User login
Reduction of Opioid Use With Enhanced Recovery Program for Total Knee Arthroplasty
Total knee arthroplasty (TKA) is one of the most common surgical procedures in the United States. The volume of TKAs is projected to substantially increase over the next 30 years.1 Adequate pain control after TKA is critically important to achieve early mobilization, shorten the length of hospital stay, and reduce postoperative complications. The evolution and inclusion of multimodal pain-management protocols have had a major impact on the clinical outcomes for TKA patients.2,3
Pain-management protocols typically use several modalities to control pain throughout the perioperative period. Multimodal opioid and nonopioid oral medications are administered during the pre- and postoperative periods and often involve a combination of acetaminophen, gabapentinoids, and cyclooxygenase-2 inhibitors.4 Peripheral nerve blocks and central neuraxial blockades are widely used and have been shown to be effective in reducing postoperative pain as well as overall opioid consumption.5,6 Finally, intraoperative periarticular injections have been shown to reduce postoperative pain and opioid consumption as well as improve patient satisfaction scores.7-9 These strategies are routinely used in TKA with the goal of minimizing overall opioid consumption and adverse events, reducing perioperative complications, and improving patient satisfaction.
Periarticular injections during surgery are an integral part of the multimodal pain-management protocols, though no consensus has been reached on proper injection formulation or technique. Liposomal bupivacaine is a local anesthetic depot formulation approved by the US Food and Drug Administration for surgical patients. The reported results have been discrepant regarding the efficacy of using liposomal bupivacaine injection in patients with TKA. Several studies have reported no added benefit of liposomal bupivacaine in contrast to a mixture of local anesthetics.10,11 Other studies have demonstrated superior pain relief.12 Many factors may contribute to the discrepant data, such as injection techniques, infiltration volume, and the assessment tools used to measure efficacy and safety.13
The US Department of Veterans Affairs (VA) Veterans Health Administration (VHA) provides care to a large patient population. Many of the patients in that system have high-risk profiles, including medical comorbidities; exposure to chronic pain and opioid use; and psychological and central nervous system injuries, including posttraumatic stress disorder and traumatic brain injury. Hadlandsmyth and colleagues reported increased risk of prolonged opioid use in VA patients after TKA surgery.14 They found that 20% of the patients were still on long-term opioids more than 90 days after TKA.
The purpose of this study was to evaluate the efficacy of the implementation of a comprehensive enhanced recovery after surgery (ERAS) protocol at a regional VA medical center. We hypothesize that the addition of liposomal bupivacaine in a multidisciplinary ERAS protocol would reduce the length of hospital stay and opioid consumption without any deleterious effects on postoperative outcomes.
Methods
A postoperative recovery protocol was implemented in 2013 at VA North Texas Health Care System (VANTHCS) in Dallas, and many of the patients continued to have issues with satisfactory pain control, prolonged length of stay, and extended opioid consumption postoperatively. A multimodal pain-management protocol and multidisciplinary perioperative case-management protocol were implemented in 2016 to further improve the clinical outcomes of patients undergoing TKA surgery. The senior surgeon (JM) organized a multidisciplinary team of health care providers to identify and implement potential solutions. This task force met weekly and consisted of surgeons, anesthesiologists, certified registered nurse anesthetists, orthopedic physician assistants, a nurse coordinator, a physical therapist, and an occupational therapist, as well as operating room, postanesthesia care unit (PACU), and surgical ward nurses. In addition, the staff from the home health agencies and social services attended the weekly meetings.
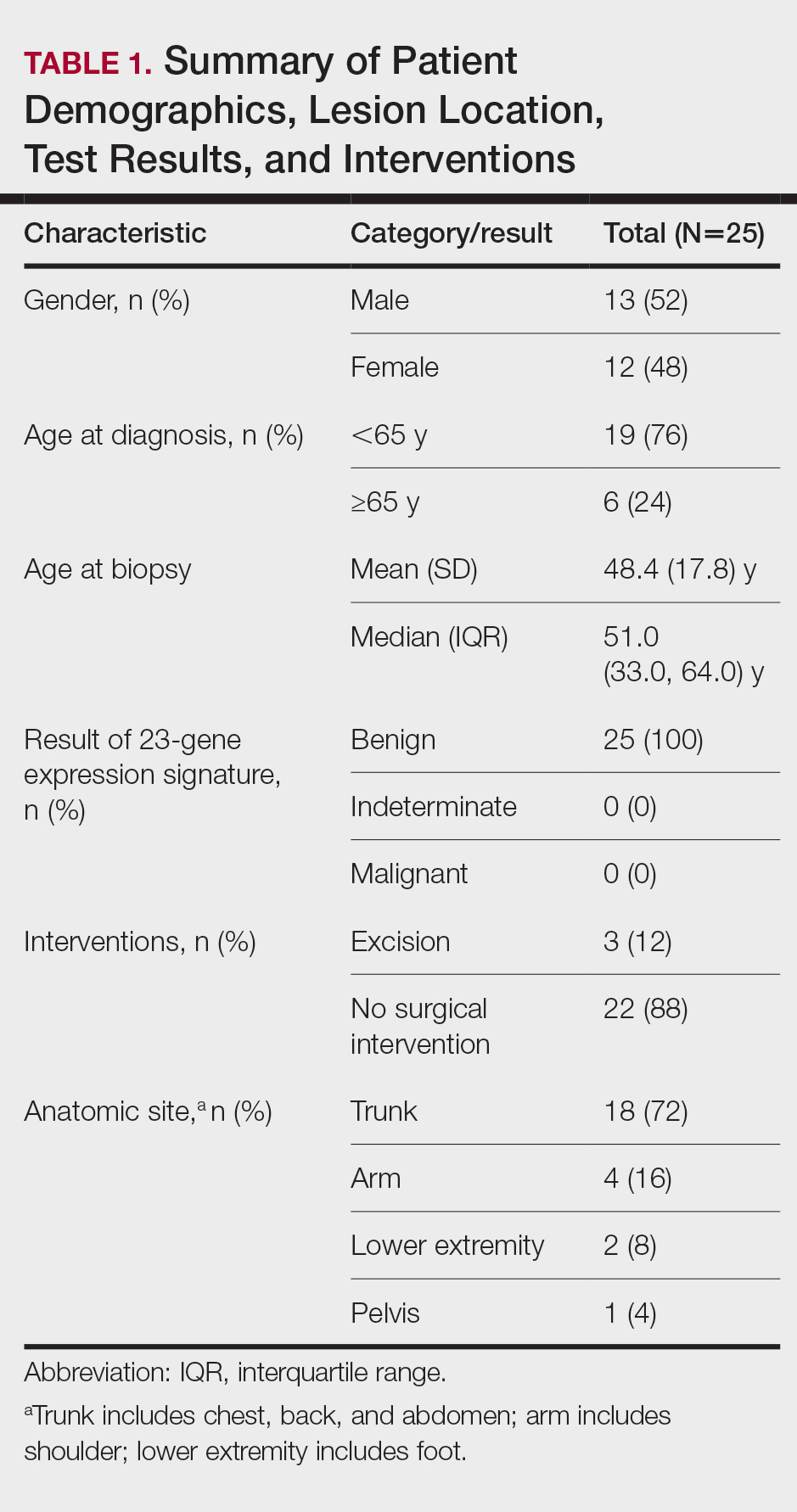
We conducted a retrospective review of all patients who had undergone unilateral TKA from 2013 to 2018 at VANTHCS. This was a consecutive, unselected cohort. All patients were under the care of a single surgeon using identical implant systems and identical surgical techniques. This study was approved by the institutional review board at VANTHCS. Patients were divided into 2 distinct and consecutive cohorts. The standard of care (SOC) group included all patients from 2013 to 2016. The ERAS group included all patients after the institution of the standardized protocol until the end of the study period.
Data on patient demographics, the American Society of Anesthesiologists risk classification, and preoperative functional status were extracted. Anesthesia techniques included either general endotracheal anesthesia or subarachnoid block with monitored anesthesia care. The quantity of the opioids given during surgery, in the PACU, during the inpatient stay, as discharge prescriptions, and as refills of the narcotic prescriptions up to 3 months postsurgery were recorded. All opioids were converted into morphine equivalent dosages (MED) in order to be properly analyzed using the statistical methodologies described in the statistical section.15 The VHA is a closed health care delivery system; therefore, all of the prescriptions ordered by surgery providers were recorded in the electronic health record.
ERAS Protocol
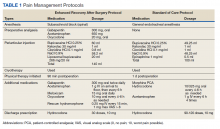
The SOC cohort was predominantly managed with general endotracheal anesthesia. The ERAS group was predominantly managed with subarachnoid blocks (Table 1). For the ERAS protocol preoperatively, the patients were administered oral gabapentin 300 mg, acetaminophen 650 mg, and oxycodone 20 mg, and IV ondansetron 4 mg. Intraoperatively, minimal opioids were used. In the PACU, the patients received dilaudid 0.25 mg IV as needed every 15 minutes for up to 1 mg/h. The nursing staff was trained to use the visual analog pain scale scores to titrate the medication. During the inpatient stay, patients received 1 g IV acetaminophen every 6 hours for 3 doses. The patients thereafter received oral acetaminophen as needed. Other medications in the multimodal pain-management protocol included gabapentin 300 mg twice daily, meloxicam 15 mg daily, and oxycodone 10 mg every 4 hours as needed. Rescue medication for insufficient pain relief was dilaudid 0.25 mg IV every 15 minutes for visual analog pain scale > 8. On discharge, the patients received a prescription of 30 tablets of hydrocodone 10 mg.
Periarticular Injections
Intraoperatively, all patients in the SOC and ERAS groups received periarticular injections. The liposomal bupivacaine injection was added to the standard injection mixture for the ERAS group. For the SOC group, the total volume of 100 ml was divided into 10 separate 10 cc syringes, and for the ERAS group, the total volume of 140 ml was divided into 14 separate 10 cc syringes. The SOC group injections were performed with an 18-gauge needle and the periarticular soft tissues grossly infiltrated. The ERAS group injections were done with more attention to anatomical detail. Injection sites for the ERAS group included the posterior joint capsule, the medial compartment, the lateral compartment, the tibial fat pad, the quadriceps and the patellar tendon, the femoral and tibial periosteum circumferentially, and the anterior joint capsule. Each needle-stick in the ERAS group delivered 1 to 1.5 ml through a 22-gauge needle to each compartment of the knee.
Outcome Variable
The primary outcome measure was total oral MED intraoperatively, in the PACU, during the hospital inpatient stay, in the hospital discharge prescription, and during the 3-month period after hospital discharge. Incidence of nausea and vomiting during the inpatient stay and any narcotic use at 6 months postsurgery were secondary binary outcomes.
Statistical Analysis
Demographic data and the clinical characteristics for the entire group were described using the sample mean and SD for continuous variables and the frequency and percentage for categorical variables. Differences between the 2 cohorts were analyzed using a 2-independent-sample t test and Fisher exact test.
The estimation of the total oral MED throughout all phases of care was done using a separate Poisson model due to the data being not normally distributed. A log-linear regression model was used to evaluate the main effect of ERAS vs the SOC cohort on the total oral MED used. Finally, a separate multiple logistic regression model was used to estimate the odds of postoperative nausea and vomiting and narcotic use at 6 months postsurgery between the cohorts. The adjusted odds ratio (OR) was estimated from the logistic model. Age, sex, body mass index, preoperative functional independence score, narcotic use within 3 months prior to surgery, anesthesia type used (subarachnoid block with monitored anesthesia care vs general endotracheal anesthesia), and postoperative complications (yes/no) were included as covariates in each model. The length of hospital stay and the above-mentioned factors were also included as covariates in the model estimating the total oral MED during the hospital stay, on hospital discharge, during the 3-month period after hospital discharge, and at 6 months following hospital discharge.
Statistical analysis was done using SAS version 9.4. The level of significance was set at α = 0.05 (2 tailed), and we implemented the false discovery rate (FDR) procedure to control false positives over multiple tests.16
Results
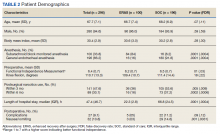
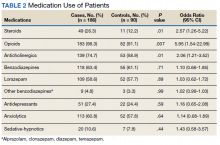
Two hundred forty-nine patients had 296 elective unilateral TKAs in this study from 2013 through 2018. Thirty-one patients had both unilateral TKAs under the SOC protocol; 5 patients had both unilateral TKAs under the ERAS protocol. Eleven of the patients who eventually had both knees replaced had 1 operation under each protocol The SOC group included 196 TKAs and the ERAS group included 100 TKAs. Of the 196 SOC patients, 94% were male. The mean age was 68.2 years (range, 48-86). The length of hospital stay ranged from 36.6 to 664.3 hours. Of the 100 ERAS patients, 96% were male (Table 2). The mean age was 66.7 years (range, 48-85). The length of hospital stay ranged from 12.5 to 45 hours.
Perioperative Opioid Use
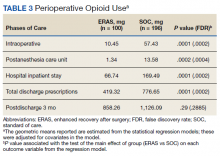
Of the SOC patients, 99.0% received narcotics intraoperatively (range, 0-198 mg MED), and 74.5% received narcotics during PACU recovery (range, 0-141 mg MED). The total oral MED during the hospital stay for the SOC patients ranged from 10 to 2,946 mg. Of the ERAS patients, 86% received no narcotics during surgery (range, 0-110 mg MED), and 98% received no narcotics during PACU recovery (range, 0-65 mg MED). The total oral MED during the hospital stay for the ERAS patients ranged from 10 to 240 mg.
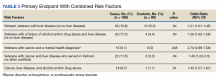
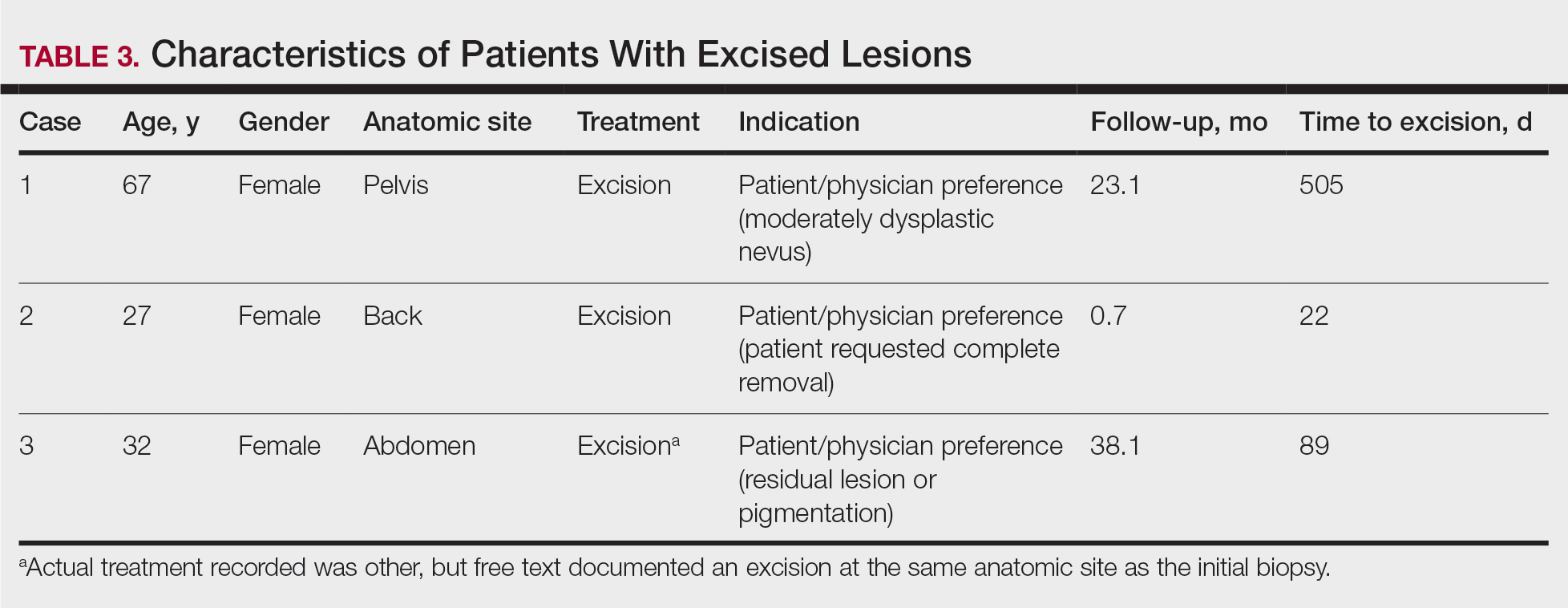
The MED used was significantly lower for the ERAS patients than it was for the SOC patients during surgery (10.5 mg vs 57.4 mg, P = .0001, FDR = .0002) and in the PACU (1.3 mg vs 13.6 mg, P = .0002, FDR = .0004), during the inpatient stay (66.7 mg vs 169.5 mg, P = .0001, FDR = .0002), and on hospital discharge (419.3 mg vs 776.7 mg, P = .0001, FDR = .0002). However, there was no significant difference in the total MED prescriptions filled between patients on the ERAS protocol vs those who received SOC during the 3-month period after hospital discharge (858.3 mg vs 1126.1 mg, P = .29, FDR = .29)(Table 3).
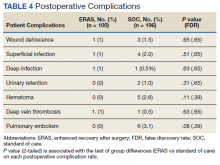
Finally, the logistic regression analysis, adjusting for the covariates demonstrated that the ERAS patients were less likely to take narcotics at 6 months following hospital discharge (OR, 0.23; P = .013; FDR = .018) and less likely to have postoperative nausea and vomiting (OR, 0.18; P = .019; FDR = .02) than SOC patients. There was no statistically significant difference between complication rates for the SOC and ERAS groups, which were 11.2% and 5.0%, respectively, with an overall complication rate of 9.1% (P = .09)(Table 4).
Discussion
Orthopedic surgery has been associated with long-term opioid use and misuse. Orthopedic surgeons are frequently among the highest prescribers of narcotics. According to Volkow and colleagues, orthopedic surgeons were the fourth largest prescribers of opioids in 2009, behind primary care physicians, internists, and dentists.17 The opioid crisis in the United States is well recognized. In 2017, > 70,000 deaths occurred due to drug overdoses, with 68% involving a prescription or illicit opioid. The Centers for Disease Control and Prevention has estimated a total economic burden of $78.5 billion per year as a direct result of misused prescribed opioids.18 This includes the cost of health care, lost productivity, addiction treatment, and the impact on the criminal justice system.
The current opioid crisis places further emphasis on opioid-reducing or sparing techniques in patients undergoing TKA. The use of liposomal bupivacaine for intraoperative periarticular injection is debated in the literature regarding its efficacy and whether it should be included in multimodal protocols. Researchers have argued that liposomal bupivacaine is not superior to regular bupivacaine and because of its increased cost is not justified.19,20 A meta-analysis from Zhao and colleagues showed no difference in pain control and functional recovery when comparing liposomal bupivacaine and control.21 In a randomized clinical trial, Schroer and colleagues matched liposomal bupivacaine against regular bupivacaine and found no difference in pain scores and similar narcotic use during hospitalization.22
Studies evaluating liposomal bupivacaine have demonstrated postoperative benefits in pain relief and potential opioid consumption.23 In a multicenter randomized controlled trial, Barrington and colleagues noted improved pain control at 6 and 12 hours after surgery with liposomal bupivacaine as a periarticular injection vs ropivacaine, though results were similar when compared with intrathecal morphine.24 Snyder and colleagues reported higher patient satisfaction in pain control and overall experience as well as decreased MED consumption in the PACU and on postoperative days 0 to 2 when using liposomal bupivacaine vs a multidrug cocktail for periarticular injection.25
The PILLAR trial, an industry-sponsored study, was designed to compare the effects of local infiltration anesthesia with and without liposomal bupivacaine with emphasis on a meticulous standardized infiltration technique. In our study, we used a similar technique with an expanded volume of injection solution to 140 ml that was delivered throughout the knee in a series of 14 syringes. Each needle-stick delivered 1 to 1.5 ml through a 22-gauge needle to each compartment of the knee. Infiltration technique has varied among the literature focused on periarticular injections.
In our experience, a standard infiltration technique is critical to the effective delivery of liposomal bupivacaine throughout all compartments of the knee and to obtaining reproducible pain control. The importance of injection technique cannot be overemphasized, and variations can be seen in studies published to date.26 Well-designed trials are needed to address this key component.
There have been limited data focused on the veteran population regarding postoperative pain-management strategies and recovery pathways either with or without liposomal bupivacaine. In a retrospective review, Sakamoto and colleagues found VA patients undergoing TKA had reduced opioid use in the first 24 hours after primary TKA with the use of intraoperative liposomal bupivacaine.27 The VA population has been shown to be at high risk for opioid misuse. The prevalence of comorbidities such as traumatic brain injury, posttraumatic stress disorder, and depression in the VA population also places them at risk for polypharmacy of central nervous system–acting medications.28 This emphasizes the importance of multimodal strategies, which can limit or eliminate narcotics in the perioperative period. The implementation of our ERAS protocol reduced opioid use during intraoperative, PACU, and inpatient hospital stay.
While the financial implications of our recovery protocol were not a primary focus of this study, there are many notable benefits on the overall inpatient cost to the VHA. According to the Health Economics Resource Center, the average daily cost of stay while under VA care for an inpatient surgical bed increased from $4,831 in 2013 to $6,220 in 2018.29 Our reduction in length of stay between our cohorts is 44.5 hours, which translates to a substantial financial savings per patient after protocol implementation. A more detailed look at the financial aspect of our protocol would need to be performed to evaluate the financial impact of other aspects of our protocol, such as the elimination of patient-controlled anesthesia and the reduction in total narcotics prescribed in the postoperative global period.
Limitations
The limitations of this study include its retrospective study design. With the VHA patient population, it may be subject to selection bias, as the population is mostly older and predominantly male compared with that of the general population. This could potentially influence the efficacy of our protocol on a population of patients with more women. In a recent study by Perruccio and colleagues, sex was found to moderate the effects of comorbidities, low back pain, and depressive symptoms on postoperative pain in patients undergoing TKA.30
With regard to outpatient narcotic prescriptions, although we cannot fully know whether these filled prescriptions were used for pain control, it is a reasonable assumption that patients who are dealing with continued postoperative or chronic pain issues will fill these prescriptions or seek refills. It is important to note that the data on prescriptions and refills in the 3-month postoperative period include all narcotic prescriptions filled by any VHA prescriber and are not specifically limited to our orthopedic team. For outpatient narcotic use, we were not able to access accurate pill counts for any discharge prescriptions or subsequent refills that were given throughout the VA system. We were able to report on total prescriptions filled in the first 3 months following TKA.
We calculated total oral MEDs to better understand the amount of narcotics being distributed throughout our population of patients. We believe this provides important information about the overall narcotic burden in the veteran population. There was no significant difference between the SOC and ERAS groups regarding oral MED prescribed in the 3-month postoperative period; however, at the 6-month follow-up visit, only 16% of patients in the ERAS group were taking any type of narcotic vs 37.2% in the SOC group (P = .0002).
Conclusions
A multidisciplinary ERAS protocol implemented at VANTHCS was effective in reducing length of stay and opioid burden throughout all phases of surgical care in our patients undergoing primary TKA. Patient and nursing education seem to be critical components to the implementation of a successful multimodal pain protocol. Reducing the narcotic burden has valuable financial and medical benefits in this at-risk population.
1. Inacio MCS, Paxton EW, Graves SE, Namba RS, Nemes S. Projected increase in total knee arthroplasty in the United States - an alternative projection model. Osteoarthritis Cartilage. 2017;25(11):1797-1803. doi:10.1016/j.joca.2017.07.022
2. Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of Postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council [published correction appears in J Pain. 2016 Apr;17(4):508-10. Dosage error in article text]. J Pain. 2016;17(2):131-157. doi:10.1016/j.jpain.2015.12.008
3. Moucha CS, Weiser MC, Levin EJ. Current Strategies in anesthesia and analgesia for total knee arthroplasty. J Am Acad Orthop Surg. 2016;24(2):60-73. doi:10.5435/JAAOS-D-14-00259
4. Parvizi J, Miller AG, Gandhi K. Multimodal pain management after total joint arthroplasty. J Bone Joint Surg Am. 2011;93(11):1075-1084. doi:10.2106/JBJS.J.01095
5. Jenstrup MT, Jæger P, Lund J, et al. Effects of adductor-canal-blockade on pain and ambulation after total knee arthroplasty: a randomized study. Acta Anaesthesiol Scand. 2012;56(3):357-364. doi:10.1111/j.1399-6576.2011.02621.x
6. Macfarlane AJ, Prasad GA, Chan VW, Brull R. Does regional anesthesia improve outcome after total knee arthroplasty?. Clin Orthop Relat Res. 2009;467(9):2379-2402. doi:10.1007/s11999-008-0666-9
7. Parvataneni HK, Shah VP, Howard H, Cole N, Ranawat AS, Ranawat CS. Controlling pain after total hip and knee arthroplasty using a multimodal protocol with local periarticular injections: a prospective randomized study. J Arthroplasty. 2007;22(6)(suppl 2):33-38. doi:10.1016/j.arth.2007.03.034
8. Busch CA, Shore BJ, Bhandari R, et al. Efficacy of periarticular multimodal drug injection in total knee arthroplasty. A randomized trial. J Bone Joint Surg Am. 2006;88(5):959-963. doi:10.2106/JBJS.E.00344
9. Lamplot JD, Wagner ER, Manning DW. Multimodal pain management in total knee arthroplasty: a prospective randomized controlled trial. J Arthroplasty. 2014;29(2):329-334. doi:10.1016/j.arth.2013.06.005
10. Hyland SJ, Deliberato DG, Fada RA, Romanelli MJ, Collins CL, Wasielewski RC. Liposomal bupivacaine versus standard periarticular injection in total knee arthroplasty with regional anesthesia: a prospective randomized controlled trial. J Arthroplasty. 2019;34(3):488-494. doi:10.1016/j.arth.2018.11.026
11. Barrington JW, Lovald ST, Ong KL, Watson HN, Emerson RH Jr. Postoperative pain after primary total knee arthroplasty: comparison of local injection analgesic cocktails and the role of demographic and surgical factors. J Arthroplasty. 2016;31(9) (suppl):288-292. doi:10.1016/j.arth.2016.05.002
12. Bramlett K, Onel E, Viscusi ER, Jones K. A randomized, double-blind, dose-ranging study comparing wound infiltration of DepoFoam bupivacaine, an extended-release liposomal bupivacaine, to bupivacaine HCl for postsurgical analgesia in total knee arthroplasty. Knee. 2012;19(5):530-536. doi:10.1016/j.knee.2011.12.004
13. Mont MA, Beaver WB, Dysart SH, Barrington JW, Del Gaizo D. Local infiltration analgesia with liposomal bupivacaine improves pain scores and reduces opioid use after total knee arthroplasty: results of a randomized controlled trial. J Arthroplasty. 2018;33(1):90-96. doi:10.1016/j.arth.2017.07.024
14. Hadlandsmyth K, Vander Weg MW, McCoy KD, Mosher HJ, Vaughan-Sarrazin MS, Lund BC. Risk for prolonged opioid use following total knee arthroplasty in veterans. J Arthroplasty. 2018;33(1):119-123. doi:10.1016/j.arth.2017.08.022
15. Nielsen S, Degenhardt L, Hoban B, Gisev N. A synthesis of oral morphine equivalents (OME) for opioid utilisation studies. Pharmacoepidemiol Drug Saf. 2016;25(6):733-737. doi:10.1002/pds.3945
16. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statist Soc B. 1995;57(1):289-300. doi:10.1111/j.2517-6161.1995.tb02031.x
17. Volkow ND, McLellan TA, Cotto JH, Karithanom M, Weiss SRB. Characteristics of opioid prescriptions in 2009. JAMA. 2011;305(13):1299-1301. doi:10.1001/jama.2011.401
18. Scholl L, Seth P, Kariisa M, Wilson N, Baldwin G. Drug and opioid-involved overdose deaths - United States, 2013-2017. MMWR Morb Mortal Wkly Rep. 2018;67(5152):1419-1427. doi:10.15585/mmwr.mm675152e1
19. Pichler L, Poeran J, Zubizarreta N, et al. Liposomal bupivacaine does not reduce inpatient opioid prescription or related complications after knee arthroplasty: a database analysis. Anesthesiology. 2018;129(4):689-699. doi:10.1097/ALN.0000000000002267
20. Jain RK, Porat MD, Klingenstein GG, Reid JJ, Post RE, Schoifet SD. The AAHKS Clinical Research Award: liposomal bupivacaine and periarticular injection are not superior to single-shot intra-articular injection for pain control in total knee arthroplasty. J Arthroplasty. 2016;31(9)(suppl):22-25. doi:10.1016/j.arth.2016.03.036
21. Zhao B, Ma X, Zhang J, Ma J, Cao Q. The efficacy of local liposomal bupivacaine infiltration on pain and recovery after total joint arthroplasty: a systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore). 2019;98(3):e14092. doi:10.1097/MD.0000000000014092
22. Schroer WC, Diesfeld PG, LeMarr AR, Morton DJ, Reedy ME. Does extended-release liposomal bupivacaine better control pain than bupivacaine after total knee arthroplasty (TKA)? A prospective, randomized clinical trial. J Arthroplasty. 2015;30(9)(suppl):64-67. doi:10.1016/j.arth.2015.01.059
23. Ma J, Zhang W, Yao S. Liposomal bupivacaine infiltration versus femoral nerve block for pain control in total knee arthroplasty: a systematic review and meta-analysis. Int J Surg. 2016;36(Pt A): 44-55. doi:10.1016/j.ijsu.2016.10.007
24. Barrington JW, Emerson RH, Lovald ST, Lombardi AV, Berend KR. No difference in early analgesia between liposomal bupivacaine injection and intrathecal morphine after TKA. Clin Orthop Relat Res. 2017;475(1):94-105. doi:10.1007/s11999-016-4931-z
25. Snyder MA, Scheuerman CM, Gregg JL, Ruhnke CJ, Eten K. Improving total knee arthroplasty perioperative pain management using a periarticular injection with bupivacaine liposomal suspension. Arthroplast Today. 2016;2(1):37-42. doi:10.1016/j.artd.2015.05.005
26. Kuang MJ,Du Y, Ma JX, He W, Fu L, Ma XL. The efficacy of liposomal bupivacaine using periarticular injection in total knee arthroplasty: a systematic review and meta-analysis. J Arthroplasty. 2017;32(4):1395-1402. doi:10.1016/j.arth.2016.12.025
27. Sakamoto B, Keiser S, Meldrum R, Harker G, Freese A. Efficacy of liposomal bupivacaine infiltration on the management of total knee arthroplasty. JAMA Surg. 2017;152(1):90-95. doi:10.1001/jamasurg.2016.3474
28. Collett GA, Song K, Jaramillo CA, Potter JS, Finley EP, Pugh MJ. Prevalence of central nervous system polypharmacy and associations with overdose and suicide-related behaviors in Iraq and Afghanistan war veterans in VA care 2010-2011. Drugs Real World Outcomes. 2016;3(1):45-52. doi:10.1007/s40801-015-0055-0
29. US Department of Veterans Affairs. HERC inpatient average cost data. Updated April 2, 2021. Accessed April 16, 2021. https://www.herc.research.va.gov/include/page.asp?id=inpatient#herc-inpat-avg-cost
30. Perruccio AV, Fitzpatrick J, Power JD, et al. Sex-modified effects of depression, low back pain, and comorbidities on pain after total knee arthroplasty for osteoarthritis. Arthritis Care Res (Hoboken). 2020;72(8):1074-1080. doi:10.1002/acr.24002
Total knee arthroplasty (TKA) is one of the most common surgical procedures in the United States. The volume of TKAs is projected to substantially increase over the next 30 years.1 Adequate pain control after TKA is critically important to achieve early mobilization, shorten the length of hospital stay, and reduce postoperative complications. The evolution and inclusion of multimodal pain-management protocols have had a major impact on the clinical outcomes for TKA patients.2,3
Pain-management protocols typically use several modalities to control pain throughout the perioperative period. Multimodal opioid and nonopioid oral medications are administered during the pre- and postoperative periods and often involve a combination of acetaminophen, gabapentinoids, and cyclooxygenase-2 inhibitors.4 Peripheral nerve blocks and central neuraxial blockades are widely used and have been shown to be effective in reducing postoperative pain as well as overall opioid consumption.5,6 Finally, intraoperative periarticular injections have been shown to reduce postoperative pain and opioid consumption as well as improve patient satisfaction scores.7-9 These strategies are routinely used in TKA with the goal of minimizing overall opioid consumption and adverse events, reducing perioperative complications, and improving patient satisfaction.
Periarticular injections during surgery are an integral part of the multimodal pain-management protocols, though no consensus has been reached on proper injection formulation or technique. Liposomal bupivacaine is a local anesthetic depot formulation approved by the US Food and Drug Administration for surgical patients. The reported results have been discrepant regarding the efficacy of using liposomal bupivacaine injection in patients with TKA. Several studies have reported no added benefit of liposomal bupivacaine in contrast to a mixture of local anesthetics.10,11 Other studies have demonstrated superior pain relief.12 Many factors may contribute to the discrepant data, such as injection techniques, infiltration volume, and the assessment tools used to measure efficacy and safety.13
The US Department of Veterans Affairs (VA) Veterans Health Administration (VHA) provides care to a large patient population. Many of the patients in that system have high-risk profiles, including medical comorbidities; exposure to chronic pain and opioid use; and psychological and central nervous system injuries, including posttraumatic stress disorder and traumatic brain injury. Hadlandsmyth and colleagues reported increased risk of prolonged opioid use in VA patients after TKA surgery.14 They found that 20% of the patients were still on long-term opioids more than 90 days after TKA.
The purpose of this study was to evaluate the efficacy of the implementation of a comprehensive enhanced recovery after surgery (ERAS) protocol at a regional VA medical center. We hypothesize that the addition of liposomal bupivacaine in a multidisciplinary ERAS protocol would reduce the length of hospital stay and opioid consumption without any deleterious effects on postoperative outcomes.
Methods
A postoperative recovery protocol was implemented in 2013 at VA North Texas Health Care System (VANTHCS) in Dallas, and many of the patients continued to have issues with satisfactory pain control, prolonged length of stay, and extended opioid consumption postoperatively. A multimodal pain-management protocol and multidisciplinary perioperative case-management protocol were implemented in 2016 to further improve the clinical outcomes of patients undergoing TKA surgery. The senior surgeon (JM) organized a multidisciplinary team of health care providers to identify and implement potential solutions. This task force met weekly and consisted of surgeons, anesthesiologists, certified registered nurse anesthetists, orthopedic physician assistants, a nurse coordinator, a physical therapist, and an occupational therapist, as well as operating room, postanesthesia care unit (PACU), and surgical ward nurses. In addition, the staff from the home health agencies and social services attended the weekly meetings.
We conducted a retrospective review of all patients who had undergone unilateral TKA from 2013 to 2018 at VANTHCS. This was a consecutive, unselected cohort. All patients were under the care of a single surgeon using identical implant systems and identical surgical techniques. This study was approved by the institutional review board at VANTHCS. Patients were divided into 2 distinct and consecutive cohorts. The standard of care (SOC) group included all patients from 2013 to 2016. The ERAS group included all patients after the institution of the standardized protocol until the end of the study period.
Data on patient demographics, the American Society of Anesthesiologists risk classification, and preoperative functional status were extracted. Anesthesia techniques included either general endotracheal anesthesia or subarachnoid block with monitored anesthesia care. The quantity of the opioids given during surgery, in the PACU, during the inpatient stay, as discharge prescriptions, and as refills of the narcotic prescriptions up to 3 months postsurgery were recorded. All opioids were converted into morphine equivalent dosages (MED) in order to be properly analyzed using the statistical methodologies described in the statistical section.15 The VHA is a closed health care delivery system; therefore, all of the prescriptions ordered by surgery providers were recorded in the electronic health record.
ERAS Protocol
The SOC cohort was predominantly managed with general endotracheal anesthesia. The ERAS group was predominantly managed with subarachnoid blocks (Table 1). For the ERAS protocol preoperatively, the patients were administered oral gabapentin 300 mg, acetaminophen 650 mg, and oxycodone 20 mg, and IV ondansetron 4 mg. Intraoperatively, minimal opioids were used. In the PACU, the patients received dilaudid 0.25 mg IV as needed every 15 minutes for up to 1 mg/h. The nursing staff was trained to use the visual analog pain scale scores to titrate the medication. During the inpatient stay, patients received 1 g IV acetaminophen every 6 hours for 3 doses. The patients thereafter received oral acetaminophen as needed. Other medications in the multimodal pain-management protocol included gabapentin 300 mg twice daily, meloxicam 15 mg daily, and oxycodone 10 mg every 4 hours as needed. Rescue medication for insufficient pain relief was dilaudid 0.25 mg IV every 15 minutes for visual analog pain scale > 8. On discharge, the patients received a prescription of 30 tablets of hydrocodone 10 mg.
Periarticular Injections
Intraoperatively, all patients in the SOC and ERAS groups received periarticular injections. The liposomal bupivacaine injection was added to the standard injection mixture for the ERAS group. For the SOC group, the total volume of 100 ml was divided into 10 separate 10 cc syringes, and for the ERAS group, the total volume of 140 ml was divided into 14 separate 10 cc syringes. The SOC group injections were performed with an 18-gauge needle and the periarticular soft tissues grossly infiltrated. The ERAS group injections were done with more attention to anatomical detail. Injection sites for the ERAS group included the posterior joint capsule, the medial compartment, the lateral compartment, the tibial fat pad, the quadriceps and the patellar tendon, the femoral and tibial periosteum circumferentially, and the anterior joint capsule. Each needle-stick in the ERAS group delivered 1 to 1.5 ml through a 22-gauge needle to each compartment of the knee.
Outcome Variable
The primary outcome measure was total oral MED intraoperatively, in the PACU, during the hospital inpatient stay, in the hospital discharge prescription, and during the 3-month period after hospital discharge. Incidence of nausea and vomiting during the inpatient stay and any narcotic use at 6 months postsurgery were secondary binary outcomes.
Statistical Analysis
Demographic data and the clinical characteristics for the entire group were described using the sample mean and SD for continuous variables and the frequency and percentage for categorical variables. Differences between the 2 cohorts were analyzed using a 2-independent-sample t test and Fisher exact test.
The estimation of the total oral MED throughout all phases of care was done using a separate Poisson model due to the data being not normally distributed. A log-linear regression model was used to evaluate the main effect of ERAS vs the SOC cohort on the total oral MED used. Finally, a separate multiple logistic regression model was used to estimate the odds of postoperative nausea and vomiting and narcotic use at 6 months postsurgery between the cohorts. The adjusted odds ratio (OR) was estimated from the logistic model. Age, sex, body mass index, preoperative functional independence score, narcotic use within 3 months prior to surgery, anesthesia type used (subarachnoid block with monitored anesthesia care vs general endotracheal anesthesia), and postoperative complications (yes/no) were included as covariates in each model. The length of hospital stay and the above-mentioned factors were also included as covariates in the model estimating the total oral MED during the hospital stay, on hospital discharge, during the 3-month period after hospital discharge, and at 6 months following hospital discharge.
Statistical analysis was done using SAS version 9.4. The level of significance was set at α = 0.05 (2 tailed), and we implemented the false discovery rate (FDR) procedure to control false positives over multiple tests.16
Results
Two hundred forty-nine patients had 296 elective unilateral TKAs in this study from 2013 through 2018. Thirty-one patients had both unilateral TKAs under the SOC protocol; 5 patients had both unilateral TKAs under the ERAS protocol. Eleven of the patients who eventually had both knees replaced had 1 operation under each protocol The SOC group included 196 TKAs and the ERAS group included 100 TKAs. Of the 196 SOC patients, 94% were male. The mean age was 68.2 years (range, 48-86). The length of hospital stay ranged from 36.6 to 664.3 hours. Of the 100 ERAS patients, 96% were male (Table 2). The mean age was 66.7 years (range, 48-85). The length of hospital stay ranged from 12.5 to 45 hours.
Perioperative Opioid Use
Of the SOC patients, 99.0% received narcotics intraoperatively (range, 0-198 mg MED), and 74.5% received narcotics during PACU recovery (range, 0-141 mg MED). The total oral MED during the hospital stay for the SOC patients ranged from 10 to 2,946 mg. Of the ERAS patients, 86% received no narcotics during surgery (range, 0-110 mg MED), and 98% received no narcotics during PACU recovery (range, 0-65 mg MED). The total oral MED during the hospital stay for the ERAS patients ranged from 10 to 240 mg.
The MED used was significantly lower for the ERAS patients than it was for the SOC patients during surgery (10.5 mg vs 57.4 mg, P = .0001, FDR = .0002) and in the PACU (1.3 mg vs 13.6 mg, P = .0002, FDR = .0004), during the inpatient stay (66.7 mg vs 169.5 mg, P = .0001, FDR = .0002), and on hospital discharge (419.3 mg vs 776.7 mg, P = .0001, FDR = .0002). However, there was no significant difference in the total MED prescriptions filled between patients on the ERAS protocol vs those who received SOC during the 3-month period after hospital discharge (858.3 mg vs 1126.1 mg, P = .29, FDR = .29)(Table 3).
Finally, the logistic regression analysis, adjusting for the covariates demonstrated that the ERAS patients were less likely to take narcotics at 6 months following hospital discharge (OR, 0.23; P = .013; FDR = .018) and less likely to have postoperative nausea and vomiting (OR, 0.18; P = .019; FDR = .02) than SOC patients. There was no statistically significant difference between complication rates for the SOC and ERAS groups, which were 11.2% and 5.0%, respectively, with an overall complication rate of 9.1% (P = .09)(Table 4).
Discussion
Orthopedic surgery has been associated with long-term opioid use and misuse. Orthopedic surgeons are frequently among the highest prescribers of narcotics. According to Volkow and colleagues, orthopedic surgeons were the fourth largest prescribers of opioids in 2009, behind primary care physicians, internists, and dentists.17 The opioid crisis in the United States is well recognized. In 2017, > 70,000 deaths occurred due to drug overdoses, with 68% involving a prescription or illicit opioid. The Centers for Disease Control and Prevention has estimated a total economic burden of $78.5 billion per year as a direct result of misused prescribed opioids.18 This includes the cost of health care, lost productivity, addiction treatment, and the impact on the criminal justice system.
The current opioid crisis places further emphasis on opioid-reducing or sparing techniques in patients undergoing TKA. The use of liposomal bupivacaine for intraoperative periarticular injection is debated in the literature regarding its efficacy and whether it should be included in multimodal protocols. Researchers have argued that liposomal bupivacaine is not superior to regular bupivacaine and because of its increased cost is not justified.19,20 A meta-analysis from Zhao and colleagues showed no difference in pain control and functional recovery when comparing liposomal bupivacaine and control.21 In a randomized clinical trial, Schroer and colleagues matched liposomal bupivacaine against regular bupivacaine and found no difference in pain scores and similar narcotic use during hospitalization.22
Studies evaluating liposomal bupivacaine have demonstrated postoperative benefits in pain relief and potential opioid consumption.23 In a multicenter randomized controlled trial, Barrington and colleagues noted improved pain control at 6 and 12 hours after surgery with liposomal bupivacaine as a periarticular injection vs ropivacaine, though results were similar when compared with intrathecal morphine.24 Snyder and colleagues reported higher patient satisfaction in pain control and overall experience as well as decreased MED consumption in the PACU and on postoperative days 0 to 2 when using liposomal bupivacaine vs a multidrug cocktail for periarticular injection.25
The PILLAR trial, an industry-sponsored study, was designed to compare the effects of local infiltration anesthesia with and without liposomal bupivacaine with emphasis on a meticulous standardized infiltration technique. In our study, we used a similar technique with an expanded volume of injection solution to 140 ml that was delivered throughout the knee in a series of 14 syringes. Each needle-stick delivered 1 to 1.5 ml through a 22-gauge needle to each compartment of the knee. Infiltration technique has varied among the literature focused on periarticular injections.
In our experience, a standard infiltration technique is critical to the effective delivery of liposomal bupivacaine throughout all compartments of the knee and to obtaining reproducible pain control. The importance of injection technique cannot be overemphasized, and variations can be seen in studies published to date.26 Well-designed trials are needed to address this key component.
There have been limited data focused on the veteran population regarding postoperative pain-management strategies and recovery pathways either with or without liposomal bupivacaine. In a retrospective review, Sakamoto and colleagues found VA patients undergoing TKA had reduced opioid use in the first 24 hours after primary TKA with the use of intraoperative liposomal bupivacaine.27 The VA population has been shown to be at high risk for opioid misuse. The prevalence of comorbidities such as traumatic brain injury, posttraumatic stress disorder, and depression in the VA population also places them at risk for polypharmacy of central nervous system–acting medications.28 This emphasizes the importance of multimodal strategies, which can limit or eliminate narcotics in the perioperative period. The implementation of our ERAS protocol reduced opioid use during intraoperative, PACU, and inpatient hospital stay.
While the financial implications of our recovery protocol were not a primary focus of this study, there are many notable benefits on the overall inpatient cost to the VHA. According to the Health Economics Resource Center, the average daily cost of stay while under VA care for an inpatient surgical bed increased from $4,831 in 2013 to $6,220 in 2018.29 Our reduction in length of stay between our cohorts is 44.5 hours, which translates to a substantial financial savings per patient after protocol implementation. A more detailed look at the financial aspect of our protocol would need to be performed to evaluate the financial impact of other aspects of our protocol, such as the elimination of patient-controlled anesthesia and the reduction in total narcotics prescribed in the postoperative global period.
Limitations
The limitations of this study include its retrospective study design. With the VHA patient population, it may be subject to selection bias, as the population is mostly older and predominantly male compared with that of the general population. This could potentially influence the efficacy of our protocol on a population of patients with more women. In a recent study by Perruccio and colleagues, sex was found to moderate the effects of comorbidities, low back pain, and depressive symptoms on postoperative pain in patients undergoing TKA.30
With regard to outpatient narcotic prescriptions, although we cannot fully know whether these filled prescriptions were used for pain control, it is a reasonable assumption that patients who are dealing with continued postoperative or chronic pain issues will fill these prescriptions or seek refills. It is important to note that the data on prescriptions and refills in the 3-month postoperative period include all narcotic prescriptions filled by any VHA prescriber and are not specifically limited to our orthopedic team. For outpatient narcotic use, we were not able to access accurate pill counts for any discharge prescriptions or subsequent refills that were given throughout the VA system. We were able to report on total prescriptions filled in the first 3 months following TKA.
We calculated total oral MEDs to better understand the amount of narcotics being distributed throughout our population of patients. We believe this provides important information about the overall narcotic burden in the veteran population. There was no significant difference between the SOC and ERAS groups regarding oral MED prescribed in the 3-month postoperative period; however, at the 6-month follow-up visit, only 16% of patients in the ERAS group were taking any type of narcotic vs 37.2% in the SOC group (P = .0002).
Conclusions
A multidisciplinary ERAS protocol implemented at VANTHCS was effective in reducing length of stay and opioid burden throughout all phases of surgical care in our patients undergoing primary TKA. Patient and nursing education seem to be critical components to the implementation of a successful multimodal pain protocol. Reducing the narcotic burden has valuable financial and medical benefits in this at-risk population.
Total knee arthroplasty (TKA) is one of the most common surgical procedures in the United States. The volume of TKAs is projected to substantially increase over the next 30 years.1 Adequate pain control after TKA is critically important to achieve early mobilization, shorten the length of hospital stay, and reduce postoperative complications. The evolution and inclusion of multimodal pain-management protocols have had a major impact on the clinical outcomes for TKA patients.2,3
Pain-management protocols typically use several modalities to control pain throughout the perioperative period. Multimodal opioid and nonopioid oral medications are administered during the pre- and postoperative periods and often involve a combination of acetaminophen, gabapentinoids, and cyclooxygenase-2 inhibitors.4 Peripheral nerve blocks and central neuraxial blockades are widely used and have been shown to be effective in reducing postoperative pain as well as overall opioid consumption.5,6 Finally, intraoperative periarticular injections have been shown to reduce postoperative pain and opioid consumption as well as improve patient satisfaction scores.7-9 These strategies are routinely used in TKA with the goal of minimizing overall opioid consumption and adverse events, reducing perioperative complications, and improving patient satisfaction.
Periarticular injections during surgery are an integral part of the multimodal pain-management protocols, though no consensus has been reached on proper injection formulation or technique. Liposomal bupivacaine is a local anesthetic depot formulation approved by the US Food and Drug Administration for surgical patients. The reported results have been discrepant regarding the efficacy of using liposomal bupivacaine injection in patients with TKA. Several studies have reported no added benefit of liposomal bupivacaine in contrast to a mixture of local anesthetics.10,11 Other studies have demonstrated superior pain relief.12 Many factors may contribute to the discrepant data, such as injection techniques, infiltration volume, and the assessment tools used to measure efficacy and safety.13
The US Department of Veterans Affairs (VA) Veterans Health Administration (VHA) provides care to a large patient population. Many of the patients in that system have high-risk profiles, including medical comorbidities; exposure to chronic pain and opioid use; and psychological and central nervous system injuries, including posttraumatic stress disorder and traumatic brain injury. Hadlandsmyth and colleagues reported increased risk of prolonged opioid use in VA patients after TKA surgery.14 They found that 20% of the patients were still on long-term opioids more than 90 days after TKA.
The purpose of this study was to evaluate the efficacy of the implementation of a comprehensive enhanced recovery after surgery (ERAS) protocol at a regional VA medical center. We hypothesize that the addition of liposomal bupivacaine in a multidisciplinary ERAS protocol would reduce the length of hospital stay and opioid consumption without any deleterious effects on postoperative outcomes.
Methods
A postoperative recovery protocol was implemented in 2013 at VA North Texas Health Care System (VANTHCS) in Dallas, and many of the patients continued to have issues with satisfactory pain control, prolonged length of stay, and extended opioid consumption postoperatively. A multimodal pain-management protocol and multidisciplinary perioperative case-management protocol were implemented in 2016 to further improve the clinical outcomes of patients undergoing TKA surgery. The senior surgeon (JM) organized a multidisciplinary team of health care providers to identify and implement potential solutions. This task force met weekly and consisted of surgeons, anesthesiologists, certified registered nurse anesthetists, orthopedic physician assistants, a nurse coordinator, a physical therapist, and an occupational therapist, as well as operating room, postanesthesia care unit (PACU), and surgical ward nurses. In addition, the staff from the home health agencies and social services attended the weekly meetings.
We conducted a retrospective review of all patients who had undergone unilateral TKA from 2013 to 2018 at VANTHCS. This was a consecutive, unselected cohort. All patients were under the care of a single surgeon using identical implant systems and identical surgical techniques. This study was approved by the institutional review board at VANTHCS. Patients were divided into 2 distinct and consecutive cohorts. The standard of care (SOC) group included all patients from 2013 to 2016. The ERAS group included all patients after the institution of the standardized protocol until the end of the study period.
Data on patient demographics, the American Society of Anesthesiologists risk classification, and preoperative functional status were extracted. Anesthesia techniques included either general endotracheal anesthesia or subarachnoid block with monitored anesthesia care. The quantity of the opioids given during surgery, in the PACU, during the inpatient stay, as discharge prescriptions, and as refills of the narcotic prescriptions up to 3 months postsurgery were recorded. All opioids were converted into morphine equivalent dosages (MED) in order to be properly analyzed using the statistical methodologies described in the statistical section.15 The VHA is a closed health care delivery system; therefore, all of the prescriptions ordered by surgery providers were recorded in the electronic health record.
ERAS Protocol
The SOC cohort was predominantly managed with general endotracheal anesthesia. The ERAS group was predominantly managed with subarachnoid blocks (Table 1). For the ERAS protocol preoperatively, the patients were administered oral gabapentin 300 mg, acetaminophen 650 mg, and oxycodone 20 mg, and IV ondansetron 4 mg. Intraoperatively, minimal opioids were used. In the PACU, the patients received dilaudid 0.25 mg IV as needed every 15 minutes for up to 1 mg/h. The nursing staff was trained to use the visual analog pain scale scores to titrate the medication. During the inpatient stay, patients received 1 g IV acetaminophen every 6 hours for 3 doses. The patients thereafter received oral acetaminophen as needed. Other medications in the multimodal pain-management protocol included gabapentin 300 mg twice daily, meloxicam 15 mg daily, and oxycodone 10 mg every 4 hours as needed. Rescue medication for insufficient pain relief was dilaudid 0.25 mg IV every 15 minutes for visual analog pain scale > 8. On discharge, the patients received a prescription of 30 tablets of hydrocodone 10 mg.
Periarticular Injections
Intraoperatively, all patients in the SOC and ERAS groups received periarticular injections. The liposomal bupivacaine injection was added to the standard injection mixture for the ERAS group. For the SOC group, the total volume of 100 ml was divided into 10 separate 10 cc syringes, and for the ERAS group, the total volume of 140 ml was divided into 14 separate 10 cc syringes. The SOC group injections were performed with an 18-gauge needle and the periarticular soft tissues grossly infiltrated. The ERAS group injections were done with more attention to anatomical detail. Injection sites for the ERAS group included the posterior joint capsule, the medial compartment, the lateral compartment, the tibial fat pad, the quadriceps and the patellar tendon, the femoral and tibial periosteum circumferentially, and the anterior joint capsule. Each needle-stick in the ERAS group delivered 1 to 1.5 ml through a 22-gauge needle to each compartment of the knee.
Outcome Variable
The primary outcome measure was total oral MED intraoperatively, in the PACU, during the hospital inpatient stay, in the hospital discharge prescription, and during the 3-month period after hospital discharge. Incidence of nausea and vomiting during the inpatient stay and any narcotic use at 6 months postsurgery were secondary binary outcomes.
Statistical Analysis
Demographic data and the clinical characteristics for the entire group were described using the sample mean and SD for continuous variables and the frequency and percentage for categorical variables. Differences between the 2 cohorts were analyzed using a 2-independent-sample t test and Fisher exact test.
The estimation of the total oral MED throughout all phases of care was done using a separate Poisson model due to the data being not normally distributed. A log-linear regression model was used to evaluate the main effect of ERAS vs the SOC cohort on the total oral MED used. Finally, a separate multiple logistic regression model was used to estimate the odds of postoperative nausea and vomiting and narcotic use at 6 months postsurgery between the cohorts. The adjusted odds ratio (OR) was estimated from the logistic model. Age, sex, body mass index, preoperative functional independence score, narcotic use within 3 months prior to surgery, anesthesia type used (subarachnoid block with monitored anesthesia care vs general endotracheal anesthesia), and postoperative complications (yes/no) were included as covariates in each model. The length of hospital stay and the above-mentioned factors were also included as covariates in the model estimating the total oral MED during the hospital stay, on hospital discharge, during the 3-month period after hospital discharge, and at 6 months following hospital discharge.
Statistical analysis was done using SAS version 9.4. The level of significance was set at α = 0.05 (2 tailed), and we implemented the false discovery rate (FDR) procedure to control false positives over multiple tests.16
Results
Two hundred forty-nine patients had 296 elective unilateral TKAs in this study from 2013 through 2018. Thirty-one patients had both unilateral TKAs under the SOC protocol; 5 patients had both unilateral TKAs under the ERAS protocol. Eleven of the patients who eventually had both knees replaced had 1 operation under each protocol The SOC group included 196 TKAs and the ERAS group included 100 TKAs. Of the 196 SOC patients, 94% were male. The mean age was 68.2 years (range, 48-86). The length of hospital stay ranged from 36.6 to 664.3 hours. Of the 100 ERAS patients, 96% were male (Table 2). The mean age was 66.7 years (range, 48-85). The length of hospital stay ranged from 12.5 to 45 hours.
Perioperative Opioid Use
Of the SOC patients, 99.0% received narcotics intraoperatively (range, 0-198 mg MED), and 74.5% received narcotics during PACU recovery (range, 0-141 mg MED). The total oral MED during the hospital stay for the SOC patients ranged from 10 to 2,946 mg. Of the ERAS patients, 86% received no narcotics during surgery (range, 0-110 mg MED), and 98% received no narcotics during PACU recovery (range, 0-65 mg MED). The total oral MED during the hospital stay for the ERAS patients ranged from 10 to 240 mg.
The MED used was significantly lower for the ERAS patients than it was for the SOC patients during surgery (10.5 mg vs 57.4 mg, P = .0001, FDR = .0002) and in the PACU (1.3 mg vs 13.6 mg, P = .0002, FDR = .0004), during the inpatient stay (66.7 mg vs 169.5 mg, P = .0001, FDR = .0002), and on hospital discharge (419.3 mg vs 776.7 mg, P = .0001, FDR = .0002). However, there was no significant difference in the total MED prescriptions filled between patients on the ERAS protocol vs those who received SOC during the 3-month period after hospital discharge (858.3 mg vs 1126.1 mg, P = .29, FDR = .29)(Table 3).
Finally, the logistic regression analysis, adjusting for the covariates demonstrated that the ERAS patients were less likely to take narcotics at 6 months following hospital discharge (OR, 0.23; P = .013; FDR = .018) and less likely to have postoperative nausea and vomiting (OR, 0.18; P = .019; FDR = .02) than SOC patients. There was no statistically significant difference between complication rates for the SOC and ERAS groups, which were 11.2% and 5.0%, respectively, with an overall complication rate of 9.1% (P = .09)(Table 4).
Discussion
Orthopedic surgery has been associated with long-term opioid use and misuse. Orthopedic surgeons are frequently among the highest prescribers of narcotics. According to Volkow and colleagues, orthopedic surgeons were the fourth largest prescribers of opioids in 2009, behind primary care physicians, internists, and dentists.17 The opioid crisis in the United States is well recognized. In 2017, > 70,000 deaths occurred due to drug overdoses, with 68% involving a prescription or illicit opioid. The Centers for Disease Control and Prevention has estimated a total economic burden of $78.5 billion per year as a direct result of misused prescribed opioids.18 This includes the cost of health care, lost productivity, addiction treatment, and the impact on the criminal justice system.
The current opioid crisis places further emphasis on opioid-reducing or sparing techniques in patients undergoing TKA. The use of liposomal bupivacaine for intraoperative periarticular injection is debated in the literature regarding its efficacy and whether it should be included in multimodal protocols. Researchers have argued that liposomal bupivacaine is not superior to regular bupivacaine and because of its increased cost is not justified.19,20 A meta-analysis from Zhao and colleagues showed no difference in pain control and functional recovery when comparing liposomal bupivacaine and control.21 In a randomized clinical trial, Schroer and colleagues matched liposomal bupivacaine against regular bupivacaine and found no difference in pain scores and similar narcotic use during hospitalization.22
Studies evaluating liposomal bupivacaine have demonstrated postoperative benefits in pain relief and potential opioid consumption.23 In a multicenter randomized controlled trial, Barrington and colleagues noted improved pain control at 6 and 12 hours after surgery with liposomal bupivacaine as a periarticular injection vs ropivacaine, though results were similar when compared with intrathecal morphine.24 Snyder and colleagues reported higher patient satisfaction in pain control and overall experience as well as decreased MED consumption in the PACU and on postoperative days 0 to 2 when using liposomal bupivacaine vs a multidrug cocktail for periarticular injection.25
The PILLAR trial, an industry-sponsored study, was designed to compare the effects of local infiltration anesthesia with and without liposomal bupivacaine with emphasis on a meticulous standardized infiltration technique. In our study, we used a similar technique with an expanded volume of injection solution to 140 ml that was delivered throughout the knee in a series of 14 syringes. Each needle-stick delivered 1 to 1.5 ml through a 22-gauge needle to each compartment of the knee. Infiltration technique has varied among the literature focused on periarticular injections.
In our experience, a standard infiltration technique is critical to the effective delivery of liposomal bupivacaine throughout all compartments of the knee and to obtaining reproducible pain control. The importance of injection technique cannot be overemphasized, and variations can be seen in studies published to date.26 Well-designed trials are needed to address this key component.
There have been limited data focused on the veteran population regarding postoperative pain-management strategies and recovery pathways either with or without liposomal bupivacaine. In a retrospective review, Sakamoto and colleagues found VA patients undergoing TKA had reduced opioid use in the first 24 hours after primary TKA with the use of intraoperative liposomal bupivacaine.27 The VA population has been shown to be at high risk for opioid misuse. The prevalence of comorbidities such as traumatic brain injury, posttraumatic stress disorder, and depression in the VA population also places them at risk for polypharmacy of central nervous system–acting medications.28 This emphasizes the importance of multimodal strategies, which can limit or eliminate narcotics in the perioperative period. The implementation of our ERAS protocol reduced opioid use during intraoperative, PACU, and inpatient hospital stay.
While the financial implications of our recovery protocol were not a primary focus of this study, there are many notable benefits on the overall inpatient cost to the VHA. According to the Health Economics Resource Center, the average daily cost of stay while under VA care for an inpatient surgical bed increased from $4,831 in 2013 to $6,220 in 2018.29 Our reduction in length of stay between our cohorts is 44.5 hours, which translates to a substantial financial savings per patient after protocol implementation. A more detailed look at the financial aspect of our protocol would need to be performed to evaluate the financial impact of other aspects of our protocol, such as the elimination of patient-controlled anesthesia and the reduction in total narcotics prescribed in the postoperative global period.
Limitations
The limitations of this study include its retrospective study design. With the VHA patient population, it may be subject to selection bias, as the population is mostly older and predominantly male compared with that of the general population. This could potentially influence the efficacy of our protocol on a population of patients with more women. In a recent study by Perruccio and colleagues, sex was found to moderate the effects of comorbidities, low back pain, and depressive symptoms on postoperative pain in patients undergoing TKA.30
With regard to outpatient narcotic prescriptions, although we cannot fully know whether these filled prescriptions were used for pain control, it is a reasonable assumption that patients who are dealing with continued postoperative or chronic pain issues will fill these prescriptions or seek refills. It is important to note that the data on prescriptions and refills in the 3-month postoperative period include all narcotic prescriptions filled by any VHA prescriber and are not specifically limited to our orthopedic team. For outpatient narcotic use, we were not able to access accurate pill counts for any discharge prescriptions or subsequent refills that were given throughout the VA system. We were able to report on total prescriptions filled in the first 3 months following TKA.
We calculated total oral MEDs to better understand the amount of narcotics being distributed throughout our population of patients. We believe this provides important information about the overall narcotic burden in the veteran population. There was no significant difference between the SOC and ERAS groups regarding oral MED prescribed in the 3-month postoperative period; however, at the 6-month follow-up visit, only 16% of patients in the ERAS group were taking any type of narcotic vs 37.2% in the SOC group (P = .0002).
Conclusions
A multidisciplinary ERAS protocol implemented at VANTHCS was effective in reducing length of stay and opioid burden throughout all phases of surgical care in our patients undergoing primary TKA. Patient and nursing education seem to be critical components to the implementation of a successful multimodal pain protocol. Reducing the narcotic burden has valuable financial and medical benefits in this at-risk population.
