User login
Role of Speech Pathology in a Multidisciplinary Approach to a Patient With Mild Traumatic Brain Injury
Speech-language pathologists can fill a unique need in the treatment of patients with several conditions that are seen regularly in primary care.
Speech-language pathologists (SLPs) are integral to the comprehensive treatment of mild traumatic brain injury (mTBI), yet the evaluation and treatment options they offer may not be known to all primary care providers (PCPs). As the research on the management and treatment of mTBI continues to evolve, the PCPs role in referring patients with mTBI to the appropriate resources becomes imperative.
mTBI is a common injury in both military and civilian settings, but it can be difficult to diagnose and is not always well understood. Long-term debilitating effects have been associated with mTBI, with literature linking it to an increased risk of developing Alzheimer disease, motor neuron disease, and Parkinson disease.1 In addition, mTBI is a strong predictor for the development of posttraumatic stress disorder (PTSD). Among returning Iraq and Afghanistan service members, the incidence of mTBI associated mental health conditions have been reported to be as high as 22.8%, affecting > 320,000 veterans.2-5
The US Department of Veteran Affairs (VA) health care system offers these returning veterans a comprehensive, multidisciplinary treatment strategy. The care is often coordinated by the veteran’s patient aligned care team (PACT) that consists of a PCP, nurses, and a medical support associate. The US Department of Defense (DoD) and VA also facilitated the development of a clinical practice guideline (CPG) that can be used by the PACT and other health care providers to support evidence based patient-centered care. This CPG is extensive and has recommendations for evaluation and treatment of mTBI and the symptoms associated such as impaired memory and alterations in executive function.6
The following hypothetical case is based on an actual patient. This case illustrates the role of speech pathology in caring for patients with mTBI.
Case Presentation
A 25-year-old male combat veteran presented to his VA PACT team for a new patient visit. As part of the screening of his medical history, mTBI was fully defined for the patient to include “alteration” in consciousness. This reminded the patient of an injury that occurred 1 year prior to presentation during a routine convoy mission. He was riding in the back of a Humvee when it hit a large pothole slamming his head into the side of the vehicle. He reported that he felt “dazed and dizzy” with “ringing” in his ears immediately following the event, without an overt loss of consciousness. He was unable to seek medical attention secondary to the urgency of the convoy mission, so he “shook it off” and kept going. Later that week he noted headache and insomnia. He was seen and evaluated by his health care provider for insomnia, but when questioned he reported no head trauma as he had forgotten the incident. Upon follow-up with his PCP, he reported his headaches were manageable, and his insomnia was somewhat improved with recommended life-style modifications and good sleep hygiene.
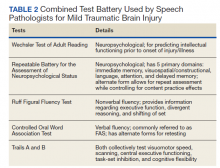
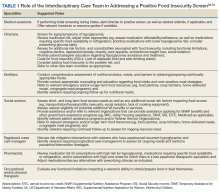
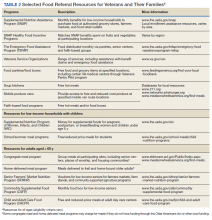
He still had frequent headaches, dizziness, and some insomnia. However, his chief concern was that he was struggling with new schoolwork. He noted that he was a straight-A student prior to his military service. A review of his medical history in his medical chart showed that a previous PCP had treated his associated symptoms of insomnia and headache without improvement. In addition, he had recently been diagnosed with PTSD. As his symptoms had lasted > 90 days, not resolved with initial treatment in primary care, and were causing a significant impact on his activities of daily living, his PCP placed a consult to Speech Pathology for cognitive-linguistic assessment and treatment, if indicated, noting that he may have had a mTBI.6 Although not intended to be comprehensive, Table 1 describes several clinical areas where a speech pathology referral may be appropriate.
The Role of the Speech-Language Pathologist
The speech-language pathologist takes an additional history of the patient. This better quantifies specific details of the veteran’s functional concerns pertaining to possible difficulty with attention, memory, executive function, visuospatial awareness, etc. Examples might include difficulty with attention/memory, including not remembering what to get at the store, forgetting to take medications, forgetting appointments, and difficulty in school, among many others. Reports of feeling “stupid” also are common. Assessment varies by clinician, but it is not uncommon for the SLP to administer a battery of evaluations to help identify a range of possible impairments. Choosing testing that is sensitive to even mild impairment is important and should be used in combination with subjective complaints. Mild deficits can sometimes be missed in those with average performance, but whose premorbid intelligence was above average. One combination of test batteries sometimes utilized is the Wechsler Test of Adult Reading (WTAR), the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS), the Ruff Figural Fluency Test (RFFT), the Controlled Oral Word Association Test (COWAT), and Trails A and B (Table 2).
The initial testing results are discussed with the veteran. If patient concerns and/or testing reveal impairment that is amenable to treatment and the veteran wishes to proceed, subsequent treatment sessions are scheduled. The first treatment session is spent establishing and prioritizing functional goals specific to that individual and their needs (eg, for daily life, work, school). In a case of subacute or older mTBI, as is often seen in veterans coming to the VA, intervention often targets strategies and techniques that can help the individual compensate for current deficits.
Many patients already own a smartphone, so this device often is used functionally as a cognitive prosthetic as early as the first treatment session. In an effort to immediately start addressing important issues like medication management and attending appointments, the veteran is educated to the benefit of entering important information into the calendar and/or reminder apps on their phone and setting associated alarms that would serve as a reminder for what was entered. Patients are often encouraged by the positive impact of these initial strategies and look forward to future treatment sessions to address compensation for their functional deficits.
If a veteran with TBI has numerous needs, it can be beneficial for the care team to discuss the care plan at an interdisciplinary team meeting. It is not uncommon for veterans like the one discussed above to be referred to neurology (persistent headaches and further neurological evaluation); mental health (PTSD treatment and family support/counseling options); occupational therapy (visuospatial needs); and audiology (vestibular concerns). Social work involvement is often extremely beneficial for coordination of care in more complex cases. If patient is having difficulty making healthy eating choices or with meal preparation, a consult to a dietitian may prove invaluable. Concerns related to trouble with medication adherence (beyond memory-related adherence issues that speech pathology would address) or polypharmacy can be directed to a clinical pharmacy specialist, who could prepare a medication chart, review optimal medication timing, and provide education on adverse effects. A veteran's communication with the team can be facilitated through secure messaging (a method of secure emailing) and encouraging use of the My HealtheVet portal. With this modality, patients could review chart notes and results and share them with non-VA health care providers and/or family members as indicated.
A whole health approach also may appeal to some mTBI patients. This approach focuses on the totality of patient needs for healthy living and on patient-centered goal setting. Services provided may differ at various VA medical centers, but the PACT team can connect the veteran to the services of interest.
Conclusions
A team approach to veterans with mTBI provides a comprehensive way to treat the various problems associated with the condition. Further research into the role of multidisciplinary teams in the management of mTBI was recommended in the 2016 CPG.6 The unique role that the speech-language pathologist plays as part of this team has been highlighted, as it is important that PCP’s be aware of the extent of evaluation and treatment services they offer. Beyond mTBI, speech pathologists evaluate and treat patients with several conditions that are seen regularly in primary care.
1. McKee AC, Robinson ME. Military-related traumatic brain injury and neurodegeneration. Alzheimers Dement. 2014;10(3 suppl):S242-S253. doi:10.1016/j.jalz.2014.04.003
2. Yurgil KA, Barkauskas DA, Vasterling JJ, et al. Association between traumatic brain injury and risk of posttraumatic stress disorder in active-duty Marines. JAMA Psychiatry. 2014;71(2):149-157. doi:10.1001/jamapsychiatry.2013.3080
3. Chin DL, Zeber JE. Mental Health Outcomes Among Military Service Members After Severe Injury in Combat and TBI. Mil Med. 2020;185(5-6):e711-e718. doi:10.1093/milmed/usz440
4. Hoge CW, Auchterlonie JL, Milliken CS. Mental health problems, use of mental health services, and attrition from military service after returning from deployment to Iraq or Afghanistan. JAMA. 2006;295(9):1023-1032. doi:10.1001/jama.295.9.1023
5. Miles SR, Harik JM, Hundt NE, et al. Delivery of mental health treatment to combat veterans with psychiatric diagnoses and TBI histories. PLoS One. 2017;12(9):e0184265. Published 2017 Sep 8. doi:10.1371/journal.pone.0184265
6. US Department of Defense, US Department of Veterans Affairs; Management of Concussion/mTBI Working Group. VA/DoD clinical practice guideline for management of concussion/mild traumatic brain injury. Version 2.0. Published February 2016. Accessed February 8, 2021. https://www.healthquality.va.gov/guidelines/Rehab/mtbi/mTBICPGFullCPG50821816.pdf
Speech-language pathologists can fill a unique need in the treatment of patients with several conditions that are seen regularly in primary care.
Speech-language pathologists can fill a unique need in the treatment of patients with several conditions that are seen regularly in primary care.
Speech-language pathologists (SLPs) are integral to the comprehensive treatment of mild traumatic brain injury (mTBI), yet the evaluation and treatment options they offer may not be known to all primary care providers (PCPs). As the research on the management and treatment of mTBI continues to evolve, the PCPs role in referring patients with mTBI to the appropriate resources becomes imperative.
mTBI is a common injury in both military and civilian settings, but it can be difficult to diagnose and is not always well understood. Long-term debilitating effects have been associated with mTBI, with literature linking it to an increased risk of developing Alzheimer disease, motor neuron disease, and Parkinson disease.1 In addition, mTBI is a strong predictor for the development of posttraumatic stress disorder (PTSD). Among returning Iraq and Afghanistan service members, the incidence of mTBI associated mental health conditions have been reported to be as high as 22.8%, affecting > 320,000 veterans.2-5
The US Department of Veteran Affairs (VA) health care system offers these returning veterans a comprehensive, multidisciplinary treatment strategy. The care is often coordinated by the veteran’s patient aligned care team (PACT) that consists of a PCP, nurses, and a medical support associate. The US Department of Defense (DoD) and VA also facilitated the development of a clinical practice guideline (CPG) that can be used by the PACT and other health care providers to support evidence based patient-centered care. This CPG is extensive and has recommendations for evaluation and treatment of mTBI and the symptoms associated such as impaired memory and alterations in executive function.6
The following hypothetical case is based on an actual patient. This case illustrates the role of speech pathology in caring for patients with mTBI.
Case Presentation
A 25-year-old male combat veteran presented to his VA PACT team for a new patient visit. As part of the screening of his medical history, mTBI was fully defined for the patient to include “alteration” in consciousness. This reminded the patient of an injury that occurred 1 year prior to presentation during a routine convoy mission. He was riding in the back of a Humvee when it hit a large pothole slamming his head into the side of the vehicle. He reported that he felt “dazed and dizzy” with “ringing” in his ears immediately following the event, without an overt loss of consciousness. He was unable to seek medical attention secondary to the urgency of the convoy mission, so he “shook it off” and kept going. Later that week he noted headache and insomnia. He was seen and evaluated by his health care provider for insomnia, but when questioned he reported no head trauma as he had forgotten the incident. Upon follow-up with his PCP, he reported his headaches were manageable, and his insomnia was somewhat improved with recommended life-style modifications and good sleep hygiene.
He still had frequent headaches, dizziness, and some insomnia. However, his chief concern was that he was struggling with new schoolwork. He noted that he was a straight-A student prior to his military service. A review of his medical history in his medical chart showed that a previous PCP had treated his associated symptoms of insomnia and headache without improvement. In addition, he had recently been diagnosed with PTSD. As his symptoms had lasted > 90 days, not resolved with initial treatment in primary care, and were causing a significant impact on his activities of daily living, his PCP placed a consult to Speech Pathology for cognitive-linguistic assessment and treatment, if indicated, noting that he may have had a mTBI.6 Although not intended to be comprehensive, Table 1 describes several clinical areas where a speech pathology referral may be appropriate.
The Role of the Speech-Language Pathologist
The speech-language pathologist takes an additional history of the patient. This better quantifies specific details of the veteran’s functional concerns pertaining to possible difficulty with attention, memory, executive function, visuospatial awareness, etc. Examples might include difficulty with attention/memory, including not remembering what to get at the store, forgetting to take medications, forgetting appointments, and difficulty in school, among many others. Reports of feeling “stupid” also are common. Assessment varies by clinician, but it is not uncommon for the SLP to administer a battery of evaluations to help identify a range of possible impairments. Choosing testing that is sensitive to even mild impairment is important and should be used in combination with subjective complaints. Mild deficits can sometimes be missed in those with average performance, but whose premorbid intelligence was above average. One combination of test batteries sometimes utilized is the Wechsler Test of Adult Reading (WTAR), the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS), the Ruff Figural Fluency Test (RFFT), the Controlled Oral Word Association Test (COWAT), and Trails A and B (Table 2).
The initial testing results are discussed with the veteran. If patient concerns and/or testing reveal impairment that is amenable to treatment and the veteran wishes to proceed, subsequent treatment sessions are scheduled. The first treatment session is spent establishing and prioritizing functional goals specific to that individual and their needs (eg, for daily life, work, school). In a case of subacute or older mTBI, as is often seen in veterans coming to the VA, intervention often targets strategies and techniques that can help the individual compensate for current deficits.
Many patients already own a smartphone, so this device often is used functionally as a cognitive prosthetic as early as the first treatment session. In an effort to immediately start addressing important issues like medication management and attending appointments, the veteran is educated to the benefit of entering important information into the calendar and/or reminder apps on their phone and setting associated alarms that would serve as a reminder for what was entered. Patients are often encouraged by the positive impact of these initial strategies and look forward to future treatment sessions to address compensation for their functional deficits.
If a veteran with TBI has numerous needs, it can be beneficial for the care team to discuss the care plan at an interdisciplinary team meeting. It is not uncommon for veterans like the one discussed above to be referred to neurology (persistent headaches and further neurological evaluation); mental health (PTSD treatment and family support/counseling options); occupational therapy (visuospatial needs); and audiology (vestibular concerns). Social work involvement is often extremely beneficial for coordination of care in more complex cases. If patient is having difficulty making healthy eating choices or with meal preparation, a consult to a dietitian may prove invaluable. Concerns related to trouble with medication adherence (beyond memory-related adherence issues that speech pathology would address) or polypharmacy can be directed to a clinical pharmacy specialist, who could prepare a medication chart, review optimal medication timing, and provide education on adverse effects. A veteran's communication with the team can be facilitated through secure messaging (a method of secure emailing) and encouraging use of the My HealtheVet portal. With this modality, patients could review chart notes and results and share them with non-VA health care providers and/or family members as indicated.
A whole health approach also may appeal to some mTBI patients. This approach focuses on the totality of patient needs for healthy living and on patient-centered goal setting. Services provided may differ at various VA medical centers, but the PACT team can connect the veteran to the services of interest.
Conclusions
A team approach to veterans with mTBI provides a comprehensive way to treat the various problems associated with the condition. Further research into the role of multidisciplinary teams in the management of mTBI was recommended in the 2016 CPG.6 The unique role that the speech-language pathologist plays as part of this team has been highlighted, as it is important that PCP’s be aware of the extent of evaluation and treatment services they offer. Beyond mTBI, speech pathologists evaluate and treat patients with several conditions that are seen regularly in primary care.
Speech-language pathologists (SLPs) are integral to the comprehensive treatment of mild traumatic brain injury (mTBI), yet the evaluation and treatment options they offer may not be known to all primary care providers (PCPs). As the research on the management and treatment of mTBI continues to evolve, the PCPs role in referring patients with mTBI to the appropriate resources becomes imperative.
mTBI is a common injury in both military and civilian settings, but it can be difficult to diagnose and is not always well understood. Long-term debilitating effects have been associated with mTBI, with literature linking it to an increased risk of developing Alzheimer disease, motor neuron disease, and Parkinson disease.1 In addition, mTBI is a strong predictor for the development of posttraumatic stress disorder (PTSD). Among returning Iraq and Afghanistan service members, the incidence of mTBI associated mental health conditions have been reported to be as high as 22.8%, affecting > 320,000 veterans.2-5
The US Department of Veteran Affairs (VA) health care system offers these returning veterans a comprehensive, multidisciplinary treatment strategy. The care is often coordinated by the veteran’s patient aligned care team (PACT) that consists of a PCP, nurses, and a medical support associate. The US Department of Defense (DoD) and VA also facilitated the development of a clinical practice guideline (CPG) that can be used by the PACT and other health care providers to support evidence based patient-centered care. This CPG is extensive and has recommendations for evaluation and treatment of mTBI and the symptoms associated such as impaired memory and alterations in executive function.6
The following hypothetical case is based on an actual patient. This case illustrates the role of speech pathology in caring for patients with mTBI.
Case Presentation
A 25-year-old male combat veteran presented to his VA PACT team for a new patient visit. As part of the screening of his medical history, mTBI was fully defined for the patient to include “alteration” in consciousness. This reminded the patient of an injury that occurred 1 year prior to presentation during a routine convoy mission. He was riding in the back of a Humvee when it hit a large pothole slamming his head into the side of the vehicle. He reported that he felt “dazed and dizzy” with “ringing” in his ears immediately following the event, without an overt loss of consciousness. He was unable to seek medical attention secondary to the urgency of the convoy mission, so he “shook it off” and kept going. Later that week he noted headache and insomnia. He was seen and evaluated by his health care provider for insomnia, but when questioned he reported no head trauma as he had forgotten the incident. Upon follow-up with his PCP, he reported his headaches were manageable, and his insomnia was somewhat improved with recommended life-style modifications and good sleep hygiene.
He still had frequent headaches, dizziness, and some insomnia. However, his chief concern was that he was struggling with new schoolwork. He noted that he was a straight-A student prior to his military service. A review of his medical history in his medical chart showed that a previous PCP had treated his associated symptoms of insomnia and headache without improvement. In addition, he had recently been diagnosed with PTSD. As his symptoms had lasted > 90 days, not resolved with initial treatment in primary care, and were causing a significant impact on his activities of daily living, his PCP placed a consult to Speech Pathology for cognitive-linguistic assessment and treatment, if indicated, noting that he may have had a mTBI.6 Although not intended to be comprehensive, Table 1 describes several clinical areas where a speech pathology referral may be appropriate.
The Role of the Speech-Language Pathologist
The speech-language pathologist takes an additional history of the patient. This better quantifies specific details of the veteran’s functional concerns pertaining to possible difficulty with attention, memory, executive function, visuospatial awareness, etc. Examples might include difficulty with attention/memory, including not remembering what to get at the store, forgetting to take medications, forgetting appointments, and difficulty in school, among many others. Reports of feeling “stupid” also are common. Assessment varies by clinician, but it is not uncommon for the SLP to administer a battery of evaluations to help identify a range of possible impairments. Choosing testing that is sensitive to even mild impairment is important and should be used in combination with subjective complaints. Mild deficits can sometimes be missed in those with average performance, but whose premorbid intelligence was above average. One combination of test batteries sometimes utilized is the Wechsler Test of Adult Reading (WTAR), the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS), the Ruff Figural Fluency Test (RFFT), the Controlled Oral Word Association Test (COWAT), and Trails A and B (Table 2).
The initial testing results are discussed with the veteran. If patient concerns and/or testing reveal impairment that is amenable to treatment and the veteran wishes to proceed, subsequent treatment sessions are scheduled. The first treatment session is spent establishing and prioritizing functional goals specific to that individual and their needs (eg, for daily life, work, school). In a case of subacute or older mTBI, as is often seen in veterans coming to the VA, intervention often targets strategies and techniques that can help the individual compensate for current deficits.
Many patients already own a smartphone, so this device often is used functionally as a cognitive prosthetic as early as the first treatment session. In an effort to immediately start addressing important issues like medication management and attending appointments, the veteran is educated to the benefit of entering important information into the calendar and/or reminder apps on their phone and setting associated alarms that would serve as a reminder for what was entered. Patients are often encouraged by the positive impact of these initial strategies and look forward to future treatment sessions to address compensation for their functional deficits.
If a veteran with TBI has numerous needs, it can be beneficial for the care team to discuss the care plan at an interdisciplinary team meeting. It is not uncommon for veterans like the one discussed above to be referred to neurology (persistent headaches and further neurological evaluation); mental health (PTSD treatment and family support/counseling options); occupational therapy (visuospatial needs); and audiology (vestibular concerns). Social work involvement is often extremely beneficial for coordination of care in more complex cases. If patient is having difficulty making healthy eating choices or with meal preparation, a consult to a dietitian may prove invaluable. Concerns related to trouble with medication adherence (beyond memory-related adherence issues that speech pathology would address) or polypharmacy can be directed to a clinical pharmacy specialist, who could prepare a medication chart, review optimal medication timing, and provide education on adverse effects. A veteran's communication with the team can be facilitated through secure messaging (a method of secure emailing) and encouraging use of the My HealtheVet portal. With this modality, patients could review chart notes and results and share them with non-VA health care providers and/or family members as indicated.
A whole health approach also may appeal to some mTBI patients. This approach focuses on the totality of patient needs for healthy living and on patient-centered goal setting. Services provided may differ at various VA medical centers, but the PACT team can connect the veteran to the services of interest.
Conclusions
A team approach to veterans with mTBI provides a comprehensive way to treat the various problems associated with the condition. Further research into the role of multidisciplinary teams in the management of mTBI was recommended in the 2016 CPG.6 The unique role that the speech-language pathologist plays as part of this team has been highlighted, as it is important that PCP’s be aware of the extent of evaluation and treatment services they offer. Beyond mTBI, speech pathologists evaluate and treat patients with several conditions that are seen regularly in primary care.
1. McKee AC, Robinson ME. Military-related traumatic brain injury and neurodegeneration. Alzheimers Dement. 2014;10(3 suppl):S242-S253. doi:10.1016/j.jalz.2014.04.003
2. Yurgil KA, Barkauskas DA, Vasterling JJ, et al. Association between traumatic brain injury and risk of posttraumatic stress disorder in active-duty Marines. JAMA Psychiatry. 2014;71(2):149-157. doi:10.1001/jamapsychiatry.2013.3080
3. Chin DL, Zeber JE. Mental Health Outcomes Among Military Service Members After Severe Injury in Combat and TBI. Mil Med. 2020;185(5-6):e711-e718. doi:10.1093/milmed/usz440
4. Hoge CW, Auchterlonie JL, Milliken CS. Mental health problems, use of mental health services, and attrition from military service after returning from deployment to Iraq or Afghanistan. JAMA. 2006;295(9):1023-1032. doi:10.1001/jama.295.9.1023
5. Miles SR, Harik JM, Hundt NE, et al. Delivery of mental health treatment to combat veterans with psychiatric diagnoses and TBI histories. PLoS One. 2017;12(9):e0184265. Published 2017 Sep 8. doi:10.1371/journal.pone.0184265
6. US Department of Defense, US Department of Veterans Affairs; Management of Concussion/mTBI Working Group. VA/DoD clinical practice guideline for management of concussion/mild traumatic brain injury. Version 2.0. Published February 2016. Accessed February 8, 2021. https://www.healthquality.va.gov/guidelines/Rehab/mtbi/mTBICPGFullCPG50821816.pdf
1. McKee AC, Robinson ME. Military-related traumatic brain injury and neurodegeneration. Alzheimers Dement. 2014;10(3 suppl):S242-S253. doi:10.1016/j.jalz.2014.04.003
2. Yurgil KA, Barkauskas DA, Vasterling JJ, et al. Association between traumatic brain injury and risk of posttraumatic stress disorder in active-duty Marines. JAMA Psychiatry. 2014;71(2):149-157. doi:10.1001/jamapsychiatry.2013.3080
3. Chin DL, Zeber JE. Mental Health Outcomes Among Military Service Members After Severe Injury in Combat and TBI. Mil Med. 2020;185(5-6):e711-e718. doi:10.1093/milmed/usz440
4. Hoge CW, Auchterlonie JL, Milliken CS. Mental health problems, use of mental health services, and attrition from military service after returning from deployment to Iraq or Afghanistan. JAMA. 2006;295(9):1023-1032. doi:10.1001/jama.295.9.1023
5. Miles SR, Harik JM, Hundt NE, et al. Delivery of mental health treatment to combat veterans with psychiatric diagnoses and TBI histories. PLoS One. 2017;12(9):e0184265. Published 2017 Sep 8. doi:10.1371/journal.pone.0184265
6. US Department of Defense, US Department of Veterans Affairs; Management of Concussion/mTBI Working Group. VA/DoD clinical practice guideline for management of concussion/mild traumatic brain injury. Version 2.0. Published February 2016. Accessed February 8, 2021. https://www.healthquality.va.gov/guidelines/Rehab/mtbi/mTBICPGFullCPG50821816.pdf
Validation of the Timberlawn Couple and Family Evaluation Scales–Self-Report in Veterans with PTSD
Although about 8.3% of the general adult civilian population will be diagnosed with posttraumatic stress disorder (PTSD) in their lifetime, rates of PTSD are even higher in the veteran population.1,2 PTSD is associated with a number of psychosocial consequences in veterans, including decreased intimate partner relationship functioning.3,4 For example, Cloitre and colleagues reported that PTSD is associated with difficulty with socializing, intimacy, responsibility, and control, all of which increase difficulties in intimate partner relationships.5 Similarly, researchers also have noted that traumatic experiences can affect an individual’s attachment style, resulting in progressive avoidance of interpersonal relationships, which can lead to marked difficulties in maintaining and beginning intimate partner relationships.6,7 Despite these known consequences of PTSD, as Dekel and Monson noted in a review,further research is still needed regarding the mechanisms by which trauma and PTSD result in decreased intimate partner relationship functioning among veterans.8 Nonetheless, as positive interpersonal relationships are associated with decreased PTSD symptom severity9,10 and increased engagement in PTSD treatment,11 determining methods of measuring intimate partner relationship functioning in veterans with PTSD is important to inform future research and aid the provision of care.
To date, limited research has examined the valid measurement of intimate partner relationship functioning among veterans with PTSD. Many existing measures that comprehensively assess intimate partner relationship functioning are time and resource intensive. One such measure, the Timberlawn Couple and Family Evaluation Scales (TCFES), comprehensively assesses multiple pertinent domains of intimate partner relationship functioning (ie, structure, autonomy, problem solving, affect regulation, and disagreement/conflict).12 By assessing multiple domains, the TCFES offers a method of understanding the specific components of an individual’s intimate partner relationship in need of increased clinical attention.12 However, the TCFES is a time- and labor-intensive observational measure that requires a couple to interact while a blinded, independent rater observes and rates their interactions using an intricate coding process. This survey structure precludes the ability to quickly and comprehensively assess a veteran’s intimate partner functioning in settings such as mental health outpatient clinics where mental health providers engage in brief, time-limited psychotherapy. As such, brief measures of intimate partner relationship functioning are needed to best inform clinical care among veterans with PTSD.
The primary aim of the current study was to create a psychometrically valid, yet brief, self-report version of the TCFES to assess multiple domains of intimate partner relationship functioning. The psychometric properties of this measure were assessed among a sample of US veterans with PTSD who were in an intimate partner relationship. We specifically examined factor structure, reliability, and associations to established measures of specific domains of relational functioning.
Methods
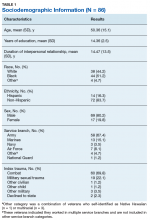
Ninety-four veterans were recruited via posted advertisements, promotion in PTSD therapy groups/staff meetings, and word of mouth at the Dallas Veterans Affairs Medical Center (VAMC). Participants were eligible if they had a documented diagnosis of PTSD as confirmed in the veteran’s electronic medical record and an affirmative response to currently being involved in an intimate partner relationship (ie, legally married, common-law spouse, involved in a relationship/partnership). There were no exclusion criteria.
Interested veterans were invited to complete several study-related self-report measures concerning their intimate partner relationships that would take about an hour. They were informed that the surveys were voluntary and confidential, and that they would be compensated for their participation. All veterans who participated provided written consent and the study was approved by the Dallas VAMC institutional review board.
Of the 94 veterans recruited, 3 veterans’ data were removed from current analyses after informed consent but before completing the surveys when they indicated they were not currently in a relationship or were divorced. After consent, the 91 participants were administered several study-related self-report measures. The measures took between 30 and 55 minutes to complete. Participants were then compensated $25 for their participation.
Intimate Partner Relationship Functioning
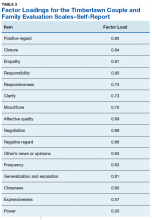
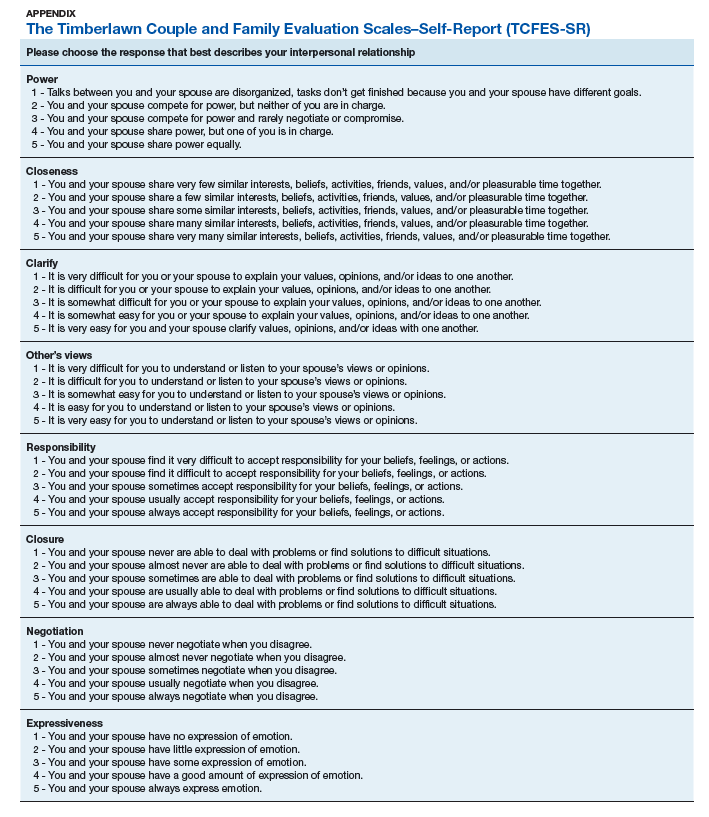
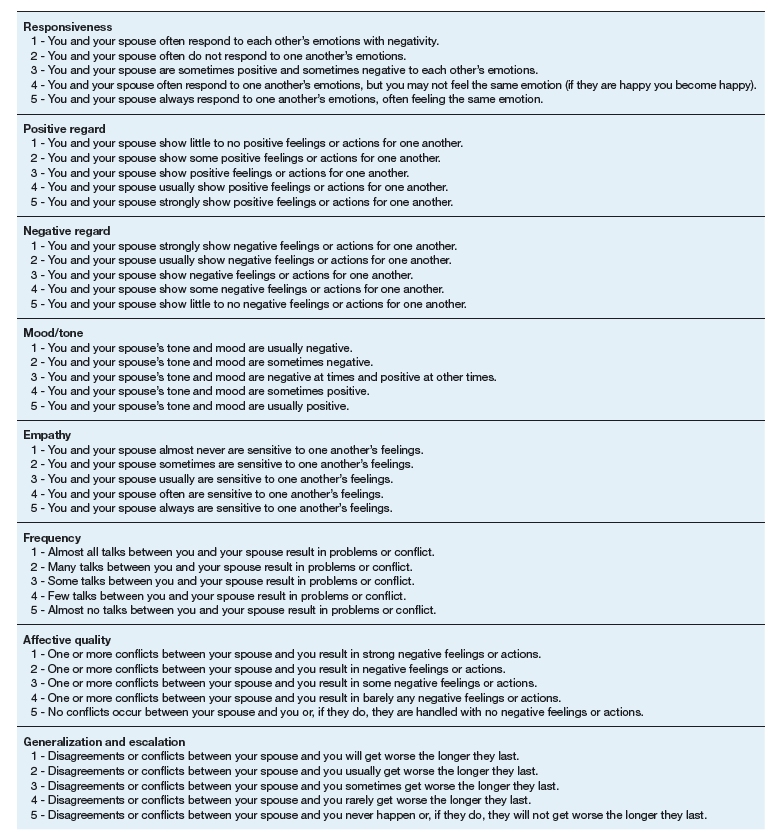
The 16-item TCFES self-report version (TCFES-SR) was developed to assess multiple domains of interpersonal functioning (Appendix). The observational TCFES assesses 5 intimate partner relationship characteristic domains (ie, structure, autonomy, problem solving, affect regulation, and disagreement/conflict) during a couple’s interaction by an independent trained rater.12 Each of the 16 TCFES-SR items were modeled after original constructs measured by the TCFES, including power, closeness, clarify, other’s views, responsibility, closure, negotiation, expressiveness, responsiveness, positive regard, negative regard, mood/tone, empathy, frequency, affective quality, and generalization and escalation. To maintain consistency with the TCFES, each item of the TCFES-SR was scored from 1 (severely dysfunctional) to 5 (highly functional). Additionally, all item wording for the TCFES-SR was based on wording in the TCFES manual after consultation with an expert who facilitated the development of the TCFES.12 On average, the TCFES-SR took 5 to 10 minutes to complete.
To measure concurrent validity of the modified TCFES-SR, several additional interpersonal measures were selected and administered based on prior research and established domains of the TCFES. The Positive and Negative Quality in Marriage Scale (PANQIMS) was administered to assess perceived attitudes toward a relationship.13,14 The PANQIMS generates 2 subscales: positive quality and negative quality in the relationship. Because the PANQIMS specifically assesses married relationships and our sample included married and nonmarried participants, wording was modified (eg, “spouse/partner”).
The relative power subscale of the Network Relationships Inventory–Relationship Qualities Version (NRI-RQV) measure was administered to assess the unequal/shared role romantic partners have in power equality (ie, relative power).15
The Revised Dyadic Adjustment Scale (RDAS) is a self-report measure that assesses multiple dimensions of marital adjustment and functioning.16 Six subscales of the RDAS were chosen based on items of the TCFES-SR: decision making, values, affection, conflict, activities, and discussion.
The Interpersonal Reactivity Index (IRI) empathetic concern subscale was administered to assess empathy across multiple contexts and situations17 and the Experiences in Close Relationships-Revised Questionnaire (ECR-R) was administered to assess relational functioning by determining attachment-related anxiety and avoidance.18
Sociodemographic Information
A sociodemographic questionnaire also was administered. The questionnaire assessed gender, age, education, service branch, length of interpersonal relationship, race, and ethnicity of the veteran as well as gender of the veteran’s partner.
Statistical Analysis
Factor structure of the TCFES-SR was determined by conducting an exploratory factor analysis. To allow for correlation between items, the Promax oblique rotation method was chosen.19 Number of factors was determined by agreement between number of eigenvalues ≥ 1, visual inspection of the scree plot, and a parallel analysis. Factor loadings of ≥ 0.3 were used to determine which items loaded on to which factors.
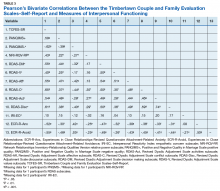
Convergent validity was assessed by conducting Pearson’s bivariate correlations between identified TCFES-SR factor(s) and other administered measures of interpersonal functioning (ie, PANQIMS positive and negative quality; NRI-RQV relative power subscale; RDAS decision making, values, affection, conflict, activities, and discussion subscales; IRI-empathetic concern subscale; and ECR-R attachment-related anxiety and avoidance subscales). Strength of relationship was determined based on the following guidelines: ± 0.3 to 0.49 = small, ± 0.5 to 0.69 = moderate, and ± 0.7 to 1.00 = large. Internal consistency was also determined for TCFES-SR factor(s) using Cronbach’s α. A standard level of significance (α=.05) was used for all statistical analyses.
Results
Eighty-six veterans provided complete data (Table 1). The Kaiser-Meyer-Olkin measure of sampling adequacy was indicative that sample size was adequate (.91), while Bartlett’s test of sphericity found the variables were suitable for structure detection, χ2 (120) = 800.00, P < .001. While 2 eigenvalues were ≥ 1, visual inspection of the scree plot and subsequent parallel analysis identified a unidimensional structure (ie, 1 factor) for the TCFES-SR. All items were found to load to this single factor, with all loadings being ≥ 0.5 (Table 2). Additionally, internal consistency was excellent for the scale (α = .93).
Pearson’s bivariate correlations were significant (P < .05) between TCFES-SR total score, and almost all administered interpersonal functioning measures (Table 3). Interestingly, no significant associations were found between any of the administered measures, including the TCFES-SR total score, and the IRI-empathetic concern subscale (P > .05).
Discussion
These findings provide initial support for the psychometric properties of the TCFES-SR, including excellent internal consistency and the adequate association of its total score to established measures of interpersonal functioning. Contrary to the TCFES, the TCFES-SR was shown to best fit a unidimensional factor rather than a multidimensional measure of relationship functioning. However, the TCFES-SR was also shown to have strong convergent validity with multiple domains of relationship functioning, indicating that the measure of overall intimate partner relationship functioning encompasses a number of relational domains (ie, structure, autonomy, problem solving, affect regulation, and disagreement/conflict). Critically, the TCFES-SR is brief and was administered easily in our sample, providing utility as clinical tool to be used in time-sensitive outpatient settings.
A unidimensional factor has particular strength in providing a global portrait of perceived intimate partner relationship functioning, and mental health providers can administer the TCFES-SR to assess for overall perceptions of intimate partner relationship functioning rather than administering a number of measures focusing on specific interpersonal domains (eg, decision making processes or positive/negative attitudes towards one’s relationship). This allows for the quick assessment (ie, 5-10 minutes) of overall intimate partner relationship functioning rather than administration of multiple self-report measures which can be time-intensive and expensive. However, the TCFES-SR also is limited by a lack of nuanced understanding of perceptions of functioning specific to particular domains. For example, the TCFES-SR score cannot describe intimate partner functioning in the domain of problem solving. Therefore, brief screening tools need to be developed that assess multiple intimate partner relationship domains.
Importantly, overall intimate partner relationship functioning as measured by the TCFES-SR may not incorporate perceptions of relationship empathy, as the total score did not correlate with a measure of empathetic concern (ie, the IRI-empathetic concern subscale). As empathy was based on one item in the TCFES-SR vs 7 in the IRI-empathetic concern subscale, it is unclear if the TCFES-SR only captures a portion of the construct of empathy (ie, sensitivity to partner) vs the comprehensive assessment of trait empathy that the IRI subscale measures. Additionally, the IRI-empathetic concern subscale did not significantly correlate with any of the other administered measures of relationship functioning. Given the role of empathy in positive, healthy intimate partner relationships, future research should explore the role of empathetic concern among veterans with PTSD as it relates to overall (eg, TCFES-SR) and specific aspects of intimate partner relationship functioning.20
While the clinical applicability of the TCFES-SR requires further examination, this measure has a number of potential uses. Information captured quickly by the TCFES-SR may help to inform appropriate referral for treatment. For instance, veterans reporting low total scores on the TCFES-SR may indicate a need for a referral for intervention focused on improving overall relationship functioning (eg, Integrative Behavioral Couple Therapy).21,22 Measurement-based care (ie, tracking and discussing changes in symptoms during treatment using validated self-report measures) is now required by the Joint Commission as a standard of care,and has been shown to improve outcomes in couples therapy.23,24 As a brief self-report measure, the TCFES-SR may be able to facilitate measurement-based care and assist providers in tracking changes in overall relationship functioning over the course of treatment. However, the purpose of the current study was to validate the TCFES-SR and not to examine the utility of the TCFES-SR in clinical care; additional research is needed to determine standardized cutoff scores to indicate a need for clinical intervention.
Limitations
Several limitations should be noted. The current study only assessed perceived intimate partner relationship functioning from the perspective of the veteran, thus limiting implications as it pertains to the spouse/partner of the veteran. PTSD diagnosis was based on chart review rather than a psychodiagnostic measure (eg, Clinician Administered PTSD Scale); therefore, whether this diagnosis was current or in remission was unclear. Although our sample was adequate to conduct an exploratory factor analysis,the overall sample size was modest, and results should be considered preliminary with need for further replication.25 The sample was also primarily male, white or black, and non-Hispanic; therefore, results may not generalize to a more sociodemographically diverse population. Finally, given the focus of the study to develop a self-report measure, we did not compare the TCFES-SR to the original TCFES. Thus, further research examining the relationship between the TCFES-SR and TCFES may be needed to better understand overlap and potential incongruence in these measures, and to ascertain any differences in their factor structures.
Conclusion
This study is novel in that it adapted a comprehensive observational measure of relationship functioning to a self-report measure piloted among a sample of veterans with PTSD in an intimate partner relationship, a clinical population that remains largely understudied. Although findings are preliminary, the TCFES-SR was found to be a reliable and valid measure of overall intimate partner relationship functioning. Given the rapid administration of this self-report measure, the TCFES-SR may hold clinical utility as a screen of intimate partner relationship deficits in need of clinical intervention. Replication in a larger, more diverse sample is needed to further examine the generalizability and confirm psychometric properties of the TCFES-SR. Additionally, further understanding of the clinical utility of the TCFES-SR in treatment settings remains critical to promote the development and maintenance of healthy intimate partner relationships among veterans with PTSD. Finally, development of effective self-report measures of intimate partner relationship functioning, such as the TCFES-SR, may help to facilitate needed research to understand the effect of PTSD on establishing and maintaining healthy intimate partner relationships among veterans.
Acknowledgments
The current study was funded by the Timberlawn Psychiatric Research Foundation. This material is the result of work supported in part by the US Department of Veterans Affairs; the Rocky Mountain Mental Illness Research, Education and Clinical Center (MIRECC) for Suicide Prevention; Sierra Pacific MIRECC; and the Office of Academic Affiliations, Advanced Fellowship Program in Mental Illness Research and Treatment, Department of Veterans Affairs.
1. Kilpatrick DG, Resnick HS, Milanak ME, Miller MW, Keyes KM, Friedman MJ. National estimates of exposure to traumatic events and PTSD prevalence using DSM-IV and DSM-5 criteria. J Trauma Stress. 2013;26(5):537-547.
2. Lehavot K, Goldberg SB, Chen JA, et al. Do trauma type, stressful life events, and social support explain women veterans’ high prevalence of PTSD? Soc Psychiatry Psychiatr Epidemiol. 2018;53(9):943-953.
3. Galovski T, Lyons JA. Psychological sequelae of combat violence: a review of the impact of PTSD on the veteran’s family and possible interventions. Aggress Violent Behav. 2004;9(5):477-501.
4. Ray SL, Vanstone M. The impact of PTSD on veterans’ family relationships: an interpretative phenomenological inquiry. Int J Nurs Stud. 2009;46(6):838-847.
5. Cloitre M, Miranda R, Stovall-McClough KC, Han H. Beyond PTSD: emotion regulation and interpersonal problems as predictors of functional impairment in survivors of childhood abuse. Behav Ther. 2005;36(2):119-124.
6. McFarlane AC, Bookless C. The effect of PTSD on interpersonal relationships: issues for emergency service works. Sex Relation Ther. 2001;16(3):261-267.
7. Itzhaky L, Stein JY, Levin Y, Solomon Z. Posttraumatic stress symptoms and marital adjustment among Israeli combat veterans: the role of loneliness and attachment. Psychol Trauma. 2017;9(6):655-662.
8. Dekel R, Monson CM. Military-related post-traumatic stress disorder and family relations: current knowledge and future directions. Aggress Violent Behav. 2010;15(4):303-309.
9. Allen ES, Rhoades GK, Stanley SM, Markman HJ. Hitting home: relationships between recent deployment, posttraumatic stress symptoms, and marital functioning for Army couples. J Fam Psychol. 2010;24(3):280-288.
10. Laffaye C, Cavella S, Drescher K, Rosen C. Relationships among PTSD symptoms, social support, and support source in veterans with chronic PTSD. J Trauma Stress. 2008;21(4):394-401.
11. Meis LA, Noorbaloochi S, Hagel Campbell EM, et al. Sticking it out in trauma-focused treatment for PTSD: it takes a village. J Consult Clin Psychol. 2019;87(3):246-256.
12. Lewis JM, Gossett JT, Housson MM, Owen MT. Timberlawn Couple and Family Evaluation Scales. Dallas, TX: Timberlawn Psychiatric Research Foundation; 1999.
13. Fincham FD, Linfield KJ. A new look at marital quality: can spouses feel positive and negative about their marriage? J Fam Psychol. 1997;11(4):489-502.
14. Kaplan KJ. On the ambivalence-indifference problem in attitude theory and measurement: a suggested modification of the semantic differential technique. Psychol Bull. 1972;77(5):361-372.
15. Buhrmester D, Furman W. The Network of Relationship Inventory: Relationship Qualities Version [unpublished measure]. University of Texas at Dallas; 2008.
16. Busby DM, Christensen C, Crane DR, Larson JH. A revision of the Dyadic Adjustment Scale for use with distressed and nondistressed couples: construct hierarchy and multidimensional scales. J Marital Fam Ther. 1995;21(3):289-308.
17. Davis MH. A multidimensional approach to individual differences in empathy. JSAS Catalog Sel Doc Psychol. 1980;10:85.
18. Fraley RC, Waller NG, Brennan KA. An item-response theory analysis of self-report measures of adult attachment. J Pers Soc Psychol. 2000;78(2):350-365.
19. Tabachnick BG, Fidell L. Using Multivariate Statistics. 6th ed. Boston, MA: Pearson; 2013.
20. Sautter FJ, Armelie AP, Glynn SM, Wielt DB. The development of a couple-based treatment for PTSD in returning veterans. Prof Psychol Res Pr. 2011;42(1):63-69.
21. Jacobson NS, Christensen A, Prince SE, Cordova J, Eldridge K. Integrative behavioral couple therapy: an acceptance-based, promising new treatment of couple discord. J Consult Clin Psychol. 2000;9(2):351-355.
22. Makin-Byrd K, Gifford E, McCutcheon S, Glynn S. Family and couples treatment for newly returning veterans. Prof Psychol Res Pr. 2011;42(1):47-55.
23. Peterson K, Anderson J, Bourne D. Evidence Brief: Use of Patient Reported Outcome Measures for Measurement Based Care in Mental Health Shared Decision Making. Washington, DC: Department of Veterans Affairs; 2018. https://www.ncbi.nlm.nih.gov/books/NBK536143. Accessed September 13, 2019.
24. Fortney JC, Unützer J, Wrenn G, et al. A tipping point for measurement-based care. Psychiatr Serv. 2017;68(2):179-188.
25. Costello AB, Osborne JW. Best practices in exploratory factor analysis: four recommendations for getting the most from your analysis. Pract Assess Res Eval. 2005;10(7):1-9.
Although about 8.3% of the general adult civilian population will be diagnosed with posttraumatic stress disorder (PTSD) in their lifetime, rates of PTSD are even higher in the veteran population.1,2 PTSD is associated with a number of psychosocial consequences in veterans, including decreased intimate partner relationship functioning.3,4 For example, Cloitre and colleagues reported that PTSD is associated with difficulty with socializing, intimacy, responsibility, and control, all of which increase difficulties in intimate partner relationships.5 Similarly, researchers also have noted that traumatic experiences can affect an individual’s attachment style, resulting in progressive avoidance of interpersonal relationships, which can lead to marked difficulties in maintaining and beginning intimate partner relationships.6,7 Despite these known consequences of PTSD, as Dekel and Monson noted in a review,further research is still needed regarding the mechanisms by which trauma and PTSD result in decreased intimate partner relationship functioning among veterans.8 Nonetheless, as positive interpersonal relationships are associated with decreased PTSD symptom severity9,10 and increased engagement in PTSD treatment,11 determining methods of measuring intimate partner relationship functioning in veterans with PTSD is important to inform future research and aid the provision of care.
To date, limited research has examined the valid measurement of intimate partner relationship functioning among veterans with PTSD. Many existing measures that comprehensively assess intimate partner relationship functioning are time and resource intensive. One such measure, the Timberlawn Couple and Family Evaluation Scales (TCFES), comprehensively assesses multiple pertinent domains of intimate partner relationship functioning (ie, structure, autonomy, problem solving, affect regulation, and disagreement/conflict).12 By assessing multiple domains, the TCFES offers a method of understanding the specific components of an individual’s intimate partner relationship in need of increased clinical attention.12 However, the TCFES is a time- and labor-intensive observational measure that requires a couple to interact while a blinded, independent rater observes and rates their interactions using an intricate coding process. This survey structure precludes the ability to quickly and comprehensively assess a veteran’s intimate partner functioning in settings such as mental health outpatient clinics where mental health providers engage in brief, time-limited psychotherapy. As such, brief measures of intimate partner relationship functioning are needed to best inform clinical care among veterans with PTSD.
The primary aim of the current study was to create a psychometrically valid, yet brief, self-report version of the TCFES to assess multiple domains of intimate partner relationship functioning. The psychometric properties of this measure were assessed among a sample of US veterans with PTSD who were in an intimate partner relationship. We specifically examined factor structure, reliability, and associations to established measures of specific domains of relational functioning.
Methods
Ninety-four veterans were recruited via posted advertisements, promotion in PTSD therapy groups/staff meetings, and word of mouth at the Dallas Veterans Affairs Medical Center (VAMC). Participants were eligible if they had a documented diagnosis of PTSD as confirmed in the veteran’s electronic medical record and an affirmative response to currently being involved in an intimate partner relationship (ie, legally married, common-law spouse, involved in a relationship/partnership). There were no exclusion criteria.
Interested veterans were invited to complete several study-related self-report measures concerning their intimate partner relationships that would take about an hour. They were informed that the surveys were voluntary and confidential, and that they would be compensated for their participation. All veterans who participated provided written consent and the study was approved by the Dallas VAMC institutional review board.
Of the 94 veterans recruited, 3 veterans’ data were removed from current analyses after informed consent but before completing the surveys when they indicated they were not currently in a relationship or were divorced. After consent, the 91 participants were administered several study-related self-report measures. The measures took between 30 and 55 minutes to complete. Participants were then compensated $25 for their participation.
Intimate Partner Relationship Functioning
The 16-item TCFES self-report version (TCFES-SR) was developed to assess multiple domains of interpersonal functioning (Appendix). The observational TCFES assesses 5 intimate partner relationship characteristic domains (ie, structure, autonomy, problem solving, affect regulation, and disagreement/conflict) during a couple’s interaction by an independent trained rater.12 Each of the 16 TCFES-SR items were modeled after original constructs measured by the TCFES, including power, closeness, clarify, other’s views, responsibility, closure, negotiation, expressiveness, responsiveness, positive regard, negative regard, mood/tone, empathy, frequency, affective quality, and generalization and escalation. To maintain consistency with the TCFES, each item of the TCFES-SR was scored from 1 (severely dysfunctional) to 5 (highly functional). Additionally, all item wording for the TCFES-SR was based on wording in the TCFES manual after consultation with an expert who facilitated the development of the TCFES.12 On average, the TCFES-SR took 5 to 10 minutes to complete.
To measure concurrent validity of the modified TCFES-SR, several additional interpersonal measures were selected and administered based on prior research and established domains of the TCFES. The Positive and Negative Quality in Marriage Scale (PANQIMS) was administered to assess perceived attitudes toward a relationship.13,14 The PANQIMS generates 2 subscales: positive quality and negative quality in the relationship. Because the PANQIMS specifically assesses married relationships and our sample included married and nonmarried participants, wording was modified (eg, “spouse/partner”).
The relative power subscale of the Network Relationships Inventory–Relationship Qualities Version (NRI-RQV) measure was administered to assess the unequal/shared role romantic partners have in power equality (ie, relative power).15
The Revised Dyadic Adjustment Scale (RDAS) is a self-report measure that assesses multiple dimensions of marital adjustment and functioning.16 Six subscales of the RDAS were chosen based on items of the TCFES-SR: decision making, values, affection, conflict, activities, and discussion.
The Interpersonal Reactivity Index (IRI) empathetic concern subscale was administered to assess empathy across multiple contexts and situations17 and the Experiences in Close Relationships-Revised Questionnaire (ECR-R) was administered to assess relational functioning by determining attachment-related anxiety and avoidance.18
Sociodemographic Information
A sociodemographic questionnaire also was administered. The questionnaire assessed gender, age, education, service branch, length of interpersonal relationship, race, and ethnicity of the veteran as well as gender of the veteran’s partner.
Statistical Analysis
Factor structure of the TCFES-SR was determined by conducting an exploratory factor analysis. To allow for correlation between items, the Promax oblique rotation method was chosen.19 Number of factors was determined by agreement between number of eigenvalues ≥ 1, visual inspection of the scree plot, and a parallel analysis. Factor loadings of ≥ 0.3 were used to determine which items loaded on to which factors.
Convergent validity was assessed by conducting Pearson’s bivariate correlations between identified TCFES-SR factor(s) and other administered measures of interpersonal functioning (ie, PANQIMS positive and negative quality; NRI-RQV relative power subscale; RDAS decision making, values, affection, conflict, activities, and discussion subscales; IRI-empathetic concern subscale; and ECR-R attachment-related anxiety and avoidance subscales). Strength of relationship was determined based on the following guidelines: ± 0.3 to 0.49 = small, ± 0.5 to 0.69 = moderate, and ± 0.7 to 1.00 = large. Internal consistency was also determined for TCFES-SR factor(s) using Cronbach’s α. A standard level of significance (α=.05) was used for all statistical analyses.
Results
Eighty-six veterans provided complete data (Table 1). The Kaiser-Meyer-Olkin measure of sampling adequacy was indicative that sample size was adequate (.91), while Bartlett’s test of sphericity found the variables were suitable for structure detection, χ2 (120) = 800.00, P < .001. While 2 eigenvalues were ≥ 1, visual inspection of the scree plot and subsequent parallel analysis identified a unidimensional structure (ie, 1 factor) for the TCFES-SR. All items were found to load to this single factor, with all loadings being ≥ 0.5 (Table 2). Additionally, internal consistency was excellent for the scale (α = .93).
Pearson’s bivariate correlations were significant (P < .05) between TCFES-SR total score, and almost all administered interpersonal functioning measures (Table 3). Interestingly, no significant associations were found between any of the administered measures, including the TCFES-SR total score, and the IRI-empathetic concern subscale (P > .05).
Discussion
These findings provide initial support for the psychometric properties of the TCFES-SR, including excellent internal consistency and the adequate association of its total score to established measures of interpersonal functioning. Contrary to the TCFES, the TCFES-SR was shown to best fit a unidimensional factor rather than a multidimensional measure of relationship functioning. However, the TCFES-SR was also shown to have strong convergent validity with multiple domains of relationship functioning, indicating that the measure of overall intimate partner relationship functioning encompasses a number of relational domains (ie, structure, autonomy, problem solving, affect regulation, and disagreement/conflict). Critically, the TCFES-SR is brief and was administered easily in our sample, providing utility as clinical tool to be used in time-sensitive outpatient settings.
A unidimensional factor has particular strength in providing a global portrait of perceived intimate partner relationship functioning, and mental health providers can administer the TCFES-SR to assess for overall perceptions of intimate partner relationship functioning rather than administering a number of measures focusing on specific interpersonal domains (eg, decision making processes or positive/negative attitudes towards one’s relationship). This allows for the quick assessment (ie, 5-10 minutes) of overall intimate partner relationship functioning rather than administration of multiple self-report measures which can be time-intensive and expensive. However, the TCFES-SR also is limited by a lack of nuanced understanding of perceptions of functioning specific to particular domains. For example, the TCFES-SR score cannot describe intimate partner functioning in the domain of problem solving. Therefore, brief screening tools need to be developed that assess multiple intimate partner relationship domains.
Importantly, overall intimate partner relationship functioning as measured by the TCFES-SR may not incorporate perceptions of relationship empathy, as the total score did not correlate with a measure of empathetic concern (ie, the IRI-empathetic concern subscale). As empathy was based on one item in the TCFES-SR vs 7 in the IRI-empathetic concern subscale, it is unclear if the TCFES-SR only captures a portion of the construct of empathy (ie, sensitivity to partner) vs the comprehensive assessment of trait empathy that the IRI subscale measures. Additionally, the IRI-empathetic concern subscale did not significantly correlate with any of the other administered measures of relationship functioning. Given the role of empathy in positive, healthy intimate partner relationships, future research should explore the role of empathetic concern among veterans with PTSD as it relates to overall (eg, TCFES-SR) and specific aspects of intimate partner relationship functioning.20
While the clinical applicability of the TCFES-SR requires further examination, this measure has a number of potential uses. Information captured quickly by the TCFES-SR may help to inform appropriate referral for treatment. For instance, veterans reporting low total scores on the TCFES-SR may indicate a need for a referral for intervention focused on improving overall relationship functioning (eg, Integrative Behavioral Couple Therapy).21,22 Measurement-based care (ie, tracking and discussing changes in symptoms during treatment using validated self-report measures) is now required by the Joint Commission as a standard of care,and has been shown to improve outcomes in couples therapy.23,24 As a brief self-report measure, the TCFES-SR may be able to facilitate measurement-based care and assist providers in tracking changes in overall relationship functioning over the course of treatment. However, the purpose of the current study was to validate the TCFES-SR and not to examine the utility of the TCFES-SR in clinical care; additional research is needed to determine standardized cutoff scores to indicate a need for clinical intervention.
Limitations
Several limitations should be noted. The current study only assessed perceived intimate partner relationship functioning from the perspective of the veteran, thus limiting implications as it pertains to the spouse/partner of the veteran. PTSD diagnosis was based on chart review rather than a psychodiagnostic measure (eg, Clinician Administered PTSD Scale); therefore, whether this diagnosis was current or in remission was unclear. Although our sample was adequate to conduct an exploratory factor analysis,the overall sample size was modest, and results should be considered preliminary with need for further replication.25 The sample was also primarily male, white or black, and non-Hispanic; therefore, results may not generalize to a more sociodemographically diverse population. Finally, given the focus of the study to develop a self-report measure, we did not compare the TCFES-SR to the original TCFES. Thus, further research examining the relationship between the TCFES-SR and TCFES may be needed to better understand overlap and potential incongruence in these measures, and to ascertain any differences in their factor structures.
Conclusion
This study is novel in that it adapted a comprehensive observational measure of relationship functioning to a self-report measure piloted among a sample of veterans with PTSD in an intimate partner relationship, a clinical population that remains largely understudied. Although findings are preliminary, the TCFES-SR was found to be a reliable and valid measure of overall intimate partner relationship functioning. Given the rapid administration of this self-report measure, the TCFES-SR may hold clinical utility as a screen of intimate partner relationship deficits in need of clinical intervention. Replication in a larger, more diverse sample is needed to further examine the generalizability and confirm psychometric properties of the TCFES-SR. Additionally, further understanding of the clinical utility of the TCFES-SR in treatment settings remains critical to promote the development and maintenance of healthy intimate partner relationships among veterans with PTSD. Finally, development of effective self-report measures of intimate partner relationship functioning, such as the TCFES-SR, may help to facilitate needed research to understand the effect of PTSD on establishing and maintaining healthy intimate partner relationships among veterans.
