User login
Review clarifies depression, anxiety risk among hidradenitis suppurativa patients
and meta-analysis of more than 40,000 adults.
Previous studies of psychiatric comorbidities in HS patients have suggested an increased rate of depression, anxiety, and suicide risk, but have varied in methodology. “Therefore, the exact magnitude of the prevalence and odds of depression and anxiety in patients with HS is unclear,” wrote Myrela O. Machado, MD, of the division of dermatology, Women’s College Hospital, Toronto, and colleagues.
In a review of PubMed/MEDLINE, Embase, and PsycINFO electronic databases through July 2018, the researchers identified 10 studies comprising 40,307 adults with HS. The overall prevalence of depression was 16.9%, and in the studies that included a comparison group, the odds ratio for depression was 1.84 for HS patients, compared with controls who did not have HS. The overall prevalence of anxiety was 4.9%, but data were insufficient to determine an odds ratio for anxiety. The study was published in JAMA Dermatology.
All 10 studies assessed depression; 4 assessed anxiety. In subgroup analyses, the prevalence of depression was 11.9% in studies with a diagnosis based on clinical criteria and 26.8% in studies that used a screening instrument. The prevalence was 25.9% for outpatients with HS.
“Although our findings indicate that depression and anxiety may be common among people with HS, whether there is a causal relationship in those associations remains to be proved,” the researchers wrote.
However, they noted, “the consequences of depression and anxiety on HS-related outcomes have recently received more attention.”
The study findings were limited by several factors including the lack of data from structured diagnostic interviews, variation in methodological quality, and variation in comparison groups across studies, as well as a high level of heterogeneity across studies, the researchers noted. However, the results support the need to recognize and treat psychiatric conditions in HS patients, and to develop new management strategies, they said.
Dr. Machado had no financial conflicts to disclose. Several coauthors disclosed relationships with Galderma, LEO Pharma, Janssen, Novartis, AbbVie, Celgene, Naos, Lilly, Sanofi, Valeant, and La Roche-Posay.
SOURCE: Machado M et al. JAMA Dermatol. 2019 June 5. doi: 10.1001/jamadermatol.2019.0759.
and meta-analysis of more than 40,000 adults.
Previous studies of psychiatric comorbidities in HS patients have suggested an increased rate of depression, anxiety, and suicide risk, but have varied in methodology. “Therefore, the exact magnitude of the prevalence and odds of depression and anxiety in patients with HS is unclear,” wrote Myrela O. Machado, MD, of the division of dermatology, Women’s College Hospital, Toronto, and colleagues.
In a review of PubMed/MEDLINE, Embase, and PsycINFO electronic databases through July 2018, the researchers identified 10 studies comprising 40,307 adults with HS. The overall prevalence of depression was 16.9%, and in the studies that included a comparison group, the odds ratio for depression was 1.84 for HS patients, compared with controls who did not have HS. The overall prevalence of anxiety was 4.9%, but data were insufficient to determine an odds ratio for anxiety. The study was published in JAMA Dermatology.
All 10 studies assessed depression; 4 assessed anxiety. In subgroup analyses, the prevalence of depression was 11.9% in studies with a diagnosis based on clinical criteria and 26.8% in studies that used a screening instrument. The prevalence was 25.9% for outpatients with HS.
“Although our findings indicate that depression and anxiety may be common among people with HS, whether there is a causal relationship in those associations remains to be proved,” the researchers wrote.
However, they noted, “the consequences of depression and anxiety on HS-related outcomes have recently received more attention.”
The study findings were limited by several factors including the lack of data from structured diagnostic interviews, variation in methodological quality, and variation in comparison groups across studies, as well as a high level of heterogeneity across studies, the researchers noted. However, the results support the need to recognize and treat psychiatric conditions in HS patients, and to develop new management strategies, they said.
Dr. Machado had no financial conflicts to disclose. Several coauthors disclosed relationships with Galderma, LEO Pharma, Janssen, Novartis, AbbVie, Celgene, Naos, Lilly, Sanofi, Valeant, and La Roche-Posay.
SOURCE: Machado M et al. JAMA Dermatol. 2019 June 5. doi: 10.1001/jamadermatol.2019.0759.
and meta-analysis of more than 40,000 adults.
Previous studies of psychiatric comorbidities in HS patients have suggested an increased rate of depression, anxiety, and suicide risk, but have varied in methodology. “Therefore, the exact magnitude of the prevalence and odds of depression and anxiety in patients with HS is unclear,” wrote Myrela O. Machado, MD, of the division of dermatology, Women’s College Hospital, Toronto, and colleagues.
In a review of PubMed/MEDLINE, Embase, and PsycINFO electronic databases through July 2018, the researchers identified 10 studies comprising 40,307 adults with HS. The overall prevalence of depression was 16.9%, and in the studies that included a comparison group, the odds ratio for depression was 1.84 for HS patients, compared with controls who did not have HS. The overall prevalence of anxiety was 4.9%, but data were insufficient to determine an odds ratio for anxiety. The study was published in JAMA Dermatology.
All 10 studies assessed depression; 4 assessed anxiety. In subgroup analyses, the prevalence of depression was 11.9% in studies with a diagnosis based on clinical criteria and 26.8% in studies that used a screening instrument. The prevalence was 25.9% for outpatients with HS.
“Although our findings indicate that depression and anxiety may be common among people with HS, whether there is a causal relationship in those associations remains to be proved,” the researchers wrote.
However, they noted, “the consequences of depression and anxiety on HS-related outcomes have recently received more attention.”
The study findings were limited by several factors including the lack of data from structured diagnostic interviews, variation in methodological quality, and variation in comparison groups across studies, as well as a high level of heterogeneity across studies, the researchers noted. However, the results support the need to recognize and treat psychiatric conditions in HS patients, and to develop new management strategies, they said.
Dr. Machado had no financial conflicts to disclose. Several coauthors disclosed relationships with Galderma, LEO Pharma, Janssen, Novartis, AbbVie, Celgene, Naos, Lilly, Sanofi, Valeant, and La Roche-Posay.
SOURCE: Machado M et al. JAMA Dermatol. 2019 June 5. doi: 10.1001/jamadermatol.2019.0759.
FROM JAMA DERMATOLOGY
Mental illness in MS: ‘Follow the why’
SEATTLE – , a neuropsychiatrist cautioned colleagues who treat MS.
For example, depression may strike a patient as a primary condition, just as it could in anyone. But it may also be a manifestation of MS itself, or a side effect of an MS medication, or spurred by the fatigue and pain caused by MS, said Laura T. Safar, MD, a psychiatrist affiliated with Brigham and Women's Hospital, Boston*. As a result, popular psychiatric treatments such as SSRIs might not necessarily be the best approach, said Dr. Safar, who spoke in an interview and during a presentation at the annual meeting of the Consortium of Multiple Sclerosis Centers.
“You need to follow the why,” she said in the interview, adding that it is crucial to view neurologic and mental health as one and the same in MS. “More integration,” she said, “continues to be the way to go.”
Here are some pearls and tips from Dr. Safar’s presentation on treating psychiatric conditions in patients with MS:
Mental illness incidence
Depression is estimated to affect 25%-45% of people with MS over their lifetimes, while bipolar disorder is thought to affect 6% of patients and a quarter are estimated to have anxiety.
Researchers also believe as many as 10% of patients are affected by pathological laughing and crying during their lives.
Psychiatric side effects
Interferon drugs are notoriously linked to depression and psychosis. Glatiramer acetate (Copaxone) and natalizumab (Tysabri) are also thought to cause psychiatric side effects in some cases – anxiety and depression, respectively. But drug-modifying therapies can also provide relief on the psychiatric front, Dr. Safar said.
Meanwhile, dozens of other drugs used to treat aspects of MS such as spasticity, pain, and fatigue have possible psychiatric side effects.
Alternatives to SSRIs
SSRIs are often a first option in psychiatric patients, but those with MS may need another option because so many – an estimated 80% – also have fatigue, Dr. Safar said.
Alternatives for patients with MS include serotonin and norepinephrine reuptake inhibitors (SNRIs), which may have an advantage over SSRIs, she said. Specifically, SNRIs and bupropion (Wellbutrin) may be better for patients with fatigue and cognitive problems, she said, while vortioxetine (Trintellix) may benefit cognition.
Treating anxiety
There are no data regarding the best drug treatment for anxiety in patients with MS, she said, and SSRIs are typically the starting point. Consider SNRIs and duloxetine, respectively, when patients also have significant fatigue and cognitive symptoms. Use benzodiazepines only in occasional cases (such as anxiety regarding an MRI) and severe cases, she said.
MS-specific side effects
Beware of MS-specific side effects, Dr. Safar said. Some common psychiatric drugs, especially citalopram (Celexa) and escitalopram (Lexapro), may increase the QTc interval and shouldn’t be used in combination with the MS drug fingolimod (Gilenya).
And, she said, bupropion is “a very helpful agent” but poses a rare risk of seizures. Dr. Safar said she has seen this side effect a couple times over 10 years, but both were in patients with “other factors involved.” Still, “it’s something to keep in mind.”
Also understand that serotonergic agents can worsen restless legs syndrome, which is more common in patients with MS. Dr. Safar advises monitoring for the condition.
Pathological laughing, crying
Episodes of so-called pathological laughing, crying, or both tend to be brief, frequent, and intense. They may be sparked by nothing at all, and more often feature crying.
Certain SSRIs have proved helpful for the condition in MS, Dr. Safar said. Research also supports a combination of dextromethorphan (cough suppressant) and quinidine (a drug used to treat arrhythmias and malaria). The combination is sold together as Nuedexta.
Other agents such as venlafaxine (Effexor) and duloxetine (Cymbalta) have very limited data and shouldn’t be first-line treatment, she said.
Dr. Safar reports no relevant disclosures.
Correction, 5/31/19: An earlier version of this article misstated Dr. Safar's hospital affiliation.
SEATTLE – , a neuropsychiatrist cautioned colleagues who treat MS.
For example, depression may strike a patient as a primary condition, just as it could in anyone. But it may also be a manifestation of MS itself, or a side effect of an MS medication, or spurred by the fatigue and pain caused by MS, said Laura T. Safar, MD, a psychiatrist affiliated with Brigham and Women's Hospital, Boston*. As a result, popular psychiatric treatments such as SSRIs might not necessarily be the best approach, said Dr. Safar, who spoke in an interview and during a presentation at the annual meeting of the Consortium of Multiple Sclerosis Centers.
“You need to follow the why,” she said in the interview, adding that it is crucial to view neurologic and mental health as one and the same in MS. “More integration,” she said, “continues to be the way to go.”
Here are some pearls and tips from Dr. Safar’s presentation on treating psychiatric conditions in patients with MS:
Mental illness incidence
Depression is estimated to affect 25%-45% of people with MS over their lifetimes, while bipolar disorder is thought to affect 6% of patients and a quarter are estimated to have anxiety.
Researchers also believe as many as 10% of patients are affected by pathological laughing and crying during their lives.
Psychiatric side effects
Interferon drugs are notoriously linked to depression and psychosis. Glatiramer acetate (Copaxone) and natalizumab (Tysabri) are also thought to cause psychiatric side effects in some cases – anxiety and depression, respectively. But drug-modifying therapies can also provide relief on the psychiatric front, Dr. Safar said.
Meanwhile, dozens of other drugs used to treat aspects of MS such as spasticity, pain, and fatigue have possible psychiatric side effects.
Alternatives to SSRIs
SSRIs are often a first option in psychiatric patients, but those with MS may need another option because so many – an estimated 80% – also have fatigue, Dr. Safar said.
Alternatives for patients with MS include serotonin and norepinephrine reuptake inhibitors (SNRIs), which may have an advantage over SSRIs, she said. Specifically, SNRIs and bupropion (Wellbutrin) may be better for patients with fatigue and cognitive problems, she said, while vortioxetine (Trintellix) may benefit cognition.
Treating anxiety
There are no data regarding the best drug treatment for anxiety in patients with MS, she said, and SSRIs are typically the starting point. Consider SNRIs and duloxetine, respectively, when patients also have significant fatigue and cognitive symptoms. Use benzodiazepines only in occasional cases (such as anxiety regarding an MRI) and severe cases, she said.
MS-specific side effects
Beware of MS-specific side effects, Dr. Safar said. Some common psychiatric drugs, especially citalopram (Celexa) and escitalopram (Lexapro), may increase the QTc interval and shouldn’t be used in combination with the MS drug fingolimod (Gilenya).
And, she said, bupropion is “a very helpful agent” but poses a rare risk of seizures. Dr. Safar said she has seen this side effect a couple times over 10 years, but both were in patients with “other factors involved.” Still, “it’s something to keep in mind.”
Also understand that serotonergic agents can worsen restless legs syndrome, which is more common in patients with MS. Dr. Safar advises monitoring for the condition.
Pathological laughing, crying
Episodes of so-called pathological laughing, crying, or both tend to be brief, frequent, and intense. They may be sparked by nothing at all, and more often feature crying.
Certain SSRIs have proved helpful for the condition in MS, Dr. Safar said. Research also supports a combination of dextromethorphan (cough suppressant) and quinidine (a drug used to treat arrhythmias and malaria). The combination is sold together as Nuedexta.
Other agents such as venlafaxine (Effexor) and duloxetine (Cymbalta) have very limited data and shouldn’t be first-line treatment, she said.
Dr. Safar reports no relevant disclosures.
Correction, 5/31/19: An earlier version of this article misstated Dr. Safar's hospital affiliation.
SEATTLE – , a neuropsychiatrist cautioned colleagues who treat MS.
For example, depression may strike a patient as a primary condition, just as it could in anyone. But it may also be a manifestation of MS itself, or a side effect of an MS medication, or spurred by the fatigue and pain caused by MS, said Laura T. Safar, MD, a psychiatrist affiliated with Brigham and Women's Hospital, Boston*. As a result, popular psychiatric treatments such as SSRIs might not necessarily be the best approach, said Dr. Safar, who spoke in an interview and during a presentation at the annual meeting of the Consortium of Multiple Sclerosis Centers.
“You need to follow the why,” she said in the interview, adding that it is crucial to view neurologic and mental health as one and the same in MS. “More integration,” she said, “continues to be the way to go.”
Here are some pearls and tips from Dr. Safar’s presentation on treating psychiatric conditions in patients with MS:
Mental illness incidence
Depression is estimated to affect 25%-45% of people with MS over their lifetimes, while bipolar disorder is thought to affect 6% of patients and a quarter are estimated to have anxiety.
Researchers also believe as many as 10% of patients are affected by pathological laughing and crying during their lives.
