User login
The art and science of detecting allergic contact dermatitis
WOODINVILLE, WASH. – Identifying the culprit in allergic contact dermatitis often requires careful sleuthing and tailored patch testing, according to Dr. James G. Marks Jr.
Dr. Marks reviewed the current options for patch testing trays, described several cases of allergic contact dermatitis, and shared pointers for diagnosis and management at the annual Coastal Dermatology Symposium.
Allergen-screening series
Dermatologists can now choose from a variety of standard screening trays for patch testing, said Dr. Marks, professor and chair of the department of dermatology, Pennsylvania State University, Hershey. The TRUE test (the only one approved by the Food and Drug Administration) contains 35 antigens.
However, dermatologists can supplement and customize these screening trays to create a system specific to their practice and geographic area. Dr. Marks said he uses a customized tray with 100 antigens.
"The important point is there is no universal standard screening tray, so pick what works for you," he said.
"Those of you who use the TRUE test, great; as you know in the last year you have had another panel [added], so you get more screening antigens," he said. "It makes sense intuitively, and it’s proven by publications of the North American [Contact Dermatitis] Group and others that the more antigens you test with, the more positives you get and the more relevant reactions you can get," he explained.
"Then create your own," Dr. Marks advised. "So if you use the TRUE test, maybe supplement ... with a few more allergens."
Alpha-methylene-gamma-butyrolactone
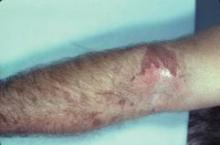
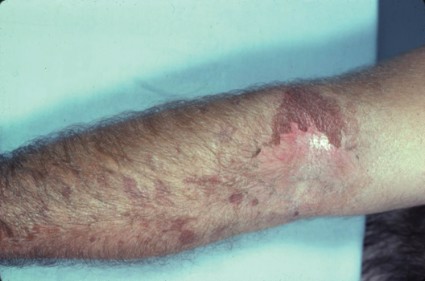
Florists may come in contact with alpha-methylene-gamma-butyrolactone through handling Alstroemeria (also known as Peruvian lily), a flower popular because of its long-lasting blooms.
The compound is found in the sap that leaks out from cut stems; thus, the presentation is typically finger dermatitis, Dr. Marks said at the meeting, which was presented by the Caribbean Dermatology Symposium.
"It is the most common cause of allergic contact dermatitis in florists. So if you see florists, this is the allergen until proven otherwise," he said.
The compound is also found in the white epidermis of tulips, in which case it is known as tuliposide A. About half of tulip bulb sorters are allergic to it.
"You either patch test with parts of the Alstroemeria plant or get the allergen alpha-methylene-gamma-butyrolactone" commercially, Dr. Marks said. "I test everyone to alpha-methylene-gamma-butyrolactone, even though it’s a small subset. That’s one of my 100 [antigens]."
Methylisothiazolinone
"Methylisothiazolinone has become a very important and hot allergen," Dr. Marks commented. This allergen is increasingly used in personal care products and requires a special approach to patch testing. It is found in many wet wipes, use of which can produce, for example, perioral dermatitis.
The standard combination test antigen, applied at 100 parts per million, contains 3 parts methylchloroisothiazolinone (MCI) and 1 part methylisothiazolinone (MI), he noted. Thus, "you are really only patch testing to 25 parts per million of MI."
"The recommended concentration of patch test to MI is a bit in flux," said Dr. Marks; the North American group currently uses 2,000 parts per million but is considering halving that number, he noted.
"You can see how you can miss patients who are allergic to MI if you only patch test to MCI/MI," he commented. "So those of you who are using the TRUE test, you are going to miss patients who are allergic to MI."
"The important point is you’ve got to test both – MCI/MI and MI alone. ... You should supplement what you are patch testing with MI, certainly at least at 1,000 parts per million, if not at 2,000," Dr. Marks advised.
"The Cosmetic Ingredient Review, which sets limits for [MI] in the U.S., is going to be reevaluating, and I’m sure will be having lower limits in leave-on and rinse-off products," he noted.
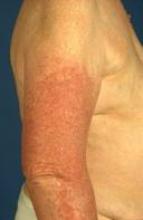
Rubber accelerators
Surgeons may develop particularly problematic allergic contact dermatitis as a reaction to the rubber accelerators used in the manufacturing of many surgical gloves, Dr. Marks noted.
He described the case of a surgeon who developed severe hand dermatitis and eventually a generalized dermatitis. "In this case, if you used the TRUE test, you would make the diagnosis; he was positive to thiuram and carba mix."
Allergen avoidance entailed finding an alternative, rubber-free surgical glove, the Derma Prene Ultra glove (manufactured by Ansell), which is made of neoprene. Also, the surgeon switched to vinyl exam gloves for outpatient care.
"There may be other surgical gloves ...," Dr. Marks acknowledged. "But be sure and keep this some place because some time in the future when you have your surgeon friend with hand dermatitis, you can recommend that glove after you patch test them and prove that they are rubber-accelerator positive."
Cocamidopropyl betaine contaminants
Patients may develop allergic contact dermatitis after using shampoos and bath gels containing cocamidopropyl betaine, a surfactant.
In fact, they are actually reacting to a contaminant or impurity generated in the manufacturing process, either 3-dimethylaminopropylamine or amidoamine, according to Dr. Marks.
"So if you have pure cocamidopropyl betaine, there will be no allergy," he noted. But if you test for "cocamidopropyl betaine, and what you are patch testing with is from, say, Chemotechnique or Allergeaze, it’s going to have presumably the contaminants or the impurities in it, 3-dimethylaminopropylamine and amidoamine."
Treatment entails careful reading of labels on personal care products and avoidance of those containing cocamidopropyl betaine.
Acrylates
Don’t rule out acrylates – either acrylic or methacrylic acid – monomers that are polymerized with heat or light to form solid plastics that can cause reactions.
"The monomers are both irritants and allergens, so you need the right concentration to patch test to," Dr. Marks noted. "They are found in all sorts of things – adhesives, inks, artificial nails, dental resins, bone cement, and plastics."
Presentations may vary widely, including, for example, finger dermatitis in patients who have sculptured nails, and gum stomatitis in patients who have undergone procedures involving dental resin, said Dr. Marks.
"If you have workers or patients who have exposure to acrylates, you need more extensive screening," Dr. Marks advised, noting that his acrylate patch test series contains six compounds.
"No one is a screen for all of them," he commented. "Some [experts] feel that ethyl acrylate is the best screen; certainly, for sculptured nails it’s good." Others in his series include methyl methacrylate (found in bone cement) and ethyl cyanoacrylate (found in Super Glue adhesive).
Glyceryl thioglycolate
Allergy to glyceryl thioglycolate, found in acid permanent waves, can manifest as hand dermatitis in hairdressers and as dermatitis of the face, neck, and ears in their clients.
"If you see hairdressers [in your practice], you should consider strongly having this antigen as part of your [patch test] armamentarium," Dr. Marks recommended.
Alkaline perms, by contrast, do not contain glyceryl thioglycolate and thus provide a simple solution. "You can cure that hairdresser, and she or he can continue to do perms just by switching from an acid to an alkaline perm," he explained.
Dr. Marks said he had no relevant financial disclosures.
WOODINVILLE, WASH. – Identifying the culprit in allergic contact dermatitis often requires careful sleuthing and tailored patch testing, according to Dr. James G. Marks Jr.
Dr. Marks reviewed the current options for patch testing trays, described several cases of allergic contact dermatitis, and shared pointers for diagnosis and management at the annual Coastal Dermatology Symposium.
Allergen-screening series
Dermatologists can now choose from a variety of standard screening trays for patch testing, said Dr. Marks, professor and chair of the department of dermatology, Pennsylvania State University, Hershey. The TRUE test (the only one approved by the Food and Drug Administration) contains 35 antigens.
However, dermatologists can supplement and customize these screening trays to create a system specific to their practice and geographic area. Dr. Marks said he uses a customized tray with 100 antigens.
"The important point is there is no universal standard screening tray, so pick what works for you," he said.
"Those of you who use the TRUE test, great; as you know in the last year you have had another panel [added], so you get more screening antigens," he said. "It makes sense intuitively, and it’s proven by publications of the North American [Contact Dermatitis] Group and others that the more antigens you test with, the more positives you get and the more relevant reactions you can get," he explained.
"Then create your own," Dr. Marks advised. "So if you use the TRUE test, maybe supplement ... with a few more allergens."
Alpha-methylene-gamma-butyrolactone
Florists may come in contact with alpha-methylene-gamma-butyrolactone through handling Alstroemeria (also known as Peruvian lily), a flower popular because of its long-lasting blooms.
The compound is found in the sap that leaks out from cut stems; thus, the presentation is typically finger dermatitis, Dr. Marks said at the meeting, which was presented by the Caribbean Dermatology Symposium.
"It is the most common cause of allergic contact dermatitis in florists. So if you see florists, this is the allergen until proven otherwise," he said.
The compound is also found in the white epidermis of tulips, in which case it is known as tuliposide A. About half of tulip bulb sorters are allergic to it.
"You either patch test with parts of the Alstroemeria plant or get the allergen alpha-methylene-gamma-butyrolactone" commercially, Dr. Marks said. "I test everyone to alpha-methylene-gamma-butyrolactone, even though it’s a small subset. That’s one of my 100 [antigens]."
Methylisothiazolinone
"Methylisothiazolinone has become a very important and hot allergen," Dr. Marks commented. This allergen is increasingly used in personal care products and requires a special approach to patch testing. It is found in many wet wipes, use of which can produce, for example, perioral dermatitis.
The standard combination test antigen, applied at 100 parts per million, contains 3 parts methylchloroisothiazolinone (MCI) and 1 part methylisothiazolinone (MI), he noted. Thus, "you are really only patch testing to 25 parts per million of MI."
"The recommended concentration of patch test to MI is a bit in flux," said Dr. Marks; the North American group currently uses 2,000 parts per million but is considering halving that number, he noted.
"You can see how you can miss patients who are allergic to MI if you only patch test to MCI/MI," he commented. "So those of you who are using the TRUE test, you are going to miss patients who are allergic to MI."
"The important point is you’ve got to test both – MCI/MI and MI alone. ... You should supplement what you are patch testing with MI, certainly at least at 1,000 parts per million, if not at 2,000," Dr. Marks advised.
"The Cosmetic Ingredient Review, which sets limits for [MI] in the U.S., is going to be reevaluating, and I’m sure will be having lower limits in leave-on and rinse-off products," he noted.
Rubber accelerators
Surgeons may develop particularly problematic allergic contact dermatitis as a reaction to the rubber accelerators used in the manufacturing of many surgical gloves, Dr. Marks noted.
He described the case of a surgeon who developed severe hand dermatitis and eventually a generalized dermatitis. "In this case, if you used the TRUE test, you would make the diagnosis; he was positive to thiuram and carba mix."
Allergen avoidance entailed finding an alternative, rubber-free surgical glove, the Derma Prene Ultra glove (manufactured by Ansell), which is made of neoprene. Also, the surgeon switched to vinyl exam gloves for outpatient care.
"There may be other surgical gloves ...," Dr. Marks acknowledged. "But be sure and keep this some place because some time in the future when you have your surgeon friend with hand dermatitis, you can recommend that glove after you patch test them and prove that they are rubber-accelerator positive."
Cocamidopropyl betaine contaminants
Patients may develop allergic contact dermatitis after using shampoos and bath gels containing cocamidopropyl betaine, a surfactant.
In fact, they are actually reacting to a contaminant or impurity generated in the manufacturing process, either 3-dimethylaminopropylamine or amidoamine, according to Dr. Marks.
"So if you have pure cocamidopropyl betaine, there will be no allergy," he noted. But if you test for "cocamidopropyl betaine, and what you are patch testing with is from, say, Chemotechnique or Allergeaze, it’s going to have presumably the contaminants or the impurities in it, 3-dimethylaminopropylamine and amidoamine."
Treatment entails careful reading of labels on personal care products and avoidance of those containing cocamidopropyl betaine.
Acrylates
Don’t rule out acrylates – either acrylic or methacrylic acid – monomers that are polymerized with heat or light to form solid plastics that can cause reactions.
"The monomers are both irritants and allergens, so you need the right concentration to patch test to," Dr. Marks noted. "They are found in all sorts of things – adhesives, inks, artificial nails, dental resins, bone cement, and plastics."
Presentations may vary widely, including, for example, finger dermatitis in patients who have sculptured nails, and gum stomatitis in patients who have undergone procedures involving dental resin, said Dr. Marks.
"If you have workers or patients who have exposure to acrylates, you need more extensive screening," Dr. Marks advised, noting that his acrylate patch test series contains six compounds.
"No one is a screen for all of them," he commented. "Some [experts] feel that ethyl acrylate is the best screen; certainly, for sculptured nails it’s good." Others in his series include methyl methacrylate (found in bone cement) and ethyl cyanoacrylate (found in Super Glue adhesive).
Glyceryl thioglycolate
Allergy to glyceryl thioglycolate, found in acid permanent waves, can manifest as hand dermatitis in hairdressers and as dermatitis of the face, neck, and ears in their clients.
"If you see hairdressers [in your practice], you should consider strongly having this antigen as part of your [patch test] armamentarium," Dr. Marks recommended.
Alkaline perms, by contrast, do not contain glyceryl thioglycolate and thus provide a simple solution. "You can cure that hairdresser, and she or he can continue to do perms just by switching from an acid to an alkaline perm," he explained.
Dr. Marks said he had no relevant financial disclosures.
WOODINVILLE, WASH. – Identifying the culprit in allergic contact dermatitis often requires careful sleuthing and tailored patch testing, according to Dr. James G. Marks Jr.
Dr. Marks reviewed the current options for patch testing trays, described several cases of allergic contact dermatitis, and shared pointers for diagnosis and management at the annual Coastal Dermatology Symposium.
Allergen-screening series
Dermatologists can now choose from a variety of standard screening trays for patch testing, said Dr. Marks, professor and chair of the department of dermatology, Pennsylvania State University, Hershey. The TRUE test (the only one approved by the Food and Drug Administration) contains 35 antigens.
However, dermatologists can supplement and customize these screening trays to create a system specific to their practice and geographic area. Dr. Marks said he uses a customized tray with 100 antigens.
"The important point is there is no universal standard screening tray, so pick what works for you," he said.
"Those of you who use the TRUE test, great; as you know in the last year you have had another panel [added], so you get more screening antigens," he said. "It makes sense intuitively, and it’s proven by publications of the North American [Contact Dermatitis] Group and others that the more antigens you test with, the more positives you get and the more relevant reactions you can get," he explained.
"Then create your own," Dr. Marks advised. "So if you use the TRUE test, maybe supplement ... with a few more allergens."
Alpha-methylene-gamma-butyrolactone
Florists may come in contact with alpha-methylene-gamma-butyrolactone through handling Alstroemeria (also known as Peruvian lily), a flower popular because of its long-lasting blooms.
The compound is found in the sap that leaks out from cut stems; thus, the presentation is typically finger dermatitis, Dr. Marks said at the meeting, which was presented by the Caribbean Dermatology Symposium.
"It is the most common cause of allergic contact dermatitis in florists. So if you see florists, this is the allergen until proven otherwise," he said.
The compound is also found in the white epidermis of tulips, in which case it is known as tuliposide A. About half of tulip bulb sorters are allergic to it.