1. Inacio MCS, Paxton EW, Graves SE, Namba RS, Nemes S. Projected increase in total knee arthroplasty in the United States - an alternative projection model. Osteoarthritis Cartilage. 2017;25(11):1797-1803. doi:10.1016/j.joca.2017.07.022
2. Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of Postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council [published correction appears in J Pain. 2016 Apr;17(4):508-10. Dosage error in article text]. J Pain. 2016;17(2):131-157. doi:10.1016/j.jpain.2015.12.008
3. Moucha CS, Weiser MC, Levin EJ. Current Strategies in anesthesia and analgesia for total knee arthroplasty. J Am Acad Orthop Surg. 2016;24(2):60-73. doi:10.5435/JAAOS-D-14-00259
4. Parvizi J, Miller AG, Gandhi K. Multimodal pain management after total joint arthroplasty. J Bone Joint Surg Am. 2011;93(11):1075-1084. doi:10.2106/JBJS.J.01095
5. Jenstrup MT, Jæger P, Lund J, et al. Effects of adductor-canal-blockade on pain and ambulation after total knee arthroplasty: a randomized study. Acta Anaesthesiol Scand. 2012;56(3):357-364. doi:10.1111/j.1399-6576.2011.02621.x
6. Macfarlane AJ, Prasad GA, Chan VW, Brull R. Does regional anesthesia improve outcome after total knee arthroplasty?. Clin Orthop Relat Res. 2009;467(9):2379-2402. doi:10.1007/s11999-008-0666-9
7. Parvataneni HK, Shah VP, Howard H, Cole N, Ranawat AS, Ranawat CS. Controlling pain after total hip and knee arthroplasty using a multimodal protocol with local periarticular injections: a prospective randomized study. J Arthroplasty. 2007;22(6)(suppl 2):33-38. doi:10.1016/j.arth.2007.03.034
8. Busch CA, Shore BJ, Bhandari R, et al. Efficacy of periarticular multimodal drug injection in total knee arthroplasty. A randomized trial. J Bone Joint Surg Am. 2006;88(5):959-963. doi:10.2106/JBJS.E.00344
9. Lamplot JD, Wagner ER, Manning DW. Multimodal pain management in total knee arthroplasty: a prospective randomized controlled trial. J Arthroplasty. 2014;29(2):329-334. doi:10.1016/j.arth.2013.06.005
10. Hyland SJ, Deliberato DG, Fada RA, Romanelli MJ, Collins CL, Wasielewski RC. Liposomal bupivacaine versus standard periarticular injection in total knee arthroplasty with regional anesthesia: a prospective randomized controlled trial. J Arthroplasty. 2019;34(3):488-494. doi:10.1016/j.arth.2018.11.026
11. Barrington JW, Lovald ST, Ong KL, Watson HN, Emerson RH Jr. Postoperative pain after primary total knee arthroplasty: comparison of local injection analgesic cocktails and the role of demographic and surgical factors. J Arthroplasty. 2016;31(9) (suppl):288-292. doi:10.1016/j.arth.2016.05.002
12. Bramlett K, Onel E, Viscusi ER, Jones K. A randomized, double-blind, dose-ranging study comparing wound infiltration of DepoFoam bupivacaine, an extended-release liposomal bupivacaine, to bupivacaine HCl for postsurgical analgesia in total knee arthroplasty. Knee. 2012;19(5):530-536. doi:10.1016/j.knee.2011.12.004
13. Mont MA, Beaver WB, Dysart SH, Barrington JW, Del Gaizo D. Local infiltration analgesia with liposomal bupivacaine improves pain scores and reduces opioid use after total knee arthroplasty: results of a randomized controlled trial. J Arthroplasty. 2018;33(1):90-96. doi:10.1016/j.arth.2017.07.024
14. Hadlandsmyth K, Vander Weg MW, McCoy KD, Mosher HJ, Vaughan-Sarrazin MS, Lund BC. Risk for prolonged opioid use following total knee arthroplasty in veterans. J Arthroplasty. 2018;33(1):119-123. doi:10.1016/j.arth.2017.08.022
15. Nielsen S, Degenhardt L, Hoban B, Gisev N. A synthesis of oral morphine equivalents (OME) for opioid utilisation studies. Pharmacoepidemiol Drug Saf. 2016;25(6):733-737. doi:10.1002/pds.3945
16. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statist Soc B. 1995;57(1):289-300. doi:10.1111/j.2517-6161.1995.tb02031.x
17. Volkow ND, McLellan TA, Cotto JH, Karithanom M, Weiss SRB. Characteristics of opioid prescriptions in 2009. JAMA. 2011;305(13):1299-1301. doi:10.1001/jama.2011.401
18. Scholl L, Seth P, Kariisa M, Wilson N, Baldwin G. Drug and opioid-involved overdose deaths - United States, 2013-2017. MMWR Morb Mortal Wkly Rep. 2018;67(5152):1419-1427. doi:10.15585/mmwr.mm675152e1
19. Pichler L, Poeran J, Zubizarreta N, et al. Liposomal bupivacaine does not reduce inpatient opioid prescription or related complications after knee arthroplasty: a database analysis. Anesthesiology. 2018;129(4):689-699. doi:10.1097/ALN.0000000000002267
20. Jain RK, Porat MD, Klingenstein GG, Reid JJ, Post RE, Schoifet SD. The AAHKS Clinical Research Award: liposomal bupivacaine and periarticular injection are not superior to single-shot intra-articular injection for pain control in total knee arthroplasty. J Arthroplasty. 2016;31(9)(suppl):22-25. doi:10.1016/j.arth.2016.03.036
21. Zhao B, Ma X, Zhang J, Ma J, Cao Q. The efficacy of local liposomal bupivacaine infiltration on pain and recovery after total joint arthroplasty: a systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore). 2019;98(3):e14092. doi:10.1097/MD.0000000000014092
22. Schroer WC, Diesfeld PG, LeMarr AR, Morton DJ, Reedy ME. Does extended-release liposomal bupivacaine better control pain than bupivacaine after total knee arthroplasty (TKA)? A prospective, randomized clinical trial. J Arthroplasty. 2015;30(9)(suppl):64-67. doi:10.1016/j.arth.2015.01.059
23. Ma J, Zhang W, Yao S. Liposomal bupivacaine infiltration versus femoral nerve block for pain control in total knee arthroplasty: a systematic review and meta-analysis. Int J Surg. 2016;36(Pt A): 44-55. doi:10.1016/j.ijsu.2016.10.007
24. Barrington JW, Emerson RH, Lovald ST, Lombardi AV, Berend KR. No difference in early analgesia between liposomal bupivacaine injection and intrathecal morphine after TKA. Clin Orthop Relat Res. 2017;475(1):94-105. doi:10.1007/s11999-016-4931-z
25. Snyder MA, Scheuerman CM, Gregg JL, Ruhnke CJ, Eten K. Improving total knee arthroplasty perioperative pain management using a periarticular injection with bupivacaine liposomal suspension. Arthroplast Today. 2016;2(1):37-42. doi:10.1016/j.artd.2015.05.005
26. Kuang MJ,Du Y, Ma JX, He W, Fu L, Ma XL. The efficacy of liposomal bupivacaine using periarticular injection in total knee arthroplasty: a systematic review and meta-analysis. J Arthroplasty. 2017;32(4):1395-1402. doi:10.1016/j.arth.2016.12.025
27. Sakamoto B, Keiser S, Meldrum R, Harker G, Freese A. Efficacy of liposomal bupivacaine infiltration on the management of total knee arthroplasty. JAMA Surg. 2017;152(1):90-95. doi:10.1001/jamasurg.2016.3474
28. Collett GA, Song K, Jaramillo CA, Potter JS, Finley EP, Pugh MJ. Prevalence of central nervous system polypharmacy and associations with overdose and suicide-related behaviors in Iraq and Afghanistan war veterans in VA care 2010-2011. Drugs Real World Outcomes. 2016;3(1):45-52. doi:10.1007/s40801-015-0055-0
29. US Department of Veterans Affairs. HERC inpatient average cost data. Updated April 2, 2021. Accessed April 16, 2021. https://www.herc.research.va.gov/include/page.asp?id=inpatient#herc-inpat-avg-cost
30. Perruccio AV, Fitzpatrick J, Power JD, et al. Sex-modified effects of depression, low back pain, and comorbidities on pain after total knee arthroplasty for osteoarthritis. Arthritis Care Res (Hoboken). 2020;72(8):1074-1080. doi:10.1002/acr.24002
1. Inacio MCS, Paxton EW, Graves SE, Namba RS, Nemes S. Projected increase in total knee arthroplasty in the United States - an alternative projection model. Osteoarthritis Cartilage. 2017;25(11):1797-1803. doi:10.1016/j.joca.2017.07.022
2. Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of Postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council [published correction appears in J Pain. 2016 Apr;17(4):508-10. Dosage error in article text]. J Pain. 2016;17(2):131-157. doi:10.1016/j.jpain.2015.12.008
3. Moucha CS, Weiser MC, Levin EJ. Current Strategies in anesthesia and analgesia for total knee arthroplasty. J Am Acad Orthop Surg. 2016;24(2):60-73. doi:10.5435/JAAOS-D-14-00259
4. Parvizi J, Miller AG, Gandhi K. Multimodal pain management after total joint arthroplasty. J Bone Joint Surg Am. 2011;93(11):1075-1084. doi:10.2106/JBJS.J.01095
5. Jenstrup MT, Jæger P, Lund J, et al. Effects of adductor-canal-blockade on pain and ambulation after total knee arthroplasty: a randomized study. Acta Anaesthesiol Scand. 2012;56(3):357-364. doi:10.1111/j.1399-6576.2011.02621.x
6. Macfarlane AJ, Prasad GA, Chan VW, Brull R. Does regional anesthesia improve outcome after total knee arthroplasty?. Clin Orthop Relat Res. 2009;467(9):2379-2402. doi:10.1007/s11999-008-0666-9
7. Parvataneni HK, Shah VP, Howard H, Cole N, Ranawat AS, Ranawat CS. Controlling pain after total hip and knee arthroplasty using a multimodal protocol with local periarticular injections: a prospective randomized study. J Arthroplasty. 2007;22(6)(suppl 2):33-38. doi:10.1016/j.arth.2007.03.034
8. Busch CA, Shore BJ, Bhandari R, et al. Efficacy of periarticular multimodal drug injection in total knee arthroplasty. A randomized trial. J Bone Joint Surg Am. 2006;88(5):959-963. doi:10.2106/JBJS.E.00344
9. Lamplot JD, Wagner ER, Manning DW. Multimodal pain management in total knee arthroplasty: a prospective randomized controlled trial. J Arthroplasty. 2014;29(2):329-334. doi:10.1016/j.arth.2013.06.005
10. Hyland SJ, Deliberato DG, Fada RA, Romanelli MJ, Collins CL, Wasielewski RC. Liposomal bupivacaine versus standard periarticular injection in total knee arthroplasty with regional anesthesia: a prospective randomized controlled trial. J Arthroplasty. 2019;34(3):488-494. doi:10.1016/j.arth.2018.11.026
11. Barrington JW, Lovald ST, Ong KL, Watson HN, Emerson RH Jr. Postoperative pain after primary total knee arthroplasty: comparison of local injection analgesic cocktails and the role of demographic and surgical factors. J Arthroplasty. 2016;31(9) (suppl):288-292. doi:10.1016/j.arth.2016.05.002
12. Bramlett K, Onel E, Viscusi ER, Jones K. A randomized, double-blind, dose-ranging study comparing wound infiltration of DepoFoam bupivacaine, an extended-release liposomal bupivacaine, to bupivacaine HCl for postsurgical analgesia in total knee arthroplasty. Knee. 2012;19(5):530-536. doi:10.1016/j.knee.2011.12.004
13. Mont MA, Beaver WB, Dysart SH, Barrington JW, Del Gaizo D. Local infiltration analgesia with liposomal bupivacaine improves pain scores and reduces opioid use after total knee arthroplasty: results of a randomized controlled trial. J Arthroplasty. 2018;33(1):90-96. doi:10.1016/j.arth.2017.07.024
14. Hadlandsmyth K, Vander Weg MW, McCoy KD, Mosher HJ, Vaughan-Sarrazin MS, Lund BC. Risk for prolonged opioid use following total knee arthroplasty in veterans. J Arthroplasty. 2018;33(1):119-123. doi:10.1016/j.arth.2017.08.022
15. Nielsen S, Degenhardt L, Hoban B, Gisev N. A synthesis of oral morphine equivalents (OME) for opioid utilisation studies. Pharmacoepidemiol Drug Saf. 2016;25(6):733-737. doi:10.1002/pds.3945
16. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statist Soc B. 1995;57(1):289-300. doi:10.1111/j.2517-6161.1995.tb02031.x
17. Volkow ND, McLellan TA, Cotto JH, Karithanom M, Weiss SRB. Characteristics of opioid prescriptions in 2009. JAMA. 2011;305(13):1299-1301. doi:10.1001/jama.2011.401
18. Scholl L, Seth P, Kariisa M, Wilson N, Baldwin G. Drug and opioid-involved overdose deaths - United States, 2013-2017. MMWR Morb Mortal Wkly Rep. 2018;67(5152):1419-1427. doi:10.15585/mmwr.mm675152e1
19. Pichler L, Poeran J, Zubizarreta N, et al. Liposomal bupivacaine does not reduce inpatient opioid prescription or related complications after knee arthroplasty: a database analysis. Anesthesiology. 2018;129(4):689-699. doi:10.1097/ALN.0000000000002267
20. Jain RK, Porat MD, Klingenstein GG, Reid JJ, Post RE, Schoifet SD. The AAHKS Clinical Research Award: liposomal bupivacaine and periarticular injection are not superior to single-shot intra-articular injection for pain control in total knee arthroplasty. J Arthroplasty. 2016;31(9)(suppl):22-25. doi:10.1016/j.arth.2016.03.036
21. Zhao B, Ma X, Zhang J, Ma J, Cao Q. The efficacy of local liposomal bupivacaine infiltration on pain and recovery after total joint arthroplasty: a systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore). 2019;98(3):e14092. doi:10.1097/MD.0000000000014092
22. Schroer WC, Diesfeld PG, LeMarr AR, Morton DJ, Reedy ME. Does extended-release liposomal bupivacaine better control pain than bupivacaine after total knee arthroplasty (TKA)? A prospective, randomized clinical trial. J Arthroplasty. 2015;30(9)(suppl):64-67. doi:10.1016/j.arth.2015.01.059
23. Ma J, Zhang W, Yao S. Liposomal bupivacaine infiltration versus femoral nerve block for pain control in total knee arthroplasty: a systematic review and meta-analysis. Int J Surg. 2016;36(Pt A): 44-55. doi:10.1016/j.ijsu.2016.10.007
24. Barrington JW, Emerson RH, Lovald ST, Lombardi AV, Berend KR. No difference in early analgesia between liposomal bupivacaine injection and intrathecal morphine after TKA. Clin Orthop Relat Res. 2017;475(1):94-105. doi:10.1007/s11999-016-4931-z
25. Snyder MA, Scheuerman CM, Gregg JL, Ruhnke CJ, Eten K. Improving total knee arthroplasty perioperative pain management using a periarticular injection with bupivacaine liposomal suspension. Arthroplast Today. 2016;2(1):37-42. doi:10.1016/j.artd.2015.05.005
26. Kuang MJ,Du Y, Ma JX, He W, Fu L, Ma XL. The efficacy of liposomal bupivacaine using periarticular injection in total knee arthroplasty: a systematic review and meta-analysis. J Arthroplasty. 2017;32(4):1395-1402. doi:10.1016/j.arth.2016.12.025
27. Sakamoto B, Keiser S, Meldrum R, Harker G, Freese A. Efficacy of liposomal bupivacaine infiltration on the management of total knee arthroplasty. JAMA Surg. 2017;152(1):90-95. doi:10.1001/jamasurg.2016.3474
28. Collett GA, Song K, Jaramillo CA, Potter JS, Finley EP, Pugh MJ. Prevalence of central nervous system polypharmacy and associations with overdose and suicide-related behaviors in Iraq and Afghanistan war veterans in VA care 2010-2011. Drugs Real World Outcomes. 2016;3(1):45-52. doi:10.1007/s40801-015-0055-0
29. US Department of Veterans Affairs. HERC inpatient average cost data. Updated April 2, 2021. Accessed April 16, 2021. https://www.herc.research.va.gov/include/page.asp?id=inpatient#herc-inpat-avg-cost
30. Perruccio AV, Fitzpatrick J, Power JD, et al. Sex-modified effects of depression, low back pain, and comorbidities on pain after total knee arthroplasty for osteoarthritis. Arthritis Care Res (Hoboken). 2020;72(8):1074-1080. doi:10.1002/acr.24002
Reducing False-Positive Results With Fourth-Generation HIV Testing at a Veterans Affairs Medical Center
Ever since the first clinical reports of patients with AIDS in 1981, there have been improvements both in the knowledge base of the pathogenesis of HIV in causing AIDS as well as a progressive refinement in the test methodologies used to diagnose this illness.1-3 Given that there are both public health and clinical benefits in earlier diagnosis and treatment of patients with available antiretroviral therapies, universal screening with opt-out consent has been a standard of practice recommendation by the Centers of Disease Control and Prevention (CDC) since 2006; universal screening with opt-out consent also has been recommended by the US Preventative Task Force and has been widely implemented.4-7
HIV Screening
While HIV screening assays have evolved to be accurate with very high sensitivities and specificities, false-positive results are a significant issue both currently and historically.8-16 The use of an HIV assay on a low prevalence population predictably reduces the positive predictive value (PPV) of even an otherwise accurate assay.8-23 In light of this, laboratory HIV testing algorithms include confirmatory testing to increase the likelihood that the correct diagnosis is being rendered.
The fourth-generation assay has been shown to be more sensitive and specific compared with that of the third-generation assay due to the addition of detection of p24 antigen and the refinement of the antigenic targets for the antibody detection.6,8,11-13,18-20,22 Due to these improvements, in the general population, increased sensitivity/specificity with a reduction in both false positives and false negatives have been reported.
It has been observed in the nonveteran population that switching from the older third-generation to a more sensitive and specific fourth-generation HIV screening assay has reduced the false-positive screening rate.18,19,22 For instance, Muthukumar and colleagues demonstrated a false-positive rate of only 2 out of 99 (2%) tested specimens for the fourth-generation ARCHITECT HIV Ag/Ab Combo assay vs 9 out of 99 tested specimens (9%) for the third-generation ADVIA Centaur HIV 1/O/2 Enhanced assay.18 In addition, it has been noted that fourth-generation HIV screening assays can reduce the window period by detecting HIV infection sooner after initial acute infection.19 Mitchell and colleagues demonstrated even highly specific fourth-generation HIV assays with specificities estimated at 99.7% can have PPVs as low as 25.0% if used in a population of low HIV prevalence (such as a 0.1% prevalence population).19 However, the veteran population has been documented to differ significantly on a number of population variables, including severity of disease and susceptibility to infections, and as a result extrapolation of these data from the general population may be limited.24-26 To our knowledge, this article represents the first study directly examining the reduction in false-positive results with the switch to a fourth-generation HIV generation assay from a third-generation assay for the veteran patient population at a regional US Department of Veterans Affairs (VA) medical center (VAMC).8,11
Methods
Quality assurance documents on test volume were retrospectively reviewed to obtain the number of HIV screening tests that were performed by the laboratory at the Corporal Michael J. Crescenz VAMC (CMJCVAMC) in Philadelphia, Pennsylvania, between March 1, 2016 and February 28, 2017, prior to implementation of the fourth-generation assay. The study also include results from the first year of use of the fourth-generation assay (March 1, 2017 to February 28, 2018). In addition, paper quality assurance records of all positive screening results during those periods were reviewed and manually counted for the abstract presentation of these data.
For assurance of accuracy, a search of all HIV testing assays using Veterans Health Information Systems and Technology Architecture and FileMan also was performed, and the results were compared to records in the Computerized Patient Record System (CPRS). Any discrepancies in the numbers of test results generated by both searches were investigated, and data for the manuscript were derived from records associating tests with particular patients. Only results from patient samples were considered for the electronic search. Quality samples that did not correspond to a true patient as identified in CPRS or same time patient sample duplicates were excluded from the calculations. Basic demographic data (age, ethnicity, and gender) were obtained from this FileMan search. The third-generation assay was the Ortho-Clinical Diagnostics Vitros, and the fourth-generation assay was the Abbott Architect.
To interpret the true HIV result of each sample with a reactive or positive screening result, the CDC laboratory HIV testing algorithm was followed and reviewed with a clinical pathologist or microbiologist director.12,13 All specimens interpreted as HIV positive by the pathologist or microbiologist director were discussed with the clinical health care provider at the time of the test with results added to CPRS after all testing was complete and discussions had taken place. All initially reactive specimens (confirmed with retesting in duplicate on the screening platform with at least 1 repeat reactive result) were further tested with the Bio-Rad Geenius HIV 1/2 Supplemental Assay, which screens for both HIV-1 and HIV-2 antibodies. Specimens with reactive results by this supplemental assay were interpreted as positive for HIV based on the CDC laboratory HIV testing algorithm. Specimens with negative or indeterminant results by the supplemental assay then underwent HIV-1 nucleic acid testing (NAT) using the Roche Diagnostics COBAS AmpliPrep/COBAS TaqMan HIV-1 Test v2.0. Specimens with viral load detected on NAT were positive for HIV infection, while specimens with viral load not detected on NAT testing were interpreted as negative for HIV-1 infection. Although there were no HIV-2 positive or indeterminant specimens during the study period, HIV-2 reactivity also would have been interpreted per the CDC laboratory HIV testing algorithm. Specimens with inadequate volume to complete all testing steps would be interpreted as indeterminant for HIV with request for additional specimen to complete testing. All testing platforms used for HIV testing in the laboratory had been properly validated prior to use.
The number of false positives and indeterminant results was tabulated in Microsoft Excel by month throughout the study period alongside the total number of HIV screening tests performed. Statistical analyses to verify statistical significance was performed by 1-tailed homoscedastic t test calculation using Excel.
Results
From March 1, 2016 to February 28, 2017, 7,516 specimens were screened for HIV, using the third-generation assay, and 52 specimens tested positive for HIV. On further review of these reactive specimens per the CDC laboratory testing algorithm, 24 tests were true positive and 28 were false positives with a PPV of 46% (24/52) (Figure 1).
From March 1, 2017 to February 28, 2018, 7,802 specimens were screened for HIV using a fourth-generation assay and 23 tested positive for HIV. On further review of these reactive specimens per the CDC laboratory testing algorithm, 16 were true positive and 7 were false positives with a PPV of 70% (16/23).
The fourth-generation assay was more specific when compared with the third-generation assay (0.09% vs 0.37%, respectively) with a 75.7% decrease in the false-positivity rate after the implementation of fourth-generation testing. The decreased number of false-positive test results per month with the fourth-generation test implementation was statistically significant (P = .002). The mean (SD) number of false-positive test results for the third-generation assay was 2.3 (1.7) per month, while the fourth-generation assay only had a mean (SD) of 0.58 (0.9) false positives monthly. The decrease in the percentage of false positives per month with the implementation of the fourth-generation assay also was statistically significant (P = .002) (Figure 2).
For population-based reference of the tested population at CMJCVAMC, there was a FileMan search for basic demographic data of patients for the HIV specimens screened by the third- or fourth-generation test (Table). For the population tested by the third-generation assay, 1,114 out of the 7,516 total tested population did not have readily available demographic information by the FileMan search as the specimens originated outside of the facility. For 6,402 of 7,516 patients tested by the third-generation assay with demographic information, the age ranged from 25 to 97 years with a mean of 57 years. This population of 6,402 was 88% male (n = 5,639), 50% African American (n = 3,220) and 43% White (n = 2,756). For the population tested by the fourth-generation assay, 993 of 7,802 total tested population did not have readily available demographic information by the FileMan search as the specimens originated outside of the facility. For the 6,809 of 7,802 patients tested by the fourth-generation assay with demographic information, the age ranged from 24 to 97 years with a mean age of 56 years. This population was 88% male (n = 5,971), 47% African American (n = 3,189), and 46% White (n = 3,149).
Discussion
Current practice guidelines from the CDC and the US Preventive Services Task Force recommend universal screening of the population for HIV infection.5,6 As the general population to be screened would normally have a low prevalence of HIV infection, the risk of a false positive on the initial screen is significant.17 Indeed, the CMJCVAMC experience has been that with the third-generation screening assay, the number of false-positive test results outnumbered the number of true-positive test results. Even with the fourth-generation assay, approximately one-third of the results were false positives. These results are similar to those observed in studies involving nonveteran populations in which the implementation of a fourth-generation screening assay led to significantly fewer false-positive results.18
For laboratories that do not follows CDC testing algorithm guidelines, each false-positive screening result represents a potential opportunity for a HIV misdiagnosis.Even in laboratories with proper procedures in place, false-positive results have consequences for the patients and for the cost-effectiveness of laboratory operations.9-11,18 As per CDC HIV testing guidelines, all positive screening results should be retested, which leads to additional use of technologist time and reagents. After this additional testing is performed and reviewed appropriately, only then can an appropriate final laboratory diagnosis be rendered that meets the standard of laboratory care.
Cost Savings
As observed at CMJCVAMC, the use of a fourth-generation assay with increased sensitivity/specificity led to a reduction in these false-positive results, which improved laboratory efficiency and avoided wasted resources for confirmatory tests.11,18 Cost savings at CMJCVAMC from the implementation of the fourth-generation assay would include technologist time and reagent cost. Generalizable technologist time costs at any institution would include the time needed to perform the confirmatory HIV-1/HIV-2 antibody differentiation assay (slightly less than 1 hour at CMJCVAMC per specimen) and the time needed to perform the viral load assay (about 6 hours to run a batch of 24 tests at CMJCVAMC). We calculated that confirmatory testing cost $184.51 per test at CMJCVAMC. Replacing the third-generation assay with the more sensitive and specific fourth-generation test saved an estimated $3,875 annually. This cost savings does not even consider savings in the pathologist/director’s time for reviewing HIV results after the completion of the algorithm or the clinician/patient costs or anxiety while waiting for results of the confirmatory sequence of tests.
As diagnosis of HIV can have a significant psychological impact on the patient, it is important to ensure the diagnosis conveyed is correct.27 The provision of an HIV diagnosis to a patient has been described as a traumatic stressor capable of causing psychological harm; this harm should ideally be avoided if the HIV diagnosis is not accurate. There can be a temptation, when presented with a positive or reactive screening test that is known to come from an instrument or assay with a very high sensitivity and specificity, to present this result as a diagnosis to the patient. However, a false diagnosis from a false-positive screen would not only be harmful, but given the low prevalence of the disease in the screened population, would happen fairly frequently; in some settings the number of false positives may actually outnumber the number of true positive test results.
Better screening assays with greater specificity (even fractions of a percentage, given that specificities are already > 99%) would help reduce the number of false positives and reduce the number of potential enticements to convey an incorrect diagnosis. Therefore, by adding an additional layer of safety through greater specificity, the fourth-generation assay implementation helped improve the diagnostic safety of the laboratory and reduced the significant error risk to the clinician who would ultimately bear responsibility for conveying the HIV diagnoses to the patient. Given the increased prevalence of psychological and physical ailments in veterans, it may be even more important to ensure the diagnosis is correct to avoid increased psychological harm.27,28
Veteran Population
For the general population, the fourth-generation assay has been shown to be more sensitive and specific when compared with the third-generation assay due to the addition of detection of p24 antigen and the refinement of the antigenic targets for the antibody detection.6,8,11-13,18-20,22 However, the veteran population that receives VA medical care differs significantly from the nonveteran general population. Compared with nonveterans, veterans tend to have generally poorer health status, more comorbid conditions, and greater need to use medical resources.24-26 In addition, veterans also may differ in sociodemographic status, race, ethnicity, and gender.24-26
VA research in the veteran population is unique, and veterans who use VA health care services are an even more highly selected subpopulation.26 Conclusions made from studies of the general population may not always be applicable to the veteran population treated by VA health care services due to these population differences. Therefore, specific studies tailored to this special veteran population in the specific VA health care setting are essential to ensure that the results of the general population truly and definitively apply to the veteran population.
While the false-positive risk is most closely associated with testing in a population of low prevalence, it also should be noted that false-positive screening results also can occur in high-risk individuals, such as an individual on preexposure prophylaxis (PrEP) for continuous behavior that places the individual at high risk of HIV acquisition.8,29 The false-positive result in these cases can lead to a conundrum for the clinician, and the differential diagnosis should consider both detection of very early infection as well as false positive. Interventions could include either stopping PrEP and treating for presumed early primary infection with HIV or continuing the PrEP. These interventions all have the potential to impact the patient whether through the production of resistant HIV virus due to the inadvertent provision of an inadequate treatment regimen, increased risk of infection if taken off PrEP as the patient may likely continue the behavior regardless, or the risks carried by the administration of additional antiretroviral therapies for the complete empiric therapy. Cases of an individual on PrEP who had a false-positive HIV screening test has been reported previously both within and outside the veteran population.8 Better screening tests with greater sensitivity/specificity can only help in guiding better patient care.
Limitations
This quality assurance study was limited to retrospectively identifying the improvement in the false-positive rate on the transition from the third-generation to the more advanced fourth-generation HIV screen. False-positive screen cases could be easily picked up on review of the confirmatory testing per the CDC laboratory HIV testing algorithm.12,13 This study also was a retrospective review of clinically ordered and indicated testing; as a result, without confirmatory testing performed on all negative screen cases, a false-negative rate would not be calculable.
This study also was restricted to only the population being treated in a VA health care setting. This population is known to be different from the general population.24-26
Conclusions
The switch to a fourth-generation assay resulted in a significant reduction in false-positive test results for veteran patients at CMJCVAMC. This reduction in false-positive screening not only reduced laboratory workload due to the necessary confirmatory testing and subsequent review, but also saved costs for technologist’s time and reagents. While this reduction in false-positive results has been documented in nonveteran populations, this is the first study specifically on a veteran population treated at a VAMC.8,11,18 This study confirms previously documented findings of improvement in the false-positive rate of HIV screening tests with the change from third-generation to fourth-generation assay for a veteran population.24
1. Feinberg MB. Changing the natural history of HIV disease. Lancet. 1996;348(9022):239-246. doi:10.1016/s0140-6736(96)06231-9.
2. Alexander TS. Human immunodeficiency virus diagnostic testing: 30 years of evolution. Clin Vaccine Immunol. 2016;23(4):249-253. Published 2016 Apr 4. doi:10.1128/CVI.00053-16
3. Mortimer PP, Parry JV, Mortimer JY. Which anti-HTLV III/LAV assays for screening and confirmatory testing?. Lancet. 1985;2(8460):873-877. doi:10.1016/s0140-6736(85)90136-9
4. Holmberg SD, Palella FJ Jr, Lichtenstein KA, Havlir DV. The case for earlier treatment of HIV infection [published correction appears in Clin Infect Dis. 2004 Dec 15;39(12):1869]. Clin Infect Dis. 2004;39(11):1699-1704. doi:10.1086/425743
5. US Preventive Services Task Force, Owens DK, Davidson KW, et al. Screening for HIV Infection: US Preventive Services Task Force Recommendation Statement. JAMA. 2019;321(23):2326-2336. doi:10.1001/jama.2019.6587
6. Branson BM, Handsfield HH, Lampe MA, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55(RR-14):1-CE4.
7. Bayer R, Philbin M, Remien RH. The end of written informed consent for HIV testing: not with a bang but a whimper. Am J Public Health. 2017;107(8):1259-1265. doi:10.2105/AJPH.2017.303819
8. Petersen J, Jhala D. Its not HIV! The pitfall of unconfirmed positive HIV screening assays. Abstract presented at: Annual Meeting Pennsylvania Association of Pathologists; April 14, 2018.
9. Wood RW, Dunphy C, Okita K, Swenson P. Two “HIV-infected” persons not really infected. Arch Intern Med. 2003;163(15):1857-1859. doi:10.1001/archinte.163.15.1857
10. Permpalung N, Ungprasert P, Chongnarungsin D, Okoli A, Hyman CL. A diagnostic blind spot: acute infectious mononucleosis or acute retroviral syndrome. Am J Med. 2013;126(9):e5-e6. doi:10.1016/j.amjmed.2013.03.017
11. Dalal S, Petersen J, Luta D, Jhala D. Third- to fourth-generation HIV testing: reduction in false-positive results with the new way of testing, the Corporal Michael J. Crescenz Veteran Affairs Medical Center (CMCVAMC) Experience. Am J Clin Pathol.2018;150(suppl 1):S70-S71. doi:10.1093/ajcp/aqy093.172
12. Centers for Disease Control and Prevention. Laboratory testing for the diagnosis of HIV infection: updated recommendations. Published June 27, 2014. Accessed April 14, 2021. doi:10.15620/cdc.23447
13. Centers for Disease Control and Prevention. 2018 quick reference guide: recommended laboratory HIV testing algorithm for serum or plasma specimens. Updated January 2018. Accessed April 14, 202. https://stacks.cdc.gov/view/cdc/50872
14. Masciotra S, McDougal JS, Feldman J, Sprinkle P, Wesolowski L, Owen SM. Evaluation of an alternative HIV diagnostic algorithm using specimens from seroconversion panels and persons with established HIV infections. J Clin Virol. 2011;52(suppl 1):S17-S22. doi:10.1016/j.jcv.2011.09.011
15. Morton A. When lab tests lie … heterophile antibodies. Aust Fam Physician. 2014;43(6):391-393.
16. Spencer DV, Nolte FS, Zhu Y. Heterophilic antibody interference causing false-positive rapid human immunodeficiency virus antibody testing. Clin Chim Acta. 2009;399(1-2):121-122. doi:10.1016/j.cca.2008.09.030
17. Kim S, Lee JH, Choi JY, Kim JM, Kim HS. False-positive rate of a “fourth-generation” HIV antigen/antibody combination assay in an area of low HIV prevalence. Clin Vaccine Immunol. 2010;17(10):1642-1644. doi:10.1128/CVI.00258-10
18. Muthukumar A, Alatoom A, Burns S, et al. Comparison of 4th-generation HIV antigen/antibody combination assay with 3rd-generation HIV antibody assays for the occurrence of false-positive and false-negative results. Lab Med. 2015;46(2):84-e29. doi:10.1309/LMM3X37NSWUCMVRS
19. Mitchell EO, Stewart G, Bajzik O, Ferret M, Bentsen C, Shriver MK. Performance comparison of the 4th generation Bio-Rad Laboratories GS HIV Combo Ag/Ab EIA on the EVOLIS™ automated system versus Abbott ARCHITECT HIV Ag/Ab Combo, Ortho Anti-HIV 1+2 EIA on Vitros ECi and Siemens HIV-1/O/2 enhanced on Advia Centaur. J Clin Virol. 2013;58(suppl 1):e79-e84. doi:10.1016/j.jcv.2013.08.009
20. Dubravac T, Gahan TF, Pentella MA. Use of the Abbott Architect HIV antigen/antibody assay in a low incidence population. J Clin Virol. 2013;58(suppl 1):e76-e78. doi:10.1016/j.jcv.2013.10.020
21. Montesinos I, Eykmans J, Delforge ML. Evaluation of the Bio-Rad Geenius HIV-1/2 test as a confirmatory assay. J Clin Virol. 2014;60(4):399-401. doi:10.1016/j.jcv.2014.04.025
22. van Binsbergen J, Siebelink A, Jacobs A, et al. Improved performance of seroconversion with a 4th generation HIV antigen/antibody assay. J Virol Methods. 1999;82(1):77-84. doi:10.1016/s0166-0934(99)00086-5
23. CLSI. User Protocol for Evaluation of Qualitative Test Performance: Approved Guideline. Second ed. EP12-A2. CLSI; 2008:1-46.
24. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252-3257. doi:10.1001/archinte.160.21.3252
25. Eibner C, Krull H, Brown KM, et al. Current and projected characteristics and unique health care needs of the patient population served by the Department of Veterans Affairs. Rand Health Q. 2016;5(4):13. Published 2016 May 9.
26. Morgan RO, Teal CR, Reddy SG, Ford ME, Ashton CM. Measurement in Veterans Affairs Health Services Research: veterans as a special population. Health Serv Res. 2005;40(5, pt 2):1573-1583. doi:10.1111/j.1475-6773.2005.00448.x
27. Nightingale VR, Sher TG, Hansen NB. The impact of receiving an HIV diagnosis and cognitive processing on psychological distress and posttraumatic growth. J Trauma Stress. 2010;23(4):452-460. doi:10.1002/jts.20554
28. Spelman JF, Hunt SC, Seal KH, Burgo-Black AL. Post deployment care for returning combat veterans. J Gen Intern Med. 2012;27(9):1200-1209. doi:10.1007/s11606-012-2061-1
29. Ndase P, Celum C, Kidoguchi L, et al. Frequency of false positive rapid HIV serologic tests in African men and women receiving PrEP for HIV prevention: implications for programmatic roll-out of biomedical interventions. PLoS One. 2015;10(4):e0123005. Published 2015 Apr 17. doi:10.1371/journal.pone.0123005
Ever since the first clinical reports of patients with AIDS in 1981, there have been improvements both in the knowledge base of the pathogenesis of HIV in causing AIDS as well as a progressive refinement in the test methodologies used to diagnose this illness.1-3 Given that there are both public health and clinical benefits in earlier diagnosis and treatment of patients with available antiretroviral therapies, universal screening with opt-out consent has been a standard of practice recommendation by the Centers of Disease Control and Prevention (CDC) since 2006; universal screening with opt-out consent also has been recommended by the US Preventative Task Force and has been widely implemented.4-7
HIV Screening
While HIV screening assays have evolved to be accurate with very high sensitivities and specificities, false-positive results are a significant issue both currently and historically.8-16 The use of an HIV assay on a low prevalence population predictably reduces the positive predictive value (PPV) of even an otherwise accurate assay.8-23 In light of this, laboratory HIV testing algorithms include confirmatory testing to increase the likelihood that the correct diagnosis is being rendered.
The fourth-generation assay has been shown to be more sensitive and specific compared with that of the third-generation assay due to the addition of detection of p24 antigen and the refinement of the antigenic targets for the antibody detection.6,8,11-13,18-20,22 Due to these improvements, in the general population, increased sensitivity/specificity with a reduction in both false positives and false negatives have been reported.
It has been observed in the nonveteran population that switching from the older third-generation to a more sensitive and specific fourth-generation HIV screening assay has reduced the false-positive screening rate.18,19,22 For instance, Muthukumar and colleagues demonstrated a false-positive rate of only 2 out of 99 (2%) tested specimens for the fourth-generation ARCHITECT HIV Ag/Ab Combo assay vs 9 out of 99 tested specimens (9%) for the third-generation ADVIA Centaur HIV 1/O/2 Enhanced assay.18 In addition, it has been noted that fourth-generation HIV screening assays can reduce the window period by detecting HIV infection sooner after initial acute infection.19 Mitchell and colleagues demonstrated even highly specific fourth-generation HIV assays with specificities estimated at 99.7% can have PPVs as low as 25.0% if used in a population of low HIV prevalence (such as a 0.1% prevalence population).19 However, the veteran population has been documented to differ significantly on a number of population variables, including severity of disease and susceptibility to infections, and as a result extrapolation of these data from the general population may be limited.24-26 To our knowledge, this article represents the first study directly examining the reduction in false-positive results with the switch to a fourth-generation HIV generation assay from a third-generation assay for the veteran patient population at a regional US Department of Veterans Affairs (VA) medical center (VAMC).8,11
Methods
Quality assurance documents on test volume were retrospectively reviewed to obtain the number of HIV screening tests that were performed by the laboratory at the Corporal Michael J. Crescenz VAMC (CMJCVAMC) in Philadelphia, Pennsylvania, between March 1, 2016 and February 28, 2017, prior to implementation of the fourth-generation assay. The study also include results from the first year of use of the fourth-generation assay (March 1, 2017 to February 28, 2018). In addition, paper quality assurance records of all positive screening results during those periods were reviewed and manually counted for the abstract presentation of these data.
For assurance of accuracy, a search of all HIV testing assays using Veterans Health Information Systems and Technology Architecture and FileMan also was performed, and the results were compared to records in the Computerized Patient Record System (CPRS). Any discrepancies in the numbers of test results generated by both searches were investigated, and data for the manuscript were derived from records associating tests with particular patients. Only results from patient samples were considered for the electronic search. Quality samples that did not correspond to a true patient as identified in CPRS or same time patient sample duplicates were excluded from the calculations. Basic demographic data (age, ethnicity, and gender) were obtained from this FileMan search. The third-generation assay was the Ortho-Clinical Diagnostics Vitros, and the fourth-generation assay was the Abbott Architect.
To interpret the true HIV result of each sample with a reactive or positive screening result, the CDC laboratory HIV testing algorithm was followed and reviewed with a clinical pathologist or microbiologist director.12,13 All specimens interpreted as HIV positive by the pathologist or microbiologist director were discussed with the clinical health care provider at the time of the test with results added to CPRS after all testing was complete and discussions had taken place. All initially reactive specimens (confirmed with retesting in duplicate on the screening platform with at least 1 repeat reactive result) were further tested with the Bio-Rad Geenius HIV 1/2 Supplemental Assay, which screens for both HIV-1 and HIV-2 antibodies. Specimens with reactive results by this supplemental assay were interpreted as positive for HIV based on the CDC laboratory HIV testing algorithm. Specimens with negative or indeterminant results by the supplemental assay then underwent HIV-1 nucleic acid testing (NAT) using the Roche Diagnostics COBAS AmpliPrep/COBAS TaqMan HIV-1 Test v2.0. Specimens with viral load detected on NAT were positive for HIV infection, while specimens with viral load not detected on NAT testing were interpreted as negative for HIV-1 infection. Although there were no HIV-2 positive or indeterminant specimens during the study period, HIV-2 reactivity also would have been interpreted per the CDC laboratory HIV testing algorithm. Specimens with inadequate volume to complete all testing steps would be interpreted as indeterminant for HIV with request for additional specimen to complete testing. All testing platforms used for HIV testing in the laboratory had been properly validated prior to use.
The number of false positives and indeterminant results was tabulated in Microsoft Excel by month throughout the study period alongside the total number of HIV screening tests performed. Statistical analyses to verify statistical significance was performed by 1-tailed homoscedastic t test calculation using Excel.
Results
From March 1, 2016 to February 28, 2017, 7,516 specimens were screened for HIV, using the third-generation assay, and 52 specimens tested positive for HIV. On further review of these reactive specimens per the CDC laboratory testing algorithm, 24 tests were true positive and 28 were false positives with a PPV of 46% (24/52) (Figure 1).
From March 1, 2017 to February 28, 2018, 7,802 specimens were screened for HIV using a fourth-generation assay and 23 tested positive for HIV. On further review of these reactive specimens per the CDC laboratory testing algorithm, 16 were true positive and 7 were false positives with a PPV of 70% (16/23).
The fourth-generation assay was more specific when compared with the third-generation assay (0.09% vs 0.37%, respectively) with a 75.7% decrease in the false-positivity rate after the implementation of fourth-generation testing. The decreased number of false-positive test results per month with the fourth-generation test implementation was statistically significant (P = .002). The mean (SD) number of false-positive test results for the third-generation assay was 2.3 (1.7) per month, while the fourth-generation assay only had a mean (SD) of 0.58 (0.9) false positives monthly. The decrease in the percentage of false positives per month with the implementation of the fourth-generation assay also was statistically significant (P = .002) (Figure 2).
For population-based reference of the tested population at CMJCVAMC, there was a FileMan search for basic demographic data of patients for the HIV specimens screened by the third- or fourth-generation test (Table). For the population tested by the third-generation assay, 1,114 out of the 7,516 total tested population did not have readily available demographic information by the FileMan search as the specimens originated outside of the facility. For 6,402 of 7,516 patients tested by the third-generation assay with demographic information, the age ranged from 25 to 97 years with a mean of 57 years. This population of 6,402 was 88% male (n = 5,639), 50% African American (n = 3,220) and 43% White (n = 2,756). For the population tested by the fourth-generation assay, 993 of 7,802 total tested population did not have readily available demographic information by the FileMan search as the specimens originated outside of the facility. For the 6,809 of 7,802 patients tested by the fourth-generation assay with demographic information, the age ranged from 24 to 97 years with a mean age of 56 years. This population was 88% male (n = 5,971), 47% African American (n = 3,189), and 46% White (n = 3,149).
Discussion
Current practice guidelines from the CDC and the US Preventive Services Task Force recommend universal screening of the population for HIV infection.5,6 As the general population to be screened would normally have a low prevalence of HIV infection, the risk of a false positive on the initial screen is significant.17 Indeed, the CMJCVAMC experience has been that with the third-generation screening assay, the number of false-positive test results outnumbered the number of true-positive test results. Even with the fourth-generation assay, approximately one-third of the results were false positives. These results are similar to those observed in studies involving nonveteran populations in which the implementation of a fourth-generation screening assay led to significantly fewer false-positive results.18
For laboratories that do not follows CDC testing algorithm guidelines, each false-positive screening result represents a potential opportunity for a HIV misdiagnosis.Even in laboratories with proper procedures in place, false-positive results have consequences for the patients and for the cost-effectiveness of laboratory operations.9-11,18 As per CDC HIV testing guidelines, all positive screening results should be retested, which leads to additional use of technologist time and reagents. After this additional testing is performed and reviewed appropriately, only then can an appropriate final laboratory diagnosis be rendered that meets the standard of laboratory care.
Cost Savings
As observed at CMJCVAMC, the use of a fourth-generation assay with increased sensitivity/specificity led to a reduction in these false-positive results, which improved laboratory efficiency and avoided wasted resources for confirmatory tests.11,18 Cost savings at CMJCVAMC from the implementation of the fourth-generation assay would include technologist time and reagent cost. Generalizable technologist time costs at any institution would include the time needed to perform the confirmatory HIV-1/HIV-2 antibody differentiation assay (slightly less than 1 hour at CMJCVAMC per specimen) and the time needed to perform the viral load assay (about 6 hours to run a batch of 24 tests at CMJCVAMC). We calculated that confirmatory testing cost $184.51 per test at CMJCVAMC. Replacing the third-generation assay with the more sensitive and specific fourth-generation test saved an estimated $3,875 annually. This cost savings does not even consider savings in the pathologist/director’s time for reviewing HIV results after the completion of the algorithm or the clinician/patient costs or anxiety while waiting for results of the confirmatory sequence of tests.
As diagnosis of HIV can have a significant psychological impact on the patient, it is important to ensure the diagnosis conveyed is correct.27 The provision of an HIV diagnosis to a patient has been described as a traumatic stressor capable of causing psychological harm; this harm should ideally be avoided if the HIV diagnosis is not accurate. There can be a temptation, when presented with a positive or reactive screening test that is known to come from an instrument or assay with a very high sensitivity and specificity, to present this result as a diagnosis to the patient. However, a false diagnosis from a false-positive screen would not only be harmful, but given the low prevalence of the disease in the screened population, would happen fairly frequently; in some settings the number of false positives may actually outnumber the number of true positive test results.
Better screening assays with greater specificity (even fractions of a percentage, given that specificities are already > 99%) would help reduce the number of false positives and reduce the number of potential enticements to convey an incorrect diagnosis. Therefore, by adding an additional layer of safety through greater specificity, the fourth-generation assay implementation helped improve the diagnostic safety of the laboratory and reduced the significant error risk to the clinician who would ultimately bear responsibility for conveying the HIV diagnoses to the patient. Given the increased prevalence of psychological and physical ailments in veterans, it may be even more important to ensure the diagnosis is correct to avoid increased psychological harm.27,28
Veteran Population
For the general population, the fourth-generation assay has been shown to be more sensitive and specific when compared with the third-generation assay due to the addition of detection of p24 antigen and the refinement of the antigenic targets for the antibody detection.6,8,11-13,18-20,22 However, the veteran population that receives VA medical care differs significantly from the nonveteran general population. Compared with nonveterans, veterans tend to have generally poorer health status, more comorbid conditions, and greater need to use medical resources.24-26 In addition, veterans also may differ in sociodemographic status, race, ethnicity, and gender.24-26
VA research in the veteran population is unique, and veterans who use VA health care services are an even more highly selected subpopulation.26 Conclusions made from studies of the general population may not always be applicable to the veteran population treated by VA health care services due to these population differences. Therefore, specific studies tailored to this special veteran population in the specific VA health care setting are essential to ensure that the results of the general population truly and definitively apply to the veteran population.
While the false-positive risk is most closely associated with testing in a population of low prevalence, it also should be noted that false-positive screening results also can occur in high-risk individuals, such as an individual on preexposure prophylaxis (PrEP) for continuous behavior that places the individual at high risk of HIV acquisition.8,29 The false-positive result in these cases can lead to a conundrum for the clinician, and the differential diagnosis should consider both detection of very early infection as well as false positive. Interventions could include either stopping PrEP and treating for presumed early primary infection with HIV or continuing the PrEP. These interventions all have the potential to impact the patient whether through the production of resistant HIV virus due to the inadvertent provision of an inadequate treatment regimen, increased risk of infection if taken off PrEP as the patient may likely continue the behavior regardless, or the risks carried by the administration of additional antiretroviral therapies for the complete empiric therapy. Cases of an individual on PrEP who had a false-positive HIV screening test has been reported previously both within and outside the veteran population.8 Better screening tests with greater sensitivity/specificity can only help in guiding better patient care.
Limitations
This quality assurance study was limited to retrospectively identifying the improvement in the false-positive rate on the transition from the third-generation to the more advanced fourth-generation HIV screen. False-positive screen cases could be easily picked up on review of the confirmatory testing per the CDC laboratory HIV testing algorithm.12,13 This study also was a retrospective review of clinically ordered and indicated testing; as a result, without confirmatory testing performed on all negative screen cases, a false-negative rate would not be calculable.
This study also was restricted to only the population being treated in a VA health care setting. This population is known to be different from the general population.24-26
Conclusions
The switch to a fourth-generation assay resulted in a significant reduction in false-positive test results for veteran patients at CMJCVAMC. This reduction in false-positive screening not only reduced laboratory workload due to the necessary confirmatory testing and subsequent review, but also saved costs for technologist’s time and reagents. While this reduction in false-positive results has been documented in nonveteran populations, this is the first study specifically on a veteran population treated at a VAMC.8,11,18 This study confirms previously documented findings of improvement in the false-positive rate of HIV screening tests with the change from third-generation to fourth-generation assay for a veteran population.24
Ever since the first clinical reports of patients with AIDS in 1981, there have been improvements both in the knowledge base of the pathogenesis of HIV in causing AIDS as well as a progressive refinement in the test methodologies used to diagnose this illness.1-3 Given that there are both public health and clinical benefits in earlier diagnosis and treatment of patients with available antiretroviral therapies, universal screening with opt-out consent has been a standard of practice recommendation by the Centers of Disease Control and Prevention (CDC) since 2006; universal screening with opt-out consent also has been recommended by the US Preventative Task Force and has been widely implemented.4-7
HIV Screening
While HIV screening assays have evolved to be accurate with very high sensitivities and specificities, false-positive results are a significant issue both currently and historically.8-16 The use of an HIV assay on a low prevalence population predictably reduces the positive predictive value (PPV) of even an otherwise accurate assay.8-23 In light of this, laboratory HIV testing algorithms include confirmatory testing to increase the likelihood that the correct diagnosis is being rendered.
The fourth-generation assay has been shown to be more sensitive and specific compared with that of the third-generation assay due to the addition of detection of p24 antigen and the refinement of the antigenic targets for the antibody detection.6,8,11-13,18-20,22 Due to these improvements, in the general population, increased sensitivity/specificity with a reduction in both false positives and false negatives have been reported.
It has been observed in the nonveteran population that switching from the older third-generation to a more sensitive and specific fourth-generation HIV screening assay has reduced the false-positive screening rate.18,19,22 For instance, Muthukumar and colleagues demonstrated a false-positive rate of only 2 out of 99 (2%) tested specimens for the fourth-generation ARCHITECT HIV Ag/Ab Combo assay vs 9 out of 99 tested specimens (9%) for the third-generation ADVIA Centaur HIV 1/O/2 Enhanced assay.18 In addition, it has been noted that fourth-generation HIV screening assays can reduce the window period by detecting HIV infection sooner after initial acute infection.19 Mitchell and colleagues demonstrated even highly specific fourth-generation HIV assays with specificities estimated at 99.7% can have PPVs as low as 25.0% if used in a population of low HIV prevalence (such as a 0.1% prevalence population).19 However, the veteran population has been documented to differ significantly on a number of population variables, including severity of disease and susceptibility to infections, and as a result extrapolation of these data from the general population may be limited.24-26 To our knowledge, this article represents the first study directly examining the reduction in false-positive results with the switch to a fourth-generation HIV generation assay from a third-generation assay for the veteran patient population at a regional US Department of Veterans Affairs (VA) medical center (VAMC).8,11
Methods
Quality assurance documents on test volume were retrospectively reviewed to obtain the number of HIV screening tests that were performed by the laboratory at the Corporal Michael J. Crescenz VAMC (CMJCVAMC) in Philadelphia, Pennsylvania, between March 1, 2016 and February 28, 2017, prior to implementation of the fourth-generation assay. The study also include results from the first year of use of the fourth-generation assay (March 1, 2017 to February 28, 2018). In addition, paper quality assurance records of all positive screening results during those periods were reviewed and manually counted for the abstract presentation of these data.
For assurance of accuracy, a search of all HIV testing assays using Veterans Health Information Systems and Technology Architecture and FileMan also was performed, and the results were compared to records in the Computerized Patient Record System (CPRS). Any discrepancies in the numbers of test results generated by both searches were investigated, and data for the manuscript were derived from records associating tests with particular patients. Only results from patient samples were considered for the electronic search. Quality samples that did not correspond to a true patient as identified in CPRS or same time patient sample duplicates were excluded from the calculations. Basic demographic data (age, ethnicity, and gender) were obtained from this FileMan search. The third-generation assay was the Ortho-Clinical Diagnostics Vitros, and the fourth-generation assay was the Abbott Architect.
To interpret the true HIV result of each sample with a reactive or positive screening result, the CDC laboratory HIV testing algorithm was followed and reviewed with a clinical pathologist or microbiologist director.12,13 All specimens interpreted as HIV positive by the pathologist or microbiologist director were discussed with the clinical health care provider at the time of the test with results added to CPRS after all testing was complete and discussions had taken place. All initially reactive specimens (confirmed with retesting in duplicate on the screening platform with at least 1 repeat reactive result) were further tested with the Bio-Rad Geenius HIV 1/2 Supplemental Assay, which screens for both HIV-1 and HIV-2 antibodies. Specimens with reactive results by this supplemental assay were interpreted as positive for HIV based on the CDC laboratory HIV testing algorithm. Specimens with negative or indeterminant results by the supplemental assay then underwent HIV-1 nucleic acid testing (NAT) using the Roche Diagnostics COBAS AmpliPrep/COBAS TaqMan HIV-1 Test v2.0. Specimens with viral load detected on NAT were positive for HIV infection, while specimens with viral load not detected on NAT testing were interpreted as negative for HIV-1 infection. Although there were no HIV-2 positive or indeterminant specimens during the study period, HIV-2 reactivity also would have been interpreted per the CDC laboratory HIV testing algorithm. Specimens with inadequate volume to complete all testing steps would be interpreted as indeterminant for HIV with request for additional specimen to complete testing. All testing platforms used for HIV testing in the laboratory had been properly validated prior to use.
The number of false positives and indeterminant results was tabulated in Microsoft Excel by month throughout the study period alongside the total number of HIV screening tests performed. Statistical analyses to verify statistical significance was performed by 1-tailed homoscedastic t test calculation using Excel.
Results
From March 1, 2016 to February 28, 2017, 7,516 specimens were screened for HIV, using the third-generation assay, and 52 specimens tested positive for HIV. On further review of these reactive specimens per the CDC laboratory testing algorithm, 24 tests were true positive and 28 were false positives with a PPV of 46% (24/52) (Figure 1).
From March 1, 2017 to February 28, 2018, 7,802 specimens were screened for HIV using a fourth-generation assay and 23 tested positive for HIV. On further review of these reactive specimens per the CDC laboratory testing algorithm, 16 were true positive and 7 were false positives with a PPV of 70% (16/23).
The fourth-generation assay was more specific when compared with the third-generation assay (0.09% vs 0.37%, respectively) with a 75.7% decrease in the false-positivity rate after the implementation of fourth-generation testing. The decreased number of false-positive test results per month with the fourth-generation test implementation was statistically significant (P = .002). The mean (SD) number of false-positive test results for the third-generation assay was 2.3 (1.7) per month, while the fourth-generation assay only had a mean (SD) of 0.58 (0.9) false positives monthly. The decrease in the percentage of false positives per month with the implementation of the fourth-generation assay also was statistically significant (P = .002) (Figure 2).
For population-based reference of the tested population at CMJCVAMC, there was a FileMan search for basic demographic data of patients for the HIV specimens screened by the third- or fourth-generation test (Table). For the population tested by the third-generation assay, 1,114 out of the 7,516 total tested population did not have readily available demographic information by the FileMan search as the specimens originated outside of the facility. For 6,402 of 7,516 patients tested by the third-generation assay with demographic information, the age ranged from 25 to 97 years with a mean of 57 years. This population of 6,402 was 88% male (n = 5,639), 50% African American (n = 3,220) and 43% White (n = 2,756). For the population tested by the fourth-generation assay, 993 of 7,802 total tested population did not have readily available demographic information by the FileMan search as the specimens originated outside of the facility. For the 6,809 of 7,802 patients tested by the fourth-generation assay with demographic information, the age ranged from 24 to 97 years with a mean age of 56 years. This population was 88% male (n = 5,971), 47% African American (n = 3,189), and 46% White (n = 3,149).
Discussion
Current practice guidelines from the CDC and the US Preventive Services Task Force recommend universal screening of the population for HIV infection.5,6 As the general population to be screened would normally have a low prevalence of HIV infection, the risk of a false positive on the initial screen is significant.17 Indeed, the CMJCVAMC experience has been that with the third-generation screening assay, the number of false-positive test results outnumbered the number of true-positive test results. Even with the fourth-generation assay, approximately one-third of the results were false positives. These results are similar to those observed in studies involving nonveteran populations in which the implementation of a fourth-generation screening assay led to significantly fewer false-positive results.18
For laboratories that do not follows CDC testing algorithm guidelines, each false-positive screening result represents a potential opportunity for a HIV misdiagnosis.Even in laboratories with proper procedures in place, false-positive results have consequences for the patients and for the cost-effectiveness of laboratory operations.9-11,18 As per CDC HIV testing guidelines, all positive screening results should be retested, which leads to additional use of technologist time and reagents. After this additional testing is performed and reviewed appropriately, only then can an appropriate final laboratory diagnosis be rendered that meets the standard of laboratory care.
Cost Savings
As observed at CMJCVAMC, the use of a fourth-generation assay with increased sensitivity/specificity led to a reduction in these false-positive results, which improved laboratory efficiency and avoided wasted resources for confirmatory tests.11,18 Cost savings at CMJCVAMC from the implementation of the fourth-generation assay would include technologist time and reagent cost. Generalizable technologist time costs at any institution would include the time needed to perform the confirmatory HIV-1/HIV-2 antibody differentiation assay (slightly less than 1 hour at CMJCVAMC per specimen) and the time needed to perform the viral load assay (about 6 hours to run a batch of 24 tests at CMJCVAMC). We calculated that confirmatory testing cost $184.51 per test at CMJCVAMC. Replacing the third-generation assay with the more sensitive and specific fourth-generation test saved an estimated $3,875 annually. This cost savings does not even consider savings in the pathologist/director’s time for reviewing HIV results after the completion of the algorithm or the clinician/patient costs or anxiety while waiting for results of the confirmatory sequence of tests.
As diagnosis of HIV can have a significant psychological impact on the patient, it is important to ensure the diagnosis conveyed is correct.27 The provision of an HIV diagnosis to a patient has been described as a traumatic stressor capable of causing psychological harm; this harm should ideally be avoided if the HIV diagnosis is not accurate. There can be a temptation, when presented with a positive or reactive screening test that is known to come from an instrument or assay with a very high sensitivity and specificity, to present this result as a diagnosis to the patient. However, a false diagnosis from a false-positive screen would not only be harmful, but given the low prevalence of the disease in the screened population, would happen fairly frequently; in some settings the number of false positives may actually outnumber the number of true positive test results.
Better screening assays with greater specificity (even fractions of a percentage, given that specificities are already > 99%) would help reduce the number of false positives and reduce the number of potential enticements to convey an incorrect diagnosis. Therefore, by adding an additional layer of safety through greater specificity, the fourth-generation assay implementation helped improve the diagnostic safety of the laboratory and reduced the significant error risk to the clinician who would ultimately bear responsibility for conveying the HIV diagnoses to the patient. Given the increased prevalence of psychological and physical ailments in veterans, it may be even more important to ensure the diagnosis is correct to avoid increased psychological harm.27,28
Veteran Population
For the general population, the fourth-generation assay has been shown to be more sensitive and specific when compared with the third-generation assay due to the addition of detection of p24 antigen and the refinement of the antigenic targets for the antibody detection.6,8,11-13,18-20,22 However, the veteran population that receives VA medical care differs significantly from the nonveteran general population. Compared with nonveterans, veterans tend to have generally poorer health status, more comorbid conditions, and greater need to use medical resources.24-26 In addition, veterans also may differ in sociodemographic status, race, ethnicity, and gender.24-26
VA research in the veteran population is unique, and veterans who use VA health care services are an even more highly selected subpopulation.26 Conclusions made from studies of the general population may not always be applicable to the veteran population treated by VA health care services due to these population differences. Therefore, specific studies tailored to this special veteran population in the specific VA health care setting are essential to ensure that the results of the general population truly and definitively apply to the veteran population.
While the false-positive risk is most closely associated with testing in a population of low prevalence, it also should be noted that false-positive screening results also can occur in high-risk individuals, such as an individual on preexposure prophylaxis (PrEP) for continuous behavior that places the individual at high risk of HIV acquisition.8,29 The false-positive result in these cases can lead to a conundrum for the clinician, and the differential diagnosis should consider both detection of very early infection as well as false positive. Interventions could include either stopping PrEP and treating for presumed early primary infection with HIV or continuing the PrEP. These interventions all have the potential to impact the patient whether through the production of resistant HIV virus due to the inadvertent provision of an inadequate treatment regimen, increased risk of infection if taken off PrEP as the patient may likely continue the behavior regardless, or the risks carried by the administration of additional antiretroviral therapies for the complete empiric therapy. Cases of an individual on PrEP who had a false-positive HIV screening test has been reported previously both within and outside the veteran population.8 Better screening tests with greater sensitivity/specificity can only help in guiding better patient care.
Limitations
This quality assurance study was limited to retrospectively identifying the improvement in the false-positive rate on the transition from the third-generation to the more advanced fourth-generation HIV screen. False-positive screen cases could be easily picked up on review of the confirmatory testing per the CDC laboratory HIV testing algorithm.12,13 This study also was a retrospective review of clinically ordered and indicated testing; as a result, without confirmatory testing performed on all negative screen cases, a false-negative rate would not be calculable.
This study also was restricted to only the population being treated in a VA health care setting. This population is known to be different from the general population.24-26
Conclusions
The switch to a fourth-generation assay resulted in a significant reduction in false-positive test results for veteran patients at CMJCVAMC. This reduction in false-positive screening not only reduced laboratory workload due to the necessary confirmatory testing and subsequent review, but also saved costs for technologist’s time and reagents. While this reduction in false-positive results has been documented in nonveteran populations, this is the first study specifically on a veteran population treated at a VAMC.8,11,18 This study confirms previously documented findings of improvement in the false-positive rate of HIV screening tests with the change from third-generation to fourth-generation assay for a veteran population.24
1. Feinberg MB. Changing the natural history of HIV disease. Lancet. 1996;348(9022):239-246. doi:10.1016/s0140-6736(96)06231-9.
2. Alexander TS. Human immunodeficiency virus diagnostic testing: 30 years of evolution. Clin Vaccine Immunol. 2016;23(4):249-253. Published 2016 Apr 4. doi:10.1128/CVI.00053-16
3. Mortimer PP, Parry JV, Mortimer JY. Which anti-HTLV III/LAV assays for screening and confirmatory testing?. Lancet. 1985;2(8460):873-877. doi:10.1016/s0140-6736(85)90136-9
4. Holmberg SD, Palella FJ Jr, Lichtenstein KA, Havlir DV. The case for earlier treatment of HIV infection [published correction appears in Clin Infect Dis. 2004 Dec 15;39(12):1869]. Clin Infect Dis. 2004;39(11):1699-1704. doi:10.1086/425743
5. US Preventive Services Task Force, Owens DK, Davidson KW, et al. Screening for HIV Infection: US Preventive Services Task Force Recommendation Statement. JAMA. 2019;321(23):2326-2336. doi:10.1001/jama.2019.6587
6. Branson BM, Handsfield HH, Lampe MA, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55(RR-14):1-CE4.
7. Bayer R, Philbin M, Remien RH. The end of written informed consent for HIV testing: not with a bang but a whimper. Am J Public Health. 2017;107(8):1259-1265. doi:10.2105/AJPH.2017.303819
8. Petersen J, Jhala D. Its not HIV! The pitfall of unconfirmed positive HIV screening assays. Abstract presented at: Annual Meeting Pennsylvania Association of Pathologists; April 14, 2018.
