a procedure to remove painful hardware that had been implanted, during the primary procedure, to repair the medial malleolar osteotomy used to access the lesion. Recurrence rates for RFA (n = 1) and surgical excision (n = 0) were similar.
Discussion
OOs are relatively common bone tumors that account for about 13% of all benign bone tumors.4,13 OOs typically occur in children or young adults—the majority of patients are younger than 25 years—and are 3 times more common in males than females.4,13 Our findings for all patients with a lower extremity OO are consistent with those previously reported: male predominance (75 males, 42 females) and mean age under 25 years (mean age, 18.7 years). In patients with foot or ankle OO, male predominance was substantially greater (12 males, 1 female), though mean age at presentation (20.1 years) was similar.
Local pain is the most common complaint in patients who present with OO.4,13 Pain is thought to be generated by a combination of multiple nerve endings in the tumor14 and prostaglandin production by the tumor nidus (prostaglandins E2 and I2)3 causing an inflammatory reaction.6 In accord with previous studies,4 localized foot or ankle pain was the most common complaint at time of presentation in our study; 100% of our patients had it. All but 1 patient (92%) in our study described pain that was worse at night and relieved by aspirin or other nonsteroidal anti-inflammatory medications. Pain reduction after NSAID use was observed in 92% (12/13) of our patients as well; the 1 patient who did not report pain relief had not used NSAIDs before being evaluated at our institution. Our patient population reported night pain and pain relief with NSAID use more frequently than patients in other studies did.15,16
The bone most commonly involved in our patients’ foot and ankle OOs was the talus (5/13, 38%). This is in accord with 1 study1 but contradicts another, in which the most common foot and ankle site was the calcaneus.17 The site of the lesion in the bone can be subclassified as cortical, cancellous, or subperiosteal.11,12 Cortical OOs were the most common in our study, but in previous reports the most common were subperiosteal and cancellous.1,11 As all our OOs were cortical, we classified them (on the basis of the relationship of the nidus to the cortex) as intracortical, periosteal, or subcortical (endosteal) instead of subperiosteal or cancellous. Three of our patients’ lesions were intracortical, 8 were subcortical, and 2 patients did not have radiographs available for review at the time of the study.
Although the classic clinical presentation of OO is often sufficient to raise suspicion for the diagnosis, imaging studies play a crucial role in accurate diagnosis. An accurate diagnosis of OO in the long bones can be made if the lesion presents with characteristic imaging features, as a small round lytic lesion with associated cortical thickening, medullary sclerosis, and chronic benign periosteal new bone formation.15 In some cases, however, the nidus may be obscured by the extensive associated reactive changes on the radiographs, and therefore the differential diagnosis may also include stress fracture, Brodie abscess, or even osteosarcoma. High-resolution CT is the imaging modality of choice for accurate diagnosis of OO, and it often plays an instrumental role in making the diagnosis and excluding other diagnostic possibilities.15-17
As foot OOs often occur near the joint (7 intra-articular lesions in our study), they often lack the exuberant periosteal reaction, cortical thickening, and reactive medullary sclerosis that characterize these lesions in the appendicular skeleton.17 In addition, the anatomical complexity of the small bones of the foot and ankle, particularly the hindfoot, where the bones are flat and irregular, makes identifying the lesions difficult.17 Conventional radiographs are the initial imaging modality of choice for evaluating patients with a clinical suspicion of OO, and they may identify the tumor. However, if radiographs are nondiagnostic, and the diagnosis of OO is suspected, high-resolution CT should be performed.
MRI is commonly used to assess for ligamentous, tendinous, and articular cartilage injuries in patients with ankle and hindfoot pain. However, as already discussed, and as reported in previous studies,17 accurate diagnosis of OO can be challenging with MRI (Figure 5A), and often the patients who had MRI scans then underwent CT (Table) for the definitive diagnosis (Figure 5B). In only 1 patient in our study was MRI used to make the preoperative diagnosis of OO (Table). In 2 patients (15%), even advanced imaging did not result in OO being included in the differential diagnosis. This is consistent with other reports, which found that a diagnosis was not made in 11% of patients.16 Although almost a quarter of patients did not have radiographic features diagnostic of OO, CT is the modality of choice for all patients who have clinical features suggestive of a diagnosis of OO.
Surgical treatment of OO is effective when the entire nidus is removed, with excision providing rapid pain relief.4,6,7,11,12 Historically, the tumor was often treated with wide, en bloc resection, but this is a large operation involving removal of a substantial amount of surrounding normal bone, as the lesion is often difficult to identify intraoperatively without preoperative localization.4,6,13 Curettage was performed on the lesion to reduce the amount of bone removed.4 Both techniques are reportedly very successful in treating OOs, with recurrence rates ranging from 0% to 15%.18,19 In our study, none of the surgically treated lesions recurred, and their AOFAS score improved from 67.11 (range, 54-80) before surgery to 98.33 (range, 93-100) after surgery. However, all surgically treated patients required a mean of 3 weeks (0-2.5 months) of either partial weight-bearing or non-weight-bearing of the affected extremity. A variety of treatment techniques have been used as alternatives to surgical resection in an attempt to treat OOs effectively and minimize damage to the surrounding normal bone.4,6,13 These techniques have included percutaneous CT-guided tumor excision with a trephine; percutaneous or surgical ablation using laser, cryotherapy, or ethanol; CT-guided localization followed by operative excision; and CT-guided percutaneous RFA.4,6,13,20 Over the past 2 decades, CT-guided percutaneous RFA has evolved to become the treatment of choice for painful OOs of the appendicular skeleton.15,21,22 The success of this procedure depends on accurate preprocedure diagnosis and precise anatomical localization with CT. Our results correlate with those in series reported in the literature, showing no significant difference in tumor recurrence rates between this technique and surgical excision.22
In our study, 3 patients were treated with CT-guided RFA. Because of recurrent pain, 1 of these patients had a repeat RFA 4 months after the initial procedure. After the second procedure, the patient was asymptomatic. Pain recurrence rates have ranged from 2% to 11% in large series of treated nonspinal OOs.21-23 Our RFA patients’ mean AOFAS score notably improved from 60.33 (range, 60-61) before surgery to 96.66 (range, 90-100) after surgery.
One of the distinct advantages of CT-guided RFA of OO is that it provides a minimally invasive technique for curative treatment with minimal damage to the adjacent normal bone by providing selective and controlled ablation of the tumor nidus.15 Additional advantages are that it can be performed as an outpatient procedure, and patients convalesce quickly with unrestricted weight-bearing and immediate return to activities of daily living.21-23 In addition, when RFA and surgical excision were compared on their average costs of hospitalization and treatment for OO, RFA was found to be less expensive.24
There were no RFA-related complications in our study population, but complications have been reported (albeit rarely) in other large studies of using RFA throughout the appendicular skeleton.21,25 Reported complications include skin burns, nerve damage, reflex sympathetic dystrophy, cellulitis, and thrombophlebitis.21,25 To reduce the risk for these complications, the investigators emphasized the importance of avoiding use of RFA for lesions near a neurovascular bundle (<1.5 cm away) or in a superficial location near the surface of the skin (<1.0 cm away).21,25
We believe that surgical resection and RFA provide equally effective treatment outcomes for patients with foot and ankle OOs. The major contraindication to RFA is anatomical proximity (<1.5 cm) to a major neurovascular bundle. Theoretically, articular cartilage can be damaged during RFA.21,25 To our knowledge, there have been no reported complications involving articular cartilage damage. However, surgeons should carefully measure the distance from lesion to articular cartilage and select the treatment option that will cause the least amount of damage to the cartilage.
Two limitations of this study are its retrospective nature and relatively small number of patients. As all the lesions in the study were treated surgically or with RFA, we are unable to comment on the natural history of untreated foot and ankle OOs. Although there were no recurrences, late recurrence is possible with longer follow-up. However, we think this study will not only increase familiarity with the imaging features of OOs involving the bones of the foot and ankle, but it will help clinicians formulate optimal treatment plans.
Overall, OOs are relatively common benign bone tumors, with limited reports of their occurrence in the foot and ankle. There should be a high index of suspicion for the diagnosis if a patient presents with the symptoms classically associated with the tumor, but in some cases the diagnosis can be challenging. Proper imaging is essential for prompt and accurate diagnosis.
1. Shereff MJ, Cullivan WT, Johnson KA. Osteoid-osteoma of the foot. J Bone Joint Surg Am. 1983;65(5):638-641.
2. Snow SW, Sobel M, DiCarlo EF, Thompson FM, Deland JT. Chronic ankle pain caused by osteoid osteoma of the neck of the talus. Foot Ankle Int. 1997;18(2):98-101.
3. Greco F, Tamburrelli F, Ciabattoni G. Prostaglandins in osteoid osteoma. Int Orthop. 1991;15(1):35-37.
4. Lee EH, Shafi M, Hui JH. Osteoid osteoma: a current review. J Ped Orthop. 2006;26(5):695-700.
5. Jaffe HL. Osteoid-osteoma: a benign osteoblastic tumour composed of osteoid and atypical bone. Arch Surg. 1935;31:19.
6. Ghanem I. The management of osteoid osteoma: updates and controversies. Curr Opin Pediatr. 2006;18(1):36-41.
7. Klein MH, Shankman S. Osteoid osteoma: radiologic and pathologic correlation. Skeletal Radiol. 1992;21(1):23-31.
8. Casadei R, Ferraro A, Ferruzzi A, Biagini R, Ruggieri P. Bone tumors of the foot: epidemiology and diagnosis. Chir Organi Mov. 1991;76(1):47-62.
9. Ebrahimzadeh MH, Ahmadzadeh-Chabock H, Ebrahimzadeh AR. Osteoid osteoma: a diagnosis for radicular pain of extremities. Orthopedics. 2009;32(11):821.
10. Lander PH, Azouz EM, Marton D. Subperiosteal osteoid osteoma of the talus. Clin Radiol. 1986;37(5):491-493.
11. Oztürk A, Yalçinkaya U, Ozkan Y, Yalçin N. Subperiosteal osteoid osteoma in the hallux of a 9-year-old female. J Foot Ankle Surg. 2008;47(6):579-582.
12. Sproule JA, Khan F, Fogarty EE. Osteoid osteoma: painful enlargement of the second toe. Arch Orthop Trauma Surg. 2004;124(5):354-356.
13. Atesok KI, Alman BA, Schemitsch EH, Peyser A, Mankin H. Osteoid osteoma and osteoblastoma. J Am Acad Orthop Surg. 2011;19(11):678-689.
14. Schulman L, Dorfman HD. Nerve fibers in osteoid osteoma. J Bone Joint Surg Am. 1970;52(7):1351-1356.
15. Rosenthal DI, Alexander A, Rosenberg AE, Springfield D. Ablation of osteoid osteomas with a percutaneously placed electrode: a new procedure. Radiology. 1992;183(1):29-33.
16. Gamba JL, Martinez S, Apple J, Harrelson JM, Nunley JA. Computed tomography of axial skeletal osteoid osteomas. AJR Am J Roentgenol. 1984;142(4):769-772.
17. Shukla S, Clarke AW, Saifuddin A. Imaging features of foot osteoid osteoma. Skeletal Radiol. 2010;39(7):683-689.
18. Sluga M, Windhager R, Pfeiffer M, Dominkus M, Kotz R. Peripheral osteoid osteoma. Is there still a place for traditional surgery? J Bone Joint Surg Br. 2002;84(2):249-251.
19. Ward WG, Eckardt JJ, Shayestehfar S, et al. Osteoid osteoma diagnosis and management with low morbidity. Clin Orthop. 1993;(291):229-235.
20. Donahue F, Ahmad A, Mnaymneh W, Pevsner NH. Osteoid osteoma. Computed tomography guided percutaneous excision. Clin Orthop. 1999;(366):191-196.
21. Rosenthal DI, Hornicek FJ, Torriani M, Gebhardt MC, Mankin HJ. Osteoid osteoma: percutaneous treatment with radiofrequency energy. Radiology. 2003;229(1):171-175.
22. Rosenthal DI, Hornicek FJ, Wolfe MW, et al. Percutaneous radiofrequency coagulation of osteoid osteoma compared with operative treatment. J Bone Joint Surg Am. 1998;80(6):815-821.
23. Rosenthal DI, Hornicek FJ, Wolfe MW, Jennings LC, Gebhardt MC, Mankin HJ. Decreasing length of hospital stay in treatment of osteoid osteoma. Clin Orthop. 1999;(361):186-191.
24. Lindner NJ, Scarborough M, Ciccarelli JM, Enneking WF. CT-controlled thermocoagulation of osteoid osteoma in comparison with traditional methods [in German]. Z Orthop Ihre Grenzgeb. 1997;135(6):522-527.
25. Rimondi E, Mavrogenis AF, Rossi G, et al. Radiofrequency ablation for non-spinal osteoid osteomas in 557 patients. Eur Radiol. 2012;22(1):181-188.
1. Shereff MJ, Cullivan WT, Johnson KA. Osteoid-osteoma of the foot. J Bone Joint Surg Am. 1983;65(5):638-641.
2. Snow SW, Sobel M, DiCarlo EF, Thompson FM, Deland JT. Chronic ankle pain caused by osteoid osteoma of the neck of the talus. Foot Ankle Int. 1997;18(2):98-101.
3. Greco F, Tamburrelli F, Ciabattoni G. Prostaglandins in osteoid osteoma. Int Orthop. 1991;15(1):35-37.
4. Lee EH, Shafi M, Hui JH. Osteoid osteoma: a current review. J Ped Orthop. 2006;26(5):695-700.
5. Jaffe HL. Osteoid-osteoma: a benign osteoblastic tumour composed of osteoid and atypical bone. Arch Surg. 1935;31:19.
6. Ghanem I. The management of osteoid osteoma: updates and controversies. Curr Opin Pediatr. 2006;18(1):36-41.
7. Klein MH, Shankman S. Osteoid osteoma: radiologic and pathologic correlation. Skeletal Radiol. 1992;21(1):23-31.
8. Casadei R, Ferraro A, Ferruzzi A, Biagini R, Ruggieri P. Bone tumors of the foot: epidemiology and diagnosis. Chir Organi Mov. 1991;76(1):47-62.
9. Ebrahimzadeh MH, Ahmadzadeh-Chabock H, Ebrahimzadeh AR. Osteoid osteoma: a diagnosis for radicular pain of extremities. Orthopedics. 2009;32(11):821.
10. Lander PH, Azouz EM, Marton D. Subperiosteal osteoid osteoma of the talus. Clin Radiol. 1986;37(5):491-493.
11. Oztürk A, Yalçinkaya U, Ozkan Y, Yalçin N. Subperiosteal osteoid osteoma in the hallux of a 9-year-old female. J Foot Ankle Surg. 2008;47(6):579-582.
12. Sproule JA, Khan F, Fogarty EE. Osteoid osteoma: painful enlargement of the second toe. Arch Orthop Trauma Surg. 2004;124(5):354-356.
13. Atesok KI, Alman BA, Schemitsch EH, Peyser A, Mankin H. Osteoid osteoma and osteoblastoma. J Am Acad Orthop Surg. 2011;19(11):678-689.
14. Schulman L, Dorfman HD. Nerve fibers in osteoid osteoma. J Bone Joint Surg Am. 1970;52(7):1351-1356.
15. Rosenthal DI, Alexander A, Rosenberg AE, Springfield D. Ablation of osteoid osteomas with a percutaneously placed electrode: a new procedure. Radiology. 1992;183(1):29-33.
16. Gamba JL, Martinez S, Apple J, Harrelson JM, Nunley JA. Computed tomography of axial skeletal osteoid osteomas. AJR Am J Roentgenol. 1984;142(4):769-772.
17. Shukla S, Clarke AW, Saifuddin A. Imaging features of foot osteoid osteoma. Skeletal Radiol. 2010;39(7):683-689.
18. Sluga M, Windhager R, Pfeiffer M, Dominkus M, Kotz R. Peripheral osteoid osteoma. Is there still a place for traditional surgery? J Bone Joint Surg Br. 2002;84(2):249-251.
19. Ward WG, Eckardt JJ, Shayestehfar S, et al. Osteoid osteoma diagnosis and management with low morbidity. Clin Orthop. 1993;(291):229-235.
20. Donahue F, Ahmad A, Mnaymneh W, Pevsner NH. Osteoid osteoma. Computed tomography guided percutaneous excision. Clin Orthop. 1999;(366):191-196.
21. Rosenthal DI, Hornicek FJ, Torriani M, Gebhardt MC, Mankin HJ. Osteoid osteoma: percutaneous treatment with radiofrequency energy. Radiology. 2003;229(1):171-175.
22. Rosenthal DI, Hornicek FJ, Wolfe MW, et al. Percutaneous radiofrequency coagulation of osteoid osteoma compared with operative treatment. J Bone Joint Surg Am. 1998;80(6):815-821.
23. Rosenthal DI, Hornicek FJ, Wolfe MW, Jennings LC, Gebhardt MC, Mankin HJ. Decreasing length of hospital stay in treatment of osteoid osteoma. Clin Orthop. 1999;(361):186-191.
24. Lindner NJ, Scarborough M, Ciccarelli JM, Enneking WF. CT-controlled thermocoagulation of osteoid osteoma in comparison with traditional methods [in German]. Z Orthop Ihre Grenzgeb. 1997;135(6):522-527.
25. Rimondi E, Mavrogenis AF, Rossi G, et al. Radiofrequency ablation for non-spinal osteoid osteomas in 557 patients. Eur Radiol. 2012;22(1):181-188.
Factors Affecting Heart Failure Readmission Rates in VA Patients
Heart failure (HF) continues to grow as a significant health problem in the U.S., accounting for 1.1 million hospitalizations annually.1 About 5.8 million Americans have HF, and 670,000 new cases are diagnosed each year.1 The prevalence of HF increases with age. Persons aged > 65 years comprise the largest group of patients hospitalized for the condition. Heart failure-related hospitalizations place a major financial burden on patients, caregivers, and the national health care system. In 2010, the estimated cost of health care for HF was $35 billion with hospitalizations accounting for 1% to 2% of the total annual health care costs.1-3 Furthermore, > 50% of patients with HF are rehospitalized before their first outpatient follow-up.4
To ensure patients are ready for discharge, HF guidelines recommend specific interventions for all hospitalized patients. These recommendations include successful transition from IV to oral diuretic therapy as well as the initiation of a ß-blocker and an angiotensin-converting enzyme inhibitor (ACE-I) or an angiotensin receptor blocker (ARB) in stable patients with a left ventricular ejection fraction (LVEF) < 40% and without contraindications. Additionally, patients and their caregivers should receive comprehensive discharge instructions regarding medications, the importance of adherence and regular follow-up, sodium and fluid restriction, weight monitoring, physical activity, and a plan for worsening symptoms. When available, assistance with the hospital-to-home transition should also be provided.5,6
Heart Failure Measures
Recognizing common factors essential to HF care, the Joint Commission has implemented HF core measures that all U.S. hospitals are required to meet to maintain accreditation status. These guideline-supported measures include receipt of diet, weight, and medication instructions; measured or scheduled assessment of LVEF; ACE-I or an ARB prescribed in patients with LVEF < 40%; and smoking cessation counseling before discharge for all patients with HF.
In addition to HF core measures, 30-day HF readmission rates have also become available to the general public as another hospital quality indicator. In 2009, the Centers for Medicare & Medicaid Services began publicly reporting 30-day HF readmission rates for Medicare patients. A CMS report indicated a 24.8% national 30-day HF readmission rate from July 1, 2007, through June 30, 2010.7 Unfortunately, even with the increased quality improvement effort, national HF rehospitalization rates have remained relatively steady in recent years.3
The VA health care system has a growing number of veterans with HF, and it is the leading discharge diagnosis in patients treated at VA hospitals. The number of HF-related hospitalizations at the VA health care system increased from just over 74,000 in fiscal year 2002 to 96,000 in 2009.8
To advance the care of veterans with HF and implement best practices, the VA launched the Chronic HF-Quality Enhancement Research Initiative (CHF-QUERI). The major goals of this initiative are to reduce hospitalization rates, increase use of life-prolonging care, empower patients and their caregivers in self-management, and improve appropriateness of HF therapies and tests. As part of its efforts, CHF-QUERI launched the HF Provider Network (HF Network), involving more than 712 VA health care providers (as of July 2014 there were more than 900 providers) committed to improving HF management throughout the entire VA health care system. The HF Network has already put into practice several quality improvement initiatives.
The National Hospital to Home initiative led by the American College of Cardiology and the Institute for Healthcare Improvement was launched throughout the VA system in January 2010.9 The main goal of this initiative is to reduce all-cause hospital readmission rates in patients with a discharge diagnosis of HF by improving medication management, early follow-up after discharge, and symptom management.
The Jesse Brown VAMC (JBVAMC) is an active participant of the Hospital to Home initiative, embracing the goals of reducing HF readmission rates and improving the transition of veterans from inpatient to outpatient care. The JBVAMC also has been successfully meeting or exceeding HF core measures except for providing discharge instructions. In May 2011, 91% of patients received discharge instructions, falling just slightly below the 93% target goal. Despite the implementation of HF care improvement initiatives and successful core measure performance, from July 1, 2007, to June 30, 2010, the average HF 30-day readmission rate at JBVAMC was reported to be 28.4%, compared with the national average of 24.8%. Additionally, the average readmission rate for fiscal year 2011 was 31% at JBVAMC, showing a further increase in readmission rates.
The cost of a hospital bed at JBVAMC ranges from about $2,000 to $5,000 per day. According to the American Heart Association’s Get With the Guidelines-HF registry, the mean hospital length of stay for HF in 2009 was 5.5 days.1 Consequently, HF hospitalizations could potentially cost JBVAMC nearly $7 million annually. Therefore, HF readmissions not only affect patients and caregivers, but also represent a financial burden for JBVAMC.
METHODS
The purpose of this study was to identify factors contributing to the high HF readmission rates in veterans enrolled at JBVAMC. This study was an Institutional Review Board and VA Research and Development Committee-approved retrospective, electronic chart review of patients with an ICD-9 principal discharge diagnosis code for HF and hospitalization for HF exacerbation anytime between October 1, 2010, and March 1, 2011. A patient chart was reviewed for 6 months after inclusion. A report was generated to identify patients discharged from JBVAMC with a principal discharge diagnosis of HF between October 1, 2010, and March 1, 2011, using the following ICD-9 HF codes: 402.01, 402.11, 402.91, 404.01, 404.03, 404.11, 404.13, 404.91, 404.93, 428.0, 428.1, 428.20, 428.21, 428.22, 428.23, 428.30, 428.31, 428.32, 428.33, 428.40, 428.41, 428.42, 428.43, and 428.9.
Patients were included if aged ≥ 18 years with one of the ICD-9 HF codes as the principal discharge diagnosis within the study period. Patients were excluded from the study if transferred to or from an outside hospital, discharged without an ICD-9 principal diagnosis code for HF, electively admitted for HF, not treated for HF during hospitalization, left the hospital against medical advice, had chart documentation with comfort measures only, were discharged/transferred to hospice, had active HF medications listed under non-VA medications in the electronic medication profile, or did not receive follow-up at JBVAMC. Study participants were included in the study once, which was classified as their index HF hospitalization, and were followed for 6 months thereafter.
The primary endpoint was the difference in patient characteristics between 2 groups of patients: those readmitted for HF within 30 days of the index hospitalization and those readmitted after 30 days or not at all.
The study had multiple secondary endpoints. One was the difference in patient characteristics between 2 groups of patients: those readmitted for HF within 90 days of the index hospitalization and those readmitted after 90 days or not at all. Another secondary endpoint was the difference in patient characteristics between 2 groups of patients: those with ≥ 2 readmissions for HF within 6 months and those with < 2 HF readmissions within 6 months. Additional secondary endpoints included percentage of patients readmitted for HF within 30 days of the index HF hospitalization, time to readmission if applicable, time to death if applicable, and average number of readmissions per patient within 6 months.
Index data collected included age, gender, ethnicity, prior diagnosis of HF, date of diagnosis, hospitalization for HF within 30 days of the index HF admission, in-hospital cardiac arrest, comorbid conditions, systolic blood pressure (BP), heart rate, respiratory rate, weight, serum sodium, blood urea nitrogen, serum creatinine, hematocrit, and glucose. For this study, comorbid conditions gathered were diabetes mellitus, coronary artery disease, prior percutaneous coronary intervention, aortic stenosis, stroke, chronic obstructive pulmonary disease, and dementia.
Medication profiles were reviewed at the time of admission to determine whether the patient was prescribed an ACE-I/ARB, ß-blocker, diuretic, hydralazine and isosorbide dinitrate, aldosterone antagonist, digoxin, NSAIDs, nonvasoselective calcium channel blocker, and an antiarrhytmic other than amiodarone and dofetilide. Hospitalization data included the most recent LVEF, the number of days on oral diuretic therapy after stopping IV diuretics, the number of days admitted, and documentation of an in-person inpatient dietitian consultation.
Data collected at discharge included diet/weight/medication instructions, weight, BP, American College of Cardiology/American Heart Association HF stage and New York Heart Association (NYHA) HF functional class, if documented. Discharge medication profiles were assessed for the number of medications (< 9 or ≥ 9), documentation of active prescriptions for an ACE-I/ARB and a ß-blocker (or contraindication documented), diuretic, hydralazine and isosorbide dinitrate, aldosterone antagonist, and digoxin. Other data collected were documentation of a scheduled follow-up appointment with primary care physician, urgent care, chronic HF (CHF) clinic, or cardiologist, and whether the patient was discharged to home, skilled nursing facility, shelter, or homeless. Additionally, if the patient was discharged on a diuretic, the dose was compared with the baseline diuretic. If the diuretic at discharge was different from the home diuretic, equivalent doses were used for comparison with that of the baseline diuretic.
Postdischarge data collection included telephone follow-up within 48 hours of discharge, medication compliance since the initial hospitalization, date of first outpatient follow-up after initial hospital discharge, enrollment in CHF clinic/CHF-PharmD/Care Coordination Home Telehealth (CCHT) program, outpatient dietitian consultations, and date of death if applicable. Medication adherence was defined as ≥ 80% of lowest percentage filled medication of all HF medications, determined by the refill history in the computerized patient record system (CPRS). First outpatient follow-up was defined as a visit in which HF was addressed in the assessment and plan.
If readmitted within the study period, data collection included the date of first nonelective hospital readmission for HF, BP, heart rate, weight, serum digoxin level, serum creatinine, serum potassium, and whether the patient was on a target dose of HF recommended medications (if LVEF < 40% and no contraindication). Heart failure recommended medications for which target doses are established include ACE-I/ARB and ß-blockers. For this study, target doses of ACE-Is were captopril 50 mg 3 times daily, enalapril 10 mg twice daily, fosinopril 40 mg daily, lisinopril 20 mg daily, ramipril 10 mg daily, and trandolapril 4 mg daily. Target doses for ARBs were candesartan 32 mg daily, losartan 50 mg daily, and valsartan 160 mg twice daily. ß-blocker target doses were bisoprolol 10 mg daily, carvedilol 25 mg twice daily (50 mg twice daily if patients’ weight was > 85 kg), and metoprolol succinate 200 mg daily.5,6 A statistical analysis was not performed on the data.
RESULTS
A total of 137 patient charts were reviewed, and 109 patients were included in the study. Patients were excluded if they transferred to or from an outside hospital (n = 8), had no follow-up at JBVAMC (n = 8), left the hospital against medical advice (n = 4), were electively admitted (n = 4), were not treated for HF (n = 3), or only had comfort measures documented in the chart (n = 1). The patients included were predominantly male (99%) and African American (78%) and had a mean age of 70 years. The majority of the patients had a prior diagnosis of HF (87%) and a history of systolic HF (58%). Most patients were previously prescribed an ACE-I/ARB (83%) and a ß-blocker (76%) at the time of admission (Table 1).
Six patients were readmitted within 30 days of the index hospitalization, whereas 103 patients were readmitted after 30 days or not at all. With respect to secondary endpoints, there were 21 patients readmitted within 90 days of the index hospitalization, whereas 88 patients were readmitted after 90 days or not at all. Additionally, 6 patients were readmitted ≥ 2 times within 6 months of the index hospitalization, whereas 103 patients were readmitted < 2 times within 6 months.
Baseline characteristics seemed similar across the study groups, except a greater percentage of patients readmitted within 30 days of the index HF hospitalization had a prior history of systolic HF and were hospitalized for HF 30 days prior to the index hospitalization (Table 2). In addition, patients readmitted within 30 days tended to receive a shorter duration of oral diuretic therapy after discontinuation of IV diuretics (mean 0.2 days vs 1.1 days). Patients in this group with an LVEF < 40% were less likely to be discharged on an ACE-I/ARB (75% vs 95%) and a ß-blocker (50% vs 85%) than were the patients who were readmitted after 30 days or not at all. These trends continued for patients readmitted within 90 days of the index hospitalization and for those readmitted after 90 days or not at all. The mean length of stay for the index HF hospitalization was about 5 days and was comparable among all study groups.
From the evaluation of postdischarge characteristics, no patients readmitted within 30 days had a follow-up appointment scheduled with the CHF clinic. In comparison with patients readmitted after 30 days or not at all, more patients had follow-up at an urgent care clinic (33% vs 6%) or no follow-up appointment scheduled at the time of discharge (17% vs 2%). Half of all the patients with a scheduled follow-up missed their appointment. Additionally, medication adherence was lower (33% vs 80%), and none of the patients were enrolled in the CHF-PharmD clinic (0% vs 5%). A similar trend continued for the secondary endpoint groups (Table 3). Last, none of the study patients had an outpatient dietitian consultation.
On readmission, the majority of patients readmitted within 30 days were not on a target dose of an ACE-I/ARB (75%), and none were on a target dose of a ß-blocker. The same trend continued for the secondary endpoint groups. None of the study patients had a serum digoxin level > 0.9 ng/mL. However, serum digoxin level was not measured in all readmitted patients prescribed digoxin (Table 4).
In regard to other secondary endpoints, 6 patients (5.5%) were readmitted for HF within 30 days of the index HF hospitalization. The average number of readmissions per patient in 6 months was < 1, mean time to readmission was 85 days (n = 33), and mean time to death was 88 days (n = 5) when applicable.
DISCUSSION
Based on the trends observed in this study, multiple recommendations can be made to further improve the quality of care and reduce HF readmissions at JBVAMC. The medical center physicians currently use a discharge note template, which already includes sections such as HF discharge instructions and follow-up appointments. The template also prompts providers to prescribe an ACE-I in appropriate patients.
When JBVAMC providers are ready to enter discharge notes into the CPRS, they first select the discharge note template from available note template options. The electronic template contains spaces for the provider to enter a patient’s primary reason for hospitalization, date of admission, discharge medication list, specific or suggested dates for follow-up with outpatient provider(s), general diet/weight/medication instructions, a space to answer whether the patient has HF, a space to record NYHA HF class if applicable, and a space to record whether the patient is prescribed or will be prescribed an ACE-I if appropriate, or whether ACE-I is contraindicated. The providers are able to modify and add information to the discharge note template as they see appropriate.
The findings of this study suggest that modifying the existing discharge template to include additional provider prompts in a form of designated spaces asking for specific information may help improve HF care outcomes. If providers are prompted to answer whether an oral diuretic was continued for at least 24 hours after stopping IV diuretics for HF, adherence to the HF guideline-recommended duration of oral diuretic therapy may improve. Additionally, ß-blocker prescribing in appropriate systolic HF patients may increase if providers are prompted. To enhance continuity of care, the discharge note template may be modified to include a section in which the providers can document patients followed by outside providers. This can be done by incorporating a space in the discharge template to enter the patient’s non-VA provider information if applicable and may help further coordinate the care of such patients to ensure that they are not lost.
Furthermore, the discharge template may be modified to include a prompt to place a CHF clinic consult to increase provider awareness about the availability of CHF and CHF-PharmD clinics at JBVAMC. CHF and CHF-PharmD clinics collaborate to provide comprehensive care to HF patients. After an initial evaluation at the CHF clinic, patients are referred to the clinical pharmacist for further medication therapy management when necessary. Currently, the physicians are encouraged to refer HF patients to the CHF clinic after discharge, but not all providers know that such a service is available. The prompt within the discharge note template would provide CHF/CHF-PharmD clinic provider contact information, clinic times, and a link that would take the provider to an appropriate screen for placing the consult.
Limitations
There are several limitations to this study, including its retrospective design and small sample size. Another source of potential study limitation was the initial process for creating a study patient list. The study list was designed to use ICD-9 codes to capture readmissions only for HF and only at JBVAMC. This was achieved by specifying any of the HF ICD-9 codes as the principal discharge diagnosis. However, the providers may not have always used a HF specific ICD-9 code for the principal discharge diagnosis, even if a patient was admitted primarily for HF. The provider may have chosen another principal discharge diagnosis for which the patient received treatment during the hospitalization.
There are multiple ways to obtain HF patient lists, one includes using the diagnosis-related group codes instead of ICD-9 codes. Due to the way the patient list was obtained and an inherent possibility that some patients admitted for HF had a non-HF ICD-9 code recorded as their principal discharge diagnosis, some eligible patients may not have appeared on the generated list. Additionally, this study captured readmission rates for only HF whereas the national HF 30-day readmission rate represents all-cause readmissions for HF patients. This difference may be reflected in the low 30-day readmission rate observed.
Another possible limitation was the timing of the launch of the CHF-PharmD clinic and the initiative for telephone follow-up 48 hours postdischarge. The CHF-PharmD clinic was launched in April 2011, and the initiative for telephone follow-up 48 hours postdischarge began in January 2011. As the start dates fell within the study period, these services may not have been available to all patients. Therefore, the data describing patient enrollment in CHF-PharmD clinic and those who received postdischarge telephone follow-up may not accurately reflect current practice. Last, statistical tests were not used in the study data analysis leaving any differences found open to interpretation. To minimize these limitations, larger prospective studies with statistical analysis capturing all-cause readmissions are necessary to further evaluate patient characteristics that may be contributing to HF readmissions at JBVAMC.
Conclusions
In general, earlier and more frequent readmissions were more common in patients who were converted to oral diuretic therapy for < 24 hours before discharge and were not discharged on an ACE-I/ARB and a b-blocker when appropriate. Additionally, most of the readmitted patients had no follow-up scheduled at discharge, were nonadherent with medications and follow-up appointments, and were not enrolled in the CHF and/or CHF-PharmD clinic. The majority of patients with systolic HF were not at target doses of either the ACE-I/ARB or the ß-blocker when readmitted. Overall, JBVAMC had a low percentage of patients readmitted for HF within 30 days, but there is still room for improvement in reducing HF readmissions.
At the time of discharge, all JBVAMC patients receive printed instructions and recommendations for their care after hospitalization. The patient handout includes the most current medications, diet/weight/medication instructions, and actual or suggested dates for follow-up appointments and/or tests. It may enhance awareness regarding dietician services to patients if the current discharge instruction template can be modified to provide information regarding the outpatient dietitian class. This could include date, time, and location of classes as well as dietician contact information. (See Appendixes 1 and 2.)
When these recommendations have been implemented, further studies will be warranted to assess the impact of the interventions.
Acknowledgments
The authors thank Ms. Yvette Bloodson for her assistance in generating the initial patient list.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Roger VL, Go AS, Lloyd-Jones DM, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics—2011 Update. A Report from the American Heart Association. Circulation. 2011;123(4):e18-e209.
2. National Heart, Lung, and Blood Institute. Incidence and Prevalence: 2009 Chart Book on Cardiovascular and Lung Diseases. Bethesda, MD: National Institutes of Health; 2009.
3. Ross JS, Chen J, Lin ZQ, et al. Recent national trends in readmission rates after HF hospitalization. Circ Heart Fail. 2010;3(1):97-103.
4. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428.
5. Hunt SA, Abraham WT, Chin MH., et al. 2009 focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: Developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119(14):e391-e479.
6. Lindenfeld J, Albert NM, Boehmer JP, et al. Executive Summary: HFSA 2010 Comprehensive HF Practice Guideline. J Card Fail. 2010;16(6):475-539.
7. U.S. Department of Health and Human Services. Hospital Compare. https://data.medicare.gov/data/archives/hospital-compare. Updated August 22, 2011. Accessed October 15, 2014.
8. Heidenreich PA. Chronic HF QUERI Center Application: Strategic Plan 2009. U.S. Department of Veterans Affairs Quality Enhancement Research Initiative (QUERI) Website. http://www.queri.research.va.gov/about/strategic_plans/chf.pdf. Updated August 22, 2011. Accessed September 2, 2014.
9. U.S. Department of Veterans Affairs. Chronic HF Quality Enhancement Research Initiative: VA Hospital to Home (H2H) Initiative. U.S. Department of Veterans Affairs Quality Enhancement Research Initiative (QUERI) Website. http://www.queri.research.va.gov/chf/products/h2h. Updated August 19, 2011. Accessed September 2, 2014.
Heart failure (HF) continues to grow as a significant health problem in the U.S., accounting for 1.1 million hospitalizations annually.1 About 5.8 million Americans have HF, and 670,000 new cases are diagnosed each year.1 The prevalence of HF increases with age. Persons aged > 65 years comprise the largest group of patients hospitalized for the condition. Heart failure-related hospitalizations place a major financial burden on patients, caregivers, and the national health care system. In 2010, the estimated cost of health care for HF was $35 billion with hospitalizations accounting for 1% to 2% of the total annual health care costs.1-3 Furthermore, > 50% of patients with HF are rehospitalized before their first outpatient follow-up.4
To ensure patients are ready for discharge, HF guidelines recommend specific interventions for all hospitalized patients. These recommendations include successful transition from IV to oral diuretic therapy as well as the initiation of a ß-blocker and an angiotensin-converting enzyme inhibitor (ACE-I) or an angiotensin receptor blocker (ARB) in stable patients with a left ventricular ejection fraction (LVEF) < 40% and without contraindications. Additionally, patients and their caregivers should receive comprehensive discharge instructions regarding medications, the importance of adherence and regular follow-up, sodium and fluid restriction, weight monitoring, physical activity, and a plan for worsening symptoms. When available, assistance with the hospital-to-home transition should also be provided.5,6
Heart Failure Measures
Recognizing common factors essential to HF care, the Joint Commission has implemented HF core measures that all U.S. hospitals are required to meet to maintain accreditation status. These guideline-supported measures include receipt of diet, weight, and medication instructions; measured or scheduled assessment of LVEF; ACE-I or an ARB prescribed in patients with LVEF < 40%; and smoking cessation counseling before discharge for all patients with HF.
In addition to HF core measures, 30-day HF readmission rates have also become available to the general public as another hospital quality indicator. In 2009, the Centers for Medicare & Medicaid Services began publicly reporting 30-day HF readmission rates for Medicare patients. A CMS report indicated a 24.8% national 30-day HF readmission rate from July 1, 2007, through June 30, 2010.7 Unfortunately, even with the increased quality improvement effort, national HF rehospitalization rates have remained relatively steady in recent years.3
The VA health care system has a growing number of veterans with HF, and it is the leading discharge diagnosis in patients treated at VA hospitals. The number of HF-related hospitalizations at the VA health care system increased from just over 74,000 in fiscal year 2002 to 96,000 in 2009.8
To advance the care of veterans with HF and implement best practices, the VA launched the Chronic HF-Quality Enhancement Research Initiative (CHF-QUERI). The major goals of this initiative are to reduce hospitalization rates, increase use of life-prolonging care, empower patients and their caregivers in self-management, and improve appropriateness of HF therapies and tests. As part of its efforts, CHF-QUERI launched the HF Provider Network (HF Network), involving more than 712 VA health care providers (as of July 2014 there were more than 900 providers) committed to improving HF management throughout the entire VA health care system. The HF Network has already put into practice several quality improvement initiatives.
The National Hospital to Home initiative led by the American College of Cardiology and the Institute for Healthcare Improvement was launched throughout the VA system in January 2010.9 The main goal of this initiative is to reduce all-cause hospital readmission rates in patients with a discharge diagnosis of HF by improving medication management, early follow-up after discharge, and symptom management.
The Jesse Brown VAMC (JBVAMC) is an active participant of the Hospital to Home initiative, embracing the goals of reducing HF readmission rates and improving the transition of veterans from inpatient to outpatient care. The JBVAMC also has been successfully meeting or exceeding HF core measures except for providing discharge instructions. In May 2011, 91% of patients received discharge instructions, falling just slightly below the 93% target goal. Despite the implementation of HF care improvement initiatives and successful core measure performance, from July 1, 2007, to June 30, 2010, the average HF 30-day readmission rate at JBVAMC was reported to be 28.4%, compared with the national average of 24.8%. Additionally, the average readmission rate for fiscal year 2011 was 31% at JBVAMC, showing a further increase in readmission rates.
The cost of a hospital bed at JBVAMC ranges from about $2,000 to $5,000 per day. According to the American Heart Association’s Get With the Guidelines-HF registry, the mean hospital length of stay for HF in 2009 was 5.5 days.1 Consequently, HF hospitalizations could potentially cost JBVAMC nearly $7 million annually. Therefore, HF readmissions not only affect patients and caregivers, but also represent a financial burden for JBVAMC.
METHODS
The purpose of this study was to identify factors contributing to the high HF readmission rates in veterans enrolled at JBVAMC. This study was an Institutional Review Board and VA Research and Development Committee-approved retrospective, electronic chart review of patients with an ICD-9 principal discharge diagnosis code for HF and hospitalization for HF exacerbation anytime between October 1, 2010, and March 1, 2011. A patient chart was reviewed for 6 months after inclusion. A report was generated to identify patients discharged from JBVAMC with a principal discharge diagnosis of HF between October 1, 2010, and March 1, 2011, using the following ICD-9 HF codes: 402.01, 402.11, 402.91, 404.01, 404.03, 404.11, 404.13, 404.91, 404.93, 428.0, 428.1, 428.20, 428.21, 428.22, 428.23, 428.30, 428.31, 428.32, 428.33, 428.40, 428.41, 428.42, 428.43, and 428.9.
Patients were included if aged ≥ 18 years with one of the ICD-9 HF codes as the principal discharge diagnosis within the study period. Patients were excluded from the study if transferred to or from an outside hospital, discharged without an ICD-9 principal diagnosis code for HF, electively admitted for HF, not treated for HF during hospitalization, left the hospital against medical advice, had chart documentation with comfort measures only, were discharged/transferred to hospice, had active HF medications listed under non-VA medications in the electronic medication profile, or did not receive follow-up at JBVAMC. Study participants were included in the study once, which was classified as their index HF hospitalization, and were followed for 6 months thereafter.
The primary endpoint was the difference in patient characteristics between 2 groups of patients: those readmitted for HF within 30 days of the index hospitalization and those readmitted after 30 days or not at all.
The study had multiple secondary endpoints. One was the difference in patient characteristics between 2 groups of patients: those readmitted for HF within 90 days of the index hospitalization and those readmitted after 90 days or not at all. Another secondary endpoint was the difference in patient characteristics between 2 groups of patients: those with ≥ 2 readmissions for HF within 6 months and those with < 2 HF readmissions within 6 months. Additional secondary endpoints included percentage of patients readmitted for HF within 30 days of the index HF hospitalization, time to readmission if applicable, time to death if applicable, and average number of readmissions per patient within 6 months.
Index data collected included age, gender, ethnicity, prior diagnosis of HF, date of diagnosis, hospitalization for HF within 30 days of the index HF admission, in-hospital cardiac arrest, comorbid conditions, systolic blood pressure (BP), heart rate, respiratory rate, weight, serum sodium, blood urea nitrogen, serum creatinine, hematocrit, and glucose. For this study, comorbid conditions gathered were diabetes mellitus, coronary artery disease, prior percutaneous coronary intervention, aortic stenosis, stroke, chronic obstructive pulmonary disease, and dementia.
Medication profiles were reviewed at the time of admission to determine whether the patient was prescribed an ACE-I/ARB, ß-blocker, diuretic, hydralazine and isosorbide dinitrate, aldosterone antagonist, digoxin, NSAIDs, nonvasoselective calcium channel blocker, and an antiarrhytmic other than amiodarone and dofetilide. Hospitalization data included the most recent LVEF, the number of days on oral diuretic therapy after stopping IV diuretics, the number of days admitted, and documentation of an in-person inpatient dietitian consultation.
Data collected at discharge included diet/weight/medication instructions, weight, BP, American College of Cardiology/American Heart Association HF stage and New York Heart Association (NYHA) HF functional class, if documented. Discharge medication profiles were assessed for the number of medications (< 9 or ≥ 9), documentation of active prescriptions for an ACE-I/ARB and a ß-blocker (or contraindication documented), diuretic, hydralazine and isosorbide dinitrate, aldosterone antagonist, and digoxin. Other data collected were documentation of a scheduled follow-up appointment with primary care physician, urgent care, chronic HF (CHF) clinic, or cardiologist, and whether the patient was discharged to home, skilled nursing facility, shelter, or homeless. Additionally, if the patient was discharged on a diuretic, the dose was compared with the baseline diuretic. If the diuretic at discharge was different from the home diuretic, equivalent doses were used for comparison with that of the baseline diuretic.
Postdischarge data collection included telephone follow-up within 48 hours of discharge, medication compliance since the initial hospitalization, date of first outpatient follow-up after initial hospital discharge, enrollment in CHF clinic/CHF-PharmD/Care Coordination Home Telehealth (CCHT) program, outpatient dietitian consultations, and date of death if applicable. Medication adherence was defined as ≥ 80% of lowest percentage filled medication of all HF medications, determined by the refill history in the computerized patient record system (CPRS). First outpatient follow-up was defined as a visit in which HF was addressed in the assessment and plan.
If readmitted within the study period, data collection included the date of first nonelective hospital readmission for HF, BP, heart rate, weight, serum digoxin level, serum creatinine, serum potassium, and whether the patient was on a target dose of HF recommended medications (if LVEF < 40% and no contraindication). Heart failure recommended medications for which target doses are established include ACE-I/ARB and ß-blockers. For this study, target doses of ACE-Is were captopril 50 mg 3 times daily, enalapril 10 mg twice daily, fosinopril 40 mg daily, lisinopril 20 mg daily, ramipril 10 mg daily, and trandolapril 4 mg daily. Target doses for ARBs were candesartan 32 mg daily, losartan 50 mg daily, and valsartan 160 mg twice daily. ß-blocker target doses were bisoprolol 10 mg daily, carvedilol 25 mg twice daily (50 mg twice daily if patients’ weight was > 85 kg), and metoprolol succinate 200 mg daily.5,6 A statistical analysis was not performed on the data.
RESULTS
A total of 137 patient charts were reviewed, and 109 patients were included in the study. Patients were excluded if they transferred to or from an outside hospital (n = 8), had no follow-up at JBVAMC (n = 8), left the hospital against medical advice (n = 4), were electively admitted (n = 4), were not treated for HF (n = 3), or only had comfort measures documented in the chart (n = 1). The patients included were predominantly male (99%) and African American (78%) and had a mean age of 70 years. The majority of the patients had a prior diagnosis of HF (87%) and a history of systolic HF (58%). Most patients were previously prescribed an ACE-I/ARB (83%) and a ß-blocker (76%) at the time of admission (Table 1).
Six patients were readmitted within 30 days of the index hospitalization, whereas 103 patients were readmitted after 30 days or not at all. With respect to secondary endpoints, there were 21 patients readmitted within 90 days of the index hospitalization, whereas 88 patients were readmitted after 90 days or not at all. Additionally, 6 patients were readmitted ≥ 2 times within 6 months of the index hospitalization, whereas 103 patients were readmitted < 2 times within 6 months.
Baseline characteristics seemed similar across the study groups, except a greater percentage of patients readmitted within 30 days of the index HF hospitalization had a prior history of systolic HF and were hospitalized for HF 30 days prior to the index hospitalization (Table 2). In addition, patients readmitted within 30 days tended to receive a shorter duration of oral diuretic therapy after discontinuation of IV diuretics (mean 0.2 days vs 1.1 days). Patients in this group with an LVEF < 40% were less likely to be discharged on an ACE-I/ARB (75% vs 95%) and a ß-blocker (50% vs 85%) than were the patients who were readmitted after 30 days or not at all. These trends continued for patients readmitted within 90 days of the index hospitalization and for those readmitted after 90 days or not at all. The mean length of stay for the index HF hospitalization was about 5 days and was comparable among all study groups.
From the evaluation of postdischarge characteristics, no patients readmitted within 30 days had a follow-up appointment scheduled with the CHF clinic. In comparison with patients readmitted after 30 days or not at all, more patients had follow-up at an urgent care clinic (33% vs 6%) or no follow-up appointment scheduled at the time of discharge (17% vs 2%). Half of all the patients with a scheduled follow-up missed their appointment. Additionally, medication adherence was lower (33% vs 80%), and none of the patients were enrolled in the CHF-PharmD clinic (0% vs 5%). A similar trend continued for the secondary endpoint groups (Table 3). Last, none of the study patients had an outpatient dietitian consultation.
On readmission, the majority of patients readmitted within 30 days were not on a target dose of an ACE-I/ARB (75%), and none were on a target dose of a ß-blocker. The same trend continued for the secondary endpoint groups. None of the study patients had a serum digoxin level > 0.9 ng/mL. However, serum digoxin level was not measured in all readmitted patients prescribed digoxin (Table 4).
In regard to other secondary endpoints, 6 patients (5.5%) were readmitted for HF within 30 days of the index HF hospitalization. The average number of readmissions per patient in 6 months was < 1, mean time to readmission was 85 days (n = 33), and mean time to death was 88 days (n = 5) when applicable.
DISCUSSION
Based on the trends observed in this study, multiple recommendations can be made to further improve the quality of care and reduce HF readmissions at JBVAMC. The medical center physicians currently use a discharge note template, which already includes sections such as HF discharge instructions and follow-up appointments. The template also prompts providers to prescribe an ACE-I in appropriate patients.
When JBVAMC providers are ready to enter discharge notes into the CPRS, they first select the discharge note template from available note template options. The electronic template contains spaces for the provider to enter a patient’s primary reason for hospitalization, date of admission, discharge medication list, specific or suggested dates for follow-up with outpatient provider(s), general diet/weight/medication instructions, a space to answer whether the patient has HF, a space to record NYHA HF class if applicable, and a space to record whether the patient is prescribed or will be prescribed an ACE-I if appropriate, or whether ACE-I is contraindicated. The providers are able to modify and add information to the discharge note template as they see appropriate.
The findings of this study suggest that modifying the existing discharge template to include additional provider prompts in a form of designated spaces asking for specific information may help improve HF care outcomes. If providers are prompted to answer whether an oral diuretic was continued for at least 24 hours after stopping IV diuretics for HF, adherence to the HF guideline-recommended duration of oral diuretic therapy may improve. Additionally, ß-blocker prescribing in appropriate systolic HF patients may increase if providers are prompted. To enhance continuity of care, the discharge note template may be modified to include a section in which the providers can document patients followed by outside providers. This can be done by incorporating a space in the discharge template to enter the patient’s non-VA provider information if applicable and may help further coordinate the care of such patients to ensure that they are not lost.
Furthermore, the discharge template may be modified to include a prompt to place a CHF clinic consult to increase provider awareness about the availability of CHF and CHF-PharmD clinics at JBVAMC. CHF and CHF-PharmD clinics collaborate to provide comprehensive care to HF patients. After an initial evaluation at the CHF clinic, patients are referred to the clinical pharmacist for further medication therapy management when necessary. Currently, the physicians are encouraged to refer HF patients to the CHF clinic after discharge, but not all providers know that such a service is available. The prompt within the discharge note template would provide CHF/CHF-PharmD clinic provider contact information, clinic times, and a link that would take the provider to an appropriate screen for placing the consult.
Limitations
There are several limitations to this study, including its retrospective design and small sample size. Another source of potential study limitation was the initial process for creating a study patient list. The study list was designed to use ICD-9 codes to capture readmissions only for HF and only at JBVAMC. This was achieved by specifying any of the HF ICD-9 codes as the principal discharge diagnosis. However, the providers may not have always used a HF specific ICD-9 code for the principal discharge diagnosis, even if a patient was admitted primarily for HF. The provider may have chosen another principal discharge diagnosis for which the patient received treatment during the hospitalization.
There are multiple ways to obtain HF patient lists, one includes using the diagnosis-related group codes instead of ICD-9 codes. Due to the way the patient list was obtained and an inherent possibility that some patients admitted for HF had a non-HF ICD-9 code recorded as their principal discharge diagnosis, some eligible patients may not have appeared on the generated list. Additionally, this study captured readmission rates for only HF whereas the national HF 30-day readmission rate represents all-cause readmissions for HF patients. This difference may be reflected in the low 30-day readmission rate observed.
Another possible limitation was the timing of the launch of the CHF-PharmD clinic and the initiative for telephone follow-up 48 hours postdischarge. The CHF-PharmD clinic was launched in April 2011, and the initiative for telephone follow-up 48 hours postdischarge began in January 2011. As the start dates fell within the study period, these services may not have been available to all patients. Therefore, the data describing patient enrollment in CHF-PharmD clinic and those who received postdischarge telephone follow-up may not accurately reflect current practice. Last, statistical tests were not used in the study data analysis leaving any differences found open to interpretation. To minimize these limitations, larger prospective studies with statistical analysis capturing all-cause readmissions are necessary to further evaluate patient characteristics that may be contributing to HF readmissions at JBVAMC.
Conclusions
In general, earlier and more frequent readmissions were more common in patients who were converted to oral diuretic therapy for < 24 hours before discharge and were not discharged on an ACE-I/ARB and a b-blocker when appropriate. Additionally, most of the readmitted patients had no follow-up scheduled at discharge, were nonadherent with medications and follow-up appointments, and were not enrolled in the CHF and/or CHF-PharmD clinic. The majority of patients with systolic HF were not at target doses of either the ACE-I/ARB or the ß-blocker when readmitted. Overall, JBVAMC had a low percentage of patients readmitted for HF within 30 days, but there is still room for improvement in reducing HF readmissions.
At the time of discharge, all JBVAMC patients receive printed instructions and recommendations for their care after hospitalization. The patient handout includes the most current medications, diet/weight/medication instructions, and actual or suggested dates for follow-up appointments and/or tests. It may enhance awareness regarding dietician services to patients if the current discharge instruction template can be modified to provide information regarding the outpatient dietitian class. This could include date, time, and location of classes as well as dietician contact information. (See Appendixes 1 and 2.)
When these recommendations have been implemented, further studies will be warranted to assess the impact of the interventions.
Acknowledgments
The authors thank Ms. Yvette Bloodson for her assistance in generating the initial patient list.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Heart failure (HF) continues to grow as a significant health problem in the U.S., accounting for 1.1 million hospitalizations annually.1 About 5.8 million Americans have HF, and 670,000 new cases are diagnosed each year.1 The prevalence of HF increases with age. Persons aged > 65 years comprise the largest group of patients hospitalized for the condition. Heart failure-related hospitalizations place a major financial burden on patients, caregivers, and the national health care system. In 2010, the estimated cost of health care for HF was $35 billion with hospitalizations accounting for 1% to 2% of the total annual health care costs.1-3 Furthermore, > 50% of patients with HF are rehospitalized before their first outpatient follow-up.4
To ensure patients are ready for discharge, HF guidelines recommend specific interventions for all hospitalized patients. These recommendations include successful transition from IV to oral diuretic therapy as well as the initiation of a ß-blocker and an angiotensin-converting enzyme inhibitor (ACE-I) or an angiotensin receptor blocker (ARB) in stable patients with a left ventricular ejection fraction (LVEF) < 40% and without contraindications. Additionally, patients and their caregivers should receive comprehensive discharge instructions regarding medications, the importance of adherence and regular follow-up, sodium and fluid restriction, weight monitoring, physical activity, and a plan for worsening symptoms. When available, assistance with the hospital-to-home transition should also be provided.5,6
Heart Failure Measures
Recognizing common factors essential to HF care, the Joint Commission has implemented HF core measures that all U.S. hospitals are required to meet to maintain accreditation status. These guideline-supported measures include receipt of diet, weight, and medication instructions; measured or scheduled assessment of LVEF; ACE-I or an ARB prescribed in patients with LVEF < 40%; and smoking cessation counseling before discharge for all patients with HF.
In addition to HF core measures, 30-day HF readmission rates have also become available to the general public as another hospital quality indicator. In 2009, the Centers for Medicare & Medicaid Services began publicly reporting 30-day HF readmission rates for Medicare patients. A CMS report indicated a 24.8% national 30-day HF readmission rate from July 1, 2007, through June 30, 2010.7 Unfortunately, even with the increased quality improvement effort, national HF rehospitalization rates have remained relatively steady in recent years.3
The VA health care system has a growing number of veterans with HF, and it is the leading discharge diagnosis in patients treated at VA hospitals. The number of HF-related hospitalizations at the VA health care system increased from just over 74,000 in fiscal year 2002 to 96,000 in 2009.8
To advance the care of veterans with HF and implement best practices, the VA launched the Chronic HF-Quality Enhancement Research Initiative (CHF-QUERI). The major goals of this initiative are to reduce hospitalization rates, increase use of life-prolonging care, empower patients and their caregivers in self-management, and improve appropriateness of HF therapies and tests. As part of its efforts, CHF-QUERI launched the HF Provider Network (HF Network), involving more than 712 VA health care providers (as of July 2014 there were more than 900 providers) committed to improving HF management throughout the entire VA health care system. The HF Network has already put into practice several quality improvement initiatives.
The National Hospital to Home initiative led by the American College of Cardiology and the Institute for Healthcare Improvement was launched throughout the VA system in January 2010.9 The main goal of this initiative is to reduce all-cause hospital readmission rates in patients with a discharge diagnosis of HF by improving medication management, early follow-up after discharge, and symptom management.
The Jesse Brown VAMC (JBVAMC) is an active participant of the Hospital to Home initiative, embracing the goals of reducing HF readmission rates and improving the transition of veterans from inpatient to outpatient care. The JBVAMC also has been successfully meeting or exceeding HF core measures except for providing discharge instructions. In May 2011, 91% of patients received discharge instructions, falling just slightly below the 93% target goal. Despite the implementation of HF care improvement initiatives and successful core measure performance, from July 1, 2007, to June 30, 2010, the average HF 30-day readmission rate at JBVAMC was reported to be 28.4%, compared with the national average of 24.8%. Additionally, the average readmission rate for fiscal year 2011 was 31% at JBVAMC, showing a further increase in readmission rates.
The cost of a hospital bed at JBVAMC ranges from about $2,000 to $5,000 per day. According to the American Heart Association’s Get With the Guidelines-HF registry, the mean hospital length of stay for HF in 2009 was 5.5 days.1 Consequently, HF hospitalizations could potentially cost JBVAMC nearly $7 million annually. Therefore, HF readmissions not only affect patients and caregivers, but also represent a financial burden for JBVAMC.
METHODS
The purpose of this study was to identify factors contributing to the high HF readmission rates in veterans enrolled at JBVAMC. This study was an Institutional Review Board and VA Research and Development Committee-approved retrospective, electronic chart review of patients with an ICD-9 principal discharge diagnosis code for HF and hospitalization for HF exacerbation anytime between October 1, 2010, and March 1, 2011. A patient chart was reviewed for 6 months after inclusion. A report was generated to identify patients discharged from JBVAMC with a principal discharge diagnosis of HF between October 1, 2010, and March 1, 2011, using the following ICD-9 HF codes: 402.01, 402.11, 402.91, 404.01, 404.03, 404.11, 404.13, 404.91, 404.93, 428.0, 428.1, 428.20, 428.21, 428.22, 428.23, 428.30, 428.31, 428.32, 428.33, 428.40, 428.41, 428.42, 428.43, and 428.9.
Patients were included if aged ≥ 18 years with one of the ICD-9 HF codes as the principal discharge diagnosis within the study period. Patients were excluded from the study if transferred to or from an outside hospital, discharged without an ICD-9 principal diagnosis code for HF, electively admitted for HF, not treated for HF during hospitalization, left the hospital against medical advice, had chart documentation with comfort measures only, were discharged/transferred to hospice, had active HF medications listed under non-VA medications in the electronic medication profile, or did not receive follow-up at JBVAMC. Study participants were included in the study once, which was classified as their index HF hospitalization, and were followed for 6 months thereafter.
The primary endpoint was the difference in patient characteristics between 2 groups of patients: those readmitted for HF within 30 days of the index hospitalization and those readmitted after 30 days or not at all.
The study had multiple secondary endpoints. One was the difference in patient characteristics between 2 groups of patients: those readmitted for HF within 90 days of the index hospitalization and those readmitted after 90 days or not at all. Another secondary endpoint was the difference in patient characteristics between 2 groups of patients: those with ≥ 2 readmissions for HF within 6 months and those with < 2 HF readmissions within 6 months. Additional secondary endpoints included percentage of patients readmitted for HF within 30 days of the index HF hospitalization, time to readmission if applicable, time to death if applicable, and average number of readmissions per patient within 6 months.
Index data collected included age, gender, ethnicity, prior diagnosis of HF, date of diagnosis, hospitalization for HF within 30 days of the index HF admission, in-hospital cardiac arrest, comorbid conditions, systolic blood pressure (BP), heart rate, respiratory rate, weight, serum sodium, blood urea nitrogen, serum creatinine, hematocrit, and glucose. For this study, comorbid conditions gathered were diabetes mellitus, coronary artery disease, prior percutaneous coronary intervention, aortic stenosis, stroke, chronic obstructive pulmonary disease, and dementia.
Medication profiles were reviewed at the time of admission to determine whether the patient was prescribed an ACE-I/ARB, ß-blocker, diuretic, hydralazine and isosorbide dinitrate, aldosterone antagonist, digoxin, NSAIDs, nonvasoselective calcium channel blocker, and an antiarrhytmic other than amiodarone and dofetilide. Hospitalization data included the most recent LVEF, the number of days on oral diuretic therapy after stopping IV diuretics, the number of days admitted, and documentation of an in-person inpatient dietitian consultation.