"You either patch test with parts of the Alstroemeria plant or get the allergen alpha-methylene-gamma-butyrolactone" commercially, Dr. Marks said. "I test everyone to alpha-methylene-gamma-butyrolactone, even though it’s a small subset. That’s one of my 100 [antigens]."
Methylisothiazolinone
"Methylisothiazolinone has become a very important and hot allergen," Dr. Marks commented. This allergen is increasingly used in personal care products and requires a special approach to patch testing. It is found in many wet wipes, use of which can produce, for example, perioral dermatitis.
The standard combination test antigen, applied at 100 parts per million, contains 3 parts methylchloroisothiazolinone (MCI) and 1 part methylisothiazolinone (MI), he noted. Thus, "you are really only patch testing to 25 parts per million of MI."
"The recommended concentration of patch test to MI is a bit in flux," said Dr. Marks; the North American group currently uses 2,000 parts per million but is considering halving that number, he noted.
"You can see how you can miss patients who are allergic to MI if you only patch test to MCI/MI," he commented. "So those of you who are using the TRUE test, you are going to miss patients who are allergic to MI."
"The important point is you’ve got to test both – MCI/MI and MI alone. ... You should supplement what you are patch testing with MI, certainly at least at 1,000 parts per million, if not at 2,000," Dr. Marks advised.
"The Cosmetic Ingredient Review, which sets limits for [MI] in the U.S., is going to be reevaluating, and I’m sure will be having lower limits in leave-on and rinse-off products," he noted.
Rubber accelerators
Surgeons may develop particularly problematic allergic contact dermatitis as a reaction to the rubber accelerators used in the manufacturing of many surgical gloves, Dr. Marks noted.
He described the case of a surgeon who developed severe hand dermatitis and eventually a generalized dermatitis. "In this case, if you used the TRUE test, you would make the diagnosis; he was positive to thiuram and carba mix."
Allergen avoidance entailed finding an alternative, rubber-free surgical glove, the Derma Prene Ultra glove (manufactured by Ansell), which is made of neoprene. Also, the surgeon switched to vinyl exam gloves for outpatient care.
"There may be other surgical gloves ...," Dr. Marks acknowledged. "But be sure and keep this some place because some time in the future when you have your surgeon friend with hand dermatitis, you can recommend that glove after you patch test them and prove that they are rubber-accelerator positive."
Cocamidopropyl betaine contaminants
Patients may develop allergic contact dermatitis after using shampoos and bath gels containing cocamidopropyl betaine, a surfactant.
In fact, they are actually reacting to a contaminant or impurity generated in the manufacturing process, either 3-dimethylaminopropylamine or amidoamine, according to Dr. Marks.
"So if you have pure cocamidopropyl betaine, there will be no allergy," he noted. But if you test for "cocamidopropyl betaine, and what you are patch testing with is from, say, Chemotechnique or Allergeaze, it’s going to have presumably the contaminants or the impurities in it, 3-dimethylaminopropylamine and amidoamine."
Treatment entails careful reading of labels on personal care products and avoidance of those containing cocamidopropyl betaine.
Acrylates
Don’t rule out acrylates – either acrylic or methacrylic acid – monomers that are polymerized with heat or light to form solid plastics that can cause reactions.
"The monomers are both irritants and allergens, so you need the right concentration to patch test to," Dr. Marks noted. "They are found in all sorts of things – adhesives, inks, artificial nails, dental resins, bone cement, and plastics."
Presentations may vary widely, including, for example, finger dermatitis in patients who have sculptured nails, and gum stomatitis in patients who have undergone procedures involving dental resin, said Dr. Marks.
"If you have workers or patients who have exposure to acrylates, you need more extensive screening," Dr. Marks advised, noting that his acrylate patch test series contains six compounds.
"No one is a screen for all of them," he commented. "Some [experts] feel that ethyl acrylate is the best screen; certainly, for sculptured nails it’s good." Others in his series include methyl methacrylate (found in bone cement) and ethyl cyanoacrylate (found in Super Glue adhesive).
Glyceryl thioglycolate
Allergy to glyceryl thioglycolate, found in acid permanent waves, can manifest as hand dermatitis in hairdressers and as dermatitis of the face, neck, and ears in their clients.
"If you see hairdressers [in your practice], you should consider strongly having this antigen as part of your [patch test] armamentarium," Dr. Marks recommended.
Alkaline perms, by contrast, do not contain glyceryl thioglycolate and thus provide a simple solution. "You can cure that hairdresser, and she or he can continue to do perms just by switching from an acid to an alkaline perm," he explained.
Dr. Marks said he had no relevant financial disclosures.
AT THE COASTAL DERMATOLOGY SYMPOSIUM
Palm lines predict worse outcomes in atopic dermatitis
LAS VEGAS – Hyperlinearity in the palms of atopic dermatitis patients may indicate filaggrin deficiency, which increases the likelihood of more severe and persistent disease and associated problems, according to Dr. Jacob Thyssen of the National Allergy Research Centre at Copenhagen University.
"If you can’t genotype" the filaggrin gene to check for mutations that decrease or eliminate the protective skin barrier protein, "check the palms of your patients and their parents" for deeper, more numerous, and slightly erythematous lines, said Dr. Thyssen. "I do that routinely. It’s harder in children, but it gets easier as they age," he said at the Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar.
When such lines are present, it’s probably a good idea to tell patients they may have a more severe case of atopic dermatitis and need to follow treatments and advice carefully, he said.
Filaggrin mutations were first identified in 2006 as the cause of ichthyosis vulgaris – a related condition associated with palm and plantar hyperlinearity – plus keratosis pilaris and fissured, dry, and scaly skin. The mutations have since been identified in about half of all severe atopic dermatitis patients, and risk of the disease increases 150-fold in homozygous mutation carriers who produce no filaggrin at all. Perhaps 10% of people of European ancestry carry filaggrin mutations, and it is less common in people of Asian or African descent, Dr. Thyssen noted.
"The filaggrin deficient group needs emollients," perhaps more so than other atopic dermatitis patients, and may benefit less from anti-inflammatory treatments, he said. "Barrier restoration [with hypoallergenic emollients] should be the main issue," he emphasized.
Moisturizers with ceramides and filaggrin breakdown products haven’t been heavily promoted in Europe. Although they may be effective, at this point "we just try to use very lipid-rich emollients," Dr. Thyssen said.
Filaggrin mutations in atopic dermatitis patients have been associated with allergic rhinitis and asthma, Dr. Thyssen said. Deficient patients also may be more susceptible to sun sensitivity, skin dehydration, skin infections, food allergies, and. contact dermatitis. The reason for these associations remains unclear; perhaps allergens have an easier time penetrating the skin and engaging the immune system when filaggrin is lacking, he said. Patients also seem to self-select for careers that avoid skin irritants such as nickel, he added.
Research is ongoing to devise treatments that directly correct the deficiency. However, it is likely that atopic dermatitis represents "many diseases with one face," said Dr. Thyssen. "Today, we treat all cases the same, but we are beginning to dissect" the various factors at play in individuals, and may one day be able to individualize treatment approaches, he said.
Dr. Thyssen had no relevant disclosures.
SDEF and this news organization are owned by Frontline Medical Communications.
LAS VEGAS – Hyperlinearity in the palms of atopic dermatitis patients may indicate filaggrin deficiency, which increases the likelihood of more severe and persistent disease and associated problems, according to Dr. Jacob Thyssen of the National Allergy Research Centre at Copenhagen University.
"If you can’t genotype" the filaggrin gene to check for mutations that decrease or eliminate the protective skin barrier protein, "check the palms of your patients and their parents" for deeper, more numerous, and slightly erythematous lines, said Dr. Thyssen. "I do that routinely. It’s harder in children, but it gets easier as they age," he said at the Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar.
When such lines are present, it’s probably a good idea to tell patients they may have a more severe case of atopic dermatitis and need to follow treatments and advice carefully, he said.
Filaggrin mutations were first identified in 2006 as the cause of ichthyosis vulgaris – a related condition associated with palm and plantar hyperlinearity – plus keratosis pilaris and fissured, dry, and scaly skin. The mutations have since been identified in about half of all severe atopic dermatitis patients, and risk of the disease increases 150-fold in homozygous mutation carriers who produce no filaggrin at all. Perhaps 10% of people of European ancestry carry filaggrin mutations, and it is less common in people of Asian or African descent, Dr. Thyssen noted.
"The filaggrin deficient group needs emollients," perhaps more so than other atopic dermatitis patients, and may benefit less from anti-inflammatory treatments, he said. "Barrier restoration [with hypoallergenic emollients] should be the main issue," he emphasized.
Moisturizers with ceramides and filaggrin breakdown products haven’t been heavily promoted in Europe. Although they may be effective, at this point "we just try to use very lipid-rich emollients," Dr. Thyssen said.
Filaggrin mutations in atopic dermatitis patients have been associated with allergic rhinitis and asthma, Dr. Thyssen said. Deficient patients also may be more susceptible to sun sensitivity, skin dehydration, skin infections, food allergies, and. contact dermatitis. The reason for these associations remains unclear; perhaps allergens have an easier time penetrating the skin and engaging the immune system when filaggrin is lacking, he said. Patients also seem to self-select for careers that avoid skin irritants such as nickel, he added.
Research is ongoing to devise treatments that directly correct the deficiency. However, it is likely that atopic dermatitis represents "many diseases with one face," said Dr. Thyssen. "Today, we treat all cases the same, but we are beginning to dissect" the various factors at play in individuals, and may one day be able to individualize treatment approaches, he said.
Dr. Thyssen had no relevant disclosures.
SDEF and this news organization are owned by Frontline Medical Communications.
LAS VEGAS – Hyperlinearity in the palms of atopic dermatitis patients may indicate filaggrin deficiency, which increases the likelihood of more severe and persistent disease and associated problems, according to Dr. Jacob Thyssen of the National Allergy Research Centre at Copenhagen University.
"If you can’t genotype" the filaggrin gene to check for mutations that decrease or eliminate the protective skin barrier protein, "check the palms of your patients and their parents" for deeper, more numerous, and slightly erythematous lines, said Dr. Thyssen. "I do that routinely. It’s harder in children, but it gets easier as they age," he said at the Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar.
When such lines are present, it’s probably a good idea to tell patients they may have a more severe case of atopic dermatitis and need to follow treatments and advice carefully, he said.
Filaggrin mutations were first identified in 2006 as the cause of ichthyosis vulgaris – a related condition associated with palm and plantar hyperlinearity – plus keratosis pilaris and fissured, dry, and scaly skin. The mutations have since been identified in about half of all severe atopic dermatitis patients, and risk of the disease increases 150-fold in homozygous mutation carriers who produce no filaggrin at all. Perhaps 10% of people of European ancestry carry filaggrin mutations, and it is less common in people of Asian or African descent, Dr. Thyssen noted.
"The filaggrin deficient group needs emollients," perhaps more so than other atopic dermatitis patients, and may benefit less from anti-inflammatory treatments, he said. "Barrier restoration [with hypoallergenic emollients] should be the main issue," he emphasized.
Moisturizers with ceramides and filaggrin breakdown products haven’t been heavily promoted in Europe. Although they may be effective, at this point "we just try to use very lipid-rich emollients," Dr. Thyssen said.
Filaggrin mutations in atopic dermatitis patients have been associated with allergic rhinitis and asthma, Dr. Thyssen said. Deficient patients also may be more susceptible to sun sensitivity, skin dehydration, skin infections, food allergies, and. contact dermatitis. The reason for these associations remains unclear; perhaps allergens have an easier time penetrating the skin and engaging the immune system when filaggrin is lacking, he said. Patients also seem to self-select for careers that avoid skin irritants such as nickel, he added.
Research is ongoing to devise treatments that directly correct the deficiency. However, it is likely that atopic dermatitis represents "many diseases with one face," said Dr. Thyssen. "Today, we treat all cases the same, but we are beginning to dissect" the various factors at play in individuals, and may one day be able to individualize treatment approaches, he said.
Dr. Thyssen had no relevant disclosures.
SDEF and this news organization are owned by Frontline Medical Communications.
AT SDEF LAS VEGAS DERMATOLOGY SEMINAR
Canadian agency reports rare, severe skin reactions to cancer drug
A notice about the risk of severe skin reactions associated with the cancer drug capecitabine – including Stevens-Johnson syndrome – has been released by the Canadian health department.
"Very rare cases of severe cutaneous reactions such as Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN), in some cases with fatal outcome, have been reported during treatment with" capecitabine, according to a letter to health care professionals, posted on the Health Canada website on Dec. 3. The letter states that treatment with capecitabine should be "immediately discontinued" if a patient develops signs and symptoms of SJS or TEN.
No such notice has been posted by the U.S. Food and Drug Administration. The FDA "constantly monitors and reviews reports from regulatory agencies, especially those concerning products that affect Americans’ health," and is reviewing related scientific information on this issue, an FDA spokesperson said in response to a query on the notice. The agency "will determine what, if any, actions to take," he added.
Capecitabine is a nucleoside metabolic inhibitor, and is marketed as Xeloda in Canada and the United States for treatment of colorectal cancer and breast cancer. In the United States, it was initially approved in 1998 for treating metastatic breast cancer, and is now also approved as a first-line treatment for metastatic colon cancer, as well as for adjuvant colon cancer in patients with Dukes’ C colon cancer. It is marketed by Roche, which the letter says is working with Health Canada to revise the product monograph in Canada. In the United States, a generic formulation is also available.
The U.S. label includes hand-and-foot syndrome (including grade 2 and 3 events), dermatitis, rash, and erythema among the skin and subcutaneous adverse events reported in clinical trials, but there are no reports of SJS or TEN listed.
Serious adverse events associated with capecitabine should be reported to MedWatch, the FDA Safety Information and Adverse Event Reporting Program, at www.fda.gov/Safety/MedWatch/default.htm.
A notice about the risk of severe skin reactions associated with the cancer drug capecitabine – including Stevens-Johnson syndrome – has been released by the Canadian health department.
"Very rare cases of severe cutaneous reactions such as Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN), in some cases with fatal outcome, have been reported during treatment with" capecitabine, according to a letter to health care professionals, posted on the Health Canada website on Dec. 3. The letter states that treatment with capecitabine should be "immediately discontinued" if a patient develops signs and symptoms of SJS or TEN.
No such notice has been posted by the U.S. Food and Drug Administration. The FDA "constantly monitors and reviews reports from regulatory agencies, especially those concerning products that affect Americans’ health," and is reviewing related scientific information on this issue, an FDA spokesperson said in response to a query on the notice. The agency "will determine what, if any, actions to take," he added.
Capecitabine is a nucleoside metabolic inhibitor, and is marketed as Xeloda in Canada and the United States for treatment of colorectal cancer and breast cancer. In the United States, it was initially approved in 1998 for treating metastatic breast cancer, and is now also approved as a first-line treatment for metastatic colon cancer, as well as for adjuvant colon cancer in patients with Dukes’ C colon cancer. It is marketed by Roche, which the letter says is working with Health Canada to revise the product monograph in Canada. In the United States, a generic formulation is also available.
The U.S. label includes hand-and-foot syndrome (including grade 2 and 3 events), dermatitis, rash, and erythema among the skin and subcutaneous adverse events reported in clinical trials, but there are no reports of SJS or TEN listed.
