User login
Nalbuphine reduced uremic pruritus in hemodialysis
SAN DIEGO – The oral opioid nalbuphine was safe and significantly reduced itching intensity in hemodialysis patients with uremic pruritus, a randomized, placebo-controlled trial showed.
“Uremic pruritus is a common problem in dialysis patients,” Dr. Vandana S. Mathur said during a press briefing at a meeting sponsored by the American Society of Nephrology. “A recent study in over 73,000 patients found that 60% of them experience pruritus, and 30% of them experience moderate to severe pruritus. Uremic pruritus is associated with worsening quality of life, sleep, mood, social functioning, higher use of IV antibiotics, higher erythropoiesis-stimulating agent and iron doses, and higher mortality.”
Endogenous opioids are important in the pathogenesis of itch, including itch related to systemic disease, noted Dr. Mathur, a nephrologist and clinical and regulatory drug development consultant based in Woodside, Calif.
“Mu receptors mediate mast cell degranulation and have direct central and peripheral pruritogenic effects, while kappa receptors mediate opposing, antipruritic effects,” she said. “Hemodialysis patients have an increased ratio of beta-endorphin to dynorphin A, and the ratio is associated with increased itch intensity.”
Extended-release nalbuphine is a mu receptor antagonist and a kappa receptor agonist being developed by Trevi Therapeutics. “This dual mechanism of action suggests that it may be effective in the treatment of uremic pruritus,” Dr. Mathur said.
The researchers enrolled 373 hemodialysis patients at 46 sites who had moderate or severe uremic pruritus in a phase II/III study. The primary objectives were to evaluate the drug’s effects on itching intensity as assessed by the Worst Itching Numerical Rating Scale (NRS), as well as safety and tolerability. The patients were randomized 1:1:1 to nalbuphine 60 mg b.i.d., nalbuphine 120 mg b.i.d., or placebo b.i.d. for 8 weeks.
Patients’ mean age was 55 years, and 57% were male. They had been on hemodialysis for nearly 5 years and had experienced pruritus for an average of 3.2 years.
The mean NRS in the 120-mg nalbuphine group declined by 3.5 (from 6.9) on the 10-point scale, which represented a 49% decrease in symptoms and was statistically significant from the least squares mean NRS observed in the placebo group (P = .017), Dr. Mathur reported.
The mean NRS in the 60-mg nalbuphine group declined by 3.1 (from 6.9), which was not statistically different from the least squares mean NRS in the placebo group (P = .432).
“The effect of 120-mg nalbuphine b.i.d. was evident within 1 week following titration and was durable for the full 8-week treatment period,” Dr. Mathur said.
The most common adverse events resulting in discontinuation were nausea, vomiting, somnolence, dizziness, and hallucination, with the incidence rate of these events quickly approaching that of placebo after the first week of titration.
The study’s secondary endpoints of itch-related quality of life and sleep were not significantly improved, “but directional trends supported the primary endpoint findings,” she said.
Trevi Therapeutics supported the study. Dr. Mathur disclosed that she is a consultant for the company.
SAN DIEGO – The oral opioid nalbuphine was safe and significantly reduced itching intensity in hemodialysis patients with uremic pruritus, a randomized, placebo-controlled trial showed.
“Uremic pruritus is a common problem in dialysis patients,” Dr. Vandana S. Mathur said during a press briefing at a meeting sponsored by the American Society of Nephrology. “A recent study in over 73,000 patients found that 60% of them experience pruritus, and 30% of them experience moderate to severe pruritus. Uremic pruritus is associated with worsening quality of life, sleep, mood, social functioning, higher use of IV antibiotics, higher erythropoiesis-stimulating agent and iron doses, and higher mortality.”
Endogenous opioids are important in the pathogenesis of itch, including itch related to systemic disease, noted Dr. Mathur, a nephrologist and clinical and regulatory drug development consultant based in Woodside, Calif.
“Mu receptors mediate mast cell degranulation and have direct central and peripheral pruritogenic effects, while kappa receptors mediate opposing, antipruritic effects,” she said. “Hemodialysis patients have an increased ratio of beta-endorphin to dynorphin A, and the ratio is associated with increased itch intensity.”
Extended-release nalbuphine is a mu receptor antagonist and a kappa receptor agonist being developed by Trevi Therapeutics. “This dual mechanism of action suggests that it may be effective in the treatment of uremic pruritus,” Dr. Mathur said.
The researchers enrolled 373 hemodialysis patients at 46 sites who had moderate or severe uremic pruritus in a phase II/III study. The primary objectives were to evaluate the drug’s effects on itching intensity as assessed by the Worst Itching Numerical Rating Scale (NRS), as well as safety and tolerability. The patients were randomized 1:1:1 to nalbuphine 60 mg b.i.d., nalbuphine 120 mg b.i.d., or placebo b.i.d. for 8 weeks.
Patients’ mean age was 55 years, and 57% were male. They had been on hemodialysis for nearly 5 years and had experienced pruritus for an average of 3.2 years.
The mean NRS in the 120-mg nalbuphine group declined by 3.5 (from 6.9) on the 10-point scale, which represented a 49% decrease in symptoms and was statistically significant from the least squares mean NRS observed in the placebo group (P = .017), Dr. Mathur reported.
The mean NRS in the 60-mg nalbuphine group declined by 3.1 (from 6.9), which was not statistically different from the least squares mean NRS in the placebo group (P = .432).
“The effect of 120-mg nalbuphine b.i.d. was evident within 1 week following titration and was durable for the full 8-week treatment period,” Dr. Mathur said.
The most common adverse events resulting in discontinuation were nausea, vomiting, somnolence, dizziness, and hallucination, with the incidence rate of these events quickly approaching that of placebo after the first week of titration.
The study’s secondary endpoints of itch-related quality of life and sleep were not significantly improved, “but directional trends supported the primary endpoint findings,” she said.
Trevi Therapeutics supported the study. Dr. Mathur disclosed that she is a consultant for the company.
SAN DIEGO – The oral opioid nalbuphine was safe and significantly reduced itching intensity in hemodialysis patients with uremic pruritus, a randomized, placebo-controlled trial showed.
“Uremic pruritus is a common problem in dialysis patients,” Dr. Vandana S. Mathur said during a press briefing at a meeting sponsored by the American Society of Nephrology. “A recent study in over 73,000 patients found that 60% of them experience pruritus, and 30% of them experience moderate to severe pruritus. Uremic pruritus is associated with worsening quality of life, sleep, mood, social functioning, higher use of IV antibiotics, higher erythropoiesis-stimulating agent and iron doses, and higher mortality.”
Endogenous opioids are important in the pathogenesis of itch, including itch related to systemic disease, noted Dr. Mathur, a nephrologist and clinical and regulatory drug development consultant based in Woodside, Calif.
“Mu receptors mediate mast cell degranulation and have direct central and peripheral pruritogenic effects, while kappa receptors mediate opposing, antipruritic effects,” she said. “Hemodialysis patients have an increased ratio of beta-endorphin to dynorphin A, and the ratio is associated with increased itch intensity.”
Extended-release nalbuphine is a mu receptor antagonist and a kappa receptor agonist being developed by Trevi Therapeutics. “This dual mechanism of action suggests that it may be effective in the treatment of uremic pruritus,” Dr. Mathur said.
The researchers enrolled 373 hemodialysis patients at 46 sites who had moderate or severe uremic pruritus in a phase II/III study. The primary objectives were to evaluate the drug’s effects on itching intensity as assessed by the Worst Itching Numerical Rating Scale (NRS), as well as safety and tolerability. The patients were randomized 1:1:1 to nalbuphine 60 mg b.i.d., nalbuphine 120 mg b.i.d., or placebo b.i.d. for 8 weeks.
Patients’ mean age was 55 years, and 57% were male. They had been on hemodialysis for nearly 5 years and had experienced pruritus for an average of 3.2 years.
The mean NRS in the 120-mg nalbuphine group declined by 3.5 (from 6.9) on the 10-point scale, which represented a 49% decrease in symptoms and was statistically significant from the least squares mean NRS observed in the placebo group (P = .017), Dr. Mathur reported.
The mean NRS in the 60-mg nalbuphine group declined by 3.1 (from 6.9), which was not statistically different from the least squares mean NRS in the placebo group (P = .432).
“The effect of 120-mg nalbuphine b.i.d. was evident within 1 week following titration and was durable for the full 8-week treatment period,” Dr. Mathur said.
The most common adverse events resulting in discontinuation were nausea, vomiting, somnolence, dizziness, and hallucination, with the incidence rate of these events quickly approaching that of placebo after the first week of titration.
The study’s secondary endpoints of itch-related quality of life and sleep were not significantly improved, “but directional trends supported the primary endpoint findings,” she said.
Trevi Therapeutics supported the study. Dr. Mathur disclosed that she is a consultant for the company.
AT KIDNEY WEEK 2015
Key clinical point: Extended-release oral nalbuphine at 120 mg twice daily provided relief to hemodialysis patients with uremic pruritus.
Major finding: The mean Worst Itch Numerical Rating Scale (NRS) in the 120-mg nalbuphine group declined by 3.5 (from 6.9) on the 10-point scale, which represented a 49% decrease in symptoms.
Data source: A multicenter study of 373 hemodialysis patients with uremic pruritus who were randomized 1:1:1 to nalbuphine 60 mg b.i.d., nalbuphine 120 mg b.i.d., or placebo b.i.d. for 8 weeks.
Disclosures: Trevi Therapeutics supported the study. Dr. Mathur disclosed that she is a consultant for the company.
A Review of Patient Adherence to Topical Therapies for Treatment of Atopic Dermatitis
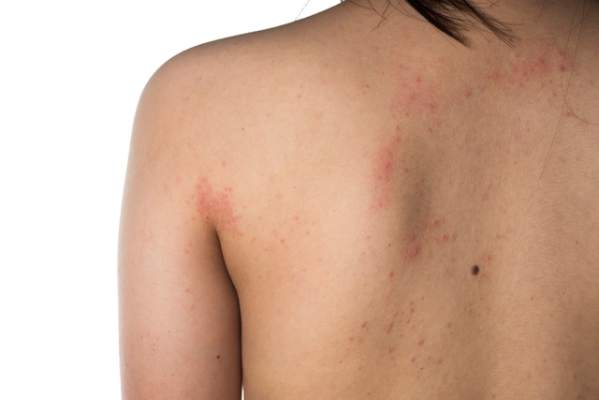
Atopic dermatitis (AD) is a chronic inflammatory skin disease that typically begins in early childhood (Figure). It is one of the most commonly diagnosed dermatologic conditions, affecting up to 25% of children and 2% to 3% of adults in the United States.1,2 The mainstays of treatment for AD are topical emollients and topical medications, of which corticosteroids are most commonly prescribed.3 Although treatments for AD generally are straightforward and efficacious when used correctly, poor adherence to treatment often prevents patients from achieving disease control.4 Patient adherence to therapy is a familiar challenge in dermatology, especially for diseases like AD that require long-term treatment with topical medications.4,5 In some instances, poor adherence may be misconstrued as poor response to treatment, which may lead to escalation to more powerful and potentially dangerous systemic medications.6 Ensuring good adherence to treatment leads to better outcomes and disease control, averts unnecessary treatment, prevents disease complications, improves quality of life, and decreases treatment cost.4,5 This article provides a review of the literature on patient adherence to topical therapies for AD as well as a discussion of methods to improve patient adherence to treatment in the clinical setting.
Methods
A PubMed search of articles indexed for MEDLINE from January 2005 to May 2015 was conducted to identify studies that focused on treatment adherence in AD using the search terms atopic dermatitis and medication adherence and atopic dermatitis and patient compliance After excluding duplicate results and those that were not in the English language, a final list of clinical trials that investigated patient adherence/compliance to topical medications for the treatment of AD was extracted for evaluation.
Results
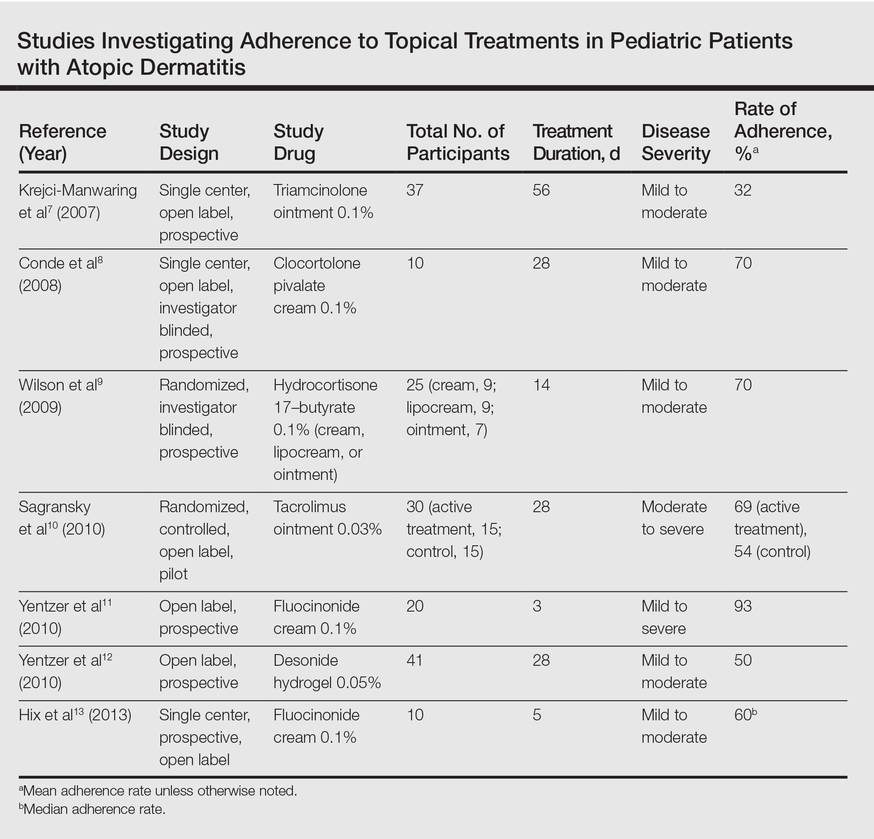
Our review of the literature yielded 7 quantitative studies that evaluated adherence to topical medications in AD using electronic monitoring and/or self-reporting (Table).7-13 Participant demographics, disease severity, drug and vehicle used, duration of treatment, and number of follow-up visits varied. All studies used medication event monitoring system caps on medication jars to objectively track patient adherence by recording the date and time when the cap was removed. To assess disease response, the studies used such measures as the Investigator Global Assessment scale, Eczema Area and Severity Index score, or other visual analog scales.
In all of the studies, treatment proved effective and disease severity declined from baseline regardless of the rate of adherence, with benefit continuing after treatment had ended.7-13 Some results suggested that better adherence increased treatment efficacy and reduced disease severity.8,9 However, one 10-day trial found no difference in severity and efficacy among participants who applied the medication at least once daily, missed applications some days, or applied the medication more than twice daily.13
Study participants typically overestimated their adherence to treatment compared to actual adherence rates, with most reporting near 100% adherence.7-9,11,12 Average measured adherence rates ranged from 32% to 93% (Table). Adherence rates typically were highest at the beginning of the study and decreased as the study continued.7-13 The study with the best average adherence rate of 93% had the shortest treatment period of 3 days,11 and the study with the lowest average adherence rate of 32% had the longest treatment period of 8 weeks.7 The study with the lowest adherence rate was the only study wherein participants were blinded to their enrollment in the study, which would most closely mimic adherence rates in clinical practice.7 The participants in the other studies were not aware that their adherence was being monitored, but their behavior may have been influenced since they were aware of their enrollment in the study.
Many variables affect treatment adherence in patients with AD. Average adherence rates were significantly higher (P=.03) in participants with greater disease severity.7 There is conflicting evidence regarding the role of medication vehicle in treatment adherence. While Wilson et al9 did not find any difference in adherence based on medication vehicle, Yentzer et al12 found vehicle characteristics and medication side effects were among patients’ top-ranked concerns about using topical medications. Sagransky et al10 compared treatment adherence between 2 groups of AD patients: one control group received a standard-of-care 4-week follow-up, and an active group received an additional 1-week follow-up. The mean adherence rate of the treatment group was 69% compared with 54% in the control group.10
Comment
Poor adherence to treatment is a pervasive problem in patients with AD. Our review of the literature confirmed that patients generally are not accurate historians of their medication usage, often reporting near-perfect treatment adherence even when actual adherence is poor. Rates of adherence from clinical trials are likely higher than those seen in clinical practice due in part to study incentives and differences between how patients in a study are treated compared to those in a physician’s clinic; for example, research study participants often have additional follow-up visits compared to those being treated in the clinical population and by virtue of being enrolled in a study are aware that their behavior is being monitored, which can increase treatment adherence.7
The dogma suggesting that tachyphylaxis can occur with long-term use of topical corticosteroids is not supported by clinical trials.14 Furthermore, in our review of the literature patient adherence was highest in the shortest study11 and lowest in the longest study.7 Given that AD patients cannot benefit from a treatment if they do not use it, the supposed decrease in efficacy of topical corticosteroids over time may be because patients fail to use them consistently.
