User login
FDA head calls for investigation into agency’s approval of aducanumab (Aduhelm)
After several weeks of outcry and heated debate over the Food and Drug Administration’s controversial approval of the Alzheimer’s drug aducanumab (Aduhelm), the head of the agency is now calling for a federal investigation into its own approval proceedings.
Janet Woodcock, MD, the FDA’s acting commissioner, sent a letter to the Office of the Inspector General on July 9, she announced in a tweet.
Dr. Woodcock is asking for an investigation into questionable meetings and other interactions between Biogen and FDA staff members prior to the drug’s approval that “may have occurred outside of the formal correspondence process.”
The letter explains that concerns around these issues “could undermine the public’s confidence in the FDA’s decision.” Therefore, an independent investigation is needed to determine whether anything occurred that was “inconsistent with FDA policies and procedures.”
Dr. Woodcock noted that she has “tremendous confidence in the integrity of the staff and leadership of the Center for Drug Evaluation and Research” involved in the review process.
However, “FDA is dedicated to scientific integrity, to reviewing data without bias, and to basing its regulatory decisions on data,” she wrote. “You have my personal commitment that the Agency will fully cooperate should your office undertake a review.”
Dr. Woodcock concluded by urging that a review be conducted as soon as possible, noting that “should such a review result in actionable items, you also have my commitment to addressing these issues.”
A version of this article first appeared on Medscape.com.
After several weeks of outcry and heated debate over the Food and Drug Administration’s controversial approval of the Alzheimer’s drug aducanumab (Aduhelm), the head of the agency is now calling for a federal investigation into its own approval proceedings.
Janet Woodcock, MD, the FDA’s acting commissioner, sent a letter to the Office of the Inspector General on July 9, she announced in a tweet.
Dr. Woodcock is asking for an investigation into questionable meetings and other interactions between Biogen and FDA staff members prior to the drug’s approval that “may have occurred outside of the formal correspondence process.”
The letter explains that concerns around these issues “could undermine the public’s confidence in the FDA’s decision.” Therefore, an independent investigation is needed to determine whether anything occurred that was “inconsistent with FDA policies and procedures.”
Dr. Woodcock noted that she has “tremendous confidence in the integrity of the staff and leadership of the Center for Drug Evaluation and Research” involved in the review process.
However, “FDA is dedicated to scientific integrity, to reviewing data without bias, and to basing its regulatory decisions on data,” she wrote. “You have my personal commitment that the Agency will fully cooperate should your office undertake a review.”
Dr. Woodcock concluded by urging that a review be conducted as soon as possible, noting that “should such a review result in actionable items, you also have my commitment to addressing these issues.”
A version of this article first appeared on Medscape.com.
After several weeks of outcry and heated debate over the Food and Drug Administration’s controversial approval of the Alzheimer’s drug aducanumab (Aduhelm), the head of the agency is now calling for a federal investigation into its own approval proceedings.
Janet Woodcock, MD, the FDA’s acting commissioner, sent a letter to the Office of the Inspector General on July 9, she announced in a tweet.
Dr. Woodcock is asking for an investigation into questionable meetings and other interactions between Biogen and FDA staff members prior to the drug’s approval that “may have occurred outside of the formal correspondence process.”
The letter explains that concerns around these issues “could undermine the public’s confidence in the FDA’s decision.” Therefore, an independent investigation is needed to determine whether anything occurred that was “inconsistent with FDA policies and procedures.”
Dr. Woodcock noted that she has “tremendous confidence in the integrity of the staff and leadership of the Center for Drug Evaluation and Research” involved in the review process.
However, “FDA is dedicated to scientific integrity, to reviewing data without bias, and to basing its regulatory decisions on data,” she wrote. “You have my personal commitment that the Agency will fully cooperate should your office undertake a review.”
Dr. Woodcock concluded by urging that a review be conducted as soon as possible, noting that “should such a review result in actionable items, you also have my commitment to addressing these issues.”
A version of this article first appeared on Medscape.com.
FDA updates label for controversial Alzheimer’s drug aducanumab (Aduhelm)
– the group studied in the clinical trials.
The FDA approved aducanumab in early June amid significant controversy and disregarding the recommendation by its own advisory panel not to approve the drug. The original prescribing information implied that the drug – which is administered intravenously and costs around $56,000 a year – could be used for treatment of any patient with Alzheimer’s disease.
The updated label now states that aducanumab should be initiated only in patients with mild cognitive impairment (MCI) or mild dementia stage of disease – the population in which treatment was initiated in the clinical trials leading to approval of the anti-amyloid drug.
The FDA granted accelerated approval of the drug based on data from clinical trials showing a reduction in amyloid beta plaques observed in patients with MCI or mild dementia stage of disease.
“Continued approval for the indication may be contingent upon verification of clinical benefit in confirmatory trial(s),” the label states. It emphasizes that there are no safety or effectiveness data on starting aducanumab treatment at earlier or later stages of the disease than were studied.
“Based on our ongoing conversations with prescribing physicians, FDA, and patient advocates, we submitted this label update with the goal to further clarify the patient population that was studied across the three Aduhelm clinical trials that supported approval,” Alfred Sandrock Jr., MD, PhD, Biogen’s head of research and development, said in a statement announcing the label update.
“We are committed to continue to listen to the community’s needs as clinical practice adapts to this important, first-in-class treatment option,” said Dr. Sandrock.
A version of this article first appeared on Medscape.com.
– the group studied in the clinical trials.
The FDA approved aducanumab in early June amid significant controversy and disregarding the recommendation by its own advisory panel not to approve the drug. The original prescribing information implied that the drug – which is administered intravenously and costs around $56,000 a year – could be used for treatment of any patient with Alzheimer’s disease.
The updated label now states that aducanumab should be initiated only in patients with mild cognitive impairment (MCI) or mild dementia stage of disease – the population in which treatment was initiated in the clinical trials leading to approval of the anti-amyloid drug.
The FDA granted accelerated approval of the drug based on data from clinical trials showing a reduction in amyloid beta plaques observed in patients with MCI or mild dementia stage of disease.
“Continued approval for the indication may be contingent upon verification of clinical benefit in confirmatory trial(s),” the label states. It emphasizes that there are no safety or effectiveness data on starting aducanumab treatment at earlier or later stages of the disease than were studied.
“Based on our ongoing conversations with prescribing physicians, FDA, and patient advocates, we submitted this label update with the goal to further clarify the patient population that was studied across the three Aduhelm clinical trials that supported approval,” Alfred Sandrock Jr., MD, PhD, Biogen’s head of research and development, said in a statement announcing the label update.
“We are committed to continue to listen to the community’s needs as clinical practice adapts to this important, first-in-class treatment option,” said Dr. Sandrock.
A version of this article first appeared on Medscape.com.
– the group studied in the clinical trials.
The FDA approved aducanumab in early June amid significant controversy and disregarding the recommendation by its own advisory panel not to approve the drug. The original prescribing information implied that the drug – which is administered intravenously and costs around $56,000 a year – could be used for treatment of any patient with Alzheimer’s disease.
The updated label now states that aducanumab should be initiated only in patients with mild cognitive impairment (MCI) or mild dementia stage of disease – the population in which treatment was initiated in the clinical trials leading to approval of the anti-amyloid drug.
The FDA granted accelerated approval of the drug based on data from clinical trials showing a reduction in amyloid beta plaques observed in patients with MCI or mild dementia stage of disease.
“Continued approval for the indication may be contingent upon verification of clinical benefit in confirmatory trial(s),” the label states. It emphasizes that there are no safety or effectiveness data on starting aducanumab treatment at earlier or later stages of the disease than were studied.
“Based on our ongoing conversations with prescribing physicians, FDA, and patient advocates, we submitted this label update with the goal to further clarify the patient population that was studied across the three Aduhelm clinical trials that supported approval,” Alfred Sandrock Jr., MD, PhD, Biogen’s head of research and development, said in a statement announcing the label update.
“We are committed to continue to listen to the community’s needs as clinical practice adapts to this important, first-in-class treatment option,” said Dr. Sandrock.
A version of this article first appeared on Medscape.com.
Chronic stress and genetics can raise the risk of Alzheimer’s disease
The researchers also proposed a mechanism to account for how genetic factors may affect HPA axis reactivity and lead to inflammation, which is a core component of neurodegeneration.
“Chronic stress can impact the way immune cells in the brain function and increase inflammation. Genetic variants within that stress response can further affect the function of immune cells,” lead author Ayeisha Milligan Armstrong, a PhD candidate at Curtin Health Innovation Research Institute in Perth, Australia, said in an interview.
The findings were published online June 22 in Biological Reviews).
Research has found that long-term stress during early and mid-life is increasingly associated with cognitive decline and neurodegeneration. There is already evidence to suggest that chronic stress is a risk factor for the “sporadic” or late-onset subtype of Alzheimer’s disease.
A cascade of events
Stress activates the HPA, which in turn regulates bodily levels of cortisol, a glucocorticoid stress hormone. Increased levels of cortisol are frequently observed in patients with Alzheimer’s disease and “make a major contribution to the disease process,” the authors wrote. For example, the hippocampus – a part of the brain involved in processing and forming memories – has numerous glucocorticoid receptors and is “therefore particularly sensitive to the effects of glucocorticoids.” However, the molecular mechanisms involved remain poorly understood.
“There is an intimate interplay between exposure to chronic stress and pathways influencing the body’s reaction to such stress,” senior author David Groth, PhD, said in a statement. Dr. Groth is an associate professor at Curtin University in Perth, Australia.
There is variation between individuals with regard to how sensitive they are to stress and glucocorticoid responses. Environmental factors such as stress are thought to be at least partly responsible, as are genetic factors such as genetic polymorphisms and epigenetics. “Genetic variations within these pathways can influence the way the brain’s immune system behaves, leading to a dysfunctional response. In the brain, this leads to a chronic disruption of normal brain processes, increasing the risk of subsequent neurodegeneration and ultimately dementia,” Dr. Groth said.
The researchers suggested that these variations may prime the immune cells of the brain, the microglia, to cause inflammation in the brain. Normally, microglia are involved in monitoring the brain tissue for and responding to damage and infections to keep the brain healthy. However, in an inflammatory state, the microglia instead contribute to a “more neurotoxic environment through the production of proinflammatory cytokines, altered synaptic pruning, and the reduced production of protective neurotrophic factors,” the authors wrote. Microglia may also promote the accumulation of amyloid beta and tau protein, which damage the brain tissue and can cause neurodegeneration. There are different groups of microglia in the brain, each of which may respond differently to genetic and environmental stressors.
“Genome-wide association studies have found that of the genes identified as being associated with Alzheimer’s disease, 60.5% are expressed in microglia,” the authors noted.
To connect the roles of chronic stress and brain inflammation in Alzheimer’s disease, the researchers proposed a “two-hit” hypothesis: Early or mid-life exposure to stress primes the microglia to enter an inflammatory state in response to a secondary stimulus later in life.
Pay attention to stress
For clinicians, this paper highlights the importance of managing stress in patients and their families.
“Clinicians need to be attuned to the effects of stress on patients and their caregivers, and how that [stress] can affect their morbidity and mortality,” Cynthia Munro, PhD, associate professor of psychiatry and behavioral sciences at Johns Hopkins University, Baltimore, said in an interview. She added that attention must be paid to modifiable risk factors such as poor sleep and diet.
Although managing stress is important, that doesn’t mean that everyone who’s experienced chronic stress will develop Alzheimer’s disease. “Chronic stress can alter the HPA axis but it doesn’t necessarily do so in everyone. A cascade of events needs to occur,” said Dr. Munro. “People should always try to reduce the effects of stress to the extent that they can. Stress can lead to a whole host of negative health outcomes, not just Alzheimer’s disease.”
Next steps
Moving forward, the researchers plan to further investigate the molecular mechanisms responsible for the role of stress in Alzheimer’s disease and how genetic variants affect neurodegeneration, Ms. Armstrong said. Ultimately, understanding how stress and genetics contribute to Alzheimer’s disease may lead to the identification of possible therapeutic targets.
Ms. Armstrong and Dr. Munro declared no relevant financial relationships. The study was independently funded.
The researchers also proposed a mechanism to account for how genetic factors may affect HPA axis reactivity and lead to inflammation, which is a core component of neurodegeneration.
“Chronic stress can impact the way immune cells in the brain function and increase inflammation. Genetic variants within that stress response can further affect the function of immune cells,” lead author Ayeisha Milligan Armstrong, a PhD candidate at Curtin Health Innovation Research Institute in Perth, Australia, said in an interview.
The findings were published online June 22 in Biological Reviews).
Research has found that long-term stress during early and mid-life is increasingly associated with cognitive decline and neurodegeneration. There is already evidence to suggest that chronic stress is a risk factor for the “sporadic” or late-onset subtype of Alzheimer’s disease.
A cascade of events
Stress activates the HPA, which in turn regulates bodily levels of cortisol, a glucocorticoid stress hormone. Increased levels of cortisol are frequently observed in patients with Alzheimer’s disease and “make a major contribution to the disease process,” the authors wrote. For example, the hippocampus – a part of the brain involved in processing and forming memories – has numerous glucocorticoid receptors and is “therefore particularly sensitive to the effects of glucocorticoids.” However, the molecular mechanisms involved remain poorly understood.
“There is an intimate interplay between exposure to chronic stress and pathways influencing the body’s reaction to such stress,” senior author David Groth, PhD, said in a statement. Dr. Groth is an associate professor at Curtin University in Perth, Australia.
There is variation between individuals with regard to how sensitive they are to stress and glucocorticoid responses. Environmental factors such as stress are thought to be at least partly responsible, as are genetic factors such as genetic polymorphisms and epigenetics. “Genetic variations within these pathways can influence the way the brain’s immune system behaves, leading to a dysfunctional response. In the brain, this leads to a chronic disruption of normal brain processes, increasing the risk of subsequent neurodegeneration and ultimately dementia,” Dr. Groth said.
The researchers suggested that these variations may prime the immune cells of the brain, the microglia, to cause inflammation in the brain. Normally, microglia are involved in monitoring the brain tissue for and responding to damage and infections to keep the brain healthy. However, in an inflammatory state, the microglia instead contribute to a “more neurotoxic environment through the production of proinflammatory cytokines, altered synaptic pruning, and the reduced production of protective neurotrophic factors,” the authors wrote. Microglia may also promote the accumulation of amyloid beta and tau protein, which damage the brain tissue and can cause neurodegeneration. There are different groups of microglia in the brain, each of which may respond differently to genetic and environmental stressors.
“Genome-wide association studies have found that of the genes identified as being associated with Alzheimer’s disease, 60.5% are expressed in microglia,” the authors noted.
To connect the roles of chronic stress and brain inflammation in Alzheimer’s disease, the researchers proposed a “two-hit” hypothesis: Early or mid-life exposure to stress primes the microglia to enter an inflammatory state in response to a secondary stimulus later in life.
Pay attention to stress
For clinicians, this paper highlights the importance of managing stress in patients and their families.
“Clinicians need to be attuned to the effects of stress on patients and their caregivers, and how that [stress] can affect their morbidity and mortality,” Cynthia Munro, PhD, associate professor of psychiatry and behavioral sciences at Johns Hopkins University, Baltimore, said in an interview. She added that attention must be paid to modifiable risk factors such as poor sleep and diet.
