User login
Peanut reactivity returned if exposure delayed after immunotherapy
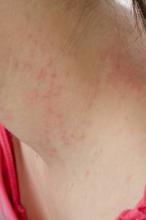
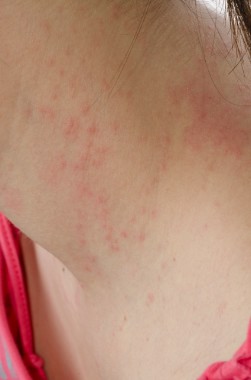
SAN DIEGO – Waiting 3 months after successful oral immunotherapy for peanut allergy before exposure to peanuts significantly increased the odds of reactivity returning in a prospective study of 20 children.
All 20 patients became desensitized to peanuts while on daily oral immunotherapy and successfully passed double-blind, placebo-controlled food challenges at the end of immunotherapy. All 16 patients who then avoided peanuts for 1 month passed a second food challenge, but only 1 of 4 patients who avoided peanuts for 3 months after immunotherapy passed a second one.
"If you wait long enough, the desensitization effect may well wear off," Dr. Brian P. Vickery said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
He and his associates looked at levels of basophil activation and conducted skin prick tests to peanut to get a better sense of why this was happening. Basophil activation to peanut antigen and anti-IgE stimulation increased significantly between the times of the first and second food challenges in the patients who avoided peanuts for 3 months but not in those who avoided the nuts for only 1 month.
The basophil activation levels had been similar between groups at the end of immunotherapy, suggesting that desensitization succeeded in all patients, but the length of peanut avoidance affected basophil responses, leading to revival of clinical reactivity.
Skin prick test results returned to baseline levels in three of the four patients who avoided peanuts for 3 months after the end of immunotherapy.
The amount of time on oral immunotherapy did not seem to be a key factor in the likelihood of sustained suppression of allergic disease, reported Dr. Vickery of the department of pediatrics at the University of North Carolina at Chapel Hill. Patients who waited 1 month before exposure to peanut had been on approximately 20-50 months of oral immunotherapy, and patients who waited 3 months before exposure had been on approximately 60 months of immunotherapy.
The lead author of the study was Michael D. Kulis Jr., Ph.D., also of the university.
Prolonged avoidance of peanut after peanut oral immunotherapy appears to be detrimental and may reverse the effects of the treatment, Dr. A. Wesley Burks said at a press briefing.
The findings may be applicable to other food allergies, but "we don’t know that because there have not been enough studies that long with other foods," said Dr. Burks, a coinvestigator in the study and chairman of the department of pediatrics at the university. "I wouldn’t anticipate that it would be different."
The average age of children in the study was 6 years.
This exploratory study was not controlled and was limited by its small size. The Peanut Oral Immunotherapy in Children (IMPACT) study is underway and should answer the question of how long the clinical effects of oral immunotherapy last, Dr. Vickery said. The study is being sponsored by the Immune Tolerance Network and the National Institute of Allergy and Infectious Diseases.
Dr. Vickery, Dr. Kulis, and Dr. Burks reported having no financial disclosures. The National Institutes of Health funded the study.
On Twitter @sherryboschert
SAN DIEGO – Waiting 3 months after successful oral immunotherapy for peanut allergy before exposure to peanuts significantly increased the odds of reactivity returning in a prospective study of 20 children.
All 20 patients became desensitized to peanuts while on daily oral immunotherapy and successfully passed double-blind, placebo-controlled food challenges at the end of immunotherapy. All 16 patients who then avoided peanuts for 1 month passed a second food challenge, but only 1 of 4 patients who avoided peanuts for 3 months after immunotherapy passed a second one.
"If you wait long enough, the desensitization effect may well wear off," Dr. Brian P. Vickery said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
He and his associates looked at levels of basophil activation and conducted skin prick tests to peanut to get a better sense of why this was happening. Basophil activation to peanut antigen and anti-IgE stimulation increased significantly between the times of the first and second food challenges in the patients who avoided peanuts for 3 months but not in those who avoided the nuts for only 1 month.
The basophil activation levels had been similar between groups at the end of immunotherapy, suggesting that desensitization succeeded in all patients, but the length of peanut avoidance affected basophil responses, leading to revival of clinical reactivity.
Skin prick test results returned to baseline levels in three of the four patients who avoided peanuts for 3 months after the end of immunotherapy.
The amount of time on oral immunotherapy did not seem to be a key factor in the likelihood of sustained suppression of allergic disease, reported Dr. Vickery of the department of pediatrics at the University of North Carolina at Chapel Hill. Patients who waited 1 month before exposure to peanut had been on approximately 20-50 months of oral immunotherapy, and patients who waited 3 months before exposure had been on approximately 60 months of immunotherapy.
The lead author of the study was Michael D. Kulis Jr., Ph.D., also of the university.
Prolonged avoidance of peanut after peanut oral immunotherapy appears to be detrimental and may reverse the effects of the treatment, Dr. A. Wesley Burks said at a press briefing.
The findings may be applicable to other food allergies, but "we don’t know that because there have not been enough studies that long with other foods," said Dr. Burks, a coinvestigator in the study and chairman of the department of pediatrics at the university. "I wouldn’t anticipate that it would be different."
The average age of children in the study was 6 years.
This exploratory study was not controlled and was limited by its small size. The Peanut Oral Immunotherapy in Children (IMPACT) study is underway and should answer the question of how long the clinical effects of oral immunotherapy last, Dr. Vickery said. The study is being sponsored by the Immune Tolerance Network and the National Institute of Allergy and Infectious Diseases.
Dr. Vickery, Dr. Kulis, and Dr. Burks reported having no financial disclosures. The National Institutes of Health funded the study.
On Twitter @sherryboschert
SAN DIEGO – Waiting 3 months after successful oral immunotherapy for peanut allergy before exposure to peanuts significantly increased the odds of reactivity returning in a prospective study of 20 children.
All 20 patients became desensitized to peanuts while on daily oral immunotherapy and successfully passed double-blind, placebo-controlled food challenges at the end of immunotherapy. All 16 patients who then avoided peanuts for 1 month passed a second food challenge, but only 1 of 4 patients who avoided peanuts for 3 months after immunotherapy passed a second one.
"If you wait long enough, the desensitization effect may well wear off," Dr. Brian P. Vickery said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
He and his associates looked at levels of basophil activation and conducted skin prick tests to peanut to get a better sense of why this was happening. Basophil activation to peanut antigen and anti-IgE stimulation increased significantly between the times of the first and second food challenges in the patients who avoided peanuts for 3 months but not in those who avoided the nuts for only 1 month.
The basophil activation levels had been similar between groups at the end of immunotherapy, suggesting that desensitization succeeded in all patients, but the length of peanut avoidance affected basophil responses, leading to revival of clinical reactivity.
Skin prick test results returned to baseline levels in three of the four patients who avoided peanuts for 3 months after the end of immunotherapy.
The amount of time on oral immunotherapy did not seem to be a key factor in the likelihood of sustained suppression of allergic disease, reported Dr. Vickery of the department of pediatrics at the University of North Carolina at Chapel Hill. Patients who waited 1 month before exposure to peanut had been on approximately 20-50 months of oral immunotherapy, and patients who waited 3 months before exposure had been on approximately 60 months of immunotherapy.
The lead author of the study was Michael D. Kulis Jr., Ph.D., also of the university.
Prolonged avoidance of peanut after peanut oral immunotherapy appears to be detrimental and may reverse the effects of the treatment, Dr. A. Wesley Burks said at a press briefing.
The findings may be applicable to other food allergies, but "we don’t know that because there have not been enough studies that long with other foods," said Dr. Burks, a coinvestigator in the study and chairman of the department of pediatrics at the university. "I wouldn’t anticipate that it would be different."
The average age of children in the study was 6 years.
This exploratory study was not controlled and was limited by its small size. The Peanut Oral Immunotherapy in Children (IMPACT) study is underway and should answer the question of how long the clinical effects of oral immunotherapy last, Dr. Vickery said. The study is being sponsored by the Immune Tolerance Network and the National Institute of Allergy and Infectious Diseases.
Dr. Vickery, Dr. Kulis, and Dr. Burks reported having no financial disclosures. The National Institutes of Health funded the study.
On Twitter @sherryboschert
AT 2014 AAAAI ANNUAL MEETING
Major finding: Reactivity to peanuts returned in none of the 16 patients who avoided peanuts for 1 month after oral immunotherapy and in three of four patients who avoided peanuts for 3 months.
Data source: A prospective study of 20 children who underwent food challenges after oral immunotherapy treatment for peanut allergy.
Disclosures: Dr. Vickery, Dr. Kulis, and Dr. Burks reported having no financial disclosures.
Food allergy overdiagnosed with IgE tests
SAN DIEGO – Unwarranted food allergy testing in 67% of 274 patients who underwent testing identified a new food allergen in only 4 patients, in a retrospective study.
