User login
Evaluation of Micrographic Surgery and Dermatologic Oncology Fellowship Program Websites
To the Editor:
Micrographic surgery and dermatologic oncology (MSDO) is a highly competitive subspecialty fellowship in dermatology. Prospective applicants often depend on the Internet to obtain pertinent information about fellowship programs to navigate the application process. An up-to-date and comprehensive fellowship website has the potential to be advantageous for both applicants and programs—applicants can more readily identify programs that align with their goals and values, and programs can effectively attract compatible applicants. These advantages are increasingly relevant with the virtual application process that has become essential considering the COVID-19 pandemic. At the height of the COVID-19 pandemic in 2020, we sought to evaluate the comprehensiveness of the content of Accreditation Council for Graduate Medical Education (ACGME) MSDO fellowship program websites to identify possible areas for improvement.
We obtained a list of all ACGME MSDO fellowships from the ACGME website (https://www.acgme.org/) and verified it against the list of MSDO programs in FREIDA, the American Medical Association residency and fellowship database (https://freida.ama-assn.org/). All programs without a website were excluded from further analysis. All data collection from currently accessible fellowship websites and evaluation occurred in April 2020.
The remaining MSDO fellowship program websites were evaluated using 25 criteria distributed among 5 domains: education/research, clinical training, program information, application process, and incentives. These criteria were determined based on earlier studies that similarly evaluated the website content of fellowship programs with inclusion of information that was considered valuable in the appraisal of fellowship programs.1,2 Criteria were further refined by direct consideration of relevance and importance to MSDO fellowship applicants (eg, inclusion of case volume, exclusion of call schedule).
Each criterion was independently assessed by 2 investigators (J.Y.C. and S.J.E.S.). A third investigator (J.R.P.) then independently evaluated those 2 assessments for agreement. Where disagreement was discovered, the third evaluator (J.R.P.) provided a final appraisal. Cohen’s kappa (κ) was conducted to evaluate for concordance between the 2 primary website evaluators. We found there to be substantial agreement between the reviewers within the education/research (κ [SD]=0.772 [0.077]), clinical training (κ [SD]=0.740 [0.051]), application process (κ [SD]=0.726 [0.103]), and incentives domains (κ [SD]=0.730 [0.110]). There was moderate agreement (κ [SD]=0.603 [0.128]) between the reviewers within the program information domain.
We identified 77 active MSDO fellowship programs. Sixty of those 77 programs (77.9%) had a dedicated fellowship website that was readily accessible. Most programs that had a dedicated fellowship website had a core or affiliated residency program (49/60 [81.7%]).
Websites that we evaluated fulfilled a mean (SD) of 9.37 (4.17) of the 25 identified criteria. Only 13 of 60 (21.7%) websites fulfilled more than 50% of evaluated criteria.
There was no statistical difference in the number of criteria fulfilled based on whether the fellowship program had a core or affiliated residency program.
Upon reviewing website accessibility directly from FREIDA, only 5 of 60 programs (8.3%) provided applicants with a link directly to their fellowship page (Table). Most programs (41 [68.3%]) provided a link to the dermatology department website, not to the specific fellowship program page, thus requiring a multistep process to find the fellowship-specific page. The remaining programs had an inaccessible (4 [6.7%]) or absent (10 [16.7%]) link on FREIDA, though a fellowship website could be identified by an Internet search of the program name.
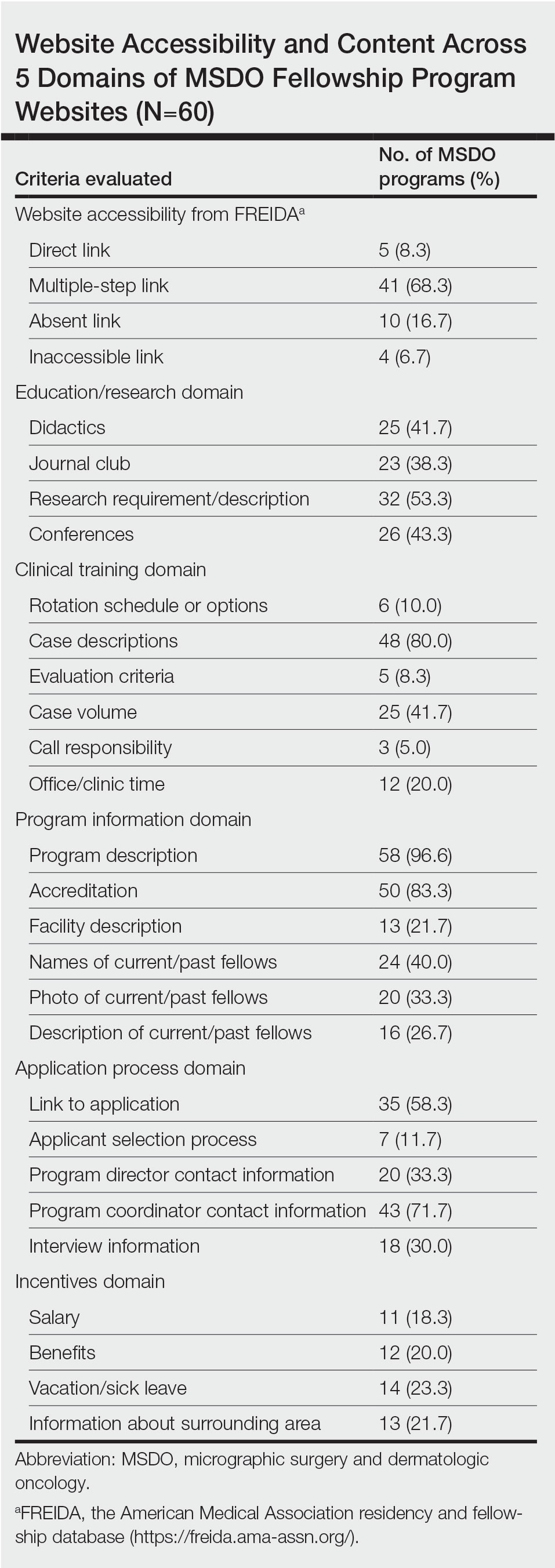
The domain most fulfilled was program information with an average of 51.1% of programs satisfying the criteria, whereas the incentives domain was least fulfilled with an average of only 20.8% of programs satisfying the criteria. Across the various criteria, websites more often included a description of the program (58 [96.6%]), mentioned accreditation (53 [88.3%]), and provided case descriptions (48 [80.0%]). They less often reported information regarding a fellow’s call responsibility (3 [5%]); evaluation criteria (5 [8.3%]); and rotation schedule or options (6 [10.0%]).
The highest number of criteria fulfilled by a single program was 19 (76%). The lowest number of criteria met was 2 (8%). These findings suggest a large variation in comprehensiveness across fellowship websites.
Our research suggests that many current MSDO fellowship programs have room to maximize the information provided to applicants through their websites, which is particularly relevant following the COVID-19 pandemic, as the value of providing comprehensive and transparent information through an online platform is greater than ever. Given the ongoing desire to limit travel, virtual methods for navigating the application process have been readily used, including online videoconferencing for interviews and virtual program visits. This scenario has placed applicants in a challenging situation—their ability to directly evaluate their compatibility with a given program has been limited.3
Earlier studies that analyzed rheumatology fellowship recruitment during the COVID-19 pandemic found that programs may have more difficulty highlighting the strengths of their institution (eg, clinical facilities, professional opportunities, educational environment).4 An updated and comprehensive fellowship website was recommended4 as a key part in facing these new challenges. On the other hand, given the large number of applicants each year for fellowship positions in any given program, we acknowledge the potential benefit programs may obtain from limiting electronic information that is readily accessible to all applicants, as doing so may encourage applicants to communicate directly with a program and allow programs to identify candidates who are more interested.
In light of the movement to a more virtual-friendly and technology-driven fellowship application process, we identified 25 content areas that fellowships may want to include on their websites so that potential applicants can be well informed about the program before submitting an application and scheduling an interview. Efforts to improve accessibility and maximize the content of these websites may help programs attract compatible candidates, improve transparency, and guide applicants throughout the application process.
- Lu F, Vijayasarathi A, Murray N, et al. Evaluation of pediatric radiology fellowship website content in USA and Canada. Curr Prob Diagn Radiol. 2021;50:151-155. doi:10.1067/j.cpradiol.2020.01.007
- Cantrell CK, Bergstresser SL, Schuh AC, et al. Accessibility and content of abdominal transplant fellowship program websites in the United States. J Surg Res. 2018;232:271-274. doi:10.1016/j.jss.2018.06.052
- Nesemeier BR, Lebo NL, Schmalbach CE, et al. Impact of the COVID-19 global pandemic on the otolaryngology fellowship application process. Otolaryngol Head Neck Surg. 2020;163:712-713. doi:10.1177/0194599820934370
- Kilian A, Dua AB, Bolster MB, et al. Rheumatology fellowship recruitment in 2020: benefits, challenges, and adaptations. Arthritis Care Res (Hoboken). 2021;73:459-461. doi:10.1002/acr.24445
To the Editor:
Micrographic surgery and dermatologic oncology (MSDO) is a highly competitive subspecialty fellowship in dermatology. Prospective applicants often depend on the Internet to obtain pertinent information about fellowship programs to navigate the application process. An up-to-date and comprehensive fellowship website has the potential to be advantageous for both applicants and programs—applicants can more readily identify programs that align with their goals and values, and programs can effectively attract compatible applicants. These advantages are increasingly relevant with the virtual application process that has become essential considering the COVID-19 pandemic. At the height of the COVID-19 pandemic in 2020, we sought to evaluate the comprehensiveness of the content of Accreditation Council for Graduate Medical Education (ACGME) MSDO fellowship program websites to identify possible areas for improvement.
We obtained a list of all ACGME MSDO fellowships from the ACGME website (https://www.acgme.org/) and verified it against the list of MSDO programs in FREIDA, the American Medical Association residency and fellowship database (https://freida.ama-assn.org/). All programs without a website were excluded from further analysis. All data collection from currently accessible fellowship websites and evaluation occurred in April 2020.
The remaining MSDO fellowship program websites were evaluated using 25 criteria distributed among 5 domains: education/research, clinical training, program information, application process, and incentives. These criteria were determined based on earlier studies that similarly evaluated the website content of fellowship programs with inclusion of information that was considered valuable in the appraisal of fellowship programs.1,2 Criteria were further refined by direct consideration of relevance and importance to MSDO fellowship applicants (eg, inclusion of case volume, exclusion of call schedule).
Each criterion was independently assessed by 2 investigators (J.Y.C. and S.J.E.S.). A third investigator (J.R.P.) then independently evaluated those 2 assessments for agreement. Where disagreement was discovered, the third evaluator (J.R.P.) provided a final appraisal. Cohen’s kappa (κ) was conducted to evaluate for concordance between the 2 primary website evaluators. We found there to be substantial agreement between the reviewers within the education/research (κ [SD]=0.772 [0.077]), clinical training (κ [SD]=0.740 [0.051]), application process (κ [SD]=0.726 [0.103]), and incentives domains (κ [SD]=0.730 [0.110]). There was moderate agreement (κ [SD]=0.603 [0.128]) between the reviewers within the program information domain.
We identified 77 active MSDO fellowship programs. Sixty of those 77 programs (77.9%) had a dedicated fellowship website that was readily accessible. Most programs that had a dedicated fellowship website had a core or affiliated residency program (49/60 [81.7%]).
Websites that we evaluated fulfilled a mean (SD) of 9.37 (4.17) of the 25 identified criteria. Only 13 of 60 (21.7%) websites fulfilled more than 50% of evaluated criteria.
There was no statistical difference in the number of criteria fulfilled based on whether the fellowship program had a core or affiliated residency program.
Upon reviewing website accessibility directly from FREIDA, only 5 of 60 programs (8.3%) provided applicants with a link directly to their fellowship page (Table). Most programs (41 [68.3%]) provided a link to the dermatology department website, not to the specific fellowship program page, thus requiring a multistep process to find the fellowship-specific page. The remaining programs had an inaccessible (4 [6.7%]) or absent (10 [16.7%]) link on FREIDA, though a fellowship website could be identified by an Internet search of the program name.

The domain most fulfilled was program information with an average of 51.1% of programs satisfying the criteria, whereas the incentives domain was least fulfilled with an average of only 20.8% of programs satisfying the criteria. Across the various criteria, websites more often included a description of the program (58 [96.6%]), mentioned accreditation (53 [88.3%]), and provided case descriptions (48 [80.0%]). They less often reported information regarding a fellow’s call responsibility (3 [5%]); evaluation criteria (5 [8.3%]); and rotation schedule or options (6 [10.0%]).
The highest number of criteria fulfilled by a single program was 19 (76%). The lowest number of criteria met was 2 (8%). These findings suggest a large variation in comprehensiveness across fellowship websites.
Our research suggests that many current MSDO fellowship programs have room to maximize the information provided to applicants through their websites, which is particularly relevant following the COVID-19 pandemic, as the value of providing comprehensive and transparent information through an online platform is greater than ever. Given the ongoing desire to limit travel, virtual methods for navigating the application process have been readily used, including online videoconferencing for interviews and virtual program visits. This scenario has placed applicants in a challenging situation—their ability to directly evaluate their compatibility with a given program has been limited.3
Earlier studies that analyzed rheumatology fellowship recruitment during the COVID-19 pandemic found that programs may have more difficulty highlighting the strengths of their institution (eg, clinical facilities, professional opportunities, educational environment).4 An updated and comprehensive fellowship website was recommended4 as a key part in facing these new challenges. On the other hand, given the large number of applicants each year for fellowship positions in any given program, we acknowledge the potential benefit programs may obtain from limiting electronic information that is readily accessible to all applicants, as doing so may encourage applicants to communicate directly with a program and allow programs to identify candidates who are more interested.
In light of the movement to a more virtual-friendly and technology-driven fellowship application process, we identified 25 content areas that fellowships may want to include on their websites so that potential applicants can be well informed about the program before submitting an application and scheduling an interview. Efforts to improve accessibility and maximize the content of these websites may help programs attract compatible candidates, improve transparency, and guide applicants throughout the application process.
To the Editor:
Micrographic surgery and dermatologic oncology (MSDO) is a highly competitive subspecialty fellowship in dermatology. Prospective applicants often depend on the Internet to obtain pertinent information about fellowship programs to navigate the application process. An up-to-date and comprehensive fellowship website has the potential to be advantageous for both applicants and programs—applicants can more readily identify programs that align with their goals and values, and programs can effectively attract compatible applicants. These advantages are increasingly relevant with the virtual application process that has become essential considering the COVID-19 pandemic. At the height of the COVID-19 pandemic in 2020, we sought to evaluate the comprehensiveness of the content of Accreditation Council for Graduate Medical Education (ACGME) MSDO fellowship program websites to identify possible areas for improvement.
We obtained a list of all ACGME MSDO fellowships from the ACGME website (https://www.acgme.org/) and verified it against the list of MSDO programs in FREIDA, the American Medical Association residency and fellowship database (https://freida.ama-assn.org/). All programs without a website were excluded from further analysis. All data collection from currently accessible fellowship websites and evaluation occurred in April 2020.
The remaining MSDO fellowship program websites were evaluated using 25 criteria distributed among 5 domains: education/research, clinical training, program information, application process, and incentives. These criteria were determined based on earlier studies that similarly evaluated the website content of fellowship programs with inclusion of information that was considered valuable in the appraisal of fellowship programs.1,2 Criteria were further refined by direct consideration of relevance and importance to MSDO fellowship applicants (eg, inclusion of case volume, exclusion of call schedule).
Each criterion was independently assessed by 2 investigators (J.Y.C. and S.J.E.S.). A third investigator (J.R.P.) then independently evaluated those 2 assessments for agreement. Where disagreement was discovered, the third evaluator (J.R.P.) provided a final appraisal. Cohen’s kappa (κ) was conducted to evaluate for concordance between the 2 primary website evaluators. We found there to be substantial agreement between the reviewers within the education/research (κ [SD]=0.772 [0.077]), clinical training (κ [SD]=0.740 [0.051]), application process (κ [SD]=0.726 [0.103]), and incentives domains (κ [SD]=0.730 [0.110]). There was moderate agreement (κ [SD]=0.603 [0.128]) between the reviewers within the program information domain.
We identified 77 active MSDO fellowship programs. Sixty of those 77 programs (77.9%) had a dedicated fellowship website that was readily accessible. Most programs that had a dedicated fellowship website had a core or affiliated residency program (49/60 [81.7%]).
Websites that we evaluated fulfilled a mean (SD) of 9.37 (4.17) of the 25 identified criteria. Only 13 of 60 (21.7%) websites fulfilled more than 50% of evaluated criteria.
There was no statistical difference in the number of criteria fulfilled based on whether the fellowship program had a core or affiliated residency program.
Upon reviewing website accessibility directly from FREIDA, only 5 of 60 programs (8.3%) provided applicants with a link directly to their fellowship page (Table). Most programs (41 [68.3%]) provided a link to the dermatology department website, not to the specific fellowship program page, thus requiring a multistep process to find the fellowship-specific page. The remaining programs had an inaccessible (4 [6.7%]) or absent (10 [16.7%]) link on FREIDA, though a fellowship website could be identified by an Internet search of the program name.

The domain most fulfilled was program information with an average of 51.1% of programs satisfying the criteria, whereas the incentives domain was least fulfilled with an average of only 20.8% of programs satisfying the criteria. Across the various criteria, websites more often included a description of the program (58 [96.6%]), mentioned accreditation (53 [88.3%]), and provided case descriptions (48 [80.0%]). They less often reported information regarding a fellow’s call responsibility (3 [5%]); evaluation criteria (5 [8.3%]); and rotation schedule or options (6 [10.0%]).
The highest number of criteria fulfilled by a single program was 19 (76%). The lowest number of criteria met was 2 (8%). These findings suggest a large variation in comprehensiveness across fellowship websites.
Our research suggests that many current MSDO fellowship programs have room to maximize the information provided to applicants through their websites, which is particularly relevant following the COVID-19 pandemic, as the value of providing comprehensive and transparent information through an online platform is greater than ever. Given the ongoing desire to limit travel, virtual methods for navigating the application process have been readily used, including online videoconferencing for interviews and virtual program visits. This scenario has placed applicants in a challenging situation—their ability to directly evaluate their compatibility with a given program has been limited.3
Earlier studies that analyzed rheumatology fellowship recruitment during the COVID-19 pandemic found that programs may have more difficulty highlighting the strengths of their institution (eg, clinical facilities, professional opportunities, educational environment).4 An updated and comprehensive fellowship website was recommended4 as a key part in facing these new challenges. On the other hand, given the large number of applicants each year for fellowship positions in any given program, we acknowledge the potential benefit programs may obtain from limiting electronic information that is readily accessible to all applicants, as doing so may encourage applicants to communicate directly with a program and allow programs to identify candidates who are more interested.
In light of the movement to a more virtual-friendly and technology-driven fellowship application process, we identified 25 content areas that fellowships may want to include on their websites so that potential applicants can be well informed about the program before submitting an application and scheduling an interview. Efforts to improve accessibility and maximize the content of these websites may help programs attract compatible candidates, improve transparency, and guide applicants throughout the application process.
- Lu F, Vijayasarathi A, Murray N, et al. Evaluation of pediatric radiology fellowship website content in USA and Canada. Curr Prob Diagn Radiol. 2021;50:151-155. doi:10.1067/j.cpradiol.2020.01.007
- Cantrell CK, Bergstresser SL, Schuh AC, et al. Accessibility and content of abdominal transplant fellowship program websites in the United States. J Surg Res. 2018;232:271-274. doi:10.1016/j.jss.2018.06.052
- Nesemeier BR, Lebo NL, Schmalbach CE, et al. Impact of the COVID-19 global pandemic on the otolaryngology fellowship application process. Otolaryngol Head Neck Surg. 2020;163:712-713. doi:10.1177/0194599820934370
- Kilian A, Dua AB, Bolster MB, et al. Rheumatology fellowship recruitment in 2020: benefits, challenges, and adaptations. Arthritis Care Res (Hoboken). 2021;73:459-461. doi:10.1002/acr.24445
- Lu F, Vijayasarathi A, Murray N, et al. Evaluation of pediatric radiology fellowship website content in USA and Canada. Curr Prob Diagn Radiol. 2021;50:151-155. doi:10.1067/j.cpradiol.2020.01.007
- Cantrell CK, Bergstresser SL, Schuh AC, et al. Accessibility and content of abdominal transplant fellowship program websites in the United States. J Surg Res. 2018;232:271-274. doi:10.1016/j.jss.2018.06.052
- Nesemeier BR, Lebo NL, Schmalbach CE, et al. Impact of the COVID-19 global pandemic on the otolaryngology fellowship application process. Otolaryngol Head Neck Surg. 2020;163:712-713. doi:10.1177/0194599820934370
- Kilian A, Dua AB, Bolster MB, et al. Rheumatology fellowship recruitment in 2020: benefits, challenges, and adaptations. Arthritis Care Res (Hoboken). 2021;73:459-461. doi:10.1002/acr.24445
Practice Points
- With the COVID-19 pandemic and the movement to a virtual fellowship application process, fellowship program websites that are comprehensive and accessible may help programs attract compatible candidates, improve transparency, and guide applicants through the application process.
- There is variation in the content of current micrographic surgery and dermatologic oncology fellowship program websites and areas upon which programs may seek to augment their website content to better reflect program strengths while attracting competitive candidates best suited for their program.
Economic Burden and Quality of Life of Patients With Moderate to Severe Atopic Dermatitis in a Tertiary Care Hospital in Helsinki, Finland: A Survey-Based Study
Atopic dermatitis (AD) is a common inflammatory skin disease that may severely decrease quality of life (QOL) and lead to psychiatric comorbidities.1-3 Prior studies have indicated that AD causes a substantial economic burden, and disease severity has been proportionally linked to medical costs.4,5 Results of a multicenter cost-of-illness study from Germany estimated that a relapse of AD costs approximately €123 (US $136). The authors calculated the average annual cost of AD per patient to be €1425 (US $1580), whereas it is €956 (US $1060) in moderate disease and €2068 (US $2293) in severe disease (direct and indirect medical costs included).6 An observational cohort study from the Netherlands found that total direct cost per patient-year (PPY) was €4401 (US $4879) for patients with controlled AD vs €6993 (US $7756) for patients with uncontrolled AD.7
In a retrospective survey-based study, it was estimated that the annual cost of AD in Canada was approximately CAD $1.4 billion. The cost per patient varied from CAD $282 to CAD $1242 depending on disease severity.8 In another retrospective cohort study from the Netherlands, the average direct medical cost per patient with AD seeing a general practitioner was US $71 during follow-up in primary care. If the patient needed specialist consultation, the cost increased to an average of US $186.9
We aimed to assess the direct and indirect medical costs in adult patients with moderate to severe AD who attended a tertiary health care center in Finland. In addition, we evaluated the impact of AD on QOL in this patient cohort.
Methods
Study Design—Patients with AD who were treated at the Department of Dermatology and Allergology, Helsinki University Hospital, Finland, between February 2018 and December 2019 were randomly selected to participate in our survey study. All participants provided written informed consent. In Finland, patients with mild AD generally are treated in primary health care centers, and only patients with moderate to severe AD are referred to specialists and tertiary care centers. Patients were excluded if they were younger than 18 years, had AD confined to the hands, or reported the presence of other concomitant skin diseases that were being treated with topical or systemic therapies. The protocol for the study was approved by the local ethics committee of the University of Helsinki.
Questionnaire and Analysis of Disease Severity—The survey included the medical history, signs of atopy, former treatment(s) for AD, skin infections, visits to dermatologists or general practitioners, questions on mental health and hospitalization, and absence from work due to AD in the last 12 months. Disease severity was evaluated using the patient-oriented Rajka & Langeland eczema severity score and Patient Oriented Eczema Measure (POEM).10,11 The impact on QOL was evaluated by the Dermatology Life Quality Index (DLQI).12
Medication Costs—The cost of prescription drugs was based on data from the Finnish national electronic prescription center. In Finland, all prescriptions are made electronically in the database. We analyzed all topical medications (eg, topical corticosteroids [TCSs], topical calcineurin inhibitors [TCIs], and emollients) and systemic medicaments (eg, antibiotics, antihistamines, cyclosporine, methotrexate, and corticosteroids) prescribed for the treatment of AD. In Finland, dupilumab was introduced for the treatment of severe AD in early 2019, and patients receiving dupilumab were excluded from the study. Over-the-counter medications were not included. The costs for laboratory testing were estimations based on the standard monitoring protocols of the Helsinki University Hospital. All costs were based on the Finnish price level standard for the year 2019.
Inpatient/Outpatient Visits and Sick Leave Due to AD—The number of inpatient and outpatient visits due to AD in the last 12 months was evaluated. Outpatient specialist consultations or nurse appointments at Helsinki University Hospital were verified from electronic patient records. In addition, inpatient treatment and phototherapy sessions were calculated from the database.
We assessed the number of sick leave days from work or educational activities during the last year. All costs of transportation for doctors’ appointments, laboratory monitoring, and phototherapy treatments were summed together to estimate the total transportation cost. Visits to nurse and inpatient visits were not included in the total transportation cost because patients often were hospitalized directly after consultation visits, and nurse appointments often were combined with inpatient and outpatient visits. To calculate the total transportation cost, we used a rate of €0.43 per kilometer measured from the patients’ home addresses, which was the official compensation rate of the Finnish Tax Administration for 2019.13
Statistical Analysis—Statistical analyses were performed using SPSS Statistics 25 (IBM). Descriptive analyses were used to describe baseline characteristics and to evaluate the mean costs of AD. The patients were divided into 2 groups according to POEM: (1) controlled AD (patients with clear skin or only mild AD; POEM score 0–7) and (2) uncontrolled AD (patients with moderate to very severe AD; POEM score 8–28). The Mann-Whitney U statistic was used to evaluate differences between the study groups.
Results
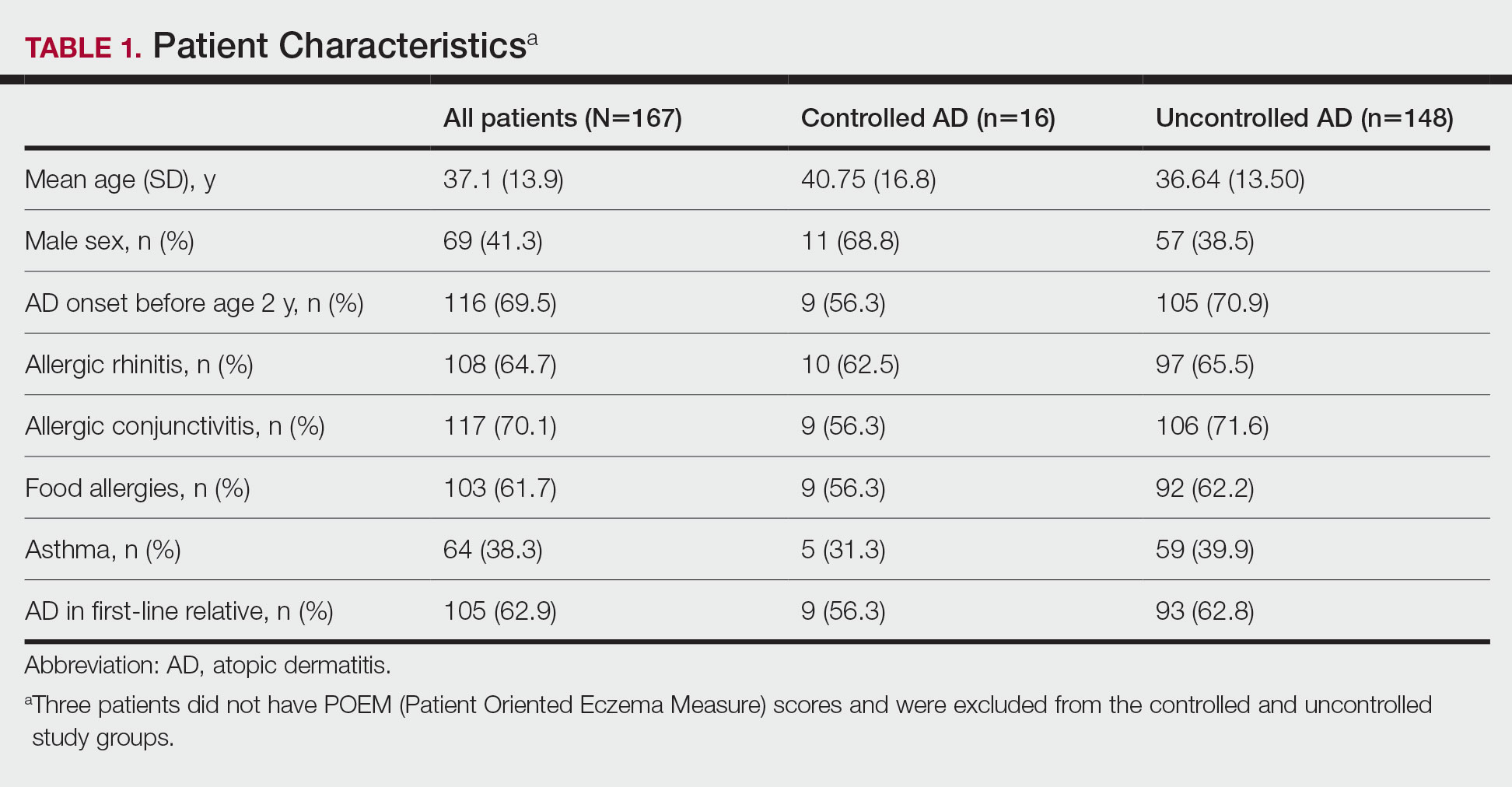
Patient Characteristics—One hundred sixty-seven patients answered the survey, of which 69 (41.3%) were males and 98 (58.7%) were females. There were 16 patients with controlled AD and 148 patients with uncontrolled AD. Three patients did not answer to POEM and were excluded. The baseline characteristics are presented in Table 1 and include self-reported symptoms related to atopy.
The most-used topical treatments were TCSs (n=155; 92.8%) and emollients (n=166; 99.4%). One hundred sixteen (69.5%) patients had used TCIs. The median amount of TCSs used was 300 g/y vs 30 g/y for TCIs (range, 0-5160 g/y) and 1200 g/y for emollients.
Fifteen (9.0%) patients had been hospitalized for AD in the last year. The mean (SD) length of hospitalization was 6.5 (2.8) days. Thirty-four (20.4%) patients received UVB phototherapy. Thirty-four (20.4%) patients were treated with at least 1 antibiotic course for secondary AD infection. Thirty-six (21.6%) patients needed at least 1 oral corticosteroid course for the treatment of an AD flare.
Fifteen (9.0%) patients reported a diagnosed psychiatric illness, and 17 (10.2%) patients were using prescription drugs for psychiatric illness. Forty-nine (29.3%) patients reported anxiety or depression often or very often, 54 (32.3%) patients reported sometimes, 33 (19.8%) patients reported rarely, and only 30 (18.0%) patients reported none.
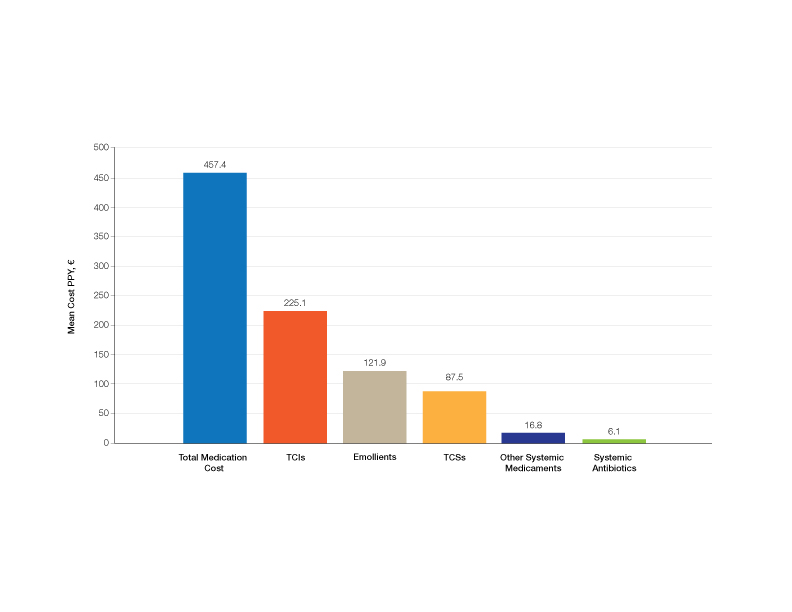
Medication Costs—Mean medication cost PPY was €457.40 (US $507.34)(Figure 1 and Table 2). On average, one patient spent €87.50 (US $97.05) for TCSs, €121.90 (US $135.21) for emollients, and €225.10 (US $249.68) for TCIs. The average cost PPY for antibiotics was €6.10 (US $6.77). Other systemic treatments, including (US $18.65). Seventeen patients (10.2%) were on methotrexate therapy for AD in the last year, and 1 patient also used cyclosporine. The costs for laboratory monitoring in these patients were included in the direct cost calculations. The mean cost PPY of laboratory monitoring in the whole study cohort was €6.60 (US $7.32). In patients with systemic immunosuppressive therapy, the mean cost PPY for laboratory monitoring was €65.00 (US $72.09). Five patients had been tested for contact dermatitis; the costs of patch tests or other diagnostic tests were not included.
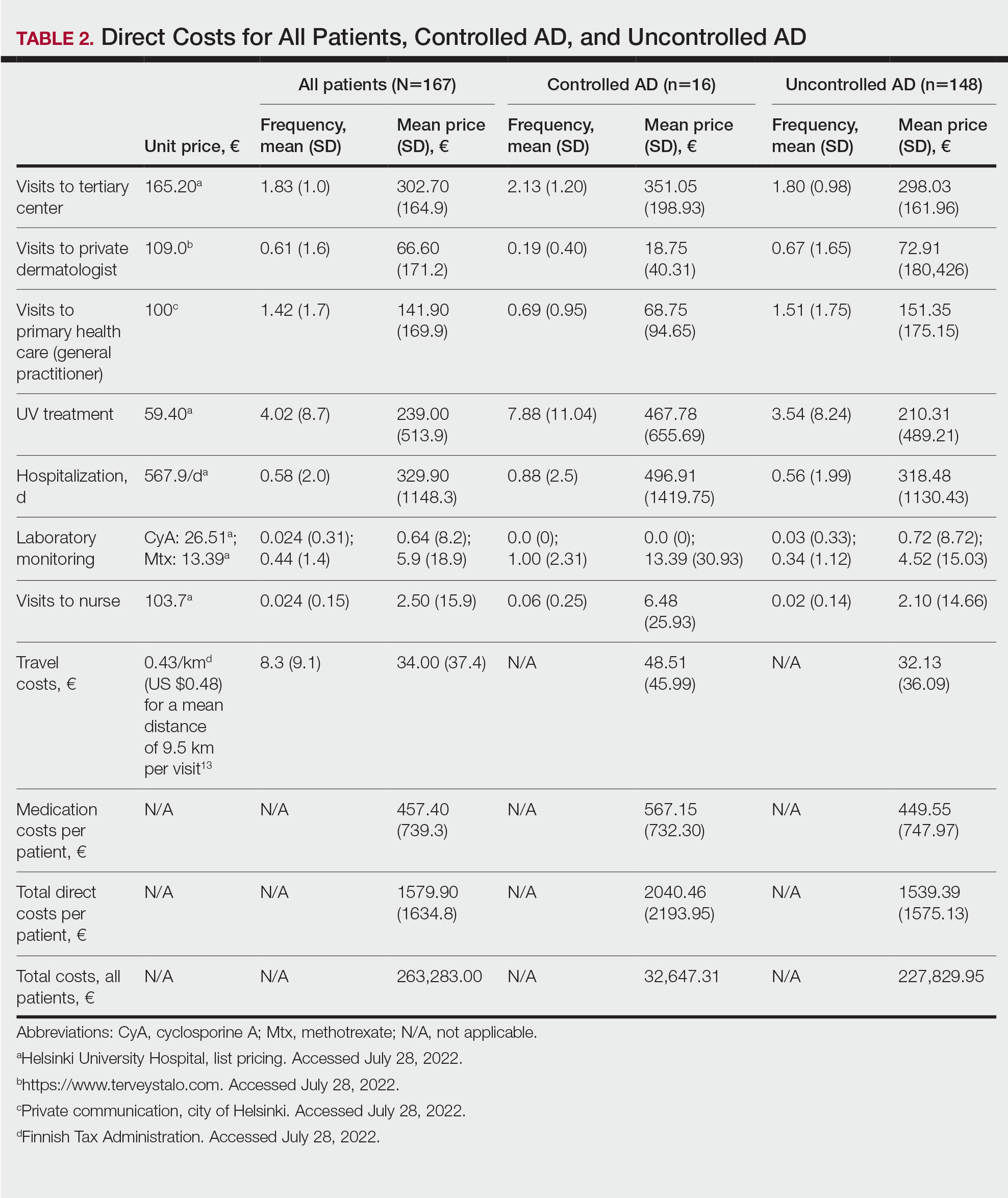
Visits to Health Care Providers—In the last year, patients had an average of 1.83 dermatologist consultations in the tertiary center (Table 2). In addition, the mean number of visits to private dermatologists was 0.61 and 1.42 visits to general practitioners. The mean cost of physician visits was €302.70 (US $335.75) in the tertiary center, €66.60 (US $73.87) in the private sector, and €141.90 (US $157.39) in primary health care. In total, the average cost of doctors’ appointments PPY was €506.30 (US $561.57). The mean estimated distance traveled per visit was 9.5 km.
The mean cost PPY of inpatient treatments was €329.90 (US $365.92) and €239.00 (US $265.09) for UV phototherapy. Only 4 patients had visited a nurse in the last year, with an average cost PPY of €2.50 (US $2.78).
In total, the cost PPY for health care provider visits was €1084.20, which included specialist consultations in a tertiary center and private sector, visits in primary health care, inpatient treatments, UV phototherapy sessions, nurse appointments in a tertiary center, and laboratory monitoring. The average transportation cost PPY was €34.00 (US $37.71). The mean number of visits to health care providers was 8.3 per year. Altogether, the direct cost PPY in the study cohort was €1580.60 (US $1752.39)(Table 2 and Figure 2).

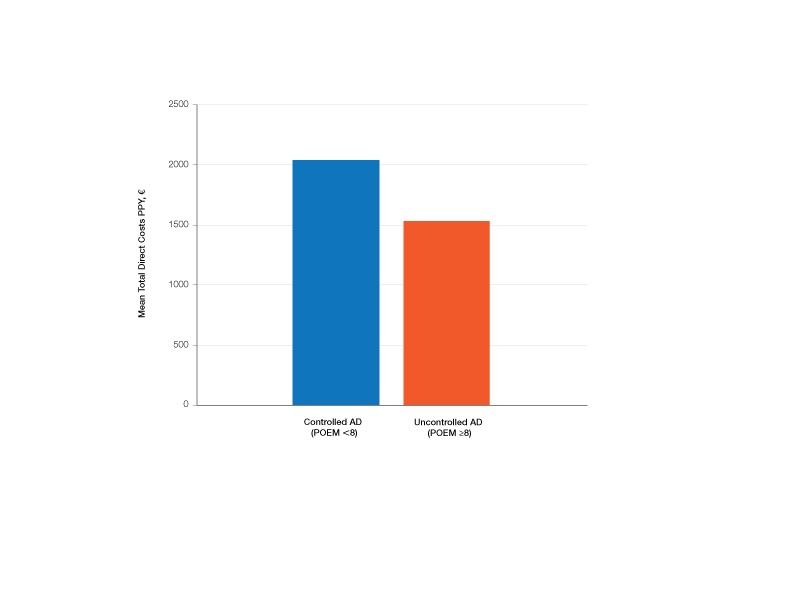
Comparison of Medical Costs in Controlled vs Uncontrolled AD—In the controlled AD group (POEM score <8), the mean medication cost PPY was €567.15 (US $629.13), and the mean total direct cost PPY was €2040.46 (US $2263.24). In the uncontrolled AD group (POEM score ≥8), the mean medication cost PPY was €449.55 (US $498.63), and the mean total direct cost PPY was €1539.39 (US $1707.36)(Table 2). The comparisons of the study groups—controlled vs uncontrolled AD—showed no significant differences regarding medication costs PPY (P=.305, Mann-Whitney U statistic) and total direct costs PPY (P=.361, Mann-Whitney U statistic)(Figure 3). Thus, the distribution of medical costs was similar across all categories of the POEM score.
AD Severity and QOL—The mean (SD) POEM score in the study cohort was 17.9 (6.9). Sixteen (9.6%) patients had clear to almost clear skin or mild AD (POEM score 0–7). Forty-two (25.1%) patients had moderate AD (POEM score 8–16). Most of the patients (106; 63.5%) had severe or very severe AD (POEM score 17–28). According to the Rajka & Langeland score, 5 (3.0%) patients had mild disease (score 34), 81 (48.5%) patients had moderate disease (score 5–7), and 81 (48.5%) patients had severe disease (score 8–9). Eighty-one (48.5%) patients answered that AD affects their lives greatly, and 58 (34.7%) patients answered that it affects their lives extremely. Twenty-five (15.0%) patients answered that AD affects their everyday life to some extent, and only 2 (1.2%) patients answered that AD had little or no effect.
The mean (SD) DLQI was 13 (7.2). Based on the DLQI, 31 (18.6%) patients answered that AD had no effect or only a small effect on QOL (DLQI 0–5). In 36 (21.6%) patients, AD had a moderate effect on QOL (DLQI 6–10). The QOL impact was large (DLQI 11–20) and very large (DLQI 21–30) in 67 (40.1%) and 33 (19.8%) patients, respectively.
There was no significant difference in the impact of disease severity (POEM score) on the decrease of QOL (severe or very severe disease; P=.305, Mann-Whitney U statistic).
Absence From Work or Studies—At the study inclusion, 12 (7.2%) patients were not working or studying. Of the remaining 155 patients, 73 (47.1%) reported absence from work or educational activities due to AD in the last 12 months. The mean (SD) length of absence was 11.6 (10.2) days.
Comment
In this survey-based study of Finnish patients with moderate to severe AD, we observed that AD creates a substantial economic burden14 and negative impact on everyday life and QOL. According to DLQI, AD had a large or very large effect on most of the patients’ (59.9%) lives, and 90.2% of the included patients had self-reported moderate to very severe symptoms (POEM score 8–28). Our observations can partly be explained by characteristics of the Finnish health care system, in which patients with moderate to severe AD mainly are referred to specialist consultation. In the investigated cohort, many patients had used antibiotics (20.4%) and/or oral corticosteroids (21.6%) in the last year for the treatment of AD, which might indicate inadequate treatment of AD in the Finnish health care system.
Motivating patients to remain compliant is one of the main challenges in AD therapy.15 Fear of adverse effects from TCSs is common among patients and may cause poor treatment adherence.16 In a prospective study from the United Kingdom, the use of emollients in moderate to severe AD was considerably lower than AD guidelines recommend—approximately 10 g/d on average in adult patients. The median use of TCSs was between 35 and 38 g/mo.17 In our Finnish patient cohort, the amount of topical treatments was even lower, with a median use of emollients of 3.3 g/d and median use of TCSs of 25 g/mo. In another study from Denmark (N=322), 31% of patients with AD did not redeem their topical prescription medicaments, indicating poor adherence to topical treatment.18
It has been demonstrated that most of the patients’ habituation (tachyphylaxis) to TCSs is due to poor adherence instead of physiologic changes in tissue corticosteroid receptors.19,20 Treatment adherence may be increased by scheduling early follow-up visits and providing adequate therapeutic patient education,21 which requires major efforts by the health care system and a financial investment.
Inadequate treatment will lead to more frequent disease flares and subsequently increase the medical costs for the patients and the health care system.22 In our Finnish patient cohort, a large part of direct treatment costs was due to inpatient treatment (Figure 2) even though only a small proportion of patients had been hospitalized. The patients were frequently young and otherwise in good general health, and they did not necessarily need continuous inpatient treatment and monitoring. In Finland, it will be necessary to develop more cost-effective treatment regimens for patients with AD with severe and frequent flares. Many patients would benefit from subsequent and regular sessions of topical treatment in an outpatient setting. In addition, the prevention of flares in moderate to severe AD will decrease medical costs.23
The mean medication cost PPY was €457.40 (US $507.34), and mean total direct cost PPY was €1579.90 (US $1752.40), which indicates that AD causes a major economic burden to Finnish patients and to the Finnish health care system (Figures 1 and 2).24 We did not observe significant differences between controlled and uncontrolled AD medical costs in our patient cohort (Figure 3), which may have been due to the relatively small sample size of only 16 patients in the controlled AD group. All patients attending the tertiary care hospital had moderate to severe AD, so it is likely that the patients with lower POEM scores had better-controlled disease. The POEM score estimates the grade of AD in the last 7 days, but based on the relapsing course of the disease, the grading score may differ substantially during the year in the same patient depending on the timing.25,26
Topical calcineurin inhibitors comprised almost half of the medication costs (Figure 1), which may be caused by their higher prices compared with TCSs in Finland. In the beginning of 2019, a 50% less expensive biosimilar of tacrolimus ointment 0.1% was introduced to the Finnish market, which might decrease future treatment costs of TCIs. However, availability problems in both topical tacrolimus products were seen throughout 2019, which also may have affected the results in our study cohort. The median use of TCIs was unexpectedly low (only 30 g/y), which may be explained by different application habits. The use of large TCI amounts in some patients may have elevated mean costs.27
In the Finnish public health care system, 40% of the cost for prescription medication and emollients is reimbursed after an initial deductible of €50. Emollients are reimbursed up to an amount of 1500 g/mo. Therefore, patients mostly acquired emollients as prescription medicine and not over-the-counter. Nonprescription medicaments were not included in our study, so the actual costs of topical treatment may have been higher.28
In our cohort, 61.7% of the patients reported food allergies, and 70.1% reported allergic conjunctivitis. However, the study included only questionnaire-based data, and many of these patients probably had symptoms not associated with IgE-mediated allergies. The high prevalence indicates a substantial concomitant burden of more than skin symptoms in patients with AD.29 Nine percent of patients reported a diagnosed psychiatric disorder, and 29.3% had self-reported anxiety or depression often or very often in the last year. Based on these findings, there may be high percentages of undiagnosed psychiatric comorbidities such as depression and anxiety disorders in patients with moderate to severe AD in Finland.30 An important limitation of our study was that the patient data were based on a voluntary and anonymous survey and that depression and anxiety were addressed solely by a single question. In addition, the response rate cannot be analyzed correctly, and the demographics of the survey responders likely will differ substantially from all patients with AD at the university hospital.
Atopic dermatitis had a substantial effect on QOL in our patient cohort. Inadequate treatment of AD is known to negatively affect patient QOL and may lead to hospitalization or frequent oral corticosteroid courses.31,32 In most cases, structured patient education and early follow-up visits may improve patient adherence to treatment and should be considered as an integral part of AD treatment.33 In the investigated Finnish tertiary care hospital, a structured patient education system unfortunately was still lacking, though it has been proven effective elsewhere.34 In addition, patient-centred educational programs are recommended in European guidelines for the treatment of AD.35
Medical costs of AD may increase in the future as new treatments with higher direct costs, such as dupilumab, are introduced. Eichenfeld et al36 analyzed electronic health plan claims in patients with AD with newly introduced systemic therapies and phototherapies after the availability of dupilumab in the United States (March 2017). Mean annualized total cost in all patients was $20,722; the highest in the dupilumab group with $36,505. Compared to our data, the total costs are much higher, but these are likely to rise in Finland in the future if a substantial amount (eg, 1%–5%) of patients will be on advanced therapies, including dupilumab. If advanced therapies will be introduced more broadly in Finland (eg, in the treatment of moderate AD [10%–20% of patients]), they will represent a major direct cost to the health care system. Zimmermann et al37 showed in a cost-utility analysis that dupilumab improves health outcomes but with additional direct costs, and it is likely more cost-effective in patients with severe AD. Conversely, more efficient treatments may improve severe AD, reduce the need for hospitalization and recurrent doctors’ appointments as well as absence from work, and improve patient QOL,38 consequently decreasing indirect medical costs and disease burden. Ariëns et al39 showed in a recent registry-based study that dupilumab treatment induces a notable rise in work productivity and reduction of associated costs in patients with difficult-to-treat AD.
Conclusion
We aimed to analyze the economic burden of AD in Finland before the introduction of dupilumab. It will be interesting to see what the introduction of dupilumab and other novel systemic therapies have on total economic burden and medical costs. Most patients with AD in Finland can achieve disease control with topical treatments, but it is important to efficiently manage the patients who require additional supportive measures and specialist consultations, which may be challenging in the primary health care system because of the relapsing and remitting nature of the disease.
- Nutten S. Atopic dermatitis: global epidemiology and risk factors. Ann Nutr Metab. 2015;66(suppl 1):8-16.
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351.
- Yang EJ, Beck KM, Sekhon S, et al. The impact of pediatric atopic dermatitis on families: a review. Pediatr Dermatol. 2019;36:66-71.
- Eckert L, Gupta S, Amand C, et al. Impact of atopic dermatitis on health-related quality of life and productivity in adults in the United States: an analysis using the National Health and Wellness Survey. J Am Acad Dermatol. 2017;77:274-279.
- Drucker AM, Wang AR, Li WQ, et al. The burden of atopic dermatitis: summary of a report for the National Eczema Association. J Invest Dermatol. 2017;137:26-30.
- Ehlken B, Möhrenschlager M, Kugland B, et al. Cost-of-illness study in patients suffering from atopic eczema in Germany. Der Hautarzt. 2006;56:1144-1151.
- Ariëns LFM, van Nimwegen KJM, Shams M, et al. Economic burden of adult patients with moderate to severe atopic dermatitis indicated for systemic treatment. Acta Derm Venereol. 2019;99:762-768.
- Barbeau M, Bpharm HL. Burden of atopic dermatitis in Canada. Int J Dermatol. 2006;45:31-36.
- Verboom P, Hakkaart‐Van Roijen L, Sturkenboom M, et al. The cost of atopic dermatitis in the Netherlands: an international comparison. Br J Dermatol. 2002;147:716-724.
- Gånemo A, Svensson Å, Svedman C, et al. Usefulness of Rajka & Langeland eczema severity score in clinical practice. Acta Derm Venereol. 2016;96:521-524.
- Charman CR, Venn AJ, Williams HC. The Patient-Oriented Eczema Measure: development and initial validation of a new tool for measuring atopic eczema severity from the patients’ perspective. Arch Dermatol. 2004;140:1513-1519.
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI): a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19:210-216.
- Rehunen A, Reissell E, Honkatukia J, et al. Social and health services: regional changes in need, use and production and future options. Accessed July 20, 2023. http://urn.fi/URN:ISBN:978-952-287-294-4
- Reed B, Blaiss MS. The burden of atopic dermatitis. Allergy Asthma Proc. 2018;39:406-410.
- Koszorú K, Borza J, Gulácsi L, et al. Quality of life in patients with atopic dermatitis. Cutis. 2019;104:174-177.
- Li AW, Yin ES, Antaya RJ. Topical corticosteroid phobia in atopic dermatitis: a systematic review. JAMA Dermatol. 2017;153:1036-1042.
- Choi J, Dawe R, Ibbotson S, et al. Quantitative analysis of topical treatments in atopic dermatitis: unexpectedly low use of emollients and strong correlation of topical corticosteroid use both with depression and concurrent asthma. Br J Dermatol. 2020;182:1017-1025.
- Storm A, Andersen SE, Benfeldt E, et al. One in 3 prescriptions are never redeemed: primary nonadherence in an outpatient clinic. J Am Acad Dermatol. 2008;59:27-33.
- Okwundu N, Cardwell LA, Cline A, et al. Topical corticosteroids for treatment-resistant atopic dermatitis. Cutis. 2018;102:205-209.
- Eicher L, Knop M, Aszodi N, et al. A systematic review of factors influencing treatment adherence in chronic inflammatory skin disease—strategies for optimizing treatment outcome. J Eur Acad Dermatol Venereol. 2019;33:2253-2263.
- Heratizadeh A, Werfel T, Wollenberg A, et al; Arbeitsgemeinschaft Neurodermitisschulung für Erwachsene (ARNE) Study Group. Effects of structured patient education in adults with atopic dermatitis: multicenter randomized controlled trial. J Allergy Clin Immunol. 2017;140:845-853.
- Dierick BJH, van der Molen T, Flokstra-de Blok BMJ, et al. Burden and socioeconomics of asthma, allergic rhinitis, atopic dermatitis and food allergy. Expert Rev Pharmacoecon Outcomes Res. 2020;20:437-453.
- Olsson M, Bajpai R, Yew YW, et al. Associations between health-related quality of life and health care costs among children with atopic dermatitis and their caregivers: a cross-sectional study. Pediatr Dermatol. 2020;37:284-293.
- Bruin-Weller M, Pink AE, Patrizi A, et al. Disease burden and treatment history among adults with atopic dermatitis receiving systemic therapy: baseline characteristics of participants on the EUROSTAD prospective observational study. J Dermatolog Treat. 2021;32:164-173.
- Silverberg JI, Lei D, Yousaf M, et al. Comparison of Patient-Oriented Eczema Measure and Patient-Oriented Scoring Atopic Dermatitis vs Eczema Area and Severity Index and other measures of atopic dermatitis: a validation study. Ann Allergy Asthma Immunol. 2020;125:78-83.
- Kido-Nakahara M, Nakahara T, Yasukochi Y, et al. Patient-oriented eczema measure score: a useful tool for web-based surveys in patients with atopic dermatitis. Acta Derm Venereol. 2020;47:924-925.
- Komura Y, Kogure T, Kawahara K, et al. Economic assessment of actual prescription of drugs for treatment of atopic dermatitis: differences between dermatology and pediatrics in large-scale receipt data. J Dermatol. 2018;45:165-174.
- Thompson AM, Chan A, Torabi M, et al. Eczema moisturizers: allergenic potential, marketing claims, and costs. Dermatol Ther. 2020;33:E14228.
- Egeberg A, Andersen YM, Gislason GH, et al. Prevalence of comorbidity and associated risk factors in adults with atopic dermatitis. Allergy. 2017;72:783-791.
- Kauppi S, Jokelainen J, Timonen M, et al. Adult patients with atopic eczema have a high burden of psychiatric disease: a Finnish nationwide registry study. Acta Derm Venereol. 2019;99:647-651.
- Ali F, Vyas J, Finlay AY. Counting the burden: atopic dermatitis and health-related quality of life. Acta Derm Venereol. 2020;100:adv00161.
- Birdi G, Cooke R, Knibb RC. Impact of atopic dermatitis on quality of life in adults: a systematic review and meta-analysis. Int J Dermatol. 2020;59:E75-E91.
- Gabes M, Tischer C, Apfelbacher C; quality of life working group of the Harmonising Outcome Measures for Eczema (HOME) initiative. Measurement properties of quality-of-life outcome measures for children and adults with eczema: an updated systematic review. Pediatr Allergy Immunol. 2020;31:66-77.
- Staab D, Diepgen TL, Fartasch M, et al. Age related, structured educational programmes for the management of atopic dermatitis in children and adolescents: multicentre, randomised controlled trial. BMJ. 2006;332:933-938.
- Wollenberg A, Barbarot S, Bieber T, et al; European Dermatology Forum (EDF), the European Academy of Dermatology and Venereology (EADV), the European Academy of Allergy and Clinical Immunology (EAACI), the European Task Force on Atopic Dermatitis (ETFAD), European Federation of Allergy and Airways Diseases Patients’ Associations (EFA), the European Society for Dermatology and Psychiatry (ESDaP), the European Society of Pediatric Dermatology (ESPD), Global Allergy and Asthma European Network (GA2LEN) and the European Union of Medical Specialists (UEMS). Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part II. J Eur Acad Dermatol Venereol. 2018;32:850-878.
- Eichenfield LF, DiBonaventura M, Xenakis J, et al. Costs and treatment patterns among patients with atopic dermatitis using advanced therapies in the United States: analysis of a retrospective claims database. Dermatol Ther (Heidelb). 2020;10:791-806.
- Zimmermann M, Rind D, Chapman R, et al. Economic evaluation of dupilumab for moderate-to-severe atopic dermatitis: a cost-utility analysis. J Drugs Dermatol. 2018;17:750-756.
- Mata E, Loh TY, Ludwig C, et al. Pharmacy costs of systemic and topical medications for atopic dermatitis. J Dermatolog Treat. 2019;12:1-3.
- Ariëns LFM, Bakker DS, Spekhorst LS, et al. Rapid and sustained effect of dupilumab on work productivity in patients with difficult-to-treat atopic dermatitis: results from the Dutch BioDay Registry. Acta Derm Venereol. 2021;19;101:adv00573.
Atopic dermatitis (AD) is a common inflammatory skin disease that may severely decrease quality of life (QOL) and lead to psychiatric comorbidities.1-3 Prior studies have indicated that AD causes a substantial economic burden, and disease severity has been proportionally linked to medical costs.4,5 Results of a multicenter cost-of-illness study from Germany estimated that a relapse of AD costs approximately €123 (US $136). The authors calculated the average annual cost of AD per patient to be €1425 (US $1580), whereas it is €956 (US $1060) in moderate disease and €2068 (US $2293) in severe disease (direct and indirect medical costs included).6 An observational cohort study from the Netherlands found that total direct cost per patient-year (PPY) was €4401 (US $4879) for patients with controlled AD vs €6993 (US $7756) for patients with uncontrolled AD.7
In a retrospective survey-based study, it was estimated that the annual cost of AD in Canada was approximately CAD $1.4 billion. The cost per patient varied from CAD $282 to CAD $1242 depending on disease severity.8 In another retrospective cohort study from the Netherlands, the average direct medical cost per patient with AD seeing a general practitioner was US $71 during follow-up in primary care. If the patient needed specialist consultation, the cost increased to an average of US $186.9
We aimed to assess the direct and indirect medical costs in adult patients with moderate to severe AD who attended a tertiary health care center in Finland. In addition, we evaluated the impact of AD on QOL in this patient cohort.
Methods
Study Design—Patients with AD who were treated at the Department of Dermatology and Allergology, Helsinki University Hospital, Finland, between February 2018 and December 2019 were randomly selected to participate in our survey study. All participants provided written informed consent. In Finland, patients with mild AD generally are treated in primary health care centers, and only patients with moderate to severe AD are referred to specialists and tertiary care centers. Patients were excluded if they were younger than 18 years, had AD confined to the hands, or reported the presence of other concomitant skin diseases that were being treated with topical or systemic therapies. The protocol for the study was approved by the local ethics committee of the University of Helsinki.
Questionnaire and Analysis of Disease Severity—The survey included the medical history, signs of atopy, former treatment(s) for AD, skin infections, visits to dermatologists or general practitioners, questions on mental health and hospitalization, and absence from work due to AD in the last 12 months. Disease severity was evaluated using the patient-oriented Rajka & Langeland eczema severity score and Patient Oriented Eczema Measure (POEM).10,11 The impact on QOL was evaluated by the Dermatology Life Quality Index (DLQI).12
Medication Costs—The cost of prescription drugs was based on data from the Finnish national electronic prescription center. In Finland, all prescriptions are made electronically in the database. We analyzed all topical medications (eg, topical corticosteroids [TCSs], topical calcineurin inhibitors [TCIs], and emollients) and systemic medicaments (eg, antibiotics, antihistamines, cyclosporine, methotrexate, and corticosteroids) prescribed for the treatment of AD. In Finland, dupilumab was introduced for the treatment of severe AD in early 2019, and patients receiving dupilumab were excluded from the study. Over-the-counter medications were not included. The costs for laboratory testing were estimations based on the standard monitoring protocols of the Helsinki University Hospital. All costs were based on the Finnish price level standard for the year 2019.
Inpatient/Outpatient Visits and Sick Leave Due to AD—The number of inpatient and outpatient visits due to AD in the last 12 months was evaluated. Outpatient specialist consultations or nurse appointments at Helsinki University Hospital were verified from electronic patient records. In addition, inpatient treatment and phototherapy sessions were calculated from the database.
We assessed the number of sick leave days from work or educational activities during the last year. All costs of transportation for doctors’ appointments, laboratory monitoring, and phototherapy treatments were summed together to estimate the total transportation cost. Visits to nurse and inpatient visits were not included in the total transportation cost because patients often were hospitalized directly after consultation visits, and nurse appointments often were combined with inpatient and outpatient visits. To calculate the total transportation cost, we used a rate of €0.43 per kilometer measured from the patients’ home addresses, which was the official compensation rate of the Finnish Tax Administration for 2019.13
Statistical Analysis—Statistical analyses were performed using SPSS Statistics 25 (IBM). Descriptive analyses were used to describe baseline characteristics and to evaluate the mean costs of AD. The patients were divided into 2 groups according to POEM: (1) controlled AD (patients with clear skin or only mild AD; POEM score 0–7) and (2) uncontrolled AD (patients with moderate to very severe AD; POEM score 8–28). The Mann-Whitney U statistic was used to evaluate differences between the study groups.
Results
Patient Characteristics—One hundred sixty-seven patients answered the survey, of which 69 (41.3%) were males and 98 (58.7%) were females. There were 16 patients with controlled AD and 148 patients with uncontrolled AD. Three patients did not answer to POEM and were excluded. The baseline characteristics are presented in Table 1 and include self-reported symptoms related to atopy.