Psychiatric side effects
Interferon drugs are notoriously linked to depression and psychosis. Glatiramer acetate (Copaxone) and natalizumab (Tysabri) are also thought to cause psychiatric side effects in some cases – anxiety and depression, respectively. But drug-modifying therapies can also provide relief on the psychiatric front, Dr. Safar said.
Meanwhile, dozens of other drugs used to treat aspects of MS such as spasticity, pain, and fatigue have possible psychiatric side effects.
Alternatives to SSRIs
SSRIs are often a first option in psychiatric patients, but those with MS may need another option because so many – an estimated 80% – also have fatigue, Dr. Safar said.
Alternatives for patients with MS include serotonin and norepinephrine reuptake inhibitors (SNRIs), which may have an advantage over SSRIs, she said. Specifically, SNRIs and bupropion (Wellbutrin) may be better for patients with fatigue and cognitive problems, she said, while vortioxetine (Trintellix) may benefit cognition.
Treating anxiety
There are no data regarding the best drug treatment for anxiety in patients with MS, she said, and SSRIs are typically the starting point. Consider SNRIs and duloxetine, respectively, when patients also have significant fatigue and cognitive symptoms. Use benzodiazepines only in occasional cases (such as anxiety regarding an MRI) and severe cases, she said.
MS-specific side effects
Beware of MS-specific side effects, Dr. Safar said. Some common psychiatric drugs, especially citalopram (Celexa) and escitalopram (Lexapro), may increase the QTc interval and shouldn’t be used in combination with the MS drug fingolimod (Gilenya).
And, she said, bupropion is “a very helpful agent” but poses a rare risk of seizures. Dr. Safar said she has seen this side effect a couple times over 10 years, but both were in patients with “other factors involved.” Still, “it’s something to keep in mind.”
Also understand that serotonergic agents can worsen restless legs syndrome, which is more common in patients with MS. Dr. Safar advises monitoring for the condition.
Pathological laughing, crying
Episodes of so-called pathological laughing, crying, or both tend to be brief, frequent, and intense. They may be sparked by nothing at all, and more often feature crying.
Certain SSRIs have proved helpful for the condition in MS, Dr. Safar said. Research also supports a combination of dextromethorphan (cough suppressant) and quinidine (a drug used to treat arrhythmias and malaria). The combination is sold together as Nuedexta.
Other agents such as venlafaxine (Effexor) and duloxetine (Cymbalta) have very limited data and shouldn’t be first-line treatment, she said.
Dr. Safar reports no relevant disclosures.
Correction, 5/31/19: An earlier version of this article misstated Dr. Safar's hospital affiliation.
EXPERT ANALYSIS FROM CMSC 2019
Music shows promise for inpatient agitation
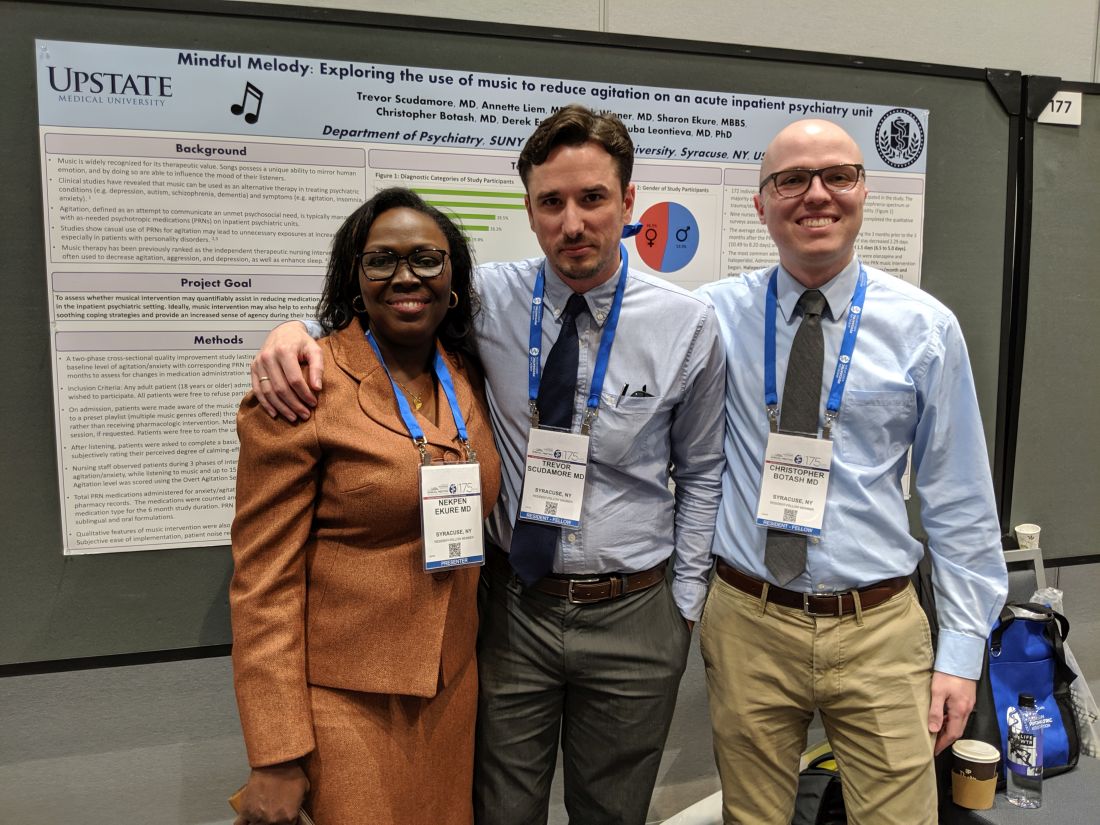
SAN FRANCISCO – In a proof-of-concept study, music provided an alternative to oral psychotropic medication in calming agitated patients at an inpatient psychiatric facility. Music has been studied as a treatment for agitation in dementia patients but not so much in psychiatric patients, according to Trevor Scudamore, MD, who is a resident fellow at State University of New York, Syracuse.
“The other thing is that music has been more looked at in group therapy settings than as adjunct therapy, or as an option as an as-needed medication for agitation,” Dr. Scudamore said in an interview. He presented the study at a poster session at the annual meeting of the American Psychiatric Association.
When agitation arose, the program allowed patients to choose between an oral medication or music, which entailed a 30-minute session listening to a preset playlist using a wireless headphone. Playlist options included a variety of musical genres, and participants could sit in one place or roam around while listening.
Traditionally, agitated patients had the choice of an oral medication. If the patient refused and then escalated, they had to accept an intravenous medication. “Now there’s a choice between an oral medication and music, almost like a third layer in defusing agitation in the patient. If they refuse music, then they could go for oral medication, and then [IV medication]. They’re given a little bit more options. Maybe they don’t feel so confined, which is an interesting way of helping possibly defuse anxiety from a situation. That needs to be explored further,” Dr. Scudamore said.
The study had a two-phase, cross-sectional design. The first 3 months, the study included 71 patients, who were used to establish a baseline of agitation and psychotropic medication use. They introduced the music intervention during the second 3-month period, with 101 participants. After they listened to music, the patients completed a self-report form using the Likert scale, and nursing staff observed the patients status during the initial anxiety/agitation, while they listened to music, and 15 minutes after the listening session.
That need for commitment from nurses presented a challenge to implementation. They had to hand out headphones, keep track of them, and make sure they got the headphones back. “It is a lot more work than just giving 50 mg of hydroxyzine,” said coauthor Christopher Botash, MD, who also is a resident at the university.
Study participants had a range of psychiatric ills, including substance abuse (61%), depression (51%), psychosis (28%), trauma (26%), personality disorder (20%), and anxiety (17%).
As part of a preliminary report, the researchers presented data from surveys completed by nine nurses and 31 patients. The two most commonly prescribed antipsychotics saw a decrease in administrations, from 3.37 to 2.93/month for haloperidol, and from 3.83 to 2.73 administrations/month for olanzapine.
A total of 56% of the nursing staff stated that the music therapy program helped calm down the patients. Nurses who disagreed cited the tendency for patients to intrude at the nursing station asking for the music, though this improved as patients learned the routine. Ninety-six percent of the patients reported satisfaction with the experience.
It was challenging for the researchers to implement the study, since offering music was a break in the routine. “The staff really does rely a lot on the meds, so oftentimes we would have to say, ‘Hey, have you offered the music yet?’ It’s a bit of a culture change,” Dr. Botash said.
The study, which Dr. Scudamore and Dr. Botash coauthored with Nekpen S. Ekure, MD, did not receive external funding. Dr. Scudamore and Dr. Botash had no relevant financial disclosures.
SAN FRANCISCO – In a proof-of-concept study, music provided an alternative to oral psychotropic medication in calming agitated patients at an inpatient psychiatric facility. Music has been studied as a treatment for agitation in dementia patients but not so much in psychiatric patients, according to Trevor Scudamore, MD, who is a resident fellow at State University of New York, Syracuse.
“The other thing is that music has been more looked at in group therapy settings than as adjunct therapy, or as an option as an as-needed medication for agitation,” Dr. Scudamore said in an interview. He presented the study at a poster session at the annual meeting of the American Psychiatric Association.
When agitation arose, the program allowed patients to choose between an oral medication or music, which entailed a 30-minute session listening to a preset playlist using a wireless headphone. Playlist options included a variety of musical genres, and participants could sit in one place or roam around while listening.
Traditionally, agitated patients had the choice of an oral medication. If the patient refused and then escalated, they had to accept an intravenous medication. “Now there’s a choice between an oral medication and music, almost like a third layer in defusing agitation in the patient. If they refuse music, then they could go for oral medication, and then [IV medication]. They’re given a little bit more options. Maybe they don’t feel so confined, which is an interesting way of helping possibly defuse anxiety from a situation. That needs to be explored further,” Dr. Scudamore said.
The study had a two-phase, cross-sectional design. The first 3 months, the study included 71 patients, who were used to establish a baseline of agitation and psychotropic medication use. They introduced the music intervention during the second 3-month period, with 101 participants. After they listened to music, the patients completed a self-report form using the Likert scale, and nursing staff observed the patients status during the initial anxiety/agitation, while they listened to music, and 15 minutes after the listening session.
That need for commitment from nurses presented a challenge to implementation. They had to hand out headphones, keep track of them, and make sure they got the headphones back. “It is a lot more work than just giving 50 mg of hydroxyzine,” said coauthor Christopher Botash, MD, who also is a resident at the university.
Study participants had a range of psychiatric ills, including substance abuse (61%), depression (51%), psychosis (28%), trauma (26%), personality disorder (20%), and anxiety (17%).
As part of a preliminary report, the researchers presented data from surveys completed by nine nurses and 31 patients. The two most commonly prescribed antipsychotics saw a decrease in administrations, from 3.37 to 2.93/month for haloperidol, and from 3.83 to 2.73 administrations/month for olanzapine.
A total of 56% of the nursing staff stated that the music therapy program helped calm down the patients. Nurses who disagreed cited the tendency for patients to intrude at the nursing station asking for the music, though this improved as patients learned the routine. Ninety-six percent of the patients reported satisfaction with the experience.
It was challenging for the researchers to implement the study, since offering music was a break in the routine. “The staff really does rely a lot on the meds, so oftentimes we would have to say, ‘Hey, have you offered the music yet?’ It’s a bit of a culture change,” Dr. Botash said.
The study, which Dr. Scudamore and Dr. Botash coauthored with Nekpen S. Ekure, MD, did not receive external funding. Dr. Scudamore and Dr. Botash had no relevant financial disclosures.
SAN FRANCISCO – In a proof-of-concept study, music provided an alternative to oral psychotropic medication in calming agitated patients at an inpatient psychiatric facility. Music has been studied as a treatment for agitation in dementia patients but not so much in psychiatric patients, according to Trevor Scudamore, MD, who is a resident fellow at State University of New York, Syracuse.
“The other thing is that music has been more looked at in group therapy settings than as adjunct therapy, or as an option as an as-needed medication for agitation,” Dr. Scudamore said in an interview. He presented the study at a poster session at the annual meeting of the American Psychiatric Association.
When agitation arose, the program allowed patients to choose between an oral medication or music, which entailed a 30-minute session listening to a preset playlist using a wireless headphone. Playlist options included a variety of musical genres, and participants could sit in one place or roam around while listening.
Traditionally, agitated patients had the choice of an oral medication. If the patient refused and then escalated, they had to accept an intravenous medication. “Now there’s a choice between an oral medication and music, almost like a third layer in defusing agitation in the patient. If they refuse music, then they could go for oral medication, and then [IV medication]. They’re given a little bit more options. Maybe they don’t feel so confined, which is an interesting way of helping possibly defuse anxiety from a situation. That needs to be explored further,” Dr. Scudamore said.
The study had a two-phase, cross-sectional design. The first 3 months, the study included 71 patients, who were used to establish a baseline of agitation and psychotropic medication use. They introduced the music intervention during the second 3-month period, with 101 participants. After they listened to music, the patients completed a self-report form using the Likert scale, and nursing staff observed the patients status during the initial anxiety/agitation, while they listened to music, and 15 minutes after the listening session.
That need for commitment from nurses presented a challenge to implementation. They had to hand out headphones, keep track of them, and make sure they got the headphones back. “It is a lot more work than just giving 50 mg of hydroxyzine,” said coauthor Christopher Botash, MD, who also is a resident at the university.
Study participants had a range of psychiatric ills, including substance abuse (61%), depression (51%), psychosis (28%), trauma (26%), personality disorder (20%), and anxiety (17%).
As part of a preliminary report, the researchers presented data from surveys completed by nine nurses and 31 patients. The two most commonly prescribed antipsychotics saw a decrease in administrations, from 3.37 to 2.93/month for haloperidol, and from 3.83 to 2.73 administrations/month for olanzapine.
A total of 56% of the nursing staff stated that the music therapy program helped calm down the patients. Nurses who disagreed cited the tendency for patients to intrude at the nursing station asking for the music, though this improved as patients learned the routine. Ninety-six percent of the patients reported satisfaction with the experience.
It was challenging for the researchers to implement the study, since offering music was a break in the routine. “The staff really does rely a lot on the meds, so oftentimes we would have to say, ‘Hey, have you offered the music yet?’ It’s a bit of a culture change,” Dr. Botash said.
The study, which Dr. Scudamore and Dr. Botash coauthored with Nekpen S. Ekure, MD, did not receive external funding. Dr. Scudamore and Dr. Botash had no relevant financial disclosures.
