User login
Hospital readmission remains common for teens with nonfatal drug overdose
Approximately 1 in 5 adolescents hospitalized for nonfatal drug overdoses were readmitted within 6 months, based on data from more than 12,000 individuals.
Previous studies suggest that many adolescents fail to receive timely treatment for addiction after a nonfatal overdose, but the rates of hospital readmission in this population have not been examined, according to Julie Gaither, PhD, of Yale University, New Haven, Conn.
In a study presented at the annual meeting of the Pediatric Academic Societies, Dr. Gaither and her colleague, John M. Leventhal, MD, also of Yale University, used data from the 2016 Nationwide Readmissions Database to examine incidence and recurrent hospitalizations for nonfatal drug overdoses in adolescents. The study population included 12,952 patients aged 11-21 years who were admitted to a hospital after a nonfatal drug overdose in 2016. Of these, 15% were younger than 15 years, and 52.1% were females.
Overall, 76.2% of the overdoses involved opioids; 77.9% involved a prescription opioid, 15.3% involved heroin, and 7.9% involved fentanyl.
Across all drug overdoses, the majority (86.5%) were attributed to accidental intent and 11.8% were attributed to self-harm. Notably, females were nearly four times more likely than males to attempt suicide (odds ratio, 3.57). After the initial hospitalization, 79.3% of the patients were discharged home, and 11.5% went to a short-term care facility.
The 6-month hospital readmission rate was 21.4%. Of the patients readmitted for any cause, 18.2% of readmissions were for recurrent overdoses, and 92.1% of these were attributed to opioids.
The median cost of the initial hospital admission was $23,705 (ranging from $11,902 to $54,682) and the median cost of the first readmission was $25,416 (ranging from $13,905 to $48,810). In 42.1% of all hospitalizations, Medicaid was the primary payer.
The study findings were limited by the relatively high number of Medicaid patients, which may limit generalizability, but is strengthened by the large sample size.
The findings highlight not only the need for prevention efforts to limit opioid use among adolescents, but also “speak to the need for timely evidenced-based addiction treatment and appropriate follow-up care for teens following hospitalization for a nonfatal drug overdose,” the researchers wrote in their abstract.
Potential for postpandemic surge in drug use
Interestingly, some recent research has shown a decline in teens’ substance use during the pandemic, Kelly Curran, MD, of the University of Oklahoma Health Sciences Center, Oklahoma City, said in an interview.
“However, as the world begins ‘opening up’ again, I suspect rates of drug use will rise – especially with the significant burden of mental health issues adolescents have struggled with during the last few years,” said Dr. Curran, who was not involved with the current study.
“Sadly, I am not surprised by this study’s findings. Too often, teens with substance abuse issues are not connected to effective, evidenced-based treatment, and for those who are, the wait list can be long,” she said.
“Teens who are misusing drugs – either to get high or to attempt suicide – who are admitted for nonfatal overdose have a high rate of readmission for recurrent drug overdose,” Dr. Curran said. “This high rate of readmission has serious social and financial implications,” she added. “This study is part of a growing body of literature that supports the importance of getting adolescents into effective, evidence-based substance abuse treatment, such as medication-assisted treatment in opioid abuse. However, we also should be advocating for improved funding for and access to these treatments for all individuals.”
The study received no outside funding. Dr. Gaither had no financial conflicts to disclose. Dr. Curran had no financial conflicts to disclose and serves on the editorial advisory board of Pediatric News.
Approximately 1 in 5 adolescents hospitalized for nonfatal drug overdoses were readmitted within 6 months, based on data from more than 12,000 individuals.
Previous studies suggest that many adolescents fail to receive timely treatment for addiction after a nonfatal overdose, but the rates of hospital readmission in this population have not been examined, according to Julie Gaither, PhD, of Yale University, New Haven, Conn.
In a study presented at the annual meeting of the Pediatric Academic Societies, Dr. Gaither and her colleague, John M. Leventhal, MD, also of Yale University, used data from the 2016 Nationwide Readmissions Database to examine incidence and recurrent hospitalizations for nonfatal drug overdoses in adolescents. The study population included 12,952 patients aged 11-21 years who were admitted to a hospital after a nonfatal drug overdose in 2016. Of these, 15% were younger than 15 years, and 52.1% were females.
Overall, 76.2% of the overdoses involved opioids; 77.9% involved a prescription opioid, 15.3% involved heroin, and 7.9% involved fentanyl.
Across all drug overdoses, the majority (86.5%) were attributed to accidental intent and 11.8% were attributed to self-harm. Notably, females were nearly four times more likely than males to attempt suicide (odds ratio, 3.57). After the initial hospitalization, 79.3% of the patients were discharged home, and 11.5% went to a short-term care facility.
The 6-month hospital readmission rate was 21.4%. Of the patients readmitted for any cause, 18.2% of readmissions were for recurrent overdoses, and 92.1% of these were attributed to opioids.
The median cost of the initial hospital admission was $23,705 (ranging from $11,902 to $54,682) and the median cost of the first readmission was $25,416 (ranging from $13,905 to $48,810). In 42.1% of all hospitalizations, Medicaid was the primary payer.
The study findings were limited by the relatively high number of Medicaid patients, which may limit generalizability, but is strengthened by the large sample size.
The findings highlight not only the need for prevention efforts to limit opioid use among adolescents, but also “speak to the need for timely evidenced-based addiction treatment and appropriate follow-up care for teens following hospitalization for a nonfatal drug overdose,” the researchers wrote in their abstract.
Potential for postpandemic surge in drug use
Interestingly, some recent research has shown a decline in teens’ substance use during the pandemic, Kelly Curran, MD, of the University of Oklahoma Health Sciences Center, Oklahoma City, said in an interview.
“However, as the world begins ‘opening up’ again, I suspect rates of drug use will rise – especially with the significant burden of mental health issues adolescents have struggled with during the last few years,” said Dr. Curran, who was not involved with the current study.
“Sadly, I am not surprised by this study’s findings. Too often, teens with substance abuse issues are not connected to effective, evidenced-based treatment, and for those who are, the wait list can be long,” she said.
“Teens who are misusing drugs – either to get high or to attempt suicide – who are admitted for nonfatal overdose have a high rate of readmission for recurrent drug overdose,” Dr. Curran said. “This high rate of readmission has serious social and financial implications,” she added. “This study is part of a growing body of literature that supports the importance of getting adolescents into effective, evidence-based substance abuse treatment, such as medication-assisted treatment in opioid abuse. However, we also should be advocating for improved funding for and access to these treatments for all individuals.”
The study received no outside funding. Dr. Gaither had no financial conflicts to disclose. Dr. Curran had no financial conflicts to disclose and serves on the editorial advisory board of Pediatric News.
Approximately 1 in 5 adolescents hospitalized for nonfatal drug overdoses were readmitted within 6 months, based on data from more than 12,000 individuals.
Previous studies suggest that many adolescents fail to receive timely treatment for addiction after a nonfatal overdose, but the rates of hospital readmission in this population have not been examined, according to Julie Gaither, PhD, of Yale University, New Haven, Conn.
In a study presented at the annual meeting of the Pediatric Academic Societies, Dr. Gaither and her colleague, John M. Leventhal, MD, also of Yale University, used data from the 2016 Nationwide Readmissions Database to examine incidence and recurrent hospitalizations for nonfatal drug overdoses in adolescents. The study population included 12,952 patients aged 11-21 years who were admitted to a hospital after a nonfatal drug overdose in 2016. Of these, 15% were younger than 15 years, and 52.1% were females.
Overall, 76.2% of the overdoses involved opioids; 77.9% involved a prescription opioid, 15.3% involved heroin, and 7.9% involved fentanyl.
Across all drug overdoses, the majority (86.5%) were attributed to accidental intent and 11.8% were attributed to self-harm. Notably, females were nearly four times more likely than males to attempt suicide (odds ratio, 3.57). After the initial hospitalization, 79.3% of the patients were discharged home, and 11.5% went to a short-term care facility.
The 6-month hospital readmission rate was 21.4%. Of the patients readmitted for any cause, 18.2% of readmissions were for recurrent overdoses, and 92.1% of these were attributed to opioids.
The median cost of the initial hospital admission was $23,705 (ranging from $11,902 to $54,682) and the median cost of the first readmission was $25,416 (ranging from $13,905 to $48,810). In 42.1% of all hospitalizations, Medicaid was the primary payer.
The study findings were limited by the relatively high number of Medicaid patients, which may limit generalizability, but is strengthened by the large sample size.
The findings highlight not only the need for prevention efforts to limit opioid use among adolescents, but also “speak to the need for timely evidenced-based addiction treatment and appropriate follow-up care for teens following hospitalization for a nonfatal drug overdose,” the researchers wrote in their abstract.
Potential for postpandemic surge in drug use
Interestingly, some recent research has shown a decline in teens’ substance use during the pandemic, Kelly Curran, MD, of the University of Oklahoma Health Sciences Center, Oklahoma City, said in an interview.
“However, as the world begins ‘opening up’ again, I suspect rates of drug use will rise – especially with the significant burden of mental health issues adolescents have struggled with during the last few years,” said Dr. Curran, who was not involved with the current study.
“Sadly, I am not surprised by this study’s findings. Too often, teens with substance abuse issues are not connected to effective, evidenced-based treatment, and for those who are, the wait list can be long,” she said.
“Teens who are misusing drugs – either to get high or to attempt suicide – who are admitted for nonfatal overdose have a high rate of readmission for recurrent drug overdose,” Dr. Curran said. “This high rate of readmission has serious social and financial implications,” she added. “This study is part of a growing body of literature that supports the importance of getting adolescents into effective, evidence-based substance abuse treatment, such as medication-assisted treatment in opioid abuse. However, we also should be advocating for improved funding for and access to these treatments for all individuals.”
The study received no outside funding. Dr. Gaither had no financial conflicts to disclose. Dr. Curran had no financial conflicts to disclose and serves on the editorial advisory board of Pediatric News.
FROM PAS 2022
Substance use disorders increase risk for death from COVID-19
MADRID, Spain – – compared with the general population. Such are the findings of a line of research led by Mexican psychiatrist Nora Volkow, MD, director of the U.S. National Institute on Drug Abuse (NIDA).
A pioneer in the use of brain imaging to investigate how substance use affects brain functions and one of Time magazine’s “Top 100 People Who Shape Our World,” she led the Inaugural Conference at the XXXI Congress of the Spanish Society of Clinical Pharmacology “Drugs and Actions During the Pandemic.” Dr. Volkow spoke about the effects that the current health crisis has had on drug use and the social challenges that arose from lockdowns. She also presented and discussed the results of studies being conducted at NIDA that “are aimed at reviewing what we’ve learned and what the consequences of COVID-19 have been with respect to substance abuse disorder.”
As Dr. Volkow pointed out, drugs affect much more than just the brain. “In particular, the heart, the lungs, the immune system – all of these are significantly harmed by substances like tobacco, alcohol, cocaine, and methamphetamine. This is why, since the beginning of the pandemic, we’ve been worried about seeing what consequences SARS-CoV-2 was going to have on users of these substances, especially in light of the great toll this disease takes on the respiratory system and the vascular system.”
Pulmonary ‘predisposition’ and race
Dr. Volkow and her team launched several studies to get a more thorough understanding of the link between substance abuse disorders and poor COVID-19 prognoses. One of them was based on analyses from electronic health records in the United States. The purpose was to determine COVID-19 risk and outcomes in patients based on the type of use disorder (for example, alcohol, opioid, cannabis, cocaine).
“The results showed that regardless of the drug type, all users of these substances had both a higher risk of being infected by COVID-19 and a higher death rate in comparison with the rest of the population,” said Dr. Volkow. “This surprised us, because there’s no evidence that drugs themselves make the virus more infectious. However, what the results did clearly indicate to us was that using these substances was associated with behavior that put these individuals at a greater risk for infection,” Dr. Volkow explained.
“In addition,” she continued, “using, for example, tobacco or cannabis causes inflammation in the lungs. It seems that, as a result, they end up being more vulnerable to infection by COVID. And this has consequences, above all, in terms of mortality.”
Another finding was that, among patients with substance use disorders, race had the largest effect on COVID risk. “From the very start, we saw that, compared with White individuals, Black individuals showed a much higher risk of not only getting COVID, but also dying from it,” said Dr. Volkow. “Therefore, on the one hand, our data show that drug users are more vulnerable to COVID-19 and, on the other hand, they reflect that within this group, Black individuals are even more vulnerable.”
In her presentation, Dr. Volkow drew particular attention to the impact that social surroundings have on these patients and the decisive role they played in terms of vulnerability. “It’s a very complex issue, what with the various factors at play: family, social environment. ... A person living in an at-risk situation can more easily get drugs or even prescription medication, which can also be abused.”
The psychiatrist stressed that when it comes to addictive disorders (and related questions such as prevention, treatment, and social reintegration), one of the most crucial factors has to do with the individual’s social support structures. “The studies also brought to light the role that social interaction has as an inhibitory factor with regard to drug use,” said Dr. Volkow. “And indeed, adequate adherence to treatment requires that the necessary support systems be maintained.”
In the context of the pandemic, this social aspect was also key, especially concerning the high death rate among substance use disorder patients with COVID-19. “There are very significant social determinants, such as the stigma associated with these groups – a stigma that makes these individuals more likely to hesitate to seek out treatment for diseases that may be starting to take hold, in this case COVID-19.”
On that note, Dr. Volkow emphasized the importance of treating drug addicts as though they had a chronic disease in need of treatment. “In fact, the prevalence of pathologies such as hypertension, diabetes, cancer, and dementia is much higher in these individuals than in the general population,” she said. “However, this isn’t widely known. The data reflect that not only the prevalence of these diseases, but also the severity of the symptoms, is higher, and this has a lot to do with these individuals’ reticence when it comes to reaching out for medical care. Added to that are the effects of their economic situation and other factors, such as stress (which can trigger a relapse), lack of ready access to medications, and limited access to community support or other sources of social connection.”
Opioids and COVID-19
As for drug use during the pandemic, Dr. Volkow provided context by mentioning that in the United States, the experts and authorities have spent two decades fighting the epidemic of opioid-related drug overdoses, which has caused many deaths. “And on top of this epidemic – one that we still haven’t been able to get control of – there’s the situation brought about by COVID-19. So, we had to see the consequences of a pandemic crossing paths with an epidemic.”
The United States’s epidemic of overdose deaths started with the use of opioid painkillers, medications which are overprescribed. Another issue that the United States faces is that many drugs are contaminated with fentanyl. This contamination has caused an increase in deaths.
“In the United States, fentanyl is everywhere,” said Dr. Volkow. “And what’s more concerning: almost a third of this fentanyl comes in pills that are sold as benzodiazepines. With this comes a high risk for overdose. In line with this, we saw overdose deaths among adolescents nearly double in 1 year, an increase which is likely related to these contaminated pills. It’s a risk that’s just below the surface. We’ve got to be vigilant, because this phenomenon is expected to eventually spread to Europe. After all, these pills are very cheap, hence the rapid increase in their use.”
To provide figures on drug use and overdose deaths since the beginning of the pandemic, Dr. Volkow referred to COVID-19 data provided by the National Center for Health Statistics (NCHS) at the U.S. Centers for Disease Control and Prevention. The data indicate that of the 70,630 drug overdose deaths that occurred in 2019, 49,860 involved opioids (whether prescribed or illicit). “And these numbers have continued to rise, so much so that the current situation can be classified as catastrophic – because this increase has been even greater during the pandemic due to the rise in the use of all drugs,” said Dr. Volkow.
Dr. Volkow referred to an NCHS study that looked at the period between September 2020 and September 2021, finding a 15.9% increase in the number of drug overdose deaths. A breakdown of these data shows that the highest percentage corresponds to deaths from “other psychostimulants,” primarily methamphetamines (35.7%). This category is followed by deaths involving synthetic opioids, mostly illicit fentanyl (25.8%), and deaths from cocaine (13.4%).
“These figures indicate that, for the first time in history, the United States had over 100,000 overdose deaths in 1 year,” said Dr. Volkow. “This is something that has never happened. We can only infer that the pandemic had a hand in making the overdose crisis even worse than it already was.”
As Dr. Volkow explained, policies related to handling overdoses and prescribing medications have been changed in the context of COVID-19. Addiction treatment consequently has been provided through a larger number of telehealth services, and measures such as greater access to treatment for comorbid conditions, expanded access to behavioral treatments, and the establishment of mental health hotlines have been undertaken.
Children’s cognitive development
Dr. Volkow also spoke about another of NIDA’s current subjects of research: The role that damage or compromise from drugs has on the neural circuits involved in reinforcement systems. “It’s important that we make people aware of the significance of what’s at play there, because the greatest damage that can be inflicted on the brain comes from using any type of drug during adolescence. In these cases, the likelihood of having an addictive disorder as an adult significantly increases.”
Within this framework, her team has also investigated the impact of the pandemic on the cognitive development of infants under 1 year of age. One of these studies was a pilot program in which pregnant women participated. “We found that children born during the pandemic had lower cognitive development: n = 112 versus n = 554 of those born before January 2019.”
“None of the mothers or children in the study had been infected with SARS-CoV-2,” Dr. Volkow explained. “But the results clearly reflect the negative effect of the circumstances brought about by the pandemic, especially the high level of stress, the isolation, and the lack of stimuli. Another study, currently in preprint, is based on imaging. It analyzed the impact on myelination in children not exposed to COVID-19 but born during the pandemic, compared with pre-pandemic infants. The data showed significantly reduced areas of myelin development (P < .05) in those born after 2019. And the researchers didn’t find significant differences in gestation duration or birth weight.”
The longitudinal characteristics of these studies will let us see whether a change in these individuals’ social circumstances over time also brings to light cognitive changes, even the recovery of lost or underdeveloped cognitive processes, Dr. Volkow concluded.
Dr. Volkow has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
MADRID, Spain – – compared with the general population. Such are the findings of a line of research led by Mexican psychiatrist Nora Volkow, MD, director of the U.S. National Institute on Drug Abuse (NIDA).
A pioneer in the use of brain imaging to investigate how substance use affects brain functions and one of Time magazine’s “Top 100 People Who Shape Our World,” she led the Inaugural Conference at the XXXI Congress of the Spanish Society of Clinical Pharmacology “Drugs and Actions During the Pandemic.” Dr. Volkow spoke about the effects that the current health crisis has had on drug use and the social challenges that arose from lockdowns. She also presented and discussed the results of studies being conducted at NIDA that “are aimed at reviewing what we’ve learned and what the consequences of COVID-19 have been with respect to substance abuse disorder.”
As Dr. Volkow pointed out, drugs affect much more than just the brain. “In particular, the heart, the lungs, the immune system – all of these are significantly harmed by substances like tobacco, alcohol, cocaine, and methamphetamine. This is why, since the beginning of the pandemic, we’ve been worried about seeing what consequences SARS-CoV-2 was going to have on users of these substances, especially in light of the great toll this disease takes on the respiratory system and the vascular system.”
Pulmonary ‘predisposition’ and race
Dr. Volkow and her team launched several studies to get a more thorough understanding of the link between substance abuse disorders and poor COVID-19 prognoses. One of them was based on analyses from electronic health records in the United States. The purpose was to determine COVID-19 risk and outcomes in patients based on the type of use disorder (for example, alcohol, opioid, cannabis, cocaine).
“The results showed that regardless of the drug type, all users of these substances had both a higher risk of being infected by COVID-19 and a higher death rate in comparison with the rest of the population,” said Dr. Volkow. “This surprised us, because there’s no evidence that drugs themselves make the virus more infectious. However, what the results did clearly indicate to us was that using these substances was associated with behavior that put these individuals at a greater risk for infection,” Dr. Volkow explained.
“In addition,” she continued, “using, for example, tobacco or cannabis causes inflammation in the lungs. It seems that, as a result, they end up being more vulnerable to infection by COVID. And this has consequences, above all, in terms of mortality.”
Another finding was that, among patients with substance use disorders, race had the largest effect on COVID risk. “From the very start, we saw that, compared with White individuals, Black individuals showed a much higher risk of not only getting COVID, but also dying from it,” said Dr. Volkow. “Therefore, on the one hand, our data show that drug users are more vulnerable to COVID-19 and, on the other hand, they reflect that within this group, Black individuals are even more vulnerable.”
In her presentation, Dr. Volkow drew particular attention to the impact that social surroundings have on these patients and the decisive role they played in terms of vulnerability. “It’s a very complex issue, what with the various factors at play: family, social environment. ... A person living in an at-risk situation can more easily get drugs or even prescription medication, which can also be abused.”
The psychiatrist stressed that when it comes to addictive disorders (and related questions such as prevention, treatment, and social reintegration), one of the most crucial factors has to do with the individual’s social support structures. “The studies also brought to light the role that social interaction has as an inhibitory factor with regard to drug use,” said Dr. Volkow. “And indeed, adequate adherence to treatment requires that the necessary support systems be maintained.”
In the context of the pandemic, this social aspect was also key, especially concerning the high death rate among substance use disorder patients with COVID-19. “There are very significant social determinants, such as the stigma associated with these groups – a stigma that makes these individuals more likely to hesitate to seek out treatment for diseases that may be starting to take hold, in this case COVID-19.”
On that note, Dr. Volkow emphasized the importance of treating drug addicts as though they had a chronic disease in need of treatment. “In fact, the prevalence of pathologies such as hypertension, diabetes, cancer, and dementia is much higher in these individuals than in the general population,” she said. “However, this isn’t widely known. The data reflect that not only the prevalence of these diseases, but also the severity of the symptoms, is higher, and this has a lot to do with these individuals’ reticence when it comes to reaching out for medical care. Added to that are the effects of their economic situation and other factors, such as stress (which can trigger a relapse), lack of ready access to medications, and limited access to community support or other sources of social connection.”
Opioids and COVID-19
As for drug use during the pandemic, Dr. Volkow provided context by mentioning that in the United States, the experts and authorities have spent two decades fighting the epidemic of opioid-related drug overdoses, which has caused many deaths. “And on top of this epidemic – one that we still haven’t been able to get control of – there’s the situation brought about by COVID-19. So, we had to see the consequences of a pandemic crossing paths with an epidemic.”
The United States’s epidemic of overdose deaths started with the use of opioid painkillers, medications which are overprescribed. Another issue that the United States faces is that many drugs are contaminated with fentanyl. This contamination has caused an increase in deaths.
“In the United States, fentanyl is everywhere,” said Dr. Volkow. “And what’s more concerning: almost a third of this fentanyl comes in pills that are sold as benzodiazepines. With this comes a high risk for overdose. In line with this, we saw overdose deaths among adolescents nearly double in 1 year, an increase which is likely related to these contaminated pills. It’s a risk that’s just below the surface. We’ve got to be vigilant, because this phenomenon is expected to eventually spread to Europe. After all, these pills are very cheap, hence the rapid increase in their use.”
To provide figures on drug use and overdose deaths since the beginning of the pandemic, Dr. Volkow referred to COVID-19 data provided by the National Center for Health Statistics (NCHS) at the U.S. Centers for Disease Control and Prevention. The data indicate that of the 70,630 drug overdose deaths that occurred in 2019, 49,860 involved opioids (whether prescribed or illicit). “And these numbers have continued to rise, so much so that the current situation can be classified as catastrophic – because this increase has been even greater during the pandemic due to the rise in the use of all drugs,” said Dr. Volkow.
Dr. Volkow referred to an NCHS study that looked at the period between September 2020 and September 2021, finding a 15.9% increase in the number of drug overdose deaths. A breakdown of these data shows that the highest percentage corresponds to deaths from “other psychostimulants,” primarily methamphetamines (35.7%). This category is followed by deaths involving synthetic opioids, mostly illicit fentanyl (25.8%), and deaths from cocaine (13.4%).
“These figures indicate that, for the first time in history, the United States had over 100,000 overdose deaths in 1 year,” said Dr. Volkow. “This is something that has never happened. We can only infer that the pandemic had a hand in making the overdose crisis even worse than it already was.”
As Dr. Volkow explained, policies related to handling overdoses and prescribing medications have been changed in the context of COVID-19. Addiction treatment consequently has been provided through a larger number of telehealth services, and measures such as greater access to treatment for comorbid conditions, expanded access to behavioral treatments, and the establishment of mental health hotlines have been undertaken.
Children’s cognitive development
Dr. Volkow also spoke about another of NIDA’s current subjects of research: The role that damage or compromise from drugs has on the neural circuits involved in reinforcement systems. “It’s important that we make people aware of the significance of what’s at play there, because the greatest damage that can be inflicted on the brain comes from using any type of drug during adolescence. In these cases, the likelihood of having an addictive disorder as an adult significantly increases.”
Within this framework, her team has also investigated the impact of the pandemic on the cognitive development of infants under 1 year of age. One of these studies was a pilot program in which pregnant women participated. “We found that children born during the pandemic had lower cognitive development: n = 112 versus n = 554 of those born before January 2019.”
“None of the mothers or children in the study had been infected with SARS-CoV-2,” Dr. Volkow explained. “But the results clearly reflect the negative effect of the circumstances brought about by the pandemic, especially the high level of stress, the isolation, and the lack of stimuli. Another study, currently in preprint, is based on imaging. It analyzed the impact on myelination in children not exposed to COVID-19 but born during the pandemic, compared with pre-pandemic infants. The data showed significantly reduced areas of myelin development (P < .05) in those born after 2019. And the researchers didn’t find significant differences in gestation duration or birth weight.”
The longitudinal characteristics of these studies will let us see whether a change in these individuals’ social circumstances over time also brings to light cognitive changes, even the recovery of lost or underdeveloped cognitive processes, Dr. Volkow concluded.
Dr. Volkow has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
MADRID, Spain – – compared with the general population. Such are the findings of a line of research led by Mexican psychiatrist Nora Volkow, MD, director of the U.S. National Institute on Drug Abuse (NIDA).
A pioneer in the use of brain imaging to investigate how substance use affects brain functions and one of Time magazine’s “Top 100 People Who Shape Our World,” she led the Inaugural Conference at the XXXI Congress of the Spanish Society of Clinical Pharmacology “Drugs and Actions During the Pandemic.” Dr. Volkow spoke about the effects that the current health crisis has had on drug use and the social challenges that arose from lockdowns. She also presented and discussed the results of studies being conducted at NIDA that “are aimed at reviewing what we’ve learned and what the consequences of COVID-19 have been with respect to substance abuse disorder.”
As Dr. Volkow pointed out, drugs affect much more than just the brain. “In particular, the heart, the lungs, the immune system – all of these are significantly harmed by substances like tobacco, alcohol, cocaine, and methamphetamine. This is why, since the beginning of the pandemic, we’ve been worried about seeing what consequences SARS-CoV-2 was going to have on users of these substances, especially in light of the great toll this disease takes on the respiratory system and the vascular system.”
Pulmonary ‘predisposition’ and race
Dr. Volkow and her team launched several studies to get a more thorough understanding of the link between substance abuse disorders and poor COVID-19 prognoses. One of them was based on analyses from electronic health records in the United States. The purpose was to determine COVID-19 risk and outcomes in patients based on the type of use disorder (for example, alcohol, opioid, cannabis, cocaine).