The most-used topical treatments were TCSs (n=155; 92.8%) and emollients (n=166; 99.4%). One hundred sixteen (69.5%) patients had used TCIs. The median amount of TCSs used was 300 g/y vs 30 g/y for TCIs (range, 0-5160 g/y) and 1200 g/y for emollients.
Fifteen (9.0%) patients had been hospitalized for AD in the last year. The mean (SD) length of hospitalization was 6.5 (2.8) days. Thirty-four (20.4%) patients received UVB phototherapy. Thirty-four (20.4%) patients were treated with at least 1 antibiotic course for secondary AD infection. Thirty-six (21.6%) patients needed at least 1 oral corticosteroid course for the treatment of an AD flare.
Fifteen (9.0%) patients reported a diagnosed psychiatric illness, and 17 (10.2%) patients were using prescription drugs for psychiatric illness. Forty-nine (29.3%) patients reported anxiety or depression often or very often, 54 (32.3%) patients reported sometimes, 33 (19.8%) patients reported rarely, and only 30 (18.0%) patients reported none.

Medication Costs—Mean medication cost PPY was €457.40 (US $507.34)(Figure 1 and Table 2). On average, one patient spent €87.50 (US $97.05) for TCSs, €121.90 (US $135.21) for emollients, and €225.10 (US $249.68) for TCIs. The average cost PPY for antibiotics was €6.10 (US $6.77). Other systemic treatments, including (US $18.65). Seventeen patients (10.2%) were on methotrexate therapy for AD in the last year, and 1 patient also used cyclosporine. The costs for laboratory monitoring in these patients were included in the direct cost calculations. The mean cost PPY of laboratory monitoring in the whole study cohort was €6.60 (US $7.32). In patients with systemic immunosuppressive therapy, the mean cost PPY for laboratory monitoring was €65.00 (US $72.09). Five patients had been tested for contact dermatitis; the costs of patch tests or other diagnostic tests were not included.

Visits to Health Care Providers—In the last year, patients had an average of 1.83 dermatologist consultations in the tertiary center (Table 2). In addition, the mean number of visits to private dermatologists was 0.61 and 1.42 visits to general practitioners. The mean cost of physician visits was €302.70 (US $335.75) in the tertiary center, €66.60 (US $73.87) in the private sector, and €141.90 (US $157.39) in primary health care. In total, the average cost of doctors’ appointments PPY was €506.30 (US $561.57). The mean estimated distance traveled per visit was 9.5 km.
The mean cost PPY of inpatient treatments was €329.90 (US $365.92) and €239.00 (US $265.09) for UV phototherapy. Only 4 patients had visited a nurse in the last year, with an average cost PPY of €2.50 (US $2.78).
In total, the cost PPY for health care provider visits was €1084.20, which included specialist consultations in a tertiary center and private sector, visits in primary health care, inpatient treatments, UV phototherapy sessions, nurse appointments in a tertiary center, and laboratory monitoring. The average transportation cost PPY was €34.00 (US $37.71). The mean number of visits to health care providers was 8.3 per year. Altogether, the direct cost PPY in the study cohort was €1580.60 (US $1752.39)(Table 2 and Figure 2).

Comparison of Medical Costs in Controlled vs Uncontrolled AD—In the controlled AD group (POEM score <8), the mean medication cost PPY was €567.15 (US $629.13), and the mean total direct cost PPY was €2040.46 (US $2263.24). In the uncontrolled AD group (POEM score ≥8), the mean medication cost PPY was €449.55 (US $498.63), and the mean total direct cost PPY was €1539.39 (US $1707.36)(Table 2). The comparisons of the study groups—controlled vs uncontrolled AD—showed no significant differences regarding medication costs PPY (P=.305, Mann-Whitney U statistic) and total direct costs PPY (P=.361, Mann-Whitney U statistic)(Figure 3). Thus, the distribution of medical costs was similar across all categories of the POEM score.

AD Severity and QOL—The mean (SD) POEM score in the study cohort was 17.9 (6.9). Sixteen (9.6%) patients had clear to almost clear skin or mild AD (POEM score 0–7). Forty-two (25.1%) patients had moderate AD (POEM score 8–16). Most of the patients (106; 63.5%) had severe or very severe AD (POEM score 17–28). According to the Rajka & Langeland score, 5 (3.0%) patients had mild disease (score 34), 81 (48.5%) patients had moderate disease (score 5–7), and 81 (48.5%) patients had severe disease (score 8–9). Eighty-one (48.5%) patients answered that AD affects their lives greatly, and 58 (34.7%) patients answered that it affects their lives extremely. Twenty-five (15.0%) patients answered that AD affects their everyday life to some extent, and only 2 (1.2%) patients answered that AD had little or no effect.
The mean (SD) DLQI was 13 (7.2). Based on the DLQI, 31 (18.6%) patients answered that AD had no effect or only a small effect on QOL (DLQI 0–5). In 36 (21.6%) patients, AD had a moderate effect on QOL (DLQI 6–10). The QOL impact was large (DLQI 11–20) and very large (DLQI 21–30) in 67 (40.1%) and 33 (19.8%) patients, respectively.
There was no significant difference in the impact of disease severity (POEM score) on the decrease of QOL (severe or very severe disease; P=.305, Mann-Whitney U statistic).
Absence From Work or Studies—At the study inclusion, 12 (7.2%) patients were not working or studying. Of the remaining 155 patients, 73 (47.1%) reported absence from work or educational activities due to AD in the last 12 months. The mean (SD) length of absence was 11.6 (10.2) days.
Comment
In this survey-based study of Finnish patients with moderate to severe AD, we observed that AD creates a substantial economic burden14 and negative impact on everyday life and QOL. According to DLQI, AD had a large or very large effect on most of the patients’ (59.9%) lives, and 90.2% of the included patients had self-reported moderate to very severe symptoms (POEM score 8–28). Our observations can partly be explained by characteristics of the Finnish health care system, in which patients with moderate to severe AD mainly are referred to specialist consultation. In the investigated cohort, many patients had used antibiotics (20.4%) and/or oral corticosteroids (21.6%) in the last year for the treatment of AD, which might indicate inadequate treatment of AD in the Finnish health care system.
Motivating patients to remain compliant is one of the main challenges in AD therapy.15 Fear of adverse effects from TCSs is common among patients and may cause poor treatment adherence.16 In a prospective study from the United Kingdom, the use of emollients in moderate to severe AD was considerably lower than AD guidelines recommend—approximately 10 g/d on average in adult patients. The median use of TCSs was between 35 and 38 g/mo.17 In our Finnish patient cohort, the amount of topical treatments was even lower, with a median use of emollients of 3.3 g/d and median use of TCSs of 25 g/mo. In another study from Denmark (N=322), 31% of patients with AD did not redeem their topical prescription medicaments, indicating poor adherence to topical treatment.18
It has been demonstrated that most of the patients’ habituation (tachyphylaxis) to TCSs is due to poor adherence instead of physiologic changes in tissue corticosteroid receptors.19,20 Treatment adherence may be increased by scheduling early follow-up visits and providing adequate therapeutic patient education,21 which requires major efforts by the health care system and a financial investment.
Inadequate treatment will lead to more frequent disease flares and subsequently increase the medical costs for the patients and the health care system.22 In our Finnish patient cohort, a large part of direct treatment costs was due to inpatient treatment (Figure 2) even though only a small proportion of patients had been hospitalized. The patients were frequently young and otherwise in good general health, and they did not necessarily need continuous inpatient treatment and monitoring. In Finland, it will be necessary to develop more cost-effective treatment regimens for patients with AD with severe and frequent flares. Many patients would benefit from subsequent and regular sessions of topical treatment in an outpatient setting. In addition, the prevention of flares in moderate to severe AD will decrease medical costs.23
The mean medication cost PPY was €457.40 (US $507.34), and mean total direct cost PPY was €1579.90 (US $1752.40), which indicates that AD causes a major economic burden to Finnish patients and to the Finnish health care system (Figures 1 and 2).24 We did not observe significant differences between controlled and uncontrolled AD medical costs in our patient cohort (Figure 3), which may have been due to the relatively small sample size of only 16 patients in the controlled AD group. All patients attending the tertiary care hospital had moderate to severe AD, so it is likely that the patients with lower POEM scores had better-controlled disease. The POEM score estimates the grade of AD in the last 7 days, but based on the relapsing course of the disease, the grading score may differ substantially during the year in the same patient depending on the timing.25,26
Topical calcineurin inhibitors comprised almost half of the medication costs (Figure 1), which may be caused by their higher prices compared with TCSs in Finland. In the beginning of 2019, a 50% less expensive biosimilar of tacrolimus ointment 0.1% was introduced to the Finnish market, which might decrease future treatment costs of TCIs. However, availability problems in both topical tacrolimus products were seen throughout 2019, which also may have affected the results in our study cohort. The median use of TCIs was unexpectedly low (only 30 g/y), which may be explained by different application habits. The use of large TCI amounts in some patients may have elevated mean costs.27
In the Finnish public health care system, 40% of the cost for prescription medication and emollients is reimbursed after an initial deductible of €50. Emollients are reimbursed up to an amount of 1500 g/mo. Therefore, patients mostly acquired emollients as prescription medicine and not over-the-counter. Nonprescription medicaments were not included in our study, so the actual costs of topical treatment may have been higher.28
In our cohort, 61.7% of the patients reported food allergies, and 70.1% reported allergic conjunctivitis. However, the study included only questionnaire-based data, and many of these patients probably had symptoms not associated with IgE-mediated allergies. The high prevalence indicates a substantial concomitant burden of more than skin symptoms in patients with AD.29 Nine percent of patients reported a diagnosed psychiatric disorder, and 29.3% had self-reported anxiety or depression often or very often in the last year. Based on these findings, there may be high percentages of undiagnosed psychiatric comorbidities such as depression and anxiety disorders in patients with moderate to severe AD in Finland.30 An important limitation of our study was that the patient data were based on a voluntary and anonymous survey and that depression and anxiety were addressed solely by a single question. In addition, the response rate cannot be analyzed correctly, and the demographics of the survey responders likely will differ substantially from all patients with AD at the university hospital.
Atopic dermatitis had a substantial effect on QOL in our patient cohort. Inadequate treatment of AD is known to negatively affect patient QOL and may lead to hospitalization or frequent oral corticosteroid courses.31,32 In most cases, structured patient education and early follow-up visits may improve patient adherence to treatment and should be considered as an integral part of AD treatment.33 In the investigated Finnish tertiary care hospital, a structured patient education system unfortunately was still lacking, though it has been proven effective elsewhere.34 In addition, patient-centred educational programs are recommended in European guidelines for the treatment of AD.35
Medical costs of AD may increase in the future as new treatments with higher direct costs, such as dupilumab, are introduced. Eichenfeld et al36 analyzed electronic health plan claims in patients with AD with newly introduced systemic therapies and phototherapies after the availability of dupilumab in the United States (March 2017). Mean annualized total cost in all patients was $20,722; the highest in the dupilumab group with $36,505. Compared to our data, the total costs are much higher, but these are likely to rise in Finland in the future if a substantial amount (eg, 1%–5%) of patients will be on advanced therapies, including dupilumab. If advanced therapies will be introduced more broadly in Finland (eg, in the treatment of moderate AD [10%–20% of patients]), they will represent a major direct cost to the health care system. Zimmermann et al37 showed in a cost-utility analysis that dupilumab improves health outcomes but with additional direct costs, and it is likely more cost-effective in patients with severe AD. Conversely, more efficient treatments may improve severe AD, reduce the need for hospitalization and recurrent doctors’ appointments as well as absence from work, and improve patient QOL,38 consequently decreasing indirect medical costs and disease burden. Ariëns et al39 showed in a recent registry-based study that dupilumab treatment induces a notable rise in work productivity and reduction of associated costs in patients with difficult-to-treat AD.
Conclusion
We aimed to analyze the economic burden of AD in Finland before the introduction of dupilumab. It will be interesting to see what the introduction of dupilumab and other novel systemic therapies have on total economic burden and medical costs. Most patients with AD in Finland can achieve disease control with topical treatments, but it is important to efficiently manage the patients who require additional supportive measures and specialist consultations, which may be challenging in the primary health care system because of the relapsing and remitting nature of the disease.
Atopic dermatitis (AD) is a common inflammatory skin disease that may severely decrease quality of life (QOL) and lead to psychiatric comorbidities.1-3 Prior studies have indicated that AD causes a substantial economic burden, and disease severity has been proportionally linked to medical costs.4,5 Results of a multicenter cost-of-illness study from Germany estimated that a relapse of AD costs approximately €123 (US $136). The authors calculated the average annual cost of AD per patient to be €1425 (US $1580), whereas it is €956 (US $1060) in moderate disease and €2068 (US $2293) in severe disease (direct and indirect medical costs included).6 An observational cohort study from the Netherlands found that total direct cost per patient-year (PPY) was €4401 (US $4879) for patients with controlled AD vs €6993 (US $7756) for patients with uncontrolled AD.7
In a retrospective survey-based study, it was estimated that the annual cost of AD in Canada was approximately CAD $1.4 billion. The cost per patient varied from CAD $282 to CAD $1242 depending on disease severity.8 In another retrospective cohort study from the Netherlands, the average direct medical cost per patient with AD seeing a general practitioner was US $71 during follow-up in primary care. If the patient needed specialist consultation, the cost increased to an average of US $186.9
We aimed to assess the direct and indirect medical costs in adult patients with moderate to severe AD who attended a tertiary health care center in Finland. In addition, we evaluated the impact of AD on QOL in this patient cohort.
Methods
Study Design—Patients with AD who were treated at the Department of Dermatology and Allergology, Helsinki University Hospital, Finland, between February 2018 and December 2019 were randomly selected to participate in our survey study. All participants provided written informed consent. In Finland, patients with mild AD generally are treated in primary health care centers, and only patients with moderate to severe AD are referred to specialists and tertiary care centers. Patients were excluded if they were younger than 18 years, had AD confined to the hands, or reported the presence of other concomitant skin diseases that were being treated with topical or systemic therapies. The protocol for the study was approved by the local ethics committee of the University of Helsinki.
Questionnaire and Analysis of Disease Severity—The survey included the medical history, signs of atopy, former treatment(s) for AD, skin infections, visits to dermatologists or general practitioners, questions on mental health and hospitalization, and absence from work due to AD in the last 12 months. Disease severity was evaluated using the patient-oriented Rajka & Langeland eczema severity score and Patient Oriented Eczema Measure (POEM).10,11 The impact on QOL was evaluated by the Dermatology Life Quality Index (DLQI).12
Medication Costs—The cost of prescription drugs was based on data from the Finnish national electronic prescription center. In Finland, all prescriptions are made electronically in the database. We analyzed all topical medications (eg, topical corticosteroids [TCSs], topical calcineurin inhibitors [TCIs], and emollients) and systemic medicaments (eg, antibiotics, antihistamines, cyclosporine, methotrexate, and corticosteroids) prescribed for the treatment of AD. In Finland, dupilumab was introduced for the treatment of severe AD in early 2019, and patients receiving dupilumab were excluded from the study. Over-the-counter medications were not included. The costs for laboratory testing were estimations based on the standard monitoring protocols of the Helsinki University Hospital. All costs were based on the Finnish price level standard for the year 2019.
Inpatient/Outpatient Visits and Sick Leave Due to AD—The number of inpatient and outpatient visits due to AD in the last 12 months was evaluated. Outpatient specialist consultations or nurse appointments at Helsinki University Hospital were verified from electronic patient records. In addition, inpatient treatment and phototherapy sessions were calculated from the database.
We assessed the number of sick leave days from work or educational activities during the last year. All costs of transportation for doctors’ appointments, laboratory monitoring, and phototherapy treatments were summed together to estimate the total transportation cost. Visits to nurse and inpatient visits were not included in the total transportation cost because patients often were hospitalized directly after consultation visits, and nurse appointments often were combined with inpatient and outpatient visits. To calculate the total transportation cost, we used a rate of €0.43 per kilometer measured from the patients’ home addresses, which was the official compensation rate of the Finnish Tax Administration for 2019.13
Statistical Analysis—Statistical analyses were performed using SPSS Statistics 25 (IBM). Descriptive analyses were used to describe baseline characteristics and to evaluate the mean costs of AD. The patients were divided into 2 groups according to POEM: (1) controlled AD (patients with clear skin or only mild AD; POEM score 0–7) and (2) uncontrolled AD (patients with moderate to very severe AD; POEM score 8–28). The Mann-Whitney U statistic was used to evaluate differences between the study groups.
Results
Patient Characteristics—One hundred sixty-seven patients answered the survey, of which 69 (41.3%) were males and 98 (58.7%) were females. There were 16 patients with controlled AD and 148 patients with uncontrolled AD. Three patients did not answer to POEM and were excluded. The baseline characteristics are presented in Table 1 and include self-reported symptoms related to atopy.

The most-used topical treatments were TCSs (n=155; 92.8%) and emollients (n=166; 99.4%). One hundred sixteen (69.5%) patients had used TCIs. The median amount of TCSs used was 300 g/y vs 30 g/y for TCIs (range, 0-5160 g/y) and 1200 g/y for emollients.
Fifteen (9.0%) patients had been hospitalized for AD in the last year. The mean (SD) length of hospitalization was 6.5 (2.8) days. Thirty-four (20.4%) patients received UVB phototherapy. Thirty-four (20.4%) patients were treated with at least 1 antibiotic course for secondary AD infection. Thirty-six (21.6%) patients needed at least 1 oral corticosteroid course for the treatment of an AD flare.
Fifteen (9.0%) patients reported a diagnosed psychiatric illness, and 17 (10.2%) patients were using prescription drugs for psychiatric illness. Forty-nine (29.3%) patients reported anxiety or depression often or very often, 54 (32.3%) patients reported sometimes, 33 (19.8%) patients reported rarely, and only 30 (18.0%) patients reported none.

Medication Costs—Mean medication cost PPY was €457.40 (US $507.34)(Figure 1 and Table 2). On average, one patient spent €87.50 (US $97.05) for TCSs, €121.90 (US $135.21) for emollients, and €225.10 (US $249.68) for TCIs. The average cost PPY for antibiotics was €6.10 (US $6.77). Other systemic treatments, including (US $18.65). Seventeen patients (10.2%) were on methotrexate therapy for AD in the last year, and 1 patient also used cyclosporine. The costs for laboratory monitoring in these patients were included in the direct cost calculations. The mean cost PPY of laboratory monitoring in the whole study cohort was €6.60 (US $7.32). In patients with systemic immunosuppressive therapy, the mean cost PPY for laboratory monitoring was €65.00 (US $72.09). Five patients had been tested for contact dermatitis; the costs of patch tests or other diagnostic tests were not included.

Visits to Health Care Providers—In the last year, patients had an average of 1.83 dermatologist consultations in the tertiary center (Table 2). In addition, the mean number of visits to private dermatologists was 0.61 and 1.42 visits to general practitioners. The mean cost of physician visits was €302.70 (US $335.75) in the tertiary center, €66.60 (US $73.87) in the private sector, and €141.90 (US $157.39) in primary health care. In total, the average cost of doctors’ appointments PPY was €506.30 (US $561.57). The mean estimated distance traveled per visit was 9.5 km.
The mean cost PPY of inpatient treatments was €329.90 (US $365.92) and €239.00 (US $265.09) for UV phototherapy. Only 4 patients had visited a nurse in the last year, with an average cost PPY of €2.50 (US $2.78).
In total, the cost PPY for health care provider visits was €1084.20, which included specialist consultations in a tertiary center and private sector, visits in primary health care, inpatient treatments, UV phototherapy sessions, nurse appointments in a tertiary center, and laboratory monitoring. The average transportation cost PPY was €34.00 (US $37.71). The mean number of visits to health care providers was 8.3 per year. Altogether, the direct cost PPY in the study cohort was €1580.60 (US $1752.39)(Table 2 and Figure 2).

Comparison of Medical Costs in Controlled vs Uncontrolled AD—In the controlled AD group (POEM score <8), the mean medication cost PPY was €567.15 (US $629.13), and the mean total direct cost PPY was €2040.46 (US $2263.24). In the uncontrolled AD group (POEM score ≥8), the mean medication cost PPY was €449.55 (US $498.63), and the mean total direct cost PPY was €1539.39 (US $1707.36)(Table 2). The comparisons of the study groups—controlled vs uncontrolled AD—showed no significant differences regarding medication costs PPY (P=.305, Mann-Whitney U statistic) and total direct costs PPY (P=.361, Mann-Whitney U statistic)(Figure 3). Thus, the distribution of medical costs was similar across all categories of the POEM score.