9. Wood RW, Dunphy C, Okita K, Swenson P. Two “HIV-infected” persons not really infected. Arch Intern Med. 2003;163(15):1857-1859. doi:10.1001/archinte.163.15.1857
10. Permpalung N, Ungprasert P, Chongnarungsin D, Okoli A, Hyman CL. A diagnostic blind spot: acute infectious mononucleosis or acute retroviral syndrome. Am J Med. 2013;126(9):e5-e6. doi:10.1016/j.amjmed.2013.03.017
11. Dalal S, Petersen J, Luta D, Jhala D. Third- to fourth-generation HIV testing: reduction in false-positive results with the new way of testing, the Corporal Michael J. Crescenz Veteran Affairs Medical Center (CMCVAMC) Experience. Am J Clin Pathol.2018;150(suppl 1):S70-S71. doi:10.1093/ajcp/aqy093.172
12. Centers for Disease Control and Prevention. Laboratory testing for the diagnosis of HIV infection: updated recommendations. Published June 27, 2014. Accessed April 14, 2021. doi:10.15620/cdc.23447
13. Centers for Disease Control and Prevention. 2018 quick reference guide: recommended laboratory HIV testing algorithm for serum or plasma specimens. Updated January 2018. Accessed April 14, 202. https://stacks.cdc.gov/view/cdc/50872
14. Masciotra S, McDougal JS, Feldman J, Sprinkle P, Wesolowski L, Owen SM. Evaluation of an alternative HIV diagnostic algorithm using specimens from seroconversion panels and persons with established HIV infections. J Clin Virol. 2011;52(suppl 1):S17-S22. doi:10.1016/j.jcv.2011.09.011
15. Morton A. When lab tests lie … heterophile antibodies. Aust Fam Physician. 2014;43(6):391-393.
16. Spencer DV, Nolte FS, Zhu Y. Heterophilic antibody interference causing false-positive rapid human immunodeficiency virus antibody testing. Clin Chim Acta. 2009;399(1-2):121-122. doi:10.1016/j.cca.2008.09.030
17. Kim S, Lee JH, Choi JY, Kim JM, Kim HS. False-positive rate of a “fourth-generation” HIV antigen/antibody combination assay in an area of low HIV prevalence. Clin Vaccine Immunol. 2010;17(10):1642-1644. doi:10.1128/CVI.00258-10
18. Muthukumar A, Alatoom A, Burns S, et al. Comparison of 4th-generation HIV antigen/antibody combination assay with 3rd-generation HIV antibody assays for the occurrence of false-positive and false-negative results. Lab Med. 2015;46(2):84-e29. doi:10.1309/LMM3X37NSWUCMVRS
19. Mitchell EO, Stewart G, Bajzik O, Ferret M, Bentsen C, Shriver MK. Performance comparison of the 4th generation Bio-Rad Laboratories GS HIV Combo Ag/Ab EIA on the EVOLIS™ automated system versus Abbott ARCHITECT HIV Ag/Ab Combo, Ortho Anti-HIV 1+2 EIA on Vitros ECi and Siemens HIV-1/O/2 enhanced on Advia Centaur. J Clin Virol. 2013;58(suppl 1):e79-e84. doi:10.1016/j.jcv.2013.08.009
20. Dubravac T, Gahan TF, Pentella MA. Use of the Abbott Architect HIV antigen/antibody assay in a low incidence population. J Clin Virol. 2013;58(suppl 1):e76-e78. doi:10.1016/j.jcv.2013.10.020
21. Montesinos I, Eykmans J, Delforge ML. Evaluation of the Bio-Rad Geenius HIV-1/2 test as a confirmatory assay. J Clin Virol. 2014;60(4):399-401. doi:10.1016/j.jcv.2014.04.025
22. van Binsbergen J, Siebelink A, Jacobs A, et al. Improved performance of seroconversion with a 4th generation HIV antigen/antibody assay. J Virol Methods. 1999;82(1):77-84. doi:10.1016/s0166-0934(99)00086-5
23. CLSI. User Protocol for Evaluation of Qualitative Test Performance: Approved Guideline. Second ed. EP12-A2. CLSI; 2008:1-46.
24. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252-3257. doi:10.1001/archinte.160.21.3252
25. Eibner C, Krull H, Brown KM, et al. Current and projected characteristics and unique health care needs of the patient population served by the Department of Veterans Affairs. Rand Health Q. 2016;5(4):13. Published 2016 May 9.
26. Morgan RO, Teal CR, Reddy SG, Ford ME, Ashton CM. Measurement in Veterans Affairs Health Services Research: veterans as a special population. Health Serv Res. 2005;40(5, pt 2):1573-1583. doi:10.1111/j.1475-6773.2005.00448.x
27. Nightingale VR, Sher TG, Hansen NB. The impact of receiving an HIV diagnosis and cognitive processing on psychological distress and posttraumatic growth. J Trauma Stress. 2010;23(4):452-460. doi:10.1002/jts.20554
28. Spelman JF, Hunt SC, Seal KH, Burgo-Black AL. Post deployment care for returning combat veterans. J Gen Intern Med. 2012;27(9):1200-1209. doi:10.1007/s11606-012-2061-1
29. Ndase P, Celum C, Kidoguchi L, et al. Frequency of false positive rapid HIV serologic tests in African men and women receiving PrEP for HIV prevention: implications for programmatic roll-out of biomedical interventions. PLoS One. 2015;10(4):e0123005. Published 2015 Apr 17. doi:10.1371/journal.pone.0123005
1. Feinberg MB. Changing the natural history of HIV disease. Lancet. 1996;348(9022):239-246. doi:10.1016/s0140-6736(96)06231-9.
2. Alexander TS. Human immunodeficiency virus diagnostic testing: 30 years of evolution. Clin Vaccine Immunol. 2016;23(4):249-253. Published 2016 Apr 4. doi:10.1128/CVI.00053-16
3. Mortimer PP, Parry JV, Mortimer JY. Which anti-HTLV III/LAV assays for screening and confirmatory testing?. Lancet. 1985;2(8460):873-877. doi:10.1016/s0140-6736(85)90136-9
4. Holmberg SD, Palella FJ Jr, Lichtenstein KA, Havlir DV. The case for earlier treatment of HIV infection [published correction appears in Clin Infect Dis. 2004 Dec 15;39(12):1869]. Clin Infect Dis. 2004;39(11):1699-1704. doi:10.1086/425743
5. US Preventive Services Task Force, Owens DK, Davidson KW, et al. Screening for HIV Infection: US Preventive Services Task Force Recommendation Statement. JAMA. 2019;321(23):2326-2336. doi:10.1001/jama.2019.6587
6. Branson BM, Handsfield HH, Lampe MA, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55(RR-14):1-CE4.
7. Bayer R, Philbin M, Remien RH. The end of written informed consent for HIV testing: not with a bang but a whimper. Am J Public Health. 2017;107(8):1259-1265. doi:10.2105/AJPH.2017.303819
8. Petersen J, Jhala D. Its not HIV! The pitfall of unconfirmed positive HIV screening assays. Abstract presented at: Annual Meeting Pennsylvania Association of Pathologists; April 14, 2018.
9. Wood RW, Dunphy C, Okita K, Swenson P. Two “HIV-infected” persons not really infected. Arch Intern Med. 2003;163(15):1857-1859. doi:10.1001/archinte.163.15.1857
10. Permpalung N, Ungprasert P, Chongnarungsin D, Okoli A, Hyman CL. A diagnostic blind spot: acute infectious mononucleosis or acute retroviral syndrome. Am J Med. 2013;126(9):e5-e6. doi:10.1016/j.amjmed.2013.03.017
11. Dalal S, Petersen J, Luta D, Jhala D. Third- to fourth-generation HIV testing: reduction in false-positive results with the new way of testing, the Corporal Michael J. Crescenz Veteran Affairs Medical Center (CMCVAMC) Experience. Am J Clin Pathol.2018;150(suppl 1):S70-S71. doi:10.1093/ajcp/aqy093.172
12. Centers for Disease Control and Prevention. Laboratory testing for the diagnosis of HIV infection: updated recommendations. Published June 27, 2014. Accessed April 14, 2021. doi:10.15620/cdc.23447
13. Centers for Disease Control and Prevention. 2018 quick reference guide: recommended laboratory HIV testing algorithm for serum or plasma specimens. Updated January 2018. Accessed April 14, 202. https://stacks.cdc.gov/view/cdc/50872
14. Masciotra S, McDougal JS, Feldman J, Sprinkle P, Wesolowski L, Owen SM. Evaluation of an alternative HIV diagnostic algorithm using specimens from seroconversion panels and persons with established HIV infections. J Clin Virol. 2011;52(suppl 1):S17-S22. doi:10.1016/j.jcv.2011.09.011
15. Morton A. When lab tests lie … heterophile antibodies. Aust Fam Physician. 2014;43(6):391-393.
16. Spencer DV, Nolte FS, Zhu Y. Heterophilic antibody interference causing false-positive rapid human immunodeficiency virus antibody testing. Clin Chim Acta. 2009;399(1-2):121-122. doi:10.1016/j.cca.2008.09.030
17. Kim S, Lee JH, Choi JY, Kim JM, Kim HS. False-positive rate of a “fourth-generation” HIV antigen/antibody combination assay in an area of low HIV prevalence. Clin Vaccine Immunol. 2010;17(10):1642-1644. doi:10.1128/CVI.00258-10
18. Muthukumar A, Alatoom A, Burns S, et al. Comparison of 4th-generation HIV antigen/antibody combination assay with 3rd-generation HIV antibody assays for the occurrence of false-positive and false-negative results. Lab Med. 2015;46(2):84-e29. doi:10.1309/LMM3X37NSWUCMVRS
19. Mitchell EO, Stewart G, Bajzik O, Ferret M, Bentsen C, Shriver MK. Performance comparison of the 4th generation Bio-Rad Laboratories GS HIV Combo Ag/Ab EIA on the EVOLIS™ automated system versus Abbott ARCHITECT HIV Ag/Ab Combo, Ortho Anti-HIV 1+2 EIA on Vitros ECi and Siemens HIV-1/O/2 enhanced on Advia Centaur. J Clin Virol. 2013;58(suppl 1):e79-e84. doi:10.1016/j.jcv.2013.08.009
20. Dubravac T, Gahan TF, Pentella MA. Use of the Abbott Architect HIV antigen/antibody assay in a low incidence population. J Clin Virol. 2013;58(suppl 1):e76-e78. doi:10.1016/j.jcv.2013.10.020
21. Montesinos I, Eykmans J, Delforge ML. Evaluation of the Bio-Rad Geenius HIV-1/2 test as a confirmatory assay. J Clin Virol. 2014;60(4):399-401. doi:10.1016/j.jcv.2014.04.025
22. van Binsbergen J, Siebelink A, Jacobs A, et al. Improved performance of seroconversion with a 4th generation HIV antigen/antibody assay. J Virol Methods. 1999;82(1):77-84. doi:10.1016/s0166-0934(99)00086-5
23. CLSI. User Protocol for Evaluation of Qualitative Test Performance: Approved Guideline. Second ed. EP12-A2. CLSI; 2008:1-46.
24. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252-3257. doi:10.1001/archinte.160.21.3252
25. Eibner C, Krull H, Brown KM, et al. Current and projected characteristics and unique health care needs of the patient population served by the Department of Veterans Affairs. Rand Health Q. 2016;5(4):13. Published 2016 May 9.
26. Morgan RO, Teal CR, Reddy SG, Ford ME, Ashton CM. Measurement in Veterans Affairs Health Services Research: veterans as a special population. Health Serv Res. 2005;40(5, pt 2):1573-1583. doi:10.1111/j.1475-6773.2005.00448.x
27. Nightingale VR, Sher TG, Hansen NB. The impact of receiving an HIV diagnosis and cognitive processing on psychological distress and posttraumatic growth. J Trauma Stress. 2010;23(4):452-460. doi:10.1002/jts.20554
28. Spelman JF, Hunt SC, Seal KH, Burgo-Black AL. Post deployment care for returning combat veterans. J Gen Intern Med. 2012;27(9):1200-1209. doi:10.1007/s11606-012-2061-1
29. Ndase P, Celum C, Kidoguchi L, et al. Frequency of false positive rapid HIV serologic tests in African men and women receiving PrEP for HIV prevention: implications for programmatic roll-out of biomedical interventions. PLoS One. 2015;10(4):e0123005. Published 2015 Apr 17. doi:10.1371/journal.pone.0123005
Risk Factors and Antipsychotic Usage Patterns Associated With Terminal Delirium in a Veteran Long-Term Care Hospice Population
Delirium is a condition commonly exhibited by hospitalized patients and by those who are approaching the end of life.1 Patients who experience a disturbance in attention that develops over a relatively short period and represents an acute change may have delirium.2 Furthermore, there is often an additional cognitive disturbance, such as disorientation, memory deficit, language deficits, visuospatial deficit, or perception. Terminal delirium is defined as delirium that occurs in the dying process and implies that reversal is less likely.3 When death is anticipated, diagnostic workups are not recommended, and treatment of the physiologic abnormalities that contribute to delirium is generally ineffective.4
Background
Delirium is often underdiagnosed and undetected by the clinician. Some studies have shown that delirium is not detected in 22 to 50% of cases.5 Factors that contribute to the underdetection of delirium include preexisting dementia, older age, presence of visual or hearing impairment, and hypoactive presentation of delirium. Other possible reasons for nondetection of delirium are its fluctuating nature and lack of formal cognitive assessment as part of a routine screening across care settings.5 Another study found that 41% of health care providers (HCPs) felt that screening for delirium was burdensome.6
To date, there are no veteran-focused studies that investigate prevalence or risk factors for terminal delirium in US Department of Veterans Affairs (VA) long-term care hospice units. Most long-term care hospice units in the VA are in community living centers (CLCs) that follow regulatory guidelines for using antipsychotic medications. The Centers for Medicare and Medicaid Services state that if antipsychotics are prescribed, documentation must clearly show the indication for the antipsychotic medication, the multiple attempts to implement planned care, nonpharmacologic approaches, and ongoing evaluation of the effectiveness of these interventions.7 The symptoms of terminal delirium cause significant distress to patients, family and caregivers, and nursing staff. Literature suggests that delirium poses significant relational challenges for patients, families, and HCPs in end-of-life situations.8,9 We hypothesize that the early identification of risk factors for the development of terminal delirium in this population may lead to increased use of nonpharmacologic measures to prevent terminal delirium, increase nursing vigilance for development of symptoms, and reduce symptom burden should terminal delirium develop.
Prevalence of delirium in the long-term care setting has ranged between 1.4 and 70.3%.10 The rate was found to be much higher in institutionalized populations compared with that of patients classified as at-home. In a study of the prevalence, severity, and natural history of neuropsychiatric syndromes in terminally ill veterans enrolled in community hospice, delirium was found to be present in only 4.1% on the initial visit and 42.5% during last visit. Also, more than half had at least 1 episode of delirium during the 90-day study period.11 In a study of the prevalence of delirium in terminal cancer patients admitted to hospice, 80% experienced delirium in their final days.12
Risk factors for the development of delirium that have been identified in actively dying patients include bowel or bladder obstruction, fluid and electrolyte imbalances, suboptimal pain management, medication adverse effects and toxicity (eg, benzodiazepines, opioids, anticholinergics, and steroids), the addition of ≥ 3 medications, infection, hepatic and renal failure, poor glycemic control, hypoxia, and hematologic disturbances.4,5,13 A high percentage of patients with a previous diagnosis of dementia were found to exhibit terminal delirium.14
There are 2 major subtypes of delirium: hyperactive and hypoactive.4 Patients with hypoactive delirium exhibit lethargy, reduced motor activity, lack of interest, and/or incoherent speech. There is currently little evidence to guide the treatment of hypoactive delirium. By contrast, hyperactive delirium is associated with hallucinations, agitation, heightened arousal, and inappropriate behavior. Many studies suggest both nonpharmacologic and pharmacologic treatment modalities for the treatment of hyperactive delirium.4,13 Nonpharmacologic interventions may minimize the risk and severity of symptoms associated with delirium. Current guidelines recommend these interventions before pharmacologic treatment.4 Nonpharmacologic interventions include but are not limited to the following: engaging the patient in mentally stimulating activities; surrounding the patient with familiar materials (eg, photos); ensuring that all individuals identify themselves when they encounter a patient; minimizing the intensity of stimulation, providing family or volunteer presence, soft lighting and warm blankets; and ensuring the patient uses hearing aids and glasses if needed.4,14
Although there are no US Food and Drug Administration-approved medications to treat hyperactive delirium, first-generation antipsychotics (eg, haloperidol, chlorpromazine) are considered the first-line treatment for patients exhibiting psychosis and psychomotor agitation.3,4,14-16 In terminally ill patients, there is limited evidence from clinical trials to support the efficacy of drug therapy.14 One study showed lack of efficacy with hydration and opioid rotation.17 In terminally ill patients experiencing hyperactive delirium, there is a significant increased risk of muscle tension, myoclonic seizures, and distress to the patient, family, and caregiver.1 Benzodiazepines can be considered first-line treatment for dying patients with terminal delirium in which the goals of treatment are to relieve muscle tension, ensure amnesia, reduce the risk of seizures, and decrease psychosis and agitation.18,19 Furthermore, in patients with history of alcohol misuse who are experiencing terminal delirium, benzodiazepines also may be the preferred pharmacologic treatment.20 Caution must be exercised with the use of benzodiazepines because they can also cause oversedation, increased confusion, and/or a paradoxical worsening of delirium.3,4,14
Methods
This was a retrospective case-control study of patients who died in the Edward Hines Jr. Veterans Affairs Hospital CLC in Hines, Illinois, under the treating specialty nursing home hospice from October 1, 2013 to September 30, 2015. Due to the retrospective nature of this trial, the use of antipsychotics within the last 2 weeks of life was a surrogate marker for development of terminal delirium. Cases were defined as patients who were treated with antipsychotics for terminal delirium within the last 2 weeks of their lives. Controls were defined as patients who were not treated with antipsychotics for terminal delirium within the last 2 weeks of their lives. Living hospice patients and patients who were discharged from the CLC before death were excluded.
The goals of this study were to (1) determine risk factors in the VA CLC hospice veteran population for the development of terminal delirium; (2) evaluate documentation by the nursing staff of nonpharmacologic interventions and indications for antipsychotic use in the treatment of terminal delirium; and (3) examine the current usage patterns of antipsychotics for the treatment of terminal delirium.
Veterans’ medical records were reviewed from 2 weeks before death until the recorded death date. Factors that were assessed included age, war era of service, date of death, terminal diagnosis, time interval from cancer diagnosis to death, comorbid conditions, prescribed antipsychotic medications, and other medications potentially contributing to delirium. Nursing documentation was reviewed for indications for administration of antipsychotic medications and nonpharmacologic interventions used to mitigate the symptoms of terminal delirium.
Statistical analysis was conducted in SAS Version 9.3. Cases were compared with controls using univariate and multivariate statistics as appropriate. Comparisons for continuous variables (eg, age) were conducted with Student t tests. Categorical variables (eg, PTSD diagnosis) were compared using χ2 analysis or Fisher exact test as appropriate. Variables with a P value < .1 in the univariate analysis were included in logistic regression models. Independent variables were removed from the models, using a backward selection process. Interaction terms were tested based on significance and clinical relevance. A P value < .05 was considered statistically significant.
Results
From October 1, 2013 to September 30, 2015, 307 patients were analyzed for inclusion in this study. Within this population, 186 received antipsychotic medications for the treatment of terminal delirium (cases), while 90 did not receive antipsychotics (controls). Of the 31 excluded patients, 13 were discharged to receive home hospice care, 11 were discharged to community nursing homes, 5 died in acute care units of Edward Hines, Jr. VA Hospital, and 2 died outside of the study period.
The mean age of all included patients was 75.5 years, and the most common terminal diagnosis was cancer, which occurred in 156 patients (56.5%) (Table 1). The baseline characteristics were similar between the cases and controls, including war era of veteran, terminal diagnosis, and comorbid conditions. The mean time between cancer diagnosis and death was not notably longer in the control group compared with that of the case group (25 vs 16 mo, respectively). There was no statistically significant difference in terminal diagnoses between cases and controls. Veterans in the control group spent more days (mean [SD]) in the hospice unit compared with veterans who experienced terminal delirium (48.5 [168.4] vs 28.2 [46.9]; P = .01). Patients with suspected infections were more likely found in the control group (P = .04; odds ratio [OR] = 1.70; 95% CI, 1.02-2.82).
The most common antipsychotic administered in the last 14 days of life was haloperidol. In the case group, 175 (94%) received haloperidol at least once in the last 2 weeks of life. Four (4.4%) veterans in the control group received haloperidol for the indication of nausea/vomiting; not terminal delirium. Atypical antipsychotics were infrequently used and included risperidone, olanzapine, quetiapine, and aripiprazole.
A total of 186 veterans received at least 1 dose of an antipsychotic for terminal delirium: 97 (52.2% ) veterans requiring antipsychotics for the treatment of terminal delirium required both scheduled and as-needed doses; 75 (40.3%) received only as-needed doses, and 14 (7.5%) required only scheduled doses. When the number of as-needed and scheduled doses were combined, each veteran received a mean 14.9 doses. However, for those veterans with antipsychotics ordered only as needed, a mean 5.8 doses were received per patient. Administration of antipsychotic doses was split evenly among the 3 nursing shifts (day-evening-night) with about 30% of doses administered on each shift.
Nurses were expected to document nonpharmacologic interventions that preceded the administration of each antipsychotic dose. Of the 1,028 doses administered to the 186 veterans who received at least 1 dose of an antipsychotic for terminal delirium, most of the doses (99.4%) had inadequate documentation based on current long-term care guidelines for prudent antipsychotic use.9
Several risk factors for terminal delirium were identified in this veteran population. Veterans with a history of drug or alcohol abuse were found to be at a significantly higher risk for terminal delirium (P = .04; OR, 1.87; 95% CI, 1.03-3.37). As noted in previous studies, steroid use (P = .01; OR, 2.57; 95% CI, 1.26-5.22); opioids (P = .007; OR, 5.94; 95% CI, 1.54-22.99), and anticholinergic medications (P = .01; OR, 2.06; 95% CI, 1.21-3.52) also increased the risk of delirium (Table 2).
When risk factors were combined, interaction terms were identified (Table 3). Those patients found to be at a higher risk of terminal delirium included Vietnam-era veterans with liver disease (P = .04; OR, 1.21; 95% CI, 1.01-1.45) and veterans with a history of drug or alcohol abuse plus comorbid liver disease (P = .03; OR, 1.26; 95% CI, 1.02-1.56). In a stratified analysis in veterans with a terminal diagnosis of cancer, those with a mental health condition (eg, PTSD, bipolar disorder, or schizophrenia) (P = .048; OR, 2.73; 95% CI, 0.98-7.58) also had higher risk of delirium, though not statistically significant. Within the cancer cohort, veterans with liver disease and a history of drug/alcohol abuse had increased risk of delirium (P = .01; OR, 1.43; 95% CI, 1.07-1.91).
Discussion
Terminal delirium is experienced by many individuals in their last days to weeks of life. Symptoms can present as hyperactive (eg, agitation, hallucinations, heightened arousal) or hypoactive (lethargy, reduced motor activity, incoherent speech). Hyperactive terminal delirium is particularly problematic because it causes increased distress to the patient, family, and caregivers. Delirium can lead to safety concerns, such as fall risk, due to patients’ decreased insight into functional decline.
Many studies suggest both nonpharmacologic and pharmacologic treatments for nonterminal delirium that may also apply to terminal delirium. Nonpharmacologic methods, such as providing a quiet and familiar environment, relieving urinary retention or constipation, and attending to sensory deficits may help prevent or minimize delirium. Pharmacologic interventions, such as antipsychotics or benzodiazepines, may benefit when other modalities have failed to assuage distressing symptoms of delirium. Because hypoactive delirium is usually accompanied by somnolence and reduced motor activity, medication is most often administered to individuals with hyperactive delirium.
The VA provides long-term care hospice beds in their CLCs for veterans who are nearing end of life and have inadequate caregiver support for comprehensive end-of-life care in the home (Case Presentation). Because of their military service and other factors common in their life histories, they may have a unique set of characteristics that are predictive of developing terminal delirium. Awareness of the propensity for terminal delirium will allow for early identification of symptoms, timely initiation of nonpharmacologic interventions, and potentially a decreased need for use of antipsychotic medications.
In this study, as noted in previous studies, certain medications (eg, steroids, opioids, and anticholinergics) increased the risk of developing terminal delirium in this veteran population. Steroids and opioids are commonly used in management of neoplasm-related pain and are prescribed throughout the course of terminal illness. The utility of these medications often outweighs potential adverse effects but should be considered when assessing the risk for development of delirium. Anticholinergics (eg, glycopyrrolate or scopolamine) are often prescribed in the last days of life for terminal secretions despite lack of evidence of patient benefit. Nonetheless, anticholinergics are used to reduce family and caregiver distress resulting from bothersome sounds from terminal secretions, referred to as the death rattle.21
It was found that veterans in the control group lived longer on the hospice unit. It is unclear whether the severity of illness was related to the development of terminal delirium or whether the development of terminal delirium contributed to a hastened death. Veterans with a suspected infection were identified by the use of antibiotics on admission to the hospice unit or when antibiotics were prescribed during the last 2 weeks of life. Thus, treatment of the underlying infection may have contributed to the finding of less delirium in the control group.
More than half the veterans in this study received at least 1 dose of an antipsychotic in the last 2 weeks of life for the treatment of terminal delirium. The most commonly administered medication was haloperidol, given either orally or subcutaneously. Atypical antipsychotics were used less often and were sometimes transitioned to subcutaneous haloperidol as the ability to swallow declined if symptoms persisted.
In this veteran population, having a history of drug or alcohol abuse (even if not recent) increased the risk of terminal delirium. Comorbid cancer and history of mental health disease (eg, PTSD, schizophrenia, bipolar disorder) and Vietnam-era veterans with liver disease (primary cancer, metastases, or cirrhosis) also were more likely to develop terminal delirium.
Just as hospice care is being provided in community settings, nurses are at the forefront of symptom management for veterans residing in VA CLCs under hospice care. Nonpharmacologic interventions are provided by the around-the-clock bedside team to provide comfort for veterans, families, and caregivers throughout the dying process. Nurses’ assessment skills and documentation inform the plan of care for the entire interdisciplinary hospice team. Because the treatment of terminal delirium often involves the administration of antipsychotic medications, scrutiny is applied to documentation surrounding these medications.7 This study suggested that there is a need for a more rigorous and consistent method of documenting the assessment of, and interventions for, terminal delirium.
Limitations
Limitations to the current study include hyperactive delirium that was misinterpreted and treated as pain; the probable underreporting of hypoactive delirium and associated symptoms; the use of antipsychotics as a surrogate marker for the development of terminal delirium; and lack of nursing documentation of assessment and interventions of terminal delirium. In addition, the total milligrams of antipsychotics administered per patient were not collected. Finally, there was the potential that other risk factors were not identified due to low numbers of veterans with certain diagnoses (eg, dementia).
Conclusions
Based on the findings in this study, several steps have been implemented to enhance the care of veterans under hospice care in this CLC: (1) Nurses providing direct patient care have been educated on the assessment by use of the mRASS and treatment of terminal delirium;22 (2) A hospice delirium note template has been created that details symptoms of terminal delirium, nonpharmacologic interventions, the use of antipsychotic medications if indicated, and the outcome of interventions; (3) Providers (eg, physician, advanced practice nurses) review each veteran’s medical history for the risk factors noted above; (4) Any risk factor(s) identified by this study will lead to a nursing order for delirium precautions, which requires completion of the delirium note template by nurses each shift.
The goal for this enhanced process is to identify veterans at risk for terminal delirium, observe changes that may indicate the onset of delirium, and intervene promptly to decrease symptom burden and improve quality of life and safety. Potentially, there will be less requirement for the use of antipsychotic medications to control the more severe symptoms of terminal delirium. A future study will evaluate the outcome of this enhanced process for the assessment and treatment of terminal delirium in this veteran population.
Acknowledgment
We thank Martin J. Gorbien, MD, associate chief of staff of Geriatrics and Extended Care, for his continued support throughout this project.
1. Casarett DJ, Inouye SK. Diagnosis and management of delirium near the end of life. Ann Intern Med. 2001;135(1):32-40.
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC; 2013.
3. Grassi L, Caraceni A, Mitchell A, et al. Management of delirium in palliative care: a review. Curr Psychiatry Rep. 2015;17(13):1-9. doi:10.1007/s11920-015-0550-8
4. Bush S, Leonard M, Agar M, et al. End-of-life delirium: issues regarding the recognition, optimal management, and role of sedation in the dying phase. J Pain Symptom Manage. 2014;48 (2):215-230. doi:10.1016/j.jpainsymman. 2014.05.009
5. Moyer D. Terminal delirium in geriatric patients with cancer at end of life. Am J Hosp Palliat Med. 2010;28(1):44-51. doi:10.1177/1049909110376755
6. Lai X, Huang Z, Chen C, et al. Delirium screening in patients in a palliative care ward: a best practice implementation project. JBI Database System Rev Implement Rep. 2019;17(3):429-441. doi:10.11124/JBISRIR-2017-003646
7. Centers for Medicare and Medicaid Services. Medicare and Medicaid Programs; reform of requirements for long-term care facilities. Final rule. Fed Regist. 2016;81 (192):68688-68872. Accessed April 17, 2021. https://pubmed.ncbi.nlm.nih.gov/27731960
8. Wright D, Brajtman S, Macdonald M. A relational ethical approach to end-of-life delirium. J Pain Symptom Manage. 2014;48(2):191-198. doi:10.1016/j.jpainsymman.2013.08.015
9. Brajtman S, Higuchi K, McPherson C. Caring for patients with terminal delirium: palliative care unit and home care nurses’ experience. Int J Palliat Nurs. 2006;12(4):150-156. doi:10.12968/ijpn.2006.12.4.21010
10. Lange E, Verhaak P, Meer K. Prevalence, presentation, and prognosis of delirium in older people in the population, at home and in long-term care: a review. Int J Geriatr Psychiatry. 2013;28(2):127-134. doi:10.1002/gps.3814
11. Goy E, Ganzini L. Prevalence and natural history of neuropsychiatric syndromes in veteran hospice patients. J Pain Symptom Manage. 2011;41(12):394-401. doi:10.1016/j.jpainsymman.2010.04.015
12. Bush S, Bruera E. The assessment and management of delirium in cancer patients. Oncologist. 2009;4(10):1039-1049. doi:10.1634/theoncologist.2009-0122
13. Clary P, Lawson P. Pharmacologic pearls for end-of-life care. Am Fam Physician. 2009;79(12):1059-1065.
14. Blinderman CD, Billings J. Comfort for patients dying in the hospital. N Engl J Med. 2015;373(26):2549-2561. doi:10.1056/NEJMra1411746
15. Irwin SA, Pirrello RD, Hirst JM, Buckholz GT, Ferris F.D. Clarifying delirium management: practical evidence-based, expert recommendation for clinical practice. J Palliat Med. 2013;16(4):423-435. doi:10.1089/jpm.2012.0319
16. Bobb B. Dyspnea and delirium at the end of life. Clin J Oncol Nurs. 2016;20(3):244-246. doi:10.1188/16.CJON.244-246
17. Morita T, Tei Y, Inoue S. Agitated terminal delirium and association with partial opioid substitution and hydration. J Palliat Med. 2003;6(4):557-563. doi:10.1089/109662103768253669
18. Attard A, Ranjith G, Taylor D. Delirium and its treatment. CNS Drugs. 2008;22(8):631-644-649. doi:10.2165/00023210-200822080-00002
19. Hui D. Benzodiazepines for agitation in patients with delirium: selecting the right patient, right time, and right indication. Curr Opin Support Palliat Care. 2018;12(4):489-494. doi:10.1097/SPC.0000000000000395
20. Irwin P, Murray S, Bilinski A, Chern B, Stafford B. Alcohol withdrawal as an underrated cause of agitated delirium and terminal restlessness in patients with advanced malignancy. J Pain Symptom Manage. 2005;29(1):104-108. doi:10.1016/j.jpainsymman.2004.04.010
21. Lokker ME, van Zuylen L, van der Rijt CCD, van der Heide A. Prevalence, impact, and treatment of death rattle: a systematic review. J Pain Symptom Manage. 2014;48:2-12. doi:10.1016/j.jpainsymman.2013.03.011
22. Sessler C, Gosnell M, Grap M, et al. The Richmond Agitation–Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002:166(10):1338-1344. doi:10.1164/rccm.2107138
Delirium is a condition commonly exhibited by hospitalized patients and by those who are approaching the end of life.1 Patients who experience a disturbance in attention that develops over a relatively short period and represents an acute change may have delirium.2 Furthermore, there is often an additional cognitive disturbance, such as disorientation, memory deficit, language deficits, visuospatial deficit, or perception. Terminal delirium is defined as delirium that occurs in the dying process and implies that reversal is less likely.3 When death is anticipated, diagnostic workups are not recommended, and treatment of the physiologic abnormalities that contribute to delirium is generally ineffective.4
Background
Delirium is often underdiagnosed and undetected by the clinician. Some studies have shown that delirium is not detected in 22 to 50% of cases.5 Factors that contribute to the underdetection of delirium include preexisting dementia, older age, presence of visual or hearing impairment, and hypoactive presentation of delirium. Other possible reasons for nondetection of delirium are its fluctuating nature and lack of formal cognitive assessment as part of a routine screening across care settings.5 Another study found that 41% of health care providers (HCPs) felt that screening for delirium was burdensome.6
To date, there are no veteran-focused studies that investigate prevalence or risk factors for terminal delirium in US Department of Veterans Affairs (VA) long-term care hospice units. Most long-term care hospice units in the VA are in community living centers (CLCs) that follow regulatory guidelines for using antipsychotic medications. The Centers for Medicare and Medicaid Services state that if antipsychotics are prescribed, documentation must clearly show the indication for the antipsychotic medication, the multiple attempts to implement planned care, nonpharmacologic approaches, and ongoing evaluation of the effectiveness of these interventions.7 The symptoms of terminal delirium cause significant distress to patients, family and caregivers, and nursing staff. Literature suggests that delirium poses significant relational challenges for patients, families, and HCPs in end-of-life situations.8,9 We hypothesize that the early identification of risk factors for the development of terminal delirium in this population may lead to increased use of nonpharmacologic measures to prevent terminal delirium, increase nursing vigilance for development of symptoms, and reduce symptom burden should terminal delirium develop.
Prevalence of delirium in the long-term care setting has ranged between 1.4 and 70.3%.10 The rate was found to be much higher in institutionalized populations compared with that of patients classified as at-home. In a study of the prevalence, severity, and natural history of neuropsychiatric syndromes in terminally ill veterans enrolled in community hospice, delirium was found to be present in only 4.1% on the initial visit and 42.5% during last visit. Also, more than half had at least 1 episode of delirium during the 90-day study period.11 In a study of the prevalence of delirium in terminal cancer patients admitted to hospice, 80% experienced delirium in their final days.12
Risk factors for the development of delirium that have been identified in actively dying patients include bowel or bladder obstruction, fluid and electrolyte imbalances, suboptimal pain management, medication adverse effects and toxicity (eg, benzodiazepines, opioids, anticholinergics, and steroids), the addition of ≥ 3 medications, infection, hepatic and renal failure, poor glycemic control, hypoxia, and hematologic disturbances.4,5,13 A high percentage of patients with a previous diagnosis of dementia were found to exhibit terminal delirium.14
There are 2 major subtypes of delirium: hyperactive and hypoactive.4 Patients with hypoactive delirium exhibit lethargy, reduced motor activity, lack of interest, and/or incoherent speech. There is currently little evidence to guide the treatment of hypoactive delirium. By contrast, hyperactive delirium is associated with hallucinations, agitation, heightened arousal, and inappropriate behavior. Many studies suggest both nonpharmacologic and pharmacologic treatment modalities for the treatment of hyperactive delirium.4,13 Nonpharmacologic interventions may minimize the risk and severity of symptoms associated with delirium. Current guidelines recommend these interventions before pharmacologic treatment.4 Nonpharmacologic interventions include but are not limited to the following: engaging the patient in mentally stimulating activities; surrounding the patient with familiar materials (eg, photos); ensuring that all individuals identify themselves when they encounter a patient; minimizing the intensity of stimulation, providing family or volunteer presence, soft lighting and warm blankets; and ensuring the patient uses hearing aids and glasses if needed.4,14
Although there are no US Food and Drug Administration-approved medications to treat hyperactive delirium, first-generation antipsychotics (eg, haloperidol, chlorpromazine) are considered the first-line treatment for patients exhibiting psychosis and psychomotor agitation.3,4,14-16 In terminally ill patients, there is limited evidence from clinical trials to support the efficacy of drug therapy.14 One study showed lack of efficacy with hydration and opioid rotation.17 In terminally ill patients experiencing hyperactive delirium, there is a significant increased risk of muscle tension, myoclonic seizures, and distress to the patient, family, and caregiver.1 Benzodiazepines can be considered first-line treatment for dying patients with terminal delirium in which the goals of treatment are to relieve muscle tension, ensure amnesia, reduce the risk of seizures, and decrease psychosis and agitation.18,19 Furthermore, in patients with history of alcohol misuse who are experiencing terminal delirium, benzodiazepines also may be the preferred pharmacologic treatment.20 Caution must be exercised with the use of benzodiazepines because they can also cause oversedation, increased confusion, and/or a paradoxical worsening of delirium.3,4,14
Methods
This was a retrospective case-control study of patients who died in the Edward Hines Jr. Veterans Affairs Hospital CLC in Hines, Illinois, under the treating specialty nursing home hospice from October 1, 2013 to September 30, 2015. Due to the retrospective nature of this trial, the use of antipsychotics within the last 2 weeks of life was a surrogate marker for development of terminal delirium. Cases were defined as patients who were treated with antipsychotics for terminal delirium within the last 2 weeks of their lives. Controls were defined as patients who were not treated with antipsychotics for terminal delirium within the last 2 weeks of their lives. Living hospice patients and patients who were discharged from the CLC before death were excluded.
The goals of this study were to (1) determine risk factors in the VA CLC hospice veteran population for the development of terminal delirium; (2) evaluate documentation by the nursing staff of nonpharmacologic interventions and indications for antipsychotic use in the treatment of terminal delirium; and (3) examine the current usage patterns of antipsychotics for the treatment of terminal delirium.
Veterans’ medical records were reviewed from 2 weeks before death until the recorded death date. Factors that were assessed included age, war era of service, date of death, terminal diagnosis, time interval from cancer diagnosis to death, comorbid conditions, prescribed antipsychotic medications, and other medications potentially contributing to delirium. Nursing documentation was reviewed for indications for administration of antipsychotic medications and nonpharmacologic interventions used to mitigate the symptoms of terminal delirium.
Statistical analysis was conducted in SAS Version 9.3. Cases were compared with controls using univariate and multivariate statistics as appropriate. Comparisons for continuous variables (eg, age) were conducted with Student t tests. Categorical variables (eg, PTSD diagnosis) were compared using χ2 analysis or Fisher exact test as appropriate. Variables with a P value < .1 in the univariate analysis were included in logistic regression models. Independent variables were removed from the models, using a backward selection process. Interaction terms were tested based on significance and clinical relevance. A P value < .05 was considered statistically significant.
Results
From October 1, 2013 to September 30, 2015, 307 patients were analyzed for inclusion in this study. Within this population, 186 received antipsychotic medications for the treatment of terminal delirium (cases), while 90 did not receive antipsychotics (controls). Of the 31 excluded patients, 13 were discharged to receive home hospice care, 11 were discharged to community nursing homes, 5 died in acute care units of Edward Hines, Jr. VA Hospital, and 2 died outside of the study period.
The mean age of all included patients was 75.5 years, and the most common terminal diagnosis was cancer, which occurred in 156 patients (56.5%) (Table 1). The baseline characteristics were similar between the cases and controls, including war era of veteran, terminal diagnosis, and comorbid conditions. The mean time between cancer diagnosis and death was not notably longer in the control group compared with that of the case group (25 vs 16 mo, respectively). There was no statistically significant difference in terminal diagnoses between cases and controls. Veterans in the control group spent more days (mean [SD]) in the hospice unit compared with veterans who experienced terminal delirium (48.5 [168.4] vs 28.2 [46.9]; P = .01). Patients with suspected infections were more likely found in the control group (P = .04; odds ratio [OR] = 1.70; 95% CI, 1.02-2.82).
The most common antipsychotic administered in the last 14 days of life was haloperidol. In the case group, 175 (94%) received haloperidol at least once in the last 2 weeks of life. Four (4.4%) veterans in the control group received haloperidol for the indication of nausea/vomiting; not terminal delirium. Atypical antipsychotics were infrequently used and included risperidone, olanzapine, quetiapine, and aripiprazole.
A total of 186 veterans received at least 1 dose of an antipsychotic for terminal delirium: 97 (52.2% ) veterans requiring antipsychotics for the treatment of terminal delirium required both scheduled and as-needed doses; 75 (40.3%) received only as-needed doses, and 14 (7.5%) required only scheduled doses. When the number of as-needed and scheduled doses were combined, each veteran received a mean 14.9 doses. However, for those veterans with antipsychotics ordered only as needed, a mean 5.8 doses were received per patient. Administration of antipsychotic doses was split evenly among the 3 nursing shifts (day-evening-night) with about 30% of doses administered on each shift.
Nurses were expected to document nonpharmacologic interventions that preceded the administration of each antipsychotic dose. Of the 1,028 doses administered to the 186 veterans who received at least 1 dose of an antipsychotic for terminal delirium, most of the doses (99.4%) had inadequate documentation based on current long-term care guidelines for prudent antipsychotic use.9
Several risk factors for terminal delirium were identified in this veteran population. Veterans with a history of drug or alcohol abuse were found to be at a significantly higher risk for terminal delirium (P = .04; OR, 1.87; 95% CI, 1.03-3.37). As noted in previous studies, steroid use (P = .01; OR, 2.57; 95% CI, 1.26-5.22); opioids (P = .007; OR, 5.94; 95% CI, 1.54-22.99), and anticholinergic medications (P = .01; OR, 2.06; 95% CI, 1.21-3.52) also increased the risk of delirium (Table 2).
When risk factors were combined, interaction terms were identified (Table 3). Those patients found to be at a higher risk of terminal delirium included Vietnam-era veterans with liver disease (P = .04; OR, 1.21; 95% CI, 1.01-1.45) and veterans with a history of drug or alcohol abuse plus comorbid liver disease (P = .03; OR, 1.26; 95% CI, 1.02-1.56). In a stratified analysis in veterans with a terminal diagnosis of cancer, those with a mental health condition (eg, PTSD, bipolar disorder, or schizophrenia) (P = .048; OR, 2.73; 95% CI, 0.98-7.58) also had higher risk of delirium, though not statistically significant. Within the cancer cohort, veterans with liver disease and a history of drug/alcohol abuse had increased risk of delirium (P = .01; OR, 1.43; 95% CI, 1.07-1.91).
Discussion
Terminal delirium is experienced by many individuals in their last days to weeks of life. Symptoms can present as hyperactive (eg, agitation, hallucinations, heightened arousal) or hypoactive (lethargy, reduced motor activity, incoherent speech). Hyperactive terminal delirium is particularly problematic because it causes increased distress to the patient, family, and caregivers. Delirium can lead to safety concerns, such as fall risk, due to patients’ decreased insight into functional decline.
Many studies suggest both nonpharmacologic and pharmacologic treatments for nonterminal delirium that may also apply to terminal delirium. Nonpharmacologic methods, such as providing a quiet and familiar environment, relieving urinary retention or constipation, and attending to sensory deficits may help prevent or minimize delirium. Pharmacologic interventions, such as antipsychotics or benzodiazepines, may benefit when other modalities have failed to assuage distressing symptoms of delirium. Because hypoactive delirium is usually accompanied by somnolence and reduced motor activity, medication is most often administered to individuals with hyperactive delirium.
The VA provides long-term care hospice beds in their CLCs for veterans who are nearing end of life and have inadequate caregiver support for comprehensive end-of-life care in the home (Case Presentation). Because of their military service and other factors common in their life histories, they may have a unique set of characteristics that are predictive of developing terminal delirium. Awareness of the propensity for terminal delirium will allow for early identification of symptoms, timely initiation of nonpharmacologic interventions, and potentially a decreased need for use of antipsychotic medications.
In this study, as noted in previous studies, certain medications (eg, steroids, opioids, and anticholinergics) increased the risk of developing terminal delirium in this veteran population. Steroids and opioids are commonly used in management of neoplasm-related pain and are prescribed throughout the course of terminal illness. The utility of these medications often outweighs potential adverse effects but should be considered when assessing the risk for development of delirium. Anticholinergics (eg, glycopyrrolate or scopolamine) are often prescribed in the last days of life for terminal secretions despite lack of evidence of patient benefit. Nonetheless, anticholinergics are used to reduce family and caregiver distress resulting from bothersome sounds from terminal secretions, referred to as the death rattle.21
It was found that veterans in the control group lived longer on the hospice unit. It is unclear whether the severity of illness was related to the development of terminal delirium or whether the development of terminal delirium contributed to a hastened death. Veterans with a suspected infection were identified by the use of antibiotics on admission to the hospice unit or when antibiotics were prescribed during the last 2 weeks of life. Thus, treatment of the underlying infection may have contributed to the finding of less delirium in the control group.
More than half the veterans in this study received at least 1 dose of an antipsychotic in the last 2 weeks of life for the treatment of terminal delirium. The most commonly administered medication was haloperidol, given either orally or subcutaneously. Atypical antipsychotics were used less often and were sometimes transitioned to subcutaneous haloperidol as the ability to swallow declined if symptoms persisted.
In this veteran population, having a history of drug or alcohol abuse (even if not recent) increased the risk of terminal delirium. Comorbid cancer and history of mental health disease (eg, PTSD, schizophrenia, bipolar disorder) and Vietnam-era veterans with liver disease (primary cancer, metastases, or cirrhosis) also were more likely to develop terminal delirium.
Just as hospice care is being provided in community settings, nurses are at the forefront of symptom management for veterans residing in VA CLCs under hospice care. Nonpharmacologic interventions are provided by the around-the-clock bedside team to provide comfort for veterans, families, and caregivers throughout the dying process. Nurses’ assessment skills and documentation inform the plan of care for the entire interdisciplinary hospice team. Because the treatment of terminal delirium often involves the administration of antipsychotic medications, scrutiny is applied to documentation surrounding these medications.7 This study suggested that there is a need for a more rigorous and consistent method of documenting the assessment of, and interventions for, terminal delirium.
Limitations
Limitations to the current study include hyperactive delirium that was misinterpreted and treated as pain; the probable underreporting of hypoactive delirium and associated symptoms; the use of antipsychotics as a surrogate marker for the development of terminal delirium; and lack of nursing documentation of assessment and interventions of terminal delirium. In addition, the total milligrams of antipsychotics administered per patient were not collected. Finally, there was the potential that other risk factors were not identified due to low numbers of veterans with certain diagnoses (eg, dementia).
Conclusions
Based on the findings in this study, several steps have been implemented to enhance the care of veterans under hospice care in this CLC: (1) Nurses providing direct patient care have been educated on the assessment by use of the mRASS and treatment of terminal delirium;22 (2) A hospice delirium note template has been created that details symptoms of terminal delirium, nonpharmacologic interventions, the use of antipsychotic medications if indicated, and the outcome of interventions; (3) Providers (eg, physician, advanced practice nurses) review each veteran’s medical history for the risk factors noted above; (4) Any risk factor(s) identified by this study will lead to a nursing order for delirium precautions, which requires completion of the delirium note template by nurses each shift.
The goal for this enhanced process is to identify veterans at risk for terminal delirium, observe changes that may indicate the onset of delirium, and intervene promptly to decrease symptom burden and improve quality of life and safety. Potentially, there will be less requirement for the use of antipsychotic medications to control the more severe symptoms of terminal delirium. A future study will evaluate the outcome of this enhanced process for the assessment and treatment of terminal delirium in this veteran population.
Acknowledgment
We thank Martin J. Gorbien, MD, associate chief of staff of Geriatrics and Extended Care, for his continued support throughout this project.
Delirium is a condition commonly exhibited by hospitalized patients and by those who are approaching the end of life.1 Patients who experience a disturbance in attention that develops over a relatively short period and represents an acute change may have delirium.2 Furthermore, there is often an additional cognitive disturbance, such as disorientation, memory deficit, language deficits, visuospatial deficit, or perception. Terminal delirium is defined as delirium that occurs in the dying process and implies that reversal is less likely.3 When death is anticipated, diagnostic workups are not recommended, and treatment of the physiologic abnormalities that contribute to delirium is generally ineffective.4
Background
Delirium is often underdiagnosed and undetected by the clinician. Some studies have shown that delirium is not detected in 22 to 50% of cases.5 Factors that contribute to the underdetection of delirium include preexisting dementia, older age, presence of visual or hearing impairment, and hypoactive presentation of delirium. Other possible reasons for nondetection of delirium are its fluctuating nature and lack of formal cognitive assessment as part of a routine screening across care settings.5 Another study found that 41% of health care providers (HCPs) felt that screening for delirium was burdensome.6
To date, there are no veteran-focused studies that investigate prevalence or risk factors for terminal delirium in US Department of Veterans Affairs (VA) long-term care hospice units. Most long-term care hospice units in the VA are in community living centers (CLCs) that follow regulatory guidelines for using antipsychotic medications. The Centers for Medicare and Medicaid Services state that if antipsychotics are prescribed, documentation must clearly show the indication for the antipsychotic medication, the multiple attempts to implement planned care, nonpharmacologic approaches, and ongoing evaluation of the effectiveness of these interventions.7 The symptoms of terminal delirium cause significant distress to patients, family and caregivers, and nursing staff. Literature suggests that delirium poses significant relational challenges for patients, families, and HCPs in end-of-life situations.8,9 We hypothesize that the early identification of risk factors for the development of terminal delirium in this population may lead to increased use of nonpharmacologic measures to prevent terminal delirium, increase nursing vigilance for development of symptoms, and reduce symptom burden should terminal delirium develop.
Prevalence of delirium in the long-term care setting has ranged between 1.4 and 70.3%.10 The rate was found to be much higher in institutionalized populations compared with that of patients classified as at-home. In a study of the prevalence, severity, and natural history of neuropsychiatric syndromes in terminally ill veterans enrolled in community hospice, delirium was found to be present in only 4.1% on the initial visit and 42.5% during last visit. Also, more than half had at least 1 episode of delirium during the 90-day study period.11 In a study of the prevalence of delirium in terminal cancer patients admitted to hospice, 80% experienced delirium in their final days.12
Risk factors for the development of delirium that have been identified in actively dying patients include bowel or bladder obstruction, fluid and electrolyte imbalances, suboptimal pain management, medication adverse effects and toxicity (eg, benzodiazepines, opioids, anticholinergics, and steroids), the addition of ≥ 3 medications, infection, hepatic and renal failure, poor glycemic control, hypoxia, and hematologic disturbances.4,5,13 A high percentage of patients with a previous diagnosis of dementia were found to exhibit terminal delirium.14
There are 2 major subtypes of delirium: hyperactive and hypoactive.4 Patients with hypoactive delirium exhibit lethargy, reduced motor activity, lack of interest, and/or incoherent speech. There is currently little evidence to guide the treatment of hypoactive delirium. By contrast, hyperactive delirium is associated with hallucinations, agitation, heightened arousal, and inappropriate behavior. Many studies suggest both nonpharmacologic and pharmacologic treatment modalities for the treatment of hyperactive delirium.4,13 Nonpharmacologic interventions may minimize the risk and severity of symptoms associated with delirium. Current guidelines recommend these interventions before pharmacologic treatment.4 Nonpharmacologic interventions include but are not limited to the following: engaging the patient in mentally stimulating activities; surrounding the patient with familiar materials (eg, photos); ensuring that all individuals identify themselves when they encounter a patient; minimizing the intensity of stimulation, providing family or volunteer presence, soft lighting and warm blankets; and ensuring the patient uses hearing aids and glasses if needed.4,14
Although there are no US Food and Drug Administration-approved medications to treat hyperactive delirium, first-generation antipsychotics (eg, haloperidol, chlorpromazine) are considered the first-line treatment for patients exhibiting psychosis and psychomotor agitation.3,4,14-16 In terminally ill patients, there is limited evidence from clinical trials to support the efficacy of drug therapy.14 One study showed lack of efficacy with hydration and opioid rotation.17 In terminally ill patients experiencing hyperactive delirium, there is a significant increased risk of muscle tension, myoclonic seizures, and distress to the patient, family, and caregiver.1 Benzodiazepines can be considered first-line treatment for dying patients with terminal delirium in which the goals of treatment are to relieve muscle tension, ensure amnesia, reduce the risk of seizures, and decrease psychosis and agitation.18,19 Furthermore, in patients with history of alcohol misuse who are experiencing terminal delirium, benzodiazepines also may be the preferred pharmacologic treatment.20 Caution must be exercised with the use of benzodiazepines because they can also cause oversedation, increased confusion, and/or a paradoxical worsening of delirium.3,4,14
Methods
This was a retrospective case-control study of patients who died in the Edward Hines Jr. Veterans Affairs Hospital CLC in Hines, Illinois, under the treating specialty nursing home hospice from October 1, 2013 to September 30, 2015. Due to the retrospective nature of this trial, the use of antipsychotics within the last 2 weeks of life was a surrogate marker for development of terminal delirium. Cases were defined as patients who were treated with antipsychotics for terminal delirium within the last 2 weeks of their lives. Controls were defined as patients who were not treated with antipsychotics for terminal delirium within the last 2 weeks of their lives. Living hospice patients and patients who were discharged from the CLC before death were excluded.
The goals of this study were to (1) determine risk factors in the VA CLC hospice veteran population for the development of terminal delirium; (2) evaluate documentation by the nursing staff of nonpharmacologic interventions and indications for antipsychotic use in the treatment of terminal delirium; and (3) examine the current usage patterns of antipsychotics for the treatment of terminal delirium.
Veterans’ medical records were reviewed from 2 weeks before death until the recorded death date. Factors that were assessed included age, war era of service, date of death, terminal diagnosis, time interval from cancer diagnosis to death, comorbid conditions, prescribed antipsychotic medications, and other medications potentially contributing to delirium. Nursing documentation was reviewed for indications for administration of antipsychotic medications and nonpharmacologic interventions used to mitigate the symptoms of terminal delirium.
Statistical analysis was conducted in SAS Version 9.3. Cases were compared with controls using univariate and multivariate statistics as appropriate. Comparisons for continuous variables (eg, age) were conducted with Student t tests. Categorical variables (eg, PTSD diagnosis) were compared using χ2 analysis or Fisher exact test as appropriate. Variables with a P value < .1 in the univariate analysis were included in logistic regression models. Independent variables were removed from the models, using a backward selection process. Interaction terms were tested based on significance and clinical relevance. A P value < .05 was considered statistically significant.
Results
From October 1, 2013 to September 30, 2015, 307 patients were analyzed for inclusion in this study. Within this population, 186 received antipsychotic medications for the treatment of terminal delirium (cases), while 90 did not receive antipsychotics (controls). Of the 31 excluded patients, 13 were discharged to receive home hospice care, 11 were discharged to community nursing homes, 5 died in acute care units of Edward Hines, Jr. VA Hospital, and 2 died outside of the study period.
The mean age of all included patients was 75.5 years, and the most common terminal diagnosis was cancer, which occurred in 156 patients (56.5%) (Table 1). The baseline characteristics were similar between the cases and controls, including war era of veteran, terminal diagnosis, and comorbid conditions. The mean time between cancer diagnosis and death was not notably longer in the control group compared with that of the case group (25 vs 16 mo, respectively). There was no statistically significant difference in terminal diagnoses between cases and controls. Veterans in the control group spent more days (mean [SD]) in the hospice unit compared with veterans who experienced terminal delirium (48.5 [168.4] vs 28.2 [46.9]; P = .01). Patients with suspected infections were more likely found in the control group (P = .04; odds ratio [OR] = 1.70; 95% CI, 1.02-2.82).
The most common antipsychotic administered in the last 14 days of life was haloperidol. In the case group, 175 (94%) received haloperidol at least once in the last 2 weeks of life. Four (4.4%) veterans in the control group received haloperidol for the indication of nausea/vomiting; not terminal delirium. Atypical antipsychotics were infrequently used and included risperidone, olanzapine, quetiapine, and aripiprazole.
A total of 186 veterans received at least 1 dose of an antipsychotic for terminal delirium: 97 (52.2% ) veterans requiring antipsychotics for the treatment of terminal delirium required both scheduled and as-needed doses; 75 (40.3%) received only as-needed doses, and 14 (7.5%) required only scheduled doses. When the number of as-needed and scheduled doses were combined, each veteran received a mean 14.9 doses. However, for those veterans with antipsychotics ordered only as needed, a mean 5.8 doses were received per patient. Administration of antipsychotic doses was split evenly among the 3 nursing shifts (day-evening-night) with about 30% of doses administered on each shift.
Nurses were expected to document nonpharmacologic interventions that preceded the administration of each antipsychotic dose. Of the 1,028 doses administered to the 186 veterans who received at least 1 dose of an antipsychotic for terminal delirium, most of the doses (99.4%) had inadequate documentation based on current long-term care guidelines for prudent antipsychotic use.9
Several risk factors for terminal delirium were identified in this veteran population. Veterans with a history of drug or alcohol abuse were found to be at a significantly higher risk for terminal delirium (P = .04; OR, 1.87; 95% CI, 1.03-3.37). As noted in previous studies, steroid use (P = .01; OR, 2.57; 95% CI, 1.26-5.22); opioids (P = .007; OR, 5.94; 95% CI, 1.54-22.99), and anticholinergic medications (P = .01; OR, 2.06; 95% CI, 1.21-3.52) also increased the risk of delirium (Table 2).
When risk factors were combined, interaction terms were identified (Table 3). Those patients found to be at a higher risk of terminal delirium included Vietnam-era veterans with liver disease (P = .04; OR, 1.21; 95% CI, 1.01-1.45) and veterans with a history of drug or alcohol abuse plus comorbid liver disease (P = .03; OR, 1.26; 95% CI, 1.02-1.56). In a stratified analysis in veterans with a terminal diagnosis of cancer, those with a mental health condition (eg, PTSD, bipolar disorder, or schizophrenia) (P = .048; OR, 2.73; 95% CI, 0.98-7.58) also had higher risk of delirium, though not statistically significant. Within the cancer cohort, veterans with liver disease and a history of drug/alcohol abuse had increased risk of delirium (P = .01; OR, 1.43; 95% CI, 1.07-1.91).
Discussion
Terminal delirium is experienced by many individuals in their last days to weeks of life. Symptoms can present as hyperactive (eg, agitation, hallucinations, heightened arousal) or hypoactive (lethargy, reduced motor activity, incoherent speech). Hyperactive terminal delirium is particularly problematic because it causes increased distress to the patient, family, and caregivers. Delirium can lead to safety concerns, such as fall risk, due to patients’ decreased insight into functional decline.
Many studies suggest both nonpharmacologic and pharmacologic treatments for nonterminal delirium that may also apply to terminal delirium. Nonpharmacologic methods, such as providing a quiet and familiar environment, relieving urinary retention or constipation, and attending to sensory deficits may help prevent or minimize delirium. Pharmacologic interventions, such as antipsychotics or benzodiazepines, may benefit when other modalities have failed to assuage distressing symptoms of delirium. Because hypoactive delirium is usually accompanied by somnolence and reduced motor activity, medication is most often administered to individuals with hyperactive delirium.
The VA provides long-term care hospice beds in their CLCs for veterans who are nearing end of life and have inadequate caregiver support for comprehensive end-of-life care in the home (Case Presentation). Because of their military service and other factors common in their life histories, they may have a unique set of characteristics that are predictive of developing terminal delirium. Awareness of the propensity for terminal delirium will allow for early identification of symptoms, timely initiation of nonpharmacologic interventions, and potentially a decreased need for use of antipsychotic medications.
In this study, as noted in previous studies, certain medications (eg, steroids, opioids, and anticholinergics) increased the risk of developing terminal delirium in this veteran population. Steroids and opioids are commonly used in management of neoplasm-related pain and are prescribed throughout the course of terminal illness. The utility of these medications often outweighs potential adverse effects but should be considered when assessing the risk for development of delirium. Anticholinergics (eg, glycopyrrolate or scopolamine) are often prescribed in the last days of life for terminal secretions despite lack of evidence of patient benefit. Nonetheless, anticholinergics are used to reduce family and caregiver distress resulting from bothersome sounds from terminal secretions, referred to as the death rattle.21
It was found that veterans in the control group lived longer on the hospice unit. It is unclear whether the severity of illness was related to the development of terminal delirium or whether the development of terminal delirium contributed to a hastened death. Veterans with a suspected infection were identified by the use of antibiotics on admission to the hospice unit or when antibiotics were prescribed during the last 2 weeks of life. Thus, treatment of the underlying infection may have contributed to the finding of less delirium in the control group.
More than half the veterans in this study received at least 1 dose of an antipsychotic in the last 2 weeks of life for the treatment of terminal delirium. The most commonly administered medication was haloperidol, given either orally or subcutaneously. Atypical antipsychotics were used less often and were sometimes transitioned to subcutaneous haloperidol as the ability to swallow declined if symptoms persisted.
In this veteran population, having a history of drug or alcohol abuse (even if not recent) increased the risk of terminal delirium. Comorbid cancer and history of mental health disease (eg, PTSD, schizophrenia, bipolar disorder) and Vietnam-era veterans with liver disease (primary cancer, metastases, or cirrhosis) also were more likely to develop terminal delirium.
Just as hospice care is being provided in community settings, nurses are at the forefront of symptom management for veterans residing in VA CLCs under hospice care. Nonpharmacologic interventions are provided by the around-the-clock bedside team to provide comfort for veterans, families, and caregivers throughout the dying process. Nurses’ assessment skills and documentation inform the plan of care for the entire interdisciplinary hospice team. Because the treatment of terminal delirium often involves the administration of antipsychotic medications, scrutiny is applied to documentation surrounding these medications.7 This study suggested that there is a need for a more rigorous and consistent method of documenting the assessment of, and interventions for, terminal delirium.
Limitations
Limitations to the current study include hyperactive delirium that was misinterpreted and treated as pain; the probable underreporting of hypoactive delirium and associated symptoms; the use of antipsychotics as a surrogate marker for the development of terminal delirium; and lack of nursing documentation of assessment and interventions of terminal delirium. In addition, the total milligrams of antipsychotics administered per patient were not collected. Finally, there was the potential that other risk factors were not identified due to low numbers of veterans with certain diagnoses (eg, dementia).
Conclusions
Based on the findings in this study, several steps have been implemented to enhance the care of veterans under hospice care in this CLC: (1) Nurses providing direct patient care have been educated on the assessment by use of the mRASS and treatment of terminal delirium;22 (2) A hospice delirium note template has been created that details symptoms of terminal delirium, nonpharmacologic interventions, the use of antipsychotic medications if indicated, and the outcome of interventions; (3) Providers (eg, physician, advanced practice nurses) review each veteran’s medical history for the risk factors noted above; (4) Any risk factor(s) identified by this study will lead to a nursing order for delirium precautions, which requires completion of the delirium note template by nurses each shift.
The goal for this enhanced process is to identify veterans at risk for terminal delirium, observe changes that may indicate the onset of delirium, and intervene promptly to decrease symptom burden and improve quality of life and safety. Potentially, there will be less requirement for the use of antipsychotic medications to control the more severe symptoms of terminal delirium. A future study will evaluate the outcome of this enhanced process for the assessment and treatment of terminal delirium in this veteran population.