REPORTING FROM APA 2019
Hazardous cannabis use in MS linked to anxiety, depression
SEATTLE – A small new study suggests that although it is not clear whether there is a cause-and-effect relationship. “We highly recommend screening for hazardous cannabis use in clinical settings,” said study lead author and rehabilitation psychologist Abbey J. Hughes, PhD, an assistant professor at Johns Hopkins University, Baltimore, in an interview. She spoke prior to the presentation of the study findings at the annual meeting of the Consortium of Multiple Sclerosis Centers.
According to Dr. Hughes, research suggests that patients with MS are using cannabis more now than in the past, especially for medical reasons. It is not clear, however, how cannabis is affecting neurobehavior in patients with MS who use it, said Dr. Hughes, who works with patients with MS.
For the new study, researchers gave surveys to 100 patients with MS (76% female; mean age, 46 years) who sought outpatient care at an MS center. Of those, 31 said they had used cannabis within the past month.
The patients were screened via several tools: the Cannabis Use Disorders Identification Test–Revised (CUDIT-R) Fatigue Severity Scale; Patient Health Questionnaire–8; Generalized Anxiety Disorders Scale–7; and Brief International Cognitive Assessment for Multiple Sclerosis.
Subjects were considered to have a problem with “hazardous cannabis use” if they met or exceeded the CUDIT-R’s clinical cut-off of 8 points. The test asks about topics such as hazardous behavior while using cannabis, problems with memory or concentration after using it, and inability to stop using it. Twelve participants met this criteria, and they were more likely to have more symptoms of depression (beta = 0.32; P less than .01) and anxiety (beta = 0.24; P = .02), after researchers controlled for age, years of education, and MS subtype.
They also were slightly more likely to have more severe fatigue (beta = 0.20; P = .07) and poor sleep (beta = 0.20; P = .07).
The researchers found no link between cannabis use and scores on the cognitive test, although Dr. Hughes noted that other research has suggested such a link.
The study is cross-sectional and does not offer insight into cause and effect, Dr. Hughes said. She noted that it is possible that patients used cannabis because they had higher levels of anxiety and depression.
No study funding was reported and the authors report no relevant disclosures.
SEATTLE – A small new study suggests that although it is not clear whether there is a cause-and-effect relationship. “We highly recommend screening for hazardous cannabis use in clinical settings,” said study lead author and rehabilitation psychologist Abbey J. Hughes, PhD, an assistant professor at Johns Hopkins University, Baltimore, in an interview. She spoke prior to the presentation of the study findings at the annual meeting of the Consortium of Multiple Sclerosis Centers.
According to Dr. Hughes, research suggests that patients with MS are using cannabis more now than in the past, especially for medical reasons. It is not clear, however, how cannabis is affecting neurobehavior in patients with MS who use it, said Dr. Hughes, who works with patients with MS.
For the new study, researchers gave surveys to 100 patients with MS (76% female; mean age, 46 years) who sought outpatient care at an MS center. Of those, 31 said they had used cannabis within the past month.
The patients were screened via several tools: the Cannabis Use Disorders Identification Test–Revised (CUDIT-R) Fatigue Severity Scale; Patient Health Questionnaire–8; Generalized Anxiety Disorders Scale–7; and Brief International Cognitive Assessment for Multiple Sclerosis.
Subjects were considered to have a problem with “hazardous cannabis use” if they met or exceeded the CUDIT-R’s clinical cut-off of 8 points. The test asks about topics such as hazardous behavior while using cannabis, problems with memory or concentration after using it, and inability to stop using it. Twelve participants met this criteria, and they were more likely to have more symptoms of depression (beta = 0.32; P less than .01) and anxiety (beta = 0.24; P = .02), after researchers controlled for age, years of education, and MS subtype.
They also were slightly more likely to have more severe fatigue (beta = 0.20; P = .07) and poor sleep (beta = 0.20; P = .07).
The researchers found no link between cannabis use and scores on the cognitive test, although Dr. Hughes noted that other research has suggested such a link.
The study is cross-sectional and does not offer insight into cause and effect, Dr. Hughes said. She noted that it is possible that patients used cannabis because they had higher levels of anxiety and depression.
No study funding was reported and the authors report no relevant disclosures.
SEATTLE – A small new study suggests that although it is not clear whether there is a cause-and-effect relationship. “We highly recommend screening for hazardous cannabis use in clinical settings,” said study lead author and rehabilitation psychologist Abbey J. Hughes, PhD, an assistant professor at Johns Hopkins University, Baltimore, in an interview. She spoke prior to the presentation of the study findings at the annual meeting of the Consortium of Multiple Sclerosis Centers.
According to Dr. Hughes, research suggests that patients with MS are using cannabis more now than in the past, especially for medical reasons. It is not clear, however, how cannabis is affecting neurobehavior in patients with MS who use it, said Dr. Hughes, who works with patients with MS.
For the new study, researchers gave surveys to 100 patients with MS (76% female; mean age, 46 years) who sought outpatient care at an MS center. Of those, 31 said they had used cannabis within the past month.
The patients were screened via several tools: the Cannabis Use Disorders Identification Test–Revised (CUDIT-R) Fatigue Severity Scale; Patient Health Questionnaire–8; Generalized Anxiety Disorders Scale–7; and Brief International Cognitive Assessment for Multiple Sclerosis.
Subjects were considered to have a problem with “hazardous cannabis use” if they met or exceeded the CUDIT-R’s clinical cut-off of 8 points. The test asks about topics such as hazardous behavior while using cannabis, problems with memory or concentration after using it, and inability to stop using it. Twelve participants met this criteria, and they were more likely to have more symptoms of depression (beta = 0.32; P less than .01) and anxiety (beta = 0.24; P = .02), after researchers controlled for age, years of education, and MS subtype.
They also were slightly more likely to have more severe fatigue (beta = 0.20; P = .07) and poor sleep (beta = 0.20; P = .07).
The researchers found no link between cannabis use and scores on the cognitive test, although Dr. Hughes noted that other research has suggested such a link.
The study is cross-sectional and does not offer insight into cause and effect, Dr. Hughes said. She noted that it is possible that patients used cannabis because they had higher levels of anxiety and depression.
No study funding was reported and the authors report no relevant disclosures.
REPORTING FROM CMSC 2019
Team sports may mitigate tough childhoods
Individuals who experienced adverse childhood experiences but also played team sports as teens were less likely to have mental health problems in adulthood than those with childhood challenges who did not play sports, based on data from nearly 5,000 individuals.
Physical and mental health problems are more prominent throughout life among those exposed to adverse childhood experiences (ACEs), and physical activity in general and team sports in particular have been shown to improve mental health, wrote Molly C. Easterlin, MD, of the University of California, Los Angeles, and colleagues.
In a study published in JAMA Pediatrics, the researchers used data from the National Longitudinal Study of Adolescent to Adult Health to compare the development of depression, anxiety, or depressive symptoms among those with childhood ACEs who did and did not participate in team sports in adolescence.
Overall, team sports participation was significantly associated with reduced odds of depression (adjusted odds ratio, 0.76), anxiety (aOR, 0.70), and depressive symptoms (aOR, 0.85) in young adulthood for individuals with ACEs, compared with those with ACEs who did not play team sports.
Of 9,668 adolescents in the study, 4,888 individuals reported one or more ACEs and 2,084 reported two or more ACEs. The researchers compared data from the 1994-1995 school year when participants were in grades 7-12 and in 2008 to assess their mental health as young adults (aged 24-32 years).
No significant differences in associations appeared between sports participation and mental health between males and females.
The results were limited by several factors including the study design that did not allow for causality and the potential social desirability bias that might lead to underreporting ACEs, Dr. Easterlin and associates noted.
Nonetheless, “given that participation in team sports was associated with improved adult mental health among those with ACEs, and parents might consider enrolling their children with ACEs in team sports,” they wrote.
Dr. Easterlin is supported by the Cedars-Sinai Medical Center via the UCLA National Clinician Scholars Program. The authors reported no conflicts of interest.
SOURCE: Easterlin MC et al. JAMA Pediatr. 2019 May 28. doi: 10.1001/jamapediatrics.2019.1212.
Approximately half of children suffer an adverse childhood experience (ACE) that can negatively affect their mental health throughout life, and “team sports can be an avenue to interrupt these negative sequelae and address the important public health burden of depression,” wrote Amanda E. Paluch, PhD; Nia Heard-Garris, MD, MSc; and Mercedes R. Carnethon, PhD.
However, a significant socioeconomic disparity in team sports for children continues to grow in the United States, driven in part by a youth sports industry and culture that caters to high-income families looking to improve their children’s performance. “Although unintentional, these expenses leave behind lower-income children,” many of whom may be at increased risk for ACEs, the editorialists noted. Many inexpensive, community-based recreation leagues, especially in low-income areas, are often underfunded and unable to update facilities and attract more participants.
The benefits of team sports appear to go beyond the physical, as the study by Easterlin et al. suggests that feeling accepted and connected as part of a team has an impact on mental health. Also, the winning and losing of sports helps build emotional resilience that carries over to other areas of life, the editorialists added.
“Optimizing the opportunities for sports during adolescence requires relatively few resources and is a low-cost way to improve quality of life and reduce the population burden of mental health disorders, especially for adolescents and young adults with histories of ACEs,” they concluded.
Dr. Paluch and Dr. Carnethon are affiliated with the department of preventive medicine and Dr. Heard-Garris is affiliated with the department of pediatrics at Northwestern University, Chicago. They commented on the study by Easterlin et al (JAMA Pediatr. 2019 May 28. doi:10.1001/jamapediatrics.2019.1209). They reported no conflicts of interest.
Approximately half of children suffer an adverse childhood experience (ACE) that can negatively affect their mental health throughout life, and “team sports can be an avenue to interrupt these negative sequelae and address the important public health burden of depression,” wrote Amanda E. Paluch, PhD; Nia Heard-Garris, MD, MSc; and Mercedes R. Carnethon, PhD.
However, a significant socioeconomic disparity in team sports for children continues to grow in the United States, driven in part by a youth sports industry and culture that caters to high-income families looking to improve their children’s performance. “Although unintentional, these expenses leave behind lower-income children,” many of whom may be at increased risk for ACEs, the editorialists noted. Many inexpensive, community-based recreation leagues, especially in low-income areas, are often underfunded and unable to update facilities and attract more participants.
The benefits of team sports appear to go beyond the physical, as the study by Easterlin et al. suggests that feeling accepted and connected as part of a team has an impact on mental health. Also, the winning and losing of sports helps build emotional resilience that carries over to other areas of life, the editorialists added.
“Optimizing the opportunities for sports during adolescence requires relatively few resources and is a low-cost way to improve quality of life and reduce the population burden of mental health disorders, especially for adolescents and young adults with histories of ACEs,” they concluded.
Dr. Paluch and Dr. Carnethon are affiliated with the department of preventive medicine and Dr. Heard-Garris is affiliated with the department of pediatrics at Northwestern University, Chicago. They commented on the study by Easterlin et al (JAMA Pediatr. 2019 May 28. doi:10.1001/jamapediatrics.2019.1209). They reported no conflicts of interest.
Approximately half of children suffer an adverse childhood experience (ACE) that can negatively affect their mental health throughout life, and “team sports can be an avenue to interrupt these negative sequelae and address the important public health burden of depression,” wrote Amanda E. Paluch, PhD; Nia Heard-Garris, MD, MSc; and Mercedes R. Carnethon, PhD.
However, a significant socioeconomic disparity in team sports for children continues to grow in the United States, driven in part by a youth sports industry and culture that caters to high-income families looking to improve their children’s performance. “Although unintentional, these expenses leave behind lower-income children,” many of whom may be at increased risk for ACEs, the editorialists noted. Many inexpensive, community-based recreation leagues, especially in low-income areas, are often underfunded and unable to update facilities and attract more participants.
The benefits of team sports appear to go beyond the physical, as the study by Easterlin et al. suggests that feeling accepted and connected as part of a team has an impact on mental health. Also, the winning and losing of sports helps build emotional resilience that carries over to other areas of life, the editorialists added.
“Optimizing the opportunities for sports during adolescence requires relatively few resources and is a low-cost way to improve quality of life and reduce the population burden of mental health disorders, especially for adolescents and young adults with histories of ACEs,” they concluded.
Dr. Paluch and Dr. Carnethon are affiliated with the department of preventive medicine and Dr. Heard-Garris is affiliated with the department of pediatrics at Northwestern University, Chicago. They commented on the study by Easterlin et al (JAMA Pediatr. 2019 May 28. doi:10.1001/jamapediatrics.2019.1209). They reported no conflicts of interest.
Individuals who experienced adverse childhood experiences but also played team sports as teens were less likely to have mental health problems in adulthood than those with childhood challenges who did not play sports, based on data from nearly 5,000 individuals.
Physical and mental health problems are more prominent throughout life among those exposed to adverse childhood experiences (ACEs), and physical activity in general and team sports in particular have been shown to improve mental health, wrote Molly C. Easterlin, MD, of the University of California, Los Angeles, and colleagues.
In a study published in JAMA Pediatrics, the researchers used data from the National Longitudinal Study of Adolescent to Adult Health to compare the development of depression, anxiety, or depressive symptoms among those with childhood ACEs who did and did not participate in team sports in adolescence.
Overall, team sports participation was significantly associated with reduced odds of depression (adjusted odds ratio, 0.76), anxiety (aOR, 0.70), and depressive symptoms (aOR, 0.85) in young adulthood for individuals with ACEs, compared with those with ACEs who did not play team sports.
Of 9,668 adolescents in the study, 4,888 individuals reported one or more ACEs and 2,084 reported two or more ACEs. The researchers compared data from the 1994-1995 school year when participants were in grades 7-12 and in 2008 to assess their mental health as young adults (aged 24-32 years).
No significant differences in associations appeared between sports participation and mental health between males and females.
The results were limited by several factors including the study design that did not allow for causality and the potential social desirability bias that might lead to underreporting ACEs, Dr. Easterlin and associates noted.
Nonetheless, “given that participation in team sports was associated with improved adult mental health among those with ACEs, and parents might consider enrolling their children with ACEs in team sports,” they wrote.
Dr. Easterlin is supported by the Cedars-Sinai Medical Center via the UCLA National Clinician Scholars Program. The authors reported no conflicts of interest.
SOURCE: Easterlin MC et al. JAMA Pediatr. 2019 May 28. doi: 10.1001/jamapediatrics.2019.1212.
Individuals who experienced adverse childhood experiences but also played team sports as teens were less likely to have mental health problems in adulthood than those with childhood challenges who did not play sports, based on data from nearly 5,000 individuals.
Physical and mental health problems are more prominent throughout life among those exposed to adverse childhood experiences (ACEs), and physical activity in general and team sports in particular have been shown to improve mental health, wrote Molly C. Easterlin, MD, of the University of California, Los Angeles, and colleagues.