AD Severity and QOL—The mean (SD) POEM score in the study cohort was 17.9 (6.9). Sixteen (9.6%) patients had clear to almost clear skin or mild AD (POEM score 0–7). Forty-two (25.1%) patients had moderate AD (POEM score 8–16). Most of the patients (106; 63.5%) had severe or very severe AD (POEM score 17–28). According to the Rajka & Langeland score, 5 (3.0%) patients had mild disease (score 34), 81 (48.5%) patients had moderate disease (score 5–7), and 81 (48.5%) patients had severe disease (score 8–9). Eighty-one (48.5%) patients answered that AD affects their lives greatly, and 58 (34.7%) patients answered that it affects their lives extremely. Twenty-five (15.0%) patients answered that AD affects their everyday life to some extent, and only 2 (1.2%) patients answered that AD had little or no effect.
The mean (SD) DLQI was 13 (7.2). Based on the DLQI, 31 (18.6%) patients answered that AD had no effect or only a small effect on QOL (DLQI 0–5). In 36 (21.6%) patients, AD had a moderate effect on QOL (DLQI 6–10). The QOL impact was large (DLQI 11–20) and very large (DLQI 21–30) in 67 (40.1%) and 33 (19.8%) patients, respectively.
There was no significant difference in the impact of disease severity (POEM score) on the decrease of QOL (severe or very severe disease; P=.305, Mann-Whitney U statistic).
Absence From Work or Studies—At the study inclusion, 12 (7.2%) patients were not working or studying. Of the remaining 155 patients, 73 (47.1%) reported absence from work or educational activities due to AD in the last 12 months. The mean (SD) length of absence was 11.6 (10.2) days.
Comment
In this survey-based study of Finnish patients with moderate to severe AD, we observed that AD creates a substantial economic burden14 and negative impact on everyday life and QOL. According to DLQI, AD had a large or very large effect on most of the patients’ (59.9%) lives, and 90.2% of the included patients had self-reported moderate to very severe symptoms (POEM score 8–28). Our observations can partly be explained by characteristics of the Finnish health care system, in which patients with moderate to severe AD mainly are referred to specialist consultation. In the investigated cohort, many patients had used antibiotics (20.4%) and/or oral corticosteroids (21.6%) in the last year for the treatment of AD, which might indicate inadequate treatment of AD in the Finnish health care system.
Motivating patients to remain compliant is one of the main challenges in AD therapy.15 Fear of adverse effects from TCSs is common among patients and may cause poor treatment adherence.16 In a prospective study from the United Kingdom, the use of emollients in moderate to severe AD was considerably lower than AD guidelines recommend—approximately 10 g/d on average in adult patients. The median use of TCSs was between 35 and 38 g/mo.17 In our Finnish patient cohort, the amount of topical treatments was even lower, with a median use of emollients of 3.3 g/d and median use of TCSs of 25 g/mo. In another study from Denmark (N=322), 31% of patients with AD did not redeem their topical prescription medicaments, indicating poor adherence to topical treatment.18
It has been demonstrated that most of the patients’ habituation (tachyphylaxis) to TCSs is due to poor adherence instead of physiologic changes in tissue corticosteroid receptors.19,20 Treatment adherence may be increased by scheduling early follow-up visits and providing adequate therapeutic patient education,21 which requires major efforts by the health care system and a financial investment.
Inadequate treatment will lead to more frequent disease flares and subsequently increase the medical costs for the patients and the health care system.22 In our Finnish patient cohort, a large part of direct treatment costs was due to inpatient treatment (Figure 2) even though only a small proportion of patients had been hospitalized. The patients were frequently young and otherwise in good general health, and they did not necessarily need continuous inpatient treatment and monitoring. In Finland, it will be necessary to develop more cost-effective treatment regimens for patients with AD with severe and frequent flares. Many patients would benefit from subsequent and regular sessions of topical treatment in an outpatient setting. In addition, the prevention of flares in moderate to severe AD will decrease medical costs.23
The mean medication cost PPY was €457.40 (US $507.34), and mean total direct cost PPY was €1579.90 (US $1752.40), which indicates that AD causes a major economic burden to Finnish patients and to the Finnish health care system (Figures 1 and 2).24 We did not observe significant differences between controlled and uncontrolled AD medical costs in our patient cohort (Figure 3), which may have been due to the relatively small sample size of only 16 patients in the controlled AD group. All patients attending the tertiary care hospital had moderate to severe AD, so it is likely that the patients with lower POEM scores had better-controlled disease. The POEM score estimates the grade of AD in the last 7 days, but based on the relapsing course of the disease, the grading score may differ substantially during the year in the same patient depending on the timing.25,26
Topical calcineurin inhibitors comprised almost half of the medication costs (Figure 1), which may be caused by their higher prices compared with TCSs in Finland. In the beginning of 2019, a 50% less expensive biosimilar of tacrolimus ointment 0.1% was introduced to the Finnish market, which might decrease future treatment costs of TCIs. However, availability problems in both topical tacrolimus products were seen throughout 2019, which also may have affected the results in our study cohort. The median use of TCIs was unexpectedly low (only 30 g/y), which may be explained by different application habits. The use of large TCI amounts in some patients may have elevated mean costs.27
In the Finnish public health care system, 40% of the cost for prescription medication and emollients is reimbursed after an initial deductible of €50. Emollients are reimbursed up to an amount of 1500 g/mo. Therefore, patients mostly acquired emollients as prescription medicine and not over-the-counter. Nonprescription medicaments were not included in our study, so the actual costs of topical treatment may have been higher.28
In our cohort, 61.7% of the patients reported food allergies, and 70.1% reported allergic conjunctivitis. However, the study included only questionnaire-based data, and many of these patients probably had symptoms not associated with IgE-mediated allergies. The high prevalence indicates a substantial concomitant burden of more than skin symptoms in patients with AD.29 Nine percent of patients reported a diagnosed psychiatric disorder, and 29.3% had self-reported anxiety or depression often or very often in the last year. Based on these findings, there may be high percentages of undiagnosed psychiatric comorbidities such as depression and anxiety disorders in patients with moderate to severe AD in Finland.30 An important limitation of our study was that the patient data were based on a voluntary and anonymous survey and that depression and anxiety were addressed solely by a single question. In addition, the response rate cannot be analyzed correctly, and the demographics of the survey responders likely will differ substantially from all patients with AD at the university hospital.
Atopic dermatitis had a substantial effect on QOL in our patient cohort. Inadequate treatment of AD is known to negatively affect patient QOL and may lead to hospitalization or frequent oral corticosteroid courses.31,32 In most cases, structured patient education and early follow-up visits may improve patient adherence to treatment and should be considered as an integral part of AD treatment.33 In the investigated Finnish tertiary care hospital, a structured patient education system unfortunately was still lacking, though it has been proven effective elsewhere.34 In addition, patient-centred educational programs are recommended in European guidelines for the treatment of AD.35
Medical costs of AD may increase in the future as new treatments with higher direct costs, such as dupilumab, are introduced. Eichenfeld et al36 analyzed electronic health plan claims in patients with AD with newly introduced systemic therapies and phototherapies after the availability of dupilumab in the United States (March 2017). Mean annualized total cost in all patients was $20,722; the highest in the dupilumab group with $36,505. Compared to our data, the total costs are much higher, but these are likely to rise in Finland in the future if a substantial amount (eg, 1%–5%) of patients will be on advanced therapies, including dupilumab. If advanced therapies will be introduced more broadly in Finland (eg, in the treatment of moderate AD [10%–20% of patients]), they will represent a major direct cost to the health care system. Zimmermann et al37 showed in a cost-utility analysis that dupilumab improves health outcomes but with additional direct costs, and it is likely more cost-effective in patients with severe AD. Conversely, more efficient treatments may improve severe AD, reduce the need for hospitalization and recurrent doctors’ appointments as well as absence from work, and improve patient QOL,38 consequently decreasing indirect medical costs and disease burden. Ariëns et al39 showed in a recent registry-based study that dupilumab treatment induces a notable rise in work productivity and reduction of associated costs in patients with difficult-to-treat AD.
Conclusion
We aimed to analyze the economic burden of AD in Finland before the introduction of dupilumab. It will be interesting to see what the introduction of dupilumab and other novel systemic therapies have on total economic burden and medical costs. Most patients with AD in Finland can achieve disease control with topical treatments, but it is important to efficiently manage the patients who require additional supportive measures and specialist consultations, which may be challenging in the primary health care system because of the relapsing and remitting nature of the disease.
- Nutten S. Atopic dermatitis: global epidemiology and risk factors. Ann Nutr Metab. 2015;66(suppl 1):8-16.
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351.
- Yang EJ, Beck KM, Sekhon S, et al. The impact of pediatric atopic dermatitis on families: a review. Pediatr Dermatol. 2019;36:66-71.
- Eckert L, Gupta S, Amand C, et al. Impact of atopic dermatitis on health-related quality of life and productivity in adults in the United States: an analysis using the National Health and Wellness Survey. J Am Acad Dermatol. 2017;77:274-279.
- Drucker AM, Wang AR, Li WQ, et al. The burden of atopic dermatitis: summary of a report for the National Eczema Association. J Invest Dermatol. 2017;137:26-30.
- Ehlken B, Möhrenschlager M, Kugland B, et al. Cost-of-illness study in patients suffering from atopic eczema in Germany. Der Hautarzt. 2006;56:1144-1151.
- Ariëns LFM, van Nimwegen KJM, Shams M, et al. Economic burden of adult patients with moderate to severe atopic dermatitis indicated for systemic treatment. Acta Derm Venereol. 2019;99:762-768.
- Barbeau M, Bpharm HL. Burden of atopic dermatitis in Canada. Int J Dermatol. 2006;45:31-36.
- Verboom P, Hakkaart‐Van Roijen L, Sturkenboom M, et al. The cost of atopic dermatitis in the Netherlands: an international comparison. Br J Dermatol. 2002;147:716-724.
- Gånemo A, Svensson Å, Svedman C, et al. Usefulness of Rajka & Langeland eczema severity score in clinical practice. Acta Derm Venereol. 2016;96:521-524.
- Charman CR, Venn AJ, Williams HC. The Patient-Oriented Eczema Measure: development and initial validation of a new tool for measuring atopic eczema severity from the patients’ perspective. Arch Dermatol. 2004;140:1513-1519.
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI): a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19:210-216.
- Rehunen A, Reissell E, Honkatukia J, et al. Social and health services: regional changes in need, use and production and future options. Accessed July 20, 2023. http://urn.fi/URN:ISBN:978-952-287-294-4
- Reed B, Blaiss MS. The burden of atopic dermatitis. Allergy Asthma Proc. 2018;39:406-410.
- Koszorú K, Borza J, Gulácsi L, et al. Quality of life in patients with atopic dermatitis. Cutis. 2019;104:174-177.
- Li AW, Yin ES, Antaya RJ. Topical corticosteroid phobia in atopic dermatitis: a systematic review. JAMA Dermatol. 2017;153:1036-1042.
- Choi J, Dawe R, Ibbotson S, et al. Quantitative analysis of topical treatments in atopic dermatitis: unexpectedly low use of emollients and strong correlation of topical corticosteroid use both with depression and concurrent asthma. Br J Dermatol. 2020;182:1017-1025.
- Storm A, Andersen SE, Benfeldt E, et al. One in 3 prescriptions are never redeemed: primary nonadherence in an outpatient clinic. J Am Acad Dermatol. 2008;59:27-33.
- Okwundu N, Cardwell LA, Cline A, et al. Topical corticosteroids for treatment-resistant atopic dermatitis. Cutis. 2018;102:205-209.
- Eicher L, Knop M, Aszodi N, et al. A systematic review of factors influencing treatment adherence in chronic inflammatory skin disease—strategies for optimizing treatment outcome. J Eur Acad Dermatol Venereol. 2019;33:2253-2263.
- Heratizadeh A, Werfel T, Wollenberg A, et al; Arbeitsgemeinschaft Neurodermitisschulung für Erwachsene (ARNE) Study Group. Effects of structured patient education in adults with atopic dermatitis: multicenter randomized controlled trial. J Allergy Clin Immunol. 2017;140:845-853.
- Dierick BJH, van der Molen T, Flokstra-de Blok BMJ, et al. Burden and socioeconomics of asthma, allergic rhinitis, atopic dermatitis and food allergy. Expert Rev Pharmacoecon Outcomes Res. 2020;20:437-453.
- Olsson M, Bajpai R, Yew YW, et al. Associations between health-related quality of life and health care costs among children with atopic dermatitis and their caregivers: a cross-sectional study. Pediatr Dermatol. 2020;37:284-293.
- Bruin-Weller M, Pink AE, Patrizi A, et al. Disease burden and treatment history among adults with atopic dermatitis receiving systemic therapy: baseline characteristics of participants on the EUROSTAD prospective observational study. J Dermatolog Treat. 2021;32:164-173.
- Silverberg JI, Lei D, Yousaf M, et al. Comparison of Patient-Oriented Eczema Measure and Patient-Oriented Scoring Atopic Dermatitis vs Eczema Area and Severity Index and other measures of atopic dermatitis: a validation study. Ann Allergy Asthma Immunol. 2020;125:78-83.
- Kido-Nakahara M, Nakahara T, Yasukochi Y, et al. Patient-oriented eczema measure score: a useful tool for web-based surveys in patients with atopic dermatitis. Acta Derm Venereol. 2020;47:924-925.
- Komura Y, Kogure T, Kawahara K, et al. Economic assessment of actual prescription of drugs for treatment of atopic dermatitis: differences between dermatology and pediatrics in large-scale receipt data. J Dermatol. 2018;45:165-174.
- Thompson AM, Chan A, Torabi M, et al. Eczema moisturizers: allergenic potential, marketing claims, and costs. Dermatol Ther. 2020;33:E14228.
- Egeberg A, Andersen YM, Gislason GH, et al. Prevalence of comorbidity and associated risk factors in adults with atopic dermatitis. Allergy. 2017;72:783-791.
- Kauppi S, Jokelainen J, Timonen M, et al. Adult patients with atopic eczema have a high burden of psychiatric disease: a Finnish nationwide registry study. Acta Derm Venereol. 2019;99:647-651.
- Ali F, Vyas J, Finlay AY. Counting the burden: atopic dermatitis and health-related quality of life. Acta Derm Venereol. 2020;100:adv00161.
- Birdi G, Cooke R, Knibb RC. Impact of atopic dermatitis on quality of life in adults: a systematic review and meta-analysis. Int J Dermatol. 2020;59:E75-E91.
- Gabes M, Tischer C, Apfelbacher C; quality of life working group of the Harmonising Outcome Measures for Eczema (HOME) initiative. Measurement properties of quality-of-life outcome measures for children and adults with eczema: an updated systematic review. Pediatr Allergy Immunol. 2020;31:66-77.
- Staab D, Diepgen TL, Fartasch M, et al. Age related, structured educational programmes for the management of atopic dermatitis in children and adolescents: multicentre, randomised controlled trial. BMJ. 2006;332:933-938.
- Wollenberg A, Barbarot S, Bieber T, et al; European Dermatology Forum (EDF), the European Academy of Dermatology and Venereology (EADV), the European Academy of Allergy and Clinical Immunology (EAACI), the European Task Force on Atopic Dermatitis (ETFAD), European Federation of Allergy and Airways Diseases Patients’ Associations (EFA), the European Society for Dermatology and Psychiatry (ESDaP), the European Society of Pediatric Dermatology (ESPD), Global Allergy and Asthma European Network (GA2LEN) and the European Union of Medical Specialists (UEMS). Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part II. J Eur Acad Dermatol Venereol. 2018;32:850-878.
- Eichenfield LF, DiBonaventura M, Xenakis J, et al. Costs and treatment patterns among patients with atopic dermatitis using advanced therapies in the United States: analysis of a retrospective claims database. Dermatol Ther (Heidelb). 2020;10:791-806.
- Zimmermann M, Rind D, Chapman R, et al. Economic evaluation of dupilumab for moderate-to-severe atopic dermatitis: a cost-utility analysis. J Drugs Dermatol. 2018;17:750-756.
- Mata E, Loh TY, Ludwig C, et al. Pharmacy costs of systemic and topical medications for atopic dermatitis. J Dermatolog Treat. 2019;12:1-3.
- Ariëns LFM, Bakker DS, Spekhorst LS, et al. Rapid and sustained effect of dupilumab on work productivity in patients with difficult-to-treat atopic dermatitis: results from the Dutch BioDay Registry. Acta Derm Venereol. 2021;19;101:adv00573.
- Nutten S. Atopic dermatitis: global epidemiology and risk factors. Ann Nutr Metab. 2015;66(suppl 1):8-16.
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351.
- Yang EJ, Beck KM, Sekhon S, et al. The impact of pediatric atopic dermatitis on families: a review. Pediatr Dermatol. 2019;36:66-71.
- Eckert L, Gupta S, Amand C, et al. Impact of atopic dermatitis on health-related quality of life and productivity in adults in the United States: an analysis using the National Health and Wellness Survey. J Am Acad Dermatol. 2017;77:274-279.
- Drucker AM, Wang AR, Li WQ, et al. The burden of atopic dermatitis: summary of a report for the National Eczema Association. J Invest Dermatol. 2017;137:26-30.
- Ehlken B, Möhrenschlager M, Kugland B, et al. Cost-of-illness study in patients suffering from atopic eczema in Germany. Der Hautarzt. 2006;56:1144-1151.
- Ariëns LFM, van Nimwegen KJM, Shams M, et al. Economic burden of adult patients with moderate to severe atopic dermatitis indicated for systemic treatment. Acta Derm Venereol. 2019;99:762-768.
- Barbeau M, Bpharm HL. Burden of atopic dermatitis in Canada. Int J Dermatol. 2006;45:31-36.
- Verboom P, Hakkaart‐Van Roijen L, Sturkenboom M, et al. The cost of atopic dermatitis in the Netherlands: an international comparison. Br J Dermatol. 2002;147:716-724.
- Gånemo A, Svensson Å, Svedman C, et al. Usefulness of Rajka & Langeland eczema severity score in clinical practice. Acta Derm Venereol. 2016;96:521-524.
- Charman CR, Venn AJ, Williams HC. The Patient-Oriented Eczema Measure: development and initial validation of a new tool for measuring atopic eczema severity from the patients’ perspective. Arch Dermatol. 2004;140:1513-1519.
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI): a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19:210-216.
- Rehunen A, Reissell E, Honkatukia J, et al. Social and health services: regional changes in need, use and production and future options. Accessed July 20, 2023. http://urn.fi/URN:ISBN:978-952-287-294-4
- Reed B, Blaiss MS. The burden of atopic dermatitis. Allergy Asthma Proc. 2018;39:406-410.
- Koszorú K, Borza J, Gulácsi L, et al. Quality of life in patients with atopic dermatitis. Cutis. 2019;104:174-177.
- Li AW, Yin ES, Antaya RJ. Topical corticosteroid phobia in atopic dermatitis: a systematic review. JAMA Dermatol. 2017;153:1036-1042.
- Choi J, Dawe R, Ibbotson S, et al. Quantitative analysis of topical treatments in atopic dermatitis: unexpectedly low use of emollients and strong correlation of topical corticosteroid use both with depression and concurrent asthma. Br J Dermatol. 2020;182:1017-1025.
- Storm A, Andersen SE, Benfeldt E, et al. One in 3 prescriptions are never redeemed: primary nonadherence in an outpatient clinic. J Am Acad Dermatol. 2008;59:27-33.
- Okwundu N, Cardwell LA, Cline A, et al. Topical corticosteroids for treatment-resistant atopic dermatitis. Cutis. 2018;102:205-209.
- Eicher L, Knop M, Aszodi N, et al. A systematic review of factors influencing treatment adherence in chronic inflammatory skin disease—strategies for optimizing treatment outcome. J Eur Acad Dermatol Venereol. 2019;33:2253-2263.
- Heratizadeh A, Werfel T, Wollenberg A, et al; Arbeitsgemeinschaft Neurodermitisschulung für Erwachsene (ARNE) Study Group. Effects of structured patient education in adults with atopic dermatitis: multicenter randomized controlled trial. J Allergy Clin Immunol. 2017;140:845-853.
- Dierick BJH, van der Molen T, Flokstra-de Blok BMJ, et al. Burden and socioeconomics of asthma, allergic rhinitis, atopic dermatitis and food allergy. Expert Rev Pharmacoecon Outcomes Res. 2020;20:437-453.
- Olsson M, Bajpai R, Yew YW, et al. Associations between health-related quality of life and health care costs among children with atopic dermatitis and their caregivers: a cross-sectional study. Pediatr Dermatol. 2020;37:284-293.
- Bruin-Weller M, Pink AE, Patrizi A, et al. Disease burden and treatment history among adults with atopic dermatitis receiving systemic therapy: baseline characteristics of participants on the EUROSTAD prospective observational study. J Dermatolog Treat. 2021;32:164-173.
- Silverberg JI, Lei D, Yousaf M, et al. Comparison of Patient-Oriented Eczema Measure and Patient-Oriented Scoring Atopic Dermatitis vs Eczema Area and Severity Index and other measures of atopic dermatitis: a validation study. Ann Allergy Asthma Immunol. 2020;125:78-83.
- Kido-Nakahara M, Nakahara T, Yasukochi Y, et al. Patient-oriented eczema measure score: a useful tool for web-based surveys in patients with atopic dermatitis. Acta Derm Venereol. 2020;47:924-925.
- Komura Y, Kogure T, Kawahara K, et al. Economic assessment of actual prescription of drugs for treatment of atopic dermatitis: differences between dermatology and pediatrics in large-scale receipt data. J Dermatol. 2018;45:165-174.
- Thompson AM, Chan A, Torabi M, et al. Eczema moisturizers: allergenic potential, marketing claims, and costs. Dermatol Ther. 2020;33:E14228.
- Egeberg A, Andersen YM, Gislason GH, et al. Prevalence of comorbidity and associated risk factors in adults with atopic dermatitis. Allergy. 2017;72:783-791.
- Kauppi S, Jokelainen J, Timonen M, et al. Adult patients with atopic eczema have a high burden of psychiatric disease: a Finnish nationwide registry study. Acta Derm Venereol. 2019;99:647-651.
- Ali F, Vyas J, Finlay AY. Counting the burden: atopic dermatitis and health-related quality of life. Acta Derm Venereol. 2020;100:adv00161.
- Birdi G, Cooke R, Knibb RC. Impact of atopic dermatitis on quality of life in adults: a systematic review and meta-analysis. Int J Dermatol. 2020;59:E75-E91.
- Gabes M, Tischer C, Apfelbacher C; quality of life working group of the Harmonising Outcome Measures for Eczema (HOME) initiative. Measurement properties of quality-of-life outcome measures for children and adults with eczema: an updated systematic review. Pediatr Allergy Immunol. 2020;31:66-77.
- Staab D, Diepgen TL, Fartasch M, et al. Age related, structured educational programmes for the management of atopic dermatitis in children and adolescents: multicentre, randomised controlled trial. BMJ. 2006;332:933-938.
- Wollenberg A, Barbarot S, Bieber T, et al; European Dermatology Forum (EDF), the European Academy of Dermatology and Venereology (EADV), the European Academy of Allergy and Clinical Immunology (EAACI), the European Task Force on Atopic Dermatitis (ETFAD), European Federation of Allergy and Airways Diseases Patients’ Associations (EFA), the European Society for Dermatology and Psychiatry (ESDaP), the European Society of Pediatric Dermatology (ESPD), Global Allergy and Asthma European Network (GA2LEN) and the European Union of Medical Specialists (UEMS). Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part II. J Eur Acad Dermatol Venereol. 2018;32:850-878.
- Eichenfield LF, DiBonaventura M, Xenakis J, et al. Costs and treatment patterns among patients with atopic dermatitis using advanced therapies in the United States: analysis of a retrospective claims database. Dermatol Ther (Heidelb). 2020;10:791-806.
- Zimmermann M, Rind D, Chapman R, et al. Economic evaluation of dupilumab for moderate-to-severe atopic dermatitis: a cost-utility analysis. J Drugs Dermatol. 2018;17:750-756.
- Mata E, Loh TY, Ludwig C, et al. Pharmacy costs of systemic and topical medications for atopic dermatitis. J Dermatolog Treat. 2019;12:1-3.
- Ariëns LFM, Bakker DS, Spekhorst LS, et al. Rapid and sustained effect of dupilumab on work productivity in patients with difficult-to-treat atopic dermatitis: results from the Dutch BioDay Registry. Acta Derm Venereol. 2021;19;101:adv00573.
Practice Points
- Atopic dermatitis (AD) causes a substantial economic burden.
- Atopic dermatitis profoundly affects quality of life and is associated with psychiatric comorbidities. With effective treatments, AD-associated comorbidities may be decreased and the economic burden for the patient and health care system reduced.
Cancer Screening for Dermatomyositis: A Survey of Indirect Costs, Burden, and Patient Willingness to Pay
Dermatomyositis (DM) is an uncommon idiopathic inflammatory myopathy (IIM) characterized by muscle inflammation; proximal muscle weakness; and dermatologic findings, such as the heliotrope eruption and Gottron papules.1-3 Dermatomyositis is associated with an increased malignancy risk compared to other IIMs, with a 13% to 42% lifetime risk for malignancy development.4,5 The incidence for malignancy peaks during the first year following diagnosis and falls gradually over 5 years but remains increased compared to the general population.6-11 Adenocarcinoma represents the majority of cancers associated with DM, particularly of the ovaries, lungs, breasts, gastrointestinal tract, pancreas, bladder, and prostate. The lymphatic system (non-Hodgkin lymphoma) also is overrepresented among cancers in DM.12
Because of the increased malignancy risk and cancer-related mortality in patients with DM, cancer screening generally is recommended following diagnosis.13,14 However, consensus guidelines for screening modalities and frequency currently do not exist, resulting in widely varying practice patterns.15 Some experts advocate for a conventional cancer screening panel (CSP), as summarized in Table 1.15-18 These tests may be repeated annually for 3 to 5 years following the diagnosis of DM. Although the use of myositis-specific antibodies (MSAs) recently has helped to risk-stratify DM patients, up to half of patients are MSA negative,19 and broad malignancy screening remains essential. Individualized discussions with patients about their risk factors, screening options, and risks and benefits of screening also are strongly encouraged.19-22 Studies of the direct costs and effectiveness of streamlined screening with positron emission tomography/computed tomography (PET/CT) compared with a CSP have shown similar efficacy and lower out-of-pocket costs for patients receiving PET/CT imaging.16-18
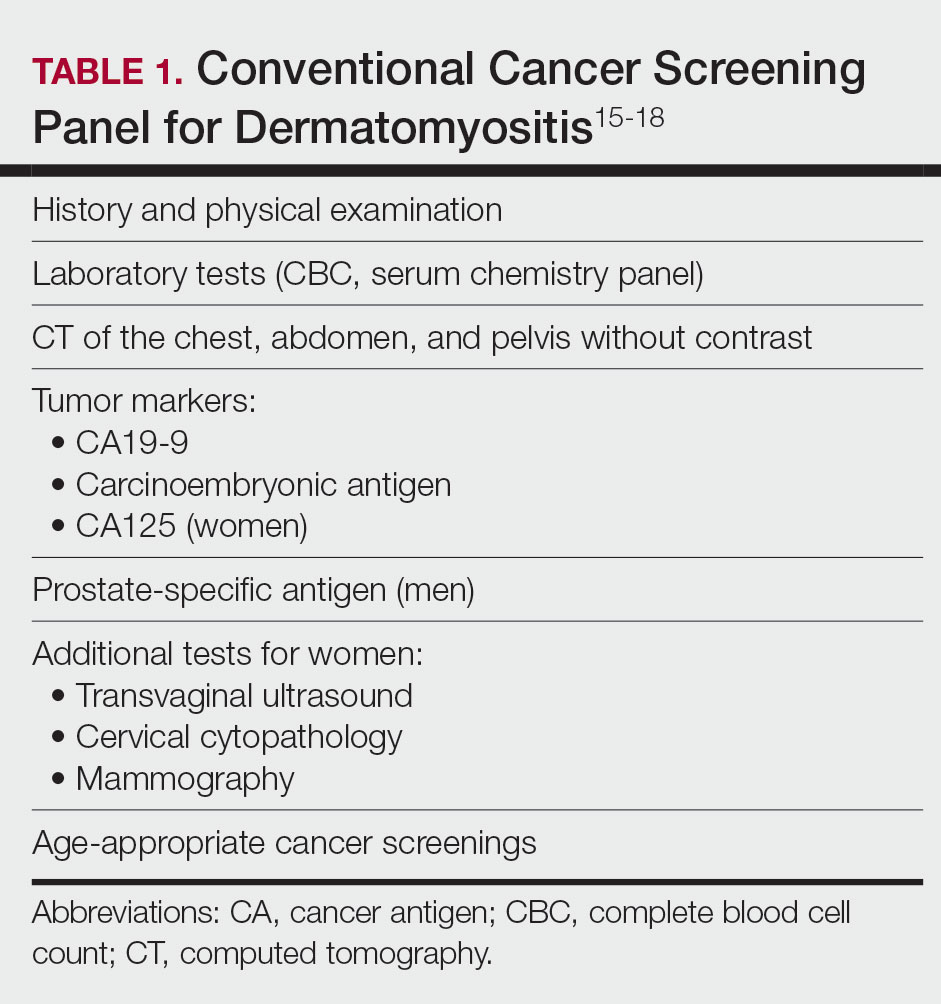
The goal of our study was to further characterize patients’ perspectives and experience of cancer screening in DM as well as indirect costs, both of which must be taken into consideration when developing consensus guidelines for DM malignancy screening. Inclusion of patient voice is essential given the similar efficacy of both screening methods. We assessed the indirect costs (eg, travel, lost work or wages, childcare) of a CSP in patients with DM. We theorized that the large quantity of tests involved in a CSP, which are performed at various locations on multiple days over the course of several years, may have substantial costs to patients beyond the co-pay and deductible. We also sought to measure patients’ perception of the burden associated with an annual CSP, which we defined to participants as the inconvenience or unpleasantness experienced by the patient, compared with an annual whole-body PET/CT. Finally, we examined the relative value of these screening methods to patients using a willingness-to-pay (WTP) analysis.
Materials and Methods
Patient Eligibility—Our study included Penn State Health (Hershey, Pennsylvania) patients 18 years or older with a recent diagnosis of DM—International Classification of Diseases, Ninth Revision code 710.3 or International Classification of Diseases, Tenth Revision codes M33.10 or M33.90—who were undergoing or had recently completed a CSP. Patients were excluded from the study if they had a concurrent or preceding diagnosis of malignancy (excluding nonmelanoma skin cancers) or had another IIM. The institutional review board at Penn State Health College of Medicine approved the study. Data for all patients were prospectively obtained.
Survey Design—A survey was generated to assess the burden and indirect costs associated with a CSP, which was modified from work done by Tchuenche et al23 and Teni et al.24 Focus groups were held in 2018 and 2019 with patients who met our inclusion criteria with the purpose of refining the survey instrument based on patient input. A summary explanation of research was provided to all participants, and informed consent was obtained. Patients were compensated for their time for focus groups. Audio of each focus group was then transcribed and analyzed for common themes. Following focus group feedback, a finalized survey was generated for assessing burden and indirect costs (survey instrument provided in the Supplementary Information). REDCap (Vanderbilt University), a secure web application, was used to construct the finalized survey and to collect and manage data.25
Patients who fit our inclusion criteria were identified and recruited in multiple ways. Patients with appointments at the Penn State Milton S. Hershey Medical Center Department of Dermatology were presented with the opportunity to participate, Penn State Health records with the appropriate billing codes were collected and patients were contacted, and an advertisement for the study was posted on StudyFinder. Surveys constructed on REDCap were then sent electronically to patients who agreed to participate in the study. A second summary explanation of research was included on the first page of the survey to describe the process.
The survey had 3 main sections. The first section collected demographic information. In the second section, we surveyed patients regarding the various aspects of a CSP that focus groups identified as burdensome. In addition, patients were asked to compare their feelings regarding an annual CSP vs whole-body PET/CT for a 3-year period utilizing a rating scale of strongly disagree, somewhat disagree, somewhat agree, and strongly agree. This section also included a willingness-to-pay (WTP) analysis for each modality. We defined WTP as the maximum out-of-pocket cost that the patient would be willing to pay to receive testing, which was measured in a hypothetical scenario where neither whole-body PET/CT nor CSP was covered by insurance.26 Although WTP may be influenced by external factors such as patient income, it can serve as a numerical measure of how much the patient values each service. Furthermore, these external factors become less relevant when comparing the relative value of 2 separate tests, as such factors apply equally in both scenarios. In the third section of the survey, patients were queried regarding various indirect costs associated with a CSP. Descriptions for a CSP and whole-body PET/CT, including risks and benefits, were provided to allow patients to make informed decisions.
Statistical Analysis—Because of the rarity of DM and the subsequently limited sample size, summary and descriptive statistics were utilized to characterize the sample and identify patterns in the results. Continuous variables are presented with means and standard deviations, and proportions are presented with frequencies and percentages. All analyses were done using SAS Version 9.4 (SAS Institute Inc).
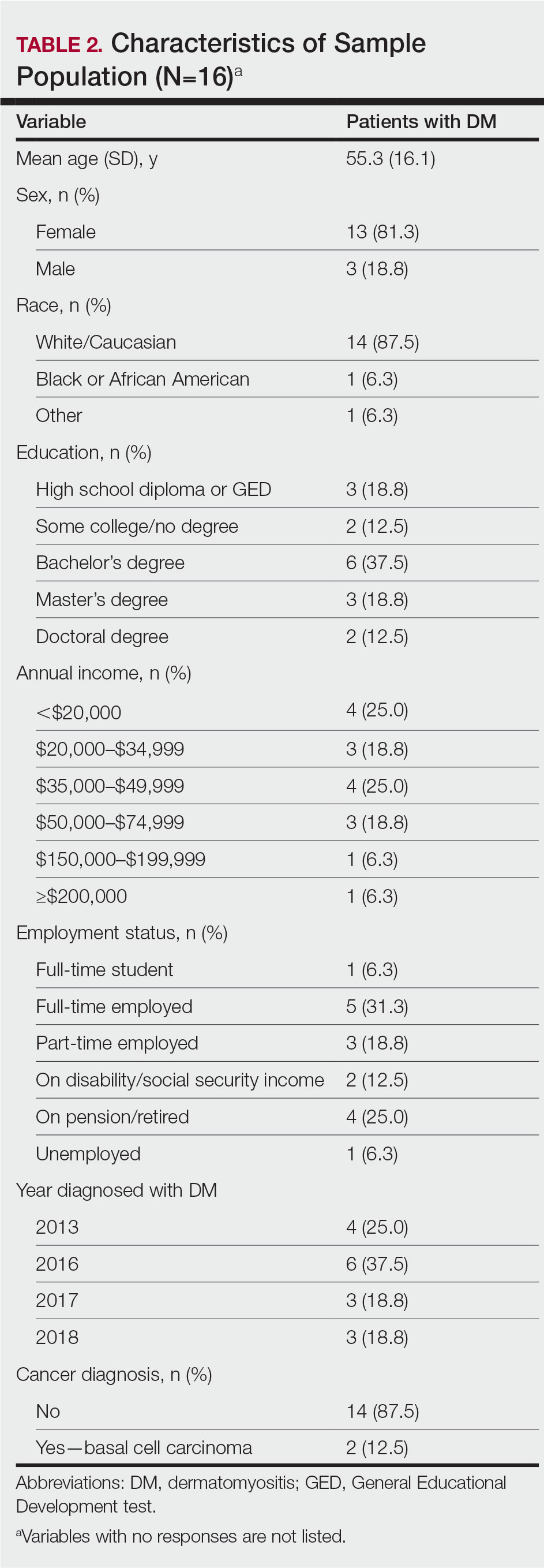
Results
Patient Demographics—Fifty-four patients were identified using StudyFinder, physician referral, and search of the electronic health record. Nine patients agreed to take part in the focus groups, and 27 offered email addresses to be contacted for the survey. Of those 27 patients, 16 (59.3%) fit our inclusion criteria and completed the survey. Patient demographics are detailed in Table 2. The mean age was 55 years, and most patients were White (88% [14/16]), female (81% [13/16]), and had at least a bachelor’s degree (69% [11/16]). Most patients (69% [11/16]) had an annual income of less than $50,000, and half (50% [8/16]) were employed. All patients had been diagnosed with DM in or after 2013. Two patients were diagnosed with basal cell carcinoma during or after cancer screening.
Patient Preference for Screening and WTP—A majority (81% [13/16]) of patients desired some form of screening for occult malignancy following the diagnosis of DM, even in the hypothetical situation in which screening did not provide survival benefit (Figure 1). Twenty-five percent (4/16) of patients expressed that a CSP was burdensome, and 12.5% of patients (2/16) missed a CSP appointment; all of these patients rescheduled or were planning to reschedule. Assuming that both screening methods had similar predictive value in detecting malignancy, all 16 patients felt annual whole-body PET/CT for a 3-year period would be less burdensome than a CSP, and most (73% [11/15]) felt that it would decrease the likelihood of missed appointments. Overall, 93% (13/14) of patients preferred whole-body PET/CT over a CSP when given the choice between the 2 options (Figure 2). This preference was consistent with the patients’ WTP for these tests; patients reliably reported that they would pay more for annual whole-body PET/CT than for a CSP (Figure 3). Specifically, 75% (12/16) and 38% (6/16) of patients were willing to spend $250 or more and $1000 or more for annual whole-body PET/CT, respectively, compared with 56% (9/16) and 19% (3/16), respectively, for an annual CSP. Many patients (38% [6/16]) reported that they would not be willing to pay any out-of-pocket cost for a CSP compared with 13% (2/16) for PET/CT.Indirect Costs of Screening for Patients—Indirect costs incurred by patients undergoing a CSP are summarized in Table 3. Specifically, a large percentage of employed patients missed work (63% [5/8]) or had family miss work (38% [3/8]), necessitating the use of vacation and/or sick days to attend CSP appointments. A subset (25% [2/8]) lost income (average, $1500), and 1 patient reported that a family member lost income due to attending a CSP appointment. Most (75% [12/16]) patients also incurred substantial transportation costs (average, $243), with 1 patient spending $1000. No patients incurred child or elder care costs. One patient paid a small sum for lodging/meals while traveling to attend a CSP appointment.
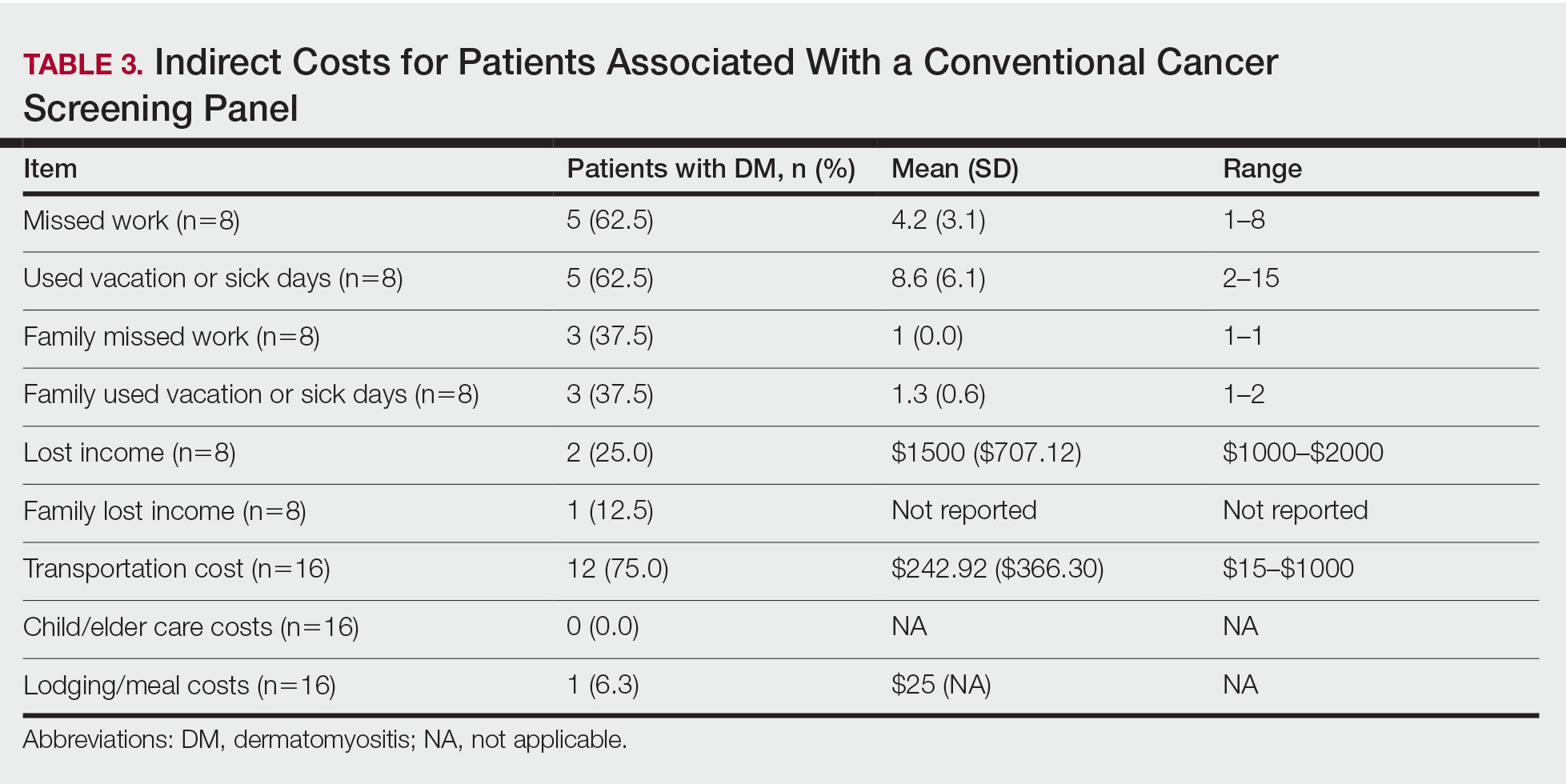
Comment
Patients with DM have an increased incidence of malignancy, thus cancer screening serves a crucial role in the detection of occult disease.13 Up to half of DM patients are MSA negative, and most cancers in these patients are found with blind screening. Whole-body PET/CT has emerged as an alternative to a CSP. Evidence suggests that it has similar efficacy in detecting malignancy and may be particularly useful for identifying malignancies not routinely screened for in a CSP. In a prospective study of patients diagnosed with DM and polymyositis (N=55), whole-body PET/CT had a positive predictive value of 85.7% and negative predictive value for detecting occult malignancy of 93.8% compared with 77.8% and 95.7%, respectively, for a CSP.17
The results of our study showed that cancer screening is important to patients diagnosed with DM and that most of these patients desire some form of cancer screening. This finding held true even when patients were presented with a hypothetical situation in which screening was proven to have no survival benefit. Based on focus group data, this desire was likely driven by the fear generated by not knowing whether cancer is present, as reported by the following DM patients:
“I mean [cancer screening] is peace of mind. It is ultimately worth it. You know, better than . . . not doing the screenings and finding 3 years down the road that you have, you know, a serious problem . . . you had the cancer, and you didn’t have the screenings.” (DM patient 1)
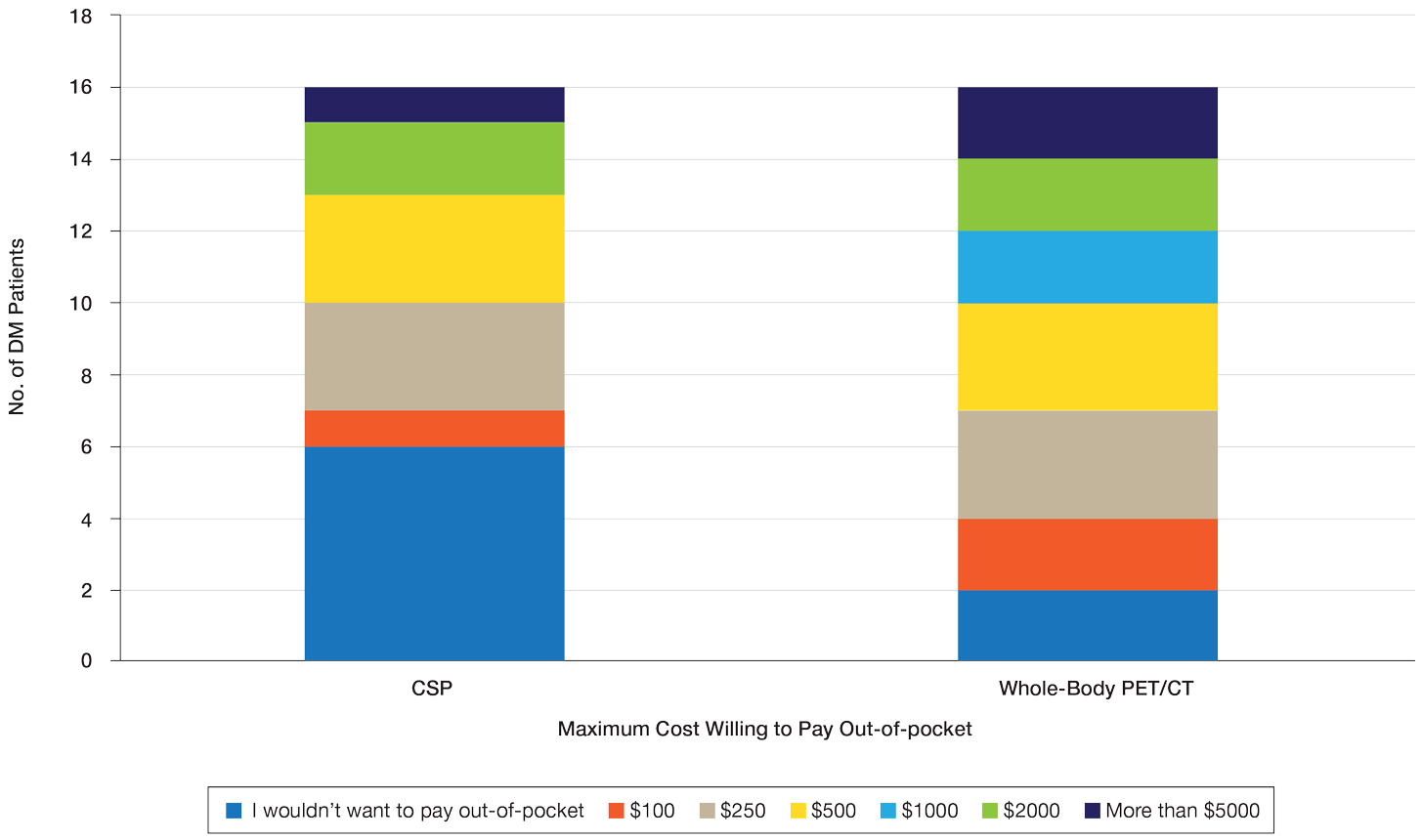
“I would rather know than not know, even if it is bad news, just tell me. The sooner the better, and give me the whole spiel . . . maybe all the screenings don’t need to be done, done so much, so often afterwards if the initial ones are ok, but I think too, for peace of mind, I would rather know it all up front.” (DM patient 2)
Further, when presented with the hypothetical situation that insurance would not cover screenings, a few patients remarked they would relocate to obtain them:
“I would find a place where the screenings were done. I’d move.” (DM patient 4)
“If it was just sky high and [insurance companies] weren’t willing to negotiate, I would consider moving.” (DM patient 3).
Sentiments such as these emphasize the importance and value that DM patients place on being screened for cancer and also may explain why only 25% of patients felt a CSP was burdensome and only 13% reported missing appointments, all of whom planned on making them up at a later time.
When presented with the choice of a CSP or annual whole-body PET/CT for a 3-year period following the diagnosis of DM, all patients expressed that whole-body PET/CT would be less burdensome. Most preferred annual whole-body PET/CT despite the slightly increased radiation exposure associated and thought that it would limit missed appointments. Accordingly, more patients responded that they would pay more money out-of-pocket for annual whole-body PET/CT. Given that WTP can function as a numerical measure of value, our results showed that patients placed a higher value on whole-body PET/CT compared with a CSP. The indirect costs associated with a CSP also were substantial, particularly regarding missed work, use of vacation and/or sick days, and travel expenses, which is particularly important because most patients reported an annual income less than $50,000.
The direct costs of a CSP and whole-body PET/CT have been studied. Specifically, Kundrick et al18 found that whole-body PET/CT was less expensive for patients (by approximately $111) out-of-pocket compared with a CSP, though cost to insurance companies was slightly greater. The present study adds to these findings by better illustrating the burden and indirect costs that patients experience while undergoing a CSP and by characterizing the patient’s perception and preference of these 2 screening methods.
Limitations of our study include a small sample size willing to complete the survey. There also was a predominance of White and female participants, partially attributed to the greater number of female patients who develop DM compared to male patients. However, this still may limit applicability of this study to males and patients of other races. Another limitation includes recall bias on survey responses, particularly regarding indirect costs incurred with a CSP. A final limitation was that only patients with a recent diagnosis of DM who were actively undergoing screening or had recently completed malignancy screening were included in the study. Given that these patients were receiving (or had completed) exclusively a CSP, patients were comparing their personal experience with a described experience. In addition, only 2 patients were diagnosed with cancer—both with basal cell carcinoma diagnosed on physical examination—which may have influenced their perception of a CSP, given that nothing was found on an extensive number of tests. However, these patients still greatly valued their screening, as evidenced in the survey.
Conclusion
- Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet. 2003;362:971-982. doi:10.1016/S0140-6736(03)14368-1
- Schmidt J. Current classification and management of inflammatory myopathies. J Neuromuscul Dis. 2018;5:109-129. doi:10.3233/JND-180308
- Lazarou IN, Guerne PA. Classification, diagnosis, and management of idiopathic inflammatory myopathies. J Rheumatol. 201;40:550-564. doi:10.3899/jrheum.120682
- Wang J, Guo G, Chen G, et al. Meta-analysis of the association of dermatomyositis and polymyositis with cancer. Br J Dermatol. 2013;169:838-847. doi:10.1111/bjd.12564
- Zampieri S, Valente M, Adami N, et al. Polymyositis, dermatomyositis and malignancy: a further intriguing link. Autoimmun Rev. 2010;9:449-453. doi:10.1016/j.autrev.2009.12.005
- Sigurgeirsson B, Lindelöf B, Edhag O, et al. Risk of cancer in patients with dermatomyositis or polymyositis. a population-based study. N Engl J Med. 1992;326:363-367. doi:10.1056/nejm199202063260602
- Chen YJ, Wu CY, Huang YL, et al. Cancer risks of dermatomyositis and polymyositis: a nationwide cohort study in Taiwan. Arthritis Res Ther. 2010;12:R70. doi:10.1186/ar2987
- Chen YJ, Wu CY, Shen JL. Predicting factors of malignancy in dermatomyositis and polymyositis: a case-control study. Br J Dermatol. 2001;144:825-831. doi:10.1046/j.1365-2133.2001.04140.x
- Targoff IN, Mamyrova G, Trieu EP, et al. A novel autoantibody to a 155-kd protein is associated with dermatomyositis. Arthritis Rheum. 2006;54:3682-3689. doi:10.1002/art.22164
- Chow WH, Gridley G, Mellemkjær L, et al. Cancer risk following polymyositis and dermatomyositis: a nationwide cohort study in Denmark. Cancer Causes Control. 1995;6:9-13. doi:10.1007/BF00051675
- Buchbinder R, Forbes A, Hall S, et al. Incidence of malignant disease in biopsy-proven inflammatory myopathy: a population-based cohort study. Ann Intern Med. 2001;134:1087-1095. doi:10.7326/0003-4819-134-12-200106190-00008
- Hill CL, Zhang Y, Sigurgeirsson B, et al. Frequency of specific cancer types in dermatomyositis and polymyositis: a population-based study. Lancet. 2001;357:96-100. doi:10.1016/S0140-6736(00)03540-6
- Leatham H, Schadt C, Chisolm S, et al. Evidence supports blind screening for internal malignancy in dermatomyositis: data from 2 large US dermatology cohorts. Medicine (Baltimore). 2018;97:E9639. doi:10.1097/MD.0000000000009639
- Sparsa A, Liozon E, Herrmann F, et al. Routine vs extensive malignancy search for adult dermatomyositis and polymyositis: a study of 40 patients. Arch Dermatol. 2002;138:885-890.
- Dutton K, Soden M. Malignancy screening in autoimmune myositis among Australian rheumatologists. Intern Med J. 2017;47:1367-1375. doi:10.1111/imj.13556
- Selva-O’Callaghan A, Martinez-Gómez X, Trallero-Araguás E, et al. The diagnostic work-up of cancer-associated myositis. Curr Opin Rheumatol. 2018;30:630-636. doi:10.1097/BOR.0000000000000535
- Selva-O’Callaghan A, Grau JM, Gámez-Cenzano C, et al. Conventional cancer screening versus PET/CT in dermatomyositis/polymyositis. Am J Med. 2010;123:558-562. doi:10.1016/j.amjmed.2009.11.012
- Kundrick A, Kirby J, Ba D, et al. Positron emission tomography costs less to patients than conventional screening for malignancy in dermatomyositis. Semin Arthritis Rheum. 2019;49:140-144. doi:10.1016/j.semarthrit.2018.10.021
- Satoh M, Tanaka S, Ceribelli A, et al. A comprehensive overview on myositis-specific antibodies: new and old biomarkers in idiopathic inflammatory myopathy. Clin Rev Allergy Immunol. 2017;52:1-19. doi:10.1007/s12016-015-8510-y
- Vaughan H, Rugo HS, Haemel A. Risk-based screening for cancer in patients with dermatomyositis: toward a more individualized approach. JAMA Dermatol. 2022;158:244-247. doi:10.1001/jamadermatol.2021.5841
- Khanna U, Galimberti F, Li Y, et al. Dermatomyositis and malignancy: should all patients with dermatomyositis undergo malignancy screening? Ann Transl Med. 2021;9:432. doi:10.21037/atm-20-5215
- Oldroyd AGS, Allard AB, Callen JP, et al. Corrigendum to: A systematic review and meta-analysis to inform cancer screening guidelines in idiopathic inflammatory myopathies. Rheumatology (Oxford). 2021;60:5483. doi:10.1093/rheumatology/keab616
- Tchuenche M, Haté V, McPherson D, et al. Estimating client out-of-pocket costs for accessing voluntary medical male circumcision in South Africa. PLoS One. 2016;11:E0164147. doi:10.1371/journal.pone.0164147
- Teni FS, Gebresillassie BM, Birru EM, et al. Costs incurred by outpatients at a university hospital in northwestern Ethiopia: a cross-sectional study. BMC Health Serv Res. 2018;18:842. doi:10.1186/s12913-018-3628-2
- Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377-381. doi:10.1016/j.jbi.2008.08.010
- Bala MV, Mauskopf JA, Wood LL. Willingness to pay as a measure of health benefits. Pharmacoeconomics. 1999;15:9-18. doi:10.2165/00019053-199915010-00002
Dermatomyositis (DM) is an uncommon idiopathic inflammatory myopathy (IIM) characterized by muscle inflammation; proximal muscle weakness; and dermatologic findings, such as the heliotrope eruption and Gottron papules.1-3 Dermatomyositis is associated with an increased malignancy risk compared to other IIMs, with a 13% to 42% lifetime risk for malignancy development.4,5 The incidence for malignancy peaks during the first year following diagnosis and falls gradually over 5 years but remains increased compared to the general population.6-11 Adenocarcinoma represents the majority of cancers associated with DM, particularly of the ovaries, lungs, breasts, gastrointestinal tract, pancreas, bladder, and prostate. The lymphatic system (non-Hodgkin lymphoma) also is overrepresented among cancers in DM.12
Because of the increased malignancy risk and cancer-related mortality in patients with DM, cancer screening generally is recommended following diagnosis.13,14 However, consensus guidelines for screening modalities and frequency currently do not exist, resulting in widely varying practice patterns.15 Some experts advocate for a conventional cancer screening panel (CSP), as summarized in Table 1.15-18 These tests may be repeated annually for 3 to 5 years following the diagnosis of DM. Although the use of myositis-specific antibodies (MSAs) recently has helped to risk-stratify DM patients, up to half of patients are MSA negative,19 and broad malignancy screening remains essential. Individualized discussions with patients about their risk factors, screening options, and risks and benefits of screening also are strongly encouraged.19-22 Studies of the direct costs and effectiveness of streamlined screening with positron emission tomography/computed tomography (PET/CT) compared with a CSP have shown similar efficacy and lower out-of-pocket costs for patients receiving PET/CT imaging.16-18

The goal of our study was to further characterize patients’ perspectives and experience of cancer screening in DM as well as indirect costs, both of which must be taken into consideration when developing consensus guidelines for DM malignancy screening. Inclusion of patient voice is essential given the similar efficacy of both screening methods. We assessed the indirect costs (eg, travel, lost work or wages, childcare) of a CSP in patients with DM. We theorized that the large quantity of tests involved in a CSP, which are performed at various locations on multiple days over the course of several years, may have substantial costs to patients beyond the co-pay and deductible. We also sought to measure patients’ perception of the burden associated with an annual CSP, which we defined to participants as the inconvenience or unpleasantness experienced by the patient, compared with an annual whole-body PET/CT. Finally, we examined the relative value of these screening methods to patients using a willingness-to-pay (WTP) analysis.
Materials and Methods
Patient Eligibility—Our study included Penn State Health (Hershey, Pennsylvania) patients 18 years or older with a recent diagnosis of DM—International Classification of Diseases, Ninth Revision code 710.3 or International Classification of Diseases, Tenth Revision codes M33.10 or M33.90—who were undergoing or had recently completed a CSP. Patients were excluded from the study if they had a concurrent or preceding diagnosis of malignancy (excluding nonmelanoma skin cancers) or had another IIM. The institutional review board at Penn State Health College of Medicine approved the study. Data for all patients were prospectively obtained.
Survey Design—A survey was generated to assess the burden and indirect costs associated with a CSP, which was modified from work done by Tchuenche et al23 and Teni et al.24 Focus groups were held in 2018 and 2019 with patients who met our inclusion criteria with the purpose of refining the survey instrument based on patient input. A summary explanation of research was provided to all participants, and informed consent was obtained. Patients were compensated for their time for focus groups. Audio of each focus group was then transcribed and analyzed for common themes. Following focus group feedback, a finalized survey was generated for assessing burden and indirect costs (survey instrument provided in the Supplementary Information). REDCap (Vanderbilt University), a secure web application, was used to construct the finalized survey and to collect and manage data.25
Patients who fit our inclusion criteria were identified and recruited in multiple ways. Patients with appointments at the Penn State Milton S. Hershey Medical Center Department of Dermatology were presented with the opportunity to participate, Penn State Health records with the appropriate billing codes were collected and patients were contacted, and an advertisement for the study was posted on StudyFinder. Surveys constructed on REDCap were then sent electronically to patients who agreed to participate in the study. A second summary explanation of research was included on the first page of the survey to describe the process.
The survey had 3 main sections. The first section collected demographic information. In the second section, we surveyed patients regarding the various aspects of a CSP that focus groups identified as burdensome. In addition, patients were asked to compare their feelings regarding an annual CSP vs whole-body PET/CT for a 3-year period utilizing a rating scale of strongly disagree, somewhat disagree, somewhat agree, and strongly agree. This section also included a willingness-to-pay (WTP) analysis for each modality. We defined WTP as the maximum out-of-pocket cost that the patient would be willing to pay to receive testing, which was measured in a hypothetical scenario where neither whole-body PET/CT nor CSP was covered by insurance.26 Although WTP may be influenced by external factors such as patient income, it can serve as a numerical measure of how much the patient values each service. Furthermore, these external factors become less relevant when comparing the relative value of 2 separate tests, as such factors apply equally in both scenarios. In the third section of the survey, patients were queried regarding various indirect costs associated with a CSP. Descriptions for a CSP and whole-body PET/CT, including risks and benefits, were provided to allow patients to make informed decisions.
Statistical Analysis—Because of the rarity of DM and the subsequently limited sample size, summary and descriptive statistics were utilized to characterize the sample and identify patterns in the results. Continuous variables are presented with means and standard deviations, and proportions are presented with frequencies and percentages. All analyses were done using SAS Version 9.4 (SAS Institute Inc).

Results
Patient Demographics—Fifty-four patients were identified using StudyFinder, physician referral, and search of the electronic health record. Nine patients agreed to take part in the focus groups, and 27 offered email addresses to be contacted for the survey. Of those 27 patients, 16 (59.3%) fit our inclusion criteria and completed the survey. Patient demographics are detailed in Table 2. The mean age was 55 years, and most patients were White (88% [14/16]), female (81% [13/16]), and had at least a bachelor’s degree (69% [11/16]). Most patients (69% [11/16]) had an annual income of less than $50,000, and half (50% [8/16]) were employed. All patients had been diagnosed with DM in or after 2013. Two patients were diagnosed with basal cell carcinoma during or after cancer screening.

Patient Preference for Screening and WTP—A majority (81% [13/16]) of patients desired some form of screening for occult malignancy following the diagnosis of DM, even in the hypothetical situation in which screening did not provide survival benefit (Figure 1). Twenty-five percent (4/16) of patients expressed that a CSP was burdensome, and 12.5% of patients (2/16) missed a CSP appointment; all of these patients rescheduled or were planning to reschedule. Assuming that both screening methods had similar predictive value in detecting malignancy, all 16 patients felt annual whole-body PET/CT for a 3-year period would be less burdensome than a CSP, and most (73% [11/15]) felt that it would decrease the likelihood of missed appointments. Overall, 93% (13/14) of patients preferred whole-body PET/CT over a CSP when given the choice between the 2 options (Figure 2). This preference was consistent with the patients’ WTP for these tests; patients reliably reported that they would pay more for annual whole-body PET/CT than for a CSP (Figure 3). Specifically, 75% (12/16) and 38% (6/16) of patients were willing to spend $250 or more and $1000 or more for annual whole-body PET/CT, respectively, compared with 56% (9/16) and 19% (3/16), respectively, for an annual CSP. Many patients (38% [6/16]) reported that they would not be willing to pay any out-of-pocket cost for a CSP compared with 13% (2/16) for PET/CT.Indirect Costs of Screening for Patients—Indirect costs incurred by patients undergoing a CSP are summarized in Table 3. Specifically, a large percentage of employed patients missed work (63% [5/8]) or had family miss work (38% [3/8]), necessitating the use of vacation and/or sick days to attend CSP appointments. A subset (25% [2/8]) lost income (average, $1500), and 1 patient reported that a family member lost income due to attending a CSP appointment. Most (75% [12/16]) patients also incurred substantial transportation costs (average, $243), with 1 patient spending $1000. No patients incurred child or elder care costs. One patient paid a small sum for lodging/meals while traveling to attend a CSP appointment.

Comment
Patients with DM have an increased incidence of malignancy, thus cancer screening serves a crucial role in the detection of occult disease.13 Up to half of DM patients are MSA negative, and most cancers in these patients are found with blind screening. Whole-body PET/CT has emerged as an alternative to a CSP. Evidence suggests that it has similar efficacy in detecting malignancy and may be particularly useful for identifying malignancies not routinely screened for in a CSP. In a prospective study of patients diagnosed with DM and polymyositis (N=55), whole-body PET/CT had a positive predictive value of 85.7% and negative predictive value for detecting occult malignancy of 93.8% compared with 77.8% and 95.7%, respectively, for a CSP.17

The results of our study showed that cancer screening is important to patients diagnosed with DM and that most of these patients desire some form of cancer screening. This finding held true even when patients were presented with a hypothetical situation in which screening was proven to have no survival benefit. Based on focus group data, this desire was likely driven by the fear generated by not knowing whether cancer is present, as reported by the following DM patients:
“I mean [cancer screening] is peace of mind. It is ultimately worth it. You know, better than . . . not doing the screenings and finding 3 years down the road that you have, you know, a serious problem . . . you had the cancer, and you didn’t have the screenings.” (DM patient 1)

“I would rather know than not know, even if it is bad news, just tell me. The sooner the better, and give me the whole spiel . . . maybe all the screenings don’t need to be done, done so much, so often afterwards if the initial ones are ok, but I think too, for peace of mind, I would rather know it all up front.” (DM patient 2)
Further, when presented with the hypothetical situation that insurance would not cover screenings, a few patients remarked they would relocate to obtain them:
“I would find a place where the screenings were done. I’d move.” (DM patient 4)
“If it was just sky high and [insurance companies] weren’t willing to negotiate, I would consider moving.” (DM patient 3).
Sentiments such as these emphasize the importance and value that DM patients place on being screened for cancer and also may explain why only 25% of patients felt a CSP was burdensome and only 13% reported missing appointments, all of whom planned on making them up at a later time.
When presented with the choice of a CSP or annual whole-body PET/CT for a 3-year period following the diagnosis of DM, all patients expressed that whole-body PET/CT would be less burdensome. Most preferred annual whole-body PET/CT despite the slightly increased radiation exposure associated and thought that it would limit missed appointments. Accordingly, more patients responded that they would pay more money out-of-pocket for annual whole-body PET/CT. Given that WTP can function as a numerical measure of value, our results showed that patients placed a higher value on whole-body PET/CT compared with a CSP. The indirect costs associated with a CSP also were substantial, particularly regarding missed work, use of vacation and/or sick days, and travel expenses, which is particularly important because most patients reported an annual income less than $50,000.
The direct costs of a CSP and whole-body PET/CT have been studied. Specifically, Kundrick et al18 found that whole-body PET/CT was less expensive for patients (by approximately $111) out-of-pocket compared with a CSP, though cost to insurance companies was slightly greater. The present study adds to these findings by better illustrating the burden and indirect costs that patients experience while undergoing a CSP and by characterizing the patient’s perception and preference of these 2 screening methods.
Limitations of our study include a small sample size willing to complete the survey. There also was a predominance of White and female participants, partially attributed to the greater number of female patients who develop DM compared to male patients. However, this still may limit applicability of this study to males and patients of other races. Another limitation includes recall bias on survey responses, particularly regarding indirect costs incurred with a CSP. A final limitation was that only patients with a recent diagnosis of DM who were actively undergoing screening or had recently completed malignancy screening were included in the study. Given that these patients were receiving (or had completed) exclusively a CSP, patients were comparing their personal experience with a described experience. In addition, only 2 patients were diagnosed with cancer—both with basal cell carcinoma diagnosed on physical examination—which may have influenced their perception of a CSP, given that nothing was found on an extensive number of tests. However, these patients still greatly valued their screening, as evidenced in the survey.
Conclusion
Dermatomyositis (DM) is an uncommon idiopathic inflammatory myopathy (IIM) characterized by muscle inflammation; proximal muscle weakness; and dermatologic findings, such as the heliotrope eruption and Gottron papules.1-3 Dermatomyositis is associated with an increased malignancy risk compared to other IIMs, with a 13% to 42% lifetime risk for malignancy development.4,5 The incidence for malignancy peaks during the first year following diagnosis and falls gradually over 5 years but remains increased compared to the general population.6-11 Adenocarcinoma represents the majority of cancers associated with DM, particularly of the ovaries, lungs, breasts, gastrointestinal tract, pancreas, bladder, and prostate. The lymphatic system (non-Hodgkin lymphoma) also is overrepresented among cancers in DM.12
Because of the increased malignancy risk and cancer-related mortality in patients with DM, cancer screening generally is recommended following diagnosis.13,14 However, consensus guidelines for screening modalities and frequency currently do not exist, resulting in widely varying practice patterns.15 Some experts advocate for a conventional cancer screening panel (CSP), as summarized in Table 1.15-18 These tests may be repeated annually for 3 to 5 years following the diagnosis of DM. Although the use of myositis-specific antibodies (MSAs) recently has helped to risk-stratify DM patients, up to half of patients are MSA negative,19 and broad malignancy screening remains essential. Individualized discussions with patients about their risk factors, screening options, and risks and benefits of screening also are strongly encouraged.19-22 Studies of the direct costs and effectiveness of streamlined screening with positron emission tomography/computed tomography (PET/CT) compared with a CSP have shown similar efficacy and lower out-of-pocket costs for patients receiving PET/CT imaging.16-18

The goal of our study was to further characterize patients’ perspectives and experience of cancer screening in DM as well as indirect costs, both of which must be taken into consideration when developing consensus guidelines for DM malignancy screening. Inclusion of patient voice is essential given the similar efficacy of both screening methods. We assessed the indirect costs (eg, travel, lost work or wages, childcare) of a CSP in patients with DM. We theorized that the large quantity of tests involved in a CSP, which are performed at various locations on multiple days over the course of several years, may have substantial costs to patients beyond the co-pay and deductible. We also sought to measure patients’ perception of the burden associated with an annual CSP, which we defined to participants as the inconvenience or unpleasantness experienced by the patient, compared with an annual whole-body PET/CT. Finally, we examined the relative value of these screening methods to patients using a willingness-to-pay (WTP) analysis.
Materials and Methods
Patient Eligibility—Our study included Penn State Health (Hershey, Pennsylvania) patients 18 years or older with a recent diagnosis of DM—International Classification of Diseases, Ninth Revision code 710.3 or International Classification of Diseases, Tenth Revision codes M33.10 or M33.90—who were undergoing or had recently completed a CSP. Patients were excluded from the study if they had a concurrent or preceding diagnosis of malignancy (excluding nonmelanoma skin cancers) or had another IIM. The institutional review board at Penn State Health College of Medicine approved the study. Data for all patients were prospectively obtained.
Survey Design—A survey was generated to assess the burden and indirect costs associated with a CSP, which was modified from work done by Tchuenche et al23 and Teni et al.24 Focus groups were held in 2018 and 2019 with patients who met our inclusion criteria with the purpose of refining the survey instrument based on patient input. A summary explanation of research was provided to all participants, and informed consent was obtained. Patients were compensated for their time for focus groups. Audio of each focus group was then transcribed and analyzed for common themes. Following focus group feedback, a finalized survey was generated for assessing burden and indirect costs (survey instrument provided in the Supplementary Information). REDCap (Vanderbilt University), a secure web application, was used to construct the finalized survey and to collect and manage data.25
Patients who fit our inclusion criteria were identified and recruited in multiple ways. Patients with appointments at the Penn State Milton S. Hershey Medical Center Department of Dermatology were presented with the opportunity to participate, Penn State Health records with the appropriate billing codes were collected and patients were contacted, and an advertisement for the study was posted on StudyFinder. Surveys constructed on REDCap were then sent electronically to patients who agreed to participate in the study. A second summary explanation of research was included on the first page of the survey to describe the process.
The survey had 3 main sections. The first section collected demographic information. In the second section, we surveyed patients regarding the various aspects of a CSP that focus groups identified as burdensome. In addition, patients were asked to compare their feelings regarding an annual CSP vs whole-body PET/CT for a 3-year period utilizing a rating scale of strongly disagree, somewhat disagree, somewhat agree, and strongly agree. This section also included a willingness-to-pay (WTP) analysis for each modality. We defined WTP as the maximum out-of-pocket cost that the patient would be willing to pay to receive testing, which was measured in a hypothetical scenario where neither whole-body PET/CT nor CSP was covered by insurance.26 Although WTP may be influenced by external factors such as patient income, it can serve as a numerical measure of how much the patient values each service. Furthermore, these external factors become less relevant when comparing the relative value of 2 separate tests, as such factors apply equally in both scenarios. In the third section of the survey, patients were queried regarding various indirect costs associated with a CSP. Descriptions for a CSP and whole-body PET/CT, including risks and benefits, were provided to allow patients to make informed decisions.
Statistical Analysis—Because of the rarity of DM and the subsequently limited sample size, summary and descriptive statistics were utilized to characterize the sample and identify patterns in the results. Continuous variables are presented with means and standard deviations, and proportions are presented with frequencies and percentages. All analyses were done using SAS Version 9.4 (SAS Institute Inc).

