User login
Celiac disease ‘as common as psoriasis’
PARK CITY, UTAH – Dr. John J. Zone first began to study gluten sensitivity in 1977, an interest that left some of his clinician colleagues wondering why.
“Everybody told me I was crazy – that this was extremely rare. So I always say I was gluten when gluten wasn’t cool,” Dr. Zone, professor and chairman of dermatology at the University of Utah, Salt Lake City, told attendees at the annual meeting of the Pacific Dermatologic Association.
These days, it’s hard to shop in a food market without noticing all the gluten-free foods available, from pizza dough to beer. Many restaurants also serve gluten-free dishes. But is it hype, or is gluten sensitivity that common? Five percent of people in the United States “will say they are gluten sensitive,” he said. “In fact, 1% of Caucasians actually have celiac disease and 1% of Caucasians have gluten sensitivity that can be documented by challenge but don’t have celiac disease, while 3% have nothing.”
Gluten is a group of proteins contained in wheat, barley, and rye that is insoluble in water. Dr. Zone described celiac disease as a “spectrum of disease” characterized by inflammation of the small intestinal mucosa that occurs with the ingestion of gluten. The condition improves when gluten is removed from the diet. From a genetic standpoint, having a predisposition to express human leukocyte antigen-DQ2 or HLA-DQ8 is required for a diagnosis of celiac disease (CD). An estimated 20%-25% of whites “have that HLA background, but it is rare in Asians,” he said. “The receptors coded by HLA genes are essential for the processing of the gliadin antigen in CD.”
The hallmark for CD is a blood test for immunoglobulin A (IgA) anti-tissue transglutaminase antibodies, which are detectable in patients with untreated disease. “You should be able to get that test for $50 or $60 in any laboratory in the country,” Dr. Zone said. “It’s about 98% reliable. You also want to do a total serum IgA to rule out IgA-deficiency.”
CD clusters with other autoimmune disorders such as Addison’s disease, autoimmune thyroiditis, atrophic gastritis, systemic lupus erythematosus, rheumatoid arthritis, myasthenia gravis, and vitiligo. It’s also common in Down syndrome. “Many patients with histological inflammation have atypical intestinal symptoms or none at all,” he said. “Clinical studies have shown that only 15%-20% of CD patients identified by serology and confirmed by biopsy have classical symptoms of diarrhea and malabsorption.” The presenting symptom in patients with celiac disease may be limited to only aphthous stomatitis, eczema, alopecia areata, psoriasis, or diabetes, along with fatigue or anemia.
Researchers who analyzed the prevalence of CD in the United States estimated the risk to be 1:133 among individuals deemed not to be at risk, 1:56 in symptomatic patients, 1:39 in second-degree relatives, and 1:22 in first-degree relatives (Arch Intern Med. 2003;163[3]:286-92.). “We tested 2,100 people in Utah and found the prevalence among first-degree relatives was 1:12,” Dr. Zone said. “The point is that CD is common, not rare. It’s as common as psoriasis. It profoundly affects the immune system, which is the modulator of inflammatory skin disease.”
Dr. Zone reported having no financial disclosures.
PARK CITY, UTAH – Dr. John J. Zone first began to study gluten sensitivity in 1977, an interest that left some of his clinician colleagues wondering why.
“Everybody told me I was crazy – that this was extremely rare. So I always say I was gluten when gluten wasn’t cool,” Dr. Zone, professor and chairman of dermatology at the University of Utah, Salt Lake City, told attendees at the annual meeting of the Pacific Dermatologic Association.
These days, it’s hard to shop in a food market without noticing all the gluten-free foods available, from pizza dough to beer. Many restaurants also serve gluten-free dishes. But is it hype, or is gluten sensitivity that common? Five percent of people in the United States “will say they are gluten sensitive,” he said. “In fact, 1% of Caucasians actually have celiac disease and 1% of Caucasians have gluten sensitivity that can be documented by challenge but don’t have celiac disease, while 3% have nothing.”
Gluten is a group of proteins contained in wheat, barley, and rye that is insoluble in water. Dr. Zone described celiac disease as a “spectrum of disease” characterized by inflammation of the small intestinal mucosa that occurs with the ingestion of gluten. The condition improves when gluten is removed from the diet. From a genetic standpoint, having a predisposition to express human leukocyte antigen-DQ2 or HLA-DQ8 is required for a diagnosis of celiac disease (CD). An estimated 20%-25% of whites “have that HLA background, but it is rare in Asians,” he said. “The receptors coded by HLA genes are essential for the processing of the gliadin antigen in CD.”
The hallmark for CD is a blood test for immunoglobulin A (IgA) anti-tissue transglutaminase antibodies, which are detectable in patients with untreated disease. “You should be able to get that test for $50 or $60 in any laboratory in the country,” Dr. Zone said. “It’s about 98% reliable. You also want to do a total serum IgA to rule out IgA-deficiency.”
CD clusters with other autoimmune disorders such as Addison’s disease, autoimmune thyroiditis, atrophic gastritis, systemic lupus erythematosus, rheumatoid arthritis, myasthenia gravis, and vitiligo. It’s also common in Down syndrome. “Many patients with histological inflammation have atypical intestinal symptoms or none at all,” he said. “Clinical studies have shown that only 15%-20% of CD patients identified by serology and confirmed by biopsy have classical symptoms of diarrhea and malabsorption.” The presenting symptom in patients with celiac disease may be limited to only aphthous stomatitis, eczema, alopecia areata, psoriasis, or diabetes, along with fatigue or anemia.
Researchers who analyzed the prevalence of CD in the United States estimated the risk to be 1:133 among individuals deemed not to be at risk, 1:56 in symptomatic patients, 1:39 in second-degree relatives, and 1:22 in first-degree relatives (Arch Intern Med. 2003;163[3]:286-92.). “We tested 2,100 people in Utah and found the prevalence among first-degree relatives was 1:12,” Dr. Zone said. “The point is that CD is common, not rare. It’s as common as psoriasis. It profoundly affects the immune system, which is the modulator of inflammatory skin disease.”
Dr. Zone reported having no financial disclosures.
PARK CITY, UTAH – Dr. John J. Zone first began to study gluten sensitivity in 1977, an interest that left some of his clinician colleagues wondering why.
“Everybody told me I was crazy – that this was extremely rare. So I always say I was gluten when gluten wasn’t cool,” Dr. Zone, professor and chairman of dermatology at the University of Utah, Salt Lake City, told attendees at the annual meeting of the Pacific Dermatologic Association.
These days, it’s hard to shop in a food market without noticing all the gluten-free foods available, from pizza dough to beer. Many restaurants also serve gluten-free dishes. But is it hype, or is gluten sensitivity that common? Five percent of people in the United States “will say they are gluten sensitive,” he said. “In fact, 1% of Caucasians actually have celiac disease and 1% of Caucasians have gluten sensitivity that can be documented by challenge but don’t have celiac disease, while 3% have nothing.”
Gluten is a group of proteins contained in wheat, barley, and rye that is insoluble in water. Dr. Zone described celiac disease as a “spectrum of disease” characterized by inflammation of the small intestinal mucosa that occurs with the ingestion of gluten. The condition improves when gluten is removed from the diet. From a genetic standpoint, having a predisposition to express human leukocyte antigen-DQ2 or HLA-DQ8 is required for a diagnosis of celiac disease (CD). An estimated 20%-25% of whites “have that HLA background, but it is rare in Asians,” he said. “The receptors coded by HLA genes are essential for the processing of the gliadin antigen in CD.”
The hallmark for CD is a blood test for immunoglobulin A (IgA) anti-tissue transglutaminase antibodies, which are detectable in patients with untreated disease. “You should be able to get that test for $50 or $60 in any laboratory in the country,” Dr. Zone said. “It’s about 98% reliable. You also want to do a total serum IgA to rule out IgA-deficiency.”
CD clusters with other autoimmune disorders such as Addison’s disease, autoimmune thyroiditis, atrophic gastritis, systemic lupus erythematosus, rheumatoid arthritis, myasthenia gravis, and vitiligo. It’s also common in Down syndrome. “Many patients with histological inflammation have atypical intestinal symptoms or none at all,” he said. “Clinical studies have shown that only 15%-20% of CD patients identified by serology and confirmed by biopsy have classical symptoms of diarrhea and malabsorption.” The presenting symptom in patients with celiac disease may be limited to only aphthous stomatitis, eczema, alopecia areata, psoriasis, or diabetes, along with fatigue or anemia.
Researchers who analyzed the prevalence of CD in the United States estimated the risk to be 1:133 among individuals deemed not to be at risk, 1:56 in symptomatic patients, 1:39 in second-degree relatives, and 1:22 in first-degree relatives (Arch Intern Med. 2003;163[3]:286-92.). “We tested 2,100 people in Utah and found the prevalence among first-degree relatives was 1:12,” Dr. Zone said. “The point is that CD is common, not rare. It’s as common as psoriasis. It profoundly affects the immune system, which is the modulator of inflammatory skin disease.”
Dr. Zone reported having no financial disclosures.
EXPERT ANALYSIS FROM PDA 2015
AAP: Give peanut products to high-risk infants to cut allergy risk
Infants at high risk for peanut allergy should start a peanut-based diet by age 4-11 months, experts from the American Academy of Pediatrics and nine other medical groups advised in the September issue of Pediatrics.*
The consensus communication upends traditional views about preventing childhood peanut allergy and highlights the landmark LEAP study in which high-risk infants fed peanut-based foods had about an 80% lower risk of developing peanut allergy, compared with those fed a peanut-free diet.
“Early intervention will prevent peanut allergy, and the pediatrician’s involvement is absolutely essential to the success of this approach,” said Dr. Hugh Sampson, who contributed to the guidance and is at the Icahn School of Medicine at Mount Sinai, New York. “Without very early evaluation and implementation, we won’t change anything.”
LEAP investigators defined “high risk” for peanut allergy as severe eczema with or without egg allergy, “but many other infants are likely at risk, and thus would benefit from early peanut introduction,” added Dr. David Fleischer, who also contributed to the guidance and is at the University of Colorado at Denver, Aurora. “Many feel that given the potential benefit, all infants, regardless of risk level, should have peanut introduced early into the diet,” he said.
Peanut allergy affects more than 2% of American children and is about twice as prevalent in Western countries as it was a decade ago. It’s not clear why rates have increased, but pediatricians can help stem the rising tide, Dr. Sampson said. “When a pediatrician suspects a ‘high-risk’ baby, he or she needs to explain to parents the risks involved in their baby developing peanut allergy, and the benefits of early evaluation and introduction. Once peanut allergy is established, the vast majority of young children will retain the allergy for life.”
Because “high-risk” children might already be allergic to peanuts, they could benefit from evaluation by an allergist, according to the consensus communication (Pediatrics 2015;136[3]:601-4). Expert consultation might also benefit those who feel reluctant to introduce peanuts for other reasons, Dr. Fleischer said.
Dr. Sampson recommends peanut-based skin prick testing for high-risk infants aged 4-8 months. Patients with a negative result should receive 2 grams of peanut protein three times a week for the next 3 years. Those who are mildly sensitive (wheal diameter less than 4 mm) should undergo a peanut challenge observed by an experienced physician. Infants who do not react can start the peanut-based diet.
