User login
Frailty Trends in an Older Veteran Subpopulation 1 Year Prior and Into the COVID-19 Pandemic Using CAN Scores
Frailty is an age-associated, nonspecific vulnerability to adverse health outcomes. Frailty can also be described as a complex of symptoms characterized by impaired stress tolerance due to a decline in the functionality of different organs.1 The prevalence of frailty varies widely depending on the method of measurement and the population studied.2-4 It is a nonconstant factor that increases with age. A deficit accumulation frailty index (FI) is one method used to measure frailty.5 This approach sees frailty as a multidimensional risk state measured by quantity rather than the nature of health concerns. A deficit accumulation FI does not require physical testing but correlates well with other phenotypic FIs.6 It is, however, time consuming, as ≥ 30 deficits need to be measured to offer greater stability to the frailty estimate.
Health care is seeing increasing utilization of big data analytics to derive predictive models and help with resource allocation. There are currently 2 existing automated tools to predict health care utilization and mortality at the US Department of Veterans Affairs (VA): the VA Frailty Index (VA-FI-10) and the Care Assessment Need (CAN). VA-FI-10 is an International Statistical Classification of Diseases, Tenth Revision (ICD-10) update of the VA-FI that was created in March 2021. The VA-FI-10 is a claims-based frailty assessment tool using 31 health deficits. Calculating the VA-FI-10 requires defining an index date and lookback period (typically 3 years) relative to which it will be calculated.7
CAN is a set of risk-stratifying statistical models run on veterans receiving VA primary care services as part of a patient aligned care team (PACT) using electronic health record data.8 Each veteran is stratified based on the individual’s risks of hospitalization, death, and hospitalization or death. These 3 events are predicted for 90-day and 1-year time periods for a total of 6 distinct outcomes. CAN is currently on its third iteration (CAN 2.5) and scores range from 0 (low) to 99 (high). CAN scores are updated weekly. The 1-year hospitalization probabilities for all patients range from 0.8% to 93.1%. For patients with a CAN score of 50, the probability of being hospitalized within a year ranges from 4.5% to 5.2%, which increases to 32.2% to 36% for veterans with a CAN score of 95. The probability range widens significantly (32.2%-93.1%) for patients in the top 5 CAN scores (95-99).
CAN scores are a potential screening tool for frailty among older adults; they are generated automatically and provide acceptable diagnostic accuracy. Hence, the CAN score may be a useful tool for primary care practitioners for the detection of frailty in their patients. The CAN score has shown a moderate positive association with the FRAIL Scale.9,10 The population-based studies that have used the FI approach (differing FIs, depending on the data available) give robust results: People accumulate an average of 0.03 deficits per year after the age of 70 years.11 Interventions to delay or reverse frailty have not been clearly defined with heterogeneity in the definition of frailty and measurement of frailty outcomes.12,13 The prevalence of frailty in the veteran population is substantially higher than the prevalence in community populations with a similar age distribution. There is also mounting evidence that veterans accumulate deficits more rapidly than their civilian counterparts.14
COVID-19 was declared a pandemic in March 2020 and had many impacts on global health that were most marked in the first year. These included reductions in hospital visits for non-COVID-19 health concerns, a reduction in completed screening tests, an initial reduction in other infectious diseases (attributable to quarantines), and an increase or worsening of mental health concerns.15,16
We aimed to investigate whether frailty increased disproportionately in a subset of older veterans in the first year of the COVID-19 pandemic when compared with the previous year using CAN scores. This single institution, longitudinal cohort study was determined to be exempt from institutional review board review but was approved by the Phoenix VA Health Care System (PVAHCS) Research and Development Committee.
Methods
The Office of Clinical Systems Development and Evaluation (CSDE–10E2A) produces a weekly CAN Score Report to help identify the highest-risk patients in a primary care panel or cohort. CAN scores range from 0 (lowest risk) to 99 (highest risk), indicating how likely a patient is to experience hospitalization or death compared with other VA patients. CAN scores are calculated with statistical prediction models that use data elements from the following Corporate Data Warehouse (CDW) domains: demographics, health care utilization, laboratory tests, medical conditions, medications, and vital signs (eAppendix available online at 10.12788/fp.0385).
The CAN Score Report is generated weekly and stored on a CDW server. A patient will receive all 6 distinct CAN scores if they are: (1) assigned to a primary care PACT on the risk date; (2) a veteran; (3) not hospitalized in a VA facility on the risk date; and (4) alive as of the risk date. New to CAN 2.5 is that patients who meet criteria 1, 2, and 4 but are hospitalized in a VA facility on the risk date will receive CAN scores for the 1-year and 90-day mortality models.
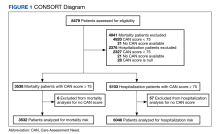
Utilizing VA Informatics and Computing Infrastructure (VA HSR RES 13-457, US Department of Veterans Affairs [2008]), we obtained 2 lists of veterans aged 70 to 75 years on February 8, 2019, with a calculated CAN score of ≥ 75 for 1-year mortality and 1-year hospitalization on that date. A veteran with a CAN score of ≥ 75 is likely to be prefrail or frail.9,10 Veterans who did not have a corresponding calculated CAN score on February 7, 2020, and February 12, 2021, were excluded. COVID-19 was declared a public health emergency in the United States on January 31, 2020, and the World Health Organization declared COVID-19 a pandemic on March 11, 2020.17 We picked February 7, 2020, within this time frame and without any other special significance. We picked additional CAN score calculation dates approximately 1 year prior and 1 year after this date. Veterans had to be alive on February 12, 2021, (the last date of the CAN score) to be included in the cohorts.
Statistical Analyses
The difference in CAN score from one year to the next was calculated for each patient. The difference between 2019 and 2020 was compared with the difference between 2020 to 2021 using a paired t test. Yearly CAN score values were analyzed using repeated measures analysis of variance. The number of patients that showed an increase in CAN score (ie, increased risk of either mortality or hospitalization within the year) or a decrease (lower risk) was compared using the χ2 test. IBM SPSS v26 and GraphPad Prism v18 were used for statistical analysis. P < .05 was considered statistically significant.
Results
There were 3538 veterans at PVAHCS who met the inclusion criteria and had a 1-year mortality CAN score ≥ 75 on February 8, 2019.
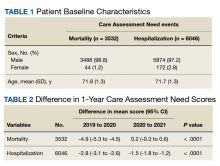
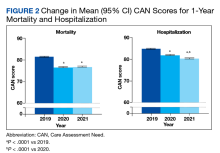
In the hospitalization group, there were 6046 veterans in the analysis; 57 veterans missing a 1-year hospitalization CAN score that were excluded. The mean age was 71.7 (1.3) years and included 5874 male (97.2%) and 172 female (2.8%) veterans. There was a decline in mean 1-year hospitalization CAN scores in our subset of frail older veterans by 2.8 (95% CI, -3.1 to -2.6) in the year preceding the COVID-19 pandemic. This mean decline slowed significantly to 1.5 (95% CI, -1.8 to -1.2; P < .0001) after the first year of the COVID-19 pandemic. Mean CAN scores for 1-year hospitalization were 84.6 (95% CI, 84.4 to 84.8), 81.8 (95% CI, 81.5 to 82.1), and 80.2 (95% CI, 79.9 to 80.6)
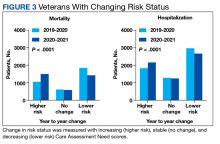
We also calculated the number of veterans with increasing, stable, and decreasing CAN scores across each of our defined periods in both the 1-year mortality and hospitalization groups.
A previous study used a 1-year combined hospitalization or mortality event CAN score as the most all-inclusive measure of frailty but determined that it was possible that 1 of the other 5 CAN risk measures could perform better in predicting frailty.10 We collected and presented data for 1-year mortality and hospitalization CAN scores. There were declines in pandemic-related US hospitalizations for illnesses not related to COVID-19 during the first few months of the pandemic.18 This may or may not have affected the 1-year hospitalization CAN score data; thus, we used the 1-year mortality CAN score data to predict frailty.
Discussion
We studied frailty trends in an older veteran subpopulation enrolled at the PVAHCS 1 year prior and into the COVID-19 pandemic using CAN scores. Frailty is a dynamic state. Previous frailty assessments aimed to identify patients at the highest risk of death. With the advent of advanced therapeutics for several diseases, the number of medical conditions that are now managed as chronic illnesses continues to grow. There is a role for repeated measures of frailty to try to identify frailty trends.19 These trends may assist us in resource allocation, identifying interventions that work and those that do not.
Some studies have shown an overall declining lethality of frailty. This may reflect improvements in the care and management of chronic conditions, screening tests, and increased awareness of healthy lifestyles.20 Another study of frailty trajectories in a veteran population in the 5 years preceding death showed multiple trajectories (stable, gradually increasing, rapidly increasing, and recovering).19
The PACT is a primary care model implemented at VA medical centers in April 2010. It is a patient-centered medical home model (PCMH) with several components. The VA treats a population of socioeconomically vulnerable patients with complex chronic illness management needs. Some of the components of a PACT model relevant to our study include facilitated self-management support for veterans in between practitioner visits via care partners, peer-to-peer and transitional care programs, physical activity and diet programs, primary care mental health, integration between primary and specialty care, and telehealth.21 A previous study has shown that VA primary care clinics with the most PCMH components in place had greater improvements in several chronic disease quality measures than in clinics with a lower number of PCMH components.22
Limitations
Our study is limited by our older veteran population demographics. We chose only a subset of older veterans at a single VA center for this study and cannot extrapolate the results to all older frail veterans or community dwelling older adults. Robust individuals may also transition to prefrailty and frailty over longer periods; our study monitored frailty trends over 2 years.
CAN scores are not quality measures to improve upon. Allocation and utilization of additional resources may clinically benefit a patient but increase their CAN scores. Although our results are statistically significant, we are unable to make any conclusions about clinical significance.
Conclusions
Our study results indicate frailty as determined by 1-year mortality CAN scores significantly increased in a subset of older veterans during the first year of the COVID-19 pandemic when compared with the previous year. Whether this change in frailty is temporary or long lasting remains to be seen. Automated CAN scores can be effectively utilized to monitor frailty trends in certain veteran populations over longer periods.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the Phoenix Veterans Affairs Health Care System.
1. Rohrmann S. Epidemiology of frailty in older people. Adv Exp Med Biol. 2020;1216:21-27. doi:10.1007/978-3-030-33330-0_3
2. Bandeen-Roche K, Seplaki CL, Huang J, et al. Frailty in older adults: a nationally representative profile in the United States. J Gerontol A Biol Sci Med Sci. 2015;70(11):1427-1434. doi:10.1093/gerona/glv133
3. Siriwardhana DD, Hardoon S, Rait G, Weerasinghe MC, Walters KR. Prevalence of frailty and prefrailty among community-dwelling older adults in low-income and middle-income countries: a systematic review and meta-analysis. BMJ Open. 2018;8(3):e018195. Published 2018 Mar 1. doi:10.1136/bmjopen-2017-018195
4. Song X, Mitnitski A, Rockwood K. Prevalence and 10-year outcomes of frailty in older adults in relation to deficit accumulation. J Am Geriatr Soc. 2010;58(4):681-687. doi:10.1111/j.1532-5415.2010.02764.x
5. Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci. 2007;62(7):722-727. doi:10.1093/gerona/62.7.722
6. Buta BJ, Walston JD, Godino JG, et al. Frailty assessment instruments: Systematic characterization of the uses and contexts of highly-cited instruments. Ageing Res Rev. 2016;26:53-61. doi:10.1016/j.arr.2015.12.003
7. Cheng D, DuMontier C, Yildirim C, et al. Updating and validating the U.S. Veterans Affairs Frailty Index: transitioning From ICD-9 to ICD-10. J Gerontol A Biol Sci Med Sci. 2021;76(7):1318-1325. doi:10.1093/gerona/glab071
8. Fihn SD, Francis J, Clancy C, et al. Insights from advanced analytics at the Veterans Health Administration. Health Aff (Millwood). 2014;33(7):1203-1211. doi:10.1377/hlthaff.2014.0054
9. Ruiz JG, Priyadarshni S, Rahaman Z, et al. Validation of an automatically generated screening score for frailty: the care assessment need (CAN) score. BMC Geriatr. 2018;18(1):106. doi:10.1186/s12877-018-0802-7
10. Ruiz JG, Rahaman Z, Dang S, Anam R, Valencia WM, Mintzer MJ. Association of the CAN score with the FRAIL scale in community dwelling older adults. Aging Clin Exp Res. 2018;30(10):1241-1245. doi:10.1007/s40520-018-0910-4
11. Ofori-Asenso R, Chin KL, Mazidi M, et al. Global incidence of frailty and prefrailty among community-dwelling older adults: a systematic review and meta-analysis. JAMA Netw Open. 2019;2(8):e198398. Published 2019 Aug 2. doi:10.1001/jamanetworkopen.2019.8398
12. Marcucci M, Damanti S, Germini F, et al. Interventions to prevent, delay or reverse frailty in older people: a journey towards clinical guidelines. BMC Med. 2019;17(1):193. Published 2019 Oct 29. doi:10.1186/s12916-019-1434-2
13. Travers J, Romero-Ortuno R, Bailey J, Cooney MT. Delaying and reversing frailty: a systematic review of primary care interventions. Br J Gen Pract. 2019;69(678):e61-e69. doi:10.3399/bjgp18X700241
14. Orkaby AR, Nussbaum L, Ho YL, et al. The burden of frailty among U.S. veterans and its association with mortality, 2002-2012. J Gerontol A Biol Sci Med Sci. 2019;74(8):1257-1264. doi:10.1093/gerona/gly232
15. Bakouny Z, Paciotti M, Schmidt AL, Lipsitz SR, Choueiri TK, Trinh QD. Cancer screening tests and cancer diagnoses during the COVID-19 pandemic. JAMA Oncol. 2021;7(3):458-460. doi:10.1001/jamaoncol.2020.7600
16. Steffen R, Lautenschlager S, Fehr J. Travel restrictions and lockdown during the COVID-19 pandemic-impact on notified infectious diseases in Switzerland. J Travel Med. 2020;27(8):taaa180. doi:10.1093/jtm/taaa180
17. CDC Museum COVID-19 Timeline. Centers for Disease Control and Prevention. Updated March 15, 2023. Accessed May 12, 2023. https://www.cdc.gov/museum/timeline/covid19.html18. Nguyen JL, Benigno M, Malhotra D, et al. Pandemic-related declines in hospitalization for non-COVID-19-related illness in the United States from January through July 2020. PLoS One. 2022;17(1):e0262347. Published 2022 Jan 6. doi:10.1371/journal.pone.0262347
19. Ward RE, Orkaby AR, Dumontier C, et al. Trajectories of frailty in the 5 years prior to death among U.S. veterans born 1927-1934. J Gerontol A Biol Sci Med Sci. 2021;76(11):e347-e353. doi:10.1093/gerona/glab196
20. Bäckman K, Joas E, Falk H, Mitnitski A, Rockwood K, Skoog I. Changes in the lethality of frailty over 30 years: evidence from two cohorts of 70-year-olds in Gothenburg Sweden. J Gerontol A Biol Sci Med Sci. 2017;72(7):945-950. doi:10.1093/gerona/glw160
21. Piette JD, Holtz B, Beard AJ, et al. Improving chronic illness care for veterans within the framework of the Patient-Centered Medical Home: experiences from the Ann Arbor Patient-Aligned Care Team Laboratory. Transl Behav Med. 2011;1(4):615-623. doi:10.1007/s13142-011-0065-8
22. Rosland AM, Nelson K, Sun H, et al. The patient-centered medical home in the Veterans Health Administration. Am J Manag Care. 2013;19(7):e263-e272. Published 2013 Jul 1.
Frailty is an age-associated, nonspecific vulnerability to adverse health outcomes. Frailty can also be described as a complex of symptoms characterized by impaired stress tolerance due to a decline in the functionality of different organs.1 The prevalence of frailty varies widely depending on the method of measurement and the population studied.2-4 It is a nonconstant factor that increases with age. A deficit accumulation frailty index (FI) is one method used to measure frailty.5 This approach sees frailty as a multidimensional risk state measured by quantity rather than the nature of health concerns. A deficit accumulation FI does not require physical testing but correlates well with other phenotypic FIs.6 It is, however, time consuming, as ≥ 30 deficits need to be measured to offer greater stability to the frailty estimate.
Health care is seeing increasing utilization of big data analytics to derive predictive models and help with resource allocation. There are currently 2 existing automated tools to predict health care utilization and mortality at the US Department of Veterans Affairs (VA): the VA Frailty Index (VA-FI-10) and the Care Assessment Need (CAN). VA-FI-10 is an International Statistical Classification of Diseases, Tenth Revision (ICD-10) update of the VA-FI that was created in March 2021. The VA-FI-10 is a claims-based frailty assessment tool using 31 health deficits. Calculating the VA-FI-10 requires defining an index date and lookback period (typically 3 years) relative to which it will be calculated.7
CAN is a set of risk-stratifying statistical models run on veterans receiving VA primary care services as part of a patient aligned care team (PACT) using electronic health record data.8 Each veteran is stratified based on the individual’s risks of hospitalization, death, and hospitalization or death. These 3 events are predicted for 90-day and 1-year time periods for a total of 6 distinct outcomes. CAN is currently on its third iteration (CAN 2.5) and scores range from 0 (low) to 99 (high). CAN scores are updated weekly. The 1-year hospitalization probabilities for all patients range from 0.8% to 93.1%. For patients with a CAN score of 50, the probability of being hospitalized within a year ranges from 4.5% to 5.2%, which increases to 32.2% to 36% for veterans with a CAN score of 95. The probability range widens significantly (32.2%-93.1%) for patients in the top 5 CAN scores (95-99).
CAN scores are a potential screening tool for frailty among older adults; they are generated automatically and provide acceptable diagnostic accuracy. Hence, the CAN score may be a useful tool for primary care practitioners for the detection of frailty in their patients. The CAN score has shown a moderate positive association with the FRAIL Scale.9,10 The population-based studies that have used the FI approach (differing FIs, depending on the data available) give robust results: People accumulate an average of 0.03 deficits per year after the age of 70 years.11 Interventions to delay or reverse frailty have not been clearly defined with heterogeneity in the definition of frailty and measurement of frailty outcomes.12,13 The prevalence of frailty in the veteran population is substantially higher than the prevalence in community populations with a similar age distribution. There is also mounting evidence that veterans accumulate deficits more rapidly than their civilian counterparts.14
COVID-19 was declared a pandemic in March 2020 and had many impacts on global health that were most marked in the first year. These included reductions in hospital visits for non-COVID-19 health concerns, a reduction in completed screening tests, an initial reduction in other infectious diseases (attributable to quarantines), and an increase or worsening of mental health concerns.15,16
We aimed to investigate whether frailty increased disproportionately in a subset of older veterans in the first year of the COVID-19 pandemic when compared with the previous year using CAN scores. This single institution, longitudinal cohort study was determined to be exempt from institutional review board review but was approved by the Phoenix VA Health Care System (PVAHCS) Research and Development Committee.
Methods
The Office of Clinical Systems Development and Evaluation (CSDE–10E2A) produces a weekly CAN Score Report to help identify the highest-risk patients in a primary care panel or cohort. CAN scores range from 0 (lowest risk) to 99 (highest risk), indicating how likely a patient is to experience hospitalization or death compared with other VA patients. CAN scores are calculated with statistical prediction models that use data elements from the following Corporate Data Warehouse (CDW) domains: demographics, health care utilization, laboratory tests, medical conditions, medications, and vital signs (eAppendix available online at 10.12788/fp.0385).
The CAN Score Report is generated weekly and stored on a CDW server. A patient will receive all 6 distinct CAN scores if they are: (1) assigned to a primary care PACT on the risk date; (2) a veteran; (3) not hospitalized in a VA facility on the risk date; and (4) alive as of the risk date. New to CAN 2.5 is that patients who meet criteria 1, 2, and 4 but are hospitalized in a VA facility on the risk date will receive CAN scores for the 1-year and 90-day mortality models.
Utilizing VA Informatics and Computing Infrastructure (VA HSR RES 13-457, US Department of Veterans Affairs [2008]), we obtained 2 lists of veterans aged 70 to 75 years on February 8, 2019, with a calculated CAN score of ≥ 75 for 1-year mortality and 1-year hospitalization on that date. A veteran with a CAN score of ≥ 75 is likely to be prefrail or frail.9,10 Veterans who did not have a corresponding calculated CAN score on February 7, 2020, and February 12, 2021, were excluded. COVID-19 was declared a public health emergency in the United States on January 31, 2020, and the World Health Organization declared COVID-19 a pandemic on March 11, 2020.17 We picked February 7, 2020, within this time frame and without any other special significance. We picked additional CAN score calculation dates approximately 1 year prior and 1 year after this date. Veterans had to be alive on February 12, 2021, (the last date of the CAN score) to be included in the cohorts.
Statistical Analyses
The difference in CAN score from one year to the next was calculated for each patient. The difference between 2019 and 2020 was compared with the difference between 2020 to 2021 using a paired t test. Yearly CAN score values were analyzed using repeated measures analysis of variance. The number of patients that showed an increase in CAN score (ie, increased risk of either mortality or hospitalization within the year) or a decrease (lower risk) was compared using the χ2 test. IBM SPSS v26 and GraphPad Prism v18 were used for statistical analysis. P < .05 was considered statistically significant.
Results
There were 3538 veterans at PVAHCS who met the inclusion criteria and had a 1-year mortality CAN score ≥ 75 on February 8, 2019.
In the hospitalization group, there were 6046 veterans in the analysis; 57 veterans missing a 1-year hospitalization CAN score that were excluded. The mean age was 71.7 (1.3) years and included 5874 male (97.2%) and 172 female (2.8%) veterans. There was a decline in mean 1-year hospitalization CAN scores in our subset of frail older veterans by 2.8 (95% CI, -3.1 to -2.6) in the year preceding the COVID-19 pandemic. This mean decline slowed significantly to 1.5 (95% CI, -1.8 to -1.2; P < .0001) after the first year of the COVID-19 pandemic. Mean CAN scores for 1-year hospitalization were 84.6 (95% CI, 84.4 to 84.8), 81.8 (95% CI, 81.5 to 82.1), and 80.2 (95% CI, 79.9 to 80.6)
We also calculated the number of veterans with increasing, stable, and decreasing CAN scores across each of our defined periods in both the 1-year mortality and hospitalization groups.
A previous study used a 1-year combined hospitalization or mortality event CAN score as the most all-inclusive measure of frailty but determined that it was possible that 1 of the other 5 CAN risk measures could perform better in predicting frailty.10 We collected and presented data for 1-year mortality and hospitalization CAN scores. There were declines in pandemic-related US hospitalizations for illnesses not related to COVID-19 during the first few months of the pandemic.18 This may or may not have affected the 1-year hospitalization CAN score data; thus, we used the 1-year mortality CAN score data to predict frailty.
Discussion
We studied frailty trends in an older veteran subpopulation enrolled at the PVAHCS 1 year prior and into the COVID-19 pandemic using CAN scores. Frailty is a dynamic state. Previous frailty assessments aimed to identify patients at the highest risk of death. With the advent of advanced therapeutics for several diseases, the number of medical conditions that are now managed as chronic illnesses continues to grow. There is a role for repeated measures of frailty to try to identify frailty trends.19 These trends may assist us in resource allocation, identifying interventions that work and those that do not.
Some studies have shown an overall declining lethality of frailty. This may reflect improvements in the care and management of chronic conditions, screening tests, and increased awareness of healthy lifestyles.20 Another study of frailty trajectories in a veteran population in the 5 years preceding death showed multiple trajectories (stable, gradually increasing, rapidly increasing, and recovering).19
The PACT is a primary care model implemented at VA medical centers in April 2010. It is a patient-centered medical home model (PCMH) with several components. The VA treats a population of socioeconomically vulnerable patients with complex chronic illness management needs. Some of the components of a PACT model relevant to our study include facilitated self-management support for veterans in between practitioner visits via care partners, peer-to-peer and transitional care programs, physical activity and diet programs, primary care mental health, integration between primary and specialty care, and telehealth.21 A previous study has shown that VA primary care clinics with the most PCMH components in place had greater improvements in several chronic disease quality measures than in clinics with a lower number of PCMH components.22
Limitations
Our study is limited by our older veteran population demographics. We chose only a subset of older veterans at a single VA center for this study and cannot extrapolate the results to all older frail veterans or community dwelling older adults. Robust individuals may also transition to prefrailty and frailty over longer periods; our study monitored frailty trends over 2 years.
CAN scores are not quality measures to improve upon. Allocation and utilization of additional resources may clinically benefit a patient but increase their CAN scores. Although our results are statistically significant, we are unable to make any conclusions about clinical significance.
Conclusions
Our study results indicate frailty as determined by 1-year mortality CAN scores significantly increased in a subset of older veterans during the first year of the COVID-19 pandemic when compared with the previous year. Whether this change in frailty is temporary or long lasting remains to be seen. Automated CAN scores can be effectively utilized to monitor frailty trends in certain veteran populations over longer periods.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the Phoenix Veterans Affairs Health Care System.
Frailty is an age-associated, nonspecific vulnerability to adverse health outcomes. Frailty can also be described as a complex of symptoms characterized by impaired stress tolerance due to a decline in the functionality of different organs.1 The prevalence of frailty varies widely depending on the method of measurement and the population studied.2-4 It is a nonconstant factor that increases with age. A deficit accumulation frailty index (FI) is one method used to measure frailty.5 This approach sees frailty as a multidimensional risk state measured by quantity rather than the nature of health concerns. A deficit accumulation FI does not require physical testing but correlates well with other phenotypic FIs.6 It is, however, time consuming, as ≥ 30 deficits need to be measured to offer greater stability to the frailty estimate.
Health care is seeing increasing utilization of big data analytics to derive predictive models and help with resource allocation. There are currently 2 existing automated tools to predict health care utilization and mortality at the US Department of Veterans Affairs (VA): the VA Frailty Index (VA-FI-10) and the Care Assessment Need (CAN). VA-FI-10 is an International Statistical Classification of Diseases, Tenth Revision (ICD-10) update of the VA-FI that was created in March 2021. The VA-FI-10 is a claims-based frailty assessment tool using 31 health deficits. Calculating the VA-FI-10 requires defining an index date and lookback period (typically 3 years) relative to which it will be calculated.7
CAN is a set of risk-stratifying statistical models run on veterans receiving VA primary care services as part of a patient aligned care team (PACT) using electronic health record data.8 Each veteran is stratified based on the individual’s risks of hospitalization, death, and hospitalization or death. These 3 events are predicted for 90-day and 1-year time periods for a total of 6 distinct outcomes. CAN is currently on its third iteration (CAN 2.5) and scores range from 0 (low) to 99 (high). CAN scores are updated weekly. The 1-year hospitalization probabilities for all patients range from 0.8% to 93.1%. For patients with a CAN score of 50, the probability of being hospitalized within a year ranges from 4.5% to 5.2%, which increases to 32.2% to 36% for veterans with a CAN score of 95. The probability range widens significantly (32.2%-93.1%) for patients in the top 5 CAN scores (95-99).
CAN scores are a potential screening tool for frailty among older adults; they are generated automatically and provide acceptable diagnostic accuracy. Hence, the CAN score may be a useful tool for primary care practitioners for the detection of frailty in their patients. The CAN score has shown a moderate positive association with the FRAIL Scale.9,10 The population-based studies that have used the FI approach (differing FIs, depending on the data available) give robust results: People accumulate an average of 0.03 deficits per year after the age of 70 years.11 Interventions to delay or reverse frailty have not been clearly defined with heterogeneity in the definition of frailty and measurement of frailty outcomes.12,13 The prevalence of frailty in the veteran population is substantially higher than the prevalence in community populations with a similar age distribution. There is also mounting evidence that veterans accumulate deficits more rapidly than their civilian counterparts.14
COVID-19 was declared a pandemic in March 2020 and had many impacts on global health that were most marked in the first year. These included reductions in hospital visits for non-COVID-19 health concerns, a reduction in completed screening tests, an initial reduction in other infectious diseases (attributable to quarantines), and an increase or worsening of mental health concerns.15,16
We aimed to investigate whether frailty increased disproportionately in a subset of older veterans in the first year of the COVID-19 pandemic when compared with the previous year using CAN scores. This single institution, longitudinal cohort study was determined to be exempt from institutional review board review but was approved by the Phoenix VA Health Care System (PVAHCS) Research and Development Committee.
Methods
The Office of Clinical Systems Development and Evaluation (CSDE–10E2A) produces a weekly CAN Score Report to help identify the highest-risk patients in a primary care panel or cohort. CAN scores range from 0 (lowest risk) to 99 (highest risk), indicating how likely a patient is to experience hospitalization or death compared with other VA patients. CAN scores are calculated with statistical prediction models that use data elements from the following Corporate Data Warehouse (CDW) domains: demographics, health care utilization, laboratory tests, medical conditions, medications, and vital signs (eAppendix available online at 10.12788/fp.0385).
The CAN Score Report is generated weekly and stored on a CDW server. A patient will receive all 6 distinct CAN scores if they are: (1) assigned to a primary care PACT on the risk date; (2) a veteran; (3) not hospitalized in a VA facility on the risk date; and (4) alive as of the risk date. New to CAN 2.5 is that patients who meet criteria 1, 2, and 4 but are hospitalized in a VA facility on the risk date will receive CAN scores for the 1-year and 90-day mortality models.
Utilizing VA Informatics and Computing Infrastructure (VA HSR RES 13-457, US Department of Veterans Affairs [2008]), we obtained 2 lists of veterans aged 70 to 75 years on February 8, 2019, with a calculated CAN score of ≥ 75 for 1-year mortality and 1-year hospitalization on that date. A veteran with a CAN score of ≥ 75 is likely to be prefrail or frail.9,10 Veterans who did not have a corresponding calculated CAN score on February 7, 2020, and February 12, 2021, were excluded. COVID-19 was declared a public health emergency in the United States on January 31, 2020, and the World Health Organization declared COVID-19 a pandemic on March 11, 2020.17 We picked February 7, 2020, within this time frame and without any other special significance. We picked additional CAN score calculation dates approximately 1 year prior and 1 year after this date. Veterans had to be alive on February 12, 2021, (the last date of the CAN score) to be included in the cohorts.
Statistical Analyses
The difference in CAN score from one year to the next was calculated for each patient. The difference between 2019 and 2020 was compared with the difference between 2020 to 2021 using a paired t test. Yearly CAN score values were analyzed using repeated measures analysis of variance. The number of patients that showed an increase in CAN score (ie, increased risk of either mortality or hospitalization within the year) or a decrease (lower risk) was compared using the χ2 test. IBM SPSS v26 and GraphPad Prism v18 were used for statistical analysis. P < .05 was considered statistically significant.
Results
There were 3538 veterans at PVAHCS who met the inclusion criteria and had a 1-year mortality CAN score ≥ 75 on February 8, 2019.
In the hospitalization group, there were 6046 veterans in the analysis; 57 veterans missing a 1-year hospitalization CAN score that were excluded. The mean age was 71.7 (1.3) years and included 5874 male (97.2%) and 172 female (2.8%) veterans. There was a decline in mean 1-year hospitalization CAN scores in our subset of frail older veterans by 2.8 (95% CI, -3.1 to -2.6) in the year preceding the COVID-19 pandemic. This mean decline slowed significantly to 1.5 (95% CI, -1.8 to -1.2; P < .0001) after the first year of the COVID-19 pandemic. Mean CAN scores for 1-year hospitalization were 84.6 (95% CI, 84.4 to 84.8), 81.8 (95% CI, 81.5 to 82.1), and 80.2 (95% CI, 79.9 to 80.6)
We also calculated the number of veterans with increasing, stable, and decreasing CAN scores across each of our defined periods in both the 1-year mortality and hospitalization groups.
A previous study used a 1-year combined hospitalization or mortality event CAN score as the most all-inclusive measure of frailty but determined that it was possible that 1 of the other 5 CAN risk measures could perform better in predicting frailty.10 We collected and presented data for 1-year mortality and hospitalization CAN scores. There were declines in pandemic-related US hospitalizations for illnesses not related to COVID-19 during the first few months of the pandemic.18 This may or may not have affected the 1-year hospitalization CAN score data; thus, we used the 1-year mortality CAN score data to predict frailty.
Discussion
We studied frailty trends in an older veteran subpopulation enrolled at the PVAHCS 1 year prior and into the COVID-19 pandemic using CAN scores. Frailty is a dynamic state. Previous frailty assessments aimed to identify patients at the highest risk of death. With the advent of advanced therapeutics for several diseases, the number of medical conditions that are now managed as chronic illnesses continues to grow. There is a role for repeated measures of frailty to try to identify frailty trends.19 These trends may assist us in resource allocation, identifying interventions that work and those that do not.
Some studies have shown an overall declining lethality of frailty. This may reflect improvements in the care and management of chronic conditions, screening tests, and increased awareness of healthy lifestyles.20 Another study of frailty trajectories in a veteran population in the 5 years preceding death showed multiple trajectories (stable, gradually increasing, rapidly increasing, and recovering).19
The PACT is a primary care model implemented at VA medical centers in April 2010. It is a patient-centered medical home model (PCMH) with several components. The VA treats a population of socioeconomically vulnerable patients with complex chronic illness management needs. Some of the components of a PACT model relevant to our study include facilitated self-management support for veterans in between practitioner visits via care partners, peer-to-peer and transitional care programs, physical activity and diet programs, primary care mental health, integration between primary and specialty care, and telehealth.21 A previous study has shown that VA primary care clinics with the most PCMH components in place had greater improvements in several chronic disease quality measures than in clinics with a lower number of PCMH components.22
Limitations
Our study is limited by our older veteran population demographics. We chose only a subset of older veterans at a single VA center for this study and cannot extrapolate the results to all older frail veterans or community dwelling older adults. Robust individuals may also transition to prefrailty and frailty over longer periods; our study monitored frailty trends over 2 years.
CAN scores are not quality measures to improve upon. Allocation and utilization of additional resources may clinically benefit a patient but increase their CAN scores. Although our results are statistically significant, we are unable to make any conclusions about clinical significance.
Conclusions
Our study results indicate frailty as determined by 1-year mortality CAN scores significantly increased in a subset of older veterans during the first year of the COVID-19 pandemic when compared with the previous year. Whether this change in frailty is temporary or long lasting remains to be seen. Automated CAN scores can be effectively utilized to monitor frailty trends in certain veteran populations over longer periods.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the Phoenix Veterans Affairs Health Care System.
1. Rohrmann S. Epidemiology of frailty in older people. Adv Exp Med Biol. 2020;1216:21-27. doi:10.1007/978-3-030-33330-0_3
2. Bandeen-Roche K, Seplaki CL, Huang J, et al. Frailty in older adults: a nationally representative profile in the United States. J Gerontol A Biol Sci Med Sci. 2015;70(11):1427-1434. doi:10.1093/gerona/glv133
3. Siriwardhana DD, Hardoon S, Rait G, Weerasinghe MC, Walters KR. Prevalence of frailty and prefrailty among community-dwelling older adults in low-income and middle-income countries: a systematic review and meta-analysis. BMJ Open. 2018;8(3):e018195. Published 2018 Mar 1. doi:10.1136/bmjopen-2017-018195
4. Song X, Mitnitski A, Rockwood K. Prevalence and 10-year outcomes of frailty in older adults in relation to deficit accumulation. J Am Geriatr Soc. 2010;58(4):681-687. doi:10.1111/j.1532-5415.2010.02764.x
5. Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci. 2007;62(7):722-727. doi:10.1093/gerona/62.7.722
6. Buta BJ, Walston JD, Godino JG, et al. Frailty assessment instruments: Systematic characterization of the uses and contexts of highly-cited instruments. Ageing Res Rev. 2016;26:53-61. doi:10.1016/j.arr.2015.12.003
7. Cheng D, DuMontier C, Yildirim C, et al. Updating and validating the U.S. Veterans Affairs Frailty Index: transitioning From ICD-9 to ICD-10. J Gerontol A Biol Sci Med Sci. 2021;76(7):1318-1325. doi:10.1093/gerona/glab071
8. Fihn SD, Francis J, Clancy C, et al. Insights from advanced analytics at the Veterans Health Administration. Health Aff (Millwood). 2014;33(7):1203-1211. doi:10.1377/hlthaff.2014.0054
9. Ruiz JG, Priyadarshni S, Rahaman Z, et al. Validation of an automatically generated screening score for frailty: the care assessment need (CAN) score. BMC Geriatr. 2018;18(1):106. doi:10.1186/s12877-018-0802-7
10. Ruiz JG, Rahaman Z, Dang S, Anam R, Valencia WM, Mintzer MJ. Association of the CAN score with the FRAIL scale in community dwelling older adults. Aging Clin Exp Res. 2018;30(10):1241-1245. doi:10.1007/s40520-018-0910-4
11. Ofori-Asenso R, Chin KL, Mazidi M, et al. Global incidence of frailty and prefrailty among community-dwelling older adults: a systematic review and meta-analysis. JAMA Netw Open. 2019;2(8):e198398. Published 2019 Aug 2. doi:10.1001/jamanetworkopen.2019.8398
12. Marcucci M, Damanti S, Germini F, et al. Interventions to prevent, delay or reverse frailty in older people: a journey towards clinical guidelines. BMC Med. 2019;17(1):193. Published 2019 Oct 29. doi:10.1186/s12916-019-1434-2
13. Travers J, Romero-Ortuno R, Bailey J, Cooney MT. Delaying and reversing frailty: a systematic review of primary care interventions. Br J Gen Pract. 2019;69(678):e61-e69. doi:10.3399/bjgp18X700241
14. Orkaby AR, Nussbaum L, Ho YL, et al. The burden of frailty among U.S. veterans and its association with mortality, 2002-2012. J Gerontol A Biol Sci Med Sci. 2019;74(8):1257-1264. doi:10.1093/gerona/gly232
15. Bakouny Z, Paciotti M, Schmidt AL, Lipsitz SR, Choueiri TK, Trinh QD. Cancer screening tests and cancer diagnoses during the COVID-19 pandemic. JAMA Oncol. 2021;7(3):458-460. doi:10.1001/jamaoncol.2020.7600
16. Steffen R, Lautenschlager S, Fehr J. Travel restrictions and lockdown during the COVID-19 pandemic-impact on notified infectious diseases in Switzerland. J Travel Med. 2020;27(8):taaa180. doi:10.1093/jtm/taaa180
17. CDC Museum COVID-19 Timeline. Centers for Disease Control and Prevention. Updated March 15, 2023. Accessed May 12, 2023. https://www.cdc.gov/museum/timeline/covid19.html18. Nguyen JL, Benigno M, Malhotra D, et al. Pandemic-related declines in hospitalization for non-COVID-19-related illness in the United States from January through July 2020. PLoS One. 2022;17(1):e0262347. Published 2022 Jan 6. doi:10.1371/journal.pone.0262347
19. Ward RE, Orkaby AR, Dumontier C, et al. Trajectories of frailty in the 5 years prior to death among U.S. veterans born 1927-1934. J Gerontol A Biol Sci Med Sci. 2021;76(11):e347-e353. doi:10.1093/gerona/glab196
20. Bäckman K, Joas E, Falk H, Mitnitski A, Rockwood K, Skoog I. Changes in the lethality of frailty over 30 years: evidence from two cohorts of 70-year-olds in Gothenburg Sweden. J Gerontol A Biol Sci Med Sci. 2017;72(7):945-950. doi:10.1093/gerona/glw160
21. Piette JD, Holtz B, Beard AJ, et al. Improving chronic illness care for veterans within the framework of the Patient-Centered Medical Home: experiences from the Ann Arbor Patient-Aligned Care Team Laboratory. Transl Behav Med. 2011;1(4):615-623. doi:10.1007/s13142-011-0065-8
22. Rosland AM, Nelson K, Sun H, et al. The patient-centered medical home in the Veterans Health Administration. Am J Manag Care. 2013;19(7):e263-e272. Published 2013 Jul 1.
1. Rohrmann S. Epidemiology of frailty in older people. Adv Exp Med Biol. 2020;1216:21-27. doi:10.1007/978-3-030-33330-0_3
2. Bandeen-Roche K, Seplaki CL, Huang J, et al. Frailty in older adults: a nationally representative profile in the United States. J Gerontol A Biol Sci Med Sci. 2015;70(11):1427-1434. doi:10.1093/gerona/glv133
3. Siriwardhana DD, Hardoon S, Rait G, Weerasinghe MC, Walters KR. Prevalence of frailty and prefrailty among community-dwelling older adults in low-income and middle-income countries: a systematic review and meta-analysis. BMJ Open. 2018;8(3):e018195. Published 2018 Mar 1. doi:10.1136/bmjopen-2017-018195
4. Song X, Mitnitski A, Rockwood K. Prevalence and 10-year outcomes of frailty in older adults in relation to deficit accumulation. J Am Geriatr Soc. 2010;58(4):681-687. doi:10.1111/j.1532-5415.2010.02764.x
5. Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci. 2007;62(7):722-727. doi:10.1093/gerona/62.7.722
6. Buta BJ, Walston JD, Godino JG, et al. Frailty assessment instruments: Systematic characterization of the uses and contexts of highly-cited instruments. Ageing Res Rev. 2016;26:53-61. doi:10.1016/j.arr.2015.12.003
7. Cheng D, DuMontier C, Yildirim C, et al. Updating and validating the U.S. Veterans Affairs Frailty Index: transitioning From ICD-9 to ICD-10. J Gerontol A Biol Sci Med Sci. 2021;76(7):1318-1325. doi:10.1093/gerona/glab071
8. Fihn SD, Francis J, Clancy C, et al. Insights from advanced analytics at the Veterans Health Administration. Health Aff (Millwood). 2014;33(7):1203-1211. doi:10.1377/hlthaff.2014.0054
9. Ruiz JG, Priyadarshni S, Rahaman Z, et al. Validation of an automatically generated screening score for frailty: the care assessment need (CAN) score. BMC Geriatr. 2018;18(1):106. doi:10.1186/s12877-018-0802-7
10. Ruiz JG, Rahaman Z, Dang S, Anam R, Valencia WM, Mintzer MJ. Association of the CAN score with the FRAIL scale in community dwelling older adults. Aging Clin Exp Res. 2018;30(10):1241-1245. doi:10.1007/s40520-018-0910-4
11. Ofori-Asenso R, Chin KL, Mazidi M, et al. Global incidence of frailty and prefrailty among community-dwelling older adults: a systematic review and meta-analysis. JAMA Netw Open. 2019;2(8):e198398. Published 2019 Aug 2. doi:10.1001/jamanetworkopen.2019.8398
12. Marcucci M, Damanti S, Germini F, et al. Interventions to prevent, delay or reverse frailty in older people: a journey towards clinical guidelines. BMC Med. 2019;17(1):193. Published 2019 Oct 29. doi:10.1186/s12916-019-1434-2
13. Travers J, Romero-Ortuno R, Bailey J, Cooney MT. Delaying and reversing frailty: a systematic review of primary care interventions. Br J Gen Pract. 2019;69(678):e61-e69. doi:10.3399/bjgp18X700241
14. Orkaby AR, Nussbaum L, Ho YL, et al. The burden of frailty among U.S. veterans and its association with mortality, 2002-2012. J Gerontol A Biol Sci Med Sci. 2019;74(8):1257-1264. doi:10.1093/gerona/gly232
15. Bakouny Z, Paciotti M, Schmidt AL, Lipsitz SR, Choueiri TK, Trinh QD. Cancer screening tests and cancer diagnoses during the COVID-19 pandemic. JAMA Oncol. 2021;7(3):458-460. doi:10.1001/jamaoncol.2020.7600
16. Steffen R, Lautenschlager S, Fehr J. Travel restrictions and lockdown during the COVID-19 pandemic-impact on notified infectious diseases in Switzerland. J Travel Med. 2020;27(8):taaa180. doi:10.1093/jtm/taaa180
17. CDC Museum COVID-19 Timeline. Centers for Disease Control and Prevention. Updated March 15, 2023. Accessed May 12, 2023. https://www.cdc.gov/museum/timeline/covid19.html18. Nguyen JL, Benigno M, Malhotra D, et al. Pandemic-related declines in hospitalization for non-COVID-19-related illness in the United States from January through July 2020. PLoS One. 2022;17(1):e0262347. Published 2022 Jan 6. doi:10.1371/journal.pone.0262347
19. Ward RE, Orkaby AR, Dumontier C, et al. Trajectories of frailty in the 5 years prior to death among U.S. veterans born 1927-1934. J Gerontol A Biol Sci Med Sci. 2021;76(11):e347-e353. doi:10.1093/gerona/glab196
20. Bäckman K, Joas E, Falk H, Mitnitski A, Rockwood K, Skoog I. Changes in the lethality of frailty over 30 years: evidence from two cohorts of 70-year-olds in Gothenburg Sweden. J Gerontol A Biol Sci Med Sci. 2017;72(7):945-950. doi:10.1093/gerona/glw160
21. Piette JD, Holtz B, Beard AJ, et al. Improving chronic illness care for veterans within the framework of the Patient-Centered Medical Home: experiences from the Ann Arbor Patient-Aligned Care Team Laboratory. Transl Behav Med. 2011;1(4):615-623. doi:10.1007/s13142-011-0065-8
22. Rosland AM, Nelson K, Sun H, et al. The patient-centered medical home in the Veterans Health Administration. Am J Manag Care. 2013;19(7):e263-e272. Published 2013 Jul 1.
Impact of Pharmacist Interventions at an Outpatient US Coast Guard Clinic
The US Coast Guard (USCG) operates within the US Department of Homeland Security during times of peace and represents a force of > 55,000 active-duty service members (ADSMs), civilians, and reservists. ADSMs account for about 40,000 USCG personnel. The missions of the USCG include activities such as maritime law enforcement (drug interdiction), search and rescue, and defense readiness.1 Akin to other US Department of Defense (DoD) services, USCG ADSMs are required to maintain medical readiness to maximize operational success.
Whereas the DoD centralizes its health care services at military treatment facilities, USCG health care tends to be dispersed to smaller clinics and sickbays across large geographic areas. The USCG operates 42 clinics of varying sizes and medical capabilities, providing outpatient, dentistry, pharmacy, laboratory, radiology, physical therapy, optometry, and other health care services. Many ADSMs are evaluated by a USCG medical officer in these outpatient clinics, and ADSMs may choose to fill prescriptions at the in-house pharmacy if present at that clinic.
The USCG has 14 field pharmacists. In addition to the standard dispensing role at their respective clinics, USCG pharmacists provide regional oversight of pharmaceutical services for USCG units within their area of responsibility (AOR). Therefore, USCG pharmacists clinically, operationally, and logistically support these regional assets within their AOR while serving the traditional pharmacist role. USCG pharmacists have access to ADSM electronic health records (EHRs) when evaluating prescription orders, similar to other ambulatory care settings.
New recruits and accessions into the USCG are first screened for disqualifying health conditions, and ADSMs are required to maintain medical readiness throughout their careers.2 Therefore, this population tends to be younger and overall healthier compared with the general population. Equally important, medication errors or inappropriate prescribing in the ADSM group could negatively affect their duty status and mission readiness of the USCG in addition to exposing the ADSM to medication-related harms.
Duty status is an important and unique consideration in this population. ADSMs are expected to be deployable worldwide and physically and mentally capable of executing all duties associated with their position. Duty status implications and the perceived ability to stand watch are tied to an ADMS’s specialty, training, and unit role. Duty status is based on various frameworks like the USCG Medical Manual, Aeromedical Policy Letters, and other governing documents.3 Duty status determinations are initiated by privileged USCG medical practitioners and may be executed in consultation with relevant commands and other subject matter experts. An inappropriately dosed antibiotic prescription, for example, can extend the duration that an ADSM would be considered unfit for full duty due to prolonged illness. Accordingly, being on a limited duty status may negatively affect USCG total mission readiness as a whole. USCG pharmacists play a vital role in optimizing ADSMs’ medication therapies to ensure safety and efficacy.
Currently no published literature explores the number of medication interventions or the impact of those interventions made by USCG pharmacists. This study aimed to quantify the number, duty status impact, and replicability of medication interventions made by one pharmacist at the USCG Base Alameda clinic over 6 months.
Methods
As part of a USCG quality improvement study, a pharmacist tracked all medication interventions on a spreadsheet at USCG Base Alameda clinic from July 1, 2021, to December 31, 2021. The study defined a medication intervention as a communication with the prescriber with the intention to change the medication, strength, dose, dosage form, quantity, or instructions. Each intervention was subcategorized as either a drug therapy problem (DTP) or a non-DTP intervention. Interventions were divided into 7 categories.
Each DTP intervention was evaluated in a retrospective chart review by a panel of USCG pharmacists to assess for duty status severity and replicability. For duty status severity, the panel reviewed the intervention after considering patient-specific factors and determined whether the original prescribing (had there not been an intervention) could have reasonably resulted in a change of duty status for the ADSM from a fit for full duty (FFFD) status to a different duty status (eg, fit for limited duty [FFLD]). This duty status review factored in potential impacts across multiple positions and billets, including aviators (pilots) and divers. In addition, the panel, whose members all have prior community pharmacy experience, assessed replicability by determining whether the same intervention could have reasonably been made in the absence of access to the patient EHR, as would be common in a community pharmacy setting.
Interventions without an identified DTP were considered non-DTP interventions. These interventions involved recommendations for a more cost-effective medication or a similar in stock therapeutic option to minimize delay of patient care. The spreadsheet also included the date, medication name, medication class, specific intervention made, outcome, and other descriptive comments.
Results
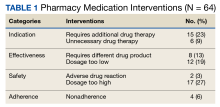
During the 6-month period, 1751 prescriptions were dispensed at USCG Base Alameda pharmacy with 116 interventions (7%).
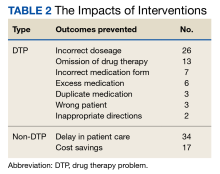
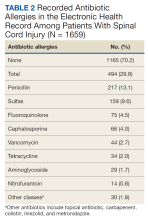
Among the DTP interventions, 26 (41%) dealt with an inappropriate dose, 13 (20%) were for medication omission, 7 (11%) for inappropriate dosage form, and 6 (9%) for excess medication (Table 2).
Discussion
This study is novel in examining the impact of a pharmacist’s medication interventions in a USCG ambulatory care practice setting. A PubMed literature search of the phrases “Coast Guard AND pharmacy” or “Coast Guard AND pharmacy AND intervention” yielded no results specific to pharmacy interventions in a USCG setting. However, the 2021 implementation of the enterprise-wide MHS GENESIS EHR may support additional tracking and analysis tools in the future.
Pharmacist interventions have been studied in diverse patient populations and practice settings, and most conclude that pharmacists make meaningful interventions at their respective organizations.4-7 Many of these studies were conducted at open-door health care systems, whereas USCG clinics serve ADSMs nearly exclusively. The ADSM population tends to be younger and healthier due to age requirements and medical accession and retention standards.
It is important to recognize the value of a USCG pharmacist in identifying and rectifying potential medication errors, particularly those that may affect the ability to stand duty for ADSMs. An example intervention includes changing the daily starting dose of citalopram from the ordered 30 mg to the intended 10 mg. Inappropriately prescribed medication regimens may increase the incidence of adverse effects or prolong duration to therapeutic efficacy, which impairs the ability to stand duty. There were 3 circumstances where the prescriber had ordered the medication for an incorrect ADSM that were rectified by the pharmacist. If left unchanged, these errors could negatively affect the ADSM’s overall health, well-being, and duty status.
The acceptance rate for interventions in this study was 96%. The literature suggests a highly variable acceptance rate of pharmacist interventions when examined across various practice settings, health systems, and geographic locations.8-10 This study’s comparatively high rate could be due to the pharmacist-prescriber relationships at USCG clinics. By virtue of colocatation and teamwork initiatives, the pharmacist has the opportunity to develop positive rapport with physicians, physician assistants, and other clinic staff.
Having access to EHRs allowed the pharmacist to make 18 of the DTP interventions. Chart access is not unique to the USCG and is common in other ambulatory care settings. Those 18 interventions, such as reconciling a prescription ordered as fluticasone/salmeterol but recorded in the EHR as “will prescribe montelukast,” were deemed possible because of EHR access. Such interventions could potentially be lost if ADSMs solely received their pharmaceutical care elsewhere.
USCG uses independent duty health services technicians (IDHSs) who practice in settings where a medical officer is not present, such as at smaller sickbays or aboard Coast Guard cutters. In this study, an IDHS had mistakenly created a medication order for the medical officer to sign for bupropion SR, when the ADSM had been taking and was intended to continue taking bupropion XL. This order was signed off by the medical officer, but this oversight was identified and corrected by the pharmacist before dispensing. This indicates that there is a vital educational role that the USCG pharmacist fulfills when working with health care team members within the AOR.
Equally important to consider are the non-DTP interventions. In a military setting, minimizations of delay in care are a high priority. There were 34 instances where the pharmacist made an intervention to recommend a similar therapeutic medication that was in stock to ensure that the ADSM had timely access to the medication without the need for prior authorization. In the context of short-notice, mission-critical deployments that may last for multiple months, recognizing medication shortages or other inventory constraints and recommending therapeutic alternatives ensures that the USCG can maintain a ready posture for missions in addition to providing timely and quality patient care.
Saving about $1700 over 6 months is also important. While this was not explicitly evaluated in the study, prescribers may not be acutely aware of medication pricing. There are often significant price differences between different formulations of the same medication (eg, naproxen delayed-release vs tablets). Because USCG pharmacists are responsible for ordering medications and managing their regional budget within the AOR, they are best poised to make cost-savings recommendations. These interventions suggest that USCG pharmacists must continue to remain actively involved in the patient care team alongside physicians, physician assistants, nurses, and corpsmen. Throughout this setting and in so many others, patients’ health outcomes improve when pharmacists are more engaged in the pharmacotherapy care plan.
Limitations
Currently, the USCG does not publish ADSM demographic or health-related data, making it difficult to evaluate these interventions in the context of age, gender, or type of disease. Accordingly, potential directions for future research include how USCG pharmacists’ interventions are stratified by duty station and initial diagnosis. Such studies may support future models where USCG pharmacists are providing targeted education to prescribers based on disease or medication classes.
This analysis may have limited applicability to other practice settings even within USCG. Most USCG clinics have a limited number of medical officers; indeed, many have only one, and clinics with pharmacies typically have 1 to 5 medical officers aboard. USCG medical officers have a multitude of other duties, which may impact prescribing patterns and pharmacist interventions. Statistical analyses were limited by the dearth of baseline data or comparative literature. Finally, the assessment of DTP interventions’ impact did not use an official measurement tool like the US Department of Veterans Affairs’ Safety Assessment Code matrix.11 Instead, the study used the internal USCG pharmacist panel for the fitness for duty consideration as the main stratification of the DTP interventions’ duty status severity, because maintaining medical readiness is the top priority for a USCG clinic.
Conclusions
The multifaceted role of pharmacists in USCG clinics includes collaborating with the patient care team to make pharmacy interventions that have significant impacts on ADSMs’ wellness and the USCG mission. The ADSMs of this nation deserve quality medical care that translates into mission readiness, and the USCG pharmacy force stands ready to support that goal.
Acknowledgments
The authors acknowledge the contributions of CDR Christopher Janik, US Coast Guard Headquarters, and LCDR Darin Schneider, US Coast Guard D11 Regional Practice Manager, in the drafting of the manuscript.
1. US Coast Guard. Missions. Accessed May 4, 2023. https://www.uscg.mil/About/Missions
2. US Coast Guard. Coast Guard Medical Manual. Updated September 13, 2022. Accessed May 4, 2023. https://media.defense.gov/2022/Sep/14/2003076969/-1/-1/0/CIM_6000_1F.PDF
3. US Coast Guard. USCG Aeromedical Policy Letters. Accessed May 5, 2023. https://www.dcms.uscg.mil/Portals/10/CG-1/cg112/cg1121/docs/pdf/USCG_Aeromedical_Policy_Letters.pdf
4. Bedouch P, Sylvoz N, Charpiat B, et al. Trends in pharmacists’ medication order review in French hospitals from 2006 to 2009: analysis of pharmacists’ interventions from the Act-IP website observatory. J Clin Pharm Ther. 2015;40(1):32-40. doi:10.1111/jcpt.12214
5. Ooi PL, Zainal H, Lean QY, Ming LC, Ibrahim B. Pharmacists’ interventions on electronic prescriptions from various specialty wards in a Malaysian public hospital: a cross-sectional study. Pharmacy (Basel). 2021;9(4):161. Published 2021 Oct 1. doi:10.3390/pharmacy9040161
6. Alomi YA, El-Bahnasawi M, Kamran M, Shaweesh T, Alhaj S, Radwan RA. The clinical outcomes of pharmacist interventions at critical care services of private hospital in Riyadh City, Saudi Arabia. PTB Report. 2019;5(1):16-19. doi:10.5530/ptb.2019.5.4
7. Garin N, Sole N, Lucas B, et al. Drug related problems in clinical practice: a cross-sectional study on their prevalence, risk factors and associated pharmaceutical interventions. Sci Rep. 2021;11(1):883. Published 2021 Jan 13. doi:10.1038/s41598-020-80560-2
8. Zaal RJ, den Haak EW, Andrinopoulou ER, van Gelder T, Vulto AG, van den Bemt PMLA. Physicians’ acceptance of pharmacists’ interventions in daily hospital practice. Int J Clin Pharm. 2020;42(1):141-149. doi:10.1007/s11096-020-00970-0
9. Carson GL, Crosby K, Huxall GR, Brahm NC. Acceptance rates for pharmacist-initiated interventions in long-term care facilities. Inov Pharm. 2013;4(4):Article 135.
10. Bondesson A, Holmdahl L, Midlöv P, Höglund P, Andersson E, Eriksson T. Acceptance and importance of clinical pharmacists’ LIMM-based recommendations. Int J Clin Pharm. 2012;34(2):272-276. doi:10.1007/s11096-012-9609-3
11. US Department of Veterans Affairs. Safety assessment code (SAC) matrix. Updated June 3, 2015. Accessed May 4, 2023. https://www.patientsafety.va.gov/professionals/publications/matrix.asp
The US Coast Guard (USCG) operates within the US Department of Homeland Security during times of peace and represents a force of > 55,000 active-duty service members (ADSMs), civilians, and reservists. ADSMs account for about 40,000 USCG personnel. The missions of the USCG include activities such as maritime law enforcement (drug interdiction), search and rescue, and defense readiness.1 Akin to other US Department of Defense (DoD) services, USCG ADSMs are required to maintain medical readiness to maximize operational success.
Whereas the DoD centralizes its health care services at military treatment facilities, USCG health care tends to be dispersed to smaller clinics and sickbays across large geographic areas. The USCG operates 42 clinics of varying sizes and medical capabilities, providing outpatient, dentistry, pharmacy, laboratory, radiology, physical therapy, optometry, and other health care services. Many ADSMs are evaluated by a USCG medical officer in these outpatient clinics, and ADSMs may choose to fill prescriptions at the in-house pharmacy if present at that clinic.
The USCG has 14 field pharmacists. In addition to the standard dispensing role at their respective clinics, USCG pharmacists provide regional oversight of pharmaceutical services for USCG units within their area of responsibility (AOR). Therefore, USCG pharmacists clinically, operationally, and logistically support these regional assets within their AOR while serving the traditional pharmacist role. USCG pharmacists have access to ADSM electronic health records (EHRs) when evaluating prescription orders, similar to other ambulatory care settings.
New recruits and accessions into the USCG are first screened for disqualifying health conditions, and ADSMs are required to maintain medical readiness throughout their careers.2 Therefore, this population tends to be younger and overall healthier compared with the general population. Equally important, medication errors or inappropriate prescribing in the ADSM group could negatively affect their duty status and mission readiness of the USCG in addition to exposing the ADSM to medication-related harms.
Duty status is an important and unique consideration in this population. ADSMs are expected to be deployable worldwide and physically and mentally capable of executing all duties associated with their position. Duty status implications and the perceived ability to stand watch are tied to an ADMS’s specialty, training, and unit role. Duty status is based on various frameworks like the USCG Medical Manual, Aeromedical Policy Letters, and other governing documents.3 Duty status determinations are initiated by privileged USCG medical practitioners and may be executed in consultation with relevant commands and other subject matter experts. An inappropriately dosed antibiotic prescription, for example, can extend the duration that an ADSM would be considered unfit for full duty due to prolonged illness. Accordingly, being on a limited duty status may negatively affect USCG total mission readiness as a whole. USCG pharmacists play a vital role in optimizing ADSMs’ medication therapies to ensure safety and efficacy.
Currently no published literature explores the number of medication interventions or the impact of those interventions made by USCG pharmacists. This study aimed to quantify the number, duty status impact, and replicability of medication interventions made by one pharmacist at the USCG Base Alameda clinic over 6 months.
Methods
As part of a USCG quality improvement study, a pharmacist tracked all medication interventions on a spreadsheet at USCG Base Alameda clinic from July 1, 2021, to December 31, 2021. The study defined a medication intervention as a communication with the prescriber with the intention to change the medication, strength, dose, dosage form, quantity, or instructions. Each intervention was subcategorized as either a drug therapy problem (DTP) or a non-DTP intervention. Interventions were divided into 7 categories.
Each DTP intervention was evaluated in a retrospective chart review by a panel of USCG pharmacists to assess for duty status severity and replicability. For duty status severity, the panel reviewed the intervention after considering patient-specific factors and determined whether the original prescribing (had there not been an intervention) could have reasonably resulted in a change of duty status for the ADSM from a fit for full duty (FFFD) status to a different duty status (eg, fit for limited duty [FFLD]). This duty status review factored in potential impacts across multiple positions and billets, including aviators (pilots) and divers. In addition, the panel, whose members all have prior community pharmacy experience, assessed replicability by determining whether the same intervention could have reasonably been made in the absence of access to the patient EHR, as would be common in a community pharmacy setting.
Interventions without an identified DTP were considered non-DTP interventions. These interventions involved recommendations for a more cost-effective medication or a similar in stock therapeutic option to minimize delay of patient care. The spreadsheet also included the date, medication name, medication class, specific intervention made, outcome, and other descriptive comments.
Results
During the 6-month period, 1751 prescriptions were dispensed at USCG Base Alameda pharmacy with 116 interventions (7%).
Among the DTP interventions, 26 (41%) dealt with an inappropriate dose, 13 (20%) were for medication omission, 7 (11%) for inappropriate dosage form, and 6 (9%) for excess medication (Table 2).
Discussion
This study is novel in examining the impact of a pharmacist’s medication interventions in a USCG ambulatory care practice setting. A PubMed literature search of the phrases “Coast Guard AND pharmacy” or “Coast Guard AND pharmacy AND intervention” yielded no results specific to pharmacy interventions in a USCG setting. However, the 2021 implementation of the enterprise-wide MHS GENESIS EHR may support additional tracking and analysis tools in the future.
Pharmacist interventions have been studied in diverse patient populations and practice settings, and most conclude that pharmacists make meaningful interventions at their respective organizations.4-7 Many of these studies were conducted at open-door health care systems, whereas USCG clinics serve ADSMs nearly exclusively. The ADSM population tends to be younger and healthier due to age requirements and medical accession and retention standards.
It is important to recognize the value of a USCG pharmacist in identifying and rectifying potential medication errors, particularly those that may affect the ability to stand duty for ADSMs. An example intervention includes changing the daily starting dose of citalopram from the ordered 30 mg to the intended 10 mg. Inappropriately prescribed medication regimens may increase the incidence of adverse effects or prolong duration to therapeutic efficacy, which impairs the ability to stand duty. There were 3 circumstances where the prescriber had ordered the medication for an incorrect ADSM that were rectified by the pharmacist. If left unchanged, these errors could negatively affect the ADSM’s overall health, well-being, and duty status.
The acceptance rate for interventions in this study was 96%. The literature suggests a highly variable acceptance rate of pharmacist interventions when examined across various practice settings, health systems, and geographic locations.8-10 This study’s comparatively high rate could be due to the pharmacist-prescriber relationships at USCG clinics. By virtue of colocatation and teamwork initiatives, the pharmacist has the opportunity to develop positive rapport with physicians, physician assistants, and other clinic staff.
Having access to EHRs allowed the pharmacist to make 18 of the DTP interventions. Chart access is not unique to the USCG and is common in other ambulatory care settings. Those 18 interventions, such as reconciling a prescription ordered as fluticasone/salmeterol but recorded in the EHR as “will prescribe montelukast,” were deemed possible because of EHR access. Such interventions could potentially be lost if ADSMs solely received their pharmaceutical care elsewhere.
USCG uses independent duty health services technicians (IDHSs) who practice in settings where a medical officer is not present, such as at smaller sickbays or aboard Coast Guard cutters. In this study, an IDHS had mistakenly created a medication order for the medical officer to sign for bupropion SR, when the ADSM had been taking and was intended to continue taking bupropion XL. This order was signed off by the medical officer, but this oversight was identified and corrected by the pharmacist before dispensing. This indicates that there is a vital educational role that the USCG pharmacist fulfills when working with health care team members within the AOR.
Equally important to consider are the non-DTP interventions. In a military setting, minimizations of delay in care are a high priority. There were 34 instances where the pharmacist made an intervention to recommend a similar therapeutic medication that was in stock to ensure that the ADSM had timely access to the medication without the need for prior authorization. In the context of short-notice, mission-critical deployments that may last for multiple months, recognizing medication shortages or other inventory constraints and recommending therapeutic alternatives ensures that the USCG can maintain a ready posture for missions in addition to providing timely and quality patient care.
Saving about $1700 over 6 months is also important. While this was not explicitly evaluated in the study, prescribers may not be acutely aware of medication pricing. There are often significant price differences between different formulations of the same medication (eg, naproxen delayed-release vs tablets). Because USCG pharmacists are responsible for ordering medications and managing their regional budget within the AOR, they are best poised to make cost-savings recommendations. These interventions suggest that USCG pharmacists must continue to remain actively involved in the patient care team alongside physicians, physician assistants, nurses, and corpsmen. Throughout this setting and in so many others, patients’ health outcomes improve when pharmacists are more engaged in the pharmacotherapy care plan.
Limitations
Currently, the USCG does not publish ADSM demographic or health-related data, making it difficult to evaluate these interventions in the context of age, gender, or type of disease. Accordingly, potential directions for future research include how USCG pharmacists’ interventions are stratified by duty station and initial diagnosis. Such studies may support future models where USCG pharmacists are providing targeted education to prescribers based on disease or medication classes.
This analysis may have limited applicability to other practice settings even within USCG. Most USCG clinics have a limited number of medical officers; indeed, many have only one, and clinics with pharmacies typically have 1 to 5 medical officers aboard. USCG medical officers have a multitude of other duties, which may impact prescribing patterns and pharmacist interventions. Statistical analyses were limited by the dearth of baseline data or comparative literature. Finally, the assessment of DTP interventions’ impact did not use an official measurement tool like the US Department of Veterans Affairs’ Safety Assessment Code matrix.11 Instead, the study used the internal USCG pharmacist panel for the fitness for duty consideration as the main stratification of the DTP interventions’ duty status severity, because maintaining medical readiness is the top priority for a USCG clinic.
Conclusions
The multifaceted role of pharmacists in USCG clinics includes collaborating with the patient care team to make pharmacy interventions that have significant impacts on ADSMs’ wellness and the USCG mission. The ADSMs of this nation deserve quality medical care that translates into mission readiness, and the USCG pharmacy force stands ready to support that goal.
Acknowledgments
The authors acknowledge the contributions of CDR Christopher Janik, US Coast Guard Headquarters, and LCDR Darin Schneider, US Coast Guard D11 Regional Practice Manager, in the drafting of the manuscript.
The US Coast Guard (USCG) operates within the US Department of Homeland Security during times of peace and represents a force of > 55,000 active-duty service members (ADSMs), civilians, and reservists. ADSMs account for about 40,000 USCG personnel. The missions of the USCG include activities such as maritime law enforcement (drug interdiction), search and rescue, and defense readiness.1 Akin to other US Department of Defense (DoD) services, USCG ADSMs are required to maintain medical readiness to maximize operational success.
Whereas the DoD centralizes its health care services at military treatment facilities, USCG health care tends to be dispersed to smaller clinics and sickbays across large geographic areas. The USCG operates 42 clinics of varying sizes and medical capabilities, providing outpatient, dentistry, pharmacy, laboratory, radiology, physical therapy, optometry, and other health care services. Many ADSMs are evaluated by a USCG medical officer in these outpatient clinics, and ADSMs may choose to fill prescriptions at the in-house pharmacy if present at that clinic.
The USCG has 14 field pharmacists. In addition to the standard dispensing role at their respective clinics, USCG pharmacists provide regional oversight of pharmaceutical services for USCG units within their area of responsibility (AOR). Therefore, USCG pharmacists clinically, operationally, and logistically support these regional assets within their AOR while serving the traditional pharmacist role. USCG pharmacists have access to ADSM electronic health records (EHRs) when evaluating prescription orders, similar to other ambulatory care settings.
New recruits and accessions into the USCG are first screened for disqualifying health conditions, and ADSMs are required to maintain medical readiness throughout their careers.2 Therefore, this population tends to be younger and overall healthier compared with the general population. Equally important, medication errors or inappropriate prescribing in the ADSM group could negatively affect their duty status and mission readiness of the USCG in addition to exposing the ADSM to medication-related harms.
Duty status is an important and unique consideration in this population. ADSMs are expected to be deployable worldwide and physically and mentally capable of executing all duties associated with their position. Duty status implications and the perceived ability to stand watch are tied to an ADMS’s specialty, training, and unit role. Duty status is based on various frameworks like the USCG Medical Manual, Aeromedical Policy Letters, and other governing documents.3 Duty status determinations are initiated by privileged USCG medical practitioners and may be executed in consultation with relevant commands and other subject matter experts. An inappropriately dosed antibiotic prescription, for example, can extend the duration that an ADSM would be considered unfit for full duty due to prolonged illness. Accordingly, being on a limited duty status may negatively affect USCG total mission readiness as a whole. USCG pharmacists play a vital role in optimizing ADSMs’ medication therapies to ensure safety and efficacy.
Currently no published literature explores the number of medication interventions or the impact of those interventions made by USCG pharmacists. This study aimed to quantify the number, duty status impact, and replicability of medication interventions made by one pharmacist at the USCG Base Alameda clinic over 6 months.
Methods
As part of a USCG quality improvement study, a pharmacist tracked all medication interventions on a spreadsheet at USCG Base Alameda clinic from July 1, 2021, to December 31, 2021. The study defined a medication intervention as a communication with the prescriber with the intention to change the medication, strength, dose, dosage form, quantity, or instructions. Each intervention was subcategorized as either a drug therapy problem (DTP) or a non-DTP intervention. Interventions were divided into 7 categories.
Each DTP intervention was evaluated in a retrospective chart review by a panel of USCG pharmacists to assess for duty status severity and replicability. For duty status severity, the panel reviewed the intervention after considering patient-specific factors and determined whether the original prescribing (had there not been an intervention) could have reasonably resulted in a change of duty status for the ADSM from a fit for full duty (FFFD) status to a different duty status (eg, fit for limited duty [FFLD]). This duty status review factored in potential impacts across multiple positions and billets, including aviators (pilots) and divers. In addition, the panel, whose members all have prior community pharmacy experience, assessed replicability by determining whether the same intervention could have reasonably been made in the absence of access to the patient EHR, as would be common in a community pharmacy setting.
Interventions without an identified DTP were considered non-DTP interventions. These interventions involved recommendations for a more cost-effective medication or a similar in stock therapeutic option to minimize delay of patient care. The spreadsheet also included the date, medication name, medication class, specific intervention made, outcome, and other descriptive comments.
Results
During the 6-month period, 1751 prescriptions were dispensed at USCG Base Alameda pharmacy with 116 interventions (7%).
Among the DTP interventions, 26 (41%) dealt with an inappropriate dose, 13 (20%) were for medication omission, 7 (11%) for inappropriate dosage form, and 6 (9%) for excess medication (Table 2).
Discussion
This study is novel in examining the impact of a pharmacist’s medication interventions in a USCG ambulatory care practice setting. A PubMed literature search of the phrases “Coast Guard AND pharmacy” or “Coast Guard AND pharmacy AND intervention” yielded no results specific to pharmacy interventions in a USCG setting. However, the 2021 implementation of the enterprise-wide MHS GENESIS EHR may support additional tracking and analysis tools in the future.
Pharmacist interventions have been studied in diverse patient populations and practice settings, and most conclude that pharmacists make meaningful interventions at their respective organizations.4-7 Many of these studies were conducted at open-door health care systems, whereas USCG clinics serve ADSMs nearly exclusively. The ADSM population tends to be younger and healthier due to age requirements and medical accession and retention standards.
It is important to recognize the value of a USCG pharmacist in identifying and rectifying potential medication errors, particularly those that may affect the ability to stand duty for ADSMs. An example intervention includes changing the daily starting dose of citalopram from the ordered 30 mg to the intended 10 mg. Inappropriately prescribed medication regimens may increase the incidence of adverse effects or prolong duration to therapeutic efficacy, which impairs the ability to stand duty. There were 3 circumstances where the prescriber had ordered the medication for an incorrect ADSM that were rectified by the pharmacist. If left unchanged, these errors could negatively affect the ADSM’s overall health, well-being, and duty status.
The acceptance rate for interventions in this study was 96%. The literature suggests a highly variable acceptance rate of pharmacist interventions when examined across various practice settings, health systems, and geographic locations.8-10 This study’s comparatively high rate could be due to the pharmacist-prescriber relationships at USCG clinics. By virtue of colocatation and teamwork initiatives, the pharmacist has the opportunity to develop positive rapport with physicians, physician assistants, and other clinic staff.
Having access to EHRs allowed the pharmacist to make 18 of the DTP interventions. Chart access is not unique to the USCG and is common in other ambulatory care settings. Those 18 interventions, such as reconciling a prescription ordered as fluticasone/salmeterol but recorded in the EHR as “will prescribe montelukast,” were deemed possible because of EHR access. Such interventions could potentially be lost if ADSMs solely received their pharmaceutical care elsewhere.
USCG uses independent duty health services technicians (IDHSs) who practice in settings where a medical officer is not present, such as at smaller sickbays or aboard Coast Guard cutters. In this study, an IDHS had mistakenly created a medication order for the medical officer to sign for bupropion SR, when the ADSM had been taking and was intended to continue taking bupropion XL. This order was signed off by the medical officer, but this oversight was identified and corrected by the pharmacist before dispensing. This indicates that there is a vital educational role that the USCG pharmacist fulfills when working with health care team members within the AOR.
Equally important to consider are the non-DTP interventions. In a military setting, minimizations of delay in care are a high priority. There were 34 instances where the pharmacist made an intervention to recommend a similar therapeutic medication that was in stock to ensure that the ADSM had timely access to the medication without the need for prior authorization. In the context of short-notice, mission-critical deployments that may last for multiple months, recognizing medication shortages or other inventory constraints and recommending therapeutic alternatives ensures that the USCG can maintain a ready posture for missions in addition to providing timely and quality patient care.
Saving about $1700 over 6 months is also important. While this was not explicitly evaluated in the study, prescribers may not be acutely aware of medication pricing. There are often significant price differences between different formulations of the same medication (eg, naproxen delayed-release vs tablets). Because USCG pharmacists are responsible for ordering medications and managing their regional budget within the AOR, they are best poised to make cost-savings recommendations. These interventions suggest that USCG pharmacists must continue to remain actively involved in the patient care team alongside physicians, physician assistants, nurses, and corpsmen. Throughout this setting and in so many others, patients’ health outcomes improve when pharmacists are more engaged in the pharmacotherapy care plan.
Limitations
Currently, the USCG does not publish ADSM demographic or health-related data, making it difficult to evaluate these interventions in the context of age, gender, or type of disease. Accordingly, potential directions for future research include how USCG pharmacists’ interventions are stratified by duty station and initial diagnosis. Such studies may support future models where USCG pharmacists are providing targeted education to prescribers based on disease or medication classes.
This analysis may have limited applicability to other practice settings even within USCG. Most USCG clinics have a limited number of medical officers; indeed, many have only one, and clinics with pharmacies typically have 1 to 5 medical officers aboard. USCG medical officers have a multitude of other duties, which may impact prescribing patterns and pharmacist interventions. Statistical analyses were limited by the dearth of baseline data or comparative literature. Finally, the assessment of DTP interventions’ impact did not use an official measurement tool like the US Department of Veterans Affairs’ Safety Assessment Code matrix.11 Instead, the study used the internal USCG pharmacist panel for the fitness for duty consideration as the main stratification of the DTP interventions’ duty status severity, because maintaining medical readiness is the top priority for a USCG clinic.
Conclusions
The multifaceted role of pharmacists in USCG clinics includes collaborating with the patient care team to make pharmacy interventions that have significant impacts on ADSMs’ wellness and the USCG mission. The ADSMs of this nation deserve quality medical care that translates into mission readiness, and the USCG pharmacy force stands ready to support that goal.
Acknowledgments
The authors acknowledge the contributions of CDR Christopher Janik, US Coast Guard Headquarters, and LCDR Darin Schneider, US Coast Guard D11 Regional Practice Manager, in the drafting of the manuscript.
1. US Coast Guard. Missions. Accessed May 4, 2023. https://www.uscg.mil/About/Missions
2. US Coast Guard. Coast Guard Medical Manual. Updated September 13, 2022. Accessed May 4, 2023. https://media.defense.gov/2022/Sep/14/2003076969/-1/-1/0/CIM_6000_1F.PDF
3. US Coast Guard. USCG Aeromedical Policy Letters. Accessed May 5, 2023. https://www.dcms.uscg.mil/Portals/10/CG-1/cg112/cg1121/docs/pdf/USCG_Aeromedical_Policy_Letters.pdf
4. Bedouch P, Sylvoz N, Charpiat B, et al. Trends in pharmacists’ medication order review in French hospitals from 2006 to 2009: analysis of pharmacists’ interventions from the Act-IP website observatory. J Clin Pharm Ther. 2015;40(1):32-40. doi:10.1111/jcpt.12214
5. Ooi PL, Zainal H, Lean QY, Ming LC, Ibrahim B. Pharmacists’ interventions on electronic prescriptions from various specialty wards in a Malaysian public hospital: a cross-sectional study. Pharmacy (Basel). 2021;9(4):161. Published 2021 Oct 1. doi:10.3390/pharmacy9040161
6. Alomi YA, El-Bahnasawi M, Kamran M, Shaweesh T, Alhaj S, Radwan RA. The clinical outcomes of pharmacist interventions at critical care services of private hospital in Riyadh City, Saudi Arabia. PTB Report. 2019;5(1):16-19. doi:10.5530/ptb.2019.5.4
7. Garin N, Sole N, Lucas B, et al. Drug related problems in clinical practice: a cross-sectional study on their prevalence, risk factors and associated pharmaceutical interventions. Sci Rep. 2021;11(1):883. Published 2021 Jan 13. doi:10.1038/s41598-020-80560-2
8. Zaal RJ, den Haak EW, Andrinopoulou ER, van Gelder T, Vulto AG, van den Bemt PMLA. Physicians’ acceptance of pharmacists’ interventions in daily hospital practice. Int J Clin Pharm. 2020;42(1):141-149. doi:10.1007/s11096-020-00970-0
9. Carson GL, Crosby K, Huxall GR, Brahm NC. Acceptance rates for pharmacist-initiated interventions in long-term care facilities. Inov Pharm. 2013;4(4):Article 135.
10. Bondesson A, Holmdahl L, Midlöv P, Höglund P, Andersson E, Eriksson T. Acceptance and importance of clinical pharmacists’ LIMM-based recommendations. Int J Clin Pharm. 2012;34(2):272-276. doi:10.1007/s11096-012-9609-3
11. US Department of Veterans Affairs. Safety assessment code (SAC) matrix. Updated June 3, 2015. Accessed May 4, 2023. https://www.patientsafety.va.gov/professionals/publications/matrix.asp
1. US Coast Guard. Missions. Accessed May 4, 2023. https://www.uscg.mil/About/Missions
2. US Coast Guard. Coast Guard Medical Manual. Updated September 13, 2022. Accessed May 4, 2023. https://media.defense.gov/2022/Sep/14/2003076969/-1/-1/0/CIM_6000_1F.PDF
3. US Coast Guard. USCG Aeromedical Policy Letters. Accessed May 5, 2023. https://www.dcms.uscg.mil/Portals/10/CG-1/cg112/cg1121/docs/pdf/USCG_Aeromedical_Policy_Letters.pdf
4. Bedouch P, Sylvoz N, Charpiat B, et al. Trends in pharmacists’ medication order review in French hospitals from 2006 to 2009: analysis of pharmacists’ interventions from the Act-IP website observatory. J Clin Pharm Ther. 2015;40(1):32-40. doi:10.1111/jcpt.12214
5. Ooi PL, Zainal H, Lean QY, Ming LC, Ibrahim B. Pharmacists’ interventions on electronic prescriptions from various specialty wards in a Malaysian public hospital: a cross-sectional study. Pharmacy (Basel). 2021;9(4):161. Published 2021 Oct 1. doi:10.3390/pharmacy9040161
6. Alomi YA, El-Bahnasawi M, Kamran M, Shaweesh T, Alhaj S, Radwan RA. The clinical outcomes of pharmacist interventions at critical care services of private hospital in Riyadh City, Saudi Arabia. PTB Report. 2019;5(1):16-19. doi:10.5530/ptb.2019.5.4
7. Garin N, Sole N, Lucas B, et al. Drug related problems in clinical practice: a cross-sectional study on their prevalence, risk factors and associated pharmaceutical interventions. Sci Rep. 2021;11(1):883. Published 2021 Jan 13. doi:10.1038/s41598-020-80560-2
8. Zaal RJ, den Haak EW, Andrinopoulou ER, van Gelder T, Vulto AG, van den Bemt PMLA. Physicians’ acceptance of pharmacists’ interventions in daily hospital practice. Int J Clin Pharm. 2020;42(1):141-149. doi:10.1007/s11096-020-00970-0
9. Carson GL, Crosby K, Huxall GR, Brahm NC. Acceptance rates for pharmacist-initiated interventions in long-term care facilities. Inov Pharm. 2013;4(4):Article 135.
10. Bondesson A, Holmdahl L, Midlöv P, Höglund P, Andersson E, Eriksson T. Acceptance and importance of clinical pharmacists’ LIMM-based recommendations. Int J Clin Pharm. 2012;34(2):272-276. doi:10.1007/s11096-012-9609-3
11. US Department of Veterans Affairs. Safety assessment code (SAC) matrix. Updated June 3, 2015. Accessed May 4, 2023. https://www.patientsafety.va.gov/professionals/publications/matrix.asp
Cross-sectional Analysis of Matched Dermatology Residency Applicants Without US Home Programs
To the Editor:
Dermatology is one of the most competitive residencies for matching, with a 57.5% match rate in 2022.1 Our prior study of research-mentor relationships among matched dermatology applicants corroborated the importance of home programs (HPs) and program connections.2 Therefore, our current objective was to compare profiles of matched dermatology applicants without HPs vs those with HPs.
We searched websites of 139 dermatology programs nationwide and found 1736 matched applicants from 2016 to 2020; of them, 323 did not have HPs. We determined program rank by research output using Doximity Residency Navigator (https://www.doximity.com/residency/). Advanced degrees (ADs) of applicants were identified using program websites and LinkedIn. A PubMed search was conducted for number of articles published by each applicant before September 15 of their match year. For applicants without HPs, we identified the senior author on each publication. The senior author publishing with an applicant most often was considered the research mentor. Two-tailed independent t tests and χ2 tests were used to determine statistical significance (P<.05).
On average, matched applicants without HPs matched in lower-ranked (74.4) and smaller (12.4) programs compared with matched applicants with HPs (45.3 [P<.0001] and 15.1 [P<.0001], respectively)(eTable). The mean number of publications was similar between matched applicants with HPs and without HPs (5.64 and 4.80, respectively; P=.0525) as well as the percentage with ADs (14.7% and 11.5%, respectively; P=.0953). Overall, 14.8% of matched applicants without HPs matched at their mentors’ institutions.
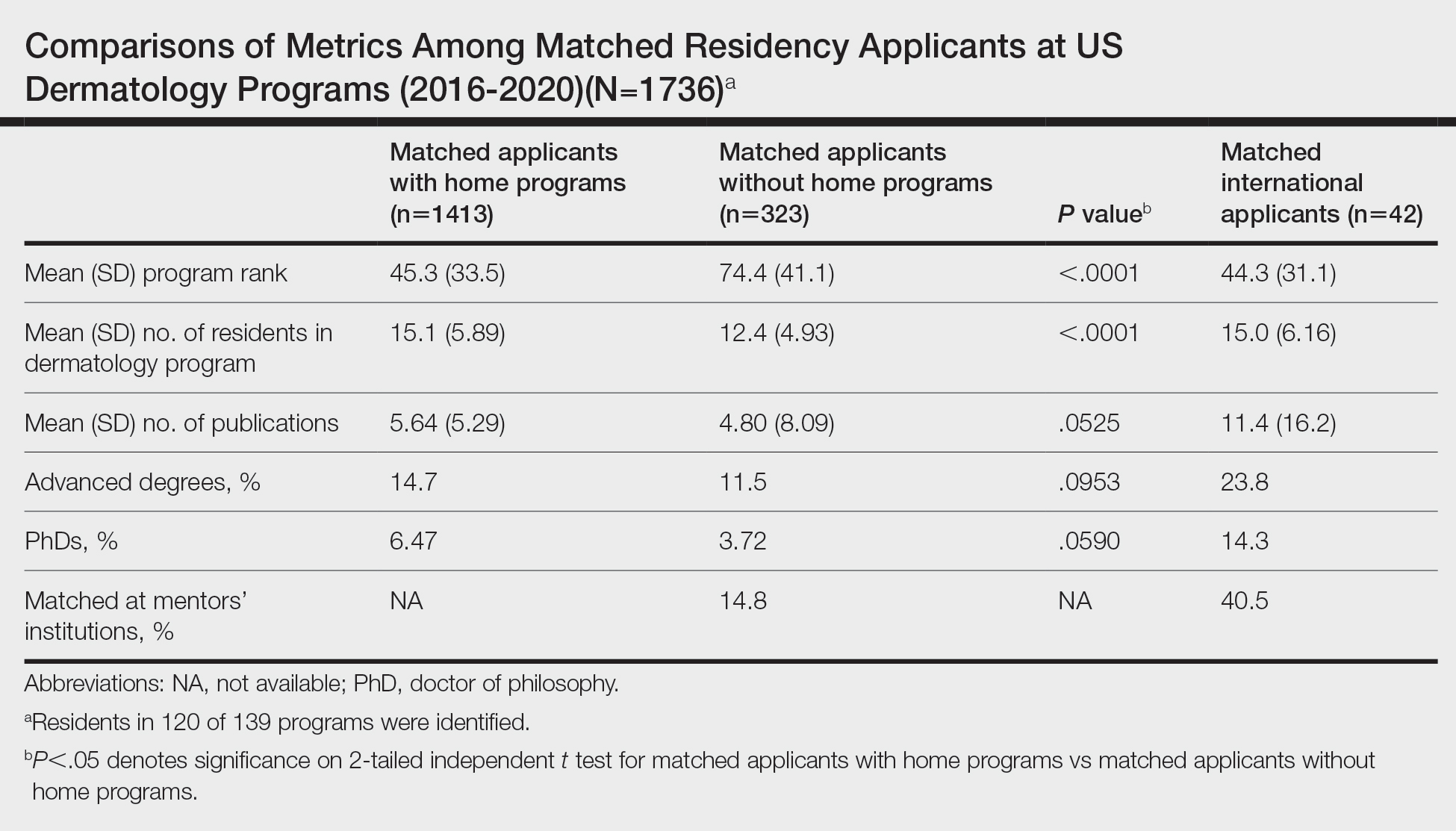
Data were obtained for matched international applicants as a subset of non-HP applicants. Despite attending medical schools without associated HPs in the United States, international applicants matched at similarly ranked (44.3) and sized (15.0) programs, on average, compared with HP applicants. The mean number of publications was higher for international applicants (11.4) vs domestic applicants (5.33). International applicants more often had ADs (23.8%) and 60.1% of them held doctor of philosophy degrees. Overall, 40.5% of international applicants matched at their mentors’ institutions.
Our study suggests that matched dermatology applicants with and without HPs had similar achievements, on average, for the number of publications and percentage with ADs. However, non-HP applicants matched at lower-ranked programs than HP applicants. Therefore, applicants without HPs should strongly consider cultivating program connections, especially if they desire to match at higher-ranked dermatology programs. To illustrate, the rate of matching at research mentors’ institutions was approximately 3-times higher for international applicants than non-HP applicants overall. Despite the disadvantages of applying as international applicants, they were able to match at substantially higher-ranked dermatology programs than non-HP applicants. International applicants may have a longer time investment—the number of years from obtaining their medical degree or US medical license to matching—giving them time to produce quality research and develop meaningful relationships at an institution. Additionally, our prior study of the top 25 dermatology residencies showed that 26.2% of successful applicants matched at their research mentors’ institutions, with almost half of this subset matching at their HPs, where their mentors also practiced.2 Because of the potential benefits of having program connections, applicants without HPs should seek dermatology research mentors, especially via highly beneficial in-person networking opportunities (eg, away rotations, conferences) that had previously been limited during the COVID-19 pandemic.3 Formal mentorship programs giving priority to students without HPs recently have been developed, which only begins to address the inequities in the dermatology residency application process.4
Study limitations include lack of resident information on 15 program websites, missed publications due to applicant name changes, not accounting for abstracts and posters, and inability to collect data on unmatched applicants.
We hope that our study alleviates some concerns that applicants without HPs may have regarding applying for dermatology residency and encourages those with a genuine interest in dermatology to pursue the specialty, provided they find a strong research mentor. Residency programs should be cognizant of the unique challenges that non-HP applicants face for matching.
- National Resident Matching Program. Results and Data: 2022 Main Residency Match. National Resident Matching Program; May 2022. Accessed May 30, 2023. https://www.nrmp.org/wp-content/uploads/2022/11 /2022-Main-Match-Results-and-Data-Final-Revised.pdf
- Yeh C, Desai AD, Wilson BN, et al. Cross-sectional analysis of scholarly work and mentor relationships in matched dermatology residency applicants. J Am Acad Dermatol. 2022;86:1437-1439.
- Association of American Medical Colleges. Specialty recommendations on away rotations for 2021-22 academic year. Accessed May 24, 2023. https://students-residents.aamc.org/researching-residency-programs -and-building-application-strategy/specialty-response-covid-19
- derminterest Instagram page. DIGA is excited for the second year of our mentor-mentee program! Mentors are dermatology residents. Please keep in mind due to the current circumstances, dermatology residency 2021-2022 applicants without home programs will be prioritized as mentees. Please refrain from signing up if you were paired with a faculty mentor for the APD-DIGA Mentorship Program in May 2021. Contact @suryasweetie123 only if you have specific questions, otherwise all information is on our website and the link is here. Link is below and in our bio! #DIGA #derm #mentee #residencyapplication. Accessed May 24, 2023. https://www.instagram.com/p/CSrq0exMchY/
To the Editor:
Dermatology is one of the most competitive residencies for matching, with a 57.5% match rate in 2022.1 Our prior study of research-mentor relationships among matched dermatology applicants corroborated the importance of home programs (HPs) and program connections.2 Therefore, our current objective was to compare profiles of matched dermatology applicants without HPs vs those with HPs.
We searched websites of 139 dermatology programs nationwide and found 1736 matched applicants from 2016 to 2020; of them, 323 did not have HPs. We determined program rank by research output using Doximity Residency Navigator (https://www.doximity.com/residency/). Advanced degrees (ADs) of applicants were identified using program websites and LinkedIn. A PubMed search was conducted for number of articles published by each applicant before September 15 of their match year. For applicants without HPs, we identified the senior author on each publication. The senior author publishing with an applicant most often was considered the research mentor. Two-tailed independent t tests and χ2 tests were used to determine statistical significance (P<.05).
On average, matched applicants without HPs matched in lower-ranked (74.4) and smaller (12.4) programs compared with matched applicants with HPs (45.3 [P<.0001] and 15.1 [P<.0001], respectively)(eTable). The mean number of publications was similar between matched applicants with HPs and without HPs (5.64 and 4.80, respectively; P=.0525) as well as the percentage with ADs (14.7% and 11.5%, respectively; P=.0953). Overall, 14.8% of matched applicants without HPs matched at their mentors’ institutions.