Serious adverse events associated with capecitabine should be reported to MedWatch, the FDA Safety Information and Adverse Event Reporting Program, at www.fda.gov/Safety/MedWatch/default.htm.
A notice about the risk of severe skin reactions associated with the cancer drug capecitabine – including Stevens-Johnson syndrome – has been released by the Canadian health department.
"Very rare cases of severe cutaneous reactions such as Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN), in some cases with fatal outcome, have been reported during treatment with" capecitabine, according to a letter to health care professionals, posted on the Health Canada website on Dec. 3. The letter states that treatment with capecitabine should be "immediately discontinued" if a patient develops signs and symptoms of SJS or TEN.
No such notice has been posted by the U.S. Food and Drug Administration. The FDA "constantly monitors and reviews reports from regulatory agencies, especially those concerning products that affect Americans’ health," and is reviewing related scientific information on this issue, an FDA spokesperson said in response to a query on the notice. The agency "will determine what, if any, actions to take," he added.
Capecitabine is a nucleoside metabolic inhibitor, and is marketed as Xeloda in Canada and the United States for treatment of colorectal cancer and breast cancer. In the United States, it was initially approved in 1998 for treating metastatic breast cancer, and is now also approved as a first-line treatment for metastatic colon cancer, as well as for adjuvant colon cancer in patients with Dukes’ C colon cancer. It is marketed by Roche, which the letter says is working with Health Canada to revise the product monograph in Canada. In the United States, a generic formulation is also available.
The U.S. label includes hand-and-foot syndrome (including grade 2 and 3 events), dermatitis, rash, and erythema among the skin and subcutaneous adverse events reported in clinical trials, but there are no reports of SJS or TEN listed.
Serious adverse events associated with capecitabine should be reported to MedWatch, the FDA Safety Information and Adverse Event Reporting Program, at www.fda.gov/Safety/MedWatch/default.htm.
Expert offers insight on photocontact dermatitis
WOODINVILLE, WASH. – The diagnosis of photocontact dermatitis can be challenging and often requires careful photopatch testing, according to Dr. Vincent A. DeLeo.
He reviewed the finer points of diagnosing the two main types of photocontact dermatitis – photoirritant and photoallergic – and differentiating them from other conditions with a similar clinical presentation at the annual Coastal Dermatology Symposium.
Photoirritant contact dermatitis
In photoirritant contact dermatitis, a chemical deposited on the skin absorbs radiation and then causes damage directly, without any immune reaction. The diagnosis is a clinical one, said Dr. DeLeo, chairman of the department of dermatology at St. Luke’s–Roosevelt Hospital Center and Beth Israel Medical Center in New York.
"You as a clinician know it is a photodistributed disease," he said. "You take a good history, you get a history of the patient being exposed topically to the chemical, and that’s how you make the diagnosis," he commented. "This is universal; it will happen to any of us if we get exposed to these photosensitizing chemicals in enough concentration and we get enough light to develop this kind of reaction."
Photoirritant contact dermatitis is almost always induced by UVA radiation; therefore, it can occur even if a patient is wearing a sunscreen that protects against UVB light.
The reaction has the appearance of a sunburn, with erythema, edema, and possibly blistering. It usually resolves with hyperpigmentation. "The interesting thing is that a lot of these reactions are subclinical, at least the erythema component that you don’t see. All you do see is the resulting hyperpigmentation," he said at the meeting, presented by the Caribbean Dermatology Symposium.
A common culprit is tar, in which case the condition is known as tar smarts. These cases usually result from occupational exposure in roofers, but can be seen as well in people who use tar shampoos and then go out in the sun.
The furocoumarins (psoralens) also can produce photoirritant contact dermatitis. The most common form is phytophotodermatitis, which involves plant compounds, especially those in the exocorp (green peel) of limes. The hallmark is tiny blisters on involved skin.
"It’s a delayed reaction. So people are squeezing limes into their gin and tonics on the weekend, and then Monday or Tuesday, when they are back at work, all of a sudden they develop these blisters, and they don’t associate it with what they did on weekend," Dr. DeLeo noted.
Phytophotodermatitis also can result from contact with celery that has been bred to have a high psoralen content so that it stays fresh longer. "You see this in grocery workers primarily, people who are exposed to a lot of celery, handling it and then going into the sun and getting the reaction," Dr. DeLeo said.
Photoallergic contact dermatitis
In photoallergic contact dermatitis, the chemical deposited on the skin – most commonly a sunscreen, fragrance, or medication – absorbs radiation and becomes an allergen. Only sensitized people have a reaction.
This dermatitis is exematous and pruritic, and shows a sun-exposed distribution, although it is usually patchy. The reaction is delayed, typically starting 1-2 days after sun exposure.
"The photodistributed eruption will usually be in all photoexposed areas. And this is important in making the distinction between this and other photosensitivities," Dr. DeLeo said. "Usually it will occur on all areas including the face ... and usually it spares upper lids and beneath the chin, although not always," he noted.
"The diagnosis of photoallergic contact dermatitis is confirmed by photopatch testing," he said. "Almost all photosensitive patients with eczematous morphology should be photopatch tested."
However, survey data suggest that only about two-thirds of dermatologists do this testing (Am. J. Contact Dermat. 2003;14:5-11), largely because this dermatitis is uncommon today as most sensitizing agents are identified before products come to market.
Photopatch testing can be done in the office; it requires at least a UVA source, photoantigens, and general patch test supplies. A UVB source is helpful for distinguishing photoallergic contact dermatitis from conditions such as chronic actinic dermatitis.
"If I were to buy four photoantigens, I would probably buy oxybenzone, musk ambrette, padimate O, and avobenzone," Dr. DeLeo said. "You can also test directly to the patient’s products, and usually it’s going to be sunscreens, so you can just have them bring in whatever they are using."
In a study by the North American Contact Dermatitis Group in which 363 consecutive patients with a history of photosensitivity were given a final diagnosis after photopatch testing, the leading diagnosis was allergic contact dermatitis (122 patients), followed by photoallergic contact dermatitis (75 patients) and polymorphous light eruption (72 patients).
The most common photoallergen was oxybenzone. Others included ketoprofen, avobenzone, sulisobenzone, and musk ambrette.
"In the Northwest, mainly in Washington and Oregon, people are compounding ketoprofen, which is a nonsteroidal anti-inflammatory agent, into a topical agent ... that can induce a photoallergic contact dermatitis," Dr. DeLeo explained.
Newer photoallergens include the sunscreen methylene bis-benzotriazolyl tetramethylbutylphenol (bisoctrizole, brand name Tinosorb M) (Dermatitis 2011;22:106-11).
"This [product] is not available in the U.S., but is available in Europe," Dr. DeLeo noted. However, reactions have been seen among patients using Ahava, an Israeli product sold in the United States. The manufacturers "are apparently saying they are not using it as a sunscreen but as a sort of preservative. So you need to watch out for this one," he said.
In fact, the Food and Drug Administration is considering approval of several new sunscreens, and bisoctrizole is among them, so more cases may be seen, according to Dr. DeLeo.
Another emerging photoallergen is the sunscreen octocrylene. "We haven’t seen any positives to this as yet, but it is being reported in Europe as a very important new photoallergic agent. It may be that they are using a lot more of that as a photostabilizer for avobenzone in Europe than we do in our own cosmetics," he said.
Importantly, there is cross-reactivity among oxybenzone, octocrylene, and ketoprofen, said Dr. DeLeo. Therefore, "those of you who live in the Northwest and see this reaction to ketoprofen may want your patients to avoid oxybenzone and octocrylene as well," he added.
Dr. DeLeo disclosed that he is a consultant for Pfizer, Prous Science, L’Oréal, Limited Brands, Goodyear, Estée Lauder, Mary Kay, and La Roche-Posay.
WOODINVILLE, WASH. – The diagnosis of photocontact dermatitis can be challenging and often requires careful photopatch testing, according to Dr. Vincent A. DeLeo.
He reviewed the finer points of diagnosing the two main types of photocontact dermatitis – photoirritant and photoallergic – and differentiating them from other conditions with a similar clinical presentation at the annual Coastal Dermatology Symposium.
Photoirritant contact dermatitis
In photoirritant contact dermatitis, a chemical deposited on the skin absorbs radiation and then causes damage directly, without any immune reaction. The diagnosis is a clinical one, said Dr. DeLeo, chairman of the department of dermatology at St. Luke’s–Roosevelt Hospital Center and Beth Israel Medical Center in New York.
"You as a clinician know it is a photodistributed disease," he said. "You take a good history, you get a history of the patient being exposed topically to the chemical, and that’s how you make the diagnosis," he commented. "This is universal; it will happen to any of us if we get exposed to these photosensitizing chemicals in enough concentration and we get enough light to develop this kind of reaction."
Photoirritant contact dermatitis is almost always induced by UVA radiation; therefore, it can occur even if a patient is wearing a sunscreen that protects against UVB light.
The reaction has the appearance of a sunburn, with erythema, edema, and possibly blistering. It usually resolves with hyperpigmentation. "The interesting thing is that a lot of these reactions are subclinical, at least the erythema component that you don’t see. All you do see is the resulting hyperpigmentation," he said at the meeting, presented by the Caribbean Dermatology Symposium.
A common culprit is tar, in which case the condition is known as tar smarts. These cases usually result from occupational exposure in roofers, but can be seen as well in people who use tar shampoos and then go out in the sun.
The furocoumarins (psoralens) also can produce photoirritant contact dermatitis. The most common form is phytophotodermatitis, which involves plant compounds, especially those in the exocorp (green peel) of limes. The hallmark is tiny blisters on involved skin.
"It’s a delayed reaction. So people are squeezing limes into their gin and tonics on the weekend, and then Monday or Tuesday, when they are back at work, all of a sudden they develop these blisters, and they don’t associate it with what they did on weekend," Dr. DeLeo noted.
Phytophotodermatitis also can result from contact with celery that has been bred to have a high psoralen content so that it stays fresh longer. "You see this in grocery workers primarily, people who are exposed to a lot of celery, handling it and then going into the sun and getting the reaction," Dr. DeLeo said.
Photoallergic contact dermatitis
In photoallergic contact dermatitis, the chemical deposited on the skin – most commonly a sunscreen, fragrance, or medication – absorbs radiation and becomes an allergen. Only sensitized people have a reaction.
This dermatitis is exematous and pruritic, and shows a sun-exposed distribution, although it is usually patchy. The reaction is delayed, typically starting 1-2 days after sun exposure.
"The photodistributed eruption will usually be in all photoexposed areas. And this is important in making the distinction between this and other photosensitivities," Dr. DeLeo said. "Usually it will occur on all areas including the face ... and usually it spares upper lids and beneath the chin, although not always," he noted.
"The diagnosis of photoallergic contact dermatitis is confirmed by photopatch testing," he said. "Almost all photosensitive patients with eczematous morphology should be photopatch tested."
However, survey data suggest that only about two-thirds of dermatologists do this testing (Am. J. Contact Dermat. 2003;14:5-11), largely because this dermatitis is uncommon today as most sensitizing agents are identified before products come to market.
Photopatch testing can be done in the office; it requires at least a UVA source, photoantigens, and general patch test supplies. A UVB source is helpful for distinguishing photoallergic contact dermatitis from conditions such as chronic actinic dermatitis.
"If I were to buy four photoantigens, I would probably buy oxybenzone, musk ambrette, padimate O, and avobenzone," Dr. DeLeo said. "You can also test directly to the patient’s products, and usually it’s going to be sunscreens, so you can just have them bring in whatever they are using."
In a study by the North American Contact Dermatitis Group in which 363 consecutive patients with a history of photosensitivity were given a final diagnosis after photopatch testing, the leading diagnosis was allergic contact dermatitis (122 patients), followed by photoallergic contact dermatitis (75 patients) and polymorphous light eruption (72 patients).
The most common photoallergen was oxybenzone. Others included ketoprofen, avobenzone, sulisobenzone, and musk ambrette.
"In the Northwest, mainly in Washington and Oregon, people are compounding ketoprofen, which is a nonsteroidal anti-inflammatory agent, into a topical agent ... that can induce a photoallergic contact dermatitis," Dr. DeLeo explained.
Newer photoallergens include the sunscreen methylene bis-benzotriazolyl tetramethylbutylphenol (bisoctrizole, brand name Tinosorb M) (Dermatitis 2011;22:106-11).
"This [product] is not available in the U.S., but is available in Europe," Dr. DeLeo noted. However, reactions have been seen among patients using Ahava, an Israeli product sold in the United States. The manufacturers "are apparently saying they are not using it as a sunscreen but as a sort of preservative. So you need to watch out for this one," he said.
In fact, the Food and Drug Administration is considering approval of several new sunscreens, and bisoctrizole is among them, so more cases may be seen, according to Dr. DeLeo.
Another emerging photoallergen is the sunscreen octocrylene. "We haven’t seen any positives to this as yet, but it is being reported in Europe as a very important new photoallergic agent. It may be that they are using a lot more of that as a photostabilizer for avobenzone in Europe than we do in our own cosmetics," he said.
Importantly, there is cross-reactivity among oxybenzone, octocrylene, and ketoprofen, said Dr. DeLeo. Therefore, "those of you who live in the Northwest and see this reaction to ketoprofen may want your patients to avoid oxybenzone and octocrylene as well," he added.
Dr. DeLeo disclosed that he is a consultant for Pfizer, Prous Science, L’Oréal, Limited Brands, Goodyear, Estée Lauder, Mary Kay, and La Roche-Posay.
WOODINVILLE, WASH. – The diagnosis of photocontact dermatitis can be challenging and often requires careful photopatch testing, according to Dr. Vincent A. DeLeo.
He reviewed the finer points of diagnosing the two main types of photocontact dermatitis – photoirritant and photoallergic – and differentiating them from other conditions with a similar clinical presentation at the annual Coastal Dermatology Symposium.
Photoirritant contact dermatitis
In photoirritant contact dermatitis, a chemical deposited on the skin absorbs radiation and then causes damage directly, without any immune reaction. The diagnosis is a clinical one, said Dr. DeLeo, chairman of the department of dermatology at St. Luke’s–Roosevelt Hospital Center and Beth Israel Medical Center in New York.
"You as a clinician know it is a photodistributed disease," he said. "You take a good history, you get a history of the patient being exposed topically to the chemical, and that’s how you make the diagnosis," he commented. "This is universal; it will happen to any of us if we get exposed to these photosensitizing chemicals in enough concentration and we get enough light to develop this kind of reaction."
Photoirritant contact dermatitis is almost always induced by UVA radiation; therefore, it can occur even if a patient is wearing a sunscreen that protects against UVB light.
The reaction has the appearance of a sunburn, with erythema, edema, and possibly blistering. It usually resolves with hyperpigmentation. "The interesting thing is that a lot of these reactions are subclinical, at least the erythema component that you don’t see. All you do see is the resulting hyperpigmentation," he said at the meeting, presented by the Caribbean Dermatology Symposium.
A common culprit is tar, in which case the condition is known as tar smarts. These cases usually result from occupational exposure in roofers, but can be seen as well in people who use tar shampoos and then go out in the sun.
The furocoumarins (psoralens) also can produce photoirritant contact dermatitis. The most common form is phytophotodermatitis, which involves plant compounds, especially those in the exocorp (green peel) of limes. The hallmark is tiny blisters on involved skin.