Although managing stress is important, that doesn’t mean that everyone who’s experienced chronic stress will develop Alzheimer’s disease. “Chronic stress can alter the HPA axis but it doesn’t necessarily do so in everyone. A cascade of events needs to occur,” said Dr. Munro. “People should always try to reduce the effects of stress to the extent that they can. Stress can lead to a whole host of negative health outcomes, not just Alzheimer’s disease.”
Next steps
Moving forward, the researchers plan to further investigate the molecular mechanisms responsible for the role of stress in Alzheimer’s disease and how genetic variants affect neurodegeneration, Ms. Armstrong said. Ultimately, understanding how stress and genetics contribute to Alzheimer’s disease may lead to the identification of possible therapeutic targets.
Ms. Armstrong and Dr. Munro declared no relevant financial relationships. The study was independently funded.
The researchers also proposed a mechanism to account for how genetic factors may affect HPA axis reactivity and lead to inflammation, which is a core component of neurodegeneration.
“Chronic stress can impact the way immune cells in the brain function and increase inflammation. Genetic variants within that stress response can further affect the function of immune cells,” lead author Ayeisha Milligan Armstrong, a PhD candidate at Curtin Health Innovation Research Institute in Perth, Australia, said in an interview.
The findings were published online June 22 in Biological Reviews).
Research has found that long-term stress during early and mid-life is increasingly associated with cognitive decline and neurodegeneration. There is already evidence to suggest that chronic stress is a risk factor for the “sporadic” or late-onset subtype of Alzheimer’s disease.
A cascade of events
Stress activates the HPA, which in turn regulates bodily levels of cortisol, a glucocorticoid stress hormone. Increased levels of cortisol are frequently observed in patients with Alzheimer’s disease and “make a major contribution to the disease process,” the authors wrote. For example, the hippocampus – a part of the brain involved in processing and forming memories – has numerous glucocorticoid receptors and is “therefore particularly sensitive to the effects of glucocorticoids.” However, the molecular mechanisms involved remain poorly understood.
“There is an intimate interplay between exposure to chronic stress and pathways influencing the body’s reaction to such stress,” senior author David Groth, PhD, said in a statement. Dr. Groth is an associate professor at Curtin University in Perth, Australia.
There is variation between individuals with regard to how sensitive they are to stress and glucocorticoid responses. Environmental factors such as stress are thought to be at least partly responsible, as are genetic factors such as genetic polymorphisms and epigenetics. “Genetic variations within these pathways can influence the way the brain’s immune system behaves, leading to a dysfunctional response. In the brain, this leads to a chronic disruption of normal brain processes, increasing the risk of subsequent neurodegeneration and ultimately dementia,” Dr. Groth said.
The researchers suggested that these variations may prime the immune cells of the brain, the microglia, to cause inflammation in the brain. Normally, microglia are involved in monitoring the brain tissue for and responding to damage and infections to keep the brain healthy. However, in an inflammatory state, the microglia instead contribute to a “more neurotoxic environment through the production of proinflammatory cytokines, altered synaptic pruning, and the reduced production of protective neurotrophic factors,” the authors wrote. Microglia may also promote the accumulation of amyloid beta and tau protein, which damage the brain tissue and can cause neurodegeneration. There are different groups of microglia in the brain, each of which may respond differently to genetic and environmental stressors.
“Genome-wide association studies have found that of the genes identified as being associated with Alzheimer’s disease, 60.5% are expressed in microglia,” the authors noted.
To connect the roles of chronic stress and brain inflammation in Alzheimer’s disease, the researchers proposed a “two-hit” hypothesis: Early or mid-life exposure to stress primes the microglia to enter an inflammatory state in response to a secondary stimulus later in life.
Pay attention to stress
For clinicians, this paper highlights the importance of managing stress in patients and their families.
“Clinicians need to be attuned to the effects of stress on patients and their caregivers, and how that [stress] can affect their morbidity and mortality,” Cynthia Munro, PhD, associate professor of psychiatry and behavioral sciences at Johns Hopkins University, Baltimore, said in an interview. She added that attention must be paid to modifiable risk factors such as poor sleep and diet.
Although managing stress is important, that doesn’t mean that everyone who’s experienced chronic stress will develop Alzheimer’s disease. “Chronic stress can alter the HPA axis but it doesn’t necessarily do so in everyone. A cascade of events needs to occur,” said Dr. Munro. “People should always try to reduce the effects of stress to the extent that they can. Stress can lead to a whole host of negative health outcomes, not just Alzheimer’s disease.”
Next steps
Moving forward, the researchers plan to further investigate the molecular mechanisms responsible for the role of stress in Alzheimer’s disease and how genetic variants affect neurodegeneration, Ms. Armstrong said. Ultimately, understanding how stress and genetics contribute to Alzheimer’s disease may lead to the identification of possible therapeutic targets.
Ms. Armstrong and Dr. Munro declared no relevant financial relationships. The study was independently funded.
FROM BIOLOGICAL REVIEWS
Hearing loss tied to decline in physical functioning
published online in JAMA Network Open.
Hearing loss is associated with slower gait and, in particular, worse balance, the data suggest.
“Because hearing impairment is amenable to prevention and management, it potentially serves as a target for interventions to slow physical decline with aging,” the researchers said.
To examine how hearing impairment relates to physical function in older adults, Pablo Martinez-Amezcua, MD, PhD, MHS, a researcher in the department of epidemiology at Johns Hopkins University, Baltimore, and colleagues analyzed data from the ongoing Atherosclerosis Risk in Communities (ARIC) study.
ARIC initially enrolled more than 15,000 adults in Maryland, Minnesota, Mississippi, and North Carolina between 1987 and 1989. In the present study, the researchers focused on data from 2,956 participants who attended a study visit between 2016 and 2017, during which researchers assessed their hearing using pure tone audiometry.
Hearing-study participants had an average age of 79 years, about 58% were women, and 80% were White. Approximately 33% of the participants had normal hearing, 40% had mild hearing impairment, 23% had moderate hearing impairment, and 4% had severe hearing impairment.
Participants had also undergone assessment of physical functioning at study visits between 2011 and 2019, including a fast-paced 2-minute walk test to measure their walking endurance. Another assessment, the Short Physical Performance Battery (SPPB), tests balance, gait speed, and chair stands (seated participants stand up and sit back down five times as quickly as possible while their arms are crossed).
Dr. Martinez-Amezcua and colleagues found that severe hearing impairment was associated with a lower average SPPB score compared with normal hearing in a regression analysis. Specifically, compared with those with normal hearing, participants with severe hearing impairment were more likely to have low scores on the SPPB (odds ratio, 2.72), balance (OR, 2.72), and gait speed (OR, 2.16).
However, hearing impairment was not significantly associated with the chair stand test results. The researchers note that chair stands may rely more on strength, whereas balance and gait speed may rely more on coordination and movement.
The team also found that people with worse hearing tended to walk a shorter distance during the 2-minute walk test. Compared with participants with normal hearing, participants with moderate hearing impairment walked 2.81 meters less and those with severe hearing impairment walked 5.31 meters less on average, after adjustment for variables including age, sex, and health conditions.
Participants with hearing impairment also tended to have faster declines in physical function over time.
Various mechanisms could explain associations between hearing and physical function, the authors said. For example, an underlying condition such as cardiovascular disease might affect both hearing and physical function. Damage to the inner ear could affect vestibular and auditory systems at the same time. In addition, hearing impairment may relate to cognition, depression, or social isolation, which could influence physical activity.
“Age-related hearing loss is traditionally seen as a barrier for communication,” Dr. Martinez-Amezcua told this news organization. “In the past decade, research on the consequences of hearing loss has identified it as a risk factor for cognitive decline and dementia. Our findings contribute to our understanding of other negative outcomes associated with hearing loss.”
Randomized clinical trials are the best way to assess whether addressing hearing loss might improve physical function, Dr. Martinez-Amezcua said. “Currently there is one clinical trial (ACHIEVE) that will, among other outcomes, study the impact of hearing aids on cognitive and physical function,” he said.
Although interventions may not reverse hearing loss, hearing rehabilitation strategies, including hearing aids and cochlear implants, may help, he added. Educating caregivers and changing a person’s environment can also reduce the effects hearing loss has on daily life, Dr. Martinez-Amezcua said.
“We rely so much in our sense of vision for activities of daily living that we tend to underestimate how important hearing is, and the consequences of hearing loss go beyond having trouble communicating with someone,” he said.
This study and prior research “raise the intriguing idea that hearing may provide essential information to the neural circuits underpinning movement in our environment and that correction for hearing loss may help promote physical well-being,” Willa D. Brenowitz, PhD, MPH, and Margaret I. Wallhagen, PhD, GNP-BC, both at the University of California, San Francisco, wrote in an accompanying commentary. “While this hypothesis is appealing and warrants further investigation, there are multiple other potential explanations of such an association, including potential sources of bias that may affect observational studies such as this one.”
Beyond treating hearing loss, interventions such as physical therapy or tai chi may benefit patients, they suggested.
Because many changes occur during older age, it can be difficult to understand which factor is influencing another, Dr. Brenowitz said in an interview. There are potentially relevant mechanisms through which hearing could affect cognition and physical functioning. Still another explanation could be that some people are “aging in a faster way” than others, Dr. Brenowitz said.
Dr. Martinez-Amezcua and a coauthor disclosed receiving sponsorship from the Cochlear Center for Hearing and Public Health. Another author, Frank R. Lin, MD, PhD, directs the research center, which is partly funded by a philanthropic gift from Cochlear to the Johns Hopkins Bloomberg School of Public Health. Dr. Lin also disclosed personal fees from Frequency Therapeutics and Caption Call. One author serves on a scientific advisory board for Shoebox and Good Machine Studio.
Dr. Wallhagen has served on the board of trustees of the Hearing Loss Association of America and is a member of the board of the Hearing Loss Association of America–California. Dr. Wallhagen also received funding for a pilot project on the impact of hearing loss on communication in the context of chronic serious illness from the National Palliative Care Research Center outside the submitted work.
A version of this article first appeared on Medscape.com.
published online in JAMA Network Open.
Hearing loss is associated with slower gait and, in particular, worse balance, the data suggest.
“Because hearing impairment is amenable to prevention and management, it potentially serves as a target for interventions to slow physical decline with aging,” the researchers said.
To examine how hearing impairment relates to physical function in older adults, Pablo Martinez-Amezcua, MD, PhD, MHS, a researcher in the department of epidemiology at Johns Hopkins University, Baltimore, and colleagues analyzed data from the ongoing Atherosclerosis Risk in Communities (ARIC) study.
ARIC initially enrolled more than 15,000 adults in Maryland, Minnesota, Mississippi, and North Carolina between 1987 and 1989. In the present study, the researchers focused on data from 2,956 participants who attended a study visit between 2016 and 2017, during which researchers assessed their hearing using pure tone audiometry.
Hearing-study participants had an average age of 79 years, about 58% were women, and 80% were White. Approximately 33% of the participants had normal hearing, 40% had mild hearing impairment, 23% had moderate hearing impairment, and 4% had severe hearing impairment.
Participants had also undergone assessment of physical functioning at study visits between 2011 and 2019, including a fast-paced 2-minute walk test to measure their walking endurance. Another assessment, the Short Physical Performance Battery (SPPB), tests balance, gait speed, and chair stands (seated participants stand up and sit back down five times as quickly as possible while their arms are crossed).
Dr. Martinez-Amezcua and colleagues found that severe hearing impairment was associated with a lower average SPPB score compared with normal hearing in a regression analysis. Specifically, compared with those with normal hearing, participants with severe hearing impairment were more likely to have low scores on the SPPB (odds ratio, 2.72), balance (OR, 2.72), and gait speed (OR, 2.16).
However, hearing impairment was not significantly associated with the chair stand test results. The researchers note that chair stands may rely more on strength, whereas balance and gait speed may rely more on coordination and movement.
The team also found that people with worse hearing tended to walk a shorter distance during the 2-minute walk test. Compared with participants with normal hearing, participants with moderate hearing impairment walked 2.81 meters less and those with severe hearing impairment walked 5.31 meters less on average, after adjustment for variables including age, sex, and health conditions.
Participants with hearing impairment also tended to have faster declines in physical function over time.
Various mechanisms could explain associations between hearing and physical function, the authors said. For example, an underlying condition such as cardiovascular disease might affect both hearing and physical function. Damage to the inner ear could affect vestibular and auditory systems at the same time. In addition, hearing impairment may relate to cognition, depression, or social isolation, which could influence physical activity.
“Age-related hearing loss is traditionally seen as a barrier for communication,” Dr. Martinez-Amezcua told this news organization. “In the past decade, research on the consequences of hearing loss has identified it as a risk factor for cognitive decline and dementia. Our findings contribute to our understanding of other negative outcomes associated with hearing loss.”
Randomized clinical trials are the best way to assess whether addressing hearing loss might improve physical function, Dr. Martinez-Amezcua said. “Currently there is one clinical trial (ACHIEVE) that will, among other outcomes, study the impact of hearing aids on cognitive and physical function,” he said.
Although interventions may not reverse hearing loss, hearing rehabilitation strategies, including hearing aids and cochlear implants, may help, he added. Educating caregivers and changing a person’s environment can also reduce the effects hearing loss has on daily life, Dr. Martinez-Amezcua said.
“We rely so much in our sense of vision for activities of daily living that we tend to underestimate how important hearing is, and the consequences of hearing loss go beyond having trouble communicating with someone,” he said.
This study and prior research “raise the intriguing idea that hearing may provide essential information to the neural circuits underpinning movement in our environment and that correction for hearing loss may help promote physical well-being,” Willa D. Brenowitz, PhD, MPH, and Margaret I. Wallhagen, PhD, GNP-BC, both at the University of California, San Francisco, wrote in an accompanying commentary. “While this hypothesis is appealing and warrants further investigation, there are multiple other potential explanations of such an association, including potential sources of bias that may affect observational studies such as this one.”
Beyond treating hearing loss, interventions such as physical therapy or tai chi may benefit patients, they suggested.
Because many changes occur during older age, it can be difficult to understand which factor is influencing another, Dr. Brenowitz said in an interview. There are potentially relevant mechanisms through which hearing could affect cognition and physical functioning. Still another explanation could be that some people are “aging in a faster way” than others, Dr. Brenowitz said.
Dr. Martinez-Amezcua and a coauthor disclosed receiving sponsorship from the Cochlear Center for Hearing and Public Health. Another author, Frank R. Lin, MD, PhD, directs the research center, which is partly funded by a philanthropic gift from Cochlear to the Johns Hopkins Bloomberg School of Public Health. Dr. Lin also disclosed personal fees from Frequency Therapeutics and Caption Call. One author serves on a scientific advisory board for Shoebox and Good Machine Studio.
Dr. Wallhagen has served on the board of trustees of the Hearing Loss Association of America and is a member of the board of the Hearing Loss Association of America–California. Dr. Wallhagen also received funding for a pilot project on the impact of hearing loss on communication in the context of chronic serious illness from the National Palliative Care Research Center outside the submitted work.
A version of this article first appeared on Medscape.com.
published online in JAMA Network Open.
Hearing loss is associated with slower gait and, in particular, worse balance, the data suggest.