Acknowledgments
The current study was funded by the Timberlawn Psychiatric Research Foundation. This material is the result of work supported in part by the US Department of Veterans Affairs; the Rocky Mountain Mental Illness Research, Education and Clinical Center (MIRECC) for Suicide Prevention; Sierra Pacific MIRECC; and the Office of Academic Affiliations, Advanced Fellowship Program in Mental Illness Research and Treatment, Department of Veterans Affairs.


Although about 8.3% of the general adult civilian population will be diagnosed with posttraumatic stress disorder (PTSD) in their lifetime, rates of PTSD are even higher in the veteran population.1,2 PTSD is associated with a number of psychosocial consequences in veterans, including decreased intimate partner relationship functioning.3,4 For example, Cloitre and colleagues reported that PTSD is associated with difficulty with socializing, intimacy, responsibility, and control, all of which increase difficulties in intimate partner relationships.5 Similarly, researchers also have noted that traumatic experiences can affect an individual’s attachment style, resulting in progressive avoidance of interpersonal relationships, which can lead to marked difficulties in maintaining and beginning intimate partner relationships.6,7 Despite these known consequences of PTSD, as Dekel and Monson noted in a review,further research is still needed regarding the mechanisms by which trauma and PTSD result in decreased intimate partner relationship functioning among veterans.8 Nonetheless, as positive interpersonal relationships are associated with decreased PTSD symptom severity9,10 and increased engagement in PTSD treatment,11 determining methods of measuring intimate partner relationship functioning in veterans with PTSD is important to inform future research and aid the provision of care.
To date, limited research has examined the valid measurement of intimate partner relationship functioning among veterans with PTSD. Many existing measures that comprehensively assess intimate partner relationship functioning are time and resource intensive. One such measure, the Timberlawn Couple and Family Evaluation Scales (TCFES), comprehensively assesses multiple pertinent domains of intimate partner relationship functioning (ie, structure, autonomy, problem solving, affect regulation, and disagreement/conflict).12 By assessing multiple domains, the TCFES offers a method of understanding the specific components of an individual’s intimate partner relationship in need of increased clinical attention.12 However, the TCFES is a time- and labor-intensive observational measure that requires a couple to interact while a blinded, independent rater observes and rates their interactions using an intricate coding process. This survey structure precludes the ability to quickly and comprehensively assess a veteran’s intimate partner functioning in settings such as mental health outpatient clinics where mental health providers engage in brief, time-limited psychotherapy. As such, brief measures of intimate partner relationship functioning are needed to best inform clinical care among veterans with PTSD.
The primary aim of the current study was to create a psychometrically valid, yet brief, self-report version of the TCFES to assess multiple domains of intimate partner relationship functioning. The psychometric properties of this measure were assessed among a sample of US veterans with PTSD who were in an intimate partner relationship. We specifically examined factor structure, reliability, and associations to established measures of specific domains of relational functioning.
Methods
Ninety-four veterans were recruited via posted advertisements, promotion in PTSD therapy groups/staff meetings, and word of mouth at the Dallas Veterans Affairs Medical Center (VAMC). Participants were eligible if they had a documented diagnosis of PTSD as confirmed in the veteran’s electronic medical record and an affirmative response to currently being involved in an intimate partner relationship (ie, legally married, common-law spouse, involved in a relationship/partnership). There were no exclusion criteria.
Interested veterans were invited to complete several study-related self-report measures concerning their intimate partner relationships that would take about an hour. They were informed that the surveys were voluntary and confidential, and that they would be compensated for their participation. All veterans who participated provided written consent and the study was approved by the Dallas VAMC institutional review board.
Of the 94 veterans recruited, 3 veterans’ data were removed from current analyses after informed consent but before completing the surveys when they indicated they were not currently in a relationship or were divorced. After consent, the 91 participants were administered several study-related self-report measures. The measures took between 30 and 55 minutes to complete. Participants were then compensated $25 for their participation.
Intimate Partner Relationship Functioning
The 16-item TCFES self-report version (TCFES-SR) was developed to assess multiple domains of interpersonal functioning (Appendix). The observational TCFES assesses 5 intimate partner relationship characteristic domains (ie, structure, autonomy, problem solving, affect regulation, and disagreement/conflict) during a couple’s interaction by an independent trained rater.12 Each of the 16 TCFES-SR items were modeled after original constructs measured by the TCFES, including power, closeness, clarify, other’s views, responsibility, closure, negotiation, expressiveness, responsiveness, positive regard, negative regard, mood/tone, empathy, frequency, affective quality, and generalization and escalation. To maintain consistency with the TCFES, each item of the TCFES-SR was scored from 1 (severely dysfunctional) to 5 (highly functional). Additionally, all item wording for the TCFES-SR was based on wording in the TCFES manual after consultation with an expert who facilitated the development of the TCFES.12 On average, the TCFES-SR took 5 to 10 minutes to complete.
To measure concurrent validity of the modified TCFES-SR, several additional interpersonal measures were selected and administered based on prior research and established domains of the TCFES. The Positive and Negative Quality in Marriage Scale (PANQIMS) was administered to assess perceived attitudes toward a relationship.13,14 The PANQIMS generates 2 subscales: positive quality and negative quality in the relationship. Because the PANQIMS specifically assesses married relationships and our sample included married and nonmarried participants, wording was modified (eg, “spouse/partner”).
The relative power subscale of the Network Relationships Inventory–Relationship Qualities Version (NRI-RQV) measure was administered to assess the unequal/shared role romantic partners have in power equality (ie, relative power).15
The Revised Dyadic Adjustment Scale (RDAS) is a self-report measure that assesses multiple dimensions of marital adjustment and functioning.16 Six subscales of the RDAS were chosen based on items of the TCFES-SR: decision making, values, affection, conflict, activities, and discussion.
The Interpersonal Reactivity Index (IRI) empathetic concern subscale was administered to assess empathy across multiple contexts and situations17 and the Experiences in Close Relationships-Revised Questionnaire (ECR-R) was administered to assess relational functioning by determining attachment-related anxiety and avoidance.18
Sociodemographic Information
A sociodemographic questionnaire also was administered. The questionnaire assessed gender, age, education, service branch, length of interpersonal relationship, race, and ethnicity of the veteran as well as gender of the veteran’s partner.
Statistical Analysis
Factor structure of the TCFES-SR was determined by conducting an exploratory factor analysis. To allow for correlation between items, the Promax oblique rotation method was chosen.19 Number of factors was determined by agreement between number of eigenvalues ≥ 1, visual inspection of the scree plot, and a parallel analysis. Factor loadings of ≥ 0.3 were used to determine which items loaded on to which factors.
Convergent validity was assessed by conducting Pearson’s bivariate correlations between identified TCFES-SR factor(s) and other administered measures of interpersonal functioning (ie, PANQIMS positive and negative quality; NRI-RQV relative power subscale; RDAS decision making, values, affection, conflict, activities, and discussion subscales; IRI-empathetic concern subscale; and ECR-R attachment-related anxiety and avoidance subscales). Strength of relationship was determined based on the following guidelines: ± 0.3 to 0.49 = small, ± 0.5 to 0.69 = moderate, and ± 0.7 to 1.00 = large. Internal consistency was also determined for TCFES-SR factor(s) using Cronbach’s α. A standard level of significance (α=.05) was used for all statistical analyses.
Results
Eighty-six veterans provided complete data (Table 1). The Kaiser-Meyer-Olkin measure of sampling adequacy was indicative that sample size was adequate (.91), while Bartlett’s test of sphericity found the variables were suitable for structure detection, χ2 (120) = 800.00, P < .001. While 2 eigenvalues were ≥ 1, visual inspection of the scree plot and subsequent parallel analysis identified a unidimensional structure (ie, 1 factor) for the TCFES-SR. All items were found to load to this single factor, with all loadings being ≥ 0.5 (Table 2). Additionally, internal consistency was excellent for the scale (α = .93).
Pearson’s bivariate correlations were significant (P < .05) between TCFES-SR total score, and almost all administered interpersonal functioning measures (Table 3). Interestingly, no significant associations were found between any of the administered measures, including the TCFES-SR total score, and the IRI-empathetic concern subscale (P > .05).
Discussion
These findings provide initial support for the psychometric properties of the TCFES-SR, including excellent internal consistency and the adequate association of its total score to established measures of interpersonal functioning. Contrary to the TCFES, the TCFES-SR was shown to best fit a unidimensional factor rather than a multidimensional measure of relationship functioning. However, the TCFES-SR was also shown to have strong convergent validity with multiple domains of relationship functioning, indicating that the measure of overall intimate partner relationship functioning encompasses a number of relational domains (ie, structure, autonomy, problem solving, affect regulation, and disagreement/conflict). Critically, the TCFES-SR is brief and was administered easily in our sample, providing utility as clinical tool to be used in time-sensitive outpatient settings.
A unidimensional factor has particular strength in providing a global portrait of perceived intimate partner relationship functioning, and mental health providers can administer the TCFES-SR to assess for overall perceptions of intimate partner relationship functioning rather than administering a number of measures focusing on specific interpersonal domains (eg, decision making processes or positive/negative attitudes towards one’s relationship). This allows for the quick assessment (ie, 5-10 minutes) of overall intimate partner relationship functioning rather than administration of multiple self-report measures which can be time-intensive and expensive. However, the TCFES-SR also is limited by a lack of nuanced understanding of perceptions of functioning specific to particular domains. For example, the TCFES-SR score cannot describe intimate partner functioning in the domain of problem solving. Therefore, brief screening tools need to be developed that assess multiple intimate partner relationship domains.
Importantly, overall intimate partner relationship functioning as measured by the TCFES-SR may not incorporate perceptions of relationship empathy, as the total score did not correlate with a measure of empathetic concern (ie, the IRI-empathetic concern subscale). As empathy was based on one item in the TCFES-SR vs 7 in the IRI-empathetic concern subscale, it is unclear if the TCFES-SR only captures a portion of the construct of empathy (ie, sensitivity to partner) vs the comprehensive assessment of trait empathy that the IRI subscale measures. Additionally, the IRI-empathetic concern subscale did not significantly correlate with any of the other administered measures of relationship functioning. Given the role of empathy in positive, healthy intimate partner relationships, future research should explore the role of empathetic concern among veterans with PTSD as it relates to overall (eg, TCFES-SR) and specific aspects of intimate partner relationship functioning.20
While the clinical applicability of the TCFES-SR requires further examination, this measure has a number of potential uses. Information captured quickly by the TCFES-SR may help to inform appropriate referral for treatment. For instance, veterans reporting low total scores on the TCFES-SR may indicate a need for a referral for intervention focused on improving overall relationship functioning (eg, Integrative Behavioral Couple Therapy).21,22 Measurement-based care (ie, tracking and discussing changes in symptoms during treatment using validated self-report measures) is now required by the Joint Commission as a standard of care,and has been shown to improve outcomes in couples therapy.23,24 As a brief self-report measure, the TCFES-SR may be able to facilitate measurement-based care and assist providers in tracking changes in overall relationship functioning over the course of treatment. However, the purpose of the current study was to validate the TCFES-SR and not to examine the utility of the TCFES-SR in clinical care; additional research is needed to determine standardized cutoff scores to indicate a need for clinical intervention.
Limitations
Several limitations should be noted. The current study only assessed perceived intimate partner relationship functioning from the perspective of the veteran, thus limiting implications as it pertains to the spouse/partner of the veteran. PTSD diagnosis was based on chart review rather than a psychodiagnostic measure (eg, Clinician Administered PTSD Scale); therefore, whether this diagnosis was current or in remission was unclear. Although our sample was adequate to conduct an exploratory factor analysis,the overall sample size was modest, and results should be considered preliminary with need for further replication.25 The sample was also primarily male, white or black, and non-Hispanic; therefore, results may not generalize to a more sociodemographically diverse population. Finally, given the focus of the study to develop a self-report measure, we did not compare the TCFES-SR to the original TCFES. Thus, further research examining the relationship between the TCFES-SR and TCFES may be needed to better understand overlap and potential incongruence in these measures, and to ascertain any differences in their factor structures.
Conclusion
This study is novel in that it adapted a comprehensive observational measure of relationship functioning to a self-report measure piloted among a sample of veterans with PTSD in an intimate partner relationship, a clinical population that remains largely understudied. Although findings are preliminary, the TCFES-SR was found to be a reliable and valid measure of overall intimate partner relationship functioning. Given the rapid administration of this self-report measure, the TCFES-SR may hold clinical utility as a screen of intimate partner relationship deficits in need of clinical intervention. Replication in a larger, more diverse sample is needed to further examine the generalizability and confirm psychometric properties of the TCFES-SR. Additionally, further understanding of the clinical utility of the TCFES-SR in treatment settings remains critical to promote the development and maintenance of healthy intimate partner relationships among veterans with PTSD. Finally, development of effective self-report measures of intimate partner relationship functioning, such as the TCFES-SR, may help to facilitate needed research to understand the effect of PTSD on establishing and maintaining healthy intimate partner relationships among veterans.
Acknowledgments
The current study was funded by the Timberlawn Psychiatric Research Foundation. This material is the result of work supported in part by the US Department of Veterans Affairs; the Rocky Mountain Mental Illness Research, Education and Clinical Center (MIRECC) for Suicide Prevention; Sierra Pacific MIRECC; and the Office of Academic Affiliations, Advanced Fellowship Program in Mental Illness Research and Treatment, Department of Veterans Affairs.


1. Kilpatrick DG, Resnick HS, Milanak ME, Miller MW, Keyes KM, Friedman MJ. National estimates of exposure to traumatic events and PTSD prevalence using DSM-IV and DSM-5 criteria. J Trauma Stress. 2013;26(5):537-547.
2. Lehavot K, Goldberg SB, Chen JA, et al. Do trauma type, stressful life events, and social support explain women veterans’ high prevalence of PTSD? Soc Psychiatry Psychiatr Epidemiol. 2018;53(9):943-953.
3. Galovski T, Lyons JA. Psychological sequelae of combat violence: a review of the impact of PTSD on the veteran’s family and possible interventions. Aggress Violent Behav. 2004;9(5):477-501.
4. Ray SL, Vanstone M. The impact of PTSD on veterans’ family relationships: an interpretative phenomenological inquiry. Int J Nurs Stud. 2009;46(6):838-847.
5. Cloitre M, Miranda R, Stovall-McClough KC, Han H. Beyond PTSD: emotion regulation and interpersonal problems as predictors of functional impairment in survivors of childhood abuse. Behav Ther. 2005;36(2):119-124.
6. McFarlane AC, Bookless C. The effect of PTSD on interpersonal relationships: issues for emergency service works. Sex Relation Ther. 2001;16(3):261-267.
7. Itzhaky L, Stein JY, Levin Y, Solomon Z. Posttraumatic stress symptoms and marital adjustment among Israeli combat veterans: the role of loneliness and attachment. Psychol Trauma. 2017;9(6):655-662.
8. Dekel R, Monson CM. Military-related post-traumatic stress disorder and family relations: current knowledge and future directions. Aggress Violent Behav. 2010;15(4):303-309.
9. Allen ES, Rhoades GK, Stanley SM, Markman HJ. Hitting home: relationships between recent deployment, posttraumatic stress symptoms, and marital functioning for Army couples. J Fam Psychol. 2010;24(3):280-288.
10. Laffaye C, Cavella S, Drescher K, Rosen C. Relationships among PTSD symptoms, social support, and support source in veterans with chronic PTSD. J Trauma Stress. 2008;21(4):394-401.
11. Meis LA, Noorbaloochi S, Hagel Campbell EM, et al. Sticking it out in trauma-focused treatment for PTSD: it takes a village. J Consult Clin Psychol. 2019;87(3):246-256.
12. Lewis JM, Gossett JT, Housson MM, Owen MT. Timberlawn Couple and Family Evaluation Scales. Dallas, TX: Timberlawn Psychiatric Research Foundation; 1999.
13. Fincham FD, Linfield KJ. A new look at marital quality: can spouses feel positive and negative about their marriage? J Fam Psychol. 1997;11(4):489-502.
14. Kaplan KJ. On the ambivalence-indifference problem in attitude theory and measurement: a suggested modification of the semantic differential technique. Psychol Bull. 1972;77(5):361-372.
15. Buhrmester D, Furman W. The Network of Relationship Inventory: Relationship Qualities Version [unpublished measure]. University of Texas at Dallas; 2008.
16. Busby DM, Christensen C, Crane DR, Larson JH. A revision of the Dyadic Adjustment Scale for use with distressed and nondistressed couples: construct hierarchy and multidimensional scales. J Marital Fam Ther. 1995;21(3):289-308.
17. Davis MH. A multidimensional approach to individual differences in empathy. JSAS Catalog Sel Doc Psychol. 1980;10:85.
18. Fraley RC, Waller NG, Brennan KA. An item-response theory analysis of self-report measures of adult attachment. J Pers Soc Psychol. 2000;78(2):350-365.
19. Tabachnick BG, Fidell L. Using Multivariate Statistics. 6th ed. Boston, MA: Pearson; 2013.
20. Sautter FJ, Armelie AP, Glynn SM, Wielt DB. The development of a couple-based treatment for PTSD in returning veterans. Prof Psychol Res Pr. 2011;42(1):63-69.
21. Jacobson NS, Christensen A, Prince SE, Cordova J, Eldridge K. Integrative behavioral couple therapy: an acceptance-based, promising new treatment of couple discord. J Consult Clin Psychol. 2000;9(2):351-355.
22. Makin-Byrd K, Gifford E, McCutcheon S, Glynn S. Family and couples treatment for newly returning veterans. Prof Psychol Res Pr. 2011;42(1):47-55.
23. Peterson K, Anderson J, Bourne D. Evidence Brief: Use of Patient Reported Outcome Measures for Measurement Based Care in Mental Health Shared Decision Making. Washington, DC: Department of Veterans Affairs; 2018. https://www.ncbi.nlm.nih.gov/books/NBK536143. Accessed September 13, 2019.
24. Fortney JC, Unützer J, Wrenn G, et al. A tipping point for measurement-based care. Psychiatr Serv. 2017;68(2):179-188.
25. Costello AB, Osborne JW. Best practices in exploratory factor analysis: four recommendations for getting the most from your analysis. Pract Assess Res Eval. 2005;10(7):1-9.
1. Kilpatrick DG, Resnick HS, Milanak ME, Miller MW, Keyes KM, Friedman MJ. National estimates of exposure to traumatic events and PTSD prevalence using DSM-IV and DSM-5 criteria. J Trauma Stress. 2013;26(5):537-547.
2. Lehavot K, Goldberg SB, Chen JA, et al. Do trauma type, stressful life events, and social support explain women veterans’ high prevalence of PTSD? Soc Psychiatry Psychiatr Epidemiol. 2018;53(9):943-953.
3. Galovski T, Lyons JA. Psychological sequelae of combat violence: a review of the impact of PTSD on the veteran’s family and possible interventions. Aggress Violent Behav. 2004;9(5):477-501.
4. Ray SL, Vanstone M. The impact of PTSD on veterans’ family relationships: an interpretative phenomenological inquiry. Int J Nurs Stud. 2009;46(6):838-847.
5. Cloitre M, Miranda R, Stovall-McClough KC, Han H. Beyond PTSD: emotion regulation and interpersonal problems as predictors of functional impairment in survivors of childhood abuse. Behav Ther. 2005;36(2):119-124.
6. McFarlane AC, Bookless C. The effect of PTSD on interpersonal relationships: issues for emergency service works. Sex Relation Ther. 2001;16(3):261-267.
7. Itzhaky L, Stein JY, Levin Y, Solomon Z. Posttraumatic stress symptoms and marital adjustment among Israeli combat veterans: the role of loneliness and attachment. Psychol Trauma. 2017;9(6):655-662.
8. Dekel R, Monson CM. Military-related post-traumatic stress disorder and family relations: current knowledge and future directions. Aggress Violent Behav. 2010;15(4):303-309.
9. Allen ES, Rhoades GK, Stanley SM, Markman HJ. Hitting home: relationships between recent deployment, posttraumatic stress symptoms, and marital functioning for Army couples. J Fam Psychol. 2010;24(3):280-288.
10. Laffaye C, Cavella S, Drescher K, Rosen C. Relationships among PTSD symptoms, social support, and support source in veterans with chronic PTSD. J Trauma Stress. 2008;21(4):394-401.
11. Meis LA, Noorbaloochi S, Hagel Campbell EM, et al. Sticking it out in trauma-focused treatment for PTSD: it takes a village. J Consult Clin Psychol. 2019;87(3):246-256.
12. Lewis JM, Gossett JT, Housson MM, Owen MT. Timberlawn Couple and Family Evaluation Scales. Dallas, TX: Timberlawn Psychiatric Research Foundation; 1999.
13. Fincham FD, Linfield KJ. A new look at marital quality: can spouses feel positive and negative about their marriage? J Fam Psychol. 1997;11(4):489-502.
14. Kaplan KJ. On the ambivalence-indifference problem in attitude theory and measurement: a suggested modification of the semantic differential technique. Psychol Bull. 1972;77(5):361-372.
15. Buhrmester D, Furman W. The Network of Relationship Inventory: Relationship Qualities Version [unpublished measure]. University of Texas at Dallas; 2008.
16. Busby DM, Christensen C, Crane DR, Larson JH. A revision of the Dyadic Adjustment Scale for use with distressed and nondistressed couples: construct hierarchy and multidimensional scales. J Marital Fam Ther. 1995;21(3):289-308.
17. Davis MH. A multidimensional approach to individual differences in empathy. JSAS Catalog Sel Doc Psychol. 1980;10:85.
18. Fraley RC, Waller NG, Brennan KA. An item-response theory analysis of self-report measures of adult attachment. J Pers Soc Psychol. 2000;78(2):350-365.
19. Tabachnick BG, Fidell L. Using Multivariate Statistics. 6th ed. Boston, MA: Pearson; 2013.
20. Sautter FJ, Armelie AP, Glynn SM, Wielt DB. The development of a couple-based treatment for PTSD in returning veterans. Prof Psychol Res Pr. 2011;42(1):63-69.
21. Jacobson NS, Christensen A, Prince SE, Cordova J, Eldridge K. Integrative behavioral couple therapy: an acceptance-based, promising new treatment of couple discord. J Consult Clin Psychol. 2000;9(2):351-355.
22. Makin-Byrd K, Gifford E, McCutcheon S, Glynn S. Family and couples treatment for newly returning veterans. Prof Psychol Res Pr. 2011;42(1):47-55.
23. Peterson K, Anderson J, Bourne D. Evidence Brief: Use of Patient Reported Outcome Measures for Measurement Based Care in Mental Health Shared Decision Making. Washington, DC: Department of Veterans Affairs; 2018. https://www.ncbi.nlm.nih.gov/books/NBK536143. Accessed September 13, 2019.
24. Fortney JC, Unützer J, Wrenn G, et al. A tipping point for measurement-based care. Psychiatr Serv. 2017;68(2):179-188.
25. Costello AB, Osborne JW. Best practices in exploratory factor analysis: four recommendations for getting the most from your analysis. Pract Assess Res Eval. 2005;10(7):1-9.
Assessment of Consolidated Mail Outpatient Pharmacy Utilization in the Indian Health Service
Consolidated mail outpatient pharmacy (CMOP) is an automated prescription order processing and delivery system developed by the US Department of Veterans Affairs (VA) in 1994 to provide medications to VA patients.1 In fiscal year (FY) 2016, CMOP filled about 80% of VA outpatient prescriptions.2
Formalized by the 2010 Memorandum of Understanding between Indian Health Service (IHS) and VA, CMOP is a partnership undertaken to improve the delivery of care to patients by both agencies.3 The number of prescriptions filled by CMOP for IHS patients increased from 1,972 in FY 2010 to 840,109 in FY 2018.4 In the fourth quarter of FY 2018, there were 94 CMOP-enrolled IHS federal and tribal sites.5 It is only appropriate that a growing number of IHS sites are adopting CMOP considering the evidence for mail-order pharmacy on better patient adherence, improved health outcomes, and potential cost savings.6-9 Furthermore, using a centralized pharmacy operation, such as CMOP, can lead to better quality services.10
Crownpoint Health Care Facility (CHCF) serves > 30,000 American Indians and is in Crownpoint, New Mexico, a small community of about 3,000 people.11 Most of the patients served by the facility live in distant places. Many of these underserved patients do not have a stable means of transportation.12 Therefore, these patients may have difficulty traveling to the facility for their health care needs, including medication pickups. More than 2.5 million American Indians and Alaska Natives IHS beneficiaries face similar challenges due to the rurality of their communities.13 CMOP can be a method to increase access to care for this vulnerable population. However, the utilization of CMOP varies significantly among IHS facilities. While some IHS facilities process large numbers of prescriptions through CMOP, other facilities process few, if any. There also are IHS facilities, such as CHCF, which are at the initial stage of implementing CMOP or trying to increase the volume of prescriptions processed through CMOP. Although the utilization of CMOP has grown exponentially among IHS facilities, there is currently no available resource that summarizes the relative advantages and disadvantages, the challenges and opportunities, and the strengths and weaknesses of implementing CMOP for IHS facilities
Methods
A questionnaire encompassing various aspects of CMOP prescription processing was developed and distributed to the primary CMOP contacts for IHS facilities. The questionnaire was first distributed by e-mail on December 19, 2018. It was e-mailed for a second time on January 16, 2019, and the questionnaire was open for responses until the end of January 2019 (Table).
Results
Forty-four of 94 CMOP-enrolled IHS sites responded to the questionnaire. Most sites train the majority of their pharmacists in CMOP prescription processing. Overall, 310 of 347 pharmacists (89%) in these 44 IHS sites can process prescriptions through CMOP. Thirty-one sites have all their pharmacists trained in CMOP prescription processing. Only 1 facility had less than half (2 of 17 pharmacists) of its pharmacists trained in CMOP prescription processing. More than half the total number of pharmacists, 185 out of 347 (53%), check electronic messages via Resource and Patient Management System (RPMS) MailMan to get information about prescriptions rejected by CMOP. Twenty sites have all their pharmacists check messages about CMOP rejections. However, 2 facilities reported that they do not check the rejection messages at all. Twenty-six of the 44 responding sites (59%) transmit prescriptions to CMOP manually in the electronic system. The rest (18 of 44) rely on the auto-transmission (AT) setup to transmit the CMOP-suspended prescriptions at specified times of the day.
Half the sites (8 of 16) that rely on patients asking for prescriptions to be mailed at the time of refill request do not use any method to designate a CMOP patient. Twenty-four sites use the narrative field on the patient’s profile in RPMS, the health information system used by most IHS facilities, to designate CMOP patients. Eighteen sites use pop-up messages on ScriptPro, a pharmacy automation system, as a designation method. Most of the sites (12 of 15) that use both RPMS and ScriptPro designation methods do not require patients to ask for prescriptions to be mailed at the time of refill request; prescriptions for these patients are routed through CMOP unless patients request otherwise. Only 3 of 44 sites use both methods and rely on patients asking for prescriptions to be mailed at the time of refill request. Some other reported designation methods were using the electronic health record (EHR) posting box, keeping a manual list of CMOP patients, and solely utilizing the Prescription Mail Delivery field in RPMS. Three sites also noted that they keep manual lists to auto-refill prescriptions through CMOP.
Thirty sites (68%) reported that they process every prescription through CMOP even if the patient had prescriptions with specified CMOP quantities. Only 8 sites (18%) said that they used the local mail-out program to keep the same days’ supply for all medication orders. For patients with CMOP-ineligible prescriptions, 34 of the 44 sites (77%) process the eligible prescriptions through CMOP and refill the rest of the prescriptions locally. Six sites (14%) process all medication orders locally for patients with any CMOP-ineligible prescriptions.
Only 12 of 44 sites (27%) involve pharmacy technicians in CMOP prescription processing. Five sites have technicians process prescription refills through CMOP. Two of these sites mentioned the strategy of technicians suspending the prescriptions to be sent to CMOP on the refill due date. Other technician roles included tracking CMOP packages, checking electronic messages for CMOP rejections, and signing up patients for CMOP.
Only 3 of the 44 sites (7%) have measured patient satisfaction with the CMOP program. One of these 3 sites reported that the overall satisfaction was high with CMOP. This site administered the survey to patients who came to the clinic for appointments. The second facility called patients and asked for their feedback. The third site conducted the survey by using student pharmacists. Two sites reported that they use the survey results from the CMOP-conducted patient satisfaction surveys, although they have not measured patient satisfaction at their specific facilities.
Most sites have not assessed CMOP’s impact on their insurance (point of sale) collections. However, 13 sites (30%) reported that they believe they are losing on collections by utilizing CMOP. The use of repackaged products by CMOP, which are usually nonreimbursable, is an issue that was mentioned multiple times. In contrast, 2 sites mentioned that CMOP has led to increased insurance collections for their facilities.
Discussion
The utility of CMOP among the responding IHS sites varies quite significantly. Some sites appreciate the convenience of CMOP while acknowledging its limitations, such as the possible decrease in insurance collections, lengthy prescription processing time, or medication backorders. However, some sites have reserved CMOP for special circumstances (eg, mailing refrigerated items to the patient’s street address) due to various complexities that may come with CMOP. One site reported that it compares IHS contract drug prices with VA contract drug prices quarterly to determine which prescriptions should be sent through CMOP.
Most of the IHS pharmacists (89%) are trained in CMOP prescription processing. If an IHS site wants to increase its volume of CMOP prescriptions, it is sensible to train as many pharmacists as possible so that the responsibility does not fall on a few pharmacists. Newly hired pharmacists can receive guidance from trained pharmacists. Designation methods for CMOP patients can be beneficial for these pharmacists to identify CMOP-enrolled patients, especially if the site does not require patients to ask for prescriptions to be mailed at the time of refill request. Only 3 sites (7%) use multiple designation methods in addition to relying on patients to ask for prescriptions to be mailed. Proper implementation of designation methods can remove this extra burden on patients. Conversely, requiring patients to ask for prescriptions to be sent through CMOP can prevent spontaneous mail-outs if a CMOP-designated patient wants to pick up prescriptions locally. Overall, 16 sites (36%) rely on patients asking for prescriptions to be mailed.
One of the main benefits of CMOP is the ability to mail refrigerated items. Local pharmacy mail-out programs may not have this ability. Patients at rural locations often use post office (PO) boxes because they are unable to receive postal services at their physical addresses; however, they may receive packages through United Parcel Service (UPS) at their physical addresses. CMOP uses UPS to send refrigerated items, but UPS does not deliver to PO boxes. Therefore, remotely located sites like CHCF have difficulty in fully optimizing this benefit. One solution is documenting both the physical and mailing addresses on the patient’s EHR, which enables CMOP to send refrigerated items to the patient’s home address via UPS and mail the rest of the prescriptions to the patient’s PO box address with the US Postal Service. The physical address must be listed above the PO box address to ensure that refrigerated items are not rejected by CMOP. Furthermore, both the physical address and the PO box address must be in the same city for this method to work. Two sites noted mailing refrigerated items as one of the major challenges in CMOP prescription processing.
CMOP-enrolled patients must be educated about requesting medications 7 to 10 days before they run out. There is no standard time line for prescriptions filled by CMOP. However, 1 site reported that it may take up to “10 days from time requested to mailbox.” This delay leads to pharmacies facing a dilemma as processing prescriptions too early can lead to insurance rejections, but processing them too late can lead to the patient not receiving the medication by the time they run out of their current supply. However, CMOP provides the ability to track prescriptions sent through CMOP. Pharmacists and technicians need to have access to BestWay Parcel Services Client Portal (genco-mms.bestwayparcel.com) to track CMOP packages. Tracking CMOP prescriptions is a way pharmacy technicians can be involved in CMOP prescription processing. Technicians seem to be underutilized, as only 27% of the responding sites utilize them to some degree in the CMOP process. One site delegated the responsibility of checking CMOP rejection messages to pharmacy technicians. Since 2 of the responding sites do not check CMOP rejection messages at all, this is an excellent opportunity to get pharmacy technicians involved.
A CMOP auto-refill program can potentially be utilized to avoid missed or late medications. In an auto-refill program, a pharmacist can refill prescriptions through CMOP on the due date without a patient request. They may get rejected by insurance the first time they are processed through CMOP for refilling too early if the processing time is taken into account. However, the subsequent refills do not have to consider the CMOP processing time as they would already be synchronized based on the last refill date. Though, if CMOP is out of stock on a medication and it is expected to be available soon, CMOP may take a few extra days to either fill the prescription or reject it if the drug stays unavailable. One of the sites reported “the amount of time [CMOP] holds medications if they are out of stock” as “the hardest thing to work around.” A couple of sites also mentioned the longer than usual delay in processing prescriptions by CMOP during the holidays as one of the major challenges.
CMOP use of repackaged products also may lead insurance companies to deny reimbursement. Repackaged products are usually cheaper to buy.14 However, most insurances do not reimburse for prescriptions filled with these products.15 The local drug file on RPMS may have a national drug code (NDC) that is reimbursable by insurance, but CMOP will change it to the repackaged NDC if they are filling the prescription with a repackaged product. One potential solution to this problem would be filling these prescriptions locally. Furthermore, insurance claims are processed when the prescriptions are filled by CMOP. Sites cannot return/cancel the prescription anymore at that point. Therefore, the inability to see real-time rejections as the medication orders are processed on-site makes it challenging to prevent avoidable insurance rejections, such as a refill too soon. One site calculated that it lost $26,386.45 by utilizing CMOP from January 9, 2018 to December 12, 2018. However, it is unclear whether this loss was representative of other sites. It is also worth noting that IHS sites can save a substantial amount of money on certain products by utilizing CMOP because VA buys these products at a reduced price.16
CMOP-transmitted prescriptions can be rejected for various reasons, such as CMOP manufacturer’s backorder, a different quantity from CMOP stock size, etc. Information about these rejected prescriptions is accessed through electronic messages on RPMS. CMOP does not dispense less than a full, unopened package for most over-the-counter (OTC) medications. The quantity on these prescriptions must be equal to or multiples of the package size for them to be filled by CMOP. This can lead to a patient having prescriptions with different days’ supplies, which results in various refill due dates. If a site has a local mail-out program available, it can potentially keep the same days’ supply for all prescriptions by mailing these OTC medications locally rather than utilizing CMOP. However, this can partially negate CMOP’s benefit of reduced workload.
CMOP also has specified quantities on some prescription medications. One survey respondent viewed “the quantity and day supply required by CMOP” as a negative influence on the site’s insurance collection. It is possible that CMOP does not carry all the medications that a CMOP-enrolled patient is prescribed. Most sites (77%) still send eligible prescriptions through CMOP for the patients who also have CMOP-ineligible prescriptions. There are a small number of sites (14%) that utilize local mail-out program for the patients with any CMOP-ineligible prescriptions, possibly to simplify the process. Schedule II controlled substances cannot be processed through CMOP either; however, facilities may have local policies that prohibit mailing any controlled substances.
Prescriptions can be manually transmitted to CMOP, or they can be automatically transmitted based on the run time and frequency of the auto-transmission setup. The prescriptions that are waiting to be transmitted to CMOP must be in the “suspended” status. The apparent advantage of relying on auto-transmission is that you do not have to complete the steps manually to transmit suspended CMOP prescriptions, thereby making the process more convenient. However, the manual transmission can be utilized as a checkpoint to verify that prescriptions were properly suspended for CMOP, as the prescription status changes from “S” (suspended) to “AT” once the transmission is completed. If a prescription is not properly suspended for CMOP, the status will remain as S even after manual transmission. More than half (59%) of the responding sites must find the manual transmission feature useful as they use it either over or in addition to the auto-transmission setup.
Despite the challenges, many IHS sites process thousands of monthly prescriptions through CMOP. Of the 94 CMOP-enrolled IHS sites, 17 processed > 1,000 prescriptions from March 27, 2019 to April 25, 2019.17 Five sites processed > 5,000 prescriptions.17 At the rate of > 5,000 prescriptions per month, the yearly CMOP prescription count will be > 60,000. That is more than one-third of the prescriptions processed by CHCF in 2018. By handling these prescriptions through CMOP, it can decrease pharmacy filling and dispensing workload, thereby freeing pharmacists to participate in other services.18 Furthermore, implementing CMOP does not incur any cost for the IHS site. There is a nondrug cost for each prescription that is filled through CMOP. This cost was $2.67 during FY 2016.19 The fee covers prescription vial, label, packaging for mail, postage, personnel, building overhead, and equipment capitalization.19 The nondrug cost of filling a prescription locally at the site can potentially exceed the cost charged by CMOP.19
A lack of objective data exists to assess the net impact of CMOP on patients. Different theoretical assumptions can be made, such as CMOP resulting in better patient adherence. However, there is no objective information about how much CMOP improves patient adherence if it does at all. Though J.D. Power US Pharmacy Study ranks CMOP as “among the best” mail-order pharmacies in customer satisfaction, only 3 of the 44 responding sites have measured patient satisfaction locally.20 Only 1 site had objective data about CMOP’s impact on the point of sale. Therefore, it is currently difficult to perform a cost-benefit analysis of the CMOP program. There are opportunities for further studies on these topics.
Limitations
One limitation of this study is that < 50% of the CMOP-enrolled sites (44 of 94) responded to the questionnaire. It is possible that the facilities that had a significantly positive or negative experience with CMOP were more inclined to share their views. Therefore, it is difficult to conclude whether the responding sites are an accurate representative sample. Another limitation of the study was the questionnaire design and the reliance on free-text responses as opposed to structured data. The free-text responses had to be analyzed manually to determine whether they fall in the same category, thereby increasing the risk of interpretation error.
Conclusion
CMOP has its unique challenges but provides many benefits that local pharmacy mail-out programs may not possess, such as the abilities to mail refrigerated items and track packages. One must be familiar with CMOP’s various idiosyncrasies to make the best use of the program. Extensive staff education and orientation for new staff members must be done to familiarize them with the program. Nevertheless, the successful implementation of CMOP can lead to reduced pharmacy workload while increasing access to care for patients with transportation issues.
Acknowledgments
The authors thank LCDR Karsten Smith, PharmD, BCGP, the IHS CMOP Coordinator for providing the list of primary CMOP contacts and CDR Kendall Van Tyle, PharmD, BCPS, for proofreading the article.
1. US Department of Veterans Affairs, Office of Inspector General. Audit of Consolidated Mail Outpatient Pharmacy contract management. https://www.va.gov/oig/52/reports/2009/VAOIG-09-00026-143.pdf. Published June 10, 2009. Accessed June 11, 2020.
2. US Department of Veterans Affairs. Pharmacy Benefits Management Services. VA mail order pharmacy. https://www.pbm.va.gov/PBM/CMOP/VA_Mail_Order_Pharmacy.asp. Updated July 18, 2018. Accessed July 16, 2019.
3. US Department of Veterans Affairs. Memorandum of understanding between the Department of Veterans Affairs (VA) and Indian Health Service (IHS). https://www.va.gov/TRIBALGOVERNMENT/docs/Signed2010VA-IHSMOU.pdf. Published October 1, 2010. Accessed June 11, 2020.
4. US Department of Veterans Affairs, Office of Tribal Government Relations, Office of Rural Health, US Department of Health and Human Services, Indian Health Service. U.S. Department of Veterans Affairs and Indian Health Service memorandum of understanding annual report fiscal year 2018. https://www.ruralhealth.va.gov/docs/VA-IHS_MOU_AnnualReport_FY2018_FINAL.pdf. Published December 2018. Accessed June 11, 2020.
5. Karsten S. CMOP items of interest. Published October 12, 2018. [Nonpublic document]
6. Fernandez EV, McDaniel JA, Carroll NV. Examination of the link between medication adherence and use of mail-order pharmacies in chronic disease states. J Manag Care Spec Pharm. 2016;22(11):1247‐1259. doi:10.18553/jmcp.2016.22.11.1247
7. Schwab P, Racsa P, Rascati K, Mourer M, Meah Y, Worley K. A retrospective database study comparing diabetes-related medication adherence and health outcomes for mail-order versus community pharmacy. J Manag Care Spec Pharm. 2019;25(3):332‐340. doi:10.18553/jmcp.2019.25.3.332
8. Schmittdiel JA, Karter AJ, Dyer W, et al. The comparative effectiveness of mail order pharmacy use vs. local pharmacy use on LDL-C control in new statin users. J Gen Intern Med. 2011;26(12):1396‐1402. doi:10.1007/s11606-011-1805-7
9. Devine S, Vlahiotis A, Sundar H. A comparison of diabetes medication adherence and healthcare costs in patients using mail order pharmacy and retail pharmacy. J Med Econ. 2010;13(2):203‐211. doi:10.3111/13696991003741801
10. Kappenman AM, Ragsdale R, Rim MH, Tyler LS, Nickman NA. Implementation of a centralized mail-order pharmacy service. Am J Health Syst Pharm. 2019;76(suppl 3):S74‐S78. doi:10.1093/ajhp/zxz138
11. US Department of Health and Human Services, Indian Health Service. Crownpoint service unit. www.ihs.gov/crownpoint. Accessed June 11, 2020.
12. Chaco P. Roads and transportation on the Navajo Nation. https://obamawhitehouse.archives.gov/microsite/blog/31387?page=135. Published February 15, 2012. Accessed June 11, 2020.
13. US Department of Health and Human Services, Indian Health Service. Disparities. www.ihs.gov/newsroom/factsheets/disparities. Updated October 2019. Accessed June 11, 2020.
14. Golden State Medical Supply. National contracts. www.gsms.us/wp-content/uploads/2018/10/National-Contracts-Flyer.pdf. Updated October 4, 2018. Accessed June 11, 2020.
15. Arizona Health Care Cost Containment System. IHS/Tribal provider billing manual chapter 9, hospital and clinic services. www.azahcccs.gov/PlansProviders/Downloads/IHS-TribalManual/IHS-Chap09HospClinic.pdf. Updated February 28, 2019. Accessed June 11, 2020.
16. US Department of Veterans Affairs, Office of Inspector General. The impact of VA allowing government agencies to be excluded from temporary price reductions on federal supply schedule pharmaceutical contracts. www.va.gov/oig/pubs/VAOIG-18-04451-06.pdf. Published October 30, 2019. Accessed June 11, 2020.
17. Karsten S. IHS Billing Report-Apr. Indian Health Service SharePoint. Published May 3, 2019. [Nonpublic document]
18. Aragon BR, Pierce RA 2nd, Jones WN. VA CMOPs: producing a pattern of quality and efficiency in government. J Am Pharm Assoc (2003). 2012;52(6):810‐815. doi:10.1331/JAPhA.2012.11075
19. Todd W. VA-IHS Consolidated Mail Outpatient Pharmacy program (CMOP). www.npaihb.org/wp-content/uploads/2017/01/CMOP-Slides-for-Portland-Area-Tribal-Sites.pdf. Published 2017. Accessed June 11, 2020.
20. J.D. Power. Pharmacy customers slow to adopt digital offerings but satisfaction increases when they do, J.D. Power finds. www.jdpower.com/business/press-releases/2019-us-pharmacy-study. Published August 20, 2019. Accessed June 11, 2020.
Consolidated mail outpatient pharmacy (CMOP) is an automated prescription order processing and delivery system developed by the US Department of Veterans Affairs (VA) in 1994 to provide medications to VA patients.1 In fiscal year (FY) 2016, CMOP filled about 80% of VA outpatient prescriptions.2
Formalized by the 2010 Memorandum of Understanding between Indian Health Service (IHS) and VA, CMOP is a partnership undertaken to improve the delivery of care to patients by both agencies.3 The number of prescriptions filled by CMOP for IHS patients increased from 1,972 in FY 2010 to 840,109 in FY 2018.4 In the fourth quarter of FY 2018, there were 94 CMOP-enrolled IHS federal and tribal sites.5 It is only appropriate that a growing number of IHS sites are adopting CMOP considering the evidence for mail-order pharmacy on better patient adherence, improved health outcomes, and potential cost savings.6-9 Furthermore, using a centralized pharmacy operation, such as CMOP, can lead to better quality services.10
Crownpoint Health Care Facility (CHCF) serves > 30,000 American Indians and is in Crownpoint, New Mexico, a small community of about 3,000 people.11 Most of the patients served by the facility live in distant places. Many of these underserved patients do not have a stable means of transportation.12 Therefore, these patients may have difficulty traveling to the facility for their health care needs, including medication pickups. More than 2.5 million American Indians and Alaska Natives IHS beneficiaries face similar challenges due to the rurality of their communities.13 CMOP can be a method to increase access to care for this vulnerable population. However, the utilization of CMOP varies significantly among IHS facilities. While some IHS facilities process large numbers of prescriptions through CMOP, other facilities process few, if any. There also are IHS facilities, such as CHCF, which are at the initial stage of implementing CMOP or trying to increase the volume of prescriptions processed through CMOP. Although the utilization of CMOP has grown exponentially among IHS facilities, there is currently no available resource that summarizes the relative advantages and disadvantages, the challenges and opportunities, and the strengths and weaknesses of implementing CMOP for IHS facilities
Methods
A questionnaire encompassing various aspects of CMOP prescription processing was developed and distributed to the primary CMOP contacts for IHS facilities. The questionnaire was first distributed by e-mail on December 19, 2018. It was e-mailed for a second time on January 16, 2019, and the questionnaire was open for responses until the end of January 2019 (Table).
Results
Forty-four of 94 CMOP-enrolled IHS sites responded to the questionnaire. Most sites train the majority of their pharmacists in CMOP prescription processing. Overall, 310 of 347 pharmacists (89%) in these 44 IHS sites can process prescriptions through CMOP. Thirty-one sites have all their pharmacists trained in CMOP prescription processing. Only 1 facility had less than half (2 of 17 pharmacists) of its pharmacists trained in CMOP prescription processing. More than half the total number of pharmacists, 185 out of 347 (53%), check electronic messages via Resource and Patient Management System (RPMS) MailMan to get information about prescriptions rejected by CMOP. Twenty sites have all their pharmacists check messages about CMOP rejections. However, 2 facilities reported that they do not check the rejection messages at all. Twenty-six of the 44 responding sites (59%) transmit prescriptions to CMOP manually in the electronic system. The rest (18 of 44) rely on the auto-transmission (AT) setup to transmit the CMOP-suspended prescriptions at specified times of the day.
Half the sites (8 of 16) that rely on patients asking for prescriptions to be mailed at the time of refill request do not use any method to designate a CMOP patient. Twenty-four sites use the narrative field on the patient’s profile in RPMS, the health information system used by most IHS facilities, to designate CMOP patients. Eighteen sites use pop-up messages on ScriptPro, a pharmacy automation system, as a designation method. Most of the sites (12 of 15) that use both RPMS and ScriptPro designation methods do not require patients to ask for prescriptions to be mailed at the time of refill request; prescriptions for these patients are routed through CMOP unless patients request otherwise. Only 3 of 44 sites use both methods and rely on patients asking for prescriptions to be mailed at the time of refill request. Some other reported designation methods were using the electronic health record (EHR) posting box, keeping a manual list of CMOP patients, and solely utilizing the Prescription Mail Delivery field in RPMS. Three sites also noted that they keep manual lists to auto-refill prescriptions through CMOP.
Thirty sites (68%) reported that they process every prescription through CMOP even if the patient had prescriptions with specified CMOP quantities. Only 8 sites (18%) said that they used the local mail-out program to keep the same days’ supply for all medication orders. For patients with CMOP-ineligible prescriptions, 34 of the 44 sites (77%) process the eligible prescriptions through CMOP and refill the rest of the prescriptions locally. Six sites (14%) process all medication orders locally for patients with any CMOP-ineligible prescriptions.
Only 12 of 44 sites (27%) involve pharmacy technicians in CMOP prescription processing. Five sites have technicians process prescription refills through CMOP. Two of these sites mentioned the strategy of technicians suspending the prescriptions to be sent to CMOP on the refill due date. Other technician roles included tracking CMOP packages, checking electronic messages for CMOP rejections, and signing up patients for CMOP.
Only 3 of the 44 sites (7%) have measured patient satisfaction with the CMOP program. One of these 3 sites reported that the overall satisfaction was high with CMOP. This site administered the survey to patients who came to the clinic for appointments. The second facility called patients and asked for their feedback. The third site conducted the survey by using student pharmacists. Two sites reported that they use the survey results from the CMOP-conducted patient satisfaction surveys, although they have not measured patient satisfaction at their specific facilities.
Most sites have not assessed CMOP’s impact on their insurance (point of sale) collections. However, 13 sites (30%) reported that they believe they are losing on collections by utilizing CMOP. The use of repackaged products by CMOP, which are usually nonreimbursable, is an issue that was mentioned multiple times. In contrast, 2 sites mentioned that CMOP has led to increased insurance collections for their facilities.
Discussion
The utility of CMOP among the responding IHS sites varies quite significantly. Some sites appreciate the convenience of CMOP while acknowledging its limitations, such as the possible decrease in insurance collections, lengthy prescription processing time, or medication backorders. However, some sites have reserved CMOP for special circumstances (eg, mailing refrigerated items to the patient’s street address) due to various complexities that may come with CMOP. One site reported that it compares IHS contract drug prices with VA contract drug prices quarterly to determine which prescriptions should be sent through CMOP.
Most of the IHS pharmacists (89%) are trained in CMOP prescription processing. If an IHS site wants to increase its volume of CMOP prescriptions, it is sensible to train as many pharmacists as possible so that the responsibility does not fall on a few pharmacists. Newly hired pharmacists can receive guidance from trained pharmacists. Designation methods for CMOP patients can be beneficial for these pharmacists to identify CMOP-enrolled patients, especially if the site does not require patients to ask for prescriptions to be mailed at the time of refill request. Only 3 sites (7%) use multiple designation methods in addition to relying on patients to ask for prescriptions to be mailed. Proper implementation of designation methods can remove this extra burden on patients. Conversely, requiring patients to ask for prescriptions to be sent through CMOP can prevent spontaneous mail-outs if a CMOP-designated patient wants to pick up prescriptions locally. Overall, 16 sites (36%) rely on patients asking for prescriptions to be mailed.
One of the main benefits of CMOP is the ability to mail refrigerated items. Local pharmacy mail-out programs may not have this ability. Patients at rural locations often use post office (PO) boxes because they are unable to receive postal services at their physical addresses; however, they may receive packages through United Parcel Service (UPS) at their physical addresses. CMOP uses UPS to send refrigerated items, but UPS does not deliver to PO boxes. Therefore, remotely located sites like CHCF have difficulty in fully optimizing this benefit. One solution is documenting both the physical and mailing addresses on the patient’s EHR, which enables CMOP to send refrigerated items to the patient’s home address via UPS and mail the rest of the prescriptions to the patient’s PO box address with the US Postal Service. The physical address must be listed above the PO box address to ensure that refrigerated items are not rejected by CMOP. Furthermore, both the physical address and the PO box address must be in the same city for this method to work. Two sites noted mailing refrigerated items as one of the major challenges in CMOP prescription processing.
CMOP-enrolled patients must be educated about requesting medications 7 to 10 days before they run out. There is no standard time line for prescriptions filled by CMOP. However, 1 site reported that it may take up to “10 days from time requested to mailbox.” This delay leads to pharmacies facing a dilemma as processing prescriptions too early can lead to insurance rejections, but processing them too late can lead to the patient not receiving the medication by the time they run out of their current supply. However, CMOP provides the ability to track prescriptions sent through CMOP. Pharmacists and technicians need to have access to BestWay Parcel Services Client Portal (genco-mms.bestwayparcel.com) to track CMOP packages. Tracking CMOP prescriptions is a way pharmacy technicians can be involved in CMOP prescription processing. Technicians seem to be underutilized, as only 27% of the responding sites utilize them to some degree in the CMOP process. One site delegated the responsibility of checking CMOP rejection messages to pharmacy technicians. Since 2 of the responding sites do not check CMOP rejection messages at all, this is an excellent opportunity to get pharmacy technicians involved.
A CMOP auto-refill program can potentially be utilized to avoid missed or late medications. In an auto-refill program, a pharmacist can refill prescriptions through CMOP on the due date without a patient request. They may get rejected by insurance the first time they are processed through CMOP for refilling too early if the processing time is taken into account. However, the subsequent refills do not have to consider the CMOP processing time as they would already be synchronized based on the last refill date. Though, if CMOP is out of stock on a medication and it is expected to be available soon, CMOP may take a few extra days to either fill the prescription or reject it if the drug stays unavailable. One of the sites reported “the amount of time [CMOP] holds medications if they are out of stock” as “the hardest thing to work around.” A couple of sites also mentioned the longer than usual delay in processing prescriptions by CMOP during the holidays as one of the major challenges.
CMOP use of repackaged products also may lead insurance companies to deny reimbursement. Repackaged products are usually cheaper to buy.14 However, most insurances do not reimburse for prescriptions filled with these products.15 The local drug file on RPMS may have a national drug code (NDC) that is reimbursable by insurance, but CMOP will change it to the repackaged NDC if they are filling the prescription with a repackaged product. One potential solution to this problem would be filling these prescriptions locally. Furthermore, insurance claims are processed when the prescriptions are filled by CMOP. Sites cannot return/cancel the prescription anymore at that point. Therefore, the inability to see real-time rejections as the medication orders are processed on-site makes it challenging to prevent avoidable insurance rejections, such as a refill too soon. One site calculated that it lost $26,386.45 by utilizing CMOP from January 9, 2018 to December 12, 2018. However, it is unclear whether this loss was representative of other sites. It is also worth noting that IHS sites can save a substantial amount of money on certain products by utilizing CMOP because VA buys these products at a reduced price.16
CMOP-transmitted prescriptions can be rejected for various reasons, such as CMOP manufacturer’s backorder, a different quantity from CMOP stock size, etc. Information about these rejected prescriptions is accessed through electronic messages on RPMS. CMOP does not dispense less than a full, unopened package for most over-the-counter (OTC) medications. The quantity on these prescriptions must be equal to or multiples of the package size for them to be filled by CMOP. This can lead to a patient having prescriptions with different days’ supplies, which results in various refill due dates. If a site has a local mail-out program available, it can potentially keep the same days’ supply for all prescriptions by mailing these OTC medications locally rather than utilizing CMOP. However, this can partially negate CMOP’s benefit of reduced workload.
CMOP also has specified quantities on some prescription medications. One survey respondent viewed “the quantity and day supply required by CMOP” as a negative influence on the site’s insurance collection. It is possible that CMOP does not carry all the medications that a CMOP-enrolled patient is prescribed. Most sites (77%) still send eligible prescriptions through CMOP for the patients who also have CMOP-ineligible prescriptions. There are a small number of sites (14%) that utilize local mail-out program for the patients with any CMOP-ineligible prescriptions, possibly to simplify the process. Schedule II controlled substances cannot be processed through CMOP either; however, facilities may have local policies that prohibit mailing any controlled substances.
Prescriptions can be manually transmitted to CMOP, or they can be automatically transmitted based on the run time and frequency of the auto-transmission setup. The prescriptions that are waiting to be transmitted to CMOP must be in the “suspended” status. The apparent advantage of relying on auto-transmission is that you do not have to complete the steps manually to transmit suspended CMOP prescriptions, thereby making the process more convenient. However, the manual transmission can be utilized as a checkpoint to verify that prescriptions were properly suspended for CMOP, as the prescription status changes from “S” (suspended) to “AT” once the transmission is completed. If a prescription is not properly suspended for CMOP, the status will remain as S even after manual transmission. More than half (59%) of the responding sites must find the manual transmission feature useful as they use it either over or in addition to the auto-transmission setup.
Despite the challenges, many IHS sites process thousands of monthly prescriptions through CMOP. Of the 94 CMOP-enrolled IHS sites, 17 processed > 1,000 prescriptions from March 27, 2019 to April 25, 2019.17 Five sites processed > 5,000 prescriptions.17 At the rate of > 5,000 prescriptions per month, the yearly CMOP prescription count will be > 60,000. That is more than one-third of the prescriptions processed by CHCF in 2018. By handling these prescriptions through CMOP, it can decrease pharmacy filling and dispensing workload, thereby freeing pharmacists to participate in other services.18 Furthermore, implementing CMOP does not incur any cost for the IHS site. There is a nondrug cost for each prescription that is filled through CMOP. This cost was $2.67 during FY 2016.19 The fee covers prescription vial, label, packaging for mail, postage, personnel, building overhead, and equipment capitalization.19 The nondrug cost of filling a prescription locally at the site can potentially exceed the cost charged by CMOP.19
A lack of objective data exists to assess the net impact of CMOP on patients. Different theoretical assumptions can be made, such as CMOP resulting in better patient adherence. However, there is no objective information about how much CMOP improves patient adherence if it does at all. Though J.D. Power US Pharmacy Study ranks CMOP as “among the best” mail-order pharmacies in customer satisfaction, only 3 of the 44 responding sites have measured patient satisfaction locally.20 Only 1 site had objective data about CMOP’s impact on the point of sale. Therefore, it is currently difficult to perform a cost-benefit analysis of the CMOP program. There are opportunities for further studies on these topics.
Limitations
One limitation of this study is that < 50% of the CMOP-enrolled sites (44 of 94) responded to the questionnaire. It is possible that the facilities that had a significantly positive or negative experience with CMOP were more inclined to share their views. Therefore, it is difficult to conclude whether the responding sites are an accurate representative sample. Another limitation of the study was the questionnaire design and the reliance on free-text responses as opposed to structured data. The free-text responses had to be analyzed manually to determine whether they fall in the same category, thereby increasing the risk of interpretation error.
Conclusion
CMOP has its unique challenges but provides many benefits that local pharmacy mail-out programs may not possess, such as the abilities to mail refrigerated items and track packages. One must be familiar with CMOP’s various idiosyncrasies to make the best use of the program. Extensive staff education and orientation for new staff members must be done to familiarize them with the program. Nevertheless, the successful implementation of CMOP can lead to reduced pharmacy workload while increasing access to care for patients with transportation issues.
Acknowledgments
The authors thank LCDR Karsten Smith, PharmD, BCGP, the IHS CMOP Coordinator for providing the list of primary CMOP contacts and CDR Kendall Van Tyle, PharmD, BCPS, for proofreading the article.
Consolidated mail outpatient pharmacy (CMOP) is an automated prescription order processing and delivery system developed by the US Department of Veterans Affairs (VA) in 1994 to provide medications to VA patients.1 In fiscal year (FY) 2016, CMOP filled about 80% of VA outpatient prescriptions.2
Formalized by the 2010 Memorandum of Understanding between Indian Health Service (IHS) and VA, CMOP is a partnership undertaken to improve the delivery of care to patients by both agencies.3 The number of prescriptions filled by CMOP for IHS patients increased from 1,972 in FY 2010 to 840,109 in FY 2018.4 In the fourth quarter of FY 2018, there were 94 CMOP-enrolled IHS federal and tribal sites.5 It is only appropriate that a growing number of IHS sites are adopting CMOP considering the evidence for mail-order pharmacy on better patient adherence, improved health outcomes, and potential cost savings.6-9 Furthermore, using a centralized pharmacy operation, such as CMOP, can lead to better quality services.10
Crownpoint Health Care Facility (CHCF) serves > 30,000 American Indians and is in Crownpoint, New Mexico, a small community of about 3,000 people.11 Most of the patients served by the facility live in distant places. Many of these underserved patients do not have a stable means of transportation.12 Therefore, these patients may have difficulty traveling to the facility for their health care needs, including medication pickups. More than 2.5 million American Indians and Alaska Natives IHS beneficiaries face similar challenges due to the rurality of their communities.13 CMOP can be a method to increase access to care for this vulnerable population. However, the utilization of CMOP varies significantly among IHS facilities. While some IHS facilities process large numbers of prescriptions through CMOP, other facilities process few, if any. There also are IHS facilities, such as CHCF, which are at the initial stage of implementing CMOP or trying to increase the volume of prescriptions processed through CMOP. Although the utilization of CMOP has grown exponentially among IHS facilities, there is currently no available resource that summarizes the relative advantages and disadvantages, the challenges and opportunities, and the strengths and weaknesses of implementing CMOP for IHS facilities
Methods
A questionnaire encompassing various aspects of CMOP prescription processing was developed and distributed to the primary CMOP contacts for IHS facilities. The questionnaire was first distributed by e-mail on December 19, 2018. It was e-mailed for a second time on January 16, 2019, and the questionnaire was open for responses until the end of January 2019 (Table).