Our review of the literature was limited by the small body of research that exists on treatment adherence in AD patients, especially relating to topical medications, and did not reveal any studies evaluating systemic medications in AD. Of the studies we examined, sample sizes were small and treatment and follow-up periods were short. Our review only covered adherence to prescribed topical medications in AD, chiefly corticosteroids; thus, we did not evaluate adherence to other therapies (eg, emollients) in this patient population.
The existing research also is limited by the relative paucity of data showing a correlation between improved adherence to topical treatment and improved disease outcomes, which may be due to the methodological limitations of the study designs that have been used; for instance, studies may use objective monitors to describe daily adherence to treatment, but disease severity typically is measured over longer periods of time, usually every few weeks or months. Short-term data may not be an accurate demonstration of how participants’ actual treatment adherence impacts disease outcome, as the data does not account for more complex adherence factors; for example, participants who achieve good disease control using topical corticosteroids for an 8-week study period may actually demonstrate poor treatment adherence overall, as topical corticosteroids have good short-term efficacy and the patient may have stopped using the product after the first few weeks of the treatment period. In contrast, poorly adherent patients may never use the medication well enough to achieve improvement and may continue low-level use throughout the study period. Therefore, studies that measure disease severity at more regular intervals are required to show the true effect of treatment adherence on disease outcomes.
Since AD mainly affects children, family issues can pose special challenges to attaining good treatment adherence.15,16 The physician–patient (or parent) relationship and the family’s perception of the patient’s disease severity are strong predictors of adherence to topical treatment.16 Potential barriers to adherence in the pediatric population are caregivers with negative beliefs about treatment, the time-consuming nature of applying topical therapies, or a child who is uncooperative.15,17 In the treatment of infants, critical factors are caregiver availability and beliefs and fears about medications and their side effects, while in the teenage population, the desire to “fit in” and oppositional behavior can lead to poor adherence to treatment.17 Regardless of age, other barriers to treatment adherence are forgetfulness, belief that the drug is not working, and the messiness of treatment.17
Educational tools (eg, action plans, instructions about how to apply topical medications correctly) may be underutilized in patients with AD. If consistently implemented, these tools could have a positive impact on adherence to medication in patients with AD. For example, written action plans pioneered in the asthma community have shown to improve quality of life and reduce disease severity and may offer the same benefits for AD patients due to the similarities of the diseases.18 Since AD patients and their caregivers often are not well versed in how to apply topical medications correctly, efforts to educate patients could potentially increase adherence to treatment. In one study, AD patients began to use medications more effectively after applying a fluorescent cream to reveal affected areas they had missed, and clinicians were able to provide additional instruction based on the findings.19
Adherence to topical treatments among AD patients is a multifactorial issue. Regimens often are complex and inconvenient due to the need for multiple medications, the topical nature of the products, and the need for frequent application. To optimize prescription treatments, patients also must be diligent with preventive measures such as application of topical emollients and use of bathing techniques (eg, bleach baths). A way to overcome treatment complexity and increase adherence may be to provide a written action plan and involve the patient and caregiver in the plan’s development. If a drug formulation is not aesthetically acceptable to the patient (eg, the greasiness of an ointment), allowing the patient to choose the medication vehicle may increase satisfaction and use.12 Fear of steroid side effects also is common among patients and caregivers and could be overcome with education about the product.20
Conclusion
Treatment adherence can have a dramatic effect on diseases outcomes and can be particularly challenging in AD due to the use of topical medications with complex treatment regimens. Additionally, a large majority of patients with AD are children, from infants to teenagers, adding another layer of treatment challenges. Further research is needed to more definitively develop effective methods for enhancing treatment adherence in this patient population. Although enormous amounts of money are being spent to develop improved treatments for AD, we may be able to achieve far more benefit at a much lower cost by figuring out how to get patients to adhere to the treatments that are already available.
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351.
- Landis ET, Davis SA, Taheri A, et al. Top dermatologic diagnoses by age. Dermatol Online J. 2014;20:22368.
- Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2. management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-132.
- Lee IA, Maibach HI. Pharmionics in dermatology: a review of topical medication adherence. Am J Clin Dermatol. 2006;7:231-236.
- Tan X, Feldman SR, Chang J, et al. Topical drug delivery systems in dermatology: a review of patient adherence issues. Expert Opin Drug Deliv. 2012;9:1263-1271.
- Sidbury R, Davis DM, Cohen DE, et al. Guidelines of care for the management of atopic dermatitis: section 3. Management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71:327-349.
- Krejci-Manwaring J, Tusa MG, Carroll C, et al. Stealth monitoring of adherence to topical medication: adherence is very poor in children with atopic dermatitis. J Am Acad Dermatol. 2007;56:211-216.
- Conde JF, Kaur M, Fleischer AB Jr, et al. Adherence to clocortolone pivalate cream 0.1% in a pediatric population with atopic dermatitis. Cutis. 2008;81:435-441.
- Wilson R, Camacho F, Clark AR, et al. Adherence to topical hydrocortisone 17-butyrate 0.1% in different vehicles in adults with atopic dermatitis. J Am Acad Dermatol. 2009;60:166-168.
- Sagransky MJ, Yentzer BA, Williams LL, et al. A randomized controlled pilot study of the effects of an extra office visit on adherence and outcomes in atopic dermatitis. Arch Dermatol. 2010;146:1428-1430.
- Yentzer BA, Ade RA, Fountain JM, et al. Improvement in treatment adherence with a 3-day course of fluocinonide cream 0.1% for atopic dermatitis. Cutis. 2010;86:208-213.
- Yentzer BA, Camacho FT, Young T, et al. Good adherence and early efficacy using desonide hydrogel for atopic dermatitis: results from a program addressing patient compliance. J Drugs Dermatol. 2010;9:324-329.
- Hix E, Gustafson CJ, O’Neill JL, et al. Adherence to a five day treatment course of topical fluocinonide 0.1% cream in atopic dermatitis. Dermatol Online J. 2013;19:20029.
- Taheri A, Cantrell J, Feldman SR. Tachyphylaxis to topical glucocorticoids; what is the evidence? Dermatol Online J. 2013;19:18954.
- Santer M, Burgess H, Yardley L, et al. Managing childhood eczema: qualitative study exploring carers’ experiences of barriers and facilitators to treatment adherence. J Adv Nurs. 2013;69:2493-2501.
- Ohya Y, Williams H, Steptoe A, et al. Psychosocial factors and adherence to treatment advice in childhood atopic dermatitis. J Invest Dermatol. 2001;117:852-857.
- Ou HT, Feldman SR, Balkrishnan R. Understanding and improving treatment adherence in pediatric patients. Semin Cutan Med Surg. 2010;29:137-140.
- Chisolm SS, Taylor SL, Balkrishnan R, et al. Written action plans: potential for improving outcomes in children with atopic dermatitis. J Am Acad Dermatol. 2008;59:677-683.
- Ulff E, Maroti M, Serup J. Fluorescent cream used as an educational intervention to improve the effectiveness of self-application by patients with atopic dermatitis. J Dermatolog Treat. 2013;24:268-271.
- Aubert-Wastiaux H, Moret L, Le Rhun A, et al. Topical corticosteroid phobia in atopic dermatitis: a study of its nature, origins and frequency. Br J Dermatol. 2011;165:808-814.
Atopic dermatitis (AD) is a chronic inflammatory skin disease that typically begins in early childhood (Figure). It is one of the most commonly diagnosed dermatologic conditions, affecting up to 25% of children and 2% to 3% of adults in the United States.1,2 The mainstays of treatment for AD are topical emollients and topical medications, of which corticosteroids are most commonly prescribed.3 Although treatments for AD generally are straightforward and efficacious when used correctly, poor adherence to treatment often prevents patients from achieving disease control.4 Patient adherence to therapy is a familiar challenge in dermatology, especially for diseases like AD that require long-term treatment with topical medications.4,5 In some instances, poor adherence may be misconstrued as poor response to treatment, which may lead to escalation to more powerful and potentially dangerous systemic medications.6 Ensuring good adherence to treatment leads to better outcomes and disease control, averts unnecessary treatment, prevents disease complications, improves quality of life, and decreases treatment cost.4,5 This article provides a review of the literature on patient adherence to topical therapies for AD as well as a discussion of methods to improve patient adherence to treatment in the clinical setting.

Methods
A PubMed search of articles indexed for MEDLINE from January 2005 to May 2015 was conducted to identify studies that focused on treatment adherence in AD using the search terms atopic dermatitis and medication adherence and atopic dermatitis and patient compliance After excluding duplicate results and those that were not in the English language, a final list of clinical trials that investigated patient adherence/compliance to topical medications for the treatment of AD was extracted for evaluation.
Results
Our review of the literature yielded 7 quantitative studies that evaluated adherence to topical medications in AD using electronic monitoring and/or self-reporting (Table).7-13 Participant demographics, disease severity, drug and vehicle used, duration of treatment, and number of follow-up visits varied. All studies used medication event monitoring system caps on medication jars to objectively track patient adherence by recording the date and time when the cap was removed. To assess disease response, the studies used such measures as the Investigator Global Assessment scale, Eczema Area and Severity Index score, or other visual analog scales.
In all of the studies, treatment proved effective and disease severity declined from baseline regardless of the rate of adherence, with benefit continuing after treatment had ended.7-13 Some results suggested that better adherence increased treatment efficacy and reduced disease severity.8,9 However, one 10-day trial found no difference in severity and efficacy among participants who applied the medication at least once daily, missed applications some days, or applied the medication more than twice daily.13
Study participants typically overestimated their adherence to treatment compared to actual adherence rates, with most reporting near 100% adherence.7-9,11,12 Average measured adherence rates ranged from 32% to 93% (Table). Adherence rates typically were highest at the beginning of the study and decreased as the study continued.7-13 The study with the best average adherence rate of 93% had the shortest treatment period of 3 days,11 and the study with the lowest average adherence rate of 32% had the longest treatment period of 8 weeks.7 The study with the lowest adherence rate was the only study wherein participants were blinded to their enrollment in the study, which would most closely mimic adherence rates in clinical practice.7 The participants in the other studies were not aware that their adherence was being monitored, but their behavior may have been influenced since they were aware of their enrollment in the study.
Many variables affect treatment adherence in patients with AD. Average adherence rates were significantly higher (P=.03) in participants with greater disease severity.7 There is conflicting evidence regarding the role of medication vehicle in treatment adherence. While Wilson et al9 did not find any difference in adherence based on medication vehicle, Yentzer et al12 found vehicle characteristics and medication side effects were among patients’ top-ranked concerns about using topical medications. Sagransky et al10 compared treatment adherence between 2 groups of AD patients: one control group received a standard-of-care 4-week follow-up, and an active group received an additional 1-week follow-up. The mean adherence rate of the treatment group was 69% compared with 54% in the control group.10
Comment
Poor adherence to treatment is a pervasive problem in patients with AD. Our review of the literature confirmed that patients generally are not accurate historians of their medication usage, often reporting near-perfect treatment adherence even when actual adherence is poor. Rates of adherence from clinical trials are likely higher than those seen in clinical practice due in part to study incentives and differences between how patients in a study are treated compared to those in a physician’s clinic; for example, research study participants often have additional follow-up visits compared to those being treated in the clinical population and by virtue of being enrolled in a study are aware that their behavior is being monitored, which can increase treatment adherence.7
The dogma suggesting that tachyphylaxis can occur with long-term use of topical corticosteroids is not supported by clinical trials.14 Furthermore, in our review of the literature patient adherence was highest in the shortest study11 and lowest in the longest study.7 Given that AD patients cannot benefit from a treatment if they do not use it, the supposed decrease in efficacy of topical corticosteroids over time may be because patients fail to use them consistently.
Our review of the literature was limited by the small body of research that exists on treatment adherence in AD patients, especially relating to topical medications, and did not reveal any studies evaluating systemic medications in AD. Of the studies we examined, sample sizes were small and treatment and follow-up periods were short. Our review only covered adherence to prescribed topical medications in AD, chiefly corticosteroids; thus, we did not evaluate adherence to other therapies (eg, emollients) in this patient population.
The existing research also is limited by the relative paucity of data showing a correlation between improved adherence to topical treatment and improved disease outcomes, which may be due to the methodological limitations of the study designs that have been used; for instance, studies may use objective monitors to describe daily adherence to treatment, but disease severity typically is measured over longer periods of time, usually every few weeks or months. Short-term data may not be an accurate demonstration of how participants’ actual treatment adherence impacts disease outcome, as the data does not account for more complex adherence factors; for example, participants who achieve good disease control using topical corticosteroids for an 8-week study period may actually demonstrate poor treatment adherence overall, as topical corticosteroids have good short-term efficacy and the patient may have stopped using the product after the first few weeks of the treatment period. In contrast, poorly adherent patients may never use the medication well enough to achieve improvement and may continue low-level use throughout the study period. Therefore, studies that measure disease severity at more regular intervals are required to show the true effect of treatment adherence on disease outcomes.
Since AD mainly affects children, family issues can pose special challenges to attaining good treatment adherence.15,16 The physician–patient (or parent) relationship and the family’s perception of the patient’s disease severity are strong predictors of adherence to topical treatment.16 Potential barriers to adherence in the pediatric population are caregivers with negative beliefs about treatment, the time-consuming nature of applying topical therapies, or a child who is uncooperative.15,17 In the treatment of infants, critical factors are caregiver availability and beliefs and fears about medications and their side effects, while in the teenage population, the desire to “fit in” and oppositional behavior can lead to poor adherence to treatment.17 Regardless of age, other barriers to treatment adherence are forgetfulness, belief that the drug is not working, and the messiness of treatment.17
Educational tools (eg, action plans, instructions about how to apply topical medications correctly) may be underutilized in patients with AD. If consistently implemented, these tools could have a positive impact on adherence to medication in patients with AD. For example, written action plans pioneered in the asthma community have shown to improve quality of life and reduce disease severity and may offer the same benefits for AD patients due to the similarities of the diseases.18 Since AD patients and their caregivers often are not well versed in how to apply topical medications correctly, efforts to educate patients could potentially increase adherence to treatment. In one study, AD patients began to use medications more effectively after applying a fluorescent cream to reveal affected areas they had missed, and clinicians were able to provide additional instruction based on the findings.19
Adherence to topical treatments among AD patients is a multifactorial issue. Regimens often are complex and inconvenient due to the need for multiple medications, the topical nature of the products, and the need for frequent application. To optimize prescription treatments, patients also must be diligent with preventive measures such as application of topical emollients and use of bathing techniques (eg, bleach baths). A way to overcome treatment complexity and increase adherence may be to provide a written action plan and involve the patient and caregiver in the plan’s development. If a drug formulation is not aesthetically acceptable to the patient (eg, the greasiness of an ointment), allowing the patient to choose the medication vehicle may increase satisfaction and use.12 Fear of steroid side effects also is common among patients and caregivers and could be overcome with education about the product.20
Conclusion
Treatment adherence can have a dramatic effect on diseases outcomes and can be particularly challenging in AD due to the use of topical medications with complex treatment regimens. Additionally, a large majority of patients with AD are children, from infants to teenagers, adding another layer of treatment challenges. Further research is needed to more definitively develop effective methods for enhancing treatment adherence in this patient population. Although enormous amounts of money are being spent to develop improved treatments for AD, we may be able to achieve far more benefit at a much lower cost by figuring out how to get patients to adhere to the treatments that are already available.
Atopic dermatitis (AD) is a chronic inflammatory skin disease that typically begins in early childhood (Figure). It is one of the most commonly diagnosed dermatologic conditions, affecting up to 25% of children and 2% to 3% of adults in the United States.1,2 The mainstays of treatment for AD are topical emollients and topical medications, of which corticosteroids are most commonly prescribed.3 Although treatments for AD generally are straightforward and efficacious when used correctly, poor adherence to treatment often prevents patients from achieving disease control.4 Patient adherence to therapy is a familiar challenge in dermatology, especially for diseases like AD that require long-term treatment with topical medications.4,5 In some instances, poor adherence may be misconstrued as poor response to treatment, which may lead to escalation to more powerful and potentially dangerous systemic medications.6 Ensuring good adherence to treatment leads to better outcomes and disease control, averts unnecessary treatment, prevents disease complications, improves quality of life, and decreases treatment cost.4,5 This article provides a review of the literature on patient adherence to topical therapies for AD as well as a discussion of methods to improve patient adherence to treatment in the clinical setting.

Methods
A PubMed search of articles indexed for MEDLINE from January 2005 to May 2015 was conducted to identify studies that focused on treatment adherence in AD using the search terms atopic dermatitis and medication adherence and atopic dermatitis and patient compliance After excluding duplicate results and those that were not in the English language, a final list of clinical trials that investigated patient adherence/compliance to topical medications for the treatment of AD was extracted for evaluation.
Results
Our review of the literature yielded 7 quantitative studies that evaluated adherence to topical medications in AD using electronic monitoring and/or self-reporting (Table).7-13 Participant demographics, disease severity, drug and vehicle used, duration of treatment, and number of follow-up visits varied. All studies used medication event monitoring system caps on medication jars to objectively track patient adherence by recording the date and time when the cap was removed. To assess disease response, the studies used such measures as the Investigator Global Assessment scale, Eczema Area and Severity Index score, or other visual analog scales.