The LEAP study randomized 640 high-risk infants to either avoid peanuts or consume at least 6 grams per week of the allergen in foods such as smooth peanut butter mixed with mashed fruit, peanut soup, and ground peanuts in other foods. Five-year-olds in the peanut group had significantly lower rates of peanut allergy, regardless of whether their skin prick test had been positive at baseline (N. Engl. J. Med. 2015;372[9]:803-13). While the consensus communication provides interim guidance, a panel sponsored by the National Institute of Allergy and Infectious Diseases is reviewing food allergy data in preparation for updating its guidelines, Dr. Sampson noted. “Several major questions remain,” he said. “Do we need to give such large amounts of peanut to induce tolerance? Is it necessary to give this amount of peanut for such an extended period? What happens if parents don’t give peanut to their infants on a regular basis, as done in the LEAP trial? Could this put them at higher risk? And will this approach apply to other foods?”
Another key knowledge gap is whether the results from one single-center study can be applied elsewhere, said Dr. Fleischer. “We do not know the effects of early peanut introduction in other risk populations.”
Authors of the consensus communication reported no funding sources or conflicts of interest.
*Correction, 8/31/2015: An earlier version of this article misstated the journal in which the study was published.
Infants at high risk for peanut allergy should start a peanut-based diet by age 4-11 months, experts from the American Academy of Pediatrics and nine other medical groups advised in the September issue of Pediatrics.*
The consensus communication upends traditional views about preventing childhood peanut allergy and highlights the landmark LEAP study in which high-risk infants fed peanut-based foods had about an 80% lower risk of developing peanut allergy, compared with those fed a peanut-free diet.
“Early intervention will prevent peanut allergy, and the pediatrician’s involvement is absolutely essential to the success of this approach,” said Dr. Hugh Sampson, who contributed to the guidance and is at the Icahn School of Medicine at Mount Sinai, New York. “Without very early evaluation and implementation, we won’t change anything.”
LEAP investigators defined “high risk” for peanut allergy as severe eczema with or without egg allergy, “but many other infants are likely at risk, and thus would benefit from early peanut introduction,” added Dr. David Fleischer, who also contributed to the guidance and is at the University of Colorado at Denver, Aurora. “Many feel that given the potential benefit, all infants, regardless of risk level, should have peanut introduced early into the diet,” he said.
Peanut allergy affects more than 2% of American children and is about twice as prevalent in Western countries as it was a decade ago. It’s not clear why rates have increased, but pediatricians can help stem the rising tide, Dr. Sampson said. “When a pediatrician suspects a ‘high-risk’ baby, he or she needs to explain to parents the risks involved in their baby developing peanut allergy, and the benefits of early evaluation and introduction. Once peanut allergy is established, the vast majority of young children will retain the allergy for life.”
Because “high-risk” children might already be allergic to peanuts, they could benefit from evaluation by an allergist, according to the consensus communication (Pediatrics 2015;136[3]:601-4). Expert consultation might also benefit those who feel reluctant to introduce peanuts for other reasons, Dr. Fleischer said.
Dr. Sampson recommends peanut-based skin prick testing for high-risk infants aged 4-8 months. Patients with a negative result should receive 2 grams of peanut protein three times a week for the next 3 years. Those who are mildly sensitive (wheal diameter less than 4 mm) should undergo a peanut challenge observed by an experienced physician. Infants who do not react can start the peanut-based diet.
The LEAP study randomized 640 high-risk infants to either avoid peanuts or consume at least 6 grams per week of the allergen in foods such as smooth peanut butter mixed with mashed fruit, peanut soup, and ground peanuts in other foods. Five-year-olds in the peanut group had significantly lower rates of peanut allergy, regardless of whether their skin prick test had been positive at baseline (N. Engl. J. Med. 2015;372[9]:803-13). While the consensus communication provides interim guidance, a panel sponsored by the National Institute of Allergy and Infectious Diseases is reviewing food allergy data in preparation for updating its guidelines, Dr. Sampson noted. “Several major questions remain,” he said. “Do we need to give such large amounts of peanut to induce tolerance? Is it necessary to give this amount of peanut for such an extended period? What happens if parents don’t give peanut to their infants on a regular basis, as done in the LEAP trial? Could this put them at higher risk? And will this approach apply to other foods?”
Another key knowledge gap is whether the results from one single-center study can be applied elsewhere, said Dr. Fleischer. “We do not know the effects of early peanut introduction in other risk populations.”
Authors of the consensus communication reported no funding sources or conflicts of interest.
*Correction, 8/31/2015: An earlier version of this article misstated the journal in which the study was published.
Infants at high risk for peanut allergy should start a peanut-based diet by age 4-11 months, experts from the American Academy of Pediatrics and nine other medical groups advised in the September issue of Pediatrics.*
The consensus communication upends traditional views about preventing childhood peanut allergy and highlights the landmark LEAP study in which high-risk infants fed peanut-based foods had about an 80% lower risk of developing peanut allergy, compared with those fed a peanut-free diet.
“Early intervention will prevent peanut allergy, and the pediatrician’s involvement is absolutely essential to the success of this approach,” said Dr. Hugh Sampson, who contributed to the guidance and is at the Icahn School of Medicine at Mount Sinai, New York. “Without very early evaluation and implementation, we won’t change anything.”
LEAP investigators defined “high risk” for peanut allergy as severe eczema with or without egg allergy, “but many other infants are likely at risk, and thus would benefit from early peanut introduction,” added Dr. David Fleischer, who also contributed to the guidance and is at the University of Colorado at Denver, Aurora. “Many feel that given the potential benefit, all infants, regardless of risk level, should have peanut introduced early into the diet,” he said.
Peanut allergy affects more than 2% of American children and is about twice as prevalent in Western countries as it was a decade ago. It’s not clear why rates have increased, but pediatricians can help stem the rising tide, Dr. Sampson said. “When a pediatrician suspects a ‘high-risk’ baby, he or she needs to explain to parents the risks involved in their baby developing peanut allergy, and the benefits of early evaluation and introduction. Once peanut allergy is established, the vast majority of young children will retain the allergy for life.”
Because “high-risk” children might already be allergic to peanuts, they could benefit from evaluation by an allergist, according to the consensus communication (Pediatrics 2015;136[3]:601-4). Expert consultation might also benefit those who feel reluctant to introduce peanuts for other reasons, Dr. Fleischer said.
Dr. Sampson recommends peanut-based skin prick testing for high-risk infants aged 4-8 months. Patients with a negative result should receive 2 grams of peanut protein three times a week for the next 3 years. Those who are mildly sensitive (wheal diameter less than 4 mm) should undergo a peanut challenge observed by an experienced physician. Infants who do not react can start the peanut-based diet.
The LEAP study randomized 640 high-risk infants to either avoid peanuts or consume at least 6 grams per week of the allergen in foods such as smooth peanut butter mixed with mashed fruit, peanut soup, and ground peanuts in other foods. Five-year-olds in the peanut group had significantly lower rates of peanut allergy, regardless of whether their skin prick test had been positive at baseline (N. Engl. J. Med. 2015;372[9]:803-13). While the consensus communication provides interim guidance, a panel sponsored by the National Institute of Allergy and Infectious Diseases is reviewing food allergy data in preparation for updating its guidelines, Dr. Sampson noted. “Several major questions remain,” he said. “Do we need to give such large amounts of peanut to induce tolerance? Is it necessary to give this amount of peanut for such an extended period? What happens if parents don’t give peanut to their infants on a regular basis, as done in the LEAP trial? Could this put them at higher risk? And will this approach apply to other foods?”
Another key knowledge gap is whether the results from one single-center study can be applied elsewhere, said Dr. Fleischer. “We do not know the effects of early peanut introduction in other risk populations.”
Authors of the consensus communication reported no funding sources or conflicts of interest.
*Correction, 8/31/2015: An earlier version of this article misstated the journal in which the study was published.
FROM PEDIATRICS
New treatments explored for prurigo nodularis
NEW YORK – Once it reaches the plaque stage, prurigo nodularis becomes extremely difficult to control with currently available topical and systemic therapies, according to a comprehensive summary presented at the American Academy of Dermatology summer meeting.
When treatment begins at an advanced stage, “it takes years until we see a complete relief,” reported Dr. Sonja Stander of the Center for Chronic Pruritus, University Hospital Muenster (Germany). Conveying this information to the patient is “very essential” to develop realistic expectations and to enlist cooperation in the frequent treatment modifications needed to achieve maximum relief.
Prurigo nodularis is characterized by a symmetrical distribution of very itchy, hyperkeratotic, erosive nodules and papules. Scratching contributes to the “chronification” and the progression that typically takes patients from papules to nodules to plaques, according to Dr. Stander, an expert who has joined with others to create the Prurigo Nodularis League (PNL) to raise awareness about the condition.
Information on more than 600 prurigo nodularis patients has been collected in a database created by the PNL, which is designed to consolidate information on the epidemiology, pathology, and treatments for a condition that Dr. Stander said many patients describe as “agonizing” or “horrendous.” The condition has been linked to a variety of systemic and neurologic diseases, but the underlying mechanism of this condition remains poorly understood.
Until recently, commonly used first-line treatment strategies, which include PUVA, topical steroids, and the immunomodulatory agent pimecrolimus, were employed primarily on the basis of case series and expert opinion. She reported that only three randomized, controlled trials have been published relating to these approaches. All involved topical treatments.
“Do you think this is enough for treating the prurigo patient? Topical treatment? No way, but we do not have data from trials with systemic therapies,” reported Dr. Stander, who provided an algorithm that begins with topical therapies but quickly moves to second- and third-line systemic agents. Dr. Stander noted, however, that independent phase 2 trials were recently initiated with the NK1 receptor antagonists aprepitant and serlopitant, providing hope that there are “some new drugs on the horizon.”
According to Dr. Stander, European guidelines for prurigo nodularis recommend topical capsaicin and naltrexone for initial therapy. Both are used off label, but she believes both produce benefit. Other therapies supported by case studies include the neuromodulator gabapentin, the immunomodulator methotrexate, and selective serotonin receptor inhibitors. The side effects of most of these therapies, particularly drugs such as methotrexate, typically precede the benefits, according to Dr. Stander, who reiterated that patients must be warned about the slow pace of improvement.
It is hoped that the PNL database will provide enough patients to permit patterns in disease etiology to be discerned. One of the questions is where atopy, which Dr. Stander said is a prominent feature in about 50% of prurigo nodularis patients, plays a role. She explored the theory that this prurigo nodularis represents a neuropathy of the skin, citing evidence of differences in nerve fiber density in the dermal and epidermal layers of these patients when compared with individuals without this condition.
The differential diagnosis includes autoimmune diseases, such as bullous pemphigoid. In fact, Dr. Stander reported that she screens for bullous pemphigoid in every patient suspected of prurigo nodularis.
One of the peculiar features of prurigo nodularis is that there appears to be no correlation between the duration of this condition and its intensity. Rather, most patients “start with a very severe pruritus” with skin lesions exacerbated by scratching, according to Dr. Stander. She said that although it was once thought that a plaque presentation might represent a different subtype, it is now believed that this is simply an advanced stage.
