User login
Investigational laser for acne treatment targets sebaceous gland, spares dermis
DENVER – An investigational 1726-nm without histologic disruption to the surrounding dermis, results from a small study showed.
“It is our belief that if we can destruct the sebaceous glands, it will cure acne,” Emil A. Tanghetti, MD, said at the annual conference of the American Society for Laser Medicine and Surgery. “High-dose red light PDT [photodynamic therapy] has been used, but it causes epidermal damage and an unacceptably long recovery time. Gold and silver nanoshells with infrared light have also been used. Usually these particles do not get into the sebaceous glands and appear to result in temporary improvement by wounding the infrainfundibular region of the sebaceous gland complex. It’s been shown that the 1726-nm light can target sebum and might be able to selectively damage sebaceous glands.”
Dr. Tanghetti, of the Sacramento-based Center for Dermatology and Laser Surgery, along with R. Rox Anderson, MD, and Fernanda H. Sakamoto, MD, PhD, of the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston, are studying a 1726-nm laser being developed by Accure. This device uses robust and precise air cooling, a creative pulsing strategy to enhance the differential sebum to water absorption, and active, real-time monitoring via thermal imaging.
“The device is constructed to turn off before an unsafe temperature is reached,” Dr. Tanghetti said. “The thermal camera is looking at the treatment site as it happening. If it gets too hot, it can turn the whole device off. You can only do real-time monitoring with air cooling. You cannot do it with contract cooling or cryogen cooling.”
In a study of 10 patients with acne, the researchers developed a multiple pulse strategy to slowly and preferentially heat sebaceous glands while sparing the epidermis and the surrounding dermis. They performed 3.5-mm punch biopsies at 24, 48, and 72 hours after treatment, and evaluated them with hematoxylin and eosin staining.
From a clinical standpoint, Dr. Tanghetti and his colleagues noted small papules in the treated areas immediately, 24 hours and 72 hours after treatment. “You don’t see the epidermal damage that you would see with someone who had PDT,” he said.
Histologic evaluation of tissue specimens at 24 and 72 hours revealed destruction of sebaceous glands in the dermis characterized by loss of the definition of the sebocytes, and eosinophilic changes of the basal cell layer of these glands. The collagen surrounding the gland appeared to be preserved, with occasional small clots observed in the adjacent blood vessels. “There was no obvious damage to the surrounding dermis or other follicular structures,” Dr. Tanghetti said. “The hard thing is to differentiate complete damage [of the sebaceous gland] from partial damage. That’s something we’re working on by looking at sebum production, which is going to be the ultimate outcome.”
The study authors reported having numerous financial ties to medical device and pharmaceutical companies.
dbrunk@mdedge.com
DENVER – An investigational 1726-nm without histologic disruption to the surrounding dermis, results from a small study showed.
“It is our belief that if we can destruct the sebaceous glands, it will cure acne,” Emil A. Tanghetti, MD, said at the annual conference of the American Society for Laser Medicine and Surgery. “High-dose red light PDT [photodynamic therapy] has been used, but it causes epidermal damage and an unacceptably long recovery time. Gold and silver nanoshells with infrared light have also been used. Usually these particles do not get into the sebaceous glands and appear to result in temporary improvement by wounding the infrainfundibular region of the sebaceous gland complex. It’s been shown that the 1726-nm light can target sebum and might be able to selectively damage sebaceous glands.”
Dr. Tanghetti, of the Sacramento-based Center for Dermatology and Laser Surgery, along with R. Rox Anderson, MD, and Fernanda H. Sakamoto, MD, PhD, of the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston, are studying a 1726-nm laser being developed by Accure. This device uses robust and precise air cooling, a creative pulsing strategy to enhance the differential sebum to water absorption, and active, real-time monitoring via thermal imaging.
“The device is constructed to turn off before an unsafe temperature is reached,” Dr. Tanghetti said. “The thermal camera is looking at the treatment site as it happening. If it gets too hot, it can turn the whole device off. You can only do real-time monitoring with air cooling. You cannot do it with contract cooling or cryogen cooling.”
In a study of 10 patients with acne, the researchers developed a multiple pulse strategy to slowly and preferentially heat sebaceous glands while sparing the epidermis and the surrounding dermis. They performed 3.5-mm punch biopsies at 24, 48, and 72 hours after treatment, and evaluated them with hematoxylin and eosin staining.
From a clinical standpoint, Dr. Tanghetti and his colleagues noted small papules in the treated areas immediately, 24 hours and 72 hours after treatment. “You don’t see the epidermal damage that you would see with someone who had PDT,” he said.
Histologic evaluation of tissue specimens at 24 and 72 hours revealed destruction of sebaceous glands in the dermis characterized by loss of the definition of the sebocytes, and eosinophilic changes of the basal cell layer of these glands. The collagen surrounding the gland appeared to be preserved, with occasional small clots observed in the adjacent blood vessels. “There was no obvious damage to the surrounding dermis or other follicular structures,” Dr. Tanghetti said. “The hard thing is to differentiate complete damage [of the sebaceous gland] from partial damage. That’s something we’re working on by looking at sebum production, which is going to be the ultimate outcome.”
The study authors reported having numerous financial ties to medical device and pharmaceutical companies.
dbrunk@mdedge.com
DENVER – An investigational 1726-nm without histologic disruption to the surrounding dermis, results from a small study showed.
“It is our belief that if we can destruct the sebaceous glands, it will cure acne,” Emil A. Tanghetti, MD, said at the annual conference of the American Society for Laser Medicine and Surgery. “High-dose red light PDT [photodynamic therapy] has been used, but it causes epidermal damage and an unacceptably long recovery time. Gold and silver nanoshells with infrared light have also been used. Usually these particles do not get into the sebaceous glands and appear to result in temporary improvement by wounding the infrainfundibular region of the sebaceous gland complex. It’s been shown that the 1726-nm light can target sebum and might be able to selectively damage sebaceous glands.”
Dr. Tanghetti, of the Sacramento-based Center for Dermatology and Laser Surgery, along with R. Rox Anderson, MD, and Fernanda H. Sakamoto, MD, PhD, of the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston, are studying a 1726-nm laser being developed by Accure. This device uses robust and precise air cooling, a creative pulsing strategy to enhance the differential sebum to water absorption, and active, real-time monitoring via thermal imaging.
“The device is constructed to turn off before an unsafe temperature is reached,” Dr. Tanghetti said. “The thermal camera is looking at the treatment site as it happening. If it gets too hot, it can turn the whole device off. You can only do real-time monitoring with air cooling. You cannot do it with contract cooling or cryogen cooling.”
In a study of 10 patients with acne, the researchers developed a multiple pulse strategy to slowly and preferentially heat sebaceous glands while sparing the epidermis and the surrounding dermis. They performed 3.5-mm punch biopsies at 24, 48, and 72 hours after treatment, and evaluated them with hematoxylin and eosin staining.
From a clinical standpoint, Dr. Tanghetti and his colleagues noted small papules in the treated areas immediately, 24 hours and 72 hours after treatment. “You don’t see the epidermal damage that you would see with someone who had PDT,” he said.
Histologic evaluation of tissue specimens at 24 and 72 hours revealed destruction of sebaceous glands in the dermis characterized by loss of the definition of the sebocytes, and eosinophilic changes of the basal cell layer of these glands. The collagen surrounding the gland appeared to be preserved, with occasional small clots observed in the adjacent blood vessels. “There was no obvious damage to the surrounding dermis or other follicular structures,” Dr. Tanghetti said. “The hard thing is to differentiate complete damage [of the sebaceous gland] from partial damage. That’s something we’re working on by looking at sebum production, which is going to be the ultimate outcome.”
The study authors reported having numerous financial ties to medical device and pharmaceutical companies.
dbrunk@mdedge.com
EXPERT ANALYSIS FROM ASLMS 2019
Role of Diet in Treating Skin Conditions



Topical Natural Products in Managing Dermatologic Conditions: Observations and Recommendations
Patients seek healthy skin that conveys overall health and well-being. Cosmeceuticals claim to therapeutically affect the structure and function of the skin, and it is rational to hold them to scientific standards that substantiate efficacy claims.1 Notably, it is increasingly important to consider nature-based products in helping patients and consumers to achieve healthier skin. Despite the availability of sophisticated efficacy testing, explanations of the underlying physiologic and pharmacologic principles of nature-based products lag behind those of conventional formulations. In many instances, simple form and function information cannot adequately support their desired use and expected benefits. In addition, cosmetic regulations do not even permit structure-function claims that are allowed for dietary supplements.
Physicians whose patients want recommendations for nature-based products often do not know where to turn for definitive product and use information. Unlike prescription medications or even beauty-from-within dietary supplement products, natural cosmetics and cosmeceuticals are barred from communicating scientific evidence and experience of use to form proper opinions for recommendations. Without the benefit of full product labeling, physicians are left to mine sparse, confusing, and often contradictory literature in an effort to self-educate. Here, we share our experiences with patients, our operating knowledge base, and our recommendations for investigation to improve the available information and ensure practicing physicians have the information they need to appropriately recommend nature-based products.
General Observations Pertaining to Patients and Nature-Based Products
Ethnic and cultural customs and traditions have accepted and employed nature-based products for skin health for millennia (eTables 1–3).2-20 African and the derived Caribbean cultures frequently use shea butter, black soap, or coconut oil. East Asian ethnobotanical practices include the use of ginseng, green tea, almond, and angelica root in skin care. Indian culture employs Ayurvedic medicine principles that include herbal remedies comprised of ground chickpeas, rice, turmeric, neem, ashwagandha, moringa, and kutki. These cultural traditions continue into modern times, and patients regularly use these products. Modern social trends that focus on a healthy lifestyle also create demand for nature-based products for skin health. In our opinion, the current growing interest in nature-based products implies continued growth in their use as patients become more familiar and comfortable with them.
For beauty and skin health, a new trend has evolved in which the first source of advice is rarely a dermatologist. Social media, nonphysician influencers, and pseudoscience have created an authority previously reserved for dermatologists among patients and consumers. Bloggers and social media influencers, posting their individual real-world experiences, shape the perceptions of consumers and patients.21,22 Nonphysician influencers leverage their celebrity to provide guidance and advice on beauty and cosmetic tips.23 Much of the evidence supporting cosmetic and especially nature-based products for skin care and health often is believed to be less rigorous and of lower quality than that typically supporting physician recommendations.24-26
Nature-Based Products in Skin Health and Dermatologic Conditions
Patients turn to nature-based products for skin care and health for many reasons. The simplest reason is that they grew up with such products and continue their use. Many patients find nature-based products themselves, have favorable experiences, and seek advice on their efficacy and safety for continued use. Patients also use these products as part of a holistic approach to health in which diet and exercise coincide with the idea of ministering to the whole self instead of preventing or treating an illness. These nature-based treatment options fit their natural lifestyles. Patients sometimes express concerns about synthetic products that lead them to seek out nature-based products. Chemicals and preservatives (eg, parabens, sunscreens, nanoparticles) may evoke concerns about negative health consequences, which can be a cause of great anxiety to patients.
Nature-based products, when recommended by physicians, can fulfill important roles. As healthier alternatives, they can address health concerns in the belief that plant-based ingredients may be more compatible with overall health than synthetic ingredients. This compatibility may have resulted from the human species coevolving with plant species containing therapeutic utility, leading to the development of specific receptors for many natural products, such as digoxin from foxglove (Digitalis purpurea), opioids from poppies (Papaver somniferum), and cannabinoids (Cannabis sativa and hybrids). Natural products can become alternatives to synthetic products or adjuncts to prescription medications. Often, inclusion of nature-based products into a treatment plan enables patients to feel that they are a more integral part of the care team treating their conditions. By virtue of physician recommendations, patients may have expectations on product efficacy being as robust as prescription products with the safety profile of plant-based products. Patients should be advised to accept a realistic view of the efficacy and tolerability profiles. In the end, patients consider physician recommendations based on the assumption that they are credible and derived from experience and knowledge.
Physician Perceptions of Nature-Based Products
Physicians recommend nature-based products based on several factors. Central to the recommendation is an understanding, through appropriate documentation, that the product will be reasonably efficacious. Critical to this point, physicians must understand what ingredients are in nature-based products, their concentrations or amounts, and why they are present. However, our experience with nature-based products suggests that many of these factors are not met. Limited or unclear information on the efficacy of nature-based products fails to satisfy a physician’s need for adequate information to support recommendations. Although natural ingredients are listed on product labels, their intended benefit and efficacy characteristics often are unclear or poorly stated, in some cases resulting from improper labeling and in other cases due to claim restrictions imposed on cosmetics. In addition, insufficient details on formulation, such as type and percentages of oils, antioxidants, and vitamins, hinder the physician’s ability to identify and explain mechanisms that bring benefit to the patient. Universal benchmarks do not exist for amounts or concentrations of ingredients that are required for a stated benefit.27 Currently, no standards exist for assurances that product quality, control, and efficacy are consistently reproducible. For example, angel dusting is a practice that discloses that an active ingredient is present, yet these ingredients may be present in quantities that are insufficient to provide measurable benefit. Sourcing of ingredients also can be concerning, as they may not always meet manufacturer, physician, or patient expectations for characterization or efficacy.28,29 Dry testing, which is when a manufacturer contracts a laboratory to certify their ingredients without performing assays, has been increasingly reported in lay and botanical literature over the last few years.30
It is unknown if many nature-based products clinically exhibit their stated efficacy. Empirical evidence or well-conducted clinical studies on which to base recommendations of these products are limited. Individual natural ingredients, however, do have some supporting evidence of efficacy: shea butter moisturizes31; coconut oil exhibits anti-inflammatory properties32,33; and vinegar, yogurt, and diluted tea tree oil exhibit antibacterial properties in postprocedure care and fungal infections, and as adjuvants to prescription antibiotics in atopic dermatitis, acne, and rosacea.34-41 Honey also has been shown to improve wound healing and is even available as a medical device for wounds.42,43 Although nature-based products are an interesting alternative to synthetic products, they require a fulsome understanding of characteristics and efficacy properties to support physician recommendations.
Physician Recommendations
Physicians must be educated to understand when and how to recommend nature-based products. Although we recommend increased product information to guide physicians, current laws, including the Federal Food, Drug, and Cosmetic Act and the Fair Packaging and Labeling Act, are satisfactory from a regulatory standpoint.44 Here, we discuss the information physicians could use to support an informed recommendation of nature-based products.
A clear specific explanation of natural ingredient sources, their intended efficacy, and rigorous scientific clinical evidence supporting their use should be given. Manufacturers are needed to document and report the structure and function of natural ingredients, leading to a common understanding by practicing dermatologists.45 For this reason, manufacturers must provide nonambiguous and standardized methods and measures to demonstrate the mechanism of ingredient efficacy and the limits of safety and tolerability.