“The results showed that regardless of the drug type, all users of these substances had both a higher risk of being infected by COVID-19 and a higher death rate in comparison with the rest of the population,” said Dr. Volkow. “This surprised us, because there’s no evidence that drugs themselves make the virus more infectious. However, what the results did clearly indicate to us was that using these substances was associated with behavior that put these individuals at a greater risk for infection,” Dr. Volkow explained.
“In addition,” she continued, “using, for example, tobacco or cannabis causes inflammation in the lungs. It seems that, as a result, they end up being more vulnerable to infection by COVID. And this has consequences, above all, in terms of mortality.”
Another finding was that, among patients with substance use disorders, race had the largest effect on COVID risk. “From the very start, we saw that, compared with White individuals, Black individuals showed a much higher risk of not only getting COVID, but also dying from it,” said Dr. Volkow. “Therefore, on the one hand, our data show that drug users are more vulnerable to COVID-19 and, on the other hand, they reflect that within this group, Black individuals are even more vulnerable.”
In her presentation, Dr. Volkow drew particular attention to the impact that social surroundings have on these patients and the decisive role they played in terms of vulnerability. “It’s a very complex issue, what with the various factors at play: family, social environment. ... A person living in an at-risk situation can more easily get drugs or even prescription medication, which can also be abused.”
The psychiatrist stressed that when it comes to addictive disorders (and related questions such as prevention, treatment, and social reintegration), one of the most crucial factors has to do with the individual’s social support structures. “The studies also brought to light the role that social interaction has as an inhibitory factor with regard to drug use,” said Dr. Volkow. “And indeed, adequate adherence to treatment requires that the necessary support systems be maintained.”
In the context of the pandemic, this social aspect was also key, especially concerning the high death rate among substance use disorder patients with COVID-19. “There are very significant social determinants, such as the stigma associated with these groups – a stigma that makes these individuals more likely to hesitate to seek out treatment for diseases that may be starting to take hold, in this case COVID-19.”
On that note, Dr. Volkow emphasized the importance of treating drug addicts as though they had a chronic disease in need of treatment. “In fact, the prevalence of pathologies such as hypertension, diabetes, cancer, and dementia is much higher in these individuals than in the general population,” she said. “However, this isn’t widely known. The data reflect that not only the prevalence of these diseases, but also the severity of the symptoms, is higher, and this has a lot to do with these individuals’ reticence when it comes to reaching out for medical care. Added to that are the effects of their economic situation and other factors, such as stress (which can trigger a relapse), lack of ready access to medications, and limited access to community support or other sources of social connection.”
Opioids and COVID-19
As for drug use during the pandemic, Dr. Volkow provided context by mentioning that in the United States, the experts and authorities have spent two decades fighting the epidemic of opioid-related drug overdoses, which has caused many deaths. “And on top of this epidemic – one that we still haven’t been able to get control of – there’s the situation brought about by COVID-19. So, we had to see the consequences of a pandemic crossing paths with an epidemic.”
The United States’s epidemic of overdose deaths started with the use of opioid painkillers, medications which are overprescribed. Another issue that the United States faces is that many drugs are contaminated with fentanyl. This contamination has caused an increase in deaths.
“In the United States, fentanyl is everywhere,” said Dr. Volkow. “And what’s more concerning: almost a third of this fentanyl comes in pills that are sold as benzodiazepines. With this comes a high risk for overdose. In line with this, we saw overdose deaths among adolescents nearly double in 1 year, an increase which is likely related to these contaminated pills. It’s a risk that’s just below the surface. We’ve got to be vigilant, because this phenomenon is expected to eventually spread to Europe. After all, these pills are very cheap, hence the rapid increase in their use.”
To provide figures on drug use and overdose deaths since the beginning of the pandemic, Dr. Volkow referred to COVID-19 data provided by the National Center for Health Statistics (NCHS) at the U.S. Centers for Disease Control and Prevention. The data indicate that of the 70,630 drug overdose deaths that occurred in 2019, 49,860 involved opioids (whether prescribed or illicit). “And these numbers have continued to rise, so much so that the current situation can be classified as catastrophic – because this increase has been even greater during the pandemic due to the rise in the use of all drugs,” said Dr. Volkow.
Dr. Volkow referred to an NCHS study that looked at the period between September 2020 and September 2021, finding a 15.9% increase in the number of drug overdose deaths. A breakdown of these data shows that the highest percentage corresponds to deaths from “other psychostimulants,” primarily methamphetamines (35.7%). This category is followed by deaths involving synthetic opioids, mostly illicit fentanyl (25.8%), and deaths from cocaine (13.4%).
“These figures indicate that, for the first time in history, the United States had over 100,000 overdose deaths in 1 year,” said Dr. Volkow. “This is something that has never happened. We can only infer that the pandemic had a hand in making the overdose crisis even worse than it already was.”
As Dr. Volkow explained, policies related to handling overdoses and prescribing medications have been changed in the context of COVID-19. Addiction treatment consequently has been provided through a larger number of telehealth services, and measures such as greater access to treatment for comorbid conditions, expanded access to behavioral treatments, and the establishment of mental health hotlines have been undertaken.
Children’s cognitive development
Dr. Volkow also spoke about another of NIDA’s current subjects of research: The role that damage or compromise from drugs has on the neural circuits involved in reinforcement systems. “It’s important that we make people aware of the significance of what’s at play there, because the greatest damage that can be inflicted on the brain comes from using any type of drug during adolescence. In these cases, the likelihood of having an addictive disorder as an adult significantly increases.”
Within this framework, her team has also investigated the impact of the pandemic on the cognitive development of infants under 1 year of age. One of these studies was a pilot program in which pregnant women participated. “We found that children born during the pandemic had lower cognitive development: n = 112 versus n = 554 of those born before January 2019.”
“None of the mothers or children in the study had been infected with SARS-CoV-2,” Dr. Volkow explained. “But the results clearly reflect the negative effect of the circumstances brought about by the pandemic, especially the high level of stress, the isolation, and the lack of stimuli. Another study, currently in preprint, is based on imaging. It analyzed the impact on myelination in children not exposed to COVID-19 but born during the pandemic, compared with pre-pandemic infants. The data showed significantly reduced areas of myelin development (P < .05) in those born after 2019. And the researchers didn’t find significant differences in gestation duration or birth weight.”
The longitudinal characteristics of these studies will let us see whether a change in these individuals’ social circumstances over time also brings to light cognitive changes, even the recovery of lost or underdeveloped cognitive processes, Dr. Volkow concluded.
Dr. Volkow has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ANNUAL MEETING OF SPANISH SOCIETY OF CLINICAL PHARMACOLOGY
Alcohol dependence drug the next antianxiety med?
, early research suggests.
Japanese researchers, headed by Akiyoshi Saitoh, PhD, professor in the department of pharmacy, Tokyo University of Science, compared the reactions of mice that received a classic anxiolytic agent (diazepam) to those that received disulfiram while performing a maze task and found comparable reductions in anxiety in both groups of mice.
Moreover, unlike diazepam, disulfiram caused no sedation, amnesia, or impairments in coordination.
“These results indicate that disulfiram can be used safely by elderly patients suffering from anxiety and insomnia and has the potential to become a breakthrough psychotropic drug,” Dr. Saitoh said in a press release.
The study was published online in Frontiers in Pharmacology.
Inhibitory function
Disulfiram inhibits the enzyme aldehyde dehydrogenase (ALDH), which is responsible for alcohol metabolism. Recent research suggests that disulfiram may have broader inhibitory functions.
In particular, it inhibits the cytoplasmic protein FROUNT, preventing it from interacting with two chemokine receptors (CCR2 and CCRs) that are involved in cellular signaling pathways and are associated with regulating behaviors, including anxiety, in rodents, the authors write.
“Although the functions of FROUNT-chemokines signaling in the immune system are well documented, the potential role of CNS-expressed FROUNT chemokine–related molecules as neuromodulators remains largely unknown,” they write.
The researchers had been conducting preclinical research on the secondary pharmacologic properties of disulfiram and “coincidentally discovered” its “anxiolytic-like effects.” They investigated these effects further because currently used anxiolytics – i.e., benzodiazepines – have unwanted side effects.
The researchers utilized an elevated plus-maze (EPM) test to investigate the effects of disulfiram in mice. The EPM apparatus consists of four arms set in a cross pattern and are connected to a central square. Of these, two arms are protected by vertical boundaries, while the other two have unprotected edges. Typically, mice with anxiety prefer to spend time in the closed arms. The mice also underwent other tests of coordination and the ability to navigate a Y-maze.
Some mice received disulfiram, others received a benzodiazepine, and others received merely a “vehicle,” which served as a control.
Disulfiram “significantly and dose-dependently” increased the time spent in the open arms of the EPM, compared with the vehicle-treated group, at 30 minutes after administration (F [3, 30] = 16.64; P < .0001), suggesting less anxiety. The finding was confirmed by a Bonferroni analysis that showed a significant effect of disulfiram, compared with the vehicle-treated group, at all three doses (20 mg/kg: t = 0.9894; P > .05; 40 mg/kg: t = 3.863; P < .01; 80 mg/kg: t = 6.417; P < .001).
A Student’s t-test analysis showed that diazepam likewise had a significant effect, compared to the vehicle (t = 5.038; P < .001).
Disulfiram also “significantly and dose-dependently” increased the percentage of open-arm entries (F [3, 30] = 14.24; P < .0001). The Bonferroni analysis showed this effect at all three doses (20 mg/kg: t = 0.3999; P > .05; 40 mg/kg: t = 2.693; P > .05; 80 mg/kg: t = 5.864; P < .001).
Diazepam similarly showed a significant effect, compared to the vehicle condition (t = 3.733; P < .005).
In particular, the 40 mg/kg dose of disulfiram significantly increased the percentage of time spent in the open arms at 15, 30, and 60 minutes after administration, with the peak effect occurring at 30 minutes.
The researchers examined the effect of cyanamide, another ALDH inhibitor, on the anxiety behaviors of mice and found no effect on the number of open-arm entries or percentage of time the mice spent in the open arm, compared with the vehicle condition.
In contrast to diazepam, disulfiram had no effect on the amount of spontaneous locomotor activity, time spent on the rotarod, or activity on the Y-maze test displayed by the mice, “suggesting that there were no apparent sedative effects at the dosages used.” Moreover, unlike the mice treated with diazepam, there were no increases in the number of falls the mice experienced on the rotarod.
Glutamate levels in the prelimbic-prefrontal cortex (PL-PFC) “play an important role in the development of anxiety-like behavior in mice,” the authors state. Disulfiram “significantly and completely attenuated increased extracellular glutamate levels in the PL-PFC during stress exposure” on the EPM.
“We propose that DSF inhibits FROUNT protein and the chemokine signaling pathways under its influence, which may suppress presynaptic glutamatergic transmission in the brain,” said Dr. Saitoh. “This, in turn, attenuates the levels of glutamate in the brain, reducing overall anxiety.”
Humanity’s most common affliction
Commenting for this news organization, Roger McIntyre, MD, professor of psychiatry and pharmacology, University of Toronto, and head of the mood disorders psychopharmacology unit, noted that there is a “renewed interest in psychiatry in excitatory and inhibitory balance – for example, ketamine represents a treatment that facilitates excitatory activity, while neurosteroids are candidate medicines now for inhibitory activity.”
Dr. McIntyre, who is the chairman and executive director of the Brain and Cognitive Discover Foundation, Toronto, and was not involved with the study, said it is believed “that the excitatory-inhibitory balance may be relevant to brain health and disease.”
Dr. McIntyre also pointed out that the study “highlights not only the repurposing of a well-known medicine but also exploit[s] the potential brain therapeutic effects of immune targets that indirectly affect inhibitory systems, resulting in potentially a safer treatment for anxiety – the most common affliction of humanity.”
Also commenting for this article, Wilfrid Noel Raby, MD, PhD, a psychiatrist in private practice in Teaneck, N.J., called disulfiram “grossly underused for alcohol use disorders and even more so when people use alcohol and cocaine.”
Dr. Raby, who was not involved with the study, has found that patients withdrawing from cocaine, cannabis, or stimulants “can respond very well to disulfiram [not only] in terms of their cravings but also in terms of mood stabilization and anxiolysis.”
He has also found that for patients with bipolar disorder or attention-deficit/hyperactivity disorder with depression disulfiram and low-dose lithium “can provide anxiolysis and mood stabilization, especially if a rapid effect is required, usually within a week.”
However, Dr. Raby cautioned that “it is probably not advisable to maintain patients on disulfiram for periods long than 3 months consecutively because there is a risk of neuropathy and hepatopathology that are not common but are seen often enough.” He usually interrupts treatment for a month and then resumes if necessary.
The research was partially supported by the Tsukuba Clinical Research and Development Organization from the Japan Agency for Medical Research and Development. The authors and Dr. Raby have disclosed no relevant financial relationships. Dr. McIntyre reports receiving research grant support from CIHR/GACD/National Natural Science Foundation of China; speaker/consultation fees from Lundbeck, Janssen, Alkermes, Mitsubishi Tanabe, Purdue, Pfizer, Otsuka, Takeda, Neurocrine, Sunovion, Bausch Health, Axsome, Novo Nordisk, Kris, Sanofi, Eisai, Intra-Cellular, NewBridge Pharmaceuticals, AbbVie, and Atai Life Sciences. Dr. McIntyre is CEO of Braxia Scientific.
A version of this article first appeared on Medscape.com.
, early research suggests.
Japanese researchers, headed by Akiyoshi Saitoh, PhD, professor in the department of pharmacy, Tokyo University of Science, compared the reactions of mice that received a classic anxiolytic agent (diazepam) to those that received disulfiram while performing a maze task and found comparable reductions in anxiety in both groups of mice.
Moreover, unlike diazepam, disulfiram caused no sedation, amnesia, or impairments in coordination.
“These results indicate that disulfiram can be used safely by elderly patients suffering from anxiety and insomnia and has the potential to become a breakthrough psychotropic drug,” Dr. Saitoh said in a press release.
The study was published online in Frontiers in Pharmacology.
Inhibitory function
Disulfiram inhibits the enzyme aldehyde dehydrogenase (ALDH), which is responsible for alcohol metabolism. Recent research suggests that disulfiram may have broader inhibitory functions.
In particular, it inhibits the cytoplasmic protein FROUNT, preventing it from interacting with two chemokine receptors (CCR2 and CCRs) that are involved in cellular signaling pathways and are associated with regulating behaviors, including anxiety, in rodents, the authors write.
“Although the functions of FROUNT-chemokines signaling in the immune system are well documented, the potential role of CNS-expressed FROUNT chemokine–related molecules as neuromodulators remains largely unknown,” they write.
The researchers had been conducting preclinical research on the secondary pharmacologic properties of disulfiram and “coincidentally discovered” its “anxiolytic-like effects.” They investigated these effects further because currently used anxiolytics – i.e., benzodiazepines – have unwanted side effects.
The researchers utilized an elevated plus-maze (EPM) test to investigate the effects of disulfiram in mice. The EPM apparatus consists of four arms set in a cross pattern and are connected to a central square. Of these, two arms are protected by vertical boundaries, while the other two have unprotected edges. Typically, mice with anxiety prefer to spend time in the closed arms. The mice also underwent other tests of coordination and the ability to navigate a Y-maze.
Some mice received disulfiram, others received a benzodiazepine, and others received merely a “vehicle,” which served as a control.
Disulfiram “significantly and dose-dependently” increased the time spent in the open arms of the EPM, compared with the vehicle-treated group, at 30 minutes after administration (F [3, 30] = 16.64; P < .0001), suggesting less anxiety. The finding was confirmed by a Bonferroni analysis that showed a significant effect of disulfiram, compared with the vehicle-treated group, at all three doses (20 mg/kg: t = 0.9894; P > .05; 40 mg/kg: t = 3.863; P < .01; 80 mg/kg: t = 6.417; P < .001).
A Student’s t-test analysis showed that diazepam likewise had a significant effect, compared to the vehicle (t = 5.038; P < .001).
Disulfiram also “significantly and dose-dependently” increased the percentage of open-arm entries (F [3, 30] = 14.24; P < .0001). The Bonferroni analysis showed this effect at all three doses (20 mg/kg: t = 0.3999; P > .05; 40 mg/kg: t = 2.693; P > .05; 80 mg/kg: t = 5.864; P < .001).
Diazepam similarly showed a significant effect, compared to the vehicle condition (t = 3.733; P < .005).
In particular, the 40 mg/kg dose of disulfiram significantly increased the percentage of time spent in the open arms at 15, 30, and 60 minutes after administration, with the peak effect occurring at 30 minutes.
The researchers examined the effect of cyanamide, another ALDH inhibitor, on the anxiety behaviors of mice and found no effect on the number of open-arm entries or percentage of time the mice spent in the open arm, compared with the vehicle condition.
In contrast to diazepam, disulfiram had no effect on the amount of spontaneous locomotor activity, time spent on the rotarod, or activity on the Y-maze test displayed by the mice, “suggesting that there were no apparent sedative effects at the dosages used.” Moreover, unlike the mice treated with diazepam, there were no increases in the number of falls the mice experienced on the rotarod.
Glutamate levels in the prelimbic-prefrontal cortex (PL-PFC) “play an important role in the development of anxiety-like behavior in mice,” the authors state. Disulfiram “significantly and completely attenuated increased extracellular glutamate levels in the PL-PFC during stress exposure” on the EPM.
“We propose that DSF inhibits FROUNT protein and the chemokine signaling pathways under its influence, which may suppress presynaptic glutamatergic transmission in the brain,” said Dr. Saitoh. “This, in turn, attenuates the levels of glutamate in the brain, reducing overall anxiety.”
Humanity’s most common affliction
Commenting for this news organization, Roger McIntyre, MD, professor of psychiatry and pharmacology, University of Toronto, and head of the mood disorders psychopharmacology unit, noted that there is a “renewed interest in psychiatry in excitatory and inhibitory balance – for example, ketamine represents a treatment that facilitates excitatory activity, while neurosteroids are candidate medicines now for inhibitory activity.”
Dr. McIntyre, who is the chairman and executive director of the Brain and Cognitive Discover Foundation, Toronto, and was not involved with the study, said it is believed “that the excitatory-inhibitory balance may be relevant to brain health and disease.”
Dr. McIntyre also pointed out that the study “highlights not only the repurposing of a well-known medicine but also exploit[s] the potential brain therapeutic effects of immune targets that indirectly affect inhibitory systems, resulting in potentially a safer treatment for anxiety – the most common affliction of humanity.”
Also commenting for this article, Wilfrid Noel Raby, MD, PhD, a psychiatrist in private practice in Teaneck, N.J., called disulfiram “grossly underused for alcohol use disorders and even more so when people use alcohol and cocaine.”
Dr. Raby, who was not involved with the study, has found that patients withdrawing from cocaine, cannabis, or stimulants “can respond very well to disulfiram [not only] in terms of their cravings but also in terms of mood stabilization and anxiolysis.”
He has also found that for patients with bipolar disorder or attention-deficit/hyperactivity disorder with depression disulfiram and low-dose lithium “can provide anxiolysis and mood stabilization, especially if a rapid effect is required, usually within a week.”
However, Dr. Raby cautioned that “it is probably not advisable to maintain patients on disulfiram for periods long than 3 months consecutively because there is a risk of neuropathy and hepatopathology that are not common but are seen often enough.” He usually interrupts treatment for a month and then resumes if necessary.
The research was partially supported by the Tsukuba Clinical Research and Development Organization from the Japan Agency for Medical Research and Development. The authors and Dr. Raby have disclosed no relevant financial relationships. Dr. McIntyre reports receiving research grant support from CIHR/GACD/National Natural Science Foundation of China; speaker/consultation fees from Lundbeck, Janssen, Alkermes, Mitsubishi Tanabe, Purdue, Pfizer, Otsuka, Takeda, Neurocrine, Sunovion, Bausch Health, Axsome, Novo Nordisk, Kris, Sanofi, Eisai, Intra-Cellular, NewBridge Pharmaceuticals, AbbVie, and Atai Life Sciences. Dr. McIntyre is CEO of Braxia Scientific.
A version of this article first appeared on Medscape.com.
, early research suggests.
Japanese researchers, headed by Akiyoshi Saitoh, PhD, professor in the department of pharmacy, Tokyo University of Science, compared the reactions of mice that received a classic anxiolytic agent (diazepam) to those that received disulfiram while performing a maze task and found comparable reductions in anxiety in both groups of mice.
Moreover, unlike diazepam, disulfiram caused no sedation, amnesia, or impairments in coordination.
“These results indicate that disulfiram can be used safely by elderly patients suffering from anxiety and insomnia and has the potential to become a breakthrough psychotropic drug,” Dr. Saitoh said in a press release.
The study was published online in Frontiers in Pharmacology.
Inhibitory function
Disulfiram inhibits the enzyme aldehyde dehydrogenase (ALDH), which is responsible for alcohol metabolism. Recent research suggests that disulfiram may have broader inhibitory functions.
In particular, it inhibits the cytoplasmic protein FROUNT, preventing it from interacting with two chemokine receptors (CCR2 and CCRs) that are involved in cellular signaling pathways and are associated with regulating behaviors, including anxiety, in rodents, the authors write.
“Although the functions of FROUNT-chemokines signaling in the immune system are well documented, the potential role of CNS-expressed FROUNT chemokine–related molecules as neuromodulators remains largely unknown,” they write.
The researchers had been conducting preclinical research on the secondary pharmacologic properties of disulfiram and “coincidentally discovered” its “anxiolytic-like effects.” They investigated these effects further because currently used anxiolytics – i.e., benzodiazepines – have unwanted side effects.
The researchers utilized an elevated plus-maze (EPM) test to investigate the effects of disulfiram in mice. The EPM apparatus consists of four arms set in a cross pattern and are connected to a central square. Of these, two arms are protected by vertical boundaries, while the other two have unprotected edges. Typically, mice with anxiety prefer to spend time in the closed arms. The mice also underwent other tests of coordination and the ability to navigate a Y-maze.
Some mice received disulfiram, others received a benzodiazepine, and others received merely a “vehicle,” which served as a control.
Disulfiram “significantly and dose-dependently” increased the time spent in the open arms of the EPM, compared with the vehicle-treated group, at 30 minutes after administration (F [3, 30] = 16.64; P < .0001), suggesting less anxiety. The finding was confirmed by a Bonferroni analysis that showed a significant effect of disulfiram, compared with the vehicle-treated group, at all three doses (20 mg/kg: t = 0.9894; P > .05; 40 mg/kg: t = 3.863; P < .01; 80 mg/kg: t = 6.417; P < .001).
A Student’s t-test analysis showed that diazepam likewise had a significant effect, compared to the vehicle (t = 5.038; P < .001).
Disulfiram also “significantly and dose-dependently” increased the percentage of open-arm entries (F [3, 30] = 14.24; P < .0001). The Bonferroni analysis showed this effect at all three doses (20 mg/kg: t = 0.3999; P > .05; 40 mg/kg: t = 2.693; P > .05; 80 mg/kg: t = 5.864; P < .001).
Diazepam similarly showed a significant effect, compared to the vehicle condition (t = 3.733; P < .005).
In particular, the 40 mg/kg dose of disulfiram significantly increased the percentage of time spent in the open arms at 15, 30, and 60 minutes after administration, with the peak effect occurring at 30 minutes.
The researchers examined the effect of cyanamide, another ALDH inhibitor, on the anxiety behaviors of mice and found no effect on the number of open-arm entries or percentage of time the mice spent in the open arm, compared with the vehicle condition.
In contrast to diazepam, disulfiram had no effect on the amount of spontaneous locomotor activity, time spent on the rotarod, or activity on the Y-maze test displayed by the mice, “suggesting that there were no apparent sedative effects at the dosages used.” Moreover, unlike the mice treated with diazepam, there were no increases in the number of falls the mice experienced on the rotarod.
Glutamate levels in the prelimbic-prefrontal cortex (PL-PFC) “play an important role in the development of anxiety-like behavior in mice,” the authors state. Disulfiram “significantly and completely attenuated increased extracellular glutamate levels in the PL-PFC during stress exposure” on the EPM.
“We propose that DSF inhibits FROUNT protein and the chemokine signaling pathways under its influence, which may suppress presynaptic glutamatergic transmission in the brain,” said Dr. Saitoh. “This, in turn, attenuates the levels of glutamate in the brain, reducing overall anxiety.”
Humanity’s most common affliction
Commenting for this news organization, Roger McIntyre, MD, professor of psychiatry and pharmacology, University of Toronto, and head of the mood disorders psychopharmacology unit, noted that there is a “renewed interest in psychiatry in excitatory and inhibitory balance – for example, ketamine represents a treatment that facilitates excitatory activity, while neurosteroids are candidate medicines now for inhibitory activity.”
Dr. McIntyre, who is the chairman and executive director of the Brain and Cognitive Discover Foundation, Toronto, and was not involved with the study, said it is believed “that the excitatory-inhibitory balance may be relevant to brain health and disease.”
Dr. McIntyre also pointed out that the study “highlights not only the repurposing of a well-known medicine but also exploit[s] the potential brain therapeutic effects of immune targets that indirectly affect inhibitory systems, resulting in potentially a safer treatment for anxiety – the most common affliction of humanity.”
Also commenting for this article, Wilfrid Noel Raby, MD, PhD, a psychiatrist in private practice in Teaneck, N.J., called disulfiram “grossly underused for alcohol use disorders and even more so when people use alcohol and cocaine.”
Dr. Raby, who was not involved with the study, has found that patients withdrawing from cocaine, cannabis, or stimulants “can respond very well to disulfiram [not only] in terms of their cravings but also in terms of mood stabilization and anxiolysis.”
He has also found that for patients with bipolar disorder or attention-deficit/hyperactivity disorder with depression disulfiram and low-dose lithium “can provide anxiolysis and mood stabilization, especially if a rapid effect is required, usually within a week.”
However, Dr. Raby cautioned that “it is probably not advisable to maintain patients on disulfiram for periods long than 3 months consecutively because there is a risk of neuropathy and hepatopathology that are not common but are seen often enough.” He usually interrupts treatment for a month and then resumes if necessary.
The research was partially supported by the Tsukuba Clinical Research and Development Organization from the Japan Agency for Medical Research and Development. The authors and Dr. Raby have disclosed no relevant financial relationships. Dr. McIntyre reports receiving research grant support from CIHR/GACD/National Natural Science Foundation of China; speaker/consultation fees from Lundbeck, Janssen, Alkermes, Mitsubishi Tanabe, Purdue, Pfizer, Otsuka, Takeda, Neurocrine, Sunovion, Bausch Health, Axsome, Novo Nordisk, Kris, Sanofi, Eisai, Intra-Cellular, NewBridge Pharmaceuticals, AbbVie, and Atai Life Sciences. Dr. McIntyre is CEO of Braxia Scientific.
A version of this article first appeared on Medscape.com.
FROM FRONTIERS IN PHARMACOLOGY
Mental illness tied to COVID-19 breakthrough infection
“Psychiatric disorders remained significantly associated with incident breakthrough infections above and beyond sociodemographic and medical factors, suggesting that mental health is important to consider in conjunction with other risk factors,” wrote the investigators, led by Aoife O’Donovan, PhD, University of California, San Francisco.