“Because hearing impairment is amenable to prevention and management, it potentially serves as a target for interventions to slow physical decline with aging,” the researchers said.
To examine how hearing impairment relates to physical function in older adults, Pablo Martinez-Amezcua, MD, PhD, MHS, a researcher in the department of epidemiology at Johns Hopkins University, Baltimore, and colleagues analyzed data from the ongoing Atherosclerosis Risk in Communities (ARIC) study.
ARIC initially enrolled more than 15,000 adults in Maryland, Minnesota, Mississippi, and North Carolina between 1987 and 1989. In the present study, the researchers focused on data from 2,956 participants who attended a study visit between 2016 and 2017, during which researchers assessed their hearing using pure tone audiometry.
Hearing-study participants had an average age of 79 years, about 58% were women, and 80% were White. Approximately 33% of the participants had normal hearing, 40% had mild hearing impairment, 23% had moderate hearing impairment, and 4% had severe hearing impairment.
Participants had also undergone assessment of physical functioning at study visits between 2011 and 2019, including a fast-paced 2-minute walk test to measure their walking endurance. Another assessment, the Short Physical Performance Battery (SPPB), tests balance, gait speed, and chair stands (seated participants stand up and sit back down five times as quickly as possible while their arms are crossed).
Dr. Martinez-Amezcua and colleagues found that severe hearing impairment was associated with a lower average SPPB score compared with normal hearing in a regression analysis. Specifically, compared with those with normal hearing, participants with severe hearing impairment were more likely to have low scores on the SPPB (odds ratio, 2.72), balance (OR, 2.72), and gait speed (OR, 2.16).
However, hearing impairment was not significantly associated with the chair stand test results. The researchers note that chair stands may rely more on strength, whereas balance and gait speed may rely more on coordination and movement.
The team also found that people with worse hearing tended to walk a shorter distance during the 2-minute walk test. Compared with participants with normal hearing, participants with moderate hearing impairment walked 2.81 meters less and those with severe hearing impairment walked 5.31 meters less on average, after adjustment for variables including age, sex, and health conditions.
Participants with hearing impairment also tended to have faster declines in physical function over time.
Various mechanisms could explain associations between hearing and physical function, the authors said. For example, an underlying condition such as cardiovascular disease might affect both hearing and physical function. Damage to the inner ear could affect vestibular and auditory systems at the same time. In addition, hearing impairment may relate to cognition, depression, or social isolation, which could influence physical activity.
“Age-related hearing loss is traditionally seen as a barrier for communication,” Dr. Martinez-Amezcua told this news organization. “In the past decade, research on the consequences of hearing loss has identified it as a risk factor for cognitive decline and dementia. Our findings contribute to our understanding of other negative outcomes associated with hearing loss.”
Randomized clinical trials are the best way to assess whether addressing hearing loss might improve physical function, Dr. Martinez-Amezcua said. “Currently there is one clinical trial (ACHIEVE) that will, among other outcomes, study the impact of hearing aids on cognitive and physical function,” he said.
Although interventions may not reverse hearing loss, hearing rehabilitation strategies, including hearing aids and cochlear implants, may help, he added. Educating caregivers and changing a person’s environment can also reduce the effects hearing loss has on daily life, Dr. Martinez-Amezcua said.
“We rely so much in our sense of vision for activities of daily living that we tend to underestimate how important hearing is, and the consequences of hearing loss go beyond having trouble communicating with someone,” he said.
This study and prior research “raise the intriguing idea that hearing may provide essential information to the neural circuits underpinning movement in our environment and that correction for hearing loss may help promote physical well-being,” Willa D. Brenowitz, PhD, MPH, and Margaret I. Wallhagen, PhD, GNP-BC, both at the University of California, San Francisco, wrote in an accompanying commentary. “While this hypothesis is appealing and warrants further investigation, there are multiple other potential explanations of such an association, including potential sources of bias that may affect observational studies such as this one.”
Beyond treating hearing loss, interventions such as physical therapy or tai chi may benefit patients, they suggested.
Because many changes occur during older age, it can be difficult to understand which factor is influencing another, Dr. Brenowitz said in an interview. There are potentially relevant mechanisms through which hearing could affect cognition and physical functioning. Still another explanation could be that some people are “aging in a faster way” than others, Dr. Brenowitz said.
Dr. Martinez-Amezcua and a coauthor disclosed receiving sponsorship from the Cochlear Center for Hearing and Public Health. Another author, Frank R. Lin, MD, PhD, directs the research center, which is partly funded by a philanthropic gift from Cochlear to the Johns Hopkins Bloomberg School of Public Health. Dr. Lin also disclosed personal fees from Frequency Therapeutics and Caption Call. One author serves on a scientific advisory board for Shoebox and Good Machine Studio.
Dr. Wallhagen has served on the board of trustees of the Hearing Loss Association of America and is a member of the board of the Hearing Loss Association of America–California. Dr. Wallhagen also received funding for a pilot project on the impact of hearing loss on communication in the context of chronic serious illness from the National Palliative Care Research Center outside the submitted work.
A version of this article first appeared on Medscape.com.
FDA fast-tracks lecanemab for Alzheimer’s disease
Lecanemab (formerly BAN2401) is a humanized monoclonal antibody that selectively binds to large, soluble aggregated Abeta protofibrils. The antibody was developed following the discovery of a mutation in amyloid precursor protein that leads to a form of Alzheimer’s disease that is marked by particularly high levels of Abeta protofibrils.
“As such, lecanemab may have the potential to have an effect on disease pathology and to slow down the progression of the disease,” Eisai and Biogen said in a joint news release.
The breakthrough therapy designation for lecanemab is based on results of a randomized, double-blind, phase 2b proof-of-concept study published April 17 in Alzheimer’s Research & Therapy.
The study enrolled 856 patients with mild cognitive impairment (MCI) due to Alzheimer’s disease and mild Alzheimer’s disease with confirmed presence of amyloid pathology.
At the highest doses, treatment with lecanemab led to a reduction in brain amyloid accompanied by a consistent reduction of clinical decline across several clinical and biomarker endpoints.
Phase 3 testing underway
In March, Eisai and Biogen completed enrollment in a phase 3 study designed to confirm the safety and efficacy of lecanemab in patients with symptomatic early Alzheimer’s disease.
The CLARITY AD study includes 1,795 patients with early Alzheimer’s disease, and initial results are expected by the end of September 2022. The core study will compare lecanemab against placebo on the change from baseline in the Clinical Dementia Rating-Sum of Boxes (CDR-SB) at 18 months. The study will also evaluate the long-term safety and tolerability of lecanemab in the extension phase and whether the long-term effects of lecanemab, as measured by the CDR-SB at the end of the core study, are maintained over time.
Additionally, the phase 3 AHEAD 3-45 clinical study is currently exploring lecanemab in adults with preclinical Alzheimer’s disease (clinically normal but with intermediate or elevated brain amyloid).
On June 7, the FDA – amid significant controversy – approved aducanumab (Aduhelm), the first anti-amyloid agent for the treatment Alzheimer’s disease, disregarding the recommendation by its own advisory panel not to approve the drug. Three members of the FDA’s Peripheral and Central Nervous System Drugs Advisory Committee subsequently resigned in protest following the agency’s approval of aducanumab.
In addition, the high-profile consumer advocacy group Public Citizen sent a letter to the secretary of the U.S. Department of Health & Human Services demanding the removal of three FDA officials, including acting FDA Commissioner Janet Woodcock, MD.
A version of this article first appeared on Medscape.com.
Lecanemab (formerly BAN2401) is a humanized monoclonal antibody that selectively binds to large, soluble aggregated Abeta protofibrils. The antibody was developed following the discovery of a mutation in amyloid precursor protein that leads to a form of Alzheimer’s disease that is marked by particularly high levels of Abeta protofibrils.
“As such, lecanemab may have the potential to have an effect on disease pathology and to slow down the progression of the disease,” Eisai and Biogen said in a joint news release.
The breakthrough therapy designation for lecanemab is based on results of a randomized, double-blind, phase 2b proof-of-concept study published April 17 in Alzheimer’s Research & Therapy.
The study enrolled 856 patients with mild cognitive impairment (MCI) due to Alzheimer’s disease and mild Alzheimer’s disease with confirmed presence of amyloid pathology.
At the highest doses, treatment with lecanemab led to a reduction in brain amyloid accompanied by a consistent reduction of clinical decline across several clinical and biomarker endpoints.
Phase 3 testing underway
In March, Eisai and Biogen completed enrollment in a phase 3 study designed to confirm the safety and efficacy of lecanemab in patients with symptomatic early Alzheimer’s disease.
The CLARITY AD study includes 1,795 patients with early Alzheimer’s disease, and initial results are expected by the end of September 2022. The core study will compare lecanemab against placebo on the change from baseline in the Clinical Dementia Rating-Sum of Boxes (CDR-SB) at 18 months. The study will also evaluate the long-term safety and tolerability of lecanemab in the extension phase and whether the long-term effects of lecanemab, as measured by the CDR-SB at the end of the core study, are maintained over time.
Additionally, the phase 3 AHEAD 3-45 clinical study is currently exploring lecanemab in adults with preclinical Alzheimer’s disease (clinically normal but with intermediate or elevated brain amyloid).
On June 7, the FDA – amid significant controversy – approved aducanumab (Aduhelm), the first anti-amyloid agent for the treatment Alzheimer’s disease, disregarding the recommendation by its own advisory panel not to approve the drug. Three members of the FDA’s Peripheral and Central Nervous System Drugs Advisory Committee subsequently resigned in protest following the agency’s approval of aducanumab.
In addition, the high-profile consumer advocacy group Public Citizen sent a letter to the secretary of the U.S. Department of Health & Human Services demanding the removal of three FDA officials, including acting FDA Commissioner Janet Woodcock, MD.
A version of this article first appeared on Medscape.com.
Lecanemab (formerly BAN2401) is a humanized monoclonal antibody that selectively binds to large, soluble aggregated Abeta protofibrils. The antibody was developed following the discovery of a mutation in amyloid precursor protein that leads to a form of Alzheimer’s disease that is marked by particularly high levels of Abeta protofibrils.
“As such, lecanemab may have the potential to have an effect on disease pathology and to slow down the progression of the disease,” Eisai and Biogen said in a joint news release.
The breakthrough therapy designation for lecanemab is based on results of a randomized, double-blind, phase 2b proof-of-concept study published April 17 in Alzheimer’s Research & Therapy.
The study enrolled 856 patients with mild cognitive impairment (MCI) due to Alzheimer’s disease and mild Alzheimer’s disease with confirmed presence of amyloid pathology.
At the highest doses, treatment with lecanemab led to a reduction in brain amyloid accompanied by a consistent reduction of clinical decline across several clinical and biomarker endpoints.
Phase 3 testing underway
In March, Eisai and Biogen completed enrollment in a phase 3 study designed to confirm the safety and efficacy of lecanemab in patients with symptomatic early Alzheimer’s disease.
The CLARITY AD study includes 1,795 patients with early Alzheimer’s disease, and initial results are expected by the end of September 2022. The core study will compare lecanemab against placebo on the change from baseline in the Clinical Dementia Rating-Sum of Boxes (CDR-SB) at 18 months. The study will also evaluate the long-term safety and tolerability of lecanemab in the extension phase and whether the long-term effects of lecanemab, as measured by the CDR-SB at the end of the core study, are maintained over time.
Additionally, the phase 3 AHEAD 3-45 clinical study is currently exploring lecanemab in adults with preclinical Alzheimer’s disease (clinically normal but with intermediate or elevated brain amyloid).
On June 7, the FDA – amid significant controversy – approved aducanumab (Aduhelm), the first anti-amyloid agent for the treatment Alzheimer’s disease, disregarding the recommendation by its own advisory panel not to approve the drug. Three members of the FDA’s Peripheral and Central Nervous System Drugs Advisory Committee subsequently resigned in protest following the agency’s approval of aducanumab.
In addition, the high-profile consumer advocacy group Public Citizen sent a letter to the secretary of the U.S. Department of Health & Human Services demanding the removal of three FDA officials, including acting FDA Commissioner Janet Woodcock, MD.
A version of this article first appeared on Medscape.com.
FDA leader explains rationale leading to controversial Alzheimer’s drug approval
, including the release of several internal documents.
In a letter sent to members of the FDA’s Center for Drug Evaluation Research (CDER), CDER Director Patrizia Cavazzoni, MD, noted that in view of the “fierce public debate” that erupted immediately following the drug’s approval, she felt compelled to explain how the agency came to its decision.
Also publicly released today on the FDA’s updated aducanumab landing page was “the first set of review memos,” for the drug.
“We’re releasing these documents with the intent of informing public discourse – providing interested parties with the opportunity to explore the data that helped shape our decision to grant accelerated approval,” Dr. Cavazzoni wrote. “The rest of the approval package will be released over the next several days,” she added.
Immediate backlash
The FDA’s June 7 approval of aducanumab was met with instant backlash. In November 2020, the agency’s Peripheral and Central Nervous System Drugs Advisory Committee voted nearly unanimously to not vote in favor of approval because of a lack of evidence proving its efficacy.
Since the drug was approved, three of the advisory committee’s members resigned in protest. In addition, the high-profile consumer advocacy group Public Citizen sent a letter to the secretary of the U.S. Department of Health & Human Services demanding the removal of three FDA officials, including acting FDA Commissioner Janet Woodcock, MD.
In its letter, the group noted that the FDA’s decision “showed a stunning disregard for science, eviscerated the agency’s standards for approving new drugs, and ranks as one of the most irresponsible and egregious decisions in the history of the agency.”
Even the Alzheimer’s Association, which was a staunch supporter of the drug throughout its development process and applauded its approval, expressed outrage over its more than $56,000-a-year cost to patients and called the price “simply unacceptable” in a statement.
In the June 23 letter, the CDER director noted, “this was one of the most complex applications in recent history” and admitted that deliberations were lengthy and difficult.
“It’s also not surprising, in fact it was to be expected, that there would be different viewpoints about the data, including dissenting opinions about the approval decision,” Dr. Cavazzoni wrote.
However, this “is what scientific debate is all about, and while difficult at times, it should be celebrated,” she added. “Please know that every opinion was heard, and the approval is a direct reflection of this open and robust scientific and regulatory debate.”
Accelerated approval pathway
Documents newly posted to the FDA’s aducanumab landing page include CDER’s Office of Neurology’s Summary Review Memorandum, which includes details on the basis for the approval; the Concurrence Memorandum from the director of CDER’s Office of New Drugs; and the Concurrence Memorandum from Dr. Cavazzoni.
“The remaining scientific review documents in the Aduhelm action package are not yet available but will be made available to the public as soon as the internal process of review and redaction is complete,” the FDA noted on its site.
In the document FDA’s Decision to Approve New Treatment for Alzheimer’s Disease, Dr. Cavazzoni noted that the “highly complex” data included in the submission package for the drug “left residual uncertainties regarding clinical benefit.”
However, after listening to the patient community and reviewing all the data, the FDA chose to use the Accelerated Approval pathway, deciding that the potential benefit to patients outweighed the drug’s risks.
Of two phase 3 trials, only one met its primary endpoint. However, in all trials, including earlier studies, “Aduhelm consistently and very convincingly reduced the level of amyloid plaques in the brain in a dose- and time-dependent fashion,” Dr. Cavazzoni wrote.