We recommend that manufacturers provide standardized transparency into the composition of nature-based formulations, including amounts and concentrations of ingredients; geographic sources; parts of plants used; and if extracted, what agent(s) this standard is based on (eg, hypericin in Saint-John’s-wort or kavalactones in kava kava). Most natural products contain an aqueous phase and therefore will likely require preservatives such as synthetic parabens or alcohols to avoid degradation. Unnecessary ingredients, including fragrances, fillers, and support chemicals, should be absent since inert agents may exhibit biologic effects, obscuring the boundary between active and inert. A clear explanation of the origins of these nature-based ingredients and the concentration, purity, and activity assessment should be provided. In the context of an authoritative review with standardized measures, labels that provide the common name, plant name, part used, how it was obtained, concentrations and/or amounts, and standardized activity measures can be helpful to the recommending physician, who will then know the efficacy patients should expect from the ingredients. They also can assess the expected tolerability based on the concentrations and their own experience managing a particular disorder, tempered by the patient’s experiences with prior therapies. Transparent and standardized labeling describing the formulation, quantities of ingredients, and intended activity will help inform expectations of efficacy.
We recommend clear preclinical and clinical demonstrations of the efficacy and benefits that are claimed by nature-based formulations. Properly designed placebo- or active-controlled, blinded, randomized studies with standardized measures and end points are recommended to determine efficacy and safety. These demonstrations of efficacy can provide physicians with credible evidence on which to base their recommendations and guide the use of products for the patient’s best experience. Given sufficient involvement from manufacturers and publication of the information in peer-reviewed journals, the relative benefits for each nature-based product can be cataloged as a resource for physicians.
Conclusion
Patients turn to nature-based products for many reasons. They have high expectations but also harbor concerns as to the efficacy of these products for skin and health care. Physicians seek to recommend nature-based products for these patients but often find themselves disadvantaged by limited published evidence and insufficient labeling information on composition and efficacy, which should support recommendations for use. To remedy this situation, we suggest research to allow a clear explanation of the activity of natural ingredients, clear demonstrations of the efficacy of nature-based formulas using clinical standardized measures and end points, and clear education and disclosure of ingredients contained within nature-based products.
Acknowledgments—Burt’s Bees (Durham, North Carolina) provided funding for editorial support by Medical Dynamics, Inc (New York, New York).
- Levin J, Momin SB. How much do we really know about our favorite cosmeceutical ingredients? J Clin Aesthet Dermatol. 2010;3:22-41.
- Ajala EO, Aberuagba F, Olaniyan AM, et al. Optimization of solvent extraction of shea butter (Vitellaria paradoxa) using response surface methodology and its characterization. J Food Sci Technol. 2016;53:730-738.
- Lin A, Nabatian A, Halverstam CP. Discovering black soap: a survey on the attitudes and practices of black soap users. J Clin Aesthet Dermatol. 2017;10:18-22.
- Lin TK, Zhong L, Santiago JL. Anti-inflammatory and skin barrier repair effects of topical application of some plant oils. Int J Mol Sci. 2017;19. pii:E70. doi:10.3390/ijms19010070.
- Dua K, Sheshala R, Ling TY, et al. Anti-inflammatory, antibacterial and analgesic potential of cocos nucifera linn.: a review. Antiinflamm Antiallergy Agents Med Chem. 2013;12:158-164.
- Hyun TK, Jang KI. Are berries useless by-products of ginseng? recent research on the potential health benefits of ginseng berry. EXCLI J. 2017;16:780-784.
- Truong VL, Bak MJ, Lee C, et al. Hair regenerative mechanisms of red ginseng oil and its major components in the testosterone-induced delay of anagen entry in C57BL/6 mice. Molecules. 2017;22. pii:E1505. doi:10.3390/molecules22091505.
- Hussain M, Habib Ur R, Akhtar L. Therapeutic benefits of green tea extract on various parameters in non-alcoholic fatty liver disease patients. Pak J Med Sci. 2017;33:931-936.
- Yi M, Fu J, Zhou L, et al. The effect of almond consumption on elements of endurance exercise performance in trained athletes. J Int Soc Sports Nutr. 2014;11:18.
- Sowndhararajan K, Deepa P, Kim M, et al. A review of the composition of the essential oils and biological activities of angelica species. Sci Pharm. 2017;85. pii:E33. doi:10.3390/scipharm85030033.
- Mahjour M, Khoushabi A, Noras M, et al. Effectiveness of Cicer arietinum in cutaneous problems: viewpoint of Avicenna and Razi. Curr Drug Discov Technol. 2018;15:243-250.
- Kanlayavattanakul M, Laurits N, Chaikul P. Jasmine rice panicle: a safe and efficient natural ingredient for skin aging treatments. J Ethnopharmacol. 2016;193:607-616.
- Aggarwal BB, Yuan W, Li S, et al. Curcumin-free turmeric exhibits anti-inflammatory and anticancer activities: identification of novel components of turmeric. Mol Nutr Food Res. 2013;57:1529-1542.
- Mohanty C, Sahoo SK. Curcumin and its topical formulations for wound healing applications. Drug Discov Today. 2017;22:1582-1592.
- Gupta SC, Prasad S, Tyagi AK, et al. Neem (Azadirachta indica): an Indian traditional panacea with modern molecular basis. Phytomedicine. 2017;34:14-20.
- Choudhary D, Bhattacharyya S, Bose S. Efficacy and safety of ashwagandha (Withania somnifera (L.) Dunal) root extract in improving memory and cognitive functions. J Diet Suppl. 2017;14:599-612.
- Halder B, Singh S, Thakur SS. Withania somnifera root extract has potent cytotoxic effect against human malignant melanoma cells. PLoS One. 2015;10:E0137498.
- Nadeem M, Imran M. Promising features of Moringa oleifera oil: recent updates and perspectives. Lipids Health Dis. 2016;15:212.
- Sultan P, Jan A, Pervaiz Q. Phytochemical studies for quantitative estimation of iridoid glycosides in Picrorhiza kurroa Royle. Bot Stud. 2016;57:7.
- Gianfaldoni S, Wollina U, Tirant M, et al. Herbal compounds for the treatment of vitiligo: a review. Open Access Maced J Med Sci. 2018;6:203-207.
- Diamantoglou M, Platz J, Vienken J. Cellulose carbamates and derivatives as hemocompatible membrane materials for hemodialysis. Artif Organs. 1999;23:15-22.
- Respiratory syncytial virus (RSV). Centers for Disease Control and Prevention website. http://www.cdc.gov/rsv/research/us-surveillance.html. Updated June 26, 2018. Accessed February 1, 2019.
- Dembo G, Park SB, Kharasch ED. Central nervous system concentrations of cyclooxygenase-2 inhibitors in humans. Anesthesiology. 2005;102:409-415.
- Fong P. CFTR-SLC26 transporter interactions in epithelia. Biophys Rev. 2012;4:107-116.
- Liu Z. How cosmeceuticals companies get away with pseudoscience. Pacific Standard website. https://psmag.com/environment/cosmetic-companies-get-away-pseudoscience-placebo-week-92455. Published October 15, 2014. Accessed February 1, 2019.
- Beyerstein BL. Alternative medicine and common errors of reasoning. Acad Med. 2001;76:230-237.
- Topical antimicrobial drug products for over-the-counter human use. US Food and Drug Administration website. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=333.310. Accessed February 1, 2019.
- Natural personal care. Natural Products Association website. https://www.npanational.org/certifications/natural-seal/natural-seal-personal-care/. Accessed March 27, 2019.
- Natural Cosmetics Standard. GFaW Web site. https://gfaw.eu/en/ncs-for-all-who-love-nature-and-cosmetics/ncs-information-for-consumer/. Accessed February 1, 2019.
- Brown PN, Betz JM, Jasch F. How to qualify an analytical laboratory for analysis of herbal dietary ingredients and avoid using a “dry lab”: a review of issues related to using a contract analytical laboratory by industry, academia, and regulatory agencies. HerbalGram. 2013:52-59.
- Oh MJ, Cho YH, Cha SY, et al. Novel phytoceramides containing fatty acids of diverse chain lengths are better than a single C18-ceramide N-stearoyl phytosphingosine to improve the physiological properties of human stratum corneum. Clin Cosmet Investig Dermatol. 2017;10:363-371.
- Famurewa AC, Aja PM, Maduagwuna EK, et al. Antioxidant and anti-inflammatory effects of virgin coconut oil supplementation abrogate acute chemotherapy oxidative nephrotoxicity induced by anticancer drug methotrexate in rats. Biomed Pharmacother. 2017;96:905-911.
- Intahphuak S, Khonsung P, Panthong A. Anti-inflammatory, analgesic, and antipyretic activities of virgin coconut oil. Pharm Biol. 2010;48:151-157.
- McKenna PJ, Lehr GS, Leist P, et al. Antiseptic effectiveness with fibroblast preservation. Ann Plast Surg. 1991;27:265-268.
- Brockow K, Grabenhorst P, Abeck D, et al. Effect of gentian violet, corticosteroid and tar preparations in Staphylococcus aureus-colonized atopic eczema. Dermatology. 1999;199:231-236.
- Larson D, Jacob SE. Tea tree oil. Dermatitis. 2012;23:48-49.
- Misner BD. A novel aromatic oil compound inhibits microbial overgrowth on feet: a case study. J Int Soc Sports Nutr. 2007;4:3.
- D’Auria FD, Laino L, Strippoli V, et al. In vitro activity of tea tree oil against Candida albicans mycelial conversion and other pathogenic fungi. J Chemother. 2001;13:377-383.
- Fuchs-Tarlovsky V, Marquez-Barba MF, Sriram K. Probiotics in dermatologic practice. Nutrition. 2016;32:289-295.
- Bowe W, Patel NB, Logan AC. Acne vulgaris, probiotics and the gut-brain-skin axis: from anecdote to translational medicine. Benef Microbes. 2014;5:185-199.
- Baquerizo Nole KL, Yim E, Keri JE. Probiotics and prebiotics in dermatology. J Am Acad Dermatol. 2014;71:814-821.
- Saikaly SK, Khachemoune A. Honey and wound healing: an update. Am J Clin Dermatol. 2017;18:237-251.
- Aziz Z, Abdul Rasool Hassan B. The effects of honey compared to silver sulfadiazine for the treatment of burns: a systematic review of randomized controlled trials. Burns. 2017;43:50-57.
- FDA authority over cosmetics: how cosmetics are not FDA-approved, but are FDA-regulated. US Food and Drug AdministrationWeb site. https://www.fda.gov/cosmetics/guidanceregulation/lawsregulations/ucm074162.htm. Updated July 24, 2018. Accessed February 1, 2019.
- Wohlrab J. Topical preparations and their use in dermatology. J Dtsch Dermatol Ges. 2016;4:1061-1070
Patients seek healthy skin that conveys overall health and well-being. Cosmeceuticals claim to therapeutically affect the structure and function of the skin, and it is rational to hold them to scientific standards that substantiate efficacy claims.1 Notably, it is increasingly important to consider nature-based products in helping patients and consumers to achieve healthier skin. Despite the availability of sophisticated efficacy testing, explanations of the underlying physiologic and pharmacologic principles of nature-based products lag behind those of conventional formulations. In many instances, simple form and function information cannot adequately support their desired use and expected benefits. In addition, cosmetic regulations do not even permit structure-function claims that are allowed for dietary supplements.
Physicians whose patients want recommendations for nature-based products often do not know where to turn for definitive product and use information. Unlike prescription medications or even beauty-from-within dietary supplement products, natural cosmetics and cosmeceuticals are barred from communicating scientific evidence and experience of use to form proper opinions for recommendations. Without the benefit of full product labeling, physicians are left to mine sparse, confusing, and often contradictory literature in an effort to self-educate. Here, we share our experiences with patients, our operating knowledge base, and our recommendations for investigation to improve the available information and ensure practicing physicians have the information they need to appropriately recommend nature-based products.
General Observations Pertaining to Patients and Nature-Based Products
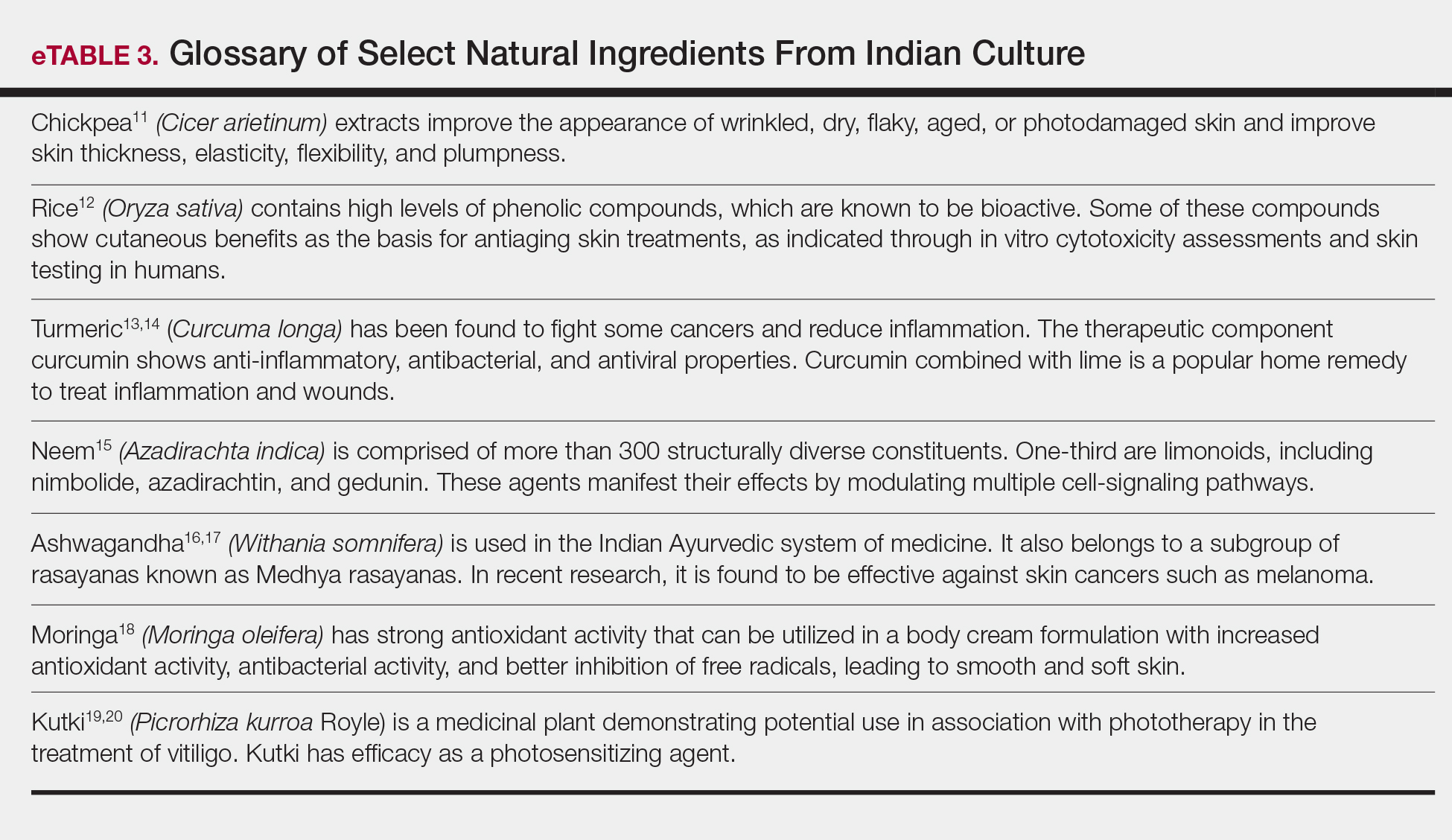
Ethnic and cultural customs and traditions have accepted and employed nature-based products for skin health for millennia (eTables 1–3).2-20 African and the derived Caribbean cultures frequently use shea butter, black soap, or coconut oil. East Asian ethnobotanical practices include the use of ginseng, green tea, almond, and angelica root in skin care. Indian culture employs Ayurvedic medicine principles that include herbal remedies comprised of ground chickpeas, rice, turmeric, neem, ashwagandha, moringa, and kutki. These cultural traditions continue into modern times, and patients regularly use these products. Modern social trends that focus on a healthy lifestyle also create demand for nature-based products for skin health. In our opinion, the current growing interest in nature-based products implies continued growth in their use as patients become more familiar and comfortable with them.