Individuals with psychiatric disorders “should be prioritized for booster vaccinations and other critical preventive efforts, including increased SARS-CoV-2 screening, public health campaigns, or COVID-19 discussions during clinical care,” they added.
The study was published online in JAMA Network Open.
Elderly most vulnerable
The researchers reviewed the records of 263,697 veterans who were fully vaccinated against COVID-19.
Just over a half (51.4%) had one or more psychiatric diagnoses within the last 5 years and 14.8% developed breakthrough COVID-19 infections, confirmed by a positive SARS-CoV-2 test.
Psychiatric diagnoses among the veterans included depression, posttraumatic stress, anxiety, adjustment disorder, substance use disorder, bipolar disorder, psychosis, ADHD, dissociation, and eating disorders.
In the overall sample, a history of any psychiatric disorder was associated with a 7% higher incidence of breakthrough COVID-19 infection in models adjusted for potential confounders (adjusted relative risk, 1.07; 95% confidence interval, 1.05-1.09) and a 3% higher incidence in models additionally adjusted for underlying medical comorbidities and smoking (aRR, 1.03; 95% CI, 1.01-1.05).
Most psychiatric disorders were associated with a higher incidence of breakthrough infection, with the highest relative risk observed for substance use disorders (aRR, 1.16; 95% CI, 1.12 -1.21) and adjustment disorder (aRR, 1.13; 95% CI, 1.10-1.16) in fully adjusted models.
Older vaccinated veterans with psychiatric illnesses appear to be most vulnerable to COVID-19 reinfection.
In veterans aged 65 and older, all psychiatric disorders were associated with an increased incidence of breakthrough infection, with increases in the incidence rate ranging from 3% to 24% in fully adjusted models.
In the younger veterans, in contrast, only anxiety, adjustment, and substance use disorders were associated with an increased incidence of breakthrough infection in fully adjusted models.
Psychotic disorders were associated with a 10% lower incidence of breakthrough infection among younger veterans, perhaps because of greater social isolation, the researchers said.
Risky behavior or impaired immunity?
“Although some of the larger observed effect sizes are compelling at an individual level, even the relatively modest effect sizes may have a large effect at the population level when considering the high prevalence of psychiatric disorders and the global reach and scale of the pandemic,” Dr. O’Donovan and colleagues wrote.
They noted that psychiatric disorders, including depression, schizophrenia, and bipolar disorders, have been associated with impaired cellular immunity and blunted response to vaccines. Therefore, it’s possible that those with psychiatric disorders have poorer responses to COVID-19 vaccination.
It’s also possible that immunity following vaccination wanes more quickly or more strongly in people with psychiatric disorders and they could have less protection against new variants, they added.
Patients with psychiatric disorders could be more apt to engage in risky behaviors for contracting COVID-19, which could also increase the risk for breakthrough infection, they said.
The study was supported by a UCSF Department of Psychiatry Rapid Award and UCSF Faculty Resource Fund Award. Dr. O’Donovan reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
“Psychiatric disorders remained significantly associated with incident breakthrough infections above and beyond sociodemographic and medical factors, suggesting that mental health is important to consider in conjunction with other risk factors,” wrote the investigators, led by Aoife O’Donovan, PhD, University of California, San Francisco.
Individuals with psychiatric disorders “should be prioritized for booster vaccinations and other critical preventive efforts, including increased SARS-CoV-2 screening, public health campaigns, or COVID-19 discussions during clinical care,” they added.
The study was published online in JAMA Network Open.
Elderly most vulnerable
The researchers reviewed the records of 263,697 veterans who were fully vaccinated against COVID-19.
Just over a half (51.4%) had one or more psychiatric diagnoses within the last 5 years and 14.8% developed breakthrough COVID-19 infections, confirmed by a positive SARS-CoV-2 test.
Psychiatric diagnoses among the veterans included depression, posttraumatic stress, anxiety, adjustment disorder, substance use disorder, bipolar disorder, psychosis, ADHD, dissociation, and eating disorders.
In the overall sample, a history of any psychiatric disorder was associated with a 7% higher incidence of breakthrough COVID-19 infection in models adjusted for potential confounders (adjusted relative risk, 1.07; 95% confidence interval, 1.05-1.09) and a 3% higher incidence in models additionally adjusted for underlying medical comorbidities and smoking (aRR, 1.03; 95% CI, 1.01-1.05).
Most psychiatric disorders were associated with a higher incidence of breakthrough infection, with the highest relative risk observed for substance use disorders (aRR, 1.16; 95% CI, 1.12 -1.21) and adjustment disorder (aRR, 1.13; 95% CI, 1.10-1.16) in fully adjusted models.
Older vaccinated veterans with psychiatric illnesses appear to be most vulnerable to COVID-19 reinfection.
In veterans aged 65 and older, all psychiatric disorders were associated with an increased incidence of breakthrough infection, with increases in the incidence rate ranging from 3% to 24% in fully adjusted models.
In the younger veterans, in contrast, only anxiety, adjustment, and substance use disorders were associated with an increased incidence of breakthrough infection in fully adjusted models.
Psychotic disorders were associated with a 10% lower incidence of breakthrough infection among younger veterans, perhaps because of greater social isolation, the researchers said.
Risky behavior or impaired immunity?
“Although some of the larger observed effect sizes are compelling at an individual level, even the relatively modest effect sizes may have a large effect at the population level when considering the high prevalence of psychiatric disorders and the global reach and scale of the pandemic,” Dr. O’Donovan and colleagues wrote.
They noted that psychiatric disorders, including depression, schizophrenia, and bipolar disorders, have been associated with impaired cellular immunity and blunted response to vaccines. Therefore, it’s possible that those with psychiatric disorders have poorer responses to COVID-19 vaccination.
It’s also possible that immunity following vaccination wanes more quickly or more strongly in people with psychiatric disorders and they could have less protection against new variants, they added.
Patients with psychiatric disorders could be more apt to engage in risky behaviors for contracting COVID-19, which could also increase the risk for breakthrough infection, they said.
The study was supported by a UCSF Department of Psychiatry Rapid Award and UCSF Faculty Resource Fund Award. Dr. O’Donovan reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
“Psychiatric disorders remained significantly associated with incident breakthrough infections above and beyond sociodemographic and medical factors, suggesting that mental health is important to consider in conjunction with other risk factors,” wrote the investigators, led by Aoife O’Donovan, PhD, University of California, San Francisco.
Individuals with psychiatric disorders “should be prioritized for booster vaccinations and other critical preventive efforts, including increased SARS-CoV-2 screening, public health campaigns, or COVID-19 discussions during clinical care,” they added.
The study was published online in JAMA Network Open.
Elderly most vulnerable
The researchers reviewed the records of 263,697 veterans who were fully vaccinated against COVID-19.
Just over a half (51.4%) had one or more psychiatric diagnoses within the last 5 years and 14.8% developed breakthrough COVID-19 infections, confirmed by a positive SARS-CoV-2 test.
Psychiatric diagnoses among the veterans included depression, posttraumatic stress, anxiety, adjustment disorder, substance use disorder, bipolar disorder, psychosis, ADHD, dissociation, and eating disorders.
In the overall sample, a history of any psychiatric disorder was associated with a 7% higher incidence of breakthrough COVID-19 infection in models adjusted for potential confounders (adjusted relative risk, 1.07; 95% confidence interval, 1.05-1.09) and a 3% higher incidence in models additionally adjusted for underlying medical comorbidities and smoking (aRR, 1.03; 95% CI, 1.01-1.05).
Most psychiatric disorders were associated with a higher incidence of breakthrough infection, with the highest relative risk observed for substance use disorders (aRR, 1.16; 95% CI, 1.12 -1.21) and adjustment disorder (aRR, 1.13; 95% CI, 1.10-1.16) in fully adjusted models.
Older vaccinated veterans with psychiatric illnesses appear to be most vulnerable to COVID-19 reinfection.
In veterans aged 65 and older, all psychiatric disorders were associated with an increased incidence of breakthrough infection, with increases in the incidence rate ranging from 3% to 24% in fully adjusted models.
In the younger veterans, in contrast, only anxiety, adjustment, and substance use disorders were associated with an increased incidence of breakthrough infection in fully adjusted models.
Psychotic disorders were associated with a 10% lower incidence of breakthrough infection among younger veterans, perhaps because of greater social isolation, the researchers said.
Risky behavior or impaired immunity?
“Although some of the larger observed effect sizes are compelling at an individual level, even the relatively modest effect sizes may have a large effect at the population level when considering the high prevalence of psychiatric disorders and the global reach and scale of the pandemic,” Dr. O’Donovan and colleagues wrote.
They noted that psychiatric disorders, including depression, schizophrenia, and bipolar disorders, have been associated with impaired cellular immunity and blunted response to vaccines. Therefore, it’s possible that those with psychiatric disorders have poorer responses to COVID-19 vaccination.
It’s also possible that immunity following vaccination wanes more quickly or more strongly in people with psychiatric disorders and they could have less protection against new variants, they added.
Patients with psychiatric disorders could be more apt to engage in risky behaviors for contracting COVID-19, which could also increase the risk for breakthrough infection, they said.
The study was supported by a UCSF Department of Psychiatry Rapid Award and UCSF Faculty Resource Fund Award. Dr. O’Donovan reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
52-year-old man • hematemesis • history of cirrhosis • persistent fevers • Dx?
THE CASE
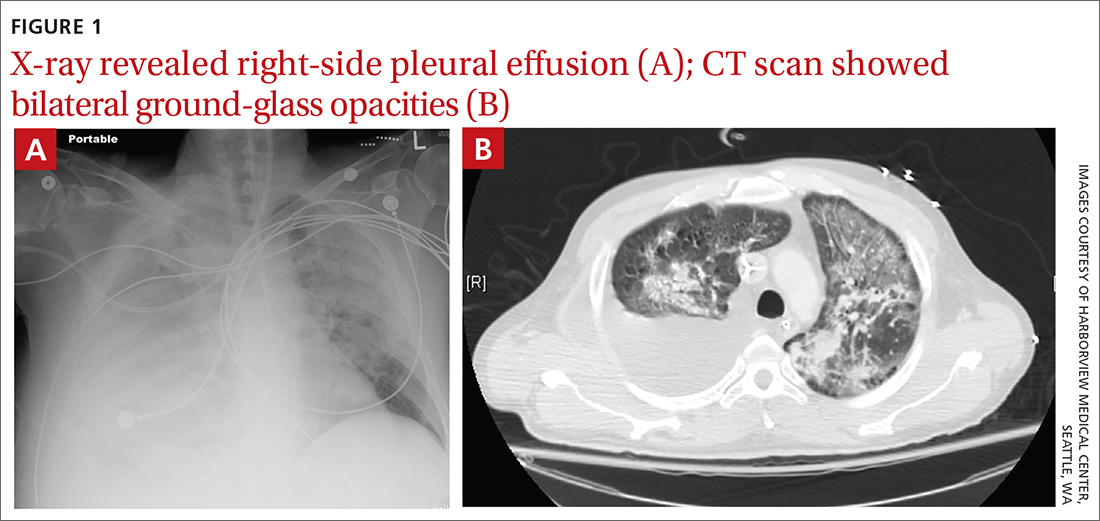
A 52-year-old man presented to the emergency department after vomiting a large volume of blood and was admitted to the intensive care unit. His past medical history was remarkable for untreated chronic hepatitis C resulting from injection drug use and cirrhosis without prior history of esophageal varices.
Due to ongoing hematemesis, he was intubated for airway protection and underwent esophagogastroduodenoscopy with banding of large esophageal varices on hospital day (HD) 1. He was extubated on HD 2 after clinical stability was achieved; however, he became encephalopathic over the subsequent days despite treatment with lactulose. On HD 4, the patient required re-intubation for progressive respiratory failure. Chest imaging revealed a large, simple-appearing right pleural effusion and extensive bilateral patchy ground-glass opacities (FIGURE 1).
Thoracentesis was ordered and revealed transudative pleural fluid; this finding, along with negative infectious studies, was consistent with hepatic hydrothorax. In the setting of initial decompensation, empiric treatment with vancomycin and meropenem was started for suspected hospital-acquired pneumonia.
The patient had persistent fevers that had developed during his hospital stay and pulmonary opacities, despite 72 hours of treatment with broad-spectrum antibiotics. Thus, a diagnostic bronchoscopy with bronchoalveolar lavage (BAL) was performed. BAL cell count and differential revealed 363 nucleated cells/µL, with profound eosinophilia (42% eosinophils, 44% macrophages, 14% neutrophils).
Bacterial and fungal cultures and a viral polymerase chain reaction panel were negative. HIV antibody-antigen and RNA testing were also negative. The patient had no evidence or history of underlying malignancy, autoimmune disease, or recent immunosuppressive therapy, including corticosteroids. Due to consistent imaging findings and lack of improvement with appropriate treatment for bacterial pneumonia, further work-up was pursued.
THE DIAGNOSIS
Given the consistent radiographic pattern, the differential diagnosis for this patient included pneumocystis pneumonia (PCP), a potentially life-threatening opportunistic infection. Work-up therefore included direct fluorescent antibody testing, which was positive for Pneumocystis jirovecii, a fungus that can cause PCP.
Of note, the patient’s white blood cell count was elevated on admission (11.44 × 103/µL) but low for much of his hospital stay (nadir = 1.97 × 103/µL), with associated lymphopenia (nadir = 0.22 × 103/µl). No peripheral eosinophilia was noted.
Continue to: DISCUSSION
DISCUSSION
PCP typically occurs in immunocompromised individuals and may be related to HIV infection, malignancy, or exposure to immunosuppressive therapies.1,2 While rare cases of PCP have been described in adults without predisposing factors, many of these cases occurred at the beginning of the AIDS epidemic, prior to reliable HIV testing.3-5
Uncharted territory. We were confident in our diagnosis because immunofluorescence testing has very few false-positives and a high specificity.6-8 But there were informational gaps. The eosinophilia recorded on BAL is poorly described in HIV-negative patients with PCP but well-described in HIV-positive patients, with the level of eosinophilia associated with disease severity.9,10 Eosinophils are thought to contribute to pulmonary inflammation, which may explain the severity of our patient’s course.10
A first of its kind case?
To our knowledge, this is the first report of PCP in a patient with cirrhosis from chronic hepatitis C virus infection and no other predisposing conditions or preceding immunosuppressive therapy. We suspect that his lymphopenia, which was noted during his critical illness, predisposed him to PCP.
Lymphocytes (in particular CD4+ T cells) have been shown to play an important role, along with alveolar macrophages and neutrophils, in directing the host defense against
Typical risk factors for lymphopenia had not been observed in this patient. However, cirrhosis has been associated with low CD4+ T-cell counts and disruption of cell-mediated immunity, even in HIV-seronegative patients.14,15 There are several postulated mechanisms for low CD4+ T-cell counts in cirrhosis, including splenic sequestration, impaired T-cell production (due to impaired thymopoiesis), increased T-cell consumption, and apoptosis (due to persistent immune system activation from bacterial translocation and an overall pro-inflammatory state).16,17
Continue to: Predisposing factors guide treatment
Predisposing factors guide treatment
Routine treatment for PCP in patients without HIV is a 21-day course of trimethoprim/sulfamethoxazole (Bactrim). Dosing for patients with normal renal function is 15 to 20 mg/kg orally or intravenously per day. Patients with allergy to trimethoprim/sulfamethoxazole should ideally undergo desensitization, given its effectiveness against PCP.
Due to a sulfonamide allergy, our patient was started on primaquine 30 mg/d, clindamycin 600 mg tid, and prednisone 40 mg bid. (The corticosteroid was added because of the severity of the disease.) Three days after starting treatment—and 10 days into his hospital stay—the patient had significant improvement in his respiratory status and was successfully extubated. He underwent trimethoprim/sulfamethoxazole desensitization and completed a 21-day course of treatment for PCP with complete resolution of respiratory symptoms. Follow-up chest radiograph 2 months later (FIGURE 2) confirmed clearance of opacities.
THE TAKEAWAY
PCP remains a rare disease in patients without the typical immunosuppressive risk factors. However, it should be considered in patients with cirrhosis who develop respiratory failure, especially those with compatible radiographic findings and negative microbiologic evaluation for other, more typical, organisms.
CORRESPONDENCE
Tyler Albert, MD, VA Puget Sound Healthcare System, 1660 South Columbian Way, S-111-Pulm, Seattle, WA 98108; talbert@uw.edu
1. Thomas CF Jr, Limper AH. Pneumocystis pneumonia. N Engl J Med. 2004;350:2487-2498. doi: 10.1056/NEJMra032588
2. Walzer PD, Perl DP, Krogstad DJ, et al. Pneumocystis carinii pneumonia in the United States. Epidemiologic, diagnostic, and clinical features. Ann Intern Med. 1974;80:83-93. doi: 10.7326/0003-4819-80-1-83
3. Sepkowitz KA. Pneumocystis carinii pneumonia in patients without AIDS. Clin Infect Dis. 1993;17 suppl 2:S416-422. doi: 10.1093/clinids/17.supplement_2.s416
4. Al Soub H, Taha RY, El Deeb Y, et al. Pneumocystis carinii pneumonia in a patient without a predisposing illness: case report and review. Scand J Infect Dis. 2004;36:618-621. doi: 10.1080/00365540410017608
5. Jacobs JL, Libby DM, Winters RA, et al. A cluster of Pneumocystis carinii pneumonia in adults without predisposing illnesses. N Engl J Med. 1991;324:246-250. doi: 10.1056/NEJM199101243240407
6. Ng VL, Yajko DM, McPhaul LW, et al. Evaluation of an indirect fluorescent-antibody stain for detection of Pneumocystis carinii in respiratory specimens. J Clin Microbiol. 1990;28:975-979. doi: 10.1128/jcm.28.5.975-979.1990
7. Cregan P, Yamamoto A, Lum A, et al. Comparison of four methods for rapid detection of Pneumocystis carinii in respiratory specimens. J Clin Microbiol. 1990;28:2432-2436. doi: 10.1128/jcm.28.11.2432-2436.1990
8. Turner D, Schwarz Y, Yust I. Induced sputum for diagnosing Pneumocystis carinii pneumonia in HIV patients: new data, new issues. Eur Respir J. 2003;21:204-208. doi: 10.1183/09031936.03.00035303
9. Smith RL, el-Sadr WM, Lewis ML. Correlation of bronchoalveolar lavage cell populations with clinical severity of Pneumocystis carinii pneumonia. Chest. 1988;93:60-64. doi: 10.1378/chest.93.1.60
10. Fleury-Feith J, Van Nhieu JT, Picard C, et al. Bronchoalveolar lavage eosinophilia associated with Pneumocystis carinii pneumonitis in AIDS patients. Comparative study with non-AIDS patients. Chest. 1989;95:1198-1201. doi: 10.1378/chest.95.6.1198
11. Thomas CF Jr, Limper AH. Current insights into the biology and pathogenesis of Pneumocystis pneumonia. Nat Rev Microbiol. 2007;5:298-308. doi: 10.1038/nrmicro1621
12. Toh BH, Roberts-Thomson IC, Mathews JD, et al. Depression of cell-mediated immunity in old age and the immunopathic diseases, lupus erythematosus, chronic hepatitis and rheumatoid arthritis. Clin Exp Immunol. 1973;14:193-202.
13. Mansharamani NG, Balachandran D, Vernovsky I, et al. Peripheral blood CD4 + T-lymphocyte counts during Pneumocystis carinii pneumonia in immunocompromised patients without HIV infection. Chest. 2000;118:712-720. doi: 10.1378/chest.118.3.712
14. McGovern BH, Golan Y, Lopez M, et al. The impact of cirrhosis on CD4+ T cell counts in HIV-seronegative patients. Clin Infect Dis. 2007;44:431-437. doi: 10.1086/509580
15. Bienvenu AL, Traore K, Plekhanova I, et al. Pneumocystis pneumonia suspected cases in 604 non-HIV and HIV patients. Int J Infect Dis. 2016;46:11-17. doi: 10.1016/j.ijid.2016.03.018
16. Albillos A, Lario M, Álvarez-Mon M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J Hepatol. 2014;61:1385-1396. doi: 10.1016/j.jhep.2014.08.010
17. Lario M, Muñoz L, Ubeda M, et al. Defective thymopoiesis and poor peripheral homeostatic replenishment of T-helper cells cause T-cell lymphopenia in cirrhosis. J Hepatol. 2013;59:723-730. doi: 10.1016/j.jhep.2013.05.042
THE CASE
A 52-year-old man presented to the emergency department after vomiting a large volume of blood and was admitted to the intensive care unit. His past medical history was remarkable for untreated chronic hepatitis C resulting from injection drug use and cirrhosis without prior history of esophageal varices.
Due to ongoing hematemesis, he was intubated for airway protection and underwent esophagogastroduodenoscopy with banding of large esophageal varices on hospital day (HD) 1. He was extubated on HD 2 after clinical stability was achieved; however, he became encephalopathic over the subsequent days despite treatment with lactulose. On HD 4, the patient required re-intubation for progressive respiratory failure. Chest imaging revealed a large, simple-appearing right pleural effusion and extensive bilateral patchy ground-glass opacities (FIGURE 1).
Thoracentesis was ordered and revealed transudative pleural fluid; this finding, along with negative infectious studies, was consistent with hepatic hydrothorax. In the setting of initial decompensation, empiric treatment with vancomycin and meropenem was started for suspected hospital-acquired pneumonia.
The patient had persistent fevers that had developed during his hospital stay and pulmonary opacities, despite 72 hours of treatment with broad-spectrum antibiotics. Thus, a diagnostic bronchoscopy with bronchoalveolar lavage (BAL) was performed. BAL cell count and differential revealed 363 nucleated cells/µL, with profound eosinophilia (42% eosinophils, 44% macrophages, 14% neutrophils).
Bacterial and fungal cultures and a viral polymerase chain reaction panel were negative. HIV antibody-antigen and RNA testing were also negative. The patient had no evidence or history of underlying malignancy, autoimmune disease, or recent immunosuppressive therapy, including corticosteroids. Due to consistent imaging findings and lack of improvement with appropriate treatment for bacterial pneumonia, further work-up was pursued.
THE DIAGNOSIS
Given the consistent radiographic pattern, the differential diagnosis for this patient included pneumocystis pneumonia (PCP), a potentially life-threatening opportunistic infection. Work-up therefore included direct fluorescent antibody testing, which was positive for Pneumocystis jirovecii, a fungus that can cause PCP.
Of note, the patient’s white blood cell count was elevated on admission (11.44 × 103/µL) but low for much of his hospital stay (nadir = 1.97 × 103/µL), with associated lymphopenia (nadir = 0.22 × 103/µl). No peripheral eosinophilia was noted.
Continue to: DISCUSSION
DISCUSSION
PCP typically occurs in immunocompromised individuals and may be related to HIV infection, malignancy, or exposure to immunosuppressive therapies.1,2 While rare cases of PCP have been described in adults without predisposing factors, many of these cases occurred at the beginning of the AIDS epidemic, prior to reliable HIV testing.3-5
Uncharted territory. We were confident in our diagnosis because immunofluorescence testing has very few false-positives and a high specificity.6-8 But there were informational gaps. The eosinophilia recorded on BAL is poorly described in HIV-negative patients with PCP but well-described in HIV-positive patients, with the level of eosinophilia associated with disease severity.9,10 Eosinophils are thought to contribute to pulmonary inflammation, which may explain the severity of our patient’s course.10
A first of its kind case?
To our knowledge, this is the first report of PCP in a patient with cirrhosis from chronic hepatitis C virus infection and no other predisposing conditions or preceding immunosuppressive therapy. We suspect that his lymphopenia, which was noted during his critical illness, predisposed him to PCP.
Lymphocytes (in particular CD4+ T cells) have been shown to play an important role, along with alveolar macrophages and neutrophils, in directing the host defense against
Typical risk factors for lymphopenia had not been observed in this patient. However, cirrhosis has been associated with low CD4+ T-cell counts and disruption of cell-mediated immunity, even in HIV-seronegative patients.14,15 There are several postulated mechanisms for low CD4+ T-cell counts in cirrhosis, including splenic sequestration, impaired T-cell production (due to impaired thymopoiesis), increased T-cell consumption, and apoptosis (due to persistent immune system activation from bacterial translocation and an overall pro-inflammatory state).16,17
Continue to: Predisposing factors guide treatment
Predisposing factors guide treatment
Routine treatment for PCP in patients without HIV is a 21-day course of trimethoprim/sulfamethoxazole (Bactrim). Dosing for patients with normal renal function is 15 to 20 mg/kg orally or intravenously per day. Patients with allergy to trimethoprim/sulfamethoxazole should ideally undergo desensitization, given its effectiveness against PCP.
Due to a sulfonamide allergy, our patient was started on primaquine 30 mg/d, clindamycin 600 mg tid, and prednisone 40 mg bid. (The corticosteroid was added because of the severity of the disease.) Three days after starting treatment—and 10 days into his hospital stay—the patient had significant improvement in his respiratory status and was successfully extubated. He underwent trimethoprim/sulfamethoxazole desensitization and completed a 21-day course of treatment for PCP with complete resolution of respiratory symptoms. Follow-up chest radiograph 2 months later (FIGURE 2) confirmed clearance of opacities.
THE TAKEAWAY
PCP remains a rare disease in patients without the typical immunosuppressive risk factors. However, it should be considered in patients with cirrhosis who develop respiratory failure, especially those with compatible radiographic findings and negative microbiologic evaluation for other, more typical, organisms.
CORRESPONDENCE
Tyler Albert, MD, VA Puget Sound Healthcare System, 1660 South Columbian Way, S-111-Pulm, Seattle, WA 98108; talbert@uw.edu
THE CASE
A 52-year-old man presented to the emergency department after vomiting a large volume of blood and was admitted to the intensive care unit. His past medical history was remarkable for untreated chronic hepatitis C resulting from injection drug use and cirrhosis without prior history of esophageal varices.
Due to ongoing hematemesis, he was intubated for airway protection and underwent esophagogastroduodenoscopy with banding of large esophageal varices on hospital day (HD) 1. He was extubated on HD 2 after clinical stability was achieved; however, he became encephalopathic over the subsequent days despite treatment with lactulose. On HD 4, the patient required re-intubation for progressive respiratory failure. Chest imaging revealed a large, simple-appearing right pleural effusion and extensive bilateral patchy ground-glass opacities (FIGURE 1).
Thoracentesis was ordered and revealed transudative pleural fluid; this finding, along with negative infectious studies, was consistent with hepatic hydrothorax. In the setting of initial decompensation, empiric treatment with vancomycin and meropenem was started for suspected hospital-acquired pneumonia.
The patient had persistent fevers that had developed during his hospital stay and pulmonary opacities, despite 72 hours of treatment with broad-spectrum antibiotics. Thus, a diagnostic bronchoscopy with bronchoalveolar lavage (BAL) was performed. BAL cell count and differential revealed 363 nucleated cells/µL, with profound eosinophilia (42% eosinophils, 44% macrophages, 14% neutrophils).
Bacterial and fungal cultures and a viral polymerase chain reaction panel were negative. HIV antibody-antigen and RNA testing were also negative. The patient had no evidence or history of underlying malignancy, autoimmune disease, or recent immunosuppressive therapy, including corticosteroids. Due to consistent imaging findings and lack of improvement with appropriate treatment for bacterial pneumonia, further work-up was pursued.