Acknowledgment
We thank Martin J. Gorbien, MD, associate chief of staff of Geriatrics and Extended Care, for his continued support throughout this project.
1. Casarett DJ, Inouye SK. Diagnosis and management of delirium near the end of life. Ann Intern Med. 2001;135(1):32-40.
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC; 2013.
3. Grassi L, Caraceni A, Mitchell A, et al. Management of delirium in palliative care: a review. Curr Psychiatry Rep. 2015;17(13):1-9. doi:10.1007/s11920-015-0550-8
4. Bush S, Leonard M, Agar M, et al. End-of-life delirium: issues regarding the recognition, optimal management, and role of sedation in the dying phase. J Pain Symptom Manage. 2014;48 (2):215-230. doi:10.1016/j.jpainsymman. 2014.05.009
5. Moyer D. Terminal delirium in geriatric patients with cancer at end of life. Am J Hosp Palliat Med. 2010;28(1):44-51. doi:10.1177/1049909110376755
6. Lai X, Huang Z, Chen C, et al. Delirium screening in patients in a palliative care ward: a best practice implementation project. JBI Database System Rev Implement Rep. 2019;17(3):429-441. doi:10.11124/JBISRIR-2017-003646
7. Centers for Medicare and Medicaid Services. Medicare and Medicaid Programs; reform of requirements for long-term care facilities. Final rule. Fed Regist. 2016;81 (192):68688-68872. Accessed April 17, 2021. https://pubmed.ncbi.nlm.nih.gov/27731960
8. Wright D, Brajtman S, Macdonald M. A relational ethical approach to end-of-life delirium. J Pain Symptom Manage. 2014;48(2):191-198. doi:10.1016/j.jpainsymman.2013.08.015
9. Brajtman S, Higuchi K, McPherson C. Caring for patients with terminal delirium: palliative care unit and home care nurses’ experience. Int J Palliat Nurs. 2006;12(4):150-156. doi:10.12968/ijpn.2006.12.4.21010
10. Lange E, Verhaak P, Meer K. Prevalence, presentation, and prognosis of delirium in older people in the population, at home and in long-term care: a review. Int J Geriatr Psychiatry. 2013;28(2):127-134. doi:10.1002/gps.3814
11. Goy E, Ganzini L. Prevalence and natural history of neuropsychiatric syndromes in veteran hospice patients. J Pain Symptom Manage. 2011;41(12):394-401. doi:10.1016/j.jpainsymman.2010.04.015
12. Bush S, Bruera E. The assessment and management of delirium in cancer patients. Oncologist. 2009;4(10):1039-1049. doi:10.1634/theoncologist.2009-0122
13. Clary P, Lawson P. Pharmacologic pearls for end-of-life care. Am Fam Physician. 2009;79(12):1059-1065.
14. Blinderman CD, Billings J. Comfort for patients dying in the hospital. N Engl J Med. 2015;373(26):2549-2561. doi:10.1056/NEJMra1411746
15. Irwin SA, Pirrello RD, Hirst JM, Buckholz GT, Ferris F.D. Clarifying delirium management: practical evidence-based, expert recommendation for clinical practice. J Palliat Med. 2013;16(4):423-435. doi:10.1089/jpm.2012.0319
16. Bobb B. Dyspnea and delirium at the end of life. Clin J Oncol Nurs. 2016;20(3):244-246. doi:10.1188/16.CJON.244-246
17. Morita T, Tei Y, Inoue S. Agitated terminal delirium and association with partial opioid substitution and hydration. J Palliat Med. 2003;6(4):557-563. doi:10.1089/109662103768253669
18. Attard A, Ranjith G, Taylor D. Delirium and its treatment. CNS Drugs. 2008;22(8):631-644-649. doi:10.2165/00023210-200822080-00002
19. Hui D. Benzodiazepines for agitation in patients with delirium: selecting the right patient, right time, and right indication. Curr Opin Support Palliat Care. 2018;12(4):489-494. doi:10.1097/SPC.0000000000000395
20. Irwin P, Murray S, Bilinski A, Chern B, Stafford B. Alcohol withdrawal as an underrated cause of agitated delirium and terminal restlessness in patients with advanced malignancy. J Pain Symptom Manage. 2005;29(1):104-108. doi:10.1016/j.jpainsymman.2004.04.010
21. Lokker ME, van Zuylen L, van der Rijt CCD, van der Heide A. Prevalence, impact, and treatment of death rattle: a systematic review. J Pain Symptom Manage. 2014;48:2-12. doi:10.1016/j.jpainsymman.2013.03.011
22. Sessler C, Gosnell M, Grap M, et al. The Richmond Agitation–Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002:166(10):1338-1344. doi:10.1164/rccm.2107138
1. Casarett DJ, Inouye SK. Diagnosis and management of delirium near the end of life. Ann Intern Med. 2001;135(1):32-40.
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC; 2013.
3. Grassi L, Caraceni A, Mitchell A, et al. Management of delirium in palliative care: a review. Curr Psychiatry Rep. 2015;17(13):1-9. doi:10.1007/s11920-015-0550-8
4. Bush S, Leonard M, Agar M, et al. End-of-life delirium: issues regarding the recognition, optimal management, and role of sedation in the dying phase. J Pain Symptom Manage. 2014;48 (2):215-230. doi:10.1016/j.jpainsymman. 2014.05.009
5. Moyer D. Terminal delirium in geriatric patients with cancer at end of life. Am J Hosp Palliat Med. 2010;28(1):44-51. doi:10.1177/1049909110376755
6. Lai X, Huang Z, Chen C, et al. Delirium screening in patients in a palliative care ward: a best practice implementation project. JBI Database System Rev Implement Rep. 2019;17(3):429-441. doi:10.11124/JBISRIR-2017-003646
7. Centers for Medicare and Medicaid Services. Medicare and Medicaid Programs; reform of requirements for long-term care facilities. Final rule. Fed Regist. 2016;81 (192):68688-68872. Accessed April 17, 2021. https://pubmed.ncbi.nlm.nih.gov/27731960
8. Wright D, Brajtman S, Macdonald M. A relational ethical approach to end-of-life delirium. J Pain Symptom Manage. 2014;48(2):191-198. doi:10.1016/j.jpainsymman.2013.08.015
9. Brajtman S, Higuchi K, McPherson C. Caring for patients with terminal delirium: palliative care unit and home care nurses’ experience. Int J Palliat Nurs. 2006;12(4):150-156. doi:10.12968/ijpn.2006.12.4.21010
10. Lange E, Verhaak P, Meer K. Prevalence, presentation, and prognosis of delirium in older people in the population, at home and in long-term care: a review. Int J Geriatr Psychiatry. 2013;28(2):127-134. doi:10.1002/gps.3814
11. Goy E, Ganzini L. Prevalence and natural history of neuropsychiatric syndromes in veteran hospice patients. J Pain Symptom Manage. 2011;41(12):394-401. doi:10.1016/j.jpainsymman.2010.04.015
12. Bush S, Bruera E. The assessment and management of delirium in cancer patients. Oncologist. 2009;4(10):1039-1049. doi:10.1634/theoncologist.2009-0122
13. Clary P, Lawson P. Pharmacologic pearls for end-of-life care. Am Fam Physician. 2009;79(12):1059-1065.
14. Blinderman CD, Billings J. Comfort for patients dying in the hospital. N Engl J Med. 2015;373(26):2549-2561. doi:10.1056/NEJMra1411746
15. Irwin SA, Pirrello RD, Hirst JM, Buckholz GT, Ferris F.D. Clarifying delirium management: practical evidence-based, expert recommendation for clinical practice. J Palliat Med. 2013;16(4):423-435. doi:10.1089/jpm.2012.0319
16. Bobb B. Dyspnea and delirium at the end of life. Clin J Oncol Nurs. 2016;20(3):244-246. doi:10.1188/16.CJON.244-246
17. Morita T, Tei Y, Inoue S. Agitated terminal delirium and association with partial opioid substitution and hydration. J Palliat Med. 2003;6(4):557-563. doi:10.1089/109662103768253669
18. Attard A, Ranjith G, Taylor D. Delirium and its treatment. CNS Drugs. 2008;22(8):631-644-649. doi:10.2165/00023210-200822080-00002
19. Hui D. Benzodiazepines for agitation in patients with delirium: selecting the right patient, right time, and right indication. Curr Opin Support Palliat Care. 2018;12(4):489-494. doi:10.1097/SPC.0000000000000395
20. Irwin P, Murray S, Bilinski A, Chern B, Stafford B. Alcohol withdrawal as an underrated cause of agitated delirium and terminal restlessness in patients with advanced malignancy. J Pain Symptom Manage. 2005;29(1):104-108. doi:10.1016/j.jpainsymman.2004.04.010
21. Lokker ME, van Zuylen L, van der Rijt CCD, van der Heide A. Prevalence, impact, and treatment of death rattle: a systematic review. J Pain Symptom Manage. 2014;48:2-12. doi:10.1016/j.jpainsymman.2013.03.011
22. Sessler C, Gosnell M, Grap M, et al. The Richmond Agitation–Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002:166(10):1338-1344. doi:10.1164/rccm.2107138
Impact of the COVID-19 Pandemic on Multiple Sclerosis Care for Veterans
The following is a lightly edited transcript of a teleconference recorded in February 2021.
How has COVID impacted Veterans with multiple sclerosis?
Mitchell Wallin, MD, MPH: There has been a lot of concern in the multiple sclerosis (MS) patient community about getting infected with COVID-19 and what to do about it. Now that there are vaccines, the concern is whether and how to take a vaccine. At least here, in the Washington DC/Baltimore area where I practice, we have seen many veterans being hospitalized with COVID-19, some with multiple sclerosis (MS), and some who have died of COVID-19. So, there has been a lot of fear, especially in veterans that are older with comorbid diseases.
Rebecca Spain, MD, MSPH: There also has been an impact on our ability to provide care to our veterans with MS. There are challenges having them come into the office or providing virtual care. There are additional challenges and concerns this year about making changes in MS medications because we can’t see patients in person to or understand their needs or current status of their MS. So, providing care has been a challenge this year as well.
There has also been an impact on our day to day lives, like there has been for all of us, from the lockdown particularly not being able to exercise and socialize as much. There have been physical and social and emotional tolls that this disease has taken on veterans with MS.
Jodie Haselkorn, MD, MPH: The survivors of COVID-19, that are transferred to an inpatient multidisciplinary rehabilitation program unit to address impairments related to the cardiopulmonary, immobility, psychological impacts and other medical complications are highly motivated to work with the team to achieve a safe discharge. The US Department of Veterans Affairs (VA) Rehabilitation Services has much to offer them.
Heidi Maloni, PhD, NP: Veterans with MS are not at greater risk because they are diagnosed with MS. But, their comorbidities such as hypertension, obesity, or factors such as older age and increased disability can increase the risk of COVID-19 infection and poorer outcomes if infected. might place them at greater risk.
Veterans have asked “Am I at greater risk? Do I need to do something more to protect myself?” I have had innumerable veterans call and ask whether I can write them letters for their employer to ensure that they work at home longer rather than go into the workplace because they’re very nervous and don’t feel confident that masking and distancing is really going to be protective.
Mitchell Wallin: We are analyzing some of our data in the VA health care system related to COVID-19 infections in the MS population. We can’t say for sure what are numbers are, but our rates of infection and hospitalization are higher than the general population and we will soon have a report. We have a majority male population, which is different from the general MS population, which is predominantly female. The proportion of minority patients in VA mirrors those of the US population. These demographic factors along with a high level of comorbid disease put veterans at high risk for acquiring COVID-19. So, in some ways it’s hard to compare when you look at reports from other countries or the US National MS-COVID-19 Registry, which captures a population that is predominantly female. In the VA, our age range spans from the 20s to almost 100 years. We must understand our population to prevent COVID-19 and better care for the most vulnerable.
Rebecca Spain: Heidi, my understanding, although the numbers are small, that for the most part, Veterans with MS who are older are at higher risk of complications and death, which is also true of the general population. But that there is an additional risk for people with MS who have higher disability levels. My understanding from reading the literature, was that people with MS needing or requiring a cane to walk or greater assistance for mobility were at a higher risk for COVID-19 complications, including mortality. I have been particularly encouraged that in many places this special population of people with MS are getting vaccinated sooner.
Heidi Maloni: I completely agree, you said it very clearly, Becca. Their disability level puts them at risk
Rebecca Spain: Disability is a comorbidity.
Heidi Maloni: Yes. Just sitting in a wheelchair and not being able to get a full breath or having problems with respiratory effort really does put you at risk for doing well if you were to have COVID-19.
Are there other ancillary impacts from COVID-19 for patients with MS?
Jodie Haselkorn: Individuals who are hospitalized with COVID-19 miss social touch and social support from family and friends. They miss familiar conversations, a hug and having someone hold their hand. The acute phase of the infection limits professional face-to-face interaction with patients due to time and protective garments. There are reports of negative consequences with isolation and social reintegration of the COVID-19 survivors is necessary and a necessary part of rehabilitation.
Mitchell Wallin: For certain procedures (eg, magnetic resonance imaging [MRI]) or consultations, we need to bring people into the medical center. Many clinical encounters, however, can be done through telemedicine and both the VA and the US Department of Defense systems were set up to execute this type of visit. We had been doing telemedicine for a long time before the pandemic and we were in a better position than a lot of other health systems to shift to a virtual format with COVID-19. We had to ramp up a little bit and get our tools working a little more effectively for all clinics, but I think we were prepared to broadly execute telemedicine clinics for the pandemic.
Jodie Haselkorn: I agree that the he VA infrastructure was ahead of most other health system in terms of readiness for telehealth and maintaining access to care. Not all health care providers (HCPs) were using it, but the system was there, and included a telehealth coordinator in all of the facilities who could gear health care professionals up quickly. Additionally, a system was in place to provide veterans and caregivers with telehealth home equipment and provide training. Another thing that really helped was the MISSION Act. Veterans who have difficulty travelling for an appointment may have the ability to seek care outside of the VA within their own community. They may be able to go into a local facility to get laboratory or radiologic studies done or continue rehabilitation closer to home.
VA MS Registry Data
Rebecca Spain: Mitch, there are many interesting things we can learn about the interplay between COVID-19 and MS using registries such as how it affects people based on rural vs metropolitan living, whether people are living in single family homes or not as a proxy marker for social support, and so on.
Mitchell Wallin: We have both an MS registry to track and follow patients through our clinical network and a specific COVID-19 registry as well in VA. We have identified the MS cases infected with CoVID-19 and are putting them together.
Jodie Haselkorn: There are a number of efforts in mental health that are moving forward to examine depression and in anxiety during COVID-19. Individuals with MS have increased rates of depression and anxiety above that of the general population during usual times. The literature reports an increase in anxiety and depression in general population associated with the pandemic and veterans with MS seem to be reporting these symptoms more frequently as well. We will be able to track use the registry to assess the impacts of COVID-19 on depression and anxiety in Veterans with MS.
Providing MS Care During COVID-19
Jodie Haselkorn: The transition to telehealth in COVID-19 has been surprisingly seamless with some additional training for veterans and HCPs. I initially experienced an inefficiency in my clinic visit productivity. It took me longer to see a veteran because I wasn’t doing telehealth in our clinic with support staff and residents, my examination had to change, my documentation template needed to be restructured, and the coding was different. Sometimes I saw a veteran in clinic the and my next appointment required me to move back to my office in another building for a telehealth appointment. Teaching virtual trainees who also participated in the clinic encounters had its own challenges and rewards. My ‘motor routine’ was disrupted.
Rebecca Spain: There’s a real learning curve for telehealth in terms of how comfortable you feel with the data you get by telephone or video and how reliable that is. There are issues based on technology factors—like the patient’s bandwidth—because determining how smooth their motions are is challenging if you have a jerky, intermittent signal. I learned quickly to always do the physical examination first because I might lose video connection partway through and have to switch to a phone visit!
It’s still an open question, how much are we missing by using a video and not in-person visits. And what are the long-term health outcomes and implications of that? That is something that needs to be studied in neurology where we pride ourselves on the physical examination. When move to a virtual physical examination, is there cost? There are incredible gains using telehealth in terms of convenience and access to care, which may outweigh some of the drawbacks in particular cases.
There are also pandemic challenge in terms of clinic workflow. At VA Portland Health Care System in Oregon, I have 3 clinics for Friday morning: telephone, virtual, and face-to-face clinics. It’s a real struggle for the schedulers. And because of that transition to new system workflows to accommodate this, some patient visits have been dropped, lost, or scheduled incorrectly.
Heidi Maloni: As the nurse in this group, I agree with everything that Becca and Jodie have said about telehealth. But, I have found some benefits, and one of them is a greater intimacy with my patients. What do I mean by that? For instance, if a patient has taken me to their kitchen and opened their cupboard to show me the breakfast cereal, I’m also observing that there’s nothing else in that cupboard other than cereal. I’m also putting some things together about health and wellness. Or, for the first time, I might meet their significant other who can’t come to clinic because they’re working, but they are at home with the patient. And then having that 3-way conversation with the patient and the significant other, that’s kind of opened up my sense of who that person is.
You are right about the neurological examination. It’s challenging to make exacting assessments. When gathering household objects, ice bags and pronged forks to assess sensation, you remember that this exam is subjective and there is meaning in this remote evaluation. But all in all, I have been blessed with telehealth. Patients don’t mind it at all. They’re completely open to the idea. They like the telehealth for the contact they are able to have with their HCP.
Jodie Haselkorn: As you were saying that, Heidi, I thought, I’ve been inside my veterans’ bathrooms virtually and have seen all of their equipment that they have at home. In a face-to-face clinic visit, you don’t have an opportunity to see all their canes and walkers, braces, and other assistive technology. Some of it’s stashed in a closet, some of it under the bed. In a virtual visit, I get to understand why some is not used, what veterans prefer, and see their own innovations for mobility and self-care.
Mitchell Wallin: There’s a typical ritual that patients talk about when they go to a clinic. They check in, sit down, and wait for the nurse to give them their vital signs and set them up in the room. And then they meet with their HCP, and finally they complete the tasks on the checklist. And part of that may mean scheduling an MRI or going to the lab. But some of these handoffs don’t happen as well on telehealth. Maybe we haven’t integrated these segments of a clinical visit into telehealth platforms. But it could be developed, and there could be new neurologic tools to improve the interview and physical examination. Twenty years ago, you couldn’t deposit a check on your phone; but now you can do everything on your phone you could do in a physical bank. With some creativity, we can improve parts of the neurological exam that are currently difficult to assess remotely.
Jodie Haselkorn: I have not used peripherals in video telehealth to home and I would need to become accustomed to their use with current technology and train patients and caregivers. I would like telehealth peripherals such as a stethoscope to listen to the abdomen of a veteran with neurogenic bowel or a user-friendly ultrasound probe to measure postvoid residual urine in an individual with symptoms of neurogenic bladder, in addition to devices that measure walking speed and pulmonary function. I look forward to the development, use, and the incorporation peripherals that will enable a more extensive virtual exam within the home.
What are the MS Centers of Excellence working on now?
Jodie Haselkorn: We are working to understand the healthcare needs of veterans with MS by evaluating not only care for MS within the VA, but also the types and quantity of MS specialty care VA that is being received in the community during the pandemic. Dr. Wallin is also using the registry to lead a telehealth study to capture the variety of different codes that VA health professionals in MS have used to document workload by telehealth, and face-to-face, and telephone encounters.
Rebecca Spain: The MS Center of Excellence (MSCoE) is coming out with note templates to be available for HCPs, which we can refine as we get experience. This is s one way we can promote high standards in MS care by making these ancillary tools more productive.
Jodie Haselkorn: We are looking at different ways to achieve a high-quality virtual examination using standardized examination strategies and patient and caregiver information to prepare for a specialty MS visit.
Rebecca Spain: I would like to, in more of a research setting, study health outcomes using telehealth vs in person and start tracking that long term.
Mitchell Wallin: We can probably do more in terms of standardization, such as the routine patient reported surveys and implementing the new Consortium of Multiple Sclerosis Centers’ International MRI criteria. The COVID pandemic has affected everything in medical care. But we want to have a regular standardized outcome to assess, and if we can start to do some of the standard data collection through telemedicine, it becomes part of our regular clinic data.
Heidi Maloni: We need better technology. You can do electrocardiograms on your watch. Could we do Dinamaps? Could we figure out strength? That’s a wish list.
Jodie Haselkorn: Since the MSCoE is a national program, we were set up to do what we needed to do for education. We were able to continue on with all of our HCP webinars, including the series with the National MS Society (NMSS). We also have a Specialty Care Access Network-Extension for Community Healthcare Outcomes (SCAN-ECHO) series with the Northwest ECHO VA program and collaborated with the Can Do MS program on patient education as well. We’ve sent out 2 printed newsletters for veterans. The training of HCPs for the future has continued as well. All of our postdoctoral fellows who have finished their programs on time and moved on to either clinical practice or received career development grants to continue their VA careers, a new fellow has joined, and our other fellows are continuing as planned.
The loss that we sustained was in-person meetings. We held MSCoE Regional Program meetings in the East and West that combined education and administrative goals. Both of these were well attended and successful. There was a lot of virtual education available from multiple sources. It was challenging this year was to anticipate what education programming people wanted from MSCoE. Interestingly, a lot of our regional HCPs did not want much more COVID-19 education. They wanted other education and we were able to meet those needs.
Did the pandemic impact the VA MS registry?
Mitchell Wallin: Like any electronic product, the VA MS Surveillance Registry must be maintained, and we have tried to encourage people to use it. Our biggest concern was to identify cases of MS that got infected with COVID-19 and to put those people into the registry. In some cases, Veterans with MS were in locations without a MS clinic. So, we’ve spent a lot more time identifying those cases and adjudicating them to make sure their infection and MS were documented correctly.
During the COVID-19 pandemic, the VA healthcare system has been taxed like others and so HCPs have been a lot busier than normal, forcing new workflows. It has been a hard year that way because a lot of health care providers have been doing many other jobs to help maintain patient care during the COVID-19 pandemic.
Heidi Maloni: The impact of COVID-19 has been positive for the registry because we’ve had more opportunities to populate it.
Jodie Haselkorn: Dr. Wallin and the COVID-19 Registry group began building the combined registry at the onset of the pandemic. We have developed the capacity to identify COVID-19 infections in veterans who have MS and receive care in the VA. We entered these cases in the MS Surveillance Registry and have developed a linkage with the COVID-19 national VA registry. We are in the middle of the grunt work part case entry, but it is a rich resource.
How has the pandemic impacted MS research?
Rebecca Spain: COVID-19 has put a big damper on clinical research progress, including some of our MSCoE studies. It has been difficult to have subjects come in for clinical visits. It’s been difficult to get approval for new studies. It’s shifted timelines dramatically, and then that always increases budgets in a time when there’s not a lot of extra money. So, for clinical research, it’s been a real struggle and a strain and an ever-moving target. For laboratory research most, if not all, centers that have laboratory research at some point were closed and have only slowly reopened. Some still haven’t reopened to any kind of research or laboratory. So, it’s been tough, I think, on research in general.
Heidi Maloni: I would say the word is devastating. The pandemic essentially put a stop to in-person research studies. Our hospital was in research phase I, meaning human subjects can only participate in a research study if they are an inpatient or outpatient with an established clinic visit (clinics open to 25% occupancy) or involved in a study requiring safety monitoring, This plan limits risk of COVID-19 exposure.
Rebecca Spain: There is risk for a higher dropout rate of subjects from studies meaning there’s less chance of success for finding answers if enough people don’t stay in. At a certain point, you have to say, “Is this going to be a successful study?”
Jodie Haselkorn: Dr. Spain has done an amazing job leading a multisite, international clinical trial funded by the VA and the NMSS and kept it afloat, despite challenges. The pandemic has had impacts, but the study continues to move towards completion. I’ve appreciated the efforts of the Research Service at VA Puget Sound to ensure that we could safely obtain many of the 12-month outcomes for all the participants enrolled in that study.
Mitchell Wallin: The funding for some of our nonprofit partners, including the Paralyzed Veterans Association (PVA) and the NMSS, has suffered as well and so a lot of their funding programs have closed or been cut back during the pandemic. Despite that, we still have been able to use televideo technology for our clinical and educational programs with our network.
Jodie Haselkorn: MSCoE also does health services and epidemiological studies in addition to clinical trials and that work has continued. Quite a few of the studies that had human subjects in them were completed in terms of data collection, and so those are being analyzed. There will be a drop in funded studies, publications and posters as the pandemic continues and for a recovery period. We have a robust baseline for research productivity and a talented team. We’ll be able to track drop off and recovery over time.
Rebecca Spain: There’s going to be long-term consequences that we don’t see right now, especially for young researchers who have missed getting pilot data which would have led to additional small grants and then later large grants. There’s going to be an education gap that’s going on with all of the kids who are not able to go to school properly. It’s part of that whole swath of lost time and lost opportunity that we will have to deal with.
However, there are going to be some positive changes. We’re now busy designing clinical trials that can be done virtually to minimize any contact with the health facility, and then looking at things like shifting to research ideas that are more focused around health services.
Jodie Haselkorn: Given the current impacts of the pandemic on delivery of health care there is a strong interest in looking at how we can deliver health care in ways that accommodates the consumers and the providers perspectives. In the future we see marked impacts in our abilities to deliver care to Veterans with MS.
As a final thought, I wanted to put in a plug for this talented team. One of our pandemic resolutions was to innovatively find new possibilities and avoid negative focus on small changes. We are fortunate that all our staff have remained healthy and been supportive and compassionate with each other throughout this period. We have met our goals and are still moving forward.
MSCoE has benefited from the supportive leadership of Sharyl Martini, MD, PhD, and Glenn Graham, MD, PhD, in VA Specialty Care Neurology and leadership and space from VA Puget Sound, VA Portland Health Care System, the Washington DC VA Medical Center and VA Maryland Health Care System in Baltimore.
We also have a national advisory system that is actively involved, sets high standards and performs a rigorous annual review. We have rich inputs from the VA National Regional Programs and Veterans. Additionally, we have had the leadership and opportunities to collaborate with outside organizations including, the Consortium of MS Centers, the NMSS, and the PVA. We have been fortunate.
The following is a lightly edited transcript of a teleconference recorded in February 2021.
How has COVID impacted Veterans with multiple sclerosis?
Mitchell Wallin, MD, MPH: There has been a lot of concern in the multiple sclerosis (MS) patient community about getting infected with COVID-19 and what to do about it. Now that there are vaccines, the concern is whether and how to take a vaccine. At least here, in the Washington DC/Baltimore area where I practice, we have seen many veterans being hospitalized with COVID-19, some with multiple sclerosis (MS), and some who have died of COVID-19. So, there has been a lot of fear, especially in veterans that are older with comorbid diseases.
Rebecca Spain, MD, MSPH: There also has been an impact on our ability to provide care to our veterans with MS. There are challenges having them come into the office or providing virtual care. There are additional challenges and concerns this year about making changes in MS medications because we can’t see patients in person to or understand their needs or current status of their MS. So, providing care has been a challenge this year as well.
There has also been an impact on our day to day lives, like there has been for all of us, from the lockdown particularly not being able to exercise and socialize as much. There have been physical and social and emotional tolls that this disease has taken on veterans with MS.
Jodie Haselkorn, MD, MPH: The survivors of COVID-19, that are transferred to an inpatient multidisciplinary rehabilitation program unit to address impairments related to the cardiopulmonary, immobility, psychological impacts and other medical complications are highly motivated to work with the team to achieve a safe discharge. The US Department of Veterans Affairs (VA) Rehabilitation Services has much to offer them.
Heidi Maloni, PhD, NP: Veterans with MS are not at greater risk because they are diagnosed with MS. But, their comorbidities such as hypertension, obesity, or factors such as older age and increased disability can increase the risk of COVID-19 infection and poorer outcomes if infected. might place them at greater risk.
Veterans have asked “Am I at greater risk? Do I need to do something more to protect myself?” I have had innumerable veterans call and ask whether I can write them letters for their employer to ensure that they work at home longer rather than go into the workplace because they’re very nervous and don’t feel confident that masking and distancing is really going to be protective.
Mitchell Wallin: We are analyzing some of our data in the VA health care system related to COVID-19 infections in the MS population. We can’t say for sure what are numbers are, but our rates of infection and hospitalization are higher than the general population and we will soon have a report. We have a majority male population, which is different from the general MS population, which is predominantly female. The proportion of minority patients in VA mirrors those of the US population. These demographic factors along with a high level of comorbid disease put veterans at high risk for acquiring COVID-19. So, in some ways it’s hard to compare when you look at reports from other countries or the US National MS-COVID-19 Registry, which captures a population that is predominantly female. In the VA, our age range spans from the 20s to almost 100 years. We must understand our population to prevent COVID-19 and better care for the most vulnerable.
Rebecca Spain: Heidi, my understanding, although the numbers are small, that for the most part, Veterans with MS who are older are at higher risk of complications and death, which is also true of the general population. But that there is an additional risk for people with MS who have higher disability levels. My understanding from reading the literature, was that people with MS needing or requiring a cane to walk or greater assistance for mobility were at a higher risk for COVID-19 complications, including mortality. I have been particularly encouraged that in many places this special population of people with MS are getting vaccinated sooner.
Heidi Maloni: I completely agree, you said it very clearly, Becca. Their disability level puts them at risk
Rebecca Spain: Disability is a comorbidity.
Heidi Maloni: Yes. Just sitting in a wheelchair and not being able to get a full breath or having problems with respiratory effort really does put you at risk for doing well if you were to have COVID-19.
Are there other ancillary impacts from COVID-19 for patients with MS?
Jodie Haselkorn: Individuals who are hospitalized with COVID-19 miss social touch and social support from family and friends. They miss familiar conversations, a hug and having someone hold their hand. The acute phase of the infection limits professional face-to-face interaction with patients due to time and protective garments. There are reports of negative consequences with isolation and social reintegration of the COVID-19 survivors is necessary and a necessary part of rehabilitation.
Mitchell Wallin: For certain procedures (eg, magnetic resonance imaging [MRI]) or consultations, we need to bring people into the medical center. Many clinical encounters, however, can be done through telemedicine and both the VA and the US Department of Defense systems were set up to execute this type of visit. We had been doing telemedicine for a long time before the pandemic and we were in a better position than a lot of other health systems to shift to a virtual format with COVID-19. We had to ramp up a little bit and get our tools working a little more effectively for all clinics, but I think we were prepared to broadly execute telemedicine clinics for the pandemic.
Jodie Haselkorn: I agree that the he VA infrastructure was ahead of most other health system in terms of readiness for telehealth and maintaining access to care. Not all health care providers (HCPs) were using it, but the system was there, and included a telehealth coordinator in all of the facilities who could gear health care professionals up quickly. Additionally, a system was in place to provide veterans and caregivers with telehealth home equipment and provide training. Another thing that really helped was the MISSION Act. Veterans who have difficulty travelling for an appointment may have the ability to seek care outside of the VA within their own community. They may be able to go into a local facility to get laboratory or radiologic studies done or continue rehabilitation closer to home.
VA MS Registry Data
Rebecca Spain: Mitch, there are many interesting things we can learn about the interplay between COVID-19 and MS using registries such as how it affects people based on rural vs metropolitan living, whether people are living in single family homes or not as a proxy marker for social support, and so on.
Mitchell Wallin: We have both an MS registry to track and follow patients through our clinical network and a specific COVID-19 registry as well in VA. We have identified the MS cases infected with CoVID-19 and are putting them together.
Jodie Haselkorn: There are a number of efforts in mental health that are moving forward to examine depression and in anxiety during COVID-19. Individuals with MS have increased rates of depression and anxiety above that of the general population during usual times. The literature reports an increase in anxiety and depression in general population associated with the pandemic and veterans with MS seem to be reporting these symptoms more frequently as well. We will be able to track use the registry to assess the impacts of COVID-19 on depression and anxiety in Veterans with MS.
Providing MS Care During COVID-19
Jodie Haselkorn: The transition to telehealth in COVID-19 has been surprisingly seamless with some additional training for veterans and HCPs. I initially experienced an inefficiency in my clinic visit productivity. It took me longer to see a veteran because I wasn’t doing telehealth in our clinic with support staff and residents, my examination had to change, my documentation template needed to be restructured, and the coding was different. Sometimes I saw a veteran in clinic the and my next appointment required me to move back to my office in another building for a telehealth appointment. Teaching virtual trainees who also participated in the clinic encounters had its own challenges and rewards. My ‘motor routine’ was disrupted.
Rebecca Spain: There’s a real learning curve for telehealth in terms of how comfortable you feel with the data you get by telephone or video and how reliable that is. There are issues based on technology factors—like the patient’s bandwidth—because determining how smooth their motions are is challenging if you have a jerky, intermittent signal. I learned quickly to always do the physical examination first because I might lose video connection partway through and have to switch to a phone visit!
It’s still an open question, how much are we missing by using a video and not in-person visits. And what are the long-term health outcomes and implications of that? That is something that needs to be studied in neurology where we pride ourselves on the physical examination. When move to a virtual physical examination, is there cost? There are incredible gains using telehealth in terms of convenience and access to care, which may outweigh some of the drawbacks in particular cases.
There are also pandemic challenge in terms of clinic workflow. At VA Portland Health Care System in Oregon, I have 3 clinics for Friday morning: telephone, virtual, and face-to-face clinics. It’s a real struggle for the schedulers. And because of that transition to new system workflows to accommodate this, some patient visits have been dropped, lost, or scheduled incorrectly.
Heidi Maloni: As the nurse in this group, I agree with everything that Becca and Jodie have said about telehealth. But, I have found some benefits, and one of them is a greater intimacy with my patients. What do I mean by that? For instance, if a patient has taken me to their kitchen and opened their cupboard to show me the breakfast cereal, I’m also observing that there’s nothing else in that cupboard other than cereal. I’m also putting some things together about health and wellness. Or, for the first time, I might meet their significant other who can’t come to clinic because they’re working, but they are at home with the patient. And then having that 3-way conversation with the patient and the significant other, that’s kind of opened up my sense of who that person is.
You are right about the neurological examination. It’s challenging to make exacting assessments. When gathering household objects, ice bags and pronged forks to assess sensation, you remember that this exam is subjective and there is meaning in this remote evaluation. But all in all, I have been blessed with telehealth. Patients don’t mind it at all. They’re completely open to the idea. They like the telehealth for the contact they are able to have with their HCP.
Jodie Haselkorn: As you were saying that, Heidi, I thought, I’ve been inside my veterans’ bathrooms virtually and have seen all of their equipment that they have at home. In a face-to-face clinic visit, you don’t have an opportunity to see all their canes and walkers, braces, and other assistive technology. Some of it’s stashed in a closet, some of it under the bed. In a virtual visit, I get to understand why some is not used, what veterans prefer, and see their own innovations for mobility and self-care.
Mitchell Wallin: There’s a typical ritual that patients talk about when they go to a clinic. They check in, sit down, and wait for the nurse to give them their vital signs and set them up in the room. And then they meet with their HCP, and finally they complete the tasks on the checklist. And part of that may mean scheduling an MRI or going to the lab. But some of these handoffs don’t happen as well on telehealth. Maybe we haven’t integrated these segments of a clinical visit into telehealth platforms. But it could be developed, and there could be new neurologic tools to improve the interview and physical examination. Twenty years ago, you couldn’t deposit a check on your phone; but now you can do everything on your phone you could do in a physical bank. With some creativity, we can improve parts of the neurological exam that are currently difficult to assess remotely.
Jodie Haselkorn: I have not used peripherals in video telehealth to home and I would need to become accustomed to their use with current technology and train patients and caregivers. I would like telehealth peripherals such as a stethoscope to listen to the abdomen of a veteran with neurogenic bowel or a user-friendly ultrasound probe to measure postvoid residual urine in an individual with symptoms of neurogenic bladder, in addition to devices that measure walking speed and pulmonary function. I look forward to the development, use, and the incorporation peripherals that will enable a more extensive virtual exam within the home.
What are the MS Centers of Excellence working on now?
Jodie Haselkorn: We are working to understand the healthcare needs of veterans with MS by evaluating not only care for MS within the VA, but also the types and quantity of MS specialty care VA that is being received in the community during the pandemic. Dr. Wallin is also using the registry to lead a telehealth study to capture the variety of different codes that VA health professionals in MS have used to document workload by telehealth, and face-to-face, and telephone encounters.
Rebecca Spain: The MS Center of Excellence (MSCoE) is coming out with note templates to be available for HCPs, which we can refine as we get experience. This is s one way we can promote high standards in MS care by making these ancillary tools more productive.
Jodie Haselkorn: We are looking at different ways to achieve a high-quality virtual examination using standardized examination strategies and patient and caregiver information to prepare for a specialty MS visit.
Rebecca Spain: I would like to, in more of a research setting, study health outcomes using telehealth vs in person and start tracking that long term.
Mitchell Wallin: We can probably do more in terms of standardization, such as the routine patient reported surveys and implementing the new Consortium of Multiple Sclerosis Centers’ International MRI criteria. The COVID pandemic has affected everything in medical care. But we want to have a regular standardized outcome to assess, and if we can start to do some of the standard data collection through telemedicine, it becomes part of our regular clinic data.
Heidi Maloni: We need better technology. You can do electrocardiograms on your watch. Could we do Dinamaps? Could we figure out strength? That’s a wish list.
Jodie Haselkorn: Since the MSCoE is a national program, we were set up to do what we needed to do for education. We were able to continue on with all of our HCP webinars, including the series with the National MS Society (NMSS). We also have a Specialty Care Access Network-Extension for Community Healthcare Outcomes (SCAN-ECHO) series with the Northwest ECHO VA program and collaborated with the Can Do MS program on patient education as well. We’ve sent out 2 printed newsletters for veterans. The training of HCPs for the future has continued as well. All of our postdoctoral fellows who have finished their programs on time and moved on to either clinical practice or received career development grants to continue their VA careers, a new fellow has joined, and our other fellows are continuing as planned.
The loss that we sustained was in-person meetings. We held MSCoE Regional Program meetings in the East and West that combined education and administrative goals. Both of these were well attended and successful. There was a lot of virtual education available from multiple sources. It was challenging this year was to anticipate what education programming people wanted from MSCoE. Interestingly, a lot of our regional HCPs did not want much more COVID-19 education. They wanted other education and we were able to meet those needs.
Did the pandemic impact the VA MS registry?
Mitchell Wallin: Like any electronic product, the VA MS Surveillance Registry must be maintained, and we have tried to encourage people to use it. Our biggest concern was to identify cases of MS that got infected with COVID-19 and to put those people into the registry. In some cases, Veterans with MS were in locations without a MS clinic. So, we’ve spent a lot more time identifying those cases and adjudicating them to make sure their infection and MS were documented correctly.
During the COVID-19 pandemic, the VA healthcare system has been taxed like others and so HCPs have been a lot busier than normal, forcing new workflows. It has been a hard year that way because a lot of health care providers have been doing many other jobs to help maintain patient care during the COVID-19 pandemic.
Heidi Maloni: The impact of COVID-19 has been positive for the registry because we’ve had more opportunities to populate it.
Jodie Haselkorn: Dr. Wallin and the COVID-19 Registry group began building the combined registry at the onset of the pandemic. We have developed the capacity to identify COVID-19 infections in veterans who have MS and receive care in the VA. We entered these cases in the MS Surveillance Registry and have developed a linkage with the COVID-19 national VA registry. We are in the middle of the grunt work part case entry, but it is a rich resource.
How has the pandemic impacted MS research?
Rebecca Spain: COVID-19 has put a big damper on clinical research progress, including some of our MSCoE studies. It has been difficult to have subjects come in for clinical visits. It’s been difficult to get approval for new studies. It’s shifted timelines dramatically, and then that always increases budgets in a time when there’s not a lot of extra money. So, for clinical research, it’s been a real struggle and a strain and an ever-moving target. For laboratory research most, if not all, centers that have laboratory research at some point were closed and have only slowly reopened. Some still haven’t reopened to any kind of research or laboratory. So, it’s been tough, I think, on research in general.
Heidi Maloni: I would say the word is devastating. The pandemic essentially put a stop to in-person research studies. Our hospital was in research phase I, meaning human subjects can only participate in a research study if they are an inpatient or outpatient with an established clinic visit (clinics open to 25% occupancy) or involved in a study requiring safety monitoring, This plan limits risk of COVID-19 exposure.
Rebecca Spain: There is risk for a higher dropout rate of subjects from studies meaning there’s less chance of success for finding answers if enough people don’t stay in. At a certain point, you have to say, “Is this going to be a successful study?”
Jodie Haselkorn: Dr. Spain has done an amazing job leading a multisite, international clinical trial funded by the VA and the NMSS and kept it afloat, despite challenges. The pandemic has had impacts, but the study continues to move towards completion. I’ve appreciated the efforts of the Research Service at VA Puget Sound to ensure that we could safely obtain many of the 12-month outcomes for all the participants enrolled in that study.
Mitchell Wallin: The funding for some of our nonprofit partners, including the Paralyzed Veterans Association (PVA) and the NMSS, has suffered as well and so a lot of their funding programs have closed or been cut back during the pandemic. Despite that, we still have been able to use televideo technology for our clinical and educational programs with our network.
Jodie Haselkorn: MSCoE also does health services and epidemiological studies in addition to clinical trials and that work has continued. Quite a few of the studies that had human subjects in them were completed in terms of data collection, and so those are being analyzed. There will be a drop in funded studies, publications and posters as the pandemic continues and for a recovery period. We have a robust baseline for research productivity and a talented team. We’ll be able to track drop off and recovery over time.
Rebecca Spain: There’s going to be long-term consequences that we don’t see right now, especially for young researchers who have missed getting pilot data which would have led to additional small grants and then later large grants. There’s going to be an education gap that’s going on with all of the kids who are not able to go to school properly. It’s part of that whole swath of lost time and lost opportunity that we will have to deal with.
However, there are going to be some positive changes. We’re now busy designing clinical trials that can be done virtually to minimize any contact with the health facility, and then looking at things like shifting to research ideas that are more focused around health services.
Jodie Haselkorn: Given the current impacts of the pandemic on delivery of health care there is a strong interest in looking at how we can deliver health care in ways that accommodates the consumers and the providers perspectives. In the future we see marked impacts in our abilities to deliver care to Veterans with MS.
As a final thought, I wanted to put in a plug for this talented team. One of our pandemic resolutions was to innovatively find new possibilities and avoid negative focus on small changes. We are fortunate that all our staff have remained healthy and been supportive and compassionate with each other throughout this period. We have met our goals and are still moving forward.
MSCoE has benefited from the supportive leadership of Sharyl Martini, MD, PhD, and Glenn Graham, MD, PhD, in VA Specialty Care Neurology and leadership and space from VA Puget Sound, VA Portland Health Care System, the Washington DC VA Medical Center and VA Maryland Health Care System in Baltimore.
We also have a national advisory system that is actively involved, sets high standards and performs a rigorous annual review. We have rich inputs from the VA National Regional Programs and Veterans. Additionally, we have had the leadership and opportunities to collaborate with outside organizations including, the Consortium of MS Centers, the NMSS, and the PVA. We have been fortunate.
The following is a lightly edited transcript of a teleconference recorded in February 2021.
How has COVID impacted Veterans with multiple sclerosis?
Mitchell Wallin, MD, MPH: There has been a lot of concern in the multiple sclerosis (MS) patient community about getting infected with COVID-19 and what to do about it. Now that there are vaccines, the concern is whether and how to take a vaccine. At least here, in the Washington DC/Baltimore area where I practice, we have seen many veterans being hospitalized with COVID-19, some with multiple sclerosis (MS), and some who have died of COVID-19. So, there has been a lot of fear, especially in veterans that are older with comorbid diseases.
Rebecca Spain, MD, MSPH: There also has been an impact on our ability to provide care to our veterans with MS. There are challenges having them come into the office or providing virtual care. There are additional challenges and concerns this year about making changes in MS medications because we can’t see patients in person to or understand their needs or current status of their MS. So, providing care has been a challenge this year as well.
There has also been an impact on our day to day lives, like there has been for all of us, from the lockdown particularly not being able to exercise and socialize as much. There have been physical and social and emotional tolls that this disease has taken on veterans with MS.
Jodie Haselkorn, MD, MPH: The survivors of COVID-19, that are transferred to an inpatient multidisciplinary rehabilitation program unit to address impairments related to the cardiopulmonary, immobility, psychological impacts and other medical complications are highly motivated to work with the team to achieve a safe discharge. The US Department of Veterans Affairs (VA) Rehabilitation Services has much to offer them.
Heidi Maloni, PhD, NP: Veterans with MS are not at greater risk because they are diagnosed with MS. But, their comorbidities such as hypertension, obesity, or factors such as older age and increased disability can increase the risk of COVID-19 infection and poorer outcomes if infected. might place them at greater risk.
Veterans have asked “Am I at greater risk? Do I need to do something more to protect myself?” I have had innumerable veterans call and ask whether I can write them letters for their employer to ensure that they work at home longer rather than go into the workplace because they’re very nervous and don’t feel confident that masking and distancing is really going to be protective.
Mitchell Wallin: We are analyzing some of our data in the VA health care system related to COVID-19 infections in the MS population. We can’t say for sure what are numbers are, but our rates of infection and hospitalization are higher than the general population and we will soon have a report. We have a majority male population, which is different from the general MS population, which is predominantly female. The proportion of minority patients in VA mirrors those of the US population. These demographic factors along with a high level of comorbid disease put veterans at high risk for acquiring COVID-19. So, in some ways it’s hard to compare when you look at reports from other countries or the US National MS-COVID-19 Registry, which captures a population that is predominantly female. In the VA, our age range spans from the 20s to almost 100 years. We must understand our population to prevent COVID-19 and better care for the most vulnerable.
Rebecca Spain: Heidi, my understanding, although the numbers are small, that for the most part, Veterans with MS who are older are at higher risk of complications and death, which is also true of the general population. But that there is an additional risk for people with MS who have higher disability levels. My understanding from reading the literature, was that people with MS needing or requiring a cane to walk or greater assistance for mobility were at a higher risk for COVID-19 complications, including mortality. I have been particularly encouraged that in many places this special population of people with MS are getting vaccinated sooner.
Heidi Maloni: I completely agree, you said it very clearly, Becca. Their disability level puts them at risk
Rebecca Spain: Disability is a comorbidity.
Heidi Maloni: Yes. Just sitting in a wheelchair and not being able to get a full breath or having problems with respiratory effort really does put you at risk for doing well if you were to have COVID-19.
Are there other ancillary impacts from COVID-19 for patients with MS?
Jodie Haselkorn: Individuals who are hospitalized with COVID-19 miss social touch and social support from family and friends. They miss familiar conversations, a hug and having someone hold their hand. The acute phase of the infection limits professional face-to-face interaction with patients due to time and protective garments. There are reports of negative consequences with isolation and social reintegration of the COVID-19 survivors is necessary and a necessary part of rehabilitation.
Mitchell Wallin: For certain procedures (eg, magnetic resonance imaging [MRI]) or consultations, we need to bring people into the medical center. Many clinical encounters, however, can be done through telemedicine and both the VA and the US Department of Defense systems were set up to execute this type of visit. We had been doing telemedicine for a long time before the pandemic and we were in a better position than a lot of other health systems to shift to a virtual format with COVID-19. We had to ramp up a little bit and get our tools working a little more effectively for all clinics, but I think we were prepared to broadly execute telemedicine clinics for the pandemic.
Jodie Haselkorn: I agree that the he VA infrastructure was ahead of most other health system in terms of readiness for telehealth and maintaining access to care. Not all health care providers (HCPs) were using it, but the system was there, and included a telehealth coordinator in all of the facilities who could gear health care professionals up quickly. Additionally, a system was in place to provide veterans and caregivers with telehealth home equipment and provide training. Another thing that really helped was the MISSION Act. Veterans who have difficulty travelling for an appointment may have the ability to seek care outside of the VA within their own community. They may be able to go into a local facility to get laboratory or radiologic studies done or continue rehabilitation closer to home.
VA MS Registry Data
Rebecca Spain: Mitch, there are many interesting things we can learn about the interplay between COVID-19 and MS using registries such as how it affects people based on rural vs metropolitan living, whether people are living in single family homes or not as a proxy marker for social support, and so on.
Mitchell Wallin: We have both an MS registry to track and follow patients through our clinical network and a specific COVID-19 registry as well in VA. We have identified the MS cases infected with CoVID-19 and are putting them together.
Jodie Haselkorn: There are a number of efforts in mental health that are moving forward to examine depression and in anxiety during COVID-19. Individuals with MS have increased rates of depression and anxiety above that of the general population during usual times. The literature reports an increase in anxiety and depression in general population associated with the pandemic and veterans with MS seem to be reporting these symptoms more frequently as well. We will be able to track use the registry to assess the impacts of COVID-19 on depression and anxiety in Veterans with MS.
Providing MS Care During COVID-19
Jodie Haselkorn: The transition to telehealth in COVID-19 has been surprisingly seamless with some additional training for veterans and HCPs. I initially experienced an inefficiency in my clinic visit productivity. It took me longer to see a veteran because I wasn’t doing telehealth in our clinic with support staff and residents, my examination had to change, my documentation template needed to be restructured, and the coding was different. Sometimes I saw a veteran in clinic the and my next appointment required me to move back to my office in another building for a telehealth appointment. Teaching virtual trainees who also participated in the clinic encounters had its own challenges and rewards. My ‘motor routine’ was disrupted.
Rebecca Spain: There’s a real learning curve for telehealth in terms of how comfortable you feel with the data you get by telephone or video and how reliable that is. There are issues based on technology factors—like the patient’s bandwidth—because determining how smooth their motions are is challenging if you have a jerky, intermittent signal. I learned quickly to always do the physical examination first because I might lose video connection partway through and have to switch to a phone visit!
It’s still an open question, how much are we missing by using a video and not in-person visits. And what are the long-term health outcomes and implications of that? That is something that needs to be studied in neurology where we pride ourselves on the physical examination. When move to a virtual physical examination, is there cost? There are incredible gains using telehealth in terms of convenience and access to care, which may outweigh some of the drawbacks in particular cases.
There are also pandemic challenge in terms of clinic workflow. At VA Portland Health Care System in Oregon, I have 3 clinics for Friday morning: telephone, virtual, and face-to-face clinics. It’s a real struggle for the schedulers. And because of that transition to new system workflows to accommodate this, some patient visits have been dropped, lost, or scheduled incorrectly.
Heidi Maloni: As the nurse in this group, I agree with everything that Becca and Jodie have said about telehealth. But, I have found some benefits, and one of them is a greater intimacy with my patients. What do I mean by that? For instance, if a patient has taken me to their kitchen and opened their cupboard to show me the breakfast cereal, I’m also observing that there’s nothing else in that cupboard other than cereal. I’m also putting some things together about health and wellness. Or, for the first time, I might meet their significant other who can’t come to clinic because they’re working, but they are at home with the patient. And then having that 3-way conversation with the patient and the significant other, that’s kind of opened up my sense of who that person is.
You are right about the neurological examination. It’s challenging to make exacting assessments. When gathering household objects, ice bags and pronged forks to assess sensation, you remember that this exam is subjective and there is meaning in this remote evaluation. But all in all, I have been blessed with telehealth. Patients don’t mind it at all. They’re completely open to the idea. They like the telehealth for the contact they are able to have with their HCP.
Jodie Haselkorn: As you were saying that, Heidi, I thought, I’ve been inside my veterans’ bathrooms virtually and have seen all of their equipment that they have at home. In a face-to-face clinic visit, you don’t have an opportunity to see all their canes and walkers, braces, and other assistive technology. Some of it’s stashed in a closet, some of it under the bed. In a virtual visit, I get to understand why some is not used, what veterans prefer, and see their own innovations for mobility and self-care.
Mitchell Wallin: There’s a typical ritual that patients talk about when they go to a clinic. They check in, sit down, and wait for the nurse to give them their vital signs and set them up in the room. And then they meet with their HCP, and finally they complete the tasks on the checklist. And part of that may mean scheduling an MRI or going to the lab. But some of these handoffs don’t happen as well on telehealth. Maybe we haven’t integrated these segments of a clinical visit into telehealth platforms. But it could be developed, and there could be new neurologic tools to improve the interview and physical examination. Twenty years ago, you couldn’t deposit a check on your phone; but now you can do everything on your phone you could do in a physical bank. With some creativity, we can improve parts of the neurological exam that are currently difficult to assess remotely.
Jodie Haselkorn: I have not used peripherals in video telehealth to home and I would need to become accustomed to their use with current technology and train patients and caregivers. I would like telehealth peripherals such as a stethoscope to listen to the abdomen of a veteran with neurogenic bowel or a user-friendly ultrasound probe to measure postvoid residual urine in an individual with symptoms of neurogenic bladder, in addition to devices that measure walking speed and pulmonary function. I look forward to the development, use, and the incorporation peripherals that will enable a more extensive virtual exam within the home.
What are the MS Centers of Excellence working on now?
Jodie Haselkorn: We are working to understand the healthcare needs of veterans with MS by evaluating not only care for MS within the VA, but also the types and quantity of MS specialty care VA that is being received in the community during the pandemic. Dr. Wallin is also using the registry to lead a telehealth study to capture the variety of different codes that VA health professionals in MS have used to document workload by telehealth, and face-to-face, and telephone encounters.
Rebecca Spain: The MS Center of Excellence (MSCoE) is coming out with note templates to be available for HCPs, which we can refine as we get experience. This is s one way we can promote high standards in MS care by making these ancillary tools more productive.
Jodie Haselkorn: We are looking at different ways to achieve a high-quality virtual examination using standardized examination strategies and patient and caregiver information to prepare for a specialty MS visit.
Rebecca Spain: I would like to, in more of a research setting, study health outcomes using telehealth vs in person and start tracking that long term.
Mitchell Wallin: We can probably do more in terms of standardization, such as the routine patient reported surveys and implementing the new Consortium of Multiple Sclerosis Centers’ International MRI criteria. The COVID pandemic has affected everything in medical care. But we want to have a regular standardized outcome to assess, and if we can start to do some of the standard data collection through telemedicine, it becomes part of our regular clinic data.
Heidi Maloni: We need better technology. You can do electrocardiograms on your watch. Could we do Dinamaps? Could we figure out strength? That’s a wish list.
Jodie Haselkorn: Since the MSCoE is a national program, we were set up to do what we needed to do for education. We were able to continue on with all of our HCP webinars, including the series with the National MS Society (NMSS). We also have a Specialty Care Access Network-Extension for Community Healthcare Outcomes (SCAN-ECHO) series with the Northwest ECHO VA program and collaborated with the Can Do MS program on patient education as well. We’ve sent out 2 printed newsletters for veterans. The training of HCPs for the future has continued as well. All of our postdoctoral fellows who have finished their programs on time and moved on to either clinical practice or received career development grants to continue their VA careers, a new fellow has joined, and our other fellows are continuing as planned.
The loss that we sustained was in-person meetings. We held MSCoE Regional Program meetings in the East and West that combined education and administrative goals. Both of these were well attended and successful. There was a lot of virtual education available from multiple sources. It was challenging this year was to anticipate what education programming people wanted from MSCoE. Interestingly, a lot of our regional HCPs did not want much more COVID-19 education. They wanted other education and we were able to meet those needs.
Did the pandemic impact the VA MS registry?
Mitchell Wallin: Like any electronic product, the VA MS Surveillance Registry must be maintained, and we have tried to encourage people to use it. Our biggest concern was to identify cases of MS that got infected with COVID-19 and to put those people into the registry. In some cases, Veterans with MS were in locations without a MS clinic. So, we’ve spent a lot more time identifying those cases and adjudicating them to make sure their infection and MS were documented correctly.
During the COVID-19 pandemic, the VA healthcare system has been taxed like others and so HCPs have been a lot busier than normal, forcing new workflows. It has been a hard year that way because a lot of health care providers have been doing many other jobs to help maintain patient care during the COVID-19 pandemic.
Heidi Maloni: The impact of COVID-19 has been positive for the registry because we’ve had more opportunities to populate it.
Jodie Haselkorn: Dr. Wallin and the COVID-19 Registry group began building the combined registry at the onset of the pandemic. We have developed the capacity to identify COVID-19 infections in veterans who have MS and receive care in the VA. We entered these cases in the MS Surveillance Registry and have developed a linkage with the COVID-19 national VA registry. We are in the middle of the grunt work part case entry, but it is a rich resource.
How has the pandemic impacted MS research?
Rebecca Spain: COVID-19 has put a big damper on clinical research progress, including some of our MSCoE studies. It has been difficult to have subjects come in for clinical visits. It’s been difficult to get approval for new studies. It’s shifted timelines dramatically, and then that always increases budgets in a time when there’s not a lot of extra money. So, for clinical research, it’s been a real struggle and a strain and an ever-moving target. For laboratory research most, if not all, centers that have laboratory research at some point were closed and have only slowly reopened. Some still haven’t reopened to any kind of research or laboratory. So, it’s been tough, I think, on research in general.
Heidi Maloni: I would say the word is devastating. The pandemic essentially put a stop to in-person research studies. Our hospital was in research phase I, meaning human subjects can only participate in a research study if they are an inpatient or outpatient with an established clinic visit (clinics open to 25% occupancy) or involved in a study requiring safety monitoring, This plan limits risk of COVID-19 exposure.
Rebecca Spain: There is risk for a higher dropout rate of subjects from studies meaning there’s less chance of success for finding answers if enough people don’t stay in. At a certain point, you have to say, “Is this going to be a successful study?”
Jodie Haselkorn: Dr. Spain has done an amazing job leading a multisite, international clinical trial funded by the VA and the NMSS and kept it afloat, despite challenges. The pandemic has had impacts, but the study continues to move towards completion. I’ve appreciated the efforts of the Research Service at VA Puget Sound to ensure that we could safely obtain many of the 12-month outcomes for all the participants enrolled in that study.
Mitchell Wallin: The funding for some of our nonprofit partners, including the Paralyzed Veterans Association (PVA) and the NMSS, has suffered as well and so a lot of their funding programs have closed or been cut back during the pandemic. Despite that, we still have been able to use televideo technology for our clinical and educational programs with our network.
Jodie Haselkorn: MSCoE also does health services and epidemiological studies in addition to clinical trials and that work has continued. Quite a few of the studies that had human subjects in them were completed in terms of data collection, and so those are being analyzed. There will be a drop in funded studies, publications and posters as the pandemic continues and for a recovery period. We have a robust baseline for research productivity and a talented team. We’ll be able to track drop off and recovery over time.
Rebecca Spain: There’s going to be long-term consequences that we don’t see right now, especially for young researchers who have missed getting pilot data which would have led to additional small grants and then later large grants. There’s going to be an education gap that’s going on with all of the kids who are not able to go to school properly. It’s part of that whole swath of lost time and lost opportunity that we will have to deal with.
However, there are going to be some positive changes. We’re now busy designing clinical trials that can be done virtually to minimize any contact with the health facility, and then looking at things like shifting to research ideas that are more focused around health services.
Jodie Haselkorn: Given the current impacts of the pandemic on delivery of health care there is a strong interest in looking at how we can deliver health care in ways that accommodates the consumers and the providers perspectives. In the future we see marked impacts in our abilities to deliver care to Veterans with MS.
As a final thought, I wanted to put in a plug for this talented team. One of our pandemic resolutions was to innovatively find new possibilities and avoid negative focus on small changes. We are fortunate that all our staff have remained healthy and been supportive and compassionate with each other throughout this period. We have met our goals and are still moving forward.
MSCoE has benefited from the supportive leadership of Sharyl Martini, MD, PhD, and Glenn Graham, MD, PhD, in VA Specialty Care Neurology and leadership and space from VA Puget Sound, VA Portland Health Care System, the Washington DC VA Medical Center and VA Maryland Health Care System in Baltimore.
We also have a national advisory system that is actively involved, sets high standards and performs a rigorous annual review. We have rich inputs from the VA National Regional Programs and Veterans. Additionally, we have had the leadership and opportunities to collaborate with outside organizations including, the Consortium of MS Centers, the NMSS, and the PVA. We have been fortunate.
Screening High-Risk Women Veterans for Breast Cancer
The number of women seeking care from the Veterans Health Administration (VHA) is increasing.1 In 2015, there were 2 million women veterans in the United States, which is 9.4% of the total veteran population. This group is expected to increase at an average of about 18,000 women per year for the next 10 years.2 The percentage of women veterans who are US Department of Veterans Affairs (VA) users aged 45 to 64 years rose 46% from 2000 to 2015.1,3-4 It is estimated that 15% of veterans who used VA services in 2020 were women.1 Nineteen percent of women veterans are Black.1 The median age of women veterans in 2015 was 50 years.5 Breast cancer is the leading cancer affecting female veterans, and data suggest they have an increased risk of breast cancer based on unique service-related exposures.1,6-9
In the US, about 10 million women are eligible for breast cancer preventive therapy, including, but not limited to, medications, surgery, or lifestyle changes.10 Secondary prevention options include change in surveillance that can reduce their risk or identify cancer at an earlier stage when treatment is more effective. The United States Preventive Services Task Force, the National Comprehensive Cancer Network, the American Society for Clinical Oncology, the National Institute for Health and Care Excellence, and the Oncology Nursing Society recommend screening women aged ≥ 35 years to assess breast cancer risk.11-18 If a woman is at increased risk, she may be a candidate for chemoprevention, prozphylactic surgery, and possibly an enhanced screening regimen.
Urban and minority women are an understudied population. Most veterans (75%) live in urban or suburban settings.19,20 Urban veteran women constitute an important potential study population.
Chemoprevention measures have been underused because of factors involving both women and their health care providers. A large proportion of women are unaware of their higher risk status due to lack of adequate screening and risk assessment.21,22 In addition to patient lack of awareness of their high-risk status, primary care physicians are also reluctant to prescribe chemopreventive agents due to a lack of comfort or familiarity with the risks and benefits.23-26 The STAR2015, BCPT2005, IBIS2014, MAP3 2011, IBIS-I 2014, and IBIS II 2014 studies clearly demonstrate a 49 to 62% reduction in risk for women using chemoprevention such as selective estrogen receptor modulators or aromatase inhibitors, respectively.27-32 Yet only 4 to 9% of high-risk women not enrolled in a clinical trial are using chemoprevention.33-39
The possibility of developing breast cancer also may be increased because of a positive family history or being a member of a family in which there is a known susceptibility gene mutation.40 Based on these risk factors, women may be eligible for tailored follow-up and genetic counseling.41-44
Nationally, 7 to 10% of the civilian US population will experience posttraumatic stress disorder (PTSD).45 The rates are remarkably higher for women veterans, with roughly 20% diagnosed with PTSD.46,47 Anxiety and PTSD have been implicated in poor adherence to medical advice.48,49
In 2014, a national VA multidisciplinary group focused on breast cancer prevention, detection, treatment, and research to address breast health in the growing population of women veterans. High-risk breast cancer screenings are not routinely carried out by the VA in primary care, women’s health, or oncology services. Furthermore, the recording of screening questionnaire results was not synchronized until a standard questionnaire was created and approved as a template by this group in the VA electronic medical record (EMR) in 2015.
Several prediction models can identify which women are at an increased risk of developing breast cancer. The most commonly used risk assessment model, the Gail breast cancer risk assessment tool (BCRAT), has been refined to include women of additional ethnicities (https://www.cancer.gov/bcrisktool).
This pilot project was launched to identify an effective manner to screen women veterans regarding their risk of developing breast cancer and refer them for chemoprevention education or genetic counseling as appropriate.
Methods
A high-risk breast cancer screening questionnaire based on the Gail BCRAT and including lifestyle questions was developed and included as a note template in the VA EMR. The James J. Peters VA Medical Center, Bronx, NY (JJPVAMC) and the Washington DC VA Medical Center (DCVAMC) ran a pilot study between 2015 and 2018 using this breast cancer screening questionnaire to collect data from women veterans. Quality Executive Committee and institutional review board approvals were granted respectively.
