User login
Patient Preferences for Physician Attire: A Multicenter Study in Japan
The patient-physician relationship is critical for ensuring the delivery of high-quality healthcare. Successful patient-physician relationships arise from shared trust, knowledge, mutual respect, and effective verbal and nonverbal communication. The ways in which patients experience healthcare and their satisfaction with physicians affect a myriad of important health outcomes, such as adherence to treatment and outcomes for conditions such as hypertension and diabetes mellitus.1-5 One method for potentially enhancing patient satisfaction is through understanding how patients wish their physicians to dress6-8 and tailoring attire to match these expectations. In addition to our systematic review,9 a recent large-scale, multicenter study in the United States revealed that most patients perceive physician attire as important, but that preferences for specific types of attire are contextual.9,10 For example, elderly patients preferred physicians in formal attire and white coat, while scrubs with white coat or scrubs alone were preferred for emergency department (ED) physicians and surgeons, respectively. Moreover, regional variation regarding attire preference was also observed in the US, with preferences for more formal attire in the South and less formal in the Midwest.
Geographic variation, regarding patient preferences for physician dress, is perhaps even more relevant internationally. In particular, Japan is considered to have a highly contextualized culture that relies on nonverbal and implicit communication. However, medical professionals have no specific dress code and, thus, don many different kinds of attire. In part, this may be because it is not clear whether or how physician attire impacts patient satisfaction and perceived healthcare quality in Japan.11-13 Although previous studies in Japan have suggested that physician attire has a considerable influence on patient satisfaction, these studies either involved a single department in one hospital or a small number of respondents.14-17 Therefore, we performed a multicenter, cross-sectional study to understand patients’ preferences for physician attire in different clinical settings and in different geographic regions in Japan.
METHODS
Study Population
We conducted a cross-sectional, questionnaire-based study from 2015 to 2017, in four geographically diverse hospitals in Japan. Two of these hospitals, Tokyo Joto Hospital and Juntendo University Hospital, are located in eastern Japan whereas the others, Kurashiki Central Hospital and Akashi Medical Center, are in western Japan.
Questionnaires were printed and randomly distributed by research staff to outpatients in waiting rooms and inpatients in medical wards who were 20 years of age or older. We placed no restriction on ward site or time of questionnaire distribution. Research staff, including physicians, nurses, and medical clerks, were instructed to avoid guiding or influencing participants’ responses. Informed consent was obtained by the staff; only those who provided informed consent participated in the study. Respondents could request assistance with form completion from persons accompanying them if they had difficulties, such as physical, visual, or hearing impairments. All responses were collected anonymously. The study was approved by the ethics committees of all four hospitals.
Questionnaire
We used a modified version of the survey instrument from a prior study.10 The first section of the survey showed photographs of either a male or female physician with 7 unique forms of attire, including casual, casual with white coat, scrubs, scrubs with white coat, formal, formal with white coat, and business suit (Figure 1). Given the Japanese context of this study, the language was translated to Japanese and photographs of physicians of Japanese descent were used. Photographs were taken with attention paid to achieving constant facial expressions on the physicians as well as in other visual cues (eg, lighting, background, pose). The physician’s gender and attire in the first photograph seen by each respondent were randomized to prevent bias in ordering, priming, and anchoring; all other sections of the survey were identical.
Respondents were first asked to rate the standalone, randomized physician photograph using a 1-10 scale across five domains (ie, how knowledgeable, trustworthy, caring, and approachable the physician appeared and how comfortable the physician’s appearance made the respondent feel), with a score of 10 representing the highest rating. Respondents were subsequently given 7 photographs of the same physician wearing various forms of attire. Questions were asked regarding preference of attire in varied clinical settings (ie, primary care, ED, hospital, surgery, overall preference). To identify the influence of and respondent preferences for physician dress and white coats, a Likert scale ranging from 1 (strongly disagree) to 5 (strongly agree) was employed. The scale was trichotomized into “disagree” (1, 2), “neither agree nor disagree” (3), and “agree” (4, 5) for analysis. Demographic data, including age, gender, education level, nationality (Japanese or non-Japanese), and number of physicians seen in the past year were collected.
Outcomes and Sample Size Calculation
The primary outcome of attire preference was calculated as the mean composite score of the five individual rating domains (ie, knowledgeable, trustworthy, caring, approachable, and comfortable), with the highest score representing the most preferred form of attire. We also assessed variation in preferences for physician attire by respondent characteristics, such as age and gender.
Sample size estimation was based on previous survey methodology.10 The Likert scale range for identifying influence of and respondent preferences for physician dress and white coats was 1-5 (“strongly disagree” to “strongly agree”). The scale range for measuring preferences for the randomized attire photograph was 1-10. An assumption of normality was made regarding responses on the 1-10 scale. An estimated standard deviation of 2.2 was assumed, based on prior findings.10 Based on these assumptions and the inclusion of at least 816 respondents (assuming a two-sided alpha error of 0.05), we expected to have 90% capacity to detect differences for effect sizes of 0.50 on the 1-10 scale.
Statistical Analyses
Paper-based survey data were entered independently and in duplicate by the study team. Respondents were not required to answer all questions; therefore, the denominator for each question varied. Data were reported as mean and standard deviation (SD) or percentages, where appropriate. Differences in the mean composite rating scores were assessed using one-way ANOVA with the Tukey method for pairwise comparisons. Differences in proportions for categorical data were compared using the Z-test. Chi-squared tests were used for bivariate comparisons between respondent age, gender, and level of education and corresponding respondent preferences. All analyses were performed using Stata 14 MP/SE (Stata Corp., College Station, Texas, USA).
RESULTS
Characteristics of Participants
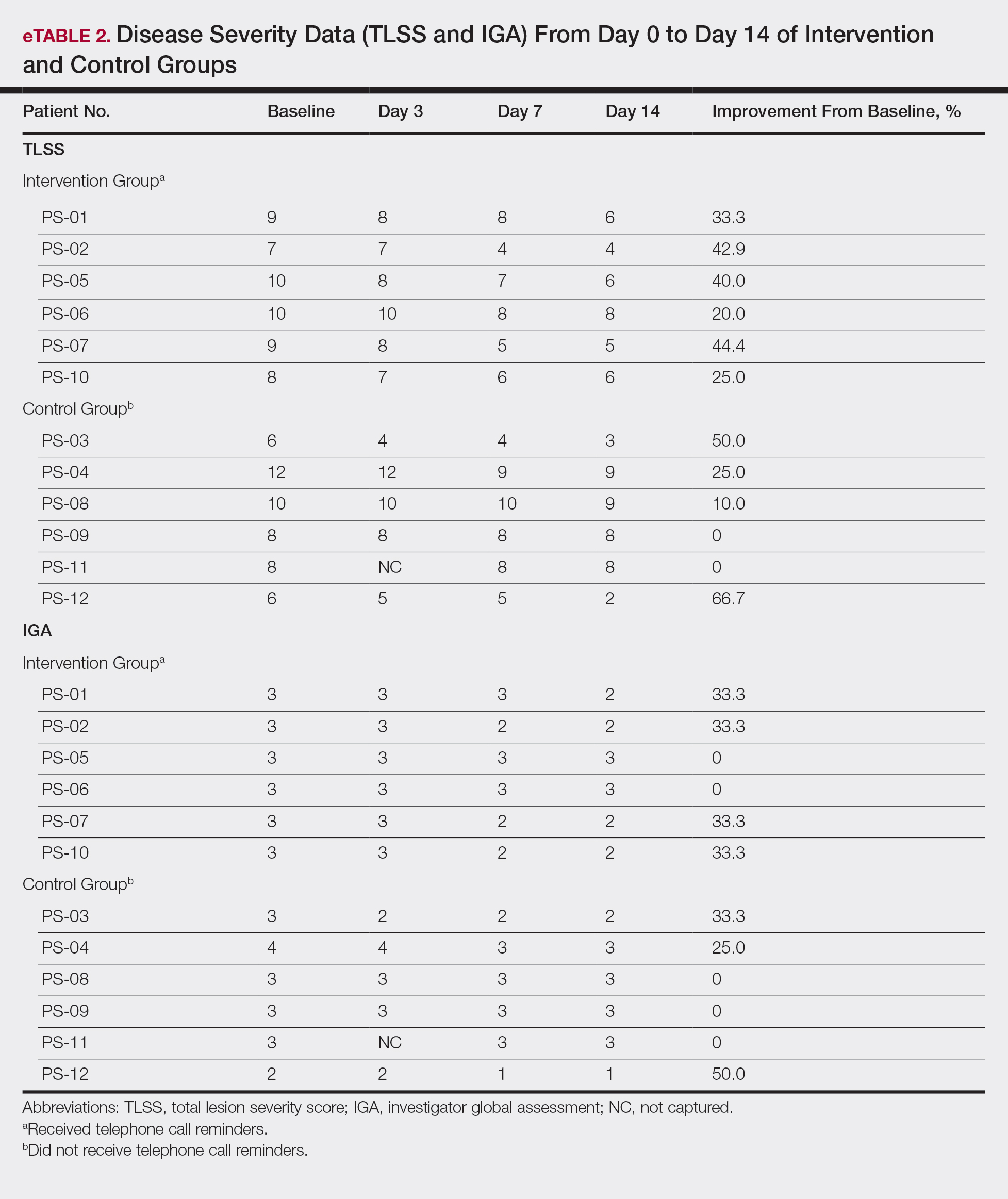
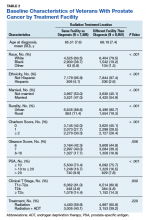
Between December 1, 2015 and October 30, 2017, a total of 2,020 surveys were completed by patients across four academic hospitals in Japan. Of those, 1,960 patients (97.0%) completed the survey in its entirety. Approximately half of the respondents were 65 years of age or older (49%), of female gender (52%), and reported receiving care in the outpatient setting (53%). Regarding use of healthcare, 91% had seen more than one physician in the year preceding the time of survey completion (Table 1).
Ratings of Physician Attire
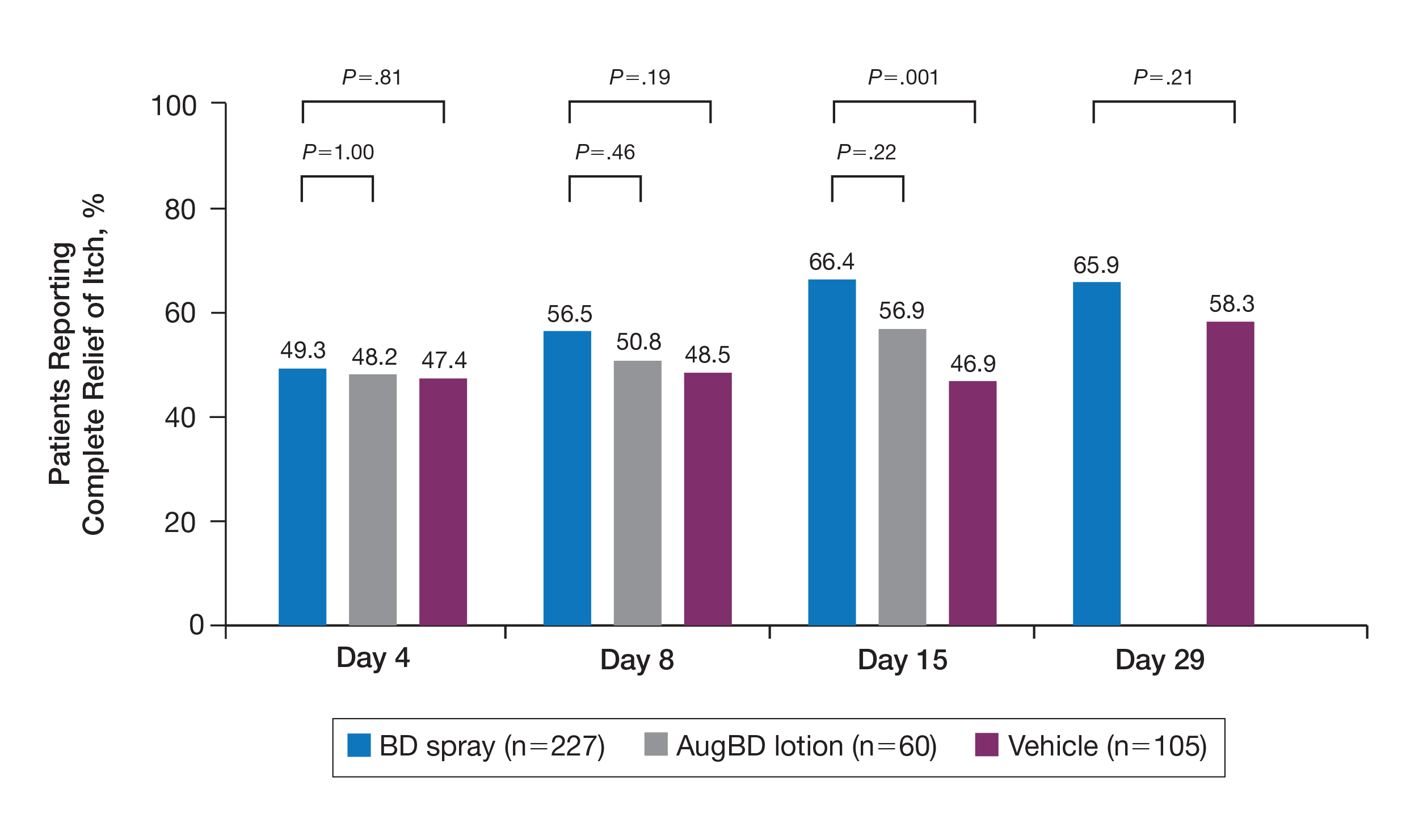
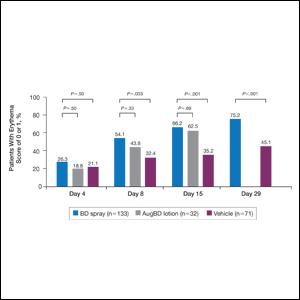
Compared with all forms of attire depicted in the survey’s first standalone photograph, respondents rated “casual attire with white coat” the highest (Figure 2). The mean composite score for “casual attire with white coat” was 7.1 (standard deviation [SD] = 1.8), and this attire was set as the referent group. Cronbach’s alpha, for the five items included in the composite score, was 0.95. However, “formal attire with white coat” was rated almost as highly as “casual attire with white coat” with an overall mean composite score of 7.0 (SD = 1.6).
Variation in Preference for Physician Attire by Clinical Setting
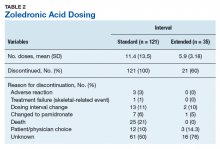
Preferences for physician attire varied by clinical care setting. Most respondents preferred “casual attire with white coat” or “formal attire with white coat” in both primary care and hospital settings, with a slight preference for “casual attire with white coat.” In contrast, respondents preferred “scrubs without white coat” in the ED and surgical settings. When asked about their overall preference, respondents reported they felt their physician should wear “formal attire with white coat” (35%) or “casual attire with white coat” (30%; Table 2). When comparing the group of photographs of physicians with white coats to the group without white coats (Figure 1), respondents preferred physicians wearing white coats overall and specifically when providing care in primary care and hospital settings. However, they preferred physicians without white coats when providing care in the ED (P < .001). With respect to surgeons, there was no statistically significant difference between preference for white coats and no white coats. These results were similar for photographs of both male and female physicians.
When asked whether physician dress was important to them and if physician attire influenced their satisfaction with the care received, 61% of participants agreed that physician dress was important, and 47% agreed that physician attire influenced satisfaction (Appendix Table 1). With respect to appropriateness of physicians dressing casually over the weekend in clinical settings, 52% responded that casual wear was inappropriate, while 31% had a neutral opinion.
Participants were asked whether physicians should wear a white coat in different clinical settings. Nearly two-thirds indicated a preference for white coats in the office and hospital (65% and 64%, respectively). Responses regarding whether emergency physicians should wear white coats were nearly equally divided (Agree, 37%; Disagree, 32%; Neither Agree nor Disagree, 31%). However, “scrubs without white coat” was most preferred (56%) when patients were given photographs of various attire and asked, “Which physician would you prefer to see when visiting the ER?” Responses to the question “Physicians should always wear a white coat when seeing patients in any setting” varied equally (Agree, 32%; Disagree, 34%; Neither Agree nor Disagree, 34%).
Variation in Preference for Physician Attire by Respondent Demographics
When comparing respondents by age, those 65 years or older preferred “formal attire with white coat” more so than respondents younger than 65 years (Appendix Table 2). This finding was identified in both primary care (36% vs 31%, P < .001) and hospital settings (37% vs 30%, P < .001). Additionally, physician attire had a greater impact on older respondents’ satisfaction and experience (Appendix Table 3). For example, 67% of respondents 65 years and older agreed that physician attire was important, and 54% agreed that attire influenced satisfaction. Conversely, for respondents younger than 65 years, the proportion agreeing with these statements was lower (56% and 41%, both P < .001). When comparing older and younger respondents, those 65 years and older more often preferred physicians wearing white coats in any setting (39% vs 26%, P < .001) and specifically in their office (68% vs 61%, P = .002), the ED (40% vs 34%, P < .001), and the hospital (69% vs 60%, P < .001).
When comparing male and female respondents, male respondents more often stated that physician dress was important to them (men, 64%; women, 58%; P = .002). When comparing responses to the question “Overall, which clothes do you feel a doctor should wear?”, between the eastern and western Japanese hospitals, preferences for physician attire varied.
Variation in Expectations Between Male and Female Physicians
When comparing the ratings of male and female physicians, female physicians were rated higher in how caring (P = .005) and approachable (P < .001) they appeared. However, there were no significant differences in the ratings of the three remaining domains (ie, knowledgeable, trustworthy, and comfortable) or the composite score.
DISCUSSION
Since we employed the same methodology as previous studies conducted in the US10 and Switzerland,18 a notable strength of our approach is that comparisons among these countries can be drawn. For example, physician attire appears to hold greater importance in Japan than in the US and Switzerland. Among Japanese participants, 61% agreed that physician dress is important (US, 53%; Switzerland, 36%), and 47% agreed that physician dress influenced how satisfied they were with their care (US, 36%; Switzerland, 23%).10 This result supports the notion that nonverbal and implicit communications (such as physician dress) may carry more importance among Japanese people.11-13
Regarding preference ratings for type of dress among respondents in Japan, “casual attire with white coat” received the highest mean composite score rating, with “formal attire with white coat” rated second overall. In contrast, US respondents rated “formal attire with white coat” highest and “scrubs with white coat” second.10 Our result runs counter to our expectation in that we expected Japanese respondents to prefer formal attire, since Japan is one of the most formal cultures in the world. One potential explanation for this difference is that the casual style chosen for this study was close to the smart casual style (slightly casual). Most hospitals and clinics in Japan do not allow physicians to wear jeans or polo shirts, which were chosen as the casual attire in the previous US study.
When examining various care settings and physician types, both Japanese and US respondents were more likely to prefer physicians wearing a white coat in the office or hospital.10 However, Japanese participants preferred both “casual attire with white coat” and “formal attire with white coat” equally in primary care or hospital settings. A smaller proportion of US respondents preferred “casual attire with white coat” in primary care (11%) and hospital settings (9%), but more preferred “formal attire with white coat” for primary care (44%) and hospital physicians (39%). In the ED setting, 32% of participants in Japan and 18% in the US disagreed with the idea that physicians should wear a white coat. Among Japanese participants, “scrubs without white coat” was rated highest for emergency physicians (56%) and surgeons (47%), while US preferences were 40% and 42%, respectively.10 One potential explanation is that scrubs-based attire became popular among Japanese ED and surgical contexts as a result of cultural influence and spread from western countries.19, 20
With respect to perceptions regarding physician attire on weekends, 52% of participants considered it inappropriate for a physician to dress casually over the weekend, compared with only 30% in Switzerland and 21% in the US.11,12 Given Japan’s level of formality and the fact that most Japanese physicians continue to work over the weekend,21-23 Japanese patients tend to expect their physicians to dress in more formal attire during these times.
Previous studies in Japan have demonstrated that older patients gave low ratings to scrubs and high ratings to white coat with any attire,15,17 and this was also the case in our study. Perhaps elderly patients reflect conservative values in their preferences of physician dress. Their perceptions may be less influenced by scenes portraying physicians in popular media when compared with the perceptions of younger patients. Though a 2015 systematic review and studies in other countries revealed white coats were preferred regardless of exact dress,9,24-26 they also showed variation in preferences for physician attire. For example, patients in Saudi Arabia preferred white coat and traditional ethnic dress,25 whereas mothers of pediatric patients in Saudi Arabia preferred scrubs for their pediatricians.27 Therefore, it is recommended for internationally mobile physicians to choose their dress depending on a variety of factors including country, context, and patient age group.
Our study has limitations. First, because some physicians presented the surveys to the patients, participants may have responded differently. Second, participants may have identified photographs of the male physician model as their personal healthcare provider (one author, K.K.). To avoid this possible bias, we randomly distributed 14 different versions of physician photographs in the questionnaire. Third, although physician photographs were strictly controlled, the “formal attire and white coat” and “casual attire and white coat” photographs appeared similar, especially given that the white coats were buttoned. Also, the female physician depicted in the photographs did not have the scrub shirt tucked in, while the male physician did. These nuances may have affected participant ratings between groups. Fourth,
In conclusion, patient preferences for physician attire were examined using a multicenter survey with a large sample size and robust survey methodology, thus overcoming weaknesses of previous studies into Japanese attire. Japanese patients perceive that physician attire is important and influences satisfaction with their care, more so than patients in other countries, like the US and Switzerland. Geography, settings of care, and patient age play a role in preferences. As a result, hospitals and health systems may use these findings to inform dress code policy based on patient population and context, recognizing that the appearance of their providers affects the patient-physician relationship. Future research should focus on better understanding the various cultural and societal customs that lead to patient expectations of physician attire.
Acknowledgments
The authors thank Drs. Fumi Takemoto, Masayuki Ueno, Kazuya Sakai, Saori Kinami, and Toshio Naito for their assistance with data collection at their respective sites. Additionally, the authors thank Dr. Yoko Kanamitsu for serving as a model for photographs.
1. Manary MP, Boulding W, Staelin R, Glickman SW. The patient experience and health outcomes. N Engl J Med. 2013;368(3):201-203. https://doi.org/ 10.1056/NEJMp1211775.
2. Boulding W, Glickman SW, Manary MP, Schulman KA, Staelin R. Relationship between patient satisfaction with inpatient care and hospital readmission within 30 days. Am J Manag Care. 2011;17(1):41-48.
3. Barbosa CD, Balp MM, Kulich K, Germain N, Rofail D. A literature review to explore the link between treatment satisfaction and adherence, compliance, and persistence. Patient Prefer Adherence. 2012;6:39-48. https://doi.org/10.2147/PPA.S24752.
4. Jha AK, Orav EJ, Zheng J, Epstein AM. Patients’ perception of hospital care in the United States. N Engl J Med. 2008;359(18):1921-31. https://doi.org/10.1056/NEJMsa080411.
5. O’Malley AS, Forrest CB, Mandelblatt J. Adherence of low-income women to cancer screening recommendations. J Gen Intern Med. 2002;17(2):144-54. https://doi.org/10.1046/j.1525-1497.2002.10431.x.
6. Chung H, Lee H, Chang DS, Kim HS, Park HJ, Chae Y. Doctor’s attire influences perceived empathy in the patient-doctor relationship. Patient Educ Couns. 2012;89(3):387-391. https://doi.org/10.1016/j.pec.2012.02.017.
7. Bianchi MT. Desiderata or dogma: what the evidence reveals about physician attire. J Gen Intern Med. 2008;23(5):641-643. https://doi.org/10.1007/s11606-008-0546-8.
8. Brandt LJ. On the value of an old dress code in the new millennium. Arch Intern Med. 2003;163(11):1277-1281. https://doi.org/10.1001/archinte.163.11.1277.
9. Petrilli CM, Mack M, Petrilli JJ, Hickner A, Saint S, Chopra V. Understanding the role of physician attire on patient perceptions: a systematic review of the literature--targeting attire to improve likelihood of rapport (TAILOR) investigators. BMJ Open. 2015;5(1):e006578. https://doi.org/10.1136/bmjopen-2014-006578.
10. Petrilli CM, Saint S, Jennings JJ, et al. Understanding patient preference for physician attire: a cross-sectional observational study of 10 academic medical centres in the USA. BMJ Open. 2018;8(5):e021239. https://doi.org/10.1136/bmjopen-2017-021239.
11. Rowbury R. The need for more proactive communications. Low trust and changing values mean Japan can no longer fall back on its homogeneity. The Japan Times. 2017, Oct 15;Sect. Opinion. https://www.japantimes.co.jp/opinion/2017/10/15/commentary/japan-commentary/need-proactive-communications/#.Xej7lC3MzUI. Accessed December 5, 2019.
12. Shoji Nishimura ANaST. Communication Style and Cultural Features in High/Low Context Communication Cultures: A Case Study of Finland, Japan and India. Nov 22nd, 2009.
13. Smith RMRSW. The influence of high/low-context culture and power distance on choice of communication media: Students’ media choice to communicate with Professors in Japan and America. Int J Intercultural Relations. 2007;31(4):479-501.
14. Yamada Y, Takahashi O, Ohde S, Deshpande GA, Fukui T. Patients’ preferences for doctors’ attire in Japan. Intern Med. 2010;49(15):1521-1526. https://doi.org/10.2169/internalmedicine.49.3572.
15. Ikusaka M, Kamegai M, Sunaga T, et al. Patients’ attitude toward consultations by a physician without a white coat in Japan. Intern Med. 1999;38(7):533-536. https://doi.org/10.2169/internalmedicine.38.533.
16. Lefor AK, Ohnuma T, Nunomiya S, Yokota S, Makino J, Sanui M. Physician attire in the intensive care unit in Japan influences visitors’ perception of care. J Crit Care. 2018;43:288-293.
17. Kurihara H, Maeno T. Importance of physicians’ attire: factors influencing the impression it makes on patients, a cross-sectional study. Asia Pac Fam Med. 2014;13(1):2. https://doi.org/10.1186/1447-056X-13-2.
18. Zollinger M, Houchens N, Chopra V, et al. Understanding patient preference for physician attire in ambulatory clinics: a cross-sectional observational study. BMJ Open. 2019;9(5):e026009. https://doi.org/10.1136/bmjopen-2018-026009.
19. Chung JE. Medical Dramas and Viewer Perception of Health: Testing Cultivation Effects. Hum Commun Res. 2014;40(3):333-349.
20. Michael Pfau LJM, Kirsten Garrow. The influence of television viewing on public perceptions of physicians. J Broadcast Electron Media. 1995;39(4):441-458.
21. Suzuki S. Exhausting physicians employed in hospitals in Japan assessed by a health questionnaire [in Japanese]. Sangyo Eiseigaku Zasshi. 2017;59(4):107-118. https://doi.org/10.1539/sangyoeisei.
22. Ogawa R, Seo E, Maeno T, Ito M, Sanuki M. The relationship between long working hours and depression among first-year residents in Japan. BMC Med Educ. 2018;18(1):50. https://doi.org/10.1186/s12909-018-1171-9.
23. Saijo Y, Chiba S, Yoshioka E, et al. Effects of work burden, job strain and support on depressive symptoms and burnout among Japanese physicians. Int J Occup Med Environ Health. 2014;27(6):980-992. https://doi.org/10.2478/s13382-014-0324-2.
24. Tiang KW, Razack AH, Ng KL. The ‘auxiliary’ white coat effect in hospitals: perceptions of patients and doctors. Singapore Med J. 2017;58(10):574-575. https://doi.org/10.11622/smedj.2017023.
25. Al Amry KM, Al Farrah M, Ur Rahman S, Abdulmajeed I. Patient perceptions and preferences of physicians’ attire in Saudi primary healthcare setting. J Community Hosp Intern Med Perspect. 2018;8(6):326-330. https://doi.org/10.1080/20009666.2018.1551026.
26. Healy WL. Letter to the editor: editor’s spotlight/take 5: physicians’ attire influences patients’ perceptions in the urban outpatient orthopaedic surgery setting. Clin Orthop Relat Res. 2016;474(11):2545-2546. https://doi.org/10.1007/s11999-016-5049-z.
27. Aldrees T, Alsuhaibani R, Alqaryan S, et al. Physicians’ attire. Parents preferences in a tertiary hospital. Saudi Med J. 2017;38(4):435-439. https://doi.org/10.15537/smj.2017.4.15853.
The patient-physician relationship is critical for ensuring the delivery of high-quality healthcare. Successful patient-physician relationships arise from shared trust, knowledge, mutual respect, and effective verbal and nonverbal communication. The ways in which patients experience healthcare and their satisfaction with physicians affect a myriad of important health outcomes, such as adherence to treatment and outcomes for conditions such as hypertension and diabetes mellitus.1-5 One method for potentially enhancing patient satisfaction is through understanding how patients wish their physicians to dress6-8 and tailoring attire to match these expectations. In addition to our systematic review,9 a recent large-scale, multicenter study in the United States revealed that most patients perceive physician attire as important, but that preferences for specific types of attire are contextual.9,10 For example, elderly patients preferred physicians in formal attire and white coat, while scrubs with white coat or scrubs alone were preferred for emergency department (ED) physicians and surgeons, respectively. Moreover, regional variation regarding attire preference was also observed in the US, with preferences for more formal attire in the South and less formal in the Midwest.
Geographic variation, regarding patient preferences for physician dress, is perhaps even more relevant internationally. In particular, Japan is considered to have a highly contextualized culture that relies on nonverbal and implicit communication. However, medical professionals have no specific dress code and, thus, don many different kinds of attire. In part, this may be because it is not clear whether or how physician attire impacts patient satisfaction and perceived healthcare quality in Japan.11-13 Although previous studies in Japan have suggested that physician attire has a considerable influence on patient satisfaction, these studies either involved a single department in one hospital or a small number of respondents.14-17 Therefore, we performed a multicenter, cross-sectional study to understand patients’ preferences for physician attire in different clinical settings and in different geographic regions in Japan.
METHODS
Study Population
We conducted a cross-sectional, questionnaire-based study from 2015 to 2017, in four geographically diverse hospitals in Japan. Two of these hospitals, Tokyo Joto Hospital and Juntendo University Hospital, are located in eastern Japan whereas the others, Kurashiki Central Hospital and Akashi Medical Center, are in western Japan.
Questionnaires were printed and randomly distributed by research staff to outpatients in waiting rooms and inpatients in medical wards who were 20 years of age or older. We placed no restriction on ward site or time of questionnaire distribution. Research staff, including physicians, nurses, and medical clerks, were instructed to avoid guiding or influencing participants’ responses. Informed consent was obtained by the staff; only those who provided informed consent participated in the study. Respondents could request assistance with form completion from persons accompanying them if they had difficulties, such as physical, visual, or hearing impairments. All responses were collected anonymously. The study was approved by the ethics committees of all four hospitals.
Questionnaire
We used a modified version of the survey instrument from a prior study.10 The first section of the survey showed photographs of either a male or female physician with 7 unique forms of attire, including casual, casual with white coat, scrubs, scrubs with white coat, formal, formal with white coat, and business suit (Figure 1). Given the Japanese context of this study, the language was translated to Japanese and photographs of physicians of Japanese descent were used. Photographs were taken with attention paid to achieving constant facial expressions on the physicians as well as in other visual cues (eg, lighting, background, pose). The physician’s gender and attire in the first photograph seen by each respondent were randomized to prevent bias in ordering, priming, and anchoring; all other sections of the survey were identical.
Respondents were first asked to rate the standalone, randomized physician photograph using a 1-10 scale across five domains (ie, how knowledgeable, trustworthy, caring, and approachable the physician appeared and how comfortable the physician’s appearance made the respondent feel), with a score of 10 representing the highest rating. Respondents were subsequently given 7 photographs of the same physician wearing various forms of attire. Questions were asked regarding preference of attire in varied clinical settings (ie, primary care, ED, hospital, surgery, overall preference). To identify the influence of and respondent preferences for physician dress and white coats, a Likert scale ranging from 1 (strongly disagree) to 5 (strongly agree) was employed. The scale was trichotomized into “disagree” (1, 2), “neither agree nor disagree” (3), and “agree” (4, 5) for analysis. Demographic data, including age, gender, education level, nationality (Japanese or non-Japanese), and number of physicians seen in the past year were collected.
Outcomes and Sample Size Calculation
The primary outcome of attire preference was calculated as the mean composite score of the five individual rating domains (ie, knowledgeable, trustworthy, caring, approachable, and comfortable), with the highest score representing the most preferred form of attire. We also assessed variation in preferences for physician attire by respondent characteristics, such as age and gender.
Sample size estimation was based on previous survey methodology.10 The Likert scale range for identifying influence of and respondent preferences for physician dress and white coats was 1-5 (“strongly disagree” to “strongly agree”). The scale range for measuring preferences for the randomized attire photograph was 1-10. An assumption of normality was made regarding responses on the 1-10 scale. An estimated standard deviation of 2.2 was assumed, based on prior findings.10 Based on these assumptions and the inclusion of at least 816 respondents (assuming a two-sided alpha error of 0.05), we expected to have 90% capacity to detect differences for effect sizes of 0.50 on the 1-10 scale.
Statistical Analyses
Paper-based survey data were entered independently and in duplicate by the study team. Respondents were not required to answer all questions; therefore, the denominator for each question varied. Data were reported as mean and standard deviation (SD) or percentages, where appropriate. Differences in the mean composite rating scores were assessed using one-way ANOVA with the Tukey method for pairwise comparisons. Differences in proportions for categorical data were compared using the Z-test. Chi-squared tests were used for bivariate comparisons between respondent age, gender, and level of education and corresponding respondent preferences. All analyses were performed using Stata 14 MP/SE (Stata Corp., College Station, Texas, USA).
RESULTS
Characteristics of Participants
Between December 1, 2015 and October 30, 2017, a total of 2,020 surveys were completed by patients across four academic hospitals in Japan. Of those, 1,960 patients (97.0%) completed the survey in its entirety. Approximately half of the respondents were 65 years of age or older (49%), of female gender (52%), and reported receiving care in the outpatient setting (53%). Regarding use of healthcare, 91% had seen more than one physician in the year preceding the time of survey completion (Table 1).
Ratings of Physician Attire
Compared with all forms of attire depicted in the survey’s first standalone photograph, respondents rated “casual attire with white coat” the highest (Figure 2). The mean composite score for “casual attire with white coat” was 7.1 (standard deviation [SD] = 1.8), and this attire was set as the referent group. Cronbach’s alpha, for the five items included in the composite score, was 0.95. However, “formal attire with white coat” was rated almost as highly as “casual attire with white coat” with an overall mean composite score of 7.0 (SD = 1.6).
Variation in Preference for Physician Attire by Clinical Setting
Preferences for physician attire varied by clinical care setting. Most respondents preferred “casual attire with white coat” or “formal attire with white coat” in both primary care and hospital settings, with a slight preference for “casual attire with white coat.” In contrast, respondents preferred “scrubs without white coat” in the ED and surgical settings. When asked about their overall preference, respondents reported they felt their physician should wear “formal attire with white coat” (35%) or “casual attire with white coat” (30%; Table 2). When comparing the group of photographs of physicians with white coats to the group without white coats (Figure 1), respondents preferred physicians wearing white coats overall and specifically when providing care in primary care and hospital settings. However, they preferred physicians without white coats when providing care in the ED (P < .001). With respect to surgeons, there was no statistically significant difference between preference for white coats and no white coats. These results were similar for photographs of both male and female physicians.
When asked whether physician dress was important to them and if physician attire influenced their satisfaction with the care received, 61% of participants agreed that physician dress was important, and 47% agreed that physician attire influenced satisfaction (Appendix Table 1). With respect to appropriateness of physicians dressing casually over the weekend in clinical settings, 52% responded that casual wear was inappropriate, while 31% had a neutral opinion.
Participants were asked whether physicians should wear a white coat in different clinical settings. Nearly two-thirds indicated a preference for white coats in the office and hospital (65% and 64%, respectively). Responses regarding whether emergency physicians should wear white coats were nearly equally divided (Agree, 37%; Disagree, 32%; Neither Agree nor Disagree, 31%). However, “scrubs without white coat” was most preferred (56%) when patients were given photographs of various attire and asked, “Which physician would you prefer to see when visiting the ER?” Responses to the question “Physicians should always wear a white coat when seeing patients in any setting” varied equally (Agree, 32%; Disagree, 34%; Neither Agree nor Disagree, 34%).
Variation in Preference for Physician Attire by Respondent Demographics
When comparing respondents by age, those 65 years or older preferred “formal attire with white coat” more so than respondents younger than 65 years (Appendix Table 2). This finding was identified in both primary care (36% vs 31%, P < .001) and hospital settings (37% vs 30%, P < .001). Additionally, physician attire had a greater impact on older respondents’ satisfaction and experience (Appendix Table 3). For example, 67% of respondents 65 years and older agreed that physician attire was important, and 54% agreed that attire influenced satisfaction. Conversely, for respondents younger than 65 years, the proportion agreeing with these statements was lower (56% and 41%, both P < .001). When comparing older and younger respondents, those 65 years and older more often preferred physicians wearing white coats in any setting (39% vs 26%, P < .001) and specifically in their office (68% vs 61%, P = .002), the ED (40% vs 34%, P < .001), and the hospital (69% vs 60%, P < .001).
When comparing male and female respondents, male respondents more often stated that physician dress was important to them (men, 64%; women, 58%; P = .002). When comparing responses to the question “Overall, which clothes do you feel a doctor should wear?”, between the eastern and western Japanese hospitals, preferences for physician attire varied.
Variation in Expectations Between Male and Female Physicians
When comparing the ratings of male and female physicians, female physicians were rated higher in how caring (P = .005) and approachable (P < .001) they appeared. However, there were no significant differences in the ratings of the three remaining domains (ie, knowledgeable, trustworthy, and comfortable) or the composite score.
DISCUSSION
Since we employed the same methodology as previous studies conducted in the US10 and Switzerland,18 a notable strength of our approach is that comparisons among these countries can be drawn. For example, physician attire appears to hold greater importance in Japan than in the US and Switzerland. Among Japanese participants, 61% agreed that physician dress is important (US, 53%; Switzerland, 36%), and 47% agreed that physician dress influenced how satisfied they were with their care (US, 36%; Switzerland, 23%).10 This result supports the notion that nonverbal and implicit communications (such as physician dress) may carry more importance among Japanese people.11-13
Regarding preference ratings for type of dress among respondents in Japan, “casual attire with white coat” received the highest mean composite score rating, with “formal attire with white coat” rated second overall. In contrast, US respondents rated “formal attire with white coat” highest and “scrubs with white coat” second.10 Our result runs counter to our expectation in that we expected Japanese respondents to prefer formal attire, since Japan is one of the most formal cultures in the world. One potential explanation for this difference is that the casual style chosen for this study was close to the smart casual style (slightly casual). Most hospitals and clinics in Japan do not allow physicians to wear jeans or polo shirts, which were chosen as the casual attire in the previous US study.
When examining various care settings and physician types, both Japanese and US respondents were more likely to prefer physicians wearing a white coat in the office or hospital.10 However, Japanese participants preferred both “casual attire with white coat” and “formal attire with white coat” equally in primary care or hospital settings. A smaller proportion of US respondents preferred “casual attire with white coat” in primary care (11%) and hospital settings (9%), but more preferred “formal attire with white coat” for primary care (44%) and hospital physicians (39%). In the ED setting, 32% of participants in Japan and 18% in the US disagreed with the idea that physicians should wear a white coat. Among Japanese participants, “scrubs without white coat” was rated highest for emergency physicians (56%) and surgeons (47%), while US preferences were 40% and 42%, respectively.10 One potential explanation is that scrubs-based attire became popular among Japanese ED and surgical contexts as a result of cultural influence and spread from western countries.19, 20
With respect to perceptions regarding physician attire on weekends, 52% of participants considered it inappropriate for a physician to dress casually over the weekend, compared with only 30% in Switzerland and 21% in the US.11,12 Given Japan’s level of formality and the fact that most Japanese physicians continue to work over the weekend,21-23 Japanese patients tend to expect their physicians to dress in more formal attire during these times.
Previous studies in Japan have demonstrated that older patients gave low ratings to scrubs and high ratings to white coat with any attire,15,17 and this was also the case in our study. Perhaps elderly patients reflect conservative values in their preferences of physician dress. Their perceptions may be less influenced by scenes portraying physicians in popular media when compared with the perceptions of younger patients. Though a 2015 systematic review and studies in other countries revealed white coats were preferred regardless of exact dress,9,24-26 they also showed variation in preferences for physician attire. For example, patients in Saudi Arabia preferred white coat and traditional ethnic dress,25 whereas mothers of pediatric patients in Saudi Arabia preferred scrubs for their pediatricians.27 Therefore, it is recommended for internationally mobile physicians to choose their dress depending on a variety of factors including country, context, and patient age group.
Our study has limitations. First, because some physicians presented the surveys to the patients, participants may have responded differently. Second, participants may have identified photographs of the male physician model as their personal healthcare provider (one author, K.K.). To avoid this possible bias, we randomly distributed 14 different versions of physician photographs in the questionnaire. Third, although physician photographs were strictly controlled, the “formal attire and white coat” and “casual attire and white coat” photographs appeared similar, especially given that the white coats were buttoned. Also, the female physician depicted in the photographs did not have the scrub shirt tucked in, while the male physician did. These nuances may have affected participant ratings between groups. Fourth,
In conclusion, patient preferences for physician attire were examined using a multicenter survey with a large sample size and robust survey methodology, thus overcoming weaknesses of previous studies into Japanese attire. Japanese patients perceive that physician attire is important and influences satisfaction with their care, more so than patients in other countries, like the US and Switzerland. Geography, settings of care, and patient age play a role in preferences. As a result, hospitals and health systems may use these findings to inform dress code policy based on patient population and context, recognizing that the appearance of their providers affects the patient-physician relationship. Future research should focus on better understanding the various cultural and societal customs that lead to patient expectations of physician attire.
Acknowledgments
The authors thank Drs. Fumi Takemoto, Masayuki Ueno, Kazuya Sakai, Saori Kinami, and Toshio Naito for their assistance with data collection at their respective sites. Additionally, the authors thank Dr. Yoko Kanamitsu for serving as a model for photographs.
The patient-physician relationship is critical for ensuring the delivery of high-quality healthcare. Successful patient-physician relationships arise from shared trust, knowledge, mutual respect, and effective verbal and nonverbal communication. The ways in which patients experience healthcare and their satisfaction with physicians affect a myriad of important health outcomes, such as adherence to treatment and outcomes for conditions such as hypertension and diabetes mellitus.1-5 One method for potentially enhancing patient satisfaction is through understanding how patients wish their physicians to dress6-8 and tailoring attire to match these expectations. In addition to our systematic review,9 a recent large-scale, multicenter study in the United States revealed that most patients perceive physician attire as important, but that preferences for specific types of attire are contextual.9,10 For example, elderly patients preferred physicians in formal attire and white coat, while scrubs with white coat or scrubs alone were preferred for emergency department (ED) physicians and surgeons, respectively. Moreover, regional variation regarding attire preference was also observed in the US, with preferences for more formal attire in the South and less formal in the Midwest.
Geographic variation, regarding patient preferences for physician dress, is perhaps even more relevant internationally. In particular, Japan is considered to have a highly contextualized culture that relies on nonverbal and implicit communication. However, medical professionals have no specific dress code and, thus, don many different kinds of attire. In part, this may be because it is not clear whether or how physician attire impacts patient satisfaction and perceived healthcare quality in Japan.11-13 Although previous studies in Japan have suggested that physician attire has a considerable influence on patient satisfaction, these studies either involved a single department in one hospital or a small number of respondents.14-17 Therefore, we performed a multicenter, cross-sectional study to understand patients’ preferences for physician attire in different clinical settings and in different geographic regions in Japan.
METHODS
Study Population
We conducted a cross-sectional, questionnaire-based study from 2015 to 2017, in four geographically diverse hospitals in Japan. Two of these hospitals, Tokyo Joto Hospital and Juntendo University Hospital, are located in eastern Japan whereas the others, Kurashiki Central Hospital and Akashi Medical Center, are in western Japan.
Questionnaires were printed and randomly distributed by research staff to outpatients in waiting rooms and inpatients in medical wards who were 20 years of age or older. We placed no restriction on ward site or time of questionnaire distribution. Research staff, including physicians, nurses, and medical clerks, were instructed to avoid guiding or influencing participants’ responses. Informed consent was obtained by the staff; only those who provided informed consent participated in the study. Respondents could request assistance with form completion from persons accompanying them if they had difficulties, such as physical, visual, or hearing impairments. All responses were collected anonymously. The study was approved by the ethics committees of all four hospitals.
Questionnaire
We used a modified version of the survey instrument from a prior study.10 The first section of the survey showed photographs of either a male or female physician with 7 unique forms of attire, including casual, casual with white coat, scrubs, scrubs with white coat, formal, formal with white coat, and business suit (Figure 1). Given the Japanese context of this study, the language was translated to Japanese and photographs of physicians of Japanese descent were used. Photographs were taken with attention paid to achieving constant facial expressions on the physicians as well as in other visual cues (eg, lighting, background, pose). The physician’s gender and attire in the first photograph seen by each respondent were randomized to prevent bias in ordering, priming, and anchoring; all other sections of the survey were identical.
Respondents were first asked to rate the standalone, randomized physician photograph using a 1-10 scale across five domains (ie, how knowledgeable, trustworthy, caring, and approachable the physician appeared and how comfortable the physician’s appearance made the respondent feel), with a score of 10 representing the highest rating. Respondents were subsequently given 7 photographs of the same physician wearing various forms of attire. Questions were asked regarding preference of attire in varied clinical settings (ie, primary care, ED, hospital, surgery, overall preference). To identify the influence of and respondent preferences for physician dress and white coats, a Likert scale ranging from 1 (strongly disagree) to 5 (strongly agree) was employed. The scale was trichotomized into “disagree” (1, 2), “neither agree nor disagree” (3), and “agree” (4, 5) for analysis. Demographic data, including age, gender, education level, nationality (Japanese or non-Japanese), and number of physicians seen in the past year were collected.
Outcomes and Sample Size Calculation
The primary outcome of attire preference was calculated as the mean composite score of the five individual rating domains (ie, knowledgeable, trustworthy, caring, approachable, and comfortable), with the highest score representing the most preferred form of attire. We also assessed variation in preferences for physician attire by respondent characteristics, such as age and gender.
Sample size estimation was based on previous survey methodology.10 The Likert scale range for identifying influence of and respondent preferences for physician dress and white coats was 1-5 (“strongly disagree” to “strongly agree”). The scale range for measuring preferences for the randomized attire photograph was 1-10. An assumption of normality was made regarding responses on the 1-10 scale. An estimated standard deviation of 2.2 was assumed, based on prior findings.10 Based on these assumptions and the inclusion of at least 816 respondents (assuming a two-sided alpha error of 0.05), we expected to have 90% capacity to detect differences for effect sizes of 0.50 on the 1-10 scale.
Statistical Analyses
Paper-based survey data were entered independently and in duplicate by the study team. Respondents were not required to answer all questions; therefore, the denominator for each question varied. Data were reported as mean and standard deviation (SD) or percentages, where appropriate. Differences in the mean composite rating scores were assessed using one-way ANOVA with the Tukey method for pairwise comparisons. Differences in proportions for categorical data were compared using the Z-test. Chi-squared tests were used for bivariate comparisons between respondent age, gender, and level of education and corresponding respondent preferences. All analyses were performed using Stata 14 MP/SE (Stata Corp., College Station, Texas, USA).
RESULTS
Characteristics of Participants
Between December 1, 2015 and October 30, 2017, a total of 2,020 surveys were completed by patients across four academic hospitals in Japan. Of those, 1,960 patients (97.0%) completed the survey in its entirety. Approximately half of the respondents were 65 years of age or older (49%), of female gender (52%), and reported receiving care in the outpatient setting (53%). Regarding use of healthcare, 91% had seen more than one physician in the year preceding the time of survey completion (Table 1).
Ratings of Physician Attire
Compared with all forms of attire depicted in the survey’s first standalone photograph, respondents rated “casual attire with white coat” the highest (Figure 2). The mean composite score for “casual attire with white coat” was 7.1 (standard deviation [SD] = 1.8), and this attire was set as the referent group. Cronbach’s alpha, for the five items included in the composite score, was 0.95. However, “formal attire with white coat” was rated almost as highly as “casual attire with white coat” with an overall mean composite score of 7.0 (SD = 1.6).
Variation in Preference for Physician Attire by Clinical Setting
Preferences for physician attire varied by clinical care setting. Most respondents preferred “casual attire with white coat” or “formal attire with white coat” in both primary care and hospital settings, with a slight preference for “casual attire with white coat.” In contrast, respondents preferred “scrubs without white coat” in the ED and surgical settings. When asked about their overall preference, respondents reported they felt their physician should wear “formal attire with white coat” (35%) or “casual attire with white coat” (30%; Table 2). When comparing the group of photographs of physicians with white coats to the group without white coats (Figure 1), respondents preferred physicians wearing white coats overall and specifically when providing care in primary care and hospital settings. However, they preferred physicians without white coats when providing care in the ED (P < .001). With respect to surgeons, there was no statistically significant difference between preference for white coats and no white coats. These results were similar for photographs of both male and female physicians.
When asked whether physician dress was important to them and if physician attire influenced their satisfaction with the care received, 61% of participants agreed that physician dress was important, and 47% agreed that physician attire influenced satisfaction (Appendix Table 1). With respect to appropriateness of physicians dressing casually over the weekend in clinical settings, 52% responded that casual wear was inappropriate, while 31% had a neutral opinion.
Participants were asked whether physicians should wear a white coat in different clinical settings. Nearly two-thirds indicated a preference for white coats in the office and hospital (65% and 64%, respectively). Responses regarding whether emergency physicians should wear white coats were nearly equally divided (Agree, 37%; Disagree, 32%; Neither Agree nor Disagree, 31%). However, “scrubs without white coat” was most preferred (56%) when patients were given photographs of various attire and asked, “Which physician would you prefer to see when visiting the ER?” Responses to the question “Physicians should always wear a white coat when seeing patients in any setting” varied equally (Agree, 32%; Disagree, 34%; Neither Agree nor Disagree, 34%).
Variation in Preference for Physician Attire by Respondent Demographics
When comparing respondents by age, those 65 years or older preferred “formal attire with white coat” more so than respondents younger than 65 years (Appendix Table 2). This finding was identified in both primary care (36% vs 31%, P < .001) and hospital settings (37% vs 30%, P < .001). Additionally, physician attire had a greater impact on older respondents’ satisfaction and experience (Appendix Table 3). For example, 67% of respondents 65 years and older agreed that physician attire was important, and 54% agreed that attire influenced satisfaction. Conversely, for respondents younger than 65 years, the proportion agreeing with these statements was lower (56% and 41%, both P < .001). When comparing older and younger respondents, those 65 years and older more often preferred physicians wearing white coats in any setting (39% vs 26%, P < .001) and specifically in their office (68% vs 61%, P = .002), the ED (40% vs 34%, P < .001), and the hospital (69% vs 60%, P < .001).
When comparing male and female respondents, male respondents more often stated that physician dress was important to them (men, 64%; women, 58%; P = .002). When comparing responses to the question “Overall, which clothes do you feel a doctor should wear?”, between the eastern and western Japanese hospitals, preferences for physician attire varied.
Variation in Expectations Between Male and Female Physicians
When comparing the ratings of male and female physicians, female physicians were rated higher in how caring (P = .005) and approachable (P < .001) they appeared. However, there were no significant differences in the ratings of the three remaining domains (ie, knowledgeable, trustworthy, and comfortable) or the composite score.
DISCUSSION
Since we employed the same methodology as previous studies conducted in the US10 and Switzerland,18 a notable strength of our approach is that comparisons among these countries can be drawn. For example, physician attire appears to hold greater importance in Japan than in the US and Switzerland. Among Japanese participants, 61% agreed that physician dress is important (US, 53%; Switzerland, 36%), and 47% agreed that physician dress influenced how satisfied they were with their care (US, 36%; Switzerland, 23%).10 This result supports the notion that nonverbal and implicit communications (such as physician dress) may carry more importance among Japanese people.11-13
Regarding preference ratings for type of dress among respondents in Japan, “casual attire with white coat” received the highest mean composite score rating, with “formal attire with white coat” rated second overall. In contrast, US respondents rated “formal attire with white coat” highest and “scrubs with white coat” second.10 Our result runs counter to our expectation in that we expected Japanese respondents to prefer formal attire, since Japan is one of the most formal cultures in the world. One potential explanation for this difference is that the casual style chosen for this study was close to the smart casual style (slightly casual). Most hospitals and clinics in Japan do not allow physicians to wear jeans or polo shirts, which were chosen as the casual attire in the previous US study.
When examining various care settings and physician types, both Japanese and US respondents were more likely to prefer physicians wearing a white coat in the office or hospital.10 However, Japanese participants preferred both “casual attire with white coat” and “formal attire with white coat” equally in primary care or hospital settings. A smaller proportion of US respondents preferred “casual attire with white coat” in primary care (11%) and hospital settings (9%), but more preferred “formal attire with white coat” for primary care (44%) and hospital physicians (39%). In the ED setting, 32% of participants in Japan and 18% in the US disagreed with the idea that physicians should wear a white coat. Among Japanese participants, “scrubs without white coat” was rated highest for emergency physicians (56%) and surgeons (47%), while US preferences were 40% and 42%, respectively.10 One potential explanation is that scrubs-based attire became popular among Japanese ED and surgical contexts as a result of cultural influence and spread from western countries.19, 20
With respect to perceptions regarding physician attire on weekends, 52% of participants considered it inappropriate for a physician to dress casually over the weekend, compared with only 30% in Switzerland and 21% in the US.11,12 Given Japan’s level of formality and the fact that most Japanese physicians continue to work over the weekend,21-23 Japanese patients tend to expect their physicians to dress in more formal attire during these times.
Previous studies in Japan have demonstrated that older patients gave low ratings to scrubs and high ratings to white coat with any attire,15,17 and this was also the case in our study. Perhaps elderly patients reflect conservative values in their preferences of physician dress. Their perceptions may be less influenced by scenes portraying physicians in popular media when compared with the perceptions of younger patients. Though a 2015 systematic review and studies in other countries revealed white coats were preferred regardless of exact dress,9,24-26 they also showed variation in preferences for physician attire. For example, patients in Saudi Arabia preferred white coat and traditional ethnic dress,25 whereas mothers of pediatric patients in Saudi Arabia preferred scrubs for their pediatricians.27 Therefore, it is recommended for internationally mobile physicians to choose their dress depending on a variety of factors including country, context, and patient age group.
Our study has limitations. First, because some physicians presented the surveys to the patients, participants may have responded differently. Second, participants may have identified photographs of the male physician model as their personal healthcare provider (one author, K.K.). To avoid this possible bias, we randomly distributed 14 different versions of physician photographs in the questionnaire. Third, although physician photographs were strictly controlled, the “formal attire and white coat” and “casual attire and white coat” photographs appeared similar, especially given that the white coats were buttoned. Also, the female physician depicted in the photographs did not have the scrub shirt tucked in, while the male physician did. These nuances may have affected participant ratings between groups. Fourth,
In conclusion, patient preferences for physician attire were examined using a multicenter survey with a large sample size and robust survey methodology, thus overcoming weaknesses of previous studies into Japanese attire. Japanese patients perceive that physician attire is important and influences satisfaction with their care, more so than patients in other countries, like the US and Switzerland. Geography, settings of care, and patient age play a role in preferences. As a result, hospitals and health systems may use these findings to inform dress code policy based on patient population and context, recognizing that the appearance of their providers affects the patient-physician relationship. Future research should focus on better understanding the various cultural and societal customs that lead to patient expectations of physician attire.
Acknowledgments
The authors thank Drs. Fumi Takemoto, Masayuki Ueno, Kazuya Sakai, Saori Kinami, and Toshio Naito for their assistance with data collection at their respective sites. Additionally, the authors thank Dr. Yoko Kanamitsu for serving as a model for photographs.
1. Manary MP, Boulding W, Staelin R, Glickman SW. The patient experience and health outcomes. N Engl J Med. 2013;368(3):201-203. https://doi.org/ 10.1056/NEJMp1211775.
2. Boulding W, Glickman SW, Manary MP, Schulman KA, Staelin R. Relationship between patient satisfaction with inpatient care and hospital readmission within 30 days. Am J Manag Care. 2011;17(1):41-48.
3. Barbosa CD, Balp MM, Kulich K, Germain N, Rofail D. A literature review to explore the link between treatment satisfaction and adherence, compliance, and persistence. Patient Prefer Adherence. 2012;6:39-48. https://doi.org/10.2147/PPA.S24752.
4. Jha AK, Orav EJ, Zheng J, Epstein AM. Patients’ perception of hospital care in the United States. N Engl J Med. 2008;359(18):1921-31. https://doi.org/10.1056/NEJMsa080411.
5. O’Malley AS, Forrest CB, Mandelblatt J. Adherence of low-income women to cancer screening recommendations. J Gen Intern Med. 2002;17(2):144-54. https://doi.org/10.1046/j.1525-1497.2002.10431.x.
6. Chung H, Lee H, Chang DS, Kim HS, Park HJ, Chae Y. Doctor’s attire influences perceived empathy in the patient-doctor relationship. Patient Educ Couns. 2012;89(3):387-391. https://doi.org/10.1016/j.pec.2012.02.017.
7. Bianchi MT. Desiderata or dogma: what the evidence reveals about physician attire. J Gen Intern Med. 2008;23(5):641-643. https://doi.org/10.1007/s11606-008-0546-8.