Data collected at discharge included diet/weight/medication instructions, weight, BP, American College of Cardiology/American Heart Association HF stage and New York Heart Association (NYHA) HF functional class, if documented. Discharge medication profiles were assessed for the number of medications (< 9 or ≥ 9), documentation of active prescriptions for an ACE-I/ARB and a ß-blocker (or contraindication documented), diuretic, hydralazine and isosorbide dinitrate, aldosterone antagonist, and digoxin. Other data collected were documentation of a scheduled follow-up appointment with primary care physician, urgent care, chronic HF (CHF) clinic, or cardiologist, and whether the patient was discharged to home, skilled nursing facility, shelter, or homeless. Additionally, if the patient was discharged on a diuretic, the dose was compared with the baseline diuretic. If the diuretic at discharge was different from the home diuretic, equivalent doses were used for comparison with that of the baseline diuretic.
Postdischarge data collection included telephone follow-up within 48 hours of discharge, medication compliance since the initial hospitalization, date of first outpatient follow-up after initial hospital discharge, enrollment in CHF clinic/CHF-PharmD/Care Coordination Home Telehealth (CCHT) program, outpatient dietitian consultations, and date of death if applicable. Medication adherence was defined as ≥ 80% of lowest percentage filled medication of all HF medications, determined by the refill history in the computerized patient record system (CPRS). First outpatient follow-up was defined as a visit in which HF was addressed in the assessment and plan.
If readmitted within the study period, data collection included the date of first nonelective hospital readmission for HF, BP, heart rate, weight, serum digoxin level, serum creatinine, serum potassium, and whether the patient was on a target dose of HF recommended medications (if LVEF < 40% and no contraindication). Heart failure recommended medications for which target doses are established include ACE-I/ARB and ß-blockers. For this study, target doses of ACE-Is were captopril 50 mg 3 times daily, enalapril 10 mg twice daily, fosinopril 40 mg daily, lisinopril 20 mg daily, ramipril 10 mg daily, and trandolapril 4 mg daily. Target doses for ARBs were candesartan 32 mg daily, losartan 50 mg daily, and valsartan 160 mg twice daily. ß-blocker target doses were bisoprolol 10 mg daily, carvedilol 25 mg twice daily (50 mg twice daily if patients’ weight was > 85 kg), and metoprolol succinate 200 mg daily.5,6 A statistical analysis was not performed on the data.
RESULTS
A total of 137 patient charts were reviewed, and 109 patients were included in the study. Patients were excluded if they transferred to or from an outside hospital (n = 8), had no follow-up at JBVAMC (n = 8), left the hospital against medical advice (n = 4), were electively admitted (n = 4), were not treated for HF (n = 3), or only had comfort measures documented in the chart (n = 1). The patients included were predominantly male (99%) and African American (78%) and had a mean age of 70 years. The majority of the patients had a prior diagnosis of HF (87%) and a history of systolic HF (58%). Most patients were previously prescribed an ACE-I/ARB (83%) and a ß-blocker (76%) at the time of admission (Table 1).
Six patients were readmitted within 30 days of the index hospitalization, whereas 103 patients were readmitted after 30 days or not at all. With respect to secondary endpoints, there were 21 patients readmitted within 90 days of the index hospitalization, whereas 88 patients were readmitted after 90 days or not at all. Additionally, 6 patients were readmitted ≥ 2 times within 6 months of the index hospitalization, whereas 103 patients were readmitted < 2 times within 6 months.
Baseline characteristics seemed similar across the study groups, except a greater percentage of patients readmitted within 30 days of the index HF hospitalization had a prior history of systolic HF and were hospitalized for HF 30 days prior to the index hospitalization (Table 2). In addition, patients readmitted within 30 days tended to receive a shorter duration of oral diuretic therapy after discontinuation of IV diuretics (mean 0.2 days vs 1.1 days). Patients in this group with an LVEF < 40% were less likely to be discharged on an ACE-I/ARB (75% vs 95%) and a ß-blocker (50% vs 85%) than were the patients who were readmitted after 30 days or not at all. These trends continued for patients readmitted within 90 days of the index hospitalization and for those readmitted after 90 days or not at all. The mean length of stay for the index HF hospitalization was about 5 days and was comparable among all study groups.
From the evaluation of postdischarge characteristics, no patients readmitted within 30 days had a follow-up appointment scheduled with the CHF clinic. In comparison with patients readmitted after 30 days or not at all, more patients had follow-up at an urgent care clinic (33% vs 6%) or no follow-up appointment scheduled at the time of discharge (17% vs 2%). Half of all the patients with a scheduled follow-up missed their appointment. Additionally, medication adherence was lower (33% vs 80%), and none of the patients were enrolled in the CHF-PharmD clinic (0% vs 5%). A similar trend continued for the secondary endpoint groups (Table 3). Last, none of the study patients had an outpatient dietitian consultation.
On readmission, the majority of patients readmitted within 30 days were not on a target dose of an ACE-I/ARB (75%), and none were on a target dose of a ß-blocker. The same trend continued for the secondary endpoint groups. None of the study patients had a serum digoxin level > 0.9 ng/mL. However, serum digoxin level was not measured in all readmitted patients prescribed digoxin (Table 4).
In regard to other secondary endpoints, 6 patients (5.5%) were readmitted for HF within 30 days of the index HF hospitalization. The average number of readmissions per patient in 6 months was < 1, mean time to readmission was 85 days (n = 33), and mean time to death was 88 days (n = 5) when applicable.
DISCUSSION
Based on the trends observed in this study, multiple recommendations can be made to further improve the quality of care and reduce HF readmissions at JBVAMC. The medical center physicians currently use a discharge note template, which already includes sections such as HF discharge instructions and follow-up appointments. The template also prompts providers to prescribe an ACE-I in appropriate patients.
When JBVAMC providers are ready to enter discharge notes into the CPRS, they first select the discharge note template from available note template options. The electronic template contains spaces for the provider to enter a patient’s primary reason for hospitalization, date of admission, discharge medication list, specific or suggested dates for follow-up with outpatient provider(s), general diet/weight/medication instructions, a space to answer whether the patient has HF, a space to record NYHA HF class if applicable, and a space to record whether the patient is prescribed or will be prescribed an ACE-I if appropriate, or whether ACE-I is contraindicated. The providers are able to modify and add information to the discharge note template as they see appropriate.
The findings of this study suggest that modifying the existing discharge template to include additional provider prompts in a form of designated spaces asking for specific information may help improve HF care outcomes. If providers are prompted to answer whether an oral diuretic was continued for at least 24 hours after stopping IV diuretics for HF, adherence to the HF guideline-recommended duration of oral diuretic therapy may improve. Additionally, ß-blocker prescribing in appropriate systolic HF patients may increase if providers are prompted. To enhance continuity of care, the discharge note template may be modified to include a section in which the providers can document patients followed by outside providers. This can be done by incorporating a space in the discharge template to enter the patient’s non-VA provider information if applicable and may help further coordinate the care of such patients to ensure that they are not lost.
Furthermore, the discharge template may be modified to include a prompt to place a CHF clinic consult to increase provider awareness about the availability of CHF and CHF-PharmD clinics at JBVAMC. CHF and CHF-PharmD clinics collaborate to provide comprehensive care to HF patients. After an initial evaluation at the CHF clinic, patients are referred to the clinical pharmacist for further medication therapy management when necessary. Currently, the physicians are encouraged to refer HF patients to the CHF clinic after discharge, but not all providers know that such a service is available. The prompt within the discharge note template would provide CHF/CHF-PharmD clinic provider contact information, clinic times, and a link that would take the provider to an appropriate screen for placing the consult.
Limitations
There are several limitations to this study, including its retrospective design and small sample size. Another source of potential study limitation was the initial process for creating a study patient list. The study list was designed to use ICD-9 codes to capture readmissions only for HF and only at JBVAMC. This was achieved by specifying any of the HF ICD-9 codes as the principal discharge diagnosis. However, the providers may not have always used a HF specific ICD-9 code for the principal discharge diagnosis, even if a patient was admitted primarily for HF. The provider may have chosen another principal discharge diagnosis for which the patient received treatment during the hospitalization.
There are multiple ways to obtain HF patient lists, one includes using the diagnosis-related group codes instead of ICD-9 codes. Due to the way the patient list was obtained and an inherent possibility that some patients admitted for HF had a non-HF ICD-9 code recorded as their principal discharge diagnosis, some eligible patients may not have appeared on the generated list. Additionally, this study captured readmission rates for only HF whereas the national HF 30-day readmission rate represents all-cause readmissions for HF patients. This difference may be reflected in the low 30-day readmission rate observed.
Another possible limitation was the timing of the launch of the CHF-PharmD clinic and the initiative for telephone follow-up 48 hours postdischarge. The CHF-PharmD clinic was launched in April 2011, and the initiative for telephone follow-up 48 hours postdischarge began in January 2011. As the start dates fell within the study period, these services may not have been available to all patients. Therefore, the data describing patient enrollment in CHF-PharmD clinic and those who received postdischarge telephone follow-up may not accurately reflect current practice. Last, statistical tests were not used in the study data analysis leaving any differences found open to interpretation. To minimize these limitations, larger prospective studies with statistical analysis capturing all-cause readmissions are necessary to further evaluate patient characteristics that may be contributing to HF readmissions at JBVAMC.
Conclusions
In general, earlier and more frequent readmissions were more common in patients who were converted to oral diuretic therapy for < 24 hours before discharge and were not discharged on an ACE-I/ARB and a b-blocker when appropriate. Additionally, most of the readmitted patients had no follow-up scheduled at discharge, were nonadherent with medications and follow-up appointments, and were not enrolled in the CHF and/or CHF-PharmD clinic. The majority of patients with systolic HF were not at target doses of either the ACE-I/ARB or the ß-blocker when readmitted. Overall, JBVAMC had a low percentage of patients readmitted for HF within 30 days, but there is still room for improvement in reducing HF readmissions.
At the time of discharge, all JBVAMC patients receive printed instructions and recommendations for their care after hospitalization. The patient handout includes the most current medications, diet/weight/medication instructions, and actual or suggested dates for follow-up appointments and/or tests. It may enhance awareness regarding dietician services to patients if the current discharge instruction template can be modified to provide information regarding the outpatient dietitian class. This could include date, time, and location of classes as well as dietician contact information. (See Appendixes 1 and 2.)
When these recommendations have been implemented, further studies will be warranted to assess the impact of the interventions.
Acknowledgments
The authors thank Ms. Yvette Bloodson for her assistance in generating the initial patient list.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Roger VL, Go AS, Lloyd-Jones DM, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics—2011 Update. A Report from the American Heart Association. Circulation. 2011;123(4):e18-e209.
2. National Heart, Lung, and Blood Institute. Incidence and Prevalence: 2009 Chart Book on Cardiovascular and Lung Diseases. Bethesda, MD: National Institutes of Health; 2009.
3. Ross JS, Chen J, Lin ZQ, et al. Recent national trends in readmission rates after HF hospitalization. Circ Heart Fail. 2010;3(1):97-103.
4. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428.
5. Hunt SA, Abraham WT, Chin MH., et al. 2009 focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: Developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119(14):e391-e479.
6. Lindenfeld J, Albert NM, Boehmer JP, et al. Executive Summary: HFSA 2010 Comprehensive HF Practice Guideline. J Card Fail. 2010;16(6):475-539.
7. U.S. Department of Health and Human Services. Hospital Compare. https://data.medicare.gov/data/archives/hospital-compare. Updated August 22, 2011. Accessed October 15, 2014.
8. Heidenreich PA. Chronic HF QUERI Center Application: Strategic Plan 2009. U.S. Department of Veterans Affairs Quality Enhancement Research Initiative (QUERI) Website. http://www.queri.research.va.gov/about/strategic_plans/chf.pdf. Updated August 22, 2011. Accessed September 2, 2014.
9. U.S. Department of Veterans Affairs. Chronic HF Quality Enhancement Research Initiative: VA Hospital to Home (H2H) Initiative. U.S. Department of Veterans Affairs Quality Enhancement Research Initiative (QUERI) Website. http://www.queri.research.va.gov/chf/products/h2h. Updated August 19, 2011. Accessed September 2, 2014.
1. Roger VL, Go AS, Lloyd-Jones DM, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics—2011 Update. A Report from the American Heart Association. Circulation. 2011;123(4):e18-e209.
2. National Heart, Lung, and Blood Institute. Incidence and Prevalence: 2009 Chart Book on Cardiovascular and Lung Diseases. Bethesda, MD: National Institutes of Health; 2009.
3. Ross JS, Chen J, Lin ZQ, et al. Recent national trends in readmission rates after HF hospitalization. Circ Heart Fail. 2010;3(1):97-103.
4. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428.
5. Hunt SA, Abraham WT, Chin MH., et al. 2009 focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: Developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119(14):e391-e479.
6. Lindenfeld J, Albert NM, Boehmer JP, et al. Executive Summary: HFSA 2010 Comprehensive HF Practice Guideline. J Card Fail. 2010;16(6):475-539.
7. U.S. Department of Health and Human Services. Hospital Compare. https://data.medicare.gov/data/archives/hospital-compare. Updated August 22, 2011. Accessed October 15, 2014.
8. Heidenreich PA. Chronic HF QUERI Center Application: Strategic Plan 2009. U.S. Department of Veterans Affairs Quality Enhancement Research Initiative (QUERI) Website. http://www.queri.research.va.gov/about/strategic_plans/chf.pdf. Updated August 22, 2011. Accessed September 2, 2014.
9. U.S. Department of Veterans Affairs. Chronic HF Quality Enhancement Research Initiative: VA Hospital to Home (H2H) Initiative. U.S. Department of Veterans Affairs Quality Enhancement Research Initiative (QUERI) Website. http://www.queri.research.va.gov/chf/products/h2h. Updated August 19, 2011. Accessed September 2, 2014.
Veterans’ Use of Designer Cathinones and Cannabinoids
Although the elevated risks and rates of veterans’ substance abuse patterns are well documented, little has been written about veterans’ use of designer drugs.1-6 In recent months throughout Europe and the U.S., there has been a flurry of media attention for 2 classes of designer drugs: synthetic cathinones and synthetic cannabinoids.7,8 In the U.S., the popularity of these drugs has surged, and a disproportionate amount of use of these 2 drug classes is coming from locations near military instillations.9,10
The purpose of this article is to raise awareness regarding these 2 burgeoning designer drug classes and their impact on veterans. Designer drugs affecting vulnerable populations are not a new phenomenon, yet many providers are unfamiliar with the effects of these unique drugs of abuse on their veteran populations.11-13
Many designer drugs begin their existence as variations of other addictive or psychoactive drugs. Others begin in laboratories as investigative research compounds that end up on the street, often promising a novel mind-altering experience as a “legal high.”14-18 The Designer Drug Enforcement Act of 1986 was an initial attempt in the U.S. to define and control the early rise of copycat drugs that appeared on the streets and mimicked the effects of other illicit substances. More recent legislation enacted in the U.S. has imposed Schedule I controls on the manufacture, distribution, possession, importation, and exportation of these types of drugs, including both synthetic cathinones and synthetic cannabinoids. State laws are perennially in flux trying to keep up with the latest drug trends.19-21
Similar efforts have been made by the European Union to control mephedrone, a synthetic cathinone, citing multiple fatalities, seizures, related crime, lack of medical use, and risk of dependence.22 Although uniform levels of control do not exist in Europe for synthetic cannabinoids, many countries have independently acted to limit their use.23
In its recent World Drug Report 2013, the United Nations Office on Drugs and Crime documents its growing concern about the “new psychoactive substances” category of illicit recreational substances (in which synthetic cannabinoids and cathinones are included) that has increased by 50% since 2009.24 Alone, this category now outnumbers the total number of substances controlled by international drug conventions.
The novelty and variability of designer drugs causes difficulties with detection and regulation. Innovative chemists can legally manufacture new versions of known molecules intended for illicit use with a rapidity that outpaces bureaucratic control. Local law enforcement officials may be unaware of the latest designer drug trends, stifling efforts at public education or restriction. Designer drugs are often deceptively packaged and are available in convenience stores, tobacco outlets, gas stations, pawnshops, tattoo parlors, and truck stops.25-28 The Internet may be the singular reason, however, that designer drugs continue to be widely available to veterans.11,18
Innumerable websites discuss, promote, and sell designer drugs or deceitfully market them as safe, legitimate household products (“not for human consumption”), which can be ordered online and shipped by commercial carriers.12 Little accurate information is known about their effects or about the specific compounds they contain. When the recreational nature of the drugs is actually acknowledged, information on how the buyer can evade prosecution is often provided in tandem. The suppliers’ inventory of the drugs has been shown to be variable and inconsistent, and the product ingredients can be similarly unpredictable despite comparatively more stable naming and labeling.14,29
In the clinical setting, a reliable patient drug history may not be available. This ensures that the diagnosis of designer drug use will be an exclusionary process involving routine laboratory work, physical examination, and at times electroencephalogram and/or neuroimaging. Psychiatric consultation is often useful in this setting. Routine immunoassay tests do not detect either synthetic cathinones or synthetic cannabinoids.30
Both cannabinoids and cathinones can be identified using gas chromatography-mass spectroscopy (GC-MS) or liquid chromatography-mass spectroscopy (LC-MS). However, this technology is limited to specialized laboratories.31,32 The laboratory results often are not immediately available, potentially limiting the tests’ use in emergency or inpatient settings, as the patient may have left the hospital by the time the results are available. Additionally, these drugs’ prevalence of use, while increasing, often does not justify the cost of these tests.
The inability to routinely detect metabolites in urine may increase the enticement of these drugs given the likelihood that active-duty personnel could use them surreptitiously. Further, these compounds are evolving and seemingly limitless in their variability, and there is often a paucity of pure reference materials. As such, it is impossible to guarantee reliable test results.
The following profiles of each of these drug classes will be accompanied by clinical cases depicting the drugs’ effects and how an affected veteran might present clinically. The severe effects of these novel agents illustrate the value in maintaining a functional knowledge base about emerging drug trends. The accuracy of diagnosis as well as the outcome of a veteran’s treatment may depend on the provider’s ability to identify the presence of a drug and manage its effects.
Synthetic Cathinones
Mr. H is a 28-year-old Iraq War veteran with a history of posttraumatic stress disorder (PTSD), alcohol abuse, and opioid dependence who presented for inpatient psychiatric admission after making suicidal statements to his wife in the context of 2 weeks of “bath salts” use. A family member initially introduced him to the drug. His first drug purchase had been 1 gram ($30) at a local movie rental store.
After discharge from the hospital, Mr. H began purchasing increasing amounts online with a credit card. Although he initially had been insufflating and inhaling the substance, he later began injecting it (dissolving it in tap water and loading it through a cotton filter in a syringe). The patient admitted to finding the drug significantly more addictive than any others he had used, and his use resulted in leaving his job and abandoning his family.
Severe cravings and depression were present between episodes of use. He spent $40,000 over 6 months of use. Insomnia lasted for several days, his appearance changed dramatically (including persistent skin infections), and he became paranoid, believing that everyone around him was an undercover police officer. He remained on medications for persistent anxiety. His daily drug cravings continued,
although he remained uncertain about the actual ingredients of bath salts.
Cathinone is a naturally occurring stimulant from the khat plant (Catha edulis), which grows indigenously in Egypt and on the Arabian Peninsula. The recreational and religious use of this plant has occurred for thousands of years, though it is not without risk: The chewing of the leaves containing natural cathinones has been associated with esophagitis, gastritis, oral keratosis, myocardial infarction, dilated cardiomyopathy, hypertension, cerebral ischemia, thromboembolism, diabetes, sexual dysfunction, duodenal ulcer, and hepatitis.33,34
The stimulants known colloquially as bath salts are synthetic cathinones, which have become more widely available within the past 10 years: first in the Middle East, then Europe, and now in the U.S.5,9,10,14,19,25,35-41,44 Although the current rise in use has occurred in the past few years, the first documented abuse of synthetic cathinones in the U.S. dates to the early 1990s in Michigan.42
Bath salts is the most common of the many names used to denote synthetic cathinones. The compounds have no utility when used as such but often are marketed as research chemicals, plant fertilizer, or shoe polish. It is this deliberate counterfeit of household product names that allows many distributors to avoid classifying the compounds according to the true, intended use. More appealing brand names may also be used to entice the user (Table 1).25
Synthetic cathinones owe their popularity to similarities with cocaine and methamphetamine. They are sympathomimetic with synaptic increases of monoamines after use: Surges in norepinephrine and dopamine account for the stimulant qualities, and serotonergic changes mediate distinct psychoactive effects (Table 2).40 Users are interested in the drugs for many of the same reasons that other recreational stimulants have appeal: euphoria, energy, empathy, heightened sexuality, sociability, and an overall intensification of senses. Synthetic cathinones have become preferred to cocaine for some users.43
The drugs can be used via oral and anal routes. Using methods known as “bombing” or “keystering,” users deliver boluses of the powder wrapped in cigarette paper, which they swallow or insert into the rectum. Insufflation and IV injection are also common methods of administration with a quicker onset of action expected.40 The prices of the drugs range from $25 to $50 per 500-mg packet (though the cost is increasing with more regulation). Users typically use 500 mg to 2 g in one session.
The 2 most commonly abused synthetic cathinones are mephedrone and MDPV (methylenedioxypyrovalerone). There is some regional variability about which ingredient is present; mephedrone tends to be more prevalent in Europe, whereas MDPV is noted to be more common in the U.S.10,44
When ordering a laboratory test to evaluate for the presence of these drugs, a specific request should be given to the technicians to look for signals of MDPV (most common metabolite is dimethylenyl-methyl-MDPV), mephedrone (4-methylmethcathinone), 3-bromomethcathinone (3-BMC), or 3-fluoromethcathinone (fluphedrone).45-48 The study testing (both in VA and civilian settings) for Mr. H was done by a commercial laboratory several states away where patented techniques can screen for more than 30 compounds via LC-MS. The laboratory offered bath salts panels for urine, serum/plasma, and blood samples.
Synthetic cathinones are dangerous, and as the body of medical literature continues to expand, reports of significant morbidity and death related to their use are appearing. The harmful effects of recreational synthetic cathinone use has been documented across the globe in the form of serotonin syndrome, intoxication delirium, hyperthermia and multi-organ failure, myocarditis, hypo-osmotic hyponatremia with encephalopathy, agitation, psychosis, and death after cardiac arrest.5,12,38,39,49-53 Published treatment methods are largely supportive with the available literature, suggesting that benzodiazepines, antipsychotics (both typical and atypical), restraints to maintain safety, and IV fluids may be indicated.5,9,50
Synthetic Cannabinoids
Mr. W is a 58-year-old veteran with a history of alcohol dependence and PTSD who reported use of the synthetic cannabinoid “Spice” during intake assessment for treatment of alcohol dependence. He reported using Spice about 4 times over a 2-month period. He purchased a small jar of the substance from a party store for $15 per gram and understood its contents to be synthetic marijuana, which he appreciated for its low cost and assumed legality. He denied having any understanding of the package’s contents beyond “synthetic marijuana.”
The patient ingested the drug by smoking and inhaling from a pipe. For the first 3 times that he used the substance, Mr. W reported feeling a pleasant sensation that started quickly and lasted about 30 minutes. The fourth time that he used synthetic cannabis he felt nauseated and vomited several times, had auditory hallucinations, and increased anxiety; he also reported a hangover effect after this use. He identified that the effects may have been different the fourth time “because the brands were changing.”
Mr. W also reported that his neighbor—a daily user of synthetic cannabinoids for several months—became paranoid, suspicious, and developed incomprehensible speech. His neighbor’s symptoms and his own unpleasant experiences prompted a discontinuation of use.
Synthetic cannabinoids are a diverse group of agents numbering in excess of 100 artificial compounds that act as agonists at cannabinoid receptors, mimicking the effects of tetrahydrocannabinol (THC), an active ingredient in marijuana.28,54 The availability of these drugs online and in specialty shops has been documented since the mid-2000s.27,28,32 Their packaging often describes the contents as incense or herbal blends, using various names. Spice is a common name, but these products are also known by a myriad of other designations (Table 1).28 A single packet usually contains several grams of the drug and costs about $30.55
To the user, who may already be familiar with marijuana, the contents intentionally appear similar to the dried buds of cannabis.30,56 In reality, the drug has just been sprayed onto inert plant material.57 The drug is smoked, and the psychoactive dose can be as little as 1 mg.30 Users describe potent drug effects (Table 2). There is a rapid onset of action, and duration of effects last 1 to 2 hours.58
The compounds’ mechanism of action and appeal are derived from their high affinity for the cannabinoid receptors. The CB1 receptor is located primarily in the central nervous system and is responsible for the psychoactive component of the drugs’ actions.27,30,58,59 Two particular synthetic cannabinoids, cannibicyclohexanol and JWH-018, are potent cannabinoid CB1 agonists with affinity exceeding their natural counterparts.27,30, 32,56,58,59
Chemically, these drugs are varied. The largest structural family of these compounds is the JWH group, which includes JWH-018.60 Also common are CP 47,497 and other CP compounds.58 HU compounds, such as HU-210, have also been identified and have been shown to be 100 to 800 times more potent at the CB1 and CB2 receptors than is THC.60,61 A final group includes the benzoylindoles, such as AM-964 and RCS-4, which also bind strongly to CB1 and CB2.60,62
Constitutional symptoms of synthetic cannabinoid intoxication include disorientation, anxiety, tremulousness, palpitations, tachycardia, agitation, injected conjunctivae, hyperreflexia, nausea, vomiting, lateral gaze nystagmus, and myoclonic jerks, which have been mistaken for seizure activity.27,30,55 Pupils are often normal sized.55 Withdrawal phenomena are similar to those of cannabis withdrawal: irritability, anxiety, tremor, palpitations, diaphoresis, insomnia, headache, diarrhea, nausea, and vomiting.59
Given the established link between cannabis use and psychosis, synthetic cannabinoids may stand as a precipitant of psychotic symptoms, which may include visual hallucinations, auditory hallucinations, disorganized speech, paranoia, grandiose delusions, disorganization, or bizarre behavior.58,63-66 These symptoms may represent a relapse of a primary thought disorder or, for some unfortunate individuals, a de novo psychotic illness.58,65,66 Symptoms can linger for months after drug use.65
A key risk in the use of synthetic cannabinoid moieties may involve the absence of cannabidiol. Cannabidiol naturally occurs in many strains of cannabis and is thought to have antipsychotic, neuroprotective properties.67 The absence of this molecule in synthetic cannabinoids may at least partially explain their severe psychoactive effects. Treatment for synthetic cannabinoid intoxication and related psychosis is largely supportive and may include the use of antipsychotic medication.66
Detection of synthetic cannabinoids in urine is difficult, yet many compounds can be detected via GC-MS or LC-MS. Molecules of significance include JWH-018, JWH-073, JWH-015, JWH-250, CP-47 497, HU-210, cannabicyclohexanol, and oleamide; however, these compounds are rarely excreted in urine in their pure form. The many hydroxylated or dealkylated metabolites of these compounds, mostly unnamed, are more consistently detected in urine.68,69 One author has noted that the pentanoic acid metabolite of JWH-018 seems to appear most reliably in urine specimens.68
Many synthetic cannabinoid herbal mixes also contain a detectable compound called tocopherol, seemingly added as an antioxidant.69,70 Synthetic cannabinoids are an evolving drug class, and reliable detection will require that laboratories stay up-to-date in their detection methods. As stated earlier, a commercial laboratory in the region accepted the civilian and veteran patient samples for these case studies. The synthetic cannabinoid panels offered evaluation of the drug itself (GC-MS), an oral fluid screen (LC-MS), and isolation of metabolites in urine (enzyme-linked immunosorbent assay).
Conclusion
Designer drugs will remain a challenge for providers caring for veterans for several key reasons: (1) Veterans are a vulnerable population who abuse substances at higher rates than do their civilian counterparts; (2) Chemists are able to manufacture variations of known habit-forming substances; (3) Modern technology facilitates the purchase and wide distribution of addictive substances; (4) Many designer drugs are deceptively packaged and marketed; (5) The effects of the drugs are often severe; (6) No standardized treatment guidelines exist; and (7) Detection of the drugs is difficult, and new versions of the molecules may evade even cutting-edge techniques.
Due to the high cost of detecting synthetic cathinones and synthetic cannabinoids in body fluids, screening should be considered only in settings where severe symptoms are accompanied by reasonable clinical suspicion of use and an otherwise negative toxicologic workup. As more designer drugs inevitably emerge, research will be needed on their pharmacology, toxidromes, and detection. Military and civilian practitioners must remain abreast of the dynamic trends in designer drugs to ensure that their patients receive the highest level of medical care.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Bray RM, Fairbank JA, Marsden ME. Stress and substance use among military women and men. J Drug Alcohol Abuse. 1999;25(2):239-256.
2. Bray RM, Hourani LL. Substance use trends among active duty military personnel: Findings from the United States Department of Defense Health Related Behavior Surveys, 1980-2005. Addiction. 2007;102(7):1092-1101.
3. Hankin CS, Spiro A 3rd, Miller DR, Kazis L. Mental disorders and mental health treatment among U.S. Department of Veterans Affairs outpatients: The Veterans Health Study. Am J Psychiatry. 1999;156(12):1924-1930.
4. Sirratt D, Ozanian A, Traenkner B. Epidemiology and prevention of substance use disorders in the military. Mil Med. 2012;177(suppl 8):21-28.
5. Winder GS, Stern N, Hosanagar A. Are “bath salts” the next generation of stimulant abuse? J Subst Abuse Treat. 2013;44(1):42-45.
6. Bobes J, Sáiz PA, González MP, et al. Use of MDMA and other illicit drugs by young adult males in northern Spain. A five-year study. Eur Addict Res. 2002;8(3):147-154.
7. D.E.A. Cracks Down on Designer Drug Operations. The New York Times Website. http://www.nytimes.com/2013/06/27/us/dea-cracks-down-on-designer-drug-operations.html. Published June 26, 2013. Accessed October 6, 2014.
8. Travis A. Mushrooming legal highs leave drug control system floundering, UN warns. The Guardian Website. http://www.guardian.co.uk/world/2013/jun/26/legal-highs-drug-control. Published June 26, 2013. Accessed October 6, 2014.
9. Jerry J, Collins G, Streem D. Synthetic legal intoxicating drugs: The emerging ‘incense’ and ‘bath salt’ phenomenon. Cleve Clin J Med. 2012;79(4):258-264.
10. Murphy CM, Dulaney AR, Beuhler MC, Kacinko S. “Bath salts” and “plant food” products: The experience of one regional US poison center. J Med Toxicol. 2013;9(1):42-48.
11. Wax PM. Just a click away: Recreational drug web sites on the Internet. Pediatrics. 2002;109(6):e96.
12. Vardakou I, Pistos C, Spiliopoulou C. Drugs for youth via Internet and the example of mephedrone. Toxicol Lett. 2011;201(3):191-195.
13. Winickoff JP, Houck CS, Rothman EL, Bauchner H. Verve and jolt: Deadly new Internet drugs. Pediatrics. 2000;106(4):829-830.
14. Camilleri A, Johnston MR, Brennan M, Davis S, Caldicott DG. Chemical analysis of four capsules containing the controlled substance analogues 4-methylmethcathinone, 2-fluoromethamphetamine, alpha-phthalimidopropiophenone and N-ethylcathinone. Forensic Sci Int. 2010;197(1-3):59-66.
15. Carroll FI, Lewin AH, Mascarella SW, Seltzman HH, Reddy PA. Designer drugs: A medicinal chemistry perspective. Ann N Y Acad Sci. 2012;1248:18-38.
16. Christophersen AS. Amphetamine designer drugs—An overview and epidemiology. Toxicol Lett. 2000;112-113:127-131.
17. Buchanan JF, Brown CR. ‘Designer drugs.’ A problem in clinical toxicology. Med Toxicol Adverse Drug Exp. 1988;3(1):1-17.
18. Griffiths P, Sedefov R, Gallegos A, Lopez D. How globalization and market innovation challenge how we think about and respond to drug use: ‘Spice’ a case study. Addiction. 2010;105(6):951-953.
19. Drug Enforcement Administration, Department of Justice. Schedules of controlled substances: Temporary placement of three synthetic cathinones in Schedule I. Final Order. Fed Regist. 2011;76(204):65371-65375.
20. Drug Enforcement Administration, Department of Justice. Schedules of controlled substances: Placement of five synthetic cannabinoids into Schedule I. Fed Regist. 2012;77(41):12508-12514.
21. Drug Enforcement Administration, Department of Justice. Schedules of controlled substances: Temporary placement of three synthetic cannabinoids into Schedule I. Fed Regist. 2013;78(95):28735-28739.
22. Council of the European Union. Council Decision of 2 December 2010 on submitting 4-methylmethcathinone (mephedrone) to control measures. EUR-Lex Website. eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2010:322:0044:0045:en:PDF. Published December 8, 2010. Accessed October 7, 2014.
23. European Monitoring Centre for Drugs and Drug Addiction. Synthetic cannabinoids and ‘spice’. European Monitoring Centre for Drugs and Drug Addiction Website. http://www.emcdda.europa.eu/publications/drug-profiles/synthetic-cannabinoids. Updated September 15, 2011. Accessed October 7, 2014.
24. United Nations Office on Drugs and Crime. World Drug Report 2014. United Nations Office on Drugs and Crime Website. http://www.unodc.org/wdr2014. Accessed October 3, 2014.
25. Fass JA, Fass AD, Garcia AS. Synthetic cathinones (bath salts): Legal status and patterns of abuse. Ann Pharmacother. 2012;46(3):436-441.
26. Gunderson EW, Haughey HM, Ait-Daoud N, Joshi AS, Hart CL. “Spice” and “K2” herbal highs: A case series and systematic review of the clinical effects and biopsychosocial implications of synthetic cannabinoid use in humans. Am J Addict. 2012;21(4):320-326.
27. Loeffler G, Hurst D, Penn A, Yung K. Spice, bath salts, and the U.S. military: The emergence of synthetic cannabinoid receptor agonists and cathinones in the U.S. Armed Forces. Mil Med. 2012;177(9):1041-1048.
28. European Monitoring Centre for Drugs and Drug Addiction. Understanding the spice phenomenon. The National Documentation Centre on Drug Use Website. http://www.drugsandalcohol.ie/12597/1/Understanding_the_Spice_phenomenon.pdf. Published 2009. Accessed October 7, 2014.
29. Davies S, Wood DM, Smith G, et al. Purchasing ‘legal highs’ on the Internet—Is there consistency in what you get? QJM. 2010;103(7):489-493.
30. Harris CR, Brown A. Synthetic cannabinoid intoxication: A case series and review. J Emerg Med. 2013;44(2):360-366.
31. Ammann D, McLaren JM, Gerostamoulos D, Beyer J. Detection and quantification of new designer drugs in human blood: Part 2--Designer cathinones. J Anal Toxicol. 2012;36(6):381-389.
32. Hudson S, Ramsey J. The emergence and analysis of synthetic cannabinoids. Drug Test Anal. 2011;3(7-8):466-478.
33. Al-Habori M. The potential adverse effects of habitual use of Catha edulis (khat). Expert Opin Drug Saf. 2005;4(6):1145-1154.
34. Al-Motarreb A, Al-Habori M, Broadley KJ. Khat chewing, cardiovascular diseases and other internal medical problems: The current situation and directions for future research. J Ethnopharmacol. 2010;132(3):540-548.
35. Dargan PI, Sedefov R, Gallegos A, Wood DM. The pharmacology and toxicology of the synthetic cathinone mephedrone (4-methylmethcathinone). Drug Test Anal. 2011;3(7-8):454-463.
36. Kriikku P, Wilhelm L, Schwarz O, Rintatalo J. New designer drug of abuse: 3,4-Methylenedioxypyrovalerone (MDPV). Findings from apprehended drivers in Finland. Forensic Sci Int. 2011;210(1-3):195-200.
37. Morris K. UK places generic ban on mephedrone drug family. Lancet. 2010;375(9723):1333-1334.
38. Garrett G, Sweeney M. The serotonin syndrome as a result of mephedrone toxicity. BMJ Case Rep. 2010.
39. Sammler EM, Foley PL, Lauder GD, Wilson SJ, Goudie AR, O’Riordan JI. A harmless high? Lancet. 2010;376(9742):742.
40. Prosser JM, Nelson LS. The toxicology of bath salts: A review of synthetic cathinones. J Med Toxicol. 2012;8(1):33-42.
41. Thornton SL, Gerona RR, Tomaszewski CA. Psychosis from a bath salt product containing flephedrone and MDPV with serum, urine, and product quantification. J Med Toxicol. 2012;8(3):310-313.
42. Emerson TS, Cisek JE. Methcathinone: a Russian designer amphetamine infiltrates the rural midwest. Ann Emerg Med. 1993;22(12):1897-1903.
43. Winstock AR, Mitcheson LR, Deluca P, Davey Z, Corazza O, Schifano F. Mephedrone, new kid for the chop? Addict. 2011;106(1):154-161.
44. Spiller HA, Ryan ML, Weston RG, Jansen J. Clinical experience with and analytical confirmation of “bath salts” and “legal highs” (synthetic cathinones) in the United States. Clin Toxicol (Phila). 2011;49(6):499-505.
45. Ojanperä IA, Heikman PK, Rasanen IJ. Urine analysis of 3,4-methylenedioxypyrovalerone in opioid-dependent patients by gas chromatography-mass spectrometry. Ther Drug Monit. 2011;33(2):257-263.
46. Meyer MR, Du P, Schuster F, Maurer HH. Studies on the metabolism of the alpha-pyrrolidinophenone designer drug methylenedioxy-pyrovalerone (MDPV) in rat and human urine and human liver microsomes using GC-MS and LC-high-resolution MS and its detectability in urine by GC-MS. JMS. 2010;45(12):1426-1442.
47. Santali EY, Cadogan AK, Daeid NN, Savage KA, Sutcliffe OB. Synthesis, full chemical characterisation and development of validated methods for the quantification of (+/-)-4’-methylmethcathinone (mephedrone): A new “legal high”. J Pharm Biomed Anal. 2011;56(2):246-255.
48. Meyer MR, Vollmar C, Schwaninger AE, Wolf E, Maurer HH. New cathinone-derived designer drugs 3-bromomethcathinone and 3-fluoromethcathinone: Studies on their metabolism in rat urine and human liver microsomes using GC-MS and LC-high-resolution MS and their detectability in urine. JMS. 2012;47(2):253-262.
49. Borek HA, Holstege CP. Hyperthermia and multiorgan failure after abuse of “bath salts” containing 3,4-methylenedioxypyrovalerone. Ann Emerg Med. 2012;60(1):103-105.
50. Kasick DP, McKnight CA, Klisovic E. “Bath salt” ingestion leading to severe intoxication delirium: Two cases and a brief review of the emergence of mephedrone use. Am J Drug Alcohol Abuse. 2012;38(2):176-180.
51. Penders TM, Gestring RE, Vilensky DA. Excited delirium following use of synthetic cathinones (bath salts). Gen Hosp Psychiatry. 2012;34(6):647-650.
52. Nicholson PJ, Quinn MJ, Dodd JD. Headshop heartache: Acute mephedrone ‘meow’ myocarditis. Heart. 2010;96(24):2051-2052.
53. Murray BL, Murphy CM, Beuhler MC. Death following recreational use of designer drug “bath salts” containing 3,4-Methylenedioxypyrovalerone (MDPV). J Med Toxicol. 2012;8(1):69-75.
54. Gunderson EW, Haughey HM, Ait-Daoud N, Joshi AS, Hart CL. Spice” and “K2” herbal highs: A case series and systematic review of the clinical effects and biopsychosocial implications of synthetic cannabinoid use in humans. Am J Addict. 2012;21(4):320-326.
55. Schneir AB, Cullen J, Ly BT. “Spice” girls: Synthetic cannabinoid intoxication. J Emerg Med. 2011;40(3):296-299.
56. Atwood BK, Huffman J, Straiker A, Mackie K. JWH018, a common constituent of ‘Spice’ herbal blends, is a potent and efficacious cannabinoid CB receptor agonist. Br J Pharmacol. 2010;160(3):585-593.
57. Uchiyama N, Kikura-Hanajiri R, Ogata J, Goda Y. Chemical analysis of synthetic cannabinoids as designer drugs in herbal products. Forensic Sci Int. 2010;198(1-3):31-38.
58. Every-Palmer S. Synthetic cannabinoid JWH-018 and psychosis: An explorative study. Drug Alcohol Depend. 2011;117(2-3):152-157.
59. Zimmermann US, Winkelmann PR, Pilhatsch M, Nees JA, Spanagel R, Schulz K. Withdrawal phenomena and dependence syndrome after the consumption of “spice gold.” Deutsches Ärzteblatt Int. 2009;106(27):464-467.
60. Loeffler G, Hurst D, Penn A, Yung K. Spice, Bath salts, and the US military: The emergence of synthetic cannabinoid receptor agonists and cathinones in the US armed forces. Mil Med. 2012;177(9):1041-1048.
61. Devane WA, Breuer A, Sheskin T, Jäerbe TU, Eisen MS, Mechoulam R. A novel probe for the cannabinoid receptor. J Med Chem. 1992;35(11):2065-2069.
62. Gottardo R, Chiarini A, Dal Prà I, et al. Direct screening of herbal blends for new synthetic cannabinoids by MALDI-TOF MS. JMS. 2012;47(1):141-146.
63. McGrath J, Welham J, Scott J, et al. Association between cannabis use and psychosis-related outcomes using sibling pair analysis in a cohort of young adults. Arch Gen Psychiatry. 2010;67(5):440-447.
64. Every-Palmer S. Warning: Legal synthetic cannabinoid-receptor agonists such as JWH-018 may precipitate psychosis in vulnerable individuals. Addict. 2010;105(10):1859-1860.
65. Hurst D, Loeffler G, McLay R. Psychosis associated with synthetic cannabinoid agonists: A case series. Am J Psychiatry. 2011;168(10):1119.
66. Peglow S, Buchner J, Briscoe G. Synthetic cannabinoid induced psychosis in a previously nonpsychotic patient. Am J Addict. 2012;21(3):287-288.
67. Morgan CJ, Curran HV. Effects of cannabidiol on schizophrenia-like symptoms in people who use cannabis. Br J Psychiatry. 2008;192(4):306-307.
68. ElSohly MA, Gul W, ElSohly KM, Murphy TP, Madgula VL, Khan SI. Liquid chromatography-tandem mass spectrometry analysis of urine specimens for K2 (JWH-018) metabolites. J Anal Toxicol. 2011;35(7):487-495.
69. Grigoryev A, Savchuk S, Melnik A, et al. Chromatography–mass spectrometry studies on the metabolism of synthetic cannabinoids JWH-018 and JWH-073, psychoactive components of smoking mixtures. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879(15-16):1126-1136.
70. Sobolevsky T, Prasolov I, Rodchenkov G. Detection of JWH-018 metabolites in smoking mixture post-administration urine. Forensic Sci Int. 2010;200(1):141-147.
Although the elevated risks and rates of veterans’ substance abuse patterns are well documented, little has been written about veterans’ use of designer drugs.1-6 In recent months throughout Europe and the U.S., there has been a flurry of media attention for 2 classes of designer drugs: synthetic cathinones and synthetic cannabinoids.7,8 In the U.S., the popularity of these drugs has surged, and a disproportionate amount of use of these 2 drug classes is coming from locations near military instillations.9,10
The purpose of this article is to raise awareness regarding these 2 burgeoning designer drug classes and their impact on veterans. Designer drugs affecting vulnerable populations are not a new phenomenon, yet many providers are unfamiliar with the effects of these unique drugs of abuse on their veteran populations.11-13
Many designer drugs begin their existence as variations of other addictive or psychoactive drugs. Others begin in laboratories as investigative research compounds that end up on the street, often promising a novel mind-altering experience as a “legal high.”14-18 The Designer Drug Enforcement Act of 1986 was an initial attempt in the U.S. to define and control the early rise of copycat drugs that appeared on the streets and mimicked the effects of other illicit substances. More recent legislation enacted in the U.S. has imposed Schedule I controls on the manufacture, distribution, possession, importation, and exportation of these types of drugs, including both synthetic cathinones and synthetic cannabinoids. State laws are perennially in flux trying to keep up with the latest drug trends.19-21
Similar efforts have been made by the European Union to control mephedrone, a synthetic cathinone, citing multiple fatalities, seizures, related crime, lack of medical use, and risk of dependence.22 Although uniform levels of control do not exist in Europe for synthetic cannabinoids, many countries have independently acted to limit their use.23
In its recent World Drug Report 2013, the United Nations Office on Drugs and Crime documents its growing concern about the “new psychoactive substances” category of illicit recreational substances (in which synthetic cannabinoids and cathinones are included) that has increased by 50% since 2009.24 Alone, this category now outnumbers the total number of substances controlled by international drug conventions.
The novelty and variability of designer drugs causes difficulties with detection and regulation. Innovative chemists can legally manufacture new versions of known molecules intended for illicit use with a rapidity that outpaces bureaucratic control. Local law enforcement officials may be unaware of the latest designer drug trends, stifling efforts at public education or restriction. Designer drugs are often deceptively packaged and are available in convenience stores, tobacco outlets, gas stations, pawnshops, tattoo parlors, and truck stops.25-28 The Internet may be the singular reason, however, that designer drugs continue to be widely available to veterans.11,18
Innumerable websites discuss, promote, and sell designer drugs or deceitfully market them as safe, legitimate household products (“not for human consumption”), which can be ordered online and shipped by commercial carriers.12 Little accurate information is known about their effects or about the specific compounds they contain. When the recreational nature of the drugs is actually acknowledged, information on how the buyer can evade prosecution is often provided in tandem. The suppliers’ inventory of the drugs has been shown to be variable and inconsistent, and the product ingredients can be similarly unpredictable despite comparatively more stable naming and labeling.14,29
In the clinical setting, a reliable patient drug history may not be available. This ensures that the diagnosis of designer drug use will be an exclusionary process involving routine laboratory work, physical examination, and at times electroencephalogram and/or neuroimaging. Psychiatric consultation is often useful in this setting. Routine immunoassay tests do not detect either synthetic cathinones or synthetic cannabinoids.30
Both cannabinoids and cathinones can be identified using gas chromatography-mass spectroscopy (GC-MS) or liquid chromatography-mass spectroscopy (LC-MS). However, this technology is limited to specialized laboratories.31,32 The laboratory results often are not immediately available, potentially limiting the tests’ use in emergency or inpatient settings, as the patient may have left the hospital by the time the results are available. Additionally, these drugs’ prevalence of use, while increasing, often does not justify the cost of these tests.
The inability to routinely detect metabolites in urine may increase the enticement of these drugs given the likelihood that active-duty personnel could use them surreptitiously. Further, these compounds are evolving and seemingly limitless in their variability, and there is often a paucity of pure reference materials. As such, it is impossible to guarantee reliable test results.
The following profiles of each of these drug classes will be accompanied by clinical cases depicting the drugs’ effects and how an affected veteran might present clinically. The severe effects of these novel agents illustrate the value in maintaining a functional knowledge base about emerging drug trends. The accuracy of diagnosis as well as the outcome of a veteran’s treatment may depend on the provider’s ability to identify the presence of a drug and manage its effects.
Synthetic Cathinones
Mr. H is a 28-year-old Iraq War veteran with a history of posttraumatic stress disorder (PTSD), alcohol abuse, and opioid dependence who presented for inpatient psychiatric admission after making suicidal statements to his wife in the context of 2 weeks of “bath salts” use. A family member initially introduced him to the drug. His first drug purchase had been 1 gram ($30) at a local movie rental store.
After discharge from the hospital, Mr. H began purchasing increasing amounts online with a credit card. Although he initially had been insufflating and inhaling the substance, he later began injecting it (dissolving it in tap water and loading it through a cotton filter in a syringe). The patient admitted to finding the drug significantly more addictive than any others he had used, and his use resulted in leaving his job and abandoning his family.
Severe cravings and depression were present between episodes of use. He spent $40,000 over 6 months of use. Insomnia lasted for several days, his appearance changed dramatically (including persistent skin infections), and he became paranoid, believing that everyone around him was an undercover police officer. He remained on medications for persistent anxiety. His daily drug cravings continued,
although he remained uncertain about the actual ingredients of bath salts.
Cathinone is a naturally occurring stimulant from the khat plant (Catha edulis), which grows indigenously in Egypt and on the Arabian Peninsula. The recreational and religious use of this plant has occurred for thousands of years, though it is not without risk: The chewing of the leaves containing natural cathinones has been associated with esophagitis, gastritis, oral keratosis, myocardial infarction, dilated cardiomyopathy, hypertension, cerebral ischemia, thromboembolism, diabetes, sexual dysfunction, duodenal ulcer, and hepatitis.33,34
The stimulants known colloquially as bath salts are synthetic cathinones, which have become more widely available within the past 10 years: first in the Middle East, then Europe, and now in the U.S.5,9,10,14,19,25,35-41,44 Although the current rise in use has occurred in the past few years, the first documented abuse of synthetic cathinones in the U.S. dates to the early 1990s in Michigan.42
Bath salts is the most common of the many names used to denote synthetic cathinones. The compounds have no utility when used as such but often are marketed as research chemicals, plant fertilizer, or shoe polish. It is this deliberate counterfeit of household product names that allows many distributors to avoid classifying the compounds according to the true, intended use. More appealing brand names may also be used to entice the user (Table 1).25
Synthetic cathinones owe their popularity to similarities with cocaine and methamphetamine. They are sympathomimetic with synaptic increases of monoamines after use: Surges in norepinephrine and dopamine account for the stimulant qualities, and serotonergic changes mediate distinct psychoactive effects (Table 2).40 Users are interested in the drugs for many of the same reasons that other recreational stimulants have appeal: euphoria, energy, empathy, heightened sexuality, sociability, and an overall intensification of senses. Synthetic cathinones have become preferred to cocaine for some users.43
The drugs can be used via oral and anal routes. Using methods known as “bombing” or “keystering,” users deliver boluses of the powder wrapped in cigarette paper, which they swallow or insert into the rectum. Insufflation and IV injection are also common methods of administration with a quicker onset of action expected.40 The prices of the drugs range from $25 to $50 per 500-mg packet (though the cost is increasing with more regulation). Users typically use 500 mg to 2 g in one session.
The 2 most commonly abused synthetic cathinones are mephedrone and MDPV (methylenedioxypyrovalerone). There is some regional variability about which ingredient is present; mephedrone tends to be more prevalent in Europe, whereas MDPV is noted to be more common in the U.S.10,44
When ordering a laboratory test to evaluate for the presence of these drugs, a specific request should be given to the technicians to look for signals of MDPV (most common metabolite is dimethylenyl-methyl-MDPV), mephedrone (4-methylmethcathinone), 3-bromomethcathinone (3-BMC), or 3-fluoromethcathinone (fluphedrone).45-48 The study testing (both in VA and civilian settings) for Mr. H was done by a commercial laboratory several states away where patented techniques can screen for more than 30 compounds via LC-MS. The laboratory offered bath salts panels for urine, serum/plasma, and blood samples.
Synthetic cathinones are dangerous, and as the body of medical literature continues to expand, reports of significant morbidity and death related to their use are appearing. The harmful effects of recreational synthetic cathinone use has been documented across the globe in the form of serotonin syndrome, intoxication delirium, hyperthermia and multi-organ failure, myocarditis, hypo-osmotic hyponatremia with encephalopathy, agitation, psychosis, and death after cardiac arrest.5,12,38,39,49-53 Published treatment methods are largely supportive with the available literature, suggesting that benzodiazepines, antipsychotics (both typical and atypical), restraints to maintain safety, and IV fluids may be indicated.5,9,50
Synthetic Cannabinoids
Mr. W is a 58-year-old veteran with a history of alcohol dependence and PTSD who reported use of the synthetic cannabinoid “Spice” during intake assessment for treatment of alcohol dependence. He reported using Spice about 4 times over a 2-month period. He purchased a small jar of the substance from a party store for $15 per gram and understood its contents to be synthetic marijuana, which he appreciated for its low cost and assumed legality. He denied having any understanding of the package’s contents beyond “synthetic marijuana.”
The patient ingested the drug by smoking and inhaling from a pipe. For the first 3 times that he used the substance, Mr. W reported feeling a pleasant sensation that started quickly and lasted about 30 minutes. The fourth time that he used synthetic cannabis he felt nauseated and vomited several times, had auditory hallucinations, and increased anxiety; he also reported a hangover effect after this use. He identified that the effects may have been different the fourth time “because the brands were changing.”
Mr. W also reported that his neighbor—a daily user of synthetic cannabinoids for several months—became paranoid, suspicious, and developed incomprehensible speech. His neighbor’s symptoms and his own unpleasant experiences prompted a discontinuation of use.
Synthetic cannabinoids are a diverse group of agents numbering in excess of 100 artificial compounds that act as agonists at cannabinoid receptors, mimicking the effects of tetrahydrocannabinol (THC), an active ingredient in marijuana.28,54 The availability of these drugs online and in specialty shops has been documented since the mid-2000s.27,28,32 Their packaging often describes the contents as incense or herbal blends, using various names. Spice is a common name, but these products are also known by a myriad of other designations (Table 1).28 A single packet usually contains several grams of the drug and costs about $30.55
To the user, who may already be familiar with marijuana, the contents intentionally appear similar to the dried buds of cannabis.30,56 In reality, the drug has just been sprayed onto inert plant material.57 The drug is smoked, and the psychoactive dose can be as little as 1 mg.30 Users describe potent drug effects (Table 2). There is a rapid onset of action, and duration of effects last 1 to 2 hours.58
The compounds’ mechanism of action and appeal are derived from their high affinity for the cannabinoid receptors. The CB1 receptor is located primarily in the central nervous system and is responsible for the psychoactive component of the drugs’ actions.27,30,58,59 Two particular synthetic cannabinoids, cannibicyclohexanol and JWH-018, are potent cannabinoid CB1 agonists with affinity exceeding their natural counterparts.27,30, 32,56,58,59
Chemically, these drugs are varied. The largest structural family of these compounds is the JWH group, which includes JWH-018.60 Also common are CP 47,497 and other CP compounds.58 HU compounds, such as HU-210, have also been identified and have been shown to be 100 to 800 times more potent at the CB1 and CB2 receptors than is THC.60,61 A final group includes the benzoylindoles, such as AM-964 and RCS-4, which also bind strongly to CB1 and CB2.60,62
Constitutional symptoms of synthetic cannabinoid intoxication include disorientation, anxiety, tremulousness, palpitations, tachycardia, agitation, injected conjunctivae, hyperreflexia, nausea, vomiting, lateral gaze nystagmus, and myoclonic jerks, which have been mistaken for seizure activity.27,30,55 Pupils are often normal sized.55 Withdrawal phenomena are similar to those of cannabis withdrawal: irritability, anxiety, tremor, palpitations, diaphoresis, insomnia, headache, diarrhea, nausea, and vomiting.59
Given the established link between cannabis use and psychosis, synthetic cannabinoids may stand as a precipitant of psychotic symptoms, which may include visual hallucinations, auditory hallucinations, disorganized speech, paranoia, grandiose delusions, disorganization, or bizarre behavior.58,63-66 These symptoms may represent a relapse of a primary thought disorder or, for some unfortunate individuals, a de novo psychotic illness.58,65,66 Symptoms can linger for months after drug use.65
A key risk in the use of synthetic cannabinoid moieties may involve the absence of cannabidiol. Cannabidiol naturally occurs in many strains of cannabis and is thought to have antipsychotic, neuroprotective properties.67 The absence of this molecule in synthetic cannabinoids may at least partially explain their severe psychoactive effects. Treatment for synthetic cannabinoid intoxication and related psychosis is largely supportive and may include the use of antipsychotic medication.66
Detection of synthetic cannabinoids in urine is difficult, yet many compounds can be detected via GC-MS or LC-MS. Molecules of significance include JWH-018, JWH-073, JWH-015, JWH-250, CP-47 497, HU-210, cannabicyclohexanol, and oleamide; however, these compounds are rarely excreted in urine in their pure form. The many hydroxylated or dealkylated metabolites of these compounds, mostly unnamed, are more consistently detected in urine.68,69 One author has noted that the pentanoic acid metabolite of JWH-018 seems to appear most reliably in urine specimens.68
Many synthetic cannabinoid herbal mixes also contain a detectable compound called tocopherol, seemingly added as an antioxidant.69,70 Synthetic cannabinoids are an evolving drug class, and reliable detection will require that laboratories stay up-to-date in their detection methods. As stated earlier, a commercial laboratory in the region accepted the civilian and veteran patient samples for these case studies. The synthetic cannabinoid panels offered evaluation of the drug itself (GC-MS), an oral fluid screen (LC-MS), and isolation of metabolites in urine (enzyme-linked immunosorbent assay).
Conclusion
Designer drugs will remain a challenge for providers caring for veterans for several key reasons: (1) Veterans are a vulnerable population who abuse substances at higher rates than do their civilian counterparts; (2) Chemists are able to manufacture variations of known habit-forming substances; (3) Modern technology facilitates the purchase and wide distribution of addictive substances; (4) Many designer drugs are deceptively packaged and marketed; (5) The effects of the drugs are often severe; (6) No standardized treatment guidelines exist; and (7) Detection of the drugs is difficult, and new versions of the molecules may evade even cutting-edge techniques.
Due to the high cost of detecting synthetic cathinones and synthetic cannabinoids in body fluids, screening should be considered only in settings where severe symptoms are accompanied by reasonable clinical suspicion of use and an otherwise negative toxicologic workup. As more designer drugs inevitably emerge, research will be needed on their pharmacology, toxidromes, and detection. Military and civilian practitioners must remain abreast of the dynamic trends in designer drugs to ensure that their patients receive the highest level of medical care.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Although the elevated risks and rates of veterans’ substance abuse patterns are well documented, little has been written about veterans’ use of designer drugs.1-6 In recent months throughout Europe and the U.S., there has been a flurry of media attention for 2 classes of designer drugs: synthetic cathinones and synthetic cannabinoids.7,8 In the U.S., the popularity of these drugs has surged, and a disproportionate amount of use of these 2 drug classes is coming from locations near military instillations.9,10
The purpose of this article is to raise awareness regarding these 2 burgeoning designer drug classes and their impact on veterans. Designer drugs affecting vulnerable populations are not a new phenomenon, yet many providers are unfamiliar with the effects of these unique drugs of abuse on their veteran populations.11-13
Many designer drugs begin their existence as variations of other addictive or psychoactive drugs. Others begin in laboratories as investigative research compounds that end up on the street, often promising a novel mind-altering experience as a “legal high.”14-18 The Designer Drug Enforcement Act of 1986 was an initial attempt in the U.S. to define and control the early rise of copycat drugs that appeared on the streets and mimicked the effects of other illicit substances. More recent legislation enacted in the U.S. has imposed Schedule I controls on the manufacture, distribution, possession, importation, and exportation of these types of drugs, including both synthetic cathinones and synthetic cannabinoids. State laws are perennially in flux trying to keep up with the latest drug trends.19-21
Similar efforts have been made by the European Union to control mephedrone, a synthetic cathinone, citing multiple fatalities, seizures, related crime, lack of medical use, and risk of dependence.22 Although uniform levels of control do not exist in Europe for synthetic cannabinoids, many countries have independently acted to limit their use.23
In its recent World Drug Report 2013, the United Nations Office on Drugs and Crime documents its growing concern about the “new psychoactive substances” category of illicit recreational substances (in which synthetic cannabinoids and cathinones are included) that has increased by 50% since 2009.24 Alone, this category now outnumbers the total number of substances controlled by international drug conventions.
The novelty and variability of designer drugs causes difficulties with detection and regulation. Innovative chemists can legally manufacture new versions of known molecules intended for illicit use with a rapidity that outpaces bureaucratic control. Local law enforcement officials may be unaware of the latest designer drug trends, stifling efforts at public education or restriction. Designer drugs are often deceptively packaged and are available in convenience stores, tobacco outlets, gas stations, pawnshops, tattoo parlors, and truck stops.25-28 The Internet may be the singular reason, however, that designer drugs continue to be widely available to veterans.11,18
Innumerable websites discuss, promote, and sell designer drugs or deceitfully market them as safe, legitimate household products (“not for human consumption”), which can be ordered online and shipped by commercial carriers.12 Little accurate information is known about their effects or about the specific compounds they contain. When the recreational nature of the drugs is actually acknowledged, information on how the buyer can evade prosecution is often provided in tandem. The suppliers’ inventory of the drugs has been shown to be variable and inconsistent, and the product ingredients can be similarly unpredictable despite comparatively more stable naming and labeling.14,29
In the clinical setting, a reliable patient drug history may not be available. This ensures that the diagnosis of designer drug use will be an exclusionary process involving routine laboratory work, physical examination, and at times electroencephalogram and/or neuroimaging. Psychiatric consultation is often useful in this setting. Routine immunoassay tests do not detect either synthetic cathinones or synthetic cannabinoids.30
Both cannabinoids and cathinones can be identified using gas chromatography-mass spectroscopy (GC-MS) or liquid chromatography-mass spectroscopy (LC-MS). However, this technology is limited to specialized laboratories.31,32 The laboratory results often are not immediately available, potentially limiting the tests’ use in emergency or inpatient settings, as the patient may have left the hospital by the time the results are available. Additionally, these drugs’ prevalence of use, while increasing, often does not justify the cost of these tests.
The inability to routinely detect metabolites in urine may increase the enticement of these drugs given the likelihood that active-duty personnel could use them surreptitiously. Further, these compounds are evolving and seemingly limitless in their variability, and there is often a paucity of pure reference materials. As such, it is impossible to guarantee reliable test results.
The following profiles of each of these drug classes will be accompanied by clinical cases depicting the drugs’ effects and how an affected veteran might present clinically. The severe effects of these novel agents illustrate the value in maintaining a functional knowledge base about emerging drug trends. The accuracy of diagnosis as well as the outcome of a veteran’s treatment may depend on the provider’s ability to identify the presence of a drug and manage its effects.