For beauty and skin health, a new trend has evolved in which the first source of advice is rarely a dermatologist. Social media, nonphysician influencers, and pseudoscience have created an authority previously reserved for dermatologists among patients and consumers. Bloggers and social media influencers, posting their individual real-world experiences, shape the perceptions of consumers and patients.21,22 Nonphysician influencers leverage their celebrity to provide guidance and advice on beauty and cosmetic tips.23 Much of the evidence supporting cosmetic and especially nature-based products for skin care and health often is believed to be less rigorous and of lower quality than that typically supporting physician recommendations.24-26
Nature-Based Products in Skin Health and Dermatologic Conditions
Patients turn to nature-based products for skin care and health for many reasons. The simplest reason is that they grew up with such products and continue their use. Many patients find nature-based products themselves, have favorable experiences, and seek advice on their efficacy and safety for continued use. Patients also use these products as part of a holistic approach to health in which diet and exercise coincide with the idea of ministering to the whole self instead of preventing or treating an illness. These nature-based treatment options fit their natural lifestyles. Patients sometimes express concerns about synthetic products that lead them to seek out nature-based products. Chemicals and preservatives (eg, parabens, sunscreens, nanoparticles) may evoke concerns about negative health consequences, which can be a cause of great anxiety to patients.
Nature-based products, when recommended by physicians, can fulfill important roles. As healthier alternatives, they can address health concerns in the belief that plant-based ingredients may be more compatible with overall health than synthetic ingredients. This compatibility may have resulted from the human species coevolving with plant species containing therapeutic utility, leading to the development of specific receptors for many natural products, such as digoxin from foxglove (Digitalis purpurea), opioids from poppies (Papaver somniferum), and cannabinoids (Cannabis sativa and hybrids). Natural products can become alternatives to synthetic products or adjuncts to prescription medications. Often, inclusion of nature-based products into a treatment plan enables patients to feel that they are a more integral part of the care team treating their conditions. By virtue of physician recommendations, patients may have expectations on product efficacy being as robust as prescription products with the safety profile of plant-based products. Patients should be advised to accept a realistic view of the efficacy and tolerability profiles. In the end, patients consider physician recommendations based on the assumption that they are credible and derived from experience and knowledge.
Physician Perceptions of Nature-Based Products
Physicians recommend nature-based products based on several factors. Central to the recommendation is an understanding, through appropriate documentation, that the product will be reasonably efficacious. Critical to this point, physicians must understand what ingredients are in nature-based products, their concentrations or amounts, and why they are present. However, our experience with nature-based products suggests that many of these factors are not met. Limited or unclear information on the efficacy of nature-based products fails to satisfy a physician’s need for adequate information to support recommendations. Although natural ingredients are listed on product labels, their intended benefit and efficacy characteristics often are unclear or poorly stated, in some cases resulting from improper labeling and in other cases due to claim restrictions imposed on cosmetics. In addition, insufficient details on formulation, such as type and percentages of oils, antioxidants, and vitamins, hinder the physician’s ability to identify and explain mechanisms that bring benefit to the patient. Universal benchmarks do not exist for amounts or concentrations of ingredients that are required for a stated benefit.27 Currently, no standards exist for assurances that product quality, control, and efficacy are consistently reproducible. For example, angel dusting is a practice that discloses that an active ingredient is present, yet these ingredients may be present in quantities that are insufficient to provide measurable benefit. Sourcing of ingredients also can be concerning, as they may not always meet manufacturer, physician, or patient expectations for characterization or efficacy.28,29 Dry testing, which is when a manufacturer contracts a laboratory to certify their ingredients without performing assays, has been increasingly reported in lay and botanical literature over the last few years.30
It is unknown if many nature-based products clinically exhibit their stated efficacy. Empirical evidence or well-conducted clinical studies on which to base recommendations of these products are limited. Individual natural ingredients, however, do have some supporting evidence of efficacy: shea butter moisturizes31; coconut oil exhibits anti-inflammatory properties32,33; and vinegar, yogurt, and diluted tea tree oil exhibit antibacterial properties in postprocedure care and fungal infections, and as adjuvants to prescription antibiotics in atopic dermatitis, acne, and rosacea.34-41 Honey also has been shown to improve wound healing and is even available as a medical device for wounds.42,43 Although nature-based products are an interesting alternative to synthetic products, they require a fulsome understanding of characteristics and efficacy properties to support physician recommendations.
Physician Recommendations
Physicians must be educated to understand when and how to recommend nature-based products. Although we recommend increased product information to guide physicians, current laws, including the Federal Food, Drug, and Cosmetic Act and the Fair Packaging and Labeling Act, are satisfactory from a regulatory standpoint.44 Here, we discuss the information physicians could use to support an informed recommendation of nature-based products.
A clear specific explanation of natural ingredient sources, their intended efficacy, and rigorous scientific clinical evidence supporting their use should be given. Manufacturers are needed to document and report the structure and function of natural ingredients, leading to a common understanding by practicing dermatologists.45 For this reason, manufacturers must provide nonambiguous and standardized methods and measures to demonstrate the mechanism of ingredient efficacy and the limits of safety and tolerability.
We recommend that manufacturers provide standardized transparency into the composition of nature-based formulations, including amounts and concentrations of ingredients; geographic sources; parts of plants used; and if extracted, what agent(s) this standard is based on (eg, hypericin in Saint-John’s-wort or kavalactones in kava kava). Most natural products contain an aqueous phase and therefore will likely require preservatives such as synthetic parabens or alcohols to avoid degradation. Unnecessary ingredients, including fragrances, fillers, and support chemicals, should be absent since inert agents may exhibit biologic effects, obscuring the boundary between active and inert. A clear explanation of the origins of these nature-based ingredients and the concentration, purity, and activity assessment should be provided. In the context of an authoritative review with standardized measures, labels that provide the common name, plant name, part used, how it was obtained, concentrations and/or amounts, and standardized activity measures can be helpful to the recommending physician, who will then know the efficacy patients should expect from the ingredients. They also can assess the expected tolerability based on the concentrations and their own experience managing a particular disorder, tempered by the patient’s experiences with prior therapies. Transparent and standardized labeling describing the formulation, quantities of ingredients, and intended activity will help inform expectations of efficacy.
We recommend clear preclinical and clinical demonstrations of the efficacy and benefits that are claimed by nature-based formulations. Properly designed placebo- or active-controlled, blinded, randomized studies with standardized measures and end points are recommended to determine efficacy and safety. These demonstrations of efficacy can provide physicians with credible evidence on which to base their recommendations and guide the use of products for the patient’s best experience. Given sufficient involvement from manufacturers and publication of the information in peer-reviewed journals, the relative benefits for each nature-based product can be cataloged as a resource for physicians.
Conclusion
Patients turn to nature-based products for many reasons. They have high expectations but also harbor concerns as to the efficacy of these products for skin and health care. Physicians seek to recommend nature-based products for these patients but often find themselves disadvantaged by limited published evidence and insufficient labeling information on composition and efficacy, which should support recommendations for use. To remedy this situation, we suggest research to allow a clear explanation of the activity of natural ingredients, clear demonstrations of the efficacy of nature-based formulas using clinical standardized measures and end points, and clear education and disclosure of ingredients contained within nature-based products.
Acknowledgments—Burt’s Bees (Durham, North Carolina) provided funding for editorial support by Medical Dynamics, Inc (New York, New York).
Patients seek healthy skin that conveys overall health and well-being. Cosmeceuticals claim to therapeutically affect the structure and function of the skin, and it is rational to hold them to scientific standards that substantiate efficacy claims.1 Notably, it is increasingly important to consider nature-based products in helping patients and consumers to achieve healthier skin. Despite the availability of sophisticated efficacy testing, explanations of the underlying physiologic and pharmacologic principles of nature-based products lag behind those of conventional formulations. In many instances, simple form and function information cannot adequately support their desired use and expected benefits. In addition, cosmetic regulations do not even permit structure-function claims that are allowed for dietary supplements.
Physicians whose patients want recommendations for nature-based products often do not know where to turn for definitive product and use information. Unlike prescription medications or even beauty-from-within dietary supplement products, natural cosmetics and cosmeceuticals are barred from communicating scientific evidence and experience of use to form proper opinions for recommendations. Without the benefit of full product labeling, physicians are left to mine sparse, confusing, and often contradictory literature in an effort to self-educate. Here, we share our experiences with patients, our operating knowledge base, and our recommendations for investigation to improve the available information and ensure practicing physicians have the information they need to appropriately recommend nature-based products.
General Observations Pertaining to Patients and Nature-Based Products
Ethnic and cultural customs and traditions have accepted and employed nature-based products for skin health for millennia (eTables 1–3).2-20 African and the derived Caribbean cultures frequently use shea butter, black soap, or coconut oil. East Asian ethnobotanical practices include the use of ginseng, green tea, almond, and angelica root in skin care. Indian culture employs Ayurvedic medicine principles that include herbal remedies comprised of ground chickpeas, rice, turmeric, neem, ashwagandha, moringa, and kutki. These cultural traditions continue into modern times, and patients regularly use these products. Modern social trends that focus on a healthy lifestyle also create demand for nature-based products for skin health. In our opinion, the current growing interest in nature-based products implies continued growth in their use as patients become more familiar and comfortable with them.
For beauty and skin health, a new trend has evolved in which the first source of advice is rarely a dermatologist. Social media, nonphysician influencers, and pseudoscience have created an authority previously reserved for dermatologists among patients and consumers. Bloggers and social media influencers, posting their individual real-world experiences, shape the perceptions of consumers and patients.21,22 Nonphysician influencers leverage their celebrity to provide guidance and advice on beauty and cosmetic tips.23 Much of the evidence supporting cosmetic and especially nature-based products for skin care and health often is believed to be less rigorous and of lower quality than that typically supporting physician recommendations.24-26
Nature-Based Products in Skin Health and Dermatologic Conditions
Patients turn to nature-based products for skin care and health for many reasons. The simplest reason is that they grew up with such products and continue their use. Many patients find nature-based products themselves, have favorable experiences, and seek advice on their efficacy and safety for continued use. Patients also use these products as part of a holistic approach to health in which diet and exercise coincide with the idea of ministering to the whole self instead of preventing or treating an illness. These nature-based treatment options fit their natural lifestyles. Patients sometimes express concerns about synthetic products that lead them to seek out nature-based products. Chemicals and preservatives (eg, parabens, sunscreens, nanoparticles) may evoke concerns about negative health consequences, which can be a cause of great anxiety to patients.
Nature-based products, when recommended by physicians, can fulfill important roles. As healthier alternatives, they can address health concerns in the belief that plant-based ingredients may be more compatible with overall health than synthetic ingredients. This compatibility may have resulted from the human species coevolving with plant species containing therapeutic utility, leading to the development of specific receptors for many natural products, such as digoxin from foxglove (Digitalis purpurea), opioids from poppies (Papaver somniferum), and cannabinoids (Cannabis sativa and hybrids). Natural products can become alternatives to synthetic products or adjuncts to prescription medications. Often, inclusion of nature-based products into a treatment plan enables patients to feel that they are a more integral part of the care team treating their conditions. By virtue of physician recommendations, patients may have expectations on product efficacy being as robust as prescription products with the safety profile of plant-based products. Patients should be advised to accept a realistic view of the efficacy and tolerability profiles. In the end, patients consider physician recommendations based on the assumption that they are credible and derived from experience and knowledge.
Physician Perceptions of Nature-Based Products
Physicians recommend nature-based products based on several factors. Central to the recommendation is an understanding, through appropriate documentation, that the product will be reasonably efficacious. Critical to this point, physicians must understand what ingredients are in nature-based products, their concentrations or amounts, and why they are present. However, our experience with nature-based products suggests that many of these factors are not met. Limited or unclear information on the efficacy of nature-based products fails to satisfy a physician’s need for adequate information to support recommendations. Although natural ingredients are listed on product labels, their intended benefit and efficacy characteristics often are unclear or poorly stated, in some cases resulting from improper labeling and in other cases due to claim restrictions imposed on cosmetics. In addition, insufficient details on formulation, such as type and percentages of oils, antioxidants, and vitamins, hinder the physician’s ability to identify and explain mechanisms that bring benefit to the patient. Universal benchmarks do not exist for amounts or concentrations of ingredients that are required for a stated benefit.27 Currently, no standards exist for assurances that product quality, control, and efficacy are consistently reproducible. For example, angel dusting is a practice that discloses that an active ingredient is present, yet these ingredients may be present in quantities that are insufficient to provide measurable benefit. Sourcing of ingredients also can be concerning, as they may not always meet manufacturer, physician, or patient expectations for characterization or efficacy.28,29 Dry testing, which is when a manufacturer contracts a laboratory to certify their ingredients without performing assays, has been increasingly reported in lay and botanical literature over the last few years.30
It is unknown if many nature-based products clinically exhibit their stated efficacy. Empirical evidence or well-conducted clinical studies on which to base recommendations of these products are limited. Individual natural ingredients, however, do have some supporting evidence of efficacy: shea butter moisturizes31; coconut oil exhibits anti-inflammatory properties32,33; and vinegar, yogurt, and diluted tea tree oil exhibit antibacterial properties in postprocedure care and fungal infections, and as adjuvants to prescription antibiotics in atopic dermatitis, acne, and rosacea.34-41 Honey also has been shown to improve wound healing and is even available as a medical device for wounds.42,43 Although nature-based products are an interesting alternative to synthetic products, they require a fulsome understanding of characteristics and efficacy properties to support physician recommendations.