THE DIAGNOSIS
Given the consistent radiographic pattern, the differential diagnosis for this patient included pneumocystis pneumonia (PCP), a potentially life-threatening opportunistic infection. Work-up therefore included direct fluorescent antibody testing, which was positive for Pneumocystis jirovecii, a fungus that can cause PCP.
Of note, the patient’s white blood cell count was elevated on admission (11.44 × 103/µL) but low for much of his hospital stay (nadir = 1.97 × 103/µL), with associated lymphopenia (nadir = 0.22 × 103/µl). No peripheral eosinophilia was noted.
Continue to: DISCUSSION
DISCUSSION
PCP typically occurs in immunocompromised individuals and may be related to HIV infection, malignancy, or exposure to immunosuppressive therapies.1,2 While rare cases of PCP have been described in adults without predisposing factors, many of these cases occurred at the beginning of the AIDS epidemic, prior to reliable HIV testing.3-5
Uncharted territory. We were confident in our diagnosis because immunofluorescence testing has very few false-positives and a high specificity.6-8 But there were informational gaps. The eosinophilia recorded on BAL is poorly described in HIV-negative patients with PCP but well-described in HIV-positive patients, with the level of eosinophilia associated with disease severity.9,10 Eosinophils are thought to contribute to pulmonary inflammation, which may explain the severity of our patient’s course.10
A first of its kind case?
To our knowledge, this is the first report of PCP in a patient with cirrhosis from chronic hepatitis C virus infection and no other predisposing conditions or preceding immunosuppressive therapy. We suspect that his lymphopenia, which was noted during his critical illness, predisposed him to PCP.
Lymphocytes (in particular CD4+ T cells) have been shown to play an important role, along with alveolar macrophages and neutrophils, in directing the host defense against
Typical risk factors for lymphopenia had not been observed in this patient. However, cirrhosis has been associated with low CD4+ T-cell counts and disruption of cell-mediated immunity, even in HIV-seronegative patients.14,15 There are several postulated mechanisms for low CD4+ T-cell counts in cirrhosis, including splenic sequestration, impaired T-cell production (due to impaired thymopoiesis), increased T-cell consumption, and apoptosis (due to persistent immune system activation from bacterial translocation and an overall pro-inflammatory state).16,17
Continue to: Predisposing factors guide treatment
Predisposing factors guide treatment
Routine treatment for PCP in patients without HIV is a 21-day course of trimethoprim/sulfamethoxazole (Bactrim). Dosing for patients with normal renal function is 15 to 20 mg/kg orally or intravenously per day. Patients with allergy to trimethoprim/sulfamethoxazole should ideally undergo desensitization, given its effectiveness against PCP.
Due to a sulfonamide allergy, our patient was started on primaquine 30 mg/d, clindamycin 600 mg tid, and prednisone 40 mg bid. (The corticosteroid was added because of the severity of the disease.) Three days after starting treatment—and 10 days into his hospital stay—the patient had significant improvement in his respiratory status and was successfully extubated. He underwent trimethoprim/sulfamethoxazole desensitization and completed a 21-day course of treatment for PCP with complete resolution of respiratory symptoms. Follow-up chest radiograph 2 months later (FIGURE 2) confirmed clearance of opacities.
THE TAKEAWAY
PCP remains a rare disease in patients without the typical immunosuppressive risk factors. However, it should be considered in patients with cirrhosis who develop respiratory failure, especially those with compatible radiographic findings and negative microbiologic evaluation for other, more typical, organisms.
CORRESPONDENCE
Tyler Albert, MD, VA Puget Sound Healthcare System, 1660 South Columbian Way, S-111-Pulm, Seattle, WA 98108; talbert@uw.edu
1. Thomas CF Jr, Limper AH. Pneumocystis pneumonia. N Engl J Med. 2004;350:2487-2498. doi: 10.1056/NEJMra032588
2. Walzer PD, Perl DP, Krogstad DJ, et al. Pneumocystis carinii pneumonia in the United States. Epidemiologic, diagnostic, and clinical features. Ann Intern Med. 1974;80:83-93. doi: 10.7326/0003-4819-80-1-83
3. Sepkowitz KA. Pneumocystis carinii pneumonia in patients without AIDS. Clin Infect Dis. 1993;17 suppl 2:S416-422. doi: 10.1093/clinids/17.supplement_2.s416
4. Al Soub H, Taha RY, El Deeb Y, et al. Pneumocystis carinii pneumonia in a patient without a predisposing illness: case report and review. Scand J Infect Dis. 2004;36:618-621. doi: 10.1080/00365540410017608
5. Jacobs JL, Libby DM, Winters RA, et al. A cluster of Pneumocystis carinii pneumonia in adults without predisposing illnesses. N Engl J Med. 1991;324:246-250. doi: 10.1056/NEJM199101243240407
6. Ng VL, Yajko DM, McPhaul LW, et al. Evaluation of an indirect fluorescent-antibody stain for detection of Pneumocystis carinii in respiratory specimens. J Clin Microbiol. 1990;28:975-979. doi: 10.1128/jcm.28.5.975-979.1990
7. Cregan P, Yamamoto A, Lum A, et al. Comparison of four methods for rapid detection of Pneumocystis carinii in respiratory specimens. J Clin Microbiol. 1990;28:2432-2436. doi: 10.1128/jcm.28.11.2432-2436.1990
8. Turner D, Schwarz Y, Yust I. Induced sputum for diagnosing Pneumocystis carinii pneumonia in HIV patients: new data, new issues. Eur Respir J. 2003;21:204-208. doi: 10.1183/09031936.03.00035303
9. Smith RL, el-Sadr WM, Lewis ML. Correlation of bronchoalveolar lavage cell populations with clinical severity of Pneumocystis carinii pneumonia. Chest. 1988;93:60-64. doi: 10.1378/chest.93.1.60
10. Fleury-Feith J, Van Nhieu JT, Picard C, et al. Bronchoalveolar lavage eosinophilia associated with Pneumocystis carinii pneumonitis in AIDS patients. Comparative study with non-AIDS patients. Chest. 1989;95:1198-1201. doi: 10.1378/chest.95.6.1198
11. Thomas CF Jr, Limper AH. Current insights into the biology and pathogenesis of Pneumocystis pneumonia. Nat Rev Microbiol. 2007;5:298-308. doi: 10.1038/nrmicro1621
12. Toh BH, Roberts-Thomson IC, Mathews JD, et al. Depression of cell-mediated immunity in old age and the immunopathic diseases, lupus erythematosus, chronic hepatitis and rheumatoid arthritis. Clin Exp Immunol. 1973;14:193-202.
13. Mansharamani NG, Balachandran D, Vernovsky I, et al. Peripheral blood CD4 + T-lymphocyte counts during Pneumocystis carinii pneumonia in immunocompromised patients without HIV infection. Chest. 2000;118:712-720. doi: 10.1378/chest.118.3.712
14. McGovern BH, Golan Y, Lopez M, et al. The impact of cirrhosis on CD4+ T cell counts in HIV-seronegative patients. Clin Infect Dis. 2007;44:431-437. doi: 10.1086/509580
15. Bienvenu AL, Traore K, Plekhanova I, et al. Pneumocystis pneumonia suspected cases in 604 non-HIV and HIV patients. Int J Infect Dis. 2016;46:11-17. doi: 10.1016/j.ijid.2016.03.018
16. Albillos A, Lario M, Álvarez-Mon M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J Hepatol. 2014;61:1385-1396. doi: 10.1016/j.jhep.2014.08.010
17. Lario M, Muñoz L, Ubeda M, et al. Defective thymopoiesis and poor peripheral homeostatic replenishment of T-helper cells cause T-cell lymphopenia in cirrhosis. J Hepatol. 2013;59:723-730. doi: 10.1016/j.jhep.2013.05.042
1. Thomas CF Jr, Limper AH. Pneumocystis pneumonia. N Engl J Med. 2004;350:2487-2498. doi: 10.1056/NEJMra032588
2. Walzer PD, Perl DP, Krogstad DJ, et al. Pneumocystis carinii pneumonia in the United States. Epidemiologic, diagnostic, and clinical features. Ann Intern Med. 1974;80:83-93. doi: 10.7326/0003-4819-80-1-83
3. Sepkowitz KA. Pneumocystis carinii pneumonia in patients without AIDS. Clin Infect Dis. 1993;17 suppl 2:S416-422. doi: 10.1093/clinids/17.supplement_2.s416
4. Al Soub H, Taha RY, El Deeb Y, et al. Pneumocystis carinii pneumonia in a patient without a predisposing illness: case report and review. Scand J Infect Dis. 2004;36:618-621. doi: 10.1080/00365540410017608
5. Jacobs JL, Libby DM, Winters RA, et al. A cluster of Pneumocystis carinii pneumonia in adults without predisposing illnesses. N Engl J Med. 1991;324:246-250. doi: 10.1056/NEJM199101243240407
6. Ng VL, Yajko DM, McPhaul LW, et al. Evaluation of an indirect fluorescent-antibody stain for detection of Pneumocystis carinii in respiratory specimens. J Clin Microbiol. 1990;28:975-979. doi: 10.1128/jcm.28.5.975-979.1990
7. Cregan P, Yamamoto A, Lum A, et al. Comparison of four methods for rapid detection of Pneumocystis carinii in respiratory specimens. J Clin Microbiol. 1990;28:2432-2436. doi: 10.1128/jcm.28.11.2432-2436.1990
8. Turner D, Schwarz Y, Yust I. Induced sputum for diagnosing Pneumocystis carinii pneumonia in HIV patients: new data, new issues. Eur Respir J. 2003;21:204-208. doi: 10.1183/09031936.03.00035303
9. Smith RL, el-Sadr WM, Lewis ML. Correlation of bronchoalveolar lavage cell populations with clinical severity of Pneumocystis carinii pneumonia. Chest. 1988;93:60-64. doi: 10.1378/chest.93.1.60
10. Fleury-Feith J, Van Nhieu JT, Picard C, et al. Bronchoalveolar lavage eosinophilia associated with Pneumocystis carinii pneumonitis in AIDS patients. Comparative study with non-AIDS patients. Chest. 1989;95:1198-1201. doi: 10.1378/chest.95.6.1198
11. Thomas CF Jr, Limper AH. Current insights into the biology and pathogenesis of Pneumocystis pneumonia. Nat Rev Microbiol. 2007;5:298-308. doi: 10.1038/nrmicro1621
12. Toh BH, Roberts-Thomson IC, Mathews JD, et al. Depression of cell-mediated immunity in old age and the immunopathic diseases, lupus erythematosus, chronic hepatitis and rheumatoid arthritis. Clin Exp Immunol. 1973;14:193-202.
13. Mansharamani NG, Balachandran D, Vernovsky I, et al. Peripheral blood CD4 + T-lymphocyte counts during Pneumocystis carinii pneumonia in immunocompromised patients without HIV infection. Chest. 2000;118:712-720. doi: 10.1378/chest.118.3.712
14. McGovern BH, Golan Y, Lopez M, et al. The impact of cirrhosis on CD4+ T cell counts in HIV-seronegative patients. Clin Infect Dis. 2007;44:431-437. doi: 10.1086/509580
15. Bienvenu AL, Traore K, Plekhanova I, et al. Pneumocystis pneumonia suspected cases in 604 non-HIV and HIV patients. Int J Infect Dis. 2016;46:11-17. doi: 10.1016/j.ijid.2016.03.018
16. Albillos A, Lario M, Álvarez-Mon M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J Hepatol. 2014;61:1385-1396. doi: 10.1016/j.jhep.2014.08.010
17. Lario M, Muñoz L, Ubeda M, et al. Defective thymopoiesis and poor peripheral homeostatic replenishment of T-helper cells cause T-cell lymphopenia in cirrhosis. J Hepatol. 2013;59:723-730. doi: 10.1016/j.jhep.2013.05.042
Benzodiazepine and Z-hypnotic stewardship
Benzodiazepines (BZDs) and Z-hypnotics have been available for decades, yet uncertainties about their use remain. They are prescribed and overprescribed most often for anxiety and insomnia, for which they have value but also the potential for significant adverse consequences, notably physiologic dependence. Use of these agents should be limited, and planned deprescribing is a fundamental aspect of prescribing.
A brief history. BZDs are a subset of benzodiazepine receptor agonists (BZRAs), which enhance the inhibitory effect of centrally acting γ-amino butyric acid (GABA) at the GABAA receptor through allosteric modulation. In 1960, the first BZD, chlordiazepoxide, was marketed for clinical use, and as other agents in the class became available, BZDs supplanted the more toxic barbiturates, another BZRA subset (TABLE 1). By the late 1970s, BZDs had risen to the top of most prescribed medications, with one agent in particular—diazepam (Valium)—earning a reputation as “mother’s little helper,” a phrase derived from a Rolling Stones' song with that title produced in 1966.1
With recognition of the problems associated with BZDs, their popularity diminished somewhat but remained high. BZDs were listed under Schedule IV by the Drug Enforcement Administration in 1975 due to the risk for addiction, and on the American Geriatrics Society Beers Criteria list in 1991 because of significant adverse consequences in the elderly. Researchers began to question their use as early as the 1970s, and the landmark Ashton Manual, guidance for patients and clinicians alike, was published in 2002.2
Currently, there are 14 BZDs approved by the Food and Drug Administration (FDA) as well as 3 Z-hypnotics, termed such as they include the letter “z” in their generic names (TABLE 1). In recent years, BZD prescribing has risen; a 2019 study found that 1 of 8 American adults reported using a BZD in the previous year.3
Limited benefits of benzodiazepine receptor agonists
BZRAs can be of benefit in a limited range of medical conditions, including some for which they are first-line considerations. (See TABLE 2 for a list of indications for BZDs.) They are most often prescribed for anxiety and insomnia, although they are not first-line treatments for these conditions and should be prescribed only when symptoms limit a patient’s daily functioning.
BZRAs are not intended for long-term use. In recent decades, the percentage of patients prescribed BZRAs has doubled, and more than 80% of these patients indicate usage for more than 6 months.4 Evidence, however, does not support long-term daily use.
Observation periods in most studies are far shorter than the number of years over which BZDs are actually prescribed, and flawed research methodology has introduced the risk of bias. Specifically, the generalizability of reported outcomes must be qualified, since efficacy trials performed under ideal study conditions (eg, exclusion criteria to minimize confounders) differ from circumstances seen in clinical practice. Conclusions are also limited by the inherent bias of pharmaceutical industry sponsorship and unavailability of unpublished trials that may have demonstrated unfavorable results.
Continue to: Insomnia
Insomnia, a current (past 30 days) complaint in more than 40% of US adults, is associated with a variety of symptoms.5 About 20% of adults have an insomnia disorder, defined as a predominant problem for at least 1 month involving sleep initiation, maintenance, or nonrestorative sleep along with daytime function-limiting fatigue.5 Meta-analyses indicate BZRAs can reduce sleep latency (BZDs, by 4 minutes; Z-hypnotics, 22 minutes) and may increase sleep duration (BZDs, 62 minutes per limited data; Z-hypnotics, data insufficient).6,7 Definitive evidence for long-term (> 2-4 weeks) BZD benefit is lacking, and cognitive behavioral therapy for insomnia (CBT-I) is well established as first-line treatment yielding improvements that may last at least 18 months after completion of therapy. 8,9
Although CBT-I is generally provided by behavioral health specialists, elements of CBT-I and sleep hygiene measures can be effectively used by primary care clinicians.10 Data indicate other nonpharmacologic interventions are also effective,11 including acceptance and commitment therapy,12 meditation,13 and acupuncture.14
Episodic fear and anxiety are universal and essential for survival. Fear is an alarm warning of an immediate hazard. Anxiety (the emotion) paired with worry (the thought) relate to a perceived future threat. Transient (state) anxiety should not be suppressed altogether if self-management can curb its intensity and thereby allow effective problem engagement. However, when individuals are incapacitated by crisis anxiety or sporadic specific phobias such as flight anxiety, episodic BZDs do have a role.
Ongoing anxiety is a more complex treatment situation. Obsessive-compulsive disorder and posttraumatic stress disorder are no longer categorized as anxiety disorders, but they often involve anxiety. Here, BZDs have no indication aside from exceptional acute crisis presentations. Anxiety disorders are defined by a core persistent (trait) anxiety disproportionate to the actual threat, limited daily functioning, and more than 6 months’ duration. One of 3 Americans older than 13 years meet the criteria for anxiety in their lifetime; 1 of 5 meet the criteria in any single year.15
BZDs are effective in treating anxiety disorders in the short term (2-4 weeks)2,16,17; however, benefit may fade over time.18-21 For some individuals, data suggest BZDs themselves might actually generate anxiety, as evidenced by reduced symptom intensity following discontinuation.22,23 Recommended first-line medications for anxiety disorders include certain antidepressants and pregabalin, which exhibit efficacy similar to that of BZDs.24 Mindfulness and various psychotherapies have value, as well.16 Among the latter, CBT is considered first line with benefit comparable to BZDs in the short term; yet unlike BZDs, CBT gains can last 12 months or longer after the conclusion of therapy. 25,26 Because there may be a delay between the start of CBT and the onset of benefit, BZDs, which work quickly, may be used to bridge functionally impaired patients in the short term.
Continue to: Risks with benzodiazepine receptor agonists
Risks with benzodiazepine receptor agonists
Harms from BZRA use are common, tempering their utility. Sedation, dyscognition, and psychomotor impairments are often seen upon initiation of BZRA use. These adverse effects can—although not always—improve with continuous BZRA exposure, an effect known as tolerance, which is due to neuropharmacologic adaptation.
Cognitive issues include problems with memory, judgment, and decision making. These may be unrecognized or, if noted, attributed to other issues such as aging, and may become clear only when BZRAs are discontinued. Anterograde amnesia and parasomnias occur less often.
Psychomotor impairment can result in falls, fractures, and other injuries, especially in the elderly. Decrements in mood, including emergent depression and paradoxical anxiety, can occur. Some individuals experience disinhibition that is expressed through irritability, agitation, aggression, and violence.
Misuse of BZRAs is not unusual and can be related to dosing errors or attempts to ease intrusive symptoms. Nonmedical use almost always occurs in the context of an underlying use disorder, whereby BZRAs serve to amplify euphoria or ameliorate withdrawal from opioids or alcohol. Addiction per se, which entails BZRA craving and compulsive use leading to adverse consequences, is unusual.
BZRAs are associated with increased mortality, including all-cause mortality and suicide. They are respiratory depressants, although when taken alone in excess rarely result in death. They are, however, strongly implicated in opioid-related overdose fatalities, as their presence has been identified in 1 of 3 such decedents.27
Continue to: Physiologic dependence with BZRAs
Physiologic dependence with BZRAs
Among the more important adverse outcomes with ongoing BZRA exposure is physiologic dependence. This occurs primarily because of neuroadaptation of GABAA and glutaminergic receptors, but dependence probably also involves changes in the adenosine A2A, serotonergic, and peripheral benzodiazepine receptors, the latter being present on mitochondrial membranes. The hypothalamic-pituitary-adrenal axis also appears to be involved.
Physiologic dependence is expressed through BZRA tolerance and characteristic physical and psychological symptoms upon withdrawal. Tolerance refers to a reduced effect with continued substance exposure or the need for an increased dose to get the same effect. Drug withdrawal can result in manifestations distinctive to addiction-prone substances, as well as to some medications without addiction liability, such as corticosteroids and antidepressants. Tolerance and withdrawal are not applicable criteria in the diagnosis of sedative-hypnotic use disorder when BZRAs are prescribed.28
Withdrawal. Reported prevalence of BZRA physiologic dependence differs according to populations studied, criteria used, and the deprescribing process employed. Some researchers have found rates of one-third and others exceeding one-half among individuals using BZRAs for longer than a month.23,29
Deprescribing BZRAs
Because benefits are limited and adverse outcomes including physiologic dependence are common, it is recommended that clinicians urge deprescribing of BZRAs for any patient taking them consistently for more than 1 month. Published deprescribing investigations and guidance are insufficient, heterogenous, and confusing. Still, some approaches can work well, and success rates as high as 80% have been achieved among the elderly, for example.35 Brief interventions such as providing individualized advice, support, and management are effective.36,37 Abrupt discontinuation is inappropriate and can be life threatening.38 Forced cessation is also inappropriate unless significant respiratory depression is identified.
The Ashton Manual is a useful guide, readable by patients. Proceed with tapering slowly at a rate led by the patient’s response.2,39 Avoid discrediting patients’ reports of unusual withdrawal symptoms, as this can lead to misdiagnosis (eg, somatic symptom disorder) or ineffective treatment (eg, addiction recovery approaches). Adding CBT to tapering improves outcomes, and adjunctive medications may be helpful, although not without their own problems.29 Consistent support of patients by others involved in treatment (prescriber, pharmacist, behavioral health specialists, peer coach, significant others) is essential. Complex challenges generally resolve through authentic listening and response but may require referral to others with necessary skills and experience. Complete cessation may take 12 to 18 months (or longer). Even if complete cessation is not possible, the least dose necessary can be achieved.
CORRESPONDENCE
Steven Wright, MD, 1975 Ashland Mine Road, Ashland, OR 97520; sleighwright@gmail.com
- Chandler A, Whittaker A, Williams N, et al. Mother’s little helper? Contrasting accounts of benzodiazepine and methadone use among drug-dependent parents in the UK. Drugs (Abingdon Engl). 2014;21:470-475. doi: 10.3109/09687637.2014.930814Ash
- ton CH. Benzodiazepines: How They Work & How to Withdraw (the Ashton Manual). 2002. Accessed March 17, 2022. www.benzoinfo.com/ashtonmanual/
- Maust DT, Lin LA, Blow FC. Benzodiazepine use and misuse among adults in the United States. Psychiatr Serv. 2019;70:97-106. doi: 10.1176/appi.ps.201800321
- Kaufmann CN, Spira AP, Depp CA, et al. Long-term use of benzodiazepines and non-benzodiazepine hypnotics from 1999 to 2014: results from the National Health and Nutrition Examination Survey. Psychiatr Serv. 2018;69:235-238. doi: 10.1176/appi.ps.201700095
- Walsh JK, Coulouvrat C, Hajak G, et al. Nighttime insomnia symptoms and perceived health in the America Insomnia Survey (AIS). Sleep. 2011;34:997-1011. doi: 10.5665/SLEEP.1150
- Holbrook AM, Crowther R, Lotter A, et al. Meta-analysis of benzodiazepine use in the treatment of insomnia. CMAJ. 2000;162:225-233.
- Huedo-Medina TB, Kirsch I, Middlemass J, et al. Effectiveness of non-benzodiazepine hypnotics in treatment of adult insomnia: meta-analysis of data submitted to the Food and Drug Administration. BMJ. 2012;345:e8343. doi: 10.1136/bmj.e8343
- Curran HV, Collins R, Fletcher S, et al. Older adults and withdrawal from benzodiazepine hypnotics in general practice: effects on cognitive function, sleep, mood and quality of life. Psychol Med. 2003;33:1223-1237. doi: 10.1017/s0033291703008213
- Geiger-Brown JM, Rogers VE, Liu W, et al. Cognitive behavioral therapy in persons with comorbid insomnia: a meta-analysis. Sleep Med Rev. 2015;23:54-67. doi: 10.1016/j.smrv.2014.11.007
- Sorscher AJ. Insomnia: getting to the cause, facilitating relief. J Fam Pract. 2017;66:216-225
- Laura Hrehová L, Mezian K. Non-pharmacologic treatment of insomnia in primary care settings. Int J Clin Pract. 2021;75:e14084. doi: 10.1111/ijcp.14084.
- Daly-Eichenhardt A, Scott W, Howard-Jones M, et al. Changes in sleep problems and psychological flexibility following interdisciplinary acceptance and commitment therapy for chronic pain: an observational cohort study. Front Psychol. 2016;7:1326. doi: 10.3389/fpsyg.2016.01326
- Rusch HL, Rosario M, Levison LM, et al. The effect of mindfulness meditation on sleep quality: a systematic review and meta-analysis of randomized controlled trials. Ann N Y Acad Sci. 2019;1445:5-16. doi: 10.1111/nyas.13996
- Cao H, Pan X, Li H, et al. Acupuncture for treatment of insomnia: a systematic review of randomized controlled trials. J Altern Complement Med. 2009;15:1171-1186. doi: 10.1089/acm.2009.0041
- Kessler RC, Petukhova M, Sampson NA, et al. Twelve‐month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int J Methods Psychiatr Res. 2012;21:169-184. doi: 10.1002/mpr.1359
- Bandelow B, Reitt M, Röver C, et al. Efficacy of treatments for anxiety disorders: a meta-analysis. Int Clin Psychopharmacol. 2015;30:183-192. doi: 10.1097/YIC.0000000000000078
- Lader M. Benzodiazepines revisited—will we ever learn? Addiction. 2011;106:2086-2109. doi: 10.1111/j.1360-0443.2011.03563.x
- Fava GA. Fading of therapeutic effects of alprazolam in agoraphobia. Case reports. Prog Neuropsychopharmacol Biol Psychiatry. 1988;12:109-112. doi: 10.1016/0278-5846(88)90066-8
- Fava GA, Grandi S, Belluardo P, et al. Benzodiazepines and anxiety sensitivity in panic disorder. Prog Neuropsychopharmacol Biol Psychiatry. 1994;18:1163-1168. doi: 10.1016/0278-5846(94)90118-x
- Pélissolo A, Maniere F, Boutges B, et al. Anxiety and depressive disorders in 4,425 long term benzodiazepine users in general practice. Encephale. 2007;33:32-38. doi: 10.1016/s0013-7006(07)91556-0
- Gale C, Glue P, Guaiana G, et al. Influence of covariates on heterogeneity in Hamilton Anxiety Scale ratings in placebo-controlled trials of benzodiazepines in generalized anxiety disorder: systematic review and meta-analysis. J Psychopharmacol. 2019;33:543-547. doi: 10.1177/0269881118822146
- Ashton CH. Benzodiazepine withdrawal: outcome in 50 patients. Br J Addict. 1987;82:655-671. Accessed February 22, 2022. www.benzo.org.uk/ashbzoc.htm
- Rickels K, Schweizer E, Case WG, et al. Long-term therapeutic use of benzodiazepines. I. Effects of abrupt discontinuation. Arch Gen Psychiatry. 1990;47:899-907. doi: 10.1001/archpsyc.1990.01810220015002
- Bandelow B, Sher L, Bunevicius R, et al. Guidelines for the pharmacological treatment of anxiety disorders, obsessive-compulsive disorder and posttraumatic stress disorder in primary care. Int J Psychiatry Clin Pract. 2012;16:77-84. Accessed March 17, 2022. www.wfsbp.org/fileadmin/user_upload/Treatment_Guidelines/Bandelow_et_al_01.pdf
- Imai H, Tajika A, Chen P, et al. Psychological therapies versus pharmacological interventions for panic disorder with or without agoraphobia in adults. Cochrane Database Syst Rev. 2016;10:CD011170. doi: 10.1002/14651858.CD011170.pub2
- van Dis EAM, van Veen SC, Hagenaars MA, et al. Long-term outcomes of cognitive behavioral therapy for anxiety-related disorders. A systematic review and meta-analysis. JAMA Psychiatry. 2020;77:265-273. doi:10.1001/jamapsychiatry.2019.3986
- Chen LH, Hedegaard H, Warner M. Drug-poisoning deaths Involving opioid analgesics: United States, 1999-2011. NCHS Data Brief. 2014;(166):1-8. Accessed March 17, 2022. www.cdc.gov/nchs/data/databriefs/db166.pdf
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th edition (DSM-5). American Psychiatric Publishing; 2013:550-555.