Synthetic Cathinones
Mr. H is a 28-year-old Iraq War veteran with a history of posttraumatic stress disorder (PTSD), alcohol abuse, and opioid dependence who presented for inpatient psychiatric admission after making suicidal statements to his wife in the context of 2 weeks of “bath salts” use. A family member initially introduced him to the drug. His first drug purchase had been 1 gram ($30) at a local movie rental store.
After discharge from the hospital, Mr. H began purchasing increasing amounts online with a credit card. Although he initially had been insufflating and inhaling the substance, he later began injecting it (dissolving it in tap water and loading it through a cotton filter in a syringe). The patient admitted to finding the drug significantly more addictive than any others he had used, and his use resulted in leaving his job and abandoning his family.
Severe cravings and depression were present between episodes of use. He spent $40,000 over 6 months of use. Insomnia lasted for several days, his appearance changed dramatically (including persistent skin infections), and he became paranoid, believing that everyone around him was an undercover police officer. He remained on medications for persistent anxiety. His daily drug cravings continued,
although he remained uncertain about the actual ingredients of bath salts.
Cathinone is a naturally occurring stimulant from the khat plant (Catha edulis), which grows indigenously in Egypt and on the Arabian Peninsula. The recreational and religious use of this plant has occurred for thousands of years, though it is not without risk: The chewing of the leaves containing natural cathinones has been associated with esophagitis, gastritis, oral keratosis, myocardial infarction, dilated cardiomyopathy, hypertension, cerebral ischemia, thromboembolism, diabetes, sexual dysfunction, duodenal ulcer, and hepatitis.33,34
The stimulants known colloquially as bath salts are synthetic cathinones, which have become more widely available within the past 10 years: first in the Middle East, then Europe, and now in the U.S.5,9,10,14,19,25,35-41,44 Although the current rise in use has occurred in the past few years, the first documented abuse of synthetic cathinones in the U.S. dates to the early 1990s in Michigan.42
Bath salts is the most common of the many names used to denote synthetic cathinones. The compounds have no utility when used as such but often are marketed as research chemicals, plant fertilizer, or shoe polish. It is this deliberate counterfeit of household product names that allows many distributors to avoid classifying the compounds according to the true, intended use. More appealing brand names may also be used to entice the user (Table 1).25
Synthetic cathinones owe their popularity to similarities with cocaine and methamphetamine. They are sympathomimetic with synaptic increases of monoamines after use: Surges in norepinephrine and dopamine account for the stimulant qualities, and serotonergic changes mediate distinct psychoactive effects (Table 2).40 Users are interested in the drugs for many of the same reasons that other recreational stimulants have appeal: euphoria, energy, empathy, heightened sexuality, sociability, and an overall intensification of senses. Synthetic cathinones have become preferred to cocaine for some users.43
The drugs can be used via oral and anal routes. Using methods known as “bombing” or “keystering,” users deliver boluses of the powder wrapped in cigarette paper, which they swallow or insert into the rectum. Insufflation and IV injection are also common methods of administration with a quicker onset of action expected.40 The prices of the drugs range from $25 to $50 per 500-mg packet (though the cost is increasing with more regulation). Users typically use 500 mg to 2 g in one session.
The 2 most commonly abused synthetic cathinones are mephedrone and MDPV (methylenedioxypyrovalerone). There is some regional variability about which ingredient is present; mephedrone tends to be more prevalent in Europe, whereas MDPV is noted to be more common in the U.S.10,44
When ordering a laboratory test to evaluate for the presence of these drugs, a specific request should be given to the technicians to look for signals of MDPV (most common metabolite is dimethylenyl-methyl-MDPV), mephedrone (4-methylmethcathinone), 3-bromomethcathinone (3-BMC), or 3-fluoromethcathinone (fluphedrone).45-48 The study testing (both in VA and civilian settings) for Mr. H was done by a commercial laboratory several states away where patented techniques can screen for more than 30 compounds via LC-MS. The laboratory offered bath salts panels for urine, serum/plasma, and blood samples.
Synthetic cathinones are dangerous, and as the body of medical literature continues to expand, reports of significant morbidity and death related to their use are appearing. The harmful effects of recreational synthetic cathinone use has been documented across the globe in the form of serotonin syndrome, intoxication delirium, hyperthermia and multi-organ failure, myocarditis, hypo-osmotic hyponatremia with encephalopathy, agitation, psychosis, and death after cardiac arrest.5,12,38,39,49-53 Published treatment methods are largely supportive with the available literature, suggesting that benzodiazepines, antipsychotics (both typical and atypical), restraints to maintain safety, and IV fluids may be indicated.5,9,50
Synthetic Cannabinoids
Mr. W is a 58-year-old veteran with a history of alcohol dependence and PTSD who reported use of the synthetic cannabinoid “Spice” during intake assessment for treatment of alcohol dependence. He reported using Spice about 4 times over a 2-month period. He purchased a small jar of the substance from a party store for $15 per gram and understood its contents to be synthetic marijuana, which he appreciated for its low cost and assumed legality. He denied having any understanding of the package’s contents beyond “synthetic marijuana.”
The patient ingested the drug by smoking and inhaling from a pipe. For the first 3 times that he used the substance, Mr. W reported feeling a pleasant sensation that started quickly and lasted about 30 minutes. The fourth time that he used synthetic cannabis he felt nauseated and vomited several times, had auditory hallucinations, and increased anxiety; he also reported a hangover effect after this use. He identified that the effects may have been different the fourth time “because the brands were changing.”
Mr. W also reported that his neighbor—a daily user of synthetic cannabinoids for several months—became paranoid, suspicious, and developed incomprehensible speech. His neighbor’s symptoms and his own unpleasant experiences prompted a discontinuation of use.
Synthetic cannabinoids are a diverse group of agents numbering in excess of 100 artificial compounds that act as agonists at cannabinoid receptors, mimicking the effects of tetrahydrocannabinol (THC), an active ingredient in marijuana.28,54 The availability of these drugs online and in specialty shops has been documented since the mid-2000s.27,28,32 Their packaging often describes the contents as incense or herbal blends, using various names. Spice is a common name, but these products are also known by a myriad of other designations (Table 1).28 A single packet usually contains several grams of the drug and costs about $30.55
To the user, who may already be familiar with marijuana, the contents intentionally appear similar to the dried buds of cannabis.30,56 In reality, the drug has just been sprayed onto inert plant material.57 The drug is smoked, and the psychoactive dose can be as little as 1 mg.30 Users describe potent drug effects (Table 2). There is a rapid onset of action, and duration of effects last 1 to 2 hours.58
The compounds’ mechanism of action and appeal are derived from their high affinity for the cannabinoid receptors. The CB1 receptor is located primarily in the central nervous system and is responsible for the psychoactive component of the drugs’ actions.27,30,58,59 Two particular synthetic cannabinoids, cannibicyclohexanol and JWH-018, are potent cannabinoid CB1 agonists with affinity exceeding their natural counterparts.27,30, 32,56,58,59
Chemically, these drugs are varied. The largest structural family of these compounds is the JWH group, which includes JWH-018.60 Also common are CP 47,497 and other CP compounds.58 HU compounds, such as HU-210, have also been identified and have been shown to be 100 to 800 times more potent at the CB1 and CB2 receptors than is THC.60,61 A final group includes the benzoylindoles, such as AM-964 and RCS-4, which also bind strongly to CB1 and CB2.60,62
Constitutional symptoms of synthetic cannabinoid intoxication include disorientation, anxiety, tremulousness, palpitations, tachycardia, agitation, injected conjunctivae, hyperreflexia, nausea, vomiting, lateral gaze nystagmus, and myoclonic jerks, which have been mistaken for seizure activity.27,30,55 Pupils are often normal sized.55 Withdrawal phenomena are similar to those of cannabis withdrawal: irritability, anxiety, tremor, palpitations, diaphoresis, insomnia, headache, diarrhea, nausea, and vomiting.59
Given the established link between cannabis use and psychosis, synthetic cannabinoids may stand as a precipitant of psychotic symptoms, which may include visual hallucinations, auditory hallucinations, disorganized speech, paranoia, grandiose delusions, disorganization, or bizarre behavior.58,63-66 These symptoms may represent a relapse of a primary thought disorder or, for some unfortunate individuals, a de novo psychotic illness.58,65,66 Symptoms can linger for months after drug use.65
A key risk in the use of synthetic cannabinoid moieties may involve the absence of cannabidiol. Cannabidiol naturally occurs in many strains of cannabis and is thought to have antipsychotic, neuroprotective properties.67 The absence of this molecule in synthetic cannabinoids may at least partially explain their severe psychoactive effects. Treatment for synthetic cannabinoid intoxication and related psychosis is largely supportive and may include the use of antipsychotic medication.66
Detection of synthetic cannabinoids in urine is difficult, yet many compounds can be detected via GC-MS or LC-MS. Molecules of significance include JWH-018, JWH-073, JWH-015, JWH-250, CP-47 497, HU-210, cannabicyclohexanol, and oleamide; however, these compounds are rarely excreted in urine in their pure form. The many hydroxylated or dealkylated metabolites of these compounds, mostly unnamed, are more consistently detected in urine.68,69 One author has noted that the pentanoic acid metabolite of JWH-018 seems to appear most reliably in urine specimens.68
Many synthetic cannabinoid herbal mixes also contain a detectable compound called tocopherol, seemingly added as an antioxidant.69,70 Synthetic cannabinoids are an evolving drug class, and reliable detection will require that laboratories stay up-to-date in their detection methods. As stated earlier, a commercial laboratory in the region accepted the civilian and veteran patient samples for these case studies. The synthetic cannabinoid panels offered evaluation of the drug itself (GC-MS), an oral fluid screen (LC-MS), and isolation of metabolites in urine (enzyme-linked immunosorbent assay).
Conclusion
Designer drugs will remain a challenge for providers caring for veterans for several key reasons: (1) Veterans are a vulnerable population who abuse substances at higher rates than do their civilian counterparts; (2) Chemists are able to manufacture variations of known habit-forming substances; (3) Modern technology facilitates the purchase and wide distribution of addictive substances; (4) Many designer drugs are deceptively packaged and marketed; (5) The effects of the drugs are often severe; (6) No standardized treatment guidelines exist; and (7) Detection of the drugs is difficult, and new versions of the molecules may evade even cutting-edge techniques.
Due to the high cost of detecting synthetic cathinones and synthetic cannabinoids in body fluids, screening should be considered only in settings where severe symptoms are accompanied by reasonable clinical suspicion of use and an otherwise negative toxicologic workup. As more designer drugs inevitably emerge, research will be needed on their pharmacology, toxidromes, and detection. Military and civilian practitioners must remain abreast of the dynamic trends in designer drugs to ensure that their patients receive the highest level of medical care.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Bray RM, Fairbank JA, Marsden ME. Stress and substance use among military women and men. J Drug Alcohol Abuse. 1999;25(2):239-256.
2. Bray RM, Hourani LL. Substance use trends among active duty military personnel: Findings from the United States Department of Defense Health Related Behavior Surveys, 1980-2005. Addiction. 2007;102(7):1092-1101.
3. Hankin CS, Spiro A 3rd, Miller DR, Kazis L. Mental disorders and mental health treatment among U.S. Department of Veterans Affairs outpatients: The Veterans Health Study. Am J Psychiatry. 1999;156(12):1924-1930.
4. Sirratt D, Ozanian A, Traenkner B. Epidemiology and prevention of substance use disorders in the military. Mil Med. 2012;177(suppl 8):21-28.
5. Winder GS, Stern N, Hosanagar A. Are “bath salts” the next generation of stimulant abuse? J Subst Abuse Treat. 2013;44(1):42-45.
6. Bobes J, Sáiz PA, González MP, et al. Use of MDMA and other illicit drugs by young adult males in northern Spain. A five-year study. Eur Addict Res. 2002;8(3):147-154.
7. D.E.A. Cracks Down on Designer Drug Operations. The New York Times Website. http://www.nytimes.com/2013/06/27/us/dea-cracks-down-on-designer-drug-operations.html. Published June 26, 2013. Accessed October 6, 2014.
8. Travis A. Mushrooming legal highs leave drug control system floundering, UN warns. The Guardian Website. http://www.guardian.co.uk/world/2013/jun/26/legal-highs-drug-control. Published June 26, 2013. Accessed October 6, 2014.
9. Jerry J, Collins G, Streem D. Synthetic legal intoxicating drugs: The emerging ‘incense’ and ‘bath salt’ phenomenon. Cleve Clin J Med. 2012;79(4):258-264.
10. Murphy CM, Dulaney AR, Beuhler MC, Kacinko S. “Bath salts” and “plant food” products: The experience of one regional US poison center. J Med Toxicol. 2013;9(1):42-48.
11. Wax PM. Just a click away: Recreational drug web sites on the Internet. Pediatrics. 2002;109(6):e96.
12. Vardakou I, Pistos C, Spiliopoulou C. Drugs for youth via Internet and the example of mephedrone. Toxicol Lett. 2011;201(3):191-195.
13. Winickoff JP, Houck CS, Rothman EL, Bauchner H. Verve and jolt: Deadly new Internet drugs. Pediatrics. 2000;106(4):829-830.
14. Camilleri A, Johnston MR, Brennan M, Davis S, Caldicott DG. Chemical analysis of four capsules containing the controlled substance analogues 4-methylmethcathinone, 2-fluoromethamphetamine, alpha-phthalimidopropiophenone and N-ethylcathinone. Forensic Sci Int. 2010;197(1-3):59-66.
15. Carroll FI, Lewin AH, Mascarella SW, Seltzman HH, Reddy PA. Designer drugs: A medicinal chemistry perspective. Ann N Y Acad Sci. 2012;1248:18-38.
16. Christophersen AS. Amphetamine designer drugs—An overview and epidemiology. Toxicol Lett. 2000;112-113:127-131.
17. Buchanan JF, Brown CR. ‘Designer drugs.’ A problem in clinical toxicology. Med Toxicol Adverse Drug Exp. 1988;3(1):1-17.
18. Griffiths P, Sedefov R, Gallegos A, Lopez D. How globalization and market innovation challenge how we think about and respond to drug use: ‘Spice’ a case study. Addiction. 2010;105(6):951-953.
19. Drug Enforcement Administration, Department of Justice. Schedules of controlled substances: Temporary placement of three synthetic cathinones in Schedule I. Final Order. Fed Regist. 2011;76(204):65371-65375.
20. Drug Enforcement Administration, Department of Justice. Schedules of controlled substances: Placement of five synthetic cannabinoids into Schedule I. Fed Regist. 2012;77(41):12508-12514.
21. Drug Enforcement Administration, Department of Justice. Schedules of controlled substances: Temporary placement of three synthetic cannabinoids into Schedule I. Fed Regist. 2013;78(95):28735-28739.
22. Council of the European Union. Council Decision of 2 December 2010 on submitting 4-methylmethcathinone (mephedrone) to control measures. EUR-Lex Website. eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2010:322:0044:0045:en:PDF. Published December 8, 2010. Accessed October 7, 2014.
23. European Monitoring Centre for Drugs and Drug Addiction. Synthetic cannabinoids and ‘spice’. European Monitoring Centre for Drugs and Drug Addiction Website. http://www.emcdda.europa.eu/publications/drug-profiles/synthetic-cannabinoids. Updated September 15, 2011. Accessed October 7, 2014.
24. United Nations Office on Drugs and Crime. World Drug Report 2014. United Nations Office on Drugs and Crime Website. http://www.unodc.org/wdr2014. Accessed October 3, 2014.
25. Fass JA, Fass AD, Garcia AS. Synthetic cathinones (bath salts): Legal status and patterns of abuse. Ann Pharmacother. 2012;46(3):436-441.
26. Gunderson EW, Haughey HM, Ait-Daoud N, Joshi AS, Hart CL. “Spice” and “K2” herbal highs: A case series and systematic review of the clinical effects and biopsychosocial implications of synthetic cannabinoid use in humans. Am J Addict. 2012;21(4):320-326.
27. Loeffler G, Hurst D, Penn A, Yung K. Spice, bath salts, and the U.S. military: The emergence of synthetic cannabinoid receptor agonists and cathinones in the U.S. Armed Forces. Mil Med. 2012;177(9):1041-1048.
28. European Monitoring Centre for Drugs and Drug Addiction. Understanding the spice phenomenon. The National Documentation Centre on Drug Use Website. http://www.drugsandalcohol.ie/12597/1/Understanding_the_Spice_phenomenon.pdf. Published 2009. Accessed October 7, 2014.
29. Davies S, Wood DM, Smith G, et al. Purchasing ‘legal highs’ on the Internet—Is there consistency in what you get? QJM. 2010;103(7):489-493.
30. Harris CR, Brown A. Synthetic cannabinoid intoxication: A case series and review. J Emerg Med. 2013;44(2):360-366.
31. Ammann D, McLaren JM, Gerostamoulos D, Beyer J. Detection and quantification of new designer drugs in human blood: Part 2--Designer cathinones. J Anal Toxicol. 2012;36(6):381-389.
32. Hudson S, Ramsey J. The emergence and analysis of synthetic cannabinoids. Drug Test Anal. 2011;3(7-8):466-478.
33. Al-Habori M. The potential adverse effects of habitual use of Catha edulis (khat). Expert Opin Drug Saf. 2005;4(6):1145-1154.
34. Al-Motarreb A, Al-Habori M, Broadley KJ. Khat chewing, cardiovascular diseases and other internal medical problems: The current situation and directions for future research. J Ethnopharmacol. 2010;132(3):540-548.
35. Dargan PI, Sedefov R, Gallegos A, Wood DM. The pharmacology and toxicology of the synthetic cathinone mephedrone (4-methylmethcathinone). Drug Test Anal. 2011;3(7-8):454-463.
36. Kriikku P, Wilhelm L, Schwarz O, Rintatalo J. New designer drug of abuse: 3,4-Methylenedioxypyrovalerone (MDPV). Findings from apprehended drivers in Finland. Forensic Sci Int. 2011;210(1-3):195-200.
37. Morris K. UK places generic ban on mephedrone drug family. Lancet. 2010;375(9723):1333-1334.
38. Garrett G, Sweeney M. The serotonin syndrome as a result of mephedrone toxicity. BMJ Case Rep. 2010.
39. Sammler EM, Foley PL, Lauder GD, Wilson SJ, Goudie AR, O’Riordan JI. A harmless high? Lancet. 2010;376(9742):742.
40. Prosser JM, Nelson LS. The toxicology of bath salts: A review of synthetic cathinones. J Med Toxicol. 2012;8(1):33-42.
41. Thornton SL, Gerona RR, Tomaszewski CA. Psychosis from a bath salt product containing flephedrone and MDPV with serum, urine, and product quantification. J Med Toxicol. 2012;8(3):310-313.
42. Emerson TS, Cisek JE. Methcathinone: a Russian designer amphetamine infiltrates the rural midwest. Ann Emerg Med. 1993;22(12):1897-1903.
43. Winstock AR, Mitcheson LR, Deluca P, Davey Z, Corazza O, Schifano F. Mephedrone, new kid for the chop? Addict. 2011;106(1):154-161.
44. Spiller HA, Ryan ML, Weston RG, Jansen J. Clinical experience with and analytical confirmation of “bath salts” and “legal highs” (synthetic cathinones) in the United States. Clin Toxicol (Phila). 2011;49(6):499-505.
45. Ojanperä IA, Heikman PK, Rasanen IJ. Urine analysis of 3,4-methylenedioxypyrovalerone in opioid-dependent patients by gas chromatography-mass spectrometry. Ther Drug Monit. 2011;33(2):257-263.
46. Meyer MR, Du P, Schuster F, Maurer HH. Studies on the metabolism of the alpha-pyrrolidinophenone designer drug methylenedioxy-pyrovalerone (MDPV) in rat and human urine and human liver microsomes using GC-MS and LC-high-resolution MS and its detectability in urine by GC-MS. JMS. 2010;45(12):1426-1442.
47. Santali EY, Cadogan AK, Daeid NN, Savage KA, Sutcliffe OB. Synthesis, full chemical characterisation and development of validated methods for the quantification of (+/-)-4’-methylmethcathinone (mephedrone): A new “legal high”. J Pharm Biomed Anal. 2011;56(2):246-255.
48. Meyer MR, Vollmar C, Schwaninger AE, Wolf E, Maurer HH. New cathinone-derived designer drugs 3-bromomethcathinone and 3-fluoromethcathinone: Studies on their metabolism in rat urine and human liver microsomes using GC-MS and LC-high-resolution MS and their detectability in urine. JMS. 2012;47(2):253-262.
49. Borek HA, Holstege CP. Hyperthermia and multiorgan failure after abuse of “bath salts” containing 3,4-methylenedioxypyrovalerone. Ann Emerg Med. 2012;60(1):103-105.
50. Kasick DP, McKnight CA, Klisovic E. “Bath salt” ingestion leading to severe intoxication delirium: Two cases and a brief review of the emergence of mephedrone use. Am J Drug Alcohol Abuse. 2012;38(2):176-180.
51. Penders TM, Gestring RE, Vilensky DA. Excited delirium following use of synthetic cathinones (bath salts). Gen Hosp Psychiatry. 2012;34(6):647-650.
52. Nicholson PJ, Quinn MJ, Dodd JD. Headshop heartache: Acute mephedrone ‘meow’ myocarditis. Heart. 2010;96(24):2051-2052.
53. Murray BL, Murphy CM, Beuhler MC. Death following recreational use of designer drug “bath salts” containing 3,4-Methylenedioxypyrovalerone (MDPV). J Med Toxicol. 2012;8(1):69-75.
54. Gunderson EW, Haughey HM, Ait-Daoud N, Joshi AS, Hart CL. Spice” and “K2” herbal highs: A case series and systematic review of the clinical effects and biopsychosocial implications of synthetic cannabinoid use in humans. Am J Addict. 2012;21(4):320-326.
55. Schneir AB, Cullen J, Ly BT. “Spice” girls: Synthetic cannabinoid intoxication. J Emerg Med. 2011;40(3):296-299.
56. Atwood BK, Huffman J, Straiker A, Mackie K. JWH018, a common constituent of ‘Spice’ herbal blends, is a potent and efficacious cannabinoid CB receptor agonist. Br J Pharmacol. 2010;160(3):585-593.
57. Uchiyama N, Kikura-Hanajiri R, Ogata J, Goda Y. Chemical analysis of synthetic cannabinoids as designer drugs in herbal products. Forensic Sci Int. 2010;198(1-3):31-38.
58. Every-Palmer S. Synthetic cannabinoid JWH-018 and psychosis: An explorative study. Drug Alcohol Depend. 2011;117(2-3):152-157.
59. Zimmermann US, Winkelmann PR, Pilhatsch M, Nees JA, Spanagel R, Schulz K. Withdrawal phenomena and dependence syndrome after the consumption of “spice gold.” Deutsches Ärzteblatt Int. 2009;106(27):464-467.
60. Loeffler G, Hurst D, Penn A, Yung K. Spice, Bath salts, and the US military: The emergence of synthetic cannabinoid receptor agonists and cathinones in the US armed forces. Mil Med. 2012;177(9):1041-1048.
61. Devane WA, Breuer A, Sheskin T, Jäerbe TU, Eisen MS, Mechoulam R. A novel probe for the cannabinoid receptor. J Med Chem. 1992;35(11):2065-2069.
62. Gottardo R, Chiarini A, Dal Prà I, et al. Direct screening of herbal blends for new synthetic cannabinoids by MALDI-TOF MS. JMS. 2012;47(1):141-146.
63. McGrath J, Welham J, Scott J, et al. Association between cannabis use and psychosis-related outcomes using sibling pair analysis in a cohort of young adults. Arch Gen Psychiatry. 2010;67(5):440-447.
64. Every-Palmer S. Warning: Legal synthetic cannabinoid-receptor agonists such as JWH-018 may precipitate psychosis in vulnerable individuals. Addict. 2010;105(10):1859-1860.
65. Hurst D, Loeffler G, McLay R. Psychosis associated with synthetic cannabinoid agonists: A case series. Am J Psychiatry. 2011;168(10):1119.
66. Peglow S, Buchner J, Briscoe G. Synthetic cannabinoid induced psychosis in a previously nonpsychotic patient. Am J Addict. 2012;21(3):287-288.
67. Morgan CJ, Curran HV. Effects of cannabidiol on schizophrenia-like symptoms in people who use cannabis. Br J Psychiatry. 2008;192(4):306-307.
68. ElSohly MA, Gul W, ElSohly KM, Murphy TP, Madgula VL, Khan SI. Liquid chromatography-tandem mass spectrometry analysis of urine specimens for K2 (JWH-018) metabolites. J Anal Toxicol. 2011;35(7):487-495.
69. Grigoryev A, Savchuk S, Melnik A, et al. Chromatography–mass spectrometry studies on the metabolism of synthetic cannabinoids JWH-018 and JWH-073, psychoactive components of smoking mixtures. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879(15-16):1126-1136.
70. Sobolevsky T, Prasolov I, Rodchenkov G. Detection of JWH-018 metabolites in smoking mixture post-administration urine. Forensic Sci Int. 2010;200(1):141-147.
1. Bray RM, Fairbank JA, Marsden ME. Stress and substance use among military women and men. J Drug Alcohol Abuse. 1999;25(2):239-256.
2. Bray RM, Hourani LL. Substance use trends among active duty military personnel: Findings from the United States Department of Defense Health Related Behavior Surveys, 1980-2005. Addiction. 2007;102(7):1092-1101.
3. Hankin CS, Spiro A 3rd, Miller DR, Kazis L. Mental disorders and mental health treatment among U.S. Department of Veterans Affairs outpatients: The Veterans Health Study. Am J Psychiatry. 1999;156(12):1924-1930.
4. Sirratt D, Ozanian A, Traenkner B. Epidemiology and prevention of substance use disorders in the military. Mil Med. 2012;177(suppl 8):21-28.
5. Winder GS, Stern N, Hosanagar A. Are “bath salts” the next generation of stimulant abuse? J Subst Abuse Treat. 2013;44(1):42-45.
6. Bobes J, Sáiz PA, González MP, et al. Use of MDMA and other illicit drugs by young adult males in northern Spain. A five-year study. Eur Addict Res. 2002;8(3):147-154.
7. D.E.A. Cracks Down on Designer Drug Operations. The New York Times Website. http://www.nytimes.com/2013/06/27/us/dea-cracks-down-on-designer-drug-operations.html. Published June 26, 2013. Accessed October 6, 2014.
8. Travis A. Mushrooming legal highs leave drug control system floundering, UN warns. The Guardian Website. http://www.guardian.co.uk/world/2013/jun/26/legal-highs-drug-control. Published June 26, 2013. Accessed October 6, 2014.
9. Jerry J, Collins G, Streem D. Synthetic legal intoxicating drugs: The emerging ‘incense’ and ‘bath salt’ phenomenon. Cleve Clin J Med. 2012;79(4):258-264.
10. Murphy CM, Dulaney AR, Beuhler MC, Kacinko S. “Bath salts” and “plant food” products: The experience of one regional US poison center. J Med Toxicol. 2013;9(1):42-48.
11. Wax PM. Just a click away: Recreational drug web sites on the Internet. Pediatrics. 2002;109(6):e96.
12. Vardakou I, Pistos C, Spiliopoulou C. Drugs for youth via Internet and the example of mephedrone. Toxicol Lett. 2011;201(3):191-195.
13. Winickoff JP, Houck CS, Rothman EL, Bauchner H. Verve and jolt: Deadly new Internet drugs. Pediatrics. 2000;106(4):829-830.
14. Camilleri A, Johnston MR, Brennan M, Davis S, Caldicott DG. Chemical analysis of four capsules containing the controlled substance analogues 4-methylmethcathinone, 2-fluoromethamphetamine, alpha-phthalimidopropiophenone and N-ethylcathinone. Forensic Sci Int. 2010;197(1-3):59-66.
15. Carroll FI, Lewin AH, Mascarella SW, Seltzman HH, Reddy PA. Designer drugs: A medicinal chemistry perspective. Ann N Y Acad Sci. 2012;1248:18-38.
16. Christophersen AS. Amphetamine designer drugs—An overview and epidemiology. Toxicol Lett. 2000;112-113:127-131.
17. Buchanan JF, Brown CR. ‘Designer drugs.’ A problem in clinical toxicology. Med Toxicol Adverse Drug Exp. 1988;3(1):1-17.
18. Griffiths P, Sedefov R, Gallegos A, Lopez D. How globalization and market innovation challenge how we think about and respond to drug use: ‘Spice’ a case study. Addiction. 2010;105(6):951-953.
19. Drug Enforcement Administration, Department of Justice. Schedules of controlled substances: Temporary placement of three synthetic cathinones in Schedule I. Final Order. Fed Regist. 2011;76(204):65371-65375.
20. Drug Enforcement Administration, Department of Justice. Schedules of controlled substances: Placement of five synthetic cannabinoids into Schedule I. Fed Regist. 2012;77(41):12508-12514.
21. Drug Enforcement Administration, Department of Justice. Schedules of controlled substances: Temporary placement of three synthetic cannabinoids into Schedule I. Fed Regist. 2013;78(95):28735-28739.
22. Council of the European Union. Council Decision of 2 December 2010 on submitting 4-methylmethcathinone (mephedrone) to control measures. EUR-Lex Website. eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2010:322:0044:0045:en:PDF. Published December 8, 2010. Accessed October 7, 2014.
23. European Monitoring Centre for Drugs and Drug Addiction. Synthetic cannabinoids and ‘spice’. European Monitoring Centre for Drugs and Drug Addiction Website. http://www.emcdda.europa.eu/publications/drug-profiles/synthetic-cannabinoids. Updated September 15, 2011. Accessed October 7, 2014.
24. United Nations Office on Drugs and Crime. World Drug Report 2014. United Nations Office on Drugs and Crime Website. http://www.unodc.org/wdr2014. Accessed October 3, 2014.
25. Fass JA, Fass AD, Garcia AS. Synthetic cathinones (bath salts): Legal status and patterns of abuse. Ann Pharmacother. 2012;46(3):436-441.
26. Gunderson EW, Haughey HM, Ait-Daoud N, Joshi AS, Hart CL. “Spice” and “K2” herbal highs: A case series and systematic review of the clinical effects and biopsychosocial implications of synthetic cannabinoid use in humans. Am J Addict. 2012;21(4):320-326.
27. Loeffler G, Hurst D, Penn A, Yung K. Spice, bath salts, and the U.S. military: The emergence of synthetic cannabinoid receptor agonists and cathinones in the U.S. Armed Forces. Mil Med. 2012;177(9):1041-1048.
28. European Monitoring Centre for Drugs and Drug Addiction. Understanding the spice phenomenon. The National Documentation Centre on Drug Use Website. http://www.drugsandalcohol.ie/12597/1/Understanding_the_Spice_phenomenon.pdf. Published 2009. Accessed October 7, 2014.
29. Davies S, Wood DM, Smith G, et al. Purchasing ‘legal highs’ on the Internet—Is there consistency in what you get? QJM. 2010;103(7):489-493.
30. Harris CR, Brown A. Synthetic cannabinoid intoxication: A case series and review. J Emerg Med. 2013;44(2):360-366.
31. Ammann D, McLaren JM, Gerostamoulos D, Beyer J. Detection and quantification of new designer drugs in human blood: Part 2--Designer cathinones. J Anal Toxicol. 2012;36(6):381-389.
32. Hudson S, Ramsey J. The emergence and analysis of synthetic cannabinoids. Drug Test Anal. 2011;3(7-8):466-478.
33. Al-Habori M. The potential adverse effects of habitual use of Catha edulis (khat). Expert Opin Drug Saf. 2005;4(6):1145-1154.
34. Al-Motarreb A, Al-Habori M, Broadley KJ. Khat chewing, cardiovascular diseases and other internal medical problems: The current situation and directions for future research. J Ethnopharmacol. 2010;132(3):540-548.
35. Dargan PI, Sedefov R, Gallegos A, Wood DM. The pharmacology and toxicology of the synthetic cathinone mephedrone (4-methylmethcathinone). Drug Test Anal. 2011;3(7-8):454-463.
36. Kriikku P, Wilhelm L, Schwarz O, Rintatalo J. New designer drug of abuse: 3,4-Methylenedioxypyrovalerone (MDPV). Findings from apprehended drivers in Finland. Forensic Sci Int. 2011;210(1-3):195-200.
37. Morris K. UK places generic ban on mephedrone drug family. Lancet. 2010;375(9723):1333-1334.
38. Garrett G, Sweeney M. The serotonin syndrome as a result of mephedrone toxicity. BMJ Case Rep. 2010.
39. Sammler EM, Foley PL, Lauder GD, Wilson SJ, Goudie AR, O’Riordan JI. A harmless high? Lancet. 2010;376(9742):742.
40. Prosser JM, Nelson LS. The toxicology of bath salts: A review of synthetic cathinones. J Med Toxicol. 2012;8(1):33-42.
41. Thornton SL, Gerona RR, Tomaszewski CA. Psychosis from a bath salt product containing flephedrone and MDPV with serum, urine, and product quantification. J Med Toxicol. 2012;8(3):310-313.
42. Emerson TS, Cisek JE. Methcathinone: a Russian designer amphetamine infiltrates the rural midwest. Ann Emerg Med. 1993;22(12):1897-1903.
43. Winstock AR, Mitcheson LR, Deluca P, Davey Z, Corazza O, Schifano F. Mephedrone, new kid for the chop? Addict. 2011;106(1):154-161.
44. Spiller HA, Ryan ML, Weston RG, Jansen J. Clinical experience with and analytical confirmation of “bath salts” and “legal highs” (synthetic cathinones) in the United States. Clin Toxicol (Phila). 2011;49(6):499-505.
45. Ojanperä IA, Heikman PK, Rasanen IJ. Urine analysis of 3,4-methylenedioxypyrovalerone in opioid-dependent patients by gas chromatography-mass spectrometry. Ther Drug Monit. 2011;33(2):257-263.
46. Meyer MR, Du P, Schuster F, Maurer HH. Studies on the metabolism of the alpha-pyrrolidinophenone designer drug methylenedioxy-pyrovalerone (MDPV) in rat and human urine and human liver microsomes using GC-MS and LC-high-resolution MS and its detectability in urine by GC-MS. JMS. 2010;45(12):1426-1442.
47. Santali EY, Cadogan AK, Daeid NN, Savage KA, Sutcliffe OB. Synthesis, full chemical characterisation and development of validated methods for the quantification of (+/-)-4’-methylmethcathinone (mephedrone): A new “legal high”. J Pharm Biomed Anal. 2011;56(2):246-255.
48. Meyer MR, Vollmar C, Schwaninger AE, Wolf E, Maurer HH. New cathinone-derived designer drugs 3-bromomethcathinone and 3-fluoromethcathinone: Studies on their metabolism in rat urine and human liver microsomes using GC-MS and LC-high-resolution MS and their detectability in urine. JMS. 2012;47(2):253-262.
49. Borek HA, Holstege CP. Hyperthermia and multiorgan failure after abuse of “bath salts” containing 3,4-methylenedioxypyrovalerone. Ann Emerg Med. 2012;60(1):103-105.
50. Kasick DP, McKnight CA, Klisovic E. “Bath salt” ingestion leading to severe intoxication delirium: Two cases and a brief review of the emergence of mephedrone use. Am J Drug Alcohol Abuse. 2012;38(2):176-180.
51. Penders TM, Gestring RE, Vilensky DA. Excited delirium following use of synthetic cathinones (bath salts). Gen Hosp Psychiatry. 2012;34(6):647-650.
52. Nicholson PJ, Quinn MJ, Dodd JD. Headshop heartache: Acute mephedrone ‘meow’ myocarditis. Heart. 2010;96(24):2051-2052.
53. Murray BL, Murphy CM, Beuhler MC. Death following recreational use of designer drug “bath salts” containing 3,4-Methylenedioxypyrovalerone (MDPV). J Med Toxicol. 2012;8(1):69-75.
54. Gunderson EW, Haughey HM, Ait-Daoud N, Joshi AS, Hart CL. Spice” and “K2” herbal highs: A case series and systematic review of the clinical effects and biopsychosocial implications of synthetic cannabinoid use in humans. Am J Addict. 2012;21(4):320-326.
55. Schneir AB, Cullen J, Ly BT. “Spice” girls: Synthetic cannabinoid intoxication. J Emerg Med. 2011;40(3):296-299.
56. Atwood BK, Huffman J, Straiker A, Mackie K. JWH018, a common constituent of ‘Spice’ herbal blends, is a potent and efficacious cannabinoid CB receptor agonist. Br J Pharmacol. 2010;160(3):585-593.
57. Uchiyama N, Kikura-Hanajiri R, Ogata J, Goda Y. Chemical analysis of synthetic cannabinoids as designer drugs in herbal products. Forensic Sci Int. 2010;198(1-3):31-38.
58. Every-Palmer S. Synthetic cannabinoid JWH-018 and psychosis: An explorative study. Drug Alcohol Depend. 2011;117(2-3):152-157.
59. Zimmermann US, Winkelmann PR, Pilhatsch M, Nees JA, Spanagel R, Schulz K. Withdrawal phenomena and dependence syndrome after the consumption of “spice gold.” Deutsches Ärzteblatt Int. 2009;106(27):464-467.
60. Loeffler G, Hurst D, Penn A, Yung K. Spice, Bath salts, and the US military: The emergence of synthetic cannabinoid receptor agonists and cathinones in the US armed forces. Mil Med. 2012;177(9):1041-1048.
61. Devane WA, Breuer A, Sheskin T, Jäerbe TU, Eisen MS, Mechoulam R. A novel probe for the cannabinoid receptor. J Med Chem. 1992;35(11):2065-2069.
62. Gottardo R, Chiarini A, Dal Prà I, et al. Direct screening of herbal blends for new synthetic cannabinoids by MALDI-TOF MS. JMS. 2012;47(1):141-146.
63. McGrath J, Welham J, Scott J, et al. Association between cannabis use and psychosis-related outcomes using sibling pair analysis in a cohort of young adults. Arch Gen Psychiatry. 2010;67(5):440-447.
64. Every-Palmer S. Warning: Legal synthetic cannabinoid-receptor agonists such as JWH-018 may precipitate psychosis in vulnerable individuals. Addict. 2010;105(10):1859-1860.
65. Hurst D, Loeffler G, McLay R. Psychosis associated with synthetic cannabinoid agonists: A case series. Am J Psychiatry. 2011;168(10):1119.
66. Peglow S, Buchner J, Briscoe G. Synthetic cannabinoid induced psychosis in a previously nonpsychotic patient. Am J Addict. 2012;21(3):287-288.
67. Morgan CJ, Curran HV. Effects of cannabidiol on schizophrenia-like symptoms in people who use cannabis. Br J Psychiatry. 2008;192(4):306-307.
68. ElSohly MA, Gul W, ElSohly KM, Murphy TP, Madgula VL, Khan SI. Liquid chromatography-tandem mass spectrometry analysis of urine specimens for K2 (JWH-018) metabolites. J Anal Toxicol. 2011;35(7):487-495.
69. Grigoryev A, Savchuk S, Melnik A, et al. Chromatography–mass spectrometry studies on the metabolism of synthetic cannabinoids JWH-018 and JWH-073, psychoactive components of smoking mixtures. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879(15-16):1126-1136.
70. Sobolevsky T, Prasolov I, Rodchenkov G. Detection of JWH-018 metabolites in smoking mixture post-administration urine. Forensic Sci Int. 2010;200(1):141-147.
Infusing Gerontologic Practice Into PACT
The older adult population in the VA is growing. Adults aged > 85 years are the fastest growing segment of the older veteran population and many are afflicted with multiple medical problems and functional impairments.1,2 The majority of older veterans (94.6%, or about 1.9 million veterans) who seek care at the VA obtain care through primary care providers (PCPs) who are often not formally trained in geriatrics.1,3 With the increasing number of older patients, new models of care are needed to provide coordinated, comprehensive, efficient, and patient-centered care.4,5
Common themes found in successful models of care for older patients include a team approach, care management (comprehensive and coordinated), and patients who are active partners.4 These themes are reflected in the VA Patient Aligned Care Team (PACT) primary care program. PACT, a model of care that was initiated in 2010 and is built on a foundation of patient-centered care, encompasses a team approach to provide comprehensive, coordinated, and personalized care.6-8 The challenge for the VA is to integrate gerontologic principles and tools into the daily practice of all PACTs in order to improve care provided for older veterans.9
This article discusses current challenges in caring for older veterans in the VA system and recommends tools that can be used to infuse geriatric care principles into VA primary care by the PACT, to improve the quality of care provided to older veterans. In addition, the article also describes VA geriatric programs that PACT clinicians can access to supplement older veterans’ care.
Challenges of Caring for Older Veterans
One concern when caring for older veterans arises when the veteran accesses both VA and non-VA health care services to offset medication costs and obtain services not covered by Medicare or other insurance companies.2,3 This “dual care” can exacerbate polypharmacy issues and increase confusion regarding plans of care. Problems may arise when multiple providers from different systems of care prescribe medications available only within their own formulary and/or order diagnostic and laboratory tests with results available only within their own health care system.
The VA is also challenged by health care delivery for rural veterans. Thirty-six percent of all veterans live in rural areas, and they often depend on non-VA services to meet their health care needs due to difficulty traveling to the nearest VA facility.10 Seasonal residency also presents challenges. An increasing number of older veterans are seen at different VA facilities when they “winter” in a different section of the country.
Fortunately, a VA provider in one facility can access a patient’s electronic medical records in another facility, using the VA Computerized Patient Record System (CPRS). However, it is unclear to what extent busy VA PCPs use this function when seeing patients. Although individual pilot programs have shown promise, integrated electronic health records between VA and non-VA health care have not advanced to the point of sharing data or reconciling care plans (R. Rupper, personal communication, March 1, 2013).
Many PCPs and other PACT staff are not formally trained in geriatrics and may have had limited exposure to geriatric principles.3 Clinic time pressures, multiple clinical reminders (eg, vaccinations), and panel management of specific diseases make it challenging to find time to focus on complex geriatric syndromes. Current PACT performance measures also do not routinely include geriatric-specific quality of care criteria or focus on patient function (K. Shay, personal communication, February 12, 2013), a hallmark of geriatric care.8 Furthermore, with increasing complexity of the health care system and limited availability of resources, it is often time consuming to identify and collaborate with non-VA resources to ensure patients’ needs are met in their communities.
Opportunities for Improvement in Care
The VA transformation to PACTs has led to process changes in clinic workflow that may aid in addressing the aforementioned challenges in caring for older veterans. Each patient is assigned to a PCP-led team that includes a registered nurse care manager, a clinical associate, and an administrative associate. The PACT model of care has increased access to care by redesigning face-to-face visits, increasingly moving toward open access, and through the increased use of virtual access via secured e-mail, telephone visits, and telehealth.8
In addition to process changes, the VA has created new tools to assist teams in patient management. One of these is the Care Assessment Need (CAN) score, a risk stratification tool available for use by PACTs to identify patients at highest risk for hospital admission and/or death for focused care management.11 It is based on statistical prediction models of veterans enrolled in primary care, using patient characteristics and health care use information.11 Although the CAN score looks promising, more research is needed to evaluate its effectiveness in improving care for older veterans and its association with better patient functioning—an important focus in quality geriatric care.
A tool that takes into account daily function is the Vulnerable Elders Survey-13 (VES-13). As measured by the VES-13, functional ability has been shown to be a strong predictor of decline and death in older adults independent of gender or comorbidities.12 Integration of the VES-13 into the evaluation of older veterans could assist PACTs in considering patients’ current function and life expectancy in their care plans along with patient and family goals.
Another potentially useful tool for the PACT team is the SPICES mnemonic (Sleeping, Problems with feeding/eating, Incontinence or urinary problems, Confusion, Evidence of falls, and Skin breakdown).13 Although SPICES is not comprehensive, this mnemonic highlights potential problems facing older patients that may not be brought up routinely. It provides a concise, formalized format that can be used by clerks or patient support assistants as part of the check-in process.
This tool has been used successfully by the Geriatric Evaluation and Management Clinic of the South Texas Veterans Health Care System (STVHCS) to improve communication between the PCP and nurse so that pertinent patient information is relayed concisely. SPICES was helpful in identifying patients needing interventions for fall risk. In a retrospective chart review of 100 randomly selected patients aged 75 to 90 years enrolled in the clinic, a 75% reduction in falls was noted during the first year of implementation (STVHCS unpublished data, 2012).
Additional tools that focus on identifying specific geriatric syndromes are available online from the Hartford Institute for Geriatric Nursing, which provides evidence-based information and training on how to assess, evaluate, and manage common geriatric syndromes such as depression, dementia, and delirium.14 The site also includes videos on how to use common brief geriatric assessment tools that can be performed by nurses and health care associates while the patient is in the waiting room. Though promising, further research is needed to study the effects of these tools on patient, provider, and system outcomes.
Infusing quality of care indicators (QI) can play an integral role in achieving PACT goals while improving the older veterans’ quality of life. For example, polypharmacy and medication-related injuries in older adults continue to pose both a safety and economic challenge to patients and the health care system.15-17 The 2012 Beers criteria for Potentially Inappropriate Medications in Older Adults lists 53 medication classes that have been identified as potentially inappropriate medications for use in older adults.17 Use of this tool by PACTs in the development of patient care plans has the potential to reduce medication-related adverse reactions and improper prescribing.18,19
Assessing Care of Vulnerable Elders (ACOVE ) also provides QIs that are specific to vulnerable older persons.20-24 The most recent version, ACOVE-3, includes 392 QIs for 26 conditions and 14 types of care processes and covers all domains of care.20 Findings from a study applying QIs involving vulnerable elderly patients in 2 managed care programs revealed that recipients of better-quality care had a 10% higher survival rate over 3 years.25
The VA currently monitors 6 frail elderly QIs based on ACOVE criteria via reviews of medical records in veterans aged > 75 years. These QIs cover falls, incontinence, functional assessment, and the presence of a surrogate decision maker. PACT staff, unfortunately, do not receive feedback on these, because they are still QIs and not part of the performance measures (K. Shay, personal communication, February 12, 2013). Though some VA sites have adopted these QIs to some extent, until these frail elderly QIs become performance measures throughout VA, other competing priorities may be more at the forefront of quality improvement projects done by PACT teams.5
The American Geriatrics Society recently published recommendations on the care of older adults with multiple chronic conditions, to aid PCPs in practicing a more individualized, patient-centered care in complex cases.26 In addition to focusing on a patient’s primary concern during a clinic visit and eliciting preferences, considering prognosis in deciding on treatment options allows patients to better weigh the potential benefits and burdens in their daily living.26 A discussion on how aggressive potential treatments are and what the patient is willing to undertake is an important component of patient-centered care and should be incorporated during routine PACT clinic visits.
VA Geriatric Programs
It is important for PACT clinicians to be familiar with the geriatric programs and resources available within the VA medical home “neighborhood,” which can supplement care. One such resource is the Geriatric Research Education and Clinical Centers (GRECCs). There are currently 19 GRECCs throughout the nation that serve as Centers of Excellence in the care of older veterans.27 The GRECCs provide training for clinicians, test innovative ways to care for older veterans, and collaborate with other staff to improve the care provided. Some have also developed Geriatric Primary Care Clinics (or Geri PACTs) to provide team care to very frail and high-risk older veterans. Since not all VA facilities have access to Geri PACTs, the GRECCs play an important role in making geriatric expertise and training available to the PACTs.3
To address this limitation in access, VA programs have begun using telehealth technology to increase competencies of PCPs in caring for older veterans. For example, the VA Geriatric Scholars Program is a national educational program with different avenues to “geriatricize” VA primary care services and improve knowledge and care provided to older veterans.28 It consists of several subprograms: Geriatric Scholars Program for Rural Community Based Outpatient Clinics; Geriatric Scholars Program for Primary Care Providers; Rural Interdisciplinary Team Training; and the Geriatric Assessment Pocket Guide.29 These components may include didactics both face-to-face and online, clinical experience with performing common geriatric screening tools, and a quality improvement project.
Some local VAMCs have also developed programs to address this need to improve care provided to older veterans in PACT. The VA Greater Los Angeles Healthcare System (GLA) GRECC, for example, has started several programs to infuse geriatrics into PACTs, including the Geri Specialty Care Access Network-Extension for Community Healthcare Outcomes (SCAN-ECHO). VA SCAN-ECHO was developed to increase access to specialty care in rural/underserved areas. The PCP presents a case and a specialty provider gives guidance in the assessment and/or management of a specific clinical problem.30 Unlike many other SCAN-ECHO programs, the GLA Geri SCAN-ECHO program encourages not only PCPs, but also nurses and social workers to submit consults for discussion and encourages team management (a hallmark of quality geriatric care). Another important GLA GRECC project is the Veterans Cognitive Assessment and Management Program (V-CAMP), which uses videoconferencing to assess and manage veterans with cognitive impairment/dementia who reside in underserved areas in the GLA region. The program provides dementia care management and access to neuropsychological examinations—services that are often not available in rural areas.31
Various VA program offices have also published useful resources to help PACT clinicians infuse gerontologic principles into their practice. The VA Office of Nursing Services has a Geriatrics and Extended Care Field Advisory Committee, which recently produced on-demand lectures in the virtual VA eHealth University (also known as myVeHU campus) on improving the PACT’s management of progressive chronic diseases and dementia recognition and initial evaluation. They also produced a resource guide for VA clinicians (nursing and non-nursing), based on a team consensus of what the workgroup thinks a clinician would find helpful in clinical practice to improve care of older veterans. The VA Office of Geriatrics and Extended Care Service also identified a list of clinical and educational resources to help PACT clinicians. These include the Geriatrics Evaluation and management (GEM) Tools Booklet (http://geriatricscareonline.org) and a SharePoint site to improve dementia care in all settings.
The VA Office of Geriatrics and Extended Care provides additional geriatric-specific programs (http://va.gov/geriatrics). These programs may be useful for consultation and collaboration for patients whom the PACT teams have found to be more challenging and require more assistance to meet performance measures and patient needs. A recent evidence synthesis notes that direct involvement of geriatricians (as opposed to indirect care with limited contact) is more likely to result in positive patient outcomes and should be considered for those patients who are the most frail and/or high utilizers of services.32
Conclusion
The PACT initiative in the VA health care system may prove to be an important vehicle for improving and standardizing the care provided to older veterans. Use of reliable and valid tools in the identification and assessment of geriatric syndromes, provision of quality standards, and use of innovative telehealth practices are promising enhancements for the primary care of older veterans.
Acknowledgements
We would like to thank the following contributors for their thoughtful review of the initial drafts of this article: Dr. Balmatee Bidassie; Dr. Kathryn Corrigan; Dr. Gail McNut; Dr. Linda Kinsinger, chief consultant for preventive medicine in the Office of Patient Care Services; Dr. Theodore Hahn, GRECC deputy director from VA Greater Los Angeles Healthcare System; Dr. James Hallenbeck, associate chief of staff, Extended Care at VA Palo Alto Health Care System; Ms. Storm Morgan, VA Office of Nursing Services PACT program manager; and Dr. Kenneth Shay, director of Geriatric Programs for the VA Office of Geriatrics and Extended Care.
The authors also would like to express their gratitude to the VA Office of Nursing Services, Clinical Practice Program, Geriatrics and Extended Care Field Advisory Committee for the opportunity to work on this manuscript.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. U.S. Department of Veterans Affairs. Geriatric Ambulatory Care. VHA Handbook 1140.10. U.S. Department of Veterans Affairs Website. http://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=2202. Published April 26, 2010. Accessed September 29, 2014.
2. Federal Interagency Forum on Aging-Related Statistics. Older Americans 2012: Key indicators of well-being. AgingStats.gov Website. http://www.agingstats.gov/agingstatsdotnet/main_site/default.aspx. Accessed September 29, 2014.
3. Shay K, Schectman G. Primary care for older veterans. Generations. 2010;34(2):35-42.
4. Institute of Medicine. Retooling for an Aging America: Building the Health Care Workforce. Washington, DC: The National Academies Press; 2008.
5. Shay K, Hyduke B, Burris JF. Strategic plan for geriatrics and extended care in the veterans health administration: Background, plan, and progress to date. J Am Geriatr Soc. 2013;61(4):632-638.
6. Berenson RA, Devers KJ, Burton RA. Will the patient-centered medical home transform the delivery of health care? Timely analysis of immediate health policy issues. Urban Institute Website. http://www.urban.org/uploadedpdf/412373-will-patient-centered-medical-home-transform-delivery-health-care.pdf. Published August 2011. Accessed September 29, 2014.
7. U.S. Department of Veterans Affairs. VA Primary Care Services. Patient-centered medical home model concept paper. U.S. Department of Veterans Affairs Website. http://www.va.gov/health/services/PrimaryCare/docs/pcmh_ConceptPaper.doc. Accessed October 7, 2014.
8. VA Undersecretary for Health. What is PACT? U.S. Department of Veterans Affairs Website http://www.va.gov/health/services/primarycare/pact/index.asp. Updated February 18, 2014. Accessed October 7, 2014.
9. Askari M, Wierenga PC, Eslami S, Medlock S, de Rooij SE, Abu-Hanna A. Assessing quality of care of elderly patients using the ACOVE quality indicator set: A systematic review. PLoS ONE. 2011;6(12):e28631.
10. U.S. Department of Veterans Affairs. Office of Rural Health. About the office of rural health. U.S. Department of Veterans Affairs Website. http://www.ruralhealth.va.gov/about/index.asp. Update June 12, 2014. Accessed October 10, 2014.
11. Schectman G, Stark R, Fihn S, VanEe H, Box T. Care assessment need score: A tool for care management. Presented on March 29, 2012. http://www.myvehucampus.com/#loc=auditoriumRoom. Accessed October 14, 2014.
12. Min L, Yoon W, Mariano J, et al. The vulnerable elders-13 survey predicts 5-year functional decline and mortality outcomes in older ambulatory care patients. J Am Geriatr Soc. 2009;57(11):2070-2076.
13. Fulmer T, Wallace M. Fulmer SPICES: An overall assessment tool for older adults. http://consultgerirn.org/uploads/File/trythis/try_this_1.pdf. Revised 2012. Accessed October 1, 2014.
14. Hartford Institute for Geriatric Nursing. ConsultGeriRN.org Website. http://consultgerirn.org. Accessed September 30, 2014.
15. Opondo D, Eslami S, Visscher S, et al. Inappropriateness of medication prescriptions to elderly patients in the primary care setting: A systemic Review. PLoS One. 2012;7(8):e43617.
16. Fick DM, Cooper JW, Wade WE, Waller JL, Maclean JR, Beers MH. Updating the Beers criteria for potentially inappropriate medication use in older adults: Results of a US consensus panel of experts. Arch Intern Med. 2003;163(22):2716-2724.
17. The American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60(4):616-631.
18. Higashi T, Shekelle PG, Solomon DH, et al. The quality of pharmacologic care for vulnerable older patients. Ann Intern Med. 2004;140(9):714-720.
19. Lund BC, Steinman MA, Chrischilles EA, Kaboli PJ. Beers criteria as a proxy for inappropriate prescribing of other medications among older adults. Ann Pharmacother. 2011;45(11):1363-1370.
20. RAND. Assessing care of vulnerable elders. Quality indicators- ACOVE 3. RAND Website. http://www.rand.org/health/projects/acove/acove3.html. Accessed October 7, 2014.
21. Wenger NS, Shekelle PG. Assessing care of vulnerable elders: ACOVE project overview. Ann Intern Med. 2001;135(8, pt 2):642-646.
22. Shekelle PG, MacLean CH, Morton SC, Wenger NS. Assessing care of vulnerable elders: Methods for developing quality indicators. Ann Intern Med. 2001;135(8, pt 2):647-652.
23. Reuben DB, Roth C, Kamberg C, Wenger NS. Restructuring primary care practices to manage geriatric syndromes: The ACOVE-2 intervention. J Am Geriatr Soc. 2003;51(12):1787-1793.
24. Wenger NS, Roth CP, Shekelle P; ACOVE Investigators. Introduction to the assessing care of vulnerable elders-3 quality indicator measurement set. J Am Geriatr Soc. 2007;55(suppl 2):S247-S252.
25. Higashi T, Shekelle PG, Solomon DH, et al. The quality of pharmacologic care for vulnerable older patients. Ann Intern Med. 2004;140(9):714-720.
26. American Geriatrics Society Expert Panel on the Care of Older Adults with Multimorbidity. Patient-centered care for older adults with multiple chronic conditions: A stepwise approach from the American Geriatrics Society. J Am Geriatr Soc. 2012;60(10):1957-1968.
27. U.S. Department of Veterans Affairs. Geriatric Research Education and Clinical Centers. GRECC. U.S. Department of Veterans Affairs Website. http://www.va.gov/GRECC/index.asp . Updated August 17, 2012. Accessed October 10, 2014.
28. Tumosa N, Horvath KJ, Huh T, et al. Health care workforce development in rural America: When geriatrics expertise is 100 miles away. Gerontol Geriatr Educ. 2012;33(2):133-151.
29. U.S. Department of Veterans Affairs. Geriatric Research Education and Clinical Centers. The VA Geriatrics Scholars Program. U.S. Department of Veterans Affairs Website. http://www.va.gov/GRECC/GRECC_Educational_Events_and_Products.asp. Updated February 21, 2013. Accessed October 1, 2014.
30. U.S. Department of Veterans Affairs. Office of Public and Intergovernmental Affairs. VA uses technology to provide rural veterans greater access to specialty care services. U.S. Department of Veterans Affairs Website. http://www.va.gov/opa/pressrel/pressrelease.cfm?id=2353. Updated July 10, 2012. Accessed October 1, 2014.
31. Harrell KM, Wilkins SS, Connor MK, Chodosh J. Telemedicine and the evaluation of cognitive impairment: The additive value of neuropsychological assessment. J Am Med Dir Assoc. 2014;15(8):600-606.
32. Totten A, Carson S, Peterson K, Low A, Christense V, Tiwari A. Evidence brief: Effect of geriatricians on outcomes of inpatient and outpatient care, VA-ESP Project #09-199. U.S. Department of Veterans Affairs Website. http://www.hsrd.research.va.gov/publications/esp/Geriatricians.pdf. Accessed October 1, 2014.
The older adult population in the VA is growing. Adults aged > 85 years are the fastest growing segment of the older veteran population and many are afflicted with multiple medical problems and functional impairments.1,2 The majority of older veterans (94.6%, or about 1.9 million veterans) who seek care at the VA obtain care through primary care providers (PCPs) who are often not formally trained in geriatrics.1,3 With the increasing number of older patients, new models of care are needed to provide coordinated, comprehensive, efficient, and patient-centered care.4,5
Common themes found in successful models of care for older patients include a team approach, care management (comprehensive and coordinated), and patients who are active partners.4 These themes are reflected in the VA Patient Aligned Care Team (PACT) primary care program. PACT, a model of care that was initiated in 2010 and is built on a foundation of patient-centered care, encompasses a team approach to provide comprehensive, coordinated, and personalized care.6-8 The challenge for the VA is to integrate gerontologic principles and tools into the daily practice of all PACTs in order to improve care provided for older veterans.9
This article discusses current challenges in caring for older veterans in the VA system and recommends tools that can be used to infuse geriatric care principles into VA primary care by the PACT, to improve the quality of care provided to older veterans. In addition, the article also describes VA geriatric programs that PACT clinicians can access to supplement older veterans’ care.
Challenges of Caring for Older Veterans
One concern when caring for older veterans arises when the veteran accesses both VA and non-VA health care services to offset medication costs and obtain services not covered by Medicare or other insurance companies.2,3 This “dual care” can exacerbate polypharmacy issues and increase confusion regarding plans of care. Problems may arise when multiple providers from different systems of care prescribe medications available only within their own formulary and/or order diagnostic and laboratory tests with results available only within their own health care system.
The VA is also challenged by health care delivery for rural veterans. Thirty-six percent of all veterans live in rural areas, and they often depend on non-VA services to meet their health care needs due to difficulty traveling to the nearest VA facility.10 Seasonal residency also presents challenges. An increasing number of older veterans are seen at different VA facilities when they “winter” in a different section of the country.
Fortunately, a VA provider in one facility can access a patient’s electronic medical records in another facility, using the VA Computerized Patient Record System (CPRS). However, it is unclear to what extent busy VA PCPs use this function when seeing patients. Although individual pilot programs have shown promise, integrated electronic health records between VA and non-VA health care have not advanced to the point of sharing data or reconciling care plans (R. Rupper, personal communication, March 1, 2013).
Many PCPs and other PACT staff are not formally trained in geriatrics and may have had limited exposure to geriatric principles.3 Clinic time pressures, multiple clinical reminders (eg, vaccinations), and panel management of specific diseases make it challenging to find time to focus on complex geriatric syndromes. Current PACT performance measures also do not routinely include geriatric-specific quality of care criteria or focus on patient function (K. Shay, personal communication, February 12, 2013), a hallmark of geriatric care.8 Furthermore, with increasing complexity of the health care system and limited availability of resources, it is often time consuming to identify and collaborate with non-VA resources to ensure patients’ needs are met in their communities.
Opportunities for Improvement in Care
The VA transformation to PACTs has led to process changes in clinic workflow that may aid in addressing the aforementioned challenges in caring for older veterans. Each patient is assigned to a PCP-led team that includes a registered nurse care manager, a clinical associate, and an administrative associate. The PACT model of care has increased access to care by redesigning face-to-face visits, increasingly moving toward open access, and through the increased use of virtual access via secured e-mail, telephone visits, and telehealth.8
In addition to process changes, the VA has created new tools to assist teams in patient management. One of these is the Care Assessment Need (CAN) score, a risk stratification tool available for use by PACTs to identify patients at highest risk for hospital admission and/or death for focused care management.11 It is based on statistical prediction models of veterans enrolled in primary care, using patient characteristics and health care use information.11 Although the CAN score looks promising, more research is needed to evaluate its effectiveness in improving care for older veterans and its association with better patient functioning—an important focus in quality geriatric care.
