User login
The Role of Inpatient Dermatology Consultations
Dermatology is an often-underutilized resource in the hospital setting. As the health care landscape has evolved, so has the role of the inpatient dermatologist.1-3 Structural changes in the health system and advances in therapies have shifted dermatology from an admitting service to an almost exclusively outpatient practice. Improved treatment modalities led to decreases in the number of patients requiring admission for chronic dermatoses, and outpatient clinics began offering therapies once limited to hospitals.1,4 Inpatient dermatology consultations emerged and continue to have profound effects on hospitalized patients regardless of their reason for admission.1-11
Inpatient dermatologists supply knowledge in areas primary medical teams lack, and there is evidence that dermatology consultations improve the quality of care while decreasing cost.2,5-7 Establishing correct diagnoses, preventing exposure to unnecessary medications, and reducing hospitalization duration and readmission rates are a few ways dermatology consultations positively impact hospitalized patients.2,5-7,9,10 This study highlights the role of the dermatologist in the care of hospitalized patients at a large academic medical center in an urban setting and reveals how consultation supports the efficiency and efficacy of other services.
Materials and Methods
Study Design—This single-institution, cross-sectional retrospective study included all hospitalized patients at the Thomas Jefferson University Hospital (Philadelphia, Pennsylvania), who received an inpatient dermatology consultation completed by physicians of Jefferson Dermatology Associates between January 1, 2019, and December 31, 2019. The institutional review board at Thomas Jefferson University approved this study.
Data Collection—A list of all inpatient dermatology consultations in 2019 was provided by Jefferson Dermatology Associates. Through a retrospective chart review, data regarding the consultations were collected from the electronic medical record (Epic Systems) and recorded into the Research Electronic Data Capture system. Data on patient demographics, the primary medical team, the dermatology evaluation, and the hospital course of the patient were collected.
Results
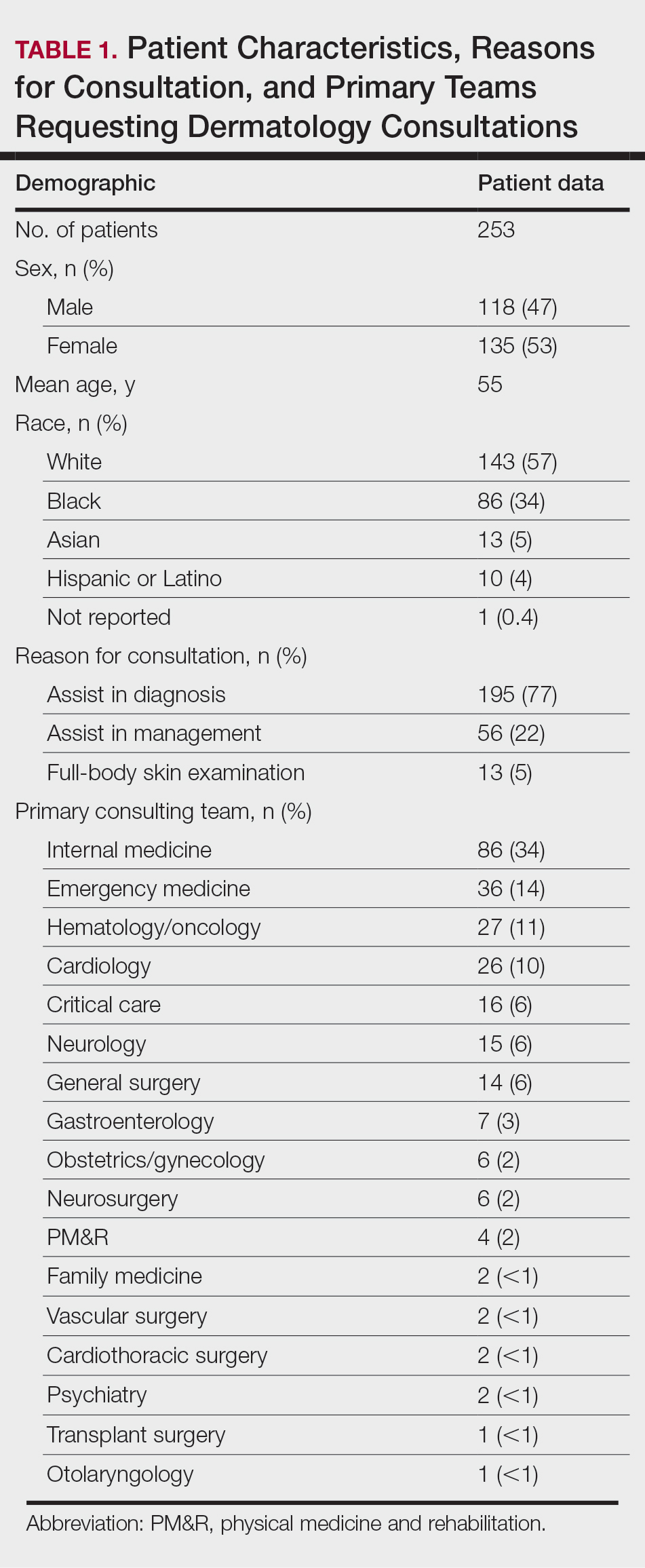
Patient Characteristics—Dermatology received 253 inpatient consultation requests during this time period; 53% of patients were female and 47% were male, with a mean age of 55 years. Most patients were White (57%), while 34% were Black. Five percent and 4% of patients were Asian and Hispanic or Latino, respectively (Table 1). The mean duration of hospitalization for all patients was 15 days, and the average number of days to discharge following the first encounter with dermatology was 10 days.
Requesting Team and Reason for Consultation—Internal medicine consulted dermatology most frequently (34% of all consultations), followed by emergency medicine (14%) and a variety of other services (Table 1). Most dermatology consultations were placed to assist in achieving a diagnosis of a cutaneous condition (77%), while a minority were to assist in the management of a previously diagnosed disease (22%). A small fraction of consultations (5%) were to complete full-body skin examinations (FBSEs) to rule out infection or malignancy in candidates for organ transplantation, left ventricular assist devices, or certain chemotherapies. One FBSE was conducted to search for a primary tumor in a patient diagnosed with metastatic melanoma.