“It is expected that the reduction in amyloid plaque will result in a reduction in clinical decline,” she added.
Dr. Cavazzoni noted that although the Advisory Committee did not agree that clinical benefit from one trial meeting its primary endpoint was enough for approval, “the option of Accelerated Approval was not discussed” at that time.
This type of approval “is based on a surrogate or intermediate clinical endpoint, in this case reduction of amyloid plaque in the brain” and requires post-approval studies to verify clinical benefit.
Dr. Cavazzoni added that the drug could still be removed from the market if its confirmatory trial does not verify this type of benefit.
A version of this article first appeared on Medscape.com.
, including the release of several internal documents.
In a letter sent to members of the FDA’s Center for Drug Evaluation Research (CDER), CDER Director Patrizia Cavazzoni, MD, noted that in view of the “fierce public debate” that erupted immediately following the drug’s approval, she felt compelled to explain how the agency came to its decision.
Also publicly released today on the FDA’s updated aducanumab landing page was “the first set of review memos,” for the drug.
“We’re releasing these documents with the intent of informing public discourse – providing interested parties with the opportunity to explore the data that helped shape our decision to grant accelerated approval,” Dr. Cavazzoni wrote. “The rest of the approval package will be released over the next several days,” she added.
Immediate backlash
The FDA’s June 7 approval of aducanumab was met with instant backlash. In November 2020, the agency’s Peripheral and Central Nervous System Drugs Advisory Committee voted nearly unanimously to not vote in favor of approval because of a lack of evidence proving its efficacy.
Since the drug was approved, three of the advisory committee’s members resigned in protest. In addition, the high-profile consumer advocacy group Public Citizen sent a letter to the secretary of the U.S. Department of Health & Human Services demanding the removal of three FDA officials, including acting FDA Commissioner Janet Woodcock, MD.
In its letter, the group noted that the FDA’s decision “showed a stunning disregard for science, eviscerated the agency’s standards for approving new drugs, and ranks as one of the most irresponsible and egregious decisions in the history of the agency.”
Even the Alzheimer’s Association, which was a staunch supporter of the drug throughout its development process and applauded its approval, expressed outrage over its more than $56,000-a-year cost to patients and called the price “simply unacceptable” in a statement.
In the June 23 letter, the CDER director noted, “this was one of the most complex applications in recent history” and admitted that deliberations were lengthy and difficult.
“It’s also not surprising, in fact it was to be expected, that there would be different viewpoints about the data, including dissenting opinions about the approval decision,” Dr. Cavazzoni wrote.
However, this “is what scientific debate is all about, and while difficult at times, it should be celebrated,” she added. “Please know that every opinion was heard, and the approval is a direct reflection of this open and robust scientific and regulatory debate.”
Accelerated approval pathway
Documents newly posted to the FDA’s aducanumab landing page include CDER’s Office of Neurology’s Summary Review Memorandum, which includes details on the basis for the approval; the Concurrence Memorandum from the director of CDER’s Office of New Drugs; and the Concurrence Memorandum from Dr. Cavazzoni.
“The remaining scientific review documents in the Aduhelm action package are not yet available but will be made available to the public as soon as the internal process of review and redaction is complete,” the FDA noted on its site.
In the document FDA’s Decision to Approve New Treatment for Alzheimer’s Disease, Dr. Cavazzoni noted that the “highly complex” data included in the submission package for the drug “left residual uncertainties regarding clinical benefit.”
However, after listening to the patient community and reviewing all the data, the FDA chose to use the Accelerated Approval pathway, deciding that the potential benefit to patients outweighed the drug’s risks.
Of two phase 3 trials, only one met its primary endpoint. However, in all trials, including earlier studies, “Aduhelm consistently and very convincingly reduced the level of amyloid plaques in the brain in a dose- and time-dependent fashion,” Dr. Cavazzoni wrote.
“It is expected that the reduction in amyloid plaque will result in a reduction in clinical decline,” she added.
Dr. Cavazzoni noted that although the Advisory Committee did not agree that clinical benefit from one trial meeting its primary endpoint was enough for approval, “the option of Accelerated Approval was not discussed” at that time.
This type of approval “is based on a surrogate or intermediate clinical endpoint, in this case reduction of amyloid plaque in the brain” and requires post-approval studies to verify clinical benefit.
Dr. Cavazzoni added that the drug could still be removed from the market if its confirmatory trial does not verify this type of benefit.
A version of this article first appeared on Medscape.com.
, including the release of several internal documents.
In a letter sent to members of the FDA’s Center for Drug Evaluation Research (CDER), CDER Director Patrizia Cavazzoni, MD, noted that in view of the “fierce public debate” that erupted immediately following the drug’s approval, she felt compelled to explain how the agency came to its decision.
Also publicly released today on the FDA’s updated aducanumab landing page was “the first set of review memos,” for the drug.
“We’re releasing these documents with the intent of informing public discourse – providing interested parties with the opportunity to explore the data that helped shape our decision to grant accelerated approval,” Dr. Cavazzoni wrote. “The rest of the approval package will be released over the next several days,” she added.
Immediate backlash
The FDA’s June 7 approval of aducanumab was met with instant backlash. In November 2020, the agency’s Peripheral and Central Nervous System Drugs Advisory Committee voted nearly unanimously to not vote in favor of approval because of a lack of evidence proving its efficacy.
Since the drug was approved, three of the advisory committee’s members resigned in protest. In addition, the high-profile consumer advocacy group Public Citizen sent a letter to the secretary of the U.S. Department of Health & Human Services demanding the removal of three FDA officials, including acting FDA Commissioner Janet Woodcock, MD.
In its letter, the group noted that the FDA’s decision “showed a stunning disregard for science, eviscerated the agency’s standards for approving new drugs, and ranks as one of the most irresponsible and egregious decisions in the history of the agency.”
Even the Alzheimer’s Association, which was a staunch supporter of the drug throughout its development process and applauded its approval, expressed outrage over its more than $56,000-a-year cost to patients and called the price “simply unacceptable” in a statement.
In the June 23 letter, the CDER director noted, “this was one of the most complex applications in recent history” and admitted that deliberations were lengthy and difficult.
“It’s also not surprising, in fact it was to be expected, that there would be different viewpoints about the data, including dissenting opinions about the approval decision,” Dr. Cavazzoni wrote.
However, this “is what scientific debate is all about, and while difficult at times, it should be celebrated,” she added. “Please know that every opinion was heard, and the approval is a direct reflection of this open and robust scientific and regulatory debate.”
Accelerated approval pathway
Documents newly posted to the FDA’s aducanumab landing page include CDER’s Office of Neurology’s Summary Review Memorandum, which includes details on the basis for the approval; the Concurrence Memorandum from the director of CDER’s Office of New Drugs; and the Concurrence Memorandum from Dr. Cavazzoni.
“The remaining scientific review documents in the Aduhelm action package are not yet available but will be made available to the public as soon as the internal process of review and redaction is complete,” the FDA noted on its site.
In the document FDA’s Decision to Approve New Treatment for Alzheimer’s Disease, Dr. Cavazzoni noted that the “highly complex” data included in the submission package for the drug “left residual uncertainties regarding clinical benefit.”
However, after listening to the patient community and reviewing all the data, the FDA chose to use the Accelerated Approval pathway, deciding that the potential benefit to patients outweighed the drug’s risks.
Of two phase 3 trials, only one met its primary endpoint. However, in all trials, including earlier studies, “Aduhelm consistently and very convincingly reduced the level of amyloid plaques in the brain in a dose- and time-dependent fashion,” Dr. Cavazzoni wrote.
“It is expected that the reduction in amyloid plaque will result in a reduction in clinical decline,” she added.
Dr. Cavazzoni noted that although the Advisory Committee did not agree that clinical benefit from one trial meeting its primary endpoint was enough for approval, “the option of Accelerated Approval was not discussed” at that time.
This type of approval “is based on a surrogate or intermediate clinical endpoint, in this case reduction of amyloid plaque in the brain” and requires post-approval studies to verify clinical benefit.
Dr. Cavazzoni added that the drug could still be removed from the market if its confirmatory trial does not verify this type of benefit.
A version of this article first appeared on Medscape.com.
No overall statin effect seen on dementia, cognition in ASPREE analysis
Statin therapy likely didn’t lead to dementia or even mild cognitive impairment (MCI) in older patients taking the drugs for cardiovascular (CV) primary prevention in a post hoc analysis of a trial that required normal cognitive ability for entry.
Nor did statins, whether lipophilic or hydrophilic, appear to influence changes in cognition or affect separate domains of mental performance, such as memory, language ability, or executive function, over the trial’s follow-up, which averaged almost 5 years.
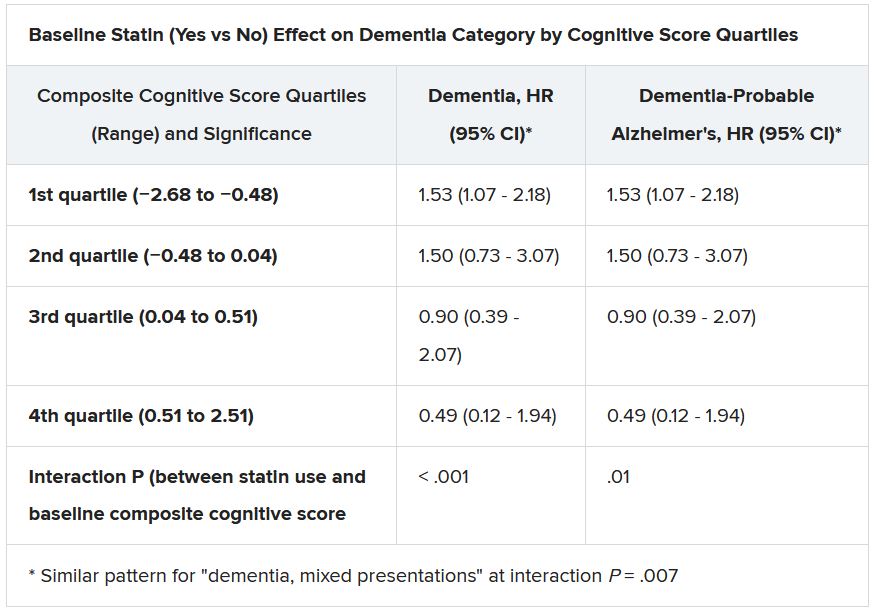
Although such findings aren’t novel – they are consistent with observations from a number of earlier studies – the new analysis included a possible signal for a statin association with new-onset dementia in a subgroup of more than 18,000 patients. Researchers attribute the retrospective finding, from a trial not designed to explore the issue, to confounding or chance.
Still, the adjusted risk for dementia seemed to go up by a third among statin users who at baseline placed in the lowest quartile for cognitive function, based on a composite test score, in the ASPREE trial, a test of primary-prevention low-dose aspirin in patients 65 or older. The better the baseline cognitive score by quartile, the lower the risk for dementia ( interaction P < .001).
The bottom-quartile association of statins with dementia was driven by new diagnoses of Alzheimer’s disease, as opposed to the study’s other “mixed presentation” dementia subtype, wrote the authors of analysis, published June 21, 2021, in the Journal of the American College of Cardiology), led by Zhen Zhou, PhD, Menzies Institute for Medical Research, University of Tasmania, Hobart, Australia.
“I wouldn’t overinterpret that,” said senior author Mark R. Nelson, MBBS, PhD, of the same institution. Indeed, it should be “reassuring” for physicians prescribing statins to older patients that there was no overall statin effect on cognition or new-onset dementia, he said in an interview.
“This is a post hoc analysis within a dataset, although a very-high-quality dataset, it must be said.” The patients were prospectively followed for a range of cognition domains, and the results were adjudicated, Dr. Nelson observed. Although the question of statins and dementia risk is thought to be largely settled, the analysis “was just too tempting not to do.”
On the basis of the current analysis and the bulk of preceding evidence, “lipid lowering in the short term does not appear to result in improvement or deterioration of cognition irrespective of baseline LDL cholesterol levels and medication used,” Christie M. Ballantyne, MD, and Vijay Nambi, MD, PhD, both from Baylor College of Medicine, Houston, wrote in an accompanying editorial.
The current study “provides additional information that the lipo- or hydrophilicity of the statin does not affect changes in cognition. However, the potential increased risk for Alzheimer’s disease, especially among patients with baseline cognitive impairment, requires further investigation.”
The current analysis is reassuring that the likelihood of such statin effects on cognition “is vanishingly small,” Neil J. Stone MD, Northwestern University, Chicago, said in an interview. In fact, its primary finding of no such association “best summarizes what we know in 2021 about statin therapy” after exploration of the issue in a number of prospective trials and systematic reviews, said Dr. Stone, who was not a coauthor on the report.
The observed interaction between statin use and baseline neurocognitive ability “is hypothesis raising at best. It should be explored in randomized, controlled trials that can look at this question in an unbiased manner,” he agreed.
If patients believe or suspect that a statin is causing symptoms that suggest cognitive dysfunction, “what they really need to do is to stop it for 3 weeks and check out other causes. And in rechallenging, the guidelines say, if they think that it’s causing a memory problem that occurs anecdotally, then they can be given another statin, usually, which doesn’t cause it.”
ASPREE compared daily low-dose aspirin with placebo in a community-based older population numbering about 19,000 in Australia and the United States. Patients were initially without known CV disease, dementia, or physical disabilities. It did not randomize patients by statin therapy.
Of note, entry to the trial required a score of at least 78 on the Modified Mini-Mental State Examination (3MS), corresponding to normal cognition.
Aspirin showed no significant benefit for disability-free survival, an endpoint that included death and dementia, or CV events over a median of 4.7 years. It was associated with slightly more cases of major hemorrhage, as previously reported.
A subsequent ASPREE analysis suggested that the aspirin had no effect on risks of mild cognitive impairment, cognitive decline, or dementia.
Of the 18,846 patients in the current post hoc analysis, the average age of the patients was 74 years, and 56.4% were women; 31.3% were taking statins at baseline. The incidence of dementia per 1,000 person-years for those taking statins in comparison with those not taking statins was 6.91 and 6.48, respectively. Any cognitive changes were tracked by the 3MS and three other validated tests in different domains of cognition, with results contributing to the composite score.
The corresponding incidence of dementia considered probable Alzheimer’s disease was 2.97 and 2.65 for those receiving versus not receiving statins, respectively. The incidence of dementia with mixed presentation was 3.94 and 3.84, respectively.
There were no significant differences in risk for dementia overall or for either dementia subtype in multivariate analyses. Adjustments included demographics, CV lifestyle risk factors, family medical history, including dementia, ASPREE randomization group, and individual scores on the four tests of cognition.
Results for development of MCI mirrored those for dementia, as did results stratified for baseline lipids and for use of lipophilic statins, such as atorvastatin or simvastatin versus hydrophilic statins, including pravastatin and rosuvastatin.
Significant interactions were observed between composite cognitive scores and statin therapy at baseline; as scores increased, indicating better cognitive performance, the risks for dementia and its subtypes went down. Statins were associated with incident dementia at the lowest cognitive performance quartile.
That association is probably a function of the cohort’s advanced age, Dr. Nelson said. “If you get into old age, and you’ve got high cognitive scores, you’ve probably got protective factors. That’s how I would interpret that.”