A tool that takes into account daily function is the Vulnerable Elders Survey-13 (VES-13). As measured by the VES-13, functional ability has been shown to be a strong predictor of decline and death in older adults independent of gender or comorbidities.12 Integration of the VES-13 into the evaluation of older veterans could assist PACTs in considering patients’ current function and life expectancy in their care plans along with patient and family goals.
Another potentially useful tool for the PACT team is the SPICES mnemonic (Sleeping, Problems with feeding/eating, Incontinence or urinary problems, Confusion, Evidence of falls, and Skin breakdown).13 Although SPICES is not comprehensive, this mnemonic highlights potential problems facing older patients that may not be brought up routinely. It provides a concise, formalized format that can be used by clerks or patient support assistants as part of the check-in process.
This tool has been used successfully by the Geriatric Evaluation and Management Clinic of the South Texas Veterans Health Care System (STVHCS) to improve communication between the PCP and nurse so that pertinent patient information is relayed concisely. SPICES was helpful in identifying patients needing interventions for fall risk. In a retrospective chart review of 100 randomly selected patients aged 75 to 90 years enrolled in the clinic, a 75% reduction in falls was noted during the first year of implementation (STVHCS unpublished data, 2012).
Additional tools that focus on identifying specific geriatric syndromes are available online from the Hartford Institute for Geriatric Nursing, which provides evidence-based information and training on how to assess, evaluate, and manage common geriatric syndromes such as depression, dementia, and delirium.14 The site also includes videos on how to use common brief geriatric assessment tools that can be performed by nurses and health care associates while the patient is in the waiting room. Though promising, further research is needed to study the effects of these tools on patient, provider, and system outcomes.
Infusing quality of care indicators (QI) can play an integral role in achieving PACT goals while improving the older veterans’ quality of life. For example, polypharmacy and medication-related injuries in older adults continue to pose both a safety and economic challenge to patients and the health care system.15-17 The 2012 Beers criteria for Potentially Inappropriate Medications in Older Adults lists 53 medication classes that have been identified as potentially inappropriate medications for use in older adults.17 Use of this tool by PACTs in the development of patient care plans has the potential to reduce medication-related adverse reactions and improper prescribing.18,19
Assessing Care of Vulnerable Elders (ACOVE ) also provides QIs that are specific to vulnerable older persons.20-24 The most recent version, ACOVE-3, includes 392 QIs for 26 conditions and 14 types of care processes and covers all domains of care.20 Findings from a study applying QIs involving vulnerable elderly patients in 2 managed care programs revealed that recipients of better-quality care had a 10% higher survival rate over 3 years.25
The VA currently monitors 6 frail elderly QIs based on ACOVE criteria via reviews of medical records in veterans aged > 75 years. These QIs cover falls, incontinence, functional assessment, and the presence of a surrogate decision maker. PACT staff, unfortunately, do not receive feedback on these, because they are still QIs and not part of the performance measures (K. Shay, personal communication, February 12, 2013). Though some VA sites have adopted these QIs to some extent, until these frail elderly QIs become performance measures throughout VA, other competing priorities may be more at the forefront of quality improvement projects done by PACT teams.5
The American Geriatrics Society recently published recommendations on the care of older adults with multiple chronic conditions, to aid PCPs in practicing a more individualized, patient-centered care in complex cases.26 In addition to focusing on a patient’s primary concern during a clinic visit and eliciting preferences, considering prognosis in deciding on treatment options allows patients to better weigh the potential benefits and burdens in their daily living.26 A discussion on how aggressive potential treatments are and what the patient is willing to undertake is an important component of patient-centered care and should be incorporated during routine PACT clinic visits.
VA Geriatric Programs
It is important for PACT clinicians to be familiar with the geriatric programs and resources available within the VA medical home “neighborhood,” which can supplement care. One such resource is the Geriatric Research Education and Clinical Centers (GRECCs). There are currently 19 GRECCs throughout the nation that serve as Centers of Excellence in the care of older veterans.27 The GRECCs provide training for clinicians, test innovative ways to care for older veterans, and collaborate with other staff to improve the care provided. Some have also developed Geriatric Primary Care Clinics (or Geri PACTs) to provide team care to very frail and high-risk older veterans. Since not all VA facilities have access to Geri PACTs, the GRECCs play an important role in making geriatric expertise and training available to the PACTs.3
To address this limitation in access, VA programs have begun using telehealth technology to increase competencies of PCPs in caring for older veterans. For example, the VA Geriatric Scholars Program is a national educational program with different avenues to “geriatricize” VA primary care services and improve knowledge and care provided to older veterans.28 It consists of several subprograms: Geriatric Scholars Program for Rural Community Based Outpatient Clinics; Geriatric Scholars Program for Primary Care Providers; Rural Interdisciplinary Team Training; and the Geriatric Assessment Pocket Guide.29 These components may include didactics both face-to-face and online, clinical experience with performing common geriatric screening tools, and a quality improvement project.
Some local VAMCs have also developed programs to address this need to improve care provided to older veterans in PACT. The VA Greater Los Angeles Healthcare System (GLA) GRECC, for example, has started several programs to infuse geriatrics into PACTs, including the Geri Specialty Care Access Network-Extension for Community Healthcare Outcomes (SCAN-ECHO). VA SCAN-ECHO was developed to increase access to specialty care in rural/underserved areas. The PCP presents a case and a specialty provider gives guidance in the assessment and/or management of a specific clinical problem.30 Unlike many other SCAN-ECHO programs, the GLA Geri SCAN-ECHO program encourages not only PCPs, but also nurses and social workers to submit consults for discussion and encourages team management (a hallmark of quality geriatric care). Another important GLA GRECC project is the Veterans Cognitive Assessment and Management Program (V-CAMP), which uses videoconferencing to assess and manage veterans with cognitive impairment/dementia who reside in underserved areas in the GLA region. The program provides dementia care management and access to neuropsychological examinations—services that are often not available in rural areas.31
Various VA program offices have also published useful resources to help PACT clinicians infuse gerontologic principles into their practice. The VA Office of Nursing Services has a Geriatrics and Extended Care Field Advisory Committee, which recently produced on-demand lectures in the virtual VA eHealth University (also known as myVeHU campus) on improving the PACT’s management of progressive chronic diseases and dementia recognition and initial evaluation. They also produced a resource guide for VA clinicians (nursing and non-nursing), based on a team consensus of what the workgroup thinks a clinician would find helpful in clinical practice to improve care of older veterans. The VA Office of Geriatrics and Extended Care Service also identified a list of clinical and educational resources to help PACT clinicians. These include the Geriatrics Evaluation and management (GEM) Tools Booklet (http://geriatricscareonline.org) and a SharePoint site to improve dementia care in all settings.
The VA Office of Geriatrics and Extended Care provides additional geriatric-specific programs (http://va.gov/geriatrics). These programs may be useful for consultation and collaboration for patients whom the PACT teams have found to be more challenging and require more assistance to meet performance measures and patient needs. A recent evidence synthesis notes that direct involvement of geriatricians (as opposed to indirect care with limited contact) is more likely to result in positive patient outcomes and should be considered for those patients who are the most frail and/or high utilizers of services.32
Conclusion
The PACT initiative in the VA health care system may prove to be an important vehicle for improving and standardizing the care provided to older veterans. Use of reliable and valid tools in the identification and assessment of geriatric syndromes, provision of quality standards, and use of innovative telehealth practices are promising enhancements for the primary care of older veterans.
Acknowledgements
We would like to thank the following contributors for their thoughtful review of the initial drafts of this article: Dr. Balmatee Bidassie; Dr. Kathryn Corrigan; Dr. Gail McNut; Dr. Linda Kinsinger, chief consultant for preventive medicine in the Office of Patient Care Services; Dr. Theodore Hahn, GRECC deputy director from VA Greater Los Angeles Healthcare System; Dr. James Hallenbeck, associate chief of staff, Extended Care at VA Palo Alto Health Care System; Ms. Storm Morgan, VA Office of Nursing Services PACT program manager; and Dr. Kenneth Shay, director of Geriatric Programs for the VA Office of Geriatrics and Extended Care.
The authors also would like to express their gratitude to the VA Office of Nursing Services, Clinical Practice Program, Geriatrics and Extended Care Field Advisory Committee for the opportunity to work on this manuscript.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
The older adult population in the VA is growing. Adults aged > 85 years are the fastest growing segment of the older veteran population and many are afflicted with multiple medical problems and functional impairments.1,2 The majority of older veterans (94.6%, or about 1.9 million veterans) who seek care at the VA obtain care through primary care providers (PCPs) who are often not formally trained in geriatrics.1,3 With the increasing number of older patients, new models of care are needed to provide coordinated, comprehensive, efficient, and patient-centered care.4,5
Common themes found in successful models of care for older patients include a team approach, care management (comprehensive and coordinated), and patients who are active partners.4 These themes are reflected in the VA Patient Aligned Care Team (PACT) primary care program. PACT, a model of care that was initiated in 2010 and is built on a foundation of patient-centered care, encompasses a team approach to provide comprehensive, coordinated, and personalized care.6-8 The challenge for the VA is to integrate gerontologic principles and tools into the daily practice of all PACTs in order to improve care provided for older veterans.9
This article discusses current challenges in caring for older veterans in the VA system and recommends tools that can be used to infuse geriatric care principles into VA primary care by the PACT, to improve the quality of care provided to older veterans. In addition, the article also describes VA geriatric programs that PACT clinicians can access to supplement older veterans’ care.
Challenges of Caring for Older Veterans
One concern when caring for older veterans arises when the veteran accesses both VA and non-VA health care services to offset medication costs and obtain services not covered by Medicare or other insurance companies.2,3 This “dual care” can exacerbate polypharmacy issues and increase confusion regarding plans of care. Problems may arise when multiple providers from different systems of care prescribe medications available only within their own formulary and/or order diagnostic and laboratory tests with results available only within their own health care system.
The VA is also challenged by health care delivery for rural veterans. Thirty-six percent of all veterans live in rural areas, and they often depend on non-VA services to meet their health care needs due to difficulty traveling to the nearest VA facility.10 Seasonal residency also presents challenges. An increasing number of older veterans are seen at different VA facilities when they “winter” in a different section of the country.
Fortunately, a VA provider in one facility can access a patient’s electronic medical records in another facility, using the VA Computerized Patient Record System (CPRS). However, it is unclear to what extent busy VA PCPs use this function when seeing patients. Although individual pilot programs have shown promise, integrated electronic health records between VA and non-VA health care have not advanced to the point of sharing data or reconciling care plans (R. Rupper, personal communication, March 1, 2013).
Many PCPs and other PACT staff are not formally trained in geriatrics and may have had limited exposure to geriatric principles.3 Clinic time pressures, multiple clinical reminders (eg, vaccinations), and panel management of specific diseases make it challenging to find time to focus on complex geriatric syndromes. Current PACT performance measures also do not routinely include geriatric-specific quality of care criteria or focus on patient function (K. Shay, personal communication, February 12, 2013), a hallmark of geriatric care.8 Furthermore, with increasing complexity of the health care system and limited availability of resources, it is often time consuming to identify and collaborate with non-VA resources to ensure patients’ needs are met in their communities.
Opportunities for Improvement in Care
The VA transformation to PACTs has led to process changes in clinic workflow that may aid in addressing the aforementioned challenges in caring for older veterans. Each patient is assigned to a PCP-led team that includes a registered nurse care manager, a clinical associate, and an administrative associate. The PACT model of care has increased access to care by redesigning face-to-face visits, increasingly moving toward open access, and through the increased use of virtual access via secured e-mail, telephone visits, and telehealth.8
In addition to process changes, the VA has created new tools to assist teams in patient management. One of these is the Care Assessment Need (CAN) score, a risk stratification tool available for use by PACTs to identify patients at highest risk for hospital admission and/or death for focused care management.11 It is based on statistical prediction models of veterans enrolled in primary care, using patient characteristics and health care use information.11 Although the CAN score looks promising, more research is needed to evaluate its effectiveness in improving care for older veterans and its association with better patient functioning—an important focus in quality geriatric care.
A tool that takes into account daily function is the Vulnerable Elders Survey-13 (VES-13). As measured by the VES-13, functional ability has been shown to be a strong predictor of decline and death in older adults independent of gender or comorbidities.12 Integration of the VES-13 into the evaluation of older veterans could assist PACTs in considering patients’ current function and life expectancy in their care plans along with patient and family goals.
Another potentially useful tool for the PACT team is the SPICES mnemonic (Sleeping, Problems with feeding/eating, Incontinence or urinary problems, Confusion, Evidence of falls, and Skin breakdown).13 Although SPICES is not comprehensive, this mnemonic highlights potential problems facing older patients that may not be brought up routinely. It provides a concise, formalized format that can be used by clerks or patient support assistants as part of the check-in process.
This tool has been used successfully by the Geriatric Evaluation and Management Clinic of the South Texas Veterans Health Care System (STVHCS) to improve communication between the PCP and nurse so that pertinent patient information is relayed concisely. SPICES was helpful in identifying patients needing interventions for fall risk. In a retrospective chart review of 100 randomly selected patients aged 75 to 90 years enrolled in the clinic, a 75% reduction in falls was noted during the first year of implementation (STVHCS unpublished data, 2012).
Additional tools that focus on identifying specific geriatric syndromes are available online from the Hartford Institute for Geriatric Nursing, which provides evidence-based information and training on how to assess, evaluate, and manage common geriatric syndromes such as depression, dementia, and delirium.14 The site also includes videos on how to use common brief geriatric assessment tools that can be performed by nurses and health care associates while the patient is in the waiting room. Though promising, further research is needed to study the effects of these tools on patient, provider, and system outcomes.
Infusing quality of care indicators (QI) can play an integral role in achieving PACT goals while improving the older veterans’ quality of life. For example, polypharmacy and medication-related injuries in older adults continue to pose both a safety and economic challenge to patients and the health care system.15-17 The 2012 Beers criteria for Potentially Inappropriate Medications in Older Adults lists 53 medication classes that have been identified as potentially inappropriate medications for use in older adults.17 Use of this tool by PACTs in the development of patient care plans has the potential to reduce medication-related adverse reactions and improper prescribing.18,19
Assessing Care of Vulnerable Elders (ACOVE ) also provides QIs that are specific to vulnerable older persons.20-24 The most recent version, ACOVE-3, includes 392 QIs for 26 conditions and 14 types of care processes and covers all domains of care.20 Findings from a study applying QIs involving vulnerable elderly patients in 2 managed care programs revealed that recipients of better-quality care had a 10% higher survival rate over 3 years.25
The VA currently monitors 6 frail elderly QIs based on ACOVE criteria via reviews of medical records in veterans aged > 75 years. These QIs cover falls, incontinence, functional assessment, and the presence of a surrogate decision maker. PACT staff, unfortunately, do not receive feedback on these, because they are still QIs and not part of the performance measures (K. Shay, personal communication, February 12, 2013). Though some VA sites have adopted these QIs to some extent, until these frail elderly QIs become performance measures throughout VA, other competing priorities may be more at the forefront of quality improvement projects done by PACT teams.5
The American Geriatrics Society recently published recommendations on the care of older adults with multiple chronic conditions, to aid PCPs in practicing a more individualized, patient-centered care in complex cases.26 In addition to focusing on a patient’s primary concern during a clinic visit and eliciting preferences, considering prognosis in deciding on treatment options allows patients to better weigh the potential benefits and burdens in their daily living.26 A discussion on how aggressive potential treatments are and what the patient is willing to undertake is an important component of patient-centered care and should be incorporated during routine PACT clinic visits.
VA Geriatric Programs
It is important for PACT clinicians to be familiar with the geriatric programs and resources available within the VA medical home “neighborhood,” which can supplement care. One such resource is the Geriatric Research Education and Clinical Centers (GRECCs). There are currently 19 GRECCs throughout the nation that serve as Centers of Excellence in the care of older veterans.27 The GRECCs provide training for clinicians, test innovative ways to care for older veterans, and collaborate with other staff to improve the care provided. Some have also developed Geriatric Primary Care Clinics (or Geri PACTs) to provide team care to very frail and high-risk older veterans. Since not all VA facilities have access to Geri PACTs, the GRECCs play an important role in making geriatric expertise and training available to the PACTs.3
To address this limitation in access, VA programs have begun using telehealth technology to increase competencies of PCPs in caring for older veterans. For example, the VA Geriatric Scholars Program is a national educational program with different avenues to “geriatricize” VA primary care services and improve knowledge and care provided to older veterans.28 It consists of several subprograms: Geriatric Scholars Program for Rural Community Based Outpatient Clinics; Geriatric Scholars Program for Primary Care Providers; Rural Interdisciplinary Team Training; and the Geriatric Assessment Pocket Guide.29 These components may include didactics both face-to-face and online, clinical experience with performing common geriatric screening tools, and a quality improvement project.
Some local VAMCs have also developed programs to address this need to improve care provided to older veterans in PACT. The VA Greater Los Angeles Healthcare System (GLA) GRECC, for example, has started several programs to infuse geriatrics into PACTs, including the Geri Specialty Care Access Network-Extension for Community Healthcare Outcomes (SCAN-ECHO). VA SCAN-ECHO was developed to increase access to specialty care in rural/underserved areas. The PCP presents a case and a specialty provider gives guidance in the assessment and/or management of a specific clinical problem.30 Unlike many other SCAN-ECHO programs, the GLA Geri SCAN-ECHO program encourages not only PCPs, but also nurses and social workers to submit consults for discussion and encourages team management (a hallmark of quality geriatric care). Another important GLA GRECC project is the Veterans Cognitive Assessment and Management Program (V-CAMP), which uses videoconferencing to assess and manage veterans with cognitive impairment/dementia who reside in underserved areas in the GLA region. The program provides dementia care management and access to neuropsychological examinations—services that are often not available in rural areas.31
Various VA program offices have also published useful resources to help PACT clinicians infuse gerontologic principles into their practice. The VA Office of Nursing Services has a Geriatrics and Extended Care Field Advisory Committee, which recently produced on-demand lectures in the virtual VA eHealth University (also known as myVeHU campus) on improving the PACT’s management of progressive chronic diseases and dementia recognition and initial evaluation. They also produced a resource guide for VA clinicians (nursing and non-nursing), based on a team consensus of what the workgroup thinks a clinician would find helpful in clinical practice to improve care of older veterans. The VA Office of Geriatrics and Extended Care Service also identified a list of clinical and educational resources to help PACT clinicians. These include the Geriatrics Evaluation and management (GEM) Tools Booklet (http://geriatricscareonline.org) and a SharePoint site to improve dementia care in all settings.
The VA Office of Geriatrics and Extended Care provides additional geriatric-specific programs (http://va.gov/geriatrics). These programs may be useful for consultation and collaboration for patients whom the PACT teams have found to be more challenging and require more assistance to meet performance measures and patient needs. A recent evidence synthesis notes that direct involvement of geriatricians (as opposed to indirect care with limited contact) is more likely to result in positive patient outcomes and should be considered for those patients who are the most frail and/or high utilizers of services.32
Conclusion
The PACT initiative in the VA health care system may prove to be an important vehicle for improving and standardizing the care provided to older veterans. Use of reliable and valid tools in the identification and assessment of geriatric syndromes, provision of quality standards, and use of innovative telehealth practices are promising enhancements for the primary care of older veterans.
Acknowledgements
We would like to thank the following contributors for their thoughtful review of the initial drafts of this article: Dr. Balmatee Bidassie; Dr. Kathryn Corrigan; Dr. Gail McNut; Dr. Linda Kinsinger, chief consultant for preventive medicine in the Office of Patient Care Services; Dr. Theodore Hahn, GRECC deputy director from VA Greater Los Angeles Healthcare System; Dr. James Hallenbeck, associate chief of staff, Extended Care at VA Palo Alto Health Care System; Ms. Storm Morgan, VA Office of Nursing Services PACT program manager; and Dr. Kenneth Shay, director of Geriatric Programs for the VA Office of Geriatrics and Extended Care.
The authors also would like to express their gratitude to the VA Office of Nursing Services, Clinical Practice Program, Geriatrics and Extended Care Field Advisory Committee for the opportunity to work on this manuscript.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. U.S. Department of Veterans Affairs. Geriatric Ambulatory Care. VHA Handbook 1140.10. U.S. Department of Veterans Affairs Website. http://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=2202. Published April 26, 2010. Accessed September 29, 2014.
2. Federal Interagency Forum on Aging-Related Statistics. Older Americans 2012: Key indicators of well-being. AgingStats.gov Website. http://www.agingstats.gov/agingstatsdotnet/main_site/default.aspx. Accessed September 29, 2014.
3. Shay K, Schectman G. Primary care for older veterans. Generations. 2010;34(2):35-42.
4. Institute of Medicine. Retooling for an Aging America: Building the Health Care Workforce. Washington, DC: The National Academies Press; 2008.
5. Shay K, Hyduke B, Burris JF. Strategic plan for geriatrics and extended care in the veterans health administration: Background, plan, and progress to date. J Am Geriatr Soc. 2013;61(4):632-638.
6. Berenson RA, Devers KJ, Burton RA. Will the patient-centered medical home transform the delivery of health care? Timely analysis of immediate health policy issues. Urban Institute Website. http://www.urban.org/uploadedpdf/412373-will-patient-centered-medical-home-transform-delivery-health-care.pdf. Published August 2011. Accessed September 29, 2014.
7. U.S. Department of Veterans Affairs. VA Primary Care Services. Patient-centered medical home model concept paper. U.S. Department of Veterans Affairs Website. http://www.va.gov/health/services/PrimaryCare/docs/pcmh_ConceptPaper.doc. Accessed October 7, 2014.
8. VA Undersecretary for Health. What is PACT? U.S. Department of Veterans Affairs Website http://www.va.gov/health/services/primarycare/pact/index.asp. Updated February 18, 2014. Accessed October 7, 2014.
9. Askari M, Wierenga PC, Eslami S, Medlock S, de Rooij SE, Abu-Hanna A. Assessing quality of care of elderly patients using the ACOVE quality indicator set: A systematic review. PLoS ONE. 2011;6(12):e28631.
10. U.S. Department of Veterans Affairs. Office of Rural Health. About the office of rural health. U.S. Department of Veterans Affairs Website. http://www.ruralhealth.va.gov/about/index.asp. Update June 12, 2014. Accessed October 10, 2014.
11. Schectman G, Stark R, Fihn S, VanEe H, Box T. Care assessment need score: A tool for care management. Presented on March 29, 2012. http://www.myvehucampus.com/#loc=auditoriumRoom. Accessed October 14, 2014.
12. Min L, Yoon W, Mariano J, et al. The vulnerable elders-13 survey predicts 5-year functional decline and mortality outcomes in older ambulatory care patients. J Am Geriatr Soc. 2009;57(11):2070-2076.
13. Fulmer T, Wallace M. Fulmer SPICES: An overall assessment tool for older adults. http://consultgerirn.org/uploads/File/trythis/try_this_1.pdf. Revised 2012. Accessed October 1, 2014.
14. Hartford Institute for Geriatric Nursing. ConsultGeriRN.org Website. http://consultgerirn.org. Accessed September 30, 2014.
15. Opondo D, Eslami S, Visscher S, et al. Inappropriateness of medication prescriptions to elderly patients in the primary care setting: A systemic Review. PLoS One. 2012;7(8):e43617.
16. Fick DM, Cooper JW, Wade WE, Waller JL, Maclean JR, Beers MH. Updating the Beers criteria for potentially inappropriate medication use in older adults: Results of a US consensus panel of experts. Arch Intern Med. 2003;163(22):2716-2724.
17. The American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60(4):616-631.
18. Higashi T, Shekelle PG, Solomon DH, et al. The quality of pharmacologic care for vulnerable older patients. Ann Intern Med. 2004;140(9):714-720.
19. Lund BC, Steinman MA, Chrischilles EA, Kaboli PJ. Beers criteria as a proxy for inappropriate prescribing of other medications among older adults. Ann Pharmacother. 2011;45(11):1363-1370.
20. RAND. Assessing care of vulnerable elders. Quality indicators- ACOVE 3. RAND Website. http://www.rand.org/health/projects/acove/acove3.html. Accessed October 7, 2014.
21. Wenger NS, Shekelle PG. Assessing care of vulnerable elders: ACOVE project overview. Ann Intern Med. 2001;135(8, pt 2):642-646.
22. Shekelle PG, MacLean CH, Morton SC, Wenger NS. Assessing care of vulnerable elders: Methods for developing quality indicators. Ann Intern Med. 2001;135(8, pt 2):647-652.
23. Reuben DB, Roth C, Kamberg C, Wenger NS. Restructuring primary care practices to manage geriatric syndromes: The ACOVE-2 intervention. J Am Geriatr Soc. 2003;51(12):1787-1793.
24. Wenger NS, Roth CP, Shekelle P; ACOVE Investigators. Introduction to the assessing care of vulnerable elders-3 quality indicator measurement set. J Am Geriatr Soc. 2007;55(suppl 2):S247-S252.
25. Higashi T, Shekelle PG, Solomon DH, et al. The quality of pharmacologic care for vulnerable older patients. Ann Intern Med. 2004;140(9):714-720.
26. American Geriatrics Society Expert Panel on the Care of Older Adults with Multimorbidity. Patient-centered care for older adults with multiple chronic conditions: A stepwise approach from the American Geriatrics Society. J Am Geriatr Soc. 2012;60(10):1957-1968.
27. U.S. Department of Veterans Affairs. Geriatric Research Education and Clinical Centers. GRECC. U.S. Department of Veterans Affairs Website. http://www.va.gov/GRECC/index.asp . Updated August 17, 2012. Accessed October 10, 2014.
28. Tumosa N, Horvath KJ, Huh T, et al. Health care workforce development in rural America: When geriatrics expertise is 100 miles away. Gerontol Geriatr Educ. 2012;33(2):133-151.
29. U.S. Department of Veterans Affairs. Geriatric Research Education and Clinical Centers. The VA Geriatrics Scholars Program. U.S. Department of Veterans Affairs Website. http://www.va.gov/GRECC/GRECC_Educational_Events_and_Products.asp. Updated February 21, 2013. Accessed October 1, 2014.
30. U.S. Department of Veterans Affairs. Office of Public and Intergovernmental Affairs. VA uses technology to provide rural veterans greater access to specialty care services. U.S. Department of Veterans Affairs Website. http://www.va.gov/opa/pressrel/pressrelease.cfm?id=2353. Updated July 10, 2012. Accessed October 1, 2014.
31. Harrell KM, Wilkins SS, Connor MK, Chodosh J. Telemedicine and the evaluation of cognitive impairment: The additive value of neuropsychological assessment. J Am Med Dir Assoc. 2014;15(8):600-606.
32. Totten A, Carson S, Peterson K, Low A, Christense V, Tiwari A. Evidence brief: Effect of geriatricians on outcomes of inpatient and outpatient care, VA-ESP Project #09-199. U.S. Department of Veterans Affairs Website. http://www.hsrd.research.va.gov/publications/esp/Geriatricians.pdf. Accessed October 1, 2014.
1. U.S. Department of Veterans Affairs. Geriatric Ambulatory Care. VHA Handbook 1140.10. U.S. Department of Veterans Affairs Website. http://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=2202. Published April 26, 2010. Accessed September 29, 2014.
2. Federal Interagency Forum on Aging-Related Statistics. Older Americans 2012: Key indicators of well-being. AgingStats.gov Website. http://www.agingstats.gov/agingstatsdotnet/main_site/default.aspx. Accessed September 29, 2014.
3. Shay K, Schectman G. Primary care for older veterans. Generations. 2010;34(2):35-42.
4. Institute of Medicine. Retooling for an Aging America: Building the Health Care Workforce. Washington, DC: The National Academies Press; 2008.
5. Shay K, Hyduke B, Burris JF. Strategic plan for geriatrics and extended care in the veterans health administration: Background, plan, and progress to date. J Am Geriatr Soc. 2013;61(4):632-638.
6. Berenson RA, Devers KJ, Burton RA. Will the patient-centered medical home transform the delivery of health care? Timely analysis of immediate health policy issues. Urban Institute Website. http://www.urban.org/uploadedpdf/412373-will-patient-centered-medical-home-transform-delivery-health-care.pdf. Published August 2011. Accessed September 29, 2014.
7. U.S. Department of Veterans Affairs. VA Primary Care Services. Patient-centered medical home model concept paper. U.S. Department of Veterans Affairs Website. http://www.va.gov/health/services/PrimaryCare/docs/pcmh_ConceptPaper.doc. Accessed October 7, 2014.
8. VA Undersecretary for Health. What is PACT? U.S. Department of Veterans Affairs Website http://www.va.gov/health/services/primarycare/pact/index.asp. Updated February 18, 2014. Accessed October 7, 2014.
9. Askari M, Wierenga PC, Eslami S, Medlock S, de Rooij SE, Abu-Hanna A. Assessing quality of care of elderly patients using the ACOVE quality indicator set: A systematic review. PLoS ONE. 2011;6(12):e28631.
10. U.S. Department of Veterans Affairs. Office of Rural Health. About the office of rural health. U.S. Department of Veterans Affairs Website. http://www.ruralhealth.va.gov/about/index.asp. Update June 12, 2014. Accessed October 10, 2014.
11. Schectman G, Stark R, Fihn S, VanEe H, Box T. Care assessment need score: A tool for care management. Presented on March 29, 2012. http://www.myvehucampus.com/#loc=auditoriumRoom. Accessed October 14, 2014.
12. Min L, Yoon W, Mariano J, et al. The vulnerable elders-13 survey predicts 5-year functional decline and mortality outcomes in older ambulatory care patients. J Am Geriatr Soc. 2009;57(11):2070-2076.
13. Fulmer T, Wallace M. Fulmer SPICES: An overall assessment tool for older adults. http://consultgerirn.org/uploads/File/trythis/try_this_1.pdf. Revised 2012. Accessed October 1, 2014.
14. Hartford Institute for Geriatric Nursing. ConsultGeriRN.org Website. http://consultgerirn.org. Accessed September 30, 2014.
15. Opondo D, Eslami S, Visscher S, et al. Inappropriateness of medication prescriptions to elderly patients in the primary care setting: A systemic Review. PLoS One. 2012;7(8):e43617.
16. Fick DM, Cooper JW, Wade WE, Waller JL, Maclean JR, Beers MH. Updating the Beers criteria for potentially inappropriate medication use in older adults: Results of a US consensus panel of experts. Arch Intern Med. 2003;163(22):2716-2724.
17. The American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60(4):616-631.
18. Higashi T, Shekelle PG, Solomon DH, et al. The quality of pharmacologic care for vulnerable older patients. Ann Intern Med. 2004;140(9):714-720.
19. Lund BC, Steinman MA, Chrischilles EA, Kaboli PJ. Beers criteria as a proxy for inappropriate prescribing of other medications among older adults. Ann Pharmacother. 2011;45(11):1363-1370.
20. RAND. Assessing care of vulnerable elders. Quality indicators- ACOVE 3. RAND Website. http://www.rand.org/health/projects/acove/acove3.html. Accessed October 7, 2014.
21. Wenger NS, Shekelle PG. Assessing care of vulnerable elders: ACOVE project overview. Ann Intern Med. 2001;135(8, pt 2):642-646.
22. Shekelle PG, MacLean CH, Morton SC, Wenger NS. Assessing care of vulnerable elders: Methods for developing quality indicators. Ann Intern Med. 2001;135(8, pt 2):647-652.
23. Reuben DB, Roth C, Kamberg C, Wenger NS. Restructuring primary care practices to manage geriatric syndromes: The ACOVE-2 intervention. J Am Geriatr Soc. 2003;51(12):1787-1793.
24. Wenger NS, Roth CP, Shekelle P; ACOVE Investigators. Introduction to the assessing care of vulnerable elders-3 quality indicator measurement set. J Am Geriatr Soc. 2007;55(suppl 2):S247-S252.
25. Higashi T, Shekelle PG, Solomon DH, et al. The quality of pharmacologic care for vulnerable older patients. Ann Intern Med. 2004;140(9):714-720.
26. American Geriatrics Society Expert Panel on the Care of Older Adults with Multimorbidity. Patient-centered care for older adults with multiple chronic conditions: A stepwise approach from the American Geriatrics Society. J Am Geriatr Soc. 2012;60(10):1957-1968.
27. U.S. Department of Veterans Affairs. Geriatric Research Education and Clinical Centers. GRECC. U.S. Department of Veterans Affairs Website. http://www.va.gov/GRECC/index.asp . Updated August 17, 2012. Accessed October 10, 2014.
28. Tumosa N, Horvath KJ, Huh T, et al. Health care workforce development in rural America: When geriatrics expertise is 100 miles away. Gerontol Geriatr Educ. 2012;33(2):133-151.
29. U.S. Department of Veterans Affairs. Geriatric Research Education and Clinical Centers. The VA Geriatrics Scholars Program. U.S. Department of Veterans Affairs Website. http://www.va.gov/GRECC/GRECC_Educational_Events_and_Products.asp. Updated February 21, 2013. Accessed October 1, 2014.
30. U.S. Department of Veterans Affairs. Office of Public and Intergovernmental Affairs. VA uses technology to provide rural veterans greater access to specialty care services. U.S. Department of Veterans Affairs Website. http://www.va.gov/opa/pressrel/pressrelease.cfm?id=2353. Updated July 10, 2012. Accessed October 1, 2014.
31. Harrell KM, Wilkins SS, Connor MK, Chodosh J. Telemedicine and the evaluation of cognitive impairment: The additive value of neuropsychological assessment. J Am Med Dir Assoc. 2014;15(8):600-606.
32. Totten A, Carson S, Peterson K, Low A, Christense V, Tiwari A. Evidence brief: Effect of geriatricians on outcomes of inpatient and outpatient care, VA-ESP Project #09-199. U.S. Department of Veterans Affairs Website. http://www.hsrd.research.va.gov/publications/esp/Geriatricians.pdf. Accessed October 1, 2014.
E-Consults in Gastroenterology: A Quality Improvement Project
Although the VA has the largest health care system in the U.S., not every VA facility offers medical subspecialty care. As a result, patients are often separated by long distances from services they need.
At the VA Pittsburgh Healthcare System (VAPHS) in Pennsylvania, about 15,700 veterans received care in 2011. The Gastroenterology Department (GD) served many of these patients: 5,800 patients were seen in clinic appointments, 2,500 underwent colonoscopy, and 1,700 underwent esophagogastroduodenoscopy (EGD). Patients traveled up to 150 miles from 3 states for appointments and procedures. Prior to each procedure, a face-to-face appointment was standard practice for most patients to plan procedures and ensure medical stability. Patients expressed dissatisfaction with transportation, cost, time, and inconvenience, particularly when they were required to attend both the preprocedure and procedure appointments.
Patient satisfaction, timely care, and appropriate use of resources are important VA goals of care, so the VAPHS developed an electronic consultation (e-consult) program as a component of its long-term strategic plan. The goal was to increase access to care through the use of an e-consult in lieu of a face-to-face appointment for select patients. The e-consult program established guidelines and benchmark goals (Table). The program also established a database to track the benchmarks.
The purpose of this quality improvement (QI) project was to evaluate e-consults in the GD over a 6-month period from January 2012, when e-consults were implemented in the GD, to July 2012. Based on the outcomes, recommendations for program continuation, change, and sustainability were made.
Background
Telemedicine using information technologies has been reported as a viable solution to support health care delivery when distance limits patients’ access to care.1,2 Telemedicine has also been shown to improve efficacy in clinical decision making and reduce costs. It also can increase patient satisfaction by reducing travel and time, minimize duplication of diagnostic testing, and integrate services effectively across multiple sites when an electronic medical record (EMR) is in place.1-5
A randomized controlled trial in 2004 compared a standard outpatient referral appointment with a joint teleconsultation between provider, specialist, and patient.3 In the teleconsultation arm, there was higher patient satisfaction, fewer diagnostic tests (particularly in gastroenterology), and lower patient costs.
A study published in 2009 examined the impact of cardiac, dermatologic, and diabetes teleconsultations on organization and patient outcomes in 950 patients in 30 rural communities.2 Rapid access to care was provided for 85% of the patients. Organizational benefits included resource savings and efficacy improvement measured by a provider opinion Likert scale. Patient benefits included reductions in wait times, transportation savings, avoidance of unnecessary office visits, and ease of use.
A large systematic review of telemedicine services across all medical specialties in 2006 included 106 published studies.4 Clinical outcomes (decision making, diagnosis, and management) were similar between conventional care and telemedicine in specialty care, particularly in neurology and psychiatry. Virtual consults provided equal care to traditional specialty visits.6
Communication and coordination of care via an e-consult instead of a face-to-face clinic visit was evaluated by Horner and colleagues.5 The authors identified e-consult benefits for patients and specialists and that e-consults can reduce unnecessary referrals and appointments by 30%. They concluded that reserved time to complete e-consults must be built into workflow systems and that an advanced EMR was necessary for successful use of e-consults.
Two studies have evaluated satisfaction with e-consults. A preliminary analysis of satisfaction with e-consults was conducted in 2009 by K. L. Rodriguez, PhD, and colleagues (unpublished data). Patients, primary care providers (PCPs), and medical specialists reported overall satisfaction in 8 satisfaction domains. A pilot study of 34 patients in 2011 with inflammatory bowel disease compared a standard patient-GD physician encounter with a video encounter.7 The authors reported patient satisfaction, appointment time, wait times, and quality of care were similar for the 2 groups.
Methods
The GD where this QI project was completed consisted of a clinic staffed by nurse practitioners (NPs) and a procedure lab staffed by gastroenterology physicians. Before e-consult implementation, a NP reviewed and triaged new referrals daily. During the project period, an average of 25 to 35 new referrals were received daily via the EMR. Referrals came mostly from PCPs requesting an evaluation of their patients’ gastroenterology problem. Patients were triaged either to an appointment for evaluation or directly to the GD procedure lab for EGD or colonoscopy.
When e-consults were implemented, several changes occurred. Two providers were assigned to new referral triage, and they were expected to process 20% as e-consults. In the EMR, e-consult note titles, templates, and an e-consult encounter form were created, and staff was given access to the e-consult tracking database. The EMR referral template was amended so the entering provider could say whether a face-to-face appointment was desired or whether an e-consult was acceptable. The patient was to be included in decision making about this choice. Department staff had permission to triage according to judgment and expertise; thus, appointment requests could be triaged to e-consults, and e-consult requests could be triaged to appointments.
To complete an e-consult, the EMR was reviewed for medical diagnoses, medications, diagnostics, and recent physical exam. A summary note outlining an impression, treatment recommendations, and follow-up was entered in the EMR and communicated to the PCP. In most cases, no discussion with the patient occurred. The encounter form was completed, and information was entered into the tracking database. The database was installed on each provider’s computer who processed e-consults. If EGD or colonoscopy was indicated, the scheduler was notified to call the patient. Once a procedure date was established, procedure orders were completed in the EMR, and instructions were mailed to the patient.
Project Description and Evaluation
The project was reviewed by the Institutional Review Board and determined to be a QI project. VA organizational policies were followed for data collection and security. Benchmarks were identified from the e-consult program and from the GD, where available. Process variables were determined to measure benchmark outcomes. (Table)
To identify participants, a retrospective chart review was performed. A total of 203 potential patients were identified from the e-consult program database for the 6-month period between January and July 2012. For comparison, 50 patients who attended an appointment during the same time frame were systematically identified in the EMR. Although this comparison group was eligible for e-consults, they were triaged to in-person appointments and subsequently had colonoscopies completed.
Outcome data were extracted from the e-consult program database and the EMR. The data analysis was descriptive. Summary aggregate data were compared with the benchmarks and comparison patient outcomes. The Table summarizes the process variables, how they were measured, where they came from, and what the comparisons were.
Discussion
Figure 1 illustrates the volume of completed e-consults from January to July 2012. A gastroenterology procedure was not indicated in 43 patients. A procedure was indicated for 160 patients and completed in 116 patients (72%). One hundred procedures were colonoscopies, and 16 were EGDs. Figure 2 provides reasons why procedures were not completed in 44 patients (27%). Group comparisons of colon prep quality and preprocedure reminder calls are displayed in Figures 3 and 4, respectively.
This project sought to evaluate VAPHS GD e-consults beginning in January 2012. Process variables were established to measure benchmarks in the e-consult program and in the GD. Some benchmarks were met with outcomes that were comparable between the groups, while others were not. To our knowledge, this project is the first to evaluate e-consults in the subspecialty of gastroenterology.
Volume of Completed E-Consults
The benchmark for 20% e-consults was not met (Figure 1). For weeks 1 through 8, the volume was between 10% and 20%. Lower volume in weeks 9 through 15 (late March and April) may have been due to staff vacations. Not only do the outcomes show a downward trend in e-consult volume, they also show a precipitous fall in volume at week 15 to almost no e-consults for the remainder of the project. To explore reasons for this outcome, the workflow process of new referral triage and e-consults was reviewed.
Two providers (1 NP, 1 physician) were assigned to new referral triage and e-consults from weeks 1 through 14. At week 15, the physician was reassigned to perform procedures. From this point, only 1 NP worked on e-consults and referral triage. Competing time demands included an e-consult encounter form, tracking database entry, procedure orders, patient instructions, appointment changes, phone calls, and resolution of medications issues for procedures. The triage NP was also required to see patients in the clinic. Each day, only a half-day was allotted to complete e-consults, new referral triage (25-35/day), and the aforementioned tasks.
Therefore, it became clear that a half-day was not sufficient to meet the 20% benchmark for e-consults. Horner and colleagues also found that dedicated time in workflow processes was necessary to allow for e-consult completion.5
E-Consults vs Appointment Groups
All patients in both groups were offered the choice of e-consult or appointment; this benchmark was met. Of the 203 e-consult patients, 70% requested an appointment, but their evaluation was completed as an e-consult. By design, the appointment group patients chose e-consult but were triaged to appointment due to time constraints and the high volume of new referrals.
Evaluations via e-consult were completed within 2 to 3 days, whereas the mean for appointments was 19 days, with the longest time frame of 44 days. Thus, e-consult evaluations were completed sooner. Rapid access to care was also found by Zanaboni and colleagues.2
When appointments are delayed, patient complaints or status may change, which in turn may affect treatment plans. In addition, the reason for referral may have already resolved by the actual appointment, rendering the appointment unnecessary. This can be viewed as a missed opportunity for another patient to be seen. Ideally, it is best for a patient to be evaluated soon after a new referral is made.
The VA encounter form for an e-consult had only one 5-digit code, which allotted only 15 minutes of work credit. Encounter form codes were established by the Center for Medicare and Medicaid Services (CMS) for billing purposes in the private sector, with coding levels based on information documented in a chart note: review of systems, physical exam, and diagnoses decision making. Because all criteria could not be met in an e-consult, only 1 code was assigned for VAPHS e-consults. The CMS has specific telemedicine codes; it was unknown why they were not used for e-consults.
E-consults took an average of 19 minutes to complete, with 91 completed in ≤ 15 minutes and over half (112) having taken > 15 minutes. Therefore, the actual workload was not captured, and more work was done than was credited. To speculate, e-consults were in their infancy; a learning curve may have existed as staff became accustomed to this new process.
The EMRs were reviewed for the 7 e-consults that took > 30 minutes to complete. Two were in the early stage of e-consult implementation, but the remainder were scattered throughout. Patients in these e-consults had complicated medical histories and perhaps should not have been triaged to e-consult. Theoretically, only uncomplicated patients with simple reasons for gastroenterology referral should be triaged to e-consult, allowing for a shorter time frame and higher volume.
The wait times to procedure were 58 days for the e-consult group and 39 days in the appointment group. Although wait time was originally identified as an outcome, its relevance is questionable after looking at the outcome data. The procedure appointment date was a subjective decision by the patient; many factors affected what date the patient established, including weekday preference, time off from work, caretaker availability, season, and staffing. Many patients rescheduled their initial procedure dates, often several times. These factors are reflected in the variable ranges of wait times.
Colon Prep Quality
Colon prep refers to patient instructions on the day before the procedure and includes a clear liquid diet, drinking a liquid solution to empty bowel contents, and no food or liquid after midnight. Prep quality is stated in the colonoscopy report. During the procedure, the physician makes a visual decision based on presence or absence of stool inside the colon. Prep quality is important, because retained stool can preclude thorough visualization of the colon wall for polyps or abnormalities. In the event of fair and poor preps, the colonoscopy might be aborted and rescheduled or completed, but with the recommendation for another colonoscopy in a short time frame, such as 1 to 3 years.
Forty-four percent of the e-consult group and 62% of the appointment group had good or adequate preps. Thus, more patients in the appointment group achieved good and adequate preps, and far fewer achieved fair or poor preps. One important point was that almost half (47%) of the e-consult group had only a fair prep (Figure 3).
A number of reasons have been identified in the literature, which might help us understand these findings. First, patients may not fully understand or adhere to prep instructions.8-10 Furthermore, certain medical diagnoses are known to affect prep quality (ie, diabetes, thyroid disease, constipation).11,12 Another potential factor is the manner in which prep quality is determined.13,14 However, due to the focus of this QI study, the influential drivers of prep quality can only be inferred from the literature; thus, a future research or QI study is warranted to ascertain the underlying mechanism of colon prep quality in our specific veteran population.
Preprocedure Reminder Calls
Outcomes were essentially reversed between the 2 groups (Figure 4). Between 50% and 60% of the e-consult group received a call, while the same percentage of appointment patients did not. All patients did attend their procedure appointment. A GD goal was to call every patient before their procedure, but the ability to make the calls was staffing-dependent, which may explain these findings.
Most Relevant Findings
Although this project provides a thorough analysis of various benchmarks within this recently implemented e-consult gastroenterology program, 3 findings emerged that were identified as most relevant. First, the benchmark of 20% volume of completed e-consults was not met. A review of the workflow processes revealed that a daily allotment of only a half-day was not sufficient to complete e-consults, referral triage, and related tasks.
Second, outcomes for colon prep quality and preprocedure reminder calls were also relevant. Although beyond the scope of this project, the question arose of a relationship between prep quality and the reminder call: Does the reminder call affect prep quality? The goal of colon prep is to achieve a good or adequate prep. The purpose of the reminder call was to confirm the appointment and to review the colon prep. Among patients in both groups who achieved only a fair prep, 62% in the e-consult group did receive the reminder call; thus, the call seemed to have failed in helping these patients achieve an adequate or good prep. The actual content of colon prep review during the call was unknown, but certainly bears improvement.
Another speculation concerned the prep instruction sheet. Although all patients received the same sheet, e-consult patients received it in the mail and read it themselves, while it was directly reviewed face-to-face with the appointment patients. Questions remain whether a face-to-face review increases the likelihood for a better prep and how to help e-consult patients achieve optimal prep, since they are not seen face-to-face.
Practice Implications and Sustaining Measures
Theoretically, e-consults are a viable alternative to face-to-face appointments. Potential advantages include efficient use of an EMR, avoidance of unnecessary appointments, and improved access to care for patients who require an appointment. Although patient satisfaction was not measured in this project, the literature review revealed that satisfaction was increased through use of various virtual health care modalities, including a preliminary analysis in this facility by the aforementioned 2009 study by K. L. Rodriguez (unpublished data). Based on findings in this project, the following 4 recommendations were made to improve benchmark outcomes and quality of care.
- Provide dedicated time to complete e-consults and related tasks. In this setting, a full day was recommended. An alternative was to hire a NP whose sole responsibility was e-consults.
- Develop a selection process to determine which new referrals are best suited for e-consult. This process will increase e-consult efficiency and decrease the time to complete an e-consult. Recommended selection criteria were (A) gastroenterology referrals only for simple symptoms or issues; (B) referrals only for a procedure; and (C) stable patients with uncomplicated medical histories.
- Sustain the preprocedure reminder phone call. The reminder call helps patients keep appointments and thus reduces a missed opportunity for care.
- Plan a future QI project or research study on patient colon prep quality for colonoscopy. Such a project might evaluate types of colon prep, how prep quality is measured, patient instructions, and the timing/content of pre-procedure reminder phone calls, particularly for e-consult patients.
Conclusion
This QI project provided outcomes for e-consults in the subspecialty of gastroenterology at the VAPHS. Although some benchmark outcomes were met and favorable, others were less favorable. By conducting this benchmark analysis, the areas of needed improvement are now clear. This analysis provides information so recommendations for process improvements can be made.
Quality of care improvement is an ongoing process at VAPHS. Since completion of this project, several processes have been adjusted so that outcomes will be improved. For example, corrective actions were taken for patients who did not complete their gastroenterology procedure. The process for scheduling gastroenterology procedures was adjusted for appointments and cancellations. Ongoing efforts to sustain the reminder phone call were put in place. Changes in NP staffing and time assigned for both clinical and nonclinical work were proposed and are currently under review.
It is the mission of the VA to provide access to care, patient satisfaction, timely care, and appropriate use of resources. Having the ability to highlight our strengths, as well as the willingness to recognize weaknesses, allows us to create new improved processes to provide the best care possible for our veterans.
Acknowledgments
This project was Elena Swann’s capstone for the Doctor of Nursing Practice Program, University of Pittsburgh School of Nursing in Pennsylvania. Rich Laufer, Larry Priscella, and Janie Fleming assisted with the VA Gastroenterology Clinic and procedure metrics. Dr. Melissa Taylor, VA associate chief nurse for research provided project guidance and manuscript revisions. Dr. Sandra Engberg, University of Pittsburgh School of Nursing faculty, assisted with project and manuscript development.
Author disclosures
The author reports no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the author and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Bashshur RL, Shannon GW, Krupinski EA, et al. National telemedicine initiatives: Essential to healthcare reform. Telemed J E Health. 2009;15(6):600-610.
2. Zanaboni P, Scalvini S, Bernocchi P, Borghi G, Tridico C, Masella C. Teleconsultation service to improve healthcare in rural areas: Acceptance, organizational impact and appropriateness. BMC Health Serv Res. 2009;9:238.
3. Wallace P, Barber J, Clayton W, et al. Virtual outreach: A randomised controlled trial and economic evaluation of joint teleconferenced medical consultations. Health Technol Assess. 2004;8(50):1-106, iii-iv.
4. Hersh WR, Hickam DH, Severance SM, Dana TL, Pyle Krages K, Helfand M. Diagnosis, access and outcomes: Update of a systematic review of telemedicine services. J Telemed Telecare. 2006;12(suppl 2):S3-S31.
5. Horner K, Wagner E, Tufano J. Electronic consultations between primary and specialty care clinicians: Early insights. Issue Brief (Commonw Fund). 2011;23:1-14.
6. Angstman KB, Adamson SC, Furst JW, Houston MS, Rohrer JE. Provider satisfaction with virtual specialist consultations in a family medicine department. Health Care Manag (Frederick). 2009;28(1):14-18.
7. Krier M, Kaltenbach T, McQuaid K, Soetikno R. Potential use of telemedicine to provide outpatient care for inflammatory bowel disease. Am J Gastroenterol. 2011;106(12):2063-2067.
8. Calderwood AH, Lai EJ, Fix OK, Jacobson BC. An endoscopist-blinded, randomized, controlled trial of a simple visual aid to improve bowel preparation for screening colonoscopy. Gastrointest Endosc. 2011;73(2):307-314.
9. Hillyer GC, Basch CH, Basch CE, et al. Gastroenterologists’ perceived barriers to optimal pre-colonoscopy bowel preparation: Results of a national survey. J Cancer Educ. 2012;27(3):526-532.
10. Smith SG, von Wagner C, McGregor LM, et al. The influence of health literacy on comprehension of a colonoscopy preparation information leaflet. Dis Colon Rectum. 2012;55(10):1074-1080.
11. Mittal S, Lin YL, Tan A, Kuo YF, El-Serag HB, Goodwin JS. Limited life expectancy among a subgroup of Medicare beneficiaries receiving screening colonoscopies. Clin Gastroenterol Hepatol. 2014;12(3):443-450.e1.
12. Ko CW, Sonnenberg A. Comparing risks and benefits of colorectal cancer screening in elderly patients. Gastroenterology. 2005;129(4):1163-1170.
13. Ell C, Fischbach W, Keller R, et al; Hintertux Study Group. A randomized, blinded, prospective trial to compare the safety and efficacy of three bowel-cleansing solutions for colonoscopy (HSG-01*). Endoscopy. 2003;35(4):300-304.
14. Jansen SV, Goedhard JG, Winkens B, van Deursen CT. Preparation before colonoscopy: A randomized controlled trial comparing different regimes. Eur J Gastroenterol Hepatol. 2011;23(10):897-902.
Although the VA has the largest health care system in the U.S., not every VA facility offers medical subspecialty care. As a result, patients are often separated by long distances from services they need.
At the VA Pittsburgh Healthcare System (VAPHS) in Pennsylvania, about 15,700 veterans received care in 2011. The Gastroenterology Department (GD) served many of these patients: 5,800 patients were seen in clinic appointments, 2,500 underwent colonoscopy, and 1,700 underwent esophagogastroduodenoscopy (EGD). Patients traveled up to 150 miles from 3 states for appointments and procedures. Prior to each procedure, a face-to-face appointment was standard practice for most patients to plan procedures and ensure medical stability. Patients expressed dissatisfaction with transportation, cost, time, and inconvenience, particularly when they were required to attend both the preprocedure and procedure appointments.
Patient satisfaction, timely care, and appropriate use of resources are important VA goals of care, so the VAPHS developed an electronic consultation (e-consult) program as a component of its long-term strategic plan. The goal was to increase access to care through the use of an e-consult in lieu of a face-to-face appointment for select patients. The e-consult program established guidelines and benchmark goals (Table). The program also established a database to track the benchmarks.
The purpose of this quality improvement (QI) project was to evaluate e-consults in the GD over a 6-month period from January 2012, when e-consults were implemented in the GD, to July 2012. Based on the outcomes, recommendations for program continuation, change, and sustainability were made.
Background
Telemedicine using information technologies has been reported as a viable solution to support health care delivery when distance limits patients’ access to care.1,2 Telemedicine has also been shown to improve efficacy in clinical decision making and reduce costs. It also can increase patient satisfaction by reducing travel and time, minimize duplication of diagnostic testing, and integrate services effectively across multiple sites when an electronic medical record (EMR) is in place.1-5
A randomized controlled trial in 2004 compared a standard outpatient referral appointment with a joint teleconsultation between provider, specialist, and patient.3 In the teleconsultation arm, there was higher patient satisfaction, fewer diagnostic tests (particularly in gastroenterology), and lower patient costs.
A study published in 2009 examined the impact of cardiac, dermatologic, and diabetes teleconsultations on organization and patient outcomes in 950 patients in 30 rural communities.2 Rapid access to care was provided for 85% of the patients. Organizational benefits included resource savings and efficacy improvement measured by a provider opinion Likert scale. Patient benefits included reductions in wait times, transportation savings, avoidance of unnecessary office visits, and ease of use.
A large systematic review of telemedicine services across all medical specialties in 2006 included 106 published studies.4 Clinical outcomes (decision making, diagnosis, and management) were similar between conventional care and telemedicine in specialty care, particularly in neurology and psychiatry. Virtual consults provided equal care to traditional specialty visits.6
Communication and coordination of care via an e-consult instead of a face-to-face clinic visit was evaluated by Horner and colleagues.5 The authors identified e-consult benefits for patients and specialists and that e-consults can reduce unnecessary referrals and appointments by 30%. They concluded that reserved time to complete e-consults must be built into workflow systems and that an advanced EMR was necessary for successful use of e-consults.
Two studies have evaluated satisfaction with e-consults. A preliminary analysis of satisfaction with e-consults was conducted in 2009 by K. L. Rodriguez, PhD, and colleagues (unpublished data). Patients, primary care providers (PCPs), and medical specialists reported overall satisfaction in 8 satisfaction domains. A pilot study of 34 patients in 2011 with inflammatory bowel disease compared a standard patient-GD physician encounter with a video encounter.7 The authors reported patient satisfaction, appointment time, wait times, and quality of care were similar for the 2 groups.
Methods
The GD where this QI project was completed consisted of a clinic staffed by nurse practitioners (NPs) and a procedure lab staffed by gastroenterology physicians. Before e-consult implementation, a NP reviewed and triaged new referrals daily. During the project period, an average of 25 to 35 new referrals were received daily via the EMR. Referrals came mostly from PCPs requesting an evaluation of their patients’ gastroenterology problem. Patients were triaged either to an appointment for evaluation or directly to the GD procedure lab for EGD or colonoscopy.
When e-consults were implemented, several changes occurred. Two providers were assigned to new referral triage, and they were expected to process 20% as e-consults. In the EMR, e-consult note titles, templates, and an e-consult encounter form were created, and staff was given access to the e-consult tracking database. The EMR referral template was amended so the entering provider could say whether a face-to-face appointment was desired or whether an e-consult was acceptable. The patient was to be included in decision making about this choice. Department staff had permission to triage according to judgment and expertise; thus, appointment requests could be triaged to e-consults, and e-consult requests could be triaged to appointments.
To complete an e-consult, the EMR was reviewed for medical diagnoses, medications, diagnostics, and recent physical exam. A summary note outlining an impression, treatment recommendations, and follow-up was entered in the EMR and communicated to the PCP. In most cases, no discussion with the patient occurred. The encounter form was completed, and information was entered into the tracking database. The database was installed on each provider’s computer who processed e-consults. If EGD or colonoscopy was indicated, the scheduler was notified to call the patient. Once a procedure date was established, procedure orders were completed in the EMR, and instructions were mailed to the patient.
Project Description and Evaluation
The project was reviewed by the Institutional Review Board and determined to be a QI project. VA organizational policies were followed for data collection and security. Benchmarks were identified from the e-consult program and from the GD, where available. Process variables were determined to measure benchmark outcomes. (Table)
To identify participants, a retrospective chart review was performed. A total of 203 potential patients were identified from the e-consult program database for the 6-month period between January and July 2012. For comparison, 50 patients who attended an appointment during the same time frame were systematically identified in the EMR. Although this comparison group was eligible for e-consults, they were triaged to in-person appointments and subsequently had colonoscopies completed.
Outcome data were extracted from the e-consult program database and the EMR. The data analysis was descriptive. Summary aggregate data were compared with the benchmarks and comparison patient outcomes. The Table summarizes the process variables, how they were measured, where they came from, and what the comparisons were.
Discussion
Figure 1 illustrates the volume of completed e-consults from January to July 2012. A gastroenterology procedure was not indicated in 43 patients. A procedure was indicated for 160 patients and completed in 116 patients (72%). One hundred procedures were colonoscopies, and 16 were EGDs. Figure 2 provides reasons why procedures were not completed in 44 patients (27%). Group comparisons of colon prep quality and preprocedure reminder calls are displayed in Figures 3 and 4, respectively.
This project sought to evaluate VAPHS GD e-consults beginning in January 2012. Process variables were established to measure benchmarks in the e-consult program and in the GD. Some benchmarks were met with outcomes that were comparable between the groups, while others were not. To our knowledge, this project is the first to evaluate e-consults in the subspecialty of gastroenterology.
Volume of Completed E-Consults
The benchmark for 20% e-consults was not met (Figure 1). For weeks 1 through 8, the volume was between 10% and 20%. Lower volume in weeks 9 through 15 (late March and April) may have been due to staff vacations. Not only do the outcomes show a downward trend in e-consult volume, they also show a precipitous fall in volume at week 15 to almost no e-consults for the remainder of the project. To explore reasons for this outcome, the workflow process of new referral triage and e-consults was reviewed.
Two providers (1 NP, 1 physician) were assigned to new referral triage and e-consults from weeks 1 through 14. At week 15, the physician was reassigned to perform procedures. From this point, only 1 NP worked on e-consults and referral triage. Competing time demands included an e-consult encounter form, tracking database entry, procedure orders, patient instructions, appointment changes, phone calls, and resolution of medications issues for procedures. The triage NP was also required to see patients in the clinic. Each day, only a half-day was allotted to complete e-consults, new referral triage (25-35/day), and the aforementioned tasks.
Therefore, it became clear that a half-day was not sufficient to meet the 20% benchmark for e-consults. Horner and colleagues also found that dedicated time in workflow processes was necessary to allow for e-consult completion.5
E-Consults vs Appointment Groups
All patients in both groups were offered the choice of e-consult or appointment; this benchmark was met. Of the 203 e-consult patients, 70% requested an appointment, but their evaluation was completed as an e-consult. By design, the appointment group patients chose e-consult but were triaged to appointment due to time constraints and the high volume of new referrals.
Evaluations via e-consult were completed within 2 to 3 days, whereas the mean for appointments was 19 days, with the longest time frame of 44 days. Thus, e-consult evaluations were completed sooner. Rapid access to care was also found by Zanaboni and colleagues.2
When appointments are delayed, patient complaints or status may change, which in turn may affect treatment plans. In addition, the reason for referral may have already resolved by the actual appointment, rendering the appointment unnecessary. This can be viewed as a missed opportunity for another patient to be seen. Ideally, it is best for a patient to be evaluated soon after a new referral is made.
The VA encounter form for an e-consult had only one 5-digit code, which allotted only 15 minutes of work credit. Encounter form codes were established by the Center for Medicare and Medicaid Services (CMS) for billing purposes in the private sector, with coding levels based on information documented in a chart note: review of systems, physical exam, and diagnoses decision making. Because all criteria could not be met in an e-consult, only 1 code was assigned for VAPHS e-consults. The CMS has specific telemedicine codes; it was unknown why they were not used for e-consults.
E-consults took an average of 19 minutes to complete, with 91 completed in ≤ 15 minutes and over half (112) having taken > 15 minutes. Therefore, the actual workload was not captured, and more work was done than was credited. To speculate, e-consults were in their infancy; a learning curve may have existed as staff became accustomed to this new process.