In a study published in JAMA Pediatrics, the researchers used data from the National Longitudinal Study of Adolescent to Adult Health to compare the development of depression, anxiety, or depressive symptoms among those with childhood ACEs who did and did not participate in team sports in adolescence.
Overall, team sports participation was significantly associated with reduced odds of depression (adjusted odds ratio, 0.76), anxiety (aOR, 0.70), and depressive symptoms (aOR, 0.85) in young adulthood for individuals with ACEs, compared with those with ACEs who did not play team sports.
Of 9,668 adolescents in the study, 4,888 individuals reported one or more ACEs and 2,084 reported two or more ACEs. The researchers compared data from the 1994-1995 school year when participants were in grades 7-12 and in 2008 to assess their mental health as young adults (aged 24-32 years).
No significant differences in associations appeared between sports participation and mental health between males and females.
The results were limited by several factors including the study design that did not allow for causality and the potential social desirability bias that might lead to underreporting ACEs, Dr. Easterlin and associates noted.
Nonetheless, “given that participation in team sports was associated with improved adult mental health among those with ACEs, and parents might consider enrolling their children with ACEs in team sports,” they wrote.
Dr. Easterlin is supported by the Cedars-Sinai Medical Center via the UCLA National Clinician Scholars Program. The authors reported no conflicts of interest.
SOURCE: Easterlin MC et al. JAMA Pediatr. 2019 May 28. doi: 10.1001/jamapediatrics.2019.1212.
FROM JAMA PEDIATRICS
About one-third of anxiety patients relapse after stopping antidepressants
SAN FRANCISCO – Relapse is more likely in the absence of medication and, if they resume their antidepressant after relapse, some patients experience adverse events or drug resistance.
“It’s important that we realize that anxiety disorders can be treated effectively in the short term, but it’s very difficult to treat them for the long term. We know that within a year, it’s better to continue the medication. There’s a lack of data to give evidence-based advice after 1 year,” Neeltje Batelaan, MD, PhD, a psychiatrist and senior researcher at VU University Medical Center, Amsterdam, said in an interview.
Dr. Batelaan moderated a session at the annual meeting of the American Psychiatric Association on discontinuation of antidepressant medications in these patients.
Anxiety disorders can often be successfully treated with antidepressants, but their adverse effects can become less tolerable over time, especially after patients go into remission. When patients begin treatment, they are willing to endure side effects in the service of resolving their symptoms. But when they go into remission, “they want to get on with their lives, so their sexual side effects or their weight gain rates a lot worse,” Dr. Batelaan said.
A meta-analysis of 28 studies, with follow-up periods ranging from 8 to 52 weeks, found that the anxiety relapse risk after discontinuation of antidepressants was 36.4%, compared with 16.4% in those who stayed on medication. Even continuing antidepressants isn’t completely protective, she noted. The study found a number needed to treat of five to prevent one relapse.
Researchers at the VU University Medical Center developed a cognitive-behavioral therapy (CBT) regimen aimed at reducing anxiety relapses. In their study, 87 patients with a remitted anxiety disorder who wanted to stop their antidepressants were randomized to do so either with or without CBT intervention.
Unfortunately, the study had to be stopped when an interim analysis showed a lack of efficacy. In fact, patients who received CBT actually had higher relapse rates. Surprisingly, just 37% of patients succeeded in completely discontinuing medication, which hints at the inherent challenges of the transition.
“Unfortunately, building a CBT relapse prevention did not come true, but we learned some valuable lessons that will guide further studies,” Willemijn Scholten, PhD, a postdoc researcher at VU University Medical Center, said during one of the presentations.
In his presentation, Anton (Ton) Van Balkom, MD, PhD, professor of psychiatry at VU University Medical Center, recounted the case of a woman who had been functioning well with an antidepressant but grew tired of the sexual side effects. She carefully discontinued her medication under his guidance, but in 2 months she experienced her first panic attack in 30 years. Reintroducing the medication failed to resolve the issue, and it took years of effort before cognitive behavioral therapy resulted in a remission.
Further, a meta-analysis of nine studies showed that 17% of patients with remitted anxiety who went off and then restarted their antidepressants experienced tachycardia.
To help reduce tachycardia, Dr. Van Balkom suggested alternative options to antidepressants in less-complicated patients with anxiety, and to anticipate long-term use of antidepressants once those medications are employed.
Dr. Batelaan agreed with that assessment, drawing an analogy with type 2 diabetes. “You first start with a diet, and advise the patient to lose weight, and then if that’s not successful you go to [medication] and you realize it’s lifelong. Maybe we have to differentiate antidepressant therapies and [not start them until necessary]. But if you have to start them, realize that there’s a difficult decision waiting ahead.”
SAN FRANCISCO – Relapse is more likely in the absence of medication and, if they resume their antidepressant after relapse, some patients experience adverse events or drug resistance.
“It’s important that we realize that anxiety disorders can be treated effectively in the short term, but it’s very difficult to treat them for the long term. We know that within a year, it’s better to continue the medication. There’s a lack of data to give evidence-based advice after 1 year,” Neeltje Batelaan, MD, PhD, a psychiatrist and senior researcher at VU University Medical Center, Amsterdam, said in an interview.
Dr. Batelaan moderated a session at the annual meeting of the American Psychiatric Association on discontinuation of antidepressant medications in these patients.
Anxiety disorders can often be successfully treated with antidepressants, but their adverse effects can become less tolerable over time, especially after patients go into remission. When patients begin treatment, they are willing to endure side effects in the service of resolving their symptoms. But when they go into remission, “they want to get on with their lives, so their sexual side effects or their weight gain rates a lot worse,” Dr. Batelaan said.
A meta-analysis of 28 studies, with follow-up periods ranging from 8 to 52 weeks, found that the anxiety relapse risk after discontinuation of antidepressants was 36.4%, compared with 16.4% in those who stayed on medication. Even continuing antidepressants isn’t completely protective, she noted. The study found a number needed to treat of five to prevent one relapse.
Researchers at the VU University Medical Center developed a cognitive-behavioral therapy (CBT) regimen aimed at reducing anxiety relapses. In their study, 87 patients with a remitted anxiety disorder who wanted to stop their antidepressants were randomized to do so either with or without CBT intervention.
Unfortunately, the study had to be stopped when an interim analysis showed a lack of efficacy. In fact, patients who received CBT actually had higher relapse rates. Surprisingly, just 37% of patients succeeded in completely discontinuing medication, which hints at the inherent challenges of the transition.
“Unfortunately, building a CBT relapse prevention did not come true, but we learned some valuable lessons that will guide further studies,” Willemijn Scholten, PhD, a postdoc researcher at VU University Medical Center, said during one of the presentations.
In his presentation, Anton (Ton) Van Balkom, MD, PhD, professor of psychiatry at VU University Medical Center, recounted the case of a woman who had been functioning well with an antidepressant but grew tired of the sexual side effects. She carefully discontinued her medication under his guidance, but in 2 months she experienced her first panic attack in 30 years. Reintroducing the medication failed to resolve the issue, and it took years of effort before cognitive behavioral therapy resulted in a remission.
Further, a meta-analysis of nine studies showed that 17% of patients with remitted anxiety who went off and then restarted their antidepressants experienced tachycardia.
To help reduce tachycardia, Dr. Van Balkom suggested alternative options to antidepressants in less-complicated patients with anxiety, and to anticipate long-term use of antidepressants once those medications are employed.
Dr. Batelaan agreed with that assessment, drawing an analogy with type 2 diabetes. “You first start with a diet, and advise the patient to lose weight, and then if that’s not successful you go to [medication] and you realize it’s lifelong. Maybe we have to differentiate antidepressant therapies and [not start them until necessary]. But if you have to start them, realize that there’s a difficult decision waiting ahead.”
SAN FRANCISCO – Relapse is more likely in the absence of medication and, if they resume their antidepressant after relapse, some patients experience adverse events or drug resistance.
“It’s important that we realize that anxiety disorders can be treated effectively in the short term, but it’s very difficult to treat them for the long term. We know that within a year, it’s better to continue the medication. There’s a lack of data to give evidence-based advice after 1 year,” Neeltje Batelaan, MD, PhD, a psychiatrist and senior researcher at VU University Medical Center, Amsterdam, said in an interview.
Dr. Batelaan moderated a session at the annual meeting of the American Psychiatric Association on discontinuation of antidepressant medications in these patients.
Anxiety disorders can often be successfully treated with antidepressants, but their adverse effects can become less tolerable over time, especially after patients go into remission. When patients begin treatment, they are willing to endure side effects in the service of resolving their symptoms. But when they go into remission, “they want to get on with their lives, so their sexual side effects or their weight gain rates a lot worse,” Dr. Batelaan said.
A meta-analysis of 28 studies, with follow-up periods ranging from 8 to 52 weeks, found that the anxiety relapse risk after discontinuation of antidepressants was 36.4%, compared with 16.4% in those who stayed on medication. Even continuing antidepressants isn’t completely protective, she noted. The study found a number needed to treat of five to prevent one relapse.
Researchers at the VU University Medical Center developed a cognitive-behavioral therapy (CBT) regimen aimed at reducing anxiety relapses. In their study, 87 patients with a remitted anxiety disorder who wanted to stop their antidepressants were randomized to do so either with or without CBT intervention.
Unfortunately, the study had to be stopped when an interim analysis showed a lack of efficacy. In fact, patients who received CBT actually had higher relapse rates. Surprisingly, just 37% of patients succeeded in completely discontinuing medication, which hints at the inherent challenges of the transition.
“Unfortunately, building a CBT relapse prevention did not come true, but we learned some valuable lessons that will guide further studies,” Willemijn Scholten, PhD, a postdoc researcher at VU University Medical Center, said during one of the presentations.
In his presentation, Anton (Ton) Van Balkom, MD, PhD, professor of psychiatry at VU University Medical Center, recounted the case of a woman who had been functioning well with an antidepressant but grew tired of the sexual side effects. She carefully discontinued her medication under his guidance, but in 2 months she experienced her first panic attack in 30 years. Reintroducing the medication failed to resolve the issue, and it took years of effort before cognitive behavioral therapy resulted in a remission.
Further, a meta-analysis of nine studies showed that 17% of patients with remitted anxiety who went off and then restarted their antidepressants experienced tachycardia.
To help reduce tachycardia, Dr. Van Balkom suggested alternative options to antidepressants in less-complicated patients with anxiety, and to anticipate long-term use of antidepressants once those medications are employed.
Dr. Batelaan agreed with that assessment, drawing an analogy with type 2 diabetes. “You first start with a diet, and advise the patient to lose weight, and then if that’s not successful you go to [medication] and you realize it’s lifelong. Maybe we have to differentiate antidepressant therapies and [not start them until necessary]. But if you have to start them, realize that there’s a difficult decision waiting ahead.”
REPORTING FROM APA 2019
Benzodiazepines nearly double the odds of spontaneous abortion
Early-pregnancy spontaneous abortion was almost twice as common among women who used benzodiazepines, according to 17 years’ worth of data from the Quebec Pregnancy Cohort, which prospectively collects data on all pregnancies of women covered by the Quebec Public Prescription Drug Insurance Plan.
The findings suggest the need for caution before prescribing benzodiazepines to treat insomnia and mood or anxiety disorders in early pregnancy. “Alternative nonpharmacologic treatments exist and are recommended, but if benzodiazepines are needed, they should be prescribed for short durations,” wrote Odile Sheehy, MSc, of the Research Center at Centre Hospitalier Universitaire Sainte-Justine, Montreal, and colleagues, in a study published in JAMA Psychiatry.
The researchers evaluated data from 27,149 study-eligible women who had a spontaneous abortion after 6 weeks’ gestation and before 20 weeks’ gestation between Jan. 1, 1998, and Dec. 31, 2015. Among filled prescriptions, at least one benzodiazepine was used by 375 (1.4%) of the women. These women were matched with five randomly selected control pregnancies per case. The data were adjusted for diagnoses of mood and anxiety disorders and insomnia as well as for several documented proxies of these diseases, such as concomitant exposure to antidepressants or antipsychotics, visits to a psychiatrist, comorbidities, and hospitalizations.
The investigators found an adjusted odds ratio (aOR) of 1.85 (95% confidence interval, 1.61-2.12) for benzodiazepine use. The odds of spontaneous abortion was increased with use of all types of benzodiazepines evaluated in the study, with aORs as low as 1.13 and as high as 3.43, as well as similar aORs between long-acting and short-acting benzodiazepines (1.81 vs. 1.73, respectively).
While the information is accurate regarding filled prescriptions, the findings might not apply to women with private drug insurance as the study included only women in a prescription drug program, the researchers said. They noted, however, that pregnant women receiving medication insurance from Quebec’s public system have characteristics and comorbidities similar to those of women who are covered by private medication insurance.
One author reported being a consultant for plaintiffs in litigations involving antidepressants and birth defects. No other disclosures were reported.
SOURCE: Sheehy O et al. JAMA Psychiatry. 2019 May 15. doi: 10.1001/jamapsychiatry.2019.0963.
Early-pregnancy spontaneous abortion was almost twice as common among women who used benzodiazepines, according to 17 years’ worth of data from the Quebec Pregnancy Cohort, which prospectively collects data on all pregnancies of women covered by the Quebec Public Prescription Drug Insurance Plan.
The findings suggest the need for caution before prescribing benzodiazepines to treat insomnia and mood or anxiety disorders in early pregnancy. “Alternative nonpharmacologic treatments exist and are recommended, but if benzodiazepines are needed, they should be prescribed for short durations,” wrote Odile Sheehy, MSc, of the Research Center at Centre Hospitalier Universitaire Sainte-Justine, Montreal, and colleagues, in a study published in JAMA Psychiatry.
The researchers evaluated data from 27,149 study-eligible women who had a spontaneous abortion after 6 weeks’ gestation and before 20 weeks’ gestation between Jan. 1, 1998, and Dec. 31, 2015. Among filled prescriptions, at least one benzodiazepine was used by 375 (1.4%) of the women. These women were matched with five randomly selected control pregnancies per case. The data were adjusted for diagnoses of mood and anxiety disorders and insomnia as well as for several documented proxies of these diseases, such as concomitant exposure to antidepressants or antipsychotics, visits to a psychiatrist, comorbidities, and hospitalizations.
The investigators found an adjusted odds ratio (aOR) of 1.85 (95% confidence interval, 1.61-2.12) for benzodiazepine use. The odds of spontaneous abortion was increased with use of all types of benzodiazepines evaluated in the study, with aORs as low as 1.13 and as high as 3.43, as well as similar aORs between long-acting and short-acting benzodiazepines (1.81 vs. 1.73, respectively).