Data were obtained for matched international applicants as a subset of non-HP applicants. Despite attending medical schools without associated HPs in the United States, international applicants matched at similarly ranked (44.3) and sized (15.0) programs, on average, compared with HP applicants. The mean number of publications was higher for international applicants (11.4) vs domestic applicants (5.33). International applicants more often had ADs (23.8%) and 60.1% of them held doctor of philosophy degrees. Overall, 40.5% of international applicants matched at their mentors’ institutions.
Our study suggests that matched dermatology applicants with and without HPs had similar achievements, on average, for the number of publications and percentage with ADs. However, non-HP applicants matched at lower-ranked programs than HP applicants. Therefore, applicants without HPs should strongly consider cultivating program connections, especially if they desire to match at higher-ranked dermatology programs. To illustrate, the rate of matching at research mentors’ institutions was approximately 3-times higher for international applicants than non-HP applicants overall. Despite the disadvantages of applying as international applicants, they were able to match at substantially higher-ranked dermatology programs than non-HP applicants. International applicants may have a longer time investment—the number of years from obtaining their medical degree or US medical license to matching—giving them time to produce quality research and develop meaningful relationships at an institution. Additionally, our prior study of the top 25 dermatology residencies showed that 26.2% of successful applicants matched at their research mentors’ institutions, with almost half of this subset matching at their HPs, where their mentors also practiced.2 Because of the potential benefits of having program connections, applicants without HPs should seek dermatology research mentors, especially via highly beneficial in-person networking opportunities (eg, away rotations, conferences) that had previously been limited during the COVID-19 pandemic.3 Formal mentorship programs giving priority to students without HPs recently have been developed, which only begins to address the inequities in the dermatology residency application process.4
Study limitations include lack of resident information on 15 program websites, missed publications due to applicant name changes, not accounting for abstracts and posters, and inability to collect data on unmatched applicants.
We hope that our study alleviates some concerns that applicants without HPs may have regarding applying for dermatology residency and encourages those with a genuine interest in dermatology to pursue the specialty, provided they find a strong research mentor. Residency programs should be cognizant of the unique challenges that non-HP applicants face for matching.
To the Editor:
Dermatology is one of the most competitive residencies for matching, with a 57.5% match rate in 2022.1 Our prior study of research-mentor relationships among matched dermatology applicants corroborated the importance of home programs (HPs) and program connections.2 Therefore, our current objective was to compare profiles of matched dermatology applicants without HPs vs those with HPs.
We searched websites of 139 dermatology programs nationwide and found 1736 matched applicants from 2016 to 2020; of them, 323 did not have HPs. We determined program rank by research output using Doximity Residency Navigator (https://www.doximity.com/residency/). Advanced degrees (ADs) of applicants were identified using program websites and LinkedIn. A PubMed search was conducted for number of articles published by each applicant before September 15 of their match year. For applicants without HPs, we identified the senior author on each publication. The senior author publishing with an applicant most often was considered the research mentor. Two-tailed independent t tests and χ2 tests were used to determine statistical significance (P<.05).
On average, matched applicants without HPs matched in lower-ranked (74.4) and smaller (12.4) programs compared with matched applicants with HPs (45.3 [P<.0001] and 15.1 [P<.0001], respectively)(eTable). The mean number of publications was similar between matched applicants with HPs and without HPs (5.64 and 4.80, respectively; P=.0525) as well as the percentage with ADs (14.7% and 11.5%, respectively; P=.0953). Overall, 14.8% of matched applicants without HPs matched at their mentors’ institutions.