Results
Patient Demographics—Fifty-four patients were identified using StudyFinder, physician referral, and search of the electronic health record. Nine patients agreed to take part in the focus groups, and 27 offered email addresses to be contacted for the survey. Of those 27 patients, 16 (59.3%) fit our inclusion criteria and completed the survey. Patient demographics are detailed in Table 2. The mean age was 55 years, and most patients were White (88% [14/16]), female (81% [13/16]), and had at least a bachelor’s degree (69% [11/16]). Most patients (69% [11/16]) had an annual income of less than $50,000, and half (50% [8/16]) were employed. All patients had been diagnosed with DM in or after 2013. Two patients were diagnosed with basal cell carcinoma during or after cancer screening.

Patient Preference for Screening and WTP—A majority (81% [13/16]) of patients desired some form of screening for occult malignancy following the diagnosis of DM, even in the hypothetical situation in which screening did not provide survival benefit (Figure 1). Twenty-five percent (4/16) of patients expressed that a CSP was burdensome, and 12.5% of patients (2/16) missed a CSP appointment; all of these patients rescheduled or were planning to reschedule. Assuming that both screening methods had similar predictive value in detecting malignancy, all 16 patients felt annual whole-body PET/CT for a 3-year period would be less burdensome than a CSP, and most (73% [11/15]) felt that it would decrease the likelihood of missed appointments. Overall, 93% (13/14) of patients preferred whole-body PET/CT over a CSP when given the choice between the 2 options (Figure 2). This preference was consistent with the patients’ WTP for these tests; patients reliably reported that they would pay more for annual whole-body PET/CT than for a CSP (Figure 3). Specifically, 75% (12/16) and 38% (6/16) of patients were willing to spend $250 or more and $1000 or more for annual whole-body PET/CT, respectively, compared with 56% (9/16) and 19% (3/16), respectively, for an annual CSP. Many patients (38% [6/16]) reported that they would not be willing to pay any out-of-pocket cost for a CSP compared with 13% (2/16) for PET/CT.Indirect Costs of Screening for Patients—Indirect costs incurred by patients undergoing a CSP are summarized in Table 3. Specifically, a large percentage of employed patients missed work (63% [5/8]) or had family miss work (38% [3/8]), necessitating the use of vacation and/or sick days to attend CSP appointments. A subset (25% [2/8]) lost income (average, $1500), and 1 patient reported that a family member lost income due to attending a CSP appointment. Most (75% [12/16]) patients also incurred substantial transportation costs (average, $243), with 1 patient spending $1000. No patients incurred child or elder care costs. One patient paid a small sum for lodging/meals while traveling to attend a CSP appointment.

Comment
Patients with DM have an increased incidence of malignancy, thus cancer screening serves a crucial role in the detection of occult disease.13 Up to half of DM patients are MSA negative, and most cancers in these patients are found with blind screening. Whole-body PET/CT has emerged as an alternative to a CSP. Evidence suggests that it has similar efficacy in detecting malignancy and may be particularly useful for identifying malignancies not routinely screened for in a CSP. In a prospective study of patients diagnosed with DM and polymyositis (N=55), whole-body PET/CT had a positive predictive value of 85.7% and negative predictive value for detecting occult malignancy of 93.8% compared with 77.8% and 95.7%, respectively, for a CSP.17

The results of our study showed that cancer screening is important to patients diagnosed with DM and that most of these patients desire some form of cancer screening. This finding held true even when patients were presented with a hypothetical situation in which screening was proven to have no survival benefit. Based on focus group data, this desire was likely driven by the fear generated by not knowing whether cancer is present, as reported by the following DM patients:
“I mean [cancer screening] is peace of mind. It is ultimately worth it. You know, better than . . . not doing the screenings and finding 3 years down the road that you have, you know, a serious problem . . . you had the cancer, and you didn’t have the screenings.” (DM patient 1)

“I would rather know than not know, even if it is bad news, just tell me. The sooner the better, and give me the whole spiel . . . maybe all the screenings don’t need to be done, done so much, so often afterwards if the initial ones are ok, but I think too, for peace of mind, I would rather know it all up front.” (DM patient 2)
Further, when presented with the hypothetical situation that insurance would not cover screenings, a few patients remarked they would relocate to obtain them:
“I would find a place where the screenings were done. I’d move.” (DM patient 4)
“If it was just sky high and [insurance companies] weren’t willing to negotiate, I would consider moving.” (DM patient 3).
Sentiments such as these emphasize the importance and value that DM patients place on being screened for cancer and also may explain why only 25% of patients felt a CSP was burdensome and only 13% reported missing appointments, all of whom planned on making them up at a later time.
When presented with the choice of a CSP or annual whole-body PET/CT for a 3-year period following the diagnosis of DM, all patients expressed that whole-body PET/CT would be less burdensome. Most preferred annual whole-body PET/CT despite the slightly increased radiation exposure associated and thought that it would limit missed appointments. Accordingly, more patients responded that they would pay more money out-of-pocket for annual whole-body PET/CT. Given that WTP can function as a numerical measure of value, our results showed that patients placed a higher value on whole-body PET/CT compared with a CSP. The indirect costs associated with a CSP also were substantial, particularly regarding missed work, use of vacation and/or sick days, and travel expenses, which is particularly important because most patients reported an annual income less than $50,000.
The direct costs of a CSP and whole-body PET/CT have been studied. Specifically, Kundrick et al18 found that whole-body PET/CT was less expensive for patients (by approximately $111) out-of-pocket compared with a CSP, though cost to insurance companies was slightly greater. The present study adds to these findings by better illustrating the burden and indirect costs that patients experience while undergoing a CSP and by characterizing the patient’s perception and preference of these 2 screening methods.
Limitations of our study include a small sample size willing to complete the survey. There also was a predominance of White and female participants, partially attributed to the greater number of female patients who develop DM compared to male patients. However, this still may limit applicability of this study to males and patients of other races. Another limitation includes recall bias on survey responses, particularly regarding indirect costs incurred with a CSP. A final limitation was that only patients with a recent diagnosis of DM who were actively undergoing screening or had recently completed malignancy screening were included in the study. Given that these patients were receiving (or had completed) exclusively a CSP, patients were comparing their personal experience with a described experience. In addition, only 2 patients were diagnosed with cancer—both with basal cell carcinoma diagnosed on physical examination—which may have influenced their perception of a CSP, given that nothing was found on an extensive number of tests. However, these patients still greatly valued their screening, as evidenced in the survey.
Conclusion
- Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet. 2003;362:971-982. doi:10.1016/S0140-6736(03)14368-1
- Schmidt J. Current classification and management of inflammatory myopathies. J Neuromuscul Dis. 2018;5:109-129. doi:10.3233/JND-180308
- Lazarou IN, Guerne PA. Classification, diagnosis, and management of idiopathic inflammatory myopathies. J Rheumatol. 201;40:550-564. doi:10.3899/jrheum.120682
- Wang J, Guo G, Chen G, et al. Meta-analysis of the association of dermatomyositis and polymyositis with cancer. Br J Dermatol. 2013;169:838-847. doi:10.1111/bjd.12564
- Zampieri S, Valente M, Adami N, et al. Polymyositis, dermatomyositis and malignancy: a further intriguing link. Autoimmun Rev. 2010;9:449-453. doi:10.1016/j.autrev.2009.12.005
- Sigurgeirsson B, Lindelöf B, Edhag O, et al. Risk of cancer in patients with dermatomyositis or polymyositis. a population-based study. N Engl J Med. 1992;326:363-367. doi:10.1056/nejm199202063260602
- Chen YJ, Wu CY, Huang YL, et al. Cancer risks of dermatomyositis and polymyositis: a nationwide cohort study in Taiwan. Arthritis Res Ther. 2010;12:R70. doi:10.1186/ar2987
- Chen YJ, Wu CY, Shen JL. Predicting factors of malignancy in dermatomyositis and polymyositis: a case-control study. Br J Dermatol. 2001;144:825-831. doi:10.1046/j.1365-2133.2001.04140.x
- Targoff IN, Mamyrova G, Trieu EP, et al. A novel autoantibody to a 155-kd protein is associated with dermatomyositis. Arthritis Rheum. 2006;54:3682-3689. doi:10.1002/art.22164
- Chow WH, Gridley G, Mellemkjær L, et al. Cancer risk following polymyositis and dermatomyositis: a nationwide cohort study in Denmark. Cancer Causes Control. 1995;6:9-13. doi:10.1007/BF00051675
- Buchbinder R, Forbes A, Hall S, et al. Incidence of malignant disease in biopsy-proven inflammatory myopathy: a population-based cohort study. Ann Intern Med. 2001;134:1087-1095. doi:10.7326/0003-4819-134-12-200106190-00008
- Hill CL, Zhang Y, Sigurgeirsson B, et al. Frequency of specific cancer types in dermatomyositis and polymyositis: a population-based study. Lancet. 2001;357:96-100. doi:10.1016/S0140-6736(00)03540-6
- Leatham H, Schadt C, Chisolm S, et al. Evidence supports blind screening for internal malignancy in dermatomyositis: data from 2 large US dermatology cohorts. Medicine (Baltimore). 2018;97:E9639. doi:10.1097/MD.0000000000009639
- Sparsa A, Liozon E, Herrmann F, et al. Routine vs extensive malignancy search for adult dermatomyositis and polymyositis: a study of 40 patients. Arch Dermatol. 2002;138:885-890.
- Dutton K, Soden M. Malignancy screening in autoimmune myositis among Australian rheumatologists. Intern Med J. 2017;47:1367-1375. doi:10.1111/imj.13556
- Selva-O’Callaghan A, Martinez-Gómez X, Trallero-Araguás E, et al. The diagnostic work-up of cancer-associated myositis. Curr Opin Rheumatol. 2018;30:630-636. doi:10.1097/BOR.0000000000000535
- Selva-O’Callaghan A, Grau JM, Gámez-Cenzano C, et al. Conventional cancer screening versus PET/CT in dermatomyositis/polymyositis. Am J Med. 2010;123:558-562. doi:10.1016/j.amjmed.2009.11.012
- Kundrick A, Kirby J, Ba D, et al. Positron emission tomography costs less to patients than conventional screening for malignancy in dermatomyositis. Semin Arthritis Rheum. 2019;49:140-144. doi:10.1016/j.semarthrit.2018.10.021
- Satoh M, Tanaka S, Ceribelli A, et al. A comprehensive overview on myositis-specific antibodies: new and old biomarkers in idiopathic inflammatory myopathy. Clin Rev Allergy Immunol. 2017;52:1-19. doi:10.1007/s12016-015-8510-y
- Vaughan H, Rugo HS, Haemel A. Risk-based screening for cancer in patients with dermatomyositis: toward a more individualized approach. JAMA Dermatol. 2022;158:244-247. doi:10.1001/jamadermatol.2021.5841
- Khanna U, Galimberti F, Li Y, et al. Dermatomyositis and malignancy: should all patients with dermatomyositis undergo malignancy screening? Ann Transl Med. 2021;9:432. doi:10.21037/atm-20-5215
- Oldroyd AGS, Allard AB, Callen JP, et al. Corrigendum to: A systematic review and meta-analysis to inform cancer screening guidelines in idiopathic inflammatory myopathies. Rheumatology (Oxford). 2021;60:5483. doi:10.1093/rheumatology/keab616
- Tchuenche M, Haté V, McPherson D, et al. Estimating client out-of-pocket costs for accessing voluntary medical male circumcision in South Africa. PLoS One. 2016;11:E0164147. doi:10.1371/journal.pone.0164147
- Teni FS, Gebresillassie BM, Birru EM, et al. Costs incurred by outpatients at a university hospital in northwestern Ethiopia: a cross-sectional study. BMC Health Serv Res. 2018;18:842. doi:10.1186/s12913-018-3628-2
- Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377-381. doi:10.1016/j.jbi.2008.08.010
- Bala MV, Mauskopf JA, Wood LL. Willingness to pay as a measure of health benefits. Pharmacoeconomics. 1999;15:9-18. doi:10.2165/00019053-199915010-00002
- Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet. 2003;362:971-982. doi:10.1016/S0140-6736(03)14368-1
- Schmidt J. Current classification and management of inflammatory myopathies. J Neuromuscul Dis. 2018;5:109-129. doi:10.3233/JND-180308
- Lazarou IN, Guerne PA. Classification, diagnosis, and management of idiopathic inflammatory myopathies. J Rheumatol. 201;40:550-564. doi:10.3899/jrheum.120682
- Wang J, Guo G, Chen G, et al. Meta-analysis of the association of dermatomyositis and polymyositis with cancer. Br J Dermatol. 2013;169:838-847. doi:10.1111/bjd.12564
- Zampieri S, Valente M, Adami N, et al. Polymyositis, dermatomyositis and malignancy: a further intriguing link. Autoimmun Rev. 2010;9:449-453. doi:10.1016/j.autrev.2009.12.005
- Sigurgeirsson B, Lindelöf B, Edhag O, et al. Risk of cancer in patients with dermatomyositis or polymyositis. a population-based study. N Engl J Med. 1992;326:363-367. doi:10.1056/nejm199202063260602
- Chen YJ, Wu CY, Huang YL, et al. Cancer risks of dermatomyositis and polymyositis: a nationwide cohort study in Taiwan. Arthritis Res Ther. 2010;12:R70. doi:10.1186/ar2987
- Chen YJ, Wu CY, Shen JL. Predicting factors of malignancy in dermatomyositis and polymyositis: a case-control study. Br J Dermatol. 2001;144:825-831. doi:10.1046/j.1365-2133.2001.04140.x
- Targoff IN, Mamyrova G, Trieu EP, et al. A novel autoantibody to a 155-kd protein is associated with dermatomyositis. Arthritis Rheum. 2006;54:3682-3689. doi:10.1002/art.22164
- Chow WH, Gridley G, Mellemkjær L, et al. Cancer risk following polymyositis and dermatomyositis: a nationwide cohort study in Denmark. Cancer Causes Control. 1995;6:9-13. doi:10.1007/BF00051675
- Buchbinder R, Forbes A, Hall S, et al. Incidence of malignant disease in biopsy-proven inflammatory myopathy: a population-based cohort study. Ann Intern Med. 2001;134:1087-1095. doi:10.7326/0003-4819-134-12-200106190-00008
- Hill CL, Zhang Y, Sigurgeirsson B, et al. Frequency of specific cancer types in dermatomyositis and polymyositis: a population-based study. Lancet. 2001;357:96-100. doi:10.1016/S0140-6736(00)03540-6
- Leatham H, Schadt C, Chisolm S, et al. Evidence supports blind screening for internal malignancy in dermatomyositis: data from 2 large US dermatology cohorts. Medicine (Baltimore). 2018;97:E9639. doi:10.1097/MD.0000000000009639
- Sparsa A, Liozon E, Herrmann F, et al. Routine vs extensive malignancy search for adult dermatomyositis and polymyositis: a study of 40 patients. Arch Dermatol. 2002;138:885-890.
- Dutton K, Soden M. Malignancy screening in autoimmune myositis among Australian rheumatologists. Intern Med J. 2017;47:1367-1375. doi:10.1111/imj.13556
- Selva-O’Callaghan A, Martinez-Gómez X, Trallero-Araguás E, et al. The diagnostic work-up of cancer-associated myositis. Curr Opin Rheumatol. 2018;30:630-636. doi:10.1097/BOR.0000000000000535
- Selva-O’Callaghan A, Grau JM, Gámez-Cenzano C, et al. Conventional cancer screening versus PET/CT in dermatomyositis/polymyositis. Am J Med. 2010;123:558-562. doi:10.1016/j.amjmed.2009.11.012
- Kundrick A, Kirby J, Ba D, et al. Positron emission tomography costs less to patients than conventional screening for malignancy in dermatomyositis. Semin Arthritis Rheum. 2019;49:140-144. doi:10.1016/j.semarthrit.2018.10.021
- Satoh M, Tanaka S, Ceribelli A, et al. A comprehensive overview on myositis-specific antibodies: new and old biomarkers in idiopathic inflammatory myopathy. Clin Rev Allergy Immunol. 2017;52:1-19. doi:10.1007/s12016-015-8510-y
- Vaughan H, Rugo HS, Haemel A. Risk-based screening for cancer in patients with dermatomyositis: toward a more individualized approach. JAMA Dermatol. 2022;158:244-247. doi:10.1001/jamadermatol.2021.5841
- Khanna U, Galimberti F, Li Y, et al. Dermatomyositis and malignancy: should all patients with dermatomyositis undergo malignancy screening? Ann Transl Med. 2021;9:432. doi:10.21037/atm-20-5215
- Oldroyd AGS, Allard AB, Callen JP, et al. Corrigendum to: A systematic review and meta-analysis to inform cancer screening guidelines in idiopathic inflammatory myopathies. Rheumatology (Oxford). 2021;60:5483. doi:10.1093/rheumatology/keab616
- Tchuenche M, Haté V, McPherson D, et al. Estimating client out-of-pocket costs for accessing voluntary medical male circumcision in South Africa. PLoS One. 2016;11:E0164147. doi:10.1371/journal.pone.0164147
- Teni FS, Gebresillassie BM, Birru EM, et al. Costs incurred by outpatients at a university hospital in northwestern Ethiopia: a cross-sectional study. BMC Health Serv Res. 2018;18:842. doi:10.1186/s12913-018-3628-2
- Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377-381. doi:10.1016/j.jbi.2008.08.010
- Bala MV, Mauskopf JA, Wood LL. Willingness to pay as a measure of health benefits. Pharmacoeconomics. 1999;15:9-18. doi:10.2165/00019053-199915010-00002
Practice Points
- Dermatomyositis (DM) is associated with an increased risk for malignancy. Patient perspective needs to be considered in developing cancer screening guidelines for patients with DM, particularly given the similar efficacy of available screening modalities.
- Current modalities for cancer screening in DM include whole-body positron emission tomography/computed tomography (PET/CT) and a conventional cancer screening panel (CSP), which includes a battery of tests typically requiring multiple visits. Patients may find the simplicity of PET/CT more preferrable than the more complex CSP.
- Indirect costs of cancer screening include missed work, travel and childcare expenses, and lost wages. Conventional cancer screening has greater indirect costs than PET/CT.
Analysis of a Pilot Curriculum for Business Education in Dermatology Residency
To the Editor:
With health care constituting one of the larger segments of the US economy, medical practice is increasingly subject to business considerations.1 Patients, providers, and organizations are all required to make decisions that reflect choices beyond clinical needs alone. Given the impact of market forces, clinicians often are asked to navigate operational and business decisions. Accordingly, education about the policy and systems that shape care delivery can improve quality and help patients.2
The ability to understand the ecosystem of health care is of utmost importance for medical providers and can be achieved through resident education. Teaching fundamental business concepts enables residents to deliver care that is responsive to the constraints and opportunities encountered by patients and organizations, which ultimately will better prepare them to serve as advocates in alignment with their principal duties as physicians.
Despite the recognizable relationship between business and medicine, training has not yet been standardized to include topics in business education, and clinicians in dermatology are remarkably positioned to benefit because of the variety of practice settings and services they can provide. In dermatology, the diversity of services provided gives rise to complex coding and use of modifiers. Proper utilization of coding and billing is critical to create accurate documentation and receive appropriate reimbursement.3 Furthermore, clinicians in dermatology have to contend with the influence of insurance at many points of care, such as with coverage of pharmaceuticals. Formularies often have wide variability in coverage and are changing as new drugs come to market in the dermatologic space.4
The landscape of practice structure also has undergone change with increasing consolidation and mergers. The acquisition of practices by private equity firms has induced changes in practice infrastructure. The impact of changing organizational and managerial influences continues to be a topic of debate, with disparate opinions on how these developments shape standards of physician satisfaction and patient care.5
The convergence of these factors points to an important question that is gaining popularity: How will young dermatologists work within the context of all these parameters to best advocate and care for their patients? These questions are garnering more attention and were recently investigated through a survey of participants in a pilot program to evaluate the importance of business education in dermatology residency.
A survey of residency program directors was created by Patrinley and Dewan,6 which found that business education during residency was important and additional training should be implemented. Despite the perceived importance of business education, only half of the programs represented by survey respondents offered any structured educational opportunities, revealing a discrepancy between believed importance and practical implementation of business training, which suggests the need to develop a standardized, dermatology-specific curriculum that could be accessed by all residents in training.6
We performed a search of the medical literature to identify models of business education in residency programs. Only a few programs were identified, in which courses were predominantly instructed to trainees in primary care–based fields. According to course graduates, the programs were beneficial.7,8 Programs that had descriptive information about curriculum structure and content were chosen for further investigation and included internal medicine programs at the University of California San Francisco (UCSF) and Columbia University Vagelos College of Physicians and Surgeons (New York, New York). UCSF implemented a Program in Residency Investigation Methods and Epidemiology (PRIME program) to deliver seven 90-minute sessions dedicated to introducing residents to medical economics. Sessions were constructed with the intent of being interactive seminars that took on a variety of forms, including reading-based discussions, case-based analysis, and simulation-based learning.7 Columbia University developed a pilot program of week-long didactic sessions that were delivered to third-year internal medicine residents. These seminars featured discussions on health policy and economics, health insurance, technology and cost assessment, legal medicine, public health, community-oriented primary care, and local health department initiatives.8 We drew on both courses to build a lecture series focused on the business of dermatology that was delivered to dermatology residents at UMass Chan Medical School (Worcester, Massachusetts). Topic selection also was informed by qualitative input collected via email from recent graduates of the UMass dermatology residency program, focusing on the following areas: the US medical economy and health care costs; billing, coding, and claims processing; quality, relative value units (RVUs), reimbursement, and the merit-based incentive payment system; coverage of pharmaceuticals and teledermatology; and management. Residents were not required to prepare for any of the sessions; they were provided with handouts and slideshow presentations for reference to review at their convenience if desired. Five seminars were virtually conducted by an MD/MBA candidate at the institution (E.H.). They were recorded over the course of an academic year at 1- to 2-month intervals. Each 45-minute session was conducted in a lecture-discussion format and included case examples to help illustrate key principles and stimulate conversation. For example, the lecture on reimbursement incorporated a fee schedule calculation for a shave biopsy, using RVU and geographic pricing cost index (GCPI) multipliers. This demonstrated the variation in Centers for Medicare & Medicaid Services reimbursement in relation to (1) constituents of the RVU calculation (ie, work, practice expense, and malpractice) and (2) practice in a particular location (ie, the GCPI). Following this example, a conversation ensued among participants regarding the factors that drive valuation, with particular interest in variation based on urban vs suburban locations across the United States. Participants also found it of interest to examine the percentage of the valuation dedicated to each constituent and how features such as lesion size informed the final assessment of the charge. Another stylistic choice in developing the model was to include prompts for further consideration prior to transitioning topics in the lectures. For example: when examining the burden of skin disease, the audience was prompted to consider: “What is driving cost escalations, and how will services of the clinical domain meet these evolving needs?” At another point in the introductory lecture, residents were asked: “How do different types of insurance plans impact the management of patients with dermatologic concerns?” These questions were intended to transition residents to the next topic of discussion and highlight take-home points of consideration for medical practice. The project was reviewed by the UMass institutional review board and met criteria for exemption.
Residents who participated in at least 1 lecture (N=10) were surveyed after attendance; there were 7 responses (70% response rate). Residents were asked to rate a series of statements on a scale of 1 (strongly disagree) to 5 (strongly agree) and to provide commentary via an online form. Respondents indicated that the course was enjoyable (average score, 4.00), provided an appropriate level of detail (average score, 4.00), would be beneficial to integrate into a dermatology residency curriculum (average score, 3.86), and informed how they would practice as a clinician (average score, 3.86)(Figure). The respondents agreed that the course met the main goals of this initiative: it helped them develop knowledge about the interface between business and dermatology (4.14) and exposed residents to topics they had not learned about previously (4.71).
Although the course generally was well received, areas for improvement were identified from respondents’ comments, relating to audience engagement and refining the level of detail in the lectures. Recommendations included “less technical jargon and more focus on ‘big picture’ concepts, given audience’s low baseline knowledge”; “more case examples in each module”; and “more diagrams or interactive activities (polls, quizzes, break-out rooms) because the lectures were a bit dense.” This input was taken into consideration when revising the lectures for future use; they were reconstructed to have more case-based examples and prompts to encourage participation.
Resident commentary also demonstrated appreciation for education in this subject material. Statements such as “this is an important topic for future dermatologists” and “thank you so much for taking the time to implement this course” reflected the perceived value of this material during critical academic time. Another resident remarked: “This was great, thanks for putting it together.”
Given the positive experience of the residents and successful implementation of the series, this course was made available to all dermatology trainees on a network server with accompanying written documents. It is planned to be offered on a 3-year cycle in the future and will be updated to reflect inevitable changes in health care.
Although the relationship between business and medicine is increasingly important, teaching business principles has not become standardized or required in medical training. Despite the perception that this content is of value, implementation of programming has lagged behind that recognition, likely due to challenges in designing the curriculum and diffusing content into an already-saturated schedule. A model course that can be replicated in other residency programs would be valuable. We introduced a dermatology-specific lecture series to help prepare trainees for dermatology practice in a variety of clinical settings and train them with the language of business and operations that will equip them to respond to the needs of their patients, their practice, and the medical environment. Findings of this pilot study may not be generalizable to all dermatology residency programs because the sample size was small; the study was conducted at a single institution; and the content was delivered entirely online.
1. Tan S, Seiger K, Renehan P, et al. Trends in private equity acquisition of dermatology practices in the United States. JAMA Dermatol. 2019;155:1013-1021. doi:10.1001/jamadermatol.2019.1634
2. The business of health care in the United States. Harvard Online [Internet]. June 27, 2022. Accessed July 24, 2023. https://www.harvardonline.harvard.edu/blog/business-health-care-united-states
3. Ranpariya V, Cull D, Feldman SR, et al. Evaluation and management 2021 coding guidelines: key changes and implications. The Dermatologist. December 2020. Accessed July 24, 2023. https://www.hmpgloballearningnetwork.com/site/thederm/article/evaluation-and-management-2021-coding-guidelines-key-changes-and-implications?key=Ranpariya&elastic%5B0%5D=brand%3A73468
4. Lim HW, Collins SAB, Resneck JS Jr, et al. The burden of skin disease in the United States. J Am Acad Dermatol. 2017;76:958-972.e2. doi:10.1016/j.jaad.2016.12.043
5. Resneck JS Jr. Dermatology practice consolidation fueled by private equity investment: potential consequences for the specialty and patients. JAMA Dermatol. 2018;154:13-14. doi:10.1001/jamadermatol.2017.5558
6. Patrinely JR Jr, Dewan AK. Business education in dermatology residency: a survey of program directors. Cutis. 2021;108:E7-E19. doi:10.12788/cutis.0331
7. Kohlwes RJ, Chou CL. A curriculum in medical economics for residents. Acad Med. 2002;77:465-466. doi:10.1097/00001888-200205000-00040
8. Fiebach NH, Rao D, Hamm ME. A curriculum in health systems and public health for internal medicine residents. Am J Prev Med. 2011;41(4 suppl 3):S264-S269. doi:10.1016/j.amepre.2011.05.025
To the Editor:
With health care constituting one of the larger segments of the US economy, medical practice is increasingly subject to business considerations.1 Patients, providers, and organizations are all required to make decisions that reflect choices beyond clinical needs alone. Given the impact of market forces, clinicians often are asked to navigate operational and business decisions. Accordingly, education about the policy and systems that shape care delivery can improve quality and help patients.2
The ability to understand the ecosystem of health care is of utmost importance for medical providers and can be achieved through resident education. Teaching fundamental business concepts enables residents to deliver care that is responsive to the constraints and opportunities encountered by patients and organizations, which ultimately will better prepare them to serve as advocates in alignment with their principal duties as physicians.
Despite the recognizable relationship between business and medicine, training has not yet been standardized to include topics in business education, and clinicians in dermatology are remarkably positioned to benefit because of the variety of practice settings and services they can provide. In dermatology, the diversity of services provided gives rise to complex coding and use of modifiers. Proper utilization of coding and billing is critical to create accurate documentation and receive appropriate reimbursement.3 Furthermore, clinicians in dermatology have to contend with the influence of insurance at many points of care, such as with coverage of pharmaceuticals. Formularies often have wide variability in coverage and are changing as new drugs come to market in the dermatologic space.4
The landscape of practice structure also has undergone change with increasing consolidation and mergers. The acquisition of practices by private equity firms has induced changes in practice infrastructure. The impact of changing organizational and managerial influences continues to be a topic of debate, with disparate opinions on how these developments shape standards of physician satisfaction and patient care.5
The convergence of these factors points to an important question that is gaining popularity: How will young dermatologists work within the context of all these parameters to best advocate and care for their patients? These questions are garnering more attention and were recently investigated through a survey of participants in a pilot program to evaluate the importance of business education in dermatology residency.
A survey of residency program directors was created by Patrinley and Dewan,6 which found that business education during residency was important and additional training should be implemented. Despite the perceived importance of business education, only half of the programs represented by survey respondents offered any structured educational opportunities, revealing a discrepancy between believed importance and practical implementation of business training, which suggests the need to develop a standardized, dermatology-specific curriculum that could be accessed by all residents in training.6
We performed a search of the medical literature to identify models of business education in residency programs. Only a few programs were identified, in which courses were predominantly instructed to trainees in primary care–based fields. According to course graduates, the programs were beneficial.7,8 Programs that had descriptive information about curriculum structure and content were chosen for further investigation and included internal medicine programs at the University of California San Francisco (UCSF) and Columbia University Vagelos College of Physicians and Surgeons (New York, New York). UCSF implemented a Program in Residency Investigation Methods and Epidemiology (PRIME program) to deliver seven 90-minute sessions dedicated to introducing residents to medical economics. Sessions were constructed with the intent of being interactive seminars that took on a variety of forms, including reading-based discussions, case-based analysis, and simulation-based learning.7 Columbia University developed a pilot program of week-long didactic sessions that were delivered to third-year internal medicine residents. These seminars featured discussions on health policy and economics, health insurance, technology and cost assessment, legal medicine, public health, community-oriented primary care, and local health department initiatives.8 We drew on both courses to build a lecture series focused on the business of dermatology that was delivered to dermatology residents at UMass Chan Medical School (Worcester, Massachusetts). Topic selection also was informed by qualitative input collected via email from recent graduates of the UMass dermatology residency program, focusing on the following areas: the US medical economy and health care costs; billing, coding, and claims processing; quality, relative value units (RVUs), reimbursement, and the merit-based incentive payment system; coverage of pharmaceuticals and teledermatology; and management. Residents were not required to prepare for any of the sessions; they were provided with handouts and slideshow presentations for reference to review at their convenience if desired. Five seminars were virtually conducted by an MD/MBA candidate at the institution (E.H.). They were recorded over the course of an academic year at 1- to 2-month intervals. Each 45-minute session was conducted in a lecture-discussion format and included case examples to help illustrate key principles and stimulate conversation. For example, the lecture on reimbursement incorporated a fee schedule calculation for a shave biopsy, using RVU and geographic pricing cost index (GCPI) multipliers. This demonstrated the variation in Centers for Medicare & Medicaid Services reimbursement in relation to (1) constituents of the RVU calculation (ie, work, practice expense, and malpractice) and (2) practice in a particular location (ie, the GCPI). Following this example, a conversation ensued among participants regarding the factors that drive valuation, with particular interest in variation based on urban vs suburban locations across the United States. Participants also found it of interest to examine the percentage of the valuation dedicated to each constituent and how features such as lesion size informed the final assessment of the charge. Another stylistic choice in developing the model was to include prompts for further consideration prior to transitioning topics in the lectures. For example: when examining the burden of skin disease, the audience was prompted to consider: “What is driving cost escalations, and how will services of the clinical domain meet these evolving needs?” At another point in the introductory lecture, residents were asked: “How do different types of insurance plans impact the management of patients with dermatologic concerns?” These questions were intended to transition residents to the next topic of discussion and highlight take-home points of consideration for medical practice. The project was reviewed by the UMass institutional review board and met criteria for exemption.
Residents who participated in at least 1 lecture (N=10) were surveyed after attendance; there were 7 responses (70% response rate). Residents were asked to rate a series of statements on a scale of 1 (strongly disagree) to 5 (strongly agree) and to provide commentary via an online form. Respondents indicated that the course was enjoyable (average score, 4.00), provided an appropriate level of detail (average score, 4.00), would be beneficial to integrate into a dermatology residency curriculum (average score, 3.86), and informed how they would practice as a clinician (average score, 3.86)(Figure). The respondents agreed that the course met the main goals of this initiative: it helped them develop knowledge about the interface between business and dermatology (4.14) and exposed residents to topics they had not learned about previously (4.71).

Although the course generally was well received, areas for improvement were identified from respondents’ comments, relating to audience engagement and refining the level of detail in the lectures. Recommendations included “less technical jargon and more focus on ‘big picture’ concepts, given audience’s low baseline knowledge”; “more case examples in each module”; and “more diagrams or interactive activities (polls, quizzes, break-out rooms) because the lectures were a bit dense.” This input was taken into consideration when revising the lectures for future use; they were reconstructed to have more case-based examples and prompts to encourage participation.
Resident commentary also demonstrated appreciation for education in this subject material. Statements such as “this is an important topic for future dermatologists” and “thank you so much for taking the time to implement this course” reflected the perceived value of this material during critical academic time. Another resident remarked: “This was great, thanks for putting it together.”
Given the positive experience of the residents and successful implementation of the series, this course was made available to all dermatology trainees on a network server with accompanying written documents. It is planned to be offered on a 3-year cycle in the future and will be updated to reflect inevitable changes in health care.
Although the relationship between business and medicine is increasingly important, teaching business principles has not become standardized or required in medical training. Despite the perception that this content is of value, implementation of programming has lagged behind that recognition, likely due to challenges in designing the curriculum and diffusing content into an already-saturated schedule. A model course that can be replicated in other residency programs would be valuable. We introduced a dermatology-specific lecture series to help prepare trainees for dermatology practice in a variety of clinical settings and train them with the language of business and operations that will equip them to respond to the needs of their patients, their practice, and the medical environment. Findings of this pilot study may not be generalizable to all dermatology residency programs because the sample size was small; the study was conducted at a single institution; and the content was delivered entirely online.
To the Editor:
With health care constituting one of the larger segments of the US economy, medical practice is increasingly subject to business considerations.1 Patients, providers, and organizations are all required to make decisions that reflect choices beyond clinical needs alone. Given the impact of market forces, clinicians often are asked to navigate operational and business decisions. Accordingly, education about the policy and systems that shape care delivery can improve quality and help patients.2
The ability to understand the ecosystem of health care is of utmost importance for medical providers and can be achieved through resident education. Teaching fundamental business concepts enables residents to deliver care that is responsive to the constraints and opportunities encountered by patients and organizations, which ultimately will better prepare them to serve as advocates in alignment with their principal duties as physicians.
Despite the recognizable relationship between business and medicine, training has not yet been standardized to include topics in business education, and clinicians in dermatology are remarkably positioned to benefit because of the variety of practice settings and services they can provide. In dermatology, the diversity of services provided gives rise to complex coding and use of modifiers. Proper utilization of coding and billing is critical to create accurate documentation and receive appropriate reimbursement.3 Furthermore, clinicians in dermatology have to contend with the influence of insurance at many points of care, such as with coverage of pharmaceuticals. Formularies often have wide variability in coverage and are changing as new drugs come to market in the dermatologic space.4
The landscape of practice structure also has undergone change with increasing consolidation and mergers. The acquisition of practices by private equity firms has induced changes in practice infrastructure. The impact of changing organizational and managerial influences continues to be a topic of debate, with disparate opinions on how these developments shape standards of physician satisfaction and patient care.5
The convergence of these factors points to an important question that is gaining popularity: How will young dermatologists work within the context of all these parameters to best advocate and care for their patients? These questions are garnering more attention and were recently investigated through a survey of participants in a pilot program to evaluate the importance of business education in dermatology residency.
A survey of residency program directors was created by Patrinley and Dewan,6 which found that business education during residency was important and additional training should be implemented. Despite the perceived importance of business education, only half of the programs represented by survey respondents offered any structured educational opportunities, revealing a discrepancy between believed importance and practical implementation of business training, which suggests the need to develop a standardized, dermatology-specific curriculum that could be accessed by all residents in training.6
We performed a search of the medical literature to identify models of business education in residency programs. Only a few programs were identified, in which courses were predominantly instructed to trainees in primary care–based fields. According to course graduates, the programs were beneficial.7,8 Programs that had descriptive information about curriculum structure and content were chosen for further investigation and included internal medicine programs at the University of California San Francisco (UCSF) and Columbia University Vagelos College of Physicians and Surgeons (New York, New York). UCSF implemented a Program in Residency Investigation Methods and Epidemiology (PRIME program) to deliver seven 90-minute sessions dedicated to introducing residents to medical economics. Sessions were constructed with the intent of being interactive seminars that took on a variety of forms, including reading-based discussions, case-based analysis, and simulation-based learning.7 Columbia University developed a pilot program of week-long didactic sessions that were delivered to third-year internal medicine residents. These seminars featured discussions on health policy and economics, health insurance, technology and cost assessment, legal medicine, public health, community-oriented primary care, and local health department initiatives.8 We drew on both courses to build a lecture series focused on the business of dermatology that was delivered to dermatology residents at UMass Chan Medical School (Worcester, Massachusetts). Topic selection also was informed by qualitative input collected via email from recent graduates of the UMass dermatology residency program, focusing on the following areas: the US medical economy and health care costs; billing, coding, and claims processing; quality, relative value units (RVUs), reimbursement, and the merit-based incentive payment system; coverage of pharmaceuticals and teledermatology; and management. Residents were not required to prepare for any of the sessions; they were provided with handouts and slideshow presentations for reference to review at their convenience if desired. Five seminars were virtually conducted by an MD/MBA candidate at the institution (E.H.). They were recorded over the course of an academic year at 1- to 2-month intervals. Each 45-minute session was conducted in a lecture-discussion format and included case examples to help illustrate key principles and stimulate conversation. For example, the lecture on reimbursement incorporated a fee schedule calculation for a shave biopsy, using RVU and geographic pricing cost index (GCPI) multipliers. This demonstrated the variation in Centers for Medicare & Medicaid Services reimbursement in relation to (1) constituents of the RVU calculation (ie, work, practice expense, and malpractice) and (2) practice in a particular location (ie, the GCPI). Following this example, a conversation ensued among participants regarding the factors that drive valuation, with particular interest in variation based on urban vs suburban locations across the United States. Participants also found it of interest to examine the percentage of the valuation dedicated to each constituent and how features such as lesion size informed the final assessment of the charge. Another stylistic choice in developing the model was to include prompts for further consideration prior to transitioning topics in the lectures. For example: when examining the burden of skin disease, the audience was prompted to consider: “What is driving cost escalations, and how will services of the clinical domain meet these evolving needs?” At another point in the introductory lecture, residents were asked: “How do different types of insurance plans impact the management of patients with dermatologic concerns?” These questions were intended to transition residents to the next topic of discussion and highlight take-home points of consideration for medical practice. The project was reviewed by the UMass institutional review board and met criteria for exemption.
Residents who participated in at least 1 lecture (N=10) were surveyed after attendance; there were 7 responses (70% response rate). Residents were asked to rate a series of statements on a scale of 1 (strongly disagree) to 5 (strongly agree) and to provide commentary via an online form. Respondents indicated that the course was enjoyable (average score, 4.00), provided an appropriate level of detail (average score, 4.00), would be beneficial to integrate into a dermatology residency curriculum (average score, 3.86), and informed how they would practice as a clinician (average score, 3.86)(Figure). The respondents agreed that the course met the main goals of this initiative: it helped them develop knowledge about the interface between business and dermatology (4.14) and exposed residents to topics they had not learned about previously (4.71).

Although the course generally was well received, areas for improvement were identified from respondents’ comments, relating to audience engagement and refining the level of detail in the lectures. Recommendations included “less technical jargon and more focus on ‘big picture’ concepts, given audience’s low baseline knowledge”; “more case examples in each module”; and “more diagrams or interactive activities (polls, quizzes, break-out rooms) because the lectures were a bit dense.” This input was taken into consideration when revising the lectures for future use; they were reconstructed to have more case-based examples and prompts to encourage participation.
Resident commentary also demonstrated appreciation for education in this subject material. Statements such as “this is an important topic for future dermatologists” and “thank you so much for taking the time to implement this course” reflected the perceived value of this material during critical academic time. Another resident remarked: “This was great, thanks for putting it together.”
Given the positive experience of the residents and successful implementation of the series, this course was made available to all dermatology trainees on a network server with accompanying written documents. It is planned to be offered on a 3-year cycle in the future and will be updated to reflect inevitable changes in health care.
Although the relationship between business and medicine is increasingly important, teaching business principles has not become standardized or required in medical training. Despite the perception that this content is of value, implementation of programming has lagged behind that recognition, likely due to challenges in designing the curriculum and diffusing content into an already-saturated schedule. A model course that can be replicated in other residency programs would be valuable. We introduced a dermatology-specific lecture series to help prepare trainees for dermatology practice in a variety of clinical settings and train them with the language of business and operations that will equip them to respond to the needs of their patients, their practice, and the medical environment. Findings of this pilot study may not be generalizable to all dermatology residency programs because the sample size was small; the study was conducted at a single institution; and the content was delivered entirely online.
1. Tan S, Seiger K, Renehan P, et al. Trends in private equity acquisition of dermatology practices in the United States. JAMA Dermatol. 2019;155:1013-1021. doi:10.1001/jamadermatol.2019.1634
2. The business of health care in the United States. Harvard Online [Internet]. June 27, 2022. Accessed July 24, 2023. https://www.harvardonline.harvard.edu/blog/business-health-care-united-states
3. Ranpariya V, Cull D, Feldman SR, et al. Evaluation and management 2021 coding guidelines: key changes and implications. The Dermatologist. December 2020. Accessed July 24, 2023. https://www.hmpgloballearningnetwork.com/site/thederm/article/evaluation-and-management-2021-coding-guidelines-key-changes-and-implications?key=Ranpariya&elastic%5B0%5D=brand%3A73468
4. Lim HW, Collins SAB, Resneck JS Jr, et al. The burden of skin disease in the United States. J Am Acad Dermatol. 2017;76:958-972.e2. doi:10.1016/j.jaad.2016.12.043
5. Resneck JS Jr. Dermatology practice consolidation fueled by private equity investment: potential consequences for the specialty and patients. JAMA Dermatol. 2018;154:13-14. doi:10.1001/jamadermatol.2017.5558
6. Patrinely JR Jr, Dewan AK. Business education in dermatology residency: a survey of program directors. Cutis. 2021;108:E7-E19. doi:10.12788/cutis.0331
7. Kohlwes RJ, Chou CL. A curriculum in medical economics for residents. Acad Med. 2002;77:465-466. doi:10.1097/00001888-200205000-00040
8. Fiebach NH, Rao D, Hamm ME. A curriculum in health systems and public health for internal medicine residents. Am J Prev Med. 2011;41(4 suppl 3):S264-S269. doi:10.1016/j.amepre.2011.05.025
1. Tan S, Seiger K, Renehan P, et al. Trends in private equity acquisition of dermatology practices in the United States. JAMA Dermatol. 2019;155:1013-1021. doi:10.1001/jamadermatol.2019.1634
2. The business of health care in the United States. Harvard Online [Internet]. June 27, 2022. Accessed July 24, 2023. https://www.harvardonline.harvard.edu/blog/business-health-care-united-states
3. Ranpariya V, Cull D, Feldman SR, et al. Evaluation and management 2021 coding guidelines: key changes and implications. The Dermatologist. December 2020. Accessed July 24, 2023. https://www.hmpgloballearningnetwork.com/site/thederm/article/evaluation-and-management-2021-coding-guidelines-key-changes-and-implications?key=Ranpariya&elastic%5B0%5D=brand%3A73468
4. Lim HW, Collins SAB, Resneck JS Jr, et al. The burden of skin disease in the United States. J Am Acad Dermatol. 2017;76:958-972.e2. doi:10.1016/j.jaad.2016.12.043
5. Resneck JS Jr. Dermatology practice consolidation fueled by private equity investment: potential consequences for the specialty and patients. JAMA Dermatol. 2018;154:13-14. doi:10.1001/jamadermatol.2017.5558
6. Patrinely JR Jr, Dewan AK. Business education in dermatology residency: a survey of program directors. Cutis. 2021;108:E7-E19. doi:10.12788/cutis.0331
7. Kohlwes RJ, Chou CL. A curriculum in medical economics for residents. Acad Med. 2002;77:465-466. doi:10.1097/00001888-200205000-00040
8. Fiebach NH, Rao D, Hamm ME. A curriculum in health systems and public health for internal medicine residents. Am J Prev Med. 2011;41(4 suppl 3):S264-S269. doi:10.1016/j.amepre.2011.05.025
Practice Points
- Business education in dermatology residency promotes understanding of the health care ecosystem and can enable residents to more effectively deliver care that is responsive to the needs of their patients.
- Teaching fundamental business principles to residents can inform decision-making on patient, provider, and systems levels.
- A pilot curriculum supports implementation of business education teaching and will be particularly helpful in dermatology.
Top 50 Authors in Dermatology by Publication Rate (2017-2022)
To the Editor:
Citation number and Hirsch index (h-index) have long been employed as metrics of productivity for academic scholarship. The h-index is defined as the highest number of publications (the maximum h value) of an author who has published at least h papers, each cited by other authors at least h times.1 In a bibliometric analysis of the most frequently cited authors in dermatology from 1974 to 2019 (N=378,276), females comprised 12% of first and 11% of senior authors of the most cited publications, and 6 of the most cited authors in dermatology were women.2 In another study analyzing the most prolific dermatologic authors based on h-index, 0% from 1980 to 1989 and 19% from 2010 to 2019 were female (N=393,488).3 Because citation number and h-index favor longer-practicing dermatologists, we examined dermatology author productivity and gender trends by recent publication rates.
The Scopus database was searched for dermatology publications by using the field category “dermatology”from January 1, 2017, to October 7, 2022. Nondermatologists and authors with the same initials were excluded. Authors were ranked by number of publications, including original articles, case reports, letters, and reviews. Sex, degree, and years of experience were determined via a Google search of the author’s name. The h-index; number of citations; and percentages of first, middle, and last authorship were recorded.
Of the top 50 published dermatologists, 30% were female (n=15) and 56% (n=28) held both MD and PhD degrees (Table). The mean years of experience was 26.27 years (range, 6–44 years), with a mean of 29.23 years in females and 25.87 years in males. The mean h-index was 27.96 (range, 8–88), with 24.87 for females and 29.29 for males. The mean number of citations was 4032.64 (range, 235–36,908), with 2891.13 for females and 4521.86 for males. Thirty-one authors were most frequently middle authors, 18 were senior authors, and 1 was a first author. On average (SD), authors were senior or first author in 47.97% (20.08%) of their publications (range, 6.32%–94.93%).
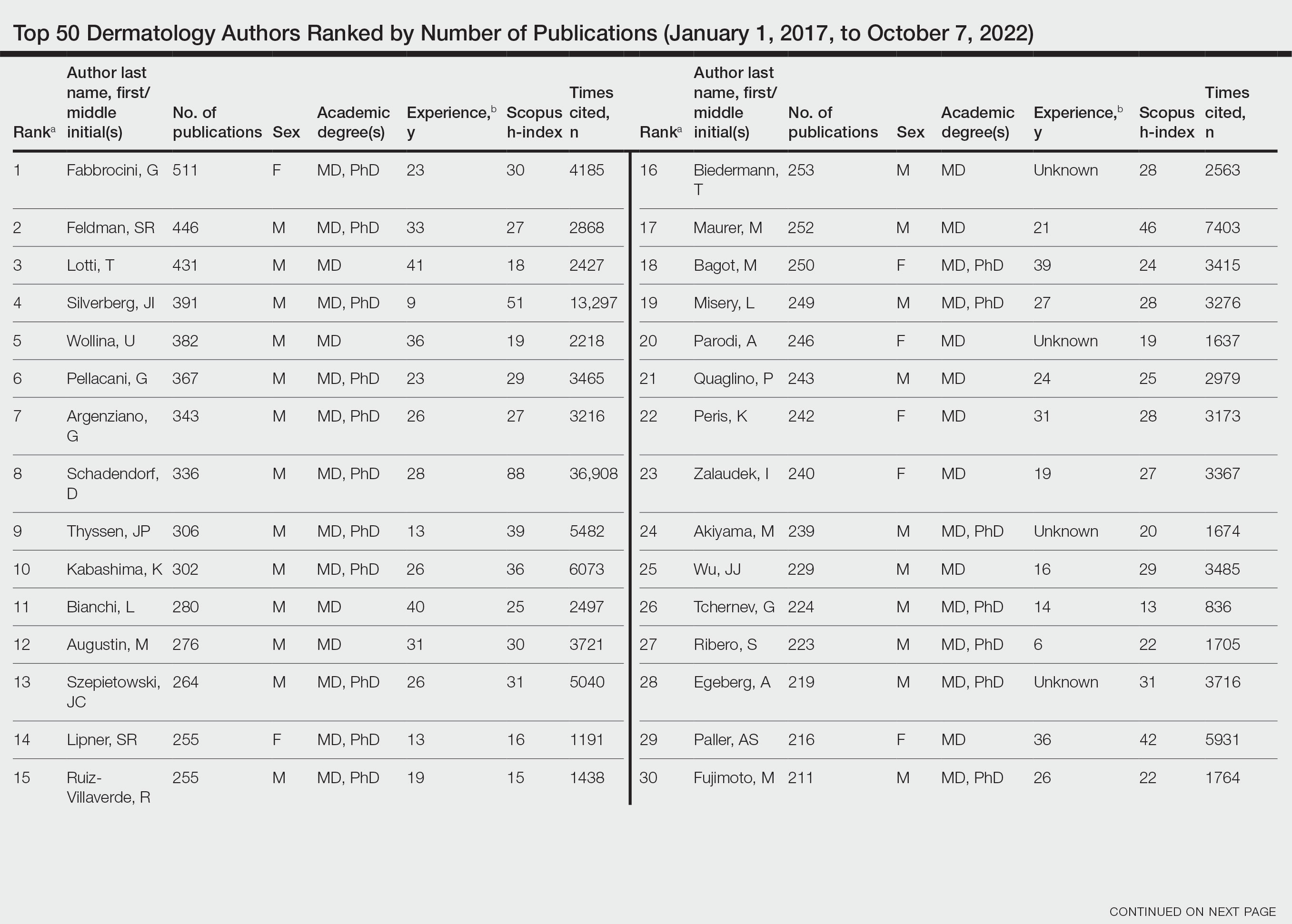
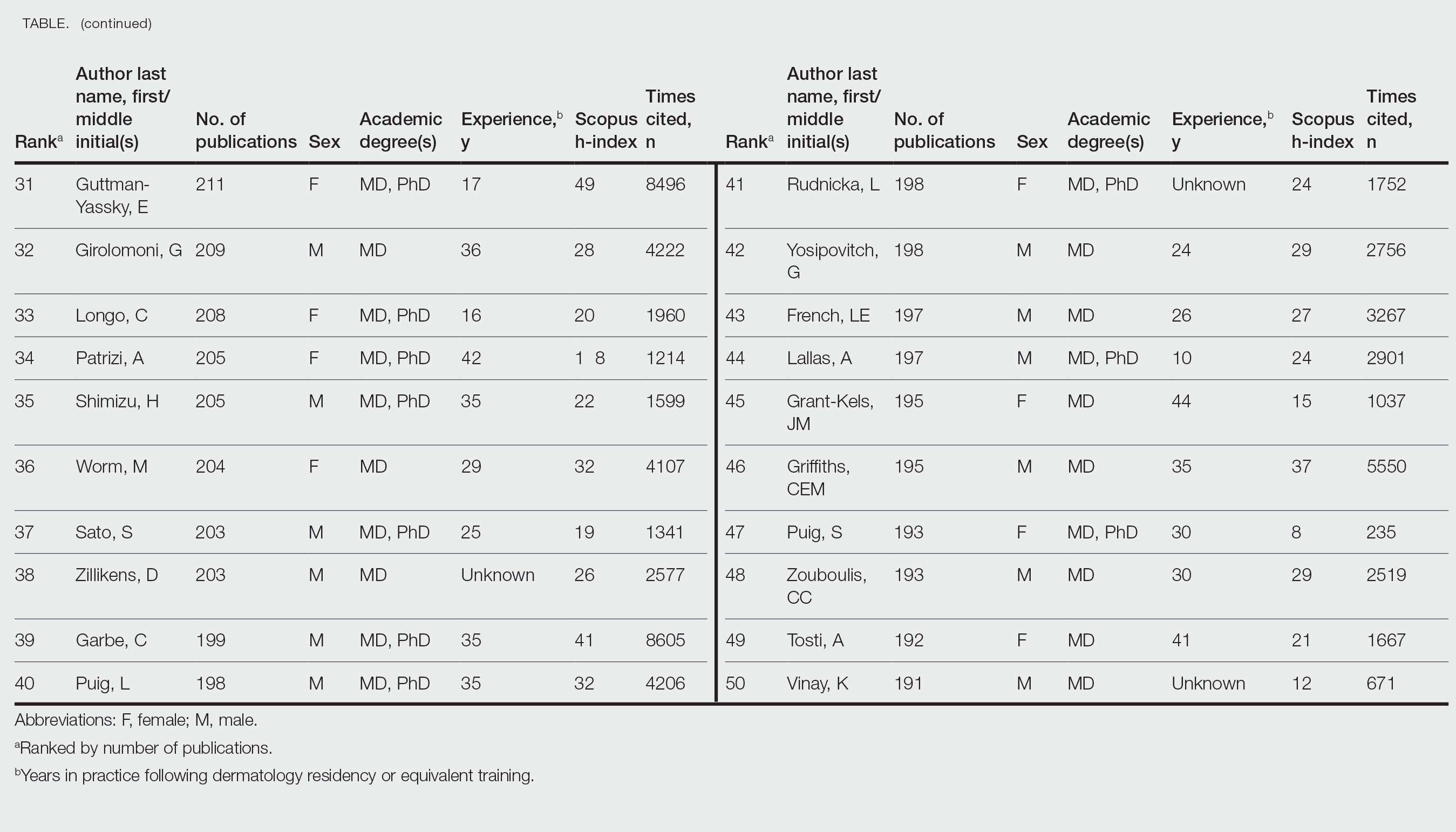
Our study shows that females were more highly represented as top dermatology authors (30%) as measured by publication numbers from 2017 to 2022 than in studies measuring citation rate from 1974 to 2019 (12%)2 or h-index from 2010 to 2019 (19%).3 Similarly, in a study of dermatology authorship from 2009 to 2019, on average, females represented 51.06% first and 38.18% last authors.4
The proportion of females in the dermatology workforce has increased, with 3964 of 10,385 (38.2%) active dermatologists in 20075 being female vs 6372 of 12,505 (51.0%) in 2019.6 The lower proportion of practicing female dermatologists in earlier years likely accounts for the lower percentage of females in dermatology citations and h-index top lists during that time, given that citation and h-index metrics are biased to dermatologists with longer careers.
Although our data are encouraging, females still accounted for less than one-third of the top 50 authors by publication numbers. Gender inequalities persist, with only one-third of a total of 1292 National Institutes of Health dermatology grants and one-fourth of Research Project Grant Program (R01) grants being awarded to females in the years 2009 to 2014.7 Therefore, formal and informal mentorship, protected time for research, resources for childcare, and opportunities for funding will be critical in supporting female dermatologists to both publish highly impactful research and obtain research grants.
Limitations of our study include the omission of authors with identical initials and the inability to account for name changes. Furthermore, Scopus does not include all articles published by each author. Finally, publication number reflects quantity but may not reflect quality.
By quantitating dermatology author publication numbers, we found better representation of female authors compared with studies measuring citation number and h-index. With higher proportions of female dermatology trainees and efforts to increase mentorship and research support for female dermatologists, we expect improved equality in top lists of dermatology citations and h-index values.
- Dysart J. Measuring research impact and quality: h-index. Accessed July 11, 2023. https://libraryguides.missouri.edu/impact/hindex
- Maymone MBC, Laughter M, Vashi NA, et al. The most cited articles and authors in dermatology: a bibliometric analysis of 1974-2019. J Am Acad Dermatol. 2020;83:201-205. doi:10.1016/j.jaad.2019.06.1308
- Szeto MD, Presley CL, Maymone MBC, et al. Top authors in dermatology by h-index: a bibliometric analysis of 1980-2020. J Am Acad Dermatol. 2021;85:1573-1579. doi:10.1016/j.jaad.2020.10.087
- Laughter MR, Yemc MG, Presley CL, et al. Gender representation in the authorship of dermatology publications. J Am Acad Dermatol. 2022;86:698-700. doi:10.1016/j.jaad.2021.03.019
- Association of American Medical Colleges. 2008 physician specialty data report. Accessed July 11, 2023. https://www.aamc.org/media/33491/download
- Association of American Medical Colleges. 2019 physician specialty data report. Accessed July 11, 2023. https://www.aamc.org/data-reports/workforce/data/active-physicians-sex-and-specialty-2019
- Cheng MY, Sukhov A, Sultani H, et al. Trends in National Institutes of Health funding of principal investigators in dermatology research by academic degree and sex. JAMA Dermatol. 2016;152:883-888. doi:10.1001/jamadermatol.2016.0271
To the Editor:
Citation number and Hirsch index (h-index) have long been employed as metrics of productivity for academic scholarship. The h-index is defined as the highest number of publications (the maximum h value) of an author who has published at least h papers, each cited by other authors at least h times.1 In a bibliometric analysis of the most frequently cited authors in dermatology from 1974 to 2019 (N=378,276), females comprised 12% of first and 11% of senior authors of the most cited publications, and 6 of the most cited authors in dermatology were women.2 In another study analyzing the most prolific dermatologic authors based on h-index, 0% from 1980 to 1989 and 19% from 2010 to 2019 were female (N=393,488).3 Because citation number and h-index favor longer-practicing dermatologists, we examined dermatology author productivity and gender trends by recent publication rates.
The Scopus database was searched for dermatology publications by using the field category “dermatology”from January 1, 2017, to October 7, 2022. Nondermatologists and authors with the same initials were excluded. Authors were ranked by number of publications, including original articles, case reports, letters, and reviews. Sex, degree, and years of experience were determined via a Google search of the author’s name. The h-index; number of citations; and percentages of first, middle, and last authorship were recorded.
Of the top 50 published dermatologists, 30% were female (n=15) and 56% (n=28) held both MD and PhD degrees (Table). The mean years of experience was 26.27 years (range, 6–44 years), with a mean of 29.23 years in females and 25.87 years in males. The mean h-index was 27.96 (range, 8–88), with 24.87 for females and 29.29 for males. The mean number of citations was 4032.64 (range, 235–36,908), with 2891.13 for females and 4521.86 for males. Thirty-one authors were most frequently middle authors, 18 were senior authors, and 1 was a first author. On average (SD), authors were senior or first author in 47.97% (20.08%) of their publications (range, 6.32%–94.93%).