In all of the studies, treatment proved effective and disease severity declined from baseline regardless of the rate of adherence, with benefit continuing after treatment had ended.7-13 Some results suggested that better adherence increased treatment efficacy and reduced disease severity.8,9 However, one 10-day trial found no difference in severity and efficacy among participants who applied the medication at least once daily, missed applications some days, or applied the medication more than twice daily.13
Study participants typically overestimated their adherence to treatment compared to actual adherence rates, with most reporting near 100% adherence.7-9,11,12 Average measured adherence rates ranged from 32% to 93% (Table). Adherence rates typically were highest at the beginning of the study and decreased as the study continued.7-13 The study with the best average adherence rate of 93% had the shortest treatment period of 3 days,11 and the study with the lowest average adherence rate of 32% had the longest treatment period of 8 weeks.7 The study with the lowest adherence rate was the only study wherein participants were blinded to their enrollment in the study, which would most closely mimic adherence rates in clinical practice.7 The participants in the other studies were not aware that their adherence was being monitored, but their behavior may have been influenced since they were aware of their enrollment in the study.
Many variables affect treatment adherence in patients with AD. Average adherence rates were significantly higher (P=.03) in participants with greater disease severity.7 There is conflicting evidence regarding the role of medication vehicle in treatment adherence. While Wilson et al9 did not find any difference in adherence based on medication vehicle, Yentzer et al12 found vehicle characteristics and medication side effects were among patients’ top-ranked concerns about using topical medications. Sagransky et al10 compared treatment adherence between 2 groups of AD patients: one control group received a standard-of-care 4-week follow-up, and an active group received an additional 1-week follow-up. The mean adherence rate of the treatment group was 69% compared with 54% in the control group.10
Comment
Poor adherence to treatment is a pervasive problem in patients with AD. Our review of the literature confirmed that patients generally are not accurate historians of their medication usage, often reporting near-perfect treatment adherence even when actual adherence is poor. Rates of adherence from clinical trials are likely higher than those seen in clinical practice due in part to study incentives and differences between how patients in a study are treated compared to those in a physician’s clinic; for example, research study participants often have additional follow-up visits compared to those being treated in the clinical population and by virtue of being enrolled in a study are aware that their behavior is being monitored, which can increase treatment adherence.7
The dogma suggesting that tachyphylaxis can occur with long-term use of topical corticosteroids is not supported by clinical trials.14 Furthermore, in our review of the literature patient adherence was highest in the shortest study11 and lowest in the longest study.7 Given that AD patients cannot benefit from a treatment if they do not use it, the supposed decrease in efficacy of topical corticosteroids over time may be because patients fail to use them consistently.
Our review of the literature was limited by the small body of research that exists on treatment adherence in AD patients, especially relating to topical medications, and did not reveal any studies evaluating systemic medications in AD. Of the studies we examined, sample sizes were small and treatment and follow-up periods were short. Our review only covered adherence to prescribed topical medications in AD, chiefly corticosteroids; thus, we did not evaluate adherence to other therapies (eg, emollients) in this patient population.
The existing research also is limited by the relative paucity of data showing a correlation between improved adherence to topical treatment and improved disease outcomes, which may be due to the methodological limitations of the study designs that have been used; for instance, studies may use objective monitors to describe daily adherence to treatment, but disease severity typically is measured over longer periods of time, usually every few weeks or months. Short-term data may not be an accurate demonstration of how participants’ actual treatment adherence impacts disease outcome, as the data does not account for more complex adherence factors; for example, participants who achieve good disease control using topical corticosteroids for an 8-week study period may actually demonstrate poor treatment adherence overall, as topical corticosteroids have good short-term efficacy and the patient may have stopped using the product after the first few weeks of the treatment period. In contrast, poorly adherent patients may never use the medication well enough to achieve improvement and may continue low-level use throughout the study period. Therefore, studies that measure disease severity at more regular intervals are required to show the true effect of treatment adherence on disease outcomes.
Since AD mainly affects children, family issues can pose special challenges to attaining good treatment adherence.15,16 The physician–patient (or parent) relationship and the family’s perception of the patient’s disease severity are strong predictors of adherence to topical treatment.16 Potential barriers to adherence in the pediatric population are caregivers with negative beliefs about treatment, the time-consuming nature of applying topical therapies, or a child who is uncooperative.15,17 In the treatment of infants, critical factors are caregiver availability and beliefs and fears about medications and their side effects, while in the teenage population, the desire to “fit in” and oppositional behavior can lead to poor adherence to treatment.17 Regardless of age, other barriers to treatment adherence are forgetfulness, belief that the drug is not working, and the messiness of treatment.17
Educational tools (eg, action plans, instructions about how to apply topical medications correctly) may be underutilized in patients with AD. If consistently implemented, these tools could have a positive impact on adherence to medication in patients with AD. For example, written action plans pioneered in the asthma community have shown to improve quality of life and reduce disease severity and may offer the same benefits for AD patients due to the similarities of the diseases.18 Since AD patients and their caregivers often are not well versed in how to apply topical medications correctly, efforts to educate patients could potentially increase adherence to treatment. In one study, AD patients began to use medications more effectively after applying a fluorescent cream to reveal affected areas they had missed, and clinicians were able to provide additional instruction based on the findings.19
Adherence to topical treatments among AD patients is a multifactorial issue. Regimens often are complex and inconvenient due to the need for multiple medications, the topical nature of the products, and the need for frequent application. To optimize prescription treatments, patients also must be diligent with preventive measures such as application of topical emollients and use of bathing techniques (eg, bleach baths). A way to overcome treatment complexity and increase adherence may be to provide a written action plan and involve the patient and caregiver in the plan’s development. If a drug formulation is not aesthetically acceptable to the patient (eg, the greasiness of an ointment), allowing the patient to choose the medication vehicle may increase satisfaction and use.12 Fear of steroid side effects also is common among patients and caregivers and could be overcome with education about the product.20
Conclusion
Treatment adherence can have a dramatic effect on diseases outcomes and can be particularly challenging in AD due to the use of topical medications with complex treatment regimens. Additionally, a large majority of patients with AD are children, from infants to teenagers, adding another layer of treatment challenges. Further research is needed to more definitively develop effective methods for enhancing treatment adherence in this patient population. Although enormous amounts of money are being spent to develop improved treatments for AD, we may be able to achieve far more benefit at a much lower cost by figuring out how to get patients to adhere to the treatments that are already available.
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351.
- Landis ET, Davis SA, Taheri A, et al. Top dermatologic diagnoses by age. Dermatol Online J. 2014;20:22368.
- Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2. management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-132.
- Lee IA, Maibach HI. Pharmionics in dermatology: a review of topical medication adherence. Am J Clin Dermatol. 2006;7:231-236.
- Tan X, Feldman SR, Chang J, et al. Topical drug delivery systems in dermatology: a review of patient adherence issues. Expert Opin Drug Deliv. 2012;9:1263-1271.
- Sidbury R, Davis DM, Cohen DE, et al. Guidelines of care for the management of atopic dermatitis: section 3. Management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71:327-349.
- Krejci-Manwaring J, Tusa MG, Carroll C, et al. Stealth monitoring of adherence to topical medication: adherence is very poor in children with atopic dermatitis. J Am Acad Dermatol. 2007;56:211-216.
- Conde JF, Kaur M, Fleischer AB Jr, et al. Adherence to clocortolone pivalate cream 0.1% in a pediatric population with atopic dermatitis. Cutis. 2008;81:435-441.
- Wilson R, Camacho F, Clark AR, et al. Adherence to topical hydrocortisone 17-butyrate 0.1% in different vehicles in adults with atopic dermatitis. J Am Acad Dermatol. 2009;60:166-168.
- Sagransky MJ, Yentzer BA, Williams LL, et al. A randomized controlled pilot study of the effects of an extra office visit on adherence and outcomes in atopic dermatitis. Arch Dermatol. 2010;146:1428-1430.
- Yentzer BA, Ade RA, Fountain JM, et al. Improvement in treatment adherence with a 3-day course of fluocinonide cream 0.1% for atopic dermatitis. Cutis. 2010;86:208-213.
- Yentzer BA, Camacho FT, Young T, et al. Good adherence and early efficacy using desonide hydrogel for atopic dermatitis: results from a program addressing patient compliance. J Drugs Dermatol. 2010;9:324-329.
- Hix E, Gustafson CJ, O’Neill JL, et al. Adherence to a five day treatment course of topical fluocinonide 0.1% cream in atopic dermatitis. Dermatol Online J. 2013;19:20029.
- Taheri A, Cantrell J, Feldman SR. Tachyphylaxis to topical glucocorticoids; what is the evidence? Dermatol Online J. 2013;19:18954.
- Santer M, Burgess H, Yardley L, et al. Managing childhood eczema: qualitative study exploring carers’ experiences of barriers and facilitators to treatment adherence. J Adv Nurs. 2013;69:2493-2501.
- Ohya Y, Williams H, Steptoe A, et al. Psychosocial factors and adherence to treatment advice in childhood atopic dermatitis. J Invest Dermatol. 2001;117:852-857.
- Ou HT, Feldman SR, Balkrishnan R. Understanding and improving treatment adherence in pediatric patients. Semin Cutan Med Surg. 2010;29:137-140.
- Chisolm SS, Taylor SL, Balkrishnan R, et al. Written action plans: potential for improving outcomes in children with atopic dermatitis. J Am Acad Dermatol. 2008;59:677-683.
- Ulff E, Maroti M, Serup J. Fluorescent cream used as an educational intervention to improve the effectiveness of self-application by patients with atopic dermatitis. J Dermatolog Treat. 2013;24:268-271.
- Aubert-Wastiaux H, Moret L, Le Rhun A, et al. Topical corticosteroid phobia in atopic dermatitis: a study of its nature, origins and frequency. Br J Dermatol. 2011;165:808-814.
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351.
- Landis ET, Davis SA, Taheri A, et al. Top dermatologic diagnoses by age. Dermatol Online J. 2014;20:22368.
- Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2. management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-132.
- Lee IA, Maibach HI. Pharmionics in dermatology: a review of topical medication adherence. Am J Clin Dermatol. 2006;7:231-236.
- Tan X, Feldman SR, Chang J, et al. Topical drug delivery systems in dermatology: a review of patient adherence issues. Expert Opin Drug Deliv. 2012;9:1263-1271.
- Sidbury R, Davis DM, Cohen DE, et al. Guidelines of care for the management of atopic dermatitis: section 3. Management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71:327-349.
- Krejci-Manwaring J, Tusa MG, Carroll C, et al. Stealth monitoring of adherence to topical medication: adherence is very poor in children with atopic dermatitis. J Am Acad Dermatol. 2007;56:211-216.
- Conde JF, Kaur M, Fleischer AB Jr, et al. Adherence to clocortolone pivalate cream 0.1% in a pediatric population with atopic dermatitis. Cutis. 2008;81:435-441.
- Wilson R, Camacho F, Clark AR, et al. Adherence to topical hydrocortisone 17-butyrate 0.1% in different vehicles in adults with atopic dermatitis. J Am Acad Dermatol. 2009;60:166-168.
- Sagransky MJ, Yentzer BA, Williams LL, et al. A randomized controlled pilot study of the effects of an extra office visit on adherence and outcomes in atopic dermatitis. Arch Dermatol. 2010;146:1428-1430.
- Yentzer BA, Ade RA, Fountain JM, et al. Improvement in treatment adherence with a 3-day course of fluocinonide cream 0.1% for atopic dermatitis. Cutis. 2010;86:208-213.
- Yentzer BA, Camacho FT, Young T, et al. Good adherence and early efficacy using desonide hydrogel for atopic dermatitis: results from a program addressing patient compliance. J Drugs Dermatol. 2010;9:324-329.
- Hix E, Gustafson CJ, O’Neill JL, et al. Adherence to a five day treatment course of topical fluocinonide 0.1% cream in atopic dermatitis. Dermatol Online J. 2013;19:20029.
- Taheri A, Cantrell J, Feldman SR. Tachyphylaxis to topical glucocorticoids; what is the evidence? Dermatol Online J. 2013;19:18954.
- Santer M, Burgess H, Yardley L, et al. Managing childhood eczema: qualitative study exploring carers’ experiences of barriers and facilitators to treatment adherence. J Adv Nurs. 2013;69:2493-2501.
- Ohya Y, Williams H, Steptoe A, et al. Psychosocial factors and adherence to treatment advice in childhood atopic dermatitis. J Invest Dermatol. 2001;117:852-857.
- Ou HT, Feldman SR, Balkrishnan R. Understanding and improving treatment adherence in pediatric patients. Semin Cutan Med Surg. 2010;29:137-140.
- Chisolm SS, Taylor SL, Balkrishnan R, et al. Written action plans: potential for improving outcomes in children with atopic dermatitis. J Am Acad Dermatol. 2008;59:677-683.
- Ulff E, Maroti M, Serup J. Fluorescent cream used as an educational intervention to improve the effectiveness of self-application by patients with atopic dermatitis. J Dermatolog Treat. 2013;24:268-271.
- Aubert-Wastiaux H, Moret L, Le Rhun A, et al. Topical corticosteroid phobia in atopic dermatitis: a study of its nature, origins and frequency. Br J Dermatol. 2011;165:808-814.
Practice Points
- When used correctly, topical treatments for atopic dermatitis (AD) generally are straightforward and efficacious, but poor adherence to treatment can prevent patients from achieving disease control.
- Patients tend to overestimate their adherence to topical treatment regimens for AD compared to actual adherence rates.
- Improved treatment adherence in this patient population may be achieved by allowing patients to choose their preferred topical vehicle and providing patient education about how to apply medications effectively; for pediatric patients, AD action plans also may be useful.
Acquired Port-wine Stain With Superimposed Eczema Following Penetrating Abdominal Trauma
Port-wine stains (PWSs) are common congenital capillary vascular malformations with an incidence of 3 per 1000 neonates.1 Rarely, acquired PWSs are seen, sometimes appearing following trauma.2-5 Port-wine stains are diagnosed clinically and present as painless, partially or entirely blanchable pink patches that respect the median (midline) plane.6 Although histopathologic examination is not necessary for diagnosis of PWS, typical findings include dilated, ectatic capillaries.7,8 Since it was first reported by Traub9 in 1939, more than 60 cases of acquired PWSs have been reported.10 A PubMed search of articles indexed for MEDLINE using the search terms acquired port-wine stain and port-wine stain and eczema yielded no cases of acquired PWS with associated eczematous changes and only 30 cases of congenital PWS with superimposed eczema.11-18 We report the case of an acquired PWS with superimposed eczema in an 18-year-old man following penetrating abdominal trauma.
Case Report
An otherwise healthy 18-year-old man presented to our dermatology office for evaluation of an eruption that had developed at the site of an abdominal stab wound he sustained 2 to 3 years prior. One year after he was stabbed, the patient developed a nonpruritic, painless red patch located 1 cm anterior to the healed wound on the left abdomen. The patch gradually grew larger to involve the entire left abdomen, extending to the left lower back. The site of the healed stab wound also became raised and pruritic, and the patient noted another pruritic plaque that formed within the larger patch. The patient reported no other skin conditions prior to the current eruption. His medical history was notable for seasonal allergies and asthma, but no childhood eczema.
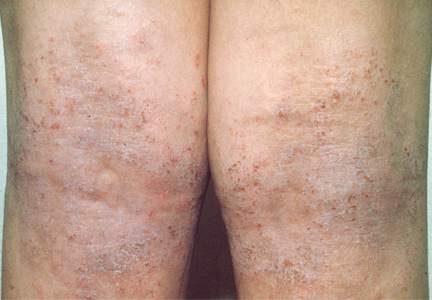
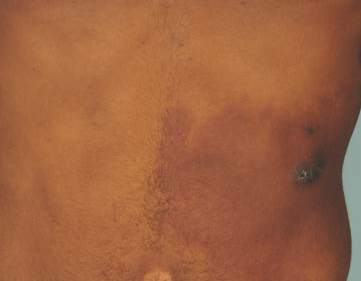
Physical examination revealed a healthy, well-nourished man with Fitzpatrick skin type IV. A red, purpuric, coalescent patch with slightly arcuate borders extending from the mid abdomen to the left posterior flank was noted. The left lateral aspect of the patch blanched with pressure and respected the median plane. Within the larger patch, a 4-cm×2-cm lichenified, slightly macerated, hyperpigmented plaque was noted at the site of the stab wound (Figure 1). Based on these clinical findings, a presumptive diagnosis of an acquired PWS with superimposed eczema was made.
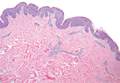
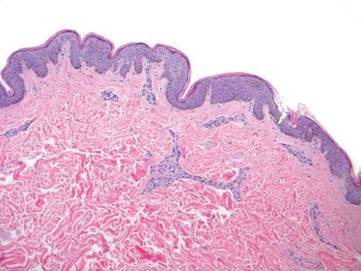
Punch biopsy specimens were taken from the large vascular patch and the smaller lichenified plaque. Histopathologic examination of the vascular patch showed an increased number of small vessels in the superficial dermis with thickened vessel walls, ectatic lumens, and no vasculopathy, consistent with a vascular malformation or a reactive vascular proliferation (Figure 2). On histopathology, the plaque showed epidermal spongiosis and hyperplasia with serum crust and a papillary dermis containing a mixed inflammatory infiltrate with occasional eosinophils, consistent with an eczematous dermatitis (Figure 3). The histologic findings confirmed the clinical diagnosis.