"It’s a delayed reaction. So people are squeezing limes into their gin and tonics on the weekend, and then Monday or Tuesday, when they are back at work, all of a sudden they develop these blisters, and they don’t associate it with what they did on weekend," Dr. DeLeo noted.
Phytophotodermatitis also can result from contact with celery that has been bred to have a high psoralen content so that it stays fresh longer. "You see this in grocery workers primarily, people who are exposed to a lot of celery, handling it and then going into the sun and getting the reaction," Dr. DeLeo said.
Photoallergic contact dermatitis
In photoallergic contact dermatitis, the chemical deposited on the skin – most commonly a sunscreen, fragrance, or medication – absorbs radiation and becomes an allergen. Only sensitized people have a reaction.
This dermatitis is exematous and pruritic, and shows a sun-exposed distribution, although it is usually patchy. The reaction is delayed, typically starting 1-2 days after sun exposure.
"The photodistributed eruption will usually be in all photoexposed areas. And this is important in making the distinction between this and other photosensitivities," Dr. DeLeo said. "Usually it will occur on all areas including the face ... and usually it spares upper lids and beneath the chin, although not always," he noted.
"The diagnosis of photoallergic contact dermatitis is confirmed by photopatch testing," he said. "Almost all photosensitive patients with eczematous morphology should be photopatch tested."
However, survey data suggest that only about two-thirds of dermatologists do this testing (Am. J. Contact Dermat. 2003;14:5-11), largely because this dermatitis is uncommon today as most sensitizing agents are identified before products come to market.
Photopatch testing can be done in the office; it requires at least a UVA source, photoantigens, and general patch test supplies. A UVB source is helpful for distinguishing photoallergic contact dermatitis from conditions such as chronic actinic dermatitis.
"If I were to buy four photoantigens, I would probably buy oxybenzone, musk ambrette, padimate O, and avobenzone," Dr. DeLeo said. "You can also test directly to the patient’s products, and usually it’s going to be sunscreens, so you can just have them bring in whatever they are using."
In a study by the North American Contact Dermatitis Group in which 363 consecutive patients with a history of photosensitivity were given a final diagnosis after photopatch testing, the leading diagnosis was allergic contact dermatitis (122 patients), followed by photoallergic contact dermatitis (75 patients) and polymorphous light eruption (72 patients).
The most common photoallergen was oxybenzone. Others included ketoprofen, avobenzone, sulisobenzone, and musk ambrette.
"In the Northwest, mainly in Washington and Oregon, people are compounding ketoprofen, which is a nonsteroidal anti-inflammatory agent, into a topical agent ... that can induce a photoallergic contact dermatitis," Dr. DeLeo explained.
Newer photoallergens include the sunscreen methylene bis-benzotriazolyl tetramethylbutylphenol (bisoctrizole, brand name Tinosorb M) (Dermatitis 2011;22:106-11).
"This [product] is not available in the U.S., but is available in Europe," Dr. DeLeo noted. However, reactions have been seen among patients using Ahava, an Israeli product sold in the United States. The manufacturers "are apparently saying they are not using it as a sunscreen but as a sort of preservative. So you need to watch out for this one," he said.
In fact, the Food and Drug Administration is considering approval of several new sunscreens, and bisoctrizole is among them, so more cases may be seen, according to Dr. DeLeo.
Another emerging photoallergen is the sunscreen octocrylene. "We haven’t seen any positives to this as yet, but it is being reported in Europe as a very important new photoallergic agent. It may be that they are using a lot more of that as a photostabilizer for avobenzone in Europe than we do in our own cosmetics," he said.
Importantly, there is cross-reactivity among oxybenzone, octocrylene, and ketoprofen, said Dr. DeLeo. Therefore, "those of you who live in the Northwest and see this reaction to ketoprofen may want your patients to avoid oxybenzone and octocrylene as well," he added.
Dr. DeLeo disclosed that he is a consultant for Pfizer, Prous Science, L’Oréal, Limited Brands, Goodyear, Estée Lauder, Mary Kay, and La Roche-Posay.
AT THE COASTAL DERMATOLOGY SYMPOSIUM
Capsaicin 8% patch offers relief for brachioradial pruritus
ISTANBUL, TURKEY – The capsaicin 8% patch known as Qutenza appears to be a novel and effective treatment for patients with brachioradial pruritus, according to a preliminary observational study.
"The big advantage of this new treatment strategy is the patient only needs one application of the patch instead of taking drugs daily. Most patients experience relief for 5-6 weeks afterward," Dr. Claudia Zeidler said at the annual congress of the European Academy of Dermatology and Venereology.
Brachioradial pruritus (BRP) is a neuropathic itch often accompanied by pain, burning, and stinging. The condition is localized to the dorsolateral forearms along the distribution of the C-5 or C-6 dermatomes. The condition is more common in women. Imaging studies of patients typically show spinal stenosis or other cervical spinal pathology entailing cervical nerve root compression, according to Dr. Zeidler of the University of Müenster, Germany.
She and her colleagues found that patients with BRP have significantly reduced intraepidermal nerve fiber density in lesional skin, compared with nonlesional skin on the ventral forearm. Patients also have reduced expression of transient receptor potential cation channel subfamily V, member 1 (TRPV1) in lesional skin, compared with nonlesional skin. TRPV1 affects the balance between keratinocyte differentiation and proliferation, and thus can influence epidermal barrier function.
Dr. Zeidler’s study enrolled 16 patients with an average BRP duration of 91 months who had undergone unsuccessful treatment attempts with various combinations of anticonvulsants, antidepressants, and antihistamines. Thirteen of the 16 patients were women, and ranged in age from 47 to 69 years. Affected skin areas were normal in appearance, aside from hyperpigmented scratch lesions that looked like prurigo nodularis in 12 patients. Magnetic resonance imaging showed 15 of the 16 patients had cervical nerve compression or degenerative changes.
Itching relief typically began the day after the in-office Qutenza treatment session. Three weeks post treatment, mean pruritus intensity scores on a 0-10 visual analog scale had dropped from 5.8 pretreatment to 1.1. Mean pain intensity scores fell from 4.2 to 0.8. Significant improvement in quality of life was documented via a reduction in Dermatology Life Quality Index scores from 8.9 pretreatment to 4.3. Levels of TRPV1 in lesional skin were significantly increased 3 weeks post treatment compared with baseline; however, intraepithelial nerve fiber density in lesional skin was not significantly different.
Side effects of the capsaicin 8% patch consisted of mild localized pain and burning lasting for 20 minutes to 2 days in 14 patients.
Additional patient follow-up at 2, 3, and 4 months indicated that the pruritus and pain relief typically lasted 5-6 weeks. Since the conclusion of the study, when symptoms return, patients come in for retreatment.
The 14-by-20-cm capsaicin 8% patch is approved in Europe for the treatment of nondiabetic neuropathic pain and in the United States for management of neuropathic pain associated with postherpetic neuralgia. The patch contains 179 mg of capsaicin. Dr. Zeidler and coworkers were careful to follow the detailed labeling instructions for the patch’s use.
The patch has to be administered by a physician or another health care professional. Self-treatment is not permitted. Removal of hair to promote patch adherence should be accomplished with the use of scissors, not by shaving. To reduce discomfort, the researchers applied topical lidocaine 2.5% for 1 hour prior to putting the patch directly on the itchy area. Upon removing the patch after 1 hour, a cleansing gel that comes with the patch was applied for 1 minute to remove any residual capsaicin.
Dr. Zeidler plans to conduct a randomized controlled trial in patients with BRP to confirm the observational study findings, and also to look at the long-term effects of repeated treatments. Her observational study was supported by Astellas Pharma. She reported having no financial conflicts.
ISTANBUL, TURKEY – The capsaicin 8% patch known as Qutenza appears to be a novel and effective treatment for patients with brachioradial pruritus, according to a preliminary observational study.
"The big advantage of this new treatment strategy is the patient only needs one application of the patch instead of taking drugs daily. Most patients experience relief for 5-6 weeks afterward," Dr. Claudia Zeidler said at the annual congress of the European Academy of Dermatology and Venereology.
Brachioradial pruritus (BRP) is a neuropathic itch often accompanied by pain, burning, and stinging. The condition is localized to the dorsolateral forearms along the distribution of the C-5 or C-6 dermatomes. The condition is more common in women. Imaging studies of patients typically show spinal stenosis or other cervical spinal pathology entailing cervical nerve root compression, according to Dr. Zeidler of the University of Müenster, Germany.
She and her colleagues found that patients with BRP have significantly reduced intraepidermal nerve fiber density in lesional skin, compared with nonlesional skin on the ventral forearm. Patients also have reduced expression of transient receptor potential cation channel subfamily V, member 1 (TRPV1) in lesional skin, compared with nonlesional skin. TRPV1 affects the balance between keratinocyte differentiation and proliferation, and thus can influence epidermal barrier function.
Dr. Zeidler’s study enrolled 16 patients with an average BRP duration of 91 months who had undergone unsuccessful treatment attempts with various combinations of anticonvulsants, antidepressants, and antihistamines. Thirteen of the 16 patients were women, and ranged in age from 47 to 69 years. Affected skin areas were normal in appearance, aside from hyperpigmented scratch lesions that looked like prurigo nodularis in 12 patients. Magnetic resonance imaging showed 15 of the 16 patients had cervical nerve compression or degenerative changes.
Itching relief typically began the day after the in-office Qutenza treatment session. Three weeks post treatment, mean pruritus intensity scores on a 0-10 visual analog scale had dropped from 5.8 pretreatment to 1.1. Mean pain intensity scores fell from 4.2 to 0.8. Significant improvement in quality of life was documented via a reduction in Dermatology Life Quality Index scores from 8.9 pretreatment to 4.3. Levels of TRPV1 in lesional skin were significantly increased 3 weeks post treatment compared with baseline; however, intraepithelial nerve fiber density in lesional skin was not significantly different.
Side effects of the capsaicin 8% patch consisted of mild localized pain and burning lasting for 20 minutes to 2 days in 14 patients.
Additional patient follow-up at 2, 3, and 4 months indicated that the pruritus and pain relief typically lasted 5-6 weeks. Since the conclusion of the study, when symptoms return, patients come in for retreatment.
The 14-by-20-cm capsaicin 8% patch is approved in Europe for the treatment of nondiabetic neuropathic pain and in the United States for management of neuropathic pain associated with postherpetic neuralgia. The patch contains 179 mg of capsaicin. Dr. Zeidler and coworkers were careful to follow the detailed labeling instructions for the patch’s use.
The patch has to be administered by a physician or another health care professional. Self-treatment is not permitted. Removal of hair to promote patch adherence should be accomplished with the use of scissors, not by shaving. To reduce discomfort, the researchers applied topical lidocaine 2.5% for 1 hour prior to putting the patch directly on the itchy area. Upon removing the patch after 1 hour, a cleansing gel that comes with the patch was applied for 1 minute to remove any residual capsaicin.
Dr. Zeidler plans to conduct a randomized controlled trial in patients with BRP to confirm the observational study findings, and also to look at the long-term effects of repeated treatments. Her observational study was supported by Astellas Pharma. She reported having no financial conflicts.
ISTANBUL, TURKEY – The capsaicin 8% patch known as Qutenza appears to be a novel and effective treatment for patients with brachioradial pruritus, according to a preliminary observational study.
"The big advantage of this new treatment strategy is the patient only needs one application of the patch instead of taking drugs daily. Most patients experience relief for 5-6 weeks afterward," Dr. Claudia Zeidler said at the annual congress of the European Academy of Dermatology and Venereology.
Brachioradial pruritus (BRP) is a neuropathic itch often accompanied by pain, burning, and stinging. The condition is localized to the dorsolateral forearms along the distribution of the C-5 or C-6 dermatomes. The condition is more common in women. Imaging studies of patients typically show spinal stenosis or other cervical spinal pathology entailing cervical nerve root compression, according to Dr. Zeidler of the University of Müenster, Germany.
She and her colleagues found that patients with BRP have significantly reduced intraepidermal nerve fiber density in lesional skin, compared with nonlesional skin on the ventral forearm. Patients also have reduced expression of transient receptor potential cation channel subfamily V, member 1 (TRPV1) in lesional skin, compared with nonlesional skin. TRPV1 affects the balance between keratinocyte differentiation and proliferation, and thus can influence epidermal barrier function.
Dr. Zeidler’s study enrolled 16 patients with an average BRP duration of 91 months who had undergone unsuccessful treatment attempts with various combinations of anticonvulsants, antidepressants, and antihistamines. Thirteen of the 16 patients were women, and ranged in age from 47 to 69 years. Affected skin areas were normal in appearance, aside from hyperpigmented scratch lesions that looked like prurigo nodularis in 12 patients. Magnetic resonance imaging showed 15 of the 16 patients had cervical nerve compression or degenerative changes.
Itching relief typically began the day after the in-office Qutenza treatment session. Three weeks post treatment, mean pruritus intensity scores on a 0-10 visual analog scale had dropped from 5.8 pretreatment to 1.1. Mean pain intensity scores fell from 4.2 to 0.8. Significant improvement in quality of life was documented via a reduction in Dermatology Life Quality Index scores from 8.9 pretreatment to 4.3. Levels of TRPV1 in lesional skin were significantly increased 3 weeks post treatment compared with baseline; however, intraepithelial nerve fiber density in lesional skin was not significantly different.
Side effects of the capsaicin 8% patch consisted of mild localized pain and burning lasting for 20 minutes to 2 days in 14 patients.
Additional patient follow-up at 2, 3, and 4 months indicated that the pruritus and pain relief typically lasted 5-6 weeks. Since the conclusion of the study, when symptoms return, patients come in for retreatment.
The 14-by-20-cm capsaicin 8% patch is approved in Europe for the treatment of nondiabetic neuropathic pain and in the United States for management of neuropathic pain associated with postherpetic neuralgia. The patch contains 179 mg of capsaicin. Dr. Zeidler and coworkers were careful to follow the detailed labeling instructions for the patch’s use.
The patch has to be administered by a physician or another health care professional. Self-treatment is not permitted. Removal of hair to promote patch adherence should be accomplished with the use of scissors, not by shaving. To reduce discomfort, the researchers applied topical lidocaine 2.5% for 1 hour prior to putting the patch directly on the itchy area. Upon removing the patch after 1 hour, a cleansing gel that comes with the patch was applied for 1 minute to remove any residual capsaicin.
Dr. Zeidler plans to conduct a randomized controlled trial in patients with BRP to confirm the observational study findings, and also to look at the long-term effects of repeated treatments. Her observational study was supported by Astellas Pharma. She reported having no financial conflicts.
AT THE EADV CONGRESS
Major finding: Three weeks after a single hour-long application of the capsaicin 8% patch known as Qutenza, mean itch intensity on a 0-10 visual analog scale in patients with brachioradial pruritus was 1.1, compared with 5.8 pretreatment.
Data source: An uncontrolled prospective observational study involving 16 treated patients with longstanding brachioradial pruritus refractory to various combinations of anticonvulsants, antihistamines, and antidepressants.
Disclosures: The investigator-initiated study was supported by Astellas Pharma. The presenter reported having no financial conflicts.
Oxidized limonene: New culprit in allergic dermatitis
ISTANBUL, TURKEY – Oxidized R-limonene, found in more than 60% of personal care and hygiene products, has been found to be a major cause of fragrance allergy, according to the findings of a large, international, patch test study.