Our study shows that females were more highly represented as top dermatology authors (30%) as measured by publication numbers from 2017 to 2022 than in studies measuring citation rate from 1974 to 2019 (12%)2 or h-index from 2010 to 2019 (19%).3 Similarly, in a study of dermatology authorship from 2009 to 2019, on average, females represented 51.06% first and 38.18% last authors.4
The proportion of females in the dermatology workforce has increased, with 3964 of 10,385 (38.2%) active dermatologists in 20075 being female vs 6372 of 12,505 (51.0%) in 2019.6 The lower proportion of practicing female dermatologists in earlier years likely accounts for the lower percentage of females in dermatology citations and h-index top lists during that time, given that citation and h-index metrics are biased to dermatologists with longer careers.
Although our data are encouraging, females still accounted for less than one-third of the top 50 authors by publication numbers. Gender inequalities persist, with only one-third of a total of 1292 National Institutes of Health dermatology grants and one-fourth of Research Project Grant Program (R01) grants being awarded to females in the years 2009 to 2014.7 Therefore, formal and informal mentorship, protected time for research, resources for childcare, and opportunities for funding will be critical in supporting female dermatologists to both publish highly impactful research and obtain research grants.
Limitations of our study include the omission of authors with identical initials and the inability to account for name changes. Furthermore, Scopus does not include all articles published by each author. Finally, publication number reflects quantity but may not reflect quality.
By quantitating dermatology author publication numbers, we found better representation of female authors compared with studies measuring citation number and h-index. With higher proportions of female dermatology trainees and efforts to increase mentorship and research support for female dermatologists, we expect improved equality in top lists of dermatology citations and h-index values.
To the Editor:
Citation number and Hirsch index (h-index) have long been employed as metrics of productivity for academic scholarship. The h-index is defined as the highest number of publications (the maximum h value) of an author who has published at least h papers, each cited by other authors at least h times.1 In a bibliometric analysis of the most frequently cited authors in dermatology from 1974 to 2019 (N=378,276), females comprised 12% of first and 11% of senior authors of the most cited publications, and 6 of the most cited authors in dermatology were women.2 In another study analyzing the most prolific dermatologic authors based on h-index, 0% from 1980 to 1989 and 19% from 2010 to 2019 were female (N=393,488).3 Because citation number and h-index favor longer-practicing dermatologists, we examined dermatology author productivity and gender trends by recent publication rates.
The Scopus database was searched for dermatology publications by using the field category “dermatology”from January 1, 2017, to October 7, 2022. Nondermatologists and authors with the same initials were excluded. Authors were ranked by number of publications, including original articles, case reports, letters, and reviews. Sex, degree, and years of experience were determined via a Google search of the author’s name. The h-index; number of citations; and percentages of first, middle, and last authorship were recorded.
Of the top 50 published dermatologists, 30% were female (n=15) and 56% (n=28) held both MD and PhD degrees (Table). The mean years of experience was 26.27 years (range, 6–44 years), with a mean of 29.23 years in females and 25.87 years in males. The mean h-index was 27.96 (range, 8–88), with 24.87 for females and 29.29 for males. The mean number of citations was 4032.64 (range, 235–36,908), with 2891.13 for females and 4521.86 for males. Thirty-one authors were most frequently middle authors, 18 were senior authors, and 1 was a first author. On average (SD), authors were senior or first author in 47.97% (20.08%) of their publications (range, 6.32%–94.93%).


Our study shows that females were more highly represented as top dermatology authors (30%) as measured by publication numbers from 2017 to 2022 than in studies measuring citation rate from 1974 to 2019 (12%)2 or h-index from 2010 to 2019 (19%).3 Similarly, in a study of dermatology authorship from 2009 to 2019, on average, females represented 51.06% first and 38.18% last authors.4
The proportion of females in the dermatology workforce has increased, with 3964 of 10,385 (38.2%) active dermatologists in 20075 being female vs 6372 of 12,505 (51.0%) in 2019.6 The lower proportion of practicing female dermatologists in earlier years likely accounts for the lower percentage of females in dermatology citations and h-index top lists during that time, given that citation and h-index metrics are biased to dermatologists with longer careers.
Although our data are encouraging, females still accounted for less than one-third of the top 50 authors by publication numbers. Gender inequalities persist, with only one-third of a total of 1292 National Institutes of Health dermatology grants and one-fourth of Research Project Grant Program (R01) grants being awarded to females in the years 2009 to 2014.7 Therefore, formal and informal mentorship, protected time for research, resources for childcare, and opportunities for funding will be critical in supporting female dermatologists to both publish highly impactful research and obtain research grants.
Limitations of our study include the omission of authors with identical initials and the inability to account for name changes. Furthermore, Scopus does not include all articles published by each author. Finally, publication number reflects quantity but may not reflect quality.
By quantitating dermatology author publication numbers, we found better representation of female authors compared with studies measuring citation number and h-index. With higher proportions of female dermatology trainees and efforts to increase mentorship and research support for female dermatologists, we expect improved equality in top lists of dermatology citations and h-index values.
- Dysart J. Measuring research impact and quality: h-index. Accessed July 11, 2023. https://libraryguides.missouri.edu/impact/hindex
- Maymone MBC, Laughter M, Vashi NA, et al. The most cited articles and authors in dermatology: a bibliometric analysis of 1974-2019. J Am Acad Dermatol. 2020;83:201-205. doi:10.1016/j.jaad.2019.06.1308
- Szeto MD, Presley CL, Maymone MBC, et al. Top authors in dermatology by h-index: a bibliometric analysis of 1980-2020. J Am Acad Dermatol. 2021;85:1573-1579. doi:10.1016/j.jaad.2020.10.087
- Laughter MR, Yemc MG, Presley CL, et al. Gender representation in the authorship of dermatology publications. J Am Acad Dermatol. 2022;86:698-700. doi:10.1016/j.jaad.2021.03.019
- Association of American Medical Colleges. 2008 physician specialty data report. Accessed July 11, 2023. https://www.aamc.org/media/33491/download
- Association of American Medical Colleges. 2019 physician specialty data report. Accessed July 11, 2023. https://www.aamc.org/data-reports/workforce/data/active-physicians-sex-and-specialty-2019
- Cheng MY, Sukhov A, Sultani H, et al. Trends in National Institutes of Health funding of principal investigators in dermatology research by academic degree and sex. JAMA Dermatol. 2016;152:883-888. doi:10.1001/jamadermatol.2016.0271
- Dysart J. Measuring research impact and quality: h-index. Accessed July 11, 2023. https://libraryguides.missouri.edu/impact/hindex
- Maymone MBC, Laughter M, Vashi NA, et al. The most cited articles and authors in dermatology: a bibliometric analysis of 1974-2019. J Am Acad Dermatol. 2020;83:201-205. doi:10.1016/j.jaad.2019.06.1308
- Szeto MD, Presley CL, Maymone MBC, et al. Top authors in dermatology by h-index: a bibliometric analysis of 1980-2020. J Am Acad Dermatol. 2021;85:1573-1579. doi:10.1016/j.jaad.2020.10.087
- Laughter MR, Yemc MG, Presley CL, et al. Gender representation in the authorship of dermatology publications. J Am Acad Dermatol. 2022;86:698-700. doi:10.1016/j.jaad.2021.03.019
- Association of American Medical Colleges. 2008 physician specialty data report. Accessed July 11, 2023. https://www.aamc.org/media/33491/download
- Association of American Medical Colleges. 2019 physician specialty data report. Accessed July 11, 2023. https://www.aamc.org/data-reports/workforce/data/active-physicians-sex-and-specialty-2019
- Cheng MY, Sukhov A, Sultani H, et al. Trends in National Institutes of Health funding of principal investigators in dermatology research by academic degree and sex. JAMA Dermatol. 2016;152:883-888. doi:10.1001/jamadermatol.2016.0271
Practice Points
- Academic scholarship often is measured by number of citations and h-index. Using these measures, female dermatologists are infrequently represented on top author lists.
- Using the Scopus database to search for the 50 most published dermatology authors from January 1, 2017, to October 7, 2022, 30% were female.
- Higher proportions of female dermatology trainees as well as efforts to increase mentorship and research support for female dermatologists may improve equality in top lists of dermatology citations and h-index values.
Racial Disparities in Hidradenitis Suppurativa–Related Pain: A Cross-sectional Analysis
Hidradenitis suppurativa (HS), a chronic inflammatory disease that is characterized by tender inflamed nodules of the skin and subcutaneous tissue, disproportionately affects postpubertal females as well as Black/African American individuals. The nodules can rupture, form sinus tracts, and scar. 1 Hidradenitis suppurativa has been associated with cardiovascular disease, type 2 diabetes mellitus, polycystic ovary syndrome, depression, suicide, and substance use disorders. Because of the symptom burden and associated conditions, HS can be a painful and distressing disease that substantially impairs the quality of life for individuals with this condition. 2
Pain is a commonly reported symptom in HS that often goes untreated. Furthermore, HS-related pain is complex due to the involvement of different pain types that require various treatment modalities.3 According to Savage et al,4 recognizing whether HS-related pain is acute, chronic, neuropathic, or nociceptive is vital in establishing a framework for an effective pain management scheme. Currently, such established multimodal pain management strategies in dermatology do not exist. In 2021, dermatology-specific pain management strategies proposed the use of a multimodal regimen to address the multifaceted nature of HS-related pain.4 However, these strategies failed to recognize the systemic racial and ethnic biases in the US health care system that undermine pain management care for minority groups.5,6 One approach to combatting racial disparities in pain management is determining average pain levels across racial groups.7 This study sought to compare HS-related pain scores by racial groups. Furthermore, we assessed differences in perception of patients’ respective pain management regimens by race. We hypothesized that the average HS-related pain intensities and pain management would differ between self-reported racial groups.
Methods
This cross-sectional study took place over 5 months (August through December 2021). A survey was emailed to 2198 adult patients with HS in the University of Alabama Health System. The survey consisted of demographic and general questions about a patient’s HS. Pain scores were captured using the numeric rating scale (NRS), a measurement tool for pain intensity on a scale from 0 to 10. 8 Age at diagnosis, gender, education level, household income, total body areas affected by HS, disease severity (categorized as mild, moderate, and severe), comorbidities including mood disorders, tobacco use, and HS and HS-related pain medication regimens also were collected. Additionally, participants were asked about their level of agreement with the following statements: “I am satisfied with how my pain related to HS is being managed by my doctors” and “My pain related to HS is under control.” The level of agreement was measured using a 5-point Likert scale, with responses ranging from strongly disagree to strongly agree. All data included in the analysis were self-reported. The study received institutional review board approval for the University of Alabama at Birmingham.
Statistical Analysis—Descriptive statistics were used to assess statistical differences in patient characteristics of Black/African American participants compared to other participants, including White, Asian, and Hispanic/Latino participants. Thirteen participants were excluded from the final analysis: 2 participants were missing data, and 11 biracial participants were excluded due to overlapping White and Black/African American races that may have confounded the analysis. Categorical variables were reported as frequencies and percentages, and χ2 and Fisher exact tests, when necessary, were used to test for statistically significant differences. Continuous variables were summarized with means and standard deviations, and a t test was used for statistically significant differences.
Logistic regression was performed to assess the relationship between race and pain after adjusting for confounding variables such as obesity, current tobacco use, self-reported HS severity, and the presence of comorbidities. A total of 204 patient records were included in the analysis, of which 70 (34.3%) had a pain score of 8 or higher, which indicates very severe pain intensity levels on the NRS,8 and were selected as a cut point based on the distribution of responses. For this cross-sectional cohort, our approach was to compare characteristics of those classified with a top score of 8 or higher (n=70) vs a top score of 0 to 7 (n=134)(cases vs noncases). Statistical analyses were performed using JMP Pro 16 (JMP Statistical Discovery LLC) at an α=.05 significance level; logistic regression was performed using SPSS Statistics (IBM). For the logistic regression, we grouped patient race into 2 categories: Black/African American and Other, which included White, Asian, and Hispanic/Latino participants.
Crude and adjusted multivariable logistic regression analyses were used to calculate prevalence odds ratios with 95% confidence intervals. Covariate inclusion in the multivariable logistic regression was based on a priori hypothesis/knowledge and was meant to estimate the independent effect of race after adjustment for income, HS severity, and history of prescription pain medication use. Other variables, including tobacco use, obesity, mood disorders, and current HS treatments, were all individually tested in the multivariate analysis and did not significantly impact the odds ratio for high pain. Statistical adjustment slightly decreased (19%) the magnitude between crude and adjusted prevalence odds ratios for the association between Black/African American race and high pain score.
Results
Survey Demographics —The final analysis included 204 survey respondents. Most respondents were Black/African American (58.82%), and nearly all were female (89.71%)(Table 1). The mean age (SD) of respondents was 37.37 (11.29) years (range, 19-70 years). Many respondents reported having completed some college (36.27%) or receiving a bachelor’s degree (19.12%). Of patients who were not Black/African American, 10.71% had higher than a master’s degree, whereas no Black/African American patients held a degree higher than a master’s ( P = .0052). Additionally, more Black/African American respondents (35.83%) reported an annual household income level of less than $25,000 compared with respondents who were not Black/African American (19.05%, P = .0001). Most respondents rated the severity of their HS as moderate or severe (46.57% and 41.67%, respectively), and there was no significant difference in reported severity of HS between racial groups ( P = .5395).


Pain Scores—As documented in the Methods, respondents were asked to rate their HS-related pain intensity from 0 to 10 using the NRS. The average pain score (SD)—the level of pain intensity over the prior month—was 6.39 (2.56)(range, 0–10). The mean pain score (SD) at the time of the survey was 3.61 (2.98)(range, 0–10)(Table 1). These data revealed that Black/African American patients had a significantly higher average pain score (SD) than patients with HS who were not Black/African American (7.08 [2.49] and 5.40 [2.35], respectively; P<.0001). After adjustment with multivariable logistical regression, Black/African American patients had 4-fold increased odds for very severe levels of pain (score of ≥8) compared with patients who were not Black/African American.
Pain Management—Although pain scores were higher for Black/African American patients with HS, there was no significant difference in the perception of pain control between racial groups (P=.0761). Additionally, we found low income (adjusted prevalence odds ratio [POR], 0.22; 95% CI, 0.05-0.91), a history of prescription pain medication use (adjusted POR, 2.25; 95% CI, 1.13-4.51), and HS severity (adjusted POR, 4.40; 95% CI, 1.11-17.36) all to be independent risk factors contributing to higher pain scores in patients with HS (Table 2). Lastly, we noted current or reported history of pain medication use was significantly correlated with higher pain scores (P=.0280 and P=.0213, respectively).
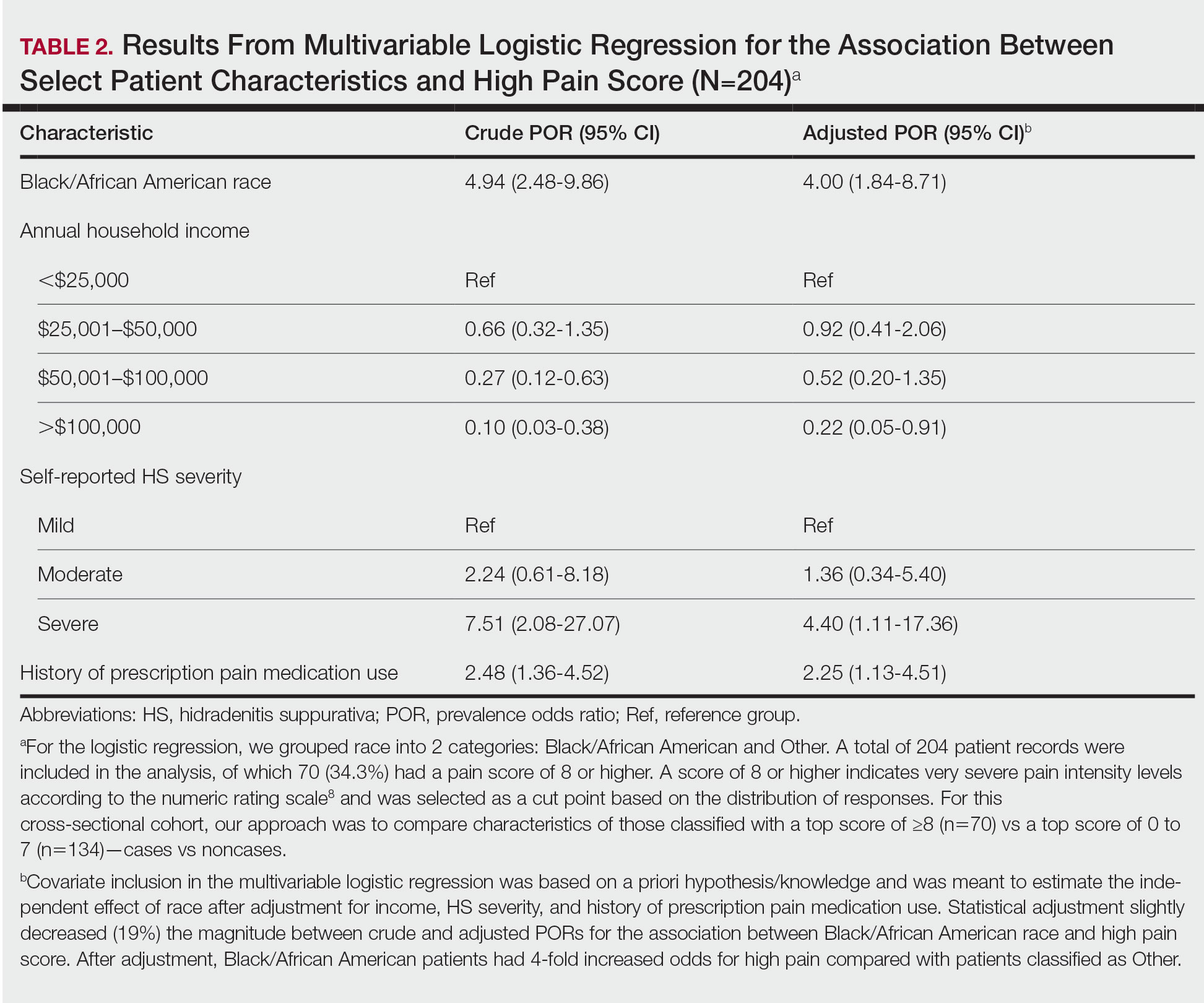
Satisfaction With Pain Management—The level of satisfaction with physician management of HS-related pain was significantly different between Black/African American patients and those who were not Black/African American (P=.0129). Of those who identified as Black/African American, 26.7% (n=32) strongly disagreed with the statement, “I am satisfied with how my pain related to HS is being managed by my doctors,” whereas only 15.5% (n=13) of patients who were not Black/African American strongly disagreed.
Comment
There is no cure for HS, and a large focus of treatment is pain management. Because racial disparities in the treatment of chronic pain will affect those with HS, we conducted a cross-sectional analysis of pain and pain management among HS patients. We found that Black/African American patients with HS have higher average pain scores than those who are not Black/African American and were 4 times more likely to experience very severe pain. Prior studies have established that patients with HS often report higher pain levels than patients with other chronic inflammatory skin conditions, 7,8 and our study identified racial disparities in HS-related pain management.
Measuring pain is challenging because of its multidimensional and subjective nature, making it essential to consider underlying causes and patients’ emotional responses to pain.9 By adjusting for confounding factors that may influence pain, such as mood disorders, disease severity, comorbidities, and medication use, we were able to gain better insight into fundamental differences in average pain intensity levels among racial groups and assess what factors may be contributing to a patient’s pain perception. Our study determined that lower income levels, higher HS disease severity, and a history of prescription pain medication use were all independent risk factors for high pain. Of note, obesity, tobacco use, and mood disorders such as anxiety and depression did not significantly differ between racial groups or increase the odds of high pain between racial groups identified.
With low income being an independent risk factor for high pain, we must consider the social determinants of health and how they may influence the pain experience in HS. We speculate that low income may be associated with other social determinants of health for the patients assessed in this study, such as lack of social and community support or limited health care access that contribute to worse health outcomes.10,11 In addition, low income contributes to limited access to medical care or treatments12; without access to effective HS management, lower-income patients may be at risk for higher disease severity and thus higher pain levels. However, economic stability is only a part of the whole picture; therefore, assessing the other social determinants of health in patients with HS may lead to better health outcomes and quality of life.
Another identified risk factor for high pain was a reported history of prescription pain medication use. This finding suggests that patients with moderate to severe pain likely have required stronger analgesic medications in the past. We further speculate that high pain levels in patients who have received prescription pain medications indicate either undertreatment, mistreatment, or recalcitrant pain. More research is needed to assess the relationship between HS-related pain intensity, analgesic medications, and providers who manage HS-related pain.
We also found that Black/African American patients with HS had a significantly higher dissatisfaction with their physician’s management of their pain, which could be attributable to several factors, including biological differences in medication metabolism (in which the patient has medication-resistant HS), undertreatment of pain, and/or poor doctor-patient relations. These reasons coincide with other diseases where health disparities are found.13-15 Recognizing these factors will be key to dismantling the disparities in HS that are noted within this study. The limitations of this work include the cross-sectional study design and its inability to evaluate causal factors of high pain levels across racial groups, the NRS lack of insight on pain chronicity or pain experience,7 the lack of provider or institution perspectives, and self-reported data. Additionally, only patients with email access were included, which may have excluded vulnerable populations with more pain associated with their HS.
Our findings highlight an area for further investigation to assess why these racial differences exist in HS-related pain. The results also emphasize the need for research evaluating whether systemic or health care provider biases contribute to racial differences in HS-related pain management.
Acknowledgment — Dr. Weir was supported by the Predoctoral Clinical/Translational Research Program (TL1), a National Institutes of Health Ruth L. Kirschstein National Research Service Award (NRSA), through the University of Alabama at Birmingham (UAB) Center for Clinical and Translational Science (CCTS).
- Garg A, Kirby JS, Lavian J, et al. Sex- and age-adjusted population analysis of prevalence estimates for hidradenitis suppurativa in the United States. JAMA Dermatol. 2017;153:760-764. doi:10.1001/jamadermatol.2017.0201
- Nguyen TV, Damiani G, Orenstein LAV, et al. Hidradenitis suppurativa: an update on epidemiology, phenotypes, diagnosis, pathogenesis, comorbidities and quality of life. J Eur Acad Dermatol Venereol. 2021;35:50-61. doi:10.1111/jdv.16677
- Krajewski PK, Matusiak Ł, von Stebut E, et al. Pain in hidradenitis suppurativa: a cross-sectional study of 1,795 patients. Acta Derm Venereol. 2021;101:adv00364. doi:10.2340/00015555-3724
- Savage KT, Singh V, Patel ZS, et al. Pain management in hidradenitis suppurativa and a proposed treatment algorithm. J Am Acad Dermatol. 2021;85:187-199. doi:10.1016/j.jaad.2020.09.039
- Morales ME, Yong RJ. Racial and ethnic disparities in the treatment of chronic pain. Pain Med. 2021;22:75-90. doi:10.1093/pm/pnaa427
- US Department of Health and Human Services. 2019 National Healthcare Quality and Disparities Report. December 2020. Accessed June 21, 2023. https://www.ahrq.gov/sites/default/files/wysiwyg/research/findings/nhqrdr/2019qdr.pdf
- Hoffman KM, Trawalter S, Axt JR, et al. Racial bias in pain assessment and treatment recommendations, and false beliefs about biological differences between blacks and whites. Proc Natl Acad Sci U S A. 2016;113:4296-4301. doi:10.1073/pnas.1516047113
- Patel ZS, Hoffman LK, Buse DC, et al. Pain, psychological comorbidities, disability, and impaired quality of life in hidradenitis suppurativa. Curr Pain Headache Rep. 2017;21:49. doi:10.1007/s11916-017-0647-3. Published correction appears in Curr Pain Headache Rep. 2017;21:52.
- McDowell I. Pain measurements. In: Measuring Health: A Guide to Rating Scales and Questionnaires. Oxford University Press; 2006:477-478.
- Singh GK, Daus GP, Allender M, et al. Social determinants of health in the United States: addressing major health inequality trends for the nation, 1935-2016. Int J MCH AIDS. 2017;6:139-164. doi:10.21106/ijma.236
- Sulley S, Bayssie M. Social determinants of health: an evaluation of risk factors associated with inpatient presentations in the United States. Cureus. 2021;13:E13287. doi:10.7759/cureus.13287
- Lazar M, Davenport L. Barriers to health care access for low income families: a review of literature. J Community Health Nurs. 2018;35:28-37. doi:10.1080/07370016.2018.1404832
- Ghoshal M, Shapiro H, Todd K, et al. Chronic noncancer pain management and systemic racism: time to move toward equal care standards.J Pain Res. 2020;13:2825-2836. doi:10.214/JPR.S287314
- Cintron A, Morrison RS. Pain and ethnicity in the United States: a systematic review. J Palliat Med. 2006;9:1454-1473. doi:10.1089/jpm.2006.9.1454
- Green CR, Anderson KO, Baker TA, et al. The unequal burden of pain: confronting racial and ethnic disparities in pain. Pain Med. 2003;4:277-294. doi:10.1046/j.1526-4637.2003.03034.x. Published correction appears in Pain Med. 2005;6:99.
Hidradenitis suppurativa (HS), a chronic inflammatory disease that is characterized by tender inflamed nodules of the skin and subcutaneous tissue, disproportionately affects postpubertal females as well as Black/African American individuals. The nodules can rupture, form sinus tracts, and scar. 1 Hidradenitis suppurativa has been associated with cardiovascular disease, type 2 diabetes mellitus, polycystic ovary syndrome, depression, suicide, and substance use disorders. Because of the symptom burden and associated conditions, HS can be a painful and distressing disease that substantially impairs the quality of life for individuals with this condition. 2
Pain is a commonly reported symptom in HS that often goes untreated. Furthermore, HS-related pain is complex due to the involvement of different pain types that require various treatment modalities.3 According to Savage et al,4 recognizing whether HS-related pain is acute, chronic, neuropathic, or nociceptive is vital in establishing a framework for an effective pain management scheme. Currently, such established multimodal pain management strategies in dermatology do not exist. In 2021, dermatology-specific pain management strategies proposed the use of a multimodal regimen to address the multifaceted nature of HS-related pain.4 However, these strategies failed to recognize the systemic racial and ethnic biases in the US health care system that undermine pain management care for minority groups.5,6 One approach to combatting racial disparities in pain management is determining average pain levels across racial groups.7 This study sought to compare HS-related pain scores by racial groups. Furthermore, we assessed differences in perception of patients’ respective pain management regimens by race. We hypothesized that the average HS-related pain intensities and pain management would differ between self-reported racial groups.
Methods
This cross-sectional study took place over 5 months (August through December 2021). A survey was emailed to 2198 adult patients with HS in the University of Alabama Health System. The survey consisted of demographic and general questions about a patient’s HS. Pain scores were captured using the numeric rating scale (NRS), a measurement tool for pain intensity on a scale from 0 to 10. 8 Age at diagnosis, gender, education level, household income, total body areas affected by HS, disease severity (categorized as mild, moderate, and severe), comorbidities including mood disorders, tobacco use, and HS and HS-related pain medication regimens also were collected. Additionally, participants were asked about their level of agreement with the following statements: “I am satisfied with how my pain related to HS is being managed by my doctors” and “My pain related to HS is under control.” The level of agreement was measured using a 5-point Likert scale, with responses ranging from strongly disagree to strongly agree. All data included in the analysis were self-reported. The study received institutional review board approval for the University of Alabama at Birmingham.
Statistical Analysis—Descriptive statistics were used to assess statistical differences in patient characteristics of Black/African American participants compared to other participants, including White, Asian, and Hispanic/Latino participants. Thirteen participants were excluded from the final analysis: 2 participants were missing data, and 11 biracial participants were excluded due to overlapping White and Black/African American races that may have confounded the analysis. Categorical variables were reported as frequencies and percentages, and χ2 and Fisher exact tests, when necessary, were used to test for statistically significant differences. Continuous variables were summarized with means and standard deviations, and a t test was used for statistically significant differences.
Logistic regression was performed to assess the relationship between race and pain after adjusting for confounding variables such as obesity, current tobacco use, self-reported HS severity, and the presence of comorbidities. A total of 204 patient records were included in the analysis, of which 70 (34.3%) had a pain score of 8 or higher, which indicates very severe pain intensity levels on the NRS,8 and were selected as a cut point based on the distribution of responses. For this cross-sectional cohort, our approach was to compare characteristics of those classified with a top score of 8 or higher (n=70) vs a top score of 0 to 7 (n=134)(cases vs noncases). Statistical analyses were performed using JMP Pro 16 (JMP Statistical Discovery LLC) at an α=.05 significance level; logistic regression was performed using SPSS Statistics (IBM). For the logistic regression, we grouped patient race into 2 categories: Black/African American and Other, which included White, Asian, and Hispanic/Latino participants.
Crude and adjusted multivariable logistic regression analyses were used to calculate prevalence odds ratios with 95% confidence intervals. Covariate inclusion in the multivariable logistic regression was based on a priori hypothesis/knowledge and was meant to estimate the independent effect of race after adjustment for income, HS severity, and history of prescription pain medication use. Other variables, including tobacco use, obesity, mood disorders, and current HS treatments, were all individually tested in the multivariate analysis and did not significantly impact the odds ratio for high pain. Statistical adjustment slightly decreased (19%) the magnitude between crude and adjusted prevalence odds ratios for the association between Black/African American race and high pain score.
Results
Survey Demographics —The final analysis included 204 survey respondents. Most respondents were Black/African American (58.82%), and nearly all were female (89.71%)(Table 1). The mean age (SD) of respondents was 37.37 (11.29) years (range, 19-70 years). Many respondents reported having completed some college (36.27%) or receiving a bachelor’s degree (19.12%). Of patients who were not Black/African American, 10.71% had higher than a master’s degree, whereas no Black/African American patients held a degree higher than a master’s ( P = .0052). Additionally, more Black/African American respondents (35.83%) reported an annual household income level of less than $25,000 compared with respondents who were not Black/African American (19.05%, P = .0001). Most respondents rated the severity of their HS as moderate or severe (46.57% and 41.67%, respectively), and there was no significant difference in reported severity of HS between racial groups ( P = .5395).


Pain Scores—As documented in the Methods, respondents were asked to rate their HS-related pain intensity from 0 to 10 using the NRS. The average pain score (SD)—the level of pain intensity over the prior month—was 6.39 (2.56)(range, 0–10). The mean pain score (SD) at the time of the survey was 3.61 (2.98)(range, 0–10)(Table 1). These data revealed that Black/African American patients had a significantly higher average pain score (SD) than patients with HS who were not Black/African American (7.08 [2.49] and 5.40 [2.35], respectively; P<.0001). After adjustment with multivariable logistical regression, Black/African American patients had 4-fold increased odds for very severe levels of pain (score of ≥8) compared with patients who were not Black/African American.
Pain Management—Although pain scores were higher for Black/African American patients with HS, there was no significant difference in the perception of pain control between racial groups (P=.0761). Additionally, we found low income (adjusted prevalence odds ratio [POR], 0.22; 95% CI, 0.05-0.91), a history of prescription pain medication use (adjusted POR, 2.25; 95% CI, 1.13-4.51), and HS severity (adjusted POR, 4.40; 95% CI, 1.11-17.36) all to be independent risk factors contributing to higher pain scores in patients with HS (Table 2). Lastly, we noted current or reported history of pain medication use was significantly correlated with higher pain scores (P=.0280 and P=.0213, respectively).

Satisfaction With Pain Management—The level of satisfaction with physician management of HS-related pain was significantly different between Black/African American patients and those who were not Black/African American (P=.0129). Of those who identified as Black/African American, 26.7% (n=32) strongly disagreed with the statement, “I am satisfied with how my pain related to HS is being managed by my doctors,” whereas only 15.5% (n=13) of patients who were not Black/African American strongly disagreed.
Comment
There is no cure for HS, and a large focus of treatment is pain management. Because racial disparities in the treatment of chronic pain will affect those with HS, we conducted a cross-sectional analysis of pain and pain management among HS patients. We found that Black/African American patients with HS have higher average pain scores than those who are not Black/African American and were 4 times more likely to experience very severe pain. Prior studies have established that patients with HS often report higher pain levels than patients with other chronic inflammatory skin conditions, 7,8 and our study identified racial disparities in HS-related pain management.
Measuring pain is challenging because of its multidimensional and subjective nature, making it essential to consider underlying causes and patients’ emotional responses to pain.9 By adjusting for confounding factors that may influence pain, such as mood disorders, disease severity, comorbidities, and medication use, we were able to gain better insight into fundamental differences in average pain intensity levels among racial groups and assess what factors may be contributing to a patient’s pain perception. Our study determined that lower income levels, higher HS disease severity, and a history of prescription pain medication use were all independent risk factors for high pain. Of note, obesity, tobacco use, and mood disorders such as anxiety and depression did not significantly differ between racial groups or increase the odds of high pain between racial groups identified.
With low income being an independent risk factor for high pain, we must consider the social determinants of health and how they may influence the pain experience in HS. We speculate that low income may be associated with other social determinants of health for the patients assessed in this study, such as lack of social and community support or limited health care access that contribute to worse health outcomes.10,11 In addition, low income contributes to limited access to medical care or treatments12; without access to effective HS management, lower-income patients may be at risk for higher disease severity and thus higher pain levels. However, economic stability is only a part of the whole picture; therefore, assessing the other social determinants of health in patients with HS may lead to better health outcomes and quality of life.
Another identified risk factor for high pain was a reported history of prescription pain medication use. This finding suggests that patients with moderate to severe pain likely have required stronger analgesic medications in the past. We further speculate that high pain levels in patients who have received prescription pain medications indicate either undertreatment, mistreatment, or recalcitrant pain. More research is needed to assess the relationship between HS-related pain intensity, analgesic medications, and providers who manage HS-related pain.
We also found that Black/African American patients with HS had a significantly higher dissatisfaction with their physician’s management of their pain, which could be attributable to several factors, including biological differences in medication metabolism (in which the patient has medication-resistant HS), undertreatment of pain, and/or poor doctor-patient relations. These reasons coincide with other diseases where health disparities are found.13-15 Recognizing these factors will be key to dismantling the disparities in HS that are noted within this study. The limitations of this work include the cross-sectional study design and its inability to evaluate causal factors of high pain levels across racial groups, the NRS lack of insight on pain chronicity or pain experience,7 the lack of provider or institution perspectives, and self-reported data. Additionally, only patients with email access were included, which may have excluded vulnerable populations with more pain associated with their HS.
Our findings highlight an area for further investigation to assess why these racial differences exist in HS-related pain. The results also emphasize the need for research evaluating whether systemic or health care provider biases contribute to racial differences in HS-related pain management.
Acknowledgment — Dr. Weir was supported by the Predoctoral Clinical/Translational Research Program (TL1), a National Institutes of Health Ruth L. Kirschstein National Research Service Award (NRSA), through the University of Alabama at Birmingham (UAB) Center for Clinical and Translational Science (CCTS).
Hidradenitis suppurativa (HS), a chronic inflammatory disease that is characterized by tender inflamed nodules of the skin and subcutaneous tissue, disproportionately affects postpubertal females as well as Black/African American individuals. The nodules can rupture, form sinus tracts, and scar. 1 Hidradenitis suppurativa has been associated with cardiovascular disease, type 2 diabetes mellitus, polycystic ovary syndrome, depression, suicide, and substance use disorders. Because of the symptom burden and associated conditions, HS can be a painful and distressing disease that substantially impairs the quality of life for individuals with this condition. 2
Pain is a commonly reported symptom in HS that often goes untreated. Furthermore, HS-related pain is complex due to the involvement of different pain types that require various treatment modalities.3 According to Savage et al,4 recognizing whether HS-related pain is acute, chronic, neuropathic, or nociceptive is vital in establishing a framework for an effective pain management scheme. Currently, such established multimodal pain management strategies in dermatology do not exist. In 2021, dermatology-specific pain management strategies proposed the use of a multimodal regimen to address the multifaceted nature of HS-related pain.4 However, these strategies failed to recognize the systemic racial and ethnic biases in the US health care system that undermine pain management care for minority groups.5,6 One approach to combatting racial disparities in pain management is determining average pain levels across racial groups.7 This study sought to compare HS-related pain scores by racial groups. Furthermore, we assessed differences in perception of patients’ respective pain management regimens by race. We hypothesized that the average HS-related pain intensities and pain management would differ between self-reported racial groups.
Methods
This cross-sectional study took place over 5 months (August through December 2021). A survey was emailed to 2198 adult patients with HS in the University of Alabama Health System. The survey consisted of demographic and general questions about a patient’s HS. Pain scores were captured using the numeric rating scale (NRS), a measurement tool for pain intensity on a scale from 0 to 10. 8 Age at diagnosis, gender, education level, household income, total body areas affected by HS, disease severity (categorized as mild, moderate, and severe), comorbidities including mood disorders, tobacco use, and HS and HS-related pain medication regimens also were collected. Additionally, participants were asked about their level of agreement with the following statements: “I am satisfied with how my pain related to HS is being managed by my doctors” and “My pain related to HS is under control.” The level of agreement was measured using a 5-point Likert scale, with responses ranging from strongly disagree to strongly agree. All data included in the analysis were self-reported. The study received institutional review board approval for the University of Alabama at Birmingham.
Statistical Analysis—Descriptive statistics were used to assess statistical differences in patient characteristics of Black/African American participants compared to other participants, including White, Asian, and Hispanic/Latino participants. Thirteen participants were excluded from the final analysis: 2 participants were missing data, and 11 biracial participants were excluded due to overlapping White and Black/African American races that may have confounded the analysis. Categorical variables were reported as frequencies and percentages, and χ2 and Fisher exact tests, when necessary, were used to test for statistically significant differences. Continuous variables were summarized with means and standard deviations, and a t test was used for statistically significant differences.
Logistic regression was performed to assess the relationship between race and pain after adjusting for confounding variables such as obesity, current tobacco use, self-reported HS severity, and the presence of comorbidities. A total of 204 patient records were included in the analysis, of which 70 (34.3%) had a pain score of 8 or higher, which indicates very severe pain intensity levels on the NRS,8 and were selected as a cut point based on the distribution of responses. For this cross-sectional cohort, our approach was to compare characteristics of those classified with a top score of 8 or higher (n=70) vs a top score of 0 to 7 (n=134)(cases vs noncases). Statistical analyses were performed using JMP Pro 16 (JMP Statistical Discovery LLC) at an α=.05 significance level; logistic regression was performed using SPSS Statistics (IBM). For the logistic regression, we grouped patient race into 2 categories: Black/African American and Other, which included White, Asian, and Hispanic/Latino participants.
Crude and adjusted multivariable logistic regression analyses were used to calculate prevalence odds ratios with 95% confidence intervals. Covariate inclusion in the multivariable logistic regression was based on a priori hypothesis/knowledge and was meant to estimate the independent effect of race after adjustment for income, HS severity, and history of prescription pain medication use. Other variables, including tobacco use, obesity, mood disorders, and current HS treatments, were all individually tested in the multivariate analysis and did not significantly impact the odds ratio for high pain. Statistical adjustment slightly decreased (19%) the magnitude between crude and adjusted prevalence odds ratios for the association between Black/African American race and high pain score.
Results
Survey Demographics —The final analysis included 204 survey respondents. Most respondents were Black/African American (58.82%), and nearly all were female (89.71%)(Table 1). The mean age (SD) of respondents was 37.37 (11.29) years (range, 19-70 years). Many respondents reported having completed some college (36.27%) or receiving a bachelor’s degree (19.12%). Of patients who were not Black/African American, 10.71% had higher than a master’s degree, whereas no Black/African American patients held a degree higher than a master’s ( P = .0052). Additionally, more Black/African American respondents (35.83%) reported an annual household income level of less than $25,000 compared with respondents who were not Black/African American (19.05%, P = .0001). Most respondents rated the severity of their HS as moderate or severe (46.57% and 41.67%, respectively), and there was no significant difference in reported severity of HS between racial groups ( P = .5395).


Pain Scores—As documented in the Methods, respondents were asked to rate their HS-related pain intensity from 0 to 10 using the NRS. The average pain score (SD)—the level of pain intensity over the prior month—was 6.39 (2.56)(range, 0–10). The mean pain score (SD) at the time of the survey was 3.61 (2.98)(range, 0–10)(Table 1). These data revealed that Black/African American patients had a significantly higher average pain score (SD) than patients with HS who were not Black/African American (7.08 [2.49] and 5.40 [2.35], respectively; P<.0001). After adjustment with multivariable logistical regression, Black/African American patients had 4-fold increased odds for very severe levels of pain (score of ≥8) compared with patients who were not Black/African American.
Pain Management—Although pain scores were higher for Black/African American patients with HS, there was no significant difference in the perception of pain control between racial groups (P=.0761). Additionally, we found low income (adjusted prevalence odds ratio [POR], 0.22; 95% CI, 0.05-0.91), a history of prescription pain medication use (adjusted POR, 2.25; 95% CI, 1.13-4.51), and HS severity (adjusted POR, 4.40; 95% CI, 1.11-17.36) all to be independent risk factors contributing to higher pain scores in patients with HS (Table 2). Lastly, we noted current or reported history of pain medication use was significantly correlated with higher pain scores (P=.0280 and P=.0213, respectively).