8. Brandt LJ. On the value of an old dress code in the new millennium. Arch Intern Med. 2003;163(11):1277-1281. https://doi.org/10.1001/archinte.163.11.1277.
9. Petrilli CM, Mack M, Petrilli JJ, Hickner A, Saint S, Chopra V. Understanding the role of physician attire on patient perceptions: a systematic review of the literature--targeting attire to improve likelihood of rapport (TAILOR) investigators. BMJ Open. 2015;5(1):e006578. https://doi.org/10.1136/bmjopen-2014-006578.
10. Petrilli CM, Saint S, Jennings JJ, et al. Understanding patient preference for physician attire: a cross-sectional observational study of 10 academic medical centres in the USA. BMJ Open. 2018;8(5):e021239. https://doi.org/10.1136/bmjopen-2017-021239.
11. Rowbury R. The need for more proactive communications. Low trust and changing values mean Japan can no longer fall back on its homogeneity. The Japan Times. 2017, Oct 15;Sect. Opinion. https://www.japantimes.co.jp/opinion/2017/10/15/commentary/japan-commentary/need-proactive-communications/#.Xej7lC3MzUI. Accessed December 5, 2019.
12. Shoji Nishimura ANaST. Communication Style and Cultural Features in High/Low Context Communication Cultures: A Case Study of Finland, Japan and India. Nov 22nd, 2009.
13. Smith RMRSW. The influence of high/low-context culture and power distance on choice of communication media: Students’ media choice to communicate with Professors in Japan and America. Int J Intercultural Relations. 2007;31(4):479-501.
14. Yamada Y, Takahashi O, Ohde S, Deshpande GA, Fukui T. Patients’ preferences for doctors’ attire in Japan. Intern Med. 2010;49(15):1521-1526. https://doi.org/10.2169/internalmedicine.49.3572.
15. Ikusaka M, Kamegai M, Sunaga T, et al. Patients’ attitude toward consultations by a physician without a white coat in Japan. Intern Med. 1999;38(7):533-536. https://doi.org/10.2169/internalmedicine.38.533.
16. Lefor AK, Ohnuma T, Nunomiya S, Yokota S, Makino J, Sanui M. Physician attire in the intensive care unit in Japan influences visitors’ perception of care. J Crit Care. 2018;43:288-293.
17. Kurihara H, Maeno T. Importance of physicians’ attire: factors influencing the impression it makes on patients, a cross-sectional study. Asia Pac Fam Med. 2014;13(1):2. https://doi.org/10.1186/1447-056X-13-2.
18. Zollinger M, Houchens N, Chopra V, et al. Understanding patient preference for physician attire in ambulatory clinics: a cross-sectional observational study. BMJ Open. 2019;9(5):e026009. https://doi.org/10.1136/bmjopen-2018-026009.
19. Chung JE. Medical Dramas and Viewer Perception of Health: Testing Cultivation Effects. Hum Commun Res. 2014;40(3):333-349.
20. Michael Pfau LJM, Kirsten Garrow. The influence of television viewing on public perceptions of physicians. J Broadcast Electron Media. 1995;39(4):441-458.
21. Suzuki S. Exhausting physicians employed in hospitals in Japan assessed by a health questionnaire [in Japanese]. Sangyo Eiseigaku Zasshi. 2017;59(4):107-118. https://doi.org/10.1539/sangyoeisei.
22. Ogawa R, Seo E, Maeno T, Ito M, Sanuki M. The relationship between long working hours and depression among first-year residents in Japan. BMC Med Educ. 2018;18(1):50. https://doi.org/10.1186/s12909-018-1171-9.
23. Saijo Y, Chiba S, Yoshioka E, et al. Effects of work burden, job strain and support on depressive symptoms and burnout among Japanese physicians. Int J Occup Med Environ Health. 2014;27(6):980-992. https://doi.org/10.2478/s13382-014-0324-2.
24. Tiang KW, Razack AH, Ng KL. The ‘auxiliary’ white coat effect in hospitals: perceptions of patients and doctors. Singapore Med J. 2017;58(10):574-575. https://doi.org/10.11622/smedj.2017023.
25. Al Amry KM, Al Farrah M, Ur Rahman S, Abdulmajeed I. Patient perceptions and preferences of physicians’ attire in Saudi primary healthcare setting. J Community Hosp Intern Med Perspect. 2018;8(6):326-330. https://doi.org/10.1080/20009666.2018.1551026.
26. Healy WL. Letter to the editor: editor’s spotlight/take 5: physicians’ attire influences patients’ perceptions in the urban outpatient orthopaedic surgery setting. Clin Orthop Relat Res. 2016;474(11):2545-2546. https://doi.org/10.1007/s11999-016-5049-z.
27. Aldrees T, Alsuhaibani R, Alqaryan S, et al. Physicians’ attire. Parents preferences in a tertiary hospital. Saudi Med J. 2017;38(4):435-439. https://doi.org/10.15537/smj.2017.4.15853.
1. Manary MP, Boulding W, Staelin R, Glickman SW. The patient experience and health outcomes. N Engl J Med. 2013;368(3):201-203. https://doi.org/ 10.1056/NEJMp1211775.
2. Boulding W, Glickman SW, Manary MP, Schulman KA, Staelin R. Relationship between patient satisfaction with inpatient care and hospital readmission within 30 days. Am J Manag Care. 2011;17(1):41-48.
3. Barbosa CD, Balp MM, Kulich K, Germain N, Rofail D. A literature review to explore the link between treatment satisfaction and adherence, compliance, and persistence. Patient Prefer Adherence. 2012;6:39-48. https://doi.org/10.2147/PPA.S24752.
4. Jha AK, Orav EJ, Zheng J, Epstein AM. Patients’ perception of hospital care in the United States. N Engl J Med. 2008;359(18):1921-31. https://doi.org/10.1056/NEJMsa080411.
5. O’Malley AS, Forrest CB, Mandelblatt J. Adherence of low-income women to cancer screening recommendations. J Gen Intern Med. 2002;17(2):144-54. https://doi.org/10.1046/j.1525-1497.2002.10431.x.
6. Chung H, Lee H, Chang DS, Kim HS, Park HJ, Chae Y. Doctor’s attire influences perceived empathy in the patient-doctor relationship. Patient Educ Couns. 2012;89(3):387-391. https://doi.org/10.1016/j.pec.2012.02.017.
7. Bianchi MT. Desiderata or dogma: what the evidence reveals about physician attire. J Gen Intern Med. 2008;23(5):641-643. https://doi.org/10.1007/s11606-008-0546-8.
8. Brandt LJ. On the value of an old dress code in the new millennium. Arch Intern Med. 2003;163(11):1277-1281. https://doi.org/10.1001/archinte.163.11.1277.
9. Petrilli CM, Mack M, Petrilli JJ, Hickner A, Saint S, Chopra V. Understanding the role of physician attire on patient perceptions: a systematic review of the literature--targeting attire to improve likelihood of rapport (TAILOR) investigators. BMJ Open. 2015;5(1):e006578. https://doi.org/10.1136/bmjopen-2014-006578.
10. Petrilli CM, Saint S, Jennings JJ, et al. Understanding patient preference for physician attire: a cross-sectional observational study of 10 academic medical centres in the USA. BMJ Open. 2018;8(5):e021239. https://doi.org/10.1136/bmjopen-2017-021239.
11. Rowbury R. The need for more proactive communications. Low trust and changing values mean Japan can no longer fall back on its homogeneity. The Japan Times. 2017, Oct 15;Sect. Opinion. https://www.japantimes.co.jp/opinion/2017/10/15/commentary/japan-commentary/need-proactive-communications/#.Xej7lC3MzUI. Accessed December 5, 2019.
12. Shoji Nishimura ANaST. Communication Style and Cultural Features in High/Low Context Communication Cultures: A Case Study of Finland, Japan and India. Nov 22nd, 2009.
13. Smith RMRSW. The influence of high/low-context culture and power distance on choice of communication media: Students’ media choice to communicate with Professors in Japan and America. Int J Intercultural Relations. 2007;31(4):479-501.
14. Yamada Y, Takahashi O, Ohde S, Deshpande GA, Fukui T. Patients’ preferences for doctors’ attire in Japan. Intern Med. 2010;49(15):1521-1526. https://doi.org/10.2169/internalmedicine.49.3572.
15. Ikusaka M, Kamegai M, Sunaga T, et al. Patients’ attitude toward consultations by a physician without a white coat in Japan. Intern Med. 1999;38(7):533-536. https://doi.org/10.2169/internalmedicine.38.533.
16. Lefor AK, Ohnuma T, Nunomiya S, Yokota S, Makino J, Sanui M. Physician attire in the intensive care unit in Japan influences visitors’ perception of care. J Crit Care. 2018;43:288-293.
17. Kurihara H, Maeno T. Importance of physicians’ attire: factors influencing the impression it makes on patients, a cross-sectional study. Asia Pac Fam Med. 2014;13(1):2. https://doi.org/10.1186/1447-056X-13-2.
18. Zollinger M, Houchens N, Chopra V, et al. Understanding patient preference for physician attire in ambulatory clinics: a cross-sectional observational study. BMJ Open. 2019;9(5):e026009. https://doi.org/10.1136/bmjopen-2018-026009.
19. Chung JE. Medical Dramas and Viewer Perception of Health: Testing Cultivation Effects. Hum Commun Res. 2014;40(3):333-349.
20. Michael Pfau LJM, Kirsten Garrow. The influence of television viewing on public perceptions of physicians. J Broadcast Electron Media. 1995;39(4):441-458.
21. Suzuki S. Exhausting physicians employed in hospitals in Japan assessed by a health questionnaire [in Japanese]. Sangyo Eiseigaku Zasshi. 2017;59(4):107-118. https://doi.org/10.1539/sangyoeisei.
22. Ogawa R, Seo E, Maeno T, Ito M, Sanuki M. The relationship between long working hours and depression among first-year residents in Japan. BMC Med Educ. 2018;18(1):50. https://doi.org/10.1186/s12909-018-1171-9.
23. Saijo Y, Chiba S, Yoshioka E, et al. Effects of work burden, job strain and support on depressive symptoms and burnout among Japanese physicians. Int J Occup Med Environ Health. 2014;27(6):980-992. https://doi.org/10.2478/s13382-014-0324-2.
24. Tiang KW, Razack AH, Ng KL. The ‘auxiliary’ white coat effect in hospitals: perceptions of patients and doctors. Singapore Med J. 2017;58(10):574-575. https://doi.org/10.11622/smedj.2017023.
25. Al Amry KM, Al Farrah M, Ur Rahman S, Abdulmajeed I. Patient perceptions and preferences of physicians’ attire in Saudi primary healthcare setting. J Community Hosp Intern Med Perspect. 2018;8(6):326-330. https://doi.org/10.1080/20009666.2018.1551026.
26. Healy WL. Letter to the editor: editor’s spotlight/take 5: physicians’ attire influences patients’ perceptions in the urban outpatient orthopaedic surgery setting. Clin Orthop Relat Res. 2016;474(11):2545-2546. https://doi.org/10.1007/s11999-016-5049-z.
27. Aldrees T, Alsuhaibani R, Alqaryan S, et al. Physicians’ attire. Parents preferences in a tertiary hospital. Saudi Med J. 2017;38(4):435-439. https://doi.org/10.15537/smj.2017.4.15853.
© 2020 Society of Hospital Medicine
Portable Ultrasound Device Usage and Learning Outcomes Among Internal Medicine Trainees: A Parallel-Group Randomized Trial
Point-of-care ultrasonography (POCUS) can transform healthcare delivery through its diagnostic and therapeutic expediency.1 POCUS has been shown to bolster diagnostic accuracy, reduce procedural complications, decrease inpatient length of stay, and improve patient satisfaction by encouraging the physician to be present at the bedside.2-8
POCUS has become widespread across a variety of clinical settings as more investigations have demonstrated its positive impact on patient care.1,9-12 This includes the use of POCUS by trainees, who are now utilizing this technology as part of their assessments of patients.13,14 However, trainees may be performing these examinations with minimal oversight, and outside of emergency medicine, there are few guidelines on how to effectively teach POCUS or measure competency.13,14 While POCUS is rapidly becoming a part of inpatient care, teaching physicians may have little experience in ultrasound or the expertise to adequately supervise trainees.14 There is a growing need to study what trainees can learn and how this knowledge is acquired.
Previous investigations have demonstrated that inexperienced users can be taught to use POCUS to identify a variety of pathological states.2,3,15-23 Most of these curricula used a single lecture series as their pedagogical vehicle, and they variably included junior medical trainees. More importantly, the investigations did not explore whether personal access to handheld ultrasound devices (HUDs) improved learning. In theory, improved access to POCUS devices increases opportunities for authentic and deliberate practice, which may be needed to improve trainee skill with POCUS beyond the classroom setting.14
This study aimed to address several ongoing gaps in knowledge related to learning POCUS. First, we hypothesized that personal HUD access would improve trainees’ POCUS-related knowledge and interpretive ability as a result of increased practice opportunities. Second, we hypothesized that trainees who receive personal access to HUDs would be more likely to perform POCUS examinations and feel more confident in their interpretations. Finally, we hypothesized that repeated exposure to POCUS-related lectures would result in greater improvements in knowledge as compared with a single lecture series.
METHODS
Participants and Setting
The 2017 intern class (n = 47) at an academic internal medicine residency program participated in the study. Control data were obtained from the 2016 intern class (historical control; n = 50) and the 2018 intern class (contemporaneous control; n = 52). The Stanford University Institutional Review Board approved this study.
Study Design
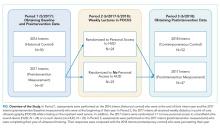
The 2017 intern class (n = 47) received POCUS didactics from June 2017 to June 2018. To evaluate if increased access to HUDs improved learning outcomes, the 2017 interns were randomized 1:1 to receive their own personal HUD that could be used for patient care and/or self-directed learning (n = 24) vs no-HUD (n = 23; Figure). Learning outcomes were assessed over the course of 1 year (see “Outcomes” below) and were compared with the 2016 and 2018 controls. The 2016 intern class had completed a year of training but had not received formalized POCUS didactics (historical control), whereas the 2018 intern class was assessed at the beginning of their year (contemporaneous control; Figure). In order to make comparisons based on intern experience, baseline data for the 2017 intern class were compared with the 2018 intern class, whereas end-of-study data for 2017 interns were compared with 2016 interns.
Outcomes
The primary outcome was the difference in assessment scores at the end of the study period between interns randomized to receive a HUD and those who were not. Secondary outcomes included differences in HUD usage rates, lecture attendance, and assessment scores. To assess whether repeated lecture exposure resulted in greater amounts of learning, this study evaluated for assessment score improvements after each lecture block. Finally, trainee attitudes toward POCUS and their confidence in their interpretative ability were measured at the beginning and end of the study period.
Curriculum Implementation
The lectures were administered as once-weekly didactics of 1-hour duration to interns rotating on the inpatient wards rotation. This rotation is 4 weeks long, and each intern will experience the rotation two to four times per year. Each lecture contained two parts: (1) 20-30 minutes of didactics via Microsoft PowerPointTM and (2) 30-40 minutes of supervised practice using HUDs on standardized patients. Four lectures were given each month: (1) introduction to POCUS and ultrasound physics, (2) thoracic/lung ultrasound, (3) echocardiography, and (4) abdominal POCUS. The lectures consisted of contrasting cases of normal/abnormal videos and clinical vignettes. These four lectures were repeated each month as new interns rotated on service. Some interns experienced the same content multiple times, which was intentional in order to assess their rates of learning over time. Lecture contents were based on previously published guidelines and expert consensus for teaching POCUS in internal medicine.13, 24-26 Content from the Accreditation Council for Graduate Medical Education (ACGME) and the American College of Emergency Physicians (ACEP) was also incorporated because these organizations had published relevant guidelines for teaching POCUS.13,26 Further development of the lectures occurred through review of previously described POCUS-relevant curricula.27-32
Handheld Ultrasound Devices
This study used the Philips LumifyTM, a United States Food and Drug Administration–approved device. Interns randomized to HUDs received their own device at the start of the rotation. It was at their discretion to use the device outside of the course. All devices were approved for patient use and were encrypted in compliance with our information security office. For privacy reasons, any saved patient images were not reviewed by the researchers. Interns were encouraged to share their findings with supervising physicians during rounds, but actual oversight was not measured. Interns not randomized to HUDs could access a single community device that was shared among all residents and fellows in the hospital. Interns reported the average number of POCUS examinations performed each week via a survey sent during the last week of the rotation.
Assessment Design and Implementation
Assessments evaluating trainee knowledge were administered before, during, and after the study period (Figure). For the 2017 cohort, assessments were also administered at the start and end of the ward month to track knowledge acquisition. Assessment contents were selected from POCUS guidelines for internal medicine and adaptation of the ACGME and ACEP guidelines.13,24,26 Additional content was obtained from major society POCUS tutorials and deidentified images collected by the study authors.13,24,33 In keeping with previously described methodology, the images were shown for approximately 12 seconds, followed by five additional seconds to allow the learner to answer the question.32 Final assessment contents were determined by the authors using the Delphi method.34 A sample assessment can be found in the Appendix Material.
Surveys
Surveys were administered alongside the assessments to the 2016-2018 intern classes. These surveys assessed trainee attitudes toward POCUS and were based on previously validated assessments.27,28,30 Attitudes were measured using 5-point Likert scales.
Statistical Analysis
For the primary outcome, we performed generalized binomial mixed-effect regressions using the survey periods, randomization group, and the interaction of the two as independent variables after adjusting for attendance and controlling of intra-intern correlations. The bivariate unadjusted analysis was performed to display the distribution of overall correctness on the assessments. Wilcoxon signed rank test was used to determine score significance for dependent score variables (R-Statistical Programming Language, Vienna, Austria).
RESULTS
Baseline Characteristics
There were 149 interns who participated in this study (Figure). Assessment/survey completion rates were as follows: 2016 control: 68.0%; 2017 preintervention: 97.9%; 2017 postintervention: 89.4%; and 2018 control: 100%. The 2017 interns reported similar amounts of prior POCUS exposure in medical school (Table 1).
Primary Outcome: Assessment Scores (HUD vs no HUD)
There were no significant differences in assessment scores at the end of the study between interns randomized to personal HUD access vs those to no-HUD access (Table 1). HUD interns reported performing POCUS assessments on patients a mean 6.8 (standard deviation [SD] 2.2) times per week vs 6.4 (SD 2.9) times per week in the no-HUD arm (P = .66). The mean lecture attendance was 75.0% and did not significantly differ between the HUD arms (Table 1).
Secondary Outcomes
Impact of Repeating Lectures
The 2017 interns demonstrated significant increases in preblock vs postblock assessment scores after first-time exposure to the lectures (median preblock score 0.61 [interquartile range (IQR), 0.53-0.70] vs postblock score 0.81 [IQR, 0.72-0.86]; P < .001; Table 2). However, intern performance on the preblock vs postblock assessments after second-time exposure to the curriculum failed to improve (median second preblock score 0.78 [IQR, 0.69-0.83] vs postblock score 0.81 [IQR, 0.64-0.89]; P = .94). Intern performance on individual domains of knowledge for each block is listed in Appendix Table 1.
Intervention Performance vs Controls
The 2016 historical control had significantly higher scores compared with the 2017 preintervention group (P < .001; Appendix Table 2). The year-long lecture series resulted in significant increases in median scores for the 2017 group (median preintervention score 0.55 [0.41-0.61] vs median postintervention score 0.84 [0.71-0.90]; P = .006; Appendix Table 1). At the end of the study, the 2017 postintervention scores were significantly higher across multiple knowledge domains compared with the 2016 historical control (Appendix Table 2).
Survey Results
Notably, the 2017 intern class at the end of the intervention did not have significantly different assessment scores for several disease-specific domains, compared with the 2016 control (Appendix Table 2). Nonetheless, the 2017 intern class reported higher levels of confidence in these same domains despite similar scores (Supplementary Figure). The HUD group seldomly cited a lack of confidence in their abilities as a barrier to performing POCUS examinations (17.6%), compared with the no-HUD group (50.0%), despite nearly identical assessment scores between the two groups (Table 1).
DISCUSSION
Previous guidelines have recommended increased HUD access for learners,13,24,35,36 but there have been few investigations that have evaluated the impact of such access on learning POCUS. One previous investigation found that hospitalists who carried HUDs were more likely to identify heart failure on bedside examination.37 In contrast, our study found no improvement in interpretative ability when randomizing interns to carry HUDs for patient care. Notably, interns did not perform more POCUS examinations when given HUDs. We offer several explanations for this finding. First, time-motion studies have demonstrated that internal medicine interns spend less than 15% of their time toward direct patient care.38 It is possible that the demands of being an intern impeded their ability to perform more POCUS examinations on their patients, regardless of HUD access. Alternatively, the interns randomized to no personal access may have used the community device more frequently as a result of the lecture series. Given the cost of HUDs, further studies are needed to assess the degree to which HUD access will improve trainee interpretive ability, especially as more training programs consider the creation of ultrasound curricula.10,11,24,39,40
This study was unique because it followed interns over a year-long course that repeated the same material to assess rates of learning with repeated exposure. Learners improved their scores after the first, but not second, block. Furthermore, the median scores were nearly identical between the first postblock assessment and second preblock assessment (0.81 vs 0.78), suggesting that knowledge was retained between blocks. Together, these findings suggest there may be limitations of traditional lectures that use standardized patient models for practice. Supplementary pedagogies, such as in-the-moment feedback with actual patients, may be needed to promote mastery.14,35
Despite no formal curriculum, the 2016 intern class (historical control) had learned POCUS to some degree based on their higher assessment scores compared with the 2017 intern class during the preintervention period. Such learning may be informal, and yet, trainees may feel confident in making clinical decisions without formalized training, accreditation, or oversight. As suggested by this study, adding regular didactics or giving trainees HUDs may not immediately solve this issue. For assessment items in which the 2017 interns did not significantly differ from the controls, they nonetheless reported higher confidence in their abilities. Similarly, interns randomized to HUDs less frequently cited a lack of confidence in their abilities, despite similar scores to the no-HUD group. Such confidence may be incongruent with their actual knowledge or ability to safely use POCUS. This phenomenon of misplaced confidence is known as the Dunning–Kruger effect, and it may be common with ultrasound learning.41 While confidence can be part of a holistic definition of competency,14 these results raise the concern that trainees may have difficulty assessing their own competency level with POCUS.35
There are several limitations to this study. It was performed at a single institution with limited sample size. It examined only intern physicians because of funding constraints, which limits the generalizability of these findings among medical trainees. Technical ability assessments (including obtaining and interpreting images) were not included. We were unable to track the timing or location of the devices’ usage, and the interns’ self-reported usage rates may be subject to recall bias. To our knowledge, there were no significant lapses in device availability/functionality. Intern physicians in the HUD arm did not receive formal feedback on personally acquired patient images, which may have limited the intervention’s impact.
In conclusion, internal medicine interns who received personal HUDs were not better at recognizing normal/abnormal findings on image assessments, and they did not report performing more POCUS examinations. Since the minority of a trainee’s time is spent toward direct patient care, offering trainees HUDs without substantial guidance may not be enough to promote mastery. Notably, trainees who received HUDs felt more confident in their abilities, despite no objective increase in their actual skill. Finally, interns who received POCUS-related lectures experienced significant benefit upon first exposure to the material, while repeated exposures did not improve performance. Future investigations should stringently track trainee POCUS usage rates with HUDs and assess whether image acquisition ability improves as a result of personal access.
1. Moore CL, Copel JA. Point-of-care ultrasonography. N Engl J Med. 2011;364(8):749-757. https://doi.org/10.1056/NEJMra0909487.
2. Akkaya A, Yesilaras M, Aksay E, Sever M, Atilla OD. The interrater reliability of ultrasound imaging of the inferior vena cava performed by emergency residents. Am J Emerg Med. 2013;31(10):1509-1511. https://doi.org/10.1016/j.ajem.2013.07.006.
3. Razi R, Estrada JR, Doll J, Spencer KT. Bedside hand-carried ultrasound by internal medicine residents versus traditional clinical assessment for the identification of systolic dysfunction in patients admitted with decompensated heart failure. J Am Soc Echocardiogr. 2011;24(12):1319-1324. https://doi.org/10.1016/j.echo.2011.07.013.
4. Dodge KL, Lynch CA, Moore CL, Biroscak BJ, Evans LV. Use of ultrasound guidance improves central venous catheter insertion success rates among junior residents. J Ultrasound Med. 2012;31(10):1519-1526. https://doi.org/10.7863/jum.2012.31.10.1519.
5. Cavanna L, Mordenti P, Bertè R, et al. Ultrasound guidance reduces pneumothorax rate and improves safety of thoracentesis in malignant pleural effusion: Report on 445 consecutive patients with advanced cancer. World J Surg Oncol. 2014;12:139. https://doi.org/10.1186/1477-7819-12-139.
6. Testa A, Francesconi A, Giannuzzi R, Berardi S, Sbraccia P. Economic analysis of bedside ultrasonography (US) implementation in an Internal Medicine department. Intern Emerg Med. 2015;10(8):1015-1024. https://doi.org/10.1007/s11739-015-1320-7.
7. Howard ZD, Noble VE, Marill KA, et al. Bedside ultrasound maximizes patient satisfaction. J Emerg Med. 2014;46(1):46-53. https://doi.org/10.1016/j.jemermed.2013.05.044.
8. Park YH, Jung RB, Lee YG, et al. Does the use of bedside ultrasonography reduce emergency department length of stay for patients with renal colic? A pilot study. Clin Exp Emerg Med. 2016;3(4):197-203. https://doi.org/10.15441/ceem.15.109.
9. Glomb N, D’Amico B, Rus M, Chen C. Point-of-care ultrasound in resource-limited settings. Clin Pediatr Emerg Med. 2015;16(4):256-261. https://doi.org/10.1016/j.cpem.2015.10.001.
10. Bahner DP, Goldman E, Way D, Royall NA, Liu YT. The state of ultrasound education in U.S. medical schools: results of a national survey. Acad Med. 2014;89(12):1681-1686. https://doi.org/10.1097/ACM.0000000000000414.
11. Hall JWW, Holman H, Bornemann P, et al. Point of care ultrasound in family medicine residency programs: A CERA study. Fam Med. 2015;47(9):706-711.
12. Schnobrich DJ, Gladding S, Olson APJ, Duran-Nelson A. Point-of-care ultrasound in internal medicine: A national survey of educational leadership. J Grad Med Educ. 2013;5(3):498-502. https://doi.org/10.4300/JGME-D-12-00215.1.
13. Stolz LA, Stolz U, Fields JM, et al. Emergency medicine resident assessment of the emergency ultrasound milestones and current training recommendations. Acad Emerg Med. 2017;24(3):353-361. https://doi.org/10.1111/acem.13113.
14. Kumar, A., Jensen, T., Kugler, J. Evaluation of trainee competency with point-of-care ultrasonography (POCUS): A conceptual framework and review of existing assessments. J Gen Intern Med. 2019;34(6):1025-1031. https://doi.org/10.1007/s11606-019-04945-4.
15. Levitov A, Frankel HL, Blaivas M, et al. Guidelines for the appropriate use of bedside general and cardiac ultrasonography in the evaluation of critically ill patients—part ii: Cardiac ultrasonography. Crit Care Med. 2016;44(6):1206-1227. https://doi.org/10.1097/CCM.0000000000001847.
16. Kobal SL, Trento L, Baharami S, et al. Comparison of effectiveness of hand-carried ultrasound to bedside cardiovascular physical examination. Am J Cardiol. 2005;96(7):1002-1006. https://doi.org/10.1016/j.amjcard.2005.05.060.
17. Ceriani E, Cogliati C. Update on bedside ultrasound diagnosis of pericardial effusion. Intern Emerg Med. 2016;11(3):477-480. https://doi.org/10.1007/s11739-015-1372-8.
18. Labovitz AJ, Noble VE, Bierig M, et al. Focused cardiac ultrasound in the emergent setting: A consensus statement of the American Society of Echocardiography and American College of Emergency Physicians. J Am Soc Echocardiogr. 2010;23(12):1225-1230. https://doi.org/10.1016/j.echo.2010.10.005.
19. Keil-Ríos D, Terrazas-Solís H, González-Garay A, Sánchez-Ávila JF, García-Juárez I. Pocket ultrasound device as a complement to physical examination for ascites evaluation and guided paracentesis. Intern Emerg Med. 2016;11(3):461-466. https://doi.org/10.1007/s11739-016-1406-x.
20. Riddell J, Case A, Wopat R, et al. Sensitivity of emergency bedside ultrasound to detect hydronephrosis in patients with computed tomography–proven stones. West J Emerg Med. 2014;15(1):96-100. https://doi.org/10.5811/westjem.2013.9.15874.
21. Dalziel PJ, Noble VE. Bedside ultrasound and the assessment of renal colic: A review. Emerg Med J. 2013;30(1):3-8. https://doi.org/10.1136/emermed-2012-201375.
22. Whitson MR, Mayo PH. Ultrasonography in the emergency department. Crit Care. 2016;20(1):227. https://doi.org/10.1186/s13054-016-1399-x.
23. Kumar A, Liu G, Chi J, Kugler J. The role of technology in the bedside encounter. Med Clin North Am. 2018;102(3):443-451. https://doi.org/10.1016/j.mcna.2017.12.006.
24. Ma IWY, Arishenkoff S, Wiseman J, et al. Internal medicine point-of-care ultrasound curriculum: Consensus recommendations from the Canadian Internal Medicine Ultrasound (CIMUS) Group. J Gen Intern Med. 2017;32(9):1052-1057. https://doi.org/10.1007/s11606-017-4071-5.
15. Sabath BF, Singh G. Point-of-care ultrasonography as a training milestone for internal medicine residents: The time is now. J Community Hosp Intern Med Perspect. 2016;6(5):33094. https://doi.org/10.3402/jchimp.v6.33094.
26. American College of Emergency Physicians. Ultrasound guidelines: emergency, point-of-care and clinical ultrasound guidelines in medicine. Ann Emerg Med. 2017;69(5):e27-e54. https://doi.org/10.1016/j.annemergmed.2016.08.457.
27. Ramsingh D, Rinehart J, Kain Z, et al. Impact assessment of perioperative point-of-care ultrasound training on anesthesiology residents. Anesthesiology. 2015;123(3):670-682. https://doi.org/10.1097/ALN.0000000000000776.
28. Keddis MT, Cullen MW, Reed DA, et al. Effectiveness of an ultrasound training module for internal medicine residents. BMC Med Educ. 2011;11:75. https://doi.org/10.1186/1472-6920-11-75.
29. Townsend NT, Kendall J, Barnett C, Robinson T. An effective curriculum for focused assessment diagnostic echocardiography: Establishing the learning curve in surgical residents. J Surg Educ. 2016;73(2):190-196. https://doi.org/10.1016/j.jsurg.2015.10.009.
30. Hoppmann RA, Rao VV, Bell F, et al. The evolution of an integrated ultrasound curriculum (iUSC) for medical students: 9-year experience. Crit Ultrasound J. 2015;7(1):18. https://doi.org/10.1186/s13089-015-0035-3.
31. Skalski JH, Elrashidi M, Reed DA, McDonald FS, Bhagra A. Using standardized patients to teach point-of-care ultrasound–guided physical examination skills to internal medicine residents. J Grad Med Educ. 2015;7(1):95-97. https://doi.org/10.4300/JGME-D-14-00178.1.
32. Chisholm CB, Dodge WR, Balise RR, Williams SR, Gharahbaghian L, Beraud A-S. Focused cardiac ultrasound training: How much is enough? J Emerg Med. 2013;44(4):818-822. https://doi.org/10.1016/j.jemermed.2012.07.092.
33. Schmidt GA, Schraufnagel D. Introduction to ATS seminars: Intensive care ultrasound. Ann Am Thorac Soc. 2013;10(5):538-539. https://doi.org/10.1513/AnnalsATS.201306-203ED.
34. Skaarup SH, Laursen CB, Bjerrum AS, Hilberg O. Objective and structured assessment of lung ultrasound competence. A multispecialty Delphi consensus and construct validity study. Ann Am Thorac Soc. 2017;14(4):555-560. https://doi.org/10.1513/AnnalsATS.201611-894OC.
35. Lucas BP, Tierney DM, Jensen TP, et al. Credentialing of hospitalists in ultrasound-guided bedside procedures: A position statement of the Society of Hospital Medicine. J Hosp Med. 2018;13(2):117-125. https://doi.org/10.12788/jhm.2917.
36. Frankel HL, Kirkpatrick AW, Elbarbary M, et al. Guidelines for the appropriate use of bedside general and cardiac ultrasonography in the evaluation of critically ill patients-part i: General ultrasonography. Crit Care Med. 2015;43(11):2479-2502. https://doi.org/10.1097/CCM.0000000000001216.
37. Martin LD, Howell EE, Ziegelstein RC, et al. Hand-carried ultrasound performed by hospitalists: Does it improve the cardiac physical examination? Am J Med. 2009;122(1):35-41. https://doi.org/10.1016/j.amjmed.2008.07.022.
38. Desai SV, Asch DA, Bellini LM, et al. Education outcomes in a duty-hour flexibility trial in internal medicine. N Engl J Med. 2018;378(16):1494-1508. https://doi.org/10.1056/NEJMoa1800965.
39. Baltarowich OH, Di Salvo DN, Scoutt LM, et al. National ultrasound curriculum for medical students. Ultrasound Q. 2014;30(1):13-19. https://doi.org/10.1097/RUQ.0000000000000066.
40. Beal EW, Sigmond BR, Sage-Silski L, Lahey S, Nguyen V, Bahner DP. Point-of-care ultrasound in general surgery residency training: A proposal for milestones in graduate medical education ultrasound. J Ultrasound Med. 2017;36(12):2577-2584. https://doi.org/10.1002/jum.14298.
41. Kruger J, Dunning D. Unskilled and unaware of it: how difficulties in recognizing one’s own incompetence lead to inflated self-assessments. J Pers Soc Psychol. 1999;77(6):1121-1134. https://doi.org/10.1037//0022-3514.77.6.1121.
Point-of-care ultrasonography (POCUS) can transform healthcare delivery through its diagnostic and therapeutic expediency.1 POCUS has been shown to bolster diagnostic accuracy, reduce procedural complications, decrease inpatient length of stay, and improve patient satisfaction by encouraging the physician to be present at the bedside.2-8
POCUS has become widespread across a variety of clinical settings as more investigations have demonstrated its positive impact on patient care.1,9-12 This includes the use of POCUS by trainees, who are now utilizing this technology as part of their assessments of patients.13,14 However, trainees may be performing these examinations with minimal oversight, and outside of emergency medicine, there are few guidelines on how to effectively teach POCUS or measure competency.13,14 While POCUS is rapidly becoming a part of inpatient care, teaching physicians may have little experience in ultrasound or the expertise to adequately supervise trainees.14 There is a growing need to study what trainees can learn and how this knowledge is acquired.
Previous investigations have demonstrated that inexperienced users can be taught to use POCUS to identify a variety of pathological states.2,3,15-23 Most of these curricula used a single lecture series as their pedagogical vehicle, and they variably included junior medical trainees. More importantly, the investigations did not explore whether personal access to handheld ultrasound devices (HUDs) improved learning. In theory, improved access to POCUS devices increases opportunities for authentic and deliberate practice, which may be needed to improve trainee skill with POCUS beyond the classroom setting.14
This study aimed to address several ongoing gaps in knowledge related to learning POCUS. First, we hypothesized that personal HUD access would improve trainees’ POCUS-related knowledge and interpretive ability as a result of increased practice opportunities. Second, we hypothesized that trainees who receive personal access to HUDs would be more likely to perform POCUS examinations and feel more confident in their interpretations. Finally, we hypothesized that repeated exposure to POCUS-related lectures would result in greater improvements in knowledge as compared with a single lecture series.
METHODS
Participants and Setting
The 2017 intern class (n = 47) at an academic internal medicine residency program participated in the study. Control data were obtained from the 2016 intern class (historical control; n = 50) and the 2018 intern class (contemporaneous control; n = 52). The Stanford University Institutional Review Board approved this study.
Study Design
The 2017 intern class (n = 47) received POCUS didactics from June 2017 to June 2018. To evaluate if increased access to HUDs improved learning outcomes, the 2017 interns were randomized 1:1 to receive their own personal HUD that could be used for patient care and/or self-directed learning (n = 24) vs no-HUD (n = 23; Figure). Learning outcomes were assessed over the course of 1 year (see “Outcomes” below) and were compared with the 2016 and 2018 controls. The 2016 intern class had completed a year of training but had not received formalized POCUS didactics (historical control), whereas the 2018 intern class was assessed at the beginning of their year (contemporaneous control; Figure). In order to make comparisons based on intern experience, baseline data for the 2017 intern class were compared with the 2018 intern class, whereas end-of-study data for 2017 interns were compared with 2016 interns.
Outcomes
The primary outcome was the difference in assessment scores at the end of the study period between interns randomized to receive a HUD and those who were not. Secondary outcomes included differences in HUD usage rates, lecture attendance, and assessment scores. To assess whether repeated lecture exposure resulted in greater amounts of learning, this study evaluated for assessment score improvements after each lecture block. Finally, trainee attitudes toward POCUS and their confidence in their interpretative ability were measured at the beginning and end of the study period.
Curriculum Implementation
The lectures were administered as once-weekly didactics of 1-hour duration to interns rotating on the inpatient wards rotation. This rotation is 4 weeks long, and each intern will experience the rotation two to four times per year. Each lecture contained two parts: (1) 20-30 minutes of didactics via Microsoft PowerPointTM and (2) 30-40 minutes of supervised practice using HUDs on standardized patients. Four lectures were given each month: (1) introduction to POCUS and ultrasound physics, (2) thoracic/lung ultrasound, (3) echocardiography, and (4) abdominal POCUS. The lectures consisted of contrasting cases of normal/abnormal videos and clinical vignettes. These four lectures were repeated each month as new interns rotated on service. Some interns experienced the same content multiple times, which was intentional in order to assess their rates of learning over time. Lecture contents were based on previously published guidelines and expert consensus for teaching POCUS in internal medicine.13, 24-26 Content from the Accreditation Council for Graduate Medical Education (ACGME) and the American College of Emergency Physicians (ACEP) was also incorporated because these organizations had published relevant guidelines for teaching POCUS.13,26 Further development of the lectures occurred through review of previously described POCUS-relevant curricula.27-32
Handheld Ultrasound Devices
This study used the Philips LumifyTM, a United States Food and Drug Administration–approved device. Interns randomized to HUDs received their own device at the start of the rotation. It was at their discretion to use the device outside of the course. All devices were approved for patient use and were encrypted in compliance with our information security office. For privacy reasons, any saved patient images were not reviewed by the researchers. Interns were encouraged to share their findings with supervising physicians during rounds, but actual oversight was not measured. Interns not randomized to HUDs could access a single community device that was shared among all residents and fellows in the hospital. Interns reported the average number of POCUS examinations performed each week via a survey sent during the last week of the rotation.
Assessment Design and Implementation
Assessments evaluating trainee knowledge were administered before, during, and after the study period (Figure). For the 2017 cohort, assessments were also administered at the start and end of the ward month to track knowledge acquisition. Assessment contents were selected from POCUS guidelines for internal medicine and adaptation of the ACGME and ACEP guidelines.13,24,26 Additional content was obtained from major society POCUS tutorials and deidentified images collected by the study authors.13,24,33 In keeping with previously described methodology, the images were shown for approximately 12 seconds, followed by five additional seconds to allow the learner to answer the question.32 Final assessment contents were determined by the authors using the Delphi method.34 A sample assessment can be found in the Appendix Material.
Surveys
Surveys were administered alongside the assessments to the 2016-2018 intern classes. These surveys assessed trainee attitudes toward POCUS and were based on previously validated assessments.27,28,30 Attitudes were measured using 5-point Likert scales.
Statistical Analysis
For the primary outcome, we performed generalized binomial mixed-effect regressions using the survey periods, randomization group, and the interaction of the two as independent variables after adjusting for attendance and controlling of intra-intern correlations. The bivariate unadjusted analysis was performed to display the distribution of overall correctness on the assessments. Wilcoxon signed rank test was used to determine score significance for dependent score variables (R-Statistical Programming Language, Vienna, Austria).
RESULTS
Baseline Characteristics
There were 149 interns who participated in this study (Figure). Assessment/survey completion rates were as follows: 2016 control: 68.0%; 2017 preintervention: 97.9%; 2017 postintervention: 89.4%; and 2018 control: 100%. The 2017 interns reported similar amounts of prior POCUS exposure in medical school (Table 1).
Primary Outcome: Assessment Scores (HUD vs no HUD)
There were no significant differences in assessment scores at the end of the study between interns randomized to personal HUD access vs those to no-HUD access (Table 1). HUD interns reported performing POCUS assessments on patients a mean 6.8 (standard deviation [SD] 2.2) times per week vs 6.4 (SD 2.9) times per week in the no-HUD arm (P = .66). The mean lecture attendance was 75.0% and did not significantly differ between the HUD arms (Table 1).
Secondary Outcomes
Impact of Repeating Lectures
The 2017 interns demonstrated significant increases in preblock vs postblock assessment scores after first-time exposure to the lectures (median preblock score 0.61 [interquartile range (IQR), 0.53-0.70] vs postblock score 0.81 [IQR, 0.72-0.86]; P < .001; Table 2). However, intern performance on the preblock vs postblock assessments after second-time exposure to the curriculum failed to improve (median second preblock score 0.78 [IQR, 0.69-0.83] vs postblock score 0.81 [IQR, 0.64-0.89]; P = .94). Intern performance on individual domains of knowledge for each block is listed in Appendix Table 1.
Intervention Performance vs Controls
The 2016 historical control had significantly higher scores compared with the 2017 preintervention group (P < .001; Appendix Table 2). The year-long lecture series resulted in significant increases in median scores for the 2017 group (median preintervention score 0.55 [0.41-0.61] vs median postintervention score 0.84 [0.71-0.90]; P = .006; Appendix Table 1). At the end of the study, the 2017 postintervention scores were significantly higher across multiple knowledge domains compared with the 2016 historical control (Appendix Table 2).
Survey Results
Notably, the 2017 intern class at the end of the intervention did not have significantly different assessment scores for several disease-specific domains, compared with the 2016 control (Appendix Table 2). Nonetheless, the 2017 intern class reported higher levels of confidence in these same domains despite similar scores (Supplementary Figure). The HUD group seldomly cited a lack of confidence in their abilities as a barrier to performing POCUS examinations (17.6%), compared with the no-HUD group (50.0%), despite nearly identical assessment scores between the two groups (Table 1).
DISCUSSION
Previous guidelines have recommended increased HUD access for learners,13,24,35,36 but there have been few investigations that have evaluated the impact of such access on learning POCUS. One previous investigation found that hospitalists who carried HUDs were more likely to identify heart failure on bedside examination.37 In contrast, our study found no improvement in interpretative ability when randomizing interns to carry HUDs for patient care. Notably, interns did not perform more POCUS examinations when given HUDs. We offer several explanations for this finding. First, time-motion studies have demonstrated that internal medicine interns spend less than 15% of their time toward direct patient care.38 It is possible that the demands of being an intern impeded their ability to perform more POCUS examinations on their patients, regardless of HUD access. Alternatively, the interns randomized to no personal access may have used the community device more frequently as a result of the lecture series. Given the cost of HUDs, further studies are needed to assess the degree to which HUD access will improve trainee interpretive ability, especially as more training programs consider the creation of ultrasound curricula.10,11,24,39,40
This study was unique because it followed interns over a year-long course that repeated the same material to assess rates of learning with repeated exposure. Learners improved their scores after the first, but not second, block. Furthermore, the median scores were nearly identical between the first postblock assessment and second preblock assessment (0.81 vs 0.78), suggesting that knowledge was retained between blocks. Together, these findings suggest there may be limitations of traditional lectures that use standardized patient models for practice. Supplementary pedagogies, such as in-the-moment feedback with actual patients, may be needed to promote mastery.14,35
Despite no formal curriculum, the 2016 intern class (historical control) had learned POCUS to some degree based on their higher assessment scores compared with the 2017 intern class during the preintervention period. Such learning may be informal, and yet, trainees may feel confident in making clinical decisions without formalized training, accreditation, or oversight. As suggested by this study, adding regular didactics or giving trainees HUDs may not immediately solve this issue. For assessment items in which the 2017 interns did not significantly differ from the controls, they nonetheless reported higher confidence in their abilities. Similarly, interns randomized to HUDs less frequently cited a lack of confidence in their abilities, despite similar scores to the no-HUD group. Such confidence may be incongruent with their actual knowledge or ability to safely use POCUS. This phenomenon of misplaced confidence is known as the Dunning–Kruger effect, and it may be common with ultrasound learning.41 While confidence can be part of a holistic definition of competency,14 these results raise the concern that trainees may have difficulty assessing their own competency level with POCUS.35
There are several limitations to this study. It was performed at a single institution with limited sample size. It examined only intern physicians because of funding constraints, which limits the generalizability of these findings among medical trainees. Technical ability assessments (including obtaining and interpreting images) were not included. We were unable to track the timing or location of the devices’ usage, and the interns’ self-reported usage rates may be subject to recall bias. To our knowledge, there were no significant lapses in device availability/functionality. Intern physicians in the HUD arm did not receive formal feedback on personally acquired patient images, which may have limited the intervention’s impact.
In conclusion, internal medicine interns who received personal HUDs were not better at recognizing normal/abnormal findings on image assessments, and they did not report performing more POCUS examinations. Since the minority of a trainee’s time is spent toward direct patient care, offering trainees HUDs without substantial guidance may not be enough to promote mastery. Notably, trainees who received HUDs felt more confident in their abilities, despite no objective increase in their actual skill. Finally, interns who received POCUS-related lectures experienced significant benefit upon first exposure to the material, while repeated exposures did not improve performance. Future investigations should stringently track trainee POCUS usage rates with HUDs and assess whether image acquisition ability improves as a result of personal access.
Point-of-care ultrasonography (POCUS) can transform healthcare delivery through its diagnostic and therapeutic expediency.1 POCUS has been shown to bolster diagnostic accuracy, reduce procedural complications, decrease inpatient length of stay, and improve patient satisfaction by encouraging the physician to be present at the bedside.2-8
POCUS has become widespread across a variety of clinical settings as more investigations have demonstrated its positive impact on patient care.1,9-12 This includes the use of POCUS by trainees, who are now utilizing this technology as part of their assessments of patients.13,14 However, trainees may be performing these examinations with minimal oversight, and outside of emergency medicine, there are few guidelines on how to effectively teach POCUS or measure competency.13,14 While POCUS is rapidly becoming a part of inpatient care, teaching physicians may have little experience in ultrasound or the expertise to adequately supervise trainees.14 There is a growing need to study what trainees can learn and how this knowledge is acquired.
Previous investigations have demonstrated that inexperienced users can be taught to use POCUS to identify a variety of pathological states.2,3,15-23 Most of these curricula used a single lecture series as their pedagogical vehicle, and they variably included junior medical trainees. More importantly, the investigations did not explore whether personal access to handheld ultrasound devices (HUDs) improved learning. In theory, improved access to POCUS devices increases opportunities for authentic and deliberate practice, which may be needed to improve trainee skill with POCUS beyond the classroom setting.14
This study aimed to address several ongoing gaps in knowledge related to learning POCUS. First, we hypothesized that personal HUD access would improve trainees’ POCUS-related knowledge and interpretive ability as a result of increased practice opportunities. Second, we hypothesized that trainees who receive personal access to HUDs would be more likely to perform POCUS examinations and feel more confident in their interpretations. Finally, we hypothesized that repeated exposure to POCUS-related lectures would result in greater improvements in knowledge as compared with a single lecture series.
METHODS
Participants and Setting
The 2017 intern class (n = 47) at an academic internal medicine residency program participated in the study. Control data were obtained from the 2016 intern class (historical control; n = 50) and the 2018 intern class (contemporaneous control; n = 52). The Stanford University Institutional Review Board approved this study.
Study Design
The 2017 intern class (n = 47) received POCUS didactics from June 2017 to June 2018. To evaluate if increased access to HUDs improved learning outcomes, the 2017 interns were randomized 1:1 to receive their own personal HUD that could be used for patient care and/or self-directed learning (n = 24) vs no-HUD (n = 23; Figure). Learning outcomes were assessed over the course of 1 year (see “Outcomes” below) and were compared with the 2016 and 2018 controls. The 2016 intern class had completed a year of training but had not received formalized POCUS didactics (historical control), whereas the 2018 intern class was assessed at the beginning of their year (contemporaneous control; Figure). In order to make comparisons based on intern experience, baseline data for the 2017 intern class were compared with the 2018 intern class, whereas end-of-study data for 2017 interns were compared with 2016 interns.
Outcomes
The primary outcome was the difference in assessment scores at the end of the study period between interns randomized to receive a HUD and those who were not. Secondary outcomes included differences in HUD usage rates, lecture attendance, and assessment scores. To assess whether repeated lecture exposure resulted in greater amounts of learning, this study evaluated for assessment score improvements after each lecture block. Finally, trainee attitudes toward POCUS and their confidence in their interpretative ability were measured at the beginning and end of the study period.
Curriculum Implementation
The lectures were administered as once-weekly didactics of 1-hour duration to interns rotating on the inpatient wards rotation. This rotation is 4 weeks long, and each intern will experience the rotation two to four times per year. Each lecture contained two parts: (1) 20-30 minutes of didactics via Microsoft PowerPointTM and (2) 30-40 minutes of supervised practice using HUDs on standardized patients. Four lectures were given each month: (1) introduction to POCUS and ultrasound physics, (2) thoracic/lung ultrasound, (3) echocardiography, and (4) abdominal POCUS. The lectures consisted of contrasting cases of normal/abnormal videos and clinical vignettes. These four lectures were repeated each month as new interns rotated on service. Some interns experienced the same content multiple times, which was intentional in order to assess their rates of learning over time. Lecture contents were based on previously published guidelines and expert consensus for teaching POCUS in internal medicine.13, 24-26 Content from the Accreditation Council for Graduate Medical Education (ACGME) and the American College of Emergency Physicians (ACEP) was also incorporated because these organizations had published relevant guidelines for teaching POCUS.13,26 Further development of the lectures occurred through review of previously described POCUS-relevant curricula.27-32
Handheld Ultrasound Devices
This study used the Philips LumifyTM, a United States Food and Drug Administration–approved device. Interns randomized to HUDs received their own device at the start of the rotation. It was at their discretion to use the device outside of the course. All devices were approved for patient use and were encrypted in compliance with our information security office. For privacy reasons, any saved patient images were not reviewed by the researchers. Interns were encouraged to share their findings with supervising physicians during rounds, but actual oversight was not measured. Interns not randomized to HUDs could access a single community device that was shared among all residents and fellows in the hospital. Interns reported the average number of POCUS examinations performed each week via a survey sent during the last week of the rotation.
Assessment Design and Implementation
Assessments evaluating trainee knowledge were administered before, during, and after the study period (Figure). For the 2017 cohort, assessments were also administered at the start and end of the ward month to track knowledge acquisition. Assessment contents were selected from POCUS guidelines for internal medicine and adaptation of the ACGME and ACEP guidelines.13,24,26 Additional content was obtained from major society POCUS tutorials and deidentified images collected by the study authors.13,24,33 In keeping with previously described methodology, the images were shown for approximately 12 seconds, followed by five additional seconds to allow the learner to answer the question.32 Final assessment contents were determined by the authors using the Delphi method.34 A sample assessment can be found in the Appendix Material.
Surveys
Surveys were administered alongside the assessments to the 2016-2018 intern classes. These surveys assessed trainee attitudes toward POCUS and were based on previously validated assessments.27,28,30 Attitudes were measured using 5-point Likert scales.
Statistical Analysis
For the primary outcome, we performed generalized binomial mixed-effect regressions using the survey periods, randomization group, and the interaction of the two as independent variables after adjusting for attendance and controlling of intra-intern correlations. The bivariate unadjusted analysis was performed to display the distribution of overall correctness on the assessments. Wilcoxon signed rank test was used to determine score significance for dependent score variables (R-Statistical Programming Language, Vienna, Austria).
RESULTS
Baseline Characteristics
There were 149 interns who participated in this study (Figure). Assessment/survey completion rates were as follows: 2016 control: 68.0%; 2017 preintervention: 97.9%; 2017 postintervention: 89.4%; and 2018 control: 100%. The 2017 interns reported similar amounts of prior POCUS exposure in medical school (Table 1).
Primary Outcome: Assessment Scores (HUD vs no HUD)
There were no significant differences in assessment scores at the end of the study between interns randomized to personal HUD access vs those to no-HUD access (Table 1). HUD interns reported performing POCUS assessments on patients a mean 6.8 (standard deviation [SD] 2.2) times per week vs 6.4 (SD 2.9) times per week in the no-HUD arm (P = .66). The mean lecture attendance was 75.0% and did not significantly differ between the HUD arms (Table 1).
Secondary Outcomes
Impact of Repeating Lectures
The 2017 interns demonstrated significant increases in preblock vs postblock assessment scores after first-time exposure to the lectures (median preblock score 0.61 [interquartile range (IQR), 0.53-0.70] vs postblock score 0.81 [IQR, 0.72-0.86]; P < .001; Table 2). However, intern performance on the preblock vs postblock assessments after second-time exposure to the curriculum failed to improve (median second preblock score 0.78 [IQR, 0.69-0.83] vs postblock score 0.81 [IQR, 0.64-0.89]; P = .94). Intern performance on individual domains of knowledge for each block is listed in Appendix Table 1.
Intervention Performance vs Controls
The 2016 historical control had significantly higher scores compared with the 2017 preintervention group (P < .001; Appendix Table 2). The year-long lecture series resulted in significant increases in median scores for the 2017 group (median preintervention score 0.55 [0.41-0.61] vs median postintervention score 0.84 [0.71-0.90]; P = .006; Appendix Table 1). At the end of the study, the 2017 postintervention scores were significantly higher across multiple knowledge domains compared with the 2016 historical control (Appendix Table 2).
Survey Results
Notably, the 2017 intern class at the end of the intervention did not have significantly different assessment scores for several disease-specific domains, compared with the 2016 control (Appendix Table 2). Nonetheless, the 2017 intern class reported higher levels of confidence in these same domains despite similar scores (Supplementary Figure). The HUD group seldomly cited a lack of confidence in their abilities as a barrier to performing POCUS examinations (17.6%), compared with the no-HUD group (50.0%), despite nearly identical assessment scores between the two groups (Table 1).
DISCUSSION
Previous guidelines have recommended increased HUD access for learners,13,24,35,36 but there have been few investigations that have evaluated the impact of such access on learning POCUS. One previous investigation found that hospitalists who carried HUDs were more likely to identify heart failure on bedside examination.37 In contrast, our study found no improvement in interpretative ability when randomizing interns to carry HUDs for patient care. Notably, interns did not perform more POCUS examinations when given HUDs. We offer several explanations for this finding. First, time-motion studies have demonstrated that internal medicine interns spend less than 15% of their time toward direct patient care.38 It is possible that the demands of being an intern impeded their ability to perform more POCUS examinations on their patients, regardless of HUD access. Alternatively, the interns randomized to no personal access may have used the community device more frequently as a result of the lecture series. Given the cost of HUDs, further studies are needed to assess the degree to which HUD access will improve trainee interpretive ability, especially as more training programs consider the creation of ultrasound curricula.10,11,24,39,40
This study was unique because it followed interns over a year-long course that repeated the same material to assess rates of learning with repeated exposure. Learners improved their scores after the first, but not second, block. Furthermore, the median scores were nearly identical between the first postblock assessment and second preblock assessment (0.81 vs 0.78), suggesting that knowledge was retained between blocks. Together, these findings suggest there may be limitations of traditional lectures that use standardized patient models for practice. Supplementary pedagogies, such as in-the-moment feedback with actual patients, may be needed to promote mastery.14,35
Despite no formal curriculum, the 2016 intern class (historical control) had learned POCUS to some degree based on their higher assessment scores compared with the 2017 intern class during the preintervention period. Such learning may be informal, and yet, trainees may feel confident in making clinical decisions without formalized training, accreditation, or oversight. As suggested by this study, adding regular didactics or giving trainees HUDs may not immediately solve this issue. For assessment items in which the 2017 interns did not significantly differ from the controls, they nonetheless reported higher confidence in their abilities. Similarly, interns randomized to HUDs less frequently cited a lack of confidence in their abilities, despite similar scores to the no-HUD group. Such confidence may be incongruent with their actual knowledge or ability to safely use POCUS. This phenomenon of misplaced confidence is known as the Dunning–Kruger effect, and it may be common with ultrasound learning.41 While confidence can be part of a holistic definition of competency,14 these results raise the concern that trainees may have difficulty assessing their own competency level with POCUS.35
There are several limitations to this study. It was performed at a single institution with limited sample size. It examined only intern physicians because of funding constraints, which limits the generalizability of these findings among medical trainees. Technical ability assessments (including obtaining and interpreting images) were not included. We were unable to track the timing or location of the devices’ usage, and the interns’ self-reported usage rates may be subject to recall bias. To our knowledge, there were no significant lapses in device availability/functionality. Intern physicians in the HUD arm did not receive formal feedback on personally acquired patient images, which may have limited the intervention’s impact.