- Marriott S, Tyrer P. Benzodiazepine dependence: avoidance and withdrawal. Drug Safety. 1993;9:93-103. doi: 10.2165/00002018-199309020-00003
- Griffiths RR, Evans SM, Guarino JJ, et al. Intravenous flumazenil following acute and repeated exposure to lorazepam in healthy volunteers: antagonism and precipitated withdrawal. J Pharmacol Exp Ther. 1993;265:1163-1174.
- Ashton H. Benzodiazepine withdrawal: an unfinished story. Br Med J. 1984;288:1135-1140. Accessed March 17, 2022. www.ncbi.nlm.nih.gov/pmc/articles/PMC1441411/pdf/bmjcred00496-0031.pdf
- Lugoboni F, Quaglio G. Exploring the dark side of the moon: the treatment of benzodiazepine tolerance. Br J Clin Pharmacol. 2014;77:239-41. doi: 10.1111/bcp.12148
- Ashton CH. Protracted withdrawal from benzodiazepines: the post-withdrawal syndrome. Psychiatr Ann. 1995;25:174-179. Accessed March 17, 2022. https://benzo.org.uk/pha-1.htm
- Fixsen AM, Ridge D. Stories of hell and healing: internet users’ construction of benzodiazepine distress and withdrawal. Qual Health Res. 2017;27:2030-2041. doi: 10.1177/1049732317728053
- Ng BJ, Le Couteur DG, Hilmer SN. Deprescribing benzodiazepines in older patients: impact of interventions targeting physicians, pharmacists, and patients. Drugs Aging. 2018;35:493-521. doi: 10.1007/s40266-018-0544-4
- Lynch T, Ryan C, Hughes CM, et al. Brief interventions targeting long-term benzodiazepine and Z-drug use in primary care: a systematic review and meta-analysis. Addiction. 2020;115:1618-1639. doi: 10.1111/add.14981
- Darker CD, Sweeney BP, Barry JM, et al. Psychosocial interventions for benzodiazepine harmful use, abuse or dependence. Cochrane Database Syst Rev. 2015;(5):CD009652. doi: 10.1002/14651858.CD009652.pub2
- Hu X. Benzodiazepine withdrawal seizures and management. J Okla State Med Assoc. 2011;104:62-65.
- Wright SL. Benzodiazepine withdrawal: clinical aspects. In Peppin J, Raffa R, Pergolizzi J, Wright SL, eds. The Benzodiazepines Crisis: The Ramifications of an Overused Drug Class. Oxford University Press. 2020:117-148.
Benzodiazepines (BZDs) and Z-hypnotics have been available for decades, yet uncertainties about their use remain. They are prescribed and overprescribed most often for anxiety and insomnia, for which they have value but also the potential for significant adverse consequences, notably physiologic dependence. Use of these agents should be limited, and planned deprescribing is a fundamental aspect of prescribing.
A brief history. BZDs are a subset of benzodiazepine receptor agonists (BZRAs), which enhance the inhibitory effect of centrally acting γ-amino butyric acid (GABA) at the GABAA receptor through allosteric modulation. In 1960, the first BZD, chlordiazepoxide, was marketed for clinical use, and as other agents in the class became available, BZDs supplanted the more toxic barbiturates, another BZRA subset (TABLE 1). By the late 1970s, BZDs had risen to the top of most prescribed medications, with one agent in particular—diazepam (Valium)—earning a reputation as “mother’s little helper,” a phrase derived from a Rolling Stones' song with that title produced in 1966.1
With recognition of the problems associated with BZDs, their popularity diminished somewhat but remained high. BZDs were listed under Schedule IV by the Drug Enforcement Administration in 1975 due to the risk for addiction, and on the American Geriatrics Society Beers Criteria list in 1991 because of significant adverse consequences in the elderly. Researchers began to question their use as early as the 1970s, and the landmark Ashton Manual, guidance for patients and clinicians alike, was published in 2002.2
Currently, there are 14 BZDs approved by the Food and Drug Administration (FDA) as well as 3 Z-hypnotics, termed such as they include the letter “z” in their generic names (TABLE 1). In recent years, BZD prescribing has risen; a 2019 study found that 1 of 8 American adults reported using a BZD in the previous year.3
Limited benefits of benzodiazepine receptor agonists
BZRAs can be of benefit in a limited range of medical conditions, including some for which they are first-line considerations. (See TABLE 2 for a list of indications for BZDs.) They are most often prescribed for anxiety and insomnia, although they are not first-line treatments for these conditions and should be prescribed only when symptoms limit a patient’s daily functioning.
BZRAs are not intended for long-term use. In recent decades, the percentage of patients prescribed BZRAs has doubled, and more than 80% of these patients indicate usage for more than 6 months.4 Evidence, however, does not support long-term daily use.
Observation periods in most studies are far shorter than the number of years over which BZDs are actually prescribed, and flawed research methodology has introduced the risk of bias. Specifically, the generalizability of reported outcomes must be qualified, since efficacy trials performed under ideal study conditions (eg, exclusion criteria to minimize confounders) differ from circumstances seen in clinical practice. Conclusions are also limited by the inherent bias of pharmaceutical industry sponsorship and unavailability of unpublished trials that may have demonstrated unfavorable results.
Continue to: Insomnia
Insomnia, a current (past 30 days) complaint in more than 40% of US adults, is associated with a variety of symptoms.5 About 20% of adults have an insomnia disorder, defined as a predominant problem for at least 1 month involving sleep initiation, maintenance, or nonrestorative sleep along with daytime function-limiting fatigue.5 Meta-analyses indicate BZRAs can reduce sleep latency (BZDs, by 4 minutes; Z-hypnotics, 22 minutes) and may increase sleep duration (BZDs, 62 minutes per limited data; Z-hypnotics, data insufficient).6,7 Definitive evidence for long-term (> 2-4 weeks) BZD benefit is lacking, and cognitive behavioral therapy for insomnia (CBT-I) is well established as first-line treatment yielding improvements that may last at least 18 months after completion of therapy. 8,9
Although CBT-I is generally provided by behavioral health specialists, elements of CBT-I and sleep hygiene measures can be effectively used by primary care clinicians.10 Data indicate other nonpharmacologic interventions are also effective,11 including acceptance and commitment therapy,12 meditation,13 and acupuncture.14
Episodic fear and anxiety are universal and essential for survival. Fear is an alarm warning of an immediate hazard. Anxiety (the emotion) paired with worry (the thought) relate to a perceived future threat. Transient (state) anxiety should not be suppressed altogether if self-management can curb its intensity and thereby allow effective problem engagement. However, when individuals are incapacitated by crisis anxiety or sporadic specific phobias such as flight anxiety, episodic BZDs do have a role.
Ongoing anxiety is a more complex treatment situation. Obsessive-compulsive disorder and posttraumatic stress disorder are no longer categorized as anxiety disorders, but they often involve anxiety. Here, BZDs have no indication aside from exceptional acute crisis presentations. Anxiety disorders are defined by a core persistent (trait) anxiety disproportionate to the actual threat, limited daily functioning, and more than 6 months’ duration. One of 3 Americans older than 13 years meet the criteria for anxiety in their lifetime; 1 of 5 meet the criteria in any single year.15
BZDs are effective in treating anxiety disorders in the short term (2-4 weeks)2,16,17; however, benefit may fade over time.18-21 For some individuals, data suggest BZDs themselves might actually generate anxiety, as evidenced by reduced symptom intensity following discontinuation.22,23 Recommended first-line medications for anxiety disorders include certain antidepressants and pregabalin, which exhibit efficacy similar to that of BZDs.24 Mindfulness and various psychotherapies have value, as well.16 Among the latter, CBT is considered first line with benefit comparable to BZDs in the short term; yet unlike BZDs, CBT gains can last 12 months or longer after the conclusion of therapy. 25,26 Because there may be a delay between the start of CBT and the onset of benefit, BZDs, which work quickly, may be used to bridge functionally impaired patients in the short term.
Continue to: Risks with benzodiazepine receptor agonists
Risks with benzodiazepine receptor agonists
Harms from BZRA use are common, tempering their utility. Sedation, dyscognition, and psychomotor impairments are often seen upon initiation of BZRA use. These adverse effects can—although not always—improve with continuous BZRA exposure, an effect known as tolerance, which is due to neuropharmacologic adaptation.
Cognitive issues include problems with memory, judgment, and decision making. These may be unrecognized or, if noted, attributed to other issues such as aging, and may become clear only when BZRAs are discontinued. Anterograde amnesia and parasomnias occur less often.
Psychomotor impairment can result in falls, fractures, and other injuries, especially in the elderly. Decrements in mood, including emergent depression and paradoxical anxiety, can occur. Some individuals experience disinhibition that is expressed through irritability, agitation, aggression, and violence.
Misuse of BZRAs is not unusual and can be related to dosing errors or attempts to ease intrusive symptoms. Nonmedical use almost always occurs in the context of an underlying use disorder, whereby BZRAs serve to amplify euphoria or ameliorate withdrawal from opioids or alcohol. Addiction per se, which entails BZRA craving and compulsive use leading to adverse consequences, is unusual.
BZRAs are associated with increased mortality, including all-cause mortality and suicide. They are respiratory depressants, although when taken alone in excess rarely result in death. They are, however, strongly implicated in opioid-related overdose fatalities, as their presence has been identified in 1 of 3 such decedents.27
Continue to: Physiologic dependence with BZRAs
Physiologic dependence with BZRAs
Among the more important adverse outcomes with ongoing BZRA exposure is physiologic dependence. This occurs primarily because of neuroadaptation of GABAA and glutaminergic receptors, but dependence probably also involves changes in the adenosine A2A, serotonergic, and peripheral benzodiazepine receptors, the latter being present on mitochondrial membranes. The hypothalamic-pituitary-adrenal axis also appears to be involved.
Physiologic dependence is expressed through BZRA tolerance and characteristic physical and psychological symptoms upon withdrawal. Tolerance refers to a reduced effect with continued substance exposure or the need for an increased dose to get the same effect. Drug withdrawal can result in manifestations distinctive to addiction-prone substances, as well as to some medications without addiction liability, such as corticosteroids and antidepressants. Tolerance and withdrawal are not applicable criteria in the diagnosis of sedative-hypnotic use disorder when BZRAs are prescribed.28
Withdrawal. Reported prevalence of BZRA physiologic dependence differs according to populations studied, criteria used, and the deprescribing process employed. Some researchers have found rates of one-third and others exceeding one-half among individuals using BZRAs for longer than a month.23,29
Deprescribing BZRAs
Because benefits are limited and adverse outcomes including physiologic dependence are common, it is recommended that clinicians urge deprescribing of BZRAs for any patient taking them consistently for more than 1 month. Published deprescribing investigations and guidance are insufficient, heterogenous, and confusing. Still, some approaches can work well, and success rates as high as 80% have been achieved among the elderly, for example.35 Brief interventions such as providing individualized advice, support, and management are effective.36,37 Abrupt discontinuation is inappropriate and can be life threatening.38 Forced cessation is also inappropriate unless significant respiratory depression is identified.
The Ashton Manual is a useful guide, readable by patients. Proceed with tapering slowly at a rate led by the patient’s response.2,39 Avoid discrediting patients’ reports of unusual withdrawal symptoms, as this can lead to misdiagnosis (eg, somatic symptom disorder) or ineffective treatment (eg, addiction recovery approaches). Adding CBT to tapering improves outcomes, and adjunctive medications may be helpful, although not without their own problems.29 Consistent support of patients by others involved in treatment (prescriber, pharmacist, behavioral health specialists, peer coach, significant others) is essential. Complex challenges generally resolve through authentic listening and response but may require referral to others with necessary skills and experience. Complete cessation may take 12 to 18 months (or longer). Even if complete cessation is not possible, the least dose necessary can be achieved.
CORRESPONDENCE
Steven Wright, MD, 1975 Ashland Mine Road, Ashland, OR 97520; sleighwright@gmail.com
Benzodiazepines (BZDs) and Z-hypnotics have been available for decades, yet uncertainties about their use remain. They are prescribed and overprescribed most often for anxiety and insomnia, for which they have value but also the potential for significant adverse consequences, notably physiologic dependence. Use of these agents should be limited, and planned deprescribing is a fundamental aspect of prescribing.
A brief history. BZDs are a subset of benzodiazepine receptor agonists (BZRAs), which enhance the inhibitory effect of centrally acting γ-amino butyric acid (GABA) at the GABAA receptor through allosteric modulation. In 1960, the first BZD, chlordiazepoxide, was marketed for clinical use, and as other agents in the class became available, BZDs supplanted the more toxic barbiturates, another BZRA subset (TABLE 1). By the late 1970s, BZDs had risen to the top of most prescribed medications, with one agent in particular—diazepam (Valium)—earning a reputation as “mother’s little helper,” a phrase derived from a Rolling Stones' song with that title produced in 1966.1
With recognition of the problems associated with BZDs, their popularity diminished somewhat but remained high. BZDs were listed under Schedule IV by the Drug Enforcement Administration in 1975 due to the risk for addiction, and on the American Geriatrics Society Beers Criteria list in 1991 because of significant adverse consequences in the elderly. Researchers began to question their use as early as the 1970s, and the landmark Ashton Manual, guidance for patients and clinicians alike, was published in 2002.2
Currently, there are 14 BZDs approved by the Food and Drug Administration (FDA) as well as 3 Z-hypnotics, termed such as they include the letter “z” in their generic names (TABLE 1). In recent years, BZD prescribing has risen; a 2019 study found that 1 of 8 American adults reported using a BZD in the previous year.3
Limited benefits of benzodiazepine receptor agonists
BZRAs can be of benefit in a limited range of medical conditions, including some for which they are first-line considerations. (See TABLE 2 for a list of indications for BZDs.) They are most often prescribed for anxiety and insomnia, although they are not first-line treatments for these conditions and should be prescribed only when symptoms limit a patient’s daily functioning.
BZRAs are not intended for long-term use. In recent decades, the percentage of patients prescribed BZRAs has doubled, and more than 80% of these patients indicate usage for more than 6 months.4 Evidence, however, does not support long-term daily use.
Observation periods in most studies are far shorter than the number of years over which BZDs are actually prescribed, and flawed research methodology has introduced the risk of bias. Specifically, the generalizability of reported outcomes must be qualified, since efficacy trials performed under ideal study conditions (eg, exclusion criteria to minimize confounders) differ from circumstances seen in clinical practice. Conclusions are also limited by the inherent bias of pharmaceutical industry sponsorship and unavailability of unpublished trials that may have demonstrated unfavorable results.
Continue to: Insomnia
Insomnia, a current (past 30 days) complaint in more than 40% of US adults, is associated with a variety of symptoms.5 About 20% of adults have an insomnia disorder, defined as a predominant problem for at least 1 month involving sleep initiation, maintenance, or nonrestorative sleep along with daytime function-limiting fatigue.5 Meta-analyses indicate BZRAs can reduce sleep latency (BZDs, by 4 minutes; Z-hypnotics, 22 minutes) and may increase sleep duration (BZDs, 62 minutes per limited data; Z-hypnotics, data insufficient).6,7 Definitive evidence for long-term (> 2-4 weeks) BZD benefit is lacking, and cognitive behavioral therapy for insomnia (CBT-I) is well established as first-line treatment yielding improvements that may last at least 18 months after completion of therapy. 8,9
Although CBT-I is generally provided by behavioral health specialists, elements of CBT-I and sleep hygiene measures can be effectively used by primary care clinicians.10 Data indicate other nonpharmacologic interventions are also effective,11 including acceptance and commitment therapy,12 meditation,13 and acupuncture.14
Episodic fear and anxiety are universal and essential for survival. Fear is an alarm warning of an immediate hazard. Anxiety (the emotion) paired with worry (the thought) relate to a perceived future threat. Transient (state) anxiety should not be suppressed altogether if self-management can curb its intensity and thereby allow effective problem engagement. However, when individuals are incapacitated by crisis anxiety or sporadic specific phobias such as flight anxiety, episodic BZDs do have a role.
Ongoing anxiety is a more complex treatment situation. Obsessive-compulsive disorder and posttraumatic stress disorder are no longer categorized as anxiety disorders, but they often involve anxiety. Here, BZDs have no indication aside from exceptional acute crisis presentations. Anxiety disorders are defined by a core persistent (trait) anxiety disproportionate to the actual threat, limited daily functioning, and more than 6 months’ duration. One of 3 Americans older than 13 years meet the criteria for anxiety in their lifetime; 1 of 5 meet the criteria in any single year.15
BZDs are effective in treating anxiety disorders in the short term (2-4 weeks)2,16,17; however, benefit may fade over time.18-21 For some individuals, data suggest BZDs themselves might actually generate anxiety, as evidenced by reduced symptom intensity following discontinuation.22,23 Recommended first-line medications for anxiety disorders include certain antidepressants and pregabalin, which exhibit efficacy similar to that of BZDs.24 Mindfulness and various psychotherapies have value, as well.16 Among the latter, CBT is considered first line with benefit comparable to BZDs in the short term; yet unlike BZDs, CBT gains can last 12 months or longer after the conclusion of therapy. 25,26 Because there may be a delay between the start of CBT and the onset of benefit, BZDs, which work quickly, may be used to bridge functionally impaired patients in the short term.
Continue to: Risks with benzodiazepine receptor agonists
Risks with benzodiazepine receptor agonists
Harms from BZRA use are common, tempering their utility. Sedation, dyscognition, and psychomotor impairments are often seen upon initiation of BZRA use. These adverse effects can—although not always—improve with continuous BZRA exposure, an effect known as tolerance, which is due to neuropharmacologic adaptation.
Cognitive issues include problems with memory, judgment, and decision making. These may be unrecognized or, if noted, attributed to other issues such as aging, and may become clear only when BZRAs are discontinued. Anterograde amnesia and parasomnias occur less often.
Psychomotor impairment can result in falls, fractures, and other injuries, especially in the elderly. Decrements in mood, including emergent depression and paradoxical anxiety, can occur. Some individuals experience disinhibition that is expressed through irritability, agitation, aggression, and violence.
Misuse of BZRAs is not unusual and can be related to dosing errors or attempts to ease intrusive symptoms. Nonmedical use almost always occurs in the context of an underlying use disorder, whereby BZRAs serve to amplify euphoria or ameliorate withdrawal from opioids or alcohol. Addiction per se, which entails BZRA craving and compulsive use leading to adverse consequences, is unusual.
BZRAs are associated with increased mortality, including all-cause mortality and suicide. They are respiratory depressants, although when taken alone in excess rarely result in death. They are, however, strongly implicated in opioid-related overdose fatalities, as their presence has been identified in 1 of 3 such decedents.27
Continue to: Physiologic dependence with BZRAs
Physiologic dependence with BZRAs
Among the more important adverse outcomes with ongoing BZRA exposure is physiologic dependence. This occurs primarily because of neuroadaptation of GABAA and glutaminergic receptors, but dependence probably also involves changes in the adenosine A2A, serotonergic, and peripheral benzodiazepine receptors, the latter being present on mitochondrial membranes. The hypothalamic-pituitary-adrenal axis also appears to be involved.
Physiologic dependence is expressed through BZRA tolerance and characteristic physical and psychological symptoms upon withdrawal. Tolerance refers to a reduced effect with continued substance exposure or the need for an increased dose to get the same effect. Drug withdrawal can result in manifestations distinctive to addiction-prone substances, as well as to some medications without addiction liability, such as corticosteroids and antidepressants. Tolerance and withdrawal are not applicable criteria in the diagnosis of sedative-hypnotic use disorder when BZRAs are prescribed.28
Withdrawal. Reported prevalence of BZRA physiologic dependence differs according to populations studied, criteria used, and the deprescribing process employed. Some researchers have found rates of one-third and others exceeding one-half among individuals using BZRAs for longer than a month.23,29
Deprescribing BZRAs
Because benefits are limited and adverse outcomes including physiologic dependence are common, it is recommended that clinicians urge deprescribing of BZRAs for any patient taking them consistently for more than 1 month. Published deprescribing investigations and guidance are insufficient, heterogenous, and confusing. Still, some approaches can work well, and success rates as high as 80% have been achieved among the elderly, for example.35 Brief interventions such as providing individualized advice, support, and management are effective.36,37 Abrupt discontinuation is inappropriate and can be life threatening.38 Forced cessation is also inappropriate unless significant respiratory depression is identified.
The Ashton Manual is a useful guide, readable by patients. Proceed with tapering slowly at a rate led by the patient’s response.2,39 Avoid discrediting patients’ reports of unusual withdrawal symptoms, as this can lead to misdiagnosis (eg, somatic symptom disorder) or ineffective treatment (eg, addiction recovery approaches). Adding CBT to tapering improves outcomes, and adjunctive medications may be helpful, although not without their own problems.29 Consistent support of patients by others involved in treatment (prescriber, pharmacist, behavioral health specialists, peer coach, significant others) is essential. Complex challenges generally resolve through authentic listening and response but may require referral to others with necessary skills and experience. Complete cessation may take 12 to 18 months (or longer). Even if complete cessation is not possible, the least dose necessary can be achieved.
CORRESPONDENCE
Steven Wright, MD, 1975 Ashland Mine Road, Ashland, OR 97520; sleighwright@gmail.com
- Chandler A, Whittaker A, Williams N, et al. Mother’s little helper? Contrasting accounts of benzodiazepine and methadone use among drug-dependent parents in the UK. Drugs (Abingdon Engl). 2014;21:470-475. doi: 10.3109/09687637.2014.930814Ash
- ton CH. Benzodiazepines: How They Work & How to Withdraw (the Ashton Manual). 2002. Accessed March 17, 2022. www.benzoinfo.com/ashtonmanual/
- Maust DT, Lin LA, Blow FC. Benzodiazepine use and misuse among adults in the United States. Psychiatr Serv. 2019;70:97-106. doi: 10.1176/appi.ps.201800321
- Kaufmann CN, Spira AP, Depp CA, et al. Long-term use of benzodiazepines and non-benzodiazepine hypnotics from 1999 to 2014: results from the National Health and Nutrition Examination Survey. Psychiatr Serv. 2018;69:235-238. doi: 10.1176/appi.ps.201700095
- Walsh JK, Coulouvrat C, Hajak G, et al. Nighttime insomnia symptoms and perceived health in the America Insomnia Survey (AIS). Sleep. 2011;34:997-1011. doi: 10.5665/SLEEP.1150
- Holbrook AM, Crowther R, Lotter A, et al. Meta-analysis of benzodiazepine use in the treatment of insomnia. CMAJ. 2000;162:225-233.
- Huedo-Medina TB, Kirsch I, Middlemass J, et al. Effectiveness of non-benzodiazepine hypnotics in treatment of adult insomnia: meta-analysis of data submitted to the Food and Drug Administration. BMJ. 2012;345:e8343. doi: 10.1136/bmj.e8343
- Curran HV, Collins R, Fletcher S, et al. Older adults and withdrawal from benzodiazepine hypnotics in general practice: effects on cognitive function, sleep, mood and quality of life. Psychol Med. 2003;33:1223-1237. doi: 10.1017/s0033291703008213
- Geiger-Brown JM, Rogers VE, Liu W, et al. Cognitive behavioral therapy in persons with comorbid insomnia: a meta-analysis. Sleep Med Rev. 2015;23:54-67. doi: 10.1016/j.smrv.2014.11.007
- Sorscher AJ. Insomnia: getting to the cause, facilitating relief. J Fam Pract. 2017;66:216-225
- Laura Hrehová L, Mezian K. Non-pharmacologic treatment of insomnia in primary care settings. Int J Clin Pract. 2021;75:e14084. doi: 10.1111/ijcp.14084.
- Daly-Eichenhardt A, Scott W, Howard-Jones M, et al. Changes in sleep problems and psychological flexibility following interdisciplinary acceptance and commitment therapy for chronic pain: an observational cohort study. Front Psychol. 2016;7:1326. doi: 10.3389/fpsyg.2016.01326
- Rusch HL, Rosario M, Levison LM, et al. The effect of mindfulness meditation on sleep quality: a systematic review and meta-analysis of randomized controlled trials. Ann N Y Acad Sci. 2019;1445:5-16. doi: 10.1111/nyas.13996
- Cao H, Pan X, Li H, et al. Acupuncture for treatment of insomnia: a systematic review of randomized controlled trials. J Altern Complement Med. 2009;15:1171-1186. doi: 10.1089/acm.2009.0041
- Kessler RC, Petukhova M, Sampson NA, et al. Twelve‐month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int J Methods Psychiatr Res. 2012;21:169-184. doi: 10.1002/mpr.1359
- Bandelow B, Reitt M, Röver C, et al. Efficacy of treatments for anxiety disorders: a meta-analysis. Int Clin Psychopharmacol. 2015;30:183-192. doi: 10.1097/YIC.0000000000000078
- Lader M. Benzodiazepines revisited—will we ever learn? Addiction. 2011;106:2086-2109. doi: 10.1111/j.1360-0443.2011.03563.x
- Fava GA. Fading of therapeutic effects of alprazolam in agoraphobia. Case reports. Prog Neuropsychopharmacol Biol Psychiatry. 1988;12:109-112. doi: 10.1016/0278-5846(88)90066-8
- Fava GA, Grandi S, Belluardo P, et al. Benzodiazepines and anxiety sensitivity in panic disorder. Prog Neuropsychopharmacol Biol Psychiatry. 1994;18:1163-1168. doi: 10.1016/0278-5846(94)90118-x
- Pélissolo A, Maniere F, Boutges B, et al. Anxiety and depressive disorders in 4,425 long term benzodiazepine users in general practice. Encephale. 2007;33:32-38. doi: 10.1016/s0013-7006(07)91556-0
- Gale C, Glue P, Guaiana G, et al. Influence of covariates on heterogeneity in Hamilton Anxiety Scale ratings in placebo-controlled trials of benzodiazepines in generalized anxiety disorder: systematic review and meta-analysis. J Psychopharmacol. 2019;33:543-547. doi: 10.1177/0269881118822146
- Ashton CH. Benzodiazepine withdrawal: outcome in 50 patients. Br J Addict. 1987;82:655-671. Accessed February 22, 2022. www.benzo.org.uk/ashbzoc.htm
- Rickels K, Schweizer E, Case WG, et al. Long-term therapeutic use of benzodiazepines. I. Effects of abrupt discontinuation. Arch Gen Psychiatry. 1990;47:899-907. doi: 10.1001/archpsyc.1990.01810220015002
- Bandelow B, Sher L, Bunevicius R, et al. Guidelines for the pharmacological treatment of anxiety disorders, obsessive-compulsive disorder and posttraumatic stress disorder in primary care. Int J Psychiatry Clin Pract. 2012;16:77-84. Accessed March 17, 2022. www.wfsbp.org/fileadmin/user_upload/Treatment_Guidelines/Bandelow_et_al_01.pdf
- Imai H, Tajika A, Chen P, et al. Psychological therapies versus pharmacological interventions for panic disorder with or without agoraphobia in adults. Cochrane Database Syst Rev. 2016;10:CD011170. doi: 10.1002/14651858.CD011170.pub2
- van Dis EAM, van Veen SC, Hagenaars MA, et al. Long-term outcomes of cognitive behavioral therapy for anxiety-related disorders. A systematic review and meta-analysis. JAMA Psychiatry. 2020;77:265-273. doi:10.1001/jamapsychiatry.2019.3986
- Chen LH, Hedegaard H, Warner M. Drug-poisoning deaths Involving opioid analgesics: United States, 1999-2011. NCHS Data Brief. 2014;(166):1-8. Accessed March 17, 2022. www.cdc.gov/nchs/data/databriefs/db166.pdf
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th edition (DSM-5). American Psychiatric Publishing; 2013:550-555.