Dr. Ballantyne and Dr. Nambi also emphasized the difficulties of controlling for potential biases even with extensive covariate adjustments. The statin dosages at which patients were treated were not part of the analysis, “and achieved LDL [cholesterol levels over the study period were not known,” they wrote.
“Furthermore, patients who were treated with statins were more likely to have diabetes, hypertension, chronic kidney disease, and obesity, all of which are known to increase risk for cognitive decline, and, as might have been predicted, statin users therefore had significantly lower scores for global cognition and episodic memory.”
Dr. Nelson pointed to an ongoing prospective atorvastatin trial that includes dementia in its primary endpoint and should be “the definitive study.” STAREE (Statin Therapy for Reducing Events in the Elderly) is running throughout Australia with a projected enrollment of 18,000 and primary completion by the end of 2022. “We’ve already enrolled 8,000 patients.”
Less far along is the PREVENTABLE (Pragmatic Evaluation of Events and Benefits of Lipid-Lowering in Older Adults) trial, based in the United States and also randomizing to atorvastatin or placebo, that will have an estimated 20,000 older patients and completion in 5 years. The primary endpoint is new dementia or persistent disability.
Both trials “are powered to enable firm conclusions concerning any statin effects,” said Dr. Ballantyne and Dr. Nambi. “In the meantime, practicing clinicians can have confidence and share with their patients that short-term lipid-lowering therapy in older patients, including with statins, is unlikely to have a major impact on cognition.”
ASPREE was supported by grants from the U.S. National Institute on Aging and the National Cancer Institute and the National Health and Medical Research Council of Australia, by Monash University, and by the Victorian Cancer Agency. Dr. Nelson reported receiving honoraria from Sanofi and Amgen; support from Bayer for ASPREE; and grant support for STAREE. Disclosures for the other authors are in the report. Dr. Ballantyne disclosed grant and research support from Abbott Diagnostic, Akcea, Amgen, Esperion, Ionis, Novartis, Regeneron, and Roche Diagnostics; and consulting for Abbott Diagnostics, Althera, Amarin, Amgen, Arrowhead, AstraZeneca, Corvidia, Denka Seiken, Esperion, Genentech, Gilead, Matinas BioPharma, New Amsterdam, Novartis, Novo Nordisk, Pfizer, Regeneron, Roche Diagnostics, and Sanofi-Synthelabo. Dr. Nambi is a coinvestigator on a provisional patent along with Baylor College of Medicine and Roche on the use of biomarkers to predict heart failure, and a site principal investigator for studies sponsored by Amgen and Merck. Dr. Stone had no disclosures.
A version of this article first appeared on Medscape.com.
Statin therapy likely didn’t lead to dementia or even mild cognitive impairment (MCI) in older patients taking the drugs for cardiovascular (CV) primary prevention in a post hoc analysis of a trial that required normal cognitive ability for entry.
Nor did statins, whether lipophilic or hydrophilic, appear to influence changes in cognition or affect separate domains of mental performance, such as memory, language ability, or executive function, over the trial’s follow-up, which averaged almost 5 years.
Although such findings aren’t novel – they are consistent with observations from a number of earlier studies – the new analysis included a possible signal for a statin association with new-onset dementia in a subgroup of more than 18,000 patients. Researchers attribute the retrospective finding, from a trial not designed to explore the issue, to confounding or chance.
Still, the adjusted risk for dementia seemed to go up by a third among statin users who at baseline placed in the lowest quartile for cognitive function, based on a composite test score, in the ASPREE trial, a test of primary-prevention low-dose aspirin in patients 65 or older. The better the baseline cognitive score by quartile, the lower the risk for dementia ( interaction P < .001).
The bottom-quartile association of statins with dementia was driven by new diagnoses of Alzheimer’s disease, as opposed to the study’s other “mixed presentation” dementia subtype, wrote the authors of analysis, published June 21, 2021, in the Journal of the American College of Cardiology), led by Zhen Zhou, PhD, Menzies Institute for Medical Research, University of Tasmania, Hobart, Australia.
“I wouldn’t overinterpret that,” said senior author Mark R. Nelson, MBBS, PhD, of the same institution. Indeed, it should be “reassuring” for physicians prescribing statins to older patients that there was no overall statin effect on cognition or new-onset dementia, he said in an interview.
“This is a post hoc analysis within a dataset, although a very-high-quality dataset, it must be said.” The patients were prospectively followed for a range of cognition domains, and the results were adjudicated, Dr. Nelson observed. Although the question of statins and dementia risk is thought to be largely settled, the analysis “was just too tempting not to do.”
On the basis of the current analysis and the bulk of preceding evidence, “lipid lowering in the short term does not appear to result in improvement or deterioration of cognition irrespective of baseline LDL cholesterol levels and medication used,” Christie M. Ballantyne, MD, and Vijay Nambi, MD, PhD, both from Baylor College of Medicine, Houston, wrote in an accompanying editorial.
The current study “provides additional information that the lipo- or hydrophilicity of the statin does not affect changes in cognition. However, the potential increased risk for Alzheimer’s disease, especially among patients with baseline cognitive impairment, requires further investigation.”
The current analysis is reassuring that the likelihood of such statin effects on cognition “is vanishingly small,” Neil J. Stone MD, Northwestern University, Chicago, said in an interview. In fact, its primary finding of no such association “best summarizes what we know in 2021 about statin therapy” after exploration of the issue in a number of prospective trials and systematic reviews, said Dr. Stone, who was not a coauthor on the report.
The observed interaction between statin use and baseline neurocognitive ability “is hypothesis raising at best. It should be explored in randomized, controlled trials that can look at this question in an unbiased manner,” he agreed.
If patients believe or suspect that a statin is causing symptoms that suggest cognitive dysfunction, “what they really need to do is to stop it for 3 weeks and check out other causes. And in rechallenging, the guidelines say, if they think that it’s causing a memory problem that occurs anecdotally, then they can be given another statin, usually, which doesn’t cause it.”
ASPREE compared daily low-dose aspirin with placebo in a community-based older population numbering about 19,000 in Australia and the United States. Patients were initially without known CV disease, dementia, or physical disabilities. It did not randomize patients by statin therapy.
Of note, entry to the trial required a score of at least 78 on the Modified Mini-Mental State Examination (3MS), corresponding to normal cognition.
Aspirin showed no significant benefit for disability-free survival, an endpoint that included death and dementia, or CV events over a median of 4.7 years. It was associated with slightly more cases of major hemorrhage, as previously reported.
A subsequent ASPREE analysis suggested that the aspirin had no effect on risks of mild cognitive impairment, cognitive decline, or dementia.
Of the 18,846 patients in the current post hoc analysis, the average age of the patients was 74 years, and 56.4% were women; 31.3% were taking statins at baseline. The incidence of dementia per 1,000 person-years for those taking statins in comparison with those not taking statins was 6.91 and 6.48, respectively. Any cognitive changes were tracked by the 3MS and three other validated tests in different domains of cognition, with results contributing to the composite score.
The corresponding incidence of dementia considered probable Alzheimer’s disease was 2.97 and 2.65 for those receiving versus not receiving statins, respectively. The incidence of dementia with mixed presentation was 3.94 and 3.84, respectively.
There were no significant differences in risk for dementia overall or for either dementia subtype in multivariate analyses. Adjustments included demographics, CV lifestyle risk factors, family medical history, including dementia, ASPREE randomization group, and individual scores on the four tests of cognition.
Results for development of MCI mirrored those for dementia, as did results stratified for baseline lipids and for use of lipophilic statins, such as atorvastatin or simvastatin versus hydrophilic statins, including pravastatin and rosuvastatin.
Significant interactions were observed between composite cognitive scores and statin therapy at baseline; as scores increased, indicating better cognitive performance, the risks for dementia and its subtypes went down. Statins were associated with incident dementia at the lowest cognitive performance quartile.
That association is probably a function of the cohort’s advanced age, Dr. Nelson said. “If you get into old age, and you’ve got high cognitive scores, you’ve probably got protective factors. That’s how I would interpret that.”
Dr. Ballantyne and Dr. Nambi also emphasized the difficulties of controlling for potential biases even with extensive covariate adjustments. The statin dosages at which patients were treated were not part of the analysis, “and achieved LDL [cholesterol levels over the study period were not known,” they wrote.
“Furthermore, patients who were treated with statins were more likely to have diabetes, hypertension, chronic kidney disease, and obesity, all of which are known to increase risk for cognitive decline, and, as might have been predicted, statin users therefore had significantly lower scores for global cognition and episodic memory.”
Dr. Nelson pointed to an ongoing prospective atorvastatin trial that includes dementia in its primary endpoint and should be “the definitive study.” STAREE (Statin Therapy for Reducing Events in the Elderly) is running throughout Australia with a projected enrollment of 18,000 and primary completion by the end of 2022. “We’ve already enrolled 8,000 patients.”
Less far along is the PREVENTABLE (Pragmatic Evaluation of Events and Benefits of Lipid-Lowering in Older Adults) trial, based in the United States and also randomizing to atorvastatin or placebo, that will have an estimated 20,000 older patients and completion in 5 years. The primary endpoint is new dementia or persistent disability.
Both trials “are powered to enable firm conclusions concerning any statin effects,” said Dr. Ballantyne and Dr. Nambi. “In the meantime, practicing clinicians can have confidence and share with their patients that short-term lipid-lowering therapy in older patients, including with statins, is unlikely to have a major impact on cognition.”
ASPREE was supported by grants from the U.S. National Institute on Aging and the National Cancer Institute and the National Health and Medical Research Council of Australia, by Monash University, and by the Victorian Cancer Agency. Dr. Nelson reported receiving honoraria from Sanofi and Amgen; support from Bayer for ASPREE; and grant support for STAREE. Disclosures for the other authors are in the report. Dr. Ballantyne disclosed grant and research support from Abbott Diagnostic, Akcea, Amgen, Esperion, Ionis, Novartis, Regeneron, and Roche Diagnostics; and consulting for Abbott Diagnostics, Althera, Amarin, Amgen, Arrowhead, AstraZeneca, Corvidia, Denka Seiken, Esperion, Genentech, Gilead, Matinas BioPharma, New Amsterdam, Novartis, Novo Nordisk, Pfizer, Regeneron, Roche Diagnostics, and Sanofi-Synthelabo. Dr. Nambi is a coinvestigator on a provisional patent along with Baylor College of Medicine and Roche on the use of biomarkers to predict heart failure, and a site principal investigator for studies sponsored by Amgen and Merck. Dr. Stone had no disclosures.
A version of this article first appeared on Medscape.com.
Statin therapy likely didn’t lead to dementia or even mild cognitive impairment (MCI) in older patients taking the drugs for cardiovascular (CV) primary prevention in a post hoc analysis of a trial that required normal cognitive ability for entry.
Nor did statins, whether lipophilic or hydrophilic, appear to influence changes in cognition or affect separate domains of mental performance, such as memory, language ability, or executive function, over the trial’s follow-up, which averaged almost 5 years.
Although such findings aren’t novel – they are consistent with observations from a number of earlier studies – the new analysis included a possible signal for a statin association with new-onset dementia in a subgroup of more than 18,000 patients. Researchers attribute the retrospective finding, from a trial not designed to explore the issue, to confounding or chance.
Still, the adjusted risk for dementia seemed to go up by a third among statin users who at baseline placed in the lowest quartile for cognitive function, based on a composite test score, in the ASPREE trial, a test of primary-prevention low-dose aspirin in patients 65 or older. The better the baseline cognitive score by quartile, the lower the risk for dementia ( interaction P < .001).
The bottom-quartile association of statins with dementia was driven by new diagnoses of Alzheimer’s disease, as opposed to the study’s other “mixed presentation” dementia subtype, wrote the authors of analysis, published June 21, 2021, in the Journal of the American College of Cardiology), led by Zhen Zhou, PhD, Menzies Institute for Medical Research, University of Tasmania, Hobart, Australia.
“I wouldn’t overinterpret that,” said senior author Mark R. Nelson, MBBS, PhD, of the same institution. Indeed, it should be “reassuring” for physicians prescribing statins to older patients that there was no overall statin effect on cognition or new-onset dementia, he said in an interview.
“This is a post hoc analysis within a dataset, although a very-high-quality dataset, it must be said.” The patients were prospectively followed for a range of cognition domains, and the results were adjudicated, Dr. Nelson observed. Although the question of statins and dementia risk is thought to be largely settled, the analysis “was just too tempting not to do.”
On the basis of the current analysis and the bulk of preceding evidence, “lipid lowering in the short term does not appear to result in improvement or deterioration of cognition irrespective of baseline LDL cholesterol levels and medication used,” Christie M. Ballantyne, MD, and Vijay Nambi, MD, PhD, both from Baylor College of Medicine, Houston, wrote in an accompanying editorial.
The current study “provides additional information that the lipo- or hydrophilicity of the statin does not affect changes in cognition. However, the potential increased risk for Alzheimer’s disease, especially among patients with baseline cognitive impairment, requires further investigation.”
The current analysis is reassuring that the likelihood of such statin effects on cognition “is vanishingly small,” Neil J. Stone MD, Northwestern University, Chicago, said in an interview. In fact, its primary finding of no such association “best summarizes what we know in 2021 about statin therapy” after exploration of the issue in a number of prospective trials and systematic reviews, said Dr. Stone, who was not a coauthor on the report.
The observed interaction between statin use and baseline neurocognitive ability “is hypothesis raising at best. It should be explored in randomized, controlled trials that can look at this question in an unbiased manner,” he agreed.
If patients believe or suspect that a statin is causing symptoms that suggest cognitive dysfunction, “what they really need to do is to stop it for 3 weeks and check out other causes. And in rechallenging, the guidelines say, if they think that it’s causing a memory problem that occurs anecdotally, then they can be given another statin, usually, which doesn’t cause it.”
ASPREE compared daily low-dose aspirin with placebo in a community-based older population numbering about 19,000 in Australia and the United States. Patients were initially without known CV disease, dementia, or physical disabilities. It did not randomize patients by statin therapy.
Of note, entry to the trial required a score of at least 78 on the Modified Mini-Mental State Examination (3MS), corresponding to normal cognition.
Aspirin showed no significant benefit for disability-free survival, an endpoint that included death and dementia, or CV events over a median of 4.7 years. It was associated with slightly more cases of major hemorrhage, as previously reported.
A subsequent ASPREE analysis suggested that the aspirin had no effect on risks of mild cognitive impairment, cognitive decline, or dementia.
Of the 18,846 patients in the current post hoc analysis, the average age of the patients was 74 years, and 56.4% were women; 31.3% were taking statins at baseline. The incidence of dementia per 1,000 person-years for those taking statins in comparison with those not taking statins was 6.91 and 6.48, respectively. Any cognitive changes were tracked by the 3MS and three other validated tests in different domains of cognition, with results contributing to the composite score.
The corresponding incidence of dementia considered probable Alzheimer’s disease was 2.97 and 2.65 for those receiving versus not receiving statins, respectively. The incidence of dementia with mixed presentation was 3.94 and 3.84, respectively.
There were no significant differences in risk for dementia overall or for either dementia subtype in multivariate analyses. Adjustments included demographics, CV lifestyle risk factors, family medical history, including dementia, ASPREE randomization group, and individual scores on the four tests of cognition.