Results
Forty-four of 94 CMOP-enrolled IHS sites responded to the questionnaire. Most sites train the majority of their pharmacists in CMOP prescription processing. Overall, 310 of 347 pharmacists (89%) in these 44 IHS sites can process prescriptions through CMOP. Thirty-one sites have all their pharmacists trained in CMOP prescription processing. Only 1 facility had less than half (2 of 17 pharmacists) of its pharmacists trained in CMOP prescription processing. More than half the total number of pharmacists, 185 out of 347 (53%), check electronic messages via Resource and Patient Management System (RPMS) MailMan to get information about prescriptions rejected by CMOP. Twenty sites have all their pharmacists check messages about CMOP rejections. However, 2 facilities reported that they do not check the rejection messages at all. Twenty-six of the 44 responding sites (59%) transmit prescriptions to CMOP manually in the electronic system. The rest (18 of 44) rely on the auto-transmission (AT) setup to transmit the CMOP-suspended prescriptions at specified times of the day.
Half the sites (8 of 16) that rely on patients asking for prescriptions to be mailed at the time of refill request do not use any method to designate a CMOP patient. Twenty-four sites use the narrative field on the patient’s profile in RPMS, the health information system used by most IHS facilities, to designate CMOP patients. Eighteen sites use pop-up messages on ScriptPro, a pharmacy automation system, as a designation method. Most of the sites (12 of 15) that use both RPMS and ScriptPro designation methods do not require patients to ask for prescriptions to be mailed at the time of refill request; prescriptions for these patients are routed through CMOP unless patients request otherwise. Only 3 of 44 sites use both methods and rely on patients asking for prescriptions to be mailed at the time of refill request. Some other reported designation methods were using the electronic health record (EHR) posting box, keeping a manual list of CMOP patients, and solely utilizing the Prescription Mail Delivery field in RPMS. Three sites also noted that they keep manual lists to auto-refill prescriptions through CMOP.
Thirty sites (68%) reported that they process every prescription through CMOP even if the patient had prescriptions with specified CMOP quantities. Only 8 sites (18%) said that they used the local mail-out program to keep the same days’ supply for all medication orders. For patients with CMOP-ineligible prescriptions, 34 of the 44 sites (77%) process the eligible prescriptions through CMOP and refill the rest of the prescriptions locally. Six sites (14%) process all medication orders locally for patients with any CMOP-ineligible prescriptions.
Only 12 of 44 sites (27%) involve pharmacy technicians in CMOP prescription processing. Five sites have technicians process prescription refills through CMOP. Two of these sites mentioned the strategy of technicians suspending the prescriptions to be sent to CMOP on the refill due date. Other technician roles included tracking CMOP packages, checking electronic messages for CMOP rejections, and signing up patients for CMOP.
Only 3 of the 44 sites (7%) have measured patient satisfaction with the CMOP program. One of these 3 sites reported that the overall satisfaction was high with CMOP. This site administered the survey to patients who came to the clinic for appointments. The second facility called patients and asked for their feedback. The third site conducted the survey by using student pharmacists. Two sites reported that they use the survey results from the CMOP-conducted patient satisfaction surveys, although they have not measured patient satisfaction at their specific facilities.
Most sites have not assessed CMOP’s impact on their insurance (point of sale) collections. However, 13 sites (30%) reported that they believe they are losing on collections by utilizing CMOP. The use of repackaged products by CMOP, which are usually nonreimbursable, is an issue that was mentioned multiple times. In contrast, 2 sites mentioned that CMOP has led to increased insurance collections for their facilities.
Discussion
The utility of CMOP among the responding IHS sites varies quite significantly. Some sites appreciate the convenience of CMOP while acknowledging its limitations, such as the possible decrease in insurance collections, lengthy prescription processing time, or medication backorders. However, some sites have reserved CMOP for special circumstances (eg, mailing refrigerated items to the patient’s street address) due to various complexities that may come with CMOP. One site reported that it compares IHS contract drug prices with VA contract drug prices quarterly to determine which prescriptions should be sent through CMOP.
Most of the IHS pharmacists (89%) are trained in CMOP prescription processing. If an IHS site wants to increase its volume of CMOP prescriptions, it is sensible to train as many pharmacists as possible so that the responsibility does not fall on a few pharmacists. Newly hired pharmacists can receive guidance from trained pharmacists. Designation methods for CMOP patients can be beneficial for these pharmacists to identify CMOP-enrolled patients, especially if the site does not require patients to ask for prescriptions to be mailed at the time of refill request. Only 3 sites (7%) use multiple designation methods in addition to relying on patients to ask for prescriptions to be mailed. Proper implementation of designation methods can remove this extra burden on patients. Conversely, requiring patients to ask for prescriptions to be sent through CMOP can prevent spontaneous mail-outs if a CMOP-designated patient wants to pick up prescriptions locally. Overall, 16 sites (36%) rely on patients asking for prescriptions to be mailed.
One of the main benefits of CMOP is the ability to mail refrigerated items. Local pharmacy mail-out programs may not have this ability. Patients at rural locations often use post office (PO) boxes because they are unable to receive postal services at their physical addresses; however, they may receive packages through United Parcel Service (UPS) at their physical addresses. CMOP uses UPS to send refrigerated items, but UPS does not deliver to PO boxes. Therefore, remotely located sites like CHCF have difficulty in fully optimizing this benefit. One solution is documenting both the physical and mailing addresses on the patient’s EHR, which enables CMOP to send refrigerated items to the patient’s home address via UPS and mail the rest of the prescriptions to the patient’s PO box address with the US Postal Service. The physical address must be listed above the PO box address to ensure that refrigerated items are not rejected by CMOP. Furthermore, both the physical address and the PO box address must be in the same city for this method to work. Two sites noted mailing refrigerated items as one of the major challenges in CMOP prescription processing.
CMOP-enrolled patients must be educated about requesting medications 7 to 10 days before they run out. There is no standard time line for prescriptions filled by CMOP. However, 1 site reported that it may take up to “10 days from time requested to mailbox.” This delay leads to pharmacies facing a dilemma as processing prescriptions too early can lead to insurance rejections, but processing them too late can lead to the patient not receiving the medication by the time they run out of their current supply. However, CMOP provides the ability to track prescriptions sent through CMOP. Pharmacists and technicians need to have access to BestWay Parcel Services Client Portal (genco-mms.bestwayparcel.com) to track CMOP packages. Tracking CMOP prescriptions is a way pharmacy technicians can be involved in CMOP prescription processing. Technicians seem to be underutilized, as only 27% of the responding sites utilize them to some degree in the CMOP process. One site delegated the responsibility of checking CMOP rejection messages to pharmacy technicians. Since 2 of the responding sites do not check CMOP rejection messages at all, this is an excellent opportunity to get pharmacy technicians involved.
A CMOP auto-refill program can potentially be utilized to avoid missed or late medications. In an auto-refill program, a pharmacist can refill prescriptions through CMOP on the due date without a patient request. They may get rejected by insurance the first time they are processed through CMOP for refilling too early if the processing time is taken into account. However, the subsequent refills do not have to consider the CMOP processing time as they would already be synchronized based on the last refill date. Though, if CMOP is out of stock on a medication and it is expected to be available soon, CMOP may take a few extra days to either fill the prescription or reject it if the drug stays unavailable. One of the sites reported “the amount of time [CMOP] holds medications if they are out of stock” as “the hardest thing to work around.” A couple of sites also mentioned the longer than usual delay in processing prescriptions by CMOP during the holidays as one of the major challenges.
CMOP use of repackaged products also may lead insurance companies to deny reimbursement. Repackaged products are usually cheaper to buy.14 However, most insurances do not reimburse for prescriptions filled with these products.15 The local drug file on RPMS may have a national drug code (NDC) that is reimbursable by insurance, but CMOP will change it to the repackaged NDC if they are filling the prescription with a repackaged product. One potential solution to this problem would be filling these prescriptions locally. Furthermore, insurance claims are processed when the prescriptions are filled by CMOP. Sites cannot return/cancel the prescription anymore at that point. Therefore, the inability to see real-time rejections as the medication orders are processed on-site makes it challenging to prevent avoidable insurance rejections, such as a refill too soon. One site calculated that it lost $26,386.45 by utilizing CMOP from January 9, 2018 to December 12, 2018. However, it is unclear whether this loss was representative of other sites. It is also worth noting that IHS sites can save a substantial amount of money on certain products by utilizing CMOP because VA buys these products at a reduced price.16
CMOP-transmitted prescriptions can be rejected for various reasons, such as CMOP manufacturer’s backorder, a different quantity from CMOP stock size, etc. Information about these rejected prescriptions is accessed through electronic messages on RPMS. CMOP does not dispense less than a full, unopened package for most over-the-counter (OTC) medications. The quantity on these prescriptions must be equal to or multiples of the package size for them to be filled by CMOP. This can lead to a patient having prescriptions with different days’ supplies, which results in various refill due dates. If a site has a local mail-out program available, it can potentially keep the same days’ supply for all prescriptions by mailing these OTC medications locally rather than utilizing CMOP. However, this can partially negate CMOP’s benefit of reduced workload.
CMOP also has specified quantities on some prescription medications. One survey respondent viewed “the quantity and day supply required by CMOP” as a negative influence on the site’s insurance collection. It is possible that CMOP does not carry all the medications that a CMOP-enrolled patient is prescribed. Most sites (77%) still send eligible prescriptions through CMOP for the patients who also have CMOP-ineligible prescriptions. There are a small number of sites (14%) that utilize local mail-out program for the patients with any CMOP-ineligible prescriptions, possibly to simplify the process. Schedule II controlled substances cannot be processed through CMOP either; however, facilities may have local policies that prohibit mailing any controlled substances.
Prescriptions can be manually transmitted to CMOP, or they can be automatically transmitted based on the run time and frequency of the auto-transmission setup. The prescriptions that are waiting to be transmitted to CMOP must be in the “suspended” status. The apparent advantage of relying on auto-transmission is that you do not have to complete the steps manually to transmit suspended CMOP prescriptions, thereby making the process more convenient. However, the manual transmission can be utilized as a checkpoint to verify that prescriptions were properly suspended for CMOP, as the prescription status changes from “S” (suspended) to “AT” once the transmission is completed. If a prescription is not properly suspended for CMOP, the status will remain as S even after manual transmission. More than half (59%) of the responding sites must find the manual transmission feature useful as they use it either over or in addition to the auto-transmission setup.
Despite the challenges, many IHS sites process thousands of monthly prescriptions through CMOP. Of the 94 CMOP-enrolled IHS sites, 17 processed > 1,000 prescriptions from March 27, 2019 to April 25, 2019.17 Five sites processed > 5,000 prescriptions.17 At the rate of > 5,000 prescriptions per month, the yearly CMOP prescription count will be > 60,000. That is more than one-third of the prescriptions processed by CHCF in 2018. By handling these prescriptions through CMOP, it can decrease pharmacy filling and dispensing workload, thereby freeing pharmacists to participate in other services.18 Furthermore, implementing CMOP does not incur any cost for the IHS site. There is a nondrug cost for each prescription that is filled through CMOP. This cost was $2.67 during FY 2016.19 The fee covers prescription vial, label, packaging for mail, postage, personnel, building overhead, and equipment capitalization.19 The nondrug cost of filling a prescription locally at the site can potentially exceed the cost charged by CMOP.19
A lack of objective data exists to assess the net impact of CMOP on patients. Different theoretical assumptions can be made, such as CMOP resulting in better patient adherence. However, there is no objective information about how much CMOP improves patient adherence if it does at all. Though J.D. Power US Pharmacy Study ranks CMOP as “among the best” mail-order pharmacies in customer satisfaction, only 3 of the 44 responding sites have measured patient satisfaction locally.20 Only 1 site had objective data about CMOP’s impact on the point of sale. Therefore, it is currently difficult to perform a cost-benefit analysis of the CMOP program. There are opportunities for further studies on these topics.
Limitations
One limitation of this study is that < 50% of the CMOP-enrolled sites (44 of 94) responded to the questionnaire. It is possible that the facilities that had a significantly positive or negative experience with CMOP were more inclined to share their views. Therefore, it is difficult to conclude whether the responding sites are an accurate representative sample. Another limitation of the study was the questionnaire design and the reliance on free-text responses as opposed to structured data. The free-text responses had to be analyzed manually to determine whether they fall in the same category, thereby increasing the risk of interpretation error.
Conclusion
CMOP has its unique challenges but provides many benefits that local pharmacy mail-out programs may not possess, such as the abilities to mail refrigerated items and track packages. One must be familiar with CMOP’s various idiosyncrasies to make the best use of the program. Extensive staff education and orientation for new staff members must be done to familiarize them with the program. Nevertheless, the successful implementation of CMOP can lead to reduced pharmacy workload while increasing access to care for patients with transportation issues.
Acknowledgments
The authors thank LCDR Karsten Smith, PharmD, BCGP, the IHS CMOP Coordinator for providing the list of primary CMOP contacts and CDR Kendall Van Tyle, PharmD, BCPS, for proofreading the article.
1. US Department of Veterans Affairs, Office of Inspector General. Audit of Consolidated Mail Outpatient Pharmacy contract management. https://www.va.gov/oig/52/reports/2009/VAOIG-09-00026-143.pdf. Published June 10, 2009. Accessed June 11, 2020.
2. US Department of Veterans Affairs. Pharmacy Benefits Management Services. VA mail order pharmacy. https://www.pbm.va.gov/PBM/CMOP/VA_Mail_Order_Pharmacy.asp. Updated July 18, 2018. Accessed July 16, 2019.
3. US Department of Veterans Affairs. Memorandum of understanding between the Department of Veterans Affairs (VA) and Indian Health Service (IHS). https://www.va.gov/TRIBALGOVERNMENT/docs/Signed2010VA-IHSMOU.pdf. Published October 1, 2010. Accessed June 11, 2020.
4. US Department of Veterans Affairs, Office of Tribal Government Relations, Office of Rural Health, US Department of Health and Human Services, Indian Health Service. U.S. Department of Veterans Affairs and Indian Health Service memorandum of understanding annual report fiscal year 2018. https://www.ruralhealth.va.gov/docs/VA-IHS_MOU_AnnualReport_FY2018_FINAL.pdf. Published December 2018. Accessed June 11, 2020.
5. Karsten S. CMOP items of interest. Published October 12, 2018. [Nonpublic document]
6. Fernandez EV, McDaniel JA, Carroll NV. Examination of the link between medication adherence and use of mail-order pharmacies in chronic disease states. J Manag Care Spec Pharm. 2016;22(11):1247‐1259. doi:10.18553/jmcp.2016.22.11.1247
7. Schwab P, Racsa P, Rascati K, Mourer M, Meah Y, Worley K. A retrospective database study comparing diabetes-related medication adherence and health outcomes for mail-order versus community pharmacy. J Manag Care Spec Pharm. 2019;25(3):332‐340. doi:10.18553/jmcp.2019.25.3.332
8. Schmittdiel JA, Karter AJ, Dyer W, et al. The comparative effectiveness of mail order pharmacy use vs. local pharmacy use on LDL-C control in new statin users. J Gen Intern Med. 2011;26(12):1396‐1402. doi:10.1007/s11606-011-1805-7
9. Devine S, Vlahiotis A, Sundar H. A comparison of diabetes medication adherence and healthcare costs in patients using mail order pharmacy and retail pharmacy. J Med Econ. 2010;13(2):203‐211. doi:10.3111/13696991003741801
10. Kappenman AM, Ragsdale R, Rim MH, Tyler LS, Nickman NA. Implementation of a centralized mail-order pharmacy service. Am J Health Syst Pharm. 2019;76(suppl 3):S74‐S78. doi:10.1093/ajhp/zxz138
11. US Department of Health and Human Services, Indian Health Service. Crownpoint service unit. www.ihs.gov/crownpoint. Accessed June 11, 2020.
12. Chaco P. Roads and transportation on the Navajo Nation. https://obamawhitehouse.archives.gov/microsite/blog/31387?page=135. Published February 15, 2012. Accessed June 11, 2020.
13. US Department of Health and Human Services, Indian Health Service. Disparities. www.ihs.gov/newsroom/factsheets/disparities. Updated October 2019. Accessed June 11, 2020.
14. Golden State Medical Supply. National contracts. www.gsms.us/wp-content/uploads/2018/10/National-Contracts-Flyer.pdf. Updated October 4, 2018. Accessed June 11, 2020.
15. Arizona Health Care Cost Containment System. IHS/Tribal provider billing manual chapter 9, hospital and clinic services. www.azahcccs.gov/PlansProviders/Downloads/IHS-TribalManual/IHS-Chap09HospClinic.pdf. Updated February 28, 2019. Accessed June 11, 2020.
16. US Department of Veterans Affairs, Office of Inspector General. The impact of VA allowing government agencies to be excluded from temporary price reductions on federal supply schedule pharmaceutical contracts. www.va.gov/oig/pubs/VAOIG-18-04451-06.pdf. Published October 30, 2019. Accessed June 11, 2020.
17. Karsten S. IHS Billing Report-Apr. Indian Health Service SharePoint. Published May 3, 2019. [Nonpublic document]
18. Aragon BR, Pierce RA 2nd, Jones WN. VA CMOPs: producing a pattern of quality and efficiency in government. J Am Pharm Assoc (2003). 2012;52(6):810‐815. doi:10.1331/JAPhA.2012.11075
19. Todd W. VA-IHS Consolidated Mail Outpatient Pharmacy program (CMOP). www.npaihb.org/wp-content/uploads/2017/01/CMOP-Slides-for-Portland-Area-Tribal-Sites.pdf. Published 2017. Accessed June 11, 2020.
20. J.D. Power. Pharmacy customers slow to adopt digital offerings but satisfaction increases when they do, J.D. Power finds. www.jdpower.com/business/press-releases/2019-us-pharmacy-study. Published August 20, 2019. Accessed June 11, 2020.
1. US Department of Veterans Affairs, Office of Inspector General. Audit of Consolidated Mail Outpatient Pharmacy contract management. https://www.va.gov/oig/52/reports/2009/VAOIG-09-00026-143.pdf. Published June 10, 2009. Accessed June 11, 2020.
2. US Department of Veterans Affairs. Pharmacy Benefits Management Services. VA mail order pharmacy. https://www.pbm.va.gov/PBM/CMOP/VA_Mail_Order_Pharmacy.asp. Updated July 18, 2018. Accessed July 16, 2019.
3. US Department of Veterans Affairs. Memorandum of understanding between the Department of Veterans Affairs (VA) and Indian Health Service (IHS). https://www.va.gov/TRIBALGOVERNMENT/docs/Signed2010VA-IHSMOU.pdf. Published October 1, 2010. Accessed June 11, 2020.
4. US Department of Veterans Affairs, Office of Tribal Government Relations, Office of Rural Health, US Department of Health and Human Services, Indian Health Service. U.S. Department of Veterans Affairs and Indian Health Service memorandum of understanding annual report fiscal year 2018. https://www.ruralhealth.va.gov/docs/VA-IHS_MOU_AnnualReport_FY2018_FINAL.pdf. Published December 2018. Accessed June 11, 2020.
5. Karsten S. CMOP items of interest. Published October 12, 2018. [Nonpublic document]
6. Fernandez EV, McDaniel JA, Carroll NV. Examination of the link between medication adherence and use of mail-order pharmacies in chronic disease states. J Manag Care Spec Pharm. 2016;22(11):1247‐1259. doi:10.18553/jmcp.2016.22.11.1247
7. Schwab P, Racsa P, Rascati K, Mourer M, Meah Y, Worley K. A retrospective database study comparing diabetes-related medication adherence and health outcomes for mail-order versus community pharmacy. J Manag Care Spec Pharm. 2019;25(3):332‐340. doi:10.18553/jmcp.2019.25.3.332
8. Schmittdiel JA, Karter AJ, Dyer W, et al. The comparative effectiveness of mail order pharmacy use vs. local pharmacy use on LDL-C control in new statin users. J Gen Intern Med. 2011;26(12):1396‐1402. doi:10.1007/s11606-011-1805-7
9. Devine S, Vlahiotis A, Sundar H. A comparison of diabetes medication adherence and healthcare costs in patients using mail order pharmacy and retail pharmacy. J Med Econ. 2010;13(2):203‐211. doi:10.3111/13696991003741801
10. Kappenman AM, Ragsdale R, Rim MH, Tyler LS, Nickman NA. Implementation of a centralized mail-order pharmacy service. Am J Health Syst Pharm. 2019;76(suppl 3):S74‐S78. doi:10.1093/ajhp/zxz138
11. US Department of Health and Human Services, Indian Health Service. Crownpoint service unit. www.ihs.gov/crownpoint. Accessed June 11, 2020.
12. Chaco P. Roads and transportation on the Navajo Nation. https://obamawhitehouse.archives.gov/microsite/blog/31387?page=135. Published February 15, 2012. Accessed June 11, 2020.
13. US Department of Health and Human Services, Indian Health Service. Disparities. www.ihs.gov/newsroom/factsheets/disparities. Updated October 2019. Accessed June 11, 2020.
14. Golden State Medical Supply. National contracts. www.gsms.us/wp-content/uploads/2018/10/National-Contracts-Flyer.pdf. Updated October 4, 2018. Accessed June 11, 2020.
15. Arizona Health Care Cost Containment System. IHS/Tribal provider billing manual chapter 9, hospital and clinic services. www.azahcccs.gov/PlansProviders/Downloads/IHS-TribalManual/IHS-Chap09HospClinic.pdf. Updated February 28, 2019. Accessed June 11, 2020.
16. US Department of Veterans Affairs, Office of Inspector General. The impact of VA allowing government agencies to be excluded from temporary price reductions on federal supply schedule pharmaceutical contracts. www.va.gov/oig/pubs/VAOIG-18-04451-06.pdf. Published October 30, 2019. Accessed June 11, 2020.
17. Karsten S. IHS Billing Report-Apr. Indian Health Service SharePoint. Published May 3, 2019. [Nonpublic document]
18. Aragon BR, Pierce RA 2nd, Jones WN. VA CMOPs: producing a pattern of quality and efficiency in government. J Am Pharm Assoc (2003). 2012;52(6):810‐815. doi:10.1331/JAPhA.2012.11075
19. Todd W. VA-IHS Consolidated Mail Outpatient Pharmacy program (CMOP). www.npaihb.org/wp-content/uploads/2017/01/CMOP-Slides-for-Portland-Area-Tribal-Sites.pdf. Published 2017. Accessed June 11, 2020.
20. J.D. Power. Pharmacy customers slow to adopt digital offerings but satisfaction increases when they do, J.D. Power finds. www.jdpower.com/business/press-releases/2019-us-pharmacy-study. Published August 20, 2019. Accessed June 11, 2020.
Trauma-Informed Telehealth in the COVID-19 Era and Beyond
COVID-19 has created stressors that are unprecedented in our modern era, prompting health care systems to adapt rapidly. Demand for telehealth has skyrocketed, and clinicians, many of whom had planned to adopt virtual practices in the future, have been pressured to do so immediately.1 In March 2020, the Centers for Medicare and Medicaid Services (CMS) expanded telehealth services, removing many barriers to virtual care.2 Similar remedy was not necessary for the Veterans Health Administration (VHA) which reported more than 2.6 million episodes of telehealth care in 2019.3 By the time the pandemic was underway in the US, use of telehealth was widespread across the agency. In late March 2020, VHA released a COVID-19 Response Plan, in which telehealth played a critical role in safe, uninterrupted delivery of services.4 While telehealth has been widely used in VHA, the call for replacement of most in-person outpatient visits with telehealth visits was a fundamental paradigm shift for many patients and clinicians.4
The Coronavirus Aid, Relief, and Economic Security (CARES) Act (HR 748) gave the US Department of Veterans Affairs (VA) funding to expand coronavirus-related telehealth services, including the purchase of mobile devices and broadband expansion. CARES authorized the agency to expand telemental health services, enter into short-term agreements with telecommunications companies to provide temporary broadband services to veterans, temporarily waived an in-person home visit requirement (accepting video and phone calls as an alternative), and provided means to make telehealth available for homeless veterans and case managers through the HUD-VASH (US Department of Housing and Urban Development-VA Supportive Housing) program.
VHA is a national telehealth exemplar, initiating telehealth by use of closed-circuit televisions as early as 1968, and continuing to expand through 2017 with the implementation of the Veterans Video Connect (VVC) platform.5 VVC has enabled veterans to participate in virtual visits from distant locations, including their homes. VVC was used successfully during hurricanes Sandy, Harvey, Irma, and Maria and is being widely deployed in the current crisis.6-8
While telehealth can take many forms, the current discussion will focus on live (synchronous) videoconferencing: a 2-way audiovisual link between a patient and clinician, such as VVC, which enables patients to maintain a safe and social distance from others while connecting with the health care team and receiving urgent as well as ongoing medical care for both new and established conditions.9 VHA has developed multiple training resources for use of VVC across many settings, including primary care, mental health, and specialties. In this review, we will make the novel case for applying a trauma-informed lens to telehealth care across VHA and beyond to other health care systems.
Trauma-Informed Care
Although our current focus is rightly on mitigating the health effects of a pandemic, we must recognize that stressful phenomena like COVID-19 occur against a backdrop of widespread physical, sexual, psychological, and racial trauma in our communities. The Substance Abuse and Mental Health Services Administration (SAMHSA) describes trauma as resulting from “an event, series of events, or set of circumstances that is experienced by an individual as physically or emotionally harmful or life threatening and that has lasting adverse effects on the individual’s functioning and mental, physical, social, emotional, or spiritual well-being.”10 Trauma exposure is both ubiquitous worldwide and inequitably distributed, with vulnerable populations disproportionately impacted.11,12
Veterans as a population are often highly trauma exposed, and while VHA routinely screens for experiences of trauma, such as military sexual trauma (MST) and intimate partner violence (IPV), and potential mental health sequelae of trauma, including posttraumatic stress disorder (PTSD) and suicidality, veterans may experience other forms of trauma or be unwilling or unable to talk about past exposures.13 One common example is that of adverse childhood experiences (ACEs), which include household dysfunction, neglect, and physical and sexual abuse before the age of 18 years.14 ACEs have been associated with a wide range of risk behaviors and poor health outcomes in adulthood.14 In population-based data, both male and female veterans have reported higher ACE scores.15 In addition, ACE scores are higher overall for those serving in the all-volunteer era (after July 1, 1973).16 Because trauma may be unseen, unmeasured, and unnamed, it is important to deliver all medical care with sensitivity to its potential presence.
It is important to distinguish the concept of trauma-informed care (TIC) from trauma-focused services. Trauma-focused or trauma-specific treatment refers to evidence-based and best practice treatment models that have been proven to facilitate recovery from problems resulting from the experience of trauma, such as PTSD.17 These treatments directly address the emotional, behavioral, and physiologic impact of trauma on an individual’s life and facilitate improvement in related symptoms and functioning: They are designed to treat the consequences of trauma. VHA offers a wide range of trauma-specific treatments, and considerable experience in delivering evidence-based trauma-focused treatment through telehealth exists.18,19 Given the range of possible responses to the experience of trauma, not all veterans with trauma histories need to, chose to, or feel ready to access trauma-specific treatments.20
In contrast, TIC is a global, universal precautions approach to providing quality care that can be applied to all aspects of health care and to all patients.21 TIC is a strengths-based service delivery framework that is grounded in an understanding of, and responsiveness to, the disempowering impact of experiencing trauma. It seeks to maximize physical, psychological, and emotional safety in all health care encounters, not just those that are specifically trauma-focused, and creates opportunities to rebuild a sense of control and empowerment while fostering healing through safe and collaborative patient-clinician relationships.22 TIC is not accomplished through any single technique or checklist but through continuous appraisal of approaches to care delivery. SAMHSA has elucidated 6 fundamental principles of TIC: safety; trustworthiness and transparency; peer support; collaboration and mutuality; empowerment; voice and choice; and sensitivity to cultural, historical, and gender issues.10
TIC is based on the understanding that often traditional service delivery models of care may trigger, silence, or disempower survivors of trauma, exacerbating physical and mental health symptoms and potentially increasing disengagement from care and poorer outcomes.23 Currier and colleagues aptly noted, “TIC assumes that trustworthiness is not something that an organization creates in a veteran client, but something that he or she will freely grant to an organization.”24 Given the global prevalence of trauma, its well-established and deleterious impact on lifelong health, and the potential for health care itself to be traumatizing, TIC is a fundamental construct to apply universally with any patient at any time, especially in the context of a large-scale community trauma, such as a pandemic.12
Trauma-Informed COVID-19 Care
Catastrophic events, such as natural disasters and pandemics, may serve as both newly traumatic and as potential triggers for survivors who have endured prior trauma.25,26 Increases in depression, PTSD, and substance use disorder (SUD) are common sequalae, occurring during the event, the immediate aftermath, and beyond.25,27 In 2003, quarantine contained the spread of Severe acute respiratory syndrome (SARS) but resulted in a high prevalence of psychological distress, including PTSD and depression.27 Many veterans may have deployed in support of humanitarian assistance/disaster relief missions, which typically do not involve armed combat but may expose service members to warlike situations, including social insecurity and suffering populations.28 COVID-19 may be reminiscent of some of these deployments as well.
The impact of the current COVID-19 pandemic on patients is pervasive. Those with preexisting financial insecurity now face additional economic hardship and health challenges, which are amplified by loneliness and loss of social support networks.26 Widespread unemployment and closures of many businesses add to stress and may exacerbate preexisting mental and physical health concerns for many; some veterans also may be at increased risk.29 While previous postdisaster research suggests that psychopathology in the general population will significantly remit over time, high-risk groups remain vulnerable to PTSD and bear the brunt of social and economic consequences associated with the crisis.25 Veterans with preexisting trauma histories and mental health conditions are at increased risk for being retraumatized by the current pandemic and impacted by isolation and unplanned job or wage loss from it.29 Compounding this, social distancing serves to protect communities but may amplify isolation and danger in abusive relationships or exacerbate underlying mental illness.26,30
Thus, as we expand our use of telehealth, replacing our face-to-face visits with virtual encounters, it is critical for clinicians to be mindful that the pandemic and public health responses to it may result in trauma and retraumatization for veterans and other vulnerable patients, which in turn can impact both access and response to care. The application of trauma-informed principles to our virtual encounters has the potential to mitigate some of these health impacts, increase engagement in care, and provide opportunities for protective, healing connections.
In the setting of the continued fear and uncertainty of the COVID-19 pandemic, we believe that application of a trauma-informed lens to telehealth efforts is timely. While virtual visits may seem to lack the warmth and immediacy of traditional medical encounters, accumulated experience suggests otherwise.19 Telehealth is fundamentally more patient-focused than traditional encounters, overcomes service delivery barriers, offers a greater range of options for treatment engagement, and can enhance clinician-patient partnerships.6,31,32 Although the rapid transition to telehealth may be challenging for those new to it, experienced clinicians and patients express high degrees of satisfaction with virtual care because direct communication is unhampered by in-office challenges and travel logistics.33
While it may feel daunting to integrate principles of TIC into telehealth during a crisis-driven scale-up, a growing practice and body of research can inform these efforts. To help better understand how trauma-exposed patients respond to telehealth, we reviewed findings from trauma-focused telemental health (TMH) treatment. This research demonstrates that telehealth promotes safety and collaboration—fundamental principles of TIC—that can, in turn, be applied to telehealth visits in primary care and other medical and surgical specialties. When compared with traditional in-person treatment, studies of both individual and group formats of TMH found no significant differences in satisfaction, acceptability, or outcomes (such as reduction in PTSD symptom severity scores34), and TMH did not impede development of rapport.19,35
Although counterintuitive, the virtual space created by the combined physical and psychological distance of videoconferencing has been shown to promote safety and transparency. In TMH, patients have reported greater honesty due to the protection afforded by this virtual space.31 Engaging in telehealth visits from the comfort of one’s home can feel emotionally safer than having to travel to a medical office, resulting in feeling more at ease during encounters.31 In one TMH study, veterans with PTSD described high comfort levels and ability to let their guard down during virtual treatment.19 Similarly, in palliative telehealth care, patients reported that clinicians successfully nurtured an experience of intimacy, expressed empathy verbally and nonverbally, and responded to the patient’s unique situation and emotions.33
Trauma-Informed Telehealth
We have discussed how telehealth’s greater flexibility may create an ideal environment in which to implement principles of TIC. It may allow increased collaboration and closeness between patients and clinicians, empowering patients to codesign their care.31,33 The Table reviews 6 core SAMHSA principles of TIC and offers examples of their application to telehealth visits. The following case illustrates the application of trauma-informed telehealth care.
Case Presentation
S is a 45-year-old male veteran of Operation Enduring Freedom (OEF) who served as a combat medic. He has a history of osteoarthritis and PTSD related to combat experiences like caring for traumatic amputees. Before the pandemic began, he was employed as a server at a local restaurant but was laid off as the business transitioned to takeout orders only. The patient worked near a VA primary care clinic and frequently dropped by to see the staff and to pick up prescriptions. He had never agreed to video visits despite receiving encouragement from his medical team. He was reluctant to try telehealth, but he had developed a painful, itchy rash on his lower leg and was concerned about getting care.
For patients like S who may be reluctant to try telehealth, it is important to understand the cause. Potential barriers to telehealth may include lack of Internet access or familiarity with technology, discomfort with being on video, shame about the appearance of one’s home, or a strong cultural preference for face-to-face medical visits. Some may miss the social support benefit of coming into a clinic, particularly in VHA, which is designed specifically for veteran patients. For these reasons it is important to offer the patient a choice and to begin with a supportive phone call that explores and strives to address the patient’s concerns about videoconferencing.
The clinic nurse called S who agreed to try a VVC visit with gentle encouragement. He shared that he was embarrassed about the appearance of his apartment and fearful about pictures being recorded of his body due to “a bad experience in my past.” The patient was reassured that visits are private and will not be recorded. The nurse also reminded him that he can choose the location in which the visit will take place and can turn his camera off at any time. Importantly, the nurse did not ask him to recount additional details of what happened in his past. Next, the nurse verified his location and contact information and explained why obtaining this information was necessary. Next, she asked his consent to proceed with the visit, reminding him that the visit can end at any point if he feels uncomfortable. After finishing this initial discussion, the nurse told him that his primary care physician (PCP) would join the visit and address his concerns with his leg.
S was happy to see his PCP despite his hesitations about video care. The PCP noticed that he seemed anxious and was avoiding talking about the rash. Knowing that he was anxious about this VVC visit, the PCP was careful to look directly at the camera to make eye contact and to be sure her face was well lit and not in shadows. She gave him some time to acclimate to the virtual environment and thanked him for joining the visit. Knowing that he was a combat veteran, she warned him that there have been sudden, loud construction noises outside her window. Although the PCP was pressed for time, she was aware that S may have had a previous difficult experience around images of his body or even combat-related trauma. She gently brought up the rash and asked for permission to examine it, avoiding commands or personalizing language such as “show me your leg” or “take off your pants for me.”36After some hesitation, the patient revealed his leg that appeared to have multiple excoriations and old scars from picking. After the examination, the PCP waited until the patient’s leg was fully covered before beginning a discussion of the care plan. Together they collaboratively reviewed treatments that would soothe the skin. They decided to virtually consult a social worker to obtain emergency economic assistance and to speak with the patient’s care team psychologist to reduce some of the anxiety that may be leading to his leg scratching.
Case Discussion
This case illustrates the ways in which TIC can be applied to telehealth for a veteran with combat-related PTSD who may have experienced additional interpersonal trauma. It was not necessary to know more detail about the veteran’s trauma history to conduct the visit in a trauma-informed manner. Connecting to patients at home while considering these principles may thus foster mutuality, mitigate retraumatization, and cultivate enhanced collaboration with health care teams in this era of social distancing.
While a virtual physical examination creates both limitations and opportunity in telehealth, patients may find the greater degree of choice over their clothing and surroundings to be empowering. Telehealth also can allow for a greater portion of time to be dedicated to quality discussion and collaborative planning, with the clinician hearing and responding to the patient’s needs with reduced distraction. This may include opportunities to discuss mental health concerns openly, normalize emotional reactions, and offer connection to mental health and support services available through telehealth, including for patients who have not previously engaged in such care.
Conclusions
1. Wosik J, Fudim M, Cameron B, et al. Telehealth transformation: COVID-19 and the rise of virtual care. J Am Med Inform Assoc. 2020;27(6):957-962. doi:10.1093/jamia/ocaa067
2. Centers for Medicare and Medicaid Services. Medicare and Medicaid programs; policy and regulatory revisions in response to the COVID-19 public health emergency. CMS-1744-IFC. https://www.cms.gov/files/document/covid-final-ifc.pdf. Published March 24, 2020. Accessed April 8, 2020.
3. Eddy N. VA sees a surge in veterans’ use of telehealth services. https://www.healthcareitnews.com/news/va-sees-surge-veterans-use-telehealth-services. Published November 25, 2019. Accessed June 17, 2020.
4. Veterans Health Administration, Office of Emergency Management. COVID-19 response plan. Version 1.6. Published March 23, 2020. Accessed June 17, 2020.
5. Caudill RL, Sager Z. Institutionally based videoconferencing. Int Rev Psychiatry. 2015;27(6):496-503. doi:10.3109/09540261.2015.1085369
6. Heyworth L. Sharing Connections [published correction appears in JAMA. 2018 May 8;319(18):1939]. JAMA. 2018;319(13):1323-1324. doi:10.1001/jama.2018.2717
7. Dobalian A. U.S. Department of Veterans Affairs’ (VA’s) response to the 2017 hurricanes. Presented at: American Public Health Association 2019 Annual Meeting and Exposition; November 2-6, 2019; Philadelphia, PA. https://apha.confex.com/apha/2019/meetingapp.cgi/Session/58543. Accessed June 16, 2020.
8. Der-Martirosian C, Griffin AR, Chu K, Dobalian A. Telehealth at the US Department of Veterans Affairs after Hurricane Sandy. J Telemed Telecare. 2019;25(5):310-317. doi:10.1177/1357633X17751005
9. The Office of the National Coordinator for Health Information Technology. Telemedicine and telehealth. https://www.healthit.gov/topic/health-it-initiatives/telemedicine-and-telehealth. Updated September 28, 2017. Accessed June 16, 2020.
10. Substance Abuse and Mental Health Services Administration, Trauma and Justice Strategic Initiative. SAMHSA’s concept of trauma and guidance for a trauma-informed approach. https://ncsacw.samhsa.gov/userfiles/files/SAMHSA_Trauma.pdf. Published July 2014. Accessed June 16, 2020.
11. Kilpatrick DG, Resnick HS, Milanak ME, Miller MW, Keyes KM, Friedman MJ. National estimates of exposure to traumatic events and PTSD prevalence using DSM-IV and DSM-5 criteria. J Trauma Stress. 2013;26(5):537-547. doi:10.1002/jts.21848
12. Kimberg L, Wheeler M. Trauma and Trauma-informed Care. In: Gerber MR, ed. Trauma-informed Healthcare Approaches: A Guide for Primary Care. Cham, Switzerland: Springer Nature; 2019:25-56.
13. Gerber MR. Trauma-informed care of veterans. In: Gerber MR, ed. Trauma-informed Healthcare Approaches: A Guide for Primary Care. Cham, Switzerland: Springer Nature; 2019:25-56.
14. Felitti VJ, Anda RF, Nordenberg D, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med. 1998;14(4):245-258. doi:10.1016/s0749-3797(98)00017-8
15. Katon JG, Lehavot K, Simpson TL, et al. Adverse childhood experiences, Military service, and adult health. Am J Prev Med. 2015;49(4):573-582. doi:10.1016/j.amepre.2015.03.020
16. Blosnich JR, Dichter ME, Cerulli C, Batten SV, Bossarte RM. Disparities in adverse childhood experiences among individuals with a history of military service. JAMA Psychiatry. 2014;71(9):1041-1048. doi:10.1001/jamapsychiatry.2014.724
17. Center for Substance Abuse Treatment. Treatment improvement protocol (TIP). Series, No. 57. In: SAMHSA, ed. Trauma-Informed Care in Behavioral Health Services. SAMHSA: Rockville, MD; 2014:137-155.
18. US Department of Veterans Affairs, Veterans Health Administration, National Center for PTSD. Trauma, PTSD and treatment. https://www.ptsd.va.gov/PTSD/professional/treat/index.asp. Updated July 5, 2019. Accessed June 17, 2020.
19. Turgoose D, Ashwick R, Murphy D. Systematic review of lessons learned from delivering tele-therapy to veterans with post-traumatic stress disorder. J Telemed Telecare. 2018;24(9):575-585. doi:10.1177/1357633X17730443
20. Cook JM, Simiola V, Hamblen JL, Bernardy N, Schnurr PP. The influence of patient readiness on implementation of evidence-based PTSD treatments in Veterans Affairs residential programs. Psychol Trauma. 2017;9(suppl 1):51-58. doi:10.1037/tra0000162
21. Raja S, Hasnain M, Hoersch M, Gove-Yin S, Rajagopalan C. Trauma informed care in medicine: current knowledge and future research directions. Fam Community Health. 2015;38(3):216-226. doi:10.1097/FCH.0000000000000071
22. Hopper EK, Bassuk EL, Olivet J. Shelter from the storm: trauma-informed care in homeless service settings. Open Health Serv Policy J. 2009;2:131-151.
23. Kelly U, Boyd MA, Valente SM, Czekanski E. Trauma-informed care: keeping mental health settings safe for veterans [published correction appears in Issues Ment Health Nurs. 2015 Jun;36(6):482]. Issues Ment Health Nurs. 2014;35(6):413-419. doi:10.3109/01612840.2014.881941
24. Currier JM, Stefurak T, Carroll TD, Shatto EH. Applying trauma-informed care to community-based mental health services for military veterans. Best Pract Ment Health. 2017;13(1):47-64.
25. Neria Y, Nandi A, Galea S. Post-traumatic stress disorder following disasters: a systematic review. Psychol Med. 2008;38(4):467-480. doi:10.1017/S0033291707001353
26. Galea S, Merchant RM, Lurie N. the mental health consequences of COVID-19 and physical distancing: the need for prevention and early intervention [published online ahead of print, 2020 Apr 10]. JAMA Intern Med. 2020;10.1001/jamainternmed.2020.1562. doi:10.1001/jamainternmed.2020.1562
27. Hawryluck L, Gold WL, Robinson S, Pogorski S, Galea S, Styra R. SARS control and psychological effects of quarantine, Toronto, Canada. Emerg Infect Dis. 2004;10(7):1206-1212. doi:10.3201/eid1007.030703
28. Cunha JM, Shen YC, Burke ZR. Contrasting the impacts of combat and humanitarian assistance/disaster relief missions on the mental health of military service members. Def Peace Economics. 2018;29(1):62-77. doi: 10.1080/10242694.2017.1349365
29. Ramchand R, Harrell MC, Berglass N, Lauck M. Veterans and COVID-19: Projecting the Economic, Social and Mental Health Needs of America’s Veterans. New York, NY: The Bob Woodruff Foundation; 2020.
30. van Gelder N, Peterman A, Potts A, et al. COVID-19: reducing the risk of infection might increase the risk of intimate partner violence [published online ahead of print, 2020 Apr 11]. EClinicalMedicine. 2020;21:100348. doi:10.1016/j.eclinm.2020.100348
31. Azarang A, Pakyurek M, Giroux C, Nordahl TE, Yellowlees P. Information technologies: an augmentation to post-traumatic stress disorder treatment among trauma survivors. Telemed J E Health. 2019;25(4):263-271. doi:10.1089/tmj.2018.0068.
32. Gilmore AK, Davis MT, Grubaugh A, et al. “Do you expect me to receive PTSD care in a setting where most of the other patients remind me of the perpetrator?”: Home-based telemedicine to address barriers to care unique to military sexual trauma and veterans affairs hospitals. Contemp Clin Trials. 2016;48:59-64. doi:10.1016/j.cct.2016.03.004.
33. van Gurp J, van Selm M, Vissers K, van Leeuwen E, Hasselaar J. How outpatient palliative care teleconsultation facilitates empathic patient-professional relationships: a qualitative study. PLoS One. 2015;10(4):e0124387. Published 2015 Apr 22. doi:10.1371/journal.pone.0124387
34. Morland LA, Mackintosh MA, Glassman LH, et al. Home-based delivery of variable length prolonged exposure therapy: a comparison of clinical efficacy between service modalities. Depress Anxiety. 2020;37(4):346-355. doi:10.1002/da.22979
35. Morland LA, Hynes AK, Mackintosh MA, Resick PA, Chard KM. Group cognitive processing therapy delivered to veterans via telehealth: a pilot cohort. J Trauma Stress. 2011;24(4):465-469. doi:10.1002/jts.20661
36. Elisseou S, Puranam S, Nandi M. A novel, trauma-informed physical examination curriculum. Med Educ. 2018;52(5):555-556. doi:10.1111/medu.13569
COVID-19 has created stressors that are unprecedented in our modern era, prompting health care systems to adapt rapidly. Demand for telehealth has skyrocketed, and clinicians, many of whom had planned to adopt virtual practices in the future, have been pressured to do so immediately.1 In March 2020, the Centers for Medicare and Medicaid Services (CMS) expanded telehealth services, removing many barriers to virtual care.2 Similar remedy was not necessary for the Veterans Health Administration (VHA) which reported more than 2.6 million episodes of telehealth care in 2019.3 By the time the pandemic was underway in the US, use of telehealth was widespread across the agency. In late March 2020, VHA released a COVID-19 Response Plan, in which telehealth played a critical role in safe, uninterrupted delivery of services.4 While telehealth has been widely used in VHA, the call for replacement of most in-person outpatient visits with telehealth visits was a fundamental paradigm shift for many patients and clinicians.4
The Coronavirus Aid, Relief, and Economic Security (CARES) Act (HR 748) gave the US Department of Veterans Affairs (VA) funding to expand coronavirus-related telehealth services, including the purchase of mobile devices and broadband expansion. CARES authorized the agency to expand telemental health services, enter into short-term agreements with telecommunications companies to provide temporary broadband services to veterans, temporarily waived an in-person home visit requirement (accepting video and phone calls as an alternative), and provided means to make telehealth available for homeless veterans and case managers through the HUD-VASH (US Department of Housing and Urban Development-VA Supportive Housing) program.
VHA is a national telehealth exemplar, initiating telehealth by use of closed-circuit televisions as early as 1968, and continuing to expand through 2017 with the implementation of the Veterans Video Connect (VVC) platform.5 VVC has enabled veterans to participate in virtual visits from distant locations, including their homes. VVC was used successfully during hurricanes Sandy, Harvey, Irma, and Maria and is being widely deployed in the current crisis.6-8
While telehealth can take many forms, the current discussion will focus on live (synchronous) videoconferencing: a 2-way audiovisual link between a patient and clinician, such as VVC, which enables patients to maintain a safe and social distance from others while connecting with the health care team and receiving urgent as well as ongoing medical care for both new and established conditions.9 VHA has developed multiple training resources for use of VVC across many settings, including primary care, mental health, and specialties. In this review, we will make the novel case for applying a trauma-informed lens to telehealth care across VHA and beyond to other health care systems.
Trauma-Informed Care
Although our current focus is rightly on mitigating the health effects of a pandemic, we must recognize that stressful phenomena like COVID-19 occur against a backdrop of widespread physical, sexual, psychological, and racial trauma in our communities. The Substance Abuse and Mental Health Services Administration (SAMHSA) describes trauma as resulting from “an event, series of events, or set of circumstances that is experienced by an individual as physically or emotionally harmful or life threatening and that has lasting adverse effects on the individual’s functioning and mental, physical, social, emotional, or spiritual well-being.”10 Trauma exposure is both ubiquitous worldwide and inequitably distributed, with vulnerable populations disproportionately impacted.11,12
Veterans as a population are often highly trauma exposed, and while VHA routinely screens for experiences of trauma, such as military sexual trauma (MST) and intimate partner violence (IPV), and potential mental health sequelae of trauma, including posttraumatic stress disorder (PTSD) and suicidality, veterans may experience other forms of trauma or be unwilling or unable to talk about past exposures.13 One common example is that of adverse childhood experiences (ACEs), which include household dysfunction, neglect, and physical and sexual abuse before the age of 18 years.14 ACEs have been associated with a wide range of risk behaviors and poor health outcomes in adulthood.14 In population-based data, both male and female veterans have reported higher ACE scores.15 In addition, ACE scores are higher overall for those serving in the all-volunteer era (after July 1, 1973).16 Because trauma may be unseen, unmeasured, and unnamed, it is important to deliver all medical care with sensitivity to its potential presence.
It is important to distinguish the concept of trauma-informed care (TIC) from trauma-focused services. Trauma-focused or trauma-specific treatment refers to evidence-based and best practice treatment models that have been proven to facilitate recovery from problems resulting from the experience of trauma, such as PTSD.17 These treatments directly address the emotional, behavioral, and physiologic impact of trauma on an individual’s life and facilitate improvement in related symptoms and functioning: They are designed to treat the consequences of trauma. VHA offers a wide range of trauma-specific treatments, and considerable experience in delivering evidence-based trauma-focused treatment through telehealth exists.18,19 Given the range of possible responses to the experience of trauma, not all veterans with trauma histories need to, chose to, or feel ready to access trauma-specific treatments.20
In contrast, TIC is a global, universal precautions approach to providing quality care that can be applied to all aspects of health care and to all patients.21 TIC is a strengths-based service delivery framework that is grounded in an understanding of, and responsiveness to, the disempowering impact of experiencing trauma. It seeks to maximize physical, psychological, and emotional safety in all health care encounters, not just those that are specifically trauma-focused, and creates opportunities to rebuild a sense of control and empowerment while fostering healing through safe and collaborative patient-clinician relationships.22 TIC is not accomplished through any single technique or checklist but through continuous appraisal of approaches to care delivery. SAMHSA has elucidated 6 fundamental principles of TIC: safety; trustworthiness and transparency; peer support; collaboration and mutuality; empowerment; voice and choice; and sensitivity to cultural, historical, and gender issues.10
TIC is based on the understanding that often traditional service delivery models of care may trigger, silence, or disempower survivors of trauma, exacerbating physical and mental health symptoms and potentially increasing disengagement from care and poorer outcomes.23 Currier and colleagues aptly noted, “TIC assumes that trustworthiness is not something that an organization creates in a veteran client, but something that he or she will freely grant to an organization.”24 Given the global prevalence of trauma, its well-established and deleterious impact on lifelong health, and the potential for health care itself to be traumatizing, TIC is a fundamental construct to apply universally with any patient at any time, especially in the context of a large-scale community trauma, such as a pandemic.12
Trauma-Informed COVID-19 Care
Catastrophic events, such as natural disasters and pandemics, may serve as both newly traumatic and as potential triggers for survivors who have endured prior trauma.25,26 Increases in depression, PTSD, and substance use disorder (SUD) are common sequalae, occurring during the event, the immediate aftermath, and beyond.25,27 In 2003, quarantine contained the spread of Severe acute respiratory syndrome (SARS) but resulted in a high prevalence of psychological distress, including PTSD and depression.27 Many veterans may have deployed in support of humanitarian assistance/disaster relief missions, which typically do not involve armed combat but may expose service members to warlike situations, including social insecurity and suffering populations.28 COVID-19 may be reminiscent of some of these deployments as well.
The impact of the current COVID-19 pandemic on patients is pervasive. Those with preexisting financial insecurity now face additional economic hardship and health challenges, which are amplified by loneliness and loss of social support networks.26 Widespread unemployment and closures of many businesses add to stress and may exacerbate preexisting mental and physical health concerns for many; some veterans also may be at increased risk.29 While previous postdisaster research suggests that psychopathology in the general population will significantly remit over time, high-risk groups remain vulnerable to PTSD and bear the brunt of social and economic consequences associated with the crisis.25 Veterans with preexisting trauma histories and mental health conditions are at increased risk for being retraumatized by the current pandemic and impacted by isolation and unplanned job or wage loss from it.29 Compounding this, social distancing serves to protect communities but may amplify isolation and danger in abusive relationships or exacerbate underlying mental illness.26,30
Thus, as we expand our use of telehealth, replacing our face-to-face visits with virtual encounters, it is critical for clinicians to be mindful that the pandemic and public health responses to it may result in trauma and retraumatization for veterans and other vulnerable patients, which in turn can impact both access and response to care. The application of trauma-informed principles to our virtual encounters has the potential to mitigate some of these health impacts, increase engagement in care, and provide opportunities for protective, healing connections.
In the setting of the continued fear and uncertainty of the COVID-19 pandemic, we believe that application of a trauma-informed lens to telehealth efforts is timely. While virtual visits may seem to lack the warmth and immediacy of traditional medical encounters, accumulated experience suggests otherwise.19 Telehealth is fundamentally more patient-focused than traditional encounters, overcomes service delivery barriers, offers a greater range of options for treatment engagement, and can enhance clinician-patient partnerships.6,31,32 Although the rapid transition to telehealth may be challenging for those new to it, experienced clinicians and patients express high degrees of satisfaction with virtual care because direct communication is unhampered by in-office challenges and travel logistics.33
While it may feel daunting to integrate principles of TIC into telehealth during a crisis-driven scale-up, a growing practice and body of research can inform these efforts. To help better understand how trauma-exposed patients respond to telehealth, we reviewed findings from trauma-focused telemental health (TMH) treatment. This research demonstrates that telehealth promotes safety and collaboration—fundamental principles of TIC—that can, in turn, be applied to telehealth visits in primary care and other medical and surgical specialties. When compared with traditional in-person treatment, studies of both individual and group formats of TMH found no significant differences in satisfaction, acceptability, or outcomes (such as reduction in PTSD symptom severity scores34), and TMH did not impede development of rapport.19,35
Although counterintuitive, the virtual space created by the combined physical and psychological distance of videoconferencing has been shown to promote safety and transparency. In TMH, patients have reported greater honesty due to the protection afforded by this virtual space.31 Engaging in telehealth visits from the comfort of one’s home can feel emotionally safer than having to travel to a medical office, resulting in feeling more at ease during encounters.31 In one TMH study, veterans with PTSD described high comfort levels and ability to let their guard down during virtual treatment.19 Similarly, in palliative telehealth care, patients reported that clinicians successfully nurtured an experience of intimacy, expressed empathy verbally and nonverbally, and responded to the patient’s unique situation and emotions.33
Trauma-Informed Telehealth
We have discussed how telehealth’s greater flexibility may create an ideal environment in which to implement principles of TIC. It may allow increased collaboration and closeness between patients and clinicians, empowering patients to codesign their care.31,33 The Table reviews 6 core SAMHSA principles of TIC and offers examples of their application to telehealth visits. The following case illustrates the application of trauma-informed telehealth care.
Case Presentation
S is a 45-year-old male veteran of Operation Enduring Freedom (OEF) who served as a combat medic. He has a history of osteoarthritis and PTSD related to combat experiences like caring for traumatic amputees. Before the pandemic began, he was employed as a server at a local restaurant but was laid off as the business transitioned to takeout orders only. The patient worked near a VA primary care clinic and frequently dropped by to see the staff and to pick up prescriptions. He had never agreed to video visits despite receiving encouragement from his medical team. He was reluctant to try telehealth, but he had developed a painful, itchy rash on his lower leg and was concerned about getting care.
For patients like S who may be reluctant to try telehealth, it is important to understand the cause. Potential barriers to telehealth may include lack of Internet access or familiarity with technology, discomfort with being on video, shame about the appearance of one’s home, or a strong cultural preference for face-to-face medical visits. Some may miss the social support benefit of coming into a clinic, particularly in VHA, which is designed specifically for veteran patients. For these reasons it is important to offer the patient a choice and to begin with a supportive phone call that explores and strives to address the patient’s concerns about videoconferencing.
The clinic nurse called S who agreed to try a VVC visit with gentle encouragement. He shared that he was embarrassed about the appearance of his apartment and fearful about pictures being recorded of his body due to “a bad experience in my past.” The patient was reassured that visits are private and will not be recorded. The nurse also reminded him that he can choose the location in which the visit will take place and can turn his camera off at any time. Importantly, the nurse did not ask him to recount additional details of what happened in his past. Next, the nurse verified his location and contact information and explained why obtaining this information was necessary. Next, she asked his consent to proceed with the visit, reminding him that the visit can end at any point if he feels uncomfortable. After finishing this initial discussion, the nurse told him that his primary care physician (PCP) would join the visit and address his concerns with his leg.
S was happy to see his PCP despite his hesitations about video care. The PCP noticed that he seemed anxious and was avoiding talking about the rash. Knowing that he was anxious about this VVC visit, the PCP was careful to look directly at the camera to make eye contact and to be sure her face was well lit and not in shadows. She gave him some time to acclimate to the virtual environment and thanked him for joining the visit. Knowing that he was a combat veteran, she warned him that there have been sudden, loud construction noises outside her window. Although the PCP was pressed for time, she was aware that S may have had a previous difficult experience around images of his body or even combat-related trauma. She gently brought up the rash and asked for permission to examine it, avoiding commands or personalizing language such as “show me your leg” or “take off your pants for me.”36After some hesitation, the patient revealed his leg that appeared to have multiple excoriations and old scars from picking. After the examination, the PCP waited until the patient’s leg was fully covered before beginning a discussion of the care plan. Together they collaboratively reviewed treatments that would soothe the skin. They decided to virtually consult a social worker to obtain emergency economic assistance and to speak with the patient’s care team psychologist to reduce some of the anxiety that may be leading to his leg scratching.
Case Discussion
This case illustrates the ways in which TIC can be applied to telehealth for a veteran with combat-related PTSD who may have experienced additional interpersonal trauma. It was not necessary to know more detail about the veteran’s trauma history to conduct the visit in a trauma-informed manner. Connecting to patients at home while considering these principles may thus foster mutuality, mitigate retraumatization, and cultivate enhanced collaboration with health care teams in this era of social distancing.