Satisfaction With Pain Management—The level of satisfaction with physician management of HS-related pain was significantly different between Black/African American patients and those who were not Black/African American (P=.0129). Of those who identified as Black/African American, 26.7% (n=32) strongly disagreed with the statement, “I am satisfied with how my pain related to HS is being managed by my doctors,” whereas only 15.5% (n=13) of patients who were not Black/African American strongly disagreed.
Comment
There is no cure for HS, and a large focus of treatment is pain management. Because racial disparities in the treatment of chronic pain will affect those with HS, we conducted a cross-sectional analysis of pain and pain management among HS patients. We found that Black/African American patients with HS have higher average pain scores than those who are not Black/African American and were 4 times more likely to experience very severe pain. Prior studies have established that patients with HS often report higher pain levels than patients with other chronic inflammatory skin conditions, 7,8 and our study identified racial disparities in HS-related pain management.
Measuring pain is challenging because of its multidimensional and subjective nature, making it essential to consider underlying causes and patients’ emotional responses to pain.9 By adjusting for confounding factors that may influence pain, such as mood disorders, disease severity, comorbidities, and medication use, we were able to gain better insight into fundamental differences in average pain intensity levels among racial groups and assess what factors may be contributing to a patient’s pain perception. Our study determined that lower income levels, higher HS disease severity, and a history of prescription pain medication use were all independent risk factors for high pain. Of note, obesity, tobacco use, and mood disorders such as anxiety and depression did not significantly differ between racial groups or increase the odds of high pain between racial groups identified.
With low income being an independent risk factor for high pain, we must consider the social determinants of health and how they may influence the pain experience in HS. We speculate that low income may be associated with other social determinants of health for the patients assessed in this study, such as lack of social and community support or limited health care access that contribute to worse health outcomes.10,11 In addition, low income contributes to limited access to medical care or treatments12; without access to effective HS management, lower-income patients may be at risk for higher disease severity and thus higher pain levels. However, economic stability is only a part of the whole picture; therefore, assessing the other social determinants of health in patients with HS may lead to better health outcomes and quality of life.
Another identified risk factor for high pain was a reported history of prescription pain medication use. This finding suggests that patients with moderate to severe pain likely have required stronger analgesic medications in the past. We further speculate that high pain levels in patients who have received prescription pain medications indicate either undertreatment, mistreatment, or recalcitrant pain. More research is needed to assess the relationship between HS-related pain intensity, analgesic medications, and providers who manage HS-related pain.
We also found that Black/African American patients with HS had a significantly higher dissatisfaction with their physician’s management of their pain, which could be attributable to several factors, including biological differences in medication metabolism (in which the patient has medication-resistant HS), undertreatment of pain, and/or poor doctor-patient relations. These reasons coincide with other diseases where health disparities are found.13-15 Recognizing these factors will be key to dismantling the disparities in HS that are noted within this study. The limitations of this work include the cross-sectional study design and its inability to evaluate causal factors of high pain levels across racial groups, the NRS lack of insight on pain chronicity or pain experience,7 the lack of provider or institution perspectives, and self-reported data. Additionally, only patients with email access were included, which may have excluded vulnerable populations with more pain associated with their HS.
Our findings highlight an area for further investigation to assess why these racial differences exist in HS-related pain. The results also emphasize the need for research evaluating whether systemic or health care provider biases contribute to racial differences in HS-related pain management.
Acknowledgment — Dr. Weir was supported by the Predoctoral Clinical/Translational Research Program (TL1), a National Institutes of Health Ruth L. Kirschstein National Research Service Award (NRSA), through the University of Alabama at Birmingham (UAB) Center for Clinical and Translational Science (CCTS).
- Garg A, Kirby JS, Lavian J, et al. Sex- and age-adjusted population analysis of prevalence estimates for hidradenitis suppurativa in the United States. JAMA Dermatol. 2017;153:760-764. doi:10.1001/jamadermatol.2017.0201
- Nguyen TV, Damiani G, Orenstein LAV, et al. Hidradenitis suppurativa: an update on epidemiology, phenotypes, diagnosis, pathogenesis, comorbidities and quality of life. J Eur Acad Dermatol Venereol. 2021;35:50-61. doi:10.1111/jdv.16677
- Krajewski PK, Matusiak Ł, von Stebut E, et al. Pain in hidradenitis suppurativa: a cross-sectional study of 1,795 patients. Acta Derm Venereol. 2021;101:adv00364. doi:10.2340/00015555-3724
- Savage KT, Singh V, Patel ZS, et al. Pain management in hidradenitis suppurativa and a proposed treatment algorithm. J Am Acad Dermatol. 2021;85:187-199. doi:10.1016/j.jaad.2020.09.039
- Morales ME, Yong RJ. Racial and ethnic disparities in the treatment of chronic pain. Pain Med. 2021;22:75-90. doi:10.1093/pm/pnaa427
- US Department of Health and Human Services. 2019 National Healthcare Quality and Disparities Report. December 2020. Accessed June 21, 2023. https://www.ahrq.gov/sites/default/files/wysiwyg/research/findings/nhqrdr/2019qdr.pdf
- Hoffman KM, Trawalter S, Axt JR, et al. Racial bias in pain assessment and treatment recommendations, and false beliefs about biological differences between blacks and whites. Proc Natl Acad Sci U S A. 2016;113:4296-4301. doi:10.1073/pnas.1516047113
- Patel ZS, Hoffman LK, Buse DC, et al. Pain, psychological comorbidities, disability, and impaired quality of life in hidradenitis suppurativa. Curr Pain Headache Rep. 2017;21:49. doi:10.1007/s11916-017-0647-3. Published correction appears in Curr Pain Headache Rep. 2017;21:52.
- McDowell I. Pain measurements. In: Measuring Health: A Guide to Rating Scales and Questionnaires. Oxford University Press; 2006:477-478.
- Singh GK, Daus GP, Allender M, et al. Social determinants of health in the United States: addressing major health inequality trends for the nation, 1935-2016. Int J MCH AIDS. 2017;6:139-164. doi:10.21106/ijma.236
- Sulley S, Bayssie M. Social determinants of health: an evaluation of risk factors associated with inpatient presentations in the United States. Cureus. 2021;13:E13287. doi:10.7759/cureus.13287
- Lazar M, Davenport L. Barriers to health care access for low income families: a review of literature. J Community Health Nurs. 2018;35:28-37. doi:10.1080/07370016.2018.1404832
- Ghoshal M, Shapiro H, Todd K, et al. Chronic noncancer pain management and systemic racism: time to move toward equal care standards.J Pain Res. 2020;13:2825-2836. doi:10.214/JPR.S287314
- Cintron A, Morrison RS. Pain and ethnicity in the United States: a systematic review. J Palliat Med. 2006;9:1454-1473. doi:10.1089/jpm.2006.9.1454
- Green CR, Anderson KO, Baker TA, et al. The unequal burden of pain: confronting racial and ethnic disparities in pain. Pain Med. 2003;4:277-294. doi:10.1046/j.1526-4637.2003.03034.x. Published correction appears in Pain Med. 2005;6:99.
- Garg A, Kirby JS, Lavian J, et al. Sex- and age-adjusted population analysis of prevalence estimates for hidradenitis suppurativa in the United States. JAMA Dermatol. 2017;153:760-764. doi:10.1001/jamadermatol.2017.0201
- Nguyen TV, Damiani G, Orenstein LAV, et al. Hidradenitis suppurativa: an update on epidemiology, phenotypes, diagnosis, pathogenesis, comorbidities and quality of life. J Eur Acad Dermatol Venereol. 2021;35:50-61. doi:10.1111/jdv.16677
- Krajewski PK, Matusiak Ł, von Stebut E, et al. Pain in hidradenitis suppurativa: a cross-sectional study of 1,795 patients. Acta Derm Venereol. 2021;101:adv00364. doi:10.2340/00015555-3724
- Savage KT, Singh V, Patel ZS, et al. Pain management in hidradenitis suppurativa and a proposed treatment algorithm. J Am Acad Dermatol. 2021;85:187-199. doi:10.1016/j.jaad.2020.09.039
- Morales ME, Yong RJ. Racial and ethnic disparities in the treatment of chronic pain. Pain Med. 2021;22:75-90. doi:10.1093/pm/pnaa427
- US Department of Health and Human Services. 2019 National Healthcare Quality and Disparities Report. December 2020. Accessed June 21, 2023. https://www.ahrq.gov/sites/default/files/wysiwyg/research/findings/nhqrdr/2019qdr.pdf
- Hoffman KM, Trawalter S, Axt JR, et al. Racial bias in pain assessment and treatment recommendations, and false beliefs about biological differences between blacks and whites. Proc Natl Acad Sci U S A. 2016;113:4296-4301. doi:10.1073/pnas.1516047113
- Patel ZS, Hoffman LK, Buse DC, et al. Pain, psychological comorbidities, disability, and impaired quality of life in hidradenitis suppurativa. Curr Pain Headache Rep. 2017;21:49. doi:10.1007/s11916-017-0647-3. Published correction appears in Curr Pain Headache Rep. 2017;21:52.
- McDowell I. Pain measurements. In: Measuring Health: A Guide to Rating Scales and Questionnaires. Oxford University Press; 2006:477-478.
- Singh GK, Daus GP, Allender M, et al. Social determinants of health in the United States: addressing major health inequality trends for the nation, 1935-2016. Int J MCH AIDS. 2017;6:139-164. doi:10.21106/ijma.236
- Sulley S, Bayssie M. Social determinants of health: an evaluation of risk factors associated with inpatient presentations in the United States. Cureus. 2021;13:E13287. doi:10.7759/cureus.13287
- Lazar M, Davenport L. Barriers to health care access for low income families: a review of literature. J Community Health Nurs. 2018;35:28-37. doi:10.1080/07370016.2018.1404832
- Ghoshal M, Shapiro H, Todd K, et al. Chronic noncancer pain management and systemic racism: time to move toward equal care standards.J Pain Res. 2020;13:2825-2836. doi:10.214/JPR.S287314
- Cintron A, Morrison RS. Pain and ethnicity in the United States: a systematic review. J Palliat Med. 2006;9:1454-1473. doi:10.1089/jpm.2006.9.1454
- Green CR, Anderson KO, Baker TA, et al. The unequal burden of pain: confronting racial and ethnic disparities in pain. Pain Med. 2003;4:277-294. doi:10.1046/j.1526-4637.2003.03034.x. Published correction appears in Pain Med. 2005;6:99.
Practice Points
- Racial disparities exist in the management of hidradenitis suppurativa (HS)–related pain.
- Black/African American patients with HS are 4 times more likely to experience very severe pain than patients of other races or ethnicities.
- Lower income levels, higher HS disease severity, and a history of prescription pain medication use are all independent risk factors for very severe pain in patients with HS.
Association Between Psoriasis and Obesity Among US Adults in the 2009-2014 National Health and Nutrition Examination Survey
To the Editor:
Psoriasis is an immune-mediated dermatologic condition that is associated with various comorbidities, including obesity.1 The underlying pathophysiology of psoriasis has been extensively studied, and recent research has discussed the role of obesity in IL-17 secretion.2 The relationship between being overweight/obese and having psoriasis has been documented in the literature.1,2 However, this association in a recent population is lacking. We sought to investigate the association between psoriasis and obesity utilizing a representative US population of adults—the 2009-2014 National Health and Nutrition Examination Survey (NHANES) data,3 which contains the most recent psoriasis data.
We conducted a population-based, cross-sectional study focused on patients 20 years and older with psoriasis from the 2009-2014 NHANES database. Three 2-year cycles of NHANES data were combined to create our 2009 to 2014 dataset. In the Table, numerous variables including age, sex, household income, race/ethnicity, education, diabetes status, tobacco use, body mass index (BMI), waist circumference, and being called overweight by a health care provider were analyzed using χ2 or t test analyses to evaluate for differences among those with and without psoriasis. Diabetes status was assessed by the question “Other than during pregnancy, have you ever been told by a doctor or health professional that you have diabetes or sugar diabetes?” Tobacco use was assessed by the question “Have you smoked at least 100 cigarettes in your entire life?” Psoriasis status was assessed by a self-reported response to the question “Have you ever been told by a doctor or other health care professional that you had psoriasis?” Three different outcome variables were used to determine if patients were overweight or obese: BMI, waist circumference, and response to the question “Has a doctor or other health professional ever told you that you were overweight?” Obesity was defined as having a BMI of 30 or higher or waist circumference of 102 cm or more in males and 88 cm or more in females.4 Being overweight was defined as having a BMI of 25 to 29.99 or response of Yes to “Has a doctor or other health professional ever told you that you were overweight?”
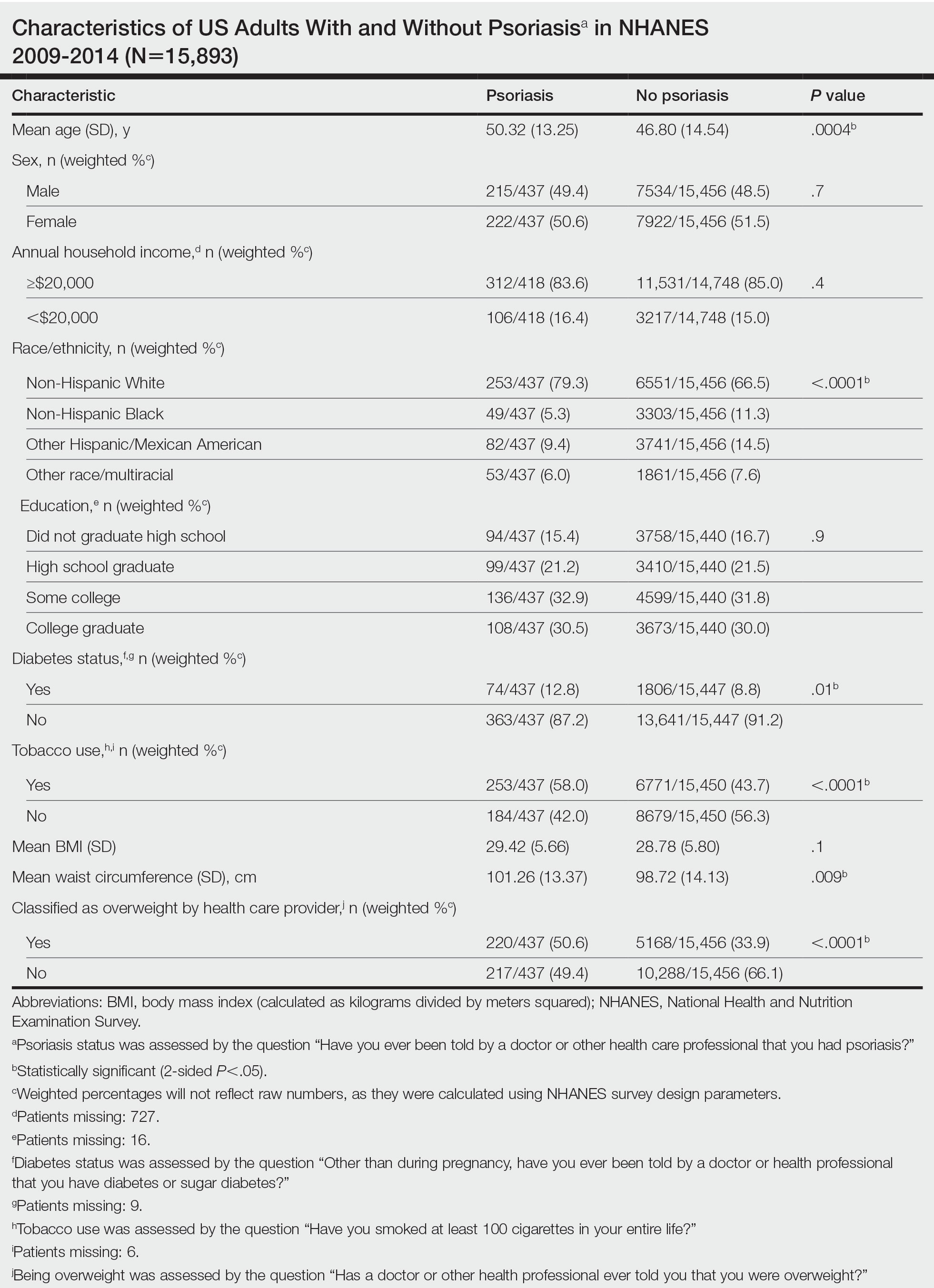
Initially, there were 17,547 participants 20 years and older from 2009 to 2014, but 1654 participants were excluded because of missing data for obesity or psoriasis; therefore, 15,893 patients were included in our analysis. Multivariable logistic regressions were utilized to examine the association between psoriasis and being overweight/obese (eTable). Additionally, the models were adjusted based on age, sex, household income, race/ethnicity, diabetes status, and tobacco use. All data processing and analysis were performed in Stata/MP 17 (StataCorp LLC). P<.05 was considered statistically significant.
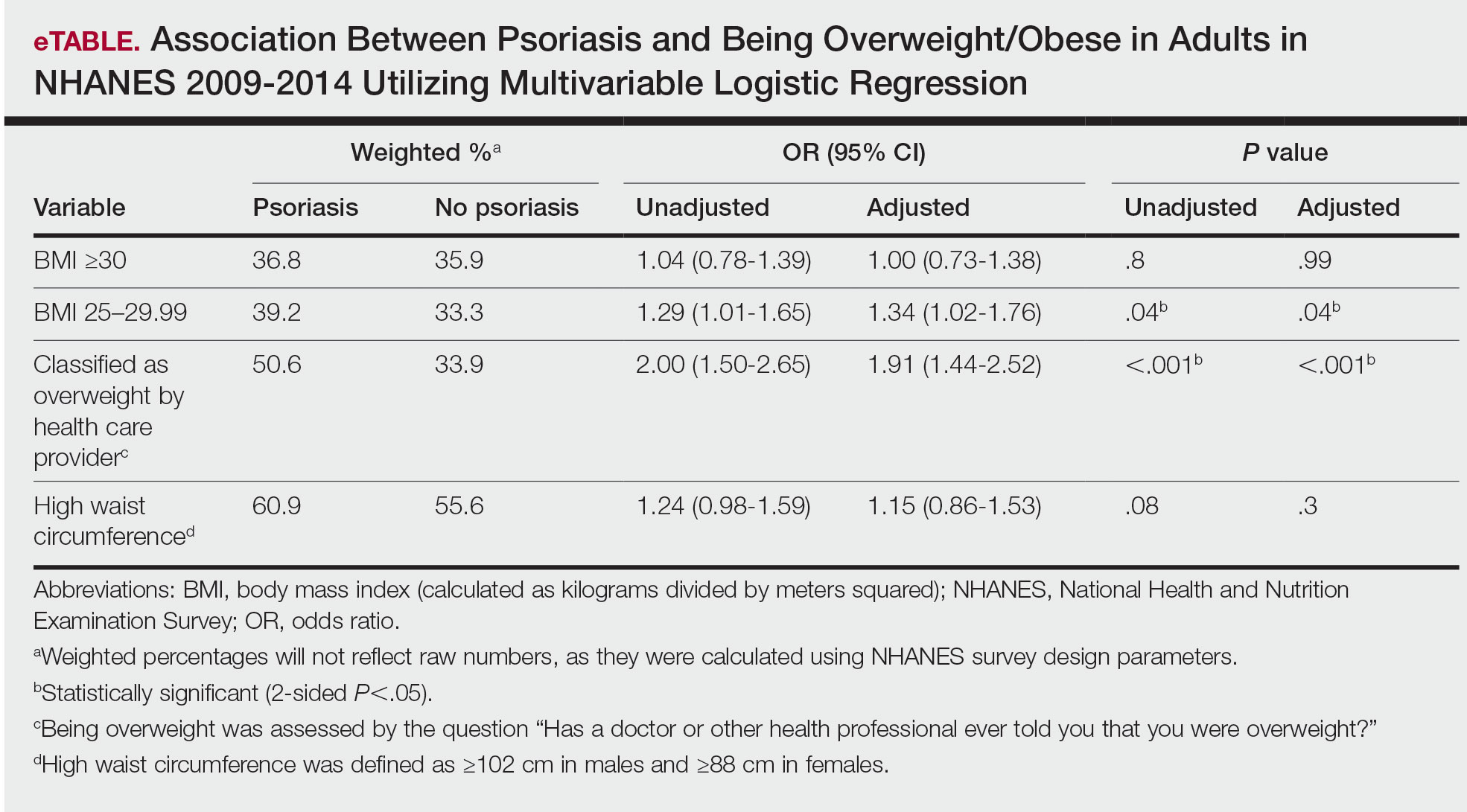
The Table shows characteristics of US adults with and without psoriasis in NHANES 2009-2014. We found that the variables of interest evaluating body weight that were significantly different on analysis between patients with and without psoriasis included waist circumference—patients with psoriasis had a significantly higher waist circumference (P=.009)—and being told by a health care provider that they are overweight (P<.0001), which supports the findings by Love et al,5 who reported abdominal obesity was the most common feature of metabolic syndrome exhibited among patients with psoriasis.
Multivariable logistic regression analysis (eTable) revealed that there was a significant association between psoriasis and BMI of 25 to 29.99 (adjusted odds ratio [AOR], 1.34; 95% CI, 1.02-1.76; P=.04) and being told by a health care provider that they are overweight (AOR, 1.91; 95% CI, 1.44-2.52; P<.001). After adjusting for confounding variables, there was no significant association between psoriasis and a BMI of 30 or higher (AOR, 1.00; 95% CI, 0.73-1.38; P=.99) or a waist circumference of 102 cm or more in males and 88 cm or more in females (AOR, 1.15; 95% CI, 0.86-1.53; P=.3).
Our findings suggest that a few variables indicative of being overweight or obese are associated with psoriasis. This relationship most likely is due to increased adipokine, including resistin, levels in overweight individuals, resulting in a proinflammatory state.6 It has been suggested that BMI alone is not a definitive marker for measuring fat storage levels in individuals. People can have a normal or slightly elevated BMI but possess excessive adiposity, resulting in chronic inflammation.6 Therefore, our findings of a significant association between psoriasis and being told by a health care provider that they are overweight might be a stronger measurement for possessing excessive fat, as this is likely due to clinical judgment rather than BMI measurement.
Moreover, it should be noted that the potential reason for the lack of association between BMI of 30 or higher and psoriasis in our analysis may be a result of BMI serving as a poor measurement for adiposity. Additionally, Armstrong and colleagues7 discussed that the association between BMI and psoriasis was stronger for patients with moderate to severe psoriasis. Our study consisted of NHANES data for self-reported psoriasis diagnoses without a psoriasis severity index, making it difficult to extrapolate which individuals had mild or moderate to severe psoriasis, which may have contributed to our finding of no association between BMI of 30 or higher and psoriasis.
The self-reported nature of the survey questions and lack of questions regarding psoriasis severity serve as limitations to the study. Both obesity and psoriasis can have various systemic consequences, such as cardiovascular disease, due to the development of an inflammatory state.8 Future studies may explore other body measurements that indicate being overweight or obese and the potential synergistic relationship of obesity and psoriasis severity, optimizing the development of effective treatment plans.
- Jensen P, Skov L. Psoriasis and obesity. Dermatology. 2016;232:633-639.
- Xu C, Ji J, Su T, et al. The association of psoriasis and obesity: focusing on IL-17A-related immunological mechanisms. Int J Dermatol Venereol. 2021;4:116-121.
- National Center for Health Statistics. NHANES questionnaires, datasets, and related documentation. Centers for Disease Control and Prevention website. Accessed June 22, 2023. https://wwwn.cdc.govnchs/nhanes/Default.aspx
- Ross R, Neeland IJ, Yamashita S, et al. Waist circumference as a vital sign in clinical practice: a Consensus Statement from the IAS and ICCR Working Group on Visceral Obesity. Nat Rev Endocrinol. 2020;16:177-189.
- Love TJ, Qureshi AA, Karlson EW, et al. Prevalence of the metabolic syndrome in psoriasis: results from the National Health and Nutrition Examination Survey, 2003-2006. Arch Dermatol. 2011;147:419-424.
- Paroutoglou K, Papadavid E, Christodoulatos GS, et al. Deciphering the association between psoriasis and obesity: current evidence and treatment considerations. Curr Obes Rep. 2020;9:165-178.
- Armstrong AW, Harskamp CT, Armstrong EJ. The association between psoriasis and obesity: a systematic review and meta-analysis of observational studies. Nutr Diabetes. 2012;2:E54.
- Hamminga EA, van der Lely AJ, Neumann HAM, et al. Chronic inflammation in psoriasis and obesity: implications for therapy. Med Hypotheses. 2006;67:768-773.
To the Editor:
Psoriasis is an immune-mediated dermatologic condition that is associated with various comorbidities, including obesity.1 The underlying pathophysiology of psoriasis has been extensively studied, and recent research has discussed the role of obesity in IL-17 secretion.2 The relationship between being overweight/obese and having psoriasis has been documented in the literature.1,2 However, this association in a recent population is lacking. We sought to investigate the association between psoriasis and obesity utilizing a representative US population of adults—the 2009-2014 National Health and Nutrition Examination Survey (NHANES) data,3 which contains the most recent psoriasis data.
We conducted a population-based, cross-sectional study focused on patients 20 years and older with psoriasis from the 2009-2014 NHANES database. Three 2-year cycles of NHANES data were combined to create our 2009 to 2014 dataset. In the Table, numerous variables including age, sex, household income, race/ethnicity, education, diabetes status, tobacco use, body mass index (BMI), waist circumference, and being called overweight by a health care provider were analyzed using χ2 or t test analyses to evaluate for differences among those with and without psoriasis. Diabetes status was assessed by the question “Other than during pregnancy, have you ever been told by a doctor or health professional that you have diabetes or sugar diabetes?” Tobacco use was assessed by the question “Have you smoked at least 100 cigarettes in your entire life?” Psoriasis status was assessed by a self-reported response to the question “Have you ever been told by a doctor or other health care professional that you had psoriasis?” Three different outcome variables were used to determine if patients were overweight or obese: BMI, waist circumference, and response to the question “Has a doctor or other health professional ever told you that you were overweight?” Obesity was defined as having a BMI of 30 or higher or waist circumference of 102 cm or more in males and 88 cm or more in females.4 Being overweight was defined as having a BMI of 25 to 29.99 or response of Yes to “Has a doctor or other health professional ever told you that you were overweight?”

Initially, there were 17,547 participants 20 years and older from 2009 to 2014, but 1654 participants were excluded because of missing data for obesity or psoriasis; therefore, 15,893 patients were included in our analysis. Multivariable logistic regressions were utilized to examine the association between psoriasis and being overweight/obese (eTable). Additionally, the models were adjusted based on age, sex, household income, race/ethnicity, diabetes status, and tobacco use. All data processing and analysis were performed in Stata/MP 17 (StataCorp LLC). P<.05 was considered statistically significant.

The Table shows characteristics of US adults with and without psoriasis in NHANES 2009-2014. We found that the variables of interest evaluating body weight that were significantly different on analysis between patients with and without psoriasis included waist circumference—patients with psoriasis had a significantly higher waist circumference (P=.009)—and being told by a health care provider that they are overweight (P<.0001), which supports the findings by Love et al,5 who reported abdominal obesity was the most common feature of metabolic syndrome exhibited among patients with psoriasis.
Multivariable logistic regression analysis (eTable) revealed that there was a significant association between psoriasis and BMI of 25 to 29.99 (adjusted odds ratio [AOR], 1.34; 95% CI, 1.02-1.76; P=.04) and being told by a health care provider that they are overweight (AOR, 1.91; 95% CI, 1.44-2.52; P<.001). After adjusting for confounding variables, there was no significant association between psoriasis and a BMI of 30 or higher (AOR, 1.00; 95% CI, 0.73-1.38; P=.99) or a waist circumference of 102 cm or more in males and 88 cm or more in females (AOR, 1.15; 95% CI, 0.86-1.53; P=.3).
Our findings suggest that a few variables indicative of being overweight or obese are associated with psoriasis. This relationship most likely is due to increased adipokine, including resistin, levels in overweight individuals, resulting in a proinflammatory state.6 It has been suggested that BMI alone is not a definitive marker for measuring fat storage levels in individuals. People can have a normal or slightly elevated BMI but possess excessive adiposity, resulting in chronic inflammation.6 Therefore, our findings of a significant association between psoriasis and being told by a health care provider that they are overweight might be a stronger measurement for possessing excessive fat, as this is likely due to clinical judgment rather than BMI measurement.
Moreover, it should be noted that the potential reason for the lack of association between BMI of 30 or higher and psoriasis in our analysis may be a result of BMI serving as a poor measurement for adiposity. Additionally, Armstrong and colleagues7 discussed that the association between BMI and psoriasis was stronger for patients with moderate to severe psoriasis. Our study consisted of NHANES data for self-reported psoriasis diagnoses without a psoriasis severity index, making it difficult to extrapolate which individuals had mild or moderate to severe psoriasis, which may have contributed to our finding of no association between BMI of 30 or higher and psoriasis.
The self-reported nature of the survey questions and lack of questions regarding psoriasis severity serve as limitations to the study. Both obesity and psoriasis can have various systemic consequences, such as cardiovascular disease, due to the development of an inflammatory state.8 Future studies may explore other body measurements that indicate being overweight or obese and the potential synergistic relationship of obesity and psoriasis severity, optimizing the development of effective treatment plans.
To the Editor:
Psoriasis is an immune-mediated dermatologic condition that is associated with various comorbidities, including obesity.1 The underlying pathophysiology of psoriasis has been extensively studied, and recent research has discussed the role of obesity in IL-17 secretion.2 The relationship between being overweight/obese and having psoriasis has been documented in the literature.1,2 However, this association in a recent population is lacking. We sought to investigate the association between psoriasis and obesity utilizing a representative US population of adults—the 2009-2014 National Health and Nutrition Examination Survey (NHANES) data,3 which contains the most recent psoriasis data.
We conducted a population-based, cross-sectional study focused on patients 20 years and older with psoriasis from the 2009-2014 NHANES database. Three 2-year cycles of NHANES data were combined to create our 2009 to 2014 dataset. In the Table, numerous variables including age, sex, household income, race/ethnicity, education, diabetes status, tobacco use, body mass index (BMI), waist circumference, and being called overweight by a health care provider were analyzed using χ2 or t test analyses to evaluate for differences among those with and without psoriasis. Diabetes status was assessed by the question “Other than during pregnancy, have you ever been told by a doctor or health professional that you have diabetes or sugar diabetes?” Tobacco use was assessed by the question “Have you smoked at least 100 cigarettes in your entire life?” Psoriasis status was assessed by a self-reported response to the question “Have you ever been told by a doctor or other health care professional that you had psoriasis?” Three different outcome variables were used to determine if patients were overweight or obese: BMI, waist circumference, and response to the question “Has a doctor or other health professional ever told you that you were overweight?” Obesity was defined as having a BMI of 30 or higher or waist circumference of 102 cm or more in males and 88 cm or more in females.4 Being overweight was defined as having a BMI of 25 to 29.99 or response of Yes to “Has a doctor or other health professional ever told you that you were overweight?”

Initially, there were 17,547 participants 20 years and older from 2009 to 2014, but 1654 participants were excluded because of missing data for obesity or psoriasis; therefore, 15,893 patients were included in our analysis. Multivariable logistic regressions were utilized to examine the association between psoriasis and being overweight/obese (eTable). Additionally, the models were adjusted based on age, sex, household income, race/ethnicity, diabetes status, and tobacco use. All data processing and analysis were performed in Stata/MP 17 (StataCorp LLC). P<.05 was considered statistically significant.

The Table shows characteristics of US adults with and without psoriasis in NHANES 2009-2014. We found that the variables of interest evaluating body weight that were significantly different on analysis between patients with and without psoriasis included waist circumference—patients with psoriasis had a significantly higher waist circumference (P=.009)—and being told by a health care provider that they are overweight (P<.0001), which supports the findings by Love et al,5 who reported abdominal obesity was the most common feature of metabolic syndrome exhibited among patients with psoriasis.
Multivariable logistic regression analysis (eTable) revealed that there was a significant association between psoriasis and BMI of 25 to 29.99 (adjusted odds ratio [AOR], 1.34; 95% CI, 1.02-1.76; P=.04) and being told by a health care provider that they are overweight (AOR, 1.91; 95% CI, 1.44-2.52; P<.001). After adjusting for confounding variables, there was no significant association between psoriasis and a BMI of 30 or higher (AOR, 1.00; 95% CI, 0.73-1.38; P=.99) or a waist circumference of 102 cm or more in males and 88 cm or more in females (AOR, 1.15; 95% CI, 0.86-1.53; P=.3).
Our findings suggest that a few variables indicative of being overweight or obese are associated with psoriasis. This relationship most likely is due to increased adipokine, including resistin, levels in overweight individuals, resulting in a proinflammatory state.6 It has been suggested that BMI alone is not a definitive marker for measuring fat storage levels in individuals. People can have a normal or slightly elevated BMI but possess excessive adiposity, resulting in chronic inflammation.6 Therefore, our findings of a significant association between psoriasis and being told by a health care provider that they are overweight might be a stronger measurement for possessing excessive fat, as this is likely due to clinical judgment rather than BMI measurement.
Moreover, it should be noted that the potential reason for the lack of association between BMI of 30 or higher and psoriasis in our analysis may be a result of BMI serving as a poor measurement for adiposity. Additionally, Armstrong and colleagues7 discussed that the association between BMI and psoriasis was stronger for patients with moderate to severe psoriasis. Our study consisted of NHANES data for self-reported psoriasis diagnoses without a psoriasis severity index, making it difficult to extrapolate which individuals had mild or moderate to severe psoriasis, which may have contributed to our finding of no association between BMI of 30 or higher and psoriasis.
The self-reported nature of the survey questions and lack of questions regarding psoriasis severity serve as limitations to the study. Both obesity and psoriasis can have various systemic consequences, such as cardiovascular disease, due to the development of an inflammatory state.8 Future studies may explore other body measurements that indicate being overweight or obese and the potential synergistic relationship of obesity and psoriasis severity, optimizing the development of effective treatment plans.
- Jensen P, Skov L. Psoriasis and obesity. Dermatology. 2016;232:633-639.
- Xu C, Ji J, Su T, et al. The association of psoriasis and obesity: focusing on IL-17A-related immunological mechanisms. Int J Dermatol Venereol. 2021;4:116-121.
- National Center for Health Statistics. NHANES questionnaires, datasets, and related documentation. Centers for Disease Control and Prevention website. Accessed June 22, 2023. https://wwwn.cdc.govnchs/nhanes/Default.aspx
- Ross R, Neeland IJ, Yamashita S, et al. Waist circumference as a vital sign in clinical practice: a Consensus Statement from the IAS and ICCR Working Group on Visceral Obesity. Nat Rev Endocrinol. 2020;16:177-189.
- Love TJ, Qureshi AA, Karlson EW, et al. Prevalence of the metabolic syndrome in psoriasis: results from the National Health and Nutrition Examination Survey, 2003-2006. Arch Dermatol. 2011;147:419-424.
- Paroutoglou K, Papadavid E, Christodoulatos GS, et al. Deciphering the association between psoriasis and obesity: current evidence and treatment considerations. Curr Obes Rep. 2020;9:165-178.
- Armstrong AW, Harskamp CT, Armstrong EJ. The association between psoriasis and obesity: a systematic review and meta-analysis of observational studies. Nutr Diabetes. 2012;2:E54.
- Hamminga EA, van der Lely AJ, Neumann HAM, et al. Chronic inflammation in psoriasis and obesity: implications for therapy. Med Hypotheses. 2006;67:768-773.
- Jensen P, Skov L. Psoriasis and obesity. Dermatology. 2016;232:633-639.
- Xu C, Ji J, Su T, et al. The association of psoriasis and obesity: focusing on IL-17A-related immunological mechanisms. Int J Dermatol Venereol. 2021;4:116-121.
- National Center for Health Statistics. NHANES questionnaires, datasets, and related documentation. Centers for Disease Control and Prevention website. Accessed June 22, 2023. https://wwwn.cdc.govnchs/nhanes/Default.aspx
- Ross R, Neeland IJ, Yamashita S, et al. Waist circumference as a vital sign in clinical practice: a Consensus Statement from the IAS and ICCR Working Group on Visceral Obesity. Nat Rev Endocrinol. 2020;16:177-189.
- Love TJ, Qureshi AA, Karlson EW, et al. Prevalence of the metabolic syndrome in psoriasis: results from the National Health and Nutrition Examination Survey, 2003-2006. Arch Dermatol. 2011;147:419-424.
- Paroutoglou K, Papadavid E, Christodoulatos GS, et al. Deciphering the association between psoriasis and obesity: current evidence and treatment considerations. Curr Obes Rep. 2020;9:165-178.
- Armstrong AW, Harskamp CT, Armstrong EJ. The association between psoriasis and obesity: a systematic review and meta-analysis of observational studies. Nutr Diabetes. 2012;2:E54.
- Hamminga EA, van der Lely AJ, Neumann HAM, et al. Chronic inflammation in psoriasis and obesity: implications for therapy. Med Hypotheses. 2006;67:768-773.
Practice Points
- There are many comorbidities that are associated with psoriasis, making it crucial to evaluate for these diseases in patients with psoriasis.
- Obesity may be a contributing factor to psoriasis development due to the role of IL-17 secretion.
Evaluation of Laboratory Follow-up in Acne Patients Treated With Isotretinoin
Isotretinoin is used in the treatment of nodulocystic and severe papulopustular acne. During the treatment period, laboratory monitoring is recommended to identify the risk for complications such as hepatotoxicity, teratogenicity, rhabdomyolysis, hyperlipidemia, and pancreatitis.1 There is a lack of consensus of the frequency of follow-up of laboratory parameters during isotretinoin treatment. This study evaluated the changes in laboratory parameters used in daily practice for patients with acne who were treated with isotretinoin to determine the optimum test repetition frequency.
Materials and Methods
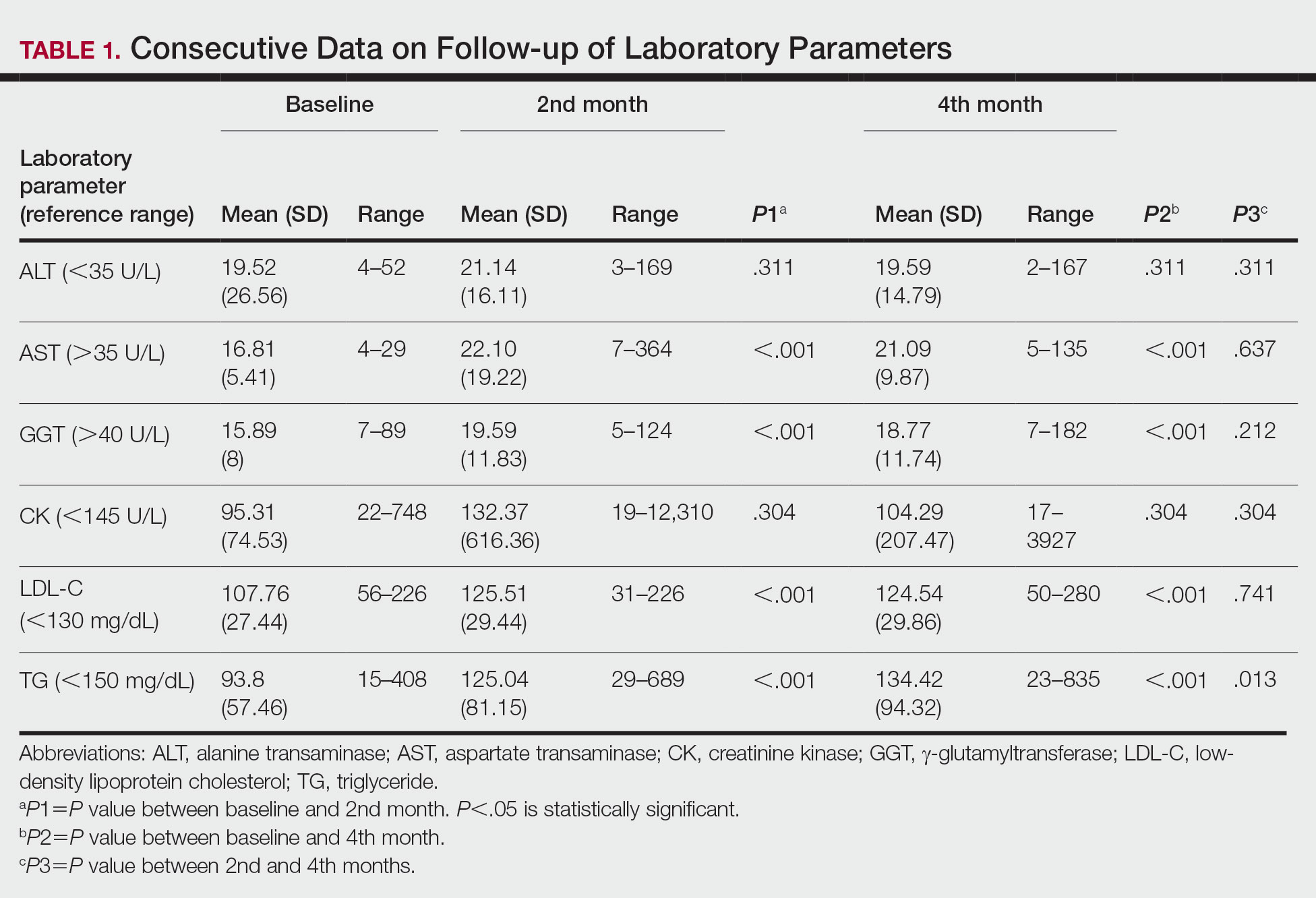
Statistical Analysis—The descriptive statistics of the measurements were presented as means, standard deviations, or medians (first and third quartiles). With respect to the normal distribution, the consistency of the measurements was evaluated with the Kolmogorov-Smirnov test, and small deviations from the normal distribution were observed. Changes in laboratory measurements were evaluated with simple repeated-measures analysis of variance, and changes that differed significantly were determined by a Holm-Sidak post hoc test. Relationships between total cumulative doses and laboratory measurements at second visits were evaluated by the Pearson correlation analysis. The statistical significance level was P<.05. SPSS Statistics 23 (IBM) was used in the calculations.
Results
Consecutive Data at Baseline and Follow-up—A total of 415 patients with a mean age (SD) of 21.49 (7.25) years (range, 12–53 years) were included in our study. The mean total cumulative dose (SD) of the patients was 7267.27 (1878.4) mg. The consecutive data of the means of the laboratory parameters are shown in Table 1 and Figure 1. There was no significant change in the ALT levels between baseline and the fourth month as well as between the second- and fourth-month assessments (both P=.311).
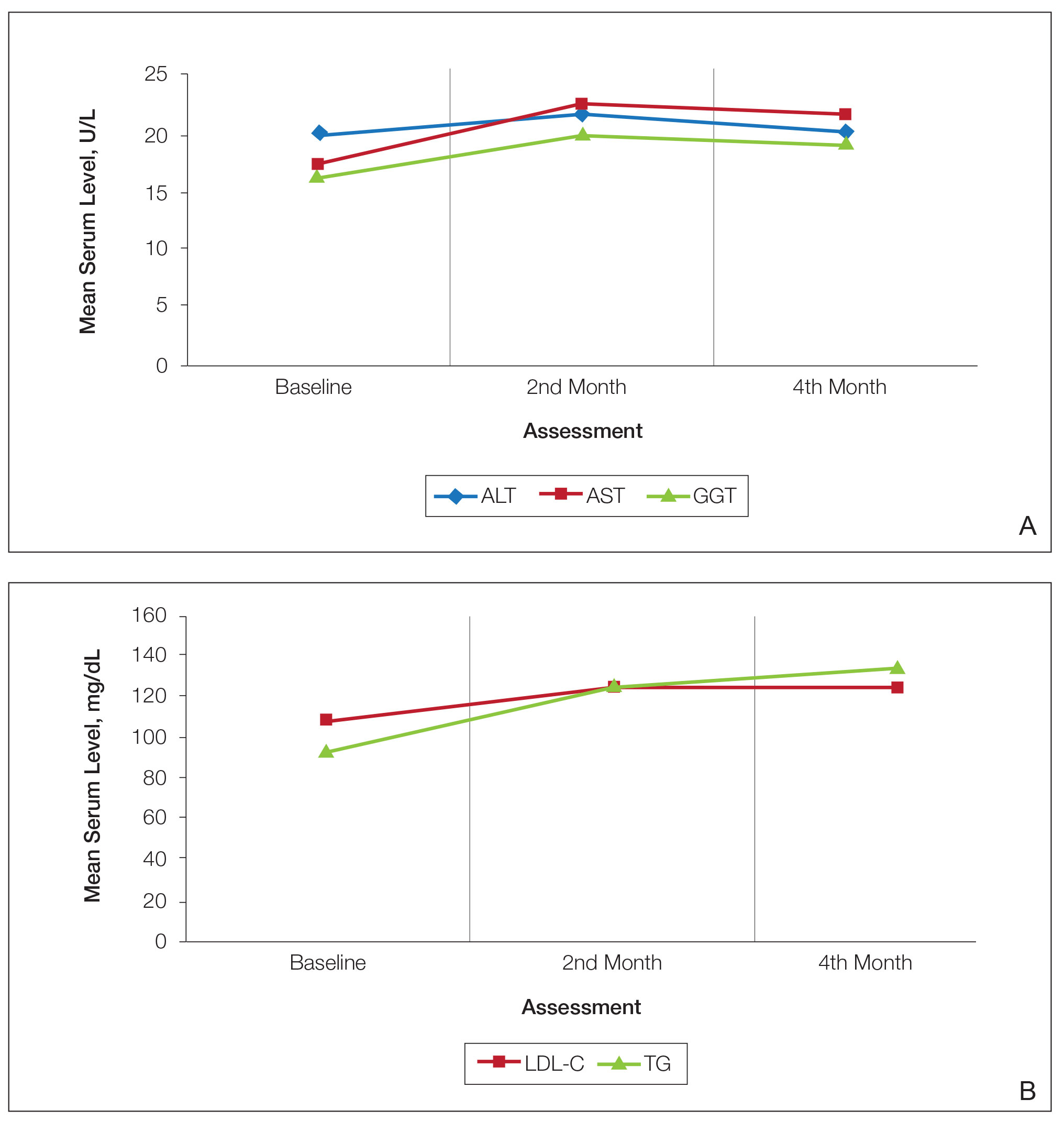
Abnormal Laboratory Measurements—The distribution of abnormal laboratory measurements during treatment is shown in Table 2 and Figure 2. Grade 3 or higher elevations of liver transaminases (ALT, AST) and GGT were observed in fewer than 2% of patients during treatment compared with baseline (grade 3 elevations of ALT and AST together in 2 patients; grade 4 AST elevation in 1 patient; grade 3 elevations of ALT, AST, and GGT combined in 1 patient; isolated grade 3 GGT elevation in 1 patient). All of the patients who developed grade 3 liver transaminases and isolated grade 3 GGT elevation had improved values when these were rechecked within 2 weeks.
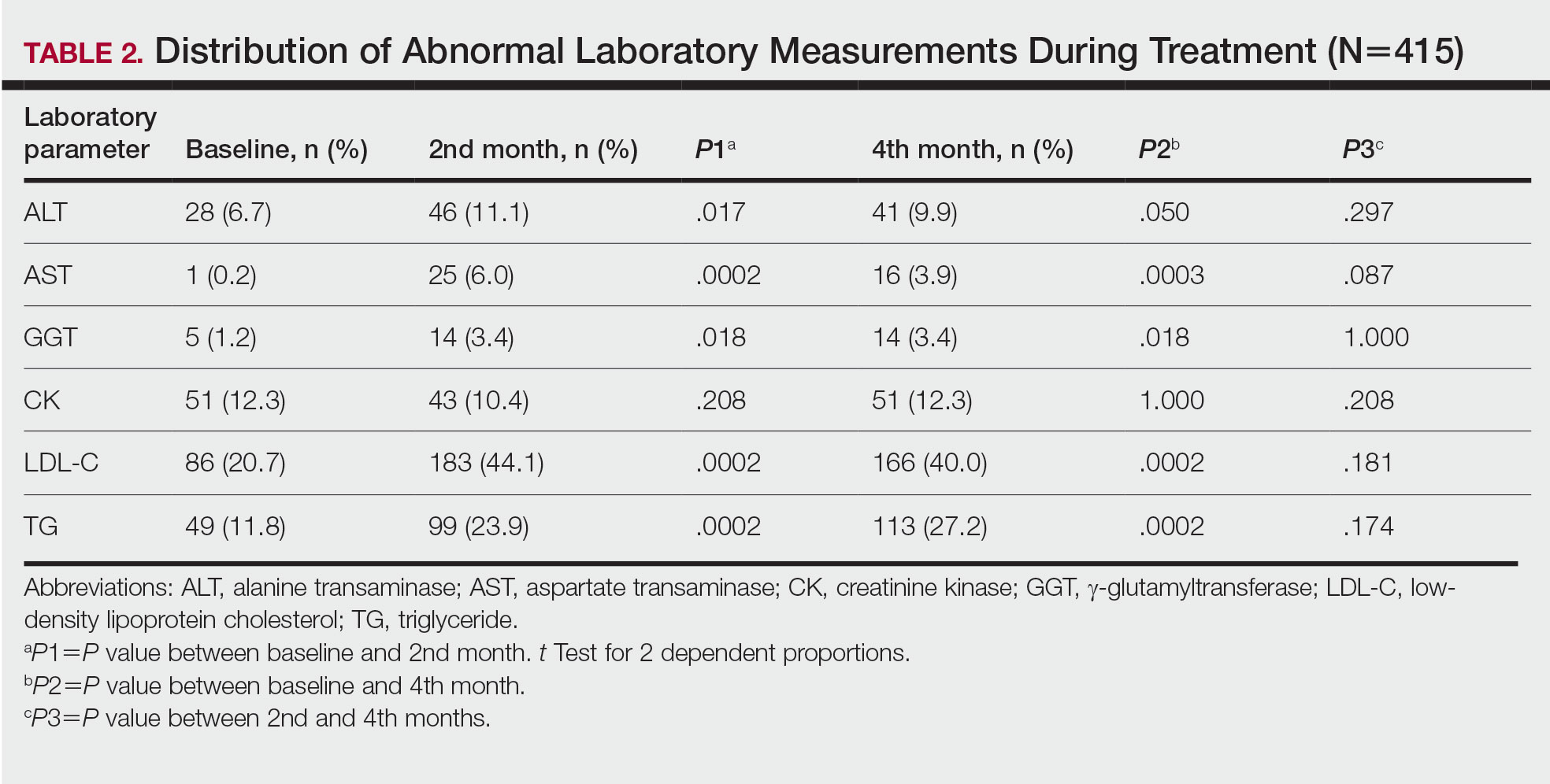
In the patient who developed hepatotoxicity in the second month, the ALT level rose from a baseline of 19 U/L to 169 U/L, the AST level from a baseline of 19 U/L to 61 U/L, and the GGT level from a baseline of 24 U/L to 124 U/L. The patient was asymptomatic. Liver function test levels returned to reference range 4 weeks after discontinuation of therapy. Hepatotoxicity did not recur after treatment was re-administered.
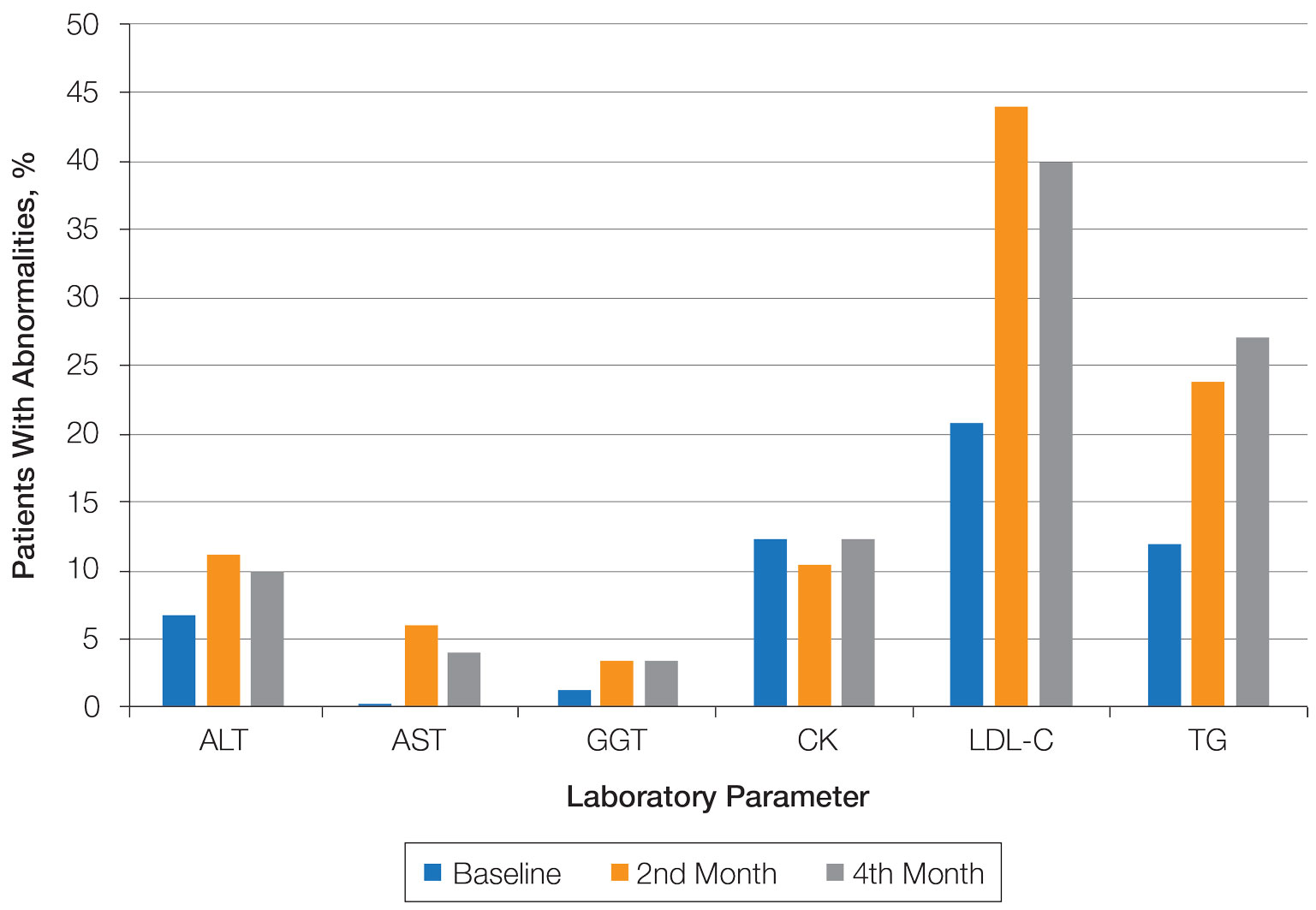
The patient who developed grade 4 AST elevation (364 U/L) experienced fatigue and myalgia. He had done vigorous exercise up to 2 days before the test and also had a grade 4 CK elevation (12,310 U/L). He was thought to have isotretinoin-related rhabdomyolysis. His treatment was discontinued, and he was advised to hydrate and rest. Treatment was re-started after 2 weeks. With frequent laboratory monitoring and avoidance of vigorous physical activity, the patient completed the remaining course of isotretinoin without any laboratory abnormalities or symptoms.
Creatinine kinase abnormalities in the second and fourth months compared with baseline were not statistically significant. The patients with grade 3 or higher CK elevations, except for the case with rhabdomyolysis, had no clinical signs or other characteristic laboratory findings of rhabdomyolysis.
Hypercholesterolemia (LDL-C ≥130 mg/dL) occurred most frequently, with a maximum of 280 mg/dL in 1 patient (in the fourth month) and less than 250 mg/dL in all other patients. Hypercholesterolemia occurred in 183 (44.1%) patients in the second month and in 166 (40.0%) patients in the fourth month. However, baseline abnormalities also were frequent (86 [20.7%]), and hypercholesterolemia persisted in the second and fourth months in all of these patients.
It was observed that the patients with TG abnormalities increased continuously in the second (99 [23.9%]) and fourth (113 [27.2%]) months compared with baseline (49 [11.8%]). Grade 3 TG elevations were observed in 2.2% of patients (n=9; 5 patients in the second month, 4 patients in the fourth month) during treatment compared with baseline, and all patients had grade 1 or 2 hypertriglyceridemia at baseline. Of the patients with grade 3 TG elevation, 3 patients in the second month and 2 patients in the fourth month were obese at baseline. No grade 4 TG elevations were observed. Complications related to hyperlipidemia, such as pancreatitis, were observed in 1 patient. No patient terminated treatment because of lipid abnormalities. The treatment of our patients with major hypercholesterolemia and/or grade 3 hypertriglyceridemia was interrupted. The hyperlipidemia of these patients was controlled by a low-fat diet and a short-term dose reduction.
Relationship Between Total Cumulative Dose and Laboratory Parameters—The relationships between the total cumulative dose and changes up to the fourth month are presented in Table 3. As the total dose increased, the changes in TG and LDL-C levels significantly increased in the fourth month (both P=.001). However, the degree of these relationships was weak. No significant correlation was found between the periodic changes of other laboratory parameters and the total dose.
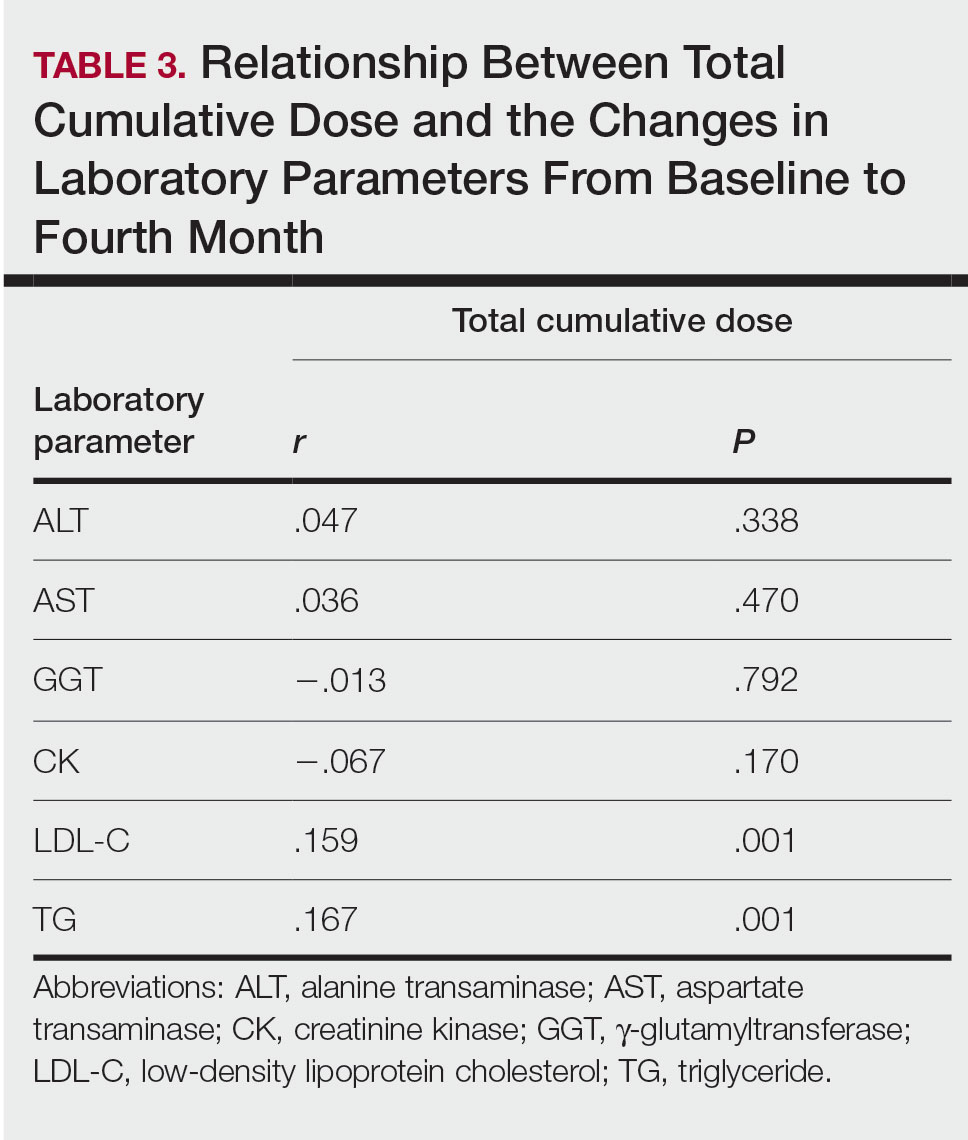
Comment
The parameters followed in our study show that TG levels tend to increase continuously from baseline during isotretinoin treatment, while ALT, AST, GGT, and LDL-C levels increase in the second month and decrease at 4 months. Although this same trend occurs with CK levels, the change was not statistically significant. The most common laboratory abnormality in our study was hyperlipidemia. Levels of LDL-C and TG were both found to be statistically elevated in the second and fourth months of treatment compared with baseline. Parthasarathy et al3 reported that obesity had an important role in the increase of lipid levels in patients using isotretinoin at baseline. In our study, 5 of 9 patients (55.6%) with grade 3 TG elevation were obese, which supports the theory that obesity plays an important role in the increase in lipid levels. Up-to-date laboratory follow-up of lipids suggests that there is no need to follow up serum lipids after the second month of treatment. Patients with risk factors for hyperlipidemia, such as abdominal obesity and familial hyperlipidemia, do not require further follow-up if there is no increase in serum lipids in the first month of treatment.1 The presence of grade 1 or 2 hypertriglyceridemia at baseline in all our patients with grade 3 TG elevation may suggest that periodic laboratory follow-up during isotretinoin treatment is necessary to detect patients with grade 3 and higher TG levels.
The lack of knowledge of other risk factors (eg, familial hyperlipidemia, insulin resistance) for hyperlipidemia in all patients at baseline may be a limitation of our study. Although hypercholesterolemia persisted in the follow-up of our patients with initial LDL-C abnormalities, hypercholesterolemia over 250 mg/dL was very rare (1 patient). Possible complications associated with serum lipid abnormalities are pancreatitis and metabolic syndrome.4 In our study, none of the patients with lipid abnormalities had any relevant clinical sequelae. The dose-dependent elevation of the changes in LDL-C and TG (Table 3) may be important to predict the significant elevation of lipids and the associated complications in patients with a high total cumulative dose target that may require a long treatment duration. However, considering the short follow-up periods in our patients, the absence of clinical sequelae may be misleading. There are differences in recommendations between the US and European guidelines for isotretinoin dosage. Although the US guidelines recommend a total cumulative dose target, the European guidelines recommend low-dose isotretinoin daily for at least 6 months instead of a cumulative dose.
Most liver transaminase abnormalities were detected in the second month. Abnormalities in GGT were seen in the second month and remained elevated at the next follow-up. However, clinically important grade 3 transaminase and GGT elevations were rare. It has been reported that GGT levels are more specific than transaminases in measuring hepatotoxicity.7 The fact that our patient with hepatotoxicity had a grade 3 GGT elevation in addition to grade 3 transaminase elevations supports that GGT elevation is more specific than transaminase levels in measuring hepatotoxicity. When these parameters were rechecked in our patients with grade 3 transaminase elevations, except in the case of hepatotoxicity, transaminase elevations did not recur, and GGT elevations did not accompany elevated transaminases, which suggested that transaminases may be elevated due to an extrahepatic origin (eg, hemolysis, exercise).
Rhabdomyolysis secondary to isotretinoin is rare in the literature of acne studies. In addition to clinical findings such as myalgia and fatigue, increased CK and abnormal liver enzymes, specifically AST, suggest the development of rhabdomyolysis.8 Our patient who developed rhabdomyolysis also had a recent history of vigorous exercise, grade 4 CK, and AST elevations. Other patients with isolated grade 3 CK elevations were informed about possible clinical signs of rhabdomyolysis, and they were able to complete their courses without any incident. According to a study by Landau et al,9 isotretinoin-associated hyperCKemia has been reported as benign. Similarly, our study found that isolated CK elevation during isotretinoin treatment may be misleading as a sign of rhabdomyolysis. Instead, CK monitoring may be more appropriate and cost-effective in patients with suspected clinical signs of rhabdomyolysis or in those with major elevations in transaminases, especially AST.
Conclusion
According to our study, hyperlipidemia was the most common complication in acne patients using isotretinoin. It may be appropriate to monitor the TG level at 2-month intervals in patients with grade 1 or 2 TG elevation at baseline to detect the possible risk for developing grade 3 hyperlipidemia. Periodic monitoring of LDL-C and TG levels may be appropriate, especially in patients who require a high total cumulative dose of isotretinoin. Clinically important liver enzyme abnormalities were rare in our study. Our findings support the idea that routine monthly monitoring of normal laboratory parameters is unnecessary and wasteful. Additionally, periodic monitoring of abnormal laboratory parameters should be considered on an individual basis.
- Affleck A, Jackson D, Williams HC, et al. Is routine laboratory testing in healthy young patients taking isotretinoin necessary: a critically appraised topic. Br J Dermatol. 2022;187:857-865.
- National Cancer Institute. Common Terminology Criteria for Adverse Events v3.0 (CTCAE). August 9, 2006. Accessed June 12, 2023. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcaev3.pdf
- Parthasarathy V, Shah N, Kirkorian AY. The utility of laboratory testing for pediatric patients undergoing isotretinoin treatment. Pediatr Dermatol. 2022;39:731-733.
- Sarkar T, Sarkar S, Patra A. Low-dose isotretinoin therapy and blood lipid abnormality: a case series with sixty patients. J Family Med Prim Care. 2018;7:171-174.
- Nast A, Dréno B, Bettoli V, et al. European evidence-based (S3) guideline for the treatment of acne - update 2016 - short version. J Eur Acad Dermatol Venereol. 2016;30:1261-1268.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.
- Webster GF, Webster TG, Grimes LR. Laboratory tests in patients treated with isotretinoin: occurrence of liver and muscle abnormalities and failure of AST and ALT to predict liver abnormality. Dermatol Online J. 2017;23:13030/qt7rv7j80p.
- Raneses E, Schmidgal EC. Rhabdomyolysis caused by isotretinoin and exercise in an otherwise healthy female patient. Cureus. 2022;14:E25981.
- Landau M, Mesterman R, Ophir J, et al. Clinical significance of markedly elevated serum creatine kinase levels in patients with acne on isotretinoin. Acta Derm Venereol. 2001;81:350-352.
Isotretinoin is used in the treatment of nodulocystic and severe papulopustular acne. During the treatment period, laboratory monitoring is recommended to identify the risk for complications such as hepatotoxicity, teratogenicity, rhabdomyolysis, hyperlipidemia, and pancreatitis.1 There is a lack of consensus of the frequency of follow-up of laboratory parameters during isotretinoin treatment. This study evaluated the changes in laboratory parameters used in daily practice for patients with acne who were treated with isotretinoin to determine the optimum test repetition frequency.
Materials and Methods