Eligibility criteria included women aged ≥ 35 years with no personal history of breast cancer. Most patients were self-referred, but participants also were recruited during VA Breast Cancer Awareness month events, health fairs, or at informational tables in the hospital lobbies. After completing the 20 multiple choice questionnaire with a study team member, either in person or over the phone, a 5-year and lifetime risk of invasive breast cancer was calculated using the Gail BCRAT. A woman is considered high risk and eligible for chemoprevention if her 5-year risk is > 1.66% or her lifetime risk is ≥ 20%. Eligibility for genetic counseling is based on the Breast Cancer Referral Screening Tool, which includes a personal or family history of breast or ovarian cancer and Jewish ancestry.
All patients were notified of their average or high risk status by a clinician. Those who were deemed to be average risk received a follow-up letter in the mail with instructions (eg, to follow-up with a yearly mammogram). Those who were deemed to be high risk for developing breast cancer were asked to come in for an appointment with the study principal investigator (a VA oncologist/breast cancer specialist) to discuss prevention options, further screening, or referrals to genetic counseling. Depending on a patient’s other health factors, a woman at high risk for developing breast cancer also may be a candidate for chemoprevention with tamoxifen, raloxifene, exemestane, anastrozole, or letrozole.
Data on the participant’s lifestyle, including exercise, diet, and smoking, were evaluated to determine whether these factors had an impact on risk status.
Results
The JJP and DC VAMCs screened 103 women veterans between 2015 and 2018. Four patients were excluded for nonveteran (spousal) status, leaving 99 women veterans with a mean age of 54 years. The most common self-reported races were Black (60%), non-Hispanic White (14%), and Hispanic or Latino (13%) (Table 1).
Women veterans in our study were nearly 3-times more likely than the general population were to receive a high-risk Gail Score/BCRAT (35% vs 13%, respectively).50,51 Of this subset, 46% had breast biopsies, and 86% had a positive family history. Thirty-one percent of Black women in our study were high risk, while nationally, 8.2 to 13.3% of Black women aged 50 to 59 years are considered high risk.50,51 Of the Black high-risk group with a high Gail/BCRAT score, 94% had a positive family history, and 33% had a history of breast biopsy (Table 2).
Of the 35 high-risk patients 26 (74%) patients accepted consultations for chemoprevention and 5 (19%) started chemoprevention. Of this high-risk group, 13 (37%) patients were referred for genetic counseling (Table 3).44 The prevalence of PTSD was present in 31% of high-risk women and 29% of the cohort (Figure).The lifestyle questions indicated that, among all participants, 79% had an overweight or obese body mass index; 58% exercised weekly; 51% consumed alcohol; 14% were smokers; and 21% consumed 3 to 4 servings of fruits/vegetables daily.
Discussion
Breast cancer is the most common cancer in women.52 The number of women with breast cancer in the VHA has more than tripled from 1995 to 2012.1 The lifetime risk of developing breast cancer in the general population is about 13%.50 This rate can be affected by risk factors including age, hormone exposure, family history, radiation exposure, and lifestyle factors, such as weight and alcohol use.6,52-56 In the United States, invasive breast cancer affects 1 in 8 women.50,52,57
Our screened population showed nearly 3 times as many women veterans were at an increased risk for breast cancer when compared with historical averages in US women. This difference may be based on a high rate of prior breast biopsies or positive family history, although a provocative study using the Surveillance, Epidemiology, and End Results database showed military women to have higher rates of breast cancer as well.9 Historically, Blacks are vastly understudied in clinical research with only 5% representation on a national level.5,58 The urban locations of both pilot sites (Washington, DC and Bronx, NY) allowed for the inclusion of minority patients in our study. We found that the rates of breast cancer in Black women veterans to be higher than seen nationally, possibly prompting further screening initiatives for this understudied population.
Our pilot study’s chemoprevention utilization (19%) was double the < 10% seen in the national population.33-35 The presence of a knowledgeable breast health practitioner to recruit study participants and offer personalized counseling to women veterans is a likely factor in overcoming barriers to chemopreventive acceptance. These participants may have been motivated to seek care for their high-risk status given a strong family history and prior breast biopsies.
Interestingly, a 3-fold higher PTSD rate was seen in this pilot population (29%) when compared with PTSD rates in the general female population (7-10%) and still one-third higher than the general population of women veterans (20%).45-47 Mental health, anxiety, and PTSD have been barriers to patients who sought treatment and have been implicated in poor adherence to medical advice.48,49 Cancer screening can induce anxiety in patients, and it may be amplified in patients with PTSD. It was remarkable that although adherence with screening recommendations is decreased when PTSD is present, our patient population demonstrated a higher rate of screening adherence.
Women who are seen at the VA often use multiple clinical specialties, and their EMR can be accessed across VA medical centers nationwide. Therefore, identifying women veterans who meet screening criteria is easily attainable within the VA.
When comparing high-risk with average risk women, the lifestyle results (BMI, smoking history, exercise and consumption of fruits, vegetables and alcohol) were essentially the same. Lifestyle factors were similar to national population rates and were unlikely to impact risk levels.
Limitations
Study limitations included a high number of self-referrals and the large percentage of patients with a family history of breast cancer, making them more likely to seek screening. The higher-than-average risk of breast cancer may be driven by a high rate of breast biopsies and a strong family history. Lifestyle metrics could not be accurately compared to other national assessments of lifestyle factors due to the difference in data points that we used or the format of our questions.
Conclusions
As the number of women veterans increases and the incidence of breast cancer in women veterans rise, chemoprevention options should follow national guidelines. To our knowledge, this is the only oncology study with 60% Black women veterans. This study had a higher participation rate for Black women veterans than is typically seen in national research studies and shows the VA to be a germane source for further understanding of an understudied population that may benefit from increased screening for breast cancer.
A team-based, multidisciplinary model that meets the unique healthcare needs of women veterans results in a patient-centric delivery of care for assessing breast cancer risk status and prevention options. This model can be replicated nationally by directing primary care physicians and women’s health practitioners to a risk-assessment questionnaire and referring high-risk women for appropriate preventative care. Given that these results show chemoprevention adherence rates doubled those seen nationally, perhaps techniques used within this VA pilot study may be adapted to decrease breast cancer incidence nationally.
Since the rate of PTSD among women veterans is triple the national average, we would expect adherence rates to be lower in our patient cohort. However, the multidisciplinary approach we used in this study (eg, 1:1 consultation with oncologist; genetic counseling referrals; mental health support available), may have improved adherence rates. Perhaps the high rates of PTSD seen in the VA patient population can be a useful way to explore patient adherence rates in those with mental illness and medical conditions.
Future research with a larger cohort may lead to greater insight into the correlation between PTSD and adherence to treatment. Exploring the connection between breast cancer, epigenetics, and specific military service-related exposures could be an area of analysis among this veteran population exhibiting increased breast cancer rates. VAMCs are situated in rural, suburban, and urban locations across the United States and offers a diverse socioeconomic and ethnic patient population for inclusion in clinical investigations. Women veterans make up a small subpopulation of women in the United States, but it is worth considering VA patients as an untapped resource for research collaboration.
Acknowledgements
The authors thank Steven Sanchez and Marissa Vallette, PhD, Breast Health Research Group. This research project was approved by the James J. Peters VA Medical Center Quality Executive Committee and the Washington, DC VA Medical Center Institutional Review Board. This work was supported by the US Department of Veterans Affairs. This work did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the US Government, or any of its agencies.
1. US Department of Veterans Affairs. National Center for Veterans Analysis and Statistics. The past, present and future of women veterans. Published February 2017. Accessed April 28, 2021. https://www.va.gov/vetdata/docs/specialreports/women_veterans_2015_final.pdf.
2. Frayne SM, Carney DV, Bastian L, et al. The VA Women’s Health Practice-Based Research Network: amplifying women veterans’ voices in VA research. J Gen Intern Med. 2013;28 Suppl 2(Suppl 2):S504-S509. doi:10.1007/s11606-013-2476-3
3. US Department of Veterans Affairs, Veterans Health Administration, Women’s Health Evaluation Initiative, Women Veterans Health Strategic Health Care Group. Sourcebook: women veterans in the Veterans Health Administration. Volume 1: Sociodemographic characteristics and use of VHA care. Published December 2010. Accessed April 12, 2021. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=2455
4. Bean-Mayberry B, Yano EM, Bayliss N, Navratil J, Weisman CS, Scholle SH. Federally funded comprehensive women’s health centers: leading innovation in women’s healthcare delivery. J Womens Health (Larchmt). 2007;16(9):1281-1290. doi:10.1089/jwh.2006.0284
5. US Department of Veterans Affairs. National Center for Veterans Analysis and Statistics.VA utilization profile FY 2016. Published November 2017. Accessed April 12, 2021. https://www.va.gov/vetdata/docs/QuickFacts/VA_Utilization_Profile.PDF
6. Ekenga CC, Parks CG, Sandler DP. Chemical exposures in the workplace and breast cancer risk: a prospective cohort study. Int J Cancer. 2015;137(7):1765-1774. doi:10.1002/ijc.29545
7. Rennix CP, Quinn MM, Amoroso PJ, Eisen EA, Wegman DH. Risk of breast cancer among enlisted Army women occupationally exposed to volatile organic compounds. Am J Ind Med. 2005;48(3):157-167. doi:10.1002/ajim.20201
8. Ritz B. Cancer mortality among workers exposed to chemicals during uranium processing. J Occup Environ Med. 1999;41(7):556-566. doi:10.1097/00043764-199907000-00004
9. Zhu K, Devesa SS, Wu H, et al. Cancer incidence in the U.S. military population: comparison with rates from the SEER program. Cancer Epidemiol Biomarkers Prev. 2009;18(6):1740-1745. doi:10.1158/1055-9965.EPI-09-0041
10. Freedman AN, Yu B, Gail MH, et al. Benefit/risk assessment for breast cancer chemoprevention with raloxifene or tamoxifen for women age 50 years or older [published correction appears in J Clin Oncol. 2013 Nov 10;31(32):4167]. J Clin Oncol. 2011;29(17):2327-2333. doi:10.1200/JCO.2010.33.0258
11. Greene, H. Cancer prevention, screening and early detection. In: Gobel BH, Triest-Robertson S, Vogel WH, eds. Advanced Oncology Nursing Certification Review and Resource Manual. 3rd ed. Oncology Nursing Society; 2016:1-34. https://www.ons.org/sites/default/files/publication_pdfs/2%20ADVPrac%20chapter%201.pdf
12. National Comprehensive Cancer Network. NCCN Breast Cancer Risk Reduction. Version 1.2021 NCCN Clinical Practice Guidelines in Oncology. Updated March 24, 2021 Accessed April 12, 2021. https://www.nccn.org/professionals/physician_gls/pdf/breast_risk.pdf
13. US Preventive Services Task Force. Breast cancer: Medications use to reduce risk. Updated September 3, 2019. Accessed April 12, 2021. https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/breast-cancer-medications-for-risk-reduction
14. Moyer VA; U.S. Preventive Services Task Force. Medications to decrease the risk for breast cancer in women: recommendations from the U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159(10):698-708. doi:10.7326/0003-4819-159-10-201311190-00717
15. Boucher JE. Chemoprevention: an overview of pharmacologic agents and nursing considerations. Clin J Oncol Nurs. 2018;22(3):350-353. doi:10.1188/18.CJON.350-353
16. Nichols HB, Stürmer T, Lee VS, et al. Breast cancer chemoprevention in an integrated health care setting. JCO Clin Cancer Inform. 2017;1:1-12. doi:10.1200/CCI.16.00059
17. Bevers TB, Helvie M, Bonaccio E, et al. Breast cancer screening and diagnosis, Version 3.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16(11):1362-1389. doi:10.6004/jnccn.2018.0083
18. Visvanathan K, Hurley P, Bantug E, et al. Use of pharmacologic interventions for breast cancer risk reduction: American Society of Clinical Oncology clinical practice guideline [published correction appears in J Clin Oncol. 2013 Dec 1;31(34):4383]. J Clin Oncol. 2013;31(23):2942-2962. doi:10.1200/JCO.2013.49.3122
19. Sealy-Jefferson S, Roseland ME, Cote ML, et al. rural-urban residence and stage at breast cancer diagnosis among postmenopausal women: The Women’s Health Initiative. J Womens Health (Larchmt). 2019;28(2):276-283. doi:10.1089/jwh.2017.6884
20. Holder KA. Veterans in rural America: 2011-2015. Published January 25, 2017. Accessed April 12, 2021. https://www.census.gov/library/publications/2017/acs/acs-36.html
21. Owens WL, Gallagher TJ, Kincheloe MJ, Ruetten VL. Implementation in a large health system of a program to identify women at high risk for breast cancer. J Oncol Pract. 2011;7(2):85-88. doi:10.1200/JOP.2010.000107
2. Pivot X, Viguier J, Touboul C, et al. Breast cancer screening controversy: too much or not enough?. Eur J Cancer Prev. 2015;24 Suppl:S73-S76. doi:10.1097/CEJ.0000000000000145
23. Bidassie B, Kovach A, Vallette MA, et al. Breast Cancer risk assessment and chemoprevention use among veterans affairs primary care providers: a national online survey. Mil Med. 2020;185(3-4):512-518. doi:10.1093/milmed/usz291
24. Brewster AM, Davidson NE, McCaskill-Stevens W. Chemoprevention for breast cancer: overcoming barriers to treatment. Am Soc Clin Oncol Educ Book. 2012;85-90. doi:10.14694/EdBook_AM.2012.32.152
25. Meyskens FL Jr, Curt GA, Brenner DE, et al. Regulatory approval of cancer risk-reducing (chemopreventive) drugs: moving what we have learned into the clinic. Cancer Prev Res (Phila). 2011;4(3):311-323. doi:10.1158/1940-6207.CAPR-09-0014
26. Tice JA, Kerlikowske K. Screening and prevention of breast cancer in primary care. Prim Care. 2009;36(3):533-558. doi:10.1016/j.pop.2009.04.003
27. Vogel VG. Selective estrogen receptor modulators and aromatase inhibitors for breast cancer chemoprevention. Curr Drug Targets. 2011;12(13):1874-1887. doi:10.2174/138945011798184164
28. Vogel VG, Costantino JP, Wickerham DL, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial [published correction appears in JAMA. 2006 Dec 27;296(24):2926] [published correction appears in JAMA. 2007 Sep 5;298(9):973]. JAMA. 2006;295(23):2727-2741. doi:10.1001/jama.295.23.joc60074
29. Pruthi S, Heisey RE, Bevers TB. Chemoprevention for breast cancer. Ann Surg Oncol. 2015;22(10):3230-3235. doi:10.1245/s10434-015-4715-9
30. Cuzick J, Sestak I, Forbes JF, et al. Anastrozole for prevention of breast cancer in high-risk postmenopausal women (IBIS-II): an international, double-blind, randomised placebo-controlled trial [published correction appears in Lancet. 2014 Mar 22;383(9922):1040] [published correction appears in Lancet. 2017 Mar 11;389(10073):1010]. Lancet. 2014;383(9922):1041-1048. doi:10.1016/S0140-6736(13)62292-8
31. Bozovic-Spasojevic I, Azambuja E, McCaskill-Stevens W, Dinh P, Cardoso F. Chemoprevention for breast cancer. Cancer Treat Rev. 2012;38(5):329-339. doi:10.1016/j.ctrv.2011.07.005
32. Gabriel EM, Jatoi I. Breast cancer chemoprevention. Expert Rev Anticancer Ther. 2012;12(2):223-228. doi:10.1586/era.11.206

33. Crew KD, Albain KS, Hershman DL, Unger JM, Lo SS. How do we increase uptake of tamoxifen and other anti-estrogens for breast cancer prevention?. NPJ Breast Cancer. 2017;3:20. Published 2017 May 19. doi:10.1038/s41523-017-0021-y
34. Ropka ME, Keim J, Philbrick JT. Patient decisions about breast cancer chemoprevention: a systematic review and meta-analysis. J Clin Oncol. 2010;28(18):3090-3095. doi:10.1200/JCO.2009.27.8077
35. Smith SG, Sestak I, Forster A, et al. Factors affecting uptake and adherence to breast cancer chemoprevention: a systematic review and meta-analysis. Ann Oncol. 2016;27(4):575-590. doi:10.1093/annonc/mdv590
36. Grann VR, Patel PR, Jacobson JS, et al. Comparative effectiveness of screening and prevention strategies among BRCA1/2-affected mutation carriers. Breast Cancer Res Treat. 2011 Feb;125(3):837-847. doi:10.1007/s10549-010-1043-4
37. Goss PE, Ingle JN, Alés-Martínez JE, et al. Exemestane for breast-cancer prevention in postmenopausal women [published correction appears in N Engl J Med. 2011 Oct 6;365(14):1361]. N Engl J Med. 2011;364(25):2381-2391. doi:10.1056/NEJMoa1103507
38. Kmietowicz Z. Five in six women reject drugs that could reduce their risk of breast cancer. BMJ. 2015;351:h6650. Published 2015 Dec 8. doi:10.1136/bmj.h6650
39. Nelson HD, Fu R, Griffin JC, Nygren P, Smith ME, Humphrey L. Systematic review: comparative effectiveness of medications to reduce risk for primary breast cancer. Ann Intern Med. 2009;151(10):703-235. doi:10.7326/0003-4819-151-10-200911170-00147
40. Dahabreh IJ, Wieland LS, Adam GP, Halladay C, Lau J, Trikalinos TA. Core needle and open surgery biopsy for diagnosis of breast lesions: an update to the 2009 report. Published September 2014. Accessed April 12, 2021. https://www.ncbi.nlm.nih.gov/books/NBK246878
41. National Cancer Institute. Genetics of breast and ovarian cancer (PDQ)—health profession version. Updated February 12, 2021. Accessed April 12, 2021. http://www.cancer.gov/cancertopics/pdq/genetics/breast-and-ovarian/HealthProfessional
42. US Department of Health and Human Services. National Institutes of Health, National Institute of Environmental Health Sciences The sister study. Accessed April 12, 2021. https://sisterstudy.niehs.nih.gov/english/NIEHS.htm
43. Tutt A, Ashworth A. Can genetic testing guide treatment in breast cancer?. Eur J Cancer. 2008;44(18):2774-2780. doi:10.1016/j.ejca.2008.10.009
44. Katz SJ, Ward KC, Hamilton AS, et al. Gaps in receipt of clinically indicated genetic counseling after diagnosis of breast cancer. J Clin Oncol. 2018;36(12):1218-1224. doi:10.1200/JCO.2017.76.2369
45. US Department of Veterans Affairs. PTSD: National Center for PTSD. How common is PTSD in adults? Updated October 17, 2019. Accessed April 12, 2021. https://www.ptsd.va.gov/understand/common/common_adults.asp
46. US Department of Veterans Affairs. PTSD: National Center for PTSD. How common is PTSD in women? Updated October 16, 2019. Accessed April 12, 2021. https://www.ptsd.va.gov/understand/common/common_women.asp
47. US Department of Veterans Affairs. PTSD: National Center for PTSD. How common is PTSD in veterans? Updated September 24, 2018. Accessed April 12, 2021. https://www.ptsd.va.gov/understand/common/common_veterans.asp
48. Lindberg NM, Wellisch D. Anxiety and compliance among women at high risk for breast cancer. Ann Behav Med. 2001;23(4):298-303. doi:10.1207/S15324796ABM2304_9
49. DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160(14):2101-2107. doi:10.1001/archinte.160.14.2101
50. Centers for Disease Control and Prevention. MMWR appendix: breast cancer rates among black women and white women. Updated October 13, 2016. Accessed April 12, 2021. https://www.cdc.gov/cancer/breast/statistics/trends_invasive.htm
51. Richardson LC, Henley SJ, Miller JW, Massetti G, Thomas CC. Patterns and trends in age-specific black-white differences in breast cancer incidence and mortality - United States, 1999-2014. MMWR Morb Mortal Wkly Rep. 2016;65(40):1093-1098. Published 2016 Oct 14. doi:10.15585/mmwr.mm6540a1
52. Brody JG, Moysich KB, Humblet O, Attfield KR, Beehler GP, Rudel RA. Environmental pollutants and breast cancer: epidemiologic studies. Cancer. 2007;109(12 Suppl):2667-2711. doi:10.1002/cncr.22655
53. Brophy JT, Keith MM, Watterson A, et al. Breast cancer risk in relation to occupations with exposure to carcinogens and endocrine disruptors: a Canadian case-control study. Environ Health. 2012;11:87. Published 2012 Nov 19. doi:10.1186/1476-069X-11-87
54. Labrèche F, Goldberg MS, Valois MF, Nadon L. Postmenopausal breast cancer and occupational exposures. Occup Environ Med. 2010;67(4):263-269. doi:10.1136/oem.2009.049817
55. National Institute of Environmental Health Sciences, Interagency Breast Cancer & Environmental Research Coordinating Committee. Breast cancer and the environment: prioritizing prevention. Updated March 8, 2013. Accessed April 12, 2021. https://www.niehs.nih.gov/about/boards/ibcercc/index.cfm
56. Gail MH, Costantino JP, Pee D, et al. Projecting individualized absolute invasive breast cancer risk in African American women [published correction appears in J Natl Cancer Inst. 2008 Aug 6;100(15):1118] [published correction appears in J Natl Cancer Inst. 2008 Mar 5;100(5):373]. J Natl Cancer Inst. 2007;99(23):1782-1792. doi:10.1093/jnci/djm223
57. Corbie-Smith G, Thomas SB, Williams MV, Moody-Ayers S. Attitudes and beliefs of African Americans toward participation in medical research. J Gen Intern Med. 1999;14(9):537-546. doi:10.1046/j.1525-1497.1999.07048.x
58. Braunstein JB, Sherber NS, Schulman SP, Ding EL, Powe NR. Race, medical researcher distrust, perceived harm, and willingness to participate in cardiovascular prevention trials. Medicine (Baltimore). 2008;87(1):1-9. doi:10.1097/MD.0b013e3181625d78
The number of women seeking care from the Veterans Health Administration (VHA) is increasing.1 In 2015, there were 2 million women veterans in the United States, which is 9.4% of the total veteran population. This group is expected to increase at an average of about 18,000 women per year for the next 10 years.2 The percentage of women veterans who are US Department of Veterans Affairs (VA) users aged 45 to 64 years rose 46% from 2000 to 2015.1,3-4 It is estimated that 15% of veterans who used VA services in 2020 were women.1 Nineteen percent of women veterans are Black.1 The median age of women veterans in 2015 was 50 years.5 Breast cancer is the leading cancer affecting female veterans, and data suggest they have an increased risk of breast cancer based on unique service-related exposures.1,6-9
In the US, about 10 million women are eligible for breast cancer preventive therapy, including, but not limited to, medications, surgery, or lifestyle changes.10 Secondary prevention options include change in surveillance that can reduce their risk or identify cancer at an earlier stage when treatment is more effective. The United States Preventive Services Task Force, the National Comprehensive Cancer Network, the American Society for Clinical Oncology, the National Institute for Health and Care Excellence, and the Oncology Nursing Society recommend screening women aged ≥ 35 years to assess breast cancer risk.11-18 If a woman is at increased risk, she may be a candidate for chemoprevention, prozphylactic surgery, and possibly an enhanced screening regimen.
Urban and minority women are an understudied population. Most veterans (75%) live in urban or suburban settings.19,20 Urban veteran women constitute an important potential study population.
Chemoprevention measures have been underused because of factors involving both women and their health care providers. A large proportion of women are unaware of their higher risk status due to lack of adequate screening and risk assessment.21,22 In addition to patient lack of awareness of their high-risk status, primary care physicians are also reluctant to prescribe chemopreventive agents due to a lack of comfort or familiarity with the risks and benefits.23-26 The STAR2015, BCPT2005, IBIS2014, MAP3 2011, IBIS-I 2014, and IBIS II 2014 studies clearly demonstrate a 49 to 62% reduction in risk for women using chemoprevention such as selective estrogen receptor modulators or aromatase inhibitors, respectively.27-32 Yet only 4 to 9% of high-risk women not enrolled in a clinical trial are using chemoprevention.33-39
The possibility of developing breast cancer also may be increased because of a positive family history or being a member of a family in which there is a known susceptibility gene mutation.40 Based on these risk factors, women may be eligible for tailored follow-up and genetic counseling.41-44
Nationally, 7 to 10% of the civilian US population will experience posttraumatic stress disorder (PTSD).45 The rates are remarkably higher for women veterans, with roughly 20% diagnosed with PTSD.46,47 Anxiety and PTSD have been implicated in poor adherence to medical advice.48,49
In 2014, a national VA multidisciplinary group focused on breast cancer prevention, detection, treatment, and research to address breast health in the growing population of women veterans. High-risk breast cancer screenings are not routinely carried out by the VA in primary care, women’s health, or oncology services. Furthermore, the recording of screening questionnaire results was not synchronized until a standard questionnaire was created and approved as a template by this group in the VA electronic medical record (EMR) in 2015.
Several prediction models can identify which women are at an increased risk of developing breast cancer. The most commonly used risk assessment model, the Gail breast cancer risk assessment tool (BCRAT), has been refined to include women of additional ethnicities (https://www.cancer.gov/bcrisktool).
This pilot project was launched to identify an effective manner to screen women veterans regarding their risk of developing breast cancer and refer them for chemoprevention education or genetic counseling as appropriate.
Methods
A high-risk breast cancer screening questionnaire based on the Gail BCRAT and including lifestyle questions was developed and included as a note template in the VA EMR. The James J. Peters VA Medical Center, Bronx, NY (JJPVAMC) and the Washington DC VA Medical Center (DCVAMC) ran a pilot study between 2015 and 2018 using this breast cancer screening questionnaire to collect data from women veterans. Quality Executive Committee and institutional review board approvals were granted respectively.
Eligibility criteria included women aged ≥ 35 years with no personal history of breast cancer. Most patients were self-referred, but participants also were recruited during VA Breast Cancer Awareness month events, health fairs, or at informational tables in the hospital lobbies. After completing the 20 multiple choice questionnaire with a study team member, either in person or over the phone, a 5-year and lifetime risk of invasive breast cancer was calculated using the Gail BCRAT. A woman is considered high risk and eligible for chemoprevention if her 5-year risk is > 1.66% or her lifetime risk is ≥ 20%. Eligibility for genetic counseling is based on the Breast Cancer Referral Screening Tool, which includes a personal or family history of breast or ovarian cancer and Jewish ancestry.
All patients were notified of their average or high risk status by a clinician. Those who were deemed to be average risk received a follow-up letter in the mail with instructions (eg, to follow-up with a yearly mammogram). Those who were deemed to be high risk for developing breast cancer were asked to come in for an appointment with the study principal investigator (a VA oncologist/breast cancer specialist) to discuss prevention options, further screening, or referrals to genetic counseling. Depending on a patient’s other health factors, a woman at high risk for developing breast cancer also may be a candidate for chemoprevention with tamoxifen, raloxifene, exemestane, anastrozole, or letrozole.
Data on the participant’s lifestyle, including exercise, diet, and smoking, were evaluated to determine whether these factors had an impact on risk status.
Results
The JJP and DC VAMCs screened 103 women veterans between 2015 and 2018. Four patients were excluded for nonveteran (spousal) status, leaving 99 women veterans with a mean age of 54 years. The most common self-reported races were Black (60%), non-Hispanic White (14%), and Hispanic or Latino (13%) (Table 1).
Women veterans in our study were nearly 3-times more likely than the general population were to receive a high-risk Gail Score/BCRAT (35% vs 13%, respectively).50,51 Of this subset, 46% had breast biopsies, and 86% had a positive family history. Thirty-one percent of Black women in our study were high risk, while nationally, 8.2 to 13.3% of Black women aged 50 to 59 years are considered high risk.50,51 Of the Black high-risk group with a high Gail/BCRAT score, 94% had a positive family history, and 33% had a history of breast biopsy (Table 2).
Of the 35 high-risk patients 26 (74%) patients accepted consultations for chemoprevention and 5 (19%) started chemoprevention. Of this high-risk group, 13 (37%) patients were referred for genetic counseling (Table 3).44 The prevalence of PTSD was present in 31% of high-risk women and 29% of the cohort (Figure).The lifestyle questions indicated that, among all participants, 79% had an overweight or obese body mass index; 58% exercised weekly; 51% consumed alcohol; 14% were smokers; and 21% consumed 3 to 4 servings of fruits/vegetables daily.
Discussion
Breast cancer is the most common cancer in women.52 The number of women with breast cancer in the VHA has more than tripled from 1995 to 2012.1 The lifetime risk of developing breast cancer in the general population is about 13%.50 This rate can be affected by risk factors including age, hormone exposure, family history, radiation exposure, and lifestyle factors, such as weight and alcohol use.6,52-56 In the United States, invasive breast cancer affects 1 in 8 women.50,52,57
Our screened population showed nearly 3 times as many women veterans were at an increased risk for breast cancer when compared with historical averages in US women. This difference may be based on a high rate of prior breast biopsies or positive family history, although a provocative study using the Surveillance, Epidemiology, and End Results database showed military women to have higher rates of breast cancer as well.9 Historically, Blacks are vastly understudied in clinical research with only 5% representation on a national level.5,58 The urban locations of both pilot sites (Washington, DC and Bronx, NY) allowed for the inclusion of minority patients in our study. We found that the rates of breast cancer in Black women veterans to be higher than seen nationally, possibly prompting further screening initiatives for this understudied population.
Our pilot study’s chemoprevention utilization (19%) was double the < 10% seen in the national population.33-35 The presence of a knowledgeable breast health practitioner to recruit study participants and offer personalized counseling to women veterans is a likely factor in overcoming barriers to chemopreventive acceptance. These participants may have been motivated to seek care for their high-risk status given a strong family history and prior breast biopsies.
Interestingly, a 3-fold higher PTSD rate was seen in this pilot population (29%) when compared with PTSD rates in the general female population (7-10%) and still one-third higher than the general population of women veterans (20%).45-47 Mental health, anxiety, and PTSD have been barriers to patients who sought treatment and have been implicated in poor adherence to medical advice.48,49 Cancer screening can induce anxiety in patients, and it may be amplified in patients with PTSD. It was remarkable that although adherence with screening recommendations is decreased when PTSD is present, our patient population demonstrated a higher rate of screening adherence.
Women who are seen at the VA often use multiple clinical specialties, and their EMR can be accessed across VA medical centers nationwide. Therefore, identifying women veterans who meet screening criteria is easily attainable within the VA.
When comparing high-risk with average risk women, the lifestyle results (BMI, smoking history, exercise and consumption of fruits, vegetables and alcohol) were essentially the same. Lifestyle factors were similar to national population rates and were unlikely to impact risk levels.
Limitations
Study limitations included a high number of self-referrals and the large percentage of patients with a family history of breast cancer, making them more likely to seek screening. The higher-than-average risk of breast cancer may be driven by a high rate of breast biopsies and a strong family history. Lifestyle metrics could not be accurately compared to other national assessments of lifestyle factors due to the difference in data points that we used or the format of our questions.
Conclusions
As the number of women veterans increases and the incidence of breast cancer in women veterans rise, chemoprevention options should follow national guidelines. To our knowledge, this is the only oncology study with 60% Black women veterans. This study had a higher participation rate for Black women veterans than is typically seen in national research studies and shows the VA to be a germane source for further understanding of an understudied population that may benefit from increased screening for breast cancer.
A team-based, multidisciplinary model that meets the unique healthcare needs of women veterans results in a patient-centric delivery of care for assessing breast cancer risk status and prevention options. This model can be replicated nationally by directing primary care physicians and women’s health practitioners to a risk-assessment questionnaire and referring high-risk women for appropriate preventative care. Given that these results show chemoprevention adherence rates doubled those seen nationally, perhaps techniques used within this VA pilot study may be adapted to decrease breast cancer incidence nationally.
Since the rate of PTSD among women veterans is triple the national average, we would expect adherence rates to be lower in our patient cohort. However, the multidisciplinary approach we used in this study (eg, 1:1 consultation with oncologist; genetic counseling referrals; mental health support available), may have improved adherence rates. Perhaps the high rates of PTSD seen in the VA patient population can be a useful way to explore patient adherence rates in those with mental illness and medical conditions.
Future research with a larger cohort may lead to greater insight into the correlation between PTSD and adherence to treatment. Exploring the connection between breast cancer, epigenetics, and specific military service-related exposures could be an area of analysis among this veteran population exhibiting increased breast cancer rates. VAMCs are situated in rural, suburban, and urban locations across the United States and offers a diverse socioeconomic and ethnic patient population for inclusion in clinical investigations. Women veterans make up a small subpopulation of women in the United States, but it is worth considering VA patients as an untapped resource for research collaboration.
Acknowledgements
The authors thank Steven Sanchez and Marissa Vallette, PhD, Breast Health Research Group. This research project was approved by the James J. Peters VA Medical Center Quality Executive Committee and the Washington, DC VA Medical Center Institutional Review Board. This work was supported by the US Department of Veterans Affairs. This work did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the US Government, or any of its agencies.
The number of women seeking care from the Veterans Health Administration (VHA) is increasing.1 In 2015, there were 2 million women veterans in the United States, which is 9.4% of the total veteran population. This group is expected to increase at an average of about 18,000 women per year for the next 10 years.2 The percentage of women veterans who are US Department of Veterans Affairs (VA) users aged 45 to 64 years rose 46% from 2000 to 2015.1,3-4 It is estimated that 15% of veterans who used VA services in 2020 were women.1 Nineteen percent of women veterans are Black.1 The median age of women veterans in 2015 was 50 years.5 Breast cancer is the leading cancer affecting female veterans, and data suggest they have an increased risk of breast cancer based on unique service-related exposures.1,6-9
In the US, about 10 million women are eligible for breast cancer preventive therapy, including, but not limited to, medications, surgery, or lifestyle changes.10 Secondary prevention options include change in surveillance that can reduce their risk or identify cancer at an earlier stage when treatment is more effective. The United States Preventive Services Task Force, the National Comprehensive Cancer Network, the American Society for Clinical Oncology, the National Institute for Health and Care Excellence, and the Oncology Nursing Society recommend screening women aged ≥ 35 years to assess breast cancer risk.11-18 If a woman is at increased risk, she may be a candidate for chemoprevention, prozphylactic surgery, and possibly an enhanced screening regimen.
Urban and minority women are an understudied population. Most veterans (75%) live in urban or suburban settings.19,20 Urban veteran women constitute an important potential study population.
Chemoprevention measures have been underused because of factors involving both women and their health care providers. A large proportion of women are unaware of their higher risk status due to lack of adequate screening and risk assessment.21,22 In addition to patient lack of awareness of their high-risk status, primary care physicians are also reluctant to prescribe chemopreventive agents due to a lack of comfort or familiarity with the risks and benefits.23-26 The STAR2015, BCPT2005, IBIS2014, MAP3 2011, IBIS-I 2014, and IBIS II 2014 studies clearly demonstrate a 49 to 62% reduction in risk for women using chemoprevention such as selective estrogen receptor modulators or aromatase inhibitors, respectively.27-32 Yet only 4 to 9% of high-risk women not enrolled in a clinical trial are using chemoprevention.33-39
The possibility of developing breast cancer also may be increased because of a positive family history or being a member of a family in which there is a known susceptibility gene mutation.40 Based on these risk factors, women may be eligible for tailored follow-up and genetic counseling.41-44
Nationally, 7 to 10% of the civilian US population will experience posttraumatic stress disorder (PTSD).45 The rates are remarkably higher for women veterans, with roughly 20% diagnosed with PTSD.46,47 Anxiety and PTSD have been implicated in poor adherence to medical advice.48,49
In 2014, a national VA multidisciplinary group focused on breast cancer prevention, detection, treatment, and research to address breast health in the growing population of women veterans. High-risk breast cancer screenings are not routinely carried out by the VA in primary care, women’s health, or oncology services. Furthermore, the recording of screening questionnaire results was not synchronized until a standard questionnaire was created and approved as a template by this group in the VA electronic medical record (EMR) in 2015.
Several prediction models can identify which women are at an increased risk of developing breast cancer. The most commonly used risk assessment model, the Gail breast cancer risk assessment tool (BCRAT), has been refined to include women of additional ethnicities (https://www.cancer.gov/bcrisktool).
This pilot project was launched to identify an effective manner to screen women veterans regarding their risk of developing breast cancer and refer them for chemoprevention education or genetic counseling as appropriate.
Methods
A high-risk breast cancer screening questionnaire based on the Gail BCRAT and including lifestyle questions was developed and included as a note template in the VA EMR. The James J. Peters VA Medical Center, Bronx, NY (JJPVAMC) and the Washington DC VA Medical Center (DCVAMC) ran a pilot study between 2015 and 2018 using this breast cancer screening questionnaire to collect data from women veterans. Quality Executive Committee and institutional review board approvals were granted respectively.
Eligibility criteria included women aged ≥ 35 years with no personal history of breast cancer. Most patients were self-referred, but participants also were recruited during VA Breast Cancer Awareness month events, health fairs, or at informational tables in the hospital lobbies. After completing the 20 multiple choice questionnaire with a study team member, either in person or over the phone, a 5-year and lifetime risk of invasive breast cancer was calculated using the Gail BCRAT. A woman is considered high risk and eligible for chemoprevention if her 5-year risk is > 1.66% or her lifetime risk is ≥ 20%. Eligibility for genetic counseling is based on the Breast Cancer Referral Screening Tool, which includes a personal or family history of breast or ovarian cancer and Jewish ancestry.
All patients were notified of their average or high risk status by a clinician. Those who were deemed to be average risk received a follow-up letter in the mail with instructions (eg, to follow-up with a yearly mammogram). Those who were deemed to be high risk for developing breast cancer were asked to come in for an appointment with the study principal investigator (a VA oncologist/breast cancer specialist) to discuss prevention options, further screening, or referrals to genetic counseling. Depending on a patient’s other health factors, a woman at high risk for developing breast cancer also may be a candidate for chemoprevention with tamoxifen, raloxifene, exemestane, anastrozole, or letrozole.
Data on the participant’s lifestyle, including exercise, diet, and smoking, were evaluated to determine whether these factors had an impact on risk status.
Results
The JJP and DC VAMCs screened 103 women veterans between 2015 and 2018. Four patients were excluded for nonveteran (spousal) status, leaving 99 women veterans with a mean age of 54 years. The most common self-reported races were Black (60%), non-Hispanic White (14%), and Hispanic or Latino (13%) (Table 1).
Women veterans in our study were nearly 3-times more likely than the general population were to receive a high-risk Gail Score/BCRAT (35% vs 13%, respectively).50,51 Of this subset, 46% had breast biopsies, and 86% had a positive family history. Thirty-one percent of Black women in our study were high risk, while nationally, 8.2 to 13.3% of Black women aged 50 to 59 years are considered high risk.50,51 Of the Black high-risk group with a high Gail/BCRAT score, 94% had a positive family history, and 33% had a history of breast biopsy (Table 2).
Of the 35 high-risk patients 26 (74%) patients accepted consultations for chemoprevention and 5 (19%) started chemoprevention. Of this high-risk group, 13 (37%) patients were referred for genetic counseling (Table 3).44 The prevalence of PTSD was present in 31% of high-risk women and 29% of the cohort (Figure).The lifestyle questions indicated that, among all participants, 79% had an overweight or obese body mass index; 58% exercised weekly; 51% consumed alcohol; 14% were smokers; and 21% consumed 3 to 4 servings of fruits/vegetables daily.
Discussion
Breast cancer is the most common cancer in women.52 The number of women with breast cancer in the VHA has more than tripled from 1995 to 2012.1 The lifetime risk of developing breast cancer in the general population is about 13%.50 This rate can be affected by risk factors including age, hormone exposure, family history, radiation exposure, and lifestyle factors, such as weight and alcohol use.6,52-56 In the United States, invasive breast cancer affects 1 in 8 women.50,52,57
Our screened population showed nearly 3 times as many women veterans were at an increased risk for breast cancer when compared with historical averages in US women. This difference may be based on a high rate of prior breast biopsies or positive family history, although a provocative study using the Surveillance, Epidemiology, and End Results database showed military women to have higher rates of breast cancer as well.9 Historically, Blacks are vastly understudied in clinical research with only 5% representation on a national level.5,58 The urban locations of both pilot sites (Washington, DC and Bronx, NY) allowed for the inclusion of minority patients in our study. We found that the rates of breast cancer in Black women veterans to be higher than seen nationally, possibly prompting further screening initiatives for this understudied population.
Our pilot study’s chemoprevention utilization (19%) was double the < 10% seen in the national population.33-35 The presence of a knowledgeable breast health practitioner to recruit study participants and offer personalized counseling to women veterans is a likely factor in overcoming barriers to chemopreventive acceptance. These participants may have been motivated to seek care for their high-risk status given a strong family history and prior breast biopsies.
Interestingly, a 3-fold higher PTSD rate was seen in this pilot population (29%) when compared with PTSD rates in the general female population (7-10%) and still one-third higher than the general population of women veterans (20%).45-47 Mental health, anxiety, and PTSD have been barriers to patients who sought treatment and have been implicated in poor adherence to medical advice.48,49 Cancer screening can induce anxiety in patients, and it may be amplified in patients with PTSD. It was remarkable that although adherence with screening recommendations is decreased when PTSD is present, our patient population demonstrated a higher rate of screening adherence.
Women who are seen at the VA often use multiple clinical specialties, and their EMR can be accessed across VA medical centers nationwide. Therefore, identifying women veterans who meet screening criteria is easily attainable within the VA.
When comparing high-risk with average risk women, the lifestyle results (BMI, smoking history, exercise and consumption of fruits, vegetables and alcohol) were essentially the same. Lifestyle factors were similar to national population rates and were unlikely to impact risk levels.
Limitations
Study limitations included a high number of self-referrals and the large percentage of patients with a family history of breast cancer, making them more likely to seek screening. The higher-than-average risk of breast cancer may be driven by a high rate of breast biopsies and a strong family history. Lifestyle metrics could not be accurately compared to other national assessments of lifestyle factors due to the difference in data points that we used or the format of our questions.
Conclusions
As the number of women veterans increases and the incidence of breast cancer in women veterans rise, chemoprevention options should follow national guidelines. To our knowledge, this is the only oncology study with 60% Black women veterans. This study had a higher participation rate for Black women veterans than is typically seen in national research studies and shows the VA to be a germane source for further understanding of an understudied population that may benefit from increased screening for breast cancer.
A team-based, multidisciplinary model that meets the unique healthcare needs of women veterans results in a patient-centric delivery of care for assessing breast cancer risk status and prevention options. This model can be replicated nationally by directing primary care physicians and women’s health practitioners to a risk-assessment questionnaire and referring high-risk women for appropriate preventative care. Given that these results show chemoprevention adherence rates doubled those seen nationally, perhaps techniques used within this VA pilot study may be adapted to decrease breast cancer incidence nationally.
Since the rate of PTSD among women veterans is triple the national average, we would expect adherence rates to be lower in our patient cohort. However, the multidisciplinary approach we used in this study (eg, 1:1 consultation with oncologist; genetic counseling referrals; mental health support available), may have improved adherence rates. Perhaps the high rates of PTSD seen in the VA patient population can be a useful way to explore patient adherence rates in those with mental illness and medical conditions.
Future research with a larger cohort may lead to greater insight into the correlation between PTSD and adherence to treatment. Exploring the connection between breast cancer, epigenetics, and specific military service-related exposures could be an area of analysis among this veteran population exhibiting increased breast cancer rates. VAMCs are situated in rural, suburban, and urban locations across the United States and offers a diverse socioeconomic and ethnic patient population for inclusion in clinical investigations. Women veterans make up a small subpopulation of women in the United States, but it is worth considering VA patients as an untapped resource for research collaboration.
Acknowledgements
The authors thank Steven Sanchez and Marissa Vallette, PhD, Breast Health Research Group. This research project was approved by the James J. Peters VA Medical Center Quality Executive Committee and the Washington, DC VA Medical Center Institutional Review Board. This work was supported by the US Department of Veterans Affairs. This work did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the US Government, or any of its agencies.
1. US Department of Veterans Affairs. National Center for Veterans Analysis and Statistics. The past, present and future of women veterans. Published February 2017. Accessed April 28, 2021. https://www.va.gov/vetdata/docs/specialreports/women_veterans_2015_final.pdf.
2. Frayne SM, Carney DV, Bastian L, et al. The VA Women’s Health Practice-Based Research Network: amplifying women veterans’ voices in VA research. J Gen Intern Med. 2013;28 Suppl 2(Suppl 2):S504-S509. doi:10.1007/s11606-013-2476-3
3. US Department of Veterans Affairs, Veterans Health Administration, Women’s Health Evaluation Initiative, Women Veterans Health Strategic Health Care Group. Sourcebook: women veterans in the Veterans Health Administration. Volume 1: Sociodemographic characteristics and use of VHA care. Published December 2010. Accessed April 12, 2021. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=2455
4. Bean-Mayberry B, Yano EM, Bayliss N, Navratil J, Weisman CS, Scholle SH. Federally funded comprehensive women’s health centers: leading innovation in women’s healthcare delivery. J Womens Health (Larchmt). 2007;16(9):1281-1290. doi:10.1089/jwh.2006.0284
5. US Department of Veterans Affairs. National Center for Veterans Analysis and Statistics.VA utilization profile FY 2016. Published November 2017. Accessed April 12, 2021. https://www.va.gov/vetdata/docs/QuickFacts/VA_Utilization_Profile.PDF
6. Ekenga CC, Parks CG, Sandler DP. Chemical exposures in the workplace and breast cancer risk: a prospective cohort study. Int J Cancer. 2015;137(7):1765-1774. doi:10.1002/ijc.29545
7. Rennix CP, Quinn MM, Amoroso PJ, Eisen EA, Wegman DH. Risk of breast cancer among enlisted Army women occupationally exposed to volatile organic compounds. Am J Ind Med. 2005;48(3):157-167. doi:10.1002/ajim.20201
8. Ritz B. Cancer mortality among workers exposed to chemicals during uranium processing. J Occup Environ Med. 1999;41(7):556-566. doi:10.1097/00043764-199907000-00004
9. Zhu K, Devesa SS, Wu H, et al. Cancer incidence in the U.S. military population: comparison with rates from the SEER program. Cancer Epidemiol Biomarkers Prev. 2009;18(6):1740-1745. doi:10.1158/1055-9965.EPI-09-0041
10. Freedman AN, Yu B, Gail MH, et al. Benefit/risk assessment for breast cancer chemoprevention with raloxifene or tamoxifen for women age 50 years or older [published correction appears in J Clin Oncol. 2013 Nov 10;31(32):4167]. J Clin Oncol. 2011;29(17):2327-2333. doi:10.1200/JCO.2010.33.0258
11. Greene, H. Cancer prevention, screening and early detection. In: Gobel BH, Triest-Robertson S, Vogel WH, eds. Advanced Oncology Nursing Certification Review and Resource Manual. 3rd ed. Oncology Nursing Society; 2016:1-34. https://www.ons.org/sites/default/files/publication_pdfs/2%20ADVPrac%20chapter%201.pdf
12. National Comprehensive Cancer Network. NCCN Breast Cancer Risk Reduction. Version 1.2021 NCCN Clinical Practice Guidelines in Oncology. Updated March 24, 2021 Accessed April 12, 2021. https://www.nccn.org/professionals/physician_gls/pdf/breast_risk.pdf
13. US Preventive Services Task Force. Breast cancer: Medications use to reduce risk. Updated September 3, 2019. Accessed April 12, 2021. https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/breast-cancer-medications-for-risk-reduction
14. Moyer VA; U.S. Preventive Services Task Force. Medications to decrease the risk for breast cancer in women: recommendations from the U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159(10):698-708. doi:10.7326/0003-4819-159-10-201311190-00717
15. Boucher JE. Chemoprevention: an overview of pharmacologic agents and nursing considerations. Clin J Oncol Nurs. 2018;22(3):350-353. doi:10.1188/18.CJON.350-353
16. Nichols HB, Stürmer T, Lee VS, et al. Breast cancer chemoprevention in an integrated health care setting. JCO Clin Cancer Inform. 2017;1:1-12. doi:10.1200/CCI.16.00059
17. Bevers TB, Helvie M, Bonaccio E, et al. Breast cancer screening and diagnosis, Version 3.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16(11):1362-1389. doi:10.6004/jnccn.2018.0083
18. Visvanathan K, Hurley P, Bantug E, et al. Use of pharmacologic interventions for breast cancer risk reduction: American Society of Clinical Oncology clinical practice guideline [published correction appears in J Clin Oncol. 2013 Dec 1;31(34):4383]. J Clin Oncol. 2013;31(23):2942-2962. doi:10.1200/JCO.2013.49.3122
19. Sealy-Jefferson S, Roseland ME, Cote ML, et al. rural-urban residence and stage at breast cancer diagnosis among postmenopausal women: The Women’s Health Initiative. J Womens Health (Larchmt). 2019;28(2):276-283. doi:10.1089/jwh.2017.6884
20. Holder KA. Veterans in rural America: 2011-2015. Published January 25, 2017. Accessed April 12, 2021. https://www.census.gov/library/publications/2017/acs/acs-36.html
21. Owens WL, Gallagher TJ, Kincheloe MJ, Ruetten VL. Implementation in a large health system of a program to identify women at high risk for breast cancer. J Oncol Pract. 2011;7(2):85-88. doi:10.1200/JOP.2010.000107
2. Pivot X, Viguier J, Touboul C, et al. Breast cancer screening controversy: too much or not enough?. Eur J Cancer Prev. 2015;24 Suppl:S73-S76. doi:10.1097/CEJ.0000000000000145
23. Bidassie B, Kovach A, Vallette MA, et al. Breast Cancer risk assessment and chemoprevention use among veterans affairs primary care providers: a national online survey. Mil Med. 2020;185(3-4):512-518. doi:10.1093/milmed/usz291
24. Brewster AM, Davidson NE, McCaskill-Stevens W. Chemoprevention for breast cancer: overcoming barriers to treatment. Am Soc Clin Oncol Educ Book. 2012;85-90. doi:10.14694/EdBook_AM.2012.32.152
25. Meyskens FL Jr, Curt GA, Brenner DE, et al. Regulatory approval of cancer risk-reducing (chemopreventive) drugs: moving what we have learned into the clinic. Cancer Prev Res (Phila). 2011;4(3):311-323. doi:10.1158/1940-6207.CAPR-09-0014
26. Tice JA, Kerlikowske K. Screening and prevention of breast cancer in primary care. Prim Care. 2009;36(3):533-558. doi:10.1016/j.pop.2009.04.003
27. Vogel VG. Selective estrogen receptor modulators and aromatase inhibitors for breast cancer chemoprevention. Curr Drug Targets. 2011;12(13):1874-1887. doi:10.2174/138945011798184164
28. Vogel VG, Costantino JP, Wickerham DL, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial [published correction appears in JAMA. 2006 Dec 27;296(24):2926] [published correction appears in JAMA. 2007 Sep 5;298(9):973]. JAMA. 2006;295(23):2727-2741. doi:10.1001/jama.295.23.joc60074
29. Pruthi S, Heisey RE, Bevers TB. Chemoprevention for breast cancer. Ann Surg Oncol. 2015;22(10):3230-3235. doi:10.1245/s10434-015-4715-9
30. Cuzick J, Sestak I, Forbes JF, et al. Anastrozole for prevention of breast cancer in high-risk postmenopausal women (IBIS-II): an international, double-blind, randomised placebo-controlled trial [published correction appears in Lancet. 2014 Mar 22;383(9922):1040] [published correction appears in Lancet. 2017 Mar 11;389(10073):1010]. Lancet. 2014;383(9922):1041-1048. doi:10.1016/S0140-6736(13)62292-8
31. Bozovic-Spasojevic I, Azambuja E, McCaskill-Stevens W, Dinh P, Cardoso F. Chemoprevention for breast cancer. Cancer Treat Rev. 2012;38(5):329-339. doi:10.1016/j.ctrv.2011.07.005
32. Gabriel EM, Jatoi I. Breast cancer chemoprevention. Expert Rev Anticancer Ther. 2012;12(2):223-228. doi:10.1586/era.11.206

33. Crew KD, Albain KS, Hershman DL, Unger JM, Lo SS. How do we increase uptake of tamoxifen and other anti-estrogens for breast cancer prevention?. NPJ Breast Cancer. 2017;3:20. Published 2017 May 19. doi:10.1038/s41523-017-0021-y
34. Ropka ME, Keim J, Philbrick JT. Patient decisions about breast cancer chemoprevention: a systematic review and meta-analysis. J Clin Oncol. 2010;28(18):3090-3095. doi:10.1200/JCO.2009.27.8077
35. Smith SG, Sestak I, Forster A, et al. Factors affecting uptake and adherence to breast cancer chemoprevention: a systematic review and meta-analysis. Ann Oncol. 2016;27(4):575-590. doi:10.1093/annonc/mdv590
36. Grann VR, Patel PR, Jacobson JS, et al. Comparative effectiveness of screening and prevention strategies among BRCA1/2-affected mutation carriers. Breast Cancer Res Treat. 2011 Feb;125(3):837-847. doi:10.1007/s10549-010-1043-4
37. Goss PE, Ingle JN, Alés-Martínez JE, et al. Exemestane for breast-cancer prevention in postmenopausal women [published correction appears in N Engl J Med. 2011 Oct 6;365(14):1361]. N Engl J Med. 2011;364(25):2381-2391. doi:10.1056/NEJMoa1103507
38. Kmietowicz Z. Five in six women reject drugs that could reduce their risk of breast cancer. BMJ. 2015;351:h6650. Published 2015 Dec 8. doi:10.1136/bmj.h6650
39. Nelson HD, Fu R, Griffin JC, Nygren P, Smith ME, Humphrey L. Systematic review: comparative effectiveness of medications to reduce risk for primary breast cancer. Ann Intern Med. 2009;151(10):703-235. doi:10.7326/0003-4819-151-10-200911170-00147
40. Dahabreh IJ, Wieland LS, Adam GP, Halladay C, Lau J, Trikalinos TA. Core needle and open surgery biopsy for diagnosis of breast lesions: an update to the 2009 report. Published September 2014. Accessed April 12, 2021. https://www.ncbi.nlm.nih.gov/books/NBK246878
41. National Cancer Institute. Genetics of breast and ovarian cancer (PDQ)—health profession version. Updated February 12, 2021. Accessed April 12, 2021. http://www.cancer.gov/cancertopics/pdq/genetics/breast-and-ovarian/HealthProfessional
42. US Department of Health and Human Services. National Institutes of Health, National Institute of Environmental Health Sciences The sister study. Accessed April 12, 2021. https://sisterstudy.niehs.nih.gov/english/NIEHS.htm
43. Tutt A, Ashworth A. Can genetic testing guide treatment in breast cancer?. Eur J Cancer. 2008;44(18):2774-2780. doi:10.1016/j.ejca.2008.10.009
44. Katz SJ, Ward KC, Hamilton AS, et al. Gaps in receipt of clinically indicated genetic counseling after diagnosis of breast cancer. J Clin Oncol. 2018;36(12):1218-1224. doi:10.1200/JCO.2017.76.2369
45. US Department of Veterans Affairs. PTSD: National Center for PTSD. How common is PTSD in adults? Updated October 17, 2019. Accessed April 12, 2021. https://www.ptsd.va.gov/understand/common/common_adults.asp
46. US Department of Veterans Affairs. PTSD: National Center for PTSD. How common is PTSD in women? Updated October 16, 2019. Accessed April 12, 2021. https://www.ptsd.va.gov/understand/common/common_women.asp
47. US Department of Veterans Affairs. PTSD: National Center for PTSD. How common is PTSD in veterans? Updated September 24, 2018. Accessed April 12, 2021. https://www.ptsd.va.gov/understand/common/common_veterans.asp
48. Lindberg NM, Wellisch D. Anxiety and compliance among women at high risk for breast cancer. Ann Behav Med. 2001;23(4):298-303. doi:10.1207/S15324796ABM2304_9
49. DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160(14):2101-2107. doi:10.1001/archinte.160.14.2101
50. Centers for Disease Control and Prevention. MMWR appendix: breast cancer rates among black women and white women. Updated October 13, 2016. Accessed April 12, 2021. https://www.cdc.gov/cancer/breast/statistics/trends_invasive.htm
51. Richardson LC, Henley SJ, Miller JW, Massetti G, Thomas CC. Patterns and trends in age-specific black-white differences in breast cancer incidence and mortality - United States, 1999-2014. MMWR Morb Mortal Wkly Rep. 2016;65(40):1093-1098. Published 2016 Oct 14. doi:10.15585/mmwr.mm6540a1
52. Brody JG, Moysich KB, Humblet O, Attfield KR, Beehler GP, Rudel RA. Environmental pollutants and breast cancer: epidemiologic studies. Cancer. 2007;109(12 Suppl):2667-2711. doi:10.1002/cncr.22655
53. Brophy JT, Keith MM, Watterson A, et al. Breast cancer risk in relation to occupations with exposure to carcinogens and endocrine disruptors: a Canadian case-control study. Environ Health. 2012;11:87. Published 2012 Nov 19. doi:10.1186/1476-069X-11-87
54. Labrèche F, Goldberg MS, Valois MF, Nadon L. Postmenopausal breast cancer and occupational exposures. Occup Environ Med. 2010;67(4):263-269. doi:10.1136/oem.2009.049817
55. National Institute of Environmental Health Sciences, Interagency Breast Cancer & Environmental Research Coordinating Committee. Breast cancer and the environment: prioritizing prevention. Updated March 8, 2013. Accessed April 12, 2021. https://www.niehs.nih.gov/about/boards/ibcercc/index.cfm
56. Gail MH, Costantino JP, Pee D, et al. Projecting individualized absolute invasive breast cancer risk in African American women [published correction appears in J Natl Cancer Inst. 2008 Aug 6;100(15):1118] [published correction appears in J Natl Cancer Inst. 2008 Mar 5;100(5):373]. J Natl Cancer Inst. 2007;99(23):1782-1792. doi:10.1093/jnci/djm223
57. Corbie-Smith G, Thomas SB, Williams MV, Moody-Ayers S. Attitudes and beliefs of African Americans toward participation in medical research. J Gen Intern Med. 1999;14(9):537-546. doi:10.1046/j.1525-1497.1999.07048.x
58. Braunstein JB, Sherber NS, Schulman SP, Ding EL, Powe NR. Race, medical researcher distrust, perceived harm, and willingness to participate in cardiovascular prevention trials. Medicine (Baltimore). 2008;87(1):1-9. doi:10.1097/MD.0b013e3181625d78
1. US Department of Veterans Affairs. National Center for Veterans Analysis and Statistics. The past, present and future of women veterans. Published February 2017. Accessed April 28, 2021. https://www.va.gov/vetdata/docs/specialreports/women_veterans_2015_final.pdf.