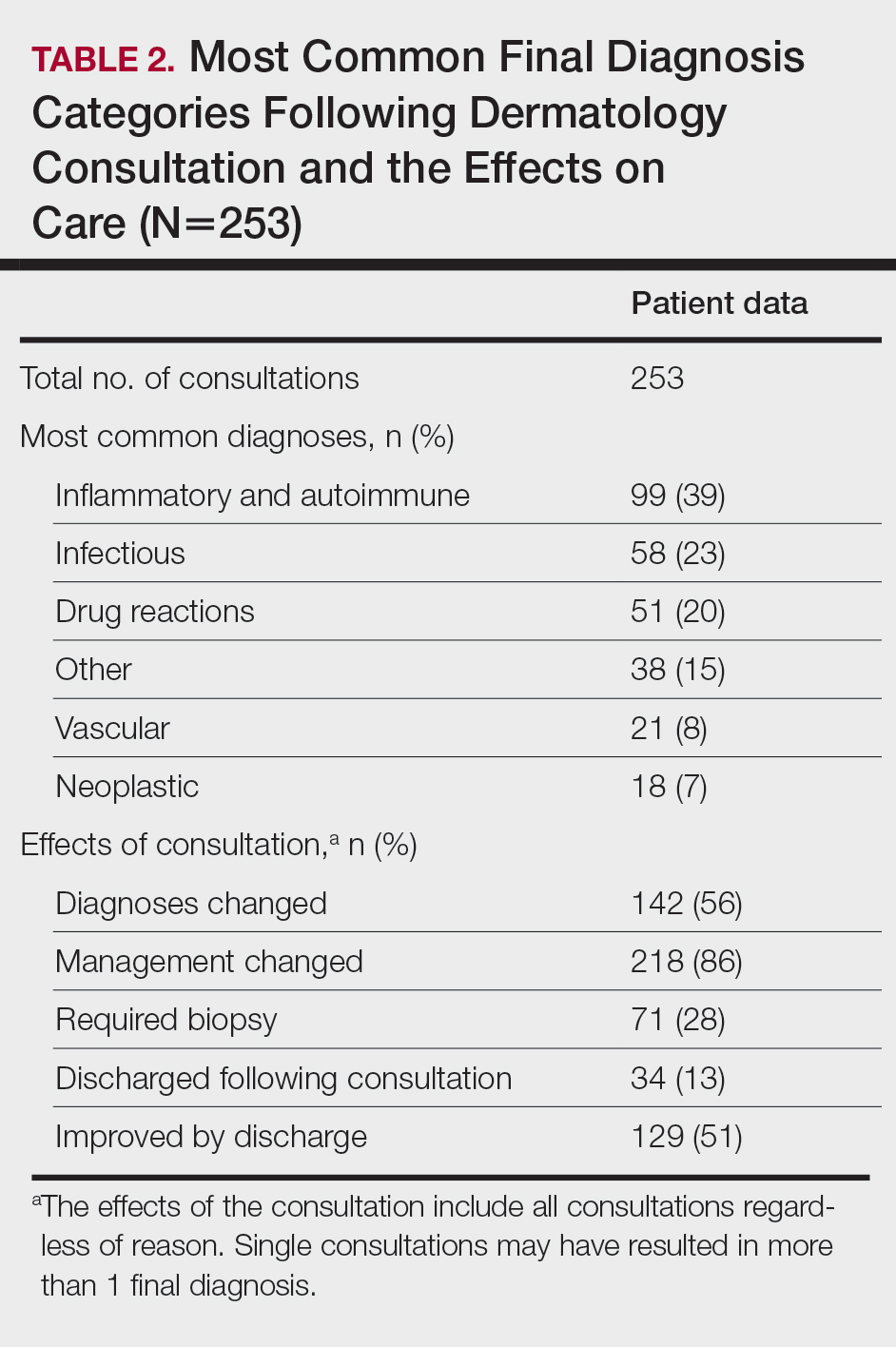
Most Common Final Diagnoses and Consultation Impact—Table 2 lists the most common final diagnosis categories, as well as the effects of the consultation on diagnosis, management, biopsies, hospitalization, and clinical improvement as documented by the primary medical provider. The most common final diagnoses were inflammatory and autoimmune (39%), such as contact dermatitis and seborrheic dermatitis; infectious (23%), such as varicella (primary or zoster) and bacterial furunculosis; drug reactions (20%), such as morbilliform drug eruptions; vascular (8%), such as vasculitis and calciphylaxis; neoplastic (7%), such as keratinocyte carcinomas and leukemia cutis; and other (15%), such as xerosis, keratosis pilaris, and miliaria rubra.
Impact on Diagnosis—Fifty-six percent of all consultations resulted in a change in diagnosis. When dermatology was consulted specifically to assist in the diagnosis of a patient (195 consultations), the working diagnosis of the primary team was changed 69% of the time. Thirty-five of these consultation requests had no preliminary diagnosis, and the primary team listed the working diagnosis as either rash or a morphologic description of the lesion(s). Sixty-three percent of suspected drug eruptions ended with a diagnosis of a form of drug eruption, while 20% of consultations for suspected cellulitis or bacterial infections were confirmed to be cellulitis or soft tissue infections.
Impact on Management—Regardless of the reason for the consultation, most consultations (86%) resulted in a change in management. The remaining 14% consisted of FBSEs with benign findings; cases of cutaneous metastases and leukemia cutis managed by oncology; as well as select cases of purpura fulminans, postfebrile desquamation, and postinflammatory hyperpigmentation.
Changes in management included alterations in medications, requests for additional laboratory work or imaging, additional consultation requests, biopsies, or specific wound care instructions. Seventy-five percent of all consultations were given specific medication recommendations by dermatology. Most (61%) were recommended to be given a topical steroid, antibiotic, or both. However, 45% of all consultations were recommended to initiate a systemic medication, most commonly antihistamines, antibiotics, steroids, antivirals, or immunomodulators. Dermatology recommended discontinuing specific medications in 16% of all consultations, with antibiotics being the most frequent culprit (17 antibiotics discontinued), owing to drug eruptions or misdiagnosed infections. Vancomycin, piperacillin-tazobactam, and trimethoprim-sulfamethoxazole were the most frequently discontinued antibiotics.
Dermatology was consulted for assistance in management of previously diagnosed cutaneous conditions 56 times (22% of all consultations), often regarding complicated cases of hidradenitis suppurativa (9 cases), pyoderma gangrenosum (5 cases), bullous pemphigoid (4 cases), or erythroderma (4 cases). Most of these cases required a single dermatology encounter to provide recommendations (71%), and 21% required 1 additional follow-up. Sixty-three percent of patients consulted for management assistance were noted to have improvement in their cutaneous condition by time of discharge, as documented by the primary provider in the medical record.
Twenty-eight percent of all consultations required at least 1 biopsy. Seventy-two percent of all biopsies were consistent with the dermatologist’s working diagnosis or highest-ranked differential diagnosis, and 16% of biopsy results were consistent with the second- or third-ranked diagnosis. The primary teams requested a biopsy 38 times to assist in diagnosis, as documented in the progress note or consultation request. Only 21 of these consultations (55% of requests) received at least 1 biopsy, as the remaining consultations did not require a biopsy to establish a diagnosis. The most common final diagnoses of consultations receiving biopsies included drug eruptions (5), leukemia cutis (4), vasculopathies (4), vasculitis (4), and calciphylaxis (3).