The review of charts on patients referred to a tertiary food allergy center from September 2011 to December 2012 found 274 children with results from a standard panel of food-specific IgE tests obtained prior to referral. Only 33% of cases warranted evaluation for food allergy according to criteria set by the National Institute of Allergy and Infectious Diseases, according to Dr. Maryam Saifi and her associates.
In the 90 patients who underwent food allergy testing appropriately, testing identified a previously unknown allergen in 38 patients (42%) and found no new allergen in 52 (58%). When food allergy testing was not warranted, however, testing identified a previously unknown allergen in only 4 of 184 patients (5%) and no new allergen in 180 patients (95%), Dr. Saifi reported in a poster presentation at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
The most common reason for conducting food-specific IgE panel testing in patients who did not meet criteria for testing was allergic rhinitis, followed by mild atopic dermatitis, urticaria, GI complaints, rash (not otherwise specified), angioedema without urticaria, and cough.
Test results and recommendations from primary care providers led 126 of the 274 patients to alter their diets (46%), yet only 54 food-avoiding patients had a history warranting evaluation for food allergy (20% of the whole cohort), reported Dr. Saifi, a pediatrician at the University of Texas Southwestern Medical Center, Dallas.
Diet-altering patients were avoiding foods such as milk, eggs, peanuts, tree nuts, soy, wheat, fish, shellfish, sesame seed, corn, chocolate, or beef. After being seen at the referral clinic for a history, repeat testing, and observed challenges when necessary, patients were able to reintroduce an average of two foods per person, most commonly among milk, eggs, peanuts, soy, and wheat. All patients who had been avoiding corn and chocolate were able to reintroduce those foods.
Serum allergy testing should be used judiciously and only when indicated by history and physical exam, Dr. Saifi concluded. IgE panels for food allergy seem to have little utility as screening tests and often lead to misdiagnosis of food allergy, resulting in inappropriate food avoidance that could cause nutritional deficiencies, increased anxiety, and lower quality of life, she said.
The study excluded patients diagnosed with eosinophilic esophagitis and patients whose records lacked results from food-specific IgE tests prior to referral.
The investigators’ financial disclosures were not available.
On Twitter @sherryboschert
SAN DIEGO – Unwarranted food allergy testing in 67% of 274 patients who underwent testing identified a new food allergen in only 4 patients, in a retrospective study.
The review of charts on patients referred to a tertiary food allergy center from September 2011 to December 2012 found 274 children with results from a standard panel of food-specific IgE tests obtained prior to referral. Only 33% of cases warranted evaluation for food allergy according to criteria set by the National Institute of Allergy and Infectious Diseases, according to Dr. Maryam Saifi and her associates.
In the 90 patients who underwent food allergy testing appropriately, testing identified a previously unknown allergen in 38 patients (42%) and found no new allergen in 52 (58%). When food allergy testing was not warranted, however, testing identified a previously unknown allergen in only 4 of 184 patients (5%) and no new allergen in 180 patients (95%), Dr. Saifi reported in a poster presentation at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
The most common reason for conducting food-specific IgE panel testing in patients who did not meet criteria for testing was allergic rhinitis, followed by mild atopic dermatitis, urticaria, GI complaints, rash (not otherwise specified), angioedema without urticaria, and cough.
Test results and recommendations from primary care providers led 126 of the 274 patients to alter their diets (46%), yet only 54 food-avoiding patients had a history warranting evaluation for food allergy (20% of the whole cohort), reported Dr. Saifi, a pediatrician at the University of Texas Southwestern Medical Center, Dallas.
Diet-altering patients were avoiding foods such as milk, eggs, peanuts, tree nuts, soy, wheat, fish, shellfish, sesame seed, corn, chocolate, or beef. After being seen at the referral clinic for a history, repeat testing, and observed challenges when necessary, patients were able to reintroduce an average of two foods per person, most commonly among milk, eggs, peanuts, soy, and wheat. All patients who had been avoiding corn and chocolate were able to reintroduce those foods.
Serum allergy testing should be used judiciously and only when indicated by history and physical exam, Dr. Saifi concluded. IgE panels for food allergy seem to have little utility as screening tests and often lead to misdiagnosis of food allergy, resulting in inappropriate food avoidance that could cause nutritional deficiencies, increased anxiety, and lower quality of life, she said.
The study excluded patients diagnosed with eosinophilic esophagitis and patients whose records lacked results from food-specific IgE tests prior to referral.
The investigators’ financial disclosures were not available.
On Twitter @sherryboschert
SAN DIEGO – Unwarranted food allergy testing in 67% of 274 patients who underwent testing identified a new food allergen in only 4 patients, in a retrospective study.
The review of charts on patients referred to a tertiary food allergy center from September 2011 to December 2012 found 274 children with results from a standard panel of food-specific IgE tests obtained prior to referral. Only 33% of cases warranted evaluation for food allergy according to criteria set by the National Institute of Allergy and Infectious Diseases, according to Dr. Maryam Saifi and her associates.
In the 90 patients who underwent food allergy testing appropriately, testing identified a previously unknown allergen in 38 patients (42%) and found no new allergen in 52 (58%). When food allergy testing was not warranted, however, testing identified a previously unknown allergen in only 4 of 184 patients (5%) and no new allergen in 180 patients (95%), Dr. Saifi reported in a poster presentation at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
The most common reason for conducting food-specific IgE panel testing in patients who did not meet criteria for testing was allergic rhinitis, followed by mild atopic dermatitis, urticaria, GI complaints, rash (not otherwise specified), angioedema without urticaria, and cough.
Test results and recommendations from primary care providers led 126 of the 274 patients to alter their diets (46%), yet only 54 food-avoiding patients had a history warranting evaluation for food allergy (20% of the whole cohort), reported Dr. Saifi, a pediatrician at the University of Texas Southwestern Medical Center, Dallas.
Diet-altering patients were avoiding foods such as milk, eggs, peanuts, tree nuts, soy, wheat, fish, shellfish, sesame seed, corn, chocolate, or beef. After being seen at the referral clinic for a history, repeat testing, and observed challenges when necessary, patients were able to reintroduce an average of two foods per person, most commonly among milk, eggs, peanuts, soy, and wheat. All patients who had been avoiding corn and chocolate were able to reintroduce those foods.
Serum allergy testing should be used judiciously and only when indicated by history and physical exam, Dr. Saifi concluded. IgE panels for food allergy seem to have little utility as screening tests and often lead to misdiagnosis of food allergy, resulting in inappropriate food avoidance that could cause nutritional deficiencies, increased anxiety, and lower quality of life, she said.
The study excluded patients diagnosed with eosinophilic esophagitis and patients whose records lacked results from food-specific IgE tests prior to referral.
The investigators’ financial disclosures were not available.
On Twitter @sherryboschert
AT THE 2014 AAAAI ANNUAL MEETING
Major finding: Food allergy testing identified a new allergen in 5% of 184 patients in whom testing was not warranted and in 42% of 90 patients in whom testing was warranted.
Data source: Retrospective study of 274 patients referred to a tertiary allergy center who had results from a panel of food-specific IgE tests before referral.
Disclosures: The investigators’ financial disclosures were not available.
Using cyclosporine A: Study sheds light on inflammatory mechanisms in atopic dermatitis
ALBUQUERQUE – Symptoms decreased by 51% at 2 weeks and by 73% at 12 weeks in adults with atopic dermatitis who received cyclosporine A therapy in an open-label, single-arm study.
Clinical improvements correlated with suppression of epidermal hyperplasia and decreases in inflammatory biomarkers including S100A7, IL-13, and IL-22, reported Mariya Rozenblit of Mount Sinai Medical Center in New York and her associates. Ms. Rozenblit presented the results at the annual meeting of the Society for Investigative Dermatology, and the findings were published online in the Journal of Allergy and Clinical Immunology (J. Allergy Clin. Immunol. 2014 [doi:10.1016/j.jaci.2014.03.003]).
"This is the first study that establishes a link between Th2/Th22 cytokines and molecular epidermal alterations as well as correlations between clinical improvement and skin biomarkers" in atopic dermatitis, said Ms. Rozenblit and her associates.
To characterize these mechanisms and compare them with clinical outcomes in atopic dermatitis, the researchers treated 19 affected adults, aged 18-69 years, with 5 mg/kg/day of cyclosporine A. Patients were assessed at baseline, week 2, and week 12 using gene expression and immunohistochemistry studies, biopsies of lesional and nonlesional skin, and the Scoring of Atopic Dermatitis (SCORAD) measure.
"Patients had decreases in erythema and edema at week 2, with further reductions at week 12," said Ms. Rozenblit. By week 12, 17 of the 19 patients had SCORAD reductions of at least 50%, the threshold for therapeutic response, she said. The cohort’s SCORAD scores improved by an average of 51% (standard deviation, 23%) at week 2 and by 73% (SD, 18%) at week 12, she added. SCORAD results ranged from 44 to 98 (mean, 65; standard deviation, 16) at baseline, from 5.9 to 76 (mean, 33; SD, 20) at week 2, and from 0 to 59 (mean, 18; SD, 15) at week 12.