While the information is accurate regarding filled prescriptions, the findings might not apply to women with private drug insurance as the study included only women in a prescription drug program, the researchers said. They noted, however, that pregnant women receiving medication insurance from Quebec’s public system have characteristics and comorbidities similar to those of women who are covered by private medication insurance.
One author reported being a consultant for plaintiffs in litigations involving antidepressants and birth defects. No other disclosures were reported.
SOURCE: Sheehy O et al. JAMA Psychiatry. 2019 May 15. doi: 10.1001/jamapsychiatry.2019.0963.
Early-pregnancy spontaneous abortion was almost twice as common among women who used benzodiazepines, according to 17 years’ worth of data from the Quebec Pregnancy Cohort, which prospectively collects data on all pregnancies of women covered by the Quebec Public Prescription Drug Insurance Plan.
The findings suggest the need for caution before prescribing benzodiazepines to treat insomnia and mood or anxiety disorders in early pregnancy. “Alternative nonpharmacologic treatments exist and are recommended, but if benzodiazepines are needed, they should be prescribed for short durations,” wrote Odile Sheehy, MSc, of the Research Center at Centre Hospitalier Universitaire Sainte-Justine, Montreal, and colleagues, in a study published in JAMA Psychiatry.
The researchers evaluated data from 27,149 study-eligible women who had a spontaneous abortion after 6 weeks’ gestation and before 20 weeks’ gestation between Jan. 1, 1998, and Dec. 31, 2015. Among filled prescriptions, at least one benzodiazepine was used by 375 (1.4%) of the women. These women were matched with five randomly selected control pregnancies per case. The data were adjusted for diagnoses of mood and anxiety disorders and insomnia as well as for several documented proxies of these diseases, such as concomitant exposure to antidepressants or antipsychotics, visits to a psychiatrist, comorbidities, and hospitalizations.
The investigators found an adjusted odds ratio (aOR) of 1.85 (95% confidence interval, 1.61-2.12) for benzodiazepine use. The odds of spontaneous abortion was increased with use of all types of benzodiazepines evaluated in the study, with aORs as low as 1.13 and as high as 3.43, as well as similar aORs between long-acting and short-acting benzodiazepines (1.81 vs. 1.73, respectively).
While the information is accurate regarding filled prescriptions, the findings might not apply to women with private drug insurance as the study included only women in a prescription drug program, the researchers said. They noted, however, that pregnant women receiving medication insurance from Quebec’s public system have characteristics and comorbidities similar to those of women who are covered by private medication insurance.
One author reported being a consultant for plaintiffs in litigations involving antidepressants and birth defects. No other disclosures were reported.
SOURCE: Sheehy O et al. JAMA Psychiatry. 2019 May 15. doi: 10.1001/jamapsychiatry.2019.0963.
FROM JAMA PSYCHIATRY
More than 40% of U.K. physicians report binge drinking
Many doctors say they cope with job-related stress by drinking alcohol or taking drugs
Occupational distress among physicians is tied to increased odds of substance use, sleep disturbance, binge eating, and poor health in general, a cross-sectional study of 417 U.K. doctors shows.
Burned-out or depressed doctors had higher risks of those health problems regardless of whether or not they worked in a hospital setting, according to Asta Medisauskaite, PhD, of University College London and Caroline Kamau, PhD, of the University of London. The study was published in BMJ Open.
The investigators asked the participants to answer a battery of validated questionnaires online, including the Alcohol Use Disorders Identification Test, the Eating Disorder Diagnostic Scale, and the Insomnia Severity Index.
The odds of many health problems were increased among the physicians with emotional exhaustion, as indicated by the Maslach Burnout Inventory, as was also the case with psychiatric disorders, according to the General Health Questionnaire-12, which investigators noted has been used extensively to examine medical doctors and other working populations.
Sleep disturbances were, for example, more likely in physicians with burnout or psychiatric morbidity, with odds ratios ranging from 1.344 to 3.826, the investigators reported. Likewise, these indicators of occupational distress increased the risk of suffering from frequent poor health, with odds ratios from 1.050 to 3.544, and of binge eating, with odds ratios from 1.311 to 1.841, Dr. Medisauskaite and Dr. Kamau reported.
Distressed doctors more often used alcohol, according to the researchers, who said that they found a higher risk of alcohol dependence (odds ratio, 6.165) among physicians reporting that they used substances to feel better or cope with stress. Those doctors also had higher risk of binge drinking, drinking larger quantities, and using alcohol more often, the data show. In fact, 44% of the physicians reported binge drinking, and 5% met the criteria for alcohol dependence, Dr. Medisauskaite and Dr. Kamau wrote. Binge drinking was defined as consuming more than six drinks on a single occasion.
Previous studies have indicated that occupational distress among physicians has negative effects on quality of care and patient safety, the authors noted. This latest cross-sectional study builds on those findings by showing that occupational distress increases risk of health problems among doctors. “The impact of occupational distress or ill health could increase levels of sickness-absence among doctors, thus reducing patient safety because of understaffing,” Dr. Medisauskaite and Dr. Kamau wrote.
Similarly, physicians with sleep problems or substance use related to occupational distress could perform poorly on the job because of being groggy, intoxicated, or hung over, they said.
“We recommend that doctors’ mentors, supervisors, peers, and occupational health support services recognize and act on (1) the prevalence of occupational distress and health problems among doctors; (2) the possibility that occupational distress raises the risk of several health problems; and (3) the need to provide early interventions,” Dr. Medisauskaite and Dr. Kamau wrote. Such interventions could help prevent physicians who are experiencing occupational distress from suffering the long-term health effects from sleep disturbances, binge drinking and binge eating, and ill health, they suggested.
One limitation cited was the study’s cross-sectional design, which makes it impossible to draw conclusions about causation. The researchers also conceded that some participants might not have been comfortable answering questions about illicit use of drugs or alcohol. Nevertheless, they said,
Dr. Medisauskaite and Dr. Kamau declared no competing interests related to the study.
SOURCE: Medisauskaite A, Kamau C. BMJ Open. 2019 May 15. doi: 10.1136/bmjopen-2018-027362.
Many doctors say they cope with job-related stress by drinking alcohol or taking drugs
Many doctors say they cope with job-related stress by drinking alcohol or taking drugs
Occupational distress among physicians is tied to increased odds of substance use, sleep disturbance, binge eating, and poor health in general, a cross-sectional study of 417 U.K. doctors shows.
Burned-out or depressed doctors had higher risks of those health problems regardless of whether or not they worked in a hospital setting, according to Asta Medisauskaite, PhD, of University College London and Caroline Kamau, PhD, of the University of London. The study was published in BMJ Open.
The investigators asked the participants to answer a battery of validated questionnaires online, including the Alcohol Use Disorders Identification Test, the Eating Disorder Diagnostic Scale, and the Insomnia Severity Index.
The odds of many health problems were increased among the physicians with emotional exhaustion, as indicated by the Maslach Burnout Inventory, as was also the case with psychiatric disorders, according to the General Health Questionnaire-12, which investigators noted has been used extensively to examine medical doctors and other working populations.
Sleep disturbances were, for example, more likely in physicians with burnout or psychiatric morbidity, with odds ratios ranging from 1.344 to 3.826, the investigators reported. Likewise, these indicators of occupational distress increased the risk of suffering from frequent poor health, with odds ratios from 1.050 to 3.544, and of binge eating, with odds ratios from 1.311 to 1.841, Dr. Medisauskaite and Dr. Kamau reported.
Distressed doctors more often used alcohol, according to the researchers, who said that they found a higher risk of alcohol dependence (odds ratio, 6.165) among physicians reporting that they used substances to feel better or cope with stress. Those doctors also had higher risk of binge drinking, drinking larger quantities, and using alcohol more often, the data show. In fact, 44% of the physicians reported binge drinking, and 5% met the criteria for alcohol dependence, Dr. Medisauskaite and Dr. Kamau wrote. Binge drinking was defined as consuming more than six drinks on a single occasion.
Previous studies have indicated that occupational distress among physicians has negative effects on quality of care and patient safety, the authors noted. This latest cross-sectional study builds on those findings by showing that occupational distress increases risk of health problems among doctors. “The impact of occupational distress or ill health could increase levels of sickness-absence among doctors, thus reducing patient safety because of understaffing,” Dr. Medisauskaite and Dr. Kamau wrote.
Similarly, physicians with sleep problems or substance use related to occupational distress could perform poorly on the job because of being groggy, intoxicated, or hung over, they said.
“We recommend that doctors’ mentors, supervisors, peers, and occupational health support services recognize and act on (1) the prevalence of occupational distress and health problems among doctors; (2) the possibility that occupational distress raises the risk of several health problems; and (3) the need to provide early interventions,” Dr. Medisauskaite and Dr. Kamau wrote. Such interventions could help prevent physicians who are experiencing occupational distress from suffering the long-term health effects from sleep disturbances, binge drinking and binge eating, and ill health, they suggested.
One limitation cited was the study’s cross-sectional design, which makes it impossible to draw conclusions about causation. The researchers also conceded that some participants might not have been comfortable answering questions about illicit use of drugs or alcohol. Nevertheless, they said,
Dr. Medisauskaite and Dr. Kamau declared no competing interests related to the study.
SOURCE: Medisauskaite A, Kamau C. BMJ Open. 2019 May 15. doi: 10.1136/bmjopen-2018-027362.
Occupational distress among physicians is tied to increased odds of substance use, sleep disturbance, binge eating, and poor health in general, a cross-sectional study of 417 U.K. doctors shows.
Burned-out or depressed doctors had higher risks of those health problems regardless of whether or not they worked in a hospital setting, according to Asta Medisauskaite, PhD, of University College London and Caroline Kamau, PhD, of the University of London. The study was published in BMJ Open.
The investigators asked the participants to answer a battery of validated questionnaires online, including the Alcohol Use Disorders Identification Test, the Eating Disorder Diagnostic Scale, and the Insomnia Severity Index.
The odds of many health problems were increased among the physicians with emotional exhaustion, as indicated by the Maslach Burnout Inventory, as was also the case with psychiatric disorders, according to the General Health Questionnaire-12, which investigators noted has been used extensively to examine medical doctors and other working populations.
Sleep disturbances were, for example, more likely in physicians with burnout or psychiatric morbidity, with odds ratios ranging from 1.344 to 3.826, the investigators reported. Likewise, these indicators of occupational distress increased the risk of suffering from frequent poor health, with odds ratios from 1.050 to 3.544, and of binge eating, with odds ratios from 1.311 to 1.841, Dr. Medisauskaite and Dr. Kamau reported.
Distressed doctors more often used alcohol, according to the researchers, who said that they found a higher risk of alcohol dependence (odds ratio, 6.165) among physicians reporting that they used substances to feel better or cope with stress. Those doctors also had higher risk of binge drinking, drinking larger quantities, and using alcohol more often, the data show. In fact, 44% of the physicians reported binge drinking, and 5% met the criteria for alcohol dependence, Dr. Medisauskaite and Dr. Kamau wrote. Binge drinking was defined as consuming more than six drinks on a single occasion.
Previous studies have indicated that occupational distress among physicians has negative effects on quality of care and patient safety, the authors noted. This latest cross-sectional study builds on those findings by showing that occupational distress increases risk of health problems among doctors. “The impact of occupational distress or ill health could increase levels of sickness-absence among doctors, thus reducing patient safety because of understaffing,” Dr. Medisauskaite and Dr. Kamau wrote.
Similarly, physicians with sleep problems or substance use related to occupational distress could perform poorly on the job because of being groggy, intoxicated, or hung over, they said.
“We recommend that doctors’ mentors, supervisors, peers, and occupational health support services recognize and act on (1) the prevalence of occupational distress and health problems among doctors; (2) the possibility that occupational distress raises the risk of several health problems; and (3) the need to provide early interventions,” Dr. Medisauskaite and Dr. Kamau wrote. Such interventions could help prevent physicians who are experiencing occupational distress from suffering the long-term health effects from sleep disturbances, binge drinking and binge eating, and ill health, they suggested.
One limitation cited was the study’s cross-sectional design, which makes it impossible to draw conclusions about causation. The researchers also conceded that some participants might not have been comfortable answering questions about illicit use of drugs or alcohol. Nevertheless, they said,
Dr. Medisauskaite and Dr. Kamau declared no competing interests related to the study.
SOURCE: Medisauskaite A, Kamau C. BMJ Open. 2019 May 15. doi: 10.1136/bmjopen-2018-027362.
FROM BMJ OPEN
Key clinical point: Occupational distress increased the odds of substance use, sleep disturbance, binge eating, and poor health among medical doctors in the United Kingdom.
Major finding: Distressed medical doctors had a higher risk of binge drinking. In fact, 44% reported binge drinking, defined as consuming more than six drinks on a single occasion.
Study details: A U.K. cross-sectional study of 417 doctors who answered a series of validated health-related questionnaires online.
Disclosures: The authors declared no competing interests related to the study.
Source: Medisauskaite A, Kamau C. BMJ Open. 2019 May 15. doi: 10.1136/bmjopen-2018-027362.
An insidious onset of symptoms
CASE Tremors, increasing anxiety
Ms. S, age 56, has a history of depression and anxiety.
The authors’ observations
The incidence of serotonin syndrome has increased because of increasing use of serotonergic agents.1-3 Although the severity could range from benign to life-threatening, the potential lethality combined with difficulty of diagnosis makes this condition of continued interest. Stimulation of the 5-hydroxytryptamine (5-HT) receptor subtypes, specifically 5-HT1A and 5-HT2, are implicated in this syndrome.4,5
Serotonin syndrome is a clinical diagnosis with a triad of symptoms that includes mental status changes, autonomic hyperactivity, and neuromuscular abnormalities.1,2 However, because of the varied presentation and similarity to other syndromes such as NMS, serotonin syndrome often is undiagnosed.5
TREATMENT Discontinue fluoxetine
Several months after the fluoxetine increase, Ms. S's physical symptoms emerge. Several weeks later, she notices significant hypertension of 162/102 mm Hg by checking her blood pressure at home. She had no history of hypertension before taking fluoxetine. She then tells her psychiatrist she has been experiencing confusion, shakiness, loss of balance, forgetfulness, joint pain, sweating, and fatigue, along with worsening anxiety. The treatment team makes a diagnosis of serotonin syndrome and recommends discontinuing fluoxetine and starting cyproheptadine, 4 mg initially, and then repeating the cyproheptadine dose in several hours if her symptoms do not resolve. Approximately, 2.5 months after the serotonin syndrome reaction, Ms. S receives hydroxyzine, 10 mg every 8 hours, as needed for anxiety.