Encouraging physicians who encounter prurigo nodularis to contribute cases to the database, Dr. Stander reported that “we want to raise awareness for this severe entity” through the PNL. She said the PNL website is a repository for information and invites comments to allow information to be exchanged.
Dr. Stander reported no financial relationships to disclose.
NEW YORK – Once it reaches the plaque stage, prurigo nodularis becomes extremely difficult to control with currently available topical and systemic therapies, according to a comprehensive summary presented at the American Academy of Dermatology summer meeting.
When treatment begins at an advanced stage, “it takes years until we see a complete relief,” reported Dr. Sonja Stander of the Center for Chronic Pruritus, University Hospital Muenster (Germany). Conveying this information to the patient is “very essential” to develop realistic expectations and to enlist cooperation in the frequent treatment modifications needed to achieve maximum relief.
Prurigo nodularis is characterized by a symmetrical distribution of very itchy, hyperkeratotic, erosive nodules and papules. Scratching contributes to the “chronification” and the progression that typically takes patients from papules to nodules to plaques, according to Dr. Stander, an expert who has joined with others to create the Prurigo Nodularis League (PNL) to raise awareness about the condition.
Information on more than 600 prurigo nodularis patients has been collected in a database created by the PNL, which is designed to consolidate information on the epidemiology, pathology, and treatments for a condition that Dr. Stander said many patients describe as “agonizing” or “horrendous.” The condition has been linked to a variety of systemic and neurologic diseases, but the underlying mechanism of this condition remains poorly understood.
Until recently, commonly used first-line treatment strategies, which include PUVA, topical steroids, and the immunomodulatory agent pimecrolimus, were employed primarily on the basis of case series and expert opinion. She reported that only three randomized, controlled trials have been published relating to these approaches. All involved topical treatments.
“Do you think this is enough for treating the prurigo patient? Topical treatment? No way, but we do not have data from trials with systemic therapies,” reported Dr. Stander, who provided an algorithm that begins with topical therapies but quickly moves to second- and third-line systemic agents. Dr. Stander noted, however, that independent phase 2 trials were recently initiated with the NK1 receptor antagonists aprepitant and serlopitant, providing hope that there are “some new drugs on the horizon.”
According to Dr. Stander, European guidelines for prurigo nodularis recommend topical capsaicin and naltrexone for initial therapy. Both are used off label, but she believes both produce benefit. Other therapies supported by case studies include the neuromodulator gabapentin, the immunomodulator methotrexate, and selective serotonin receptor inhibitors. The side effects of most of these therapies, particularly drugs such as methotrexate, typically precede the benefits, according to Dr. Stander, who reiterated that patients must be warned about the slow pace of improvement.
It is hoped that the PNL database will provide enough patients to permit patterns in disease etiology to be discerned. One of the questions is where atopy, which Dr. Stander said is a prominent feature in about 50% of prurigo nodularis patients, plays a role. She explored the theory that this prurigo nodularis represents a neuropathy of the skin, citing evidence of differences in nerve fiber density in the dermal and epidermal layers of these patients when compared with individuals without this condition.
The differential diagnosis includes autoimmune diseases, such as bullous pemphigoid. In fact, Dr. Stander reported that she screens for bullous pemphigoid in every patient suspected of prurigo nodularis.
One of the peculiar features of prurigo nodularis is that there appears to be no correlation between the duration of this condition and its intensity. Rather, most patients “start with a very severe pruritus” with skin lesions exacerbated by scratching, according to Dr. Stander. She said that although it was once thought that a plaque presentation might represent a different subtype, it is now believed that this is simply an advanced stage.
Encouraging physicians who encounter prurigo nodularis to contribute cases to the database, Dr. Stander reported that “we want to raise awareness for this severe entity” through the PNL. She said the PNL website is a repository for information and invites comments to allow information to be exchanged.
Dr. Stander reported no financial relationships to disclose.
NEW YORK – Once it reaches the plaque stage, prurigo nodularis becomes extremely difficult to control with currently available topical and systemic therapies, according to a comprehensive summary presented at the American Academy of Dermatology summer meeting.
When treatment begins at an advanced stage, “it takes years until we see a complete relief,” reported Dr. Sonja Stander of the Center for Chronic Pruritus, University Hospital Muenster (Germany). Conveying this information to the patient is “very essential” to develop realistic expectations and to enlist cooperation in the frequent treatment modifications needed to achieve maximum relief.
Prurigo nodularis is characterized by a symmetrical distribution of very itchy, hyperkeratotic, erosive nodules and papules. Scratching contributes to the “chronification” and the progression that typically takes patients from papules to nodules to plaques, according to Dr. Stander, an expert who has joined with others to create the Prurigo Nodularis League (PNL) to raise awareness about the condition.
Information on more than 600 prurigo nodularis patients has been collected in a database created by the PNL, which is designed to consolidate information on the epidemiology, pathology, and treatments for a condition that Dr. Stander said many patients describe as “agonizing” or “horrendous.” The condition has been linked to a variety of systemic and neurologic diseases, but the underlying mechanism of this condition remains poorly understood.
Until recently, commonly used first-line treatment strategies, which include PUVA, topical steroids, and the immunomodulatory agent pimecrolimus, were employed primarily on the basis of case series and expert opinion. She reported that only three randomized, controlled trials have been published relating to these approaches. All involved topical treatments.
“Do you think this is enough for treating the prurigo patient? Topical treatment? No way, but we do not have data from trials with systemic therapies,” reported Dr. Stander, who provided an algorithm that begins with topical therapies but quickly moves to second- and third-line systemic agents. Dr. Stander noted, however, that independent phase 2 trials were recently initiated with the NK1 receptor antagonists aprepitant and serlopitant, providing hope that there are “some new drugs on the horizon.”
According to Dr. Stander, European guidelines for prurigo nodularis recommend topical capsaicin and naltrexone for initial therapy. Both are used off label, but she believes both produce benefit. Other therapies supported by case studies include the neuromodulator gabapentin, the immunomodulator methotrexate, and selective serotonin receptor inhibitors. The side effects of most of these therapies, particularly drugs such as methotrexate, typically precede the benefits, according to Dr. Stander, who reiterated that patients must be warned about the slow pace of improvement.
It is hoped that the PNL database will provide enough patients to permit patterns in disease etiology to be discerned. One of the questions is where atopy, which Dr. Stander said is a prominent feature in about 50% of prurigo nodularis patients, plays a role. She explored the theory that this prurigo nodularis represents a neuropathy of the skin, citing evidence of differences in nerve fiber density in the dermal and epidermal layers of these patients when compared with individuals without this condition.
The differential diagnosis includes autoimmune diseases, such as bullous pemphigoid. In fact, Dr. Stander reported that she screens for bullous pemphigoid in every patient suspected of prurigo nodularis.
One of the peculiar features of prurigo nodularis is that there appears to be no correlation between the duration of this condition and its intensity. Rather, most patients “start with a very severe pruritus” with skin lesions exacerbated by scratching, according to Dr. Stander. She said that although it was once thought that a plaque presentation might represent a different subtype, it is now believed that this is simply an advanced stage.
Encouraging physicians who encounter prurigo nodularis to contribute cases to the database, Dr. Stander reported that “we want to raise awareness for this severe entity” through the PNL. She said the PNL website is a repository for information and invites comments to allow information to be exchanged.
Dr. Stander reported no financial relationships to disclose.
AT THE AAD SUMMER ACADEMY 2015
Pruritus in the elderly often linked to atopic-like dermatosis
PARK CITY, UTAH – When an otherwise healthy 75-year-old patient presents with persistent pruritus as the chief complaint, the first thing to do is rule out specific dermatologic disorders, according to Dr. Kevin C. Wang.
“Thankfully, most of the patients complaining of pruritus have visible dermatoses,” Dr. Wang said at the annual meeting of the Pacific Dermatologic Association. “According to a review of more than 150 elderly patients in the outpatient setting who presented with a chief complaint of persistent pruritus, the five most common diagnoses were atopic-like dermatosis, lichen simplex chronicus/prurigo nodularis, subacute prurigo, transient acantholytic dermatosis, and neuropathic disease.”
Treatment directed at the primary element triggering the pruritus is most effective. “The best treatment for these patients will likely involve multiple modalities/combination therapy, as there is no one major pathway pathophysiologically,” said Dr. Wang of the department of dermatology at Stanford (Calif.) University and the Palo Alto VA Hospital.
“Pruritus can be quite debilitating,” said Dr. Wang, who also is the principal investigator of a research lab at Stanford. “I have not met an itchy patient who has said that it has not ruined their lives somehow: whether it’s their work, social life, things that they like to do. Also, many elderly veterans are already quite debilitated functionally in the first place, so it is a huge problem.”
An estimated 2% of all dermatology visits are for pruritus, “but it’s probably more than that, because a lot of those complaints don’t come up until you ask the patient in person,” he said. “This issue is also important because as physicians we really don’t have any specific ‘itch blockers.’ We just use drugs developed for other conditions that happen to work, but not often enough.”
Dr. Wang then went on to share some of the concepts raised in a 2011 article by Dr. Timothy G. Berger and Dr. Martin Steinhoff of the University of California, San Francisco. They suggested that pruritic conditions afflicting the elderly are the results of a variety of age-related changes they termed “eruptions of senescence” (Semin Cutan Med Surg. 2011;30[2]:113-7).
As people age, Dr. Wang said, the immune system “becomes much more proinflammatory, including significant aberration of T- and B-cell populations. More importantly, the immune system develops an allergic Th2 phenotype, where you have loss of naive T cells as the immune repertoire becomes populated with ‘committed’ T and B cells, and a preponderance of Th2 cells. This means you have an impaired ability to respond to new antigens, with a greater propensity for autoimmune responses, and lingering, low-grade inflammation.”
Aging also brings structural changes to the epidermal barrier, he continued. Specifically, the surface pH becomes less acidic. This is problematic because enzymes that are required to process lipids function best at acidic pH. “You also have a reduction in the rate of barrier repair, and decreased production of filaggrin and aquaporin-3,” he said. “In combination, this impaired barrier has two direct consequences: Barrier failure may lead to increased development of contact dermatitis, because the impaired barrier may not prevent penetration of potential antigens into the epidermis, and when the barrier fails, the cytokines released to induce barrier repair are proinflammatory, resulting in dermatitis.”
He reported having no financial disclosures.
PARK CITY, UTAH – When an otherwise healthy 75-year-old patient presents with persistent pruritus as the chief complaint, the first thing to do is rule out specific dermatologic disorders, according to Dr. Kevin C. Wang.
“Thankfully, most of the patients complaining of pruritus have visible dermatoses,” Dr. Wang said at the annual meeting of the Pacific Dermatologic Association. “According to a review of more than 150 elderly patients in the outpatient setting who presented with a chief complaint of persistent pruritus, the five most common diagnoses were atopic-like dermatosis, lichen simplex chronicus/prurigo nodularis, subacute prurigo, transient acantholytic dermatosis, and neuropathic disease.”
Treatment directed at the primary element triggering the pruritus is most effective. “The best treatment for these patients will likely involve multiple modalities/combination therapy, as there is no one major pathway pathophysiologically,” said Dr. Wang of the department of dermatology at Stanford (Calif.) University and the Palo Alto VA Hospital.