Statistical Analysis—The descriptive statistics of the measurements were presented as means, standard deviations, or medians (first and third quartiles). With respect to the normal distribution, the consistency of the measurements was evaluated with the Kolmogorov-Smirnov test, and small deviations from the normal distribution were observed. Changes in laboratory measurements were evaluated with simple repeated-measures analysis of variance, and changes that differed significantly were determined by a Holm-Sidak post hoc test. Relationships between total cumulative doses and laboratory measurements at second visits were evaluated by the Pearson correlation analysis. The statistical significance level was P<.05. SPSS Statistics 23 (IBM) was used in the calculations.
Results
Consecutive Data at Baseline and Follow-up—A total of 415 patients with a mean age (SD) of 21.49 (7.25) years (range, 12–53 years) were included in our study. The mean total cumulative dose (SD) of the patients was 7267.27 (1878.4) mg. The consecutive data of the means of the laboratory parameters are shown in Table 1 and Figure 1. There was no significant change in the ALT levels between baseline and the fourth month as well as between the second- and fourth-month assessments (both P=.311).

Abnormal Laboratory Measurements—The distribution of abnormal laboratory measurements during treatment is shown in Table 2 and Figure 2. Grade 3 or higher elevations of liver transaminases (ALT, AST) and GGT were observed in fewer than 2% of patients during treatment compared with baseline (grade 3 elevations of ALT and AST together in 2 patients; grade 4 AST elevation in 1 patient; grade 3 elevations of ALT, AST, and GGT combined in 1 patient; isolated grade 3 GGT elevation in 1 patient). All of the patients who developed grade 3 liver transaminases and isolated grade 3 GGT elevation had improved values when these were rechecked within 2 weeks.

In the patient who developed hepatotoxicity in the second month, the ALT level rose from a baseline of 19 U/L to 169 U/L, the AST level from a baseline of 19 U/L to 61 U/L, and the GGT level from a baseline of 24 U/L to 124 U/L. The patient was asymptomatic. Liver function test levels returned to reference range 4 weeks after discontinuation of therapy. Hepatotoxicity did not recur after treatment was re-administered.

The patient who developed grade 4 AST elevation (364 U/L) experienced fatigue and myalgia. He had done vigorous exercise up to 2 days before the test and also had a grade 4 CK elevation (12,310 U/L). He was thought to have isotretinoin-related rhabdomyolysis. His treatment was discontinued, and he was advised to hydrate and rest. Treatment was re-started after 2 weeks. With frequent laboratory monitoring and avoidance of vigorous physical activity, the patient completed the remaining course of isotretinoin without any laboratory abnormalities or symptoms.
Creatinine kinase abnormalities in the second and fourth months compared with baseline were not statistically significant. The patients with grade 3 or higher CK elevations, except for the case with rhabdomyolysis, had no clinical signs or other characteristic laboratory findings of rhabdomyolysis.
Hypercholesterolemia (LDL-C ≥130 mg/dL) occurred most frequently, with a maximum of 280 mg/dL in 1 patient (in the fourth month) and less than 250 mg/dL in all other patients. Hypercholesterolemia occurred in 183 (44.1%) patients in the second month and in 166 (40.0%) patients in the fourth month. However, baseline abnormalities also were frequent (86 [20.7%]), and hypercholesterolemia persisted in the second and fourth months in all of these patients.
It was observed that the patients with TG abnormalities increased continuously in the second (99 [23.9%]) and fourth (113 [27.2%]) months compared with baseline (49 [11.8%]). Grade 3 TG elevations were observed in 2.2% of patients (n=9; 5 patients in the second month, 4 patients in the fourth month) during treatment compared with baseline, and all patients had grade 1 or 2 hypertriglyceridemia at baseline. Of the patients with grade 3 TG elevation, 3 patients in the second month and 2 patients in the fourth month were obese at baseline. No grade 4 TG elevations were observed. Complications related to hyperlipidemia, such as pancreatitis, were observed in 1 patient. No patient terminated treatment because of lipid abnormalities. The treatment of our patients with major hypercholesterolemia and/or grade 3 hypertriglyceridemia was interrupted. The hyperlipidemia of these patients was controlled by a low-fat diet and a short-term dose reduction.
Relationship Between Total Cumulative Dose and Laboratory Parameters—The relationships between the total cumulative dose and changes up to the fourth month are presented in Table 3. As the total dose increased, the changes in TG and LDL-C levels significantly increased in the fourth month (both P=.001). However, the degree of these relationships was weak. No significant correlation was found between the periodic changes of other laboratory parameters and the total dose.

Comment
The parameters followed in our study show that TG levels tend to increase continuously from baseline during isotretinoin treatment, while ALT, AST, GGT, and LDL-C levels increase in the second month and decrease at 4 months. Although this same trend occurs with CK levels, the change was not statistically significant. The most common laboratory abnormality in our study was hyperlipidemia. Levels of LDL-C and TG were both found to be statistically elevated in the second and fourth months of treatment compared with baseline. Parthasarathy et al3 reported that obesity had an important role in the increase of lipid levels in patients using isotretinoin at baseline. In our study, 5 of 9 patients (55.6%) with grade 3 TG elevation were obese, which supports the theory that obesity plays an important role in the increase in lipid levels. Up-to-date laboratory follow-up of lipids suggests that there is no need to follow up serum lipids after the second month of treatment. Patients with risk factors for hyperlipidemia, such as abdominal obesity and familial hyperlipidemia, do not require further follow-up if there is no increase in serum lipids in the first month of treatment.1 The presence of grade 1 or 2 hypertriglyceridemia at baseline in all our patients with grade 3 TG elevation may suggest that periodic laboratory follow-up during isotretinoin treatment is necessary to detect patients with grade 3 and higher TG levels.
The lack of knowledge of other risk factors (eg, familial hyperlipidemia, insulin resistance) for hyperlipidemia in all patients at baseline may be a limitation of our study. Although hypercholesterolemia persisted in the follow-up of our patients with initial LDL-C abnormalities, hypercholesterolemia over 250 mg/dL was very rare (1 patient). Possible complications associated with serum lipid abnormalities are pancreatitis and metabolic syndrome.4 In our study, none of the patients with lipid abnormalities had any relevant clinical sequelae. The dose-dependent elevation of the changes in LDL-C and TG (Table 3) may be important to predict the significant elevation of lipids and the associated complications in patients with a high total cumulative dose target that may require a long treatment duration. However, considering the short follow-up periods in our patients, the absence of clinical sequelae may be misleading. There are differences in recommendations between the US and European guidelines for isotretinoin dosage. Although the US guidelines recommend a total cumulative dose target, the European guidelines recommend low-dose isotretinoin daily for at least 6 months instead of a cumulative dose.
Most liver transaminase abnormalities were detected in the second month. Abnormalities in GGT were seen in the second month and remained elevated at the next follow-up. However, clinically important grade 3 transaminase and GGT elevations were rare. It has been reported that GGT levels are more specific than transaminases in measuring hepatotoxicity.7 The fact that our patient with hepatotoxicity had a grade 3 GGT elevation in addition to grade 3 transaminase elevations supports that GGT elevation is more specific than transaminase levels in measuring hepatotoxicity. When these parameters were rechecked in our patients with grade 3 transaminase elevations, except in the case of hepatotoxicity, transaminase elevations did not recur, and GGT elevations did not accompany elevated transaminases, which suggested that transaminases may be elevated due to an extrahepatic origin (eg, hemolysis, exercise).
Rhabdomyolysis secondary to isotretinoin is rare in the literature of acne studies. In addition to clinical findings such as myalgia and fatigue, increased CK and abnormal liver enzymes, specifically AST, suggest the development of rhabdomyolysis.8 Our patient who developed rhabdomyolysis also had a recent history of vigorous exercise, grade 4 CK, and AST elevations. Other patients with isolated grade 3 CK elevations were informed about possible clinical signs of rhabdomyolysis, and they were able to complete their courses without any incident. According to a study by Landau et al,9 isotretinoin-associated hyperCKemia has been reported as benign. Similarly, our study found that isolated CK elevation during isotretinoin treatment may be misleading as a sign of rhabdomyolysis. Instead, CK monitoring may be more appropriate and cost-effective in patients with suspected clinical signs of rhabdomyolysis or in those with major elevations in transaminases, especially AST.
Conclusion
According to our study, hyperlipidemia was the most common complication in acne patients using isotretinoin. It may be appropriate to monitor the TG level at 2-month intervals in patients with grade 1 or 2 TG elevation at baseline to detect the possible risk for developing grade 3 hyperlipidemia. Periodic monitoring of LDL-C and TG levels may be appropriate, especially in patients who require a high total cumulative dose of isotretinoin. Clinically important liver enzyme abnormalities were rare in our study. Our findings support the idea that routine monthly monitoring of normal laboratory parameters is unnecessary and wasteful. Additionally, periodic monitoring of abnormal laboratory parameters should be considered on an individual basis.
Isotretinoin is used in the treatment of nodulocystic and severe papulopustular acne. During the treatment period, laboratory monitoring is recommended to identify the risk for complications such as hepatotoxicity, teratogenicity, rhabdomyolysis, hyperlipidemia, and pancreatitis.1 There is a lack of consensus of the frequency of follow-up of laboratory parameters during isotretinoin treatment. This study evaluated the changes in laboratory parameters used in daily practice for patients with acne who were treated with isotretinoin to determine the optimum test repetition frequency.
Materials and Methods

Statistical Analysis—The descriptive statistics of the measurements were presented as means, standard deviations, or medians (first and third quartiles). With respect to the normal distribution, the consistency of the measurements was evaluated with the Kolmogorov-Smirnov test, and small deviations from the normal distribution were observed. Changes in laboratory measurements were evaluated with simple repeated-measures analysis of variance, and changes that differed significantly were determined by a Holm-Sidak post hoc test. Relationships between total cumulative doses and laboratory measurements at second visits were evaluated by the Pearson correlation analysis. The statistical significance level was P<.05. SPSS Statistics 23 (IBM) was used in the calculations.
Results
Consecutive Data at Baseline and Follow-up—A total of 415 patients with a mean age (SD) of 21.49 (7.25) years (range, 12–53 years) were included in our study. The mean total cumulative dose (SD) of the patients was 7267.27 (1878.4) mg. The consecutive data of the means of the laboratory parameters are shown in Table 1 and Figure 1. There was no significant change in the ALT levels between baseline and the fourth month as well as between the second- and fourth-month assessments (both P=.311).

Abnormal Laboratory Measurements—The distribution of abnormal laboratory measurements during treatment is shown in Table 2 and Figure 2. Grade 3 or higher elevations of liver transaminases (ALT, AST) and GGT were observed in fewer than 2% of patients during treatment compared with baseline (grade 3 elevations of ALT and AST together in 2 patients; grade 4 AST elevation in 1 patient; grade 3 elevations of ALT, AST, and GGT combined in 1 patient; isolated grade 3 GGT elevation in 1 patient). All of the patients who developed grade 3 liver transaminases and isolated grade 3 GGT elevation had improved values when these were rechecked within 2 weeks.

In the patient who developed hepatotoxicity in the second month, the ALT level rose from a baseline of 19 U/L to 169 U/L, the AST level from a baseline of 19 U/L to 61 U/L, and the GGT level from a baseline of 24 U/L to 124 U/L. The patient was asymptomatic. Liver function test levels returned to reference range 4 weeks after discontinuation of therapy. Hepatotoxicity did not recur after treatment was re-administered.

The patient who developed grade 4 AST elevation (364 U/L) experienced fatigue and myalgia. He had done vigorous exercise up to 2 days before the test and also had a grade 4 CK elevation (12,310 U/L). He was thought to have isotretinoin-related rhabdomyolysis. His treatment was discontinued, and he was advised to hydrate and rest. Treatment was re-started after 2 weeks. With frequent laboratory monitoring and avoidance of vigorous physical activity, the patient completed the remaining course of isotretinoin without any laboratory abnormalities or symptoms.
Creatinine kinase abnormalities in the second and fourth months compared with baseline were not statistically significant. The patients with grade 3 or higher CK elevations, except for the case with rhabdomyolysis, had no clinical signs or other characteristic laboratory findings of rhabdomyolysis.
Hypercholesterolemia (LDL-C ≥130 mg/dL) occurred most frequently, with a maximum of 280 mg/dL in 1 patient (in the fourth month) and less than 250 mg/dL in all other patients. Hypercholesterolemia occurred in 183 (44.1%) patients in the second month and in 166 (40.0%) patients in the fourth month. However, baseline abnormalities also were frequent (86 [20.7%]), and hypercholesterolemia persisted in the second and fourth months in all of these patients.
It was observed that the patients with TG abnormalities increased continuously in the second (99 [23.9%]) and fourth (113 [27.2%]) months compared with baseline (49 [11.8%]). Grade 3 TG elevations were observed in 2.2% of patients (n=9; 5 patients in the second month, 4 patients in the fourth month) during treatment compared with baseline, and all patients had grade 1 or 2 hypertriglyceridemia at baseline. Of the patients with grade 3 TG elevation, 3 patients in the second month and 2 patients in the fourth month were obese at baseline. No grade 4 TG elevations were observed. Complications related to hyperlipidemia, such as pancreatitis, were observed in 1 patient. No patient terminated treatment because of lipid abnormalities. The treatment of our patients with major hypercholesterolemia and/or grade 3 hypertriglyceridemia was interrupted. The hyperlipidemia of these patients was controlled by a low-fat diet and a short-term dose reduction.
Relationship Between Total Cumulative Dose and Laboratory Parameters—The relationships between the total cumulative dose and changes up to the fourth month are presented in Table 3. As the total dose increased, the changes in TG and LDL-C levels significantly increased in the fourth month (both P=.001). However, the degree of these relationships was weak. No significant correlation was found between the periodic changes of other laboratory parameters and the total dose.