Results for development of MCI mirrored those for dementia, as did results stratified for baseline lipids and for use of lipophilic statins, such as atorvastatin or simvastatin versus hydrophilic statins, including pravastatin and rosuvastatin.
Significant interactions were observed between composite cognitive scores and statin therapy at baseline; as scores increased, indicating better cognitive performance, the risks for dementia and its subtypes went down. Statins were associated with incident dementia at the lowest cognitive performance quartile.
That association is probably a function of the cohort’s advanced age, Dr. Nelson said. “If you get into old age, and you’ve got high cognitive scores, you’ve probably got protective factors. That’s how I would interpret that.”
Dr. Ballantyne and Dr. Nambi also emphasized the difficulties of controlling for potential biases even with extensive covariate adjustments. The statin dosages at which patients were treated were not part of the analysis, “and achieved LDL [cholesterol levels over the study period were not known,” they wrote.
“Furthermore, patients who were treated with statins were more likely to have diabetes, hypertension, chronic kidney disease, and obesity, all of which are known to increase risk for cognitive decline, and, as might have been predicted, statin users therefore had significantly lower scores for global cognition and episodic memory.”
Dr. Nelson pointed to an ongoing prospective atorvastatin trial that includes dementia in its primary endpoint and should be “the definitive study.” STAREE (Statin Therapy for Reducing Events in the Elderly) is running throughout Australia with a projected enrollment of 18,000 and primary completion by the end of 2022. “We’ve already enrolled 8,000 patients.”
Less far along is the PREVENTABLE (Pragmatic Evaluation of Events and Benefits of Lipid-Lowering in Older Adults) trial, based in the United States and also randomizing to atorvastatin or placebo, that will have an estimated 20,000 older patients and completion in 5 years. The primary endpoint is new dementia or persistent disability.
Both trials “are powered to enable firm conclusions concerning any statin effects,” said Dr. Ballantyne and Dr. Nambi. “In the meantime, practicing clinicians can have confidence and share with their patients that short-term lipid-lowering therapy in older patients, including with statins, is unlikely to have a major impact on cognition.”
ASPREE was supported by grants from the U.S. National Institute on Aging and the National Cancer Institute and the National Health and Medical Research Council of Australia, by Monash University, and by the Victorian Cancer Agency. Dr. Nelson reported receiving honoraria from Sanofi and Amgen; support from Bayer for ASPREE; and grant support for STAREE. Disclosures for the other authors are in the report. Dr. Ballantyne disclosed grant and research support from Abbott Diagnostic, Akcea, Amgen, Esperion, Ionis, Novartis, Regeneron, and Roche Diagnostics; and consulting for Abbott Diagnostics, Althera, Amarin, Amgen, Arrowhead, AstraZeneca, Corvidia, Denka Seiken, Esperion, Genentech, Gilead, Matinas BioPharma, New Amsterdam, Novartis, Novo Nordisk, Pfizer, Regeneron, Roche Diagnostics, and Sanofi-Synthelabo. Dr. Nambi is a coinvestigator on a provisional patent along with Baylor College of Medicine and Roche on the use of biomarkers to predict heart failure, and a site principal investigator for studies sponsored by Amgen and Merck. Dr. Stone had no disclosures.
A version of this article first appeared on Medscape.com.
Memory benefit seen with antihypertensives crossing blood-brain barrier
Over a 3-year period, cognitively normal older adults taking BBB-crossing antihypertensives demonstrated superior verbal memory, compared with similar individuals receiving non–BBB-crossing antihypertensives, reported lead author Jean K. Ho, PhD, of the Institute for Memory Impairments and Neurological Disorders at the University of California, Irvine, and colleagues.
According to the investigators, the findings add color to a known link between hypertension and neurologic degeneration, and may aid the search for new therapeutic targets.
“Hypertension is a well-established risk factor for cognitive decline and dementia, possibly through its effects on both cerebrovascular disease and Alzheimer’s disease,” Dr. Ho and colleagues wrote in Hypertension. “Studies of antihypertensive treatments have reported possible salutary effects on cognition and cerebrovascular disease, as well as Alzheimer’s disease neuropathology.”
In a previous study, individuals younger than 75 years exposed to antihypertensives had an 8% decreased risk of dementia per year of use, while another trial showed that intensive blood pressure–lowering therapy reduced mild cognitive impairment by 19%.
“Despite these encouraging findings ... larger meta-analytic studies have been hampered by the fact that pharmacokinetic properties are typically not considered in existing studies or routine clinical practice,” wrote Dr. Ho and colleagues. “The present study sought to fill this gap [in that it was] a large and longitudinal meta-analytic study of existing data recoded to assess the effects of BBB-crossing potential in renin-angiotensin system [RAS] treatments among hypertensive adults.”
Methods and results
The meta-analysis included randomized clinical trials, prospective cohort studies, and retrospective observational studies. The researchers assessed data on 12,849 individuals from 14 cohorts that received either BBB-crossing or non–BBB-crossing antihypertensives.
The BBB-crossing properties of RAS treatments were identified by a literature review. Of ACE inhibitors, captopril, fosinopril, lisinopril, perindopril, ramipril, and trandolapril were classified as BBB crossing, and benazepril, enalapril, moexipril, and quinapril were classified as non–BBB-crossing. Of ARBs, telmisartan and candesartan were considered BBB-crossing, and olmesartan, eprosartan, irbesartan, and losartan were tagged as non–BBB-crossing.
Cognition was assessed via the following seven domains: executive function, attention, verbal memory learning, language, mental status, recall, and processing speed.
Compared with individuals taking non–BBB-crossing antihypertensives, those taking BBB-crossing agents had significantly superior verbal memory (recall), with a maximum effect size of 0.07 (P = .03).
According to the investigators, this finding was particularly noteworthy, as the BBB-crossing group had relatively higher vascular risk burden and lower mean education level.
“These differences make it all the more remarkable that the BBB-crossing group displayed better memory ability over time despite these cognitive disadvantages,” the investigators wrote.
Still, not all the findings favored BBB-crossing agents. Individuals in the BBB-crossing group had relatively inferior attention ability, with a minimum effect size of –0.17 (P = .02).
The other cognitive measures were not significantly different between groups.
Clinicians may consider findings after accounting for other factors
Principal investigator Daniel A. Nation, PhD, associate professor of psychological science and a faculty member of the Institute for Memory Impairments and Neurological Disorders at the University of California, Irvine, suggested that the small difference in verbal memory between groups could be clinically significant over a longer period of time.
“Although the overall effect size was pretty small, if you look at how long it would take for someone [with dementia] to progress over many years of decline, it would actually end up being a pretty big effect,” Dr. Nation said in an interview. “Small effect sizes could actually end up preventing a lot of cases of dementia,” he added.
The conflicting results in the BBB-crossing group – better verbal memory but worse attention ability – were “surprising,” he noted.
“I sort of didn’t believe it at first,” Dr. Nation said, “because the memory finding is sort of replication – we’d observed the same exact effect on memory in a smaller sample in another study. ... The attention [finding], going another way, was a new thing.”
Dr. Nation suggested that the intergroup differences in attention ability may stem from idiosyncrasies of the tests used to measure that domain, which can be impacted by cardiovascular or brain vascular disease. Or it could be caused by something else entirely, he said, noting that further investigation is needed.
He added that the improvements in verbal memory within the BBB-crossing group could be caused by direct effects on the brain. He pointed out that certain ACE polymorphisms have been linked with Alzheimer’s disease risk, and those same polymorphisms, in animal models, lead to neurodegeneration, with reversal possible through administration of ACE inhibitors.
“It could be that what we’re observing has nothing really to do with blood pressure,” Dr. Nation explained. “This could be a neuronal effect on learning memory systems.”
He went on to suggest that clinicians may consider these findings when selecting antihypertensive agents for their patients, with the caveat that all other prescribing factors have already been taking to account.
“In the event that you’re going to give an ACE inhibitor or an angiotensin receptor blocker anyway, and it ends up being a somewhat arbitrary decision in terms of which specific drug you’re going to give, then perhaps this is a piece of information you would take into account – that one gets in the brain and one doesn’t – in somebody at risk for cognitive decline,” Dr. Nation said.
Exact mechanisms of action unknown
Hélène Girouard, PhD, assistant professor of pharmacology and physiology at the University of Montreal, said in an interview that the findings are “of considerable importance, knowing that brain alterations could begin as much as 30 years before manifestation of dementia.”
Since 2003, Dr. Girouard has been studying the cognitive effects of antihypertensive medications. She noted that previous studies involving rodents “have shown beneficial effects [of BBB-crossing antihypertensive drugs] on cognition independent of their effects on blood pressure.”
The drugs’ exact mechanisms of action, however, remain elusive, according to Dr. Girouard, who offered several possible explanations, including amelioration of BBB disruption, brain inflammation, cerebral blood flow dysregulation, cholinergic dysfunction, and neurologic deficits. “Whether these mechanisms may explain Ho and colleagues’ observations remains to be established,” she added.
Andrea L. Schneider, MD, PhD, assistant professor of neurology at the University of Pennsylvania, Philadelphia, applauded the study, but ultimately suggested that more research is needed to impact clinical decision-making.
“The results of this important and well-done study suggest that further investigation into targeted mechanism-based approaches to selecting hypertension treatment agents, with a specific focus on cognitive outcomes, is warranted,” Dr. Schneider said in an interview. “Before changing clinical practice, further work is necessary to disentangle contributions of medication mechanism, comorbid vascular risk factors, and achieved blood pressure reduction, among others.”
The investigators disclosed support from the National Institutes of Health, the Alzheimer’s Association, the Waksman Foundation of Japan, and others. The interviewees reported no relevant conflicts of interest.
Over a 3-year period, cognitively normal older adults taking BBB-crossing antihypertensives demonstrated superior verbal memory, compared with similar individuals receiving non–BBB-crossing antihypertensives, reported lead author Jean K. Ho, PhD, of the Institute for Memory Impairments and Neurological Disorders at the University of California, Irvine, and colleagues.
According to the investigators, the findings add color to a known link between hypertension and neurologic degeneration, and may aid the search for new therapeutic targets.
“Hypertension is a well-established risk factor for cognitive decline and dementia, possibly through its effects on both cerebrovascular disease and Alzheimer’s disease,” Dr. Ho and colleagues wrote in Hypertension. “Studies of antihypertensive treatments have reported possible salutary effects on cognition and cerebrovascular disease, as well as Alzheimer’s disease neuropathology.”
In a previous study, individuals younger than 75 years exposed to antihypertensives had an 8% decreased risk of dementia per year of use, while another trial showed that intensive blood pressure–lowering therapy reduced mild cognitive impairment by 19%.
“Despite these encouraging findings ... larger meta-analytic studies have been hampered by the fact that pharmacokinetic properties are typically not considered in existing studies or routine clinical practice,” wrote Dr. Ho and colleagues. “The present study sought to fill this gap [in that it was] a large and longitudinal meta-analytic study of existing data recoded to assess the effects of BBB-crossing potential in renin-angiotensin system [RAS] treatments among hypertensive adults.”
Methods and results
The meta-analysis included randomized clinical trials, prospective cohort studies, and retrospective observational studies. The researchers assessed data on 12,849 individuals from 14 cohorts that received either BBB-crossing or non–BBB-crossing antihypertensives.
The BBB-crossing properties of RAS treatments were identified by a literature review. Of ACE inhibitors, captopril, fosinopril, lisinopril, perindopril, ramipril, and trandolapril were classified as BBB crossing, and benazepril, enalapril, moexipril, and quinapril were classified as non–BBB-crossing. Of ARBs, telmisartan and candesartan were considered BBB-crossing, and olmesartan, eprosartan, irbesartan, and losartan were tagged as non–BBB-crossing.
Cognition was assessed via the following seven domains: executive function, attention, verbal memory learning, language, mental status, recall, and processing speed.
Compared with individuals taking non–BBB-crossing antihypertensives, those taking BBB-crossing agents had significantly superior verbal memory (recall), with a maximum effect size of 0.07 (P = .03).
According to the investigators, this finding was particularly noteworthy, as the BBB-crossing group had relatively higher vascular risk burden and lower mean education level.
“These differences make it all the more remarkable that the BBB-crossing group displayed better memory ability over time despite these cognitive disadvantages,” the investigators wrote.
Still, not all the findings favored BBB-crossing agents. Individuals in the BBB-crossing group had relatively inferior attention ability, with a minimum effect size of –0.17 (P = .02).
The other cognitive measures were not significantly different between groups.
Clinicians may consider findings after accounting for other factors
Principal investigator Daniel A. Nation, PhD, associate professor of psychological science and a faculty member of the Institute for Memory Impairments and Neurological Disorders at the University of California, Irvine, suggested that the small difference in verbal memory between groups could be clinically significant over a longer period of time.
“Although the overall effect size was pretty small, if you look at how long it would take for someone [with dementia] to progress over many years of decline, it would actually end up being a pretty big effect,” Dr. Nation said in an interview. “Small effect sizes could actually end up preventing a lot of cases of dementia,” he added.
The conflicting results in the BBB-crossing group – better verbal memory but worse attention ability – were “surprising,” he noted.
“I sort of didn’t believe it at first,” Dr. Nation said, “because the memory finding is sort of replication – we’d observed the same exact effect on memory in a smaller sample in another study. ... The attention [finding], going another way, was a new thing.”
Dr. Nation suggested that the intergroup differences in attention ability may stem from idiosyncrasies of the tests used to measure that domain, which can be impacted by cardiovascular or brain vascular disease. Or it could be caused by something else entirely, he said, noting that further investigation is needed.
He added that the improvements in verbal memory within the BBB-crossing group could be caused by direct effects on the brain. He pointed out that certain ACE polymorphisms have been linked with Alzheimer’s disease risk, and those same polymorphisms, in animal models, lead to neurodegeneration, with reversal possible through administration of ACE inhibitors.
“It could be that what we’re observing has nothing really to do with blood pressure,” Dr. Nation explained. “This could be a neuronal effect on learning memory systems.”
He went on to suggest that clinicians may consider these findings when selecting antihypertensive agents for their patients, with the caveat that all other prescribing factors have already been taking to account.
“In the event that you’re going to give an ACE inhibitor or an angiotensin receptor blocker anyway, and it ends up being a somewhat arbitrary decision in terms of which specific drug you’re going to give, then perhaps this is a piece of information you would take into account – that one gets in the brain and one doesn’t – in somebody at risk for cognitive decline,” Dr. Nation said.
Exact mechanisms of action unknown
Hélène Girouard, PhD, assistant professor of pharmacology and physiology at the University of Montreal, said in an interview that the findings are “of considerable importance, knowing that brain alterations could begin as much as 30 years before manifestation of dementia.”
Since 2003, Dr. Girouard has been studying the cognitive effects of antihypertensive medications. She noted that previous studies involving rodents “have shown beneficial effects [of BBB-crossing antihypertensive drugs] on cognition independent of their effects on blood pressure.”
The drugs’ exact mechanisms of action, however, remain elusive, according to Dr. Girouard, who offered several possible explanations, including amelioration of BBB disruption, brain inflammation, cerebral blood flow dysregulation, cholinergic dysfunction, and neurologic deficits. “Whether these mechanisms may explain Ho and colleagues’ observations remains to be established,” she added.