Clinical improvements correlated with significant genomic improvements as measured by gene arrays and reverse transcriptase polymerase chain reaction (PCR) testing. In particular, the researchers observed reductions of Th2, Th22, and some Th17-related molecules such as IL-13 and IL-22; modulation of epidermal hyperplasia and proliferation markers K16 and Ki67 also were noted. IL-13 as well as several S100 proteins and IL-22 were among the top genes that were decreased after treatment, said Ms. Rozenblit.
After 12 weeks of treatment, lesional skin biopsies also indicated that the granular layer had improved to the point that it resembled nonlesional skin, Ms. Rozenblit noted. "Baseline lesional skin had substantial inflammatory cell infiltrates, with decreases essentially to baseline nonlesional levels by week 12," she said. Nonlesional skin showed similar but less marked reversal of inflammatory and epidermal measures after cyclosporine A treatment.
As clinical trials investigate better-tolerated targeted agents for atopic dermatitis, understanding the biomarkers involved will provide a reference point for evaluating improvements in tissue inflammation.
Ms. Rozenblit reported no conflicts of interest. The National Institutes of Health, Rockefeller University, and a Dermatology Foundation Physician Scientist Career Development Award helped fund the research. Novartis provided cyclosporine A for U.S. patients.
ALBUQUERQUE – Symptoms decreased by 51% at 2 weeks and by 73% at 12 weeks in adults with atopic dermatitis who received cyclosporine A therapy in an open-label, single-arm study.
Clinical improvements correlated with suppression of epidermal hyperplasia and decreases in inflammatory biomarkers including S100A7, IL-13, and IL-22, reported Mariya Rozenblit of Mount Sinai Medical Center in New York and her associates. Ms. Rozenblit presented the results at the annual meeting of the Society for Investigative Dermatology, and the findings were published online in the Journal of Allergy and Clinical Immunology (J. Allergy Clin. Immunol. 2014 [doi:10.1016/j.jaci.2014.03.003]).
"This is the first study that establishes a link between Th2/Th22 cytokines and molecular epidermal alterations as well as correlations between clinical improvement and skin biomarkers" in atopic dermatitis, said Ms. Rozenblit and her associates.
To characterize these mechanisms and compare them with clinical outcomes in atopic dermatitis, the researchers treated 19 affected adults, aged 18-69 years, with 5 mg/kg/day of cyclosporine A. Patients were assessed at baseline, week 2, and week 12 using gene expression and immunohistochemistry studies, biopsies of lesional and nonlesional skin, and the Scoring of Atopic Dermatitis (SCORAD) measure.
"Patients had decreases in erythema and edema at week 2, with further reductions at week 12," said Ms. Rozenblit. By week 12, 17 of the 19 patients had SCORAD reductions of at least 50%, the threshold for therapeutic response, she said. The cohort’s SCORAD scores improved by an average of 51% (standard deviation, 23%) at week 2 and by 73% (SD, 18%) at week 12, she added. SCORAD results ranged from 44 to 98 (mean, 65; standard deviation, 16) at baseline, from 5.9 to 76 (mean, 33; SD, 20) at week 2, and from 0 to 59 (mean, 18; SD, 15) at week 12.
Clinical improvements correlated with significant genomic improvements as measured by gene arrays and reverse transcriptase polymerase chain reaction (PCR) testing. In particular, the researchers observed reductions of Th2, Th22, and some Th17-related molecules such as IL-13 and IL-22; modulation of epidermal hyperplasia and proliferation markers K16 and Ki67 also were noted. IL-13 as well as several S100 proteins and IL-22 were among the top genes that were decreased after treatment, said Ms. Rozenblit.
After 12 weeks of treatment, lesional skin biopsies also indicated that the granular layer had improved to the point that it resembled nonlesional skin, Ms. Rozenblit noted. "Baseline lesional skin had substantial inflammatory cell infiltrates, with decreases essentially to baseline nonlesional levels by week 12," she said. Nonlesional skin showed similar but less marked reversal of inflammatory and epidermal measures after cyclosporine A treatment.
As clinical trials investigate better-tolerated targeted agents for atopic dermatitis, understanding the biomarkers involved will provide a reference point for evaluating improvements in tissue inflammation.
Ms. Rozenblit reported no conflicts of interest. The National Institutes of Health, Rockefeller University, and a Dermatology Foundation Physician Scientist Career Development Award helped fund the research. Novartis provided cyclosporine A for U.S. patients.
ALBUQUERQUE – Symptoms decreased by 51% at 2 weeks and by 73% at 12 weeks in adults with atopic dermatitis who received cyclosporine A therapy in an open-label, single-arm study.
Clinical improvements correlated with suppression of epidermal hyperplasia and decreases in inflammatory biomarkers including S100A7, IL-13, and IL-22, reported Mariya Rozenblit of Mount Sinai Medical Center in New York and her associates. Ms. Rozenblit presented the results at the annual meeting of the Society for Investigative Dermatology, and the findings were published online in the Journal of Allergy and Clinical Immunology (J. Allergy Clin. Immunol. 2014 [doi:10.1016/j.jaci.2014.03.003]).
"This is the first study that establishes a link between Th2/Th22 cytokines and molecular epidermal alterations as well as correlations between clinical improvement and skin biomarkers" in atopic dermatitis, said Ms. Rozenblit and her associates.
To characterize these mechanisms and compare them with clinical outcomes in atopic dermatitis, the researchers treated 19 affected adults, aged 18-69 years, with 5 mg/kg/day of cyclosporine A. Patients were assessed at baseline, week 2, and week 12 using gene expression and immunohistochemistry studies, biopsies of lesional and nonlesional skin, and the Scoring of Atopic Dermatitis (SCORAD) measure.
"Patients had decreases in erythema and edema at week 2, with further reductions at week 12," said Ms. Rozenblit. By week 12, 17 of the 19 patients had SCORAD reductions of at least 50%, the threshold for therapeutic response, she said. The cohort’s SCORAD scores improved by an average of 51% (standard deviation, 23%) at week 2 and by 73% (SD, 18%) at week 12, she added. SCORAD results ranged from 44 to 98 (mean, 65; standard deviation, 16) at baseline, from 5.9 to 76 (mean, 33; SD, 20) at week 2, and from 0 to 59 (mean, 18; SD, 15) at week 12.
Clinical improvements correlated with significant genomic improvements as measured by gene arrays and reverse transcriptase polymerase chain reaction (PCR) testing. In particular, the researchers observed reductions of Th2, Th22, and some Th17-related molecules such as IL-13 and IL-22; modulation of epidermal hyperplasia and proliferation markers K16 and Ki67 also were noted. IL-13 as well as several S100 proteins and IL-22 were among the top genes that were decreased after treatment, said Ms. Rozenblit.
After 12 weeks of treatment, lesional skin biopsies also indicated that the granular layer had improved to the point that it resembled nonlesional skin, Ms. Rozenblit noted. "Baseline lesional skin had substantial inflammatory cell infiltrates, with decreases essentially to baseline nonlesional levels by week 12," she said. Nonlesional skin showed similar but less marked reversal of inflammatory and epidermal measures after cyclosporine A treatment.
As clinical trials investigate better-tolerated targeted agents for atopic dermatitis, understanding the biomarkers involved will provide a reference point for evaluating improvements in tissue inflammation.
Ms. Rozenblit reported no conflicts of interest. The National Institutes of Health, Rockefeller University, and a Dermatology Foundation Physician Scientist Career Development Award helped fund the research. Novartis provided cyclosporine A for U.S. patients.
AT THE 2014 SID ANNUAL MEETING
Key clinical point: Clinical improvements in atopic dermatitis correlated with reductions in inflammatory markers.
Major finding: SCORAD scores decreased by 51% at week 2 and 73% at week 12 of treatment with 5 mg/kg cyclosporine A daily.
Data source: Single-arm cohort study of 19 adults with atopic dermatitis.
Disclosures: Ms. Rozenblit reported no conflicts of interest. The National Institutes of Health, Rockefeller University, and a Dermatology Foundation Physician Scientist Career Development Award helped fund the research. Novartis provided cyclosporine A for U.S. patients.
Childhood atopic dermatitis persists into adolescence and beyond
Contrary to conventional wisdom, atopic dermatitis persists well into the second decade of life and probably longer, based on data from a prospective longitudinal study published online April 3 in JAMA Dermatology.
At every age studied (2-26 years), more than 80% of patients reported having symptoms of atopic dermatitis (AD) or taking AD medication in the past 6 months, reported Dr. David J. Margolis and his associates at the University of Pennsylvania, Philadelphia.