Physician Recommendations
Physicians must be educated to understand when and how to recommend nature-based products. Although we recommend increased product information to guide physicians, current laws, including the Federal Food, Drug, and Cosmetic Act and the Fair Packaging and Labeling Act, are satisfactory from a regulatory standpoint.44 Here, we discuss the information physicians could use to support an informed recommendation of nature-based products.
A clear specific explanation of natural ingredient sources, their intended efficacy, and rigorous scientific clinical evidence supporting their use should be given. Manufacturers are needed to document and report the structure and function of natural ingredients, leading to a common understanding by practicing dermatologists.45 For this reason, manufacturers must provide nonambiguous and standardized methods and measures to demonstrate the mechanism of ingredient efficacy and the limits of safety and tolerability.
We recommend that manufacturers provide standardized transparency into the composition of nature-based formulations, including amounts and concentrations of ingredients; geographic sources; parts of plants used; and if extracted, what agent(s) this standard is based on (eg, hypericin in Saint-John’s-wort or kavalactones in kava kava). Most natural products contain an aqueous phase and therefore will likely require preservatives such as synthetic parabens or alcohols to avoid degradation. Unnecessary ingredients, including fragrances, fillers, and support chemicals, should be absent since inert agents may exhibit biologic effects, obscuring the boundary between active and inert. A clear explanation of the origins of these nature-based ingredients and the concentration, purity, and activity assessment should be provided. In the context of an authoritative review with standardized measures, labels that provide the common name, plant name, part used, how it was obtained, concentrations and/or amounts, and standardized activity measures can be helpful to the recommending physician, who will then know the efficacy patients should expect from the ingredients. They also can assess the expected tolerability based on the concentrations and their own experience managing a particular disorder, tempered by the patient’s experiences with prior therapies. Transparent and standardized labeling describing the formulation, quantities of ingredients, and intended activity will help inform expectations of efficacy.
We recommend clear preclinical and clinical demonstrations of the efficacy and benefits that are claimed by nature-based formulations. Properly designed placebo- or active-controlled, blinded, randomized studies with standardized measures and end points are recommended to determine efficacy and safety. These demonstrations of efficacy can provide physicians with credible evidence on which to base their recommendations and guide the use of products for the patient’s best experience. Given sufficient involvement from manufacturers and publication of the information in peer-reviewed journals, the relative benefits for each nature-based product can be cataloged as a resource for physicians.
Conclusion
Patients turn to nature-based products for many reasons. They have high expectations but also harbor concerns as to the efficacy of these products for skin and health care. Physicians seek to recommend nature-based products for these patients but often find themselves disadvantaged by limited published evidence and insufficient labeling information on composition and efficacy, which should support recommendations for use. To remedy this situation, we suggest research to allow a clear explanation of the activity of natural ingredients, clear demonstrations of the efficacy of nature-based formulas using clinical standardized measures and end points, and clear education and disclosure of ingredients contained within nature-based products.
Acknowledgments—Burt’s Bees (Durham, North Carolina) provided funding for editorial support by Medical Dynamics, Inc (New York, New York).
- Levin J, Momin SB. How much do we really know about our favorite cosmeceutical ingredients? J Clin Aesthet Dermatol. 2010;3:22-41.
- Ajala EO, Aberuagba F, Olaniyan AM, et al. Optimization of solvent extraction of shea butter (Vitellaria paradoxa) using response surface methodology and its characterization. J Food Sci Technol. 2016;53:730-738.
- Lin A, Nabatian A, Halverstam CP. Discovering black soap: a survey on the attitudes and practices of black soap users. J Clin Aesthet Dermatol. 2017;10:18-22.
- Lin TK, Zhong L, Santiago JL. Anti-inflammatory and skin barrier repair effects of topical application of some plant oils. Int J Mol Sci. 2017;19. pii:E70. doi:10.3390/ijms19010070.
- Dua K, Sheshala R, Ling TY, et al. Anti-inflammatory, antibacterial and analgesic potential of cocos nucifera linn.: a review. Antiinflamm Antiallergy Agents Med Chem. 2013;12:158-164.
- Hyun TK, Jang KI. Are berries useless by-products of ginseng? recent research on the potential health benefits of ginseng berry. EXCLI J. 2017;16:780-784.
- Truong VL, Bak MJ, Lee C, et al. Hair regenerative mechanisms of red ginseng oil and its major components in the testosterone-induced delay of anagen entry in C57BL/6 mice. Molecules. 2017;22. pii:E1505. doi:10.3390/molecules22091505.
- Hussain M, Habib Ur R, Akhtar L. Therapeutic benefits of green tea extract on various parameters in non-alcoholic fatty liver disease patients. Pak J Med Sci. 2017;33:931-936.
- Yi M, Fu J, Zhou L, et al. The effect of almond consumption on elements of endurance exercise performance in trained athletes. J Int Soc Sports Nutr. 2014;11:18.
- Sowndhararajan K, Deepa P, Kim M, et al. A review of the composition of the essential oils and biological activities of angelica species. Sci Pharm. 2017;85. pii:E33. doi:10.3390/scipharm85030033.
- Mahjour M, Khoushabi A, Noras M, et al. Effectiveness of Cicer arietinum in cutaneous problems: viewpoint of Avicenna and Razi. Curr Drug Discov Technol. 2018;15:243-250.
- Kanlayavattanakul M, Laurits N, Chaikul P. Jasmine rice panicle: a safe and efficient natural ingredient for skin aging treatments. J Ethnopharmacol. 2016;193:607-616.
- Aggarwal BB, Yuan W, Li S, et al. Curcumin-free turmeric exhibits anti-inflammatory and anticancer activities: identification of novel components of turmeric. Mol Nutr Food Res. 2013;57:1529-1542.
- Mohanty C, Sahoo SK. Curcumin and its topical formulations for wound healing applications. Drug Discov Today. 2017;22:1582-1592.
- Gupta SC, Prasad S, Tyagi AK, et al. Neem (Azadirachta indica): an Indian traditional panacea with modern molecular basis. Phytomedicine. 2017;34:14-20.
- Choudhary D, Bhattacharyya S, Bose S. Efficacy and safety of ashwagandha (Withania somnifera (L.) Dunal) root extract in improving memory and cognitive functions. J Diet Suppl. 2017;14:599-612.
- Halder B, Singh S, Thakur SS. Withania somnifera root extract has potent cytotoxic effect against human malignant melanoma cells. PLoS One. 2015;10:E0137498.
- Nadeem M, Imran M. Promising features of Moringa oleifera oil: recent updates and perspectives. Lipids Health Dis. 2016;15:212.
- Sultan P, Jan A, Pervaiz Q. Phytochemical studies for quantitative estimation of iridoid glycosides in Picrorhiza kurroa Royle. Bot Stud. 2016;57:7.
- Gianfaldoni S, Wollina U, Tirant M, et al. Herbal compounds for the treatment of vitiligo: a review. Open Access Maced J Med Sci. 2018;6:203-207.
- Diamantoglou M, Platz J, Vienken J. Cellulose carbamates and derivatives as hemocompatible membrane materials for hemodialysis. Artif Organs. 1999;23:15-22.
- Respiratory syncytial virus (RSV). Centers for Disease Control and Prevention website. http://www.cdc.gov/rsv/research/us-surveillance.html. Updated June 26, 2018. Accessed February 1, 2019.
- Dembo G, Park SB, Kharasch ED. Central nervous system concentrations of cyclooxygenase-2 inhibitors in humans. Anesthesiology. 2005;102:409-415.
- Fong P. CFTR-SLC26 transporter interactions in epithelia. Biophys Rev. 2012;4:107-116.
- Liu Z. How cosmeceuticals companies get away with pseudoscience. Pacific Standard website. https://psmag.com/environment/cosmetic-companies-get-away-pseudoscience-placebo-week-92455. Published October 15, 2014. Accessed February 1, 2019.
- Beyerstein BL. Alternative medicine and common errors of reasoning. Acad Med. 2001;76:230-237.
- Topical antimicrobial drug products for over-the-counter human use. US Food and Drug Administration website. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=333.310. Accessed February 1, 2019.
- Natural personal care. Natural Products Association website. https://www.npanational.org/certifications/natural-seal/natural-seal-personal-care/. Accessed March 27, 2019.
- Natural Cosmetics Standard. GFaW Web site. https://gfaw.eu/en/ncs-for-all-who-love-nature-and-cosmetics/ncs-information-for-consumer/. Accessed February 1, 2019.
- Brown PN, Betz JM, Jasch F. How to qualify an analytical laboratory for analysis of herbal dietary ingredients and avoid using a “dry lab”: a review of issues related to using a contract analytical laboratory by industry, academia, and regulatory agencies. HerbalGram. 2013:52-59.
- Oh MJ, Cho YH, Cha SY, et al. Novel phytoceramides containing fatty acids of diverse chain lengths are better than a single C18-ceramide N-stearoyl phytosphingosine to improve the physiological properties of human stratum corneum. Clin Cosmet Investig Dermatol. 2017;10:363-371.
- Famurewa AC, Aja PM, Maduagwuna EK, et al. Antioxidant and anti-inflammatory effects of virgin coconut oil supplementation abrogate acute chemotherapy oxidative nephrotoxicity induced by anticancer drug methotrexate in rats. Biomed Pharmacother. 2017;96:905-911.
- Intahphuak S, Khonsung P, Panthong A. Anti-inflammatory, analgesic, and antipyretic activities of virgin coconut oil. Pharm Biol. 2010;48:151-157.
- McKenna PJ, Lehr GS, Leist P, et al. Antiseptic effectiveness with fibroblast preservation. Ann Plast Surg. 1991;27:265-268.
- Brockow K, Grabenhorst P, Abeck D, et al. Effect of gentian violet, corticosteroid and tar preparations in Staphylococcus aureus-colonized atopic eczema. Dermatology. 1999;199:231-236.
- Larson D, Jacob SE. Tea tree oil. Dermatitis. 2012;23:48-49.
- Misner BD. A novel aromatic oil compound inhibits microbial overgrowth on feet: a case study. J Int Soc Sports Nutr. 2007;4:3.
- D’Auria FD, Laino L, Strippoli V, et al. In vitro activity of tea tree oil against Candida albicans mycelial conversion and other pathogenic fungi. J Chemother. 2001;13:377-383.
- Fuchs-Tarlovsky V, Marquez-Barba MF, Sriram K. Probiotics in dermatologic practice. Nutrition. 2016;32:289-295.
- Bowe W, Patel NB, Logan AC. Acne vulgaris, probiotics and the gut-brain-skin axis: from anecdote to translational medicine. Benef Microbes. 2014;5:185-199.
- Baquerizo Nole KL, Yim E, Keri JE. Probiotics and prebiotics in dermatology. J Am Acad Dermatol. 2014;71:814-821.
- Saikaly SK, Khachemoune A. Honey and wound healing: an update. Am J Clin Dermatol. 2017;18:237-251.
- Aziz Z, Abdul Rasool Hassan B. The effects of honey compared to silver sulfadiazine for the treatment of burns: a systematic review of randomized controlled trials. Burns. 2017;43:50-57.
- FDA authority over cosmetics: how cosmetics are not FDA-approved, but are FDA-regulated. US Food and Drug AdministrationWeb site. https://www.fda.gov/cosmetics/guidanceregulation/lawsregulations/ucm074162.htm. Updated July 24, 2018. Accessed February 1, 2019.
- Wohlrab J. Topical preparations and their use in dermatology. J Dtsch Dermatol Ges. 2016;4:1061-1070
- Levin J, Momin SB. How much do we really know about our favorite cosmeceutical ingredients? J Clin Aesthet Dermatol. 2010;3:22-41.
- Ajala EO, Aberuagba F, Olaniyan AM, et al. Optimization of solvent extraction of shea butter (Vitellaria paradoxa) using response surface methodology and its characterization. J Food Sci Technol. 2016;53:730-738.
- Lin A, Nabatian A, Halverstam CP. Discovering black soap: a survey on the attitudes and practices of black soap users. J Clin Aesthet Dermatol. 2017;10:18-22.
- Lin TK, Zhong L, Santiago JL. Anti-inflammatory and skin barrier repair effects of topical application of some plant oils. Int J Mol Sci. 2017;19. pii:E70. doi:10.3390/ijms19010070.
- Dua K, Sheshala R, Ling TY, et al. Anti-inflammatory, antibacterial and analgesic potential of cocos nucifera linn.: a review. Antiinflamm Antiallergy Agents Med Chem. 2013;12:158-164.
- Hyun TK, Jang KI. Are berries useless by-products of ginseng? recent research on the potential health benefits of ginseng berry. EXCLI J. 2017;16:780-784.
- Truong VL, Bak MJ, Lee C, et al. Hair regenerative mechanisms of red ginseng oil and its major components in the testosterone-induced delay of anagen entry in C57BL/6 mice. Molecules. 2017;22. pii:E1505. doi:10.3390/molecules22091505.
- Hussain M, Habib Ur R, Akhtar L. Therapeutic benefits of green tea extract on various parameters in non-alcoholic fatty liver disease patients. Pak J Med Sci. 2017;33:931-936.
- Yi M, Fu J, Zhou L, et al. The effect of almond consumption on elements of endurance exercise performance in trained athletes. J Int Soc Sports Nutr. 2014;11:18.
- Sowndhararajan K, Deepa P, Kim M, et al. A review of the composition of the essential oils and biological activities of angelica species. Sci Pharm. 2017;85. pii:E33. doi:10.3390/scipharm85030033.
- Mahjour M, Khoushabi A, Noras M, et al. Effectiveness of Cicer arietinum in cutaneous problems: viewpoint of Avicenna and Razi. Curr Drug Discov Technol. 2018;15:243-250.
- Kanlayavattanakul M, Laurits N, Chaikul P. Jasmine rice panicle: a safe and efficient natural ingredient for skin aging treatments. J Ethnopharmacol. 2016;193:607-616.
- Aggarwal BB, Yuan W, Li S, et al. Curcumin-free turmeric exhibits anti-inflammatory and anticancer activities: identification of novel components of turmeric. Mol Nutr Food Res. 2013;57:1529-1542.
- Mohanty C, Sahoo SK. Curcumin and its topical formulations for wound healing applications. Drug Discov Today. 2017;22:1582-1592.
- Gupta SC, Prasad S, Tyagi AK, et al. Neem (Azadirachta indica): an Indian traditional panacea with modern molecular basis. Phytomedicine. 2017;34:14-20.