Impact on Hospitalization and Efficacy—Dermatology performed 217 consultations regarding patients already admitted to the hospital, and 92% remained hospitalized either due to comorbidities or complicated cutaneous conditions following the consultation. The remaining 8% were cleared for discharge. Dermatology received 36 consultation requests from emergency medicine physicians. Fifty-three percent of these patients were admitted, while the remaining 47% were discharged from the emergency department or its observation unit following evaluation.
Fifty-one percent of all consultations were noted to have improvement in their cutaneous condition by the time of discharge, as noted in the physical examination, progress note, or discharge summary of the primary team. Thirty percent of cases remained stable, where improvement was not noted in in the medical record. Most of these cases involved keratinocyte carcinomas scheduled for outpatient excision, benign melanocytic nevi found on FBSE, and benign etiologies that led to immediate discharge following consultation. Three percent of all consultations were noted to have worsened following consultation, including cases of calciphylaxis, vasculopathies, and purpura fulminans, as well as patients who elected for palliative care and hospice. The cutaneous condition by the time of discharge could not be determined from the medical record in 16% of all consultations.
Eighty-five percent of all consultations required a single encounter with dermatology. An additional 10% required a single follow-up with dermatology, while only 5% of patients required 3 or more encounters. Notably, these cases included patients with 1 or more severe cutaneous diseases, such as Sweet syndrome, calciphylaxis, Stevens-Johnson syndrome/toxic epidermal necrolysis, and hidradenitis suppurativa.
Comment
Although dermatology often is viewed as an outpatient specialty, this study provides a glimpse into the ways inpatient dermatology consultations optimize the care of hospitalized patients. Most consultations involved assistance in diagnosing an unknown condition, but several regarded pre-existing skin disorders requiring management aid. As a variety of medical specialties requested consultations, dermatology was able to provide care to a diverse group of patients with conditions varying in complexity and severity. Several specialties benefited from niche dermatologic expertise: hematology and oncology frequently requested dermatology to assist in diagnosis and management of the toxic effects of chemotherapy, cutaneous metastasis, or suspected cutaneous infections in immunocompromised patients. Cardiology patients were frequently evaluated for potential malignancy or infection prior to heart transplantation and initiation of antirejection immunosuppressants. Dermatology was consulted to differentiate cutaneous manifestations of critical illness from underlying systemic disease in the intensive care unit, and patients presenting to the emergency department often were examined to determine if hospital admission was necessary, with 47% of these consultations resulting in a discharge following evaluation by a dermatologist.
Our results were consistent with prior studies1,5,6 that have reported frequent changes in final diagnosis following dermatology consultation, with 69% of working diagnoses changed in this study when consultation was requested for diagnostic assistance. When dermatology was consulted for diagnostic assistance, several of these cases lacked a preliminary differential diagnosis. Although the absence of a documented differential diagnosis may not necessarily reflect a lack of suspicion for a particular etiology, 86% of all consultations included a ranked differential or working diagnosis either in the consultation request or progress note prior to consultation. The final diagnoses of consultations without a preliminary diagnosis varied from the mild and localized to systemic and severe, further suggesting these cases reflected knowledge gaps of the primary medical team.
Integration of dermatology into the care of hospitalized patients could provide an opportunity for education of primary medical teams. With frequent consultation, primary medical teams may become more comfortable diagnosing and managing common cutaneous conditions specific to their specialty or extended hospitalizations.
Several consultations were requested to aid in management of cases of hidradenitis suppurativa, pyoderma gangrenosum, or bullous pemphigoid that either failed outpatient therapy or were complicated by superinfections. Despite the ranges in complexity, the majority of all consultations required a single encounter and led to improvement by the time of discharge, demonstrating the efficacy and efficiency of inpatient dermatologists.
Dermatology consultations often led to changes in management involving medications and additional workup. Changes in management also extended to specific wound care instructions provided by dermatology, as expected for cases of Stevens-Johnson syndrome/toxic epidermal necrolysis, Sweet syndrome, hidradenitis suppurativa, and pyoderma gangrenosum. However, patients with the sequelae of extended hospitalizations, such as chronic wounds, pressure ulcers, and edema bullae, also benefited from this expertise.
When patients required a biopsy, the final diagnoses were consistent with the dermatologist’s number one differential diagnosis or top 3 differential diagnoses 72% and 88% of the time, respectively. Only 55% of cases where the primary team requested a biopsy ultimately required a biopsy, as many involved clinical diagnoses such as urticaria. Not only was dermatology accurate in their preliminary diagnoses, but they decreased cost and morbidity by avoiding unnecessary procedures.
This study provided additional evidence to support the integration of dermatology into the hospital setting for the benefit of patients, primary medical teams, and hospital systems. Dermatology offers high-value care through the efficient diagnosis and management of hospitalized patients, which contributes to decreased cost and improved outcomes.2,5-7,9,10 This study highlighted lesser-known areas of impact, such as the various specialty-specific services dermatology provides as well as the high rates of reported improvement following consultation. Future studies should continue to explore the field’s unique impact on hospitalized medicine as well as other avenues of care delivery, such as telemedicine, that may encourage dermatologists to participate in consultations and increase the volume of patients who may benefit from their care.