“Pruritus can be quite debilitating,” said Dr. Wang, who also is the principal investigator of a research lab at Stanford. “I have not met an itchy patient who has said that it has not ruined their lives somehow: whether it’s their work, social life, things that they like to do. Also, many elderly veterans are already quite debilitated functionally in the first place, so it is a huge problem.”
An estimated 2% of all dermatology visits are for pruritus, “but it’s probably more than that, because a lot of those complaints don’t come up until you ask the patient in person,” he said. “This issue is also important because as physicians we really don’t have any specific ‘itch blockers.’ We just use drugs developed for other conditions that happen to work, but not often enough.”
Dr. Wang then went on to share some of the concepts raised in a 2011 article by Dr. Timothy G. Berger and Dr. Martin Steinhoff of the University of California, San Francisco. They suggested that pruritic conditions afflicting the elderly are the results of a variety of age-related changes they termed “eruptions of senescence” (Semin Cutan Med Surg. 2011;30[2]:113-7).
As people age, Dr. Wang said, the immune system “becomes much more proinflammatory, including significant aberration of T- and B-cell populations. More importantly, the immune system develops an allergic Th2 phenotype, where you have loss of naive T cells as the immune repertoire becomes populated with ‘committed’ T and B cells, and a preponderance of Th2 cells. This means you have an impaired ability to respond to new antigens, with a greater propensity for autoimmune responses, and lingering, low-grade inflammation.”
Aging also brings structural changes to the epidermal barrier, he continued. Specifically, the surface pH becomes less acidic. This is problematic because enzymes that are required to process lipids function best at acidic pH. “You also have a reduction in the rate of barrier repair, and decreased production of filaggrin and aquaporin-3,” he said. “In combination, this impaired barrier has two direct consequences: Barrier failure may lead to increased development of contact dermatitis, because the impaired barrier may not prevent penetration of potential antigens into the epidermis, and when the barrier fails, the cytokines released to induce barrier repair are proinflammatory, resulting in dermatitis.”
He reported having no financial disclosures.
PARK CITY, UTAH – When an otherwise healthy 75-year-old patient presents with persistent pruritus as the chief complaint, the first thing to do is rule out specific dermatologic disorders, according to Dr. Kevin C. Wang.
“Thankfully, most of the patients complaining of pruritus have visible dermatoses,” Dr. Wang said at the annual meeting of the Pacific Dermatologic Association. “According to a review of more than 150 elderly patients in the outpatient setting who presented with a chief complaint of persistent pruritus, the five most common diagnoses were atopic-like dermatosis, lichen simplex chronicus/prurigo nodularis, subacute prurigo, transient acantholytic dermatosis, and neuropathic disease.”
Treatment directed at the primary element triggering the pruritus is most effective. “The best treatment for these patients will likely involve multiple modalities/combination therapy, as there is no one major pathway pathophysiologically,” said Dr. Wang of the department of dermatology at Stanford (Calif.) University and the Palo Alto VA Hospital.
“Pruritus can be quite debilitating,” said Dr. Wang, who also is the principal investigator of a research lab at Stanford. “I have not met an itchy patient who has said that it has not ruined their lives somehow: whether it’s their work, social life, things that they like to do. Also, many elderly veterans are already quite debilitated functionally in the first place, so it is a huge problem.”
An estimated 2% of all dermatology visits are for pruritus, “but it’s probably more than that, because a lot of those complaints don’t come up until you ask the patient in person,” he said. “This issue is also important because as physicians we really don’t have any specific ‘itch blockers.’ We just use drugs developed for other conditions that happen to work, but not often enough.”
Dr. Wang then went on to share some of the concepts raised in a 2011 article by Dr. Timothy G. Berger and Dr. Martin Steinhoff of the University of California, San Francisco. They suggested that pruritic conditions afflicting the elderly are the results of a variety of age-related changes they termed “eruptions of senescence” (Semin Cutan Med Surg. 2011;30[2]:113-7).
As people age, Dr. Wang said, the immune system “becomes much more proinflammatory, including significant aberration of T- and B-cell populations. More importantly, the immune system develops an allergic Th2 phenotype, where you have loss of naive T cells as the immune repertoire becomes populated with ‘committed’ T and B cells, and a preponderance of Th2 cells. This means you have an impaired ability to respond to new antigens, with a greater propensity for autoimmune responses, and lingering, low-grade inflammation.”
Aging also brings structural changes to the epidermal barrier, he continued. Specifically, the surface pH becomes less acidic. This is problematic because enzymes that are required to process lipids function best at acidic pH. “You also have a reduction in the rate of barrier repair, and decreased production of filaggrin and aquaporin-3,” he said. “In combination, this impaired barrier has two direct consequences: Barrier failure may lead to increased development of contact dermatitis, because the impaired barrier may not prevent penetration of potential antigens into the epidermis, and when the barrier fails, the cytokines released to induce barrier repair are proinflammatory, resulting in dermatitis.”
He reported having no financial disclosures.
EXPERT ANALYSIS FROM PDA 2015
Beware of skin complications of newer antidiabetic agents
VANCOUVER, B.C. – Each of the newer classes of antidiabetic medications – the DPP-IV inhibitors, GLP-1 agonists, and SGLT2 inhibitors – has its own characteristic cutaneous side effects, Dr. Justin Endo said at the World Congress of Dermatology.
These drugs aren’t first-line agents for patients with type 2 diabetes, but they have gained solid standing as second-tier medications for the vast number of patients with inadequate glucose control or limiting side effects on first-line therapies, said Dr. Endo of the department of dermatology at the University of Wisconsin–Madison.
DPP-IV inhibitors
The dipeptidyl peptidase–IV inhibitors are the newer drugs linked to the most potentially serious side effects, namely, drug-induced bullous pemphigoid and angioedema.
Investigators at Western Michigan University, Kalamazoo, conducted a literature review and an analysis of the Food and Drug Administration’s Adverse Event Reporting System database and concluded there is a link between DPP-IV inhibitors and the development of bullous pemphigoid, a potentially fatal cutaneous autoimmune blistering disorder. The skin disease appeared after an average of 6 months on DPP-IV inhibitor therapy in their series. In most cases the bullous pemphigoid remitted in response to discontinuation of the drug, often in conjunction with a course of topical or less frequently, oral corticosteroid therapy (J Dermatol Case Rep. 2014 Mar 31;8[1]:24-8).
The mechanism for the development of bullous pemphigoid in patients on a DPP-IV inhibitor is unknown; however, the enzyme DPP-IV is expressed in skin and most other organs.
“I think a key take home is if patients in their 60s or 70s come in with pemphigoid, even though they’re in the usual age group for non–drug-induced pemphigoid, ask whether they are taking a DPP-IV inhibitor. And if they are, get it stopped,” Dr. Endo said.
An initial postmarketing flurry of case reports of angioedema in patients on the then-new DPP-IV inhibitors caused enormous concern until clarification was provided in a study by investigators at Vanderbilt University, Nashville, Tenn., and Novartis. They dove deep into the phase III randomized clinical trial data for one of these agents, vildagliptin (Galvus, manufactured by Novartis), and concluded there was no association between the drug and angioedema, except in patients simultaneously taking an ACE inhibitor, a known risk factor for angioedema. It seems that the dual therapy may cause a double hit, multiplying risk.
The bad news was that patients on an ACE inhibitor and vildagliptin had a 4.57-fold greater risk of angioedema than did those on a non–DPP-IV inhibitor comparator medication. The good news was that the absolute risk was very low: 14 confirmed cases in 2,754 subjects. And most cases of angioedema were of the non–life-threatening variety (Hypertension. 2009 Sep;54[3]:516-23).
“The evidence is really limited, but I have to agree with one expert who said, ‘Hey, if somebody comes into the hospital with angioedema, and they happen to be on a DPP-IV inhibitor and an ACE inhibitor, stop both.’ Whether or not patients can be safely rechallenged with a DPP-IV inhibitor isn’t yet known,” Dr. Endo said.
Another cutaneous side effect associated with DPP-IV inhibitor therapy is peripheral edema. It occurs in 1%-2% of treated patients and could contribute to the refractory nature of some diabetic foot ulcers, according to the dermatologist. Documented cases of photosensitivity, urticaria, and eosinophilic drug rashes have also been reported.
The DPP-IV inhibitors are oral drugs. Those approved in the United States are alogliptin (Nesina), linagliptin (Trajenta), saxagliptin (Onglyza), and sitagliptin (Januvia).
GLP-1 agonists
The chief cutaneous side effects of the glucagonlike peptide–1 receptor agonists are injection site reactions, reported in up to 20% of treated patients in some studies. These reactions are typically mild, itchy, erythematous, and transient.
The GLP-1 agonists are administered subcutaneously once or twice weekly. Those approved in the United States are albiglutide (Tanzeum), dulaglutide (Trulicity), exenatide (Byetta, Bydureon), and liraglutide (Saxenda, Victoza).
SGLT2 inhibitors
The sodium-glucose cotransporter–2 inhibitors are the newest of these three antidiabetic drug classes. They are oral agents. Their most common cutaneous side effect is candidiasis, occurring in about 10% of patients. Women and uncircumcized men are at greatest risk. Fortunately, most cases are mild, episodic, and successfully treated with topical or oral antimycotic therapy, Dr. Endo said.
The SGLT2 inhibitors approved in the United States are canagliflozin (Invokana), dapagliflozin (Farxiga), and enpagliflozin (Jardiance).
Dr. Endo reported having no relevant financial conflicts.
VANCOUVER, B.C. – Each of the newer classes of antidiabetic medications – the DPP-IV inhibitors, GLP-1 agonists, and SGLT2 inhibitors – has its own characteristic cutaneous side effects, Dr. Justin Endo said at the World Congress of Dermatology.
These drugs aren’t first-line agents for patients with type 2 diabetes, but they have gained solid standing as second-tier medications for the vast number of patients with inadequate glucose control or limiting side effects on first-line therapies, said Dr. Endo of the department of dermatology at the University of Wisconsin–Madison.
DPP-IV inhibitors
The dipeptidyl peptidase–IV inhibitors are the newer drugs linked to the most potentially serious side effects, namely, drug-induced bullous pemphigoid and angioedema.
Investigators at Western Michigan University, Kalamazoo, conducted a literature review and an analysis of the Food and Drug Administration’s Adverse Event Reporting System database and concluded there is a link between DPP-IV inhibitors and the development of bullous pemphigoid, a potentially fatal cutaneous autoimmune blistering disorder. The skin disease appeared after an average of 6 months on DPP-IV inhibitor therapy in their series. In most cases the bullous pemphigoid remitted in response to discontinuation of the drug, often in conjunction with a course of topical or less frequently, oral corticosteroid therapy (J Dermatol Case Rep. 2014 Mar 31;8[1]:24-8).
The mechanism for the development of bullous pemphigoid in patients on a DPP-IV inhibitor is unknown; however, the enzyme DPP-IV is expressed in skin and most other organs.
“I think a key take home is if patients in their 60s or 70s come in with pemphigoid, even though they’re in the usual age group for non–drug-induced pemphigoid, ask whether they are taking a DPP-IV inhibitor. And if they are, get it stopped,” Dr. Endo said.