The authors’ observations
The diagnosis of serotonin syndrome is most accurately made using Hunter Serotonin Toxicity Criteria (Table 16). Because Ms. S had an insidious onset of symptoms, and treatment was initiated before full evaluation, it is unknown if she met Hunter criteria. To meet these criteria, a patient must have ≥1 of the following6:
- spontaneous clonus
- inducible clonus plus agitation or diaphoresis
- ocular clonus plus agitation or diaphoresis
- tremor plus hyperreflexia
- hypertonia plus temperature >38°C plus ocular or inducible clonus.
The Sternbach diagnostic criteria for serotonin syndrome (Table 23) is another commonly used tool.3 These criteria include the addition or increase of a serotonin agent and absence of substances or metabolic derangements that could account for symptoms and at least 3 of the following 10 symptoms3:
- mental status changes (confusion, hypomania)
- agitation
- myoclonus
- hyperreflexia
- diaphoresis
- shivering
- tremor
- diarrhea
- incoordination
- fever.
Continue to: Ms. S met the Sternbach diagnostic criteria...
Ms. S met the Sternbach diagnostic criteria for serotonin syndrome.
Ms. S was taking a single serotonin agent and initially had mild symptoms. More commonly, a patient who presents with serotonin syndrome has been receiving ≥2 serotonergic agents or toxic levels of a single agent, and these agents usually include a psychotropic medication such as a monoamine oxidase inhibitor, tricyclic antidepressant, or SSRI, as well as a medication from a different class, such as dextromethorphan, linezolid, tramadol, methylene blue, and/or St. John’s wort.1,7-13 However, in this case, Ms. S also was taking bupropion, a known inhibitor of cytochrome P450 2D6. Bupropion might have increased Ms. S’s fluoxetine levels.
Ms. S was a healthy, middle-age patient who took no medications other than those listed, had no medical comorbidities, and had a straightforward psychiatric history, which makes the diagnosis of serotonin syndrome clearer. However, other potential differential diagnoses, such as NMS, delirium tremens, and anticholinergic toxicity, might cloud the clinical picture. When differentiating NMS and serotonin syndrome, it is helpful to note whether a patient shows tremor, diarrhea, and myoclonus present in the absence of muscular, “lead-pipe” rigidity, which suggests a diagnosis of serotonin syndrome.2,3,5
[polldaddy:10279173]
The authors’ observations
Treating serotonin syndrome includes supportive care, discontinuing offending agents, administering benzodiazepines, and using a serotonin antagonist as an antidote for patients with moderate-to-severe cases. Cyproheptadine is an antihistaminergic medication with non-specific 5-HT1A and 5-HT2 antagonism. It is FDA-approved for specific allergic reactions, urticaria, and anaphylaxis adjunctive therapy, but not for serotonin syndrome. Case series support the use of cyproheptadine for acute management of serotonin syndrome, with rapid symptom improvement.4,7,14-18 We observed a similar outcome with Ms. S. Her significant autonomic symptoms resolved rapidly, although she experienced some residual, mild symptoms that took weeks to resolve.
Continue to: Because serotonergic agents...
Because serotonergic agents are used frequently and readily by primary care clinicians as well as psychiatrists, the ability to properly diagnose this syndrome is vital, particularly because severe cases can rapidly deteriorate.1,9,16,17 This presentation of a single serotonergic agent causing significant symptoms that worsened over months is not typical, but important to recognize as a patient begins to experience autonomic instability. As was the case with Ms. S, it is important to remain vigilant when changing dosages or adding medications. Symptoms of serotonin syndrome might be vague and difficult to diagnose, especially if the clinician is not aware of the variability of presentation of this syndrome. Cyproheptadine can be used safely and rapidly and should be considered a treatment option for serotonin syndrome.
OUTCOME Hypertension resolves
After her first dose of cyproheptadine, Ms. S’s blood pressure drops to 146/86 mm Hg. Three hours later, she repeats the cyproheptadine dose and her blood pressure drops to 106/60 mm Hg. She reports that her anxiety has lessened, although she is still tremulous. Overall, she says she feels better. She experiences improvement of her condition with a pharmacologic regimen of bupropion, gabapentin, and hydroxyzine.
Several weeks later, her health returns to baseline, with complete resolution of hypertension.
Bottom Line
Although serotonin syndrome is most commonly associated with co-administered serotonergic medications, symptoms can emerge with a single, moderately dosed agent. Treatment includes withdrawing the offending agent, and administering a serotonin antagonist. Mild cases of serotonin syndrome usually resolve.
Related Resources
- Turner AH, Kim JJ, McCarron RM. Differentiating serotonin syndrome and neuroleptic malignant syndrome. Current Psychiatry. 2019;18(2):30-36.
- Iqbal MM, Basil MJ, Kaplan J, et al. Overview of serotonin syndrome. Ann Clin Psychiatry. 2012;24(4):310-318.
Drug Brand Names
Bupropion • Wellbutrin, Zyban
Cyproheptadine • Periactin
Dextromethorphan • Benylin
Duloxetine • Cymbalta
Fluoxetine • Prozac
Gabapentin • Neurontin
Hydroxyzine • Atarax
Linezolid • Zyvox
Tramadol • Ultram, Ryzolt
1. Mason PJ, Morris VA, Balcezak TJ. Serotonin syndrome: presentation of 2 cases and review of literature. Medicine (Baltimore). 2000;79(4):201-209.
2. Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005;352(11):1112-1120.
3. Sternbach H. The serotonin syndrome. Am J Psychiatry. 1991;148(6):705-713.
4. Graudins A, Stearman A, Chan B. Treatment of the serotonin syndrome with cyproheptadine. J Emerg Med. 1998;16(4):615-619.
5. Mills KC. Serotonin syndrome a clinical update. Crit Care Clin. 1997;13(4):763-783.
6. Dunkley EJ, Isbister GK, Sibbritt D, et al. The Hunter Serotonin Toxicity Criteria: simple and accurate diagnostic decision rules for serotonin toxicity. QJM. 2003;96:635-642.
7. Horowitz BZ, Mullins ME. Cyproheptadine for serotonin syndrome in an accidental pediatric sertraline ingestion. Pediatr Emerg Care. 1999;15(5):325-327.
8. Kolecki P. Isolated venlafaxine-induced serotonin syndrome. J Emerg Med. 1996;15:491-493.
9. Pan J, Shen W. Serotonin syndrome induced by low-dose venlafaxine. Ann Pharmacother. 2003;37(2):209-211.
10. Hernández JL, Ramos FJ, Infante J, et al. Severe serotonin syndrome induced by mirtazapine monotherapy. Ann Pharmacother. 2002;36(4):641-643.
11. Patel DD, Galarneau D. Serotonin syndrome with fluoxetine: two case reports. Ochsner J. 2016;16(4):554-557.
12. Frank C. Recognition and treatment of serotonin syndrome. Can Fam Physician. 2008;54(7):988-992.
13. Zuschlag ZD, Warren MW, K Schultz S. Serotonin toxicity and urinary analgesics: a case report and systematic literature review of methylene blue-induced serotonin syndrome. Psychosomatics. 2018;59(6):539-546.
14. Kapur S, Zipursky RB, Jones C, et al. Cyproheptadine: a potent in vivo serotonin antagonist. Am J Psychiatry. 1997;154(6):884.
15. Baigel GD. Cyproheptadine and the treatment of an unconscious patient with the serotonin syndrome. Eur J Anaesthesiol. 2003;20(7):586-588.
16. Lappin RI, Auchincloss EL. Treatment of the serotonin syndrome with cyproheptadine. N Engl J Med. 1994;331:1021-1022.
17. McDaniel WW. Serotonin syndrome: early management with cyproheptadine. Ann Pharmacother. 2001;35(7-8):870-873.
18. Kolecki P. Venlafaxine induced serotonin syndrome occurring after abstinence from phenelzine for more than two weeks. J Toxicol Clin Toxicol. 1997;35(2):211-212.
CASE Tremors, increasing anxiety
Ms. S, age 56, has a history of depression and anxiety.
The authors’ observations
The incidence of serotonin syndrome has increased because of increasing use of serotonergic agents.1-3 Although the severity could range from benign to life-threatening, the potential lethality combined with difficulty of diagnosis makes this condition of continued interest. Stimulation of the 5-hydroxytryptamine (5-HT) receptor subtypes, specifically 5-HT1A and 5-HT2, are implicated in this syndrome.4,5
Serotonin syndrome is a clinical diagnosis with a triad of symptoms that includes mental status changes, autonomic hyperactivity, and neuromuscular abnormalities.1,2 However, because of the varied presentation and similarity to other syndromes such as NMS, serotonin syndrome often is undiagnosed.5
TREATMENT Discontinue fluoxetine
Several months after the fluoxetine increase, Ms. S's physical symptoms emerge. Several weeks later, she notices significant hypertension of 162/102 mm Hg by checking her blood pressure at home. She had no history of hypertension before taking fluoxetine. She then tells her psychiatrist she has been experiencing confusion, shakiness, loss of balance, forgetfulness, joint pain, sweating, and fatigue, along with worsening anxiety. The treatment team makes a diagnosis of serotonin syndrome and recommends discontinuing fluoxetine and starting cyproheptadine, 4 mg initially, and then repeating the cyproheptadine dose in several hours if her symptoms do not resolve. Approximately, 2.5 months after the serotonin syndrome reaction, Ms. S receives hydroxyzine, 10 mg every 8 hours, as needed for anxiety.
The authors’ observations
The diagnosis of serotonin syndrome is most accurately made using Hunter Serotonin Toxicity Criteria (Table 16). Because Ms. S had an insidious onset of symptoms, and treatment was initiated before full evaluation, it is unknown if she met Hunter criteria. To meet these criteria, a patient must have ≥1 of the following6:
- spontaneous clonus
- inducible clonus plus agitation or diaphoresis
- ocular clonus plus agitation or diaphoresis
- tremor plus hyperreflexia
- hypertonia plus temperature >38°C plus ocular or inducible clonus.
The Sternbach diagnostic criteria for serotonin syndrome (Table 23) is another commonly used tool.3 These criteria include the addition or increase of a serotonin agent and absence of substances or metabolic derangements that could account for symptoms and at least 3 of the following 10 symptoms3:
- mental status changes (confusion, hypomania)
- agitation
- myoclonus
- hyperreflexia
- diaphoresis
- shivering
- tremor
- diarrhea
- incoordination
- fever.
Continue to: Ms. S met the Sternbach diagnostic criteria...
Ms. S met the Sternbach diagnostic criteria for serotonin syndrome.
Ms. S was taking a single serotonin agent and initially had mild symptoms. More commonly, a patient who presents with serotonin syndrome has been receiving ≥2 serotonergic agents or toxic levels of a single agent, and these agents usually include a psychotropic medication such as a monoamine oxidase inhibitor, tricyclic antidepressant, or SSRI, as well as a medication from a different class, such as dextromethorphan, linezolid, tramadol, methylene blue, and/or St. John’s wort.1,7-13 However, in this case, Ms. S also was taking bupropion, a known inhibitor of cytochrome P450 2D6. Bupropion might have increased Ms. S’s fluoxetine levels.
Ms. S was a healthy, middle-age patient who took no medications other than those listed, had no medical comorbidities, and had a straightforward psychiatric history, which makes the diagnosis of serotonin syndrome clearer. However, other potential differential diagnoses, such as NMS, delirium tremens, and anticholinergic toxicity, might cloud the clinical picture. When differentiating NMS and serotonin syndrome, it is helpful to note whether a patient shows tremor, diarrhea, and myoclonus present in the absence of muscular, “lead-pipe” rigidity, which suggests a diagnosis of serotonin syndrome.2,3,5
[polldaddy:10279173]
The authors’ observations
Treating serotonin syndrome includes supportive care, discontinuing offending agents, administering benzodiazepines, and using a serotonin antagonist as an antidote for patients with moderate-to-severe cases. Cyproheptadine is an antihistaminergic medication with non-specific 5-HT1A and 5-HT2 antagonism. It is FDA-approved for specific allergic reactions, urticaria, and anaphylaxis adjunctive therapy, but not for serotonin syndrome. Case series support the use of cyproheptadine for acute management of serotonin syndrome, with rapid symptom improvement.4,7,14-18 We observed a similar outcome with Ms. S. Her significant autonomic symptoms resolved rapidly, although she experienced some residual, mild symptoms that took weeks to resolve.
Continue to: Because serotonergic agents...
Because serotonergic agents are used frequently and readily by primary care clinicians as well as psychiatrists, the ability to properly diagnose this syndrome is vital, particularly because severe cases can rapidly deteriorate.1,9,16,17 This presentation of a single serotonergic agent causing significant symptoms that worsened over months is not typical, but important to recognize as a patient begins to experience autonomic instability. As was the case with Ms. S, it is important to remain vigilant when changing dosages or adding medications. Symptoms of serotonin syndrome might be vague and difficult to diagnose, especially if the clinician is not aware of the variability of presentation of this syndrome. Cyproheptadine can be used safely and rapidly and should be considered a treatment option for serotonin syndrome.
OUTCOME Hypertension resolves
After her first dose of cyproheptadine, Ms. S’s blood pressure drops to 146/86 mm Hg. Three hours later, she repeats the cyproheptadine dose and her blood pressure drops to 106/60 mm Hg. She reports that her anxiety has lessened, although she is still tremulous. Overall, she says she feels better. She experiences improvement of her condition with a pharmacologic regimen of bupropion, gabapentin, and hydroxyzine.
Several weeks later, her health returns to baseline, with complete resolution of hypertension.
Bottom Line
Although serotonin syndrome is most commonly associated with co-administered serotonergic medications, symptoms can emerge with a single, moderately dosed agent. Treatment includes withdrawing the offending agent, and administering a serotonin antagonist. Mild cases of serotonin syndrome usually resolve.
Related Resources
- Turner AH, Kim JJ, McCarron RM. Differentiating serotonin syndrome and neuroleptic malignant syndrome. Current Psychiatry. 2019;18(2):30-36.
- Iqbal MM, Basil MJ, Kaplan J, et al. Overview of serotonin syndrome. Ann Clin Psychiatry. 2012;24(4):310-318.
Drug Brand Names
Bupropion • Wellbutrin, Zyban
Cyproheptadine • Periactin
Dextromethorphan • Benylin
Duloxetine • Cymbalta
Fluoxetine • Prozac
Gabapentin • Neurontin
Hydroxyzine • Atarax
Linezolid • Zyvox
Tramadol • Ultram, Ryzolt
CASE Tremors, increasing anxiety
Ms. S, age 56, has a history of depression and anxiety.