- Marriott S, Tyrer P. Benzodiazepine dependence: avoidance and withdrawal. Drug Safety. 1993;9:93-103. doi: 10.2165/00002018-199309020-00003
- Griffiths RR, Evans SM, Guarino JJ, et al. Intravenous flumazenil following acute and repeated exposure to lorazepam in healthy volunteers: antagonism and precipitated withdrawal. J Pharmacol Exp Ther. 1993;265:1163-1174.
- Ashton H. Benzodiazepine withdrawal: an unfinished story. Br Med J. 1984;288:1135-1140. Accessed March 17, 2022. www.ncbi.nlm.nih.gov/pmc/articles/PMC1441411/pdf/bmjcred00496-0031.pdf
- Lugoboni F, Quaglio G. Exploring the dark side of the moon: the treatment of benzodiazepine tolerance. Br J Clin Pharmacol. 2014;77:239-41. doi: 10.1111/bcp.12148
- Ashton CH. Protracted withdrawal from benzodiazepines: the post-withdrawal syndrome. Psychiatr Ann. 1995;25:174-179. Accessed March 17, 2022. https://benzo.org.uk/pha-1.htm
- Fixsen AM, Ridge D. Stories of hell and healing: internet users’ construction of benzodiazepine distress and withdrawal. Qual Health Res. 2017;27:2030-2041. doi: 10.1177/1049732317728053
- Ng BJ, Le Couteur DG, Hilmer SN. Deprescribing benzodiazepines in older patients: impact of interventions targeting physicians, pharmacists, and patients. Drugs Aging. 2018;35:493-521. doi: 10.1007/s40266-018-0544-4
- Lynch T, Ryan C, Hughes CM, et al. Brief interventions targeting long-term benzodiazepine and Z-drug use in primary care: a systematic review and meta-analysis. Addiction. 2020;115:1618-1639. doi: 10.1111/add.14981
- Darker CD, Sweeney BP, Barry JM, et al. Psychosocial interventions for benzodiazepine harmful use, abuse or dependence. Cochrane Database Syst Rev. 2015;(5):CD009652. doi: 10.1002/14651858.CD009652.pub2
- Hu X. Benzodiazepine withdrawal seizures and management. J Okla State Med Assoc. 2011;104:62-65.
- Wright SL. Benzodiazepine withdrawal: clinical aspects. In Peppin J, Raffa R, Pergolizzi J, Wright SL, eds. The Benzodiazepines Crisis: The Ramifications of an Overused Drug Class. Oxford University Press. 2020:117-148.
- Chandler A, Whittaker A, Williams N, et al. Mother’s little helper? Contrasting accounts of benzodiazepine and methadone use among drug-dependent parents in the UK. Drugs (Abingdon Engl). 2014;21:470-475. doi: 10.3109/09687637.2014.930814Ash
- ton CH. Benzodiazepines: How They Work & How to Withdraw (the Ashton Manual). 2002. Accessed March 17, 2022. www.benzoinfo.com/ashtonmanual/
- Maust DT, Lin LA, Blow FC. Benzodiazepine use and misuse among adults in the United States. Psychiatr Serv. 2019;70:97-106. doi: 10.1176/appi.ps.201800321
- Kaufmann CN, Spira AP, Depp CA, et al. Long-term use of benzodiazepines and non-benzodiazepine hypnotics from 1999 to 2014: results from the National Health and Nutrition Examination Survey. Psychiatr Serv. 2018;69:235-238. doi: 10.1176/appi.ps.201700095
- Walsh JK, Coulouvrat C, Hajak G, et al. Nighttime insomnia symptoms and perceived health in the America Insomnia Survey (AIS). Sleep. 2011;34:997-1011. doi: 10.5665/SLEEP.1150
- Holbrook AM, Crowther R, Lotter A, et al. Meta-analysis of benzodiazepine use in the treatment of insomnia. CMAJ. 2000;162:225-233.
- Huedo-Medina TB, Kirsch I, Middlemass J, et al. Effectiveness of non-benzodiazepine hypnotics in treatment of adult insomnia: meta-analysis of data submitted to the Food and Drug Administration. BMJ. 2012;345:e8343. doi: 10.1136/bmj.e8343
- Curran HV, Collins R, Fletcher S, et al. Older adults and withdrawal from benzodiazepine hypnotics in general practice: effects on cognitive function, sleep, mood and quality of life. Psychol Med. 2003;33:1223-1237. doi: 10.1017/s0033291703008213
- Geiger-Brown JM, Rogers VE, Liu W, et al. Cognitive behavioral therapy in persons with comorbid insomnia: a meta-analysis. Sleep Med Rev. 2015;23:54-67. doi: 10.1016/j.smrv.2014.11.007
- Sorscher AJ. Insomnia: getting to the cause, facilitating relief. J Fam Pract. 2017;66:216-225
- Laura Hrehová L, Mezian K. Non-pharmacologic treatment of insomnia in primary care settings. Int J Clin Pract. 2021;75:e14084. doi: 10.1111/ijcp.14084.
- Daly-Eichenhardt A, Scott W, Howard-Jones M, et al. Changes in sleep problems and psychological flexibility following interdisciplinary acceptance and commitment therapy for chronic pain: an observational cohort study. Front Psychol. 2016;7:1326. doi: 10.3389/fpsyg.2016.01326
- Rusch HL, Rosario M, Levison LM, et al. The effect of mindfulness meditation on sleep quality: a systematic review and meta-analysis of randomized controlled trials. Ann N Y Acad Sci. 2019;1445:5-16. doi: 10.1111/nyas.13996
- Cao H, Pan X, Li H, et al. Acupuncture for treatment of insomnia: a systematic review of randomized controlled trials. J Altern Complement Med. 2009;15:1171-1186. doi: 10.1089/acm.2009.0041
- Kessler RC, Petukhova M, Sampson NA, et al. Twelve‐month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int J Methods Psychiatr Res. 2012;21:169-184. doi: 10.1002/mpr.1359
- Bandelow B, Reitt M, Röver C, et al. Efficacy of treatments for anxiety disorders: a meta-analysis. Int Clin Psychopharmacol. 2015;30:183-192. doi: 10.1097/YIC.0000000000000078
- Lader M. Benzodiazepines revisited—will we ever learn? Addiction. 2011;106:2086-2109. doi: 10.1111/j.1360-0443.2011.03563.x
- Fava GA. Fading of therapeutic effects of alprazolam in agoraphobia. Case reports. Prog Neuropsychopharmacol Biol Psychiatry. 1988;12:109-112. doi: 10.1016/0278-5846(88)90066-8
- Fava GA, Grandi S, Belluardo P, et al. Benzodiazepines and anxiety sensitivity in panic disorder. Prog Neuropsychopharmacol Biol Psychiatry. 1994;18:1163-1168. doi: 10.1016/0278-5846(94)90118-x
- Pélissolo A, Maniere F, Boutges B, et al. Anxiety and depressive disorders in 4,425 long term benzodiazepine users in general practice. Encephale. 2007;33:32-38. doi: 10.1016/s0013-7006(07)91556-0
- Gale C, Glue P, Guaiana G, et al. Influence of covariates on heterogeneity in Hamilton Anxiety Scale ratings in placebo-controlled trials of benzodiazepines in generalized anxiety disorder: systematic review and meta-analysis. J Psychopharmacol. 2019;33:543-547. doi: 10.1177/0269881118822146
- Ashton CH. Benzodiazepine withdrawal: outcome in 50 patients. Br J Addict. 1987;82:655-671. Accessed February 22, 2022. www.benzo.org.uk/ashbzoc.htm
- Rickels K, Schweizer E, Case WG, et al. Long-term therapeutic use of benzodiazepines. I. Effects of abrupt discontinuation. Arch Gen Psychiatry. 1990;47:899-907. doi: 10.1001/archpsyc.1990.01810220015002
- Bandelow B, Sher L, Bunevicius R, et al. Guidelines for the pharmacological treatment of anxiety disorders, obsessive-compulsive disorder and posttraumatic stress disorder in primary care. Int J Psychiatry Clin Pract. 2012;16:77-84. Accessed March 17, 2022. www.wfsbp.org/fileadmin/user_upload/Treatment_Guidelines/Bandelow_et_al_01.pdf
- Imai H, Tajika A, Chen P, et al. Psychological therapies versus pharmacological interventions for panic disorder with or without agoraphobia in adults. Cochrane Database Syst Rev. 2016;10:CD011170. doi: 10.1002/14651858.CD011170.pub2
- van Dis EAM, van Veen SC, Hagenaars MA, et al. Long-term outcomes of cognitive behavioral therapy for anxiety-related disorders. A systematic review and meta-analysis. JAMA Psychiatry. 2020;77:265-273. doi:10.1001/jamapsychiatry.2019.3986
- Chen LH, Hedegaard H, Warner M. Drug-poisoning deaths Involving opioid analgesics: United States, 1999-2011. NCHS Data Brief. 2014;(166):1-8. Accessed March 17, 2022. www.cdc.gov/nchs/data/databriefs/db166.pdf
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th edition (DSM-5). American Psychiatric Publishing; 2013:550-555.
- Marriott S, Tyrer P. Benzodiazepine dependence: avoidance and withdrawal. Drug Safety. 1993;9:93-103. doi: 10.2165/00002018-199309020-00003
- Griffiths RR, Evans SM, Guarino JJ, et al. Intravenous flumazenil following acute and repeated exposure to lorazepam in healthy volunteers: antagonism and precipitated withdrawal. J Pharmacol Exp Ther. 1993;265:1163-1174.
- Ashton H. Benzodiazepine withdrawal: an unfinished story. Br Med J. 1984;288:1135-1140. Accessed March 17, 2022. www.ncbi.nlm.nih.gov/pmc/articles/PMC1441411/pdf/bmjcred00496-0031.pdf
- Lugoboni F, Quaglio G. Exploring the dark side of the moon: the treatment of benzodiazepine tolerance. Br J Clin Pharmacol. 2014;77:239-41. doi: 10.1111/bcp.12148
- Ashton CH. Protracted withdrawal from benzodiazepines: the post-withdrawal syndrome. Psychiatr Ann. 1995;25:174-179. Accessed March 17, 2022. https://benzo.org.uk/pha-1.htm
- Fixsen AM, Ridge D. Stories of hell and healing: internet users’ construction of benzodiazepine distress and withdrawal. Qual Health Res. 2017;27:2030-2041. doi: 10.1177/1049732317728053
- Ng BJ, Le Couteur DG, Hilmer SN. Deprescribing benzodiazepines in older patients: impact of interventions targeting physicians, pharmacists, and patients. Drugs Aging. 2018;35:493-521. doi: 10.1007/s40266-018-0544-4
- Lynch T, Ryan C, Hughes CM, et al. Brief interventions targeting long-term benzodiazepine and Z-drug use in primary care: a systematic review and meta-analysis. Addiction. 2020;115:1618-1639. doi: 10.1111/add.14981
- Darker CD, Sweeney BP, Barry JM, et al. Psychosocial interventions for benzodiazepine harmful use, abuse or dependence. Cochrane Database Syst Rev. 2015;(5):CD009652. doi: 10.1002/14651858.CD009652.pub2
- Hu X. Benzodiazepine withdrawal seizures and management. J Okla State Med Assoc. 2011;104:62-65.
- Wright SL. Benzodiazepine withdrawal: clinical aspects. In Peppin J, Raffa R, Pergolizzi J, Wright SL, eds. The Benzodiazepines Crisis: The Ramifications of an Overused Drug Class. Oxford University Press. 2020:117-148.
PRACTICE RECOMMENDATIONS
› Recommend cognitive behavioral therapy as first-line treatment for anxiety and insomnia. A
› Limit benzodiazepine prescribing to ≤ 2 to 4 weeks for anxiety and insomnia. B
› Taper benzodiazepines slowly and flexibly. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Has the anti-benzodiazepine backlash gone too far?
When benzodiazepines were first introduced, they were greeted with enthusiasm. Librium came first, in 1960, followed by Valium in 1962, and they were seen as an improvement over barbiturates for the treatment of anxiety, insomnia, and seizures. From 1968 to 1982, Valium (diazepam) was the No. 1–selling U.S. pharmaceutical: 2.3 billion tablets of Valium were sold in 1978 alone. Valium was even the subject of a 1966 Rolling Stones hit, “Mother’s Little Helper.”
By the 1980s, it became apparent that there was a downside to these medications: patients became tolerant, dependent, and some became addicted to the medications. In older patients an association was noted with falls and cognitive impairment. And while safe in overdoses when they are the only agent, combined with alcohol or opioids, benzodiazepines can be lethal and have played a significant role in the current overdose crisis.
Because of the problems that are associated with their use, benzodiazepines and their relatives, the Z-drugs used for sleep, have become stigmatized, as have the patients who use them and perhaps even the doctors who prescribe them. Still, there are circumstances where patients find these medications to be helpful, where other medications don’t work, or don’t work quickly enough. They provide fast relief in conditions where there are not always good alternatives.
In the Facebook group, “Psychiatry for All Physicians,” it’s not uncommon for physicians to ask what to do with older patients who are transferred to them on therapeutic doses of benzodiazepines or zolpidem. These are outpatients coming for routine care, and they find the medications helpful and don’t want to discontinue them. They have tried other medications that were not helpful. I’ve been surprised at how often the respondents insist the patient should be told he must taper off the medication. “Just say no,” is often the advice, and perhaps it’s more about the doctor’s discomfort than it is about the individual patient. For sleep issues, cognitive-behavioral therapy is given as the gold-standard treatment, while in my practice I have found it difficult to motivate patients to engage in it, and of those who do, it is sometimes helpful, but not a panacea. Severe anxiety and sleepless nights, however, are not benign conditions.
This “just say no, hold the line” sentiment has me wondering if our pendulum has swung too far with respect to prescribing benzodiazepines. Is this just one more issue that has become strongly polarized? Certainly the literature would support that idea, with some physicians writing about how benzodiazepines are underused, and others urging avoidance.
I posted a poll on Twitter: Has the anti-benzo movement gone too far? In addition, I started a Twitter thread of my own thoughts about prescribing and deprescribing these medications and will give a synopsis of those ideas here.
Clearly, benzodiazepines are harmful to some patients, they have side effects, can be difficult to stop because of withdrawal symptoms, and they carry the risk of addiction. That’s not in question. Many medications, however, have the potential to do more harm than good, for example ibuprofen can cause bleeding or renal problems, and Fosamax, used to treat osteoporosis, can cause osteonecrosis of the jaw and femur, to name just two.
It would be so much easier if we could know in advance who benzodiazepines will harm, just as it would be good if we could know in advance who will get tardive dyskinesia or dyslipidemia from antipsychotic medications, or who will have life-threatening adverse reactions from cancer chemotherapy with no tumor response. There are risks to both starting and stopping sedatives, and if we insist a patient stop a medication because of potential risk, then we are cutting them off from being a partner in their own care. It also creates an adversarial relationship that can be draining for the doctor and upsetting for the patient.
By definition, if someone needs hospitalization for a psychiatric condition, their outpatient benzodiazepine is not keeping them stable and stopping it may be a good idea. If someone is seen in an ED for a fall, it’s common to blame the benzodiazepine, but older people who are not on these medications also fall and have memory problems. In his book, “Being Mortal: Illness, Medicine, and What Matters Most in the End” (New York: Picador, 2014), Atul Gawande, MD, makes the point that taking more than four prescriptions medications increases the risk for falls in the elderly. Still, no one is suggesting patients be taken off their antidepressants, antihypertensives, or blood thinners.
Finally, the question is not should we be giving benzodiazepines out without careful consideration – the answer is clearly no. Physicians don’t pass out benzodiazepines “like candy” for all the above reasons. They are initiated because the patient is suffering and sometimes desperate. Anxiety, panic, intractable insomnia, and severe agitation are all miserable, and alternative treatments may take weeks to work, or not work at all. Yet these subjective symptoms may be dismissed by physicians.
So what do I do in my own practice? I don’t encourage patients to take potentially addictive medications, but I do sometimes use them. I give ‘as needed’ benzodiazepines to people in distress who don’t have a history of misusing them. I never plan to start them as a permanent standing medication, though once in a while that ends up happening. As with other medications, it is best to use the minimally effective dose.
There is some controversy as to whether it is best to use anxiety medications on an “as-needed” basis or as a standing dosage. Psychiatrists who prescribe benzodiazepines more liberally often feel it’s better to give standing doses and prevent breakthrough anxiety. Patients may appear to be ‘medication seeking’ not because they are addicted, but because the doses used are too low to adequately treat their anxiety.
My hope is that there is less risk of tolerance, dependence, or addiction with less-frequent dosing, and I prescribe as-needed benzodiazepines for panic attacks, agitated major depression while we wait for the antidepressant to “kick in,” insomnia during manic episodes, and to people who get very anxious in specific situations such as flying or for medical procedures. I sometimes prescribe them for people with insomnia that does not respond to other treatments, or for disabling generalized anxiety.
For patients who have taken benzodiazepines for many years, I continue to discuss the risks, but often they are not looking to fix something that isn’t broken, or to live a risk-free life. A few of the patients who have come to me on low standing doses of sedatives are now in their 80’s, yet they remain active, live independently, drive, travel, and have busy social lives. One could argue either that the medications are working, or that the patient has become dependent on them and needs them to prevent withdrawal.
These medications present a quandary: by denying patients treatment with benzodiazepines, we are sparing some people addictions (this is good, we should be careful), but we are leaving some people to suffer. There is no perfect answer.
What I do know is that doctors should think carefully and consider the patient in front of them. “No Benzos Ever For Anyone” or “you must come off because there is risk and people will think I am a bad doctor for prescribing them to you” can be done by a robot.
So, yes, I think the pendulum has swung a bit too far; there is a place for these medications in acute treatment for those at low risk of addiction, and there are people who benefit from them over the long run. At times, they provide immense relief to someone who is really struggling.
So what was the result of my Twitter poll? Of the 219 voters, 34.2% voted: “No, the pendulum has not swung too far, and these medications are harmful”; 65.8% voted: “Yes, these medications are helpful.” There were many comments expressing a wide variety of sentiments. Of those who had taken prescription benzodiazepines, some felt they had been harmed and wished they had never been started on them, and others continue to find them helpful. Psychiatrists, it seems, see them from the vantage point of the populations they treat.
People who are uncomfortable search for answers, and those answers may come in the form of meditation or exercise, medicines, or illicit drugs. It’s interesting that these same patients can now easily obtain “medical” marijuana, and the Rolling Stones’ “Mother’s Little Helper” is often replaced by a gin and tonic.
Dr. Dinah Miller is a coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins in Baltimore. Dr. Miller has no conflicts of interest.
When benzodiazepines were first introduced, they were greeted with enthusiasm. Librium came first, in 1960, followed by Valium in 1962, and they were seen as an improvement over barbiturates for the treatment of anxiety, insomnia, and seizures. From 1968 to 1982, Valium (diazepam) was the No. 1–selling U.S. pharmaceutical: 2.3 billion tablets of Valium were sold in 1978 alone. Valium was even the subject of a 1966 Rolling Stones hit, “Mother’s Little Helper.”
By the 1980s, it became apparent that there was a downside to these medications: patients became tolerant, dependent, and some became addicted to the medications. In older patients an association was noted with falls and cognitive impairment. And while safe in overdoses when they are the only agent, combined with alcohol or opioids, benzodiazepines can be lethal and have played a significant role in the current overdose crisis.
Because of the problems that are associated with their use, benzodiazepines and their relatives, the Z-drugs used for sleep, have become stigmatized, as have the patients who use them and perhaps even the doctors who prescribe them. Still, there are circumstances where patients find these medications to be helpful, where other medications don’t work, or don’t work quickly enough. They provide fast relief in conditions where there are not always good alternatives.
In the Facebook group, “Psychiatry for All Physicians,” it’s not uncommon for physicians to ask what to do with older patients who are transferred to them on therapeutic doses of benzodiazepines or zolpidem. These are outpatients coming for routine care, and they find the medications helpful and don’t want to discontinue them. They have tried other medications that were not helpful. I’ve been surprised at how often the respondents insist the patient should be told he must taper off the medication. “Just say no,” is often the advice, and perhaps it’s more about the doctor’s discomfort than it is about the individual patient. For sleep issues, cognitive-behavioral therapy is given as the gold-standard treatment, while in my practice I have found it difficult to motivate patients to engage in it, and of those who do, it is sometimes helpful, but not a panacea. Severe anxiety and sleepless nights, however, are not benign conditions.
This “just say no, hold the line” sentiment has me wondering if our pendulum has swung too far with respect to prescribing benzodiazepines. Is this just one more issue that has become strongly polarized? Certainly the literature would support that idea, with some physicians writing about how benzodiazepines are underused, and others urging avoidance.
I posted a poll on Twitter: Has the anti-benzo movement gone too far? In addition, I started a Twitter thread of my own thoughts about prescribing and deprescribing these medications and will give a synopsis of those ideas here.
Clearly, benzodiazepines are harmful to some patients, they have side effects, can be difficult to stop because of withdrawal symptoms, and they carry the risk of addiction. That’s not in question. Many medications, however, have the potential to do more harm than good, for example ibuprofen can cause bleeding or renal problems, and Fosamax, used to treat osteoporosis, can cause osteonecrosis of the jaw and femur, to name just two.
It would be so much easier if we could know in advance who benzodiazepines will harm, just as it would be good if we could know in advance who will get tardive dyskinesia or dyslipidemia from antipsychotic medications, or who will have life-threatening adverse reactions from cancer chemotherapy with no tumor response. There are risks to both starting and stopping sedatives, and if we insist a patient stop a medication because of potential risk, then we are cutting them off from being a partner in their own care. It also creates an adversarial relationship that can be draining for the doctor and upsetting for the patient.
By definition, if someone needs hospitalization for a psychiatric condition, their outpatient benzodiazepine is not keeping them stable and stopping it may be a good idea. If someone is seen in an ED for a fall, it’s common to blame the benzodiazepine, but older people who are not on these medications also fall and have memory problems. In his book, “Being Mortal: Illness, Medicine, and What Matters Most in the End” (New York: Picador, 2014), Atul Gawande, MD, makes the point that taking more than four prescriptions medications increases the risk for falls in the elderly. Still, no one is suggesting patients be taken off their antidepressants, antihypertensives, or blood thinners.
Finally, the question is not should we be giving benzodiazepines out without careful consideration – the answer is clearly no. Physicians don’t pass out benzodiazepines “like candy” for all the above reasons. They are initiated because the patient is suffering and sometimes desperate. Anxiety, panic, intractable insomnia, and severe agitation are all miserable, and alternative treatments may take weeks to work, or not work at all. Yet these subjective symptoms may be dismissed by physicians.
So what do I do in my own practice? I don’t encourage patients to take potentially addictive medications, but I do sometimes use them. I give ‘as needed’ benzodiazepines to people in distress who don’t have a history of misusing them. I never plan to start them as a permanent standing medication, though once in a while that ends up happening. As with other medications, it is best to use the minimally effective dose.
There is some controversy as to whether it is best to use anxiety medications on an “as-needed” basis or as a standing dosage. Psychiatrists who prescribe benzodiazepines more liberally often feel it’s better to give standing doses and prevent breakthrough anxiety. Patients may appear to be ‘medication seeking’ not because they are addicted, but because the doses used are too low to adequately treat their anxiety.
My hope is that there is less risk of tolerance, dependence, or addiction with less-frequent dosing, and I prescribe as-needed benzodiazepines for panic attacks, agitated major depression while we wait for the antidepressant to “kick in,” insomnia during manic episodes, and to people who get very anxious in specific situations such as flying or for medical procedures. I sometimes prescribe them for people with insomnia that does not respond to other treatments, or for disabling generalized anxiety.
For patients who have taken benzodiazepines for many years, I continue to discuss the risks, but often they are not looking to fix something that isn’t broken, or to live a risk-free life. A few of the patients who have come to me on low standing doses of sedatives are now in their 80’s, yet they remain active, live independently, drive, travel, and have busy social lives. One could argue either that the medications are working, or that the patient has become dependent on them and needs them to prevent withdrawal.
These medications present a quandary: by denying patients treatment with benzodiazepines, we are sparing some people addictions (this is good, we should be careful), but we are leaving some people to suffer. There is no perfect answer.
What I do know is that doctors should think carefully and consider the patient in front of them. “No Benzos Ever For Anyone” or “you must come off because there is risk and people will think I am a bad doctor for prescribing them to you” can be done by a robot.
So, yes, I think the pendulum has swung a bit too far; there is a place for these medications in acute treatment for those at low risk of addiction, and there are people who benefit from them over the long run. At times, they provide immense relief to someone who is really struggling.
So what was the result of my Twitter poll? Of the 219 voters, 34.2% voted: “No, the pendulum has not swung too far, and these medications are harmful”; 65.8% voted: “Yes, these medications are helpful.” There were many comments expressing a wide variety of sentiments. Of those who had taken prescription benzodiazepines, some felt they had been harmed and wished they had never been started on them, and others continue to find them helpful. Psychiatrists, it seems, see them from the vantage point of the populations they treat.
People who are uncomfortable search for answers, and those answers may come in the form of meditation or exercise, medicines, or illicit drugs. It’s interesting that these same patients can now easily obtain “medical” marijuana, and the Rolling Stones’ “Mother’s Little Helper” is often replaced by a gin and tonic.
Dr. Dinah Miller is a coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins in Baltimore. Dr. Miller has no conflicts of interest.
When benzodiazepines were first introduced, they were greeted with enthusiasm. Librium came first, in 1960, followed by Valium in 1962, and they were seen as an improvement over barbiturates for the treatment of anxiety, insomnia, and seizures. From 1968 to 1982, Valium (diazepam) was the No. 1–selling U.S. pharmaceutical: 2.3 billion tablets of Valium were sold in 1978 alone. Valium was even the subject of a 1966 Rolling Stones hit, “Mother’s Little Helper.”
By the 1980s, it became apparent that there was a downside to these medications: patients became tolerant, dependent, and some became addicted to the medications. In older patients an association was noted with falls and cognitive impairment. And while safe in overdoses when they are the only agent, combined with alcohol or opioids, benzodiazepines can be lethal and have played a significant role in the current overdose crisis.