Data were obtained for matched international applicants as a subset of non-HP applicants. Despite attending medical schools without associated HPs in the United States, international applicants matched at similarly ranked (44.3) and sized (15.0) programs, on average, compared with HP applicants. The mean number of publications was higher for international applicants (11.4) vs domestic applicants (5.33). International applicants more often had ADs (23.8%) and 60.1% of them held doctor of philosophy degrees. Overall, 40.5% of international applicants matched at their mentors’ institutions.
Our study suggests that matched dermatology applicants with and without HPs had similar achievements, on average, for the number of publications and percentage with ADs. However, non-HP applicants matched at lower-ranked programs than HP applicants. Therefore, applicants without HPs should strongly consider cultivating program connections, especially if they desire to match at higher-ranked dermatology programs. To illustrate, the rate of matching at research mentors’ institutions was approximately 3-times higher for international applicants than non-HP applicants overall. Despite the disadvantages of applying as international applicants, they were able to match at substantially higher-ranked dermatology programs than non-HP applicants. International applicants may have a longer time investment—the number of years from obtaining their medical degree or US medical license to matching—giving them time to produce quality research and develop meaningful relationships at an institution. Additionally, our prior study of the top 25 dermatology residencies showed that 26.2% of successful applicants matched at their research mentors’ institutions, with almost half of this subset matching at their HPs, where their mentors also practiced.2 Because of the potential benefits of having program connections, applicants without HPs should seek dermatology research mentors, especially via highly beneficial in-person networking opportunities (eg, away rotations, conferences) that had previously been limited during the COVID-19 pandemic.3 Formal mentorship programs giving priority to students without HPs recently have been developed, which only begins to address the inequities in the dermatology residency application process.4
Study limitations include lack of resident information on 15 program websites, missed publications due to applicant name changes, not accounting for abstracts and posters, and inability to collect data on unmatched applicants.
We hope that our study alleviates some concerns that applicants without HPs may have regarding applying for dermatology residency and encourages those with a genuine interest in dermatology to pursue the specialty, provided they find a strong research mentor. Residency programs should be cognizant of the unique challenges that non-HP applicants face for matching.
- National Resident Matching Program. Results and Data: 2022 Main Residency Match. National Resident Matching Program; May 2022. Accessed May 30, 2023. https://www.nrmp.org/wp-content/uploads/2022/11 /2022-Main-Match-Results-and-Data-Final-Revised.pdf
- Yeh C, Desai AD, Wilson BN, et al. Cross-sectional analysis of scholarly work and mentor relationships in matched dermatology residency applicants. J Am Acad Dermatol. 2022;86:1437-1439.
- Association of American Medical Colleges. Specialty recommendations on away rotations for 2021-22 academic year. Accessed May 24, 2023. https://students-residents.aamc.org/researching-residency-programs -and-building-application-strategy/specialty-response-covid-19
- derminterest Instagram page. DIGA is excited for the second year of our mentor-mentee program! Mentors are dermatology residents. Please keep in mind due to the current circumstances, dermatology residency 2021-2022 applicants without home programs will be prioritized as mentees. Please refrain from signing up if you were paired with a faculty mentor for the APD-DIGA Mentorship Program in May 2021. Contact @suryasweetie123 only if you have specific questions, otherwise all information is on our website and the link is here. Link is below and in our bio! #DIGA #derm #mentee #residencyapplication. Accessed May 24, 2023. https://www.instagram.com/p/CSrq0exMchY/
- National Resident Matching Program. Results and Data: 2022 Main Residency Match. National Resident Matching Program; May 2022. Accessed May 30, 2023. https://www.nrmp.org/wp-content/uploads/2022/11 /2022-Main-Match-Results-and-Data-Final-Revised.pdf
- Yeh C, Desai AD, Wilson BN, et al. Cross-sectional analysis of scholarly work and mentor relationships in matched dermatology residency applicants. J Am Acad Dermatol. 2022;86:1437-1439.
- Association of American Medical Colleges. Specialty recommendations on away rotations for 2021-22 academic year. Accessed May 24, 2023. https://students-residents.aamc.org/researching-residency-programs -and-building-application-strategy/specialty-response-covid-19
- derminterest Instagram page. DIGA is excited for the second year of our mentor-mentee program! Mentors are dermatology residents. Please keep in mind due to the current circumstances, dermatology residency 2021-2022 applicants without home programs will be prioritized as mentees. Please refrain from signing up if you were paired with a faculty mentor for the APD-DIGA Mentorship Program in May 2021. Contact @suryasweetie123 only if you have specific questions, otherwise all information is on our website and the link is here. Link is below and in our bio! #DIGA #derm #mentee #residencyapplication. Accessed May 24, 2023. https://www.instagram.com/p/CSrq0exMchY/
Practice Points
- Our study suggests that matched dermatology applicants with and without home programs (HPs) had similar achievements, on average, for number of publications and holding advanced degrees.
- Because of the potential benefits of having program connections for matching in dermatology, applicants without HPs should seek dermatology research mentors.
Interacting With Dermatology Patients Online: Private Practice vs Academic Institute Website Content
Patients are finding it easier to use online resources to discover health care providers who fit their personalized needs. In the United States, approximately 70% of individuals use the internet to find health care information, and 80% are influenced by the information presented to them on health care websites.1 Patients utilize the internet to better understand treatments offered by providers and their prices as well as how other patients have rated their experience. Providers in private practice also have noticed that many patients are referring themselves vs obtaining a referral from another provider.2 As a result, it is critical for practice websites to have information that is of value to their patients, including the unique qualities and treatments offered. The purpose of this study was to analyze the differences between the content presented on dermatology private practice websites and academic institutional websites.
Methods
Websites Searched —All 140 academic dermatology programs, including both allopathic and osteopathic programs, were queried from the Association of American Medical Colleges (AAMC) database in March 2022. 3 First, the dermatology departmental websites for each program were analyzed to see if they contained information pertinent to patients. Any website that lacked this information or only had information relevant to the dermatology residency program was excluded from the study. After exclusion, a total of 113 websites were used in the academic website cohort. The private practices were found through an incognito Google search with the search term dermatologist and matched to be within 5 miles of each academic institution. The private practices that included at least one board-certified dermatologist and received the highest number of reviews on Google compared to other practices in the same region—a measure of online reputation—were selected to be in the private practice cohort (N = 113). Any duplicate practices, practices belonging to the same conglomerate company, or multispecialty clinics were excluded from the study. Board-certified dermatologists were confirmed using the Find a Dermatologist tool on the American Academy of Dermatology (AAD) website. 4
Website Assessments —Each website was assessed using 23 criteria divided into 4 categories: practice, physician(s), patient, and treatment/procedure (Table). Criteria for social media and publicity were further assessed. Criteria for social media included links on the website to a Facebook page, an Instagram account, a Twitter account, a Pinterest account, a LinkedIn account, a blog, a Yelp page, a YouTube channel, and/or any other social media. Criteria for publicity included links on the website to local television news, national news, newspapers, and/or magazines. 5-8 Ease of site access was determined if the website was the first search result found on Google when searching for each website. Nondermatology professionals included listing of mid-level providers or researchers.
Four individuals (V.S.J., A.C.B., M.E.O., and M.B.B.) independently assessed each of the websites using the established criteria. Each criterion was defined and discussed prior to data collection to maintain consistency. The criteria were determined as being present if the website clearly displayed, stated, explained, or linked to the relevant content. If the website did not directly contain the content, it was determined that the criteria were absent. One other individual (J.P.) independently cross-examined the data for consistency and evaluated for any discrepancies. 8
A raw analysis was done between each cohort. Another analysis was done that controlled for population density and the proportionate population age in each city 9 in which an academic institution/private practice was located. We proposed that more densely populated cities naturally may have more competition between practices, which may result in more optimized websites. 10 We also anticipated similar findings in cities with younger populations, as the younger demographic may be more likely to utilize and value online information when compared to older populations. 11 The websites for each cohort were equally divided into 3 tiers of population density (not shown) and population age (not shown).
Statistical Analysis —Statistical analysis was completed using descriptive statistics, χ 2 testing, and Fisher exact tests where appropriate with a predetermined level of significance of P < .05 in Microsoft Excel.
Results
Demographics —A total of 226 websites from both private practices and academic institutions were evaluated. Of them, only 108 private practices and 108 academic institutions listed practicing dermatologists on their site. Of 108 private practices, 76 (70.4%) had more than one practicing board-certified dermatologist. Of 108 academic institutions, all 108 (100%) institutions had more than one practicing board-certified dermatologist.
Of the dermatologists who practiced at academic institutions (n=2014) and private practices (n=817), 1157 (57.4%) and 419 (51.2%) were females, respectively. The population density of the cities with each of these practices/institutions ranged from 137 individuals per square kilometer to 11,232 individuals per square kilometer (mean [SD] population density, 2579 [2485] individuals per square kilometer). Densely populated, moderately populated, and sparsely populated cities had a median population density of 4618, 1708, and 760 individuals per square kilometer, respectively. The data also were divided into 3 age groups. In the older population tier, the median percentage of individuals older than 64 years was 14.2%, the median percentage of individuals aged 18 to 64 years was 63.8%, and the median percentage of individuals aged 5 to 17 years was 14.9%. In the moderately aged population tier, the median percentage of individuals older than 64 years was 10.2%, the median percentage of individuals aged 18 to 64 years was 70.3%, and the median percentage of individuals aged 5 to 17 years was 13.6%. In the younger population tier, the median percentage of individuals older than 64 years was 12%, the median percentage of individuals aged 18 to 64 years was 66.8%, and the median percentage of individuals aged 5 to 17 years was 15%.
Practice and Physician Content—In the raw analysis (Figure), the most commonly listed types of content (>90% of websites) in both private practice and academic sites was address (range, 95% to 100%), telephone number (range, 97% to 100%), and dermatologist profiles (both 92%). The least commonly listed types of content in both cohorts was publicity (range, 20% to 23%). Private practices were more likely to list profiles of nondermatology professionals (73% vs 56%; P<.02), email (47% vs 17%; P<.0001), and social media (29% vs 8%; P<.0001) compared with academic institution websites. Although Facebook was the most-linked social media account for both groups, 75% of private practice sites included the link compared with 16% of academic institutions. Academic institutions were more likely to list fellowship availability (66% vs 1%; P<.0001). Accessing each website was significantly easier in the private practice cohort (99% vs 61%; P<.0001).
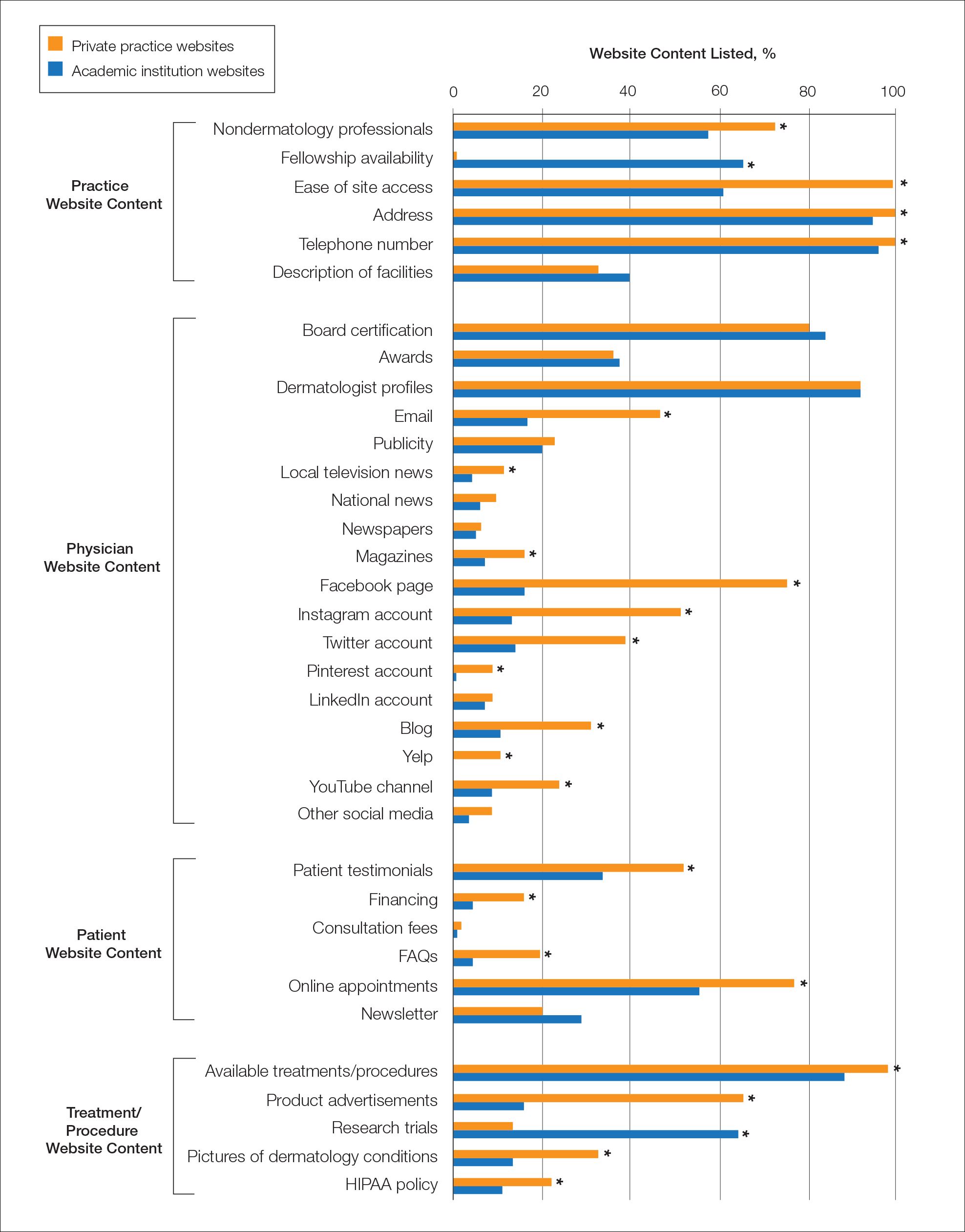
When controlling for population density, private practices were only more likely to list nondermatology professionals’ profiles in densely populated cities when compared with academic institutions (73% vs 41%; P<.01). Academic institutions continued to list fellowship availability more often than private practices regardless of population density. The same trend was observed for private practices with ease of site access and listing of social media.
When controlling for population age, similar trends were seen as when controlling for population density. However, private practices listing nondermatology professionals’ profiles was only more likely in the cities with a proportionately younger population when compared with academic institutions (74% vs 47%; P<.04).
Patient and Treatment/Procedure—The most commonly listed content types on both private practice websites and academic institution websites were available treatments/procedures (range, 89% to 98%). The least commonly listed content included financing for elective procedures (range, 4% to 16%), consultation fees (range, 1% to 2%), FAQs (frequently asked questions)(range, 4% to 20%), and HIPAA (Health Insurance Portability and Accountability Act) policy (range, 12% to 22%). Private practices were more likely to list patient testimonials (52% vs 35%; P<.005), financing (16% vs 4%; P<.005), FAQs (20% vs 4%; P<.001), online appointments (77% vs 56%; P<.001), available treatments/procedures (98% vs 86%; P<.004), product advertisements (66% vs 16%; P<.0001), pictures of dermatology conditions (33% vs 13%; P<.001), and HIPAA policy (22% vs 12%; P<.04). Academic institutions were more likely to list research trials (65% vs 13%; P<.0001).
When controlling for population density, private practices were only more likely to list patient testimonials in densely populated (P=.035) and moderately populated cities (P=.019). The same trend was observed for online appointments in densely populated (P=.0023) and moderately populated cities (P=.037). Private practices continued to list product availability more often than academic institutions regardless of population density or population age. Academic institutions also continued to list research trials more often than private practices regardless of population density or population age.
Comment
Our study uniquely analyzed the differences in website content between private practices and academic institutions in dermatology. Of the 140 academic institutions accredited by the Accreditation Council for Graduate Medical Education (ACGME), only 113 had patient-pertinent websites.
Access to Websites —There was a significant difference in many website content criteria between the 2 groups. Private practice sites were easier to access via a Google search when compared with academic sites, which likely is influenced by the Google search algorithm that ranks websites higher based on several criteria including but not limited to keyword use in the title tag, link popularity of the site, and historic ranking. 12,13 Academic sites often were only accessible through portals found on their main institutional site or institution’s residency site.
Role of Social Media —Social media has been found to assist in educating patients on medical practices as well as selecting a physician. 14,15 Our study found that private practice websites listed links to social media more often than their academic counterparts. Social media consumption is increasing, in part due to the COVID-19 pandemic, and it may be optimal for patients and practices alike to include links on their websites. 16 Facebook and Instagram were listed more often on private practice sites when compared with academic institution sites, which was similar to a recent study analyzing the websites of plastic surgery private practices (N = 310) in which 90% of private practices included some type of social media, with Instagram and Facebook being the most used. 8 Social networking accounts can act as convenient platforms for marketing, providing patient education, and generating referrals, which suggests that the prominence of their usage in private practice poses benefits in patient decision-making when seeking care. 17-19 A study analyzing the impact of Facebook in medicine concluded that a Facebook page can serve as an effective vehicle for medical education, particularly in younger generations that favor technology-oriented teaching methods. 20 A survey on trends in cosmetic facial procedures in plastic surgery found that the most influential online methods patients used for choosing their providers were social media platforms and practice websites. Front-page placement on Google also was commonly associated with the number of social media followers. 21,22 A lack of social media prominence could hinder a website’s potential to reach patients.
Communication With Practices —Our study also found significant differences in other metrics related to a patient’s ability to directly communicate with a practice, such as physical addresses, telephone numbers, products available for direct purchase, and online appointment booking, all of which were listed more often on private practice websites compared with academic institution websites. Online appointment booking also was found more frequently on private practice websites. Although physical addresses and telephone numbers were listed significantly more often on private practice sites, this information was ubiquitous and easily accessible elsewhere. Academic institution websites listed research trials and fellowship training significantly more often than private practices. These differences imply a divergence in focus between private practices and academic institutions, likely because academic institutions are funded in large part from research grants, begetting a cycle of academic contribution. 23 In contrast, private practices may not rely as heavily on academic revenue and may be more likely to prioritize other revenue streams such as product sales. 24
HIPAA Policy —Surprisingly, HIPAA policy rarely was listed on any private (22%) or academic site (12%). Conversely, in the plastic surgery study, HIPAA policy was listed much more often, with more than half of private practices with board-certified plastic surgeons accredited in the year 2015 including it on their website, 8 which may suggest that surgically oriented specialties, particularly cosmetic subspecialties, aim to more noticeably display their privacy policies for patient reassurance.
Study Limitations —There are several limitations of our study. First, it is common for a conglomerate company to own multiple private practices in different specialties. As with academic sites, private practice sites may be limited by the hosting platforms, which often are tedious to navigate. Also noteworthy is the emergence of designated social media management positions—both by practice employees and by third-party firms 25 —but the impact of these positions in private practices and academic institutions has not been fully explored. Finally, inclusion criteria and standardized criteria definitions were chosen based on the precedent established by the authors of similar analyses in plastic surgery and radiology. 5-8 Further investigation into the most valued aspects of care by patients within the context of the type of practice chosen would be valuable in refining inclusion criteria. Additionally, this study did not stratify the data collected based on factors such as gender, race, and geographical location; studies conducted on website traffic analysis patterns that focus on these aspects likely would further explain the significance of these findings. Differences in the length of time to the next available appointment between private practices and academic institutions also may help support our findings. Finally, there is a need for further investigation into the preferences of patients themselves garnered from website traffic alone.
Conclusion
Our study examined a diverse compilation of private practice and academic institution websites and uncovered numerous differences in content. As technology and health care continuously evolve, it is imperative that both private practices and academic institutions are actively adapting to optimize their online presence. In doing so, patients will be better equipped at accessing provider information, gaining familiarity with the practice, and understanding treatment options.
- Gentry ZL, Ananthasekar S, Yeatts M, et al. Can patients find an endocrine surgeon? how hospital websites hide the expertise of these medical professionals. Am J Surg . 2021;221:101-105.
- Pollack CE, Rastegar A, Keating NL, et al. Is self-referral associated with higher quality care? Health Serv Res . 2015;50:1472-1490.
- Association of American Medical Colleges. Residency Explorer TM tool. Accessed May 15, 2023. https://students-residents.aamc.org/apply-smart-residency/residency-explorer-tool
- Find a dermatologist. American Academy of Dermatology website. Accessed May 15, 2023. https://find-a-derm.aad.org/
- Johnson EJ, Doshi AM, Rosenkrantz AB. Strengths and deficiencies in the content of US radiology private practices’ websites. J Am Coll Radiol. 2017;14:431-435.
- Brunk D. Medical website expert shares design tips. Dermatology News . February 9, 2012. Accessed May 15, 2023. https://www.mdedge.com/dermatology/article/47413/health-policy/medical-website-expert-shares-design-tips
- Kuhnigk O, Ramuschkat M, Schreiner J, et al. Internet presence of neurologists, psychiatrists and medical psychotherapists in private practice [in German]. Psychiatr Prax . 2013;41:142-147.
- Ananthasekar S, Patel JJ, Patel NJ, et al. The content of US plastic surgery private practices’ websites. Ann Plast Surg . 2021;86(6S suppl 5):S578-S584.
- US Census Bureau. Age and Sex: 2021. Updated December 2, 2021. Accessed March 15, 2023. https://www.census.gov/topics/population/age-and-sex/data/tables.2021.List_897222059.html#list-tab-List_897222059
- Porter ME. The competitive advantage of the inner city. Harvard Business Review . Published August 1, 2014. https://hbr.org/1995/05/the-competitive-advantage-of-the-inner-city
- Clark PG. The social allocation of health care resources: ethical dilemmas in age-group competition. Gerontologist. 1985;25:119-125.
- Su A-J, Hu YC, Kuzmanovic A, et al. How to improve your Google ranking: myths and reality. ACM Transactions on the Web . 2014;8. https://dl.acm.org/doi/abs/10.1145/2579990
- McCormick K. 39 ways to increase traffic to your website. WordStream website. Published March 28, 2023. Accessed May 22, 2023. https://www.wordstream.com/blog/ws/2014/08/14/increase-traffic-to-my-website
- Montemurro P, Porcnik A, Hedén P, et al. The influence of social media and easily accessible online information on the aesthetic plastic surgery practice: literature review and our own experience. Aesthetic Plast Surg . 2015;39:270-277.
- Steehler KR, Steehler MK, Pierce ML, et al. Social media’s role in otolaryngology–head and neck surgery. Otolaryngol Head Neck Surg . 2013;149:521-524.
- Tsao S-F, Chen H, Tisseverasinghe T, et al. What social media told us in the time of COVID-19: a scoping review. Lancet Digit Health . 2021;3:E175-E194.
- Geist R, Militello M, Albrecht JM, et al. Social media and clinical research in dermatology. Curr Dermatol Rep . 2021;10:105-111.
- McLawhorn AS, De Martino I, Fehring KA, et al. Social media and your practice: navigating the surgeon-patient relationship. Curr Rev Musculoskelet Med . 2016;9:487-495.
- Thomas RB, Johnson PT, Fishman EK. Social media for global education: pearls and pitfalls of using Facebook, Twitter, and Instagram. J Am Coll Radiol . 2018;15:1513-1516.
- Lugo-Fagundo C, Johnson MB, Thomas RB, et al. New frontiers in education: Facebook as a vehicle for medical information delivery. J Am Coll Radiol . 2016;13:316-319.
- Ho T-VT, Dayan SH. How to leverage social media in private practice. Facial Plast Surg Clin North Am . 2020;28:515-522.
- Fan KL, Graziano F, Economides JM, et al. The public’s preferences on plastic surgery social media engagement and professionalism. Plast Reconstr Surg . 2019;143:619-630.
- Jacob BA, Lefgren L. The impact of research grant funding on scientific productivity. J Public Econ. 2011;95:1168-1177.
- Baumann L. Ethics in cosmetic dermatology. Clin Dermatol. 2012;30:522-527.
- Miller AR, Tucker C. Active social media management: the case of health care. Info Sys Res . 2013;24:52-70.
Patients are finding it easier to use online resources to discover health care providers who fit their personalized needs. In the United States, approximately 70% of individuals use the internet to find health care information, and 80% are influenced by the information presented to them on health care websites.1 Patients utilize the internet to better understand treatments offered by providers and their prices as well as how other patients have rated their experience. Providers in private practice also have noticed that many patients are referring themselves vs obtaining a referral from another provider.2 As a result, it is critical for practice websites to have information that is of value to their patients, including the unique qualities and treatments offered. The purpose of this study was to analyze the differences between the content presented on dermatology private practice websites and academic institutional websites.
Methods
Websites Searched —All 140 academic dermatology programs, including both allopathic and osteopathic programs, were queried from the Association of American Medical Colleges (AAMC) database in March 2022. 3 First, the dermatology departmental websites for each program were analyzed to see if they contained information pertinent to patients. Any website that lacked this information or only had information relevant to the dermatology residency program was excluded from the study. After exclusion, a total of 113 websites were used in the academic website cohort. The private practices were found through an incognito Google search with the search term dermatologist and matched to be within 5 miles of each academic institution. The private practices that included at least one board-certified dermatologist and received the highest number of reviews on Google compared to other practices in the same region—a measure of online reputation—were selected to be in the private practice cohort (N = 113). Any duplicate practices, practices belonging to the same conglomerate company, or multispecialty clinics were excluded from the study. Board-certified dermatologists were confirmed using the Find a Dermatologist tool on the American Academy of Dermatology (AAD) website. 4
Website Assessments —Each website was assessed using 23 criteria divided into 4 categories: practice, physician(s), patient, and treatment/procedure (Table). Criteria for social media and publicity were further assessed. Criteria for social media included links on the website to a Facebook page, an Instagram account, a Twitter account, a Pinterest account, a LinkedIn account, a blog, a Yelp page, a YouTube channel, and/or any other social media. Criteria for publicity included links on the website to local television news, national news, newspapers, and/or magazines. 5-8 Ease of site access was determined if the website was the first search result found on Google when searching for each website. Nondermatology professionals included listing of mid-level providers or researchers.

Four individuals (V.S.J., A.C.B., M.E.O., and M.B.B.) independently assessed each of the websites using the established criteria. Each criterion was defined and discussed prior to data collection to maintain consistency. The criteria were determined as being present if the website clearly displayed, stated, explained, or linked to the relevant content. If the website did not directly contain the content, it was determined that the criteria were absent. One other individual (J.P.) independently cross-examined the data for consistency and evaluated for any discrepancies. 8
A raw analysis was done between each cohort. Another analysis was done that controlled for population density and the proportionate population age in each city 9 in which an academic institution/private practice was located. We proposed that more densely populated cities naturally may have more competition between practices, which may result in more optimized websites. 10 We also anticipated similar findings in cities with younger populations, as the younger demographic may be more likely to utilize and value online information when compared to older populations. 11 The websites for each cohort were equally divided into 3 tiers of population density (not shown) and population age (not shown).
Statistical Analysis —Statistical analysis was completed using descriptive statistics, χ 2 testing, and Fisher exact tests where appropriate with a predetermined level of significance of P < .05 in Microsoft Excel.
Results
Demographics —A total of 226 websites from both private practices and academic institutions were evaluated. Of them, only 108 private practices and 108 academic institutions listed practicing dermatologists on their site. Of 108 private practices, 76 (70.4%) had more than one practicing board-certified dermatologist. Of 108 academic institutions, all 108 (100%) institutions had more than one practicing board-certified dermatologist.
Of the dermatologists who practiced at academic institutions (n=2014) and private practices (n=817), 1157 (57.4%) and 419 (51.2%) were females, respectively. The population density of the cities with each of these practices/institutions ranged from 137 individuals per square kilometer to 11,232 individuals per square kilometer (mean [SD] population density, 2579 [2485] individuals per square kilometer). Densely populated, moderately populated, and sparsely populated cities had a median population density of 4618, 1708, and 760 individuals per square kilometer, respectively. The data also were divided into 3 age groups. In the older population tier, the median percentage of individuals older than 64 years was 14.2%, the median percentage of individuals aged 18 to 64 years was 63.8%, and the median percentage of individuals aged 5 to 17 years was 14.9%. In the moderately aged population tier, the median percentage of individuals older than 64 years was 10.2%, the median percentage of individuals aged 18 to 64 years was 70.3%, and the median percentage of individuals aged 5 to 17 years was 13.6%. In the younger population tier, the median percentage of individuals older than 64 years was 12%, the median percentage of individuals aged 18 to 64 years was 66.8%, and the median percentage of individuals aged 5 to 17 years was 15%.
Practice and Physician Content—In the raw analysis (Figure), the most commonly listed types of content (>90% of websites) in both private practice and academic sites was address (range, 95% to 100%), telephone number (range, 97% to 100%), and dermatologist profiles (both 92%). The least commonly listed types of content in both cohorts was publicity (range, 20% to 23%). Private practices were more likely to list profiles of nondermatology professionals (73% vs 56%; P<.02), email (47% vs 17%; P<.0001), and social media (29% vs 8%; P<.0001) compared with academic institution websites. Although Facebook was the most-linked social media account for both groups, 75% of private practice sites included the link compared with 16% of academic institutions. Academic institutions were more likely to list fellowship availability (66% vs 1%; P<.0001). Accessing each website was significantly easier in the private practice cohort (99% vs 61%; P<.0001).

When controlling for population density, private practices were only more likely to list nondermatology professionals’ profiles in densely populated cities when compared with academic institutions (73% vs 41%; P<.01). Academic institutions continued to list fellowship availability more often than private practices regardless of population density. The same trend was observed for private practices with ease of site access and listing of social media.
When controlling for population age, similar trends were seen as when controlling for population density. However, private practices listing nondermatology professionals’ profiles was only more likely in the cities with a proportionately younger population when compared with academic institutions (74% vs 47%; P<.04).
Patient and Treatment/Procedure—The most commonly listed content types on both private practice websites and academic institution websites were available treatments/procedures (range, 89% to 98%). The least commonly listed content included financing for elective procedures (range, 4% to 16%), consultation fees (range, 1% to 2%), FAQs (frequently asked questions)(range, 4% to 20%), and HIPAA (Health Insurance Portability and Accountability Act) policy (range, 12% to 22%). Private practices were more likely to list patient testimonials (52% vs 35%; P<.005), financing (16% vs 4%; P<.005), FAQs (20% vs 4%; P<.001), online appointments (77% vs 56%; P<.001), available treatments/procedures (98% vs 86%; P<.004), product advertisements (66% vs 16%; P<.0001), pictures of dermatology conditions (33% vs 13%; P<.001), and HIPAA policy (22% vs 12%; P<.04). Academic institutions were more likely to list research trials (65% vs 13%; P<.0001).
When controlling for population density, private practices were only more likely to list patient testimonials in densely populated (P=.035) and moderately populated cities (P=.019). The same trend was observed for online appointments in densely populated (P=.0023) and moderately populated cities (P=.037). Private practices continued to list product availability more often than academic institutions regardless of population density or population age. Academic institutions also continued to list research trials more often than private practices regardless of population density or population age.
Comment
Our study uniquely analyzed the differences in website content between private practices and academic institutions in dermatology. Of the 140 academic institutions accredited by the Accreditation Council for Graduate Medical Education (ACGME), only 113 had patient-pertinent websites.
Access to Websites —There was a significant difference in many website content criteria between the 2 groups. Private practice sites were easier to access via a Google search when compared with academic sites, which likely is influenced by the Google search algorithm that ranks websites higher based on several criteria including but not limited to keyword use in the title tag, link popularity of the site, and historic ranking. 12,13 Academic sites often were only accessible through portals found on their main institutional site or institution’s residency site.
Role of Social Media —Social media has been found to assist in educating patients on medical practices as well as selecting a physician. 14,15 Our study found that private practice websites listed links to social media more often than their academic counterparts. Social media consumption is increasing, in part due to the COVID-19 pandemic, and it may be optimal for patients and practices alike to include links on their websites. 16 Facebook and Instagram were listed more often on private practice sites when compared with academic institution sites, which was similar to a recent study analyzing the websites of plastic surgery private practices (N = 310) in which 90% of private practices included some type of social media, with Instagram and Facebook being the most used. 8 Social networking accounts can act as convenient platforms for marketing, providing patient education, and generating referrals, which suggests that the prominence of their usage in private practice poses benefits in patient decision-making when seeking care. 17-19 A study analyzing the impact of Facebook in medicine concluded that a Facebook page can serve as an effective vehicle for medical education, particularly in younger generations that favor technology-oriented teaching methods. 20 A survey on trends in cosmetic facial procedures in plastic surgery found that the most influential online methods patients used for choosing their providers were social media platforms and practice websites. Front-page placement on Google also was commonly associated with the number of social media followers. 21,22 A lack of social media prominence could hinder a website’s potential to reach patients.
Communication With Practices —Our study also found significant differences in other metrics related to a patient’s ability to directly communicate with a practice, such as physical addresses, telephone numbers, products available for direct purchase, and online appointment booking, all of which were listed more often on private practice websites compared with academic institution websites. Online appointment booking also was found more frequently on private practice websites. Although physical addresses and telephone numbers were listed significantly more often on private practice sites, this information was ubiquitous and easily accessible elsewhere. Academic institution websites listed research trials and fellowship training significantly more often than private practices. These differences imply a divergence in focus between private practices and academic institutions, likely because academic institutions are funded in large part from research grants, begetting a cycle of academic contribution. 23 In contrast, private practices may not rely as heavily on academic revenue and may be more likely to prioritize other revenue streams such as product sales. 24
HIPAA Policy —Surprisingly, HIPAA policy rarely was listed on any private (22%) or academic site (12%). Conversely, in the plastic surgery study, HIPAA policy was listed much more often, with more than half of private practices with board-certified plastic surgeons accredited in the year 2015 including it on their website, 8 which may suggest that surgically oriented specialties, particularly cosmetic subspecialties, aim to more noticeably display their privacy policies for patient reassurance.
Study Limitations —There are several limitations of our study. First, it is common for a conglomerate company to own multiple private practices in different specialties. As with academic sites, private practice sites may be limited by the hosting platforms, which often are tedious to navigate. Also noteworthy is the emergence of designated social media management positions—both by practice employees and by third-party firms 25 —but the impact of these positions in private practices and academic institutions has not been fully explored. Finally, inclusion criteria and standardized criteria definitions were chosen based on the precedent established by the authors of similar analyses in plastic surgery and radiology. 5-8 Further investigation into the most valued aspects of care by patients within the context of the type of practice chosen would be valuable in refining inclusion criteria. Additionally, this study did not stratify the data collected based on factors such as gender, race, and geographical location; studies conducted on website traffic analysis patterns that focus on these aspects likely would further explain the significance of these findings. Differences in the length of time to the next available appointment between private practices and academic institutions also may help support our findings. Finally, there is a need for further investigation into the preferences of patients themselves garnered from website traffic alone.
Conclusion
Our study examined a diverse compilation of private practice and academic institution websites and uncovered numerous differences in content. As technology and health care continuously evolve, it is imperative that both private practices and academic institutions are actively adapting to optimize their online presence. In doing so, patients will be better equipped at accessing provider information, gaining familiarity with the practice, and understanding treatment options.
Patients are finding it easier to use online resources to discover health care providers who fit their personalized needs. In the United States, approximately 70% of individuals use the internet to find health care information, and 80% are influenced by the information presented to them on health care websites.1 Patients utilize the internet to better understand treatments offered by providers and their prices as well as how other patients have rated their experience. Providers in private practice also have noticed that many patients are referring themselves vs obtaining a referral from another provider.2 As a result, it is critical for practice websites to have information that is of value to their patients, including the unique qualities and treatments offered. The purpose of this study was to analyze the differences between the content presented on dermatology private practice websites and academic institutional websites.
Methods
Websites Searched —All 140 academic dermatology programs, including both allopathic and osteopathic programs, were queried from the Association of American Medical Colleges (AAMC) database in March 2022. 3 First, the dermatology departmental websites for each program were analyzed to see if they contained information pertinent to patients. Any website that lacked this information or only had information relevant to the dermatology residency program was excluded from the study. After exclusion, a total of 113 websites were used in the academic website cohort. The private practices were found through an incognito Google search with the search term dermatologist and matched to be within 5 miles of each academic institution. The private practices that included at least one board-certified dermatologist and received the highest number of reviews on Google compared to other practices in the same region—a measure of online reputation—were selected to be in the private practice cohort (N = 113). Any duplicate practices, practices belonging to the same conglomerate company, or multispecialty clinics were excluded from the study. Board-certified dermatologists were confirmed using the Find a Dermatologist tool on the American Academy of Dermatology (AAD) website. 4
Website Assessments —Each website was assessed using 23 criteria divided into 4 categories: practice, physician(s), patient, and treatment/procedure (Table). Criteria for social media and publicity were further assessed. Criteria for social media included links on the website to a Facebook page, an Instagram account, a Twitter account, a Pinterest account, a LinkedIn account, a blog, a Yelp page, a YouTube channel, and/or any other social media. Criteria for publicity included links on the website to local television news, national news, newspapers, and/or magazines. 5-8 Ease of site access was determined if the website was the first search result found on Google when searching for each website. Nondermatology professionals included listing of mid-level providers or researchers.

Four individuals (V.S.J., A.C.B., M.E.O., and M.B.B.) independently assessed each of the websites using the established criteria. Each criterion was defined and discussed prior to data collection to maintain consistency. The criteria were determined as being present if the website clearly displayed, stated, explained, or linked to the relevant content. If the website did not directly contain the content, it was determined that the criteria were absent. One other individual (J.P.) independently cross-examined the data for consistency and evaluated for any discrepancies. 8
A raw analysis was done between each cohort. Another analysis was done that controlled for population density and the proportionate population age in each city 9 in which an academic institution/private practice was located. We proposed that more densely populated cities naturally may have more competition between practices, which may result in more optimized websites. 10 We also anticipated similar findings in cities with younger populations, as the younger demographic may be more likely to utilize and value online information when compared to older populations. 11 The websites for each cohort were equally divided into 3 tiers of population density (not shown) and population age (not shown).
Statistical Analysis —Statistical analysis was completed using descriptive statistics, χ 2 testing, and Fisher exact tests where appropriate with a predetermined level of significance of P < .05 in Microsoft Excel.
Results
Demographics —A total of 226 websites from both private practices and academic institutions were evaluated. Of them, only 108 private practices and 108 academic institutions listed practicing dermatologists on their site. Of 108 private practices, 76 (70.4%) had more than one practicing board-certified dermatologist. Of 108 academic institutions, all 108 (100%) institutions had more than one practicing board-certified dermatologist.
Of the dermatologists who practiced at academic institutions (n=2014) and private practices (n=817), 1157 (57.4%) and 419 (51.2%) were females, respectively. The population density of the cities with each of these practices/institutions ranged from 137 individuals per square kilometer to 11,232 individuals per square kilometer (mean [SD] population density, 2579 [2485] individuals per square kilometer). Densely populated, moderately populated, and sparsely populated cities had a median population density of 4618, 1708, and 760 individuals per square kilometer, respectively. The data also were divided into 3 age groups. In the older population tier, the median percentage of individuals older than 64 years was 14.2%, the median percentage of individuals aged 18 to 64 years was 63.8%, and the median percentage of individuals aged 5 to 17 years was 14.9%. In the moderately aged population tier, the median percentage of individuals older than 64 years was 10.2%, the median percentage of individuals aged 18 to 64 years was 70.3%, and the median percentage of individuals aged 5 to 17 years was 13.6%. In the younger population tier, the median percentage of individuals older than 64 years was 12%, the median percentage of individuals aged 18 to 64 years was 66.8%, and the median percentage of individuals aged 5 to 17 years was 15%.
Practice and Physician Content—In the raw analysis (Figure), the most commonly listed types of content (>90% of websites) in both private practice and academic sites was address (range, 95% to 100%), telephone number (range, 97% to 100%), and dermatologist profiles (both 92%). The least commonly listed types of content in both cohorts was publicity (range, 20% to 23%). Private practices were more likely to list profiles of nondermatology professionals (73% vs 56%; P<.02), email (47% vs 17%; P<.0001), and social media (29% vs 8%; P<.0001) compared with academic institution websites. Although Facebook was the most-linked social media account for both groups, 75% of private practice sites included the link compared with 16% of academic institutions. Academic institutions were more likely to list fellowship availability (66% vs 1%; P<.0001). Accessing each website was significantly easier in the private practice cohort (99% vs 61%; P<.0001).