"Past teaching that nearly 50%-70% of children with AD will achieve a resolution of their AD by age 12 years was not achieved in our study," the researchers said (JAMA Dermatol. 2014 April 3 [doi:10.1001/ jamadermatol.2013.10271]).
The study enrolled 7,157 children aged 2-17 years who had physician-diagnosed mild to moderate AD. In a Kaplan-Meier survival estimate of 2,416 patients followed for at least 5 years, only by age 20 years did 50% of patients report at least 6 months free of AD symptoms or medications.
The findings are consistent with recent data suggesting that AD or eczema affects similar proportions of children and adults in the United States, the researchers said.
The study was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases and by Valeant Pharmaceuticals, maker of the atopic dermatitis drug pimecrolimus. The authors reported no individual conflicts of interest.
The findings "challenge the conventional dogma that most childhood AD ‘burns out’ and dissipates by late adolescence and adulthood," said Dr. Jonathan I. Silverberg.
"It seems that children with eczema do not really outgrow their eczema," he said. "Rather, eczema seems to be a lifelong disease with a variable phenotype and/or reduced expression in adulthood."
Adult eczema’s diverse symptoms could have led researchers to underestimate its prevalence, said Dr. Silverberg, adding that many clinical studies of the disease defined cases based on classical criteria, such as flexural eczema. "Thus, the use of self-reported outcomes may be more sensitive and actually better suited for some studies of adult eczema."
The current study’s response rate of 70% is considered very good for mail-based surveys, he said. He noted that participants were geographically diverse, even though the study did not use random, population-based sampling.
Dr. Silverberg is a dermatologist at Northwestern University, Chicago. He reported that he has no financial conflicts of interest. These remarks were taken from his editorial accompanying Dr. Margolis’s report (JAMA Dermatol. 2014 April 3 [doi:10.1001/jamadermatol.2013.10267]).
The findings "challenge the conventional dogma that most childhood AD ‘burns out’ and dissipates by late adolescence and adulthood," said Dr. Jonathan I. Silverberg.
"It seems that children with eczema do not really outgrow their eczema," he said. "Rather, eczema seems to be a lifelong disease with a variable phenotype and/or reduced expression in adulthood."
Adult eczema’s diverse symptoms could have led researchers to underestimate its prevalence, said Dr. Silverberg, adding that many clinical studies of the disease defined cases based on classical criteria, such as flexural eczema. "Thus, the use of self-reported outcomes may be more sensitive and actually better suited for some studies of adult eczema."
The current study’s response rate of 70% is considered very good for mail-based surveys, he said. He noted that participants were geographically diverse, even though the study did not use random, population-based sampling.
Dr. Silverberg is a dermatologist at Northwestern University, Chicago. He reported that he has no financial conflicts of interest. These remarks were taken from his editorial accompanying Dr. Margolis’s report (JAMA Dermatol. 2014 April 3 [doi:10.1001/jamadermatol.2013.10267]).
The findings "challenge the conventional dogma that most childhood AD ‘burns out’ and dissipates by late adolescence and adulthood," said Dr. Jonathan I. Silverberg.
"It seems that children with eczema do not really outgrow their eczema," he said. "Rather, eczema seems to be a lifelong disease with a variable phenotype and/or reduced expression in adulthood."
Adult eczema’s diverse symptoms could have led researchers to underestimate its prevalence, said Dr. Silverberg, adding that many clinical studies of the disease defined cases based on classical criteria, such as flexural eczema. "Thus, the use of self-reported outcomes may be more sensitive and actually better suited for some studies of adult eczema."
The current study’s response rate of 70% is considered very good for mail-based surveys, he said. He noted that participants were geographically diverse, even though the study did not use random, population-based sampling.
Dr. Silverberg is a dermatologist at Northwestern University, Chicago. He reported that he has no financial conflicts of interest. These remarks were taken from his editorial accompanying Dr. Margolis’s report (JAMA Dermatol. 2014 April 3 [doi:10.1001/jamadermatol.2013.10267]).
Contrary to conventional wisdom, atopic dermatitis persists well into the second decade of life and probably longer, based on data from a prospective longitudinal study published online April 3 in JAMA Dermatology.
At every age studied (2-26 years), more than 80% of patients reported having symptoms of atopic dermatitis (AD) or taking AD medication in the past 6 months, reported Dr. David J. Margolis and his associates at the University of Pennsylvania, Philadelphia.
"Past teaching that nearly 50%-70% of children with AD will achieve a resolution of their AD by age 12 years was not achieved in our study," the researchers said (JAMA Dermatol. 2014 April 3 [doi:10.1001/ jamadermatol.2013.10271]).
The study enrolled 7,157 children aged 2-17 years who had physician-diagnosed mild to moderate AD. In a Kaplan-Meier survival estimate of 2,416 patients followed for at least 5 years, only by age 20 years did 50% of patients report at least 6 months free of AD symptoms or medications.
The findings are consistent with recent data suggesting that AD or eczema affects similar proportions of children and adults in the United States, the researchers said.
The study was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases and by Valeant Pharmaceuticals, maker of the atopic dermatitis drug pimecrolimus. The authors reported no individual conflicts of interest.
Contrary to conventional wisdom, atopic dermatitis persists well into the second decade of life and probably longer, based on data from a prospective longitudinal study published online April 3 in JAMA Dermatology.
At every age studied (2-26 years), more than 80% of patients reported having symptoms of atopic dermatitis (AD) or taking AD medication in the past 6 months, reported Dr. David J. Margolis and his associates at the University of Pennsylvania, Philadelphia.
"Past teaching that nearly 50%-70% of children with AD will achieve a resolution of their AD by age 12 years was not achieved in our study," the researchers said (JAMA Dermatol. 2014 April 3 [doi:10.1001/ jamadermatol.2013.10271]).
The study enrolled 7,157 children aged 2-17 years who had physician-diagnosed mild to moderate AD. In a Kaplan-Meier survival estimate of 2,416 patients followed for at least 5 years, only by age 20 years did 50% of patients report at least 6 months free of AD symptoms or medications.
The findings are consistent with recent data suggesting that AD or eczema affects similar proportions of children and adults in the United States, the researchers said.
The study was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases and by Valeant Pharmaceuticals, maker of the atopic dermatitis drug pimecrolimus. The authors reported no individual conflicts of interest.
FROM JAMA DERMATOLOGY
Major finding: At every age studied (2-26 years), more than 80% of participants had experienced symptoms of atopic dermatitis or had used atopic dermatitis medication in the past 6 months.
Data source: A prospective longitudinal cohort study of 7,157 patients with atopic dermatitis who were aged 2-17 years at enrollment.
Disclosures: The study was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases and by Valeant Pharmaceuticals, maker of the atopic dermatitis drug pimecrolimus. The authors reported no individual conflicts of interest.
Psoriatic pruritus improves with TrkA-blocking drug
An investigational topical drug that targets a nerve sensitization pathway showed good efficacy against chronic itch in patients with pruritic psoriasis.
CT327 works by inhibiting the kinase pathway of TrkA, a receptor that controls uptake of nerve growth factor, Dr. Gil Yosipovitch said at the annual meeting of the American Academy of Dermatology.
In a placebo-controlled trial of 160 patients with pruritic psoriasis, CT327 reduced itch by a mean of 60%, said Dr. Yosipovitch, chair of dermatology at the Temple University, Philadelphia.
"Sixty percent is not 90%," he said. "But when we consider that most of the antipruritic drugs have about a 15% efficacy in comparison to a vehicle, this is an effect we should consider. It’s well tolerated, and there were no application site reactions. I see potential in this drug."
CT327, developed by Creabilis, is a selective kinase inhibitor that targets TrkA, the receptor for nerve growth factor (NGF). NGF, which is overexpressed in atopic dermatitis and psoriatic skin, sensitizes neural networks that transmit the itch sensation to the brain, Dr. Yosipovitch said.
The phase IIb study comprised 160 adults with mild to moderate psoriasis, with at least a 10% body surface area involvement. Their mean score on the modified Psoriasis Area and Severity Index (mPASI) was about 9. Most (97%) had pruritus; 69% reported this as at least moderate, with a score of 40 on a 100-point visual analog scale (VAS). A third of the group reported severe pruritus, with a VAS of more than 70. They were randomized to four groups: an emollient placebo or CT327 in concentrations of 0.05%, 0.1%, or 0.5%.
The primary endpoint was reduction on a validated 100-point pruritus visual analog scale. The secondary endpoint was change on the mPASI.
By the end of 8 weeks, most patients (108) had experienced a significant reduction on the pruritus VAS. Reductions were about 35 points for the 0.1% group and 38 points for the 0.05% and 0.5% groups. These changes represented about a 60% decrease overall, Dr. Yosipovitch said.
Changes began early and continued along a steep trajectory, reaching a significant difference from baseline by week 2. However, he pointed out, by that point, the placebo group was similarly improved. This was probably because of the benefit of the daily emollient vehicle, he noted.