An initial postmarketing flurry of case reports of angioedema in patients on the then-new DPP-IV inhibitors caused enormous concern until clarification was provided in a study by investigators at Vanderbilt University, Nashville, Tenn., and Novartis. They dove deep into the phase III randomized clinical trial data for one of these agents, vildagliptin (Galvus, manufactured by Novartis), and concluded there was no association between the drug and angioedema, except in patients simultaneously taking an ACE inhibitor, a known risk factor for angioedema. It seems that the dual therapy may cause a double hit, multiplying risk.
The bad news was that patients on an ACE inhibitor and vildagliptin had a 4.57-fold greater risk of angioedema than did those on a non–DPP-IV inhibitor comparator medication. The good news was that the absolute risk was very low: 14 confirmed cases in 2,754 subjects. And most cases of angioedema were of the non–life-threatening variety (Hypertension. 2009 Sep;54[3]:516-23).
“The evidence is really limited, but I have to agree with one expert who said, ‘Hey, if somebody comes into the hospital with angioedema, and they happen to be on a DPP-IV inhibitor and an ACE inhibitor, stop both.’ Whether or not patients can be safely rechallenged with a DPP-IV inhibitor isn’t yet known,” Dr. Endo said.
Another cutaneous side effect associated with DPP-IV inhibitor therapy is peripheral edema. It occurs in 1%-2% of treated patients and could contribute to the refractory nature of some diabetic foot ulcers, according to the dermatologist. Documented cases of photosensitivity, urticaria, and eosinophilic drug rashes have also been reported.
The DPP-IV inhibitors are oral drugs. Those approved in the United States are alogliptin (Nesina), linagliptin (Trajenta), saxagliptin (Onglyza), and sitagliptin (Januvia).
GLP-1 agonists
The chief cutaneous side effects of the glucagonlike peptide–1 receptor agonists are injection site reactions, reported in up to 20% of treated patients in some studies. These reactions are typically mild, itchy, erythematous, and transient.
The GLP-1 agonists are administered subcutaneously once or twice weekly. Those approved in the United States are albiglutide (Tanzeum), dulaglutide (Trulicity), exenatide (Byetta, Bydureon), and liraglutide (Saxenda, Victoza).
SGLT2 inhibitors
The sodium-glucose cotransporter–2 inhibitors are the newest of these three antidiabetic drug classes. They are oral agents. Their most common cutaneous side effect is candidiasis, occurring in about 10% of patients. Women and uncircumcized men are at greatest risk. Fortunately, most cases are mild, episodic, and successfully treated with topical or oral antimycotic therapy, Dr. Endo said.
The SGLT2 inhibitors approved in the United States are canagliflozin (Invokana), dapagliflozin (Farxiga), and enpagliflozin (Jardiance).
Dr. Endo reported having no relevant financial conflicts.
VANCOUVER, B.C. – Each of the newer classes of antidiabetic medications – the DPP-IV inhibitors, GLP-1 agonists, and SGLT2 inhibitors – has its own characteristic cutaneous side effects, Dr. Justin Endo said at the World Congress of Dermatology.
These drugs aren’t first-line agents for patients with type 2 diabetes, but they have gained solid standing as second-tier medications for the vast number of patients with inadequate glucose control or limiting side effects on first-line therapies, said Dr. Endo of the department of dermatology at the University of Wisconsin–Madison.
DPP-IV inhibitors
The dipeptidyl peptidase–IV inhibitors are the newer drugs linked to the most potentially serious side effects, namely, drug-induced bullous pemphigoid and angioedema.
Investigators at Western Michigan University, Kalamazoo, conducted a literature review and an analysis of the Food and Drug Administration’s Adverse Event Reporting System database and concluded there is a link between DPP-IV inhibitors and the development of bullous pemphigoid, a potentially fatal cutaneous autoimmune blistering disorder. The skin disease appeared after an average of 6 months on DPP-IV inhibitor therapy in their series. In most cases the bullous pemphigoid remitted in response to discontinuation of the drug, often in conjunction with a course of topical or less frequently, oral corticosteroid therapy (J Dermatol Case Rep. 2014 Mar 31;8[1]:24-8).
The mechanism for the development of bullous pemphigoid in patients on a DPP-IV inhibitor is unknown; however, the enzyme DPP-IV is expressed in skin and most other organs.
“I think a key take home is if patients in their 60s or 70s come in with pemphigoid, even though they’re in the usual age group for non–drug-induced pemphigoid, ask whether they are taking a DPP-IV inhibitor. And if they are, get it stopped,” Dr. Endo said.
An initial postmarketing flurry of case reports of angioedema in patients on the then-new DPP-IV inhibitors caused enormous concern until clarification was provided in a study by investigators at Vanderbilt University, Nashville, Tenn., and Novartis. They dove deep into the phase III randomized clinical trial data for one of these agents, vildagliptin (Galvus, manufactured by Novartis), and concluded there was no association between the drug and angioedema, except in patients simultaneously taking an ACE inhibitor, a known risk factor for angioedema. It seems that the dual therapy may cause a double hit, multiplying risk.
The bad news was that patients on an ACE inhibitor and vildagliptin had a 4.57-fold greater risk of angioedema than did those on a non–DPP-IV inhibitor comparator medication. The good news was that the absolute risk was very low: 14 confirmed cases in 2,754 subjects. And most cases of angioedema were of the non–life-threatening variety (Hypertension. 2009 Sep;54[3]:516-23).
“The evidence is really limited, but I have to agree with one expert who said, ‘Hey, if somebody comes into the hospital with angioedema, and they happen to be on a DPP-IV inhibitor and an ACE inhibitor, stop both.’ Whether or not patients can be safely rechallenged with a DPP-IV inhibitor isn’t yet known,” Dr. Endo said.
Another cutaneous side effect associated with DPP-IV inhibitor therapy is peripheral edema. It occurs in 1%-2% of treated patients and could contribute to the refractory nature of some diabetic foot ulcers, according to the dermatologist. Documented cases of photosensitivity, urticaria, and eosinophilic drug rashes have also been reported.
The DPP-IV inhibitors are oral drugs. Those approved in the United States are alogliptin (Nesina), linagliptin (Trajenta), saxagliptin (Onglyza), and sitagliptin (Januvia).
GLP-1 agonists
The chief cutaneous side effects of the glucagonlike peptide–1 receptor agonists are injection site reactions, reported in up to 20% of treated patients in some studies. These reactions are typically mild, itchy, erythematous, and transient.
The GLP-1 agonists are administered subcutaneously once or twice weekly. Those approved in the United States are albiglutide (Tanzeum), dulaglutide (Trulicity), exenatide (Byetta, Bydureon), and liraglutide (Saxenda, Victoza).
SGLT2 inhibitors
The sodium-glucose cotransporter–2 inhibitors are the newest of these three antidiabetic drug classes. They are oral agents. Their most common cutaneous side effect is candidiasis, occurring in about 10% of patients. Women and uncircumcized men are at greatest risk. Fortunately, most cases are mild, episodic, and successfully treated with topical or oral antimycotic therapy, Dr. Endo said.
The SGLT2 inhibitors approved in the United States are canagliflozin (Invokana), dapagliflozin (Farxiga), and enpagliflozin (Jardiance).
Dr. Endo reported having no relevant financial conflicts.
EXPERT ANALYSIS FROM WCD 2015
New potential atopic dermatitis biomarkers found
Several new biomarkers for atopic dermatitis have been identified that may serve as targets for future drugs, according to a research letter from Angelo Landriscina at Albert Einstein College of Medicine, New York, and his associates.
In a study of 40 biopsy specimens from patients with atopic dermatitis (AD) and 40 matched controls from banked abdominoplasty tissue, interleukin-2 was found to produce pruritus in all study subjects, but this process was accelerated in AD patients. The pruritigens in the arachidonic acid pathways, leukotriene B4 and 5-lipoxygenase, also were increased in AD patients. In addition, the investigators found increased matrix metalloproteinase (MMP)-7, a product of mast cell degradation. In AD, mast cells localize in the epidermis and are stimulated, releasing MMP-7. Finally, alpha-2 macroglobulin was significantly elevated in AD skin.
“Interestingly, all mediators investigated are inhibited by hypochlorous acid, formed when sodium hypochlorite (bleach) mixes with water at pH 5-7,” the investigators noted.
Find the full research letter in Journal of the American Academy of Dermatology (doi:10.1016/j.jaad.2015.06.036).
Several new biomarkers for atopic dermatitis have been identified that may serve as targets for future drugs, according to a research letter from Angelo Landriscina at Albert Einstein College of Medicine, New York, and his associates.
In a study of 40 biopsy specimens from patients with atopic dermatitis (AD) and 40 matched controls from banked abdominoplasty tissue, interleukin-2 was found to produce pruritus in all study subjects, but this process was accelerated in AD patients. The pruritigens in the arachidonic acid pathways, leukotriene B4 and 5-lipoxygenase, also were increased in AD patients. In addition, the investigators found increased matrix metalloproteinase (MMP)-7, a product of mast cell degradation. In AD, mast cells localize in the epidermis and are stimulated, releasing MMP-7. Finally, alpha-2 macroglobulin was significantly elevated in AD skin.
“Interestingly, all mediators investigated are inhibited by hypochlorous acid, formed when sodium hypochlorite (bleach) mixes with water at pH 5-7,” the investigators noted.
Find the full research letter in Journal of the American Academy of Dermatology (doi:10.1016/j.jaad.2015.06.036).
Several new biomarkers for atopic dermatitis have been identified that may serve as targets for future drugs, according to a research letter from Angelo Landriscina at Albert Einstein College of Medicine, New York, and his associates.
In a study of 40 biopsy specimens from patients with atopic dermatitis (AD) and 40 matched controls from banked abdominoplasty tissue, interleukin-2 was found to produce pruritus in all study subjects, but this process was accelerated in AD patients. The pruritigens in the arachidonic acid pathways, leukotriene B4 and 5-lipoxygenase, also were increased in AD patients. In addition, the investigators found increased matrix metalloproteinase (MMP)-7, a product of mast cell degradation. In AD, mast cells localize in the epidermis and are stimulated, releasing MMP-7. Finally, alpha-2 macroglobulin was significantly elevated in AD skin.
“Interestingly, all mediators investigated are inhibited by hypochlorous acid, formed when sodium hypochlorite (bleach) mixes with water at pH 5-7,” the investigators noted.
Find the full research letter in Journal of the American Academy of Dermatology (doi:10.1016/j.jaad.2015.06.036).
Naringenin
The flavanone naringenin (5,7,4-trihydroxyflavanone) is known to exhibit anticarcinogenic, antioxidative, antiatherogenic, estrogenic, and immunomodulatory activity (Nutr. Cancer. 2012;64:714-24; J. Nutr. 2001;131:235-41; Life Sci. 2013;93:516-24). Naringenin can be found in high concentrations in grapefruits, oranges, and other citrus fruits as well as tomatoes (skin), with grapefruit juice found to yield much higher levels in plasma than orange juice (J. Nutr. 2001;131:235-41; Am. J. Physiol. Gastrointest. Liver Physiol. 2000;279:G1148-54; Nutr. Cancer 2012;64:714-24). Naringenin has been shown, along with other flavanones such as hesperetin and ponciretin, to strongly inhibit IgE-induced beta-hexosaminidase release from RBL-2H3 cells. Sung-Hwan Park and associates have suggested that the glycosides of these substances have potential as agents for treating IgE-induced atopic allergies (Planta Med. 2005;71:24-7).