Comment
The parameters followed in our study show that TG levels tend to increase continuously from baseline during isotretinoin treatment, while ALT, AST, GGT, and LDL-C levels increase in the second month and decrease at 4 months. Although this same trend occurs with CK levels, the change was not statistically significant. The most common laboratory abnormality in our study was hyperlipidemia. Levels of LDL-C and TG were both found to be statistically elevated in the second and fourth months of treatment compared with baseline. Parthasarathy et al3 reported that obesity had an important role in the increase of lipid levels in patients using isotretinoin at baseline. In our study, 5 of 9 patients (55.6%) with grade 3 TG elevation were obese, which supports the theory that obesity plays an important role in the increase in lipid levels. Up-to-date laboratory follow-up of lipids suggests that there is no need to follow up serum lipids after the second month of treatment. Patients with risk factors for hyperlipidemia, such as abdominal obesity and familial hyperlipidemia, do not require further follow-up if there is no increase in serum lipids in the first month of treatment.1 The presence of grade 1 or 2 hypertriglyceridemia at baseline in all our patients with grade 3 TG elevation may suggest that periodic laboratory follow-up during isotretinoin treatment is necessary to detect patients with grade 3 and higher TG levels.
The lack of knowledge of other risk factors (eg, familial hyperlipidemia, insulin resistance) for hyperlipidemia in all patients at baseline may be a limitation of our study. Although hypercholesterolemia persisted in the follow-up of our patients with initial LDL-C abnormalities, hypercholesterolemia over 250 mg/dL was very rare (1 patient). Possible complications associated with serum lipid abnormalities are pancreatitis and metabolic syndrome.4 In our study, none of the patients with lipid abnormalities had any relevant clinical sequelae. The dose-dependent elevation of the changes in LDL-C and TG (Table 3) may be important to predict the significant elevation of lipids and the associated complications in patients with a high total cumulative dose target that may require a long treatment duration. However, considering the short follow-up periods in our patients, the absence of clinical sequelae may be misleading. There are differences in recommendations between the US and European guidelines for isotretinoin dosage. Although the US guidelines recommend a total cumulative dose target, the European guidelines recommend low-dose isotretinoin daily for at least 6 months instead of a cumulative dose.
Most liver transaminase abnormalities were detected in the second month. Abnormalities in GGT were seen in the second month and remained elevated at the next follow-up. However, clinically important grade 3 transaminase and GGT elevations were rare. It has been reported that GGT levels are more specific than transaminases in measuring hepatotoxicity.7 The fact that our patient with hepatotoxicity had a grade 3 GGT elevation in addition to grade 3 transaminase elevations supports that GGT elevation is more specific than transaminase levels in measuring hepatotoxicity. When these parameters were rechecked in our patients with grade 3 transaminase elevations, except in the case of hepatotoxicity, transaminase elevations did not recur, and GGT elevations did not accompany elevated transaminases, which suggested that transaminases may be elevated due to an extrahepatic origin (eg, hemolysis, exercise).
Rhabdomyolysis secondary to isotretinoin is rare in the literature of acne studies. In addition to clinical findings such as myalgia and fatigue, increased CK and abnormal liver enzymes, specifically AST, suggest the development of rhabdomyolysis.8 Our patient who developed rhabdomyolysis also had a recent history of vigorous exercise, grade 4 CK, and AST elevations. Other patients with isolated grade 3 CK elevations were informed about possible clinical signs of rhabdomyolysis, and they were able to complete their courses without any incident. According to a study by Landau et al,9 isotretinoin-associated hyperCKemia has been reported as benign. Similarly, our study found that isolated CK elevation during isotretinoin treatment may be misleading as a sign of rhabdomyolysis. Instead, CK monitoring may be more appropriate and cost-effective in patients with suspected clinical signs of rhabdomyolysis or in those with major elevations in transaminases, especially AST.
Conclusion
According to our study, hyperlipidemia was the most common complication in acne patients using isotretinoin. It may be appropriate to monitor the TG level at 2-month intervals in patients with grade 1 or 2 TG elevation at baseline to detect the possible risk for developing grade 3 hyperlipidemia. Periodic monitoring of LDL-C and TG levels may be appropriate, especially in patients who require a high total cumulative dose of isotretinoin. Clinically important liver enzyme abnormalities were rare in our study. Our findings support the idea that routine monthly monitoring of normal laboratory parameters is unnecessary and wasteful. Additionally, periodic monitoring of abnormal laboratory parameters should be considered on an individual basis.
- Affleck A, Jackson D, Williams HC, et al. Is routine laboratory testing in healthy young patients taking isotretinoin necessary: a critically appraised topic. Br J Dermatol. 2022;187:857-865.
- National Cancer Institute. Common Terminology Criteria for Adverse Events v3.0 (CTCAE). August 9, 2006. Accessed June 12, 2023. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcaev3.pdf
- Parthasarathy V, Shah N, Kirkorian AY. The utility of laboratory testing for pediatric patients undergoing isotretinoin treatment. Pediatr Dermatol. 2022;39:731-733.
- Sarkar T, Sarkar S, Patra A. Low-dose isotretinoin therapy and blood lipid abnormality: a case series with sixty patients. J Family Med Prim Care. 2018;7:171-174.
- Nast A, Dréno B, Bettoli V, et al. European evidence-based (S3) guideline for the treatment of acne - update 2016 - short version. J Eur Acad Dermatol Venereol. 2016;30:1261-1268.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.
- Webster GF, Webster TG, Grimes LR. Laboratory tests in patients treated with isotretinoin: occurrence of liver and muscle abnormalities and failure of AST and ALT to predict liver abnormality. Dermatol Online J. 2017;23:13030/qt7rv7j80p.
- Raneses E, Schmidgal EC. Rhabdomyolysis caused by isotretinoin and exercise in an otherwise healthy female patient. Cureus. 2022;14:E25981.
- Landau M, Mesterman R, Ophir J, et al. Clinical significance of markedly elevated serum creatine kinase levels in patients with acne on isotretinoin. Acta Derm Venereol. 2001;81:350-352.
- Affleck A, Jackson D, Williams HC, et al. Is routine laboratory testing in healthy young patients taking isotretinoin necessary: a critically appraised topic. Br J Dermatol. 2022;187:857-865.
- National Cancer Institute. Common Terminology Criteria for Adverse Events v3.0 (CTCAE). August 9, 2006. Accessed June 12, 2023. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcaev3.pdf
- Parthasarathy V, Shah N, Kirkorian AY. The utility of laboratory testing for pediatric patients undergoing isotretinoin treatment. Pediatr Dermatol. 2022;39:731-733.
- Sarkar T, Sarkar S, Patra A. Low-dose isotretinoin therapy and blood lipid abnormality: a case series with sixty patients. J Family Med Prim Care. 2018;7:171-174.
- Nast A, Dréno B, Bettoli V, et al. European evidence-based (S3) guideline for the treatment of acne - update 2016 - short version. J Eur Acad Dermatol Venereol. 2016;30:1261-1268.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.
- Webster GF, Webster TG, Grimes LR. Laboratory tests in patients treated with isotretinoin: occurrence of liver and muscle abnormalities and failure of AST and ALT to predict liver abnormality. Dermatol Online J. 2017;23:13030/qt7rv7j80p.
- Raneses E, Schmidgal EC. Rhabdomyolysis caused by isotretinoin and exercise in an otherwise healthy female patient. Cureus. 2022;14:E25981.
- Landau M, Mesterman R, Ophir J, et al. Clinical significance of markedly elevated serum creatine kinase levels in patients with acne on isotretinoin. Acta Derm Venereol. 2001;81:350-352.
Practice Points
- Hyperlipidemia was the most common complication in patients with acne using isotretinoin.
- It may be appropriate to monitor triglyceride levels at 2-month intervals in patients with grade 1 or 2 triglyceride elevation at baseline to detect the possible risk for developing grade 3 hyperlipidemia.
- Routine monthly monitoring of normal laboratory parameters is unnecessary and wasteful. Periodic monitoring of abnormal laboratory parameters should be considered on an individual basis.
Barriers to Implementation of Telehealth Pre-anesthesia Evaluation Visits in the Department of Veterans Affairs
Days or weeks before a scheduled surgical or invasive procedure involving anesthesia, evaluations are conducted to assess a patient’s condition and risk, optimize their status, and prepare them for their procedure. A comprehensive pre-anesthesia evaluation visit includes a history of present illness, the evaluation of comorbidities and medication use, the assessment of health habits such as alcohol and tobacco use, functional capacity and nutritional assessments, and the identification of social support deficiencies that may influence recovery. It also includes a focused physical examination and laboratory and other ancillary testing as needed and may include optimization interventions such as anemia management or prehabilitation. Conducting pre-anesthesia evaluations before surgery has been shown to reduce delays and cancellations, unnecessary preprocedure testing, hospital length of stay, and in-hospital mortality.1-4
The pre-anesthesia evaluation is usually conducted in person, although other modalities have been in use for several years and have accelerated since the advent of the COVID-19 pandemic. Specifically, audio-only telephone visits are used in many settings to conduct abbreviated forms of a pre-anesthesia evaluation, typically for less-invasive procedures. When patients are evaluated over the telephone, the physical examination and testing are deferred until the day of the procedure. Another modality is the use of synchronous video telehealth. Emerging evidence for the use of video-based care in anesthesiology provides encouraging results. Several institutions have proven the technological feasibility of performing preoperative evaluations via video.5,6 Compared with in-person evaluations, these visits seem to have similar surgery cancellation rates, improved patient satisfaction, and reduced wait times and costs.7-9
As part of a quality improvement project, we studied the use of telehealth for pre-anesthesia evaluations within the US Department of Veterans Affairs (VA). An internal review found overall low utilization of these modalities before the COVID-19 pandemic that accelerated toward telehealth during the pandemic: The largest uptake was with telephone visits. Given the increasing adoption of telehealth for pre-anesthesia evaluations and the marked preference for telephone over video modalities among VA practitioners during the COVID-19 pandemic, we sought to understand the barriers and facilitators to the adoption of telephone- and video-based pre-anesthesia evaluation visits within the VA.
Methods
Our objective was to assess health care practitioners’ (HCPs) preferences regarding pre-anesthesia evaluation modalities (in-person, telephone, or video), and the perceived advantages and barriers to adoption for each modality. We followed the Strengthening the Reporting of Observational studies in Epidemiology (STROBE) guideline and Checklist for statistical Assessment of Medical Papers (CHAMP) statement.10,11 The survey was deemed a quality improvement activity that was exempt from institutional review board oversight by the VA National Anesthesia Program Office and the VA Office of Connected Care.
A survey was distributed to all VA anesthesiology service chiefs via email between April 27, 2022, and May 3, 2022. Three emails were sent to each participant (initial invitation and 2 reminders). The respondents were asked to identify themselves by facility and role and to indicate whether their anesthesiology service performed any pre-anesthesia evaluations, including any telephone- or video-based evaluations; and whether their service has a dedicated pre-anesthesia evaluation clinic.
A second set of questions referred to the use of telephone- and video-based preprocedure evaluations. The questions were based on branch logic and depended on the respondent’s answers concerning their use of telephone- and video-based evaluations. Questions included statements about perceived barriers to the adoption of these pre-anesthesia evaluation modalities. Each item was rated on a 5-point Likert scale, (completely disagree [1] to completely agree [5]). A third section measured acceptability and feasibility of video using the validated Acceptability of Intervention Measure (AIM) and Feasibility of Intervention Measure (FIM)questionnaires.12 These instruments are 4-item measures of implementation outcomes that are often considered indicators of implementation success.13Acceptability is the perception among implementation stakeholders that a given treatment, service, practice, or innovation is agreeable, palatable, or satisfactory. Feasibility is defined as the extent to which a new treatment or an innovation can be successfully used or carried out within a given agency or setting.13 The criterion for acceptability is personal, meaning that different HCPs may have differing needs, preferences, and expectations regarding the same intervention. The criterion for feasibility is practical. An intervention may be considered to be feasible if the required tasks can be performed easily or conveniently. Finally, 2 open-ended questions allowed respondents to identify the most important factor that allowed the implementation of telehealth for pre-anesthesia evaluations in their service, and provide comments about the use of telehealth for pre-anesthesia evaluations at the VA. All questions were developed by the authors except for the 2 implementation measure instruments.
The survey was administered using an electronic survey platform (Qualtrics, version April 2022) and sent by email alongside a brief introductory video. Participation was voluntary and anonymous, as no personal information was collected. Responses were attributed to each facility, using the self-declared affiliation. When an affiliation was not provided, we deduced it using the latitude/longitude of the respondent, a feature included in the survey software. No incentives were provided. Data were stored and maintained in a secure VA server. All completed surveys were included. Some facilities had > 1 complete response, and all were included. Facilities that provided > 1 response and where responses were discordant, we clarified with the facility service chief. Incomplete responses were excluded from the analysis.
Statistics
For this analysis, the 2 positive sentiment responses (agree and completely agree) and the 2 negative sentiment responses (disagree and completely disagree) in the Likert scale were collapsed into single categories (good and poor, respectively). The neither agree nor disagree responses were coded as neutral. Our analysis began with a visual exploration of all variables to evaluate the frequency, percentage, and near-zero variance for categorical variables.14 Near-zero variance occurs when a categorical variable has a low frequency of unique values over the sample size (ie, the variable is almost constant), and we addressed it by combining different variable categorizations. We handled missing values through imputation algorithms followed by sensitivity analyses to verify whether our results were stable with and without imputation. We performed comparisons for the exploratory analysis using P values for one-way analysis of variance tests for numeric variables and χ2tests for categorical variables. We considered P values < .05 to be statistically significant. We also used correlation matrices and plots as exploratory analysis tools to better understand all items’ correlations. We used Pearson, polychoric, and polyserial correlation tests as appropriate for numeric, ordinal, and logical items.
Our modeling strategy involved a series of generalized linear models (GLMs) with a Gaussian family, ie, multiple linear regression models, to assess the association between (1) facilities’ preferences regarding pre-anesthesia evaluation modalities; (2) advantages between modalities; and (3) barriers to the adoption of telehealth and the ability to perform different pre-anesthesia evaluation-related tasks. In addition, we used backward deletion to reach the most parsimonious model based on a series of likelihood-ratio tests comparing nested models. Results are reported as predicted means with 95% confidence intervals, with results being interpreted as significant when any 2 predicted means do not overlap between different estimates along with P for trends < .001. We performed all analyses using the R language.15
Results
Of 109 surveyed facilities, 50 (46%) responded to the survey. The final study sample included 67 responses, and 55 were included in the analysis. Twelve responses were excluded from the analysis as they were either incomplete or test responses. Three facilities had > 1 complete response (2 facilities had 2 responses and 1 facility had 4 responses), and these were all included in the analysis.
Thirty-six locations were complex inpatient facilities, and 32 (89%) had pre-anesthesia evaluation clinics (Table 1).
The ability to obtain a history of present illness was rated good/very good via telephone for 34 respondents (92%) and 25 for video (86%). Assessing comorbidities and health habits was rated good/very good via telephone for 32 respondents (89%) and 31 respondents (86%), respectively, and via video for 24 respondents (83%) and 23 respondents (79%), respectively (Figure 1).
To compare differences between the 2 remote pre-anesthesia evaluation modalities, we created GLMs evaluating the association between each modality and the perceived ability to perform the tasks. For GLMs, we transformed the values of the categories into numerical (ie, 1, poor; 2, neutral; 3, good). Compared with telephone, video was rated more favorably regarding the assessment of nutritional status (mean, 2.1; 95% CI, 1.8-2.3 vs mean, 2.4; 95% CI, 2.2-2.7; P = .04) (eAppendix 1, available at doi:10.12788/fp.0387). No other significant differences in ratings existed between the 2 remote pre-anesthesia evaluation modalities.
The most significant barriers (cited as significant or very significant in the survey) included the inability to perform a physical examination, which was noted by 13 respondents (72%) and 15 respondents (60%) for telephone and video, respectively. The inability to obtain vital signs was rated as a significant barrier for telephone by 12 respondents (67%) and for video by 15 respondents (60%)(Figure 2).
The average FIM score was 3.7, with the highest score among respondents who used both phone and video (Table 2). The average AIM score was 3.4, with the highest score among respondents who used both telehealth modalities. The internal consistency of the implementation measures was excellent (Cronbach’s α 0.95 and 0.975 for FIM and AIM, respectively).
Discussion
We surveyed 109 anesthesiology services across the VA regarding barriers to implementing telephone- and video-based pre-anesthesia evaluation visits. We found that 12 (23%) of the 50 anesthesiology services responding to this survey still conduct the totality of their pre-anesthesia evaluations in person. This represents an opportunity to further disseminate the appropriate use of telehealth and potentially reduce travel time, costs, and low-value testing, as it is well established that remote pre-anesthesia evaluations for low-risk procedures are safe and effective.6
We also found no difference between telephone and video regarding users’ perceived ability to perform any of the basic pre-anesthesia evaluation tasks except for assessing patients’ nutritional status, which was rated as easier using video than telephone. According to those not using telephone and/or video, the biggest barriers to implementation of telehealth visits were the inability to obtain vital signs and to perform a physical examination. This finding was unexpected, as facilities that conduct remote evaluations typically defer these tasks to the day of surgery, a practice that has been well established and shown to be safe and efficient. Respondents also identified patient-level factors (eg, patient preference, lack of telephone or computer) as significant barriers. Finally, feasibility ratings were higher than acceptability ratings with regards to the implementation of telehealth.
In 2004, the first use of telehealth for pre-anesthesia evaluations was reported by Wong and colleagues.16 Since then, several case series and a literature review have documented the efficacy, safety, and patient and HCP satisfaction with the use of telehealth for pre-anesthesia evaluations. A study by Mullen-Fortino and colleagues showed reduced visit times when telehealth was used for pre-anesthesia evaluation.8 Another study at VA hospitals showed that 88% of veterans reported that telemedicine saved them time and money.17 A report of 35 patients in rural Australia reported 98% satisfaction with the video quality of the visit, 95% perceived efficacy, and 87% preference for telehealth compared with driving to be seen in person.18 These reports conflict with the perceptions of the respondents of our survey, who identified patient preference as an important barrier to adoption of telehealth. Given these findings, research is needed on veterans’ perceptions on the use of telehealth modalities for pre-anesthesia evaluations; if their perceptions are similarly favorable, it will be important to communicate this information to HCPs and leadership, which may help increase subsequent telehealth adoption.
Despite the reported safety, efficacy, and high satisfaction of video visits among anesthesiology teams conducting pre-anesthesia evaluations, its use remains low at VA. We have found that most facilities in the VA system chose telephone platforms during the COVID-19 pandemic. One possibility is that the adoption of video modalities among pre-anesthesia evaluation clinics in the VA system is resource intensive or difficult from the HCP’s perspective. When combined with the lack of perceived advantages over telephone as we found in our survey, most practitioners resort to the technologically less demanding and more familiar telephone platform. The results from FIM and AIM support this. While both telephone and video have high feasibility scores, acceptability scores are lower for video, even among those currently using this technology. Our findings do not rule out the utility of video-based care in perioperative medicine. Rather than a yes/no proposition, future studies need to establish the precise indications for video for pre-anesthesia evaluations; that is, situations where video visits offer an advantage over telephone. For example, video could be used to deliver preoperative optimization therapies, such as supervised exercise or mental health interventions or to guide the achievement of certain milestones before surgery in patients with chronic conditions, such as target glucose values or the treatment of anemia. Future studies should explore the perceived benefits of video over telephone among centers offering these more advanced optimization interventions.
Limitations
We received responses from a subset of VA anesthesiology services; therefore, they may not be representative of the entire VA system. Facilities designated by the VA as inpatient complex were overrepresented (72% of our sample vs 50% of the total facilities nationally), and ambulatory centers (those designed by the VA as ambulatory procedural center with basic or advanced capabilities) were underrepresented (2% of our sample vs 22% nationally). Despite this, the response rate was high, and no geographic area appeared to be underrepresented. In addition, we surveyed pre-anesthesia evaluation facilities led by anesthesiologists, and the results may not be representative of the preferences of HCPs working in nonanesthesiology led pre-anesthesia evaluation clinics. Finally, just 11 facilities used both telephone and video; therefore, a true direct comparison between these 2 platforms was limited. The VA serves a unique patient population, and the findings may not be completely applicable to the non-VA population.
Conclusions
We found no significant perceived advantages of video over telephone in the ability to conduct routine pre-anesthesia evaluations among a sample of anesthesiology HCPs in the VA except for the perceived ability to assess nutritional status. HCPs with no telehealth experience cited the inability to perform a physical examination and obtain vital signs as the most significant barriers to implementation. Respondents not using telephone cited concerns about safety. Video visits in this clinical setting had additional perceived barriers to implementation, such as lack of information technology and staff support and patient-level barriers. Video had lower acceptability by HCPs. Given findings that pre-anesthesia evaluations can be conducted effectively via telehealth and have high levels of patient satisfaction, future work should focus on increasing uptake of these remote modalities. Additionally, research on the most appropriate uses of video visits within perioperative care is also needed.
1. Starsnic MA, Guarnieri DM, Norris MC. Efficacy and financial benefit of an anesthesiologist-directed university preadmission evaluation center. J Clin Anesth. 1997;9(4):299-305. doi:10.1016/s0952-8180(97)00007-x
2. Kristoffersen EW, Opsal A, Tveit TO, Berg RC, Fossum M. Effectiveness of pre-anaesthetic assessment clinic: a systematic review of randomised and non-randomised prospective controlled studies. BMJ Open. 2022;12(5):e054206. doi:10.1136/bmjopen-2021-054206
3. Ferschl MB, Tung A, Sweitzer B, Huo D, Glick DB. Preoperative clinic visits reduce operating room cancellations and delays. Anesthesiology. 2005;103(4):855-9. doi:10.1097/00000542-200510000-00025
4. Blitz JD, Kendale SM, Jain SK, Cuff GE, Kim JT, Rosenberg AD. preoperative evaluation clinic visit is associated with decreased risk of in-hospital postoperative mortality. Anesthesiology. 2016;125(2):280-294. doi:10.1097/ALN.0000000000001193
5. Dilisio RP, Dilisio AJ, Weiner MM. Preoperative virtual screening examination of the airway. J Clin Anesth. 2014;26(4):315-317. doi:10.1016/j.jclinane.2013.12.010
6. Kamdar NV, Huverserian A, Jalilian L, et al. Development, implementation, and evaluation of a telemedicine preoperative evaluation initiative at a major academic medical center. Anesth Analg. 2020;131(6):1647-1656. doi:10.1213/ANE.0000000000005208
7. Azizad O, Joshi GP. Telemedicine for preanesthesia evaluation: review of current literature and recommendations for future implementation. Curr Opin Anaesthesiol. 2021;34(6):672-677. doi:10.1097/ACO.0000000000001064
8. Mullen-Fortino M, Rising KL, Duckworth J, Gwynn V, Sites FD, Hollander JE. Presurgical assessment using telemedicine technology: impact on efficiency, effectiveness, and patient experience of care. Telemed J E Health. 2019;25(2):137-142. doi:10.1089/tmj.2017.0133
9. Zhang K, Rashid-Kolvear M, Waseem R, Englesakis M, Chung F. Virtual preoperative assessment in surgical patients: a systematic review and meta-analysis. J Clin Anesth. 2021;75:110540. doi:10.1016/j.jclinane.2021.110540
10. Mansournia MA, Collins GS, Nielsen RO, et al. A CHecklist for statistical Assessment of Medical Papers (the CHAMP statement): explanation and elaboration. Br J Sports Med. 2021;55(18):1009-1017. doi:10.1136/bjsports-2020-103652
11. von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg. 2014;12(12):1495-1499. doi:10.1016/j.ijsu.2014.07.013
12. Weiner BJ, Lewis CC, Stanick C, et al. Psychometric assessment of three newly developed implementation outcome measures. Implement Sci. 2017;12(1):108. doi:10.1186/s13012-017-0635-3
13. Proctor E, Silmere H, Raghavan R, et al. Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health. 2011;38(2):65-76. doi:10.1007/s10488-010-0319-7
14. Kuhn M, Johnson K. Applied Predictive Modeling. Springer; 2013.
15. Team RC. A language and environment for statistical computing. 2018. Accessed December 16, 2022. https://www.R-project.org
16. Wong DT, Kamming D, Salenieks ME, Go K, Kohm C, Chung F. Preadmission anesthesia consultation using telemedicine technology: a pilot study. Anesthesiology. 2004;100(6):1605-1607. doi:10.1097/00000542-200406000-00038
17. Zetterman CV, Sweitzer BJ, Webb B, Barak-Bernhagen MA, Boedeker BH. Validation of a virtual preoperative evaluation clinic: a pilot study. Stud Health Technol Inform. 2011;163:737-739. doi: 10.3233/978-1-60750-706-2-737
18. Roberts S, Spain B, Hicks C, London J, Tay S. Telemedicine in the Northern Territory: an assessment of patient perceptions in the preoperative anaesthetic clinic. Aust J Rural Health. 2015;23(3):136-141. doi:10.1111/ajr.12140
Days or weeks before a scheduled surgical or invasive procedure involving anesthesia, evaluations are conducted to assess a patient’s condition and risk, optimize their status, and prepare them for their procedure. A comprehensive pre-anesthesia evaluation visit includes a history of present illness, the evaluation of comorbidities and medication use, the assessment of health habits such as alcohol and tobacco use, functional capacity and nutritional assessments, and the identification of social support deficiencies that may influence recovery. It also includes a focused physical examination and laboratory and other ancillary testing as needed and may include optimization interventions such as anemia management or prehabilitation. Conducting pre-anesthesia evaluations before surgery has been shown to reduce delays and cancellations, unnecessary preprocedure testing, hospital length of stay, and in-hospital mortality.1-4
The pre-anesthesia evaluation is usually conducted in person, although other modalities have been in use for several years and have accelerated since the advent of the COVID-19 pandemic. Specifically, audio-only telephone visits are used in many settings to conduct abbreviated forms of a pre-anesthesia evaluation, typically for less-invasive procedures. When patients are evaluated over the telephone, the physical examination and testing are deferred until the day of the procedure. Another modality is the use of synchronous video telehealth. Emerging evidence for the use of video-based care in anesthesiology provides encouraging results. Several institutions have proven the technological feasibility of performing preoperative evaluations via video.5,6 Compared with in-person evaluations, these visits seem to have similar surgery cancellation rates, improved patient satisfaction, and reduced wait times and costs.7-9
As part of a quality improvement project, we studied the use of telehealth for pre-anesthesia evaluations within the US Department of Veterans Affairs (VA). An internal review found overall low utilization of these modalities before the COVID-19 pandemic that accelerated toward telehealth during the pandemic: The largest uptake was with telephone visits. Given the increasing adoption of telehealth for pre-anesthesia evaluations and the marked preference for telephone over video modalities among VA practitioners during the COVID-19 pandemic, we sought to understand the barriers and facilitators to the adoption of telephone- and video-based pre-anesthesia evaluation visits within the VA.
Methods
Our objective was to assess health care practitioners’ (HCPs) preferences regarding pre-anesthesia evaluation modalities (in-person, telephone, or video), and the perceived advantages and barriers to adoption for each modality. We followed the Strengthening the Reporting of Observational studies in Epidemiology (STROBE) guideline and Checklist for statistical Assessment of Medical Papers (CHAMP) statement.10,11 The survey was deemed a quality improvement activity that was exempt from institutional review board oversight by the VA National Anesthesia Program Office and the VA Office of Connected Care.
A survey was distributed to all VA anesthesiology service chiefs via email between April 27, 2022, and May 3, 2022. Three emails were sent to each participant (initial invitation and 2 reminders). The respondents were asked to identify themselves by facility and role and to indicate whether their anesthesiology service performed any pre-anesthesia evaluations, including any telephone- or video-based evaluations; and whether their service has a dedicated pre-anesthesia evaluation clinic.
A second set of questions referred to the use of telephone- and video-based preprocedure evaluations. The questions were based on branch logic and depended on the respondent’s answers concerning their use of telephone- and video-based evaluations. Questions included statements about perceived barriers to the adoption of these pre-anesthesia evaluation modalities. Each item was rated on a 5-point Likert scale, (completely disagree [1] to completely agree [5]). A third section measured acceptability and feasibility of video using the validated Acceptability of Intervention Measure (AIM) and Feasibility of Intervention Measure (FIM)questionnaires.12 These instruments are 4-item measures of implementation outcomes that are often considered indicators of implementation success.13Acceptability is the perception among implementation stakeholders that a given treatment, service, practice, or innovation is agreeable, palatable, or satisfactory. Feasibility is defined as the extent to which a new treatment or an innovation can be successfully used or carried out within a given agency or setting.13 The criterion for acceptability is personal, meaning that different HCPs may have differing needs, preferences, and expectations regarding the same intervention. The criterion for feasibility is practical. An intervention may be considered to be feasible if the required tasks can be performed easily or conveniently. Finally, 2 open-ended questions allowed respondents to identify the most important factor that allowed the implementation of telehealth for pre-anesthesia evaluations in their service, and provide comments about the use of telehealth for pre-anesthesia evaluations at the VA. All questions were developed by the authors except for the 2 implementation measure instruments.
The survey was administered using an electronic survey platform (Qualtrics, version April 2022) and sent by email alongside a brief introductory video. Participation was voluntary and anonymous, as no personal information was collected. Responses were attributed to each facility, using the self-declared affiliation. When an affiliation was not provided, we deduced it using the latitude/longitude of the respondent, a feature included in the survey software. No incentives were provided. Data were stored and maintained in a secure VA server. All completed surveys were included. Some facilities had > 1 complete response, and all were included. Facilities that provided > 1 response and where responses were discordant, we clarified with the facility service chief. Incomplete responses were excluded from the analysis.
Statistics
For this analysis, the 2 positive sentiment responses (agree and completely agree) and the 2 negative sentiment responses (disagree and completely disagree) in the Likert scale were collapsed into single categories (good and poor, respectively). The neither agree nor disagree responses were coded as neutral. Our analysis began with a visual exploration of all variables to evaluate the frequency, percentage, and near-zero variance for categorical variables.14 Near-zero variance occurs when a categorical variable has a low frequency of unique values over the sample size (ie, the variable is almost constant), and we addressed it by combining different variable categorizations. We handled missing values through imputation algorithms followed by sensitivity analyses to verify whether our results were stable with and without imputation. We performed comparisons for the exploratory analysis using P values for one-way analysis of variance tests for numeric variables and χ2tests for categorical variables. We considered P values < .05 to be statistically significant. We also used correlation matrices and plots as exploratory analysis tools to better understand all items’ correlations. We used Pearson, polychoric, and polyserial correlation tests as appropriate for numeric, ordinal, and logical items.
Our modeling strategy involved a series of generalized linear models (GLMs) with a Gaussian family, ie, multiple linear regression models, to assess the association between (1) facilities’ preferences regarding pre-anesthesia evaluation modalities; (2) advantages between modalities; and (3) barriers to the adoption of telehealth and the ability to perform different pre-anesthesia evaluation-related tasks. In addition, we used backward deletion to reach the most parsimonious model based on a series of likelihood-ratio tests comparing nested models. Results are reported as predicted means with 95% confidence intervals, with results being interpreted as significant when any 2 predicted means do not overlap between different estimates along with P for trends < .001. We performed all analyses using the R language.15
Results
Of 109 surveyed facilities, 50 (46%) responded to the survey. The final study sample included 67 responses, and 55 were included in the analysis. Twelve responses were excluded from the analysis as they were either incomplete or test responses. Three facilities had > 1 complete response (2 facilities had 2 responses and 1 facility had 4 responses), and these were all included in the analysis.
Thirty-six locations were complex inpatient facilities, and 32 (89%) had pre-anesthesia evaluation clinics (Table 1).
The ability to obtain a history of present illness was rated good/very good via telephone for 34 respondents (92%) and 25 for video (86%). Assessing comorbidities and health habits was rated good/very good via telephone for 32 respondents (89%) and 31 respondents (86%), respectively, and via video for 24 respondents (83%) and 23 respondents (79%), respectively (Figure 1).
To compare differences between the 2 remote pre-anesthesia evaluation modalities, we created GLMs evaluating the association between each modality and the perceived ability to perform the tasks. For GLMs, we transformed the values of the categories into numerical (ie, 1, poor; 2, neutral; 3, good). Compared with telephone, video was rated more favorably regarding the assessment of nutritional status (mean, 2.1; 95% CI, 1.8-2.3 vs mean, 2.4; 95% CI, 2.2-2.7; P = .04) (eAppendix 1, available at doi:10.12788/fp.0387). No other significant differences in ratings existed between the 2 remote pre-anesthesia evaluation modalities.
The most significant barriers (cited as significant or very significant in the survey) included the inability to perform a physical examination, which was noted by 13 respondents (72%) and 15 respondents (60%) for telephone and video, respectively. The inability to obtain vital signs was rated as a significant barrier for telephone by 12 respondents (67%) and for video by 15 respondents (60%)(Figure 2).
The average FIM score was 3.7, with the highest score among respondents who used both phone and video (Table 2). The average AIM score was 3.4, with the highest score among respondents who used both telehealth modalities. The internal consistency of the implementation measures was excellent (Cronbach’s α 0.95 and 0.975 for FIM and AIM, respectively).
Discussion
We surveyed 109 anesthesiology services across the VA regarding barriers to implementing telephone- and video-based pre-anesthesia evaluation visits. We found that 12 (23%) of the 50 anesthesiology services responding to this survey still conduct the totality of their pre-anesthesia evaluations in person. This represents an opportunity to further disseminate the appropriate use of telehealth and potentially reduce travel time, costs, and low-value testing, as it is well established that remote pre-anesthesia evaluations for low-risk procedures are safe and effective.6
We also found no difference between telephone and video regarding users’ perceived ability to perform any of the basic pre-anesthesia evaluation tasks except for assessing patients’ nutritional status, which was rated as easier using video than telephone. According to those not using telephone and/or video, the biggest barriers to implementation of telehealth visits were the inability to obtain vital signs and to perform a physical examination. This finding was unexpected, as facilities that conduct remote evaluations typically defer these tasks to the day of surgery, a practice that has been well established and shown to be safe and efficient. Respondents also identified patient-level factors (eg, patient preference, lack of telephone or computer) as significant barriers. Finally, feasibility ratings were higher than acceptability ratings with regards to the implementation of telehealth.
In 2004, the first use of telehealth for pre-anesthesia evaluations was reported by Wong and colleagues.16 Since then, several case series and a literature review have documented the efficacy, safety, and patient and HCP satisfaction with the use of telehealth for pre-anesthesia evaluations. A study by Mullen-Fortino and colleagues showed reduced visit times when telehealth was used for pre-anesthesia evaluation.8 Another study at VA hospitals showed that 88% of veterans reported that telemedicine saved them time and money.17 A report of 35 patients in rural Australia reported 98% satisfaction with the video quality of the visit, 95% perceived efficacy, and 87% preference for telehealth compared with driving to be seen in person.18 These reports conflict with the perceptions of the respondents of our survey, who identified patient preference as an important barrier to adoption of telehealth. Given these findings, research is needed on veterans’ perceptions on the use of telehealth modalities for pre-anesthesia evaluations; if their perceptions are similarly favorable, it will be important to communicate this information to HCPs and leadership, which may help increase subsequent telehealth adoption.
Despite the reported safety, efficacy, and high satisfaction of video visits among anesthesiology teams conducting pre-anesthesia evaluations, its use remains low at VA. We have found that most facilities in the VA system chose telephone platforms during the COVID-19 pandemic. One possibility is that the adoption of video modalities among pre-anesthesia evaluation clinics in the VA system is resource intensive or difficult from the HCP’s perspective. When combined with the lack of perceived advantages over telephone as we found in our survey, most practitioners resort to the technologically less demanding and more familiar telephone platform. The results from FIM and AIM support this. While both telephone and video have high feasibility scores, acceptability scores are lower for video, even among those currently using this technology. Our findings do not rule out the utility of video-based care in perioperative medicine. Rather than a yes/no proposition, future studies need to establish the precise indications for video for pre-anesthesia evaluations; that is, situations where video visits offer an advantage over telephone. For example, video could be used to deliver preoperative optimization therapies, such as supervised exercise or mental health interventions or to guide the achievement of certain milestones before surgery in patients with chronic conditions, such as target glucose values or the treatment of anemia. Future studies should explore the perceived benefits of video over telephone among centers offering these more advanced optimization interventions.
Limitations
We received responses from a subset of VA anesthesiology services; therefore, they may not be representative of the entire VA system. Facilities designated by the VA as inpatient complex were overrepresented (72% of our sample vs 50% of the total facilities nationally), and ambulatory centers (those designed by the VA as ambulatory procedural center with basic or advanced capabilities) were underrepresented (2% of our sample vs 22% nationally). Despite this, the response rate was high, and no geographic area appeared to be underrepresented. In addition, we surveyed pre-anesthesia evaluation facilities led by anesthesiologists, and the results may not be representative of the preferences of HCPs working in nonanesthesiology led pre-anesthesia evaluation clinics. Finally, just 11 facilities used both telephone and video; therefore, a true direct comparison between these 2 platforms was limited. The VA serves a unique patient population, and the findings may not be completely applicable to the non-VA population.
Conclusions
We found no significant perceived advantages of video over telephone in the ability to conduct routine pre-anesthesia evaluations among a sample of anesthesiology HCPs in the VA except for the perceived ability to assess nutritional status. HCPs with no telehealth experience cited the inability to perform a physical examination and obtain vital signs as the most significant barriers to implementation. Respondents not using telephone cited concerns about safety. Video visits in this clinical setting had additional perceived barriers to implementation, such as lack of information technology and staff support and patient-level barriers. Video had lower acceptability by HCPs. Given findings that pre-anesthesia evaluations can be conducted effectively via telehealth and have high levels of patient satisfaction, future work should focus on increasing uptake of these remote modalities. Additionally, research on the most appropriate uses of video visits within perioperative care is also needed.
Days or weeks before a scheduled surgical or invasive procedure involving anesthesia, evaluations are conducted to assess a patient’s condition and risk, optimize their status, and prepare them for their procedure. A comprehensive pre-anesthesia evaluation visit includes a history of present illness, the evaluation of comorbidities and medication use, the assessment of health habits such as alcohol and tobacco use, functional capacity and nutritional assessments, and the identification of social support deficiencies that may influence recovery. It also includes a focused physical examination and laboratory and other ancillary testing as needed and may include optimization interventions such as anemia management or prehabilitation. Conducting pre-anesthesia evaluations before surgery has been shown to reduce delays and cancellations, unnecessary preprocedure testing, hospital length of stay, and in-hospital mortality.1-4
The pre-anesthesia evaluation is usually conducted in person, although other modalities have been in use for several years and have accelerated since the advent of the COVID-19 pandemic. Specifically, audio-only telephone visits are used in many settings to conduct abbreviated forms of a pre-anesthesia evaluation, typically for less-invasive procedures. When patients are evaluated over the telephone, the physical examination and testing are deferred until the day of the procedure. Another modality is the use of synchronous video telehealth. Emerging evidence for the use of video-based care in anesthesiology provides encouraging results. Several institutions have proven the technological feasibility of performing preoperative evaluations via video.5,6 Compared with in-person evaluations, these visits seem to have similar surgery cancellation rates, improved patient satisfaction, and reduced wait times and costs.7-9
As part of a quality improvement project, we studied the use of telehealth for pre-anesthesia evaluations within the US Department of Veterans Affairs (VA). An internal review found overall low utilization of these modalities before the COVID-19 pandemic that accelerated toward telehealth during the pandemic: The largest uptake was with telephone visits. Given the increasing adoption of telehealth for pre-anesthesia evaluations and the marked preference for telephone over video modalities among VA practitioners during the COVID-19 pandemic, we sought to understand the barriers and facilitators to the adoption of telephone- and video-based pre-anesthesia evaluation visits within the VA.
Methods
Our objective was to assess health care practitioners’ (HCPs) preferences regarding pre-anesthesia evaluation modalities (in-person, telephone, or video), and the perceived advantages and barriers to adoption for each modality. We followed the Strengthening the Reporting of Observational studies in Epidemiology (STROBE) guideline and Checklist for statistical Assessment of Medical Papers (CHAMP) statement.10,11 The survey was deemed a quality improvement activity that was exempt from institutional review board oversight by the VA National Anesthesia Program Office and the VA Office of Connected Care.
A survey was distributed to all VA anesthesiology service chiefs via email between April 27, 2022, and May 3, 2022. Three emails were sent to each participant (initial invitation and 2 reminders). The respondents were asked to identify themselves by facility and role and to indicate whether their anesthesiology service performed any pre-anesthesia evaluations, including any telephone- or video-based evaluations; and whether their service has a dedicated pre-anesthesia evaluation clinic.
A second set of questions referred to the use of telephone- and video-based preprocedure evaluations. The questions were based on branch logic and depended on the respondent’s answers concerning their use of telephone- and video-based evaluations. Questions included statements about perceived barriers to the adoption of these pre-anesthesia evaluation modalities. Each item was rated on a 5-point Likert scale, (completely disagree [1] to completely agree [5]). A third section measured acceptability and feasibility of video using the validated Acceptability of Intervention Measure (AIM) and Feasibility of Intervention Measure (FIM)questionnaires.12 These instruments are 4-item measures of implementation outcomes that are often considered indicators of implementation success.13Acceptability is the perception among implementation stakeholders that a given treatment, service, practice, or innovation is agreeable, palatable, or satisfactory. Feasibility is defined as the extent to which a new treatment or an innovation can be successfully used or carried out within a given agency or setting.13 The criterion for acceptability is personal, meaning that different HCPs may have differing needs, preferences, and expectations regarding the same intervention. The criterion for feasibility is practical. An intervention may be considered to be feasible if the required tasks can be performed easily or conveniently. Finally, 2 open-ended questions allowed respondents to identify the most important factor that allowed the implementation of telehealth for pre-anesthesia evaluations in their service, and provide comments about the use of telehealth for pre-anesthesia evaluations at the VA. All questions were developed by the authors except for the 2 implementation measure instruments.
The survey was administered using an electronic survey platform (Qualtrics, version April 2022) and sent by email alongside a brief introductory video. Participation was voluntary and anonymous, as no personal information was collected. Responses were attributed to each facility, using the self-declared affiliation. When an affiliation was not provided, we deduced it using the latitude/longitude of the respondent, a feature included in the survey software. No incentives were provided. Data were stored and maintained in a secure VA server. All completed surveys were included. Some facilities had > 1 complete response, and all were included. Facilities that provided > 1 response and where responses were discordant, we clarified with the facility service chief. Incomplete responses were excluded from the analysis.
Statistics
For this analysis, the 2 positive sentiment responses (agree and completely agree) and the 2 negative sentiment responses (disagree and completely disagree) in the Likert scale were collapsed into single categories (good and poor, respectively). The neither agree nor disagree responses were coded as neutral. Our analysis began with a visual exploration of all variables to evaluate the frequency, percentage, and near-zero variance for categorical variables.14 Near-zero variance occurs when a categorical variable has a low frequency of unique values over the sample size (ie, the variable is almost constant), and we addressed it by combining different variable categorizations. We handled missing values through imputation algorithms followed by sensitivity analyses to verify whether our results were stable with and without imputation. We performed comparisons for the exploratory analysis using P values for one-way analysis of variance tests for numeric variables and χ2tests for categorical variables. We considered P values < .05 to be statistically significant. We also used correlation matrices and plots as exploratory analysis tools to better understand all items’ correlations. We used Pearson, polychoric, and polyserial correlation tests as appropriate for numeric, ordinal, and logical items.
Our modeling strategy involved a series of generalized linear models (GLMs) with a Gaussian family, ie, multiple linear regression models, to assess the association between (1) facilities’ preferences regarding pre-anesthesia evaluation modalities; (2) advantages between modalities; and (3) barriers to the adoption of telehealth and the ability to perform different pre-anesthesia evaluation-related tasks. In addition, we used backward deletion to reach the most parsimonious model based on a series of likelihood-ratio tests comparing nested models. Results are reported as predicted means with 95% confidence intervals, with results being interpreted as significant when any 2 predicted means do not overlap between different estimates along with P for trends < .001. We performed all analyses using the R language.15
Results
Of 109 surveyed facilities, 50 (46%) responded to the survey. The final study sample included 67 responses, and 55 were included in the analysis. Twelve responses were excluded from the analysis as they were either incomplete or test responses. Three facilities had > 1 complete response (2 facilities had 2 responses and 1 facility had 4 responses), and these were all included in the analysis.
Thirty-six locations were complex inpatient facilities, and 32 (89%) had pre-anesthesia evaluation clinics (Table 1).
The ability to obtain a history of present illness was rated good/very good via telephone for 34 respondents (92%) and 25 for video (86%). Assessing comorbidities and health habits was rated good/very good via telephone for 32 respondents (89%) and 31 respondents (86%), respectively, and via video for 24 respondents (83%) and 23 respondents (79%), respectively (Figure 1).
To compare differences between the 2 remote pre-anesthesia evaluation modalities, we created GLMs evaluating the association between each modality and the perceived ability to perform the tasks. For GLMs, we transformed the values of the categories into numerical (ie, 1, poor; 2, neutral; 3, good). Compared with telephone, video was rated more favorably regarding the assessment of nutritional status (mean, 2.1; 95% CI, 1.8-2.3 vs mean, 2.4; 95% CI, 2.2-2.7; P = .04) (eAppendix 1, available at doi:10.12788/fp.0387). No other significant differences in ratings existed between the 2 remote pre-anesthesia evaluation modalities.
The most significant barriers (cited as significant or very significant in the survey) included the inability to perform a physical examination, which was noted by 13 respondents (72%) and 15 respondents (60%) for telephone and video, respectively. The inability to obtain vital signs was rated as a significant barrier for telephone by 12 respondents (67%) and for video by 15 respondents (60%)(Figure 2).
The average FIM score was 3.7, with the highest score among respondents who used both phone and video (Table 2). The average AIM score was 3.4, with the highest score among respondents who used both telehealth modalities. The internal consistency of the implementation measures was excellent (Cronbach’s α 0.95 and 0.975 for FIM and AIM, respectively).
Discussion
We surveyed 109 anesthesiology services across the VA regarding barriers to implementing telephone- and video-based pre-anesthesia evaluation visits. We found that 12 (23%) of the 50 anesthesiology services responding to this survey still conduct the totality of their pre-anesthesia evaluations in person. This represents an opportunity to further disseminate the appropriate use of telehealth and potentially reduce travel time, costs, and low-value testing, as it is well established that remote pre-anesthesia evaluations for low-risk procedures are safe and effective.6
We also found no difference between telephone and video regarding users’ perceived ability to perform any of the basic pre-anesthesia evaluation tasks except for assessing patients’ nutritional status, which was rated as easier using video than telephone. According to those not using telephone and/or video, the biggest barriers to implementation of telehealth visits were the inability to obtain vital signs and to perform a physical examination. This finding was unexpected, as facilities that conduct remote evaluations typically defer these tasks to the day of surgery, a practice that has been well established and shown to be safe and efficient. Respondents also identified patient-level factors (eg, patient preference, lack of telephone or computer) as significant barriers. Finally, feasibility ratings were higher than acceptability ratings with regards to the implementation of telehealth.
In 2004, the first use of telehealth for pre-anesthesia evaluations was reported by Wong and colleagues.16 Since then, several case series and a literature review have documented the efficacy, safety, and patient and HCP satisfaction with the use of telehealth for pre-anesthesia evaluations. A study by Mullen-Fortino and colleagues showed reduced visit times when telehealth was used for pre-anesthesia evaluation.8 Another study at VA hospitals showed that 88% of veterans reported that telemedicine saved them time and money.17 A report of 35 patients in rural Australia reported 98% satisfaction with the video quality of the visit, 95% perceived efficacy, and 87% preference for telehealth compared with driving to be seen in person.18 These reports conflict with the perceptions of the respondents of our survey, who identified patient preference as an important barrier to adoption of telehealth. Given these findings, research is needed on veterans’ perceptions on the use of telehealth modalities for pre-anesthesia evaluations; if their perceptions are similarly favorable, it will be important to communicate this information to HCPs and leadership, which may help increase subsequent telehealth adoption.
Despite the reported safety, efficacy, and high satisfaction of video visits among anesthesiology teams conducting pre-anesthesia evaluations, its use remains low at VA. We have found that most facilities in the VA system chose telephone platforms during the COVID-19 pandemic. One possibility is that the adoption of video modalities among pre-anesthesia evaluation clinics in the VA system is resource intensive or difficult from the HCP’s perspective. When combined with the lack of perceived advantages over telephone as we found in our survey, most practitioners resort to the technologically less demanding and more familiar telephone platform. The results from FIM and AIM support this. While both telephone and video have high feasibility scores, acceptability scores are lower for video, even among those currently using this technology. Our findings do not rule out the utility of video-based care in perioperative medicine. Rather than a yes/no proposition, future studies need to establish the precise indications for video for pre-anesthesia evaluations; that is, situations where video visits offer an advantage over telephone. For example, video could be used to deliver preoperative optimization therapies, such as supervised exercise or mental health interventions or to guide the achievement of certain milestones before surgery in patients with chronic conditions, such as target glucose values or the treatment of anemia. Future studies should explore the perceived benefits of video over telephone among centers offering these more advanced optimization interventions.
Limitations
We received responses from a subset of VA anesthesiology services; therefore, they may not be representative of the entire VA system. Facilities designated by the VA as inpatient complex were overrepresented (72% of our sample vs 50% of the total facilities nationally), and ambulatory centers (those designed by the VA as ambulatory procedural center with basic or advanced capabilities) were underrepresented (2% of our sample vs 22% nationally). Despite this, the response rate was high, and no geographic area appeared to be underrepresented. In addition, we surveyed pre-anesthesia evaluation facilities led by anesthesiologists, and the results may not be representative of the preferences of HCPs working in nonanesthesiology led pre-anesthesia evaluation clinics. Finally, just 11 facilities used both telephone and video; therefore, a true direct comparison between these 2 platforms was limited. The VA serves a unique patient population, and the findings may not be completely applicable to the non-VA population.
Conclusions
We found no significant perceived advantages of video over telephone in the ability to conduct routine pre-anesthesia evaluations among a sample of anesthesiology HCPs in the VA except for the perceived ability to assess nutritional status. HCPs with no telehealth experience cited the inability to perform a physical examination and obtain vital signs as the most significant barriers to implementation. Respondents not using telephone cited concerns about safety. Video visits in this clinical setting had additional perceived barriers to implementation, such as lack of information technology and staff support and patient-level barriers. Video had lower acceptability by HCPs. Given findings that pre-anesthesia evaluations can be conducted effectively via telehealth and have high levels of patient satisfaction, future work should focus on increasing uptake of these remote modalities. Additionally, research on the most appropriate uses of video visits within perioperative care is also needed.
1. Starsnic MA, Guarnieri DM, Norris MC. Efficacy and financial benefit of an anesthesiologist-directed university preadmission evaluation center. J Clin Anesth. 1997;9(4):299-305. doi:10.1016/s0952-8180(97)00007-x
2. Kristoffersen EW, Opsal A, Tveit TO, Berg RC, Fossum M. Effectiveness of pre-anaesthetic assessment clinic: a systematic review of randomised and non-randomised prospective controlled studies. BMJ Open. 2022;12(5):e054206. doi:10.1136/bmjopen-2021-054206
3. Ferschl MB, Tung A, Sweitzer B, Huo D, Glick DB. Preoperative clinic visits reduce operating room cancellations and delays. Anesthesiology. 2005;103(4):855-9. doi:10.1097/00000542-200510000-00025
4. Blitz JD, Kendale SM, Jain SK, Cuff GE, Kim JT, Rosenberg AD. preoperative evaluation clinic visit is associated with decreased risk of in-hospital postoperative mortality. Anesthesiology. 2016;125(2):280-294. doi:10.1097/ALN.0000000000001193
5. Dilisio RP, Dilisio AJ, Weiner MM. Preoperative virtual screening examination of the airway. J Clin Anesth. 2014;26(4):315-317. doi:10.1016/j.jclinane.2013.12.010
6. Kamdar NV, Huverserian A, Jalilian L, et al. Development, implementation, and evaluation of a telemedicine preoperative evaluation initiative at a major academic medical center. Anesth Analg. 2020;131(6):1647-1656. doi:10.1213/ANE.0000000000005208
7. Azizad O, Joshi GP. Telemedicine for preanesthesia evaluation: review of current literature and recommendations for future implementation. Curr Opin Anaesthesiol. 2021;34(6):672-677. doi:10.1097/ACO.0000000000001064
8. Mullen-Fortino M, Rising KL, Duckworth J, Gwynn V, Sites FD, Hollander JE. Presurgical assessment using telemedicine technology: impact on efficiency, effectiveness, and patient experience of care. Telemed J E Health. 2019;25(2):137-142. doi:10.1089/tmj.2017.0133
9. Zhang K, Rashid-Kolvear M, Waseem R, Englesakis M, Chung F. Virtual preoperative assessment in surgical patients: a systematic review and meta-analysis. J Clin Anesth. 2021;75:110540. doi:10.1016/j.jclinane.2021.110540
10. Mansournia MA, Collins GS, Nielsen RO, et al. A CHecklist for statistical Assessment of Medical Papers (the CHAMP statement): explanation and elaboration. Br J Sports Med. 2021;55(18):1009-1017. doi:10.1136/bjsports-2020-103652
11. von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg. 2014;12(12):1495-1499. doi:10.1016/j.ijsu.2014.07.013
12. Weiner BJ, Lewis CC, Stanick C, et al. Psychometric assessment of three newly developed implementation outcome measures. Implement Sci. 2017;12(1):108. doi:10.1186/s13012-017-0635-3
13. Proctor E, Silmere H, Raghavan R, et al. Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health. 2011;38(2):65-76. doi:10.1007/s10488-010-0319-7
14. Kuhn M, Johnson K. Applied Predictive Modeling. Springer; 2013.
15. Team RC. A language and environment for statistical computing. 2018. Accessed December 16, 2022. https://www.R-project.org
16. Wong DT, Kamming D, Salenieks ME, Go K, Kohm C, Chung F. Preadmission anesthesia consultation using telemedicine technology: a pilot study. Anesthesiology. 2004;100(6):1605-1607. doi:10.1097/00000542-200406000-00038
17. Zetterman CV, Sweitzer BJ, Webb B, Barak-Bernhagen MA, Boedeker BH. Validation of a virtual preoperative evaluation clinic: a pilot study. Stud Health Technol Inform. 2011;163:737-739. doi: 10.3233/978-1-60750-706-2-737
18. Roberts S, Spain B, Hicks C, London J, Tay S. Telemedicine in the Northern Territory: an assessment of patient perceptions in the preoperative anaesthetic clinic. Aust J Rural Health. 2015;23(3):136-141. doi:10.1111/ajr.12140
1. Starsnic MA, Guarnieri DM, Norris MC. Efficacy and financial benefit of an anesthesiologist-directed university preadmission evaluation center. J Clin Anesth. 1997;9(4):299-305. doi:10.1016/s0952-8180(97)00007-x
2. Kristoffersen EW, Opsal A, Tveit TO, Berg RC, Fossum M. Effectiveness of pre-anaesthetic assessment clinic: a systematic review of randomised and non-randomised prospective controlled studies. BMJ Open. 2022;12(5):e054206. doi:10.1136/bmjopen-2021-054206
3. Ferschl MB, Tung A, Sweitzer B, Huo D, Glick DB. Preoperative clinic visits reduce operating room cancellations and delays. Anesthesiology. 2005;103(4):855-9. doi:10.1097/00000542-200510000-00025
4. Blitz JD, Kendale SM, Jain SK, Cuff GE, Kim JT, Rosenberg AD. preoperative evaluation clinic visit is associated with decreased risk of in-hospital postoperative mortality. Anesthesiology. 2016;125(2):280-294. doi:10.1097/ALN.0000000000001193
5. Dilisio RP, Dilisio AJ, Weiner MM. Preoperative virtual screening examination of the airway. J Clin Anesth. 2014;26(4):315-317. doi:10.1016/j.jclinane.2013.12.010
6. Kamdar NV, Huverserian A, Jalilian L, et al. Development, implementation, and evaluation of a telemedicine preoperative evaluation initiative at a major academic medical center. Anesth Analg. 2020;131(6):1647-1656. doi:10.1213/ANE.0000000000005208
7. Azizad O, Joshi GP. Telemedicine for preanesthesia evaluation: review of current literature and recommendations for future implementation. Curr Opin Anaesthesiol. 2021;34(6):672-677. doi:10.1097/ACO.0000000000001064
8. Mullen-Fortino M, Rising KL, Duckworth J, Gwynn V, Sites FD, Hollander JE. Presurgical assessment using telemedicine technology: impact on efficiency, effectiveness, and patient experience of care. Telemed J E Health. 2019;25(2):137-142. doi:10.1089/tmj.2017.0133
9. Zhang K, Rashid-Kolvear M, Waseem R, Englesakis M, Chung F. Virtual preoperative assessment in surgical patients: a systematic review and meta-analysis. J Clin Anesth. 2021;75:110540. doi:10.1016/j.jclinane.2021.110540
10. Mansournia MA, Collins GS, Nielsen RO, et al. A CHecklist for statistical Assessment of Medical Papers (the CHAMP statement): explanation and elaboration. Br J Sports Med. 2021;55(18):1009-1017. doi:10.1136/bjsports-2020-103652
11. von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg. 2014;12(12):1495-1499. doi:10.1016/j.ijsu.2014.07.013
12. Weiner BJ, Lewis CC, Stanick C, et al. Psychometric assessment of three newly developed implementation outcome measures. Implement Sci. 2017;12(1):108. doi:10.1186/s13012-017-0635-3
13. Proctor E, Silmere H, Raghavan R, et al. Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health. 2011;38(2):65-76. doi:10.1007/s10488-010-0319-7
14. Kuhn M, Johnson K. Applied Predictive Modeling. Springer; 2013.
15. Team RC. A language and environment for statistical computing. 2018. Accessed December 16, 2022. https://www.R-project.org
16. Wong DT, Kamming D, Salenieks ME, Go K, Kohm C, Chung F. Preadmission anesthesia consultation using telemedicine technology: a pilot study. Anesthesiology. 2004;100(6):1605-1607. doi:10.1097/00000542-200406000-00038
17. Zetterman CV, Sweitzer BJ, Webb B, Barak-Bernhagen MA, Boedeker BH. Validation of a virtual preoperative evaluation clinic: a pilot study. Stud Health Technol Inform. 2011;163:737-739. doi: 10.3233/978-1-60750-706-2-737
18. Roberts S, Spain B, Hicks C, London J, Tay S. Telemedicine in the Northern Territory: an assessment of patient perceptions in the preoperative anaesthetic clinic. Aust J Rural Health. 2015;23(3):136-141. doi:10.1111/ajr.12140
Dermatology Author Gender Trends During the COVID-19 Pandemic
To the Editor:
Peer-reviewed publications are important determinants for promotions, academic leadership, and grants in dermatology.1 The impact of the COVID-19 pandemic on dermatology research productivity remains an area of investigation. We sought to determine authorship trends for males and females during the pandemic.
A cross-sectional retrospective study of the top 20 dermatology journals—determined by impact factor and Google Scholar H5-index—was conducted to identify manuscripts with submission date specified prepandemic (May 1, 2019–October 31, 2019) and during the pandemic (May 1, 2020–October 31, 2020). Submission date, first/last author name, sex, and affiliated country were extracted. Single authors were designated as first authors. Gender API (https://gender-api.com/en/) classified gender. A χ2 test (P<.05) compared differences in proportions of female first/last authors from 2019 to 2020.
Overall, 811 and 1061 articles submitted in 2019 and 2020, respectively, were included. There were 1517 articles submitted to clinical journals and 355 articles submitted to basic science journals (Table). For the 7 clinical journals included, there was a 7.7% decrease in the proportion of female last authors in 2020 vs 2019 (P=.002), with the largest decrease between August and September 2020. Although other comparisons did not yield statistically significant differences (P>.05 all)(Table), several trends were observed. For clinical journals, there was a 1.8% decrease in the proportion of female first authors. For the 4 basic science journals included, there was a 4.9% increase and a 0.3% decrease in percentages of female first and last authors, respectively, for 2020 vs 2019.
Our findings indicate that the COVID-19 pandemic may have impacted female authors’ productivity in clinical dermatology publications. In a survey-based study for 2010 to 2011, female physician-researchers (n=437) spent 8.5 more hours per week on domestic activities and childcare and were more likely to take time off for childcare if their partner worked full time compared with males (n=612)(42.6% vs 12.4%, respectively).2 Our observation that female last authors had a significant decrease in publications may suggest that this population had a disproportionate burden of domestic labor and childcare during the pandemic. It is possible that last authors, who generally are more senior researchers, may be more likely to have childcare, eldercare, and other types of domestic responsibilities. Similarly, in a study of surgery submissions (n=1068), there were 6%, 7%, and 4% decreases in percentages of female last, corresponding, and first authors, respectively, from 2019 to 2020.3Our study had limitations. Only 11 journals were analyzed because others did not have specified submission dates. Some journals only provided submission information for a subset of articles (eg, those published in the In Press section), which may have accounted for the large discrepancy in submission numbers for 2019 to 2020. Gender could not be determined for 1% of authors and was limited to female and male. Although our study submission time frame (May–October 2020) aimed at identifying research conducted during the height of the COVID-19 pandemic, some of these studies may have been conducted months or years before the pandemic. Future studies should focus on longer and more comprehensive time frames. Finally, estimated dates of stay-at-home orders fail to consider differences within countries.
The proportion of female US-affiliated first and last authors publishing in dermatology journals increased from 12% to 48% in 1976 and from 6% to 31% in 2006,4 which is encouraging. However, a gender gap persists, with one-third of National Institutes of Health grants in dermatology and one-fourth of research project grants in dermatology awarded to women.5 Consequences of the pandemic on academic productivity may include fewer women represented in higher academic ranks, lower compensation, and lower career satisfaction compared with men.1 We urge academic institutions and funding agencies to recognize and take action to mitigate long-term sequelae. Extended grant end dates and submission periods, funding opportunities dedicated to women, and prioritization of female-authored submissions are some strategies that can safeguard equitable career progression in dermatology research.
- Stewart C, Lipner SR. Gender and race trends in academic rank of dermatologists at top U.S. institutions: a cross-sectional study. Int J Womens Dermatol. 2020;6:283-285. doi:10.1016/j .ijwd.2020.04.010
- Jolly S, Griffith KA, DeCastro R, et al. Gender differences in time spent on parenting and domestic responsibilities by highachieving young physician-researchers. Ann Intern Med. 2014; 160:344-353. doi:10.7326/M13-0974
- Kibbe MR. Consequences of the COVID-19 pandemic on manuscript submissions by women. JAMA Surg. 2020;155:803-804. doi:10.1001/jamasurg.2020.3917
- Feramisco JD, Leitenberger JJ, Redfern SI, et al. A gender gap in the dermatology literature? cross-sectional analysis of manuscript authorship trends in dermatology journals during 3 decades. J Am Acad Dermatol. 2009;6:63-69. doi:10.1016/j.jaad.2008.06.044
- Cheng MY, Sukhov A, Sultani H, et al. Trends in national institutes of health funding of principal investigators in dermatology research by academic degree and sex. JAMA Dermatol. 2016;152:883-888. doi:10.1001/jamadermatol.2016.0271
To the Editor:
Peer-reviewed publications are important determinants for promotions, academic leadership, and grants in dermatology.1 The impact of the COVID-19 pandemic on dermatology research productivity remains an area of investigation. We sought to determine authorship trends for males and females during the pandemic.
A cross-sectional retrospective study of the top 20 dermatology journals—determined by impact factor and Google Scholar H5-index—was conducted to identify manuscripts with submission date specified prepandemic (May 1, 2019–October 31, 2019) and during the pandemic (May 1, 2020–October 31, 2020). Submission date, first/last author name, sex, and affiliated country were extracted. Single authors were designated as first authors. Gender API (https://gender-api.com/en/) classified gender. A χ2 test (P<.05) compared differences in proportions of female first/last authors from 2019 to 2020.
Overall, 811 and 1061 articles submitted in 2019 and 2020, respectively, were included. There were 1517 articles submitted to clinical journals and 355 articles submitted to basic science journals (Table). For the 7 clinical journals included, there was a 7.7% decrease in the proportion of female last authors in 2020 vs 2019 (P=.002), with the largest decrease between August and September 2020. Although other comparisons did not yield statistically significant differences (P>.05 all)(Table), several trends were observed. For clinical journals, there was a 1.8% decrease in the proportion of female first authors. For the 4 basic science journals included, there was a 4.9% increase and a 0.3% decrease in percentages of female first and last authors, respectively, for 2020 vs 2019.

Our findings indicate that the COVID-19 pandemic may have impacted female authors’ productivity in clinical dermatology publications. In a survey-based study for 2010 to 2011, female physician-researchers (n=437) spent 8.5 more hours per week on domestic activities and childcare and were more likely to take time off for childcare if their partner worked full time compared with males (n=612)(42.6% vs 12.4%, respectively).2 Our observation that female last authors had a significant decrease in publications may suggest that this population had a disproportionate burden of domestic labor and childcare during the pandemic. It is possible that last authors, who generally are more senior researchers, may be more likely to have childcare, eldercare, and other types of domestic responsibilities. Similarly, in a study of surgery submissions (n=1068), there were 6%, 7%, and 4% decreases in percentages of female last, corresponding, and first authors, respectively, from 2019 to 2020.3Our study had limitations. Only 11 journals were analyzed because others did not have specified submission dates. Some journals only provided submission information for a subset of articles (eg, those published in the In Press section), which may have accounted for the large discrepancy in submission numbers for 2019 to 2020. Gender could not be determined for 1% of authors and was limited to female and male. Although our study submission time frame (May–October 2020) aimed at identifying research conducted during the height of the COVID-19 pandemic, some of these studies may have been conducted months or years before the pandemic. Future studies should focus on longer and more comprehensive time frames. Finally, estimated dates of stay-at-home orders fail to consider differences within countries.
The proportion of female US-affiliated first and last authors publishing in dermatology journals increased from 12% to 48% in 1976 and from 6% to 31% in 2006,4 which is encouraging. However, a gender gap persists, with one-third of National Institutes of Health grants in dermatology and one-fourth of research project grants in dermatology awarded to women.5 Consequences of the pandemic on academic productivity may include fewer women represented in higher academic ranks, lower compensation, and lower career satisfaction compared with men.1 We urge academic institutions and funding agencies to recognize and take action to mitigate long-term sequelae. Extended grant end dates and submission periods, funding opportunities dedicated to women, and prioritization of female-authored submissions are some strategies that can safeguard equitable career progression in dermatology research.
To the Editor:
Peer-reviewed publications are important determinants for promotions, academic leadership, and grants in dermatology.1 The impact of the COVID-19 pandemic on dermatology research productivity remains an area of investigation. We sought to determine authorship trends for males and females during the pandemic.
A cross-sectional retrospective study of the top 20 dermatology journals—determined by impact factor and Google Scholar H5-index—was conducted to identify manuscripts with submission date specified prepandemic (May 1, 2019–October 31, 2019) and during the pandemic (May 1, 2020–October 31, 2020). Submission date, first/last author name, sex, and affiliated country were extracted. Single authors were designated as first authors. Gender API (https://gender-api.com/en/) classified gender. A χ2 test (P<.05) compared differences in proportions of female first/last authors from 2019 to 2020.
Overall, 811 and 1061 articles submitted in 2019 and 2020, respectively, were included. There were 1517 articles submitted to clinical journals and 355 articles submitted to basic science journals (Table). For the 7 clinical journals included, there was a 7.7% decrease in the proportion of female last authors in 2020 vs 2019 (P=.002), with the largest decrease between August and September 2020. Although other comparisons did not yield statistically significant differences (P>.05 all)(Table), several trends were observed. For clinical journals, there was a 1.8% decrease in the proportion of female first authors. For the 4 basic science journals included, there was a 4.9% increase and a 0.3% decrease in percentages of female first and last authors, respectively, for 2020 vs 2019.

Our findings indicate that the COVID-19 pandemic may have impacted female authors’ productivity in clinical dermatology publications. In a survey-based study for 2010 to 2011, female physician-researchers (n=437) spent 8.5 more hours per week on domestic activities and childcare and were more likely to take time off for childcare if their partner worked full time compared with males (n=612)(42.6% vs 12.4%, respectively).2 Our observation that female last authors had a significant decrease in publications may suggest that this population had a disproportionate burden of domestic labor and childcare during the pandemic. It is possible that last authors, who generally are more senior researchers, may be more likely to have childcare, eldercare, and other types of domestic responsibilities. Similarly, in a study of surgery submissions (n=1068), there were 6%, 7%, and 4% decreases in percentages of female last, corresponding, and first authors, respectively, from 2019 to 2020.3Our study had limitations. Only 11 journals were analyzed because others did not have specified submission dates. Some journals only provided submission information for a subset of articles (eg, those published in the In Press section), which may have accounted for the large discrepancy in submission numbers for 2019 to 2020. Gender could not be determined for 1% of authors and was limited to female and male. Although our study submission time frame (May–October 2020) aimed at identifying research conducted during the height of the COVID-19 pandemic, some of these studies may have been conducted months or years before the pandemic. Future studies should focus on longer and more comprehensive time frames. Finally, estimated dates of stay-at-home orders fail to consider differences within countries.
The proportion of female US-affiliated first and last authors publishing in dermatology journals increased from 12% to 48% in 1976 and from 6% to 31% in 2006,4 which is encouraging. However, a gender gap persists, with one-third of National Institutes of Health grants in dermatology and one-fourth of research project grants in dermatology awarded to women.5 Consequences of the pandemic on academic productivity may include fewer women represented in higher academic ranks, lower compensation, and lower career satisfaction compared with men.1 We urge academic institutions and funding agencies to recognize and take action to mitigate long-term sequelae. Extended grant end dates and submission periods, funding opportunities dedicated to women, and prioritization of female-authored submissions are some strategies that can safeguard equitable career progression in dermatology research.
- Stewart C, Lipner SR. Gender and race trends in academic rank of dermatologists at top U.S. institutions: a cross-sectional study. Int J Womens Dermatol. 2020;6:283-285. doi:10.1016/j .ijwd.2020.04.010
- Jolly S, Griffith KA, DeCastro R, et al. Gender differences in time spent on parenting and domestic responsibilities by highachieving young physician-researchers. Ann Intern Med. 2014; 160:344-353. doi:10.7326/M13-0974
- Kibbe MR. Consequences of the COVID-19 pandemic on manuscript submissions by women. JAMA Surg. 2020;155:803-804. doi:10.1001/jamasurg.2020.3917
- Feramisco JD, Leitenberger JJ, Redfern SI, et al. A gender gap in the dermatology literature? cross-sectional analysis of manuscript authorship trends in dermatology journals during 3 decades. J Am Acad Dermatol. 2009;6:63-69. doi:10.1016/j.jaad.2008.06.044
- Cheng MY, Sukhov A, Sultani H, et al. Trends in national institutes of health funding of principal investigators in dermatology research by academic degree and sex. JAMA Dermatol. 2016;152:883-888. doi:10.1001/jamadermatol.2016.0271
- Stewart C, Lipner SR. Gender and race trends in academic rank of dermatologists at top U.S. institutions: a cross-sectional study. Int J Womens Dermatol. 2020;6:283-285. doi:10.1016/j .ijwd.2020.04.010
- Jolly S, Griffith KA, DeCastro R, et al. Gender differences in time spent on parenting and domestic responsibilities by highachieving young physician-researchers. Ann Intern Med. 2014; 160:344-353. doi:10.7326/M13-0974
- Kibbe MR. Consequences of the COVID-19 pandemic on manuscript submissions by women. JAMA Surg. 2020;155:803-804. doi:10.1001/jamasurg.2020.3917
- Feramisco JD, Leitenberger JJ, Redfern SI, et al. A gender gap in the dermatology literature? cross-sectional analysis of manuscript authorship trends in dermatology journals during 3 decades. J Am Acad Dermatol. 2009;6:63-69. doi:10.1016/j.jaad.2008.06.044
- Cheng MY, Sukhov A, Sultani H, et al. Trends in national institutes of health funding of principal investigators in dermatology research by academic degree and sex. JAMA Dermatol. 2016;152:883-888. doi:10.1001/jamadermatol.2016.0271
Practice Points
- The academic productivity of female dermatologists as last authors in dermatology clinical journals has potentially been impacted by the COVID-19 pandemic.
- To potentially aid in the resurgence of female dermatologist authors impacted by the pandemic, academic institutions and funding agencies may consider implementing strategies such as extending grant end dates, providing dedicated funding opportunities, and prioritizing female-authored submissions in dermatology research.