In conclusion, internal medicine interns who received personal HUDs were not better at recognizing normal/abnormal findings on image assessments, and they did not report performing more POCUS examinations. Since the minority of a trainee’s time is spent toward direct patient care, offering trainees HUDs without substantial guidance may not be enough to promote mastery. Notably, trainees who received HUDs felt more confident in their abilities, despite no objective increase in their actual skill. Finally, interns who received POCUS-related lectures experienced significant benefit upon first exposure to the material, while repeated exposures did not improve performance. Future investigations should stringently track trainee POCUS usage rates with HUDs and assess whether image acquisition ability improves as a result of personal access.
1. Moore CL, Copel JA. Point-of-care ultrasonography. N Engl J Med. 2011;364(8):749-757. https://doi.org/10.1056/NEJMra0909487.
2. Akkaya A, Yesilaras M, Aksay E, Sever M, Atilla OD. The interrater reliability of ultrasound imaging of the inferior vena cava performed by emergency residents. Am J Emerg Med. 2013;31(10):1509-1511. https://doi.org/10.1016/j.ajem.2013.07.006.
3. Razi R, Estrada JR, Doll J, Spencer KT. Bedside hand-carried ultrasound by internal medicine residents versus traditional clinical assessment for the identification of systolic dysfunction in patients admitted with decompensated heart failure. J Am Soc Echocardiogr. 2011;24(12):1319-1324. https://doi.org/10.1016/j.echo.2011.07.013.
4. Dodge KL, Lynch CA, Moore CL, Biroscak BJ, Evans LV. Use of ultrasound guidance improves central venous catheter insertion success rates among junior residents. J Ultrasound Med. 2012;31(10):1519-1526. https://doi.org/10.7863/jum.2012.31.10.1519.
5. Cavanna L, Mordenti P, Bertè R, et al. Ultrasound guidance reduces pneumothorax rate and improves safety of thoracentesis in malignant pleural effusion: Report on 445 consecutive patients with advanced cancer. World J Surg Oncol. 2014;12:139. https://doi.org/10.1186/1477-7819-12-139.
6. Testa A, Francesconi A, Giannuzzi R, Berardi S, Sbraccia P. Economic analysis of bedside ultrasonography (US) implementation in an Internal Medicine department. Intern Emerg Med. 2015;10(8):1015-1024. https://doi.org/10.1007/s11739-015-1320-7.
7. Howard ZD, Noble VE, Marill KA, et al. Bedside ultrasound maximizes patient satisfaction. J Emerg Med. 2014;46(1):46-53. https://doi.org/10.1016/j.jemermed.2013.05.044.
8. Park YH, Jung RB, Lee YG, et al. Does the use of bedside ultrasonography reduce emergency department length of stay for patients with renal colic? A pilot study. Clin Exp Emerg Med. 2016;3(4):197-203. https://doi.org/10.15441/ceem.15.109.
9. Glomb N, D’Amico B, Rus M, Chen C. Point-of-care ultrasound in resource-limited settings. Clin Pediatr Emerg Med. 2015;16(4):256-261. https://doi.org/10.1016/j.cpem.2015.10.001.
10. Bahner DP, Goldman E, Way D, Royall NA, Liu YT. The state of ultrasound education in U.S. medical schools: results of a national survey. Acad Med. 2014;89(12):1681-1686. https://doi.org/10.1097/ACM.0000000000000414.
11. Hall JWW, Holman H, Bornemann P, et al. Point of care ultrasound in family medicine residency programs: A CERA study. Fam Med. 2015;47(9):706-711.
12. Schnobrich DJ, Gladding S, Olson APJ, Duran-Nelson A. Point-of-care ultrasound in internal medicine: A national survey of educational leadership. J Grad Med Educ. 2013;5(3):498-502. https://doi.org/10.4300/JGME-D-12-00215.1.
13. Stolz LA, Stolz U, Fields JM, et al. Emergency medicine resident assessment of the emergency ultrasound milestones and current training recommendations. Acad Emerg Med. 2017;24(3):353-361. https://doi.org/10.1111/acem.13113.
14. Kumar, A., Jensen, T., Kugler, J. Evaluation of trainee competency with point-of-care ultrasonography (POCUS): A conceptual framework and review of existing assessments. J Gen Intern Med. 2019;34(6):1025-1031. https://doi.org/10.1007/s11606-019-04945-4.
15. Levitov A, Frankel HL, Blaivas M, et al. Guidelines for the appropriate use of bedside general and cardiac ultrasonography in the evaluation of critically ill patients—part ii: Cardiac ultrasonography. Crit Care Med. 2016;44(6):1206-1227. https://doi.org/10.1097/CCM.0000000000001847.
16. Kobal SL, Trento L, Baharami S, et al. Comparison of effectiveness of hand-carried ultrasound to bedside cardiovascular physical examination. Am J Cardiol. 2005;96(7):1002-1006. https://doi.org/10.1016/j.amjcard.2005.05.060.
17. Ceriani E, Cogliati C. Update on bedside ultrasound diagnosis of pericardial effusion. Intern Emerg Med. 2016;11(3):477-480. https://doi.org/10.1007/s11739-015-1372-8.
18. Labovitz AJ, Noble VE, Bierig M, et al. Focused cardiac ultrasound in the emergent setting: A consensus statement of the American Society of Echocardiography and American College of Emergency Physicians. J Am Soc Echocardiogr. 2010;23(12):1225-1230. https://doi.org/10.1016/j.echo.2010.10.005.
19. Keil-Ríos D, Terrazas-Solís H, González-Garay A, Sánchez-Ávila JF, García-Juárez I. Pocket ultrasound device as a complement to physical examination for ascites evaluation and guided paracentesis. Intern Emerg Med. 2016;11(3):461-466. https://doi.org/10.1007/s11739-016-1406-x.
20. Riddell J, Case A, Wopat R, et al. Sensitivity of emergency bedside ultrasound to detect hydronephrosis in patients with computed tomography–proven stones. West J Emerg Med. 2014;15(1):96-100. https://doi.org/10.5811/westjem.2013.9.15874.
21. Dalziel PJ, Noble VE. Bedside ultrasound and the assessment of renal colic: A review. Emerg Med J. 2013;30(1):3-8. https://doi.org/10.1136/emermed-2012-201375.
22. Whitson MR, Mayo PH. Ultrasonography in the emergency department. Crit Care. 2016;20(1):227. https://doi.org/10.1186/s13054-016-1399-x.
23. Kumar A, Liu G, Chi J, Kugler J. The role of technology in the bedside encounter. Med Clin North Am. 2018;102(3):443-451. https://doi.org/10.1016/j.mcna.2017.12.006.
24. Ma IWY, Arishenkoff S, Wiseman J, et al. Internal medicine point-of-care ultrasound curriculum: Consensus recommendations from the Canadian Internal Medicine Ultrasound (CIMUS) Group. J Gen Intern Med. 2017;32(9):1052-1057. https://doi.org/10.1007/s11606-017-4071-5.
15. Sabath BF, Singh G. Point-of-care ultrasonography as a training milestone for internal medicine residents: The time is now. J Community Hosp Intern Med Perspect. 2016;6(5):33094. https://doi.org/10.3402/jchimp.v6.33094.
26. American College of Emergency Physicians. Ultrasound guidelines: emergency, point-of-care and clinical ultrasound guidelines in medicine. Ann Emerg Med. 2017;69(5):e27-e54. https://doi.org/10.1016/j.annemergmed.2016.08.457.
27. Ramsingh D, Rinehart J, Kain Z, et al. Impact assessment of perioperative point-of-care ultrasound training on anesthesiology residents. Anesthesiology. 2015;123(3):670-682. https://doi.org/10.1097/ALN.0000000000000776.
28. Keddis MT, Cullen MW, Reed DA, et al. Effectiveness of an ultrasound training module for internal medicine residents. BMC Med Educ. 2011;11:75. https://doi.org/10.1186/1472-6920-11-75.
29. Townsend NT, Kendall J, Barnett C, Robinson T. An effective curriculum for focused assessment diagnostic echocardiography: Establishing the learning curve in surgical residents. J Surg Educ. 2016;73(2):190-196. https://doi.org/10.1016/j.jsurg.2015.10.009.
30. Hoppmann RA, Rao VV, Bell F, et al. The evolution of an integrated ultrasound curriculum (iUSC) for medical students: 9-year experience. Crit Ultrasound J. 2015;7(1):18. https://doi.org/10.1186/s13089-015-0035-3.
31. Skalski JH, Elrashidi M, Reed DA, McDonald FS, Bhagra A. Using standardized patients to teach point-of-care ultrasound–guided physical examination skills to internal medicine residents. J Grad Med Educ. 2015;7(1):95-97. https://doi.org/10.4300/JGME-D-14-00178.1.
32. Chisholm CB, Dodge WR, Balise RR, Williams SR, Gharahbaghian L, Beraud A-S. Focused cardiac ultrasound training: How much is enough? J Emerg Med. 2013;44(4):818-822. https://doi.org/10.1016/j.jemermed.2012.07.092.
33. Schmidt GA, Schraufnagel D. Introduction to ATS seminars: Intensive care ultrasound. Ann Am Thorac Soc. 2013;10(5):538-539. https://doi.org/10.1513/AnnalsATS.201306-203ED.
34. Skaarup SH, Laursen CB, Bjerrum AS, Hilberg O. Objective and structured assessment of lung ultrasound competence. A multispecialty Delphi consensus and construct validity study. Ann Am Thorac Soc. 2017;14(4):555-560. https://doi.org/10.1513/AnnalsATS.201611-894OC.
35. Lucas BP, Tierney DM, Jensen TP, et al. Credentialing of hospitalists in ultrasound-guided bedside procedures: A position statement of the Society of Hospital Medicine. J Hosp Med. 2018;13(2):117-125. https://doi.org/10.12788/jhm.2917.
36. Frankel HL, Kirkpatrick AW, Elbarbary M, et al. Guidelines for the appropriate use of bedside general and cardiac ultrasonography in the evaluation of critically ill patients-part i: General ultrasonography. Crit Care Med. 2015;43(11):2479-2502. https://doi.org/10.1097/CCM.0000000000001216.
37. Martin LD, Howell EE, Ziegelstein RC, et al. Hand-carried ultrasound performed by hospitalists: Does it improve the cardiac physical examination? Am J Med. 2009;122(1):35-41. https://doi.org/10.1016/j.amjmed.2008.07.022.
38. Desai SV, Asch DA, Bellini LM, et al. Education outcomes in a duty-hour flexibility trial in internal medicine. N Engl J Med. 2018;378(16):1494-1508. https://doi.org/10.1056/NEJMoa1800965.
39. Baltarowich OH, Di Salvo DN, Scoutt LM, et al. National ultrasound curriculum for medical students. Ultrasound Q. 2014;30(1):13-19. https://doi.org/10.1097/RUQ.0000000000000066.
40. Beal EW, Sigmond BR, Sage-Silski L, Lahey S, Nguyen V, Bahner DP. Point-of-care ultrasound in general surgery residency training: A proposal for milestones in graduate medical education ultrasound. J Ultrasound Med. 2017;36(12):2577-2584. https://doi.org/10.1002/jum.14298.
41. Kruger J, Dunning D. Unskilled and unaware of it: how difficulties in recognizing one’s own incompetence lead to inflated self-assessments. J Pers Soc Psychol. 1999;77(6):1121-1134. https://doi.org/10.1037//0022-3514.77.6.1121.
1. Moore CL, Copel JA. Point-of-care ultrasonography. N Engl J Med. 2011;364(8):749-757. https://doi.org/10.1056/NEJMra0909487.
2. Akkaya A, Yesilaras M, Aksay E, Sever M, Atilla OD. The interrater reliability of ultrasound imaging of the inferior vena cava performed by emergency residents. Am J Emerg Med. 2013;31(10):1509-1511. https://doi.org/10.1016/j.ajem.2013.07.006.
3. Razi R, Estrada JR, Doll J, Spencer KT. Bedside hand-carried ultrasound by internal medicine residents versus traditional clinical assessment for the identification of systolic dysfunction in patients admitted with decompensated heart failure. J Am Soc Echocardiogr. 2011;24(12):1319-1324. https://doi.org/10.1016/j.echo.2011.07.013.
4. Dodge KL, Lynch CA, Moore CL, Biroscak BJ, Evans LV. Use of ultrasound guidance improves central venous catheter insertion success rates among junior residents. J Ultrasound Med. 2012;31(10):1519-1526. https://doi.org/10.7863/jum.2012.31.10.1519.
5. Cavanna L, Mordenti P, Bertè R, et al. Ultrasound guidance reduces pneumothorax rate and improves safety of thoracentesis in malignant pleural effusion: Report on 445 consecutive patients with advanced cancer. World J Surg Oncol. 2014;12:139. https://doi.org/10.1186/1477-7819-12-139.
6. Testa A, Francesconi A, Giannuzzi R, Berardi S, Sbraccia P. Economic analysis of bedside ultrasonography (US) implementation in an Internal Medicine department. Intern Emerg Med. 2015;10(8):1015-1024. https://doi.org/10.1007/s11739-015-1320-7.
7. Howard ZD, Noble VE, Marill KA, et al. Bedside ultrasound maximizes patient satisfaction. J Emerg Med. 2014;46(1):46-53. https://doi.org/10.1016/j.jemermed.2013.05.044.
8. Park YH, Jung RB, Lee YG, et al. Does the use of bedside ultrasonography reduce emergency department length of stay for patients with renal colic? A pilot study. Clin Exp Emerg Med. 2016;3(4):197-203. https://doi.org/10.15441/ceem.15.109.
9. Glomb N, D’Amico B, Rus M, Chen C. Point-of-care ultrasound in resource-limited settings. Clin Pediatr Emerg Med. 2015;16(4):256-261. https://doi.org/10.1016/j.cpem.2015.10.001.
10. Bahner DP, Goldman E, Way D, Royall NA, Liu YT. The state of ultrasound education in U.S. medical schools: results of a national survey. Acad Med. 2014;89(12):1681-1686. https://doi.org/10.1097/ACM.0000000000000414.
11. Hall JWW, Holman H, Bornemann P, et al. Point of care ultrasound in family medicine residency programs: A CERA study. Fam Med. 2015;47(9):706-711.
12. Schnobrich DJ, Gladding S, Olson APJ, Duran-Nelson A. Point-of-care ultrasound in internal medicine: A national survey of educational leadership. J Grad Med Educ. 2013;5(3):498-502. https://doi.org/10.4300/JGME-D-12-00215.1.
13. Stolz LA, Stolz U, Fields JM, et al. Emergency medicine resident assessment of the emergency ultrasound milestones and current training recommendations. Acad Emerg Med. 2017;24(3):353-361. https://doi.org/10.1111/acem.13113.
14. Kumar, A., Jensen, T., Kugler, J. Evaluation of trainee competency with point-of-care ultrasonography (POCUS): A conceptual framework and review of existing assessments. J Gen Intern Med. 2019;34(6):1025-1031. https://doi.org/10.1007/s11606-019-04945-4.
15. Levitov A, Frankel HL, Blaivas M, et al. Guidelines for the appropriate use of bedside general and cardiac ultrasonography in the evaluation of critically ill patients—part ii: Cardiac ultrasonography. Crit Care Med. 2016;44(6):1206-1227. https://doi.org/10.1097/CCM.0000000000001847.
16. Kobal SL, Trento L, Baharami S, et al. Comparison of effectiveness of hand-carried ultrasound to bedside cardiovascular physical examination. Am J Cardiol. 2005;96(7):1002-1006. https://doi.org/10.1016/j.amjcard.2005.05.060.
17. Ceriani E, Cogliati C. Update on bedside ultrasound diagnosis of pericardial effusion. Intern Emerg Med. 2016;11(3):477-480. https://doi.org/10.1007/s11739-015-1372-8.
18. Labovitz AJ, Noble VE, Bierig M, et al. Focused cardiac ultrasound in the emergent setting: A consensus statement of the American Society of Echocardiography and American College of Emergency Physicians. J Am Soc Echocardiogr. 2010;23(12):1225-1230. https://doi.org/10.1016/j.echo.2010.10.005.
19. Keil-Ríos D, Terrazas-Solís H, González-Garay A, Sánchez-Ávila JF, García-Juárez I. Pocket ultrasound device as a complement to physical examination for ascites evaluation and guided paracentesis. Intern Emerg Med. 2016;11(3):461-466. https://doi.org/10.1007/s11739-016-1406-x.
20. Riddell J, Case A, Wopat R, et al. Sensitivity of emergency bedside ultrasound to detect hydronephrosis in patients with computed tomography–proven stones. West J Emerg Med. 2014;15(1):96-100. https://doi.org/10.5811/westjem.2013.9.15874.
21. Dalziel PJ, Noble VE. Bedside ultrasound and the assessment of renal colic: A review. Emerg Med J. 2013;30(1):3-8. https://doi.org/10.1136/emermed-2012-201375.
22. Whitson MR, Mayo PH. Ultrasonography in the emergency department. Crit Care. 2016;20(1):227. https://doi.org/10.1186/s13054-016-1399-x.
23. Kumar A, Liu G, Chi J, Kugler J. The role of technology in the bedside encounter. Med Clin North Am. 2018;102(3):443-451. https://doi.org/10.1016/j.mcna.2017.12.006.
24. Ma IWY, Arishenkoff S, Wiseman J, et al. Internal medicine point-of-care ultrasound curriculum: Consensus recommendations from the Canadian Internal Medicine Ultrasound (CIMUS) Group. J Gen Intern Med. 2017;32(9):1052-1057. https://doi.org/10.1007/s11606-017-4071-5.
15. Sabath BF, Singh G. Point-of-care ultrasonography as a training milestone for internal medicine residents: The time is now. J Community Hosp Intern Med Perspect. 2016;6(5):33094. https://doi.org/10.3402/jchimp.v6.33094.
26. American College of Emergency Physicians. Ultrasound guidelines: emergency, point-of-care and clinical ultrasound guidelines in medicine. Ann Emerg Med. 2017;69(5):e27-e54. https://doi.org/10.1016/j.annemergmed.2016.08.457.
27. Ramsingh D, Rinehart J, Kain Z, et al. Impact assessment of perioperative point-of-care ultrasound training on anesthesiology residents. Anesthesiology. 2015;123(3):670-682. https://doi.org/10.1097/ALN.0000000000000776.
28. Keddis MT, Cullen MW, Reed DA, et al. Effectiveness of an ultrasound training module for internal medicine residents. BMC Med Educ. 2011;11:75. https://doi.org/10.1186/1472-6920-11-75.
29. Townsend NT, Kendall J, Barnett C, Robinson T. An effective curriculum for focused assessment diagnostic echocardiography: Establishing the learning curve in surgical residents. J Surg Educ. 2016;73(2):190-196. https://doi.org/10.1016/j.jsurg.2015.10.009.
30. Hoppmann RA, Rao VV, Bell F, et al. The evolution of an integrated ultrasound curriculum (iUSC) for medical students: 9-year experience. Crit Ultrasound J. 2015;7(1):18. https://doi.org/10.1186/s13089-015-0035-3.
31. Skalski JH, Elrashidi M, Reed DA, McDonald FS, Bhagra A. Using standardized patients to teach point-of-care ultrasound–guided physical examination skills to internal medicine residents. J Grad Med Educ. 2015;7(1):95-97. https://doi.org/10.4300/JGME-D-14-00178.1.
32. Chisholm CB, Dodge WR, Balise RR, Williams SR, Gharahbaghian L, Beraud A-S. Focused cardiac ultrasound training: How much is enough? J Emerg Med. 2013;44(4):818-822. https://doi.org/10.1016/j.jemermed.2012.07.092.
33. Schmidt GA, Schraufnagel D. Introduction to ATS seminars: Intensive care ultrasound. Ann Am Thorac Soc. 2013;10(5):538-539. https://doi.org/10.1513/AnnalsATS.201306-203ED.
34. Skaarup SH, Laursen CB, Bjerrum AS, Hilberg O. Objective and structured assessment of lung ultrasound competence. A multispecialty Delphi consensus and construct validity study. Ann Am Thorac Soc. 2017;14(4):555-560. https://doi.org/10.1513/AnnalsATS.201611-894OC.
35. Lucas BP, Tierney DM, Jensen TP, et al. Credentialing of hospitalists in ultrasound-guided bedside procedures: A position statement of the Society of Hospital Medicine. J Hosp Med. 2018;13(2):117-125. https://doi.org/10.12788/jhm.2917.
36. Frankel HL, Kirkpatrick AW, Elbarbary M, et al. Guidelines for the appropriate use of bedside general and cardiac ultrasonography in the evaluation of critically ill patients-part i: General ultrasonography. Crit Care Med. 2015;43(11):2479-2502. https://doi.org/10.1097/CCM.0000000000001216.
37. Martin LD, Howell EE, Ziegelstein RC, et al. Hand-carried ultrasound performed by hospitalists: Does it improve the cardiac physical examination? Am J Med. 2009;122(1):35-41. https://doi.org/10.1016/j.amjmed.2008.07.022.
38. Desai SV, Asch DA, Bellini LM, et al. Education outcomes in a duty-hour flexibility trial in internal medicine. N Engl J Med. 2018;378(16):1494-1508. https://doi.org/10.1056/NEJMoa1800965.
39. Baltarowich OH, Di Salvo DN, Scoutt LM, et al. National ultrasound curriculum for medical students. Ultrasound Q. 2014;30(1):13-19. https://doi.org/10.1097/RUQ.0000000000000066.
40. Beal EW, Sigmond BR, Sage-Silski L, Lahey S, Nguyen V, Bahner DP. Point-of-care ultrasound in general surgery residency training: A proposal for milestones in graduate medical education ultrasound. J Ultrasound Med. 2017;36(12):2577-2584. https://doi.org/10.1002/jum.14298.
41. Kruger J, Dunning D. Unskilled and unaware of it: how difficulties in recognizing one’s own incompetence lead to inflated self-assessments. J Pers Soc Psychol. 1999;77(6):1121-1134. https://doi.org/10.1037//0022-3514.77.6.1121.
© 2020 Society of Hospital Medicine
Adherence to Topical Treatment Can Improve Treatment-Resistant Moderate Psoriasis
High-potency topical corticosteroids are first-line treatments for psoriasis, but many patients report that they are ineffective or lose effectiveness over time.1-5 The mechanism underlying the lack or loss of activity is not well characterized but may be due to poor adherence to treatment. Adherence to topical treatment is poor in the short run and even worse in the long run.6,7 We evaluated 12 patients with psoriasis resistant to topical corticosteroids to determine if they would respond to topical corticosteroids under conditions designed to promote adherence to treatment.
Methods
This open-label, randomized, single-center clinical study recruited 12 patients with plaque psoriasis that previously failed treatment with topical corticosteroids and other therapies (Table). We stratified disease by body surface area: mild (<3%), moderate (3%–10%), and severe (>10%). Inclusion criteria included adult patients with plaque psoriasis amenable to topical corticosteroid therapy, ability to comply with requirements of the study, and a history of failed topical corticosteroid treatment (Figure). Patients were excluded if they were pregnant, breastfeeding, had conditions that would affect adherence or potentially bias results (eg, dementia, Alzheimer disease), had a history of allergy or sensitivity to corticosteroids, and had a history of drug hypersensitivity.
All patients received desoximetasone spray 0.25% twice daily for 14 days. At the baseline visit, 6 patients were randomly selected to also receive a twice-daily reminder telephone call. Study visits occurred frequently—at baseline and on days 3, 7, and 14—to further assure good adherence to the treatment regimen.
During visits, disease severity was scored using the visual analog scale for pruritus, psoriasis area and severity index (PASI), total lesion severity score (TLSS), and investigator global assessment (IGA). Descriptive statistics were used to report the outcomes for each patient.
The study was designed to assess the number of topical treatment–resistant patients who would improve with topical treatment but was not designed or powered to test if the telephone call reminders increased adherence.
Results
All patients completed the study; 10 of 12 patients (83.3%) had previously used topical clobetasol and it failed (Table). At the 2-week end-of-study visit, most patients improved on all measures. Patients who received telephone call reminders improved more than patients who did not. All 12 patients (100%) reported relief of itching; 11 of 12 (91.7%) had an improved PASI; 10 of 12 (83.3%) had an improved TLSS; and 7 of 12 (58.3%) had an improved IGA (eTables 1 and 2).
The percentage reduction in pruritus ranged from 66.7% to 100% and 50.0% to 85.7% with and without telephone call reminders, respectively. Improvement in PASI ranged from 18.0% to 62.8% and 0% to 54.5% with and without telephone call reminders, respectively. Improvement in TLSS and IGA was of lower magnitude but showed a similar pattern, with numerically greater improvement in the telephone call reminders group compared to the group that was not called (eTable 2). No patients showed a worse score for pruritus on the visual analog scale, PASI, TLSS, or IGA.
Discussion
Topical corticosteroids are highly effective for psoriasis in clinical trials, with clearance in 2 to 4 weeks in 60% to 80% of patients, a rapidity of response not matched by even the most potent biologic treatments.8,9 However, topical corticosteroids are not always effective in clinical practice. There may be primary inefficacy (they do not work at first) or secondary inefficacy (a previously effective treatment loses efficacy over time).10 Poor adherence can explain both phenomena. Primary adherence occurs when patients fill their prescription; secondary adherence occurs when patients follow the medication recommendations.11 Primary nonadherence is common in patients with psoriasis; in one study, 50% of psoriasis prescriptions were not filled.12 Secondary adherence also is poor and declines over time; electronic monitoring revealed adherence to topical treatments in psoriasis patients decreased from 85% initially to 51% at the end of 8 weeks.7 Given the high efficacy of topical corticosteroids in clinical trials and the poor adherence to topical treatment in patients with psoriasis, we anticipated that psoriasis that is resistant to topical corticosteroids would improve rapidly under conditions designed to promote adherence.
As expected, disease improved in almost every patient in this small cohort when they were given a potent topical corticosteroid, even though they previously reported that their psoriasis was resistant to potent topical corticosteroids. Although this study enrolled only a small cohort, it appears that the majority of patients with limited psoriasis that was reported to be resistant to topical treatment can see a response to topical treatment under conditions designed to encourage good adherence.
We believe that the good outcomes seen in our study were a result of good adherence. Although the desoximetasone spray 0.25% used in this study is a superpotent topical corticosteroid,8 the response to treatment was unlikely due to changing corticosteroid potency because 10 of 12 patients had tried another superpotent topical corticosteroid (clobetasol) and it failed. We chose a spray product for this study rather than an ointment to promote adherence; however, this choice limited the ability to assess adherence directly, as adherence-monitoring devices for spray delivery systems are not readily available.
Our study was limited by the small sample size and brief duration of treatment. However, the effect size is so large (ie, the topical treatment was so effective) that only a small sample size and brief treatment duration were needed to show that a high percentage of patients with psoriasis that had previously failed treatment with topical corticosteroids can in fact respond to this treatment.
We used telephone calls as reminders in 50% of patients to further encourage adherence. The study was not designed or powered to assess the effect of the telephone call reminders, but patients receiving those calls appeared to have slightly greater reduction in disease severity. Nonetheless, twice-daily telephone call reminders are unlikely to be a wanted or practical intervention; other approaches to encourage adherence are needed.
Frequent follow-up visits were incorporated in our study design to maximize adherence. Although it might not be feasible for clinical practices to schedule follow-up visits as often as in our study, other approaches such as virtual visits and electronic interaction might provide a practical alternative. Multifaceted approaches to increasing adherence include encouraging patients to participate in the treatment plan, prescribing therapy consistent with a patient’s preferred vehicle, and extensive patient education.13 If patients do not respond as expected, poor adherence can be considered. Other potential causes of poor outcomes include error in diagnosis; resistance to the prescribed treatment; concomitant infection; irritant exposure; and, in the case of biologics, antidrug antibody formation.14,15
- Feldman SR, Fleischer AB Jr, Cooper JZ. New topical treatments change the pattern of treatment of psoriasis: dermatologists remain the primary providers of this care. Int J Dermatol. 2000;39:41-44.
- Menter A. Topical monotherapy with clobetasol propionate spray 0.05% in the COBRA trial. Cutis. 2007;80(suppl 5):12-19.
- Saleem MD, Negus D, Feldman SR. Topical 0.25% desoximetasone spray efficacy for moderate to severe plaque psoriasis: a randomized clinical trial. J Dermatolog Treat. 2018;29:32-35.
- Mraz S, Leonardi C, Colón LE, et al. Different treatment outcomes with different formulations of clobetasol propionate 0.05% for the treatment of plaque psoriasis. J Dermatolog Treat. 2008;19:354-359.
- Chiricozzi A, Pimpinelli N, Ricceri F, et al. Treatment of psoriasis with topical agents: recommendations from a Tuscany Consensus. Dermatol Ther. 2017;30:e12549.
- Carroll CL, Feldman SR, Camacho FT, et al. Adherence to topical therapy decreases during the course of an 8-week psoriasis clinical trial: commonly used methods of measuring adherence to topical therapy overestimate actual use. J Am Acad Dermatol. 2004;51:212-216.
- Alinia H, Moradi Tuchayi S, Smith JA, et al. Long-term adherence to topical psoriasis treatment can be abysmal: a 1-year randomized intervention study using objective electronic adherence monitoring. Br J Dermatol. 2017;176:759-764.
- Keegan BR. Desoximetasone 0.25% spray for the relief of scaling in adults with plaque psoriasis. J Drugs Dermatol. 2015;14:835-840.
- Beutner K, Chakrabarty A, Lemke S, et al. An intra-individual randomized safety and efficacy comparison of clobetasol propionate 0.05% spray and its vehicle in the treatment of plaque psoriasis. J Drugs Dermatol. 2006;5:357-360.
- Mehta AB, Nadkarni NJ, Patil SP, et al. Topical corticosteroids in dermatology. Indian J Dermatol Venereol Leprol. 2016;82:371-378.
- Blais L, Kettani FZ, Forget A, et al. Assessing adherence to inhaled corticosteroids in asthma patients using an integrated measure based on primary and secondary adherence. Eur J Clin Pharmacol. 2016;73:91-97.
- Storm A, Andersen SE, Benfeldt E, et al. One in 3 prescriptions are never redeemed: primary nonadherence in an outpatient clinic. J Am Acad Dermatol. 2008;59:27-33.
- Zschocke I, Mrowietz U, Karakasili E, et al. Non-adherence and measures to improve adherence in the topical treatment of psoriasis. J Eur Acad Dermatol Venereol. 2014;28(Suppl 2):4-9.
- Mooney E, Rademaker M, Dailey R, et al. Adverse effects of topical corticosteroids in paediatric eczema: Australasian consensus statement. Australas J Dermatol. 2015;56:241-251.
- Varada S, Tintle SJ, Gottlieb AB. Apremilast for the treatment of psoriatic arthritis. Expert Rev Clin Pharmacol. 2014;7:239-250.
High-potency topical corticosteroids are first-line treatments for psoriasis, but many patients report that they are ineffective or lose effectiveness over time.1-5 The mechanism underlying the lack or loss of activity is not well characterized but may be due to poor adherence to treatment. Adherence to topical treatment is poor in the short run and even worse in the long run.6,7 We evaluated 12 patients with psoriasis resistant to topical corticosteroids to determine if they would respond to topical corticosteroids under conditions designed to promote adherence to treatment.
Methods
This open-label, randomized, single-center clinical study recruited 12 patients with plaque psoriasis that previously failed treatment with topical corticosteroids and other therapies (Table). We stratified disease by body surface area: mild (<3%), moderate (3%–10%), and severe (>10%). Inclusion criteria included adult patients with plaque psoriasis amenable to topical corticosteroid therapy, ability to comply with requirements of the study, and a history of failed topical corticosteroid treatment (Figure). Patients were excluded if they were pregnant, breastfeeding, had conditions that would affect adherence or potentially bias results (eg, dementia, Alzheimer disease), had a history of allergy or sensitivity to corticosteroids, and had a history of drug hypersensitivity.
All patients received desoximetasone spray 0.25% twice daily for 14 days. At the baseline visit, 6 patients were randomly selected to also receive a twice-daily reminder telephone call. Study visits occurred frequently—at baseline and on days 3, 7, and 14—to further assure good adherence to the treatment regimen.
During visits, disease severity was scored using the visual analog scale for pruritus, psoriasis area and severity index (PASI), total lesion severity score (TLSS), and investigator global assessment (IGA). Descriptive statistics were used to report the outcomes for each patient.
The study was designed to assess the number of topical treatment–resistant patients who would improve with topical treatment but was not designed or powered to test if the telephone call reminders increased adherence.
Results
All patients completed the study; 10 of 12 patients (83.3%) had previously used topical clobetasol and it failed (Table). At the 2-week end-of-study visit, most patients improved on all measures. Patients who received telephone call reminders improved more than patients who did not. All 12 patients (100%) reported relief of itching; 11 of 12 (91.7%) had an improved PASI; 10 of 12 (83.3%) had an improved TLSS; and 7 of 12 (58.3%) had an improved IGA (eTables 1 and 2).
The percentage reduction in pruritus ranged from 66.7% to 100% and 50.0% to 85.7% with and without telephone call reminders, respectively. Improvement in PASI ranged from 18.0% to 62.8% and 0% to 54.5% with and without telephone call reminders, respectively. Improvement in TLSS and IGA was of lower magnitude but showed a similar pattern, with numerically greater improvement in the telephone call reminders group compared to the group that was not called (eTable 2). No patients showed a worse score for pruritus on the visual analog scale, PASI, TLSS, or IGA.
Discussion
Topical corticosteroids are highly effective for psoriasis in clinical trials, with clearance in 2 to 4 weeks in 60% to 80% of patients, a rapidity of response not matched by even the most potent biologic treatments.8,9 However, topical corticosteroids are not always effective in clinical practice. There may be primary inefficacy (they do not work at first) or secondary inefficacy (a previously effective treatment loses efficacy over time).10 Poor adherence can explain both phenomena. Primary adherence occurs when patients fill their prescription; secondary adherence occurs when patients follow the medication recommendations.11 Primary nonadherence is common in patients with psoriasis; in one study, 50% of psoriasis prescriptions were not filled.12 Secondary adherence also is poor and declines over time; electronic monitoring revealed adherence to topical treatments in psoriasis patients decreased from 85% initially to 51% at the end of 8 weeks.7 Given the high efficacy of topical corticosteroids in clinical trials and the poor adherence to topical treatment in patients with psoriasis, we anticipated that psoriasis that is resistant to topical corticosteroids would improve rapidly under conditions designed to promote adherence.
As expected, disease improved in almost every patient in this small cohort when they were given a potent topical corticosteroid, even though they previously reported that their psoriasis was resistant to potent topical corticosteroids. Although this study enrolled only a small cohort, it appears that the majority of patients with limited psoriasis that was reported to be resistant to topical treatment can see a response to topical treatment under conditions designed to encourage good adherence.
We believe that the good outcomes seen in our study were a result of good adherence. Although the desoximetasone spray 0.25% used in this study is a superpotent topical corticosteroid,8 the response to treatment was unlikely due to changing corticosteroid potency because 10 of 12 patients had tried another superpotent topical corticosteroid (clobetasol) and it failed. We chose a spray product for this study rather than an ointment to promote adherence; however, this choice limited the ability to assess adherence directly, as adherence-monitoring devices for spray delivery systems are not readily available.
Our study was limited by the small sample size and brief duration of treatment. However, the effect size is so large (ie, the topical treatment was so effective) that only a small sample size and brief treatment duration were needed to show that a high percentage of patients with psoriasis that had previously failed treatment with topical corticosteroids can in fact respond to this treatment.
We used telephone calls as reminders in 50% of patients to further encourage adherence. The study was not designed or powered to assess the effect of the telephone call reminders, but patients receiving those calls appeared to have slightly greater reduction in disease severity. Nonetheless, twice-daily telephone call reminders are unlikely to be a wanted or practical intervention; other approaches to encourage adherence are needed.
Frequent follow-up visits were incorporated in our study design to maximize adherence. Although it might not be feasible for clinical practices to schedule follow-up visits as often as in our study, other approaches such as virtual visits and electronic interaction might provide a practical alternative. Multifaceted approaches to increasing adherence include encouraging patients to participate in the treatment plan, prescribing therapy consistent with a patient’s preferred vehicle, and extensive patient education.13 If patients do not respond as expected, poor adherence can be considered. Other potential causes of poor outcomes include error in diagnosis; resistance to the prescribed treatment; concomitant infection; irritant exposure; and, in the case of biologics, antidrug antibody formation.14,15
High-potency topical corticosteroids are first-line treatments for psoriasis, but many patients report that they are ineffective or lose effectiveness over time.1-5 The mechanism underlying the lack or loss of activity is not well characterized but may be due to poor adherence to treatment. Adherence to topical treatment is poor in the short run and even worse in the long run.6,7 We evaluated 12 patients with psoriasis resistant to topical corticosteroids to determine if they would respond to topical corticosteroids under conditions designed to promote adherence to treatment.
Methods
This open-label, randomized, single-center clinical study recruited 12 patients with plaque psoriasis that previously failed treatment with topical corticosteroids and other therapies (Table). We stratified disease by body surface area: mild (<3%), moderate (3%–10%), and severe (>10%). Inclusion criteria included adult patients with plaque psoriasis amenable to topical corticosteroid therapy, ability to comply with requirements of the study, and a history of failed topical corticosteroid treatment (Figure). Patients were excluded if they were pregnant, breastfeeding, had conditions that would affect adherence or potentially bias results (eg, dementia, Alzheimer disease), had a history of allergy or sensitivity to corticosteroids, and had a history of drug hypersensitivity.
All patients received desoximetasone spray 0.25% twice daily for 14 days. At the baseline visit, 6 patients were randomly selected to also receive a twice-daily reminder telephone call. Study visits occurred frequently—at baseline and on days 3, 7, and 14—to further assure good adherence to the treatment regimen.
During visits, disease severity was scored using the visual analog scale for pruritus, psoriasis area and severity index (PASI), total lesion severity score (TLSS), and investigator global assessment (IGA). Descriptive statistics were used to report the outcomes for each patient.
The study was designed to assess the number of topical treatment–resistant patients who would improve with topical treatment but was not designed or powered to test if the telephone call reminders increased adherence.
Results
All patients completed the study; 10 of 12 patients (83.3%) had previously used topical clobetasol and it failed (Table). At the 2-week end-of-study visit, most patients improved on all measures. Patients who received telephone call reminders improved more than patients who did not. All 12 patients (100%) reported relief of itching; 11 of 12 (91.7%) had an improved PASI; 10 of 12 (83.3%) had an improved TLSS; and 7 of 12 (58.3%) had an improved IGA (eTables 1 and 2).
The percentage reduction in pruritus ranged from 66.7% to 100% and 50.0% to 85.7% with and without telephone call reminders, respectively. Improvement in PASI ranged from 18.0% to 62.8% and 0% to 54.5% with and without telephone call reminders, respectively. Improvement in TLSS and IGA was of lower magnitude but showed a similar pattern, with numerically greater improvement in the telephone call reminders group compared to the group that was not called (eTable 2). No patients showed a worse score for pruritus on the visual analog scale, PASI, TLSS, or IGA.
Discussion
Topical corticosteroids are highly effective for psoriasis in clinical trials, with clearance in 2 to 4 weeks in 60% to 80% of patients, a rapidity of response not matched by even the most potent biologic treatments.8,9 However, topical corticosteroids are not always effective in clinical practice. There may be primary inefficacy (they do not work at first) or secondary inefficacy (a previously effective treatment loses efficacy over time).10 Poor adherence can explain both phenomena. Primary adherence occurs when patients fill their prescription; secondary adherence occurs when patients follow the medication recommendations.11 Primary nonadherence is common in patients with psoriasis; in one study, 50% of psoriasis prescriptions were not filled.12 Secondary adherence also is poor and declines over time; electronic monitoring revealed adherence to topical treatments in psoriasis patients decreased from 85% initially to 51% at the end of 8 weeks.7 Given the high efficacy of topical corticosteroids in clinical trials and the poor adherence to topical treatment in patients with psoriasis, we anticipated that psoriasis that is resistant to topical corticosteroids would improve rapidly under conditions designed to promote adherence.
As expected, disease improved in almost every patient in this small cohort when they were given a potent topical corticosteroid, even though they previously reported that their psoriasis was resistant to potent topical corticosteroids. Although this study enrolled only a small cohort, it appears that the majority of patients with limited psoriasis that was reported to be resistant to topical treatment can see a response to topical treatment under conditions designed to encourage good adherence.
We believe that the good outcomes seen in our study were a result of good adherence. Although the desoximetasone spray 0.25% used in this study is a superpotent topical corticosteroid,8 the response to treatment was unlikely due to changing corticosteroid potency because 10 of 12 patients had tried another superpotent topical corticosteroid (clobetasol) and it failed. We chose a spray product for this study rather than an ointment to promote adherence; however, this choice limited the ability to assess adherence directly, as adherence-monitoring devices for spray delivery systems are not readily available.
Our study was limited by the small sample size and brief duration of treatment. However, the effect size is so large (ie, the topical treatment was so effective) that only a small sample size and brief treatment duration were needed to show that a high percentage of patients with psoriasis that had previously failed treatment with topical corticosteroids can in fact respond to this treatment.
We used telephone calls as reminders in 50% of patients to further encourage adherence. The study was not designed or powered to assess the effect of the telephone call reminders, but patients receiving those calls appeared to have slightly greater reduction in disease severity. Nonetheless, twice-daily telephone call reminders are unlikely to be a wanted or practical intervention; other approaches to encourage adherence are needed.
Frequent follow-up visits were incorporated in our study design to maximize adherence. Although it might not be feasible for clinical practices to schedule follow-up visits as often as in our study, other approaches such as virtual visits and electronic interaction might provide a practical alternative. Multifaceted approaches to increasing adherence include encouraging patients to participate in the treatment plan, prescribing therapy consistent with a patient’s preferred vehicle, and extensive patient education.13 If patients do not respond as expected, poor adherence can be considered. Other potential causes of poor outcomes include error in diagnosis; resistance to the prescribed treatment; concomitant infection; irritant exposure; and, in the case of biologics, antidrug antibody formation.14,15
- Feldman SR, Fleischer AB Jr, Cooper JZ. New topical treatments change the pattern of treatment of psoriasis: dermatologists remain the primary providers of this care. Int J Dermatol. 2000;39:41-44.
- Menter A. Topical monotherapy with clobetasol propionate spray 0.05% in the COBRA trial. Cutis. 2007;80(suppl 5):12-19.
- Saleem MD, Negus D, Feldman SR. Topical 0.25% desoximetasone spray efficacy for moderate to severe plaque psoriasis: a randomized clinical trial. J Dermatolog Treat. 2018;29:32-35.
- Mraz S, Leonardi C, Colón LE, et al. Different treatment outcomes with different formulations of clobetasol propionate 0.05% for the treatment of plaque psoriasis. J Dermatolog Treat. 2008;19:354-359.
- Chiricozzi A, Pimpinelli N, Ricceri F, et al. Treatment of psoriasis with topical agents: recommendations from a Tuscany Consensus. Dermatol Ther. 2017;30:e12549.
- Carroll CL, Feldman SR, Camacho FT, et al. Adherence to topical therapy decreases during the course of an 8-week psoriasis clinical trial: commonly used methods of measuring adherence to topical therapy overestimate actual use. J Am Acad Dermatol. 2004;51:212-216.
- Alinia H, Moradi Tuchayi S, Smith JA, et al. Long-term adherence to topical psoriasis treatment can be abysmal: a 1-year randomized intervention study using objective electronic adherence monitoring. Br J Dermatol. 2017;176:759-764.
- Keegan BR. Desoximetasone 0.25% spray for the relief of scaling in adults with plaque psoriasis. J Drugs Dermatol. 2015;14:835-840.
- Beutner K, Chakrabarty A, Lemke S, et al. An intra-individual randomized safety and efficacy comparison of clobetasol propionate 0.05% spray and its vehicle in the treatment of plaque psoriasis. J Drugs Dermatol. 2006;5:357-360.
- Mehta AB, Nadkarni NJ, Patil SP, et al. Topical corticosteroids in dermatology. Indian J Dermatol Venereol Leprol. 2016;82:371-378.
- Blais L, Kettani FZ, Forget A, et al. Assessing adherence to inhaled corticosteroids in asthma patients using an integrated measure based on primary and secondary adherence. Eur J Clin Pharmacol. 2016;73:91-97.
- Storm A, Andersen SE, Benfeldt E, et al. One in 3 prescriptions are never redeemed: primary nonadherence in an outpatient clinic. J Am Acad Dermatol. 2008;59:27-33.
- Zschocke I, Mrowietz U, Karakasili E, et al. Non-adherence and measures to improve adherence in the topical treatment of psoriasis. J Eur Acad Dermatol Venereol. 2014;28(Suppl 2):4-9.
- Mooney E, Rademaker M, Dailey R, et al. Adverse effects of topical corticosteroids in paediatric eczema: Australasian consensus statement. Australas J Dermatol. 2015;56:241-251.
- Varada S, Tintle SJ, Gottlieb AB. Apremilast for the treatment of psoriatic arthritis. Expert Rev Clin Pharmacol. 2014;7:239-250.
- Feldman SR, Fleischer AB Jr, Cooper JZ. New topical treatments change the pattern of treatment of psoriasis: dermatologists remain the primary providers of this care. Int J Dermatol. 2000;39:41-44.
- Menter A. Topical monotherapy with clobetasol propionate spray 0.05% in the COBRA trial. Cutis. 2007;80(suppl 5):12-19.
- Saleem MD, Negus D, Feldman SR. Topical 0.25% desoximetasone spray efficacy for moderate to severe plaque psoriasis: a randomized clinical trial. J Dermatolog Treat. 2018;29:32-35.
- Mraz S, Leonardi C, Colón LE, et al. Different treatment outcomes with different formulations of clobetasol propionate 0.05% for the treatment of plaque psoriasis. J Dermatolog Treat. 2008;19:354-359.
- Chiricozzi A, Pimpinelli N, Ricceri F, et al. Treatment of psoriasis with topical agents: recommendations from a Tuscany Consensus. Dermatol Ther. 2017;30:e12549.
- Carroll CL, Feldman SR, Camacho FT, et al. Adherence to topical therapy decreases during the course of an 8-week psoriasis clinical trial: commonly used methods of measuring adherence to topical therapy overestimate actual use. J Am Acad Dermatol. 2004;51:212-216.
- Alinia H, Moradi Tuchayi S, Smith JA, et al. Long-term adherence to topical psoriasis treatment can be abysmal: a 1-year randomized intervention study using objective electronic adherence monitoring. Br J Dermatol. 2017;176:759-764.
- Keegan BR. Desoximetasone 0.25% spray for the relief of scaling in adults with plaque psoriasis. J Drugs Dermatol. 2015;14:835-840.
- Beutner K, Chakrabarty A, Lemke S, et al. An intra-individual randomized safety and efficacy comparison of clobetasol propionate 0.05% spray and its vehicle in the treatment of plaque psoriasis. J Drugs Dermatol. 2006;5:357-360.
- Mehta AB, Nadkarni NJ, Patil SP, et al. Topical corticosteroids in dermatology. Indian J Dermatol Venereol Leprol. 2016;82:371-378.
- Blais L, Kettani FZ, Forget A, et al. Assessing adherence to inhaled corticosteroids in asthma patients using an integrated measure based on primary and secondary adherence. Eur J Clin Pharmacol. 2016;73:91-97.
- Storm A, Andersen SE, Benfeldt E, et al. One in 3 prescriptions are never redeemed: primary nonadherence in an outpatient clinic. J Am Acad Dermatol. 2008;59:27-33.
- Zschocke I, Mrowietz U, Karakasili E, et al. Non-adherence and measures to improve adherence in the topical treatment of psoriasis. J Eur Acad Dermatol Venereol. 2014;28(Suppl 2):4-9.
- Mooney E, Rademaker M, Dailey R, et al. Adverse effects of topical corticosteroids in paediatric eczema: Australasian consensus statement. Australas J Dermatol. 2015;56:241-251.
- Varada S, Tintle SJ, Gottlieb AB. Apremilast for the treatment of psoriatic arthritis. Expert Rev Clin Pharmacol. 2014;7:239-250.
Practice Points
- Most patients with psoriasis are good candidates for topical treatment.
- Topical treatment of psoriasis often is ineffective.
- Topical treatment of psoriasis can be rapidly effective, even in patients who reported disease that was resistant to topical treatment.
Betamethasone Dipropionate Spray 0.05% Alleviates Troublesome Symptoms of Plaque Psoriasis
Psoriasis affects approximately 2% to 3% of the US population and is characterized by plaques that are red, scaly, and elevated.1 Cutaneous symptoms of the disease are described by patients as itching, burning, and stinging sensations. Large multinational and US surveys have reported pruritus as patients’ most bothersome symptom, with scaling/flaking reported as the second most bothersome.2,3 Reported incidence rates for itching range from 60.4% to 98.3%, with at least half of these patients reporting daily or constant pruritus.2,4-7 Consequent effects on quality of life include impaired sleep,6 difficulty concentrating, lower sex drive, and depression.7 Despite these findings, pruritus is rarely included in the efficacy assessments of psoriasis treatments. In addition, 2 of the most commonly reported but difficult-to-treat locations for plaques are the outside of the elbows (45%) and the knees (32%),1,2,8 areas where the stratum corneum typically is thicker, less hydrated, and less likely to absorb topical products.9-11 Clinical studies have not focused specifically on these areas when assessing treatments.
Topical corticosteroids have been the mainstay of psoriasis therapy for decades because of their anti-inflammatory and antiproliferative properties.7 One large multinational physician survey indicated that 75% of patients are prescribed topical steroids,12 which are important for first-line treatment and are often maintained as adjunctive therapy in combination with other treatments for patients with extensive disease or recalcitrant lesions.13 Topical corticosteroids are ranked into different classes based on their vasoconstrictor assay (VCA), a measure of skin blanching used as a marker for vasoconstriction. Topical agents with VCA ratings of mid-potency or superpotency are generally recommended for initial therapy, with superpotent agents required for the treatment of thick chronic plaques. However, longer durations of use may contribute to systemic absorption and adverse events.13 The vehicle composition is important for corticosteroid delivery and retention at the site of pathology, contributing to the efficacy of the steroid.13,14 Selecting the appropriate steroid and vehicle is important to maximize efficacy and minimize adverse events.
Betamethasone dipropionate (BD) spray 0.05% is an emollient formulation of 0.05% BD that can be sprayed onto psoriatic plaques. The BD spray formulation was designed to penetrate the stratum corneum and be retained within the dermis and epidermis, the site of T-cell activity that drives the psoriatic disease process.14 In 2 phase 3 studies, BD spray demonstrated the ability to reduce the signs of plaque psoriasis with indication of improvement by day 4.15,16 These studies also showed improvement in the local cutaneous symptoms of itching, burning and stinging, and pain. As a mid-potent steroid, BD spray displays less systemic absorption but similar efficacy compared to a superpotent augmented BD (AugBD) lotion in relieving the signs and symptoms of plaque psoriasis.15-17
The objective of the current investigation was to assess the ability of BD spray to relieve itching and to clear plaque psoriasis on the knees and elbows utilizing post hoc analyses of the 2 phase 3 trials. The goal of these analyses was to demonstrate BD spray as effective at relieving the most troublesome signs and symptoms affecting patients with plaque psoriasis.
Methods
Two phase 3 studies were conducted to demonstrate the efficacy and safety of BD spray.15,16 The design of the studies was similar15,16 to allow the data to be pooled for post hoc analyses.
Both were US multicenter, randomized, vehicle-controlled, double-blind, parallel-group studies comparing the safety and efficacy of BD spray 0.05% (Sernivo, Promius Pharma) with its vehicle formulation spray (identical to BD spray, but lacking the active steroid component).15,16 One of the studies also compared BD spray with an AugBD lotion 0.05% (Diprolene,Merck & Co). Adults with moderate plaque psoriasis (investigator global assessment of 3; 10%–20% body surface area) were randomized to apply BD spray, vehicle spray, or AugBD lotion (1 study only) twice daily to all affected areas, excluding the face, scalp, and intertriginous areas for 28 days (BD spray and vehicle) or 14 days (AugBD lotion, per product label).15
Assessments
Two post hoc analyses were conducted on data pooled from the 2 phase 3 trials: (1) incidence of itching, and (2) total sign score (TSS) for lesions located on the knees and elbows.
Itching
Itching was assessed proactively by asking patients if they were experiencing itching (yes/no) at each visit (baseline and days 4, 8, 15, and 29) or had experienced itching since their last visit. As itching could be an adverse event of topical application, application-site pruritus was also recorded.
Total Sign Score
For each patient, a target plaque was selected that was representative of their psoriasis. The plaque was assessed on a 3-point grading scale for each of 3 key signs of plaque psoriasis: erythema, scaling, and plaque elevation (Table 1) at baseline and days 4, 8, 15, and 29. Total sign score was calculated by summing the scores for these 3 signs, resulting in a score ranging from 0 to 9. Treatment success was measured as (1) achieving a score of 0 or 1 (ie, reducing the plaque to clear or slight to mild) for the individual signs of erythema, scaling, and plaque elevation; and (2) achieving a TSS of 0 or 1 for all 3 signs—erythema, scaling, and plaque elevation—for each target lesion. Total sign score was assessed proactively for all patients.15,16 The post hoc analysis reported here examined patients whose target lesion was located on either the knee or the elbow.
Statistical Analyses
Because both study protocols were identical, data were pooled from the 2 phase 3 trials. All statistical analyses were performed using SAS software (SAS Institute). Two-sided hypothesis testing was conducted for all analyses using a significance level of P=.05. Post hoc analyses used Fisher exact test. No imputations were made for missing data.
Statistical analyses of itching compared the incidence of itching at each assessment time point (baseline and days 4, 8, 15, and 29) between BD spray and vehicle and between BD spray and AugBD lotion. Additional analysis included a statistical test on the incidence of itching in the subgroup of patients who reported itching at baseline.
Statistical analyses for the knees and elbows included only patients with their target lesion located on either the knee or the elbow. Analyses compared BD spray with vehicle and BD spray with AugBD lotion at days 4, 8, 15, and 29. Comparison with AugBD lotion treatment was up to day 14 only, consistent with application time limits in the AugBD lotion product label.18
Results
These analyses included data from the 628 patients enrolled in the 2 phase 3 trials. Patients had similar baseline characteristics across treatment groups (Table 2). Itching was the most common cutaneous symptom at baseline, reported by almost two-thirds (n=392, 62.4%) of patients. Of the 628 patients, 236 (37.6%) had a target lesion located on the elbow or knee selected for assessment. The mean baseline body surface area was 13% to 14% across groups.
A post hoc analysis was performed on the subgroup of patients who reported itching at baseline (N=392)(eFigure 1). For these patients, almost half were itch free by day 4 across all groups (49.3% BD spray, 48.2% AugBD lotion, and 47.4% vehicle). By the end of treatment, 65.9% of patients using BD spray and 58.3% of patients using vehicle were itch free at day 29, with 56.9% of AugBD lotion patients itch free at day 15.
Application-site pruritus recorded as a treatment-emergent adverse event was seen in low numbers and was similar in proportion between the 2 steroid treatments (7.7% BD spray, 6.7% AugBD lotion, and 14.4% vehicle).
Psoriasis Individual Sign Scores for Knee and Elbow Plaques
Target lesions located on the knee or elbow represented 37.6% of all target lesions assessed. Efficacy analysis of the pooled data on knee and elbow lesions revealed that BD spray was similar to AugBD lotion in reducing sign scores to 0 or 1 (Figures 1 and 2).
The percentage of patients reporting improvements in erythema, scaling, and plaque elevation scores at day 4
The proportion of patients achieving treatment success (defined as a score of 0 or 1) was comparable for the2 products on day 15 for erythema (66.2% BD spray vs 62.5% AugBD lotion), scaling (70.7% BD spray vs 62.5% AugBD lotion), and plaque elevation (65.4% BD spray vs 62.5% AugBD lotion)(Figure 1). From day 8, BD spray reduced erythema and scaling in significantly more patients than vehicle (P=.003 for both), and BD spray reduced erythema, scaling, and plaque elevation in more patients than vehicle from day 15 (P<.001 for all). No statistically significant difference was found between BD spray and AugBD lotion on erythema, scaling, and plaque elevation scores.
Total Sign Score
Total sign score results showed that the mean percentage of patients achieving a TSS of 0 or 1 for all signs for lesions located on the knees or elbows was numerically higher for BD spray vs AugBD lotion at day 4, but this difference was not statistically significant (Figure 2). Day 15 outcomes for TSS also showed a numerically greater success rate for BD spray, but again this difference was not statistically significant (53.4% BD spray vs 43.8% AugBD lotion). At days 15 and 29, significantly more patients treated with BD spray achieved TSS of 0 or 1 for all 3 signs compared to those treated with vehicle (P<.001). Improvement in TSS with BD spray continued through to day 29 of the study.
Comment
In these post hoc analyses, mid-potency BD spray demonstrated early relief of itching and early efficacy in the treatment of psoriasis plaques on the elbows and knees with minimal systemic absorption and a low rate of adverse events.
Betamethasone dipropionate spray and its vehicle formulation relieved psoriatic itching with similar efficacy to the superpotent AugBD steroid lotion. Notably, relief was rapid, with approximately half of responding patients reporting relief of itching by day 4. The results seen with vehicle suggest that the emollient formulation of BD spray is responsible for hydrating dry skin, contributing to the relief of this cutaneous symptom. Dry skin can exacerbate itching, and emollients are recognized as being able to alleviate itching by hydrating and soothing the skin.7
The second set of post hoc analyses reported here demonstrated that BD spray was efficacious in clearing the signs of psoriatic lesions on the difficult-to-treat areas of the knees and elbows. Efficacy with BD spray was similar to the superpotent steroid AugBD lotion, with no statistical difference between the 2 products at any time point. Betamethasone dipropionate spray was significantly more effective than its vehicle in reducing the signs of erythema and scaling from day 8 and plaque elevation from day 15.