While a virtual physical examination creates both limitations and opportunity in telehealth, patients may find the greater degree of choice over their clothing and surroundings to be empowering. Telehealth also can allow for a greater portion of time to be dedicated to quality discussion and collaborative planning, with the clinician hearing and responding to the patient’s needs with reduced distraction. This may include opportunities to discuss mental health concerns openly, normalize emotional reactions, and offer connection to mental health and support services available through telehealth, including for patients who have not previously engaged in such care.
Conclusions
COVID-19 has created stressors that are unprecedented in our modern era, prompting health care systems to adapt rapidly. Demand for telehealth has skyrocketed, and clinicians, many of whom had planned to adopt virtual practices in the future, have been pressured to do so immediately.1 In March 2020, the Centers for Medicare and Medicaid Services (CMS) expanded telehealth services, removing many barriers to virtual care.2 Similar remedy was not necessary for the Veterans Health Administration (VHA) which reported more than 2.6 million episodes of telehealth care in 2019.3 By the time the pandemic was underway in the US, use of telehealth was widespread across the agency. In late March 2020, VHA released a COVID-19 Response Plan, in which telehealth played a critical role in safe, uninterrupted delivery of services.4 While telehealth has been widely used in VHA, the call for replacement of most in-person outpatient visits with telehealth visits was a fundamental paradigm shift for many patients and clinicians.4
The Coronavirus Aid, Relief, and Economic Security (CARES) Act (HR 748) gave the US Department of Veterans Affairs (VA) funding to expand coronavirus-related telehealth services, including the purchase of mobile devices and broadband expansion. CARES authorized the agency to expand telemental health services, enter into short-term agreements with telecommunications companies to provide temporary broadband services to veterans, temporarily waived an in-person home visit requirement (accepting video and phone calls as an alternative), and provided means to make telehealth available for homeless veterans and case managers through the HUD-VASH (US Department of Housing and Urban Development-VA Supportive Housing) program.
VHA is a national telehealth exemplar, initiating telehealth by use of closed-circuit televisions as early as 1968, and continuing to expand through 2017 with the implementation of the Veterans Video Connect (VVC) platform.5 VVC has enabled veterans to participate in virtual visits from distant locations, including their homes. VVC was used successfully during hurricanes Sandy, Harvey, Irma, and Maria and is being widely deployed in the current crisis.6-8
While telehealth can take many forms, the current discussion will focus on live (synchronous) videoconferencing: a 2-way audiovisual link between a patient and clinician, such as VVC, which enables patients to maintain a safe and social distance from others while connecting with the health care team and receiving urgent as well as ongoing medical care for both new and established conditions.9 VHA has developed multiple training resources for use of VVC across many settings, including primary care, mental health, and specialties. In this review, we will make the novel case for applying a trauma-informed lens to telehealth care across VHA and beyond to other health care systems.
Trauma-Informed Care
Although our current focus is rightly on mitigating the health effects of a pandemic, we must recognize that stressful phenomena like COVID-19 occur against a backdrop of widespread physical, sexual, psychological, and racial trauma in our communities. The Substance Abuse and Mental Health Services Administration (SAMHSA) describes trauma as resulting from “an event, series of events, or set of circumstances that is experienced by an individual as physically or emotionally harmful or life threatening and that has lasting adverse effects on the individual’s functioning and mental, physical, social, emotional, or spiritual well-being.”10 Trauma exposure is both ubiquitous worldwide and inequitably distributed, with vulnerable populations disproportionately impacted.11,12
Veterans as a population are often highly trauma exposed, and while VHA routinely screens for experiences of trauma, such as military sexual trauma (MST) and intimate partner violence (IPV), and potential mental health sequelae of trauma, including posttraumatic stress disorder (PTSD) and suicidality, veterans may experience other forms of trauma or be unwilling or unable to talk about past exposures.13 One common example is that of adverse childhood experiences (ACEs), which include household dysfunction, neglect, and physical and sexual abuse before the age of 18 years.14 ACEs have been associated with a wide range of risk behaviors and poor health outcomes in adulthood.14 In population-based data, both male and female veterans have reported higher ACE scores.15 In addition, ACE scores are higher overall for those serving in the all-volunteer era (after July 1, 1973).16 Because trauma may be unseen, unmeasured, and unnamed, it is important to deliver all medical care with sensitivity to its potential presence.
It is important to distinguish the concept of trauma-informed care (TIC) from trauma-focused services. Trauma-focused or trauma-specific treatment refers to evidence-based and best practice treatment models that have been proven to facilitate recovery from problems resulting from the experience of trauma, such as PTSD.17 These treatments directly address the emotional, behavioral, and physiologic impact of trauma on an individual’s life and facilitate improvement in related symptoms and functioning: They are designed to treat the consequences of trauma. VHA offers a wide range of trauma-specific treatments, and considerable experience in delivering evidence-based trauma-focused treatment through telehealth exists.18,19 Given the range of possible responses to the experience of trauma, not all veterans with trauma histories need to, chose to, or feel ready to access trauma-specific treatments.20
In contrast, TIC is a global, universal precautions approach to providing quality care that can be applied to all aspects of health care and to all patients.21 TIC is a strengths-based service delivery framework that is grounded in an understanding of, and responsiveness to, the disempowering impact of experiencing trauma. It seeks to maximize physical, psychological, and emotional safety in all health care encounters, not just those that are specifically trauma-focused, and creates opportunities to rebuild a sense of control and empowerment while fostering healing through safe and collaborative patient-clinician relationships.22 TIC is not accomplished through any single technique or checklist but through continuous appraisal of approaches to care delivery. SAMHSA has elucidated 6 fundamental principles of TIC: safety; trustworthiness and transparency; peer support; collaboration and mutuality; empowerment; voice and choice; and sensitivity to cultural, historical, and gender issues.10
TIC is based on the understanding that often traditional service delivery models of care may trigger, silence, or disempower survivors of trauma, exacerbating physical and mental health symptoms and potentially increasing disengagement from care and poorer outcomes.23 Currier and colleagues aptly noted, “TIC assumes that trustworthiness is not something that an organization creates in a veteran client, but something that he or she will freely grant to an organization.”24 Given the global prevalence of trauma, its well-established and deleterious impact on lifelong health, and the potential for health care itself to be traumatizing, TIC is a fundamental construct to apply universally with any patient at any time, especially in the context of a large-scale community trauma, such as a pandemic.12
Trauma-Informed COVID-19 Care
Catastrophic events, such as natural disasters and pandemics, may serve as both newly traumatic and as potential triggers for survivors who have endured prior trauma.25,26 Increases in depression, PTSD, and substance use disorder (SUD) are common sequalae, occurring during the event, the immediate aftermath, and beyond.25,27 In 2003, quarantine contained the spread of Severe acute respiratory syndrome (SARS) but resulted in a high prevalence of psychological distress, including PTSD and depression.27 Many veterans may have deployed in support of humanitarian assistance/disaster relief missions, which typically do not involve armed combat but may expose service members to warlike situations, including social insecurity and suffering populations.28 COVID-19 may be reminiscent of some of these deployments as well.
The impact of the current COVID-19 pandemic on patients is pervasive. Those with preexisting financial insecurity now face additional economic hardship and health challenges, which are amplified by loneliness and loss of social support networks.26 Widespread unemployment and closures of many businesses add to stress and may exacerbate preexisting mental and physical health concerns for many; some veterans also may be at increased risk.29 While previous postdisaster research suggests that psychopathology in the general population will significantly remit over time, high-risk groups remain vulnerable to PTSD and bear the brunt of social and economic consequences associated with the crisis.25 Veterans with preexisting trauma histories and mental health conditions are at increased risk for being retraumatized by the current pandemic and impacted by isolation and unplanned job or wage loss from it.29 Compounding this, social distancing serves to protect communities but may amplify isolation and danger in abusive relationships or exacerbate underlying mental illness.26,30
Thus, as we expand our use of telehealth, replacing our face-to-face visits with virtual encounters, it is critical for clinicians to be mindful that the pandemic and public health responses to it may result in trauma and retraumatization for veterans and other vulnerable patients, which in turn can impact both access and response to care. The application of trauma-informed principles to our virtual encounters has the potential to mitigate some of these health impacts, increase engagement in care, and provide opportunities for protective, healing connections.
In the setting of the continued fear and uncertainty of the COVID-19 pandemic, we believe that application of a trauma-informed lens to telehealth efforts is timely. While virtual visits may seem to lack the warmth and immediacy of traditional medical encounters, accumulated experience suggests otherwise.19 Telehealth is fundamentally more patient-focused than traditional encounters, overcomes service delivery barriers, offers a greater range of options for treatment engagement, and can enhance clinician-patient partnerships.6,31,32 Although the rapid transition to telehealth may be challenging for those new to it, experienced clinicians and patients express high degrees of satisfaction with virtual care because direct communication is unhampered by in-office challenges and travel logistics.33
While it may feel daunting to integrate principles of TIC into telehealth during a crisis-driven scale-up, a growing practice and body of research can inform these efforts. To help better understand how trauma-exposed patients respond to telehealth, we reviewed findings from trauma-focused telemental health (TMH) treatment. This research demonstrates that telehealth promotes safety and collaboration—fundamental principles of TIC—that can, in turn, be applied to telehealth visits in primary care and other medical and surgical specialties. When compared with traditional in-person treatment, studies of both individual and group formats of TMH found no significant differences in satisfaction, acceptability, or outcomes (such as reduction in PTSD symptom severity scores34), and TMH did not impede development of rapport.19,35
Although counterintuitive, the virtual space created by the combined physical and psychological distance of videoconferencing has been shown to promote safety and transparency. In TMH, patients have reported greater honesty due to the protection afforded by this virtual space.31 Engaging in telehealth visits from the comfort of one’s home can feel emotionally safer than having to travel to a medical office, resulting in feeling more at ease during encounters.31 In one TMH study, veterans with PTSD described high comfort levels and ability to let their guard down during virtual treatment.19 Similarly, in palliative telehealth care, patients reported that clinicians successfully nurtured an experience of intimacy, expressed empathy verbally and nonverbally, and responded to the patient’s unique situation and emotions.33
Trauma-Informed Telehealth
We have discussed how telehealth’s greater flexibility may create an ideal environment in which to implement principles of TIC. It may allow increased collaboration and closeness between patients and clinicians, empowering patients to codesign their care.31,33 The Table reviews 6 core SAMHSA principles of TIC and offers examples of their application to telehealth visits. The following case illustrates the application of trauma-informed telehealth care.
Case Presentation
S is a 45-year-old male veteran of Operation Enduring Freedom (OEF) who served as a combat medic. He has a history of osteoarthritis and PTSD related to combat experiences like caring for traumatic amputees. Before the pandemic began, he was employed as a server at a local restaurant but was laid off as the business transitioned to takeout orders only. The patient worked near a VA primary care clinic and frequently dropped by to see the staff and to pick up prescriptions. He had never agreed to video visits despite receiving encouragement from his medical team. He was reluctant to try telehealth, but he had developed a painful, itchy rash on his lower leg and was concerned about getting care.
For patients like S who may be reluctant to try telehealth, it is important to understand the cause. Potential barriers to telehealth may include lack of Internet access or familiarity with technology, discomfort with being on video, shame about the appearance of one’s home, or a strong cultural preference for face-to-face medical visits. Some may miss the social support benefit of coming into a clinic, particularly in VHA, which is designed specifically for veteran patients. For these reasons it is important to offer the patient a choice and to begin with a supportive phone call that explores and strives to address the patient’s concerns about videoconferencing.
The clinic nurse called S who agreed to try a VVC visit with gentle encouragement. He shared that he was embarrassed about the appearance of his apartment and fearful about pictures being recorded of his body due to “a bad experience in my past.” The patient was reassured that visits are private and will not be recorded. The nurse also reminded him that he can choose the location in which the visit will take place and can turn his camera off at any time. Importantly, the nurse did not ask him to recount additional details of what happened in his past. Next, the nurse verified his location and contact information and explained why obtaining this information was necessary. Next, she asked his consent to proceed with the visit, reminding him that the visit can end at any point if he feels uncomfortable. After finishing this initial discussion, the nurse told him that his primary care physician (PCP) would join the visit and address his concerns with his leg.
S was happy to see his PCP despite his hesitations about video care. The PCP noticed that he seemed anxious and was avoiding talking about the rash. Knowing that he was anxious about this VVC visit, the PCP was careful to look directly at the camera to make eye contact and to be sure her face was well lit and not in shadows. She gave him some time to acclimate to the virtual environment and thanked him for joining the visit. Knowing that he was a combat veteran, she warned him that there have been sudden, loud construction noises outside her window. Although the PCP was pressed for time, she was aware that S may have had a previous difficult experience around images of his body or even combat-related trauma. She gently brought up the rash and asked for permission to examine it, avoiding commands or personalizing language such as “show me your leg” or “take off your pants for me.”36After some hesitation, the patient revealed his leg that appeared to have multiple excoriations and old scars from picking. After the examination, the PCP waited until the patient’s leg was fully covered before beginning a discussion of the care plan. Together they collaboratively reviewed treatments that would soothe the skin. They decided to virtually consult a social worker to obtain emergency economic assistance and to speak with the patient’s care team psychologist to reduce some of the anxiety that may be leading to his leg scratching.
Case Discussion
This case illustrates the ways in which TIC can be applied to telehealth for a veteran with combat-related PTSD who may have experienced additional interpersonal trauma. It was not necessary to know more detail about the veteran’s trauma history to conduct the visit in a trauma-informed manner. Connecting to patients at home while considering these principles may thus foster mutuality, mitigate retraumatization, and cultivate enhanced collaboration with health care teams in this era of social distancing.
While a virtual physical examination creates both limitations and opportunity in telehealth, patients may find the greater degree of choice over their clothing and surroundings to be empowering. Telehealth also can allow for a greater portion of time to be dedicated to quality discussion and collaborative planning, with the clinician hearing and responding to the patient’s needs with reduced distraction. This may include opportunities to discuss mental health concerns openly, normalize emotional reactions, and offer connection to mental health and support services available through telehealth, including for patients who have not previously engaged in such care.
Conclusions
1. Wosik J, Fudim M, Cameron B, et al. Telehealth transformation: COVID-19 and the rise of virtual care. J Am Med Inform Assoc. 2020;27(6):957-962. doi:10.1093/jamia/ocaa067
2. Centers for Medicare and Medicaid Services. Medicare and Medicaid programs; policy and regulatory revisions in response to the COVID-19 public health emergency. CMS-1744-IFC. https://www.cms.gov/files/document/covid-final-ifc.pdf. Published March 24, 2020. Accessed April 8, 2020.
3. Eddy N. VA sees a surge in veterans’ use of telehealth services. https://www.healthcareitnews.com/news/va-sees-surge-veterans-use-telehealth-services. Published November 25, 2019. Accessed June 17, 2020.
4. Veterans Health Administration, Office of Emergency Management. COVID-19 response plan. Version 1.6. Published March 23, 2020. Accessed June 17, 2020.
5. Caudill RL, Sager Z. Institutionally based videoconferencing. Int Rev Psychiatry. 2015;27(6):496-503. doi:10.3109/09540261.2015.1085369
6. Heyworth L. Sharing Connections [published correction appears in JAMA. 2018 May 8;319(18):1939]. JAMA. 2018;319(13):1323-1324. doi:10.1001/jama.2018.2717
7. Dobalian A. U.S. Department of Veterans Affairs’ (VA’s) response to the 2017 hurricanes. Presented at: American Public Health Association 2019 Annual Meeting and Exposition; November 2-6, 2019; Philadelphia, PA. https://apha.confex.com/apha/2019/meetingapp.cgi/Session/58543. Accessed June 16, 2020.
8. Der-Martirosian C, Griffin AR, Chu K, Dobalian A. Telehealth at the US Department of Veterans Affairs after Hurricane Sandy. J Telemed Telecare. 2019;25(5):310-317. doi:10.1177/1357633X17751005
9. The Office of the National Coordinator for Health Information Technology. Telemedicine and telehealth. https://www.healthit.gov/topic/health-it-initiatives/telemedicine-and-telehealth. Updated September 28, 2017. Accessed June 16, 2020.
10. Substance Abuse and Mental Health Services Administration, Trauma and Justice Strategic Initiative. SAMHSA’s concept of trauma and guidance for a trauma-informed approach. https://ncsacw.samhsa.gov/userfiles/files/SAMHSA_Trauma.pdf. Published July 2014. Accessed June 16, 2020.
11. Kilpatrick DG, Resnick HS, Milanak ME, Miller MW, Keyes KM, Friedman MJ. National estimates of exposure to traumatic events and PTSD prevalence using DSM-IV and DSM-5 criteria. J Trauma Stress. 2013;26(5):537-547. doi:10.1002/jts.21848
12. Kimberg L, Wheeler M. Trauma and Trauma-informed Care. In: Gerber MR, ed. Trauma-informed Healthcare Approaches: A Guide for Primary Care. Cham, Switzerland: Springer Nature; 2019:25-56.
13. Gerber MR. Trauma-informed care of veterans. In: Gerber MR, ed. Trauma-informed Healthcare Approaches: A Guide for Primary Care. Cham, Switzerland: Springer Nature; 2019:25-56.
14. Felitti VJ, Anda RF, Nordenberg D, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med. 1998;14(4):245-258. doi:10.1016/s0749-3797(98)00017-8
15. Katon JG, Lehavot K, Simpson TL, et al. Adverse childhood experiences, Military service, and adult health. Am J Prev Med. 2015;49(4):573-582. doi:10.1016/j.amepre.2015.03.020
16. Blosnich JR, Dichter ME, Cerulli C, Batten SV, Bossarte RM. Disparities in adverse childhood experiences among individuals with a history of military service. JAMA Psychiatry. 2014;71(9):1041-1048. doi:10.1001/jamapsychiatry.2014.724
17. Center for Substance Abuse Treatment. Treatment improvement protocol (TIP). Series, No. 57. In: SAMHSA, ed. Trauma-Informed Care in Behavioral Health Services. SAMHSA: Rockville, MD; 2014:137-155.
18. US Department of Veterans Affairs, Veterans Health Administration, National Center for PTSD. Trauma, PTSD and treatment. https://www.ptsd.va.gov/PTSD/professional/treat/index.asp. Updated July 5, 2019. Accessed June 17, 2020.
19. Turgoose D, Ashwick R, Murphy D. Systematic review of lessons learned from delivering tele-therapy to veterans with post-traumatic stress disorder. J Telemed Telecare. 2018;24(9):575-585. doi:10.1177/1357633X17730443
20. Cook JM, Simiola V, Hamblen JL, Bernardy N, Schnurr PP. The influence of patient readiness on implementation of evidence-based PTSD treatments in Veterans Affairs residential programs. Psychol Trauma. 2017;9(suppl 1):51-58. doi:10.1037/tra0000162
21. Raja S, Hasnain M, Hoersch M, Gove-Yin S, Rajagopalan C. Trauma informed care in medicine: current knowledge and future research directions. Fam Community Health. 2015;38(3):216-226. doi:10.1097/FCH.0000000000000071
22. Hopper EK, Bassuk EL, Olivet J. Shelter from the storm: trauma-informed care in homeless service settings. Open Health Serv Policy J. 2009;2:131-151.
23. Kelly U, Boyd MA, Valente SM, Czekanski E. Trauma-informed care: keeping mental health settings safe for veterans [published correction appears in Issues Ment Health Nurs. 2015 Jun;36(6):482]. Issues Ment Health Nurs. 2014;35(6):413-419. doi:10.3109/01612840.2014.881941
24. Currier JM, Stefurak T, Carroll TD, Shatto EH. Applying trauma-informed care to community-based mental health services for military veterans. Best Pract Ment Health. 2017;13(1):47-64.
25. Neria Y, Nandi A, Galea S. Post-traumatic stress disorder following disasters: a systematic review. Psychol Med. 2008;38(4):467-480. doi:10.1017/S0033291707001353
26. Galea S, Merchant RM, Lurie N. the mental health consequences of COVID-19 and physical distancing: the need for prevention and early intervention [published online ahead of print, 2020 Apr 10]. JAMA Intern Med. 2020;10.1001/jamainternmed.2020.1562. doi:10.1001/jamainternmed.2020.1562
27. Hawryluck L, Gold WL, Robinson S, Pogorski S, Galea S, Styra R. SARS control and psychological effects of quarantine, Toronto, Canada. Emerg Infect Dis. 2004;10(7):1206-1212. doi:10.3201/eid1007.030703
28. Cunha JM, Shen YC, Burke ZR. Contrasting the impacts of combat and humanitarian assistance/disaster relief missions on the mental health of military service members. Def Peace Economics. 2018;29(1):62-77. doi: 10.1080/10242694.2017.1349365
29. Ramchand R, Harrell MC, Berglass N, Lauck M. Veterans and COVID-19: Projecting the Economic, Social and Mental Health Needs of America’s Veterans. New York, NY: The Bob Woodruff Foundation; 2020.
30. van Gelder N, Peterman A, Potts A, et al. COVID-19: reducing the risk of infection might increase the risk of intimate partner violence [published online ahead of print, 2020 Apr 11]. EClinicalMedicine. 2020;21:100348. doi:10.1016/j.eclinm.2020.100348
31. Azarang A, Pakyurek M, Giroux C, Nordahl TE, Yellowlees P. Information technologies: an augmentation to post-traumatic stress disorder treatment among trauma survivors. Telemed J E Health. 2019;25(4):263-271. doi:10.1089/tmj.2018.0068.
32. Gilmore AK, Davis MT, Grubaugh A, et al. “Do you expect me to receive PTSD care in a setting where most of the other patients remind me of the perpetrator?”: Home-based telemedicine to address barriers to care unique to military sexual trauma and veterans affairs hospitals. Contemp Clin Trials. 2016;48:59-64. doi:10.1016/j.cct.2016.03.004.
33. van Gurp J, van Selm M, Vissers K, van Leeuwen E, Hasselaar J. How outpatient palliative care teleconsultation facilitates empathic patient-professional relationships: a qualitative study. PLoS One. 2015;10(4):e0124387. Published 2015 Apr 22. doi:10.1371/journal.pone.0124387
34. Morland LA, Mackintosh MA, Glassman LH, et al. Home-based delivery of variable length prolonged exposure therapy: a comparison of clinical efficacy between service modalities. Depress Anxiety. 2020;37(4):346-355. doi:10.1002/da.22979
35. Morland LA, Hynes AK, Mackintosh MA, Resick PA, Chard KM. Group cognitive processing therapy delivered to veterans via telehealth: a pilot cohort. J Trauma Stress. 2011;24(4):465-469. doi:10.1002/jts.20661
36. Elisseou S, Puranam S, Nandi M. A novel, trauma-informed physical examination curriculum. Med Educ. 2018;52(5):555-556. doi:10.1111/medu.13569
1. Wosik J, Fudim M, Cameron B, et al. Telehealth transformation: COVID-19 and the rise of virtual care. J Am Med Inform Assoc. 2020;27(6):957-962. doi:10.1093/jamia/ocaa067
2. Centers for Medicare and Medicaid Services. Medicare and Medicaid programs; policy and regulatory revisions in response to the COVID-19 public health emergency. CMS-1744-IFC. https://www.cms.gov/files/document/covid-final-ifc.pdf. Published March 24, 2020. Accessed April 8, 2020.
3. Eddy N. VA sees a surge in veterans’ use of telehealth services. https://www.healthcareitnews.com/news/va-sees-surge-veterans-use-telehealth-services. Published November 25, 2019. Accessed June 17, 2020.
4. Veterans Health Administration, Office of Emergency Management. COVID-19 response plan. Version 1.6. Published March 23, 2020. Accessed June 17, 2020.
5. Caudill RL, Sager Z. Institutionally based videoconferencing. Int Rev Psychiatry. 2015;27(6):496-503. doi:10.3109/09540261.2015.1085369
6. Heyworth L. Sharing Connections [published correction appears in JAMA. 2018 May 8;319(18):1939]. JAMA. 2018;319(13):1323-1324. doi:10.1001/jama.2018.2717
7. Dobalian A. U.S. Department of Veterans Affairs’ (VA’s) response to the 2017 hurricanes. Presented at: American Public Health Association 2019 Annual Meeting and Exposition; November 2-6, 2019; Philadelphia, PA. https://apha.confex.com/apha/2019/meetingapp.cgi/Session/58543. Accessed June 16, 2020.
8. Der-Martirosian C, Griffin AR, Chu K, Dobalian A. Telehealth at the US Department of Veterans Affairs after Hurricane Sandy. J Telemed Telecare. 2019;25(5):310-317. doi:10.1177/1357633X17751005
9. The Office of the National Coordinator for Health Information Technology. Telemedicine and telehealth. https://www.healthit.gov/topic/health-it-initiatives/telemedicine-and-telehealth. Updated September 28, 2017. Accessed June 16, 2020.
10. Substance Abuse and Mental Health Services Administration, Trauma and Justice Strategic Initiative. SAMHSA’s concept of trauma and guidance for a trauma-informed approach. https://ncsacw.samhsa.gov/userfiles/files/SAMHSA_Trauma.pdf. Published July 2014. Accessed June 16, 2020.
11. Kilpatrick DG, Resnick HS, Milanak ME, Miller MW, Keyes KM, Friedman MJ. National estimates of exposure to traumatic events and PTSD prevalence using DSM-IV and DSM-5 criteria. J Trauma Stress. 2013;26(5):537-547. doi:10.1002/jts.21848
12. Kimberg L, Wheeler M. Trauma and Trauma-informed Care. In: Gerber MR, ed. Trauma-informed Healthcare Approaches: A Guide for Primary Care. Cham, Switzerland: Springer Nature; 2019:25-56.
13. Gerber MR. Trauma-informed care of veterans. In: Gerber MR, ed. Trauma-informed Healthcare Approaches: A Guide for Primary Care. Cham, Switzerland: Springer Nature; 2019:25-56.
14. Felitti VJ, Anda RF, Nordenberg D, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med. 1998;14(4):245-258. doi:10.1016/s0749-3797(98)00017-8
15. Katon JG, Lehavot K, Simpson TL, et al. Adverse childhood experiences, Military service, and adult health. Am J Prev Med. 2015;49(4):573-582. doi:10.1016/j.amepre.2015.03.020
16. Blosnich JR, Dichter ME, Cerulli C, Batten SV, Bossarte RM. Disparities in adverse childhood experiences among individuals with a history of military service. JAMA Psychiatry. 2014;71(9):1041-1048. doi:10.1001/jamapsychiatry.2014.724
17. Center for Substance Abuse Treatment. Treatment improvement protocol (TIP). Series, No. 57. In: SAMHSA, ed. Trauma-Informed Care in Behavioral Health Services. SAMHSA: Rockville, MD; 2014:137-155.
18. US Department of Veterans Affairs, Veterans Health Administration, National Center for PTSD. Trauma, PTSD and treatment. https://www.ptsd.va.gov/PTSD/professional/treat/index.asp. Updated July 5, 2019. Accessed June 17, 2020.
19. Turgoose D, Ashwick R, Murphy D. Systematic review of lessons learned from delivering tele-therapy to veterans with post-traumatic stress disorder. J Telemed Telecare. 2018;24(9):575-585. doi:10.1177/1357633X17730443
20. Cook JM, Simiola V, Hamblen JL, Bernardy N, Schnurr PP. The influence of patient readiness on implementation of evidence-based PTSD treatments in Veterans Affairs residential programs. Psychol Trauma. 2017;9(suppl 1):51-58. doi:10.1037/tra0000162
21. Raja S, Hasnain M, Hoersch M, Gove-Yin S, Rajagopalan C. Trauma informed care in medicine: current knowledge and future research directions. Fam Community Health. 2015;38(3):216-226. doi:10.1097/FCH.0000000000000071
22. Hopper EK, Bassuk EL, Olivet J. Shelter from the storm: trauma-informed care in homeless service settings. Open Health Serv Policy J. 2009;2:131-151.
23. Kelly U, Boyd MA, Valente SM, Czekanski E. Trauma-informed care: keeping mental health settings safe for veterans [published correction appears in Issues Ment Health Nurs. 2015 Jun;36(6):482]. Issues Ment Health Nurs. 2014;35(6):413-419. doi:10.3109/01612840.2014.881941
24. Currier JM, Stefurak T, Carroll TD, Shatto EH. Applying trauma-informed care to community-based mental health services for military veterans. Best Pract Ment Health. 2017;13(1):47-64.
25. Neria Y, Nandi A, Galea S. Post-traumatic stress disorder following disasters: a systematic review. Psychol Med. 2008;38(4):467-480. doi:10.1017/S0033291707001353
26. Galea S, Merchant RM, Lurie N. the mental health consequences of COVID-19 and physical distancing: the need for prevention and early intervention [published online ahead of print, 2020 Apr 10]. JAMA Intern Med. 2020;10.1001/jamainternmed.2020.1562. doi:10.1001/jamainternmed.2020.1562
27. Hawryluck L, Gold WL, Robinson S, Pogorski S, Galea S, Styra R. SARS control and psychological effects of quarantine, Toronto, Canada. Emerg Infect Dis. 2004;10(7):1206-1212. doi:10.3201/eid1007.030703
28. Cunha JM, Shen YC, Burke ZR. Contrasting the impacts of combat and humanitarian assistance/disaster relief missions on the mental health of military service members. Def Peace Economics. 2018;29(1):62-77. doi: 10.1080/10242694.2017.1349365
29. Ramchand R, Harrell MC, Berglass N, Lauck M. Veterans and COVID-19: Projecting the Economic, Social and Mental Health Needs of America’s Veterans. New York, NY: The Bob Woodruff Foundation; 2020.
30. van Gelder N, Peterman A, Potts A, et al. COVID-19: reducing the risk of infection might increase the risk of intimate partner violence [published online ahead of print, 2020 Apr 11]. EClinicalMedicine. 2020;21:100348. doi:10.1016/j.eclinm.2020.100348
31. Azarang A, Pakyurek M, Giroux C, Nordahl TE, Yellowlees P. Information technologies: an augmentation to post-traumatic stress disorder treatment among trauma survivors. Telemed J E Health. 2019;25(4):263-271. doi:10.1089/tmj.2018.0068.
32. Gilmore AK, Davis MT, Grubaugh A, et al. “Do you expect me to receive PTSD care in a setting where most of the other patients remind me of the perpetrator?”: Home-based telemedicine to address barriers to care unique to military sexual trauma and veterans affairs hospitals. Contemp Clin Trials. 2016;48:59-64. doi:10.1016/j.cct.2016.03.004.
33. van Gurp J, van Selm M, Vissers K, van Leeuwen E, Hasselaar J. How outpatient palliative care teleconsultation facilitates empathic patient-professional relationships: a qualitative study. PLoS One. 2015;10(4):e0124387. Published 2015 Apr 22. doi:10.1371/journal.pone.0124387
34. Morland LA, Mackintosh MA, Glassman LH, et al. Home-based delivery of variable length prolonged exposure therapy: a comparison of clinical efficacy between service modalities. Depress Anxiety. 2020;37(4):346-355. doi:10.1002/da.22979
35. Morland LA, Hynes AK, Mackintosh MA, Resick PA, Chard KM. Group cognitive processing therapy delivered to veterans via telehealth: a pilot cohort. J Trauma Stress. 2011;24(4):465-469. doi:10.1002/jts.20661
36. Elisseou S, Puranam S, Nandi M. A novel, trauma-informed physical examination curriculum. Med Educ. 2018;52(5):555-556. doi:10.1111/medu.13569
A Veteran With a Solitary Pulmonary Nodule
Case Presentation. A 69-year-old veteran presented with an intermittent, waxing and waning cough. He had never smoked and had no family history of lung cancer. His primary care physician ordered a chest radiograph, which revealed a nodular opacity within the lingula concerning for a parenchymal nodule. Further characterization with a chest computed tomography (CT) demonstrated a 1.4-cm left upper lobe subpleural nodule with small satellite nodules (Figure 1). Given these imaging findings, the patient was referred to the pulmonary clinic.
►Lauren Kearney, MD, Medical Resident, VA Boston Healthcare System (VABHS) and Boston Medical Center. What is the differential diagnosis of a solitary pulmonary nodule? What characteristics of the nodule do you consider to differentiate these diagnoses?
►Renda Wiener, MD, Pulmonary and Critical Care, VABHS, and Assistant Professor of Medicine, Boston University School of Medicine. Pulmonary nodules are well-defined lesions < 3 cm in diameter that are surrounded by lung parenchyma. Although cancer is a possibility (including primary lung cancers, metastatic cancers, or carcinoid tumors), most small nodules do not turn out to be malignant.1 Benign etiologies include infections, benign tumors, vascular malformations, and inflammatory conditions. Infectious causes of nodules are often granulomatous in nature, including fungi, Mycobacterium tuberculosis, and nontuberculous mycobacteria. Benign tumors are most commonly hamartomas, and these may be clearly distinguished based on imaging characteristics. Pulmonary arteriovenous malformations, hematomas, and infarcts may present as nodules as well. Inflammatory causes of nodules are important and relatively common, including granulomatosis with polyangiitis, rheumatoid arthritis, sarcoidosis, amyloidosis, and rounded atelectasis.
To distinguish benign from malignant etiologies, we look for several features of pulmonary nodules on imaging. Larger size, irregular borders, and upper lobe location all increase the likelihood of cancer, whereas solid attenuation and calcification make cancer less likely. One of the most reassuring findings that suggests a benign etiology is lack of growth over a period of surveillance; after 2 years without growth we typically consider a nodule benign.1 And of course, we also consider the patient’s symptoms and risk factors: weight loss, hemoptysis, a history of cigarette smoking or asbestos exposure, or family history of cancer all increase the likelihood of malignancy.
►Dr. Kearney. Given that the differential diagnosis is so broad, how do you think about the next step in evaluating a pulmonary nodule? How do you approach shared decision making with the patient?
►Dr. Wiener. The characteristics of the patient, the nodule, and the circumstances in which the nodule were discovered are all important to consider. Incidental pulmonary nodules are often found on chest imaging. The imaging characteristics of the nodule are important, as are the patient’s risk factors. A similarly appearing nodule can have very different implications if the patient is a never-smoker exposed to endemic fungi, or a long-time smoker enrolled in a lung cancer screening program. Consultation with a pulmonologist is often appropriate.
It’s important to note that we lack high-quality evidence on the optimal strategy to evaluate pulmonary nodules, and there is no single “right answer“ for all patients. For patients with a low risk of malignancy (< 5%-10%)—which comprises the majority of the incidental nodules discovered—we typically favor serial CT surveillance of the nodule over a period of a few years, whereas for patients at high risk of malignancy (> 65%), we favor early surgical resection if the patient is able to tolerate that. For patients with an intermediate risk of malignancy (~5%-65%), we might consider serial CT surveillance, positron emission tomography (PET) scan, or biopsy.1 The American College of Chest Physicians guidelines for pulmonary nodule evaluation recommend discussing with patients the different options and the trade-offs of these options in a shared decision-making process.1
►Dr. Kearney. The patient’s pulmonologist laid out options, including monitoring with serial CT scans, obtaining a PET scan, performing CT-guided needle biopsy, or referring for surgical excision. In this case, the patient elected to undergo CT-guided needle biopsy. Dr. Huang, can you discuss the pathology results?
►Qin Huang, MD, Pathology and Laboratory Medicine, VABHS, and Assistant Professor of Pathology, Harvard Medical School (HMS). The microscopic examination of the needle biopsy of the lung mass revealed rare clusters of atypical cells with crushed cells adjacent to an extensive area of necrosis with scarring. The atypical cells were suspicious for carcinoma. The Gomori methenamine silver (GMS) and periodic acid-Schiff (PAS) stains were negative for common bacterial and fungal microorganisms.
►Dr. Kearney. The tumor board, pulmonologist, and patient decide to move forward with video-assisted excisional biopsy with lymphadenectomy. Dr. Huang, can you interpret the pathology?
►Dr. Huang. Figure 2 showed an hemotoxylin and eosin (H&E)-stained lung resection tissue section with multiple caseating necrotic granulomas. No foreign bodies were identified. There was no evidence of malignancy. The GMS stain revealed a fungal microorganism oval with morphology typical of histoplasma capsulatum (Figure 3).
►Dr. Kearney. What are some of the different ways histoplasmosis can present? Which of these diagnoses fits this patient’s presentation?
►Judy Strymish, MD, Infectious Disease, VABHS, and Assistant Professor of Medicine, HMS. Most patients who inhale histoplasmosis spores develop asymptomatic or self-limited infection that is usually not detected. Patients at risk of symptomatic and clinically relevant disease include those who are immunocompromised, at extremes of ages, or exposed to larger inoculums. Acute pulmonary histoplasmosis can present with cough, shortness of breath, fever, chills, and less commonly, rheumatologic complaints such as erythema nodosum or erythema multiforme. Imaging often shows patchy infiltrates and enlarged mediastinal and hilar lymphadenopathy. Patients can go on to develop subacute or chronic pulmonary disease with focal opacities and mediastinal and hilar lymphadenopathy. Those with chronic disease can have cavitary lesions similar to patients with tuberculosis. Progressive disseminated histoplasmosis can develop in immunocompromised patients and disseminate through the reticuloendothelial system to other organs with the gastrointestinal tract, central nervous system, and adrenal glands.2
Pulmonary nodules are common incidental finding on chest imaging of patients who reside in histoplasmosis endemic regions, and they are often hard to differentiate from malignancies. There are 3 mediastinal manifestations: adenitis, granuloma, and fibrosis. Usually the syndromes are subclinical, but occasionally the nodes cause symptoms by impinging on other structures.2
This patient had a solitary pulmonary nodule with none of the associated features mentioned above. Pathology showed caseating granuloma and confirmed histoplasmosis.
►Dr. Kearney. Given the diagnosis of solitary histoplasmoma, how should this patient be managed?
►Dr. Strymish. The optimal therapy for histoplasmosis depends on the patient’s clinical syndrome. Most infections are self-limited and require no therapy. However, patients who are immunocompromised, exposed to large inoculum, and have progressive disease require antifungal treatment, usually with itraconazole for mild-to-moderate disease and a combination of azole therapy and amphotericin B with extensive disease. Patients with few solitary pulmonary nodules do not benefit from antifungal therapy as the nodule could represent quiescent disease that is unlikely to have clinical impact; in this case, the treatment would be higher risk than the nodule.3
►Dr. Kearney. While the discussion of the diagnosis is interesting, it is also important to acknowledge what the patient went through to arrive at this, an essentially benign diagnosis: 8 months, multiple imaging studies, and 2 invasive diagnostic procedures. Further, the patient had to grapple with the possibility of a diagnosis of cancer. Dr. Wiener, can you talk about the challenges in communicating with patients about pulmonary nodules when cancer is on the differential? What are some of the harms patients face and how can clinicians work to mitigate these harms?
►Dr. Wiener. My colleague Dr. Christopher Slatore of the Portland VA Medical Center and I studied communication about pulmonary nodules in a series of surveys and qualitative studies of patients with pulmonary nodules and the clinicians who take care of them. We found that there seems to be a disconnect between patients’ perceptions of pulmonary nodules and their clinicians, often due to inadequate communication about the nodule. Many clinicians indicated that they do not tell patients about the chance that a nodule may be cancer, because the clinicians know that cancer is unlikely (< 5% of incidentally detected pulmonary nodules turn out to be malignant), and they do not want to alarm patients unnecessarily. However, we found that patients almost immediately wondered about cancer when they learned about their pulmonary nodule, and without hearing explicitly from their clinician that cancer was unlikely, patients tended to overestimate the likelihood of a malignant nodule. Moreover, patients often were not told much about the evaluation plan for the nodule or the rationale for CT surveillance of small nodules instead of biopsy. This uncertainty about the risk of cancer and the plan for evaluating the nodule was difficult for some patients to live with; we found that about one-quarter of patients with a small pulmonary nodule experienced mild-moderate distress during the period of radiographic surveillance. Reassuringly, high-quality patient-clinician communication was associated with lower distress and higher adherence to pulmonary nodule evaluation.4
►Dr. Kearney. The patient was educated about his diagnosis of solitary histoplasmoma. Given that the patient was otherwise well appearing with no complicating factors, he was not treated with antifungal therapy. After an 8-month-long workup, the patient was relieved to receive a diagnosis that excluded cancer and did not require any further treatment. His case provides a good example of how to proceed in the workup of a solitary pulmonary nodule and on the importance of communication and shared decision making with our patients.
1. Gould MK, Donington J, Lynch WR, et al. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(suppl 5):e93S-e120S.
2. Azar MM, Hage CA. Clinical perspectives in the diagnosis and management of histoplasmosis. Clin Chest Med. 2017;38(3):403-415.
3. Wheat LJ, Freifeld A, Kleiman MB, et al. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis. 2007;45(7):807-825.
4. Slatore CG, Wiener RS. Pulmonary nodules: a small problem for many, severe distress for some, and how to communicate about it. Chest. 2018;153(4):1004-1015.
Case Presentation. A 69-year-old veteran presented with an intermittent, waxing and waning cough. He had never smoked and had no family history of lung cancer. His primary care physician ordered a chest radiograph, which revealed a nodular opacity within the lingula concerning for a parenchymal nodule. Further characterization with a chest computed tomography (CT) demonstrated a 1.4-cm left upper lobe subpleural nodule with small satellite nodules (Figure 1). Given these imaging findings, the patient was referred to the pulmonary clinic.
►Lauren Kearney, MD, Medical Resident, VA Boston Healthcare System (VABHS) and Boston Medical Center. What is the differential diagnosis of a solitary pulmonary nodule? What characteristics of the nodule do you consider to differentiate these diagnoses?
►Renda Wiener, MD, Pulmonary and Critical Care, VABHS, and Assistant Professor of Medicine, Boston University School of Medicine. Pulmonary nodules are well-defined lesions < 3 cm in diameter that are surrounded by lung parenchyma. Although cancer is a possibility (including primary lung cancers, metastatic cancers, or carcinoid tumors), most small nodules do not turn out to be malignant.1 Benign etiologies include infections, benign tumors, vascular malformations, and inflammatory conditions. Infectious causes of nodules are often granulomatous in nature, including fungi, Mycobacterium tuberculosis, and nontuberculous mycobacteria. Benign tumors are most commonly hamartomas, and these may be clearly distinguished based on imaging characteristics. Pulmonary arteriovenous malformations, hematomas, and infarcts may present as nodules as well. Inflammatory causes of nodules are important and relatively common, including granulomatosis with polyangiitis, rheumatoid arthritis, sarcoidosis, amyloidosis, and rounded atelectasis.
To distinguish benign from malignant etiologies, we look for several features of pulmonary nodules on imaging. Larger size, irregular borders, and upper lobe location all increase the likelihood of cancer, whereas solid attenuation and calcification make cancer less likely. One of the most reassuring findings that suggests a benign etiology is lack of growth over a period of surveillance; after 2 years without growth we typically consider a nodule benign.1 And of course, we also consider the patient’s symptoms and risk factors: weight loss, hemoptysis, a history of cigarette smoking or asbestos exposure, or family history of cancer all increase the likelihood of malignancy.
►Dr. Kearney. Given that the differential diagnosis is so broad, how do you think about the next step in evaluating a pulmonary nodule? How do you approach shared decision making with the patient?
►Dr. Wiener. The characteristics of the patient, the nodule, and the circumstances in which the nodule were discovered are all important to consider. Incidental pulmonary nodules are often found on chest imaging. The imaging characteristics of the nodule are important, as are the patient’s risk factors. A similarly appearing nodule can have very different implications if the patient is a never-smoker exposed to endemic fungi, or a long-time smoker enrolled in a lung cancer screening program. Consultation with a pulmonologist is often appropriate.
It’s important to note that we lack high-quality evidence on the optimal strategy to evaluate pulmonary nodules, and there is no single “right answer“ for all patients. For patients with a low risk of malignancy (< 5%-10%)—which comprises the majority of the incidental nodules discovered—we typically favor serial CT surveillance of the nodule over a period of a few years, whereas for patients at high risk of malignancy (> 65%), we favor early surgical resection if the patient is able to tolerate that. For patients with an intermediate risk of malignancy (~5%-65%), we might consider serial CT surveillance, positron emission tomography (PET) scan, or biopsy.1 The American College of Chest Physicians guidelines for pulmonary nodule evaluation recommend discussing with patients the different options and the trade-offs of these options in a shared decision-making process.1
►Dr. Kearney. The patient’s pulmonologist laid out options, including monitoring with serial CT scans, obtaining a PET scan, performing CT-guided needle biopsy, or referring for surgical excision. In this case, the patient elected to undergo CT-guided needle biopsy. Dr. Huang, can you discuss the pathology results?
►Qin Huang, MD, Pathology and Laboratory Medicine, VABHS, and Assistant Professor of Pathology, Harvard Medical School (HMS). The microscopic examination of the needle biopsy of the lung mass revealed rare clusters of atypical cells with crushed cells adjacent to an extensive area of necrosis with scarring. The atypical cells were suspicious for carcinoma. The Gomori methenamine silver (GMS) and periodic acid-Schiff (PAS) stains were negative for common bacterial and fungal microorganisms.
►Dr. Kearney. The tumor board, pulmonologist, and patient decide to move forward with video-assisted excisional biopsy with lymphadenectomy. Dr. Huang, can you interpret the pathology?
►Dr. Huang. Figure 2 showed an hemotoxylin and eosin (H&E)-stained lung resection tissue section with multiple caseating necrotic granulomas. No foreign bodies were identified. There was no evidence of malignancy. The GMS stain revealed a fungal microorganism oval with morphology typical of histoplasma capsulatum (Figure 3).
►Dr. Kearney. What are some of the different ways histoplasmosis can present? Which of these diagnoses fits this patient’s presentation?
►Judy Strymish, MD, Infectious Disease, VABHS, and Assistant Professor of Medicine, HMS. Most patients who inhale histoplasmosis spores develop asymptomatic or self-limited infection that is usually not detected. Patients at risk of symptomatic and clinically relevant disease include those who are immunocompromised, at extremes of ages, or exposed to larger inoculums. Acute pulmonary histoplasmosis can present with cough, shortness of breath, fever, chills, and less commonly, rheumatologic complaints such as erythema nodosum or erythema multiforme. Imaging often shows patchy infiltrates and enlarged mediastinal and hilar lymphadenopathy. Patients can go on to develop subacute or chronic pulmonary disease with focal opacities and mediastinal and hilar lymphadenopathy. Those with chronic disease can have cavitary lesions similar to patients with tuberculosis. Progressive disseminated histoplasmosis can develop in immunocompromised patients and disseminate through the reticuloendothelial system to other organs with the gastrointestinal tract, central nervous system, and adrenal glands.2
Pulmonary nodules are common incidental finding on chest imaging of patients who reside in histoplasmosis endemic regions, and they are often hard to differentiate from malignancies. There are 3 mediastinal manifestations: adenitis, granuloma, and fibrosis. Usually the syndromes are subclinical, but occasionally the nodes cause symptoms by impinging on other structures.2
This patient had a solitary pulmonary nodule with none of the associated features mentioned above. Pathology showed caseating granuloma and confirmed histoplasmosis.
►Dr. Kearney. Given the diagnosis of solitary histoplasmoma, how should this patient be managed?
►Dr. Strymish. The optimal therapy for histoplasmosis depends on the patient’s clinical syndrome. Most infections are self-limited and require no therapy. However, patients who are immunocompromised, exposed to large inoculum, and have progressive disease require antifungal treatment, usually with itraconazole for mild-to-moderate disease and a combination of azole therapy and amphotericin B with extensive disease. Patients with few solitary pulmonary nodules do not benefit from antifungal therapy as the nodule could represent quiescent disease that is unlikely to have clinical impact; in this case, the treatment would be higher risk than the nodule.3
►Dr. Kearney. While the discussion of the diagnosis is interesting, it is also important to acknowledge what the patient went through to arrive at this, an essentially benign diagnosis: 8 months, multiple imaging studies, and 2 invasive diagnostic procedures. Further, the patient had to grapple with the possibility of a diagnosis of cancer. Dr. Wiener, can you talk about the challenges in communicating with patients about pulmonary nodules when cancer is on the differential? What are some of the harms patients face and how can clinicians work to mitigate these harms?
►Dr. Wiener. My colleague Dr. Christopher Slatore of the Portland VA Medical Center and I studied communication about pulmonary nodules in a series of surveys and qualitative studies of patients with pulmonary nodules and the clinicians who take care of them. We found that there seems to be a disconnect between patients’ perceptions of pulmonary nodules and their clinicians, often due to inadequate communication about the nodule. Many clinicians indicated that they do not tell patients about the chance that a nodule may be cancer, because the clinicians know that cancer is unlikely (< 5% of incidentally detected pulmonary nodules turn out to be malignant), and they do not want to alarm patients unnecessarily. However, we found that patients almost immediately wondered about cancer when they learned about their pulmonary nodule, and without hearing explicitly from their clinician that cancer was unlikely, patients tended to overestimate the likelihood of a malignant nodule. Moreover, patients often were not told much about the evaluation plan for the nodule or the rationale for CT surveillance of small nodules instead of biopsy. This uncertainty about the risk of cancer and the plan for evaluating the nodule was difficult for some patients to live with; we found that about one-quarter of patients with a small pulmonary nodule experienced mild-moderate distress during the period of radiographic surveillance. Reassuringly, high-quality patient-clinician communication was associated with lower distress and higher adherence to pulmonary nodule evaluation.4
►Dr. Kearney. The patient was educated about his diagnosis of solitary histoplasmoma. Given that the patient was otherwise well appearing with no complicating factors, he was not treated with antifungal therapy. After an 8-month-long workup, the patient was relieved to receive a diagnosis that excluded cancer and did not require any further treatment. His case provides a good example of how to proceed in the workup of a solitary pulmonary nodule and on the importance of communication and shared decision making with our patients.
Case Presentation. A 69-year-old veteran presented with an intermittent, waxing and waning cough. He had never smoked and had no family history of lung cancer. His primary care physician ordered a chest radiograph, which revealed a nodular opacity within the lingula concerning for a parenchymal nodule. Further characterization with a chest computed tomography (CT) demonstrated a 1.4-cm left upper lobe subpleural nodule with small satellite nodules (Figure 1). Given these imaging findings, the patient was referred to the pulmonary clinic.
►Lauren Kearney, MD, Medical Resident, VA Boston Healthcare System (VABHS) and Boston Medical Center. What is the differential diagnosis of a solitary pulmonary nodule? What characteristics of the nodule do you consider to differentiate these diagnoses?
►Renda Wiener, MD, Pulmonary and Critical Care, VABHS, and Assistant Professor of Medicine, Boston University School of Medicine. Pulmonary nodules are well-defined lesions < 3 cm in diameter that are surrounded by lung parenchyma. Although cancer is a possibility (including primary lung cancers, metastatic cancers, or carcinoid tumors), most small nodules do not turn out to be malignant.1 Benign etiologies include infections, benign tumors, vascular malformations, and inflammatory conditions. Infectious causes of nodules are often granulomatous in nature, including fungi, Mycobacterium tuberculosis, and nontuberculous mycobacteria. Benign tumors are most commonly hamartomas, and these may be clearly distinguished based on imaging characteristics. Pulmonary arteriovenous malformations, hematomas, and infarcts may present as nodules as well. Inflammatory causes of nodules are important and relatively common, including granulomatosis with polyangiitis, rheumatoid arthritis, sarcoidosis, amyloidosis, and rounded atelectasis.
To distinguish benign from malignant etiologies, we look for several features of pulmonary nodules on imaging. Larger size, irregular borders, and upper lobe location all increase the likelihood of cancer, whereas solid attenuation and calcification make cancer less likely. One of the most reassuring findings that suggests a benign etiology is lack of growth over a period of surveillance; after 2 years without growth we typically consider a nodule benign.1 And of course, we also consider the patient’s symptoms and risk factors: weight loss, hemoptysis, a history of cigarette smoking or asbestos exposure, or family history of cancer all increase the likelihood of malignancy.
►Dr. Kearney. Given that the differential diagnosis is so broad, how do you think about the next step in evaluating a pulmonary nodule? How do you approach shared decision making with the patient?
►Dr. Wiener. The characteristics of the patient, the nodule, and the circumstances in which the nodule were discovered are all important to consider. Incidental pulmonary nodules are often found on chest imaging. The imaging characteristics of the nodule are important, as are the patient’s risk factors. A similarly appearing nodule can have very different implications if the patient is a never-smoker exposed to endemic fungi, or a long-time smoker enrolled in a lung cancer screening program. Consultation with a pulmonologist is often appropriate.
It’s important to note that we lack high-quality evidence on the optimal strategy to evaluate pulmonary nodules, and there is no single “right answer“ for all patients. For patients with a low risk of malignancy (< 5%-10%)—which comprises the majority of the incidental nodules discovered—we typically favor serial CT surveillance of the nodule over a period of a few years, whereas for patients at high risk of malignancy (> 65%), we favor early surgical resection if the patient is able to tolerate that. For patients with an intermediate risk of malignancy (~5%-65%), we might consider serial CT surveillance, positron emission tomography (PET) scan, or biopsy.1 The American College of Chest Physicians guidelines for pulmonary nodule evaluation recommend discussing with patients the different options and the trade-offs of these options in a shared decision-making process.1
►Dr. Kearney. The patient’s pulmonologist laid out options, including monitoring with serial CT scans, obtaining a PET scan, performing CT-guided needle biopsy, or referring for surgical excision. In this case, the patient elected to undergo CT-guided needle biopsy. Dr. Huang, can you discuss the pathology results?
►Qin Huang, MD, Pathology and Laboratory Medicine, VABHS, and Assistant Professor of Pathology, Harvard Medical School (HMS). The microscopic examination of the needle biopsy of the lung mass revealed rare clusters of atypical cells with crushed cells adjacent to an extensive area of necrosis with scarring. The atypical cells were suspicious for carcinoma. The Gomori methenamine silver (GMS) and periodic acid-Schiff (PAS) stains were negative for common bacterial and fungal microorganisms.
►Dr. Kearney. The tumor board, pulmonologist, and patient decide to move forward with video-assisted excisional biopsy with lymphadenectomy. Dr. Huang, can you interpret the pathology?
►Dr. Huang. Figure 2 showed an hemotoxylin and eosin (H&E)-stained lung resection tissue section with multiple caseating necrotic granulomas. No foreign bodies were identified. There was no evidence of malignancy. The GMS stain revealed a fungal microorganism oval with morphology typical of histoplasma capsulatum (Figure 3).
►Dr. Kearney. What are some of the different ways histoplasmosis can present? Which of these diagnoses fits this patient’s presentation?
►Judy Strymish, MD, Infectious Disease, VABHS, and Assistant Professor of Medicine, HMS. Most patients who inhale histoplasmosis spores develop asymptomatic or self-limited infection that is usually not detected. Patients at risk of symptomatic and clinically relevant disease include those who are immunocompromised, at extremes of ages, or exposed to larger inoculums. Acute pulmonary histoplasmosis can present with cough, shortness of breath, fever, chills, and less commonly, rheumatologic complaints such as erythema nodosum or erythema multiforme. Imaging often shows patchy infiltrates and enlarged mediastinal and hilar lymphadenopathy. Patients can go on to develop subacute or chronic pulmonary disease with focal opacities and mediastinal and hilar lymphadenopathy. Those with chronic disease can have cavitary lesions similar to patients with tuberculosis. Progressive disseminated histoplasmosis can develop in immunocompromised patients and disseminate through the reticuloendothelial system to other organs with the gastrointestinal tract, central nervous system, and adrenal glands.2
Pulmonary nodules are common incidental finding on chest imaging of patients who reside in histoplasmosis endemic regions, and they are often hard to differentiate from malignancies. There are 3 mediastinal manifestations: adenitis, granuloma, and fibrosis. Usually the syndromes are subclinical, but occasionally the nodes cause symptoms by impinging on other structures.2
This patient had a solitary pulmonary nodule with none of the associated features mentioned above. Pathology showed caseating granuloma and confirmed histoplasmosis.
►Dr. Kearney. Given the diagnosis of solitary histoplasmoma, how should this patient be managed?
►Dr. Strymish. The optimal therapy for histoplasmosis depends on the patient’s clinical syndrome. Most infections are self-limited and require no therapy. However, patients who are immunocompromised, exposed to large inoculum, and have progressive disease require antifungal treatment, usually with itraconazole for mild-to-moderate disease and a combination of azole therapy and amphotericin B with extensive disease. Patients with few solitary pulmonary nodules do not benefit from antifungal therapy as the nodule could represent quiescent disease that is unlikely to have clinical impact; in this case, the treatment would be higher risk than the nodule.3
►Dr. Kearney. While the discussion of the diagnosis is interesting, it is also important to acknowledge what the patient went through to arrive at this, an essentially benign diagnosis: 8 months, multiple imaging studies, and 2 invasive diagnostic procedures. Further, the patient had to grapple with the possibility of a diagnosis of cancer. Dr. Wiener, can you talk about the challenges in communicating with patients about pulmonary nodules when cancer is on the differential? What are some of the harms patients face and how can clinicians work to mitigate these harms?
►Dr. Wiener. My colleague Dr. Christopher Slatore of the Portland VA Medical Center and I studied communication about pulmonary nodules in a series of surveys and qualitative studies of patients with pulmonary nodules and the clinicians who take care of them. We found that there seems to be a disconnect between patients’ perceptions of pulmonary nodules and their clinicians, often due to inadequate communication about the nodule. Many clinicians indicated that they do not tell patients about the chance that a nodule may be cancer, because the clinicians know that cancer is unlikely (< 5% of incidentally detected pulmonary nodules turn out to be malignant), and they do not want to alarm patients unnecessarily. However, we found that patients almost immediately wondered about cancer when they learned about their pulmonary nodule, and without hearing explicitly from their clinician that cancer was unlikely, patients tended to overestimate the likelihood of a malignant nodule. Moreover, patients often were not told much about the evaluation plan for the nodule or the rationale for CT surveillance of small nodules instead of biopsy. This uncertainty about the risk of cancer and the plan for evaluating the nodule was difficult for some patients to live with; we found that about one-quarter of patients with a small pulmonary nodule experienced mild-moderate distress during the period of radiographic surveillance. Reassuringly, high-quality patient-clinician communication was associated with lower distress and higher adherence to pulmonary nodule evaluation.4
►Dr. Kearney. The patient was educated about his diagnosis of solitary histoplasmoma. Given that the patient was otherwise well appearing with no complicating factors, he was not treated with antifungal therapy. After an 8-month-long workup, the patient was relieved to receive a diagnosis that excluded cancer and did not require any further treatment. His case provides a good example of how to proceed in the workup of a solitary pulmonary nodule and on the importance of communication and shared decision making with our patients.
1. Gould MK, Donington J, Lynch WR, et al. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(suppl 5):e93S-e120S.
2. Azar MM, Hage CA. Clinical perspectives in the diagnosis and management of histoplasmosis. Clin Chest Med. 2017;38(3):403-415.
3. Wheat LJ, Freifeld A, Kleiman MB, et al. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis. 2007;45(7):807-825.
4. Slatore CG, Wiener RS. Pulmonary nodules: a small problem for many, severe distress for some, and how to communicate about it. Chest. 2018;153(4):1004-1015.