Overall, 5.2% of almost 3,000 patients had a positive patch test result for oxidized limonene, and 37% of those reactions were deemed to be clinically relevant. Shampoos, soaps, perfumes, domestic cleaners, sunscreens, and massage creams are among the household products likely to contain limonene, Dr. Johanna Bråred Christensson said at the annual congress of the European Academy of Dermatology and Venereology. R-limonene is also used in high concentrations in industry as a solvent and degreaser.
R-limonene is structurally a fragrance terpene, with a citrus odor. It is found in nature and produced industrially in mass quantities. In its pure form, R-limonene is nonallergenic or at most a weak allergen. However, when R-limonene is oxidized, it includes limonene hydroperoxides, which Dr. Christensson found to be a contact allergen. This oxidation can occur during storage or handling of R-limonene. The hydroperoxides are often already present in containers of R-limonene when shipped from chemical plants to manufacturers of consumer products, according to Dr. Christensson, a dermatologist at the University of Gothenburg (Sweden).
A stable oxidized R-limonene 3.0% in petrolatum with a controlled 0.33% concentration of limonene hydroperoxides, which is commercially available as a patch test material, was used in the patch test study of 2,900 consecutive dermatitis patients who presented to contact dermatitis clinics in Sweden, Denmark, the United Kingdom, Spain, Australia, and Singapore. Participants completed a questionnaire assessing the relevance of a positive patch test result as indicated by exposure to limonene-containing products on the area of their dermatitis.
The study was funded by grants from national allergy research centers. Dr. Christensson reported having no relevant financial conflicts.
ISTANBUL, TURKEY – Oxidized R-limonene, found in more than 60% of personal care and hygiene products, has been found to be a major cause of fragrance allergy, according to the findings of a large, international, patch test study.
Overall, 5.2% of almost 3,000 patients had a positive patch test result for oxidized limonene, and 37% of those reactions were deemed to be clinically relevant. Shampoos, soaps, perfumes, domestic cleaners, sunscreens, and massage creams are among the household products likely to contain limonene, Dr. Johanna Bråred Christensson said at the annual congress of the European Academy of Dermatology and Venereology. R-limonene is also used in high concentrations in industry as a solvent and degreaser.
R-limonene is structurally a fragrance terpene, with a citrus odor. It is found in nature and produced industrially in mass quantities. In its pure form, R-limonene is nonallergenic or at most a weak allergen. However, when R-limonene is oxidized, it includes limonene hydroperoxides, which Dr. Christensson found to be a contact allergen. This oxidation can occur during storage or handling of R-limonene. The hydroperoxides are often already present in containers of R-limonene when shipped from chemical plants to manufacturers of consumer products, according to Dr. Christensson, a dermatologist at the University of Gothenburg (Sweden).
A stable oxidized R-limonene 3.0% in petrolatum with a controlled 0.33% concentration of limonene hydroperoxides, which is commercially available as a patch test material, was used in the patch test study of 2,900 consecutive dermatitis patients who presented to contact dermatitis clinics in Sweden, Denmark, the United Kingdom, Spain, Australia, and Singapore. Participants completed a questionnaire assessing the relevance of a positive patch test result as indicated by exposure to limonene-containing products on the area of their dermatitis.
The study was funded by grants from national allergy research centers. Dr. Christensson reported having no relevant financial conflicts.
ISTANBUL, TURKEY – Oxidized R-limonene, found in more than 60% of personal care and hygiene products, has been found to be a major cause of fragrance allergy, according to the findings of a large, international, patch test study.
Overall, 5.2% of almost 3,000 patients had a positive patch test result for oxidized limonene, and 37% of those reactions were deemed to be clinically relevant. Shampoos, soaps, perfumes, domestic cleaners, sunscreens, and massage creams are among the household products likely to contain limonene, Dr. Johanna Bråred Christensson said at the annual congress of the European Academy of Dermatology and Venereology. R-limonene is also used in high concentrations in industry as a solvent and degreaser.
R-limonene is structurally a fragrance terpene, with a citrus odor. It is found in nature and produced industrially in mass quantities. In its pure form, R-limonene is nonallergenic or at most a weak allergen. However, when R-limonene is oxidized, it includes limonene hydroperoxides, which Dr. Christensson found to be a contact allergen. This oxidation can occur during storage or handling of R-limonene. The hydroperoxides are often already present in containers of R-limonene when shipped from chemical plants to manufacturers of consumer products, according to Dr. Christensson, a dermatologist at the University of Gothenburg (Sweden).
A stable oxidized R-limonene 3.0% in petrolatum with a controlled 0.33% concentration of limonene hydroperoxides, which is commercially available as a patch test material, was used in the patch test study of 2,900 consecutive dermatitis patients who presented to contact dermatitis clinics in Sweden, Denmark, the United Kingdom, Spain, Australia, and Singapore. Participants completed a questionnaire assessing the relevance of a positive patch test result as indicated by exposure to limonene-containing products on the area of their dermatitis.
The study was funded by grants from national allergy research centers. Dr. Christensson reported having no relevant financial conflicts.
AT THE EADV CONGRESS
Major finding: More than 5% of 2,900 consecutive patch test patients had a positive result for oxidized limonene. More than one-third of the positive results were deemed clinically relevant.
Data source: A prospective study involving 2,900 consecutive dermatitis patients who underwent patch testing at contact dermatitis clinics in six countries.
Disclosures: The study was funded by grants from national allergy research centers. Dr. Christensson reported having no relevant financial conflicts.
Novel topical agent reduces chronic itch
ISTANBUL, TURKEY – A first-in-class topical tyrosine kinase inhibitor markedly reduced chronic pruritus in psoriasis patients in a phase-IIb study.
Moreover, the investigational agent, known for now as CT327, produced no application site reactions or indeed any other adverse events. Nor was it absorbed systemically, Dr. David Roblin reported at the annual congress of the European Academy of Dermatology and Venereology.
A significant unmet need exists for safe and effective therapies for chronic itching, not only in psoriasis but in other diseases for which chronic pruritus figures prominently and has a debilitating effect on quality of life. At present, there is no medication with an indication for treatment of chronic pruritus, noted Dr. Roblin, chief medical officer at Creabilis in Canterbury, England.
Tyrosine kinase is a high-affinity receptor of nerve growth factor. Thus, CT327 targets the sensory neurons implicated in the pathogenesis of chronic pruritis, he explained.
The multicenter, randomized, double-blind clinical trial included 160 patients with mild-to-moderate psoriasis affecting up to 10% of their body surface area. They were assigned to twice-daily application of CT327 at 0.05%, 0.1%, or 0.5% or to the vehicle for 8 weeks. Participants treated all their plaques except those on the face or scalp.
The safety assessment included all 160 patients; however, efficacy endpoints were assessed only in the 108 with at least moderate baseline pruritus as defined by a score greater than 40 mm on a 0-100 mm visual analog scale (VAS).
From a baseline mean pruritus VAS of 65.2, scores decreased at week 8 by 37.1 mm in the CT327 0.05% group, 31.5 mm in patients using CT327 0.1%, 36.4 mm in the 0.5% group, and 16.1 mm in vehicle-treated controls. A 20-mm reduction on the VAS is considered clinically meaningful. Four weeks after treatment stopped, pruritus scores had returned to baseline.
The 108 patients with at least moderate baseline pruritus had a baseline mean Psoriasis Area and Severity Index score, modified to exclude the untreated face and scalp (mPASI), of 9.3. At 8 weeks, patients in the CT327 0.05%, 0.1%, and 0.5% groups averaged mPASI score reductions of 46%, 36%, and 37%, respectively, compared with a 17% drop in the control group.
Of note, there was no correlation between baseline mPASI and pruritus severity scores, which suggests that trying to treat pruritus with agents directed at the inflammatory aspect of psoriasis may be a suboptimal strategy. It’s better to target the sensory neurons involved in the itch, according to Dr. Roblin.
The study was sponsored by Creabilis, which is developing CT327. Dr. Roblin is the company’s medical director.
ISTANBUL, TURKEY – A first-in-class topical tyrosine kinase inhibitor markedly reduced chronic pruritus in psoriasis patients in a phase-IIb study.
Moreover, the investigational agent, known for now as CT327, produced no application site reactions or indeed any other adverse events. Nor was it absorbed systemically, Dr. David Roblin reported at the annual congress of the European Academy of Dermatology and Venereology.
A significant unmet need exists for safe and effective therapies for chronic itching, not only in psoriasis but in other diseases for which chronic pruritus figures prominently and has a debilitating effect on quality of life. At present, there is no medication with an indication for treatment of chronic pruritus, noted Dr. Roblin, chief medical officer at Creabilis in Canterbury, England.
Tyrosine kinase is a high-affinity receptor of nerve growth factor. Thus, CT327 targets the sensory neurons implicated in the pathogenesis of chronic pruritis, he explained.
The multicenter, randomized, double-blind clinical trial included 160 patients with mild-to-moderate psoriasis affecting up to 10% of their body surface area. They were assigned to twice-daily application of CT327 at 0.05%, 0.1%, or 0.5% or to the vehicle for 8 weeks. Participants treated all their plaques except those on the face or scalp.
The safety assessment included all 160 patients; however, efficacy endpoints were assessed only in the 108 with at least moderate baseline pruritus as defined by a score greater than 40 mm on a 0-100 mm visual analog scale (VAS).
From a baseline mean pruritus VAS of 65.2, scores decreased at week 8 by 37.1 mm in the CT327 0.05% group, 31.5 mm in patients using CT327 0.1%, 36.4 mm in the 0.5% group, and 16.1 mm in vehicle-treated controls. A 20-mm reduction on the VAS is considered clinically meaningful. Four weeks after treatment stopped, pruritus scores had returned to baseline.
The 108 patients with at least moderate baseline pruritus had a baseline mean Psoriasis Area and Severity Index score, modified to exclude the untreated face and scalp (mPASI), of 9.3. At 8 weeks, patients in the CT327 0.05%, 0.1%, and 0.5% groups averaged mPASI score reductions of 46%, 36%, and 37%, respectively, compared with a 17% drop in the control group.
Of note, there was no correlation between baseline mPASI and pruritus severity scores, which suggests that trying to treat pruritus with agents directed at the inflammatory aspect of psoriasis may be a suboptimal strategy. It’s better to target the sensory neurons involved in the itch, according to Dr. Roblin.
The study was sponsored by Creabilis, which is developing CT327. Dr. Roblin is the company’s medical director.
ISTANBUL, TURKEY – A first-in-class topical tyrosine kinase inhibitor markedly reduced chronic pruritus in psoriasis patients in a phase-IIb study.
Moreover, the investigational agent, known for now as CT327, produced no application site reactions or indeed any other adverse events. Nor was it absorbed systemically, Dr. David Roblin reported at the annual congress of the European Academy of Dermatology and Venereology.
A significant unmet need exists for safe and effective therapies for chronic itching, not only in psoriasis but in other diseases for which chronic pruritus figures prominently and has a debilitating effect on quality of life. At present, there is no medication with an indication for treatment of chronic pruritus, noted Dr. Roblin, chief medical officer at Creabilis in Canterbury, England.
Tyrosine kinase is a high-affinity receptor of nerve growth factor. Thus, CT327 targets the sensory neurons implicated in the pathogenesis of chronic pruritis, he explained.
The multicenter, randomized, double-blind clinical trial included 160 patients with mild-to-moderate psoriasis affecting up to 10% of their body surface area. They were assigned to twice-daily application of CT327 at 0.05%, 0.1%, or 0.5% or to the vehicle for 8 weeks. Participants treated all their plaques except those on the face or scalp.
The safety assessment included all 160 patients; however, efficacy endpoints were assessed only in the 108 with at least moderate baseline pruritus as defined by a score greater than 40 mm on a 0-100 mm visual analog scale (VAS).
From a baseline mean pruritus VAS of 65.2, scores decreased at week 8 by 37.1 mm in the CT327 0.05% group, 31.5 mm in patients using CT327 0.1%, 36.4 mm in the 0.5% group, and 16.1 mm in vehicle-treated controls. A 20-mm reduction on the VAS is considered clinically meaningful. Four weeks after treatment stopped, pruritus scores had returned to baseline.
The 108 patients with at least moderate baseline pruritus had a baseline mean Psoriasis Area and Severity Index score, modified to exclude the untreated face and scalp (mPASI), of 9.3. At 8 weeks, patients in the CT327 0.05%, 0.1%, and 0.5% groups averaged mPASI score reductions of 46%, 36%, and 37%, respectively, compared with a 17% drop in the control group.
Of note, there was no correlation between baseline mPASI and pruritus severity scores, which suggests that trying to treat pruritus with agents directed at the inflammatory aspect of psoriasis may be a suboptimal strategy. It’s better to target the sensory neurons involved in the itch, according to Dr. Roblin.
The study was sponsored by Creabilis, which is developing CT327. Dr. Roblin is the company’s medical director.
AT THE EADV CONGRESS
Major finding: Treatment of chronic pruritus using various doses of CT327, a novel topical tyrosine kinase inhibitor, resulted in pruritus score reductions of 32-37 points from a mean baseline of 65 on a 0-100 scale.
Data source: This was a randomized, double-blind, multicenter, phase-IIb study including 160 patients with mild-to-moderate psoriasis who applied the investigational agent or its vehicle to their plaques twice daily for 8 weeks.
Disclosures: The study was sponsored by Creabilis. Dr. Roblin is the company’s chief medical officer.
Two new trials bolster omalizumab for urticaria
ISTANBUL, TURKEY – The case for omalizumab as a novel, safe, and highly effective therapy for H1-antihistamine-refractory chronic idiopathic/spontaneous urticaria received a boost in the form of two new phase III clinical trials presented at the annual congress of the European Academy of Dermatology and Venereology.
The two new multicenter, randomized, double-blind, placebo-controlled studies join another trial published earlier this year (N. Engl. J. Med. 2013;368:924-35). Together, the three trials total nearly 1,000 participants aged 12-75 years who remained severely affected by chronic idiopathic/spontaneous urticaria despite treatment with nonsedating H1-antihistamines, the sole approved form of treatment.
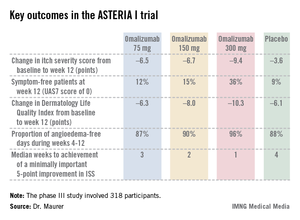
The GLACIAL trial, one of the new studies, set the bar even higher: Participants not only had to be refractory to H1-antihistamines at up to four times the approved dose, but also to off-label H2-antihistamines and/or leukotriene receptor antagonists.
The results were highly consistent across all three clinical trials – 80%-90% of patients demonstrated a dramatic clinical response to omalizumab in dose-dependent fashion. The top dose studied, 300 mg per subcutaneous injection once every 4 weeks, brought the greatest reductions in itching, wheals, and days with angioedema, Dr. Marcus Maurer at the meeting, where he presented the phase III data from the GLACIAL and ASTERIA I trials.
"Omalizumab is the new hope in urticaria treatment. The response is super-fast. We saw a massive and rapid decline in urticaria activity as early as in the first days after the first injection," he said. "In fact, the vast majority of responders respond within the first week of treatment. The disease activity then stays down as long as treatment continues," noted Dr. Maurer, professor of dermatology and allergy at Charité University Hospital, Berlin.