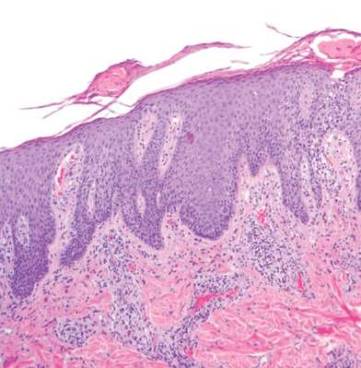
The pruritic, lichenified plaque improved with application of triamcinolone ointment 0.1% twice daily for 2 weeks. Magnetic resonance imaging to rule out an underlying arteriovenous malformation was recommended, but the patient declined.
Comment
The exact cause of PWS is unknown. There have been a multitude of genomic suspects for congenital lesions, including a somatic activating mutation (ie, a mutation acquired during fetal development) of the GNAQ (guanine nucleotide binding protein [G protein], q polypeptide) gene, which may contribute to abnormal cell proliferation including the regulation of blood vessels, and inactivating mutations in the RASA1 (RAS p21 protein activator [GTPase activating protein] 1) gene, which controls endothelial cell organization.19-22 Later mutations (ie, those occurring after the first trimester) may be more likely to result in isolated PWSs as opposed to syndromic PWSs.19 Whatever the source of genetic misinformation, it is thought that the diminished neuronal control of blood flow and the resulting alterations in dermal structure contribute to the pathogenesis of PWS and its associated histologic features.7,23
The clinical and histopathologic features of acquired PWSs are indistinguishable from those of congenital lesions, indicating that different processes may lead to the same presentation.4 Abnormal innervation and decreased supportive stroma have both been identified as contributing factors in the development of congenital and acquired PWSs.7,23-25 Rosen and Smoller23 found that diminished nerve density affects vascular tone and caliber in PWSs and had hypothesized in a prior report that decreased perivascular Schwann cells may indicate abnormal sympathetic innervation.7 Since then, PWS has been shown to lack both somatic and sensory innervation.24 Tsuji and Sawabe25 indicated that alterations to the perivascular stroma, whether congenital or as a result of trauma, decrease support for vessels, leading to ectasia.
In addition to an acquired PWS, our patient also had associated eczema within the PWS. Eczematous lesions were absent elsewhere, and he did not have a history of childhood eczema. Our review of the literature yielded 8 studies since 1996 that collectively described 30 cases of eczema within PWSs.11-18 Only 2 of these reports described adult patients with concomitant eczema and PWS and none described acquired PWS.13,18
Few studies have addressed the relationship between PWSs and eczema. It is unclear if concomitant PWS and localized eczema are collision dermatoses or if a PWS may predispose the affected skin to eczema.11-13 It has been hypothesized that the increased dermal vasculature in PWSs predisposes the skin to the development of eczema—more specifically, that ectasia may lead to increased inflammation.12,17 The concept of the “immunocompromised district” proposed by Ruocco et al26 is a unifying theory that may underlie the association noted between cases of trauma and later development of a PWS and superimposed eczematous dermatitis, such as in our case. Trauma is noted as one of a number of possible disruptive forces affecting both immunomodulation and neuromodulation within a local area of skin, leading to increased susceptibility of that district to various cutaneous diseases.26
Although our patient’s eczema responded to conservative treatment with a topical steroid, several case series have reported success with laser therapy in the treatment of PWS while preventing recurrence of associated eczematous dermatitis.12,17 Following the cessation of eczema treatment with topical steroid, which causes vasoconstriction, we suggest postponing laser therapy several weeks to allow resolution of vasoconstriction, thus providing enhanced therapeutic targeting with a vascular laser. Of particular relevance to our case, a recent study showed efficacy of the pulsed dye laser in treating PWSs in Fitzpatrick skin types IV and V.27
Conclusion
Although acquired PWS is rare, it can present later in life as an acquired lesion at a site of previous trauma.1-5 Congenital capillary malformations also can be associated with superimposed, localized eczema.11-18 We present a rarely reported case of an acquired PWS with superimposed, localized eczema. As in cases of congenital PWS with concomitant eczema, the associated eczema in our case was responsive to topical corticosteroid therapy. Additionally, pulsed dye laser has been shown to treat PWSs while preventing the recurrence of eczema, and it has been deemed effective for individuals with darker skin types.12,17, 27 Further studies are needed to explore the relationship between PWS and eczema.
- Jacobs AH, Walton RG. The incidence of birthmarks in the neonate. Pediatrics. 1976;58:218-222.
- Fegeler F. Naevus flammeus im trigeminusgebiet nach trauma im rahmen eines posttraumatisch-vegetativen syndroms. Arch Dermatol Syphilol. 1949;188:416-422.
- Kirkland CR, Mutasim DF. Acquired port-wine stain following repetitive trauma. J Am Acad Dermatol. 2011;65:462-463.
- Adams BB, Lucky AW. Acquired port-wine stains and antecedent trauma: case report and review of the literature. Arch Dermatol. 2000;136:897-899.
- Colver GB, Ryan TJ. Acquired port-wine stain. Arch Dermatol. 1986;122:1415-1416.
- Nigro J, Swerlick RA, Sepp NT, et al. Angiogenesis, vascular malformations and proliferations. In: Arndt KA, LeBoit PE, Robinson JK, Wintroub BU, eds. Cutaneous Medicine and Surgery: An Integrated Program in Dermatology. Philadelphia, PA: WB Saunders Co; 1996:1492-1521.
- Smoller BR, Rosen S. Port-wine stains. a disease of altered neural modulation of blood vessels? Arch Dermatol. 1986;122:177-179.
- Chang CJ, Yu JS, Nelson JS. Confocal microscopy study of neurovascular distribution in facial port wine stains(capillary malformation). J Formos Med Assoc. 2008;107:559-666.
- Traub EF. Naevus flammeus appearing at the age of twenty three. Arch Dermatol. 1939;39:752.
- Freysz M, Cribier B, Lipsker, D. Fegelers syndrome, acquired port-wine stain or acquired capillary malformation: three cases and a literature review [article in French]. Ann Dermatol Venereol. 2013;140:341-346.
- Tay YK, Morelli J, Weston WL. Inflammatory nuchal-occipital port-wine stains. J Am Acad Dermatol. 1996;35:811-813.
- Sidwell RU, Syed S, Harper JI. Port-wine stains and eczema. Br J Dermatol. 2001;144:1269-1270.
- Hofer T. Meyerson phenomenon within a nevus flammeus. Dermatology. 2002;205:180-183.
- Raff K, Landthaler M, Hoheleutner U. Port-wine stains with eczema. Phlebologie. 2003;32:15-17.
- Tsuboi H, Miyata T, Katsuoka K. Eczema in a port-wine stain. Clin Exp Dermatol. 2003;28:322-323.
- Rajan N, Natarahan S. Impetiginized eczema arising within a port-wine stain of the arm. J Eur Acad Dermatol Venereol. 2006;20:1009-1010.
- Fonder MA, Mamelak AJ, Kazin RA, et al. Port-wine-stain-associated dermatitis: implications for cutaneous vascular laser therapy. Pediatr Dermatol. 2007;24:376-379.
- Simon V, Wolfgan H, Katharina F. Meyerson-Phenomenon hides a nevus flammeus. J Dtsch Dermatol Ges. 2011;9:305-307.
- Shirley MD, Tang H, Gallione CJ, et al. Sturge-Weber syndrome and port-wine stains caused by somatic mutation in GNAQ. N Engl J Med. 2013;368:1971-1979.
- Hershkovitz D, Bercovich D, Sprecher E, et al. RASA1 mutations may cause hereditary capillary malformations without arteriovenous malformations. Br J Dermatol. 2008;158:1035-1040.
- Eerola I, Boon LM, Mulliken JB, et al. Capillary malformation-arteriovenous malformation, a new clinical and genetic disorder caused by RASA1 mutations. Am J Hum Genet. 2003;73:1240-1249.
- Henkemeyer M, Rossi DJ, Holmyard DP, et al. Vascular system defects and neuronal apoptosis in mice lacking ras GTPase-activating protein. Nature. 1995;377:695-701.
- Rosen S, Smoller BR. Port-wine stains: a new hypothesis. J Am Acad Dermatol. 1987;17:164-166.
- Rydh M, Malm BM, Jernmeck J, et al. Ectatic blood vessels in port-wine stains lack innervation: possible role in pathogenesis. Plast Reconstr Surg. 1991;87:419-422.
- Tsuji T, Sawabe M. A new type of telangiectasia following trauma. J Cutan Pathol. 1988;15:22-26.
- Ruocco V, Ruocco E, Brunnetti G, et al. Opportunistic localization of skin lesions on vulnerable areas. Clin Dermatol. 2011;29:483-488.
- Thajudeheen CP, Jyothy K, Pryadarshi A. Treatment of port-wine stains with flash lamp pumped pulsed dye laser on Indian skin: a six year study. J Cutan Aesthet Surg. 2014;7:32-36.
Port-wine stains (PWSs) are common congenital capillary vascular malformations with an incidence of 3 per 1000 neonates.1 Rarely, acquired PWSs are seen, sometimes appearing following trauma.2-5 Port-wine stains are diagnosed clinically and present as painless, partially or entirely blanchable pink patches that respect the median (midline) plane.6 Although histopathologic examination is not necessary for diagnosis of PWS, typical findings include dilated, ectatic capillaries.7,8 Since it was first reported by Traub9 in 1939, more than 60 cases of acquired PWSs have been reported.10 A PubMed search of articles indexed for MEDLINE using the search terms acquired port-wine stain and port-wine stain and eczema yielded no cases of acquired PWS with associated eczematous changes and only 30 cases of congenital PWS with superimposed eczema.11-18 We report the case of an acquired PWS with superimposed eczema in an 18-year-old man following penetrating abdominal trauma.
Case Report
An otherwise healthy 18-year-old man presented to our dermatology office for evaluation of an eruption that had developed at the site of an abdominal stab wound he sustained 2 to 3 years prior. One year after he was stabbed, the patient developed a nonpruritic, painless red patch located 1 cm anterior to the healed wound on the left abdomen. The patch gradually grew larger to involve the entire left abdomen, extending to the left lower back. The site of the healed stab wound also became raised and pruritic, and the patient noted another pruritic plaque that formed within the larger patch. The patient reported no other skin conditions prior to the current eruption. His medical history was notable for seasonal allergies and asthma, but no childhood eczema.
Physical examination revealed a healthy, well-nourished man with Fitzpatrick skin type IV. A red, purpuric, coalescent patch with slightly arcuate borders extending from the mid abdomen to the left posterior flank was noted. The left lateral aspect of the patch blanched with pressure and respected the median plane. Within the larger patch, a 4-cm×2-cm lichenified, slightly macerated, hyperpigmented plaque was noted at the site of the stab wound (Figure 1). Based on these clinical findings, a presumptive diagnosis of an acquired PWS with superimposed eczema was made.

Punch biopsy specimens were taken from the large vascular patch and the smaller lichenified plaque. Histopathologic examination of the vascular patch showed an increased number of small vessels in the superficial dermis with thickened vessel walls, ectatic lumens, and no vasculopathy, consistent with a vascular malformation or a reactive vascular proliferation (Figure 2). On histopathology, the plaque showed epidermal spongiosis and hyperplasia with serum crust and a papillary dermis containing a mixed inflammatory infiltrate with occasional eosinophils, consistent with an eczematous dermatitis (Figure 3). The histologic findings confirmed the clinical diagnosis.


The pruritic, lichenified plaque improved with application of triamcinolone ointment 0.1% twice daily for 2 weeks. Magnetic resonance imaging to rule out an underlying arteriovenous malformation was recommended, but the patient declined.
Comment
The exact cause of PWS is unknown. There have been a multitude of genomic suspects for congenital lesions, including a somatic activating mutation (ie, a mutation acquired during fetal development) of the GNAQ (guanine nucleotide binding protein [G protein], q polypeptide) gene, which may contribute to abnormal cell proliferation including the regulation of blood vessels, and inactivating mutations in the RASA1 (RAS p21 protein activator [GTPase activating protein] 1) gene, which controls endothelial cell organization.19-22 Later mutations (ie, those occurring after the first trimester) may be more likely to result in isolated PWSs as opposed to syndromic PWSs.19 Whatever the source of genetic misinformation, it is thought that the diminished neuronal control of blood flow and the resulting alterations in dermal structure contribute to the pathogenesis of PWS and its associated histologic features.7,23
The clinical and histopathologic features of acquired PWSs are indistinguishable from those of congenital lesions, indicating that different processes may lead to the same presentation.4 Abnormal innervation and decreased supportive stroma have both been identified as contributing factors in the development of congenital and acquired PWSs.7,23-25 Rosen and Smoller23 found that diminished nerve density affects vascular tone and caliber in PWSs and had hypothesized in a prior report that decreased perivascular Schwann cells may indicate abnormal sympathetic innervation.7 Since then, PWS has been shown to lack both somatic and sensory innervation.24 Tsuji and Sawabe25 indicated that alterations to the perivascular stroma, whether congenital or as a result of trauma, decrease support for vessels, leading to ectasia.
In addition to an acquired PWS, our patient also had associated eczema within the PWS. Eczematous lesions were absent elsewhere, and he did not have a history of childhood eczema. Our review of the literature yielded 8 studies since 1996 that collectively described 30 cases of eczema within PWSs.11-18 Only 2 of these reports described adult patients with concomitant eczema and PWS and none described acquired PWS.13,18
Few studies have addressed the relationship between PWSs and eczema. It is unclear if concomitant PWS and localized eczema are collision dermatoses or if a PWS may predispose the affected skin to eczema.11-13 It has been hypothesized that the increased dermal vasculature in PWSs predisposes the skin to the development of eczema—more specifically, that ectasia may lead to increased inflammation.12,17 The concept of the “immunocompromised district” proposed by Ruocco et al26 is a unifying theory that may underlie the association noted between cases of trauma and later development of a PWS and superimposed eczematous dermatitis, such as in our case. Trauma is noted as one of a number of possible disruptive forces affecting both immunomodulation and neuromodulation within a local area of skin, leading to increased susceptibility of that district to various cutaneous diseases.26
Although our patient’s eczema responded to conservative treatment with a topical steroid, several case series have reported success with laser therapy in the treatment of PWS while preventing recurrence of associated eczematous dermatitis.12,17 Following the cessation of eczema treatment with topical steroid, which causes vasoconstriction, we suggest postponing laser therapy several weeks to allow resolution of vasoconstriction, thus providing enhanced therapeutic targeting with a vascular laser. Of particular relevance to our case, a recent study showed efficacy of the pulsed dye laser in treating PWSs in Fitzpatrick skin types IV and V.27
Conclusion
Although acquired PWS is rare, it can present later in life as an acquired lesion at a site of previous trauma.1-5 Congenital capillary malformations also can be associated with superimposed, localized eczema.11-18 We present a rarely reported case of an acquired PWS with superimposed, localized eczema. As in cases of congenital PWS with concomitant eczema, the associated eczema in our case was responsive to topical corticosteroid therapy. Additionally, pulsed dye laser has been shown to treat PWSs while preventing the recurrence of eczema, and it has been deemed effective for individuals with darker skin types.12,17, 27 Further studies are needed to explore the relationship between PWS and eczema.
Port-wine stains (PWSs) are common congenital capillary vascular malformations with an incidence of 3 per 1000 neonates.1 Rarely, acquired PWSs are seen, sometimes appearing following trauma.2-5 Port-wine stains are diagnosed clinically and present as painless, partially or entirely blanchable pink patches that respect the median (midline) plane.6 Although histopathologic examination is not necessary for diagnosis of PWS, typical findings include dilated, ectatic capillaries.7,8 Since it was first reported by Traub9 in 1939, more than 60 cases of acquired PWSs have been reported.10 A PubMed search of articles indexed for MEDLINE using the search terms acquired port-wine stain and port-wine stain and eczema yielded no cases of acquired PWS with associated eczematous changes and only 30 cases of congenital PWS with superimposed eczema.11-18 We report the case of an acquired PWS with superimposed eczema in an 18-year-old man following penetrating abdominal trauma.
Case Report
An otherwise healthy 18-year-old man presented to our dermatology office for evaluation of an eruption that had developed at the site of an abdominal stab wound he sustained 2 to 3 years prior. One year after he was stabbed, the patient developed a nonpruritic, painless red patch located 1 cm anterior to the healed wound on the left abdomen. The patch gradually grew larger to involve the entire left abdomen, extending to the left lower back. The site of the healed stab wound also became raised and pruritic, and the patient noted another pruritic plaque that formed within the larger patch. The patient reported no other skin conditions prior to the current eruption. His medical history was notable for seasonal allergies and asthma, but no childhood eczema.
Physical examination revealed a healthy, well-nourished man with Fitzpatrick skin type IV. A red, purpuric, coalescent patch with slightly arcuate borders extending from the mid abdomen to the left posterior flank was noted. The left lateral aspect of the patch blanched with pressure and respected the median plane. Within the larger patch, a 4-cm×2-cm lichenified, slightly macerated, hyperpigmented plaque was noted at the site of the stab wound (Figure 1). Based on these clinical findings, a presumptive diagnosis of an acquired PWS with superimposed eczema was made.