1. Gould MK, Donington J, Lynch WR, et al. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(suppl 5):e93S-e120S.
2. Azar MM, Hage CA. Clinical perspectives in the diagnosis and management of histoplasmosis. Clin Chest Med. 2017;38(3):403-415.
3. Wheat LJ, Freifeld A, Kleiman MB, et al. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis. 2007;45(7):807-825.
4. Slatore CG, Wiener RS. Pulmonary nodules: a small problem for many, severe distress for some, and how to communicate about it. Chest. 2018;153(4):1004-1015.
Food Insecurity Among Veterans: Resources to Screen and Intervene
Nearly 1 in 8 households—and 1 in 6 households with children—experienced food insecurity in 2017, defined as limited or uncertain availability of nutritionally adequate and safe foods.1 Food insecurity is often even more pronounced among households with individuals with acute or chronic medical conditions.2-6 Moreover, food insecurity is independently associated with a range of adverse health outcomes, including poorer control of diabetes mellitus, hypertension, depression and other major psychiatric disorders, HIV, and chronic lung and kidney disease, as well as poorer overall health status.7-14 Food insecurity also has been associated with increased health care costs and acute care utilization as well as increased probability of delayed or missed care.15-19
The relationship between food insecurity and poor health outcomes is a complex and often cyclic phenomenon (Figure 1). Poor nutritional status is fueled by limited access to healthful foods as well as increased reliance on calorie-dense and nutrient-poor “junk” foods, which are less expensive and often more readily available in low-income neighborhoods.5,20-24 These compensatory dietary patterns place individuals at higher risk for developing cardiometabolic conditions and for poor control of these conditions.5,8,9,12,25,26 Additionally, the physiological and psychological stressors of food insecurity may precipitate depression and anxiety or worsen existing mental health conditions, resulting in feelings of overwhelm and decreased self-management capacity.5,8,27-31 Food insecurity has further been associated with poor sleep, declines in cognitive function, and increased falls, particularly among the frail and elderly.32-34
Individuals experiencing food insecurity often report having to make trade-offs between food and other necessities, such as paying rent or utilities. Additional strategies to stretch limited resources include cost-related underuse of medication and delays in needed medical care.4,17,31,35 In a nationally representative survey among adults with at least 1 chronic medical condition, 1 in 3 reported having to choose between food and medicine; 11% were unable to afford either.3 Furthermore, the inability to reliably adhere to medication regimens that need to be taken with food can result in potentially life-threatening hypoglycemia (as can lack of food regardless of medication use).5,26,36 In addition to the more obvious risks of glucose-lowering medications, such as insulin and long-acting sulfonylureas in patients experiencing food insecurity, many drugs commonly used among nondiabetic adults such as ACE-inhibitors, β blockers, quinolones, and salicylates can also precipitate hypoglycemia, and food insecurity has been associated with experiences of hypoglycemia even among individuals without diabetes mellitus.32,37 In one study the risk for hospital admissions for hypoglycemia among low-income populations increased by 27% at the end of the month when food budgets were more likely to be exhausted.38 Worsening health status and increased emergency department visits and hospitalizations may then result in lost wages and mounting medical bills, contributing to further financial strain and worsening food insecurity.
Prevalence and Importance of Food Insecurity Among US Veterans
Nearly 1.5 million veterans in the US are living below the federal poverty level (FPL).39 An additional 2.4 million veterans are living paycheck to paycheck at < 200% of the FPL.40 Veterans living in poverty are at even higher risk than nonveterans for food insecurity, homelessness, and other material hardship.41
Estimates of food insecurity among veterans vary widely, ranging from 6% to 24%—nearly twice that of the general US population.8,42-45 Higher rates of food insecurity have been reported among certain high-risk subgroups, including veterans who served in Iraq and Afghanistan (27%), female veterans (28%), homeless and formerly homeless veterans (49%), and veterans with serious mental illness (35%).6,32,43,46 Additional risk factors for food insecurity specific to veteran populations include younger age, having recently left active-duty military service, and lower final military paygrade.42,45-47 As in the general population, veteran food insecurity is associated with a range of adverse health outcomes, including poorer overall health status as well as increased probability of delayed or missed care.6,8,32,42-44,46
Even among veterans enrolled in federal food assistance programs, many still struggle to afford nutritionally adequate foods. As one example, in a study of mostly male homeless and formerly homeless veterans, O’Toole and colleagues found that nearly half of those reporting food insecurity were already receiving federal food assistance benefits, and 22% relied on emergency food resources.32 Of households served by Feeding America food pantries and meal programs, 20% have a member who has served in the US military.48
Federal Programs To Address Food Insecurity
There are several important federal food assistance programs designed to help alleviate food insecurity. The Supplemental Nutrition Assistance Program (SNAP, formerly the Food Stamp program) is the largest federal food assistance program and provides low-income Americans with cash benefits to purchase food. SNAP has been shown to substantially reduce food insecurity.7,49 The program also is associated with significant decreases in cost-related medication nonadherence as well as reductions in health care costs and both acute care and nursing home utilization.16,50-54 Although nearly 1.4 million veterans live in SNAP-enrolled households, 59% of eligible veterans are not enrolled.43,55 Closing this SNAP eligibility-enrollment gap, has been a focus of recent efforts to improve long-term food security among veterans. There also are several federal food assistance programs for households with children, including the Special Supplemental Nutrition Program for Women, Infants, and Children (WIC) and school meals programs. Among federal nutrition programs for seniors, the Older American’s Act contains designated funding to support nutrition services for older adults, including congregate meal programs in community settings like senior centers, places of worship, and housing communities, and home-delivered meals through programs like Meals on Wheels.56
VHA Response to Food Insecurity
The Veterans Health Administration (VHA) is the country’s largest integrated, federally funded health care system.57 In November 2015, congressional briefings on veteran food insecurity organized by the national non-profit organization MAZON: A Jewish Response to Hunger and hosted with bipartisan support were provided to the US House and Senate. As a result of these briefings, VHA chartered the national Ensuring Veteran Food Security Workgroup with a mandate to partner with governmental and nonprofit agencies to “focus on the issue of food insecurity, the identification of veterans at risk, the needed training of VHA staff and the coordination of resources and initiatives to support the veterans for whom we care.” Building off a pilot in US Department of Veterans Affairs (VA) Homeless Patient Aligned Care Teams (H-PACTs),32 VHA subsequently integrated a single-item food insecurity screening tool into the VA electronic health record (EHR) clinical reminder system (Figure 2). The clinical reminder, which was rolled out across VA medical centers nationally in October 2017, provides an alert to screen all noninstitutionalized veterans for food insecurity. To date, nearly 5 million veterans have been screened. When a veteran endorses food insecurity based on the initial screening question, a prompt appears to offer the veteran a referral to a social worker and/or dietitian. Positive screening results also should be communicated to the patient’s primary care provider. Depending on site-specific clinical flow, the reminders are typically completed in the outpatient setting either by nurses or medical assistants during intake or by providers as part of the clinical visit. However, any member of the health care team can complete the clinical reminder at any time. As of September 2019, approximately 74,000 veterans have been identified as food insecure.58
Addressing Food Insecurity
VHA has been a recognized leader in addressing homelessness and other social determinants of health through its integrated care and PACT delivery models.59-61 The food insecurity clinical reminder was designed to facilitate a tailored, interdisciplinary approach to identify and address food insecurity. Interdisciplinary care team members—including medical assistants, clinicians, social workers, registered dietitians, nurse care managers, occupational or physical therapists, and pharmacists—are uniquely positioned to identify veterans impacted by food insecurity, assess for associated clinical and/or social risk factors, and offer appropriate medical and nutrition interventions and resource referrals.
This interdisciplinary team-based model is essential given the range of potential drivers underlying veteran experiences of food insecurity and subsequent health outcomes. It is critically important for clinicians to review the medication list with veterans screening positive for food insecurity to assess for risk of hypoglycemia and/or cost-related nonadherence, make any necessary adjustments to therapeutic regimens, and assess for additional risk factors associated with food insecurity. Examples of tailored nutrition counseling that clinical dietitians may provide include meal preparation strategies for veterans who only have access to a microwave or hotplate, or recommendations for how veterans on medically restricted diets can best navigate food selection at soup kitchens or food pantries. Resource referrals provided by social workers or other care team members may include both emergency food resources to address immediate shortages (eg, food pantries, soup kitchens, or vouchers for free lunch) as well as resources focused on improving longer term food security (eg, federal food assistance programs or home delivered meal programs). Importantly, although providing a list of food resources may be helpful for some patients, such lists are often insufficient.62,63 Many patients require active assistance with program enrollment either onsite the day of their clinic visit or through connection with a partnering community-based organization that can, in turn, identify appropriate resources and facilitate program enrollment.63,64 Planned follow-up is also crucial to determine whether referrals are successful and to assess for ongoing need. Proposed roles for interdisciplinary care team members in addressing a positive food insecurity screen are outlined in Table 1.
VHA-Community Partnerships
In addition to services offered within VA, public and private sector partnerships can greatly enhance the range of resources available to food insecure veterans. Several VA facilities have developed formal community partnerships, such as the Veterans Pantry Pilot (VPP) program, a national partnership between Feeding America food banks and VA medical centers to establish onsite or mobile food pantries. There are currently 17 active Feeding America VPP sites, with a number of additional sites under development. Several of the VPP sites also include other “wraparound services,” such as SNAP application assistance.65,66
State Veterans Affairs offices67 and Veterans Service Organizations (VSOs)68 also can serve as valuable partners for connecting veterans with needed resources. VSOs offer a range of services, including assistancewith benefit claims, employment and housing assistance, emergency food assistance, and transportation to medical appointments. Some VSOs also have established local affiliations with Meals on Wheels focused on veteran outreach and providing hot meals for low-income, homebound, and disabled veterans.
Additional Resources
Although resources vary by regional setting, several key governmental and community-based food assistance programs are summarized in Table 2. Local community partners and online/phone-based directories, such as United Way’s 2-1-1 can help identify additional local resources. For older adults and individuals with disabilities, local Aging and Disability Resources Centers can provide information and assistance connecting to needed resources.69 Finally, there are a number of online resources available for clinicians interested in learning more about the impact of food insecurity on health and tools to use in the clinical setting (Table 3).
Conclusion
The VA has recognized food insecurity as a critical concern for the well-being of our nation’s veterans. Use of the EHR clinical reminder represents a crucial first step toward increasing provider awareness about veteran food insecurity and improving clinical efforts to address food insecurity once identified. Through the reminder, health care teams can connect veterans to needed resources and create both the individual and population-level data necessary to inform VHA and community efforts to address veteran food insecurity. Clinical reminder data are currently being used for local quality improvement efforts and have established the need nationally for formalized partnerships between VHA Social Work Services and Nutrition and Food Services to connect veterans with food and provide them with strategies to best use available food resources.
Moving forward, the Ensuring Veteran Food Security Workgroup continues to work with agencies and organizations across the country to improve food insecure veterans’ access to needed services. In addition to existing VA partnerships with Feeding America for the VPP, memorandums of understanding are currently underway to formalize partnerships with both the Food Research and Action Center (FRAC) and MAZON. Additional research is needed both to formally validate the current food insecurity clinical reminder screening question and to identify best practices and potential models for how to most effectively use VHA-community partnerships to address the unique needs of the veteran population.
Ensuring the food security of our nation’s veterans is essential to VA’s commitment to providing integrated, veteran-centered, whole person care. Toward that goal, VA health care teams are urged to use the clinical reminder and help connect food insecure veterans with relevant resources both within and outside of the VA health care system.
1. Coleman-Jensen A, Rabbitt MP, Gregory CA, Singh A. Household food security in the United States in 2017. http://www.ers.usda.gov/publications/pub-details/?pubid=90022. Published September 2018. Accessed December 9, 2019.
2. Berkowitz SA, Meigs JB, DeWalt D, et al. Material need insecurities, control of diabetes mellitus, and use of health care resources: results of the Measuring Economic Insecurity in Diabetes study. JAMA Intern Med. 2015;175(2):257-265.
3. Berkowitz SA, Seligman HK, Choudhry NK. Treat or eat: food insecurity, cost-related medication underuse, and unmet needs. Am J Med. 2014;127(4):303-310.e3.
4. Lyles CR, Seligman HK, Parker MM, et al. Financial strain and medication adherence among diabetes patients in an integrated health care delivery system: The Diabetes Study of Northern California (DISTANCE). Health Serv Res. 2016;51(2):610-624.
5. Seligman HK, Schillinger D. Hunger and socioeconomic disparities in chronic disease. N Engl J Med. 2010;363(1):6-9.
6. Narain K, Bean-Mayberry B, Washington DL, Canelo IA, Darling JE, Yano EM. Access to care and health outcomes among women veterans using veterans administration health care: association with food insufficiency. Womens Health Issues. 2018;28(3):267-272.
7. Gundersen C, Ziliak JP. Food insecurity and health outcomes. Health Aff. 2015;34(11):1830-1839.
8. Wang EA, McGinnis KA, Goulet J, et al; Veterans Aging Cohort Study Project Team. Food insecurity and health: data from the Veterans Aging Cohort Study. Public Health Rep. 2015;130(3):261-268.
9. Berkowitz SA, Berkowitz TSZ, Meigs JB, Wexler DJ. Trends in food insecurity for adults with cardiometabolic disease in the United States: 2005-2012. PloS One. 2017;12(6):e0179172.
10. Seligman HK, Laraia BA, Kushel MB. Food insecurity is associated with chronic disease among low-income NHANES participants. J Nutr. 2010;140(2):304-310.
11. Berkowitz SA, Baggett TP, Wexler DJ, Huskey KW, Wee CC. Food insecurity and metabolic control among U.S. adults with diabetes. Diabetes Care. 2013;36(10):3093-3099.
12. Seligman HK, Jacobs EA, López A, Tschann J, Fernandez A. Food insecurity and glycemic control among low-income patients with type 2 diabetes. Diabetes Care. 2012;35(2):233-238.
13. Banerjee T, Crews DC, Wesson DE, et al; CDC CKD Surveillance Team. Food insecurity, CKD, and subsequent ESRD in US adults. Am J Kidney Dis. 2017;70(1):38-47.
14. Bruening M, Dinour LM, Chavez JBR. Food insecurity and emotional health in the USA: a systematic narrative review of longitudinal research. Public Health Nutr. 2017;20(17):3200-3208.
15. Berkowitz SA, Basu S, Meigs JB, Seligman HK. Food insecurity and health care expenditures in the United States, 2011-2013. Health Serv Res. 2018;53(3):1600-1620.
16. Berkowitz SA, Seligman HK, Basu S. Impact of food insecurity and SNAP participation on healthcare utilization and expenditures. http://www.ukcpr.org/research/discussion-papers. Published 2017. Accessed December 9, 2019.
17. Kushel MB, Gupta R, Gee L, Haas JS. Housing instability and food insecurity as barriers to health care among low-income Americans. J Gen Intern Med. 2006;21(1):71-77.
18. Garcia SP, Haddix A, Barnett K. Incremental health care costs associated with food insecurity and chronic conditions among older adults. Chronic Dis. 2018;15:180058.
19. Berkowitz SA, Seligman HK, Meigs JB, Basu S. Food insecurity, healthcare utilization, and high cost: a longitudinal cohort study. Am J Manag Care. 2018;24(9):399-404.
20. Larson NI, Story MT, Nelson MC. Neighborhood environments: disparities in access to healthy foods in the U.S. Am J Prev Med. 2009;36(1):74-81.
21. Darmon N, Drewnowski A. Contribution of food prices and diet cost to socioeconomic disparities in diet quality and health: a systematic review and analysis. Nutr Rev. 2015;73(10):643-660.
22. Darmon N, Drewnowski A. Does social class predict diet quality? Am J Clin Nutr. 2008;87(5):1107-1117.
23. Drewnowski A. The cost of US foods as related to their nutritive value. Am J Clin Nutr. 2010;92(5):1181-1188.
24. Lucan SC, Maroko AR, Seitchik JL, Yoon DH, Sperry LE, Schechter CB. Unexpected neighborhood sources of food and drink: implications for research and community health. Am J Prev Med. 2018;55(2):e29-e38.
25. Castillo DC, Ramsey NL, Yu SS, Ricks M, Courville AB, Sumner AE. Inconsistent access to food and cardiometabolic disease: the effect of food insecurity. Curr Cardiovasc Risk Rep. 2012;6(3):245-250.
26. Seligman HK, Davis TC, Schillinger D, Wolf MS. Food insecurity is associated with hypoglycemia and poor diabetes self-management in a low-income sample with diabetes. J Health Care Poor Underserved. 2010;21(4):1227-1233.
27. Siefert K, Heflin CM, Corcoran ME, Williams DR. Food insufficiency and physical and mental health in a longitudinal survey of welfare recipients. J Health Soc Behav. 2004;45(2):171-186.
28. Mangurian C, Sreshta N, Seligman H. Food insecurity among adults with severe mental illness. Psychiatr Serv. 2013;64(9):931-932.
29. Melchior M, Caspi A, Howard LM, et al. Mental health context of food insecurity: a representative cohort of families with young children. Pediatrics. 2009;124(4):e564-e572.
30. Brostow DP, Gunzburger E, Abbate LM, Brenner LA, Thomas KS. Mental illness, not obesity status, is associated with food insecurity among the elderly in the health and retirement study. J Nutr Gerontol Geriatr. 2019;38(2):149-172.
31. Higashi RT, Craddock Lee SJ, Pezzia C, Quirk L, Leonard T, Pruitt SL. Family and social context contributes to the interplay of economic insecurity, food insecurity, and health. Ann Anthropol Pract. 2017;41(2):67-77.
32. O’Toole TP, Roberts CB, Johnson EE. Screening for food insecurity in six Veterans Administration clinics for the homeless, June-December 2015. Prev Chronic Dis. 2017;14:160375.
33. Feil DG, Pogach LM. Cognitive impairment is a major risk factor for serious hypoglycaemia; public health intervention is warranted. Evid Based Med. 2014;19(2):77.
34. Frith E, Loprinzi PD. Food insecurity and cognitive function in older adults: Brief report. Clin Nutr. 2018;37(5):1765-1768.
35. Herman D, Afulani P, Coleman-Jensen A, Harrison GG. Food insecurity and cost-related medication underuse among nonelderly adults in a nationally representative sample. Am J Public Health. 2015;105(10):e48-e59.
36. Tseng C-L, Soroka O, Maney M, Aron DC, Pogach LM. Assessing potential glycemic overtreatment in persons at hypoglycemic risk. JAMA Intern Med. 2014;174(2):259-268.
37. Vue MH, Setter SM. Drug-induced glucose alterations part 1: drug-induced hypoglycemia. Diabetes Spectr. 2011;24(3):171-177.
38. Seligman HK, Bolger AF, Guzman D, López A, Bibbins-Domingo K. Exhaustion of food budgets at month’s end and hospital admissions for hypoglycemia. Health Aff (Millwood). 2014;33(1):116-123.
39. US Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. Veteran poverty trends. https://www.va.gov/vetdata/docs/specialreports/veteran_poverty_trends.pdf. Published May 2015. Accessed December 9, 2019.
40. Robbins KG, Ravi A. Veterans living paycheck to paycheck are under threat during budget debates. https://www.americanprogress.org/issues/poverty/news/2017/09/19/439023/veterans-living-paycheck-paycheck-threat-budget-debates. Published September 19, 2017. Accessed December 9, 2019.
41. Wilmoth JM, London AS, Heflin CM. Economic well-being among older-adult households: variation by veteran and disability status. J Gerontol Soc Work. 2015;58(4):399-419.
42. Brostow DP, Gunzburger E, Thomas KS. Food insecurity among veterans: findings from the health and retirement study. J Nutr Health Aging. 2017;21(10):1358-1364.
43. Pooler J, Mian P, Srinivasan M, Miller Z. Veterans and food insecurity. https://www.impaqint.com/sites/default/files/issue-briefs/VeteransFoodInsecurity_IssueBrief_V1.3.pdf. Published November 2018. Accessed December 9, 2019.
44. Schure MB, Katon JG, Wong E, Liu C-F. Food and housing insecurity and health status among U.S. adults with and without prior military service. SSM Popul Health. 2016;29(2):244-248.
45. Miller DP, Larson MJ, Byrne T, DeVoe E. Food insecurity in veteran households: findings from nationally representative data. Public Health Nutr. 2016;19(10):1731-1740.
46. Widome R, Jensen A, Bangerter A, Fu SS. Food insecurity among veterans of the US wars in Iraq and Afghanistan. Public Health Nutr. 2015;18(5):844-849.
47. London AS, Heflin CM. Supplemental Nutrition Assistance Program (SNAP) use among active-duty military personnel, veterans, and reservists. Popul Res Policy Rev. 2015;34(6):805-826.
48. Weinfield NS, Mills G, Borger C, et al. Hunger in America 2014. Natl rep prepared for Feeding America. https://www.feedingamerica.org/research/hunger-in-america. Published 2014. Accessed December 9, 2019.
49. Mabli J, Ohls J, Dragoset L, Castner L, Santos B. Measuring the Effect of Supplemental Nutrition Assistance Program (SNAP) Participation on Food Security. Washington, DC: US Department of Agriculture, Food and Nutrition Service; 2013.
50. Srinivasan M, Pooler JA. Cost-related medication nonadherence for older adults participating in SNAP, 2013–2015. Am J Public Health. 2017;108(2):224-230.
51. Heflin C, Hodges L, Mueser P. Supplemental Nutrition Assistance Progam benefits and emergency room visits for hypoglycaemia. Public Health Nutr. 2017;20(7):1314-1321.
52. Samuel LJ, Szanton SL, Cahill R, et al. Does the Supplemental Nutrition Assistance Program affect hospital utilization among older adults? The case of Maryland. Popul Health Manag. 2018;21(2):88-95.
53. Szanton SL, Samuel LJ, Cahill R, et al. Food assistance is associated with decreased nursing home admissions for Maryland’s dually eligible older adults. BMC Geriatr. 2017;17(1):162.
54. Carlson S, Keith-Jennings B. SNAP is linked with improved nutritional outcomes and lower health care costs. https://www.cbpp.org/research/food-assistance/snap-is-linked-with-improved-nutritional-outcomes-and-lower-health-care. Published January 17, 2018. Accessed December 10, 2019.
55. Keith-Jennings B, Cai L. SNAP helps almost 1.4 million low-income veterans, including thousands in every state. https://www.cbpp.org/research/food-assistance/snap-helps-almost-14-million-low-income-veterans-including-thousands-in. Updated November 8, 2018. Accessed December 10, 2019.
56. US Department of Health and Human Services. Older Americans Act nutrition programs. https://acl.gov/sites/default/files/news%202017-03/OAA-Nutrition_Programs_Fact_Sheet.pdf. Accessed December 10, 2019.
57. US Department of Veterans Affairs. About VHA. https://www.va.gov/health/aboutvha.asp. Accessed December 10, 2019.
58. US Department of Veterans Affairs. VA Corporate Data Warehouse.
59. Yano EM, Bair MJ, Carrasquillo O, Krein SL, Rubenstein LV. Patient aligned care teams (PACT): VA’s journey to implement patient-centered medical homes. J Gen Intern Med. 2014;29(suppl 2):S547-s549.
60. O’Toole TP, Pape L. Innovative efforts to address homelessness among veterans. N C Med J. 2015;76(5):311-314.
61. O’Toole TP, Johnson EE, Aiello R, Kane V, Pape L. Tailoring care to vulnerable populations by incorporating social determinants of health: the Veterans Health Administration’s “Homeless Patient Aligned Care Team” Program. Prev Chronic Dis. 2016;13:150567.
62. Marpadga S, Fernandez A, Leung J, Tang A, Seligman H, Murphy EJ. Challenges and successes with food resource referrals for food-insecure patients with diabetes. Perm J. 2019;23.
63. Stenmark SH, Steiner JF, Marpadga S, Debor M, Underhill K, Seligman H. Lessons learned from implementation of the food insecurity screening and referral program at Kaiser Permanente Colorado. Perm J. 2018;22.
64. Martel ML, Klein LR, Hager KA, Cutts DB. Emergency department experience with novel electronic medical record order for referral to food resources. West J Emerg Med. 2018;19(2):232-237.
65. Going C, Cohen AJ, Bares M, Christensen M. Interdisciplinary approaches to addressing the food insecure veteran. Veterans Health Administration Employee Education System webinar; October 30, 2018.
66. Feeding America Announces New Partnership With U.S. Department Of Veterans Affairs. https://www.prnewswire.com/news-releases/feeding-america-announces-new-partnership-with-us-department-of-veterans-affairs-300481891.html. Published June 29, 2017. Accessed December 10, 2019.
67. US Department of Veterans Affairs. State Veterans Affairs offices. https://www.va.gov/statedva.htm. Updated March 20, 2019. Accessed December 10, 2019.
68. US Department of Veterans Affairs. Directory of veterans service organizations. https://www.va.gov/vso. Updated December 24, 2013. Accessed December 10, 2019.
69. ACL Administration for Community Living. Aging and disability resource centers. https://acl.gov/programs/aging-and-disability-networks/aging-and-disability-resource-centers. Updated December 13, 2017. Accessed December 10, 2019.
70. Nutrition and Obesity Policy Research and Evaluation Network (NOPREN). Clinical screening algorithms. https://nopren.org/resource/download-food-insecurity-screening-and-referral-algorithms-for-adults-patients-living-with-diabetes-and-pediatric-patients. Accessed December 10, 2019.
Nearly 1 in 8 households—and 1 in 6 households with children—experienced food insecurity in 2017, defined as limited or uncertain availability of nutritionally adequate and safe foods.1 Food insecurity is often even more pronounced among households with individuals with acute or chronic medical conditions.2-6 Moreover, food insecurity is independently associated with a range of adverse health outcomes, including poorer control of diabetes mellitus, hypertension, depression and other major psychiatric disorders, HIV, and chronic lung and kidney disease, as well as poorer overall health status.7-14 Food insecurity also has been associated with increased health care costs and acute care utilization as well as increased probability of delayed or missed care.15-19
The relationship between food insecurity and poor health outcomes is a complex and often cyclic phenomenon (Figure 1). Poor nutritional status is fueled by limited access to healthful foods as well as increased reliance on calorie-dense and nutrient-poor “junk” foods, which are less expensive and often more readily available in low-income neighborhoods.5,20-24 These compensatory dietary patterns place individuals at higher risk for developing cardiometabolic conditions and for poor control of these conditions.5,8,9,12,25,26 Additionally, the physiological and psychological stressors of food insecurity may precipitate depression and anxiety or worsen existing mental health conditions, resulting in feelings of overwhelm and decreased self-management capacity.5,8,27-31 Food insecurity has further been associated with poor sleep, declines in cognitive function, and increased falls, particularly among the frail and elderly.32-34
Individuals experiencing food insecurity often report having to make trade-offs between food and other necessities, such as paying rent or utilities. Additional strategies to stretch limited resources include cost-related underuse of medication and delays in needed medical care.4,17,31,35 In a nationally representative survey among adults with at least 1 chronic medical condition, 1 in 3 reported having to choose between food and medicine; 11% were unable to afford either.3 Furthermore, the inability to reliably adhere to medication regimens that need to be taken with food can result in potentially life-threatening hypoglycemia (as can lack of food regardless of medication use).5,26,36 In addition to the more obvious risks of glucose-lowering medications, such as insulin and long-acting sulfonylureas in patients experiencing food insecurity, many drugs commonly used among nondiabetic adults such as ACE-inhibitors, β blockers, quinolones, and salicylates can also precipitate hypoglycemia, and food insecurity has been associated with experiences of hypoglycemia even among individuals without diabetes mellitus.32,37 In one study the risk for hospital admissions for hypoglycemia among low-income populations increased by 27% at the end of the month when food budgets were more likely to be exhausted.38 Worsening health status and increased emergency department visits and hospitalizations may then result in lost wages and mounting medical bills, contributing to further financial strain and worsening food insecurity.
Prevalence and Importance of Food Insecurity Among US Veterans
Nearly 1.5 million veterans in the US are living below the federal poverty level (FPL).39 An additional 2.4 million veterans are living paycheck to paycheck at < 200% of the FPL.40 Veterans living in poverty are at even higher risk than nonveterans for food insecurity, homelessness, and other material hardship.41
Estimates of food insecurity among veterans vary widely, ranging from 6% to 24%—nearly twice that of the general US population.8,42-45 Higher rates of food insecurity have been reported among certain high-risk subgroups, including veterans who served in Iraq and Afghanistan (27%), female veterans (28%), homeless and formerly homeless veterans (49%), and veterans with serious mental illness (35%).6,32,43,46 Additional risk factors for food insecurity specific to veteran populations include younger age, having recently left active-duty military service, and lower final military paygrade.42,45-47 As in the general population, veteran food insecurity is associated with a range of adverse health outcomes, including poorer overall health status as well as increased probability of delayed or missed care.6,8,32,42-44,46
Even among veterans enrolled in federal food assistance programs, many still struggle to afford nutritionally adequate foods. As one example, in a study of mostly male homeless and formerly homeless veterans, O’Toole and colleagues found that nearly half of those reporting food insecurity were already receiving federal food assistance benefits, and 22% relied on emergency food resources.32 Of households served by Feeding America food pantries and meal programs, 20% have a member who has served in the US military.48
Federal Programs To Address Food Insecurity
There are several important federal food assistance programs designed to help alleviate food insecurity. The Supplemental Nutrition Assistance Program (SNAP, formerly the Food Stamp program) is the largest federal food assistance program and provides low-income Americans with cash benefits to purchase food. SNAP has been shown to substantially reduce food insecurity.7,49 The program also is associated with significant decreases in cost-related medication nonadherence as well as reductions in health care costs and both acute care and nursing home utilization.16,50-54 Although nearly 1.4 million veterans live in SNAP-enrolled households, 59% of eligible veterans are not enrolled.43,55 Closing this SNAP eligibility-enrollment gap, has been a focus of recent efforts to improve long-term food security among veterans. There also are several federal food assistance programs for households with children, including the Special Supplemental Nutrition Program for Women, Infants, and Children (WIC) and school meals programs. Among federal nutrition programs for seniors, the Older American’s Act contains designated funding to support nutrition services for older adults, including congregate meal programs in community settings like senior centers, places of worship, and housing communities, and home-delivered meals through programs like Meals on Wheels.56
VHA Response to Food Insecurity
The Veterans Health Administration (VHA) is the country’s largest integrated, federally funded health care system.57 In November 2015, congressional briefings on veteran food insecurity organized by the national non-profit organization MAZON: A Jewish Response to Hunger and hosted with bipartisan support were provided to the US House and Senate. As a result of these briefings, VHA chartered the national Ensuring Veteran Food Security Workgroup with a mandate to partner with governmental and nonprofit agencies to “focus on the issue of food insecurity, the identification of veterans at risk, the needed training of VHA staff and the coordination of resources and initiatives to support the veterans for whom we care.” Building off a pilot in US Department of Veterans Affairs (VA) Homeless Patient Aligned Care Teams (H-PACTs),32 VHA subsequently integrated a single-item food insecurity screening tool into the VA electronic health record (EHR) clinical reminder system (Figure 2). The clinical reminder, which was rolled out across VA medical centers nationally in October 2017, provides an alert to screen all noninstitutionalized veterans for food insecurity. To date, nearly 5 million veterans have been screened. When a veteran endorses food insecurity based on the initial screening question, a prompt appears to offer the veteran a referral to a social worker and/or dietitian. Positive screening results also should be communicated to the patient’s primary care provider. Depending on site-specific clinical flow, the reminders are typically completed in the outpatient setting either by nurses or medical assistants during intake or by providers as part of the clinical visit. However, any member of the health care team can complete the clinical reminder at any time. As of September 2019, approximately 74,000 veterans have been identified as food insecure.58
Addressing Food Insecurity
VHA has been a recognized leader in addressing homelessness and other social determinants of health through its integrated care and PACT delivery models.59-61 The food insecurity clinical reminder was designed to facilitate a tailored, interdisciplinary approach to identify and address food insecurity. Interdisciplinary care team members—including medical assistants, clinicians, social workers, registered dietitians, nurse care managers, occupational or physical therapists, and pharmacists—are uniquely positioned to identify veterans impacted by food insecurity, assess for associated clinical and/or social risk factors, and offer appropriate medical and nutrition interventions and resource referrals.
This interdisciplinary team-based model is essential given the range of potential drivers underlying veteran experiences of food insecurity and subsequent health outcomes. It is critically important for clinicians to review the medication list with veterans screening positive for food insecurity to assess for risk of hypoglycemia and/or cost-related nonadherence, make any necessary adjustments to therapeutic regimens, and assess for additional risk factors associated with food insecurity. Examples of tailored nutrition counseling that clinical dietitians may provide include meal preparation strategies for veterans who only have access to a microwave or hotplate, or recommendations for how veterans on medically restricted diets can best navigate food selection at soup kitchens or food pantries. Resource referrals provided by social workers or other care team members may include both emergency food resources to address immediate shortages (eg, food pantries, soup kitchens, or vouchers for free lunch) as well as resources focused on improving longer term food security (eg, federal food assistance programs or home delivered meal programs). Importantly, although providing a list of food resources may be helpful for some patients, such lists are often insufficient.62,63 Many patients require active assistance with program enrollment either onsite the day of their clinic visit or through connection with a partnering community-based organization that can, in turn, identify appropriate resources and facilitate program enrollment.63,64 Planned follow-up is also crucial to determine whether referrals are successful and to assess for ongoing need. Proposed roles for interdisciplinary care team members in addressing a positive food insecurity screen are outlined in Table 1.
VHA-Community Partnerships
In addition to services offered within VA, public and private sector partnerships can greatly enhance the range of resources available to food insecure veterans. Several VA facilities have developed formal community partnerships, such as the Veterans Pantry Pilot (VPP) program, a national partnership between Feeding America food banks and VA medical centers to establish onsite or mobile food pantries. There are currently 17 active Feeding America VPP sites, with a number of additional sites under development. Several of the VPP sites also include other “wraparound services,” such as SNAP application assistance.65,66
State Veterans Affairs offices67 and Veterans Service Organizations (VSOs)68 also can serve as valuable partners for connecting veterans with needed resources. VSOs offer a range of services, including assistancewith benefit claims, employment and housing assistance, emergency food assistance, and transportation to medical appointments. Some VSOs also have established local affiliations with Meals on Wheels focused on veteran outreach and providing hot meals for low-income, homebound, and disabled veterans.
Additional Resources
Although resources vary by regional setting, several key governmental and community-based food assistance programs are summarized in Table 2. Local community partners and online/phone-based directories, such as United Way’s 2-1-1 can help identify additional local resources. For older adults and individuals with disabilities, local Aging and Disability Resources Centers can provide information and assistance connecting to needed resources.69 Finally, there are a number of online resources available for clinicians interested in learning more about the impact of food insecurity on health and tools to use in the clinical setting (Table 3).
Conclusion
The VA has recognized food insecurity as a critical concern for the well-being of our nation’s veterans. Use of the EHR clinical reminder represents a crucial first step toward increasing provider awareness about veteran food insecurity and improving clinical efforts to address food insecurity once identified. Through the reminder, health care teams can connect veterans to needed resources and create both the individual and population-level data necessary to inform VHA and community efforts to address veteran food insecurity. Clinical reminder data are currently being used for local quality improvement efforts and have established the need nationally for formalized partnerships between VHA Social Work Services and Nutrition and Food Services to connect veterans with food and provide them with strategies to best use available food resources.
Moving forward, the Ensuring Veteran Food Security Workgroup continues to work with agencies and organizations across the country to improve food insecure veterans’ access to needed services. In addition to existing VA partnerships with Feeding America for the VPP, memorandums of understanding are currently underway to formalize partnerships with both the Food Research and Action Center (FRAC) and MAZON. Additional research is needed both to formally validate the current food insecurity clinical reminder screening question and to identify best practices and potential models for how to most effectively use VHA-community partnerships to address the unique needs of the veteran population.
Ensuring the food security of our nation’s veterans is essential to VA’s commitment to providing integrated, veteran-centered, whole person care. Toward that goal, VA health care teams are urged to use the clinical reminder and help connect food insecure veterans with relevant resources both within and outside of the VA health care system.
Nearly 1 in 8 households—and 1 in 6 households with children—experienced food insecurity in 2017, defined as limited or uncertain availability of nutritionally adequate and safe foods.1 Food insecurity is often even more pronounced among households with individuals with acute or chronic medical conditions.2-6 Moreover, food insecurity is independently associated with a range of adverse health outcomes, including poorer control of diabetes mellitus, hypertension, depression and other major psychiatric disorders, HIV, and chronic lung and kidney disease, as well as poorer overall health status.7-14 Food insecurity also has been associated with increased health care costs and acute care utilization as well as increased probability of delayed or missed care.15-19
The relationship between food insecurity and poor health outcomes is a complex and often cyclic phenomenon (Figure 1). Poor nutritional status is fueled by limited access to healthful foods as well as increased reliance on calorie-dense and nutrient-poor “junk” foods, which are less expensive and often more readily available in low-income neighborhoods.5,20-24 These compensatory dietary patterns place individuals at higher risk for developing cardiometabolic conditions and for poor control of these conditions.5,8,9,12,25,26 Additionally, the physiological and psychological stressors of food insecurity may precipitate depression and anxiety or worsen existing mental health conditions, resulting in feelings of overwhelm and decreased self-management capacity.5,8,27-31 Food insecurity has further been associated with poor sleep, declines in cognitive function, and increased falls, particularly among the frail and elderly.32-34
Individuals experiencing food insecurity often report having to make trade-offs between food and other necessities, such as paying rent or utilities. Additional strategies to stretch limited resources include cost-related underuse of medication and delays in needed medical care.4,17,31,35 In a nationally representative survey among adults with at least 1 chronic medical condition, 1 in 3 reported having to choose between food and medicine; 11% were unable to afford either.3 Furthermore, the inability to reliably adhere to medication regimens that need to be taken with food can result in potentially life-threatening hypoglycemia (as can lack of food regardless of medication use).5,26,36 In addition to the more obvious risks of glucose-lowering medications, such as insulin and long-acting sulfonylureas in patients experiencing food insecurity, many drugs commonly used among nondiabetic adults such as ACE-inhibitors, β blockers, quinolones, and salicylates can also precipitate hypoglycemia, and food insecurity has been associated with experiences of hypoglycemia even among individuals without diabetes mellitus.32,37 In one study the risk for hospital admissions for hypoglycemia among low-income populations increased by 27% at the end of the month when food budgets were more likely to be exhausted.38 Worsening health status and increased emergency department visits and hospitalizations may then result in lost wages and mounting medical bills, contributing to further financial strain and worsening food insecurity.
Prevalence and Importance of Food Insecurity Among US Veterans
Nearly 1.5 million veterans in the US are living below the federal poverty level (FPL).39 An additional 2.4 million veterans are living paycheck to paycheck at < 200% of the FPL.40 Veterans living in poverty are at even higher risk than nonveterans for food insecurity, homelessness, and other material hardship.41
Estimates of food insecurity among veterans vary widely, ranging from 6% to 24%—nearly twice that of the general US population.8,42-45 Higher rates of food insecurity have been reported among certain high-risk subgroups, including veterans who served in Iraq and Afghanistan (27%), female veterans (28%), homeless and formerly homeless veterans (49%), and veterans with serious mental illness (35%).6,32,43,46 Additional risk factors for food insecurity specific to veteran populations include younger age, having recently left active-duty military service, and lower final military paygrade.42,45-47 As in the general population, veteran food insecurity is associated with a range of adverse health outcomes, including poorer overall health status as well as increased probability of delayed or missed care.6,8,32,42-44,46
Even among veterans enrolled in federal food assistance programs, many still struggle to afford nutritionally adequate foods. As one example, in a study of mostly male homeless and formerly homeless veterans, O’Toole and colleagues found that nearly half of those reporting food insecurity were already receiving federal food assistance benefits, and 22% relied on emergency food resources.32 Of households served by Feeding America food pantries and meal programs, 20% have a member who has served in the US military.48
Federal Programs To Address Food Insecurity
There are several important federal food assistance programs designed to help alleviate food insecurity. The Supplemental Nutrition Assistance Program (SNAP, formerly the Food Stamp program) is the largest federal food assistance program and provides low-income Americans with cash benefits to purchase food. SNAP has been shown to substantially reduce food insecurity.7,49 The program also is associated with significant decreases in cost-related medication nonadherence as well as reductions in health care costs and both acute care and nursing home utilization.16,50-54 Although nearly 1.4 million veterans live in SNAP-enrolled households, 59% of eligible veterans are not enrolled.43,55 Closing this SNAP eligibility-enrollment gap, has been a focus of recent efforts to improve long-term food security among veterans. There also are several federal food assistance programs for households with children, including the Special Supplemental Nutrition Program for Women, Infants, and Children (WIC) and school meals programs. Among federal nutrition programs for seniors, the Older American’s Act contains designated funding to support nutrition services for older adults, including congregate meal programs in community settings like senior centers, places of worship, and housing communities, and home-delivered meals through programs like Meals on Wheels.56
VHA Response to Food Insecurity
The Veterans Health Administration (VHA) is the country’s largest integrated, federally funded health care system.57 In November 2015, congressional briefings on veteran food insecurity organized by the national non-profit organization MAZON: A Jewish Response to Hunger and hosted with bipartisan support were provided to the US House and Senate. As a result of these briefings, VHA chartered the national Ensuring Veteran Food Security Workgroup with a mandate to partner with governmental and nonprofit agencies to “focus on the issue of food insecurity, the identification of veterans at risk, the needed training of VHA staff and the coordination of resources and initiatives to support the veterans for whom we care.” Building off a pilot in US Department of Veterans Affairs (VA) Homeless Patient Aligned Care Teams (H-PACTs),32 VHA subsequently integrated a single-item food insecurity screening tool into the VA electronic health record (EHR) clinical reminder system (Figure 2). The clinical reminder, which was rolled out across VA medical centers nationally in October 2017, provides an alert to screen all noninstitutionalized veterans for food insecurity. To date, nearly 5 million veterans have been screened. When a veteran endorses food insecurity based on the initial screening question, a prompt appears to offer the veteran a referral to a social worker and/or dietitian. Positive screening results also should be communicated to the patient’s primary care provider. Depending on site-specific clinical flow, the reminders are typically completed in the outpatient setting either by nurses or medical assistants during intake or by providers as part of the clinical visit. However, any member of the health care team can complete the clinical reminder at any time. As of September 2019, approximately 74,000 veterans have been identified as food insecure.58
Addressing Food Insecurity
VHA has been a recognized leader in addressing homelessness and other social determinants of health through its integrated care and PACT delivery models.59-61 The food insecurity clinical reminder was designed to facilitate a tailored, interdisciplinary approach to identify and address food insecurity. Interdisciplinary care team members—including medical assistants, clinicians, social workers, registered dietitians, nurse care managers, occupational or physical therapists, and pharmacists—are uniquely positioned to identify veterans impacted by food insecurity, assess for associated clinical and/or social risk factors, and offer appropriate medical and nutrition interventions and resource referrals.
This interdisciplinary team-based model is essential given the range of potential drivers underlying veteran experiences of food insecurity and subsequent health outcomes. It is critically important for clinicians to review the medication list with veterans screening positive for food insecurity to assess for risk of hypoglycemia and/or cost-related nonadherence, make any necessary adjustments to therapeutic regimens, and assess for additional risk factors associated with food insecurity. Examples of tailored nutrition counseling that clinical dietitians may provide include meal preparation strategies for veterans who only have access to a microwave or hotplate, or recommendations for how veterans on medically restricted diets can best navigate food selection at soup kitchens or food pantries. Resource referrals provided by social workers or other care team members may include both emergency food resources to address immediate shortages (eg, food pantries, soup kitchens, or vouchers for free lunch) as well as resources focused on improving longer term food security (eg, federal food assistance programs or home delivered meal programs). Importantly, although providing a list of food resources may be helpful for some patients, such lists are often insufficient.62,63 Many patients require active assistance with program enrollment either onsite the day of their clinic visit or through connection with a partnering community-based organization that can, in turn, identify appropriate resources and facilitate program enrollment.63,64 Planned follow-up is also crucial to determine whether referrals are successful and to assess for ongoing need. Proposed roles for interdisciplinary care team members in addressing a positive food insecurity screen are outlined in Table 1.
VHA-Community Partnerships
In addition to services offered within VA, public and private sector partnerships can greatly enhance the range of resources available to food insecure veterans. Several VA facilities have developed formal community partnerships, such as the Veterans Pantry Pilot (VPP) program, a national partnership between Feeding America food banks and VA medical centers to establish onsite or mobile food pantries. There are currently 17 active Feeding America VPP sites, with a number of additional sites under development. Several of the VPP sites also include other “wraparound services,” such as SNAP application assistance.65,66
State Veterans Affairs offices67 and Veterans Service Organizations (VSOs)68 also can serve as valuable partners for connecting veterans with needed resources. VSOs offer a range of services, including assistancewith benefit claims, employment and housing assistance, emergency food assistance, and transportation to medical appointments. Some VSOs also have established local affiliations with Meals on Wheels focused on veteran outreach and providing hot meals for low-income, homebound, and disabled veterans.
Additional Resources
Although resources vary by regional setting, several key governmental and community-based food assistance programs are summarized in Table 2. Local community partners and online/phone-based directories, such as United Way’s 2-1-1 can help identify additional local resources. For older adults and individuals with disabilities, local Aging and Disability Resources Centers can provide information and assistance connecting to needed resources.69 Finally, there are a number of online resources available for clinicians interested in learning more about the impact of food insecurity on health and tools to use in the clinical setting (Table 3).
Conclusion
The VA has recognized food insecurity as a critical concern for the well-being of our nation’s veterans. Use of the EHR clinical reminder represents a crucial first step toward increasing provider awareness about veteran food insecurity and improving clinical efforts to address food insecurity once identified. Through the reminder, health care teams can connect veterans to needed resources and create both the individual and population-level data necessary to inform VHA and community efforts to address veteran food insecurity. Clinical reminder data are currently being used for local quality improvement efforts and have established the need nationally for formalized partnerships between VHA Social Work Services and Nutrition and Food Services to connect veterans with food and provide them with strategies to best use available food resources.
Moving forward, the Ensuring Veteran Food Security Workgroup continues to work with agencies and organizations across the country to improve food insecure veterans’ access to needed services. In addition to existing VA partnerships with Feeding America for the VPP, memorandums of understanding are currently underway to formalize partnerships with both the Food Research and Action Center (FRAC) and MAZON. Additional research is needed both to formally validate the current food insecurity clinical reminder screening question and to identify best practices and potential models for how to most effectively use VHA-community partnerships to address the unique needs of the veteran population.
Ensuring the food security of our nation’s veterans is essential to VA’s commitment to providing integrated, veteran-centered, whole person care. Toward that goal, VA health care teams are urged to use the clinical reminder and help connect food insecure veterans with relevant resources both within and outside of the VA health care system.
1. Coleman-Jensen A, Rabbitt MP, Gregory CA, Singh A. Household food security in the United States in 2017. http://www.ers.usda.gov/publications/pub-details/?pubid=90022. Published September 2018. Accessed December 9, 2019.
2. Berkowitz SA, Meigs JB, DeWalt D, et al. Material need insecurities, control of diabetes mellitus, and use of health care resources: results of the Measuring Economic Insecurity in Diabetes study. JAMA Intern Med. 2015;175(2):257-265.
3. Berkowitz SA, Seligman HK, Choudhry NK. Treat or eat: food insecurity, cost-related medication underuse, and unmet needs. Am J Med. 2014;127(4):303-310.e3.
4. Lyles CR, Seligman HK, Parker MM, et al. Financial strain and medication adherence among diabetes patients in an integrated health care delivery system: The Diabetes Study of Northern California (DISTANCE). Health Serv Res. 2016;51(2):610-624.
5. Seligman HK, Schillinger D. Hunger and socioeconomic disparities in chronic disease. N Engl J Med. 2010;363(1):6-9.
6. Narain K, Bean-Mayberry B, Washington DL, Canelo IA, Darling JE, Yano EM. Access to care and health outcomes among women veterans using veterans administration health care: association with food insufficiency. Womens Health Issues. 2018;28(3):267-272.
7. Gundersen C, Ziliak JP. Food insecurity and health outcomes. Health Aff. 2015;34(11):1830-1839.
8. Wang EA, McGinnis KA, Goulet J, et al; Veterans Aging Cohort Study Project Team. Food insecurity and health: data from the Veterans Aging Cohort Study. Public Health Rep. 2015;130(3):261-268.
9. Berkowitz SA, Berkowitz TSZ, Meigs JB, Wexler DJ. Trends in food insecurity for adults with cardiometabolic disease in the United States: 2005-2012. PloS One. 2017;12(6):e0179172.
10. Seligman HK, Laraia BA, Kushel MB. Food insecurity is associated with chronic disease among low-income NHANES participants. J Nutr. 2010;140(2):304-310.
11. Berkowitz SA, Baggett TP, Wexler DJ, Huskey KW, Wee CC. Food insecurity and metabolic control among U.S. adults with diabetes. Diabetes Care. 2013;36(10):3093-3099.
12. Seligman HK, Jacobs EA, López A, Tschann J, Fernandez A. Food insecurity and glycemic control among low-income patients with type 2 diabetes. Diabetes Care. 2012;35(2):233-238.
13. Banerjee T, Crews DC, Wesson DE, et al; CDC CKD Surveillance Team. Food insecurity, CKD, and subsequent ESRD in US adults. Am J Kidney Dis. 2017;70(1):38-47.
14. Bruening M, Dinour LM, Chavez JBR. Food insecurity and emotional health in the USA: a systematic narrative review of longitudinal research. Public Health Nutr. 2017;20(17):3200-3208.
15. Berkowitz SA, Basu S, Meigs JB, Seligman HK. Food insecurity and health care expenditures in the United States, 2011-2013. Health Serv Res. 2018;53(3):1600-1620.
16. Berkowitz SA, Seligman HK, Basu S. Impact of food insecurity and SNAP participation on healthcare utilization and expenditures. http://www.ukcpr.org/research/discussion-papers. Published 2017. Accessed December 9, 2019.
17. Kushel MB, Gupta R, Gee L, Haas JS. Housing instability and food insecurity as barriers to health care among low-income Americans. J Gen Intern Med. 2006;21(1):71-77.
18. Garcia SP, Haddix A, Barnett K. Incremental health care costs associated with food insecurity and chronic conditions among older adults. Chronic Dis. 2018;15:180058.
19. Berkowitz SA, Seligman HK, Meigs JB, Basu S. Food insecurity, healthcare utilization, and high cost: a longitudinal cohort study. Am J Manag Care. 2018;24(9):399-404.
20. Larson NI, Story MT, Nelson MC. Neighborhood environments: disparities in access to healthy foods in the U.S. Am J Prev Med. 2009;36(1):74-81.
21. Darmon N, Drewnowski A. Contribution of food prices and diet cost to socioeconomic disparities in diet quality and health: a systematic review and analysis. Nutr Rev. 2015;73(10):643-660.
22. Darmon N, Drewnowski A. Does social class predict diet quality? Am J Clin Nutr. 2008;87(5):1107-1117.
23. Drewnowski A. The cost of US foods as related to their nutritive value. Am J Clin Nutr. 2010;92(5):1181-1188.
24. Lucan SC, Maroko AR, Seitchik JL, Yoon DH, Sperry LE, Schechter CB. Unexpected neighborhood sources of food and drink: implications for research and community health. Am J Prev Med. 2018;55(2):e29-e38.
25. Castillo DC, Ramsey NL, Yu SS, Ricks M, Courville AB, Sumner AE. Inconsistent access to food and cardiometabolic disease: the effect of food insecurity. Curr Cardiovasc Risk Rep. 2012;6(3):245-250.
26. Seligman HK, Davis TC, Schillinger D, Wolf MS. Food insecurity is associated with hypoglycemia and poor diabetes self-management in a low-income sample with diabetes. J Health Care Poor Underserved. 2010;21(4):1227-1233.
27. Siefert K, Heflin CM, Corcoran ME, Williams DR. Food insufficiency and physical and mental health in a longitudinal survey of welfare recipients. J Health Soc Behav. 2004;45(2):171-186.
28. Mangurian C, Sreshta N, Seligman H. Food insecurity among adults with severe mental illness. Psychiatr Serv. 2013;64(9):931-932.
29. Melchior M, Caspi A, Howard LM, et al. Mental health context of food insecurity: a representative cohort of families with young children. Pediatrics. 2009;124(4):e564-e572.
30. Brostow DP, Gunzburger E, Abbate LM, Brenner LA, Thomas KS. Mental illness, not obesity status, is associated with food insecurity among the elderly in the health and retirement study. J Nutr Gerontol Geriatr. 2019;38(2):149-172.
31. Higashi RT, Craddock Lee SJ, Pezzia C, Quirk L, Leonard T, Pruitt SL. Family and social context contributes to the interplay of economic insecurity, food insecurity, and health. Ann Anthropol Pract. 2017;41(2):67-77.
32. O’Toole TP, Roberts CB, Johnson EE. Screening for food insecurity in six Veterans Administration clinics for the homeless, June-December 2015. Prev Chronic Dis. 2017;14:160375.
33. Feil DG, Pogach LM. Cognitive impairment is a major risk factor for serious hypoglycaemia; public health intervention is warranted. Evid Based Med. 2014;19(2):77.
34. Frith E, Loprinzi PD. Food insecurity and cognitive function in older adults: Brief report. Clin Nutr. 2018;37(5):1765-1768.
35. Herman D, Afulani P, Coleman-Jensen A, Harrison GG. Food insecurity and cost-related medication underuse among nonelderly adults in a nationally representative sample. Am J Public Health. 2015;105(10):e48-e59.
36. Tseng C-L, Soroka O, Maney M, Aron DC, Pogach LM. Assessing potential glycemic overtreatment in persons at hypoglycemic risk. JAMA Intern Med. 2014;174(2):259-268.
37. Vue MH, Setter SM. Drug-induced glucose alterations part 1: drug-induced hypoglycemia. Diabetes Spectr. 2011;24(3):171-177.
38. Seligman HK, Bolger AF, Guzman D, López A, Bibbins-Domingo K. Exhaustion of food budgets at month’s end and hospital admissions for hypoglycemia. Health Aff (Millwood). 2014;33(1):116-123.
39. US Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. Veteran poverty trends. https://www.va.gov/vetdata/docs/specialreports/veteran_poverty_trends.pdf. Published May 2015. Accessed December 9, 2019.
40. Robbins KG, Ravi A. Veterans living paycheck to paycheck are under threat during budget debates. https://www.americanprogress.org/issues/poverty/news/2017/09/19/439023/veterans-living-paycheck-paycheck-threat-budget-debates. Published September 19, 2017. Accessed December 9, 2019.
41. Wilmoth JM, London AS, Heflin CM. Economic well-being among older-adult households: variation by veteran and disability status. J Gerontol Soc Work. 2015;58(4):399-419.
42. Brostow DP, Gunzburger E, Thomas KS. Food insecurity among veterans: findings from the health and retirement study. J Nutr Health Aging. 2017;21(10):1358-1364.
43. Pooler J, Mian P, Srinivasan M, Miller Z. Veterans and food insecurity. https://www.impaqint.com/sites/default/files/issue-briefs/VeteransFoodInsecurity_IssueBrief_V1.3.pdf. Published November 2018. Accessed December 9, 2019.
44. Schure MB, Katon JG, Wong E, Liu C-F. Food and housing insecurity and health status among U.S. adults with and without prior military service. SSM Popul Health. 2016;29(2):244-248.
45. Miller DP, Larson MJ, Byrne T, DeVoe E. Food insecurity in veteran households: findings from nationally representative data. Public Health Nutr. 2016;19(10):1731-1740.
46. Widome R, Jensen A, Bangerter A, Fu SS. Food insecurity among veterans of the US wars in Iraq and Afghanistan. Public Health Nutr. 2015;18(5):844-849.
47. London AS, Heflin CM. Supplemental Nutrition Assistance Program (SNAP) use among active-duty military personnel, veterans, and reservists. Popul Res Policy Rev. 2015;34(6):805-826.
48. Weinfield NS, Mills G, Borger C, et al. Hunger in America 2014. Natl rep prepared for Feeding America. https://www.feedingamerica.org/research/hunger-in-america. Published 2014. Accessed December 9, 2019.
49. Mabli J, Ohls J, Dragoset L, Castner L, Santos B. Measuring the Effect of Supplemental Nutrition Assistance Program (SNAP) Participation on Food Security. Washington, DC: US Department of Agriculture, Food and Nutrition Service; 2013.
50. Srinivasan M, Pooler JA. Cost-related medication nonadherence for older adults participating in SNAP, 2013–2015. Am J Public Health. 2017;108(2):224-230.
51. Heflin C, Hodges L, Mueser P. Supplemental Nutrition Assistance Progam benefits and emergency room visits for hypoglycaemia. Public Health Nutr. 2017;20(7):1314-1321.
52. Samuel LJ, Szanton SL, Cahill R, et al. Does the Supplemental Nutrition Assistance Program affect hospital utilization among older adults? The case of Maryland. Popul Health Manag. 2018;21(2):88-95.
53. Szanton SL, Samuel LJ, Cahill R, et al. Food assistance is associated with decreased nursing home admissions for Maryland’s dually eligible older adults. BMC Geriatr. 2017;17(1):162.
54. Carlson S, Keith-Jennings B. SNAP is linked with improved nutritional outcomes and lower health care costs. https://www.cbpp.org/research/food-assistance/snap-is-linked-with-improved-nutritional-outcomes-and-lower-health-care. Published January 17, 2018. Accessed December 10, 2019.
55. Keith-Jennings B, Cai L. SNAP helps almost 1.4 million low-income veterans, including thousands in every state. https://www.cbpp.org/research/food-assistance/snap-helps-almost-14-million-low-income-veterans-including-thousands-in. Updated November 8, 2018. Accessed December 10, 2019.
56. US Department of Health and Human Services. Older Americans Act nutrition programs. https://acl.gov/sites/default/files/news%202017-03/OAA-Nutrition_Programs_Fact_Sheet.pdf. Accessed December 10, 2019.
57. US Department of Veterans Affairs. About VHA. https://www.va.gov/health/aboutvha.asp. Accessed December 10, 2019.
58. US Department of Veterans Affairs. VA Corporate Data Warehouse.
59. Yano EM, Bair MJ, Carrasquillo O, Krein SL, Rubenstein LV. Patient aligned care teams (PACT): VA’s journey to implement patient-centered medical homes. J Gen Intern Med. 2014;29(suppl 2):S547-s549.
60. O’Toole TP, Pape L. Innovative efforts to address homelessness among veterans. N C Med J. 2015;76(5):311-314.
61. O’Toole TP, Johnson EE, Aiello R, Kane V, Pape L. Tailoring care to vulnerable populations by incorporating social determinants of health: the Veterans Health Administration’s “Homeless Patient Aligned Care Team” Program. Prev Chronic Dis. 2016;13:150567.
62. Marpadga S, Fernandez A, Leung J, Tang A, Seligman H, Murphy EJ. Challenges and successes with food resource referrals for food-insecure patients with diabetes. Perm J. 2019;23.