Because of the problems that are associated with their use, benzodiazepines and their relatives, the Z-drugs used for sleep, have become stigmatized, as have the patients who use them and perhaps even the doctors who prescribe them. Still, there are circumstances where patients find these medications to be helpful, where other medications don’t work, or don’t work quickly enough. They provide fast relief in conditions where there are not always good alternatives.
In the Facebook group, “Psychiatry for All Physicians,” it’s not uncommon for physicians to ask what to do with older patients who are transferred to them on therapeutic doses of benzodiazepines or zolpidem. These are outpatients coming for routine care, and they find the medications helpful and don’t want to discontinue them. They have tried other medications that were not helpful. I’ve been surprised at how often the respondents insist the patient should be told he must taper off the medication. “Just say no,” is often the advice, and perhaps it’s more about the doctor’s discomfort than it is about the individual patient. For sleep issues, cognitive-behavioral therapy is given as the gold-standard treatment, while in my practice I have found it difficult to motivate patients to engage in it, and of those who do, it is sometimes helpful, but not a panacea. Severe anxiety and sleepless nights, however, are not benign conditions.
This “just say no, hold the line” sentiment has me wondering if our pendulum has swung too far with respect to prescribing benzodiazepines. Is this just one more issue that has become strongly polarized? Certainly the literature would support that idea, with some physicians writing about how benzodiazepines are underused, and others urging avoidance.
I posted a poll on Twitter: Has the anti-benzo movement gone too far? In addition, I started a Twitter thread of my own thoughts about prescribing and deprescribing these medications and will give a synopsis of those ideas here.
Clearly, benzodiazepines are harmful to some patients, they have side effects, can be difficult to stop because of withdrawal symptoms, and they carry the risk of addiction. That’s not in question. Many medications, however, have the potential to do more harm than good, for example ibuprofen can cause bleeding or renal problems, and Fosamax, used to treat osteoporosis, can cause osteonecrosis of the jaw and femur, to name just two.
It would be so much easier if we could know in advance who benzodiazepines will harm, just as it would be good if we could know in advance who will get tardive dyskinesia or dyslipidemia from antipsychotic medications, or who will have life-threatening adverse reactions from cancer chemotherapy with no tumor response. There are risks to both starting and stopping sedatives, and if we insist a patient stop a medication because of potential risk, then we are cutting them off from being a partner in their own care. It also creates an adversarial relationship that can be draining for the doctor and upsetting for the patient.
By definition, if someone needs hospitalization for a psychiatric condition, their outpatient benzodiazepine is not keeping them stable and stopping it may be a good idea. If someone is seen in an ED for a fall, it’s common to blame the benzodiazepine, but older people who are not on these medications also fall and have memory problems. In his book, “Being Mortal: Illness, Medicine, and What Matters Most in the End” (New York: Picador, 2014), Atul Gawande, MD, makes the point that taking more than four prescriptions medications increases the risk for falls in the elderly. Still, no one is suggesting patients be taken off their antidepressants, antihypertensives, or blood thinners.
Finally, the question is not should we be giving benzodiazepines out without careful consideration – the answer is clearly no. Physicians don’t pass out benzodiazepines “like candy” for all the above reasons. They are initiated because the patient is suffering and sometimes desperate. Anxiety, panic, intractable insomnia, and severe agitation are all miserable, and alternative treatments may take weeks to work, or not work at all. Yet these subjective symptoms may be dismissed by physicians.
So what do I do in my own practice? I don’t encourage patients to take potentially addictive medications, but I do sometimes use them. I give ‘as needed’ benzodiazepines to people in distress who don’t have a history of misusing them. I never plan to start them as a permanent standing medication, though once in a while that ends up happening. As with other medications, it is best to use the minimally effective dose.
There is some controversy as to whether it is best to use anxiety medications on an “as-needed” basis or as a standing dosage. Psychiatrists who prescribe benzodiazepines more liberally often feel it’s better to give standing doses and prevent breakthrough anxiety. Patients may appear to be ‘medication seeking’ not because they are addicted, but because the doses used are too low to adequately treat their anxiety.
My hope is that there is less risk of tolerance, dependence, or addiction with less-frequent dosing, and I prescribe as-needed benzodiazepines for panic attacks, agitated major depression while we wait for the antidepressant to “kick in,” insomnia during manic episodes, and to people who get very anxious in specific situations such as flying or for medical procedures. I sometimes prescribe them for people with insomnia that does not respond to other treatments, or for disabling generalized anxiety.
For patients who have taken benzodiazepines for many years, I continue to discuss the risks, but often they are not looking to fix something that isn’t broken, or to live a risk-free life. A few of the patients who have come to me on low standing doses of sedatives are now in their 80’s, yet they remain active, live independently, drive, travel, and have busy social lives. One could argue either that the medications are working, or that the patient has become dependent on them and needs them to prevent withdrawal.
These medications present a quandary: by denying patients treatment with benzodiazepines, we are sparing some people addictions (this is good, we should be careful), but we are leaving some people to suffer. There is no perfect answer.
What I do know is that doctors should think carefully and consider the patient in front of them. “No Benzos Ever For Anyone” or “you must come off because there is risk and people will think I am a bad doctor for prescribing them to you” can be done by a robot.
So, yes, I think the pendulum has swung a bit too far; there is a place for these medications in acute treatment for those at low risk of addiction, and there are people who benefit from them over the long run. At times, they provide immense relief to someone who is really struggling.
So what was the result of my Twitter poll? Of the 219 voters, 34.2% voted: “No, the pendulum has not swung too far, and these medications are harmful”; 65.8% voted: “Yes, these medications are helpful.” There were many comments expressing a wide variety of sentiments. Of those who had taken prescription benzodiazepines, some felt they had been harmed and wished they had never been started on them, and others continue to find them helpful. Psychiatrists, it seems, see them from the vantage point of the populations they treat.
People who are uncomfortable search for answers, and those answers may come in the form of meditation or exercise, medicines, or illicit drugs. It’s interesting that these same patients can now easily obtain “medical” marijuana, and the Rolling Stones’ “Mother’s Little Helper” is often replaced by a gin and tonic.
Dr. Dinah Miller is a coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins in Baltimore. Dr. Miller has no conflicts of interest.
Adolescent overdose deaths nearly doubled in 2020 and spiked again in 2021
The number of overdose deaths in adolescents nearly doubled in 2020 from the year before and increased substantially again in 2021 after nearly a decade of fairly stable rates, according to data published in a JAMA research letter.
Most of the deaths involved fentanyl, the researchers found.
Joseph Friedman, MPH, of the Center for Social Medicine and Humanities at the University of California, Los Angeles, led the study, which analyzed adolescent (14-18 years old) overdose deaths in the United States from 2010 to June 2021 in light of increasing contamination in the supply of illicit drugs.
The researchers found there were 518 deaths among adolescents (2.40 per 100,000 population) in 2010, and the rates remained stable through 2019 with 492 deaths (2.36 per 100,000).
In 2020, however, deaths spiked to 954 (4.57 per 100 000), increasing by 94.3%, compared with 2019. In 2021, they increased another 20%.
The rise in fentanyl-involved deaths was particularly striking. Fentanyl-involved deaths increased from 253 (1.21 per 100,000) in 2019 to 680 (3.26 per 100,000) in 2020. The numbers through June 2021 were annualized for 2021 and calculations predicted 884 deaths (4.23 per 100,000) for the year.
Numbers point to fentanyl potency
In 2021, more than three-fourths (77.14%) of adolescent overdose deaths involved fentanyl, compared with 13.26% for benzodiazepines, 9.77% for methamphetamine, 7.33% for cocaine, 5.76% for prescription opioids, and 2.27% for heroin.
American Indian and Alaska Native adolescents had the highest overdose rate in 2021 (n = 24; 11.79 per 100,000), followed by Latinx adolescents (n = 354; 6.98 per 100,000).
“These adolescent trends fit a wider pattern of increasing racial and ethnic inequalities in overdose that deserve further investigation and intervention efforts,” the authors wrote.
Pandemic’s role unclear
The spikes in adolescent overdoses overlap the COVID-19 pandemic, but Dr. Friedman said in an interview the pandemic “may or may not have been a big factor. “
The authors wrote that drug use had generally been stable among adolescents between 2010 and 2020. The number of 10th graders reporting any illicit drug use was 30.2% in 2010 and 30.4% in 2020.
“So it’s not that more teens are using drugs. It’s just that drug use is becoming more dangerous due to the spread of counterfeit pills containing fentanyls,” Dr. Friedman said.
The authors noted that “the illicit drug supply has increasingly become contaminated with illicitly manufactured fentanyls and other synthetic opioid and benzodiazepine analogues.”
Mr. Friedman said the pandemic may have accelerated the spread of more dangerous forms of drugs as supply chains were disrupted.
Benjamin Brady, DrPH, an assistant professor at the University of Arizona, Tucson, who also has an appointment in the university’s Comprehensive Pain and Addiction Center, said in an interview the numbers that Dr. Friedman and colleagues present represent “worst fears coming true.”
He said he and his colleagues in the field “were anticipating a rise in overdose deaths for the next 5-10 years because of the way the supply-and-demand environment exists in the U.S.”
Dr. Brady explained that restricting access to prescription opioids has had an unfortunate side effect in decreasing access to a safer supply of drugs.
“Without having solutions that would reduce demand at the same rate, supply of the safer form of the drug has been reduced; that has pushed people toward heroin and street drugs and from 2016 on those have been adulterated with fentanyl,” he said.
He said the United States, compared with other developed nations, has been slower to embrace longer-term harm-reduction strategies and to improve access to treatment and care.
COVID likely also has exacerbated the problem in terms of isolation and reduction in quality of life that has adolescents seeking to fill that void with drugs, Dr. Brady said. They may be completely unaware that the drugs they are seeking are commonly cut with counterfeit fentanyl.
“Fentanyl can be up to 50 times stronger than heroin,” he noted. “Even just a little bit of fentanyl dramatically changes the risk profile on an overdose.”
Increasing rates of mental health concerns among adolescents over decades also contribute to drug-seeking trends, Dr. Brady noted.
Overdose increases in the overall population were smaller
In the overall population, the percentage increases were not nearly as large in 2020 and 2021 as they were for adolescents.
Rates of overdose deaths in the overall population increased steadily from 2010 and reached 70,630 in 2019. In 2020, the deaths increased to 91,799 (an increase of 29.48% from 2019) and increased 11.48% in 2021.
The researchers analyzed numbers from the Centers for Disease Control and Prevention WONDER (Wide-Ranging Online Data for Epidemiologic Research) database, which has records of all U.S. deaths for which drug overdose was listed as the underlying cause.
The authors and Dr. Brady report no relevant financial relationships.
The number of overdose deaths in adolescents nearly doubled in 2020 from the year before and increased substantially again in 2021 after nearly a decade of fairly stable rates, according to data published in a JAMA research letter.
Most of the deaths involved fentanyl, the researchers found.
Joseph Friedman, MPH, of the Center for Social Medicine and Humanities at the University of California, Los Angeles, led the study, which analyzed adolescent (14-18 years old) overdose deaths in the United States from 2010 to June 2021 in light of increasing contamination in the supply of illicit drugs.
The researchers found there were 518 deaths among adolescents (2.40 per 100,000 population) in 2010, and the rates remained stable through 2019 with 492 deaths (2.36 per 100,000).
In 2020, however, deaths spiked to 954 (4.57 per 100 000), increasing by 94.3%, compared with 2019. In 2021, they increased another 20%.
The rise in fentanyl-involved deaths was particularly striking. Fentanyl-involved deaths increased from 253 (1.21 per 100,000) in 2019 to 680 (3.26 per 100,000) in 2020. The numbers through June 2021 were annualized for 2021 and calculations predicted 884 deaths (4.23 per 100,000) for the year.
Numbers point to fentanyl potency
In 2021, more than three-fourths (77.14%) of adolescent overdose deaths involved fentanyl, compared with 13.26% for benzodiazepines, 9.77% for methamphetamine, 7.33% for cocaine, 5.76% for prescription opioids, and 2.27% for heroin.
American Indian and Alaska Native adolescents had the highest overdose rate in 2021 (n = 24; 11.79 per 100,000), followed by Latinx adolescents (n = 354; 6.98 per 100,000).
“These adolescent trends fit a wider pattern of increasing racial and ethnic inequalities in overdose that deserve further investigation and intervention efforts,” the authors wrote.
Pandemic’s role unclear
The spikes in adolescent overdoses overlap the COVID-19 pandemic, but Dr. Friedman said in an interview the pandemic “may or may not have been a big factor. “
The authors wrote that drug use had generally been stable among adolescents between 2010 and 2020. The number of 10th graders reporting any illicit drug use was 30.2% in 2010 and 30.4% in 2020.
“So it’s not that more teens are using drugs. It’s just that drug use is becoming more dangerous due to the spread of counterfeit pills containing fentanyls,” Dr. Friedman said.
The authors noted that “the illicit drug supply has increasingly become contaminated with illicitly manufactured fentanyls and other synthetic opioid and benzodiazepine analogues.”
Mr. Friedman said the pandemic may have accelerated the spread of more dangerous forms of drugs as supply chains were disrupted.
Benjamin Brady, DrPH, an assistant professor at the University of Arizona, Tucson, who also has an appointment in the university’s Comprehensive Pain and Addiction Center, said in an interview the numbers that Dr. Friedman and colleagues present represent “worst fears coming true.”
He said he and his colleagues in the field “were anticipating a rise in overdose deaths for the next 5-10 years because of the way the supply-and-demand environment exists in the U.S.”
Dr. Brady explained that restricting access to prescription opioids has had an unfortunate side effect in decreasing access to a safer supply of drugs.
“Without having solutions that would reduce demand at the same rate, supply of the safer form of the drug has been reduced; that has pushed people toward heroin and street drugs and from 2016 on those have been adulterated with fentanyl,” he said.
He said the United States, compared with other developed nations, has been slower to embrace longer-term harm-reduction strategies and to improve access to treatment and care.
COVID likely also has exacerbated the problem in terms of isolation and reduction in quality of life that has adolescents seeking to fill that void with drugs, Dr. Brady said. They may be completely unaware that the drugs they are seeking are commonly cut with counterfeit fentanyl.
“Fentanyl can be up to 50 times stronger than heroin,” he noted. “Even just a little bit of fentanyl dramatically changes the risk profile on an overdose.”
Increasing rates of mental health concerns among adolescents over decades also contribute to drug-seeking trends, Dr. Brady noted.
Overdose increases in the overall population were smaller
In the overall population, the percentage increases were not nearly as large in 2020 and 2021 as they were for adolescents.
Rates of overdose deaths in the overall population increased steadily from 2010 and reached 70,630 in 2019. In 2020, the deaths increased to 91,799 (an increase of 29.48% from 2019) and increased 11.48% in 2021.
The researchers analyzed numbers from the Centers for Disease Control and Prevention WONDER (Wide-Ranging Online Data for Epidemiologic Research) database, which has records of all U.S. deaths for which drug overdose was listed as the underlying cause.
The authors and Dr. Brady report no relevant financial relationships.
The number of overdose deaths in adolescents nearly doubled in 2020 from the year before and increased substantially again in 2021 after nearly a decade of fairly stable rates, according to data published in a JAMA research letter.
Most of the deaths involved fentanyl, the researchers found.
Joseph Friedman, MPH, of the Center for Social Medicine and Humanities at the University of California, Los Angeles, led the study, which analyzed adolescent (14-18 years old) overdose deaths in the United States from 2010 to June 2021 in light of increasing contamination in the supply of illicit drugs.
The researchers found there were 518 deaths among adolescents (2.40 per 100,000 population) in 2010, and the rates remained stable through 2019 with 492 deaths (2.36 per 100,000).
In 2020, however, deaths spiked to 954 (4.57 per 100 000), increasing by 94.3%, compared with 2019. In 2021, they increased another 20%.
The rise in fentanyl-involved deaths was particularly striking. Fentanyl-involved deaths increased from 253 (1.21 per 100,000) in 2019 to 680 (3.26 per 100,000) in 2020. The numbers through June 2021 were annualized for 2021 and calculations predicted 884 deaths (4.23 per 100,000) for the year.
Numbers point to fentanyl potency
In 2021, more than three-fourths (77.14%) of adolescent overdose deaths involved fentanyl, compared with 13.26% for benzodiazepines, 9.77% for methamphetamine, 7.33% for cocaine, 5.76% for prescription opioids, and 2.27% for heroin.
American Indian and Alaska Native adolescents had the highest overdose rate in 2021 (n = 24; 11.79 per 100,000), followed by Latinx adolescents (n = 354; 6.98 per 100,000).
“These adolescent trends fit a wider pattern of increasing racial and ethnic inequalities in overdose that deserve further investigation and intervention efforts,” the authors wrote.
Pandemic’s role unclear
The spikes in adolescent overdoses overlap the COVID-19 pandemic, but Dr. Friedman said in an interview the pandemic “may or may not have been a big factor. “
The authors wrote that drug use had generally been stable among adolescents between 2010 and 2020. The number of 10th graders reporting any illicit drug use was 30.2% in 2010 and 30.4% in 2020.
“So it’s not that more teens are using drugs. It’s just that drug use is becoming more dangerous due to the spread of counterfeit pills containing fentanyls,” Dr. Friedman said.
The authors noted that “the illicit drug supply has increasingly become contaminated with illicitly manufactured fentanyls and other synthetic opioid and benzodiazepine analogues.”
Mr. Friedman said the pandemic may have accelerated the spread of more dangerous forms of drugs as supply chains were disrupted.
Benjamin Brady, DrPH, an assistant professor at the University of Arizona, Tucson, who also has an appointment in the university’s Comprehensive Pain and Addiction Center, said in an interview the numbers that Dr. Friedman and colleagues present represent “worst fears coming true.”
He said he and his colleagues in the field “were anticipating a rise in overdose deaths for the next 5-10 years because of the way the supply-and-demand environment exists in the U.S.”
Dr. Brady explained that restricting access to prescription opioids has had an unfortunate side effect in decreasing access to a safer supply of drugs.
“Without having solutions that would reduce demand at the same rate, supply of the safer form of the drug has been reduced; that has pushed people toward heroin and street drugs and from 2016 on those have been adulterated with fentanyl,” he said.
He said the United States, compared with other developed nations, has been slower to embrace longer-term harm-reduction strategies and to improve access to treatment and care.
COVID likely also has exacerbated the problem in terms of isolation and reduction in quality of life that has adolescents seeking to fill that void with drugs, Dr. Brady said. They may be completely unaware that the drugs they are seeking are commonly cut with counterfeit fentanyl.
“Fentanyl can be up to 50 times stronger than heroin,” he noted. “Even just a little bit of fentanyl dramatically changes the risk profile on an overdose.”
Increasing rates of mental health concerns among adolescents over decades also contribute to drug-seeking trends, Dr. Brady noted.
Overdose increases in the overall population were smaller
In the overall population, the percentage increases were not nearly as large in 2020 and 2021 as they were for adolescents.
Rates of overdose deaths in the overall population increased steadily from 2010 and reached 70,630 in 2019. In 2020, the deaths increased to 91,799 (an increase of 29.48% from 2019) and increased 11.48% in 2021.
The researchers analyzed numbers from the Centers for Disease Control and Prevention WONDER (Wide-Ranging Online Data for Epidemiologic Research) database, which has records of all U.S. deaths for which drug overdose was listed as the underlying cause.
The authors and Dr. Brady report no relevant financial relationships.
FROM JAMA
Trichotillomania: What you should know about this common hair-pulling disorder
Trichotillomania is a chronic psychiatric disorder that causes people to repeatedly pull out their own hair. Not only does it result in alopecia with no other underlying causes but it can have significant psychosocial ramifications and rare, but serious, complications. Though the reported prevalence rates are up to approximately 2%, it’s probable that you’ll come upon a patient suffering with this disorder at your practice, if you haven’t already.
To find out more about the best methods for diagnosing and treating this disorder, we spoke with Jon E. Grant, JD, MD, MPH, a leading trichotillomania researcher and part of the department of psychiatry and behavioral neuroscience at the University of Chicago.
Defining trichotillomania
What were the earliest descriptions of trichotillomania in medical literature?
The first real discussion of it probably goes back to Hippocrates, but from a modern medical perspective, discussion began in the 19th century with reports from the French dermatologist François Hallopeau.
They didn’t really call them disorders then – it was long before the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) – but they described this in young men who kept pulling their hair for unclear reasons. These early case reports don’t provide a lot of psychological perspective, but they seem consistent with what we see now.
What are the diagnostic criteria for trichotillomania?
The current DSM-5 criteria are recurrent pulling out of hair, an inability to stop it, the pulling resulting in some noticeable thinning or hair loss, and that it causes some level of distress or some type of impairment in functioning.
At what age do most people experience an onset of symptoms?
Generally speaking, it’s in early adolescence, post puberty, around 12-15 years of age. Having said that, we do see children as young as 1-2 years who are pulling their hair, and we occasionally see somebody far older who is doing it for the first time, a sort of geriatric onset.
Overlap and differences with other disorders
You’ve written that although trichotillomania is grouped with obsessive-compulsive disorder (OCD) in the DSM-5, the thinking around that has recently shifted. Why is that?
At first, it was noticed that many of these people pulled their hair repetitively in an almost ritualized manner, perhaps every night before bed. That looked like a compulsion of OCD.
When DSM-5 came out in 2013, they grouped it with OCD. Yet people shifted to thinking that it’s kind of a cousin of OCD because it has this compulsive quality but doesn’t really have obsessive thinking that drives it. Many people just pull their hair. They’re not even always aware of it: sometimes yes, sometimes no.
We know that it has some links to OCD. You’ll see more OCD in folks with trichotillomania, but it clearly is not just the same as OCD. One of the biggest pieces of evidence for that is that our first-line treatment for OCD – a selective serotonin reuptake inhibitor antidepressant – does not really help hair pulling.
Having said that, if people are looking for help with trichotillomania, they often are best served by therapists and doctors who have a familiarity with OCD and have kept it on their radar over the past couple of decades.
How does trichotillomania overlap with skin picking disorder, which is another condition that you’ve closely researched?
It does have some overlap with skin picking in the sense that it often seems familial. For example, the mother may pull her hair and child picks their skin.
It also has a fair amount of comorbidity with skin picking. Many people who pull will pick a little bit or did at some point. Many people who pick pulled their hair at some point. It seems closely related to nail biting as well.
Studies have also shown that one of the things that runs in the histories of most families of people with trichotillomania might be substance abuse – alcohol or drug addiction.
All of this has led people to believe that there might be subtypes of trichotillomania: one that’s more like an OCD and one that’s more like an addiction. That’s similar to the debate with other mental health conditions, that there are probably multiple types of depression, multiple types of schizophrenia.
Is there a component of this that could be defined as self-harm?
That’s been its own debate. It doesn’t seem to have the same developmental trajectory that we see with self-harm, or even some of the personality features.
However, there may be a small segment of folks with trichotillomania that might more appropriately fit that category. For example, those with family histories of trauma, higher rates of posttraumatic stress disorder, or borderline personality. But it wouldn’t be the majority.
The problem is, if you look at some of the pediatrician data, they often group picking, pulling, and cutting. I think that’s far too all-inclusive.
A gap in clinician education
Are adolescent patients likely to self-report this behavior, or is it something that physicians need to suss out for themselves?
Clearly, if child psychologists, psychiatrists, or pediatricians see young people with patches of alopecia – eyebrows or eyelashes missing, head hair with spots – in addition to a dermatologic assessment, they should simply ask, “Do you pull your hair?”
But it’s interesting that with the internet, young people are much more likely to disclose and actually come forward and tell their parents that they think they have trichotillomania.
I also hear from a lot of the adolescents that they have to educate their doctors about trichotillomania because so often physicians don’t know much about it and will assume that it’s self-injury or just a symptom of anxiety. It’s a little bit of a flip from what we might have seen 20 years ago.
I’ve seen several patients who’ve said, basically, “I’m tired of no professionals seeming to know about this. I shouldn’t have to be educating my doctors about this.” I tell them that I completely agree. It’s a shame because if a doctor doesn’t know about it, then how can they get the appropriate care?
What are the complications that accompany trichotillomania?
A small percentage, maybe about 10%, will ingest their hair, much like people who bite and swallow their fingernails. The concern there is that because hair is nondigestible, it could create an intestinal plug that could rupture and be potentially life-threatening. That makes it all the more important to ask those who pull their hair what they do with the hair once they pull it.
However, with most people, the real problem is with self-esteem. Young people may not want to socialize, go on dates, or do other things they would normally do because of it. In adults, you may find that they’re far more educated than their job allows but don’t want to go to an interview because they don’t want to have somebody sit there and look at them and notice that perhaps they don’t have any eyebrows, or that they’re wearing a wig. Those psychosocial implications are huge for so many people.
Treatment options
In a 2021 study, you showed that nearly one-quarter of people with trichotillomania do naturally recover from it. What characteristics do they seem to have?
It’s interesting because we see natural recovery across many mental health problems: alcohol addition, gambling, OCD. The question then becomes why is that some people can seemingly just stop doing a behavior? Can we learn from those people?
We did see that those who naturally recovered were less likely to have some other mental health comorbidities. It seems like when you have other things such as skin picking or OCD plus trichotillomania, that it probably speaks to something that perhaps synergistically is keeping it going. But this is just a first study; learning how to harness and understand it is the next step.
What’s the goal of treating trichotillomania?
The desired goal is zero pulling. The realistic goal is more likely significantly reduced pulling that then leads to greater function in life, greater quality-of-life.
One doesn’t have to go from 100 to 0 in order to do that. I always tell people that maybe every now and then, every few months, when something is going on in life, you might find yourself pulling a hair or two. That’s okay. If you’re not pulling every day and it’s significantly reduced, we’ll call that a success. I think that setting reasonable goals at this point is really important.
And what would the treatment pathway look like for most patients?
The standard approach is probably some type of habit-reversal therapy, of which there have been many variants over the years. It involves doing something different with your hand, identifying the triggers that may set you off, and then doing something in response to those triggers that is not pulling and might neutralize whatever that anxious or stressed feeling is. That could be different with each person.
At this point, there is no drug approved by the U.S. Food and Drug Administration for trichotillomania. Our best approaches have included N-acetylcysteine, a glutamate modulator, which we’ve done research in.
That’s kind of a go-to option for people because its side-effect profile is generally innocuous. The data show that it could be beneficial in many people with very few, if any, side effects. That would be one “medication,” although it’s actually an over-the-counter vitamin. But we’re constantly looking for better and better treatments.
Do you have any final advice for clinicians or researchers?
Given how common it is, I don’t think clinicians should just see it as an innocuous little habit that people should be able to stop on their own. Clinicians should educate themselves about trichotillomania and know where the person should get the appropriate care.
From the research perspective, given the fact that we see this in animals of multiple species – that they overgroom – this seems to be deeply ingrained in us as animals. So when it comes to the underlying neuroscience, people should pay more attention because it probably has a lot to do with our understanding of habit and compulsive behaviors. It arguably can cut across a lot of different behaviors.
A version of this article first appeared on Medscape.com.