- Choudhary D, Bhattacharyya S, Bose S. Efficacy and safety of ashwagandha (Withania somnifera (L.) Dunal) root extract in improving memory and cognitive functions. J Diet Suppl. 2017;14:599-612.
- Halder B, Singh S, Thakur SS. Withania somnifera root extract has potent cytotoxic effect against human malignant melanoma cells. PLoS One. 2015;10:E0137498.
- Nadeem M, Imran M. Promising features of Moringa oleifera oil: recent updates and perspectives. Lipids Health Dis. 2016;15:212.
- Sultan P, Jan A, Pervaiz Q. Phytochemical studies for quantitative estimation of iridoid glycosides in Picrorhiza kurroa Royle. Bot Stud. 2016;57:7.
- Gianfaldoni S, Wollina U, Tirant M, et al. Herbal compounds for the treatment of vitiligo: a review. Open Access Maced J Med Sci. 2018;6:203-207.
- Diamantoglou M, Platz J, Vienken J. Cellulose carbamates and derivatives as hemocompatible membrane materials for hemodialysis. Artif Organs. 1999;23:15-22.
- Respiratory syncytial virus (RSV). Centers for Disease Control and Prevention website. http://www.cdc.gov/rsv/research/us-surveillance.html. Updated June 26, 2018. Accessed February 1, 2019.
- Dembo G, Park SB, Kharasch ED. Central nervous system concentrations of cyclooxygenase-2 inhibitors in humans. Anesthesiology. 2005;102:409-415.
- Fong P. CFTR-SLC26 transporter interactions in epithelia. Biophys Rev. 2012;4:107-116.
- Liu Z. How cosmeceuticals companies get away with pseudoscience. Pacific Standard website. https://psmag.com/environment/cosmetic-companies-get-away-pseudoscience-placebo-week-92455. Published October 15, 2014. Accessed February 1, 2019.
- Beyerstein BL. Alternative medicine and common errors of reasoning. Acad Med. 2001;76:230-237.
- Topical antimicrobial drug products for over-the-counter human use. US Food and Drug Administration website. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=333.310. Accessed February 1, 2019.
- Natural personal care. Natural Products Association website. https://www.npanational.org/certifications/natural-seal/natural-seal-personal-care/. Accessed March 27, 2019.
- Natural Cosmetics Standard. GFaW Web site. https://gfaw.eu/en/ncs-for-all-who-love-nature-and-cosmetics/ncs-information-for-consumer/. Accessed February 1, 2019.
- Brown PN, Betz JM, Jasch F. How to qualify an analytical laboratory for analysis of herbal dietary ingredients and avoid using a “dry lab”: a review of issues related to using a contract analytical laboratory by industry, academia, and regulatory agencies. HerbalGram. 2013:52-59.
- Oh MJ, Cho YH, Cha SY, et al. Novel phytoceramides containing fatty acids of diverse chain lengths are better than a single C18-ceramide N-stearoyl phytosphingosine to improve the physiological properties of human stratum corneum. Clin Cosmet Investig Dermatol. 2017;10:363-371.
- Famurewa AC, Aja PM, Maduagwuna EK, et al. Antioxidant and anti-inflammatory effects of virgin coconut oil supplementation abrogate acute chemotherapy oxidative nephrotoxicity induced by anticancer drug methotrexate in rats. Biomed Pharmacother. 2017;96:905-911.
- Intahphuak S, Khonsung P, Panthong A. Anti-inflammatory, analgesic, and antipyretic activities of virgin coconut oil. Pharm Biol. 2010;48:151-157.
- McKenna PJ, Lehr GS, Leist P, et al. Antiseptic effectiveness with fibroblast preservation. Ann Plast Surg. 1991;27:265-268.
- Brockow K, Grabenhorst P, Abeck D, et al. Effect of gentian violet, corticosteroid and tar preparations in Staphylococcus aureus-colonized atopic eczema. Dermatology. 1999;199:231-236.
- Larson D, Jacob SE. Tea tree oil. Dermatitis. 2012;23:48-49.
- Misner BD. A novel aromatic oil compound inhibits microbial overgrowth on feet: a case study. J Int Soc Sports Nutr. 2007;4:3.
- D’Auria FD, Laino L, Strippoli V, et al. In vitro activity of tea tree oil against Candida albicans mycelial conversion and other pathogenic fungi. J Chemother. 2001;13:377-383.
- Fuchs-Tarlovsky V, Marquez-Barba MF, Sriram K. Probiotics in dermatologic practice. Nutrition. 2016;32:289-295.
- Bowe W, Patel NB, Logan AC. Acne vulgaris, probiotics and the gut-brain-skin axis: from anecdote to translational medicine. Benef Microbes. 2014;5:185-199.
- Baquerizo Nole KL, Yim E, Keri JE. Probiotics and prebiotics in dermatology. J Am Acad Dermatol. 2014;71:814-821.
- Saikaly SK, Khachemoune A. Honey and wound healing: an update. Am J Clin Dermatol. 2017;18:237-251.
- Aziz Z, Abdul Rasool Hassan B. The effects of honey compared to silver sulfadiazine for the treatment of burns: a systematic review of randomized controlled trials. Burns. 2017;43:50-57.
- FDA authority over cosmetics: how cosmetics are not FDA-approved, but are FDA-regulated. US Food and Drug AdministrationWeb site. https://www.fda.gov/cosmetics/guidanceregulation/lawsregulations/ucm074162.htm. Updated July 24, 2018. Accessed February 1, 2019.
- Wohlrab J. Topical preparations and their use in dermatology. J Dtsch Dermatol Ges. 2016;4:1061-1070
Practice Points
- Patients are increasingly interested in and asking for nature-based products and formulations to manage dermatologic conditions.
- Physicians can satisfy patient interests with nature-based formulations that are as beneficial or more so than synthetic formulations because of the physiologic activity of the ingredients within these formulations.
- Physicians should have resources available to them that adequately educate on nature-based ingredients and how to recommend them.
Treatment with gold microparticles plus lasers a viable option for acne
DENVER – Combining according to Jill S. Waibel, MD.
“Why do we need lasers and light for acne? We have compliance issues, particularly in the pubertal population,” she said at the annual conference of the American Society for Laser Medicine and Surgery. “We also have systemic side effects from medications and antibiotic resistance. Acne is still the number one reason people see a dermatologist.”
The gold microparticles were cleared by the Food and Drug Administration in September 2018 for the treatment of mild to moderate inflammatory acne. Based on European study data and ongoing studies in the United States, the optimal treatment approach begins with putting on a topical retinoid nightly and benzoyl peroxide (BPO) wash to clear follicular debris and reduce bacterial load prior to the application of gold microparticles and laser energy.
Dr. Waibel, director of the Miami Dermatology and Laser Institute, presented results from a clinical trial of 64 patients with mild to moderate acne that was conducted at seven European centers. All patients applied topical 0.1% adapalene/2.5% benzoyl peroxide daily for 2-4 weeks, then they underwent three microparticles plus laser procedures over a 2-week period. “It takes about 10 minutes to put the gold nanoparticles on,” Dr. Waibel said. “They’re rubbed in by a massager, the particles get in down into the follicle. Once it’s in the follicle, you wipe the gold completely off and you activate the FDA-approved 1064 nm Nd:YAG laser.”
Energy was delivered at a fluency of 25-34 J/cm2 and a pulse duration of 30 seconds. Concomitant rescue medications were allowed at the discretion of the investigator after the 2-month evaluation. Formal evaluations included inflammatory lesion counts at baseline, 2, 3, and 6 months; the Investigator Global Assessment scale at baseline, 2, 3, and 6 months; and safety and tolerability. Most of the patients (78%) were female, and their average age was 23 years, with a range between 13 and 38 years. The average level of pain reported by patients was 4 on a scale between 0 and 10, and mild, transient erythema and edema were reported post procedure. There were no adverse events otherwise.
Dr. Waibel reported that the interim mean improvement of inflammatory lesion counts was 66% at 3 months and 79% at 6 months. “At six months, 63% of patients were clear or almost clear according to the Investigator Global Assessment scale,” she added.
After the three treatment sessions, researchers observed a 63% reduction in acne at 2 months, a 74% reduction at 6 months, and an 85% reduction at 12 months. By comparison, a subset of 26 patients who received gold microparticle monotherapy had a 49% reduction in acne at 2 months and a 65% reduction at 6 months.
“A short course of retinoid and benzoyl peroxide prior to gold microparticles treatment improved the inflammatory lesion count reduction, compared with historical studies,” Dr. Waibel said (J Invest Dermatol. 2015;135[7]:1727-34). “Gold microparticles treatment for acne may be part of a multitherapy approach for patients with mild to moderate facial acne. Clinical outcomes are predictable and durable through 12 months.”
The approach is being further studied in the United States and researchers are exploring the potential for fewer treatments over a period of two weeks. Dr. Waibel disclosed that she has served on the advisory board for Sebacia, which developed the gold microparticles, and has received clinical trials funding from the company. She also reported having financial relationships with numerous other device and pharmaceutical companies.
DENVER – Combining according to Jill S. Waibel, MD.
“Why do we need lasers and light for acne? We have compliance issues, particularly in the pubertal population,” she said at the annual conference of the American Society for Laser Medicine and Surgery. “We also have systemic side effects from medications and antibiotic resistance. Acne is still the number one reason people see a dermatologist.”
The gold microparticles were cleared by the Food and Drug Administration in September 2018 for the treatment of mild to moderate inflammatory acne. Based on European study data and ongoing studies in the United States, the optimal treatment approach begins with putting on a topical retinoid nightly and benzoyl peroxide (BPO) wash to clear follicular debris and reduce bacterial load prior to the application of gold microparticles and laser energy.
Dr. Waibel, director of the Miami Dermatology and Laser Institute, presented results from a clinical trial of 64 patients with mild to moderate acne that was conducted at seven European centers. All patients applied topical 0.1% adapalene/2.5% benzoyl peroxide daily for 2-4 weeks, then they underwent three microparticles plus laser procedures over a 2-week period. “It takes about 10 minutes to put the gold nanoparticles on,” Dr. Waibel said. “They’re rubbed in by a massager, the particles get in down into the follicle. Once it’s in the follicle, you wipe the gold completely off and you activate the FDA-approved 1064 nm Nd:YAG laser.”
Energy was delivered at a fluency of 25-34 J/cm2 and a pulse duration of 30 seconds. Concomitant rescue medications were allowed at the discretion of the investigator after the 2-month evaluation. Formal evaluations included inflammatory lesion counts at baseline, 2, 3, and 6 months; the Investigator Global Assessment scale at baseline, 2, 3, and 6 months; and safety and tolerability. Most of the patients (78%) were female, and their average age was 23 years, with a range between 13 and 38 years. The average level of pain reported by patients was 4 on a scale between 0 and 10, and mild, transient erythema and edema were reported post procedure. There were no adverse events otherwise.
Dr. Waibel reported that the interim mean improvement of inflammatory lesion counts was 66% at 3 months and 79% at 6 months. “At six months, 63% of patients were clear or almost clear according to the Investigator Global Assessment scale,” she added.
After the three treatment sessions, researchers observed a 63% reduction in acne at 2 months, a 74% reduction at 6 months, and an 85% reduction at 12 months. By comparison, a subset of 26 patients who received gold microparticle monotherapy had a 49% reduction in acne at 2 months and a 65% reduction at 6 months.
“A short course of retinoid and benzoyl peroxide prior to gold microparticles treatment improved the inflammatory lesion count reduction, compared with historical studies,” Dr. Waibel said (J Invest Dermatol. 2015;135[7]:1727-34). “Gold microparticles treatment for acne may be part of a multitherapy approach for patients with mild to moderate facial acne. Clinical outcomes are predictable and durable through 12 months.”
The approach is being further studied in the United States and researchers are exploring the potential for fewer treatments over a period of two weeks. Dr. Waibel disclosed that she has served on the advisory board for Sebacia, which developed the gold microparticles, and has received clinical trials funding from the company. She also reported having financial relationships with numerous other device and pharmaceutical companies.
DENVER – Combining according to Jill S. Waibel, MD.
“Why do we need lasers and light for acne? We have compliance issues, particularly in the pubertal population,” she said at the annual conference of the American Society for Laser Medicine and Surgery. “We also have systemic side effects from medications and antibiotic resistance. Acne is still the number one reason people see a dermatologist.”
The gold microparticles were cleared by the Food and Drug Administration in September 2018 for the treatment of mild to moderate inflammatory acne. Based on European study data and ongoing studies in the United States, the optimal treatment approach begins with putting on a topical retinoid nightly and benzoyl peroxide (BPO) wash to clear follicular debris and reduce bacterial load prior to the application of gold microparticles and laser energy.
Dr. Waibel, director of the Miami Dermatology and Laser Institute, presented results from a clinical trial of 64 patients with mild to moderate acne that was conducted at seven European centers. All patients applied topical 0.1% adapalene/2.5% benzoyl peroxide daily for 2-4 weeks, then they underwent three microparticles plus laser procedures over a 2-week period. “It takes about 10 minutes to put the gold nanoparticles on,” Dr. Waibel said. “They’re rubbed in by a massager, the particles get in down into the follicle. Once it’s in the follicle, you wipe the gold completely off and you activate the FDA-approved 1064 nm Nd:YAG laser.”
Energy was delivered at a fluency of 25-34 J/cm2 and a pulse duration of 30 seconds. Concomitant rescue medications were allowed at the discretion of the investigator after the 2-month evaluation. Formal evaluations included inflammatory lesion counts at baseline, 2, 3, and 6 months; the Investigator Global Assessment scale at baseline, 2, 3, and 6 months; and safety and tolerability. Most of the patients (78%) were female, and their average age was 23 years, with a range between 13 and 38 years. The average level of pain reported by patients was 4 on a scale between 0 and 10, and mild, transient erythema and edema were reported post procedure. There were no adverse events otherwise.
Dr. Waibel reported that the interim mean improvement of inflammatory lesion counts was 66% at 3 months and 79% at 6 months. “At six months, 63% of patients were clear or almost clear according to the Investigator Global Assessment scale,” she added.
After the three treatment sessions, researchers observed a 63% reduction in acne at 2 months, a 74% reduction at 6 months, and an 85% reduction at 12 months. By comparison, a subset of 26 patients who received gold microparticle monotherapy had a 49% reduction in acne at 2 months and a 65% reduction at 6 months.
“A short course of retinoid and benzoyl peroxide prior to gold microparticles treatment improved the inflammatory lesion count reduction, compared with historical studies,” Dr. Waibel said (J Invest Dermatol. 2015;135[7]:1727-34). “Gold microparticles treatment for acne may be part of a multitherapy approach for patients with mild to moderate facial acne. Clinical outcomes are predictable and durable through 12 months.”