The EMRs were reviewed for the 7 e-consults that took > 30 minutes to complete. Two were in the early stage of e-consult implementation, but the remainder were scattered throughout. Patients in these e-consults had complicated medical histories and perhaps should not have been triaged to e-consult. Theoretically, only uncomplicated patients with simple reasons for gastroenterology referral should be triaged to e-consult, allowing for a shorter time frame and higher volume.
The wait times to procedure were 58 days for the e-consult group and 39 days in the appointment group. Although wait time was originally identified as an outcome, its relevance is questionable after looking at the outcome data. The procedure appointment date was a subjective decision by the patient; many factors affected what date the patient established, including weekday preference, time off from work, caretaker availability, season, and staffing. Many patients rescheduled their initial procedure dates, often several times. These factors are reflected in the variable ranges of wait times.
Colon Prep Quality
Colon prep refers to patient instructions on the day before the procedure and includes a clear liquid diet, drinking a liquid solution to empty bowel contents, and no food or liquid after midnight. Prep quality is stated in the colonoscopy report. During the procedure, the physician makes a visual decision based on presence or absence of stool inside the colon. Prep quality is important, because retained stool can preclude thorough visualization of the colon wall for polyps or abnormalities. In the event of fair and poor preps, the colonoscopy might be aborted and rescheduled or completed, but with the recommendation for another colonoscopy in a short time frame, such as 1 to 3 years.
Forty-four percent of the e-consult group and 62% of the appointment group had good or adequate preps. Thus, more patients in the appointment group achieved good and adequate preps, and far fewer achieved fair or poor preps. One important point was that almost half (47%) of the e-consult group had only a fair prep (Figure 3).
A number of reasons have been identified in the literature, which might help us understand these findings. First, patients may not fully understand or adhere to prep instructions.8-10 Furthermore, certain medical diagnoses are known to affect prep quality (ie, diabetes, thyroid disease, constipation).11,12 Another potential factor is the manner in which prep quality is determined.13,14 However, due to the focus of this QI study, the influential drivers of prep quality can only be inferred from the literature; thus, a future research or QI study is warranted to ascertain the underlying mechanism of colon prep quality in our specific veteran population.
Preprocedure Reminder Calls
Outcomes were essentially reversed between the 2 groups (Figure 4). Between 50% and 60% of the e-consult group received a call, while the same percentage of appointment patients did not. All patients did attend their procedure appointment. A GD goal was to call every patient before their procedure, but the ability to make the calls was staffing-dependent, which may explain these findings.
Most Relevant Findings
Although this project provides a thorough analysis of various benchmarks within this recently implemented e-consult gastroenterology program, 3 findings emerged that were identified as most relevant. First, the benchmark of 20% volume of completed e-consults was not met. A review of the workflow processes revealed that a daily allotment of only a half-day was not sufficient to complete e-consults, referral triage, and related tasks.
Second, outcomes for colon prep quality and preprocedure reminder calls were also relevant. Although beyond the scope of this project, the question arose of a relationship between prep quality and the reminder call: Does the reminder call affect prep quality? The goal of colon prep is to achieve a good or adequate prep. The purpose of the reminder call was to confirm the appointment and to review the colon prep. Among patients in both groups who achieved only a fair prep, 62% in the e-consult group did receive the reminder call; thus, the call seemed to have failed in helping these patients achieve an adequate or good prep. The actual content of colon prep review during the call was unknown, but certainly bears improvement.
Another speculation concerned the prep instruction sheet. Although all patients received the same sheet, e-consult patients received it in the mail and read it themselves, while it was directly reviewed face-to-face with the appointment patients. Questions remain whether a face-to-face review increases the likelihood for a better prep and how to help e-consult patients achieve optimal prep, since they are not seen face-to-face.
Practice Implications and Sustaining Measures
Theoretically, e-consults are a viable alternative to face-to-face appointments. Potential advantages include efficient use of an EMR, avoidance of unnecessary appointments, and improved access to care for patients who require an appointment. Although patient satisfaction was not measured in this project, the literature review revealed that satisfaction was increased through use of various virtual health care modalities, including a preliminary analysis in this facility by the aforementioned 2009 study by K. L. Rodriguez (unpublished data). Based on findings in this project, the following 4 recommendations were made to improve benchmark outcomes and quality of care.
- Provide dedicated time to complete e-consults and related tasks. In this setting, a full day was recommended. An alternative was to hire a NP whose sole responsibility was e-consults.
- Develop a selection process to determine which new referrals are best suited for e-consult. This process will increase e-consult efficiency and decrease the time to complete an e-consult. Recommended selection criteria were (A) gastroenterology referrals only for simple symptoms or issues; (B) referrals only for a procedure; and (C) stable patients with uncomplicated medical histories.
- Sustain the preprocedure reminder phone call. The reminder call helps patients keep appointments and thus reduces a missed opportunity for care.
- Plan a future QI project or research study on patient colon prep quality for colonoscopy. Such a project might evaluate types of colon prep, how prep quality is measured, patient instructions, and the timing/content of pre-procedure reminder phone calls, particularly for e-consult patients.
Conclusion
This QI project provided outcomes for e-consults in the subspecialty of gastroenterology at the VAPHS. Although some benchmark outcomes were met and favorable, others were less favorable. By conducting this benchmark analysis, the areas of needed improvement are now clear. This analysis provides information so recommendations for process improvements can be made.
Quality of care improvement is an ongoing process at VAPHS. Since completion of this project, several processes have been adjusted so that outcomes will be improved. For example, corrective actions were taken for patients who did not complete their gastroenterology procedure. The process for scheduling gastroenterology procedures was adjusted for appointments and cancellations. Ongoing efforts to sustain the reminder phone call were put in place. Changes in NP staffing and time assigned for both clinical and nonclinical work were proposed and are currently under review.
It is the mission of the VA to provide access to care, patient satisfaction, timely care, and appropriate use of resources. Having the ability to highlight our strengths, as well as the willingness to recognize weaknesses, allows us to create new improved processes to provide the best care possible for our veterans.
Acknowledgments
This project was Elena Swann’s capstone for the Doctor of Nursing Practice Program, University of Pittsburgh School of Nursing in Pennsylvania. Rich Laufer, Larry Priscella, and Janie Fleming assisted with the VA Gastroenterology Clinic and procedure metrics. Dr. Melissa Taylor, VA associate chief nurse for research provided project guidance and manuscript revisions. Dr. Sandra Engberg, University of Pittsburgh School of Nursing faculty, assisted with project and manuscript development.
Author disclosures
The author reports no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the author and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Although the VA has the largest health care system in the U.S., not every VA facility offers medical subspecialty care. As a result, patients are often separated by long distances from services they need.
At the VA Pittsburgh Healthcare System (VAPHS) in Pennsylvania, about 15,700 veterans received care in 2011. The Gastroenterology Department (GD) served many of these patients: 5,800 patients were seen in clinic appointments, 2,500 underwent colonoscopy, and 1,700 underwent esophagogastroduodenoscopy (EGD). Patients traveled up to 150 miles from 3 states for appointments and procedures. Prior to each procedure, a face-to-face appointment was standard practice for most patients to plan procedures and ensure medical stability. Patients expressed dissatisfaction with transportation, cost, time, and inconvenience, particularly when they were required to attend both the preprocedure and procedure appointments.
Patient satisfaction, timely care, and appropriate use of resources are important VA goals of care, so the VAPHS developed an electronic consultation (e-consult) program as a component of its long-term strategic plan. The goal was to increase access to care through the use of an e-consult in lieu of a face-to-face appointment for select patients. The e-consult program established guidelines and benchmark goals (Table). The program also established a database to track the benchmarks.
The purpose of this quality improvement (QI) project was to evaluate e-consults in the GD over a 6-month period from January 2012, when e-consults were implemented in the GD, to July 2012. Based on the outcomes, recommendations for program continuation, change, and sustainability were made.
Background
Telemedicine using information technologies has been reported as a viable solution to support health care delivery when distance limits patients’ access to care.1,2 Telemedicine has also been shown to improve efficacy in clinical decision making and reduce costs. It also can increase patient satisfaction by reducing travel and time, minimize duplication of diagnostic testing, and integrate services effectively across multiple sites when an electronic medical record (EMR) is in place.1-5
A randomized controlled trial in 2004 compared a standard outpatient referral appointment with a joint teleconsultation between provider, specialist, and patient.3 In the teleconsultation arm, there was higher patient satisfaction, fewer diagnostic tests (particularly in gastroenterology), and lower patient costs.
A study published in 2009 examined the impact of cardiac, dermatologic, and diabetes teleconsultations on organization and patient outcomes in 950 patients in 30 rural communities.2 Rapid access to care was provided for 85% of the patients. Organizational benefits included resource savings and efficacy improvement measured by a provider opinion Likert scale. Patient benefits included reductions in wait times, transportation savings, avoidance of unnecessary office visits, and ease of use.
A large systematic review of telemedicine services across all medical specialties in 2006 included 106 published studies.4 Clinical outcomes (decision making, diagnosis, and management) were similar between conventional care and telemedicine in specialty care, particularly in neurology and psychiatry. Virtual consults provided equal care to traditional specialty visits.6
Communication and coordination of care via an e-consult instead of a face-to-face clinic visit was evaluated by Horner and colleagues.5 The authors identified e-consult benefits for patients and specialists and that e-consults can reduce unnecessary referrals and appointments by 30%. They concluded that reserved time to complete e-consults must be built into workflow systems and that an advanced EMR was necessary for successful use of e-consults.
Two studies have evaluated satisfaction with e-consults. A preliminary analysis of satisfaction with e-consults was conducted in 2009 by K. L. Rodriguez, PhD, and colleagues (unpublished data). Patients, primary care providers (PCPs), and medical specialists reported overall satisfaction in 8 satisfaction domains. A pilot study of 34 patients in 2011 with inflammatory bowel disease compared a standard patient-GD physician encounter with a video encounter.7 The authors reported patient satisfaction, appointment time, wait times, and quality of care were similar for the 2 groups.
Methods
The GD where this QI project was completed consisted of a clinic staffed by nurse practitioners (NPs) and a procedure lab staffed by gastroenterology physicians. Before e-consult implementation, a NP reviewed and triaged new referrals daily. During the project period, an average of 25 to 35 new referrals were received daily via the EMR. Referrals came mostly from PCPs requesting an evaluation of their patients’ gastroenterology problem. Patients were triaged either to an appointment for evaluation or directly to the GD procedure lab for EGD or colonoscopy.
When e-consults were implemented, several changes occurred. Two providers were assigned to new referral triage, and they were expected to process 20% as e-consults. In the EMR, e-consult note titles, templates, and an e-consult encounter form were created, and staff was given access to the e-consult tracking database. The EMR referral template was amended so the entering provider could say whether a face-to-face appointment was desired or whether an e-consult was acceptable. The patient was to be included in decision making about this choice. Department staff had permission to triage according to judgment and expertise; thus, appointment requests could be triaged to e-consults, and e-consult requests could be triaged to appointments.
To complete an e-consult, the EMR was reviewed for medical diagnoses, medications, diagnostics, and recent physical exam. A summary note outlining an impression, treatment recommendations, and follow-up was entered in the EMR and communicated to the PCP. In most cases, no discussion with the patient occurred. The encounter form was completed, and information was entered into the tracking database. The database was installed on each provider’s computer who processed e-consults. If EGD or colonoscopy was indicated, the scheduler was notified to call the patient. Once a procedure date was established, procedure orders were completed in the EMR, and instructions were mailed to the patient.
Project Description and Evaluation
The project was reviewed by the Institutional Review Board and determined to be a QI project. VA organizational policies were followed for data collection and security. Benchmarks were identified from the e-consult program and from the GD, where available. Process variables were determined to measure benchmark outcomes. (Table)
To identify participants, a retrospective chart review was performed. A total of 203 potential patients were identified from the e-consult program database for the 6-month period between January and July 2012. For comparison, 50 patients who attended an appointment during the same time frame were systematically identified in the EMR. Although this comparison group was eligible for e-consults, they were triaged to in-person appointments and subsequently had colonoscopies completed.
Outcome data were extracted from the e-consult program database and the EMR. The data analysis was descriptive. Summary aggregate data were compared with the benchmarks and comparison patient outcomes. The Table summarizes the process variables, how they were measured, where they came from, and what the comparisons were.
Discussion
Figure 1 illustrates the volume of completed e-consults from January to July 2012. A gastroenterology procedure was not indicated in 43 patients. A procedure was indicated for 160 patients and completed in 116 patients (72%). One hundred procedures were colonoscopies, and 16 were EGDs. Figure 2 provides reasons why procedures were not completed in 44 patients (27%). Group comparisons of colon prep quality and preprocedure reminder calls are displayed in Figures 3 and 4, respectively.
This project sought to evaluate VAPHS GD e-consults beginning in January 2012. Process variables were established to measure benchmarks in the e-consult program and in the GD. Some benchmarks were met with outcomes that were comparable between the groups, while others were not. To our knowledge, this project is the first to evaluate e-consults in the subspecialty of gastroenterology.
Volume of Completed E-Consults
The benchmark for 20% e-consults was not met (Figure 1). For weeks 1 through 8, the volume was between 10% and 20%. Lower volume in weeks 9 through 15 (late March and April) may have been due to staff vacations. Not only do the outcomes show a downward trend in e-consult volume, they also show a precipitous fall in volume at week 15 to almost no e-consults for the remainder of the project. To explore reasons for this outcome, the workflow process of new referral triage and e-consults was reviewed.
Two providers (1 NP, 1 physician) were assigned to new referral triage and e-consults from weeks 1 through 14. At week 15, the physician was reassigned to perform procedures. From this point, only 1 NP worked on e-consults and referral triage. Competing time demands included an e-consult encounter form, tracking database entry, procedure orders, patient instructions, appointment changes, phone calls, and resolution of medications issues for procedures. The triage NP was also required to see patients in the clinic. Each day, only a half-day was allotted to complete e-consults, new referral triage (25-35/day), and the aforementioned tasks.
Therefore, it became clear that a half-day was not sufficient to meet the 20% benchmark for e-consults. Horner and colleagues also found that dedicated time in workflow processes was necessary to allow for e-consult completion.5
E-Consults vs Appointment Groups
All patients in both groups were offered the choice of e-consult or appointment; this benchmark was met. Of the 203 e-consult patients, 70% requested an appointment, but their evaluation was completed as an e-consult. By design, the appointment group patients chose e-consult but were triaged to appointment due to time constraints and the high volume of new referrals.
Evaluations via e-consult were completed within 2 to 3 days, whereas the mean for appointments was 19 days, with the longest time frame of 44 days. Thus, e-consult evaluations were completed sooner. Rapid access to care was also found by Zanaboni and colleagues.2
When appointments are delayed, patient complaints or status may change, which in turn may affect treatment plans. In addition, the reason for referral may have already resolved by the actual appointment, rendering the appointment unnecessary. This can be viewed as a missed opportunity for another patient to be seen. Ideally, it is best for a patient to be evaluated soon after a new referral is made.
The VA encounter form for an e-consult had only one 5-digit code, which allotted only 15 minutes of work credit. Encounter form codes were established by the Center for Medicare and Medicaid Services (CMS) for billing purposes in the private sector, with coding levels based on information documented in a chart note: review of systems, physical exam, and diagnoses decision making. Because all criteria could not be met in an e-consult, only 1 code was assigned for VAPHS e-consults. The CMS has specific telemedicine codes; it was unknown why they were not used for e-consults.
E-consults took an average of 19 minutes to complete, with 91 completed in ≤ 15 minutes and over half (112) having taken > 15 minutes. Therefore, the actual workload was not captured, and more work was done than was credited. To speculate, e-consults were in their infancy; a learning curve may have existed as staff became accustomed to this new process.
The EMRs were reviewed for the 7 e-consults that took > 30 minutes to complete. Two were in the early stage of e-consult implementation, but the remainder were scattered throughout. Patients in these e-consults had complicated medical histories and perhaps should not have been triaged to e-consult. Theoretically, only uncomplicated patients with simple reasons for gastroenterology referral should be triaged to e-consult, allowing for a shorter time frame and higher volume.
The wait times to procedure were 58 days for the e-consult group and 39 days in the appointment group. Although wait time was originally identified as an outcome, its relevance is questionable after looking at the outcome data. The procedure appointment date was a subjective decision by the patient; many factors affected what date the patient established, including weekday preference, time off from work, caretaker availability, season, and staffing. Many patients rescheduled their initial procedure dates, often several times. These factors are reflected in the variable ranges of wait times.
Colon Prep Quality
Colon prep refers to patient instructions on the day before the procedure and includes a clear liquid diet, drinking a liquid solution to empty bowel contents, and no food or liquid after midnight. Prep quality is stated in the colonoscopy report. During the procedure, the physician makes a visual decision based on presence or absence of stool inside the colon. Prep quality is important, because retained stool can preclude thorough visualization of the colon wall for polyps or abnormalities. In the event of fair and poor preps, the colonoscopy might be aborted and rescheduled or completed, but with the recommendation for another colonoscopy in a short time frame, such as 1 to 3 years.
Forty-four percent of the e-consult group and 62% of the appointment group had good or adequate preps. Thus, more patients in the appointment group achieved good and adequate preps, and far fewer achieved fair or poor preps. One important point was that almost half (47%) of the e-consult group had only a fair prep (Figure 3).
A number of reasons have been identified in the literature, which might help us understand these findings. First, patients may not fully understand or adhere to prep instructions.8-10 Furthermore, certain medical diagnoses are known to affect prep quality (ie, diabetes, thyroid disease, constipation).11,12 Another potential factor is the manner in which prep quality is determined.13,14 However, due to the focus of this QI study, the influential drivers of prep quality can only be inferred from the literature; thus, a future research or QI study is warranted to ascertain the underlying mechanism of colon prep quality in our specific veteran population.
Preprocedure Reminder Calls
Outcomes were essentially reversed between the 2 groups (Figure 4). Between 50% and 60% of the e-consult group received a call, while the same percentage of appointment patients did not. All patients did attend their procedure appointment. A GD goal was to call every patient before their procedure, but the ability to make the calls was staffing-dependent, which may explain these findings.
Most Relevant Findings
Although this project provides a thorough analysis of various benchmarks within this recently implemented e-consult gastroenterology program, 3 findings emerged that were identified as most relevant. First, the benchmark of 20% volume of completed e-consults was not met. A review of the workflow processes revealed that a daily allotment of only a half-day was not sufficient to complete e-consults, referral triage, and related tasks.
Second, outcomes for colon prep quality and preprocedure reminder calls were also relevant. Although beyond the scope of this project, the question arose of a relationship between prep quality and the reminder call: Does the reminder call affect prep quality? The goal of colon prep is to achieve a good or adequate prep. The purpose of the reminder call was to confirm the appointment and to review the colon prep. Among patients in both groups who achieved only a fair prep, 62% in the e-consult group did receive the reminder call; thus, the call seemed to have failed in helping these patients achieve an adequate or good prep. The actual content of colon prep review during the call was unknown, but certainly bears improvement.
Another speculation concerned the prep instruction sheet. Although all patients received the same sheet, e-consult patients received it in the mail and read it themselves, while it was directly reviewed face-to-face with the appointment patients. Questions remain whether a face-to-face review increases the likelihood for a better prep and how to help e-consult patients achieve optimal prep, since they are not seen face-to-face.
Practice Implications and Sustaining Measures
Theoretically, e-consults are a viable alternative to face-to-face appointments. Potential advantages include efficient use of an EMR, avoidance of unnecessary appointments, and improved access to care for patients who require an appointment. Although patient satisfaction was not measured in this project, the literature review revealed that satisfaction was increased through use of various virtual health care modalities, including a preliminary analysis in this facility by the aforementioned 2009 study by K. L. Rodriguez (unpublished data). Based on findings in this project, the following 4 recommendations were made to improve benchmark outcomes and quality of care.
- Provide dedicated time to complete e-consults and related tasks. In this setting, a full day was recommended. An alternative was to hire a NP whose sole responsibility was e-consults.
- Develop a selection process to determine which new referrals are best suited for e-consult. This process will increase e-consult efficiency and decrease the time to complete an e-consult. Recommended selection criteria were (A) gastroenterology referrals only for simple symptoms or issues; (B) referrals only for a procedure; and (C) stable patients with uncomplicated medical histories.
- Sustain the preprocedure reminder phone call. The reminder call helps patients keep appointments and thus reduces a missed opportunity for care.
- Plan a future QI project or research study on patient colon prep quality for colonoscopy. Such a project might evaluate types of colon prep, how prep quality is measured, patient instructions, and the timing/content of pre-procedure reminder phone calls, particularly for e-consult patients.
Conclusion
This QI project provided outcomes for e-consults in the subspecialty of gastroenterology at the VAPHS. Although some benchmark outcomes were met and favorable, others were less favorable. By conducting this benchmark analysis, the areas of needed improvement are now clear. This analysis provides information so recommendations for process improvements can be made.
Quality of care improvement is an ongoing process at VAPHS. Since completion of this project, several processes have been adjusted so that outcomes will be improved. For example, corrective actions were taken for patients who did not complete their gastroenterology procedure. The process for scheduling gastroenterology procedures was adjusted for appointments and cancellations. Ongoing efforts to sustain the reminder phone call were put in place. Changes in NP staffing and time assigned for both clinical and nonclinical work were proposed and are currently under review.
It is the mission of the VA to provide access to care, patient satisfaction, timely care, and appropriate use of resources. Having the ability to highlight our strengths, as well as the willingness to recognize weaknesses, allows us to create new improved processes to provide the best care possible for our veterans.
Acknowledgments
This project was Elena Swann’s capstone for the Doctor of Nursing Practice Program, University of Pittsburgh School of Nursing in Pennsylvania. Rich Laufer, Larry Priscella, and Janie Fleming assisted with the VA Gastroenterology Clinic and procedure metrics. Dr. Melissa Taylor, VA associate chief nurse for research provided project guidance and manuscript revisions. Dr. Sandra Engberg, University of Pittsburgh School of Nursing faculty, assisted with project and manuscript development.
Author disclosures
The author reports no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the author and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Bashshur RL, Shannon GW, Krupinski EA, et al. National telemedicine initiatives: Essential to healthcare reform. Telemed J E Health. 2009;15(6):600-610.
2. Zanaboni P, Scalvini S, Bernocchi P, Borghi G, Tridico C, Masella C. Teleconsultation service to improve healthcare in rural areas: Acceptance, organizational impact and appropriateness. BMC Health Serv Res. 2009;9:238.
3. Wallace P, Barber J, Clayton W, et al. Virtual outreach: A randomised controlled trial and economic evaluation of joint teleconferenced medical consultations. Health Technol Assess. 2004;8(50):1-106, iii-iv.
4. Hersh WR, Hickam DH, Severance SM, Dana TL, Pyle Krages K, Helfand M. Diagnosis, access and outcomes: Update of a systematic review of telemedicine services. J Telemed Telecare. 2006;12(suppl 2):S3-S31.
5. Horner K, Wagner E, Tufano J. Electronic consultations between primary and specialty care clinicians: Early insights. Issue Brief (Commonw Fund). 2011;23:1-14.
6. Angstman KB, Adamson SC, Furst JW, Houston MS, Rohrer JE. Provider satisfaction with virtual specialist consultations in a family medicine department. Health Care Manag (Frederick). 2009;28(1):14-18.
7. Krier M, Kaltenbach T, McQuaid K, Soetikno R. Potential use of telemedicine to provide outpatient care for inflammatory bowel disease. Am J Gastroenterol. 2011;106(12):2063-2067.
8. Calderwood AH, Lai EJ, Fix OK, Jacobson BC. An endoscopist-blinded, randomized, controlled trial of a simple visual aid to improve bowel preparation for screening colonoscopy. Gastrointest Endosc. 2011;73(2):307-314.
9. Hillyer GC, Basch CH, Basch CE, et al. Gastroenterologists’ perceived barriers to optimal pre-colonoscopy bowel preparation: Results of a national survey. J Cancer Educ. 2012;27(3):526-532.
10. Smith SG, von Wagner C, McGregor LM, et al. The influence of health literacy on comprehension of a colonoscopy preparation information leaflet. Dis Colon Rectum. 2012;55(10):1074-1080.
11. Mittal S, Lin YL, Tan A, Kuo YF, El-Serag HB, Goodwin JS. Limited life expectancy among a subgroup of Medicare beneficiaries receiving screening colonoscopies. Clin Gastroenterol Hepatol. 2014;12(3):443-450.e1.
12. Ko CW, Sonnenberg A. Comparing risks and benefits of colorectal cancer screening in elderly patients. Gastroenterology. 2005;129(4):1163-1170.
13. Ell C, Fischbach W, Keller R, et al; Hintertux Study Group. A randomized, blinded, prospective trial to compare the safety and efficacy of three bowel-cleansing solutions for colonoscopy (HSG-01*). Endoscopy. 2003;35(4):300-304.
14. Jansen SV, Goedhard JG, Winkens B, van Deursen CT. Preparation before colonoscopy: A randomized controlled trial comparing different regimes. Eur J Gastroenterol Hepatol. 2011;23(10):897-902.
1. Bashshur RL, Shannon GW, Krupinski EA, et al. National telemedicine initiatives: Essential to healthcare reform. Telemed J E Health. 2009;15(6):600-610.
2. Zanaboni P, Scalvini S, Bernocchi P, Borghi G, Tridico C, Masella C. Teleconsultation service to improve healthcare in rural areas: Acceptance, organizational impact and appropriateness. BMC Health Serv Res. 2009;9:238.
3. Wallace P, Barber J, Clayton W, et al. Virtual outreach: A randomised controlled trial and economic evaluation of joint teleconferenced medical consultations. Health Technol Assess. 2004;8(50):1-106, iii-iv.
4. Hersh WR, Hickam DH, Severance SM, Dana TL, Pyle Krages K, Helfand M. Diagnosis, access and outcomes: Update of a systematic review of telemedicine services. J Telemed Telecare. 2006;12(suppl 2):S3-S31.
5. Horner K, Wagner E, Tufano J. Electronic consultations between primary and specialty care clinicians: Early insights. Issue Brief (Commonw Fund). 2011;23:1-14.
6. Angstman KB, Adamson SC, Furst JW, Houston MS, Rohrer JE. Provider satisfaction with virtual specialist consultations in a family medicine department. Health Care Manag (Frederick). 2009;28(1):14-18.
7. Krier M, Kaltenbach T, McQuaid K, Soetikno R. Potential use of telemedicine to provide outpatient care for inflammatory bowel disease. Am J Gastroenterol. 2011;106(12):2063-2067.
8. Calderwood AH, Lai EJ, Fix OK, Jacobson BC. An endoscopist-blinded, randomized, controlled trial of a simple visual aid to improve bowel preparation for screening colonoscopy. Gastrointest Endosc. 2011;73(2):307-314.
9. Hillyer GC, Basch CH, Basch CE, et al. Gastroenterologists’ perceived barriers to optimal pre-colonoscopy bowel preparation: Results of a national survey. J Cancer Educ. 2012;27(3):526-532.
10. Smith SG, von Wagner C, McGregor LM, et al. The influence of health literacy on comprehension of a colonoscopy preparation information leaflet. Dis Colon Rectum. 2012;55(10):1074-1080.
11. Mittal S, Lin YL, Tan A, Kuo YF, El-Serag HB, Goodwin JS. Limited life expectancy among a subgroup of Medicare beneficiaries receiving screening colonoscopies. Clin Gastroenterol Hepatol. 2014;12(3):443-450.e1.
12. Ko CW, Sonnenberg A. Comparing risks and benefits of colorectal cancer screening in elderly patients. Gastroenterology. 2005;129(4):1163-1170.
13. Ell C, Fischbach W, Keller R, et al; Hintertux Study Group. A randomized, blinded, prospective trial to compare the safety and efficacy of three bowel-cleansing solutions for colonoscopy (HSG-01*). Endoscopy. 2003;35(4):300-304.
14. Jansen SV, Goedhard JG, Winkens B, van Deursen CT. Preparation before colonoscopy: A randomized controlled trial comparing different regimes. Eur J Gastroenterol Hepatol. 2011;23(10):897-902.
Utilization of the ICF-CY for the Classification of Therapeutic Objectives in the Treatment of Spasticity in Children with Cerebral Palsy
From the IRCCS Institute of Neurological Sciences, Bellaria Hospital, Bologna, Italy.
Abstract
- Objective: To identify objectives for treatment of spasticity with botulinum toxin type A (BTX) in children with cerebral palsy (CP), standardize the objectives according to typology, and classify them according to the International Classification of Functioning for Children and Youth (ICF-CY), as well as to analyze treatment goals in relationship to CP clinical type, severity level, and age.
- Methods: 188 children were included in the study (mean age, 12 years; 42% female, 58% male). The diplegic type made up 38% of CP cases, the tetraplegic type 35%, and the hemiplegic type 24%. Children were mainly classified in the lowest and highest levels in the Gross Motor Function Classification System (GMFCS 1, 39%; GMFCS 5, 26%). Treatment objectives for individual therapies were discussed, identified, and transcribed in the therapeutic proposals. The objectives were then collected and subjected to an internal audit in order to standardize their denomination. Two trained health care providers expert in the use of the ICF-CY classification mapped the objectives to ICF-CY domains and categories. The objectives were then analyzed in relationship to CP clinical type, GMFCS level, and age.
- Results: Of the objectives, 88% (246) were in the “Body Functions” domain. In this domain, there were 28 typologies of objectives in 6 categories. Only 12% (32) of the objectives were in the “Activity” domain; there were 11 typologies in 5 categories. In diplegic and hemiplegic patients with mild disability (GMFCS 1), objectives were aimed at improving gait pattern. For quadriplegic patients with severe disability (GMFCS 5), objectives were aimed mainly at controlling deformities and improving health care provision. Objectives concerning pain treatment were proposed principally for patients with diplegic and quadriplegic type CP.
- Conclusions: The ICF-CY can be used to categorize treatment objectives proposed for patient improvement in the domains of Body Functions and Activity. Goal setting for BTX injections occurs mainly in the Body Functions domain and aims at finding changes in the gait pattern.
Botulinum toxin type A (BTX) has been used for 20 years for the focal treatment of spasticity in patients with cerebral palsy (CP) [1–3]. While numerous studies have shown the functional benefits of BTX treatment, especially if carried out in combination with other treatments (eg, physiotherapy, occupational therapy, serial casting), studies that focus on the indications for BTX use are limited.
Patients with CP require rehabilitation that involves multiple disciplines and multiprofessional therapeutic programs (eg, pharmacologic, orthotic, physiotherapeutic). The complexity of both the program and the pathology requires choosing the appropriate treatment objectives. The International Classification of Functioning for Children and Youth (ICF-CY) [4] is a unified and standard language and framework for clinical, public health, and research applications to facilitate the documentation and measurement of health and disability in child and youth populations. As such, it can be used to inform clinical thinking, practice and research in the field of cerebral palsy [5], including being used as a tool for developing treatment plans and providing a common language for defining and sharing treatment objectives with patients and families [6]. Thamar et al [7] recently pointed out the value of adopting a standardized method of writing specific and measurable goals. Goals that are specific and clear are important not only for the evaluation of efficacy but also for systematic evaluation of the quality of health services [8,9].
In the literature regarding rehabilitation (especially in adults) and, more recently, in the literature on CP [10], core sets derived from ICF that are condition- and setting-specific are increasingly being used. They are used for the evaluation of the functional profiles of patients and documentation of the results of rehabilitative treatment, and also for defining the objectives of the treatment. Some authors [11–14] have explored in detail the possibility of using the core sets for formulating treatment objectives and assessing outcomes. However, using the core sets is complicated and their use in day-to-day clinical settings is limited. In a recent study, Preston et al [15] sought to define a sub-set of functional goals and outcomes relevant to patients with CP undergoing BTX treatment that could be more appropriate for use. In this retrospective analysis, they used the ICF-CY to classify treatment goals into corresponding domains and categories. The ICF-CY contains 4 major components (Body Structure, Body Function, Activities and Participation, and Environmental Factors), which each contain hierarchically arranged chapters and category levels. The authors found that the goals were mainly in the domain of “Body Functions,” specifically “functions of joint mobility” and “functions of gait pattern.” Those in the “Activity” domain were in the “walking” and “changing body positions” categories. This study was the first to focus on CP as a pathology and on the objectives of the individual therapeutic programs; other reports in the literature deal with the entire articulation of treatment. The authors limited themselves to the identification of the domain and the category of the objectives but did not report in detail their denomination. A greater degree of specificity and standardization in the description of the objectives would be useful from a practical point of view both for comparing results and for improving communication between the health care providers, and between these professionals and the families. The authors also did not assess for the various clinical types of CP.
The aim of the present study involving patients having CP and undergoing BTX injections was to identify the treatment objectives, standardize them according to denomination, classify them according to ICF-CY domains and categories, and establish their relative frequency. A further objective of the study was to analyze treatment goals in relationship to the clinical type (eg, hemiplegia, diplegia, quadriplegia), level of severity according to the Gross Motor Function Classification System (GMFCS) [16], and age.
Methods
Our center in Bologna, Italy, specializes in the evaluation and advanced treatment of spasticity in neuromotor disability in children and young adults. Between 2010 and the first half of 2012, 217 children were admitted to our center for evaluation and BTX treatment of spasticity in the upper or lower limbs or both. Of these, 188 children who had been diagnosed with spastic CP were included in the prospective study. Twenty-nine patients with other pathologies (epileptic and degenerative encephalopathy, spastic paraparesis) were excluded. The enrolled patients and their families were informed about the study and written informed consent was obtained.
Patients were evaluated from a functional point of view by 3 expert physiatrists and 2 pediatric physiotherapists for eligibility for BTX injection according to the recommendations of Ferrari and Cioni [17]. Functional assessment included evaluation of impairments (spasticity, contractures, deformities), main motor functions (gait pattern, manipulation pattern), and capacity of carrying out the principal motor activities (walking, maintaining and changing body position, rolling, use of upper limbs), thus enabling the identification of specific and realistic objectives for treatment with BTX. The objectives were chosen by a physiatrist and a physiotherapist, shared among the health care providers and the patients and their families, and added to the written treatment proposals. For each child more than 1 treatment objective could be proposed. These proposals were then collected and audited so as to obtain a uniform denomination of the proposed therapeutic objectives. In a series of meetings among all the members of the research group, the descriptions/denominations of the therapeutic goals were standardized and shared, eliminating inexact descriptions or adding new ones as needed. Two trained health care providers expert in the use of the ICF-CY classification mapped these to the ICF-CY domains and categories (up to the 2nd level of categorization). Each interpretative disagreement was resolved by group discussion. Finally, the objectives were analyzed in relationship to clinical type, severity according to GMFCS, and age. The frequency of the individual objectives, domains, and categories was evaluated by means of descriptive statistics.
Results
Body Functions Domain
The most represented category in the “Body Functions” domain was “b770 functions of gait pattern” (50%). There were 123 proposed objectives distributed among 11 typologies of objectives for a total of 123 proposed objectives in the functions of gait pattern category.
In the “b715 functions of joint stability” category, 25 objectives were proposed for controlling hip lateralization while, in the “b720 functions of bone mobility” category, 4 typologies of objectives were identified out of a total of 15 proposed objectives aimed at improving the position of the pelvis. The “b280 pain sensation” category was also used to indicate 15 objectives aiming at alleviating knee, hip and spinal column pain. Finally, 4 objectives were aimed at tone reduction.
Activity Domain
As concerns the “Activity” domain, 38% of objectives were classified into the “d415 maintain body position” category (3 typologies and a total of 12 proposals), 25% were in the “d540 dress oneself ” category (2 typologies and a total of 8 proposals), 19% were in the “d440 fine use of the hands” category (3 typologies and a total of 6 proposals), 13% were in the “d445 use of hands and arms” category (2 typologies and 4 proposals) and, 6% of cases were classified into the “d510 wash oneself” category (2 proposals) (Table 2).
Analysis by Type, Severity, and Age
Discussion
The results show that in the majority of cases, the objectives of treatment with BTX injections proposed by our group fell within the “Body Functions” domain, in the “b770 gait pattern” and “b710 joint mobility” categories. This focus has also been reported by other authors [18]. Furthermore, these results are analogous to those reported by Preston [15]. The objectives classifiable into the “Activity” domain were more limited in our group. The most represented categories were “d415 maintain body position,” as also reported by Preston, and “d540 dressing oneself.” Preston et al reported many more objectives in the Activity domain, also utilizing the “walking” category. A possible reason is that objectives may reflect more the expectations of professionals and less those of patients. Indeed, when objectives suggested by patients and families are taken into greater consideration, goals proposed in the Activity area notably increase [19]. It is probably necessary to evaluate the objectives relevant to the professionals and those significant to the families and children separately.
The discrepancies between our data and Preston’s also most likely reflect differences in the study population. In our study, those undergoing injections aimed at improving gait pattern are, for the most part, hemiplegic and diplegic patients with mild disabilities (GMFCS 1). Their elevated degree of autonomy in mobility probably accounts for the scarcity of objectives for improving walking autonomy. In the most severe cases, such as quadriplegia, objectives are mainly aimed at controlling deformities and facilitating health care provision. Pain reduction is another important aspect and concerned quadriplegic and diplegic patients with severe disability. In contrast, objectives related to muscle tone reduction were limited, as the main objective was not a reduction but the control of muscle shortening and the subsequent deformities. However, this can become a primary objective in cases of spastic hyperactivation (eg, in adductor muscles) or in the case of dystonia, to improve patient comfort.
From a practical point of view, the use of this methodology provides for a common language that facilitates the communication and sharing of therapeutic objectives between different professionals (physiatrists and physiotherapists) and between health care providers and families and/or patients. This is important, as physiotherapy is often complementary to BTX injections and the objectives must be shared with the family. This methodology can help the clinician in the decision-making process and allows determining with greater specificity what is to be measured to document the achievement of the objectives.
Future research in this field will be aimed at evaluating patient outcomes by means of the adoption of suitable instruments (measurement scales) in order to quantify results which are consistent, according to the ICF-CY classification, with the domain and the category undergoing analysis.
Conclusion
As it has already been pointed out by various authors [10–15], the ICF-CY is a useful instrument for the classification of proposed therapeutic objectives into domains and categories, in order to standardize the language and to increase the sharing of the aims between the health care providers and between providers and families/patients. The most commonly followed approach calls for the use of functional profiles at the beginning of the care planning process, in order to establish the priorities and objectives of the interventions to be carried out. In order to streamline and facilitate procedures in clinical practice, many have proposed the use of core sets, but the validation procedure is complex and not always possible in all centers. Recently, Preston et al were the first to propose using the ICF-CY for classifying the objectives of an individual program. The procedure utilized is simple, easily reproducible, and allows identifying and classifying the objectives into categories using the ICF-CY. Furthermore, it is focused on an individual program and not on the entire articulation of programs, making interpretation of the data more linear. Our proposal is similar because it is focused on the analysis of an individual therapeutic program and because it utilizes the ICF classification system to classify the objectives; however, it achieves a higher degree of detail and standardization of the objectives.
In conclusion, the classification structure of the ICF-CY furnishes a useful and recognized instrument for categorizing the objectives of the interventions to be carried out. The classification of the objectives is specific for each pathology and for each individual program. The standardization of the objectives themselves and the use of the ICF-CY categories only for classification represents a possible methodologic alternative to the use of ICF-CY individual categories and sub-categories for identifying these objectives (core sets), as proposed by other authors. This procedure offers greater detail and a greater degree of standardization, which is important for the successive and systematic evaluation of treatment results.
Corresponding author: Nicoletta Battisti, Via Altura 3, 40139 Bologna, Italy, nbattisti@ausilioteca.org.
1. Lukban M, Rosales RL. Effectiveness of botulinum toxin A for upper and lower limb spasticity in children with cerebral palsy: a summary of evidence. J Neural Transm 2009;116:319–31.
2. Ryll U, Bastianen C, De Bie R, Staal B. Effects of leg muscle botulinum toxin A injections on walking in children with spasticity related cerebral palsy: a systematic review. Devel Med Child Neurol 2011;53:210–6.
3. Hoare BJ, Wallen MA,Villanueva E, et al. Botulinum toxin A as an adjunct to treatment in the management of upper limb in children with spastic cerebral palsy. The Cochraine Library 2010.
4. World Health Organization. International Classification of Functioning, Disability, and Health: Children and Youth Version for Children and Youth (ICF-CY). 2007. Available at http://apps.who.int/bookorders/anglais/detart1.jsp?codlan=1&codcol=15&codcch=716#
5. Rosenbaum P, Stewart D. The World Health Organization International Classification of Functioning Disability and Health: a model to guide clinical thinking, practice, and research. Semin Pediatr Neurol 2004;11:5–10.
6. Steiner W, Ryser L, Huber E, et al. Use of the ICF model as a clinical problem solving tool in physical therapy and rehabilitation medicine. Phys Ther 2002;82:1098–107.
7. Thamar JH, Bovend’Eerdt, Botell RE, Wade DT. Writing SMART rehabilitation goals and achieving goal attainment scaling: practical guide. Clin Rehab 2009;23:352–61.
8. Program outcome evaluations. United Way of Winnipeg; 2007.
9. Main K. Program design: a practical guide. Available at www.calgaryunitedway.org.
10. Schiariti V, Selb M, Cieza A, O’Donnel M. International classification of Functioning, Disability and Health Core sets for children and youth with cerebral palsy: a consensus meeting 1. Dev Med Child Neurol 2014 Aug 6. Epub ahead of print
11. Huber EO, Tobler A, Gloor-Juzzi T, et al. The ICF as a way to specify goals and assess the outcome of physiotherapeutic interventions in the acute hospitals Rehabil Med 2011;43:174–7.
12. Mittrach R, Grill E, Walchner-Bonjean M, et al. Goals of physiotherapy interventions can be described using the International Classification Of Functioning, Disability and Health Physiotherapy 2008;94:150–7.
13. Muller MJ, Strobl R, Grill E. Goals of patients with rehabilitation needs in acute hospitals: goal achievement is an indicator for improved functioning Rehabil Med 2011;43:145–50.
14. Grill E J, Stucki G. Criteria for validating comprehensive ICF core sets and developing brief ICF core set versions. J Rehabil Med 2011;43:87–91.
15. Preston NJ, Clarke M, Bhakta B. Development of a framework to define the functional goals and outcomes of botulinum toxin A spasticity treatment relevant to the child and family living with cerebral palsy using the international classification of functioning disability and health for children and youth (ICF-CY). J Rehabil Med 2011;43:1010–5.
16. Palisano R, Rosenbaum P, Walter S, et al. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol 1997;39:214–23.
17. Ferrari A, Cioni G. The spastic forms of cerebral palsy: a guide to the assessment of adaptive functions. Springer-Verlag; 2010.
18. Franki I, De Cat J, Deschepper E, et al. A clinical decision framework for the identification of main problems and treatment goals for ambulant children with bilateral spastic cerebral palsy. Res Dev Disabil 2014;35:1160–76.
19. Lohmann S, Decker J, Müller M, et al. The ICF forms a useful framework for classifying individual patients goals in post-acute rehabilitation. Rehabil Med 2011;43:151–5.
From the IRCCS Institute of Neurological Sciences, Bellaria Hospital, Bologna, Italy.
Abstract
- Objective: To identify objectives for treatment of spasticity with botulinum toxin type A (BTX) in children with cerebral palsy (CP), standardize the objectives according to typology, and classify them according to the International Classification of Functioning for Children and Youth (ICF-CY), as well as to analyze treatment goals in relationship to CP clinical type, severity level, and age.
- Methods: 188 children were included in the study (mean age, 12 years; 42% female, 58% male). The diplegic type made up 38% of CP cases, the tetraplegic type 35%, and the hemiplegic type 24%. Children were mainly classified in the lowest and highest levels in the Gross Motor Function Classification System (GMFCS 1, 39%; GMFCS 5, 26%). Treatment objectives for individual therapies were discussed, identified, and transcribed in the therapeutic proposals. The objectives were then collected and subjected to an internal audit in order to standardize their denomination. Two trained health care providers expert in the use of the ICF-CY classification mapped the objectives to ICF-CY domains and categories. The objectives were then analyzed in relationship to CP clinical type, GMFCS level, and age.
- Results: Of the objectives, 88% (246) were in the “Body Functions” domain. In this domain, there were 28 typologies of objectives in 6 categories. Only 12% (32) of the objectives were in the “Activity” domain; there were 11 typologies in 5 categories. In diplegic and hemiplegic patients with mild disability (GMFCS 1), objectives were aimed at improving gait pattern. For quadriplegic patients with severe disability (GMFCS 5), objectives were aimed mainly at controlling deformities and improving health care provision. Objectives concerning pain treatment were proposed principally for patients with diplegic and quadriplegic type CP.
- Conclusions: The ICF-CY can be used to categorize treatment objectives proposed for patient improvement in the domains of Body Functions and Activity. Goal setting for BTX injections occurs mainly in the Body Functions domain and aims at finding changes in the gait pattern.
Botulinum toxin type A (BTX) has been used for 20 years for the focal treatment of spasticity in patients with cerebral palsy (CP) [1–3]. While numerous studies have shown the functional benefits of BTX treatment, especially if carried out in combination with other treatments (eg, physiotherapy, occupational therapy, serial casting), studies that focus on the indications for BTX use are limited.
Patients with CP require rehabilitation that involves multiple disciplines and multiprofessional therapeutic programs (eg, pharmacologic, orthotic, physiotherapeutic). The complexity of both the program and the pathology requires choosing the appropriate treatment objectives. The International Classification of Functioning for Children and Youth (ICF-CY) [4] is a unified and standard language and framework for clinical, public health, and research applications to facilitate the documentation and measurement of health and disability in child and youth populations. As such, it can be used to inform clinical thinking, practice and research in the field of cerebral palsy [5], including being used as a tool for developing treatment plans and providing a common language for defining and sharing treatment objectives with patients and families [6]. Thamar et al [7] recently pointed out the value of adopting a standardized method of writing specific and measurable goals. Goals that are specific and clear are important not only for the evaluation of efficacy but also for systematic evaluation of the quality of health services [8,9].
In the literature regarding rehabilitation (especially in adults) and, more recently, in the literature on CP [10], core sets derived from ICF that are condition- and setting-specific are increasingly being used. They are used for the evaluation of the functional profiles of patients and documentation of the results of rehabilitative treatment, and also for defining the objectives of the treatment. Some authors [11–14] have explored in detail the possibility of using the core sets for formulating treatment objectives and assessing outcomes. However, using the core sets is complicated and their use in day-to-day clinical settings is limited. In a recent study, Preston et al [15] sought to define a sub-set of functional goals and outcomes relevant to patients with CP undergoing BTX treatment that could be more appropriate for use. In this retrospective analysis, they used the ICF-CY to classify treatment goals into corresponding domains and categories. The ICF-CY contains 4 major components (Body Structure, Body Function, Activities and Participation, and Environmental Factors), which each contain hierarchically arranged chapters and category levels. The authors found that the goals were mainly in the domain of “Body Functions,” specifically “functions of joint mobility” and “functions of gait pattern.” Those in the “Activity” domain were in the “walking” and “changing body positions” categories. This study was the first to focus on CP as a pathology and on the objectives of the individual therapeutic programs; other reports in the literature deal with the entire articulation of treatment. The authors limited themselves to the identification of the domain and the category of the objectives but did not report in detail their denomination. A greater degree of specificity and standardization in the description of the objectives would be useful from a practical point of view both for comparing results and for improving communication between the health care providers, and between these professionals and the families. The authors also did not assess for the various clinical types of CP.
The aim of the present study involving patients having CP and undergoing BTX injections was to identify the treatment objectives, standardize them according to denomination, classify them according to ICF-CY domains and categories, and establish their relative frequency. A further objective of the study was to analyze treatment goals in relationship to the clinical type (eg, hemiplegia, diplegia, quadriplegia), level of severity according to the Gross Motor Function Classification System (GMFCS) [16], and age.
Methods
Our center in Bologna, Italy, specializes in the evaluation and advanced treatment of spasticity in neuromotor disability in children and young adults. Between 2010 and the first half of 2012, 217 children were admitted to our center for evaluation and BTX treatment of spasticity in the upper or lower limbs or both. Of these, 188 children who had been diagnosed with spastic CP were included in the prospective study. Twenty-nine patients with other pathologies (epileptic and degenerative encephalopathy, spastic paraparesis) were excluded. The enrolled patients and their families were informed about the study and written informed consent was obtained.
Patients were evaluated from a functional point of view by 3 expert physiatrists and 2 pediatric physiotherapists for eligibility for BTX injection according to the recommendations of Ferrari and Cioni [17]. Functional assessment included evaluation of impairments (spasticity, contractures, deformities), main motor functions (gait pattern, manipulation pattern), and capacity of carrying out the principal motor activities (walking, maintaining and changing body position, rolling, use of upper limbs), thus enabling the identification of specific and realistic objectives for treatment with BTX. The objectives were chosen by a physiatrist and a physiotherapist, shared among the health care providers and the patients and their families, and added to the written treatment proposals. For each child more than 1 treatment objective could be proposed. These proposals were then collected and audited so as to obtain a uniform denomination of the proposed therapeutic objectives. In a series of meetings among all the members of the research group, the descriptions/denominations of the therapeutic goals were standardized and shared, eliminating inexact descriptions or adding new ones as needed. Two trained health care providers expert in the use of the ICF-CY classification mapped these to the ICF-CY domains and categories (up to the 2nd level of categorization). Each interpretative disagreement was resolved by group discussion. Finally, the objectives were analyzed in relationship to clinical type, severity according to GMFCS, and age. The frequency of the individual objectives, domains, and categories was evaluated by means of descriptive statistics.
Results
Body Functions Domain
The most represented category in the “Body Functions” domain was “b770 functions of gait pattern” (50%). There were 123 proposed objectives distributed among 11 typologies of objectives for a total of 123 proposed objectives in the functions of gait pattern category.
In the “b715 functions of joint stability” category, 25 objectives were proposed for controlling hip lateralization while, in the “b720 functions of bone mobility” category, 4 typologies of objectives were identified out of a total of 15 proposed objectives aimed at improving the position of the pelvis. The “b280 pain sensation” category was also used to indicate 15 objectives aiming at alleviating knee, hip and spinal column pain. Finally, 4 objectives were aimed at tone reduction.
Activity Domain
As concerns the “Activity” domain, 38% of objectives were classified into the “d415 maintain body position” category (3 typologies and a total of 12 proposals), 25% were in the “d540 dress oneself ” category (2 typologies and a total of 8 proposals), 19% were in the “d440 fine use of the hands” category (3 typologies and a total of 6 proposals), 13% were in the “d445 use of hands and arms” category (2 typologies and 4 proposals) and, 6% of cases were classified into the “d510 wash oneself” category (2 proposals) (Table 2).
Analysis by Type, Severity, and Age
Discussion
The results show that in the majority of cases, the objectives of treatment with BTX injections proposed by our group fell within the “Body Functions” domain, in the “b770 gait pattern” and “b710 joint mobility” categories. This focus has also been reported by other authors [18]. Furthermore, these results are analogous to those reported by Preston [15]. The objectives classifiable into the “Activity” domain were more limited in our group. The most represented categories were “d415 maintain body position,” as also reported by Preston, and “d540 dressing oneself.” Preston et al reported many more objectives in the Activity domain, also utilizing the “walking” category. A possible reason is that objectives may reflect more the expectations of professionals and less those of patients. Indeed, when objectives suggested by patients and families are taken into greater consideration, goals proposed in the Activity area notably increase [19]. It is probably necessary to evaluate the objectives relevant to the professionals and those significant to the families and children separately.
The discrepancies between our data and Preston’s also most likely reflect differences in the study population. In our study, those undergoing injections aimed at improving gait pattern are, for the most part, hemiplegic and diplegic patients with mild disabilities (GMFCS 1). Their elevated degree of autonomy in mobility probably accounts for the scarcity of objectives for improving walking autonomy. In the most severe cases, such as quadriplegia, objectives are mainly aimed at controlling deformities and facilitating health care provision. Pain reduction is another important aspect and concerned quadriplegic and diplegic patients with severe disability. In contrast, objectives related to muscle tone reduction were limited, as the main objective was not a reduction but the control of muscle shortening and the subsequent deformities. However, this can become a primary objective in cases of spastic hyperactivation (eg, in adductor muscles) or in the case of dystonia, to improve patient comfort.
From a practical point of view, the use of this methodology provides for a common language that facilitates the communication and sharing of therapeutic objectives between different professionals (physiatrists and physiotherapists) and between health care providers and families and/or patients. This is important, as physiotherapy is often complementary to BTX injections and the objectives must be shared with the family. This methodology can help the clinician in the decision-making process and allows determining with greater specificity what is to be measured to document the achievement of the objectives.
Future research in this field will be aimed at evaluating patient outcomes by means of the adoption of suitable instruments (measurement scales) in order to quantify results which are consistent, according to the ICF-CY classification, with the domain and the category undergoing analysis.
Conclusion
As it has already been pointed out by various authors [10–15], the ICF-CY is a useful instrument for the classification of proposed therapeutic objectives into domains and categories, in order to standardize the language and to increase the sharing of the aims between the health care providers and between providers and families/patients. The most commonly followed approach calls for the use of functional profiles at the beginning of the care planning process, in order to establish the priorities and objectives of the interventions to be carried out. In order to streamline and facilitate procedures in clinical practice, many have proposed the use of core sets, but the validation procedure is complex and not always possible in all centers. Recently, Preston et al were the first to propose using the ICF-CY for classifying the objectives of an individual program. The procedure utilized is simple, easily reproducible, and allows identifying and classifying the objectives into categories using the ICF-CY. Furthermore, it is focused on an individual program and not on the entire articulation of programs, making interpretation of the data more linear. Our proposal is similar because it is focused on the analysis of an individual therapeutic program and because it utilizes the ICF classification system to classify the objectives; however, it achieves a higher degree of detail and standardization of the objectives.
In conclusion, the classification structure of the ICF-CY furnishes a useful and recognized instrument for categorizing the objectives of the interventions to be carried out. The classification of the objectives is specific for each pathology and for each individual program. The standardization of the objectives themselves and the use of the ICF-CY categories only for classification represents a possible methodologic alternative to the use of ICF-CY individual categories and sub-categories for identifying these objectives (core sets), as proposed by other authors. This procedure offers greater detail and a greater degree of standardization, which is important for the successive and systematic evaluation of treatment results.
Corresponding author: Nicoletta Battisti, Via Altura 3, 40139 Bologna, Italy, nbattisti@ausilioteca.org.
From the IRCCS Institute of Neurological Sciences, Bellaria Hospital, Bologna, Italy.
Abstract
- Objective: To identify objectives for treatment of spasticity with botulinum toxin type A (BTX) in children with cerebral palsy (CP), standardize the objectives according to typology, and classify them according to the International Classification of Functioning for Children and Youth (ICF-CY), as well as to analyze treatment goals in relationship to CP clinical type, severity level, and age.
- Methods: 188 children were included in the study (mean age, 12 years; 42% female, 58% male). The diplegic type made up 38% of CP cases, the tetraplegic type 35%, and the hemiplegic type 24%. Children were mainly classified in the lowest and highest levels in the Gross Motor Function Classification System (GMFCS 1, 39%; GMFCS 5, 26%). Treatment objectives for individual therapies were discussed, identified, and transcribed in the therapeutic proposals. The objectives were then collected and subjected to an internal audit in order to standardize their denomination. Two trained health care providers expert in the use of the ICF-CY classification mapped the objectives to ICF-CY domains and categories. The objectives were then analyzed in relationship to CP clinical type, GMFCS level, and age.
- Results: Of the objectives, 88% (246) were in the “Body Functions” domain. In this domain, there were 28 typologies of objectives in 6 categories. Only 12% (32) of the objectives were in the “Activity” domain; there were 11 typologies in 5 categories. In diplegic and hemiplegic patients with mild disability (GMFCS 1), objectives were aimed at improving gait pattern. For quadriplegic patients with severe disability (GMFCS 5), objectives were aimed mainly at controlling deformities and improving health care provision. Objectives concerning pain treatment were proposed principally for patients with diplegic and quadriplegic type CP.
- Conclusions: The ICF-CY can be used to categorize treatment objectives proposed for patient improvement in the domains of Body Functions and Activity. Goal setting for BTX injections occurs mainly in the Body Functions domain and aims at finding changes in the gait pattern.
Botulinum toxin type A (BTX) has been used for 20 years for the focal treatment of spasticity in patients with cerebral palsy (CP) [1–3]. While numerous studies have shown the functional benefits of BTX treatment, especially if carried out in combination with other treatments (eg, physiotherapy, occupational therapy, serial casting), studies that focus on the indications for BTX use are limited.
Patients with CP require rehabilitation that involves multiple disciplines and multiprofessional therapeutic programs (eg, pharmacologic, orthotic, physiotherapeutic). The complexity of both the program and the pathology requires choosing the appropriate treatment objectives. The International Classification of Functioning for Children and Youth (ICF-CY) [4] is a unified and standard language and framework for clinical, public health, and research applications to facilitate the documentation and measurement of health and disability in child and youth populations. As such, it can be used to inform clinical thinking, practice and research in the field of cerebral palsy [5], including being used as a tool for developing treatment plans and providing a common language for defining and sharing treatment objectives with patients and families [6]. Thamar et al [7] recently pointed out the value of adopting a standardized method of writing specific and measurable goals. Goals that are specific and clear are important not only for the evaluation of efficacy but also for systematic evaluation of the quality of health services [8,9].
In the literature regarding rehabilitation (especially in adults) and, more recently, in the literature on CP [10], core sets derived from ICF that are condition- and setting-specific are increasingly being used. They are used for the evaluation of the functional profiles of patients and documentation of the results of rehabilitative treatment, and also for defining the objectives of the treatment. Some authors [11–14] have explored in detail the possibility of using the core sets for formulating treatment objectives and assessing outcomes. However, using the core sets is complicated and their use in day-to-day clinical settings is limited. In a recent study, Preston et al [15] sought to define a sub-set of functional goals and outcomes relevant to patients with CP undergoing BTX treatment that could be more appropriate for use. In this retrospective analysis, they used the ICF-CY to classify treatment goals into corresponding domains and categories. The ICF-CY contains 4 major components (Body Structure, Body Function, Activities and Participation, and Environmental Factors), which each contain hierarchically arranged chapters and category levels. The authors found that the goals were mainly in the domain of “Body Functions,” specifically “functions of joint mobility” and “functions of gait pattern.” Those in the “Activity” domain were in the “walking” and “changing body positions” categories. This study was the first to focus on CP as a pathology and on the objectives of the individual therapeutic programs; other reports in the literature deal with the entire articulation of treatment. The authors limited themselves to the identification of the domain and the category of the objectives but did not report in detail their denomination. A greater degree of specificity and standardization in the description of the objectives would be useful from a practical point of view both for comparing results and for improving communication between the health care providers, and between these professionals and the families. The authors also did not assess for the various clinical types of CP.
The aim of the present study involving patients having CP and undergoing BTX injections was to identify the treatment objectives, standardize them according to denomination, classify them according to ICF-CY domains and categories, and establish their relative frequency. A further objective of the study was to analyze treatment goals in relationship to the clinical type (eg, hemiplegia, diplegia, quadriplegia), level of severity according to the Gross Motor Function Classification System (GMFCS) [16], and age.
Methods
Our center in Bologna, Italy, specializes in the evaluation and advanced treatment of spasticity in neuromotor disability in children and young adults. Between 2010 and the first half of 2012, 217 children were admitted to our center for evaluation and BTX treatment of spasticity in the upper or lower limbs or both. Of these, 188 children who had been diagnosed with spastic CP were included in the prospective study. Twenty-nine patients with other pathologies (epileptic and degenerative encephalopathy, spastic paraparesis) were excluded. The enrolled patients and their families were informed about the study and written informed consent was obtained.
Patients were evaluated from a functional point of view by 3 expert physiatrists and 2 pediatric physiotherapists for eligibility for BTX injection according to the recommendations of Ferrari and Cioni [17]. Functional assessment included evaluation of impairments (spasticity, contractures, deformities), main motor functions (gait pattern, manipulation pattern), and capacity of carrying out the principal motor activities (walking, maintaining and changing body position, rolling, use of upper limbs), thus enabling the identification of specific and realistic objectives for treatment with BTX. The objectives were chosen by a physiatrist and a physiotherapist, shared among the health care providers and the patients and their families, and added to the written treatment proposals. For each child more than 1 treatment objective could be proposed. These proposals were then collected and audited so as to obtain a uniform denomination of the proposed therapeutic objectives. In a series of meetings among all the members of the research group, the descriptions/denominations of the therapeutic goals were standardized and shared, eliminating inexact descriptions or adding new ones as needed. Two trained health care providers expert in the use of the ICF-CY classification mapped these to the ICF-CY domains and categories (up to the 2nd level of categorization). Each interpretative disagreement was resolved by group discussion. Finally, the objectives were analyzed in relationship to clinical type, severity according to GMFCS, and age. The frequency of the individual objectives, domains, and categories was evaluated by means of descriptive statistics.
Results
Body Functions Domain
The most represented category in the “Body Functions” domain was “b770 functions of gait pattern” (50%). There were 123 proposed objectives distributed among 11 typologies of objectives for a total of 123 proposed objectives in the functions of gait pattern category.
In the “b715 functions of joint stability” category, 25 objectives were proposed for controlling hip lateralization while, in the “b720 functions of bone mobility” category, 4 typologies of objectives were identified out of a total of 15 proposed objectives aimed at improving the position of the pelvis. The “b280 pain sensation” category was also used to indicate 15 objectives aiming at alleviating knee, hip and spinal column pain. Finally, 4 objectives were aimed at tone reduction.
Activity Domain
As concerns the “Activity” domain, 38% of objectives were classified into the “d415 maintain body position” category (3 typologies and a total of 12 proposals), 25% were in the “d540 dress oneself ” category (2 typologies and a total of 8 proposals), 19% were in the “d440 fine use of the hands” category (3 typologies and a total of 6 proposals), 13% were in the “d445 use of hands and arms” category (2 typologies and 4 proposals) and, 6% of cases were classified into the “d510 wash oneself” category (2 proposals) (Table 2).