The authors’ observations
The incidence of serotonin syndrome has increased because of increasing use of serotonergic agents.1-3 Although the severity could range from benign to life-threatening, the potential lethality combined with difficulty of diagnosis makes this condition of continued interest. Stimulation of the 5-hydroxytryptamine (5-HT) receptor subtypes, specifically 5-HT1A and 5-HT2, are implicated in this syndrome.4,5
Serotonin syndrome is a clinical diagnosis with a triad of symptoms that includes mental status changes, autonomic hyperactivity, and neuromuscular abnormalities.1,2 However, because of the varied presentation and similarity to other syndromes such as NMS, serotonin syndrome often is undiagnosed.5
TREATMENT Discontinue fluoxetine
Several months after the fluoxetine increase, Ms. S's physical symptoms emerge. Several weeks later, she notices significant hypertension of 162/102 mm Hg by checking her blood pressure at home. She had no history of hypertension before taking fluoxetine. She then tells her psychiatrist she has been experiencing confusion, shakiness, loss of balance, forgetfulness, joint pain, sweating, and fatigue, along with worsening anxiety. The treatment team makes a diagnosis of serotonin syndrome and recommends discontinuing fluoxetine and starting cyproheptadine, 4 mg initially, and then repeating the cyproheptadine dose in several hours if her symptoms do not resolve. Approximately, 2.5 months after the serotonin syndrome reaction, Ms. S receives hydroxyzine, 10 mg every 8 hours, as needed for anxiety.
The authors’ observations
The diagnosis of serotonin syndrome is most accurately made using Hunter Serotonin Toxicity Criteria (Table 16). Because Ms. S had an insidious onset of symptoms, and treatment was initiated before full evaluation, it is unknown if she met Hunter criteria. To meet these criteria, a patient must have ≥1 of the following6:
- spontaneous clonus
- inducible clonus plus agitation or diaphoresis
- ocular clonus plus agitation or diaphoresis
- tremor plus hyperreflexia
- hypertonia plus temperature >38°C plus ocular or inducible clonus.
The Sternbach diagnostic criteria for serotonin syndrome (Table 23) is another commonly used tool.3 These criteria include the addition or increase of a serotonin agent and absence of substances or metabolic derangements that could account for symptoms and at least 3 of the following 10 symptoms3:
- mental status changes (confusion, hypomania)
- agitation
- myoclonus
- hyperreflexia
- diaphoresis
- shivering
- tremor
- diarrhea
- incoordination
- fever.
Continue to: Ms. S met the Sternbach diagnostic criteria...
Ms. S met the Sternbach diagnostic criteria for serotonin syndrome.
Ms. S was taking a single serotonin agent and initially had mild symptoms. More commonly, a patient who presents with serotonin syndrome has been receiving ≥2 serotonergic agents or toxic levels of a single agent, and these agents usually include a psychotropic medication such as a monoamine oxidase inhibitor, tricyclic antidepressant, or SSRI, as well as a medication from a different class, such as dextromethorphan, linezolid, tramadol, methylene blue, and/or St. John’s wort.1,7-13 However, in this case, Ms. S also was taking bupropion, a known inhibitor of cytochrome P450 2D6. Bupropion might have increased Ms. S’s fluoxetine levels.
Ms. S was a healthy, middle-age patient who took no medications other than those listed, had no medical comorbidities, and had a straightforward psychiatric history, which makes the diagnosis of serotonin syndrome clearer. However, other potential differential diagnoses, such as NMS, delirium tremens, and anticholinergic toxicity, might cloud the clinical picture. When differentiating NMS and serotonin syndrome, it is helpful to note whether a patient shows tremor, diarrhea, and myoclonus present in the absence of muscular, “lead-pipe” rigidity, which suggests a diagnosis of serotonin syndrome.2,3,5
[polldaddy:10279173]
The authors’ observations
Treating serotonin syndrome includes supportive care, discontinuing offending agents, administering benzodiazepines, and using a serotonin antagonist as an antidote for patients with moderate-to-severe cases. Cyproheptadine is an antihistaminergic medication with non-specific 5-HT1A and 5-HT2 antagonism. It is FDA-approved for specific allergic reactions, urticaria, and anaphylaxis adjunctive therapy, but not for serotonin syndrome. Case series support the use of cyproheptadine for acute management of serotonin syndrome, with rapid symptom improvement.4,7,14-18 We observed a similar outcome with Ms. S. Her significant autonomic symptoms resolved rapidly, although she experienced some residual, mild symptoms that took weeks to resolve.
Continue to: Because serotonergic agents...
Because serotonergic agents are used frequently and readily by primary care clinicians as well as psychiatrists, the ability to properly diagnose this syndrome is vital, particularly because severe cases can rapidly deteriorate.1,9,16,17 This presentation of a single serotonergic agent causing significant symptoms that worsened over months is not typical, but important to recognize as a patient begins to experience autonomic instability. As was the case with Ms. S, it is important to remain vigilant when changing dosages or adding medications. Symptoms of serotonin syndrome might be vague and difficult to diagnose, especially if the clinician is not aware of the variability of presentation of this syndrome. Cyproheptadine can be used safely and rapidly and should be considered a treatment option for serotonin syndrome.
OUTCOME Hypertension resolves
After her first dose of cyproheptadine, Ms. S’s blood pressure drops to 146/86 mm Hg. Three hours later, she repeats the cyproheptadine dose and her blood pressure drops to 106/60 mm Hg. She reports that her anxiety has lessened, although she is still tremulous. Overall, she says she feels better. She experiences improvement of her condition with a pharmacologic regimen of bupropion, gabapentin, and hydroxyzine.
Several weeks later, her health returns to baseline, with complete resolution of hypertension.
Bottom Line
Although serotonin syndrome is most commonly associated with co-administered serotonergic medications, symptoms can emerge with a single, moderately dosed agent. Treatment includes withdrawing the offending agent, and administering a serotonin antagonist. Mild cases of serotonin syndrome usually resolve.
Related Resources
- Turner AH, Kim JJ, McCarron RM. Differentiating serotonin syndrome and neuroleptic malignant syndrome. Current Psychiatry. 2019;18(2):30-36.
- Iqbal MM, Basil MJ, Kaplan J, et al. Overview of serotonin syndrome. Ann Clin Psychiatry. 2012;24(4):310-318.
Drug Brand Names
Bupropion • Wellbutrin, Zyban
Cyproheptadine • Periactin
Dextromethorphan • Benylin
Duloxetine • Cymbalta
Fluoxetine • Prozac
Gabapentin • Neurontin
Hydroxyzine • Atarax
Linezolid • Zyvox
Tramadol • Ultram, Ryzolt
1. Mason PJ, Morris VA, Balcezak TJ. Serotonin syndrome: presentation of 2 cases and review of literature. Medicine (Baltimore). 2000;79(4):201-209.
2. Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005;352(11):1112-1120.
3. Sternbach H. The serotonin syndrome. Am J Psychiatry. 1991;148(6):705-713.
4. Graudins A, Stearman A, Chan B. Treatment of the serotonin syndrome with cyproheptadine. J Emerg Med. 1998;16(4):615-619.
5. Mills KC. Serotonin syndrome a clinical update. Crit Care Clin. 1997;13(4):763-783.
6. Dunkley EJ, Isbister GK, Sibbritt D, et al. The Hunter Serotonin Toxicity Criteria: simple and accurate diagnostic decision rules for serotonin toxicity. QJM. 2003;96:635-642.
7. Horowitz BZ, Mullins ME. Cyproheptadine for serotonin syndrome in an accidental pediatric sertraline ingestion. Pediatr Emerg Care. 1999;15(5):325-327.
8. Kolecki P. Isolated venlafaxine-induced serotonin syndrome. J Emerg Med. 1996;15:491-493.
9. Pan J, Shen W. Serotonin syndrome induced by low-dose venlafaxine. Ann Pharmacother. 2003;37(2):209-211.
10. Hernández JL, Ramos FJ, Infante J, et al. Severe serotonin syndrome induced by mirtazapine monotherapy. Ann Pharmacother. 2002;36(4):641-643.
11. Patel DD, Galarneau D. Serotonin syndrome with fluoxetine: two case reports. Ochsner J. 2016;16(4):554-557.
12. Frank C. Recognition and treatment of serotonin syndrome. Can Fam Physician. 2008;54(7):988-992.
13. Zuschlag ZD, Warren MW, K Schultz S. Serotonin toxicity and urinary analgesics: a case report and systematic literature review of methylene blue-induced serotonin syndrome. Psychosomatics. 2018;59(6):539-546.
14. Kapur S, Zipursky RB, Jones C, et al. Cyproheptadine: a potent in vivo serotonin antagonist. Am J Psychiatry. 1997;154(6):884.
15. Baigel GD. Cyproheptadine and the treatment of an unconscious patient with the serotonin syndrome. Eur J Anaesthesiol. 2003;20(7):586-588.
16. Lappin RI, Auchincloss EL. Treatment of the serotonin syndrome with cyproheptadine. N Engl J Med. 1994;331:1021-1022.
17. McDaniel WW. Serotonin syndrome: early management with cyproheptadine. Ann Pharmacother. 2001;35(7-8):870-873.
18. Kolecki P. Venlafaxine induced serotonin syndrome occurring after abstinence from phenelzine for more than two weeks. J Toxicol Clin Toxicol. 1997;35(2):211-212.
1. Mason PJ, Morris VA, Balcezak TJ. Serotonin syndrome: presentation of 2 cases and review of literature. Medicine (Baltimore). 2000;79(4):201-209.
2. Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005;352(11):1112-1120.
3. Sternbach H. The serotonin syndrome. Am J Psychiatry. 1991;148(6):705-713.
4. Graudins A, Stearman A, Chan B. Treatment of the serotonin syndrome with cyproheptadine. J Emerg Med. 1998;16(4):615-619.
5. Mills KC. Serotonin syndrome a clinical update. Crit Care Clin. 1997;13(4):763-783.
6. Dunkley EJ, Isbister GK, Sibbritt D, et al. The Hunter Serotonin Toxicity Criteria: simple and accurate diagnostic decision rules for serotonin toxicity. QJM. 2003;96:635-642.
7. Horowitz BZ, Mullins ME. Cyproheptadine for serotonin syndrome in an accidental pediatric sertraline ingestion. Pediatr Emerg Care. 1999;15(5):325-327.
8. Kolecki P. Isolated venlafaxine-induced serotonin syndrome. J Emerg Med. 1996;15:491-493.
9. Pan J, Shen W. Serotonin syndrome induced by low-dose venlafaxine. Ann Pharmacother. 2003;37(2):209-211.
10. Hernández JL, Ramos FJ, Infante J, et al. Severe serotonin syndrome induced by mirtazapine monotherapy. Ann Pharmacother. 2002;36(4):641-643.
11. Patel DD, Galarneau D. Serotonin syndrome with fluoxetine: two case reports. Ochsner J. 2016;16(4):554-557.
12. Frank C. Recognition and treatment of serotonin syndrome. Can Fam Physician. 2008;54(7):988-992.
13. Zuschlag ZD, Warren MW, K Schultz S. Serotonin toxicity and urinary analgesics: a case report and systematic literature review of methylene blue-induced serotonin syndrome. Psychosomatics. 2018;59(6):539-546.
14. Kapur S, Zipursky RB, Jones C, et al. Cyproheptadine: a potent in vivo serotonin antagonist. Am J Psychiatry. 1997;154(6):884.
15. Baigel GD. Cyproheptadine and the treatment of an unconscious patient with the serotonin syndrome. Eur J Anaesthesiol. 2003;20(7):586-588.
16. Lappin RI, Auchincloss EL. Treatment of the serotonin syndrome with cyproheptadine. N Engl J Med. 1994;331:1021-1022.
17. McDaniel WW. Serotonin syndrome: early management with cyproheptadine. Ann Pharmacother. 2001;35(7-8):870-873.
18. Kolecki P. Venlafaxine induced serotonin syndrome occurring after abstinence from phenelzine for more than two weeks. J Toxicol Clin Toxicol. 1997;35(2):211-212.
Seniors in long-term care face higher suicide risks
Seniors who move into and live in long-term care facilities are at increased risk of suicide, according to reporting by the PBS NewsHour and Kaiser Health News. The report focused on the story of Roland K. Tiedemann, a senior who, in his younger days, was an outdoorsman, traveled around the world, and served as a surrogate dad to his granddaughter. When his health deteriorated, he moved to a long-term care facility with his wife, who later was diagnosed with dementia. At age 89, Mr. Tiedemann, who was facing a third move into a facility that would take Medicaid, “locked his door ... and jumped to his death from his fourth floor window,” the report said. The death of Mr. Tiedemann led Julie A. Rickard, PhD, to start asking questions at his facility and working with other centers to identify the signs of depression. A few years earlier, after a “rash of suicides, mostly among young people,” Dr. Rickard had started developing the Suicide Prevention Coalition of North Central Washington. It is also important for families to ask whether suicide prevention and mental health protocols are in place at long-term care facilities. Ultimately, Dr. Rickard and Jane Davis – Mr. Tiedemann’s daughter – agree that stigma needs to be reduced so that residents feel free to talk their depression and anxiety. PBS NewsHour.
More and more people are using mental health apps, and a new study has found that few of those apps have a sound scientific basis for their claims. The researchers scoured iTunes and Google Play for 1,435 mental health apps. These were whittled to 73 of the most popular. The apps addressed common mental health disorders, including depression, anxiety, and substance abuse, as well as some less common illnesses, such as schizophrenia. Of the 73 apps, 47 claimed to be able to effectively diagnose the target condition, improve the user symptoms or mood, and bolster self-management. In about 40% of cases, the app site trotted out scientific language to buttress claims of effectiveness. However, when the researchers took a rigorous look at the science behind the apps, only one was based on a published study. Moreover, for about one-third of the apps, no supporting scientific at all could be found. Science speak did not translate into evidence-based science. The annual market for self-improvement products and apps, including those focusing on mental health, is $10 billion in the United States. Forbes.
Police officers trained in helping people with mental health problems can get positive results, a newspaper report shows. That’s what happened when Logan Elliott called Clive, Iowa, police to report that his fiancé had threatened suicide – and had had a prior attempt, according to the Des Moines Register. Mr. Elliott’s fiancé, Codii Lewis, was in a “depressive state,” was suffering from the loss of his dog, and was troubled by uncertainty regarding the cost of a procedure undertaken to confirm his gender as a transgender man; as a result, Mr. Lewis climbed onto a ledge. He streamed video of his encounter with the police on Facebook Live, and eventually, after kicking one of the officers, he was subdued and taken into custody. The training that Clive officers receive “calls for less aggressive behavior by police,” the article said. “It emphasizes de-escalation, especially when the subject may be suicidal. It doesn’t expect officers to be therapists or handle all mental health situations, but it does ask that they handle mental health situations differently” from the way they might handle other calls. People with mental illness that is untreated are 16 times more likely to be killed by law enforcement than are people without mental illness. Des Moines Register.