Rapid relief of symptoms is important for patient comfort and to improve treatment adherence. These analyses showed that by day 4, BD spray resulted in numerically higher percentages of patients achieving a score of 0 or 1 for the individual signs of erythema, scaling, and plaque elevation compared to AugBD lotion. Of particular note, 37.6% of patients treated with BD spray had scaling scores of clear or almost clear by day 4 compared to 25.0% of patients treated with AugBD lotion. Scaling has been consistently reported as the second most bothersome symptom experienced by patients2,3 and has been shown to be associated with decreased quality of life and work productivity.19
Betamethasone dipropionate spray has a rationally designed vehicle, with the formulation selected specifically to maximize penetration of the product through the stratum corneum and retention of BD steroid in the epidermis and upper dermis while reducing absorption into the systemic circulation.14 The reduced absorption into the systemic circulation leads to less vasoconstriction; fewer adverse events; and a “medium potent” VCA designation compared to the “superpotent” designation of the AugBD formulation, despite containing the same active ingredient.
These analyses demonstrate that BD spray is effective at addressing 2 symptoms that patients with psoriasis consider most bothersome: itching and scaling. Notably, BD spray was able to achieve these results rapidly, with many patients experiencing improvements in 4 days. In these analyses, mid-potent BD spray demonstrated similar efficacy to AugBD lotion, a superpotent steroid formulation.
This analysis is limited by being post hoc. Although the statistical methodology is valid, the AugBD lotion arm of the analyses was relatively small compared with the BD spray and vehicle arms, as it was only included in 1 of 2 studies pooled.
Conclusion
Mid-potency BD spray effectively improved the symptom of itching and cleared hard-to-treat lesions on knees and elbows with efficacy similar to a superpotent AugBD formulation but with less systemic absorption. Improvements were seen in erythema, scaling, and plaque elevation. Reductions in psoriatic signs were observed as early as day 4, with continued improvement seen throughout the study period. These findings provide evidence that BD spray can rapidly relieve 2 of the most troublesome symptoms affecting patients with psoriasis (itching and scaling), potentially improving quality of life.
Acknowledgments
The authors wish to thank Alix Bennett, PhD, formerly of Promius Pharma, a subsidiary of Dr. Reddy’s Laboratories, Inc (Princeton, New Jersey), and Jodie Macoun, PhD, of CUBE Information (Katonah, New York), for their review and assistance with the preparation of this manuscript. Manuscript preparation was supported by Promius Pharma (Princeton, New Jersey)(DRL #866).
- About psoriasis. National Psoriasis Foundation website. https://www.psoriasis.org/about-psoriasis. Accessed October 1, 2019.
- Lebwohl MG, Bachelez H, Barker J, et al. Patient perspectives in the management of psoriasis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis Survey. J Am Acad Dermatol. 2014;70:871-881.e1-30.
- Pariser D, Schenkel B, Carter C, et al; Psoriasis Patient Interview Study Group. A multicenter, non-interventional study to evaluate patient-reported experiences of living with psoriasis. J Dermatolog Treat. 2016;27:19-26.
- Dickison P, Swain G, Peek JJ, et al. Itching for answers: prevalence and severity of pruritus in psoriasis. Australas J Dermatol. 2018;59:206-209.
- Bahali AG, Onsun N, Su O, et al. The relationship between pruritus and clinical variables in patients with psoriasis. An Bras Dermatol. 2017;92:470-473.
- Prignano F, Ricceri F, Pescitelli L, et al. Itch in psoriasis: epidemiology, clinical aspects and treatment options. Clin Cosmet Investig Dermatol. 2009;2:9-13.
- Dawn A, Yosipovitch G. Treating itch in psoriasis. Dermatol Nurs. 2006;18:227-233.
- Queille-Roussel C, Rosen M, Clonier F, et al. Efficacy and safety of calcipotriol plus betamethasone dipropionate aerosol foam compared with betamethasone 17-valerate-medicated plaster for the treatment of psoriasis. Clin Drug Investig. 2017;37:355-361.
- Betesil [package insert]. Lodi, Italy: IBSA Pharmaceutici Italia S.r.I; 2013.
- Cannavò SP, Guarneri F, Giuffrida R, et al. Evaluation of cutaneous surface parameters in psoriatic patients. Skin Res Technol. 2017;23:41-47.
- Egawa M, Arimoto H, Hirao T, et al. Regional difference of water content in human skin studied by diffuse-reflectance near-infrared spectroscopy: consideration of measurement depth. Appl Spectrosc. 2006;60:24-28.
- van de Kerkhof PC, Reich K, Kavanaugh A, et al. Physician perspectives in the management of psoriasis and psoriatic arthritis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis survey. J Eur Acad Dermatol Venereol. 2015;29:2002-2010.
- Menter A, Korman NJ, Elmets CA, et al; American Academy of Dermatology. Guidelines of care for the management of psoriasis and psoriatic arthritis. section 3. guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
- Kircik L, Okumu F, Kandavilli S, et al. Rational vehicle design ensures targeted cutaneous steroid delivery. J Clin Aesthet Dermatol. 2017;10:12-19.
- Fowler JF Jr, Herbert AA, Sugarman J. DFD-01, a novel medium potency betamethasone dipropionate 0.05% emollient spray, demonstrates similar efficacy to augmented betamethasone dipropionate 0.05% lotion for the treatment of moderate plaque psoriasis. J Drugs Dermatol. 2016;15:154-162.
- Stein Gold L, Jackson JM, Knuckles ML, et al. Improvement in extensive moderate plaque psoriasis with a novel emollient spray formulation of betamethasone dipropionate 0.05. J Drugs Dermatol. 2016;15:334-342.
- Sidgiddi S, Pakunlu RI, Allenby K. Efficacy, safety, and potency of betamethasone dipropionate spray 0.05%: a treatment for adults with mild-to-moderate plaque psoriasis. J Clin Aesthet Dermatol. 2018;11:14-22.
- Diprolene Lotion (augmented betamethasone dipropionate 0.05%) [package insert]. Kenilworth, NJ: Schering Corporation; 1999.
- Korman NJ, Zhao Y, Pike J, et al. Increased severity of itching, pain, and scaling in psoriasis patients is associated with increased disease severity, reduced quality of life, and reduced work productivity. Dermatol Online J. 2015;21. pii:13030/qt1x16v3dg.
Psoriasis affects approximately 2% to 3% of the US population and is characterized by plaques that are red, scaly, and elevated.1 Cutaneous symptoms of the disease are described by patients as itching, burning, and stinging sensations. Large multinational and US surveys have reported pruritus as patients’ most bothersome symptom, with scaling/flaking reported as the second most bothersome.2,3 Reported incidence rates for itching range from 60.4% to 98.3%, with at least half of these patients reporting daily or constant pruritus.2,4-7 Consequent effects on quality of life include impaired sleep,6 difficulty concentrating, lower sex drive, and depression.7 Despite these findings, pruritus is rarely included in the efficacy assessments of psoriasis treatments. In addition, 2 of the most commonly reported but difficult-to-treat locations for plaques are the outside of the elbows (45%) and the knees (32%),1,2,8 areas where the stratum corneum typically is thicker, less hydrated, and less likely to absorb topical products.9-11 Clinical studies have not focused specifically on these areas when assessing treatments.
Topical corticosteroids have been the mainstay of psoriasis therapy for decades because of their anti-inflammatory and antiproliferative properties.7 One large multinational physician survey indicated that 75% of patients are prescribed topical steroids,12 which are important for first-line treatment and are often maintained as adjunctive therapy in combination with other treatments for patients with extensive disease or recalcitrant lesions.13 Topical corticosteroids are ranked into different classes based on their vasoconstrictor assay (VCA), a measure of skin blanching used as a marker for vasoconstriction. Topical agents with VCA ratings of mid-potency or superpotency are generally recommended for initial therapy, with superpotent agents required for the treatment of thick chronic plaques. However, longer durations of use may contribute to systemic absorption and adverse events.13 The vehicle composition is important for corticosteroid delivery and retention at the site of pathology, contributing to the efficacy of the steroid.13,14 Selecting the appropriate steroid and vehicle is important to maximize efficacy and minimize adverse events.
Betamethasone dipropionate (BD) spray 0.05% is an emollient formulation of 0.05% BD that can be sprayed onto psoriatic plaques. The BD spray formulation was designed to penetrate the stratum corneum and be retained within the dermis and epidermis, the site of T-cell activity that drives the psoriatic disease process.14 In 2 phase 3 studies, BD spray demonstrated the ability to reduce the signs of plaque psoriasis with indication of improvement by day 4.15,16 These studies also showed improvement in the local cutaneous symptoms of itching, burning and stinging, and pain. As a mid-potent steroid, BD spray displays less systemic absorption but similar efficacy compared to a superpotent augmented BD (AugBD) lotion in relieving the signs and symptoms of plaque psoriasis.15-17
The objective of the current investigation was to assess the ability of BD spray to relieve itching and to clear plaque psoriasis on the knees and elbows utilizing post hoc analyses of the 2 phase 3 trials. The goal of these analyses was to demonstrate BD spray as effective at relieving the most troublesome signs and symptoms affecting patients with plaque psoriasis.
Methods
Two phase 3 studies were conducted to demonstrate the efficacy and safety of BD spray.15,16 The design of the studies was similar15,16 to allow the data to be pooled for post hoc analyses.
Both were US multicenter, randomized, vehicle-controlled, double-blind, parallel-group studies comparing the safety and efficacy of BD spray 0.05% (Sernivo, Promius Pharma) with its vehicle formulation spray (identical to BD spray, but lacking the active steroid component).15,16 One of the studies also compared BD spray with an AugBD lotion 0.05% (Diprolene,Merck & Co). Adults with moderate plaque psoriasis (investigator global assessment of 3; 10%–20% body surface area) were randomized to apply BD spray, vehicle spray, or AugBD lotion (1 study only) twice daily to all affected areas, excluding the face, scalp, and intertriginous areas for 28 days (BD spray and vehicle) or 14 days (AugBD lotion, per product label).15
Assessments
Two post hoc analyses were conducted on data pooled from the 2 phase 3 trials: (1) incidence of itching, and (2) total sign score (TSS) for lesions located on the knees and elbows.
Itching
Itching was assessed proactively by asking patients if they were experiencing itching (yes/no) at each visit (baseline and days 4, 8, 15, and 29) or had experienced itching since their last visit. As itching could be an adverse event of topical application, application-site pruritus was also recorded.
Total Sign Score
For each patient, a target plaque was selected that was representative of their psoriasis. The plaque was assessed on a 3-point grading scale for each of 3 key signs of plaque psoriasis: erythema, scaling, and plaque elevation (Table 1) at baseline and days 4, 8, 15, and 29. Total sign score was calculated by summing the scores for these 3 signs, resulting in a score ranging from 0 to 9. Treatment success was measured as (1) achieving a score of 0 or 1 (ie, reducing the plaque to clear or slight to mild) for the individual signs of erythema, scaling, and plaque elevation; and (2) achieving a TSS of 0 or 1 for all 3 signs—erythema, scaling, and plaque elevation—for each target lesion. Total sign score was assessed proactively for all patients.15,16 The post hoc analysis reported here examined patients whose target lesion was located on either the knee or the elbow.
Statistical Analyses
Because both study protocols were identical, data were pooled from the 2 phase 3 trials. All statistical analyses were performed using SAS software (SAS Institute). Two-sided hypothesis testing was conducted for all analyses using a significance level of P=.05. Post hoc analyses used Fisher exact test. No imputations were made for missing data.
Statistical analyses of itching compared the incidence of itching at each assessment time point (baseline and days 4, 8, 15, and 29) between BD spray and vehicle and between BD spray and AugBD lotion. Additional analysis included a statistical test on the incidence of itching in the subgroup of patients who reported itching at baseline.
Statistical analyses for the knees and elbows included only patients with their target lesion located on either the knee or the elbow. Analyses compared BD spray with vehicle and BD spray with AugBD lotion at days 4, 8, 15, and 29. Comparison with AugBD lotion treatment was up to day 14 only, consistent with application time limits in the AugBD lotion product label.18
Results
These analyses included data from the 628 patients enrolled in the 2 phase 3 trials. Patients had similar baseline characteristics across treatment groups (Table 2). Itching was the most common cutaneous symptom at baseline, reported by almost two-thirds (n=392, 62.4%) of patients. Of the 628 patients, 236 (37.6%) had a target lesion located on the elbow or knee selected for assessment. The mean baseline body surface area was 13% to 14% across groups.
A post hoc analysis was performed on the subgroup of patients who reported itching at baseline (N=392)(eFigure 1). For these patients, almost half were itch free by day 4 across all groups (49.3% BD spray, 48.2% AugBD lotion, and 47.4% vehicle). By the end of treatment, 65.9% of patients using BD spray and 58.3% of patients using vehicle were itch free at day 29, with 56.9% of AugBD lotion patients itch free at day 15.
Application-site pruritus recorded as a treatment-emergent adverse event was seen in low numbers and was similar in proportion between the 2 steroid treatments (7.7% BD spray, 6.7% AugBD lotion, and 14.4% vehicle).
Psoriasis Individual Sign Scores for Knee and Elbow Plaques
Target lesions located on the knee or elbow represented 37.6% of all target lesions assessed. Efficacy analysis of the pooled data on knee and elbow lesions revealed that BD spray was similar to AugBD lotion in reducing sign scores to 0 or 1 (Figures 1 and 2).
The percentage of patients reporting improvements in erythema, scaling, and plaque elevation scores at day 4
The proportion of patients achieving treatment success (defined as a score of 0 or 1) was comparable for the2 products on day 15 for erythema (66.2% BD spray vs 62.5% AugBD lotion), scaling (70.7% BD spray vs 62.5% AugBD lotion), and plaque elevation (65.4% BD spray vs 62.5% AugBD lotion)(Figure 1). From day 8, BD spray reduced erythema and scaling in significantly more patients than vehicle (P=.003 for both), and BD spray reduced erythema, scaling, and plaque elevation in more patients than vehicle from day 15 (P<.001 for all). No statistically significant difference was found between BD spray and AugBD lotion on erythema, scaling, and plaque elevation scores.
Total Sign Score
Total sign score results showed that the mean percentage of patients achieving a TSS of 0 or 1 for all signs for lesions located on the knees or elbows was numerically higher for BD spray vs AugBD lotion at day 4, but this difference was not statistically significant (Figure 2). Day 15 outcomes for TSS also showed a numerically greater success rate for BD spray, but again this difference was not statistically significant (53.4% BD spray vs 43.8% AugBD lotion). At days 15 and 29, significantly more patients treated with BD spray achieved TSS of 0 or 1 for all 3 signs compared to those treated with vehicle (P<.001). Improvement in TSS with BD spray continued through to day 29 of the study.
Comment
In these post hoc analyses, mid-potency BD spray demonstrated early relief of itching and early efficacy in the treatment of psoriasis plaques on the elbows and knees with minimal systemic absorption and a low rate of adverse events.
Betamethasone dipropionate spray and its vehicle formulation relieved psoriatic itching with similar efficacy to the superpotent AugBD steroid lotion. Notably, relief was rapid, with approximately half of responding patients reporting relief of itching by day 4. The results seen with vehicle suggest that the emollient formulation of BD spray is responsible for hydrating dry skin, contributing to the relief of this cutaneous symptom. Dry skin can exacerbate itching, and emollients are recognized as being able to alleviate itching by hydrating and soothing the skin.7
The second set of post hoc analyses reported here demonstrated that BD spray was efficacious in clearing the signs of psoriatic lesions on the difficult-to-treat areas of the knees and elbows. Efficacy with BD spray was similar to the superpotent steroid AugBD lotion, with no statistical difference between the 2 products at any time point. Betamethasone dipropionate spray was significantly more effective than its vehicle in reducing the signs of erythema and scaling from day 8 and plaque elevation from day 15.
Rapid relief of symptoms is important for patient comfort and to improve treatment adherence. These analyses showed that by day 4, BD spray resulted in numerically higher percentages of patients achieving a score of 0 or 1 for the individual signs of erythema, scaling, and plaque elevation compared to AugBD lotion. Of particular note, 37.6% of patients treated with BD spray had scaling scores of clear or almost clear by day 4 compared to 25.0% of patients treated with AugBD lotion. Scaling has been consistently reported as the second most bothersome symptom experienced by patients2,3 and has been shown to be associated with decreased quality of life and work productivity.19
Betamethasone dipropionate spray has a rationally designed vehicle, with the formulation selected specifically to maximize penetration of the product through the stratum corneum and retention of BD steroid in the epidermis and upper dermis while reducing absorption into the systemic circulation.14 The reduced absorption into the systemic circulation leads to less vasoconstriction; fewer adverse events; and a “medium potent” VCA designation compared to the “superpotent” designation of the AugBD formulation, despite containing the same active ingredient.
These analyses demonstrate that BD spray is effective at addressing 2 symptoms that patients with psoriasis consider most bothersome: itching and scaling. Notably, BD spray was able to achieve these results rapidly, with many patients experiencing improvements in 4 days. In these analyses, mid-potent BD spray demonstrated similar efficacy to AugBD lotion, a superpotent steroid formulation.
This analysis is limited by being post hoc. Although the statistical methodology is valid, the AugBD lotion arm of the analyses was relatively small compared with the BD spray and vehicle arms, as it was only included in 1 of 2 studies pooled.
Conclusion
Mid-potency BD spray effectively improved the symptom of itching and cleared hard-to-treat lesions on knees and elbows with efficacy similar to a superpotent AugBD formulation but with less systemic absorption. Improvements were seen in erythema, scaling, and plaque elevation. Reductions in psoriatic signs were observed as early as day 4, with continued improvement seen throughout the study period. These findings provide evidence that BD spray can rapidly relieve 2 of the most troublesome symptoms affecting patients with psoriasis (itching and scaling), potentially improving quality of life.
Acknowledgments
The authors wish to thank Alix Bennett, PhD, formerly of Promius Pharma, a subsidiary of Dr. Reddy’s Laboratories, Inc (Princeton, New Jersey), and Jodie Macoun, PhD, of CUBE Information (Katonah, New York), for their review and assistance with the preparation of this manuscript. Manuscript preparation was supported by Promius Pharma (Princeton, New Jersey)(DRL #866).
Psoriasis affects approximately 2% to 3% of the US population and is characterized by plaques that are red, scaly, and elevated.1 Cutaneous symptoms of the disease are described by patients as itching, burning, and stinging sensations. Large multinational and US surveys have reported pruritus as patients’ most bothersome symptom, with scaling/flaking reported as the second most bothersome.2,3 Reported incidence rates for itching range from 60.4% to 98.3%, with at least half of these patients reporting daily or constant pruritus.2,4-7 Consequent effects on quality of life include impaired sleep,6 difficulty concentrating, lower sex drive, and depression.7 Despite these findings, pruritus is rarely included in the efficacy assessments of psoriasis treatments. In addition, 2 of the most commonly reported but difficult-to-treat locations for plaques are the outside of the elbows (45%) and the knees (32%),1,2,8 areas where the stratum corneum typically is thicker, less hydrated, and less likely to absorb topical products.9-11 Clinical studies have not focused specifically on these areas when assessing treatments.
Topical corticosteroids have been the mainstay of psoriasis therapy for decades because of their anti-inflammatory and antiproliferative properties.7 One large multinational physician survey indicated that 75% of patients are prescribed topical steroids,12 which are important for first-line treatment and are often maintained as adjunctive therapy in combination with other treatments for patients with extensive disease or recalcitrant lesions.13 Topical corticosteroids are ranked into different classes based on their vasoconstrictor assay (VCA), a measure of skin blanching used as a marker for vasoconstriction. Topical agents with VCA ratings of mid-potency or superpotency are generally recommended for initial therapy, with superpotent agents required for the treatment of thick chronic plaques. However, longer durations of use may contribute to systemic absorption and adverse events.13 The vehicle composition is important for corticosteroid delivery and retention at the site of pathology, contributing to the efficacy of the steroid.13,14 Selecting the appropriate steroid and vehicle is important to maximize efficacy and minimize adverse events.
Betamethasone dipropionate (BD) spray 0.05% is an emollient formulation of 0.05% BD that can be sprayed onto psoriatic plaques. The BD spray formulation was designed to penetrate the stratum corneum and be retained within the dermis and epidermis, the site of T-cell activity that drives the psoriatic disease process.14 In 2 phase 3 studies, BD spray demonstrated the ability to reduce the signs of plaque psoriasis with indication of improvement by day 4.15,16 These studies also showed improvement in the local cutaneous symptoms of itching, burning and stinging, and pain. As a mid-potent steroid, BD spray displays less systemic absorption but similar efficacy compared to a superpotent augmented BD (AugBD) lotion in relieving the signs and symptoms of plaque psoriasis.15-17
The objective of the current investigation was to assess the ability of BD spray to relieve itching and to clear plaque psoriasis on the knees and elbows utilizing post hoc analyses of the 2 phase 3 trials. The goal of these analyses was to demonstrate BD spray as effective at relieving the most troublesome signs and symptoms affecting patients with plaque psoriasis.
Methods
Two phase 3 studies were conducted to demonstrate the efficacy and safety of BD spray.15,16 The design of the studies was similar15,16 to allow the data to be pooled for post hoc analyses.
Both were US multicenter, randomized, vehicle-controlled, double-blind, parallel-group studies comparing the safety and efficacy of BD spray 0.05% (Sernivo, Promius Pharma) with its vehicle formulation spray (identical to BD spray, but lacking the active steroid component).15,16 One of the studies also compared BD spray with an AugBD lotion 0.05% (Diprolene,Merck & Co). Adults with moderate plaque psoriasis (investigator global assessment of 3; 10%–20% body surface area) were randomized to apply BD spray, vehicle spray, or AugBD lotion (1 study only) twice daily to all affected areas, excluding the face, scalp, and intertriginous areas for 28 days (BD spray and vehicle) or 14 days (AugBD lotion, per product label).15
Assessments
Two post hoc analyses were conducted on data pooled from the 2 phase 3 trials: (1) incidence of itching, and (2) total sign score (TSS) for lesions located on the knees and elbows.
Itching
Itching was assessed proactively by asking patients if they were experiencing itching (yes/no) at each visit (baseline and days 4, 8, 15, and 29) or had experienced itching since their last visit. As itching could be an adverse event of topical application, application-site pruritus was also recorded.
Total Sign Score
For each patient, a target plaque was selected that was representative of their psoriasis. The plaque was assessed on a 3-point grading scale for each of 3 key signs of plaque psoriasis: erythema, scaling, and plaque elevation (Table 1) at baseline and days 4, 8, 15, and 29. Total sign score was calculated by summing the scores for these 3 signs, resulting in a score ranging from 0 to 9. Treatment success was measured as (1) achieving a score of 0 or 1 (ie, reducing the plaque to clear or slight to mild) for the individual signs of erythema, scaling, and plaque elevation; and (2) achieving a TSS of 0 or 1 for all 3 signs—erythema, scaling, and plaque elevation—for each target lesion. Total sign score was assessed proactively for all patients.15,16 The post hoc analysis reported here examined patients whose target lesion was located on either the knee or the elbow.
Statistical Analyses
Because both study protocols were identical, data were pooled from the 2 phase 3 trials. All statistical analyses were performed using SAS software (SAS Institute). Two-sided hypothesis testing was conducted for all analyses using a significance level of P=.05. Post hoc analyses used Fisher exact test. No imputations were made for missing data.
Statistical analyses of itching compared the incidence of itching at each assessment time point (baseline and days 4, 8, 15, and 29) between BD spray and vehicle and between BD spray and AugBD lotion. Additional analysis included a statistical test on the incidence of itching in the subgroup of patients who reported itching at baseline.
Statistical analyses for the knees and elbows included only patients with their target lesion located on either the knee or the elbow. Analyses compared BD spray with vehicle and BD spray with AugBD lotion at days 4, 8, 15, and 29. Comparison with AugBD lotion treatment was up to day 14 only, consistent with application time limits in the AugBD lotion product label.18
Results
These analyses included data from the 628 patients enrolled in the 2 phase 3 trials. Patients had similar baseline characteristics across treatment groups (Table 2). Itching was the most common cutaneous symptom at baseline, reported by almost two-thirds (n=392, 62.4%) of patients. Of the 628 patients, 236 (37.6%) had a target lesion located on the elbow or knee selected for assessment. The mean baseline body surface area was 13% to 14% across groups.
A post hoc analysis was performed on the subgroup of patients who reported itching at baseline (N=392)(eFigure 1). For these patients, almost half were itch free by day 4 across all groups (49.3% BD spray, 48.2% AugBD lotion, and 47.4% vehicle). By the end of treatment, 65.9% of patients using BD spray and 58.3% of patients using vehicle were itch free at day 29, with 56.9% of AugBD lotion patients itch free at day 15.
Application-site pruritus recorded as a treatment-emergent adverse event was seen in low numbers and was similar in proportion between the 2 steroid treatments (7.7% BD spray, 6.7% AugBD lotion, and 14.4% vehicle).
Psoriasis Individual Sign Scores for Knee and Elbow Plaques
Target lesions located on the knee or elbow represented 37.6% of all target lesions assessed. Efficacy analysis of the pooled data on knee and elbow lesions revealed that BD spray was similar to AugBD lotion in reducing sign scores to 0 or 1 (Figures 1 and 2).
The percentage of patients reporting improvements in erythema, scaling, and plaque elevation scores at day 4
The proportion of patients achieving treatment success (defined as a score of 0 or 1) was comparable for the2 products on day 15 for erythema (66.2% BD spray vs 62.5% AugBD lotion), scaling (70.7% BD spray vs 62.5% AugBD lotion), and plaque elevation (65.4% BD spray vs 62.5% AugBD lotion)(Figure 1). From day 8, BD spray reduced erythema and scaling in significantly more patients than vehicle (P=.003 for both), and BD spray reduced erythema, scaling, and plaque elevation in more patients than vehicle from day 15 (P<.001 for all). No statistically significant difference was found between BD spray and AugBD lotion on erythema, scaling, and plaque elevation scores.
Total Sign Score
Total sign score results showed that the mean percentage of patients achieving a TSS of 0 or 1 for all signs for lesions located on the knees or elbows was numerically higher for BD spray vs AugBD lotion at day 4, but this difference was not statistically significant (Figure 2). Day 15 outcomes for TSS also showed a numerically greater success rate for BD spray, but again this difference was not statistically significant (53.4% BD spray vs 43.8% AugBD lotion). At days 15 and 29, significantly more patients treated with BD spray achieved TSS of 0 or 1 for all 3 signs compared to those treated with vehicle (P<.001). Improvement in TSS with BD spray continued through to day 29 of the study.
Comment
In these post hoc analyses, mid-potency BD spray demonstrated early relief of itching and early efficacy in the treatment of psoriasis plaques on the elbows and knees with minimal systemic absorption and a low rate of adverse events.
Betamethasone dipropionate spray and its vehicle formulation relieved psoriatic itching with similar efficacy to the superpotent AugBD steroid lotion. Notably, relief was rapid, with approximately half of responding patients reporting relief of itching by day 4. The results seen with vehicle suggest that the emollient formulation of BD spray is responsible for hydrating dry skin, contributing to the relief of this cutaneous symptom. Dry skin can exacerbate itching, and emollients are recognized as being able to alleviate itching by hydrating and soothing the skin.7
The second set of post hoc analyses reported here demonstrated that BD spray was efficacious in clearing the signs of psoriatic lesions on the difficult-to-treat areas of the knees and elbows. Efficacy with BD spray was similar to the superpotent steroid AugBD lotion, with no statistical difference between the 2 products at any time point. Betamethasone dipropionate spray was significantly more effective than its vehicle in reducing the signs of erythema and scaling from day 8 and plaque elevation from day 15.
Rapid relief of symptoms is important for patient comfort and to improve treatment adherence. These analyses showed that by day 4, BD spray resulted in numerically higher percentages of patients achieving a score of 0 or 1 for the individual signs of erythema, scaling, and plaque elevation compared to AugBD lotion. Of particular note, 37.6% of patients treated with BD spray had scaling scores of clear or almost clear by day 4 compared to 25.0% of patients treated with AugBD lotion. Scaling has been consistently reported as the second most bothersome symptom experienced by patients2,3 and has been shown to be associated with decreased quality of life and work productivity.19
Betamethasone dipropionate spray has a rationally designed vehicle, with the formulation selected specifically to maximize penetration of the product through the stratum corneum and retention of BD steroid in the epidermis and upper dermis while reducing absorption into the systemic circulation.14 The reduced absorption into the systemic circulation leads to less vasoconstriction; fewer adverse events; and a “medium potent” VCA designation compared to the “superpotent” designation of the AugBD formulation, despite containing the same active ingredient.
These analyses demonstrate that BD spray is effective at addressing 2 symptoms that patients with psoriasis consider most bothersome: itching and scaling. Notably, BD spray was able to achieve these results rapidly, with many patients experiencing improvements in 4 days. In these analyses, mid-potent BD spray demonstrated similar efficacy to AugBD lotion, a superpotent steroid formulation.
This analysis is limited by being post hoc. Although the statistical methodology is valid, the AugBD lotion arm of the analyses was relatively small compared with the BD spray and vehicle arms, as it was only included in 1 of 2 studies pooled.
Conclusion
Mid-potency BD spray effectively improved the symptom of itching and cleared hard-to-treat lesions on knees and elbows with efficacy similar to a superpotent AugBD formulation but with less systemic absorption. Improvements were seen in erythema, scaling, and plaque elevation. Reductions in psoriatic signs were observed as early as day 4, with continued improvement seen throughout the study period. These findings provide evidence that BD spray can rapidly relieve 2 of the most troublesome symptoms affecting patients with psoriasis (itching and scaling), potentially improving quality of life.
Acknowledgments
The authors wish to thank Alix Bennett, PhD, formerly of Promius Pharma, a subsidiary of Dr. Reddy’s Laboratories, Inc (Princeton, New Jersey), and Jodie Macoun, PhD, of CUBE Information (Katonah, New York), for their review and assistance with the preparation of this manuscript. Manuscript preparation was supported by Promius Pharma (Princeton, New Jersey)(DRL #866).
- About psoriasis. National Psoriasis Foundation website. https://www.psoriasis.org/about-psoriasis. Accessed October 1, 2019.
- Lebwohl MG, Bachelez H, Barker J, et al. Patient perspectives in the management of psoriasis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis Survey. J Am Acad Dermatol. 2014;70:871-881.e1-30.
- Pariser D, Schenkel B, Carter C, et al; Psoriasis Patient Interview Study Group. A multicenter, non-interventional study to evaluate patient-reported experiences of living with psoriasis. J Dermatolog Treat. 2016;27:19-26.
- Dickison P, Swain G, Peek JJ, et al. Itching for answers: prevalence and severity of pruritus in psoriasis. Australas J Dermatol. 2018;59:206-209.
- Bahali AG, Onsun N, Su O, et al. The relationship between pruritus and clinical variables in patients with psoriasis. An Bras Dermatol. 2017;92:470-473.
- Prignano F, Ricceri F, Pescitelli L, et al. Itch in psoriasis: epidemiology, clinical aspects and treatment options. Clin Cosmet Investig Dermatol. 2009;2:9-13.
- Dawn A, Yosipovitch G. Treating itch in psoriasis. Dermatol Nurs. 2006;18:227-233.
- Queille-Roussel C, Rosen M, Clonier F, et al. Efficacy and safety of calcipotriol plus betamethasone dipropionate aerosol foam compared with betamethasone 17-valerate-medicated plaster for the treatment of psoriasis. Clin Drug Investig. 2017;37:355-361.
- Betesil [package insert]. Lodi, Italy: IBSA Pharmaceutici Italia S.r.I; 2013.
- Cannavò SP, Guarneri F, Giuffrida R, et al. Evaluation of cutaneous surface parameters in psoriatic patients. Skin Res Technol. 2017;23:41-47.
- Egawa M, Arimoto H, Hirao T, et al. Regional difference of water content in human skin studied by diffuse-reflectance near-infrared spectroscopy: consideration of measurement depth. Appl Spectrosc. 2006;60:24-28.
- van de Kerkhof PC, Reich K, Kavanaugh A, et al. Physician perspectives in the management of psoriasis and psoriatic arthritis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis survey. J Eur Acad Dermatol Venereol. 2015;29:2002-2010.
- Menter A, Korman NJ, Elmets CA, et al; American Academy of Dermatology. Guidelines of care for the management of psoriasis and psoriatic arthritis. section 3. guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
- Kircik L, Okumu F, Kandavilli S, et al. Rational vehicle design ensures targeted cutaneous steroid delivery. J Clin Aesthet Dermatol. 2017;10:12-19.
- Fowler JF Jr, Herbert AA, Sugarman J. DFD-01, a novel medium potency betamethasone dipropionate 0.05% emollient spray, demonstrates similar efficacy to augmented betamethasone dipropionate 0.05% lotion for the treatment of moderate plaque psoriasis. J Drugs Dermatol. 2016;15:154-162.
- Stein Gold L, Jackson JM, Knuckles ML, et al. Improvement in extensive moderate plaque psoriasis with a novel emollient spray formulation of betamethasone dipropionate 0.05. J Drugs Dermatol. 2016;15:334-342.
- Sidgiddi S, Pakunlu RI, Allenby K. Efficacy, safety, and potency of betamethasone dipropionate spray 0.05%: a treatment for adults with mild-to-moderate plaque psoriasis. J Clin Aesthet Dermatol. 2018;11:14-22.
- Diprolene Lotion (augmented betamethasone dipropionate 0.05%) [package insert]. Kenilworth, NJ: Schering Corporation; 1999.
- Korman NJ, Zhao Y, Pike J, et al. Increased severity of itching, pain, and scaling in psoriasis patients is associated with increased disease severity, reduced quality of life, and reduced work productivity. Dermatol Online J. 2015;21. pii:13030/qt1x16v3dg.
- About psoriasis. National Psoriasis Foundation website. https://www.psoriasis.org/about-psoriasis. Accessed October 1, 2019.
- Lebwohl MG, Bachelez H, Barker J, et al. Patient perspectives in the management of psoriasis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis Survey. J Am Acad Dermatol. 2014;70:871-881.e1-30.
- Pariser D, Schenkel B, Carter C, et al; Psoriasis Patient Interview Study Group. A multicenter, non-interventional study to evaluate patient-reported experiences of living with psoriasis. J Dermatolog Treat. 2016;27:19-26.
- Dickison P, Swain G, Peek JJ, et al. Itching for answers: prevalence and severity of pruritus in psoriasis. Australas J Dermatol. 2018;59:206-209.
- Bahali AG, Onsun N, Su O, et al. The relationship between pruritus and clinical variables in patients with psoriasis. An Bras Dermatol. 2017;92:470-473.
- Prignano F, Ricceri F, Pescitelli L, et al. Itch in psoriasis: epidemiology, clinical aspects and treatment options. Clin Cosmet Investig Dermatol. 2009;2:9-13.
- Dawn A, Yosipovitch G. Treating itch in psoriasis. Dermatol Nurs. 2006;18:227-233.
- Queille-Roussel C, Rosen M, Clonier F, et al. Efficacy and safety of calcipotriol plus betamethasone dipropionate aerosol foam compared with betamethasone 17-valerate-medicated plaster for the treatment of psoriasis. Clin Drug Investig. 2017;37:355-361.
- Betesil [package insert]. Lodi, Italy: IBSA Pharmaceutici Italia S.r.I; 2013.
- Cannavò SP, Guarneri F, Giuffrida R, et al. Evaluation of cutaneous surface parameters in psoriatic patients. Skin Res Technol. 2017;23:41-47.
- Egawa M, Arimoto H, Hirao T, et al. Regional difference of water content in human skin studied by diffuse-reflectance near-infrared spectroscopy: consideration of measurement depth. Appl Spectrosc. 2006;60:24-28.
- van de Kerkhof PC, Reich K, Kavanaugh A, et al. Physician perspectives in the management of psoriasis and psoriatic arthritis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis survey. J Eur Acad Dermatol Venereol. 2015;29:2002-2010.
- Menter A, Korman NJ, Elmets CA, et al; American Academy of Dermatology. Guidelines of care for the management of psoriasis and psoriatic arthritis. section 3. guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
- Kircik L, Okumu F, Kandavilli S, et al. Rational vehicle design ensures targeted cutaneous steroid delivery. J Clin Aesthet Dermatol. 2017;10:12-19.
- Fowler JF Jr, Herbert AA, Sugarman J. DFD-01, a novel medium potency betamethasone dipropionate 0.05% emollient spray, demonstrates similar efficacy to augmented betamethasone dipropionate 0.05% lotion for the treatment of moderate plaque psoriasis. J Drugs Dermatol. 2016;15:154-162.
- Stein Gold L, Jackson JM, Knuckles ML, et al. Improvement in extensive moderate plaque psoriasis with a novel emollient spray formulation of betamethasone dipropionate 0.05. J Drugs Dermatol. 2016;15:334-342.
- Sidgiddi S, Pakunlu RI, Allenby K. Efficacy, safety, and potency of betamethasone dipropionate spray 0.05%: a treatment for adults with mild-to-moderate plaque psoriasis. J Clin Aesthet Dermatol. 2018;11:14-22.
- Diprolene Lotion (augmented betamethasone dipropionate 0.05%) [package insert]. Kenilworth, NJ: Schering Corporation; 1999.
- Korman NJ, Zhao Y, Pike J, et al. Increased severity of itching, pain, and scaling in psoriasis patients is associated with increased disease severity, reduced quality of life, and reduced work productivity. Dermatol Online J. 2015;21. pii:13030/qt1x16v3dg.
Practice Points
- Pruritus is one of the most bothersome symptoms of psoriasis; plaques located on the knees and elbows remain hard to treat.
- Topical corticosteroids are the initial form of treatment of localized plaque psoriasis.
- The choice of vehicle can change the penetration of the medication, alter the efficacy, and minimize side effects of the drug.
- Betamethasone dipropionate spray 0.05% is a mid-potent corticosteroid that provides fast symptom relief and early efficacy in clearing plaques, similar to a high-potency topical corticosteroid but with less potential for systemic absorption and adverse events.
Dermatology Residency Applications: Correlation of Applicant Personal Statement Content With Match Result
The personal statement is a narrative written by an applicant to residency programs to discuss his/her interests. It is one of the few places in the residency application process where applicants can express their personalities.1 Applicants believe the personal statement is an important opportunity to distinguish themselves from others, thus increasing their chances of successful matching, particularly in competitive specialties.1,2
Dermatology is a highly competitive specialty, with 614 medical students applying for 440 total dermatology positions in 2016.3 According to the results of the 2016 National Resident Matching program director survey, 82% (27/33) of dermatology program directors reported that the personal statement was a factor in selecting applicants to interview. Furthermore, dermatology program directors, on average, rated personal statements as more important than the Medical Student Performance Evaluation/Dean’s Letter, US Medical Licensing Examination (USMLE) Step 2 scores, and class ranking/quartile.4
Prior studies have sought to evaluate the impact of personal statements on the application process. A 2014 study of personal statements submitted by dermatology residency applicants found that the prevalence of certain themes differed according to match outcome.5 However, some of the conclusions drawn in this study were not supported by the reported results or were based on low numbers of participants. The purpose of our study was to examine personal statements from applications to a dermatology program at a major academic institution. This study identified common themes in personal statements, allowing for an analysis of their association with successful matching into dermatology.
Methods
All applications to the dermatology residency program at UNC School of Medicine (Chapel Hill, North Carolina) during the 2012 application cycle (N=422) were eligible. All submitted personal statements (N=422) were included with all personal identifiers removed prior to analysis. The investigator (D.S.M.) was blinded to other Electronic Residency Application Service data and match outcome.
The investigator initially reviewed a small, randomly selected subset of 20 personal statements to identify characteristics and common themes. The investigator then analyzed each of the personal statements to quantify the frequency of each theme. All personal statements submitted to the dermatology residency program at UNC School of Medicine were analyzed in this manner. Dermatology match outcomes for each applicant were confirmed later using dermatology program websites.
Differences in the prevalence of common themes between matched and unmatched applicants were calculated. Analysis of variance tests were used to determine if the differences in prevalence were statistically significant (P≤.05).
Results
All 422 submitted personal statements were evaluated, with 308 personal statements from applicants who matched and 114 personal statements from unmatched applicants. The screening of the initial subset of 20 personal statements resulted in a total of 9 content themes. The prevalence of each theme among matched and unmatched applicants is shown in the Table.
The most common themes among both matched and unmatched groups were personal accomplishments or attributes and positive qualities of dermatology. The prevalence of certain themes varied between matched and unmatched groups. Dermatologic cases were discussed significantly more frequently in the matched group compared to the unmatched group (60.06% vs 46.49%, P=.013). Name-dropping was more prevalent in the unmatched group (37.72%) compared to the matched group (26.95%). This difference in prevalence reached statistical significance (P=.014). Religious influences also were discussed more frequently in the unmatched group (5.26%) vs the matched group (0.65%) with statistical significance (P=.002).
Comment
This study of 422 personal statements submitted to a major academic institution showed that certain themes were common in personal statements among both matched and unmatched applicants. These themes included personal accomplishments/attributes and positive qualities of dermatology. This finding is consistent with prior studies that show common themes in the personal statements of applicants across a wide variety of specialties, including dermatology, anesthesiology, pediatrics, general surgery, internal medicine, and radiology.5-10 Most commonly, applicants feel the need to justify why they chose their particular specialty, with Olazagasti et al5 (N=332) reporting that 70% of submitted dermatology personal statements explained why the applicant chose dermatology.
Certain themes, however, varied in prevalence between matched and unmatched groups in our study. Discussion of dermatologic cases was significantly more prevalent in the matched group compared to the unmatched group (P=.013), possibly because dermatology faculty enjoy hearing about cases and how the applicant responds and interacts with the cases. These data suggest that matched applicants focus more on characteristics specific to the clinical aspects of dermatology.
Conversely, name-dropping was significantly more prevalent in the unmatched group (P=.014). Dermatology is a highly competitive specialty. In 2016, applicants who matched into dermatology had a mean USMLE Step 1 score of 249 with a mean number of 4.7 research experiences and 11.7 abstracts, presentations, or publications, which is higher than the average USMLE Step 1 score of 239 with a mean number of 3.8 research experiences and 8.7 abstracts, presentations, or publications for unmatched applicants.3 It is possible that residency selection committees may view name-dropping negatively if applicants choose to name-drop to strengthen their applications in comparison to more competitive candidates. Religious influences also were significantly more prevalent in the unmatched group (P=.002), but the overall frequency of religious influences was low (approximately 2% of all applicants).
The 422 personal statements examined in our study represent 83.1% of the total pool of applicants to postgraduate year 2 dermatology positions in 2012 (N=508).11 Our data differed somewhat from an analysis of same-year dermatology personal statements of 65% of the national applicant pool.5 Olazagasti et al5 found that themes of a family member in medicine (more in unmatched), a desire to contribute to decreasing literature gap (more in matched), and a desire to better understand dermatologic pathophysiology (more in matched) to be statistically significant (P≤.05 for all). Unfortunately, these themes were found in a small number of applicants, with each being reported in less than 7%.5 Our study included 23% more unmatched candidates and likely better estimated potential significant differences between matched and unmatched applicants.
In the Results section, Olazagasti et al5 reported that matched applicants emphasized the study of cutaneous manifestations of systemic disease significantly more frequently than unmatched applicants. However, the P value in their report did not support this statement (P=.054). In addition, their Conclusion section discussed matched candidates including themes of “why dermatology” and unmatched candidates including a “personal story” as differences between groups. Again, their results did not show any statistical significance to support these recommendations.5 When providing medical student mentorship in a field as competitive as dermatology, faculty must be careful in giving accurate advice that, if at all possible, is supported by objective data rather than personal preference or anecdotes.
Our study was limited in that only personal statements of applicants to a single program in a specific specialty were analyzed. Applicants may have submitted personalized versions of their personal statements to specific schools, which may have biased the themes present in this subset of personal statements. Given these limitations, we are unable to determine if these results are generalizable to all dermatology residency applicants. Further limitation is that the analysis of personal statements is in itself a subjective process.
This study included a larger number of personal statements representing a larger proportion of the total pool of applicants in 2012 than prior studies examining personal statements of dermatology residency applicants. In addition, this study examined the ultimate dermatology match outcome for each applicant during the 2012 application cycle. Future investigations could explore the role of other factors in the residency selection process such as USMLE Step scores, community service, research experiences, and Alpha Omega Alpha Honor Medical Society status.
Conclusion
There are common themes in the personal statements of dermatology residency applicants, including personal accomplishments/attributes and positive qualities of dermatology. In addition, discussion of dermatologic cases was statistically more prevalent in applicants who ultimately matched, whereas name-dropping and religious influences were more prevalent in applicants who did not match. This information may be useful to effectively mentor medical students about the writing process for the personal statement. Further investigation is needed to explore these associations and the role of other aspects of the application in the residency selection process.
- Arbelaez C, Ganguli I. The personal statement for residency application: review and guidance. J Natl Med Assoc. 2011;103:439-442.
- White BA, Sadoski M, Thomas S, et al. Is the evaluation of the personal statement a reliable component of the general surgery residency application? J Surg Educ. 2012;69:340-343.
- Charting Outcomes in the Match for U.S. Allopathic Seniors: Characteristics of US Allopathic Seniors Who Matched to Their Preferred Specialty in the 2016 Main Residency Match. Washington, DC: National Resident Matching Program; September 2016. https://www.nrmp.org/wp-content/uploads/2016/09/Charting-Outcomes-US-Allopathic-Seniors-2016.pdf. Accessed January 21, 2020.
- Results of the 2016 NRMP Program Director Survey. Washington, DC: National Resident Matching Program; June 2016. https://www.nrmp.org/wp-content/uploads/2016/09/NRMP-2016-Program-Director-Survey.pdf. Accessed January 21, 2020.
- Olazagasti J, Gorouhi F, Fazel N. A critical review of personal statements submitted by dermatology residency applicants. Dermatol Res Pract. 2014;2014:934874.
- Max BA, Gelfand B, Brooks MR, et al. Have personal statements become impersonal? an evaluation of personal statements in anesthesiology residency applications. J Clin Anesth. 2010;22:346-351.
- Nield LS, Nease EK, Mitra S, et al. Major themes in the personal statements of pediatric resident applicants. Clin Pediatr (Phila). 2016;55:671-672.
- Ostapenko L, Schonhardt-Bailey C, Sublette JW, et al. Textual analysis of general surgery residency personal statements: topics and gender differences. J Surg Educ. 2018;75:573-581.
- Osman NY, Schonhardt-Bailey C, Walling JL, et al. Textual analysis of internal medicine residency personal statements: themes and gender differences. Med Educ. 2015;49:93-102.
- Smith EA, Weyhing B, Mody Y, et al. A critical analysis of personal statements submitted by radiology residency applicants. Acad Radiol. 2005;12:1024-1028.
- Results and Data: 2012 Main Residency Match. Washington, DC: National Resident Matching Program; April 2012. http://www.nrmp.org/wp-content/uploads/2013/08/resultsanddata20121.pdf. Accessed January 21, 2020.
The personal statement is a narrative written by an applicant to residency programs to discuss his/her interests. It is one of the few places in the residency application process where applicants can express their personalities.1 Applicants believe the personal statement is an important opportunity to distinguish themselves from others, thus increasing their chances of successful matching, particularly in competitive specialties.1,2
Dermatology is a highly competitive specialty, with 614 medical students applying for 440 total dermatology positions in 2016.3 According to the results of the 2016 National Resident Matching program director survey, 82% (27/33) of dermatology program directors reported that the personal statement was a factor in selecting applicants to interview. Furthermore, dermatology program directors, on average, rated personal statements as more important than the Medical Student Performance Evaluation/Dean’s Letter, US Medical Licensing Examination (USMLE) Step 2 scores, and class ranking/quartile.4
Prior studies have sought to evaluate the impact of personal statements on the application process. A 2014 study of personal statements submitted by dermatology residency applicants found that the prevalence of certain themes differed according to match outcome.5 However, some of the conclusions drawn in this study were not supported by the reported results or were based on low numbers of participants. The purpose of our study was to examine personal statements from applications to a dermatology program at a major academic institution. This study identified common themes in personal statements, allowing for an analysis of their association with successful matching into dermatology.
Methods
All applications to the dermatology residency program at UNC School of Medicine (Chapel Hill, North Carolina) during the 2012 application cycle (N=422) were eligible. All submitted personal statements (N=422) were included with all personal identifiers removed prior to analysis. The investigator (D.S.M.) was blinded to other Electronic Residency Application Service data and match outcome.
The investigator initially reviewed a small, randomly selected subset of 20 personal statements to identify characteristics and common themes. The investigator then analyzed each of the personal statements to quantify the frequency of each theme. All personal statements submitted to the dermatology residency program at UNC School of Medicine were analyzed in this manner. Dermatology match outcomes for each applicant were confirmed later using dermatology program websites.
Differences in the prevalence of common themes between matched and unmatched applicants were calculated. Analysis of variance tests were used to determine if the differences in prevalence were statistically significant (P≤.05).
Results
All 422 submitted personal statements were evaluated, with 308 personal statements from applicants who matched and 114 personal statements from unmatched applicants. The screening of the initial subset of 20 personal statements resulted in a total of 9 content themes. The prevalence of each theme among matched and unmatched applicants is shown in the Table.
The most common themes among both matched and unmatched groups were personal accomplishments or attributes and positive qualities of dermatology. The prevalence of certain themes varied between matched and unmatched groups. Dermatologic cases were discussed significantly more frequently in the matched group compared to the unmatched group (60.06% vs 46.49%, P=.013). Name-dropping was more prevalent in the unmatched group (37.72%) compared to the matched group (26.95%). This difference in prevalence reached statistical significance (P=.014). Religious influences also were discussed more frequently in the unmatched group (5.26%) vs the matched group (0.65%) with statistical significance (P=.002).
Comment
This study of 422 personal statements submitted to a major academic institution showed that certain themes were common in personal statements among both matched and unmatched applicants. These themes included personal accomplishments/attributes and positive qualities of dermatology. This finding is consistent with prior studies that show common themes in the personal statements of applicants across a wide variety of specialties, including dermatology, anesthesiology, pediatrics, general surgery, internal medicine, and radiology.5-10 Most commonly, applicants feel the need to justify why they chose their particular specialty, with Olazagasti et al5 (N=332) reporting that 70% of submitted dermatology personal statements explained why the applicant chose dermatology.
Certain themes, however, varied in prevalence between matched and unmatched groups in our study. Discussion of dermatologic cases was significantly more prevalent in the matched group compared to the unmatched group (P=.013), possibly because dermatology faculty enjoy hearing about cases and how the applicant responds and interacts with the cases. These data suggest that matched applicants focus more on characteristics specific to the clinical aspects of dermatology.
Conversely, name-dropping was significantly more prevalent in the unmatched group (P=.014). Dermatology is a highly competitive specialty. In 2016, applicants who matched into dermatology had a mean USMLE Step 1 score of 249 with a mean number of 4.7 research experiences and 11.7 abstracts, presentations, or publications, which is higher than the average USMLE Step 1 score of 239 with a mean number of 3.8 research experiences and 8.7 abstracts, presentations, or publications for unmatched applicants.3 It is possible that residency selection committees may view name-dropping negatively if applicants choose to name-drop to strengthen their applications in comparison to more competitive candidates. Religious influences also were significantly more prevalent in the unmatched group (P=.002), but the overall frequency of religious influences was low (approximately 2% of all applicants).
The 422 personal statements examined in our study represent 83.1% of the total pool of applicants to postgraduate year 2 dermatology positions in 2012 (N=508).11 Our data differed somewhat from an analysis of same-year dermatology personal statements of 65% of the national applicant pool.5 Olazagasti et al5 found that themes of a family member in medicine (more in unmatched), a desire to contribute to decreasing literature gap (more in matched), and a desire to better understand dermatologic pathophysiology (more in matched) to be statistically significant (P≤.05 for all). Unfortunately, these themes were found in a small number of applicants, with each being reported in less than 7%.5 Our study included 23% more unmatched candidates and likely better estimated potential significant differences between matched and unmatched applicants.
In the Results section, Olazagasti et al5 reported that matched applicants emphasized the study of cutaneous manifestations of systemic disease significantly more frequently than unmatched applicants. However, the P value in their report did not support this statement (P=.054). In addition, their Conclusion section discussed matched candidates including themes of “why dermatology” and unmatched candidates including a “personal story” as differences between groups. Again, their results did not show any statistical significance to support these recommendations.5 When providing medical student mentorship in a field as competitive as dermatology, faculty must be careful in giving accurate advice that, if at all possible, is supported by objective data rather than personal preference or anecdotes.
Our study was limited in that only personal statements of applicants to a single program in a specific specialty were analyzed. Applicants may have submitted personalized versions of their personal statements to specific schools, which may have biased the themes present in this subset of personal statements. Given these limitations, we are unable to determine if these results are generalizable to all dermatology residency applicants. Further limitation is that the analysis of personal statements is in itself a subjective process.
This study included a larger number of personal statements representing a larger proportion of the total pool of applicants in 2012 than prior studies examining personal statements of dermatology residency applicants. In addition, this study examined the ultimate dermatology match outcome for each applicant during the 2012 application cycle. Future investigations could explore the role of other factors in the residency selection process such as USMLE Step scores, community service, research experiences, and Alpha Omega Alpha Honor Medical Society status.
Conclusion
There are common themes in the personal statements of dermatology residency applicants, including personal accomplishments/attributes and positive qualities of dermatology. In addition, discussion of dermatologic cases was statistically more prevalent in applicants who ultimately matched, whereas name-dropping and religious influences were more prevalent in applicants who did not match. This information may be useful to effectively mentor medical students about the writing process for the personal statement. Further investigation is needed to explore these associations and the role of other aspects of the application in the residency selection process.
The personal statement is a narrative written by an applicant to residency programs to discuss his/her interests. It is one of the few places in the residency application process where applicants can express their personalities.1 Applicants believe the personal statement is an important opportunity to distinguish themselves from others, thus increasing their chances of successful matching, particularly in competitive specialties.1,2
Dermatology is a highly competitive specialty, with 614 medical students applying for 440 total dermatology positions in 2016.3 According to the results of the 2016 National Resident Matching program director survey, 82% (27/33) of dermatology program directors reported that the personal statement was a factor in selecting applicants to interview. Furthermore, dermatology program directors, on average, rated personal statements as more important than the Medical Student Performance Evaluation/Dean’s Letter, US Medical Licensing Examination (USMLE) Step 2 scores, and class ranking/quartile.4
Prior studies have sought to evaluate the impact of personal statements on the application process. A 2014 study of personal statements submitted by dermatology residency applicants found that the prevalence of certain themes differed according to match outcome.5 However, some of the conclusions drawn in this study were not supported by the reported results or were based on low numbers of participants. The purpose of our study was to examine personal statements from applications to a dermatology program at a major academic institution. This study identified common themes in personal statements, allowing for an analysis of their association with successful matching into dermatology.
Methods
All applications to the dermatology residency program at UNC School of Medicine (Chapel Hill, North Carolina) during the 2012 application cycle (N=422) were eligible. All submitted personal statements (N=422) were included with all personal identifiers removed prior to analysis. The investigator (D.S.M.) was blinded to other Electronic Residency Application Service data and match outcome.
The investigator initially reviewed a small, randomly selected subset of 20 personal statements to identify characteristics and common themes. The investigator then analyzed each of the personal statements to quantify the frequency of each theme. All personal statements submitted to the dermatology residency program at UNC School of Medicine were analyzed in this manner. Dermatology match outcomes for each applicant were confirmed later using dermatology program websites.
Differences in the prevalence of common themes between matched and unmatched applicants were calculated. Analysis of variance tests were used to determine if the differences in prevalence were statistically significant (P≤.05).
Results
All 422 submitted personal statements were evaluated, with 308 personal statements from applicants who matched and 114 personal statements from unmatched applicants. The screening of the initial subset of 20 personal statements resulted in a total of 9 content themes. The prevalence of each theme among matched and unmatched applicants is shown in the Table.
The most common themes among both matched and unmatched groups were personal accomplishments or attributes and positive qualities of dermatology. The prevalence of certain themes varied between matched and unmatched groups. Dermatologic cases were discussed significantly more frequently in the matched group compared to the unmatched group (60.06% vs 46.49%, P=.013). Name-dropping was more prevalent in the unmatched group (37.72%) compared to the matched group (26.95%). This difference in prevalence reached statistical significance (P=.014). Religious influences also were discussed more frequently in the unmatched group (5.26%) vs the matched group (0.65%) with statistical significance (P=.002).
Comment
This study of 422 personal statements submitted to a major academic institution showed that certain themes were common in personal statements among both matched and unmatched applicants. These themes included personal accomplishments/attributes and positive qualities of dermatology. This finding is consistent with prior studies that show common themes in the personal statements of applicants across a wide variety of specialties, including dermatology, anesthesiology, pediatrics, general surgery, internal medicine, and radiology.5-10 Most commonly, applicants feel the need to justify why they chose their particular specialty, with Olazagasti et al5 (N=332) reporting that 70% of submitted dermatology personal statements explained why the applicant chose dermatology.
Certain themes, however, varied in prevalence between matched and unmatched groups in our study. Discussion of dermatologic cases was significantly more prevalent in the matched group compared to the unmatched group (P=.013), possibly because dermatology faculty enjoy hearing about cases and how the applicant responds and interacts with the cases. These data suggest that matched applicants focus more on characteristics specific to the clinical aspects of dermatology.