- Madigan LM, Fox LP. Where are we now with inpatient consultative dermatology?: assessing the value and evolution of this subspecialty over the past decade. J Am Acad Dermatol. 2019;80:1804-1808. doi:10.1016/j.jaad.2019.01.031
- Noe MH, Rosenbach M. Inpatient dermatologists—crucial for the management of skin diseases in hospitalized patients [editorial]. JAMA Dermatol. 2018;154:524-525. doi:10.1001/jamadermatol.2017.6195
- Strowd LC. Inpatient dermatology: a paradigm shift in the management of skin disease in the hospital. Br J Dermatol. 2019;180:966-967. doi:10.1111/bjd.17778
- Kirsner RS, Yang DG, Kerdel FA. The changing status of inpatient dermatology at American academic dermatology programs. J Am Acad Dermatol. 1999;40:755-757. doi:10.1016/s0190-9622(99)70158-1
- Kroshinsky D, Cotliar J, Hughey LC, et al. Association of dermatology consultation with accuracy of cutaneous disorder diagnoses in hospitalized patients: a multicenter analysis. JAMA Dermatol. 2016;152:477-480. doi:10.1001/jamadermatol.2015.5098
- Ko LN, Garza-Mayers AC, St John J, et al. Effect of dermatology consultation on outcomes for patients with presumed cellulitis. JAMA Dermatol. 2018;154:529-533. doi:10.1001/jamadermatol.2017.6196
- Li DG, Xia FD, Khosravi H, et al. Outcomes of early dermatology consultation for inpatients diagnosed with cellulitis. JAMA Dermatol. 2018;154:537-543. doi:10.1001/jamadermatol.2017.6197
- Milani-Nejad N, Zhang M, Kaffenberger BH. Association of dermatology consultations with patient care outcomes in hospitalized patients with inflammatory skin diseases. JAMA Dermatol. 2017;153:523-528. doi:10.1001/jamadermatol.2016.6130
- Imadojemu S, Rosenbach M. Dermatologists must take an active role in the diagnosis of cellulitis. JAMA Dermatol. 2017;153:134-135. doi:10.1001/jamadermatol.2016.4230
- Hughey LC. The impact dermatologists can have on misdiagnosis of cellulitis and overuse of antibiotics: closing the gap. JAMA Dermatol. 2014;150:1061-1062. doi:10.1001/jamadermatol.2014.1164
- Ko LN, Kroshinsky D. Dermatology hospitalists: a multicenter survey study characterizing the infrastructure of consultative dermatology in select American hospitals. Int J Dermatol. 2018;57:553-558. doi:10.1111/ijd.13939
Dermatology is an often-underutilized resource in the hospital setting. As the health care landscape has evolved, so has the role of the inpatient dermatologist.1-3 Structural changes in the health system and advances in therapies have shifted dermatology from an admitting service to an almost exclusively outpatient practice. Improved treatment modalities led to decreases in the number of patients requiring admission for chronic dermatoses, and outpatient clinics began offering therapies once limited to hospitals.1,4 Inpatient dermatology consultations emerged and continue to have profound effects on hospitalized patients regardless of their reason for admission.1-11
Inpatient dermatologists supply knowledge in areas primary medical teams lack, and there is evidence that dermatology consultations improve the quality of care while decreasing cost.2,5-7 Establishing correct diagnoses, preventing exposure to unnecessary medications, and reducing hospitalization duration and readmission rates are a few ways dermatology consultations positively impact hospitalized patients.2,5-7,9,10 This study highlights the role of the dermatologist in the care of hospitalized patients at a large academic medical center in an urban setting and reveals how consultation supports the efficiency and efficacy of other services.
Materials and Methods
Study Design—This single-institution, cross-sectional retrospective study included all hospitalized patients at the Thomas Jefferson University Hospital (Philadelphia, Pennsylvania), who received an inpatient dermatology consultation completed by physicians of Jefferson Dermatology Associates between January 1, 2019, and December 31, 2019. The institutional review board at Thomas Jefferson University approved this study.
Data Collection—A list of all inpatient dermatology consultations in 2019 was provided by Jefferson Dermatology Associates. Through a retrospective chart review, data regarding the consultations were collected from the electronic medical record (Epic Systems) and recorded into the Research Electronic Data Capture system. Data on patient demographics, the primary medical team, the dermatology evaluation, and the hospital course of the patient were collected.
Results
Patient Characteristics—Dermatology received 253 inpatient consultation requests during this time period; 53% of patients were female and 47% were male, with a mean age of 55 years. Most patients were White (57%), while 34% were Black. Five percent and 4% of patients were Asian and Hispanic or Latino, respectively (Table 1). The mean duration of hospitalization for all patients was 15 days, and the average number of days to discharge following the first encounter with dermatology was 10 days.
Requesting Team and Reason for Consultation—Internal medicine consulted dermatology most frequently (34% of all consultations), followed by emergency medicine (14%) and a variety of other services (Table 1). Most dermatology consultations were placed to assist in achieving a diagnosis of a cutaneous condition (77%), while a minority were to assist in the management of a previously diagnosed disease (22%). A small fraction of consultations (5%) were to complete full-body skin examinations (FBSEs) to rule out infection or malignancy in candidates for organ transplantation, left ventricular assist devices, or certain chemotherapies. One FBSE was conducted to search for a primary tumor in a patient diagnosed with metastatic melanoma.
Most Common Final Diagnoses and Consultation Impact—Table 2 lists the most common final diagnosis categories, as well as the effects of the consultation on diagnosis, management, biopsies, hospitalization, and clinical improvement as documented by the primary medical provider. The most common final diagnoses were inflammatory and autoimmune (39%), such as contact dermatitis and seborrheic dermatitis; infectious (23%), such as varicella (primary or zoster) and bacterial furunculosis; drug reactions (20%), such as morbilliform drug eruptions; vascular (8%), such as vasculitis and calciphylaxis; neoplastic (7%), such as keratinocyte carcinomas and leukemia cutis; and other (15%), such as xerosis, keratosis pilaris, and miliaria rubra.
Impact on Diagnosis—Fifty-six percent of all consultations resulted in a change in diagnosis. When dermatology was consulted specifically to assist in the diagnosis of a patient (195 consultations), the working diagnosis of the primary team was changed 69% of the time. Thirty-five of these consultation requests had no preliminary diagnosis, and the primary team listed the working diagnosis as either rash or a morphologic description of the lesion(s). Sixty-three percent of suspected drug eruptions ended with a diagnosis of a form of drug eruption, while 20% of consultations for suspected cellulitis or bacterial infections were confirmed to be cellulitis or soft tissue infections.
Impact on Management—Regardless of the reason for the consultation, most consultations (86%) resulted in a change in management. The remaining 14% consisted of FBSEs with benign findings; cases of cutaneous metastases and leukemia cutis managed by oncology; as well as select cases of purpura fulminans, postfebrile desquamation, and postinflammatory hyperpigmentation.
Changes in management included alterations in medications, requests for additional laboratory work or imaging, additional consultation requests, biopsies, or specific wound care instructions. Seventy-five percent of all consultations were given specific medication recommendations by dermatology. Most (61%) were recommended to be given a topical steroid, antibiotic, or both. However, 45% of all consultations were recommended to initiate a systemic medication, most commonly antihistamines, antibiotics, steroids, antivirals, or immunomodulators. Dermatology recommended discontinuing specific medications in 16% of all consultations, with antibiotics being the most frequent culprit (17 antibiotics discontinued), owing to drug eruptions or misdiagnosed infections. Vancomycin, piperacillin-tazobactam, and trimethoprim-sulfamethoxazole were the most frequently discontinued antibiotics.
Dermatology was consulted for assistance in management of previously diagnosed cutaneous conditions 56 times (22% of all consultations), often regarding complicated cases of hidradenitis suppurativa (9 cases), pyoderma gangrenosum (5 cases), bullous pemphigoid (4 cases), or erythroderma (4 cases). Most of these cases required a single dermatology encounter to provide recommendations (71%), and 21% required 1 additional follow-up. Sixty-three percent of patients consulted for management assistance were noted to have improvement in their cutaneous condition by time of discharge, as documented by the primary provider in the medical record.