Trichotillomania is a chronic psychiatric disorder that causes people to repeatedly pull out their own hair. Not only does it result in alopecia with no other underlying causes but it can have significant psychosocial ramifications and rare, but serious, complications. Though the reported prevalence rates are up to approximately 2%, it’s probable that you’ll come upon a patient suffering with this disorder at your practice, if you haven’t already.
To find out more about the best methods for diagnosing and treating this disorder, we spoke with Jon E. Grant, JD, MD, MPH, a leading trichotillomania researcher and part of the department of psychiatry and behavioral neuroscience at the University of Chicago.
Defining trichotillomania
What were the earliest descriptions of trichotillomania in medical literature?
The first real discussion of it probably goes back to Hippocrates, but from a modern medical perspective, discussion began in the 19th century with reports from the French dermatologist François Hallopeau.
They didn’t really call them disorders then – it was long before the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) – but they described this in young men who kept pulling their hair for unclear reasons. These early case reports don’t provide a lot of psychological perspective, but they seem consistent with what we see now.
What are the diagnostic criteria for trichotillomania?
The current DSM-5 criteria are recurrent pulling out of hair, an inability to stop it, the pulling resulting in some noticeable thinning or hair loss, and that it causes some level of distress or some type of impairment in functioning.
At what age do most people experience an onset of symptoms?
Generally speaking, it’s in early adolescence, post puberty, around 12-15 years of age. Having said that, we do see children as young as 1-2 years who are pulling their hair, and we occasionally see somebody far older who is doing it for the first time, a sort of geriatric onset.
Overlap and differences with other disorders
You’ve written that although trichotillomania is grouped with obsessive-compulsive disorder (OCD) in the DSM-5, the thinking around that has recently shifted. Why is that?
At first, it was noticed that many of these people pulled their hair repetitively in an almost ritualized manner, perhaps every night before bed. That looked like a compulsion of OCD.
When DSM-5 came out in 2013, they grouped it with OCD. Yet people shifted to thinking that it’s kind of a cousin of OCD because it has this compulsive quality but doesn’t really have obsessive thinking that drives it. Many people just pull their hair. They’re not even always aware of it: sometimes yes, sometimes no.
We know that it has some links to OCD. You’ll see more OCD in folks with trichotillomania, but it clearly is not just the same as OCD. One of the biggest pieces of evidence for that is that our first-line treatment for OCD – a selective serotonin reuptake inhibitor antidepressant – does not really help hair pulling.
Having said that, if people are looking for help with trichotillomania, they often are best served by therapists and doctors who have a familiarity with OCD and have kept it on their radar over the past couple of decades.
How does trichotillomania overlap with skin picking disorder, which is another condition that you’ve closely researched?
It does have some overlap with skin picking in the sense that it often seems familial. For example, the mother may pull her hair and child picks their skin.
It also has a fair amount of comorbidity with skin picking. Many people who pull will pick a little bit or did at some point. Many people who pick pulled their hair at some point. It seems closely related to nail biting as well.
Studies have also shown that one of the things that runs in the histories of most families of people with trichotillomania might be substance abuse – alcohol or drug addiction.
All of this has led people to believe that there might be subtypes of trichotillomania: one that’s more like an OCD and one that’s more like an addiction. That’s similar to the debate with other mental health conditions, that there are probably multiple types of depression, multiple types of schizophrenia.
Is there a component of this that could be defined as self-harm?
That’s been its own debate. It doesn’t seem to have the same developmental trajectory that we see with self-harm, or even some of the personality features.
However, there may be a small segment of folks with trichotillomania that might more appropriately fit that category. For example, those with family histories of trauma, higher rates of posttraumatic stress disorder, or borderline personality. But it wouldn’t be the majority.
The problem is, if you look at some of the pediatrician data, they often group picking, pulling, and cutting. I think that’s far too all-inclusive.
A gap in clinician education
Are adolescent patients likely to self-report this behavior, or is it something that physicians need to suss out for themselves?
Clearly, if child psychologists, psychiatrists, or pediatricians see young people with patches of alopecia – eyebrows or eyelashes missing, head hair with spots – in addition to a dermatologic assessment, they should simply ask, “Do you pull your hair?”
But it’s interesting that with the internet, young people are much more likely to disclose and actually come forward and tell their parents that they think they have trichotillomania.
I also hear from a lot of the adolescents that they have to educate their doctors about trichotillomania because so often physicians don’t know much about it and will assume that it’s self-injury or just a symptom of anxiety. It’s a little bit of a flip from what we might have seen 20 years ago.
I’ve seen several patients who’ve said, basically, “I’m tired of no professionals seeming to know about this. I shouldn’t have to be educating my doctors about this.” I tell them that I completely agree. It’s a shame because if a doctor doesn’t know about it, then how can they get the appropriate care?
What are the complications that accompany trichotillomania?
A small percentage, maybe about 10%, will ingest their hair, much like people who bite and swallow their fingernails. The concern there is that because hair is nondigestible, it could create an intestinal plug that could rupture and be potentially life-threatening. That makes it all the more important to ask those who pull their hair what they do with the hair once they pull it.
However, with most people, the real problem is with self-esteem. Young people may not want to socialize, go on dates, or do other things they would normally do because of it. In adults, you may find that they’re far more educated than their job allows but don’t want to go to an interview because they don’t want to have somebody sit there and look at them and notice that perhaps they don’t have any eyebrows, or that they’re wearing a wig. Those psychosocial implications are huge for so many people.
Treatment options
In a 2021 study, you showed that nearly one-quarter of people with trichotillomania do naturally recover from it. What characteristics do they seem to have?
It’s interesting because we see natural recovery across many mental health problems: alcohol addition, gambling, OCD. The question then becomes why is that some people can seemingly just stop doing a behavior? Can we learn from those people?
We did see that those who naturally recovered were less likely to have some other mental health comorbidities. It seems like when you have other things such as skin picking or OCD plus trichotillomania, that it probably speaks to something that perhaps synergistically is keeping it going. But this is just a first study; learning how to harness and understand it is the next step.
What’s the goal of treating trichotillomania?
The desired goal is zero pulling. The realistic goal is more likely significantly reduced pulling that then leads to greater function in life, greater quality-of-life.
One doesn’t have to go from 100 to 0 in order to do that. I always tell people that maybe every now and then, every few months, when something is going on in life, you might find yourself pulling a hair or two. That’s okay. If you’re not pulling every day and it’s significantly reduced, we’ll call that a success. I think that setting reasonable goals at this point is really important.
And what would the treatment pathway look like for most patients?
The standard approach is probably some type of habit-reversal therapy, of which there have been many variants over the years. It involves doing something different with your hand, identifying the triggers that may set you off, and then doing something in response to those triggers that is not pulling and might neutralize whatever that anxious or stressed feeling is. That could be different with each person.
At this point, there is no drug approved by the U.S. Food and Drug Administration for trichotillomania. Our best approaches have included N-acetylcysteine, a glutamate modulator, which we’ve done research in.
That’s kind of a go-to option for people because its side-effect profile is generally innocuous. The data show that it could be beneficial in many people with very few, if any, side effects. That would be one “medication,” although it’s actually an over-the-counter vitamin. But we’re constantly looking for better and better treatments.
Do you have any final advice for clinicians or researchers?
Given how common it is, I don’t think clinicians should just see it as an innocuous little habit that people should be able to stop on their own. Clinicians should educate themselves about trichotillomania and know where the person should get the appropriate care.
From the research perspective, given the fact that we see this in animals of multiple species – that they overgroom – this seems to be deeply ingrained in us as animals. So when it comes to the underlying neuroscience, people should pay more attention because it probably has a lot to do with our understanding of habit and compulsive behaviors. It arguably can cut across a lot of different behaviors.
A version of this article first appeared on Medscape.com.
Trichotillomania is a chronic psychiatric disorder that causes people to repeatedly pull out their own hair. Not only does it result in alopecia with no other underlying causes but it can have significant psychosocial ramifications and rare, but serious, complications. Though the reported prevalence rates are up to approximately 2%, it’s probable that you’ll come upon a patient suffering with this disorder at your practice, if you haven’t already.
To find out more about the best methods for diagnosing and treating this disorder, we spoke with Jon E. Grant, JD, MD, MPH, a leading trichotillomania researcher and part of the department of psychiatry and behavioral neuroscience at the University of Chicago.
Defining trichotillomania
What were the earliest descriptions of trichotillomania in medical literature?
The first real discussion of it probably goes back to Hippocrates, but from a modern medical perspective, discussion began in the 19th century with reports from the French dermatologist François Hallopeau.
They didn’t really call them disorders then – it was long before the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) – but they described this in young men who kept pulling their hair for unclear reasons. These early case reports don’t provide a lot of psychological perspective, but they seem consistent with what we see now.
What are the diagnostic criteria for trichotillomania?
The current DSM-5 criteria are recurrent pulling out of hair, an inability to stop it, the pulling resulting in some noticeable thinning or hair loss, and that it causes some level of distress or some type of impairment in functioning.
At what age do most people experience an onset of symptoms?
Generally speaking, it’s in early adolescence, post puberty, around 12-15 years of age. Having said that, we do see children as young as 1-2 years who are pulling their hair, and we occasionally see somebody far older who is doing it for the first time, a sort of geriatric onset.
Overlap and differences with other disorders
You’ve written that although trichotillomania is grouped with obsessive-compulsive disorder (OCD) in the DSM-5, the thinking around that has recently shifted. Why is that?
At first, it was noticed that many of these people pulled their hair repetitively in an almost ritualized manner, perhaps every night before bed. That looked like a compulsion of OCD.
When DSM-5 came out in 2013, they grouped it with OCD. Yet people shifted to thinking that it’s kind of a cousin of OCD because it has this compulsive quality but doesn’t really have obsessive thinking that drives it. Many people just pull their hair. They’re not even always aware of it: sometimes yes, sometimes no.
We know that it has some links to OCD. You’ll see more OCD in folks with trichotillomania, but it clearly is not just the same as OCD. One of the biggest pieces of evidence for that is that our first-line treatment for OCD – a selective serotonin reuptake inhibitor antidepressant – does not really help hair pulling.
Having said that, if people are looking for help with trichotillomania, they often are best served by therapists and doctors who have a familiarity with OCD and have kept it on their radar over the past couple of decades.
How does trichotillomania overlap with skin picking disorder, which is another condition that you’ve closely researched?
It does have some overlap with skin picking in the sense that it often seems familial. For example, the mother may pull her hair and child picks their skin.
It also has a fair amount of comorbidity with skin picking. Many people who pull will pick a little bit or did at some point. Many people who pick pulled their hair at some point. It seems closely related to nail biting as well.
Studies have also shown that one of the things that runs in the histories of most families of people with trichotillomania might be substance abuse – alcohol or drug addiction.
All of this has led people to believe that there might be subtypes of trichotillomania: one that’s more like an OCD and one that’s more like an addiction. That’s similar to the debate with other mental health conditions, that there are probably multiple types of depression, multiple types of schizophrenia.
Is there a component of this that could be defined as self-harm?
That’s been its own debate. It doesn’t seem to have the same developmental trajectory that we see with self-harm, or even some of the personality features.
However, there may be a small segment of folks with trichotillomania that might more appropriately fit that category. For example, those with family histories of trauma, higher rates of posttraumatic stress disorder, or borderline personality. But it wouldn’t be the majority.
The problem is, if you look at some of the pediatrician data, they often group picking, pulling, and cutting. I think that’s far too all-inclusive.
A gap in clinician education
Are adolescent patients likely to self-report this behavior, or is it something that physicians need to suss out for themselves?
Clearly, if child psychologists, psychiatrists, or pediatricians see young people with patches of alopecia – eyebrows or eyelashes missing, head hair with spots – in addition to a dermatologic assessment, they should simply ask, “Do you pull your hair?”
But it’s interesting that with the internet, young people are much more likely to disclose and actually come forward and tell their parents that they think they have trichotillomania.
I also hear from a lot of the adolescents that they have to educate their doctors about trichotillomania because so often physicians don’t know much about it and will assume that it’s self-injury or just a symptom of anxiety. It’s a little bit of a flip from what we might have seen 20 years ago.
I’ve seen several patients who’ve said, basically, “I’m tired of no professionals seeming to know about this. I shouldn’t have to be educating my doctors about this.” I tell them that I completely agree. It’s a shame because if a doctor doesn’t know about it, then how can they get the appropriate care?
What are the complications that accompany trichotillomania?
A small percentage, maybe about 10%, will ingest their hair, much like people who bite and swallow their fingernails. The concern there is that because hair is nondigestible, it could create an intestinal plug that could rupture and be potentially life-threatening. That makes it all the more important to ask those who pull their hair what they do with the hair once they pull it.
However, with most people, the real problem is with self-esteem. Young people may not want to socialize, go on dates, or do other things they would normally do because of it. In adults, you may find that they’re far more educated than their job allows but don’t want to go to an interview because they don’t want to have somebody sit there and look at them and notice that perhaps they don’t have any eyebrows, or that they’re wearing a wig. Those psychosocial implications are huge for so many people.
Treatment options
In a 2021 study, you showed that nearly one-quarter of people with trichotillomania do naturally recover from it. What characteristics do they seem to have?
It’s interesting because we see natural recovery across many mental health problems: alcohol addition, gambling, OCD. The question then becomes why is that some people can seemingly just stop doing a behavior? Can we learn from those people?
We did see that those who naturally recovered were less likely to have some other mental health comorbidities. It seems like when you have other things such as skin picking or OCD plus trichotillomania, that it probably speaks to something that perhaps synergistically is keeping it going. But this is just a first study; learning how to harness and understand it is the next step.
What’s the goal of treating trichotillomania?
The desired goal is zero pulling. The realistic goal is more likely significantly reduced pulling that then leads to greater function in life, greater quality-of-life.
One doesn’t have to go from 100 to 0 in order to do that. I always tell people that maybe every now and then, every few months, when something is going on in life, you might find yourself pulling a hair or two. That’s okay. If you’re not pulling every day and it’s significantly reduced, we’ll call that a success. I think that setting reasonable goals at this point is really important.
And what would the treatment pathway look like for most patients?
The standard approach is probably some type of habit-reversal therapy, of which there have been many variants over the years. It involves doing something different with your hand, identifying the triggers that may set you off, and then doing something in response to those triggers that is not pulling and might neutralize whatever that anxious or stressed feeling is. That could be different with each person.
At this point, there is no drug approved by the U.S. Food and Drug Administration for trichotillomania. Our best approaches have included N-acetylcysteine, a glutamate modulator, which we’ve done research in.
That’s kind of a go-to option for people because its side-effect profile is generally innocuous. The data show that it could be beneficial in many people with very few, if any, side effects. That would be one “medication,” although it’s actually an over-the-counter vitamin. But we’re constantly looking for better and better treatments.
Do you have any final advice for clinicians or researchers?
Given how common it is, I don’t think clinicians should just see it as an innocuous little habit that people should be able to stop on their own. Clinicians should educate themselves about trichotillomania and know where the person should get the appropriate care.
From the research perspective, given the fact that we see this in animals of multiple species – that they overgroom – this seems to be deeply ingrained in us as animals. So when it comes to the underlying neuroscience, people should pay more attention because it probably has a lot to do with our understanding of habit and compulsive behaviors. It arguably can cut across a lot of different behaviors.
A version of this article first appeared on Medscape.com.
Postpartum HCV treatment rare in infected mothers with opioid use disorder
Despite the availability of effective direct-acting antivirals, very few a mothers with opioid use disorder (OUD) and hepatitis C virus (HCV) during pregnancy received follow-up care or treatment for the infection within 6 months of giving birth, a retrospective study of Medicaid maternity patients found.
The study pooled data on 23,780 Medicaid-enrolled pregnant women with OUD who had a live or stillbirth during 2016-2019 and were followed for 6 months after delivery. Among these women – drawn from six states in the Medicaid Outcomes Distributed Research Network – the pooled average probability of HCV testing during pregnancy was 70.3% (95% confidence interval, 61.5%-79.1%). Of these, 30.9% (95% CI, 23.8%-38%) tested positive. At 60 days postpartum, just 3.2% (95% CI, 2.6%-3.8%) had a follow-up visit or treatment for HCV. In a subset of patients followed for 6 months, only 5.9% (95% CI, 4.9%-6.9%) had any HCV follow-up visit or medication within 6 months of delivery.
While HCV screening and diagnosis rates varied across states, postpartum follow-up rates were universally low. The results suggest a need to improve the cascade of postpartum care for HCV and, ultimately perhaps, introduce antenatal HCV treatment, as is currently given safely for HIV, if current clinical research establishes safety, according to Marian P. Jarlenski, PhD, MPH, an associate professor of public health policy and management at the University of Pittsburgh. The study was published in Obstetrics & Gynecology.
HCV infection has risen substantially in people of reproductive age in tandem with an increase in OUDs. HCV is transmitted from an infected mother to her baby in about 6% of cases, according to the Centers for Disease Control and Prevention, which in 2020 expanded its HCV screening recommendations to include all pregnant women. Currently no treatment for HCV during pregnancy has been approved.
In light of those recent recommendations, Dr. Jarlenski said in an interview that her group was “interested in looking at high-risk screened people and estimating what proportion received follow-up care and treatment for HCV. What is the promise of screening? The promise is that you can treat. Otherwise why screen?”
She acknowledged, however, that the postpartum period is a challenging time for a mother to seek health information or care for herself, whether she’s a new parent or has other children in the home. Nevertheless, the low rate of follow-up and treatment was unexpected. “Even the 70% rate of screening was low – we felt it should have been closer to 100% – but the follow-up rate was surprisingly low,” Dr. Jarlenski said.
Mishka Terplan, MD, MPH, medical director of Friends Research Institute in Baltimore, was not surprised at the low follow-up rate. “The cascade of care for hep C is demoralizing,” said Dr. Terplan, who was not involved in the study. “We know that hep C is syndemic with OUD and other opioid crises and we know that screening is effective for identifying hep C and that antiviral medications are now more effective and less toxic than ever before. But despite this, we’re failing pregnant women and their kids at every step along the cascade. We do a better job with initial testing than with the follow-up testing. We do a horrible job with postpartum medication initiation.”
He pointed to the systemic challenges mothers face in getting postpartum HCV care. “They may be transferred to a subspecialist for treatment, and this transfer is compounded by issues of insurance coverage and eligibility.” With the onus on new mothers to submit the paperwork, “the idea that mothers would be able to initiate much less continue postpartum treatment is absurd,” Dr. Terplan said.
He added that the children born to HCV-positive mothers need surveillance as well, but data suggest that the rates of newborn testing are also low. “There’s a preventable public health burden in all of this.”
The obvious way to increase eradicative therapy would be to treat women while they are getting antenatal care. A small phase 1 trial found that all pregnant participants who were HCV positive and given antivirals in their second trimester were safely treated and gave birth to healthy babies.
“If larger trials prove this treatment is safe and effective, then these results should be communicated to care providers and pregnant patients,” Dr. Jarlenski said. Otherwise, the public health potential of universal screening in pregnancy will not be realized.
This research was supported by the National Institute of Drug Abuse and by the Delaware Division of Medicaid and Medical Assistance and the University of Delaware, Center for Community Research & Service. Dr. Jarlenski disclosed no competing interests. One coauthor disclosed grant funding through her institution from Gilead Sciences and Organon unrelated to this work. Dr. Terplan reported no relevant competing interests.
Despite the availability of effective direct-acting antivirals, very few a mothers with opioid use disorder (OUD) and hepatitis C virus (HCV) during pregnancy received follow-up care or treatment for the infection within 6 months of giving birth, a retrospective study of Medicaid maternity patients found.
The study pooled data on 23,780 Medicaid-enrolled pregnant women with OUD who had a live or stillbirth during 2016-2019 and were followed for 6 months after delivery. Among these women – drawn from six states in the Medicaid Outcomes Distributed Research Network – the pooled average probability of HCV testing during pregnancy was 70.3% (95% confidence interval, 61.5%-79.1%). Of these, 30.9% (95% CI, 23.8%-38%) tested positive. At 60 days postpartum, just 3.2% (95% CI, 2.6%-3.8%) had a follow-up visit or treatment for HCV. In a subset of patients followed for 6 months, only 5.9% (95% CI, 4.9%-6.9%) had any HCV follow-up visit or medication within 6 months of delivery.
While HCV screening and diagnosis rates varied across states, postpartum follow-up rates were universally low. The results suggest a need to improve the cascade of postpartum care for HCV and, ultimately perhaps, introduce antenatal HCV treatment, as is currently given safely for HIV, if current clinical research establishes safety, according to Marian P. Jarlenski, PhD, MPH, an associate professor of public health policy and management at the University of Pittsburgh. The study was published in Obstetrics & Gynecology.
HCV infection has risen substantially in people of reproductive age in tandem with an increase in OUDs. HCV is transmitted from an infected mother to her baby in about 6% of cases, according to the Centers for Disease Control and Prevention, which in 2020 expanded its HCV screening recommendations to include all pregnant women. Currently no treatment for HCV during pregnancy has been approved.
In light of those recent recommendations, Dr. Jarlenski said in an interview that her group was “interested in looking at high-risk screened people and estimating what proportion received follow-up care and treatment for HCV. What is the promise of screening? The promise is that you can treat. Otherwise why screen?”
She acknowledged, however, that the postpartum period is a challenging time for a mother to seek health information or care for herself, whether she’s a new parent or has other children in the home. Nevertheless, the low rate of follow-up and treatment was unexpected. “Even the 70% rate of screening was low – we felt it should have been closer to 100% – but the follow-up rate was surprisingly low,” Dr. Jarlenski said.
Mishka Terplan, MD, MPH, medical director of Friends Research Institute in Baltimore, was not surprised at the low follow-up rate. “The cascade of care for hep C is demoralizing,” said Dr. Terplan, who was not involved in the study. “We know that hep C is syndemic with OUD and other opioid crises and we know that screening is effective for identifying hep C and that antiviral medications are now more effective and less toxic than ever before. But despite this, we’re failing pregnant women and their kids at every step along the cascade. We do a better job with initial testing than with the follow-up testing. We do a horrible job with postpartum medication initiation.”
He pointed to the systemic challenges mothers face in getting postpartum HCV care. “They may be transferred to a subspecialist for treatment, and this transfer is compounded by issues of insurance coverage and eligibility.” With the onus on new mothers to submit the paperwork, “the idea that mothers would be able to initiate much less continue postpartum treatment is absurd,” Dr. Terplan said.
He added that the children born to HCV-positive mothers need surveillance as well, but data suggest that the rates of newborn testing are also low. “There’s a preventable public health burden in all of this.”
The obvious way to increase eradicative therapy would be to treat women while they are getting antenatal care. A small phase 1 trial found that all pregnant participants who were HCV positive and given antivirals in their second trimester were safely treated and gave birth to healthy babies.
“If larger trials prove this treatment is safe and effective, then these results should be communicated to care providers and pregnant patients,” Dr. Jarlenski said. Otherwise, the public health potential of universal screening in pregnancy will not be realized.
This research was supported by the National Institute of Drug Abuse and by the Delaware Division of Medicaid and Medical Assistance and the University of Delaware, Center for Community Research & Service. Dr. Jarlenski disclosed no competing interests. One coauthor disclosed grant funding through her institution from Gilead Sciences and Organon unrelated to this work. Dr. Terplan reported no relevant competing interests.
Despite the availability of effective direct-acting antivirals, very few a mothers with opioid use disorder (OUD) and hepatitis C virus (HCV) during pregnancy received follow-up care or treatment for the infection within 6 months of giving birth, a retrospective study of Medicaid maternity patients found.
The study pooled data on 23,780 Medicaid-enrolled pregnant women with OUD who had a live or stillbirth during 2016-2019 and were followed for 6 months after delivery. Among these women – drawn from six states in the Medicaid Outcomes Distributed Research Network – the pooled average probability of HCV testing during pregnancy was 70.3% (95% confidence interval, 61.5%-79.1%). Of these, 30.9% (95% CI, 23.8%-38%) tested positive. At 60 days postpartum, just 3.2% (95% CI, 2.6%-3.8%) had a follow-up visit or treatment for HCV. In a subset of patients followed for 6 months, only 5.9% (95% CI, 4.9%-6.9%) had any HCV follow-up visit or medication within 6 months of delivery.
While HCV screening and diagnosis rates varied across states, postpartum follow-up rates were universally low. The results suggest a need to improve the cascade of postpartum care for HCV and, ultimately perhaps, introduce antenatal HCV treatment, as is currently given safely for HIV, if current clinical research establishes safety, according to Marian P. Jarlenski, PhD, MPH, an associate professor of public health policy and management at the University of Pittsburgh. The study was published in Obstetrics & Gynecology.
HCV infection has risen substantially in people of reproductive age in tandem with an increase in OUDs. HCV is transmitted from an infected mother to her baby in about 6% of cases, according to the Centers for Disease Control and Prevention, which in 2020 expanded its HCV screening recommendations to include all pregnant women. Currently no treatment for HCV during pregnancy has been approved.
In light of those recent recommendations, Dr. Jarlenski said in an interview that her group was “interested in looking at high-risk screened people and estimating what proportion received follow-up care and treatment for HCV. What is the promise of screening? The promise is that you can treat. Otherwise why screen?”
She acknowledged, however, that the postpartum period is a challenging time for a mother to seek health information or care for herself, whether she’s a new parent or has other children in the home. Nevertheless, the low rate of follow-up and treatment was unexpected. “Even the 70% rate of screening was low – we felt it should have been closer to 100% – but the follow-up rate was surprisingly low,” Dr. Jarlenski said.
Mishka Terplan, MD, MPH, medical director of Friends Research Institute in Baltimore, was not surprised at the low follow-up rate. “The cascade of care for hep C is demoralizing,” said Dr. Terplan, who was not involved in the study. “We know that hep C is syndemic with OUD and other opioid crises and we know that screening is effective for identifying hep C and that antiviral medications are now more effective and less toxic than ever before. But despite this, we’re failing pregnant women and their kids at every step along the cascade. We do a better job with initial testing than with the follow-up testing. We do a horrible job with postpartum medication initiation.”
He pointed to the systemic challenges mothers face in getting postpartum HCV care. “They may be transferred to a subspecialist for treatment, and this transfer is compounded by issues of insurance coverage and eligibility.” With the onus on new mothers to submit the paperwork, “the idea that mothers would be able to initiate much less continue postpartum treatment is absurd,” Dr. Terplan said.
He added that the children born to HCV-positive mothers need surveillance as well, but data suggest that the rates of newborn testing are also low. “There’s a preventable public health burden in all of this.”
The obvious way to increase eradicative therapy would be to treat women while they are getting antenatal care. A small phase 1 trial found that all pregnant participants who were HCV positive and given antivirals in their second trimester were safely treated and gave birth to healthy babies.
“If larger trials prove this treatment is safe and effective, then these results should be communicated to care providers and pregnant patients,” Dr. Jarlenski said. Otherwise, the public health potential of universal screening in pregnancy will not be realized.
This research was supported by the National Institute of Drug Abuse and by the Delaware Division of Medicaid and Medical Assistance and the University of Delaware, Center for Community Research & Service. Dr. Jarlenski disclosed no competing interests. One coauthor disclosed grant funding through her institution from Gilead Sciences and Organon unrelated to this work. Dr. Terplan reported no relevant competing interests.
FROM OBSTETRICS & GYNECOLOGY