2. Frayne SM, Carney DV, Bastian L, et al. The VA Women’s Health Practice-Based Research Network: amplifying women veterans’ voices in VA research. J Gen Intern Med. 2013;28 Suppl 2(Suppl 2):S504-S509. doi:10.1007/s11606-013-2476-3
3. US Department of Veterans Affairs, Veterans Health Administration, Women’s Health Evaluation Initiative, Women Veterans Health Strategic Health Care Group. Sourcebook: women veterans in the Veterans Health Administration. Volume 1: Sociodemographic characteristics and use of VHA care. Published December 2010. Accessed April 12, 2021. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=2455
4. Bean-Mayberry B, Yano EM, Bayliss N, Navratil J, Weisman CS, Scholle SH. Federally funded comprehensive women’s health centers: leading innovation in women’s healthcare delivery. J Womens Health (Larchmt). 2007;16(9):1281-1290. doi:10.1089/jwh.2006.0284
5. US Department of Veterans Affairs. National Center for Veterans Analysis and Statistics.VA utilization profile FY 2016. Published November 2017. Accessed April 12, 2021. https://www.va.gov/vetdata/docs/QuickFacts/VA_Utilization_Profile.PDF
6. Ekenga CC, Parks CG, Sandler DP. Chemical exposures in the workplace and breast cancer risk: a prospective cohort study. Int J Cancer. 2015;137(7):1765-1774. doi:10.1002/ijc.29545
7. Rennix CP, Quinn MM, Amoroso PJ, Eisen EA, Wegman DH. Risk of breast cancer among enlisted Army women occupationally exposed to volatile organic compounds. Am J Ind Med. 2005;48(3):157-167. doi:10.1002/ajim.20201
8. Ritz B. Cancer mortality among workers exposed to chemicals during uranium processing. J Occup Environ Med. 1999;41(7):556-566. doi:10.1097/00043764-199907000-00004
9. Zhu K, Devesa SS, Wu H, et al. Cancer incidence in the U.S. military population: comparison with rates from the SEER program. Cancer Epidemiol Biomarkers Prev. 2009;18(6):1740-1745. doi:10.1158/1055-9965.EPI-09-0041
10. Freedman AN, Yu B, Gail MH, et al. Benefit/risk assessment for breast cancer chemoprevention with raloxifene or tamoxifen for women age 50 years or older [published correction appears in J Clin Oncol. 2013 Nov 10;31(32):4167]. J Clin Oncol. 2011;29(17):2327-2333. doi:10.1200/JCO.2010.33.0258
11. Greene, H. Cancer prevention, screening and early detection. In: Gobel BH, Triest-Robertson S, Vogel WH, eds. Advanced Oncology Nursing Certification Review and Resource Manual. 3rd ed. Oncology Nursing Society; 2016:1-34. https://www.ons.org/sites/default/files/publication_pdfs/2%20ADVPrac%20chapter%201.pdf
12. National Comprehensive Cancer Network. NCCN Breast Cancer Risk Reduction. Version 1.2021 NCCN Clinical Practice Guidelines in Oncology. Updated March 24, 2021 Accessed April 12, 2021. https://www.nccn.org/professionals/physician_gls/pdf/breast_risk.pdf
13. US Preventive Services Task Force. Breast cancer: Medications use to reduce risk. Updated September 3, 2019. Accessed April 12, 2021. https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/breast-cancer-medications-for-risk-reduction
14. Moyer VA; U.S. Preventive Services Task Force. Medications to decrease the risk for breast cancer in women: recommendations from the U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159(10):698-708. doi:10.7326/0003-4819-159-10-201311190-00717
15. Boucher JE. Chemoprevention: an overview of pharmacologic agents and nursing considerations. Clin J Oncol Nurs. 2018;22(3):350-353. doi:10.1188/18.CJON.350-353
16. Nichols HB, Stürmer T, Lee VS, et al. Breast cancer chemoprevention in an integrated health care setting. JCO Clin Cancer Inform. 2017;1:1-12. doi:10.1200/CCI.16.00059
17. Bevers TB, Helvie M, Bonaccio E, et al. Breast cancer screening and diagnosis, Version 3.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16(11):1362-1389. doi:10.6004/jnccn.2018.0083
18. Visvanathan K, Hurley P, Bantug E, et al. Use of pharmacologic interventions for breast cancer risk reduction: American Society of Clinical Oncology clinical practice guideline [published correction appears in J Clin Oncol. 2013 Dec 1;31(34):4383]. J Clin Oncol. 2013;31(23):2942-2962. doi:10.1200/JCO.2013.49.3122
19. Sealy-Jefferson S, Roseland ME, Cote ML, et al. rural-urban residence and stage at breast cancer diagnosis among postmenopausal women: The Women’s Health Initiative. J Womens Health (Larchmt). 2019;28(2):276-283. doi:10.1089/jwh.2017.6884
20. Holder KA. Veterans in rural America: 2011-2015. Published January 25, 2017. Accessed April 12, 2021. https://www.census.gov/library/publications/2017/acs/acs-36.html
21. Owens WL, Gallagher TJ, Kincheloe MJ, Ruetten VL. Implementation in a large health system of a program to identify women at high risk for breast cancer. J Oncol Pract. 2011;7(2):85-88. doi:10.1200/JOP.2010.000107
2. Pivot X, Viguier J, Touboul C, et al. Breast cancer screening controversy: too much or not enough?. Eur J Cancer Prev. 2015;24 Suppl:S73-S76. doi:10.1097/CEJ.0000000000000145
23. Bidassie B, Kovach A, Vallette MA, et al. Breast Cancer risk assessment and chemoprevention use among veterans affairs primary care providers: a national online survey. Mil Med. 2020;185(3-4):512-518. doi:10.1093/milmed/usz291
24. Brewster AM, Davidson NE, McCaskill-Stevens W. Chemoprevention for breast cancer: overcoming barriers to treatment. Am Soc Clin Oncol Educ Book. 2012;85-90. doi:10.14694/EdBook_AM.2012.32.152
25. Meyskens FL Jr, Curt GA, Brenner DE, et al. Regulatory approval of cancer risk-reducing (chemopreventive) drugs: moving what we have learned into the clinic. Cancer Prev Res (Phila). 2011;4(3):311-323. doi:10.1158/1940-6207.CAPR-09-0014
26. Tice JA, Kerlikowske K. Screening and prevention of breast cancer in primary care. Prim Care. 2009;36(3):533-558. doi:10.1016/j.pop.2009.04.003
27. Vogel VG. Selective estrogen receptor modulators and aromatase inhibitors for breast cancer chemoprevention. Curr Drug Targets. 2011;12(13):1874-1887. doi:10.2174/138945011798184164
28. Vogel VG, Costantino JP, Wickerham DL, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial [published correction appears in JAMA. 2006 Dec 27;296(24):2926] [published correction appears in JAMA. 2007 Sep 5;298(9):973]. JAMA. 2006;295(23):2727-2741. doi:10.1001/jama.295.23.joc60074
29. Pruthi S, Heisey RE, Bevers TB. Chemoprevention for breast cancer. Ann Surg Oncol. 2015;22(10):3230-3235. doi:10.1245/s10434-015-4715-9
30. Cuzick J, Sestak I, Forbes JF, et al. Anastrozole for prevention of breast cancer in high-risk postmenopausal women (IBIS-II): an international, double-blind, randomised placebo-controlled trial [published correction appears in Lancet. 2014 Mar 22;383(9922):1040] [published correction appears in Lancet. 2017 Mar 11;389(10073):1010]. Lancet. 2014;383(9922):1041-1048. doi:10.1016/S0140-6736(13)62292-8
31. Bozovic-Spasojevic I, Azambuja E, McCaskill-Stevens W, Dinh P, Cardoso F. Chemoprevention for breast cancer. Cancer Treat Rev. 2012;38(5):329-339. doi:10.1016/j.ctrv.2011.07.005
32. Gabriel EM, Jatoi I. Breast cancer chemoprevention. Expert Rev Anticancer Ther. 2012;12(2):223-228. doi:10.1586/era.11.206

33. Crew KD, Albain KS, Hershman DL, Unger JM, Lo SS. How do we increase uptake of tamoxifen and other anti-estrogens for breast cancer prevention?. NPJ Breast Cancer. 2017;3:20. Published 2017 May 19. doi:10.1038/s41523-017-0021-y
34. Ropka ME, Keim J, Philbrick JT. Patient decisions about breast cancer chemoprevention: a systematic review and meta-analysis. J Clin Oncol. 2010;28(18):3090-3095. doi:10.1200/JCO.2009.27.8077
35. Smith SG, Sestak I, Forster A, et al. Factors affecting uptake and adherence to breast cancer chemoprevention: a systematic review and meta-analysis. Ann Oncol. 2016;27(4):575-590. doi:10.1093/annonc/mdv590
36. Grann VR, Patel PR, Jacobson JS, et al. Comparative effectiveness of screening and prevention strategies among BRCA1/2-affected mutation carriers. Breast Cancer Res Treat. 2011 Feb;125(3):837-847. doi:10.1007/s10549-010-1043-4
37. Goss PE, Ingle JN, Alés-Martínez JE, et al. Exemestane for breast-cancer prevention in postmenopausal women [published correction appears in N Engl J Med. 2011 Oct 6;365(14):1361]. N Engl J Med. 2011;364(25):2381-2391. doi:10.1056/NEJMoa1103507
38. Kmietowicz Z. Five in six women reject drugs that could reduce their risk of breast cancer. BMJ. 2015;351:h6650. Published 2015 Dec 8. doi:10.1136/bmj.h6650
39. Nelson HD, Fu R, Griffin JC, Nygren P, Smith ME, Humphrey L. Systematic review: comparative effectiveness of medications to reduce risk for primary breast cancer. Ann Intern Med. 2009;151(10):703-235. doi:10.7326/0003-4819-151-10-200911170-00147
40. Dahabreh IJ, Wieland LS, Adam GP, Halladay C, Lau J, Trikalinos TA. Core needle and open surgery biopsy for diagnosis of breast lesions: an update to the 2009 report. Published September 2014. Accessed April 12, 2021. https://www.ncbi.nlm.nih.gov/books/NBK246878
41. National Cancer Institute. Genetics of breast and ovarian cancer (PDQ)—health profession version. Updated February 12, 2021. Accessed April 12, 2021. http://www.cancer.gov/cancertopics/pdq/genetics/breast-and-ovarian/HealthProfessional
42. US Department of Health and Human Services. National Institutes of Health, National Institute of Environmental Health Sciences The sister study. Accessed April 12, 2021. https://sisterstudy.niehs.nih.gov/english/NIEHS.htm
43. Tutt A, Ashworth A. Can genetic testing guide treatment in breast cancer?. Eur J Cancer. 2008;44(18):2774-2780. doi:10.1016/j.ejca.2008.10.009
44. Katz SJ, Ward KC, Hamilton AS, et al. Gaps in receipt of clinically indicated genetic counseling after diagnosis of breast cancer. J Clin Oncol. 2018;36(12):1218-1224. doi:10.1200/JCO.2017.76.2369
45. US Department of Veterans Affairs. PTSD: National Center for PTSD. How common is PTSD in adults? Updated October 17, 2019. Accessed April 12, 2021. https://www.ptsd.va.gov/understand/common/common_adults.asp
46. US Department of Veterans Affairs. PTSD: National Center for PTSD. How common is PTSD in women? Updated October 16, 2019. Accessed April 12, 2021. https://www.ptsd.va.gov/understand/common/common_women.asp
47. US Department of Veterans Affairs. PTSD: National Center for PTSD. How common is PTSD in veterans? Updated September 24, 2018. Accessed April 12, 2021. https://www.ptsd.va.gov/understand/common/common_veterans.asp
48. Lindberg NM, Wellisch D. Anxiety and compliance among women at high risk for breast cancer. Ann Behav Med. 2001;23(4):298-303. doi:10.1207/S15324796ABM2304_9
49. DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160(14):2101-2107. doi:10.1001/archinte.160.14.2101
50. Centers for Disease Control and Prevention. MMWR appendix: breast cancer rates among black women and white women. Updated October 13, 2016. Accessed April 12, 2021. https://www.cdc.gov/cancer/breast/statistics/trends_invasive.htm
51. Richardson LC, Henley SJ, Miller JW, Massetti G, Thomas CC. Patterns and trends in age-specific black-white differences in breast cancer incidence and mortality - United States, 1999-2014. MMWR Morb Mortal Wkly Rep. 2016;65(40):1093-1098. Published 2016 Oct 14. doi:10.15585/mmwr.mm6540a1
52. Brody JG, Moysich KB, Humblet O, Attfield KR, Beehler GP, Rudel RA. Environmental pollutants and breast cancer: epidemiologic studies. Cancer. 2007;109(12 Suppl):2667-2711. doi:10.1002/cncr.22655
53. Brophy JT, Keith MM, Watterson A, et al. Breast cancer risk in relation to occupations with exposure to carcinogens and endocrine disruptors: a Canadian case-control study. Environ Health. 2012;11:87. Published 2012 Nov 19. doi:10.1186/1476-069X-11-87
54. Labrèche F, Goldberg MS, Valois MF, Nadon L. Postmenopausal breast cancer and occupational exposures. Occup Environ Med. 2010;67(4):263-269. doi:10.1136/oem.2009.049817
55. National Institute of Environmental Health Sciences, Interagency Breast Cancer & Environmental Research Coordinating Committee. Breast cancer and the environment: prioritizing prevention. Updated March 8, 2013. Accessed April 12, 2021. https://www.niehs.nih.gov/about/boards/ibcercc/index.cfm
56. Gail MH, Costantino JP, Pee D, et al. Projecting individualized absolute invasive breast cancer risk in African American women [published correction appears in J Natl Cancer Inst. 2008 Aug 6;100(15):1118] [published correction appears in J Natl Cancer Inst. 2008 Mar 5;100(5):373]. J Natl Cancer Inst. 2007;99(23):1782-1792. doi:10.1093/jnci/djm223
57. Corbie-Smith G, Thomas SB, Williams MV, Moody-Ayers S. Attitudes and beliefs of African Americans toward participation in medical research. J Gen Intern Med. 1999;14(9):537-546. doi:10.1046/j.1525-1497.1999.07048.x
58. Braunstein JB, Sherber NS, Schulman SP, Ding EL, Powe NR. Race, medical researcher distrust, perceived harm, and willingness to participate in cardiovascular prevention trials. Medicine (Baltimore). 2008;87(1):1-9. doi:10.1097/MD.0b013e3181625d78
Clinical Use of a Diagnostic Gene Expression Signature for Melanocytic Neoplasms
According to National Institutes of Health estimates, more than 90,000 new cases of melanoma were diagnosed in 2018.1 Overall 5-year survival for patients with melanoma exceeds 90%, but individual survival estimates are highly dependent on stage at diagnosis, and survival decreases markedly with metastasis. Therefore, early and accurate diagnosis is critical.
Diagnosis of melanocytic neoplasms usually is performed by dermatopathologists through microscopic examination of stained tissue biopsy sections, a technically simple and effective method that enables a definitive diagnosis of benign nevus or malignant melanoma to be made in most cases. However, approximately 15% of all biopsied melanocytic lesions will exhibit some degree of histopathologic ambiguity,2-4 meaning that some of their microscopic features will be characteristic of a benign nevus while others will suggest the possibility of malignant melanoma. Diagnostic interpretations often vary in these cases, even among experts, and a definitive diagnosis of benign or malignant may be difficult to achieve by microscopy alone.2-4 Because of the marked reduction in survival once a melanoma has metastasized, these diagnostically ambiguous lesions often are treated as possible malignant melanomas with complete surgical excision (or re-excision). However, some experts suggest that many histopathologically ambiguous melanocytic neoplasms are, in fact, benign,5 a notion supported by epidemiologic evidence.6,7 Therefore, excision of many ambiguous melanocytic neoplasms might be avoided if definitive diagnosis could be achieved.
A gene expression signature was developed and validated for use as an adjunct to traditional methods of differentiating malignant melanocytic neoplasms from their benign counterparts.8-11 This test quantifies the RNA transcripts produced by 14 genes known to be overexpressed in malignant melanomas by comparison to benign nevi. These values are then combined algorithmically with measurements of 9 reference genes to produce an objective numerical score that is classified as benign, malignant, or indeterminate. When used by board-certified dermatopathologists and dermatologists confronting ambiguous melanocytic lesions, the test produces substantial increases in definitive diagnoses and prompts changes in treatment recommendations.12,13 However, the long-term consequences of foregoing surgical excision of melanocytic neoplasms that are diagnostically ambiguous but classified as benign by this test have not yet been formally assessed. In the current study, prospectively tested patients whose ambiguous melanocytic neoplasms were classified as benign by the gene expression signature were followed for up to 4.5 years to evaluate the long-term safety of treatment decisions aligned with benign test results.
Methods
Study Population
As part of a prior study,12 US-based dermatopathologists submitted tissue sections from biopsied melanocytic neoplasms determined to be diagnostically ambiguous by histopathology for analysis with the gene expression signature (Myriad Genetics, Inc). Diagnostically ambiguous lesions were those lesions that were described as ambiguous, uncertain, equivocal, indeterminate, or other synonymous terms by the submitting dermatopathologist and therefore lacked a confident diagnosis of benign or malignant prior to testing. Patients initially were tested between May 2014 and August 2014, with samples submitted through a prospective clinical experience study designed to assess the impact of the test on diagnosis and treatment decisions. This study was performed under an institutional review board waiver of consent (Quorum #33403/1).
Patients were eligible for inclusion in the current study if their biopsy specimens (1) had an uncertain preliminary diagnosis according to the submitting dermatopathologist (pretest diagnosis of indeterminate); (2) received a negative (benign) score from the gene expression test; (3) were treated as benign by the dermatologist(s) involved in follow-up care; and (4) were submitted by a single site (St. Joseph Medical Center, Houston, Texas). Although a single dermatopathology site was used for this study, multiple dermatologists were involved in the final treatment of these patients. Patients with benign scores who received additional intervention were excluded, as they may have a lower rate of adverse events (ie, metastasis) than those who did not receive intervention and would therefore skew the analysis population. A total of 25 patients from the prior study met these inclusion criteria. The previously collected12 pretest and posttest de-identified data were compiled from the commercial laboratory databases, and the patients were followed from the time of testing via medical record review performed by the dermatology providers at participating sites. Clinical follow-up data were collected using study-specific case report forms (CRFs) that captured the following: (1) the dates and results of clinical follow-up visits; (2) the type(s) of treatment and interventions (if any) performed at those visits; (3) the specific indication for any intervention performed; (4) any evidence of persistent, locally recurrent, and/or distant melanocytic neoplasia (whether definitively attributable to the tested lesion or not); and (5) death from any cause. The CRF assigned interventions to 1 of 5 categories: excision, excision with sentinel lymph node biopsy, referral to dermatologic or other surgeon, examination only (without surgical intervention), and other. Selection of other required a free-text description of the treatment and indications. Pertinent information not otherwise captured by the CRF also was recordable as free text.
Gene Expression Testing
Gene expression testing was carried out at the time of specimen submission in the prior study12 as described previously.14 Briefly, formalin-fixed, paraffin-embedded, unstained tissue sections and/or tissue blocks were submitted for testing along with a single hematoxylin and eosin–stained slide used to identify and designate the representative portion(s) of the lesion to be tested. These areas were macrodissected from unstained tissue sections and pooled for RNA extraction. Expression of 14 biomarker genes and 9 reference genes was measured via
Statistical Analysis
Demographic and other baseline characteristics of the patient population were summarized. Follow-up time was calculated as the interval between the date a patient’s gene expression test result was first issued to the provider and the date of the patient’s last recorded visit during the study period. All patient dermatology office visits within the designated follow-up period were documented, with a nonstandard number of visits and follow-up time across all study patients. Statistical analyses were conducted using SAS software (SAS Institute Inc), R software version 3.5.0 (R Foundation for Statistical Computing), and IBM SPSS Statistics software (IBM SPSS Statistics for Windows, Version 25).
Results
Patient Sample
A total of 25 ambiguous melanocytic neoplasms from 25 patients met the study inclusion criteria of a benign gene expression result with subsequent treatment as a benign neoplasm during follow-up. The patient sample statistics are summarized in Table 1. Most patients were younger than 65 years, with an average age at the time of biopsy of 48.4 years. All 25 neoplasms produced negative (benign) gene expression signature scores, all were diagnosed as benign nevi posttest by the submitting dermatopathologist, and all patients were initially treated in accordance with the benign diagnosis by the dermatologist(s) involved in clinical follow-up care. Prior to testing with the gene expression signature, most of these histopathologically indeterminate lesions received differential diagnoses, the most common of which were dysplastic nevus (84%), melanoma arising from a nevus (72%), and superficial spreading melanoma (64%; eTable). After testing with the gene expression signature and receiving a benign score, most lesions received a single differential diagnosis of dysplastic nevus (88%).
Follow-up and Survival
Clinical follow-up time ranged from 0.6 to 53.3 months, with a mean duration (SD) of 38.5 (16.6) months, and patients attended an average of 4 postbiopsy dermatology appointments (mean [SD], 4.6 [3.6]). According to the participating dermatology care providers, none of the 25 patients developed any indication during follow-up that the diagnosis of benign nevus was inaccurate. No patient had evidence of locally recurrent or metastatic melanoma, and none died during the study period.
Treatment/Interventions
The treatment recorded in the CRF was examination only for 21 of 25 patients, excision for 3, and other for 1 (Table 2). Because the explanation for the selection of other in this case described an excision performed at the same anatomic location as the biopsy, this treatment also was considered an excision for purposes of the study analyses. The 3 excisions all occurred at the first postbiopsy dermatology encounter. Across all follow-up visits, no additional surgical interventions occurred (Table 2).
The first excision (case 1) involved a 67-year-old woman with a lesion on the mid pubic region described clinically as an atypical nevus that generated a pretest histopathologic differential diagnosis including dysplastic nevus, superficial spreading melanoma, and melanoma arising within a nevus (Table 3; Figure, A and B). The gene expression test result was benign (score, −5.4), and the final pathology report diagnosis was nevus with junctional dysplasia, moderate. Surgical excision was performed at the patient’s first return visit, 505 days after initial diagnosis, with moderately dysplastic nevus as the recorded indication for removal. No repigmentation or other evidence of local recurrence or progression was detected, and the treating dermatologist indicated no suspicion that the original diagnosis of benign nevus was incorrect during the 23-month follow-up period.
The second excision (case 2) involved a 27-year-old woman with a pigmented neoplasm on the mid upper back (Figure, C and D) biopsied to rule out dysplastic nevus that resulted in a pretest histopathologic differential diagnosis of dysplastic nevus vs superficial spreading melanoma or melanoma arising within a nevus. The gene expression test result classified the lesion as benign (score, −2.9), and the final pathology diagnosis was nevus, compound, with moderate dysplasia. Despite the benign diagnosis, residual neoplasm (or pigmentation) at the biopsy site prompted the patient to request excision at her first postbiopsy visit, 22 days after testing (Table 3). The CRF completed by the dermatologist reported no indication that the benign diagnosis was inaccurate, but the patient was subsequently lost to follow-up.
The third excision (case 3) involved a 32-year-old woman with a pigmented lesion on the abdomen (Table 3; Figure, E and F). The clinical description was irregular-appearing black papule, nevus with atypia, and the histopathologic differential diagnosis again included dysplastic nevus, superficial spreading melanoma, and melanoma arising within a preexisting nevus. The gene expression signature result was benign (score, −7.2), and the final diagnosis issued within the accompanying pathology report was nevus with moderate junctional dysplasia. Despite the benign diagnosis, excision was performed 89 days after test result availability, with apparent residual pigmentation as the specified indication. As with the other 2 cases, the treating dermatologist confirmed that neither clinical features nor follow-up events suggested malignancy.
Comment
This study followed a cohort of 25 patients with histopathologically ambiguous melanocytic neoplasms that were classified as benign by a diagnostic gene expression test with the intent of determining the outcomes of patients whose treatment aligned with their benign test result. All patients initially were managed according to their test result. During an average posttest clinical follow-up time of more than 3 years (38.5 months), the 25 biopsied lesions, most of which received a differential diagnosis of dysplastic nevus, were regarded as benign nevi by their dermatologists, and the vast majority (88%) received no further surgical intervention. Three patients underwent subsequent excision of the biopsied lesion, with patient or physician preference as the indication in each instance. None of the 25 patients developed evidence of local recurrence, metastasis, or other findings that prompted doubt of the benign diagnosis. The absence of adverse events during clinical follow-up, particularly given that most lesions were not subjected to further intervention, supports use of the gene expression test as a safe and effective adjunct to the diagnosis and treatment of ambiguous melanocytic neoplasms by dermatologists and dermatopathologists.
Ambiguous melanocytic neoplasms evaluated without the aid of molecular adjuncts often result in equivocal or less-than-definitive diagnoses, and further surgical intervention is commonly undertaken to mitigate against the possibility of a missed melanoma.13 In this study, treatment that was aligned with the benign test result allowed most patients to avoid further surgical intervention, which suggests that adjunctive use of the gene signature can contribute to reductions in the physical and economic burdens imposed by unnecessary surgical interventions.15,16 Moreover, any means of increasing accurate and definitive diagnoses may produce an immediate impact on health outcomes by reducing the anxiety that uncertainty often provokes in patients and health care providers alike.
Study Limitations
This study must be interpreted within the context of its limitations. Obtaining meaningful patient outcome data is a common challenge in health care research due to the requisite length of follow-up and sometimes the lack of definitive evidence of adverse events. This is particularly difficult for melanocytic neoplasms because of an apparent inclination for patients with benign diagnoses to abandon follow-up and an increasing tendency for even minimal diagnostic uncertainty to prompt complete excision. Additionally, the only definitive clinical outcome for melanocytic neoplasms is distant metastasis, which (fortunately for patients) is relatively rare. Not surprisingly, studies documenting clinical outcomes of patients with ambiguous melanocytic neoplasms tested prospectively with diagnostic adjuncts are scarce, and this study’s sample size and clinical follow-up compare favorably with the few that exist.17,18 Although most melanomas declare themselves through recurrence or metastasis within several years of initial biopsy,1,19 some are clinically dormant for as long as 10 years after initial detection.20,21 This may be particularly true for the small or early-stage lesions that now comprise the majority of biopsied neoplasms, and such events would go undetected by this study and many others. It also must be recognized that uneventful follow-up, regardless of duration, cannot prove that a biopsied melanocytic neoplasm was benign. Although only 5 patients had a follow-up time of less than 2 years (the time frame in which most recurrence or metastasis will occur), it cannot be definitively proven that a minimum of 2 years recurrence- or metastasis-free survival indicates a benign lesion. Many early-stage malignant melanomas are eradicated by complete excision or even by the initial biopsy if margins are uninvolved.
Because these limitations are intrinsic to melanocytic neoplasms and current management strategies, they pertain to all investigations seeking insights into biological potential through clinical outcomes. Similarly, all current diagnostic tools and procedures have the potential for sampling error, including histopathology. The rarity of adverse outcomes (recurrence and metastasis) in patients with benign test results within this cohort indicates that false-negative results are uncommon, which is further evidenced by a similar rarity of adverse events in prior studies of the gene expression signature.8-10,22 A particular strength of this study is that most of the ambiguous melanocytic neoplasms followed did not undergo excision after the initial biopsy, an increasingly uncommon situation that may increase their likelihood to be informative.
It must be emphasized that the gene expression test, similar to other diagnostic adjuncts, is neither a replacement for histopathologic interpretation nor a substitute for judgment. As with all tests, it can produce false-positive and false-negative results. Therefore, it should always be interpreted within the constellation of the many other data points that must be considered when making a distinction between benign nevus and malignant melanoma, including but not limited to patient age, family and personal history of melanoma, anatomic location, clinical features, and histopathologic findings. As is the case for many diseases, careful consideration of all relevant input is necessary to minimize the risk of misdiagnosis that might occur should any single data point prove inaccurate, including the results of adjunctive molecular tests.
Conclusion
Ancillary methods are emerging as useful tools for the diagnostic evaluation of melanocytic neoplasms that cannot be assigned definitive diagnoses using traditional techniques alone. This study suggests that patients with ambiguous melanocytic neoplasms may benefit from diagnoses and treatment decisions aligned with the results of a gene expression test, and that for those with a benign result, simple observation may be a safe alternative to surgical excision. This expands upon prior observations of the test’s influence on diagnoses and treatment decisions and supports its role as part of dermatopathologists’ and dermatologists’ decision-making process for histopathologically ambiguous melanocytic lesions.
- Noone AM, Howlander N, Krapcho M, et al, eds. SEER Cancer Statistics Review, 1975-2015. National Cancer Institute website. Updated September 10, 2018. Accessed April 21, 2021. https://seer.cancer.gov/archive/csr/1975_2015/
- Shoo BA, Sagebiel RW, Kashani-Sabet M. Discordance in the histopathologic diagnosis of melanoma at a melanoma referral center. J Am Acad Dermatol. 2010;62:751-756.
- Veenhuizen KC, De Wit PE, Mooi WJ, et al. Quality assessment by expert opinion in melanoma pathology: experience of the pathology panel of the Dutch Melanoma Working Party. J Pathol. 1997;182:266-272.
- Elmore JG, Barnhill RL, Elder DE, et al. Pathologists’ diagnosis of invasive melanoma and melanocytic proliferations: observer accuracy and reproducibility study. BMJ. 2017;357:j2813. doi:10.1136/bmj.j2813
- Glusac EJ. The melanoma ‘epidemic’, a dermatopathologist’s perspective. J Cutan Pathol. 2011;38:264-267.
- Welch HG, Woloshin S, Schwartz LM. Skin biopsy rates and incidence of melanoma: population based ecological study. BMJ. 2005;331:481.
- Swerlick RA, Chen S. The melanoma epidemic. Is increased surveillance the solution or the problem? Arch Dermatol. 1996;132:881-884.
- Ko JS, Matharoo-Ball B, Billings SD, et al. Diagnostic distinction of malignant melanoma and benign nevi by a gene expression signature and correlation to clinical outcomes. Cancer Epidemiol Biomarkers Prev. 2017;26:1107-1113.
- Clarke LE, Flake DD 2nd, Busam K, et al. An independent validation of a gene expression signature to differentiate malignant melanoma from benign melanocytic nevi. Cancer. 2017;123:617-628.
- Clarke LE, Warf BM, Flake DD 2nd, et al. Clinical validation of a gene expression signature that differentiates benign nevi from malignant melanoma. J Cutan Pathol. 2015;42:244-252.
- Minca EC, Al-Rohil RN, Wang M, et al. Comparison between melanoma gene expression score and fluorescence in situ hybridization for the classification of melanocytic lesions. Mod Pathol. 2016;29:832-843.
- Cockerell CJ, Tschen J, Evans B, et al. The influence of a gene expression signature on the diagnosis and recommended treatment of melanocytic tumors by dermatopathologists. Medicine (Baltimore). 2016;95:e4887. doi:10.1097/MD.0000000000004887
- Cockerell C, Tschen J, Billings SD, et al. The influence of a gene-expression signature on the treatment of diagnostically challenging melanocytic lesions. Per Med. 2017;14:123-130.
- Warf MB, Flake DD 2nd, Adams D, et al. Analytical validation of a melanoma diagnostic gene signature using formalin-fixed paraffin-embedded melanocytic lesions. Biomark Med. 2015;9:407-416.
- Guy GP Jr, Ekwueme DU, Tangka FK, et al. Melanoma treatment costs: a systematic review of the literature, 1990-2011. Am J Prev Med. 2012;43:537-545.
- Guy GP Jr, Machlin SR, Ekwueme DU, et al. Prevalence and costs of skin cancer treatment in the U.S., 2002-2006 and 2007-2011. Am J Prev Med. 2015;48:183-187.
- Egnatios GL, Ferringer TC. Clinical follow-up of atypical spitzoid tumors analyzed by fluorescence in situ hybridization. Am J Dermatopathol. 2016;38:289-296.
- Fischer AS, High WA. The difficulty in interpreting gene expression profiling in BAP-negative melanocytic tumors. J Cutan Pathol. 2018;45:659-666. doi:10.1111/cup.13277
- Vollmer RT. The dynamics of death in melanoma. J Cutan Pathol. 2012;39:1075-1082.
- Osella-Abate S, Ribero S, Sanlorenzo M, et al. Risk factors related to late metastases in 1,372 melanoma patients disease free more than 10 years. Int J Cancer. 2015;136:2453-2457.
- Faries MB, Steen S, Ye X, et al. Late recurrence in melanoma: clinical implications of lost dormancy. J Am Coll Surg. 2013;217:27-34.
- Ko JS, Clarke LE, Minca EC, et al. Correlation of melanoma gene expression score with clinical outcomes on a series of melanocytic lesions. Hum Pathol. 2019;86:213-221.
According to National Institutes of Health estimates, more than 90,000 new cases of melanoma were diagnosed in 2018.1 Overall 5-year survival for patients with melanoma exceeds 90%, but individual survival estimates are highly dependent on stage at diagnosis, and survival decreases markedly with metastasis. Therefore, early and accurate diagnosis is critical.
Diagnosis of melanocytic neoplasms usually is performed by dermatopathologists through microscopic examination of stained tissue biopsy sections, a technically simple and effective method that enables a definitive diagnosis of benign nevus or malignant melanoma to be made in most cases. However, approximately 15% of all biopsied melanocytic lesions will exhibit some degree of histopathologic ambiguity,2-4 meaning that some of their microscopic features will be characteristic of a benign nevus while others will suggest the possibility of malignant melanoma. Diagnostic interpretations often vary in these cases, even among experts, and a definitive diagnosis of benign or malignant may be difficult to achieve by microscopy alone.2-4 Because of the marked reduction in survival once a melanoma has metastasized, these diagnostically ambiguous lesions often are treated as possible malignant melanomas with complete surgical excision (or re-excision). However, some experts suggest that many histopathologically ambiguous melanocytic neoplasms are, in fact, benign,5 a notion supported by epidemiologic evidence.6,7 Therefore, excision of many ambiguous melanocytic neoplasms might be avoided if definitive diagnosis could be achieved.
A gene expression signature was developed and validated for use as an adjunct to traditional methods of differentiating malignant melanocytic neoplasms from their benign counterparts.8-11 This test quantifies the RNA transcripts produced by 14 genes known to be overexpressed in malignant melanomas by comparison to benign nevi. These values are then combined algorithmically with measurements of 9 reference genes to produce an objective numerical score that is classified as benign, malignant, or indeterminate. When used by board-certified dermatopathologists and dermatologists confronting ambiguous melanocytic lesions, the test produces substantial increases in definitive diagnoses and prompts changes in treatment recommendations.12,13 However, the long-term consequences of foregoing surgical excision of melanocytic neoplasms that are diagnostically ambiguous but classified as benign by this test have not yet been formally assessed. In the current study, prospectively tested patients whose ambiguous melanocytic neoplasms were classified as benign by the gene expression signature were followed for up to 4.5 years to evaluate the long-term safety of treatment decisions aligned with benign test results.
Methods
Study Population
As part of a prior study,12 US-based dermatopathologists submitted tissue sections from biopsied melanocytic neoplasms determined to be diagnostically ambiguous by histopathology for analysis with the gene expression signature (Myriad Genetics, Inc). Diagnostically ambiguous lesions were those lesions that were described as ambiguous, uncertain, equivocal, indeterminate, or other synonymous terms by the submitting dermatopathologist and therefore lacked a confident diagnosis of benign or malignant prior to testing. Patients initially were tested between May 2014 and August 2014, with samples submitted through a prospective clinical experience study designed to assess the impact of the test on diagnosis and treatment decisions. This study was performed under an institutional review board waiver of consent (Quorum #33403/1).
Patients were eligible for inclusion in the current study if their biopsy specimens (1) had an uncertain preliminary diagnosis according to the submitting dermatopathologist (pretest diagnosis of indeterminate); (2) received a negative (benign) score from the gene expression test; (3) were treated as benign by the dermatologist(s) involved in follow-up care; and (4) were submitted by a single site (St. Joseph Medical Center, Houston, Texas). Although a single dermatopathology site was used for this study, multiple dermatologists were involved in the final treatment of these patients. Patients with benign scores who received additional intervention were excluded, as they may have a lower rate of adverse events (ie, metastasis) than those who did not receive intervention and would therefore skew the analysis population. A total of 25 patients from the prior study met these inclusion criteria. The previously collected12 pretest and posttest de-identified data were compiled from the commercial laboratory databases, and the patients were followed from the time of testing via medical record review performed by the dermatology providers at participating sites. Clinical follow-up data were collected using study-specific case report forms (CRFs) that captured the following: (1) the dates and results of clinical follow-up visits; (2) the type(s) of treatment and interventions (if any) performed at those visits; (3) the specific indication for any intervention performed; (4) any evidence of persistent, locally recurrent, and/or distant melanocytic neoplasia (whether definitively attributable to the tested lesion or not); and (5) death from any cause. The CRF assigned interventions to 1 of 5 categories: excision, excision with sentinel lymph node biopsy, referral to dermatologic or other surgeon, examination only (without surgical intervention), and other. Selection of other required a free-text description of the treatment and indications. Pertinent information not otherwise captured by the CRF also was recordable as free text.
Gene Expression Testing
Gene expression testing was carried out at the time of specimen submission in the prior study12 as described previously.14 Briefly, formalin-fixed, paraffin-embedded, unstained tissue sections and/or tissue blocks were submitted for testing along with a single hematoxylin and eosin–stained slide used to identify and designate the representative portion(s) of the lesion to be tested. These areas were macrodissected from unstained tissue sections and pooled for RNA extraction. Expression of 14 biomarker genes and 9 reference genes was measured via
Statistical Analysis
Demographic and other baseline characteristics of the patient population were summarized. Follow-up time was calculated as the interval between the date a patient’s gene expression test result was first issued to the provider and the date of the patient’s last recorded visit during the study period. All patient dermatology office visits within the designated follow-up period were documented, with a nonstandard number of visits and follow-up time across all study patients. Statistical analyses were conducted using SAS software (SAS Institute Inc), R software version 3.5.0 (R Foundation for Statistical Computing), and IBM SPSS Statistics software (IBM SPSS Statistics for Windows, Version 25).
Results
Patient Sample
A total of 25 ambiguous melanocytic neoplasms from 25 patients met the study inclusion criteria of a benign gene expression result with subsequent treatment as a benign neoplasm during follow-up. The patient sample statistics are summarized in Table 1. Most patients were younger than 65 years, with an average age at the time of biopsy of 48.4 years. All 25 neoplasms produced negative (benign) gene expression signature scores, all were diagnosed as benign nevi posttest by the submitting dermatopathologist, and all patients were initially treated in accordance with the benign diagnosis by the dermatologist(s) involved in clinical follow-up care. Prior to testing with the gene expression signature, most of these histopathologically indeterminate lesions received differential diagnoses, the most common of which were dysplastic nevus (84%), melanoma arising from a nevus (72%), and superficial spreading melanoma (64%; eTable). After testing with the gene expression signature and receiving a benign score, most lesions received a single differential diagnosis of dysplastic nevus (88%).
Follow-up and Survival
Clinical follow-up time ranged from 0.6 to 53.3 months, with a mean duration (SD) of 38.5 (16.6) months, and patients attended an average of 4 postbiopsy dermatology appointments (mean [SD], 4.6 [3.6]). According to the participating dermatology care providers, none of the 25 patients developed any indication during follow-up that the diagnosis of benign nevus was inaccurate. No patient had evidence of locally recurrent or metastatic melanoma, and none died during the study period.
Treatment/Interventions
The treatment recorded in the CRF was examination only for 21 of 25 patients, excision for 3, and other for 1 (Table 2). Because the explanation for the selection of other in this case described an excision performed at the same anatomic location as the biopsy, this treatment also was considered an excision for purposes of the study analyses. The 3 excisions all occurred at the first postbiopsy dermatology encounter. Across all follow-up visits, no additional surgical interventions occurred (Table 2).
The first excision (case 1) involved a 67-year-old woman with a lesion on the mid pubic region described clinically as an atypical nevus that generated a pretest histopathologic differential diagnosis including dysplastic nevus, superficial spreading melanoma, and melanoma arising within a nevus (Table 3; Figure, A and B). The gene expression test result was benign (score, −5.4), and the final pathology report diagnosis was nevus with junctional dysplasia, moderate. Surgical excision was performed at the patient’s first return visit, 505 days after initial diagnosis, with moderately dysplastic nevus as the recorded indication for removal. No repigmentation or other evidence of local recurrence or progression was detected, and the treating dermatologist indicated no suspicion that the original diagnosis of benign nevus was incorrect during the 23-month follow-up period.
The second excision (case 2) involved a 27-year-old woman with a pigmented neoplasm on the mid upper back (Figure, C and D) biopsied to rule out dysplastic nevus that resulted in a pretest histopathologic differential diagnosis of dysplastic nevus vs superficial spreading melanoma or melanoma arising within a nevus. The gene expression test result classified the lesion as benign (score, −2.9), and the final pathology diagnosis was nevus, compound, with moderate dysplasia. Despite the benign diagnosis, residual neoplasm (or pigmentation) at the biopsy site prompted the patient to request excision at her first postbiopsy visit, 22 days after testing (Table 3). The CRF completed by the dermatologist reported no indication that the benign diagnosis was inaccurate, but the patient was subsequently lost to follow-up.
The third excision (case 3) involved a 32-year-old woman with a pigmented lesion on the abdomen (Table 3; Figure, E and F). The clinical description was irregular-appearing black papule, nevus with atypia, and the histopathologic differential diagnosis again included dysplastic nevus, superficial spreading melanoma, and melanoma arising within a preexisting nevus. The gene expression signature result was benign (score, −7.2), and the final diagnosis issued within the accompanying pathology report was nevus with moderate junctional dysplasia. Despite the benign diagnosis, excision was performed 89 days after test result availability, with apparent residual pigmentation as the specified indication. As with the other 2 cases, the treating dermatologist confirmed that neither clinical features nor follow-up events suggested malignancy.
Comment
This study followed a cohort of 25 patients with histopathologically ambiguous melanocytic neoplasms that were classified as benign by a diagnostic gene expression test with the intent of determining the outcomes of patients whose treatment aligned with their benign test result. All patients initially were managed according to their test result. During an average posttest clinical follow-up time of more than 3 years (38.5 months), the 25 biopsied lesions, most of which received a differential diagnosis of dysplastic nevus, were regarded as benign nevi by their dermatologists, and the vast majority (88%) received no further surgical intervention. Three patients underwent subsequent excision of the biopsied lesion, with patient or physician preference as the indication in each instance. None of the 25 patients developed evidence of local recurrence, metastasis, or other findings that prompted doubt of the benign diagnosis. The absence of adverse events during clinical follow-up, particularly given that most lesions were not subjected to further intervention, supports use of the gene expression test as a safe and effective adjunct to the diagnosis and treatment of ambiguous melanocytic neoplasms by dermatologists and dermatopathologists.
Ambiguous melanocytic neoplasms evaluated without the aid of molecular adjuncts often result in equivocal or less-than-definitive diagnoses, and further surgical intervention is commonly undertaken to mitigate against the possibility of a missed melanoma.13 In this study, treatment that was aligned with the benign test result allowed most patients to avoid further surgical intervention, which suggests that adjunctive use of the gene signature can contribute to reductions in the physical and economic burdens imposed by unnecessary surgical interventions.15,16 Moreover, any means of increasing accurate and definitive diagnoses may produce an immediate impact on health outcomes by reducing the anxiety that uncertainty often provokes in patients and health care providers alike.
Study Limitations
This study must be interpreted within the context of its limitations. Obtaining meaningful patient outcome data is a common challenge in health care research due to the requisite length of follow-up and sometimes the lack of definitive evidence of adverse events. This is particularly difficult for melanocytic neoplasms because of an apparent inclination for patients with benign diagnoses to abandon follow-up and an increasing tendency for even minimal diagnostic uncertainty to prompt complete excision. Additionally, the only definitive clinical outcome for melanocytic neoplasms is distant metastasis, which (fortunately for patients) is relatively rare. Not surprisingly, studies documenting clinical outcomes of patients with ambiguous melanocytic neoplasms tested prospectively with diagnostic adjuncts are scarce, and this study’s sample size and clinical follow-up compare favorably with the few that exist.17,18 Although most melanomas declare themselves through recurrence or metastasis within several years of initial biopsy,1,19 some are clinically dormant for as long as 10 years after initial detection.20,21 This may be particularly true for the small or early-stage lesions that now comprise the majority of biopsied neoplasms, and such events would go undetected by this study and many others. It also must be recognized that uneventful follow-up, regardless of duration, cannot prove that a biopsied melanocytic neoplasm was benign. Although only 5 patients had a follow-up time of less than 2 years (the time frame in which most recurrence or metastasis will occur), it cannot be definitively proven that a minimum of 2 years recurrence- or metastasis-free survival indicates a benign lesion. Many early-stage malignant melanomas are eradicated by complete excision or even by the initial biopsy if margins are uninvolved.
Because these limitations are intrinsic to melanocytic neoplasms and current management strategies, they pertain to all investigations seeking insights into biological potential through clinical outcomes. Similarly, all current diagnostic tools and procedures have the potential for sampling error, including histopathology. The rarity of adverse outcomes (recurrence and metastasis) in patients with benign test results within this cohort indicates that false-negative results are uncommon, which is further evidenced by a similar rarity of adverse events in prior studies of the gene expression signature.8-10,22 A particular strength of this study is that most of the ambiguous melanocytic neoplasms followed did not undergo excision after the initial biopsy, an increasingly uncommon situation that may increase their likelihood to be informative.
It must be emphasized that the gene expression test, similar to other diagnostic adjuncts, is neither a replacement for histopathologic interpretation nor a substitute for judgment. As with all tests, it can produce false-positive and false-negative results. Therefore, it should always be interpreted within the constellation of the many other data points that must be considered when making a distinction between benign nevus and malignant melanoma, including but not limited to patient age, family and personal history of melanoma, anatomic location, clinical features, and histopathologic findings. As is the case for many diseases, careful consideration of all relevant input is necessary to minimize the risk of misdiagnosis that might occur should any single data point prove inaccurate, including the results of adjunctive molecular tests.
Conclusion
Ancillary methods are emerging as useful tools for the diagnostic evaluation of melanocytic neoplasms that cannot be assigned definitive diagnoses using traditional techniques alone. This study suggests that patients with ambiguous melanocytic neoplasms may benefit from diagnoses and treatment decisions aligned with the results of a gene expression test, and that for those with a benign result, simple observation may be a safe alternative to surgical excision. This expands upon prior observations of the test’s influence on diagnoses and treatment decisions and supports its role as part of dermatopathologists’ and dermatologists’ decision-making process for histopathologically ambiguous melanocytic lesions.
According to National Institutes of Health estimates, more than 90,000 new cases of melanoma were diagnosed in 2018.1 Overall 5-year survival for patients with melanoma exceeds 90%, but individual survival estimates are highly dependent on stage at diagnosis, and survival decreases markedly with metastasis. Therefore, early and accurate diagnosis is critical.
Diagnosis of melanocytic neoplasms usually is performed by dermatopathologists through microscopic examination of stained tissue biopsy sections, a technically simple and effective method that enables a definitive diagnosis of benign nevus or malignant melanoma to be made in most cases. However, approximately 15% of all biopsied melanocytic lesions will exhibit some degree of histopathologic ambiguity,2-4 meaning that some of their microscopic features will be characteristic of a benign nevus while others will suggest the possibility of malignant melanoma. Diagnostic interpretations often vary in these cases, even among experts, and a definitive diagnosis of benign or malignant may be difficult to achieve by microscopy alone.2-4 Because of the marked reduction in survival once a melanoma has metastasized, these diagnostically ambiguous lesions often are treated as possible malignant melanomas with complete surgical excision (or re-excision). However, some experts suggest that many histopathologically ambiguous melanocytic neoplasms are, in fact, benign,5 a notion supported by epidemiologic evidence.6,7 Therefore, excision of many ambiguous melanocytic neoplasms might be avoided if definitive diagnosis could be achieved.
A gene expression signature was developed and validated for use as an adjunct to traditional methods of differentiating malignant melanocytic neoplasms from their benign counterparts.8-11 This test quantifies the RNA transcripts produced by 14 genes known to be overexpressed in malignant melanomas by comparison to benign nevi. These values are then combined algorithmically with measurements of 9 reference genes to produce an objective numerical score that is classified as benign, malignant, or indeterminate. When used by board-certified dermatopathologists and dermatologists confronting ambiguous melanocytic lesions, the test produces substantial increases in definitive diagnoses and prompts changes in treatment recommendations.12,13 However, the long-term consequences of foregoing surgical excision of melanocytic neoplasms that are diagnostically ambiguous but classified as benign by this test have not yet been formally assessed. In the current study, prospectively tested patients whose ambiguous melanocytic neoplasms were classified as benign by the gene expression signature were followed for up to 4.5 years to evaluate the long-term safety of treatment decisions aligned with benign test results.
Methods
Study Population
As part of a prior study,12 US-based dermatopathologists submitted tissue sections from biopsied melanocytic neoplasms determined to be diagnostically ambiguous by histopathology for analysis with the gene expression signature (Myriad Genetics, Inc). Diagnostically ambiguous lesions were those lesions that were described as ambiguous, uncertain, equivocal, indeterminate, or other synonymous terms by the submitting dermatopathologist and therefore lacked a confident diagnosis of benign or malignant prior to testing. Patients initially were tested between May 2014 and August 2014, with samples submitted through a prospective clinical experience study designed to assess the impact of the test on diagnosis and treatment decisions. This study was performed under an institutional review board waiver of consent (Quorum #33403/1).
Patients were eligible for inclusion in the current study if their biopsy specimens (1) had an uncertain preliminary diagnosis according to the submitting dermatopathologist (pretest diagnosis of indeterminate); (2) received a negative (benign) score from the gene expression test; (3) were treated as benign by the dermatologist(s) involved in follow-up care; and (4) were submitted by a single site (St. Joseph Medical Center, Houston, Texas). Although a single dermatopathology site was used for this study, multiple dermatologists were involved in the final treatment of these patients. Patients with benign scores who received additional intervention were excluded, as they may have a lower rate of adverse events (ie, metastasis) than those who did not receive intervention and would therefore skew the analysis population. A total of 25 patients from the prior study met these inclusion criteria. The previously collected12 pretest and posttest de-identified data were compiled from the commercial laboratory databases, and the patients were followed from the time of testing via medical record review performed by the dermatology providers at participating sites. Clinical follow-up data were collected using study-specific case report forms (CRFs) that captured the following: (1) the dates and results of clinical follow-up visits; (2) the type(s) of treatment and interventions (if any) performed at those visits; (3) the specific indication for any intervention performed; (4) any evidence of persistent, locally recurrent, and/or distant melanocytic neoplasia (whether definitively attributable to the tested lesion or not); and (5) death from any cause. The CRF assigned interventions to 1 of 5 categories: excision, excision with sentinel lymph node biopsy, referral to dermatologic or other surgeon, examination only (without surgical intervention), and other. Selection of other required a free-text description of the treatment and indications. Pertinent information not otherwise captured by the CRF also was recordable as free text.
Gene Expression Testing
Gene expression testing was carried out at the time of specimen submission in the prior study12 as described previously.14 Briefly, formalin-fixed, paraffin-embedded, unstained tissue sections and/or tissue blocks were submitted for testing along with a single hematoxylin and eosin–stained slide used to identify and designate the representative portion(s) of the lesion to be tested. These areas were macrodissected from unstained tissue sections and pooled for RNA extraction. Expression of 14 biomarker genes and 9 reference genes was measured via
Statistical Analysis
Demographic and other baseline characteristics of the patient population were summarized. Follow-up time was calculated as the interval between the date a patient’s gene expression test result was first issued to the provider and the date of the patient’s last recorded visit during the study period. All patient dermatology office visits within the designated follow-up period were documented, with a nonstandard number of visits and follow-up time across all study patients. Statistical analyses were conducted using SAS software (SAS Institute Inc), R software version 3.5.0 (R Foundation for Statistical Computing), and IBM SPSS Statistics software (IBM SPSS Statistics for Windows, Version 25).
Results
Patient Sample
A total of 25 ambiguous melanocytic neoplasms from 25 patients met the study inclusion criteria of a benign gene expression result with subsequent treatment as a benign neoplasm during follow-up. The patient sample statistics are summarized in Table 1. Most patients were younger than 65 years, with an average age at the time of biopsy of 48.4 years. All 25 neoplasms produced negative (benign) gene expression signature scores, all were diagnosed as benign nevi posttest by the submitting dermatopathologist, and all patients were initially treated in accordance with the benign diagnosis by the dermatologist(s) involved in clinical follow-up care. Prior to testing with the gene expression signature, most of these histopathologically indeterminate lesions received differential diagnoses, the most common of which were dysplastic nevus (84%), melanoma arising from a nevus (72%), and superficial spreading melanoma (64%; eTable). After testing with the gene expression signature and receiving a benign score, most lesions received a single differential diagnosis of dysplastic nevus (88%).
Follow-up and Survival
Clinical follow-up time ranged from 0.6 to 53.3 months, with a mean duration (SD) of 38.5 (16.6) months, and patients attended an average of 4 postbiopsy dermatology appointments (mean [SD], 4.6 [3.6]). According to the participating dermatology care providers, none of the 25 patients developed any indication during follow-up that the diagnosis of benign nevus was inaccurate. No patient had evidence of locally recurrent or metastatic melanoma, and none died during the study period.
Treatment/Interventions
The treatment recorded in the CRF was examination only for 21 of 25 patients, excision for 3, and other for 1 (Table 2). Because the explanation for the selection of other in this case described an excision performed at the same anatomic location as the biopsy, this treatment also was considered an excision for purposes of the study analyses. The 3 excisions all occurred at the first postbiopsy dermatology encounter. Across all follow-up visits, no additional surgical interventions occurred (Table 2).
The first excision (case 1) involved a 67-year-old woman with a lesion on the mid pubic region described clinically as an atypical nevus that generated a pretest histopathologic differential diagnosis including dysplastic nevus, superficial spreading melanoma, and melanoma arising within a nevus (Table 3; Figure, A and B). The gene expression test result was benign (score, −5.4), and the final pathology report diagnosis was nevus with junctional dysplasia, moderate. Surgical excision was performed at the patient’s first return visit, 505 days after initial diagnosis, with moderately dysplastic nevus as the recorded indication for removal. No repigmentation or other evidence of local recurrence or progression was detected, and the treating dermatologist indicated no suspicion that the original diagnosis of benign nevus was incorrect during the 23-month follow-up period.
The second excision (case 2) involved a 27-year-old woman with a pigmented neoplasm on the mid upper back (Figure, C and D) biopsied to rule out dysplastic nevus that resulted in a pretest histopathologic differential diagnosis of dysplastic nevus vs superficial spreading melanoma or melanoma arising within a nevus. The gene expression test result classified the lesion as benign (score, −2.9), and the final pathology diagnosis was nevus, compound, with moderate dysplasia. Despite the benign diagnosis, residual neoplasm (or pigmentation) at the biopsy site prompted the patient to request excision at her first postbiopsy visit, 22 days after testing (Table 3). The CRF completed by the dermatologist reported no indication that the benign diagnosis was inaccurate, but the patient was subsequently lost to follow-up.
The third excision (case 3) involved a 32-year-old woman with a pigmented lesion on the abdomen (Table 3; Figure, E and F). The clinical description was irregular-appearing black papule, nevus with atypia, and the histopathologic differential diagnosis again included dysplastic nevus, superficial spreading melanoma, and melanoma arising within a preexisting nevus. The gene expression signature result was benign (score, −7.2), and the final diagnosis issued within the accompanying pathology report was nevus with moderate junctional dysplasia. Despite the benign diagnosis, excision was performed 89 days after test result availability, with apparent residual pigmentation as the specified indication. As with the other 2 cases, the treating dermatologist confirmed that neither clinical features nor follow-up events suggested malignancy.
Comment
This study followed a cohort of 25 patients with histopathologically ambiguous melanocytic neoplasms that were classified as benign by a diagnostic gene expression test with the intent of determining the outcomes of patients whose treatment aligned with their benign test result. All patients initially were managed according to their test result. During an average posttest clinical follow-up time of more than 3 years (38.5 months), the 25 biopsied lesions, most of which received a differential diagnosis of dysplastic nevus, were regarded as benign nevi by their dermatologists, and the vast majority (88%) received no further surgical intervention. Three patients underwent subsequent excision of the biopsied lesion, with patient or physician preference as the indication in each instance. None of the 25 patients developed evidence of local recurrence, metastasis, or other findings that prompted doubt of the benign diagnosis. The absence of adverse events during clinical follow-up, particularly given that most lesions were not subjected to further intervention, supports use of the gene expression test as a safe and effective adjunct to the diagnosis and treatment of ambiguous melanocytic neoplasms by dermatologists and dermatopathologists.
Ambiguous melanocytic neoplasms evaluated without the aid of molecular adjuncts often result in equivocal or less-than-definitive diagnoses, and further surgical intervention is commonly undertaken to mitigate against the possibility of a missed melanoma.13 In this study, treatment that was aligned with the benign test result allowed most patients to avoid further surgical intervention, which suggests that adjunctive use of the gene signature can contribute to reductions in the physical and economic burdens imposed by unnecessary surgical interventions.15,16 Moreover, any means of increasing accurate and definitive diagnoses may produce an immediate impact on health outcomes by reducing the anxiety that uncertainty often provokes in patients and health care providers alike.
Study Limitations
This study must be interpreted within the context of its limitations. Obtaining meaningful patient outcome data is a common challenge in health care research due to the requisite length of follow-up and sometimes the lack of definitive evidence of adverse events. This is particularly difficult for melanocytic neoplasms because of an apparent inclination for patients with benign diagnoses to abandon follow-up and an increasing tendency for even minimal diagnostic uncertainty to prompt complete excision. Additionally, the only definitive clinical outcome for melanocytic neoplasms is distant metastasis, which (fortunately for patients) is relatively rare. Not surprisingly, studies documenting clinical outcomes of patients with ambiguous melanocytic neoplasms tested prospectively with diagnostic adjuncts are scarce, and this study’s sample size and clinical follow-up compare favorably with the few that exist.17,18 Although most melanomas declare themselves through recurrence or metastasis within several years of initial biopsy,1,19 some are clinically dormant for as long as 10 years after initial detection.20,21 This may be particularly true for the small or early-stage lesions that now comprise the majority of biopsied neoplasms, and such events would go undetected by this study and many others. It also must be recognized that uneventful follow-up, regardless of duration, cannot prove that a biopsied melanocytic neoplasm was benign. Although only 5 patients had a follow-up time of less than 2 years (the time frame in which most recurrence or metastasis will occur), it cannot be definitively proven that a minimum of 2 years recurrence- or metastasis-free survival indicates a benign lesion. Many early-stage malignant melanomas are eradicated by complete excision or even by the initial biopsy if margins are uninvolved.
Because these limitations are intrinsic to melanocytic neoplasms and current management strategies, they pertain to all investigations seeking insights into biological potential through clinical outcomes. Similarly, all current diagnostic tools and procedures have the potential for sampling error, including histopathology. The rarity of adverse outcomes (recurrence and metastasis) in patients with benign test results within this cohort indicates that false-negative results are uncommon, which is further evidenced by a similar rarity of adverse events in prior studies of the gene expression signature.8-10,22 A particular strength of this study is that most of the ambiguous melanocytic neoplasms followed did not undergo excision after the initial biopsy, an increasingly uncommon situation that may increase their likelihood to be informative.
It must be emphasized that the gene expression test, similar to other diagnostic adjuncts, is neither a replacement for histopathologic interpretation nor a substitute for judgment. As with all tests, it can produce false-positive and false-negative results. Therefore, it should always be interpreted within the constellation of the many other data points that must be considered when making a distinction between benign nevus and malignant melanoma, including but not limited to patient age, family and personal history of melanoma, anatomic location, clinical features, and histopathologic findings. As is the case for many diseases, careful consideration of all relevant input is necessary to minimize the risk of misdiagnosis that might occur should any single data point prove inaccurate, including the results of adjunctive molecular tests.
Conclusion
Ancillary methods are emerging as useful tools for the diagnostic evaluation of melanocytic neoplasms that cannot be assigned definitive diagnoses using traditional techniques alone. This study suggests that patients with ambiguous melanocytic neoplasms may benefit from diagnoses and treatment decisions aligned with the results of a gene expression test, and that for those with a benign result, simple observation may be a safe alternative to surgical excision. This expands upon prior observations of the test’s influence on diagnoses and treatment decisions and supports its role as part of dermatopathologists’ and dermatologists’ decision-making process for histopathologically ambiguous melanocytic lesions.
- Noone AM, Howlander N, Krapcho M, et al, eds. SEER Cancer Statistics Review, 1975-2015. National Cancer Institute website. Updated September 10, 2018. Accessed April 21, 2021. https://seer.cancer.gov/archive/csr/1975_2015/
- Shoo BA, Sagebiel RW, Kashani-Sabet M. Discordance in the histopathologic diagnosis of melanoma at a melanoma referral center. J Am Acad Dermatol. 2010;62:751-756.
- Veenhuizen KC, De Wit PE, Mooi WJ, et al. Quality assessment by expert opinion in melanoma pathology: experience of the pathology panel of the Dutch Melanoma Working Party. J Pathol. 1997;182:266-272.
- Elmore JG, Barnhill RL, Elder DE, et al. Pathologists’ diagnosis of invasive melanoma and melanocytic proliferations: observer accuracy and reproducibility study. BMJ. 2017;357:j2813. doi:10.1136/bmj.j2813
- Glusac EJ. The melanoma ‘epidemic’, a dermatopathologist’s perspective. J Cutan Pathol. 2011;38:264-267.
- Welch HG, Woloshin S, Schwartz LM. Skin biopsy rates and incidence of melanoma: population based ecological study. BMJ. 2005;331:481.
- Swerlick RA, Chen S. The melanoma epidemic. Is increased surveillance the solution or the problem? Arch Dermatol. 1996;132:881-884.
- Ko JS, Matharoo-Ball B, Billings SD, et al. Diagnostic distinction of malignant melanoma and benign nevi by a gene expression signature and correlation to clinical outcomes. Cancer Epidemiol Biomarkers Prev. 2017;26:1107-1113.
- Clarke LE, Flake DD 2nd, Busam K, et al. An independent validation of a gene expression signature to differentiate malignant melanoma from benign melanocytic nevi. Cancer. 2017;123:617-628.
- Clarke LE, Warf BM, Flake DD 2nd, et al. Clinical validation of a gene expression signature that differentiates benign nevi from malignant melanoma. J Cutan Pathol. 2015;42:244-252.
- Minca EC, Al-Rohil RN, Wang M, et al. Comparison between melanoma gene expression score and fluorescence in situ hybridization for the classification of melanocytic lesions. Mod Pathol. 2016;29:832-843.
- Cockerell CJ, Tschen J, Evans B, et al. The influence of a gene expression signature on the diagnosis and recommended treatment of melanocytic tumors by dermatopathologists. Medicine (Baltimore). 2016;95:e4887. doi:10.1097/MD.0000000000004887
- Cockerell C, Tschen J, Billings SD, et al. The influence of a gene-expression signature on the treatment of diagnostically challenging melanocytic lesions. Per Med. 2017;14:123-130.
- Warf MB, Flake DD 2nd, Adams D, et al. Analytical validation of a melanoma diagnostic gene signature using formalin-fixed paraffin-embedded melanocytic lesions. Biomark Med. 2015;9:407-416.
- Guy GP Jr, Ekwueme DU, Tangka FK, et al. Melanoma treatment costs: a systematic review of the literature, 1990-2011. Am J Prev Med. 2012;43:537-545.
- Guy GP Jr, Machlin SR, Ekwueme DU, et al. Prevalence and costs of skin cancer treatment in the U.S., 2002-2006 and 2007-2011. Am J Prev Med. 2015;48:183-187.
- Egnatios GL, Ferringer TC. Clinical follow-up of atypical spitzoid tumors analyzed by fluorescence in situ hybridization. Am J Dermatopathol. 2016;38:289-296.
- Fischer AS, High WA. The difficulty in interpreting gene expression profiling in BAP-negative melanocytic tumors. J Cutan Pathol. 2018;45:659-666. doi:10.1111/cup.13277
- Vollmer RT. The dynamics of death in melanoma. J Cutan Pathol. 2012;39:1075-1082.
- Osella-Abate S, Ribero S, Sanlorenzo M, et al. Risk factors related to late metastases in 1,372 melanoma patients disease free more than 10 years. Int J Cancer. 2015;136:2453-2457.
- Faries MB, Steen S, Ye X, et al. Late recurrence in melanoma: clinical implications of lost dormancy. J Am Coll Surg. 2013;217:27-34.
- Ko JS, Clarke LE, Minca EC, et al. Correlation of melanoma gene expression score with clinical outcomes on a series of melanocytic lesions. Hum Pathol. 2019;86:213-221.
- Noone AM, Howlander N, Krapcho M, et al, eds. SEER Cancer Statistics Review, 1975-2015. National Cancer Institute website. Updated September 10, 2018. Accessed April 21, 2021. https://seer.cancer.gov/archive/csr/1975_2015/
- Shoo BA, Sagebiel RW, Kashani-Sabet M. Discordance in the histopathologic diagnosis of melanoma at a melanoma referral center. J Am Acad Dermatol. 2010;62:751-756.
- Veenhuizen KC, De Wit PE, Mooi WJ, et al. Quality assessment by expert opinion in melanoma pathology: experience of the pathology panel of the Dutch Melanoma Working Party. J Pathol. 1997;182:266-272.
- Elmore JG, Barnhill RL, Elder DE, et al. Pathologists’ diagnosis of invasive melanoma and melanocytic proliferations: observer accuracy and reproducibility study. BMJ. 2017;357:j2813. doi:10.1136/bmj.j2813
- Glusac EJ. The melanoma ‘epidemic’, a dermatopathologist’s perspective. J Cutan Pathol. 2011;38:264-267.
- Welch HG, Woloshin S, Schwartz LM. Skin biopsy rates and incidence of melanoma: population based ecological study. BMJ. 2005;331:481.
- Swerlick RA, Chen S. The melanoma epidemic. Is increased surveillance the solution or the problem? Arch Dermatol. 1996;132:881-884.
- Ko JS, Matharoo-Ball B, Billings SD, et al. Diagnostic distinction of malignant melanoma and benign nevi by a gene expression signature and correlation to clinical outcomes. Cancer Epidemiol Biomarkers Prev. 2017;26:1107-1113.
- Clarke LE, Flake DD 2nd, Busam K, et al. An independent validation of a gene expression signature to differentiate malignant melanoma from benign melanocytic nevi. Cancer. 2017;123:617-628.
- Clarke LE, Warf BM, Flake DD 2nd, et al. Clinical validation of a gene expression signature that differentiates benign nevi from malignant melanoma. J Cutan Pathol. 2015;42:244-252.
- Minca EC, Al-Rohil RN, Wang M, et al. Comparison between melanoma gene expression score and fluorescence in situ hybridization for the classification of melanocytic lesions. Mod Pathol. 2016;29:832-843.
- Cockerell CJ, Tschen J, Evans B, et al. The influence of a gene expression signature on the diagnosis and recommended treatment of melanocytic tumors by dermatopathologists. Medicine (Baltimore). 2016;95:e4887. doi:10.1097/MD.0000000000004887
- Cockerell C, Tschen J, Billings SD, et al. The influence of a gene-expression signature on the treatment of diagnostically challenging melanocytic lesions. Per Med. 2017;14:123-130.
- Warf MB, Flake DD 2nd, Adams D, et al. Analytical validation of a melanoma diagnostic gene signature using formalin-fixed paraffin-embedded melanocytic lesions. Biomark Med. 2015;9:407-416.
- Guy GP Jr, Ekwueme DU, Tangka FK, et al. Melanoma treatment costs: a systematic review of the literature, 1990-2011. Am J Prev Med. 2012;43:537-545.
- Guy GP Jr, Machlin SR, Ekwueme DU, et al. Prevalence and costs of skin cancer treatment in the U.S., 2002-2006 and 2007-2011. Am J Prev Med. 2015;48:183-187.
- Egnatios GL, Ferringer TC. Clinical follow-up of atypical spitzoid tumors analyzed by fluorescence in situ hybridization. Am J Dermatopathol. 2016;38:289-296.
- Fischer AS, High WA. The difficulty in interpreting gene expression profiling in BAP-negative melanocytic tumors. J Cutan Pathol. 2018;45:659-666. doi:10.1111/cup.13277
- Vollmer RT. The dynamics of death in melanoma. J Cutan Pathol. 2012;39:1075-1082.
- Osella-Abate S, Ribero S, Sanlorenzo M, et al. Risk factors related to late metastases in 1,372 melanoma patients disease free more than 10 years. Int J Cancer. 2015;136:2453-2457.
- Faries MB, Steen S, Ye X, et al. Late recurrence in melanoma: clinical implications of lost dormancy. J Am Coll Surg. 2013;217:27-34.
- Ko JS, Clarke LE, Minca EC, et al. Correlation of melanoma gene expression score with clinical outcomes on a series of melanocytic lesions. Hum Pathol. 2019;86:213-221.
Practice Point
- Implementation of a gene expression signature in the diagnosis of histopathologically ambiguous lesions can safely increase diagnostic accuracy and optimize treatment.