Twenty-eight percent of all consultations required at least 1 biopsy. Seventy-two percent of all biopsies were consistent with the dermatologist’s working diagnosis or highest-ranked differential diagnosis, and 16% of biopsy results were consistent with the second- or third-ranked diagnosis. The primary teams requested a biopsy 38 times to assist in diagnosis, as documented in the progress note or consultation request. Only 21 of these consultations (55% of requests) received at least 1 biopsy, as the remaining consultations did not require a biopsy to establish a diagnosis. The most common final diagnoses of consultations receiving biopsies included drug eruptions (5), leukemia cutis (4), vasculopathies (4), vasculitis (4), and calciphylaxis (3).
Impact on Hospitalization and Efficacy—Dermatology performed 217 consultations regarding patients already admitted to the hospital, and 92% remained hospitalized either due to comorbidities or complicated cutaneous conditions following the consultation. The remaining 8% were cleared for discharge. Dermatology received 36 consultation requests from emergency medicine physicians. Fifty-three percent of these patients were admitted, while the remaining 47% were discharged from the emergency department or its observation unit following evaluation.
Fifty-one percent of all consultations were noted to have improvement in their cutaneous condition by the time of discharge, as noted in the physical examination, progress note, or discharge summary of the primary team. Thirty percent of cases remained stable, where improvement was not noted in in the medical record. Most of these cases involved keratinocyte carcinomas scheduled for outpatient excision, benign melanocytic nevi found on FBSE, and benign etiologies that led to immediate discharge following consultation. Three percent of all consultations were noted to have worsened following consultation, including cases of calciphylaxis, vasculopathies, and purpura fulminans, as well as patients who elected for palliative care and hospice. The cutaneous condition by the time of discharge could not be determined from the medical record in 16% of all consultations.
Eighty-five percent of all consultations required a single encounter with dermatology. An additional 10% required a single follow-up with dermatology, while only 5% of patients required 3 or more encounters. Notably, these cases included patients with 1 or more severe cutaneous diseases, such as Sweet syndrome, calciphylaxis, Stevens-Johnson syndrome/toxic epidermal necrolysis, and hidradenitis suppurativa.
Comment
Although dermatology often is viewed as an outpatient specialty, this study provides a glimpse into the ways inpatient dermatology consultations optimize the care of hospitalized patients. Most consultations involved assistance in diagnosing an unknown condition, but several regarded pre-existing skin disorders requiring management aid. As a variety of medical specialties requested consultations, dermatology was able to provide care to a diverse group of patients with conditions varying in complexity and severity. Several specialties benefited from niche dermatologic expertise: hematology and oncology frequently requested dermatology to assist in diagnosis and management of the toxic effects of chemotherapy, cutaneous metastasis, or suspected cutaneous infections in immunocompromised patients. Cardiology patients were frequently evaluated for potential malignancy or infection prior to heart transplantation and initiation of antirejection immunosuppressants. Dermatology was consulted to differentiate cutaneous manifestations of critical illness from underlying systemic disease in the intensive care unit, and patients presenting to the emergency department often were examined to determine if hospital admission was necessary, with 47% of these consultations resulting in a discharge following evaluation by a dermatologist.
Our results were consistent with prior studies1,5,6 that have reported frequent changes in final diagnosis following dermatology consultation, with 69% of working diagnoses changed in this study when consultation was requested for diagnostic assistance. When dermatology was consulted for diagnostic assistance, several of these cases lacked a preliminary differential diagnosis. Although the absence of a documented differential diagnosis may not necessarily reflect a lack of suspicion for a particular etiology, 86% of all consultations included a ranked differential or working diagnosis either in the consultation request or progress note prior to consultation. The final diagnoses of consultations without a preliminary diagnosis varied from the mild and localized to systemic and severe, further suggesting these cases reflected knowledge gaps of the primary medical team.
Integration of dermatology into the care of hospitalized patients could provide an opportunity for education of primary medical teams. With frequent consultation, primary medical teams may become more comfortable diagnosing and managing common cutaneous conditions specific to their specialty or extended hospitalizations.
Several consultations were requested to aid in management of cases of hidradenitis suppurativa, pyoderma gangrenosum, or bullous pemphigoid that either failed outpatient therapy or were complicated by superinfections. Despite the ranges in complexity, the majority of all consultations required a single encounter and led to improvement by the time of discharge, demonstrating the efficacy and efficiency of inpatient dermatologists.
Dermatology consultations often led to changes in management involving medications and additional workup. Changes in management also extended to specific wound care instructions provided by dermatology, as expected for cases of Stevens-Johnson syndrome/toxic epidermal necrolysis, Sweet syndrome, hidradenitis suppurativa, and pyoderma gangrenosum. However, patients with the sequelae of extended hospitalizations, such as chronic wounds, pressure ulcers, and edema bullae, also benefited from this expertise.
When patients required a biopsy, the final diagnoses were consistent with the dermatologist’s number one differential diagnosis or top 3 differential diagnoses 72% and 88% of the time, respectively. Only 55% of cases where the primary team requested a biopsy ultimately required a biopsy, as many involved clinical diagnoses such as urticaria. Not only was dermatology accurate in their preliminary diagnoses, but they decreased cost and morbidity by avoiding unnecessary procedures.
This study provided additional evidence to support the integration of dermatology into the hospital setting for the benefit of patients, primary medical teams, and hospital systems. Dermatology offers high-value care through the efficient diagnosis and management of hospitalized patients, which contributes to decreased cost and improved outcomes.2,5-7,9,10 This study highlighted lesser-known areas of impact, such as the various specialty-specific services dermatology provides as well as the high rates of reported improvement following consultation. Future studies should continue to explore the field’s unique impact on hospitalized medicine as well as other avenues of care delivery, such as telemedicine, that may encourage dermatologists to participate in consultations and increase the volume of patients who may benefit from their care.
Dermatology is an often-underutilized resource in the hospital setting. As the health care landscape has evolved, so has the role of the inpatient dermatologist.1-3 Structural changes in the health system and advances in therapies have shifted dermatology from an admitting service to an almost exclusively outpatient practice. Improved treatment modalities led to decreases in the number of patients requiring admission for chronic dermatoses, and outpatient clinics began offering therapies once limited to hospitals.1,4 Inpatient dermatology consultations emerged and continue to have profound effects on hospitalized patients regardless of their reason for admission.1-11
Inpatient dermatologists supply knowledge in areas primary medical teams lack, and there is evidence that dermatology consultations improve the quality of care while decreasing cost.2,5-7 Establishing correct diagnoses, preventing exposure to unnecessary medications, and reducing hospitalization duration and readmission rates are a few ways dermatology consultations positively impact hospitalized patients.2,5-7,9,10 This study highlights the role of the dermatologist in the care of hospitalized patients at a large academic medical center in an urban setting and reveals how consultation supports the efficiency and efficacy of other services.