The curves began to separate shortly thereafter. By week 4, all of the treatment groups had experienced about a 30-point reduction on the VAS, while the placebo group had actually increased its score slightly. The active groups continued to improve over the next 4 weeks, while the placebo group plateaued at about a 20-point decrease from baseline.
The study concluded with 4 weeks of non–treatment follow-up. During that time, the placebo group stayed at about a 20-point decline and the active groups rebounded to about that level, "suggesting that there really is something going on here biologically," Dr. Yosipovitch said.
Patients using CT327 also experienced significant – although not overwhelming – benefit on the mPASI score. About 45% responded at the 50% level, compared with 23% of those using the emollient placebo. The active groups had similar improvements, with reductions on the mPASI of between 3 and 4 points. Those in the placebo group experienced about a 1-point improvement.
Dr. Yosipovitch said he was "not too impressed" with the mPASI changes. However, he noted, "Breaking the itch-scratch cycle at its source may have broader effects on other symptoms of psoriasis."
Based on the study results, Creabilis has decided to move forward into phase III testing with the 0.05% concentration. "Preparations are already underway," Dr. Yosipovitch said in an interview. "We also have a second phase IIb study ongoing that is investigating CT327 in patients with atopic dermatitis, which will be used to help finalize our phase III programs. This study should be completed in the middle of this year."
The NGF/TrkA pathway is implicated in other disorders as well, Dr. Yosipovitch said. CT327 might be investigated in some of these.
"These include disorders [in which] pruritus is important, such as pruritus with cutaneous T cell lymphoma, where NGF/TrkA has been shown to play a role. The pathway has also been shown to play an important role in chronic neuropathic and inflammatory pain. Molecules targeting NGF have already shown clinical efficacy."
Creabilis is investigating a related molecule, CT340, an inhibitor of both TrkA and MAP2K kinase, for treating those chronic pain conditions.
Creabilis funded the study. Dr. Yosipovitch is on the company’s scientific advisory board.
On Twitter @alz_gal
An investigational topical drug that targets a nerve sensitization pathway showed good efficacy against chronic itch in patients with pruritic psoriasis.
CT327 works by inhibiting the kinase pathway of TrkA, a receptor that controls uptake of nerve growth factor, Dr. Gil Yosipovitch said at the annual meeting of the American Academy of Dermatology.
In a placebo-controlled trial of 160 patients with pruritic psoriasis, CT327 reduced itch by a mean of 60%, said Dr. Yosipovitch, chair of dermatology at the Temple University, Philadelphia.
"Sixty percent is not 90%," he said. "But when we consider that most of the antipruritic drugs have about a 15% efficacy in comparison to a vehicle, this is an effect we should consider. It’s well tolerated, and there were no application site reactions. I see potential in this drug."
CT327, developed by Creabilis, is a selective kinase inhibitor that targets TrkA, the receptor for nerve growth factor (NGF). NGF, which is overexpressed in atopic dermatitis and psoriatic skin, sensitizes neural networks that transmit the itch sensation to the brain, Dr. Yosipovitch said.
The phase IIb study comprised 160 adults with mild to moderate psoriasis, with at least a 10% body surface area involvement. Their mean score on the modified Psoriasis Area and Severity Index (mPASI) was about 9. Most (97%) had pruritus; 69% reported this as at least moderate, with a score of 40 on a 100-point visual analog scale (VAS). A third of the group reported severe pruritus, with a VAS of more than 70. They were randomized to four groups: an emollient placebo or CT327 in concentrations of 0.05%, 0.1%, or 0.5%.
The primary endpoint was reduction on a validated 100-point pruritus visual analog scale. The secondary endpoint was change on the mPASI.
By the end of 8 weeks, most patients (108) had experienced a significant reduction on the pruritus VAS. Reductions were about 35 points for the 0.1% group and 38 points for the 0.05% and 0.5% groups. These changes represented about a 60% decrease overall, Dr. Yosipovitch said.
Changes began early and continued along a steep trajectory, reaching a significant difference from baseline by week 2. However, he pointed out, by that point, the placebo group was similarly improved. This was probably because of the benefit of the daily emollient vehicle, he noted.
The curves began to separate shortly thereafter. By week 4, all of the treatment groups had experienced about a 30-point reduction on the VAS, while the placebo group had actually increased its score slightly. The active groups continued to improve over the next 4 weeks, while the placebo group plateaued at about a 20-point decrease from baseline.
The study concluded with 4 weeks of non–treatment follow-up. During that time, the placebo group stayed at about a 20-point decline and the active groups rebounded to about that level, "suggesting that there really is something going on here biologically," Dr. Yosipovitch said.
Patients using CT327 also experienced significant – although not overwhelming – benefit on the mPASI score. About 45% responded at the 50% level, compared with 23% of those using the emollient placebo. The active groups had similar improvements, with reductions on the mPASI of between 3 and 4 points. Those in the placebo group experienced about a 1-point improvement.
Dr. Yosipovitch said he was "not too impressed" with the mPASI changes. However, he noted, "Breaking the itch-scratch cycle at its source may have broader effects on other symptoms of psoriasis."
Based on the study results, Creabilis has decided to move forward into phase III testing with the 0.05% concentration. "Preparations are already underway," Dr. Yosipovitch said in an interview. "We also have a second phase IIb study ongoing that is investigating CT327 in patients with atopic dermatitis, which will be used to help finalize our phase III programs. This study should be completed in the middle of this year."
The NGF/TrkA pathway is implicated in other disorders as well, Dr. Yosipovitch said. CT327 might be investigated in some of these.
"These include disorders [in which] pruritus is important, such as pruritus with cutaneous T cell lymphoma, where NGF/TrkA has been shown to play a role. The pathway has also been shown to play an important role in chronic neuropathic and inflammatory pain. Molecules targeting NGF have already shown clinical efficacy."
Creabilis is investigating a related molecule, CT340, an inhibitor of both TrkA and MAP2K kinase, for treating those chronic pain conditions.
Creabilis funded the study. Dr. Yosipovitch is on the company’s scientific advisory board.
On Twitter @alz_gal
An investigational topical drug that targets a nerve sensitization pathway showed good efficacy against chronic itch in patients with pruritic psoriasis.
CT327 works by inhibiting the kinase pathway of TrkA, a receptor that controls uptake of nerve growth factor, Dr. Gil Yosipovitch said at the annual meeting of the American Academy of Dermatology.
In a placebo-controlled trial of 160 patients with pruritic psoriasis, CT327 reduced itch by a mean of 60%, said Dr. Yosipovitch, chair of dermatology at the Temple University, Philadelphia.
"Sixty percent is not 90%," he said. "But when we consider that most of the antipruritic drugs have about a 15% efficacy in comparison to a vehicle, this is an effect we should consider. It’s well tolerated, and there were no application site reactions. I see potential in this drug."
CT327, developed by Creabilis, is a selective kinase inhibitor that targets TrkA, the receptor for nerve growth factor (NGF). NGF, which is overexpressed in atopic dermatitis and psoriatic skin, sensitizes neural networks that transmit the itch sensation to the brain, Dr. Yosipovitch said.
The phase IIb study comprised 160 adults with mild to moderate psoriasis, with at least a 10% body surface area involvement. Their mean score on the modified Psoriasis Area and Severity Index (mPASI) was about 9. Most (97%) had pruritus; 69% reported this as at least moderate, with a score of 40 on a 100-point visual analog scale (VAS). A third of the group reported severe pruritus, with a VAS of more than 70. They were randomized to four groups: an emollient placebo or CT327 in concentrations of 0.05%, 0.1%, or 0.5%.
The primary endpoint was reduction on a validated 100-point pruritus visual analog scale. The secondary endpoint was change on the mPASI.
By the end of 8 weeks, most patients (108) had experienced a significant reduction on the pruritus VAS. Reductions were about 35 points for the 0.1% group and 38 points for the 0.05% and 0.5% groups. These changes represented about a 60% decrease overall, Dr. Yosipovitch said.
Changes began early and continued along a steep trajectory, reaching a significant difference from baseline by week 2. However, he pointed out, by that point, the placebo group was similarly improved. This was probably because of the benefit of the daily emollient vehicle, he noted.
The curves began to separate shortly thereafter. By week 4, all of the treatment groups had experienced about a 30-point reduction on the VAS, while the placebo group had actually increased its score slightly. The active groups continued to improve over the next 4 weeks, while the placebo group plateaued at about a 20-point decrease from baseline.
The study concluded with 4 weeks of non–treatment follow-up. During that time, the placebo group stayed at about a 20-point decline and the active groups rebounded to about that level, "suggesting that there really is something going on here biologically," Dr. Yosipovitch said.
Patients using CT327 also experienced significant – although not overwhelming – benefit on the mPASI score. About 45% responded at the 50% level, compared with 23% of those using the emollient placebo. The active groups had similar improvements, with reductions on the mPASI of between 3 and 4 points. Those in the placebo group experienced about a 1-point improvement.