Tanning Attitudes and Behaviors in Adolescents and Young Adults
Intentional tanning—through sun exposure and tanning beds—is an easily avoidable contributor to skin cancer development and an important area for public education. Since the advent of social media, a correlation between social media use and increased indoor tanning behaviors has been reported.1 In 2010, 11.3% of US adults aged 18 to 29 years reported using a tanning bed in the last 12 months.2 The American Academy of Dermatology first published their “Position Statement on Indoor Tanning” in 1998, endorsing a ban on the sale of indoor tanning equipment for nonmedical purposes.3
Although there has been no outright ban on indoor tanning, regulations have been put in place in many states—including Texas, where (as of 2013) a person younger than 18 years must have written consent from their parent(s) to use a tanning bed. Despite efforts of organizations including the American Academy of Dermatology and the government to educate the public on skin cancer prevention and sun safety, the skin cancer rate has been steadily increasing over the last 20 years.
There is a constant campaign among dermatologists to educate their patients on how to reduce or avoid the risk for skin cancer, including the use of sunscreen and avoidance of tanning. Adolescents and young adults are an especially important demographic to reach and educate because increased UV light exposure during these years leads to a greatly increased risk for skin cancer later in life.4 Data on the overall prevalence of tanning and the demographics of participation in tanning activities are important to capture and can be used to efficiently target higher-risk populations.
In this study, we aimed to investigate the attitudes and behaviors of adolescents and young adults regarding sun protection and tanning. We also aimed to determine which avenues, including social media, would be most effective at educating about skin cancer awareness and sun protection to the higher-risk younger population.
Materials and Methods
We developed an institutional review board–approved protocol for the prospective collection of data from registered patients at the dermatology clinic of the Mays Cancer Center at the University of Texas Health at San Antonio. A paper survey containing 15 rating-scale questions was administered to 60 patients aged 13 to 27 years. Surveys were administered during intake, prior to the patients’ visit with a dermatologist; all visits were of a functional (not cosmetic) nature. Data collection spanned June to August 2018. Survey results were entered into Research Electronic Data Capture (REDCap) software for qualitative analysis.
Results
Sixty patients responded to the survey. The mean age of respondents was 19.5 years. No surveys were excluded from the data set. Table 1 provides baseline characteristics of respondents. Some respondents left questions unanswered, resulting in questions with fewer than 60 responses.
Among respondents to the survey, 70% (42/60) reported it is very important to protect their skin from sun exposure, and 30% (18/60) reported it is somewhat important. Regarding sunscreen use, 70% (42/60) indicated they use sunscreen only before outdoor activities, 12% (7/60) use sunscreen daily, and 17% (10/60) never use sunscreen. Of those who use sunscreen, 52% (28/54) do so to prevent skin damage and aging and 44% (24/54) to prevent skin cancer. Twenty-three percent (13/56) of respondents reported finding tanned skin attractive; 26% (14/55) reported wanting to be tan. Looking at race, 28% (10/36) of Whites, 25% (5/20) of Spanish/Hispanic/Latinos, and 22% (2/9) of Asians found tanned skin attractive; no Black respondents found tanned skin attractive.
Regarding tanning, 12% (7/57) reported using a tanning bed in their lifetime and 4% (2/57) in the last year; 34% (19/56) reported deliberately tanning outdoors; and 9% (5/56) reported using sunless or spray-on tanning. Dermatologists (75% [42/56]), primary care physicians (69.6% [39/56]), and parents (46.4% [26/56]) were perceived as more effective sources of skin care education; among media modalities, television (33.9% [19/56]), Instagram (30.4% [17/60]), and YouTube (23.2% [13/60]) were perceived as more effective sources of skin care education (Table 2).
Comment
Perceptions of Tanning
Almost one-quarter of respondents found tanned skin attractive, which might reflect a shift from prior generations. Compared to the 11% of respondents in the 2010 survey,2 only 3.5% (2/57) of our respondents reported using a tanning bed in the last year, which could reflect the results of recent Texas legislation restricting the use of tanning beds by adolescents.
An alarming number of respondents reported going outdoors with the intention of tanning; although it appears that indoor tanning education has been successful, this finding shows that there is still a need for sun protection education because outdoor tanning is not a suitable alternative. A small number of respondents reported getting a sunless or spray-on tan, which is a risk-free alternative to indoor tanning.
Despite all respondents stating that protecting skin from the sun is important, most respondents surveyed do not use sunscreen daily. More respondents use sunscreen to prevent damage and aging than to prevent skin cancer. Young people might be more alarmed by the threat of early aging and losing their “youthful appearance” than by the possibility of developing skin cancer in the distant future. This discrepancy might indicate a lack of knowledge and be an important focus for future education efforts.
Perceptions of Trustworthiness of Education Sources
Our findings show dermatologists and primary care physicians are important educators on skin protection. Primary care physicians should remain vigilant to recognize at-risk patients who would benefit from skin protection education, especially those who do not see a dermatologist. Education of young people focusing on their concern over maintaining a youthful appearance instead of the possibility of developing skin cancer in the future might be more effective.
Although education provided by a physician is effective, using media—particularly social media—might be more efficient. Television, Instagram, and YouTube were listed by respondents as the 3 most preferred media outlets for skin health education, which shows important areas of focus for future advertising. Facebook was listed at a surprisingly low level, possibly showing the change in use of certain social media websites among this age group. According to the Pew Research Center, the most widely used social media apps among young adults aged 18 to 29 years are YouTube (91%), Facebook (63%), Instagram (67%), and Snapchat (62%). More than half of the same demographic visit Facebook (74%), Instagram (63%), Snapchat (61%), and YouTube (51%) daily.5 Although respondents to our survey were not specifically asked about the frequency of their use of social media and our data set includes patients younger than 18 years, we know that social media use has been increasing over the last decade among adolescents.1 Therefore, we assume that more than one-half of respondents to our survey use their reported social media platforms daily.
Social media is an underused medium for skin cancer prevention education and can reach those who do not regularly see a dermatologist. Unlike printed pamphlets and posters, advertisements through social media can use metrics such as age, race, gender, and interests to target high-risk individuals.
Study Limitations
This was a single-site study of currently enrolled dermatology patients who might be more aware of skin protection than the general population because they are being treated by a dermatologist. Survey questions regarding demographics, required by our institution, could not effectively differentiate Hispanic and White patients. Respondents could have been subject to the Hawthorne effect—awareness that their behavior is being observed—when responding to the survey because it was administered in the office prior to being seen by a dermatologist.
- Falzone AE, Brindis CD, Chren M-M, et al. Teens, tweets, and tanning beds: rethinking the use of social media for skin cancer prevention. Am J Prev Med. 2017;53(3 suppl 1):S86-S94.
- Centers for Disease Control and Prevention. Use of indoor tanning devices by adults—United States, 2010. MMWR Morb Mortal Wkly Rep. 2012;61:323-326.
- American Academy of Dermatology. Position statement on indoor tanning. Amended November 14, 2009. Accessed January 10, 2021. https://server.aad.org/Forms/Policies/Uploads/PS/PS-Indoor%20Tanning%2011-16-09.pdf?
- American Academy of Dermatology. Indoor tanning. Accessed January 10, 2020. https://www.aad.org/media/stats-indoor-tanning
- Perrin A, Anderson M. Share of U.S. adults using social media, including Facebook, is mostly unchanged since 2018. Pew Research Center; April 10, 2019. Accessed April 16, 2021. https://www.pewresearch.org/fact-tank/2019/04/10/share-of-u-s-adults-using-social-media-including-facebook-is-mostly-unchanged-since-2018/
Intentional tanning—through sun exposure and tanning beds—is an easily avoidable contributor to skin cancer development and an important area for public education. Since the advent of social media, a correlation between social media use and increased indoor tanning behaviors has been reported.1 In 2010, 11.3% of US adults aged 18 to 29 years reported using a tanning bed in the last 12 months.2 The American Academy of Dermatology first published their “Position Statement on Indoor Tanning” in 1998, endorsing a ban on the sale of indoor tanning equipment for nonmedical purposes.3
Although there has been no outright ban on indoor tanning, regulations have been put in place in many states—including Texas, where (as of 2013) a person younger than 18 years must have written consent from their parent(s) to use a tanning bed. Despite efforts of organizations including the American Academy of Dermatology and the government to educate the public on skin cancer prevention and sun safety, the skin cancer rate has been steadily increasing over the last 20 years.
There is a constant campaign among dermatologists to educate their patients on how to reduce or avoid the risk for skin cancer, including the use of sunscreen and avoidance of tanning. Adolescents and young adults are an especially important demographic to reach and educate because increased UV light exposure during these years leads to a greatly increased risk for skin cancer later in life.4 Data on the overall prevalence of tanning and the demographics of participation in tanning activities are important to capture and can be used to efficiently target higher-risk populations.
In this study, we aimed to investigate the attitudes and behaviors of adolescents and young adults regarding sun protection and tanning. We also aimed to determine which avenues, including social media, would be most effective at educating about skin cancer awareness and sun protection to the higher-risk younger population.
Materials and Methods
We developed an institutional review board–approved protocol for the prospective collection of data from registered patients at the dermatology clinic of the Mays Cancer Center at the University of Texas Health at San Antonio. A paper survey containing 15 rating-scale questions was administered to 60 patients aged 13 to 27 years. Surveys were administered during intake, prior to the patients’ visit with a dermatologist; all visits were of a functional (not cosmetic) nature. Data collection spanned June to August 2018. Survey results were entered into Research Electronic Data Capture (REDCap) software for qualitative analysis.
Results
Sixty patients responded to the survey. The mean age of respondents was 19.5 years. No surveys were excluded from the data set. Table 1 provides baseline characteristics of respondents. Some respondents left questions unanswered, resulting in questions with fewer than 60 responses.
Among respondents to the survey, 70% (42/60) reported it is very important to protect their skin from sun exposure, and 30% (18/60) reported it is somewhat important. Regarding sunscreen use, 70% (42/60) indicated they use sunscreen only before outdoor activities, 12% (7/60) use sunscreen daily, and 17% (10/60) never use sunscreen. Of those who use sunscreen, 52% (28/54) do so to prevent skin damage and aging and 44% (24/54) to prevent skin cancer. Twenty-three percent (13/56) of respondents reported finding tanned skin attractive; 26% (14/55) reported wanting to be tan. Looking at race, 28% (10/36) of Whites, 25% (5/20) of Spanish/Hispanic/Latinos, and 22% (2/9) of Asians found tanned skin attractive; no Black respondents found tanned skin attractive.
Regarding tanning, 12% (7/57) reported using a tanning bed in their lifetime and 4% (2/57) in the last year; 34% (19/56) reported deliberately tanning outdoors; and 9% (5/56) reported using sunless or spray-on tanning. Dermatologists (75% [42/56]), primary care physicians (69.6% [39/56]), and parents (46.4% [26/56]) were perceived as more effective sources of skin care education; among media modalities, television (33.9% [19/56]), Instagram (30.4% [17/60]), and YouTube (23.2% [13/60]) were perceived as more effective sources of skin care education (Table 2).
Comment
Perceptions of Tanning
Almost one-quarter of respondents found tanned skin attractive, which might reflect a shift from prior generations. Compared to the 11% of respondents in the 2010 survey,2 only 3.5% (2/57) of our respondents reported using a tanning bed in the last year, which could reflect the results of recent Texas legislation restricting the use of tanning beds by adolescents.
An alarming number of respondents reported going outdoors with the intention of tanning; although it appears that indoor tanning education has been successful, this finding shows that there is still a need for sun protection education because outdoor tanning is not a suitable alternative. A small number of respondents reported getting a sunless or spray-on tan, which is a risk-free alternative to indoor tanning.
Despite all respondents stating that protecting skin from the sun is important, most respondents surveyed do not use sunscreen daily. More respondents use sunscreen to prevent damage and aging than to prevent skin cancer. Young people might be more alarmed by the threat of early aging and losing their “youthful appearance” than by the possibility of developing skin cancer in the distant future. This discrepancy might indicate a lack of knowledge and be an important focus for future education efforts.
Perceptions of Trustworthiness of Education Sources
Our findings show dermatologists and primary care physicians are important educators on skin protection. Primary care physicians should remain vigilant to recognize at-risk patients who would benefit from skin protection education, especially those who do not see a dermatologist. Education of young people focusing on their concern over maintaining a youthful appearance instead of the possibility of developing skin cancer in the future might be more effective.
Although education provided by a physician is effective, using media—particularly social media—might be more efficient. Television, Instagram, and YouTube were listed by respondents as the 3 most preferred media outlets for skin health education, which shows important areas of focus for future advertising. Facebook was listed at a surprisingly low level, possibly showing the change in use of certain social media websites among this age group. According to the Pew Research Center, the most widely used social media apps among young adults aged 18 to 29 years are YouTube (91%), Facebook (63%), Instagram (67%), and Snapchat (62%). More than half of the same demographic visit Facebook (74%), Instagram (63%), Snapchat (61%), and YouTube (51%) daily.5 Although respondents to our survey were not specifically asked about the frequency of their use of social media and our data set includes patients younger than 18 years, we know that social media use has been increasing over the last decade among adolescents.1 Therefore, we assume that more than one-half of respondents to our survey use their reported social media platforms daily.
Social media is an underused medium for skin cancer prevention education and can reach those who do not regularly see a dermatologist. Unlike printed pamphlets and posters, advertisements through social media can use metrics such as age, race, gender, and interests to target high-risk individuals.
Study Limitations
This was a single-site study of currently enrolled dermatology patients who might be more aware of skin protection than the general population because they are being treated by a dermatologist. Survey questions regarding demographics, required by our institution, could not effectively differentiate Hispanic and White patients. Respondents could have been subject to the Hawthorne effect—awareness that their behavior is being observed—when responding to the survey because it was administered in the office prior to being seen by a dermatologist.
Intentional tanning—through sun exposure and tanning beds—is an easily avoidable contributor to skin cancer development and an important area for public education. Since the advent of social media, a correlation between social media use and increased indoor tanning behaviors has been reported.1 In 2010, 11.3% of US adults aged 18 to 29 years reported using a tanning bed in the last 12 months.2 The American Academy of Dermatology first published their “Position Statement on Indoor Tanning” in 1998, endorsing a ban on the sale of indoor tanning equipment for nonmedical purposes.3
Although there has been no outright ban on indoor tanning, regulations have been put in place in many states—including Texas, where (as of 2013) a person younger than 18 years must have written consent from their parent(s) to use a tanning bed. Despite efforts of organizations including the American Academy of Dermatology and the government to educate the public on skin cancer prevention and sun safety, the skin cancer rate has been steadily increasing over the last 20 years.
There is a constant campaign among dermatologists to educate their patients on how to reduce or avoid the risk for skin cancer, including the use of sunscreen and avoidance of tanning. Adolescents and young adults are an especially important demographic to reach and educate because increased UV light exposure during these years leads to a greatly increased risk for skin cancer later in life.4 Data on the overall prevalence of tanning and the demographics of participation in tanning activities are important to capture and can be used to efficiently target higher-risk populations.
In this study, we aimed to investigate the attitudes and behaviors of adolescents and young adults regarding sun protection and tanning. We also aimed to determine which avenues, including social media, would be most effective at educating about skin cancer awareness and sun protection to the higher-risk younger population.
Materials and Methods
We developed an institutional review board–approved protocol for the prospective collection of data from registered patients at the dermatology clinic of the Mays Cancer Center at the University of Texas Health at San Antonio. A paper survey containing 15 rating-scale questions was administered to 60 patients aged 13 to 27 years. Surveys were administered during intake, prior to the patients’ visit with a dermatologist; all visits were of a functional (not cosmetic) nature. Data collection spanned June to August 2018. Survey results were entered into Research Electronic Data Capture (REDCap) software for qualitative analysis.
Results
Sixty patients responded to the survey. The mean age of respondents was 19.5 years. No surveys were excluded from the data set. Table 1 provides baseline characteristics of respondents. Some respondents left questions unanswered, resulting in questions with fewer than 60 responses.
Among respondents to the survey, 70% (42/60) reported it is very important to protect their skin from sun exposure, and 30% (18/60) reported it is somewhat important. Regarding sunscreen use, 70% (42/60) indicated they use sunscreen only before outdoor activities, 12% (7/60) use sunscreen daily, and 17% (10/60) never use sunscreen. Of those who use sunscreen, 52% (28/54) do so to prevent skin damage and aging and 44% (24/54) to prevent skin cancer. Twenty-three percent (13/56) of respondents reported finding tanned skin attractive; 26% (14/55) reported wanting to be tan. Looking at race, 28% (10/36) of Whites, 25% (5/20) of Spanish/Hispanic/Latinos, and 22% (2/9) of Asians found tanned skin attractive; no Black respondents found tanned skin attractive.
Regarding tanning, 12% (7/57) reported using a tanning bed in their lifetime and 4% (2/57) in the last year; 34% (19/56) reported deliberately tanning outdoors; and 9% (5/56) reported using sunless or spray-on tanning. Dermatologists (75% [42/56]), primary care physicians (69.6% [39/56]), and parents (46.4% [26/56]) were perceived as more effective sources of skin care education; among media modalities, television (33.9% [19/56]), Instagram (30.4% [17/60]), and YouTube (23.2% [13/60]) were perceived as more effective sources of skin care education (Table 2).
Comment
Perceptions of Tanning
Almost one-quarter of respondents found tanned skin attractive, which might reflect a shift from prior generations. Compared to the 11% of respondents in the 2010 survey,2 only 3.5% (2/57) of our respondents reported using a tanning bed in the last year, which could reflect the results of recent Texas legislation restricting the use of tanning beds by adolescents.
An alarming number of respondents reported going outdoors with the intention of tanning; although it appears that indoor tanning education has been successful, this finding shows that there is still a need for sun protection education because outdoor tanning is not a suitable alternative. A small number of respondents reported getting a sunless or spray-on tan, which is a risk-free alternative to indoor tanning.
Despite all respondents stating that protecting skin from the sun is important, most respondents surveyed do not use sunscreen daily. More respondents use sunscreen to prevent damage and aging than to prevent skin cancer. Young people might be more alarmed by the threat of early aging and losing their “youthful appearance” than by the possibility of developing skin cancer in the distant future. This discrepancy might indicate a lack of knowledge and be an important focus for future education efforts.
Perceptions of Trustworthiness of Education Sources
Our findings show dermatologists and primary care physicians are important educators on skin protection. Primary care physicians should remain vigilant to recognize at-risk patients who would benefit from skin protection education, especially those who do not see a dermatologist. Education of young people focusing on their concern over maintaining a youthful appearance instead of the possibility of developing skin cancer in the future might be more effective.
Although education provided by a physician is effective, using media—particularly social media—might be more efficient. Television, Instagram, and YouTube were listed by respondents as the 3 most preferred media outlets for skin health education, which shows important areas of focus for future advertising. Facebook was listed at a surprisingly low level, possibly showing the change in use of certain social media websites among this age group. According to the Pew Research Center, the most widely used social media apps among young adults aged 18 to 29 years are YouTube (91%), Facebook (63%), Instagram (67%), and Snapchat (62%). More than half of the same demographic visit Facebook (74%), Instagram (63%), Snapchat (61%), and YouTube (51%) daily.5 Although respondents to our survey were not specifically asked about the frequency of their use of social media and our data set includes patients younger than 18 years, we know that social media use has been increasing over the last decade among adolescents.1 Therefore, we assume that more than one-half of respondents to our survey use their reported social media platforms daily.
Social media is an underused medium for skin cancer prevention education and can reach those who do not regularly see a dermatologist. Unlike printed pamphlets and posters, advertisements through social media can use metrics such as age, race, gender, and interests to target high-risk individuals.
Study Limitations
This was a single-site study of currently enrolled dermatology patients who might be more aware of skin protection than the general population because they are being treated by a dermatologist. Survey questions regarding demographics, required by our institution, could not effectively differentiate Hispanic and White patients. Respondents could have been subject to the Hawthorne effect—awareness that their behavior is being observed—when responding to the survey because it was administered in the office prior to being seen by a dermatologist.
- Falzone AE, Brindis CD, Chren M-M, et al. Teens, tweets, and tanning beds: rethinking the use of social media for skin cancer prevention. Am J Prev Med. 2017;53(3 suppl 1):S86-S94.
- Centers for Disease Control and Prevention. Use of indoor tanning devices by adults—United States, 2010. MMWR Morb Mortal Wkly Rep. 2012;61:323-326.
- American Academy of Dermatology. Position statement on indoor tanning. Amended November 14, 2009. Accessed January 10, 2021. https://server.aad.org/Forms/Policies/Uploads/PS/PS-Indoor%20Tanning%2011-16-09.pdf?
- American Academy of Dermatology. Indoor tanning. Accessed January 10, 2020. https://www.aad.org/media/stats-indoor-tanning
- Perrin A, Anderson M. Share of U.S. adults using social media, including Facebook, is mostly unchanged since 2018. Pew Research Center; April 10, 2019. Accessed April 16, 2021. https://www.pewresearch.org/fact-tank/2019/04/10/share-of-u-s-adults-using-social-media-including-facebook-is-mostly-unchanged-since-2018/
- Falzone AE, Brindis CD, Chren M-M, et al. Teens, tweets, and tanning beds: rethinking the use of social media for skin cancer prevention. Am J Prev Med. 2017;53(3 suppl 1):S86-S94.
- Centers for Disease Control and Prevention. Use of indoor tanning devices by adults—United States, 2010. MMWR Morb Mortal Wkly Rep. 2012;61:323-326.
- American Academy of Dermatology. Position statement on indoor tanning. Amended November 14, 2009. Accessed January 10, 2021. https://server.aad.org/Forms/Policies/Uploads/PS/PS-Indoor%20Tanning%2011-16-09.pdf?
- American Academy of Dermatology. Indoor tanning. Accessed January 10, 2020. https://www.aad.org/media/stats-indoor-tanning
- Perrin A, Anderson M. Share of U.S. adults using social media, including Facebook, is mostly unchanged since 2018. Pew Research Center; April 10, 2019. Accessed April 16, 2021. https://www.pewresearch.org/fact-tank/2019/04/10/share-of-u-s-adults-using-social-media-including-facebook-is-mostly-unchanged-since-2018/
PRACTICE POINTS
- Dermatologists are the preferred educators of skin care for adolescents and young adults.
- Social media is an underused medium for skin cancer prevention education and can reach those who do not regularly see a dermatologist.
- Education of young people focusing on their concerns about maintaining a youthful appearance instead of the possibility of developing skin cancer in the future might be more effective.
Communication Strategies in Mohs Micrographic Surgery: A Survey of Methods, Time Savings, and Perceived Patient Satisfaction
Mohs micrographic surgery (MMS) entails multiple time-consuming surgical and histological examinations for each patient. As surgical stages are performed and histological sections are processed, an efficient communication method among providers, medical assistants, histotechnologists, and patients is necessary to avoid delays. To address these and other communication issues, providers have focused on ways to increase clinic efficiency and improve patient-reported outcomes by utilizing new or repurposed communication technologies in their Mohs practice.
Prior reports have highlighted the utility of hands-free headsets that allow real-time communication among staff members as a means of increasing clinic efficiency and decreasing patient wait times.1-4 These systems may mediate a more rapid turnover between stages by mitigating the need for surgeons and support staff to assemble within a designated workspace.1,3,4 However, there is no single or standardized communication method that best suits all surgical suites and MMS practices. Our study aimed to identify the current communication strategies employed by Mohs surgeons and thereby ascertain which method(s) portend(s) the highest benefit in average daily time savings and provider-perceived patient satisfaction.
Materials and Methods
Survey Instrument
A new 10-question electronic survey was published on the SurveyMonkey website, and a link to the survey was provided in a quarterly email that originated from the American College of Mohs Surgery and was distributed to all 1735 active members. Responses were obtained from January 2019 to February 2019.
Statistical Analysis
A statistical analysis was done to determine any significant associations among the providers’ responses. P<.05 was used to determine statistical significance. A Cochran-Armitage test for trend was used to identify significant associations between the number of rooms and the communication systems that were used. Thus, 7 total tests—1 for each device (whiteboard, light system, flag system, wired intercom, wireless intercom, walkie-talkie, or headset)—were conducted. The Cochran-Armitage test also was used to determine whether the probability of using the device was affected by the number of stations/surgical rooms that were attended by the Mohs surgeons. To determine whether the communication devices used were associated with higher patient satisfaction, a χ2 test was conducted for each device (7 total tests), testing the categories of using that device (yes/no) and patient satisfaction (yes/no). A Fisher exact test of independence was used in any case where the proportion for the device and patient satisfaction was 25% or higher. To determine whether the communication method was associated with increased time savings, 7 total Cochran-Armitage tests were conducted, 1 for each device. A logistic regression model was used to determine whether there was a significant association between the number of stations and the likelihood of reporting patient satisfaction.
Results
Eighty-eight surgeons responded to the survey, with a response rate of 5% (88/1735). A total of 55 surgeons completed the survey in its entirety and were included in the data analysis. The most commonly used communication mediums were whiteboards (29/55 [53%]), followed by a flag system (16/55 [29%]) and a light system (13/55 [24%]). Most Mohs surgeons (52/55 [95%]) used the communication media to communicate with their staff only, and 76% (42/55) of Mohs surgeons believed that their communication media contributed to higher patient satisfaction. Overall, 58% (32/55) of Mohs surgeons stated that their communication media saved more than 15 minutes (on average) per day. The use of a whiteboard and/or flag system was reported as the least efficient method, with average daily time savings of 13 minutes. With the introduction of newer technology (wired or wireless intercoms, headsets, walkie-talkies, or internal messaging systems such as Skype) to the whiteboard and/or flag system, the time savings increased by 10 minutes per day. Nearly 25% (14/55) of surgeons utilized more than 1 communication system.
As the number of stations in an MMS suite increased, the probability of using a whiteboard to track the progress of the cases decreased. There were no statistically significant associations identified between the number of stations and the use of other communication devices (ie, flag system, light system, wireless intercom, wired intercom, walkie-talkie, headset). The stratified percentages of the amount of time savings for each communication modality are presented in the Figure (whiteboards and headsets were excluded because they did not increase time savings). The use of a light system was the only communication modality found to be statistically associated with an increase in provider-reported time savings (P=.0482; Figure). In addition, our analysis did not show an improvement in provider-reported patient satisfaction with any of the current systems used in MMS clinics.
Comment
The process of transmitting information among the medical team during MMS is a complex interplay involving the relay of crucial information, with many opportunities for the introduction of distraction and error. Despite numerous improvements in the efficiency of the preparation of histological specimens and implementation of various time-saving and tissue-saving surgical interventions, relatively little attention has been given to address the sometimes chaotic and challenging process of organizing results from each stage of multiple patients in an MMS surgical suite.5
As demonstrated by our survey, incorporation of a light-based system into an MMS clinic may improve workplace efficiency by decreasing the redundant use of support staff and allowing Mohs surgeons to transition from one station to the next seamlessly. Light-based communication systems provide an immediate notification for support staff via color-coded and/or numerically coded indicators on input switches located outside and inside the examination/surgery rooms. The switch indicators can be depressed with minimal disruption from station to station, thereby foregoing the need to interrupt an ongoing excision or closure to convey the status of the case. These systems may then permit enhanced clinic and workflow efficiency, which may help to shorten patient wait times.
Study Limitation
Although all members of the American College of Mohs Surgery were invited to participate in this online survey, only a small number (N=55) completed it in its entirety. Moreover, sample sizes for some of the communication devices were small. As a result, many of the tests might be lacking sufficient power to detect possible relationships, which might be identified in future larger-scale studies.
Conclusion
Our study supports the use of light-based communication systems in MMS suites to improve efficiency in the clinic. Based on our analysis, light-based communication methods were significantly associated with improved time savings (P=.0482). Our study did not show an improvement in provider-reported satisfaction with any of the current systems used in MMS clinics. We hope that this information will help guide providers in implementing new communication techniques to improve clinic efficiency.
Acknowledgments
The authors would like to thank Ms. Kathy Kyler (Oklahoma City, Oklahoma) for her assistance in preparing this manuscript. Support for Dr. Chen and Mr. Stubblefield was provided through National Institutes of Health, National Institute of General Medical Sciences [Grant 2U54GM104938-06, PI Judith James].
- Chen T, Vines L, Wanitphakdeedecha R, et al. Electronically linked: wireless, discrete, hands-free communication to improve surgical workflow in Mohs and dermasurgery clinic. Dermatol Surg. 2009;35:248-252.
- Lanto AB, Yano EM, Fink A, et al. Anatomy of an outpatient visit. An evaluation of clinic efficiency in general and subspecialty clinics. Med Group Manage J. 1995;42:18-25.
- Kantor J. Application of Google Glass to Mohs micrographic surgery: a pilot study in 120 patients. Dermatol Surg. 2015;41:288-289.
- Spurk PA, Mohr ML, Seroka AM, et al. The impact of a wireless telecommunication system on efficiency. J Nurs Admin. 1995;25:21-26.
- Dietert JB, MacFarlane DF. A survey of Mohs tissue tracking practices. Dermatol Surg. 2019;45:514-518.
Mohs micrographic surgery (MMS) entails multiple time-consuming surgical and histological examinations for each patient. As surgical stages are performed and histological sections are processed, an efficient communication method among providers, medical assistants, histotechnologists, and patients is necessary to avoid delays. To address these and other communication issues, providers have focused on ways to increase clinic efficiency and improve patient-reported outcomes by utilizing new or repurposed communication technologies in their Mohs practice.
Prior reports have highlighted the utility of hands-free headsets that allow real-time communication among staff members as a means of increasing clinic efficiency and decreasing patient wait times.1-4 These systems may mediate a more rapid turnover between stages by mitigating the need for surgeons and support staff to assemble within a designated workspace.1,3,4 However, there is no single or standardized communication method that best suits all surgical suites and MMS practices. Our study aimed to identify the current communication strategies employed by Mohs surgeons and thereby ascertain which method(s) portend(s) the highest benefit in average daily time savings and provider-perceived patient satisfaction.
Materials and Methods
Survey Instrument
A new 10-question electronic survey was published on the SurveyMonkey website, and a link to the survey was provided in a quarterly email that originated from the American College of Mohs Surgery and was distributed to all 1735 active members. Responses were obtained from January 2019 to February 2019.
Statistical Analysis
A statistical analysis was done to determine any significant associations among the providers’ responses. P<.05 was used to determine statistical significance. A Cochran-Armitage test for trend was used to identify significant associations between the number of rooms and the communication systems that were used. Thus, 7 total tests—1 for each device (whiteboard, light system, flag system, wired intercom, wireless intercom, walkie-talkie, or headset)—were conducted. The Cochran-Armitage test also was used to determine whether the probability of using the device was affected by the number of stations/surgical rooms that were attended by the Mohs surgeons. To determine whether the communication devices used were associated with higher patient satisfaction, a χ2 test was conducted for each device (7 total tests), testing the categories of using that device (yes/no) and patient satisfaction (yes/no). A Fisher exact test of independence was used in any case where the proportion for the device and patient satisfaction was 25% or higher. To determine whether the communication method was associated with increased time savings, 7 total Cochran-Armitage tests were conducted, 1 for each device. A logistic regression model was used to determine whether there was a significant association between the number of stations and the likelihood of reporting patient satisfaction.
Results
Eighty-eight surgeons responded to the survey, with a response rate of 5% (88/1735). A total of 55 surgeons completed the survey in its entirety and were included in the data analysis. The most commonly used communication mediums were whiteboards (29/55 [53%]), followed by a flag system (16/55 [29%]) and a light system (13/55 [24%]). Most Mohs surgeons (52/55 [95%]) used the communication media to communicate with their staff only, and 76% (42/55) of Mohs surgeons believed that their communication media contributed to higher patient satisfaction. Overall, 58% (32/55) of Mohs surgeons stated that their communication media saved more than 15 minutes (on average) per day. The use of a whiteboard and/or flag system was reported as the least efficient method, with average daily time savings of 13 minutes. With the introduction of newer technology (wired or wireless intercoms, headsets, walkie-talkies, or internal messaging systems such as Skype) to the whiteboard and/or flag system, the time savings increased by 10 minutes per day. Nearly 25% (14/55) of surgeons utilized more than 1 communication system.
As the number of stations in an MMS suite increased, the probability of using a whiteboard to track the progress of the cases decreased. There were no statistically significant associations identified between the number of stations and the use of other communication devices (ie, flag system, light system, wireless intercom, wired intercom, walkie-talkie, headset). The stratified percentages of the amount of time savings for each communication modality are presented in the Figure (whiteboards and headsets were excluded because they did not increase time savings). The use of a light system was the only communication modality found to be statistically associated with an increase in provider-reported time savings (P=.0482; Figure). In addition, our analysis did not show an improvement in provider-reported patient satisfaction with any of the current systems used in MMS clinics.
Comment
The process of transmitting information among the medical team during MMS is a complex interplay involving the relay of crucial information, with many opportunities for the introduction of distraction and error. Despite numerous improvements in the efficiency of the preparation of histological specimens and implementation of various time-saving and tissue-saving surgical interventions, relatively little attention has been given to address the sometimes chaotic and challenging process of organizing results from each stage of multiple patients in an MMS surgical suite.5
As demonstrated by our survey, incorporation of a light-based system into an MMS clinic may improve workplace efficiency by decreasing the redundant use of support staff and allowing Mohs surgeons to transition from one station to the next seamlessly. Light-based communication systems provide an immediate notification for support staff via color-coded and/or numerically coded indicators on input switches located outside and inside the examination/surgery rooms. The switch indicators can be depressed with minimal disruption from station to station, thereby foregoing the need to interrupt an ongoing excision or closure to convey the status of the case. These systems may then permit enhanced clinic and workflow efficiency, which may help to shorten patient wait times.
Study Limitation
Although all members of the American College of Mohs Surgery were invited to participate in this online survey, only a small number (N=55) completed it in its entirety. Moreover, sample sizes for some of the communication devices were small. As a result, many of the tests might be lacking sufficient power to detect possible relationships, which might be identified in future larger-scale studies.
Conclusion
Our study supports the use of light-based communication systems in MMS suites to improve efficiency in the clinic. Based on our analysis, light-based communication methods were significantly associated with improved time savings (P=.0482). Our study did not show an improvement in provider-reported satisfaction with any of the current systems used in MMS clinics. We hope that this information will help guide providers in implementing new communication techniques to improve clinic efficiency.
Acknowledgments
The authors would like to thank Ms. Kathy Kyler (Oklahoma City, Oklahoma) for her assistance in preparing this manuscript. Support for Dr. Chen and Mr. Stubblefield was provided through National Institutes of Health, National Institute of General Medical Sciences [Grant 2U54GM104938-06, PI Judith James].
Mohs micrographic surgery (MMS) entails multiple time-consuming surgical and histological examinations for each patient. As surgical stages are performed and histological sections are processed, an efficient communication method among providers, medical assistants, histotechnologists, and patients is necessary to avoid delays. To address these and other communication issues, providers have focused on ways to increase clinic efficiency and improve patient-reported outcomes by utilizing new or repurposed communication technologies in their Mohs practice.
Prior reports have highlighted the utility of hands-free headsets that allow real-time communication among staff members as a means of increasing clinic efficiency and decreasing patient wait times.1-4 These systems may mediate a more rapid turnover between stages by mitigating the need for surgeons and support staff to assemble within a designated workspace.1,3,4 However, there is no single or standardized communication method that best suits all surgical suites and MMS practices. Our study aimed to identify the current communication strategies employed by Mohs surgeons and thereby ascertain which method(s) portend(s) the highest benefit in average daily time savings and provider-perceived patient satisfaction.
Materials and Methods
Survey Instrument
A new 10-question electronic survey was published on the SurveyMonkey website, and a link to the survey was provided in a quarterly email that originated from the American College of Mohs Surgery and was distributed to all 1735 active members. Responses were obtained from January 2019 to February 2019.
Statistical Analysis
A statistical analysis was done to determine any significant associations among the providers’ responses. P<.05 was used to determine statistical significance. A Cochran-Armitage test for trend was used to identify significant associations between the number of rooms and the communication systems that were used. Thus, 7 total tests—1 for each device (whiteboard, light system, flag system, wired intercom, wireless intercom, walkie-talkie, or headset)—were conducted. The Cochran-Armitage test also was used to determine whether the probability of using the device was affected by the number of stations/surgical rooms that were attended by the Mohs surgeons. To determine whether the communication devices used were associated with higher patient satisfaction, a χ2 test was conducted for each device (7 total tests), testing the categories of using that device (yes/no) and patient satisfaction (yes/no). A Fisher exact test of independence was used in any case where the proportion for the device and patient satisfaction was 25% or higher. To determine whether the communication method was associated with increased time savings, 7 total Cochran-Armitage tests were conducted, 1 for each device. A logistic regression model was used to determine whether there was a significant association between the number of stations and the likelihood of reporting patient satisfaction.
Results
Eighty-eight surgeons responded to the survey, with a response rate of 5% (88/1735). A total of 55 surgeons completed the survey in its entirety and were included in the data analysis. The most commonly used communication mediums were whiteboards (29/55 [53%]), followed by a flag system (16/55 [29%]) and a light system (13/55 [24%]). Most Mohs surgeons (52/55 [95%]) used the communication media to communicate with their staff only, and 76% (42/55) of Mohs surgeons believed that their communication media contributed to higher patient satisfaction. Overall, 58% (32/55) of Mohs surgeons stated that their communication media saved more than 15 minutes (on average) per day. The use of a whiteboard and/or flag system was reported as the least efficient method, with average daily time savings of 13 minutes. With the introduction of newer technology (wired or wireless intercoms, headsets, walkie-talkies, or internal messaging systems such as Skype) to the whiteboard and/or flag system, the time savings increased by 10 minutes per day. Nearly 25% (14/55) of surgeons utilized more than 1 communication system.
As the number of stations in an MMS suite increased, the probability of using a whiteboard to track the progress of the cases decreased. There were no statistically significant associations identified between the number of stations and the use of other communication devices (ie, flag system, light system, wireless intercom, wired intercom, walkie-talkie, headset). The stratified percentages of the amount of time savings for each communication modality are presented in the Figure (whiteboards and headsets were excluded because they did not increase time savings). The use of a light system was the only communication modality found to be statistically associated with an increase in provider-reported time savings (P=.0482; Figure). In addition, our analysis did not show an improvement in provider-reported patient satisfaction with any of the current systems used in MMS clinics.
Comment
The process of transmitting information among the medical team during MMS is a complex interplay involving the relay of crucial information, with many opportunities for the introduction of distraction and error. Despite numerous improvements in the efficiency of the preparation of histological specimens and implementation of various time-saving and tissue-saving surgical interventions, relatively little attention has been given to address the sometimes chaotic and challenging process of organizing results from each stage of multiple patients in an MMS surgical suite.5
As demonstrated by our survey, incorporation of a light-based system into an MMS clinic may improve workplace efficiency by decreasing the redundant use of support staff and allowing Mohs surgeons to transition from one station to the next seamlessly. Light-based communication systems provide an immediate notification for support staff via color-coded and/or numerically coded indicators on input switches located outside and inside the examination/surgery rooms. The switch indicators can be depressed with minimal disruption from station to station, thereby foregoing the need to interrupt an ongoing excision or closure to convey the status of the case. These systems may then permit enhanced clinic and workflow efficiency, which may help to shorten patient wait times.
Study Limitation
Although all members of the American College of Mohs Surgery were invited to participate in this online survey, only a small number (N=55) completed it in its entirety. Moreover, sample sizes for some of the communication devices were small. As a result, many of the tests might be lacking sufficient power to detect possible relationships, which might be identified in future larger-scale studies.
Conclusion
Our study supports the use of light-based communication systems in MMS suites to improve efficiency in the clinic. Based on our analysis, light-based communication methods were significantly associated with improved time savings (P=.0482). Our study did not show an improvement in provider-reported satisfaction with any of the current systems used in MMS clinics. We hope that this information will help guide providers in implementing new communication techniques to improve clinic efficiency.
Acknowledgments
The authors would like to thank Ms. Kathy Kyler (Oklahoma City, Oklahoma) for her assistance in preparing this manuscript. Support for Dr. Chen and Mr. Stubblefield was provided through National Institutes of Health, National Institute of General Medical Sciences [Grant 2U54GM104938-06, PI Judith James].
- Chen T, Vines L, Wanitphakdeedecha R, et al. Electronically linked: wireless, discrete, hands-free communication to improve surgical workflow in Mohs and dermasurgery clinic. Dermatol Surg. 2009;35:248-252.
- Lanto AB, Yano EM, Fink A, et al. Anatomy of an outpatient visit. An evaluation of clinic efficiency in general and subspecialty clinics. Med Group Manage J. 1995;42:18-25.
- Kantor J. Application of Google Glass to Mohs micrographic surgery: a pilot study in 120 patients. Dermatol Surg. 2015;41:288-289.
- Spurk PA, Mohr ML, Seroka AM, et al. The impact of a wireless telecommunication system on efficiency. J Nurs Admin. 1995;25:21-26.
- Dietert JB, MacFarlane DF. A survey of Mohs tissue tracking practices. Dermatol Surg. 2019;45:514-518.
- Chen T, Vines L, Wanitphakdeedecha R, et al. Electronically linked: wireless, discrete, hands-free communication to improve surgical workflow in Mohs and dermasurgery clinic. Dermatol Surg. 2009;35:248-252.
- Lanto AB, Yano EM, Fink A, et al. Anatomy of an outpatient visit. An evaluation of clinic efficiency in general and subspecialty clinics. Med Group Manage J. 1995;42:18-25.
- Kantor J. Application of Google Glass to Mohs micrographic surgery: a pilot study in 120 patients. Dermatol Surg. 2015;41:288-289.
- Spurk PA, Mohr ML, Seroka AM, et al. The impact of a wireless telecommunication system on efficiency. J Nurs Admin. 1995;25:21-26.
- Dietert JB, MacFarlane DF. A survey of Mohs tissue tracking practices. Dermatol Surg. 2019;45:514-518.
Practice Points
- There are limited studies evaluating the efficacy of different communication methods in Mohs micrographic surgery (MMS) clinics.
- This study suggests that incorporation of a light-based system into an MMS clinic improves workplace efficiency.
Applying a Text-Search Algorithm to Radiology Reports Can Find More Patients With Pulmonary Nodules Than Radiology Coding Alone (FULL)
Rapid advances in imaging technology have led to better spatial resolution with lower radiation doses to patients. These advances have helped to increase the use of diagnostic chest imaging, particularly in emergency departments and oncology centers, and in screening for coronary artery disease. As a result, there has been an explosion of incidental findings on chest imaging—including indeterminate lung nodules.1,2
Lung nodules are rounded and well-circumscribed lung opacities (≤ 3 cm in diameter) that may present as solitary or multiple lesions in usually asymptomatic patients. Most lung nodules are benign, the result of an infectious or inflammatory process. Nodules that are ≤ 8 mm in diameter, unless they show increase in size over time, often can be safely followed with imaging surveillance. In contrast, lung nodules > 8 mm could represent an early-stage lung cancer, especially among patients with high-risk for developing lung cancer (ie, those with advanced age, heavy tobacco abuse, or emphysema) and should be further assessed with close imaging surveillance, either chest computed tomography (CT) alone or positron-emission tomography (PET)/CT, or tissue biopsy, based on the underlying likelihood of malignancy.
Patients who receive an early-stage lung cancer diagnosis can be offered curative treatments leading to improved 5-year survival rates.3,4 Consequently, health care systems need to be able to identify these nodules accurately, in order to categorize and manage them accordingly to the Fleischner radiographic and American College of Chest Physicians clinical guidelines.5,6 Unfortunately, many hospitals struggle to identify patients with incidental lung nodules found during diagnostic chest and abdominal imaging, due in part to poor adherence to Fleischner guidelines among radiologists for categorizing pulmonary nodules.7,8
The Veterans Health Administration (VHA) system is interested in effectively detecting patients with incidental lung nodules. Veterans have a higher risk of developing lung cancer when compared with the entire US population, mainly due to a higher incidence of tobacco use.6 The prevalence of lung nodules among veterans with significant risk factors for lung cancer is about 60% nationwide, and up to 85% in the Midwest, due to the high prevalence of histoplasmosis.7 However, only a small percentage of these nodules represent an early stage primary lung cancer.
Several Veterans Integrated Service Networks (VISNs) in the VHA use a radiology diagnostic code to systematically identify imaging studies with presence of lung nodules. In VISN 23, which includes Minnesota, North Dakota, South Dakota, Iowa, and portions of neighboring states, the code used to identify these radiology studies is 44. However, there is high variability in the reporting and coding of imaging studies among radiologists, which could lead to misclassifying patients with lung nodules.8
Some studies suggest that using an automated text search algorithm within radiology reports can be a highly effective strategy to identify patients with lung nodules.9,10 In this study, we compared the diagnostic performance of a newly developed text search algorithm applied to radiology reports with the current standard practice of using a radiology diagnostic code for identifying patients with lung nodules at the Iowa City US Department of Veterans Affairs (VA) Health Care System (ICVAHCS) hospital in Iowa.
Methods
Since 2014, The ICVAHCS has used a radiology diagnostic code to identify any imaging studies with lung nodules. The radiologist enters “44” at the end of the reading process using the Nuance Powerscribe 360 radiation reporting system. The code is uploaded into the VHA Corporate Data Warehouse (CDW), and it is located within the radiology exam domain. This strategy was created and implemented by the Minneapolis VA Health Care System in Minnesota for all the VA hospitals in VISN 23. A lung nodule registry nurse was provided with a list of radiology studies flagged with this radiology diagnostic code every 2 weeks. A chart review was then performed for all these studies to determine the presence of a lung nodule. When detected, the ordering health care provider was alerted and given recommendations for managing the nodule.
We initially searched for the radiology studies with a presumptive lung nodule using the radiology code 44 within the CDW. Separately, we applied the text search strategy only to radiology reports from chest and abdomen studies (ie, X-rays, CT, magnetic resonance imaging [MRI], and PET) that contained any of the keyword phrases. The text search strategy was modeled based on a natural language processing (NLP) algorithm developed by the Puget Sound VA Healthcare System in Seattle, Washington to identify lung nodules on radiology reports.9 Our algorithm included a series of text searches using Microsoft SQL. After several simulations using a random group of radiology reports, we chose the keywords: “lung AND nodul”; “pulm AND nodul”; “pulm AND mass”; “lung AND mass”; and “ground glass”. We selected only chest and abdomen studies because on several simulations using a random group of radiology reports, the vast majority of lung nodules were identified on chest and abdomen imaging studies. Also, it would not have been feasible to chart review the approximately 30,000 total radiology reports that were generated during the study period.
From January 1, 2016 through November 30, 2016, we applied both search strategies independently: radiology diagnostic code for lung nodules to all imaging studies, and text search to all radiology reports of chest and abdomen imaging studies in the CDW (Figure). We also collected demographic (eg, age, sex, race, rurality) and clinical (eg, medical comorbidities, tobacco use) information that were uploaded to the database automatically from CDW using International Statistical Classification of Diseases, Tenth Edition and demographic codes. The VHA uses the Rural-Urban Commuting Areas (RUCA) system to define rurality, which takes into account population density and how closely a community is linked socioeconomically to larger urban centers.11 The protocol was reviewed and approved by the institutional review board of ICVAHCS and the University of Iowa.
The presence of a lung nodule was established by having the lung nodule registry nurse manually review the charts of every patient with a radiology report identified by either code 44 or the text search algorithm. The goal was to ensure that our text search strategy identified all reports with a code 44 to be compliant with VISN expectations. Cases in which a lung nodule was described in the radiology report were considered true positives, and those without a lung nodule description were considered false positives.
We compared the sociodemographic and clinical characteristics of patients with lung nodules between those identified with both code 44 and the text search and those identified with the text search alone. We used χ2 tests for categorical variables (eg, age, gender, RUCA, chronic obstructive pulmonary disease (COPD), smoking status) and t tests for continuous variables (eg, Charlson comorbidity score). A P value ≤ .05 was considered statistically significant. To assess the yield of each search strategy, we determined the number of patients with lung nodules detected by the text search and the radiology diagnostic code. We also calculated the positive predictive value (PPV) and 95% CI of each search strategy.
Results
We identified 12,983 radiology studies that required manual review during the study period. We confirmed that 8,516 imaging studies had lung nodules, representing 2,912 patients. Subjects with lung nodules were predominantly male (96%), aged between 60 and 79 years (71%), and lived in a rural area (72%). More than 50% of these patients had COPD and over a third were current smokers (Table 1). The text search algorithm identified all of the patients identified by the radiology diagnostic code (n = 1,251). It also identified an additional 1,661 patients with lung nodules that otherwise would have been missed by the radiology code. Compared with those identified only by the text search, those identified by both the radiology coding and text search were older, had lower Charlson comorbidity scores, and were more likely to be a current smoker.
The text search algorithm identified more than twice as many patients with potential lung nodules compared with the radiology diagnostic code (4,071 vs 1,363) (Table 2). However, the text search algorithm was associated with a much higher number of false positives than was the diagnostic code (1,159 vs 112) and a lower PPV (72% [95% CI, 70.6-73.4] vs 92% [95% CI, 90.6-93.4], respectively). The text search algorithm identified 130 patients with lung nodules of moderate to high risk for malignancy (> 8 mm diameter) that were not identified by the radiology code. When the PPV of each search strategy was calculated based on imaging studies with nodules (most patients had > 1 imaging study), the results remained similar (98% for radiology code and 66% for text search). A larger proportion of the lung nodules detected by code 44 vs the text search algorithm were from CT chest studies.
Discussion
In a population of predominantly older male veterans with significant risk factors for lung cancer and high incidence of incidental lung nodules, applying a text search algorithm on radiology reports identified a substantial number of patients with lung nodules, including some with nodules > 8 mm, that were missed by the radiologist-generated code.9,10 Improving the yield of detection for lung nodules in a population with high risk for lung cancer would increase the likelihood of detecting patients with potentially curable early-stage lung cancers, decreasing lung cancer mortality.
The reasons for the high number of patients with lung nodules missed by the radiology code are unclear. Potential explanations may include the lack of standardization of imaging reports by the radiologists (ie, only 21% of chest CTs used a standardized template describing a lung nodule in our study), a problem well recognized both within and outside VHA.8,12
The text search algorithm identified more patients with lung nodules but had a higher rate of false positives when compared with the diagnostic code. The high rate of false positives resulted in more charts to review and an increased workload for the lung nodule registry team. The challenges presented by an increased workload should be balanced against the potential harms of missing nodules that develop into advanced cancer.
Text Search Adjustments
Refining the text search criteria algorithm and the chart review process may decrease the rate of false positives significantly without affecting detection of lung nodules. In subsequent simulations, we found that by adding an exclusion criteria to text search algorithm to remove reports with specific keywords we could substantially reduce the number of false positive reports without affecting the detection rate of the lung nodules. These exclusion criteria would exclude any reports that: (1) contain “nodul” within the next 8 words after mentioning “no”; (2) contain “clear” within the next 8 words after mentioning “lung” in the text (eg, “lungs appear to be clear”); (3) contain “clear” within the next 4 words after mentioning “otherwise” in the text (eg, “otherwise appear to be clear”). Based on our study results, we further refined the text search strategy by limiting the search to only chest imaging studies. When we applied the revised algorithm to a random sample of imaging reports, we found all the code 44 radiology reports were still captured, but we were able to reduce the number of radiology reports needing review by about 80%.
Although classification approaches are being refined to improve radiology performance in multiple categories of nodules, this study suggests that alternative approaches based on text algorithms can improve the capture of pulmonary nodules that require surveillance. These algorithms also can be used to augment radiologist reporting systems. This represents an investment in resources to build a team that should include a bioinformatics specialist, lung nodule registry personnel (review charts of the detected imaging studies with lung nodules, populating the lung nodule database, and determining and tracking the need of imaging follow up), a lung nodule clinic nurse coordinator, and a dedicated lung nodule clinic pulmonologist.
Radiology departments could employ this text search approach to identify missed nodules and use an audit and feedback system to train radiologists to code lung nodules consistently at the time of the initial reading to avoid delays in identifying patients with nodules. Alternatively, the more widespread use of a standardized CT chest radiology reports using Fleischner or the American College of Radiology Lung Imaging Reporting and Data System (Lung RADS) templates might improve the detection of patients with lung nodules.5,13,14
The VHA system should have an effective strategy for identifying incidental lung nodules during routine radiology examinations. Relying only on radiologists to identify and code pulmonary nodules can lead to missing a significant number of patients with lung nodules and some patients with early stage lung cancer who could receive curative therapy.12,14-16 The use of a standardized algorithm, like a text search strategy, might decrease the risk of variation in the execution and result in a more sensitive detection of patients with lung nodules. The text search strategy might be easily implemented and shared with other hospitals both within and outside the VHA.
Limitations
This study was performed in a single VHA hospital and the findings may not be generalizable to other settings of care. Second, our study design is susceptible to work-up bias because the results of a diagnostic test (eg, chest or abdomen imaging) affected whether the chart review was used to verify the test result. It was not feasible to review the patient records of all radiology studies done at the facility during the study period, consequently complete 2 × 2 tables could not be created to calculate sensitivity, specificity, and negative predictive value.
Conclusion
A text search algorithm of radiology reports increased the detection of patients with lung nodules when compared with radiology diagnostic coding alone. However, the improved detection was associated with a higher rate of false positives, which requires manually reviewing a larger number of patient’s chart reports. Future research and quality improvement should focus on standardizing the radiology reporting process and improving the efficiency and reliability of follow up and tracking of incidental lung nodules.
Acknowledgments
The work reported here was supported by a grant from the Office of Rural Health (N32-FY16Q1-S1-P01577), US Department of Veterans Affairs, Veterans Health Administration. We also had the support from the Veterans Rural Health Resource Center-Iowa City, and the Health Services Research and Development (HSR&D) Service through the Comprehensive Access and Delivery Research and Evaluation (CADRE) Center (REA 09-220).
1. Jacobs PC, Mali WP, Grobbee DE, van der Graaf Y. Prevalence of incidental findings in computed tomographic screening of the chest: a systematic review. Journal of computer assisted tomography. 2008;32(2):214-221.
2. Frank L, Quint LE. Chest CT incidentalomas: thyroid lesions, enlarged mediastinal lymph nodes, and lung nodules. Cancer Imaging. 2012;12(1):41-48.
3. National Institutes of Health, National Cancer Institute, Surveillance, Epidemiology, and End Results Program. Cancer stat facts: lung and bronchus cancer. https://seer.cancer.gov/statfacts/html/lungb.html. Accessed April 8, 2020.
4. Alberg AJ, Brock MV, Ford JG, Samet JM, Spivack SD. Epidemiology of lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e1S-e29S.
5. MacMahon H, Naidich DP, Goo JM, et al. Guidelines for Management of Incidental Pulmonary Nodules Detected on CT Images: From the Fleischner Society 2017. Radiology. 2017;284(1):228-243.
6. Zullig LL, Jackson GL, Dorn RA, et al. Cancer incidence among patients of the U.S. Veterans Affairs Health Care System. Mil Med. 2012;177(6):693-701.
7. Kinsinger LS, Anderson C, Kim J, et al. Implementation of lung cancer screening in the Veterans Health Administration. JAMA Intern Med. 2017;177(3):399-406.
8. Iqbal MN, Stott E, Huml AM, et al. What’s in a name? Factors associated with documentation and evaluation of incidental pulmonary nodules. Ann Am Thorac Soc. 2016;13(10):1704-1711.
9. Farjah F, Halgrim S, Buist DS, et al. An automated method for identifying individuals with a lung nodule can be feasibly implemented across health systems. Egems (Wash DC). 2016;4(1):1254.
10. Danforth KN, Early MI, Ngan S, Kosco AE, Zheng C, Gould MK. Automated identification of patients with pulmonary nodules in an integrated health system using administrative health plan data, radiology reports, and natural language processing. J Thorac Oncol. 2012;7(8):1257-1262.
11. US Department of Veterans Affairs, Office of Rural Health. https://www.ruralhealth.va.gov/aboutus/ruralvets.asp. Updated January 28, 2020. Accessed April 8, 2020.
12. Blagev DP, Lloyd JF, Conner K, et al. Follow-up of incidental pulmonary nodules and the radiology report. J Am Coll Radiol. 2016;13(2 suppl):R18-R24.
13. Eisenberg RL, Fleischner S. Ways to improve radiologists’ adherence to Fleischner Society guidelines for management of pulmonary nodules. J Am Coll Radiol. 2013;10(6):439-441.
14. Aberle DR. Implementing lung cancer screening: the US experience. Clin Radiol. 2017;72(5):401-406.
15. Gould MK, Donington J, Lynch WR, et al. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e93S-e120S.
16. Callister ME, Baldwin DR. How should pulmonary nodules be optimally investigated and managed? Lung Cancer. 2016;91:48-55.
Rapid advances in imaging technology have led to better spatial resolution with lower radiation doses to patients. These advances have helped to increase the use of diagnostic chest imaging, particularly in emergency departments and oncology centers, and in screening for coronary artery disease. As a result, there has been an explosion of incidental findings on chest imaging—including indeterminate lung nodules.1,2
Lung nodules are rounded and well-circumscribed lung opacities (≤ 3 cm in diameter) that may present as solitary or multiple lesions in usually asymptomatic patients. Most lung nodules are benign, the result of an infectious or inflammatory process. Nodules that are ≤ 8 mm in diameter, unless they show increase in size over time, often can be safely followed with imaging surveillance. In contrast, lung nodules > 8 mm could represent an early-stage lung cancer, especially among patients with high-risk for developing lung cancer (ie, those with advanced age, heavy tobacco abuse, or emphysema) and should be further assessed with close imaging surveillance, either chest computed tomography (CT) alone or positron-emission tomography (PET)/CT, or tissue biopsy, based on the underlying likelihood of malignancy.
Patients who receive an early-stage lung cancer diagnosis can be offered curative treatments leading to improved 5-year survival rates.3,4 Consequently, health care systems need to be able to identify these nodules accurately, in order to categorize and manage them accordingly to the Fleischner radiographic and American College of Chest Physicians clinical guidelines.5,6 Unfortunately, many hospitals struggle to identify patients with incidental lung nodules found during diagnostic chest and abdominal imaging, due in part to poor adherence to Fleischner guidelines among radiologists for categorizing pulmonary nodules.7,8
The Veterans Health Administration (VHA) system is interested in effectively detecting patients with incidental lung nodules. Veterans have a higher risk of developing lung cancer when compared with the entire US population, mainly due to a higher incidence of tobacco use.6 The prevalence of lung nodules among veterans with significant risk factors for lung cancer is about 60% nationwide, and up to 85% in the Midwest, due to the high prevalence of histoplasmosis.7 However, only a small percentage of these nodules represent an early stage primary lung cancer.
Several Veterans Integrated Service Networks (VISNs) in the VHA use a radiology diagnostic code to systematically identify imaging studies with presence of lung nodules. In VISN 23, which includes Minnesota, North Dakota, South Dakota, Iowa, and portions of neighboring states, the code used to identify these radiology studies is 44. However, there is high variability in the reporting and coding of imaging studies among radiologists, which could lead to misclassifying patients with lung nodules.8
Some studies suggest that using an automated text search algorithm within radiology reports can be a highly effective strategy to identify patients with lung nodules.9,10 In this study, we compared the diagnostic performance of a newly developed text search algorithm applied to radiology reports with the current standard practice of using a radiology diagnostic code for identifying patients with lung nodules at the Iowa City US Department of Veterans Affairs (VA) Health Care System (ICVAHCS) hospital in Iowa.
Methods
Since 2014, The ICVAHCS has used a radiology diagnostic code to identify any imaging studies with lung nodules. The radiologist enters “44” at the end of the reading process using the Nuance Powerscribe 360 radiation reporting system. The code is uploaded into the VHA Corporate Data Warehouse (CDW), and it is located within the radiology exam domain. This strategy was created and implemented by the Minneapolis VA Health Care System in Minnesota for all the VA hospitals in VISN 23. A lung nodule registry nurse was provided with a list of radiology studies flagged with this radiology diagnostic code every 2 weeks. A chart review was then performed for all these studies to determine the presence of a lung nodule. When detected, the ordering health care provider was alerted and given recommendations for managing the nodule.
We initially searched for the radiology studies with a presumptive lung nodule using the radiology code 44 within the CDW. Separately, we applied the text search strategy only to radiology reports from chest and abdomen studies (ie, X-rays, CT, magnetic resonance imaging [MRI], and PET) that contained any of the keyword phrases. The text search strategy was modeled based on a natural language processing (NLP) algorithm developed by the Puget Sound VA Healthcare System in Seattle, Washington to identify lung nodules on radiology reports.9 Our algorithm included a series of text searches using Microsoft SQL. After several simulations using a random group of radiology reports, we chose the keywords: “lung AND nodul”; “pulm AND nodul”; “pulm AND mass”; “lung AND mass”; and “ground glass”. We selected only chest and abdomen studies because on several simulations using a random group of radiology reports, the vast majority of lung nodules were identified on chest and abdomen imaging studies. Also, it would not have been feasible to chart review the approximately 30,000 total radiology reports that were generated during the study period.
From January 1, 2016 through November 30, 2016, we applied both search strategies independently: radiology diagnostic code for lung nodules to all imaging studies, and text search to all radiology reports of chest and abdomen imaging studies in the CDW (Figure). We also collected demographic (eg, age, sex, race, rurality) and clinical (eg, medical comorbidities, tobacco use) information that were uploaded to the database automatically from CDW using International Statistical Classification of Diseases, Tenth Edition and demographic codes. The VHA uses the Rural-Urban Commuting Areas (RUCA) system to define rurality, which takes into account population density and how closely a community is linked socioeconomically to larger urban centers.11 The protocol was reviewed and approved by the institutional review board of ICVAHCS and the University of Iowa.
The presence of a lung nodule was established by having the lung nodule registry nurse manually review the charts of every patient with a radiology report identified by either code 44 or the text search algorithm. The goal was to ensure that our text search strategy identified all reports with a code 44 to be compliant with VISN expectations. Cases in which a lung nodule was described in the radiology report were considered true positives, and those without a lung nodule description were considered false positives.
We compared the sociodemographic and clinical characteristics of patients with lung nodules between those identified with both code 44 and the text search and those identified with the text search alone. We used χ2 tests for categorical variables (eg, age, gender, RUCA, chronic obstructive pulmonary disease (COPD), smoking status) and t tests for continuous variables (eg, Charlson comorbidity score). A P value ≤ .05 was considered statistically significant. To assess the yield of each search strategy, we determined the number of patients with lung nodules detected by the text search and the radiology diagnostic code. We also calculated the positive predictive value (PPV) and 95% CI of each search strategy.
Results
We identified 12,983 radiology studies that required manual review during the study period. We confirmed that 8,516 imaging studies had lung nodules, representing 2,912 patients. Subjects with lung nodules were predominantly male (96%), aged between 60 and 79 years (71%), and lived in a rural area (72%). More than 50% of these patients had COPD and over a third were current smokers (Table 1). The text search algorithm identified all of the patients identified by the radiology diagnostic code (n = 1,251). It also identified an additional 1,661 patients with lung nodules that otherwise would have been missed by the radiology code. Compared with those identified only by the text search, those identified by both the radiology coding and text search were older, had lower Charlson comorbidity scores, and were more likely to be a current smoker.
The text search algorithm identified more than twice as many patients with potential lung nodules compared with the radiology diagnostic code (4,071 vs 1,363) (Table 2). However, the text search algorithm was associated with a much higher number of false positives than was the diagnostic code (1,159 vs 112) and a lower PPV (72% [95% CI, 70.6-73.4] vs 92% [95% CI, 90.6-93.4], respectively). The text search algorithm identified 130 patients with lung nodules of moderate to high risk for malignancy (> 8 mm diameter) that were not identified by the radiology code. When the PPV of each search strategy was calculated based on imaging studies with nodules (most patients had > 1 imaging study), the results remained similar (98% for radiology code and 66% for text search). A larger proportion of the lung nodules detected by code 44 vs the text search algorithm were from CT chest studies.
Discussion
In a population of predominantly older male veterans with significant risk factors for lung cancer and high incidence of incidental lung nodules, applying a text search algorithm on radiology reports identified a substantial number of patients with lung nodules, including some with nodules > 8 mm, that were missed by the radiologist-generated code.9,10 Improving the yield of detection for lung nodules in a population with high risk for lung cancer would increase the likelihood of detecting patients with potentially curable early-stage lung cancers, decreasing lung cancer mortality.