The approach is being further studied in the United States and researchers are exploring the potential for fewer treatments over a period of two weeks. Dr. Waibel disclosed that she has served on the advisory board for Sebacia, which developed the gold microparticles, and has received clinical trials funding from the company. She also reported having financial relationships with numerous other device and pharmaceutical companies.
REPORTING FROM ASLMS 2019
Spending on combination products for acne has increased significantly
WASHINGTON – Expenditures for combination acne products increased from $82 million in 1996 to $487 million in 2016, according to an analysis presented at the annual meeting of the American Academy of Dermatology.
During a late-breaking research session, David Li, a fourth-year medical student at Tufts University, Boston, presented the results of a retrospective cost analysis study, conducted to identify trends in overall spending for combination acne products. Spending measures were adjusted for inflation to 2016 U.S. dollars.
he noted. Combination products are more expensive than the sum of their component parts, and prescribing generic formulations of individual acne treatments could potentially reduce costs, but possible advantages of combination products for acne include improved patient adherence and increased efficacy.
Mr. Li, a research fellow in the department of dermatology at Brigham and Women’s Hospital in Boston, and his colleagues drafted a comprehensive list of available combination products (eight brand-name products and two generics). Most combine benzoyl peroxide with another common acne medication. To analyze trends in medication use, they performed a retrospective cost analysis using Medical Expenditure Panel Survey (MEPS) data from 1996 to 2016, searching for these combination medications to gather the annual number of prescriptions, number of users, expenditures, and aggregate demographics for each product. The data were weighted to represent national estimates.
They also used data from the National Average Drug Acquisition Cost (NADAC) database, used by the Centers for Medicare & Medicaid Services as a pricing benchmark, and calculated the difference between the unit price of each combination product and the sum of the prices of its generic components. They multiplied this difference by the median number of units prescribed annually for the given combination.
The researchers found that most users of combination acne products were younger than 18 years (23%), female (55%), and white (83%), and the most commonly prescribed combination product changed over time. From 1996 to 2002, Benzamycin (benzoyl peroxide and erythromycin) was the most frequently prescribed combination. Several years later, its place was taken by BenzaClin (benzoyl peroxide and clindamycin) from 2003 to 2010, followed by Ziana (clindamycin and tretinoin) in 2011 and Epiduo (adapalene and benzoyl peroxide) from 2012 to 2016.
“Spending has increased steadily from a little bit over $82 million in 1996 to nearly half a billion dollars in 2016,” Mr. Li said. “That’s a rise of more than 500% in the last 20 years. Based on the median pricing and utilization data that we derived from the NADAC database, we determined that substitution with component generics can provide median annual savings of at least a quarter billion dollars each year.”
Although the data indicate a trend toward increased use of and spending on new, branded combination products, the literature includes “minimal data to suggest whether one combination acne product is better than the next one, or how it compares to its component medications when used in combination,” he said. He and his colleagues found no comparative data apart from a 2001 study that examined Benzamycin and BenzaClin, which suggested that there was no difference in efficacy or tolerability between the products.
The present study is limited by reporting bias and recall bias because it relies partly on MEPS, a survey, and the NADAC pricing database had information only for 2013-2016. The researchers consequently used the most recent prices to calculate potential savings.
“Until we have more meaningful data to suggest otherwise, we’re in a state of equipoise,” said Mr. Li.
The research was funded by the National Center for Advancing Translational Sciences, part of the National Institutes of Health.
SOURCE: Li D et al. AAD 2019, Abstract 11333.
WASHINGTON – Expenditures for combination acne products increased from $82 million in 1996 to $487 million in 2016, according to an analysis presented at the annual meeting of the American Academy of Dermatology.
During a late-breaking research session, David Li, a fourth-year medical student at Tufts University, Boston, presented the results of a retrospective cost analysis study, conducted to identify trends in overall spending for combination acne products. Spending measures were adjusted for inflation to 2016 U.S. dollars.
he noted. Combination products are more expensive than the sum of their component parts, and prescribing generic formulations of individual acne treatments could potentially reduce costs, but possible advantages of combination products for acne include improved patient adherence and increased efficacy.
Mr. Li, a research fellow in the department of dermatology at Brigham and Women’s Hospital in Boston, and his colleagues drafted a comprehensive list of available combination products (eight brand-name products and two generics). Most combine benzoyl peroxide with another common acne medication. To analyze trends in medication use, they performed a retrospective cost analysis using Medical Expenditure Panel Survey (MEPS) data from 1996 to 2016, searching for these combination medications to gather the annual number of prescriptions, number of users, expenditures, and aggregate demographics for each product. The data were weighted to represent national estimates.
They also used data from the National Average Drug Acquisition Cost (NADAC) database, used by the Centers for Medicare & Medicaid Services as a pricing benchmark, and calculated the difference between the unit price of each combination product and the sum of the prices of its generic components. They multiplied this difference by the median number of units prescribed annually for the given combination.
The researchers found that most users of combination acne products were younger than 18 years (23%), female (55%), and white (83%), and the most commonly prescribed combination product changed over time. From 1996 to 2002, Benzamycin (benzoyl peroxide and erythromycin) was the most frequently prescribed combination. Several years later, its place was taken by BenzaClin (benzoyl peroxide and clindamycin) from 2003 to 2010, followed by Ziana (clindamycin and tretinoin) in 2011 and Epiduo (adapalene and benzoyl peroxide) from 2012 to 2016.
“Spending has increased steadily from a little bit over $82 million in 1996 to nearly half a billion dollars in 2016,” Mr. Li said. “That’s a rise of more than 500% in the last 20 years. Based on the median pricing and utilization data that we derived from the NADAC database, we determined that substitution with component generics can provide median annual savings of at least a quarter billion dollars each year.”
Although the data indicate a trend toward increased use of and spending on new, branded combination products, the literature includes “minimal data to suggest whether one combination acne product is better than the next one, or how it compares to its component medications when used in combination,” he said. He and his colleagues found no comparative data apart from a 2001 study that examined Benzamycin and BenzaClin, which suggested that there was no difference in efficacy or tolerability between the products.
The present study is limited by reporting bias and recall bias because it relies partly on MEPS, a survey, and the NADAC pricing database had information only for 2013-2016. The researchers consequently used the most recent prices to calculate potential savings.
“Until we have more meaningful data to suggest otherwise, we’re in a state of equipoise,” said Mr. Li.
The research was funded by the National Center for Advancing Translational Sciences, part of the National Institutes of Health.
SOURCE: Li D et al. AAD 2019, Abstract 11333.
WASHINGTON – Expenditures for combination acne products increased from $82 million in 1996 to $487 million in 2016, according to an analysis presented at the annual meeting of the American Academy of Dermatology.
During a late-breaking research session, David Li, a fourth-year medical student at Tufts University, Boston, presented the results of a retrospective cost analysis study, conducted to identify trends in overall spending for combination acne products. Spending measures were adjusted for inflation to 2016 U.S. dollars.
he noted. Combination products are more expensive than the sum of their component parts, and prescribing generic formulations of individual acne treatments could potentially reduce costs, but possible advantages of combination products for acne include improved patient adherence and increased efficacy.
Mr. Li, a research fellow in the department of dermatology at Brigham and Women’s Hospital in Boston, and his colleagues drafted a comprehensive list of available combination products (eight brand-name products and two generics). Most combine benzoyl peroxide with another common acne medication. To analyze trends in medication use, they performed a retrospective cost analysis using Medical Expenditure Panel Survey (MEPS) data from 1996 to 2016, searching for these combination medications to gather the annual number of prescriptions, number of users, expenditures, and aggregate demographics for each product. The data were weighted to represent national estimates.
They also used data from the National Average Drug Acquisition Cost (NADAC) database, used by the Centers for Medicare & Medicaid Services as a pricing benchmark, and calculated the difference between the unit price of each combination product and the sum of the prices of its generic components. They multiplied this difference by the median number of units prescribed annually for the given combination.
The researchers found that most users of combination acne products were younger than 18 years (23%), female (55%), and white (83%), and the most commonly prescribed combination product changed over time. From 1996 to 2002, Benzamycin (benzoyl peroxide and erythromycin) was the most frequently prescribed combination. Several years later, its place was taken by BenzaClin (benzoyl peroxide and clindamycin) from 2003 to 2010, followed by Ziana (clindamycin and tretinoin) in 2011 and Epiduo (adapalene and benzoyl peroxide) from 2012 to 2016.
“Spending has increased steadily from a little bit over $82 million in 1996 to nearly half a billion dollars in 2016,” Mr. Li said. “That’s a rise of more than 500% in the last 20 years. Based on the median pricing and utilization data that we derived from the NADAC database, we determined that substitution with component generics can provide median annual savings of at least a quarter billion dollars each year.”
Although the data indicate a trend toward increased use of and spending on new, branded combination products, the literature includes “minimal data to suggest whether one combination acne product is better than the next one, or how it compares to its component medications when used in combination,” he said. He and his colleagues found no comparative data apart from a 2001 study that examined Benzamycin and BenzaClin, which suggested that there was no difference in efficacy or tolerability between the products.
The present study is limited by reporting bias and recall bias because it relies partly on MEPS, a survey, and the NADAC pricing database had information only for 2013-2016. The researchers consequently used the most recent prices to calculate potential savings.
“Until we have more meaningful data to suggest otherwise, we’re in a state of equipoise,” said Mr. Li.
The research was funded by the National Center for Advancing Translational Sciences, part of the National Institutes of Health.
SOURCE: Li D et al. AAD 2019, Abstract 11333.
REPORTING FROM AAD 2019
Acne take home messages from the AAD annual meeting
WASHINGTON – In an interview at the close of the annual meeting of the American Academy of Dermatology, .
Both Dr. Harper, who practices in Birmingham, Ala., and is the immediate past president of the American Acne & Rosacea Society, and Dr. Keri, of the department of dermatology at the University of Miami and the Miami VA Healthcare System, spoke during several acne sessions. Among the topics they discussed during the interview were a relatively recent meta-analysis that provides reassuring information about depression and isotretinoin, how to start patients on spironolactone, and the use of antibiotics – and benzoyl peroxide.
They emphasized the importance of not withholding treatment for patients who need it and the psychosocial impact of acne. “Patients need to get to the treatment they need ... faster,” Dr. Harper said. “We want to treat sooner, and we want to prevent scarring,” Dr. Keri added.
Dr. Keri disclosed relationships with Hoffmann–La Roche, Ortho Dermatologics, and Pierre Fabre Dermatologie. Dr. Harper has no relevant financial disclosures.
WASHINGTON – In an interview at the close of the annual meeting of the American Academy of Dermatology, .
Both Dr. Harper, who practices in Birmingham, Ala., and is the immediate past president of the American Acne & Rosacea Society, and Dr. Keri, of the department of dermatology at the University of Miami and the Miami VA Healthcare System, spoke during several acne sessions. Among the topics they discussed during the interview were a relatively recent meta-analysis that provides reassuring information about depression and isotretinoin, how to start patients on spironolactone, and the use of antibiotics – and benzoyl peroxide.
They emphasized the importance of not withholding treatment for patients who need it and the psychosocial impact of acne. “Patients need to get to the treatment they need ... faster,” Dr. Harper said. “We want to treat sooner, and we want to prevent scarring,” Dr. Keri added.
Dr. Keri disclosed relationships with Hoffmann–La Roche, Ortho Dermatologics, and Pierre Fabre Dermatologie. Dr. Harper has no relevant financial disclosures.
WASHINGTON – In an interview at the close of the annual meeting of the American Academy of Dermatology, .
Both Dr. Harper, who practices in Birmingham, Ala., and is the immediate past president of the American Acne & Rosacea Society, and Dr. Keri, of the department of dermatology at the University of Miami and the Miami VA Healthcare System, spoke during several acne sessions. Among the topics they discussed during the interview were a relatively recent meta-analysis that provides reassuring information about depression and isotretinoin, how to start patients on spironolactone, and the use of antibiotics – and benzoyl peroxide.
They emphasized the importance of not withholding treatment for patients who need it and the psychosocial impact of acne. “Patients need to get to the treatment they need ... faster,” Dr. Harper said. “We want to treat sooner, and we want to prevent scarring,” Dr. Keri added.
Dr. Keri disclosed relationships with Hoffmann–La Roche, Ortho Dermatologics, and Pierre Fabre Dermatologie. Dr. Harper has no relevant financial disclosures.
Management of acne duration, severity can improve rate of scar repair
WASHINGTON – Jerry Tan, MD, said at the annual meeting of the American Academy of Dermatology.
A “large majority” of patients present with active acne scarring, even those with mild and moderate disease, said Dr. Tan of the University of Western Ontario, London. The stigma associated with acne and scarring is real – in one survey, 41% of respondents noted that acne was the first thing they noticed about a person’s face (Dermatol Ther. 2016;6[2]:207-18).
“Patients with scars are stigmatized because they are perceived as being less attractive,” Dr. Tan said. “They’re perceived as being less healthy, less confident, all those negative attributes because they have developed something that was not of their own making.”
Dr. Tan presented results from his own group that estimated the risk factors of scarring. They found factors such as acne severity (odds ratio, 3.68; 95% confidence interval, 2.58-5.23), a family history of acne scarring (OR, 2.14; 95% CI, 1.67-2.76), acne duration (OR, 1.63; 1.09-2.47), and manipulation (picking and squeezing) behaviors (OR, 1.70; 95% CI, 1.27-2.29) increased the risk of acne scarring (J Eur Acad Dermatol Venereol. 2017 Sep;31[9]:1547-54). However, in a separate study, they found acne scarring and repair was an ongoing process: Of 32 patients with moderate inflammatory acne studied for 6 months, 36% of scars that were discovered within the study period were not present at follow up (J Drugs Dermatol. 2017 Jun 1;16[6]:566-72).
“I came from an era where we were taught that acne scars, when they developed, were permanent. This suggests based on good evidence that it’s not,” Dr. Tan said.
Adapalene has been successful in improving the skin texture and severity of acne scars, Dr. Tan said, citing a study that showed once-daily adapalene 0.3% gel showed a moderate to complete improvement in scar severity in 77.8% of patients and a slight improvement in 22.2% of patients (Dermatol Ther. 2018;8[2]:245-57). Increasing the dose helped: 32.9% of patients using topical adapalene 0.3% combined with benzoyl peroxide 2.5% gel at 24 weeks were clear or almost clear according to Scar Global Assessment scores, compared with 16.4% of patients randomized to vehicle (Am J Clin Dermatol. 2018 Apr;19[2]:275-86).
For established scars, many treatment modalities are available, with varying levels of evidence supporting their use. Ice pick scars are commonly treated with chemical reconstruction of skin scars (CROSS), punch techniques, and radiofrequency (RF). Rolling acne scares can be treated with dermabrasion, dermal fillers, RF, skin needling, subcision as well as ablative, nonablative and fractional lasers. Shallow boxcar scars are treated with dermabrasion, dermal fillers, RF, skin needling and subcision as well as ablative, nonablative, fractional lasers; deeper boxcar scars can be treated with RF and punch techniques.