In 2012, Kushi Anand and associates also showed that the combination of curcumin and naringenin exerted antiangiogenic and antitumor effects in Swiss albino mice, adding that neither compound has been associated with reports of toxicity in animals or humans (Nutr. Cancer 2012;64:714-24).
Potential cutaneous benefits
Tae-Ho Kim and colleagues studied the effects of naringenin on 2,4-dinitrofluorobenzene (DNFB)-induced atomic dermatitis in NC/Nga mice in 2013. After repetitive skin contact with DNFB, mice received intraperitoneal injections of naringenin for 1 week, with the treatment with the fruit flavonoid significantly diminishing ear swelling and back skin lesions. The flavonoid also significantly inhibited interferon (IFN)-alpha production by activated CD4+ T cells and lowered serum IgE levels as well as DNFB-induced infiltration of eosinophils, mast cells, CD4+ T cells, and CD8+ T cells in skin lesions (Life Sci. 2013;93:516-24).
Also that year, a naringenin glucoside (naringenin-7-O-glucoside) was found in an industrial blanch water extract, a byproduct of almond processing, and believed to play a role in exerting or contributing to a photoprotective effect in a small in vivo study with 12 volunteers (Molecules 2013;18:12426-40).
In 2014, K. Murata and associates screened several Prunus species in a search for skin-whitening compounds. Using an antityrosinase assay, the investigators determined that P. persica exhibited the greatest inhibitory activity and, in additional evaluation, it was found to hinder melanogenesis in B16 rat melanoma cells. Further, they identified afzelin (3-O-alpha-L-rhamnosylkaempferol) and the flavanone naringenin as the active ingredients responsible for inhibition of tyrosinase and melanogenesis and concluded that these substances warrant attention as potential skin-whitening agents (Nat. Prod. Commun. 2014;9:185-8).
A 2014 study in the ophthalmologic literature may also shed light on the photoprotective properties of naringenin. Jun-Li Lin and colleagues, studying the effects of the flavanone in eye drops used to treat N-methyl-N-nitrosourea (MNU)-induced photoreceptor cell death in rats, found that topical naringenin dose-dependently shielded the outer nuclear layer, outer retina, and whole retina, and prevented structural and functional damages to retinal neurons (Int. J. Ophthalmol. 2014;7:391-6).
Conclusion
The antioxidative, antiatherogenic, anticarcinogenic, antiproliferative, antimutagenic, estrogenic, and immunomodulatory properties of naringenin have been established in the laboratory. It remains to be seen whether such activity can be harnessed for medical applications, particularly in the dermatologic arena. Nevertheless, this flavanone warrants watching as research into its potential cutaneous applications proceeds. Currently, there is a dearth of research, though, regarding the use of naringenin in topical products.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in the Design District in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote the textbook “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and a book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). She has contributed to the Cosmeceutical Critique column in Dermatology News since January 2001. Her latest book, “Cosmeceuticals and Cosmetic Ingredients,” was published in November 2014. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Evolus, Galderma, GlaxoSmithKline, Kythera Biopharmaceuticals, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Topix Pharmaceuticals, and Unilever.
The flavanone naringenin (5,7,4-trihydroxyflavanone) is known to exhibit anticarcinogenic, antioxidative, antiatherogenic, estrogenic, and immunomodulatory activity (Nutr. Cancer. 2012;64:714-24; J. Nutr. 2001;131:235-41; Life Sci. 2013;93:516-24). Naringenin can be found in high concentrations in grapefruits, oranges, and other citrus fruits as well as tomatoes (skin), with grapefruit juice found to yield much higher levels in plasma than orange juice (J. Nutr. 2001;131:235-41; Am. J. Physiol. Gastrointest. Liver Physiol. 2000;279:G1148-54; Nutr. Cancer 2012;64:714-24). Naringenin has been shown, along with other flavanones such as hesperetin and ponciretin, to strongly inhibit IgE-induced beta-hexosaminidase release from RBL-2H3 cells. Sung-Hwan Park and associates have suggested that the glycosides of these substances have potential as agents for treating IgE-induced atopic allergies (Planta Med. 2005;71:24-7).
In 2012, Kushi Anand and associates also showed that the combination of curcumin and naringenin exerted antiangiogenic and antitumor effects in Swiss albino mice, adding that neither compound has been associated with reports of toxicity in animals or humans (Nutr. Cancer 2012;64:714-24).
Potential cutaneous benefits
Tae-Ho Kim and colleagues studied the effects of naringenin on 2,4-dinitrofluorobenzene (DNFB)-induced atomic dermatitis in NC/Nga mice in 2013. After repetitive skin contact with DNFB, mice received intraperitoneal injections of naringenin for 1 week, with the treatment with the fruit flavonoid significantly diminishing ear swelling and back skin lesions. The flavonoid also significantly inhibited interferon (IFN)-alpha production by activated CD4+ T cells and lowered serum IgE levels as well as DNFB-induced infiltration of eosinophils, mast cells, CD4+ T cells, and CD8+ T cells in skin lesions (Life Sci. 2013;93:516-24).
Also that year, a naringenin glucoside (naringenin-7-O-glucoside) was found in an industrial blanch water extract, a byproduct of almond processing, and believed to play a role in exerting or contributing to a photoprotective effect in a small in vivo study with 12 volunteers (Molecules 2013;18:12426-40).
In 2014, K. Murata and associates screened several Prunus species in a search for skin-whitening compounds. Using an antityrosinase assay, the investigators determined that P. persica exhibited the greatest inhibitory activity and, in additional evaluation, it was found to hinder melanogenesis in B16 rat melanoma cells. Further, they identified afzelin (3-O-alpha-L-rhamnosylkaempferol) and the flavanone naringenin as the active ingredients responsible for inhibition of tyrosinase and melanogenesis and concluded that these substances warrant attention as potential skin-whitening agents (Nat. Prod. Commun. 2014;9:185-8).
A 2014 study in the ophthalmologic literature may also shed light on the photoprotective properties of naringenin. Jun-Li Lin and colleagues, studying the effects of the flavanone in eye drops used to treat N-methyl-N-nitrosourea (MNU)-induced photoreceptor cell death in rats, found that topical naringenin dose-dependently shielded the outer nuclear layer, outer retina, and whole retina, and prevented structural and functional damages to retinal neurons (Int. J. Ophthalmol. 2014;7:391-6).
Conclusion
The antioxidative, antiatherogenic, anticarcinogenic, antiproliferative, antimutagenic, estrogenic, and immunomodulatory properties of naringenin have been established in the laboratory. It remains to be seen whether such activity can be harnessed for medical applications, particularly in the dermatologic arena. Nevertheless, this flavanone warrants watching as research into its potential cutaneous applications proceeds. Currently, there is a dearth of research, though, regarding the use of naringenin in topical products.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in the Design District in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote the textbook “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and a book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). She has contributed to the Cosmeceutical Critique column in Dermatology News since January 2001. Her latest book, “Cosmeceuticals and Cosmetic Ingredients,” was published in November 2014. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Evolus, Galderma, GlaxoSmithKline, Kythera Biopharmaceuticals, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Topix Pharmaceuticals, and Unilever.
The flavanone naringenin (5,7,4-trihydroxyflavanone) is known to exhibit anticarcinogenic, antioxidative, antiatherogenic, estrogenic, and immunomodulatory activity (Nutr. Cancer. 2012;64:714-24; J. Nutr. 2001;131:235-41; Life Sci. 2013;93:516-24). Naringenin can be found in high concentrations in grapefruits, oranges, and other citrus fruits as well as tomatoes (skin), with grapefruit juice found to yield much higher levels in plasma than orange juice (J. Nutr. 2001;131:235-41; Am. J. Physiol. Gastrointest. Liver Physiol. 2000;279:G1148-54; Nutr. Cancer 2012;64:714-24). Naringenin has been shown, along with other flavanones such as hesperetin and ponciretin, to strongly inhibit IgE-induced beta-hexosaminidase release from RBL-2H3 cells. Sung-Hwan Park and associates have suggested that the glycosides of these substances have potential as agents for treating IgE-induced atopic allergies (Planta Med. 2005;71:24-7).
In 2012, Kushi Anand and associates also showed that the combination of curcumin and naringenin exerted antiangiogenic and antitumor effects in Swiss albino mice, adding that neither compound has been associated with reports of toxicity in animals or humans (Nutr. Cancer 2012;64:714-24).
Potential cutaneous benefits
Tae-Ho Kim and colleagues studied the effects of naringenin on 2,4-dinitrofluorobenzene (DNFB)-induced atomic dermatitis in NC/Nga mice in 2013. After repetitive skin contact with DNFB, mice received intraperitoneal injections of naringenin for 1 week, with the treatment with the fruit flavonoid significantly diminishing ear swelling and back skin lesions. The flavonoid also significantly inhibited interferon (IFN)-alpha production by activated CD4+ T cells and lowered serum IgE levels as well as DNFB-induced infiltration of eosinophils, mast cells, CD4+ T cells, and CD8+ T cells in skin lesions (Life Sci. 2013;93:516-24).
Also that year, a naringenin glucoside (naringenin-7-O-glucoside) was found in an industrial blanch water extract, a byproduct of almond processing, and believed to play a role in exerting or contributing to a photoprotective effect in a small in vivo study with 12 volunteers (Molecules 2013;18:12426-40).
In 2014, K. Murata and associates screened several Prunus species in a search for skin-whitening compounds. Using an antityrosinase assay, the investigators determined that P. persica exhibited the greatest inhibitory activity and, in additional evaluation, it was found to hinder melanogenesis in B16 rat melanoma cells. Further, they identified afzelin (3-O-alpha-L-rhamnosylkaempferol) and the flavanone naringenin as the active ingredients responsible for inhibition of tyrosinase and melanogenesis and concluded that these substances warrant attention as potential skin-whitening agents (Nat. Prod. Commun. 2014;9:185-8).
A 2014 study in the ophthalmologic literature may also shed light on the photoprotective properties of naringenin. Jun-Li Lin and colleagues, studying the effects of the flavanone in eye drops used to treat N-methyl-N-nitrosourea (MNU)-induced photoreceptor cell death in rats, found that topical naringenin dose-dependently shielded the outer nuclear layer, outer retina, and whole retina, and prevented structural and functional damages to retinal neurons (Int. J. Ophthalmol. 2014;7:391-6).
Conclusion
The antioxidative, antiatherogenic, anticarcinogenic, antiproliferative, antimutagenic, estrogenic, and immunomodulatory properties of naringenin have been established in the laboratory. It remains to be seen whether such activity can be harnessed for medical applications, particularly in the dermatologic arena. Nevertheless, this flavanone warrants watching as research into its potential cutaneous applications proceeds. Currently, there is a dearth of research, though, regarding the use of naringenin in topical products.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in the Design District in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote the textbook “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and a book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). She has contributed to the Cosmeceutical Critique column in Dermatology News since January 2001. Her latest book, “Cosmeceuticals and Cosmetic Ingredients,” was published in November 2014. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Evolus, Galderma, GlaxoSmithKline, Kythera Biopharmaceuticals, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Topix Pharmaceuticals, and Unilever.