The reasons for the high number of patients with lung nodules missed by the radiology code are unclear. Potential explanations may include the lack of standardization of imaging reports by the radiologists (ie, only 21% of chest CTs used a standardized template describing a lung nodule in our study), a problem well recognized both within and outside VHA.8,12
The text search algorithm identified more patients with lung nodules but had a higher rate of false positives when compared with the diagnostic code. The high rate of false positives resulted in more charts to review and an increased workload for the lung nodule registry team. The challenges presented by an increased workload should be balanced against the potential harms of missing nodules that develop into advanced cancer.
Text Search Adjustments
Refining the text search criteria algorithm and the chart review process may decrease the rate of false positives significantly without affecting detection of lung nodules. In subsequent simulations, we found that by adding an exclusion criteria to text search algorithm to remove reports with specific keywords we could substantially reduce the number of false positive reports without affecting the detection rate of the lung nodules. These exclusion criteria would exclude any reports that: (1) contain “nodul” within the next 8 words after mentioning “no”; (2) contain “clear” within the next 8 words after mentioning “lung” in the text (eg, “lungs appear to be clear”); (3) contain “clear” within the next 4 words after mentioning “otherwise” in the text (eg, “otherwise appear to be clear”). Based on our study results, we further refined the text search strategy by limiting the search to only chest imaging studies. When we applied the revised algorithm to a random sample of imaging reports, we found all the code 44 radiology reports were still captured, but we were able to reduce the number of radiology reports needing review by about 80%.
Although classification approaches are being refined to improve radiology performance in multiple categories of nodules, this study suggests that alternative approaches based on text algorithms can improve the capture of pulmonary nodules that require surveillance. These algorithms also can be used to augment radiologist reporting systems. This represents an investment in resources to build a team that should include a bioinformatics specialist, lung nodule registry personnel (review charts of the detected imaging studies with lung nodules, populating the lung nodule database, and determining and tracking the need of imaging follow up), a lung nodule clinic nurse coordinator, and a dedicated lung nodule clinic pulmonologist.
Radiology departments could employ this text search approach to identify missed nodules and use an audit and feedback system to train radiologists to code lung nodules consistently at the time of the initial reading to avoid delays in identifying patients with nodules. Alternatively, the more widespread use of a standardized CT chest radiology reports using Fleischner or the American College of Radiology Lung Imaging Reporting and Data System (Lung RADS) templates might improve the detection of patients with lung nodules.5,13,14
The VHA system should have an effective strategy for identifying incidental lung nodules during routine radiology examinations. Relying only on radiologists to identify and code pulmonary nodules can lead to missing a significant number of patients with lung nodules and some patients with early stage lung cancer who could receive curative therapy.12,14-16 The use of a standardized algorithm, like a text search strategy, might decrease the risk of variation in the execution and result in a more sensitive detection of patients with lung nodules. The text search strategy might be easily implemented and shared with other hospitals both within and outside the VHA.
Limitations
This study was performed in a single VHA hospital and the findings may not be generalizable to other settings of care. Second, our study design is susceptible to work-up bias because the results of a diagnostic test (eg, chest or abdomen imaging) affected whether the chart review was used to verify the test result. It was not feasible to review the patient records of all radiology studies done at the facility during the study period, consequently complete 2 × 2 tables could not be created to calculate sensitivity, specificity, and negative predictive value.
Conclusion
A text search algorithm of radiology reports increased the detection of patients with lung nodules when compared with radiology diagnostic coding alone. However, the improved detection was associated with a higher rate of false positives, which requires manually reviewing a larger number of patient’s chart reports. Future research and quality improvement should focus on standardizing the radiology reporting process and improving the efficiency and reliability of follow up and tracking of incidental lung nodules.
Acknowledgments
The work reported here was supported by a grant from the Office of Rural Health (N32-FY16Q1-S1-P01577), US Department of Veterans Affairs, Veterans Health Administration. We also had the support from the Veterans Rural Health Resource Center-Iowa City, and the Health Services Research and Development (HSR&D) Service through the Comprehensive Access and Delivery Research and Evaluation (CADRE) Center (REA 09-220).
Rapid advances in imaging technology have led to better spatial resolution with lower radiation doses to patients. These advances have helped to increase the use of diagnostic chest imaging, particularly in emergency departments and oncology centers, and in screening for coronary artery disease. As a result, there has been an explosion of incidental findings on chest imaging—including indeterminate lung nodules.1,2
Lung nodules are rounded and well-circumscribed lung opacities (≤ 3 cm in diameter) that may present as solitary or multiple lesions in usually asymptomatic patients. Most lung nodules are benign, the result of an infectious or inflammatory process. Nodules that are ≤ 8 mm in diameter, unless they show increase in size over time, often can be safely followed with imaging surveillance. In contrast, lung nodules > 8 mm could represent an early-stage lung cancer, especially among patients with high-risk for developing lung cancer (ie, those with advanced age, heavy tobacco abuse, or emphysema) and should be further assessed with close imaging surveillance, either chest computed tomography (CT) alone or positron-emission tomography (PET)/CT, or tissue biopsy, based on the underlying likelihood of malignancy.
Patients who receive an early-stage lung cancer diagnosis can be offered curative treatments leading to improved 5-year survival rates.3,4 Consequently, health care systems need to be able to identify these nodules accurately, in order to categorize and manage them accordingly to the Fleischner radiographic and American College of Chest Physicians clinical guidelines.5,6 Unfortunately, many hospitals struggle to identify patients with incidental lung nodules found during diagnostic chest and abdominal imaging, due in part to poor adherence to Fleischner guidelines among radiologists for categorizing pulmonary nodules.7,8
The Veterans Health Administration (VHA) system is interested in effectively detecting patients with incidental lung nodules. Veterans have a higher risk of developing lung cancer when compared with the entire US population, mainly due to a higher incidence of tobacco use.6 The prevalence of lung nodules among veterans with significant risk factors for lung cancer is about 60% nationwide, and up to 85% in the Midwest, due to the high prevalence of histoplasmosis.7 However, only a small percentage of these nodules represent an early stage primary lung cancer.
Several Veterans Integrated Service Networks (VISNs) in the VHA use a radiology diagnostic code to systematically identify imaging studies with presence of lung nodules. In VISN 23, which includes Minnesota, North Dakota, South Dakota, Iowa, and portions of neighboring states, the code used to identify these radiology studies is 44. However, there is high variability in the reporting and coding of imaging studies among radiologists, which could lead to misclassifying patients with lung nodules.8
Some studies suggest that using an automated text search algorithm within radiology reports can be a highly effective strategy to identify patients with lung nodules.9,10 In this study, we compared the diagnostic performance of a newly developed text search algorithm applied to radiology reports with the current standard practice of using a radiology diagnostic code for identifying patients with lung nodules at the Iowa City US Department of Veterans Affairs (VA) Health Care System (ICVAHCS) hospital in Iowa.
Methods
Since 2014, The ICVAHCS has used a radiology diagnostic code to identify any imaging studies with lung nodules. The radiologist enters “44” at the end of the reading process using the Nuance Powerscribe 360 radiation reporting system. The code is uploaded into the VHA Corporate Data Warehouse (CDW), and it is located within the radiology exam domain. This strategy was created and implemented by the Minneapolis VA Health Care System in Minnesota for all the VA hospitals in VISN 23. A lung nodule registry nurse was provided with a list of radiology studies flagged with this radiology diagnostic code every 2 weeks. A chart review was then performed for all these studies to determine the presence of a lung nodule. When detected, the ordering health care provider was alerted and given recommendations for managing the nodule.
We initially searched for the radiology studies with a presumptive lung nodule using the radiology code 44 within the CDW. Separately, we applied the text search strategy only to radiology reports from chest and abdomen studies (ie, X-rays, CT, magnetic resonance imaging [MRI], and PET) that contained any of the keyword phrases. The text search strategy was modeled based on a natural language processing (NLP) algorithm developed by the Puget Sound VA Healthcare System in Seattle, Washington to identify lung nodules on radiology reports.9 Our algorithm included a series of text searches using Microsoft SQL. After several simulations using a random group of radiology reports, we chose the keywords: “lung AND nodul”; “pulm AND nodul”; “pulm AND mass”; “lung AND mass”; and “ground glass”. We selected only chest and abdomen studies because on several simulations using a random group of radiology reports, the vast majority of lung nodules were identified on chest and abdomen imaging studies. Also, it would not have been feasible to chart review the approximately 30,000 total radiology reports that were generated during the study period.
From January 1, 2016 through November 30, 2016, we applied both search strategies independently: radiology diagnostic code for lung nodules to all imaging studies, and text search to all radiology reports of chest and abdomen imaging studies in the CDW (Figure). We also collected demographic (eg, age, sex, race, rurality) and clinical (eg, medical comorbidities, tobacco use) information that were uploaded to the database automatically from CDW using International Statistical Classification of Diseases, Tenth Edition and demographic codes. The VHA uses the Rural-Urban Commuting Areas (RUCA) system to define rurality, which takes into account population density and how closely a community is linked socioeconomically to larger urban centers.11 The protocol was reviewed and approved by the institutional review board of ICVAHCS and the University of Iowa.
The presence of a lung nodule was established by having the lung nodule registry nurse manually review the charts of every patient with a radiology report identified by either code 44 or the text search algorithm. The goal was to ensure that our text search strategy identified all reports with a code 44 to be compliant with VISN expectations. Cases in which a lung nodule was described in the radiology report were considered true positives, and those without a lung nodule description were considered false positives.
We compared the sociodemographic and clinical characteristics of patients with lung nodules between those identified with both code 44 and the text search and those identified with the text search alone. We used χ2 tests for categorical variables (eg, age, gender, RUCA, chronic obstructive pulmonary disease (COPD), smoking status) and t tests for continuous variables (eg, Charlson comorbidity score). A P value ≤ .05 was considered statistically significant. To assess the yield of each search strategy, we determined the number of patients with lung nodules detected by the text search and the radiology diagnostic code. We also calculated the positive predictive value (PPV) and 95% CI of each search strategy.
Results
We identified 12,983 radiology studies that required manual review during the study period. We confirmed that 8,516 imaging studies had lung nodules, representing 2,912 patients. Subjects with lung nodules were predominantly male (96%), aged between 60 and 79 years (71%), and lived in a rural area (72%). More than 50% of these patients had COPD and over a third were current smokers (Table 1). The text search algorithm identified all of the patients identified by the radiology diagnostic code (n = 1,251). It also identified an additional 1,661 patients with lung nodules that otherwise would have been missed by the radiology code. Compared with those identified only by the text search, those identified by both the radiology coding and text search were older, had lower Charlson comorbidity scores, and were more likely to be a current smoker.
The text search algorithm identified more than twice as many patients with potential lung nodules compared with the radiology diagnostic code (4,071 vs 1,363) (Table 2). However, the text search algorithm was associated with a much higher number of false positives than was the diagnostic code (1,159 vs 112) and a lower PPV (72% [95% CI, 70.6-73.4] vs 92% [95% CI, 90.6-93.4], respectively). The text search algorithm identified 130 patients with lung nodules of moderate to high risk for malignancy (> 8 mm diameter) that were not identified by the radiology code. When the PPV of each search strategy was calculated based on imaging studies with nodules (most patients had > 1 imaging study), the results remained similar (98% for radiology code and 66% for text search). A larger proportion of the lung nodules detected by code 44 vs the text search algorithm were from CT chest studies.
Discussion
In a population of predominantly older male veterans with significant risk factors for lung cancer and high incidence of incidental lung nodules, applying a text search algorithm on radiology reports identified a substantial number of patients with lung nodules, including some with nodules > 8 mm, that were missed by the radiologist-generated code.9,10 Improving the yield of detection for lung nodules in a population with high risk for lung cancer would increase the likelihood of detecting patients with potentially curable early-stage lung cancers, decreasing lung cancer mortality.
The reasons for the high number of patients with lung nodules missed by the radiology code are unclear. Potential explanations may include the lack of standardization of imaging reports by the radiologists (ie, only 21% of chest CTs used a standardized template describing a lung nodule in our study), a problem well recognized both within and outside VHA.8,12
The text search algorithm identified more patients with lung nodules but had a higher rate of false positives when compared with the diagnostic code. The high rate of false positives resulted in more charts to review and an increased workload for the lung nodule registry team. The challenges presented by an increased workload should be balanced against the potential harms of missing nodules that develop into advanced cancer.
Text Search Adjustments
Refining the text search criteria algorithm and the chart review process may decrease the rate of false positives significantly without affecting detection of lung nodules. In subsequent simulations, we found that by adding an exclusion criteria to text search algorithm to remove reports with specific keywords we could substantially reduce the number of false positive reports without affecting the detection rate of the lung nodules. These exclusion criteria would exclude any reports that: (1) contain “nodul” within the next 8 words after mentioning “no”; (2) contain “clear” within the next 8 words after mentioning “lung” in the text (eg, “lungs appear to be clear”); (3) contain “clear” within the next 4 words after mentioning “otherwise” in the text (eg, “otherwise appear to be clear”). Based on our study results, we further refined the text search strategy by limiting the search to only chest imaging studies. When we applied the revised algorithm to a random sample of imaging reports, we found all the code 44 radiology reports were still captured, but we were able to reduce the number of radiology reports needing review by about 80%.
Although classification approaches are being refined to improve radiology performance in multiple categories of nodules, this study suggests that alternative approaches based on text algorithms can improve the capture of pulmonary nodules that require surveillance. These algorithms also can be used to augment radiologist reporting systems. This represents an investment in resources to build a team that should include a bioinformatics specialist, lung nodule registry personnel (review charts of the detected imaging studies with lung nodules, populating the lung nodule database, and determining and tracking the need of imaging follow up), a lung nodule clinic nurse coordinator, and a dedicated lung nodule clinic pulmonologist.
Radiology departments could employ this text search approach to identify missed nodules and use an audit and feedback system to train radiologists to code lung nodules consistently at the time of the initial reading to avoid delays in identifying patients with nodules. Alternatively, the more widespread use of a standardized CT chest radiology reports using Fleischner or the American College of Radiology Lung Imaging Reporting and Data System (Lung RADS) templates might improve the detection of patients with lung nodules.5,13,14
The VHA system should have an effective strategy for identifying incidental lung nodules during routine radiology examinations. Relying only on radiologists to identify and code pulmonary nodules can lead to missing a significant number of patients with lung nodules and some patients with early stage lung cancer who could receive curative therapy.12,14-16 The use of a standardized algorithm, like a text search strategy, might decrease the risk of variation in the execution and result in a more sensitive detection of patients with lung nodules. The text search strategy might be easily implemented and shared with other hospitals both within and outside the VHA.
Limitations
This study was performed in a single VHA hospital and the findings may not be generalizable to other settings of care. Second, our study design is susceptible to work-up bias because the results of a diagnostic test (eg, chest or abdomen imaging) affected whether the chart review was used to verify the test result. It was not feasible to review the patient records of all radiology studies done at the facility during the study period, consequently complete 2 × 2 tables could not be created to calculate sensitivity, specificity, and negative predictive value.
Conclusion
A text search algorithm of radiology reports increased the detection of patients with lung nodules when compared with radiology diagnostic coding alone. However, the improved detection was associated with a higher rate of false positives, which requires manually reviewing a larger number of patient’s chart reports. Future research and quality improvement should focus on standardizing the radiology reporting process and improving the efficiency and reliability of follow up and tracking of incidental lung nodules.
Acknowledgments
The work reported here was supported by a grant from the Office of Rural Health (N32-FY16Q1-S1-P01577), US Department of Veterans Affairs, Veterans Health Administration. We also had the support from the Veterans Rural Health Resource Center-Iowa City, and the Health Services Research and Development (HSR&D) Service through the Comprehensive Access and Delivery Research and Evaluation (CADRE) Center (REA 09-220).
1. Jacobs PC, Mali WP, Grobbee DE, van der Graaf Y. Prevalence of incidental findings in computed tomographic screening of the chest: a systematic review. Journal of computer assisted tomography. 2008;32(2):214-221.
2. Frank L, Quint LE. Chest CT incidentalomas: thyroid lesions, enlarged mediastinal lymph nodes, and lung nodules. Cancer Imaging. 2012;12(1):41-48.
3. National Institutes of Health, National Cancer Institute, Surveillance, Epidemiology, and End Results Program. Cancer stat facts: lung and bronchus cancer. https://seer.cancer.gov/statfacts/html/lungb.html. Accessed April 8, 2020.
4. Alberg AJ, Brock MV, Ford JG, Samet JM, Spivack SD. Epidemiology of lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e1S-e29S.
5. MacMahon H, Naidich DP, Goo JM, et al. Guidelines for Management of Incidental Pulmonary Nodules Detected on CT Images: From the Fleischner Society 2017. Radiology. 2017;284(1):228-243.
6. Zullig LL, Jackson GL, Dorn RA, et al. Cancer incidence among patients of the U.S. Veterans Affairs Health Care System. Mil Med. 2012;177(6):693-701.
7. Kinsinger LS, Anderson C, Kim J, et al. Implementation of lung cancer screening in the Veterans Health Administration. JAMA Intern Med. 2017;177(3):399-406.
8. Iqbal MN, Stott E, Huml AM, et al. What’s in a name? Factors associated with documentation and evaluation of incidental pulmonary nodules. Ann Am Thorac Soc. 2016;13(10):1704-1711.
9. Farjah F, Halgrim S, Buist DS, et al. An automated method for identifying individuals with a lung nodule can be feasibly implemented across health systems. Egems (Wash DC). 2016;4(1):1254.
10. Danforth KN, Early MI, Ngan S, Kosco AE, Zheng C, Gould MK. Automated identification of patients with pulmonary nodules in an integrated health system using administrative health plan data, radiology reports, and natural language processing. J Thorac Oncol. 2012;7(8):1257-1262.
11. US Department of Veterans Affairs, Office of Rural Health. https://www.ruralhealth.va.gov/aboutus/ruralvets.asp. Updated January 28, 2020. Accessed April 8, 2020.
12. Blagev DP, Lloyd JF, Conner K, et al. Follow-up of incidental pulmonary nodules and the radiology report. J Am Coll Radiol. 2016;13(2 suppl):R18-R24.
13. Eisenberg RL, Fleischner S. Ways to improve radiologists’ adherence to Fleischner Society guidelines for management of pulmonary nodules. J Am Coll Radiol. 2013;10(6):439-441.
14. Aberle DR. Implementing lung cancer screening: the US experience. Clin Radiol. 2017;72(5):401-406.
15. Gould MK, Donington J, Lynch WR, et al. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e93S-e120S.
16. Callister ME, Baldwin DR. How should pulmonary nodules be optimally investigated and managed? Lung Cancer. 2016;91:48-55.
1. Jacobs PC, Mali WP, Grobbee DE, van der Graaf Y. Prevalence of incidental findings in computed tomographic screening of the chest: a systematic review. Journal of computer assisted tomography. 2008;32(2):214-221.
2. Frank L, Quint LE. Chest CT incidentalomas: thyroid lesions, enlarged mediastinal lymph nodes, and lung nodules. Cancer Imaging. 2012;12(1):41-48.
3. National Institutes of Health, National Cancer Institute, Surveillance, Epidemiology, and End Results Program. Cancer stat facts: lung and bronchus cancer. https://seer.cancer.gov/statfacts/html/lungb.html. Accessed April 8, 2020.
4. Alberg AJ, Brock MV, Ford JG, Samet JM, Spivack SD. Epidemiology of lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e1S-e29S.
5. MacMahon H, Naidich DP, Goo JM, et al. Guidelines for Management of Incidental Pulmonary Nodules Detected on CT Images: From the Fleischner Society 2017. Radiology. 2017;284(1):228-243.
6. Zullig LL, Jackson GL, Dorn RA, et al. Cancer incidence among patients of the U.S. Veterans Affairs Health Care System. Mil Med. 2012;177(6):693-701.
7. Kinsinger LS, Anderson C, Kim J, et al. Implementation of lung cancer screening in the Veterans Health Administration. JAMA Intern Med. 2017;177(3):399-406.
8. Iqbal MN, Stott E, Huml AM, et al. What’s in a name? Factors associated with documentation and evaluation of incidental pulmonary nodules. Ann Am Thorac Soc. 2016;13(10):1704-1711.
9. Farjah F, Halgrim S, Buist DS, et al. An automated method for identifying individuals with a lung nodule can be feasibly implemented across health systems. Egems (Wash DC). 2016;4(1):1254.
10. Danforth KN, Early MI, Ngan S, Kosco AE, Zheng C, Gould MK. Automated identification of patients with pulmonary nodules in an integrated health system using administrative health plan data, radiology reports, and natural language processing. J Thorac Oncol. 2012;7(8):1257-1262.
11. US Department of Veterans Affairs, Office of Rural Health. https://www.ruralhealth.va.gov/aboutus/ruralvets.asp. Updated January 28, 2020. Accessed April 8, 2020.
12. Blagev DP, Lloyd JF, Conner K, et al. Follow-up of incidental pulmonary nodules and the radiology report. J Am Coll Radiol. 2016;13(2 suppl):R18-R24.
13. Eisenberg RL, Fleischner S. Ways to improve radiologists’ adherence to Fleischner Society guidelines for management of pulmonary nodules. J Am Coll Radiol. 2013;10(6):439-441.
14. Aberle DR. Implementing lung cancer screening: the US experience. Clin Radiol. 2017;72(5):401-406.
15. Gould MK, Donington J, Lynch WR, et al. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e93S-e120S.
16. Callister ME, Baldwin DR. How should pulmonary nodules be optimally investigated and managed? Lung Cancer. 2016;91:48-55.
Incidental Findings of Pulmonary and Hilar Malignancy by Low-Resolution Computed Tomography Used in Myocardial Perfusion Imaging (FULL)
Single-photon emission computed tomography (SPECT) myocardial perfusion imaging (MPI) is a well-established technique for the evaluation of coronary artery disease (CAD).1 To improve image quality, low-resolution computed tomography (CT) is used commonly for anatomical correct and artifact attenuation during SPECT MPI.2 The low resolution, unenhanced CT images are considered low quality and are, therefore, labeled by the manufacturer as nondiagnostic. The CT portion of the MPI in many centers is used only for image fusion and attenuation correction, and these images are not routinely reviewed or reported by cardiologists.
Incidental findings by these low-resolution CT were frequent. However, clinically significant findings, including lung cancer, although relatively infrequent, were serious enough for major clinical management.3-5 Currently, there are no consensus recommendations for reviewing low-resolution CT images or the interpretation of such incidental findings during cardiac MPI.6 Clinically, low-dose CT were used for early detection and screening of lung cancer and were associated with reduced lung-cancer and any cause mortality in National Lung Screening Trial (NLST).7,8 Therefore, low-dose CT is recommended for lung cancer screening of high-risk patients by the US Preventive Service Task Force (USPSTF).9 In the veteran population, current and past smoking history are more common when compared with the general population; therefore, veterans are potentially at increased risk of lung cancer.10 In this study, we did not intend to use low-resolution CT for lung cancer screening or detection but rather to identify and report incidental findings of pulmonary/hilar malignancy detected during cardiac MPI.
Methods
The Siemens’ (Munich, Germany) Symbia Intevo Excel SPECT/CT MPI cameras with dedicated cardiac collimators were used at both the Dwight D. Eisenhower VA Medical Center (VAMC) in Leavenworth, Kansas and Colmery-O'Neil VAMC in Topeka, Kansas. The integrated CT scanner (x-ray tube current 30 to 240 mA; voltage 110 Kv with a 40 kW power generator) has the capability to image up to a 2-slice/rotation, each of 5.0 mm per slice with a scan time of about 30 seconds. The SPECT/CT gamma camera has a low energy (140 KeV), high resolution, parallel hole collimator with IQ SPECT capabilities.
The radiation dose received by the patients were expressed in dose length product (DLP), which reflects the total energy absorbed by the patient and represents integrated dose in terms of the total scan length. Additionally, each patients received 2 injections of Technetium Tc 99m sestamibi (1-day Protocol: 10 mCi rest injection, 30 mCi stress injection: 2-day Protocol for patients weighing > 350 pounds: 30 mCi at rest injection and 30 mCi at stress injection) for myocardial perfusion imaging.
All CT images and cardiac MPI findings were reviewed and reported contemporaneously by 1 of 2 experienced, board-certified radiologists who were blinded to patients’ clinical information except the indication for the cardiac stress testing. When suspicious pulmonary/hilar nodules or masses were detected, these findings and recommendations for further evaluation were conveyed to primary care provider or ordering physician via the electronic health record system.
All CT images were reviewed with cardiac MPI from September 1, 2017 to August 31, 2018. When pulmonary/hilar malignancies were identified, the health records were reviewed. Patients with known history of prior pulmonary malignancy were excluded from the study.
Results
A total of 1,098 patients underwent cardiac MPI during the study period. When the CT imaging and cardiac MPI were reviewed, incidental findings led to the diagnosis of lung cancer in 5 patients and hilar mantle cell lymphoma in 1 patient. Their clinical characteristics, CT findings, and types of malignancies for these 6 patients are summarized in the Table and Figure. Only 0.55% (6 of 1,098) patients were found to have incidental pulmonary/hilar malignancy with the cardiac evaluation low-resolution CT. Four patients with prior, known history of lung cancer were excluded from the study.
For the 6 patients found to have cancer, the average CT radiation dose during the cardiac MPI was 100 mGy-cm (range, 77 -133 mCy-cm). The subsequent chest CT with or without contrast delivered a radiation dose of 726.4 mGy-cm (range, 279.4 - 1,075 mGy-cm).
A total of 79 (7.2%) patients were found to have significant pulmonary nodules that required further evaluation; after CT examination, 32 patients had findings of benign nature and required no further follow-up; the other 47 patients are being followed according to the Fleischner Society 2017 guidelines for pulmonary nodules.11 The follow-up findings on these patients are not within the scope of this report.
Discussion
Although incidental findings on low-resolution CT during cardiac MPI are frequent, clinically significant findings are less common. However, some incidental findings may be of important clinical significance.3-5 A multicenter analysis by Coward and colleagues reported that 2.4% findings on low-resolution CT were significant enough to warrant follow-up tests, but only 0.2% were deemed potentially detrimental to patient outcomes (ie, pathology confirmed malignancies).12 Thus, the authors suggested that routine reporting of incidental findings on low-dose CT images was not beneficial.12,13
Currently, the majority of cardiac MPIs are reviewed and interpreted by nuclear cardiologists, the use of hybrid SPECT/CT for attenuation correction give rise of issue of reviewing and interpreting these CT images during cardiac MPI. Since low-dose, low-resolution CT are considered nondiagnostic, these images are not routinely and readily reviewed by cardiologists who are not trained or skilled in CT interpretations.
Studies of high-resolution cardiac CT (including multidetector CT with contrast) suggest that incidental extracardiac findings should always be reported as there was a 0.7% incidence of previously unknown malignancies, while others have argued against “performing large field reconstructionsfor the explicit purpose of screening as it will lead to additional cost, liability and anxiety without proven benefits.”14-16 A review of incidental findings of cardiac CT by Earls suggested that all cardiac CT should be reconstructed in the maximal field of view available and images should be adequately reviewed to detect pathological findings.17 This led to an interesting discussion by Douglas and colleagues regarding the role of cardiologists and radiologists in this issue.18 Currently there is no uniform or consensus recommendations regarding incidental findings during cardiac CT imaging. Guidances range from no recommendations to optional reporting or mandatory reporting.19-23
Risk Factors for Veterans
Lung cancer is the second most common cancer and the leading cause of cancer-related death in the US.24 Smoking is the most important risk factor for lung cancer and CAD.25 Current or past smoking are more common among the veterans.10 According to a report for the US Centers for Disease Control and Prevention report, about 29.2% US veterans use tobacco products between 2010-2015, which is similar to the rate reported in 1997.26
When low-dose CT was used for lung cancer screening, it was associated with a 20.0% reduction in lung cancer mortality and a 6.7% reduction in any cause mortality.7 Currently, the US Preventive Services Task Force (USPSTF) recommends annual low-dose CT screening for lung cancer in high-risk adults that includes patients aged 55 to 80 years who have a 30-pack-year smoking history and currently smoke or have quit within the past 15 years.8
It is likely that the cardiac patients in this study might have pulmonary malignancy mortality similar to those reported in the NLST. While other studies have shown a low incidence (0.2%) of detection of malignancy by low-resolution CT during cardiac MPI,12,13 in this study we found pulmonary or hilar malignancy in 0.55% of patients.The higher incidence of malignancy in our study might be due in part to differences in the patient population studied (ie, our veterans patients have a higher proportion of current or past smoking history).10
The CT used in this study is part of the cardiac imaging process. Therefore, there was no additional radiation exposure besides that of the cardiac MPI for patients. Despite the limitations of low-resolution CT, which may miss small lesions, this study showed 0.55% incidence of incidental detection of pulmonary/hilar malignancy. This is comparable with 0.65%/year of diagnosing lung cancer using low-dose CT for lung cancer screening in NLST.8
Two of the 5 study patients who were found to have lung cancer, had quit smoking > 15 years previously and thus would not be considered as high-risk for lung cancer screening according to USPSTF guideline. These patients would not have been candidates for annual low-dose CT lung cancer screening. This study suggests that it is appropriate and necessary to review the low-resolution CT images for incidental findings during cardiac MPI.
Limitations
The study was retrospective in nature and limited by its small number of patients. The CT modality used in the study also has limitations, including low resolution, respiratory motion artifacts, and scans that did not include the entire chest area. Therefore, small and apical lesions may have been missed. However, both sets of CT at rest and after stress were reviewed to reduce or minimize the effects of respiratory motion artifacts. The true prevalence or incidence of pulmonary/hilar malignancies may have been higher than reported here. Our study population of veterans may not be representative of the general population with regards to gender (as most of our veteran patient population are of male gender, vs general population), smoking history, or lung cancer risk, thus the results should be interpreted with caution.
Conclusion
Low-resolution CTs used for attenuation correction during cardiac MPI should be routinely reviewed and interpreted by a physician or radiologist skilled in CT interpretation in order to identify incidental findings of pulmonary/hilar malignancy. This would require close collaboration between cardiologists and radiologists in the field to ensure unfragmented and high-quality patient care.
Acknowledgements
We want to thank all the staffs in cardiology and radiology department on both campuses for their dedication for our patients. Special thanks to Laura Knox, Radiation Safety Officer, Nuclear Medicine Supervisor for her technical assistance.
1. Hendel RC, Berman DS, Di Carli MF, et al. ACCF/ASNC/ACR/AHA/ASE/SCCT/SCMR/SNM 2009 appropriate use criteria for cardiac radionuclide imaging: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the American Society of Nuclear Cardiology, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the Society of Cardiovascular Computed Tomography, the Society for Cardiovascular Magnetic Resonance, and the Society of Nuclear Medicine. Circulation. 2009;119(22):e561-e587.
2. Hendel RC, Corbett JR, Cullom SJ, DePuey EG, Garcia EV, Bateman TM. The value and practice of attenuation correction for myocardial perfusion SPECT imaging: a joint position statement from the American Society of Nuclear Cardiology and the Society of Nuclear Medicine. J Nucl Cardiol. 2002;9(1):135–143.
3. Coward J, Nightingale J, Hogg P. The clinical dilemma of incidental findings on the low-resolution CT images from SPECT/CT MPI studies. J Nucl Med Technol. 2016;44(3):167-172.
4. Osman MM, Cohade C, Fishman E, Wahl RL. Clinically significant incidental findings on the unenhanced CT portion of PET/CT studies: frequency in 250 patients. J Nucl Med. 2005;46(8):1352-1355.
5. Goetze S, Pannu HK, Wahl RL. Clinically significant abnormal findings on the “nondiagnostic” CT portion of low-amperage-CT attenuation-corrected myocardial perfusion SPECT/CT studies. J Nucl Med. 2006;47(8):1312-1318.
6. American College of Cardiology Foundation Task Force on Expert Consensus Documents, Mark DB, Berman DS, et al. ACCF/ACR/AHA/NASCI/SAIP/SCAI/SCCT 2010 expert consensus document on coronary computed tomographic angiography: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. J Am Coll Cardiol. 2010;55(23):2663-2699.
7. Diederich S, Wormanns D, Semik M, et al. Screening for early lung cancer with low-dose spiral CT: prevalence in 817 asymptomatic smokers. Radiology. 2002;222(3):773-781.
8. National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395-409.
9. Moyer VA; U.S. Preventive Services Task Force. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(5):330-338.
10. McKinney WP, McIntire DD, Carmody TJ, Joseph A. Comparing the smoking behavior of veterans and nonveterans. Public Health Rep. 1997;112(3):212-218.
11. MacMahon H, Naidich DP, Goo JM, et al. Guidelines for Management of Incidental Pulmonary Nodules Detected on CT Images: From the Fleischner Society 2017. Radiology. 2017;284(1):228-243.
12. Coward J, Lawson R, Kane T, et al. Multi-centre analysis of incidental findings on low-resolution CT attenuation correction images. Br J Radiol. 2014;87(1042):20130701.
13. Coward J, Lawson R, Kane T, et al. Multicentre analysis of incidental findings on low-resolution CT attenuation correction images: an extended study. Br J Radiol. 2015;88(1056):20150555.
14. Haller S, Kaiser C, Buser P, Bongartz G, Bremerich J. Coronary artery imaging with contrast-enhanced MDCT: extracardiac findings. AJR Am J Roentgenol. 2006;187(1):105-110.
15. Flor N, Di Leo G, Squarza SA, et al. Malignant incidental extracardiac findings on cardiac CT: systematic review and meta-analysis. AJR Am J Roentgenol. 2013;201(3):555-564.
16. Budoff MJ, Gopal A. Incidental findings on cardiac computed tomography. Should we look? J Cardiovasc Comput Tomogr. 2007;1(2):97-105.
17. Earls JP. The pros and cons of searching for extracardiac findings at cardiac CT: studies should be reconstructed in the maximum field of view and adequately reviewed to detect pathologic findings. Radiology. 2011;261(2):342-346.
18. Douglas PS, Cerqueria M, Rubin GD, Chin AS. Extracardiac findings: what is a cardiologist to do? JACC Cardiovasc Imaging. 2008;1(5):682-687.
19. Holly TA, Abbott BG, Al-Mallah M, et al. Single photon-emission computed tomography. J Nucl Cardiol. 2010;17(5):941-973.
20. Dorbala S, Ananthasubramaniam K, Armstrong IS, et al. Single photon emission computed tomography (SPECT) myocardial perfusion imaging guidelines: instrumentation, acquisition, processing, and interpretation. J Nucl Cardiol. 2018;25(5):1784-1846.
21. Tilkemeier PL, Bourque J, Doukky R, Sanghani R, Weinberg RL. ASNC imaging guidelines for nuclear cardiology procedures : Standardized reporting of nuclear cardiology procedures. J Nucl Cardiol. 2017;24(6):2064-2128.
22. Dorbala S, Di Carli MF, Delbeke D, et al. SNMMI/ASNC/SCCT guideline for cardiac SPECT/CT and PET/CT 1.0. J Nucl Med. 2013;54(8):1485-1507.
23. Dilsizian V, Bacharach SL, Beanlands RS, et al. ASNC imaging guidelines/SNMMI procedure standard for positron emission tomography (PET) nuclear cardiology procedures. J Nucl Cardiol. 2016;23(5):1187-1226.
24. Jemal A, Ward EM, Johnson CJ, et al. Annual report to the nation on the status of cancer, 1975-2014, Featuring Survival. J Natl Cancer Inst. 2017;109(9):djx030.
25. US Department of Health and Human Services. The Health Consequences of Smoking: 50 Years of Progress. A Report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. Printed with corrections, January 2014.
26. Odani S, Agaku IT, Graffunder CM, Tynan MA, Armour BS. Tobacco Product Use Among Military Veterans - United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2018;67(1):7-12.
Single-photon emission computed tomography (SPECT) myocardial perfusion imaging (MPI) is a well-established technique for the evaluation of coronary artery disease (CAD).1 To improve image quality, low-resolution computed tomography (CT) is used commonly for anatomical correct and artifact attenuation during SPECT MPI.2 The low resolution, unenhanced CT images are considered low quality and are, therefore, labeled by the manufacturer as nondiagnostic. The CT portion of the MPI in many centers is used only for image fusion and attenuation correction, and these images are not routinely reviewed or reported by cardiologists.
Incidental findings by these low-resolution CT were frequent. However, clinically significant findings, including lung cancer, although relatively infrequent, were serious enough for major clinical management.3-5 Currently, there are no consensus recommendations for reviewing low-resolution CT images or the interpretation of such incidental findings during cardiac MPI.6 Clinically, low-dose CT were used for early detection and screening of lung cancer and were associated with reduced lung-cancer and any cause mortality in National Lung Screening Trial (NLST).7,8 Therefore, low-dose CT is recommended for lung cancer screening of high-risk patients by the US Preventive Service Task Force (USPSTF).9 In the veteran population, current and past smoking history are more common when compared with the general population; therefore, veterans are potentially at increased risk of lung cancer.10 In this study, we did not intend to use low-resolution CT for lung cancer screening or detection but rather to identify and report incidental findings of pulmonary/hilar malignancy detected during cardiac MPI.
Methods
The Siemens’ (Munich, Germany) Symbia Intevo Excel SPECT/CT MPI cameras with dedicated cardiac collimators were used at both the Dwight D. Eisenhower VA Medical Center (VAMC) in Leavenworth, Kansas and Colmery-O'Neil VAMC in Topeka, Kansas. The integrated CT scanner (x-ray tube current 30 to 240 mA; voltage 110 Kv with a 40 kW power generator) has the capability to image up to a 2-slice/rotation, each of 5.0 mm per slice with a scan time of about 30 seconds. The SPECT/CT gamma camera has a low energy (140 KeV), high resolution, parallel hole collimator with IQ SPECT capabilities.
The radiation dose received by the patients were expressed in dose length product (DLP), which reflects the total energy absorbed by the patient and represents integrated dose in terms of the total scan length. Additionally, each patients received 2 injections of Technetium Tc 99m sestamibi (1-day Protocol: 10 mCi rest injection, 30 mCi stress injection: 2-day Protocol for patients weighing > 350 pounds: 30 mCi at rest injection and 30 mCi at stress injection) for myocardial perfusion imaging.
All CT images and cardiac MPI findings were reviewed and reported contemporaneously by 1 of 2 experienced, board-certified radiologists who were blinded to patients’ clinical information except the indication for the cardiac stress testing. When suspicious pulmonary/hilar nodules or masses were detected, these findings and recommendations for further evaluation were conveyed to primary care provider or ordering physician via the electronic health record system.
All CT images were reviewed with cardiac MPI from September 1, 2017 to August 31, 2018. When pulmonary/hilar malignancies were identified, the health records were reviewed. Patients with known history of prior pulmonary malignancy were excluded from the study.
Results
A total of 1,098 patients underwent cardiac MPI during the study period. When the CT imaging and cardiac MPI were reviewed, incidental findings led to the diagnosis of lung cancer in 5 patients and hilar mantle cell lymphoma in 1 patient. Their clinical characteristics, CT findings, and types of malignancies for these 6 patients are summarized in the Table and Figure. Only 0.55% (6 of 1,098) patients were found to have incidental pulmonary/hilar malignancy with the cardiac evaluation low-resolution CT. Four patients with prior, known history of lung cancer were excluded from the study.
For the 6 patients found to have cancer, the average CT radiation dose during the cardiac MPI was 100 mGy-cm (range, 77 -133 mCy-cm). The subsequent chest CT with or without contrast delivered a radiation dose of 726.4 mGy-cm (range, 279.4 - 1,075 mGy-cm).
A total of 79 (7.2%) patients were found to have significant pulmonary nodules that required further evaluation; after CT examination, 32 patients had findings of benign nature and required no further follow-up; the other 47 patients are being followed according to the Fleischner Society 2017 guidelines for pulmonary nodules.11 The follow-up findings on these patients are not within the scope of this report.
Discussion
Although incidental findings on low-resolution CT during cardiac MPI are frequent, clinically significant findings are less common. However, some incidental findings may be of important clinical significance.3-5 A multicenter analysis by Coward and colleagues reported that 2.4% findings on low-resolution CT were significant enough to warrant follow-up tests, but only 0.2% were deemed potentially detrimental to patient outcomes (ie, pathology confirmed malignancies).12 Thus, the authors suggested that routine reporting of incidental findings on low-dose CT images was not beneficial.12,13
Currently, the majority of cardiac MPIs are reviewed and interpreted by nuclear cardiologists, the use of hybrid SPECT/CT for attenuation correction give rise of issue of reviewing and interpreting these CT images during cardiac MPI. Since low-dose, low-resolution CT are considered nondiagnostic, these images are not routinely and readily reviewed by cardiologists who are not trained or skilled in CT interpretations.
Studies of high-resolution cardiac CT (including multidetector CT with contrast) suggest that incidental extracardiac findings should always be reported as there was a 0.7% incidence of previously unknown malignancies, while others have argued against “performing large field reconstructionsfor the explicit purpose of screening as it will lead to additional cost, liability and anxiety without proven benefits.”14-16 A review of incidental findings of cardiac CT by Earls suggested that all cardiac CT should be reconstructed in the maximal field of view available and images should be adequately reviewed to detect pathological findings.17 This led to an interesting discussion by Douglas and colleagues regarding the role of cardiologists and radiologists in this issue.18 Currently there is no uniform or consensus recommendations regarding incidental findings during cardiac CT imaging. Guidances range from no recommendations to optional reporting or mandatory reporting.19-23
Risk Factors for Veterans
Lung cancer is the second most common cancer and the leading cause of cancer-related death in the US.24 Smoking is the most important risk factor for lung cancer and CAD.25 Current or past smoking are more common among the veterans.10 According to a report for the US Centers for Disease Control and Prevention report, about 29.2% US veterans use tobacco products between 2010-2015, which is similar to the rate reported in 1997.26
When low-dose CT was used for lung cancer screening, it was associated with a 20.0% reduction in lung cancer mortality and a 6.7% reduction in any cause mortality.7 Currently, the US Preventive Services Task Force (USPSTF) recommends annual low-dose CT screening for lung cancer in high-risk adults that includes patients aged 55 to 80 years who have a 30-pack-year smoking history and currently smoke or have quit within the past 15 years.8
It is likely that the cardiac patients in this study might have pulmonary malignancy mortality similar to those reported in the NLST. While other studies have shown a low incidence (0.2%) of detection of malignancy by low-resolution CT during cardiac MPI,12,13 in this study we found pulmonary or hilar malignancy in 0.55% of patients.The higher incidence of malignancy in our study might be due in part to differences in the patient population studied (ie, our veterans patients have a higher proportion of current or past smoking history).10
The CT used in this study is part of the cardiac imaging process. Therefore, there was no additional radiation exposure besides that of the cardiac MPI for patients. Despite the limitations of low-resolution CT, which may miss small lesions, this study showed 0.55% incidence of incidental detection of pulmonary/hilar malignancy. This is comparable with 0.65%/year of diagnosing lung cancer using low-dose CT for lung cancer screening in NLST.8
Two of the 5 study patients who were found to have lung cancer, had quit smoking > 15 years previously and thus would not be considered as high-risk for lung cancer screening according to USPSTF guideline. These patients would not have been candidates for annual low-dose CT lung cancer screening. This study suggests that it is appropriate and necessary to review the low-resolution CT images for incidental findings during cardiac MPI.
Limitations
The study was retrospective in nature and limited by its small number of patients. The CT modality used in the study also has limitations, including low resolution, respiratory motion artifacts, and scans that did not include the entire chest area. Therefore, small and apical lesions may have been missed. However, both sets of CT at rest and after stress were reviewed to reduce or minimize the effects of respiratory motion artifacts. The true prevalence or incidence of pulmonary/hilar malignancies may have been higher than reported here. Our study population of veterans may not be representative of the general population with regards to gender (as most of our veteran patient population are of male gender, vs general population), smoking history, or lung cancer risk, thus the results should be interpreted with caution.
Conclusion
Low-resolution CTs used for attenuation correction during cardiac MPI should be routinely reviewed and interpreted by a physician or radiologist skilled in CT interpretation in order to identify incidental findings of pulmonary/hilar malignancy. This would require close collaboration between cardiologists and radiologists in the field to ensure unfragmented and high-quality patient care.
Acknowledgements
We want to thank all the staffs in cardiology and radiology department on both campuses for their dedication for our patients. Special thanks to Laura Knox, Radiation Safety Officer, Nuclear Medicine Supervisor for her technical assistance.
Single-photon emission computed tomography (SPECT) myocardial perfusion imaging (MPI) is a well-established technique for the evaluation of coronary artery disease (CAD).1 To improve image quality, low-resolution computed tomography (CT) is used commonly for anatomical correct and artifact attenuation during SPECT MPI.2 The low resolution, unenhanced CT images are considered low quality and are, therefore, labeled by the manufacturer as nondiagnostic. The CT portion of the MPI in many centers is used only for image fusion and attenuation correction, and these images are not routinely reviewed or reported by cardiologists.
Incidental findings by these low-resolution CT were frequent. However, clinically significant findings, including lung cancer, although relatively infrequent, were serious enough for major clinical management.3-5 Currently, there are no consensus recommendations for reviewing low-resolution CT images or the interpretation of such incidental findings during cardiac MPI.6 Clinically, low-dose CT were used for early detection and screening of lung cancer and were associated with reduced lung-cancer and any cause mortality in National Lung Screening Trial (NLST).7,8 Therefore, low-dose CT is recommended for lung cancer screening of high-risk patients by the US Preventive Service Task Force (USPSTF).9 In the veteran population, current and past smoking history are more common when compared with the general population; therefore, veterans are potentially at increased risk of lung cancer.10 In this study, we did not intend to use low-resolution CT for lung cancer screening or detection but rather to identify and report incidental findings of pulmonary/hilar malignancy detected during cardiac MPI.
Methods
The Siemens’ (Munich, Germany) Symbia Intevo Excel SPECT/CT MPI cameras with dedicated cardiac collimators were used at both the Dwight D. Eisenhower VA Medical Center (VAMC) in Leavenworth, Kansas and Colmery-O'Neil VAMC in Topeka, Kansas. The integrated CT scanner (x-ray tube current 30 to 240 mA; voltage 110 Kv with a 40 kW power generator) has the capability to image up to a 2-slice/rotation, each of 5.0 mm per slice with a scan time of about 30 seconds. The SPECT/CT gamma camera has a low energy (140 KeV), high resolution, parallel hole collimator with IQ SPECT capabilities.
The radiation dose received by the patients were expressed in dose length product (DLP), which reflects the total energy absorbed by the patient and represents integrated dose in terms of the total scan length. Additionally, each patients received 2 injections of Technetium Tc 99m sestamibi (1-day Protocol: 10 mCi rest injection, 30 mCi stress injection: 2-day Protocol for patients weighing > 350 pounds: 30 mCi at rest injection and 30 mCi at stress injection) for myocardial perfusion imaging.
All CT images and cardiac MPI findings were reviewed and reported contemporaneously by 1 of 2 experienced, board-certified radiologists who were blinded to patients’ clinical information except the indication for the cardiac stress testing. When suspicious pulmonary/hilar nodules or masses were detected, these findings and recommendations for further evaluation were conveyed to primary care provider or ordering physician via the electronic health record system.
All CT images were reviewed with cardiac MPI from September 1, 2017 to August 31, 2018. When pulmonary/hilar malignancies were identified, the health records were reviewed. Patients with known history of prior pulmonary malignancy were excluded from the study.
Results
A total of 1,098 patients underwent cardiac MPI during the study period. When the CT imaging and cardiac MPI were reviewed, incidental findings led to the diagnosis of lung cancer in 5 patients and hilar mantle cell lymphoma in 1 patient. Their clinical characteristics, CT findings, and types of malignancies for these 6 patients are summarized in the Table and Figure. Only 0.55% (6 of 1,098) patients were found to have incidental pulmonary/hilar malignancy with the cardiac evaluation low-resolution CT. Four patients with prior, known history of lung cancer were excluded from the study.
For the 6 patients found to have cancer, the average CT radiation dose during the cardiac MPI was 100 mGy-cm (range, 77 -133 mCy-cm). The subsequent chest CT with or without contrast delivered a radiation dose of 726.4 mGy-cm (range, 279.4 - 1,075 mGy-cm).
A total of 79 (7.2%) patients were found to have significant pulmonary nodules that required further evaluation; after CT examination, 32 patients had findings of benign nature and required no further follow-up; the other 47 patients are being followed according to the Fleischner Society 2017 guidelines for pulmonary nodules.11 The follow-up findings on these patients are not within the scope of this report.
Discussion
Although incidental findings on low-resolution CT during cardiac MPI are frequent, clinically significant findings are less common. However, some incidental findings may be of important clinical significance.3-5 A multicenter analysis by Coward and colleagues reported that 2.4% findings on low-resolution CT were significant enough to warrant follow-up tests, but only 0.2% were deemed potentially detrimental to patient outcomes (ie, pathology confirmed malignancies).12 Thus, the authors suggested that routine reporting of incidental findings on low-dose CT images was not beneficial.12,13
Currently, the majority of cardiac MPIs are reviewed and interpreted by nuclear cardiologists, the use of hybrid SPECT/CT for attenuation correction give rise of issue of reviewing and interpreting these CT images during cardiac MPI. Since low-dose, low-resolution CT are considered nondiagnostic, these images are not routinely and readily reviewed by cardiologists who are not trained or skilled in CT interpretations.
Studies of high-resolution cardiac CT (including multidetector CT with contrast) suggest that incidental extracardiac findings should always be reported as there was a 0.7% incidence of previously unknown malignancies, while others have argued against “performing large field reconstructionsfor the explicit purpose of screening as it will lead to additional cost, liability and anxiety without proven benefits.”14-16 A review of incidental findings of cardiac CT by Earls suggested that all cardiac CT should be reconstructed in the maximal field of view available and images should be adequately reviewed to detect pathological findings.17 This led to an interesting discussion by Douglas and colleagues regarding the role of cardiologists and radiologists in this issue.18 Currently there is no uniform or consensus recommendations regarding incidental findings during cardiac CT imaging. Guidances range from no recommendations to optional reporting or mandatory reporting.19-23
Risk Factors for Veterans
Lung cancer is the second most common cancer and the leading cause of cancer-related death in the US.24 Smoking is the most important risk factor for lung cancer and CAD.25 Current or past smoking are more common among the veterans.10 According to a report for the US Centers for Disease Control and Prevention report, about 29.2% US veterans use tobacco products between 2010-2015, which is similar to the rate reported in 1997.26
When low-dose CT was used for lung cancer screening, it was associated with a 20.0% reduction in lung cancer mortality and a 6.7% reduction in any cause mortality.7 Currently, the US Preventive Services Task Force (USPSTF) recommends annual low-dose CT screening for lung cancer in high-risk adults that includes patients aged 55 to 80 years who have a 30-pack-year smoking history and currently smoke or have quit within the past 15 years.8
It is likely that the cardiac patients in this study might have pulmonary malignancy mortality similar to those reported in the NLST. While other studies have shown a low incidence (0.2%) of detection of malignancy by low-resolution CT during cardiac MPI,12,13 in this study we found pulmonary or hilar malignancy in 0.55% of patients.The higher incidence of malignancy in our study might be due in part to differences in the patient population studied (ie, our veterans patients have a higher proportion of current or past smoking history).10
The CT used in this study is part of the cardiac imaging process. Therefore, there was no additional radiation exposure besides that of the cardiac MPI for patients. Despite the limitations of low-resolution CT, which may miss small lesions, this study showed 0.55% incidence of incidental detection of pulmonary/hilar malignancy. This is comparable with 0.65%/year of diagnosing lung cancer using low-dose CT for lung cancer screening in NLST.8
Two of the 5 study patients who were found to have lung cancer, had quit smoking > 15 years previously and thus would not be considered as high-risk for lung cancer screening according to USPSTF guideline. These patients would not have been candidates for annual low-dose CT lung cancer screening. This study suggests that it is appropriate and necessary to review the low-resolution CT images for incidental findings during cardiac MPI.
Limitations
The study was retrospective in nature and limited by its small number of patients. The CT modality used in the study also has limitations, including low resolution, respiratory motion artifacts, and scans that did not include the entire chest area. Therefore, small and apical lesions may have been missed. However, both sets of CT at rest and after stress were reviewed to reduce or minimize the effects of respiratory motion artifacts. The true prevalence or incidence of pulmonary/hilar malignancies may have been higher than reported here. Our study population of veterans may not be representative of the general population with regards to gender (as most of our veteran patient population are of male gender, vs general population), smoking history, or lung cancer risk, thus the results should be interpreted with caution.
Conclusion
Low-resolution CTs used for attenuation correction during cardiac MPI should be routinely reviewed and interpreted by a physician or radiologist skilled in CT interpretation in order to identify incidental findings of pulmonary/hilar malignancy. This would require close collaboration between cardiologists and radiologists in the field to ensure unfragmented and high-quality patient care.
Acknowledgements
We want to thank all the staffs in cardiology and radiology department on both campuses for their dedication for our patients. Special thanks to Laura Knox, Radiation Safety Officer, Nuclear Medicine Supervisor for her technical assistance.
1. Hendel RC, Berman DS, Di Carli MF, et al. ACCF/ASNC/ACR/AHA/ASE/SCCT/SCMR/SNM 2009 appropriate use criteria for cardiac radionuclide imaging: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the American Society of Nuclear Cardiology, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the Society of Cardiovascular Computed Tomography, the Society for Cardiovascular Magnetic Resonance, and the Society of Nuclear Medicine. Circulation. 2009;119(22):e561-e587.
2. Hendel RC, Corbett JR, Cullom SJ, DePuey EG, Garcia EV, Bateman TM. The value and practice of attenuation correction for myocardial perfusion SPECT imaging: a joint position statement from the American Society of Nuclear Cardiology and the Society of Nuclear Medicine. J Nucl Cardiol. 2002;9(1):135–143.
3. Coward J, Nightingale J, Hogg P. The clinical dilemma of incidental findings on the low-resolution CT images from SPECT/CT MPI studies. J Nucl Med Technol. 2016;44(3):167-172.
4. Osman MM, Cohade C, Fishman E, Wahl RL. Clinically significant incidental findings on the unenhanced CT portion of PET/CT studies: frequency in 250 patients. J Nucl Med. 2005;46(8):1352-1355.
5. Goetze S, Pannu HK, Wahl RL. Clinically significant abnormal findings on the “nondiagnostic” CT portion of low-amperage-CT attenuation-corrected myocardial perfusion SPECT/CT studies. J Nucl Med. 2006;47(8):1312-1318.
6. American College of Cardiology Foundation Task Force on Expert Consensus Documents, Mark DB, Berman DS, et al. ACCF/ACR/AHA/NASCI/SAIP/SCAI/SCCT 2010 expert consensus document on coronary computed tomographic angiography: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. J Am Coll Cardiol. 2010;55(23):2663-2699.
7. Diederich S, Wormanns D, Semik M, et al. Screening for early lung cancer with low-dose spiral CT: prevalence in 817 asymptomatic smokers. Radiology. 2002;222(3):773-781.
8. National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395-409.
9. Moyer VA; U.S. Preventive Services Task Force. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(5):330-338.
10. McKinney WP, McIntire DD, Carmody TJ, Joseph A. Comparing the smoking behavior of veterans and nonveterans. Public Health Rep. 1997;112(3):212-218.
11. MacMahon H, Naidich DP, Goo JM, et al. Guidelines for Management of Incidental Pulmonary Nodules Detected on CT Images: From the Fleischner Society 2017. Radiology. 2017;284(1):228-243.
12. Coward J, Lawson R, Kane T, et al. Multi-centre analysis of incidental findings on low-resolution CT attenuation correction images. Br J Radiol. 2014;87(1042):20130701.
13. Coward J, Lawson R, Kane T, et al. Multicentre analysis of incidental findings on low-resolution CT attenuation correction images: an extended study. Br J Radiol. 2015;88(1056):20150555.
14. Haller S, Kaiser C, Buser P, Bongartz G, Bremerich J. Coronary artery imaging with contrast-enhanced MDCT: extracardiac findings. AJR Am J Roentgenol. 2006;187(1):105-110.
15. Flor N, Di Leo G, Squarza SA, et al. Malignant incidental extracardiac findings on cardiac CT: systematic review and meta-analysis. AJR Am J Roentgenol. 2013;201(3):555-564.
16. Budoff MJ, Gopal A. Incidental findings on cardiac computed tomography. Should we look? J Cardiovasc Comput Tomogr. 2007;1(2):97-105.
17. Earls JP. The pros and cons of searching for extracardiac findings at cardiac CT: studies should be reconstructed in the maximum field of view and adequately reviewed to detect pathologic findings. Radiology. 2011;261(2):342-346.
18. Douglas PS, Cerqueria M, Rubin GD, Chin AS. Extracardiac findings: what is a cardiologist to do? JACC Cardiovasc Imaging. 2008;1(5):682-687.
19. Holly TA, Abbott BG, Al-Mallah M, et al. Single photon-emission computed tomography. J Nucl Cardiol. 2010;17(5):941-973.
20. Dorbala S, Ananthasubramaniam K, Armstrong IS, et al. Single photon emission computed tomography (SPECT) myocardial perfusion imaging guidelines: instrumentation, acquisition, processing, and interpretation. J Nucl Cardiol. 2018;25(5):1784-1846.
21. Tilkemeier PL, Bourque J, Doukky R, Sanghani R, Weinberg RL. ASNC imaging guidelines for nuclear cardiology procedures : Standardized reporting of nuclear cardiology procedures. J Nucl Cardiol. 2017;24(6):2064-2128.
22. Dorbala S, Di Carli MF, Delbeke D, et al. SNMMI/ASNC/SCCT guideline for cardiac SPECT/CT and PET/CT 1.0. J Nucl Med. 2013;54(8):1485-1507.
23. Dilsizian V, Bacharach SL, Beanlands RS, et al. ASNC imaging guidelines/SNMMI procedure standard for positron emission tomography (PET) nuclear cardiology procedures. J Nucl Cardiol. 2016;23(5):1187-1226.
24. Jemal A, Ward EM, Johnson CJ, et al. Annual report to the nation on the status of cancer, 1975-2014, Featuring Survival. J Natl Cancer Inst. 2017;109(9):djx030.
25. US Department of Health and Human Services. The Health Consequences of Smoking: 50 Years of Progress. A Report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. Printed with corrections, January 2014.
26. Odani S, Agaku IT, Graffunder CM, Tynan MA, Armour BS. Tobacco Product Use Among Military Veterans - United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2018;67(1):7-12.
1. Hendel RC, Berman DS, Di Carli MF, et al. ACCF/ASNC/ACR/AHA/ASE/SCCT/SCMR/SNM 2009 appropriate use criteria for cardiac radionuclide imaging: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the American Society of Nuclear Cardiology, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the Society of Cardiovascular Computed Tomography, the Society for Cardiovascular Magnetic Resonance, and the Society of Nuclear Medicine. Circulation. 2009;119(22):e561-e587.
2. Hendel RC, Corbett JR, Cullom SJ, DePuey EG, Garcia EV, Bateman TM. The value and practice of attenuation correction for myocardial perfusion SPECT imaging: a joint position statement from the American Society of Nuclear Cardiology and the Society of Nuclear Medicine. J Nucl Cardiol. 2002;9(1):135–143.
3. Coward J, Nightingale J, Hogg P. The clinical dilemma of incidental findings on the low-resolution CT images from SPECT/CT MPI studies. J Nucl Med Technol. 2016;44(3):167-172.
4. Osman MM, Cohade C, Fishman E, Wahl RL. Clinically significant incidental findings on the unenhanced CT portion of PET/CT studies: frequency in 250 patients. J Nucl Med. 2005;46(8):1352-1355.
5. Goetze S, Pannu HK, Wahl RL. Clinically significant abnormal findings on the “nondiagnostic” CT portion of low-amperage-CT attenuation-corrected myocardial perfusion SPECT/CT studies. J Nucl Med. 2006;47(8):1312-1318.
6. American College of Cardiology Foundation Task Force on Expert Consensus Documents, Mark DB, Berman DS, et al. ACCF/ACR/AHA/NASCI/SAIP/SCAI/SCCT 2010 expert consensus document on coronary computed tomographic angiography: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. J Am Coll Cardiol. 2010;55(23):2663-2699.
7. Diederich S, Wormanns D, Semik M, et al. Screening for early lung cancer with low-dose spiral CT: prevalence in 817 asymptomatic smokers. Radiology. 2002;222(3):773-781.
8. National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395-409.
9. Moyer VA; U.S. Preventive Services Task Force. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(5):330-338.
10. McKinney WP, McIntire DD, Carmody TJ, Joseph A. Comparing the smoking behavior of veterans and nonveterans. Public Health Rep. 1997;112(3):212-218.
11. MacMahon H, Naidich DP, Goo JM, et al. Guidelines for Management of Incidental Pulmonary Nodules Detected on CT Images: From the Fleischner Society 2017. Radiology. 2017;284(1):228-243.
12. Coward J, Lawson R, Kane T, et al. Multi-centre analysis of incidental findings on low-resolution CT attenuation correction images. Br J Radiol. 2014;87(1042):20130701.
13. Coward J, Lawson R, Kane T, et al. Multicentre analysis of incidental findings on low-resolution CT attenuation correction images: an extended study. Br J Radiol. 2015;88(1056):20150555.
14. Haller S, Kaiser C, Buser P, Bongartz G, Bremerich J. Coronary artery imaging with contrast-enhanced MDCT: extracardiac findings. AJR Am J Roentgenol. 2006;187(1):105-110.
15. Flor N, Di Leo G, Squarza SA, et al. Malignant incidental extracardiac findings on cardiac CT: systematic review and meta-analysis. AJR Am J Roentgenol. 2013;201(3):555-564.
16. Budoff MJ, Gopal A. Incidental findings on cardiac computed tomography. Should we look? J Cardiovasc Comput Tomogr. 2007;1(2):97-105.
17. Earls JP. The pros and cons of searching for extracardiac findings at cardiac CT: studies should be reconstructed in the maximum field of view and adequately reviewed to detect pathologic findings. Radiology. 2011;261(2):342-346.
18. Douglas PS, Cerqueria M, Rubin GD, Chin AS. Extracardiac findings: what is a cardiologist to do? JACC Cardiovasc Imaging. 2008;1(5):682-687.
19. Holly TA, Abbott BG, Al-Mallah M, et al. Single photon-emission computed tomography. J Nucl Cardiol. 2010;17(5):941-973.
20. Dorbala S, Ananthasubramaniam K, Armstrong IS, et al. Single photon emission computed tomography (SPECT) myocardial perfusion imaging guidelines: instrumentation, acquisition, processing, and interpretation. J Nucl Cardiol. 2018;25(5):1784-1846.
21. Tilkemeier PL, Bourque J, Doukky R, Sanghani R, Weinberg RL. ASNC imaging guidelines for nuclear cardiology procedures : Standardized reporting of nuclear cardiology procedures. J Nucl Cardiol. 2017;24(6):2064-2128.
22. Dorbala S, Di Carli MF, Delbeke D, et al. SNMMI/ASNC/SCCT guideline for cardiac SPECT/CT and PET/CT 1.0. J Nucl Med. 2013;54(8):1485-1507.
23. Dilsizian V, Bacharach SL, Beanlands RS, et al. ASNC imaging guidelines/SNMMI procedure standard for positron emission tomography (PET) nuclear cardiology procedures. J Nucl Cardiol. 2016;23(5):1187-1226.
24. Jemal A, Ward EM, Johnson CJ, et al. Annual report to the nation on the status of cancer, 1975-2014, Featuring Survival. J Natl Cancer Inst. 2017;109(9):djx030.
25. US Department of Health and Human Services. The Health Consequences of Smoking: 50 Years of Progress. A Report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. Printed with corrections, January 2014.
26. Odani S, Agaku IT, Graffunder CM, Tynan MA, Armour BS. Tobacco Product Use Among Military Veterans - United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2018;67(1):7-12.