Andrea L. Schneider, MD, PhD, assistant professor of neurology at the University of Pennsylvania, Philadelphia, applauded the study, but ultimately suggested that more research is needed to impact clinical decision-making.
“The results of this important and well-done study suggest that further investigation into targeted mechanism-based approaches to selecting hypertension treatment agents, with a specific focus on cognitive outcomes, is warranted,” Dr. Schneider said in an interview. “Before changing clinical practice, further work is necessary to disentangle contributions of medication mechanism, comorbid vascular risk factors, and achieved blood pressure reduction, among others.”
The investigators disclosed support from the National Institutes of Health, the Alzheimer’s Association, the Waksman Foundation of Japan, and others. The interviewees reported no relevant conflicts of interest.
Over a 3-year period, cognitively normal older adults taking BBB-crossing antihypertensives demonstrated superior verbal memory, compared with similar individuals receiving non–BBB-crossing antihypertensives, reported lead author Jean K. Ho, PhD, of the Institute for Memory Impairments and Neurological Disorders at the University of California, Irvine, and colleagues.
According to the investigators, the findings add color to a known link between hypertension and neurologic degeneration, and may aid the search for new therapeutic targets.
“Hypertension is a well-established risk factor for cognitive decline and dementia, possibly through its effects on both cerebrovascular disease and Alzheimer’s disease,” Dr. Ho and colleagues wrote in Hypertension. “Studies of antihypertensive treatments have reported possible salutary effects on cognition and cerebrovascular disease, as well as Alzheimer’s disease neuropathology.”
In a previous study, individuals younger than 75 years exposed to antihypertensives had an 8% decreased risk of dementia per year of use, while another trial showed that intensive blood pressure–lowering therapy reduced mild cognitive impairment by 19%.
“Despite these encouraging findings ... larger meta-analytic studies have been hampered by the fact that pharmacokinetic properties are typically not considered in existing studies or routine clinical practice,” wrote Dr. Ho and colleagues. “The present study sought to fill this gap [in that it was] a large and longitudinal meta-analytic study of existing data recoded to assess the effects of BBB-crossing potential in renin-angiotensin system [RAS] treatments among hypertensive adults.”
Methods and results
The meta-analysis included randomized clinical trials, prospective cohort studies, and retrospective observational studies. The researchers assessed data on 12,849 individuals from 14 cohorts that received either BBB-crossing or non–BBB-crossing antihypertensives.
The BBB-crossing properties of RAS treatments were identified by a literature review. Of ACE inhibitors, captopril, fosinopril, lisinopril, perindopril, ramipril, and trandolapril were classified as BBB crossing, and benazepril, enalapril, moexipril, and quinapril were classified as non–BBB-crossing. Of ARBs, telmisartan and candesartan were considered BBB-crossing, and olmesartan, eprosartan, irbesartan, and losartan were tagged as non–BBB-crossing.
Cognition was assessed via the following seven domains: executive function, attention, verbal memory learning, language, mental status, recall, and processing speed.
Compared with individuals taking non–BBB-crossing antihypertensives, those taking BBB-crossing agents had significantly superior verbal memory (recall), with a maximum effect size of 0.07 (P = .03).
According to the investigators, this finding was particularly noteworthy, as the BBB-crossing group had relatively higher vascular risk burden and lower mean education level.
“These differences make it all the more remarkable that the BBB-crossing group displayed better memory ability over time despite these cognitive disadvantages,” the investigators wrote.
Still, not all the findings favored BBB-crossing agents. Individuals in the BBB-crossing group had relatively inferior attention ability, with a minimum effect size of –0.17 (P = .02).
The other cognitive measures were not significantly different between groups.
Clinicians may consider findings after accounting for other factors
Principal investigator Daniel A. Nation, PhD, associate professor of psychological science and a faculty member of the Institute for Memory Impairments and Neurological Disorders at the University of California, Irvine, suggested that the small difference in verbal memory between groups could be clinically significant over a longer period of time.
“Although the overall effect size was pretty small, if you look at how long it would take for someone [with dementia] to progress over many years of decline, it would actually end up being a pretty big effect,” Dr. Nation said in an interview. “Small effect sizes could actually end up preventing a lot of cases of dementia,” he added.
The conflicting results in the BBB-crossing group – better verbal memory but worse attention ability – were “surprising,” he noted.
“I sort of didn’t believe it at first,” Dr. Nation said, “because the memory finding is sort of replication – we’d observed the same exact effect on memory in a smaller sample in another study. ... The attention [finding], going another way, was a new thing.”
Dr. Nation suggested that the intergroup differences in attention ability may stem from idiosyncrasies of the tests used to measure that domain, which can be impacted by cardiovascular or brain vascular disease. Or it could be caused by something else entirely, he said, noting that further investigation is needed.
He added that the improvements in verbal memory within the BBB-crossing group could be caused by direct effects on the brain. He pointed out that certain ACE polymorphisms have been linked with Alzheimer’s disease risk, and those same polymorphisms, in animal models, lead to neurodegeneration, with reversal possible through administration of ACE inhibitors.
“It could be that what we’re observing has nothing really to do with blood pressure,” Dr. Nation explained. “This could be a neuronal effect on learning memory systems.”
He went on to suggest that clinicians may consider these findings when selecting antihypertensive agents for their patients, with the caveat that all other prescribing factors have already been taking to account.
“In the event that you’re going to give an ACE inhibitor or an angiotensin receptor blocker anyway, and it ends up being a somewhat arbitrary decision in terms of which specific drug you’re going to give, then perhaps this is a piece of information you would take into account – that one gets in the brain and one doesn’t – in somebody at risk for cognitive decline,” Dr. Nation said.
Exact mechanisms of action unknown
Hélène Girouard, PhD, assistant professor of pharmacology and physiology at the University of Montreal, said in an interview that the findings are “of considerable importance, knowing that brain alterations could begin as much as 30 years before manifestation of dementia.”
Since 2003, Dr. Girouard has been studying the cognitive effects of antihypertensive medications. She noted that previous studies involving rodents “have shown beneficial effects [of BBB-crossing antihypertensive drugs] on cognition independent of their effects on blood pressure.”
The drugs’ exact mechanisms of action, however, remain elusive, according to Dr. Girouard, who offered several possible explanations, including amelioration of BBB disruption, brain inflammation, cerebral blood flow dysregulation, cholinergic dysfunction, and neurologic deficits. “Whether these mechanisms may explain Ho and colleagues’ observations remains to be established,” she added.
Andrea L. Schneider, MD, PhD, assistant professor of neurology at the University of Pennsylvania, Philadelphia, applauded the study, but ultimately suggested that more research is needed to impact clinical decision-making.
“The results of this important and well-done study suggest that further investigation into targeted mechanism-based approaches to selecting hypertension treatment agents, with a specific focus on cognitive outcomes, is warranted,” Dr. Schneider said in an interview. “Before changing clinical practice, further work is necessary to disentangle contributions of medication mechanism, comorbid vascular risk factors, and achieved blood pressure reduction, among others.”
The investigators disclosed support from the National Institutes of Health, the Alzheimer’s Association, the Waksman Foundation of Japan, and others. The interviewees reported no relevant conflicts of interest.
FROM HYPERTENSION
Is trouble falling asleep a modifiable risk factor for dementia?
, new research suggests.
Trouble falling asleep “may be a modifiable risk factor for later-life cognitive impairment and dementia,” said lead author Afsara Zaheed, a PhD candidate in clinical science, department of psychology, University of Michigan, Ann Arbor.
“Patients should also be aware of the importance of insomnia on cognitive functioning so that they can bring up these concerns with their providers early,” she said.
The findings were presented at Virtual SLEEP 2021, the 35th Annual Meeting of the Associated Professional Sleep Societies.
Poor sleep common with age
As many as one-half of older adults report having poor sleep quality and insomnia, and growing evidence suggests that insomnia may be a unique risk factor for cognitive decline in later life, Ms. Zaheed explained.
To investigate further, the researchers analyzed data on 2,496 adults aged 51 years and older who were participants in the Health and Retirement Study, a longitudinal study of aging in a nationally representative population of older adults.
In 2002, participants were asked how often they had trouble falling asleep, woke up during the night, woke up too early, and were not able to fall asleep again and how often they felt really rested when they woke up in the morning.
In 2016, participants’ cognition was assessed using a battery of neuropsychological tests that gauged episodic memory, executive function, language, visuospatial/construction, and processing speed.
Analyses controlled for sociodemographics, baseline global cognitive performance, and the influence of depressive symptoms and vascular disease.
Compared with other insomnia symptoms, having difficulty falling asleep in 2002 was the main insomnia symptom that was predictive of cognitive impairment 14 years later, in 2016.
More frequent trouble falling asleep was predictive of poorer episodic memory, executive function, language, processing speed, and visuospatial performance.
The associations between sleep initiation and later cognitive impairment were partially explained by depressive symptoms and vascular disease burden for all domains except episodic memory, which was only partially explained by depressive symptoms.
Unclear mechanism
Ms. Zaheed said research is needed to uncover neurophysiologic mechanisms underlying the observed associations. “It may be that chronic difficulty with falling asleep is associated with inflammatory or metabolic processes that negatively affect brain structure and function over time,” she said.
“Insomnia has also been linked with higher accumulation of protein aggregates in the brain that disrupt cell communication and are characteristic of late-life disorders such as Alzheimer’s disease,” she added.
“While our project did not directly investigate these potential causal pathways between insomnia and cognition, our results suggest that investigating these potential mechanisms is an important area for future research,” Ms. Zaheed said.
“While additional intervention research is needed to determine whether targeting insomnia in older patients can have lasting cognitive benefits, results from this study suggest that discussing insomnia symptoms at the primary care level may be beneficial for both doctors and patients,” she added.
“By targeting insomnia – for example, through an evidence-based cognitive–behavioral therapy approach – individuals may improve various mental and physical health outcomes in addition to improving their sleep quality,” Ms. Zaheed said.
Reached for comment, Shaheen E. Lakhan, MD, PhD, neurologist in Newton, Massachusetts, said, “There is a strong link between chronic sleep disturbances and cognitive impairment, including dementia.”
“This study further supports this link and specifically calls out initiating sleep (as opposed to staying asleep) as the culprit. It also raises the hypothesis that the link is primarily mediated by depression and vascular disease; however, the verdict is still out,” said Dr. Lakhan.
The study was funded by the National Institute on Aging. Ms. Zaheed and Dr. Lakhan have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
Trouble falling asleep “may be a modifiable risk factor for later-life cognitive impairment and dementia,” said lead author Afsara Zaheed, a PhD candidate in clinical science, department of psychology, University of Michigan, Ann Arbor.
“Patients should also be aware of the importance of insomnia on cognitive functioning so that they can bring up these concerns with their providers early,” she said.
The findings were presented at Virtual SLEEP 2021, the 35th Annual Meeting of the Associated Professional Sleep Societies.
Poor sleep common with age
As many as one-half of older adults report having poor sleep quality and insomnia, and growing evidence suggests that insomnia may be a unique risk factor for cognitive decline in later life, Ms. Zaheed explained.
To investigate further, the researchers analyzed data on 2,496 adults aged 51 years and older who were participants in the Health and Retirement Study, a longitudinal study of aging in a nationally representative population of older adults.
In 2002, participants were asked how often they had trouble falling asleep, woke up during the night, woke up too early, and were not able to fall asleep again and how often they felt really rested when they woke up in the morning.
In 2016, participants’ cognition was assessed using a battery of neuropsychological tests that gauged episodic memory, executive function, language, visuospatial/construction, and processing speed.
Analyses controlled for sociodemographics, baseline global cognitive performance, and the influence of depressive symptoms and vascular disease.
Compared with other insomnia symptoms, having difficulty falling asleep in 2002 was the main insomnia symptom that was predictive of cognitive impairment 14 years later, in 2016.
More frequent trouble falling asleep was predictive of poorer episodic memory, executive function, language, processing speed, and visuospatial performance.
The associations between sleep initiation and later cognitive impairment were partially explained by depressive symptoms and vascular disease burden for all domains except episodic memory, which was only partially explained by depressive symptoms.
Unclear mechanism
Ms. Zaheed said research is needed to uncover neurophysiologic mechanisms underlying the observed associations. “It may be that chronic difficulty with falling asleep is associated with inflammatory or metabolic processes that negatively affect brain structure and function over time,” she said.
“Insomnia has also been linked with higher accumulation of protein aggregates in the brain that disrupt cell communication and are characteristic of late-life disorders such as Alzheimer’s disease,” she added.
“While our project did not directly investigate these potential causal pathways between insomnia and cognition, our results suggest that investigating these potential mechanisms is an important area for future research,” Ms. Zaheed said.
“While additional intervention research is needed to determine whether targeting insomnia in older patients can have lasting cognitive benefits, results from this study suggest that discussing insomnia symptoms at the primary care level may be beneficial for both doctors and patients,” she added.
“By targeting insomnia – for example, through an evidence-based cognitive–behavioral therapy approach – individuals may improve various mental and physical health outcomes in addition to improving their sleep quality,” Ms. Zaheed said.
Reached for comment, Shaheen E. Lakhan, MD, PhD, neurologist in Newton, Massachusetts, said, “There is a strong link between chronic sleep disturbances and cognitive impairment, including dementia.”
“This study further supports this link and specifically calls out initiating sleep (as opposed to staying asleep) as the culprit. It also raises the hypothesis that the link is primarily mediated by depression and vascular disease; however, the verdict is still out,” said Dr. Lakhan.
The study was funded by the National Institute on Aging. Ms. Zaheed and Dr. Lakhan have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
Trouble falling asleep “may be a modifiable risk factor for later-life cognitive impairment and dementia,” said lead author Afsara Zaheed, a PhD candidate in clinical science, department of psychology, University of Michigan, Ann Arbor.
“Patients should also be aware of the importance of insomnia on cognitive functioning so that they can bring up these concerns with their providers early,” she said.
The findings were presented at Virtual SLEEP 2021, the 35th Annual Meeting of the Associated Professional Sleep Societies.
Poor sleep common with age
As many as one-half of older adults report having poor sleep quality and insomnia, and growing evidence suggests that insomnia may be a unique risk factor for cognitive decline in later life, Ms. Zaheed explained.
To investigate further, the researchers analyzed data on 2,496 adults aged 51 years and older who were participants in the Health and Retirement Study, a longitudinal study of aging in a nationally representative population of older adults.
In 2002, participants were asked how often they had trouble falling asleep, woke up during the night, woke up too early, and were not able to fall asleep again and how often they felt really rested when they woke up in the morning.
In 2016, participants’ cognition was assessed using a battery of neuropsychological tests that gauged episodic memory, executive function, language, visuospatial/construction, and processing speed.
Analyses controlled for sociodemographics, baseline global cognitive performance, and the influence of depressive symptoms and vascular disease.
Compared with other insomnia symptoms, having difficulty falling asleep in 2002 was the main insomnia symptom that was predictive of cognitive impairment 14 years later, in 2016.
More frequent trouble falling asleep was predictive of poorer episodic memory, executive function, language, processing speed, and visuospatial performance.
The associations between sleep initiation and later cognitive impairment were partially explained by depressive symptoms and vascular disease burden for all domains except episodic memory, which was only partially explained by depressive symptoms.