Punch biopsy specimens were taken from the large vascular patch and the smaller lichenified plaque. Histopathologic examination of the vascular patch showed an increased number of small vessels in the superficial dermis with thickened vessel walls, ectatic lumens, and no vasculopathy, consistent with a vascular malformation or a reactive vascular proliferation (Figure 2). On histopathology, the plaque showed epidermal spongiosis and hyperplasia with serum crust and a papillary dermis containing a mixed inflammatory infiltrate with occasional eosinophils, consistent with an eczematous dermatitis (Figure 3). The histologic findings confirmed the clinical diagnosis.


The pruritic, lichenified plaque improved with application of triamcinolone ointment 0.1% twice daily for 2 weeks. Magnetic resonance imaging to rule out an underlying arteriovenous malformation was recommended, but the patient declined.
Comment
The exact cause of PWS is unknown. There have been a multitude of genomic suspects for congenital lesions, including a somatic activating mutation (ie, a mutation acquired during fetal development) of the GNAQ (guanine nucleotide binding protein [G protein], q polypeptide) gene, which may contribute to abnormal cell proliferation including the regulation of blood vessels, and inactivating mutations in the RASA1 (RAS p21 protein activator [GTPase activating protein] 1) gene, which controls endothelial cell organization.19-22 Later mutations (ie, those occurring after the first trimester) may be more likely to result in isolated PWSs as opposed to syndromic PWSs.19 Whatever the source of genetic misinformation, it is thought that the diminished neuronal control of blood flow and the resulting alterations in dermal structure contribute to the pathogenesis of PWS and its associated histologic features.7,23
The clinical and histopathologic features of acquired PWSs are indistinguishable from those of congenital lesions, indicating that different processes may lead to the same presentation.4 Abnormal innervation and decreased supportive stroma have both been identified as contributing factors in the development of congenital and acquired PWSs.7,23-25 Rosen and Smoller23 found that diminished nerve density affects vascular tone and caliber in PWSs and had hypothesized in a prior report that decreased perivascular Schwann cells may indicate abnormal sympathetic innervation.7 Since then, PWS has been shown to lack both somatic and sensory innervation.24 Tsuji and Sawabe25 indicated that alterations to the perivascular stroma, whether congenital or as a result of trauma, decrease support for vessels, leading to ectasia.
In addition to an acquired PWS, our patient also had associated eczema within the PWS. Eczematous lesions were absent elsewhere, and he did not have a history of childhood eczema. Our review of the literature yielded 8 studies since 1996 that collectively described 30 cases of eczema within PWSs.11-18 Only 2 of these reports described adult patients with concomitant eczema and PWS and none described acquired PWS.13,18
Few studies have addressed the relationship between PWSs and eczema. It is unclear if concomitant PWS and localized eczema are collision dermatoses or if a PWS may predispose the affected skin to eczema.11-13 It has been hypothesized that the increased dermal vasculature in PWSs predisposes the skin to the development of eczema—more specifically, that ectasia may lead to increased inflammation.12,17 The concept of the “immunocompromised district” proposed by Ruocco et al26 is a unifying theory that may underlie the association noted between cases of trauma and later development of a PWS and superimposed eczematous dermatitis, such as in our case. Trauma is noted as one of a number of possible disruptive forces affecting both immunomodulation and neuromodulation within a local area of skin, leading to increased susceptibility of that district to various cutaneous diseases.26
Although our patient’s eczema responded to conservative treatment with a topical steroid, several case series have reported success with laser therapy in the treatment of PWS while preventing recurrence of associated eczematous dermatitis.12,17 Following the cessation of eczema treatment with topical steroid, which causes vasoconstriction, we suggest postponing laser therapy several weeks to allow resolution of vasoconstriction, thus providing enhanced therapeutic targeting with a vascular laser. Of particular relevance to our case, a recent study showed efficacy of the pulsed dye laser in treating PWSs in Fitzpatrick skin types IV and V.27
Conclusion
Although acquired PWS is rare, it can present later in life as an acquired lesion at a site of previous trauma.1-5 Congenital capillary malformations also can be associated with superimposed, localized eczema.11-18 We present a rarely reported case of an acquired PWS with superimposed, localized eczema. As in cases of congenital PWS with concomitant eczema, the associated eczema in our case was responsive to topical corticosteroid therapy. Additionally, pulsed dye laser has been shown to treat PWSs while preventing the recurrence of eczema, and it has been deemed effective for individuals with darker skin types.12,17, 27 Further studies are needed to explore the relationship between PWS and eczema.
- Jacobs AH, Walton RG. The incidence of birthmarks in the neonate. Pediatrics. 1976;58:218-222.
- Fegeler F. Naevus flammeus im trigeminusgebiet nach trauma im rahmen eines posttraumatisch-vegetativen syndroms. Arch Dermatol Syphilol. 1949;188:416-422.
- Kirkland CR, Mutasim DF. Acquired port-wine stain following repetitive trauma. J Am Acad Dermatol. 2011;65:462-463.
- Adams BB, Lucky AW. Acquired port-wine stains and antecedent trauma: case report and review of the literature. Arch Dermatol. 2000;136:897-899.
- Colver GB, Ryan TJ. Acquired port-wine stain. Arch Dermatol. 1986;122:1415-1416.
- Nigro J, Swerlick RA, Sepp NT, et al. Angiogenesis, vascular malformations and proliferations. In: Arndt KA, LeBoit PE, Robinson JK, Wintroub BU, eds. Cutaneous Medicine and Surgery: An Integrated Program in Dermatology. Philadelphia, PA: WB Saunders Co; 1996:1492-1521.
- Smoller BR, Rosen S. Port-wine stains. a disease of altered neural modulation of blood vessels? Arch Dermatol. 1986;122:177-179.
- Chang CJ, Yu JS, Nelson JS. Confocal microscopy study of neurovascular distribution in facial port wine stains(capillary malformation). J Formos Med Assoc. 2008;107:559-666.
- Traub EF. Naevus flammeus appearing at the age of twenty three. Arch Dermatol. 1939;39:752.
- Freysz M, Cribier B, Lipsker, D. Fegelers syndrome, acquired port-wine stain or acquired capillary malformation: three cases and a literature review [article in French]. Ann Dermatol Venereol. 2013;140:341-346.
- Tay YK, Morelli J, Weston WL. Inflammatory nuchal-occipital port-wine stains. J Am Acad Dermatol. 1996;35:811-813.
- Sidwell RU, Syed S, Harper JI. Port-wine stains and eczema. Br J Dermatol. 2001;144:1269-1270.
- Hofer T. Meyerson phenomenon within a nevus flammeus. Dermatology. 2002;205:180-183.
- Raff K, Landthaler M, Hoheleutner U. Port-wine stains with eczema. Phlebologie. 2003;32:15-17.
- Tsuboi H, Miyata T, Katsuoka K. Eczema in a port-wine stain. Clin Exp Dermatol. 2003;28:322-323.
- Rajan N, Natarahan S. Impetiginized eczema arising within a port-wine stain of the arm. J Eur Acad Dermatol Venereol. 2006;20:1009-1010.
- Fonder MA, Mamelak AJ, Kazin RA, et al. Port-wine-stain-associated dermatitis: implications for cutaneous vascular laser therapy. Pediatr Dermatol. 2007;24:376-379.
- Simon V, Wolfgan H, Katharina F. Meyerson-Phenomenon hides a nevus flammeus. J Dtsch Dermatol Ges. 2011;9:305-307.
- Shirley MD, Tang H, Gallione CJ, et al. Sturge-Weber syndrome and port-wine stains caused by somatic mutation in GNAQ. N Engl J Med. 2013;368:1971-1979.
- Hershkovitz D, Bercovich D, Sprecher E, et al. RASA1 mutations may cause hereditary capillary malformations without arteriovenous malformations. Br J Dermatol. 2008;158:1035-1040.
- Eerola I, Boon LM, Mulliken JB, et al. Capillary malformation-arteriovenous malformation, a new clinical and genetic disorder caused by RASA1 mutations. Am J Hum Genet. 2003;73:1240-1249.
- Henkemeyer M, Rossi DJ, Holmyard DP, et al. Vascular system defects and neuronal apoptosis in mice lacking ras GTPase-activating protein. Nature. 1995;377:695-701.
- Rosen S, Smoller BR. Port-wine stains: a new hypothesis. J Am Acad Dermatol. 1987;17:164-166.
- Rydh M, Malm BM, Jernmeck J, et al. Ectatic blood vessels in port-wine stains lack innervation: possible role in pathogenesis. Plast Reconstr Surg. 1991;87:419-422.
- Tsuji T, Sawabe M. A new type of telangiectasia following trauma. J Cutan Pathol. 1988;15:22-26.
- Ruocco V, Ruocco E, Brunnetti G, et al. Opportunistic localization of skin lesions on vulnerable areas. Clin Dermatol. 2011;29:483-488.
- Thajudeheen CP, Jyothy K, Pryadarshi A. Treatment of port-wine stains with flash lamp pumped pulsed dye laser on Indian skin: a six year study. J Cutan Aesthet Surg. 2014;7:32-36.
- Jacobs AH, Walton RG. The incidence of birthmarks in the neonate. Pediatrics. 1976;58:218-222.
- Fegeler F. Naevus flammeus im trigeminusgebiet nach trauma im rahmen eines posttraumatisch-vegetativen syndroms. Arch Dermatol Syphilol. 1949;188:416-422.
- Kirkland CR, Mutasim DF. Acquired port-wine stain following repetitive trauma. J Am Acad Dermatol. 2011;65:462-463.
- Adams BB, Lucky AW. Acquired port-wine stains and antecedent trauma: case report and review of the literature. Arch Dermatol. 2000;136:897-899.
- Colver GB, Ryan TJ. Acquired port-wine stain. Arch Dermatol. 1986;122:1415-1416.
- Nigro J, Swerlick RA, Sepp NT, et al. Angiogenesis, vascular malformations and proliferations. In: Arndt KA, LeBoit PE, Robinson JK, Wintroub BU, eds. Cutaneous Medicine and Surgery: An Integrated Program in Dermatology. Philadelphia, PA: WB Saunders Co; 1996:1492-1521.
- Smoller BR, Rosen S. Port-wine stains. a disease of altered neural modulation of blood vessels? Arch Dermatol. 1986;122:177-179.
- Chang CJ, Yu JS, Nelson JS. Confocal microscopy study of neurovascular distribution in facial port wine stains(capillary malformation). J Formos Med Assoc. 2008;107:559-666.
- Traub EF. Naevus flammeus appearing at the age of twenty three. Arch Dermatol. 1939;39:752.
- Freysz M, Cribier B, Lipsker, D. Fegelers syndrome, acquired port-wine stain or acquired capillary malformation: three cases and a literature review [article in French]. Ann Dermatol Venereol. 2013;140:341-346.
- Tay YK, Morelli J, Weston WL. Inflammatory nuchal-occipital port-wine stains. J Am Acad Dermatol. 1996;35:811-813.
- Sidwell RU, Syed S, Harper JI. Port-wine stains and eczema. Br J Dermatol. 2001;144:1269-1270.
- Hofer T. Meyerson phenomenon within a nevus flammeus. Dermatology. 2002;205:180-183.
- Raff K, Landthaler M, Hoheleutner U. Port-wine stains with eczema. Phlebologie. 2003;32:15-17.
- Tsuboi H, Miyata T, Katsuoka K. Eczema in a port-wine stain. Clin Exp Dermatol. 2003;28:322-323.
- Rajan N, Natarahan S. Impetiginized eczema arising within a port-wine stain of the arm. J Eur Acad Dermatol Venereol. 2006;20:1009-1010.
- Fonder MA, Mamelak AJ, Kazin RA, et al. Port-wine-stain-associated dermatitis: implications for cutaneous vascular laser therapy. Pediatr Dermatol. 2007;24:376-379.
- Simon V, Wolfgan H, Katharina F. Meyerson-Phenomenon hides a nevus flammeus. J Dtsch Dermatol Ges. 2011;9:305-307.
- Shirley MD, Tang H, Gallione CJ, et al. Sturge-Weber syndrome and port-wine stains caused by somatic mutation in GNAQ. N Engl J Med. 2013;368:1971-1979.
- Hershkovitz D, Bercovich D, Sprecher E, et al. RASA1 mutations may cause hereditary capillary malformations without arteriovenous malformations. Br J Dermatol. 2008;158:1035-1040.
- Eerola I, Boon LM, Mulliken JB, et al. Capillary malformation-arteriovenous malformation, a new clinical and genetic disorder caused by RASA1 mutations. Am J Hum Genet. 2003;73:1240-1249.
- Henkemeyer M, Rossi DJ, Holmyard DP, et al. Vascular system defects and neuronal apoptosis in mice lacking ras GTPase-activating protein. Nature. 1995;377:695-701.
- Rosen S, Smoller BR. Port-wine stains: a new hypothesis. J Am Acad Dermatol. 1987;17:164-166.
- Rydh M, Malm BM, Jernmeck J, et al. Ectatic blood vessels in port-wine stains lack innervation: possible role in pathogenesis. Plast Reconstr Surg. 1991;87:419-422.
- Tsuji T, Sawabe M. A new type of telangiectasia following trauma. J Cutan Pathol. 1988;15:22-26.
- Ruocco V, Ruocco E, Brunnetti G, et al. Opportunistic localization of skin lesions on vulnerable areas. Clin Dermatol. 2011;29:483-488.
- Thajudeheen CP, Jyothy K, Pryadarshi A. Treatment of port-wine stains with flash lamp pumped pulsed dye laser on Indian skin: a six year study. J Cutan Aesthet Surg. 2014;7:32-36.
Practice Points
- Port-wine stains (PWSs) most often are congenital lesions but can present later in life as acquired lesions with the same clinical and histologic findings.
- Magnetic resonance imaging of acquired PWSs should be considered to rule out underlying vascular anomalies (eg, deeper arteriovenous malformations).
- Pulsed dye laser therapy is safe for darker skin types and is the treatment of choice for acquired PWSs.
Creating an Action Plan for Eczema Patients
What does your patient need to know at the first visit?
The most essential information to share with patients at the first visit (as well as at all subsequent visits) is your eczema action plan, which should include discussion and potential modification of bathing regimens, use of topical emollients and medications, and exposure to detergents. It also is important to discuss the patient’s disease pattern (eg, triggers, seasonal flares, other forms of atopy), medication history, and past treatment responses. The eczema action plan is extremely vital for a variety of reasons. As a physician, I can’t be present every time a patient has a severe flare, and it would be difficult—both physically and financially—for patients to come in to my office every time a flare occurs. Patients and their caregivers need to develop a sense of empowerment at the first visit so they can address symptoms as they arise and actively prevent severe flares by following a gentle skin care plan that includes topical emollients and gentle cleansing.
I also like to emphasize to my eczema patients that they are not alone. In the United States, almost one-quarter of the population may have eczema. It’s also essential to explain to patients/caregivers that eczema is not caused by food allergies and cannot be cured by food elimination or other dietary modifications. Finally, I like to explain to patients that there is no true cure for eczema and that they will need to follow the action plan throughout their lifetime to help treat and prevent flares. Follow-up visits to review therapeutic response and review the patient’s eczema action plan can reinforce adherence and knowledge about the disease.
What are your go-to treatments? Are there any side effects?
Typically I prescribe 3 to 4 medications, which include an agent for the head and neck areas and/or areas of sensitive or thin skin, an agent for the body, an antihistamine to address sleep disturbances, and a rescue medication, which is a somewhat stronger topical agent for severe areas if present. Elimination of triggers such as fragrance and wool can be discussed. Review of Staphylococcus aureus as a trigger and addressing this trigger with bleach baths or other modifications (eg, topical antibacterials for crusted areas of skin) is needed.
Eczema treatment is a multistep process that varies by individual as well as by cost. For most eczema patients, treatment typically costs hundreds of dollars per year; therefore, I try to be mindful of the financial hardship that can be brought on by the need for many products. The mainstay of eczema therapy includes topical emollients along with gentle cleansers, laundry detergents, and other topical products. Topical corticosteroids are the first-line treatment and have been used for over 60 years with good outcomes in most patients when used judiciously; however, side effects including striae, glaucoma and hypothalamic-pituitary-adrenal axis suppression can occur. Topical corticosteroids should be selected by class and formulation—ointments and some newer base formulations are known to cause the least amount of stinging. In infants, the least potent agent that clears the skin effectively may maximize outcomes and minimize risk for side effects. Topical calcineurin inhibitors may be a good option in patients who do not respond to corticosteroids and are supported by excellent clinical evidence; however, be sure to consider the black box warnings.1-3 Sedating antihistamines can be prescribed for bedtime usage in pruritic patients who experience sleep disturbances.
How do you keep patients compliant with treatment?
Patients can only comply with treatment if they have an adequate supply of the treatment product. It is important to prescribe the right amount of product needed to treat the affected area. Provision of refills for recurrent disease also can ensure long-term treatment compliance.
It also is important to have a conversation with patients about the nature of their disease flares. In my practice, patients typically report having seasonal flares, especially in midsummer temperatures or when the indoor heating kicks on in late fall. Encourage patients to schedule appointments in advance of these seasons; refilling medications beforehand and liberal application of emollients also can mitigate seasonal flares.