When controlling for population density, private practices were only more likely to list nondermatology professionals’ profiles in densely populated cities when compared with academic institutions (73% vs 41%; P<.01). Academic institutions continued to list fellowship availability more often than private practices regardless of population density. The same trend was observed for private practices with ease of site access and listing of social media.
When controlling for population age, similar trends were seen as when controlling for population density. However, private practices listing nondermatology professionals’ profiles was only more likely in the cities with a proportionately younger population when compared with academic institutions (74% vs 47%; P<.04).
Patient and Treatment/Procedure—The most commonly listed content types on both private practice websites and academic institution websites were available treatments/procedures (range, 89% to 98%). The least commonly listed content included financing for elective procedures (range, 4% to 16%), consultation fees (range, 1% to 2%), FAQs (frequently asked questions)(range, 4% to 20%), and HIPAA (Health Insurance Portability and Accountability Act) policy (range, 12% to 22%). Private practices were more likely to list patient testimonials (52% vs 35%; P<.005), financing (16% vs 4%; P<.005), FAQs (20% vs 4%; P<.001), online appointments (77% vs 56%; P<.001), available treatments/procedures (98% vs 86%; P<.004), product advertisements (66% vs 16%; P<.0001), pictures of dermatology conditions (33% vs 13%; P<.001), and HIPAA policy (22% vs 12%; P<.04). Academic institutions were more likely to list research trials (65% vs 13%; P<.0001).
When controlling for population density, private practices were only more likely to list patient testimonials in densely populated (P=.035) and moderately populated cities (P=.019). The same trend was observed for online appointments in densely populated (P=.0023) and moderately populated cities (P=.037). Private practices continued to list product availability more often than academic institutions regardless of population density or population age. Academic institutions also continued to list research trials more often than private practices regardless of population density or population age.
Comment
Our study uniquely analyzed the differences in website content between private practices and academic institutions in dermatology. Of the 140 academic institutions accredited by the Accreditation Council for Graduate Medical Education (ACGME), only 113 had patient-pertinent websites.
Access to Websites —There was a significant difference in many website content criteria between the 2 groups. Private practice sites were easier to access via a Google search when compared with academic sites, which likely is influenced by the Google search algorithm that ranks websites higher based on several criteria including but not limited to keyword use in the title tag, link popularity of the site, and historic ranking. 12,13 Academic sites often were only accessible through portals found on their main institutional site or institution’s residency site.
Role of Social Media —Social media has been found to assist in educating patients on medical practices as well as selecting a physician. 14,15 Our study found that private practice websites listed links to social media more often than their academic counterparts. Social media consumption is increasing, in part due to the COVID-19 pandemic, and it may be optimal for patients and practices alike to include links on their websites. 16 Facebook and Instagram were listed more often on private practice sites when compared with academic institution sites, which was similar to a recent study analyzing the websites of plastic surgery private practices (N = 310) in which 90% of private practices included some type of social media, with Instagram and Facebook being the most used. 8 Social networking accounts can act as convenient platforms for marketing, providing patient education, and generating referrals, which suggests that the prominence of their usage in private practice poses benefits in patient decision-making when seeking care. 17-19 A study analyzing the impact of Facebook in medicine concluded that a Facebook page can serve as an effective vehicle for medical education, particularly in younger generations that favor technology-oriented teaching methods. 20 A survey on trends in cosmetic facial procedures in plastic surgery found that the most influential online methods patients used for choosing their providers were social media platforms and practice websites. Front-page placement on Google also was commonly associated with the number of social media followers. 21,22 A lack of social media prominence could hinder a website’s potential to reach patients.
Communication With Practices —Our study also found significant differences in other metrics related to a patient’s ability to directly communicate with a practice, such as physical addresses, telephone numbers, products available for direct purchase, and online appointment booking, all of which were listed more often on private practice websites compared with academic institution websites. Online appointment booking also was found more frequently on private practice websites. Although physical addresses and telephone numbers were listed significantly more often on private practice sites, this information was ubiquitous and easily accessible elsewhere. Academic institution websites listed research trials and fellowship training significantly more often than private practices. These differences imply a divergence in focus between private practices and academic institutions, likely because academic institutions are funded in large part from research grants, begetting a cycle of academic contribution. 23 In contrast, private practices may not rely as heavily on academic revenue and may be more likely to prioritize other revenue streams such as product sales. 24
HIPAA Policy —Surprisingly, HIPAA policy rarely was listed on any private (22%) or academic site (12%). Conversely, in the plastic surgery study, HIPAA policy was listed much more often, with more than half of private practices with board-certified plastic surgeons accredited in the year 2015 including it on their website, 8 which may suggest that surgically oriented specialties, particularly cosmetic subspecialties, aim to more noticeably display their privacy policies for patient reassurance.
Study Limitations —There are several limitations of our study. First, it is common for a conglomerate company to own multiple private practices in different specialties. As with academic sites, private practice sites may be limited by the hosting platforms, which often are tedious to navigate. Also noteworthy is the emergence of designated social media management positions—both by practice employees and by third-party firms 25 —but the impact of these positions in private practices and academic institutions has not been fully explored. Finally, inclusion criteria and standardized criteria definitions were chosen based on the precedent established by the authors of similar analyses in plastic surgery and radiology. 5-8 Further investigation into the most valued aspects of care by patients within the context of the type of practice chosen would be valuable in refining inclusion criteria. Additionally, this study did not stratify the data collected based on factors such as gender, race, and geographical location; studies conducted on website traffic analysis patterns that focus on these aspects likely would further explain the significance of these findings. Differences in the length of time to the next available appointment between private practices and academic institutions also may help support our findings. Finally, there is a need for further investigation into the preferences of patients themselves garnered from website traffic alone.
Conclusion
Our study examined a diverse compilation of private practice and academic institution websites and uncovered numerous differences in content. As technology and health care continuously evolve, it is imperative that both private practices and academic institutions are actively adapting to optimize their online presence. In doing so, patients will be better equipped at accessing provider information, gaining familiarity with the practice, and understanding treatment options.
- Gentry ZL, Ananthasekar S, Yeatts M, et al. Can patients find an endocrine surgeon? how hospital websites hide the expertise of these medical professionals. Am J Surg . 2021;221:101-105.
- Pollack CE, Rastegar A, Keating NL, et al. Is self-referral associated with higher quality care? Health Serv Res . 2015;50:1472-1490.
- Association of American Medical Colleges. Residency Explorer TM tool. Accessed May 15, 2023. https://students-residents.aamc.org/apply-smart-residency/residency-explorer-tool
- Find a dermatologist. American Academy of Dermatology website. Accessed May 15, 2023. https://find-a-derm.aad.org/
- Johnson EJ, Doshi AM, Rosenkrantz AB. Strengths and deficiencies in the content of US radiology private practices’ websites. J Am Coll Radiol. 2017;14:431-435.
- Brunk D. Medical website expert shares design tips. Dermatology News . February 9, 2012. Accessed May 15, 2023. https://www.mdedge.com/dermatology/article/47413/health-policy/medical-website-expert-shares-design-tips
- Kuhnigk O, Ramuschkat M, Schreiner J, et al. Internet presence of neurologists, psychiatrists and medical psychotherapists in private practice [in German]. Psychiatr Prax . 2013;41:142-147.
- Ananthasekar S, Patel JJ, Patel NJ, et al. The content of US plastic surgery private practices’ websites. Ann Plast Surg . 2021;86(6S suppl 5):S578-S584.
- US Census Bureau. Age and Sex: 2021. Updated December 2, 2021. Accessed March 15, 2023. https://www.census.gov/topics/population/age-and-sex/data/tables.2021.List_897222059.html#list-tab-List_897222059
- Porter ME. The competitive advantage of the inner city. Harvard Business Review . Published August 1, 2014. https://hbr.org/1995/05/the-competitive-advantage-of-the-inner-city
- Clark PG. The social allocation of health care resources: ethical dilemmas in age-group competition. Gerontologist. 1985;25:119-125.
- Su A-J, Hu YC, Kuzmanovic A, et al. How to improve your Google ranking: myths and reality. ACM Transactions on the Web . 2014;8. https://dl.acm.org/doi/abs/10.1145/2579990
- McCormick K. 39 ways to increase traffic to your website. WordStream website. Published March 28, 2023. Accessed May 22, 2023. https://www.wordstream.com/blog/ws/2014/08/14/increase-traffic-to-my-website
- Montemurro P, Porcnik A, Hedén P, et al. The influence of social media and easily accessible online information on the aesthetic plastic surgery practice: literature review and our own experience. Aesthetic Plast Surg . 2015;39:270-277.
- Steehler KR, Steehler MK, Pierce ML, et al. Social media’s role in otolaryngology–head and neck surgery. Otolaryngol Head Neck Surg . 2013;149:521-524.
- Tsao S-F, Chen H, Tisseverasinghe T, et al. What social media told us in the time of COVID-19: a scoping review. Lancet Digit Health . 2021;3:E175-E194.
- Geist R, Militello M, Albrecht JM, et al. Social media and clinical research in dermatology. Curr Dermatol Rep . 2021;10:105-111.
- McLawhorn AS, De Martino I, Fehring KA, et al. Social media and your practice: navigating the surgeon-patient relationship. Curr Rev Musculoskelet Med . 2016;9:487-495.
- Thomas RB, Johnson PT, Fishman EK. Social media for global education: pearls and pitfalls of using Facebook, Twitter, and Instagram. J Am Coll Radiol . 2018;15:1513-1516.
- Lugo-Fagundo C, Johnson MB, Thomas RB, et al. New frontiers in education: Facebook as a vehicle for medical information delivery. J Am Coll Radiol . 2016;13:316-319.
- Ho T-VT, Dayan SH. How to leverage social media in private practice. Facial Plast Surg Clin North Am . 2020;28:515-522.
- Fan KL, Graziano F, Economides JM, et al. The public’s preferences on plastic surgery social media engagement and professionalism. Plast Reconstr Surg . 2019;143:619-630.
- Jacob BA, Lefgren L. The impact of research grant funding on scientific productivity. J Public Econ. 2011;95:1168-1177.
- Baumann L. Ethics in cosmetic dermatology. Clin Dermatol. 2012;30:522-527.
- Miller AR, Tucker C. Active social media management: the case of health care. Info Sys Res . 2013;24:52-70.
- Gentry ZL, Ananthasekar S, Yeatts M, et al. Can patients find an endocrine surgeon? how hospital websites hide the expertise of these medical professionals. Am J Surg . 2021;221:101-105.
- Pollack CE, Rastegar A, Keating NL, et al. Is self-referral associated with higher quality care? Health Serv Res . 2015;50:1472-1490.
- Association of American Medical Colleges. Residency Explorer TM tool. Accessed May 15, 2023. https://students-residents.aamc.org/apply-smart-residency/residency-explorer-tool
- Find a dermatologist. American Academy of Dermatology website. Accessed May 15, 2023. https://find-a-derm.aad.org/
- Johnson EJ, Doshi AM, Rosenkrantz AB. Strengths and deficiencies in the content of US radiology private practices’ websites. J Am Coll Radiol. 2017;14:431-435.
- Brunk D. Medical website expert shares design tips. Dermatology News . February 9, 2012. Accessed May 15, 2023. https://www.mdedge.com/dermatology/article/47413/health-policy/medical-website-expert-shares-design-tips
- Kuhnigk O, Ramuschkat M, Schreiner J, et al. Internet presence of neurologists, psychiatrists and medical psychotherapists in private practice [in German]. Psychiatr Prax . 2013;41:142-147.
- Ananthasekar S, Patel JJ, Patel NJ, et al. The content of US plastic surgery private practices’ websites. Ann Plast Surg . 2021;86(6S suppl 5):S578-S584.
- US Census Bureau. Age and Sex: 2021. Updated December 2, 2021. Accessed March 15, 2023. https://www.census.gov/topics/population/age-and-sex/data/tables.2021.List_897222059.html#list-tab-List_897222059
- Porter ME. The competitive advantage of the inner city. Harvard Business Review . Published August 1, 2014. https://hbr.org/1995/05/the-competitive-advantage-of-the-inner-city
- Clark PG. The social allocation of health care resources: ethical dilemmas in age-group competition. Gerontologist. 1985;25:119-125.
- Su A-J, Hu YC, Kuzmanovic A, et al. How to improve your Google ranking: myths and reality. ACM Transactions on the Web . 2014;8. https://dl.acm.org/doi/abs/10.1145/2579990
- McCormick K. 39 ways to increase traffic to your website. WordStream website. Published March 28, 2023. Accessed May 22, 2023. https://www.wordstream.com/blog/ws/2014/08/14/increase-traffic-to-my-website
- Montemurro P, Porcnik A, Hedén P, et al. The influence of social media and easily accessible online information on the aesthetic plastic surgery practice: literature review and our own experience. Aesthetic Plast Surg . 2015;39:270-277.
- Steehler KR, Steehler MK, Pierce ML, et al. Social media’s role in otolaryngology–head and neck surgery. Otolaryngol Head Neck Surg . 2013;149:521-524.
- Tsao S-F, Chen H, Tisseverasinghe T, et al. What social media told us in the time of COVID-19: a scoping review. Lancet Digit Health . 2021;3:E175-E194.
- Geist R, Militello M, Albrecht JM, et al. Social media and clinical research in dermatology. Curr Dermatol Rep . 2021;10:105-111.
- McLawhorn AS, De Martino I, Fehring KA, et al. Social media and your practice: navigating the surgeon-patient relationship. Curr Rev Musculoskelet Med . 2016;9:487-495.
- Thomas RB, Johnson PT, Fishman EK. Social media for global education: pearls and pitfalls of using Facebook, Twitter, and Instagram. J Am Coll Radiol . 2018;15:1513-1516.
- Lugo-Fagundo C, Johnson MB, Thomas RB, et al. New frontiers in education: Facebook as a vehicle for medical information delivery. J Am Coll Radiol . 2016;13:316-319.
- Ho T-VT, Dayan SH. How to leverage social media in private practice. Facial Plast Surg Clin North Am . 2020;28:515-522.
- Fan KL, Graziano F, Economides JM, et al. The public’s preferences on plastic surgery social media engagement and professionalism. Plast Reconstr Surg . 2019;143:619-630.
- Jacob BA, Lefgren L. The impact of research grant funding on scientific productivity. J Public Econ. 2011;95:1168-1177.
- Baumann L. Ethics in cosmetic dermatology. Clin Dermatol. 2012;30:522-527.
- Miller AR, Tucker C. Active social media management: the case of health care. Info Sys Res . 2013;24:52-70.
Practice Points
- Dermatologists at both private practices and academic institutions should understand that website content often may be the most accessible source of information about the practice available to patients and should be as specific and detailed as possible.
- When compared to private practices, academic institutions largely fail to have a social media presence, which may limit patient interaction with their websites.
An Evaluation of Spin in the Abstracts of Systematic Reviews and Meta-analyses on the Treatment of Psoriasis: A Cross-sectional Analysis
Psoriasis is an inflammatory autoimmune skin condition that affects approximately 125 million individuals worldwide, with approximately 8 million patients in the United States.1 Psoriasis not only involves a cosmetic component but also comprises other comorbidities, such as psoriatic arthritis, cardiovascular disease, and psychiatric disorders, that can influence patient quality of life.2-4 In addition, the costs associated with psoriasis are substantial, with an estimated economic burden of $35.2 billion in the United States in 2015.5 Given the prevalence of psoriasis and its many effects on patients, it is important that providers have high-quality evidence regarding efficacious treatment options.
Systematic reviews, which compile all available evidence on a subject to answer a specific question, represent the gold standard of research.6 However, studies have demonstrated that when referencing research literature, physicians tend to read only the abstract of a study rather than the entire article.7,8 A study by Marcelo et al8 showed that residents at a tertiary care center answered clinical questions using only the abstract of a paper 69% of the time. Based on these findings, it is imperative that the results of systematic reviews be accurately reported in their abstracts because they can influence patient care.
Referencing only the abstracts of systematic reviews can be problematic if the abstract contains spin. Spin is a form of reporting that inappropriately highlights the benefits of a treatment with greater emphasis than what is shown by the results.9 Research has identified the presence of spin in the abstracts of randomized controlled trials.10-12 For example, Cooper et al10 found that 70% (33/47) of abstracts in otolaryngology randomized controlled trials contained spin. Additionally, Arthur et al11 and Austin et al12 had similar findings within abstracts of orthopedic and obesity trials, where 44.8% (112/250) and 46.7% (21/45) contained spin, respectively. Ottwell et al13 found that the presence of spin in abstracts is not limited to randomized controlled trials; they demonstrated that the abstracts of nearly one-third (31% [11/36]) of systematic reviews focused on the treatment of acne vulgaris contained spin.
In our study, we aimed to evaluate the presence of spin in the abstracts of systematic reviews focused on the treatment of psoriasis.
Methods
Reproducibility and Reporting—Our study did not meet the regulatory definition for human subjects research per the US Code of Federal Regulations because the study did not involve human research subjects. The study also was not subject to review by the institutional review board. Our protocol, data set, analysis scripts, extraction forms, and other material related to the study have been placed on Open Science Framework to provide transparency and ensure reproducibility. To further allow for analytic reproducibility, our data set was given to an independent laboratory and reanalyzed with a masked approach. Our study was carried out alongside other studies assessing spin in systematic reviews regarding different specialties and disease states. Because these studies were similar in design, this methodology also has been reported elsewhere. The Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA)14 and the guidelines for meta-epidemiological studies developed by Murad and Wang15 were used in drafting this article.
Search Strategy—The search strategies for the MEDLINE (Ovid) and Embase (Ovid) databases were created by a systematic review librarian (D.N.W.) to identify systematic reviews and meta-analyses regarding treatments for psoriasis (Figure 1). The searches were performed on June 2, 2020, and uploaded to Rayyan, a systematic review screening platform.16 After duplicates were removed, the records were screened for eligibility by 2 authors (C.H. and A.L.) using the titles and abstracts. Screening was conducted independently while each of these authors was masked to the other’s results; disagreements were resolved through discussion.
Eligibility Criteria—An article had to meet the following criteria for inclusion in our study: (1) be a systematic review with or without a meta-analysis; (2) relate to the treatment of psoriasis; and (3) be written in English and include human patients only. The PRISMA definition of systematic reviews and meta-analyses was applied.17
Training—Various training occurred throughout our study to ensure understanding of each step and mitigate subjectivity. Before beginning screening, 2 investigators (C.H. and A.L.) completed the Introduction to Systematic Review and Meta-Analysis course offered by Johns Hopkins University.18 They also underwent 2 days of online and in-person training on the definition and interpretation of the 9 most severe types of spin found in the abstracts of systematic reviews as defined by Yavchitz et al.9 Finally, they were trained to use A MeaSurement Tool to Assess systematic Reviews (AMSTAR-2) to appraise the methodological quality of each systematic review. Our protocol contained an outline of all training modules used.
Data Extraction—The investigators (C.H. and A.L.) analyzed included abstracts for the 9 most severe types of spin (Table 1). Data were extracted in a masked duplicate fashion using the Google form. AMSTAR-2 was used to assess systematic reviews for methodological quality. AMSTAR-2 is an appraisal tool consisting of a 16-item checklist for systematic reviews or meta-analyses. Scores range from critically low to high based on the methodological quality of the review. Interrater reliability of AMSTAR-2 scores has been moderate to high across studies. Construct validity coefficients have been high with the original AMSTAR instrument (r=0.91) and the Risk of Bias in Systematic Reviews instrument (r=0.84).19
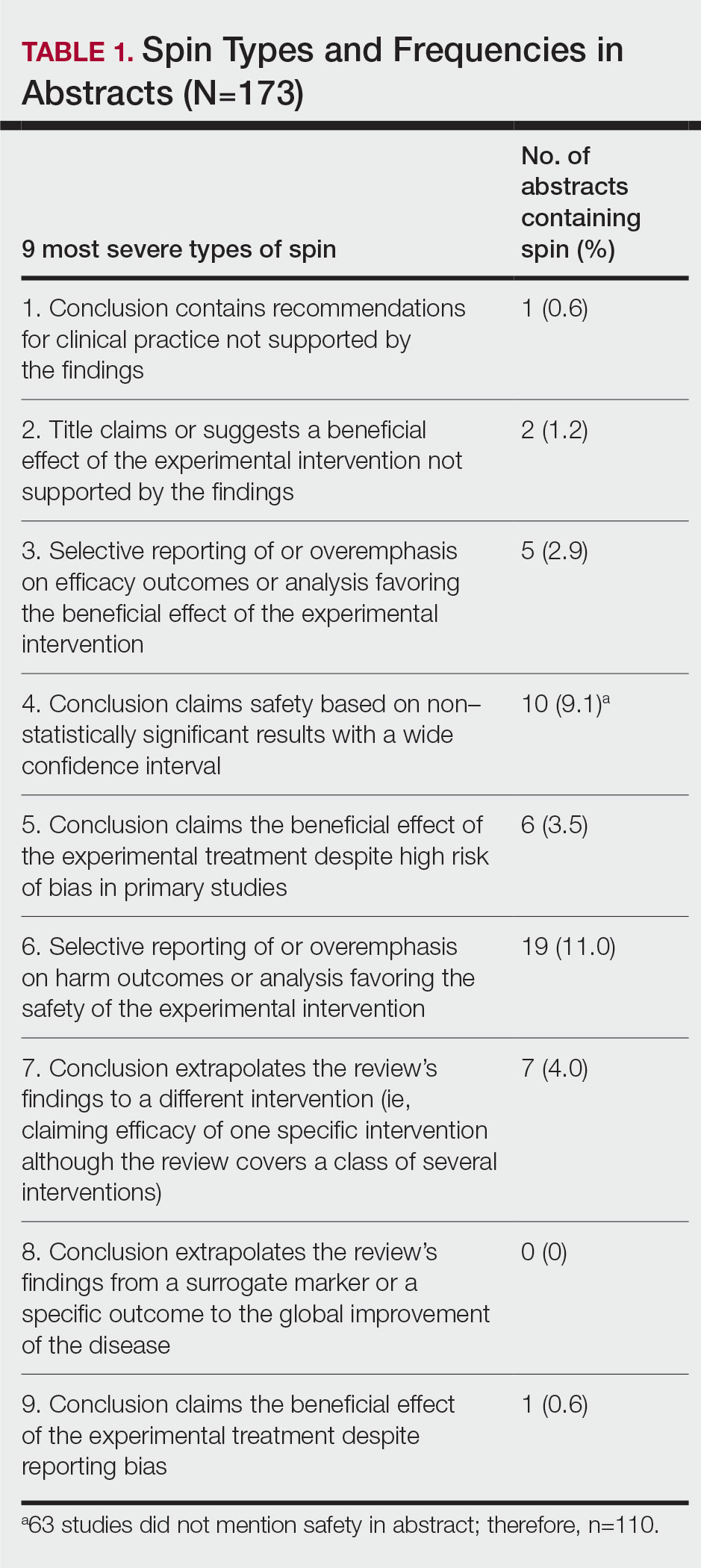
During data extraction from each included systematic review, the following additional items were obtained: (1) the date the review was received; (2) intervention type (ie, pharmacologic, nonpharmacologic, surgery, light therapy, mixed); (3) the funding source(s) for each systematic review (ie, industry, private, public, none, not mentioned, hospital, a combination of funding not including industry, a combination of funding including industry, other); (4) whether the journal submission guidelines suggested adherence to PRISMA guidelines; (5) whether the review discussed adherence to PRISMA14 or PRISMA for Abstracts20 (PRISMA-A); (6) the publishing journal’s 5-year impact factor; and (6) the country of the systematic review’s origin. When data extraction was complete, investigators (C.H. and A.L.) were unmasked and met to resolve any disagreements by discussion. Two authors (R.O. or M.V.) served as arbiters in the case that an agreement between C.H. and A.L. could not be reached.
Statistical Analysis—Frequencies and percentages were calculated to evaluate the most common types of spin found within systematic reviews and meta-analyses. One author (M.H.) prespecified the possibility of a binary logistic regression and calculated a power analysis to determine sample size, as stated in our protocol. Our final sample size of 173 was not powered to perform the multivariable logistic regression; therefore, we calculated unadjusted odds ratios to enable assessing relationships between the presence of spin in abstracts and the various study characteristics. We used Stata 16.1 for all analyses, and all analytic decisions can be found in our protocol.
Results
General Characteristics—Our systematic search of MEDLINE and Embase returned 3200 articles, of which 665 were duplicates that were removed. An additional 2253 articles were excluded during initial abstract and title screening, and full-text screening led to the exclusion of another 109 articles. In total, 173 systematic reviews were included for data extraction. Figure 2 illustrates the screening process with the rationale for all exclusions.
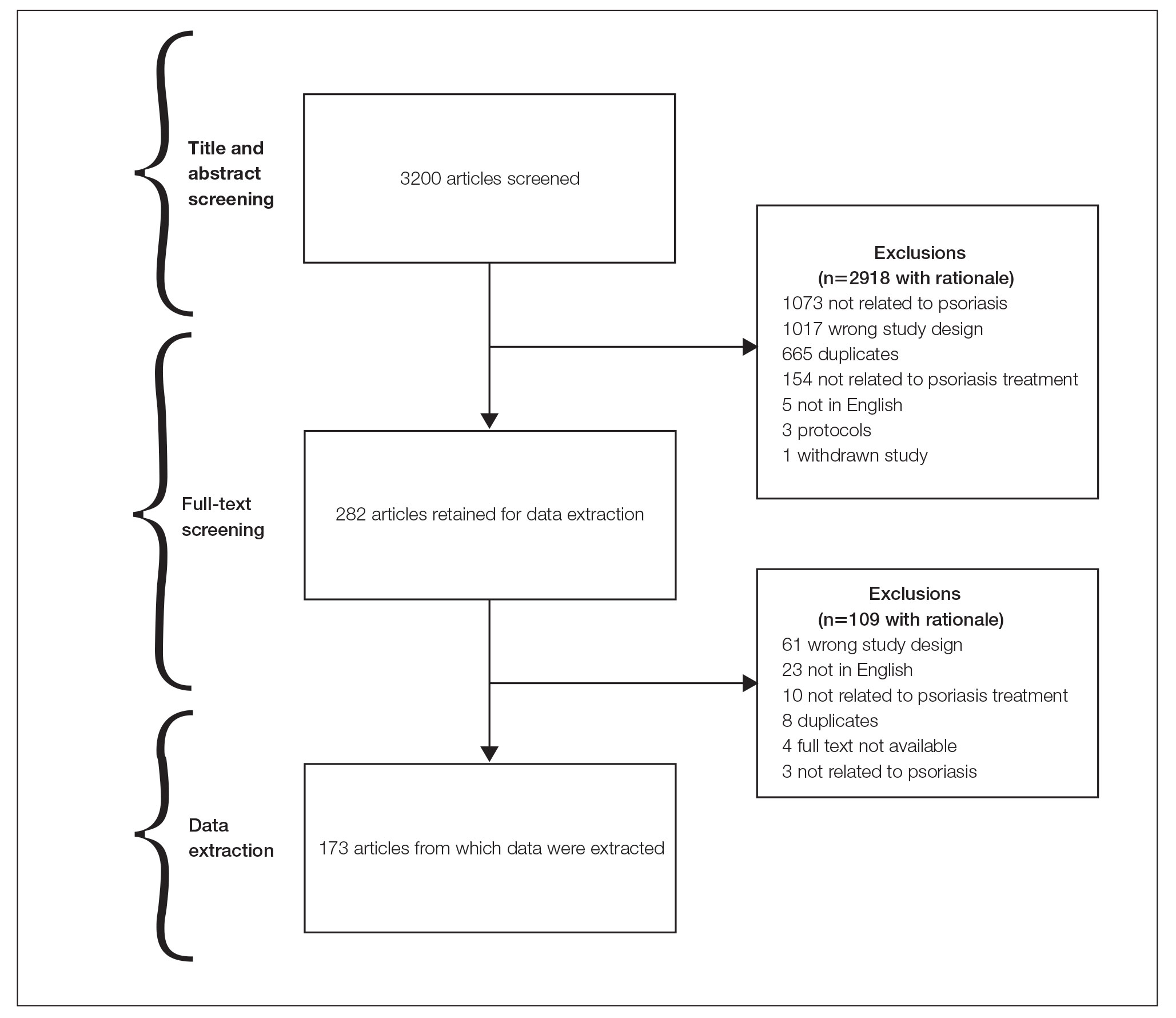
Of the 173 included systematic reviews and meta-analyses, 150 (86.7%) focused on pharmacologic interventions. The majority of studies did not mention adhering to PRISMA guidelines (125/173 [72.3%]), and the publishing journals recommended their authors adhere to PRISMA for only 66 (38.2%) of the included articles. For the articles that received funding (90/173 [52.0%]), industry sources were the most common funding source (40/90 [44.4%]), followed by private (27/90 [30%]) and public funding sources (23/90 [25.6%]). Of the remaining studies, 46 articles did not include a funding statement (46/83 [55.4%]), and 37 studies were not funded (37/83 [44.6%]). The average (SD) 5-year impact factor of our included journals was 4.68 (4.64). Systematic reviews were from 31 different countries. All studies were received by their respective journals between the years 2000 and 2020 (Table 2).
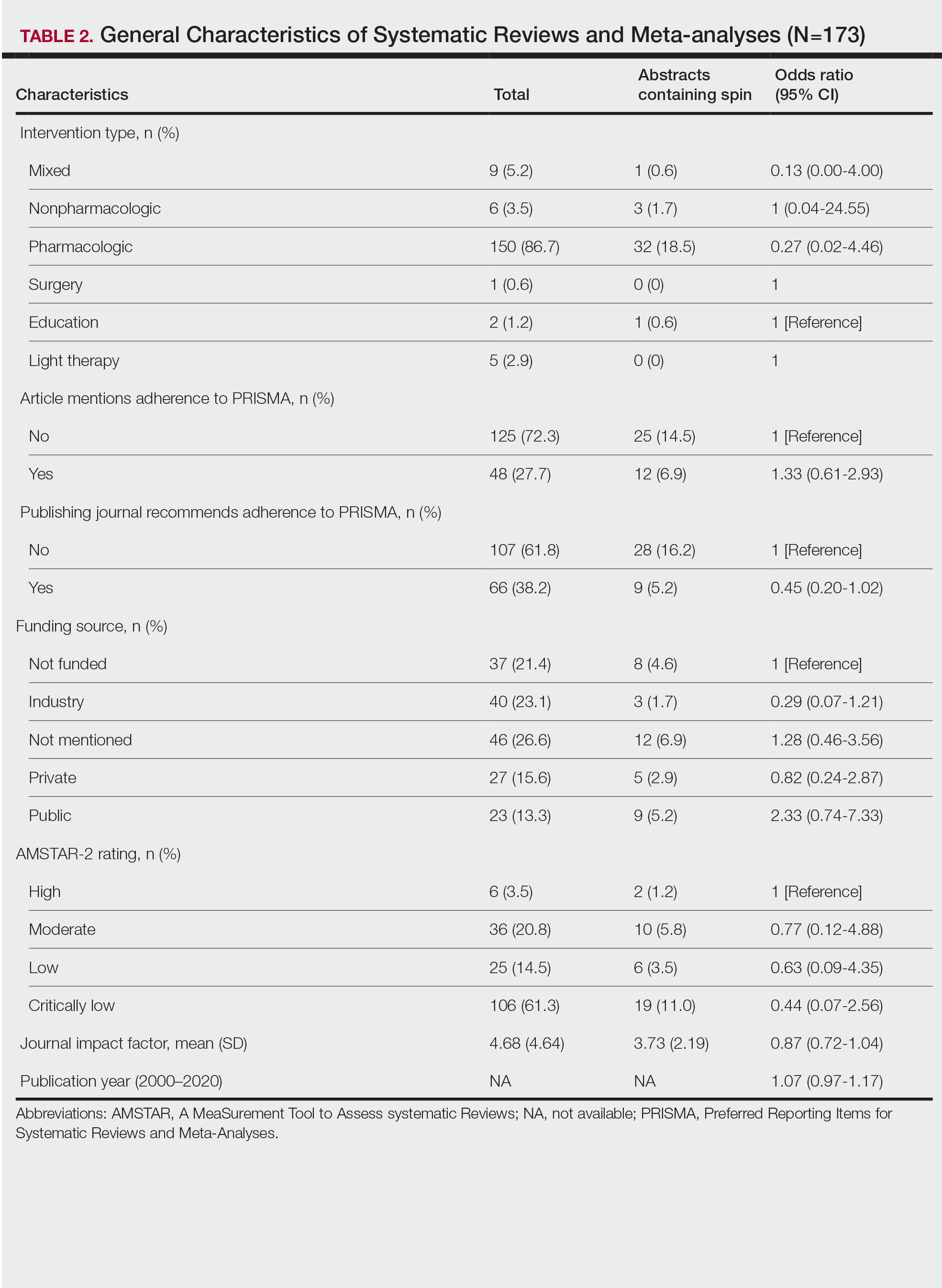
Abstracts Containing Spin—We found that 37 (21.4%) of the abstracts of systematic reviews focused on psoriasis treatments contained at least 1 type of spin. Some abstracts had more than 1 type; thus, a total of 51 different instances of spin were detected. Spin type 6—selective reporting of or overemphasis on harm outcomes or analysis favoring the safety of the experimental intervention—was the most common type ofspin, found in 19 of 173 abstracts (11.0%). The most severe type of spin—type 1 (conclusion contains recommendations for clinical practice not supported by the findings)—occurred in only 1 abstract (0.6%). Spin type 8 did not occur in any of the abstracts (Table 1). There was no statistically significant association between the presence of spin and any of the study characteristics (Table 2).
AMSTAR Ratings—After using AMSTAR-2 to appraise the included systematic reviews, we found that 6 (3.5%) of the 173 studies could be rated as high; 36 (20.8%) as moderate; 25 (14.5%) as low; and 106 (61.3%) as critically low. Of the 37 abstracts containing spin, 2 (5.4%) had an AMSTAR-2 rating of high, 10 (27%) had a rating of moderate, 6 (16.2%) had a rating of low, and 19 (51.4%) had a rating of critically low (Table 2). No statistically significant associations were seen between abstracts found to have spin and the AMSTAR-2 rating of the review.
Nearly all (160/173 [92.5%]) of the included reviews were compliant with the inclusion of Population, Intervention, Comparison, and Outcome (PICO) method. Only 17 of 173 (9.8%) reviews reported funding sources for the studies included. See Table 3 for all AMSTAR-2 items.
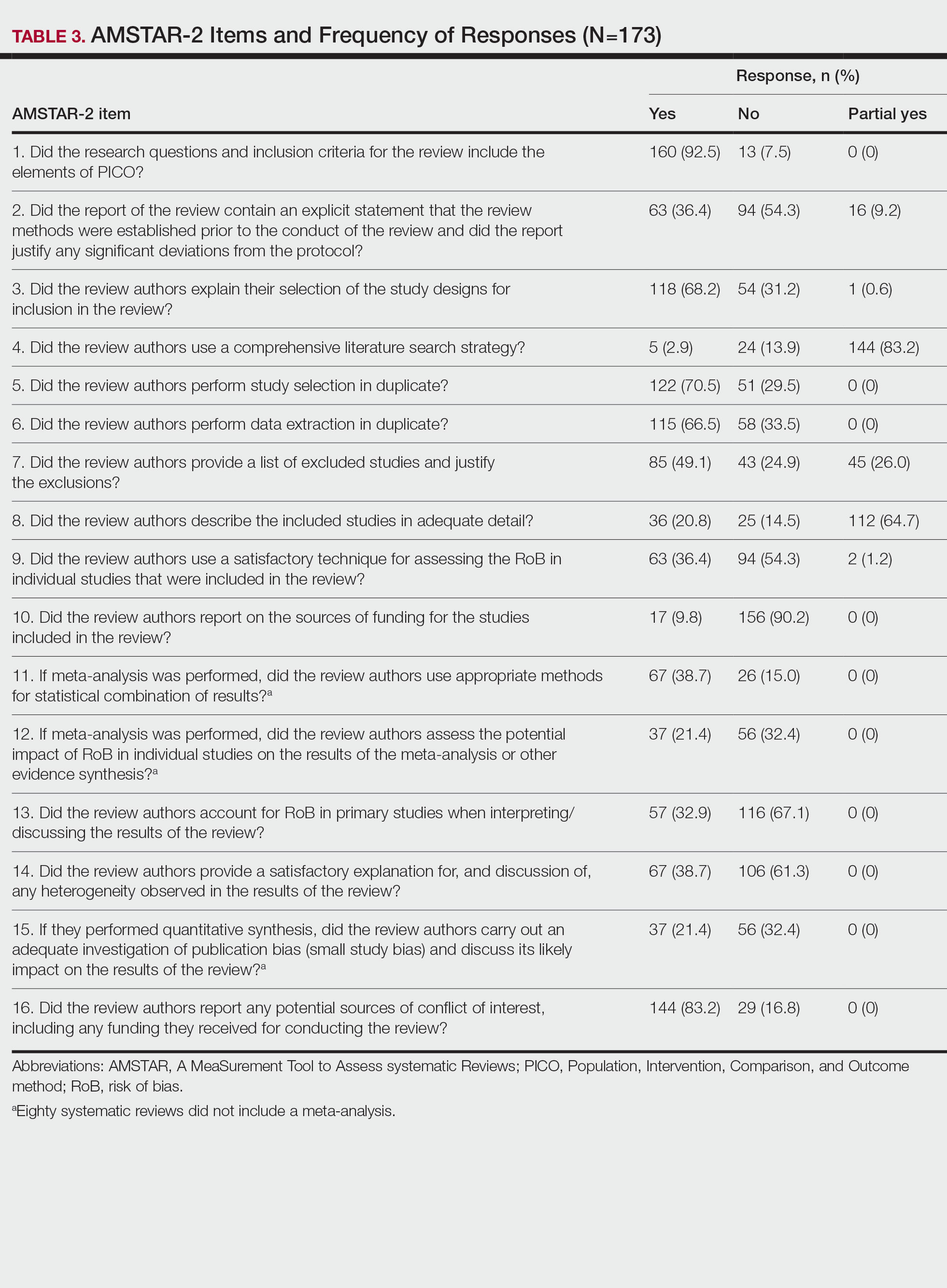
Comment
Primary Findings—We evaluated the abstracts of systematic reviews for the treatment of psoriasis and found that more than one-fifth of them contained spin. Our study contributes to the existing literature surrounding spin. Spin in randomized controlled trials is well documented across several fields of medicine, including otolaryngology,10 obesity medicine,12 dermatology,21 anesthesiology,22 psychiatry,23 orthopedics,24 emergency medicine,25 oncology,26 and cardiology.27 More recently, studies have emerged evaluating the presence of spin in systematic reviews. Specific to dermatology, one study found that 74% (84/113) of systematic reviews related to atopic dermatitis treatment contained spin.28 Additionally, Ottwell et al13 identified spin in 31% (11/36) of the systematic reviews related to the treatment of acne vulgaris, which is similar to our results for systematic reviews focused on psoriasis treatments. When comparing the presence of spin in abstracts of systematic reviews from the field of dermatology with other specialties, dermatology-focused systematic reviews appear to contain more spin in the abstract than systematic reviews focused on tinnitus and glaucoma therapies.29,30 However, systematic reviews from the field of dermatology appear to contain less spin than systematic reviews focused on therapies for lower back pain.31 For example, Nascimento et al31 found that 80% (53/66) of systematic reviews focused on low-back pain treatments contained spin.
Examples of Spin—The most common type of spin found in our study was type 6.9 An example of spin type 6 can be found in an article by Bai et al32 that investigated the short-term efficacy and safety of multiple interleukin inhibitors for the treatment of plaque psoriasis. The conclusion of the abstract states, “Risankizumab appeared to have relatively high efficacy and low risk.” However, in the results section, the authors showed that risankizumab had the highest risk of serious adverse events and was ranked highest for discontinuation because of adverse events when compared with other interleukin inhibitors. Here, the presence of spin in the abstract may mislead the reader to accept the “low risk” of risankizumab without understanding the study’s full results.32
Another example of selective reporting of harm outcomes in a systematic review can be found in the article by Wu et al,33 which focused on assessing IL-17 antagonists for the treatment of plaque psoriasis. The conclusion of the abstract indicated that IL-17 antagonists should be accepted as safe; however, in the results section, the authors discussed serious safety concerns with brodalumab, including the death of 4 patients from suicide.33 This example of spin type 6 highlights how the overgeneralization of a drug’s safety profile neglects serious harm outcomes that are critical to patient safety. In fact, against the safety claims of Wu et al,33 brodalumab later received a boxed warning from the US Food and Drug Administration after 6 patients died from suicide while receiving the drug, which led to early discontinuation of the trials.34,35 Although studies suggest this relationship is not causal,34-36 the purpose of our study was not to investigate this association but to highlight the importance of this finding. Thus, with this example of spin in mind, we offer recommendations that we believe will improve reporting in abstracts as well as quality of patient care.
Recommendations for Reporting in Abstracts—Regarding the boxed warning37 for brodalumab because of suicidal ideation and behavior, the US Food and Drug Administration recommends that prior to prescribing brodalumab, clinicians consider the potential benefits and risks in patients with a history of depression and/or suicidal ideation or behavior. However, a clinician would not adequately assess the full risks and benefits when an abstract, such as that for the article by Wu et al,33 contains spin through selectively reporting harm outcomes. Arguably, clinicians could just read the full text; however, research confirms that abstracts often are utilized by clinicians and commonly are used to guide clinical decisions.7,38 It is reasonable that clinicians would use abstracts in this fashion because they provide a quick synopsis of the full article’s findings and are widely available to clinicians who may not have access to article databases. Initiatives are in place to improve the quality of reporting in an abstract, such as PRISMA-A,20 but even this fails to address spin. In fact, it may suggest spin because checklist item 10 of PRISMA-A advises authors of systematic reviews to provide a “general interpretation of the results and important implications.” This item is concerning because it suggests that the authors interpret importance rather than the clinician who prescribes the drug and is ultimately responsible for patient safety. Therefore, we recommend a reform to abstract reporting and an update to PRISMA-A that leads authors to report all benefits and risks encountered instead of reporting what the authors define as important.
Strengths and Limitations—Our study has several strengths as well as limitations. One of these strengths is that our protocol was strictly adhered to; any deviations were noted and added as an amendment. Our protocol, data, and all study artifacts were made freely available online on the Open Science Framework to strengthen reproducibility (https://osf.io/zrxh8/). Investigators underwent training to ensure comprehension of spin and systematic review designs. All data were extracted in masked duplicate fashion per the Cochrane Handbook for Systematic Reviews of Interventions.39
Regarding limitations, only 2 databases were searched—MEDLINE and Embase. Therefore, our screening process may not have included every available systematic review on the treatment of psoriasis. Journal impact factors may be inaccurate for the systematic reviews that were published earlier in our data date range; however, we attempted to negate this limitation by using a 5-year average. Our study characteristic regarding PRISMA adherence did not account for studies published before the PRISMA statement release; we also could not access prior submission guidelines to determine when a journal began recommending PRISMA adherence. Another limitation of our study was the intrinsic subjectivity behind spin. Some may disagree with our classifications. Finally, our cross-sectional design should not be generalized to study types that are not systematic reviews or published in other journals during different periods.
Conclusion
Evidence of spin was present in many of the abstracts of systematic reviews pertaining to the treatment of psoriasis. Future clinical research should investigate any reporting of spin and search for ways to better reduce spin within literature. Continued research is necessary to evaluate the presence of spin within dermatology and other specialties.
- Psoriasis statistics. National Psoriasis Foundation. Updated December 21, 2022. Accessed March 6, 2023. https://www.psoriasis.org/content/statistics
- Greb JE, Goldminz AM, Elder JT, et al. Psoriasis. Nat Rev Dis Primers. 2016;2:16082.
- Hu SCS, Lan CCE. Psoriasis and cardiovascular comorbidities: focusing on severe vascular events, cardiovascular risk factors and implications for treatment. Int J Mol Sci. 2017;18:2211.
- Patel N, Nadkarni A, Cardwell LA, et al. Psoriasis, depression, and inflammatory overlap: a review. Am J Clin Dermatol. 2017;18:613-620.
- Brezinski EA, Dhillon JS, Armstrong AW. Economic burden of psoriasis in the United States: a systematic review. JAMA Dermatol. 2015;151:651-658.
- Gopalakrishnan S, Ganeshkumar P. Systematic reviews and meta‑analysis: understanding the best evidence in primary healthcare. J Fam Med Prim Care. 2013;2:9-14.
- Barry HC, Ebell MH, Shaughnessy AF, et al. Family physicians’ use of medical abstracts to guide decision making: style or substance? J Am Board Fam Pract. 2001;14:437-442.
- Marcelo A, Gavino A, Isip-Tan IT, et al. A comparison of the accuracy of clinical decisions based on full-text articles and on journal abstracts alone: a study among residents in a tertiary care hospital. Evid Based Med. 2013;18:48-53.
- Yavchitz A, Ravaud P, Altman DG, et al. A new classification of spin in systematic reviews and meta-analyses was developed and ranked according to the severity. J Clin Epidemiol. 2016;75:56-65.
- Cooper CM, Gray HM, Ross AE, et al. Evaluation of spin in the abstracts of otolaryngology randomized controlled trials. Laryngoscope. 2019;129:2036-2040.
- Arthur W, Zaaza Z, Checketts JX, et al. Analyzing spin in abstracts of orthopaedic randomized controlled trials with statistically insignificant primary endpoints. Arthroscopy. 2020;36:1443-1450.
- Austin J, Smith C, Natarajan K, et al. Evaluation of spin within abstracts in obesity randomized clinical trials: a cross-sectional review. Clin Obes. 2019;9:E12292.
- Ottwell R, Rogers TC, Michael Anderson J, et al. Evaluation of spin in the abstracts of systematic reviews and meta-analyses focused on the treatment of acne vulgaris: cross-sectional analysis. JMIR Dermatol. 2020;3:E16978.
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:E1000100.
- Murad MH, Wang Z. Guidelines for reporting meta-epidemiological methodology research. Evid Based Med. 2017;22:139-142.
- Rayyan QCRI. Accessed September 10, 2019. https://rayyan.qcri.org/reviews/81224
- Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350:g7647.
- Coursera. Introduction to systematic review and meta-analysis. Accessed May 18, 2023. https://www.coursera.org/learn/systematic-review
- Lorenz RC, Matthias K, Pieper D, et al. A psychometric study found AMSTAR 2 to be a valid and moderately reliable appraisal tool. J Clin Epidemiol. 2019;114:133-140.
- Beller EM, Glasziou PP, Altman DG, et al. PRISMA for abstracts: reporting systematic reviews in journal and conference abstracts. PLoS Med. 2013;10:E1001419.
- Motosko CC, Ault AK, Kimberly LL, et al. Analysis of spin in the reporting of studies of topical treatments of photoaged skin. J Am Acad Dermatol. 2019;80:516-522.e12.
- Kinder NC, Weaver MD, Wayant C, et al. Presence of “spin” in the abstracts and titles of anaesthesiology randomised controlled trials. Br J Anaesth. 2019;122:E13-E14.
- Jellison S, Roberts W, Bowers A, et al. Evaluation of spin in abstracts of papers in psychiatry and psychology journals. BMJ Evid Based Med. 2019;5:178-181.
- Checketts JX, Riddle J, Zaaza Z, et al. An evaluation of spin in lower extremity joint trials. J Arthroplasty. 2019;34:1008-1012.
- Reynolds-Vaughn V, Riddle J, Brown J, et al. Evaluation of spin in the abstracts of emergency medicine randomized controlled trials. Ann Emerg Med. 2019;14:423-431.
- Wayant C, Margalski D, Vaughn K, et al. Evaluation of spin in oncology clinical trials. Crit Rev Oncol Hematol. 2019;144:102821.
- Khan MS, Lateef N, Siddiqi TJ, et al. Level and prevalence of spin in published cardiovascular randomized clinical trial reports with statistically nonsignificant primary outcomes: a systematic review. JAMA Netw Open. 2019;2:E192622.
- Lin V, Patel R, Wirtz A, et al. Evaluation of spin in the abstracts of systematic reviews and meta-analyses of atopic dermatitis treatments and interventions. Dermatology. 2021;237:496-505.
- Rucker B, Umbarger E, Ottwell R, et al. Evaluation of spin in the abstracts of systematic reviews and meta-analyses focused on tinnitus. Otol Neurotol. 2021;10:1237-1244.
- Okonya O, Lai E, Ottwell R, et al. Evaluation of spin in the abstracts of systematic reviews and meta-analyses of treatments for glaucoma. J Glaucoma. 2021;30:235-241.
- Nascimento DP, Gonzalez GZ, Araujo AC, et al. Eight out of every ten abstracts of low back pain systematic reviews presented spin and inconsistencies with the full text: an analysis of 66 systematic reviews. J Orthop Sports Phys Ther. 2020;50:17-23.
- Bai F, Li GG, Liu Q, et al. Short-term efficacy and safety of IL-17, IL-12/23, and IL-23 inhibitors brodalumab, secukinumab, ixekizumab, ustekinumab, guselkumab, tildrakizumab, and risankizumab for the treatment of moderate to severe plaque psoriasis: a systematic review and network meta-analysis of randomized controlled trials. J Immunol Res. 2019;2019:2546161.
- Wu D, Hou SY, Zhao S, et al. Efficacy and safety of interleukin-17 antagonists in patients with plaque psoriasis: a meta-analysis from phase 3 randomized controlled trials. J Eur Acad Dermatol Venereol. 2017;31:992-1003.
- Rusta-Sallehy S, Gooderham M, Papp K. Brodalumab: a review of safety. Skin Therapy Lett. 2018;23:1-3.
- Rodrigeuz-Bolanos F, Gooderham M, Papp K. A closer look at the data regarding suicidal ideation and behavior in psoriasis patients: the case of brodalumab. Skin Therapy Lett. 2019;24:1-4.
- Danesh MJ, Kimball AB. Brodalumab and suicidal ideation in the context of a recent economic crisis in the United States. J Am Acad Dermatol. 2016;74:190-192.
- Siliq. Prescribing information. Valeant Pharmaceuticals North America LLC; 2017. Accessed May 18, 2023. chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761032lbl.pdf
- Johnson HL, Fontelo P, Olsen CH, et al. Family nurse practitioner student perception of journal abstract usefulness in clinical decision making: a randomized controlled trial. J Am Assoc Nurse Pract. 2013;25:597-603.
- Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons; 2019.
Psoriasis is an inflammatory autoimmune skin condition that affects approximately 125 million individuals worldwide, with approximately 8 million patients in the United States.1 Psoriasis not only involves a cosmetic component but also comprises other comorbidities, such as psoriatic arthritis, cardiovascular disease, and psychiatric disorders, that can influence patient quality of life.2-4 In addition, the costs associated with psoriasis are substantial, with an estimated economic burden of $35.2 billion in the United States in 2015.5 Given the prevalence of psoriasis and its many effects on patients, it is important that providers have high-quality evidence regarding efficacious treatment options.
Systematic reviews, which compile all available evidence on a subject to answer a specific question, represent the gold standard of research.6 However, studies have demonstrated that when referencing research literature, physicians tend to read only the abstract of a study rather than the entire article.7,8 A study by Marcelo et al8 showed that residents at a tertiary care center answered clinical questions using only the abstract of a paper 69% of the time. Based on these findings, it is imperative that the results of systematic reviews be accurately reported in their abstracts because they can influence patient care.
Referencing only the abstracts of systematic reviews can be problematic if the abstract contains spin. Spin is a form of reporting that inappropriately highlights the benefits of a treatment with greater emphasis than what is shown by the results.9 Research has identified the presence of spin in the abstracts of randomized controlled trials.10-12 For example, Cooper et al10 found that 70% (33/47) of abstracts in otolaryngology randomized controlled trials contained spin. Additionally, Arthur et al11 and Austin et al12 had similar findings within abstracts of orthopedic and obesity trials, where 44.8% (112/250) and 46.7% (21/45) contained spin, respectively. Ottwell et al13 found that the presence of spin in abstracts is not limited to randomized controlled trials; they demonstrated that the abstracts of nearly one-third (31% [11/36]) of systematic reviews focused on the treatment of acne vulgaris contained spin.
In our study, we aimed to evaluate the presence of spin in the abstracts of systematic reviews focused on the treatment of psoriasis.
Methods
Reproducibility and Reporting—Our study did not meet the regulatory definition for human subjects research per the US Code of Federal Regulations because the study did not involve human research subjects. The study also was not subject to review by the institutional review board. Our protocol, data set, analysis scripts, extraction forms, and other material related to the study have been placed on Open Science Framework to provide transparency and ensure reproducibility. To further allow for analytic reproducibility, our data set was given to an independent laboratory and reanalyzed with a masked approach. Our study was carried out alongside other studies assessing spin in systematic reviews regarding different specialties and disease states. Because these studies were similar in design, this methodology also has been reported elsewhere. The Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA)14 and the guidelines for meta-epidemiological studies developed by Murad and Wang15 were used in drafting this article.
Search Strategy—The search strategies for the MEDLINE (Ovid) and Embase (Ovid) databases were created by a systematic review librarian (D.N.W.) to identify systematic reviews and meta-analyses regarding treatments for psoriasis (Figure 1). The searches were performed on June 2, 2020, and uploaded to Rayyan, a systematic review screening platform.16 After duplicates were removed, the records were screened for eligibility by 2 authors (C.H. and A.L.) using the titles and abstracts. Screening was conducted independently while each of these authors was masked to the other’s results; disagreements were resolved through discussion.

Eligibility Criteria—An article had to meet the following criteria for inclusion in our study: (1) be a systematic review with or without a meta-analysis; (2) relate to the treatment of psoriasis; and (3) be written in English and include human patients only. The PRISMA definition of systematic reviews and meta-analyses was applied.17
Training—Various training occurred throughout our study to ensure understanding of each step and mitigate subjectivity. Before beginning screening, 2 investigators (C.H. and A.L.) completed the Introduction to Systematic Review and Meta-Analysis course offered by Johns Hopkins University.18 They also underwent 2 days of online and in-person training on the definition and interpretation of the 9 most severe types of spin found in the abstracts of systematic reviews as defined by Yavchitz et al.9 Finally, they were trained to use A MeaSurement Tool to Assess systematic Reviews (AMSTAR-2) to appraise the methodological quality of each systematic review. Our protocol contained an outline of all training modules used.
Data Extraction—The investigators (C.H. and A.L.) analyzed included abstracts for the 9 most severe types of spin (Table 1). Data were extracted in a masked duplicate fashion using the Google form. AMSTAR-2 was used to assess systematic reviews for methodological quality. AMSTAR-2 is an appraisal tool consisting of a 16-item checklist for systematic reviews or meta-analyses. Scores range from critically low to high based on the methodological quality of the review. Interrater reliability of AMSTAR-2 scores has been moderate to high across studies. Construct validity coefficients have been high with the original AMSTAR instrument (r=0.91) and the Risk of Bias in Systematic Reviews instrument (r=0.84).19

During data extraction from each included systematic review, the following additional items were obtained: (1) the date the review was received; (2) intervention type (ie, pharmacologic, nonpharmacologic, surgery, light therapy, mixed); (3) the funding source(s) for each systematic review (ie, industry, private, public, none, not mentioned, hospital, a combination of funding not including industry, a combination of funding including industry, other); (4) whether the journal submission guidelines suggested adherence to PRISMA guidelines; (5) whether the review discussed adherence to PRISMA14 or PRISMA for Abstracts20 (PRISMA-A); (6) the publishing journal’s 5-year impact factor; and (6) the country of the systematic review’s origin. When data extraction was complete, investigators (C.H. and A.L.) were unmasked and met to resolve any disagreements by discussion. Two authors (R.O. or M.V.) served as arbiters in the case that an agreement between C.H. and A.L. could not be reached.
Statistical Analysis—Frequencies and percentages were calculated to evaluate the most common types of spin found within systematic reviews and meta-analyses. One author (M.H.) prespecified the possibility of a binary logistic regression and calculated a power analysis to determine sample size, as stated in our protocol. Our final sample size of 173 was not powered to perform the multivariable logistic regression; therefore, we calculated unadjusted odds ratios to enable assessing relationships between the presence of spin in abstracts and the various study characteristics. We used Stata 16.1 for all analyses, and all analytic decisions can be found in our protocol.
Results
General Characteristics—Our systematic search of MEDLINE and Embase returned 3200 articles, of which 665 were duplicates that were removed. An additional 2253 articles were excluded during initial abstract and title screening, and full-text screening led to the exclusion of another 109 articles. In total, 173 systematic reviews were included for data extraction. Figure 2 illustrates the screening process with the rationale for all exclusions.

Of the 173 included systematic reviews and meta-analyses, 150 (86.7%) focused on pharmacologic interventions. The majority of studies did not mention adhering to PRISMA guidelines (125/173 [72.3%]), and the publishing journals recommended their authors adhere to PRISMA for only 66 (38.2%) of the included articles. For the articles that received funding (90/173 [52.0%]), industry sources were the most common funding source (40/90 [44.4%]), followed by private (27/90 [30%]) and public funding sources (23/90 [25.6%]). Of the remaining studies, 46 articles did not include a funding statement (46/83 [55.4%]), and 37 studies were not funded (37/83 [44.6%]). The average (SD) 5-year impact factor of our included journals was 4.68 (4.64). Systematic reviews were from 31 different countries. All studies were received by their respective journals between the years 2000 and 2020 (Table 2).

Abstracts Containing Spin—We found that 37 (21.4%) of the abstracts of systematic reviews focused on psoriasis treatments contained at least 1 type of spin. Some abstracts had more than 1 type; thus, a total of 51 different instances of spin were detected. Spin type 6—selective reporting of or overemphasis on harm outcomes or analysis favoring the safety of the experimental intervention—was the most common type ofspin, found in 19 of 173 abstracts (11.0%). The most severe type of spin—type 1 (conclusion contains recommendations for clinical practice not supported by the findings)—occurred in only 1 abstract (0.6%). Spin type 8 did not occur in any of the abstracts (Table 1). There was no statistically significant association between the presence of spin and any of the study characteristics (Table 2).
AMSTAR Ratings—After using AMSTAR-2 to appraise the included systematic reviews, we found that 6 (3.5%) of the 173 studies could be rated as high; 36 (20.8%) as moderate; 25 (14.5%) as low; and 106 (61.3%) as critically low. Of the 37 abstracts containing spin, 2 (5.4%) had an AMSTAR-2 rating of high, 10 (27%) had a rating of moderate, 6 (16.2%) had a rating of low, and 19 (51.4%) had a rating of critically low (Table 2). No statistically significant associations were seen between abstracts found to have spin and the AMSTAR-2 rating of the review.
Nearly all (160/173 [92.5%]) of the included reviews were compliant with the inclusion of Population, Intervention, Comparison, and Outcome (PICO) method. Only 17 of 173 (9.8%) reviews reported funding sources for the studies included. See Table 3 for all AMSTAR-2 items.

Comment
Primary Findings—We evaluated the abstracts of systematic reviews for the treatment of psoriasis and found that more than one-fifth of them contained spin. Our study contributes to the existing literature surrounding spin. Spin in randomized controlled trials is well documented across several fields of medicine, including otolaryngology,10 obesity medicine,12 dermatology,21 anesthesiology,22 psychiatry,23 orthopedics,24 emergency medicine,25 oncology,26 and cardiology.27 More recently, studies have emerged evaluating the presence of spin in systematic reviews. Specific to dermatology, one study found that 74% (84/113) of systematic reviews related to atopic dermatitis treatment contained spin.28 Additionally, Ottwell et al13 identified spin in 31% (11/36) of the systematic reviews related to the treatment of acne vulgaris, which is similar to our results for systematic reviews focused on psoriasis treatments. When comparing the presence of spin in abstracts of systematic reviews from the field of dermatology with other specialties, dermatology-focused systematic reviews appear to contain more spin in the abstract than systematic reviews focused on tinnitus and glaucoma therapies.29,30 However, systematic reviews from the field of dermatology appear to contain less spin than systematic reviews focused on therapies for lower back pain.31 For example, Nascimento et al31 found that 80% (53/66) of systematic reviews focused on low-back pain treatments contained spin.
Examples of Spin—The most common type of spin found in our study was type 6.9 An example of spin type 6 can be found in an article by Bai et al32 that investigated the short-term efficacy and safety of multiple interleukin inhibitors for the treatment of plaque psoriasis. The conclusion of the abstract states, “Risankizumab appeared to have relatively high efficacy and low risk.” However, in the results section, the authors showed that risankizumab had the highest risk of serious adverse events and was ranked highest for discontinuation because of adverse events when compared with other interleukin inhibitors. Here, the presence of spin in the abstract may mislead the reader to accept the “low risk” of risankizumab without understanding the study’s full results.32
Another example of selective reporting of harm outcomes in a systematic review can be found in the article by Wu et al,33 which focused on assessing IL-17 antagonists for the treatment of plaque psoriasis. The conclusion of the abstract indicated that IL-17 antagonists should be accepted as safe; however, in the results section, the authors discussed serious safety concerns with brodalumab, including the death of 4 patients from suicide.33 This example of spin type 6 highlights how the overgeneralization of a drug’s safety profile neglects serious harm outcomes that are critical to patient safety. In fact, against the safety claims of Wu et al,33 brodalumab later received a boxed warning from the US Food and Drug Administration after 6 patients died from suicide while receiving the drug, which led to early discontinuation of the trials.34,35 Although studies suggest this relationship is not causal,34-36 the purpose of our study was not to investigate this association but to highlight the importance of this finding. Thus, with this example of spin in mind, we offer recommendations that we believe will improve reporting in abstracts as well as quality of patient care.
Recommendations for Reporting in Abstracts—Regarding the boxed warning37 for brodalumab because of suicidal ideation and behavior, the US Food and Drug Administration recommends that prior to prescribing brodalumab, clinicians consider the potential benefits and risks in patients with a history of depression and/or suicidal ideation or behavior. However, a clinician would not adequately assess the full risks and benefits when an abstract, such as that for the article by Wu et al,33 contains spin through selectively reporting harm outcomes. Arguably, clinicians could just read the full text; however, research confirms that abstracts often are utilized by clinicians and commonly are used to guide clinical decisions.7,38 It is reasonable that clinicians would use abstracts in this fashion because they provide a quick synopsis of the full article’s findings and are widely available to clinicians who may not have access to article databases. Initiatives are in place to improve the quality of reporting in an abstract, such as PRISMA-A,20 but even this fails to address spin. In fact, it may suggest spin because checklist item 10 of PRISMA-A advises authors of systematic reviews to provide a “general interpretation of the results and important implications.” This item is concerning because it suggests that the authors interpret importance rather than the clinician who prescribes the drug and is ultimately responsible for patient safety. Therefore, we recommend a reform to abstract reporting and an update to PRISMA-A that leads authors to report all benefits and risks encountered instead of reporting what the authors define as important.
Strengths and Limitations—Our study has several strengths as well as limitations. One of these strengths is that our protocol was strictly adhered to; any deviations were noted and added as an amendment. Our protocol, data, and all study artifacts were made freely available online on the Open Science Framework to strengthen reproducibility (https://osf.io/zrxh8/). Investigators underwent training to ensure comprehension of spin and systematic review designs. All data were extracted in masked duplicate fashion per the Cochrane Handbook for Systematic Reviews of Interventions.39
Regarding limitations, only 2 databases were searched—MEDLINE and Embase. Therefore, our screening process may not have included every available systematic review on the treatment of psoriasis. Journal impact factors may be inaccurate for the systematic reviews that were published earlier in our data date range; however, we attempted to negate this limitation by using a 5-year average. Our study characteristic regarding PRISMA adherence did not account for studies published before the PRISMA statement release; we also could not access prior submission guidelines to determine when a journal began recommending PRISMA adherence. Another limitation of our study was the intrinsic subjectivity behind spin. Some may disagree with our classifications. Finally, our cross-sectional design should not be generalized to study types that are not systematic reviews or published in other journals during different periods.
Conclusion
Evidence of spin was present in many of the abstracts of systematic reviews pertaining to the treatment of psoriasis. Future clinical research should investigate any reporting of spin and search for ways to better reduce spin within literature. Continued research is necessary to evaluate the presence of spin within dermatology and other specialties.
Psoriasis is an inflammatory autoimmune skin condition that affects approximately 125 million individuals worldwide, with approximately 8 million patients in the United States.1 Psoriasis not only involves a cosmetic component but also comprises other comorbidities, such as psoriatic arthritis, cardiovascular disease, and psychiatric disorders, that can influence patient quality of life.2-4 In addition, the costs associated with psoriasis are substantial, with an estimated economic burden of $35.2 billion in the United States in 2015.5 Given the prevalence of psoriasis and its many effects on patients, it is important that providers have high-quality evidence regarding efficacious treatment options.
Systematic reviews, which compile all available evidence on a subject to answer a specific question, represent the gold standard of research.6 However, studies have demonstrated that when referencing research literature, physicians tend to read only the abstract of a study rather than the entire article.7,8 A study by Marcelo et al8 showed that residents at a tertiary care center answered clinical questions using only the abstract of a paper 69% of the time. Based on these findings, it is imperative that the results of systematic reviews be accurately reported in their abstracts because they can influence patient care.
Referencing only the abstracts of systematic reviews can be problematic if the abstract contains spin. Spin is a form of reporting that inappropriately highlights the benefits of a treatment with greater emphasis than what is shown by the results.9 Research has identified the presence of spin in the abstracts of randomized controlled trials.10-12 For example, Cooper et al10 found that 70% (33/47) of abstracts in otolaryngology randomized controlled trials contained spin. Additionally, Arthur et al11 and Austin et al12 had similar findings within abstracts of orthopedic and obesity trials, where 44.8% (112/250) and 46.7% (21/45) contained spin, respectively. Ottwell et al13 found that the presence of spin in abstracts is not limited to randomized controlled trials; they demonstrated that the abstracts of nearly one-third (31% [11/36]) of systematic reviews focused on the treatment of acne vulgaris contained spin.
In our study, we aimed to evaluate the presence of spin in the abstracts of systematic reviews focused on the treatment of psoriasis.
Methods
Reproducibility and Reporting—Our study did not meet the regulatory definition for human subjects research per the US Code of Federal Regulations because the study did not involve human research subjects. The study also was not subject to review by the institutional review board. Our protocol, data set, analysis scripts, extraction forms, and other material related to the study have been placed on Open Science Framework to provide transparency and ensure reproducibility. To further allow for analytic reproducibility, our data set was given to an independent laboratory and reanalyzed with a masked approach. Our study was carried out alongside other studies assessing spin in systematic reviews regarding different specialties and disease states. Because these studies were similar in design, this methodology also has been reported elsewhere. The Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA)14 and the guidelines for meta-epidemiological studies developed by Murad and Wang15 were used in drafting this article.
Search Strategy—The search strategies for the MEDLINE (Ovid) and Embase (Ovid) databases were created by a systematic review librarian (D.N.W.) to identify systematic reviews and meta-analyses regarding treatments for psoriasis (Figure 1). The searches were performed on June 2, 2020, and uploaded to Rayyan, a systematic review screening platform.16 After duplicates were removed, the records were screened for eligibility by 2 authors (C.H. and A.L.) using the titles and abstracts. Screening was conducted independently while each of these authors was masked to the other’s results; disagreements were resolved through discussion.

Eligibility Criteria—An article had to meet the following criteria for inclusion in our study: (1) be a systematic review with or without a meta-analysis; (2) relate to the treatment of psoriasis; and (3) be written in English and include human patients only. The PRISMA definition of systematic reviews and meta-analyses was applied.17
Training—Various training occurred throughout our study to ensure understanding of each step and mitigate subjectivity. Before beginning screening, 2 investigators (C.H. and A.L.) completed the Introduction to Systematic Review and Meta-Analysis course offered by Johns Hopkins University.18 They also underwent 2 days of online and in-person training on the definition and interpretation of the 9 most severe types of spin found in the abstracts of systematic reviews as defined by Yavchitz et al.9 Finally, they were trained to use A MeaSurement Tool to Assess systematic Reviews (AMSTAR-2) to appraise the methodological quality of each systematic review. Our protocol contained an outline of all training modules used.
Data Extraction—The investigators (C.H. and A.L.) analyzed included abstracts for the 9 most severe types of spin (Table 1). Data were extracted in a masked duplicate fashion using the Google form. AMSTAR-2 was used to assess systematic reviews for methodological quality. AMSTAR-2 is an appraisal tool consisting of a 16-item checklist for systematic reviews or meta-analyses. Scores range from critically low to high based on the methodological quality of the review. Interrater reliability of AMSTAR-2 scores has been moderate to high across studies. Construct validity coefficients have been high with the original AMSTAR instrument (r=0.91) and the Risk of Bias in Systematic Reviews instrument (r=0.84).19

During data extraction from each included systematic review, the following additional items were obtained: (1) the date the review was received; (2) intervention type (ie, pharmacologic, nonpharmacologic, surgery, light therapy, mixed); (3) the funding source(s) for each systematic review (ie, industry, private, public, none, not mentioned, hospital, a combination of funding not including industry, a combination of funding including industry, other); (4) whether the journal submission guidelines suggested adherence to PRISMA guidelines; (5) whether the review discussed adherence to PRISMA14 or PRISMA for Abstracts20 (PRISMA-A); (6) the publishing journal’s 5-year impact factor; and (6) the country of the systematic review’s origin. When data extraction was complete, investigators (C.H. and A.L.) were unmasked and met to resolve any disagreements by discussion. Two authors (R.O. or M.V.) served as arbiters in the case that an agreement between C.H. and A.L. could not be reached.
Statistical Analysis—Frequencies and percentages were calculated to evaluate the most common types of spin found within systematic reviews and meta-analyses. One author (M.H.) prespecified the possibility of a binary logistic regression and calculated a power analysis to determine sample size, as stated in our protocol. Our final sample size of 173 was not powered to perform the multivariable logistic regression; therefore, we calculated unadjusted odds ratios to enable assessing relationships between the presence of spin in abstracts and the various study characteristics. We used Stata 16.1 for all analyses, and all analytic decisions can be found in our protocol.
Results
General Characteristics—Our systematic search of MEDLINE and Embase returned 3200 articles, of which 665 were duplicates that were removed. An additional 2253 articles were excluded during initial abstract and title screening, and full-text screening led to the exclusion of another 109 articles. In total, 173 systematic reviews were included for data extraction. Figure 2 illustrates the screening process with the rationale for all exclusions.

Of the 173 included systematic reviews and meta-analyses, 150 (86.7%) focused on pharmacologic interventions. The majority of studies did not mention adhering to PRISMA guidelines (125/173 [72.3%]), and the publishing journals recommended their authors adhere to PRISMA for only 66 (38.2%) of the included articles. For the articles that received funding (90/173 [52.0%]), industry sources were the most common funding source (40/90 [44.4%]), followed by private (27/90 [30%]) and public funding sources (23/90 [25.6%]). Of the remaining studies, 46 articles did not include a funding statement (46/83 [55.4%]), and 37 studies were not funded (37/83 [44.6%]). The average (SD) 5-year impact factor of our included journals was 4.68 (4.64). Systematic reviews were from 31 different countries. All studies were received by their respective journals between the years 2000 and 2020 (Table 2).

Abstracts Containing Spin—We found that 37 (21.4%) of the abstracts of systematic reviews focused on psoriasis treatments contained at least 1 type of spin. Some abstracts had more than 1 type; thus, a total of 51 different instances of spin were detected. Spin type 6—selective reporting of or overemphasis on harm outcomes or analysis favoring the safety of the experimental intervention—was the most common type ofspin, found in 19 of 173 abstracts (11.0%). The most severe type of spin—type 1 (conclusion contains recommendations for clinical practice not supported by the findings)—occurred in only 1 abstract (0.6%). Spin type 8 did not occur in any of the abstracts (Table 1). There was no statistically significant association between the presence of spin and any of the study characteristics (Table 2).
AMSTAR Ratings—After using AMSTAR-2 to appraise the included systematic reviews, we found that 6 (3.5%) of the 173 studies could be rated as high; 36 (20.8%) as moderate; 25 (14.5%) as low; and 106 (61.3%) as critically low. Of the 37 abstracts containing spin, 2 (5.4%) had an AMSTAR-2 rating of high, 10 (27%) had a rating of moderate, 6 (16.2%) had a rating of low, and 19 (51.4%) had a rating of critically low (Table 2). No statistically significant associations were seen between abstracts found to have spin and the AMSTAR-2 rating of the review.
Nearly all (160/173 [92.5%]) of the included reviews were compliant with the inclusion of Population, Intervention, Comparison, and Outcome (PICO) method. Only 17 of 173 (9.8%) reviews reported funding sources for the studies included. See Table 3 for all AMSTAR-2 items.