Among these techniques, Dr. Tan said there is level I evidence to use subcision and dermal fillers with rolling acne scars and CROSS with trichloroacetic acid for ice pick and boxcar scars; and for boxcar and rolling scars, ablative and nonablative lasers, microneedling in combination with platelet-rich plasma, RF microneedle or fractional bipolar therapy.
“Remarkably atrophic acne scars can repair spontaneously. Our job is to get more willing to repair spontaneously,” Dr. Tan said. “And we think we can push that to that end with topical retinoids. Topical retinoids can reduce scar formation and certainly there are lots of corrective procedures that can help our patients.”
Dr. Tan reported relationships with Abbott Laboratories, Allergan, Avene, Bayer, Boots, Cipher Pharmaceuticals, Dermira, Eli Lilly, Galderma Laboratories, Galderma Research & Development, F. Hoffman–La Roche, Janssen Pharmaceuticals, Leo Pharma, Lilly ICOS, Pfizer, Proctor & Gamble, Stiefel, and Valeant Pharmaceuticals International.
SOURCE: Tan J et al. AAD 19, Symposium 012.
WASHINGTON – Jerry Tan, MD, said at the annual meeting of the American Academy of Dermatology.
A “large majority” of patients present with active acne scarring, even those with mild and moderate disease, said Dr. Tan of the University of Western Ontario, London. The stigma associated with acne and scarring is real – in one survey, 41% of respondents noted that acne was the first thing they noticed about a person’s face (Dermatol Ther. 2016;6[2]:207-18).
“Patients with scars are stigmatized because they are perceived as being less attractive,” Dr. Tan said. “They’re perceived as being less healthy, less confident, all those negative attributes because they have developed something that was not of their own making.”
Dr. Tan presented results from his own group that estimated the risk factors of scarring. They found factors such as acne severity (odds ratio, 3.68; 95% confidence interval, 2.58-5.23), a family history of acne scarring (OR, 2.14; 95% CI, 1.67-2.76), acne duration (OR, 1.63; 1.09-2.47), and manipulation (picking and squeezing) behaviors (OR, 1.70; 95% CI, 1.27-2.29) increased the risk of acne scarring (J Eur Acad Dermatol Venereol. 2017 Sep;31[9]:1547-54). However, in a separate study, they found acne scarring and repair was an ongoing process: Of 32 patients with moderate inflammatory acne studied for 6 months, 36% of scars that were discovered within the study period were not present at follow up (J Drugs Dermatol. 2017 Jun 1;16[6]:566-72).
“I came from an era where we were taught that acne scars, when they developed, were permanent. This suggests based on good evidence that it’s not,” Dr. Tan said.
Adapalene has been successful in improving the skin texture and severity of acne scars, Dr. Tan said, citing a study that showed once-daily adapalene 0.3% gel showed a moderate to complete improvement in scar severity in 77.8% of patients and a slight improvement in 22.2% of patients (Dermatol Ther. 2018;8[2]:245-57). Increasing the dose helped: 32.9% of patients using topical adapalene 0.3% combined with benzoyl peroxide 2.5% gel at 24 weeks were clear or almost clear according to Scar Global Assessment scores, compared with 16.4% of patients randomized to vehicle (Am J Clin Dermatol. 2018 Apr;19[2]:275-86).
For established scars, many treatment modalities are available, with varying levels of evidence supporting their use. Ice pick scars are commonly treated with chemical reconstruction of skin scars (CROSS), punch techniques, and radiofrequency (RF). Rolling acne scares can be treated with dermabrasion, dermal fillers, RF, skin needling, subcision as well as ablative, nonablative and fractional lasers. Shallow boxcar scars are treated with dermabrasion, dermal fillers, RF, skin needling and subcision as well as ablative, nonablative, fractional lasers; deeper boxcar scars can be treated with RF and punch techniques.
Among these techniques, Dr. Tan said there is level I evidence to use subcision and dermal fillers with rolling acne scars and CROSS with trichloroacetic acid for ice pick and boxcar scars; and for boxcar and rolling scars, ablative and nonablative lasers, microneedling in combination with platelet-rich plasma, RF microneedle or fractional bipolar therapy.
“Remarkably atrophic acne scars can repair spontaneously. Our job is to get more willing to repair spontaneously,” Dr. Tan said. “And we think we can push that to that end with topical retinoids. Topical retinoids can reduce scar formation and certainly there are lots of corrective procedures that can help our patients.”
Dr. Tan reported relationships with Abbott Laboratories, Allergan, Avene, Bayer, Boots, Cipher Pharmaceuticals, Dermira, Eli Lilly, Galderma Laboratories, Galderma Research & Development, F. Hoffman–La Roche, Janssen Pharmaceuticals, Leo Pharma, Lilly ICOS, Pfizer, Proctor & Gamble, Stiefel, and Valeant Pharmaceuticals International.
SOURCE: Tan J et al. AAD 19, Symposium 012.
WASHINGTON – Jerry Tan, MD, said at the annual meeting of the American Academy of Dermatology.
A “large majority” of patients present with active acne scarring, even those with mild and moderate disease, said Dr. Tan of the University of Western Ontario, London. The stigma associated with acne and scarring is real – in one survey, 41% of respondents noted that acne was the first thing they noticed about a person’s face (Dermatol Ther. 2016;6[2]:207-18).
“Patients with scars are stigmatized because they are perceived as being less attractive,” Dr. Tan said. “They’re perceived as being less healthy, less confident, all those negative attributes because they have developed something that was not of their own making.”
Dr. Tan presented results from his own group that estimated the risk factors of scarring. They found factors such as acne severity (odds ratio, 3.68; 95% confidence interval, 2.58-5.23), a family history of acne scarring (OR, 2.14; 95% CI, 1.67-2.76), acne duration (OR, 1.63; 1.09-2.47), and manipulation (picking and squeezing) behaviors (OR, 1.70; 95% CI, 1.27-2.29) increased the risk of acne scarring (J Eur Acad Dermatol Venereol. 2017 Sep;31[9]:1547-54). However, in a separate study, they found acne scarring and repair was an ongoing process: Of 32 patients with moderate inflammatory acne studied for 6 months, 36% of scars that were discovered within the study period were not present at follow up (J Drugs Dermatol. 2017 Jun 1;16[6]:566-72).
“I came from an era where we were taught that acne scars, when they developed, were permanent. This suggests based on good evidence that it’s not,” Dr. Tan said.
Adapalene has been successful in improving the skin texture and severity of acne scars, Dr. Tan said, citing a study that showed once-daily adapalene 0.3% gel showed a moderate to complete improvement in scar severity in 77.8% of patients and a slight improvement in 22.2% of patients (Dermatol Ther. 2018;8[2]:245-57). Increasing the dose helped: 32.9% of patients using topical adapalene 0.3% combined with benzoyl peroxide 2.5% gel at 24 weeks were clear or almost clear according to Scar Global Assessment scores, compared with 16.4% of patients randomized to vehicle (Am J Clin Dermatol. 2018 Apr;19[2]:275-86).
For established scars, many treatment modalities are available, with varying levels of evidence supporting their use. Ice pick scars are commonly treated with chemical reconstruction of skin scars (CROSS), punch techniques, and radiofrequency (RF). Rolling acne scares can be treated with dermabrasion, dermal fillers, RF, skin needling, subcision as well as ablative, nonablative and fractional lasers. Shallow boxcar scars are treated with dermabrasion, dermal fillers, RF, skin needling and subcision as well as ablative, nonablative, fractional lasers; deeper boxcar scars can be treated with RF and punch techniques.
Among these techniques, Dr. Tan said there is level I evidence to use subcision and dermal fillers with rolling acne scars and CROSS with trichloroacetic acid for ice pick and boxcar scars; and for boxcar and rolling scars, ablative and nonablative lasers, microneedling in combination with platelet-rich plasma, RF microneedle or fractional bipolar therapy.
“Remarkably atrophic acne scars can repair spontaneously. Our job is to get more willing to repair spontaneously,” Dr. Tan said. “And we think we can push that to that end with topical retinoids. Topical retinoids can reduce scar formation and certainly there are lots of corrective procedures that can help our patients.”
Dr. Tan reported relationships with Abbott Laboratories, Allergan, Avene, Bayer, Boots, Cipher Pharmaceuticals, Dermira, Eli Lilly, Galderma Laboratories, Galderma Research & Development, F. Hoffman–La Roche, Janssen Pharmaceuticals, Leo Pharma, Lilly ICOS, Pfizer, Proctor & Gamble, Stiefel, and Valeant Pharmaceuticals International.
SOURCE: Tan J et al. AAD 19, Symposium 012.
REPORTING FROM AAD 19
Rhymin’ pediatric dermatologist provides Demodex tips
WAIKOLOA, HAWAII – Pediatric dermatologist Andrea L. Zaenglein, MD, has composed a whimsical couplet to help physicians broaden their differential diagnosis for acneiform rashes in children.
Pustules on noses: Think demodicosis!
The classic teaching has been that Demodex carriage and its pathologic clinical expression, demodicosis, are rare in children. Not so, Dr. Zaenglein asserted at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
“One of the things that we never used to see in kids but now I see all the time, probably because I’m looking for it, is Demodex. You’re not born with Demodex. Your carriage rate will increase over the years and by the time you’re elderly, 95% of us will have it – but not me. I just decided I’m not having these. I’m not looking for them, and I don’t want to know if they’re there,” she quipped, referring to the creepiness factor surrounding these facial parasitic mites invisible to the naked eye.
The mites live in pilosebaceous units. In animals, the disease is called mange. In humans, demodicosis is caused by two species of mites: Demodex folliculorum and D. brevis. The diagnosis is made by microscopic examination of mineral oil skin scrapings.
Primary demodicosis can take the form of pityriasis folliculorum, also known as spinulate demodicosis, papulopustular perioral, periauricular, or periorbital demodicosis, or a nodulocystic/conglobate version.
More commonly, however, .
“Demodicosis can be associated with immunosuppression. Kids with Langerhans cell histiocytosis seem to be prone to it. But you can see it in kids who don’t have any underlying immunosuppression, and I think there are more and more cases of that as we go looking for it,” said Dr. Zaenglein, professor of dermatology and pediatrics at Penn State University, Hershey, and immediate past president of the Society for Pediatric Dermatology.
Look for Demodex in children with somewhat atypical versions of common acneiform eruptions: for example, the ‘pustules on noses’ variant.
“Finding Demodex can alter your treatment and make it easier in these cases,” she said.
Metronidazole and ivermectin are the systemic treatment options for pediatric demodicosis.
“I tend to use topical permethrin, just because it’s easy to get. I’ll treat them once a week for 3 weeks in a row. But also make sure to treat the underlying primary inflammatory disorder,” the pediatric dermatologist advised.
Other topical options include ivermectin cream, crotamiton cream, metronidazole gel, and salicylic acid cream.
Dr. Zaenglein reported having financial relationships with Sun Pharmaceuticals, Allergan, and Ortho Dermatologics.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – Pediatric dermatologist Andrea L. Zaenglein, MD, has composed a whimsical couplet to help physicians broaden their differential diagnosis for acneiform rashes in children.
Pustules on noses: Think demodicosis!
The classic teaching has been that Demodex carriage and its pathologic clinical expression, demodicosis, are rare in children. Not so, Dr. Zaenglein asserted at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
“One of the things that we never used to see in kids but now I see all the time, probably because I’m looking for it, is Demodex. You’re not born with Demodex. Your carriage rate will increase over the years and by the time you’re elderly, 95% of us will have it – but not me. I just decided I’m not having these. I’m not looking for them, and I don’t want to know if they’re there,” she quipped, referring to the creepiness factor surrounding these facial parasitic mites invisible to the naked eye.
The mites live in pilosebaceous units. In animals, the disease is called mange. In humans, demodicosis is caused by two species of mites: Demodex folliculorum and D. brevis. The diagnosis is made by microscopic examination of mineral oil skin scrapings.
Primary demodicosis can take the form of pityriasis folliculorum, also known as spinulate demodicosis, papulopustular perioral, periauricular, or periorbital demodicosis, or a nodulocystic/conglobate version.
More commonly, however, .
“Demodicosis can be associated with immunosuppression. Kids with Langerhans cell histiocytosis seem to be prone to it. But you can see it in kids who don’t have any underlying immunosuppression, and I think there are more and more cases of that as we go looking for it,” said Dr. Zaenglein, professor of dermatology and pediatrics at Penn State University, Hershey, and immediate past president of the Society for Pediatric Dermatology.
Look for Demodex in children with somewhat atypical versions of common acneiform eruptions: for example, the ‘pustules on noses’ variant.
“Finding Demodex can alter your treatment and make it easier in these cases,” she said.
Metronidazole and ivermectin are the systemic treatment options for pediatric demodicosis.
“I tend to use topical permethrin, just because it’s easy to get. I’ll treat them once a week for 3 weeks in a row. But also make sure to treat the underlying primary inflammatory disorder,” the pediatric dermatologist advised.
Other topical options include ivermectin cream, crotamiton cream, metronidazole gel, and salicylic acid cream.
Dr. Zaenglein reported having financial relationships with Sun Pharmaceuticals, Allergan, and Ortho Dermatologics.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – Pediatric dermatologist Andrea L. Zaenglein, MD, has composed a whimsical couplet to help physicians broaden their differential diagnosis for acneiform rashes in children.
Pustules on noses: Think demodicosis!
The classic teaching has been that Demodex carriage and its pathologic clinical expression, demodicosis, are rare in children. Not so, Dr. Zaenglein asserted at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
“One of the things that we never used to see in kids but now I see all the time, probably because I’m looking for it, is Demodex. You’re not born with Demodex. Your carriage rate will increase over the years and by the time you’re elderly, 95% of us will have it – but not me. I just decided I’m not having these. I’m not looking for them, and I don’t want to know if they’re there,” she quipped, referring to the creepiness factor surrounding these facial parasitic mites invisible to the naked eye.
The mites live in pilosebaceous units. In animals, the disease is called mange. In humans, demodicosis is caused by two species of mites: Demodex folliculorum and D. brevis. The diagnosis is made by microscopic examination of mineral oil skin scrapings.
Primary demodicosis can take the form of pityriasis folliculorum, also known as spinulate demodicosis, papulopustular perioral, periauricular, or periorbital demodicosis, or a nodulocystic/conglobate version.
More commonly, however, .
“Demodicosis can be associated with immunosuppression. Kids with Langerhans cell histiocytosis seem to be prone to it. But you can see it in kids who don’t have any underlying immunosuppression, and I think there are more and more cases of that as we go looking for it,” said Dr. Zaenglein, professor of dermatology and pediatrics at Penn State University, Hershey, and immediate past president of the Society for Pediatric Dermatology.