63. Stenmark SH, Steiner JF, Marpadga S, Debor M, Underhill K, Seligman H. Lessons learned from implementation of the food insecurity screening and referral program at Kaiser Permanente Colorado. Perm J. 2018;22.
64. Martel ML, Klein LR, Hager KA, Cutts DB. Emergency department experience with novel electronic medical record order for referral to food resources. West J Emerg Med. 2018;19(2):232-237.
65. Going C, Cohen AJ, Bares M, Christensen M. Interdisciplinary approaches to addressing the food insecure veteran. Veterans Health Administration Employee Education System webinar; October 30, 2018.
66. Feeding America Announces New Partnership With U.S. Department Of Veterans Affairs. https://www.prnewswire.com/news-releases/feeding-america-announces-new-partnership-with-us-department-of-veterans-affairs-300481891.html. Published June 29, 2017. Accessed December 10, 2019.
67. US Department of Veterans Affairs. State Veterans Affairs offices. https://www.va.gov/statedva.htm. Updated March 20, 2019. Accessed December 10, 2019.
68. US Department of Veterans Affairs. Directory of veterans service organizations. https://www.va.gov/vso. Updated December 24, 2013. Accessed December 10, 2019.
69. ACL Administration for Community Living. Aging and disability resource centers. https://acl.gov/programs/aging-and-disability-networks/aging-and-disability-resource-centers. Updated December 13, 2017. Accessed December 10, 2019.
70. Nutrition and Obesity Policy Research and Evaluation Network (NOPREN). Clinical screening algorithms. https://nopren.org/resource/download-food-insecurity-screening-and-referral-algorithms-for-adults-patients-living-with-diabetes-and-pediatric-patients. Accessed December 10, 2019.
1. Coleman-Jensen A, Rabbitt MP, Gregory CA, Singh A. Household food security in the United States in 2017. http://www.ers.usda.gov/publications/pub-details/?pubid=90022. Published September 2018. Accessed December 9, 2019.
2. Berkowitz SA, Meigs JB, DeWalt D, et al. Material need insecurities, control of diabetes mellitus, and use of health care resources: results of the Measuring Economic Insecurity in Diabetes study. JAMA Intern Med. 2015;175(2):257-265.
3. Berkowitz SA, Seligman HK, Choudhry NK. Treat or eat: food insecurity, cost-related medication underuse, and unmet needs. Am J Med. 2014;127(4):303-310.e3.
4. Lyles CR, Seligman HK, Parker MM, et al. Financial strain and medication adherence among diabetes patients in an integrated health care delivery system: The Diabetes Study of Northern California (DISTANCE). Health Serv Res. 2016;51(2):610-624.
5. Seligman HK, Schillinger D. Hunger and socioeconomic disparities in chronic disease. N Engl J Med. 2010;363(1):6-9.
6. Narain K, Bean-Mayberry B, Washington DL, Canelo IA, Darling JE, Yano EM. Access to care and health outcomes among women veterans using veterans administration health care: association with food insufficiency. Womens Health Issues. 2018;28(3):267-272.
7. Gundersen C, Ziliak JP. Food insecurity and health outcomes. Health Aff. 2015;34(11):1830-1839.
8. Wang EA, McGinnis KA, Goulet J, et al; Veterans Aging Cohort Study Project Team. Food insecurity and health: data from the Veterans Aging Cohort Study. Public Health Rep. 2015;130(3):261-268.
9. Berkowitz SA, Berkowitz TSZ, Meigs JB, Wexler DJ. Trends in food insecurity for adults with cardiometabolic disease in the United States: 2005-2012. PloS One. 2017;12(6):e0179172.
10. Seligman HK, Laraia BA, Kushel MB. Food insecurity is associated with chronic disease among low-income NHANES participants. J Nutr. 2010;140(2):304-310.
11. Berkowitz SA, Baggett TP, Wexler DJ, Huskey KW, Wee CC. Food insecurity and metabolic control among U.S. adults with diabetes. Diabetes Care. 2013;36(10):3093-3099.
12. Seligman HK, Jacobs EA, López A, Tschann J, Fernandez A. Food insecurity and glycemic control among low-income patients with type 2 diabetes. Diabetes Care. 2012;35(2):233-238.
13. Banerjee T, Crews DC, Wesson DE, et al; CDC CKD Surveillance Team. Food insecurity, CKD, and subsequent ESRD in US adults. Am J Kidney Dis. 2017;70(1):38-47.
14. Bruening M, Dinour LM, Chavez JBR. Food insecurity and emotional health in the USA: a systematic narrative review of longitudinal research. Public Health Nutr. 2017;20(17):3200-3208.
15. Berkowitz SA, Basu S, Meigs JB, Seligman HK. Food insecurity and health care expenditures in the United States, 2011-2013. Health Serv Res. 2018;53(3):1600-1620.
16. Berkowitz SA, Seligman HK, Basu S. Impact of food insecurity and SNAP participation on healthcare utilization and expenditures. http://www.ukcpr.org/research/discussion-papers. Published 2017. Accessed December 9, 2019.
17. Kushel MB, Gupta R, Gee L, Haas JS. Housing instability and food insecurity as barriers to health care among low-income Americans. J Gen Intern Med. 2006;21(1):71-77.
18. Garcia SP, Haddix A, Barnett K. Incremental health care costs associated with food insecurity and chronic conditions among older adults. Chronic Dis. 2018;15:180058.
19. Berkowitz SA, Seligman HK, Meigs JB, Basu S. Food insecurity, healthcare utilization, and high cost: a longitudinal cohort study. Am J Manag Care. 2018;24(9):399-404.
20. Larson NI, Story MT, Nelson MC. Neighborhood environments: disparities in access to healthy foods in the U.S. Am J Prev Med. 2009;36(1):74-81.
21. Darmon N, Drewnowski A. Contribution of food prices and diet cost to socioeconomic disparities in diet quality and health: a systematic review and analysis. Nutr Rev. 2015;73(10):643-660.
22. Darmon N, Drewnowski A. Does social class predict diet quality? Am J Clin Nutr. 2008;87(5):1107-1117.
23. Drewnowski A. The cost of US foods as related to their nutritive value. Am J Clin Nutr. 2010;92(5):1181-1188.
24. Lucan SC, Maroko AR, Seitchik JL, Yoon DH, Sperry LE, Schechter CB. Unexpected neighborhood sources of food and drink: implications for research and community health. Am J Prev Med. 2018;55(2):e29-e38.
25. Castillo DC, Ramsey NL, Yu SS, Ricks M, Courville AB, Sumner AE. Inconsistent access to food and cardiometabolic disease: the effect of food insecurity. Curr Cardiovasc Risk Rep. 2012;6(3):245-250.
26. Seligman HK, Davis TC, Schillinger D, Wolf MS. Food insecurity is associated with hypoglycemia and poor diabetes self-management in a low-income sample with diabetes. J Health Care Poor Underserved. 2010;21(4):1227-1233.
27. Siefert K, Heflin CM, Corcoran ME, Williams DR. Food insufficiency and physical and mental health in a longitudinal survey of welfare recipients. J Health Soc Behav. 2004;45(2):171-186.
28. Mangurian C, Sreshta N, Seligman H. Food insecurity among adults with severe mental illness. Psychiatr Serv. 2013;64(9):931-932.
29. Melchior M, Caspi A, Howard LM, et al. Mental health context of food insecurity: a representative cohort of families with young children. Pediatrics. 2009;124(4):e564-e572.
30. Brostow DP, Gunzburger E, Abbate LM, Brenner LA, Thomas KS. Mental illness, not obesity status, is associated with food insecurity among the elderly in the health and retirement study. J Nutr Gerontol Geriatr. 2019;38(2):149-172.
31. Higashi RT, Craddock Lee SJ, Pezzia C, Quirk L, Leonard T, Pruitt SL. Family and social context contributes to the interplay of economic insecurity, food insecurity, and health. Ann Anthropol Pract. 2017;41(2):67-77.
32. O’Toole TP, Roberts CB, Johnson EE. Screening for food insecurity in six Veterans Administration clinics for the homeless, June-December 2015. Prev Chronic Dis. 2017;14:160375.
33. Feil DG, Pogach LM. Cognitive impairment is a major risk factor for serious hypoglycaemia; public health intervention is warranted. Evid Based Med. 2014;19(2):77.
34. Frith E, Loprinzi PD. Food insecurity and cognitive function in older adults: Brief report. Clin Nutr. 2018;37(5):1765-1768.
35. Herman D, Afulani P, Coleman-Jensen A, Harrison GG. Food insecurity and cost-related medication underuse among nonelderly adults in a nationally representative sample. Am J Public Health. 2015;105(10):e48-e59.
36. Tseng C-L, Soroka O, Maney M, Aron DC, Pogach LM. Assessing potential glycemic overtreatment in persons at hypoglycemic risk. JAMA Intern Med. 2014;174(2):259-268.
37. Vue MH, Setter SM. Drug-induced glucose alterations part 1: drug-induced hypoglycemia. Diabetes Spectr. 2011;24(3):171-177.
38. Seligman HK, Bolger AF, Guzman D, López A, Bibbins-Domingo K. Exhaustion of food budgets at month’s end and hospital admissions for hypoglycemia. Health Aff (Millwood). 2014;33(1):116-123.
39. US Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. Veteran poverty trends. https://www.va.gov/vetdata/docs/specialreports/veteran_poverty_trends.pdf. Published May 2015. Accessed December 9, 2019.
40. Robbins KG, Ravi A. Veterans living paycheck to paycheck are under threat during budget debates. https://www.americanprogress.org/issues/poverty/news/2017/09/19/439023/veterans-living-paycheck-paycheck-threat-budget-debates. Published September 19, 2017. Accessed December 9, 2019.
41. Wilmoth JM, London AS, Heflin CM. Economic well-being among older-adult households: variation by veteran and disability status. J Gerontol Soc Work. 2015;58(4):399-419.
42. Brostow DP, Gunzburger E, Thomas KS. Food insecurity among veterans: findings from the health and retirement study. J Nutr Health Aging. 2017;21(10):1358-1364.
43. Pooler J, Mian P, Srinivasan M, Miller Z. Veterans and food insecurity. https://www.impaqint.com/sites/default/files/issue-briefs/VeteransFoodInsecurity_IssueBrief_V1.3.pdf. Published November 2018. Accessed December 9, 2019.
44. Schure MB, Katon JG, Wong E, Liu C-F. Food and housing insecurity and health status among U.S. adults with and without prior military service. SSM Popul Health. 2016;29(2):244-248.
45. Miller DP, Larson MJ, Byrne T, DeVoe E. Food insecurity in veteran households: findings from nationally representative data. Public Health Nutr. 2016;19(10):1731-1740.
46. Widome R, Jensen A, Bangerter A, Fu SS. Food insecurity among veterans of the US wars in Iraq and Afghanistan. Public Health Nutr. 2015;18(5):844-849.
47. London AS, Heflin CM. Supplemental Nutrition Assistance Program (SNAP) use among active-duty military personnel, veterans, and reservists. Popul Res Policy Rev. 2015;34(6):805-826.
48. Weinfield NS, Mills G, Borger C, et al. Hunger in America 2014. Natl rep prepared for Feeding America. https://www.feedingamerica.org/research/hunger-in-america. Published 2014. Accessed December 9, 2019.
49. Mabli J, Ohls J, Dragoset L, Castner L, Santos B. Measuring the Effect of Supplemental Nutrition Assistance Program (SNAP) Participation on Food Security. Washington, DC: US Department of Agriculture, Food and Nutrition Service; 2013.
50. Srinivasan M, Pooler JA. Cost-related medication nonadherence for older adults participating in SNAP, 2013–2015. Am J Public Health. 2017;108(2):224-230.
51. Heflin C, Hodges L, Mueser P. Supplemental Nutrition Assistance Progam benefits and emergency room visits for hypoglycaemia. Public Health Nutr. 2017;20(7):1314-1321.
52. Samuel LJ, Szanton SL, Cahill R, et al. Does the Supplemental Nutrition Assistance Program affect hospital utilization among older adults? The case of Maryland. Popul Health Manag. 2018;21(2):88-95.
53. Szanton SL, Samuel LJ, Cahill R, et al. Food assistance is associated with decreased nursing home admissions for Maryland’s dually eligible older adults. BMC Geriatr. 2017;17(1):162.
54. Carlson S, Keith-Jennings B. SNAP is linked with improved nutritional outcomes and lower health care costs. https://www.cbpp.org/research/food-assistance/snap-is-linked-with-improved-nutritional-outcomes-and-lower-health-care. Published January 17, 2018. Accessed December 10, 2019.
55. Keith-Jennings B, Cai L. SNAP helps almost 1.4 million low-income veterans, including thousands in every state. https://www.cbpp.org/research/food-assistance/snap-helps-almost-14-million-low-income-veterans-including-thousands-in. Updated November 8, 2018. Accessed December 10, 2019.
56. US Department of Health and Human Services. Older Americans Act nutrition programs. https://acl.gov/sites/default/files/news%202017-03/OAA-Nutrition_Programs_Fact_Sheet.pdf. Accessed December 10, 2019.
57. US Department of Veterans Affairs. About VHA. https://www.va.gov/health/aboutvha.asp. Accessed December 10, 2019.
58. US Department of Veterans Affairs. VA Corporate Data Warehouse.
59. Yano EM, Bair MJ, Carrasquillo O, Krein SL, Rubenstein LV. Patient aligned care teams (PACT): VA’s journey to implement patient-centered medical homes. J Gen Intern Med. 2014;29(suppl 2):S547-s549.
60. O’Toole TP, Pape L. Innovative efforts to address homelessness among veterans. N C Med J. 2015;76(5):311-314.
61. O’Toole TP, Johnson EE, Aiello R, Kane V, Pape L. Tailoring care to vulnerable populations by incorporating social determinants of health: the Veterans Health Administration’s “Homeless Patient Aligned Care Team” Program. Prev Chronic Dis. 2016;13:150567.
62. Marpadga S, Fernandez A, Leung J, Tang A, Seligman H, Murphy EJ. Challenges and successes with food resource referrals for food-insecure patients with diabetes. Perm J. 2019;23.
63. Stenmark SH, Steiner JF, Marpadga S, Debor M, Underhill K, Seligman H. Lessons learned from implementation of the food insecurity screening and referral program at Kaiser Permanente Colorado. Perm J. 2018;22.
64. Martel ML, Klein LR, Hager KA, Cutts DB. Emergency department experience with novel electronic medical record order for referral to food resources. West J Emerg Med. 2018;19(2):232-237.
65. Going C, Cohen AJ, Bares M, Christensen M. Interdisciplinary approaches to addressing the food insecure veteran. Veterans Health Administration Employee Education System webinar; October 30, 2018.
66. Feeding America Announces New Partnership With U.S. Department Of Veterans Affairs. https://www.prnewswire.com/news-releases/feeding-america-announces-new-partnership-with-us-department-of-veterans-affairs-300481891.html. Published June 29, 2017. Accessed December 10, 2019.
67. US Department of Veterans Affairs. State Veterans Affairs offices. https://www.va.gov/statedva.htm. Updated March 20, 2019. Accessed December 10, 2019.
68. US Department of Veterans Affairs. Directory of veterans service organizations. https://www.va.gov/vso. Updated December 24, 2013. Accessed December 10, 2019.
69. ACL Administration for Community Living. Aging and disability resource centers. https://acl.gov/programs/aging-and-disability-networks/aging-and-disability-resource-centers. Updated December 13, 2017. Accessed December 10, 2019.
70. Nutrition and Obesity Policy Research and Evaluation Network (NOPREN). Clinical screening algorithms. https://nopren.org/resource/download-food-insecurity-screening-and-referral-algorithms-for-adults-patients-living-with-diabetes-and-pediatric-patients. Accessed December 10, 2019.
Evaluating a Program Process Change to Improve Completion of Foot Exams and Amputation Risk Assessments for Veterans with Diabetes (FULL)
Individuals with diabetes mellitus (DM), peripheral vascular disease, or end-stage renal disease are at risk for a nontraumatic lower limb amputation.1 Veterans have a high number of risk factors and are especially vulnerable. More than 70% of veterans enrolled in US Department of Veterans Affairs (VA) healthcare are at increased risk for developing DM due to excess weight, poor eating habits, and physical inactivity.2 One in 4 veterans has DM, compared with 1 in 6 in the general population.2
DM can lead to long-term complications including limb amputations. Annually in the US about 73,000 nontraumatic lower limb amputations are performed and > 60% occur among persons with DM.3 Complications from diabetic wounds are the cause of 90% of lower limb amputations, and foot ulcers are the most prevalent complication.4 Diabetic ulcers are slow to heal due to vascular impairments and nerve damage.5 Peripheral vascular disease, a common comorbid condition, contributes to restricted blood flow and can lead to tissue death or gangrene requiring amputation.6
Between 2010 and 2014, VA Portland Healthcare System (VAPORHCS) had one of the highest national amputation rates in VA.7 A clinical chart review found that annual foot examinations and amputation risk assessments (ARAs) were not completed with all at-risk veterans. In 2013, a VA Office of Inspector General (OIG) national report found that more than one-third of veterans enrolled in VA with DM had no documentation of required annual foot exams.8 In 2017, VA released Directive 1410, which outlined the scope of care required to prevent and treat lower limb complications and amputations for veterans at risk for primary or secondary limb loss.1 This national initiative is a comprehensive approach that engages multiprofessional teams to perform routine foot examinations and amputation risk assessments; identify and promptly treat foot ulcers; track, monitor and educate at-risk veterans; and participate in clinical education to enhance staff skills.
To decrease the amputation rate, VAPORHCS redesigned its foot-care program to comply with the national initiative. As is typical in VA, VAPORHCS uses a team-based approach in primary care. The basic 4-member team patient-aligned care team (PACT) consists of a physician or nurse practitioner (NP) primary care provider (PCP), a registered nurse (RN) care manager, a licensed practical nurse (LPN), and a medical staff assistant (MSA) for administrative support. Each PACT cares for about 1,800 veterans. Formerly, LPNs completed the annual diabetic foot exams, and PCPs verified the exams and completed the ARA based on the LPNs’ findings. If patients were moderate risk or high risk, they were referred to podiatry. The VAPORHCS audit found that not all at-risk veterans had both the foot exam and ARA completed, or were referred to podiatry when indicated. There was a need for a process improvement project to develop a seamless program consisting of all recommended foot care components crucial for timely care.
This quality improvement project sought to evaluate the effectiveness of the process changes by examining PCPs’ adoption of, and consistency in completing annual diabetic foot exams and ARAs with veterans. The goals of the project were to evaluate changes in the: (1) Number of accurate diabetic foot exams and amputation risk assessments completed with veterans with DM; (2) Number and timeliness of appropriate referrals to podiatry for an in-depth assessment and treatment of veterans found to be at moderate-to-high risk for lower limb amputations; and (3) Number of administrative text orders entered by PCPs for nurse care managers to offer foot care education and the completion of the education with veterans found to be at normal-to-low risk for lower limb amputations. The institutional review boards of VAPORHCS and Gonzaga University approved the study.
Methods
Established by the American Diabetes Association and endorsed by the American Association of Clinical Endocrinologists, the comprehensive foot exam includes a visual exam, pedal pulse checks, and a sensory exam.9,10 The templated Computerized Patient Record System (CPRS) electronic health record note specifies normal and abnormal parameters of each section. On the same template, the provider assigns an ARA score based on the results of the completed foot exam. Risk scores range from 0 to 3 (0, normal or no risk; 1, low risk, 2; moderate risk; 3, high risk) If the veteran has normal or low risk, the PCP can encourage the veteran to remain at low risk by entering an administrative CPRS text order for the nurse care manager to offer education about daily foot care at the same visit or at a scheduled follow-up visit. This process facilitates nurse care managers to include routine foot care as integral to their usual duties coaching veterans to engage in self-care to manage chronic conditions. If the risk is assessed as moderate or high risk, PCPs are prompted to send a referral to podiatry to repeat the foot exam, verify the ARA score, and provide appropriate foot care treatment and follow-up.
On October 31, 2017, following training on the updated foot exam and ARA template with staff at the 13 VAPORHCS outpatient clinic sites, 2 sites piloted all components of the Comprehensive Foot Care program. An in-person training was completed with PCPs to review the changes of the foot care template in CPRS and to answer their questions about it. PCPs were required to complete both the 3-part foot exam and ARA at least once annually with veterans with DM.
An electronic clinical reminder was built to alert PCPs and PACTs that a veteran was either due or overdue for an exam and risk assessment. VA podiatrists agreed to complete the reminder with veterans under their care. One of the 2 sites was randomly selected for this study. Data were collected from August 1, 2017 to July 31, 2018. Patients were identified from the Diabetes Registry, a database established at VAPORHCS in 2008 to track veterans with DM to ensure quality care.11 Veterans’ personal health identifiers from the registry were used to access their health records to complete chart reviews and assess the completion, accuracy and timeliness of all foot care components.
The Diabetes Registry lists a veterans’ upcoming appointments and tracks their most recent clinic visits; laboratory tests; physical exams; and screening exams for foot, eye, and renal care. Newly diagnosed veterans are uploaded automatically into this registry by tracking all DM-related International Classification of Diseases (ICD-10) codes, hemoglobin A1c (HbA1c) levels ≥ 6.5%, or outpatient prescriptions for insulin or oral hypoglycemic agents.11
Study Design
This quality improvement project evaluated PCPs’ actions in a program process change intended to improve foot care provided with veterans at-risk for nontraumatic lower limb amputations. Audits of CPRS records and the Diabetes Registry determined the results of the practice change. Data on the total number of foot exams, amputation risk scores, appropriate podiatry referrals, administrative orders for nurse coaching, and completed foot care education were collected during the study period. Data collected for the 3-month period preceding the process change established preimplementation comparison vs the postimplementation data. Data were collected at 3, 6, and 9 months after implementation. The foot exams and ARAs were reviewed to determine whether exams and assessments were completed accurately during the pre- and post-implementation timeframes. Incomplete or clearly incorrectly completed documentation were considered inaccurate. For example, it was considered inaccurate if only the foot exam portion was completed in the assessment and the ARA was not.
Data Analysis
Data on the total number of accurately completed foot examinations and ARAs, total number of podiatry referrals, and total number of administrative text orders placed by PCPs, and education completed by nurse care managers were assessed. Statistical significance was evaluated using χ2 and Fisher exact test as appropriate. A Pearson correlation coefficient was used to determine whether there was a statistically significant increase in accurate foot examinations and ARAs as well as total number of podiatry referrals during the study period. Statistical analyses were performed using Stata 14.1 statistical software (College Station, TX).
Results
A total of 1,242 completed diabetic foot examinations were identified from August 1, 2017 to July 31, 2018 using the Diabetes Registry (Table). For the 3 months prior to the change, there were 191 appropriately completed foot examinations and ARAs. This number increased progressively over three 3-month periods (Figure 1). Within the 1-year study period, there was a statistically significant increase in the number of appropriate foot examinations (r = 0.495). PCPs placed 34 podiatry referrals during the prechange period. After the change, the number of appropriate referrals increased statistically significantly in the 3 following 3-month-periods (r = 0.222) (Figure 2).
To determine the accuracy of documentation and ratio of appropriate referrals, the 3-month prech
Notably, at the end of the first year of this evaluation, 119 veterans at the clinic did not show a recorded comprehensive foot examination since receiving a DM diagnosis and 299 veterans were due for an annual examination—a 25.2% gap of veterans without the recommended progression of foot care services. Of those that previously had a recorded foot examination, 51 (17.0%) veterans were found to be ≥ 2 years overdue.
Discussion
DM management requires a comprehensive team-based approach to help monitor for associated complications. At the VA, PACTs are veterans’ initial and primary point of contact for chronic condition management. PACTs have regular opportunities to engage veterans in initial and follow-up care and appropriate self-care. PCPs are critical in placing referrals for specialized care promptly to prevent and minimize complications such as foot ulcers, and ultimately, lower limb amputations.9,10,12
When PCPs assume responsibility for the entire foot examination, they are able to identify problems early.1 Left untreated, foot wounds and ulcers have the potential to grow into serious infections.9 Early risk identification and management can lead to increased patient satisfaction, improved life expectancy, quality of life, and ultimately, lower healthcare costs.12
Multiple studies have shown the clinical importance of foot examinations in preventative care. In one study, researchers found that completing foot examinations, among other early interventions, increased life expectancy and reduced foot complications.13 Diabetic foot management programs involving screening and categorizing patients into low- and high-risk groups had a 47.4% decrease in the incidence of amputations and 37.8% decrease in hospital admissions.14 Amputations were found to be inversely correlated with multidisciplinary foot care programs with reduction of lower limb amputations at 2 years.15 The Centers for Disease Control and Prevention found that comprehensive foot care programs that include a foot examination, ARA, appropriate referrals to specialists, and foot-care education and preventative services can reduce lower limb amputation rates by 45% to 85%.16
With one of the highest amputation rates in VA, VAPORHCS needed an integrated approach to ensure that appropriate foot care occurred regularly with veterans with DM. Prior to the process change, LPNs completed foot examinations and PCPs completed the ARA. Separating these clinical services resulted in few veterans receiving an amputation risk score. Of those with scores, the lack of a standardized program protocol resulted in discrepancies between ARAs from patient to patient as health care providers did not have clear or enough information to select the correct score and make the appropriate referrals. Thus, veterans previously identified as at moderate or high risk also lacked podiatric follow-up care.
The new quality-driven process change corrected the documentation process to nationally accepted standards. The goal was to create a consistent template in the electronic health record for all health care providers. The new template simplifies the documentation process and clarifies the amputation risk score assignment, which allows for proper foot care management. The PCP completes the process from assessment through referral, removing gaps in care and improving efficiency. Although this change was initially met with resistance from PCPs, it led to a significant increase in the number of patients with accurately documented examinations. Similarly, the number of appropriate referrals significantly rose during the study period. The standardized documentation process resulted in improved accurate examinations and ARAs over the past year. The new program also resulted in an increased number of appropriate podiatry referrals for those identified to be at moderate or high risk. This elevation of care is crucial for veterans to receive frequent follow-up visits for preventative care and/or treatment, including surgical modalities to promote limb salvage.
Barriers
This project identified several barriers to the Comprehensive Foot Care program. One major barrier was health care provider resistance to using the new process. For example, VAPORHCS podiatrists are not using the new template with established patients, which requires PCPs to complete the clinical reminder template for quality performance, an additional burden unrelated to clinical care. PCPs that do complete the foot examination/ARA templated note use the podiatrist’s visit note without personally assessing the patient.
PCPs also have been resistant to entering administrative text orders for preventative foot care in normal- or low-risk veterans (4.6% overall), which has resulted in decreased patient education (3.9% overall). Education for normal-risk and low-risk patients is designed to engage veterans in self-care and prevent risk progression, critical to prevention.
It was found that PCPs often did not ask nurses to coach normal- or low-risk veterans on preventative foot care, as suggested by the low rates at which patients were offered education. This is an area we will target with future quality improvement efforts. All patients with DM should have general education about risk factors and appropriate management of them to decrease their risk for complications.9 Preventative foot care education is a critical resource to share with patients during health coaching opportunities to clarify misunderstandings and support change talk when patients are ambivalent or resistant to change. Individual or group-based nurse visits can facilitate better coaching for patients.
At the VA, coaching begins with a conversation about what matters most to the veteran, facilitating the development of a personalized plan based on patients’ values, needs, preferences and goals.9,10,12,17 Coaching allows nurses to assess veterans’ knowledge and willingness to engage in healthy habits; and identify additional resources to help them achieve their goals.
Limitations
There are many limitations to this short quality improvement analysis. For example, only 1 of 2 clinics that piloted the program change was evaluated. In addition, there are 11 other clinics that need additional in-depth education on the program change. Although this analysis was overwhelmingly positive, it may not be as successful at other clinic sites and may be subject to the Hawthorne effect—since the 2 piloted locations knew they were being observed for the quality improvement program and may have made an extra effort to be compliant.18 Additionally, we were unable to track the records of veterans receiving care through the VA Choice Program for this analysis resulting in a lack of documentation of completed diabetic foot examinations and a lack of internal referrals to VA podiatry.
Another major limitation of this project involved calculating the number of referrals placed to podiatry. On January 1, 2018, about halfway through the program evaluation, a national VA decision enabled veterans to self-refer to podiatry, which may have limited the number of podiatry referrals placed by PCPs. Finally, patients could refuse podiatry referrals. In the 9-month postimplementation period, 57 (64.8%) veterans declined podiatry referrals, according to their CPRS records.
Although, there was an improvement in the accuracy of diabetic foot examinations, ARAs, and appropriate podiatry referrals, the ultimate goal of reducing diabetic foot ulcers and lower limb amputations was not tracked due to the limited timeframe of this analysis. Tracking these endpoints with continuous plan-do-study-act cycles are needed for this ongoing quality improvement project.
Conclusion
The goal of the VAPORHCS Comprehensive Foot Care program is to provide veterans with a program that is predictable, easy and consistent to prevent and treat foot ulcers to reduce the rate of lower limb amputations. It requires multidisciplinary team collaboration for success. Implementation of this new comprehensive program has increased the number of accurate annual foot exams, ARAs and podiatry referrals. Despite these improvements, areas of future improvement include emphasizing patient education and ongoing provider compliance with annual assessments.
Author contributions
MHG proposed the program evaluation project idea. TVQ collected and analyzed the data and wrote the manuscript. MHG oversaw the project and edited the manuscript. TVQ is the guarantor of this project and takes responsibility for the contents of this journal article.
Acknowledgments
The authors thank Tyra Haebe, VAPORHCS Prevention of Amputation in Veterans Everywhere (PAVE) Manager, and the entire VAPORHCS PAVE committee for their support in this program evaluation project.
1. US Department of Veterans Affairs, Veterans Health Administration. VHA directive 1410, prevention of amputation in veterans everywhere (PAVE) program. http://vaww.medical surgical.va.gov/podiatry/docs/VHADirective_1410_PAVE.pdf. Published March 31, 2017. Accessed October 11, 2019.
2. US Department of Veterans Affairs. Close to 25 percent of VA patients have diabetes http://www.va.gov/health/NewsFeatures/20111115a.asp. Accessed 14 October 2017
3. Centers for Disease Control and Prevention. National diabetes statistics report, 2017: Estimates of Diabetes and Its Burden in the United States. https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed October 11, 2019.
4. Gibson LW, Abbas A: Limb salvage for veterans with diabetes: to care for him who has borne the battle. Crit Care Nurs Clin North Am. 2012;25(1):131-134
5. Boyko EJ, Monteiro-Soares M, Wheeler SGB. “Peripheral arterial disease, foot ulcers, lower extremity amputations, and diabetes.” In: Cowie CC, Casagrande SS, Menke A, et al, eds. Diabetes in America. 3rd ed. Bethesda, MD: National Institutes of Health Publication; 2017:20-21,20-34.
6. National Institute of Health, National Institute of Neurological Disorders and Stroke. Peripheral neuropathy fact sheet. https://www.ninds.nih.gov/Disorders/Patient-Caregiver-Education/Fact-Sheets/Peripheral-Neuropathy-Fact-Sheet. Updated August 13, 2019. Accessed October 11, 2019.
7. US Department of Veterans Affairs, Veterans Health Administration, Support Services Center. Amputation cube, lower amputations 2015. http://vssc.med.va.gov/AlphaIndex. [Nonpublic source, not verified]
8. US Department of Veterans Affairs, Office of Inspector General. Healthcare inspection: Foot care for patients with diabetes and additional risk factors for amputation. https://www.va.gov/oig/pubs/VAOIG-11-00711-74.pdf. Published January 17, 2013. Accessed October 11, 2019.
9. American Diabetes Association. Standards of medical care in diabetes - 2017. Diabetes Care. 2017;40(suppl 1):1-142.
10. Boulton AJM, Armstrong DG, Albert SF, et al. Comprehensive foot examination and risk assessment: a report of the Task Force of the Foot Care Interest Group of the American Diabetes Association, with endorsement by the American Association of Clinical Endocrinologists. Diabetes Care. 2008;31(8):1679-1685.
11. Yang J, McConnachie J, Renfro R, Schreiner S, Tallett S, Winterbottom L. The diabetes registry and future panel management tool https://docplayer.net/19062632-The-diabetes-registry-and.html. Accessed October 11, 2019.
12. National Institute of Health, Centers for Disease Control and Prevention, the National Diabetes Education Program. Working together to manage diabetes: a guide for pharmcy, podiatry, optometry, and dentistry. https://www.cdc.gov/diabetes/ndep/pdfs/ppod-guide.pdf. Accessed October 11, 2019.
13. Ortegon MM, Redekop WK, Niessen LW. Cost-effectiveness of prevention and treatment of the diabetic foot: a Markov analysis. Diabetes Care. 2004;27(4):901-907.
14. Lavery LA, Wunderlich RP, Tredwell JL. Disease management for the diabetic foot: effectiveness of a diabetic foot prevention program to reduce amputations and hospitalizations. Diabetes Res Clin Pract. 2005;70(1):31-37.
15. Paisey RB, Abbott A, Levenson R, et al; South-West Cardiovascular Strategic Clinical Network peer diabetic foot service review team. Diabetes-related major lower limb amputation incidence is strongly related to diabetic foot service provision and improves with enhancement of services: peer review of the south-west of England. Diabet Med. 2017;35(1):53-62.
16. Centers for Disease Control and Prevention. National diabetes fact sheet: National estimates and general information on diabetes and prediabetes in the United States, 2011. https://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf. Published 2011. Accessed October 11, 2019.
17. US Department of Veterans Affairs. Whole health for life. https://www.va.gov/patientcenteredcare/explore/about-whole-health.asp. Updated July 20, 2017. Accessed October 11, 2019.
18. Parsons HM. What happened at Hawthorne? New evidence suggests the Hawthorne effect resulted from operant reinforcement contingencies. Science. 1974;183(4128):922–9322.
Individuals with diabetes mellitus (DM), peripheral vascular disease, or end-stage renal disease are at risk for a nontraumatic lower limb amputation.1 Veterans have a high number of risk factors and are especially vulnerable. More than 70% of veterans enrolled in US Department of Veterans Affairs (VA) healthcare are at increased risk for developing DM due to excess weight, poor eating habits, and physical inactivity.2 One in 4 veterans has DM, compared with 1 in 6 in the general population.2
DM can lead to long-term complications including limb amputations. Annually in the US about 73,000 nontraumatic lower limb amputations are performed and > 60% occur among persons with DM.3 Complications from diabetic wounds are the cause of 90% of lower limb amputations, and foot ulcers are the most prevalent complication.4 Diabetic ulcers are slow to heal due to vascular impairments and nerve damage.5 Peripheral vascular disease, a common comorbid condition, contributes to restricted blood flow and can lead to tissue death or gangrene requiring amputation.6
Between 2010 and 2014, VA Portland Healthcare System (VAPORHCS) had one of the highest national amputation rates in VA.7 A clinical chart review found that annual foot examinations and amputation risk assessments (ARAs) were not completed with all at-risk veterans. In 2013, a VA Office of Inspector General (OIG) national report found that more than one-third of veterans enrolled in VA with DM had no documentation of required annual foot exams.8 In 2017, VA released Directive 1410, which outlined the scope of care required to prevent and treat lower limb complications and amputations for veterans at risk for primary or secondary limb loss.1 This national initiative is a comprehensive approach that engages multiprofessional teams to perform routine foot examinations and amputation risk assessments; identify and promptly treat foot ulcers; track, monitor and educate at-risk veterans; and participate in clinical education to enhance staff skills.
To decrease the amputation rate, VAPORHCS redesigned its foot-care program to comply with the national initiative. As is typical in VA, VAPORHCS uses a team-based approach in primary care. The basic 4-member team patient-aligned care team (PACT) consists of a physician or nurse practitioner (NP) primary care provider (PCP), a registered nurse (RN) care manager, a licensed practical nurse (LPN), and a medical staff assistant (MSA) for administrative support. Each PACT cares for about 1,800 veterans. Formerly, LPNs completed the annual diabetic foot exams, and PCPs verified the exams and completed the ARA based on the LPNs’ findings. If patients were moderate risk or high risk, they were referred to podiatry. The VAPORHCS audit found that not all at-risk veterans had both the foot exam and ARA completed, or were referred to podiatry when indicated. There was a need for a process improvement project to develop a seamless program consisting of all recommended foot care components crucial for timely care.
This quality improvement project sought to evaluate the effectiveness of the process changes by examining PCPs’ adoption of, and consistency in completing annual diabetic foot exams and ARAs with veterans. The goals of the project were to evaluate changes in the: (1) Number of accurate diabetic foot exams and amputation risk assessments completed with veterans with DM; (2) Number and timeliness of appropriate referrals to podiatry for an in-depth assessment and treatment of veterans found to be at moderate-to-high risk for lower limb amputations; and (3) Number of administrative text orders entered by PCPs for nurse care managers to offer foot care education and the completion of the education with veterans found to be at normal-to-low risk for lower limb amputations. The institutional review boards of VAPORHCS and Gonzaga University approved the study.
Methods
Established by the American Diabetes Association and endorsed by the American Association of Clinical Endocrinologists, the comprehensive foot exam includes a visual exam, pedal pulse checks, and a sensory exam.9,10 The templated Computerized Patient Record System (CPRS) electronic health record note specifies normal and abnormal parameters of each section. On the same template, the provider assigns an ARA score based on the results of the completed foot exam. Risk scores range from 0 to 3 (0, normal or no risk; 1, low risk, 2; moderate risk; 3, high risk) If the veteran has normal or low risk, the PCP can encourage the veteran to remain at low risk by entering an administrative CPRS text order for the nurse care manager to offer education about daily foot care at the same visit or at a scheduled follow-up visit. This process facilitates nurse care managers to include routine foot care as integral to their usual duties coaching veterans to engage in self-care to manage chronic conditions. If the risk is assessed as moderate or high risk, PCPs are prompted to send a referral to podiatry to repeat the foot exam, verify the ARA score, and provide appropriate foot care treatment and follow-up.
On October 31, 2017, following training on the updated foot exam and ARA template with staff at the 13 VAPORHCS outpatient clinic sites, 2 sites piloted all components of the Comprehensive Foot Care program. An in-person training was completed with PCPs to review the changes of the foot care template in CPRS and to answer their questions about it. PCPs were required to complete both the 3-part foot exam and ARA at least once annually with veterans with DM.
An electronic clinical reminder was built to alert PCPs and PACTs that a veteran was either due or overdue for an exam and risk assessment. VA podiatrists agreed to complete the reminder with veterans under their care. One of the 2 sites was randomly selected for this study. Data were collected from August 1, 2017 to July 31, 2018. Patients were identified from the Diabetes Registry, a database established at VAPORHCS in 2008 to track veterans with DM to ensure quality care.11 Veterans’ personal health identifiers from the registry were used to access their health records to complete chart reviews and assess the completion, accuracy and timeliness of all foot care components.
The Diabetes Registry lists a veterans’ upcoming appointments and tracks their most recent clinic visits; laboratory tests; physical exams; and screening exams for foot, eye, and renal care. Newly diagnosed veterans are uploaded automatically into this registry by tracking all DM-related International Classification of Diseases (ICD-10) codes, hemoglobin A1c (HbA1c) levels ≥ 6.5%, or outpatient prescriptions for insulin or oral hypoglycemic agents.11
Study Design
This quality improvement project evaluated PCPs’ actions in a program process change intended to improve foot care provided with veterans at-risk for nontraumatic lower limb amputations. Audits of CPRS records and the Diabetes Registry determined the results of the practice change. Data on the total number of foot exams, amputation risk scores, appropriate podiatry referrals, administrative orders for nurse coaching, and completed foot care education were collected during the study period. Data collected for the 3-month period preceding the process change established preimplementation comparison vs the postimplementation data. Data were collected at 3, 6, and 9 months after implementation. The foot exams and ARAs were reviewed to determine whether exams and assessments were completed accurately during the pre- and post-implementation timeframes. Incomplete or clearly incorrectly completed documentation were considered inaccurate. For example, it was considered inaccurate if only the foot exam portion was completed in the assessment and the ARA was not.
Data Analysis
Data on the total number of accurately completed foot examinations and ARAs, total number of podiatry referrals, and total number of administrative text orders placed by PCPs, and education completed by nurse care managers were assessed. Statistical significance was evaluated using χ2 and Fisher exact test as appropriate. A Pearson correlation coefficient was used to determine whether there was a statistically significant increase in accurate foot examinations and ARAs as well as total number of podiatry referrals during the study period. Statistical analyses were performed using Stata 14.1 statistical software (College Station, TX).
Results
A total of 1,242 completed diabetic foot examinations were identified from August 1, 2017 to July 31, 2018 using the Diabetes Registry (Table). For the 3 months prior to the change, there were 191 appropriately completed foot examinations and ARAs. This number increased progressively over three 3-month periods (Figure 1). Within the 1-year study period, there was a statistically significant increase in the number of appropriate foot examinations (r = 0.495). PCPs placed 34 podiatry referrals during the prechange period. After the change, the number of appropriate referrals increased statistically significantly in the 3 following 3-month-periods (r = 0.222) (Figure 2).
To determine the accuracy of documentation and ratio of appropriate referrals, the 3-month prech
Notably, at the end of the first year of this evaluation, 119 veterans at the clinic did not show a recorded comprehensive foot examination since receiving a DM diagnosis and 299 veterans were due for an annual examination—a 25.2% gap of veterans without the recommended progression of foot care services. Of those that previously had a recorded foot examination, 51 (17.0%) veterans were found to be ≥ 2 years overdue.
Discussion
DM management requires a comprehensive team-based approach to help monitor for associated complications. At the VA, PACTs are veterans’ initial and primary point of contact for chronic condition management. PACTs have regular opportunities to engage veterans in initial and follow-up care and appropriate self-care. PCPs are critical in placing referrals for specialized care promptly to prevent and minimize complications such as foot ulcers, and ultimately, lower limb amputations.9,10,12
When PCPs assume responsibility for the entire foot examination, they are able to identify problems early.1 Left untreated, foot wounds and ulcers have the potential to grow into serious infections.9 Early risk identification and management can lead to increased patient satisfaction, improved life expectancy, quality of life, and ultimately, lower healthcare costs.12
Multiple studies have shown the clinical importance of foot examinations in preventative care. In one study, researchers found that completing foot examinations, among other early interventions, increased life expectancy and reduced foot complications.13 Diabetic foot management programs involving screening and categorizing patients into low- and high-risk groups had a 47.4% decrease in the incidence of amputations and 37.8% decrease in hospital admissions.14 Amputations were found to be inversely correlated with multidisciplinary foot care programs with reduction of lower limb amputations at 2 years.15 The Centers for Disease Control and Prevention found that comprehensive foot care programs that include a foot examination, ARA, appropriate referrals to specialists, and foot-care education and preventative services can reduce lower limb amputation rates by 45% to 85%.16
With one of the highest amputation rates in VA, VAPORHCS needed an integrated approach to ensure that appropriate foot care occurred regularly with veterans with DM. Prior to the process change, LPNs completed foot examinations and PCPs completed the ARA. Separating these clinical services resulted in few veterans receiving an amputation risk score. Of those with scores, the lack of a standardized program protocol resulted in discrepancies between ARAs from patient to patient as health care providers did not have clear or enough information to select the correct score and make the appropriate referrals. Thus, veterans previously identified as at moderate or high risk also lacked podiatric follow-up care.
The new quality-driven process change corrected the documentation process to nationally accepted standards. The goal was to create a consistent template in the electronic health record for all health care providers. The new template simplifies the documentation process and clarifies the amputation risk score assignment, which allows for proper foot care management. The PCP completes the process from assessment through referral, removing gaps in care and improving efficiency. Although this change was initially met with resistance from PCPs, it led to a significant increase in the number of patients with accurately documented examinations. Similarly, the number of appropriate referrals significantly rose during the study period. The standardized documentation process resulted in improved accurate examinations and ARAs over the past year. The new program also resulted in an increased number of appropriate podiatry referrals for those identified to be at moderate or high risk. This elevation of care is crucial for veterans to receive frequent follow-up visits for preventative care and/or treatment, including surgical modalities to promote limb salvage.
Barriers
This project identified several barriers to the Comprehensive Foot Care program. One major barrier was health care provider resistance to using the new process. For example, VAPORHCS podiatrists are not using the new template with established patients, which requires PCPs to complete the clinical reminder template for quality performance, an additional burden unrelated to clinical care. PCPs that do complete the foot examination/ARA templated note use the podiatrist’s visit note without personally assessing the patient.
PCPs also have been resistant to entering administrative text orders for preventative foot care in normal- or low-risk veterans (4.6% overall), which has resulted in decreased patient education (3.9% overall). Education for normal-risk and low-risk patients is designed to engage veterans in self-care and prevent risk progression, critical to prevention.
It was found that PCPs often did not ask nurses to coach normal- or low-risk veterans on preventative foot care, as suggested by the low rates at which patients were offered education. This is an area we will target with future quality improvement efforts. All patients with DM should have general education about risk factors and appropriate management of them to decrease their risk for complications.9 Preventative foot care education is a critical resource to share with patients during health coaching opportunities to clarify misunderstandings and support change talk when patients are ambivalent or resistant to change. Individual or group-based nurse visits can facilitate better coaching for patients.
At the VA, coaching begins with a conversation about what matters most to the veteran, facilitating the development of a personalized plan based on patients’ values, needs, preferences and goals.9,10,12,17 Coaching allows nurses to assess veterans’ knowledge and willingness to engage in healthy habits; and identify additional resources to help them achieve their goals.
Limitations
There are many limitations to this short quality improvement analysis. For example, only 1 of 2 clinics that piloted the program change was evaluated. In addition, there are 11 other clinics that need additional in-depth education on the program change. Although this analysis was overwhelmingly positive, it may not be as successful at other clinic sites and may be subject to the Hawthorne effect—since the 2 piloted locations knew they were being observed for the quality improvement program and may have made an extra effort to be compliant.18 Additionally, we were unable to track the records of veterans receiving care through the VA Choice Program for this analysis resulting in a lack of documentation of completed diabetic foot examinations and a lack of internal referrals to VA podiatry.
Another major limitation of this project involved calculating the number of referrals placed to podiatry. On January 1, 2018, about halfway through the program evaluation, a national VA decision enabled veterans to self-refer to podiatry, which may have limited the number of podiatry referrals placed by PCPs. Finally, patients could refuse podiatry referrals. In the 9-month postimplementation period, 57 (64.8%) veterans declined podiatry referrals, according to their CPRS records.
Although, there was an improvement in the accuracy of diabetic foot examinations, ARAs, and appropriate podiatry referrals, the ultimate goal of reducing diabetic foot ulcers and lower limb amputations was not tracked due to the limited timeframe of this analysis. Tracking these endpoints with continuous plan-do-study-act cycles are needed for this ongoing quality improvement project.
Conclusion
The goal of the VAPORHCS Comprehensive Foot Care program is to provide veterans with a program that is predictable, easy and consistent to prevent and treat foot ulcers to reduce the rate of lower limb amputations. It requires multidisciplinary team collaboration for success. Implementation of this new comprehensive program has increased the number of accurate annual foot exams, ARAs and podiatry referrals. Despite these improvements, areas of future improvement include emphasizing patient education and ongoing provider compliance with annual assessments.
Author contributions
MHG proposed the program evaluation project idea. TVQ collected and analyzed the data and wrote the manuscript. MHG oversaw the project and edited the manuscript. TVQ is the guarantor of this project and takes responsibility for the contents of this journal article.
Acknowledgments
The authors thank Tyra Haebe, VAPORHCS Prevention of Amputation in Veterans Everywhere (PAVE) Manager, and the entire VAPORHCS PAVE committee for their support in this program evaluation project.
Individuals with diabetes mellitus (DM), peripheral vascular disease, or end-stage renal disease are at risk for a nontraumatic lower limb amputation.1 Veterans have a high number of risk factors and are especially vulnerable. More than 70% of veterans enrolled in US Department of Veterans Affairs (VA) healthcare are at increased risk for developing DM due to excess weight, poor eating habits, and physical inactivity.2 One in 4 veterans has DM, compared with 1 in 6 in the general population.2
DM can lead to long-term complications including limb amputations. Annually in the US about 73,000 nontraumatic lower limb amputations are performed and > 60% occur among persons with DM.3 Complications from diabetic wounds are the cause of 90% of lower limb amputations, and foot ulcers are the most prevalent complication.4 Diabetic ulcers are slow to heal due to vascular impairments and nerve damage.5 Peripheral vascular disease, a common comorbid condition, contributes to restricted blood flow and can lead to tissue death or gangrene requiring amputation.6
Between 2010 and 2014, VA Portland Healthcare System (VAPORHCS) had one of the highest national amputation rates in VA.7 A clinical chart review found that annual foot examinations and amputation risk assessments (ARAs) were not completed with all at-risk veterans. In 2013, a VA Office of Inspector General (OIG) national report found that more than one-third of veterans enrolled in VA with DM had no documentation of required annual foot exams.8 In 2017, VA released Directive 1410, which outlined the scope of care required to prevent and treat lower limb complications and amputations for veterans at risk for primary or secondary limb loss.1 This national initiative is a comprehensive approach that engages multiprofessional teams to perform routine foot examinations and amputation risk assessments; identify and promptly treat foot ulcers; track, monitor and educate at-risk veterans; and participate in clinical education to enhance staff skills.
To decrease the amputation rate, VAPORHCS redesigned its foot-care program to comply with the national initiative. As is typical in VA, VAPORHCS uses a team-based approach in primary care. The basic 4-member team patient-aligned care team (PACT) consists of a physician or nurse practitioner (NP) primary care provider (PCP), a registered nurse (RN) care manager, a licensed practical nurse (LPN), and a medical staff assistant (MSA) for administrative support. Each PACT cares for about 1,800 veterans. Formerly, LPNs completed the annual diabetic foot exams, and PCPs verified the exams and completed the ARA based on the LPNs’ findings. If patients were moderate risk or high risk, they were referred to podiatry. The VAPORHCS audit found that not all at-risk veterans had both the foot exam and ARA completed, or were referred to podiatry when indicated. There was a need for a process improvement project to develop a seamless program consisting of all recommended foot care components crucial for timely care.
This quality improvement project sought to evaluate the effectiveness of the process changes by examining PCPs’ adoption of, and consistency in completing annual diabetic foot exams and ARAs with veterans. The goals of the project were to evaluate changes in the: (1) Number of accurate diabetic foot exams and amputation risk assessments completed with veterans with DM; (2) Number and timeliness of appropriate referrals to podiatry for an in-depth assessment and treatment of veterans found to be at moderate-to-high risk for lower limb amputations; and (3) Number of administrative text orders entered by PCPs for nurse care managers to offer foot care education and the completion of the education with veterans found to be at normal-to-low risk for lower limb amputations. The institutional review boards of VAPORHCS and Gonzaga University approved the study.
Methods
Established by the American Diabetes Association and endorsed by the American Association of Clinical Endocrinologists, the comprehensive foot exam includes a visual exam, pedal pulse checks, and a sensory exam.9,10 The templated Computerized Patient Record System (CPRS) electronic health record note specifies normal and abnormal parameters of each section. On the same template, the provider assigns an ARA score based on the results of the completed foot exam. Risk scores range from 0 to 3 (0, normal or no risk; 1, low risk, 2; moderate risk; 3, high risk) If the veteran has normal or low risk, the PCP can encourage the veteran to remain at low risk by entering an administrative CPRS text order for the nurse care manager to offer education about daily foot care at the same visit or at a scheduled follow-up visit. This process facilitates nurse care managers to include routine foot care as integral to their usual duties coaching veterans to engage in self-care to manage chronic conditions. If the risk is assessed as moderate or high risk, PCPs are prompted to send a referral to podiatry to repeat the foot exam, verify the ARA score, and provide appropriate foot care treatment and follow-up.
On October 31, 2017, following training on the updated foot exam and ARA template with staff at the 13 VAPORHCS outpatient clinic sites, 2 sites piloted all components of the Comprehensive Foot Care program. An in-person training was completed with PCPs to review the changes of the foot care template in CPRS and to answer their questions about it. PCPs were required to complete both the 3-part foot exam and ARA at least once annually with veterans with DM.
An electronic clinical reminder was built to alert PCPs and PACTs that a veteran was either due or overdue for an exam and risk assessment. VA podiatrists agreed to complete the reminder with veterans under their care. One of the 2 sites was randomly selected for this study. Data were collected from August 1, 2017 to July 31, 2018. Patients were identified from the Diabetes Registry, a database established at VAPORHCS in 2008 to track veterans with DM to ensure quality care.11 Veterans’ personal health identifiers from the registry were used to access their health records to complete chart reviews and assess the completion, accuracy and timeliness of all foot care components.
The Diabetes Registry lists a veterans’ upcoming appointments and tracks their most recent clinic visits; laboratory tests; physical exams; and screening exams for foot, eye, and renal care. Newly diagnosed veterans are uploaded automatically into this registry by tracking all DM-related International Classification of Diseases (ICD-10) codes, hemoglobin A1c (HbA1c) levels ≥ 6.5%, or outpatient prescriptions for insulin or oral hypoglycemic agents.11
Study Design
This quality improvement project evaluated PCPs’ actions in a program process change intended to improve foot care provided with veterans at-risk for nontraumatic lower limb amputations. Audits of CPRS records and the Diabetes Registry determined the results of the practice change. Data on the total number of foot exams, amputation risk scores, appropriate podiatry referrals, administrative orders for nurse coaching, and completed foot care education were collected during the study period. Data collected for the 3-month period preceding the process change established preimplementation comparison vs the postimplementation data. Data were collected at 3, 6, and 9 months after implementation. The foot exams and ARAs were reviewed to determine whether exams and assessments were completed accurately during the pre- and post-implementation timeframes. Incomplete or clearly incorrectly completed documentation were considered inaccurate. For example, it was considered inaccurate if only the foot exam portion was completed in the assessment and the ARA was not.
Data Analysis
Data on the total number of accurately completed foot examinations and ARAs, total number of podiatry referrals, and total number of administrative text orders placed by PCPs, and education completed by nurse care managers were assessed. Statistical significance was evaluated using χ2 and Fisher exact test as appropriate. A Pearson correlation coefficient was used to determine whether there was a statistically significant increase in accurate foot examinations and ARAs as well as total number of podiatry referrals during the study period. Statistical analyses were performed using Stata 14.1 statistical software (College Station, TX).
Results
A total of 1,242 completed diabetic foot examinations were identified from August 1, 2017 to July 31, 2018 using the Diabetes Registry (Table). For the 3 months prior to the change, there were 191 appropriately completed foot examinations and ARAs. This number increased progressively over three 3-month periods (Figure 1). Within the 1-year study period, there was a statistically significant increase in the number of appropriate foot examinations (r = 0.495). PCPs placed 34 podiatry referrals during the prechange period. After the change, the number of appropriate referrals increased statistically significantly in the 3 following 3-month-periods (r = 0.222) (Figure 2).
To determine the accuracy of documentation and ratio of appropriate referrals, the 3-month prech
Notably, at the end of the first year of this evaluation, 119 veterans at the clinic did not show a recorded comprehensive foot examination since receiving a DM diagnosis and 299 veterans were due for an annual examination—a 25.2% gap of veterans without the recommended progression of foot care services. Of those that previously had a recorded foot examination, 51 (17.0%) veterans were found to be ≥ 2 years overdue.
Discussion
DM management requires a comprehensive team-based approach to help monitor for associated complications. At the VA, PACTs are veterans’ initial and primary point of contact for chronic condition management. PACTs have regular opportunities to engage veterans in initial and follow-up care and appropriate self-care. PCPs are critical in placing referrals for specialized care promptly to prevent and minimize complications such as foot ulcers, and ultimately, lower limb amputations.9,10,12
When PCPs assume responsibility for the entire foot examination, they are able to identify problems early.1 Left untreated, foot wounds and ulcers have the potential to grow into serious infections.9 Early risk identification and management can lead to increased patient satisfaction, improved life expectancy, quality of life, and ultimately, lower healthcare costs.12
Multiple studies have shown the clinical importance of foot examinations in preventative care. In one study, researchers found that completing foot examinations, among other early interventions, increased life expectancy and reduced foot complications.13 Diabetic foot management programs involving screening and categorizing patients into low- and high-risk groups had a 47.4% decrease in the incidence of amputations and 37.8% decrease in hospital admissions.14 Amputations were found to be inversely correlated with multidisciplinary foot care programs with reduction of lower limb amputations at 2 years.15 The Centers for Disease Control and Prevention found that comprehensive foot care programs that include a foot examination, ARA, appropriate referrals to specialists, and foot-care education and preventative services can reduce lower limb amputation rates by 45% to 85%.16
With one of the highest amputation rates in VA, VAPORHCS needed an integrated approach to ensure that appropriate foot care occurred regularly with veterans with DM. Prior to the process change, LPNs completed foot examinations and PCPs completed the ARA. Separating these clinical services resulted in few veterans receiving an amputation risk score. Of those with scores, the lack of a standardized program protocol resulted in discrepancies between ARAs from patient to patient as health care providers did not have clear or enough information to select the correct score and make the appropriate referrals. Thus, veterans previously identified as at moderate or high risk also lacked podiatric follow-up care.
The new quality-driven process change corrected the documentation process to nationally accepted standards. The goal was to create a consistent template in the electronic health record for all health care providers. The new template simplifies the documentation process and clarifies the amputation risk score assignment, which allows for proper foot care management. The PCP completes the process from assessment through referral, removing gaps in care and improving efficiency. Although this change was initially met with resistance from PCPs, it led to a significant increase in the number of patients with accurately documented examinations. Similarly, the number of appropriate referrals significantly rose during the study period. The standardized documentation process resulted in improved accurate examinations and ARAs over the past year. The new program also resulted in an increased number of appropriate podiatry referrals for those identified to be at moderate or high risk. This elevation of care is crucial for veterans to receive frequent follow-up visits for preventative care and/or treatment, including surgical modalities to promote limb salvage.
Barriers
This project identified several barriers to the Comprehensive Foot Care program. One major barrier was health care provider resistance to using the new process. For example, VAPORHCS podiatrists are not using the new template with established patients, which requires PCPs to complete the clinical reminder template for quality performance, an additional burden unrelated to clinical care. PCPs that do complete the foot examination/ARA templated note use the podiatrist’s visit note without personally assessing the patient.
PCPs also have been resistant to entering administrative text orders for preventative foot care in normal- or low-risk veterans (4.6% overall), which has resulted in decreased patient education (3.9% overall). Education for normal-risk and low-risk patients is designed to engage veterans in self-care and prevent risk progression, critical to prevention.
It was found that PCPs often did not ask nurses to coach normal- or low-risk veterans on preventative foot care, as suggested by the low rates at which patients were offered education. This is an area we will target with future quality improvement efforts. All patients with DM should have general education about risk factors and appropriate management of them to decrease their risk for complications.9 Preventative foot care education is a critical resource to share with patients during health coaching opportunities to clarify misunderstandings and support change talk when patients are ambivalent or resistant to change. Individual or group-based nurse visits can facilitate better coaching for patients.