"The other important thing is, as soon as you stop the drug, you stop the benefit. Patients go back to baseline," he added. "So omalizumab is a very effective way to control disease activity, but it’s no cure. It doesn’t make the disease go away, it makes the symptoms go away. There is spontaneous remission in chronic idiopathic/spontaneous urticaria, but until that happens, patients need treatment that makes them free of symptoms. I’ve had patients on omalizumab for 5-6 years now."
As for the safety profile, Dr. Maurer called it "boring."
"This is a safe drug. This is a drug that doesn’t have any differences in its safety profile compared to placebo. The number and types of adverse events are virtually identical," he said.
Less than a week after Dr. Maurer’s presentation of the GLACIAL and ASTERIA I phase III trial results in Istanbul, Genentech filed an application with the Food and Drug Administration requesting approval of a supplemental indication for omalizumab in the treatment of chronic idiopathic/spontaneous urticaria in patients who remain symptomatic despite H1-antihistamine therapy at approved doses. A decision by the agency is expected in the spring of 2014.
Omalizumab is a humanized anti-IgE monoclonal antibody already approved as Xolair for treatment of moderate to severe allergic asthma. Unlike in allergic asthma, however, the response of chronic urticaria to omalizumab is independent of serum IgE level. Indeed, IgE levels are only modestly elevated in patients with chronic idiopathic/spontaneous urticaria – nothing like the high levels that can occur in allergic asthma. It’s not useful to measure IgE as a guide to the likelihood that chronic idiopathic/spontaneous urticaria will respond to omalizumab, Dr. Maurer advised.
The ASTERIA I trial was arguably the most clinically relevant of the three phase III studies because it employed omalizumab as add-on therapy to H1-antihistamines at approved doses. The 318 participants had a baseline mean weekly Itch Severity Scale (ISS) score of 14.3 out of a possible 21 despite being on H1-antihistamines. Their baseline urticaria activity score over 7 days (UAS7) was 31.1 on a scale of 0-42.
The study population was typical for patients with moderate to severe chronic idiopathic/spontaneous urticaria refractory to first-line therapy with H1-antihistamines. The average age was 41 years, three-quarters were female, and the mean body mass index was 29 kg/m2. The patients were diagnosed with chronic idiopathic/spontaneous urticaria an average of 4 years earlier and had been on an average of five previous unsuccessful medications for the condition. Half of the patients had angioedema.
Study participants received six subcutaneous doses of omalizumab at either 75-, 150-, or 300-mg doses; or placebo at 4-week intervals. They were then observed for 16 weeks. The primary study endpoint was the change in weekly ISS from baseline to week 12. The mean reduction was 3.6 points in the control group, significantly less than the 6.5-point fall in the low-dose omalizumab arm, the 6.7-point reduction with omalizumab 150 mg, or the 9.4-point decrease with omalizumab at 300 mg.
Omalizumab-treated patients also fared significantly better than did the placebo group in all secondary endpoints.
The anti-IgE biologic was well tolerated, with no new safety signals arising in ASTERIA I or the other two phase III trials beyond the agent’s well-established safety profile in treating allergic asthma.
The diagnosis of chronic idiopathic/spontaneous urticaria is based upon the finding of itchy hives and/or angioedema for 6 weeks or more with no specific external trigger. The estimated prevalence in the general population is 0.5%-1%. More than half of affected patients continue to experience symptoms despite treatment with H1-antihistamines.
The phase III trials were sponsored by Genentech and Novartis, which are jointly developing omalizumab for the treatment of chronic idiopathic/spontaneous urticaria. Dr. Maurer has received research grants from, and served on the advisory boards of, those pharmaceutical companies, as well as more than a dozen others.
ISTANBUL, TURKEY – The case for omalizumab as a novel, safe, and highly effective therapy for H1-antihistamine-refractory chronic idiopathic/spontaneous urticaria received a boost in the form of two new phase III clinical trials presented at the annual congress of the European Academy of Dermatology and Venereology.
The two new multicenter, randomized, double-blind, placebo-controlled studies join another trial published earlier this year (N. Engl. J. Med. 2013;368:924-35). Together, the three trials total nearly 1,000 participants aged 12-75 years who remained severely affected by chronic idiopathic/spontaneous urticaria despite treatment with nonsedating H1-antihistamines, the sole approved form of treatment.

The GLACIAL trial, one of the new studies, set the bar even higher: Participants not only had to be refractory to H1-antihistamines at up to four times the approved dose, but also to off-label H2-antihistamines and/or leukotriene receptor antagonists.
The results were highly consistent across all three clinical trials – 80%-90% of patients demonstrated a dramatic clinical response to omalizumab in dose-dependent fashion. The top dose studied, 300 mg per subcutaneous injection once every 4 weeks, brought the greatest reductions in itching, wheals, and days with angioedema, Dr. Marcus Maurer at the meeting, where he presented the phase III data from the GLACIAL and ASTERIA I trials.
"Omalizumab is the new hope in urticaria treatment. The response is super-fast. We saw a massive and rapid decline in urticaria activity as early as in the first days after the first injection," he said. "In fact, the vast majority of responders respond within the first week of treatment. The disease activity then stays down as long as treatment continues," noted Dr. Maurer, professor of dermatology and allergy at Charité University Hospital, Berlin.
"The other important thing is, as soon as you stop the drug, you stop the benefit. Patients go back to baseline," he added. "So omalizumab is a very effective way to control disease activity, but it’s no cure. It doesn’t make the disease go away, it makes the symptoms go away. There is spontaneous remission in chronic idiopathic/spontaneous urticaria, but until that happens, patients need treatment that makes them free of symptoms. I’ve had patients on omalizumab for 5-6 years now."
As for the safety profile, Dr. Maurer called it "boring."
"This is a safe drug. This is a drug that doesn’t have any differences in its safety profile compared to placebo. The number and types of adverse events are virtually identical," he said.
Less than a week after Dr. Maurer’s presentation of the GLACIAL and ASTERIA I phase III trial results in Istanbul, Genentech filed an application with the Food and Drug Administration requesting approval of a supplemental indication for omalizumab in the treatment of chronic idiopathic/spontaneous urticaria in patients who remain symptomatic despite H1-antihistamine therapy at approved doses. A decision by the agency is expected in the spring of 2014.
Omalizumab is a humanized anti-IgE monoclonal antibody already approved as Xolair for treatment of moderate to severe allergic asthma. Unlike in allergic asthma, however, the response of chronic urticaria to omalizumab is independent of serum IgE level. Indeed, IgE levels are only modestly elevated in patients with chronic idiopathic/spontaneous urticaria – nothing like the high levels that can occur in allergic asthma. It’s not useful to measure IgE as a guide to the likelihood that chronic idiopathic/spontaneous urticaria will respond to omalizumab, Dr. Maurer advised.
The ASTERIA I trial was arguably the most clinically relevant of the three phase III studies because it employed omalizumab as add-on therapy to H1-antihistamines at approved doses. The 318 participants had a baseline mean weekly Itch Severity Scale (ISS) score of 14.3 out of a possible 21 despite being on H1-antihistamines. Their baseline urticaria activity score over 7 days (UAS7) was 31.1 on a scale of 0-42.
The study population was typical for patients with moderate to severe chronic idiopathic/spontaneous urticaria refractory to first-line therapy with H1-antihistamines. The average age was 41 years, three-quarters were female, and the mean body mass index was 29 kg/m2. The patients were diagnosed with chronic idiopathic/spontaneous urticaria an average of 4 years earlier and had been on an average of five previous unsuccessful medications for the condition. Half of the patients had angioedema.
Study participants received six subcutaneous doses of omalizumab at either 75-, 150-, or 300-mg doses; or placebo at 4-week intervals. They were then observed for 16 weeks. The primary study endpoint was the change in weekly ISS from baseline to week 12. The mean reduction was 3.6 points in the control group, significantly less than the 6.5-point fall in the low-dose omalizumab arm, the 6.7-point reduction with omalizumab 150 mg, or the 9.4-point decrease with omalizumab at 300 mg.
Omalizumab-treated patients also fared significantly better than did the placebo group in all secondary endpoints.
The anti-IgE biologic was well tolerated, with no new safety signals arising in ASTERIA I or the other two phase III trials beyond the agent’s well-established safety profile in treating allergic asthma.
The diagnosis of chronic idiopathic/spontaneous urticaria is based upon the finding of itchy hives and/or angioedema for 6 weeks or more with no specific external trigger. The estimated prevalence in the general population is 0.5%-1%. More than half of affected patients continue to experience symptoms despite treatment with H1-antihistamines.
The phase III trials were sponsored by Genentech and Novartis, which are jointly developing omalizumab for the treatment of chronic idiopathic/spontaneous urticaria. Dr. Maurer has received research grants from, and served on the advisory boards of, those pharmaceutical companies, as well as more than a dozen others.
ISTANBUL, TURKEY – The case for omalizumab as a novel, safe, and highly effective therapy for H1-antihistamine-refractory chronic idiopathic/spontaneous urticaria received a boost in the form of two new phase III clinical trials presented at the annual congress of the European Academy of Dermatology and Venereology.
The two new multicenter, randomized, double-blind, placebo-controlled studies join another trial published earlier this year (N. Engl. J. Med. 2013;368:924-35). Together, the three trials total nearly 1,000 participants aged 12-75 years who remained severely affected by chronic idiopathic/spontaneous urticaria despite treatment with nonsedating H1-antihistamines, the sole approved form of treatment.

The GLACIAL trial, one of the new studies, set the bar even higher: Participants not only had to be refractory to H1-antihistamines at up to four times the approved dose, but also to off-label H2-antihistamines and/or leukotriene receptor antagonists.
The results were highly consistent across all three clinical trials – 80%-90% of patients demonstrated a dramatic clinical response to omalizumab in dose-dependent fashion. The top dose studied, 300 mg per subcutaneous injection once every 4 weeks, brought the greatest reductions in itching, wheals, and days with angioedema, Dr. Marcus Maurer at the meeting, where he presented the phase III data from the GLACIAL and ASTERIA I trials.
"Omalizumab is the new hope in urticaria treatment. The response is super-fast. We saw a massive and rapid decline in urticaria activity as early as in the first days after the first injection," he said. "In fact, the vast majority of responders respond within the first week of treatment. The disease activity then stays down as long as treatment continues," noted Dr. Maurer, professor of dermatology and allergy at Charité University Hospital, Berlin.
"The other important thing is, as soon as you stop the drug, you stop the benefit. Patients go back to baseline," he added. "So omalizumab is a very effective way to control disease activity, but it’s no cure. It doesn’t make the disease go away, it makes the symptoms go away. There is spontaneous remission in chronic idiopathic/spontaneous urticaria, but until that happens, patients need treatment that makes them free of symptoms. I’ve had patients on omalizumab for 5-6 years now."
As for the safety profile, Dr. Maurer called it "boring."
"This is a safe drug. This is a drug that doesn’t have any differences in its safety profile compared to placebo. The number and types of adverse events are virtually identical," he said.
Less than a week after Dr. Maurer’s presentation of the GLACIAL and ASTERIA I phase III trial results in Istanbul, Genentech filed an application with the Food and Drug Administration requesting approval of a supplemental indication for omalizumab in the treatment of chronic idiopathic/spontaneous urticaria in patients who remain symptomatic despite H1-antihistamine therapy at approved doses. A decision by the agency is expected in the spring of 2014.
Omalizumab is a humanized anti-IgE monoclonal antibody already approved as Xolair for treatment of moderate to severe allergic asthma. Unlike in allergic asthma, however, the response of chronic urticaria to omalizumab is independent of serum IgE level. Indeed, IgE levels are only modestly elevated in patients with chronic idiopathic/spontaneous urticaria – nothing like the high levels that can occur in allergic asthma. It’s not useful to measure IgE as a guide to the likelihood that chronic idiopathic/spontaneous urticaria will respond to omalizumab, Dr. Maurer advised.
The ASTERIA I trial was arguably the most clinically relevant of the three phase III studies because it employed omalizumab as add-on therapy to H1-antihistamines at approved doses. The 318 participants had a baseline mean weekly Itch Severity Scale (ISS) score of 14.3 out of a possible 21 despite being on H1-antihistamines. Their baseline urticaria activity score over 7 days (UAS7) was 31.1 on a scale of 0-42.
The study population was typical for patients with moderate to severe chronic idiopathic/spontaneous urticaria refractory to first-line therapy with H1-antihistamines. The average age was 41 years, three-quarters were female, and the mean body mass index was 29 kg/m2. The patients were diagnosed with chronic idiopathic/spontaneous urticaria an average of 4 years earlier and had been on an average of five previous unsuccessful medications for the condition. Half of the patients had angioedema.
Study participants received six subcutaneous doses of omalizumab at either 75-, 150-, or 300-mg doses; or placebo at 4-week intervals. They were then observed for 16 weeks. The primary study endpoint was the change in weekly ISS from baseline to week 12. The mean reduction was 3.6 points in the control group, significantly less than the 6.5-point fall in the low-dose omalizumab arm, the 6.7-point reduction with omalizumab 150 mg, or the 9.4-point decrease with omalizumab at 300 mg.
Omalizumab-treated patients also fared significantly better than did the placebo group in all secondary endpoints.
The anti-IgE biologic was well tolerated, with no new safety signals arising in ASTERIA I or the other two phase III trials beyond the agent’s well-established safety profile in treating allergic asthma.
The diagnosis of chronic idiopathic/spontaneous urticaria is based upon the finding of itchy hives and/or angioedema for 6 weeks or more with no specific external trigger. The estimated prevalence in the general population is 0.5%-1%. More than half of affected patients continue to experience symptoms despite treatment with H1-antihistamines.
The phase III trials were sponsored by Genentech and Novartis, which are jointly developing omalizumab for the treatment of chronic idiopathic/spontaneous urticaria. Dr. Maurer has received research grants from, and served on the advisory boards of, those pharmaceutical companies, as well as more than a dozen others.
AT THE EADV CONGRESS
Major finding: Among 318 patients with chronic idiopathic/spontaneous urticaria refractory to H1-antihistamines at standard doses who participated in the phase III ASTERIA I trial, add-on omalizumab at 300 mg resulted in a mean 9.4-point reduction in itch severity score at week 12 compared to a 3.6-point drop with placebo from a baseline score of 14.3.
Data source: ASTERIA I, one of three recent phase III randomized, double-blind, multicenter, placebo-controlled clinical trials of omalizumab for chronic idiopathic/spontaneous urticaria totaling nearly 1,000 participants.
Disclosures: The phase III clinical trials were jointly sponsored by Genentech and Novartis. The presenter reported having received research funds from the two companies as well as serving on their advisory boards.
Peppermint and menthol
Mentha piperita, better known as peppermint, is used worldwide in many ways. Its use for culinary and medical purposes dates back to the ancient Greek and Roman civilizations. Peppermint is used in numerous forms (i.e., oil, leaf, leaf extract, and leaf water), with the oil as the most versatile (Dermatitis 2010;21:327-9). Peppermint has long been known for its beneficial gastrointestinal effects, and it has a well-established record of antimicrobial, antifungal, and analgesic activity (Mills S., Bone K. Principles and Practice of Phytotherapy: Modern Herbal Medicine. [London: Churchill Livingstone, 2000, pp 507-13]; J. Environ. Biol. 2011;32:23-9).