Unclear mechanism
Ms. Zaheed said research is needed to uncover neurophysiologic mechanisms underlying the observed associations. “It may be that chronic difficulty with falling asleep is associated with inflammatory or metabolic processes that negatively affect brain structure and function over time,” she said.
“Insomnia has also been linked with higher accumulation of protein aggregates in the brain that disrupt cell communication and are characteristic of late-life disorders such as Alzheimer’s disease,” she added.
“While our project did not directly investigate these potential causal pathways between insomnia and cognition, our results suggest that investigating these potential mechanisms is an important area for future research,” Ms. Zaheed said.
“While additional intervention research is needed to determine whether targeting insomnia in older patients can have lasting cognitive benefits, results from this study suggest that discussing insomnia symptoms at the primary care level may be beneficial for both doctors and patients,” she added.
“By targeting insomnia – for example, through an evidence-based cognitive–behavioral therapy approach – individuals may improve various mental and physical health outcomes in addition to improving their sleep quality,” Ms. Zaheed said.
Reached for comment, Shaheen E. Lakhan, MD, PhD, neurologist in Newton, Massachusetts, said, “There is a strong link between chronic sleep disturbances and cognitive impairment, including dementia.”
“This study further supports this link and specifically calls out initiating sleep (as opposed to staying asleep) as the culprit. It also raises the hypothesis that the link is primarily mediated by depression and vascular disease; however, the verdict is still out,” said Dr. Lakhan.
The study was funded by the National Institute on Aging. Ms. Zaheed and Dr. Lakhan have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Watchdog group demands removal of FDA leaders after aducanumab approval
In a letter to the U.S. Department of Health and Human Services Secretary Xavier Becerra, Michael A. Carome, MD, director of Public Citizen’s Health Research Group, said: “The FDA’s decision to approve aducanumab for anyone with Alzheimer’s disease, regardless of severity, showed a stunning disregard for science, eviscerated the agency’s standards for approving new drugs, and ranks as one of the most irresponsible and egregious decisions in the history of the agency.”
Public Citizen urged Mr. Becerra to seek the resignations or the removal of the three FDA officials it said were most responsible for the approval – Acting FDA Commissioner Janet Woodcock, MD; Center for Drug Evaluation and Research (CDER) Director Patrizia Cavazzoni, MD; and CDER’s Office of Neuroscience Director Billy Dunn, MD.
“This decision is a disastrous blow to the agency’s credibility, public health, and the financial sustainability of the Medicare program,” writes Dr. Carome, noting that Biogen said it would charge $56,000 annually for the infusion.
Aaron Kesselheim, MD, one of three FDA Peripheral and Central Nervous System Drugs advisory committee members who resigned in the wake of the approval, agreed with Public Citizen that the agency’s credibility is suffering.
“The aducanumab decision is the worst example yet of the FDA’s movement away from its high standards,” Dr. Kesselheim, a professor of medicine at Harvard Medical School, Boston, and Harvard colleague Jerry Avorn, MD, wrote in the New York Times on June 15.
“As physicians, we know well that Alzheimer’s disease is a terrible condition,” they wrote. However, they added, “approving a drug that has such poor evidence that it works and causes such worrisome side effects is not the solution.”
In his resignation letter, Dr. Kesselheim said he had also been dismayed by the agency’s 2016 approval of eteplirsen (Exondys 51, Sarepta Therapeutics) for Duchenne muscular dystrophy. In both the eteplirsen and aducanumab approvals, the agency went against its advisers’ recommendations, Dr. Kesselheim said.
Advocates who backed approval decry cost
Aducanumab had a rocky road to approval but had unwavering backing from the Alzheimer’s Association and at least one other organization, UsAgainstAlzheimer’s.
The Alzheimer’s Association was particularly outspoken in its support and, in March, was accused of potential conflict of interest by Public Citizen and several neurologists because the association accepted at least $1.4 million from Biogen and its partner Eisai since fiscal year 2018.
The association applauded the FDA approval but, a few days later, expressed outrage over the $56,000-a-year price tag.
“This price is simply unacceptable,” the Alzheimer’s Association said in the statement. “For many, this price will pose an insurmountable barrier to access, it complicates and jeopardizes sustainable access to this treatment, and may further deepen issues of health equity,” the association said, adding, “We call on Biogen to change this price.”
UsAgainstAlzheimer’s also expressed concerns about access, even before it knew aducanumab’s price.
“Shockingly, Medicare does not reimburse patients for the expensive PET scans important to determine whether someone is appropriate for this drug,” noted George Vradenburg, chairman and cofounder of the group, in a June 7 statement. “We intend to work with Biogen and Medicare to make access to this drug affordable for every American who needs it,” Mr. Vradenburg said.
Dr. Carome said the advocates’ complaints were hard to fathom.
“This should not have come as a surprise to anyone,” Dr. Carome said, adding that “it’s essentially the ballpark figure the company threw out weeks ago.”
“Fifty-six-thousand-dollars is particularly egregiously overpriced for a drug that doesn’t work,” Dr. Carome said. “If the [Alzheimer’s Association] truly finds this objectionable, hopefully they’ll stop accepting money from Biogen and its partner Eisai,” he added.
“The Alzheimer’s Association is recognizing that the genie is out of the bottle and that they are going to have trouble reining in the inevitable run-away costs,” said Mike Greicius, MD, MPH, associate professor of neurology at Stanford University’s Wu Tsai Neurosciences Institute, Stanford, California.
“In addition to the eye-popping annual cost that Biogen has invented, I hope the Alzheimer’s Association is also concerned about the dangerously loose and broad FDA labeling which does not require screening for amyloid-positivity and does not restrict use to the milder forms of disease studied in the Phase 3 trials,” Dr. Greicius said.
Another advocacy group, Patients For Affordable Drugs, commended the Alzheimer’s Association. Its statement “was nothing short of courageous, especially in light of the Alzheimer’s Association’s reliance on funding from drug corporations, including Biogen,” said David Mitchell, a cancer patient and founder of Patients For Affordable Drugs, in a statement.
Mr. Mitchell said his members “stand with the Alzheimer’s Association in its denunciation of the price set by Biogen” and called for a new law that would allow Medicare to negotiate drug prices.
A version of this article first appeared on Medscape.com.
In a letter to the U.S. Department of Health and Human Services Secretary Xavier Becerra, Michael A. Carome, MD, director of Public Citizen’s Health Research Group, said: “The FDA’s decision to approve aducanumab for anyone with Alzheimer’s disease, regardless of severity, showed a stunning disregard for science, eviscerated the agency’s standards for approving new drugs, and ranks as one of the most irresponsible and egregious decisions in the history of the agency.”
Public Citizen urged Mr. Becerra to seek the resignations or the removal of the three FDA officials it said were most responsible for the approval – Acting FDA Commissioner Janet Woodcock, MD; Center for Drug Evaluation and Research (CDER) Director Patrizia Cavazzoni, MD; and CDER’s Office of Neuroscience Director Billy Dunn, MD.
“This decision is a disastrous blow to the agency’s credibility, public health, and the financial sustainability of the Medicare program,” writes Dr. Carome, noting that Biogen said it would charge $56,000 annually for the infusion.
Aaron Kesselheim, MD, one of three FDA Peripheral and Central Nervous System Drugs advisory committee members who resigned in the wake of the approval, agreed with Public Citizen that the agency’s credibility is suffering.
“The aducanumab decision is the worst example yet of the FDA’s movement away from its high standards,” Dr. Kesselheim, a professor of medicine at Harvard Medical School, Boston, and Harvard colleague Jerry Avorn, MD, wrote in the New York Times on June 15.
“As physicians, we know well that Alzheimer’s disease is a terrible condition,” they wrote. However, they added, “approving a drug that has such poor evidence that it works and causes such worrisome side effects is not the solution.”
In his resignation letter, Dr. Kesselheim said he had also been dismayed by the agency’s 2016 approval of eteplirsen (Exondys 51, Sarepta Therapeutics) for Duchenne muscular dystrophy. In both the eteplirsen and aducanumab approvals, the agency went against its advisers’ recommendations, Dr. Kesselheim said.
Advocates who backed approval decry cost
Aducanumab had a rocky road to approval but had unwavering backing from the Alzheimer’s Association and at least one other organization, UsAgainstAlzheimer’s.
The Alzheimer’s Association was particularly outspoken in its support and, in March, was accused of potential conflict of interest by Public Citizen and several neurologists because the association accepted at least $1.4 million from Biogen and its partner Eisai since fiscal year 2018.
The association applauded the FDA approval but, a few days later, expressed outrage over the $56,000-a-year price tag.
“This price is simply unacceptable,” the Alzheimer’s Association said in the statement. “For many, this price will pose an insurmountable barrier to access, it complicates and jeopardizes sustainable access to this treatment, and may further deepen issues of health equity,” the association said, adding, “We call on Biogen to change this price.”
UsAgainstAlzheimer’s also expressed concerns about access, even before it knew aducanumab’s price.
“Shockingly, Medicare does not reimburse patients for the expensive PET scans important to determine whether someone is appropriate for this drug,” noted George Vradenburg, chairman and cofounder of the group, in a June 7 statement. “We intend to work with Biogen and Medicare to make access to this drug affordable for every American who needs it,” Mr. Vradenburg said.
Dr. Carome said the advocates’ complaints were hard to fathom.
“This should not have come as a surprise to anyone,” Dr. Carome said, adding that “it’s essentially the ballpark figure the company threw out weeks ago.”
“Fifty-six-thousand-dollars is particularly egregiously overpriced for a drug that doesn’t work,” Dr. Carome said. “If the [Alzheimer’s Association] truly finds this objectionable, hopefully they’ll stop accepting money from Biogen and its partner Eisai,” he added.
“The Alzheimer’s Association is recognizing that the genie is out of the bottle and that they are going to have trouble reining in the inevitable run-away costs,” said Mike Greicius, MD, MPH, associate professor of neurology at Stanford University’s Wu Tsai Neurosciences Institute, Stanford, California.
“In addition to the eye-popping annual cost that Biogen has invented, I hope the Alzheimer’s Association is also concerned about the dangerously loose and broad FDA labeling which does not require screening for amyloid-positivity and does not restrict use to the milder forms of disease studied in the Phase 3 trials,” Dr. Greicius said.
Another advocacy group, Patients For Affordable Drugs, commended the Alzheimer’s Association. Its statement “was nothing short of courageous, especially in light of the Alzheimer’s Association’s reliance on funding from drug corporations, including Biogen,” said David Mitchell, a cancer patient and founder of Patients For Affordable Drugs, in a statement.
Mr. Mitchell said his members “stand with the Alzheimer’s Association in its denunciation of the price set by Biogen” and called for a new law that would allow Medicare to negotiate drug prices.
A version of this article first appeared on Medscape.com.
In a letter to the U.S. Department of Health and Human Services Secretary Xavier Becerra, Michael A. Carome, MD, director of Public Citizen’s Health Research Group, said: “The FDA’s decision to approve aducanumab for anyone with Alzheimer’s disease, regardless of severity, showed a stunning disregard for science, eviscerated the agency’s standards for approving new drugs, and ranks as one of the most irresponsible and egregious decisions in the history of the agency.”
Public Citizen urged Mr. Becerra to seek the resignations or the removal of the three FDA officials it said were most responsible for the approval – Acting FDA Commissioner Janet Woodcock, MD; Center for Drug Evaluation and Research (CDER) Director Patrizia Cavazzoni, MD; and CDER’s Office of Neuroscience Director Billy Dunn, MD.
“This decision is a disastrous blow to the agency’s credibility, public health, and the financial sustainability of the Medicare program,” writes Dr. Carome, noting that Biogen said it would charge $56,000 annually for the infusion.
Aaron Kesselheim, MD, one of three FDA Peripheral and Central Nervous System Drugs advisory committee members who resigned in the wake of the approval, agreed with Public Citizen that the agency’s credibility is suffering.
“The aducanumab decision is the worst example yet of the FDA’s movement away from its high standards,” Dr. Kesselheim, a professor of medicine at Harvard Medical School, Boston, and Harvard colleague Jerry Avorn, MD, wrote in the New York Times on June 15.
“As physicians, we know well that Alzheimer’s disease is a terrible condition,” they wrote. However, they added, “approving a drug that has such poor evidence that it works and causes such worrisome side effects is not the solution.”
In his resignation letter, Dr. Kesselheim said he had also been dismayed by the agency’s 2016 approval of eteplirsen (Exondys 51, Sarepta Therapeutics) for Duchenne muscular dystrophy. In both the eteplirsen and aducanumab approvals, the agency went against its advisers’ recommendations, Dr. Kesselheim said.
Advocates who backed approval decry cost
Aducanumab had a rocky road to approval but had unwavering backing from the Alzheimer’s Association and at least one other organization, UsAgainstAlzheimer’s.
The Alzheimer’s Association was particularly outspoken in its support and, in March, was accused of potential conflict of interest by Public Citizen and several neurologists because the association accepted at least $1.4 million from Biogen and its partner Eisai since fiscal year 2018.
The association applauded the FDA approval but, a few days later, expressed outrage over the $56,000-a-year price tag.
“This price is simply unacceptable,” the Alzheimer’s Association said in the statement. “For many, this price will pose an insurmountable barrier to access, it complicates and jeopardizes sustainable access to this treatment, and may further deepen issues of health equity,” the association said, adding, “We call on Biogen to change this price.”
UsAgainstAlzheimer’s also expressed concerns about access, even before it knew aducanumab’s price.
“Shockingly, Medicare does not reimburse patients for the expensive PET scans important to determine whether someone is appropriate for this drug,” noted George Vradenburg, chairman and cofounder of the group, in a June 7 statement. “We intend to work with Biogen and Medicare to make access to this drug affordable for every American who needs it,” Mr. Vradenburg said.
Dr. Carome said the advocates’ complaints were hard to fathom.
“This should not have come as a surprise to anyone,” Dr. Carome said, adding that “it’s essentially the ballpark figure the company threw out weeks ago.”
“Fifty-six-thousand-dollars is particularly egregiously overpriced for a drug that doesn’t work,” Dr. Carome said. “If the [Alzheimer’s Association] truly finds this objectionable, hopefully they’ll stop accepting money from Biogen and its partner Eisai,” he added.
“The Alzheimer’s Association is recognizing that the genie is out of the bottle and that they are going to have trouble reining in the inevitable run-away costs,” said Mike Greicius, MD, MPH, associate professor of neurology at Stanford University’s Wu Tsai Neurosciences Institute, Stanford, California.
“In addition to the eye-popping annual cost that Biogen has invented, I hope the Alzheimer’s Association is also concerned about the dangerously loose and broad FDA labeling which does not require screening for amyloid-positivity and does not restrict use to the milder forms of disease studied in the Phase 3 trials,” Dr. Greicius said.
Another advocacy group, Patients For Affordable Drugs, commended the Alzheimer’s Association. Its statement “was nothing short of courageous, especially in light of the Alzheimer’s Association’s reliance on funding from drug corporations, including Biogen,” said David Mitchell, a cancer patient and founder of Patients For Affordable Drugs, in a statement.
Mr. Mitchell said his members “stand with the Alzheimer’s Association in its denunciation of the price set by Biogen” and called for a new law that would allow Medicare to negotiate drug prices.
A version of this article first appeared on Medscape.com.