Finally, I try to recommend or prescribe treatments that appeal to patients both physically and emotionally. Some patients have a fear of using topical corticosteroids (known as corticophobia or steroid phobia). For these patients, I maximize the use of topical emollients and/or enhanced emollients (eg, agents with lipid additives and ceramides) to reduce the need for topical corticosteroids. I also have found that many preteen boys dislike “sticky” emollients, so light or midweight creams may be more tolerable for nightly use in this population. Another common scenario is the patient who prefers natural products. There are a variety of natural agents available that can aid in the treatment of eczema, including coconut oil, ceramide-based products, and oleodistillates. I try to refer to the literature to encourage the use of natural products that are backed by good science rather than big hype.
What do you do if patients refuse treatment?
As a physician, I can’t force patients or their caregivers to adhere to the therapies I prescribe; however, most patients are genuinely seeking a better quality of life and therefore there usually is at least some aspect of a skin care regimen they will follow to achieve relief when needed. First I make sure that serious issues (eg, bacterial infections) are addressed. I do mention to patients/caregivers that lack of treatment with topical prescription agents may have biological consequences; for example, there is evidence to support the Atopic March (ie, progression of atopic diseases to food allergies, asthma, etc). Consequences also can include discomfort, reduced quality of life, and negative effects on personal relationships; pediatric patients also may be stigmatized by their peers. Exploration of the root cause of treatment refusal usually yields a helpful discussion with the patient/caregiver about their fears as well as alternative treatment agents. Sometimes I engage the pediatrician/primary care physician, an allergist, or a family member in the discussion to enhance compliance and provide patient/caregiver support. At the very least, most patients/caregivers will adhere to trigger avoidance and barrier repair through application of emollients.
What resources do you recommend to patients for more information?
There are many resources available to patients that may enhance the overall management of eczema. I give my patients an educational handout about eczema as well as a hardcopy of their personal eczema action plan. For pediatric patients, I write the child’s first name and the date to help his or her caregivers remember when they received the plan. Examples of eczema action plans can be found in published resources ranging from simple to complex regimens and should be tailored to the physician’s own patient education and treatment patterns.4,5 The National Eczema Association Web site (https://nationaleczema.org/) provides many resources for patients, including educational tools and an online community.
- Luger T, Boguniewicz M, Carr W, et al. Pimecrolimus in atopic dermatitis: consensus on safety and the need to allow use in infants [published online ahead of print April 13, 2015]. Pediatr Allergy Immunol. 2015;26:306-315.
- Carr WW. Topical calcineurin inhibitors for atopic dermatitis: review and treatment recommendations. Paediatr Drugs. 2013;15:303-310.
- Hui RL, Lide W, Chan J, et al. Association between exposure to topical tacrolimus or pimecrolimus and cancers. Ann Pharmacother. 2009;43:1956-1963.
- Eczema action plan. University of California, San Francisco Office of Continuing Medical Education Web site. http://www.ucsfcme.com/2011/slides/MPD11001/29 Cordoro-ADD1.pdf. Accessed November 17, 2015.
- Tollefson MM, Bruckner AL; Section On Dermatology. Atopic dermatitis: skin-directed management. Pediatrics. 2014;134:e1735-e1744.
What does your patient need to know at the first visit?
The most essential information to share with patients at the first visit (as well as at all subsequent visits) is your eczema action plan, which should include discussion and potential modification of bathing regimens, use of topical emollients and medications, and exposure to detergents. It also is important to discuss the patient’s disease pattern (eg, triggers, seasonal flares, other forms of atopy), medication history, and past treatment responses. The eczema action plan is extremely vital for a variety of reasons. As a physician, I can’t be present every time a patient has a severe flare, and it would be difficult—both physically and financially—for patients to come in to my office every time a flare occurs. Patients and their caregivers need to develop a sense of empowerment at the first visit so they can address symptoms as they arise and actively prevent severe flares by following a gentle skin care plan that includes topical emollients and gentle cleansing.
I also like to emphasize to my eczema patients that they are not alone. In the United States, almost one-quarter of the population may have eczema. It’s also essential to explain to patients/caregivers that eczema is not caused by food allergies and cannot be cured by food elimination or other dietary modifications. Finally, I like to explain to patients that there is no true cure for eczema and that they will need to follow the action plan throughout their lifetime to help treat and prevent flares. Follow-up visits to review therapeutic response and review the patient’s eczema action plan can reinforce adherence and knowledge about the disease.
What are your go-to treatments? Are there any side effects?
Typically I prescribe 3 to 4 medications, which include an agent for the head and neck areas and/or areas of sensitive or thin skin, an agent for the body, an antihistamine to address sleep disturbances, and a rescue medication, which is a somewhat stronger topical agent for severe areas if present. Elimination of triggers such as fragrance and wool can be discussed. Review of Staphylococcus aureus as a trigger and addressing this trigger with bleach baths or other modifications (eg, topical antibacterials for crusted areas of skin) is needed.
Eczema treatment is a multistep process that varies by individual as well as by cost. For most eczema patients, treatment typically costs hundreds of dollars per year; therefore, I try to be mindful of the financial hardship that can be brought on by the need for many products. The mainstay of eczema therapy includes topical emollients along with gentle cleansers, laundry detergents, and other topical products. Topical corticosteroids are the first-line treatment and have been used for over 60 years with good outcomes in most patients when used judiciously; however, side effects including striae, glaucoma and hypothalamic-pituitary-adrenal axis suppression can occur. Topical corticosteroids should be selected by class and formulation—ointments and some newer base formulations are known to cause the least amount of stinging. In infants, the least potent agent that clears the skin effectively may maximize outcomes and minimize risk for side effects. Topical calcineurin inhibitors may be a good option in patients who do not respond to corticosteroids and are supported by excellent clinical evidence; however, be sure to consider the black box warnings.1-3 Sedating antihistamines can be prescribed for bedtime usage in pruritic patients who experience sleep disturbances.
How do you keep patients compliant with treatment?
Patients can only comply with treatment if they have an adequate supply of the treatment product. It is important to prescribe the right amount of product needed to treat the affected area. Provision of refills for recurrent disease also can ensure long-term treatment compliance.
It also is important to have a conversation with patients about the nature of their disease flares. In my practice, patients typically report having seasonal flares, especially in midsummer temperatures or when the indoor heating kicks on in late fall. Encourage patients to schedule appointments in advance of these seasons; refilling medications beforehand and liberal application of emollients also can mitigate seasonal flares.
Finally, I try to recommend or prescribe treatments that appeal to patients both physically and emotionally. Some patients have a fear of using topical corticosteroids (known as corticophobia or steroid phobia). For these patients, I maximize the use of topical emollients and/or enhanced emollients (eg, agents with lipid additives and ceramides) to reduce the need for topical corticosteroids. I also have found that many preteen boys dislike “sticky” emollients, so light or midweight creams may be more tolerable for nightly use in this population. Another common scenario is the patient who prefers natural products. There are a variety of natural agents available that can aid in the treatment of eczema, including coconut oil, ceramide-based products, and oleodistillates. I try to refer to the literature to encourage the use of natural products that are backed by good science rather than big hype.
What do you do if patients refuse treatment?
As a physician, I can’t force patients or their caregivers to adhere to the therapies I prescribe; however, most patients are genuinely seeking a better quality of life and therefore there usually is at least some aspect of a skin care regimen they will follow to achieve relief when needed. First I make sure that serious issues (eg, bacterial infections) are addressed. I do mention to patients/caregivers that lack of treatment with topical prescription agents may have biological consequences; for example, there is evidence to support the Atopic March (ie, progression of atopic diseases to food allergies, asthma, etc). Consequences also can include discomfort, reduced quality of life, and negative effects on personal relationships; pediatric patients also may be stigmatized by their peers. Exploration of the root cause of treatment refusal usually yields a helpful discussion with the patient/caregiver about their fears as well as alternative treatment agents. Sometimes I engage the pediatrician/primary care physician, an allergist, or a family member in the discussion to enhance compliance and provide patient/caregiver support. At the very least, most patients/caregivers will adhere to trigger avoidance and barrier repair through application of emollients.
What resources do you recommend to patients for more information?
There are many resources available to patients that may enhance the overall management of eczema. I give my patients an educational handout about eczema as well as a hardcopy of their personal eczema action plan. For pediatric patients, I write the child’s first name and the date to help his or her caregivers remember when they received the plan. Examples of eczema action plans can be found in published resources ranging from simple to complex regimens and should be tailored to the physician’s own patient education and treatment patterns.4,5 The National Eczema Association Web site (https://nationaleczema.org/) provides many resources for patients, including educational tools and an online community.
What does your patient need to know at the first visit?
The most essential information to share with patients at the first visit (as well as at all subsequent visits) is your eczema action plan, which should include discussion and potential modification of bathing regimens, use of topical emollients and medications, and exposure to detergents. It also is important to discuss the patient’s disease pattern (eg, triggers, seasonal flares, other forms of atopy), medication history, and past treatment responses. The eczema action plan is extremely vital for a variety of reasons. As a physician, I can’t be present every time a patient has a severe flare, and it would be difficult—both physically and financially—for patients to come in to my office every time a flare occurs. Patients and their caregivers need to develop a sense of empowerment at the first visit so they can address symptoms as they arise and actively prevent severe flares by following a gentle skin care plan that includes topical emollients and gentle cleansing.
I also like to emphasize to my eczema patients that they are not alone. In the United States, almost one-quarter of the population may have eczema. It’s also essential to explain to patients/caregivers that eczema is not caused by food allergies and cannot be cured by food elimination or other dietary modifications. Finally, I like to explain to patients that there is no true cure for eczema and that they will need to follow the action plan throughout their lifetime to help treat and prevent flares. Follow-up visits to review therapeutic response and review the patient’s eczema action plan can reinforce adherence and knowledge about the disease.
What are your go-to treatments? Are there any side effects?
Typically I prescribe 3 to 4 medications, which include an agent for the head and neck areas and/or areas of sensitive or thin skin, an agent for the body, an antihistamine to address sleep disturbances, and a rescue medication, which is a somewhat stronger topical agent for severe areas if present. Elimination of triggers such as fragrance and wool can be discussed. Review of Staphylococcus aureus as a trigger and addressing this trigger with bleach baths or other modifications (eg, topical antibacterials for crusted areas of skin) is needed.
Eczema treatment is a multistep process that varies by individual as well as by cost. For most eczema patients, treatment typically costs hundreds of dollars per year; therefore, I try to be mindful of the financial hardship that can be brought on by the need for many products. The mainstay of eczema therapy includes topical emollients along with gentle cleansers, laundry detergents, and other topical products. Topical corticosteroids are the first-line treatment and have been used for over 60 years with good outcomes in most patients when used judiciously; however, side effects including striae, glaucoma and hypothalamic-pituitary-adrenal axis suppression can occur. Topical corticosteroids should be selected by class and formulation—ointments and some newer base formulations are known to cause the least amount of stinging. In infants, the least potent agent that clears the skin effectively may maximize outcomes and minimize risk for side effects. Topical calcineurin inhibitors may be a good option in patients who do not respond to corticosteroids and are supported by excellent clinical evidence; however, be sure to consider the black box warnings.1-3 Sedating antihistamines can be prescribed for bedtime usage in pruritic patients who experience sleep disturbances.
How do you keep patients compliant with treatment?
Patients can only comply with treatment if they have an adequate supply of the treatment product. It is important to prescribe the right amount of product needed to treat the affected area. Provision of refills for recurrent disease also can ensure long-term treatment compliance.
It also is important to have a conversation with patients about the nature of their disease flares. In my practice, patients typically report having seasonal flares, especially in midsummer temperatures or when the indoor heating kicks on in late fall. Encourage patients to schedule appointments in advance of these seasons; refilling medications beforehand and liberal application of emollients also can mitigate seasonal flares.
Finally, I try to recommend or prescribe treatments that appeal to patients both physically and emotionally. Some patients have a fear of using topical corticosteroids (known as corticophobia or steroid phobia). For these patients, I maximize the use of topical emollients and/or enhanced emollients (eg, agents with lipid additives and ceramides) to reduce the need for topical corticosteroids. I also have found that many preteen boys dislike “sticky” emollients, so light or midweight creams may be more tolerable for nightly use in this population. Another common scenario is the patient who prefers natural products. There are a variety of natural agents available that can aid in the treatment of eczema, including coconut oil, ceramide-based products, and oleodistillates. I try to refer to the literature to encourage the use of natural products that are backed by good science rather than big hype.
What do you do if patients refuse treatment?
As a physician, I can’t force patients or their caregivers to adhere to the therapies I prescribe; however, most patients are genuinely seeking a better quality of life and therefore there usually is at least some aspect of a skin care regimen they will follow to achieve relief when needed. First I make sure that serious issues (eg, bacterial infections) are addressed. I do mention to patients/caregivers that lack of treatment with topical prescription agents may have biological consequences; for example, there is evidence to support the Atopic March (ie, progression of atopic diseases to food allergies, asthma, etc). Consequences also can include discomfort, reduced quality of life, and negative effects on personal relationships; pediatric patients also may be stigmatized by their peers. Exploration of the root cause of treatment refusal usually yields a helpful discussion with the patient/caregiver about their fears as well as alternative treatment agents. Sometimes I engage the pediatrician/primary care physician, an allergist, or a family member in the discussion to enhance compliance and provide patient/caregiver support. At the very least, most patients/caregivers will adhere to trigger avoidance and barrier repair through application of emollients.
What resources do you recommend to patients for more information?
There are many resources available to patients that may enhance the overall management of eczema. I give my patients an educational handout about eczema as well as a hardcopy of their personal eczema action plan. For pediatric patients, I write the child’s first name and the date to help his or her caregivers remember when they received the plan. Examples of eczema action plans can be found in published resources ranging from simple to complex regimens and should be tailored to the physician’s own patient education and treatment patterns.4,5 The National Eczema Association Web site (https://nationaleczema.org/) provides many resources for patients, including educational tools and an online community.
- Luger T, Boguniewicz M, Carr W, et al. Pimecrolimus in atopic dermatitis: consensus on safety and the need to allow use in infants [published online ahead of print April 13, 2015]. Pediatr Allergy Immunol. 2015;26:306-315.
- Carr WW. Topical calcineurin inhibitors for atopic dermatitis: review and treatment recommendations. Paediatr Drugs. 2013;15:303-310.
- Hui RL, Lide W, Chan J, et al. Association between exposure to topical tacrolimus or pimecrolimus and cancers. Ann Pharmacother. 2009;43:1956-1963.
- Eczema action plan. University of California, San Francisco Office of Continuing Medical Education Web site. http://www.ucsfcme.com/2011/slides/MPD11001/29 Cordoro-ADD1.pdf. Accessed November 17, 2015.
- Tollefson MM, Bruckner AL; Section On Dermatology. Atopic dermatitis: skin-directed management. Pediatrics. 2014;134:e1735-e1744.
- Luger T, Boguniewicz M, Carr W, et al. Pimecrolimus in atopic dermatitis: consensus on safety and the need to allow use in infants [published online ahead of print April 13, 2015]. Pediatr Allergy Immunol. 2015;26:306-315.
- Carr WW. Topical calcineurin inhibitors for atopic dermatitis: review and treatment recommendations. Paediatr Drugs. 2013;15:303-310.
- Hui RL, Lide W, Chan J, et al. Association between exposure to topical tacrolimus or pimecrolimus and cancers. Ann Pharmacother. 2009;43:1956-1963.
- Eczema action plan. University of California, San Francisco Office of Continuing Medical Education Web site. http://www.ucsfcme.com/2011/slides/MPD11001/29 Cordoro-ADD1.pdf. Accessed November 17, 2015.
- Tollefson MM, Bruckner AL; Section On Dermatology. Atopic dermatitis: skin-directed management. Pediatrics. 2014;134:e1735-e1744.
Anti-TNF therapy can continue for IBD patients with skin lesions
Inflammatory bowel disease (IBD) patients who experience skin lesions during anti–tumor necrosis factor therapy do not usually need to stop treatment, according to Isabelle Cleynen, Ph.D., of the University of Leuven (Belgium) and her associates.
In their retrospective study of 917 IBD patients who started treatment with infliximab at the University Hospitals Leuven between December 1994 and January 2009, 264 developed skin lesions during the follow-up period. The most common type was psoriasiform eczema, in 30.6% of the patients with lesions. Other common types included eczema (in 23.5%), xerosis cutis (10.6%), palmoplantar pustulosis (5.3%), and psoriasis (3.8%). Median cumulative doses and trough levels of infliximab were similar in people who developed skin lesions and those who did not.
Just over half of patients with skin lesions received only topical treatment, 1.9% received only systemic treatment, 28% received both, and 19.3% of patients required no specific treatment. Almost 11% of patients who developed skin lesions were forced to stop therapy. Reasons for stopping treatment included an intolerable location of lesions, concomitant itching or pain, recurring episodes, and concomitant arthralgia.
“Knowledge of the diagnostic and therapeutic criteria and the clinical course of these lesions should assist in their management. With referral to a dedicated dermatologist, most lesions can be treated and the need for interruption of anti-TNF therapy is rare,” the investigators concluded.
Find the full study in Annals of Internal Medicine (doi: 10.7326/M15-0729).
Inflammatory bowel disease (IBD) patients who experience skin lesions during anti–tumor necrosis factor therapy do not usually need to stop treatment, according to Isabelle Cleynen, Ph.D., of the University of Leuven (Belgium) and her associates.