Comment
Primary Findings—We evaluated the abstracts of systematic reviews for the treatment of psoriasis and found that more than one-fifth of them contained spin. Our study contributes to the existing literature surrounding spin. Spin in randomized controlled trials is well documented across several fields of medicine, including otolaryngology,10 obesity medicine,12 dermatology,21 anesthesiology,22 psychiatry,23 orthopedics,24 emergency medicine,25 oncology,26 and cardiology.27 More recently, studies have emerged evaluating the presence of spin in systematic reviews. Specific to dermatology, one study found that 74% (84/113) of systematic reviews related to atopic dermatitis treatment contained spin.28 Additionally, Ottwell et al13 identified spin in 31% (11/36) of the systematic reviews related to the treatment of acne vulgaris, which is similar to our results for systematic reviews focused on psoriasis treatments. When comparing the presence of spin in abstracts of systematic reviews from the field of dermatology with other specialties, dermatology-focused systematic reviews appear to contain more spin in the abstract than systematic reviews focused on tinnitus and glaucoma therapies.29,30 However, systematic reviews from the field of dermatology appear to contain less spin than systematic reviews focused on therapies for lower back pain.31 For example, Nascimento et al31 found that 80% (53/66) of systematic reviews focused on low-back pain treatments contained spin.
Examples of Spin—The most common type of spin found in our study was type 6.9 An example of spin type 6 can be found in an article by Bai et al32 that investigated the short-term efficacy and safety of multiple interleukin inhibitors for the treatment of plaque psoriasis. The conclusion of the abstract states, “Risankizumab appeared to have relatively high efficacy and low risk.” However, in the results section, the authors showed that risankizumab had the highest risk of serious adverse events and was ranked highest for discontinuation because of adverse events when compared with other interleukin inhibitors. Here, the presence of spin in the abstract may mislead the reader to accept the “low risk” of risankizumab without understanding the study’s full results.32
Another example of selective reporting of harm outcomes in a systematic review can be found in the article by Wu et al,33 which focused on assessing IL-17 antagonists for the treatment of plaque psoriasis. The conclusion of the abstract indicated that IL-17 antagonists should be accepted as safe; however, in the results section, the authors discussed serious safety concerns with brodalumab, including the death of 4 patients from suicide.33 This example of spin type 6 highlights how the overgeneralization of a drug’s safety profile neglects serious harm outcomes that are critical to patient safety. In fact, against the safety claims of Wu et al,33 brodalumab later received a boxed warning from the US Food and Drug Administration after 6 patients died from suicide while receiving the drug, which led to early discontinuation of the trials.34,35 Although studies suggest this relationship is not causal,34-36 the purpose of our study was not to investigate this association but to highlight the importance of this finding. Thus, with this example of spin in mind, we offer recommendations that we believe will improve reporting in abstracts as well as quality of patient care.
Recommendations for Reporting in Abstracts—Regarding the boxed warning37 for brodalumab because of suicidal ideation and behavior, the US Food and Drug Administration recommends that prior to prescribing brodalumab, clinicians consider the potential benefits and risks in patients with a history of depression and/or suicidal ideation or behavior. However, a clinician would not adequately assess the full risks and benefits when an abstract, such as that for the article by Wu et al,33 contains spin through selectively reporting harm outcomes. Arguably, clinicians could just read the full text; however, research confirms that abstracts often are utilized by clinicians and commonly are used to guide clinical decisions.7,38 It is reasonable that clinicians would use abstracts in this fashion because they provide a quick synopsis of the full article’s findings and are widely available to clinicians who may not have access to article databases. Initiatives are in place to improve the quality of reporting in an abstract, such as PRISMA-A,20 but even this fails to address spin. In fact, it may suggest spin because checklist item 10 of PRISMA-A advises authors of systematic reviews to provide a “general interpretation of the results and important implications.” This item is concerning because it suggests that the authors interpret importance rather than the clinician who prescribes the drug and is ultimately responsible for patient safety. Therefore, we recommend a reform to abstract reporting and an update to PRISMA-A that leads authors to report all benefits and risks encountered instead of reporting what the authors define as important.
Strengths and Limitations—Our study has several strengths as well as limitations. One of these strengths is that our protocol was strictly adhered to; any deviations were noted and added as an amendment. Our protocol, data, and all study artifacts were made freely available online on the Open Science Framework to strengthen reproducibility (https://osf.io/zrxh8/). Investigators underwent training to ensure comprehension of spin and systematic review designs. All data were extracted in masked duplicate fashion per the Cochrane Handbook for Systematic Reviews of Interventions.39
Regarding limitations, only 2 databases were searched—MEDLINE and Embase. Therefore, our screening process may not have included every available systematic review on the treatment of psoriasis. Journal impact factors may be inaccurate for the systematic reviews that were published earlier in our data date range; however, we attempted to negate this limitation by using a 5-year average. Our study characteristic regarding PRISMA adherence did not account for studies published before the PRISMA statement release; we also could not access prior submission guidelines to determine when a journal began recommending PRISMA adherence. Another limitation of our study was the intrinsic subjectivity behind spin. Some may disagree with our classifications. Finally, our cross-sectional design should not be generalized to study types that are not systematic reviews or published in other journals during different periods.
Conclusion
Evidence of spin was present in many of the abstracts of systematic reviews pertaining to the treatment of psoriasis. Future clinical research should investigate any reporting of spin and search for ways to better reduce spin within literature. Continued research is necessary to evaluate the presence of spin within dermatology and other specialties.
- Psoriasis statistics. National Psoriasis Foundation. Updated December 21, 2022. Accessed March 6, 2023. https://www.psoriasis.org/content/statistics
- Greb JE, Goldminz AM, Elder JT, et al. Psoriasis. Nat Rev Dis Primers. 2016;2:16082.
- Hu SCS, Lan CCE. Psoriasis and cardiovascular comorbidities: focusing on severe vascular events, cardiovascular risk factors and implications for treatment. Int J Mol Sci. 2017;18:2211.
- Patel N, Nadkarni A, Cardwell LA, et al. Psoriasis, depression, and inflammatory overlap: a review. Am J Clin Dermatol. 2017;18:613-620.
- Brezinski EA, Dhillon JS, Armstrong AW. Economic burden of psoriasis in the United States: a systematic review. JAMA Dermatol. 2015;151:651-658.
- Gopalakrishnan S, Ganeshkumar P. Systematic reviews and meta‑analysis: understanding the best evidence in primary healthcare. J Fam Med Prim Care. 2013;2:9-14.
- Barry HC, Ebell MH, Shaughnessy AF, et al. Family physicians’ use of medical abstracts to guide decision making: style or substance? J Am Board Fam Pract. 2001;14:437-442.
- Marcelo A, Gavino A, Isip-Tan IT, et al. A comparison of the accuracy of clinical decisions based on full-text articles and on journal abstracts alone: a study among residents in a tertiary care hospital. Evid Based Med. 2013;18:48-53.
- Yavchitz A, Ravaud P, Altman DG, et al. A new classification of spin in systematic reviews and meta-analyses was developed and ranked according to the severity. J Clin Epidemiol. 2016;75:56-65.
- Cooper CM, Gray HM, Ross AE, et al. Evaluation of spin in the abstracts of otolaryngology randomized controlled trials. Laryngoscope. 2019;129:2036-2040.
- Arthur W, Zaaza Z, Checketts JX, et al. Analyzing spin in abstracts of orthopaedic randomized controlled trials with statistically insignificant primary endpoints. Arthroscopy. 2020;36:1443-1450.
- Austin J, Smith C, Natarajan K, et al. Evaluation of spin within abstracts in obesity randomized clinical trials: a cross-sectional review. Clin Obes. 2019;9:E12292.
- Ottwell R, Rogers TC, Michael Anderson J, et al. Evaluation of spin in the abstracts of systematic reviews and meta-analyses focused on the treatment of acne vulgaris: cross-sectional analysis. JMIR Dermatol. 2020;3:E16978.
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:E1000100.
- Murad MH, Wang Z. Guidelines for reporting meta-epidemiological methodology research. Evid Based Med. 2017;22:139-142.
- Rayyan QCRI. Accessed September 10, 2019. https://rayyan.qcri.org/reviews/81224
- Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350:g7647.
- Coursera. Introduction to systematic review and meta-analysis. Accessed May 18, 2023. https://www.coursera.org/learn/systematic-review
- Lorenz RC, Matthias K, Pieper D, et al. A psychometric study found AMSTAR 2 to be a valid and moderately reliable appraisal tool. J Clin Epidemiol. 2019;114:133-140.
- Beller EM, Glasziou PP, Altman DG, et al. PRISMA for abstracts: reporting systematic reviews in journal and conference abstracts. PLoS Med. 2013;10:E1001419.
- Motosko CC, Ault AK, Kimberly LL, et al. Analysis of spin in the reporting of studies of topical treatments of photoaged skin. J Am Acad Dermatol. 2019;80:516-522.e12.
- Kinder NC, Weaver MD, Wayant C, et al. Presence of “spin” in the abstracts and titles of anaesthesiology randomised controlled trials. Br J Anaesth. 2019;122:E13-E14.
- Jellison S, Roberts W, Bowers A, et al. Evaluation of spin in abstracts of papers in psychiatry and psychology journals. BMJ Evid Based Med. 2019;5:178-181.
- Checketts JX, Riddle J, Zaaza Z, et al. An evaluation of spin in lower extremity joint trials. J Arthroplasty. 2019;34:1008-1012.
- Reynolds-Vaughn V, Riddle J, Brown J, et al. Evaluation of spin in the abstracts of emergency medicine randomized controlled trials. Ann Emerg Med. 2019;14:423-431.
- Wayant C, Margalski D, Vaughn K, et al. Evaluation of spin in oncology clinical trials. Crit Rev Oncol Hematol. 2019;144:102821.
- Khan MS, Lateef N, Siddiqi TJ, et al. Level and prevalence of spin in published cardiovascular randomized clinical trial reports with statistically nonsignificant primary outcomes: a systematic review. JAMA Netw Open. 2019;2:E192622.
- Lin V, Patel R, Wirtz A, et al. Evaluation of spin in the abstracts of systematic reviews and meta-analyses of atopic dermatitis treatments and interventions. Dermatology. 2021;237:496-505.
- Rucker B, Umbarger E, Ottwell R, et al. Evaluation of spin in the abstracts of systematic reviews and meta-analyses focused on tinnitus. Otol Neurotol. 2021;10:1237-1244.
- Okonya O, Lai E, Ottwell R, et al. Evaluation of spin in the abstracts of systematic reviews and meta-analyses of treatments for glaucoma. J Glaucoma. 2021;30:235-241.
- Nascimento DP, Gonzalez GZ, Araujo AC, et al. Eight out of every ten abstracts of low back pain systematic reviews presented spin and inconsistencies with the full text: an analysis of 66 systematic reviews. J Orthop Sports Phys Ther. 2020;50:17-23.
- Bai F, Li GG, Liu Q, et al. Short-term efficacy and safety of IL-17, IL-12/23, and IL-23 inhibitors brodalumab, secukinumab, ixekizumab, ustekinumab, guselkumab, tildrakizumab, and risankizumab for the treatment of moderate to severe plaque psoriasis: a systematic review and network meta-analysis of randomized controlled trials. J Immunol Res. 2019;2019:2546161.
- Wu D, Hou SY, Zhao S, et al. Efficacy and safety of interleukin-17 antagonists in patients with plaque psoriasis: a meta-analysis from phase 3 randomized controlled trials. J Eur Acad Dermatol Venereol. 2017;31:992-1003.
- Rusta-Sallehy S, Gooderham M, Papp K. Brodalumab: a review of safety. Skin Therapy Lett. 2018;23:1-3.
- Rodrigeuz-Bolanos F, Gooderham M, Papp K. A closer look at the data regarding suicidal ideation and behavior in psoriasis patients: the case of brodalumab. Skin Therapy Lett. 2019;24:1-4.
- Danesh MJ, Kimball AB. Brodalumab and suicidal ideation in the context of a recent economic crisis in the United States. J Am Acad Dermatol. 2016;74:190-192.
- Siliq. Prescribing information. Valeant Pharmaceuticals North America LLC; 2017. Accessed May 18, 2023. chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761032lbl.pdf
- Johnson HL, Fontelo P, Olsen CH, et al. Family nurse practitioner student perception of journal abstract usefulness in clinical decision making: a randomized controlled trial. J Am Assoc Nurse Pract. 2013;25:597-603.
- Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons; 2019.
- Psoriasis statistics. National Psoriasis Foundation. Updated December 21, 2022. Accessed March 6, 2023. https://www.psoriasis.org/content/statistics
- Greb JE, Goldminz AM, Elder JT, et al. Psoriasis. Nat Rev Dis Primers. 2016;2:16082.
- Hu SCS, Lan CCE. Psoriasis and cardiovascular comorbidities: focusing on severe vascular events, cardiovascular risk factors and implications for treatment. Int J Mol Sci. 2017;18:2211.
- Patel N, Nadkarni A, Cardwell LA, et al. Psoriasis, depression, and inflammatory overlap: a review. Am J Clin Dermatol. 2017;18:613-620.
- Brezinski EA, Dhillon JS, Armstrong AW. Economic burden of psoriasis in the United States: a systematic review. JAMA Dermatol. 2015;151:651-658.
- Gopalakrishnan S, Ganeshkumar P. Systematic reviews and meta‑analysis: understanding the best evidence in primary healthcare. J Fam Med Prim Care. 2013;2:9-14.
- Barry HC, Ebell MH, Shaughnessy AF, et al. Family physicians’ use of medical abstracts to guide decision making: style or substance? J Am Board Fam Pract. 2001;14:437-442.
- Marcelo A, Gavino A, Isip-Tan IT, et al. A comparison of the accuracy of clinical decisions based on full-text articles and on journal abstracts alone: a study among residents in a tertiary care hospital. Evid Based Med. 2013;18:48-53.
- Yavchitz A, Ravaud P, Altman DG, et al. A new classification of spin in systematic reviews and meta-analyses was developed and ranked according to the severity. J Clin Epidemiol. 2016;75:56-65.
- Cooper CM, Gray HM, Ross AE, et al. Evaluation of spin in the abstracts of otolaryngology randomized controlled trials. Laryngoscope. 2019;129:2036-2040.
- Arthur W, Zaaza Z, Checketts JX, et al. Analyzing spin in abstracts of orthopaedic randomized controlled trials with statistically insignificant primary endpoints. Arthroscopy. 2020;36:1443-1450.
- Austin J, Smith C, Natarajan K, et al. Evaluation of spin within abstracts in obesity randomized clinical trials: a cross-sectional review. Clin Obes. 2019;9:E12292.
- Ottwell R, Rogers TC, Michael Anderson J, et al. Evaluation of spin in the abstracts of systematic reviews and meta-analyses focused on the treatment of acne vulgaris: cross-sectional analysis. JMIR Dermatol. 2020;3:E16978.
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:E1000100.
- Murad MH, Wang Z. Guidelines for reporting meta-epidemiological methodology research. Evid Based Med. 2017;22:139-142.
- Rayyan QCRI. Accessed September 10, 2019. https://rayyan.qcri.org/reviews/81224
- Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350:g7647.
- Coursera. Introduction to systematic review and meta-analysis. Accessed May 18, 2023. https://www.coursera.org/learn/systematic-review
- Lorenz RC, Matthias K, Pieper D, et al. A psychometric study found AMSTAR 2 to be a valid and moderately reliable appraisal tool. J Clin Epidemiol. 2019;114:133-140.
- Beller EM, Glasziou PP, Altman DG, et al. PRISMA for abstracts: reporting systematic reviews in journal and conference abstracts. PLoS Med. 2013;10:E1001419.
- Motosko CC, Ault AK, Kimberly LL, et al. Analysis of spin in the reporting of studies of topical treatments of photoaged skin. J Am Acad Dermatol. 2019;80:516-522.e12.
- Kinder NC, Weaver MD, Wayant C, et al. Presence of “spin” in the abstracts and titles of anaesthesiology randomised controlled trials. Br J Anaesth. 2019;122:E13-E14.
- Jellison S, Roberts W, Bowers A, et al. Evaluation of spin in abstracts of papers in psychiatry and psychology journals. BMJ Evid Based Med. 2019;5:178-181.
- Checketts JX, Riddle J, Zaaza Z, et al. An evaluation of spin in lower extremity joint trials. J Arthroplasty. 2019;34:1008-1012.
- Reynolds-Vaughn V, Riddle J, Brown J, et al. Evaluation of spin in the abstracts of emergency medicine randomized controlled trials. Ann Emerg Med. 2019;14:423-431.
- Wayant C, Margalski D, Vaughn K, et al. Evaluation of spin in oncology clinical trials. Crit Rev Oncol Hematol. 2019;144:102821.
- Khan MS, Lateef N, Siddiqi TJ, et al. Level and prevalence of spin in published cardiovascular randomized clinical trial reports with statistically nonsignificant primary outcomes: a systematic review. JAMA Netw Open. 2019;2:E192622.
- Lin V, Patel R, Wirtz A, et al. Evaluation of spin in the abstracts of systematic reviews and meta-analyses of atopic dermatitis treatments and interventions. Dermatology. 2021;237:496-505.
- Rucker B, Umbarger E, Ottwell R, et al. Evaluation of spin in the abstracts of systematic reviews and meta-analyses focused on tinnitus. Otol Neurotol. 2021;10:1237-1244.
- Okonya O, Lai E, Ottwell R, et al. Evaluation of spin in the abstracts of systematic reviews and meta-analyses of treatments for glaucoma. J Glaucoma. 2021;30:235-241.
- Nascimento DP, Gonzalez GZ, Araujo AC, et al. Eight out of every ten abstracts of low back pain systematic reviews presented spin and inconsistencies with the full text: an analysis of 66 systematic reviews. J Orthop Sports Phys Ther. 2020;50:17-23.
- Bai F, Li GG, Liu Q, et al. Short-term efficacy and safety of IL-17, IL-12/23, and IL-23 inhibitors brodalumab, secukinumab, ixekizumab, ustekinumab, guselkumab, tildrakizumab, and risankizumab for the treatment of moderate to severe plaque psoriasis: a systematic review and network meta-analysis of randomized controlled trials. J Immunol Res. 2019;2019:2546161.
- Wu D, Hou SY, Zhao S, et al. Efficacy and safety of interleukin-17 antagonists in patients with plaque psoriasis: a meta-analysis from phase 3 randomized controlled trials. J Eur Acad Dermatol Venereol. 2017;31:992-1003.
- Rusta-Sallehy S, Gooderham M, Papp K. Brodalumab: a review of safety. Skin Therapy Lett. 2018;23:1-3.
- Rodrigeuz-Bolanos F, Gooderham M, Papp K. A closer look at the data regarding suicidal ideation and behavior in psoriasis patients: the case of brodalumab. Skin Therapy Lett. 2019;24:1-4.
- Danesh MJ, Kimball AB. Brodalumab and suicidal ideation in the context of a recent economic crisis in the United States. J Am Acad Dermatol. 2016;74:190-192.
- Siliq. Prescribing information. Valeant Pharmaceuticals North America LLC; 2017. Accessed May 18, 2023. chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761032lbl.pdf
- Johnson HL, Fontelo P, Olsen CH, et al. Family nurse practitioner student perception of journal abstract usefulness in clinical decision making: a randomized controlled trial. J Am Assoc Nurse Pract. 2013;25:597-603.
- Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons; 2019.
Practice Points
- Spin is defined as the intentional or unintentional misrepresentation of findings and can inappropriately highlight results and disregard results of equal importance.
- Our findings show that more than 20% of systematic reviews focused on the treatment of psoriasis contained some form of spin within the abstract.
- Because spin has the potential to misrepresent findings and distort a reader’s perception of psoriasis therapies, efforts are needed to prevent its occurrence.
Experience With Adaptive Servo-Ventilation Among Veterans in the Post-SERVE-HF Era
Sleep apnea is a heterogeneous group of conditions that may be attributable to a wide array of underlying conditions, with varying contributions of obstructive or central sleep-disordered breathing. The spectrum from obstructive sleep apnea (OSA) to central sleep apnea (CSA) includes mixed sleep apnea, treatment-emergent CSA (TECSA), and Cheyne-Stokes respiration (CSR).1 The pathophysiologic causes of CSA can be attributed to delayed cardiopulmonary circulation in heart failure, decreased brainstem ventilatory response due to stroke, blunting of central chemoreceptors in chronic opioid use, and/or stimulation of the Hering-Breuer reflex from activation of pulmonary stretch receptors after initiating positive airway pressure (PAP) for treatment of OSA.2,3 Medications are commonly implicated in many forms of sleep-disordered breathing; importantly, opioids and benzodiazepines may blunt the respiratory drive, leading to CSA, and/or impair upper airway patency, resulting in or worsening OSA.
Continuous positive airway pressure (CPAP) therapy is largely ineffective in correcting CSA or improving outcomes and is often poorly tolerated in these patients.4 Adaptive servo-ventilation (ASV) is a form of bilevel PAP (BPAP) therapy that delivers variable adjusting pressure support, primarily to treat CSA. PAP also may relieve upper airway obstructions, thereby effectively treating any comorbid obstructive component. ASV has been well documented to improve sleep-related disorders and improve apnea-hypopnea index (AHI) in patients with CSA. However, longitudinal data have demonstrated increased mortality in patients with heart failure with reduced ejection fraction (HFrEF) who were treated with ASV.5 Since the SERVE-HF trial results came to light in 2015, there has been no consensus regarding the optimal use, if any, of ASV therapy.6-8 This is partly related to the inability to fully explain the study’s major findings, which were unexpected at the time, and partly due to the absence of similar relevant mortality data in patients with CSA but without HFrEF.
TECSA may present in some patients with OSA who are new to PAP therapy. These events are frequently seen during PAP titration sleep studies, though patients can also experience significant TECSA shortly after initiating home PAP therapy. TECSA is felt to result from a combination of stimulating pulmonary stretch receptors and lowering arterial carbon dioxide below the apneic threshold. Chemoreceptors located in the medulla respond by attenuating the respiratory drive.9 Previous studies have shown most cases of mild TECSA resolve over time with CPAP treatment. However, in patients with persistent or worsening TECSA, ASV may be considered as an alternative to CPAP.
The prevalence of OSA in the veteran population is estimated to be as high as 60%, considerably higher than the general population estimation.10 Patients with more significant comorbidities may also experience a higher frequency of central events. Patients with CSA have also been shown to have a higher risk for cardiac-related hospital admissions, providing plausible justification for correcting CSA.10
In the current study, we aim to characterize the group of patients using ASV therapy in the modern era. We will assess the objective efficacy and adherence of ASV therapy in patients with primarily CSA compared with those having primarily OSA (ie, TECSA). Secondarily, we aim to identify baseline clinical and polysomnographic features that may be predictive of ASV adherence, as a surrogate for subjective benefit.11 In the wake of the SERVE-HF study, the sleep medicine community has paused prescribing ASV therapy for CSA. We hope to provide more perspective on the treatment of veterans with CSA and identify the patient groups that would benefit most from ASV therapy.
Methods
This retrospective chart review examined patients prescribed ASV therapy at the Hampton Veterans Affairs Medical Center (HVAMC) in Virginia who had therapy data between January 1, 2015, and April 30, 2020. The start date was chosen to approximate the phase-in of wireless PAP devices at HVAMC and to correspond with the release of preliminary results from the SERVE-HF trial.
Patients were initially identified through a query into commercial wireless PAP management databases and cross-referenced with HVAMC patients. Adherence and efficacy data were obtained from the most recent clinical PAP data, which allowed for the evaluation of patients who discontinued therapy for reasons other than intolerance. Clinical, demographic, and polysomnography (PSG) data were obtained from the electronic health record. One patient, identified through the database query but not found in the electronic health record, was excluded. In cases of missing PSG data, especially AHI or similar values, all attempts were made to calculate the data with other provided values. This study was determined to be exempt by the HVAMC Institutional Review Board (protocol #20-01).
Statistics
Statistical analyses were designed to compare clinical characteristics and adherence to therapy of those with primarily CSA on PSG and those with primarily OSA. Because it was not currently known how many patients would fit into each of these categories, we also planned secondary comparisons of the clinical and PSG characteristics of those patients who were adherent with therapy and those who were not. Adherence with ASV therapy was defined as device use for ≥ 4 hours for ≥ 70% of nights.
Comparisons between the means of 2 normally distributed groups were performed with an unpaired t test. Comparisons between 2 nonnormally distributed groups and groups of dates were done with the Mann-Whitney U test. The normality of a group distribution was determined using D’Agostino-Pearson omnibus normality test. Two groups of dichotomous variables were compared with the Fisher exact test. P value < .05 was considered statistically significant.
Results
Thirty-one patients were prescribed ASV therapy and had follow-up at HVAMC since 2015. All patients were male. The mean (SD) age was 67.2 (11.4) years, mean body mass index (BMI) was 34.0 (5.9), and the mean (SD) Epworth Sleepiness Scale (ESS) score was 10.9 (5.8). Patient comorbidities included 30 (97%) with hypertension, 17 (55%) with diabetes mellitus, 16 (52%) with coronary artery disease, and 11 (35%) with congestive heart failure. Three patients had no echocardiogram or other documentation of left ventricular ejection fraction (LVEF). One of these patients had voluntarily stopped using PAP therapy, another had been erroneously started on ASV (ordered for fixed BPAP), and the third had since been retitrated to CPAP. In the 28 patients with documented LVEF, the mean (SD) LVEF was 61.8% (6.9). Ten patients (32%) had opioids documented on their medication lists and 6 (19%) had benzodiazepines.
The median date of diagnostic sleep testing was January 9, 2015, and testing was completed after the release of the initial field safety notice regarding the SERVE-HF trial preliminary findings May 13, 2015, for 14 patients (45%).12 On diagnostic sleep testing, the mean (SD) AHI was 47.3 (25.6) events/h and the median (IQR) oxygen saturation (SpO2) nadir was 82% (78-84). Three patients (10%) were initially diagnosed with CSA, 19 (61%) with OSA, and 9 (29%) with both. Sixteen patients (52%) had ASV with fixed expiratory PAP (EPAP), and 15 (48%) had variable adjusting EPAP. Mean (SD) usage of ASV was 6.5 (2.6) hours and 66.0% (34.2) of nights for ≥ 4 hours. Mean (SD) titrated EPAP (set or 90th/95th percentile autotitrated) was 10.1 (3.4) cm H2O and inspiratory PAP (IPAP) (90th/95th percentile) was 17.1 (3.3) cm H2O. The median (IQR) residual AHI on ASV was 2.7 events/h (1.1-5.1), apnea index (AI) was 0.4 (0.1-1.0), and hypopnea index (HI) was 1.4 (1.0-3.2); the residual central and obstructive events were not available in most cases.
Adherence
There were no significant differences between the proportions of patients on ASV with set EPAP or the titrated EPAP and IPAP. The median (IQR) residual AHI was lower in the adherent group compared with the nonadherent group, both in absolute values (1.7 [0.9-3.2] events/h vs 4.7 [2.4-10.3] events/h, respectively [P = .004]), and as a percentage of the pretreatment AHI (3.1% [2.5-6.0] vs 10.2% [5.3-34.4], respectively; P = .002) (Figure 2).
Primarily Obstructive Sleep Apnea
Sleep apnea was a mixed picture of obstructive and central events in many patients. Only 3 patients had “pure” CSA. Thus, we were unable to define discrete comparison groups based on the sleep-disordered breathing phenotype. We identified 19 patients with primarily OSA (ie, initially diagnosed with OSA, OSA with TECSA, or complex sleep apnea). The mean (SD) age was 66.1 (12.8) years, BMI was 36.2 (4.7), and ESS was 11.4 (5.6). The mean (SD) baseline AHI was 46.9 (29.5), obstructive AHI was 40.5 (30.4), and central AHI was 0.4 (1.2); the median (IQR) SpO2 nadir was 81% (78%-84%). The mean (SD) titrated EPAP was 10.2 (3.5) cm H2O, and the 90th/95th percentile IPAP was 17.9 (3.5) cm H2O. The mean (SD) usage of ASV was 7.9 (5.3) hours with 11 patients (58%) meeting the minimum standard for adherence to ASV therapy.
No significant differences were seen between the adherent and nonadherent groups in clinical or demographic characteristics or date of diagnostic sleep testing (eAppendix, available online at doi:10.12788/fp.0374). In baseline sleep studies the mean (SD) HI was 32.3 (15.8) in the adherent group compared with 14.7 (8.8) in the nonadherent group (P = .049). In contrast, obstructive AHI was not significantly lower in the adherent group: 51.9 (30.9) in the adherent group compared with 22.2 (20.6) in the nonadherent group (P = .09). The median (IQR) residual AHI on ASV as a percentage of the pretreatment AHI was 3.0% (2.4%-6.5%) in the adherent group compared with 11.3% (5.4%-89.1%) in the nonadherent group, a statistically significant difference (P = .01). No other significant differences were seen between the groups.
Discussion
This study describes a real-world cohort of patients using ASV therapy and the characteristics associated with benefit from therapy. The patients that were prescribed and started ASV therapy most often had a significant degree of obstructive component to sleep-disordered breathing, whether primary OSA with TECSA or comorbid OSA and CSA. Moreover, we found that a higher obstructive AHI on the baseline PSG was associated with adherence to ASV therapy. Another important finding was that a lower residual AHI on ASV as a proportion of the baseline was associated with PAP adherence. Adherent patients had similar clinical characteristics as the nonadherent patients, including comorbidities, severity of sleep-disordered breathing, and obesity.
Though the results of the SERVE-HF trial have dampened the enthusiasm somewhat, ASV therapy has long been considered an effective and well-tolerated treatment for many types of CSA.13 In fact, treatments that can eliminate the central AHI are fairly limited.4,14 Our data suggest that ASV is also effective and tolerated in OSA with TECSA and/or comorbid CSA. Recent studies suggest that CSA resolves spontaneously in a majority of TECSA patients within 2 to 3 months of regular CPAP use.15 Other estimates suggest that persistent TESCA may be present in 2% of patients with OSA on treatment.16
Given the high and rising prevalence of OSA, many people are at risk for clinically significant TESCA. Another retrospective case series found that 72% of patients that failed treatment with CPAP or BPAP during PSG, met diagnostic criteria (at the time) for CSA; ASV was objectively beneficial in these patients.17 ASV can be an especially useful modality to treat OSA in patients with CSA that either prevents tolerance of standard therapies or causes clinical consequences, presuming the patient does not also have HFrEF.18 The long-term outcomes of treatment with ASV therapy remain a matter of debate.
The SERVE-HF trial remains among the only studies that have assessed the mortality effects of CSA treatments, with unfavorable findings. Treatment of OSA has been associated with favorable chronic health benefits, though recent studies have questioned the degree of benefit attributable to OSA treatment.19-24 Similar studies have not been done for comorbidities represented by our study cohort (ie, OSA with TECSA and/or comorbid CSA).
The lack of CSA diagnosis alone in our cohort may be partially attributable to changing practice patterns following the SERVE-HF trial, though it is not clear from these data why a higher baseline obstructive AHI was associated with adherence to ASV therapy. Our data in this regard are somewhat at odds with the preliminary results of the ADVENT-HF trial. In that study, adherence to ASV therapy in patients with predominantly OSA declined significantly more than in patients with predominantly CSA.25 Most of our patients were diagnosed with predominantly OSA, so a direct comparison with the CSA group is problematic; additionally, the primary brand and the pressure adjustments algorithm used in our study differed from the ADVENT-HF trial.
OSA and CSA may present with similar clinical symptoms, including sleep fragmentation, insomnia, and excessive daytime sleepiness; however, the degree of symptomatology, especially daytime sleepiness, and the response to treatment, may be less in CSA.2,26 Both the subjective report of symptoms (ESS) and PSG measures of sleep fragmentation were similar in our patients, again likely explained by the predominance of obstructive events.
The pathophysiology of CSA is more varied than OSA, which is probably relevant in this case. ASV was originally designed for the management of CSA with CSR, accomplishing this goal by stabilizing the periods of central apnea and hyperpnea characteristic of CSR.27 Although other forms of CSA demonstrate breathing patterns distinct from CSR, ASV has become an accepted treatment for most of these. It is plausible that the long-term subjective benefit and tolerance of ASV in CSA without CSR is less than for CSA with CSR or OSA. None of the patients in our study had CSA with CSR.
Ultimately, it may be the objective treatment effect that lends to adherence, as has been shown previously in OSA patients; our group of adherent patients showed a greater improvement in AHI, relative to baseline, than the nonadherent patients did.28 The technology behind ASV therapy can greatly reduce the frequencies of central apneas, yet this same treatment effectively splints the upper airway and even more effectively eliminates obstructive apneas and hypopneas. Variable adjusting EPAP devices would plausibly provide even more benefit in these patients, as has been shown in prior studies.29 To the contrary, our small sample of patients with TESCA showed a nonsignificant trend toward adherence with fixed EPAP ASV.
Opioid use was substantial in our population, without significant differences between the groups. CPAP therapy is ineffective in improving opioid-associated CSA. In a recent study, 20 patients on opioid therapy with CSA were treated with CPAP therapy; after several weeks, the average therapeutic use was 4 to 5 hours per night and CPAP was abandoned in favor of ASV therapy due to persistent central apnea. ASV treatment was associated with a considerable reduction in central apnea index, AHI, arousal index, and oxygen desaturations in a remarkable improvement over CPAP.30
Limitations and Future Directions
This retrospective, single-center study may have limited applicability to other populations. Adherence was used as a surrogate for subjective benefit from treatment, though benefit was not confirmed by the patients directly. Only patients seen in follow-up for documentation of the ASV download were identified for inclusion and data analysis. As a single center, we risk homogeneity in the treatment algorithms, though sleep medicine treatments are often decided at the time of the sleep studies. Studies and treatment recommendations were made at a variety of sites, including our sleep center, other US Department of Veterans Affairs hospitals, in the community network, and at US Department of Defense centers. Our population was homogenous in some ways; notably, 100% of our group was male, which is substantially higher than both the veteran population and the general population. Risk factors for OSA and CSA are more common in male patients, which may partially explain this anomaly. Lastly, with our small sample size, there is increased risk that the results seen occurred by chance.
There are several areas for further study. A larger multicenter study may permit these results to be generalized to the population and should include subjective measures of benefit. Patients with primarily CSA were largely absent in our group and may be the focus of future studies; data on predictors of treatment adherence in CSA are lacking. With the availability of consistent older adherence data, comparisons may be made between the efficacies of clinical practice habits, including treatment efficacy, before and after the results of the SERVE-HF trial became known.
Conclusions
In selected patients with preserved LVEF, ASV therapy appears especially effective in patients with OSA combined with CSA. Adherence to ASV treatment was associated with higher obstructive AHI during the baseline PSG and with a greater reduction in the AHI. This understanding may help guide sleep specialists in personalizing treatments for sleep-disordered breathing. Because objective efficacy appears to be important for therapy adherence, clinicians should be able to consistently determine the obstructive and central components of the residual AHI, thus taking all information into account when optimizing the treatment. Additionally, both OSA and CSA pressure requirements should be considered when developing ASV devices.
Acknowledgments
We thank Martha Harper, RRT, of Hampton Veterans Affairs Medical Center (HVAMC) for helping to identify our patients and assisting with data collection. This material is the result of work supported with resources and the use of HVAMC facilities.
1. Morgenthaler TI, Gay PC, Gordon N, Brown LK. Adaptive servoventilation versus noninvasive positive pressure ventilation for central, mixed, and complex sleep apnea syndromes. Sleep. 2007;30(4):468-475. doi:10.1093/sleep/30.4.468
2. Eckert DJ, Jordan AS, Merchia P, Malhotra A. Central sleep apnea: pathophysiology and treatment. Chest. 2007;131(2):595-607. doi:10.1378/chest.06.2287
3. Verbraecken J. Complex sleep apnoea syndrome. Breathe. 2013;9(5):372-380. doi:10.1183/20734735.042412
4. Bradley TD, Logan AG, Kimoff RJ, et al. Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med. 2005;353(19):2025-2033. doi:10.1056/NEJMoa051001
5. Cowie MR, Woehrle H, Wegscheider K, et al. Adaptive servo-ventilation for central sleep apnea in systolic heart failure. N Engl J Med. 2015;373(12):1095-1105. doi:10.1056/NEJMoa1506459
6. Imamura T, Kinugawa K. What is the optimal strategy for adaptive servo-ventilation therapy? Int Heart J. 2018;59(4):683-688. doi:10.1536/ihj.17-429
7. Javaheri S, Brown LK, Randerath W, Khayat R. SERVE-HF: more questions than answers. Chest. 2016;149(4):900-904. doi:10.1016/j.chest.2015.12.021
8. Mehra R, Gottlieb DJ. A paradigm shift in the treatment of central sleep apnea in heart failure. Chest. 2015;148(4):848-851. doi:10.1378/chest.15-1536
9. Nigam G, Riaz M, Chang E, Camacho M. Natural history of treatment-emergent central sleep apnea on positive airway pressure: a systematic review. Ann Thorac Med. 2018;13(2):86-91. doi:10.4103/atm.ATM_321_17
10. Ratz D, Wiitala W, Badr MS, Burns J, Chowdhuri S. Correlates and consequences of central sleep apnea in a national sample of US veterans. Sleep. 2018;41(9):zsy058. doi:10.1093/sleep/zsy058
11. Wolkove N, Baltzan M, Kamel H, Dabrusin R, Palayew M. Long-term compliance with continuous positive airway pressure in patients with obstructive sleep apnea. Can Respir J. 2008;15(7):365-369. doi:10.1155/2008/534372
12. Special Safety Notice: ASV therapy for central sleep apnea patients with heart failure. American Academy of Sleep Medicine. May 15, 2015. Accessed February 13, 2023. https://aasm.org/special-safety-notice-asv-therapy-for-central-sleep-apnea-patients-with-heart-failure/
13. Philippe C, Stoïca-Herman M, Drouot X, et al. Compliance with and effectiveness of adaptive servoventilation versus continuous positive airway pressure in the treatment of Cheyne-Stokes respiration in heart failure over a six month period. Heart. 2006;92(3):337-342. doi:10.1136/hrt.2005.060038
14. Randerath W, Deleanu OC, Schiza S, Pepin J-L. Central sleep apnoea and periodic breathing in heart failure: prognostic significance and treatment options. Eur Respir Rev. 2019;28(153):190084. Published 2019 Oct 11. doi:10.1183/16000617.0084-2019
15. Gay PC. Complex sleep apnea: it really is a disease. J Clin Sleep Med. 2008;4(5):403-405.
16. American Academy of Sleep Medicine. International Classification of Sleep Disorders - Third Edition (ICSD-3). 3rd ed. American Academy of Sleep Medicine; 2014.
17. Brown SE, Mosko SS, Davis JA, Pierce RA, Godfrey-Pixton TV. A retrospective case series of adaptive servoventilation for complex sleep apnea. J Clin Sleep Med. 2011;7(2):187-195.
18. Aurora RN, Bista SR, Casey KR, et al. Updated Adaptive Servo-Ventilation Recommendations for the 2012 AASM Guideline: “The Treatment of Central Sleep Apnea Syndromes in Adults: Practice Parameters with an Evidence-Based Literature Review and Meta-Analyses”. J Clin Sleep Med. 2016;12(5):757-761. doi:10.5664/jcsm.5812
19. Martínez-García MA, Soler-Cataluña JJ, Ejarque-Martínez L, et al. Continuous positive airway pressure treatment reduces mortality in patients with ischemic stroke and obstructive sleep apnea: a 5-year follow-up study. Am J Respir Crit Care Med. 2009;180(1):36-41. doi:10.1164/rccm.200808-1341OC
20. Martínez-García MA, Campos-Rodríguez F, Catalán-Serra P, et al. Cardiovascular mortality in obstructive sleep apnea in the elderly: role of long-term continuous positive airway pressure treatment: a prospective observational study. Am J Respir Crit Care Med. 2012;186(9):909-916. doi:10.1164/rccm.201203-0448OC
21. Neilan TG, Farhad H, Dodson JA, et al. Effect of sleep apnea and continuous positive airway pressure on cardiac structure and recurrence of atrial fibrillation. J Am Heart Assoc. 2013;2(6):e000421. Published 2013 Nov 25. doi:10.1161/JAHA.113.000421
22. Redline S, Adams N, Strauss ME, Roebuck T, Winters M, Rosenberg C. Improvement of mild sleep-disordered breathing with CPAP compared with conservative therapy. Am J Respir Crit Care Med. 1998;157(3):858-865. doi:10.1164/ajrccm.157.3.9709042
23. McEvoy RD, Antic NA, Heeley E, et al. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375(10):919-931. doi:10.1056/NEJMoa1606599
24. Yu J, Zhou Z, McEvoy RD, et al. Association of positive airway pressure with cardiovascular events and death in adults with sleep apnea: a systematic review and meta-analysis. JAMA. 2017;318(2):156-166. doi:10.1001/jama.2017.7967
25. Perger E, Lyons OD, Inami T, et al. Predictors of 1-year compliance with adaptive servoventilation in patients with heart failure and sleep disordered breathing: preliminary data from the ADVENT-HF trial. Eur Resp J. 2019;53(2):1801626. doi:10.1183/13993003.01626-2018
26. Lyons OD, Floras JS, Logan AG, et al. Design of the effect of adaptive servo-ventilation on survival and cardiovascular hospital admissions in patients with heart failure and sleep apnoea: the ADVENT-HF trial. Eur J Heart Fail. 2017;19(4):579-587. doi:10.1002/ejhf.790
27. Teschler H, Döhring J, Wang YM, Berthon-Jones M. Adaptive pressure support servo-ventilation: a novel treatment for Cheyne-Stokes respiration in heart failure. Am J Respir Crit Care Med. 2001;164(4):614-619. doi:10.1164/ajrccm.164.4.9908114
28. Ye L, Pack AI, Maislin G, et al. Predictors of continuous positive airway pressure use during the first week of treatment. J Sleep Res. 2012;21(4):419-426. doi:10.1111/j.1365-2869.2011.00969.x
29. Vennelle M, White S, Riha RL, Mackay TW, Engleman HM, Douglas NJ. Randomized controlled trial of variable-pressure versus fixed-pressure continuous positive airway pressure (CPAP) treatment for patients with obstructive sleep apnea/hypopnea syndrome (OSAHS). Sleep. 2010;33(2):267-271. doi:10.1093/sleep/33.2.267
30. Javaheri S, Harris N, Howard J, Chung E. Adaptive servoventilation for treatment of opioid-associated central sleep apnea. J Clin Sleep Med. 2014;10(6):637-643. Published 2014 Jun 15. doi:10.5664/jcsm.3788
Sleep apnea is a heterogeneous group of conditions that may be attributable to a wide array of underlying conditions, with varying contributions of obstructive or central sleep-disordered breathing. The spectrum from obstructive sleep apnea (OSA) to central sleep apnea (CSA) includes mixed sleep apnea, treatment-emergent CSA (TECSA), and Cheyne-Stokes respiration (CSR).1 The pathophysiologic causes of CSA can be attributed to delayed cardiopulmonary circulation in heart failure, decreased brainstem ventilatory response due to stroke, blunting of central chemoreceptors in chronic opioid use, and/or stimulation of the Hering-Breuer reflex from activation of pulmonary stretch receptors after initiating positive airway pressure (PAP) for treatment of OSA.2,3 Medications are commonly implicated in many forms of sleep-disordered breathing; importantly, opioids and benzodiazepines may blunt the respiratory drive, leading to CSA, and/or impair upper airway patency, resulting in or worsening OSA.
Continuous positive airway pressure (CPAP) therapy is largely ineffective in correcting CSA or improving outcomes and is often poorly tolerated in these patients.4 Adaptive servo-ventilation (ASV) is a form of bilevel PAP (BPAP) therapy that delivers variable adjusting pressure support, primarily to treat CSA. PAP also may relieve upper airway obstructions, thereby effectively treating any comorbid obstructive component. ASV has been well documented to improve sleep-related disorders and improve apnea-hypopnea index (AHI) in patients with CSA. However, longitudinal data have demonstrated increased mortality in patients with heart failure with reduced ejection fraction (HFrEF) who were treated with ASV.5 Since the SERVE-HF trial results came to light in 2015, there has been no consensus regarding the optimal use, if any, of ASV therapy.6-8 This is partly related to the inability to fully explain the study’s major findings, which were unexpected at the time, and partly due to the absence of similar relevant mortality data in patients with CSA but without HFrEF.
TECSA may present in some patients with OSA who are new to PAP therapy. These events are frequently seen during PAP titration sleep studies, though patients can also experience significant TECSA shortly after initiating home PAP therapy. TECSA is felt to result from a combination of stimulating pulmonary stretch receptors and lowering arterial carbon dioxide below the apneic threshold. Chemoreceptors located in the medulla respond by attenuating the respiratory drive.9 Previous studies have shown most cases of mild TECSA resolve over time with CPAP treatment. However, in patients with persistent or worsening TECSA, ASV may be considered as an alternative to CPAP.
The prevalence of OSA in the veteran population is estimated to be as high as 60%, considerably higher than the general population estimation.10 Patients with more significant comorbidities may also experience a higher frequency of central events. Patients with CSA have also been shown to have a higher risk for cardiac-related hospital admissions, providing plausible justification for correcting CSA.10
In the current study, we aim to characterize the group of patients using ASV therapy in the modern era. We will assess the objective efficacy and adherence of ASV therapy in patients with primarily CSA compared with those having primarily OSA (ie, TECSA). Secondarily, we aim to identify baseline clinical and polysomnographic features that may be predictive of ASV adherence, as a surrogate for subjective benefit.11 In the wake of the SERVE-HF study, the sleep medicine community has paused prescribing ASV therapy for CSA. We hope to provide more perspective on the treatment of veterans with CSA and identify the patient groups that would benefit most from ASV therapy.
Methods
This retrospective chart review examined patients prescribed ASV therapy at the Hampton Veterans Affairs Medical Center (HVAMC) in Virginia who had therapy data between January 1, 2015, and April 30, 2020. The start date was chosen to approximate the phase-in of wireless PAP devices at HVAMC and to correspond with the release of preliminary results from the SERVE-HF trial.
Patients were initially identified through a query into commercial wireless PAP management databases and cross-referenced with HVAMC patients. Adherence and efficacy data were obtained from the most recent clinical PAP data, which allowed for the evaluation of patients who discontinued therapy for reasons other than intolerance. Clinical, demographic, and polysomnography (PSG) data were obtained from the electronic health record. One patient, identified through the database query but not found in the electronic health record, was excluded. In cases of missing PSG data, especially AHI or similar values, all attempts were made to calculate the data with other provided values. This study was determined to be exempt by the HVAMC Institutional Review Board (protocol #20-01).
Statistics
Statistical analyses were designed to compare clinical characteristics and adherence to therapy of those with primarily CSA on PSG and those with primarily OSA. Because it was not currently known how many patients would fit into each of these categories, we also planned secondary comparisons of the clinical and PSG characteristics of those patients who were adherent with therapy and those who were not. Adherence with ASV therapy was defined as device use for ≥ 4 hours for ≥ 70% of nights.
Comparisons between the means of 2 normally distributed groups were performed with an unpaired t test. Comparisons between 2 nonnormally distributed groups and groups of dates were done with the Mann-Whitney U test. The normality of a group distribution was determined using D’Agostino-Pearson omnibus normality test. Two groups of dichotomous variables were compared with the Fisher exact test. P value < .05 was considered statistically significant.
Results
Thirty-one patients were prescribed ASV therapy and had follow-up at HVAMC since 2015. All patients were male. The mean (SD) age was 67.2 (11.4) years, mean body mass index (BMI) was 34.0 (5.9), and the mean (SD) Epworth Sleepiness Scale (ESS) score was 10.9 (5.8). Patient comorbidities included 30 (97%) with hypertension, 17 (55%) with diabetes mellitus, 16 (52%) with coronary artery disease, and 11 (35%) with congestive heart failure. Three patients had no echocardiogram or other documentation of left ventricular ejection fraction (LVEF). One of these patients had voluntarily stopped using PAP therapy, another had been erroneously started on ASV (ordered for fixed BPAP), and the third had since been retitrated to CPAP. In the 28 patients with documented LVEF, the mean (SD) LVEF was 61.8% (6.9). Ten patients (32%) had opioids documented on their medication lists and 6 (19%) had benzodiazepines.
The median date of diagnostic sleep testing was January 9, 2015, and testing was completed after the release of the initial field safety notice regarding the SERVE-HF trial preliminary findings May 13, 2015, for 14 patients (45%).12 On diagnostic sleep testing, the mean (SD) AHI was 47.3 (25.6) events/h and the median (IQR) oxygen saturation (SpO2) nadir was 82% (78-84). Three patients (10%) were initially diagnosed with CSA, 19 (61%) with OSA, and 9 (29%) with both. Sixteen patients (52%) had ASV with fixed expiratory PAP (EPAP), and 15 (48%) had variable adjusting EPAP. Mean (SD) usage of ASV was 6.5 (2.6) hours and 66.0% (34.2) of nights for ≥ 4 hours. Mean (SD) titrated EPAP (set or 90th/95th percentile autotitrated) was 10.1 (3.4) cm H2O and inspiratory PAP (IPAP) (90th/95th percentile) was 17.1 (3.3) cm H2O. The median (IQR) residual AHI on ASV was 2.7 events/h (1.1-5.1), apnea index (AI) was 0.4 (0.1-1.0), and hypopnea index (HI) was 1.4 (1.0-3.2); the residual central and obstructive events were not available in most cases.
Adherence
There were no significant differences between the proportions of patients on ASV with set EPAP or the titrated EPAP and IPAP. The median (IQR) residual AHI was lower in the adherent group compared with the nonadherent group, both in absolute values (1.7 [0.9-3.2] events/h vs 4.7 [2.4-10.3] events/h, respectively [P = .004]), and as a percentage of the pretreatment AHI (3.1% [2.5-6.0] vs 10.2% [5.3-34.4], respectively; P = .002) (Figure 2).
Primarily Obstructive Sleep Apnea
Sleep apnea was a mixed picture of obstructive and central events in many patients. Only 3 patients had “pure” CSA. Thus, we were unable to define discrete comparison groups based on the sleep-disordered breathing phenotype. We identified 19 patients with primarily OSA (ie, initially diagnosed with OSA, OSA with TECSA, or complex sleep apnea). The mean (SD) age was 66.1 (12.8) years, BMI was 36.2 (4.7), and ESS was 11.4 (5.6). The mean (SD) baseline AHI was 46.9 (29.5), obstructive AHI was 40.5 (30.4), and central AHI was 0.4 (1.2); the median (IQR) SpO2 nadir was 81% (78%-84%). The mean (SD) titrated EPAP was 10.2 (3.5) cm H2O, and the 90th/95th percentile IPAP was 17.9 (3.5) cm H2O. The mean (SD) usage of ASV was 7.9 (5.3) hours with 11 patients (58%) meeting the minimum standard for adherence to ASV therapy.
No significant differences were seen between the adherent and nonadherent groups in clinical or demographic characteristics or date of diagnostic sleep testing (eAppendix, available online at doi:10.12788/fp.0374). In baseline sleep studies the mean (SD) HI was 32.3 (15.8) in the adherent group compared with 14.7 (8.8) in the nonadherent group (P = .049). In contrast, obstructive AHI was not significantly lower in the adherent group: 51.9 (30.9) in the adherent group compared with 22.2 (20.6) in the nonadherent group (P = .09). The median (IQR) residual AHI on ASV as a percentage of the pretreatment AHI was 3.0% (2.4%-6.5%) in the adherent group compared with 11.3% (5.4%-89.1%) in the nonadherent group, a statistically significant difference (P = .01). No other significant differences were seen between the groups.
Discussion
This study describes a real-world cohort of patients using ASV therapy and the characteristics associated with benefit from therapy. The patients that were prescribed and started ASV therapy most often had a significant degree of obstructive component to sleep-disordered breathing, whether primary OSA with TECSA or comorbid OSA and CSA. Moreover, we found that a higher obstructive AHI on the baseline PSG was associated with adherence to ASV therapy. Another important finding was that a lower residual AHI on ASV as a proportion of the baseline was associated with PAP adherence. Adherent patients had similar clinical characteristics as the nonadherent patients, including comorbidities, severity of sleep-disordered breathing, and obesity.
Though the results of the SERVE-HF trial have dampened the enthusiasm somewhat, ASV therapy has long been considered an effective and well-tolerated treatment for many types of CSA.13 In fact, treatments that can eliminate the central AHI are fairly limited.4,14 Our data suggest that ASV is also effective and tolerated in OSA with TECSA and/or comorbid CSA. Recent studies suggest that CSA resolves spontaneously in a majority of TECSA patients within 2 to 3 months of regular CPAP use.15 Other estimates suggest that persistent TESCA may be present in 2% of patients with OSA on treatment.16
Given the high and rising prevalence of OSA, many people are at risk for clinically significant TESCA. Another retrospective case series found that 72% of patients that failed treatment with CPAP or BPAP during PSG, met diagnostic criteria (at the time) for CSA; ASV was objectively beneficial in these patients.17 ASV can be an especially useful modality to treat OSA in patients with CSA that either prevents tolerance of standard therapies or causes clinical consequences, presuming the patient does not also have HFrEF.18 The long-term outcomes of treatment with ASV therapy remain a matter of debate.
The SERVE-HF trial remains among the only studies that have assessed the mortality effects of CSA treatments, with unfavorable findings. Treatment of OSA has been associated with favorable chronic health benefits, though recent studies have questioned the degree of benefit attributable to OSA treatment.19-24 Similar studies have not been done for comorbidities represented by our study cohort (ie, OSA with TECSA and/or comorbid CSA).
The lack of CSA diagnosis alone in our cohort may be partially attributable to changing practice patterns following the SERVE-HF trial, though it is not clear from these data why a higher baseline obstructive AHI was associated with adherence to ASV therapy. Our data in this regard are somewhat at odds with the preliminary results of the ADVENT-HF trial. In that study, adherence to ASV therapy in patients with predominantly OSA declined significantly more than in patients with predominantly CSA.25 Most of our patients were diagnosed with predominantly OSA, so a direct comparison with the CSA group is problematic; additionally, the primary brand and the pressure adjustments algorithm used in our study differed from the ADVENT-HF trial.
OSA and CSA may present with similar clinical symptoms, including sleep fragmentation, insomnia, and excessive daytime sleepiness; however, the degree of symptomatology, especially daytime sleepiness, and the response to treatment, may be less in CSA.2,26 Both the subjective report of symptoms (ESS) and PSG measures of sleep fragmentation were similar in our patients, again likely explained by the predominance of obstructive events.
The pathophysiology of CSA is more varied than OSA, which is probably relevant in this case. ASV was originally designed for the management of CSA with CSR, accomplishing this goal by stabilizing the periods of central apnea and hyperpnea characteristic of CSR.27 Although other forms of CSA demonstrate breathing patterns distinct from CSR, ASV has become an accepted treatment for most of these. It is plausible that the long-term subjective benefit and tolerance of ASV in CSA without CSR is less than for CSA with CSR or OSA. None of the patients in our study had CSA with CSR.
Ultimately, it may be the objective treatment effect that lends to adherence, as has been shown previously in OSA patients; our group of adherent patients showed a greater improvement in AHI, relative to baseline, than the nonadherent patients did.28 The technology behind ASV therapy can greatly reduce the frequencies of central apneas, yet this same treatment effectively splints the upper airway and even more effectively eliminates obstructive apneas and hypopneas. Variable adjusting EPAP devices would plausibly provide even more benefit in these patients, as has been shown in prior studies.29 To the contrary, our small sample of patients with TESCA showed a nonsignificant trend toward adherence with fixed EPAP ASV.
Opioid use was substantial in our population, without significant differences between the groups. CPAP therapy is ineffective in improving opioid-associated CSA. In a recent study, 20 patients on opioid therapy with CSA were treated with CPAP therapy; after several weeks, the average therapeutic use was 4 to 5 hours per night and CPAP was abandoned in favor of ASV therapy due to persistent central apnea. ASV treatment was associated with a considerable reduction in central apnea index, AHI, arousal index, and oxygen desaturations in a remarkable improvement over CPAP.30
Limitations and Future Directions
This retrospective, single-center study may have limited applicability to other populations. Adherence was used as a surrogate for subjective benefit from treatment, though benefit was not confirmed by the patients directly. Only patients seen in follow-up for documentation of the ASV download were identified for inclusion and data analysis. As a single center, we risk homogeneity in the treatment algorithms, though sleep medicine treatments are often decided at the time of the sleep studies. Studies and treatment recommendations were made at a variety of sites, including our sleep center, other US Department of Veterans Affairs hospitals, in the community network, and at US Department of Defense centers. Our population was homogenous in some ways; notably, 100% of our group was male, which is substantially higher than both the veteran population and the general population. Risk factors for OSA and CSA are more common in male patients, which may partially explain this anomaly. Lastly, with our small sample size, there is increased risk that the results seen occurred by chance.