Conversely, name-dropping was significantly more prevalent in the unmatched group (P=.014). Dermatology is a highly competitive specialty. In 2016, applicants who matched into dermatology had a mean USMLE Step 1 score of 249 with a mean number of 4.7 research experiences and 11.7 abstracts, presentations, or publications, which is higher than the average USMLE Step 1 score of 239 with a mean number of 3.8 research experiences and 8.7 abstracts, presentations, or publications for unmatched applicants.3 It is possible that residency selection committees may view name-dropping negatively if applicants choose to name-drop to strengthen their applications in comparison to more competitive candidates. Religious influences also were significantly more prevalent in the unmatched group (P=.002), but the overall frequency of religious influences was low (approximately 2% of all applicants).
The 422 personal statements examined in our study represent 83.1% of the total pool of applicants to postgraduate year 2 dermatology positions in 2012 (N=508).11 Our data differed somewhat from an analysis of same-year dermatology personal statements of 65% of the national applicant pool.5 Olazagasti et al5 found that themes of a family member in medicine (more in unmatched), a desire to contribute to decreasing literature gap (more in matched), and a desire to better understand dermatologic pathophysiology (more in matched) to be statistically significant (P≤.05 for all). Unfortunately, these themes were found in a small number of applicants, with each being reported in less than 7%.5 Our study included 23% more unmatched candidates and likely better estimated potential significant differences between matched and unmatched applicants.
In the Results section, Olazagasti et al5 reported that matched applicants emphasized the study of cutaneous manifestations of systemic disease significantly more frequently than unmatched applicants. However, the P value in their report did not support this statement (P=.054). In addition, their Conclusion section discussed matched candidates including themes of “why dermatology” and unmatched candidates including a “personal story” as differences between groups. Again, their results did not show any statistical significance to support these recommendations.5 When providing medical student mentorship in a field as competitive as dermatology, faculty must be careful in giving accurate advice that, if at all possible, is supported by objective data rather than personal preference or anecdotes.
Our study was limited in that only personal statements of applicants to a single program in a specific specialty were analyzed. Applicants may have submitted personalized versions of their personal statements to specific schools, which may have biased the themes present in this subset of personal statements. Given these limitations, we are unable to determine if these results are generalizable to all dermatology residency applicants. Further limitation is that the analysis of personal statements is in itself a subjective process.
This study included a larger number of personal statements representing a larger proportion of the total pool of applicants in 2012 than prior studies examining personal statements of dermatology residency applicants. In addition, this study examined the ultimate dermatology match outcome for each applicant during the 2012 application cycle. Future investigations could explore the role of other factors in the residency selection process such as USMLE Step scores, community service, research experiences, and Alpha Omega Alpha Honor Medical Society status.
Conclusion
There are common themes in the personal statements of dermatology residency applicants, including personal accomplishments/attributes and positive qualities of dermatology. In addition, discussion of dermatologic cases was statistically more prevalent in applicants who ultimately matched, whereas name-dropping and religious influences were more prevalent in applicants who did not match. This information may be useful to effectively mentor medical students about the writing process for the personal statement. Further investigation is needed to explore these associations and the role of other aspects of the application in the residency selection process.
- Arbelaez C, Ganguli I. The personal statement for residency application: review and guidance. J Natl Med Assoc. 2011;103:439-442.
- White BA, Sadoski M, Thomas S, et al. Is the evaluation of the personal statement a reliable component of the general surgery residency application? J Surg Educ. 2012;69:340-343.
- Charting Outcomes in the Match for U.S. Allopathic Seniors: Characteristics of US Allopathic Seniors Who Matched to Their Preferred Specialty in the 2016 Main Residency Match. Washington, DC: National Resident Matching Program; September 2016. https://www.nrmp.org/wp-content/uploads/2016/09/Charting-Outcomes-US-Allopathic-Seniors-2016.pdf. Accessed January 21, 2020.
- Results of the 2016 NRMP Program Director Survey. Washington, DC: National Resident Matching Program; June 2016. https://www.nrmp.org/wp-content/uploads/2016/09/NRMP-2016-Program-Director-Survey.pdf. Accessed January 21, 2020.
- Olazagasti J, Gorouhi F, Fazel N. A critical review of personal statements submitted by dermatology residency applicants. Dermatol Res Pract. 2014;2014:934874.
- Max BA, Gelfand B, Brooks MR, et al. Have personal statements become impersonal? an evaluation of personal statements in anesthesiology residency applications. J Clin Anesth. 2010;22:346-351.
- Nield LS, Nease EK, Mitra S, et al. Major themes in the personal statements of pediatric resident applicants. Clin Pediatr (Phila). 2016;55:671-672.
- Ostapenko L, Schonhardt-Bailey C, Sublette JW, et al. Textual analysis of general surgery residency personal statements: topics and gender differences. J Surg Educ. 2018;75:573-581.
- Osman NY, Schonhardt-Bailey C, Walling JL, et al. Textual analysis of internal medicine residency personal statements: themes and gender differences. Med Educ. 2015;49:93-102.
- Smith EA, Weyhing B, Mody Y, et al. A critical analysis of personal statements submitted by radiology residency applicants. Acad Radiol. 2005;12:1024-1028.
- Results and Data: 2012 Main Residency Match. Washington, DC: National Resident Matching Program; April 2012. http://www.nrmp.org/wp-content/uploads/2013/08/resultsanddata20121.pdf. Accessed January 21, 2020.
- Arbelaez C, Ganguli I. The personal statement for residency application: review and guidance. J Natl Med Assoc. 2011;103:439-442.
- White BA, Sadoski M, Thomas S, et al. Is the evaluation of the personal statement a reliable component of the general surgery residency application? J Surg Educ. 2012;69:340-343.
- Charting Outcomes in the Match for U.S. Allopathic Seniors: Characteristics of US Allopathic Seniors Who Matched to Their Preferred Specialty in the 2016 Main Residency Match. Washington, DC: National Resident Matching Program; September 2016. https://www.nrmp.org/wp-content/uploads/2016/09/Charting-Outcomes-US-Allopathic-Seniors-2016.pdf. Accessed January 21, 2020.
- Results of the 2016 NRMP Program Director Survey. Washington, DC: National Resident Matching Program; June 2016. https://www.nrmp.org/wp-content/uploads/2016/09/NRMP-2016-Program-Director-Survey.pdf. Accessed January 21, 2020.
- Olazagasti J, Gorouhi F, Fazel N. A critical review of personal statements submitted by dermatology residency applicants. Dermatol Res Pract. 2014;2014:934874.
- Max BA, Gelfand B, Brooks MR, et al. Have personal statements become impersonal? an evaluation of personal statements in anesthesiology residency applications. J Clin Anesth. 2010;22:346-351.
- Nield LS, Nease EK, Mitra S, et al. Major themes in the personal statements of pediatric resident applicants. Clin Pediatr (Phila). 2016;55:671-672.
- Ostapenko L, Schonhardt-Bailey C, Sublette JW, et al. Textual analysis of general surgery residency personal statements: topics and gender differences. J Surg Educ. 2018;75:573-581.
- Osman NY, Schonhardt-Bailey C, Walling JL, et al. Textual analysis of internal medicine residency personal statements: themes and gender differences. Med Educ. 2015;49:93-102.
- Smith EA, Weyhing B, Mody Y, et al. A critical analysis of personal statements submitted by radiology residency applicants. Acad Radiol. 2005;12:1024-1028.
- Results and Data: 2012 Main Residency Match. Washington, DC: National Resident Matching Program; April 2012. http://www.nrmp.org/wp-content/uploads/2013/08/resultsanddata20121.pdf. Accessed January 21, 2020.
Practice Points
- The most common themes discussed in applicant personal statements include personal accomplishments/attributes and positive qualities of dermatology.
- Presentation of dermatologic cases was more prevalent in personal statements of matched applicants.
- Name-dropping was more common among unmatched applicants.
Management of Patients With Treatment-Resistant Metastatic Prostate Cancer (FULL)
Sequencing Therapies
Mark Klein, MD. The last few years, there have been several new trials in prostate cancer for people in a metastatic setting or more advanced local setting, such as the STAMPEDE, LATITUDE, and CHAARTED trials.1-4 In addition, recently a few trials have examined apalutamide and enzalutamide for people who have had PSA (prostate-specific antigen) levels rapidly rising within about 10 months or so. One of the questions that arises is, how do we wrap our heads around sequencing these therapies. Is there a sequence that we should be doing and thinking about upfront and how do the different trials compare?
Julie Graff, MD. It just got more complicated. There was news today (December 20, 2018) that using enzalutamide early on in newly diagnosed metastatic prostate cancer may have positive results. It is not yet approved by the US Food and Drug Administration (FDA), but for patients who present with metastatic prostate cancer, we may have 4 potential treatments. We could have androgen deprivation therapy (ADT) alone, ADT plus docetaxel, enzalutamide, or abiraterone.
When I see patients in this situation, I talk to them about their options, the pros and cons of each option, and try to cover all the trials that look at these combinations. It can be quite a long visit. I talk to the patient about who benefits most, whether it is patients with high-risk factors or high-volume cancers. Also, I talk with the patient about all the adverse effects (AEs), and I look at my patients’ comorbid conditions and come up with a plan.
I encourage any patient who has high-volume or high-risk disease to consider more than just ADT alone. For many patients, I have been using abiraterone plus ADT. I have a wonderful pharmacist. As a medical oncologist, I can’t do it on my own. I need someone to follow patients’ laboratory results and to be available for medication questions and complications.
Elizabeth Hansen, PharmD. With the increasing number of patients on oral antineoplastics, monitoring patients in the outpatient setting has become an increasing priority and one of my major roles as a pharmacist in the clinic at the Chalmers P. Wylie VA Ambulatory Care Center in Columbus, Ohio. This is especially important as some of these treatments require frequent laboratory monitoring, such as abiraterone with liver function tests every 2 weeks for the first 3 months of treatment and monthly thereafter. Without frequent-follow up it’s easy for these patients to get lost in the shuffle.
Abhishek Solanki, MD. You could argue that a fifth option is prostate-directed radiation for patients who have limited metastases based on the STAMPEDE trial, which we’ve started integrating into our practice at the Edward Hines, Jr. Veterans Affairs Hospital in Chicago, Illinois.4
Mark Klein. Do you have a feel for the data and using radiation in oligometastatic (≤ 5 metastatic tumors) disease in prostate cancer and how well that might work?
Abhishek Solanki. The best data we have are from the multi-arm, multistage STAMPEDE trial systemic therapies and local therapy in the setting of high-risk localized disease and metastatic disease.6 The most recent publication looked specifically at the population with newly diagnosed metastatic disease and compared standard ADT (and docetaxel in about 18% of the patients) with or without prostate-directed radiation therapy. There was no survival benefit with radiation in the overall population, but in the subgroup of patients with low metastatic burden, there was an 8% survival benefit at 3 years.
It’s difficult to know what to make of that information because, as we’ve discussed already, there are other systemic therapy options that are being used more and more upfront such as abiraterone. Can you see the same benefit of radiation in that setting? The flip side is that in this study, radiation just targeted the prostate; could survival be improved even more by targeting all sites of disease in patients with oligometastatic disease? These are still open questions in prostate cancer and there are clinical trials attempting to define the clinical benefit of radiation in the metastatic setting for patients with limited metastases.
Mark Klein. How do you select patients for radiation in this particular situation; How do you approach stratification when radiation is started upfront?
Abhishek Solanki. In the STAMPEDE trial, low metastatic burden was defined based on the definition in the CHAARTED trial, which was those patients who did not have ≥ 4 bone metastases with ≥ 1 outside the vertebral bodies or pelvis, and did not have visceral metastases.7 That’s tough, because this definition could be a patient with a solitary bone metastasis but also could include some patients who have involved nodes extending all the way up to the retroperitoneal nodes—that is a fairly heterogeneous population. What we have done at our institution is select patients who have 3 to 5 metastases, administer prostate radiation therapy, and add stereotactic body radiation therapy (SBRT) for the other sites of disease, invoking the oligometastasis approach.
We have been doing this more frequently in the last few months. Typically, we’ll do 3 to 5 fractions of SBRT to metastases. For the primary, if the patient chooses SBRT, we’ll take that approach. If the patient chooses a more standard fractionation, we’ll do 20 treatments, but from a logistic perspective, most patients would rather come in for 5 treatments than 20. We also typically would start these patients on systemic hormonal therapy.
Mark Klein. At that point, are they referred back to medical oncology for surveillance?
Abhishek Solanki. Yes, they are followed by medical oncology and radiation oncology, and typically would continue hormonal therapy.
Mark Klein. Julie, how have you thought about presenting the therapeutic options for those patients who would be either eligible for docetaxel with high-bulk disease or abiraterone? Do you find patients prefer one or the other?
Julie Graff. I try to be very open about all the possibilities, and I present both. I don’t just decide for the patient chemotherapy vs abiraterone, but after we talk about it, most of my patients do opt for the abiraterone. I had a patient referred from the community—we are seeing more and more of this because abiraterone is so expensive—whose ejection fraction was about 38%. I said to that patient, “we could do chemotherapy, but we shouldn’t do abiraterone.” But usually it’s not that clear-cut.
Elizabeth Hansen. There was also an update from the STAMPEDE trial published recently comparing upfront abiraterone and prednisone to docetaxel (18 weeks) in advanced or metastatic prostate cancer. Results from this trial indicated a nearly identical overall survival (OS) (hazard ratio [HR] = 1.16; 95% CI, 0.82-1.65; P = .40). However, the failure-free survival (HR = 0.51; 95% CI, 0.39-0.67; P < .001) and progression-free survival (PFS) (HR= 0.65; 95% CI, 0.0.48-0.88; P = .005) favored abiraterone.8,9 The authors argue that while there was no change in OS, this trial demonstrates an important difference in the pattern of treatment failure.
Julie, do you think there will be any change in the treatment paradigm between docetaxel and abiraterone with this new update?
Julie Graff. I wasn’t that impressed by that study. I do not see it as practice changing, and it makes sense to me that the PFS is different in the 2 arms because we give chemotherapy and take a break vs giving abiraterone indefinitely. For me, there’s not really a shift.
Patients With Rising PSAs
Mark Klein. Let’s discuss the data from the recent studies on enzalutamide and apalutamide for the patients with fast-rising PSAs. In your discussions with other prostate researchers, will this become a standard part of practice or not?
Julie Graff. I was one of the authors on the SPARTAN apalutamide study.10 For a long time, we have had patients without metastatic disease but with a PSA relapse after surgery or radiation; and the PSA levels climb when the cancer becomes resistant to ADT. We haven’t had many options in that setting except to use bicalutamide and some older androgen receptor (AR) antagonists. We used to use estrogen and ketoconazole as well.
But now 2 studies have come out looking at a primary endpoint of metastases-free survival. Patients whose PSA was doubling every 10 months or shorter were randomized to either apalutamide (SPARTAN10) or enzalutamide (PROSPER11), both second-generation AR antagonists. There was a placebo control arm in each of the studies. Both studies found that adding the second-generation AR targeting agent delayed the time to metastatic disease by about 2 years. There is not any signal yet for statistically significant OS benefit, so it is not entirely clear if you could wait for the first metastasis to develop and then give 1 of these treatments and have the same OS benefit.
At the VA Portland Health Care System (VAPORHCS), it took a while to make these drugs available. My fellows were excited to give these drugs right away, but I often counsel patients that we don’t know if the second-generation AR targeting agents will improve survival. They almost certainly will bring down PSAs, which helps with peace of mind, but anything we add to the ADT can cause more AEs.
I have been cautious with second-generation AR antagonists because patients, when they take one of these drugs, are going to be on it for a long time. The FDA has approved those 2 drugs regardless of PSA doubling time, but I would not give it for a PSA doubling time > 10 months. In my practice about a quarter of patients who would qualify for apalutamide or enzalutamide are actually taking one, and the others are monitored closely with computed tomography (CT) and bone scans. When the disease becomes metastatic, then we start those drugs.
Mark Klein. Why 10 months, why not 6 months, a year, or 18 months? Is there reasoning behind that?
Julie Graff. There was a publication by Matthew Smith showing that the PSA doubling time was predictive of the development of metastatic disease and cancer death or prostate cancer death, and that 10 months seemed to be the cutoff between when the prostate cancer was going to become deadly vs not.12 If you actually look at the trial data, I think the PSA doubling time was between 3 and 4 months for the participants, so pretty short.
Adverse Effects
Mark Klein. What are the AEs people are seeing from using apalutamide, enzalutamide, and abiraterone? What are they seeing in their practice vs what is in the studies? When I have had to stop people on abiraterone or drop down the dose, almost always it has been for fatigue. We check liver function tests (LFTs) repeatedly, but I can’t remember ever having to drop down the dose or take it away even for that reason.
Elizabeth Hansen.
Mark Klein. At the Minneapolis VA Health Care System (MVAHCS) when apalutamide first came out, for the PSA rapid doubling, there had already been an abstract presenting the enzalutamide data. We have chosen to recommend enzalutamide as our choice for the people with PSA doubling based on the cost. It’s significantly cheaper for the VA. Between the 2 papers there is very little difference in the efficacy data. I’m wondering what other sites have done with regard to that specific point at their VAs?
Elizabeth Hansen. In Columbus, we prefer to use either abiraterone and enzalutamide because they’re essentially cost neutral. However, this may change with generic abiraterone coming to market. Apalutamide is really cost prohibitive currently.
Julie Graff. I agree.
Patient Education
Mark Klein. At MVAHCS, the navigators handle a lot of upfront education. We have 3 navigators, including Kathleen Nelson who is on this roundtable. She works with patients and provides much of the patient education. How have you handled education for patients?
Kathleen Nelson. For the most part, our pharmacists do the drug-specific education for the oral agents, and the nurse navigators provide more generic education. We did a trial for patients on IV therapies. We learned that patients really don’t report in much detail, but if you call and ask them specific questions, then you can tease out some more detail.
Elizabeth Hansen. It is interesting that every site is different. One of my main roles is oral antineoplastic monitoring, which includes many patients on enzalutamide or abiraterone. At least initially with these patients, I try to follow them closely—abiraterone more so than enzalutamide. I typically call every 2 to 4 weeks, in between clinic visits, to follow up the laboratory tests and manage the AEs. I always try to ask direct and open-ended questions: How often are you checking your blood pressure? What is your current weight? How has your energy level changed since therapy initiation?
The VA telehealth system is amazing. For patients who need to monitor blood pressure regularly, it’s really nice for them to have those numbers come directly back to me in CPRS (Computerized Patient Record System). That has worked wonders for some of our patients to get them through therapy.
Mark Klein. What do you tend to use when the prostate cancer is progressing for a patient? And how do you determine that progression? Some studies will use PSA rise only as a marker for progression. Other studies have not used PSA rise as the only marker for progression and oftentimes require some sort of bone scan criteria or CT imaging criteria for progression.
Julie Graff. We have a limited number of treatment options. Providers typically use enzalutamide or abiraterone as there is a high degree of resistance between the 2. Then there is chemotherapy and then radium, which quite a few people don’t qualify for. We need to be very thoughtful when we change treatments. I look at the 3 factors of biochemical progression or response—PSA, radiographic progression, and clinical progression. If I don’t see 2 out of 3, I typically don’t change treatments. Then after enzalutamide or abiraterone, I wait until there are cancer-related symptoms before I consider chemotherapy and closely monitor my patients.
Imaging Modalities
Abhishek Solanki. Over the last few years the Hines VA Hospital has used fluciclovine positron emission tomography (PET), which is one of the novel imaging modalities for prostate cancer. Really the 2 novel imaging modalities that have gained the most excitement are prostate-specific membrane antigen (PSMA) PET and fluciclovine PET. Fluciclovine PET is based on a synthetic amino acid that’s taken up in multiple tissues, including prostate cancer. It has changed our practice in the localized setting for patients who have developed recurrence after radiation or radical prostatectomy. We have incorporated the scan into our workup of patients with recurrent disease, which can give us some more information at lower PSAs than historically we could get with CT, bone scan, or magnetic resonance imaging.
Our medical oncologists have started using it more and more as well. We are getting a lot of patients who have a negative CT or bone scan but have a positive fluciclovine PET. There are a few different disease settings where that becomes relevant. In patients who develop biochemical recurrence after radiation or salvage radiation after radical, we are finding that a lot of these patients who have no CT or bone scan findings of disease ultimately are found to have a PET-positive lesion. Sometimes it’s difficult to know how best to help patients with PET-only disease. Should you target the disease with an oligometastasis approach or just pursue systemic therapy or surveillance? It is challenging but more and more we are moving toward metastasis-directed therapy. There are multiple randomized trials in progress testing whether metastasis-directed therapy to the PET areas of recurrence can improve outcomes or delay systemic ADT. The STOMP trial randomized surveillance vs SBRT or surgery for patients with oligometastatic disease that showed improvement in biochemical control and ADT-free survival.13 However this was a small trial that tried to identify a signal. More definitive trials are necessary.
The other setting where we have found novel PET imaging to be helpful is in patients who have become castration resistant but don’t have clear metastases on conventional imaging. We’re identifying more patients who have only a few sites of progression, and we’ll pursue metastasis-directed therapy to those areas to try to get more mileage out of the systemic therapy that the patient is currently on and to try to avoid having to switch to the next line with the idea that, potentially, the progression site is just a limited clone that is progressing despite the current systemic therapy.
Mark Klein. I find that to be a very attractive approach. I’m assuming you do that for any systemic therapy where people have maybe 1 or 2 sites and they do not have a big PSA jump. Do you have a number of sites that you’re willing to radiate? And then, when you do that, what radiation fractionation and dosing do you use? Is there any observational data behind that for efficacy?
Abhishek Solanki. It is a patient by patient decision. Some patients, if they have a very rapid pace of progression shortly after starting systemic therapy and metastases have grown in several areas, we think that perhaps this person may benefit less from aggressive local therapy. But if it’s somebody who has been on systemic therapy for a while and has up to 3 sites of disease growth, we consider SBRT for oligoprogressive disease. Typically, we’ll use SBRT, which delivers a high dose of radiation over 3 to 5 treatments. With SBRT you can give a higher biologic dose and use more sophisticated treatment machines and image guidance for treatments to focus the radiation on the tumor area and limit exposure to normal tissue structures.
In prostate cancer to the primary site, we will typically do around 35 to 40 Gy in 5 fractions. For metastases, it depends on the site. If it’s in the lung, typically we will do 3 to 5 treatments, giving approximately 50 to 60 Gy in that course. In the spine, we use lower doses near the spinal cord and the cauda equina, typically about 30 Gy in 3 fractions. In the liver, similar to the lung, we’ll typically do 50-54 Gy in 3-5 fractions. There aren’t a lot of high-level data guiding the optimal dose/fractionation to metastases, but these are the doses we’ll use for various malignancies.
Treatment Options for Patients With Adverse Events
Mark Klein. I was just reviewing the 2004 study that randomized patients to mitoxantrone or docetaxel for up to 10 cycles.14,15 Who are good candidates for docetaxel after they have exhausted abiraterone and enzalutamide? How long do you hold to the 10-cycle rule, or do you go beyond that if they’re doing well? And if they’re not a good candidate, what are some options?
Julie Graff. The best candidates are those who are having a cancer-related AE, particularly pain, because docetaxel only improves survival over mitoxantrone by about 2.5 months. I don’t talk to patients about it as though it is a life extender, but it seems to help control pain—about 70% of patients benefited in terms of pain or some other cancer-related symptom.14
I have a lot of patients who say, “Never will I do chemotherapy.” I refer those patients to hospice, or if they’re appropriate for radium-223, I consider that. I typically give about 6 cycles of chemotherapy and then see how they’re doing. In some patients, the cancer just doesn’t respond to it.
I do tell patients about the papers that you mentioned, the 2 studies of docetaxel vs mitoxantrone where they use about 10 cycles, and some of my patients go all 10.14,15 Sometimes we have to stop because of neuropathy or some other AE. I believe in taking breaks and that you can probably start it later.
Elizabeth Hansen. I agree, our practice is similar. A lot of our patients are not very interested in chemotherapy. You have to take into consideration their ECOG (Eastern Cooperative Oncology Group) status, their goals, and quality of life when talking to them about these medications. And a lot of them tend to choose more of a palliative route. Depending on their AEs and how things are going, we will dose reduce, hold treatment, or give treatment holidays.
Mark Klein. If patients are progressing on docetaxel, what are options that people would use? Radium-223 certainly is available for patients with nonvisceral metastases, as well as cabazitaxel, mitoxantrone, estramustine and other older drugs.
Julie Graff. We have some clinical trials for patients postdocetaxel. We have the TRITON2 and TRITON3 studies open at the VA. (NCT02952534 and NCT02975934, respectively) A lot of patients would get a biopsy, and we’d look for a BRCA 1 or 2 and ATM mutation. For those patients who don’t have those mutations—and maybe 80% of them don’t—we talk about radium-223 for the patients without visceral metastases and bone pain. I have had a fair number of patients go on cabazitaxel, but I have not used mitoxantrone since cabazitaxel came out. It’s not off the table, but it hasn’t shown improvement in survival.
Elizabeth Hansen. One of our challenges, because we’re an ambulatory care center, is that we are unable to give radium-223 in house, and these services have to be sent out to a non-VA facility. It is doable, but it takes more legwork and organization on our part.
Julie Graff. We have not had radium-223, although we’re working to get that online. And we are physically connected to Oregon Health Science University (OHSU), so we send our patients there for radium. It is a pain because the doctors at OHSU don’t have CPRS access. I’m often in the middle of making sure the complete blood counts (CBCs) are sent to OHSU and to get my patients their treatments.
Mark Klein. The Minneapolis VAMC has radium-223 on site, and we have used it for patients whose cancer has progressed while on docetaxel without visceral metastases. Katie, have you had an opportunity to coordinate that care for patients?
Kathleen Nelson. Radium is administered at our facility by one of our nuclear medicine physicians. A complete blood count is checked at least 3 days prior to the infusion date but no sooner than 6 days. Due to the cost of the material, ordering without knowing the patient’s counts are within a safe range to administer is prohibitive. This adds an additional burden of 2 visits (lab with return visit) to the patient. We have treated 12 patients. Four patients stopped treatment prior to completing the 6 planned treatments citing debilitating fatigue and/or nonresolution of symptoms as their reason to stop treatment. One patient died. The 7 remaining patients subjectively reported varying degrees of pain relief.
Elizabeth Hansen. Another thing to mention is the lack of a PSA response from radium-223 as well. Patients are generally very diligent about monitoring their PSA, so this can be a bit distressing.
Mark Klein. Julie, have you noticed a PSA flare with radium-223? I know it has been reported.
Julie Graff. I haven’t. But I put little stock in PSAs in these patients. I spend 20 minutes explaining to patients that the PSA is not helpful in determining whether or not the radium is working. I tell them that the bone marker alkaline phosphatase may decrease. And I think it’s important to note, too, that radium-223 is not a treatment we have on the shelf. We order it from Denver I believe. It is weight based, and it takes 5 days to get.
Clinical Trials
Mark Klein. That leads us into clinical trials. What is the role for precision oncology in prostate cancer right now, specifically looking at particular panels? One would be the DNA repair enzyme-based genes and/or also the AR variants and any other markers.
Elizabeth Hansen. The National Comprehensive Cancer Network came out with a statement recommending germ-line and somatic-mutation testing in all patients with metastatic prostate cancer. This highlights the need to offer patients the availability of clinical trials.
Julie Graff. I agree. We occasionally get to a place in the disease where patients are feeling fine, but we don’t have anything else to offer. The studies by Robinson16 and then Matteo17 showed that (a) these DNA repair defects are present in about a quarter of patients; and (b) that PARP inhibitors can help these patients. At least it has an anticancer effect.
What’s interesting is that we have TRITON2, and TRITON3, which are sponsored by Clovis,for patients with BRCA 1/2 and ATM mutations and using the PARP-inhibitor rucaparib. Based on the data we have available, we thought a quarter of patients would have the mutation in the tumor, but they’re finding that it is more like 10% to 15%. They are screening many patients but not finding it.
I agree that clinical trials are the way to go. I am hopeful that we’ll get more treatments based on molecular markers. The approval for pembrolizumab in any tumor type with microsatellite instability is interesting, but in prostate cancer, I believe that’s about 3%. I haven’t seen anyone qualify for pembrolizumab based on that. Another plug for clinical trials: Let’s learn more and offer our patients potentially beneficial treatments earlier.
Mark Klein. The first interim analysis from the TRITON2 study found about 12% of patients had alterations in BRCA 1/2. But in those that met the RECIST criteria, they were able to have evaluable disease via that standard with about a 44% response rate so far and a 51% PSA response rate. It is promising data, but it’s only 85 patients so far. We’ll know more because the TRITON2 study is of a more pretreated population than the TRITION3 study at this point. Are there any data on precision medicine and radiation in prostate cancer?
Abhishek Solanki. In the prostate cancer setting, there are not a lot of emerging data specifically looking at using precision oncology biomarkers to help guide decisions in radiation therapy. For example, genomic classifiers, like GenomeDx Decipher (Vancouver, BC) and Myriad Genetics Prolaris (Salt Lake City, UT) are increasingly being utilized in patients with localized disease. Decipher can help predict the risk of recurrence after radical prostatectomy. The difficulty is that there are limited data that show that by using these genomic classifiers, one can improve outcomes in patients over traditional clinical characteristics.
There are 2 trials currently ongoing through NRG Oncology that are using Decipher. The GU002 is a trial for patients who had a radical prostatectomy and had a postoperative PSA that never nadired below 0.2. These patients are randomized between salvage radiation with hormone therapy with or without docetaxel. This trial is collecting Decipher results for patients enrolled in the study. The GU006 is a trial for a slightly more favorable group of patients who do nadir but still have biochemical recurrence and relatively low PSAs. This trial randomizes between radiotherapy alone and radiotherapy and 6 months of apalutamide, stratifying patients based on Decipher results, specially differentiating between patients who have a luminal vs basal subtype of prostate cancer. There are data that suggest that patients who have a luminal subtype may benefit more from the combination of radiation and hormone therapy vs patients who have basal subtype.18 However this hasn’t been validated in a prospective setting, and that’s what this trial will hopefully do.
Immunotherapies
Mark Klein. Outside of prostate cancer, there has been a lot of research trying to determine how to improve PD-L1 expression. Where are immunotherapy trials moving? How radiation might play a role in conjunction with immunotherapy.
Julie Graff. Two phase 3 studies did not show statistically improved survival or statistically significant survival improvement on ipilimumab, an immunotherapy agent that targets CTLA4. Some early studies of the PD-1 drugs nivolumab and pembrolizumab did not show much response with monotherapy. Despite the negative phase 3 studies for ipilimumab, we periodically see exceptional responses.
In prostate cancer, enzalutamide is FDA approved. And there’s currently a phase 3 study of the PD-L1 inhibitor atezolizumab plus enzalutamide in patients who have progressed on abiraterone. That trial is fully accrued, bu
I just received a Prostate Cancer Foundation Challenge Award to open a VA-only study looking at fecal microbiota transplant from responders to nonresponders to see how manipulating host factors can increase potential responses to PD-1 inhibition.
Abhishek Solanki. The classic mechanism by which radiation therapy works is direct DNA damage and indirect DNA damage through hydroxyl radicals that leads to cytotoxicity. But preclinical and clinical data suggest that radiation therapy can augment the local and systemic immunotherapy response. The radiation oncologist’s dream is what is called the abscopal effect, which is the idea that when you treat one site of disease with radiation, it can induce a response at other sites that didn’t get radiation therapy through reactivation of the immune system. I like to think of the abscopal effect like bigfoot—it’s elusive. However, it seems that the setting it is most likely to happen in is in combination with immunotherapy.
One of the ways that radiation fails locally is that it can upregulate PD-1 expression, and as a result, you can have progression of the tumor because of local immune suppression. We know that T cells are important for the activity of radiation therapy. If you combine checkpoint inhibition with radiation therapy, you can not only have better local control in the area of the tumor, but perhaps you can release tumor antigens that will then induce a systemic response.
The other potential mechanism by which radiation may work synergistically with immunotherapy is as a debulking agent. There are some data that suggest that the ratio of T-cell reinvigoration to bulk of disease, or the volume of tumor burden, is important. That is, having T-cell reinvigoration may not be sufficient to have a response to immunotherapy in patients with a large burden of disease. By using radiation to debulk disease, perhaps you could help make checkpoint inhibition more effective. Ultimately, in the setting of prostate cancer, there are not a lot of data yet showing meaningful benefits with the combination of immunotherapy and radiotherapy, but there are trials that are ongoing that will educate on potential synergy.
Pharmacy
Julie Graff. Before we end I want to make sure that we applaud the amazing pharmacists and patient care navigation teams in the VA who do such a great job of getting veterans the appropriate treatment expeditiously and keeping them safe. It’s something that is truly unique to the VA. And I want to thank the people on this call who do this every day.
Elizabeth Hansen. Thank you Julie. Compared with working in the community, at the VA I’m honestly amazed by the ease of access to these medications for our patients. Being able to deliver medications sometimes the same day to the patient is just not something that happens in the community. It’s nice to see that our veterans are getting cared for in that manner.
Author disclosures
Dr. Solanki participated in advisory boards for Blue Earth Diagnostics’ fluciclovine PET and was previously paid as a consultant. Dr. Graff is a consultant for Sanofi (docetaxel) and Astellas (enzalutamide), and has received research funding (no personal funding)from Sanofi, Merck (pembrolizumab), Astellas, and Jannsen (abiraterone, apalutamide). The other authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the US Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. James ND, de Bono JS, Spears MR, et al; STAMPEDE Investigators. Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med. 2017;377(4):338-351.
2. James ND, Sydes MR, Clarke NW, et al; STAMPEDE Investigators. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2017;387(10024):1163-1177.
3. Fizazi K, Tran N, Fein L, et al; LATITUDE Investigators. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med. 2017;377(4):352-360.
4. Kyriakopoulos CE, Chen YH, Carducci MA, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer: long-term survival analysis of the randomized Phase III E3805 CHAARTED trial. J Clin Oncol. 2018;36(11):1080-1087.
5. Tosoian JJ, Gorin MA, Ross AE, Pienta KJ, Tran PT, Schaeffer EM. Oligometastatic prostate cancer: definitions, clinical outcomes, and treatment considerations. Nat Rev Urol. 2017;14(1):15-25.
6. Parker CC, James ND, Brawley CD, et al; Systemic Therapy for Advanced or Metastatic Prostate cancer: Evaluation of Drug Efficacy (STAMPEDE) investigators. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial. Lancet. 2018;392(10162):2353-2366.
7. Sweeney CJ, Chen YH, Carducci M, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med. 2015;373(8):737-746.
8. Feyerabend S, Saad F, Li T, et al. Survival benefit, disease progression and quality-of-life outcomes of abiraterone acetate plus prednisone versus docetaxel in metastatic hormone-sensitive prostate cancer: a network meta-analysis. Eur J Cancer. 2018;103:78-87.
9. Sydes MR, Spears MR, Mason MD, et al; STAMPEDE Investigators. Adding abiraterone or docetaxel to long-term hormone therapy for prostate cancer: directly randomised data from the STAMPEDE multi-arm, multi-stage platform protocol. Ann Oncol. 2018;29(5):1235-1248.
10. Smith MR, Saad F, Chowdhury S, et al; SPARTAN Investigators. Apalutamide treatment and metastasis-free survival in prostate cancer. N Engl J Med. 2018;378(15):1408-1418.
11. Hussain M, Fizazi K, Saad F, et al. Enzalutamide in men with nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2018;378(26):2465-2474.
12. Smith MR, Kabbinavar F, Saad F, et al. Natural history of rising serum prostate-specific antigen in men with castrate nonmetastatic prostate cancer. J Clin Oncol. 2005;23(13):2918-2925.
13. Ost P, Reynders D, Decaestecker K, et al. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence: a prospective, randomized, multicenter phase II trial. J Clin Oncol. 2018;36(5):446-453.
14. Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351(15):1513-1520.
15. Tannock IF, de Wit R, Berry WR, et al; TAX 327 Investigators. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351(15):1502-1512.
16. Robinson D, Van Allen EM, Wu YM, et al. Integrative clinical genomics of advanced prostate cancer. Cell. 2015;161(5):1215-1228.
17. Mateo J, Carreira S, Sandhu S, et al. DNA-repair defects and olaparib in metastatic prostate cancer. N Engl J Med. 2015;373(18):1697-1708.
18. Zhao SG, Chang SL, Erho N, et al. Associations of luminal and basal subtyping of prostate cancer with prognosis and response to androgen deprivation therapy. JAMA Oncol. 2017;3(12):1663-1672.
Sequencing Therapies
Mark Klein, MD. The last few years, there have been several new trials in prostate cancer for people in a metastatic setting or more advanced local setting, such as the STAMPEDE, LATITUDE, and CHAARTED trials.1-4 In addition, recently a few trials have examined apalutamide and enzalutamide for people who have had PSA (prostate-specific antigen) levels rapidly rising within about 10 months or so. One of the questions that arises is, how do we wrap our heads around sequencing these therapies. Is there a sequence that we should be doing and thinking about upfront and how do the different trials compare?
Julie Graff, MD. It just got more complicated. There was news today (December 20, 2018) that using enzalutamide early on in newly diagnosed metastatic prostate cancer may have positive results. It is not yet approved by the US Food and Drug Administration (FDA), but for patients who present with metastatic prostate cancer, we may have 4 potential treatments. We could have androgen deprivation therapy (ADT) alone, ADT plus docetaxel, enzalutamide, or abiraterone.
When I see patients in this situation, I talk to them about their options, the pros and cons of each option, and try to cover all the trials that look at these combinations. It can be quite a long visit. I talk to the patient about who benefits most, whether it is patients with high-risk factors or high-volume cancers. Also, I talk with the patient about all the adverse effects (AEs), and I look at my patients’ comorbid conditions and come up with a plan.
I encourage any patient who has high-volume or high-risk disease to consider more than just ADT alone. For many patients, I have been using abiraterone plus ADT. I have a wonderful pharmacist. As a medical oncologist, I can’t do it on my own. I need someone to follow patients’ laboratory results and to be available for medication questions and complications.
Elizabeth Hansen, PharmD. With the increasing number of patients on oral antineoplastics, monitoring patients in the outpatient setting has become an increasing priority and one of my major roles as a pharmacist in the clinic at the Chalmers P. Wylie VA Ambulatory Care Center in Columbus, Ohio. This is especially important as some of these treatments require frequent laboratory monitoring, such as abiraterone with liver function tests every 2 weeks for the first 3 months of treatment and monthly thereafter. Without frequent-follow up it’s easy for these patients to get lost in the shuffle.
Abhishek Solanki, MD. You could argue that a fifth option is prostate-directed radiation for patients who have limited metastases based on the STAMPEDE trial, which we’ve started integrating into our practice at the Edward Hines, Jr. Veterans Affairs Hospital in Chicago, Illinois.4
Mark Klein. Do you have a feel for the data and using radiation in oligometastatic (≤ 5 metastatic tumors) disease in prostate cancer and how well that might work?
Abhishek Solanki. The best data we have are from the multi-arm, multistage STAMPEDE trial systemic therapies and local therapy in the setting of high-risk localized disease and metastatic disease.6 The most recent publication looked specifically at the population with newly diagnosed metastatic disease and compared standard ADT (and docetaxel in about 18% of the patients) with or without prostate-directed radiation therapy. There was no survival benefit with radiation in the overall population, but in the subgroup of patients with low metastatic burden, there was an 8% survival benefit at 3 years.
It’s difficult to know what to make of that information because, as we’ve discussed already, there are other systemic therapy options that are being used more and more upfront such as abiraterone. Can you see the same benefit of radiation in that setting? The flip side is that in this study, radiation just targeted the prostate; could survival be improved even more by targeting all sites of disease in patients with oligometastatic disease? These are still open questions in prostate cancer and there are clinical trials attempting to define the clinical benefit of radiation in the metastatic setting for patients with limited metastases.
Mark Klein. How do you select patients for radiation in this particular situation; How do you approach stratification when radiation is started upfront?
Abhishek Solanki. In the STAMPEDE trial, low metastatic burden was defined based on the definition in the CHAARTED trial, which was those patients who did not have ≥ 4 bone metastases with ≥ 1 outside the vertebral bodies or pelvis, and did not have visceral metastases.7 That’s tough, because this definition could be a patient with a solitary bone metastasis but also could include some patients who have involved nodes extending all the way up to the retroperitoneal nodes—that is a fairly heterogeneous population. What we have done at our institution is select patients who have 3 to 5 metastases, administer prostate radiation therapy, and add stereotactic body radiation therapy (SBRT) for the other sites of disease, invoking the oligometastasis approach.
We have been doing this more frequently in the last few months. Typically, we’ll do 3 to 5 fractions of SBRT to metastases. For the primary, if the patient chooses SBRT, we’ll take that approach. If the patient chooses a more standard fractionation, we’ll do 20 treatments, but from a logistic perspective, most patients would rather come in for 5 treatments than 20. We also typically would start these patients on systemic hormonal therapy.
Mark Klein. At that point, are they referred back to medical oncology for surveillance?
Abhishek Solanki. Yes, they are followed by medical oncology and radiation oncology, and typically would continue hormonal therapy.
Mark Klein. Julie, how have you thought about presenting the therapeutic options for those patients who would be either eligible for docetaxel with high-bulk disease or abiraterone? Do you find patients prefer one or the other?
Julie Graff. I try to be very open about all the possibilities, and I present both. I don’t just decide for the patient chemotherapy vs abiraterone, but after we talk about it, most of my patients do opt for the abiraterone. I had a patient referred from the community—we are seeing more and more of this because abiraterone is so expensive—whose ejection fraction was about 38%. I said to that patient, “we could do chemotherapy, but we shouldn’t do abiraterone.” But usually it’s not that clear-cut.
Elizabeth Hansen. There was also an update from the STAMPEDE trial published recently comparing upfront abiraterone and prednisone to docetaxel (18 weeks) in advanced or metastatic prostate cancer. Results from this trial indicated a nearly identical overall survival (OS) (hazard ratio [HR] = 1.16; 95% CI, 0.82-1.65; P = .40). However, the failure-free survival (HR = 0.51; 95% CI, 0.39-0.67; P < .001) and progression-free survival (PFS) (HR= 0.65; 95% CI, 0.0.48-0.88; P = .005) favored abiraterone.8,9 The authors argue that while there was no change in OS, this trial demonstrates an important difference in the pattern of treatment failure.
Julie, do you think there will be any change in the treatment paradigm between docetaxel and abiraterone with this new update?
Julie Graff. I wasn’t that impressed by that study. I do not see it as practice changing, and it makes sense to me that the PFS is different in the 2 arms because we give chemotherapy and take a break vs giving abiraterone indefinitely. For me, there’s not really a shift.
Patients With Rising PSAs
Mark Klein. Let’s discuss the data from the recent studies on enzalutamide and apalutamide for the patients with fast-rising PSAs. In your discussions with other prostate researchers, will this become a standard part of practice or not?
Julie Graff. I was one of the authors on the SPARTAN apalutamide study.10 For a long time, we have had patients without metastatic disease but with a PSA relapse after surgery or radiation; and the PSA levels climb when the cancer becomes resistant to ADT. We haven’t had many options in that setting except to use bicalutamide and some older androgen receptor (AR) antagonists. We used to use estrogen and ketoconazole as well.
But now 2 studies have come out looking at a primary endpoint of metastases-free survival. Patients whose PSA was doubling every 10 months or shorter were randomized to either apalutamide (SPARTAN10) or enzalutamide (PROSPER11), both second-generation AR antagonists. There was a placebo control arm in each of the studies. Both studies found that adding the second-generation AR targeting agent delayed the time to metastatic disease by about 2 years. There is not any signal yet for statistically significant OS benefit, so it is not entirely clear if you could wait for the first metastasis to develop and then give 1 of these treatments and have the same OS benefit.
At the VA Portland Health Care System (VAPORHCS), it took a while to make these drugs available. My fellows were excited to give these drugs right away, but I often counsel patients that we don’t know if the second-generation AR targeting agents will improve survival. They almost certainly will bring down PSAs, which helps with peace of mind, but anything we add to the ADT can cause more AEs.
I have been cautious with second-generation AR antagonists because patients, when they take one of these drugs, are going to be on it for a long time. The FDA has approved those 2 drugs regardless of PSA doubling time, but I would not give it for a PSA doubling time > 10 months. In my practice about a quarter of patients who would qualify for apalutamide or enzalutamide are actually taking one, and the others are monitored closely with computed tomography (CT) and bone scans. When the disease becomes metastatic, then we start those drugs.
Mark Klein. Why 10 months, why not 6 months, a year, or 18 months? Is there reasoning behind that?
Julie Graff. There was a publication by Matthew Smith showing that the PSA doubling time was predictive of the development of metastatic disease and cancer death or prostate cancer death, and that 10 months seemed to be the cutoff between when the prostate cancer was going to become deadly vs not.12 If you actually look at the trial data, I think the PSA doubling time was between 3 and 4 months for the participants, so pretty short.
Adverse Effects
Mark Klein. What are the AEs people are seeing from using apalutamide, enzalutamide, and abiraterone? What are they seeing in their practice vs what is in the studies? When I have had to stop people on abiraterone or drop down the dose, almost always it has been for fatigue. We check liver function tests (LFTs) repeatedly, but I can’t remember ever having to drop down the dose or take it away even for that reason.
Elizabeth Hansen.
Mark Klein. At the Minneapolis VA Health Care System (MVAHCS) when apalutamide first came out, for the PSA rapid doubling, there had already been an abstract presenting the enzalutamide data. We have chosen to recommend enzalutamide as our choice for the people with PSA doubling based on the cost. It’s significantly cheaper for the VA. Between the 2 papers there is very little difference in the efficacy data. I’m wondering what other sites have done with regard to that specific point at their VAs?
Elizabeth Hansen. In Columbus, we prefer to use either abiraterone and enzalutamide because they’re essentially cost neutral. However, this may change with generic abiraterone coming to market. Apalutamide is really cost prohibitive currently.
Julie Graff. I agree.
Patient Education
Mark Klein. At MVAHCS, the navigators handle a lot of upfront education. We have 3 navigators, including Kathleen Nelson who is on this roundtable. She works with patients and provides much of the patient education. How have you handled education for patients?
Kathleen Nelson. For the most part, our pharmacists do the drug-specific education for the oral agents, and the nurse navigators provide more generic education. We did a trial for patients on IV therapies. We learned that patients really don’t report in much detail, but if you call and ask them specific questions, then you can tease out some more detail.
Elizabeth Hansen. It is interesting that every site is different. One of my main roles is oral antineoplastic monitoring, which includes many patients on enzalutamide or abiraterone. At least initially with these patients, I try to follow them closely—abiraterone more so than enzalutamide. I typically call every 2 to 4 weeks, in between clinic visits, to follow up the laboratory tests and manage the AEs. I always try to ask direct and open-ended questions: How often are you checking your blood pressure? What is your current weight? How has your energy level changed since therapy initiation?
The VA telehealth system is amazing. For patients who need to monitor blood pressure regularly, it’s really nice for them to have those numbers come directly back to me in CPRS (Computerized Patient Record System). That has worked wonders for some of our patients to get them through therapy.
Mark Klein. What do you tend to use when the prostate cancer is progressing for a patient? And how do you determine that progression? Some studies will use PSA rise only as a marker for progression. Other studies have not used PSA rise as the only marker for progression and oftentimes require some sort of bone scan criteria or CT imaging criteria for progression.
Julie Graff. We have a limited number of treatment options. Providers typically use enzalutamide or abiraterone as there is a high degree of resistance between the 2. Then there is chemotherapy and then radium, which quite a few people don’t qualify for. We need to be very thoughtful when we change treatments. I look at the 3 factors of biochemical progression or response—PSA, radiographic progression, and clinical progression. If I don’t see 2 out of 3, I typically don’t change treatments. Then after enzalutamide or abiraterone, I wait until there are cancer-related symptoms before I consider chemotherapy and closely monitor my patients.
Imaging Modalities
Abhishek Solanki. Over the last few years the Hines VA Hospital has used fluciclovine positron emission tomography (PET), which is one of the novel imaging modalities for prostate cancer. Really the 2 novel imaging modalities that have gained the most excitement are prostate-specific membrane antigen (PSMA) PET and fluciclovine PET. Fluciclovine PET is based on a synthetic amino acid that’s taken up in multiple tissues, including prostate cancer. It has changed our practice in the localized setting for patients who have developed recurrence after radiation or radical prostatectomy. We have incorporated the scan into our workup of patients with recurrent disease, which can give us some more information at lower PSAs than historically we could get with CT, bone scan, or magnetic resonance imaging.
Our medical oncologists have started using it more and more as well. We are getting a lot of patients who have a negative CT or bone scan but have a positive fluciclovine PET. There are a few different disease settings where that becomes relevant. In patients who develop biochemical recurrence after radiation or salvage radiation after radical, we are finding that a lot of these patients who have no CT or bone scan findings of disease ultimately are found to have a PET-positive lesion. Sometimes it’s difficult to know how best to help patients with PET-only disease. Should you target the disease with an oligometastasis approach or just pursue systemic therapy or surveillance? It is challenging but more and more we are moving toward metastasis-directed therapy. There are multiple randomized trials in progress testing whether metastasis-directed therapy to the PET areas of recurrence can improve outcomes or delay systemic ADT. The STOMP trial randomized surveillance vs SBRT or surgery for patients with oligometastatic disease that showed improvement in biochemical control and ADT-free survival.13 However this was a small trial that tried to identify a signal. More definitive trials are necessary.
The other setting where we have found novel PET imaging to be helpful is in patients who have become castration resistant but don’t have clear metastases on conventional imaging. We’re identifying more patients who have only a few sites of progression, and we’ll pursue metastasis-directed therapy to those areas to try to get more mileage out of the systemic therapy that the patient is currently on and to try to avoid having to switch to the next line with the idea that, potentially, the progression site is just a limited clone that is progressing despite the current systemic therapy.
Mark Klein. I find that to be a very attractive approach. I’m assuming you do that for any systemic therapy where people have maybe 1 or 2 sites and they do not have a big PSA jump. Do you have a number of sites that you’re willing to radiate? And then, when you do that, what radiation fractionation and dosing do you use? Is there any observational data behind that for efficacy?
Abhishek Solanki. It is a patient by patient decision. Some patients, if they have a very rapid pace of progression shortly after starting systemic therapy and metastases have grown in several areas, we think that perhaps this person may benefit less from aggressive local therapy. But if it’s somebody who has been on systemic therapy for a while and has up to 3 sites of disease growth, we consider SBRT for oligoprogressive disease. Typically, we’ll use SBRT, which delivers a high dose of radiation over 3 to 5 treatments. With SBRT you can give a higher biologic dose and use more sophisticated treatment machines and image guidance for treatments to focus the radiation on the tumor area and limit exposure to normal tissue structures.
In prostate cancer to the primary site, we will typically do around 35 to 40 Gy in 5 fractions. For metastases, it depends on the site. If it’s in the lung, typically we will do 3 to 5 treatments, giving approximately 50 to 60 Gy in that course. In the spine, we use lower doses near the spinal cord and the cauda equina, typically about 30 Gy in 3 fractions. In the liver, similar to the lung, we’ll typically do 50-54 Gy in 3-5 fractions. There aren’t a lot of high-level data guiding the optimal dose/fractionation to metastases, but these are the doses we’ll use for various malignancies.
Treatment Options for Patients With Adverse Events
Mark Klein. I was just reviewing the 2004 study that randomized patients to mitoxantrone or docetaxel for up to 10 cycles.14,15 Who are good candidates for docetaxel after they have exhausted abiraterone and enzalutamide? How long do you hold to the 10-cycle rule, or do you go beyond that if they’re doing well? And if they’re not a good candidate, what are some options?
Julie Graff. The best candidates are those who are having a cancer-related AE, particularly pain, because docetaxel only improves survival over mitoxantrone by about 2.5 months. I don’t talk to patients about it as though it is a life extender, but it seems to help control pain—about 70% of patients benefited in terms of pain or some other cancer-related symptom.14
I have a lot of patients who say, “Never will I do chemotherapy.” I refer those patients to hospice, or if they’re appropriate for radium-223, I consider that. I typically give about 6 cycles of chemotherapy and then see how they’re doing. In some patients, the cancer just doesn’t respond to it.
I do tell patients about the papers that you mentioned, the 2 studies of docetaxel vs mitoxantrone where they use about 10 cycles, and some of my patients go all 10.14,15 Sometimes we have to stop because of neuropathy or some other AE. I believe in taking breaks and that you can probably start it later.
Elizabeth Hansen. I agree, our practice is similar. A lot of our patients are not very interested in chemotherapy. You have to take into consideration their ECOG (Eastern Cooperative Oncology Group) status, their goals, and quality of life when talking to them about these medications. And a lot of them tend to choose more of a palliative route. Depending on their AEs and how things are going, we will dose reduce, hold treatment, or give treatment holidays.
Mark Klein. If patients are progressing on docetaxel, what are options that people would use? Radium-223 certainly is available for patients with nonvisceral metastases, as well as cabazitaxel, mitoxantrone, estramustine and other older drugs.
Julie Graff. We have some clinical trials for patients postdocetaxel. We have the TRITON2 and TRITON3 studies open at the VA. (NCT02952534 and NCT02975934, respectively) A lot of patients would get a biopsy, and we’d look for a BRCA 1 or 2 and ATM mutation. For those patients who don’t have those mutations—and maybe 80% of them don’t—we talk about radium-223 for the patients without visceral metastases and bone pain. I have had a fair number of patients go on cabazitaxel, but I have not used mitoxantrone since cabazitaxel came out. It’s not off the table, but it hasn’t shown improvement in survival.
Elizabeth Hansen. One of our challenges, because we’re an ambulatory care center, is that we are unable to give radium-223 in house, and these services have to be sent out to a non-VA facility. It is doable, but it takes more legwork and organization on our part.
Julie Graff. We have not had radium-223, although we’re working to get that online. And we are physically connected to Oregon Health Science University (OHSU), so we send our patients there for radium. It is a pain because the doctors at OHSU don’t have CPRS access. I’m often in the middle of making sure the complete blood counts (CBCs) are sent to OHSU and to get my patients their treatments.
Mark Klein. The Minneapolis VAMC has radium-223 on site, and we have used it for patients whose cancer has progressed while on docetaxel without visceral metastases. Katie, have you had an opportunity to coordinate that care for patients?
Kathleen Nelson. Radium is administered at our facility by one of our nuclear medicine physicians. A complete blood count is checked at least 3 days prior to the infusion date but no sooner than 6 days. Due to the cost of the material, ordering without knowing the patient’s counts are within a safe range to administer is prohibitive. This adds an additional burden of 2 visits (lab with return visit) to the patient. We have treated 12 patients. Four patients stopped treatment prior to completing the 6 planned treatments citing debilitating fatigue and/or nonresolution of symptoms as their reason to stop treatment. One patient died. The 7 remaining patients subjectively reported varying degrees of pain relief.
Elizabeth Hansen. Another thing to mention is the lack of a PSA response from radium-223 as well. Patients are generally very diligent about monitoring their PSA, so this can be a bit distressing.
Mark Klein. Julie, have you noticed a PSA flare with radium-223? I know it has been reported.
Julie Graff. I haven’t. But I put little stock in PSAs in these patients. I spend 20 minutes explaining to patients that the PSA is not helpful in determining whether or not the radium is working. I tell them that the bone marker alkaline phosphatase may decrease. And I think it’s important to note, too, that radium-223 is not a treatment we have on the shelf. We order it from Denver I believe. It is weight based, and it takes 5 days to get.
Clinical Trials
Mark Klein. That leads us into clinical trials. What is the role for precision oncology in prostate cancer right now, specifically looking at particular panels? One would be the DNA repair enzyme-based genes and/or also the AR variants and any other markers.
Elizabeth Hansen. The National Comprehensive Cancer Network came out with a statement recommending germ-line and somatic-mutation testing in all patients with metastatic prostate cancer. This highlights the need to offer patients the availability of clinical trials.
Julie Graff. I agree. We occasionally get to a place in the disease where patients are feeling fine, but we don’t have anything else to offer. The studies by Robinson16 and then Matteo17 showed that (a) these DNA repair defects are present in about a quarter of patients; and (b) that PARP inhibitors can help these patients. At least it has an anticancer effect.
What’s interesting is that we have TRITON2, and TRITON3, which are sponsored by Clovis,for patients with BRCA 1/2 and ATM mutations and using the PARP-inhibitor rucaparib. Based on the data we have available, we thought a quarter of patients would have the mutation in the tumor, but they’re finding that it is more like 10% to 15%. They are screening many patients but not finding it.
I agree that clinical trials are the way to go. I am hopeful that we’ll get more treatments based on molecular markers. The approval for pembrolizumab in any tumor type with microsatellite instability is interesting, but in prostate cancer, I believe that’s about 3%. I haven’t seen anyone qualify for pembrolizumab based on that. Another plug for clinical trials: Let’s learn more and offer our patients potentially beneficial treatments earlier.
Mark Klein. The first interim analysis from the TRITON2 study found about 12% of patients had alterations in BRCA 1/2. But in those that met the RECIST criteria, they were able to have evaluable disease via that standard with about a 44% response rate so far and a 51% PSA response rate. It is promising data, but it’s only 85 patients so far. We’ll know more because the TRITON2 study is of a more pretreated population than the TRITION3 study at this point. Are there any data on precision medicine and radiation in prostate cancer?