Materials and Methods
Study Design—This single-institution, cross-sectional retrospective study included all hospitalized patients at the Thomas Jefferson University Hospital (Philadelphia, Pennsylvania), who received an inpatient dermatology consultation completed by physicians of Jefferson Dermatology Associates between January 1, 2019, and December 31, 2019. The institutional review board at Thomas Jefferson University approved this study.
Data Collection—A list of all inpatient dermatology consultations in 2019 was provided by Jefferson Dermatology Associates. Through a retrospective chart review, data regarding the consultations were collected from the electronic medical record (Epic Systems) and recorded into the Research Electronic Data Capture system. Data on patient demographics, the primary medical team, the dermatology evaluation, and the hospital course of the patient were collected.
Results
Patient Characteristics—Dermatology received 253 inpatient consultation requests during this time period; 53% of patients were female and 47% were male, with a mean age of 55 years. Most patients were White (57%), while 34% were Black. Five percent and 4% of patients were Asian and Hispanic or Latino, respectively (Table 1). The mean duration of hospitalization for all patients was 15 days, and the average number of days to discharge following the first encounter with dermatology was 10 days.
Requesting Team and Reason for Consultation—Internal medicine consulted dermatology most frequently (34% of all consultations), followed by emergency medicine (14%) and a variety of other services (Table 1). Most dermatology consultations were placed to assist in achieving a diagnosis of a cutaneous condition (77%), while a minority were to assist in the management of a previously diagnosed disease (22%). A small fraction of consultations (5%) were to complete full-body skin examinations (FBSEs) to rule out infection or malignancy in candidates for organ transplantation, left ventricular assist devices, or certain chemotherapies. One FBSE was conducted to search for a primary tumor in a patient diagnosed with metastatic melanoma.
Most Common Final Diagnoses and Consultation Impact—Table 2 lists the most common final diagnosis categories, as well as the effects of the consultation on diagnosis, management, biopsies, hospitalization, and clinical improvement as documented by the primary medical provider. The most common final diagnoses were inflammatory and autoimmune (39%), such as contact dermatitis and seborrheic dermatitis; infectious (23%), such as varicella (primary or zoster) and bacterial furunculosis; drug reactions (20%), such as morbilliform drug eruptions; vascular (8%), such as vasculitis and calciphylaxis; neoplastic (7%), such as keratinocyte carcinomas and leukemia cutis; and other (15%), such as xerosis, keratosis pilaris, and miliaria rubra.
Impact on Diagnosis—Fifty-six percent of all consultations resulted in a change in diagnosis. When dermatology was consulted specifically to assist in the diagnosis of a patient (195 consultations), the working diagnosis of the primary team was changed 69% of the time. Thirty-five of these consultation requests had no preliminary diagnosis, and the primary team listed the working diagnosis as either rash or a morphologic description of the lesion(s). Sixty-three percent of suspected drug eruptions ended with a diagnosis of a form of drug eruption, while 20% of consultations for suspected cellulitis or bacterial infections were confirmed to be cellulitis or soft tissue infections.
Impact on Management—Regardless of the reason for the consultation, most consultations (86%) resulted in a change in management. The remaining 14% consisted of FBSEs with benign findings; cases of cutaneous metastases and leukemia cutis managed by oncology; as well as select cases of purpura fulminans, postfebrile desquamation, and postinflammatory hyperpigmentation.
Changes in management included alterations in medications, requests for additional laboratory work or imaging, additional consultation requests, biopsies, or specific wound care instructions. Seventy-five percent of all consultations were given specific medication recommendations by dermatology. Most (61%) were recommended to be given a topical steroid, antibiotic, or both. However, 45% of all consultations were recommended to initiate a systemic medication, most commonly antihistamines, antibiotics, steroids, antivirals, or immunomodulators. Dermatology recommended discontinuing specific medications in 16% of all consultations, with antibiotics being the most frequent culprit (17 antibiotics discontinued), owing to drug eruptions or misdiagnosed infections. Vancomycin, piperacillin-tazobactam, and trimethoprim-sulfamethoxazole were the most frequently discontinued antibiotics.
Dermatology was consulted for assistance in management of previously diagnosed cutaneous conditions 56 times (22% of all consultations), often regarding complicated cases of hidradenitis suppurativa (9 cases), pyoderma gangrenosum (5 cases), bullous pemphigoid (4 cases), or erythroderma (4 cases). Most of these cases required a single dermatology encounter to provide recommendations (71%), and 21% required 1 additional follow-up. Sixty-three percent of patients consulted for management assistance were noted to have improvement in their cutaneous condition by time of discharge, as documented by the primary provider in the medical record.
Twenty-eight percent of all consultations required at least 1 biopsy. Seventy-two percent of all biopsies were consistent with the dermatologist’s working diagnosis or highest-ranked differential diagnosis, and 16% of biopsy results were consistent with the second- or third-ranked diagnosis. The primary teams requested a biopsy 38 times to assist in diagnosis, as documented in the progress note or consultation request. Only 21 of these consultations (55% of requests) received at least 1 biopsy, as the remaining consultations did not require a biopsy to establish a diagnosis. The most common final diagnoses of consultations receiving biopsies included drug eruptions (5), leukemia cutis (4), vasculopathies (4), vasculitis (4), and calciphylaxis (3).
Impact on Hospitalization and Efficacy—Dermatology performed 217 consultations regarding patients already admitted to the hospital, and 92% remained hospitalized either due to comorbidities or complicated cutaneous conditions following the consultation. The remaining 8% were cleared for discharge. Dermatology received 36 consultation requests from emergency medicine physicians. Fifty-three percent of these patients were admitted, while the remaining 47% were discharged from the emergency department or its observation unit following evaluation.
Fifty-one percent of all consultations were noted to have improvement in their cutaneous condition by the time of discharge, as noted in the physical examination, progress note, or discharge summary of the primary team. Thirty percent of cases remained stable, where improvement was not noted in in the medical record. Most of these cases involved keratinocyte carcinomas scheduled for outpatient excision, benign melanocytic nevi found on FBSE, and benign etiologies that led to immediate discharge following consultation. Three percent of all consultations were noted to have worsened following consultation, including cases of calciphylaxis, vasculopathies, and purpura fulminans, as well as patients who elected for palliative care and hospice. The cutaneous condition by the time of discharge could not be determined from the medical record in 16% of all consultations.
Eighty-five percent of all consultations required a single encounter with dermatology. An additional 10% required a single follow-up with dermatology, while only 5% of patients required 3 or more encounters. Notably, these cases included patients with 1 or more severe cutaneous diseases, such as Sweet syndrome, calciphylaxis, Stevens-Johnson syndrome/toxic epidermal necrolysis, and hidradenitis suppurativa.