Dr. Yosipovitch said he was "not too impressed" with the mPASI changes. However, he noted, "Breaking the itch-scratch cycle at its source may have broader effects on other symptoms of psoriasis."
Based on the study results, Creabilis has decided to move forward into phase III testing with the 0.05% concentration. "Preparations are already underway," Dr. Yosipovitch said in an interview. "We also have a second phase IIb study ongoing that is investigating CT327 in patients with atopic dermatitis, which will be used to help finalize our phase III programs. This study should be completed in the middle of this year."
The NGF/TrkA pathway is implicated in other disorders as well, Dr. Yosipovitch said. CT327 might be investigated in some of these.
"These include disorders [in which] pruritus is important, such as pruritus with cutaneous T cell lymphoma, where NGF/TrkA has been shown to play a role. The pathway has also been shown to play an important role in chronic neuropathic and inflammatory pain. Molecules targeting NGF have already shown clinical efficacy."
Creabilis is investigating a related molecule, CT340, an inhibitor of both TrkA and MAP2K kinase, for treating those chronic pain conditions.
Creabilis funded the study. Dr. Yosipovitch is on the company’s scientific advisory board.
On Twitter @alz_gal
AT THE AAD ANNUAL MEETING
Major finding: The investigational drug CT327 reduced pruritus scores by 60% in a group of pruritic psoriatic patients.
Data source: The randomized, placebo-controlled trial of 160 patients.
Disclosures: Creabilis funded the study. Dr. Gil Yosipovitch is on the company’s scientific advisory board.
Investigational antibody improves itching, quality of life in severe atopic dermatitis
DENVER – The investigational drug REGN668, a monoclonal antibody that modulates interleukins 4 and 13, significantly improved overall quality of life and pruritus in patients with severe, longstanding atopic dermatitis.
Improvements for patients in the REGN668 (dupilumab) group were obvious in the first week of treatment and achieved statistical significance by week 2 of the placebo-controlled trial, Dr. Thomas Luger said at the annual meeting of the American Academy of Dermatology.
The drug probably works in two ways, said Dr. Luger of the University of Munster, Germany.
"It can be explained by its down-regulation of the immune mediators IL 4 and IL 13, but another issue is also important. IL4 has been shown to impair barrier function by down-modulating filaggrin expression," he noted.
The 12-week, multicenter trial randomized 109 patients to weekly injections of 300 mg dupilumab or placebo. The primary endpoint was the percent change in the Eczema Area and Severity Index (EASI) scale. Secondary endpoints were change from baseline in measures of pruritus and the proportion of patients who reached the EASI 50-75 level.
On the primary endpoint of change in EASI, dupilumab was associated with significantly greater improvement than placebo. By the end of week 1, the EASI had already dropped 10%, Dr. Luger said. By week 2, it had decreased 40% from baseline compared with placebo, and the improvement continued each week, then plateaued at about 7 weeks, with a mean decrease of 74%.
Changes in pruritus and overall quality of life were secondary endpoints for 65 patients who completed the quality of life portion. The validated survey was only available in French and German, and some of the study participants did not speak those languages.
The secondary exploratory endpoint was the change in baseline on the Quality of Life Index for Atopic Dermatitis, a 25-question survey, and its association with clinical outcomes. A higher score indicates worse quality of life; a two- to three-point change is considered clinically significant.
All of the patients were white; mean age was about 40 years and the mean disease duration was 28 years. The mean baseline EASI score was 27, indicating moderate severity. Scores on measures of pruritus indicated severe itching. The mean QoLIAD score was 12, with 25 being the worst.
The quality of life score followed the same pattern as the primary endpoint, Dr. Luger said. The average quality of life score had improved by approximately 25% after 1 week and by approximately 45% after 2 weeks. The score continued to drop, plateauing at an 80% decrease by week 7 and maintaining that level through the end of the treatment period. Almost all treated patients (91%) achieved at least at 50% reduction in symptoms, with 69% achieving at least a 75% reduction.
Pruritus was measured in two QoLIAD subscales: the numeric rating scale (NRS) and the 5-D itch scale. Itching began to improve early and continued to do so until it reached a maximum reduction of 65% in week 8 on the NRS. By week 12, the mean reduction in itching was 60%.
The 5-D scale followed a similar pattern, with early improvement that reached a maximum 50% reduction by week 8. By week 12, the mean reduction from baseline was 45%.
The overall QoLIAD score reflected these changes, improving by a mean of 6.5 points by week 12. In the placebo group, this score remained virtually unchanged. QoLIAD improvements strongly correlated with clinical improvements, Dr. Luger added.
Dupilumab was well tolerated overall. The most frequent adverse events were nasopharyngitis, headache, and conjunctivitis. Skin infections occurred in significantly fewer patients taking the drug, compared with placebo (5.5% vs. 24%). There were no infection-related serious adverse events or eczema herpeticum occurrences in the dupilumab group.
Sanofi and Regeneron, which are jointly developing dupilumab, sponsored the study. Dr. Luger is a consultant for numerous pharmaceutical companies but not for Sanofi or Regeneron.
On Twitter @alz_gal
DENVER – The investigational drug REGN668, a monoclonal antibody that modulates interleukins 4 and 13, significantly improved overall quality of life and pruritus in patients with severe, longstanding atopic dermatitis.
Improvements for patients in the REGN668 (dupilumab) group were obvious in the first week of treatment and achieved statistical significance by week 2 of the placebo-controlled trial, Dr. Thomas Luger said at the annual meeting of the American Academy of Dermatology.
The drug probably works in two ways, said Dr. Luger of the University of Munster, Germany.
"It can be explained by its down-regulation of the immune mediators IL 4 and IL 13, but another issue is also important. IL4 has been shown to impair barrier function by down-modulating filaggrin expression," he noted.
The 12-week, multicenter trial randomized 109 patients to weekly injections of 300 mg dupilumab or placebo. The primary endpoint was the percent change in the Eczema Area and Severity Index (EASI) scale. Secondary endpoints were change from baseline in measures of pruritus and the proportion of patients who reached the EASI 50-75 level.
On the primary endpoint of change in EASI, dupilumab was associated with significantly greater improvement than placebo. By the end of week 1, the EASI had already dropped 10%, Dr. Luger said. By week 2, it had decreased 40% from baseline compared with placebo, and the improvement continued each week, then plateaued at about 7 weeks, with a mean decrease of 74%.
Changes in pruritus and overall quality of life were secondary endpoints for 65 patients who completed the quality of life portion. The validated survey was only available in French and German, and some of the study participants did not speak those languages.
The secondary exploratory endpoint was the change in baseline on the Quality of Life Index for Atopic Dermatitis, a 25-question survey, and its association with clinical outcomes. A higher score indicates worse quality of life; a two- to three-point change is considered clinically significant.
All of the patients were white; mean age was about 40 years and the mean disease duration was 28 years. The mean baseline EASI score was 27, indicating moderate severity. Scores on measures of pruritus indicated severe itching. The mean QoLIAD score was 12, with 25 being the worst.
The quality of life score followed the same pattern as the primary endpoint, Dr. Luger said. The average quality of life score had improved by approximately 25% after 1 week and by approximately 45% after 2 weeks. The score continued to drop, plateauing at an 80% decrease by week 7 and maintaining that level through the end of the treatment period. Almost all treated patients (91%) achieved at least at 50% reduction in symptoms, with 69% achieving at least a 75% reduction.
Pruritus was measured in two QoLIAD subscales: the numeric rating scale (NRS) and the 5-D itch scale. Itching began to improve early and continued to do so until it reached a maximum reduction of 65% in week 8 on the NRS. By week 12, the mean reduction in itching was 60%.
The 5-D scale followed a similar pattern, with early improvement that reached a maximum 50% reduction by week 8. By week 12, the mean reduction from baseline was 45%.
The overall QoLIAD score reflected these changes, improving by a mean of 6.5 points by week 12. In the placebo group, this score remained virtually unchanged. QoLIAD improvements strongly correlated with clinical improvements, Dr. Luger added.
Dupilumab was well tolerated overall. The most frequent adverse events were nasopharyngitis, headache, and conjunctivitis. Skin infections occurred in significantly fewer patients taking the drug, compared with placebo (5.5% vs. 24%). There were no infection-related serious adverse events or eczema herpeticum occurrences in the dupilumab group.
Sanofi and Regeneron, which are jointly developing dupilumab, sponsored the study. Dr. Luger is a consultant for numerous pharmaceutical companies but not for Sanofi or Regeneron.
On Twitter @alz_gal
DENVER – The investigational drug REGN668, a monoclonal antibody that modulates interleukins 4 and 13, significantly improved overall quality of life and pruritus in patients with severe, longstanding atopic dermatitis.
Improvements for patients in the REGN668 (dupilumab) group were obvious in the first week of treatment and achieved statistical significance by week 2 of the placebo-controlled trial, Dr. Thomas Luger said at the annual meeting of the American Academy of Dermatology.