In their retrospective study of 917 IBD patients who started treatment with infliximab at the University Hospitals Leuven between December 1994 and January 2009, 264 developed skin lesions during the follow-up period. The most common type was psoriasiform eczema, in 30.6% of the patients with lesions. Other common types included eczema (in 23.5%), xerosis cutis (10.6%), palmoplantar pustulosis (5.3%), and psoriasis (3.8%). Median cumulative doses and trough levels of infliximab were similar in people who developed skin lesions and those who did not.
Just over half of patients with skin lesions received only topical treatment, 1.9% received only systemic treatment, 28% received both, and 19.3% of patients required no specific treatment. Almost 11% of patients who developed skin lesions were forced to stop therapy. Reasons for stopping treatment included an intolerable location of lesions, concomitant itching or pain, recurring episodes, and concomitant arthralgia.
“Knowledge of the diagnostic and therapeutic criteria and the clinical course of these lesions should assist in their management. With referral to a dedicated dermatologist, most lesions can be treated and the need for interruption of anti-TNF therapy is rare,” the investigators concluded.
Find the full study in Annals of Internal Medicine (doi: 10.7326/M15-0729).
Inflammatory bowel disease (IBD) patients who experience skin lesions during anti–tumor necrosis factor therapy do not usually need to stop treatment, according to Isabelle Cleynen, Ph.D., of the University of Leuven (Belgium) and her associates.
In their retrospective study of 917 IBD patients who started treatment with infliximab at the University Hospitals Leuven between December 1994 and January 2009, 264 developed skin lesions during the follow-up period. The most common type was psoriasiform eczema, in 30.6% of the patients with lesions. Other common types included eczema (in 23.5%), xerosis cutis (10.6%), palmoplantar pustulosis (5.3%), and psoriasis (3.8%). Median cumulative doses and trough levels of infliximab were similar in people who developed skin lesions and those who did not.
Just over half of patients with skin lesions received only topical treatment, 1.9% received only systemic treatment, 28% received both, and 19.3% of patients required no specific treatment. Almost 11% of patients who developed skin lesions were forced to stop therapy. Reasons for stopping treatment included an intolerable location of lesions, concomitant itching or pain, recurring episodes, and concomitant arthralgia.
“Knowledge of the diagnostic and therapeutic criteria and the clinical course of these lesions should assist in their management. With referral to a dedicated dermatologist, most lesions can be treated and the need for interruption of anti-TNF therapy is rare,” the investigators concluded.
Find the full study in Annals of Internal Medicine (doi: 10.7326/M15-0729).
Higher anemia risk for children with atopic disease
Children with a history of atopic disease (AD) are at a significantly higher risk for an anemia diagnosis than are children without AD, according to an analysis of two large U.S. population-based studies.
In addition, the risk of anemia increased with the number of caregiver-reported atopic disorders, reported the investigators, Dr. Jonathan I. Silverberg and his associates in the department of dermatology, Northwestern University, Chicago. A current diagnosis of eczema or asthma was associated with a significantly increased risk of anemia, particularly microcytic anemia, in the study, published online on Nov. 30 in JAMA Pediatrics (doi: 10.1001/jamapediatrics.2015.3065).
The authors evaluated data from the U.S. National Health Interview Survey (NHIS) on about 207,000 children and adolescents collected between 1997 and 2013, and from the National Health and Nutrition Examination Survey (NHANES) on nearly 31,000 children and adolescents between 1999 and 2012.
Data from the NHIS cohort found a significantly elevated anemia risk among children with a caregiver-reported history of hay fever, eczema, asthma, and food allergy (P less than .001 for all 4 disorders). After adjustments for age, sex, ethnicity and socioeconomic factors, the anemia risk for a child with any single atopic disorder was modestly elevated (adjusted odds ratio, 1.84; 95% confidence interval, 1.60-2.11; P less than .001). The adjusted risk among those with all four disorders was markedly elevated (adjusted OR, 7.87; 95% CI, 5.17-12.00; P less than .001).
In the NHANES cohort, the investigators found a current asthma or eczema diagnosis associated with anemia, as defined by laboratory assessment (adjusted OR, 1.33; 95% CI, 1.04-1.70; P = .02 for asthma; and an adjusted OR 1.93; 95% CI, 1.04-3.59; P = .04 for eczema).
In the NHANES cohort, asthma was associated with an increased risk for microcytic anemia (adjusted OR, 1.61; 95% CI 1.09-2.38 P = .02). In the 2005-2006 NHANES cohort, a history of eczema was associated with an increased risk of microcytic anemia (adjusted OR, 2.03; 95% CI, 1.20-3.46; P = .009). But no significant associations for hay fever and any type of anemia were noted.
While the reasons for the observed association between atopic disease and anemia remain unknown and are probably multifactorial, physicians “should be aware that fatigue may be related to unrecognized anemia and not merely sleep loss owing to atopic dermatitis or airway disease,” the investigators wrote. Moreover, they noted, restricted diets to treat atopic disorders could play a role. “This finding underscores the importance of properly evaluating and ruling out suspected food allergy in children rather than placing them on empirical avoidance diets that might contribute to anemia,” they said.
The study was funded by the Agency for Healthcare Research and Quality and the Dermatology Foundation. Dr. Silverberg and his coauthors, Kerry E. Drury and Matt Schaeffer, declared no conflicts of interest.
Children with a history of atopic disease (AD) are at a significantly higher risk for an anemia diagnosis than are children without AD, according to an analysis of two large U.S. population-based studies.
In addition, the risk of anemia increased with the number of caregiver-reported atopic disorders, reported the investigators, Dr. Jonathan I. Silverberg and his associates in the department of dermatology, Northwestern University, Chicago. A current diagnosis of eczema or asthma was associated with a significantly increased risk of anemia, particularly microcytic anemia, in the study, published online on Nov. 30 in JAMA Pediatrics (doi: 10.1001/jamapediatrics.2015.3065).
The authors evaluated data from the U.S. National Health Interview Survey (NHIS) on about 207,000 children and adolescents collected between 1997 and 2013, and from the National Health and Nutrition Examination Survey (NHANES) on nearly 31,000 children and adolescents between 1999 and 2012.
Data from the NHIS cohort found a significantly elevated anemia risk among children with a caregiver-reported history of hay fever, eczema, asthma, and food allergy (P less than .001 for all 4 disorders). After adjustments for age, sex, ethnicity and socioeconomic factors, the anemia risk for a child with any single atopic disorder was modestly elevated (adjusted odds ratio, 1.84; 95% confidence interval, 1.60-2.11; P less than .001). The adjusted risk among those with all four disorders was markedly elevated (adjusted OR, 7.87; 95% CI, 5.17-12.00; P less than .001).
In the NHANES cohort, the investigators found a current asthma or eczema diagnosis associated with anemia, as defined by laboratory assessment (adjusted OR, 1.33; 95% CI, 1.04-1.70; P = .02 for asthma; and an adjusted OR 1.93; 95% CI, 1.04-3.59; P = .04 for eczema).
In the NHANES cohort, asthma was associated with an increased risk for microcytic anemia (adjusted OR, 1.61; 95% CI 1.09-2.38 P = .02). In the 2005-2006 NHANES cohort, a history of eczema was associated with an increased risk of microcytic anemia (adjusted OR, 2.03; 95% CI, 1.20-3.46; P = .009). But no significant associations for hay fever and any type of anemia were noted.
While the reasons for the observed association between atopic disease and anemia remain unknown and are probably multifactorial, physicians “should be aware that fatigue may be related to unrecognized anemia and not merely sleep loss owing to atopic dermatitis or airway disease,” the investigators wrote. Moreover, they noted, restricted diets to treat atopic disorders could play a role. “This finding underscores the importance of properly evaluating and ruling out suspected food allergy in children rather than placing them on empirical avoidance diets that might contribute to anemia,” they said.
The study was funded by the Agency for Healthcare Research and Quality and the Dermatology Foundation. Dr. Silverberg and his coauthors, Kerry E. Drury and Matt Schaeffer, declared no conflicts of interest.
Children with a history of atopic disease (AD) are at a significantly higher risk for an anemia diagnosis than are children without AD, according to an analysis of two large U.S. population-based studies.
In addition, the risk of anemia increased with the number of caregiver-reported atopic disorders, reported the investigators, Dr. Jonathan I. Silverberg and his associates in the department of dermatology, Northwestern University, Chicago. A current diagnosis of eczema or asthma was associated with a significantly increased risk of anemia, particularly microcytic anemia, in the study, published online on Nov. 30 in JAMA Pediatrics (doi: 10.1001/jamapediatrics.2015.3065).
The authors evaluated data from the U.S. National Health Interview Survey (NHIS) on about 207,000 children and adolescents collected between 1997 and 2013, and from the National Health and Nutrition Examination Survey (NHANES) on nearly 31,000 children and adolescents between 1999 and 2012.
Data from the NHIS cohort found a significantly elevated anemia risk among children with a caregiver-reported history of hay fever, eczema, asthma, and food allergy (P less than .001 for all 4 disorders). After adjustments for age, sex, ethnicity and socioeconomic factors, the anemia risk for a child with any single atopic disorder was modestly elevated (adjusted odds ratio, 1.84; 95% confidence interval, 1.60-2.11; P less than .001). The adjusted risk among those with all four disorders was markedly elevated (adjusted OR, 7.87; 95% CI, 5.17-12.00; P less than .001).
In the NHANES cohort, the investigators found a current asthma or eczema diagnosis associated with anemia, as defined by laboratory assessment (adjusted OR, 1.33; 95% CI, 1.04-1.70; P = .02 for asthma; and an adjusted OR 1.93; 95% CI, 1.04-3.59; P = .04 for eczema).
In the NHANES cohort, asthma was associated with an increased risk for microcytic anemia (adjusted OR, 1.61; 95% CI 1.09-2.38 P = .02). In the 2005-2006 NHANES cohort, a history of eczema was associated with an increased risk of microcytic anemia (adjusted OR, 2.03; 95% CI, 1.20-3.46; P = .009). But no significant associations for hay fever and any type of anemia were noted.
While the reasons for the observed association between atopic disease and anemia remain unknown and are probably multifactorial, physicians “should be aware that fatigue may be related to unrecognized anemia and not merely sleep loss owing to atopic dermatitis or airway disease,” the investigators wrote. Moreover, they noted, restricted diets to treat atopic disorders could play a role. “This finding underscores the importance of properly evaluating and ruling out suspected food allergy in children rather than placing them on empirical avoidance diets that might contribute to anemia,” they said.
The study was funded by the Agency for Healthcare Research and Quality and the Dermatology Foundation. Dr. Silverberg and his coauthors, Kerry E. Drury and Matt Schaeffer, declared no conflicts of interest.
FROM JAMA PEDIATRICS
Key clinical point: Children with atopic disorders are at a higher risk of anemia, which may be underrecognized in this population, and could be related to a restricted diet.
Major finding: Children with history of eczema, asthma, hay fever, or food allergy were at an increased risk of anemia, compared with children without these disorders (P less than .001 for all).
Data source: The study evaluated data on children and adolescents in the population-based U.S. National Health Interview Survey (207,007) and the National Health and Nutrition Examination Survey (30,673 children).
Disclosures: The study was sponsored by the Agency for Healthcare Research and Quality and the Dermatology Foundation; the authors reported no conflicts.
Food-antigen–specific immunoglobulin E is not a predictor of food allergies in atopic dermatitis
Food-antigen–specific immunoglobulin E (sIgE) levels were not clinically useful for predicting food allergy development in a study of infants with atopic dermatitis (AD).
The dual-phase study included 1,087 patients aged 3-18 months who had been diagnosed with AD for no more than 3 months prior to enrollment in the study and had at least mild disease activity. During the first phase of the study, which was a 36-month, randomized, double-blind, vehicle-controlled phase, half of patients were treated with placebo cream and the other half were treated with 1% pimecrolimus cream. In the second phase of the study, which was open-label, all patients received 1% pimecrolimus cream for up to 33 months or the patient’s 6th birthday, whichever occurred sooner. Patients were excluded if they received treatment with topical or systemic agents within 7 days before the first application of cream in the study.
The researchers followed food allergy development during both phases of the study. Other data collected by the researchers included sIgE levels for various foods at baseline and at the end of both phases of the study, with sIgE decision points having been assigned to each food.
By the end of the second phase of the trial, 15.9% of patients had developed a food allergy, with 292 days having been the median period of time that passed before the initial diagnosis of a food allergy was made. The most common food allergies were to peanuts, cow’s milk, and egg whites, occurring in 7%, 4%, and 4% of patients respectively. The percentage of patients with any allergy to food other than fish decreased over time. Higher levels of AD severity were predictive for the development of food allergy, with the percentage of patients who developed one or more food allergies by the end of the study having increased with increasing AD severity at baseline.
Total serum immunoglobulin E (IgE) and sIgE for milk, eggs, and peanuts measured at the end of the second phase also were increased in patients with increasing AD severity. Despite these findings, the positive predictive values for sIgE decision points for the foods tested were low (less than 0.6 for all values tested).
“SIgE decision points, both published values and the novel decision points used in this study, had high [negative predictive values], in particular for peanut[s], egg white[s], and cow’s milk. Thus, patients with mild AD with sIgE levels below these cutoffs would be unlikely to have or develop these specific allergies and would not benefit from food challenges or elimination diets. Similarly, elevated sIgE, as defined by the decision points tested, had very low [positive predictive values] for food allergy, both for sIgE values at baseline and at the end of the [first phase of the study] ... Thus, despite an increased likelihood of allergy development with increasing sIgE shown for cow’s milk, egg[s], and peanut[s], our data do not support the use of sIgE testing for the diagnosis of food allergy in subjects without a history of reaction to that food,” said Dr. Jonathan M. Spergelof the Children’s Hospital of Philadelphia and his colleagues.
Read the full study in Pediatrics (doi: 10.1542/peds.2015-1444).
Food-antigen–specific immunoglobulin E (sIgE) levels were not clinically useful for predicting food allergy development in a study of infants with atopic dermatitis (AD).
The dual-phase study included 1,087 patients aged 3-18 months who had been diagnosed with AD for no more than 3 months prior to enrollment in the study and had at least mild disease activity. During the first phase of the study, which was a 36-month, randomized, double-blind, vehicle-controlled phase, half of patients were treated with placebo cream and the other half were treated with 1% pimecrolimus cream. In the second phase of the study, which was open-label, all patients received 1% pimecrolimus cream for up to 33 months or the patient’s 6th birthday, whichever occurred sooner. Patients were excluded if they received treatment with topical or systemic agents within 7 days before the first application of cream in the study.
The researchers followed food allergy development during both phases of the study. Other data collected by the researchers included sIgE levels for various foods at baseline and at the end of both phases of the study, with sIgE decision points having been assigned to each food.
By the end of the second phase of the trial, 15.9% of patients had developed a food allergy, with 292 days having been the median period of time that passed before the initial diagnosis of a food allergy was made. The most common food allergies were to peanuts, cow’s milk, and egg whites, occurring in 7%, 4%, and 4% of patients respectively. The percentage of patients with any allergy to food other than fish decreased over time. Higher levels of AD severity were predictive for the development of food allergy, with the percentage of patients who developed one or more food allergies by the end of the study having increased with increasing AD severity at baseline.
Total serum immunoglobulin E (IgE) and sIgE for milk, eggs, and peanuts measured at the end of the second phase also were increased in patients with increasing AD severity. Despite these findings, the positive predictive values for sIgE decision points for the foods tested were low (less than 0.6 for all values tested).
“SIgE decision points, both published values and the novel decision points used in this study, had high [negative predictive values], in particular for peanut[s], egg white[s], and cow’s milk. Thus, patients with mild AD with sIgE levels below these cutoffs would be unlikely to have or develop these specific allergies and would not benefit from food challenges or elimination diets. Similarly, elevated sIgE, as defined by the decision points tested, had very low [positive predictive values] for food allergy, both for sIgE values at baseline and at the end of the [first phase of the study] ... Thus, despite an increased likelihood of allergy development with increasing sIgE shown for cow’s milk, egg[s], and peanut[s], our data do not support the use of sIgE testing for the diagnosis of food allergy in subjects without a history of reaction to that food,” said Dr. Jonathan M. Spergelof the Children’s Hospital of Philadelphia and his colleagues.
Read the full study in Pediatrics (doi: 10.1542/peds.2015-1444).
Food-antigen–specific immunoglobulin E (sIgE) levels were not clinically useful for predicting food allergy development in a study of infants with atopic dermatitis (AD).
The dual-phase study included 1,087 patients aged 3-18 months who had been diagnosed with AD for no more than 3 months prior to enrollment in the study and had at least mild disease activity. During the first phase of the study, which was a 36-month, randomized, double-blind, vehicle-controlled phase, half of patients were treated with placebo cream and the other half were treated with 1% pimecrolimus cream. In the second phase of the study, which was open-label, all patients received 1% pimecrolimus cream for up to 33 months or the patient’s 6th birthday, whichever occurred sooner. Patients were excluded if they received treatment with topical or systemic agents within 7 days before the first application of cream in the study.
The researchers followed food allergy development during both phases of the study. Other data collected by the researchers included sIgE levels for various foods at baseline and at the end of both phases of the study, with sIgE decision points having been assigned to each food.