WCD: Pramocaine is a common contact sensitizer
VANCOUVER – Pramocaine, a topical anesthetic long used in many OTC anti-itch preparations, turns out to itself be a common cause of contact sensitization, Dr. Mariam Abbas reported at the World Congress of Dermatology.
Pramocaine, also known as pramoxine, is an ingredient in Gold Bond cream, some forms of calamine lotion, hemorrhoid relief products, and other OTC antipruritic agents. It’s not available in commercial patch test kits, so Dr. Abbas created a test material comprised of 2% pramocaine in petrolatum.
Seven of 232 patients (3%) referred to a university patch test clinic and who underwent testing to pramocaine along with recognized contact sensitizers showed a clear positive response to pramocaine at 96 hours. The reactions were clinically relevant, according to Dr. Abbas of the University of Alberta, Edmonton.
That 3% positive rate is in the range of published figures for such well-known problem contact sensitizers as neomycin, thiuram mix, and Balsam of Peru.
Dr. Mariam Abbas reported having no financial conflicts regarding this study, conducted free of commercial support.
VANCOUVER – Pramocaine, a topical anesthetic long used in many OTC anti-itch preparations, turns out to itself be a common cause of contact sensitization, Dr. Mariam Abbas reported at the World Congress of Dermatology.
Pramocaine, also known as pramoxine, is an ingredient in Gold Bond cream, some forms of calamine lotion, hemorrhoid relief products, and other OTC antipruritic agents. It’s not available in commercial patch test kits, so Dr. Abbas created a test material comprised of 2% pramocaine in petrolatum.
Seven of 232 patients (3%) referred to a university patch test clinic and who underwent testing to pramocaine along with recognized contact sensitizers showed a clear positive response to pramocaine at 96 hours. The reactions were clinically relevant, according to Dr. Abbas of the University of Alberta, Edmonton.
That 3% positive rate is in the range of published figures for such well-known problem contact sensitizers as neomycin, thiuram mix, and Balsam of Peru.
Dr. Mariam Abbas reported having no financial conflicts regarding this study, conducted free of commercial support.
VANCOUVER – Pramocaine, a topical anesthetic long used in many OTC anti-itch preparations, turns out to itself be a common cause of contact sensitization, Dr. Mariam Abbas reported at the World Congress of Dermatology.
Pramocaine, also known as pramoxine, is an ingredient in Gold Bond cream, some forms of calamine lotion, hemorrhoid relief products, and other OTC antipruritic agents. It’s not available in commercial patch test kits, so Dr. Abbas created a test material comprised of 2% pramocaine in petrolatum.
Seven of 232 patients (3%) referred to a university patch test clinic and who underwent testing to pramocaine along with recognized contact sensitizers showed a clear positive response to pramocaine at 96 hours. The reactions were clinically relevant, according to Dr. Abbas of the University of Alberta, Edmonton.
That 3% positive rate is in the range of published figures for such well-known problem contact sensitizers as neomycin, thiuram mix, and Balsam of Peru.
Dr. Mariam Abbas reported having no financial conflicts regarding this study, conducted free of commercial support.
AT WCD 2015
Key clinical point: Pramocaine, a topical anesthetic that’s an ingredient in many widely used OTC anti-itch products, turns out to be a common cause of contact sensitization.
Major finding: Seven of 232 patients (3%) referred to a patch test clinic showed a clinically relevant positive test to pramocaine.
Data source: A prospective study of 232 patients referred to a patch test clinic.
Disclosures: Dr. Mariam Abbas reported having no financial conflicts regarding this study, conducted free of commercial support.
Nipping buds, kicking butts, being safer than sorry
Brad came in with his mother for me to treat a small wart on the sole of his left foot. “It doesn’t bother me,” he said.
“I had one of those when I was Brad’s age,” said his mother, Mary Lou. “We neglected it and it really grew! With a thing like that, you have to nip it in the bud.”
We all learn little maxims about how the world works and what to do about it. One of these is that to avoid trouble, you should nip things in the bud.
This sounds like it makes sense. Sometimes it’s actually true. But there are other times when what you’re trying to nip doesn’t have a bud.
If you have a plantar wart on the bottom of your foot and you don’t treat it, here are some things that can happen:
• It can grow and become painful.
• It can stay the same for years, never bother you, and go away.
• New ones can appear elsewhere on the sole.
• It can disappear tomorrow afternoon.
Which will happen? For the individual case, I have no idea. Like you, I’ve seen ‘em all.
There are reasons other than functional disability to treat plantar warts. For instance, they’re ugly and embarrassing. So if treatment is not too painful or expensive, why not? But sometimes we freeze it – a standard treatment – and it takes forever, visit after visit, and the wart is still there, grinning complacently. Some insurance plans don’t cover treatments unless the wart hurts, so therapy gets too expensive.
That’s when it might make sense to explain to the patient that you can nip some buds off plants to help them grow better, but you really can’t nip the buds off warts, which have neither roots nor buds.
Another maxim we all pick up is that it’s better to be safe than sorry. That sounds like plain common sense. “Can’t you take off that mole?” asks Annie. “I’m sure it’s bigger that it used to be.”
It’s just an ordinary mole, though, and it doesn’t look worrisome. All moles grow – they start out small and get a bit bigger before they stop. Plus, Annie is a young woman, and her mole is on her face. Even if a plastic surgeon takes it off, she’ll have a scar with no wrinkles to hide it in.
I explain this to Annie. “But isn’t it better to be safe than sorry?” she asks.
Well, sometimes maybe. Just not this time.
Ankur has eczema. He is really frustrated. “Doctors keep giving me creams,” he says. “The rash gets a little better,” but then it comes back. “I’d like you to give me a treatment that will kick it in the butt.”
What Ankur wants, of course, is for me to do something that will shove eczema out the door and then lock the door behind it so it can’t come back.
I would love to do that. Only I can’t. Like the many other recurring conditions we treat every day, nothing specific causes eczema, so nothing definitive gets rid of it once and for all.
In other words, eczema has no butt. So you can’t kick it.
The examples I’ve given are common and homely. There are bigger issues, in medicine and in life, to which common-sense maxims seem to apply but sometimes don’t.
The well-known public debates over prostate-specific antigen (PSA) screening for prostate cancer in older men and routine mammography in younger women attest to how tricky it is to decide whether catching things early is necessarily a good idea. It also shows how the public reacts when data contradict common sense. Of course you should catch cancer early, says the outraged public. Isn’t it always better to be safe than sorry?
No, actually it sometimes isn’t.
We all pick up maxims to live by. We hear them as children without realizing we’re learning them. That makes it hard to accept that not everything is a plant with a bud to be nipped. Or that there are situations when trying to be safe can make you sorrier.
Or that there are indeed butts, big and small. But not everything has one to kick.
Dr. Rockoff practices dermatology in Brookline, Mass., and is a longtime contributor to Dermatology News. He serves on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years.
Brad came in with his mother for me to treat a small wart on the sole of his left foot. “It doesn’t bother me,” he said.
“I had one of those when I was Brad’s age,” said his mother, Mary Lou. “We neglected it and it really grew! With a thing like that, you have to nip it in the bud.”
We all learn little maxims about how the world works and what to do about it. One of these is that to avoid trouble, you should nip things in the bud.
This sounds like it makes sense. Sometimes it’s actually true. But there are other times when what you’re trying to nip doesn’t have a bud.
If you have a plantar wart on the bottom of your foot and you don’t treat it, here are some things that can happen:
• It can grow and become painful.
• It can stay the same for years, never bother you, and go away.
• New ones can appear elsewhere on the sole.
• It can disappear tomorrow afternoon.
Which will happen? For the individual case, I have no idea. Like you, I’ve seen ‘em all.
There are reasons other than functional disability to treat plantar warts. For instance, they’re ugly and embarrassing. So if treatment is not too painful or expensive, why not? But sometimes we freeze it – a standard treatment – and it takes forever, visit after visit, and the wart is still there, grinning complacently. Some insurance plans don’t cover treatments unless the wart hurts, so therapy gets too expensive.
That’s when it might make sense to explain to the patient that you can nip some buds off plants to help them grow better, but you really can’t nip the buds off warts, which have neither roots nor buds.
Another maxim we all pick up is that it’s better to be safe than sorry. That sounds like plain common sense. “Can’t you take off that mole?” asks Annie. “I’m sure it’s bigger that it used to be.”
It’s just an ordinary mole, though, and it doesn’t look worrisome. All moles grow – they start out small and get a bit bigger before they stop. Plus, Annie is a young woman, and her mole is on her face. Even if a plastic surgeon takes it off, she’ll have a scar with no wrinkles to hide it in.
I explain this to Annie. “But isn’t it better to be safe than sorry?” she asks.
Well, sometimes maybe. Just not this time.
Ankur has eczema. He is really frustrated. “Doctors keep giving me creams,” he says. “The rash gets a little better,” but then it comes back. “I’d like you to give me a treatment that will kick it in the butt.”
What Ankur wants, of course, is for me to do something that will shove eczema out the door and then lock the door behind it so it can’t come back.
I would love to do that. Only I can’t. Like the many other recurring conditions we treat every day, nothing specific causes eczema, so nothing definitive gets rid of it once and for all.
In other words, eczema has no butt. So you can’t kick it.
The examples I’ve given are common and homely. There are bigger issues, in medicine and in life, to which common-sense maxims seem to apply but sometimes don’t.
The well-known public debates over prostate-specific antigen (PSA) screening for prostate cancer in older men and routine mammography in younger women attest to how tricky it is to decide whether catching things early is necessarily a good idea. It also shows how the public reacts when data contradict common sense. Of course you should catch cancer early, says the outraged public. Isn’t it always better to be safe than sorry?
No, actually it sometimes isn’t.
We all pick up maxims to live by. We hear them as children without realizing we’re learning them. That makes it hard to accept that not everything is a plant with a bud to be nipped. Or that there are situations when trying to be safe can make you sorrier.
Or that there are indeed butts, big and small. But not everything has one to kick.
Dr. Rockoff practices dermatology in Brookline, Mass., and is a longtime contributor to Dermatology News. He serves on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years.
Brad came in with his mother for me to treat a small wart on the sole of his left foot. “It doesn’t bother me,” he said.
“I had one of those when I was Brad’s age,” said his mother, Mary Lou. “We neglected it and it really grew! With a thing like that, you have to nip it in the bud.”
We all learn little maxims about how the world works and what to do about it. One of these is that to avoid trouble, you should nip things in the bud.
This sounds like it makes sense. Sometimes it’s actually true. But there are other times when what you’re trying to nip doesn’t have a bud.
If you have a plantar wart on the bottom of your foot and you don’t treat it, here are some things that can happen:
• It can grow and become painful.
• It can stay the same for years, never bother you, and go away.
• New ones can appear elsewhere on the sole.
• It can disappear tomorrow afternoon.
Which will happen? For the individual case, I have no idea. Like you, I’ve seen ‘em all.