The drug probably works in two ways, said Dr. Luger of the University of Munster, Germany.
"It can be explained by its down-regulation of the immune mediators IL 4 and IL 13, but another issue is also important. IL4 has been shown to impair barrier function by down-modulating filaggrin expression," he noted.
The 12-week, multicenter trial randomized 109 patients to weekly injections of 300 mg dupilumab or placebo. The primary endpoint was the percent change in the Eczema Area and Severity Index (EASI) scale. Secondary endpoints were change from baseline in measures of pruritus and the proportion of patients who reached the EASI 50-75 level.
On the primary endpoint of change in EASI, dupilumab was associated with significantly greater improvement than placebo. By the end of week 1, the EASI had already dropped 10%, Dr. Luger said. By week 2, it had decreased 40% from baseline compared with placebo, and the improvement continued each week, then plateaued at about 7 weeks, with a mean decrease of 74%.
Changes in pruritus and overall quality of life were secondary endpoints for 65 patients who completed the quality of life portion. The validated survey was only available in French and German, and some of the study participants did not speak those languages.
The secondary exploratory endpoint was the change in baseline on the Quality of Life Index for Atopic Dermatitis, a 25-question survey, and its association with clinical outcomes. A higher score indicates worse quality of life; a two- to three-point change is considered clinically significant.
All of the patients were white; mean age was about 40 years and the mean disease duration was 28 years. The mean baseline EASI score was 27, indicating moderate severity. Scores on measures of pruritus indicated severe itching. The mean QoLIAD score was 12, with 25 being the worst.
The quality of life score followed the same pattern as the primary endpoint, Dr. Luger said. The average quality of life score had improved by approximately 25% after 1 week and by approximately 45% after 2 weeks. The score continued to drop, plateauing at an 80% decrease by week 7 and maintaining that level through the end of the treatment period. Almost all treated patients (91%) achieved at least at 50% reduction in symptoms, with 69% achieving at least a 75% reduction.
Pruritus was measured in two QoLIAD subscales: the numeric rating scale (NRS) and the 5-D itch scale. Itching began to improve early and continued to do so until it reached a maximum reduction of 65% in week 8 on the NRS. By week 12, the mean reduction in itching was 60%.
The 5-D scale followed a similar pattern, with early improvement that reached a maximum 50% reduction by week 8. By week 12, the mean reduction from baseline was 45%.
The overall QoLIAD score reflected these changes, improving by a mean of 6.5 points by week 12. In the placebo group, this score remained virtually unchanged. QoLIAD improvements strongly correlated with clinical improvements, Dr. Luger added.
Dupilumab was well tolerated overall. The most frequent adverse events were nasopharyngitis, headache, and conjunctivitis. Skin infections occurred in significantly fewer patients taking the drug, compared with placebo (5.5% vs. 24%). There were no infection-related serious adverse events or eczema herpeticum occurrences in the dupilumab group.
Sanofi and Regeneron, which are jointly developing dupilumab, sponsored the study. Dr. Luger is a consultant for numerous pharmaceutical companies but not for Sanofi or Regeneron.
On Twitter @alz_gal
AT THE AAD ANNUAL MEETING
Major finding: Compared with placebo, the investigational monoclonal antibody dupilumab reduced pruritus by up to 65% and improved quality of life scores by 80%.
Data source: The placebo-controlled study involved 109 patients, 65 of whom completed quality of life measures.
Disclosures: Sanofi and Regeneron, which are jointly developing dupilumab, sponsored the study. Dr. Luger is a consultant for numerous pharmaceutical companies, but not for Sanofi or Regeneron.
Phytoestrogens may prevent, treat asthma and allergy
SAN DIEGO – Could increased consumption of phytoestrogens help prevent or treat asthma and allergy?
Dr. Jessica Savage, an allergist and immunologist at Brigham and Women’s Hospital, Boston, and her colleagues correlated one-time urinary phytoestrogen measurements from 7,909 subjects in the National Health and Nutrition Examination Survey, 2003-2010, with histories of physician-diagnosed asthma and self-reported wheezing.
The investigators also considered serum total and specific IgE levels obtained from a subset of 2,218 subjects. They defined atopy as having at least one positive IgE level (0.35 kU/L or above) to an aeroallergen.
Adjusting for a wide range of potential cofounders, including age, gender, race, urinary creatinine, poverty, body mass index, smoking, and smoke exposure, they found that, for every natural log increase in urinary enterolactone, the odds of asthma decreased by 8%. Enterolactone also was significantly inversely associated with asthma prevalence and had the strongest inverse association with wheezing.
For every natural log increase in urinary o-desmethylangolensin (ODMA), there was a 7% decrease in the odds of wheeze. The odds of atopy significantly decreased with increasing ODMA levels.
"We can’t say anything about cause and effect" yet, but if the association holds up with further investigation, it might suggest a role for phytoestrogen probiotics to help treat the conditions, Dr. Savagesaid at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
Phytoestrogens are plant-derived compounds. Gut bacteria convert lignans, which are particularly plentiful in flax seeds, into enterolactone, and the isoflavone daidzein, particularly plentiful in soybeans, into ODMA.
"Increased consumption of sources of phytoestrogens or probiotics to increase precursor conversion may help prevent or treat asthma and allergic disease," Dr. Savage said. "I was really surprised that the enterolactone signal is very strong both for asthma and for wheezing."
Although soy-derived compounds have been associated with better lung function and decreased lung symptoms in the past, "there’s really not a lot known about enterolactone," she said. "The idea is that somehow these metabolites are anti-inflammatory. Urinary levels are partly due to your diet and partly to having the right bacterial flora in your gut. Our findings could be explained by people just having different diets; they could also be explained by people with asthma having lower levels of the right kind of bacteria."
About half the subjects were female, and about 80% were over age 18 years; 70% of the study population was white.
Enterolactone tertiles were defined as 0.2-178; 179-644; and 645-122,000 ng/mL urine. ODMA tertiles were defined as 0.1-1.4; 1.5-12.8; and 12.9-18,500 ng/mL urine.
The study was funded by the National Institutes of Health. The investigators reported no relevant disclosures.
SAN DIEGO – Could increased consumption of phytoestrogens help prevent or treat asthma and allergy?
Dr. Jessica Savage, an allergist and immunologist at Brigham and Women’s Hospital, Boston, and her colleagues correlated one-time urinary phytoestrogen measurements from 7,909 subjects in the National Health and Nutrition Examination Survey, 2003-2010, with histories of physician-diagnosed asthma and self-reported wheezing.
The investigators also considered serum total and specific IgE levels obtained from a subset of 2,218 subjects. They defined atopy as having at least one positive IgE level (0.35 kU/L or above) to an aeroallergen.
Adjusting for a wide range of potential cofounders, including age, gender, race, urinary creatinine, poverty, body mass index, smoking, and smoke exposure, they found that, for every natural log increase in urinary enterolactone, the odds of asthma decreased by 8%. Enterolactone also was significantly inversely associated with asthma prevalence and had the strongest inverse association with wheezing.
For every natural log increase in urinary o-desmethylangolensin (ODMA), there was a 7% decrease in the odds of wheeze. The odds of atopy significantly decreased with increasing ODMA levels.
"We can’t say anything about cause and effect" yet, but if the association holds up with further investigation, it might suggest a role for phytoestrogen probiotics to help treat the conditions, Dr. Savagesaid at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
Phytoestrogens are plant-derived compounds. Gut bacteria convert lignans, which are particularly plentiful in flax seeds, into enterolactone, and the isoflavone daidzein, particularly plentiful in soybeans, into ODMA.
"Increased consumption of sources of phytoestrogens or probiotics to increase precursor conversion may help prevent or treat asthma and allergic disease," Dr. Savage said. "I was really surprised that the enterolactone signal is very strong both for asthma and for wheezing."
Although soy-derived compounds have been associated with better lung function and decreased lung symptoms in the past, "there’s really not a lot known about enterolactone," she said. "The idea is that somehow these metabolites are anti-inflammatory. Urinary levels are partly due to your diet and partly to having the right bacterial flora in your gut. Our findings could be explained by people just having different diets; they could also be explained by people with asthma having lower levels of the right kind of bacteria."
About half the subjects were female, and about 80% were over age 18 years; 70% of the study population was white.
Enterolactone tertiles were defined as 0.2-178; 179-644; and 645-122,000 ng/mL urine. ODMA tertiles were defined as 0.1-1.4; 1.5-12.8; and 12.9-18,500 ng/mL urine.
The study was funded by the National Institutes of Health. The investigators reported no relevant disclosures.
SAN DIEGO – Could increased consumption of phytoestrogens help prevent or treat asthma and allergy?