Abhishek Solanki. In the prostate cancer setting, there are not a lot of emerging data specifically looking at using precision oncology biomarkers to help guide decisions in radiation therapy. For example, genomic classifiers, like GenomeDx Decipher (Vancouver, BC) and Myriad Genetics Prolaris (Salt Lake City, UT) are increasingly being utilized in patients with localized disease. Decipher can help predict the risk of recurrence after radical prostatectomy. The difficulty is that there are limited data that show that by using these genomic classifiers, one can improve outcomes in patients over traditional clinical characteristics.
There are 2 trials currently ongoing through NRG Oncology that are using Decipher. The GU002 is a trial for patients who had a radical prostatectomy and had a postoperative PSA that never nadired below 0.2. These patients are randomized between salvage radiation with hormone therapy with or without docetaxel. This trial is collecting Decipher results for patients enrolled in the study. The GU006 is a trial for a slightly more favorable group of patients who do nadir but still have biochemical recurrence and relatively low PSAs. This trial randomizes between radiotherapy alone and radiotherapy and 6 months of apalutamide, stratifying patients based on Decipher results, specially differentiating between patients who have a luminal vs basal subtype of prostate cancer. There are data that suggest that patients who have a luminal subtype may benefit more from the combination of radiation and hormone therapy vs patients who have basal subtype.18 However this hasn’t been validated in a prospective setting, and that’s what this trial will hopefully do.
Immunotherapies
Mark Klein. Outside of prostate cancer, there has been a lot of research trying to determine how to improve PD-L1 expression. Where are immunotherapy trials moving? How radiation might play a role in conjunction with immunotherapy.
Julie Graff. Two phase 3 studies did not show statistically improved survival or statistically significant survival improvement on ipilimumab, an immunotherapy agent that targets CTLA4. Some early studies of the PD-1 drugs nivolumab and pembrolizumab did not show much response with monotherapy. Despite the negative phase 3 studies for ipilimumab, we periodically see exceptional responses.
In prostate cancer, enzalutamide is FDA approved. And there’s currently a phase 3 study of the PD-L1 inhibitor atezolizumab plus enzalutamide in patients who have progressed on abiraterone. That trial is fully accrued, bu
I just received a Prostate Cancer Foundation Challenge Award to open a VA-only study looking at fecal microbiota transplant from responders to nonresponders to see how manipulating host factors can increase potential responses to PD-1 inhibition.
Abhishek Solanki. The classic mechanism by which radiation therapy works is direct DNA damage and indirect DNA damage through hydroxyl radicals that leads to cytotoxicity. But preclinical and clinical data suggest that radiation therapy can augment the local and systemic immunotherapy response. The radiation oncologist’s dream is what is called the abscopal effect, which is the idea that when you treat one site of disease with radiation, it can induce a response at other sites that didn’t get radiation therapy through reactivation of the immune system. I like to think of the abscopal effect like bigfoot—it’s elusive. However, it seems that the setting it is most likely to happen in is in combination with immunotherapy.
One of the ways that radiation fails locally is that it can upregulate PD-1 expression, and as a result, you can have progression of the tumor because of local immune suppression. We know that T cells are important for the activity of radiation therapy. If you combine checkpoint inhibition with radiation therapy, you can not only have better local control in the area of the tumor, but perhaps you can release tumor antigens that will then induce a systemic response.
The other potential mechanism by which radiation may work synergistically with immunotherapy is as a debulking agent. There are some data that suggest that the ratio of T-cell reinvigoration to bulk of disease, or the volume of tumor burden, is important. That is, having T-cell reinvigoration may not be sufficient to have a response to immunotherapy in patients with a large burden of disease. By using radiation to debulk disease, perhaps you could help make checkpoint inhibition more effective. Ultimately, in the setting of prostate cancer, there are not a lot of data yet showing meaningful benefits with the combination of immunotherapy and radiotherapy, but there are trials that are ongoing that will educate on potential synergy.
Pharmacy
Julie Graff. Before we end I want to make sure that we applaud the amazing pharmacists and patient care navigation teams in the VA who do such a great job of getting veterans the appropriate treatment expeditiously and keeping them safe. It’s something that is truly unique to the VA. And I want to thank the people on this call who do this every day.
Elizabeth Hansen. Thank you Julie. Compared with working in the community, at the VA I’m honestly amazed by the ease of access to these medications for our patients. Being able to deliver medications sometimes the same day to the patient is just not something that happens in the community. It’s nice to see that our veterans are getting cared for in that manner.
Author disclosures
Dr. Solanki participated in advisory boards for Blue Earth Diagnostics’ fluciclovine PET and was previously paid as a consultant. Dr. Graff is a consultant for Sanofi (docetaxel) and Astellas (enzalutamide), and has received research funding (no personal funding)from Sanofi, Merck (pembrolizumab), Astellas, and Jannsen (abiraterone, apalutamide). The other authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the US Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Sequencing Therapies
Mark Klein, MD. The last few years, there have been several new trials in prostate cancer for people in a metastatic setting or more advanced local setting, such as the STAMPEDE, LATITUDE, and CHAARTED trials.1-4 In addition, recently a few trials have examined apalutamide and enzalutamide for people who have had PSA (prostate-specific antigen) levels rapidly rising within about 10 months or so. One of the questions that arises is, how do we wrap our heads around sequencing these therapies. Is there a sequence that we should be doing and thinking about upfront and how do the different trials compare?
Julie Graff, MD. It just got more complicated. There was news today (December 20, 2018) that using enzalutamide early on in newly diagnosed metastatic prostate cancer may have positive results. It is not yet approved by the US Food and Drug Administration (FDA), but for patients who present with metastatic prostate cancer, we may have 4 potential treatments. We could have androgen deprivation therapy (ADT) alone, ADT plus docetaxel, enzalutamide, or abiraterone.
When I see patients in this situation, I talk to them about their options, the pros and cons of each option, and try to cover all the trials that look at these combinations. It can be quite a long visit. I talk to the patient about who benefits most, whether it is patients with high-risk factors or high-volume cancers. Also, I talk with the patient about all the adverse effects (AEs), and I look at my patients’ comorbid conditions and come up with a plan.
I encourage any patient who has high-volume or high-risk disease to consider more than just ADT alone. For many patients, I have been using abiraterone plus ADT. I have a wonderful pharmacist. As a medical oncologist, I can’t do it on my own. I need someone to follow patients’ laboratory results and to be available for medication questions and complications.
Elizabeth Hansen, PharmD. With the increasing number of patients on oral antineoplastics, monitoring patients in the outpatient setting has become an increasing priority and one of my major roles as a pharmacist in the clinic at the Chalmers P. Wylie VA Ambulatory Care Center in Columbus, Ohio. This is especially important as some of these treatments require frequent laboratory monitoring, such as abiraterone with liver function tests every 2 weeks for the first 3 months of treatment and monthly thereafter. Without frequent-follow up it’s easy for these patients to get lost in the shuffle.
Abhishek Solanki, MD. You could argue that a fifth option is prostate-directed radiation for patients who have limited metastases based on the STAMPEDE trial, which we’ve started integrating into our practice at the Edward Hines, Jr. Veterans Affairs Hospital in Chicago, Illinois.4
Mark Klein. Do you have a feel for the data and using radiation in oligometastatic (≤ 5 metastatic tumors) disease in prostate cancer and how well that might work?
Abhishek Solanki. The best data we have are from the multi-arm, multistage STAMPEDE trial systemic therapies and local therapy in the setting of high-risk localized disease and metastatic disease.6 The most recent publication looked specifically at the population with newly diagnosed metastatic disease and compared standard ADT (and docetaxel in about 18% of the patients) with or without prostate-directed radiation therapy. There was no survival benefit with radiation in the overall population, but in the subgroup of patients with low metastatic burden, there was an 8% survival benefit at 3 years.
It’s difficult to know what to make of that information because, as we’ve discussed already, there are other systemic therapy options that are being used more and more upfront such as abiraterone. Can you see the same benefit of radiation in that setting? The flip side is that in this study, radiation just targeted the prostate; could survival be improved even more by targeting all sites of disease in patients with oligometastatic disease? These are still open questions in prostate cancer and there are clinical trials attempting to define the clinical benefit of radiation in the metastatic setting for patients with limited metastases.
Mark Klein. How do you select patients for radiation in this particular situation; How do you approach stratification when radiation is started upfront?
Abhishek Solanki. In the STAMPEDE trial, low metastatic burden was defined based on the definition in the CHAARTED trial, which was those patients who did not have ≥ 4 bone metastases with ≥ 1 outside the vertebral bodies or pelvis, and did not have visceral metastases.7 That’s tough, because this definition could be a patient with a solitary bone metastasis but also could include some patients who have involved nodes extending all the way up to the retroperitoneal nodes—that is a fairly heterogeneous population. What we have done at our institution is select patients who have 3 to 5 metastases, administer prostate radiation therapy, and add stereotactic body radiation therapy (SBRT) for the other sites of disease, invoking the oligometastasis approach.
We have been doing this more frequently in the last few months. Typically, we’ll do 3 to 5 fractions of SBRT to metastases. For the primary, if the patient chooses SBRT, we’ll take that approach. If the patient chooses a more standard fractionation, we’ll do 20 treatments, but from a logistic perspective, most patients would rather come in for 5 treatments than 20. We also typically would start these patients on systemic hormonal therapy.
Mark Klein. At that point, are they referred back to medical oncology for surveillance?
Abhishek Solanki. Yes, they are followed by medical oncology and radiation oncology, and typically would continue hormonal therapy.
Mark Klein. Julie, how have you thought about presenting the therapeutic options for those patients who would be either eligible for docetaxel with high-bulk disease or abiraterone? Do you find patients prefer one or the other?
Julie Graff. I try to be very open about all the possibilities, and I present both. I don’t just decide for the patient chemotherapy vs abiraterone, but after we talk about it, most of my patients do opt for the abiraterone. I had a patient referred from the community—we are seeing more and more of this because abiraterone is so expensive—whose ejection fraction was about 38%. I said to that patient, “we could do chemotherapy, but we shouldn’t do abiraterone.” But usually it’s not that clear-cut.
Elizabeth Hansen. There was also an update from the STAMPEDE trial published recently comparing upfront abiraterone and prednisone to docetaxel (18 weeks) in advanced or metastatic prostate cancer. Results from this trial indicated a nearly identical overall survival (OS) (hazard ratio [HR] = 1.16; 95% CI, 0.82-1.65; P = .40). However, the failure-free survival (HR = 0.51; 95% CI, 0.39-0.67; P < .001) and progression-free survival (PFS) (HR= 0.65; 95% CI, 0.0.48-0.88; P = .005) favored abiraterone.8,9 The authors argue that while there was no change in OS, this trial demonstrates an important difference in the pattern of treatment failure.
Julie, do you think there will be any change in the treatment paradigm between docetaxel and abiraterone with this new update?
Julie Graff. I wasn’t that impressed by that study. I do not see it as practice changing, and it makes sense to me that the PFS is different in the 2 arms because we give chemotherapy and take a break vs giving abiraterone indefinitely. For me, there’s not really a shift.
Patients With Rising PSAs
Mark Klein. Let’s discuss the data from the recent studies on enzalutamide and apalutamide for the patients with fast-rising PSAs. In your discussions with other prostate researchers, will this become a standard part of practice or not?
Julie Graff. I was one of the authors on the SPARTAN apalutamide study.10 For a long time, we have had patients without metastatic disease but with a PSA relapse after surgery or radiation; and the PSA levels climb when the cancer becomes resistant to ADT. We haven’t had many options in that setting except to use bicalutamide and some older androgen receptor (AR) antagonists. We used to use estrogen and ketoconazole as well.
But now 2 studies have come out looking at a primary endpoint of metastases-free survival. Patients whose PSA was doubling every 10 months or shorter were randomized to either apalutamide (SPARTAN10) or enzalutamide (PROSPER11), both second-generation AR antagonists. There was a placebo control arm in each of the studies. Both studies found that adding the second-generation AR targeting agent delayed the time to metastatic disease by about 2 years. There is not any signal yet for statistically significant OS benefit, so it is not entirely clear if you could wait for the first metastasis to develop and then give 1 of these treatments and have the same OS benefit.
At the VA Portland Health Care System (VAPORHCS), it took a while to make these drugs available. My fellows were excited to give these drugs right away, but I often counsel patients that we don’t know if the second-generation AR targeting agents will improve survival. They almost certainly will bring down PSAs, which helps with peace of mind, but anything we add to the ADT can cause more AEs.
I have been cautious with second-generation AR antagonists because patients, when they take one of these drugs, are going to be on it for a long time. The FDA has approved those 2 drugs regardless of PSA doubling time, but I would not give it for a PSA doubling time > 10 months. In my practice about a quarter of patients who would qualify for apalutamide or enzalutamide are actually taking one, and the others are monitored closely with computed tomography (CT) and bone scans. When the disease becomes metastatic, then we start those drugs.
Mark Klein. Why 10 months, why not 6 months, a year, or 18 months? Is there reasoning behind that?
Julie Graff. There was a publication by Matthew Smith showing that the PSA doubling time was predictive of the development of metastatic disease and cancer death or prostate cancer death, and that 10 months seemed to be the cutoff between when the prostate cancer was going to become deadly vs not.12 If you actually look at the trial data, I think the PSA doubling time was between 3 and 4 months for the participants, so pretty short.
Adverse Effects
Mark Klein. What are the AEs people are seeing from using apalutamide, enzalutamide, and abiraterone? What are they seeing in their practice vs what is in the studies? When I have had to stop people on abiraterone or drop down the dose, almost always it has been for fatigue. We check liver function tests (LFTs) repeatedly, but I can’t remember ever having to drop down the dose or take it away even for that reason.
Elizabeth Hansen.
Mark Klein. At the Minneapolis VA Health Care System (MVAHCS) when apalutamide first came out, for the PSA rapid doubling, there had already been an abstract presenting the enzalutamide data. We have chosen to recommend enzalutamide as our choice for the people with PSA doubling based on the cost. It’s significantly cheaper for the VA. Between the 2 papers there is very little difference in the efficacy data. I’m wondering what other sites have done with regard to that specific point at their VAs?
Elizabeth Hansen. In Columbus, we prefer to use either abiraterone and enzalutamide because they’re essentially cost neutral. However, this may change with generic abiraterone coming to market. Apalutamide is really cost prohibitive currently.
Julie Graff. I agree.
Patient Education
Mark Klein. At MVAHCS, the navigators handle a lot of upfront education. We have 3 navigators, including Kathleen Nelson who is on this roundtable. She works with patients and provides much of the patient education. How have you handled education for patients?
Kathleen Nelson. For the most part, our pharmacists do the drug-specific education for the oral agents, and the nurse navigators provide more generic education. We did a trial for patients on IV therapies. We learned that patients really don’t report in much detail, but if you call and ask them specific questions, then you can tease out some more detail.
Elizabeth Hansen. It is interesting that every site is different. One of my main roles is oral antineoplastic monitoring, which includes many patients on enzalutamide or abiraterone. At least initially with these patients, I try to follow them closely—abiraterone more so than enzalutamide. I typically call every 2 to 4 weeks, in between clinic visits, to follow up the laboratory tests and manage the AEs. I always try to ask direct and open-ended questions: How often are you checking your blood pressure? What is your current weight? How has your energy level changed since therapy initiation?
The VA telehealth system is amazing. For patients who need to monitor blood pressure regularly, it’s really nice for them to have those numbers come directly back to me in CPRS (Computerized Patient Record System). That has worked wonders for some of our patients to get them through therapy.
Mark Klein. What do you tend to use when the prostate cancer is progressing for a patient? And how do you determine that progression? Some studies will use PSA rise only as a marker for progression. Other studies have not used PSA rise as the only marker for progression and oftentimes require some sort of bone scan criteria or CT imaging criteria for progression.
Julie Graff. We have a limited number of treatment options. Providers typically use enzalutamide or abiraterone as there is a high degree of resistance between the 2. Then there is chemotherapy and then radium, which quite a few people don’t qualify for. We need to be very thoughtful when we change treatments. I look at the 3 factors of biochemical progression or response—PSA, radiographic progression, and clinical progression. If I don’t see 2 out of 3, I typically don’t change treatments. Then after enzalutamide or abiraterone, I wait until there are cancer-related symptoms before I consider chemotherapy and closely monitor my patients.
Imaging Modalities
Abhishek Solanki. Over the last few years the Hines VA Hospital has used fluciclovine positron emission tomography (PET), which is one of the novel imaging modalities for prostate cancer. Really the 2 novel imaging modalities that have gained the most excitement are prostate-specific membrane antigen (PSMA) PET and fluciclovine PET. Fluciclovine PET is based on a synthetic amino acid that’s taken up in multiple tissues, including prostate cancer. It has changed our practice in the localized setting for patients who have developed recurrence after radiation or radical prostatectomy. We have incorporated the scan into our workup of patients with recurrent disease, which can give us some more information at lower PSAs than historically we could get with CT, bone scan, or magnetic resonance imaging.
Our medical oncologists have started using it more and more as well. We are getting a lot of patients who have a negative CT or bone scan but have a positive fluciclovine PET. There are a few different disease settings where that becomes relevant. In patients who develop biochemical recurrence after radiation or salvage radiation after radical, we are finding that a lot of these patients who have no CT or bone scan findings of disease ultimately are found to have a PET-positive lesion. Sometimes it’s difficult to know how best to help patients with PET-only disease. Should you target the disease with an oligometastasis approach or just pursue systemic therapy or surveillance? It is challenging but more and more we are moving toward metastasis-directed therapy. There are multiple randomized trials in progress testing whether metastasis-directed therapy to the PET areas of recurrence can improve outcomes or delay systemic ADT. The STOMP trial randomized surveillance vs SBRT or surgery for patients with oligometastatic disease that showed improvement in biochemical control and ADT-free survival.13 However this was a small trial that tried to identify a signal. More definitive trials are necessary.
The other setting where we have found novel PET imaging to be helpful is in patients who have become castration resistant but don’t have clear metastases on conventional imaging. We’re identifying more patients who have only a few sites of progression, and we’ll pursue metastasis-directed therapy to those areas to try to get more mileage out of the systemic therapy that the patient is currently on and to try to avoid having to switch to the next line with the idea that, potentially, the progression site is just a limited clone that is progressing despite the current systemic therapy.
Mark Klein. I find that to be a very attractive approach. I’m assuming you do that for any systemic therapy where people have maybe 1 or 2 sites and they do not have a big PSA jump. Do you have a number of sites that you’re willing to radiate? And then, when you do that, what radiation fractionation and dosing do you use? Is there any observational data behind that for efficacy?
Abhishek Solanki. It is a patient by patient decision. Some patients, if they have a very rapid pace of progression shortly after starting systemic therapy and metastases have grown in several areas, we think that perhaps this person may benefit less from aggressive local therapy. But if it’s somebody who has been on systemic therapy for a while and has up to 3 sites of disease growth, we consider SBRT for oligoprogressive disease. Typically, we’ll use SBRT, which delivers a high dose of radiation over 3 to 5 treatments. With SBRT you can give a higher biologic dose and use more sophisticated treatment machines and image guidance for treatments to focus the radiation on the tumor area and limit exposure to normal tissue structures.
In prostate cancer to the primary site, we will typically do around 35 to 40 Gy in 5 fractions. For metastases, it depends on the site. If it’s in the lung, typically we will do 3 to 5 treatments, giving approximately 50 to 60 Gy in that course. In the spine, we use lower doses near the spinal cord and the cauda equina, typically about 30 Gy in 3 fractions. In the liver, similar to the lung, we’ll typically do 50-54 Gy in 3-5 fractions. There aren’t a lot of high-level data guiding the optimal dose/fractionation to metastases, but these are the doses we’ll use for various malignancies.
Treatment Options for Patients With Adverse Events
Mark Klein. I was just reviewing the 2004 study that randomized patients to mitoxantrone or docetaxel for up to 10 cycles.14,15 Who are good candidates for docetaxel after they have exhausted abiraterone and enzalutamide? How long do you hold to the 10-cycle rule, or do you go beyond that if they’re doing well? And if they’re not a good candidate, what are some options?
Julie Graff. The best candidates are those who are having a cancer-related AE, particularly pain, because docetaxel only improves survival over mitoxantrone by about 2.5 months. I don’t talk to patients about it as though it is a life extender, but it seems to help control pain—about 70% of patients benefited in terms of pain or some other cancer-related symptom.14
I have a lot of patients who say, “Never will I do chemotherapy.” I refer those patients to hospice, or if they’re appropriate for radium-223, I consider that. I typically give about 6 cycles of chemotherapy and then see how they’re doing. In some patients, the cancer just doesn’t respond to it.
I do tell patients about the papers that you mentioned, the 2 studies of docetaxel vs mitoxantrone where they use about 10 cycles, and some of my patients go all 10.14,15 Sometimes we have to stop because of neuropathy or some other AE. I believe in taking breaks and that you can probably start it later.
Elizabeth Hansen. I agree, our practice is similar. A lot of our patients are not very interested in chemotherapy. You have to take into consideration their ECOG (Eastern Cooperative Oncology Group) status, their goals, and quality of life when talking to them about these medications. And a lot of them tend to choose more of a palliative route. Depending on their AEs and how things are going, we will dose reduce, hold treatment, or give treatment holidays.
Mark Klein. If patients are progressing on docetaxel, what are options that people would use? Radium-223 certainly is available for patients with nonvisceral metastases, as well as cabazitaxel, mitoxantrone, estramustine and other older drugs.
Julie Graff. We have some clinical trials for patients postdocetaxel. We have the TRITON2 and TRITON3 studies open at the VA. (NCT02952534 and NCT02975934, respectively) A lot of patients would get a biopsy, and we’d look for a BRCA 1 or 2 and ATM mutation. For those patients who don’t have those mutations—and maybe 80% of them don’t—we talk about radium-223 for the patients without visceral metastases and bone pain. I have had a fair number of patients go on cabazitaxel, but I have not used mitoxantrone since cabazitaxel came out. It’s not off the table, but it hasn’t shown improvement in survival.
Elizabeth Hansen. One of our challenges, because we’re an ambulatory care center, is that we are unable to give radium-223 in house, and these services have to be sent out to a non-VA facility. It is doable, but it takes more legwork and organization on our part.
Julie Graff. We have not had radium-223, although we’re working to get that online. And we are physically connected to Oregon Health Science University (OHSU), so we send our patients there for radium. It is a pain because the doctors at OHSU don’t have CPRS access. I’m often in the middle of making sure the complete blood counts (CBCs) are sent to OHSU and to get my patients their treatments.
Mark Klein. The Minneapolis VAMC has radium-223 on site, and we have used it for patients whose cancer has progressed while on docetaxel without visceral metastases. Katie, have you had an opportunity to coordinate that care for patients?
Kathleen Nelson. Radium is administered at our facility by one of our nuclear medicine physicians. A complete blood count is checked at least 3 days prior to the infusion date but no sooner than 6 days. Due to the cost of the material, ordering without knowing the patient’s counts are within a safe range to administer is prohibitive. This adds an additional burden of 2 visits (lab with return visit) to the patient. We have treated 12 patients. Four patients stopped treatment prior to completing the 6 planned treatments citing debilitating fatigue and/or nonresolution of symptoms as their reason to stop treatment. One patient died. The 7 remaining patients subjectively reported varying degrees of pain relief.
Elizabeth Hansen. Another thing to mention is the lack of a PSA response from radium-223 as well. Patients are generally very diligent about monitoring their PSA, so this can be a bit distressing.
Mark Klein. Julie, have you noticed a PSA flare with radium-223? I know it has been reported.
Julie Graff. I haven’t. But I put little stock in PSAs in these patients. I spend 20 minutes explaining to patients that the PSA is not helpful in determining whether or not the radium is working. I tell them that the bone marker alkaline phosphatase may decrease. And I think it’s important to note, too, that radium-223 is not a treatment we have on the shelf. We order it from Denver I believe. It is weight based, and it takes 5 days to get.
Clinical Trials
Mark Klein. That leads us into clinical trials. What is the role for precision oncology in prostate cancer right now, specifically looking at particular panels? One would be the DNA repair enzyme-based genes and/or also the AR variants and any other markers.
Elizabeth Hansen. The National Comprehensive Cancer Network came out with a statement recommending germ-line and somatic-mutation testing in all patients with metastatic prostate cancer. This highlights the need to offer patients the availability of clinical trials.
Julie Graff. I agree. We occasionally get to a place in the disease where patients are feeling fine, but we don’t have anything else to offer. The studies by Robinson16 and then Matteo17 showed that (a) these DNA repair defects are present in about a quarter of patients; and (b) that PARP inhibitors can help these patients. At least it has an anticancer effect.
What’s interesting is that we have TRITON2, and TRITON3, which are sponsored by Clovis,for patients with BRCA 1/2 and ATM mutations and using the PARP-inhibitor rucaparib. Based on the data we have available, we thought a quarter of patients would have the mutation in the tumor, but they’re finding that it is more like 10% to 15%. They are screening many patients but not finding it.
I agree that clinical trials are the way to go. I am hopeful that we’ll get more treatments based on molecular markers. The approval for pembrolizumab in any tumor type with microsatellite instability is interesting, but in prostate cancer, I believe that’s about 3%. I haven’t seen anyone qualify for pembrolizumab based on that. Another plug for clinical trials: Let’s learn more and offer our patients potentially beneficial treatments earlier.
Mark Klein. The first interim analysis from the TRITON2 study found about 12% of patients had alterations in BRCA 1/2. But in those that met the RECIST criteria, they were able to have evaluable disease via that standard with about a 44% response rate so far and a 51% PSA response rate. It is promising data, but it’s only 85 patients so far. We’ll know more because the TRITON2 study is of a more pretreated population than the TRITION3 study at this point. Are there any data on precision medicine and radiation in prostate cancer?
Abhishek Solanki. In the prostate cancer setting, there are not a lot of emerging data specifically looking at using precision oncology biomarkers to help guide decisions in radiation therapy. For example, genomic classifiers, like GenomeDx Decipher (Vancouver, BC) and Myriad Genetics Prolaris (Salt Lake City, UT) are increasingly being utilized in patients with localized disease. Decipher can help predict the risk of recurrence after radical prostatectomy. The difficulty is that there are limited data that show that by using these genomic classifiers, one can improve outcomes in patients over traditional clinical characteristics.
There are 2 trials currently ongoing through NRG Oncology that are using Decipher. The GU002 is a trial for patients who had a radical prostatectomy and had a postoperative PSA that never nadired below 0.2. These patients are randomized between salvage radiation with hormone therapy with or without docetaxel. This trial is collecting Decipher results for patients enrolled in the study. The GU006 is a trial for a slightly more favorable group of patients who do nadir but still have biochemical recurrence and relatively low PSAs. This trial randomizes between radiotherapy alone and radiotherapy and 6 months of apalutamide, stratifying patients based on Decipher results, specially differentiating between patients who have a luminal vs basal subtype of prostate cancer. There are data that suggest that patients who have a luminal subtype may benefit more from the combination of radiation and hormone therapy vs patients who have basal subtype.18 However this hasn’t been validated in a prospective setting, and that’s what this trial will hopefully do.
Immunotherapies
Mark Klein. Outside of prostate cancer, there has been a lot of research trying to determine how to improve PD-L1 expression. Where are immunotherapy trials moving? How radiation might play a role in conjunction with immunotherapy.
Julie Graff. Two phase 3 studies did not show statistically improved survival or statistically significant survival improvement on ipilimumab, an immunotherapy agent that targets CTLA4. Some early studies of the PD-1 drugs nivolumab and pembrolizumab did not show much response with monotherapy. Despite the negative phase 3 studies for ipilimumab, we periodically see exceptional responses.
In prostate cancer, enzalutamide is FDA approved. And there’s currently a phase 3 study of the PD-L1 inhibitor atezolizumab plus enzalutamide in patients who have progressed on abiraterone. That trial is fully accrued, bu
I just received a Prostate Cancer Foundation Challenge Award to open a VA-only study looking at fecal microbiota transplant from responders to nonresponders to see how manipulating host factors can increase potential responses to PD-1 inhibition.
Abhishek Solanki. The classic mechanism by which radiation therapy works is direct DNA damage and indirect DNA damage through hydroxyl radicals that leads to cytotoxicity. But preclinical and clinical data suggest that radiation therapy can augment the local and systemic immunotherapy response. The radiation oncologist’s dream is what is called the abscopal effect, which is the idea that when you treat one site of disease with radiation, it can induce a response at other sites that didn’t get radiation therapy through reactivation of the immune system. I like to think of the abscopal effect like bigfoot—it’s elusive. However, it seems that the setting it is most likely to happen in is in combination with immunotherapy.
One of the ways that radiation fails locally is that it can upregulate PD-1 expression, and as a result, you can have progression of the tumor because of local immune suppression. We know that T cells are important for the activity of radiation therapy. If you combine checkpoint inhibition with radiation therapy, you can not only have better local control in the area of the tumor, but perhaps you can release tumor antigens that will then induce a systemic response.
The other potential mechanism by which radiation may work synergistically with immunotherapy is as a debulking agent. There are some data that suggest that the ratio of T-cell reinvigoration to bulk of disease, or the volume of tumor burden, is important. That is, having T-cell reinvigoration may not be sufficient to have a response to immunotherapy in patients with a large burden of disease. By using radiation to debulk disease, perhaps you could help make checkpoint inhibition more effective. Ultimately, in the setting of prostate cancer, there are not a lot of data yet showing meaningful benefits with the combination of immunotherapy and radiotherapy, but there are trials that are ongoing that will educate on potential synergy.
Pharmacy
Julie Graff. Before we end I want to make sure that we applaud the amazing pharmacists and patient care navigation teams in the VA who do such a great job of getting veterans the appropriate treatment expeditiously and keeping them safe. It’s something that is truly unique to the VA. And I want to thank the people on this call who do this every day.
Elizabeth Hansen. Thank you Julie. Compared with working in the community, at the VA I’m honestly amazed by the ease of access to these medications for our patients. Being able to deliver medications sometimes the same day to the patient is just not something that happens in the community. It’s nice to see that our veterans are getting cared for in that manner.
Author disclosures
Dr. Solanki participated in advisory boards for Blue Earth Diagnostics’ fluciclovine PET and was previously paid as a consultant. Dr. Graff is a consultant for Sanofi (docetaxel) and Astellas (enzalutamide), and has received research funding (no personal funding)from Sanofi, Merck (pembrolizumab), Astellas, and Jannsen (abiraterone, apalutamide). The other authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the US Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. James ND, de Bono JS, Spears MR, et al; STAMPEDE Investigators. Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med. 2017;377(4):338-351.
2. James ND, Sydes MR, Clarke NW, et al; STAMPEDE Investigators. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2017;387(10024):1163-1177.
3. Fizazi K, Tran N, Fein L, et al; LATITUDE Investigators. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med. 2017;377(4):352-360.
4. Kyriakopoulos CE, Chen YH, Carducci MA, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer: long-term survival analysis of the randomized Phase III E3805 CHAARTED trial. J Clin Oncol. 2018;36(11):1080-1087.
5. Tosoian JJ, Gorin MA, Ross AE, Pienta KJ, Tran PT, Schaeffer EM. Oligometastatic prostate cancer: definitions, clinical outcomes, and treatment considerations. Nat Rev Urol. 2017;14(1):15-25.
6. Parker CC, James ND, Brawley CD, et al; Systemic Therapy for Advanced or Metastatic Prostate cancer: Evaluation of Drug Efficacy (STAMPEDE) investigators. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial. Lancet. 2018;392(10162):2353-2366.
7. Sweeney CJ, Chen YH, Carducci M, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med. 2015;373(8):737-746.
8. Feyerabend S, Saad F, Li T, et al. Survival benefit, disease progression and quality-of-life outcomes of abiraterone acetate plus prednisone versus docetaxel in metastatic hormone-sensitive prostate cancer: a network meta-analysis. Eur J Cancer. 2018;103:78-87.
9. Sydes MR, Spears MR, Mason MD, et al; STAMPEDE Investigators. Adding abiraterone or docetaxel to long-term hormone therapy for prostate cancer: directly randomised data from the STAMPEDE multi-arm, multi-stage platform protocol. Ann Oncol. 2018;29(5):1235-1248.
10. Smith MR, Saad F, Chowdhury S, et al; SPARTAN Investigators. Apalutamide treatment and metastasis-free survival in prostate cancer. N Engl J Med. 2018;378(15):1408-1418.
11. Hussain M, Fizazi K, Saad F, et al. Enzalutamide in men with nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2018;378(26):2465-2474.
12. Smith MR, Kabbinavar F, Saad F, et al. Natural history of rising serum prostate-specific antigen in men with castrate nonmetastatic prostate cancer. J Clin Oncol. 2005;23(13):2918-2925.
13. Ost P, Reynders D, Decaestecker K, et al. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence: a prospective, randomized, multicenter phase II trial. J Clin Oncol. 2018;36(5):446-453.
14. Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351(15):1513-1520.
15. Tannock IF, de Wit R, Berry WR, et al; TAX 327 Investigators. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351(15):1502-1512.
16. Robinson D, Van Allen EM, Wu YM, et al. Integrative clinical genomics of advanced prostate cancer. Cell. 2015;161(5):1215-1228.
17. Mateo J, Carreira S, Sandhu S, et al. DNA-repair defects and olaparib in metastatic prostate cancer. N Engl J Med. 2015;373(18):1697-1708.
18. Zhao SG, Chang SL, Erho N, et al. Associations of luminal and basal subtyping of prostate cancer with prognosis and response to androgen deprivation therapy. JAMA Oncol. 2017;3(12):1663-1672.
1. James ND, de Bono JS, Spears MR, et al; STAMPEDE Investigators. Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med. 2017;377(4):338-351.
2. James ND, Sydes MR, Clarke NW, et al; STAMPEDE Investigators. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2017;387(10024):1163-1177.
3. Fizazi K, Tran N, Fein L, et al; LATITUDE Investigators. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med. 2017;377(4):352-360.
4. Kyriakopoulos CE, Chen YH, Carducci MA, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer: long-term survival analysis of the randomized Phase III E3805 CHAARTED trial. J Clin Oncol. 2018;36(11):1080-1087.
5. Tosoian JJ, Gorin MA, Ross AE, Pienta KJ, Tran PT, Schaeffer EM. Oligometastatic prostate cancer: definitions, clinical outcomes, and treatment considerations. Nat Rev Urol. 2017;14(1):15-25.
6. Parker CC, James ND, Brawley CD, et al; Systemic Therapy for Advanced or Metastatic Prostate cancer: Evaluation of Drug Efficacy (STAMPEDE) investigators. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial. Lancet. 2018;392(10162):2353-2366.
7. Sweeney CJ, Chen YH, Carducci M, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med. 2015;373(8):737-746.
8. Feyerabend S, Saad F, Li T, et al. Survival benefit, disease progression and quality-of-life outcomes of abiraterone acetate plus prednisone versus docetaxel in metastatic hormone-sensitive prostate cancer: a network meta-analysis. Eur J Cancer. 2018;103:78-87.
9. Sydes MR, Spears MR, Mason MD, et al; STAMPEDE Investigators. Adding abiraterone or docetaxel to long-term hormone therapy for prostate cancer: directly randomised data from the STAMPEDE multi-arm, multi-stage platform protocol. Ann Oncol. 2018;29(5):1235-1248.
10. Smith MR, Saad F, Chowdhury S, et al; SPARTAN Investigators. Apalutamide treatment and metastasis-free survival in prostate cancer. N Engl J Med. 2018;378(15):1408-1418.
11. Hussain M, Fizazi K, Saad F, et al. Enzalutamide in men with nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2018;378(26):2465-2474.
12. Smith MR, Kabbinavar F, Saad F, et al. Natural history of rising serum prostate-specific antigen in men with castrate nonmetastatic prostate cancer. J Clin Oncol. 2005;23(13):2918-2925.
13. Ost P, Reynders D, Decaestecker K, et al. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence: a prospective, randomized, multicenter phase II trial. J Clin Oncol. 2018;36(5):446-453.
14. Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351(15):1513-1520.
15. Tannock IF, de Wit R, Berry WR, et al; TAX 327 Investigators. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351(15):1502-1512.
16. Robinson D, Van Allen EM, Wu YM, et al. Integrative clinical genomics of advanced prostate cancer. Cell. 2015;161(5):1215-1228.
17. Mateo J, Carreira S, Sandhu S, et al. DNA-repair defects and olaparib in metastatic prostate cancer. N Engl J Med. 2015;373(18):1697-1708.
18. Zhao SG, Chang SL, Erho N, et al. Associations of luminal and basal subtyping of prostate cancer with prognosis and response to androgen deprivation therapy. JAMA Oncol. 2017;3(12):1663-1672.
Presentation of a Rare Malignancy: Leiomyosarcoma of the Prostate (FULL)
Prostatic leiomyosarcoma is an aggressive malignancy with a high risk of metastasis and a poor prognosis that poses unique diagnostic and treatment challenges.
Prostatic leiomyosarcoma is a rare tumor.1 This neoplasm is composed of highly aggressive prostatic smooth muscle cells that present with nonspecific signs and symptoms mimicking other forms of prostatic pathology. Of the primary prostatic sarcomas, leiomyosarcoma represents the most common subtype in adults and is found in 38% to 52% of newly diagnosed prostate sarcoma.1,2 The prognosis is poor, and no clear guidelines exist regarding the optimal treatment approach. We report a case of prostate leiomyosarcoma and describe the disease characteristics, diagnostic modalities, and treatment approach regarding these rare malignancies.
Case Presentation
A 72-year-old male presented with 6 months of progressive severe lower urinary tract symptoms (LUTS) secondary to bladder outlet obstruction. The patient was refractory to medical management with combination α-blocker and 5-α-reductase inhibitor therapy and continued to require multiple emergent bladder catheterizations. Workup with urinalysis, blood biochemistry, and prostate specific antigen (PSA) levels were persistently normal. He reported no hematuria, weight loss, or perineal pain. The patient reported no history of tobacco use, exposure to hazardous chemicals, and had no family history of genitourinary cancers. On rectal exam, the prostate was firm and nodular, with induration noted along the right upper lobe of the prostate.
The patient was referred for a urology consultation and subsequently underwent transurethral resection of the prostate (TURP) for suspected severe benign prostatic hypertrophy (BPH). A histopathologic examination demonstrated atypical cytology consistent with high- grade leiomyosarcoma. Immunohistochemical analysis revealed positive staining for vimentin, smooth muscle actin, desmin (partial), cytokeratin, smooth muscle myosin, muscle specific actin, and Ki-67 (50%-60% expression).
Fluorodeoxyglucose positron emission tomography (FDG-PET) scan revealed a 5.7 x 5.9 cm tumor with a maximum standardized uptake value (SUVmax) of 12.6 in the right posterior prostate, without evidence of metastatic disease (Figures 1A and 1B).
Discussion
Originating from prostatic interstitial cells, prostatic leiomyosarcoma is a rare tumor that accounts for < 0.1% of all primary prostatic malignancies.1 Since its first description in 1950 by Riba and colleagues, < 200 cases have been reported worldwide.2 Among the sarcomas of the prostate, it is the most common tumor, accounting for around 38% to 52% of prostate sarcoma presentations.1,2
Patients typically present between the ages of 41 and 78 years (mean age 61 years).2,3 Signs and symptoms at presentation may vary; however, the most common symptoms are related to lower urinary tract obstruction (89.4% of patients). These symptoms include urinary frequency, urgency, nocturia, and may mimic the presentation of BPH.
Symptoms commonly associated with other malignancies, including constitutional symptoms such as weight loss, tend to occur less frequently or may be absent. Perineal or rectal pain may only be present in 25.6% of patients. Hematuria, burning on ejaculation, and constitutional symptoms are a less common presentation (< 10% of patients).3,4 PSA levels typically do not rise and are found to be within normal limits. The lack of PSA elevation is related to the tumors nonepithelial origin and may contribute to a delay in diagnosis.2,4,5
Diagnosis
Diagnosis may be further eluded as digital rectal exam (DRE) findings tend to reveal nonspecific enlargement of the prostate, resembling that of BPH. DRE may show a hard and firm prostate with nodular induration at the base or over the lobes of the prostate.6 At this stage a urology consultation is useful, as diagnosis is most commonly achieved using transrectal ultrasound (TRUS) with ultrasound-guided needle biopsy or after a TURP procedure.3
Prostate sarcoma is associated with markedly enlarged prostate volume, irregular margins with invasion, or heterogenous hypoechoic lesions on TRUS.7 Transperineal biopsy, computed tomography (CT)-guided biopsy, or suprapubic prostatectomy have been less frequently employed for diagnosis in previously reported cases.8 Specialized imaging modalities, such as CT scan or bone scan, do not show any specific findings with regards to these tumors; their role is limited to evaluation of the local and distant metastasis and for follow-up assessments.9 Transabdominal ultrasound may assess hydronephrosis or enlarged prostate and its relation to nearby structures, although it has not been shown to be helpful in establishing a specific diagnosis.6
Histologically, prostatic leiomyosarcoma is a distinct subtype of prostatic sarcoma. Other subtypes include stromal tumors such as rhabdomyosarcoma, fibrosarcoma, and spindle cell sarcoma.2 The majority of leiomyosarcomas are high-grade lesions demonstrating neoplastic spindle cells with nuclear atypia, multifocal necrosis, and cystic degeneration. Low-grade leiomyosarcomas are very rare.10 Immunohistochemistry is characteristically positive for vimentin, smooth muscle actin, and desmin expression. Cytokeratin may be positive in up to 25% of cases, whereas S-100, CD34, CD117, and PSA are negative.2,3 These histopathological findings help to differentiate leiomyosarcoma from other prostatic tumors.
Tumor size may vary greatly, and measurements have been reported to range from 3 cm to 21 cm, frequently presenting with invasion of local structures.11 Advanced stage disease is commonly found at initial diagnosis and is thought to be due to the lack of early specific symptoms. Metastatic disease at presentation may be found in up to one-third of patients, with the lungs being the most common site of metastasis followed by the liver. Local extent and distant spread of disease may be determined by CT or magnetic resonance imaging (MRI) scans, which provide clear delineation of neoplastic and nonneoplastic tissues.
Treatment
Treatment regimens may include a multimodal approach of combination surgery, radiation, and chemotherapy. However, there are currently no standardized guidelines for treatment and the optimal therapy remains unknown.2,3,6 Surgery remains the mainstay of treatment, and patients with surgically resectable tumors are treated with curative intent. Surgeries performed include radical retropubic prostatectomy, radical cystoprostatectomy, suprapubic prostatectomy, and pelvic exenteration.2,5,8,12 These operations may be preceded or followed by radiation therapy and/or chemotherapy depending on extent of disease.
It has been reported that neo-adjuvant chemotherapy and/or radiotherapy can aid in decreasing tumor burden to facilitate a complete resection.2,8,13,14 Patients who are determined to not be candidates for surgery or whom have widespread disease may be offered systemic chemotherapy. Chemotherapy regimens vary, but common regimens include anthracyclines (doxorubicin or epirubicin), alkylating agents (cyclophosphamide, ifosfamide, dacarbazine), and/or vinca alkaloids (vinblastine or vincristine). Patients who do not receive surgical intervention rarely achieve a sustained remission.3,5,8
The long-term prognosis of prostatic leiomyosarcoma is poor due to the aggressive nature of the neoplasm and the high chance of disease recurrence or metastasis. Median survival is estimated at 17 months, and from 50% to 75% of patients die within 2 to 5 years of diagnosis.2,3 Prognosis may be improved in patients with localized disease at diagnosis who are candidates for complete surgical resection with negative margins.13 Adverse prognostic factors include metastatic disease at presentation and the presence of positive surgical margins after surgery.
Overall survival is very poor, and it is estimated that the 1-, 3-, and 5-year survival rates are 68%, 34%, and 26%, respectively.3 However, some studies estimate the 5-year survival to be anywhere from 0 to 60%.8,9 Due to the substantially high risk of death, prostatic leiomyosarcoma may be one of the most aggressive and poorly prognostic malignancies involving the prostate.
Conclusion
Prostatic leiomyosarcoma poses a unique diagnostic challenge, as clinical presentation alone may not always be suggestive of underlying malignancy. This challenge is further exacerbated by its aggressive nature, high risk of metastasis, and difficulties with unclear treatment. Proper history and physical examination, differential diagnosis, and a multidisciplinary approach to patient care are the foundation for early detection and promoting improved survival.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the US Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Miedler JD, MacLennan GT. Leiomyosarcoma of the prostate. J Urol. 2007;178(2):668.
2. Zazzara M, Divenuto L, Scarcia M, Cardo G, Maselli FP, Ludovico GM. Leiomyosarcoma of prostate: case report and literature review. Urol Case Rep. 2018;17:4-6.
3. Vandoros GP, Manolidis T, Karamouzis MV, et al. Leiomyosarcoma of the prostate: case report and review of 54 previously published cases. Sarcoma. 2008;2008:458709.
4. Talapatra K, Nemade B, Bhutani R, et al. Recurrent episodes of hematuria: a rare presentation of leiomyosarcoma of prostate. J Cancer Res Ther. 2006;2(4):212-214.
5. Cheville JC, Dundore PA, Nascimento AG, et al. Leiomyosarcoma of the prostate. Report of 23 cases. Cancer. 1995;76(8):1422-1427.
6. Venyo AK. A review of the literature on primary leiomyosarcoma of the prostate gland. Adv Urol. 2015;2015:485786.
7. Stilgenbauer R, Benedict M, Bamshad R, Viduetsky A. Sarcoma of the prostate: sonographic findings and pathologic correlation. J Ultrasound Med. 2007;26(12):1789-1793.
8. Sexton WJ, Lance RE, Reyes AO, Pisters PW, Tu SM, Pisters LL. Adult prostate sarcoma: the M.D. Anderson Cancer Center experience. J Urol. 2001;166(2):521-525.
9. Singh JP, Chakraborty D, Bera MK, Pal D. Leiomyosarcoma of prostate: a rare, aggressive tumor. J Cancer Res Ther. 2013;9(4):743-745.
10. Hansel DE, Herawi M, Montgomery E, Epstein JI. Spindle cell lesions of the adult prostate. Mod Pathol. 2007;20(1):148-158.
11. Punt SE, Eary JF, O'Sullivan J, Conrad EU. Fluorodeoxyglucose positron emission tomography in leiomyosarcoma: imaging characteristics. Nucl Med Commun. 2009;30(7):546-549.
12. Dotan ZA, Tal R, Golijanin D, et al. Adult genitourinary sarcoma: the 25-year Memorial Sloan-Kettering experience. J Urol. 2006;176(5):2033-2038.
13. Musser JE, Assel M, Mashni JW, Sjoberg DD, Russo P. Adult prostate sarcoma: the Memorial Sloan Kettering experience. Urology. 2014;84(3):624-628.
14. Janet NL, May AW, Akins RS. Sarcoma of the prostate: a single institutional review. Am J Clin Oncol. 2009;32:27-29
Prostatic leiomyosarcoma is an aggressive malignancy with a high risk of metastasis and a poor prognosis that poses unique diagnostic and treatment challenges.
Prostatic leiomyosarcoma is an aggressive malignancy with a high risk of metastasis and a poor prognosis that poses unique diagnostic and treatment challenges.
Prostatic leiomyosarcoma is a rare tumor.1 This neoplasm is composed of highly aggressive prostatic smooth muscle cells that present with nonspecific signs and symptoms mimicking other forms of prostatic pathology. Of the primary prostatic sarcomas, leiomyosarcoma represents the most common subtype in adults and is found in 38% to 52% of newly diagnosed prostate sarcoma.1,2 The prognosis is poor, and no clear guidelines exist regarding the optimal treatment approach. We report a case of prostate leiomyosarcoma and describe the disease characteristics, diagnostic modalities, and treatment approach regarding these rare malignancies.
Case Presentation
A 72-year-old male presented with 6 months of progressive severe lower urinary tract symptoms (LUTS) secondary to bladder outlet obstruction. The patient was refractory to medical management with combination α-blocker and 5-α-reductase inhibitor therapy and continued to require multiple emergent bladder catheterizations. Workup with urinalysis, blood biochemistry, and prostate specific antigen (PSA) levels were persistently normal. He reported no hematuria, weight loss, or perineal pain. The patient reported no history of tobacco use, exposure to hazardous chemicals, and had no family history of genitourinary cancers. On rectal exam, the prostate was firm and nodular, with induration noted along the right upper lobe of the prostate.
The patient was referred for a urology consultation and subsequently underwent transurethral resection of the prostate (TURP) for suspected severe benign prostatic hypertrophy (BPH). A histopathologic examination demonstrated atypical cytology consistent with high- grade leiomyosarcoma. Immunohistochemical analysis revealed positive staining for vimentin, smooth muscle actin, desmin (partial), cytokeratin, smooth muscle myosin, muscle specific actin, and Ki-67 (50%-60% expression).
Fluorodeoxyglucose positron emission tomography (FDG-PET) scan revealed a 5.7 x 5.9 cm tumor with a maximum standardized uptake value (SUVmax) of 12.6 in the right posterior prostate, without evidence of metastatic disease (Figures 1A and 1B).
Discussion
Originating from prostatic interstitial cells, prostatic leiomyosarcoma is a rare tumor that accounts for < 0.1% of all primary prostatic malignancies.1 Since its first description in 1950 by Riba and colleagues, < 200 cases have been reported worldwide.2 Among the sarcomas of the prostate, it is the most common tumor, accounting for around 38% to 52% of prostate sarcoma presentations.1,2
Patients typically present between the ages of 41 and 78 years (mean age 61 years).2,3 Signs and symptoms at presentation may vary; however, the most common symptoms are related to lower urinary tract obstruction (89.4% of patients). These symptoms include urinary frequency, urgency, nocturia, and may mimic the presentation of BPH.
Symptoms commonly associated with other malignancies, including constitutional symptoms such as weight loss, tend to occur less frequently or may be absent. Perineal or rectal pain may only be present in 25.6% of patients. Hematuria, burning on ejaculation, and constitutional symptoms are a less common presentation (< 10% of patients).3,4 PSA levels typically do not rise and are found to be within normal limits. The lack of PSA elevation is related to the tumors nonepithelial origin and may contribute to a delay in diagnosis.2,4,5
Diagnosis
Diagnosis may be further eluded as digital rectal exam (DRE) findings tend to reveal nonspecific enlargement of the prostate, resembling that of BPH. DRE may show a hard and firm prostate with nodular induration at the base or over the lobes of the prostate.6 At this stage a urology consultation is useful, as diagnosis is most commonly achieved using transrectal ultrasound (TRUS) with ultrasound-guided needle biopsy or after a TURP procedure.3
Prostate sarcoma is associated with markedly enlarged prostate volume, irregular margins with invasion, or heterogenous hypoechoic lesions on TRUS.7 Transperineal biopsy, computed tomography (CT)-guided biopsy, or suprapubic prostatectomy have been less frequently employed for diagnosis in previously reported cases.8 Specialized imaging modalities, such as CT scan or bone scan, do not show any specific findings with regards to these tumors; their role is limited to evaluation of the local and distant metastasis and for follow-up assessments.9 Transabdominal ultrasound may assess hydronephrosis or enlarged prostate and its relation to nearby structures, although it has not been shown to be helpful in establishing a specific diagnosis.6
Histologically, prostatic leiomyosarcoma is a distinct subtype of prostatic sarcoma. Other subtypes include stromal tumors such as rhabdomyosarcoma, fibrosarcoma, and spindle cell sarcoma.2 The majority of leiomyosarcomas are high-grade lesions demonstrating neoplastic spindle cells with nuclear atypia, multifocal necrosis, and cystic degeneration. Low-grade leiomyosarcomas are very rare.10 Immunohistochemistry is characteristically positive for vimentin, smooth muscle actin, and desmin expression. Cytokeratin may be positive in up to 25% of cases, whereas S-100, CD34, CD117, and PSA are negative.2,3 These histopathological findings help to differentiate leiomyosarcoma from other prostatic tumors.
Tumor size may vary greatly, and measurements have been reported to range from 3 cm to 21 cm, frequently presenting with invasion of local structures.11 Advanced stage disease is commonly found at initial diagnosis and is thought to be due to the lack of early specific symptoms. Metastatic disease at presentation may be found in up to one-third of patients, with the lungs being the most common site of metastasis followed by the liver. Local extent and distant spread of disease may be determined by CT or magnetic resonance imaging (MRI) scans, which provide clear delineation of neoplastic and nonneoplastic tissues.
Treatment
Treatment regimens may include a multimodal approach of combination surgery, radiation, and chemotherapy. However, there are currently no standardized guidelines for treatment and the optimal therapy remains unknown.2,3,6 Surgery remains the mainstay of treatment, and patients with surgically resectable tumors are treated with curative intent. Surgeries performed include radical retropubic prostatectomy, radical cystoprostatectomy, suprapubic prostatectomy, and pelvic exenteration.2,5,8,12 These operations may be preceded or followed by radiation therapy and/or chemotherapy depending on extent of disease.
It has been reported that neo-adjuvant chemotherapy and/or radiotherapy can aid in decreasing tumor burden to facilitate a complete resection.2,8,13,14 Patients who are determined to not be candidates for surgery or whom have widespread disease may be offered systemic chemotherapy. Chemotherapy regimens vary, but common regimens include anthracyclines (doxorubicin or epirubicin), alkylating agents (cyclophosphamide, ifosfamide, dacarbazine), and/or vinca alkaloids (vinblastine or vincristine). Patients who do not receive surgical intervention rarely achieve a sustained remission.3,5,8
The long-term prognosis of prostatic leiomyosarcoma is poor due to the aggressive nature of the neoplasm and the high chance of disease recurrence or metastasis. Median survival is estimated at 17 months, and from 50% to 75% of patients die within 2 to 5 years of diagnosis.2,3 Prognosis may be improved in patients with localized disease at diagnosis who are candidates for complete surgical resection with negative margins.13 Adverse prognostic factors include metastatic disease at presentation and the presence of positive surgical margins after surgery.
Overall survival is very poor, and it is estimated that the 1-, 3-, and 5-year survival rates are 68%, 34%, and 26%, respectively.3 However, some studies estimate the 5-year survival to be anywhere from 0 to 60%.8,9 Due to the substantially high risk of death, prostatic leiomyosarcoma may be one of the most aggressive and poorly prognostic malignancies involving the prostate.
Conclusion
Prostatic leiomyosarcoma poses a unique diagnostic challenge, as clinical presentation alone may not always be suggestive of underlying malignancy. This challenge is further exacerbated by its aggressive nature, high risk of metastasis, and difficulties with unclear treatment. Proper history and physical examination, differential diagnosis, and a multidisciplinary approach to patient care are the foundation for early detection and promoting improved survival.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the US Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Prostatic leiomyosarcoma is a rare tumor.1 This neoplasm is composed of highly aggressive prostatic smooth muscle cells that present with nonspecific signs and symptoms mimicking other forms of prostatic pathology. Of the primary prostatic sarcomas, leiomyosarcoma represents the most common subtype in adults and is found in 38% to 52% of newly diagnosed prostate sarcoma.1,2 The prognosis is poor, and no clear guidelines exist regarding the optimal treatment approach. We report a case of prostate leiomyosarcoma and describe the disease characteristics, diagnostic modalities, and treatment approach regarding these rare malignancies.
Case Presentation
A 72-year-old male presented with 6 months of progressive severe lower urinary tract symptoms (LUTS) secondary to bladder outlet obstruction. The patient was refractory to medical management with combination α-blocker and 5-α-reductase inhibitor therapy and continued to require multiple emergent bladder catheterizations. Workup with urinalysis, blood biochemistry, and prostate specific antigen (PSA) levels were persistently normal. He reported no hematuria, weight loss, or perineal pain. The patient reported no history of tobacco use, exposure to hazardous chemicals, and had no family history of genitourinary cancers. On rectal exam, the prostate was firm and nodular, with induration noted along the right upper lobe of the prostate.
The patient was referred for a urology consultation and subsequently underwent transurethral resection of the prostate (TURP) for suspected severe benign prostatic hypertrophy (BPH). A histopathologic examination demonstrated atypical cytology consistent with high- grade leiomyosarcoma. Immunohistochemical analysis revealed positive staining for vimentin, smooth muscle actin, desmin (partial), cytokeratin, smooth muscle myosin, muscle specific actin, and Ki-67 (50%-60% expression).
Fluorodeoxyglucose positron emission tomography (FDG-PET) scan revealed a 5.7 x 5.9 cm tumor with a maximum standardized uptake value (SUVmax) of 12.6 in the right posterior prostate, without evidence of metastatic disease (Figures 1A and 1B).
Discussion
Originating from prostatic interstitial cells, prostatic leiomyosarcoma is a rare tumor that accounts for < 0.1% of all primary prostatic malignancies.1 Since its first description in 1950 by Riba and colleagues, < 200 cases have been reported worldwide.2 Among the sarcomas of the prostate, it is the most common tumor, accounting for around 38% to 52% of prostate sarcoma presentations.1,2
Patients typically present between the ages of 41 and 78 years (mean age 61 years).2,3 Signs and symptoms at presentation may vary; however, the most common symptoms are related to lower urinary tract obstruction (89.4% of patients). These symptoms include urinary frequency, urgency, nocturia, and may mimic the presentation of BPH.