Analysis by Type, Severity, and Age
Discussion
The results show that in the majority of cases, the objectives of treatment with BTX injections proposed by our group fell within the “Body Functions” domain, in the “b770 gait pattern” and “b710 joint mobility” categories. This focus has also been reported by other authors [18]. Furthermore, these results are analogous to those reported by Preston [15]. The objectives classifiable into the “Activity” domain were more limited in our group. The most represented categories were “d415 maintain body position,” as also reported by Preston, and “d540 dressing oneself.” Preston et al reported many more objectives in the Activity domain, also utilizing the “walking” category. A possible reason is that objectives may reflect more the expectations of professionals and less those of patients. Indeed, when objectives suggested by patients and families are taken into greater consideration, goals proposed in the Activity area notably increase [19]. It is probably necessary to evaluate the objectives relevant to the professionals and those significant to the families and children separately.
The discrepancies between our data and Preston’s also most likely reflect differences in the study population. In our study, those undergoing injections aimed at improving gait pattern are, for the most part, hemiplegic and diplegic patients with mild disabilities (GMFCS 1). Their elevated degree of autonomy in mobility probably accounts for the scarcity of objectives for improving walking autonomy. In the most severe cases, such as quadriplegia, objectives are mainly aimed at controlling deformities and facilitating health care provision. Pain reduction is another important aspect and concerned quadriplegic and diplegic patients with severe disability. In contrast, objectives related to muscle tone reduction were limited, as the main objective was not a reduction but the control of muscle shortening and the subsequent deformities. However, this can become a primary objective in cases of spastic hyperactivation (eg, in adductor muscles) or in the case of dystonia, to improve patient comfort.
From a practical point of view, the use of this methodology provides for a common language that facilitates the communication and sharing of therapeutic objectives between different professionals (physiatrists and physiotherapists) and between health care providers and families and/or patients. This is important, as physiotherapy is often complementary to BTX injections and the objectives must be shared with the family. This methodology can help the clinician in the decision-making process and allows determining with greater specificity what is to be measured to document the achievement of the objectives.
Future research in this field will be aimed at evaluating patient outcomes by means of the adoption of suitable instruments (measurement scales) in order to quantify results which are consistent, according to the ICF-CY classification, with the domain and the category undergoing analysis.
Conclusion
As it has already been pointed out by various authors [10–15], the ICF-CY is a useful instrument for the classification of proposed therapeutic objectives into domains and categories, in order to standardize the language and to increase the sharing of the aims between the health care providers and between providers and families/patients. The most commonly followed approach calls for the use of functional profiles at the beginning of the care planning process, in order to establish the priorities and objectives of the interventions to be carried out. In order to streamline and facilitate procedures in clinical practice, many have proposed the use of core sets, but the validation procedure is complex and not always possible in all centers. Recently, Preston et al were the first to propose using the ICF-CY for classifying the objectives of an individual program. The procedure utilized is simple, easily reproducible, and allows identifying and classifying the objectives into categories using the ICF-CY. Furthermore, it is focused on an individual program and not on the entire articulation of programs, making interpretation of the data more linear. Our proposal is similar because it is focused on the analysis of an individual therapeutic program and because it utilizes the ICF classification system to classify the objectives; however, it achieves a higher degree of detail and standardization of the objectives.
In conclusion, the classification structure of the ICF-CY furnishes a useful and recognized instrument for categorizing the objectives of the interventions to be carried out. The classification of the objectives is specific for each pathology and for each individual program. The standardization of the objectives themselves and the use of the ICF-CY categories only for classification represents a possible methodologic alternative to the use of ICF-CY individual categories and sub-categories for identifying these objectives (core sets), as proposed by other authors. This procedure offers greater detail and a greater degree of standardization, which is important for the successive and systematic evaluation of treatment results.
Corresponding author: Nicoletta Battisti, Via Altura 3, 40139 Bologna, Italy, nbattisti@ausilioteca.org.
1. Lukban M, Rosales RL. Effectiveness of botulinum toxin A for upper and lower limb spasticity in children with cerebral palsy: a summary of evidence. J Neural Transm 2009;116:319–31.
2. Ryll U, Bastianen C, De Bie R, Staal B. Effects of leg muscle botulinum toxin A injections on walking in children with spasticity related cerebral palsy: a systematic review. Devel Med Child Neurol 2011;53:210–6.
3. Hoare BJ, Wallen MA,Villanueva E, et al. Botulinum toxin A as an adjunct to treatment in the management of upper limb in children with spastic cerebral palsy. The Cochraine Library 2010.
4. World Health Organization. International Classification of Functioning, Disability, and Health: Children and Youth Version for Children and Youth (ICF-CY). 2007. Available at http://apps.who.int/bookorders/anglais/detart1.jsp?codlan=1&codcol=15&codcch=716#
5. Rosenbaum P, Stewart D. The World Health Organization International Classification of Functioning Disability and Health: a model to guide clinical thinking, practice, and research. Semin Pediatr Neurol 2004;11:5–10.
6. Steiner W, Ryser L, Huber E, et al. Use of the ICF model as a clinical problem solving tool in physical therapy and rehabilitation medicine. Phys Ther 2002;82:1098–107.
7. Thamar JH, Bovend’Eerdt, Botell RE, Wade DT. Writing SMART rehabilitation goals and achieving goal attainment scaling: practical guide. Clin Rehab 2009;23:352–61.
8. Program outcome evaluations. United Way of Winnipeg; 2007.
9. Main K. Program design: a practical guide. Available at www.calgaryunitedway.org.
10. Schiariti V, Selb M, Cieza A, O’Donnel M. International classification of Functioning, Disability and Health Core sets for children and youth with cerebral palsy: a consensus meeting 1. Dev Med Child Neurol 2014 Aug 6. Epub ahead of print
11. Huber EO, Tobler A, Gloor-Juzzi T, et al. The ICF as a way to specify goals and assess the outcome of physiotherapeutic interventions in the acute hospitals Rehabil Med 2011;43:174–7.
12. Mittrach R, Grill E, Walchner-Bonjean M, et al. Goals of physiotherapy interventions can be described using the International Classification Of Functioning, Disability and Health Physiotherapy 2008;94:150–7.
13. Muller MJ, Strobl R, Grill E. Goals of patients with rehabilitation needs in acute hospitals: goal achievement is an indicator for improved functioning Rehabil Med 2011;43:145–50.
14. Grill E J, Stucki G. Criteria for validating comprehensive ICF core sets and developing brief ICF core set versions. J Rehabil Med 2011;43:87–91.
15. Preston NJ, Clarke M, Bhakta B. Development of a framework to define the functional goals and outcomes of botulinum toxin A spasticity treatment relevant to the child and family living with cerebral palsy using the international classification of functioning disability and health for children and youth (ICF-CY). J Rehabil Med 2011;43:1010–5.
16. Palisano R, Rosenbaum P, Walter S, et al. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol 1997;39:214–23.
17. Ferrari A, Cioni G. The spastic forms of cerebral palsy: a guide to the assessment of adaptive functions. Springer-Verlag; 2010.
18. Franki I, De Cat J, Deschepper E, et al. A clinical decision framework for the identification of main problems and treatment goals for ambulant children with bilateral spastic cerebral palsy. Res Dev Disabil 2014;35:1160–76.
19. Lohmann S, Decker J, Müller M, et al. The ICF forms a useful framework for classifying individual patients goals in post-acute rehabilitation. Rehabil Med 2011;43:151–5.
1. Lukban M, Rosales RL. Effectiveness of botulinum toxin A for upper and lower limb spasticity in children with cerebral palsy: a summary of evidence. J Neural Transm 2009;116:319–31.
2. Ryll U, Bastianen C, De Bie R, Staal B. Effects of leg muscle botulinum toxin A injections on walking in children with spasticity related cerebral palsy: a systematic review. Devel Med Child Neurol 2011;53:210–6.
3. Hoare BJ, Wallen MA,Villanueva E, et al. Botulinum toxin A as an adjunct to treatment in the management of upper limb in children with spastic cerebral palsy. The Cochraine Library 2010.
4. World Health Organization. International Classification of Functioning, Disability, and Health: Children and Youth Version for Children and Youth (ICF-CY). 2007. Available at http://apps.who.int/bookorders/anglais/detart1.jsp?codlan=1&codcol=15&codcch=716#
5. Rosenbaum P, Stewart D. The World Health Organization International Classification of Functioning Disability and Health: a model to guide clinical thinking, practice, and research. Semin Pediatr Neurol 2004;11:5–10.
6. Steiner W, Ryser L, Huber E, et al. Use of the ICF model as a clinical problem solving tool in physical therapy and rehabilitation medicine. Phys Ther 2002;82:1098–107.
7. Thamar JH, Bovend’Eerdt, Botell RE, Wade DT. Writing SMART rehabilitation goals and achieving goal attainment scaling: practical guide. Clin Rehab 2009;23:352–61.
8. Program outcome evaluations. United Way of Winnipeg; 2007.
9. Main K. Program design: a practical guide. Available at www.calgaryunitedway.org.
10. Schiariti V, Selb M, Cieza A, O’Donnel M. International classification of Functioning, Disability and Health Core sets for children and youth with cerebral palsy: a consensus meeting 1. Dev Med Child Neurol 2014 Aug 6. Epub ahead of print
11. Huber EO, Tobler A, Gloor-Juzzi T, et al. The ICF as a way to specify goals and assess the outcome of physiotherapeutic interventions in the acute hospitals Rehabil Med 2011;43:174–7.
12. Mittrach R, Grill E, Walchner-Bonjean M, et al. Goals of physiotherapy interventions can be described using the International Classification Of Functioning, Disability and Health Physiotherapy 2008;94:150–7.
13. Muller MJ, Strobl R, Grill E. Goals of patients with rehabilitation needs in acute hospitals: goal achievement is an indicator for improved functioning Rehabil Med 2011;43:145–50.
14. Grill E J, Stucki G. Criteria for validating comprehensive ICF core sets and developing brief ICF core set versions. J Rehabil Med 2011;43:87–91.
15. Preston NJ, Clarke M, Bhakta B. Development of a framework to define the functional goals and outcomes of botulinum toxin A spasticity treatment relevant to the child and family living with cerebral palsy using the international classification of functioning disability and health for children and youth (ICF-CY). J Rehabil Med 2011;43:1010–5.
16. Palisano R, Rosenbaum P, Walter S, et al. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol 1997;39:214–23.
17. Ferrari A, Cioni G. The spastic forms of cerebral palsy: a guide to the assessment of adaptive functions. Springer-Verlag; 2010.
18. Franki I, De Cat J, Deschepper E, et al. A clinical decision framework for the identification of main problems and treatment goals for ambulant children with bilateral spastic cerebral palsy. Res Dev Disabil 2014;35:1160–76.
19. Lohmann S, Decker J, Müller M, et al. The ICF forms a useful framework for classifying individual patients goals in post-acute rehabilitation. Rehabil Med 2011;43:151–5.
Behavioral Health Problems in Medical Patients
From Michigan State University, East Lansing, MI.
Abstract
- Objective: To describe the clinical presentations of medical patients attending a behavioral health clinic staffed by medical residents and faculty in the patients’ usual medical setting.
- Methods: We extracted the following clinical data from the patients’ electronic medical records: duration of problem; symptom presentation; symptom types; use of narcotics, antidepressants, benzodiazepines, antipsychotics, and mood stabilizers; impairment/disability; PHQ-9 scores and DSM-V diagnoses; and prior care from behavioral health professionals.
- Results: There were 64 patients, with an average age of 48.6 years. 68.8% were female, and 81.3% had had the presenting problem > 5 years. Presentation was psychological in 21/64 (32.8%), physical in 16/64 (25%), and both in 27/64 (42.2%). Patients averaged 3.3 common comorbid medical disease diagnoses. DSM-V diagnoses averaged 2.3 per patient; 30/64 (46.9%) had somatic symptom disorder, 27/64 (42.2%) had major depressive disorder, and 24/64 (37.5%) had generalized anxiety disorder. Social and economic impairment was present in > 70%. Some narcotic use occurred in 35/64 (54.7%) but only 7/35 (20.0%) were on unsafe doses; 46/64 (71.9%) took antidepressants but only 6/46 (13.0%) were on subtherapeutic doses. Averaging 71.9 months in the same clinic, only 18/64 (28.1%) had received behavioral health care for the presenting problem, and only 10.9% from psychiatrists.
- Conclusion: We described the chronic behavioral health problems of medical patients receiving behavioral care in their own medical setting from medical residents and faculty. These data can guide educators interested in training residents to manage common but now unattended behavioral health problems.
Patients with “any DSM behavioral health disorder” (mental health and substance use problems) account for 25% of patients seen in medical clinics [1]. These patients frequently present with poorly explained and sometimes confusing physical symptoms, and less often with psychological symptoms [2,3]. Common complaints in this population include chronic pain in almost any location, bowel complaints, insomnia, and fatigue [4]. Multiple somatic symptoms and increasing severity of symptoms correlate with the likelihood of an underlying depressive or anxiety disorder [3]. Unfortunately, medical physicians often do not recognize behavioral health problems and provide inadequate treatment for those they do [5].
As part of a Health Resources and Services Administration (HRSA) grant to develop behavioral health training guidelines for medical residents [6], we developed a special clinic for these patients. The clinic was located in their regular clinic area, and care was provided by medical residents and faculty. The objective of this paper is to describe the clinical presentation of patients attending the behavioral health care clinic, thus highlighting the common problems for which medical physicians are increasingly called upon to diagnose and treat.
Methods
We are in the third year of a 5-year HRSA grant to develop a method for teaching residents a primary care behavioral health care treatment model based on patient-centered, cognitive-behavioral, pharmacologic, and teamwork principles [6]. It is derived from consultation-liaison psychiatry, multidisciplinary pain management, and primary care research [7–10] and adapted for medical physicians. Described in detail elsewhere [6], we intensively train PGY-2 and PGY-3 residents in the Complex Patient Clinic (CPC), the name we applied to a behavioral health care clinic and the focus of this report.
Theoretical Base
The theoretical basis for this approach is general system theory and its medical derivative, the biopsychosocial (BPS) model [11]. In describing prevalent but overlooked behavioral health problems of patients attending our CPC, we underscore the importance of the BPS model relative to the prevailing biomedical, disease-only model. The latter does not include behavioral or psychosocial dimensions, the result being that they are largely excluded from medical education and, hence, overlooked in practice. The BPS model provides the theoretical basis for including these behavioral health patients in teaching and care.
Patients
Observations
The CPC uses 3 examination rooms for one half-day a week in the usual resident and faculty area of the Clinical Center of Michigan State University Department of Medicine. Rooms are similar to other clinic examination rooms except that a second computer attached to small audio video recorder is placed on the physician’s desk. Visible to the patient, it broadcasts live the patient-resident interaction to a nearby room where teaching faculty observe the interaction on a computer linked by a special software program (Vidyo, Hackensack, NJ) [12]. Access and control of Vidyo virtual rooms is restricted and rooms can only be entered by participating faculty using pre-assigned usernames and passwords. No recordings of the interactions are made.
Training faculty and the resident reviewed the patient’s EMR before each interaction and faculty continued to review it while observing the interaction. Both faculty and trainee documented information in the EMR in the fashion used with other patients.
Data
Two authors, RCS and AD, independently reviewed the EMR records of CPC visits, including follow-up visits and free text sources, and recorded results on an Excel spreadsheet; records of visits prior to CPC consultation were not reviewed nor were later non-CPC visits. They abstracted chart information on the first 5 patients and then updated and refined criteria. This was repeated again for the next 5 patients and near 100% agreement was obtained on all items except disability where > 90% agreement was achieved. All subsequent ratings were independently obtained and any differences were then jointly resolved in this extraction of mostly straightforward descriptive data. RCS is a senior faculty active in teaching and AD is a senior medical resident rated as superior by her faculty.
Results
Of 77 patients referred between 19 February 2013 and 10 December 2013, 13 (16.9%) did not complete the first scheduled or any subsequently scheduled appointments, while the remaining 64 patients (83.1%) completed referral to the CPC. Of the 64 attending the CPC, 6 (9.4%) missed the first appointment but made their first visit an average of 36.2 days later. The mean age was 48.6 years (range 25–75), 44/64 (68.8%) were women, 55/64 (85.9%) were Caucasian, 60/64 (93.8%) were non-Hispanic/Latino, and 63/64 (98.4%) were English speaking. All had insurance of some type, and 25/64 (39.1%) were Medicaid patients. Of 3583 total patients seen in the referring clinics during the same period, we found a mean age of 57 years (range, 17–97), 53% women, 75% Caucasian, 95% non-Hispanic/Latino, 97% English-speaking, and 9% Medicaid.
Current cigarette smokers were 22/64 (34.4%) of the population, higher than in national databases but similar to many behavioral health populations [23]. The BMI was 25 or less in 21/64 (32.8%), similar to the national distribution demonstrating that approximately 2/3 of patients are overweight or obese; 12/64 (18.8%) had a BMI of 25–30 (overweight), lower than national data, and 33/64 (48.5%) had a BMI >30 (obesity), higher than national data [24]. Similar increased rates of obesity are found in other behavioral health populations [25].
Mode of Symptom Presentation
Psychological symptoms were the sole mode of presentation in 21/64 (32.8%), while physical symptoms were the sole presenting complaint in 16/64 (25.0%). Combined psychological and physical symptoms were the predominant pattern at 27/64 (42.2%). Thus, 43/64 (67.2%) had physical symptoms and 48/64 (75.0%) had psychological symptoms at presentation. The mean duration of presenting symptoms was > 5 years in 52/64 (81.3%); only 5/64 (7.8%) had symptoms < 12 months in duration.
Presenting Symptoms
Pain symptoms were present in 53/64 (82.8%) and averaged 1.9 per patient. The details presented in Table 3 demonstrate a high frequency of musculoskeletal problems.
Non-pain physical symptoms were present in 45/64 (70.3%) and averaged 1.5 per patient. There was a very high frequency of insomnia (Table 3).
Comorbid Physical Diseases
Medications
Narcotic use was found in 35/64 (54.7%) patients; of these, 23/35 (65.7%) were using 80 or fewer morphine equivalents and 12/35 (34.3%) were using > 80 morphine equivalents, only 7/35 (20.0%) at > 120 morphine equivalents. Thus, only the latter took unsafe doses. There was no narcotic use in 29/64 (45.3%).
Antidepressant use was found in 46/64 (71.9%); only 6/46 (13.0%) were on subtherapeutic doses while 40/46 (87.0%) were on “usual therapeutic” doses. There was no antidepressant use in 18/64 (28.1%).
Benzodiazepine use was found in 31/64 (48.4%), antipsychotic use in 8/64 (12.5%), and mood stabilizer use in 10/64 (15.6%).
Impairment/Disability
Major physical impairment was present in 27/64 (42.2%), major economic impairment was present in 45/64 (70.3%), and major social impairment occurred in 49/64 (76.6%).
Diagnoses
The PHQ-9 was available in 41/64 (64.1%) of cases. Of these, it was < 5 (normal) in 3/41 (7.3%), from 5–10 (mild depression) in 11/41 (26.8%), from 10–15 (moderate depression) in 13/41 (31.7%), from 15–20 (severe depression) in 3/41 (7.3%), and > 20 (very severe depression) in 11/41 (26.8%).
Prior Care History
Behavioral health care for problems prior to the presentation problem had been received by 27/64 (42.2%): 11/27 (40.7%) from non-psychiatrists, 10/27 (37.0%) from psychiatrists, and 6/27 (22.2%) from both. Behavioral care for the presentation problem had been received by only 18/64 (28.1%): 11/18 (61.1%) from non-psychiatrists, 3/18 (16.7%) from psychiatrists, and 4/18 (22.2%) from both. Thus, of all 64 CPC patients, only 7 (10.9%) had received psychiatric care. Patients had received care in the same medical clinic for an average of 71.9 months.
Discussion
We identified the clinical profile of medical patients referred to a behavioral health care clinic. Located in the patients’ usual clinic area, care in the CPC was provided by medical residents and faculty. CPC patients were predominantly middle-aged, female, white, and non-Hispanic/Latino. Obesity and tobacco use were greater than in the general population but at levels often found in psychiatric populations [23,25]. Presenting symptoms of most patients were of > 5 years’ duration. The most common presentation was a combination of psychological and physical symptoms rather than either alone. Psychological symptoms were mainly depression and anxiety, while physical presentations primarily involved insomnia and many types of pain. These findings parallel the literature, except that psychological symptoms were more prominent than often reported [2,3]. This may indicate better recognition by referring physicians (and thus referral) of patients having a psychological presentation [26].
On average, there were 3.3 common comorbid physical disease diagnoses and 2.3 DSM-V diagnoses in each patient. The most common DSM-V diagnoses were somatic symptom disorder (46.9%), major depressive disorder (42.2%), and generalized anxiety disorder (37.5%) [22]. Representing diagnoses with which residents likely would have less recognition, several other disorders were in the 5% to 15% range: bipolar disorder, PTSD, various types of substance abuse, ADHD, psychological factors affecting medical conditions, and dysthymia.
Based on the literature and frequent comments from faculty and residents, we had expected greater narcotic use, especially at unsafe levels [27]. But, nearly half were taking none. Of those taking narcotics, only 20% received unsafe doses (more than 120 morphine equivalents). At odds with the literature citing frequent subtherapeutic antidepressant use by physicians [16], only 13.0% of the 71.9% taking antidepressants were at subtherapeutic levels. This suggests that referring physicians were not remiss when prescribing a single drug and that multiple drugs may be necessary [28]. Referring physicians may not be comfortable initiating and managing these more complex regimens. The narcotic and antidepressant practices by referring physicians suggested that the patients referred were more complex than can be addressed by good general medical care (low-dose narcotics and full-dose antidepressants). The complexity of these patients is further suggested by the PHQ-9 data, which indicated that more than one-third were in the severe to very severe range for depression [21]. The extent of economic and social impairment was striking (> 70%).
Even though these patients had been in the same medical clinic for nearly 6 years, only 28.1% had received behavioral health care for the presenting problem, and only 10.9% by a psychiatrist [5]. This suggests failure to recognize the problem [5] and/or the inability to access increasingly unavailable psychiatric consultation [29]. The latter is consistent with the literature that psychiatrists care for < 15% of all mental health patients [30], are of insufficient numbers in 96% of U.S. counties [31], and that most medical physicians find it nearly impossible to obtain a psychiatric consultation [29]. We also demonstrated behavioral health patients’ ready acceptance of behavioral health consultation in a medical setting by medical physicians. The 16.9% no-show rate for referrals to the CPC compares favorably to completion of psychiatry referrals where 50% to 60% no-show rates are not uncommon [32]. While our results may be due to decreased stigma in a medical setting [33], they likely also reflect that direct appointments were made by the referring physician at the time of the appointment (rather than the frequent psychiatry practice of having the patient make the appointment later by telephone), and that there was no more than a 1- to 2-week waiting period [34].
There were important limitations. The patient population from this small academic medical center may vary from that seen in different clinic types, and its physicians may differ in their referral practices. Although it is possible that our results are unique to the CPC and not generalizable, the similarity of our patients to those reported in the survey literature of primary care strongly suggests that these are indeed the types of patients who would be referred to and attend such clinics elsewhere. Patients also were mostly white, so the results may not apply in other populations. Further, some reports indicate using unstructured records from the EMR alone for diagnosing depression has significant limitations [35]. We did not have structured data, and the quality of documentation cannot be assured. A further limitation is that we did not verify our findings by talking with the physicians or with the patients, nor did we use formal diagnostic tools administered to patients, such as the World Health Organization Composite International Diagnostic Interview [36], to establish independently our DSM-V diagnoses [22]. Nevertheless, CPC diagnoses were made by experienced clinicians familiar with DSM-V.
Conclusion
This descriptive research demonstrated the clinical presentation of behavioral health patients when consultation was provided by medical physicians in their usual clinic. We have identified the types of patients for which educators may want to prepare their residents (and students) and for which practitioners can seek continuing education. Specifically, we demonstrated that learners will need to know how to diagnose and manage patients presenting with many different physical symptoms, often difficult to explain on a disease basis. Further, they will need to recognize that the usual mode of presentation of a primary care behavioral health problem, typically underlying depression and anxiety, is with multiple physical symptoms [37]. Learners will, in turn, need to be taught the relational, cognitive behavioral, pharmacologic, and teamwork principles that must be used in treatment [37].
Nevertheless, practically speaking, training practitioners has been ineffective [38], and training residents and students would not yield results for many years, Thus, these data also highlight the need for increased training of consultation-liaison and other psychiatrists. The well-established success of collaborative care [39] warrants increased support, as do related team efforts such as the patient-centered medical home. As well, more support for services and implementation research is badly needed to facilitate behavioral care in the medical setting.
The well-trained physician of the future can greatly complement these current efforts. If we can address all the multiple factors involved, we can look ahead to a much changed behavioral health care scene in 10 to 15 years [40].
Acknowledgements: The authors would like to acknowledge key advisory roles played by the following parts of our team in developing this project. Heather Spotts, MSW, advised and participated in team management. Jose Herrera, MD, was crucial in providing psychiatry continuity in the Complex Patient Clinic. Carmen Meerschaert, MD, played a key initial role in developing the structure of the Complex Patient Clinic. Geraud Plantegenest, MS, was responsible to developing and ensuring the function of our internet technology work in the Complex Patient Clinic.
Corresponding author: Robert C. Smith, B312 Clinical Center, 788 Service Rd., Michigan State Univ., East Lansing, MI 48824, robert.smith@ht.msu.edu.
Funding/support: We are grateful for the generous support from the Health Resources and Services Administration (HRSA) (D58HP23259) that provides the opportunity to develop this curriculum and produce papers from it. HRSA had no role in the study design; collection, analysis, and interpretation of data; writing the report; or in decision to submit the article for publication.
Financial disclosures: None.
Author contributions: conception and design, FCD, DD, JF, AD, DS, RCS; analysis and interpretation of data, FCD, AD, KGS, DS, RCS; drafting of article, FCD, HLF, LF, DD, JF, AD, KGS, DS, RCS; critical revision of the article, FCD, HLF, LF, DD, JF, AD, KGS, DS, RCS; provision of study materials or patients, FCD, HLF, LF, RCS; statistical expertise, AD, KGS, DS; obtaining of funding, FCD, LF, RCS; administrative or technical support, FCD, HLF, KGS, RCS; collection and assembly of data, AD, RCS.
1. Norquist GS, Regier DA. The epidemiology of psychiatric disorders and the de facto mental health care system. Annu Rev Med 1996;47:473–9.
2. Collins C, Hewson D, Munger R, Wade T. Evolving models of behavioral health integration in primary care. In: Fund MM, editor. New York: Milbank Memorial Fund; 2010.
3. Kroenke K. The interface between physical and psychological symptoms. Prim Care Companion J Clin Psychiatry 2003;5(Suppl 7):11–8.
4. Kroenke K, Price RK. Symptoms in the community--prevalence, classification, and psychiatric comorbidity. Arch Intern Med 1993;153:2474–80.
5. Melek S, Norris D. Chronic conditions and comorbid psychological disorders. Millman Research Report. Seattle, WA: Millman 2008:19.
6. Smith R, Laird-Fick H, D’Mello D, et al. Addressing mental health issues in primary care: an initial curriculum for medical residents. Patient Educ Couns 2013;94:33–42.
7. Cutler RB, Fishbain DA, Rosomoff HL, et al. Does nonsurgical pain center treatment of chronic pain return patients to work? -- a review and meta-analysis of the literature. Spine 1994;19:643–52.
8. Katon W, von Korff M, Lin E, et al. Distressed high utilizers of medical care: DSM-III-R diagnoses and treatment needs. Gen Hosp Psychiatry 1990;12:355–62.
9. Sharpe M, Hawton K, Simkin S, et al. Cognitive behaviour therapy for the chronic fatigue syndrome:a randomised controlled trial. BMJ 1996;312:22–6.
10. World Organization of Family Doctors. Accessed 26 Aug 2013 at www.who.int/workforcealliance/members_partners/member_list/wonca/en/index.html.
11. Engel GL. The need for a new medical model: a challenge for biomedicine. Science 1977;196:129–36.
12. Vidyo. www.vidyo.com/products/use/.
13. Allison JJ, Wall TC, Spettell CM, et al. The art and science of chart review. Jt Comm J Qual Improve 2000;26:115–36.
14. Vieweg WV, Lipps WF, Fernandez A. Opioids and methadone equivalents for clinicians. Prim Care Companion J Clin Psychiatry 2005;7:86–8.
15. Dunn KM, Saunders KW, Rutter CM, et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med 2010;152:85–92.
16. Kessler R, Stafford D. Primary care is the de facto mental health system. In: Kessler R, Stafford D, editors. Collaborative medicine case studies—evidence in practice. New York: Springer; 2008:9–21.
17. Schneider RK, Levenson JL. Psychiatry essentials for primary care. Philadelphia: American College of Physicians; 2008.
18. Von Korff M, Ormel J, Katon W, Lin EHB. Disability and depression among high utilizers of health care—a longitudinal analysis. Arch Gen Psychiatry 1992;49:91–100.
19. Von Korff M, Ustun TB, Ormel J, et al. Self-report disability in an international primary care study of psychological illness. J Clin Epidemiol 1996;49:297–303.
20. Fairbank JC, Couper J, Davies JB, O’Brien JP. The Oswestry low back pain disability questionnaire. Physiotherapy 1980;66:271–3.
21. Kroenke K, Spitzer RL, Williams JB, Lowe B. The Patient Health Questionnaire Somatic, Anxiety, and Depressive Symptom Scales: a systematic review. Gen Hosp Psychiatry 2010;32:345–59.
22. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013.
23. Lasser K, Boyd JW, Woolhandler S, et al. Smoking and mental illness: A population-based prevalence study. JAMA 2000;284:2606–10.
24. NIDDK. Overweight and obesity statistics. Accessed 30 May 2014 at win.niddk.nih.gov/statistics/
25. Allison DB, Newcomer JW, Dunn AL, et al. Obesity among those with mental disorders: a National Institute of Mental Health meeting report. Am J Prev Med 2009;36:341–50.
26. Salmon P, Humphris GM, Ring A, et al. Primary care consultations about medically unexplained symptoms: patient presentations and doctor responses that influence the probability of somatic intervention. Psychosom Med 2007;69:571–7.
27. Sullivan MD, Howe CQ. Opioid therapy for chronic pain in the United States: promises and perils. Pain 2013;154 Suppl 1:S94–100.
28. Rush AJ. STAR*D: what have we learned? Am J Psychiatry 2007;164:201–4.
29. Cunningham PJ. Beyond parity: primary care physicians’ perspectives on access to mental health care. Health Aff (Millwood) 2009;28:w490–501.
30. Wang PS, Lane M, Olfson M, et al. Twelve-month use of mental health services in the United States—results from the National Comorbidity Survey Replication. Arch Gen Psychiatry 2005;62:629–40.
31. Morrisey J, Thomas K, Ellis A, Konrad T. Development of a new method for designation of mental health professional shortage areas. Chapel Hill, NC: University of North Carolina at Chapel Hill; 2007.
32. deGruy F. Mental health care in the primary care setting. In: Donaldson MS, Yordy KD, Lohr KN, Vanselow NA, editors. Primary care—America’s health in a new era. Washington, DC: National Academy Press; 1996:285–311.
33. World Organization of Family Doctors. Companion to primary care mental health. New York: WONCA and Radcliffe Publishing; 2012.
34. Craig TJ, Huffine CL, Brooks M. Completion of referral to psychiatric services by inner city residents. Arch Gen Psychiatry 1974;31:353–7.
35. Chen Y, Li H, Li Y, et al. Resemblance of symptoms for major depression assessed at interview versus from hospital record review. PLoS ONE 2012;7:e28734.
36. World Health Organization. Composite International Diagnostic Interview (CIDI) – core version 2.1. Geneva: WHO; 1997.
37. Smith RC, Lein C, Collins C, et al. Treating patients with medically unexplained symptoms in primary care. J Gen Intern Med 2003;18:478–89.
38. Lin EH, Simon GE, Katzelnick DJ, Pearson SD. Does physician education on depression management improve treatment in primary care? J Gen Intern Med 2001;16:614–9.
39. Huffman JC, Niazi SK, Rundell JR, et al. Essential articles on collaborative care models for the treatment of psychiatric disorders in medical settings: a publication by the Academy of Psychosomatic Medicine Research and Evidence-Based Practice Committee. Psychosomatics 2014;55:109–22.
40. Summergrad P, Kathol R. A vision of integrated psychiatric and medical care for 2023. In: Summergrad P, Kathol R, editors. Integrated care in psychiatry: redefining the role of mental health professionals in the medical setting. New York: Springer; 2014.
From Michigan State University, East Lansing, MI.
Abstract
- Objective: To describe the clinical presentations of medical patients attending a behavioral health clinic staffed by medical residents and faculty in the patients’ usual medical setting.
- Methods: We extracted the following clinical data from the patients’ electronic medical records: duration of problem; symptom presentation; symptom types; use of narcotics, antidepressants, benzodiazepines, antipsychotics, and mood stabilizers; impairment/disability; PHQ-9 scores and DSM-V diagnoses; and prior care from behavioral health professionals.
- Results: There were 64 patients, with an average age of 48.6 years. 68.8% were female, and 81.3% had had the presenting problem > 5 years. Presentation was psychological in 21/64 (32.8%), physical in 16/64 (25%), and both in 27/64 (42.2%). Patients averaged 3.3 common comorbid medical disease diagnoses. DSM-V diagnoses averaged 2.3 per patient; 30/64 (46.9%) had somatic symptom disorder, 27/64 (42.2%) had major depressive disorder, and 24/64 (37.5%) had generalized anxiety disorder. Social and economic impairment was present in > 70%. Some narcotic use occurred in 35/64 (54.7%) but only 7/35 (20.0%) were on unsafe doses; 46/64 (71.9%) took antidepressants but only 6/46 (13.0%) were on subtherapeutic doses. Averaging 71.9 months in the same clinic, only 18/64 (28.1%) had received behavioral health care for the presenting problem, and only 10.9% from psychiatrists.
- Conclusion: We described the chronic behavioral health problems of medical patients receiving behavioral care in their own medical setting from medical residents and faculty. These data can guide educators interested in training residents to manage common but now unattended behavioral health problems.
Patients with “any DSM behavioral health disorder” (mental health and substance use problems) account for 25% of patients seen in medical clinics [1]. These patients frequently present with poorly explained and sometimes confusing physical symptoms, and less often with psychological symptoms [2,3]. Common complaints in this population include chronic pain in almost any location, bowel complaints, insomnia, and fatigue [4]. Multiple somatic symptoms and increasing severity of symptoms correlate with the likelihood of an underlying depressive or anxiety disorder [3]. Unfortunately, medical physicians often do not recognize behavioral health problems and provide inadequate treatment for those they do [5].
As part of a Health Resources and Services Administration (HRSA) grant to develop behavioral health training guidelines for medical residents [6], we developed a special clinic for these patients. The clinic was located in their regular clinic area, and care was provided by medical residents and faculty. The objective of this paper is to describe the clinical presentation of patients attending the behavioral health care clinic, thus highlighting the common problems for which medical physicians are increasingly called upon to diagnose and treat.
Methods
We are in the third year of a 5-year HRSA grant to develop a method for teaching residents a primary care behavioral health care treatment model based on patient-centered, cognitive-behavioral, pharmacologic, and teamwork principles [6]. It is derived from consultation-liaison psychiatry, multidisciplinary pain management, and primary care research [7–10] and adapted for medical physicians. Described in detail elsewhere [6], we intensively train PGY-2 and PGY-3 residents in the Complex Patient Clinic (CPC), the name we applied to a behavioral health care clinic and the focus of this report.
Theoretical Base
The theoretical basis for this approach is general system theory and its medical derivative, the biopsychosocial (BPS) model [11]. In describing prevalent but overlooked behavioral health problems of patients attending our CPC, we underscore the importance of the BPS model relative to the prevailing biomedical, disease-only model. The latter does not include behavioral or psychosocial dimensions, the result being that they are largely excluded from medical education and, hence, overlooked in practice. The BPS model provides the theoretical basis for including these behavioral health patients in teaching and care.
Patients
Observations
The CPC uses 3 examination rooms for one half-day a week in the usual resident and faculty area of the Clinical Center of Michigan State University Department of Medicine. Rooms are similar to other clinic examination rooms except that a second computer attached to small audio video recorder is placed on the physician’s desk. Visible to the patient, it broadcasts live the patient-resident interaction to a nearby room where teaching faculty observe the interaction on a computer linked by a special software program (Vidyo, Hackensack, NJ) [12]. Access and control of Vidyo virtual rooms is restricted and rooms can only be entered by participating faculty using pre-assigned usernames and passwords. No recordings of the interactions are made.
Training faculty and the resident reviewed the patient’s EMR before each interaction and faculty continued to review it while observing the interaction. Both faculty and trainee documented information in the EMR in the fashion used with other patients.
Data
Two authors, RCS and AD, independently reviewed the EMR records of CPC visits, including follow-up visits and free text sources, and recorded results on an Excel spreadsheet; records of visits prior to CPC consultation were not reviewed nor were later non-CPC visits. They abstracted chart information on the first 5 patients and then updated and refined criteria. This was repeated again for the next 5 patients and near 100% agreement was obtained on all items except disability where > 90% agreement was achieved. All subsequent ratings were independently obtained and any differences were then jointly resolved in this extraction of mostly straightforward descriptive data. RCS is a senior faculty active in teaching and AD is a senior medical resident rated as superior by her faculty.
Results
Of 77 patients referred between 19 February 2013 and 10 December 2013, 13 (16.9%) did not complete the first scheduled or any subsequently scheduled appointments, while the remaining 64 patients (83.1%) completed referral to the CPC. Of the 64 attending the CPC, 6 (9.4%) missed the first appointment but made their first visit an average of 36.2 days later. The mean age was 48.6 years (range 25–75), 44/64 (68.8%) were women, 55/64 (85.9%) were Caucasian, 60/64 (93.8%) were non-Hispanic/Latino, and 63/64 (98.4%) were English speaking. All had insurance of some type, and 25/64 (39.1%) were Medicaid patients. Of 3583 total patients seen in the referring clinics during the same period, we found a mean age of 57 years (range, 17–97), 53% women, 75% Caucasian, 95% non-Hispanic/Latino, 97% English-speaking, and 9% Medicaid.
Current cigarette smokers were 22/64 (34.4%) of the population, higher than in national databases but similar to many behavioral health populations [23]. The BMI was 25 or less in 21/64 (32.8%), similar to the national distribution demonstrating that approximately 2/3 of patients are overweight or obese; 12/64 (18.8%) had a BMI of 25–30 (overweight), lower than national data, and 33/64 (48.5%) had a BMI >30 (obesity), higher than national data [24]. Similar increased rates of obesity are found in other behavioral health populations [25].
Mode of Symptom Presentation
Psychological symptoms were the sole mode of presentation in 21/64 (32.8%), while physical symptoms were the sole presenting complaint in 16/64 (25.0%). Combined psychological and physical symptoms were the predominant pattern at 27/64 (42.2%). Thus, 43/64 (67.2%) had physical symptoms and 48/64 (75.0%) had psychological symptoms at presentation. The mean duration of presenting symptoms was > 5 years in 52/64 (81.3%); only 5/64 (7.8%) had symptoms < 12 months in duration.
Presenting Symptoms
Pain symptoms were present in 53/64 (82.8%) and averaged 1.9 per patient. The details presented in Table 3 demonstrate a high frequency of musculoskeletal problems.
Non-pain physical symptoms were present in 45/64 (70.3%) and averaged 1.5 per patient. There was a very high frequency of insomnia (Table 3).
Comorbid Physical Diseases
Medications
Narcotic use was found in 35/64 (54.7%) patients; of these, 23/35 (65.7%) were using 80 or fewer morphine equivalents and 12/35 (34.3%) were using > 80 morphine equivalents, only 7/35 (20.0%) at > 120 morphine equivalents. Thus, only the latter took unsafe doses. There was no narcotic use in 29/64 (45.3%).
Antidepressant use was found in 46/64 (71.9%); only 6/46 (13.0%) were on subtherapeutic doses while 40/46 (87.0%) were on “usual therapeutic” doses. There was no antidepressant use in 18/64 (28.1%).
Benzodiazepine use was found in 31/64 (48.4%), antipsychotic use in 8/64 (12.5%), and mood stabilizer use in 10/64 (15.6%).
Impairment/Disability
Major physical impairment was present in 27/64 (42.2%), major economic impairment was present in 45/64 (70.3%), and major social impairment occurred in 49/64 (76.6%).
Diagnoses
The PHQ-9 was available in 41/64 (64.1%) of cases. Of these, it was < 5 (normal) in 3/41 (7.3%), from 5–10 (mild depression) in 11/41 (26.8%), from 10–15 (moderate depression) in 13/41 (31.7%), from 15–20 (severe depression) in 3/41 (7.3%), and > 20 (very severe depression) in 11/41 (26.8%).
Prior Care History
Behavioral health care for problems prior to the presentation problem had been received by 27/64 (42.2%): 11/27 (40.7%) from non-psychiatrists, 10/27 (37.0%) from psychiatrists, and 6/27 (22.2%) from both. Behavioral care for the presentation problem had been received by only 18/64 (28.1%): 11/18 (61.1%) from non-psychiatrists, 3/18 (16.7%) from psychiatrists, and 4/18 (22.2%) from both. Thus, of all 64 CPC patients, only 7 (10.9%) had received psychiatric care. Patients had received care in the same medical clinic for an average of 71.9 months.
Discussion
We identified the clinical profile of medical patients referred to a behavioral health care clinic. Located in the patients’ usual clinic area, care in the CPC was provided by medical residents and faculty. CPC patients were predominantly middle-aged, female, white, and non-Hispanic/Latino. Obesity and tobacco use were greater than in the general population but at levels often found in psychiatric populations [23,25]. Presenting symptoms of most patients were of > 5 years’ duration. The most common presentation was a combination of psychological and physical symptoms rather than either alone. Psychological symptoms were mainly depression and anxiety, while physical presentations primarily involved insomnia and many types of pain. These findings parallel the literature, except that psychological symptoms were more prominent than often reported [2,3]. This may indicate better recognition by referring physicians (and thus referral) of patients having a psychological presentation [26].
On average, there were 3.3 common comorbid physical disease diagnoses and 2.3 DSM-V diagnoses in each patient. The most common DSM-V diagnoses were somatic symptom disorder (46.9%), major depressive disorder (42.2%), and generalized anxiety disorder (37.5%) [22]. Representing diagnoses with which residents likely would have less recognition, several other disorders were in the 5% to 15% range: bipolar disorder, PTSD, various types of substance abuse, ADHD, psychological factors affecting medical conditions, and dysthymia.
Based on the literature and frequent comments from faculty and residents, we had expected greater narcotic use, especially at unsafe levels [27]. But, nearly half were taking none. Of those taking narcotics, only 20% received unsafe doses (more than 120 morphine equivalents). At odds with the literature citing frequent subtherapeutic antidepressant use by physicians [16], only 13.0% of the 71.9% taking antidepressants were at subtherapeutic levels. This suggests that referring physicians were not remiss when prescribing a single drug and that multiple drugs may be necessary [28]. Referring physicians may not be comfortable initiating and managing these more complex regimens. The narcotic and antidepressant practices by referring physicians suggested that the patients referred were more complex than can be addressed by good general medical care (low-dose narcotics and full-dose antidepressants). The complexity of these patients is further suggested by the PHQ-9 data, which indicated that more than one-third were in the severe to very severe range for depression [21]. The extent of economic and social impairment was striking (> 70%).
Even though these patients had been in the same medical clinic for nearly 6 years, only 28.1% had received behavioral health care for the presenting problem, and only 10.9% by a psychiatrist [5]. This suggests failure to recognize the problem [5] and/or the inability to access increasingly unavailable psychiatric consultation [29]. The latter is consistent with the literature that psychiatrists care for < 15% of all mental health patients [30], are of insufficient numbers in 96% of U.S. counties [31], and that most medical physicians find it nearly impossible to obtain a psychiatric consultation [29]. We also demonstrated behavioral health patients’ ready acceptance of behavioral health consultation in a medical setting by medical physicians. The 16.9% no-show rate for referrals to the CPC compares favorably to completion of psychiatry referrals where 50% to 60% no-show rates are not uncommon [32]. While our results may be due to decreased stigma in a medical setting [33], they likely also reflect that direct appointments were made by the referring physician at the time of the appointment (rather than the frequent psychiatry practice of having the patient make the appointment later by telephone), and that there was no more than a 1- to 2-week waiting period [34].
There were important limitations. The patient population from this small academic medical center may vary from that seen in different clinic types, and its physicians may differ in their referral practices. Although it is possible that our results are unique to the CPC and not generalizable, the similarity of our patients to those reported in the survey literature of primary care strongly suggests that these are indeed the types of patients who would be referred to and attend such clinics elsewhere. Patients also were mostly white, so the results may not apply in other populations. Further, some reports indicate using unstructured records from the EMR alone for diagnosing depression has significant limitations [35]. We did not have structured data, and the quality of documentation cannot be assured. A further limitation is that we did not verify our findings by talking with the physicians or with the patients, nor did we use formal diagnostic tools administered to patients, such as the World Health Organization Composite International Diagnostic Interview [36], to establish independently our DSM-V diagnoses [22]. Nevertheless, CPC diagnoses were made by experienced clinicians familiar with DSM-V.
Conclusion
This descriptive research demonstrated the clinical presentation of behavioral health patients when consultation was provided by medical physicians in their usual clinic. We have identified the types of patients for which educators may want to prepare their residents (and students) and for which practitioners can seek continuing education. Specifically, we demonstrated that learners will need to know how to diagnose and manage patients presenting with many different physical symptoms, often difficult to explain on a disease basis. Further, they will need to recognize that the usual mode of presentation of a primary care behavioral health problem, typically underlying depression and anxiety, is with multiple physical symptoms [37]. Learners will, in turn, need to be taught the relational, cognitive behavioral, pharmacologic, and teamwork principles that must be used in treatment [37].
Nevertheless, practically speaking, training practitioners has been ineffective [38], and training residents and students would not yield results for many years, Thus, these data also highlight the need for increased training of consultation-liaison and other psychiatrists. The well-established success of collaborative care [39] warrants increased support, as do related team efforts such as the patient-centered medical home. As well, more support for services and implementation research is badly needed to facilitate behavioral care in the medical setting.
The well-trained physician of the future can greatly complement these current efforts. If we can address all the multiple factors involved, we can look ahead to a much changed behavioral health care scene in 10 to 15 years [40].
Acknowledgements: The authors would like to acknowledge key advisory roles played by the following parts of our team in developing this project. Heather Spotts, MSW, advised and participated in team management. Jose Herrera, MD, was crucial in providing psychiatry continuity in the Complex Patient Clinic. Carmen Meerschaert, MD, played a key initial role in developing the structure of the Complex Patient Clinic. Geraud Plantegenest, MS, was responsible to developing and ensuring the function of our internet technology work in the Complex Patient Clinic.
Corresponding author: Robert C. Smith, B312 Clinical Center, 788 Service Rd., Michigan State Univ., East Lansing, MI 48824, robert.smith@ht.msu.edu.
Funding/support: We are grateful for the generous support from the Health Resources and Services Administration (HRSA) (D58HP23259) that provides the opportunity to develop this curriculum and produce papers from it. HRSA had no role in the study design; collection, analysis, and interpretation of data; writing the report; or in decision to submit the article for publication.
Financial disclosures: None.
Author contributions: conception and design, FCD, DD, JF, AD, DS, RCS; analysis and interpretation of data, FCD, AD, KGS, DS, RCS; drafting of article, FCD, HLF, LF, DD, JF, AD, KGS, DS, RCS; critical revision of the article, FCD, HLF, LF, DD, JF, AD, KGS, DS, RCS; provision of study materials or patients, FCD, HLF, LF, RCS; statistical expertise, AD, KGS, DS; obtaining of funding, FCD, LF, RCS; administrative or technical support, FCD, HLF, KGS, RCS; collection and assembly of data, AD, RCS.
From Michigan State University, East Lansing, MI.
Abstract
- Objective: To describe the clinical presentations of medical patients attending a behavioral health clinic staffed by medical residents and faculty in the patients’ usual medical setting.
- Methods: We extracted the following clinical data from the patients’ electronic medical records: duration of problem; symptom presentation; symptom types; use of narcotics, antidepressants, benzodiazepines, antipsychotics, and mood stabilizers; impairment/disability; PHQ-9 scores and DSM-V diagnoses; and prior care from behavioral health professionals.
- Results: There were 64 patients, with an average age of 48.6 years. 68.8% were female, and 81.3% had had the presenting problem > 5 years. Presentation was psychological in 21/64 (32.8%), physical in 16/64 (25%), and both in 27/64 (42.2%). Patients averaged 3.3 common comorbid medical disease diagnoses. DSM-V diagnoses averaged 2.3 per patient; 30/64 (46.9%) had somatic symptom disorder, 27/64 (42.2%) had major depressive disorder, and 24/64 (37.5%) had generalized anxiety disorder. Social and economic impairment was present in > 70%. Some narcotic use occurred in 35/64 (54.7%) but only 7/35 (20.0%) were on unsafe doses; 46/64 (71.9%) took antidepressants but only 6/46 (13.0%) were on subtherapeutic doses. Averaging 71.9 months in the same clinic, only 18/64 (28.1%) had received behavioral health care for the presenting problem, and only 10.9% from psychiatrists.
- Conclusion: We described the chronic behavioral health problems of medical patients receiving behavioral care in their own medical setting from medical residents and faculty. These data can guide educators interested in training residents to manage common but now unattended behavioral health problems.
Patients with “any DSM behavioral health disorder” (mental health and substance use problems) account for 25% of patients seen in medical clinics [1]. These patients frequently present with poorly explained and sometimes confusing physical symptoms, and less often with psychological symptoms [2,3]. Common complaints in this population include chronic pain in almost any location, bowel complaints, insomnia, and fatigue [4]. Multiple somatic symptoms and increasing severity of symptoms correlate with the likelihood of an underlying depressive or anxiety disorder [3]. Unfortunately, medical physicians often do not recognize behavioral health problems and provide inadequate treatment for those they do [5].
As part of a Health Resources and Services Administration (HRSA) grant to develop behavioral health training guidelines for medical residents [6], we developed a special clinic for these patients. The clinic was located in their regular clinic area, and care was provided by medical residents and faculty. The objective of this paper is to describe the clinical presentation of patients attending the behavioral health care clinic, thus highlighting the common problems for which medical physicians are increasingly called upon to diagnose and treat.
Methods
We are in the third year of a 5-year HRSA grant to develop a method for teaching residents a primary care behavioral health care treatment model based on patient-centered, cognitive-behavioral, pharmacologic, and teamwork principles [6]. It is derived from consultation-liaison psychiatry, multidisciplinary pain management, and primary care research [7–10] and adapted for medical physicians. Described in detail elsewhere [6], we intensively train PGY-2 and PGY-3 residents in the Complex Patient Clinic (CPC), the name we applied to a behavioral health care clinic and the focus of this report.
Theoretical Base
The theoretical basis for this approach is general system theory and its medical derivative, the biopsychosocial (BPS) model [11]. In describing prevalent but overlooked behavioral health problems of patients attending our CPC, we underscore the importance of the BPS model relative to the prevailing biomedical, disease-only model. The latter does not include behavioral or psychosocial dimensions, the result being that they are largely excluded from medical education and, hence, overlooked in practice. The BPS model provides the theoretical basis for including these behavioral health patients in teaching and care.
Patients
Observations
The CPC uses 3 examination rooms for one half-day a week in the usual resident and faculty area of the Clinical Center of Michigan State University Department of Medicine. Rooms are similar to other clinic examination rooms except that a second computer attached to small audio video recorder is placed on the physician’s desk. Visible to the patient, it broadcasts live the patient-resident interaction to a nearby room where teaching faculty observe the interaction on a computer linked by a special software program (Vidyo, Hackensack, NJ) [12]. Access and control of Vidyo virtual rooms is restricted and rooms can only be entered by participating faculty using pre-assigned usernames and passwords. No recordings of the interactions are made.
Training faculty and the resident reviewed the patient’s EMR before each interaction and faculty continued to review it while observing the interaction. Both faculty and trainee documented information in the EMR in the fashion used with other patients.
Data
Two authors, RCS and AD, independently reviewed the EMR records of CPC visits, including follow-up visits and free text sources, and recorded results on an Excel spreadsheet; records of visits prior to CPC consultation were not reviewed nor were later non-CPC visits. They abstracted chart information on the first 5 patients and then updated and refined criteria. This was repeated again for the next 5 patients and near 100% agreement was obtained on all items except disability where > 90% agreement was achieved. All subsequent ratings were independently obtained and any differences were then jointly resolved in this extraction of mostly straightforward descriptive data. RCS is a senior faculty active in teaching and AD is a senior medical resident rated as superior by her faculty.
Results
Of 77 patients referred between 19 February 2013 and 10 December 2013, 13 (16.9%) did not complete the first scheduled or any subsequently scheduled appointments, while the remaining 64 patients (83.1%) completed referral to the CPC. Of the 64 attending the CPC, 6 (9.4%) missed the first appointment but made their first visit an average of 36.2 days later. The mean age was 48.6 years (range 25–75), 44/64 (68.8%) were women, 55/64 (85.9%) were Caucasian, 60/64 (93.8%) were non-Hispanic/Latino, and 63/64 (98.4%) were English speaking. All had insurance of some type, and 25/64 (39.1%) were Medicaid patients. Of 3583 total patients seen in the referring clinics during the same period, we found a mean age of 57 years (range, 17–97), 53% women, 75% Caucasian, 95% non-Hispanic/Latino, 97% English-speaking, and 9% Medicaid.
Current cigarette smokers were 22/64 (34.4%) of the population, higher than in national databases but similar to many behavioral health populations [23]. The BMI was 25 or less in 21/64 (32.8%), similar to the national distribution demonstrating that approximately 2/3 of patients are overweight or obese; 12/64 (18.8%) had a BMI of 25–30 (overweight), lower than national data, and 33/64 (48.5%) had a BMI >30 (obesity), higher than national data [24]. Similar increased rates of obesity are found in other behavioral health populations [25].
Mode of Symptom Presentation
Psychological symptoms were the sole mode of presentation in 21/64 (32.8%), while physical symptoms were the sole presenting complaint in 16/64 (25.0%). Combined psychological and physical symptoms were the predominant pattern at 27/64 (42.2%). Thus, 43/64 (67.2%) had physical symptoms and 48/64 (75.0%) had psychological symptoms at presentation. The mean duration of presenting symptoms was > 5 years in 52/64 (81.3%); only 5/64 (7.8%) had symptoms < 12 months in duration.
Presenting Symptoms
Pain symptoms were present in 53/64 (82.8%) and averaged 1.9 per patient. The details presented in Table 3 demonstrate a high frequency of musculoskeletal problems.
Non-pain physical symptoms were present in 45/64 (70.3%) and averaged 1.5 per patient. There was a very high frequency of insomnia (Table 3).
Comorbid Physical Diseases
Medications
Narcotic use was found in 35/64 (54.7%) patients; of these, 23/35 (65.7%) were using 80 or fewer morphine equivalents and 12/35 (34.3%) were using > 80 morphine equivalents, only 7/35 (20.0%) at > 120 morphine equivalents. Thus, only the latter took unsafe doses. There was no narcotic use in 29/64 (45.3%).
Antidepressant use was found in 46/64 (71.9%); only 6/46 (13.0%) were on subtherapeutic doses while 40/46 (87.0%) were on “usual therapeutic” doses. There was no antidepressant use in 18/64 (28.1%).
Benzodiazepine use was found in 31/64 (48.4%), antipsychotic use in 8/64 (12.5%), and mood stabilizer use in 10/64 (15.6%).
Impairment/Disability
Major physical impairment was present in 27/64 (42.2%), major economic impairment was present in 45/64 (70.3%), and major social impairment occurred in 49/64 (76.6%).
Diagnoses
The PHQ-9 was available in 41/64 (64.1%) of cases. Of these, it was < 5 (normal) in 3/41 (7.3%), from 5–10 (mild depression) in 11/41 (26.8%), from 10–15 (moderate depression) in 13/41 (31.7%), from 15–20 (severe depression) in 3/41 (7.3%), and > 20 (very severe depression) in 11/41 (26.8%).
Prior Care History
Behavioral health care for problems prior to the presentation problem had been received by 27/64 (42.2%): 11/27 (40.7%) from non-psychiatrists, 10/27 (37.0%) from psychiatrists, and 6/27 (22.2%) from both. Behavioral care for the presentation problem had been received by only 18/64 (28.1%): 11/18 (61.1%) from non-psychiatrists, 3/18 (16.7%) from psychiatrists, and 4/18 (22.2%) from both. Thus, of all 64 CPC patients, only 7 (10.9%) had received psychiatric care. Patients had received care in the same medical clinic for an average of 71.9 months.
Discussion
We identified the clinical profile of medical patients referred to a behavioral health care clinic. Located in the patients’ usual clinic area, care in the CPC was provided by medical residents and faculty. CPC patients were predominantly middle-aged, female, white, and non-Hispanic/Latino. Obesity and tobacco use were greater than in the general population but at levels often found in psychiatric populations [23,25]. Presenting symptoms of most patients were of > 5 years’ duration. The most common presentation was a combination of psychological and physical symptoms rather than either alone. Psychological symptoms were mainly depression and anxiety, while physical presentations primarily involved insomnia and many types of pain. These findings parallel the literature, except that psychological symptoms were more prominent than often reported [2,3]. This may indicate better recognition by referring physicians (and thus referral) of patients having a psychological presentation [26].
On average, there were 3.3 common comorbid physical disease diagnoses and 2.3 DSM-V diagnoses in each patient. The most common DSM-V diagnoses were somatic symptom disorder (46.9%), major depressive disorder (42.2%), and generalized anxiety disorder (37.5%) [22]. Representing diagnoses with which residents likely would have less recognition, several other disorders were in the 5% to 15% range: bipolar disorder, PTSD, various types of substance abuse, ADHD, psychological factors affecting medical conditions, and dysthymia.
Based on the literature and frequent comments from faculty and residents, we had expected greater narcotic use, especially at unsafe levels [27]. But, nearly half were taking none. Of those taking narcotics, only 20% received unsafe doses (more than 120 morphine equivalents). At odds with the literature citing frequent subtherapeutic antidepressant use by physicians [16], only 13.0% of the 71.9% taking antidepressants were at subtherapeutic levels. This suggests that referring physicians were not remiss when prescribing a single drug and that multiple drugs may be necessary [28]. Referring physicians may not be comfortable initiating and managing these more complex regimens. The narcotic and antidepressant practices by referring physicians suggested that the patients referred were more complex than can be addressed by good general medical care (low-dose narcotics and full-dose antidepressants). The complexity of these patients is further suggested by the PHQ-9 data, which indicated that more than one-third were in the severe to very severe range for depression [21]. The extent of economic and social impairment was striking (> 70%).
Even though these patients had been in the same medical clinic for nearly 6 years, only 28.1% had received behavioral health care for the presenting problem, and only 10.9% by a psychiatrist [5]. This suggests failure to recognize the problem [5] and/or the inability to access increasingly unavailable psychiatric consultation [29]. The latter is consistent with the literature that psychiatrists care for < 15% of all mental health patients [30], are of insufficient numbers in 96% of U.S. counties [31], and that most medical physicians find it nearly impossible to obtain a psychiatric consultation [29]. We also demonstrated behavioral health patients’ ready acceptance of behavioral health consultation in a medical setting by medical physicians. The 16.9% no-show rate for referrals to the CPC compares favorably to completion of psychiatry referrals where 50% to 60% no-show rates are not uncommon [32]. While our results may be due to decreased stigma in a medical setting [33], they likely also reflect that direct appointments were made by the referring physician at the time of the appointment (rather than the frequent psychiatry practice of having the patient make the appointment later by telephone), and that there was no more than a 1- to 2-week waiting period [34].
There were important limitations. The patient population from this small academic medical center may vary from that seen in different clinic types, and its physicians may differ in their referral practices. Although it is possible that our results are unique to the CPC and not generalizable, the similarity of our patients to those reported in the survey literature of primary care strongly suggests that these are indeed the types of patients who would be referred to and attend such clinics elsewhere. Patients also were mostly white, so the results may not apply in other populations. Further, some reports indicate using unstructured records from the EMR alone for diagnosing depression has significant limitations [35]. We did not have structured data, and the quality of documentation cannot be assured. A further limitation is that we did not verify our findings by talking with the physicians or with the patients, nor did we use formal diagnostic tools administered to patients, such as the World Health Organization Composite International Diagnostic Interview [36], to establish independently our DSM-V diagnoses [22]. Nevertheless, CPC diagnoses were made by experienced clinicians familiar with DSM-V.
Conclusion
This descriptive research demonstrated the clinical presentation of behavioral health patients when consultation was provided by medical physicians in their usual clinic. We have identified the types of patients for which educators may want to prepare their residents (and students) and for which practitioners can seek continuing education. Specifically, we demonstrated that learners will need to know how to diagnose and manage patients presenting with many different physical symptoms, often difficult to explain on a disease basis. Further, they will need to recognize that the usual mode of presentation of a primary care behavioral health problem, typically underlying depression and anxiety, is with multiple physical symptoms [37]. Learners will, in turn, need to be taught the relational, cognitive behavioral, pharmacologic, and teamwork principles that must be used in treatment [37].