At the VA, coaching begins with a conversation about what matters most to the veteran, facilitating the development of a personalized plan based on patients’ values, needs, preferences and goals.9,10,12,17 Coaching allows nurses to assess veterans’ knowledge and willingness to engage in healthy habits; and identify additional resources to help them achieve their goals.
Limitations
There are many limitations to this short quality improvement analysis. For example, only 1 of 2 clinics that piloted the program change was evaluated. In addition, there are 11 other clinics that need additional in-depth education on the program change. Although this analysis was overwhelmingly positive, it may not be as successful at other clinic sites and may be subject to the Hawthorne effect—since the 2 piloted locations knew they were being observed for the quality improvement program and may have made an extra effort to be compliant.18 Additionally, we were unable to track the records of veterans receiving care through the VA Choice Program for this analysis resulting in a lack of documentation of completed diabetic foot examinations and a lack of internal referrals to VA podiatry.
Another major limitation of this project involved calculating the number of referrals placed to podiatry. On January 1, 2018, about halfway through the program evaluation, a national VA decision enabled veterans to self-refer to podiatry, which may have limited the number of podiatry referrals placed by PCPs. Finally, patients could refuse podiatry referrals. In the 9-month postimplementation period, 57 (64.8%) veterans declined podiatry referrals, according to their CPRS records.
Although, there was an improvement in the accuracy of diabetic foot examinations, ARAs, and appropriate podiatry referrals, the ultimate goal of reducing diabetic foot ulcers and lower limb amputations was not tracked due to the limited timeframe of this analysis. Tracking these endpoints with continuous plan-do-study-act cycles are needed for this ongoing quality improvement project.
Conclusion
The goal of the VAPORHCS Comprehensive Foot Care program is to provide veterans with a program that is predictable, easy and consistent to prevent and treat foot ulcers to reduce the rate of lower limb amputations. It requires multidisciplinary team collaboration for success. Implementation of this new comprehensive program has increased the number of accurate annual foot exams, ARAs and podiatry referrals. Despite these improvements, areas of future improvement include emphasizing patient education and ongoing provider compliance with annual assessments.
Author contributions
MHG proposed the program evaluation project idea. TVQ collected and analyzed the data and wrote the manuscript. MHG oversaw the project and edited the manuscript. TVQ is the guarantor of this project and takes responsibility for the contents of this journal article.
Acknowledgments
The authors thank Tyra Haebe, VAPORHCS Prevention of Amputation in Veterans Everywhere (PAVE) Manager, and the entire VAPORHCS PAVE committee for their support in this program evaluation project.
1. US Department of Veterans Affairs, Veterans Health Administration. VHA directive 1410, prevention of amputation in veterans everywhere (PAVE) program. http://vaww.medical surgical.va.gov/podiatry/docs/VHADirective_1410_PAVE.pdf. Published March 31, 2017. Accessed October 11, 2019.
2. US Department of Veterans Affairs. Close to 25 percent of VA patients have diabetes http://www.va.gov/health/NewsFeatures/20111115a.asp. Accessed 14 October 2017
3. Centers for Disease Control and Prevention. National diabetes statistics report, 2017: Estimates of Diabetes and Its Burden in the United States. https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed October 11, 2019.
4. Gibson LW, Abbas A: Limb salvage for veterans with diabetes: to care for him who has borne the battle. Crit Care Nurs Clin North Am. 2012;25(1):131-134
5. Boyko EJ, Monteiro-Soares M, Wheeler SGB. “Peripheral arterial disease, foot ulcers, lower extremity amputations, and diabetes.” In: Cowie CC, Casagrande SS, Menke A, et al, eds. Diabetes in America. 3rd ed. Bethesda, MD: National Institutes of Health Publication; 2017:20-21,20-34.
6. National Institute of Health, National Institute of Neurological Disorders and Stroke. Peripheral neuropathy fact sheet. https://www.ninds.nih.gov/Disorders/Patient-Caregiver-Education/Fact-Sheets/Peripheral-Neuropathy-Fact-Sheet. Updated August 13, 2019. Accessed October 11, 2019.
7. US Department of Veterans Affairs, Veterans Health Administration, Support Services Center. Amputation cube, lower amputations 2015. http://vssc.med.va.gov/AlphaIndex. [Nonpublic source, not verified]
8. US Department of Veterans Affairs, Office of Inspector General. Healthcare inspection: Foot care for patients with diabetes and additional risk factors for amputation. https://www.va.gov/oig/pubs/VAOIG-11-00711-74.pdf. Published January 17, 2013. Accessed October 11, 2019.
9. American Diabetes Association. Standards of medical care in diabetes - 2017. Diabetes Care. 2017;40(suppl 1):1-142.
10. Boulton AJM, Armstrong DG, Albert SF, et al. Comprehensive foot examination and risk assessment: a report of the Task Force of the Foot Care Interest Group of the American Diabetes Association, with endorsement by the American Association of Clinical Endocrinologists. Diabetes Care. 2008;31(8):1679-1685.
11. Yang J, McConnachie J, Renfro R, Schreiner S, Tallett S, Winterbottom L. The diabetes registry and future panel management tool https://docplayer.net/19062632-The-diabetes-registry-and.html. Accessed October 11, 2019.
12. National Institute of Health, Centers for Disease Control and Prevention, the National Diabetes Education Program. Working together to manage diabetes: a guide for pharmcy, podiatry, optometry, and dentistry. https://www.cdc.gov/diabetes/ndep/pdfs/ppod-guide.pdf. Accessed October 11, 2019.
13. Ortegon MM, Redekop WK, Niessen LW. Cost-effectiveness of prevention and treatment of the diabetic foot: a Markov analysis. Diabetes Care. 2004;27(4):901-907.
14. Lavery LA, Wunderlich RP, Tredwell JL. Disease management for the diabetic foot: effectiveness of a diabetic foot prevention program to reduce amputations and hospitalizations. Diabetes Res Clin Pract. 2005;70(1):31-37.
15. Paisey RB, Abbott A, Levenson R, et al; South-West Cardiovascular Strategic Clinical Network peer diabetic foot service review team. Diabetes-related major lower limb amputation incidence is strongly related to diabetic foot service provision and improves with enhancement of services: peer review of the south-west of England. Diabet Med. 2017;35(1):53-62.
16. Centers for Disease Control and Prevention. National diabetes fact sheet: National estimates and general information on diabetes and prediabetes in the United States, 2011. https://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf. Published 2011. Accessed October 11, 2019.
17. US Department of Veterans Affairs. Whole health for life. https://www.va.gov/patientcenteredcare/explore/about-whole-health.asp. Updated July 20, 2017. Accessed October 11, 2019.
18. Parsons HM. What happened at Hawthorne? New evidence suggests the Hawthorne effect resulted from operant reinforcement contingencies. Science. 1974;183(4128):922–9322.
1. US Department of Veterans Affairs, Veterans Health Administration. VHA directive 1410, prevention of amputation in veterans everywhere (PAVE) program. http://vaww.medical surgical.va.gov/podiatry/docs/VHADirective_1410_PAVE.pdf. Published March 31, 2017. Accessed October 11, 2019.
2. US Department of Veterans Affairs. Close to 25 percent of VA patients have diabetes http://www.va.gov/health/NewsFeatures/20111115a.asp. Accessed 14 October 2017
3. Centers for Disease Control and Prevention. National diabetes statistics report, 2017: Estimates of Diabetes and Its Burden in the United States. https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed October 11, 2019.
4. Gibson LW, Abbas A: Limb salvage for veterans with diabetes: to care for him who has borne the battle. Crit Care Nurs Clin North Am. 2012;25(1):131-134
5. Boyko EJ, Monteiro-Soares M, Wheeler SGB. “Peripheral arterial disease, foot ulcers, lower extremity amputations, and diabetes.” In: Cowie CC, Casagrande SS, Menke A, et al, eds. Diabetes in America. 3rd ed. Bethesda, MD: National Institutes of Health Publication; 2017:20-21,20-34.
6. National Institute of Health, National Institute of Neurological Disorders and Stroke. Peripheral neuropathy fact sheet. https://www.ninds.nih.gov/Disorders/Patient-Caregiver-Education/Fact-Sheets/Peripheral-Neuropathy-Fact-Sheet. Updated August 13, 2019. Accessed October 11, 2019.
7. US Department of Veterans Affairs, Veterans Health Administration, Support Services Center. Amputation cube, lower amputations 2015. http://vssc.med.va.gov/AlphaIndex. [Nonpublic source, not verified]
8. US Department of Veterans Affairs, Office of Inspector General. Healthcare inspection: Foot care for patients with diabetes and additional risk factors for amputation. https://www.va.gov/oig/pubs/VAOIG-11-00711-74.pdf. Published January 17, 2013. Accessed October 11, 2019.
9. American Diabetes Association. Standards of medical care in diabetes - 2017. Diabetes Care. 2017;40(suppl 1):1-142.
10. Boulton AJM, Armstrong DG, Albert SF, et al. Comprehensive foot examination and risk assessment: a report of the Task Force of the Foot Care Interest Group of the American Diabetes Association, with endorsement by the American Association of Clinical Endocrinologists. Diabetes Care. 2008;31(8):1679-1685.
11. Yang J, McConnachie J, Renfro R, Schreiner S, Tallett S, Winterbottom L. The diabetes registry and future panel management tool https://docplayer.net/19062632-The-diabetes-registry-and.html. Accessed October 11, 2019.
12. National Institute of Health, Centers for Disease Control and Prevention, the National Diabetes Education Program. Working together to manage diabetes: a guide for pharmcy, podiatry, optometry, and dentistry. https://www.cdc.gov/diabetes/ndep/pdfs/ppod-guide.pdf. Accessed October 11, 2019.
13. Ortegon MM, Redekop WK, Niessen LW. Cost-effectiveness of prevention and treatment of the diabetic foot: a Markov analysis. Diabetes Care. 2004;27(4):901-907.
14. Lavery LA, Wunderlich RP, Tredwell JL. Disease management for the diabetic foot: effectiveness of a diabetic foot prevention program to reduce amputations and hospitalizations. Diabetes Res Clin Pract. 2005;70(1):31-37.
15. Paisey RB, Abbott A, Levenson R, et al; South-West Cardiovascular Strategic Clinical Network peer diabetic foot service review team. Diabetes-related major lower limb amputation incidence is strongly related to diabetic foot service provision and improves with enhancement of services: peer review of the south-west of England. Diabet Med. 2017;35(1):53-62.
16. Centers for Disease Control and Prevention. National diabetes fact sheet: National estimates and general information on diabetes and prediabetes in the United States, 2011. https://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf. Published 2011. Accessed October 11, 2019.
17. US Department of Veterans Affairs. Whole health for life. https://www.va.gov/patientcenteredcare/explore/about-whole-health.asp. Updated July 20, 2017. Accessed October 11, 2019.
18. Parsons HM. What happened at Hawthorne? New evidence suggests the Hawthorne effect resulted from operant reinforcement contingencies. Science. 1974;183(4128):922–9322.
Heparin Drug Shortage Conservation Strategies
Heparin is the anticoagulant of choice when a rapid anticoagulant is indicated: Onset of action is immediate when administered IV as a bolus.1 The major anticoagulant effect of heparin is mediated by heparin/antithrombin (AT) interaction. Heparin/AT inactivates factor IIa (thrombin) and factors Xa, IXa, XIa, and XIIa. Heparin is approved for multiple indications, such as venous thromboembolism (VTE) treatment and prophylaxis of medical and surgical patients; stroke prevention in atrial fibrillation (AF); acute coronary syndrome (ACS); vascular and cardiac surgeries; and various interventional procedures (eg, diagnostic angiography and percutaneous coronary intervention [PCI]). It also is used as an anticoagulant in blood transfusions, extracorporeal circulation, and for maintaining patency of central vascular access devices (CVADs).
About 60% of the crude heparin used to manufacture heparin in the US originates in China, derived from porcine mucosa. African swine fever, a contagious virus with no cure, has eliminated about 25% to 35% of China’s pig population, or about 150 million pigs. In July 2019, members of the US House of Representatives Committee on Energy and Commerce sent a letter to the US Food and Drug Administration asking for details on the potential impact of African swine fever on the supply of heparin.2
The US Department of Veterans Affairs (VA) heath care system is currently experiencing a shortage of heparin vials and syringes. It is unclear when resolution of this shortage will occur as it could resolve within several weeks or as late as January 2020.3 Although vials and syringes are the current products that are affected, it is possible the shortage may eventually include IV heparin bags as well.
Since the foremost objective of VA health care providers is to provide timely access to medications for veterans, strategies to conserve unfractionated heparin (UfH) must be used since it is a first-line therapy where few evidence-based alternatives exist. Conservation strategies may include drug rationing, therapeutic substitution, and compounding of needed products using the limited stock available in the pharmacy.4 It is important that all staff are educated on facility strategies in order to be familiar with alternatives and limit the potential for near misses, adverse events, and provider frustration.
In shortage situations, the VA-Pharmacy Benefits Management (PBM) defers decisions regarding drug preservation, processes to shift to viable alternatives, and the best practice for safe transitions to local facilities and their subject matter experts.5 At the VA Tennessee Valley Healthcare System, a 1A, tertiary, dual campus health care system, a pharmacy task force has formed to track drug shortages impacting the facility’s efficiencies and budgets. This group communicates with the Pharmacy and Therapeutics committee about potential risks to patient care and develops shortage briefs (following an SBAR [situation, background, assessment, recommendation] design) generally authored and championed by at least 1 clinical pharmacy specialist and supervising physicians who are field experts. Prior to dissemination, the SBAR undergoes a rapid peer-review process.
To date, VA PBM has not issued specific guidance on how pharmacists should proceed in case of a shortage. However, we recommend strategies that may be considered for implementation during a potential UfH shortage. For example, pharmacists can use therapeutic alternatives for which best available evidence suggests no disadvantage.4 The Table lists alternative agents according to indication and patient-specific considerations that may preclude use. Existing UfH products may also be used for drug compounding (eg, use current stock to provide an indicated aliquot) to meet the need of prioritized patients.4 In addition, we suggest prioritizing current UfH/heparinized saline for use for the following groups of patients4:
- Emergent/urgent cardiac surgery1,6;
- Hemodialysis patients1,7-9 for which the low-molecular-weight heparin (LMWH) dalteparin is deemed inappropriate or the patient is not monitored in the intensive care unit for regional citrate administration;
- VTE prophylaxis for patients with epidurals or chest tubes for which urgent invasive management may occur, recent cardiac or neurosurgery, or for patients with a creatine clearance < 15 mL/min or receiving hemodialysis10-12;
- Vascular surgery (eg, limb ischemia) and interventions (eg, carotid stenting, endarterectomy)13,14;
- Mesenteric ischemia (venous thrombosis) with a potential to proceed to laparotomy15;
- Critically ill patients with arterial lines for which normal saline is deemed inappropriate for line flushing16;
- Electrophysiology procedures (eg, AF ablation)17; and
- Contraindication to use of a long-acting alternative listed in the table or a medical necessity exists for using a rapidly reversible agent. Examples for this category include but are not limited to recent gastrointestinal bleeding, central nervous system lesion, and select neurologic diagnoses (eg, cerebral venous sinus thrombosis with hemorrhage, thrombus in vertebral basilar system or anterior circulation, intraparenchymal hemorrhage plus mechanical valve, medium to large cardioembolic stroke with intracardiac thrombus).
Conclusion
The UfH drug shortage represents a significant threat to public health and is a major challenge for US health care systems, including the Veterans Health Administration. Overreliance on a predominant source of crude heparin has affected multiple UfH manufacturers and products. Current alternatives to UfH include low-molecular-weight heparins, IV direct thrombin inhibitors, and SC fondaparinux, with selection supported by guidelines or evolving literature. However, the shortage has the potential to expand to other injectables, such as dalteparin and enoxaparin, and severely limit care for veterans. It is vital that clinicians rapidly address the current shortage by creating a plan to develop efficient and equitable access to UfH, continue to assess supply and update stakeholders, and select evidence-based alternatives while maintaining focus on efficacy and safety.
Acknowledgments
The authors thank Ashley Yost, PharmD, for her coordination of the multidisciplinary task force assigned to efficiently manage the heparin drug shortage. This material is the result of work supported with resources and the use of facilities at the VA Tennessee Valley Healthcare System in Nashville, Tennessee.
1. Hirsh J, Warkentin TE, Shaughnessy SG, et al. Heparin and low-molecular-weight heparin mechanisms of action, pharmacokinetics, dosing, monitoring, efficacy, and safety. Chest. 2001;119(1):64S-94S.
2. Bipartisan E&C leaders request FDA briefing on threat to U.S. heparin supply [press release]. Washington, DC: House Committee on Energy and Commerce; July 30, 2019. https://energycommerce.house.gov/newsroom/press-releases/bipartisan-ec-leaders-request-fda-briefing-on-threat-to-us-heparin-supply. Accessed September 19, 2019.
3. American Society of Health-System Pharmacists. Drug Shortages. Heparin injection. https://www.ashp.org/Drug-Shortages/Current-Shortages/Drug-Shortages-List?page=CurrentShortages. Accessed September 19, 2019.
4. Reed BN, Fox ER, Konig M, et al. The impact of drug shortages on patients with cardiovascular disease: causes, consequences, and a call to action. Am Heart J. 2016;175:130-141.
5. US Department of Veterans Affairs. Pharmacy Benefits Management Services, Medical Advisory Panel, VISN Pharmacist Executives, The Center For Medication Safety. Heparin supply status: frequently asked questions. PBM-2018-02. https://www.pbm.va.gov/PBM/vacenterformedicationsafety/HeparinandSalineSyringeRecallDuetoContamination_NationalPBMPati.pdf. Published May 3, 2018. Accessed September 11, 2019.
6. Shore-Lesserson I, Baker RA, Ferraris VA, et al. The Society of Thoracic Surgeons, The Society of Cardiovascular Anesthesiologists, and the American Society of ExtraCorporeal Technology: Clinical Practice Guidelines-anticoagulation during cardiopulmonary bypass. Ann Thorac Surg. 2018;105(2):650-662.
7. Soroka S, Agharazii M, Donnelly S, et al. An adjustable dalteparin sodium dose regimen for the prevention of clotting in the extracorporeal circuit in hemodialysis: a clinical trial of safety and efficacy (the PARROT Study). Can J Kidney Health Dis. 2018;5:1-12.
8. Shantha GPS, Kumar AA, Sethi M, Khanna RC, Pancholy SB. Efficacy and safety of low molecular weight heparin compared to unfractionated heparin for chronic outpatient hemodialysis in end stage renal disease: systematic review and meta-analysis. Peer J. 2015;3:e835.
9. Kessler M, Moureau F, and Nguyen P. Anticoagulation in chronic hemodialysis: progress toward an optimal approach. Semin Dial. 2015;28(5):474-489.
10. Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in nonorthopedic surgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(suppl):e227s-e277S.
11. Kaye AD, Brunk AJ, Kaye AJ, et al. Regional anesthesia in patients on anticoagulation therapies—evidence-based recommendations. Curr Pain Headache Rep. 2019;23(9):67.
12. Kahn SR, Lim W, Dunn AS, et al. Prevention of VTE in nonsurgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(suppl):e195S-e226S.
13. Naylor AR, Ricco JB, de Borst GJ, et al. Management of atherosclerotic carotid and vertebral artery disease: 2017 clinical practice guidelines of the European Society for Vascular Surgery. Eur J Vasc Endovasc Surg. 2018;55:3-81.
14. Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. JACC. 2017;69(11): e71-e126.
15. Bjorck M, Koelemaya M, Acosta S, et al. Management of diseases of mesenteric arteries and veins. Eur J Vasc Endovasc Surg. 2017;53(4):460-510.
16. Gorski L, Hadaway L, Hagle ME, McGoldrick M, Orr M, Doellman D. Infusion therapy standards of practice. J Infusion Nurs. 2016;39:S1-S156.
17. Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017;14(10):e275-e444.
18. Spyropoulos AC, Al-Badri A, Sherwood MW, Douketis JD. Periprocedural management of patients receiving a vitamin K antagonist or a direct oral anticoagulant requiring an elective procedure or surgery. J Thromb Haemost. 2016;14(5):875-885.
, . Periprocedural bridging management of anticoagulation. Circulation. 2012;126(4):486-490.
20. Douketis JD, Spyropoulos AC, Spencer FA, et al. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(suppl):e326S-e350S.
21. Sousa-Uva M, Neumann F-J, Ahlsson A, et al; ESC Scientific Document Group. 2018 ESC/EACTS Guidelines on myocardial revascularization. The Task Force on myocardial revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Developed with a special contribution of the European Association for Percutaneous Cardiovascular Interventions (EAPCI). Eur J Cardiothorac Surg. 2019;55(1):4-90.
22. Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes. JACC. 2014;64(24):e139-e228.
23. O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of patients with ST-elevation myocardial infarction. JACC. 2013;61(4):e78-e140.
24. Angiomax [package insert]. Parsippany, NJ: The Medicines Company; March 2016.
25. Sousa-Uva, Head SJ, Milojevic M, et al. 2017 EACTS guidelines on perioperative medication in adult cardiac surgery. Eur J Cardiothorac Surg. 2018;53(1):5-33.
26. Witt DM, Nieuwlaat R, Clark NP, et al. American Society of Hematology 2018 guidelines for the management of venous thromboembolism: optimal management of anticoagulation therapy. Blood Adv. 2018: 2(22):3257-3291
27. Kearon C, Akl EA, Blaivas A, et al. Antithrombotic therapy for VTE disease: Chest guideline and expert panel report. Chest. 2016;149(2):315-352.
28. US Department of Veterans Affairs, Pharmacy Benefits Manager Service. Direct oral anticoagulants criteria for use and algorithm for venous thromboembolism treatment. https://www.pbm.va.gov/PBM/clinicalguidance/criteriaforuse.asp. Updated December 2016. [Source not verified]
29. Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(suppl):e278S-e325S.
30. Raja S, Idrees JJ, Blackstone EH, et al. Routine venous thromboembolism screening after pneumonectomy: the more you look, the more you see. J Thorac Cardiovasc Surg. 2016;152(2):524-532.e2.
31. Schünemann HJ, Cushman M, Burnett AE, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized patients. Blood Adv. 2018;2(22):3198-3225.
32. Naidu SS, Aronow HD, Box LC, et al. SCAI expert consensus statement: 2016 best practices in the cardiac catheterization laboratory:(endorsed by the Cardiological Society of India, and Sociedad Latino Americana de Cardiologia Intervencionista; affirmation of value by the Canadian Association of Interventional Cardiology-Association Canadienne de Cardiologie d’intervention). Catheter Cardiovasc Interv. 2016;88(3):407-423.
33. Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. JACC. 2011;58(24):e44-e122.
34. Mason PJ, Shah B, Tamis-Holland JE, et al; American Heart Association Interventional Cardiovascular Care Committee of the Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Peripheral Vascular Disease; and Council on Genomic and Precision Medicine. AHA scientific statement: an update on radial artery access and best practices for transradial coronary angiography and intervention in acute coronary syndrome. Circ Cardiovasc Interv. 2018;11(9):e000035.
35. Rao SV, Tremmel JA, Gilchrist IC, et al; Society for Cardiovascular Angiography and Intervention’s Transradial Working Group. Best practices for transradial angiography and intervention: a consensus statement from the society for cardiovascular angiography and interventions’ transradial working group. Catheter Cardiovasc Interv. 2014;83(2):228-236. 36. Moran JE, Ash SR. Locking solutions for hemodialysis catheters; heparin and citrate: a position paper by ASDIN. Semin Dial. 2008;21(5):490-492.
Heparin is the anticoagulant of choice when a rapid anticoagulant is indicated: Onset of action is immediate when administered IV as a bolus.1 The major anticoagulant effect of heparin is mediated by heparin/antithrombin (AT) interaction. Heparin/AT inactivates factor IIa (thrombin) and factors Xa, IXa, XIa, and XIIa. Heparin is approved for multiple indications, such as venous thromboembolism (VTE) treatment and prophylaxis of medical and surgical patients; stroke prevention in atrial fibrillation (AF); acute coronary syndrome (ACS); vascular and cardiac surgeries; and various interventional procedures (eg, diagnostic angiography and percutaneous coronary intervention [PCI]). It also is used as an anticoagulant in blood transfusions, extracorporeal circulation, and for maintaining patency of central vascular access devices (CVADs).
About 60% of the crude heparin used to manufacture heparin in the US originates in China, derived from porcine mucosa. African swine fever, a contagious virus with no cure, has eliminated about 25% to 35% of China’s pig population, or about 150 million pigs. In July 2019, members of the US House of Representatives Committee on Energy and Commerce sent a letter to the US Food and Drug Administration asking for details on the potential impact of African swine fever on the supply of heparin.2
The US Department of Veterans Affairs (VA) heath care system is currently experiencing a shortage of heparin vials and syringes. It is unclear when resolution of this shortage will occur as it could resolve within several weeks or as late as January 2020.3 Although vials and syringes are the current products that are affected, it is possible the shortage may eventually include IV heparin bags as well.
Since the foremost objective of VA health care providers is to provide timely access to medications for veterans, strategies to conserve unfractionated heparin (UfH) must be used since it is a first-line therapy where few evidence-based alternatives exist. Conservation strategies may include drug rationing, therapeutic substitution, and compounding of needed products using the limited stock available in the pharmacy.4 It is important that all staff are educated on facility strategies in order to be familiar with alternatives and limit the potential for near misses, adverse events, and provider frustration.
In shortage situations, the VA-Pharmacy Benefits Management (PBM) defers decisions regarding drug preservation, processes to shift to viable alternatives, and the best practice for safe transitions to local facilities and their subject matter experts.5 At the VA Tennessee Valley Healthcare System, a 1A, tertiary, dual campus health care system, a pharmacy task force has formed to track drug shortages impacting the facility’s efficiencies and budgets. This group communicates with the Pharmacy and Therapeutics committee about potential risks to patient care and develops shortage briefs (following an SBAR [situation, background, assessment, recommendation] design) generally authored and championed by at least 1 clinical pharmacy specialist and supervising physicians who are field experts. Prior to dissemination, the SBAR undergoes a rapid peer-review process.
To date, VA PBM has not issued specific guidance on how pharmacists should proceed in case of a shortage. However, we recommend strategies that may be considered for implementation during a potential UfH shortage. For example, pharmacists can use therapeutic alternatives for which best available evidence suggests no disadvantage.4 The Table lists alternative agents according to indication and patient-specific considerations that may preclude use. Existing UfH products may also be used for drug compounding (eg, use current stock to provide an indicated aliquot) to meet the need of prioritized patients.4 In addition, we suggest prioritizing current UfH/heparinized saline for use for the following groups of patients4:
- Emergent/urgent cardiac surgery1,6;
- Hemodialysis patients1,7-9 for which the low-molecular-weight heparin (LMWH) dalteparin is deemed inappropriate or the patient is not monitored in the intensive care unit for regional citrate administration;
- VTE prophylaxis for patients with epidurals or chest tubes for which urgent invasive management may occur, recent cardiac or neurosurgery, or for patients with a creatine clearance < 15 mL/min or receiving hemodialysis10-12;
- Vascular surgery (eg, limb ischemia) and interventions (eg, carotid stenting, endarterectomy)13,14;
- Mesenteric ischemia (venous thrombosis) with a potential to proceed to laparotomy15;
- Critically ill patients with arterial lines for which normal saline is deemed inappropriate for line flushing16;
- Electrophysiology procedures (eg, AF ablation)17; and
- Contraindication to use of a long-acting alternative listed in the table or a medical necessity exists for using a rapidly reversible agent. Examples for this category include but are not limited to recent gastrointestinal bleeding, central nervous system lesion, and select neurologic diagnoses (eg, cerebral venous sinus thrombosis with hemorrhage, thrombus in vertebral basilar system or anterior circulation, intraparenchymal hemorrhage plus mechanical valve, medium to large cardioembolic stroke with intracardiac thrombus).
Conclusion
The UfH drug shortage represents a significant threat to public health and is a major challenge for US health care systems, including the Veterans Health Administration. Overreliance on a predominant source of crude heparin has affected multiple UfH manufacturers and products. Current alternatives to UfH include low-molecular-weight heparins, IV direct thrombin inhibitors, and SC fondaparinux, with selection supported by guidelines or evolving literature. However, the shortage has the potential to expand to other injectables, such as dalteparin and enoxaparin, and severely limit care for veterans. It is vital that clinicians rapidly address the current shortage by creating a plan to develop efficient and equitable access to UfH, continue to assess supply and update stakeholders, and select evidence-based alternatives while maintaining focus on efficacy and safety.
Acknowledgments
The authors thank Ashley Yost, PharmD, for her coordination of the multidisciplinary task force assigned to efficiently manage the heparin drug shortage. This material is the result of work supported with resources and the use of facilities at the VA Tennessee Valley Healthcare System in Nashville, Tennessee.
Heparin is the anticoagulant of choice when a rapid anticoagulant is indicated: Onset of action is immediate when administered IV as a bolus.1 The major anticoagulant effect of heparin is mediated by heparin/antithrombin (AT) interaction. Heparin/AT inactivates factor IIa (thrombin) and factors Xa, IXa, XIa, and XIIa. Heparin is approved for multiple indications, such as venous thromboembolism (VTE) treatment and prophylaxis of medical and surgical patients; stroke prevention in atrial fibrillation (AF); acute coronary syndrome (ACS); vascular and cardiac surgeries; and various interventional procedures (eg, diagnostic angiography and percutaneous coronary intervention [PCI]). It also is used as an anticoagulant in blood transfusions, extracorporeal circulation, and for maintaining patency of central vascular access devices (CVADs).
About 60% of the crude heparin used to manufacture heparin in the US originates in China, derived from porcine mucosa. African swine fever, a contagious virus with no cure, has eliminated about 25% to 35% of China’s pig population, or about 150 million pigs. In July 2019, members of the US House of Representatives Committee on Energy and Commerce sent a letter to the US Food and Drug Administration asking for details on the potential impact of African swine fever on the supply of heparin.2
The US Department of Veterans Affairs (VA) heath care system is currently experiencing a shortage of heparin vials and syringes. It is unclear when resolution of this shortage will occur as it could resolve within several weeks or as late as January 2020.3 Although vials and syringes are the current products that are affected, it is possible the shortage may eventually include IV heparin bags as well.
Since the foremost objective of VA health care providers is to provide timely access to medications for veterans, strategies to conserve unfractionated heparin (UfH) must be used since it is a first-line therapy where few evidence-based alternatives exist. Conservation strategies may include drug rationing, therapeutic substitution, and compounding of needed products using the limited stock available in the pharmacy.4 It is important that all staff are educated on facility strategies in order to be familiar with alternatives and limit the potential for near misses, adverse events, and provider frustration.
In shortage situations, the VA-Pharmacy Benefits Management (PBM) defers decisions regarding drug preservation, processes to shift to viable alternatives, and the best practice for safe transitions to local facilities and their subject matter experts.5 At the VA Tennessee Valley Healthcare System, a 1A, tertiary, dual campus health care system, a pharmacy task force has formed to track drug shortages impacting the facility’s efficiencies and budgets. This group communicates with the Pharmacy and Therapeutics committee about potential risks to patient care and develops shortage briefs (following an SBAR [situation, background, assessment, recommendation] design) generally authored and championed by at least 1 clinical pharmacy specialist and supervising physicians who are field experts. Prior to dissemination, the SBAR undergoes a rapid peer-review process.
To date, VA PBM has not issued specific guidance on how pharmacists should proceed in case of a shortage. However, we recommend strategies that may be considered for implementation during a potential UfH shortage. For example, pharmacists can use therapeutic alternatives for which best available evidence suggests no disadvantage.4 The Table lists alternative agents according to indication and patient-specific considerations that may preclude use. Existing UfH products may also be used for drug compounding (eg, use current stock to provide an indicated aliquot) to meet the need of prioritized patients.4 In addition, we suggest prioritizing current UfH/heparinized saline for use for the following groups of patients4:
- Emergent/urgent cardiac surgery1,6;
- Hemodialysis patients1,7-9 for which the low-molecular-weight heparin (LMWH) dalteparin is deemed inappropriate or the patient is not monitored in the intensive care unit for regional citrate administration;
- VTE prophylaxis for patients with epidurals or chest tubes for which urgent invasive management may occur, recent cardiac or neurosurgery, or for patients with a creatine clearance < 15 mL/min or receiving hemodialysis10-12;
- Vascular surgery (eg, limb ischemia) and interventions (eg, carotid stenting, endarterectomy)13,14;
- Mesenteric ischemia (venous thrombosis) with a potential to proceed to laparotomy15;
- Critically ill patients with arterial lines for which normal saline is deemed inappropriate for line flushing16;
- Electrophysiology procedures (eg, AF ablation)17; and
- Contraindication to use of a long-acting alternative listed in the table or a medical necessity exists for using a rapidly reversible agent. Examples for this category include but are not limited to recent gastrointestinal bleeding, central nervous system lesion, and select neurologic diagnoses (eg, cerebral venous sinus thrombosis with hemorrhage, thrombus in vertebral basilar system or anterior circulation, intraparenchymal hemorrhage plus mechanical valve, medium to large cardioembolic stroke with intracardiac thrombus).
Conclusion
The UfH drug shortage represents a significant threat to public health and is a major challenge for US health care systems, including the Veterans Health Administration. Overreliance on a predominant source of crude heparin has affected multiple UfH manufacturers and products. Current alternatives to UfH include low-molecular-weight heparins, IV direct thrombin inhibitors, and SC fondaparinux, with selection supported by guidelines or evolving literature. However, the shortage has the potential to expand to other injectables, such as dalteparin and enoxaparin, and severely limit care for veterans. It is vital that clinicians rapidly address the current shortage by creating a plan to develop efficient and equitable access to UfH, continue to assess supply and update stakeholders, and select evidence-based alternatives while maintaining focus on efficacy and safety.
Acknowledgments
The authors thank Ashley Yost, PharmD, for her coordination of the multidisciplinary task force assigned to efficiently manage the heparin drug shortage. This material is the result of work supported with resources and the use of facilities at the VA Tennessee Valley Healthcare System in Nashville, Tennessee.
1. Hirsh J, Warkentin TE, Shaughnessy SG, et al. Heparin and low-molecular-weight heparin mechanisms of action, pharmacokinetics, dosing, monitoring, efficacy, and safety. Chest. 2001;119(1):64S-94S.
2. Bipartisan E&C leaders request FDA briefing on threat to U.S. heparin supply [press release]. Washington, DC: House Committee on Energy and Commerce; July 30, 2019. https://energycommerce.house.gov/newsroom/press-releases/bipartisan-ec-leaders-request-fda-briefing-on-threat-to-us-heparin-supply. Accessed September 19, 2019.
3. American Society of Health-System Pharmacists. Drug Shortages. Heparin injection. https://www.ashp.org/Drug-Shortages/Current-Shortages/Drug-Shortages-List?page=CurrentShortages. Accessed September 19, 2019.
4. Reed BN, Fox ER, Konig M, et al. The impact of drug shortages on patients with cardiovascular disease: causes, consequences, and a call to action. Am Heart J. 2016;175:130-141.
5. US Department of Veterans Affairs. Pharmacy Benefits Management Services, Medical Advisory Panel, VISN Pharmacist Executives, The Center For Medication Safety. Heparin supply status: frequently asked questions. PBM-2018-02. https://www.pbm.va.gov/PBM/vacenterformedicationsafety/HeparinandSalineSyringeRecallDuetoContamination_NationalPBMPati.pdf. Published May 3, 2018. Accessed September 11, 2019.
6. Shore-Lesserson I, Baker RA, Ferraris VA, et al. The Society of Thoracic Surgeons, The Society of Cardiovascular Anesthesiologists, and the American Society of ExtraCorporeal Technology: Clinical Practice Guidelines-anticoagulation during cardiopulmonary bypass. Ann Thorac Surg. 2018;105(2):650-662.
7. Soroka S, Agharazii M, Donnelly S, et al. An adjustable dalteparin sodium dose regimen for the prevention of clotting in the extracorporeal circuit in hemodialysis: a clinical trial of safety and efficacy (the PARROT Study). Can J Kidney Health Dis. 2018;5:1-12.
8. Shantha GPS, Kumar AA, Sethi M, Khanna RC, Pancholy SB. Efficacy and safety of low molecular weight heparin compared to unfractionated heparin for chronic outpatient hemodialysis in end stage renal disease: systematic review and meta-analysis. Peer J. 2015;3:e835.
9. Kessler M, Moureau F, and Nguyen P. Anticoagulation in chronic hemodialysis: progress toward an optimal approach. Semin Dial. 2015;28(5):474-489.
10. Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in nonorthopedic surgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(suppl):e227s-e277S.
11. Kaye AD, Brunk AJ, Kaye AJ, et al. Regional anesthesia in patients on anticoagulation therapies—evidence-based recommendations. Curr Pain Headache Rep. 2019;23(9):67.
12. Kahn SR, Lim W, Dunn AS, et al. Prevention of VTE in nonsurgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(suppl):e195S-e226S.
13. Naylor AR, Ricco JB, de Borst GJ, et al. Management of atherosclerotic carotid and vertebral artery disease: 2017 clinical practice guidelines of the European Society for Vascular Surgery. Eur J Vasc Endovasc Surg. 2018;55:3-81.
14. Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. JACC. 2017;69(11): e71-e126.
15. Bjorck M, Koelemaya M, Acosta S, et al. Management of diseases of mesenteric arteries and veins. Eur J Vasc Endovasc Surg. 2017;53(4):460-510.
16. Gorski L, Hadaway L, Hagle ME, McGoldrick M, Orr M, Doellman D. Infusion therapy standards of practice. J Infusion Nurs. 2016;39:S1-S156.
17. Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017;14(10):e275-e444.
18. Spyropoulos AC, Al-Badri A, Sherwood MW, Douketis JD. Periprocedural management of patients receiving a vitamin K antagonist or a direct oral anticoagulant requiring an elective procedure or surgery. J Thromb Haemost. 2016;14(5):875-885.
, . Periprocedural bridging management of anticoagulation. Circulation. 2012;126(4):486-490.
20. Douketis JD, Spyropoulos AC, Spencer FA, et al. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(suppl):e326S-e350S.
21. Sousa-Uva M, Neumann F-J, Ahlsson A, et al; ESC Scientific Document Group. 2018 ESC/EACTS Guidelines on myocardial revascularization. The Task Force on myocardial revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Developed with a special contribution of the European Association for Percutaneous Cardiovascular Interventions (EAPCI). Eur J Cardiothorac Surg. 2019;55(1):4-90.
22. Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes. JACC. 2014;64(24):e139-e228.
23. O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of patients with ST-elevation myocardial infarction. JACC. 2013;61(4):e78-e140.
24. Angiomax [package insert]. Parsippany, NJ: The Medicines Company; March 2016.
25. Sousa-Uva, Head SJ, Milojevic M, et al. 2017 EACTS guidelines on perioperative medication in adult cardiac surgery. Eur J Cardiothorac Surg. 2018;53(1):5-33.
26. Witt DM, Nieuwlaat R, Clark NP, et al. American Society of Hematology 2018 guidelines for the management of venous thromboembolism: optimal management of anticoagulation therapy. Blood Adv. 2018: 2(22):3257-3291
27. Kearon C, Akl EA, Blaivas A, et al. Antithrombotic therapy for VTE disease: Chest guideline and expert panel report. Chest. 2016;149(2):315-352.
28. US Department of Veterans Affairs, Pharmacy Benefits Manager Service. Direct oral anticoagulants criteria for use and algorithm for venous thromboembolism treatment. https://www.pbm.va.gov/PBM/clinicalguidance/criteriaforuse.asp. Updated December 2016. [Source not verified]
29. Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(suppl):e278S-e325S.
30. Raja S, Idrees JJ, Blackstone EH, et al. Routine venous thromboembolism screening after pneumonectomy: the more you look, the more you see. J Thorac Cardiovasc Surg. 2016;152(2):524-532.e2.
31. Schünemann HJ, Cushman M, Burnett AE, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized patients. Blood Adv. 2018;2(22):3198-3225.
32. Naidu SS, Aronow HD, Box LC, et al. SCAI expert consensus statement: 2016 best practices in the cardiac catheterization laboratory:(endorsed by the Cardiological Society of India, and Sociedad Latino Americana de Cardiologia Intervencionista; affirmation of value by the Canadian Association of Interventional Cardiology-Association Canadienne de Cardiologie d’intervention). Catheter Cardiovasc Interv. 2016;88(3):407-423.
33. Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. JACC. 2011;58(24):e44-e122.
34. Mason PJ, Shah B, Tamis-Holland JE, et al; American Heart Association Interventional Cardiovascular Care Committee of the Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Peripheral Vascular Disease; and Council on Genomic and Precision Medicine. AHA scientific statement: an update on radial artery access and best practices for transradial coronary angiography and intervention in acute coronary syndrome. Circ Cardiovasc Interv. 2018;11(9):e000035.
35. Rao SV, Tremmel JA, Gilchrist IC, et al; Society for Cardiovascular Angiography and Intervention’s Transradial Working Group. Best practices for transradial angiography and intervention: a consensus statement from the society for cardiovascular angiography and interventions’ transradial working group. Catheter Cardiovasc Interv. 2014;83(2):228-236. 36. Moran JE, Ash SR. Locking solutions for hemodialysis catheters; heparin and citrate: a position paper by ASDIN. Semin Dial. 2008;21(5):490-492.
1. Hirsh J, Warkentin TE, Shaughnessy SG, et al. Heparin and low-molecular-weight heparin mechanisms of action, pharmacokinetics, dosing, monitoring, efficacy, and safety. Chest. 2001;119(1):64S-94S.
2. Bipartisan E&C leaders request FDA briefing on threat to U.S. heparin supply [press release]. Washington, DC: House Committee on Energy and Commerce; July 30, 2019. https://energycommerce.house.gov/newsroom/press-releases/bipartisan-ec-leaders-request-fda-briefing-on-threat-to-us-heparin-supply. Accessed September 19, 2019.
3. American Society of Health-System Pharmacists. Drug Shortages. Heparin injection. https://www.ashp.org/Drug-Shortages/Current-Shortages/Drug-Shortages-List?page=CurrentShortages. Accessed September 19, 2019.
4. Reed BN, Fox ER, Konig M, et al. The impact of drug shortages on patients with cardiovascular disease: causes, consequences, and a call to action. Am Heart J. 2016;175:130-141.
5. US Department of Veterans Affairs. Pharmacy Benefits Management Services, Medical Advisory Panel, VISN Pharmacist Executives, The Center For Medication Safety. Heparin supply status: frequently asked questions. PBM-2018-02. https://www.pbm.va.gov/PBM/vacenterformedicationsafety/HeparinandSalineSyringeRecallDuetoContamination_NationalPBMPati.pdf. Published May 3, 2018. Accessed September 11, 2019.
6. Shore-Lesserson I, Baker RA, Ferraris VA, et al. The Society of Thoracic Surgeons, The Society of Cardiovascular Anesthesiologists, and the American Society of ExtraCorporeal Technology: Clinical Practice Guidelines-anticoagulation during cardiopulmonary bypass. Ann Thorac Surg. 2018;105(2):650-662.
7. Soroka S, Agharazii M, Donnelly S, et al. An adjustable dalteparin sodium dose regimen for the prevention of clotting in the extracorporeal circuit in hemodialysis: a clinical trial of safety and efficacy (the PARROT Study). Can J Kidney Health Dis. 2018;5:1-12.
8. Shantha GPS, Kumar AA, Sethi M, Khanna RC, Pancholy SB. Efficacy and safety of low molecular weight heparin compared to unfractionated heparin for chronic outpatient hemodialysis in end stage renal disease: systematic review and meta-analysis. Peer J. 2015;3:e835.
9. Kessler M, Moureau F, and Nguyen P. Anticoagulation in chronic hemodialysis: progress toward an optimal approach. Semin Dial. 2015;28(5):474-489.
10. Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in nonorthopedic surgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(suppl):e227s-e277S.
11. Kaye AD, Brunk AJ, Kaye AJ, et al. Regional anesthesia in patients on anticoagulation therapies—evidence-based recommendations. Curr Pain Headache Rep. 2019;23(9):67.
12. Kahn SR, Lim W, Dunn AS, et al. Prevention of VTE in nonsurgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(suppl):e195S-e226S.
13. Naylor AR, Ricco JB, de Borst GJ, et al. Management of atherosclerotic carotid and vertebral artery disease: 2017 clinical practice guidelines of the European Society for Vascular Surgery. Eur J Vasc Endovasc Surg. 2018;55:3-81.
14. Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. JACC. 2017;69(11): e71-e126.
15. Bjorck M, Koelemaya M, Acosta S, et al. Management of diseases of mesenteric arteries and veins. Eur J Vasc Endovasc Surg. 2017;53(4):460-510.
16. Gorski L, Hadaway L, Hagle ME, McGoldrick M, Orr M, Doellman D. Infusion therapy standards of practice. J Infusion Nurs. 2016;39:S1-S156.
17. Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017;14(10):e275-e444.
18. Spyropoulos AC, Al-Badri A, Sherwood MW, Douketis JD. Periprocedural management of patients receiving a vitamin K antagonist or a direct oral anticoagulant requiring an elective procedure or surgery. J Thromb Haemost. 2016;14(5):875-885.
, . Periprocedural bridging management of anticoagulation. Circulation. 2012;126(4):486-490.
20. Douketis JD, Spyropoulos AC, Spencer FA, et al. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(suppl):e326S-e350S.
21. Sousa-Uva M, Neumann F-J, Ahlsson A, et al; ESC Scientific Document Group. 2018 ESC/EACTS Guidelines on myocardial revascularization. The Task Force on myocardial revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Developed with a special contribution of the European Association for Percutaneous Cardiovascular Interventions (EAPCI). Eur J Cardiothorac Surg. 2019;55(1):4-90.
22. Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes. JACC. 2014;64(24):e139-e228.
23. O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of patients with ST-elevation myocardial infarction. JACC. 2013;61(4):e78-e140.
24. Angiomax [package insert]. Parsippany, NJ: The Medicines Company; March 2016.
25. Sousa-Uva, Head SJ, Milojevic M, et al. 2017 EACTS guidelines on perioperative medication in adult cardiac surgery. Eur J Cardiothorac Surg. 2018;53(1):5-33.
26. Witt DM, Nieuwlaat R, Clark NP, et al. American Society of Hematology 2018 guidelines for the management of venous thromboembolism: optimal management of anticoagulation therapy. Blood Adv. 2018: 2(22):3257-3291
27. Kearon C, Akl EA, Blaivas A, et al. Antithrombotic therapy for VTE disease: Chest guideline and expert panel report. Chest. 2016;149(2):315-352.
28. US Department of Veterans Affairs, Pharmacy Benefits Manager Service. Direct oral anticoagulants criteria for use and algorithm for venous thromboembolism treatment. https://www.pbm.va.gov/PBM/clinicalguidance/criteriaforuse.asp. Updated December 2016. [Source not verified]
29. Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(suppl):e278S-e325S.
30. Raja S, Idrees JJ, Blackstone EH, et al. Routine venous thromboembolism screening after pneumonectomy: the more you look, the more you see. J Thorac Cardiovasc Surg. 2016;152(2):524-532.e2.
31. Schünemann HJ, Cushman M, Burnett AE, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized patients. Blood Adv. 2018;2(22):3198-3225.
32. Naidu SS, Aronow HD, Box LC, et al. SCAI expert consensus statement: 2016 best practices in the cardiac catheterization laboratory:(endorsed by the Cardiological Society of India, and Sociedad Latino Americana de Cardiologia Intervencionista; affirmation of value by the Canadian Association of Interventional Cardiology-Association Canadienne de Cardiologie d’intervention). Catheter Cardiovasc Interv. 2016;88(3):407-423.
33. Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. JACC. 2011;58(24):e44-e122.
34. Mason PJ, Shah B, Tamis-Holland JE, et al; American Heart Association Interventional Cardiovascular Care Committee of the Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Peripheral Vascular Disease; and Council on Genomic and Precision Medicine. AHA scientific statement: an update on radial artery access and best practices for transradial coronary angiography and intervention in acute coronary syndrome. Circ Cardiovasc Interv. 2018;11(9):e000035.
35. Rao SV, Tremmel JA, Gilchrist IC, et al; Society for Cardiovascular Angiography and Intervention’s Transradial Working Group. Best practices for transradial angiography and intervention: a consensus statement from the society for cardiovascular angiography and interventions’ transradial working group. Catheter Cardiovasc Interv. 2014;83(2):228-236. 36. Moran JE, Ash SR. Locking solutions for hemodialysis catheters; heparin and citrate: a position paper by ASDIN. Semin Dial. 2008;21(5):490-492.
Telehealth Pulmonary Rehabilitation for Patients With Severe Chronic Obstructive Pulmonary Disease
According to World Health Organization estimates, 65 million people have moderate-to-severe chronic obstructive pulmonary disease (COPD) globally, and > 20 million patients with COPD are living in the US.1 COPD is a progressive respiratory disease with a poor prognosis and a significant cause of morbidity and mortality in the US, especially within the Veterans Health Administration (VHA).2 The prevalence of COPD is higher in veterans than it is in the general population. COPD prevalence in the adult US population has been estimated to be between 5% and 15%, whereas in veterans, prevalence estimates have ranged from about 5% to 43%.3-5
COPD is associated with disabling dyspnea, muscle weakness, exercise intolerance, morbidity, and mortality. These symptoms and complications gradually and progressively compromise mobility, ability to perform daily functions, and decrease quality of life (QOL). Dyspnea, fatigue, and discomfort are the principal symptoms that negatively impact exercise tolerance.6,7 Therefore, patients often intentionally limit their activities to avoid these uncomfortable feelings and adopt a more sedentary behavior. As the disease progresses, individuals with COPD will gradually need assistance in performing activities of daily living, which eventually leads to functional dependence.
Pulmonary rehabilitation (PR) is an essential component of the management of symptomatic patients with COPD. PR is an evidence-based, multidisciplinary, comprehensive intervention that includes exercise and education for patients with chronic respiratory disease.8 The key benefits of PR are clinical improvements in dyspnea, physical capacity, QOL, and reduced disability in patients with COPD and other respiratory diseases.9-11 PR was found to improve respiratory health in veterans with COPD and decrease respiratory-related health care utilization.12
Despite the known benefits of PR, many patients with chronic respiratory diseases are not referred or do not have access to rehabilitation. Also, uptake of PR is low due to patient frailty, transportation issues, and other health care access problems.13-15 Unfortunately, in the US health care system, access to PR and other nonpharmacologic treatments can be challenging due to a shortage of available PR programs, limited physician referral to existing programs, and lack of family and social support.16
There are only a few accredited PR programs in VHA facilities, and they tend to be located in urban areas.12,17 Many patients have limited access to the PR programs due to geographic distance to the programs and transportation challenges (eg, limited ability to drive, cost of transportation). Moreover, veterans with COPD are likely to have limited mobility or are homebound due to experiencing shortness of breath with minimal exertion. Given the clear benefits of PR and the increasing impact of COPD on morbidity and mortality of the patients with COPD, strategies to improve the access and capacity of PR are needed. VA telehealth services allow for distribution of health care services in different geographic locations by providing access for the veterans who live in rural and highly rural areas. The most recent implementation of VA Video Connect (VVC) by the VHA provides a new avenue for clinicians to deliver much needed medical care into the veterans’ home.
COPD Telehealth Program
In this article, we describe the processes for developing and delivering an in-home, interactive, supervised PR program for veterans with severe COPD through VA telehealth service. The program consists of 18 sessions delivered over 6 weeks by a licensed physical therapist (PT) and a respiratory therapist (RT). The aims of the telehealth PR are to improve exercise tolerance, reduce dyspnea and fatigue, improve QOL, improve accessibility, and decrease costs and transportation burdens for patients with COPD. The program was developed, implemented and delivered by an interdisciplinary team, including a pulmonologist, PT, RT, physiatrist, and nonclinical supporting staff.
Patient Assessment
To be eligible to participate in the program the patient must: (1) have a forced expiratory volume (FEV1) < 60%; ( 2) be medically stable and be receiving optimal medical management; (3) have no severe cognitive impairments; (4) be able to use a computer and e-mail; (5) be able to ambulate with or without a walking device; (6) be willing to enroll in a smoking cessation program or to stop smoking; (7) be willing to participate without prolonged interruption; and (8) have all visual and auditory impairments corrected with medical devices.
After referral and enrollment, patients receive medical and physical examinations by the PR team, including a pulmonologist, a PT, and a RT, to ensure that the patients are medically stable to undergo rehabilitation and to develop a tailored exercise program while being mindful of the comorbidities, limitations, and precautions, (eg, loss of balance, risk of fall, limited range of motion). The preprogram assessment includes a pulmonary function test, arterial blood gas test, Montreal Cognitive Assessment, Modified Medical Research Council Scale, St. George Respiratory Questionnaire, the COPD Assessment Test, Patient Health Questionnaire-9,Generalized Anxiety Disorder Assessment-7, Epworth Sleepiness Scale, Katz Index of Independence of Activities of Daily Living, medications and inhaler use, oxygen use, breathing pattern, coughing, 6-minute walk test, Modified Borg Dyspnea Scale, grip strength, 5 Times Sit to Stand Test, manual muscle test, gait measure, Timed Up & Go test, clinical balance tests, range of motion, flexibility, sensation, pain, and fall history.18-32 Educational needs (eg, respiratory hygiene, nutrition, infection control, sleep, disease/symptom management) also are evaluated.
This thorough assessment is performed in a face-to-face outpatient visit. During the program participation, a physiatrist may be consulted for additional needs (eg, wheelchair assessment, home safety evaluation/ modifications, and mobility/disability issues). After completing the 6-week program, patients are scheduled for the postprogram evaluation in a face-to-face outpatient visit with the clinicians.
Equipment
Both clinician and the patient are equipped with a computer with Wi-Fi connectivity, a webcam, and a microphone. Patients are provided an exercise pictorial booklet, an exercise compact disk (audio and video), small exercise apparatuses (eg, assorted colors of resistance bands, hand grip exerciser, hand putty, ergometer, harmonica, and pedometer), incentive spirometer, pulse oximeter, cough assistive device (as needed), blood pressure monitor, COPD information booklets, and a diary to use at home during the program.33
Technology Preparation
Prior to starting the telehealth program, the patient is contacted 1 or 2 days before the first session for technical preparation and familiarization of the VA telehealth connection process. Either the PT or RT provides step-by-step instructions for the patient to practice connecting through VVC during this preparatory phone call. The patient also practices using the computer webcam, speaker, and microphone; checks the telehealth scheduling e-mail; and learns how to solve possible common technical issues (eg, adjusting volume and position of webcam). The patient is asked to set up a table close to the computer and to place all exercise apparatuses and respiratory devices on the table surface.
Program Delivery
A secure online VVC is used for connection during the telehealth session. The patient received an e-mail from the telehealth scheduling system with a link for VVC before each session. During the 6-week program, each telehealth session is conducted by a PT and a RT concurrently for 120 minutes, 3 days per week. The PT provides exercises for the patient to attempt, and the RT provides breathing training and monitoring during the session. After a successful connection to VVC, the therapist verifies the patient’s identity and confirms patient consent for the telehealth session.
After this check-in process, the patient performs a self-measure of resting blood pressure (BP), heart rate, respiratory rate, and blood oxygen saturation and reports to the therapists. During the exercise session, fatigue/exertion, dyspnea (Modified Borg Dyspnea Scale; Borg CR10 Scale), BP, heart rate, oxygen saturation, and other clinical symptoms and responses to exercise are monitored by the therapists, using both patient-reported measures and clinical observation by the therapists.34,35 Any medical emergency during the session is reported immediately to the pulmonologist for further management.
Structure
Prior to each exercise session, exercise precautions, fall prevention, good posture, pursed-lip breathing, pacing, and coordinated breathing are discussed with the patient. The PT demonstrates stretching and warm-up exercises in front of the webcam for the patient to follow. Then the patient performs all exercises in view of the webcam during the session (Figure 1). A RT monitors breathing patterns and corrects with verbal instructions if not properly performed.
Loss of skeletal muscle mass and cachexia are highly prevalent comorbidities of COPD and have been associated with breathlessness, functional limitation, and poor prognosis.36 To address these comorbidities, our program consists of progressive strengthening, aerobic, balance, and flexibility exercises. Resistance bands and tubes are used for strengthening exercises. Callisthenic exercises (eg, chair squat, chair stand, knee marching, bridging, single limb stances, and lunge) are used for progressive strengthening and balance exercises. Progression of strengthening and balance exercises are done through increasing the volume of exercise (ie, numbers of sets and repetitions) and increased load and level of difficulty based on the patient’s progress and comorbidity. The exercise program focuses on strengthening muscles, especially large muscle groups, to improve overall muscle strength and performance of functional activities.37
Arm/pedal ergometer and daily walking are used for daily aerobic exercise. In a study of patients with COPD by the PAC-COPD Study Group, step counter use was found to increase physical activity and improve exercise capacity, which supports its use in COPD management.38 During program participation, the patient is asked to wear a pedometer to monitor the number of steps taken per day and to report step data to the therapists during the telehealth session. The pedometer stores the previous 41 calendar days of data and displays the most recent 7 calendar days of data.
The patient is encouraged to set a realistic daily step goal. The general program goal is to increase at least 1000 steps per day. However, this goal can be adjusted depending on the patient’s health status and comorbid conditions. The PAC-COPD Study Group found that for every additional 1000 daily steps at low intensity, COPD hospitalization risk decreased by 20%.39 A magnitude of 2000 steps or about 1 mile of walking per day was found to be associated with increased physical activity and health benefits in the general population.40
Respiratory muscle training and breathing exercise are provided by the RT, using breathing and incentive spirometer techniques (Figure 2). Huff coughing, diaphragmatic deep breathing, and pursed-lip breathing are instructed by the RT during the session. Effective coughing technique with a cough assistive device is also provided during breathing training if needed.
Patient Education
In patients with COPD, there are numerous positive health benefits associated with education, including assisting the patients to become active participants in the PR program leading to satisfying outcomes; assisting the patients to better understand the lung health, disease processes, physical and psychological changes that occur with COPD; assisting the patients to explore coping strategies for those changes; building lifelong behavioral changes; and developing the self-management skills for sustainability. Through the educational process, patients with COPD can become more skilled at collaborative self-management and improve adherence to their treatment plan, which in turn can result in a reduction in hospital admissions and reduced health care costs.8,41
Education is provided with every session after the patient completes the exercise. Patients are required to record their COPD symptoms, daily activity, home exercise program, sleep, food intake, and additional physical or social activity in their COPD diary and to report during the session (Figure 3). A COPD diary assists patients in self-monitoring their COPD symptoms and provides the therapists with information about clinical changes, behavioral changes, and/or specific unmet needs for education. Several topics related to COPD are included in the education session: lung or respiratory disease/condition and self-management; smoking cessation; physical activity; energy-conserving techniques; breathing and coughing techniques; smoking cessation; nutrition/healthy eating and weight counseling; sex and intimacy; psychological counseling and/or group support; emergency planning (eg, medical, travel, and inclement weather); correct use of inhaler and medications; home oxygen; sleep and sleep hygiene; palliative care and advanced directive; infection control; and sputum clearance.42,43
Program Maintenance
After successfully completing the 6-week program, patients are referred to the VA TeleMOVE! Program or MOVE! Weight Management Program for continuous, long-term management of weight, nutrition, physical activity/exercise, and social activity needs or goals. The patients are scheduled for monthly follow-up phone visits for 6 months with the telerehabilitation team for enforcing sustainability. The phone call visit consists of reviewing breathing techniques, exercise program, physical activity, education, encouragement, and addressing any issues that arise during the self-maintained period.