Symptoms commonly associated with other malignancies, including constitutional symptoms such as weight loss, tend to occur less frequently or may be absent. Perineal or rectal pain may only be present in 25.6% of patients. Hematuria, burning on ejaculation, and constitutional symptoms are a less common presentation (< 10% of patients).3,4 PSA levels typically do not rise and are found to be within normal limits. The lack of PSA elevation is related to the tumors nonepithelial origin and may contribute to a delay in diagnosis.2,4,5
Diagnosis
Diagnosis may be further eluded as digital rectal exam (DRE) findings tend to reveal nonspecific enlargement of the prostate, resembling that of BPH. DRE may show a hard and firm prostate with nodular induration at the base or over the lobes of the prostate.6 At this stage a urology consultation is useful, as diagnosis is most commonly achieved using transrectal ultrasound (TRUS) with ultrasound-guided needle biopsy or after a TURP procedure.3
Prostate sarcoma is associated with markedly enlarged prostate volume, irregular margins with invasion, or heterogenous hypoechoic lesions on TRUS.7 Transperineal biopsy, computed tomography (CT)-guided biopsy, or suprapubic prostatectomy have been less frequently employed for diagnosis in previously reported cases.8 Specialized imaging modalities, such as CT scan or bone scan, do not show any specific findings with regards to these tumors; their role is limited to evaluation of the local and distant metastasis and for follow-up assessments.9 Transabdominal ultrasound may assess hydronephrosis or enlarged prostate and its relation to nearby structures, although it has not been shown to be helpful in establishing a specific diagnosis.6
Histologically, prostatic leiomyosarcoma is a distinct subtype of prostatic sarcoma. Other subtypes include stromal tumors such as rhabdomyosarcoma, fibrosarcoma, and spindle cell sarcoma.2 The majority of leiomyosarcomas are high-grade lesions demonstrating neoplastic spindle cells with nuclear atypia, multifocal necrosis, and cystic degeneration. Low-grade leiomyosarcomas are very rare.10 Immunohistochemistry is characteristically positive for vimentin, smooth muscle actin, and desmin expression. Cytokeratin may be positive in up to 25% of cases, whereas S-100, CD34, CD117, and PSA are negative.2,3 These histopathological findings help to differentiate leiomyosarcoma from other prostatic tumors.
Tumor size may vary greatly, and measurements have been reported to range from 3 cm to 21 cm, frequently presenting with invasion of local structures.11 Advanced stage disease is commonly found at initial diagnosis and is thought to be due to the lack of early specific symptoms. Metastatic disease at presentation may be found in up to one-third of patients, with the lungs being the most common site of metastasis followed by the liver. Local extent and distant spread of disease may be determined by CT or magnetic resonance imaging (MRI) scans, which provide clear delineation of neoplastic and nonneoplastic tissues.
Treatment
Treatment regimens may include a multimodal approach of combination surgery, radiation, and chemotherapy. However, there are currently no standardized guidelines for treatment and the optimal therapy remains unknown.2,3,6 Surgery remains the mainstay of treatment, and patients with surgically resectable tumors are treated with curative intent. Surgeries performed include radical retropubic prostatectomy, radical cystoprostatectomy, suprapubic prostatectomy, and pelvic exenteration.2,5,8,12 These operations may be preceded or followed by radiation therapy and/or chemotherapy depending on extent of disease.
It has been reported that neo-adjuvant chemotherapy and/or radiotherapy can aid in decreasing tumor burden to facilitate a complete resection.2,8,13,14 Patients who are determined to not be candidates for surgery or whom have widespread disease may be offered systemic chemotherapy. Chemotherapy regimens vary, but common regimens include anthracyclines (doxorubicin or epirubicin), alkylating agents (cyclophosphamide, ifosfamide, dacarbazine), and/or vinca alkaloids (vinblastine or vincristine). Patients who do not receive surgical intervention rarely achieve a sustained remission.3,5,8
The long-term prognosis of prostatic leiomyosarcoma is poor due to the aggressive nature of the neoplasm and the high chance of disease recurrence or metastasis. Median survival is estimated at 17 months, and from 50% to 75% of patients die within 2 to 5 years of diagnosis.2,3 Prognosis may be improved in patients with localized disease at diagnosis who are candidates for complete surgical resection with negative margins.13 Adverse prognostic factors include metastatic disease at presentation and the presence of positive surgical margins after surgery.
Overall survival is very poor, and it is estimated that the 1-, 3-, and 5-year survival rates are 68%, 34%, and 26%, respectively.3 However, some studies estimate the 5-year survival to be anywhere from 0 to 60%.8,9 Due to the substantially high risk of death, prostatic leiomyosarcoma may be one of the most aggressive and poorly prognostic malignancies involving the prostate.
Conclusion
Prostatic leiomyosarcoma poses a unique diagnostic challenge, as clinical presentation alone may not always be suggestive of underlying malignancy. This challenge is further exacerbated by its aggressive nature, high risk of metastasis, and difficulties with unclear treatment. Proper history and physical examination, differential diagnosis, and a multidisciplinary approach to patient care are the foundation for early detection and promoting improved survival.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the US Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Miedler JD, MacLennan GT. Leiomyosarcoma of the prostate. J Urol. 2007;178(2):668.
2. Zazzara M, Divenuto L, Scarcia M, Cardo G, Maselli FP, Ludovico GM. Leiomyosarcoma of prostate: case report and literature review. Urol Case Rep. 2018;17:4-6.
3. Vandoros GP, Manolidis T, Karamouzis MV, et al. Leiomyosarcoma of the prostate: case report and review of 54 previously published cases. Sarcoma. 2008;2008:458709.
4. Talapatra K, Nemade B, Bhutani R, et al. Recurrent episodes of hematuria: a rare presentation of leiomyosarcoma of prostate. J Cancer Res Ther. 2006;2(4):212-214.
5. Cheville JC, Dundore PA, Nascimento AG, et al. Leiomyosarcoma of the prostate. Report of 23 cases. Cancer. 1995;76(8):1422-1427.
6. Venyo AK. A review of the literature on primary leiomyosarcoma of the prostate gland. Adv Urol. 2015;2015:485786.
7. Stilgenbauer R, Benedict M, Bamshad R, Viduetsky A. Sarcoma of the prostate: sonographic findings and pathologic correlation. J Ultrasound Med. 2007;26(12):1789-1793.
8. Sexton WJ, Lance RE, Reyes AO, Pisters PW, Tu SM, Pisters LL. Adult prostate sarcoma: the M.D. Anderson Cancer Center experience. J Urol. 2001;166(2):521-525.
9. Singh JP, Chakraborty D, Bera MK, Pal D. Leiomyosarcoma of prostate: a rare, aggressive tumor. J Cancer Res Ther. 2013;9(4):743-745.
10. Hansel DE, Herawi M, Montgomery E, Epstein JI. Spindle cell lesions of the adult prostate. Mod Pathol. 2007;20(1):148-158.
11. Punt SE, Eary JF, O'Sullivan J, Conrad EU. Fluorodeoxyglucose positron emission tomography in leiomyosarcoma: imaging characteristics. Nucl Med Commun. 2009;30(7):546-549.
12. Dotan ZA, Tal R, Golijanin D, et al. Adult genitourinary sarcoma: the 25-year Memorial Sloan-Kettering experience. J Urol. 2006;176(5):2033-2038.
13. Musser JE, Assel M, Mashni JW, Sjoberg DD, Russo P. Adult prostate sarcoma: the Memorial Sloan Kettering experience. Urology. 2014;84(3):624-628.
14. Janet NL, May AW, Akins RS. Sarcoma of the prostate: a single institutional review. Am J Clin Oncol. 2009;32:27-29
1. Miedler JD, MacLennan GT. Leiomyosarcoma of the prostate. J Urol. 2007;178(2):668.
2. Zazzara M, Divenuto L, Scarcia M, Cardo G, Maselli FP, Ludovico GM. Leiomyosarcoma of prostate: case report and literature review. Urol Case Rep. 2018;17:4-6.
3. Vandoros GP, Manolidis T, Karamouzis MV, et al. Leiomyosarcoma of the prostate: case report and review of 54 previously published cases. Sarcoma. 2008;2008:458709.
4. Talapatra K, Nemade B, Bhutani R, et al. Recurrent episodes of hematuria: a rare presentation of leiomyosarcoma of prostate. J Cancer Res Ther. 2006;2(4):212-214.
5. Cheville JC, Dundore PA, Nascimento AG, et al. Leiomyosarcoma of the prostate. Report of 23 cases. Cancer. 1995;76(8):1422-1427.
6. Venyo AK. A review of the literature on primary leiomyosarcoma of the prostate gland. Adv Urol. 2015;2015:485786.
7. Stilgenbauer R, Benedict M, Bamshad R, Viduetsky A. Sarcoma of the prostate: sonographic findings and pathologic correlation. J Ultrasound Med. 2007;26(12):1789-1793.
8. Sexton WJ, Lance RE, Reyes AO, Pisters PW, Tu SM, Pisters LL. Adult prostate sarcoma: the M.D. Anderson Cancer Center experience. J Urol. 2001;166(2):521-525.
9. Singh JP, Chakraborty D, Bera MK, Pal D. Leiomyosarcoma of prostate: a rare, aggressive tumor. J Cancer Res Ther. 2013;9(4):743-745.
10. Hansel DE, Herawi M, Montgomery E, Epstein JI. Spindle cell lesions of the adult prostate. Mod Pathol. 2007;20(1):148-158.
11. Punt SE, Eary JF, O'Sullivan J, Conrad EU. Fluorodeoxyglucose positron emission tomography in leiomyosarcoma: imaging characteristics. Nucl Med Commun. 2009;30(7):546-549.
12. Dotan ZA, Tal R, Golijanin D, et al. Adult genitourinary sarcoma: the 25-year Memorial Sloan-Kettering experience. J Urol. 2006;176(5):2033-2038.
13. Musser JE, Assel M, Mashni JW, Sjoberg DD, Russo P. Adult prostate sarcoma: the Memorial Sloan Kettering experience. Urology. 2014;84(3):624-628.
14. Janet NL, May AW, Akins RS. Sarcoma of the prostate: a single institutional review. Am J Clin Oncol. 2009;32:27-29
Primary Urethral Carcinoma With Nodal Metastasis (FULL)
The presentation of a fungating penile mass often indicates penile carcinoma, but providers should be aware of urethral carcinoma in the differential diagnosis.
Primary urethral carcinoma (PUC) is a rare but morbid disease, representing < 1% of all urologic malignancies.1 Up to one-third of male patients may present with nodal metastases.2-4 The overall survival (OS) for all male PUC is < 50% at 5 years and is lower still in patients with nodal involvement.4
Although surgical intervention, including radical resection, has been a mainstay in disease management, the presence of high-stage disease may warrant multimodal treatment with chemotherapy, radiation, and surgery. Recent series have described success with neoadjuvant and adjuvant chemoradiation, yet the optimal regimen remains unestablished.5,6 Although nodal disease is commonly encountered with proximal, high-stage tumors, this case exhibits a rare presentation of a distal fungating penile mass with low pathologic stage but rapid progression to nodal disease.
Case Presentation
A male veteran aged 77 years with a history of diabetes mellitus and stroke presented with obstructive urinary symptoms, gross hematuria, and 15-pound weight loss. Examination revealed a distal penile mass with purulent exudate at the meatus but no inguinal lymphadenopathy. Two fragments of this mass detached during office cystoscopy, and pathology revealed high-grade urothelial cell carcinoma (UCC). A magnetic resonance image of the pelvis with and without IV contrast revealed a 2.4-cm tumor in the glans penis with possible extension into the subcutaneous connective tissue of the penis and penile skin, without invasion of the corpora cavernosa/spongiosum or lymphadenopathy (Figure 1).
Prostatic urethral and random bladder biopsies, bilateral retrograde pyelograms, and selective ureteral washings revealed no abnormalities or signs of disease. Percutaneous biopsy of the inguinal node confirmed metastatic UCC. The patient underwent radical penectomy, creation of a perineal urethrostomy, and suprapubic cystostomy tube placement. Negative margins were confirmed on the urethral stump and corpus spongiosum. Final pathology revealed high-grade UCC with squamous differentiation on hematoxylin and eosin staining, arising from the penile urethra, invading the glans and corpus spongiosum, with no invasion of the corpus cavernosa (Figures 3 and 4).
Immunohistochemical stains were performed and strongly positive for cytokeratin 7 and p63. Final pathologic stage was described as pT2N1, with negative margins, indicating an American Joint Committee on Cancer classification of Stage III disease.7 The patient was referred postoperatively for adjuvant chemoradiation.
Discussion
The low incidence of PUC, coupled with a high morbidity/mortality rate, creates a difficult scenario in choosing the best oncologic management for this disease. National guidelines stratify treatment algorithms by stage and location of primary tumor, as these were found to be the 2 most important prognostic factors for men.1 The location of the primary tumor is most often in the bulbomembranous urethra, but up to one-third occur in the pendulous urethra.2
A recent review reported that UCC is the most common histologic subtype.4 When considering the differential diagnosis, a distal penile mass may represent a malignant penile lesion, such as squamous cell carcinoma, Buschke-Lowenstein tumor, Kaposi sarcoma, or precancerous lesions. Additional benign and infectious disorders include epidermoid and retention cysts, leukoplakia, balanitis xerotica obliterans, condyloma acuminatum, chancre/chancroid, lymphogranuloma venereum, granuloma inguinale, and tuberculosis. Clinical workup typically includes physical examination, cystourethroscopy and biopsy, chest X-ray, and pelvic/abdominal cross-sectional imaging.9,10 Magnetic resonance imaging of the abdomen and pelvis is ideal in identifying soft tissue structures and extension of tumor.
In male patients with PUC, nodal metastases are commonly seen at initial presentation in up to one-third of patients, while distant metastases may be present in up to 6% at presentation.2-4 When tumors arise from the anterior urethra, the primary lymphatic drainage is first to the inguinal lymph nodes, whereas posterior tumors drain to the pelvic lymph nodes. A multivariate analysis of men with PUC within the Surveillance, Epidemiology, and End Results database demonstrated an OS across all stages to be 46.2% and 29.3% at 5 and 10 years, respectively. Increased likelihood of death was predicted by advanced age, high grade/stage, systemic metastases, non-UCC histology, and the lack of surgery.4
Surgical intervention, including radical resection via penectomy, has been the mainstay in disease management and was first described by Marshall in 1957 for bulbar urethral cancer.11 In 1998, Gheiler and colleagues demonstrated that surgical resection alone yielded excellent outcomes in patients with low-stage disease with 89% of patients disease free at mean 42 months. This was in stark contrast to patients with advanced stage disease (T3 or N+) who exhibited a disease-free survival rate of 42% at the same follow-up interval and benefited from combined chemoradiation and surgical resection.3
In the presence of high-stage disease, multimodal therapy with chemotherapy, radiation, and/or surgery is warranted. A study in 2008 reviewed chemoradiation in which patients with PUC received a 5-week protocol of external beam radiotherapy to the genitals, inguinal/pelvic lymph nodes, plus an additional radiation bolus to the primary tumor.5 In the 18 patients reported, 15 had complete response to therapy, and only 4 patients required salvage surgical resection. The 7-year survival for the cohort was 72% with chemoradiation alone, with about half the population recurring or progressing at 7 years. However, all patients that avoided surgical resection went on to develop urethral strictures that required surgical therapy, 3 of which required complex reconstructive procedures.
To place this survival into context, the 1999 study by Dalbagni and colleagues reported a 5-year OS of 42% when surgical resection alone was performed in 40/46 men with PUC.2 Last, a large retrospective series of 44 patients reported mostly advanced-stage patients with PUC and analyzed patients treated with chemotherapy based on histologic pathology. The results demonstrated a 72% overall response rate to neoadjuvant chemotherapy, with a median OS of 32 months in patients undergoing chemotherapy vs 46 months in patients who underwent subsequent surgery. This study solidified that for patients with PUC involving the lymph nodes; optimal treatment includes neoadjuvant cisplatin-based chemotherapy followed by surgical resection.6
As medicine and oncologic therapies become more individualized, physicians are looking to new immunologic agents for systemic therapy. Immune checkpoint inhibitors were approved by the US Food and Drug Administration for UCC of the bladder in 2016.12 Unfortunately, due to the rarity of PUC and the recent development of immune checkpoint inhibitors, there have been no published reports of these or other immunotherapies in PUC. However, given the histologic similarity and pathogenesis, checkpoint inhibitors may have a future indication in the systemic management of this disease.
Conclusion
This patient’s PUC represents a rare presentation of a distal urethral carcinoma, T2-staged tumor, with rapid progression to nodal metastases. Additionally, the presentation of a fungating penile mass would usually indicate penile carcinoma, but providers should be aware of urethral carcinoma in the differential diagnosis. Notably, the patient was found to have progression to lymph node involvement during a mere 2-month period.
Recent case series have published encouraging results with neoadjuvant chemotherapy or chemoradiation.5,6 However, radical resection in men with T2 to T4 disease is associated with significantly higher cancer-specific survival. Given our concern of a loss to follow-up, we felt that radical resection of the primary tumor and adjuvant chemoradiation represented the patient’s best oncologic outcomes. Therefore, he underwent radical penectomy and creation of a perineal urethrostomy. As of his 6-month follow-up, he showed no evidence of disease, had returned to his preoperative functional status, and was referred for chemoradiation.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the US Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Swartz MA, Porter MP, Lin DW, Weiss NS. Incidence of primary urethral carcinoma in the United States. Urology. 2006;68(6):1164-1168.
2. Dalbagni G, Zhang ZF, Lacombe L, Herr HW. Male urethral carcinoma: analysis of treatment outcome. Urology. 1999;53(6):1126-1132.
3. Gheiler EL, Tefilli MV, Tiguert R, de Oliveira JG, Pontes JE, Wood DP Jr. Management of primary urethral cancer. Urology. 1998;52(3):487-493.
4. Rabbani F. Prognostic factors in male urethral cancer. Cancer. 2011;117(11):2426-2434.
5. Cohen MS, Triaca V, Billmeyer B, et al. Coordinated chemoradiation therapy with genital preservation for the treatment of primary invasive carcinoma of the male urethra. J Urol. 2008;179(2):536-541; discussion 541.
6. Dayyani F, Pettaway CA, Kamat AM, Munsell MF, Sircar K, Pagliaro LC. Retrospective analysis of survival outcomes and the role of cisplatin-based chemotherapy in patients with urethral carcinomas referred to medical oncologists. Urol Oncol. 2013;31(7):1171-1177.
7. American Joint Committee on Cancer. AJCC cancer staging manual. 8th ed. https://cancerstaging.org/references-tools/deskreferences/Documents/AJCC%20Cancer%20Staging%20Form%20Supplement.pdf. Updated June 5, 2018. Accessed January 22, 2019.
8. Gakis G, Witjes JA, Compérat E, et al. European Association of Urology guidelines on primary urethral carcinoma. https://uroweb.org/wp-content/uploads/EAU-Guidelines-Primary-Urethral-Carcinoma-2016-1.pdf. Updated March 2015. Accessed January 22, 2019
9. National Comprehensive Cancer Network. Bladder Cancer. Version 1.2019. https://www.nccn.org/professionals/physician_gls/pdf/bladder.pdf. Updated December 20, 2018. Accessed January 17, 2019.
10. Dayyani F, Hoffman K, Eifel P, et al. Management of advanced primary urethral carcinomas. BJU Int. 2014;114(1):25-31.
11. Marshall VF. Radical excision of locally extensive carcinoma of the deep male urethra. J Urol. 1957;78(3):252-264.
12. Hsu FS, Su CH, Huang KH. A comprehensive review of US FDA-approved immune checkpoint inhibitors in urothelial carcinoma. J Immunol Res. 2017;2017:6940546.
The presentation of a fungating penile mass often indicates penile carcinoma, but providers should be aware of urethral carcinoma in the differential diagnosis.
The presentation of a fungating penile mass often indicates penile carcinoma, but providers should be aware of urethral carcinoma in the differential diagnosis.
Primary urethral carcinoma (PUC) is a rare but morbid disease, representing < 1% of all urologic malignancies.1 Up to one-third of male patients may present with nodal metastases.2-4 The overall survival (OS) for all male PUC is < 50% at 5 years and is lower still in patients with nodal involvement.4
Although surgical intervention, including radical resection, has been a mainstay in disease management, the presence of high-stage disease may warrant multimodal treatment with chemotherapy, radiation, and surgery. Recent series have described success with neoadjuvant and adjuvant chemoradiation, yet the optimal regimen remains unestablished.5,6 Although nodal disease is commonly encountered with proximal, high-stage tumors, this case exhibits a rare presentation of a distal fungating penile mass with low pathologic stage but rapid progression to nodal disease.
Case Presentation
A male veteran aged 77 years with a history of diabetes mellitus and stroke presented with obstructive urinary symptoms, gross hematuria, and 15-pound weight loss. Examination revealed a distal penile mass with purulent exudate at the meatus but no inguinal lymphadenopathy. Two fragments of this mass detached during office cystoscopy, and pathology revealed high-grade urothelial cell carcinoma (UCC). A magnetic resonance image of the pelvis with and without IV contrast revealed a 2.4-cm tumor in the glans penis with possible extension into the subcutaneous connective tissue of the penis and penile skin, without invasion of the corpora cavernosa/spongiosum or lymphadenopathy (Figure 1).
Prostatic urethral and random bladder biopsies, bilateral retrograde pyelograms, and selective ureteral washings revealed no abnormalities or signs of disease. Percutaneous biopsy of the inguinal node confirmed metastatic UCC. The patient underwent radical penectomy, creation of a perineal urethrostomy, and suprapubic cystostomy tube placement. Negative margins were confirmed on the urethral stump and corpus spongiosum. Final pathology revealed high-grade UCC with squamous differentiation on hematoxylin and eosin staining, arising from the penile urethra, invading the glans and corpus spongiosum, with no invasion of the corpus cavernosa (Figures 3 and 4).
Immunohistochemical stains were performed and strongly positive for cytokeratin 7 and p63. Final pathologic stage was described as pT2N1, with negative margins, indicating an American Joint Committee on Cancer classification of Stage III disease.7 The patient was referred postoperatively for adjuvant chemoradiation.
Discussion
The low incidence of PUC, coupled with a high morbidity/mortality rate, creates a difficult scenario in choosing the best oncologic management for this disease. National guidelines stratify treatment algorithms by stage and location of primary tumor, as these were found to be the 2 most important prognostic factors for men.1 The location of the primary tumor is most often in the bulbomembranous urethra, but up to one-third occur in the pendulous urethra.2
A recent review reported that UCC is the most common histologic subtype.4 When considering the differential diagnosis, a distal penile mass may represent a malignant penile lesion, such as squamous cell carcinoma, Buschke-Lowenstein tumor, Kaposi sarcoma, or precancerous lesions. Additional benign and infectious disorders include epidermoid and retention cysts, leukoplakia, balanitis xerotica obliterans, condyloma acuminatum, chancre/chancroid, lymphogranuloma venereum, granuloma inguinale, and tuberculosis. Clinical workup typically includes physical examination, cystourethroscopy and biopsy, chest X-ray, and pelvic/abdominal cross-sectional imaging.9,10 Magnetic resonance imaging of the abdomen and pelvis is ideal in identifying soft tissue structures and extension of tumor.
In male patients with PUC, nodal metastases are commonly seen at initial presentation in up to one-third of patients, while distant metastases may be present in up to 6% at presentation.2-4 When tumors arise from the anterior urethra, the primary lymphatic drainage is first to the inguinal lymph nodes, whereas posterior tumors drain to the pelvic lymph nodes. A multivariate analysis of men with PUC within the Surveillance, Epidemiology, and End Results database demonstrated an OS across all stages to be 46.2% and 29.3% at 5 and 10 years, respectively. Increased likelihood of death was predicted by advanced age, high grade/stage, systemic metastases, non-UCC histology, and the lack of surgery.4
Surgical intervention, including radical resection via penectomy, has been the mainstay in disease management and was first described by Marshall in 1957 for bulbar urethral cancer.11 In 1998, Gheiler and colleagues demonstrated that surgical resection alone yielded excellent outcomes in patients with low-stage disease with 89% of patients disease free at mean 42 months. This was in stark contrast to patients with advanced stage disease (T3 or N+) who exhibited a disease-free survival rate of 42% at the same follow-up interval and benefited from combined chemoradiation and surgical resection.3
In the presence of high-stage disease, multimodal therapy with chemotherapy, radiation, and/or surgery is warranted. A study in 2008 reviewed chemoradiation in which patients with PUC received a 5-week protocol of external beam radiotherapy to the genitals, inguinal/pelvic lymph nodes, plus an additional radiation bolus to the primary tumor.5 In the 18 patients reported, 15 had complete response to therapy, and only 4 patients required salvage surgical resection. The 7-year survival for the cohort was 72% with chemoradiation alone, with about half the population recurring or progressing at 7 years. However, all patients that avoided surgical resection went on to develop urethral strictures that required surgical therapy, 3 of which required complex reconstructive procedures.
To place this survival into context, the 1999 study by Dalbagni and colleagues reported a 5-year OS of 42% when surgical resection alone was performed in 40/46 men with PUC.2 Last, a large retrospective series of 44 patients reported mostly advanced-stage patients with PUC and analyzed patients treated with chemotherapy based on histologic pathology. The results demonstrated a 72% overall response rate to neoadjuvant chemotherapy, with a median OS of 32 months in patients undergoing chemotherapy vs 46 months in patients who underwent subsequent surgery. This study solidified that for patients with PUC involving the lymph nodes; optimal treatment includes neoadjuvant cisplatin-based chemotherapy followed by surgical resection.6
As medicine and oncologic therapies become more individualized, physicians are looking to new immunologic agents for systemic therapy. Immune checkpoint inhibitors were approved by the US Food and Drug Administration for UCC of the bladder in 2016.12 Unfortunately, due to the rarity of PUC and the recent development of immune checkpoint inhibitors, there have been no published reports of these or other immunotherapies in PUC. However, given the histologic similarity and pathogenesis, checkpoint inhibitors may have a future indication in the systemic management of this disease.
Conclusion
This patient’s PUC represents a rare presentation of a distal urethral carcinoma, T2-staged tumor, with rapid progression to nodal metastases. Additionally, the presentation of a fungating penile mass would usually indicate penile carcinoma, but providers should be aware of urethral carcinoma in the differential diagnosis. Notably, the patient was found to have progression to lymph node involvement during a mere 2-month period.
Recent case series have published encouraging results with neoadjuvant chemotherapy or chemoradiation.5,6 However, radical resection in men with T2 to T4 disease is associated with significantly higher cancer-specific survival. Given our concern of a loss to follow-up, we felt that radical resection of the primary tumor and adjuvant chemoradiation represented the patient’s best oncologic outcomes. Therefore, he underwent radical penectomy and creation of a perineal urethrostomy. As of his 6-month follow-up, he showed no evidence of disease, had returned to his preoperative functional status, and was referred for chemoradiation.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the US Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Primary urethral carcinoma (PUC) is a rare but morbid disease, representing < 1% of all urologic malignancies.1 Up to one-third of male patients may present with nodal metastases.2-4 The overall survival (OS) for all male PUC is < 50% at 5 years and is lower still in patients with nodal involvement.4
Although surgical intervention, including radical resection, has been a mainstay in disease management, the presence of high-stage disease may warrant multimodal treatment with chemotherapy, radiation, and surgery. Recent series have described success with neoadjuvant and adjuvant chemoradiation, yet the optimal regimen remains unestablished.5,6 Although nodal disease is commonly encountered with proximal, high-stage tumors, this case exhibits a rare presentation of a distal fungating penile mass with low pathologic stage but rapid progression to nodal disease.
Case Presentation
A male veteran aged 77 years with a history of diabetes mellitus and stroke presented with obstructive urinary symptoms, gross hematuria, and 15-pound weight loss. Examination revealed a distal penile mass with purulent exudate at the meatus but no inguinal lymphadenopathy. Two fragments of this mass detached during office cystoscopy, and pathology revealed high-grade urothelial cell carcinoma (UCC). A magnetic resonance image of the pelvis with and without IV contrast revealed a 2.4-cm tumor in the glans penis with possible extension into the subcutaneous connective tissue of the penis and penile skin, without invasion of the corpora cavernosa/spongiosum or lymphadenopathy (Figure 1).
Prostatic urethral and random bladder biopsies, bilateral retrograde pyelograms, and selective ureteral washings revealed no abnormalities or signs of disease. Percutaneous biopsy of the inguinal node confirmed metastatic UCC. The patient underwent radical penectomy, creation of a perineal urethrostomy, and suprapubic cystostomy tube placement. Negative margins were confirmed on the urethral stump and corpus spongiosum. Final pathology revealed high-grade UCC with squamous differentiation on hematoxylin and eosin staining, arising from the penile urethra, invading the glans and corpus spongiosum, with no invasion of the corpus cavernosa (Figures 3 and 4).
Immunohistochemical stains were performed and strongly positive for cytokeratin 7 and p63. Final pathologic stage was described as pT2N1, with negative margins, indicating an American Joint Committee on Cancer classification of Stage III disease.7 The patient was referred postoperatively for adjuvant chemoradiation.
Discussion
The low incidence of PUC, coupled with a high morbidity/mortality rate, creates a difficult scenario in choosing the best oncologic management for this disease. National guidelines stratify treatment algorithms by stage and location of primary tumor, as these were found to be the 2 most important prognostic factors for men.1 The location of the primary tumor is most often in the bulbomembranous urethra, but up to one-third occur in the pendulous urethra.2
A recent review reported that UCC is the most common histologic subtype.4 When considering the differential diagnosis, a distal penile mass may represent a malignant penile lesion, such as squamous cell carcinoma, Buschke-Lowenstein tumor, Kaposi sarcoma, or precancerous lesions. Additional benign and infectious disorders include epidermoid and retention cysts, leukoplakia, balanitis xerotica obliterans, condyloma acuminatum, chancre/chancroid, lymphogranuloma venereum, granuloma inguinale, and tuberculosis. Clinical workup typically includes physical examination, cystourethroscopy and biopsy, chest X-ray, and pelvic/abdominal cross-sectional imaging.9,10 Magnetic resonance imaging of the abdomen and pelvis is ideal in identifying soft tissue structures and extension of tumor.
In male patients with PUC, nodal metastases are commonly seen at initial presentation in up to one-third of patients, while distant metastases may be present in up to 6% at presentation.2-4 When tumors arise from the anterior urethra, the primary lymphatic drainage is first to the inguinal lymph nodes, whereas posterior tumors drain to the pelvic lymph nodes. A multivariate analysis of men with PUC within the Surveillance, Epidemiology, and End Results database demonstrated an OS across all stages to be 46.2% and 29.3% at 5 and 10 years, respectively. Increased likelihood of death was predicted by advanced age, high grade/stage, systemic metastases, non-UCC histology, and the lack of surgery.4
Surgical intervention, including radical resection via penectomy, has been the mainstay in disease management and was first described by Marshall in 1957 for bulbar urethral cancer.11 In 1998, Gheiler and colleagues demonstrated that surgical resection alone yielded excellent outcomes in patients with low-stage disease with 89% of patients disease free at mean 42 months. This was in stark contrast to patients with advanced stage disease (T3 or N+) who exhibited a disease-free survival rate of 42% at the same follow-up interval and benefited from combined chemoradiation and surgical resection.3
In the presence of high-stage disease, multimodal therapy with chemotherapy, radiation, and/or surgery is warranted. A study in 2008 reviewed chemoradiation in which patients with PUC received a 5-week protocol of external beam radiotherapy to the genitals, inguinal/pelvic lymph nodes, plus an additional radiation bolus to the primary tumor.5 In the 18 patients reported, 15 had complete response to therapy, and only 4 patients required salvage surgical resection. The 7-year survival for the cohort was 72% with chemoradiation alone, with about half the population recurring or progressing at 7 years. However, all patients that avoided surgical resection went on to develop urethral strictures that required surgical therapy, 3 of which required complex reconstructive procedures.
To place this survival into context, the 1999 study by Dalbagni and colleagues reported a 5-year OS of 42% when surgical resection alone was performed in 40/46 men with PUC.2 Last, a large retrospective series of 44 patients reported mostly advanced-stage patients with PUC and analyzed patients treated with chemotherapy based on histologic pathology. The results demonstrated a 72% overall response rate to neoadjuvant chemotherapy, with a median OS of 32 months in patients undergoing chemotherapy vs 46 months in patients who underwent subsequent surgery. This study solidified that for patients with PUC involving the lymph nodes; optimal treatment includes neoadjuvant cisplatin-based chemotherapy followed by surgical resection.6
As medicine and oncologic therapies become more individualized, physicians are looking to new immunologic agents for systemic therapy. Immune checkpoint inhibitors were approved by the US Food and Drug Administration for UCC of the bladder in 2016.12 Unfortunately, due to the rarity of PUC and the recent development of immune checkpoint inhibitors, there have been no published reports of these or other immunotherapies in PUC. However, given the histologic similarity and pathogenesis, checkpoint inhibitors may have a future indication in the systemic management of this disease.
Conclusion
This patient’s PUC represents a rare presentation of a distal urethral carcinoma, T2-staged tumor, with rapid progression to nodal metastases. Additionally, the presentation of a fungating penile mass would usually indicate penile carcinoma, but providers should be aware of urethral carcinoma in the differential diagnosis. Notably, the patient was found to have progression to lymph node involvement during a mere 2-month period.
Recent case series have published encouraging results with neoadjuvant chemotherapy or chemoradiation.5,6 However, radical resection in men with T2 to T4 disease is associated with significantly higher cancer-specific survival. Given our concern of a loss to follow-up, we felt that radical resection of the primary tumor and adjuvant chemoradiation represented the patient’s best oncologic outcomes. Therefore, he underwent radical penectomy and creation of a perineal urethrostomy. As of his 6-month follow-up, he showed no evidence of disease, had returned to his preoperative functional status, and was referred for chemoradiation.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the US Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Swartz MA, Porter MP, Lin DW, Weiss NS. Incidence of primary urethral carcinoma in the United States. Urology. 2006;68(6):1164-1168.
2. Dalbagni G, Zhang ZF, Lacombe L, Herr HW. Male urethral carcinoma: analysis of treatment outcome. Urology. 1999;53(6):1126-1132.
3. Gheiler EL, Tefilli MV, Tiguert R, de Oliveira JG, Pontes JE, Wood DP Jr. Management of primary urethral cancer. Urology. 1998;52(3):487-493.
4. Rabbani F. Prognostic factors in male urethral cancer. Cancer. 2011;117(11):2426-2434.
5. Cohen MS, Triaca V, Billmeyer B, et al. Coordinated chemoradiation therapy with genital preservation for the treatment of primary invasive carcinoma of the male urethra. J Urol. 2008;179(2):536-541; discussion 541.
6. Dayyani F, Pettaway CA, Kamat AM, Munsell MF, Sircar K, Pagliaro LC. Retrospective analysis of survival outcomes and the role of cisplatin-based chemotherapy in patients with urethral carcinomas referred to medical oncologists. Urol Oncol. 2013;31(7):1171-1177.
7. American Joint Committee on Cancer. AJCC cancer staging manual. 8th ed. https://cancerstaging.org/references-tools/deskreferences/Documents/AJCC%20Cancer%20Staging%20Form%20Supplement.pdf. Updated June 5, 2018. Accessed January 22, 2019.
8. Gakis G, Witjes JA, Compérat E, et al. European Association of Urology guidelines on primary urethral carcinoma. https://uroweb.org/wp-content/uploads/EAU-Guidelines-Primary-Urethral-Carcinoma-2016-1.pdf. Updated March 2015. Accessed January 22, 2019
9. National Comprehensive Cancer Network. Bladder Cancer. Version 1.2019. https://www.nccn.org/professionals/physician_gls/pdf/bladder.pdf. Updated December 20, 2018. Accessed January 17, 2019.
10. Dayyani F, Hoffman K, Eifel P, et al. Management of advanced primary urethral carcinomas. BJU Int. 2014;114(1):25-31.
11. Marshall VF. Radical excision of locally extensive carcinoma of the deep male urethra. J Urol. 1957;78(3):252-264.
12. Hsu FS, Su CH, Huang KH. A comprehensive review of US FDA-approved immune checkpoint inhibitors in urothelial carcinoma. J Immunol Res. 2017;2017:6940546.
1. Swartz MA, Porter MP, Lin DW, Weiss NS. Incidence of primary urethral carcinoma in the United States. Urology. 2006;68(6):1164-1168.
2. Dalbagni G, Zhang ZF, Lacombe L, Herr HW. Male urethral carcinoma: analysis of treatment outcome. Urology. 1999;53(6):1126-1132.
3. Gheiler EL, Tefilli MV, Tiguert R, de Oliveira JG, Pontes JE, Wood DP Jr. Management of primary urethral cancer. Urology. 1998;52(3):487-493.
4. Rabbani F. Prognostic factors in male urethral cancer. Cancer. 2011;117(11):2426-2434.
5. Cohen MS, Triaca V, Billmeyer B, et al. Coordinated chemoradiation therapy with genital preservation for the treatment of primary invasive carcinoma of the male urethra. J Urol. 2008;179(2):536-541; discussion 541.
6. Dayyani F, Pettaway CA, Kamat AM, Munsell MF, Sircar K, Pagliaro LC. Retrospective analysis of survival outcomes and the role of cisplatin-based chemotherapy in patients with urethral carcinomas referred to medical oncologists. Urol Oncol. 2013;31(7):1171-1177.
7. American Joint Committee on Cancer. AJCC cancer staging manual. 8th ed. https://cancerstaging.org/references-tools/deskreferences/Documents/AJCC%20Cancer%20Staging%20Form%20Supplement.pdf. Updated June 5, 2018. Accessed January 22, 2019.
8. Gakis G, Witjes JA, Compérat E, et al. European Association of Urology guidelines on primary urethral carcinoma. https://uroweb.org/wp-content/uploads/EAU-Guidelines-Primary-Urethral-Carcinoma-2016-1.pdf. Updated March 2015. Accessed January 22, 2019
9. National Comprehensive Cancer Network. Bladder Cancer. Version 1.2019. https://www.nccn.org/professionals/physician_gls/pdf/bladder.pdf. Updated December 20, 2018. Accessed January 17, 2019.
10. Dayyani F, Hoffman K, Eifel P, et al. Management of advanced primary urethral carcinomas. BJU Int. 2014;114(1):25-31.
11. Marshall VF. Radical excision of locally extensive carcinoma of the deep male urethra. J Urol. 1957;78(3):252-264.
12. Hsu FS, Su CH, Huang KH. A comprehensive review of US FDA-approved immune checkpoint inhibitors in urothelial carcinoma. J Immunol Res. 2017;2017:6940546.
Skeletal-Related Events in Patients With Multiple Myeloma and Prostate Cancer Who Receive Standard vs Extended-Interval Bisphosphonate Dosing (FULL)
In patients with multiple myeloma and prostate cancer, extending the bisphosphonatedosing interval may help decrease medication-related morbidity without compromising therapeutic benefit.
Bone pain is one of the most common causes of morbidity in multiple myeloma (MM) and metastatic prostate cancer (CaP). This pain originates with the underlying pathologic processes of the cancer and with downstream skeletal-related events (SREs). SREs—fractures, spinal cord compression, and irradiation or surgery performed in ≥ 1 bone sites—represent a significant health care burden, particularly given the incidence of the underlying malignancies. According to American Cancer Society statistics, CaP is the second most common cancer in American men, and MM the second most common hematologic malignancy, despite its relatively low overall lifetime risk.1,2 Regardless of the underlying malignancy, bisphosphonates are the cornerstone of SRE prevention, though the optimal dosing strategy is the subject of clinical debate.
Although similar in SRE incidence, MM and CaP have distinct pathophysiologic processes in the dysregulation of bone resorption. MM is a hematologic malignancy that increases the risk of SREs by osteoclast up-regulation, primarily through the RANK (receptor activator of nuclear factor α-B) signaling pathway.3 CaP is a solid tumor malignancy that metastasizes to bone. Dysregulation of the bone resorption or formation cycle and net bone loss are a result of endogenous osteoclast up-regulation in response to abnormal bone formation in osteoblastic bone metastases.4 Androgen-deprivation therapy, the cornerstone of CaP treatment, further predisposes CaP patients to osteoporosis and SREs.
Prevention of SREs is pharmacologically driven by bisphosphonates, which have antiresorptive effects on bone through promotion of osteoclast apoptosis.5 Two IV formulations, pamidronate and zoledronic acid (ZA), are US Food and Drug Administration approved for use in bone metastases from MM or solid tumors.6-10 Although generally well tolerated, bisphosphonates can cause osteonecrosis of the jaw (ONJ), an avascular death of bone tissue, particularly with prolonged use.11 With its documented incidence of 5% to 6.7% in bone metastasis, ONJ represents a significant morbidity risk in patients with MM and CaP who are treated with IV bisphosphonates.12
Investigators are exploring bisphosphonate dosing intervals to determine which is most appropriate in mitigating the risk of ONJ. Before 2006, bisphosphonates were consistently dosed once monthly in patients with MM or metastatic bone disease—a standard derived empirically rather than from comparative studies or compelling pharmacodynamic data.13-15 In a 2006 consensus statement, the Mayo Clinic issued an expert opinion recommendation for increasing the bisphosphonate dosing interval to every 3 months in patients with MM.16 The first objective evidence for the clinical applicability of extending the ZA dosing interval was reported by Himelstein and colleagues in 2017.17 The randomized clinical trial found no differences in SRE rates when ZA was dosed every 12 weeks,17 prompting a conditional recommendation for dosing interval extension in the American Society of Clinical Oncology MM treatment guidelines (2018).13 Because of the age and racial demographics of the patients in these studies, many questions remain unanswered.
For the US Department of Veterans Affairs (VA) population, the pharmacokinetic and dynamic differences imposed by age and race limit the applicability of the available data. However, in veterans with MM or CaP, extending the bisphosphonate dosing interval may help decrease medication-related morbidity (eg, ONJ, nephrotoxicity) without compromising therapeutic benefit. To this end at the Memphis VA Medical Center (VAMC), we assessed for differences in SRE rates by comparing outcomes of patients who received ZA in standard- vs extended-interval dosing.
Methods
We retrospectively reviewed the Computerized Patient Record System for veterans with MM or metastatic CaP treated with ZA at the Memphis VAMC. Study inclusion criteria were aged > 18 years and care provided by a Memphis VAMC oncologist between January 2003 and January 2018. The study was approved by the Memphis VAMC’s Institutional Review Board, and procedures were followed in accordance with the ethical standards of its committee on human experimentation.
Using Microsoft SQL 2016 (Redmond, WA), we performed a query to identify patients who were prescribed ZA during the study period. Exclusion criteria were ZA prescribed for an indication other than MM or CaP (ie, osteoporosis) and receipt of ≤ 1 dose of ZA. Once a list was compiled, patients were stratified by ZA dosing interval: standard (mean, every month) or extended (mean, every 3 months). Patients whose ZA dosing interval was changed during treatment were included as independent data points in each group.
Skeletal-related events included fractures, spinal compression, irradiation, and surgery. Fractures and spinal compression were pertinent in the presence of radiographic documentation (eg, X-ray, magnetic resonance imaging scan) during the period the patient received ZA or within 1 dosing interval of the last recorded ZA dose. Irradiation was defined as documented application of radiation therapy to ≥ 1 bone sites for palliation of pain or as an intervention in the setting of spinal compression. Surgery was defined as any procedure performed to correct a fracture or spinal compression. Each SRE was counted as a single occurrence.
Osteonecrosis of the jaw was defined as radiographically documented necrosis of the mandible or associated structures with assessment by a VA dentist. Records from non-VA dental practices were not available for assessment. Documentation of dental assessment before the first dose of ZA and any assessments during treatment were recorded.
Medication use was assessed before and during ZA treatment. Number of ZA doses and reasons for any discontinuations were documented, as was concomitant use of calcium supplements, vitamin D supplements, calcitriol, paricalcitol, calcitonin, cinacalcet, and pamidronate.
The primary study outcome was observed difference in incidence of SREs between standard- and extended-interval dosing of ZA. Secondary outcomes included difference in incidence of ONJ as well as incidence of SREs and ONJ by disease subtype (MM, CaP).
Descriptive statistics were used to summarize demographic data and assess prespecified outcomes. Differences in rates of SREs and ONJ between dosing interval groups were analyzed with the Pearson χ2 test. The predetermined a priori level of significance was .05.
Results
Of the 300 patients prescribed ZA at the Memphis VAMC, 177 were excluded (96 for indication,78 for receiving only 1 dose of ZA, 3 for not receiving any doses of ZA). The remaining 123 patients were stratified into a standard-interval dosing group (121) and an extended-interval dosing group (35). Of the 123 patients, 33 received both standard- and extended-interval dosing of ZA over the course of the study period and were included discretely in each group for the duration of each dosing strategy.
Pre-ZA dental screenings were documented in 14% of standard-interval patients and 17% of extended-interval patients, and during-ZA screenings were documented in 17% of standard-interval patients and 20% of extended-interval patients. Chi-square analysis revealed no significant difference in rates of dental screening before or during use of ZA.
Standard-interval patients received a mean (SD) 11.4 (13.5) doses of ZA (range, 2-124). Extended-interval patients received a mean (SD) of 5.9 (3.18) doses (range, 2-14). All standard-interval patients had discontinued treatment at the time of the study, most commonly because of death or for an unknown reason. Sixty percent of extended-interval patients had discontinued treatment, most commonly because of patient/physician choice or for an unknown reason (Table 2).
Skeletal-related events were observed in 31% of standard-interval patients and 23% of extended-interval patients. There were no statistically significant differences in SRE rates between groups (P = .374). The most common SRE in both groups was bone irradiation (42% and 60%, respectively), with no statistically significant difference in proportion between groups (Table 4).
Discussion
This retrospective review of patients with MM and CaP receiving ZA for bone metastasesfound no differences in the rates of SREs when ZA was dosed monthly vs every 3 months.
Earlier studies found that ZA can decrease SRE rates, but a major concern is that frequent, prolonged exposure to IV bisphosphonates may increase the risk of ONJ. No significant differences in ONJ rates existed between dosing groups, but all documented cases of ONJ occurred in the standard-interval group, suggesting a trend toward decreased incidence with an extension of the dosing interval.
Limitations
This study had several limitations. Geriatric African American men comprised the majority of the study population, and patients with MM accounted for only 22% of included regimens, limiting external validity. Patient overlap between groups may have confounded the results. The retrospective design precluded the ability to control for confounding variables, such as concomitant medication use and medication adherence, and significant heterogeneity was noted in rates of adherence with ZA infusion schedules regardless of dosing group. Use of medications associated with increased risk of osteoporosis—including corticosteroids and proton pump inhibitors—was not assessed.
Assessment of ONJ incidence was limited by the lack of access to dental records from providers outside the VA. Many patients in this review were not eligible for VA dental benefits because of requirements involving time and service connection, a reimbursement measurement that reflects health conditions “incurred or aggravated during active military service.”18
The results of this study provide further support for extended-interval dosing of ZA as a potential method of increasing patient adherence and decreasing the possibility of adverse drug reactions without compromising therapeutic benefit. Further randomized controlled trials are needed to define the potential decrease in ONJ incidence.
Conclusion
In comparisons of standard- and extended-interval dosing of ZA, there was no difference in the incidence of skeletal-related events in veteran patients with bone metastases from MM or CaP.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the US Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. American Cancer Society. Cancer Facts & Figures 2018. Atlanta, GA: American Cancer Society; 2018.
2. Howlader N, Noone AM, Krapcho M, et al, eds. SEER Cancer Statistics Review (CSR), 1975-2014 [based on November 2016 SEER data submission posted to SEER website April 2017]. Bethesda, MD: National Cancer Institute; 2017. https://seer.cancer.gov/archive/csr/1975_2014/. Accessed January 12, 2019.
3. Roodman GD. Pathogenesis of myeloma bone disease. Leukemia. 2009;23(3):435-441.
4. Sartor O, de Bono JS. Metastatic prostate cancer. N Engl J Med. 2018;378(7):645-657.
5. Drake MT, Clarke BL, Khosla S. Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clin Proc. 2008;83(9):1032-1045.
6. Zometa [package insert]. East Hanover, NJ: Novartis; 2016.
7. Aredia [package insert]. East Hanover, NJ: Novartis; 2011.
8. Berenson JR, Rosen LS, Howell A, et al. Zoledronic acid reduces skeletal-related events in patients with osteolytic metastases: a double-blind, randomized dose-response study [published correction appears in Cancer. 2001;91(10):1956]. Cancer. 2001;91(7):1191-1200.
9. Berenson JR, Lichtenstein A, Porter L, et al. Efficacy of pamidronate in reducing skeletal events in patients with advanced multiple myeloma. Myeloma Aredia Study Group. N Engl J Med. 1996;334(8):488-493.
10. Mhaskar R, Redzepovic J, Wheatley K, et al. Bisphosphonates in multiple myeloma: a network meta-analysis. Cochrane Database Syst Rev. 2012;(5):CD003188.
11. Wu S, Dahut WL, Gulley JL. The use of bisphosphonates in cancer patients. Acta Oncol. 2007;46(5):581-591.
12. Bamias A, Kastritis E, Bamia C, et al. Osteonecrosis of the jaw in cancer after treatment with bisphosphonates: incidence and risk factors. J Clin Oncol. 2005;23(34):8580-8587.
13. Anderson K, Ismaila N, Flynn PJ, et al. Role of bone-modifying agents in multiple myeloma: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2018;36(8):812-818.
14. National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines). Multiple Myeloma. Version 2.2019. https://www.nccn.org/professionals/physician_gls/pdf/myeloma.pdf. Accessed January 29, 2019.
15. National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines). Prostate Cancer. Version 4.2018. https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Accessed January 29, 2019.
16. Lacy MQ, Dispenzieri A, Gertz MA, et al. Mayo Clinic consensus statement for the use of bisphosphonates in multiple myeloma. Mayo Clin Proc. 2006;81(8):1047-1053.
17. Himelstein AL, Foster JC, Khatcheressian JL, et al. Effect of longer-interval vs. standard dosing of zoledronic acid on skeletal events in patients with bone metastases: a randomized clinical trial. JAMA. 2017;317(1):48-58.
18. Office of Public and Intergovernmental Affairs, US Department of Veterans Affairs. Service connected disabilities. In: Federal Benefits for Veterans, Dependents, and Survivors. https://www.va.gov/opa/publications/benefits_book/benefits_chap02.asp. Published April 2015. Accessed May 22, 2018.
In patients with multiple myeloma and prostate cancer, extending the bisphosphonatedosing interval may help decrease medication-related morbidity without compromising therapeutic benefit.
In patients with multiple myeloma and prostate cancer, extending the bisphosphonatedosing interval may help decrease medication-related morbidity without compromising therapeutic benefit.
Bone pain is one of the most common causes of morbidity in multiple myeloma (MM) and metastatic prostate cancer (CaP). This pain originates with the underlying pathologic processes of the cancer and with downstream skeletal-related events (SREs). SREs—fractures, spinal cord compression, and irradiation or surgery performed in ≥ 1 bone sites—represent a significant health care burden, particularly given the incidence of the underlying malignancies. According to American Cancer Society statistics, CaP is the second most common cancer in American men, and MM the second most common hematologic malignancy, despite its relatively low overall lifetime risk.1,2 Regardless of the underlying malignancy, bisphosphonates are the cornerstone of SRE prevention, though the optimal dosing strategy is the subject of clinical debate.
Although similar in SRE incidence, MM and CaP have distinct pathophysiologic processes in the dysregulation of bone resorption. MM is a hematologic malignancy that increases the risk of SREs by osteoclast up-regulation, primarily through the RANK (receptor activator of nuclear factor α-B) signaling pathway.3 CaP is a solid tumor malignancy that metastasizes to bone. Dysregulation of the bone resorption or formation cycle and net bone loss are a result of endogenous osteoclast up-regulation in response to abnormal bone formation in osteoblastic bone metastases.4 Androgen-deprivation therapy, the cornerstone of CaP treatment, further predisposes CaP patients to osteoporosis and SREs.
Prevention of SREs is pharmacologically driven by bisphosphonates, which have antiresorptive effects on bone through promotion of osteoclast apoptosis.5 Two IV formulations, pamidronate and zoledronic acid (ZA), are US Food and Drug Administration approved for use in bone metastases from MM or solid tumors.6-10 Although generally well tolerated, bisphosphonates can cause osteonecrosis of the jaw (ONJ), an avascular death of bone tissue, particularly with prolonged use.11 With its documented incidence of 5% to 6.7% in bone metastasis, ONJ represents a significant morbidity risk in patients with MM and CaP who are treated with IV bisphosphonates.12
Investigators are exploring bisphosphonate dosing intervals to determine which is most appropriate in mitigating the risk of ONJ. Before 2006, bisphosphonates were consistently dosed once monthly in patients with MM or metastatic bone disease—a standard derived empirically rather than from comparative studies or compelling pharmacodynamic data.13-15 In a 2006 consensus statement, the Mayo Clinic issued an expert opinion recommendation for increasing the bisphosphonate dosing interval to every 3 months in patients with MM.16 The first objective evidence for the clinical applicability of extending the ZA dosing interval was reported by Himelstein and colleagues in 2017.17 The randomized clinical trial found no differences in SRE rates when ZA was dosed every 12 weeks,17 prompting a conditional recommendation for dosing interval extension in the American Society of Clinical Oncology MM treatment guidelines (2018).13 Because of the age and racial demographics of the patients in these studies, many questions remain unanswered.
For the US Department of Veterans Affairs (VA) population, the pharmacokinetic and dynamic differences imposed by age and race limit the applicability of the available data. However, in veterans with MM or CaP, extending the bisphosphonate dosing interval may help decrease medication-related morbidity (eg, ONJ, nephrotoxicity) without compromising therapeutic benefit. To this end at the Memphis VA Medical Center (VAMC), we assessed for differences in SRE rates by comparing outcomes of patients who received ZA in standard- vs extended-interval dosing.
Methods
We retrospectively reviewed the Computerized Patient Record System for veterans with MM or metastatic CaP treated with ZA at the Memphis VAMC. Study inclusion criteria were aged > 18 years and care provided by a Memphis VAMC oncologist between January 2003 and January 2018. The study was approved by the Memphis VAMC’s Institutional Review Board, and procedures were followed in accordance with the ethical standards of its committee on human experimentation.
Using Microsoft SQL 2016 (Redmond, WA), we performed a query to identify patients who were prescribed ZA during the study period. Exclusion criteria were ZA prescribed for an indication other than MM or CaP (ie, osteoporosis) and receipt of ≤ 1 dose of ZA. Once a list was compiled, patients were stratified by ZA dosing interval: standard (mean, every month) or extended (mean, every 3 months). Patients whose ZA dosing interval was changed during treatment were included as independent data points in each group.
Skeletal-related events included fractures, spinal compression, irradiation, and surgery. Fractures and spinal compression were pertinent in the presence of radiographic documentation (eg, X-ray, magnetic resonance imaging scan) during the period the patient received ZA or within 1 dosing interval of the last recorded ZA dose. Irradiation was defined as documented application of radiation therapy to ≥ 1 bone sites for palliation of pain or as an intervention in the setting of spinal compression. Surgery was defined as any procedure performed to correct a fracture or spinal compression. Each SRE was counted as a single occurrence.
Osteonecrosis of the jaw was defined as radiographically documented necrosis of the mandible or associated structures with assessment by a VA dentist. Records from non-VA dental practices were not available for assessment. Documentation of dental assessment before the first dose of ZA and any assessments during treatment were recorded.
Medication use was assessed before and during ZA treatment. Number of ZA doses and reasons for any discontinuations were documented, as was concomitant use of calcium supplements, vitamin D supplements, calcitriol, paricalcitol, calcitonin, cinacalcet, and pamidronate.
The primary study outcome was observed difference in incidence of SREs between standard- and extended-interval dosing of ZA. Secondary outcomes included difference in incidence of ONJ as well as incidence of SREs and ONJ by disease subtype (MM, CaP).
Descriptive statistics were used to summarize demographic data and assess prespecified outcomes. Differences in rates of SREs and ONJ between dosing interval groups were analyzed with the Pearson χ2 test. The predetermined a priori level of significance was .05.
Results
Of the 300 patients prescribed ZA at the Memphis VAMC, 177 were excluded (96 for indication,78 for receiving only 1 dose of ZA, 3 for not receiving any doses of ZA). The remaining 123 patients were stratified into a standard-interval dosing group (121) and an extended-interval dosing group (35). Of the 123 patients, 33 received both standard- and extended-interval dosing of ZA over the course of the study period and were included discretely in each group for the duration of each dosing strategy.
Pre-ZA dental screenings were documented in 14% of standard-interval patients and 17% of extended-interval patients, and during-ZA screenings were documented in 17% of standard-interval patients and 20% of extended-interval patients. Chi-square analysis revealed no significant difference in rates of dental screening before or during use of ZA.
Standard-interval patients received a mean (SD) 11.4 (13.5) doses of ZA (range, 2-124). Extended-interval patients received a mean (SD) of 5.9 (3.18) doses (range, 2-14). All standard-interval patients had discontinued treatment at the time of the study, most commonly because of death or for an unknown reason. Sixty percent of extended-interval patients had discontinued treatment, most commonly because of patient/physician choice or for an unknown reason (Table 2).
Skeletal-related events were observed in 31% of standard-interval patients and 23% of extended-interval patients. There were no statistically significant differences in SRE rates between groups (P = .374). The most common SRE in both groups was bone irradiation (42% and 60%, respectively), with no statistically significant difference in proportion between groups (Table 4).
Discussion
This retrospective review of patients with MM and CaP receiving ZA for bone metastasesfound no differences in the rates of SREs when ZA was dosed monthly vs every 3 months.
Earlier studies found that ZA can decrease SRE rates, but a major concern is that frequent, prolonged exposure to IV bisphosphonates may increase the risk of ONJ. No significant differences in ONJ rates existed between dosing groups, but all documented cases of ONJ occurred in the standard-interval group, suggesting a trend toward decreased incidence with an extension of the dosing interval.