Nevertheless, practically speaking, training practitioners has been ineffective [38], and training residents and students would not yield results for many years, Thus, these data also highlight the need for increased training of consultation-liaison and other psychiatrists. The well-established success of collaborative care [39] warrants increased support, as do related team efforts such as the patient-centered medical home. As well, more support for services and implementation research is badly needed to facilitate behavioral care in the medical setting.
The well-trained physician of the future can greatly complement these current efforts. If we can address all the multiple factors involved, we can look ahead to a much changed behavioral health care scene in 10 to 15 years [40].
Acknowledgements: The authors would like to acknowledge key advisory roles played by the following parts of our team in developing this project. Heather Spotts, MSW, advised and participated in team management. Jose Herrera, MD, was crucial in providing psychiatry continuity in the Complex Patient Clinic. Carmen Meerschaert, MD, played a key initial role in developing the structure of the Complex Patient Clinic. Geraud Plantegenest, MS, was responsible to developing and ensuring the function of our internet technology work in the Complex Patient Clinic.
Corresponding author: Robert C. Smith, B312 Clinical Center, 788 Service Rd., Michigan State Univ., East Lansing, MI 48824, robert.smith@ht.msu.edu.
Funding/support: We are grateful for the generous support from the Health Resources and Services Administration (HRSA) (D58HP23259) that provides the opportunity to develop this curriculum and produce papers from it. HRSA had no role in the study design; collection, analysis, and interpretation of data; writing the report; or in decision to submit the article for publication.
Financial disclosures: None.
Author contributions: conception and design, FCD, DD, JF, AD, DS, RCS; analysis and interpretation of data, FCD, AD, KGS, DS, RCS; drafting of article, FCD, HLF, LF, DD, JF, AD, KGS, DS, RCS; critical revision of the article, FCD, HLF, LF, DD, JF, AD, KGS, DS, RCS; provision of study materials or patients, FCD, HLF, LF, RCS; statistical expertise, AD, KGS, DS; obtaining of funding, FCD, LF, RCS; administrative or technical support, FCD, HLF, KGS, RCS; collection and assembly of data, AD, RCS.
1. Norquist GS, Regier DA. The epidemiology of psychiatric disorders and the de facto mental health care system. Annu Rev Med 1996;47:473–9.
2. Collins C, Hewson D, Munger R, Wade T. Evolving models of behavioral health integration in primary care. In: Fund MM, editor. New York: Milbank Memorial Fund; 2010.
3. Kroenke K. The interface between physical and psychological symptoms. Prim Care Companion J Clin Psychiatry 2003;5(Suppl 7):11–8.
4. Kroenke K, Price RK. Symptoms in the community--prevalence, classification, and psychiatric comorbidity. Arch Intern Med 1993;153:2474–80.
5. Melek S, Norris D. Chronic conditions and comorbid psychological disorders. Millman Research Report. Seattle, WA: Millman 2008:19.
6. Smith R, Laird-Fick H, D’Mello D, et al. Addressing mental health issues in primary care: an initial curriculum for medical residents. Patient Educ Couns 2013;94:33–42.
7. Cutler RB, Fishbain DA, Rosomoff HL, et al. Does nonsurgical pain center treatment of chronic pain return patients to work? -- a review and meta-analysis of the literature. Spine 1994;19:643–52.
8. Katon W, von Korff M, Lin E, et al. Distressed high utilizers of medical care: DSM-III-R diagnoses and treatment needs. Gen Hosp Psychiatry 1990;12:355–62.
9. Sharpe M, Hawton K, Simkin S, et al. Cognitive behaviour therapy for the chronic fatigue syndrome:a randomised controlled trial. BMJ 1996;312:22–6.
10. World Organization of Family Doctors. Accessed 26 Aug 2013 at www.who.int/workforcealliance/members_partners/member_list/wonca/en/index.html.
11. Engel GL. The need for a new medical model: a challenge for biomedicine. Science 1977;196:129–36.
12. Vidyo. www.vidyo.com/products/use/.
13. Allison JJ, Wall TC, Spettell CM, et al. The art and science of chart review. Jt Comm J Qual Improve 2000;26:115–36.
14. Vieweg WV, Lipps WF, Fernandez A. Opioids and methadone equivalents for clinicians. Prim Care Companion J Clin Psychiatry 2005;7:86–8.
15. Dunn KM, Saunders KW, Rutter CM, et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med 2010;152:85–92.
16. Kessler R, Stafford D. Primary care is the de facto mental health system. In: Kessler R, Stafford D, editors. Collaborative medicine case studies—evidence in practice. New York: Springer; 2008:9–21.
17. Schneider RK, Levenson JL. Psychiatry essentials for primary care. Philadelphia: American College of Physicians; 2008.
18. Von Korff M, Ormel J, Katon W, Lin EHB. Disability and depression among high utilizers of health care—a longitudinal analysis. Arch Gen Psychiatry 1992;49:91–100.
19. Von Korff M, Ustun TB, Ormel J, et al. Self-report disability in an international primary care study of psychological illness. J Clin Epidemiol 1996;49:297–303.
20. Fairbank JC, Couper J, Davies JB, O’Brien JP. The Oswestry low back pain disability questionnaire. Physiotherapy 1980;66:271–3.
21. Kroenke K, Spitzer RL, Williams JB, Lowe B. The Patient Health Questionnaire Somatic, Anxiety, and Depressive Symptom Scales: a systematic review. Gen Hosp Psychiatry 2010;32:345–59.
22. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013.
23. Lasser K, Boyd JW, Woolhandler S, et al. Smoking and mental illness: A population-based prevalence study. JAMA 2000;284:2606–10.
24. NIDDK. Overweight and obesity statistics. Accessed 30 May 2014 at win.niddk.nih.gov/statistics/
25. Allison DB, Newcomer JW, Dunn AL, et al. Obesity among those with mental disorders: a National Institute of Mental Health meeting report. Am J Prev Med 2009;36:341–50.
26. Salmon P, Humphris GM, Ring A, et al. Primary care consultations about medically unexplained symptoms: patient presentations and doctor responses that influence the probability of somatic intervention. Psychosom Med 2007;69:571–7.
27. Sullivan MD, Howe CQ. Opioid therapy for chronic pain in the United States: promises and perils. Pain 2013;154 Suppl 1:S94–100.
28. Rush AJ. STAR*D: what have we learned? Am J Psychiatry 2007;164:201–4.
29. Cunningham PJ. Beyond parity: primary care physicians’ perspectives on access to mental health care. Health Aff (Millwood) 2009;28:w490–501.
30. Wang PS, Lane M, Olfson M, et al. Twelve-month use of mental health services in the United States—results from the National Comorbidity Survey Replication. Arch Gen Psychiatry 2005;62:629–40.
31. Morrisey J, Thomas K, Ellis A, Konrad T. Development of a new method for designation of mental health professional shortage areas. Chapel Hill, NC: University of North Carolina at Chapel Hill; 2007.
32. deGruy F. Mental health care in the primary care setting. In: Donaldson MS, Yordy KD, Lohr KN, Vanselow NA, editors. Primary care—America’s health in a new era. Washington, DC: National Academy Press; 1996:285–311.
33. World Organization of Family Doctors. Companion to primary care mental health. New York: WONCA and Radcliffe Publishing; 2012.
34. Craig TJ, Huffine CL, Brooks M. Completion of referral to psychiatric services by inner city residents. Arch Gen Psychiatry 1974;31:353–7.
35. Chen Y, Li H, Li Y, et al. Resemblance of symptoms for major depression assessed at interview versus from hospital record review. PLoS ONE 2012;7:e28734.
36. World Health Organization. Composite International Diagnostic Interview (CIDI) – core version 2.1. Geneva: WHO; 1997.
37. Smith RC, Lein C, Collins C, et al. Treating patients with medically unexplained symptoms in primary care. J Gen Intern Med 2003;18:478–89.
38. Lin EH, Simon GE, Katzelnick DJ, Pearson SD. Does physician education on depression management improve treatment in primary care? J Gen Intern Med 2001;16:614–9.
39. Huffman JC, Niazi SK, Rundell JR, et al. Essential articles on collaborative care models for the treatment of psychiatric disorders in medical settings: a publication by the Academy of Psychosomatic Medicine Research and Evidence-Based Practice Committee. Psychosomatics 2014;55:109–22.
40. Summergrad P, Kathol R. A vision of integrated psychiatric and medical care for 2023. In: Summergrad P, Kathol R, editors. Integrated care in psychiatry: redefining the role of mental health professionals in the medical setting. New York: Springer; 2014.
1. Norquist GS, Regier DA. The epidemiology of psychiatric disorders and the de facto mental health care system. Annu Rev Med 1996;47:473–9.
2. Collins C, Hewson D, Munger R, Wade T. Evolving models of behavioral health integration in primary care. In: Fund MM, editor. New York: Milbank Memorial Fund; 2010.
3. Kroenke K. The interface between physical and psychological symptoms. Prim Care Companion J Clin Psychiatry 2003;5(Suppl 7):11–8.
4. Kroenke K, Price RK. Symptoms in the community--prevalence, classification, and psychiatric comorbidity. Arch Intern Med 1993;153:2474–80.
5. Melek S, Norris D. Chronic conditions and comorbid psychological disorders. Millman Research Report. Seattle, WA: Millman 2008:19.
6. Smith R, Laird-Fick H, D’Mello D, et al. Addressing mental health issues in primary care: an initial curriculum for medical residents. Patient Educ Couns 2013;94:33–42.
7. Cutler RB, Fishbain DA, Rosomoff HL, et al. Does nonsurgical pain center treatment of chronic pain return patients to work? -- a review and meta-analysis of the literature. Spine 1994;19:643–52.
8. Katon W, von Korff M, Lin E, et al. Distressed high utilizers of medical care: DSM-III-R diagnoses and treatment needs. Gen Hosp Psychiatry 1990;12:355–62.
9. Sharpe M, Hawton K, Simkin S, et al. Cognitive behaviour therapy for the chronic fatigue syndrome:a randomised controlled trial. BMJ 1996;312:22–6.
10. World Organization of Family Doctors. Accessed 26 Aug 2013 at www.who.int/workforcealliance/members_partners/member_list/wonca/en/index.html.
11. Engel GL. The need for a new medical model: a challenge for biomedicine. Science 1977;196:129–36.
12. Vidyo. www.vidyo.com/products/use/.
13. Allison JJ, Wall TC, Spettell CM, et al. The art and science of chart review. Jt Comm J Qual Improve 2000;26:115–36.
14. Vieweg WV, Lipps WF, Fernandez A. Opioids and methadone equivalents for clinicians. Prim Care Companion J Clin Psychiatry 2005;7:86–8.
15. Dunn KM, Saunders KW, Rutter CM, et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med 2010;152:85–92.
16. Kessler R, Stafford D. Primary care is the de facto mental health system. In: Kessler R, Stafford D, editors. Collaborative medicine case studies—evidence in practice. New York: Springer; 2008:9–21.
17. Schneider RK, Levenson JL. Psychiatry essentials for primary care. Philadelphia: American College of Physicians; 2008.
18. Von Korff M, Ormel J, Katon W, Lin EHB. Disability and depression among high utilizers of health care—a longitudinal analysis. Arch Gen Psychiatry 1992;49:91–100.
19. Von Korff M, Ustun TB, Ormel J, et al. Self-report disability in an international primary care study of psychological illness. J Clin Epidemiol 1996;49:297–303.
20. Fairbank JC, Couper J, Davies JB, O’Brien JP. The Oswestry low back pain disability questionnaire. Physiotherapy 1980;66:271–3.
21. Kroenke K, Spitzer RL, Williams JB, Lowe B. The Patient Health Questionnaire Somatic, Anxiety, and Depressive Symptom Scales: a systematic review. Gen Hosp Psychiatry 2010;32:345–59.
22. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013.
23. Lasser K, Boyd JW, Woolhandler S, et al. Smoking and mental illness: A population-based prevalence study. JAMA 2000;284:2606–10.
24. NIDDK. Overweight and obesity statistics. Accessed 30 May 2014 at win.niddk.nih.gov/statistics/
25. Allison DB, Newcomer JW, Dunn AL, et al. Obesity among those with mental disorders: a National Institute of Mental Health meeting report. Am J Prev Med 2009;36:341–50.
26. Salmon P, Humphris GM, Ring A, et al. Primary care consultations about medically unexplained symptoms: patient presentations and doctor responses that influence the probability of somatic intervention. Psychosom Med 2007;69:571–7.
27. Sullivan MD, Howe CQ. Opioid therapy for chronic pain in the United States: promises and perils. Pain 2013;154 Suppl 1:S94–100.
28. Rush AJ. STAR*D: what have we learned? Am J Psychiatry 2007;164:201–4.
29. Cunningham PJ. Beyond parity: primary care physicians’ perspectives on access to mental health care. Health Aff (Millwood) 2009;28:w490–501.
30. Wang PS, Lane M, Olfson M, et al. Twelve-month use of mental health services in the United States—results from the National Comorbidity Survey Replication. Arch Gen Psychiatry 2005;62:629–40.
31. Morrisey J, Thomas K, Ellis A, Konrad T. Development of a new method for designation of mental health professional shortage areas. Chapel Hill, NC: University of North Carolina at Chapel Hill; 2007.
32. deGruy F. Mental health care in the primary care setting. In: Donaldson MS, Yordy KD, Lohr KN, Vanselow NA, editors. Primary care—America’s health in a new era. Washington, DC: National Academy Press; 1996:285–311.
33. World Organization of Family Doctors. Companion to primary care mental health. New York: WONCA and Radcliffe Publishing; 2012.
34. Craig TJ, Huffine CL, Brooks M. Completion of referral to psychiatric services by inner city residents. Arch Gen Psychiatry 1974;31:353–7.
35. Chen Y, Li H, Li Y, et al. Resemblance of symptoms for major depression assessed at interview versus from hospital record review. PLoS ONE 2012;7:e28734.
36. World Health Organization. Composite International Diagnostic Interview (CIDI) – core version 2.1. Geneva: WHO; 1997.
37. Smith RC, Lein C, Collins C, et al. Treating patients with medically unexplained symptoms in primary care. J Gen Intern Med 2003;18:478–89.
38. Lin EH, Simon GE, Katzelnick DJ, Pearson SD. Does physician education on depression management improve treatment in primary care? J Gen Intern Med 2001;16:614–9.
39. Huffman JC, Niazi SK, Rundell JR, et al. Essential articles on collaborative care models for the treatment of psychiatric disorders in medical settings: a publication by the Academy of Psychosomatic Medicine Research and Evidence-Based Practice Committee. Psychosomatics 2014;55:109–22.
40. Summergrad P, Kathol R. A vision of integrated psychiatric and medical care for 2023. In: Summergrad P, Kathol R, editors. Integrated care in psychiatry: redefining the role of mental health professionals in the medical setting. New York: Springer; 2014.
Efficacy of Cryosurgery and 5-Fluorouracil Cream 0.5% Combination Therapy for the Treatment of Actinic Keratosis
Actinic keratosis (AK) is regarded as a lesion on a continuum of progression to squamous cell carcinoma (SCC).1 Studies have estimated that 44% to 97% of SCCs were associated with AK lesions either in contiguous skin or within the same histologic section and that AK lesions progress to SCCs at a rate of 0.6% at 1 year.2 In 1993-1994 there were 3.7 million reported office visits for AK lesions, while in 2002 alone there were 8.2 million office visits.3,4 As the burden of disease from AKs has increased, so has the associated costs from office-based visits, treatments, and subsequent surveillance.
There are a number of highly effective approaches to AK treatment that are based on several factors such as the number of and extent of the lesions, history of skin cancer, provider practice characteristics (eg, location, appointment availability), patient preferences, cost, and tolerability. Cryosurgery is the most commonly used lesion-directed modality in the treatment of individual AKs based on its effectiveness and relative ease of use. Cryosurgery alone has been shown to have a success rate of 67% on AK lesions.5 Patients often experience erythema, edema, pain, and crusting at treated sites; there also is potential for ulceration, scarring, hypopigmentation, hyperpigmentation, and secondary infection, but these effects are less common. Recurrence may be an indicator of treatment-resistant lesions or new lesions appearing in the field.
A field-directed approach with topical 5-fluorouracil (5-FU) may be preferred in patients with a history of substantial photodamage, AKs that are resistant to cryosurgery, or multiple AKs. Field-directed treatments address multiple AKs simultaneously and treat subclinical lesions. Fluorouracil is a common therapy for AKs that often is implemented by dermatologists due to its efficacy and well-understood mechanism of action. Fluorouracil inhibits thymidylate synthase during DNA synthesis, thereby halting cellular proliferation. 5-Fluorouracil cream 0.5% has been approved for 1-, 2-, and 4-week treatment periods. In one study, resolution of AK lesions was greatest in the 4-week treatment group; however, side effects also were greatest in this group.6 Patients commonly may experience a range of local reactions including erythema, pruritus, erosions, ulcerations, scabbing, crusting, and facial irritation. For patients with substantial photodamage and AKs, a robust response can lead to perceived adverse events (AEs) and considerable downtime, possibly affecting patient satisfaction and treatment compliance.7
Many alternative and combination approaches have been studied to decrease AEs and improve compliance and efficacy in the treatment of AKs. In this study, we examined the efficacy and perceived side effects of cryosurgery and 5-FU cream 0.5% combination therapy in the treatment of AKs.
Methods
Study Design and Participants
This single-blind, single-center, comparator cream–controlled pilot study was parallel designed with a balanced randomization (1:1 frequency). The study protocol and consent form were approved by the Wake Forest University Health Sciences institutional review board (Winston-Salem, North Carolina). Participants were 18 years or older with 8 clinically typical, visible, and discrete AK lesions on the face (forehead and temples) or balding scalp. Typical inclusion and exclusion criteria were observed. No other topical agents or therapies were permitted to be applied to the affected areas at least 4 weeks prior to treatment, depending on the treatment modality.
Assessment
During the screening (baseline) visit, eligible participants provided informed consent, baseline lesion counts and investigator global assessments (IGAs) were performed, and cryosurgery was administered to all visible AK lesions in the study areas. Participants returned at weeks 3, 4, 8, and 26. Three weeks following cryosurgery, participants were randomized according to standard randomization tables into 1 of 2 treatment groups to receive once-daily treatment with either 5-FU cream 0.5% or a moisturizing comparator cream. The cream was applied at bedtime to the affected sites for 1 week. Randomization was investigator blinded, but participants and the study administrators were not blinded. Participants were instructed to record their treatment compliance in daily diary entries, which were reviewed at week 4 using the medication tolerability assessment rating for burning, stinging, and ulceration. Investigator global assessment, IGA of improvement, lesion counts, and quality of life (QOL) survey responses were gathered at weeks 3, 8, and 26. The IGA measured the overall severity of AK disease involvement on a 6-point scale (clear; very severe). The IGA of improvement measured the overall improvement from baseline on a 6-point scale (clear; worse). Adverse events were measured at each visit.
Efficacy End Points
The primary end point was 100% clearance of all AK lesions at the end of the study (week 26) relative to the baseline AK lesion count. Secondary end points included comparisons between the groups for the number of participants with greater than 75% reduction of baseline lesion counts at the end of the study as well as differences at each visit in medication tolerability assessments, QOL measures, IGA improvement scores, and medication adherence based on diary entries at week 4.
Statistical Analysis
An intention-to-treat analysis was performed. The number of participants with 100% or greater than 75% clearance of AK lesions by specified time points were compared using relative risks and risk differences with Poisson regression analysis log and identity link functions, respectively, to obtain robust error variance 95% confidence intervals. Medication tolerability assessment, QOL, and IGA improvement scores were compared between the 2 groups using the Mann-Whitney U test. The significance level was set at α=.05. All analyses were performed using SAS data analysis software.
Results
Sixty age-eligible participants were enrolled in the study with 30 participants in each treatment group. All of the participants completed the 26-week study period and were included in the intention-to-treat analysis. All of the participants were white with a median age of 67 years; the median number of baseline AK lesions was 12. Participant baseline demographics and clinical characteristics are provided in Table 1. Treatment compliance in both groups was good with only a few participants reporting missed doses.
In our evaluation of the rate of change in the number of AK lesions at week 8 compared to baseline, the 5-FU cream 0.5% group showed an 84% reduction in the number of AK lesions versus a 69% reduction in the control group. At week 26, the 5-FU cream 0.5% group showed a 72% reduction in the number of lesions versus 73% in the control group. There was no significant difference between 5-FU cream 0.5% and the comparator cream for either 100% or 75% clearance of AK lesions by the end of the study; however, comparing the AK lesion count from baseline to 8 weeks following the initiation of the study, participants in the 5-FU cream 0.5% group were more likely than the control group to achieve 75% or 100% clearance on the relative risk and risk difference scales (Table 2).
There were no significant differences between the 2 groups for the IGA of improvement at any time point (Table 3). On average, participants in the 5-FU cream 0.5% group experienced more dryness, erosion, fissuring, and redness than the control group but not more ulcerations by the end of week 4 (Table 4). All other QOL measures were statistically comparable between the 2 treatment groups for all time points.
A total of 25 AEs were reported throughout the study but none were considered to be serious. One AE (redness, burning, and itching over the eyebrow) was considered to be related to the study drug. No participants withdrew from the study due to AEs. A total of 12 participants in the 5-FU cream 0.5% group and 10 in the control group reported AEs.
Comment
After a 1-week course of 5-FU cream 0.5% following cryosurgery, a greater reduction in the number of AK lesions for a period of 2 months was noted in the treatment group compared to the control group. These findings are consistent with a similar study from 2006 that used 5-FU cream 0.5% or a vehicle 1 week prior to cryosurgery and then counted the number of AK lesions that remained.8 In the 2006 study, remarkable improvement out to week 26 was noted,8 unlike our study; however, there was insufficient power in our study to demonstrate a continued effect out to week 26.
Both the 2006 study and our current study support the benefit of using a combination treatment to clear AK lesions versus either treatment alone. Of note, these studies also show that combination treatments are equally effective, regardless of the order of treatments, in lowering AK lesion counts compared to cryosurgery alone.
Although participants in the 5-FU cream 0.5% group reported slightly more AEs on average at week 4, the rate of side effects was lower than those reported in a study documenting the side effects of a 4-week course of 5-FU.6 This rate of side effects must be considered in light of the added benefits this combination treatment has demonstrated.
Results of this pilot study suggest that a larger sample size would yield a difference in the study arms for all time periods (weeks 8 and 26). In an effort to maintain exchangeability of the study arms, patients were randomized at baseline treatment, but behaviors of patients in the 6 months following treatment, such as variation in sun exposure or other habits that promote AK lesion development, may have attenuated the results.
Key strengths of this study include no loss to follow-up and high medication adherence rates. The key limitation was the small sample size, which did not demonstrate a statistical advantage of the 5-FU cream 0.5% at 26 weeks; however, our study does show promise for larger future studies in illustrating this difference. A study by Krawtchenko et al9 noted that long-term efficacy of field therapy with 5-FU may ultimately be less than imiquimod cream 5%, suggesting that a possible alteration of the study protocol to compare the efficacy of different forms of field therapy may ultimately achieve better outcomes.
Conclusion
Overall, individuals with AK may benefit from a combination of treatment with cryosurgery and topical 5-FU to resolve lesions for longer periods than with cryosurgery alone. Although prior studies have found statistically significant differences in short-term and long-term treatment efficacy when cryosurgery is combined with an active field therapy versus a placebo vehicle,8,9 the current study aimed to find the best combination of efficacy with the fewest side effects. Therefore, the results of prior literature studies only further the feelings of the authors that with a protocol that looks at a slightly different treatment regimen within the treatment arm, the results can be extremely beneficial to patients. Further studies should be implemented to confirm the longer-term benefits of this combination therapy.
1. Lebwohl M. Actinic keratosis: epidemiology and progression to squamous cell carcinoma. Br J Dermatol. 2003;149(suppl 66):31-33.
2. Criscione VD, Weinstock MA, Naylor MF, et al. Actinic keratoses: natural history and risk of malignant transformation in the Veterans Affairs Topical Tretinoin Chemoprevention Trial. Cancer. 2009;115:2523-2530.
3. Smith ES, Feldman SR, Fleischer AB Jr, et al. Characteristics of office-based visits for skin cancer. dermatologists have more experience than other physicians in managing malignant and premalignant skin conditions. Dermatol Surg. 1998;24:981-985.
4. Shoimer I, Rosen N, Muhn C. Current management of actinic keratoses. Skin Therapy Lett. 2010;15:5-7.
5. Thai KE, Fergin P, Freeman M, et al. A prospective study of the use of cryosurgery for the treatment of actinic keratoses. Int J Dermatol. 2004;43:687-692.
6. Weiss J, Menter A, Hevia O, et al. Effective treatment of actinic keratosis with 0.5% fluorouracil cream for 1, 2, or 4 weeks. Cutis. 2002;70(suppl 2):22-29.
7. Jorizzo JL, Carney PS, Ko WT, et al. Treatment options in the management of actinic keratosis. Cutis. 2004;74 (suppl 6):9-17.
8. Jorizzo J, Weiss J, Vamvakias G. One-week treatment with 0.5% fluorouracil cream prior to cryosurgery in patients with actinic keratoses: a double-blind, vehicle-controlled, long-term study. J Drugs Dermatol. 2006;5:133-139.
9. Krawtchenko N, Roewert-Huber J, Ulrich M, et al. A randomised study of topical 5% imiquimod vs. topical 5-fluorouracil vs. cryosurgery in immunocompetent patients with actinic keratoses: a comparison of clinical and histological outcomes including 1-year follow-up. Br J Dermatol. 2007;157(suppl 2):34-40.
Actinic keratosis (AK) is regarded as a lesion on a continuum of progression to squamous cell carcinoma (SCC).1 Studies have estimated that 44% to 97% of SCCs were associated with AK lesions either in contiguous skin or within the same histologic section and that AK lesions progress to SCCs at a rate of 0.6% at 1 year.2 In 1993-1994 there were 3.7 million reported office visits for AK lesions, while in 2002 alone there were 8.2 million office visits.3,4 As the burden of disease from AKs has increased, so has the associated costs from office-based visits, treatments, and subsequent surveillance.
There are a number of highly effective approaches to AK treatment that are based on several factors such as the number of and extent of the lesions, history of skin cancer, provider practice characteristics (eg, location, appointment availability), patient preferences, cost, and tolerability. Cryosurgery is the most commonly used lesion-directed modality in the treatment of individual AKs based on its effectiveness and relative ease of use. Cryosurgery alone has been shown to have a success rate of 67% on AK lesions.5 Patients often experience erythema, edema, pain, and crusting at treated sites; there also is potential for ulceration, scarring, hypopigmentation, hyperpigmentation, and secondary infection, but these effects are less common. Recurrence may be an indicator of treatment-resistant lesions or new lesions appearing in the field.
A field-directed approach with topical 5-fluorouracil (5-FU) may be preferred in patients with a history of substantial photodamage, AKs that are resistant to cryosurgery, or multiple AKs. Field-directed treatments address multiple AKs simultaneously and treat subclinical lesions. Fluorouracil is a common therapy for AKs that often is implemented by dermatologists due to its efficacy and well-understood mechanism of action. Fluorouracil inhibits thymidylate synthase during DNA synthesis, thereby halting cellular proliferation. 5-Fluorouracil cream 0.5% has been approved for 1-, 2-, and 4-week treatment periods. In one study, resolution of AK lesions was greatest in the 4-week treatment group; however, side effects also were greatest in this group.6 Patients commonly may experience a range of local reactions including erythema, pruritus, erosions, ulcerations, scabbing, crusting, and facial irritation. For patients with substantial photodamage and AKs, a robust response can lead to perceived adverse events (AEs) and considerable downtime, possibly affecting patient satisfaction and treatment compliance.7
Many alternative and combination approaches have been studied to decrease AEs and improve compliance and efficacy in the treatment of AKs. In this study, we examined the efficacy and perceived side effects of cryosurgery and 5-FU cream 0.5% combination therapy in the treatment of AKs.
Methods
Study Design and Participants
This single-blind, single-center, comparator cream–controlled pilot study was parallel designed with a balanced randomization (1:1 frequency). The study protocol and consent form were approved by the Wake Forest University Health Sciences institutional review board (Winston-Salem, North Carolina). Participants were 18 years or older with 8 clinically typical, visible, and discrete AK lesions on the face (forehead and temples) or balding scalp. Typical inclusion and exclusion criteria were observed. No other topical agents or therapies were permitted to be applied to the affected areas at least 4 weeks prior to treatment, depending on the treatment modality.
Assessment
During the screening (baseline) visit, eligible participants provided informed consent, baseline lesion counts and investigator global assessments (IGAs) were performed, and cryosurgery was administered to all visible AK lesions in the study areas. Participants returned at weeks 3, 4, 8, and 26. Three weeks following cryosurgery, participants were randomized according to standard randomization tables into 1 of 2 treatment groups to receive once-daily treatment with either 5-FU cream 0.5% or a moisturizing comparator cream. The cream was applied at bedtime to the affected sites for 1 week. Randomization was investigator blinded, but participants and the study administrators were not blinded. Participants were instructed to record their treatment compliance in daily diary entries, which were reviewed at week 4 using the medication tolerability assessment rating for burning, stinging, and ulceration. Investigator global assessment, IGA of improvement, lesion counts, and quality of life (QOL) survey responses were gathered at weeks 3, 8, and 26. The IGA measured the overall severity of AK disease involvement on a 6-point scale (clear; very severe). The IGA of improvement measured the overall improvement from baseline on a 6-point scale (clear; worse). Adverse events were measured at each visit.
Efficacy End Points
The primary end point was 100% clearance of all AK lesions at the end of the study (week 26) relative to the baseline AK lesion count. Secondary end points included comparisons between the groups for the number of participants with greater than 75% reduction of baseline lesion counts at the end of the study as well as differences at each visit in medication tolerability assessments, QOL measures, IGA improvement scores, and medication adherence based on diary entries at week 4.
Statistical Analysis
An intention-to-treat analysis was performed. The number of participants with 100% or greater than 75% clearance of AK lesions by specified time points were compared using relative risks and risk differences with Poisson regression analysis log and identity link functions, respectively, to obtain robust error variance 95% confidence intervals. Medication tolerability assessment, QOL, and IGA improvement scores were compared between the 2 groups using the Mann-Whitney U test. The significance level was set at α=.05. All analyses were performed using SAS data analysis software.
Results
Sixty age-eligible participants were enrolled in the study with 30 participants in each treatment group. All of the participants completed the 26-week study period and were included in the intention-to-treat analysis. All of the participants were white with a median age of 67 years; the median number of baseline AK lesions was 12. Participant baseline demographics and clinical characteristics are provided in Table 1. Treatment compliance in both groups was good with only a few participants reporting missed doses.
In our evaluation of the rate of change in the number of AK lesions at week 8 compared to baseline, the 5-FU cream 0.5% group showed an 84% reduction in the number of AK lesions versus a 69% reduction in the control group. At week 26, the 5-FU cream 0.5% group showed a 72% reduction in the number of lesions versus 73% in the control group. There was no significant difference between 5-FU cream 0.5% and the comparator cream for either 100% or 75% clearance of AK lesions by the end of the study; however, comparing the AK lesion count from baseline to 8 weeks following the initiation of the study, participants in the 5-FU cream 0.5% group were more likely than the control group to achieve 75% or 100% clearance on the relative risk and risk difference scales (Table 2).
There were no significant differences between the 2 groups for the IGA of improvement at any time point (Table 3). On average, participants in the 5-FU cream 0.5% group experienced more dryness, erosion, fissuring, and redness than the control group but not more ulcerations by the end of week 4 (Table 4). All other QOL measures were statistically comparable between the 2 treatment groups for all time points.
A total of 25 AEs were reported throughout the study but none were considered to be serious. One AE (redness, burning, and itching over the eyebrow) was considered to be related to the study drug. No participants withdrew from the study due to AEs. A total of 12 participants in the 5-FU cream 0.5% group and 10 in the control group reported AEs.
Comment
After a 1-week course of 5-FU cream 0.5% following cryosurgery, a greater reduction in the number of AK lesions for a period of 2 months was noted in the treatment group compared to the control group. These findings are consistent with a similar study from 2006 that used 5-FU cream 0.5% or a vehicle 1 week prior to cryosurgery and then counted the number of AK lesions that remained.8 In the 2006 study, remarkable improvement out to week 26 was noted,8 unlike our study; however, there was insufficient power in our study to demonstrate a continued effect out to week 26.
Both the 2006 study and our current study support the benefit of using a combination treatment to clear AK lesions versus either treatment alone. Of note, these studies also show that combination treatments are equally effective, regardless of the order of treatments, in lowering AK lesion counts compared to cryosurgery alone.
Although participants in the 5-FU cream 0.5% group reported slightly more AEs on average at week 4, the rate of side effects was lower than those reported in a study documenting the side effects of a 4-week course of 5-FU.6 This rate of side effects must be considered in light of the added benefits this combination treatment has demonstrated.
Results of this pilot study suggest that a larger sample size would yield a difference in the study arms for all time periods (weeks 8 and 26). In an effort to maintain exchangeability of the study arms, patients were randomized at baseline treatment, but behaviors of patients in the 6 months following treatment, such as variation in sun exposure or other habits that promote AK lesion development, may have attenuated the results.
Key strengths of this study include no loss to follow-up and high medication adherence rates. The key limitation was the small sample size, which did not demonstrate a statistical advantage of the 5-FU cream 0.5% at 26 weeks; however, our study does show promise for larger future studies in illustrating this difference. A study by Krawtchenko et al9 noted that long-term efficacy of field therapy with 5-FU may ultimately be less than imiquimod cream 5%, suggesting that a possible alteration of the study protocol to compare the efficacy of different forms of field therapy may ultimately achieve better outcomes.
Conclusion
Overall, individuals with AK may benefit from a combination of treatment with cryosurgery and topical 5-FU to resolve lesions for longer periods than with cryosurgery alone. Although prior studies have found statistically significant differences in short-term and long-term treatment efficacy when cryosurgery is combined with an active field therapy versus a placebo vehicle,8,9 the current study aimed to find the best combination of efficacy with the fewest side effects. Therefore, the results of prior literature studies only further the feelings of the authors that with a protocol that looks at a slightly different treatment regimen within the treatment arm, the results can be extremely beneficial to patients. Further studies should be implemented to confirm the longer-term benefits of this combination therapy.
Actinic keratosis (AK) is regarded as a lesion on a continuum of progression to squamous cell carcinoma (SCC).1 Studies have estimated that 44% to 97% of SCCs were associated with AK lesions either in contiguous skin or within the same histologic section and that AK lesions progress to SCCs at a rate of 0.6% at 1 year.2 In 1993-1994 there were 3.7 million reported office visits for AK lesions, while in 2002 alone there were 8.2 million office visits.3,4 As the burden of disease from AKs has increased, so has the associated costs from office-based visits, treatments, and subsequent surveillance.
There are a number of highly effective approaches to AK treatment that are based on several factors such as the number of and extent of the lesions, history of skin cancer, provider practice characteristics (eg, location, appointment availability), patient preferences, cost, and tolerability. Cryosurgery is the most commonly used lesion-directed modality in the treatment of individual AKs based on its effectiveness and relative ease of use. Cryosurgery alone has been shown to have a success rate of 67% on AK lesions.5 Patients often experience erythema, edema, pain, and crusting at treated sites; there also is potential for ulceration, scarring, hypopigmentation, hyperpigmentation, and secondary infection, but these effects are less common. Recurrence may be an indicator of treatment-resistant lesions or new lesions appearing in the field.
A field-directed approach with topical 5-fluorouracil (5-FU) may be preferred in patients with a history of substantial photodamage, AKs that are resistant to cryosurgery, or multiple AKs. Field-directed treatments address multiple AKs simultaneously and treat subclinical lesions. Fluorouracil is a common therapy for AKs that often is implemented by dermatologists due to its efficacy and well-understood mechanism of action. Fluorouracil inhibits thymidylate synthase during DNA synthesis, thereby halting cellular proliferation. 5-Fluorouracil cream 0.5% has been approved for 1-, 2-, and 4-week treatment periods. In one study, resolution of AK lesions was greatest in the 4-week treatment group; however, side effects also were greatest in this group.6 Patients commonly may experience a range of local reactions including erythema, pruritus, erosions, ulcerations, scabbing, crusting, and facial irritation. For patients with substantial photodamage and AKs, a robust response can lead to perceived adverse events (AEs) and considerable downtime, possibly affecting patient satisfaction and treatment compliance.7
Many alternative and combination approaches have been studied to decrease AEs and improve compliance and efficacy in the treatment of AKs. In this study, we examined the efficacy and perceived side effects of cryosurgery and 5-FU cream 0.5% combination therapy in the treatment of AKs.
Methods
Study Design and Participants
This single-blind, single-center, comparator cream–controlled pilot study was parallel designed with a balanced randomization (1:1 frequency). The study protocol and consent form were approved by the Wake Forest University Health Sciences institutional review board (Winston-Salem, North Carolina). Participants were 18 years or older with 8 clinically typical, visible, and discrete AK lesions on the face (forehead and temples) or balding scalp. Typical inclusion and exclusion criteria were observed. No other topical agents or therapies were permitted to be applied to the affected areas at least 4 weeks prior to treatment, depending on the treatment modality.
Assessment
During the screening (baseline) visit, eligible participants provided informed consent, baseline lesion counts and investigator global assessments (IGAs) were performed, and cryosurgery was administered to all visible AK lesions in the study areas. Participants returned at weeks 3, 4, 8, and 26. Three weeks following cryosurgery, participants were randomized according to standard randomization tables into 1 of 2 treatment groups to receive once-daily treatment with either 5-FU cream 0.5% or a moisturizing comparator cream. The cream was applied at bedtime to the affected sites for 1 week. Randomization was investigator blinded, but participants and the study administrators were not blinded. Participants were instructed to record their treatment compliance in daily diary entries, which were reviewed at week 4 using the medication tolerability assessment rating for burning, stinging, and ulceration. Investigator global assessment, IGA of improvement, lesion counts, and quality of life (QOL) survey responses were gathered at weeks 3, 8, and 26. The IGA measured the overall severity of AK disease involvement on a 6-point scale (clear; very severe). The IGA of improvement measured the overall improvement from baseline on a 6-point scale (clear; worse). Adverse events were measured at each visit.
Efficacy End Points
The primary end point was 100% clearance of all AK lesions at the end of the study (week 26) relative to the baseline AK lesion count. Secondary end points included comparisons between the groups for the number of participants with greater than 75% reduction of baseline lesion counts at the end of the study as well as differences at each visit in medication tolerability assessments, QOL measures, IGA improvement scores, and medication adherence based on diary entries at week 4.
Statistical Analysis
An intention-to-treat analysis was performed. The number of participants with 100% or greater than 75% clearance of AK lesions by specified time points were compared using relative risks and risk differences with Poisson regression analysis log and identity link functions, respectively, to obtain robust error variance 95% confidence intervals. Medication tolerability assessment, QOL, and IGA improvement scores were compared between the 2 groups using the Mann-Whitney U test. The significance level was set at α=.05. All analyses were performed using SAS data analysis software.
Results
Sixty age-eligible participants were enrolled in the study with 30 participants in each treatment group. All of the participants completed the 26-week study period and were included in the intention-to-treat analysis. All of the participants were white with a median age of 67 years; the median number of baseline AK lesions was 12. Participant baseline demographics and clinical characteristics are provided in Table 1. Treatment compliance in both groups was good with only a few participants reporting missed doses.
In our evaluation of the rate of change in the number of AK lesions at week 8 compared to baseline, the 5-FU cream 0.5% group showed an 84% reduction in the number of AK lesions versus a 69% reduction in the control group. At week 26, the 5-FU cream 0.5% group showed a 72% reduction in the number of lesions versus 73% in the control group. There was no significant difference between 5-FU cream 0.5% and the comparator cream for either 100% or 75% clearance of AK lesions by the end of the study; however, comparing the AK lesion count from baseline to 8 weeks following the initiation of the study, participants in the 5-FU cream 0.5% group were more likely than the control group to achieve 75% or 100% clearance on the relative risk and risk difference scales (Table 2).
There were no significant differences between the 2 groups for the IGA of improvement at any time point (Table 3). On average, participants in the 5-FU cream 0.5% group experienced more dryness, erosion, fissuring, and redness than the control group but not more ulcerations by the end of week 4 (Table 4). All other QOL measures were statistically comparable between the 2 treatment groups for all time points.
A total of 25 AEs were reported throughout the study but none were considered to be serious. One AE (redness, burning, and itching over the eyebrow) was considered to be related to the study drug. No participants withdrew from the study due to AEs. A total of 12 participants in the 5-FU cream 0.5% group and 10 in the control group reported AEs.
Comment
After a 1-week course of 5-FU cream 0.5% following cryosurgery, a greater reduction in the number of AK lesions for a period of 2 months was noted in the treatment group compared to the control group. These findings are consistent with a similar study from 2006 that used 5-FU cream 0.5% or a vehicle 1 week prior to cryosurgery and then counted the number of AK lesions that remained.8 In the 2006 study, remarkable improvement out to week 26 was noted,8 unlike our study; however, there was insufficient power in our study to demonstrate a continued effect out to week 26.
Both the 2006 study and our current study support the benefit of using a combination treatment to clear AK lesions versus either treatment alone. Of note, these studies also show that combination treatments are equally effective, regardless of the order of treatments, in lowering AK lesion counts compared to cryosurgery alone.
Although participants in the 5-FU cream 0.5% group reported slightly more AEs on average at week 4, the rate of side effects was lower than those reported in a study documenting the side effects of a 4-week course of 5-FU.6 This rate of side effects must be considered in light of the added benefits this combination treatment has demonstrated.
Results of this pilot study suggest that a larger sample size would yield a difference in the study arms for all time periods (weeks 8 and 26). In an effort to maintain exchangeability of the study arms, patients were randomized at baseline treatment, but behaviors of patients in the 6 months following treatment, such as variation in sun exposure or other habits that promote AK lesion development, may have attenuated the results.
Key strengths of this study include no loss to follow-up and high medication adherence rates. The key limitation was the small sample size, which did not demonstrate a statistical advantage of the 5-FU cream 0.5% at 26 weeks; however, our study does show promise for larger future studies in illustrating this difference. A study by Krawtchenko et al9 noted that long-term efficacy of field therapy with 5-FU may ultimately be less than imiquimod cream 5%, suggesting that a possible alteration of the study protocol to compare the efficacy of different forms of field therapy may ultimately achieve better outcomes.
Conclusion
Overall, individuals with AK may benefit from a combination of treatment with cryosurgery and topical 5-FU to resolve lesions for longer periods than with cryosurgery alone. Although prior studies have found statistically significant differences in short-term and long-term treatment efficacy when cryosurgery is combined with an active field therapy versus a placebo vehicle,8,9 the current study aimed to find the best combination of efficacy with the fewest side effects. Therefore, the results of prior literature studies only further the feelings of the authors that with a protocol that looks at a slightly different treatment regimen within the treatment arm, the results can be extremely beneficial to patients. Further studies should be implemented to confirm the longer-term benefits of this combination therapy.
1. Lebwohl M. Actinic keratosis: epidemiology and progression to squamous cell carcinoma. Br J Dermatol. 2003;149(suppl 66):31-33.
2. Criscione VD, Weinstock MA, Naylor MF, et al. Actinic keratoses: natural history and risk of malignant transformation in the Veterans Affairs Topical Tretinoin Chemoprevention Trial. Cancer. 2009;115:2523-2530.
3. Smith ES, Feldman SR, Fleischer AB Jr, et al. Characteristics of office-based visits for skin cancer. dermatologists have more experience than other physicians in managing malignant and premalignant skin conditions. Dermatol Surg. 1998;24:981-985.
4. Shoimer I, Rosen N, Muhn C. Current management of actinic keratoses. Skin Therapy Lett. 2010;15:5-7.
5. Thai KE, Fergin P, Freeman M, et al. A prospective study of the use of cryosurgery for the treatment of actinic keratoses. Int J Dermatol. 2004;43:687-692.
6. Weiss J, Menter A, Hevia O, et al. Effective treatment of actinic keratosis with 0.5% fluorouracil cream for 1, 2, or 4 weeks. Cutis. 2002;70(suppl 2):22-29.
7. Jorizzo JL, Carney PS, Ko WT, et al. Treatment options in the management of actinic keratosis. Cutis. 2004;74 (suppl 6):9-17.
8. Jorizzo J, Weiss J, Vamvakias G. One-week treatment with 0.5% fluorouracil cream prior to cryosurgery in patients with actinic keratoses: a double-blind, vehicle-controlled, long-term study. J Drugs Dermatol. 2006;5:133-139.
9. Krawtchenko N, Roewert-Huber J, Ulrich M, et al. A randomised study of topical 5% imiquimod vs. topical 5-fluorouracil vs. cryosurgery in immunocompetent patients with actinic keratoses: a comparison of clinical and histological outcomes including 1-year follow-up. Br J Dermatol. 2007;157(suppl 2):34-40.
1. Lebwohl M. Actinic keratosis: epidemiology and progression to squamous cell carcinoma. Br J Dermatol. 2003;149(suppl 66):31-33.
2. Criscione VD, Weinstock MA, Naylor MF, et al. Actinic keratoses: natural history and risk of malignant transformation in the Veterans Affairs Topical Tretinoin Chemoprevention Trial. Cancer. 2009;115:2523-2530.
3. Smith ES, Feldman SR, Fleischer AB Jr, et al. Characteristics of office-based visits for skin cancer. dermatologists have more experience than other physicians in managing malignant and premalignant skin conditions. Dermatol Surg. 1998;24:981-985.
4. Shoimer I, Rosen N, Muhn C. Current management of actinic keratoses. Skin Therapy Lett. 2010;15:5-7.
5. Thai KE, Fergin P, Freeman M, et al. A prospective study of the use of cryosurgery for the treatment of actinic keratoses. Int J Dermatol. 2004;43:687-692.
6. Weiss J, Menter A, Hevia O, et al. Effective treatment of actinic keratosis with 0.5% fluorouracil cream for 1, 2, or 4 weeks. Cutis. 2002;70(suppl 2):22-29.
7. Jorizzo JL, Carney PS, Ko WT, et al. Treatment options in the management of actinic keratosis. Cutis. 2004;74 (suppl 6):9-17.
8. Jorizzo J, Weiss J, Vamvakias G. One-week treatment with 0.5% fluorouracil cream prior to cryosurgery in patients with actinic keratoses: a double-blind, vehicle-controlled, long-term study. J Drugs Dermatol. 2006;5:133-139.
9. Krawtchenko N, Roewert-Huber J, Ulrich M, et al. A randomised study of topical 5% imiquimod vs. topical 5-fluorouracil vs. cryosurgery in immunocompetent patients with actinic keratoses: a comparison of clinical and histological outcomes including 1-year follow-up. Br J Dermatol. 2007;157(suppl 2):34-40.
CAPO Aspiration Pneumonia
Pneumonia is a common clinical syndrome with well‐described epidemiology and microbiology. Aspiration pneumonia comprises 5% to 15% of patients with pneumonia acquired outside of the hospital,[1] but is less well characterized despite being a major syndrome of pneumonia in the elderly.[2, 3] Difficulties in studying aspiration pneumonia include the lack of a sensitive and specific marker for aspiration as well as the potential overlap between aspiration pneumonia and other forms of pneumonia.[4, 5, 6] Additionally, clinicians have difficulty distinguishing between aspiration pneumonia, which develops after the aspiration of oropharyngeal contents, and aspiration pneumonitis, wherein inhalation of gastric contents causes inflammation without the subsequent development of bacterial infection.[7, 8] Central to the study of aspiration pneumonia is whether it should exist as its own entity, or if aspiration is really a designation used for pneumonia in an older patient with greater comorbidities. The ability to clearly understand how a clinician diagnoses aspiration pneumonia, and whether that method has face validity with expert definitions may allow for improved future research, improved generalizability of current or past research, and possibly better clinical care.
Several validated mortality prediction models exist for community‐acquired pneumonia (CAP) using a variety of clinical predictors, but their performance in patients with aspiration pneumonia is less well characterized. Most studies validating pneumonia severity scoring systems excluded aspiration pneumonia from their study population.[9, 10, 11] Severity scoring systems for CAP may not accurately predict disease severity in patients with aspiration pneumonia. The CURB‐65[9] (confusion, uremia, respiratory rate, blood pressure, age 65 years) and the eCURB[12] scoring systems are poor predictors of mortality in patients with aspiration pneumonia, perhaps because they do not account for patient comorbidities.[13] The pneumonia severity index (PSI)[10] might predict mortality better than CURB‐65 in the aspiration population due to the inclusion of comorbidities.
Previous studies have demonstrated that patients with aspiration pneumonia are older and have greater disease severity and more comorbidities.[13, 14, 15] These single‐center studies also demonstrated greater mortality, more frequent admission to an intensive care unit (ICU), and longer hospital lengths of stay in patients with aspiration pneumonia. These studies identified aspiration pneumonia by the presence of a risk factor for aspiration[15] or by physician billing codes.[13] In practice, however, the bedside clinician diagnoses a patient as having aspiration pneumonia, but the logic is likely vague and inconsistent. Despite the potential for variability with individual judgment, an aggregate estimation from independent judgments may perform better than individual judgments.[16] Because there is no gold standard for defining aspiration pneumonia, all previous research has been limited to definitions created by investigators. This multicenter study seeks to determine what clinical characteristics lead physicians to diagnose a patient as having aspiration pneumonia, and whether or not the clinician‐derived diagnosis is distinct and clinically useful.
Our objectives were to: (1) identify covariates associated with bedside clinicians diagnosing a pneumonia patient as having aspiration pneumonia; (2) compare aspiration pneumonia and nonaspiration pneumonia in regard to disease severity, patient demographics, comorbidities, and clinical outcomes; and (3) measure the performance of the PSI in aspiration pneumonia versus nonaspiration pneumonia.
PATIENTS AND METHODS
Study Design and Setting
We performed a secondary analysis of the Community‐Acquired Pneumonia Organization (CAPO) database, which contains retrospectively collected data from 71 hospitals in 16 countries between June 2001 and December 2012. In each participating center, primary investigators selected nonconsecutive, adult hospitalized patients diagnosed with CAP. To decrease systematic selection biases, the selection of patients with CAP for enrollment in the trial was based on the date of hospital admission. Each investigator completed a case report form that was transferred via the internet to the CAPO study center at the University of Louisville (Louisville, KY). A sample of the data collection form is available at the study website (
Inclusion and Exclusion Criteria
Patients 18 years of age and satisfying criteria for CAP were included in this study. A diagnosis of CAP required a new pulmonary infiltrate at time of hospitalization, and at least 1 of the following: new or increased cough; leukocytosis; leukopenia, or left shift pattern on white blood cell count; and temperature >37.8C or <35.6 C. We excluded patients with pneumonia attributed to mycobacterial or fungal infection, and patients infected with human immunodeficiency virus, as we believed these types of pneumonia differ fundamentally from typical CAP.
Patient Variables
Patient variables included presence of aspiration pneumonia, laboratory data, comorbidities, and measures of disease severity, including the PSI. The clinician made a clinical diagnosis of the presence or absence of aspiration for each patient by marking a box on the case report form. Outcomes included in‐hospital mortality, hospital length of stay up to 14 days, and time to clinical stability up to 8 days. All variables were obtained directly from the case report form. In accordance with previously published definitions, we defined clinical stability as the day the following criteria were all met: improved clinical signs (improved cough and shortness of breath), lack of fever for >8 hours, improving leukocytosis (decreased at least 10% from the previous day), and tolerating oral intake.[17, 18]
Statistical Analysis
Baseline characteristics of patients with aspiration and nonaspiration CAP were compared using 2 or Fisher exact tests for categorical variables and the Mann‐Whitney U test for continuous variables.
To determine which patient variables were important in the physician diagnosis of aspiration pneumonia, we performed logistic regression with initial covariates comprising the demographic, comorbidity, and disease severity measurements listed in Table 1. We included interactions between cerebrovascular disease and age, nursing home status, and confusion to improve model fit. We centered all variables (including binary indicators) according to the method outlined by Kraemer and Blasey to improve interpretation of the main effects.[19]
| Aspiration Pneumonia, N=451 | Nonaspiration Pneumonia, N=4,734 | P Value | |
|---|---|---|---|
|
|||
| Demographics | |||
| Age, y | 79 (6587) | 69 (5380) | <0.001 |
| % Male | 59% | 60% | 0.58 |
| Nursing home residence | 25% | 5% | <0.001 |
| Recent (30 days) antibiotic use | 21% | 16% | 0.017 |
| Comorbidities | |||
| Cerebrovascular disease | 35% | 14% | <0.001 |
| Chronic obstructive pulmonary disease | 25% | 27% | 0.62 |
| Congestive heart failure | 23% | 19% | 0.027 |
| Diabetes | 18% | 18% | 0.85 |
| Cancer | 12% | 10% | 0.12 |
| Renal disease | 10% | 11% | 0.53 |
| Liver disease | 6% | 5% | 0.29 |
| Disease severity | |||
| Pneumonia severity index | 123 (99153) | 92 (68117) | <0.001 |
| Confusion | 49% | 12% | <0.001 |
| PaO2 <60 mm Hg | 43% | 33% | <0.001 |
| BUN >30 g/dL | 42% | 23% | <0.001 |
| Multilobar pneumonia | 34% | 28% | 0.003 |
| Pleural effusion | 25% | 21% | 0.07 |
| Respiratory rate >30 breaths/minute | 21% | 20% | 0.95 |
| pH <7.35 | 13% | 5% | <0.001 |
| Hematocrit <30% | 11% | 6% | 0.001 |
| Temperature >37.8C or <35.6C | 9% | 7% | 0.30 |
| Systolic blood pressure <90 mm Hg | 8% | 9% | 0.003 |
| Sodium <130 mEq/L | 8% | 6% | 0.08 |
| Heart rate >125 beats/minute | 8% | 5% | 0.71 |
| Glucose >250 mg/dL | 6% | 7% | 0.06 |
| Cavitary lesion | 0% | 0% | 0.67 |
| Clinical outcomes | |||
| In‐hospital mortality | 23% | 9% | <0.001 |
| Intensive care unit admission | 19% | 13% | 0.002 |
| Hospital length of stay, d | 9 (515) | 7 (412) | <0.001 |
| Time to clinical stability, d | 8 (48) | 4 (38) | <0.001 |
To determine if aspiration pneumonia had worse clinical outcomes compared to nonaspiration pneumonia, multiple methods were used. To compare the differences between the 2 groups with respect to time to clinical stability and length of hospital stay, we constructed Kaplan‐Meier survival curves and Cox proportional hazards regression models. The log‐rank test was used to determine statistical differences between the Kaplan‐Meier survival curves. To compare the impact of aspiration on mortality in patients with CAP, we conducted a propensity scorematched analysis. We chose propensity score matching over traditional logistic regression to balance variables among groups and to avoid the potential for overfit and multicollinearity. We considered a variable balanced after matching if its standardized difference was <10. All variables in the propensity scorematched analysis were balanced.
Although our dataset contained minimal missing data, we imputed any missing values to maintain the full study population in the creation of the propensity score. Missing data were imputed using the aregImpute function of the hmisc package of R (The R Foundation for Statistical Computing, Vienna, Austria).[20, 21] We built the propensity score model using a variable selection algorithm described by Bursac et al.[22] Our model included variables for region (United States/Canada, Europe, Asia/Africa or Latin America) and the variables listed in Table 1, with the exception of the PSI and the 4 clinical outcomes. Given that previous analyses accounting for clustering by physician did not substantially affect our results,[23] our model did not include physician‐level variables and did not account for the clustering effects of physicians. Using the propensity scores generated from this model, we matched a case of aspiration CAP with a case of nonaspiration CAP.[24] We then constructed a general linear model using the matched dataset to obtain the magnitude of effect of aspiration on mortality.
We used receiver operating characteristic curves to define the diagnostic accuracy of the pneumonia severity index for the prediction of mortality among patients with aspiration pneumonia and those with nonaspiration pneumonia. SAS version 9.3 (SAS Institute, Cary, NC) and R version 2.15.3 (The R Foundation for Statistical Computing) were used for all analyses. P values of 0.05 were considered statistically significant in all analyses.
RESULTS
Our initial query, after exclusion criteria, yielded a study population of 5185 patients (Figure 1). We compared 451 patients diagnosed with aspiration pneumonia to 4734 with CAP (Figure 1). Patient characteristics are summarized in Table 1. Patients with aspiration pneumonia were older, more likely to live in a nursing home, had greater disease severity, and were more likely to be admitted to an ICU. Patients with aspiration pneumonia had longer adjusted hospital lengths of stay and took more days to achieve clinical stability than patients with nonaspiration pneumonia (Figure 2). After adjusting for all variables in Table 1, the Cox proportional hazards models demonstrated that aspiration pneumonia was associated with ongoing hospitalization (hazard ratio [HR] for discharge: 0.77, 95% confidence interval [CI]: 0.65‐0.91, P=0.002) and clinical instability (HR for attaining clinical stability: 0.72, 95% CI: 0.61‐0.84, P<0.001). Patients with aspiration pneumonia presented with greater disease severity than those with nonaspiration pneumonia. Although there was no difference between groups in regard to temperature, respiratory rate, hyponatremia, or presence of pleural effusions or cavitary lesions, all other measured indices of disease severity were worse in patients with aspiration pneumonia. Patients with aspiration pneumonia were more likely to have cerebrovascular disease than those with nonaspiration pneumonia. Aspiration pneumonia patients also had increased prevalence of congestive heart failure. There was no appreciable difference between groups among other measured comorbidities.
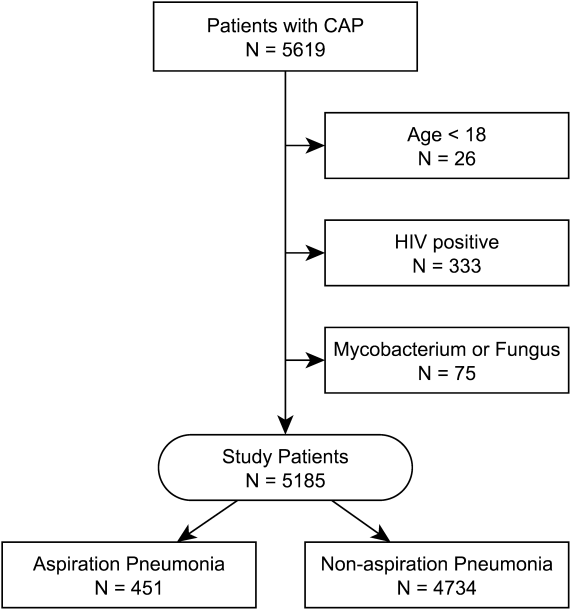
The patient characteristics most associated with a physician diagnosis of aspiration pneumonia, identified using logistic regression, were confusion, residence in nursing home, and presence of cerebrovascular disease (odds ratio [OR]: of 4.4, 2.9, and 2.3, respectively), whereas renal disease was associated with decreased physician diagnosis of aspiration pneumonia over nonaspiration pneumonia (OR: 0.58) (Table 2).
| Covariate | Odds Ratio | 95% Confidence Intervals | P Value |
|---|---|---|---|
|
|||
| Demographics | |||
| Age, y | 1.00 | 0.991.01 | 0.948 |
| Male | 1.20 | 0.941.54 | 0.148 |
| Nursing home residence | 2.93 | 2.134.00 | <0.001 |
| Comorbidities | |||
| Cerebrovascular disease | 2.26 | 1.533.32 | <0.001 |
| Renal disease | 0.58 | 0.390.85 | 0.006 |
| Disease severity | |||
| Confusion | 4.41 | 3.405.72 | <0.001 |
| Hematocrit <30% | 1.59 | 1.062.33 | 0.020 |
| pH <7.35 | 1.67 | 1.102.47 | 0.013 |
| Temperature >37.8C or <35.6C | 1.60 | 1.072.35 | 0.019 |
| Multilobar pneumonia | 1.29 | 1.001.65 | 0.047 |
| Interaction terms | |||
| Age * cerebrovascular disease | 0.98 | 0.960.99 | 0.011 |
| Nursing home * cerebrovascular disease | 0.51 | 0.270.96 | 0.037 |
| Confusion * cerebrovascular disease | 0.70 | 0.421.17 | 0.175 |
Observed in‐patient mortality of aspiration pneumonia was 23%. This mortality was considerably higher than a mean PSI score of 123 would predict (class IV risk group, with expected 30‐day mortality of 8%9%[25]). The PSI score's ability to predict inpatient mortality in patients with aspiration pneumonia was moderate, with an area under the curve (AUC) of 0.71. This was similar to its performance in patients with nonaspiration pneumonia (AUC of 0.75) (Figure 3). These values are lower than the AUC of 0.81 for the PSI in predicting mortality derived from a meta‐analysis of 31 other studies.[26]
Our regression model after propensity score matching demonstrated that aspiration pneumonia independently confers a 2.3‐fold increased odds for inpatient mortality (95% CI: 1.56‐3.45, P<0.001).
DISCUSSION
Pneumonia patients with confusion, nursing home residence, or cerebrovascular disease are more likely to be diagnosed with aspiration pneumonia by clinicians. Although this is unsurprising, it is notable that these patients are more than twice as likely to die in the inpatient setting, even after accounting for age, comorbidities, and disease severity. These findings are similar to three previously published studies comparing aspiration and nonaspiration pneumonia at single institutions, albeit using different aspiration pneumonia definitions.[13, 14, 15] This study is the first large, multicenter, multinational study to demonstrate these findings.