Comment
Although dermatology often is viewed as an outpatient specialty, this study provides a glimpse into the ways inpatient dermatology consultations optimize the care of hospitalized patients. Most consultations involved assistance in diagnosing an unknown condition, but several regarded pre-existing skin disorders requiring management aid. As a variety of medical specialties requested consultations, dermatology was able to provide care to a diverse group of patients with conditions varying in complexity and severity. Several specialties benefited from niche dermatologic expertise: hematology and oncology frequently requested dermatology to assist in diagnosis and management of the toxic effects of chemotherapy, cutaneous metastasis, or suspected cutaneous infections in immunocompromised patients. Cardiology patients were frequently evaluated for potential malignancy or infection prior to heart transplantation and initiation of antirejection immunosuppressants. Dermatology was consulted to differentiate cutaneous manifestations of critical illness from underlying systemic disease in the intensive care unit, and patients presenting to the emergency department often were examined to determine if hospital admission was necessary, with 47% of these consultations resulting in a discharge following evaluation by a dermatologist.
Our results were consistent with prior studies1,5,6 that have reported frequent changes in final diagnosis following dermatology consultation, with 69% of working diagnoses changed in this study when consultation was requested for diagnostic assistance. When dermatology was consulted for diagnostic assistance, several of these cases lacked a preliminary differential diagnosis. Although the absence of a documented differential diagnosis may not necessarily reflect a lack of suspicion for a particular etiology, 86% of all consultations included a ranked differential or working diagnosis either in the consultation request or progress note prior to consultation. The final diagnoses of consultations without a preliminary diagnosis varied from the mild and localized to systemic and severe, further suggesting these cases reflected knowledge gaps of the primary medical team.
Integration of dermatology into the care of hospitalized patients could provide an opportunity for education of primary medical teams. With frequent consultation, primary medical teams may become more comfortable diagnosing and managing common cutaneous conditions specific to their specialty or extended hospitalizations.
Several consultations were requested to aid in management of cases of hidradenitis suppurativa, pyoderma gangrenosum, or bullous pemphigoid that either failed outpatient therapy or were complicated by superinfections. Despite the ranges in complexity, the majority of all consultations required a single encounter and led to improvement by the time of discharge, demonstrating the efficacy and efficiency of inpatient dermatologists.
Dermatology consultations often led to changes in management involving medications and additional workup. Changes in management also extended to specific wound care instructions provided by dermatology, as expected for cases of Stevens-Johnson syndrome/toxic epidermal necrolysis, Sweet syndrome, hidradenitis suppurativa, and pyoderma gangrenosum. However, patients with the sequelae of extended hospitalizations, such as chronic wounds, pressure ulcers, and edema bullae, also benefited from this expertise.
When patients required a biopsy, the final diagnoses were consistent with the dermatologist’s number one differential diagnosis or top 3 differential diagnoses 72% and 88% of the time, respectively. Only 55% of cases where the primary team requested a biopsy ultimately required a biopsy, as many involved clinical diagnoses such as urticaria. Not only was dermatology accurate in their preliminary diagnoses, but they decreased cost and morbidity by avoiding unnecessary procedures.
This study provided additional evidence to support the integration of dermatology into the hospital setting for the benefit of patients, primary medical teams, and hospital systems. Dermatology offers high-value care through the efficient diagnosis and management of hospitalized patients, which contributes to decreased cost and improved outcomes.2,5-7,9,10 This study highlighted lesser-known areas of impact, such as the various specialty-specific services dermatology provides as well as the high rates of reported improvement following consultation. Future studies should continue to explore the field’s unique impact on hospitalized medicine as well as other avenues of care delivery, such as telemedicine, that may encourage dermatologists to participate in consultations and increase the volume of patients who may benefit from their care.
- Madigan LM, Fox LP. Where are we now with inpatient consultative dermatology?: assessing the value and evolution of this subspecialty over the past decade. J Am Acad Dermatol. 2019;80:1804-1808. doi:10.1016/j.jaad.2019.01.031
- Noe MH, Rosenbach M. Inpatient dermatologists—crucial for the management of skin diseases in hospitalized patients [editorial]. JAMA Dermatol. 2018;154:524-525. doi:10.1001/jamadermatol.2017.6195
- Strowd LC. Inpatient dermatology: a paradigm shift in the management of skin disease in the hospital. Br J Dermatol. 2019;180:966-967. doi:10.1111/bjd.17778
- Kirsner RS, Yang DG, Kerdel FA. The changing status of inpatient dermatology at American academic dermatology programs. J Am Acad Dermatol. 1999;40:755-757. doi:10.1016/s0190-9622(99)70158-1
- Kroshinsky D, Cotliar J, Hughey LC, et al. Association of dermatology consultation with accuracy of cutaneous disorder diagnoses in hospitalized patients: a multicenter analysis. JAMA Dermatol. 2016;152:477-480. doi:10.1001/jamadermatol.2015.5098
- Ko LN, Garza-Mayers AC, St John J, et al. Effect of dermatology consultation on outcomes for patients with presumed cellulitis. JAMA Dermatol. 2018;154:529-533. doi:10.1001/jamadermatol.2017.6196
- Li DG, Xia FD, Khosravi H, et al. Outcomes of early dermatology consultation for inpatients diagnosed with cellulitis. JAMA Dermatol. 2018;154:537-543. doi:10.1001/jamadermatol.2017.6197
- Milani-Nejad N, Zhang M, Kaffenberger BH. Association of dermatology consultations with patient care outcomes in hospitalized patients with inflammatory skin diseases. JAMA Dermatol. 2017;153:523-528. doi:10.1001/jamadermatol.2016.6130
- Imadojemu S, Rosenbach M. Dermatologists must take an active role in the diagnosis of cellulitis. JAMA Dermatol. 2017;153:134-135. doi:10.1001/jamadermatol.2016.4230
- Hughey LC. The impact dermatologists can have on misdiagnosis of cellulitis and overuse of antibiotics: closing the gap. JAMA Dermatol. 2014;150:1061-1062. doi:10.1001/jamadermatol.2014.1164
- Ko LN, Kroshinsky D. Dermatology hospitalists: a multicenter survey study characterizing the infrastructure of consultative dermatology in select American hospitals. Int J Dermatol. 2018;57:553-558. doi:10.1111/ijd.13939
- Madigan LM, Fox LP. Where are we now with inpatient consultative dermatology?: assessing the value and evolution of this subspecialty over the past decade. J Am Acad Dermatol. 2019;80:1804-1808. doi:10.1016/j.jaad.2019.01.031
- Noe MH, Rosenbach M. Inpatient dermatologists—crucial for the management of skin diseases in hospitalized patients [editorial]. JAMA Dermatol. 2018;154:524-525. doi:10.1001/jamadermatol.2017.6195