Limitations
There are several issues of concern and precautions when delivering PR through telehealth into the home. First, the patient performs exercises independently without being manually guarded by the therapists. Risk of falls are a major concern due to impaired balance, poor vision, and other possible unusual physiologic responses to exercise (eg, drop in BP, dizziness, loss of balance). The area in front of the computer needs to be cleared of fall hazards (ie, area rug, wires, objects on the floor). The patient also needs to be educated on self-measurements of BP and oxygen saturation and reports to the therapists. The therapists provide detailed instructions on how to obtain these measures correctly; otherwise, the values may not be valid for a clinical judgment during the exercise session or for other clinical management. In a home environment, there is a limited use of exercise apparatuses. For this program, we only used resistance bands/tubes, small arm/leg ergometer, hand grip, and hand putty for the exercise program. We feel that dumbbell and weight plates are not suitable due to a possible risk of injury if the patient accidently drops them.
Advanced balance training is not suitable due to an increased risk for falls. Without the presence of the PT, level of challenge/difficulty is somewhat limited for this telehealth supervision exercise program. In addition, visual and audio quality are necessary for the session. The patient and the therapists need to see each other clearly to ensure correct methods and forms of each exercise. Furthermore, rehearsal of technical skills with the therapists is very important because this population is older and often has limited computer skills. Any technical difficulty or failure can lead to undesirable situations (eg, anxiety episodes, worries, shortness of breath, upset), which compromise exercise performance during the session. Finally, a phone is needed as an alternative in case of a poor VVC connection.
Conclusion
COPD symptoms and complications greatly affect patients’ ability to perform daily activities, decrease QOL and functional ability, and result in extensive use of health services. Many patients have limited access to a PR program at hospitals or rehabilitation centers due to health conditions, lack of transportation, and/or family support. This home-based, interactive telehealth PR program can break down the geographic barriers, solve poor program accessibility, potentially increase the utilization of PR, and reduce the cost and travel required by the patients.
Acknowledgments
The Telehealth Pulmonary Rehabilitation Program was originally funded by the Veterans Health Administration VA ACCESS Program (AS, CL, HKH). We thank all the veterans for their time and effort in participating in this newly developed rehabilitation program.
1. World Health Organization. Chronic obstructive pulmonary disease (COPD). http://www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd). Published December 1, 2017. Accessed August 7, 2019.
2. Yu W, Ravelo A, Wagner TH, et al. Prevalence and costs of chronic conditions in the VA health care system. Med Care Res Rev. 2003;60(suppl 3):146S-167S.
3. Doney B, Hnizdo E, Dillon CF, et al. Prevalence of airflow obstruction in U.S. adults aged 40-79 years: NHANES data 1988-1994 and 2007-2010. COPD. 2015;12(4):355-365.
4. Murphy DE, Chaudhry Z, Almoosa KF, Panos RJ. High prevalence of chronic obstructive pulmonary disease among veterans in the urban midwest. Mil Med. 2011;176(5):552-560.
5. Cypel YS, Hines SE, Davey VJ, Eber SM, Schneiderman AI. Self-reported physician-diagnosed chronic obstructive pulmonary disease and spirometry patterns in Vietnam Era US Army Chemical Corps veterans: a retrospective cohort study. Am J Ind Med. 2018;61(10):802-814.
6. Rochester CL. Exercise training in chronic obstructive pulmonary disease. J Rehabil Res Dev. 2003;40(5)(suppl 2):59-80.
7. Cortopassi F, Gurung P, Pinto-Plata V. Chronic obstructive pulmonary disease in elderly patients. Clin Geriatr Med. 2017;33(4):539-552.
8. Spruit MA, Singh SJ, Garvey C, et al; ATS/ERS Task Force on Pulmonary Rehabilitation. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188(8):e13-e64.
9. Robinson H, Williams V, Curtis F, Bridle C, Jones AW. Facilitators and barriers to physical activity following pulmonary rehabilitation in COPD: a systematic review of qualitative studies. NPJ Prim Care Respir Med. 2018;28(1):19.
10. McCarthy B, Casey D, Devane D, Murphy K, Murphy E, Lacasse Y. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015;(2):CD003793.
11. Ries AL, Bauldoff GS, Carlin BW, et al. Pulmonary rehabilitation: joint AACP/AACVPR evidence-based clinical practice guidelines. Chest. 2007;131(suppl 5):4S-42S.
12. Major S, Moreno M, Shelton J, Panos RJ. Veterans with chronic obstructive pulmonary disease achieve clinically relevant improvements in respiratory health after pulmonary rehabilitation. J Cardiopulm Rehabil Prev. 2014;34(6):420-429.
13. Liu Y, Dickerson T, Early F, Fuld J, Clarkson PJ. Understanding influences on the uptake of pulmonary rehabilitation in the East of England: an inclusive design/mixed methods study protocol. BMJ Open. 2018;8(4):e020750.
14. Harris D, Hayter M, Allender S. Factors affecting the offer of pulmonary rehabilitation to patients with chronic obstructive pulmonary disease by primary care professionals: a qualitative study. Prim Health Care Res Dev. 2008;9(4):280-290.
15. Mathar H, Fastholm P, Hansen IR, Larsen NS. Why do patients with COPD decline rehabilitation. Scand J Caring Sci. 2016;30(3):432-441.
16. Han MK, Martinez CH, Au DH, et al. Meeting the challenge of COPD care delivery in the USA: a multiprovider perspective. Lancet Respir Med. 2016;4(6):473-526.
17. American Association of Cardiovascular and Pulmonary Rehabilitation (AACVPR). Online searchable program directory. https://www.aacvpr.org/Resources/Program-Directory Accessed July 19, 2018.
18. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695-699.
19. Fletcher CM, Elmes PC, Fairbairn AS, Wood CH. The significance of respiratory symptoms and the diagnosis of chronic bronchitis in a working population. Br Med J. 1959;2(5147):257-266.
20. O’Donnell DE, Aaron S, Bourbeau J, et al. Canadian Thoracic Society recommendations for management of chronic obstructive pulmonary disease—2007 update. Can Respir J. 2007;14(suppl B):5B-32B.
21. Jones PW, Quirk FH, Baveystock CM. The St George’s Respiratory Questionnaire. Respir Med. 1991;85(suppl B):25-31.
22. Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34(3):648-654.
23. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613.
24. Spitzer RL, Kroenke K, Williams JBW, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092-1097.
25. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540-545.
26. Katz S. Assessing self-maintenance: activities of daily living, mobility and instrumental activities of daily living. J Am Geriatr Soc. 1983;31(12):721-727.
27. Holland AE, Spruit MA, Troosters T, et al. An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J. 2014;44(6):1428-1446.
28. Mahler DA, Horowitz MB. Perception of breathlessness during exercise in patients with respiratory disease. Med Sci Sports Exerc. 1994;26(9):1078-1081.
29. Liao WC, Wang CH, Yu SY, Chen LY, Wang CY. Grip strength measurement in older adults in Taiwan: a comparison of three testing positions. Australas J Ageing. 2014;33(4):278-282.
30. Buatois S, Miljkovic D, Manckoundia P, et al. Five times sit to stand test is a predictor of recurrent falls in healthy community-living subjects aged 65 and older. J Am Geriatr Soc. 2008;56(8):1575-1577.
31. Bryant MS, Workman CD, Jackson GR. Multidirectional walk test in persons with Parkinson’s disease: a validity study. Int J Rehabil Res. 2015;38(1):88-91.
32. Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39(2):142-148.
33. University of Nebraska Medical Center. Timed Up and Go (TUG) Test. https://www.unmc.edu/media/intmed/geriatrics/nebgec/pdf/frailelderlyjuly09/toolkits/timedupandgo_w_norms.pdf. Accessed August 13, 2019.
34. Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14(5):377-381.
35. Mahler DA, Horowitz MB. Clinical evaluation of exertional dyspnea. Clin Chest Med. 1994;15(2):259-269.
36. Dudgeon D, Baracos VE. Physiological and functional failure in chronic obstructive pulmonary disease, congestive heart failure and cancer: a debilitating intersection of sarcopenia, cachexia and breathlessness. Curr Opin Support Palliat Care. 2016;10(3):236-241.
37. Lee AL, Holland AE. Time to adapt exercise training regimens in pulmonary rehabilitation—a review of the literature. Int J Chron Obstruct Pulmon Dis. 2014;9:1275-1288.
38. Qiu S, Cai X, Wang X, et al. Using step counters to promote physical activity and exercise capacity in patients with chronic obstructive pulmonary disease: a meta-analysis. Ther Adv Respir Dis. 2018;12:1753466618787386.
39. Donaire-Gonzalez D, Gimeno-Santos E, Balcells E, et al; PAC-COPD Study Group. Benefits of physical activity on COPD hospitalization depend on intensity. Eur Respir J. 2015;46(5):1281-1289.
40. Bravata DM, Smith-Spangler C, Sundaram V, et al. Using pedometers to increase physical activity and improve health: a systematic review. JAMA. 2007;298(19):2296-2304.
41. Zwerink M, Brusse-Keizer M, van der Valk PD, et al. Self-management for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014;19(3):CD002990.
42. Wilson JS, O’Neill B, Reilly J, MacMahon J, Bradley JM. Education in pulmonary rehabilitation: the patient’s perspective. Arch Phys Med Rehabil. 2007;88(12):1704-1709.
43. Bourbeau J, Nault D, Dang-Tan T. Self-management and behaviour modification in COPD. Patient Educ Couns. 2004;52(3):271-277.
According to World Health Organization estimates, 65 million people have moderate-to-severe chronic obstructive pulmonary disease (COPD) globally, and > 20 million patients with COPD are living in the US.1 COPD is a progressive respiratory disease with a poor prognosis and a significant cause of morbidity and mortality in the US, especially within the Veterans Health Administration (VHA).2 The prevalence of COPD is higher in veterans than it is in the general population. COPD prevalence in the adult US population has been estimated to be between 5% and 15%, whereas in veterans, prevalence estimates have ranged from about 5% to 43%.3-5
COPD is associated with disabling dyspnea, muscle weakness, exercise intolerance, morbidity, and mortality. These symptoms and complications gradually and progressively compromise mobility, ability to perform daily functions, and decrease quality of life (QOL). Dyspnea, fatigue, and discomfort are the principal symptoms that negatively impact exercise tolerance.6,7 Therefore, patients often intentionally limit their activities to avoid these uncomfortable feelings and adopt a more sedentary behavior. As the disease progresses, individuals with COPD will gradually need assistance in performing activities of daily living, which eventually leads to functional dependence.
Pulmonary rehabilitation (PR) is an essential component of the management of symptomatic patients with COPD. PR is an evidence-based, multidisciplinary, comprehensive intervention that includes exercise and education for patients with chronic respiratory disease.8 The key benefits of PR are clinical improvements in dyspnea, physical capacity, QOL, and reduced disability in patients with COPD and other respiratory diseases.9-11 PR was found to improve respiratory health in veterans with COPD and decrease respiratory-related health care utilization.12
Despite the known benefits of PR, many patients with chronic respiratory diseases are not referred or do not have access to rehabilitation. Also, uptake of PR is low due to patient frailty, transportation issues, and other health care access problems.13-15 Unfortunately, in the US health care system, access to PR and other nonpharmacologic treatments can be challenging due to a shortage of available PR programs, limited physician referral to existing programs, and lack of family and social support.16
There are only a few accredited PR programs in VHA facilities, and they tend to be located in urban areas.12,17 Many patients have limited access to the PR programs due to geographic distance to the programs and transportation challenges (eg, limited ability to drive, cost of transportation). Moreover, veterans with COPD are likely to have limited mobility or are homebound due to experiencing shortness of breath with minimal exertion. Given the clear benefits of PR and the increasing impact of COPD on morbidity and mortality of the patients with COPD, strategies to improve the access and capacity of PR are needed. VA telehealth services allow for distribution of health care services in different geographic locations by providing access for the veterans who live in rural and highly rural areas. The most recent implementation of VA Video Connect (VVC) by the VHA provides a new avenue for clinicians to deliver much needed medical care into the veterans’ home.
COPD Telehealth Program
In this article, we describe the processes for developing and delivering an in-home, interactive, supervised PR program for veterans with severe COPD through VA telehealth service. The program consists of 18 sessions delivered over 6 weeks by a licensed physical therapist (PT) and a respiratory therapist (RT). The aims of the telehealth PR are to improve exercise tolerance, reduce dyspnea and fatigue, improve QOL, improve accessibility, and decrease costs and transportation burdens for patients with COPD. The program was developed, implemented and delivered by an interdisciplinary team, including a pulmonologist, PT, RT, physiatrist, and nonclinical supporting staff.
Patient Assessment
To be eligible to participate in the program the patient must: (1) have a forced expiratory volume (FEV1) < 60%; ( 2) be medically stable and be receiving optimal medical management; (3) have no severe cognitive impairments; (4) be able to use a computer and e-mail; (5) be able to ambulate with or without a walking device; (6) be willing to enroll in a smoking cessation program or to stop smoking; (7) be willing to participate without prolonged interruption; and (8) have all visual and auditory impairments corrected with medical devices.
After referral and enrollment, patients receive medical and physical examinations by the PR team, including a pulmonologist, a PT, and a RT, to ensure that the patients are medically stable to undergo rehabilitation and to develop a tailored exercise program while being mindful of the comorbidities, limitations, and precautions, (eg, loss of balance, risk of fall, limited range of motion). The preprogram assessment includes a pulmonary function test, arterial blood gas test, Montreal Cognitive Assessment, Modified Medical Research Council Scale, St. George Respiratory Questionnaire, the COPD Assessment Test, Patient Health Questionnaire-9,Generalized Anxiety Disorder Assessment-7, Epworth Sleepiness Scale, Katz Index of Independence of Activities of Daily Living, medications and inhaler use, oxygen use, breathing pattern, coughing, 6-minute walk test, Modified Borg Dyspnea Scale, grip strength, 5 Times Sit to Stand Test, manual muscle test, gait measure, Timed Up & Go test, clinical balance tests, range of motion, flexibility, sensation, pain, and fall history.18-32 Educational needs (eg, respiratory hygiene, nutrition, infection control, sleep, disease/symptom management) also are evaluated.
This thorough assessment is performed in a face-to-face outpatient visit. During the program participation, a physiatrist may be consulted for additional needs (eg, wheelchair assessment, home safety evaluation/ modifications, and mobility/disability issues). After completing the 6-week program, patients are scheduled for the postprogram evaluation in a face-to-face outpatient visit with the clinicians.
Equipment
Both clinician and the patient are equipped with a computer with Wi-Fi connectivity, a webcam, and a microphone. Patients are provided an exercise pictorial booklet, an exercise compact disk (audio and video), small exercise apparatuses (eg, assorted colors of resistance bands, hand grip exerciser, hand putty, ergometer, harmonica, and pedometer), incentive spirometer, pulse oximeter, cough assistive device (as needed), blood pressure monitor, COPD information booklets, and a diary to use at home during the program.33
Technology Preparation
Prior to starting the telehealth program, the patient is contacted 1 or 2 days before the first session for technical preparation and familiarization of the VA telehealth connection process. Either the PT or RT provides step-by-step instructions for the patient to practice connecting through VVC during this preparatory phone call. The patient also practices using the computer webcam, speaker, and microphone; checks the telehealth scheduling e-mail; and learns how to solve possible common technical issues (eg, adjusting volume and position of webcam). The patient is asked to set up a table close to the computer and to place all exercise apparatuses and respiratory devices on the table surface.
Program Delivery
A secure online VVC is used for connection during the telehealth session. The patient received an e-mail from the telehealth scheduling system with a link for VVC before each session. During the 6-week program, each telehealth session is conducted by a PT and a RT concurrently for 120 minutes, 3 days per week. The PT provides exercises for the patient to attempt, and the RT provides breathing training and monitoring during the session. After a successful connection to VVC, the therapist verifies the patient’s identity and confirms patient consent for the telehealth session.
After this check-in process, the patient performs a self-measure of resting blood pressure (BP), heart rate, respiratory rate, and blood oxygen saturation and reports to the therapists. During the exercise session, fatigue/exertion, dyspnea (Modified Borg Dyspnea Scale; Borg CR10 Scale), BP, heart rate, oxygen saturation, and other clinical symptoms and responses to exercise are monitored by the therapists, using both patient-reported measures and clinical observation by the therapists.34,35 Any medical emergency during the session is reported immediately to the pulmonologist for further management.
Structure
Prior to each exercise session, exercise precautions, fall prevention, good posture, pursed-lip breathing, pacing, and coordinated breathing are discussed with the patient. The PT demonstrates stretching and warm-up exercises in front of the webcam for the patient to follow. Then the patient performs all exercises in view of the webcam during the session (Figure 1). A RT monitors breathing patterns and corrects with verbal instructions if not properly performed.
Loss of skeletal muscle mass and cachexia are highly prevalent comorbidities of COPD and have been associated with breathlessness, functional limitation, and poor prognosis.36 To address these comorbidities, our program consists of progressive strengthening, aerobic, balance, and flexibility exercises. Resistance bands and tubes are used for strengthening exercises. Callisthenic exercises (eg, chair squat, chair stand, knee marching, bridging, single limb stances, and lunge) are used for progressive strengthening and balance exercises. Progression of strengthening and balance exercises are done through increasing the volume of exercise (ie, numbers of sets and repetitions) and increased load and level of difficulty based on the patient’s progress and comorbidity. The exercise program focuses on strengthening muscles, especially large muscle groups, to improve overall muscle strength and performance of functional activities.37
Arm/pedal ergometer and daily walking are used for daily aerobic exercise. In a study of patients with COPD by the PAC-COPD Study Group, step counter use was found to increase physical activity and improve exercise capacity, which supports its use in COPD management.38 During program participation, the patient is asked to wear a pedometer to monitor the number of steps taken per day and to report step data to the therapists during the telehealth session. The pedometer stores the previous 41 calendar days of data and displays the most recent 7 calendar days of data.
The patient is encouraged to set a realistic daily step goal. The general program goal is to increase at least 1000 steps per day. However, this goal can be adjusted depending on the patient’s health status and comorbid conditions. The PAC-COPD Study Group found that for every additional 1000 daily steps at low intensity, COPD hospitalization risk decreased by 20%.39 A magnitude of 2000 steps or about 1 mile of walking per day was found to be associated with increased physical activity and health benefits in the general population.40
Respiratory muscle training and breathing exercise are provided by the RT, using breathing and incentive spirometer techniques (Figure 2). Huff coughing, diaphragmatic deep breathing, and pursed-lip breathing are instructed by the RT during the session. Effective coughing technique with a cough assistive device is also provided during breathing training if needed.
Patient Education
In patients with COPD, there are numerous positive health benefits associated with education, including assisting the patients to become active participants in the PR program leading to satisfying outcomes; assisting the patients to better understand the lung health, disease processes, physical and psychological changes that occur with COPD; assisting the patients to explore coping strategies for those changes; building lifelong behavioral changes; and developing the self-management skills for sustainability. Through the educational process, patients with COPD can become more skilled at collaborative self-management and improve adherence to their treatment plan, which in turn can result in a reduction in hospital admissions and reduced health care costs.8,41
Education is provided with every session after the patient completes the exercise. Patients are required to record their COPD symptoms, daily activity, home exercise program, sleep, food intake, and additional physical or social activity in their COPD diary and to report during the session (Figure 3). A COPD diary assists patients in self-monitoring their COPD symptoms and provides the therapists with information about clinical changes, behavioral changes, and/or specific unmet needs for education. Several topics related to COPD are included in the education session: lung or respiratory disease/condition and self-management; smoking cessation; physical activity; energy-conserving techniques; breathing and coughing techniques; smoking cessation; nutrition/healthy eating and weight counseling; sex and intimacy; psychological counseling and/or group support; emergency planning (eg, medical, travel, and inclement weather); correct use of inhaler and medications; home oxygen; sleep and sleep hygiene; palliative care and advanced directive; infection control; and sputum clearance.42,43
Program Maintenance
After successfully completing the 6-week program, patients are referred to the VA TeleMOVE! Program or MOVE! Weight Management Program for continuous, long-term management of weight, nutrition, physical activity/exercise, and social activity needs or goals. The patients are scheduled for monthly follow-up phone visits for 6 months with the telerehabilitation team for enforcing sustainability. The phone call visit consists of reviewing breathing techniques, exercise program, physical activity, education, encouragement, and addressing any issues that arise during the self-maintained period.
Limitations
There are several issues of concern and precautions when delivering PR through telehealth into the home. First, the patient performs exercises independently without being manually guarded by the therapists. Risk of falls are a major concern due to impaired balance, poor vision, and other possible unusual physiologic responses to exercise (eg, drop in BP, dizziness, loss of balance). The area in front of the computer needs to be cleared of fall hazards (ie, area rug, wires, objects on the floor). The patient also needs to be educated on self-measurements of BP and oxygen saturation and reports to the therapists. The therapists provide detailed instructions on how to obtain these measures correctly; otherwise, the values may not be valid for a clinical judgment during the exercise session or for other clinical management. In a home environment, there is a limited use of exercise apparatuses. For this program, we only used resistance bands/tubes, small arm/leg ergometer, hand grip, and hand putty for the exercise program. We feel that dumbbell and weight plates are not suitable due to a possible risk of injury if the patient accidently drops them.
Advanced balance training is not suitable due to an increased risk for falls. Without the presence of the PT, level of challenge/difficulty is somewhat limited for this telehealth supervision exercise program. In addition, visual and audio quality are necessary for the session. The patient and the therapists need to see each other clearly to ensure correct methods and forms of each exercise. Furthermore, rehearsal of technical skills with the therapists is very important because this population is older and often has limited computer skills. Any technical difficulty or failure can lead to undesirable situations (eg, anxiety episodes, worries, shortness of breath, upset), which compromise exercise performance during the session. Finally, a phone is needed as an alternative in case of a poor VVC connection.
Conclusion
COPD symptoms and complications greatly affect patients’ ability to perform daily activities, decrease QOL and functional ability, and result in extensive use of health services. Many patients have limited access to a PR program at hospitals or rehabilitation centers due to health conditions, lack of transportation, and/or family support. This home-based, interactive telehealth PR program can break down the geographic barriers, solve poor program accessibility, potentially increase the utilization of PR, and reduce the cost and travel required by the patients.
Acknowledgments
The Telehealth Pulmonary Rehabilitation Program was originally funded by the Veterans Health Administration VA ACCESS Program (AS, CL, HKH). We thank all the veterans for their time and effort in participating in this newly developed rehabilitation program.
According to World Health Organization estimates, 65 million people have moderate-to-severe chronic obstructive pulmonary disease (COPD) globally, and > 20 million patients with COPD are living in the US.1 COPD is a progressive respiratory disease with a poor prognosis and a significant cause of morbidity and mortality in the US, especially within the Veterans Health Administration (VHA).2 The prevalence of COPD is higher in veterans than it is in the general population. COPD prevalence in the adult US population has been estimated to be between 5% and 15%, whereas in veterans, prevalence estimates have ranged from about 5% to 43%.3-5
COPD is associated with disabling dyspnea, muscle weakness, exercise intolerance, morbidity, and mortality. These symptoms and complications gradually and progressively compromise mobility, ability to perform daily functions, and decrease quality of life (QOL). Dyspnea, fatigue, and discomfort are the principal symptoms that negatively impact exercise tolerance.6,7 Therefore, patients often intentionally limit their activities to avoid these uncomfortable feelings and adopt a more sedentary behavior. As the disease progresses, individuals with COPD will gradually need assistance in performing activities of daily living, which eventually leads to functional dependence.
Pulmonary rehabilitation (PR) is an essential component of the management of symptomatic patients with COPD. PR is an evidence-based, multidisciplinary, comprehensive intervention that includes exercise and education for patients with chronic respiratory disease.8 The key benefits of PR are clinical improvements in dyspnea, physical capacity, QOL, and reduced disability in patients with COPD and other respiratory diseases.9-11 PR was found to improve respiratory health in veterans with COPD and decrease respiratory-related health care utilization.12
Despite the known benefits of PR, many patients with chronic respiratory diseases are not referred or do not have access to rehabilitation. Also, uptake of PR is low due to patient frailty, transportation issues, and other health care access problems.13-15 Unfortunately, in the US health care system, access to PR and other nonpharmacologic treatments can be challenging due to a shortage of available PR programs, limited physician referral to existing programs, and lack of family and social support.16
There are only a few accredited PR programs in VHA facilities, and they tend to be located in urban areas.12,17 Many patients have limited access to the PR programs due to geographic distance to the programs and transportation challenges (eg, limited ability to drive, cost of transportation). Moreover, veterans with COPD are likely to have limited mobility or are homebound due to experiencing shortness of breath with minimal exertion. Given the clear benefits of PR and the increasing impact of COPD on morbidity and mortality of the patients with COPD, strategies to improve the access and capacity of PR are needed. VA telehealth services allow for distribution of health care services in different geographic locations by providing access for the veterans who live in rural and highly rural areas. The most recent implementation of VA Video Connect (VVC) by the VHA provides a new avenue for clinicians to deliver much needed medical care into the veterans’ home.
COPD Telehealth Program
In this article, we describe the processes for developing and delivering an in-home, interactive, supervised PR program for veterans with severe COPD through VA telehealth service. The program consists of 18 sessions delivered over 6 weeks by a licensed physical therapist (PT) and a respiratory therapist (RT). The aims of the telehealth PR are to improve exercise tolerance, reduce dyspnea and fatigue, improve QOL, improve accessibility, and decrease costs and transportation burdens for patients with COPD. The program was developed, implemented and delivered by an interdisciplinary team, including a pulmonologist, PT, RT, physiatrist, and nonclinical supporting staff.
Patient Assessment
To be eligible to participate in the program the patient must: (1) have a forced expiratory volume (FEV1) < 60%; ( 2) be medically stable and be receiving optimal medical management; (3) have no severe cognitive impairments; (4) be able to use a computer and e-mail; (5) be able to ambulate with or without a walking device; (6) be willing to enroll in a smoking cessation program or to stop smoking; (7) be willing to participate without prolonged interruption; and (8) have all visual and auditory impairments corrected with medical devices.
After referral and enrollment, patients receive medical and physical examinations by the PR team, including a pulmonologist, a PT, and a RT, to ensure that the patients are medically stable to undergo rehabilitation and to develop a tailored exercise program while being mindful of the comorbidities, limitations, and precautions, (eg, loss of balance, risk of fall, limited range of motion). The preprogram assessment includes a pulmonary function test, arterial blood gas test, Montreal Cognitive Assessment, Modified Medical Research Council Scale, St. George Respiratory Questionnaire, the COPD Assessment Test, Patient Health Questionnaire-9,Generalized Anxiety Disorder Assessment-7, Epworth Sleepiness Scale, Katz Index of Independence of Activities of Daily Living, medications and inhaler use, oxygen use, breathing pattern, coughing, 6-minute walk test, Modified Borg Dyspnea Scale, grip strength, 5 Times Sit to Stand Test, manual muscle test, gait measure, Timed Up & Go test, clinical balance tests, range of motion, flexibility, sensation, pain, and fall history.18-32 Educational needs (eg, respiratory hygiene, nutrition, infection control, sleep, disease/symptom management) also are evaluated.
This thorough assessment is performed in a face-to-face outpatient visit. During the program participation, a physiatrist may be consulted for additional needs (eg, wheelchair assessment, home safety evaluation/ modifications, and mobility/disability issues). After completing the 6-week program, patients are scheduled for the postprogram evaluation in a face-to-face outpatient visit with the clinicians.
Equipment
Both clinician and the patient are equipped with a computer with Wi-Fi connectivity, a webcam, and a microphone. Patients are provided an exercise pictorial booklet, an exercise compact disk (audio and video), small exercise apparatuses (eg, assorted colors of resistance bands, hand grip exerciser, hand putty, ergometer, harmonica, and pedometer), incentive spirometer, pulse oximeter, cough assistive device (as needed), blood pressure monitor, COPD information booklets, and a diary to use at home during the program.33
Technology Preparation
Prior to starting the telehealth program, the patient is contacted 1 or 2 days before the first session for technical preparation and familiarization of the VA telehealth connection process. Either the PT or RT provides step-by-step instructions for the patient to practice connecting through VVC during this preparatory phone call. The patient also practices using the computer webcam, speaker, and microphone; checks the telehealth scheduling e-mail; and learns how to solve possible common technical issues (eg, adjusting volume and position of webcam). The patient is asked to set up a table close to the computer and to place all exercise apparatuses and respiratory devices on the table surface.
Program Delivery
A secure online VVC is used for connection during the telehealth session. The patient received an e-mail from the telehealth scheduling system with a link for VVC before each session. During the 6-week program, each telehealth session is conducted by a PT and a RT concurrently for 120 minutes, 3 days per week. The PT provides exercises for the patient to attempt, and the RT provides breathing training and monitoring during the session. After a successful connection to VVC, the therapist verifies the patient’s identity and confirms patient consent for the telehealth session.
After this check-in process, the patient performs a self-measure of resting blood pressure (BP), heart rate, respiratory rate, and blood oxygen saturation and reports to the therapists. During the exercise session, fatigue/exertion, dyspnea (Modified Borg Dyspnea Scale; Borg CR10 Scale), BP, heart rate, oxygen saturation, and other clinical symptoms and responses to exercise are monitored by the therapists, using both patient-reported measures and clinical observation by the therapists.34,35 Any medical emergency during the session is reported immediately to the pulmonologist for further management.
Structure
Prior to each exercise session, exercise precautions, fall prevention, good posture, pursed-lip breathing, pacing, and coordinated breathing are discussed with the patient. The PT demonstrates stretching and warm-up exercises in front of the webcam for the patient to follow. Then the patient performs all exercises in view of the webcam during the session (Figure 1). A RT monitors breathing patterns and corrects with verbal instructions if not properly performed.
Loss of skeletal muscle mass and cachexia are highly prevalent comorbidities of COPD and have been associated with breathlessness, functional limitation, and poor prognosis.36 To address these comorbidities, our program consists of progressive strengthening, aerobic, balance, and flexibility exercises. Resistance bands and tubes are used for strengthening exercises. Callisthenic exercises (eg, chair squat, chair stand, knee marching, bridging, single limb stances, and lunge) are used for progressive strengthening and balance exercises. Progression of strengthening and balance exercises are done through increasing the volume of exercise (ie, numbers of sets and repetitions) and increased load and level of difficulty based on the patient’s progress and comorbidity. The exercise program focuses on strengthening muscles, especially large muscle groups, to improve overall muscle strength and performance of functional activities.37
Arm/pedal ergometer and daily walking are used for daily aerobic exercise. In a study of patients with COPD by the PAC-COPD Study Group, step counter use was found to increase physical activity and improve exercise capacity, which supports its use in COPD management.38 During program participation, the patient is asked to wear a pedometer to monitor the number of steps taken per day and to report step data to the therapists during the telehealth session. The pedometer stores the previous 41 calendar days of data and displays the most recent 7 calendar days of data.
The patient is encouraged to set a realistic daily step goal. The general program goal is to increase at least 1000 steps per day. However, this goal can be adjusted depending on the patient’s health status and comorbid conditions. The PAC-COPD Study Group found that for every additional 1000 daily steps at low intensity, COPD hospitalization risk decreased by 20%.39 A magnitude of 2000 steps or about 1 mile of walking per day was found to be associated with increased physical activity and health benefits in the general population.40
Respiratory muscle training and breathing exercise are provided by the RT, using breathing and incentive spirometer techniques (Figure 2). Huff coughing, diaphragmatic deep breathing, and pursed-lip breathing are instructed by the RT during the session. Effective coughing technique with a cough assistive device is also provided during breathing training if needed.
Patient Education
In patients with COPD, there are numerous positive health benefits associated with education, including assisting the patients to become active participants in the PR program leading to satisfying outcomes; assisting the patients to better understand the lung health, disease processes, physical and psychological changes that occur with COPD; assisting the patients to explore coping strategies for those changes; building lifelong behavioral changes; and developing the self-management skills for sustainability. Through the educational process, patients with COPD can become more skilled at collaborative self-management and improve adherence to their treatment plan, which in turn can result in a reduction in hospital admissions and reduced health care costs.8,41
Education is provided with every session after the patient completes the exercise. Patients are required to record their COPD symptoms, daily activity, home exercise program, sleep, food intake, and additional physical or social activity in their COPD diary and to report during the session (Figure 3). A COPD diary assists patients in self-monitoring their COPD symptoms and provides the therapists with information about clinical changes, behavioral changes, and/or specific unmet needs for education. Several topics related to COPD are included in the education session: lung or respiratory disease/condition and self-management; smoking cessation; physical activity; energy-conserving techniques; breathing and coughing techniques; smoking cessation; nutrition/healthy eating and weight counseling; sex and intimacy; psychological counseling and/or group support; emergency planning (eg, medical, travel, and inclement weather); correct use of inhaler and medications; home oxygen; sleep and sleep hygiene; palliative care and advanced directive; infection control; and sputum clearance.42,43
Program Maintenance
After successfully completing the 6-week program, patients are referred to the VA TeleMOVE! Program or MOVE! Weight Management Program for continuous, long-term management of weight, nutrition, physical activity/exercise, and social activity needs or goals. The patients are scheduled for monthly follow-up phone visits for 6 months with the telerehabilitation team for enforcing sustainability. The phone call visit consists of reviewing breathing techniques, exercise program, physical activity, education, encouragement, and addressing any issues that arise during the self-maintained period.
Limitations
There are several issues of concern and precautions when delivering PR through telehealth into the home. First, the patient performs exercises independently without being manually guarded by the therapists. Risk of falls are a major concern due to impaired balance, poor vision, and other possible unusual physiologic responses to exercise (eg, drop in BP, dizziness, loss of balance). The area in front of the computer needs to be cleared of fall hazards (ie, area rug, wires, objects on the floor). The patient also needs to be educated on self-measurements of BP and oxygen saturation and reports to the therapists. The therapists provide detailed instructions on how to obtain these measures correctly; otherwise, the values may not be valid for a clinical judgment during the exercise session or for other clinical management. In a home environment, there is a limited use of exercise apparatuses. For this program, we only used resistance bands/tubes, small arm/leg ergometer, hand grip, and hand putty for the exercise program. We feel that dumbbell and weight plates are not suitable due to a possible risk of injury if the patient accidently drops them.
Advanced balance training is not suitable due to an increased risk for falls. Without the presence of the PT, level of challenge/difficulty is somewhat limited for this telehealth supervision exercise program. In addition, visual and audio quality are necessary for the session. The patient and the therapists need to see each other clearly to ensure correct methods and forms of each exercise. Furthermore, rehearsal of technical skills with the therapists is very important because this population is older and often has limited computer skills. Any technical difficulty or failure can lead to undesirable situations (eg, anxiety episodes, worries, shortness of breath, upset), which compromise exercise performance during the session. Finally, a phone is needed as an alternative in case of a poor VVC connection.
Conclusion
COPD symptoms and complications greatly affect patients’ ability to perform daily activities, decrease QOL and functional ability, and result in extensive use of health services. Many patients have limited access to a PR program at hospitals or rehabilitation centers due to health conditions, lack of transportation, and/or family support. This home-based, interactive telehealth PR program can break down the geographic barriers, solve poor program accessibility, potentially increase the utilization of PR, and reduce the cost and travel required by the patients.
Acknowledgments
The Telehealth Pulmonary Rehabilitation Program was originally funded by the Veterans Health Administration VA ACCESS Program (AS, CL, HKH). We thank all the veterans for their time and effort in participating in this newly developed rehabilitation program.
1. World Health Organization. Chronic obstructive pulmonary disease (COPD). http://www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd). Published December 1, 2017. Accessed August 7, 2019.
2. Yu W, Ravelo A, Wagner TH, et al. Prevalence and costs of chronic conditions in the VA health care system. Med Care Res Rev. 2003;60(suppl 3):146S-167S.
3. Doney B, Hnizdo E, Dillon CF, et al. Prevalence of airflow obstruction in U.S. adults aged 40-79 years: NHANES data 1988-1994 and 2007-2010. COPD. 2015;12(4):355-365.
4. Murphy DE, Chaudhry Z, Almoosa KF, Panos RJ. High prevalence of chronic obstructive pulmonary disease among veterans in the urban midwest. Mil Med. 2011;176(5):552-560.
5. Cypel YS, Hines SE, Davey VJ, Eber SM, Schneiderman AI. Self-reported physician-diagnosed chronic obstructive pulmonary disease and spirometry patterns in Vietnam Era US Army Chemical Corps veterans: a retrospective cohort study. Am J Ind Med. 2018;61(10):802-814.
6. Rochester CL. Exercise training in chronic obstructive pulmonary disease. J Rehabil Res Dev. 2003;40(5)(suppl 2):59-80.
7. Cortopassi F, Gurung P, Pinto-Plata V. Chronic obstructive pulmonary disease in elderly patients. Clin Geriatr Med. 2017;33(4):539-552.
8. Spruit MA, Singh SJ, Garvey C, et al; ATS/ERS Task Force on Pulmonary Rehabilitation. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188(8):e13-e64.
9. Robinson H, Williams V, Curtis F, Bridle C, Jones AW. Facilitators and barriers to physical activity following pulmonary rehabilitation in COPD: a systematic review of qualitative studies. NPJ Prim Care Respir Med. 2018;28(1):19.
10. McCarthy B, Casey D, Devane D, Murphy K, Murphy E, Lacasse Y. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015;(2):CD003793.
11. Ries AL, Bauldoff GS, Carlin BW, et al. Pulmonary rehabilitation: joint AACP/AACVPR evidence-based clinical practice guidelines. Chest. 2007;131(suppl 5):4S-42S.
12. Major S, Moreno M, Shelton J, Panos RJ. Veterans with chronic obstructive pulmonary disease achieve clinically relevant improvements in respiratory health after pulmonary rehabilitation. J Cardiopulm Rehabil Prev. 2014;34(6):420-429.
13. Liu Y, Dickerson T, Early F, Fuld J, Clarkson PJ. Understanding influences on the uptake of pulmonary rehabilitation in the East of England: an inclusive design/mixed methods study protocol. BMJ Open. 2018;8(4):e020750.
14. Harris D, Hayter M, Allender S. Factors affecting the offer of pulmonary rehabilitation to patients with chronic obstructive pulmonary disease by primary care professionals: a qualitative study. Prim Health Care Res Dev. 2008;9(4):280-290.
15. Mathar H, Fastholm P, Hansen IR, Larsen NS. Why do patients with COPD decline rehabilitation. Scand J Caring Sci. 2016;30(3):432-441.
16. Han MK, Martinez CH, Au DH, et al. Meeting the challenge of COPD care delivery in the USA: a multiprovider perspective. Lancet Respir Med. 2016;4(6):473-526.
17. American Association of Cardiovascular and Pulmonary Rehabilitation (AACVPR). Online searchable program directory. https://www.aacvpr.org/Resources/Program-Directory Accessed July 19, 2018.
18. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695-699.
19. Fletcher CM, Elmes PC, Fairbairn AS, Wood CH. The significance of respiratory symptoms and the diagnosis of chronic bronchitis in a working population. Br Med J. 1959;2(5147):257-266.
20. O’Donnell DE, Aaron S, Bourbeau J, et al. Canadian Thoracic Society recommendations for management of chronic obstructive pulmonary disease—2007 update. Can Respir J. 2007;14(suppl B):5B-32B.
21. Jones PW, Quirk FH, Baveystock CM. The St George’s Respiratory Questionnaire. Respir Med. 1991;85(suppl B):25-31.
22. Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34(3):648-654.
23. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613.
24. Spitzer RL, Kroenke K, Williams JBW, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092-1097.
25. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540-545.
26. Katz S. Assessing self-maintenance: activities of daily living, mobility and instrumental activities of daily living. J Am Geriatr Soc. 1983;31(12):721-727.
27. Holland AE, Spruit MA, Troosters T, et al. An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J. 2014;44(6):1428-1446.
28. Mahler DA, Horowitz MB. Perception of breathlessness during exercise in patients with respiratory disease. Med Sci Sports Exerc. 1994;26(9):1078-1081.
29. Liao WC, Wang CH, Yu SY, Chen LY, Wang CY. Grip strength measurement in older adults in Taiwan: a comparison of three testing positions. Australas J Ageing. 2014;33(4):278-282.
30. Buatois S, Miljkovic D, Manckoundia P, et al. Five times sit to stand test is a predictor of recurrent falls in healthy community-living subjects aged 65 and older. J Am Geriatr Soc. 2008;56(8):1575-1577.
31. Bryant MS, Workman CD, Jackson GR. Multidirectional walk test in persons with Parkinson’s disease: a validity study. Int J Rehabil Res. 2015;38(1):88-91.
32. Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39(2):142-148.
33. University of Nebraska Medical Center. Timed Up and Go (TUG) Test. https://www.unmc.edu/media/intmed/geriatrics/nebgec/pdf/frailelderlyjuly09/toolkits/timedupandgo_w_norms.pdf. Accessed August 13, 2019.
34. Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14(5):377-381.
35. Mahler DA, Horowitz MB. Clinical evaluation of exertional dyspnea. Clin Chest Med. 1994;15(2):259-269.
36. Dudgeon D, Baracos VE. Physiological and functional failure in chronic obstructive pulmonary disease, congestive heart failure and cancer: a debilitating intersection of sarcopenia, cachexia and breathlessness. Curr Opin Support Palliat Care. 2016;10(3):236-241.
37. Lee AL, Holland AE. Time to adapt exercise training regimens in pulmonary rehabilitation—a review of the literature. Int J Chron Obstruct Pulmon Dis. 2014;9:1275-1288.
38. Qiu S, Cai X, Wang X, et al. Using step counters to promote physical activity and exercise capacity in patients with chronic obstructive pulmonary disease: a meta-analysis. Ther Adv Respir Dis. 2018;12:1753466618787386.
39. Donaire-Gonzalez D, Gimeno-Santos E, Balcells E, et al; PAC-COPD Study Group. Benefits of physical activity on COPD hospitalization depend on intensity. Eur Respir J. 2015;46(5):1281-1289.
40. Bravata DM, Smith-Spangler C, Sundaram V, et al. Using pedometers to increase physical activity and improve health: a systematic review. JAMA. 2007;298(19):2296-2304.
41. Zwerink M, Brusse-Keizer M, van der Valk PD, et al. Self-management for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014;19(3):CD002990.
42. Wilson JS, O’Neill B, Reilly J, MacMahon J, Bradley JM. Education in pulmonary rehabilitation: the patient’s perspective. Arch Phys Med Rehabil. 2007;88(12):1704-1709.
43. Bourbeau J, Nault D, Dang-Tan T. Self-management and behaviour modification in COPD. Patient Educ Couns. 2004;52(3):271-277.
1. World Health Organization. Chronic obstructive pulmonary disease (COPD). http://www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd). Published December 1, 2017. Accessed August 7, 2019.
2. Yu W, Ravelo A, Wagner TH, et al. Prevalence and costs of chronic conditions in the VA health care system. Med Care Res Rev. 2003;60(suppl 3):146S-167S.
3. Doney B, Hnizdo E, Dillon CF, et al. Prevalence of airflow obstruction in U.S. adults aged 40-79 years: NHANES data 1988-1994 and 2007-2010. COPD. 2015;12(4):355-365.
4. Murphy DE, Chaudhry Z, Almoosa KF, Panos RJ. High prevalence of chronic obstructive pulmonary disease among veterans in the urban midwest. Mil Med. 2011;176(5):552-560.
5. Cypel YS, Hines SE, Davey VJ, Eber SM, Schneiderman AI. Self-reported physician-diagnosed chronic obstructive pulmonary disease and spirometry patterns in Vietnam Era US Army Chemical Corps veterans: a retrospective cohort study. Am J Ind Med. 2018;61(10):802-814.
6. Rochester CL. Exercise training in chronic obstructive pulmonary disease. J Rehabil Res Dev. 2003;40(5)(suppl 2):59-80.
7. Cortopassi F, Gurung P, Pinto-Plata V. Chronic obstructive pulmonary disease in elderly patients. Clin Geriatr Med. 2017;33(4):539-552.
8. Spruit MA, Singh SJ, Garvey C, et al; ATS/ERS Task Force on Pulmonary Rehabilitation. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188(8):e13-e64.
9. Robinson H, Williams V, Curtis F, Bridle C, Jones AW. Facilitators and barriers to physical activity following pulmonary rehabilitation in COPD: a systematic review of qualitative studies. NPJ Prim Care Respir Med. 2018;28(1):19.
10. McCarthy B, Casey D, Devane D, Murphy K, Murphy E, Lacasse Y. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015;(2):CD003793.
11. Ries AL, Bauldoff GS, Carlin BW, et al. Pulmonary rehabilitation: joint AACP/AACVPR evidence-based clinical practice guidelines. Chest. 2007;131(suppl 5):4S-42S.
12. Major S, Moreno M, Shelton J, Panos RJ. Veterans with chronic obstructive pulmonary disease achieve clinically relevant improvements in respiratory health after pulmonary rehabilitation. J Cardiopulm Rehabil Prev. 2014;34(6):420-429.
13. Liu Y, Dickerson T, Early F, Fuld J, Clarkson PJ. Understanding influences on the uptake of pulmonary rehabilitation in the East of England: an inclusive design/mixed methods study protocol. BMJ Open. 2018;8(4):e020750.
14. Harris D, Hayter M, Allender S. Factors affecting the offer of pulmonary rehabilitation to patients with chronic obstructive pulmonary disease by primary care professionals: a qualitative study. Prim Health Care Res Dev. 2008;9(4):280-290.
15. Mathar H, Fastholm P, Hansen IR, Larsen NS. Why do patients with COPD decline rehabilitation. Scand J Caring Sci. 2016;30(3):432-441.
16. Han MK, Martinez CH, Au DH, et al. Meeting the challenge of COPD care delivery in the USA: a multiprovider perspective. Lancet Respir Med. 2016;4(6):473-526.
17. American Association of Cardiovascular and Pulmonary Rehabilitation (AACVPR). Online searchable program directory. https://www.aacvpr.org/Resources/Program-Directory Accessed July 19, 2018.
18. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695-699.
19. Fletcher CM, Elmes PC, Fairbairn AS, Wood CH. The significance of respiratory symptoms and the diagnosis of chronic bronchitis in a working population. Br Med J. 1959;2(5147):257-266.
20. O’Donnell DE, Aaron S, Bourbeau J, et al. Canadian Thoracic Society recommendations for management of chronic obstructive pulmonary disease—2007 update. Can Respir J. 2007;14(suppl B):5B-32B.
21. Jones PW, Quirk FH, Baveystock CM. The St George’s Respiratory Questionnaire. Respir Med. 1991;85(suppl B):25-31.
22. Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34(3):648-654.
23. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613.
24. Spitzer RL, Kroenke K, Williams JBW, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092-1097.
25. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540-545.
26. Katz S. Assessing self-maintenance: activities of daily living, mobility and instrumental activities of daily living. J Am Geriatr Soc. 1983;31(12):721-727.
27. Holland AE, Spruit MA, Troosters T, et al. An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J. 2014;44(6):1428-1446.
28. Mahler DA, Horowitz MB. Perception of breathlessness during exercise in patients with respiratory disease. Med Sci Sports Exerc. 1994;26(9):1078-1081.
29. Liao WC, Wang CH, Yu SY, Chen LY, Wang CY. Grip strength measurement in older adults in Taiwan: a comparison of three testing positions. Australas J Ageing. 2014;33(4):278-282.
30. Buatois S, Miljkovic D, Manckoundia P, et al. Five times sit to stand test is a predictor of recurrent falls in healthy community-living subjects aged 65 and older. J Am Geriatr Soc. 2008;56(8):1575-1577.
31. Bryant MS, Workman CD, Jackson GR. Multidirectional walk test in persons with Parkinson’s disease: a validity study. Int J Rehabil Res. 2015;38(1):88-91.
32. Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39(2):142-148.
33. University of Nebraska Medical Center. Timed Up and Go (TUG) Test. https://www.unmc.edu/media/intmed/geriatrics/nebgec/pdf/frailelderlyjuly09/toolkits/timedupandgo_w_norms.pdf. Accessed August 13, 2019.
34. Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14(5):377-381.
35. Mahler DA, Horowitz MB. Clinical evaluation of exertional dyspnea. Clin Chest Med. 1994;15(2):259-269.
36. Dudgeon D, Baracos VE. Physiological and functional failure in chronic obstructive pulmonary disease, congestive heart failure and cancer: a debilitating intersection of sarcopenia, cachexia and breathlessness. Curr Opin Support Palliat Care. 2016;10(3):236-241.
37. Lee AL, Holland AE. Time to adapt exercise training regimens in pulmonary rehabilitation—a review of the literature. Int J Chron Obstruct Pulmon Dis. 2014;9:1275-1288.
38. Qiu S, Cai X, Wang X, et al. Using step counters to promote physical activity and exercise capacity in patients with chronic obstructive pulmonary disease: a meta-analysis. Ther Adv Respir Dis. 2018;12:1753466618787386.
39. Donaire-Gonzalez D, Gimeno-Santos E, Balcells E, et al; PAC-COPD Study Group. Benefits of physical activity on COPD hospitalization depend on intensity. Eur Respir J. 2015;46(5):1281-1289.
40. Bravata DM, Smith-Spangler C, Sundaram V, et al. Using pedometers to increase physical activity and improve health: a systematic review. JAMA. 2007;298(19):2296-2304.
41. Zwerink M, Brusse-Keizer M, van der Valk PD, et al. Self-management for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014;19(3):CD002990.
42. Wilson JS, O’Neill B, Reilly J, MacMahon J, Bradley JM. Education in pulmonary rehabilitation: the patient’s perspective. Arch Phys Med Rehabil. 2007;88(12):1704-1709.
43. Bourbeau J, Nault D, Dang-Tan T. Self-management and behaviour modification in COPD. Patient Educ Couns. 2004;52(3):271-277.
Videos help chemo patients better understand their treatments
Showing cancer patients receiving chemotherapy short videos about their treatments has the potential to improve their understanding about the treatments they are receiving, new research in Cancer has shown.
Researchers showed 50 patients at an underserved hospital six 1-minute videos focused on important terms related to their chemotherapy treatments and found that the videos helped them better understand what those key terms mean. Before viewing the videos, 15 of 20 terms were misunderstood by more than one third of patients, with 98% unable to define “maintenance,” 74% unable to define “cancer,” and 58% unable to define “chemotherapy.” Six pilot educational videos describing a narrowed down list of six terms were created, and patient understanding of all six terms improved by at least 20% after watching the videos. The six terms defined in the videos were “palliative chemotherapy,” “curative,” “cancer,” “blood count,” “risk of infection,” and “chemotherapy.”
“Although a current concern is that precision medicines will not be understood due to genetic illiteracy and misunderstandings about the immune system, it is important to remember that the terminology used to describe chemotherapy, the backbone of many cancer treatments, may also be incomprehensible to some patients,” Rebecca Pentz, PhD, of Emory University, Atlanta, and colleagues wrote.
“Our video pilot suggests that multimedia can help patients understand chemotherapy terminology,” Dr. Pentz and colleagues said. “For each term, there was at least a 20% increase in patient understanding after watching the video. None of the patients could define palliative chemotherapy before watching the video, but 72% were able to provide a definition afterward.”
Researchers noted that the term most understood after the video was curative treatment (patients being able to define the phrase grew from 34% to 88%).
“Our study establishes that basic chemotherapy terminology is widely misunderstood by an underserved population, but that video-based education can significantly increase patient understanding,” the investigators concluded, but noted the research was limited by the small number of videos as well as the single underserved hospital, which may limit the generalizablility of the study.
Dr. Pentz and colleagues added that “education of physicians about the severe patient lack of understanding of basic cancer terminology and methods to improve understanding would be most helpful.”
SOURCE: Pentz R et al. Cancer. 2019 Aug 16. doi: 10.1002/cncr.32421.
Research conducted by Pentz et al. on how much the core vocabulary is misunderstood is deeply troubling, suggesting that we as oncologists are not meeting the informational needs of patients who are consenting to undergo chemotherapy.
With patients showing better understanding after the videos on the terminology, it revives the notion that informed consent is a process that may require multiple interactions to ensure that patients truly understand what they are getting into with chemotherapy treatment, counter to the current treatment environment where there is a rush to get consent, treat the patient, and move onto the next one.
Whether the results of the study improve informed consent policy is something that remains to be seen.
Kerry Kilbridge, MD , of the Dana-Farber Cancer Institute in Boston made these comments in an Aug. 16 accompanying editorial published in Cancer (doi: 10.1002/cncr.32418).
Research conducted by Pentz et al. on how much the core vocabulary is misunderstood is deeply troubling, suggesting that we as oncologists are not meeting the informational needs of patients who are consenting to undergo chemotherapy.
With patients showing better understanding after the videos on the terminology, it revives the notion that informed consent is a process that may require multiple interactions to ensure that patients truly understand what they are getting into with chemotherapy treatment, counter to the current treatment environment where there is a rush to get consent, treat the patient, and move onto the next one.
Whether the results of the study improve informed consent policy is something that remains to be seen.
Kerry Kilbridge, MD , of the Dana-Farber Cancer Institute in Boston made these comments in an Aug. 16 accompanying editorial published in Cancer (doi: 10.1002/cncr.32418).
Research conducted by Pentz et al. on how much the core vocabulary is misunderstood is deeply troubling, suggesting that we as oncologists are not meeting the informational needs of patients who are consenting to undergo chemotherapy.
With patients showing better understanding after the videos on the terminology, it revives the notion that informed consent is a process that may require multiple interactions to ensure that patients truly understand what they are getting into with chemotherapy treatment, counter to the current treatment environment where there is a rush to get consent, treat the patient, and move onto the next one.
Whether the results of the study improve informed consent policy is something that remains to be seen.
Kerry Kilbridge, MD , of the Dana-Farber Cancer Institute in Boston made these comments in an Aug. 16 accompanying editorial published in Cancer (doi: 10.1002/cncr.32418).
Showing cancer patients receiving chemotherapy short videos about their treatments has the potential to improve their understanding about the treatments they are receiving, new research in Cancer has shown.
Researchers showed 50 patients at an underserved hospital six 1-minute videos focused on important terms related to their chemotherapy treatments and found that the videos helped them better understand what those key terms mean. Before viewing the videos, 15 of 20 terms were misunderstood by more than one third of patients, with 98% unable to define “maintenance,” 74% unable to define “cancer,” and 58% unable to define “chemotherapy.” Six pilot educational videos describing a narrowed down list of six terms were created, and patient understanding of all six terms improved by at least 20% after watching the videos. The six terms defined in the videos were “palliative chemotherapy,” “curative,” “cancer,” “blood count,” “risk of infection,” and “chemotherapy.”
“Although a current concern is that precision medicines will not be understood due to genetic illiteracy and misunderstandings about the immune system, it is important to remember that the terminology used to describe chemotherapy, the backbone of many cancer treatments, may also be incomprehensible to some patients,” Rebecca Pentz, PhD, of Emory University, Atlanta, and colleagues wrote.
“Our video pilot suggests that multimedia can help patients understand chemotherapy terminology,” Dr. Pentz and colleagues said. “For each term, there was at least a 20% increase in patient understanding after watching the video. None of the patients could define palliative chemotherapy before watching the video, but 72% were able to provide a definition afterward.”
Researchers noted that the term most understood after the video was curative treatment (patients being able to define the phrase grew from 34% to 88%).
“Our study establishes that basic chemotherapy terminology is widely misunderstood by an underserved population, but that video-based education can significantly increase patient understanding,” the investigators concluded, but noted the research was limited by the small number of videos as well as the single underserved hospital, which may limit the generalizablility of the study.
Dr. Pentz and colleagues added that “education of physicians about the severe patient lack of understanding of basic cancer terminology and methods to improve understanding would be most helpful.”
SOURCE: Pentz R et al. Cancer. 2019 Aug 16. doi: 10.1002/cncr.32421.
Showing cancer patients receiving chemotherapy short videos about their treatments has the potential to improve their understanding about the treatments they are receiving, new research in Cancer has shown.
Researchers showed 50 patients at an underserved hospital six 1-minute videos focused on important terms related to their chemotherapy treatments and found that the videos helped them better understand what those key terms mean. Before viewing the videos, 15 of 20 terms were misunderstood by more than one third of patients, with 98% unable to define “maintenance,” 74% unable to define “cancer,” and 58% unable to define “chemotherapy.” Six pilot educational videos describing a narrowed down list of six terms were created, and patient understanding of all six terms improved by at least 20% after watching the videos. The six terms defined in the videos were “palliative chemotherapy,” “curative,” “cancer,” “blood count,” “risk of infection,” and “chemotherapy.”
“Although a current concern is that precision medicines will not be understood due to genetic illiteracy and misunderstandings about the immune system, it is important to remember that the terminology used to describe chemotherapy, the backbone of many cancer treatments, may also be incomprehensible to some patients,” Rebecca Pentz, PhD, of Emory University, Atlanta, and colleagues wrote.
“Our video pilot suggests that multimedia can help patients understand chemotherapy terminology,” Dr. Pentz and colleagues said. “For each term, there was at least a 20% increase in patient understanding after watching the video. None of the patients could define palliative chemotherapy before watching the video, but 72% were able to provide a definition afterward.”
Researchers noted that the term most understood after the video was curative treatment (patients being able to define the phrase grew from 34% to 88%).
“Our study establishes that basic chemotherapy terminology is widely misunderstood by an underserved population, but that video-based education can significantly increase patient understanding,” the investigators concluded, but noted the research was limited by the small number of videos as well as the single underserved hospital, which may limit the generalizablility of the study.
Dr. Pentz and colleagues added that “education of physicians about the severe patient lack of understanding of basic cancer terminology and methods to improve understanding would be most helpful.”
SOURCE: Pentz R et al. Cancer. 2019 Aug 16. doi: 10.1002/cncr.32421.
FROM CANCER
Key clinical point: Short videos on chemotherapy terms improved patient understanding of concepts.
Major finding: Patient understanding of the six terms chosen as part of the study improved by at least 20%.
Study details: 50 patients were asked to define six terms related to cancer treatment before and after seeing a 1-minute video on each term.
Disclosures: The research was sponsored by the Winship Cancer Institute and the National Cancer Institute. Research authors reported no conflicts of interest.
Source: Pentz R et al. Cancer. 2019 Aug 16. doi: 10.1002/cncr.32421.