Central to the interpretation of our results is the method of diagnosing aspiration versus nonaspiration. A bottom‐up method that relies on a clinician to check a box for aspiration may appear poorly reproducible. Because there is no diagnostic gold standard, clinicians may use different criteria to diagnose aspiration, creating potential for idiosyncratic noise. The strength of the wisdom of the crowd method used in this study is that an aggregate estimation from independent judgments may reduce the noise from individual judgments.[16] Although clinicians may vary in why they diagnose a particular patient as having aspiration pneumonia, it appears that the overwhelming reason for diagnosing a patient as having aspiration pneumonia is the presence of confusion, followed by previous nursing home residence or cerebrovascular disease. This finding has some face validity when compared with studies using an investigator definition, as altered mental status, chronic debility, and cerebrovascular disease are either prominent features of the definition of aspiration pneumonia[8] or frequently observed in patients with aspiration pneumonia.[13, 15] The distribution of cerebrovascular disease among our study's aspiration and nonaspiration pneumonia patients was similar to studies that used formal criteria in their definitions.[13, 15] Although nursing home residence was more likely in aspiration pneumonia patients, the majority of aspiration pneumonia patients were residing in the community, suggesting that aspiration is not simply a surrogate for healthcare‐associated pneumonia. Although patients with aspiration pneumonia are typically older than their nonaspiration counterparts, it appears that age is not a key determinant in the diagnosis of aspiration. With aspiration pneumonia, confusion, nursing home residence, and the presence of cerebrovascular disease are the greatest contributors in the clinical diagnosis, more than age.
Our data demonstrate that aspiration pneumonia confers increased odds for mortality, even after adjustment for age, disease severity, and comorbidities. These data suggest that aspiration pneumonia is a distinct entity from nonaspiration pneumonia, and that this disease is worse than nonaspiration CAP. If aspiration pneumonia is distinct from nonaspiration pneumonia, some unrecognized host factor other than age, disease severity, or the captured comorbidities decreases survival in aspiration pneumonia patients. However, it is also possible that aspiration pneumonia is merely a clinical designation for one end of the pneumonia spectrum, and we and others have failed to completely account for all measures of disease severity or all measures of comorbidities. Examples of unmeasured comorbidities would include presence of oropharyngeal dysphagia, which is not assessed in the database but could have a significant effect on clinical diagnosis. Unmeasured covariates can include measures beyond that of disease severity or comorbidity, such as the presence of a do not resuscitate (DNR) order, which could have a significant confounding effect on the observed association. A previous, single‐center study demonstrated that increased 30‐day mortality in aspiration pneumonia was mostly attributable to greater disease severity and comorbidities, although aspiration pneumonia independently conferred greater risk for adverse long‐term outcomes.[15] We propose that aspiration pneumonia represents a clinically distinct entity from nonaspiration pneumonia. Patients with chronic aspiration are often chronically malnourished and may have different oral flora than patients without chronic aspiration.[27, 28] Chronic aspiration has been associated with granulomatous reaction, organizing pneumonia, diffuse alveolar damage, and chronic bronchiolitis.[29] Chronic aspiration may elicit changes in the host physiology, and may render the host more susceptible to the development of secondary bacterial infection with morbid consequences.
The ability of the PSI to predict inpatient mortality was moderate (AUC only 0.7), with no significant additional discrimination between the aspiration and nonaspiration pneumonia groups. Although the PSI had moderate ability to predict inpatient mortality, the observed mortality was considerably higher than predicted. It is possible that the PSI incompletely captures clinically relevant comorbidities (eg, malnutrition). Further study to improve mortality prediction of aspiration pneumonia patients could employ sensitivity analysis to determine optimal thresholds and weighting of the PSI components.
Patients with aspiration pneumonia had longer hospital lengths of stay and took longer to achieve clinical stability than their nonaspiration counterparts. Time to clinical stability has been associated with increased posthospitalization mortality and is associated with time to switch from intravenous to oral antibiotics.[17] Although some component of hospital length‐of‐stay is subject to local practice patterns, time to clinical stability has explicit criteria for clinical improvement and failure, and therefore is less likely to be affected by local practice patterns.
We noted a relatively high (16%21%) incidence of prior antibiotic use among patients in this database. Analysis of antibiotic prescription patterns was limited, given the several different countries from which the database draws its cases. Although we used accepted criteria to define CAP cases, it is possible that this population may have a higher rate of resistant or uncommon pathogens than other studies of CAP that have populations with lower incidence of prior antibiotic use. Although not assessed, we suspect a significant component of the prior antibiotic use represented outpatient pneumonia treatment during the few days prior to visiting the hospital.
This study has several limitations, of which the most important may be that we used clinical determination for defining presence of aspiration pneumonia. This method is susceptible to the subjective perceptions of the treating clinician. We did not account for the effect of individual physicians in our model, although we did adjust for regional differences. The retrospective identification of patients allows for the possibility of selection bias, and therefore we have not attempted to make inferences regarding the relative incidence of pneumonia, nor did we adjust for temporal trends in diagnosis. The ratio of aspiration pneumonia patients to nonaspiration pneumonia patients may not necessarily reflect that observed in reality. Microbiologic and antibiotic data were unavailable for analysis. This study cannot inform on nonhospitalized patients with aspiration pneumonia, as only hospitalized patients were enrolled. The database identified cases of pneumonia, so it is possible for a patient to enter into the database more than once. Detection of mortality was limited to the inpatient setting rather than a set interval of 30 days. Inpatient mortality depends on length‐of‐stay patterns that may bias the mortality endpoint.[30] Also not assessed was the presence of a DNR order. It is possible that an older patient with greater comorbidities and disease severity may have care intentionally limited or withdrawn early by the family or clinicians.
Strengths of the study include its size and its multicenter, multinational population. The CAPO database is a large and well‐described population of patients with CAP.[17, 31] These attributes, as well as the clinician‐determined diagnosis, increase the generalizability of the study compared to a single‐center, single‐country study that employs investigator‐defined criteria.
CONCLUSION
Pneumonia patients with confusion, who are nursing home residence, and have cerebrovascular disease are more likely to be diagnosed with aspiration pneumonia by clinicians. Our clinician‐diagnosed cohort appears similar to those derived using an investigator definition. Patients with aspiration pneumonia are older, and have greater disease severity and more comorbidities than patients with nonaspiration pneumonia. They have greater mortality than their PSI score class would predict. Even after accounting for age, disease severity, and comorbidities, the presence of aspiration pneumonia independently conferred a greater than 2‐fold increase in inpatient mortality. These findings together suggest that aspiration pneumonia should be considered a distinct entity from typical pneumonia, and that additional research should be done in this field.
ACKNOWLEDGMENTS
Disclosures: M.J.L. contributed to the study design, data analysis, statistical analysis, and writing of the manuscript. P.P. contributed to the study design and revision of the manuscript for important intellectual content. T.W. and E.W. contributed to the study design, statistical analysis, and revision of the manuscript for important intellectual content. J.A.R. and N.C.D. contributed to the study design and revision of the manuscript for important intellectual content. All authors read and approved the final manuscript. M.L. takes responsibility for the integrity of the work as a whole, from inception to published article. This investigation was partly supported with funding from the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health (grant 8UL1TR000105 [formerly UL1RR025764]). The authors report no conflicts of interest.
- , , , et al. Severe community‐acquired pneumonia. Epidemiology and prognostic factors. Am Rev Respir Dis. 1991;144(2):312–318.
- , , . Risk factors for pneumonia in the elderly. Am J Med. 1994;96(4):313–320.
- , . Aspiration pneumonia and dysphagia in the elderly. Chest. 2003;124(1):328–336.
- , , . Pneumonia versus aspiration pneumonitis in nursing home residents: diagnosis and management. J Am Geriatr Soc. 2003;51(1):17–23.
- . Aspiration pneumonia: mixing apples with oranges and tangerines. Crit Care Med. 2004;32(5):1236; author reply 1236–1237.
- , , , , . Epidemiology and impact of aspiration pneumonia in patients undergoing surgery in Maryland, 1999–2000. Crit Care Med. 2003;31(7):1930–1937.
- . Aspiration syndromes: aspiration pneumonia and pneumonitis. Hosp Pract (Minneap). 2010;38(1):35–42.
- . Aspiration pneumonitis and aspiration pneumonia. N Engl J Med. 2001;344(9):665–671.
- , , , et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58(5):377–382.
- , , , et al. Comparison of a disease‐specific and a generic severity of illness measure for patients with community‐acquired pneumonia. J Gen Intern Med. 1995;10(7):359–368.
- , , , et al. Development and validation of a clinical prediction rule for severe community‐acquired pneumonia. Am J Respir Crit Care Med. 2006;174(11):1249–1256.
- , , , et al. CURB‐65 pneumonia severity assessment adapted for electronic decision support. Chest. 2011;140(1):156–163.
- , , , . Mortality, morbidity, and disease severity of patients with aspiration pneumonia. J Hosp Med. 2013;8(2):83–90.
- , , , , . Pneumonia Severity Index (PSI), CURB‐65, and mortality in hospitalized elderly patients with aspiration pneumonia [in German]. Z Gerontol Geriatr. 2011;44(4):229–234.
- , , , , . Risk factors for aspiration in community‐acquired pneumonia: analysis of a hospitalized UK cohort. Am J Med. 2013;126(11):995–1001.
- , , , . The wisdom of the crowd in combinatorial problems. Cogn Sci. 2012;36(3):452–470.
- , , , et al. Association between time to clinical stability and outcomes after discharge in hospitalized patients with community‐acquired pneumonia. Chest. 2011;140(2):482–488.
- . Clinical stability and switch therapy in hospitalised patients with community‐acquired pneumonia: are we there yet? Eur Respir J. 2013;41(1):5–6.
- , . Centring in regression analyses: a strategy to prevent errors in statistical inference. Int J Methods Psychiatr Res. 2004;13(3):141–151.
- . Hmisc: Harrell miscellaneous. Available at: http://CRAN.R‐project.org/package=Hmisc. Published Sept 12, 2014. Last accessed Oct 27, 2014.
- , . Multiple imputation for the fatal accident reporting system. J R Stat Soc Ser C Appl Stat. 1991;40(1):13–29.
- , , , . Purposeful selection of variables in logistic regression. Source Code Biol Med. 2008;3:17.
- , , , , ; CAPO authors. Mortality differences among hospitalized patients with community‐acquired pneumonia in three world regions: results from the Community‐Acquired Pneumonia Organization (CAPO) International Cohort Study. Respir Med. 2013;107(7):1101–1111.
- . Reducing bias in a propensity score matched‐pair sample using greedy matching techniques. In: Proceedings of the 26th Annual SAS Users Group International Conference. Cary, NC: SAS Institute Inc.; 2001:214–226. Available at: http://www2.sas.com/proceedings/sugi26/p214–26.pdf. Last accessed Oct 27, 2014.
- , , , et al. A prediction rule to identify low‐risk patients with community‐acquired pneumonia. N Engl J Med. 1997;336(4):243–250.
- , , , et al. Severity assessment tools for predicting mortality in hospitalised patients with community‐acquired pneumonia. Systematic review and meta‐analysis. Thorax. 2010;65(10):878–883.
- , , , , , . Prevalence and prognostic implications of dysphagia in elderly patients with pneumonia. Age Ageing. 2010;39(1):39–45.
- , . The association between oral microorgansims and aspiration pneumonia in the institutionalized elderly: review and recommendations. Dysphagia. 2010;25(4):307–322.
- , . Histopathology of aspiration pneumonia not associated with food or other particulate matter: a clinicopathologic study of 10 cases diagnosed on biopsy. Am J Surg Pathol. 2011;35(3):426–431.
- , , , , , . Interpreting hospital mortality data. The role of clinical risk adjustment. JAMA. 1988;260(24):3611–3616.
- , , , . Hospitalization for community‐acquired pneumonia: the pneumonia severity index vs clinical judgment. Chest. 2003;124(1):121–124.
Pneumonia is a common clinical syndrome with well‐described epidemiology and microbiology. Aspiration pneumonia comprises 5% to 15% of patients with pneumonia acquired outside of the hospital,[1] but is less well characterized despite being a major syndrome of pneumonia in the elderly.[2, 3] Difficulties in studying aspiration pneumonia include the lack of a sensitive and specific marker for aspiration as well as the potential overlap between aspiration pneumonia and other forms of pneumonia.[4, 5, 6] Additionally, clinicians have difficulty distinguishing between aspiration pneumonia, which develops after the aspiration of oropharyngeal contents, and aspiration pneumonitis, wherein inhalation of gastric contents causes inflammation without the subsequent development of bacterial infection.[7, 8] Central to the study of aspiration pneumonia is whether it should exist as its own entity, or if aspiration is really a designation used for pneumonia in an older patient with greater comorbidities. The ability to clearly understand how a clinician diagnoses aspiration pneumonia, and whether that method has face validity with expert definitions may allow for improved future research, improved generalizability of current or past research, and possibly better clinical care.
Several validated mortality prediction models exist for community‐acquired pneumonia (CAP) using a variety of clinical predictors, but their performance in patients with aspiration pneumonia is less well characterized. Most studies validating pneumonia severity scoring systems excluded aspiration pneumonia from their study population.[9, 10, 11] Severity scoring systems for CAP may not accurately predict disease severity in patients with aspiration pneumonia. The CURB‐65[9] (confusion, uremia, respiratory rate, blood pressure, age 65 years) and the eCURB[12] scoring systems are poor predictors of mortality in patients with aspiration pneumonia, perhaps because they do not account for patient comorbidities.[13] The pneumonia severity index (PSI)[10] might predict mortality better than CURB‐65 in the aspiration population due to the inclusion of comorbidities.
Previous studies have demonstrated that patients with aspiration pneumonia are older and have greater disease severity and more comorbidities.[13, 14, 15] These single‐center studies also demonstrated greater mortality, more frequent admission to an intensive care unit (ICU), and longer hospital lengths of stay in patients with aspiration pneumonia. These studies identified aspiration pneumonia by the presence of a risk factor for aspiration[15] or by physician billing codes.[13] In practice, however, the bedside clinician diagnoses a patient as having aspiration pneumonia, but the logic is likely vague and inconsistent. Despite the potential for variability with individual judgment, an aggregate estimation from independent judgments may perform better than individual judgments.[16] Because there is no gold standard for defining aspiration pneumonia, all previous research has been limited to definitions created by investigators. This multicenter study seeks to determine what clinical characteristics lead physicians to diagnose a patient as having aspiration pneumonia, and whether or not the clinician‐derived diagnosis is distinct and clinically useful.
Our objectives were to: (1) identify covariates associated with bedside clinicians diagnosing a pneumonia patient as having aspiration pneumonia; (2) compare aspiration pneumonia and nonaspiration pneumonia in regard to disease severity, patient demographics, comorbidities, and clinical outcomes; and (3) measure the performance of the PSI in aspiration pneumonia versus nonaspiration pneumonia.
PATIENTS AND METHODS
Study Design and Setting
We performed a secondary analysis of the Community‐Acquired Pneumonia Organization (CAPO) database, which contains retrospectively collected data from 71 hospitals in 16 countries between June 2001 and December 2012. In each participating center, primary investigators selected nonconsecutive, adult hospitalized patients diagnosed with CAP. To decrease systematic selection biases, the selection of patients with CAP for enrollment in the trial was based on the date of hospital admission. Each investigator completed a case report form that was transferred via the internet to the CAPO study center at the University of Louisville (Louisville, KY). A sample of the data collection form is available at the study website (
Inclusion and Exclusion Criteria
Patients 18 years of age and satisfying criteria for CAP were included in this study. A diagnosis of CAP required a new pulmonary infiltrate at time of hospitalization, and at least 1 of the following: new or increased cough; leukocytosis; leukopenia, or left shift pattern on white blood cell count; and temperature >37.8C or <35.6 C. We excluded patients with pneumonia attributed to mycobacterial or fungal infection, and patients infected with human immunodeficiency virus, as we believed these types of pneumonia differ fundamentally from typical CAP.
Patient Variables
Patient variables included presence of aspiration pneumonia, laboratory data, comorbidities, and measures of disease severity, including the PSI. The clinician made a clinical diagnosis of the presence or absence of aspiration for each patient by marking a box on the case report form. Outcomes included in‐hospital mortality, hospital length of stay up to 14 days, and time to clinical stability up to 8 days. All variables were obtained directly from the case report form. In accordance with previously published definitions, we defined clinical stability as the day the following criteria were all met: improved clinical signs (improved cough and shortness of breath), lack of fever for >8 hours, improving leukocytosis (decreased at least 10% from the previous day), and tolerating oral intake.[17, 18]
Statistical Analysis
Baseline characteristics of patients with aspiration and nonaspiration CAP were compared using 2 or Fisher exact tests for categorical variables and the Mann‐Whitney U test for continuous variables.
To determine which patient variables were important in the physician diagnosis of aspiration pneumonia, we performed logistic regression with initial covariates comprising the demographic, comorbidity, and disease severity measurements listed in Table 1. We included interactions between cerebrovascular disease and age, nursing home status, and confusion to improve model fit. We centered all variables (including binary indicators) according to the method outlined by Kraemer and Blasey to improve interpretation of the main effects.[19]
| Aspiration Pneumonia, N=451 | Nonaspiration Pneumonia, N=4,734 | P Value | |
|---|---|---|---|
|
|||
| Demographics | |||
| Age, y | 79 (6587) | 69 (5380) | <0.001 |
| % Male | 59% | 60% | 0.58 |
| Nursing home residence | 25% | 5% | <0.001 |
| Recent (30 days) antibiotic use | 21% | 16% | 0.017 |
| Comorbidities | |||
| Cerebrovascular disease | 35% | 14% | <0.001 |
| Chronic obstructive pulmonary disease | 25% | 27% | 0.62 |
| Congestive heart failure | 23% | 19% | 0.027 |
| Diabetes | 18% | 18% | 0.85 |
| Cancer | 12% | 10% | 0.12 |
| Renal disease | 10% | 11% | 0.53 |
| Liver disease | 6% | 5% | 0.29 |
| Disease severity | |||
| Pneumonia severity index | 123 (99153) | 92 (68117) | <0.001 |
| Confusion | 49% | 12% | <0.001 |
| PaO2 <60 mm Hg | 43% | 33% | <0.001 |
| BUN >30 g/dL | 42% | 23% | <0.001 |
| Multilobar pneumonia | 34% | 28% | 0.003 |
| Pleural effusion | 25% | 21% | 0.07 |
| Respiratory rate >30 breaths/minute | 21% | 20% | 0.95 |
| pH <7.35 | 13% | 5% | <0.001 |
| Hematocrit <30% | 11% | 6% | 0.001 |
| Temperature >37.8C or <35.6C | 9% | 7% | 0.30 |
| Systolic blood pressure <90 mm Hg | 8% | 9% | 0.003 |
| Sodium <130 mEq/L | 8% | 6% | 0.08 |
| Heart rate >125 beats/minute | 8% | 5% | 0.71 |
| Glucose >250 mg/dL | 6% | 7% | 0.06 |
| Cavitary lesion | 0% | 0% | 0.67 |
| Clinical outcomes | |||
| In‐hospital mortality | 23% | 9% | <0.001 |
| Intensive care unit admission | 19% | 13% | 0.002 |
| Hospital length of stay, d | 9 (515) | 7 (412) | <0.001 |
| Time to clinical stability, d | 8 (48) | 4 (38) | <0.001 |
To determine if aspiration pneumonia had worse clinical outcomes compared to nonaspiration pneumonia, multiple methods were used. To compare the differences between the 2 groups with respect to time to clinical stability and length of hospital stay, we constructed Kaplan‐Meier survival curves and Cox proportional hazards regression models. The log‐rank test was used to determine statistical differences between the Kaplan‐Meier survival curves. To compare the impact of aspiration on mortality in patients with CAP, we conducted a propensity scorematched analysis. We chose propensity score matching over traditional logistic regression to balance variables among groups and to avoid the potential for overfit and multicollinearity. We considered a variable balanced after matching if its standardized difference was <10. All variables in the propensity scorematched analysis were balanced.
Although our dataset contained minimal missing data, we imputed any missing values to maintain the full study population in the creation of the propensity score. Missing data were imputed using the aregImpute function of the hmisc package of R (The R Foundation for Statistical Computing, Vienna, Austria).[20, 21] We built the propensity score model using a variable selection algorithm described by Bursac et al.[22] Our model included variables for region (United States/Canada, Europe, Asia/Africa or Latin America) and the variables listed in Table 1, with the exception of the PSI and the 4 clinical outcomes. Given that previous analyses accounting for clustering by physician did not substantially affect our results,[23] our model did not include physician‐level variables and did not account for the clustering effects of physicians. Using the propensity scores generated from this model, we matched a case of aspiration CAP with a case of nonaspiration CAP.[24] We then constructed a general linear model using the matched dataset to obtain the magnitude of effect of aspiration on mortality.
We used receiver operating characteristic curves to define the diagnostic accuracy of the pneumonia severity index for the prediction of mortality among patients with aspiration pneumonia and those with nonaspiration pneumonia. SAS version 9.3 (SAS Institute, Cary, NC) and R version 2.15.3 (The R Foundation for Statistical Computing) were used for all analyses. P values of 0.05 were considered statistically significant in all analyses.
RESULTS
Our initial query, after exclusion criteria, yielded a study population of 5185 patients (Figure 1). We compared 451 patients diagnosed with aspiration pneumonia to 4734 with CAP (Figure 1). Patient characteristics are summarized in Table 1. Patients with aspiration pneumonia were older, more likely to live in a nursing home, had greater disease severity, and were more likely to be admitted to an ICU. Patients with aspiration pneumonia had longer adjusted hospital lengths of stay and took more days to achieve clinical stability than patients with nonaspiration pneumonia (Figure 2). After adjusting for all variables in Table 1, the Cox proportional hazards models demonstrated that aspiration pneumonia was associated with ongoing hospitalization (hazard ratio [HR] for discharge: 0.77, 95% confidence interval [CI]: 0.65‐0.91, P=0.002) and clinical instability (HR for attaining clinical stability: 0.72, 95% CI: 0.61‐0.84, P<0.001). Patients with aspiration pneumonia presented with greater disease severity than those with nonaspiration pneumonia. Although there was no difference between groups in regard to temperature, respiratory rate, hyponatremia, or presence of pleural effusions or cavitary lesions, all other measured indices of disease severity were worse in patients with aspiration pneumonia. Patients with aspiration pneumonia were more likely to have cerebrovascular disease than those with nonaspiration pneumonia. Aspiration pneumonia patients also had increased prevalence of congestive heart failure. There was no appreciable difference between groups among other measured comorbidities.


The patient characteristics most associated with a physician diagnosis of aspiration pneumonia, identified using logistic regression, were confusion, residence in nursing home, and presence of cerebrovascular disease (odds ratio [OR]: of 4.4, 2.9, and 2.3, respectively), whereas renal disease was associated with decreased physician diagnosis of aspiration pneumonia over nonaspiration pneumonia (OR: 0.58) (Table 2).
| Covariate | Odds Ratio | 95% Confidence Intervals | P Value |
|---|---|---|---|
|
|||
| Demographics | |||
| Age, y | 1.00 | 0.991.01 | 0.948 |
| Male | 1.20 | 0.941.54 | 0.148 |
| Nursing home residence | 2.93 | 2.134.00 | <0.001 |
| Comorbidities | |||
| Cerebrovascular disease | 2.26 | 1.533.32 | <0.001 |
| Renal disease | 0.58 | 0.390.85 | 0.006 |
| Disease severity | |||
| Confusion | 4.41 | 3.405.72 | <0.001 |
| Hematocrit <30% | 1.59 | 1.062.33 | 0.020 |
| pH <7.35 | 1.67 | 1.102.47 | 0.013 |
| Temperature >37.8C or <35.6C | 1.60 | 1.072.35 | 0.019 |
| Multilobar pneumonia | 1.29 | 1.001.65 | 0.047 |
| Interaction terms | |||
| Age * cerebrovascular disease | 0.98 | 0.960.99 | 0.011 |
| Nursing home * cerebrovascular disease | 0.51 | 0.270.96 | 0.037 |
| Confusion * cerebrovascular disease | 0.70 | 0.421.17 | 0.175 |
Observed in‐patient mortality of aspiration pneumonia was 23%. This mortality was considerably higher than a mean PSI score of 123 would predict (class IV risk group, with expected 30‐day mortality of 8%9%[25]). The PSI score's ability to predict inpatient mortality in patients with aspiration pneumonia was moderate, with an area under the curve (AUC) of 0.71. This was similar to its performance in patients with nonaspiration pneumonia (AUC of 0.75) (Figure 3). These values are lower than the AUC of 0.81 for the PSI in predicting mortality derived from a meta‐analysis of 31 other studies.[26]

Our regression model after propensity score matching demonstrated that aspiration pneumonia independently confers a 2.3‐fold increased odds for inpatient mortality (95% CI: 1.56‐3.45, P<0.001).
DISCUSSION
Pneumonia patients with confusion, nursing home residence, or cerebrovascular disease are more likely to be diagnosed with aspiration pneumonia by clinicians. Although this is unsurprising, it is notable that these patients are more than twice as likely to die in the inpatient setting, even after accounting for age, comorbidities, and disease severity. These findings are similar to three previously published studies comparing aspiration and nonaspiration pneumonia at single institutions, albeit using different aspiration pneumonia definitions.[13, 14, 15] This study is the first large, multicenter, multinational study to demonstrate these findings.
Central to the interpretation of our results is the method of diagnosing aspiration versus nonaspiration. A bottom‐up method that relies on a clinician to check a box for aspiration may appear poorly reproducible. Because there is no diagnostic gold standard, clinicians may use different criteria to diagnose aspiration, creating potential for idiosyncratic noise. The strength of the wisdom of the crowd method used in this study is that an aggregate estimation from independent judgments may reduce the noise from individual judgments.[16] Although clinicians may vary in why they diagnose a particular patient as having aspiration pneumonia, it appears that the overwhelming reason for diagnosing a patient as having aspiration pneumonia is the presence of confusion, followed by previous nursing home residence or cerebrovascular disease. This finding has some face validity when compared with studies using an investigator definition, as altered mental status, chronic debility, and cerebrovascular disease are either prominent features of the definition of aspiration pneumonia[8] or frequently observed in patients with aspiration pneumonia.[13, 15] The distribution of cerebrovascular disease among our study's aspiration and nonaspiration pneumonia patients was similar to studies that used formal criteria in their definitions.[13, 15] Although nursing home residence was more likely in aspiration pneumonia patients, the majority of aspiration pneumonia patients were residing in the community, suggesting that aspiration is not simply a surrogate for healthcare‐associated pneumonia. Although patients with aspiration pneumonia are typically older than their nonaspiration counterparts, it appears that age is not a key determinant in the diagnosis of aspiration. With aspiration pneumonia, confusion, nursing home residence, and the presence of cerebrovascular disease are the greatest contributors in the clinical diagnosis, more than age.
Our data demonstrate that aspiration pneumonia confers increased odds for mortality, even after adjustment for age, disease severity, and comorbidities. These data suggest that aspiration pneumonia is a distinct entity from nonaspiration pneumonia, and that this disease is worse than nonaspiration CAP. If aspiration pneumonia is distinct from nonaspiration pneumonia, some unrecognized host factor other than age, disease severity, or the captured comorbidities decreases survival in aspiration pneumonia patients. However, it is also possible that aspiration pneumonia is merely a clinical designation for one end of the pneumonia spectrum, and we and others have failed to completely account for all measures of disease severity or all measures of comorbidities. Examples of unmeasured comorbidities would include presence of oropharyngeal dysphagia, which is not assessed in the database but could have a significant effect on clinical diagnosis. Unmeasured covariates can include measures beyond that of disease severity or comorbidity, such as the presence of a do not resuscitate (DNR) order, which could have a significant confounding effect on the observed association. A previous, single‐center study demonstrated that increased 30‐day mortality in aspiration pneumonia was mostly attributable to greater disease severity and comorbidities, although aspiration pneumonia independently conferred greater risk for adverse long‐term outcomes.[15] We propose that aspiration pneumonia represents a clinically distinct entity from nonaspiration pneumonia. Patients with chronic aspiration are often chronically malnourished and may have different oral flora than patients without chronic aspiration.[27, 28] Chronic aspiration has been associated with granulomatous reaction, organizing pneumonia, diffuse alveolar damage, and chronic bronchiolitis.[29] Chronic aspiration may elicit changes in the host physiology, and may render the host more susceptible to the development of secondary bacterial infection with morbid consequences.
The ability of the PSI to predict inpatient mortality was moderate (AUC only 0.7), with no significant additional discrimination between the aspiration and nonaspiration pneumonia groups. Although the PSI had moderate ability to predict inpatient mortality, the observed mortality was considerably higher than predicted. It is possible that the PSI incompletely captures clinically relevant comorbidities (eg, malnutrition). Further study to improve mortality prediction of aspiration pneumonia patients could employ sensitivity analysis to determine optimal thresholds and weighting of the PSI components.
Patients with aspiration pneumonia had longer hospital lengths of stay and took longer to achieve clinical stability than their nonaspiration counterparts. Time to clinical stability has been associated with increased posthospitalization mortality and is associated with time to switch from intravenous to oral antibiotics.[17] Although some component of hospital length‐of‐stay is subject to local practice patterns, time to clinical stability has explicit criteria for clinical improvement and failure, and therefore is less likely to be affected by local practice patterns.
We noted a relatively high (16%21%) incidence of prior antibiotic use among patients in this database. Analysis of antibiotic prescription patterns was limited, given the several different countries from which the database draws its cases. Although we used accepted criteria to define CAP cases, it is possible that this population may have a higher rate of resistant or uncommon pathogens than other studies of CAP that have populations with lower incidence of prior antibiotic use. Although not assessed, we suspect a significant component of the prior antibiotic use represented outpatient pneumonia treatment during the few days prior to visiting the hospital.
This study has several limitations, of which the most important may be that we used clinical determination for defining presence of aspiration pneumonia. This method is susceptible to the subjective perceptions of the treating clinician. We did not account for the effect of individual physicians in our model, although we did adjust for regional differences. The retrospective identification of patients allows for the possibility of selection bias, and therefore we have not attempted to make inferences regarding the relative incidence of pneumonia, nor did we adjust for temporal trends in diagnosis. The ratio of aspiration pneumonia patients to nonaspiration pneumonia patients may not necessarily reflect that observed in reality. Microbiologic and antibiotic data were unavailable for analysis. This study cannot inform on nonhospitalized patients with aspiration pneumonia, as only hospitalized patients were enrolled. The database identified cases of pneumonia, so it is possible for a patient to enter into the database more than once. Detection of mortality was limited to the inpatient setting rather than a set interval of 30 days. Inpatient mortality depends on length‐of‐stay patterns that may bias the mortality endpoint.[30] Also not assessed was the presence of a DNR order. It is possible that an older patient with greater comorbidities and disease severity may have care intentionally limited or withdrawn early by the family or clinicians.
Strengths of the study include its size and its multicenter, multinational population. The CAPO database is a large and well‐described population of patients with CAP.[17, 31] These attributes, as well as the clinician‐determined diagnosis, increase the generalizability of the study compared to a single‐center, single‐country study that employs investigator‐defined criteria.
CONCLUSION
Pneumonia patients with confusion, who are nursing home residence, and have cerebrovascular disease are more likely to be diagnosed with aspiration pneumonia by clinicians. Our clinician‐diagnosed cohort appears similar to those derived using an investigator definition. Patients with aspiration pneumonia are older, and have greater disease severity and more comorbidities than patients with nonaspiration pneumonia. They have greater mortality than their PSI score class would predict. Even after accounting for age, disease severity, and comorbidities, the presence of aspiration pneumonia independently conferred a greater than 2‐fold increase in inpatient mortality. These findings together suggest that aspiration pneumonia should be considered a distinct entity from typical pneumonia, and that additional research should be done in this field.
ACKNOWLEDGMENTS
Disclosures: M.J.L. contributed to the study design, data analysis, statistical analysis, and writing of the manuscript. P.P. contributed to the study design and revision of the manuscript for important intellectual content. T.W. and E.W. contributed to the study design, statistical analysis, and revision of the manuscript for important intellectual content. J.A.R. and N.C.D. contributed to the study design and revision of the manuscript for important intellectual content. All authors read and approved the final manuscript. M.L. takes responsibility for the integrity of the work as a whole, from inception to published article. This investigation was partly supported with funding from the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health (grant 8UL1TR000105 [formerly UL1RR025764]). The authors report no conflicts of interest.
Pneumonia is a common clinical syndrome with well‐described epidemiology and microbiology. Aspiration pneumonia comprises 5% to 15% of patients with pneumonia acquired outside of the hospital,[1] but is less well characterized despite being a major syndrome of pneumonia in the elderly.[2, 3] Difficulties in studying aspiration pneumonia include the lack of a sensitive and specific marker for aspiration as well as the potential overlap between aspiration pneumonia and other forms of pneumonia.[4, 5, 6] Additionally, clinicians have difficulty distinguishing between aspiration pneumonia, which develops after the aspiration of oropharyngeal contents, and aspiration pneumonitis, wherein inhalation of gastric contents causes inflammation without the subsequent development of bacterial infection.[7, 8] Central to the study of aspiration pneumonia is whether it should exist as its own entity, or if aspiration is really a designation used for pneumonia in an older patient with greater comorbidities. The ability to clearly understand how a clinician diagnoses aspiration pneumonia, and whether that method has face validity with expert definitions may allow for improved future research, improved generalizability of current or past research, and possibly better clinical care.
Several validated mortality prediction models exist for community‐acquired pneumonia (CAP) using a variety of clinical predictors, but their performance in patients with aspiration pneumonia is less well characterized. Most studies validating pneumonia severity scoring systems excluded aspiration pneumonia from their study population.[9, 10, 11] Severity scoring systems for CAP may not accurately predict disease severity in patients with aspiration pneumonia. The CURB‐65[9] (confusion, uremia, respiratory rate, blood pressure, age 65 years) and the eCURB[12] scoring systems are poor predictors of mortality in patients with aspiration pneumonia, perhaps because they do not account for patient comorbidities.[13] The pneumonia severity index (PSI)[10] might predict mortality better than CURB‐65 in the aspiration population due to the inclusion of comorbidities.
Previous studies have demonstrated that patients with aspiration pneumonia are older and have greater disease severity and more comorbidities.[13, 14, 15] These single‐center studies also demonstrated greater mortality, more frequent admission to an intensive care unit (ICU), and longer hospital lengths of stay in patients with aspiration pneumonia. These studies identified aspiration pneumonia by the presence of a risk factor for aspiration[15] or by physician billing codes.[13] In practice, however, the bedside clinician diagnoses a patient as having aspiration pneumonia, but the logic is likely vague and inconsistent. Despite the potential for variability with individual judgment, an aggregate estimation from independent judgments may perform better than individual judgments.[16] Because there is no gold standard for defining aspiration pneumonia, all previous research has been limited to definitions created by investigators. This multicenter study seeks to determine what clinical characteristics lead physicians to diagnose a patient as having aspiration pneumonia, and whether or not the clinician‐derived diagnosis is distinct and clinically useful.
Our objectives were to: (1) identify covariates associated with bedside clinicians diagnosing a pneumonia patient as having aspiration pneumonia; (2) compare aspiration pneumonia and nonaspiration pneumonia in regard to disease severity, patient demographics, comorbidities, and clinical outcomes; and (3) measure the performance of the PSI in aspiration pneumonia versus nonaspiration pneumonia.
PATIENTS AND METHODS
Study Design and Setting
We performed a secondary analysis of the Community‐Acquired Pneumonia Organization (CAPO) database, which contains retrospectively collected data from 71 hospitals in 16 countries between June 2001 and December 2012. In each participating center, primary investigators selected nonconsecutive, adult hospitalized patients diagnosed with CAP. To decrease systematic selection biases, the selection of patients with CAP for enrollment in the trial was based on the date of hospital admission. Each investigator completed a case report form that was transferred via the internet to the CAPO study center at the University of Louisville (Louisville, KY). A sample of the data collection form is available at the study website (
Inclusion and Exclusion Criteria
Patients 18 years of age and satisfying criteria for CAP were included in this study. A diagnosis of CAP required a new pulmonary infiltrate at time of hospitalization, and at least 1 of the following: new or increased cough; leukocytosis; leukopenia, or left shift pattern on white blood cell count; and temperature >37.8C or <35.6 C. We excluded patients with pneumonia attributed to mycobacterial or fungal infection, and patients infected with human immunodeficiency virus, as we believed these types of pneumonia differ fundamentally from typical CAP.
Patient Variables
Patient variables included presence of aspiration pneumonia, laboratory data, comorbidities, and measures of disease severity, including the PSI. The clinician made a clinical diagnosis of the presence or absence of aspiration for each patient by marking a box on the case report form. Outcomes included in‐hospital mortality, hospital length of stay up to 14 days, and time to clinical stability up to 8 days. All variables were obtained directly from the case report form. In accordance with previously published definitions, we defined clinical stability as the day the following criteria were all met: improved clinical signs (improved cough and shortness of breath), lack of fever for >8 hours, improving leukocytosis (decreased at least 10% from the previous day), and tolerating oral intake.[17, 18]
Statistical Analysis
Baseline characteristics of patients with aspiration and nonaspiration CAP were compared using 2 or Fisher exact tests for categorical variables and the Mann‐Whitney U test for continuous variables.
To determine which patient variables were important in the physician diagnosis of aspiration pneumonia, we performed logistic regression with initial covariates comprising the demographic, comorbidity, and disease severity measurements listed in Table 1. We included interactions between cerebrovascular disease and age, nursing home status, and confusion to improve model fit. We centered all variables (including binary indicators) according to the method outlined by Kraemer and Blasey to improve interpretation of the main effects.[19]
| Aspiration Pneumonia, N=451 | Nonaspiration Pneumonia, N=4,734 | P Value | |
|---|---|---|---|
|
|||
| Demographics | |||
| Age, y | 79 (6587) | 69 (5380) | <0.001 |
| % Male | 59% | 60% | 0.58 |
| Nursing home residence | 25% | 5% | <0.001 |
| Recent (30 days) antibiotic use | 21% | 16% | 0.017 |
| Comorbidities | |||
| Cerebrovascular disease | 35% | 14% | <0.001 |
| Chronic obstructive pulmonary disease | 25% | 27% | 0.62 |
| Congestive heart failure | 23% | 19% | 0.027 |
| Diabetes | 18% | 18% | 0.85 |
| Cancer | 12% | 10% | 0.12 |
| Renal disease | 10% | 11% | 0.53 |
| Liver disease | 6% | 5% | 0.29 |
| Disease severity | |||
| Pneumonia severity index | 123 (99153) | 92 (68117) | <0.001 |
| Confusion | 49% | 12% | <0.001 |
| PaO2 <60 mm Hg | 43% | 33% | <0.001 |
| BUN >30 g/dL | 42% | 23% | <0.001 |
| Multilobar pneumonia | 34% | 28% | 0.003 |
| Pleural effusion | 25% | 21% | 0.07 |
| Respiratory rate >30 breaths/minute | 21% | 20% | 0.95 |
| pH <7.35 | 13% | 5% | <0.001 |
| Hematocrit <30% | 11% | 6% | 0.001 |
| Temperature >37.8C or <35.6C | 9% | 7% | 0.30 |
| Systolic blood pressure <90 mm Hg | 8% | 9% | 0.003 |
| Sodium <130 mEq/L | 8% | 6% | 0.08 |
| Heart rate >125 beats/minute | 8% | 5% | 0.71 |
| Glucose >250 mg/dL | 6% | 7% | 0.06 |
| Cavitary lesion | 0% | 0% | 0.67 |
| Clinical outcomes | |||
| In‐hospital mortality | 23% | 9% | <0.001 |
| Intensive care unit admission | 19% | 13% | 0.002 |
| Hospital length of stay, d | 9 (515) | 7 (412) | <0.001 |
| Time to clinical stability, d | 8 (48) | 4 (38) | <0.001 |
To determine if aspiration pneumonia had worse clinical outcomes compared to nonaspiration pneumonia, multiple methods were used. To compare the differences between the 2 groups with respect to time to clinical stability and length of hospital stay, we constructed Kaplan‐Meier survival curves and Cox proportional hazards regression models. The log‐rank test was used to determine statistical differences between the Kaplan‐Meier survival curves. To compare the impact of aspiration on mortality in patients with CAP, we conducted a propensity scorematched analysis. We chose propensity score matching over traditional logistic regression to balance variables among groups and to avoid the potential for overfit and multicollinearity. We considered a variable balanced after matching if its standardized difference was <10. All variables in the propensity scorematched analysis were balanced.
Although our dataset contained minimal missing data, we imputed any missing values to maintain the full study population in the creation of the propensity score. Missing data were imputed using the aregImpute function of the hmisc package of R (The R Foundation for Statistical Computing, Vienna, Austria).[20, 21] We built the propensity score model using a variable selection algorithm described by Bursac et al.[22] Our model included variables for region (United States/Canada, Europe, Asia/Africa or Latin America) and the variables listed in Table 1, with the exception of the PSI and the 4 clinical outcomes. Given that previous analyses accounting for clustering by physician did not substantially affect our results,[23] our model did not include physician‐level variables and did not account for the clustering effects of physicians. Using the propensity scores generated from this model, we matched a case of aspiration CAP with a case of nonaspiration CAP.[24] We then constructed a general linear model using the matched dataset to obtain the magnitude of effect of aspiration on mortality.
We used receiver operating characteristic curves to define the diagnostic accuracy of the pneumonia severity index for the prediction of mortality among patients with aspiration pneumonia and those with nonaspiration pneumonia. SAS version 9.3 (SAS Institute, Cary, NC) and R version 2.15.3 (The R Foundation for Statistical Computing) were used for all analyses. P values of 0.05 were considered statistically significant in all analyses.
RESULTS
Our initial query, after exclusion criteria, yielded a study population of 5185 patients (Figure 1). We compared 451 patients diagnosed with aspiration pneumonia to 4734 with CAP (Figure 1). Patient characteristics are summarized in Table 1. Patients with aspiration pneumonia were older, more likely to live in a nursing home, had greater disease severity, and were more likely to be admitted to an ICU. Patients with aspiration pneumonia had longer adjusted hospital lengths of stay and took more days to achieve clinical stability than patients with nonaspiration pneumonia (Figure 2). After adjusting for all variables in Table 1, the Cox proportional hazards models demonstrated that aspiration pneumonia was associated with ongoing hospitalization (hazard ratio [HR] for discharge: 0.77, 95% confidence interval [CI]: 0.65‐0.91, P=0.002) and clinical instability (HR for attaining clinical stability: 0.72, 95% CI: 0.61‐0.84, P<0.001). Patients with aspiration pneumonia presented with greater disease severity than those with nonaspiration pneumonia. Although there was no difference between groups in regard to temperature, respiratory rate, hyponatremia, or presence of pleural effusions or cavitary lesions, all other measured indices of disease severity were worse in patients with aspiration pneumonia. Patients with aspiration pneumonia were more likely to have cerebrovascular disease than those with nonaspiration pneumonia. Aspiration pneumonia patients also had increased prevalence of congestive heart failure. There was no appreciable difference between groups among other measured comorbidities.


The patient characteristics most associated with a physician diagnosis of aspiration pneumonia, identified using logistic regression, were confusion, residence in nursing home, and presence of cerebrovascular disease (odds ratio [OR]: of 4.4, 2.9, and 2.3, respectively), whereas renal disease was associated with decreased physician diagnosis of aspiration pneumonia over nonaspiration pneumonia (OR: 0.58) (Table 2).
| Covariate | Odds Ratio | 95% Confidence Intervals | P Value |
|---|---|---|---|
|
|||
| Demographics | |||
| Age, y | 1.00 | 0.991.01 | 0.948 |
| Male | 1.20 | 0.941.54 | 0.148 |
| Nursing home residence | 2.93 | 2.134.00 | <0.001 |
| Comorbidities | |||
| Cerebrovascular disease | 2.26 | 1.533.32 | <0.001 |
| Renal disease | 0.58 | 0.390.85 | 0.006 |
| Disease severity | |||
| Confusion | 4.41 | 3.405.72 | <0.001 |
| Hematocrit <30% | 1.59 | 1.062.33 | 0.020 |
| pH <7.35 | 1.67 | 1.102.47 | 0.013 |
| Temperature >37.8C or <35.6C | 1.60 | 1.072.35 | 0.019 |
| Multilobar pneumonia | 1.29 | 1.001.65 | 0.047 |
| Interaction terms | |||
| Age * cerebrovascular disease | 0.98 | 0.960.99 | 0.011 |
| Nursing home * cerebrovascular disease | 0.51 | 0.270.96 | 0.037 |
| Confusion * cerebrovascular disease | 0.70 | 0.421.17 | 0.175 |
Observed in‐patient mortality of aspiration pneumonia was 23%. This mortality was considerably higher than a mean PSI score of 123 would predict (class IV risk group, with expected 30‐day mortality of 8%9%[25]). The PSI score's ability to predict inpatient mortality in patients with aspiration pneumonia was moderate, with an area under the curve (AUC) of 0.71. This was similar to its performance in patients with nonaspiration pneumonia (AUC of 0.75) (Figure 3). These values are lower than the AUC of 0.81 for the PSI in predicting mortality derived from a meta‐analysis of 31 other studies.[26]

Our regression model after propensity score matching demonstrated that aspiration pneumonia independently confers a 2.3‐fold increased odds for inpatient mortality (95% CI: 1.56‐3.45, P<0.001).
DISCUSSION
Pneumonia patients with confusion, nursing home residence, or cerebrovascular disease are more likely to be diagnosed with aspiration pneumonia by clinicians. Although this is unsurprising, it is notable that these patients are more than twice as likely to die in the inpatient setting, even after accounting for age, comorbidities, and disease severity. These findings are similar to three previously published studies comparing aspiration and nonaspiration pneumonia at single institutions, albeit using different aspiration pneumonia definitions.[13, 14, 15] This study is the first large, multicenter, multinational study to demonstrate these findings.
Central to the interpretation of our results is the method of diagnosing aspiration versus nonaspiration. A bottom‐up method that relies on a clinician to check a box for aspiration may appear poorly reproducible. Because there is no diagnostic gold standard, clinicians may use different criteria to diagnose aspiration, creating potential for idiosyncratic noise. The strength of the wisdom of the crowd method used in this study is that an aggregate estimation from independent judgments may reduce the noise from individual judgments.[16] Although clinicians may vary in why they diagnose a particular patient as having aspiration pneumonia, it appears that the overwhelming reason for diagnosing a patient as having aspiration pneumonia is the presence of confusion, followed by previous nursing home residence or cerebrovascular disease. This finding has some face validity when compared with studies using an investigator definition, as altered mental status, chronic debility, and cerebrovascular disease are either prominent features of the definition of aspiration pneumonia[8] or frequently observed in patients with aspiration pneumonia.[13, 15] The distribution of cerebrovascular disease among our study's aspiration and nonaspiration pneumonia patients was similar to studies that used formal criteria in their definitions.[13, 15] Although nursing home residence was more likely in aspiration pneumonia patients, the majority of aspiration pneumonia patients were residing in the community, suggesting that aspiration is not simply a surrogate for healthcare‐associated pneumonia. Although patients with aspiration pneumonia are typically older than their nonaspiration counterparts, it appears that age is not a key determinant in the diagnosis of aspiration. With aspiration pneumonia, confusion, nursing home residence, and the presence of cerebrovascular disease are the greatest contributors in the clinical diagnosis, more than age.
Our data demonstrate that aspiration pneumonia confers increased odds for mortality, even after adjustment for age, disease severity, and comorbidities. These data suggest that aspiration pneumonia is a distinct entity from nonaspiration pneumonia, and that this disease is worse than nonaspiration CAP. If aspiration pneumonia is distinct from nonaspiration pneumonia, some unrecognized host factor other than age, disease severity, or the captured comorbidities decreases survival in aspiration pneumonia patients. However, it is also possible that aspiration pneumonia is merely a clinical designation for one end of the pneumonia spectrum, and we and others have failed to completely account for all measures of disease severity or all measures of comorbidities. Examples of unmeasured comorbidities would include presence of oropharyngeal dysphagia, which is not assessed in the database but could have a significant effect on clinical diagnosis. Unmeasured covariates can include measures beyond that of disease severity or comorbidity, such as the presence of a do not resuscitate (DNR) order, which could have a significant confounding effect on the observed association. A previous, single‐center study demonstrated that increased 30‐day mortality in aspiration pneumonia was mostly attributable to greater disease severity and comorbidities, although aspiration pneumonia independently conferred greater risk for adverse long‐term outcomes.[15] We propose that aspiration pneumonia represents a clinically distinct entity from nonaspiration pneumonia. Patients with chronic aspiration are often chronically malnourished and may have different oral flora than patients without chronic aspiration.[27, 28] Chronic aspiration has been associated with granulomatous reaction, organizing pneumonia, diffuse alveolar damage, and chronic bronchiolitis.[29] Chronic aspiration may elicit changes in the host physiology, and may render the host more susceptible to the development of secondary bacterial infection with morbid consequences.
The ability of the PSI to predict inpatient mortality was moderate (AUC only 0.7), with no significant additional discrimination between the aspiration and nonaspiration pneumonia groups. Although the PSI had moderate ability to predict inpatient mortality, the observed mortality was considerably higher than predicted. It is possible that the PSI incompletely captures clinically relevant comorbidities (eg, malnutrition). Further study to improve mortality prediction of aspiration pneumonia patients could employ sensitivity analysis to determine optimal thresholds and weighting of the PSI components.
Patients with aspiration pneumonia had longer hospital lengths of stay and took longer to achieve clinical stability than their nonaspiration counterparts. Time to clinical stability has been associated with increased posthospitalization mortality and is associated with time to switch from intravenous to oral antibiotics.[17] Although some component of hospital length‐of‐stay is subject to local practice patterns, time to clinical stability has explicit criteria for clinical improvement and failure, and therefore is less likely to be affected by local practice patterns.
We noted a relatively high (16%21%) incidence of prior antibiotic use among patients in this database. Analysis of antibiotic prescription patterns was limited, given the several different countries from which the database draws its cases. Although we used accepted criteria to define CAP cases, it is possible that this population may have a higher rate of resistant or uncommon pathogens than other studies of CAP that have populations with lower incidence of prior antibiotic use. Although not assessed, we suspect a significant component of the prior antibiotic use represented outpatient pneumonia treatment during the few days prior to visiting the hospital.
This study has several limitations, of which the most important may be that we used clinical determination for defining presence of aspiration pneumonia. This method is susceptible to the subjective perceptions of the treating clinician. We did not account for the effect of individual physicians in our model, although we did adjust for regional differences. The retrospective identification of patients allows for the possibility of selection bias, and therefore we have not attempted to make inferences regarding the relative incidence of pneumonia, nor did we adjust for temporal trends in diagnosis. The ratio of aspiration pneumonia patients to nonaspiration pneumonia patients may not necessarily reflect that observed in reality. Microbiologic and antibiotic data were unavailable for analysis. This study cannot inform on nonhospitalized patients with aspiration pneumonia, as only hospitalized patients were enrolled. The database identified cases of pneumonia, so it is possible for a patient to enter into the database more than once. Detection of mortality was limited to the inpatient setting rather than a set interval of 30 days. Inpatient mortality depends on length‐of‐stay patterns that may bias the mortality endpoint.[30] Also not assessed was the presence of a DNR order. It is possible that an older patient with greater comorbidities and disease severity may have care intentionally limited or withdrawn early by the family or clinicians.
Strengths of the study include its size and its multicenter, multinational population. The CAPO database is a large and well‐described population of patients with CAP.[17, 31] These attributes, as well as the clinician‐determined diagnosis, increase the generalizability of the study compared to a single‐center, single‐country study that employs investigator‐defined criteria.
CONCLUSION
Pneumonia patients with confusion, who are nursing home residence, and have cerebrovascular disease are more likely to be diagnosed with aspiration pneumonia by clinicians. Our clinician‐diagnosed cohort appears similar to those derived using an investigator definition. Patients with aspiration pneumonia are older, and have greater disease severity and more comorbidities than patients with nonaspiration pneumonia. They have greater mortality than their PSI score class would predict. Even after accounting for age, disease severity, and comorbidities, the presence of aspiration pneumonia independently conferred a greater than 2‐fold increase in inpatient mortality. These findings together suggest that aspiration pneumonia should be considered a distinct entity from typical pneumonia, and that additional research should be done in this field.
ACKNOWLEDGMENTS
Disclosures: M.J.L. contributed to the study design, data analysis, statistical analysis, and writing of the manuscript. P.P. contributed to the study design and revision of the manuscript for important intellectual content. T.W. and E.W. contributed to the study design, statistical analysis, and revision of the manuscript for important intellectual content. J.A.R. and N.C.D. contributed to the study design and revision of the manuscript for important intellectual content. All authors read and approved the final manuscript. M.L. takes responsibility for the integrity of the work as a whole, from inception to published article. This investigation was partly supported with funding from the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health (grant 8UL1TR000105 [formerly UL1RR025764]). The authors report no conflicts of interest.
- , , , et al. Severe community‐acquired pneumonia. Epidemiology and prognostic factors. Am Rev Respir Dis. 1991;144(2):312–318.
- , , . Risk factors for pneumonia in the elderly. Am J Med. 1994;96(4):313–320.
- , . Aspiration pneumonia and dysphagia in the elderly. Chest. 2003;124(1):328–336.
- , , . Pneumonia versus aspiration pneumonitis in nursing home residents: diagnosis and management. J Am Geriatr Soc. 2003;51(1):17–23.
- . Aspiration pneumonia: mixing apples with oranges and tangerines. Crit Care Med. 2004;32(5):1236; author reply 1236–1237.
- , , , , . Epidemiology and impact of aspiration pneumonia in patients undergoing surgery in Maryland, 1999–2000. Crit Care Med. 2003;31(7):1930–1937.
- . Aspiration syndromes: aspiration pneumonia and pneumonitis. Hosp Pract (Minneap). 2010;38(1):35–42.
- . Aspiration pneumonitis and aspiration pneumonia. N Engl J Med. 2001;344(9):665–671.
- , , , et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58(5):377–382.
- , , , et al. Comparison of a disease‐specific and a generic severity of illness measure for patients with community‐acquired pneumonia. J Gen Intern Med. 1995;10(7):359–368.
- , , , et al. Development and validation of a clinical prediction rule for severe community‐acquired pneumonia. Am J Respir Crit Care Med. 2006;174(11):1249–1256.
- , , , et al. CURB‐65 pneumonia severity assessment adapted for electronic decision support. Chest. 2011;140(1):156–163.
- , , , . Mortality, morbidity, and disease severity of patients with aspiration pneumonia. J Hosp Med. 2013;8(2):83–90.
- , , , , . Pneumonia Severity Index (PSI), CURB‐65, and mortality in hospitalized elderly patients with aspiration pneumonia [in German]. Z Gerontol Geriatr. 2011;44(4):229–234.
- , , , , . Risk factors for aspiration in community‐acquired pneumonia: analysis of a hospitalized UK cohort. Am J Med. 2013;126(11):995–1001.
- , , , . The wisdom of the crowd in combinatorial problems. Cogn Sci. 2012;36(3):452–470.
- , , , et al. Association between time to clinical stability and outcomes after discharge in hospitalized patients with community‐acquired pneumonia. Chest. 2011;140(2):482–488.
- . Clinical stability and switch therapy in hospitalised patients with community‐acquired pneumonia: are we there yet? Eur Respir J. 2013;41(1):5–6.
- , . Centring in regression analyses: a strategy to prevent errors in statistical inference. Int J Methods Psychiatr Res. 2004;13(3):141–151.
- . Hmisc: Harrell miscellaneous. Available at: http://CRAN.R‐project.org/package=Hmisc. Published Sept 12, 2014. Last accessed Oct 27, 2014.
- , . Multiple imputation for the fatal accident reporting system. J R Stat Soc Ser C Appl Stat. 1991;40(1):13–29.
- , , , . Purposeful selection of variables in logistic regression. Source Code Biol Med. 2008;3:17.
- , , , , ; CAPO authors. Mortality differences among hospitalized patients with community‐acquired pneumonia in three world regions: results from the Community‐Acquired Pneumonia Organization (CAPO) International Cohort Study. Respir Med. 2013;107(7):1101–1111.
- . Reducing bias in a propensity score matched‐pair sample using greedy matching techniques. In: Proceedings of the 26th Annual SAS Users Group International Conference. Cary, NC: SAS Institute Inc.; 2001:214–226. Available at: http://www2.sas.com/proceedings/sugi26/p214–26.pdf. Last accessed Oct 27, 2014.
- , , , et al. A prediction rule to identify low‐risk patients with community‐acquired pneumonia. N Engl J Med. 1997;336(4):243–250.
- , , , et al. Severity assessment tools for predicting mortality in hospitalised patients with community‐acquired pneumonia. Systematic review and meta‐analysis. Thorax. 2010;65(10):878–883.
- , , , , , . Prevalence and prognostic implications of dysphagia in elderly patients with pneumonia. Age Ageing. 2010;39(1):39–45.
- , . The association between oral microorgansims and aspiration pneumonia in the institutionalized elderly: review and recommendations. Dysphagia. 2010;25(4):307–322.
- , . Histopathology of aspiration pneumonia not associated with food or other particulate matter: a clinicopathologic study of 10 cases diagnosed on biopsy. Am J Surg Pathol. 2011;35(3):426–431.
- , , , , , . Interpreting hospital mortality data. The role of clinical risk adjustment. JAMA. 1988;260(24):3611–3616.
- , , , . Hospitalization for community‐acquired pneumonia: the pneumonia severity index vs clinical judgment. Chest. 2003;124(1):121–124.
- , , , et al. Severe community‐acquired pneumonia. Epidemiology and prognostic factors. Am Rev Respir Dis. 1991;144(2):312–318.
- , , . Risk factors for pneumonia in the elderly. Am J Med. 1994;96(4):313–320.
- , . Aspiration pneumonia and dysphagia in the elderly. Chest. 2003;124(1):328–336.
- , , . Pneumonia versus aspiration pneumonitis in nursing home residents: diagnosis and management. J Am Geriatr Soc. 2003;51(1):17–23.
- . Aspiration pneumonia: mixing apples with oranges and tangerines. Crit Care Med. 2004;32(5):1236; author reply 1236–1237.
- , , , , . Epidemiology and impact of aspiration pneumonia in patients undergoing surgery in Maryland, 1999–2000. Crit Care Med. 2003;31(7):1930–1937.
- . Aspiration syndromes: aspiration pneumonia and pneumonitis. Hosp Pract (Minneap). 2010;38(1):35–42.
- . Aspiration pneumonitis and aspiration pneumonia. N Engl J Med. 2001;344(9):665–671.
- , , , et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58(5):377–382.
- , , , et al. Comparison of a disease‐specific and a generic severity of illness measure for patients with community‐acquired pneumonia. J Gen Intern Med. 1995;10(7):359–368.
- , , , et al. Development and validation of a clinical prediction rule for severe community‐acquired pneumonia. Am J Respir Crit Care Med. 2006;174(11):1249–1256.
- , , , et al. CURB‐65 pneumonia severity assessment adapted for electronic decision support. Chest. 2011;140(1):156–163.
- , , , . Mortality, morbidity, and disease severity of patients with aspiration pneumonia. J Hosp Med. 2013;8(2):83–90.
- , , , , . Pneumonia Severity Index (PSI), CURB‐65, and mortality in hospitalized elderly patients with aspiration pneumonia [in German]. Z Gerontol Geriatr. 2011;44(4):229–234.
- , , , , . Risk factors for aspiration in community‐acquired pneumonia: analysis of a hospitalized UK cohort. Am J Med. 2013;126(11):995–1001.
- , , , . The wisdom of the crowd in combinatorial problems. Cogn Sci. 2012;36(3):452–470.
- , , , et al. Association between time to clinical stability and outcomes after discharge in hospitalized patients with community‐acquired pneumonia. Chest. 2011;140(2):482–488.
- . Clinical stability and switch therapy in hospitalised patients with community‐acquired pneumonia: are we there yet? Eur Respir J. 2013;41(1):5–6.
- , . Centring in regression analyses: a strategy to prevent errors in statistical inference. Int J Methods Psychiatr Res. 2004;13(3):141–151.
- . Hmisc: Harrell miscellaneous. Available at: http://CRAN.R‐project.org/package=Hmisc. Published Sept 12, 2014. Last accessed Oct 27, 2014.
- , . Multiple imputation for the fatal accident reporting system. J R Stat Soc Ser C Appl Stat. 1991;40(1):13–29.
- , , , . Purposeful selection of variables in logistic regression. Source Code Biol Med. 2008;3:17.
- , , , , ; CAPO authors. Mortality differences among hospitalized patients with community‐acquired pneumonia in three world regions: results from the Community‐Acquired Pneumonia Organization (CAPO) International Cohort Study. Respir Med. 2013;107(7):1101–1111.
- . Reducing bias in a propensity score matched‐pair sample using greedy matching techniques. In: Proceedings of the 26th Annual SAS Users Group International Conference. Cary, NC: SAS Institute Inc.; 2001:214–226. Available at: http://www2.sas.com/proceedings/sugi26/p214–26.pdf. Last accessed Oct 27, 2014.
- , , , et al. A prediction rule to identify low‐risk patients with community‐acquired pneumonia. N Engl J Med. 1997;336(4):243–250.
- , , , et al. Severity assessment tools for predicting mortality in hospitalised patients with community‐acquired pneumonia. Systematic review and meta‐analysis. Thorax. 2010;65(10):878–883.
- , , , , , . Prevalence and prognostic implications of dysphagia in elderly patients with pneumonia. Age Ageing. 2010;39(1):39–45.
- , . The association between oral microorgansims and aspiration pneumonia in the institutionalized elderly: review and recommendations. Dysphagia. 2010;25(4):307–322.
- , . Histopathology of aspiration pneumonia not associated with food or other particulate matter: a clinicopathologic study of 10 cases diagnosed on biopsy. Am J Surg Pathol. 2011;35(3):426–431.
- , , , , , . Interpreting hospital mortality data. The role of clinical risk adjustment. JAMA. 1988;260(24):3611–3616.
- , , , . Hospitalization for community‐acquired pneumonia: the pneumonia severity index vs clinical judgment. Chest. 2003;124(1):121–124.
© 2014 Society of Hospital Medicine