An exhibit now running at the Dartmouth-Hitchcock Medical Center in Lebanon, N.H., is intended to put a human face on mental illness. The 99 Faces Project: Portraits Without Labels by Boston-area artist Lynda Michaud Cutrell presents photos of people with serious mental illnesses and those who love them, the New Hampshire Union Leader reported. Portraits were taken with the help of three photographers nationwide. The long list was trimmed to 99 that mirror the ethnic makeup of the U.S. population. The roster of individuals includes 33 with schizophrenia and 33 with bipolar disorder. The remaining 33 are “chronically normal” according to Ms. Cutrell. The viewer can’t tell the difference between the mentally affected individuals and those who are not; they look like people one encounters every day. And that’s the point. Marianne Barthel, director of the Dartmouth-Hitchcock Arts Program, hopes the exhibit leads to conversations that help “normalize mental health in our society, to recognize that the people you’re looking at in these images could be you or your family member,” the article said. Ms. Barthel also hopes that the exhibit, which runs until September, will help reduce the stigma around mental illness by showing that “there are people who are living successful lives with these illnesses.” One of the 99 faces is that of actress Glenn Close, who cofounded the organization Bring Change To Mind in 2010 after her sister was diagnosed with bipolar disorder and her nephew with schizoaffective disorder. New Hampshire Union Leader.
Deliberation about the use of the death penalty for a prisoner in Kansas has implications for those with mental illness who commit crimes. As the Topeka Capital-Journal reported, James Kahler was convicted of murdering his estranged wife, her grandmother, and his two teenage daughters in 2009. Two years later, he was sentenced to death, and several years later, the Kansas Supreme Court upheld that conviction. His guilt is not in question. What is at issue is his impairment. His lawyers had earlier argued that severe depression had made his grip on reality tenuous and that he could not be executed. Now comes the news that the U.S. Supreme Court will rule whether the decision by the state of Kansas to abolish insanity as a defense was constitutional under the 8th and 14th amendments. The high court’s ruling will have profound implications for people with mental illness. In addition to those in Kansas, under state laws in Alaska, Idaho, Montana, and Utah, “a traditional insanity defense in which a person must understand the difference between right and wrong before being found guilty of a crime isn’t allowed,” the report said. An accused can cite “mental disease or defect” as a partial defense. However, in such cases, it must be proven that the person had no intention of committing a crime. In their petition to the Supreme Court, his attorneys argued that he knew he was shooting people, but that he was so disturbed at the time that he could not stop himself. “A favorable decision would make it clear that the Constitution requires that a defendant be able to understand the difference between right and wrong before being found guilty, and, in cases like Mr. Kahler’s, put to death,” his defense attorney, Meryle Carver-Allmond, told the Capital-Journal. “We’re hopeful that, in taking Mr. Kahler’s case, the United States Supreme Court has indicated a desire to find that the Constitution requires better of us in our treatment of mentally ill defendants.” The Topeka Capital-Journal.
Seniors who move into and live in long-term care facilities are at increased risk of suicide, according to reporting by the PBS NewsHour and Kaiser Health News. The report focused on the story of Roland K. Tiedemann, a senior who, in his younger days, was an outdoorsman, traveled around the world, and served as a surrogate dad to his granddaughter. When his health deteriorated, he moved to a long-term care facility with his wife, who later was diagnosed with dementia. At age 89, Mr. Tiedemann, who was facing a third move into a facility that would take Medicaid, “locked his door ... and jumped to his death from his fourth floor window,” the report said. The death of Mr. Tiedemann led Julie A. Rickard, PhD, to start asking questions at his facility and working with other centers to identify the signs of depression. A few years earlier, after a “rash of suicides, mostly among young people,” Dr. Rickard had started developing the Suicide Prevention Coalition of North Central Washington. It is also important for families to ask whether suicide prevention and mental health protocols are in place at long-term care facilities. Ultimately, Dr. Rickard and Jane Davis – Mr. Tiedemann’s daughter – agree that stigma needs to be reduced so that residents feel free to talk their depression and anxiety. PBS NewsHour.
More and more people are using mental health apps, and a new study has found that few of those apps have a sound scientific basis for their claims. The researchers scoured iTunes and Google Play for 1,435 mental health apps. These were whittled to 73 of the most popular. The apps addressed common mental health disorders, including depression, anxiety, and substance abuse, as well as some less common illnesses, such as schizophrenia. Of the 73 apps, 47 claimed to be able to effectively diagnose the target condition, improve the user symptoms or mood, and bolster self-management. In about 40% of cases, the app site trotted out scientific language to buttress claims of effectiveness. However, when the researchers took a rigorous look at the science behind the apps, only one was based on a published study. Moreover, for about one-third of the apps, no supporting scientific at all could be found. Science speak did not translate into evidence-based science. The annual market for self-improvement products and apps, including those focusing on mental health, is $10 billion in the United States. Forbes.
Police officers trained in helping people with mental health problems can get positive results, a newspaper report shows. That’s what happened when Logan Elliott called Clive, Iowa, police to report that his fiancé had threatened suicide – and had had a prior attempt, according to the Des Moines Register. Mr. Elliott’s fiancé, Codii Lewis, was in a “depressive state,” was suffering from the loss of his dog, and was troubled by uncertainty regarding the cost of a procedure undertaken to confirm his gender as a transgender man; as a result, Mr. Lewis climbed onto a ledge. He streamed video of his encounter with the police on Facebook Live, and eventually, after kicking one of the officers, he was subdued and taken into custody. The training that Clive officers receive “calls for less aggressive behavior by police,” the article said. “It emphasizes de-escalation, especially when the subject may be suicidal. It doesn’t expect officers to be therapists or handle all mental health situations, but it does ask that they handle mental health situations differently” from the way they might handle other calls. People with mental illness that is untreated are 16 times more likely to be killed by law enforcement than are people without mental illness. Des Moines Register.
An exhibit now running at the Dartmouth-Hitchcock Medical Center in Lebanon, N.H., is intended to put a human face on mental illness. The 99 Faces Project: Portraits Without Labels by Boston-area artist Lynda Michaud Cutrell presents photos of people with serious mental illnesses and those who love them, the New Hampshire Union Leader reported. Portraits were taken with the help of three photographers nationwide. The long list was trimmed to 99 that mirror the ethnic makeup of the U.S. population. The roster of individuals includes 33 with schizophrenia and 33 with bipolar disorder. The remaining 33 are “chronically normal” according to Ms. Cutrell. The viewer can’t tell the difference between the mentally affected individuals and those who are not; they look like people one encounters every day. And that’s the point. Marianne Barthel, director of the Dartmouth-Hitchcock Arts Program, hopes the exhibit leads to conversations that help “normalize mental health in our society, to recognize that the people you’re looking at in these images could be you or your family member,” the article said. Ms. Barthel also hopes that the exhibit, which runs until September, will help reduce the stigma around mental illness by showing that “there are people who are living successful lives with these illnesses.” One of the 99 faces is that of actress Glenn Close, who cofounded the organization Bring Change To Mind in 2010 after her sister was diagnosed with bipolar disorder and her nephew with schizoaffective disorder. New Hampshire Union Leader.
Deliberation about the use of the death penalty for a prisoner in Kansas has implications for those with mental illness who commit crimes. As the Topeka Capital-Journal reported, James Kahler was convicted of murdering his estranged wife, her grandmother, and his two teenage daughters in 2009. Two years later, he was sentenced to death, and several years later, the Kansas Supreme Court upheld that conviction. His guilt is not in question. What is at issue is his impairment. His lawyers had earlier argued that severe depression had made his grip on reality tenuous and that he could not be executed. Now comes the news that the U.S. Supreme Court will rule whether the decision by the state of Kansas to abolish insanity as a defense was constitutional under the 8th and 14th amendments. The high court’s ruling will have profound implications for people with mental illness. In addition to those in Kansas, under state laws in Alaska, Idaho, Montana, and Utah, “a traditional insanity defense in which a person must understand the difference between right and wrong before being found guilty of a crime isn’t allowed,” the report said. An accused can cite “mental disease or defect” as a partial defense. However, in such cases, it must be proven that the person had no intention of committing a crime. In their petition to the Supreme Court, his attorneys argued that he knew he was shooting people, but that he was so disturbed at the time that he could not stop himself. “A favorable decision would make it clear that the Constitution requires that a defendant be able to understand the difference between right and wrong before being found guilty, and, in cases like Mr. Kahler’s, put to death,” his defense attorney, Meryle Carver-Allmond, told the Capital-Journal. “We’re hopeful that, in taking Mr. Kahler’s case, the United States Supreme Court has indicated a desire to find that the Constitution requires better of us in our treatment of mentally ill defendants.” The Topeka Capital-Journal.
Seniors who move into and live in long-term care facilities are at increased risk of suicide, according to reporting by the PBS NewsHour and Kaiser Health News. The report focused on the story of Roland K. Tiedemann, a senior who, in his younger days, was an outdoorsman, traveled around the world, and served as a surrogate dad to his granddaughter. When his health deteriorated, he moved to a long-term care facility with his wife, who later was diagnosed with dementia. At age 89, Mr. Tiedemann, who was facing a third move into a facility that would take Medicaid, “locked his door ... and jumped to his death from his fourth floor window,” the report said. The death of Mr. Tiedemann led Julie A. Rickard, PhD, to start asking questions at his facility and working with other centers to identify the signs of depression. A few years earlier, after a “rash of suicides, mostly among young people,” Dr. Rickard had started developing the Suicide Prevention Coalition of North Central Washington. It is also important for families to ask whether suicide prevention and mental health protocols are in place at long-term care facilities. Ultimately, Dr. Rickard and Jane Davis – Mr. Tiedemann’s daughter – agree that stigma needs to be reduced so that residents feel free to talk their depression and anxiety. PBS NewsHour.
More and more people are using mental health apps, and a new study has found that few of those apps have a sound scientific basis for their claims. The researchers scoured iTunes and Google Play for 1,435 mental health apps. These were whittled to 73 of the most popular. The apps addressed common mental health disorders, including depression, anxiety, and substance abuse, as well as some less common illnesses, such as schizophrenia. Of the 73 apps, 47 claimed to be able to effectively diagnose the target condition, improve the user symptoms or mood, and bolster self-management. In about 40% of cases, the app site trotted out scientific language to buttress claims of effectiveness. However, when the researchers took a rigorous look at the science behind the apps, only one was based on a published study. Moreover, for about one-third of the apps, no supporting scientific at all could be found. Science speak did not translate into evidence-based science. The annual market for self-improvement products and apps, including those focusing on mental health, is $10 billion in the United States. Forbes.
Police officers trained in helping people with mental health problems can get positive results, a newspaper report shows. That’s what happened when Logan Elliott called Clive, Iowa, police to report that his fiancé had threatened suicide – and had had a prior attempt, according to the Des Moines Register. Mr. Elliott’s fiancé, Codii Lewis, was in a “depressive state,” was suffering from the loss of his dog, and was troubled by uncertainty regarding the cost of a procedure undertaken to confirm his gender as a transgender man; as a result, Mr. Lewis climbed onto a ledge. He streamed video of his encounter with the police on Facebook Live, and eventually, after kicking one of the officers, he was subdued and taken into custody. The training that Clive officers receive “calls for less aggressive behavior by police,” the article said. “It emphasizes de-escalation, especially when the subject may be suicidal. It doesn’t expect officers to be therapists or handle all mental health situations, but it does ask that they handle mental health situations differently” from the way they might handle other calls. People with mental illness that is untreated are 16 times more likely to be killed by law enforcement than are people without mental illness. Des Moines Register.
An exhibit now running at the Dartmouth-Hitchcock Medical Center in Lebanon, N.H., is intended to put a human face on mental illness. The 99 Faces Project: Portraits Without Labels by Boston-area artist Lynda Michaud Cutrell presents photos of people with serious mental illnesses and those who love them, the New Hampshire Union Leader reported. Portraits were taken with the help of three photographers nationwide. The long list was trimmed to 99 that mirror the ethnic makeup of the U.S. population. The roster of individuals includes 33 with schizophrenia and 33 with bipolar disorder. The remaining 33 are “chronically normal” according to Ms. Cutrell. The viewer can’t tell the difference between the mentally affected individuals and those who are not; they look like people one encounters every day. And that’s the point. Marianne Barthel, director of the Dartmouth-Hitchcock Arts Program, hopes the exhibit leads to conversations that help “normalize mental health in our society, to recognize that the people you’re looking at in these images could be you or your family member,” the article said. Ms. Barthel also hopes that the exhibit, which runs until September, will help reduce the stigma around mental illness by showing that “there are people who are living successful lives with these illnesses.” One of the 99 faces is that of actress Glenn Close, who cofounded the organization Bring Change To Mind in 2010 after her sister was diagnosed with bipolar disorder and her nephew with schizoaffective disorder. New Hampshire Union Leader.
Deliberation about the use of the death penalty for a prisoner in Kansas has implications for those with mental illness who commit crimes. As the Topeka Capital-Journal reported, James Kahler was convicted of murdering his estranged wife, her grandmother, and his two teenage daughters in 2009. Two years later, he was sentenced to death, and several years later, the Kansas Supreme Court upheld that conviction. His guilt is not in question. What is at issue is his impairment. His lawyers had earlier argued that severe depression had made his grip on reality tenuous and that he could not be executed. Now comes the news that the U.S. Supreme Court will rule whether the decision by the state of Kansas to abolish insanity as a defense was constitutional under the 8th and 14th amendments. The high court’s ruling will have profound implications for people with mental illness. In addition to those in Kansas, under state laws in Alaska, Idaho, Montana, and Utah, “a traditional insanity defense in which a person must understand the difference between right and wrong before being found guilty of a crime isn’t allowed,” the report said. An accused can cite “mental disease or defect” as a partial defense. However, in such cases, it must be proven that the person had no intention of committing a crime. In their petition to the Supreme Court, his attorneys argued that he knew he was shooting people, but that he was so disturbed at the time that he could not stop himself. “A favorable decision would make it clear that the Constitution requires that a defendant be able to understand the difference between right and wrong before being found guilty, and, in cases like Mr. Kahler’s, put to death,” his defense attorney, Meryle Carver-Allmond, told the Capital-Journal. “We’re hopeful that, in taking Mr. Kahler’s case, the United States Supreme Court has indicated a desire to find that the Constitution requires better of us in our treatment of mentally ill defendants.” The Topeka Capital-Journal.