There are reasons other than functional disability to treat plantar warts. For instance, they’re ugly and embarrassing. So if treatment is not too painful or expensive, why not? But sometimes we freeze it – a standard treatment – and it takes forever, visit after visit, and the wart is still there, grinning complacently. Some insurance plans don’t cover treatments unless the wart hurts, so therapy gets too expensive.
That’s when it might make sense to explain to the patient that you can nip some buds off plants to help them grow better, but you really can’t nip the buds off warts, which have neither roots nor buds.
Another maxim we all pick up is that it’s better to be safe than sorry. That sounds like plain common sense. “Can’t you take off that mole?” asks Annie. “I’m sure it’s bigger that it used to be.”
It’s just an ordinary mole, though, and it doesn’t look worrisome. All moles grow – they start out small and get a bit bigger before they stop. Plus, Annie is a young woman, and her mole is on her face. Even if a plastic surgeon takes it off, she’ll have a scar with no wrinkles to hide it in.
I explain this to Annie. “But isn’t it better to be safe than sorry?” she asks.
Well, sometimes maybe. Just not this time.
Ankur has eczema. He is really frustrated. “Doctors keep giving me creams,” he says. “The rash gets a little better,” but then it comes back. “I’d like you to give me a treatment that will kick it in the butt.”
What Ankur wants, of course, is for me to do something that will shove eczema out the door and then lock the door behind it so it can’t come back.
I would love to do that. Only I can’t. Like the many other recurring conditions we treat every day, nothing specific causes eczema, so nothing definitive gets rid of it once and for all.
In other words, eczema has no butt. So you can’t kick it.
The examples I’ve given are common and homely. There are bigger issues, in medicine and in life, to which common-sense maxims seem to apply but sometimes don’t.
The well-known public debates over prostate-specific antigen (PSA) screening for prostate cancer in older men and routine mammography in younger women attest to how tricky it is to decide whether catching things early is necessarily a good idea. It also shows how the public reacts when data contradict common sense. Of course you should catch cancer early, says the outraged public. Isn’t it always better to be safe than sorry?
No, actually it sometimes isn’t.
We all pick up maxims to live by. We hear them as children without realizing we’re learning them. That makes it hard to accept that not everything is a plant with a bud to be nipped. Or that there are situations when trying to be safe can make you sorrier.
Or that there are indeed butts, big and small. But not everything has one to kick.
Dr. Rockoff practices dermatology in Brookline, Mass., and is a longtime contributor to Dermatology News. He serves on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years.
WCD: Topical tofacitinib hits marks in atopic dermatitis
VANCOUVER, B.C. – Topical tofacitinib shows promise as a novel treatment for atopic dermatitis on the basis of a highly positive phase II randomized clinical trial.
The topical Janus kinase inhibitor hit all of its efficacy endpoints and was well tolerated, with infrequent side effects, none of them serious, Dr. Robert Bissonnette reported at the World Congress of Dermatology.
An unmet need exists for additional topical therapies for atopic dermatitis, a condition whose prevalence has been estimated at up to 20%. Existing topical agents, including corticosteroids and calcineurin inhibitors, have limitations involving long-term safety concerns and application site reactions, noted Dr. Bissonnette, president of Innovaderm Research in Montreal.
The Janus kinases have been implicated in the pathogenesis of atopic dermatitis due to their effects upon the interleukin-4, IL-5, and IL-31 signaling pathways and the resultant dysregulation of the immune response.
Dr. Bissonnette presented a phase II, 4-week, double-blind, vehicle-controlled, multicenter Canadian study involving 65 adults with mild to moderate atopic dermatitis. They applied tofacitinib ointment 2% or its vehicle twice daily for 4 weeks. Participants averaged 31 years of age, with a median 21 years since receiving the diagnosis of atopic dermatitis. Roughly three-quarters of subjects had moderate disease based upon Physician Global Assessment.
The primary study endpoint was change in Eczema Area and Severity Index (EASI) total score after 4 weeks. From a baseline EASI score of 5.4, the topical tofacitinib group experienced a mean 82% reduction, significantly outperforming the control group, which showed a 30% reduction. The difference between the two study arms reached significance at the first assessment, at week 1.
Patients on the topical Janus kinase (JAK) inhibitor also showed significantly better outcomes than controls on all secondary endpoints, with the differences reaching statistical significance at week 1 with the exception of improvement in self-assessed Itch Severity Item, where topical tofacitinib’s advantage became significant on treatment day 2.
Two patients on topical tofacitinib and five controls developed treatment area adverse events, consisting of skin irritation and/or pain, which was mild in all cases. There were no cases of herpes simplex or zoster, no opportunistic infections, no laboratory abnormalities, and no one required a dose reduction or temporary discontinuation of the topical JAK inhibitor, according to the dermatologist.
Oral tofacitinib is Food and Drug Administration–approved as Xeljanz for the treatment of rheumatoid arthritis and is currently under FDA review as a potential treatment for moderate to severe chronic plaque psoriasis, with a regulatory decision anticipated in October 2015.
The atopic dermatitis study was funded by Pfizer. Dr. Bissonnette is a consultant to and recipient of research grants from more than a dozen pharmaceutical companies, including Pfizer.
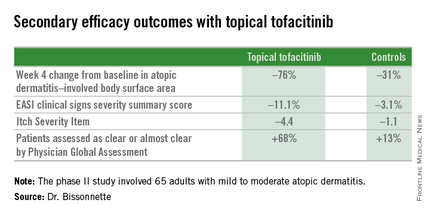
VANCOUVER, B.C. – Topical tofacitinib shows promise as a novel treatment for atopic dermatitis on the basis of a highly positive phase II randomized clinical trial.
The topical Janus kinase inhibitor hit all of its efficacy endpoints and was well tolerated, with infrequent side effects, none of them serious, Dr. Robert Bissonnette reported at the World Congress of Dermatology.
An unmet need exists for additional topical therapies for atopic dermatitis, a condition whose prevalence has been estimated at up to 20%. Existing topical agents, including corticosteroids and calcineurin inhibitors, have limitations involving long-term safety concerns and application site reactions, noted Dr. Bissonnette, president of Innovaderm Research in Montreal.
The Janus kinases have been implicated in the pathogenesis of atopic dermatitis due to their effects upon the interleukin-4, IL-5, and IL-31 signaling pathways and the resultant dysregulation of the immune response.
Dr. Bissonnette presented a phase II, 4-week, double-blind, vehicle-controlled, multicenter Canadian study involving 65 adults with mild to moderate atopic dermatitis. They applied tofacitinib ointment 2% or its vehicle twice daily for 4 weeks. Participants averaged 31 years of age, with a median 21 years since receiving the diagnosis of atopic dermatitis. Roughly three-quarters of subjects had moderate disease based upon Physician Global Assessment.
The primary study endpoint was change in Eczema Area and Severity Index (EASI) total score after 4 weeks. From a baseline EASI score of 5.4, the topical tofacitinib group experienced a mean 82% reduction, significantly outperforming the control group, which showed a 30% reduction. The difference between the two study arms reached significance at the first assessment, at week 1.
Patients on the topical Janus kinase (JAK) inhibitor also showed significantly better outcomes than controls on all secondary endpoints, with the differences reaching statistical significance at week 1 with the exception of improvement in self-assessed Itch Severity Item, where topical tofacitinib’s advantage became significant on treatment day 2.
Two patients on topical tofacitinib and five controls developed treatment area adverse events, consisting of skin irritation and/or pain, which was mild in all cases. There were no cases of herpes simplex or zoster, no opportunistic infections, no laboratory abnormalities, and no one required a dose reduction or temporary discontinuation of the topical JAK inhibitor, according to the dermatologist.
Oral tofacitinib is Food and Drug Administration–approved as Xeljanz for the treatment of rheumatoid arthritis and is currently under FDA review as a potential treatment for moderate to severe chronic plaque psoriasis, with a regulatory decision anticipated in October 2015.
The atopic dermatitis study was funded by Pfizer. Dr. Bissonnette is a consultant to and recipient of research grants from more than a dozen pharmaceutical companies, including Pfizer.

VANCOUVER, B.C. – Topical tofacitinib shows promise as a novel treatment for atopic dermatitis on the basis of a highly positive phase II randomized clinical trial.
The topical Janus kinase inhibitor hit all of its efficacy endpoints and was well tolerated, with infrequent side effects, none of them serious, Dr. Robert Bissonnette reported at the World Congress of Dermatology.
An unmet need exists for additional topical therapies for atopic dermatitis, a condition whose prevalence has been estimated at up to 20%. Existing topical agents, including corticosteroids and calcineurin inhibitors, have limitations involving long-term safety concerns and application site reactions, noted Dr. Bissonnette, president of Innovaderm Research in Montreal.
The Janus kinases have been implicated in the pathogenesis of atopic dermatitis due to their effects upon the interleukin-4, IL-5, and IL-31 signaling pathways and the resultant dysregulation of the immune response.
Dr. Bissonnette presented a phase II, 4-week, double-blind, vehicle-controlled, multicenter Canadian study involving 65 adults with mild to moderate atopic dermatitis. They applied tofacitinib ointment 2% or its vehicle twice daily for 4 weeks. Participants averaged 31 years of age, with a median 21 years since receiving the diagnosis of atopic dermatitis. Roughly three-quarters of subjects had moderate disease based upon Physician Global Assessment.
The primary study endpoint was change in Eczema Area and Severity Index (EASI) total score after 4 weeks. From a baseline EASI score of 5.4, the topical tofacitinib group experienced a mean 82% reduction, significantly outperforming the control group, which showed a 30% reduction. The difference between the two study arms reached significance at the first assessment, at week 1.
Patients on the topical Janus kinase (JAK) inhibitor also showed significantly better outcomes than controls on all secondary endpoints, with the differences reaching statistical significance at week 1 with the exception of improvement in self-assessed Itch Severity Item, where topical tofacitinib’s advantage became significant on treatment day 2.
Two patients on topical tofacitinib and five controls developed treatment area adverse events, consisting of skin irritation and/or pain, which was mild in all cases. There were no cases of herpes simplex or zoster, no opportunistic infections, no laboratory abnormalities, and no one required a dose reduction or temporary discontinuation of the topical JAK inhibitor, according to the dermatologist.
Oral tofacitinib is Food and Drug Administration–approved as Xeljanz for the treatment of rheumatoid arthritis and is currently under FDA review as a potential treatment for moderate to severe chronic plaque psoriasis, with a regulatory decision anticipated in October 2015.
The atopic dermatitis study was funded by Pfizer. Dr. Bissonnette is a consultant to and recipient of research grants from more than a dozen pharmaceutical companies, including Pfizer.

AT WCD 2015
Key clinical point: Topical tofacitinib, a Janus kinase inhibitor, may provide a novel safe and effective therapy for atopic dermatitis.
Major finding: After 4 weeks of topical tofacitinib, atopic dermatitis patients experienced a mean 82% reduction in their Eczema Area and Severity Index total score, compared with a 30% decrease in vehicle-treated controls.
Data source: A five-center, randomized, double-blind, prospective, vehicle-controlled phase II study involving 65 adults with atopic dermatitis.
Disclosures: Dr. Robert Bissonnette disclosed ties with Pfizer – the study sponsor – and more than a dozen other pharmaceutical companies.