There are several areas for further study. A larger multicenter study may permit these results to be generalized to the population and should include subjective measures of benefit. Patients with primarily CSA were largely absent in our group and may be the focus of future studies; data on predictors of treatment adherence in CSA are lacking. With the availability of consistent older adherence data, comparisons may be made between the efficacies of clinical practice habits, including treatment efficacy, before and after the results of the SERVE-HF trial became known.
Conclusions
In selected patients with preserved LVEF, ASV therapy appears especially effective in patients with OSA combined with CSA. Adherence to ASV treatment was associated with higher obstructive AHI during the baseline PSG and with a greater reduction in the AHI. This understanding may help guide sleep specialists in personalizing treatments for sleep-disordered breathing. Because objective efficacy appears to be important for therapy adherence, clinicians should be able to consistently determine the obstructive and central components of the residual AHI, thus taking all information into account when optimizing the treatment. Additionally, both OSA and CSA pressure requirements should be considered when developing ASV devices.
Acknowledgments
We thank Martha Harper, RRT, of Hampton Veterans Affairs Medical Center (HVAMC) for helping to identify our patients and assisting with data collection. This material is the result of work supported with resources and the use of HVAMC facilities.
Sleep apnea is a heterogeneous group of conditions that may be attributable to a wide array of underlying conditions, with varying contributions of obstructive or central sleep-disordered breathing. The spectrum from obstructive sleep apnea (OSA) to central sleep apnea (CSA) includes mixed sleep apnea, treatment-emergent CSA (TECSA), and Cheyne-Stokes respiration (CSR).1 The pathophysiologic causes of CSA can be attributed to delayed cardiopulmonary circulation in heart failure, decreased brainstem ventilatory response due to stroke, blunting of central chemoreceptors in chronic opioid use, and/or stimulation of the Hering-Breuer reflex from activation of pulmonary stretch receptors after initiating positive airway pressure (PAP) for treatment of OSA.2,3 Medications are commonly implicated in many forms of sleep-disordered breathing; importantly, opioids and benzodiazepines may blunt the respiratory drive, leading to CSA, and/or impair upper airway patency, resulting in or worsening OSA.
Continuous positive airway pressure (CPAP) therapy is largely ineffective in correcting CSA or improving outcomes and is often poorly tolerated in these patients.4 Adaptive servo-ventilation (ASV) is a form of bilevel PAP (BPAP) therapy that delivers variable adjusting pressure support, primarily to treat CSA. PAP also may relieve upper airway obstructions, thereby effectively treating any comorbid obstructive component. ASV has been well documented to improve sleep-related disorders and improve apnea-hypopnea index (AHI) in patients with CSA. However, longitudinal data have demonstrated increased mortality in patients with heart failure with reduced ejection fraction (HFrEF) who were treated with ASV.5 Since the SERVE-HF trial results came to light in 2015, there has been no consensus regarding the optimal use, if any, of ASV therapy.6-8 This is partly related to the inability to fully explain the study’s major findings, which were unexpected at the time, and partly due to the absence of similar relevant mortality data in patients with CSA but without HFrEF.
TECSA may present in some patients with OSA who are new to PAP therapy. These events are frequently seen during PAP titration sleep studies, though patients can also experience significant TECSA shortly after initiating home PAP therapy. TECSA is felt to result from a combination of stimulating pulmonary stretch receptors and lowering arterial carbon dioxide below the apneic threshold. Chemoreceptors located in the medulla respond by attenuating the respiratory drive.9 Previous studies have shown most cases of mild TECSA resolve over time with CPAP treatment. However, in patients with persistent or worsening TECSA, ASV may be considered as an alternative to CPAP.
The prevalence of OSA in the veteran population is estimated to be as high as 60%, considerably higher than the general population estimation.10 Patients with more significant comorbidities may also experience a higher frequency of central events. Patients with CSA have also been shown to have a higher risk for cardiac-related hospital admissions, providing plausible justification for correcting CSA.10
In the current study, we aim to characterize the group of patients using ASV therapy in the modern era. We will assess the objective efficacy and adherence of ASV therapy in patients with primarily CSA compared with those having primarily OSA (ie, TECSA). Secondarily, we aim to identify baseline clinical and polysomnographic features that may be predictive of ASV adherence, as a surrogate for subjective benefit.11 In the wake of the SERVE-HF study, the sleep medicine community has paused prescribing ASV therapy for CSA. We hope to provide more perspective on the treatment of veterans with CSA and identify the patient groups that would benefit most from ASV therapy.
Methods
This retrospective chart review examined patients prescribed ASV therapy at the Hampton Veterans Affairs Medical Center (HVAMC) in Virginia who had therapy data between January 1, 2015, and April 30, 2020. The start date was chosen to approximate the phase-in of wireless PAP devices at HVAMC and to correspond with the release of preliminary results from the SERVE-HF trial.
Patients were initially identified through a query into commercial wireless PAP management databases and cross-referenced with HVAMC patients. Adherence and efficacy data were obtained from the most recent clinical PAP data, which allowed for the evaluation of patients who discontinued therapy for reasons other than intolerance. Clinical, demographic, and polysomnography (PSG) data were obtained from the electronic health record. One patient, identified through the database query but not found in the electronic health record, was excluded. In cases of missing PSG data, especially AHI or similar values, all attempts were made to calculate the data with other provided values. This study was determined to be exempt by the HVAMC Institutional Review Board (protocol #20-01).
Statistics
Statistical analyses were designed to compare clinical characteristics and adherence to therapy of those with primarily CSA on PSG and those with primarily OSA. Because it was not currently known how many patients would fit into each of these categories, we also planned secondary comparisons of the clinical and PSG characteristics of those patients who were adherent with therapy and those who were not. Adherence with ASV therapy was defined as device use for ≥ 4 hours for ≥ 70% of nights.
Comparisons between the means of 2 normally distributed groups were performed with an unpaired t test. Comparisons between 2 nonnormally distributed groups and groups of dates were done with the Mann-Whitney U test. The normality of a group distribution was determined using D’Agostino-Pearson omnibus normality test. Two groups of dichotomous variables were compared with the Fisher exact test. P value < .05 was considered statistically significant.
Results
Thirty-one patients were prescribed ASV therapy and had follow-up at HVAMC since 2015. All patients were male. The mean (SD) age was 67.2 (11.4) years, mean body mass index (BMI) was 34.0 (5.9), and the mean (SD) Epworth Sleepiness Scale (ESS) score was 10.9 (5.8). Patient comorbidities included 30 (97%) with hypertension, 17 (55%) with diabetes mellitus, 16 (52%) with coronary artery disease, and 11 (35%) with congestive heart failure. Three patients had no echocardiogram or other documentation of left ventricular ejection fraction (LVEF). One of these patients had voluntarily stopped using PAP therapy, another had been erroneously started on ASV (ordered for fixed BPAP), and the third had since been retitrated to CPAP. In the 28 patients with documented LVEF, the mean (SD) LVEF was 61.8% (6.9). Ten patients (32%) had opioids documented on their medication lists and 6 (19%) had benzodiazepines.
The median date of diagnostic sleep testing was January 9, 2015, and testing was completed after the release of the initial field safety notice regarding the SERVE-HF trial preliminary findings May 13, 2015, for 14 patients (45%).12 On diagnostic sleep testing, the mean (SD) AHI was 47.3 (25.6) events/h and the median (IQR) oxygen saturation (SpO2) nadir was 82% (78-84). Three patients (10%) were initially diagnosed with CSA, 19 (61%) with OSA, and 9 (29%) with both. Sixteen patients (52%) had ASV with fixed expiratory PAP (EPAP), and 15 (48%) had variable adjusting EPAP. Mean (SD) usage of ASV was 6.5 (2.6) hours and 66.0% (34.2) of nights for ≥ 4 hours. Mean (SD) titrated EPAP (set or 90th/95th percentile autotitrated) was 10.1 (3.4) cm H2O and inspiratory PAP (IPAP) (90th/95th percentile) was 17.1 (3.3) cm H2O. The median (IQR) residual AHI on ASV was 2.7 events/h (1.1-5.1), apnea index (AI) was 0.4 (0.1-1.0), and hypopnea index (HI) was 1.4 (1.0-3.2); the residual central and obstructive events were not available in most cases.
Adherence
There were no significant differences between the proportions of patients on ASV with set EPAP or the titrated EPAP and IPAP. The median (IQR) residual AHI was lower in the adherent group compared with the nonadherent group, both in absolute values (1.7 [0.9-3.2] events/h vs 4.7 [2.4-10.3] events/h, respectively [P = .004]), and as a percentage of the pretreatment AHI (3.1% [2.5-6.0] vs 10.2% [5.3-34.4], respectively; P = .002) (Figure 2).
Primarily Obstructive Sleep Apnea
Sleep apnea was a mixed picture of obstructive and central events in many patients. Only 3 patients had “pure” CSA. Thus, we were unable to define discrete comparison groups based on the sleep-disordered breathing phenotype. We identified 19 patients with primarily OSA (ie, initially diagnosed with OSA, OSA with TECSA, or complex sleep apnea). The mean (SD) age was 66.1 (12.8) years, BMI was 36.2 (4.7), and ESS was 11.4 (5.6). The mean (SD) baseline AHI was 46.9 (29.5), obstructive AHI was 40.5 (30.4), and central AHI was 0.4 (1.2); the median (IQR) SpO2 nadir was 81% (78%-84%). The mean (SD) titrated EPAP was 10.2 (3.5) cm H2O, and the 90th/95th percentile IPAP was 17.9 (3.5) cm H2O. The mean (SD) usage of ASV was 7.9 (5.3) hours with 11 patients (58%) meeting the minimum standard for adherence to ASV therapy.
No significant differences were seen between the adherent and nonadherent groups in clinical or demographic characteristics or date of diagnostic sleep testing (eAppendix, available online at doi:10.12788/fp.0374). In baseline sleep studies the mean (SD) HI was 32.3 (15.8) in the adherent group compared with 14.7 (8.8) in the nonadherent group (P = .049). In contrast, obstructive AHI was not significantly lower in the adherent group: 51.9 (30.9) in the adherent group compared with 22.2 (20.6) in the nonadherent group (P = .09). The median (IQR) residual AHI on ASV as a percentage of the pretreatment AHI was 3.0% (2.4%-6.5%) in the adherent group compared with 11.3% (5.4%-89.1%) in the nonadherent group, a statistically significant difference (P = .01). No other significant differences were seen between the groups.
Discussion
This study describes a real-world cohort of patients using ASV therapy and the characteristics associated with benefit from therapy. The patients that were prescribed and started ASV therapy most often had a significant degree of obstructive component to sleep-disordered breathing, whether primary OSA with TECSA or comorbid OSA and CSA. Moreover, we found that a higher obstructive AHI on the baseline PSG was associated with adherence to ASV therapy. Another important finding was that a lower residual AHI on ASV as a proportion of the baseline was associated with PAP adherence. Adherent patients had similar clinical characteristics as the nonadherent patients, including comorbidities, severity of sleep-disordered breathing, and obesity.
Though the results of the SERVE-HF trial have dampened the enthusiasm somewhat, ASV therapy has long been considered an effective and well-tolerated treatment for many types of CSA.13 In fact, treatments that can eliminate the central AHI are fairly limited.4,14 Our data suggest that ASV is also effective and tolerated in OSA with TECSA and/or comorbid CSA. Recent studies suggest that CSA resolves spontaneously in a majority of TECSA patients within 2 to 3 months of regular CPAP use.15 Other estimates suggest that persistent TESCA may be present in 2% of patients with OSA on treatment.16
Given the high and rising prevalence of OSA, many people are at risk for clinically significant TESCA. Another retrospective case series found that 72% of patients that failed treatment with CPAP or BPAP during PSG, met diagnostic criteria (at the time) for CSA; ASV was objectively beneficial in these patients.17 ASV can be an especially useful modality to treat OSA in patients with CSA that either prevents tolerance of standard therapies or causes clinical consequences, presuming the patient does not also have HFrEF.18 The long-term outcomes of treatment with ASV therapy remain a matter of debate.
The SERVE-HF trial remains among the only studies that have assessed the mortality effects of CSA treatments, with unfavorable findings. Treatment of OSA has been associated with favorable chronic health benefits, though recent studies have questioned the degree of benefit attributable to OSA treatment.19-24 Similar studies have not been done for comorbidities represented by our study cohort (ie, OSA with TECSA and/or comorbid CSA).
The lack of CSA diagnosis alone in our cohort may be partially attributable to changing practice patterns following the SERVE-HF trial, though it is not clear from these data why a higher baseline obstructive AHI was associated with adherence to ASV therapy. Our data in this regard are somewhat at odds with the preliminary results of the ADVENT-HF trial. In that study, adherence to ASV therapy in patients with predominantly OSA declined significantly more than in patients with predominantly CSA.25 Most of our patients were diagnosed with predominantly OSA, so a direct comparison with the CSA group is problematic; additionally, the primary brand and the pressure adjustments algorithm used in our study differed from the ADVENT-HF trial.
OSA and CSA may present with similar clinical symptoms, including sleep fragmentation, insomnia, and excessive daytime sleepiness; however, the degree of symptomatology, especially daytime sleepiness, and the response to treatment, may be less in CSA.2,26 Both the subjective report of symptoms (ESS) and PSG measures of sleep fragmentation were similar in our patients, again likely explained by the predominance of obstructive events.
The pathophysiology of CSA is more varied than OSA, which is probably relevant in this case. ASV was originally designed for the management of CSA with CSR, accomplishing this goal by stabilizing the periods of central apnea and hyperpnea characteristic of CSR.27 Although other forms of CSA demonstrate breathing patterns distinct from CSR, ASV has become an accepted treatment for most of these. It is plausible that the long-term subjective benefit and tolerance of ASV in CSA without CSR is less than for CSA with CSR or OSA. None of the patients in our study had CSA with CSR.
Ultimately, it may be the objective treatment effect that lends to adherence, as has been shown previously in OSA patients; our group of adherent patients showed a greater improvement in AHI, relative to baseline, than the nonadherent patients did.28 The technology behind ASV therapy can greatly reduce the frequencies of central apneas, yet this same treatment effectively splints the upper airway and even more effectively eliminates obstructive apneas and hypopneas. Variable adjusting EPAP devices would plausibly provide even more benefit in these patients, as has been shown in prior studies.29 To the contrary, our small sample of patients with TESCA showed a nonsignificant trend toward adherence with fixed EPAP ASV.
Opioid use was substantial in our population, without significant differences between the groups. CPAP therapy is ineffective in improving opioid-associated CSA. In a recent study, 20 patients on opioid therapy with CSA were treated with CPAP therapy; after several weeks, the average therapeutic use was 4 to 5 hours per night and CPAP was abandoned in favor of ASV therapy due to persistent central apnea. ASV treatment was associated with a considerable reduction in central apnea index, AHI, arousal index, and oxygen desaturations in a remarkable improvement over CPAP.30
Limitations and Future Directions
This retrospective, single-center study may have limited applicability to other populations. Adherence was used as a surrogate for subjective benefit from treatment, though benefit was not confirmed by the patients directly. Only patients seen in follow-up for documentation of the ASV download were identified for inclusion and data analysis. As a single center, we risk homogeneity in the treatment algorithms, though sleep medicine treatments are often decided at the time of the sleep studies. Studies and treatment recommendations were made at a variety of sites, including our sleep center, other US Department of Veterans Affairs hospitals, in the community network, and at US Department of Defense centers. Our population was homogenous in some ways; notably, 100% of our group was male, which is substantially higher than both the veteran population and the general population. Risk factors for OSA and CSA are more common in male patients, which may partially explain this anomaly. Lastly, with our small sample size, there is increased risk that the results seen occurred by chance.
There are several areas for further study. A larger multicenter study may permit these results to be generalized to the population and should include subjective measures of benefit. Patients with primarily CSA were largely absent in our group and may be the focus of future studies; data on predictors of treatment adherence in CSA are lacking. With the availability of consistent older adherence data, comparisons may be made between the efficacies of clinical practice habits, including treatment efficacy, before and after the results of the SERVE-HF trial became known.
Conclusions
In selected patients with preserved LVEF, ASV therapy appears especially effective in patients with OSA combined with CSA. Adherence to ASV treatment was associated with higher obstructive AHI during the baseline PSG and with a greater reduction in the AHI. This understanding may help guide sleep specialists in personalizing treatments for sleep-disordered breathing. Because objective efficacy appears to be important for therapy adherence, clinicians should be able to consistently determine the obstructive and central components of the residual AHI, thus taking all information into account when optimizing the treatment. Additionally, both OSA and CSA pressure requirements should be considered when developing ASV devices.
Acknowledgments
We thank Martha Harper, RRT, of Hampton Veterans Affairs Medical Center (HVAMC) for helping to identify our patients and assisting with data collection. This material is the result of work supported with resources and the use of HVAMC facilities.
1. Morgenthaler TI, Gay PC, Gordon N, Brown LK. Adaptive servoventilation versus noninvasive positive pressure ventilation for central, mixed, and complex sleep apnea syndromes. Sleep. 2007;30(4):468-475. doi:10.1093/sleep/30.4.468
2. Eckert DJ, Jordan AS, Merchia P, Malhotra A. Central sleep apnea: pathophysiology and treatment. Chest. 2007;131(2):595-607. doi:10.1378/chest.06.2287
3. Verbraecken J. Complex sleep apnoea syndrome. Breathe. 2013;9(5):372-380. doi:10.1183/20734735.042412
4. Bradley TD, Logan AG, Kimoff RJ, et al. Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med. 2005;353(19):2025-2033. doi:10.1056/NEJMoa051001
5. Cowie MR, Woehrle H, Wegscheider K, et al. Adaptive servo-ventilation for central sleep apnea in systolic heart failure. N Engl J Med. 2015;373(12):1095-1105. doi:10.1056/NEJMoa1506459
6. Imamura T, Kinugawa K. What is the optimal strategy for adaptive servo-ventilation therapy? Int Heart J. 2018;59(4):683-688. doi:10.1536/ihj.17-429
7. Javaheri S, Brown LK, Randerath W, Khayat R. SERVE-HF: more questions than answers. Chest. 2016;149(4):900-904. doi:10.1016/j.chest.2015.12.021
8. Mehra R, Gottlieb DJ. A paradigm shift in the treatment of central sleep apnea in heart failure. Chest. 2015;148(4):848-851. doi:10.1378/chest.15-1536
9. Nigam G, Riaz M, Chang E, Camacho M. Natural history of treatment-emergent central sleep apnea on positive airway pressure: a systematic review. Ann Thorac Med. 2018;13(2):86-91. doi:10.4103/atm.ATM_321_17
10. Ratz D, Wiitala W, Badr MS, Burns J, Chowdhuri S. Correlates and consequences of central sleep apnea in a national sample of US veterans. Sleep. 2018;41(9):zsy058. doi:10.1093/sleep/zsy058
11. Wolkove N, Baltzan M, Kamel H, Dabrusin R, Palayew M. Long-term compliance with continuous positive airway pressure in patients with obstructive sleep apnea. Can Respir J. 2008;15(7):365-369. doi:10.1155/2008/534372
12. Special Safety Notice: ASV therapy for central sleep apnea patients with heart failure. American Academy of Sleep Medicine. May 15, 2015. Accessed February 13, 2023. https://aasm.org/special-safety-notice-asv-therapy-for-central-sleep-apnea-patients-with-heart-failure/
13. Philippe C, Stoïca-Herman M, Drouot X, et al. Compliance with and effectiveness of adaptive servoventilation versus continuous positive airway pressure in the treatment of Cheyne-Stokes respiration in heart failure over a six month period. Heart. 2006;92(3):337-342. doi:10.1136/hrt.2005.060038
14. Randerath W, Deleanu OC, Schiza S, Pepin J-L. Central sleep apnoea and periodic breathing in heart failure: prognostic significance and treatment options. Eur Respir Rev. 2019;28(153):190084. Published 2019 Oct 11. doi:10.1183/16000617.0084-2019
15. Gay PC. Complex sleep apnea: it really is a disease. J Clin Sleep Med. 2008;4(5):403-405.
16. American Academy of Sleep Medicine. International Classification of Sleep Disorders - Third Edition (ICSD-3). 3rd ed. American Academy of Sleep Medicine; 2014.
17. Brown SE, Mosko SS, Davis JA, Pierce RA, Godfrey-Pixton TV. A retrospective case series of adaptive servoventilation for complex sleep apnea. J Clin Sleep Med. 2011;7(2):187-195.
18. Aurora RN, Bista SR, Casey KR, et al. Updated Adaptive Servo-Ventilation Recommendations for the 2012 AASM Guideline: “The Treatment of Central Sleep Apnea Syndromes in Adults: Practice Parameters with an Evidence-Based Literature Review and Meta-Analyses”. J Clin Sleep Med. 2016;12(5):757-761. doi:10.5664/jcsm.5812
19. Martínez-García MA, Soler-Cataluña JJ, Ejarque-Martínez L, et al. Continuous positive airway pressure treatment reduces mortality in patients with ischemic stroke and obstructive sleep apnea: a 5-year follow-up study. Am J Respir Crit Care Med. 2009;180(1):36-41. doi:10.1164/rccm.200808-1341OC
20. Martínez-García MA, Campos-Rodríguez F, Catalán-Serra P, et al. Cardiovascular mortality in obstructive sleep apnea in the elderly: role of long-term continuous positive airway pressure treatment: a prospective observational study. Am J Respir Crit Care Med. 2012;186(9):909-916. doi:10.1164/rccm.201203-0448OC
21. Neilan TG, Farhad H, Dodson JA, et al. Effect of sleep apnea and continuous positive airway pressure on cardiac structure and recurrence of atrial fibrillation. J Am Heart Assoc. 2013;2(6):e000421. Published 2013 Nov 25. doi:10.1161/JAHA.113.000421
22. Redline S, Adams N, Strauss ME, Roebuck T, Winters M, Rosenberg C. Improvement of mild sleep-disordered breathing with CPAP compared with conservative therapy. Am J Respir Crit Care Med. 1998;157(3):858-865. doi:10.1164/ajrccm.157.3.9709042
23. McEvoy RD, Antic NA, Heeley E, et al. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375(10):919-931. doi:10.1056/NEJMoa1606599
24. Yu J, Zhou Z, McEvoy RD, et al. Association of positive airway pressure with cardiovascular events and death in adults with sleep apnea: a systematic review and meta-analysis. JAMA. 2017;318(2):156-166. doi:10.1001/jama.2017.7967
25. Perger E, Lyons OD, Inami T, et al. Predictors of 1-year compliance with adaptive servoventilation in patients with heart failure and sleep disordered breathing: preliminary data from the ADVENT-HF trial. Eur Resp J. 2019;53(2):1801626. doi:10.1183/13993003.01626-2018
26. Lyons OD, Floras JS, Logan AG, et al. Design of the effect of adaptive servo-ventilation on survival and cardiovascular hospital admissions in patients with heart failure and sleep apnoea: the ADVENT-HF trial. Eur J Heart Fail. 2017;19(4):579-587. doi:10.1002/ejhf.790
27. Teschler H, Döhring J, Wang YM, Berthon-Jones M. Adaptive pressure support servo-ventilation: a novel treatment for Cheyne-Stokes respiration in heart failure. Am J Respir Crit Care Med. 2001;164(4):614-619. doi:10.1164/ajrccm.164.4.9908114
28. Ye L, Pack AI, Maislin G, et al. Predictors of continuous positive airway pressure use during the first week of treatment. J Sleep Res. 2012;21(4):419-426. doi:10.1111/j.1365-2869.2011.00969.x
29. Vennelle M, White S, Riha RL, Mackay TW, Engleman HM, Douglas NJ. Randomized controlled trial of variable-pressure versus fixed-pressure continuous positive airway pressure (CPAP) treatment for patients with obstructive sleep apnea/hypopnea syndrome (OSAHS). Sleep. 2010;33(2):267-271. doi:10.1093/sleep/33.2.267
30. Javaheri S, Harris N, Howard J, Chung E. Adaptive servoventilation for treatment of opioid-associated central sleep apnea. J Clin Sleep Med. 2014;10(6):637-643. Published 2014 Jun 15. doi:10.5664/jcsm.3788
1. Morgenthaler TI, Gay PC, Gordon N, Brown LK. Adaptive servoventilation versus noninvasive positive pressure ventilation for central, mixed, and complex sleep apnea syndromes. Sleep. 2007;30(4):468-475. doi:10.1093/sleep/30.4.468
2. Eckert DJ, Jordan AS, Merchia P, Malhotra A. Central sleep apnea: pathophysiology and treatment. Chest. 2007;131(2):595-607. doi:10.1378/chest.06.2287
3. Verbraecken J. Complex sleep apnoea syndrome. Breathe. 2013;9(5):372-380. doi:10.1183/20734735.042412
4. Bradley TD, Logan AG, Kimoff RJ, et al. Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med. 2005;353(19):2025-2033. doi:10.1056/NEJMoa051001
5. Cowie MR, Woehrle H, Wegscheider K, et al. Adaptive servo-ventilation for central sleep apnea in systolic heart failure. N Engl J Med. 2015;373(12):1095-1105. doi:10.1056/NEJMoa1506459
6. Imamura T, Kinugawa K. What is the optimal strategy for adaptive servo-ventilation therapy? Int Heart J. 2018;59(4):683-688. doi:10.1536/ihj.17-429
7. Javaheri S, Brown LK, Randerath W, Khayat R. SERVE-HF: more questions than answers. Chest. 2016;149(4):900-904. doi:10.1016/j.chest.2015.12.021
8. Mehra R, Gottlieb DJ. A paradigm shift in the treatment of central sleep apnea in heart failure. Chest. 2015;148(4):848-851. doi:10.1378/chest.15-1536
9. Nigam G, Riaz M, Chang E, Camacho M. Natural history of treatment-emergent central sleep apnea on positive airway pressure: a systematic review. Ann Thorac Med. 2018;13(2):86-91. doi:10.4103/atm.ATM_321_17
10. Ratz D, Wiitala W, Badr MS, Burns J, Chowdhuri S. Correlates and consequences of central sleep apnea in a national sample of US veterans. Sleep. 2018;41(9):zsy058. doi:10.1093/sleep/zsy058
11. Wolkove N, Baltzan M, Kamel H, Dabrusin R, Palayew M. Long-term compliance with continuous positive airway pressure in patients with obstructive sleep apnea. Can Respir J. 2008;15(7):365-369. doi:10.1155/2008/534372
12. Special Safety Notice: ASV therapy for central sleep apnea patients with heart failure. American Academy of Sleep Medicine. May 15, 2015. Accessed February 13, 2023. https://aasm.org/special-safety-notice-asv-therapy-for-central-sleep-apnea-patients-with-heart-failure/
13. Philippe C, Stoïca-Herman M, Drouot X, et al. Compliance with and effectiveness of adaptive servoventilation versus continuous positive airway pressure in the treatment of Cheyne-Stokes respiration in heart failure over a six month period. Heart. 2006;92(3):337-342. doi:10.1136/hrt.2005.060038
14. Randerath W, Deleanu OC, Schiza S, Pepin J-L. Central sleep apnoea and periodic breathing in heart failure: prognostic significance and treatment options. Eur Respir Rev. 2019;28(153):190084. Published 2019 Oct 11. doi:10.1183/16000617.0084-2019
15. Gay PC. Complex sleep apnea: it really is a disease. J Clin Sleep Med. 2008;4(5):403-405.
16. American Academy of Sleep Medicine. International Classification of Sleep Disorders - Third Edition (ICSD-3). 3rd ed. American Academy of Sleep Medicine; 2014.
17. Brown SE, Mosko SS, Davis JA, Pierce RA, Godfrey-Pixton TV. A retrospective case series of adaptive servoventilation for complex sleep apnea. J Clin Sleep Med. 2011;7(2):187-195.
18. Aurora RN, Bista SR, Casey KR, et al. Updated Adaptive Servo-Ventilation Recommendations for the 2012 AASM Guideline: “The Treatment of Central Sleep Apnea Syndromes in Adults: Practice Parameters with an Evidence-Based Literature Review and Meta-Analyses”. J Clin Sleep Med. 2016;12(5):757-761. doi:10.5664/jcsm.5812
19. Martínez-García MA, Soler-Cataluña JJ, Ejarque-Martínez L, et al. Continuous positive airway pressure treatment reduces mortality in patients with ischemic stroke and obstructive sleep apnea: a 5-year follow-up study. Am J Respir Crit Care Med. 2009;180(1):36-41. doi:10.1164/rccm.200808-1341OC
20. Martínez-García MA, Campos-Rodríguez F, Catalán-Serra P, et al. Cardiovascular mortality in obstructive sleep apnea in the elderly: role of long-term continuous positive airway pressure treatment: a prospective observational study. Am J Respir Crit Care Med. 2012;186(9):909-916. doi:10.1164/rccm.201203-0448OC
21. Neilan TG, Farhad H, Dodson JA, et al. Effect of sleep apnea and continuous positive airway pressure on cardiac structure and recurrence of atrial fibrillation. J Am Heart Assoc. 2013;2(6):e000421. Published 2013 Nov 25. doi:10.1161/JAHA.113.000421
22. Redline S, Adams N, Strauss ME, Roebuck T, Winters M, Rosenberg C. Improvement of mild sleep-disordered breathing with CPAP compared with conservative therapy. Am J Respir Crit Care Med. 1998;157(3):858-865. doi:10.1164/ajrccm.157.3.9709042
23. McEvoy RD, Antic NA, Heeley E, et al. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375(10):919-931. doi:10.1056/NEJMoa1606599
24. Yu J, Zhou Z, McEvoy RD, et al. Association of positive airway pressure with cardiovascular events and death in adults with sleep apnea: a systematic review and meta-analysis. JAMA. 2017;318(2):156-166. doi:10.1001/jama.2017.7967
25. Perger E, Lyons OD, Inami T, et al. Predictors of 1-year compliance with adaptive servoventilation in patients with heart failure and sleep disordered breathing: preliminary data from the ADVENT-HF trial. Eur Resp J. 2019;53(2):1801626. doi:10.1183/13993003.01626-2018
26. Lyons OD, Floras JS, Logan AG, et al. Design of the effect of adaptive servo-ventilation on survival and cardiovascular hospital admissions in patients with heart failure and sleep apnoea: the ADVENT-HF trial. Eur J Heart Fail. 2017;19(4):579-587. doi:10.1002/ejhf.790
27. Teschler H, Döhring J, Wang YM, Berthon-Jones M. Adaptive pressure support servo-ventilation: a novel treatment for Cheyne-Stokes respiration in heart failure. Am J Respir Crit Care Med. 2001;164(4):614-619. doi:10.1164/ajrccm.164.4.9908114
28. Ye L, Pack AI, Maislin G, et al. Predictors of continuous positive airway pressure use during the first week of treatment. J Sleep Res. 2012;21(4):419-426. doi:10.1111/j.1365-2869.2011.00969.x
29. Vennelle M, White S, Riha RL, Mackay TW, Engleman HM, Douglas NJ. Randomized controlled trial of variable-pressure versus fixed-pressure continuous positive airway pressure (CPAP) treatment for patients with obstructive sleep apnea/hypopnea syndrome (OSAHS). Sleep. 2010;33(2):267-271. doi:10.1093/sleep/33.2.267
30. Javaheri S, Harris N, Howard J, Chung E. Adaptive servoventilation for treatment of opioid-associated central sleep apnea. J Clin Sleep Med. 2014;10(6):637-643. Published 2014 Jun 15. doi:10.5664/jcsm.3788
Pharmacist-Led Antimicrobial Stewardship and Antibiotic Use in Hospitalized Patients With COVID-19
The inappropriate use of antibiotics is associated with an increased risk of antibiotic resistance, health care costs, and risk of adverse drug reactions.1 According to the Centers for Disease Control and Prevention (CDC), a 10% decrease in overall antibiotic use across different wards was associated with a 34% decrease in Clostridioides difficile (C difficile) infections.2 In addition, antimicrobial resistance accounts for > 2.8 million infections and > 35,000 deaths each year.3 The estimated total economic costs of antibiotic resistance to the US economy have ranged as high as $20 billion in excess direct health care costs.4 A main goal of an antimicrobial stewardship program (ASP) is to optimize antibiotic use to prevent the adverse consequences of inappropriate antibiotic prescribing.
During the COVID-19 pandemic, increased use of empiric antibiotic therapy has been observed. According to the CDC, almost 80% of patients hospitalized with COVID-19 received an antibiotic from March 2020 to October 2020.5 Studies were conducted to investigate the prevalence of bacterial coinfection in patients with COVID-19 and whether antibiotics were indicated in this patient population. A United Kingdom multicenter, prospective cohort study showed a high proportion of patients hospitalized with COVID-19 received antimicrobials despite microbiologically confirmed bacterial infections being rare and more likely to be secondary infections.6
Many other studies have reported similar findings. Langord and colleagues found the prevalence of bacterial coinfection in patients with COVID-19 was 3.5% but that 71.9% received antibiotics.7 Coenen and colleagues identified 12.4% of the patients with possible and 1.1% of patients with probable bacterial coinfection, while 81% of the study population and 78% of patients were classified as unlikely bacterial coinfection received antibiotics.8
At Veterans Affairs Southern Nevada Healthcare System (VASNHS), an ASP team consisting of an infectious disease (ID) physician and 2 pharmacists provide daily prospective audits with intervention and feedback along with other interventions, such as providing restricted order menus, institutional treatment guidelines, and staff education to help improve antibiotic prescribing. The ASP pharmacists have a scope of practice to make changes to anti-infective therapies. The purpose of this study was to describe antibiotic prescribing in patients hospitalized with COVID-19 from November 1, 2020, to January 31, 2021, in an ASP setting led by pharmacists.
Methods
This retrospective descriptive study included patients who were hospitalized for the treatment of laboratory-confirmed COVID-19 infection. The Theradoc clinical surveillance system was used to retrieve a list of patients who were admitted to VASNHS from November 1, 2020, to January 31, 2021, and tested positive for COVID-19. Patients with incidental positive COVID-19 test results or those who received antibiotics for extrapulmonary indications on hospital admission were excluded.
Each patient chart was reviewed and data, including clinical presentations, procalcitonin (PCT), the requirement of supplemental oxygen, vital signs, imaging findings, antibiotic orders on admission, ASP interventions such as discontinuation or changes to antibiotic therapy during the first 72 hours of hospital admission, clinical outcomes, culture results, and readmission rate, defined as any hospital admission related to COVID-19 or respiratory tract infection within 30 days from the previous discharge, were collected.
The primary objective of the study was to describe antibiotic prescribing in patients hospitalized with COVID-19. The secondary outcomes included the prevalence of bacterial coinfection and nosocomial bacterial infection in patients hospitalized with COVID-19.
Results
A total of 199 patients were admitted to the hospital for laboratory-confirmed COVID-19 infection from November 1, 2020, to January 31, 2021. Sixty-one patients (31%) received at least 1 antibiotic on hospital admission. Among those patients who received empiric antibiotic treatment, 29 patients (48%) met the Systemic Inflammatory Response Syndrome (SIRS) criteria. Fifty-six patients (92%) had ≥ 1 PCT level obtained, and 26 of those (46%) presented with elevated PCT levels (PCT > 0.25). Fifty patients (82%) required oxygen supplement and 49 (80%) presented with remarkable imaging findings. Of 138 patients who did not receive empiric antibiotic therapy within 72 hours of hospital admission, 56 (41%) met the SIRS criteria, 31 (29%) had elevated PCT levels, 100 (72%) required oxygen supplement, and 79 (59%) presented with remarkable imaging findings.
Antibiotic Prescribing
Forty-six of 61 patients (75%) received antibiotic treatment for community-acquired pneumonia (CAP) that included ceftriaxone and azithromycin. Three patients (5%) received ≥ 1 broad-spectrum antibiotic (4th generation cephalosporin [cefepime] or piperacillin-tazobactam), 2 (3%) received vancomycin, and 1 (2%) received a fluoroquinolone (levofloxacin) on admission.
Among 61 patients who received empiric antibiotics, the readmission rate was 6%. The mortality rate was 20%, and the mean (SD) duration of hospital stay was 13.1 (12.5) days.
Six of 199 patients (3%) had microbiologically confirmed bacterial coinfection on hospital admission: 3 were Pseudomonas aeruginosa (P aeruginosa) and 2 were Klebsiella oxytoca (Table 1).
Discussion
Prospective audit and feedback and preauthorization are recommended in guidelines as “core components of any stewardship program.”9 At VASNHS, the ASP performs daily prospective audits with intervention and feedback. Efforts have been made to maintain daily ASP activities during the pandemic. This study aimed to describe antibiotic prescribing for patients hospitalized with COVID-19 in a pharmacist-led ASP setting. It was found that up to 31% of the patients received ≥ 1 antibiotic on admission for empiric treatment of bacterial coinfection. About half of these patients met the SIRS criteria. Most of these patients received ceftriaxone and azithromycin for concern of CAP. ASP discontinued antibiotics within 72 hours in most of the patients. Chart review and discussion with ID physicians and/or hospitalists determined the probability of bacterial coinfection as well as any potential complication or patient-specific risk factor. It is important to note that most patients who received antibiotics on admission had ≥ 1 PCT level and up to 46% of them had a PCT level > 0.25. However, according to Relph and colleagues, PCT may not be a reliable indicator of bacterial infection in severe viral diseases with raised interleukin-6 levels.10 An elevated PCT level should not be the sole indicator for empiric antibiotic treatment.
Study findings confirmed the low prevalence of bacterial coinfection in patients hospitalized with COVID-19. The overuse of empiric antibiotics in a patient population unlikely to present with bacterial coinfection is concerning. It is essential to continue promoting antimicrobial stewardship during the COVID-19 pandemic to ensure appropriate and responsible antimicrobial prescribing. A thorough clinical assessment consisting of comorbidities, clinical symptoms, radiologic and microbiologic findings, as well as other relevant workup or biomarker results is crucial to determine whether the antibiotic is strongly indicated in patients hospitalized with COVID-19. Empiric antibiotic therapy should be considered only in patients with clinical findings suggestive of bacterial coinfection.
Limitations
Limitations of our study included the study design (single-center, retrospective review, lack of comparative group) and small sample size with a 3-month study period. In addition, respiratory cultures are not commonly obtained in patients who present with mild-to-moderate CAP. Using culture results solely to confirm bacterial coinfection in patients with COVID-19 could have underestimated the prevalence of bacterial infection. Developing diagnostic criteria that include clinical signs and symptoms, imaging findings, and laboratory results as well as culture results would help to better assess the presence of bacterial coinfection in this patient population.
Conclusions
The study findings showed that up to 30% of patients hospitalized for COVID-19 infection received empiric antibiotic treatment for concern of bacterial coinfection. A pharmacist-led ASP provided interventions, including early discontinuation of antibiotics in 77% of these patients.
A low prevalence of bacterial coinfection (3%) in patients hospitalized with COVID-19 also was reported. A thorough clinical workup to determine the risk of bacterial coinfection in patients with COVID-19 is important before starting empiric antibiotic therapy. Continuing to promote the ASP during the COVID-19 pandemic to ensure responsible antibiotic use and prevent antimicrobial resistance is essential.
1. Demirjian A, Sanchez GV, Finkelstein JA, et al. CDC grand rounds: getting smart about antibiotics. MMWR Morb Mortal Wkly Rep. 2015;64(32):871-873. doi:10.15585/mmwr.mm6432a3
2. Nearly half a million Americans suffered from Clostridium difficile infections in a single year. Centers for Disease Control and Prevention. Updated March 22, 2017. Accessed March 21, 2023. https://www.cdc.gov/media/releases/2015/p0225-clostridium-difficile.html
3. Centers for Disease Control and Prevention. About antimicrobial resistance. Updated October 5, 2022. Accessed March 21, 2023. https://www.cdc.gov/drugresistance/about.html
4. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. Accessed March 21, 2023. https://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf
5. Centers for Disease Control and Prevention. COVID-19 & antibiotic resistance. Updated February 25, 2022. Accessed March 21, 2023. https://www.cdc.gov/drugresistance/covid19.html
6. Russell CD, Fairfield CJ, Drake TM, et al. Co-infections, secondary infections, and antimicrobial use in patients hospitalised with COVID-19 during the first pandemic wave from the ISARIC WHO CCP-UK study: a multicentre, prospective cohort study. Lancet Microbe. 2021;2(8):e354-e365. doi:10.1016/S2666-5247(21)00090-2
7. Langford BJ, So M, Raybardhan S, et al. Bacterial co-infection and secondary infection in patients with COVID-19: a living rapid review and meta-analysis. Clin Microbiol Infect. 2020;26(12):1622-1629. doi:10.1016/j.cmi.2020.07.016
8. Coenen S, de la Court JR, Buis DTP, et al. Low frequency of community-acquired bacterial co-infection in patients hospitalized for COVID-19 based on clinical, radiological and microbiological criteria: a retrospective cohort study. Antimicrob Resist Infect Control. 2021;10(1):155. doi:10.1186/s13756-021-01024-4
9. Centers for Disease Control and Prevention. The core elements of hospital antibiotic stewardship programs: 2019. Accessed March 21, 2023. https://www.cdc.gov/antibiotic-use/healthcare/pdfs/hospital-core-elements-H.pdf
10. Relph KA, Russell CD, Fairfield CJ, et al; International Severe Acute Respiratory and Emerging Infections Consortium; Coronavirus Clinical Characterisation Consortium (ISARIC4C) Investigators. Procalcitonin is not a reliable biomarker of bacterial coinfection in people with Coronavirus Disease 2019 undergoing microbiological investigation at the time of hospital admission. Open Forum Infect Dis. 2022;9(5):ofac179. doi:10.1093/ofid/ofac179
The inappropriate use of antibiotics is associated with an increased risk of antibiotic resistance, health care costs, and risk of adverse drug reactions.1 According to the Centers for Disease Control and Prevention (CDC), a 10% decrease in overall antibiotic use across different wards was associated with a 34% decrease in Clostridioides difficile (C difficile) infections.2 In addition, antimicrobial resistance accounts for > 2.8 million infections and > 35,000 deaths each year.3 The estimated total economic costs of antibiotic resistance to the US economy have ranged as high as $20 billion in excess direct health care costs.4 A main goal of an antimicrobial stewardship program (ASP) is to optimize antibiotic use to prevent the adverse consequences of inappropriate antibiotic prescribing.
During the COVID-19 pandemic, increased use of empiric antibiotic therapy has been observed. According to the CDC, almost 80% of patients hospitalized with COVID-19 received an antibiotic from March 2020 to October 2020.5 Studies were conducted to investigate the prevalence of bacterial coinfection in patients with COVID-19 and whether antibiotics were indicated in this patient population. A United Kingdom multicenter, prospective cohort study showed a high proportion of patients hospitalized with COVID-19 received antimicrobials despite microbiologically confirmed bacterial infections being rare and more likely to be secondary infections.6
Many other studies have reported similar findings. Langord and colleagues found the prevalence of bacterial coinfection in patients with COVID-19 was 3.5% but that 71.9% received antibiotics.7 Coenen and colleagues identified 12.4% of the patients with possible and 1.1% of patients with probable bacterial coinfection, while 81% of the study population and 78% of patients were classified as unlikely bacterial coinfection received antibiotics.8
At Veterans Affairs Southern Nevada Healthcare System (VASNHS), an ASP team consisting of an infectious disease (ID) physician and 2 pharmacists provide daily prospective audits with intervention and feedback along with other interventions, such as providing restricted order menus, institutional treatment guidelines, and staff education to help improve antibiotic prescribing. The ASP pharmacists have a scope of practice to make changes to anti-infective therapies. The purpose of this study was to describe antibiotic prescribing in patients hospitalized with COVID-19 from November 1, 2020, to January 31, 2021, in an ASP setting led by pharmacists.
Methods
This retrospective descriptive study included patients who were hospitalized for the treatment of laboratory-confirmed COVID-19 infection. The Theradoc clinical surveillance system was used to retrieve a list of patients who were admitted to VASNHS from November 1, 2020, to January 31, 2021, and tested positive for COVID-19. Patients with incidental positive COVID-19 test results or those who received antibiotics for extrapulmonary indications on hospital admission were excluded.
Each patient chart was reviewed and data, including clinical presentations, procalcitonin (PCT), the requirement of supplemental oxygen, vital signs, imaging findings, antibiotic orders on admission, ASP interventions such as discontinuation or changes to antibiotic therapy during the first 72 hours of hospital admission, clinical outcomes, culture results, and readmission rate, defined as any hospital admission related to COVID-19 or respiratory tract infection within 30 days from the previous discharge, were collected.
The primary objective of the study was to describe antibiotic prescribing in patients hospitalized with COVID-19. The secondary outcomes included the prevalence of bacterial coinfection and nosocomial bacterial infection in patients hospitalized with COVID-19.
Results
A total of 199 patients were admitted to the hospital for laboratory-confirmed COVID-19 infection from November 1, 2020, to January 31, 2021. Sixty-one patients (31%) received at least 1 antibiotic on hospital admission. Among those patients who received empiric antibiotic treatment, 29 patients (48%) met the Systemic Inflammatory Response Syndrome (SIRS) criteria. Fifty-six patients (92%) had ≥ 1 PCT level obtained, and 26 of those (46%) presented with elevated PCT levels (PCT > 0.25). Fifty patients (82%) required oxygen supplement and 49 (80%) presented with remarkable imaging findings. Of 138 patients who did not receive empiric antibiotic therapy within 72 hours of hospital admission, 56 (41%) met the SIRS criteria, 31 (29%) had elevated PCT levels, 100 (72%) required oxygen supplement, and 79 (59%) presented with remarkable imaging findings.
Antibiotic Prescribing
Forty-six of 61 patients (75%) received antibiotic treatment for community-acquired pneumonia (CAP) that included ceftriaxone and azithromycin. Three patients (5%) received ≥ 1 broad-spectrum antibiotic (4th generation cephalosporin [cefepime] or piperacillin-tazobactam), 2 (3%) received vancomycin, and 1 (2%) received a fluoroquinolone (levofloxacin) on admission.
Among 61 patients who received empiric antibiotics, the readmission rate was 6%. The mortality rate was 20%, and the mean (SD) duration of hospital stay was 13.1 (12.5) days.
Six of 199 patients (3%) had microbiologically confirmed bacterial coinfection on hospital admission: 3 were Pseudomonas aeruginosa (P aeruginosa) and 2 were Klebsiella oxytoca (Table 1).
Discussion
Prospective audit and feedback and preauthorization are recommended in guidelines as “core components of any stewardship program.”9 At VASNHS, the ASP performs daily prospective audits with intervention and feedback. Efforts have been made to maintain daily ASP activities during the pandemic. This study aimed to describe antibiotic prescribing for patients hospitalized with COVID-19 in a pharmacist-led ASP setting. It was found that up to 31% of the patients received ≥ 1 antibiotic on admission for empiric treatment of bacterial coinfection. About half of these patients met the SIRS criteria. Most of these patients received ceftriaxone and azithromycin for concern of CAP. ASP discontinued antibiotics within 72 hours in most of the patients. Chart review and discussion with ID physicians and/or hospitalists determined the probability of bacterial coinfection as well as any potential complication or patient-specific risk factor. It is important to note that most patients who received antibiotics on admission had ≥ 1 PCT level and up to 46% of them had a PCT level > 0.25. However, according to Relph and colleagues, PCT may not be a reliable indicator of bacterial infection in severe viral diseases with raised interleukin-6 levels.10 An elevated PCT level should not be the sole indicator for empiric antibiotic treatment.
Study findings confirmed the low prevalence of bacterial coinfection in patients hospitalized with COVID-19. The overuse of empiric antibiotics in a patient population unlikely to present with bacterial coinfection is concerning. It is essential to continue promoting antimicrobial stewardship during the COVID-19 pandemic to ensure appropriate and responsible antimicrobial prescribing. A thorough clinical assessment consisting of comorbidities, clinical symptoms, radiologic and microbiologic findings, as well as other relevant workup or biomarker results is crucial to determine whether the antibiotic is strongly indicated in patients hospitalized with COVID-19. Empiric antibiotic therapy should be considered only in patients with clinical findings suggestive of bacterial coinfection.
Limitations
Limitations of our study included the study design (single-center, retrospective review, lack of comparative group) and small sample size with a 3-month study period. In addition, respiratory cultures are not commonly obtained in patients who present with mild-to-moderate CAP. Using culture results solely to confirm bacterial coinfection in patients with COVID-19 could have underestimated the prevalence of bacterial infection. Developing diagnostic criteria that include clinical signs and symptoms, imaging findings, and laboratory results as well as culture results would help to better assess the presence of bacterial coinfection in this patient population.
Conclusions
The study findings showed that up to 30% of patients hospitalized for COVID-19 infection received empiric antibiotic treatment for concern of bacterial coinfection. A pharmacist-led ASP provided interventions, including early discontinuation of antibiotics in 77% of these patients.
A low prevalence of bacterial coinfection (3%) in patients hospitalized with COVID-19 also was reported. A thorough clinical workup to determine the risk of bacterial coinfection in patients with COVID-19 is important before starting empiric antibiotic therapy. Continuing to promote the ASP during the COVID-19 pandemic to ensure responsible antibiotic use and prevent antimicrobial resistance is essential.
The inappropriate use of antibiotics is associated with an increased risk of antibiotic resistance, health care costs, and risk of adverse drug reactions.1 According to the Centers for Disease Control and Prevention (CDC), a 10% decrease in overall antibiotic use across different wards was associated with a 34% decrease in Clostridioides difficile (C difficile) infections.2 In addition, antimicrobial resistance accounts for > 2.8 million infections and > 35,000 deaths each year.3 The estimated total economic costs of antibiotic resistance to the US economy have ranged as high as $20 billion in excess direct health care costs.4 A main goal of an antimicrobial stewardship program (ASP) is to optimize antibiotic use to prevent the adverse consequences of inappropriate antibiotic prescribing.
During the COVID-19 pandemic, increased use of empiric antibiotic therapy has been observed. According to the CDC, almost 80% of patients hospitalized with COVID-19 received an antibiotic from March 2020 to October 2020.5 Studies were conducted to investigate the prevalence of bacterial coinfection in patients with COVID-19 and whether antibiotics were indicated in this patient population. A United Kingdom multicenter, prospective cohort study showed a high proportion of patients hospitalized with COVID-19 received antimicrobials despite microbiologically confirmed bacterial infections being rare and more likely to be secondary infections.6
Many other studies have reported similar findings. Langord and colleagues found the prevalence of bacterial coinfection in patients with COVID-19 was 3.5% but that 71.9% received antibiotics.7 Coenen and colleagues identified 12.4% of the patients with possible and 1.1% of patients with probable bacterial coinfection, while 81% of the study population and 78% of patients were classified as unlikely bacterial coinfection received antibiotics.8
At Veterans Affairs Southern Nevada Healthcare System (VASNHS), an ASP team consisting of an infectious disease (ID) physician and 2 pharmacists provide daily prospective audits with intervention and feedback along with other interventions, such as providing restricted order menus, institutional treatment guidelines, and staff education to help improve antibiotic prescribing. The ASP pharmacists have a scope of practice to make changes to anti-infective therapies. The purpose of this study was to describe antibiotic prescribing in patients hospitalized with COVID-19 from November 1, 2020, to January 31, 2021, in an ASP setting led by pharmacists.
Methods
This retrospective descriptive study included patients who were hospitalized for the treatment of laboratory-confirmed COVID-19 infection. The Theradoc clinical surveillance system was used to retrieve a list of patients who were admitted to VASNHS from November 1, 2020, to January 31, 2021, and tested positive for COVID-19. Patients with incidental positive COVID-19 test results or those who received antibiotics for extrapulmonary indications on hospital admission were excluded.
Each patient chart was reviewed and data, including clinical presentations, procalcitonin (PCT), the requirement of supplemental oxygen, vital signs, imaging findings, antibiotic orders on admission, ASP interventions such as discontinuation or changes to antibiotic therapy during the first 72 hours of hospital admission, clinical outcomes, culture results, and readmission rate, defined as any hospital admission related to COVID-19 or respiratory tract infection within 30 days from the previous discharge, were collected.
The primary objective of the study was to describe antibiotic prescribing in patients hospitalized with COVID-19. The secondary outcomes included the prevalence of bacterial coinfection and nosocomial bacterial infection in patients hospitalized with COVID-19.
Results
A total of 199 patients were admitted to the hospital for laboratory-confirmed COVID-19 infection from November 1, 2020, to January 31, 2021. Sixty-one patients (31%) received at least 1 antibiotic on hospital admission. Among those patients who received empiric antibiotic treatment, 29 patients (48%) met the Systemic Inflammatory Response Syndrome (SIRS) criteria. Fifty-six patients (92%) had ≥ 1 PCT level obtained, and 26 of those (46%) presented with elevated PCT levels (PCT > 0.25). Fifty patients (82%) required oxygen supplement and 49 (80%) presented with remarkable imaging findings. Of 138 patients who did not receive empiric antibiotic therapy within 72 hours of hospital admission, 56 (41%) met the SIRS criteria, 31 (29%) had elevated PCT levels, 100 (72%) required oxygen supplement, and 79 (59%) presented with remarkable imaging findings.
Antibiotic Prescribing
Forty-six of 61 patients (75%) received antibiotic treatment for community-acquired pneumonia (CAP) that included ceftriaxone and azithromycin. Three patients (5%) received ≥ 1 broad-spectrum antibiotic (4th generation cephalosporin [cefepime] or piperacillin-tazobactam), 2 (3%) received vancomycin, and 1 (2%) received a fluoroquinolone (levofloxacin) on admission.
Among 61 patients who received empiric antibiotics, the readmission rate was 6%. The mortality rate was 20%, and the mean (SD) duration of hospital stay was 13.1 (12.5) days.
Six of 199 patients (3%) had microbiologically confirmed bacterial coinfection on hospital admission: 3 were Pseudomonas aeruginosa (P aeruginosa) and 2 were Klebsiella oxytoca (Table 1).
Discussion
Prospective audit and feedback and preauthorization are recommended in guidelines as “core components of any stewardship program.”9 At VASNHS, the ASP performs daily prospective audits with intervention and feedback. Efforts have been made to maintain daily ASP activities during the pandemic. This study aimed to describe antibiotic prescribing for patients hospitalized with COVID-19 in a pharmacist-led ASP setting. It was found that up to 31% of the patients received ≥ 1 antibiotic on admission for empiric treatment of bacterial coinfection. About half of these patients met the SIRS criteria. Most of these patients received ceftriaxone and azithromycin for concern of CAP. ASP discontinued antibiotics within 72 hours in most of the patients. Chart review and discussion with ID physicians and/or hospitalists determined the probability of bacterial coinfection as well as any potential complication or patient-specific risk factor. It is important to note that most patients who received antibiotics on admission had ≥ 1 PCT level and up to 46% of them had a PCT level > 0.25. However, according to Relph and colleagues, PCT may not be a reliable indicator of bacterial infection in severe viral diseases with raised interleukin-6 levels.10 An elevated PCT level should not be the sole indicator for empiric antibiotic treatment.
Study findings confirmed the low prevalence of bacterial coinfection in patients hospitalized with COVID-19. The overuse of empiric antibiotics in a patient population unlikely to present with bacterial coinfection is concerning. It is essential to continue promoting antimicrobial stewardship during the COVID-19 pandemic to ensure appropriate and responsible antimicrobial prescribing. A thorough clinical assessment consisting of comorbidities, clinical symptoms, radiologic and microbiologic findings, as well as other relevant workup or biomarker results is crucial to determine whether the antibiotic is strongly indicated in patients hospitalized with COVID-19. Empiric antibiotic therapy should be considered only in patients with clinical findings suggestive of bacterial coinfection.
Limitations