Menthol (C10H20O) is a naturally occurring monocyclic terpene alcohol derived from Mentha piperita as well as other mint oils (Skin Therapy Lett. 2010;15:5-9), and has been associated with several health benefits. Recently, anticancer properties have been ascribed to menthol (Biochim. Biophys. Acta 2009;1792:33-8). This column will discuss recent findings regarding the actual or potential cutaneous benefits of peppermint and menthol.
Various Mentha species, including M. piperita, have exhibited significant antioxidant activity (Toxicol. Ind. Health. 2012;28:83-9; Nat. Prod. Commun. 2009;4:1107-12; Nat. Prod. Commun. 2009;4:535-42). In a 2010 study of the antioxidant activity of the essential oils of six popular herbs, including lavender (Lavendular angustifolia), peppermint (M. piperita), rosemary (Rosmarius officinalis), lemon (Citrus limon), grapefruit (C. paradise), and frankincense (Boswellia carteri), investigators found, in testing free radical-scavenging capacity and lipid peroxidation in the linoleic acid system, that peppermint essential oil exhibited the greatest radical-scavenging activity against the 2,2\'-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) ABTS radical (Nat. Prod. Res. 2010;24:140-51).
In 2010, Baliga and Rao showed that M. piperita and M. arvensis (wild mint) protected mice against gamma-radiation–induced morbidity and mortality. Specifically, M. piperita protected murine testes as well as gastrointestinal and hemopoietic systems (J. Cancer Res. Ther. 2010;6:255-62).
Anticancer activity
Investigations by Jain et al. into the molecular mechanisms supporting the anticarcinogenic potential of M. piperita leaf extracts on six human cancer cell lines (HeLa, MCF-7, Jurkat, T24, HT-29, MIAPaCa-2) in 2011 revealed that chloroform and ethyl acetate extracts dose- and time-dependently displayed anticarcinogenic activity leading to G1 cell cycle arrest and mitochondrial-mediated apoptosis among the cascade of effects. The investigators identified their findings as the first evidence of direct anticarcinogenic activity of Mentha leaf extracts and suggested that future work might focus on isolating active constituents as a foundation for mechanistic and translational studies leading to new anticancer drugs, alone or in combination, to prevent and treat human cancers (Int. J. Toxicol. 2011;30:225-36).
Topical benefits of menthol
In a recent examination of the antibacterial and antifungal properties, as well as speculated anti-inflammatory activity of menthol as a topical treatment for diaper dermatitis, investigators conducted a pilot clinical trial in a hospital setting. The study involved 84 neonates with diagnosed candidal diaper dermatitis who required no critical care or systemic antifungal and anti-inflammatory medications. The menthol group (n = 42) received topical clotrimazole and topically applied menthol drops and the control group (n = 42) received topical clotrimazole and a placebo. Thirty-five infants in each group completed the study. The researchers found that complete healing was shorter in the menthol group, with significant relief of erythema and pustules observed in this group. They concluded that topically-applied menthol may be an effective agent in the treatment of candidal diaper dermatitis (World J. Pediatr. 2011;7:167-70).
In 2011, Qiu et al. showed, through various assays, that menthol, in low concentrations, could significantly suppress the expression of alpha-hemolysin, enterotoxins A and B, and toxic shock syndrome toxin 1 in Staphylococcus aureus. The investigators concluded that menthol may warrant inclusion in the armamentarium against S. aureus when combined with beta-lactam antibiotics, which, at subinhibitory concentrations, can actually augment S. aureus toxin secretion. They added that menthol may also have possible uses in novel anti-virulence drugs (Appl. Microbiol. Biotechnol. 2011;90:705-12). It should be noted that menthol is considered safe and effective, with concentrations up to 16% approved in OTC external products by the Food and Drug Administration (J. Am. Acad. Dermatol. 2007;57:873-8).
Pruritus, TRPM8, and melanoma
Topically applied menthol, in concentrations of 1%-3%, is often used to treat pruritus, particularly in the elderly (Skin Therapy Lett. 2010;15:5-9). In addition, recent evidence suggests that the presence of menthol can facilitate penetration of other agents in topical products (Int. J. Toxicol. 2001;20 Suppl 3:61-73; J. Am. Acad. Dermatol. 2007;57:873-8). Patel and Yosipovitch suggest that elderly patients who report diminished pruritus with cooling may stand to benefit from menthol-containing topical therapies (J. Am. Acad. Dermatol. 2007;57:873-8; Skin Therapy Lett. 2010;15:5-9). Interestingly, menthol, via the transient receptor potential melastatin subfamily 8 (TRPM8) receptor, a member of a family of excitatory ion channels, engenders the same cooling sensation as low temperature, though menthol is not linked to a reduction in skin temperature (J. Am. Acad. Dermatol. 2007;57:873-8; Skin Therapy Lett. 2010;15:5-9).
Although the exact mechanism by which menthol exerts its antipruritic and analgesic effects has yet to be determined, the discovery that the TRPM8 is its underlying receptor is proving to be significant, particularly in understanding the cooling effect of the botanical (J. Am. Acad. Dermatol. 2007;57:873-8). There are also indications that menthol has therapeutic potential for melanoma. Specifically, melanoma expresses TRPM8 receptors, the activation of which inhibits melanoma viability. Menthol appears to mediate this response through an influx of extracellular calcium ions (Am. J. Physiol. Cell Physiol. 2008;295:C296-301; Am. J. Physiol. Cell Physiol. 2008;295:C293-5).
Peppermint oil
In 2003, Schuhmacher et al. investigated the virucidal effect of peppermint oil and found that it had a direct effect against herpes simplex virus type 1 (HSV-1) and herpes simplex virus type 2 (HSV-2) as well as an acyclovir-resistant HSV-1 strain. The investigators concluded, noting the lipophilic nature of peppermint oil, that it might be an appropriate topical treatment for recurrent herpes outbreaks (Phytomedicine 2003;10:504-10).
Because of its flavor, aroma, and cooling qualities, peppermint oil is used in a wide range of products, including cosmeceuticals, personal hygiene products (e.g., bath preparations, mouthwashes, toothpastes, and topical formulations), foods, pharmaceutical products, and aromatherapy. Topical indications include pruritus, irritation, and inflammation. Peppermint oil can act as a skin sensitizer, though, particularly in impaired and sensitive skin (Dermatitis 2010;21:327-9). Although peppermint oil has been reported to be a sensitizer in isolated cases, peppermint oil 8% was not found to be a sensitizer in a recent test using a maximization protocol and the various forms of peppermint (i.e., oil, extract, leaves, and water) are considered to be safe in cosmetic formulations. In rinse-off products, peppermint oil is used in concentrations up to 3% and up to 0.2% in leave-on formulations (Int. J. Toxicol. 2001;20 Suppl 3:61-73).
Conclusion
Peppermint and menthol, its naturally occurring monocyclic terpene alcohol derivative, have long been used for medical purposes. Contemporary practice and continuing research continue to support various uses of M. piperita in the medical armamentarium, with specific and additional uses continually being found in the dermatologic realm.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in Miami Beach. She founded the cosmetic dermatology center at the University of Miami in 1997. Dr. Baumann wrote the textbook "Cosmetic Dermatology: Principles and Practice" (McGraw-Hill, April 2002), and a book for consumers, "The Skin Type Solution" (Bantam, 2006). She has contributed to the Cosmeceutical Critique column in Skin & Allergy News since January 2001 and joined the editorial advisory board in 2004. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Galderma, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Stiefel, Topix Pharmaceuticals, and Unilever.
Mentha piperita, better known as peppermint, is used worldwide in many ways. Its use for culinary and medical purposes dates back to the ancient Greek and Roman civilizations. Peppermint is used in numerous forms (i.e., oil, leaf, leaf extract, and leaf water), with the oil as the most versatile (Dermatitis 2010;21:327-9). Peppermint has long been known for its beneficial gastrointestinal effects, and it has a well-established record of antimicrobial, antifungal, and analgesic activity (Mills S., Bone K. Principles and Practice of Phytotherapy: Modern Herbal Medicine. [London: Churchill Livingstone, 2000, pp 507-13]; J. Environ. Biol. 2011;32:23-9).
Menthol (C10H20O) is a naturally occurring monocyclic terpene alcohol derived from Mentha piperita as well as other mint oils (Skin Therapy Lett. 2010;15:5-9), and has been associated with several health benefits. Recently, anticancer properties have been ascribed to menthol (Biochim. Biophys. Acta 2009;1792:33-8). This column will discuss recent findings regarding the actual or potential cutaneous benefits of peppermint and menthol.
Various Mentha species, including M. piperita, have exhibited significant antioxidant activity (Toxicol. Ind. Health. 2012;28:83-9; Nat. Prod. Commun. 2009;4:1107-12; Nat. Prod. Commun. 2009;4:535-42). In a 2010 study of the antioxidant activity of the essential oils of six popular herbs, including lavender (Lavendular angustifolia), peppermint (M. piperita), rosemary (Rosmarius officinalis), lemon (Citrus limon), grapefruit (C. paradise), and frankincense (Boswellia carteri), investigators found, in testing free radical-scavenging capacity and lipid peroxidation in the linoleic acid system, that peppermint essential oil exhibited the greatest radical-scavenging activity against the 2,2\'-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) ABTS radical (Nat. Prod. Res. 2010;24:140-51).
In 2010, Baliga and Rao showed that M. piperita and M. arvensis (wild mint) protected mice against gamma-radiation–induced morbidity and mortality. Specifically, M. piperita protected murine testes as well as gastrointestinal and hemopoietic systems (J. Cancer Res. Ther. 2010;6:255-62).
Anticancer activity
Investigations by Jain et al. into the molecular mechanisms supporting the anticarcinogenic potential of M. piperita leaf extracts on six human cancer cell lines (HeLa, MCF-7, Jurkat, T24, HT-29, MIAPaCa-2) in 2011 revealed that chloroform and ethyl acetate extracts dose- and time-dependently displayed anticarcinogenic activity leading to G1 cell cycle arrest and mitochondrial-mediated apoptosis among the cascade of effects. The investigators identified their findings as the first evidence of direct anticarcinogenic activity of Mentha leaf extracts and suggested that future work might focus on isolating active constituents as a foundation for mechanistic and translational studies leading to new anticancer drugs, alone or in combination, to prevent and treat human cancers (Int. J. Toxicol. 2011;30:225-36).
Topical benefits of menthol
In a recent examination of the antibacterial and antifungal properties, as well as speculated anti-inflammatory activity of menthol as a topical treatment for diaper dermatitis, investigators conducted a pilot clinical trial in a hospital setting. The study involved 84 neonates with diagnosed candidal diaper dermatitis who required no critical care or systemic antifungal and anti-inflammatory medications. The menthol group (n = 42) received topical clotrimazole and topically applied menthol drops and the control group (n = 42) received topical clotrimazole and a placebo. Thirty-five infants in each group completed the study. The researchers found that complete healing was shorter in the menthol group, with significant relief of erythema and pustules observed in this group. They concluded that topically-applied menthol may be an effective agent in the treatment of candidal diaper dermatitis (World J. Pediatr. 2011;7:167-70).
In 2011, Qiu et al. showed, through various assays, that menthol, in low concentrations, could significantly suppress the expression of alpha-hemolysin, enterotoxins A and B, and toxic shock syndrome toxin 1 in Staphylococcus aureus. The investigators concluded that menthol may warrant inclusion in the armamentarium against S. aureus when combined with beta-lactam antibiotics, which, at subinhibitory concentrations, can actually augment S. aureus toxin secretion. They added that menthol may also have possible uses in novel anti-virulence drugs (Appl. Microbiol. Biotechnol. 2011;90:705-12). It should be noted that menthol is considered safe and effective, with concentrations up to 16% approved in OTC external products by the Food and Drug Administration (J. Am. Acad. Dermatol. 2007;57:873-8).
Pruritus, TRPM8, and melanoma
Topically applied menthol, in concentrations of 1%-3%, is often used to treat pruritus, particularly in the elderly (Skin Therapy Lett. 2010;15:5-9). In addition, recent evidence suggests that the presence of menthol can facilitate penetration of other agents in topical products (Int. J. Toxicol. 2001;20 Suppl 3:61-73; J. Am. Acad. Dermatol. 2007;57:873-8). Patel and Yosipovitch suggest that elderly patients who report diminished pruritus with cooling may stand to benefit from menthol-containing topical therapies (J. Am. Acad. Dermatol. 2007;57:873-8; Skin Therapy Lett. 2010;15:5-9). Interestingly, menthol, via the transient receptor potential melastatin subfamily 8 (TRPM8) receptor, a member of a family of excitatory ion channels, engenders the same cooling sensation as low temperature, though menthol is not linked to a reduction in skin temperature (J. Am. Acad. Dermatol. 2007;57:873-8; Skin Therapy Lett. 2010;15:5-9).
Although the exact mechanism by which menthol exerts its antipruritic and analgesic effects has yet to be determined, the discovery that the TRPM8 is its underlying receptor is proving to be significant, particularly in understanding the cooling effect of the botanical (J. Am. Acad. Dermatol. 2007;57:873-8). There are also indications that menthol has therapeutic potential for melanoma. Specifically, melanoma expresses TRPM8 receptors, the activation of which inhibits melanoma viability. Menthol appears to mediate this response through an influx of extracellular calcium ions (Am. J. Physiol. Cell Physiol. 2008;295:C296-301; Am. J. Physiol. Cell Physiol. 2008;295:C293-5).
Peppermint oil
In 2003, Schuhmacher et al. investigated the virucidal effect of peppermint oil and found that it had a direct effect against herpes simplex virus type 1 (HSV-1) and herpes simplex virus type 2 (HSV-2) as well as an acyclovir-resistant HSV-1 strain. The investigators concluded, noting the lipophilic nature of peppermint oil, that it might be an appropriate topical treatment for recurrent herpes outbreaks (Phytomedicine 2003;10:504-10).
Because of its flavor, aroma, and cooling qualities, peppermint oil is used in a wide range of products, including cosmeceuticals, personal hygiene products (e.g., bath preparations, mouthwashes, toothpastes, and topical formulations), foods, pharmaceutical products, and aromatherapy. Topical indications include pruritus, irritation, and inflammation. Peppermint oil can act as a skin sensitizer, though, particularly in impaired and sensitive skin (Dermatitis 2010;21:327-9). Although peppermint oil has been reported to be a sensitizer in isolated cases, peppermint oil 8% was not found to be a sensitizer in a recent test using a maximization protocol and the various forms of peppermint (i.e., oil, extract, leaves, and water) are considered to be safe in cosmetic formulations. In rinse-off products, peppermint oil is used in concentrations up to 3% and up to 0.2% in leave-on formulations (Int. J. Toxicol. 2001;20 Suppl 3:61-73).
Conclusion
Peppermint and menthol, its naturally occurring monocyclic terpene alcohol derivative, have long been used for medical purposes. Contemporary practice and continuing research continue to support various uses of M. piperita in the medical armamentarium, with specific and additional uses continually being found in the dermatologic realm.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in Miami Beach. She founded the cosmetic dermatology center at the University of Miami in 1997. Dr. Baumann wrote the textbook "Cosmetic Dermatology: Principles and Practice" (McGraw-Hill, April 2002), and a book for consumers, "The Skin Type Solution" (Bantam, 2006). She has contributed to the Cosmeceutical Critique column in Skin & Allergy News since January 2001 and joined the editorial advisory board in 2004. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Galderma, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Stiefel, Topix Pharmaceuticals, and Unilever.