Dr. Jessica Savage, an allergist and immunologist at Brigham and Women’s Hospital, Boston, and her colleagues correlated one-time urinary phytoestrogen measurements from 7,909 subjects in the National Health and Nutrition Examination Survey, 2003-2010, with histories of physician-diagnosed asthma and self-reported wheezing.
The investigators also considered serum total and specific IgE levels obtained from a subset of 2,218 subjects. They defined atopy as having at least one positive IgE level (0.35 kU/L or above) to an aeroallergen.
Adjusting for a wide range of potential cofounders, including age, gender, race, urinary creatinine, poverty, body mass index, smoking, and smoke exposure, they found that, for every natural log increase in urinary enterolactone, the odds of asthma decreased by 8%. Enterolactone also was significantly inversely associated with asthma prevalence and had the strongest inverse association with wheezing.
For every natural log increase in urinary o-desmethylangolensin (ODMA), there was a 7% decrease in the odds of wheeze. The odds of atopy significantly decreased with increasing ODMA levels.
"We can’t say anything about cause and effect" yet, but if the association holds up with further investigation, it might suggest a role for phytoestrogen probiotics to help treat the conditions, Dr. Savagesaid at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
Phytoestrogens are plant-derived compounds. Gut bacteria convert lignans, which are particularly plentiful in flax seeds, into enterolactone, and the isoflavone daidzein, particularly plentiful in soybeans, into ODMA.
"Increased consumption of sources of phytoestrogens or probiotics to increase precursor conversion may help prevent or treat asthma and allergic disease," Dr. Savage said. "I was really surprised that the enterolactone signal is very strong both for asthma and for wheezing."
Although soy-derived compounds have been associated with better lung function and decreased lung symptoms in the past, "there’s really not a lot known about enterolactone," she said. "The idea is that somehow these metabolites are anti-inflammatory. Urinary levels are partly due to your diet and partly to having the right bacterial flora in your gut. Our findings could be explained by people just having different diets; they could also be explained by people with asthma having lower levels of the right kind of bacteria."
About half the subjects were female, and about 80% were over age 18 years; 70% of the study population was white.
Enterolactone tertiles were defined as 0.2-178; 179-644; and 645-122,000 ng/mL urine. ODMA tertiles were defined as 0.1-1.4; 1.5-12.8; and 12.9-18,500 ng/mL urine.
The study was funded by the National Institutes of Health. The investigators reported no relevant disclosures.
AT THE 2014 AAAAI ANNUAL MEETING
Major finding: For every natural log increase in urinary enterolactone, the odds of asthma decrease by 8%.
Data Source: The National Health and Nutrition Examination Survey 2003-2010.
Disclosures: The study was funded by the National Institutes of Health. The investigators reported no relevant disclosures.
VIDEO: Coffee Break 2: What did you learn at the meeting?
WAIKOLOA, HAWAII – Our editor, Heidi Splete, catches up with attendees at the Hawaii Dermatology Seminar to find out what they learned at the meeting that they will take back to their practices.
During a coffee break video interview, doctors said they enjoyed presentations on tips to treat fine lines around the eyes and mouth, the link between psoriasis and increased cardiovascular risks, and the "two Cs" of potential leather allergies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WAIKOLOA, HAWAII – Our editor, Heidi Splete, catches up with attendees at the Hawaii Dermatology Seminar to find out what they learned at the meeting that they will take back to their practices.
During a coffee break video interview, doctors said they enjoyed presentations on tips to treat fine lines around the eyes and mouth, the link between psoriasis and increased cardiovascular risks, and the "two Cs" of potential leather allergies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WAIKOLOA, HAWAII – Our editor, Heidi Splete, catches up with attendees at the Hawaii Dermatology Seminar to find out what they learned at the meeting that they will take back to their practices.
During a coffee break video interview, doctors said they enjoyed presentations on tips to treat fine lines around the eyes and mouth, the link between psoriasis and increased cardiovascular risks, and the "two Cs" of potential leather allergies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
FROM SDEF HAWAII DERMATOLOGY SYMPOSIUM
VIDEO: A new look at three common pediatric dermatology presentations
PALM BEACH, ARUBA – In a video interview at the Caribbean Dermatology Symposium, Dr. Fred Ghali gives expert analysis on how to approach new variants of hand-foot-mouth disease; car- and toilet-seat contact dermatitis; and off-label treatment of small hemangiomas.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
PALM BEACH, ARUBA – In a video interview at the Caribbean Dermatology Symposium, Dr. Fred Ghali gives expert analysis on how to approach new variants of hand-foot-mouth disease; car- and toilet-seat contact dermatitis; and off-label treatment of small hemangiomas.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
PALM BEACH, ARUBA – In a video interview at the Caribbean Dermatology Symposium, Dr. Fred Ghali gives expert analysis on how to approach new variants of hand-foot-mouth disease; car- and toilet-seat contact dermatitis; and off-label treatment of small hemangiomas.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ANALYSIS FROM THE CARIBBEAN DERMATOLOGY SYMPOSIUM
Perioral dermatitis and diet
Could it be the carbs?
In my practice, I have observed consistent improvements in recalcitrant perioral dermatitis when patients switch to low-carbohydrate diets. Several of my patients with perioral dermatitis that responded poorly to oral doxycycline, topical metronidazole, and topical tacrolimus – or recurred upon cessation of therapy – have proven to have gluten sensitivity or intolerance. Their skin condition improves when they go on a gluten-free diet. But I have also seen considerable improvements after patients undertake low-carbohydrate, high-protein diets, even if those patients have no diagnosed gluten sensitivity. These improvements have occurred with minimal oral and topical treatments, and these patients have not experienced recurrences.
There have been no well-controlled studies, or even case reports to my knowledge, linking carbohydrate or gluten intake to perioral dermatitis. Could the improvement be serendipitous, or is there some basis for carbohydrates contributing to inflammatory status in the oral and gastrointestinal mucosa?
Alcohol, spicy foods, and chocolate have been linked to exacerbation of erythemogenic and papulopustular rosacea. However, the precipitating ingredients in these foods have not been identified. Could the common link simply be an abundance of carbohydrates?
More studies are needed to better define the role of diet in perioral dermatitis. In the meantime, I am seeing good results with low-carb/carb-free diets and will continue to suggest them to prevent recurrences in my patients with perioral dermatitis.
Dr. Talakoub is in private practice in McLean, Va.
Could it be the carbs?
In my practice, I have observed consistent improvements in recalcitrant perioral dermatitis when patients switch to low-carbohydrate diets. Several of my patients with perioral dermatitis that responded poorly to oral doxycycline, topical metronidazole, and topical tacrolimus – or recurred upon cessation of therapy – have proven to have gluten sensitivity or intolerance. Their skin condition improves when they go on a gluten-free diet. But I have also seen considerable improvements after patients undertake low-carbohydrate, high-protein diets, even if those patients have no diagnosed gluten sensitivity. These improvements have occurred with minimal oral and topical treatments, and these patients have not experienced recurrences.
There have been no well-controlled studies, or even case reports to my knowledge, linking carbohydrate or gluten intake to perioral dermatitis. Could the improvement be serendipitous, or is there some basis for carbohydrates contributing to inflammatory status in the oral and gastrointestinal mucosa?
Alcohol, spicy foods, and chocolate have been linked to exacerbation of erythemogenic and papulopustular rosacea. However, the precipitating ingredients in these foods have not been identified. Could the common link simply be an abundance of carbohydrates?
More studies are needed to better define the role of diet in perioral dermatitis. In the meantime, I am seeing good results with low-carb/carb-free diets and will continue to suggest them to prevent recurrences in my patients with perioral dermatitis.
Dr. Talakoub is in private practice in McLean, Va.
Could it be the carbs?
In my practice, I have observed consistent improvements in recalcitrant perioral dermatitis when patients switch to low-carbohydrate diets. Several of my patients with perioral dermatitis that responded poorly to oral doxycycline, topical metronidazole, and topical tacrolimus – or recurred upon cessation of therapy – have proven to have gluten sensitivity or intolerance. Their skin condition improves when they go on a gluten-free diet. But I have also seen considerable improvements after patients undertake low-carbohydrate, high-protein diets, even if those patients have no diagnosed gluten sensitivity. These improvements have occurred with minimal oral and topical treatments, and these patients have not experienced recurrences.
There have been no well-controlled studies, or even case reports to my knowledge, linking carbohydrate or gluten intake to perioral dermatitis. Could the improvement be serendipitous, or is there some basis for carbohydrates contributing to inflammatory status in the oral and gastrointestinal mucosa?
Alcohol, spicy foods, and chocolate have been linked to exacerbation of erythemogenic and papulopustular rosacea. However, the precipitating ingredients in these foods have not been identified. Could the common link simply be an abundance of carbohydrates?
More studies are needed to better define the role of diet in perioral dermatitis. In the meantime, I am seeing good results with low-carb/carb-free diets and will continue to suggest them to prevent recurrences in my patients with perioral dermatitis.
Dr. Talakoub is in private practice in McLean, Va.