Limitations of our study included the study design (single-center, retrospective review, lack of comparative group) and small sample size with a 3-month study period. In addition, respiratory cultures are not commonly obtained in patients who present with mild-to-moderate CAP. Using culture results solely to confirm bacterial coinfection in patients with COVID-19 could have underestimated the prevalence of bacterial infection. Developing diagnostic criteria that include clinical signs and symptoms, imaging findings, and laboratory results as well as culture results would help to better assess the presence of bacterial coinfection in this patient population.
Conclusions
The study findings showed that up to 30% of patients hospitalized for COVID-19 infection received empiric antibiotic treatment for concern of bacterial coinfection. A pharmacist-led ASP provided interventions, including early discontinuation of antibiotics in 77% of these patients.
A low prevalence of bacterial coinfection (3%) in patients hospitalized with COVID-19 also was reported. A thorough clinical workup to determine the risk of bacterial coinfection in patients with COVID-19 is important before starting empiric antibiotic therapy. Continuing to promote the ASP during the COVID-19 pandemic to ensure responsible antibiotic use and prevent antimicrobial resistance is essential.
1. Demirjian A, Sanchez GV, Finkelstein JA, et al. CDC grand rounds: getting smart about antibiotics. MMWR Morb Mortal Wkly Rep. 2015;64(32):871-873. doi:10.15585/mmwr.mm6432a3
2. Nearly half a million Americans suffered from Clostridium difficile infections in a single year. Centers for Disease Control and Prevention. Updated March 22, 2017. Accessed March 21, 2023. https://www.cdc.gov/media/releases/2015/p0225-clostridium-difficile.html
3. Centers for Disease Control and Prevention. About antimicrobial resistance. Updated October 5, 2022. Accessed March 21, 2023. https://www.cdc.gov/drugresistance/about.html
4. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. Accessed March 21, 2023. https://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf
5. Centers for Disease Control and Prevention. COVID-19 & antibiotic resistance. Updated February 25, 2022. Accessed March 21, 2023. https://www.cdc.gov/drugresistance/covid19.html
6. Russell CD, Fairfield CJ, Drake TM, et al. Co-infections, secondary infections, and antimicrobial use in patients hospitalised with COVID-19 during the first pandemic wave from the ISARIC WHO CCP-UK study: a multicentre, prospective cohort study. Lancet Microbe. 2021;2(8):e354-e365. doi:10.1016/S2666-5247(21)00090-2
7. Langford BJ, So M, Raybardhan S, et al. Bacterial co-infection and secondary infection in patients with COVID-19: a living rapid review and meta-analysis. Clin Microbiol Infect. 2020;26(12):1622-1629. doi:10.1016/j.cmi.2020.07.016
8. Coenen S, de la Court JR, Buis DTP, et al. Low frequency of community-acquired bacterial co-infection in patients hospitalized for COVID-19 based on clinical, radiological and microbiological criteria: a retrospective cohort study. Antimicrob Resist Infect Control. 2021;10(1):155. doi:10.1186/s13756-021-01024-4
9. Centers for Disease Control and Prevention. The core elements of hospital antibiotic stewardship programs: 2019. Accessed March 21, 2023. https://www.cdc.gov/antibiotic-use/healthcare/pdfs/hospital-core-elements-H.pdf
10. Relph KA, Russell CD, Fairfield CJ, et al; International Severe Acute Respiratory and Emerging Infections Consortium; Coronavirus Clinical Characterisation Consortium (ISARIC4C) Investigators. Procalcitonin is not a reliable biomarker of bacterial coinfection in people with Coronavirus Disease 2019 undergoing microbiological investigation at the time of hospital admission. Open Forum Infect Dis. 2022;9(5):ofac179. doi:10.1093/ofid/ofac179
1. Demirjian A, Sanchez GV, Finkelstein JA, et al. CDC grand rounds: getting smart about antibiotics. MMWR Morb Mortal Wkly Rep. 2015;64(32):871-873. doi:10.15585/mmwr.mm6432a3
2. Nearly half a million Americans suffered from Clostridium difficile infections in a single year. Centers for Disease Control and Prevention. Updated March 22, 2017. Accessed March 21, 2023. https://www.cdc.gov/media/releases/2015/p0225-clostridium-difficile.html
3. Centers for Disease Control and Prevention. About antimicrobial resistance. Updated October 5, 2022. Accessed March 21, 2023. https://www.cdc.gov/drugresistance/about.html
4. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. Accessed March 21, 2023. https://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf
5. Centers for Disease Control and Prevention. COVID-19 & antibiotic resistance. Updated February 25, 2022. Accessed March 21, 2023. https://www.cdc.gov/drugresistance/covid19.html
6. Russell CD, Fairfield CJ, Drake TM, et al. Co-infections, secondary infections, and antimicrobial use in patients hospitalised with COVID-19 during the first pandemic wave from the ISARIC WHO CCP-UK study: a multicentre, prospective cohort study. Lancet Microbe. 2021;2(8):e354-e365. doi:10.1016/S2666-5247(21)00090-2
7. Langford BJ, So M, Raybardhan S, et al. Bacterial co-infection and secondary infection in patients with COVID-19: a living rapid review and meta-analysis. Clin Microbiol Infect. 2020;26(12):1622-1629. doi:10.1016/j.cmi.2020.07.016
8. Coenen S, de la Court JR, Buis DTP, et al. Low frequency of community-acquired bacterial co-infection in patients hospitalized for COVID-19 based on clinical, radiological and microbiological criteria: a retrospective cohort study. Antimicrob Resist Infect Control. 2021;10(1):155. doi:10.1186/s13756-021-01024-4
9. Centers for Disease Control and Prevention. The core elements of hospital antibiotic stewardship programs: 2019. Accessed March 21, 2023. https://www.cdc.gov/antibiotic-use/healthcare/pdfs/hospital-core-elements-H.pdf
10. Relph KA, Russell CD, Fairfield CJ, et al; International Severe Acute Respiratory and Emerging Infections Consortium; Coronavirus Clinical Characterisation Consortium (ISARIC4C) Investigators. Procalcitonin is not a reliable biomarker of bacterial coinfection in people with Coronavirus Disease 2019 undergoing microbiological investigation at the time of hospital admission. Open Forum Infect Dis. 2022;9(5):ofac179. doi:10.1093/ofid/ofac179
Prevalence of Antibiotic Allergy at a Spinal Cord Injury Center
Infectious diseases are the most common reason for rehospitalization among patients with spinal cord injuries (SCI), regardless of the number of years postinjury.1 The appropriate use and selection of antibiotics for properly diagnosed infectious diseases is especially important for this population. This principle helps to avoid the development of drug-resistant organisms and reduces the risk of recurrent infections, aligning with antibiotic stewardship.
Antibiotics are the most common class of drug allergies in the general population, and penicillin is the most frequently reported allergen (up to 10%).2 Prescription drug–induced anaphylaxis is severe and life threatening with a reported frequency of 1.1%. Penicillin and sulfonamide (46 and 15 per 10,000 patients, respectively) are the most common allergens.3 Although there is a significant difference between an adverse drug reaction (ADR) and true hypersensitivity, once documented in the electronic health record (EHR) as an allergy, this information deters use of the listed drugs.
Genitourinary, skin, and respiratory diseases are the leading causes for rehospitalization in patients with SCI.1 A large proportion of these are infectious in etiology and require antibiotic treatment. In fact, persons with SCI are at high risk for antibiotic overuse and hospital-acquired infection due to chronic bacteriuria, frequent health care exposure, implanted medical devices, and other factors.4 Concurrently, there is a crisis of antibiotic-resistant bacteria proliferation, described asa threat to patient safety and public health.5,6 Its severity is illustrated by the report that 38% of the cultures from patients with spinal cord injury are multidrug resistant gram-negative organisms.7
The SCI center at James A. Haley Veterans’ Hospital (JAHVH) in Tampa, Florida, serves a high concentration of active-duty military members and veterans with SCI. A study that reviews the exact frequency of antibiotic drug allergies listed on the EHR would be a key first step to identify the magnitude of this issue. The results could guide investigation into differentiating true allergies from ADRs, thereby widening the options for potentially life-saving antibiotic treatment.
Methods
We performed a retrospective chart review of patients included in the local SCI registry between October 1, 2015, and September 30, 2017. We collected data on patient demographics (age, sex, race and ethnicity) and a description of patients’ injuries (International Standards for Neurological Classification of Spinal Cord Injury [ISNCSCI] and etiology of injury [traumatic vs atraumatic]). The outcomes included antibiotic allergy and ADRs.
In the EHR, allergies can be listed toward an antibiotic class or a specific antibiotic. An allergy to each specific antibiotic would be recorded separately; however, overlap among antibiotic classes was not duplicated. For example, if a subject has a listed antibiotic allergy to ceftriaxone and cefepime with listed reactions, we would record allergies to each of these antibiotics but would only report a single allergy to the cephalosporin subclass.
Since we did not differentiate hypersensitivity reactions (HSRs) from other ADRs, the reported reactions were grouped by signs and symptoms. There is a variety of terms used to report similar reactions, and best efforts were made to record the data as accurately as possible. Patient-reported history for risk stratification is a tool we used to group these historical reactions into high- vs low-risk for severe reactions. High-risk signs are those listed as anaphylaxis; anaphylactic reactions; angioedema presenting as swelling of mouth, eyes, lips, or tongue; blisters or ulcers involving the lips, mouth, eyes, urethra, vagina, or peeling skin; respiratory changes; shortness of breath; dyspnea; hypotension; or organ involvement (kidneys, lungs, liver).6
Inclusion criteria were all veterans who were diagnosed with tetraplegia or paraplegia and received annual evaluation between October 1, 2015, and September 30, 2017. We chose this period because it was the beginning of a financial year at the JAHVH SCI department using the SCI registry. The SCI annual evaluation is a routine practitioner encounter with the veteran, along with appropriate laboratory testing and imaging to follow up potential chronic health issues specific to patients with SCI. Annual evaluations provide an opportunity to maintain routine health screening and preventive care. Patients who had significant portions of data missing or missing elements of primary outcomes were excluded from analysis. The study was reviewed and approved by the University of South Florida Institutional Review Board (VA IRBNet #1573370-4 on September 9, 2019).
Results
Of 1866 patients reviewed, 207 (11.1%) were excluded due to missing data, resulting in 1659 records that were analyzed. Mean age was 64 years, and male to female ratio was about 10 to 1. Most of the SCI or diseases were classified as incomplete (n = 1249) per ISNCSCI (absence of sensory and motor function in the lowest sacral segments) compared with 373 classified as complete.
Of the 1659 patients, 494 (29.8%) had a recorded allergy to antibiotics. The most frequently recorded were 217 penicillin (13.1%), 159 sulfa drugs (9.6%), 75 fluoroquinolone (4.5%), 66 cephalosporin (4.0%), and 44 vancomycin (2.7%) allergies.
Discussion
In this study, we evaluated the frequency and characteristics of antibiotic allergies at a single SCI center to better identify potential areas for quality improvement when recording drug allergies. A study in the general population used self-reported methods to collect such information found about a 15% prevalence of antibiotic allergy, which was lower than the 29.8% prevalence noted in our study.8
Regarding the most common antibiotic allergies, one study reported allergy to penicillin in the EHR in 12.8% of patients at a major US regional health care system, while 13.1% of patients with SCI had documented allergy to penicillin in our study.9 Regarding the other antibiotic classes, the percentage of allergies were higher than those reported in the general population: sulfonamide (9.6% vs 7.4%), fluoroquinolones (4.5% vs 1.3%), and cephalosporins (4.0% vs 1.7%).10 The EHR appears to capture a much higher rate of antibiotic allergies than that in self-reported studies, such as a study of self-reported allergy in the general adult population in Portugal, where only 4.5% of patients reported allergy to any β-lactam medications.10
The prevalence of an antibiotic allergy could be affected by the health care setting and sex distribution. For example, the Zhou and colleagues’ study conducted in the Greater Boston area showed higher reported antibiotic rates than those in a study from a Southern California medical group. The higher proportion of tertiary referral patients in that specific network was suggested to be the cause of the difference.8,9 Our results in the SCI population are more comparable to that in a tertiary setting. This is consistent with the fact that persons with SCI generally have more exposure to antibiotics and consequently a higher reported rate of allergic reactions to antibiotics.
Similarly, the same study in Southern California noted that female patients use more antibiotics than do male patients, thus potentially contributing to higher rates of reported allergy toward all classes of antibiotics.8 Our study did not investigate antibiotic allergy by sex; however, the significantly higher proportion of male sex among the veteran population would have impacted these results.
Limitations
Our study was limited as a single-center retrospective study. However, our center is one of the major SCI specialty hubs, and the results should be somewhat reflective of those in the veterans with SCI population. Veterans under the US Department of Veterans Affairs (VA) medical care have the option to seek care or procedures in non-VA facilities. If allergies to antibiotics occurred outside of the VA system, there is no mechanism to automatically merge with the VA EHR allergy list, unless they are later recorded and added to the VA EHR. Thus, there is potential for underreporting.
Drug anaphylaxis incidence was noted to change over time.4,8,9 For example, a downtrend of reported antibiotic allergy was reported between 1990 and 2013.10 Our study only reflects an overall prevalence of a single cohort, without demonstration of relationship to time.
Lastly, this study did not aim to differentiate HSRs from other ADRs. This is exactly the point of the study, which investigated the frequency of EHR-recorded antibiotic allergies in our SCI population and reflects the issue with indiscriminate recording of ADRs and HSRs under the umbrella of allergy in the EHR. Further diagnosing true allergies should be considered in the SCI population after weighing the risks and benefits of assessment, aligning with the wishes of the veteran, obtaining informed consent, and addressing the cost-effectiveness of specific tests. We suggest that primary care practitioners work closely with allergy specialists to formulate a mechanism to diagnose various antibiotic allergic reactions, including serum tryptase, epicutaneous skin testing, intradermal skin testing, patch testing, delayed intradermal testing, and drug challenge as appropriate. It is also possible that in cases where very mild reactions/adverse effects of antibiotics were recorded in the EHR, the clinicians and veterans may discuss reintroducing the same antibiotics or proceeding with further testing if necessary. In contrast, the 12% of those with a high risk of severe allergic reactions to penicillin in our study would benefit from allergist evaluation and access to epinephrine auto-injectors at all times. Differentiating true allergy is the only clear way to deter unnecessary avoidance of first-line therapies for antibiotic treatment and avoid promotion of antibiotic resistance.
Future studies can analyze antibiotic allergy based on demographics, including sex and age difference, as well as exploring outpatient vs inpatient settings. Aside from prevalence, we hope to demonstrate antibiotic allergy over time, especially after integration of diagnostic allergy testing, to evaluate the impact to EHR-recorded allergies.
Conclusions
Almost 30% of patients with SCI had a recorded allergy to at least 1 antibiotic. The most common allergy was to penicillin, which is similar to what has previously been reported for the general adult US population. However, only 12% of those with a penicillin allergy were considered high risk of true allergic reactions. Consequently, there are opportunities to examine whether approaches to confirm true reactions (such as skin testing) would help to mitigate unnecessary avoidance of certain antibiotic classes due to mild ADRs, rather than a true allergy, in persons with SCI. This would be an important effort to combat both individual safety concerns and the public health crisis of antibiotic resistance. Given the available evidence, it is reasonable for SCI health care practitioners to discuss the potential risks and benefits of allergy testing with patients with SCI; this maintains a patient-centered approach that can ensure judicious use of antibiotics when necessary.
Acknowledgments
This material is based on work supported (or supported in part) with resources and the use of facilities at the James A. Haley Veterans’ Hospital
References
1. National Spinal Cord Injury Statistical Center. Spinal Cord Injury Model Systems. 2016 Annual Report –Complete Public Version. University of Alabama at Birmingham. Accessed March 20, 2023. https://www.nscisc.uab.edu/Public/2016%20Annual%20Report%20-%20Complete%20Public%20Version.pdf
2. Macy E, Richter PK, Falkoff R, Zeiger R. Skin testing with penicilloate and penilloate prepared by an improved method: amoxicillin oral challenge in patients with negative skin test responses to penicillin reagents. J Allergy Clin Immunol. 1997;100(5):586-591. doi:10.1016/s0091-6749(97)70159-3 3. Dhopeshwarkar N, Sheikh A, Doan R, et al. Drug-induced anaphylaxis documented in electronic health records. J Allergy Clin Immunol Pract. 2019;7(1):103-111. doi:10.1016/j.jaip.2018.06.010
4. Evans CT, LaVela SL, Weaver FM, et al. Epidemiology of hospital-acquired infections in veterans with spinal cord injury and disorder. Infect Control Hosp Epidemiol. 2008;29(3):234-242. doi:10.1086/527509
5. Evans CT, Jump RL, Krein SL, et al. Setting a research agenda in prevention of healthcare-associated infections (HAIs) and multidrug-resistant organisms (MDROs) outside of acute care settings. Infect Control Hosp Epidemiol. 2018;39(2):210-213. doi:10.1017/ice.2017.291
6. Blumenthal KG, Peter JG, Trubiano JA, Phllips EJ. Antibiotic allergy. Lancet. 2019;393(10167):183-198. doi:10.1016/S0140-6736(18)32218-9 7. Evans CT, Fitzpatrick MA, Jones MM, et al. Prevalence and factors associated with multidrug-resistant gram-negative organisms in patients with spinal cord injury. Infect Control Hosp Epidemiol. 2017;38(12):1464-1471. doi:10.1017/ice.2017.238 8. Macy E, Poon KYT. Self-reported antibiotic allergy incidence and prevalence: age and sex effects. Am J Med. 2009;122(8):778.e1-778.e7. doi:10.1016/j.amjmed.2009.01.034
9. Zhou L, Dhopeshwarkar N, Blumenthal KG, et al. Drug allergies documented in electronic health records of a large healthcare system. Allergy. 2016;71(9):1305-1313. doi:10.1111/all.12881
10. Gomes E, Cardoso MF, Praça F, Gomes L, Mariño E, Demoly P. Self-reported drug allergy in a general adult Portuguese population. Clin Exp Allergy. 2004;34(10):1597-1601. doi:10.1111/j.1365-2222.2004.02070.x
Infectious diseases are the most common reason for rehospitalization among patients with spinal cord injuries (SCI), regardless of the number of years postinjury.1 The appropriate use and selection of antibiotics for properly diagnosed infectious diseases is especially important for this population. This principle helps to avoid the development of drug-resistant organisms and reduces the risk of recurrent infections, aligning with antibiotic stewardship.
Antibiotics are the most common class of drug allergies in the general population, and penicillin is the most frequently reported allergen (up to 10%).2 Prescription drug–induced anaphylaxis is severe and life threatening with a reported frequency of 1.1%. Penicillin and sulfonamide (46 and 15 per 10,000 patients, respectively) are the most common allergens.3 Although there is a significant difference between an adverse drug reaction (ADR) and true hypersensitivity, once documented in the electronic health record (EHR) as an allergy, this information deters use of the listed drugs.
Genitourinary, skin, and respiratory diseases are the leading causes for rehospitalization in patients with SCI.1 A large proportion of these are infectious in etiology and require antibiotic treatment. In fact, persons with SCI are at high risk for antibiotic overuse and hospital-acquired infection due to chronic bacteriuria, frequent health care exposure, implanted medical devices, and other factors.4 Concurrently, there is a crisis of antibiotic-resistant bacteria proliferation, described asa threat to patient safety and public health.5,6 Its severity is illustrated by the report that 38% of the cultures from patients with spinal cord injury are multidrug resistant gram-negative organisms.7
The SCI center at James A. Haley Veterans’ Hospital (JAHVH) in Tampa, Florida, serves a high concentration of active-duty military members and veterans with SCI. A study that reviews the exact frequency of antibiotic drug allergies listed on the EHR would be a key first step to identify the magnitude of this issue. The results could guide investigation into differentiating true allergies from ADRs, thereby widening the options for potentially life-saving antibiotic treatment.
Methods
We performed a retrospective chart review of patients included in the local SCI registry between October 1, 2015, and September 30, 2017. We collected data on patient demographics (age, sex, race and ethnicity) and a description of patients’ injuries (International Standards for Neurological Classification of Spinal Cord Injury [ISNCSCI] and etiology of injury [traumatic vs atraumatic]). The outcomes included antibiotic allergy and ADRs.
In the EHR, allergies can be listed toward an antibiotic class or a specific antibiotic. An allergy to each specific antibiotic would be recorded separately; however, overlap among antibiotic classes was not duplicated. For example, if a subject has a listed antibiotic allergy to ceftriaxone and cefepime with listed reactions, we would record allergies to each of these antibiotics but would only report a single allergy to the cephalosporin subclass.
Since we did not differentiate hypersensitivity reactions (HSRs) from other ADRs, the reported reactions were grouped by signs and symptoms. There is a variety of terms used to report similar reactions, and best efforts were made to record the data as accurately as possible. Patient-reported history for risk stratification is a tool we used to group these historical reactions into high- vs low-risk for severe reactions. High-risk signs are those listed as anaphylaxis; anaphylactic reactions; angioedema presenting as swelling of mouth, eyes, lips, or tongue; blisters or ulcers involving the lips, mouth, eyes, urethra, vagina, or peeling skin; respiratory changes; shortness of breath; dyspnea; hypotension; or organ involvement (kidneys, lungs, liver).6
Inclusion criteria were all veterans who were diagnosed with tetraplegia or paraplegia and received annual evaluation between October 1, 2015, and September 30, 2017. We chose this period because it was the beginning of a financial year at the JAHVH SCI department using the SCI registry. The SCI annual evaluation is a routine practitioner encounter with the veteran, along with appropriate laboratory testing and imaging to follow up potential chronic health issues specific to patients with SCI. Annual evaluations provide an opportunity to maintain routine health screening and preventive care. Patients who had significant portions of data missing or missing elements of primary outcomes were excluded from analysis. The study was reviewed and approved by the University of South Florida Institutional Review Board (VA IRBNet #1573370-4 on September 9, 2019).
Results
Of 1866 patients reviewed, 207 (11.1%) were excluded due to missing data, resulting in 1659 records that were analyzed. Mean age was 64 years, and male to female ratio was about 10 to 1. Most of the SCI or diseases were classified as incomplete (n = 1249) per ISNCSCI (absence of sensory and motor function in the lowest sacral segments) compared with 373 classified as complete.
Of the 1659 patients, 494 (29.8%) had a recorded allergy to antibiotics. The most frequently recorded were 217 penicillin (13.1%), 159 sulfa drugs (9.6%), 75 fluoroquinolone (4.5%), 66 cephalosporin (4.0%), and 44 vancomycin (2.7%) allergies.
Discussion
In this study, we evaluated the frequency and characteristics of antibiotic allergies at a single SCI center to better identify potential areas for quality improvement when recording drug allergies. A study in the general population used self-reported methods to collect such information found about a 15% prevalence of antibiotic allergy, which was lower than the 29.8% prevalence noted in our study.8
Regarding the most common antibiotic allergies, one study reported allergy to penicillin in the EHR in 12.8% of patients at a major US regional health care system, while 13.1% of patients with SCI had documented allergy to penicillin in our study.9 Regarding the other antibiotic classes, the percentage of allergies were higher than those reported in the general population: sulfonamide (9.6% vs 7.4%), fluoroquinolones (4.5% vs 1.3%), and cephalosporins (4.0% vs 1.7%).10 The EHR appears to capture a much higher rate of antibiotic allergies than that in self-reported studies, such as a study of self-reported allergy in the general adult population in Portugal, where only 4.5% of patients reported allergy to any β-lactam medications.10
The prevalence of an antibiotic allergy could be affected by the health care setting and sex distribution. For example, the Zhou and colleagues’ study conducted in the Greater Boston area showed higher reported antibiotic rates than those in a study from a Southern California medical group. The higher proportion of tertiary referral patients in that specific network was suggested to be the cause of the difference.8,9 Our results in the SCI population are more comparable to that in a tertiary setting. This is consistent with the fact that persons with SCI generally have more exposure to antibiotics and consequently a higher reported rate of allergic reactions to antibiotics.
Similarly, the same study in Southern California noted that female patients use more antibiotics than do male patients, thus potentially contributing to higher rates of reported allergy toward all classes of antibiotics.8 Our study did not investigate antibiotic allergy by sex; however, the significantly higher proportion of male sex among the veteran population would have impacted these results.
Limitations
Our study was limited as a single-center retrospective study. However, our center is one of the major SCI specialty hubs, and the results should be somewhat reflective of those in the veterans with SCI population. Veterans under the US Department of Veterans Affairs (VA) medical care have the option to seek care or procedures in non-VA facilities. If allergies to antibiotics occurred outside of the VA system, there is no mechanism to automatically merge with the VA EHR allergy list, unless they are later recorded and added to the VA EHR. Thus, there is potential for underreporting.
Drug anaphylaxis incidence was noted to change over time.4,8,9 For example, a downtrend of reported antibiotic allergy was reported between 1990 and 2013.10 Our study only reflects an overall prevalence of a single cohort, without demonstration of relationship to time.
Lastly, this study did not aim to differentiate HSRs from other ADRs. This is exactly the point of the study, which investigated the frequency of EHR-recorded antibiotic allergies in our SCI population and reflects the issue with indiscriminate recording of ADRs and HSRs under the umbrella of allergy in the EHR. Further diagnosing true allergies should be considered in the SCI population after weighing the risks and benefits of assessment, aligning with the wishes of the veteran, obtaining informed consent, and addressing the cost-effectiveness of specific tests. We suggest that primary care practitioners work closely with allergy specialists to formulate a mechanism to diagnose various antibiotic allergic reactions, including serum tryptase, epicutaneous skin testing, intradermal skin testing, patch testing, delayed intradermal testing, and drug challenge as appropriate. It is also possible that in cases where very mild reactions/adverse effects of antibiotics were recorded in the EHR, the clinicians and veterans may discuss reintroducing the same antibiotics or proceeding with further testing if necessary. In contrast, the 12% of those with a high risk of severe allergic reactions to penicillin in our study would benefit from allergist evaluation and access to epinephrine auto-injectors at all times. Differentiating true allergy is the only clear way to deter unnecessary avoidance of first-line therapies for antibiotic treatment and avoid promotion of antibiotic resistance.
Future studies can analyze antibiotic allergy based on demographics, including sex and age difference, as well as exploring outpatient vs inpatient settings. Aside from prevalence, we hope to demonstrate antibiotic allergy over time, especially after integration of diagnostic allergy testing, to evaluate the impact to EHR-recorded allergies.
Conclusions
Almost 30% of patients with SCI had a recorded allergy to at least 1 antibiotic. The most common allergy was to penicillin, which is similar to what has previously been reported for the general adult US population. However, only 12% of those with a penicillin allergy were considered high risk of true allergic reactions. Consequently, there are opportunities to examine whether approaches to confirm true reactions (such as skin testing) would help to mitigate unnecessary avoidance of certain antibiotic classes due to mild ADRs, rather than a true allergy, in persons with SCI. This would be an important effort to combat both individual safety concerns and the public health crisis of antibiotic resistance. Given the available evidence, it is reasonable for SCI health care practitioners to discuss the potential risks and benefits of allergy testing with patients with SCI; this maintains a patient-centered approach that can ensure judicious use of antibiotics when necessary.
Acknowledgments
This material is based on work supported (or supported in part) with resources and the use of facilities at the James A. Haley Veterans’ Hospital
Infectious diseases are the most common reason for rehospitalization among patients with spinal cord injuries (SCI), regardless of the number of years postinjury.1 The appropriate use and selection of antibiotics for properly diagnosed infectious diseases is especially important for this population. This principle helps to avoid the development of drug-resistant organisms and reduces the risk of recurrent infections, aligning with antibiotic stewardship.
Antibiotics are the most common class of drug allergies in the general population, and penicillin is the most frequently reported allergen (up to 10%).2 Prescription drug–induced anaphylaxis is severe and life threatening with a reported frequency of 1.1%. Penicillin and sulfonamide (46 and 15 per 10,000 patients, respectively) are the most common allergens.3 Although there is a significant difference between an adverse drug reaction (ADR) and true hypersensitivity, once documented in the electronic health record (EHR) as an allergy, this information deters use of the listed drugs.
Genitourinary, skin, and respiratory diseases are the leading causes for rehospitalization in patients with SCI.1 A large proportion of these are infectious in etiology and require antibiotic treatment. In fact, persons with SCI are at high risk for antibiotic overuse and hospital-acquired infection due to chronic bacteriuria, frequent health care exposure, implanted medical devices, and other factors.4 Concurrently, there is a crisis of antibiotic-resistant bacteria proliferation, described asa threat to patient safety and public health.5,6 Its severity is illustrated by the report that 38% of the cultures from patients with spinal cord injury are multidrug resistant gram-negative organisms.7
The SCI center at James A. Haley Veterans’ Hospital (JAHVH) in Tampa, Florida, serves a high concentration of active-duty military members and veterans with SCI. A study that reviews the exact frequency of antibiotic drug allergies listed on the EHR would be a key first step to identify the magnitude of this issue. The results could guide investigation into differentiating true allergies from ADRs, thereby widening the options for potentially life-saving antibiotic treatment.
Methods
We performed a retrospective chart review of patients included in the local SCI registry between October 1, 2015, and September 30, 2017. We collected data on patient demographics (age, sex, race and ethnicity) and a description of patients’ injuries (International Standards for Neurological Classification of Spinal Cord Injury [ISNCSCI] and etiology of injury [traumatic vs atraumatic]). The outcomes included antibiotic allergy and ADRs.
In the EHR, allergies can be listed toward an antibiotic class or a specific antibiotic. An allergy to each specific antibiotic would be recorded separately; however, overlap among antibiotic classes was not duplicated. For example, if a subject has a listed antibiotic allergy to ceftriaxone and cefepime with listed reactions, we would record allergies to each of these antibiotics but would only report a single allergy to the cephalosporin subclass.
Since we did not differentiate hypersensitivity reactions (HSRs) from other ADRs, the reported reactions were grouped by signs and symptoms. There is a variety of terms used to report similar reactions, and best efforts were made to record the data as accurately as possible. Patient-reported history for risk stratification is a tool we used to group these historical reactions into high- vs low-risk for severe reactions. High-risk signs are those listed as anaphylaxis; anaphylactic reactions; angioedema presenting as swelling of mouth, eyes, lips, or tongue; blisters or ulcers involving the lips, mouth, eyes, urethra, vagina, or peeling skin; respiratory changes; shortness of breath; dyspnea; hypotension; or organ involvement (kidneys, lungs, liver).6
Inclusion criteria were all veterans who were diagnosed with tetraplegia or paraplegia and received annual evaluation between October 1, 2015, and September 30, 2017. We chose this period because it was the beginning of a financial year at the JAHVH SCI department using the SCI registry. The SCI annual evaluation is a routine practitioner encounter with the veteran, along with appropriate laboratory testing and imaging to follow up potential chronic health issues specific to patients with SCI. Annual evaluations provide an opportunity to maintain routine health screening and preventive care. Patients who had significant portions of data missing or missing elements of primary outcomes were excluded from analysis. The study was reviewed and approved by the University of South Florida Institutional Review Board (VA IRBNet #1573370-4 on September 9, 2019).
Results
Of 1866 patients reviewed, 207 (11.1%) were excluded due to missing data, resulting in 1659 records that were analyzed. Mean age was 64 years, and male to female ratio was about 10 to 1. Most of the SCI or diseases were classified as incomplete (n = 1249) per ISNCSCI (absence of sensory and motor function in the lowest sacral segments) compared with 373 classified as complete.
Of the 1659 patients, 494 (29.8%) had a recorded allergy to antibiotics. The most frequently recorded were 217 penicillin (13.1%), 159 sulfa drugs (9.6%), 75 fluoroquinolone (4.5%), 66 cephalosporin (4.0%), and 44 vancomycin (2.7%) allergies.
Discussion
In this study, we evaluated the frequency and characteristics of antibiotic allergies at a single SCI center to better identify potential areas for quality improvement when recording drug allergies. A study in the general population used self-reported methods to collect such information found about a 15% prevalence of antibiotic allergy, which was lower than the 29.8% prevalence noted in our study.8
Regarding the most common antibiotic allergies, one study reported allergy to penicillin in the EHR in 12.8% of patients at a major US regional health care system, while 13.1% of patients with SCI had documented allergy to penicillin in our study.9 Regarding the other antibiotic classes, the percentage of allergies were higher than those reported in the general population: sulfonamide (9.6% vs 7.4%), fluoroquinolones (4.5% vs 1.3%), and cephalosporins (4.0% vs 1.7%).10 The EHR appears to capture a much higher rate of antibiotic allergies than that in self-reported studies, such as a study of self-reported allergy in the general adult population in Portugal, where only 4.5% of patients reported allergy to any β-lactam medications.10
The prevalence of an antibiotic allergy could be affected by the health care setting and sex distribution. For example, the Zhou and colleagues’ study conducted in the Greater Boston area showed higher reported antibiotic rates than those in a study from a Southern California medical group. The higher proportion of tertiary referral patients in that specific network was suggested to be the cause of the difference.8,9 Our results in the SCI population are more comparable to that in a tertiary setting. This is consistent with the fact that persons with SCI generally have more exposure to antibiotics and consequently a higher reported rate of allergic reactions to antibiotics.
Similarly, the same study in Southern California noted that female patients use more antibiotics than do male patients, thus potentially contributing to higher rates of reported allergy toward all classes of antibiotics.8 Our study did not investigate antibiotic allergy by sex; however, the significantly higher proportion of male sex among the veteran population would have impacted these results.
Limitations
Our study was limited as a single-center retrospective study. However, our center is one of the major SCI specialty hubs, and the results should be somewhat reflective of those in the veterans with SCI population. Veterans under the US Department of Veterans Affairs (VA) medical care have the option to seek care or procedures in non-VA facilities. If allergies to antibiotics occurred outside of the VA system, there is no mechanism to automatically merge with the VA EHR allergy list, unless they are later recorded and added to the VA EHR. Thus, there is potential for underreporting.
Drug anaphylaxis incidence was noted to change over time.4,8,9 For example, a downtrend of reported antibiotic allergy was reported between 1990 and 2013.10 Our study only reflects an overall prevalence of a single cohort, without demonstration of relationship to time.
Lastly, this study did not aim to differentiate HSRs from other ADRs. This is exactly the point of the study, which investigated the frequency of EHR-recorded antibiotic allergies in our SCI population and reflects the issue with indiscriminate recording of ADRs and HSRs under the umbrella of allergy in the EHR. Further diagnosing true allergies should be considered in the SCI population after weighing the risks and benefits of assessment, aligning with the wishes of the veteran, obtaining informed consent, and addressing the cost-effectiveness of specific tests. We suggest that primary care practitioners work closely with allergy specialists to formulate a mechanism to diagnose various antibiotic allergic reactions, including serum tryptase, epicutaneous skin testing, intradermal skin testing, patch testing, delayed intradermal testing, and drug challenge as appropriate. It is also possible that in cases where very mild reactions/adverse effects of antibiotics were recorded in the EHR, the clinicians and veterans may discuss reintroducing the same antibiotics or proceeding with further testing if necessary. In contrast, the 12% of those with a high risk of severe allergic reactions to penicillin in our study would benefit from allergist evaluation and access to epinephrine auto-injectors at all times. Differentiating true allergy is the only clear way to deter unnecessary avoidance of first-line therapies for antibiotic treatment and avoid promotion of antibiotic resistance.
Future studies can analyze antibiotic allergy based on demographics, including sex and age difference, as well as exploring outpatient vs inpatient settings. Aside from prevalence, we hope to demonstrate antibiotic allergy over time, especially after integration of diagnostic allergy testing, to evaluate the impact to EHR-recorded allergies.
Conclusions
Almost 30% of patients with SCI had a recorded allergy to at least 1 antibiotic. The most common allergy was to penicillin, which is similar to what has previously been reported for the general adult US population. However, only 12% of those with a penicillin allergy were considered high risk of true allergic reactions. Consequently, there are opportunities to examine whether approaches to confirm true reactions (such as skin testing) would help to mitigate unnecessary avoidance of certain antibiotic classes due to mild ADRs, rather than a true allergy, in persons with SCI. This would be an important effort to combat both individual safety concerns and the public health crisis of antibiotic resistance. Given the available evidence, it is reasonable for SCI health care practitioners to discuss the potential risks and benefits of allergy testing with patients with SCI; this maintains a patient-centered approach that can ensure judicious use of antibiotics when necessary.
Acknowledgments
This material is based on work supported (or supported in part) with resources and the use of facilities at the James A. Haley Veterans’ Hospital
References
1. National Spinal Cord Injury Statistical Center. Spinal Cord Injury Model Systems. 2016 Annual Report –Complete Public Version. University of Alabama at Birmingham. Accessed March 20, 2023. https://www.nscisc.uab.edu/Public/2016%20Annual%20Report%20-%20Complete%20Public%20Version.pdf
2. Macy E, Richter PK, Falkoff R, Zeiger R. Skin testing with penicilloate and penilloate prepared by an improved method: amoxicillin oral challenge in patients with negative skin test responses to penicillin reagents. J Allergy Clin Immunol. 1997;100(5):586-591. doi:10.1016/s0091-6749(97)70159-3 3. Dhopeshwarkar N, Sheikh A, Doan R, et al. Drug-induced anaphylaxis documented in electronic health records. J Allergy Clin Immunol Pract. 2019;7(1):103-111. doi:10.1016/j.jaip.2018.06.010
4. Evans CT, LaVela SL, Weaver FM, et al. Epidemiology of hospital-acquired infections in veterans with spinal cord injury and disorder. Infect Control Hosp Epidemiol. 2008;29(3):234-242. doi:10.1086/527509
5. Evans CT, Jump RL, Krein SL, et al. Setting a research agenda in prevention of healthcare-associated infections (HAIs) and multidrug-resistant organisms (MDROs) outside of acute care settings. Infect Control Hosp Epidemiol. 2018;39(2):210-213. doi:10.1017/ice.2017.291
6. Blumenthal KG, Peter JG, Trubiano JA, Phllips EJ. Antibiotic allergy. Lancet. 2019;393(10167):183-198. doi:10.1016/S0140-6736(18)32218-9 7. Evans CT, Fitzpatrick MA, Jones MM, et al. Prevalence and factors associated with multidrug-resistant gram-negative organisms in patients with spinal cord injury. Infect Control Hosp Epidemiol. 2017;38(12):1464-1471. doi:10.1017/ice.2017.238 8. Macy E, Poon KYT. Self-reported antibiotic allergy incidence and prevalence: age and sex effects. Am J Med. 2009;122(8):778.e1-778.e7. doi:10.1016/j.amjmed.2009.01.034
9. Zhou L, Dhopeshwarkar N, Blumenthal KG, et al. Drug allergies documented in electronic health records of a large healthcare system. Allergy. 2016;71(9):1305-1313. doi:10.1111/all.12881
10. Gomes E, Cardoso MF, Praça F, Gomes L, Mariño E, Demoly P. Self-reported drug allergy in a general adult Portuguese population. Clin Exp Allergy. 2004;34(10):1597-1601. doi:10.1111/j.1365-2222.2004.02070.x
References
1. National Spinal Cord Injury Statistical Center. Spinal Cord Injury Model Systems. 2016 Annual Report –Complete Public Version. University of Alabama at Birmingham. Accessed March 20, 2023. https://www.nscisc.uab.edu/Public/2016%20Annual%20Report%20-%20Complete%20Public%20Version.pdf
2. Macy E, Richter PK, Falkoff R, Zeiger R. Skin testing with penicilloate and penilloate prepared by an improved method: amoxicillin oral challenge in patients with negative skin test responses to penicillin reagents. J Allergy Clin Immunol. 1997;100(5):586-591. doi:10.1016/s0091-6749(97)70159-3 3. Dhopeshwarkar N, Sheikh A, Doan R, et al. Drug-induced anaphylaxis documented in electronic health records. J Allergy Clin Immunol Pract. 2019;7(1):103-111. doi:10.1016/j.jaip.2018.06.010
4. Evans CT, LaVela SL, Weaver FM, et al. Epidemiology of hospital-acquired infections in veterans with spinal cord injury and disorder. Infect Control Hosp Epidemiol. 2008;29(3):234-242. doi:10.1086/527509
5. Evans CT, Jump RL, Krein SL, et al. Setting a research agenda in prevention of healthcare-associated infections (HAIs) and multidrug-resistant organisms (MDROs) outside of acute care settings. Infect Control Hosp Epidemiol. 2018;39(2):210-213. doi:10.1017/ice.2017.291
6. Blumenthal KG, Peter JG, Trubiano JA, Phllips EJ. Antibiotic allergy. Lancet. 2019;393(10167):183-198. doi:10.1016/S0140-6736(18)32218-9 7. Evans CT, Fitzpatrick MA, Jones MM, et al. Prevalence and factors associated with multidrug-resistant gram-negative organisms in patients with spinal cord injury. Infect Control Hosp Epidemiol. 2017;38(12):1464-1471. doi:10.1017/ice.2017.238 8. Macy E, Poon KYT. Self-reported antibiotic allergy incidence and prevalence: age and sex effects. Am J Med. 2009;122(8):778.e1-778.e7. doi:10.1016/j.amjmed.2009.01.034
9. Zhou L, Dhopeshwarkar N, Blumenthal KG, et al. Drug allergies documented in electronic health records of a large healthcare system. Allergy. 2016;71(9):1305-1313. doi:10.1111/all.12881
10. Gomes E, Cardoso MF, Praça F, Gomes L, Mariño E, Demoly P. Self-reported drug allergy in a general adult Portuguese population. Clin Exp Allergy. 2004;34(10):1597-1601. doi:10.1111/j.1365-2222.2004.02070.x
Oropharyngeal Squamous Cell Carcinoma Outcomes by p16 INK4a Antigen Status in a Veteran Population
Since 1983, the correlation between head and neck squamous cell carcinoma (SCC) and human papillomavirus (HPV) has been of great interest to head and neck oncologists.1 In 1998, Smith and colleagues provided evidence of HPV as an independent risk factor for the development of head and neck SCC.2 HPV-associated head and neck SCC accounts for between 30% and 64% of oropharyngeal SCC, depending on the published study; tonsil primaries account for the majority of these cancers.3,4
The presence of HPV E6 and E7 oncoproteins leads to the inactivation of p53 and pRb tumor suppressors. Furthermore, Ragin and colleagues discussed a distinct molecular pathway specific to HPV-associated head and neck SCC, which was different from non–HPV-associated head and neck SCC, involving genetic mutations in CDKN2A/p16.5
Current methods in correlating the presence of HPV infection in head and neck SCC have centered on p16INK4a (p16) immunohistochemistry (IHC) staining and DNA in situ hybridization (ISH) for specific HPV DNA types. IHC staining for p16 involves a monoclonal antibody specific to p16. The usefulness of this test relies on p16 overexpression due to the inactivation of pRb by the HPV E7 oncoprotein. This test is readily performed on archived tissue and has a documented sensitivity and specificity of 100% and 79%, respectively, as reported by Singhi and Westra in 2010.6 HPV DNA fluorescence in situ hybridization is the gold standard for determining the presence of specific types of HPV DNA; however, p16IHC can serve as a rapid, less costly means of studying archived tissue, lending its utility to retrospective population-based studies.
METHODS
A retrospective study was designed to determine the proportion of HPV-associated oropharyngeal SCC in a US Department of Veterans Affairs (VA) population, using p16antigen IHC on paraffin-embedded tissue as the surrogate marker for the presence of HPV infection. Patients consisted of veterans who were treated for oropharyngeal SCC at Veterans Affairs Memphis Healthcare System (VAMHS) in Tennessee between January 1, 2000, and December 31, 2008. This data range allowed for at least 5 years of follow-up. Patients were excluded who lacked enough tissue specimens for analysis. Measurement outcomes included p16expression, with subset analysis by race and ethnicity, degree of tobacco and alcohol use, tumor location, stage, age at diagnosis, and survival outcome. Microsoft Excel was used to calculate Fisher exact test, Student t test, and χ2 statistics. Significance was set at P < .05. This study received institutional review board approval from the University of Tennessee Health Science Center and the VAMHS.
RESULTS
We identified 66 total cases of oropharyngeal SCC; 19 cases (29%) were positive for p16. The mean age at diagnosis for the p16-positive cohort was 59 years vs 61 years for the p16-negative cohort (P = .22; Table 1).
Although the tonsil was the most common site of tumor origin in both the p16-positive and negative cohorts (63% vs 51%, respectively), our analysis showed no statistically significant difference in sites of origin (P = .69) (Table 2).
DISCUSSION
The VAMHS population in our study had a lower proportion of HPV-associated oropharyngeal SCC compared with studies on nonveteran populations (29% vs 40%-80%, respectively).5,6 This disparity may indicate a true difference in these populations or may be related to a decreased prevalence of HPV infection in the population served by the VAMHS. This single-institution population did not completely correlate with previous population studies. Specifically, age at presentation (equivalent to patients with p16-negative status rather than earlier age at onset), disease stage at presentation (lower stage for patients with p16-positive status), and disease-specific survival (not improved compared with patients with p16-negative status in other studies) were dissimilar to previous investigations.2,3
The increased age and staging at presentation could be related in these patients with p16-positive status, which may further account for the lack of improved survival. Furthermore, both groups tended to use alcohol at a high proportion; whereas other populations have had a lesser degree of alcohol intake with p16 positivity.1-4 These differences may be due to variations in the habits and behavior of VA patients compared with non-VA patients.3,4
HPV-associated oropharyngeal SCC in published data has been associated with high-risk sexual behavior, lower age, and less tobacco and alcohol use.5,6 No difference was noted in tumor site predilection; however, the small size of our study could explain the lack of finding site preference shown in previous studies.2,3Other veteran-specific factors are absent in the at-large population, such as Agent Orange exposure. More than 8 million veterans (22%) from the Vietnam era self-reported Agent Orange exposure.7 Agent Orange exposure significantly predicted developing upper aerodigestive tract cancer. Oropharyngeal, nasopharyngeal, laryngeal, and thyroid cancers were significantly associated with Agent Orange exposure. Interestingly, these patients experienced an improved 10-year survival rate compared with patients not exposed to Agent Orange. This finding contrasts with our patients, who did not experience improved outcomes vs nonveteran patients with head and neck cancer.7
Suicide in veterans with head and neck cancer has been evaluated and was found at an incidence of 0.7%. Survivors of head and neck cancer are almost twice as likely to die by suicide compared with other cancer survivors. These patients have a higher rate of mental health disorders, substance misuse, and use of palliative care services.8 Sixty-five of 66 of our patients died during the 5-year observation period, although none died by suicide.
In a 2022 cohort study by Sun and colleagues, upfront surgical treatment was associated with a 23% reduced risk of stroke compared with definitive chemoradiotherapy in US veterans with oropharyngeal carcinoma.9 In our study, 58 of 66 patients (88%) received concurrent chemoradiation, possibly reflecting the more advanced stage of diagnosis in our study population. This was due to comorbidities and other health and economic factors. In our study, 43 patients (65%) died of factors not related to the disease, reflecting the overall comorbidity burden of this population. Seven patients (11%) in our 5-year study died of a documented stroke. In the study of veterans by Sun and colleagues, the 10-year cumulative incidence of stroke was 12.5% and death was 57.3%.9 Our veteran population experienced a similar incidence of strokes. These findings may need to be included when discussing the risk-benefit aspects of different treatment options with our veteran patients with oropharyngeal cancer.
To understand the influence of HPV infection on the course of oropharyngeal SCC in the VA patient population and to apply this understanding to future individualized treatment paradigms, this study can be expanded to a greater number of VA patients. p16 immunoexpression appears to be a useful surrogate for high-risk HPV infection in oropharyngeal SCC, and its ease of use supports its feasibility in further VA population analysis.10 While realizing that the veteran HPV-associated oropharyngeal SCC population differs from the civilian HPV-associated oropharyngeal SCC population, we also have realized that other unique considerations in the veteran population, such as chemical warfare exposure, mental illness, and vascular disease, complicate treatment decisions in these patients.
CONCLUSIONS
Disparities in racial distribution and tobacco use between patients with p16-positive and p16-negative status are similar to those reported in non-VA populations. In contrast, the frequently reported younger age at presentation and better disease outcomes seen in non-VA patients were not observed, perhaps due to the lower percentage of p16expression in VA patients with oropharyngeal SCC. Whereas de-intensification of therapy may be considered for many patients with oropharygeal cancer that is HPV-associated because of improved prognosis, this approach should be undertaken with great care in this group of patients. Personalization of therapy for these HPV-associated oropharyngeal SCC in the veteran population must be adapted to mitigate this critical disparity.
1. Syrjänen K, Syrjänen S, Lamberg M, Pyrhönen S, Nuutinen J. Morphological and immunohistochemical evidence suggesting human papillomavirus (HPV) involvement in oral squamous cell carcinogenesis. Int J Oral Surg. 1983;12(6):418-424. doi:10.1016/s0300-9785(83)80033-7
2. Smith EM, Hoffman HT, Summersgill KS, Kirchner HL, Turek LP, Haugen TH. Human papillomavirus and risk of oral cancer. Laryngoscope. 1998;108(7):1098-1103. doi:10.1097/00005537-199807000-00027
3. Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24-35. doi:10.1056/NEJMoa0912217
4. Ragin CC, Taioli E. Survival of squamous cell carcinoma of the head and neck in relation to human papillomavirus infection: review and meta-analysis. Int J Cancer. 2007;121(8):1813-1820. doi:10.1002/ijc.22851
5. Ragin CC, Taioli E, Weissfeld JL, et al. 11q13 amplification status and human papillomavirus in relation to p16 expression defines two distinct etiologies of head and neck tumours. Br J Cancer. 2006;95(10):1432-1438. doi:10.1038/sj.bjc.6603394
6. Singhi AD, Westra WH. Comparison of human papillomavirus in situ hybridization and p16 immunohistochemistry in the detection of human papillomavirus-associated head and neck cancer based on a prospective clinical experience. Cancer. 2010;116(9):2166-2173. doi:10.1002/cncr.25033
7. Mowery A, Conlin M, Clayburgh D. Increased risk of head and neck cancer in Agent Orange exposed Vietnam Era veterans. Oral Oncol. 2020;100:104483. doi:10.1016/j.oraloncology.2019.104483
8. Nugent SM, Morasco BJ, Handley R, et al. Risk of suicidal self-directed violence among US veteran survivors of head and neck cancer. JAMA Otolaryngol Head Neck Surg. 2021;147(11):981-989. doi:10.1001/jamaoto.2021.2625
9. Sun L, Brody R, Candelieri D, et al. Association between up-front surgery and risk of stroke in US veterans with oropharyngeal carcinoma. JAMA Otolaryngol Head Neck Surg. 2022;148(8):740-747. doi:10.1001/jamaoto.2022.1327
10. El-Naggar AK, Westra WH. p16 expression as a surrogate marker for HPV-related oropharyngeal carcinoma: a guide for interpretative relevance and consistency. Head Neck. 2012;34(4):459-461. doi:10.1002/hed.21974
Since 1983, the correlation between head and neck squamous cell carcinoma (SCC) and human papillomavirus (HPV) has been of great interest to head and neck oncologists.1 In 1998, Smith and colleagues provided evidence of HPV as an independent risk factor for the development of head and neck SCC.2 HPV-associated head and neck SCC accounts for between 30% and 64% of oropharyngeal SCC, depending on the published study; tonsil primaries account for the majority of these cancers.3,4
The presence of HPV E6 and E7 oncoproteins leads to the inactivation of p53 and pRb tumor suppressors. Furthermore, Ragin and colleagues discussed a distinct molecular pathway specific to HPV-associated head and neck SCC, which was different from non–HPV-associated head and neck SCC, involving genetic mutations in CDKN2A/p16.5
Current methods in correlating the presence of HPV infection in head and neck SCC have centered on p16INK4a (p16) immunohistochemistry (IHC) staining and DNA in situ hybridization (ISH) for specific HPV DNA types. IHC staining for p16 involves a monoclonal antibody specific to p16. The usefulness of this test relies on p16 overexpression due to the inactivation of pRb by the HPV E7 oncoprotein. This test is readily performed on archived tissue and has a documented sensitivity and specificity of 100% and 79%, respectively, as reported by Singhi and Westra in 2010.6 HPV DNA fluorescence in situ hybridization is the gold standard for determining the presence of specific types of HPV DNA; however, p16IHC can serve as a rapid, less costly means of studying archived tissue, lending its utility to retrospective population-based studies.
METHODS
A retrospective study was designed to determine the proportion of HPV-associated oropharyngeal SCC in a US Department of Veterans Affairs (VA) population, using p16antigen IHC on paraffin-embedded tissue as the surrogate marker for the presence of HPV infection. Patients consisted of veterans who were treated for oropharyngeal SCC at Veterans Affairs Memphis Healthcare System (VAMHS) in Tennessee between January 1, 2000, and December 31, 2008. This data range allowed for at least 5 years of follow-up. Patients were excluded who lacked enough tissue specimens for analysis. Measurement outcomes included p16expression, with subset analysis by race and ethnicity, degree of tobacco and alcohol use, tumor location, stage, age at diagnosis, and survival outcome. Microsoft Excel was used to calculate Fisher exact test, Student t test, and χ2 statistics. Significance was set at P < .05. This study received institutional review board approval from the University of Tennessee Health Science Center and the VAMHS.
RESULTS
We identified 66 total cases of oropharyngeal SCC; 19 cases (29%) were positive for p16. The mean age at diagnosis for the p16-positive cohort was 59 years vs 61 years for the p16-negative cohort (P = .22; Table 1).
Although the tonsil was the most common site of tumor origin in both the p16-positive and negative cohorts (63% vs 51%, respectively), our analysis showed no statistically significant difference in sites of origin (P = .69) (Table 2).
DISCUSSION
The VAMHS population in our study had a lower proportion of HPV-associated oropharyngeal SCC compared with studies on nonveteran populations (29% vs 40%-80%, respectively).5,6 This disparity may indicate a true difference in these populations or may be related to a decreased prevalence of HPV infection in the population served by the VAMHS. This single-institution population did not completely correlate with previous population studies. Specifically, age at presentation (equivalent to patients with p16-negative status rather than earlier age at onset), disease stage at presentation (lower stage for patients with p16-positive status), and disease-specific survival (not improved compared with patients with p16-negative status in other studies) were dissimilar to previous investigations.2,3