Limitations
This study had several limitations. Geriatric African American men comprised the majority of the study population, and patients with MM accounted for only 22% of included regimens, limiting external validity. Patient overlap between groups may have confounded the results. The retrospective design precluded the ability to control for confounding variables, such as concomitant medication use and medication adherence, and significant heterogeneity was noted in rates of adherence with ZA infusion schedules regardless of dosing group. Use of medications associated with increased risk of osteoporosis—including corticosteroids and proton pump inhibitors—was not assessed.
Assessment of ONJ incidence was limited by the lack of access to dental records from providers outside the VA. Many patients in this review were not eligible for VA dental benefits because of requirements involving time and service connection, a reimbursement measurement that reflects health conditions “incurred or aggravated during active military service.”18
The results of this study provide further support for extended-interval dosing of ZA as a potential method of increasing patient adherence and decreasing the possibility of adverse drug reactions without compromising therapeutic benefit. Further randomized controlled trials are needed to define the potential decrease in ONJ incidence.
Conclusion
In comparisons of standard- and extended-interval dosing of ZA, there was no difference in the incidence of skeletal-related events in veteran patients with bone metastases from MM or CaP.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the US Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Bone pain is one of the most common causes of morbidity in multiple myeloma (MM) and metastatic prostate cancer (CaP). This pain originates with the underlying pathologic processes of the cancer and with downstream skeletal-related events (SREs). SREs—fractures, spinal cord compression, and irradiation or surgery performed in ≥ 1 bone sites—represent a significant health care burden, particularly given the incidence of the underlying malignancies. According to American Cancer Society statistics, CaP is the second most common cancer in American men, and MM the second most common hematologic malignancy, despite its relatively low overall lifetime risk.1,2 Regardless of the underlying malignancy, bisphosphonates are the cornerstone of SRE prevention, though the optimal dosing strategy is the subject of clinical debate.
Although similar in SRE incidence, MM and CaP have distinct pathophysiologic processes in the dysregulation of bone resorption. MM is a hematologic malignancy that increases the risk of SREs by osteoclast up-regulation, primarily through the RANK (receptor activator of nuclear factor α-B) signaling pathway.3 CaP is a solid tumor malignancy that metastasizes to bone. Dysregulation of the bone resorption or formation cycle and net bone loss are a result of endogenous osteoclast up-regulation in response to abnormal bone formation in osteoblastic bone metastases.4 Androgen-deprivation therapy, the cornerstone of CaP treatment, further predisposes CaP patients to osteoporosis and SREs.
Prevention of SREs is pharmacologically driven by bisphosphonates, which have antiresorptive effects on bone through promotion of osteoclast apoptosis.5 Two IV formulations, pamidronate and zoledronic acid (ZA), are US Food and Drug Administration approved for use in bone metastases from MM or solid tumors.6-10 Although generally well tolerated, bisphosphonates can cause osteonecrosis of the jaw (ONJ), an avascular death of bone tissue, particularly with prolonged use.11 With its documented incidence of 5% to 6.7% in bone metastasis, ONJ represents a significant morbidity risk in patients with MM and CaP who are treated with IV bisphosphonates.12
Investigators are exploring bisphosphonate dosing intervals to determine which is most appropriate in mitigating the risk of ONJ. Before 2006, bisphosphonates were consistently dosed once monthly in patients with MM or metastatic bone disease—a standard derived empirically rather than from comparative studies or compelling pharmacodynamic data.13-15 In a 2006 consensus statement, the Mayo Clinic issued an expert opinion recommendation for increasing the bisphosphonate dosing interval to every 3 months in patients with MM.16 The first objective evidence for the clinical applicability of extending the ZA dosing interval was reported by Himelstein and colleagues in 2017.17 The randomized clinical trial found no differences in SRE rates when ZA was dosed every 12 weeks,17 prompting a conditional recommendation for dosing interval extension in the American Society of Clinical Oncology MM treatment guidelines (2018).13 Because of the age and racial demographics of the patients in these studies, many questions remain unanswered.
For the US Department of Veterans Affairs (VA) population, the pharmacokinetic and dynamic differences imposed by age and race limit the applicability of the available data. However, in veterans with MM or CaP, extending the bisphosphonate dosing interval may help decrease medication-related morbidity (eg, ONJ, nephrotoxicity) without compromising therapeutic benefit. To this end at the Memphis VA Medical Center (VAMC), we assessed for differences in SRE rates by comparing outcomes of patients who received ZA in standard- vs extended-interval dosing.
Methods
We retrospectively reviewed the Computerized Patient Record System for veterans with MM or metastatic CaP treated with ZA at the Memphis VAMC. Study inclusion criteria were aged > 18 years and care provided by a Memphis VAMC oncologist between January 2003 and January 2018. The study was approved by the Memphis VAMC’s Institutional Review Board, and procedures were followed in accordance with the ethical standards of its committee on human experimentation.
Using Microsoft SQL 2016 (Redmond, WA), we performed a query to identify patients who were prescribed ZA during the study period. Exclusion criteria were ZA prescribed for an indication other than MM or CaP (ie, osteoporosis) and receipt of ≤ 1 dose of ZA. Once a list was compiled, patients were stratified by ZA dosing interval: standard (mean, every month) or extended (mean, every 3 months). Patients whose ZA dosing interval was changed during treatment were included as independent data points in each group.
Skeletal-related events included fractures, spinal compression, irradiation, and surgery. Fractures and spinal compression were pertinent in the presence of radiographic documentation (eg, X-ray, magnetic resonance imaging scan) during the period the patient received ZA or within 1 dosing interval of the last recorded ZA dose. Irradiation was defined as documented application of radiation therapy to ≥ 1 bone sites for palliation of pain or as an intervention in the setting of spinal compression. Surgery was defined as any procedure performed to correct a fracture or spinal compression. Each SRE was counted as a single occurrence.
Osteonecrosis of the jaw was defined as radiographically documented necrosis of the mandible or associated structures with assessment by a VA dentist. Records from non-VA dental practices were not available for assessment. Documentation of dental assessment before the first dose of ZA and any assessments during treatment were recorded.
Medication use was assessed before and during ZA treatment. Number of ZA doses and reasons for any discontinuations were documented, as was concomitant use of calcium supplements, vitamin D supplements, calcitriol, paricalcitol, calcitonin, cinacalcet, and pamidronate.
The primary study outcome was observed difference in incidence of SREs between standard- and extended-interval dosing of ZA. Secondary outcomes included difference in incidence of ONJ as well as incidence of SREs and ONJ by disease subtype (MM, CaP).
Descriptive statistics were used to summarize demographic data and assess prespecified outcomes. Differences in rates of SREs and ONJ between dosing interval groups were analyzed with the Pearson χ2 test. The predetermined a priori level of significance was .05.
Results
Of the 300 patients prescribed ZA at the Memphis VAMC, 177 were excluded (96 for indication,78 for receiving only 1 dose of ZA, 3 for not receiving any doses of ZA). The remaining 123 patients were stratified into a standard-interval dosing group (121) and an extended-interval dosing group (35). Of the 123 patients, 33 received both standard- and extended-interval dosing of ZA over the course of the study period and were included discretely in each group for the duration of each dosing strategy.
Pre-ZA dental screenings were documented in 14% of standard-interval patients and 17% of extended-interval patients, and during-ZA screenings were documented in 17% of standard-interval patients and 20% of extended-interval patients. Chi-square analysis revealed no significant difference in rates of dental screening before or during use of ZA.
Standard-interval patients received a mean (SD) 11.4 (13.5) doses of ZA (range, 2-124). Extended-interval patients received a mean (SD) of 5.9 (3.18) doses (range, 2-14). All standard-interval patients had discontinued treatment at the time of the study, most commonly because of death or for an unknown reason. Sixty percent of extended-interval patients had discontinued treatment, most commonly because of patient/physician choice or for an unknown reason (Table 2).
Skeletal-related events were observed in 31% of standard-interval patients and 23% of extended-interval patients. There were no statistically significant differences in SRE rates between groups (P = .374). The most common SRE in both groups was bone irradiation (42% and 60%, respectively), with no statistically significant difference in proportion between groups (Table 4).
Discussion
This retrospective review of patients with MM and CaP receiving ZA for bone metastasesfound no differences in the rates of SREs when ZA was dosed monthly vs every 3 months.
Earlier studies found that ZA can decrease SRE rates, but a major concern is that frequent, prolonged exposure to IV bisphosphonates may increase the risk of ONJ. No significant differences in ONJ rates existed between dosing groups, but all documented cases of ONJ occurred in the standard-interval group, suggesting a trend toward decreased incidence with an extension of the dosing interval.
Limitations
This study had several limitations. Geriatric African American men comprised the majority of the study population, and patients with MM accounted for only 22% of included regimens, limiting external validity. Patient overlap between groups may have confounded the results. The retrospective design precluded the ability to control for confounding variables, such as concomitant medication use and medication adherence, and significant heterogeneity was noted in rates of adherence with ZA infusion schedules regardless of dosing group. Use of medications associated with increased risk of osteoporosis—including corticosteroids and proton pump inhibitors—was not assessed.
Assessment of ONJ incidence was limited by the lack of access to dental records from providers outside the VA. Many patients in this review were not eligible for VA dental benefits because of requirements involving time and service connection, a reimbursement measurement that reflects health conditions “incurred or aggravated during active military service.”18
The results of this study provide further support for extended-interval dosing of ZA as a potential method of increasing patient adherence and decreasing the possibility of adverse drug reactions without compromising therapeutic benefit. Further randomized controlled trials are needed to define the potential decrease in ONJ incidence.
Conclusion
In comparisons of standard- and extended-interval dosing of ZA, there was no difference in the incidence of skeletal-related events in veteran patients with bone metastases from MM or CaP.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the US Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. American Cancer Society. Cancer Facts & Figures 2018. Atlanta, GA: American Cancer Society; 2018.
2. Howlader N, Noone AM, Krapcho M, et al, eds. SEER Cancer Statistics Review (CSR), 1975-2014 [based on November 2016 SEER data submission posted to SEER website April 2017]. Bethesda, MD: National Cancer Institute; 2017. https://seer.cancer.gov/archive/csr/1975_2014/. Accessed January 12, 2019.
3. Roodman GD. Pathogenesis of myeloma bone disease. Leukemia. 2009;23(3):435-441.
4. Sartor O, de Bono JS. Metastatic prostate cancer. N Engl J Med. 2018;378(7):645-657.
5. Drake MT, Clarke BL, Khosla S. Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clin Proc. 2008;83(9):1032-1045.
6. Zometa [package insert]. East Hanover, NJ: Novartis; 2016.
7. Aredia [package insert]. East Hanover, NJ: Novartis; 2011.
8. Berenson JR, Rosen LS, Howell A, et al. Zoledronic acid reduces skeletal-related events in patients with osteolytic metastases: a double-blind, randomized dose-response study [published correction appears in Cancer. 2001;91(10):1956]. Cancer. 2001;91(7):1191-1200.
9. Berenson JR, Lichtenstein A, Porter L, et al. Efficacy of pamidronate in reducing skeletal events in patients with advanced multiple myeloma. Myeloma Aredia Study Group. N Engl J Med. 1996;334(8):488-493.
10. Mhaskar R, Redzepovic J, Wheatley K, et al. Bisphosphonates in multiple myeloma: a network meta-analysis. Cochrane Database Syst Rev. 2012;(5):CD003188.
11. Wu S, Dahut WL, Gulley JL. The use of bisphosphonates in cancer patients. Acta Oncol. 2007;46(5):581-591.
12. Bamias A, Kastritis E, Bamia C, et al. Osteonecrosis of the jaw in cancer after treatment with bisphosphonates: incidence and risk factors. J Clin Oncol. 2005;23(34):8580-8587.
13. Anderson K, Ismaila N, Flynn PJ, et al. Role of bone-modifying agents in multiple myeloma: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2018;36(8):812-818.
14. National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines). Multiple Myeloma. Version 2.2019. https://www.nccn.org/professionals/physician_gls/pdf/myeloma.pdf. Accessed January 29, 2019.
15. National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines). Prostate Cancer. Version 4.2018. https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Accessed January 29, 2019.
16. Lacy MQ, Dispenzieri A, Gertz MA, et al. Mayo Clinic consensus statement for the use of bisphosphonates in multiple myeloma. Mayo Clin Proc. 2006;81(8):1047-1053.
17. Himelstein AL, Foster JC, Khatcheressian JL, et al. Effect of longer-interval vs. standard dosing of zoledronic acid on skeletal events in patients with bone metastases: a randomized clinical trial. JAMA. 2017;317(1):48-58.
18. Office of Public and Intergovernmental Affairs, US Department of Veterans Affairs. Service connected disabilities. In: Federal Benefits for Veterans, Dependents, and Survivors. https://www.va.gov/opa/publications/benefits_book/benefits_chap02.asp. Published April 2015. Accessed May 22, 2018.
1. American Cancer Society. Cancer Facts & Figures 2018. Atlanta, GA: American Cancer Society; 2018.
2. Howlader N, Noone AM, Krapcho M, et al, eds. SEER Cancer Statistics Review (CSR), 1975-2014 [based on November 2016 SEER data submission posted to SEER website April 2017]. Bethesda, MD: National Cancer Institute; 2017. https://seer.cancer.gov/archive/csr/1975_2014/. Accessed January 12, 2019.
3. Roodman GD. Pathogenesis of myeloma bone disease. Leukemia. 2009;23(3):435-441.
4. Sartor O, de Bono JS. Metastatic prostate cancer. N Engl J Med. 2018;378(7):645-657.
5. Drake MT, Clarke BL, Khosla S. Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clin Proc. 2008;83(9):1032-1045.
6. Zometa [package insert]. East Hanover, NJ: Novartis; 2016.
7. Aredia [package insert]. East Hanover, NJ: Novartis; 2011.
8. Berenson JR, Rosen LS, Howell A, et al. Zoledronic acid reduces skeletal-related events in patients with osteolytic metastases: a double-blind, randomized dose-response study [published correction appears in Cancer. 2001;91(10):1956]. Cancer. 2001;91(7):1191-1200.
9. Berenson JR, Lichtenstein A, Porter L, et al. Efficacy of pamidronate in reducing skeletal events in patients with advanced multiple myeloma. Myeloma Aredia Study Group. N Engl J Med. 1996;334(8):488-493.
10. Mhaskar R, Redzepovic J, Wheatley K, et al. Bisphosphonates in multiple myeloma: a network meta-analysis. Cochrane Database Syst Rev. 2012;(5):CD003188.
11. Wu S, Dahut WL, Gulley JL. The use of bisphosphonates in cancer patients. Acta Oncol. 2007;46(5):581-591.
12. Bamias A, Kastritis E, Bamia C, et al. Osteonecrosis of the jaw in cancer after treatment with bisphosphonates: incidence and risk factors. J Clin Oncol. 2005;23(34):8580-8587.
13. Anderson K, Ismaila N, Flynn PJ, et al. Role of bone-modifying agents in multiple myeloma: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2018;36(8):812-818.
14. National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines). Multiple Myeloma. Version 2.2019. https://www.nccn.org/professionals/physician_gls/pdf/myeloma.pdf. Accessed January 29, 2019.
15. National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines). Prostate Cancer. Version 4.2018. https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Accessed January 29, 2019.
16. Lacy MQ, Dispenzieri A, Gertz MA, et al. Mayo Clinic consensus statement for the use of bisphosphonates in multiple myeloma. Mayo Clin Proc. 2006;81(8):1047-1053.
17. Himelstein AL, Foster JC, Khatcheressian JL, et al. Effect of longer-interval vs. standard dosing of zoledronic acid on skeletal events in patients with bone metastases: a randomized clinical trial. JAMA. 2017;317(1):48-58.
18. Office of Public and Intergovernmental Affairs, US Department of Veterans Affairs. Service connected disabilities. In: Federal Benefits for Veterans, Dependents, and Survivors. https://www.va.gov/opa/publications/benefits_book/benefits_chap02.asp. Published April 2015. Accessed May 22, 2018.
Prostate Cancer Surveillance After Radiation Therapy in a National Delivery System (FULL)
Guideline concordance with PSA surveillance among veterans treated with definitiveradiation therapy was generally high, but opportunities may exist to improve surveillance among select groups.
Guidelines recommend prostate-specific antigen (PSA) surveillance among men treated with definitive radiation therapy (RT) for prostate cancer. Specifically, the National Comprehensive Cancer Network recommends testing every 6 to 12 months for 5 years and annually thereafter (with no specific stopping period specified), while the American Urology Association recommends testing for at least 10 years, with the frequency to be determined by the risk of relapse and patient preferences for monitoring.1,2 Salvage treatments exist for men with localized recurrence identified early through PSA testing, so adherence to follow-up guidelines is important for quality prostate cancer survivorship care.1,2
However, few studies focus on adherence to PSA surveillance following radiation therapy. Posttreatment surveillance among surgical patients is generally high, but sociodemographic disparities exist. Racial and ethnic minorities and unmarried men are less likely to undergo guideline concordant surveillance than is the general population, potentially preventing effective salvage therapy.3,4 A recent Department of Veterans Affairs (VA) study on posttreatment surveillance included radiation therapy patients but did not examine the impact of younger age, concurrent androgen deprivation therapy (ADT), or treatment facility (ie, diagnosed and treated at the same vs different facilities, with the latter including a separate VA facility or the community) on surveillance patterns.5 The latter is particularly relevant given increasing efforts to coordinate care outside the VA delivery system supported by the 2018 VA Maintaining Systems and Strengthening Integrated Outside Networks (MISSION) Act. Furthermore, these patient, treatment, and delivery system factors may each uniquely contribute to whether patients receive guideline-recommended PSA surveillance after prostate cancer treatment.
For these reasons, we conducted a study to better understand determinants of adherence to guideline-recommended PSA surveillance among veterans undergoing definitive radiation therapy with or without concurrent ADT. Our study uniquely included both elderly and nonelderly patients as well as investigated relationships between treatment at or away from the diagnosing facility. Although we found high overall levels of adherence to PSA surveillance, our findings do offer insights into determinants associated with worse adherence and provide opportunities to improve prostate cancer survivorship care after RT.
Methods
This study population included men with biopsy-proven nonmetastatic incident prostate cancer diagnosed between January 2005 and December 2008, with follow-up through 2012, identified using the VA Central Cancer Registry. We included men who underwent definitive RT with or without concurrent ADT injections, determined using the VA pharmacy files. We excluded men with a prior diagnosis of prostate or other malignancy (given the presence of other malignancies might affect life expectancy and surveillance patterns), hospice enrollment within 30 days, diagnosis at autopsy, and those treated with radical prostatectomy. We extracted cancer registry data, including biopsy Gleason score, pretreatment PSA level, clinical tumor stage, and whether RT was delivered at the patient’s diagnosing facility. For the latter, we used data on radiation location coded by the tumor registrar. We also collected demographic information, including age at diagnosis, race, ethnicity, marital status, and ZIP code. We used diagnosis codes to determine Charlson comorbidity scores similar to prior studies.6-8
Primary Outcome
The primary outcome was receipt of guideline concordant annual PSA surveillance in the initial 5 years following RT. We used laboratory files within the VA Corporate Data Warehouse to identify the date and value for each PSA test after RT for the entire cohort. Specifically, we defined the surveillance period as 60 days after initiation of RT through December 31, 2012. We defined guideline concordance as receiving at least 1 PSA test for each 12-month period after RT.
Statistical Analysis
We used descriptive statistics to characterize our cohort of veterans with prostate cancer treated with RT with or without concurrent ADT. To handle missing data, we performed multiple imputation, generating 10 imputations using all baseline clinical and demographic variables, year of diagnosis, and the regional VA network (ie, the Veterans Integrated Services Network [VISN]) for each patient.
Next, we calculated the annual guideline concordance rate for each year of follow-up for each patient, for the overall cohort, as well as by age, race/ethnicity, and concurrent ADT use. We examined bivariable relationships between guideline concordance and baseline demographic, clinical, and delivery system factors, including year of diagnosis and whether patients were treated at the diagnosing facility, using multilevel logistic regression modeling to account for clustering at the patient level.
Analyses were performed using Stata Version 15 (College Station, TX). We considered a 2-sided P value of < .05 as statistically significant. This study was approved by the VA Ann Arbor Health Care System Institution Review Board.
Results
We evaluated annual PSA surveillance for 15,538 men treated with RT with or without concurrent ADT (Table 1).
On unadjusted analysis, annual guideline concordance was less common among patients who were at the extremes of age, white, had Gleason 6 disease, PSA ≤ 10 ng/mL, did not receive concurrent ADT, and were treated away from their diagnosing facility (P < .05) (data not shown). We did find slight differences in patient characteristics based on whether patients were treated at their diagnosing facility (Table 2).
Overall, we found annual guideline concordance was initially very high, though declined slightly over the study period. For example, guideline concordance dropped from 96% in year 1 to 85% in year 5, with an average patient-level guideline concordance of 91% during the study period. We found minimal differences in annual surveillance after RT by race/ethnicity (Figure 1).
On multilevel multivariable analysis to adjust for clustering at the patient level, we found that race and PSA level were no longer significant predictors of annual surveillance (Table 3).
Discussion
We investigated adherence to guideline-recommended annual surveillance PSA testing in a national cohort of veterans treated with definitive RT for prostate cancer. We found guideline concordance was initially high and decreased slightly over time. We also found guideline concordance with PSA surveillance varied based on a number of clinical and delivery system factors, including marital status, rurality, receipt of concurrent ADT, as well as whether the veteran was treated at his diagnosing facility. Taken together, these overall results are promising, however, also point to unique considerations for some patient groups and potentially those treated in the community.
Our finding of lower guideline concordance among nonmarried patients is consistent with prior research, including our study of patients undergoing surgery for prostate cancer.4 Addressing surveillance in this population is important, as they may have less social support than do their married counterparts. We also found surveillance was lower at the extremes of age, which may be appropriate in elderly patients with limited life expectancy but is concerning for younger men with low competing mortality risks.7 Future work should explore whether younger patients experience barriers to care, including employment challenges, as these men are at greatest risk of cancer progression if recurrence goes undetected.
Although rural patients are less likely to undergo definitive prostate cancer treatment, possibly reflecting barriers to care, in our study, surveillance was actually higher among this population than that for urban patients.9 This could reflect the VA’s success in connecting rural patients to appropriate services despite travel distances to maintain quality of cancer care.10 Given annual PSA surveillance is relatively infrequent and not particularly resource intensive, these high surveillance rates might not apply to patients with cancers who need more frequent survivorship care, such as those with head and neck cancer. Future work should examine why surveillance rates among urban patients might be slightly lower, as living in a metropolitan area does not equate to the absence of barriers to survivorship care, especially for veterans who may not be able to take time off from work or have transportation barriers.
We found guideline concordance was higher among patients with higher Gleason scores, which is important given their higher likelihood of failure. However, low- and intermediate-risk patients also are at risk for treatment failure, so annual PSA surveillance should be optimized in this population unless future studies support the safety and feasibility of less frequent surveillance.10-13 Our finding of increased surveillance in patients who receive concurrent ADT may relate to the increased frequency of survivorship care given the need for injections, often every 3 to 6 months. Future studies might examine whether surveillance decreases in this population once they complete their short or long-term ADT, typically given for a maximum of 3 years.
A particularly relevant finding given recent VA policy changes includes lower guideline concordance for patients receiving RT at a different facility than where they were diagnosed. One possible explanation is that a proportion of patients treated outside of their home facilities use Medicare or private insurance and may have surveillance performed outside of the VA, which would not have been captured in our study.14 However, it remains plausible that there are challenges related to coordination and fragmentation of survivorship care for veterans who receive care at separate VA facilities or receive their initial treatment in the community.15 Future studies can help quantify how much this difference is driven by diagnosis and treatment at separate VA sites vs treatment outside of the VA, as different strategies might be necessary to improve surveillance in these 2 populations. Moreover, electronic health record-based tracking has been proposed as a strategy to identify patients who have not received guideline concordant PSA surveillance.14 This strategy may help increase guideline concordance regardless of initial treatment location if VA survivorship care is intended.
Although our study examined receipt of PSA testing, it did not examine whether patients are physically seen back in radiation oncology clinics, or whether their PSAs have been reviewed by radiation oncology providers. Although many surgical patients return to primary care providers for PSA surveillance, surveillance after RT is more complex and likely best managed in the initial years by radiation oncologists. Unlike the postoperative setting in which the definition of PSA failure is straightforward at > 0.2 ng/mL, the definition of treatment failure after RT is more complicated as described below.
For patients who did not receive concurrent ADT, failure is defined as a PSA nadir + 2 ng/mL, which first requires establishing the nadir using the first few postradiation PSA values.15 It becomes even more complex in the setting of ADT as it causes PSA suppression even in the absence of RT due to testosterone suppression.2 At the conclusion of ADT (short term 4-6 months or long term 18-36 months), the PSA may rise as testosterone recovers.15,16 This is not necessarily indicative of treatment failure, as some normal PSA-producing prostatic tissue may remain after treatment. Given these complexities, ongoing survivorship care with radiation oncology is recommended at least in the short term.
Physical visits are a challenge for some patients undergoing prostate cancer surveillance after treatment. Therefore, exploring the safety and feasibility of automated PSA tracking15 and strategies for increasing utilization of telemedicine, including clinical video telehealth appointments that are already used for survivorship and other urologic care in a number of VA clinics, represents opportunities to systematically provide highest quality survivorship care in VA.17,18
Conclusion
Most veterans receive guideline concordant PSA surveillance after RT for prostate cancer. Nonetheless, at the beginning of treatment, providers should screen veterans for risk factors for loss to follow-up (eg, care at a different or non-VA facility), discuss geographic, financial, and other barriers, and plan to leverage existing VA resources (eg, travel support) to continue to achieve high-quality PSA surveillance and survivorship care. Future research should investigate ways to take advantage of the VA’s robust electronic health record system and telemedicine infrastructure to further optimize prostate cancer survivorship care and PSA surveillance particularly among vulnerable patient groups and those treated outside of their diagnosing facility.
Acknowledgments
Funding Sources: VA HSR&D Career Development Award: 2 (CDA 12−171) and NCI R37 R37CA222885 (TAS).
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the US Government, or any of its agencies.
1. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: prostate cancer v4.2018. https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Updated August 15, 2018. Accessed January 23, 2019.
2. Sanda MG, Chen RC, Crispino T, et al. Clinically localized prostate cancer: AUA/ASTRO/SUO guideline. https://www.auanet.org/guidelines/prostate-cancer-clinically-localized-(2017). Published 2017. Accessed January 22,2019.
3. Zeliadt SB, Penson DF, Albertsen PC, Concato J, Etzioni RD. Race independently predicts prostate specific antigen testing frequency following a prostate carcinoma diagnosis. Cancer. 2003;98(3):496-503.
4. Trantham LC, Nielsen ME, Mobley LR, Wheeler SB, Carpenter WR, Biddle AK. Use of prostate-specific antigen testing as a disease surveillance tool following radical prostatectomy. Cancer. 2013;119(19):3523-3530.
5. Shi Y, Fung KZ, John Boscardin W, et al. Individualizing PSA monitoring among older prostate cancer survivors. J Gen Intern Med. 2018;33(5):602-604.
6. Chapman C, Burns J, Caram M, Zaslavsky A, Tsodikov A, Skolarus TA. Multilevel predictors of surveillance PSA guideline concordance after radical prostatectomy: a national Veterans Affairs study. Paper presented at: Association of VA Hematology/Oncology Annual Meeting;
September 28-30, 2018; Chicago, IL. Abstract 34. https://www.mdedge.com/fedprac/avaho/article/175094/prostate-cancer/multilevel-predictors-surveillance-psa-guideline. Accessed January 22, 2019.
7. Kirk PS, Borza T, Caram MEV, et al. Characterising potential bone scan overuse amongst men treated with radical prostatectomy. BJU Int. 2018. [Epub ahead of print.]
8. Kirk PS, Borza T, Shahinian VB, et al. The implications of baseline bone-health assessment at initiation of androgen-deprivation therapy for prostate cancer. BJU Int. 2018;121(4):558-564.
9. Baldwin LM, Andrilla CH, Porter MP, Rosenblatt RA, Patel S, Doescher MP. Treatment of early-stage prostate cancer among rural and urban patients. Cancer. 2013;119(16):3067-3075.
10. Skolarus TA, Chan S, Shelton JB, et al. Quality of prostate cancer care among rural men in the Veterans Health Administration. Cancer. 2013;119(20):3629-3635.
11. Hamdy FC, Donovan JL, Lane JA, et al; ProtecT Study Group. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med. 2016;375(15):1415-1424.
12. Michalski JM, Moughan J, Purdy J, et al. Effect of standard vs dose-escalated radiation therapy for patients with intermediate-risk prostate cancer: the NRG Oncology RTOG 0126 randomized clinical trial. JAMA Oncol.2018;4(6):e180039.
13. Chang MG, DeSotto K, Taibi P, Troeschel S. Development of a PSA tracking system for patients with prostate cancer following definitive radiotherapy to enhance rural health. J Clin Oncol. 2016;34(suppl 2):39-39.
14. Skolarus TA, Zhang Y, Hollenbeck BK. Understanding fragmentation of prostate cancer survivorship care: implications for cost and quality. Cancer. 2012;118(11):2837-2845.
15. Roach M, 3rd, Hanks G, Thames H Jr, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65(4):965-974.
16. Buyyounouski MK, Hanlon AL, Horwitz EM, Uzzo RG, Pollack A. Biochemical failure and the temporal kinetics of prostate-specific antigen after radiation therapy with androgen deprivation. Int J Radiat Oncol Biol Phys. 2005;61(5):1291-1298.
17. Chu S, Boxer R, Madison P, et al. Veterans Affairs telemedicine: bringing urologic care to remote clinics. Urology. 2015;86(2):255-260.
18. Safir IJ, Gabale S, David SA, et al. Implementation of a tele-urology program for outpatient hematuria referrals: initial results and patient satisfaction. Urology. 2016;97:33-39.
Guideline concordance with PSA surveillance among veterans treated with definitiveradiation therapy was generally high, but opportunities may exist to improve surveillance among select groups.
Guideline concordance with PSA surveillance among veterans treated with definitiveradiation therapy was generally high, but opportunities may exist to improve surveillance among select groups.
Guidelines recommend prostate-specific antigen (PSA) surveillance among men treated with definitive radiation therapy (RT) for prostate cancer. Specifically, the National Comprehensive Cancer Network recommends testing every 6 to 12 months for 5 years and annually thereafter (with no specific stopping period specified), while the American Urology Association recommends testing for at least 10 years, with the frequency to be determined by the risk of relapse and patient preferences for monitoring.1,2 Salvage treatments exist for men with localized recurrence identified early through PSA testing, so adherence to follow-up guidelines is important for quality prostate cancer survivorship care.1,2
However, few studies focus on adherence to PSA surveillance following radiation therapy. Posttreatment surveillance among surgical patients is generally high, but sociodemographic disparities exist. Racial and ethnic minorities and unmarried men are less likely to undergo guideline concordant surveillance than is the general population, potentially preventing effective salvage therapy.3,4 A recent Department of Veterans Affairs (VA) study on posttreatment surveillance included radiation therapy patients but did not examine the impact of younger age, concurrent androgen deprivation therapy (ADT), or treatment facility (ie, diagnosed and treated at the same vs different facilities, with the latter including a separate VA facility or the community) on surveillance patterns.5 The latter is particularly relevant given increasing efforts to coordinate care outside the VA delivery system supported by the 2018 VA Maintaining Systems and Strengthening Integrated Outside Networks (MISSION) Act. Furthermore, these patient, treatment, and delivery system factors may each uniquely contribute to whether patients receive guideline-recommended PSA surveillance after prostate cancer treatment.
For these reasons, we conducted a study to better understand determinants of adherence to guideline-recommended PSA surveillance among veterans undergoing definitive radiation therapy with or without concurrent ADT. Our study uniquely included both elderly and nonelderly patients as well as investigated relationships between treatment at or away from the diagnosing facility. Although we found high overall levels of adherence to PSA surveillance, our findings do offer insights into determinants associated with worse adherence and provide opportunities to improve prostate cancer survivorship care after RT.
Methods
This study population included men with biopsy-proven nonmetastatic incident prostate cancer diagnosed between January 2005 and December 2008, with follow-up through 2012, identified using the VA Central Cancer Registry. We included men who underwent definitive RT with or without concurrent ADT injections, determined using the VA pharmacy files. We excluded men with a prior diagnosis of prostate or other malignancy (given the presence of other malignancies might affect life expectancy and surveillance patterns), hospice enrollment within 30 days, diagnosis at autopsy, and those treated with radical prostatectomy. We extracted cancer registry data, including biopsy Gleason score, pretreatment PSA level, clinical tumor stage, and whether RT was delivered at the patient’s diagnosing facility. For the latter, we used data on radiation location coded by the tumor registrar. We also collected demographic information, including age at diagnosis, race, ethnicity, marital status, and ZIP code. We used diagnosis codes to determine Charlson comorbidity scores similar to prior studies.6-8
Primary Outcome
The primary outcome was receipt of guideline concordant annual PSA surveillance in the initial 5 years following RT. We used laboratory files within the VA Corporate Data Warehouse to identify the date and value for each PSA test after RT for the entire cohort. Specifically, we defined the surveillance period as 60 days after initiation of RT through December 31, 2012. We defined guideline concordance as receiving at least 1 PSA test for each 12-month period after RT.
Statistical Analysis
We used descriptive statistics to characterize our cohort of veterans with prostate cancer treated with RT with or without concurrent ADT. To handle missing data, we performed multiple imputation, generating 10 imputations using all baseline clinical and demographic variables, year of diagnosis, and the regional VA network (ie, the Veterans Integrated Services Network [VISN]) for each patient.
Next, we calculated the annual guideline concordance rate for each year of follow-up for each patient, for the overall cohort, as well as by age, race/ethnicity, and concurrent ADT use. We examined bivariable relationships between guideline concordance and baseline demographic, clinical, and delivery system factors, including year of diagnosis and whether patients were treated at the diagnosing facility, using multilevel logistic regression modeling to account for clustering at the patient level.
Analyses were performed using Stata Version 15 (College Station, TX). We considered a 2-sided P value of < .05 as statistically significant. This study was approved by the VA Ann Arbor Health Care System Institution Review Board.
Results
We evaluated annual PSA surveillance for 15,538 men treated with RT with or without concurrent ADT (Table 1).
On unadjusted analysis, annual guideline concordance was less common among patients who were at the extremes of age, white, had Gleason 6 disease, PSA ≤ 10 ng/mL, did not receive concurrent ADT, and were treated away from their diagnosing facility (P < .05) (data not shown). We did find slight differences in patient characteristics based on whether patients were treated at their diagnosing facility (Table 2).
Overall, we found annual guideline concordance was initially very high, though declined slightly over the study period. For example, guideline concordance dropped from 96% in year 1 to 85% in year 5, with an average patient-level guideline concordance of 91% during the study period. We found minimal differences in annual surveillance after RT by race/ethnicity (Figure 1).
On multilevel multivariable analysis to adjust for clustering at the patient level, we found that race and PSA level were no longer significant predictors of annual surveillance (Table 3).
Discussion
We investigated adherence to guideline-recommended annual surveillance PSA testing in a national cohort of veterans treated with definitive RT for prostate cancer. We found guideline concordance was initially high and decreased slightly over time. We also found guideline concordance with PSA surveillance varied based on a number of clinical and delivery system factors, including marital status, rurality, receipt of concurrent ADT, as well as whether the veteran was treated at his diagnosing facility. Taken together, these overall results are promising, however, also point to unique considerations for some patient groups and potentially those treated in the community.
Our finding of lower guideline concordance among nonmarried patients is consistent with prior research, including our study of patients undergoing surgery for prostate cancer.4 Addressing surveillance in this population is important, as they may have less social support than do their married counterparts. We also found surveillance was lower at the extremes of age, which may be appropriate in elderly patients with limited life expectancy but is concerning for younger men with low competing mortality risks.7 Future work should explore whether younger patients experience barriers to care, including employment challenges, as these men are at greatest risk of cancer progression if recurrence goes undetected.
Although rural patients are less likely to undergo definitive prostate cancer treatment, possibly reflecting barriers to care, in our study, surveillance was actually higher among this population than that for urban patients.9 This could reflect the VA’s success in connecting rural patients to appropriate services despite travel distances to maintain quality of cancer care.10 Given annual PSA surveillance is relatively infrequent and not particularly resource intensive, these high surveillance rates might not apply to patients with cancers who need more frequent survivorship care, such as those with head and neck cancer. Future work should examine why surveillance rates among urban patients might be slightly lower, as living in a metropolitan area does not equate to the absence of barriers to survivorship care, especially for veterans who may not be able to take time off from work or have transportation barriers.
We found guideline concordance was higher among patients with higher Gleason scores, which is important given their higher likelihood of failure. However, low- and intermediate-risk patients also are at risk for treatment failure, so annual PSA surveillance should be optimized in this population unless future studies support the safety and feasibility of less frequent surveillance.10-13 Our finding of increased surveillance in patients who receive concurrent ADT may relate to the increased frequency of survivorship care given the need for injections, often every 3 to 6 months. Future studies might examine whether surveillance decreases in this population once they complete their short or long-term ADT, typically given for a maximum of 3 years.
A particularly relevant finding given recent VA policy changes includes lower guideline concordance for patients receiving RT at a different facility than where they were diagnosed. One possible explanation is that a proportion of patients treated outside of their home facilities use Medicare or private insurance and may have surveillance performed outside of the VA, which would not have been captured in our study.14 However, it remains plausible that there are challenges related to coordination and fragmentation of survivorship care for veterans who receive care at separate VA facilities or receive their initial treatment in the community.15 Future studies can help quantify how much this difference is driven by diagnosis and treatment at separate VA sites vs treatment outside of the VA, as different strategies might be necessary to improve surveillance in these 2 populations. Moreover, electronic health record-based tracking has been proposed as a strategy to identify patients who have not received guideline concordant PSA surveillance.14 This strategy may help increase guideline concordance regardless of initial treatment location if VA survivorship care is intended.
Although our study examined receipt of PSA testing, it did not examine whether patients are physically seen back in radiation oncology clinics, or whether their PSAs have been reviewed by radiation oncology providers. Although many surgical patients return to primary care providers for PSA surveillance, surveillance after RT is more complex and likely best managed in the initial years by radiation oncologists. Unlike the postoperative setting in which the definition of PSA failure is straightforward at > 0.2 ng/mL, the definition of treatment failure after RT is more complicated as described below.
For patients who did not receive concurrent ADT, failure is defined as a PSA nadir + 2 ng/mL, which first requires establishing the nadir using the first few postradiation PSA values.15 It becomes even more complex in the setting of ADT as it causes PSA suppression even in the absence of RT due to testosterone suppression.2 At the conclusion of ADT (short term 4-6 months or long term 18-36 months), the PSA may rise as testosterone recovers.15,16 This is not necessarily indicative of treatment failure, as some normal PSA-producing prostatic tissue may remain after treatment. Given these complexities, ongoing survivorship care with radiation oncology is recommended at least in the short term.
Physical visits are a challenge for some patients undergoing prostate cancer surveillance after treatment. Therefore, exploring the safety and feasibility of automated PSA tracking15 and strategies for increasing utilization of telemedicine, including clinical video telehealth appointments that are already used for survivorship and other urologic care in a number of VA clinics, represents opportunities to systematically provide highest quality survivorship care in VA.17,18
Conclusion
Most veterans receive guideline concordant PSA surveillance after RT for prostate cancer. Nonetheless, at the beginning of treatment, providers should screen veterans for risk factors for loss to follow-up (eg, care at a different or non-VA facility), discuss geographic, financial, and other barriers, and plan to leverage existing VA resources (eg, travel support) to continue to achieve high-quality PSA surveillance and survivorship care. Future research should investigate ways to take advantage of the VA’s robust electronic health record system and telemedicine infrastructure to further optimize prostate cancer survivorship care and PSA surveillance particularly among vulnerable patient groups and those treated outside of their diagnosing facility.
Acknowledgments
Funding Sources: VA HSR&D Career Development Award: 2 (CDA 12−171) and NCI R37 R37CA222885 (TAS).
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the US Government, or any of its agencies.
Guidelines recommend prostate-specific antigen (PSA) surveillance among men treated with definitive radiation therapy (RT) for prostate cancer. Specifically, the National Comprehensive Cancer Network recommends testing every 6 to 12 months for 5 years and annually thereafter (with no specific stopping period specified), while the American Urology Association recommends testing for at least 10 years, with the frequency to be determined by the risk of relapse and patient preferences for monitoring.1,2 Salvage treatments exist for men with localized recurrence identified early through PSA testing, so adherence to follow-up guidelines is important for quality prostate cancer survivorship care.1,2
However, few studies focus on adherence to PSA surveillance following radiation therapy. Posttreatment surveillance among surgical patients is generally high, but sociodemographic disparities exist. Racial and ethnic minorities and unmarried men are less likely to undergo guideline concordant surveillance than is the general population, potentially preventing effective salvage therapy.3,4 A recent Department of Veterans Affairs (VA) study on posttreatment surveillance included radiation therapy patients but did not examine the impact of younger age, concurrent androgen deprivation therapy (ADT), or treatment facility (ie, diagnosed and treated at the same vs different facilities, with the latter including a separate VA facility or the community) on surveillance patterns.5 The latter is particularly relevant given increasing efforts to coordinate care outside the VA delivery system supported by the 2018 VA Maintaining Systems and Strengthening Integrated Outside Networks (MISSION) Act. Furthermore, these patient, treatment, and delivery system factors may each uniquely contribute to whether patients receive guideline-recommended PSA surveillance after prostate cancer treatment.
For these reasons, we conducted a study to better understand determinants of adherence to guideline-recommended PSA surveillance among veterans undergoing definitive radiation therapy with or without concurrent ADT. Our study uniquely included both elderly and nonelderly patients as well as investigated relationships between treatment at or away from the diagnosing facility. Although we found high overall levels of adherence to PSA surveillance, our findings do offer insights into determinants associated with worse adherence and provide opportunities to improve prostate cancer survivorship care after RT.
Methods
This study population included men with biopsy-proven nonmetastatic incident prostate cancer diagnosed between January 2005 and December 2008, with follow-up through 2012, identified using the VA Central Cancer Registry. We included men who underwent definitive RT with or without concurrent ADT injections, determined using the VA pharmacy files. We excluded men with a prior diagnosis of prostate or other malignancy (given the presence of other malignancies might affect life expectancy and surveillance patterns), hospice enrollment within 30 days, diagnosis at autopsy, and those treated with radical prostatectomy. We extracted cancer registry data, including biopsy Gleason score, pretreatment PSA level, clinical tumor stage, and whether RT was delivered at the patient’s diagnosing facility. For the latter, we used data on radiation location coded by the tumor registrar. We also collected demographic information, including age at diagnosis, race, ethnicity, marital status, and ZIP code. We used diagnosis codes to determine Charlson comorbidity scores similar to prior studies.6-8
Primary Outcome
The primary outcome was receipt of guideline concordant annual PSA surveillance in the initial 5 years following RT. We used laboratory files within the VA Corporate Data Warehouse to identify the date and value for each PSA test after RT for the entire cohort. Specifically, we defined the surveillance period as 60 days after initiation of RT through December 31, 2012. We defined guideline concordance as receiving at least 1 PSA test for each 12-month period after RT.
Statistical Analysis
We used descriptive statistics to characterize our cohort of veterans with prostate cancer treated with RT with or without concurrent ADT. To handle missing data, we performed multiple imputation, generating 10 imputations using all baseline clinical and demographic variables, year of diagnosis, and the regional VA network (ie, the Veterans Integrated Services Network [VISN]) for each patient.
Next, we calculated the annual guideline concordance rate for each year of follow-up for each patient, for the overall cohort, as well as by age, race/ethnicity, and concurrent ADT use. We examined bivariable relationships between guideline concordance and baseline demographic, clinical, and delivery system factors, including year of diagnosis and whether patients were treated at the diagnosing facility, using multilevel logistic regression modeling to account for clustering at the patient level.
Analyses were performed using Stata Version 15 (College Station, TX). We considered a 2-sided P value of < .05 as statistically significant. This study was approved by the VA Ann Arbor Health Care System Institution Review Board.
Results
We evaluated annual PSA surveillance for 15,538 men treated with RT with or without concurrent ADT (Table 1).
On unadjusted analysis, annual guideline concordance was less common among patients who were at the extremes of age, white, had Gleason 6 disease, PSA ≤ 10 ng/mL, did not receive concurrent ADT, and were treated away from their diagnosing facility (P < .05) (data not shown). We did find slight differences in patient characteristics based on whether patients were treated at their diagnosing facility (Table 2).
Overall, we found annual guideline concordance was initially very high, though declined slightly over the study period. For example, guideline concordance dropped from 96% in year 1 to 85% in year 5, with an average patient-level guideline concordance of 91% during the study period. We found minimal differences in annual surveillance after RT by race/ethnicity (Figure 1).
On multilevel multivariable analysis to adjust for clustering at the patient level, we found that race and PSA level were no longer significant predictors of annual surveillance (Table 3).
Discussion
We investigated adherence to guideline-recommended annual surveillance PSA testing in a national cohort of veterans treated with definitive RT for prostate cancer. We found guideline concordance was initially high and decreased slightly over time. We also found guideline concordance with PSA surveillance varied based on a number of clinical and delivery system factors, including marital status, rurality, receipt of concurrent ADT, as well as whether the veteran was treated at his diagnosing facility. Taken together, these overall results are promising, however, also point to unique considerations for some patient groups and potentially those treated in the community.
Our finding of lower guideline concordance among nonmarried patients is consistent with prior research, including our study of patients undergoing surgery for prostate cancer.4 Addressing surveillance in this population is important, as they may have less social support than do their married counterparts. We also found surveillance was lower at the extremes of age, which may be appropriate in elderly patients with limited life expectancy but is concerning for younger men with low competing mortality risks.7 Future work should explore whether younger patients experience barriers to care, including employment challenges, as these men are at greatest risk of cancer progression if recurrence goes undetected.
Although rural patients are less likely to undergo definitive prostate cancer treatment, possibly reflecting barriers to care, in our study, surveillance was actually higher among this population than that for urban patients.9 This could reflect the VA’s success in connecting rural patients to appropriate services despite travel distances to maintain quality of cancer care.10 Given annual PSA surveillance is relatively infrequent and not particularly resource intensive, these high surveillance rates might not apply to patients with cancers who need more frequent survivorship care, such as those with head and neck cancer. Future work should examine why surveillance rates among urban patients might be slightly lower, as living in a metropolitan area does not equate to the absence of barriers to survivorship care, especially for veterans who may not be able to take time off from work or have transportation barriers.
We found guideline concordance was higher among patients with higher Gleason scores, which is important given their higher likelihood of failure. However, low- and intermediate-risk patients also are at risk for treatment failure, so annual PSA surveillance should be optimized in this population unless future studies support the safety and feasibility of less frequent surveillance.10-13 Our finding of increased surveillance in patients who receive concurrent ADT may relate to the increased frequency of survivorship care given the need for injections, often every 3 to 6 months. Future studies might examine whether surveillance decreases in this population once they complete their short or long-term ADT, typically given for a maximum of 3 years.
A particularly relevant finding given recent VA policy changes includes lower guideline concordance for patients receiving RT at a different facility than where they were diagnosed. One possible explanation is that a proportion of patients treated outside of their home facilities use Medicare or private insurance and may have surveillance performed outside of the VA, which would not have been captured in our study.14 However, it remains plausible that there are challenges related to coordination and fragmentation of survivorship care for veterans who receive care at separate VA facilities or receive their initial treatment in the community.15 Future studies can help quantify how much this difference is driven by diagnosis and treatment at separate VA sites vs treatment outside of the VA, as different strategies might be necessary to improve surveillance in these 2 populations. Moreover, electronic health record-based tracking has been proposed as a strategy to identify patients who have not received guideline concordant PSA surveillance.14 This strategy may help increase guideline concordance regardless of initial treatment location if VA survivorship care is intended.
Although our study examined receipt of PSA testing, it did not examine whether patients are physically seen back in radiation oncology clinics, or whether their PSAs have been reviewed by radiation oncology providers. Although many surgical patients return to primary care providers for PSA surveillance, surveillance after RT is more complex and likely best managed in the initial years by radiation oncologists. Unlike the postoperative setting in which the definition of PSA failure is straightforward at > 0.2 ng/mL, the definition of treatment failure after RT is more complicated as described below.
For patients who did not receive concurrent ADT, failure is defined as a PSA nadir + 2 ng/mL, which first requires establishing the nadir using the first few postradiation PSA values.15 It becomes even more complex in the setting of ADT as it causes PSA suppression even in the absence of RT due to testosterone suppression.2 At the conclusion of ADT (short term 4-6 months or long term 18-36 months), the PSA may rise as testosterone recovers.15,16 This is not necessarily indicative of treatment failure, as some normal PSA-producing prostatic tissue may remain after treatment. Given these complexities, ongoing survivorship care with radiation oncology is recommended at least in the short term.
Physical visits are a challenge for some patients undergoing prostate cancer surveillance after treatment. Therefore, exploring the safety and feasibility of automated PSA tracking15 and strategies for increasing utilization of telemedicine, including clinical video telehealth appointments that are already used for survivorship and other urologic care in a number of VA clinics, represents opportunities to systematically provide highest quality survivorship care in VA.17,18
Conclusion
Most veterans receive guideline concordant PSA surveillance after RT for prostate cancer. Nonetheless, at the beginning of treatment, providers should screen veterans for risk factors for loss to follow-up (eg, care at a different or non-VA facility), discuss geographic, financial, and other barriers, and plan to leverage existing VA resources (eg, travel support) to continue to achieve high-quality PSA surveillance and survivorship care. Future research should investigate ways to take advantage of the VA’s robust electronic health record system and telemedicine infrastructure to further optimize prostate cancer survivorship care and PSA surveillance particularly among vulnerable patient groups and those treated outside of their diagnosing facility.
Acknowledgments
Funding Sources: VA HSR&D Career Development Award: 2 (CDA 12−171) and NCI R37 R37CA222885 (TAS).
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the US Government, or any of its agencies.
1. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: prostate cancer v4.2018. https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Updated August 15, 2018. Accessed January 23, 2019.
2. Sanda MG, Chen RC, Crispino T, et al. Clinically localized prostate cancer: AUA/ASTRO/SUO guideline. https://www.auanet.org/guidelines/prostate-cancer-clinically-localized-(2017). Published 2017. Accessed January 22,2019.
3. Zeliadt SB, Penson DF, Albertsen PC, Concato J, Etzioni RD. Race independently predicts prostate specific antigen testing frequency following a prostate carcinoma diagnosis. Cancer. 2003;98(3):496-503.
4. Trantham LC, Nielsen ME, Mobley LR, Wheeler SB, Carpenter WR, Biddle AK. Use of prostate-specific antigen testing as a disease surveillance tool following radical prostatectomy. Cancer. 2013;119(19):3523-3530.
5. Shi Y, Fung KZ, John Boscardin W, et al. Individualizing PSA monitoring among older prostate cancer survivors. J Gen Intern Med. 2018;33(5):602-604.
6. Chapman C, Burns J, Caram M, Zaslavsky A, Tsodikov A, Skolarus TA. Multilevel predictors of surveillance PSA guideline concordance after radical prostatectomy: a national Veterans Affairs study. Paper presented at: Association of VA Hematology/Oncology Annual Meeting;
September 28-30, 2018; Chicago, IL. Abstract 34. https://www.mdedge.com/fedprac/avaho/article/175094/prostate-cancer/multilevel-predictors-surveillance-psa-guideline. Accessed January 22, 2019.
7. Kirk PS, Borza T, Caram MEV, et al. Characterising potential bone scan overuse amongst men treated with radical prostatectomy. BJU Int. 2018. [Epub ahead of print.]
8. Kirk PS, Borza T, Shahinian VB, et al. The implications of baseline bone-health assessment at initiation of androgen-deprivation therapy for prostate cancer. BJU Int. 2018;121(4):558-564.
9. Baldwin LM, Andrilla CH, Porter MP, Rosenblatt RA, Patel S, Doescher MP. Treatment of early-stage prostate cancer among rural and urban patients. Cancer. 2013;119(16):3067-3075.
10. Skolarus TA, Chan S, Shelton JB, et al. Quality of prostate cancer care among rural men in the Veterans Health Administration. Cancer. 2013;119(20):3629-3635.
11. Hamdy FC, Donovan JL, Lane JA, et al; ProtecT Study Group. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med. 2016;375(15):1415-1424.
12. Michalski JM, Moughan J, Purdy J, et al. Effect of standard vs dose-escalated radiation therapy for patients with intermediate-risk prostate cancer: the NRG Oncology RTOG 0126 randomized clinical trial. JAMA Oncol.2018;4(6):e180039.
13. Chang MG, DeSotto K, Taibi P, Troeschel S. Development of a PSA tracking system for patients with prostate cancer following definitive radiotherapy to enhance rural health. J Clin Oncol. 2016;34(suppl 2):39-39.
14. Skolarus TA, Zhang Y, Hollenbeck BK. Understanding fragmentation of prostate cancer survivorship care: implications for cost and quality. Cancer. 2012;118(11):2837-2845.
15. Roach M, 3rd, Hanks G, Thames H Jr, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65(4):965-974.
16. Buyyounouski MK, Hanlon AL, Horwitz EM, Uzzo RG, Pollack A. Biochemical failure and the temporal kinetics of prostate-specific antigen after radiation therapy with androgen deprivation. Int J Radiat Oncol Biol Phys. 2005;61(5):1291-1298.
17. Chu S, Boxer R, Madison P, et al. Veterans Affairs telemedicine: bringing urologic care to remote clinics. Urology. 2015;86(2):255-260.
18. Safir IJ, Gabale S, David SA, et al. Implementation of a tele-urology program for outpatient hematuria referrals: initial results and patient satisfaction. Urology. 2016;97:33-39.
1. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: prostate cancer v4.2018. https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Updated August 15, 2018. Accessed January 23, 2019.
2. Sanda MG, Chen RC, Crispino T, et al. Clinically localized prostate cancer: AUA/ASTRO/SUO guideline. https://www.auanet.org/guidelines/prostate-cancer-clinically-localized-(2017). Published 2017. Accessed January 22,2019.
3. Zeliadt SB, Penson DF, Albertsen PC, Concato J, Etzioni RD. Race independently predicts prostate specific antigen testing frequency following a prostate carcinoma diagnosis. Cancer. 2003;98(3):496-503.
4. Trantham LC, Nielsen ME, Mobley LR, Wheeler SB, Carpenter WR, Biddle AK. Use of prostate-specific antigen testing as a disease surveillance tool following radical prostatectomy. Cancer. 2013;119(19):3523-3530.
5. Shi Y, Fung KZ, John Boscardin W, et al. Individualizing PSA monitoring among older prostate cancer survivors. J Gen Intern Med. 2018;33(5):602-604.
6. Chapman C, Burns J, Caram M, Zaslavsky A, Tsodikov A, Skolarus TA. Multilevel predictors of surveillance PSA guideline concordance after radical prostatectomy: a national Veterans Affairs study. Paper presented at: Association of VA Hematology/Oncology Annual Meeting;
September 28-30, 2018; Chicago, IL. Abstract 34. https://www.mdedge.com/fedprac/avaho/article/175094/prostate-cancer/multilevel-predictors-surveillance-psa-guideline. Accessed January 22, 2019.
7. Kirk PS, Borza T, Caram MEV, et al. Characterising potential bone scan overuse amongst men treated with radical prostatectomy. BJU Int. 2018. [Epub ahead of print.]
8. Kirk PS, Borza T, Shahinian VB, et al. The implications of baseline bone-health assessment at initiation of androgen-deprivation therapy for prostate cancer. BJU Int. 2018;121(4):558-564.
9. Baldwin LM, Andrilla CH, Porter MP, Rosenblatt RA, Patel S, Doescher MP. Treatment of early-stage prostate cancer among rural and urban patients. Cancer. 2013;119(16):3067-3075.
10. Skolarus TA, Chan S, Shelton JB, et al. Quality of prostate cancer care among rural men in the Veterans Health Administration. Cancer. 2013;119(20):3629-3635.
11. Hamdy FC, Donovan JL, Lane JA, et al; ProtecT Study Group. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med. 2016;375(15):1415-1424.
12. Michalski JM, Moughan J, Purdy J, et al. Effect of standard vs dose-escalated radiation therapy for patients with intermediate-risk prostate cancer: the NRG Oncology RTOG 0126 randomized clinical trial. JAMA Oncol.2018;4(6):e180039.
13. Chang MG, DeSotto K, Taibi P, Troeschel S. Development of a PSA tracking system for patients with prostate cancer following definitive radiotherapy to enhance rural health. J Clin Oncol. 2016;34(suppl 2):39-39.
14. Skolarus TA, Zhang Y, Hollenbeck BK. Understanding fragmentation of prostate cancer survivorship care: implications for cost and quality. Cancer. 2012;118(11):2837-2845.
15. Roach M, 3rd, Hanks G, Thames H Jr, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65(4):965-974.
16. Buyyounouski MK, Hanlon AL, Horwitz EM, Uzzo RG, Pollack A. Biochemical failure and the temporal kinetics of prostate-specific antigen after radiation therapy with androgen deprivation. Int J Radiat Oncol Biol Phys. 2005;61(5):1291-1298.
17. Chu S, Boxer R, Madison P, et al. Veterans Affairs telemedicine: bringing urologic care to remote clinics. Urology. 2015;86(2):255-260.
18. Safir IJ, Gabale S, David SA, et al. Implementation of a tele-urology program for outpatient hematuria referrals: initial results and patient satisfaction. Urology. 2016;97:33-39.