Mentha piperita, better known as peppermint, is used worldwide in many ways. Its use for culinary and medical purposes dates back to the ancient Greek and Roman civilizations. Peppermint is used in numerous forms (i.e., oil, leaf, leaf extract, and leaf water), with the oil as the most versatile (Dermatitis 2010;21:327-9). Peppermint has long been known for its beneficial gastrointestinal effects, and it has a well-established record of antimicrobial, antifungal, and analgesic activity (Mills S., Bone K. Principles and Practice of Phytotherapy: Modern Herbal Medicine. [London: Churchill Livingstone, 2000, pp 507-13]; J. Environ. Biol. 2011;32:23-9).
Menthol (C10H20O) is a naturally occurring monocyclic terpene alcohol derived from Mentha piperita as well as other mint oils (Skin Therapy Lett. 2010;15:5-9), and has been associated with several health benefits. Recently, anticancer properties have been ascribed to menthol (Biochim. Biophys. Acta 2009;1792:33-8). This column will discuss recent findings regarding the actual or potential cutaneous benefits of peppermint and menthol.
Various Mentha species, including M. piperita, have exhibited significant antioxidant activity (Toxicol. Ind. Health. 2012;28:83-9; Nat. Prod. Commun. 2009;4:1107-12; Nat. Prod. Commun. 2009;4:535-42). In a 2010 study of the antioxidant activity of the essential oils of six popular herbs, including lavender (Lavendular angustifolia), peppermint (M. piperita), rosemary (Rosmarius officinalis), lemon (Citrus limon), grapefruit (C. paradise), and frankincense (Boswellia carteri), investigators found, in testing free radical-scavenging capacity and lipid peroxidation in the linoleic acid system, that peppermint essential oil exhibited the greatest radical-scavenging activity against the 2,2\'-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) ABTS radical (Nat. Prod. Res. 2010;24:140-51).
In 2010, Baliga and Rao showed that M. piperita and M. arvensis (wild mint) protected mice against gamma-radiation–induced morbidity and mortality. Specifically, M. piperita protected murine testes as well as gastrointestinal and hemopoietic systems (J. Cancer Res. Ther. 2010;6:255-62).
Anticancer activity
Investigations by Jain et al. into the molecular mechanisms supporting the anticarcinogenic potential of M. piperita leaf extracts on six human cancer cell lines (HeLa, MCF-7, Jurkat, T24, HT-29, MIAPaCa-2) in 2011 revealed that chloroform and ethyl acetate extracts dose- and time-dependently displayed anticarcinogenic activity leading to G1 cell cycle arrest and mitochondrial-mediated apoptosis among the cascade of effects. The investigators identified their findings as the first evidence of direct anticarcinogenic activity of Mentha leaf extracts and suggested that future work might focus on isolating active constituents as a foundation for mechanistic and translational studies leading to new anticancer drugs, alone or in combination, to prevent and treat human cancers (Int. J. Toxicol. 2011;30:225-36).
Topical benefits of menthol
In a recent examination of the antibacterial and antifungal properties, as well as speculated anti-inflammatory activity of menthol as a topical treatment for diaper dermatitis, investigators conducted a pilot clinical trial in a hospital setting. The study involved 84 neonates with diagnosed candidal diaper dermatitis who required no critical care or systemic antifungal and anti-inflammatory medications. The menthol group (n = 42) received topical clotrimazole and topically applied menthol drops and the control group (n = 42) received topical clotrimazole and a placebo. Thirty-five infants in each group completed the study. The researchers found that complete healing was shorter in the menthol group, with significant relief of erythema and pustules observed in this group. They concluded that topically-applied menthol may be an effective agent in the treatment of candidal diaper dermatitis (World J. Pediatr. 2011;7:167-70).
In 2011, Qiu et al. showed, through various assays, that menthol, in low concentrations, could significantly suppress the expression of alpha-hemolysin, enterotoxins A and B, and toxic shock syndrome toxin 1 in Staphylococcus aureus. The investigators concluded that menthol may warrant inclusion in the armamentarium against S. aureus when combined with beta-lactam antibiotics, which, at subinhibitory concentrations, can actually augment S. aureus toxin secretion. They added that menthol may also have possible uses in novel anti-virulence drugs (Appl. Microbiol. Biotechnol. 2011;90:705-12). It should be noted that menthol is considered safe and effective, with concentrations up to 16% approved in OTC external products by the Food and Drug Administration (J. Am. Acad. Dermatol. 2007;57:873-8).
Pruritus, TRPM8, and melanoma
Topically applied menthol, in concentrations of 1%-3%, is often used to treat pruritus, particularly in the elderly (Skin Therapy Lett. 2010;15:5-9). In addition, recent evidence suggests that the presence of menthol can facilitate penetration of other agents in topical products (Int. J. Toxicol. 2001;20 Suppl 3:61-73; J. Am. Acad. Dermatol. 2007;57:873-8). Patel and Yosipovitch suggest that elderly patients who report diminished pruritus with cooling may stand to benefit from menthol-containing topical therapies (J. Am. Acad. Dermatol. 2007;57:873-8; Skin Therapy Lett. 2010;15:5-9). Interestingly, menthol, via the transient receptor potential melastatin subfamily 8 (TRPM8) receptor, a member of a family of excitatory ion channels, engenders the same cooling sensation as low temperature, though menthol is not linked to a reduction in skin temperature (J. Am. Acad. Dermatol. 2007;57:873-8; Skin Therapy Lett. 2010;15:5-9).
Although the exact mechanism by which menthol exerts its antipruritic and analgesic effects has yet to be determined, the discovery that the TRPM8 is its underlying receptor is proving to be significant, particularly in understanding the cooling effect of the botanical (J. Am. Acad. Dermatol. 2007;57:873-8). There are also indications that menthol has therapeutic potential for melanoma. Specifically, melanoma expresses TRPM8 receptors, the activation of which inhibits melanoma viability. Menthol appears to mediate this response through an influx of extracellular calcium ions (Am. J. Physiol. Cell Physiol. 2008;295:C296-301; Am. J. Physiol. Cell Physiol. 2008;295:C293-5).
Peppermint oil
In 2003, Schuhmacher et al. investigated the virucidal effect of peppermint oil and found that it had a direct effect against herpes simplex virus type 1 (HSV-1) and herpes simplex virus type 2 (HSV-2) as well as an acyclovir-resistant HSV-1 strain. The investigators concluded, noting the lipophilic nature of peppermint oil, that it might be an appropriate topical treatment for recurrent herpes outbreaks (Phytomedicine 2003;10:504-10).
Because of its flavor, aroma, and cooling qualities, peppermint oil is used in a wide range of products, including cosmeceuticals, personal hygiene products (e.g., bath preparations, mouthwashes, toothpastes, and topical formulations), foods, pharmaceutical products, and aromatherapy. Topical indications include pruritus, irritation, and inflammation. Peppermint oil can act as a skin sensitizer, though, particularly in impaired and sensitive skin (Dermatitis 2010;21:327-9). Although peppermint oil has been reported to be a sensitizer in isolated cases, peppermint oil 8% was not found to be a sensitizer in a recent test using a maximization protocol and the various forms of peppermint (i.e., oil, extract, leaves, and water) are considered to be safe in cosmetic formulations. In rinse-off products, peppermint oil is used in concentrations up to 3% and up to 0.2% in leave-on formulations (Int. J. Toxicol. 2001;20 Suppl 3:61-73).
Conclusion
Peppermint and menthol, its naturally occurring monocyclic terpene alcohol derivative, have long been used for medical purposes. Contemporary practice and continuing research continue to support various uses of M. piperita in the medical armamentarium, with specific and additional uses continually being found in the dermatologic realm.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in Miami Beach. She founded the cosmetic dermatology center at the University of Miami in 1997. Dr. Baumann wrote the textbook "Cosmetic Dermatology: Principles and Practice" (McGraw-Hill, April 2002), and a book for consumers, "The Skin Type Solution" (Bantam, 2006). She has contributed to the Cosmeceutical Critique column in Skin & Allergy News since January 2001 and joined the editorial advisory board in 2004. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Galderma, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Stiefel, Topix Pharmaceuticals, and Unilever.
Wet dressings work for pruritus when other options fail
LAS VEGAS – Wet dressing, a technique forgotten in most places but in continual use at the Mayo Clinic for more than 80 years, knocks out intractable pruritus – whatever its cause – in children and adults, according to Dr. Mark Davis, chair of the division of clinical dermatology at the clinic in Rochester, Minn.
"It’s a simple technique that works extraordinarily well for any itchy condition from head to toe, and has virtually no side effects. It stops itching reliably, when nothing else has worked," including prednisone, methotrexate, phototherapy, and elimination diets, among other strategies, he said at Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
Even so, "there’s remarkably little on this in the literature, and what’s published is mostly just in kids, but in adults it works brilliantly, too, particularly for atopic dermatitis. We use it when people come in itching from anything, like psoriasis," he said (J. Am. Acad. Dermatol. 2009;60:792-800).
"The commonest question [we hear from people] is ‘I’ve been going to doctors for years. Why didn’t anybody tell me about this?’ " said Dr. Davis.
If their pruritus is severe enough, patients will be admitted to the Mayo Clinic and have wet cloths applied to wherever the itch happens to be – above the waist, below the waist, the feet, or even the entire body, including the face – with a dry blanket on top if needed to ward of the chill. Patients can get up from bed for a bathroom break when the dressings are changed every 3 hours.
Topical steroids are used with the dressings up to three times a day; 1% percent hydrocortisone for the face or genitals, 0.1% or 0.05% triamcinolone elsewhere. The Mayo Clinic has never had a case of hypothalamic-pituitary-adrenal axis suppression with the technique, and insurance usually covers the cost for 3 days, Dr. Davis said.
When outpatient treatment is sufficient, pruritic patients are instructed to put on wet pajamas or long johns, or hop into the shower in dry ones, and then leave them on for 30 minutes to an hour, 3-4 times a day. Nurses, with the help of handouts and videos, teach patients how to do this, and call them every 24 hours to see how they are getting along.
Hospital patients get the same instructions at discharge. "Initially, they have to do [it] at least once a day for a number of weeks, and then they can use [the technique] on an as-needed basis, maybe once or twice a week," Dr. Davis said.
"Wet dressings went out of favor" in most places because "they are just so much trouble," he said. At present, the technique is "largely unknown," as is the reason why it works, he added.
Dr. Davis has no relevant disclosures. SDEF and this news organization are owned by Frontline Medical Communications.
LAS VEGAS – Wet dressing, a technique forgotten in most places but in continual use at the Mayo Clinic for more than 80 years, knocks out intractable pruritus – whatever its cause – in children and adults, according to Dr. Mark Davis, chair of the division of clinical dermatology at the clinic in Rochester, Minn.
"It’s a simple technique that works extraordinarily well for any itchy condition from head to toe, and has virtually no side effects. It stops itching reliably, when nothing else has worked," including prednisone, methotrexate, phototherapy, and elimination diets, among other strategies, he said at Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
Even so, "there’s remarkably little on this in the literature, and what’s published is mostly just in kids, but in adults it works brilliantly, too, particularly for atopic dermatitis. We use it when people come in itching from anything, like psoriasis," he said (J. Am. Acad. Dermatol. 2009;60:792-800).
"The commonest question [we hear from people] is ‘I’ve been going to doctors for years. Why didn’t anybody tell me about this?’ " said Dr. Davis.
If their pruritus is severe enough, patients will be admitted to the Mayo Clinic and have wet cloths applied to wherever the itch happens to be – above the waist, below the waist, the feet, or even the entire body, including the face – with a dry blanket on top if needed to ward of the chill. Patients can get up from bed for a bathroom break when the dressings are changed every 3 hours.
Topical steroids are used with the dressings up to three times a day; 1% percent hydrocortisone for the face or genitals, 0.1% or 0.05% triamcinolone elsewhere. The Mayo Clinic has never had a case of hypothalamic-pituitary-adrenal axis suppression with the technique, and insurance usually covers the cost for 3 days, Dr. Davis said.
When outpatient treatment is sufficient, pruritic patients are instructed to put on wet pajamas or long johns, or hop into the shower in dry ones, and then leave them on for 30 minutes to an hour, 3-4 times a day. Nurses, with the help of handouts and videos, teach patients how to do this, and call them every 24 hours to see how they are getting along.
Hospital patients get the same instructions at discharge. "Initially, they have to do [it] at least once a day for a number of weeks, and then they can use [the technique] on an as-needed basis, maybe once or twice a week," Dr. Davis said.
"Wet dressings went out of favor" in most places because "they are just so much trouble," he said. At present, the technique is "largely unknown," as is the reason why it works, he added.
Dr. Davis has no relevant disclosures. SDEF and this news organization are owned by Frontline Medical Communications.
LAS VEGAS – Wet dressing, a technique forgotten in most places but in continual use at the Mayo Clinic for more than 80 years, knocks out intractable pruritus – whatever its cause – in children and adults, according to Dr. Mark Davis, chair of the division of clinical dermatology at the clinic in Rochester, Minn.
"It’s a simple technique that works extraordinarily well for any itchy condition from head to toe, and has virtually no side effects. It stops itching reliably, when nothing else has worked," including prednisone, methotrexate, phototherapy, and elimination diets, among other strategies, he said at Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
Even so, "there’s remarkably little on this in the literature, and what’s published is mostly just in kids, but in adults it works brilliantly, too, particularly for atopic dermatitis. We use it when people come in itching from anything, like psoriasis," he said (J. Am. Acad. Dermatol. 2009;60:792-800).
"The commonest question [we hear from people] is ‘I’ve been going to doctors for years. Why didn’t anybody tell me about this?’ " said Dr. Davis.
If their pruritus is severe enough, patients will be admitted to the Mayo Clinic and have wet cloths applied to wherever the itch happens to be – above the waist, below the waist, the feet, or even the entire body, including the face – with a dry blanket on top if needed to ward of the chill. Patients can get up from bed for a bathroom break when the dressings are changed every 3 hours.
Topical steroids are used with the dressings up to three times a day; 1% percent hydrocortisone for the face or genitals, 0.1% or 0.05% triamcinolone elsewhere. The Mayo Clinic has never had a case of hypothalamic-pituitary-adrenal axis suppression with the technique, and insurance usually covers the cost for 3 days, Dr. Davis said.
When outpatient treatment is sufficient, pruritic patients are instructed to put on wet pajamas or long johns, or hop into the shower in dry ones, and then leave them on for 30 minutes to an hour, 3-4 times a day. Nurses, with the help of handouts and videos, teach patients how to do this, and call them every 24 hours to see how they are getting along.
Hospital patients get the same instructions at discharge. "Initially, they have to do [it] at least once a day for a number of weeks, and then they can use [the technique] on an as-needed basis, maybe once or twice a week," Dr. Davis said.
"Wet dressings went out of favor" in most places because "they are just so much trouble," he said. At present, the technique is "largely unknown," as is the reason why it works, he added.
Dr. Davis has no relevant disclosures. SDEF and this news organization are owned by Frontline Medical Communications.
AT SDEF LAS VEGAS DERMATOLOGY SEMINAR