The increased age and staging at presentation could be related in these patients with p16-positive status, which may further account for the lack of improved survival. Furthermore, both groups tended to use alcohol at a high proportion; whereas other populations have had a lesser degree of alcohol intake with p16 positivity.1-4 These differences may be due to variations in the habits and behavior of VA patients compared with non-VA patients.3,4
HPV-associated oropharyngeal SCC in published data has been associated with high-risk sexual behavior, lower age, and less tobacco and alcohol use.5,6 No difference was noted in tumor site predilection; however, the small size of our study could explain the lack of finding site preference shown in previous studies.2,3Other veteran-specific factors are absent in the at-large population, such as Agent Orange exposure. More than 8 million veterans (22%) from the Vietnam era self-reported Agent Orange exposure.7 Agent Orange exposure significantly predicted developing upper aerodigestive tract cancer. Oropharyngeal, nasopharyngeal, laryngeal, and thyroid cancers were significantly associated with Agent Orange exposure. Interestingly, these patients experienced an improved 10-year survival rate compared with patients not exposed to Agent Orange. This finding contrasts with our patients, who did not experience improved outcomes vs nonveteran patients with head and neck cancer.7
Suicide in veterans with head and neck cancer has been evaluated and was found at an incidence of 0.7%. Survivors of head and neck cancer are almost twice as likely to die by suicide compared with other cancer survivors. These patients have a higher rate of mental health disorders, substance misuse, and use of palliative care services.8 Sixty-five of 66 of our patients died during the 5-year observation period, although none died by suicide.
In a 2022 cohort study by Sun and colleagues, upfront surgical treatment was associated with a 23% reduced risk of stroke compared with definitive chemoradiotherapy in US veterans with oropharyngeal carcinoma.9 In our study, 58 of 66 patients (88%) received concurrent chemoradiation, possibly reflecting the more advanced stage of diagnosis in our study population. This was due to comorbidities and other health and economic factors. In our study, 43 patients (65%) died of factors not related to the disease, reflecting the overall comorbidity burden of this population. Seven patients (11%) in our 5-year study died of a documented stroke. In the study of veterans by Sun and colleagues, the 10-year cumulative incidence of stroke was 12.5% and death was 57.3%.9 Our veteran population experienced a similar incidence of strokes. These findings may need to be included when discussing the risk-benefit aspects of different treatment options with our veteran patients with oropharyngeal cancer.
To understand the influence of HPV infection on the course of oropharyngeal SCC in the VA patient population and to apply this understanding to future individualized treatment paradigms, this study can be expanded to a greater number of VA patients. p16 immunoexpression appears to be a useful surrogate for high-risk HPV infection in oropharyngeal SCC, and its ease of use supports its feasibility in further VA population analysis.10 While realizing that the veteran HPV-associated oropharyngeal SCC population differs from the civilian HPV-associated oropharyngeal SCC population, we also have realized that other unique considerations in the veteran population, such as chemical warfare exposure, mental illness, and vascular disease, complicate treatment decisions in these patients.
CONCLUSIONS
Disparities in racial distribution and tobacco use between patients with p16-positive and p16-negative status are similar to those reported in non-VA populations. In contrast, the frequently reported younger age at presentation and better disease outcomes seen in non-VA patients were not observed, perhaps due to the lower percentage of p16expression in VA patients with oropharyngeal SCC. Whereas de-intensification of therapy may be considered for many patients with oropharygeal cancer that is HPV-associated because of improved prognosis, this approach should be undertaken with great care in this group of patients. Personalization of therapy for these HPV-associated oropharyngeal SCC in the veteran population must be adapted to mitigate this critical disparity.
Since 1983, the correlation between head and neck squamous cell carcinoma (SCC) and human papillomavirus (HPV) has been of great interest to head and neck oncologists.1 In 1998, Smith and colleagues provided evidence of HPV as an independent risk factor for the development of head and neck SCC.2 HPV-associated head and neck SCC accounts for between 30% and 64% of oropharyngeal SCC, depending on the published study; tonsil primaries account for the majority of these cancers.3,4
The presence of HPV E6 and E7 oncoproteins leads to the inactivation of p53 and pRb tumor suppressors. Furthermore, Ragin and colleagues discussed a distinct molecular pathway specific to HPV-associated head and neck SCC, which was different from non–HPV-associated head and neck SCC, involving genetic mutations in CDKN2A/p16.5
Current methods in correlating the presence of HPV infection in head and neck SCC have centered on p16INK4a (p16) immunohistochemistry (IHC) staining and DNA in situ hybridization (ISH) for specific HPV DNA types. IHC staining for p16 involves a monoclonal antibody specific to p16. The usefulness of this test relies on p16 overexpression due to the inactivation of pRb by the HPV E7 oncoprotein. This test is readily performed on archived tissue and has a documented sensitivity and specificity of 100% and 79%, respectively, as reported by Singhi and Westra in 2010.6 HPV DNA fluorescence in situ hybridization is the gold standard for determining the presence of specific types of HPV DNA; however, p16IHC can serve as a rapid, less costly means of studying archived tissue, lending its utility to retrospective population-based studies.
METHODS
A retrospective study was designed to determine the proportion of HPV-associated oropharyngeal SCC in a US Department of Veterans Affairs (VA) population, using p16antigen IHC on paraffin-embedded tissue as the surrogate marker for the presence of HPV infection. Patients consisted of veterans who were treated for oropharyngeal SCC at Veterans Affairs Memphis Healthcare System (VAMHS) in Tennessee between January 1, 2000, and December 31, 2008. This data range allowed for at least 5 years of follow-up. Patients were excluded who lacked enough tissue specimens for analysis. Measurement outcomes included p16expression, with subset analysis by race and ethnicity, degree of tobacco and alcohol use, tumor location, stage, age at diagnosis, and survival outcome. Microsoft Excel was used to calculate Fisher exact test, Student t test, and χ2 statistics. Significance was set at P < .05. This study received institutional review board approval from the University of Tennessee Health Science Center and the VAMHS.
RESULTS
We identified 66 total cases of oropharyngeal SCC; 19 cases (29%) were positive for p16. The mean age at diagnosis for the p16-positive cohort was 59 years vs 61 years for the p16-negative cohort (P = .22; Table 1).
Although the tonsil was the most common site of tumor origin in both the p16-positive and negative cohorts (63% vs 51%, respectively), our analysis showed no statistically significant difference in sites of origin (P = .69) (Table 2).
DISCUSSION
The VAMHS population in our study had a lower proportion of HPV-associated oropharyngeal SCC compared with studies on nonveteran populations (29% vs 40%-80%, respectively).5,6 This disparity may indicate a true difference in these populations or may be related to a decreased prevalence of HPV infection in the population served by the VAMHS. This single-institution population did not completely correlate with previous population studies. Specifically, age at presentation (equivalent to patients with p16-negative status rather than earlier age at onset), disease stage at presentation (lower stage for patients with p16-positive status), and disease-specific survival (not improved compared with patients with p16-negative status in other studies) were dissimilar to previous investigations.2,3
The increased age and staging at presentation could be related in these patients with p16-positive status, which may further account for the lack of improved survival. Furthermore, both groups tended to use alcohol at a high proportion; whereas other populations have had a lesser degree of alcohol intake with p16 positivity.1-4 These differences may be due to variations in the habits and behavior of VA patients compared with non-VA patients.3,4
HPV-associated oropharyngeal SCC in published data has been associated with high-risk sexual behavior, lower age, and less tobacco and alcohol use.5,6 No difference was noted in tumor site predilection; however, the small size of our study could explain the lack of finding site preference shown in previous studies.2,3Other veteran-specific factors are absent in the at-large population, such as Agent Orange exposure. More than 8 million veterans (22%) from the Vietnam era self-reported Agent Orange exposure.7 Agent Orange exposure significantly predicted developing upper aerodigestive tract cancer. Oropharyngeal, nasopharyngeal, laryngeal, and thyroid cancers were significantly associated with Agent Orange exposure. Interestingly, these patients experienced an improved 10-year survival rate compared with patients not exposed to Agent Orange. This finding contrasts with our patients, who did not experience improved outcomes vs nonveteran patients with head and neck cancer.7
Suicide in veterans with head and neck cancer has been evaluated and was found at an incidence of 0.7%. Survivors of head and neck cancer are almost twice as likely to die by suicide compared with other cancer survivors. These patients have a higher rate of mental health disorders, substance misuse, and use of palliative care services.8 Sixty-five of 66 of our patients died during the 5-year observation period, although none died by suicide.
In a 2022 cohort study by Sun and colleagues, upfront surgical treatment was associated with a 23% reduced risk of stroke compared with definitive chemoradiotherapy in US veterans with oropharyngeal carcinoma.9 In our study, 58 of 66 patients (88%) received concurrent chemoradiation, possibly reflecting the more advanced stage of diagnosis in our study population. This was due to comorbidities and other health and economic factors. In our study, 43 patients (65%) died of factors not related to the disease, reflecting the overall comorbidity burden of this population. Seven patients (11%) in our 5-year study died of a documented stroke. In the study of veterans by Sun and colleagues, the 10-year cumulative incidence of stroke was 12.5% and death was 57.3%.9 Our veteran population experienced a similar incidence of strokes. These findings may need to be included when discussing the risk-benefit aspects of different treatment options with our veteran patients with oropharyngeal cancer.
To understand the influence of HPV infection on the course of oropharyngeal SCC in the VA patient population and to apply this understanding to future individualized treatment paradigms, this study can be expanded to a greater number of VA patients. p16 immunoexpression appears to be a useful surrogate for high-risk HPV infection in oropharyngeal SCC, and its ease of use supports its feasibility in further VA population analysis.10 While realizing that the veteran HPV-associated oropharyngeal SCC population differs from the civilian HPV-associated oropharyngeal SCC population, we also have realized that other unique considerations in the veteran population, such as chemical warfare exposure, mental illness, and vascular disease, complicate treatment decisions in these patients.
CONCLUSIONS
Disparities in racial distribution and tobacco use between patients with p16-positive and p16-negative status are similar to those reported in non-VA populations. In contrast, the frequently reported younger age at presentation and better disease outcomes seen in non-VA patients were not observed, perhaps due to the lower percentage of p16expression in VA patients with oropharyngeal SCC. Whereas de-intensification of therapy may be considered for many patients with oropharygeal cancer that is HPV-associated because of improved prognosis, this approach should be undertaken with great care in this group of patients. Personalization of therapy for these HPV-associated oropharyngeal SCC in the veteran population must be adapted to mitigate this critical disparity.
1. Syrjänen K, Syrjänen S, Lamberg M, Pyrhönen S, Nuutinen J. Morphological and immunohistochemical evidence suggesting human papillomavirus (HPV) involvement in oral squamous cell carcinogenesis. Int J Oral Surg. 1983;12(6):418-424. doi:10.1016/s0300-9785(83)80033-7
2. Smith EM, Hoffman HT, Summersgill KS, Kirchner HL, Turek LP, Haugen TH. Human papillomavirus and risk of oral cancer. Laryngoscope. 1998;108(7):1098-1103. doi:10.1097/00005537-199807000-00027
3. Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24-35. doi:10.1056/NEJMoa0912217
4. Ragin CC, Taioli E. Survival of squamous cell carcinoma of the head and neck in relation to human papillomavirus infection: review and meta-analysis. Int J Cancer. 2007;121(8):1813-1820. doi:10.1002/ijc.22851
5. Ragin CC, Taioli E, Weissfeld JL, et al. 11q13 amplification status and human papillomavirus in relation to p16 expression defines two distinct etiologies of head and neck tumours. Br J Cancer. 2006;95(10):1432-1438. doi:10.1038/sj.bjc.6603394
6. Singhi AD, Westra WH. Comparison of human papillomavirus in situ hybridization and p16 immunohistochemistry in the detection of human papillomavirus-associated head and neck cancer based on a prospective clinical experience. Cancer. 2010;116(9):2166-2173. doi:10.1002/cncr.25033
7. Mowery A, Conlin M, Clayburgh D. Increased risk of head and neck cancer in Agent Orange exposed Vietnam Era veterans. Oral Oncol. 2020;100:104483. doi:10.1016/j.oraloncology.2019.104483
8. Nugent SM, Morasco BJ, Handley R, et al. Risk of suicidal self-directed violence among US veteran survivors of head and neck cancer. JAMA Otolaryngol Head Neck Surg. 2021;147(11):981-989. doi:10.1001/jamaoto.2021.2625
9. Sun L, Brody R, Candelieri D, et al. Association between up-front surgery and risk of stroke in US veterans with oropharyngeal carcinoma. JAMA Otolaryngol Head Neck Surg. 2022;148(8):740-747. doi:10.1001/jamaoto.2022.1327
10. El-Naggar AK, Westra WH. p16 expression as a surrogate marker for HPV-related oropharyngeal carcinoma: a guide for interpretative relevance and consistency. Head Neck. 2012;34(4):459-461. doi:10.1002/hed.21974
1. Syrjänen K, Syrjänen S, Lamberg M, Pyrhönen S, Nuutinen J. Morphological and immunohistochemical evidence suggesting human papillomavirus (HPV) involvement in oral squamous cell carcinogenesis. Int J Oral Surg. 1983;12(6):418-424. doi:10.1016/s0300-9785(83)80033-7
2. Smith EM, Hoffman HT, Summersgill KS, Kirchner HL, Turek LP, Haugen TH. Human papillomavirus and risk of oral cancer. Laryngoscope. 1998;108(7):1098-1103. doi:10.1097/00005537-199807000-00027
3. Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24-35. doi:10.1056/NEJMoa0912217
4. Ragin CC, Taioli E. Survival of squamous cell carcinoma of the head and neck in relation to human papillomavirus infection: review and meta-analysis. Int J Cancer. 2007;121(8):1813-1820. doi:10.1002/ijc.22851
5. Ragin CC, Taioli E, Weissfeld JL, et al. 11q13 amplification status and human papillomavirus in relation to p16 expression defines two distinct etiologies of head and neck tumours. Br J Cancer. 2006;95(10):1432-1438. doi:10.1038/sj.bjc.6603394
6. Singhi AD, Westra WH. Comparison of human papillomavirus in situ hybridization and p16 immunohistochemistry in the detection of human papillomavirus-associated head and neck cancer based on a prospective clinical experience. Cancer. 2010;116(9):2166-2173. doi:10.1002/cncr.25033
7. Mowery A, Conlin M, Clayburgh D. Increased risk of head and neck cancer in Agent Orange exposed Vietnam Era veterans. Oral Oncol. 2020;100:104483. doi:10.1016/j.oraloncology.2019.104483
8. Nugent SM, Morasco BJ, Handley R, et al. Risk of suicidal self-directed violence among US veteran survivors of head and neck cancer. JAMA Otolaryngol Head Neck Surg. 2021;147(11):981-989. doi:10.1001/jamaoto.2021.2625
9. Sun L, Brody R, Candelieri D, et al. Association between up-front surgery and risk of stroke in US veterans with oropharyngeal carcinoma. JAMA Otolaryngol Head Neck Surg. 2022;148(8):740-747. doi:10.1001/jamaoto.2022.1327
10. El-Naggar AK, Westra WH. p16 expression as a surrogate marker for HPV-related oropharyngeal carcinoma: a guide for interpretative relevance and consistency. Head Neck. 2012;34(4):459-461. doi:10.1002/hed.21974
Outcomes in Patients With Curative Malignancies Receiving Filgrastim as Primary Prophylaxis
Febrile neutropenia (FN) frequently occurs in patients receiving chemotherapy, with the greatest risk of complications occurring in those who experience profound and prolonged neutropenia. Although granulocyte colony-stimulating factor (G-CSF) prophylaxis has been shown to reduce the risk and duration of chemotherapy-induced neutropenia and FN, there is no well-established optimal regimen.1 The 2022 National Comprehensive Cancer Network guidelines for hematopoietic growth factors recommend prophylaxis with G-CSF in at-risk patients receiving chemotherapy, specifically in chemotherapy regimens considered high risk for FN (incidence > 20%) or intermediate risk for FN (incidence 10%-20%) with additional patient risk factors.2 The incidence of developing FN with at least 1 chemotherapy cycle is estimated at 10% to 50% of patients with solid tumors and > 80% of patients with hematologic malignancies.3 The rate of major complications (eg, hypotension, acute renal, respiratory, or heart failure) in the context of FN is 25% to 30%, and mortality is reported up to 11% in this population.4
Because of the significant consequences of neutropenia and FN, prevention is imperative due to the increase in morbidity and mortality, including chemotherapy delays, increased hospitalizations, chemotherapy dose reductions, and discontinuations that cause delays in care.5 In patients with curative malignancies, these consequences can negatively impact treatment efficacy and overall survival. Additionally, infections occur in 20% to 30% of patients with febrile episodes. Although fever is often the only clinical sign or symptom of infection, patients who are profoundly neutropenic may present with suspected infection and be afebrile or hypothermic.3
For filgrastim, the National Comprehensive Cancer Network guidelines do not specify the total days of required injections but state that a daily dose should be given until the postnadir absolute neutrophil count (ANC) recovers to normal or near normal levels by laboratory standards.2 It is uncommon in clinical practice to track postnadir ANCs due to frequent laboratory monitoring. Clinical trial data suggest an average duration of 11 days of daily filgrastim injections for ANC recovery; however, real-world data exist supporting a range from 4 to 10 days with a median of 7 injections per cycle for prevention of neutropenia or FN.6,7
At the South Texas Veterans Health Care System in San Antonio, daily filgrastim injections are preferred due to cost; patients typically receive a 7-day course for primary prophylaxis for FN.
Electronic health record reviews at the South Texas Veterans Health Care System were performed to identify patients who received filgrastim primary prophylaxis (defined as filgrastim, tbo-filgrastim, or filgrastim-sndz) for a curative cancer diagnosis. Primary prophylaxis refers to the administration of G-CSF in the first cycle of chemotherapy before the onset of neutropenia. Patients received filgrastim prophylaxis if they were undergoing treatment with a chemotherapy regimen with either high risk for FN or a chemotherapy regimen with an intermediate risk for FN and additional patient risk factors. Risk factors for patients included prior chemotherapy or radiation therapy; persistent neutropenia; bone marrow involvement by tumor; recent surgery and/or open wounds; liver dysfunction (defined as total bilirubin > 2 mg/dL); renal dysfunction (defined as creatinine clearance < 50 mL/min); and those aged > 65 years receiving full chemotherapy dose intensity. Neutropenia is defined as a decrease in ANC < 1000 neutrophils/μL, whereas FN is defined as a single temperature of > 38.3 °C or > 38.0 °C for longer than 1 hour with < 500 neutrophils/μL or < 1000 neutrophils/μL predicted to decline to < 500 neutrophils/μL over the next 48 hours. All patients had their filgrastim dispensed for home administration during their chemotherapy appointment.
Descriptive statistics were used to summarize the study population and their health outcomes. Fisher exact test was used to compare FN incidence for high- and intermediate-risk FN groups.
RESULTS
Between September 1, 2015, and September 24, 2020, 381 patients received filgrastim. Of these patients, 59 met the inclusion criteria. Patients receiving filgrastim were excluded due to stem cell transplant mobilization/engraftment (n = 145), a noncurative cancer diagnosis (n = 134), use as a secondary prophylaxis (n = 33), and nononcologic neutropenia (n = 8). Additionally, 2 patients initially received pegfilgrastim and were not included in this data set.
The median (IQR) age was 64 (55-70) years and 42 patients (71%) were male (Table 1).
Ten patients (17%) experienced dose delays despite filgrastim use (Table 2).
Nine patients (15%) had the number of filgrastim injections per chemotherapy cycle extended due to various reasons. Five patients required extended days after hospitalization for FN, 3 patients for dose delays due to neutropenia with the previous cycle, and 1 patient with an undocumented reason outside of the prespecified outcomes. Two of these patients experienced continued neutropenia and dose delays after extending filgrastim from 5 to 7 days or 7 to 10 days. One patient who experienced continued neutropenia after extending filgrastim to 10 days was subsequently transitioned to pegfilgrastim without further episodes of neutropenia. The other patient who still experienced neutropenia after extending filgrastim to 7 days was receiving the last chemotherapy cycle and did not require subsequent doses of filgrastim.
Two additional patients were not included in the hospitalizations. The first was a patient on a chemotherapy regimen with a high risk for FN who presented to the emergency department with documented FN but was never admitted since the patient elected to not be hospitalized. This patient developed oral, anal, and vaginal candidiasis, and it was noted by the oncologist at the next clinic visit that this was likely secondary to grade 4 neutropenia (ANC < 500 neutrophils/μL). The second was a patient on a chemotherapy regimen with an intermediate risk for FN who was already hospitalized but had developed FN and sepsis despite filgrastim use.
Finally, out of the hospitalized patients, 9 (15%) had infections. This included 6 patients (18%) in the high risk for FN group and 3 patients (12%) in the intermediate risk for FN group (P = .72). Six patients transitioned to pegfilgrastim for hospitalization, 2 for neutropenia, and 1 for an unspecified reason. Nine patients (15%) who received filgrastim ended up transitioning to pegfilgrastim; 6 (67%) of these patients were transitioned due to hospitalization for FN. Of all the patients who transitioned to pegfilgrastim, 1 patient on a high risk for FN regimen developed sepsis due to herpes zoster in the setting of neutropenia after the previous cycle of chemotherapy.
Real-world data are limited regarding G-CSF practice patterns; however, available data demonstrate patients may receive suboptimal treatment courses of filgrastim leading to increased complications associated with neutropenia and FN, such as dose delays and hospitalizations.8,9 At the South Texas Veterans Health Care System, 48 patients (81%) received a filgrastim course of ≥ 7 days as an initial course for primary prophylaxis. Multivariate analyses performed by Weycker and colleagues described a decreased risk of hospitalization for neutropenia or FN with each additional day of filgrastim prophylaxis; however, such analysis could not be performed in our data set due to the small sample size.8 In this review, 10 patients (17%) experienced treatment delays due to neutropenia or FN, mirroring previously published data. The hospitalization rate of 25% is higher than the published incidence of 5.2% of cancer-related hospitalizations among adults.7,10 This difference may be explained by a difference in health care access for the veteran population.
As an alternative to daily filgrastim injections, the National Comprehensive Cancer Network also recommends a single dose of pegfilgrastim for primary prevention of FN. Efficacy benefits of pegfilgrastim use include increased patient adherence due to a single injection, a reduction in FN incidence and FN-related hospitalizations, and improved time to ANC recovery compared with filgrastim.11 There are reports suggesting pegfilgrastim significantly reduces neutropenia and FN incidence to a greater extent compared with daily filgrastim injections.6 In patients with breast cancer receiving dose-dense adjuvant chemotherapy, there are data demonstrating that patients who received filgrastim were more likely to experience severe neutropenia, dose reductions, and treatment delays leading to lower dose density compared with pegfilgrastim.12 Of the 19 patients with breast cancer included in our population, 26% experienced one of the previously described outcomes leading to either extensions of daily filgrastim injections or transitions to pegfilgrastim to successfully maintain dose density. In patients with acute myeloid leukemia receiving consolidation chemotherapy, filgrastim was found to be associated with a statistically significant increased risk of hospitalizations compared with pegfilgrastim.13 The one patient with acute myeloid leukemia included in our study did not require additional hospitalizations for neutropenia or FN after transitioning to pegfilgrastim.
Given the cost advantage, the South Texas Veterans Health Care System continues to prefer daily filgrastim injections. A recent survey demonstrated that 73% of patients at 23 sites in the Veterans Health Administration used filgrastim rather than pegfilgrastim for cost savings, although it is recognized that daily filgrastim injections are less convenient for patients.14 This analysis did not review costs associated with hospitalization for FN or the appropriateness of G-CSF use. Cancer-related neutropenia accounts for 8.3% of all cancer-related hospitalization costs among adults; the average hospitalization costs nearly $25,000 per stay and about $2.3 billion among adult patients with cancer annually.10,15
Limitations
This study has limitations that affected the applicability and interpretation of the results. This included the study design since it was a retrospective, single-center, descriptive cohort study. Patient adherence to daily filgrastim injections could not be assessed due to the retrospective nature of the study. The small sample size of 59 patients was prohibitive for utilization of additional analytical tools. Additionally, the predominately male veteran population may make applicability to non-VA populations restrictive.
CONCLUSIONS
Based on the incidence of primary and secondary outcomes associated with using daily filgrastim injections as primary prophylaxis in this study, additional measures such as tracking postnadir ANCs should be performed to ensure patients receive an appropriate number of filgrastim doses to prevent complications associated with neutropenia.
Acknowledgments
We thank Eric Dougherty, PharmD, for assistance in producing granulocyte colony-stimulating factor data.
1. Hanna KS, Mancini R, Wilson D, Zuckerman D. Comparing granulocyte colony-stimulating factors prescribing practices versus guideline recommendations in a large community cancer center. J Hematol Oncol Pharm. 2019;9(3):121-126.
2. Griffiths EA, Roy V, Alwan L, et al. NCCN Guidelines insights: hematopoietic growth factors, version 1.2022. J Natl Compr Canc Netw. 2022;20(5):436-442. doi:10.6004/jnccn.2022.0026
3. Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2011;52(4):e56-e93. doi:10.1093/cid/cir073
4. Taplitz RA, Kennedy EB, Bow EJ, et al. Outpatient management of fever and neutropenia in adults treated for malignancy: American Society of Clinical Oncology and Infectious Diseases Society of America Clinical practice guideline update. J Clin Oncol. 2018;36(14):1443-1453. doi:10.1200/JCO.2017.77.6211
5. Clemons M, Fergusson D, Simos D, et al. A multicentre, randomized trial comparing schedules of G-CSF (filgrastim) administration for primary prophylaxis of chemotherapy induced febrile neutropenia in early stage breast cancer. Ann Oncol. 2020;31(7):951-957. doi:10.1016/j.annonc.2020.04.005
6. Cooper KL, Madan J, Whyte S, Stevenson MD, Akehurst RL. Granulocyte colony-stimulating factors for febrile neutropenia prophylaxis following chemotherapy: systematic review and meta-analysis. BMC Cancer. 2011;11:404. Published 2011 Sep 23. doi:10.1186/1471-2407-11-404
7. Altwairgi A, Hopman W, Mates M. Real-world impact of granulocyte-colony stimulating factor on febrile neutropenia. Curr Oncol. 2013;20(3):e171-e179. doi:10.3747/co.20.1306
8. Weycker D, Hackett J, Edelsberg JS, Oster G, Glass AG. Are shorter courses of filgrastim prophylaxis associated with increased risk of hospitalization? Ann Pharmacother. 2006;40(3):402-407. doi:10.1345/aph.1G516
9. Link H, Nietsch J, Kerkmann M, Ortner P; Supportive Care Group (ASORS) of the German Cancer Society (DKG). Adherence to granulocyte-colony stimulating factor (G-CSF) guidelines to reduce the incidence of febrile neutropenia after chemotherapy—a representative sample survey in Germany. Support Care Cancer. 2016;24(1):367-376. doi:10.1007/s00520-015-2779-5
10. Kuderer NM, Dale DC, Crawford J, Cosler LE, Lyman GH. Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer. 2006;106(10):2258-2266. doi:10.1002/cncr.21847
11. Aapro M, Boccia R, Leonard R, et al. Refining the role of pegfilgrastim (a long-acting G-CSF) for prevention of chemotherapy-induced febrile neutropenia: consensus guidance recommendations. Support Care Cancer. 2017;25(11):3295-3304. doi :10.1007/s00520-017-3842-1
12. Kourlaba G, Dimopoulos MA, Pectasides D, et al. Comparison of filgrastim and pegfilgrastim to prevent neutropenia and maintain dose intensity of adjuvant chemotherapy in patients with breast cancer. Support Care Cancer. 2015;23(7):2045-2051. doi:10.1007/s00520-014-2555-y
13. Field E, Caimi PF, Cooper B, et al. Comparison of pegfilgrastim and filgrastim to prevent neutropenic fever during consolidation with high dose cytarabine for acute myeloid leukemia. Blood. 2018;132(suppl 1):1404. doi:10.1182/blood-2018-99-118336
14. Knopf K, Hrureshky W, Love BL, Norris L, Bennett CL. Cost-effective use of white blood cell growth factors in the Veterans Administration. Blood. 2018;132(suppl 1):4761. doi:10.1182/blood-2018-99-119724
15. Tai E, Guy GP, Dunbar A, Richardson LC. Cost of cancer-related neutropenia or fever hospitalizations, United States, 2012. J Oncol Pract. 2017;13(6):e552-e561. doi:10.1200/JOP.2016.019588
Febrile neutropenia (FN) frequently occurs in patients receiving chemotherapy, with the greatest risk of complications occurring in those who experience profound and prolonged neutropenia. Although granulocyte colony-stimulating factor (G-CSF) prophylaxis has been shown to reduce the risk and duration of chemotherapy-induced neutropenia and FN, there is no well-established optimal regimen.1 The 2022 National Comprehensive Cancer Network guidelines for hematopoietic growth factors recommend prophylaxis with G-CSF in at-risk patients receiving chemotherapy, specifically in chemotherapy regimens considered high risk for FN (incidence > 20%) or intermediate risk for FN (incidence 10%-20%) with additional patient risk factors.2 The incidence of developing FN with at least 1 chemotherapy cycle is estimated at 10% to 50% of patients with solid tumors and > 80% of patients with hematologic malignancies.3 The rate of major complications (eg, hypotension, acute renal, respiratory, or heart failure) in the context of FN is 25% to 30%, and mortality is reported up to 11% in this population.4
Because of the significant consequences of neutropenia and FN, prevention is imperative due to the increase in morbidity and mortality, including chemotherapy delays, increased hospitalizations, chemotherapy dose reductions, and discontinuations that cause delays in care.5 In patients with curative malignancies, these consequences can negatively impact treatment efficacy and overall survival. Additionally, infections occur in 20% to 30% of patients with febrile episodes. Although fever is often the only clinical sign or symptom of infection, patients who are profoundly neutropenic may present with suspected infection and be afebrile or hypothermic.3
For filgrastim, the National Comprehensive Cancer Network guidelines do not specify the total days of required injections but state that a daily dose should be given until the postnadir absolute neutrophil count (ANC) recovers to normal or near normal levels by laboratory standards.2 It is uncommon in clinical practice to track postnadir ANCs due to frequent laboratory monitoring. Clinical trial data suggest an average duration of 11 days of daily filgrastim injections for ANC recovery; however, real-world data exist supporting a range from 4 to 10 days with a median of 7 injections per cycle for prevention of neutropenia or FN.6,7
At the South Texas Veterans Health Care System in San Antonio, daily filgrastim injections are preferred due to cost; patients typically receive a 7-day course for primary prophylaxis for FN.
Electronic health record reviews at the South Texas Veterans Health Care System were performed to identify patients who received filgrastim primary prophylaxis (defined as filgrastim, tbo-filgrastim, or filgrastim-sndz) for a curative cancer diagnosis. Primary prophylaxis refers to the administration of G-CSF in the first cycle of chemotherapy before the onset of neutropenia. Patients received filgrastim prophylaxis if they were undergoing treatment with a chemotherapy regimen with either high risk for FN or a chemotherapy regimen with an intermediate risk for FN and additional patient risk factors. Risk factors for patients included prior chemotherapy or radiation therapy; persistent neutropenia; bone marrow involvement by tumor; recent surgery and/or open wounds; liver dysfunction (defined as total bilirubin > 2 mg/dL); renal dysfunction (defined as creatinine clearance < 50 mL/min); and those aged > 65 years receiving full chemotherapy dose intensity. Neutropenia is defined as a decrease in ANC < 1000 neutrophils/μL, whereas FN is defined as a single temperature of > 38.3 °C or > 38.0 °C for longer than 1 hour with < 500 neutrophils/μL or < 1000 neutrophils/μL predicted to decline to < 500 neutrophils/μL over the next 48 hours. All patients had their filgrastim dispensed for home administration during their chemotherapy appointment.
Descriptive statistics were used to summarize the study population and their health outcomes. Fisher exact test was used to compare FN incidence for high- and intermediate-risk FN groups.
RESULTS
Between September 1, 2015, and September 24, 2020, 381 patients received filgrastim. Of these patients, 59 met the inclusion criteria. Patients receiving filgrastim were excluded due to stem cell transplant mobilization/engraftment (n = 145), a noncurative cancer diagnosis (n = 134), use as a secondary prophylaxis (n = 33), and nononcologic neutropenia (n = 8). Additionally, 2 patients initially received pegfilgrastim and were not included in this data set.
The median (IQR) age was 64 (55-70) years and 42 patients (71%) were male (Table 1).
Ten patients (17%) experienced dose delays despite filgrastim use (Table 2).
Nine patients (15%) had the number of filgrastim injections per chemotherapy cycle extended due to various reasons. Five patients required extended days after hospitalization for FN, 3 patients for dose delays due to neutropenia with the previous cycle, and 1 patient with an undocumented reason outside of the prespecified outcomes. Two of these patients experienced continued neutropenia and dose delays after extending filgrastim from 5 to 7 days or 7 to 10 days. One patient who experienced continued neutropenia after extending filgrastim to 10 days was subsequently transitioned to pegfilgrastim without further episodes of neutropenia. The other patient who still experienced neutropenia after extending filgrastim to 7 days was receiving the last chemotherapy cycle and did not require subsequent doses of filgrastim.
Two additional patients were not included in the hospitalizations. The first was a patient on a chemotherapy regimen with a high risk for FN who presented to the emergency department with documented FN but was never admitted since the patient elected to not be hospitalized. This patient developed oral, anal, and vaginal candidiasis, and it was noted by the oncologist at the next clinic visit that this was likely secondary to grade 4 neutropenia (ANC < 500 neutrophils/μL). The second was a patient on a chemotherapy regimen with an intermediate risk for FN who was already hospitalized but had developed FN and sepsis despite filgrastim use.
Finally, out of the hospitalized patients, 9 (15%) had infections. This included 6 patients (18%) in the high risk for FN group and 3 patients (12%) in the intermediate risk for FN group (P = .72). Six patients transitioned to pegfilgrastim for hospitalization, 2 for neutropenia, and 1 for an unspecified reason. Nine patients (15%) who received filgrastim ended up transitioning to pegfilgrastim; 6 (67%) of these patients were transitioned due to hospitalization for FN. Of all the patients who transitioned to pegfilgrastim, 1 patient on a high risk for FN regimen developed sepsis due to herpes zoster in the setting of neutropenia after the previous cycle of chemotherapy.
Real-world data are limited regarding G-CSF practice patterns; however, available data demonstrate patients may receive suboptimal treatment courses of filgrastim leading to increased complications associated with neutropenia and FN, such as dose delays and hospitalizations.8,9 At the South Texas Veterans Health Care System, 48 patients (81%) received a filgrastim course of ≥ 7 days as an initial course for primary prophylaxis. Multivariate analyses performed by Weycker and colleagues described a decreased risk of hospitalization for neutropenia or FN with each additional day of filgrastim prophylaxis; however, such analysis could not be performed in our data set due to the small sample size.8 In this review, 10 patients (17%) experienced treatment delays due to neutropenia or FN, mirroring previously published data. The hospitalization rate of 25% is higher than the published incidence of 5.2% of cancer-related hospitalizations among adults.7,10 This difference may be explained by a difference in health care access for the veteran population.
As an alternative to daily filgrastim injections, the National Comprehensive Cancer Network also recommends a single dose of pegfilgrastim for primary prevention of FN. Efficacy benefits of pegfilgrastim use include increased patient adherence due to a single injection, a reduction in FN incidence and FN-related hospitalizations, and improved time to ANC recovery compared with filgrastim.11 There are reports suggesting pegfilgrastim significantly reduces neutropenia and FN incidence to a greater extent compared with daily filgrastim injections.6 In patients with breast cancer receiving dose-dense adjuvant chemotherapy, there are data demonstrating that patients who received filgrastim were more likely to experience severe neutropenia, dose reductions, and treatment delays leading to lower dose density compared with pegfilgrastim.12 Of the 19 patients with breast cancer included in our population, 26% experienced one of the previously described outcomes leading to either extensions of daily filgrastim injections or transitions to pegfilgrastim to successfully maintain dose density. In patients with acute myeloid leukemia receiving consolidation chemotherapy, filgrastim was found to be associated with a statistically significant increased risk of hospitalizations compared with pegfilgrastim.13 The one patient with acute myeloid leukemia included in our study did not require additional hospitalizations for neutropenia or FN after transitioning to pegfilgrastim.
Given the cost advantage, the South Texas Veterans Health Care System continues to prefer daily filgrastim injections. A recent survey demonstrated that 73% of patients at 23 sites in the Veterans Health Administration used filgrastim rather than pegfilgrastim for cost savings, although it is recognized that daily filgrastim injections are less convenient for patients.14 This analysis did not review costs associated with hospitalization for FN or the appropriateness of G-CSF use. Cancer-related neutropenia accounts for 8.3% of all cancer-related hospitalization costs among adults; the average hospitalization costs nearly $25,000 per stay and about $2.3 billion among adult patients with cancer annually.10,15
Limitations
This study has limitations that affected the applicability and interpretation of the results. This included the study design since it was a retrospective, single-center, descriptive cohort study. Patient adherence to daily filgrastim injections could not be assessed due to the retrospective nature of the study. The small sample size of 59 patients was prohibitive for utilization of additional analytical tools. Additionally, the predominately male veteran population may make applicability to non-VA populations restrictive.
CONCLUSIONS
Based on the incidence of primary and secondary outcomes associated with using daily filgrastim injections as primary prophylaxis in this study, additional measures such as tracking postnadir ANCs should be performed to ensure patients receive an appropriate number of filgrastim doses to prevent complications associated with neutropenia.
Acknowledgments
We thank Eric Dougherty, PharmD, for assistance in producing granulocyte colony-stimulating factor data.
Febrile neutropenia (FN) frequently occurs in patients receiving chemotherapy, with the greatest risk of complications occurring in those who experience profound and prolonged neutropenia. Although granulocyte colony-stimulating factor (G-CSF) prophylaxis has been shown to reduce the risk and duration of chemotherapy-induced neutropenia and FN, there is no well-established optimal regimen.1 The 2022 National Comprehensive Cancer Network guidelines for hematopoietic growth factors recommend prophylaxis with G-CSF in at-risk patients receiving chemotherapy, specifically in chemotherapy regimens considered high risk for FN (incidence > 20%) or intermediate risk for FN (incidence 10%-20%) with additional patient risk factors.2 The incidence of developing FN with at least 1 chemotherapy cycle is estimated at 10% to 50% of patients with solid tumors and > 80% of patients with hematologic malignancies.3 The rate of major complications (eg, hypotension, acute renal, respiratory, or heart failure) in the context of FN is 25% to 30%, and mortality is reported up to 11% in this population.4
Because of the significant consequences of neutropenia and FN, prevention is imperative due to the increase in morbidity and mortality, including chemotherapy delays, increased hospitalizations, chemotherapy dose reductions, and discontinuations that cause delays in care.5 In patients with curative malignancies, these consequences can negatively impact treatment efficacy and overall survival. Additionally, infections occur in 20% to 30% of patients with febrile episodes. Although fever is often the only clinical sign or symptom of infection, patients who are profoundly neutropenic may present with suspected infection and be afebrile or hypothermic.3
For filgrastim, the National Comprehensive Cancer Network guidelines do not specify the total days of required injections but state that a daily dose should be given until the postnadir absolute neutrophil count (ANC) recovers to normal or near normal levels by laboratory standards.2 It is uncommon in clinical practice to track postnadir ANCs due to frequent laboratory monitoring. Clinical trial data suggest an average duration of 11 days of daily filgrastim injections for ANC recovery; however, real-world data exist supporting a range from 4 to 10 days with a median of 7 injections per cycle for prevention of neutropenia or FN.6,7
At the South Texas Veterans Health Care System in San Antonio, daily filgrastim injections are preferred due to cost; patients typically receive a 7-day course for primary prophylaxis for FN.
Electronic health record reviews at the South Texas Veterans Health Care System were performed to identify patients who received filgrastim primary prophylaxis (defined as filgrastim, tbo-filgrastim, or filgrastim-sndz) for a curative cancer diagnosis. Primary prophylaxis refers to the administration of G-CSF in the first cycle of chemotherapy before the onset of neutropenia. Patients received filgrastim prophylaxis if they were undergoing treatment with a chemotherapy regimen with either high risk for FN or a chemotherapy regimen with an intermediate risk for FN and additional patient risk factors. Risk factors for patients included prior chemotherapy or radiation therapy; persistent neutropenia; bone marrow involvement by tumor; recent surgery and/or open wounds; liver dysfunction (defined as total bilirubin > 2 mg/dL); renal dysfunction (defined as creatinine clearance < 50 mL/min); and those aged > 65 years receiving full chemotherapy dose intensity. Neutropenia is defined as a decrease in ANC < 1000 neutrophils/μL, whereas FN is defined as a single temperature of > 38.3 °C or > 38.0 °C for longer than 1 hour with < 500 neutrophils/μL or < 1000 neutrophils/μL predicted to decline to < 500 neutrophils/μL over the next 48 hours. All patients had their filgrastim dispensed for home administration during their chemotherapy appointment.
Descriptive statistics were used to summarize the study population and their health outcomes. Fisher exact test was used to compare FN incidence for high- and intermediate-risk FN groups.
RESULTS
Between September 1, 2015, and September 24, 2020, 381 patients received filgrastim. Of these patients, 59 met the inclusion criteria. Patients receiving filgrastim were excluded due to stem cell transplant mobilization/engraftment (n = 145), a noncurative cancer diagnosis (n = 134), use as a secondary prophylaxis (n = 33), and nononcologic neutropenia (n = 8). Additionally, 2 patients initially received pegfilgrastim and were not included in this data set.
The median (IQR) age was 64 (55-70) years and 42 patients (71%) were male (Table 1).
Ten patients (17%) experienced dose delays despite filgrastim use (Table 2).
Nine patients (15%) had the number of filgrastim injections per chemotherapy cycle extended due to various reasons. Five patients required extended days after hospitalization for FN, 3 patients for dose delays due to neutropenia with the previous cycle, and 1 patient with an undocumented reason outside of the prespecified outcomes. Two of these patients experienced continued neutropenia and dose delays after extending filgrastim from 5 to 7 days or 7 to 10 days. One patient who experienced continued neutropenia after extending filgrastim to 10 days was subsequently transitioned to pegfilgrastim without further episodes of neutropenia. The other patient who still experienced neutropenia after extending filgrastim to 7 days was receiving the last chemotherapy cycle and did not require subsequent doses of filgrastim.
Two additional patients were not included in the hospitalizations. The first was a patient on a chemotherapy regimen with a high risk for FN who presented to the emergency department with documented FN but was never admitted since the patient elected to not be hospitalized. This patient developed oral, anal, and vaginal candidiasis, and it was noted by the oncologist at the next clinic visit that this was likely secondary to grade 4 neutropenia (ANC < 500 neutrophils/μL). The second was a patient on a chemotherapy regimen with an intermediate risk for FN who was already hospitalized but had developed FN and sepsis despite filgrastim use.
Finally, out of the hospitalized patients, 9 (15%) had infections. This included 6 patients (18%) in the high risk for FN group and 3 patients (12%) in the intermediate risk for FN group (P = .72). Six patients transitioned to pegfilgrastim for hospitalization, 2 for neutropenia, and 1 for an unspecified reason. Nine patients (15%) who received filgrastim ended up transitioning to pegfilgrastim; 6 (67%) of these patients were transitioned due to hospitalization for FN. Of all the patients who transitioned to pegfilgrastim, 1 patient on a high risk for FN regimen developed sepsis due to herpes zoster in the setting of neutropenia after the previous cycle of chemotherapy.
Real-world data are limited regarding G-CSF practice patterns; however, available data demonstrate patients may receive suboptimal treatment courses of filgrastim leading to increased complications associated with neutropenia and FN, such as dose delays and hospitalizations.8,9 At the South Texas Veterans Health Care System, 48 patients (81%) received a filgrastim course of ≥ 7 days as an initial course for primary prophylaxis. Multivariate analyses performed by Weycker and colleagues described a decreased risk of hospitalization for neutropenia or FN with each additional day of filgrastim prophylaxis; however, such analysis could not be performed in our data set due to the small sample size.8 In this review, 10 patients (17%) experienced treatment delays due to neutropenia or FN, mirroring previously published data. The hospitalization rate of 25% is higher than the published incidence of 5.2% of cancer-related hospitalizations among adults.7,10 This difference may be explained by a difference in health care access for the veteran population.
As an alternative to daily filgrastim injections, the National Comprehensive Cancer Network also recommends a single dose of pegfilgrastim for primary prevention of FN. Efficacy benefits of pegfilgrastim use include increased patient adherence due to a single injection, a reduction in FN incidence and FN-related hospitalizations, and improved time to ANC recovery compared with filgrastim.11 There are reports suggesting pegfilgrastim significantly reduces neutropenia and FN incidence to a greater extent compared with daily filgrastim injections.6 In patients with breast cancer receiving dose-dense adjuvant chemotherapy, there are data demonstrating that patients who received filgrastim were more likely to experience severe neutropenia, dose reductions, and treatment delays leading to lower dose density compared with pegfilgrastim.12 Of the 19 patients with breast cancer included in our population, 26% experienced one of the previously described outcomes leading to either extensions of daily filgrastim injections or transitions to pegfilgrastim to successfully maintain dose density. In patients with acute myeloid leukemia receiving consolidation chemotherapy, filgrastim was found to be associated with a statistically significant increased risk of hospitalizations compared with pegfilgrastim.13 The one patient with acute myeloid leukemia included in our study did not require additional hospitalizations for neutropenia or FN after transitioning to pegfilgrastim.
Given the cost advantage, the South Texas Veterans Health Care System continues to prefer daily filgrastim injections. A recent survey demonstrated that 73% of patients at 23 sites in the Veterans Health Administration used filgrastim rather than pegfilgrastim for cost savings, although it is recognized that daily filgrastim injections are less convenient for patients.14 This analysis did not review costs associated with hospitalization for FN or the appropriateness of G-CSF use. Cancer-related neutropenia accounts for 8.3% of all cancer-related hospitalization costs among adults; the average hospitalization costs nearly $25,000 per stay and about $2.3 billion among adult patients with cancer annually.10,15
Limitations
This study has limitations that affected the applicability and interpretation of the results. This included the study design since it was a retrospective, single-center, descriptive cohort study. Patient adherence to daily filgrastim injections could not be assessed due to the retrospective nature of the study. The small sample size of 59 patients was prohibitive for utilization of additional analytical tools. Additionally, the predominately male veteran population may make applicability to non-VA populations restrictive.
CONCLUSIONS
Based on the incidence of primary and secondary outcomes associated with using daily filgrastim injections as primary prophylaxis in this study, additional measures such as tracking postnadir ANCs should be performed to ensure patients receive an appropriate number of filgrastim doses to prevent complications associated with neutropenia.
Acknowledgments
We thank Eric Dougherty, PharmD, for assistance in producing granulocyte colony-stimulating factor data.
1. Hanna KS, Mancini R, Wilson D, Zuckerman D. Comparing granulocyte colony-stimulating factors prescribing practices versus guideline recommendations in a large community cancer center. J Hematol Oncol Pharm. 2019;9(3):121-126.
2. Griffiths EA, Roy V, Alwan L, et al. NCCN Guidelines insights: hematopoietic growth factors, version 1.2022. J Natl Compr Canc Netw. 2022;20(5):436-442. doi:10.6004/jnccn.2022.0026
3. Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2011;52(4):e56-e93. doi:10.1093/cid/cir073
4. Taplitz RA, Kennedy EB, Bow EJ, et al. Outpatient management of fever and neutropenia in adults treated for malignancy: American Society of Clinical Oncology and Infectious Diseases Society of America Clinical practice guideline update. J Clin Oncol. 2018;36(14):1443-1453. doi:10.1200/JCO.2017.77.6211
5. Clemons M, Fergusson D, Simos D, et al. A multicentre, randomized trial comparing schedules of G-CSF (filgrastim) administration for primary prophylaxis of chemotherapy induced febrile neutropenia in early stage breast cancer. Ann Oncol. 2020;31(7):951-957. doi:10.1016/j.annonc.2020.04.005
6. Cooper KL, Madan J, Whyte S, Stevenson MD, Akehurst RL. Granulocyte colony-stimulating factors for febrile neutropenia prophylaxis following chemotherapy: systematic review and meta-analysis. BMC Cancer. 2011;11:404. Published 2011 Sep 23. doi:10.1186/1471-2407-11-404
7. Altwairgi A, Hopman W, Mates M. Real-world impact of granulocyte-colony stimulating factor on febrile neutropenia. Curr Oncol. 2013;20(3):e171-e179. doi:10.3747/co.20.1306
8. Weycker D, Hackett J, Edelsberg JS, Oster G, Glass AG. Are shorter courses of filgrastim prophylaxis associated with increased risk of hospitalization? Ann Pharmacother. 2006;40(3):402-407. doi:10.1345/aph.1G516
9. Link H, Nietsch J, Kerkmann M, Ortner P; Supportive Care Group (ASORS) of the German Cancer Society (DKG). Adherence to granulocyte-colony stimulating factor (G-CSF) guidelines to reduce the incidence of febrile neutropenia after chemotherapy—a representative sample survey in Germany. Support Care Cancer. 2016;24(1):367-376. doi:10.1007/s00520-015-2779-5
10. Kuderer NM, Dale DC, Crawford J, Cosler LE, Lyman GH. Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer. 2006;106(10):2258-2266. doi:10.1002/cncr.21847
11. Aapro M, Boccia R, Leonard R, et al. Refining the role of pegfilgrastim (a long-acting G-CSF) for prevention of chemotherapy-induced febrile neutropenia: consensus guidance recommendations. Support Care Cancer. 2017;25(11):3295-3304. doi :10.1007/s00520-017-3842-1
12. Kourlaba G, Dimopoulos MA, Pectasides D, et al. Comparison of filgrastim and pegfilgrastim to prevent neutropenia and maintain dose intensity of adjuvant chemotherapy in patients with breast cancer. Support Care Cancer. 2015;23(7):2045-2051. doi:10.1007/s00520-014-2555-y
13. Field E, Caimi PF, Cooper B, et al. Comparison of pegfilgrastim and filgrastim to prevent neutropenic fever during consolidation with high dose cytarabine for acute myeloid leukemia. Blood. 2018;132(suppl 1):1404. doi:10.1182/blood-2018-99-118336
14. Knopf K, Hrureshky W, Love BL, Norris L, Bennett CL. Cost-effective use of white blood cell growth factors in the Veterans Administration. Blood. 2018;132(suppl 1):4761. doi:10.1182/blood-2018-99-119724
15. Tai E, Guy GP, Dunbar A, Richardson LC. Cost of cancer-related neutropenia or fever hospitalizations, United States, 2012. J Oncol Pract. 2017;13(6):e552-e561. doi:10.1200/JOP.2016.019588
1. Hanna KS, Mancini R, Wilson D, Zuckerman D. Comparing granulocyte colony-stimulating factors prescribing practices versus guideline recommendations in a large community cancer center. J Hematol Oncol Pharm. 2019;9(3):121-126.
2. Griffiths EA, Roy V, Alwan L, et al. NCCN Guidelines insights: hematopoietic growth factors, version 1.2022. J Natl Compr Canc Netw. 2022;20(5):436-442. doi:10.6004/jnccn.2022.0026
3. Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2011;52(4):e56-e93. doi:10.1093/cid/cir073
4. Taplitz RA, Kennedy EB, Bow EJ, et al. Outpatient management of fever and neutropenia in adults treated for malignancy: American Society of Clinical Oncology and Infectious Diseases Society of America Clinical practice guideline update. J Clin Oncol. 2018;36(14):1443-1453. doi:10.1200/JCO.2017.77.6211
5. Clemons M, Fergusson D, Simos D, et al. A multicentre, randomized trial comparing schedules of G-CSF (filgrastim) administration for primary prophylaxis of chemotherapy induced febrile neutropenia in early stage breast cancer. Ann Oncol. 2020;31(7):951-957. doi:10.1016/j.annonc.2020.04.005
6. Cooper KL, Madan J, Whyte S, Stevenson MD, Akehurst RL. Granulocyte colony-stimulating factors for febrile neutropenia prophylaxis following chemotherapy: systematic review and meta-analysis. BMC Cancer. 2011;11:404. Published 2011 Sep 23. doi:10.1186/1471-2407-11-404
7. Altwairgi A, Hopman W, Mates M. Real-world impact of granulocyte-colony stimulating factor on febrile neutropenia. Curr Oncol. 2013;20(3):e171-e179. doi:10.3747/co.20.1306
8. Weycker D, Hackett J, Edelsberg JS, Oster G, Glass AG. Are shorter courses of filgrastim prophylaxis associated with increased risk of hospitalization? Ann Pharmacother. 2006;40(3):402-407. doi:10.1345/aph.1G516
9. Link H, Nietsch J, Kerkmann M, Ortner P; Supportive Care Group (ASORS) of the German Cancer Society (DKG). Adherence to granulocyte-colony stimulating factor (G-CSF) guidelines to reduce the incidence of febrile neutropenia after chemotherapy—a representative sample survey in Germany. Support Care Cancer. 2016;24(1):367-376. doi:10.1007/s00520-015-2779-5
10. Kuderer NM, Dale DC, Crawford J, Cosler LE, Lyman GH. Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer. 2006;106(10):2258-2266. doi:10.1002/cncr.21847
11. Aapro M, Boccia R, Leonard R, et al. Refining the role of pegfilgrastim (a long-acting G-CSF) for prevention of chemotherapy-induced febrile neutropenia: consensus guidance recommendations. Support Care Cancer. 2017;25(11):3295-3304. doi :10.1007/s00520-017-3842-1
12. Kourlaba G, Dimopoulos MA, Pectasides D, et al. Comparison of filgrastim and pegfilgrastim to prevent neutropenia and maintain dose intensity of adjuvant chemotherapy in patients with breast cancer. Support Care Cancer. 2015;23(7):2045-2051. doi:10.1007/s00520-014-2555-y
13. Field E, Caimi PF, Cooper B, et al. Comparison of pegfilgrastim and filgrastim to prevent neutropenic fever during consolidation with high dose cytarabine for acute myeloid leukemia. Blood. 2018;132(suppl 1):1404. doi:10.1182/blood-2018-99-118336
14. Knopf K, Hrureshky W, Love BL, Norris L, Bennett CL. Cost-effective use of white blood cell growth factors in the Veterans Administration. Blood. 2018;132(suppl 1):4761. doi:10.1182/blood-2018-99-119724
15. Tai E, Guy GP, Dunbar A, Richardson LC. Cost of cancer-related neutropenia or fever hospitalizations, United States, 2012. J Oncol Pract. 2017;13(6):e552-e561. doi:10.1200/JOP.2016.019588