Look for Demodex in children with somewhat atypical versions of common acneiform eruptions: for example, the ‘pustules on noses’ variant.
“Finding Demodex can alter your treatment and make it easier in these cases,” she said.
Metronidazole and ivermectin are the systemic treatment options for pediatric demodicosis.
“I tend to use topical permethrin, just because it’s easy to get. I’ll treat them once a week for 3 weeks in a row. But also make sure to treat the underlying primary inflammatory disorder,” the pediatric dermatologist advised.
Other topical options include ivermectin cream, crotamiton cream, metronidazole gel, and salicylic acid cream.
Dr. Zaenglein reported having financial relationships with Sun Pharmaceuticals, Allergan, and Ortho Dermatologics.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
REPORTING FROM SDEF HAWAII DERMATOLOGY SEMINAR
TCA and punch excision are two options for icepick acne scars
WAIKOLOA, HAWAII – Dermatologists can certainly improve icepick acne scars, but they have to be careful not to make them worse, according to dermatologist Nazanin Saedi, MD, director of the Jefferson Laser Surgery and Cosmetic Dermatology Center at Thomas Jefferson University, Philadelphia.
.
After about three to five TCA treatments, most patients will have a better than 50% improvement, Dr. Saedi said, but the treatment isn’t for darker skin types – Fitzpatrick types V or VI – because of the risk of pigmentation changes. Dr. Saedi uses toothpicks to apply a small amount of 80% TCA to the base of the scar, and waits for the “frost” to appear. It’s important not to reapply the TCA. “A lot of people double dip and ... keep dipping into the scar,” which causes more damage.
For patients with darker skin types, or those who don’t want to go through a series of treatments, punch excision is an option, with nonablative laser treatment a week later when sutures are removed. “Some patients heal beautifully,” but some patients may have a spread scar or a small atrophic scar at the punch site, she noted. Options to treat atrophic scarring after treatment are laser treatments and fillers.
She offered her advice in an interview at the Hawaii Dermatology Seminar, provided by the Global Academy for Medical Education/Skin Disease Education Foundation. It’s important to set realistic expectations, Dr. Saedi said.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – Dermatologists can certainly improve icepick acne scars, but they have to be careful not to make them worse, according to dermatologist Nazanin Saedi, MD, director of the Jefferson Laser Surgery and Cosmetic Dermatology Center at Thomas Jefferson University, Philadelphia.
.
After about three to five TCA treatments, most patients will have a better than 50% improvement, Dr. Saedi said, but the treatment isn’t for darker skin types – Fitzpatrick types V or VI – because of the risk of pigmentation changes. Dr. Saedi uses toothpicks to apply a small amount of 80% TCA to the base of the scar, and waits for the “frost” to appear. It’s important not to reapply the TCA. “A lot of people double dip and ... keep dipping into the scar,” which causes more damage.
For patients with darker skin types, or those who don’t want to go through a series of treatments, punch excision is an option, with nonablative laser treatment a week later when sutures are removed. “Some patients heal beautifully,” but some patients may have a spread scar or a small atrophic scar at the punch site, she noted. Options to treat atrophic scarring after treatment are laser treatments and fillers.
She offered her advice in an interview at the Hawaii Dermatology Seminar, provided by the Global Academy for Medical Education/Skin Disease Education Foundation. It’s important to set realistic expectations, Dr. Saedi said.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – Dermatologists can certainly improve icepick acne scars, but they have to be careful not to make them worse, according to dermatologist Nazanin Saedi, MD, director of the Jefferson Laser Surgery and Cosmetic Dermatology Center at Thomas Jefferson University, Philadelphia.
.
After about three to five TCA treatments, most patients will have a better than 50% improvement, Dr. Saedi said, but the treatment isn’t for darker skin types – Fitzpatrick types V or VI – because of the risk of pigmentation changes. Dr. Saedi uses toothpicks to apply a small amount of 80% TCA to the base of the scar, and waits for the “frost” to appear. It’s important not to reapply the TCA. “A lot of people double dip and ... keep dipping into the scar,” which causes more damage.
For patients with darker skin types, or those who don’t want to go through a series of treatments, punch excision is an option, with nonablative laser treatment a week later when sutures are removed. “Some patients heal beautifully,” but some patients may have a spread scar or a small atrophic scar at the punch site, she noted. Options to treat atrophic scarring after treatment are laser treatments and fillers.
She offered her advice in an interview at the Hawaii Dermatology Seminar, provided by the Global Academy for Medical Education/Skin Disease Education Foundation. It’s important to set realistic expectations, Dr. Saedi said.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
Trump bars abortion referrals from family planning program
The U.S. Department of Health & Human Services has finalized sweeping changes to the federal Title X family planning program, pulling back funds from clinics that provide abortion counseling or that refer patients for abortion services, regardless of whether the money is used for other health care services.
Under the final rule, announced Feb. 22 by HHS, women’s health clinics are ineligible for Title X funding if they offer, promote, or support abortion as a method of family planning. Title X grants generally go to health centers that provide reproductive health care – such as STD-testing, cancer screenings, and contraception – to low-income families.
In a fact sheet, HHS stated the final rule will provide for clear financial and physical separation between Title X and non-Title X activities, reduce confusion on the part of Title X clinics and the public about permissible Title X activities, and improve program transparency by requiring more complete reporting by grantees about their partnerships with referral agencies.
“The final rule ensures compliance with statutory program integrity provisions governing the program and, in particular, the statutory prohibition on funding programs where abortion is a method of family planning,” department officials said in a statement. “The final rule amends the Title X regulation, which had not been substantially updated in nearly 2 decades, and makes notable improvements designed to increase the number of patients served and improve the quality of their care.”
Lisa Hollier, MD, president for the American College of Obstetricians and Gynecologists (ACOG) said the final rule threatens the ability of women’s health care providers to deliver medically accurate and comprehensive reproductive health care and poses significant harms to women’s health.
“As the only federal program exclusively dedicated to providing low-income patients with access to family planning and preventive health services and information, [Title X] plays a vital role in the landscape of women’s health care,” Dr. Hollier said during a Feb. 22 press conference. “By weakening the requirements for the scope of contraceptive care provided by grant recipients and restricting the types of care recipients can discuss with patients, the final rule fundamentally harms the scope and purpose of this historic program.”
The American Medical Association also expressed disappointment, referring to the final requirement as a “gag rule” between physicians and patients.
“This rule interferes with and imposes restrictions on the patient-physician relationship,” Barbara L. McAneny, MD, AMA President said in a statement. “For all intents and purposes, it imposes a gag rule on what information physicians can provide to their patients. The patient-physician relationship relies on trust, open conversation and informed decision making and the government should not be telling physicians what they can and cannot say to their patients.”
Under the rule, proposed last year, physicians are prohibited from discussing abortion options with pregnant women, from sharing abortion information, and from making abortion referrals if the clinic receives Title X funds. The regulation permits, but no longer requires, nondirective pregnancy counseling, including nondirective counseling on abortion. In its statement, HHS officials said the new rule ensures “conscience protections” for Title X health providers by eliminating the requirement for providers to counsel on and refer for abortion.
Susan B. Anthony List, an anti-abortion group, praised the final rule as a measure that disentangles taxpayers from the “big abortion industry led by Planned Parenthood.”
“The Protect Life Rule does not cut family planning funding by a single dime, and instead directs tax dollars to entities that provide health care to women but do not perform abortions,” said SBA List President Marjorie Dannenfelser in a statement. “The Title X program was not intended to be a slush fund for abortion businesses like Planned Parenthood, which violently ends the lives of more than 332,000 unborn babies a year and receives almost $60 million a year in Title X taxpayer dollars.”
Emily Stewart, vice president of public policy for the Planned Parenthood Federation of America indicated that the group plans to fight the rule in court.
“Since day one, the Trump-Pence administration has aggressively targeted the health, rights, and bodily autonomy of people of color, people with low incomes, and women,” she said in a statement. “We’re going to fight this rule through every possible avenue.”
The final rule has been submitted to the Federal Register for publication.
The U.S. Department of Health & Human Services has finalized sweeping changes to the federal Title X family planning program, pulling back funds from clinics that provide abortion counseling or that refer patients for abortion services, regardless of whether the money is used for other health care services.
Under the final rule, announced Feb. 22 by HHS, women’s health clinics are ineligible for Title X funding if they offer, promote, or support abortion as a method of family planning. Title X grants generally go to health centers that provide reproductive health care – such as STD-testing, cancer screenings, and contraception – to low-income families.
In a fact sheet, HHS stated the final rule will provide for clear financial and physical separation between Title X and non-Title X activities, reduce confusion on the part of Title X clinics and the public about permissible Title X activities, and improve program transparency by requiring more complete reporting by grantees about their partnerships with referral agencies.
“The final rule ensures compliance with statutory program integrity provisions governing the program and, in particular, the statutory prohibition on funding programs where abortion is a method of family planning,” department officials said in a statement. “The final rule amends the Title X regulation, which had not been substantially updated in nearly 2 decades, and makes notable improvements designed to increase the number of patients served and improve the quality of their care.”
Lisa Hollier, MD, president for the American College of Obstetricians and Gynecologists (ACOG) said the final rule threatens the ability of women’s health care providers to deliver medically accurate and comprehensive reproductive health care and poses significant harms to women’s health.
“As the only federal program exclusively dedicated to providing low-income patients with access to family planning and preventive health services and information, [Title X] plays a vital role in the landscape of women’s health care,” Dr. Hollier said during a Feb. 22 press conference. “By weakening the requirements for the scope of contraceptive care provided by grant recipients and restricting the types of care recipients can discuss with patients, the final rule fundamentally harms the scope and purpose of this historic program.”
The American Medical Association also expressed disappointment, referring to the final requirement as a “gag rule” between physicians and patients.
“This rule interferes with and imposes restrictions on the patient-physician relationship,” Barbara L. McAneny, MD, AMA President said in a statement. “For all intents and purposes, it imposes a gag rule on what information physicians can provide to their patients. The patient-physician relationship relies on trust, open conversation and informed decision making and the government should not be telling physicians what they can and cannot say to their patients.”
Under the rule, proposed last year, physicians are prohibited from discussing abortion options with pregnant women, from sharing abortion information, and from making abortion referrals if the clinic receives Title X funds. The regulation permits, but no longer requires, nondirective pregnancy counseling, including nondirective counseling on abortion. In its statement, HHS officials said the new rule ensures “conscience protections” for Title X health providers by eliminating the requirement for providers to counsel on and refer for abortion.
Susan B. Anthony List, an anti-abortion group, praised the final rule as a measure that disentangles taxpayers from the “big abortion industry led by Planned Parenthood.”
“The Protect Life Rule does not cut family planning funding by a single dime, and instead directs tax dollars to entities that provide health care to women but do not perform abortions,” said SBA List President Marjorie Dannenfelser in a statement. “The Title X program was not intended to be a slush fund for abortion businesses like Planned Parenthood, which violently ends the lives of more than 332,000 unborn babies a year and receives almost $60 million a year in Title X taxpayer dollars.”
Emily Stewart, vice president of public policy for the Planned Parenthood Federation of America indicated that the group plans to fight the rule in court.
“Since day one, the Trump-Pence administration has aggressively targeted the health, rights, and bodily autonomy of people of color, people with low incomes, and women,” she said in a statement. “We’re going to fight this rule through every possible avenue.”
The final rule has been submitted to the Federal Register for publication.
The U.S. Department of Health & Human Services has finalized sweeping changes to the federal Title X family planning program, pulling back funds from clinics that provide abortion counseling or that refer patients for abortion services, regardless of whether the money is used for other health care services.
Under the final rule, announced Feb. 22 by HHS, women’s health clinics are ineligible for Title X funding if they offer, promote, or support abortion as a method of family planning. Title X grants generally go to health centers that provide reproductive health care – such as STD-testing, cancer screenings, and contraception – to low-income families.
In a fact sheet, HHS stated the final rule will provide for clear financial and physical separation between Title X and non-Title X activities, reduce confusion on the part of Title X clinics and the public about permissible Title X activities, and improve program transparency by requiring more complete reporting by grantees about their partnerships with referral agencies.
“The final rule ensures compliance with statutory program integrity provisions governing the program and, in particular, the statutory prohibition on funding programs where abortion is a method of family planning,” department officials said in a statement. “The final rule amends the Title X regulation, which had not been substantially updated in nearly 2 decades, and makes notable improvements designed to increase the number of patients served and improve the quality of their care.”
Lisa Hollier, MD, president for the American College of Obstetricians and Gynecologists (ACOG) said the final rule threatens the ability of women’s health care providers to deliver medically accurate and comprehensive reproductive health care and poses significant harms to women’s health.
“As the only federal program exclusively dedicated to providing low-income patients with access to family planning and preventive health services and information, [Title X] plays a vital role in the landscape of women’s health care,” Dr. Hollier said during a Feb. 22 press conference. “By weakening the requirements for the scope of contraceptive care provided by grant recipients and restricting the types of care recipients can discuss with patients, the final rule fundamentally harms the scope and purpose of this historic program.”
The American Medical Association also expressed disappointment, referring to the final requirement as a “gag rule” between physicians and patients.
“This rule interferes with and imposes restrictions on the patient-physician relationship,” Barbara L. McAneny, MD, AMA President said in a statement. “For all intents and purposes, it imposes a gag rule on what information physicians can provide to their patients. The patient-physician relationship relies on trust, open conversation and informed decision making and the government should not be telling physicians what they can and cannot say to their patients.”
Under the rule, proposed last year, physicians are prohibited from discussing abortion options with pregnant women, from sharing abortion information, and from making abortion referrals if the clinic receives Title X funds. The regulation permits, but no longer requires, nondirective pregnancy counseling, including nondirective counseling on abortion. In its statement, HHS officials said the new rule ensures “conscience protections” for Title X health providers by eliminating the requirement for providers to counsel on and refer for abortion.
Susan B. Anthony List, an anti-abortion group, praised the final rule as a measure that disentangles taxpayers from the “big abortion industry led by Planned Parenthood.”
“The Protect Life Rule does not cut family planning funding by a single dime, and instead directs tax dollars to entities that provide health care to women but do not perform abortions,” said SBA List President Marjorie Dannenfelser in a statement. “The Title X program was not intended to be a slush fund for abortion businesses like Planned Parenthood, which violently ends the lives of more than 332,000 unborn babies a year and receives almost $60 million a year in Title X taxpayer dollars.”
Emily Stewart, vice president of public policy for the Planned Parenthood Federation of America indicated that the group plans to fight the rule in court.
“Since day one, the Trump-Pence administration has aggressively targeted the health, rights, and bodily autonomy of people of color, people with low incomes, and women,” she said in a statement. “We’re going to fight this rule through every possible avenue.”
The final rule has been submitted to the Federal Register for publication.