By the end of the second phase of the trial, 15.9% of patients had developed a food allergy, with 292 days having been the median period of time that passed before the initial diagnosis of a food allergy was made. The most common food allergies were to peanuts, cow’s milk, and egg whites, occurring in 7%, 4%, and 4% of patients respectively. The percentage of patients with any allergy to food other than fish decreased over time. Higher levels of AD severity were predictive for the development of food allergy, with the percentage of patients who developed one or more food allergies by the end of the study having increased with increasing AD severity at baseline.
Total serum immunoglobulin E (IgE) and sIgE for milk, eggs, and peanuts measured at the end of the second phase also were increased in patients with increasing AD severity. Despite these findings, the positive predictive values for sIgE decision points for the foods tested were low (less than 0.6 for all values tested).
“SIgE decision points, both published values and the novel decision points used in this study, had high [negative predictive values], in particular for peanut[s], egg white[s], and cow’s milk. Thus, patients with mild AD with sIgE levels below these cutoffs would be unlikely to have or develop these specific allergies and would not benefit from food challenges or elimination diets. Similarly, elevated sIgE, as defined by the decision points tested, had very low [positive predictive values] for food allergy, both for sIgE values at baseline and at the end of the [first phase of the study] ... Thus, despite an increased likelihood of allergy development with increasing sIgE shown for cow’s milk, egg[s], and peanut[s], our data do not support the use of sIgE testing for the diagnosis of food allergy in subjects without a history of reaction to that food,” said Dr. Jonathan M. Spergelof the Children’s Hospital of Philadelphia and his colleagues.
Read the full study in Pediatrics (doi: 10.1542/peds.2015-1444).
FROM PEDIATRICS
Melatonin improves sleep, skin symptoms in pediatric AD
Oral melatonin improves both sleep disturbance and skin symptoms in children and adolescents who have atopic dermatitis, according to a report published online November 16 in JAMA Pediatrics.
“Although the magnitude of the benefit is not great, many patients might benefit from this dual effect. Sleep disturbance is highly prevalent in children with AD and . . . leads to impaired quality of life for the patients and their families,” said Dr. Yung-Sen Chang of
Taipei (Taiwan) City Hospital Renal Branch and associates.
The investigators showed in a previous study that children with AD have longer sleep-onset latency, more sleep fragmentation, less REM sleep, and reduced sleep efficiency than healthy control subjects and that these sleep disturbances are associated with scratching movements, more severe dermatitis, and decreased melatonin secretion. “Worse sleep increases the likelihood that these children will scratch, which will further exacerbate the skin inflammation. . . . We hypothesized that melatonin, with its sleep-promoting and antiinflammatory properties, could break this vicious cycle,” they noted.
Dr. Chang and associates performed a single-center randomized double-blind crossover trial involving 38 patients aged 1-18 years who had AD involving at least 5% of their body surface area and sleep problems that occurred more than 3 days per week. These study participants were randomly assigned to receive oral melatonin (3 mg/day) or a matching placebo at bedtime for 4 weeks, then crossed over to the alternate assignment for a further 4 weeks.
The primary efficacy outcome — AD severity as measured on the Scoring Atopic Dermatitis index — improved with melatonin by a mean of 9.1 points out of a possible 103 points, compared with placebo. Melatonin also significantly reduced sleep-onset latency by more than half (23.4 minutes), compared with placebo, according to objective sleep actigraphy results (JAMA Ped. 2015 November 16 [doi:10.1001/jamapediatrics.2015.3092]).
In addition, more patients and families subjectively reported that dermatitis improved when taking melatonin compared with placebo (47% vs 32%), and more reported that sleep improved when taking melatonin compared with placebo (46% vs 34%).
No adverse effects were reported. Melatonin’s good safety profile is particularly important given that all other medications used for sleep problems in patients with AD, such as antihistamines, benzodiazepines, chloral hydrate, and clonidine, are of limited benefit and carry negative adverse effects.
“We recommend melatonin supplementation for these patients, because it is a potentially safe and effective way to improve their sleep and skin condition simultaneously,” Dr. Chang and associates said.
Oral melatonin improves both sleep disturbance and skin symptoms in children and adolescents who have atopic dermatitis, according to a report published online November 16 in JAMA Pediatrics.
“Although the magnitude of the benefit is not great, many patients might benefit from this dual effect. Sleep disturbance is highly prevalent in children with AD and . . . leads to impaired quality of life for the patients and their families,” said Dr. Yung-Sen Chang of
Taipei (Taiwan) City Hospital Renal Branch and associates.
The investigators showed in a previous study that children with AD have longer sleep-onset latency, more sleep fragmentation, less REM sleep, and reduced sleep efficiency than healthy control subjects and that these sleep disturbances are associated with scratching movements, more severe dermatitis, and decreased melatonin secretion. “Worse sleep increases the likelihood that these children will scratch, which will further exacerbate the skin inflammation. . . . We hypothesized that melatonin, with its sleep-promoting and antiinflammatory properties, could break this vicious cycle,” they noted.
Dr. Chang and associates performed a single-center randomized double-blind crossover trial involving 38 patients aged 1-18 years who had AD involving at least 5% of their body surface area and sleep problems that occurred more than 3 days per week. These study participants were randomly assigned to receive oral melatonin (3 mg/day) or a matching placebo at bedtime for 4 weeks, then crossed over to the alternate assignment for a further 4 weeks.
The primary efficacy outcome — AD severity as measured on the Scoring Atopic Dermatitis index — improved with melatonin by a mean of 9.1 points out of a possible 103 points, compared with placebo. Melatonin also significantly reduced sleep-onset latency by more than half (23.4 minutes), compared with placebo, according to objective sleep actigraphy results (JAMA Ped. 2015 November 16 [doi:10.1001/jamapediatrics.2015.3092]).
In addition, more patients and families subjectively reported that dermatitis improved when taking melatonin compared with placebo (47% vs 32%), and more reported that sleep improved when taking melatonin compared with placebo (46% vs 34%).
No adverse effects were reported. Melatonin’s good safety profile is particularly important given that all other medications used for sleep problems in patients with AD, such as antihistamines, benzodiazepines, chloral hydrate, and clonidine, are of limited benefit and carry negative adverse effects.
“We recommend melatonin supplementation for these patients, because it is a potentially safe and effective way to improve their sleep and skin condition simultaneously,” Dr. Chang and associates said.
Oral melatonin improves both sleep disturbance and skin symptoms in children and adolescents who have atopic dermatitis, according to a report published online November 16 in JAMA Pediatrics.
“Although the magnitude of the benefit is not great, many patients might benefit from this dual effect. Sleep disturbance is highly prevalent in children with AD and . . . leads to impaired quality of life for the patients and their families,” said Dr. Yung-Sen Chang of
Taipei (Taiwan) City Hospital Renal Branch and associates.
The investigators showed in a previous study that children with AD have longer sleep-onset latency, more sleep fragmentation, less REM sleep, and reduced sleep efficiency than healthy control subjects and that these sleep disturbances are associated with scratching movements, more severe dermatitis, and decreased melatonin secretion. “Worse sleep increases the likelihood that these children will scratch, which will further exacerbate the skin inflammation. . . . We hypothesized that melatonin, with its sleep-promoting and antiinflammatory properties, could break this vicious cycle,” they noted.
Dr. Chang and associates performed a single-center randomized double-blind crossover trial involving 38 patients aged 1-18 years who had AD involving at least 5% of their body surface area and sleep problems that occurred more than 3 days per week. These study participants were randomly assigned to receive oral melatonin (3 mg/day) or a matching placebo at bedtime for 4 weeks, then crossed over to the alternate assignment for a further 4 weeks.
The primary efficacy outcome — AD severity as measured on the Scoring Atopic Dermatitis index — improved with melatonin by a mean of 9.1 points out of a possible 103 points, compared with placebo. Melatonin also significantly reduced sleep-onset latency by more than half (23.4 minutes), compared with placebo, according to objective sleep actigraphy results (JAMA Ped. 2015 November 16 [doi:10.1001/jamapediatrics.2015.3092]).
In addition, more patients and families subjectively reported that dermatitis improved when taking melatonin compared with placebo (47% vs 32%), and more reported that sleep improved when taking melatonin compared with placebo (46% vs 34%).
No adverse effects were reported. Melatonin’s good safety profile is particularly important given that all other medications used for sleep problems in patients with AD, such as antihistamines, benzodiazepines, chloral hydrate, and clonidine, are of limited benefit and carry negative adverse effects.
“We recommend melatonin supplementation for these patients, because it is a potentially safe and effective way to improve their sleep and skin condition simultaneously,” Dr. Chang and associates said.
FROM JAMA PEDIATRICS
Key clinical point: Oral melatonin improves both sleep and skin symptoms in children with atopic dermatitis.
Major finding: The primary efficacy outcome — AD severity as measured on the Scoring Atopic Dermatitis index — improved by a mean of 9.1 points out of a possible 103 points with melatonin, compared with placebo.
Data source: A single-center randomized double-blind crossover clinical trial involving 38 patients aged 1-18 years who had both atopic dermatitis and sleep problems.
Disclosures: This study was supported by National Taiwan University Hospital and the Yonghe Cardinal Tien Hospital. Dr. Chang and associates reported having no relevant conflicts of interest to disclose.
High IgE linked to poor treatment outcomes in AD patients
A smaller percentage of atopic dermatitis (AD) patients with high baseline serum total IgE achieved a good response to treatment, compared with AD patients with lower serum total IgE at baseline, in a retrospective study of Finnish patients.
After adjustment of the data from the multivariate analyses, “the presence of contact allergies and high baseline IgE values [greater than or equal to] 10,0000 IU/mL remained risk factors for poor long-term outcome and were statistically significantly negatively associated with a good treatment response (OR [odds ratio], 0.162 and 0.062, respectively), and with complete remission (OR 0.287 and 0.158, respectively),” wrote Ville Kiiski of Skin and Allergy Hospital, Helsinki, and his colleagues.
The study comprised 169 individuals aged 14-78 with atopic dermatitis. Patients were reevaluated a mean of 4.15 years following the first visit to the specialized AD clinic at Skin and Allergy Hospital of Helsinki University Central Hospital. They received topical treatments as either maintenance therapy with tacrolimus or maintenance treatment with either a combination of topical tacrolimus and topical corticosteroids, or topical corticosteroids alone. A dermatologist provided the patients with a long-term treatment plan, and a nurse provided “hands-on training for adequate topical therapy regimens,” when necessary.
“In patients with baseline IgE [greater than or equal to] 10,000 IU/mL, proportions achieving complete remission or a good treatment response were only 8.7% and 14.3%, compared with 51.6% and 79.7% in patients with IgE [less than] 1,000 IU/mL, and 36.9% and 58.1% in patients with IgE 1,000-10.000 IU/mL, respectively,” the researchers wrote.
While this study’s results suggest that serum total IgE is a predictor of long-term treatment outcome in AD patients, a larger, prospective study is needed to confirm this, they added.
The authors declared no conflicts of interest.
Read the study in Acta Dermato-Venereologica (doi: 10.2340/00015555-2126).
A smaller percentage of atopic dermatitis (AD) patients with high baseline serum total IgE achieved a good response to treatment, compared with AD patients with lower serum total IgE at baseline, in a retrospective study of Finnish patients.
After adjustment of the data from the multivariate analyses, “the presence of contact allergies and high baseline IgE values [greater than or equal to] 10,0000 IU/mL remained risk factors for poor long-term outcome and were statistically significantly negatively associated with a good treatment response (OR [odds ratio], 0.162 and 0.062, respectively), and with complete remission (OR 0.287 and 0.158, respectively),” wrote Ville Kiiski of Skin and Allergy Hospital, Helsinki, and his colleagues.
The study comprised 169 individuals aged 14-78 with atopic dermatitis. Patients were reevaluated a mean of 4.15 years following the first visit to the specialized AD clinic at Skin and Allergy Hospital of Helsinki University Central Hospital. They received topical treatments as either maintenance therapy with tacrolimus or maintenance treatment with either a combination of topical tacrolimus and topical corticosteroids, or topical corticosteroids alone. A dermatologist provided the patients with a long-term treatment plan, and a nurse provided “hands-on training for adequate topical therapy regimens,” when necessary.
“In patients with baseline IgE [greater than or equal to] 10,000 IU/mL, proportions achieving complete remission or a good treatment response were only 8.7% and 14.3%, compared with 51.6% and 79.7% in patients with IgE [less than] 1,000 IU/mL, and 36.9% and 58.1% in patients with IgE 1,000-10.000 IU/mL, respectively,” the researchers wrote.
While this study’s results suggest that serum total IgE is a predictor of long-term treatment outcome in AD patients, a larger, prospective study is needed to confirm this, they added.
The authors declared no conflicts of interest.
Read the study in Acta Dermato-Venereologica (doi: 10.2340/00015555-2126).
A smaller percentage of atopic dermatitis (AD) patients with high baseline serum total IgE achieved a good response to treatment, compared with AD patients with lower serum total IgE at baseline, in a retrospective study of Finnish patients.
After adjustment of the data from the multivariate analyses, “the presence of contact allergies and high baseline IgE values [greater than or equal to] 10,0000 IU/mL remained risk factors for poor long-term outcome and were statistically significantly negatively associated with a good treatment response (OR [odds ratio], 0.162 and 0.062, respectively), and with complete remission (OR 0.287 and 0.158, respectively),” wrote Ville Kiiski of Skin and Allergy Hospital, Helsinki, and his colleagues.
The study comprised 169 individuals aged 14-78 with atopic dermatitis. Patients were reevaluated a mean of 4.15 years following the first visit to the specialized AD clinic at Skin and Allergy Hospital of Helsinki University Central Hospital. They received topical treatments as either maintenance therapy with tacrolimus or maintenance treatment with either a combination of topical tacrolimus and topical corticosteroids, or topical corticosteroids alone. A dermatologist provided the patients with a long-term treatment plan, and a nurse provided “hands-on training for adequate topical therapy regimens,” when necessary.
“In patients with baseline IgE [greater than or equal to] 10,000 IU/mL, proportions achieving complete remission or a good treatment response were only 8.7% and 14.3%, compared with 51.6% and 79.7% in patients with IgE [less than] 1,000 IU/mL, and 36.9% and 58.1% in patients with IgE 1,000-10.000 IU/mL, respectively,” the researchers wrote.
While this study’s results suggest that serum total IgE is a predictor of long-term treatment outcome in AD patients, a larger, prospective study is needed to confirm this, they added.
The authors declared no conflicts of interest.
Read the study in Acta Dermato-Venereologica (doi: 10.2340/00015555-2126).
FROM ACTA DERMATO-VENEREOLOGICA
Eczema associated with childhood speech disorder
Pediatric eczema was significantly associated with a higher risk of speech disorder, reported Dr. Mark A. Strom and his coauthors from Northwestern University, Chicago.
A retrospective analysis of 354,416 children in 19 U.S. population–based cohorts found that the prevalence of speech disorder was 4.7% among the children with eczema (95% confidence interval, 4.5%-5.0%), compared with 2.2% among those without eczema (95% CI; 2.2%-2.3%).
Results from a multivariate analysis showed that eczema was associated with increased odds of speech disorder (adjusted odds ratio = 1.81 [1.57-2.05]; P less than .001). Mild (1.36 [1.02-1.81]; P = .03) and severe (3.56 [1.70-7.48]; P less than .001) eczema were associated with higher risk of speech disorder, the investigators said.
The findings were based on results from the 2003-2004 and 2007-2008 National Survey of Children’s Health, and the 1997-2013 National Health Interview Survey.
Read the study in the Journal of Pediatrics.
Pediatric eczema was significantly associated with a higher risk of speech disorder, reported Dr. Mark A. Strom and his coauthors from Northwestern University, Chicago.
A retrospective analysis of 354,416 children in 19 U.S. population–based cohorts found that the prevalence of speech disorder was 4.7% among the children with eczema (95% confidence interval, 4.5%-5.0%), compared with 2.2% among those without eczema (95% CI; 2.2%-2.3%).
Results from a multivariate analysis showed that eczema was associated with increased odds of speech disorder (adjusted odds ratio = 1.81 [1.57-2.05]; P less than .001). Mild (1.36 [1.02-1.81]; P = .03) and severe (3.56 [1.70-7.48]; P less than .001) eczema were associated with higher risk of speech disorder, the investigators said.
The findings were based on results from the 2003-2004 and 2007-2008 National Survey of Children’s Health, and the 1997-2013 National Health Interview Survey.
Read the study in the Journal of Pediatrics.
Pediatric eczema was significantly associated with a higher risk of speech disorder, reported Dr. Mark A. Strom and his coauthors from Northwestern University, Chicago.
A retrospective analysis of 354,416 children in 19 U.S. population–based cohorts found that the prevalence of speech disorder was 4.7% among the children with eczema (95% confidence interval, 4.5%-5.0%), compared with 2.2% among those without eczema (95% CI; 2.2%-2.3%).
Results from a multivariate analysis showed that eczema was associated with increased odds of speech disorder (adjusted odds ratio = 1.81 [1.57-2.05]; P less than .001). Mild (1.36 [1.02-1.81]; P = .03) and severe (3.56 [1.70-7.48]; P less than .001) eczema were associated with higher risk of speech disorder, the investigators said.
The findings were based on results from the 2003-2004 and 2007-2008 National Survey of Children’s Health, and the 1997-2013 National Health Interview Survey.
Read the study in the Journal of Pediatrics.