- Strowd LC. Inpatient dermatology: a paradigm shift in the management of skin disease in the hospital. Br J Dermatol. 2019;180:966-967. doi:10.1111/bjd.17778
- Kirsner RS, Yang DG, Kerdel FA. The changing status of inpatient dermatology at American academic dermatology programs. J Am Acad Dermatol. 1999;40:755-757. doi:10.1016/s0190-9622(99)70158-1
- Kroshinsky D, Cotliar J, Hughey LC, et al. Association of dermatology consultation with accuracy of cutaneous disorder diagnoses in hospitalized patients: a multicenter analysis. JAMA Dermatol. 2016;152:477-480. doi:10.1001/jamadermatol.2015.5098
- Ko LN, Garza-Mayers AC, St John J, et al. Effect of dermatology consultation on outcomes for patients with presumed cellulitis. JAMA Dermatol. 2018;154:529-533. doi:10.1001/jamadermatol.2017.6196
- Li DG, Xia FD, Khosravi H, et al. Outcomes of early dermatology consultation for inpatients diagnosed with cellulitis. JAMA Dermatol. 2018;154:537-543. doi:10.1001/jamadermatol.2017.6197
- Milani-Nejad N, Zhang M, Kaffenberger BH. Association of dermatology consultations with patient care outcomes in hospitalized patients with inflammatory skin diseases. JAMA Dermatol. 2017;153:523-528. doi:10.1001/jamadermatol.2016.6130
- Imadojemu S, Rosenbach M. Dermatologists must take an active role in the diagnosis of cellulitis. JAMA Dermatol. 2017;153:134-135. doi:10.1001/jamadermatol.2016.4230
- Hughey LC. The impact dermatologists can have on misdiagnosis of cellulitis and overuse of antibiotics: closing the gap. JAMA Dermatol. 2014;150:1061-1062. doi:10.1001/jamadermatol.2014.1164
- Ko LN, Kroshinsky D. Dermatology hospitalists: a multicenter survey study characterizing the infrastructure of consultative dermatology in select American hospitals. Int J Dermatol. 2018;57:553-558. doi:10.1111/ijd.13939
Practice Points
- Inpatient dermatologists fill knowledge gaps that often alter the diagnosis, management, and hospital course of hospitalized patients.
- Several medical specialties benefit from niche expertise of inpatient dermatologists specific to their patient population.
- Integration of inpatient dermatology consultations can prevent unnecessary hospital admissions and medication administration.
Extension study finds dupilumab effective for up to 1 year in teens with AD
in a phase 3, open-label extension trial, researchers reported.
At 1 year, 86% of 50 remaining patients with weights under 60 kg (132 lb) had achieved 75% improvement on the Eczema Area and Severity Index (EASI-75, and 77% of 51 remaining patients with weights over 60 kg reached that level of clearance. Only 5 (1.7%) of 294 patients had serious treatment-emergent adverse events (TEAEs).
The findings back up a perception that patients can stay on dupilumab for some time instead of having to switch from one biologic to another after a few years, study coauthor Eric Simpson, MD, professor of dermatology, Oregon Health & Science University, Portland, said in an interview. He added that the drug’s long-term safety profile is “very reassuring.”
The industry-funded findings of the study were released in a poster at the 2021 meeting of the World Congress of Pediatric Dermatology.
The FDA approved dupilumab (Dupixent), an interleukin-4 receptor alpha antagonist, for treating AD in adults in 2017; it is now approved for treating patients ages 6 years and older with moderate to severe atopic dermatitis whose disease is not adequately controlled with topicals.
The new study tracked patients who received at least 300 mg dupilumab subcutaneously every 4 weeks. The dose could be increased if needed to improve clinical response to once every 2 weeks (200 mg if baseline weight was <60 kg; 300 mg if ≥60 kg).
At 52 weeks, 37% of 52 patients with weights under 60 kg reached an Investigator Global Assessment (IGA) of 0/1, a level that had been fairly steady since week 16 (n = 146). Among 51 heavier patients, 49% reached an IGA of 0/1 at 52 weeks; this percentage grew steadily since baseline.
The mean percentage change in EASI was –87% in the lower-weight group (n = 50) at 52 weeks and –80.1% in the larger-weight group (n = 51). The majority of the reduction in EASI occurred in the first 4 weeks of treatment.
At 52 weeks, the mean Children’s Dermatology Life Quality Index level, which judges the effect of AD on life, was judged as “small” (low) in 71 patients. At baseline, the mean level among 189 patients was “moderate.” The levels dipped below “moderate” at week 4 and never rose above “small” after that.
“Treatment-emergent adverse events reported in ≥5% of patients were nasopharyngitis (21.1%), AD (19.4%), upper respiratory tract infection (12.4%), headache (9.4%), and oropharyngeal pain (5.7%),” the investigators wrote in the poster. They add that 6.7% of patients experienced injection-site reactions, and 8.7% of patients experienced treatment-emergent “narrow conjunctivitis,” which includes conjunctivitis, allergic conjunctivitis, bacterial conjunctivitis, viral conjunctivitis, and atopic keratoconjunctivitis.
Dr. Simpson noted that cases of conjunctivitis fell over time. It’s not clear why this adverse effect appears, he said.
He said that the findings reflect his own experience in clinic. Many of his adolescent patients took part in early dupilumab trials, he said, and dozens have been taking the drug for more than 5 years. “They just seem to get better and better,” he said.
University of Minnesota, Minneapolis, dermatologist Sheilagh Maguiness, MD, who wasn’t involved with the study, said in an interview that dupilumab remains “the safest, most effective and evidence-based therapy we had for children with moderate to severe atopic dermatitis.”
The new study’s findings are “very reassuring,” she said, and similar to those in a 2021 report that tracked long-term use of the drug in children aged 6-11.
Like Dr. Simpson, Dr. Maguiness said many pediatric patients at her clinic have stayed on the drug for more than 5 years. They still have “sustained improvement in skin disease and in their quality of life as well”
There are, however, still questions about dupilumab treatment. “For children who have responded well, when could we consider dose reduction or discontinuation? I have done this successfully just a handful of times, but I would love to see data about what percentage of pediatric patients experience rebound disease after coming off the drug and after what duration of treatment,” she said. “Another mystery that will be very interesting to unravel is the question as to whether or not early treatment with dupilumab may attenuate other atopic diseases.”
Dr. Maguiness added that “another issue specific to pediatric use of dupilumab is the recommendation surrounding vaccinations. This is an issue that should be studied in terms of antibody response and safety surrounding vaccinations, particularly as we are eagerly awaiting a pediatric FDA approval for the COVID-19 vaccine in children.”
She also urged colleagues to push back against insurers who resist paying for dupilumab. “Whether prescribing this medication on or off label, insurance companies are often requiring patients to try and fail other traditional immunosuppressive medications such as methotrexate, cyclosporine, or to pursue phototherapy,” she said. “Oftentimes, these are not practical or even safe options for children for a multitude of reasons. Don’t be shy about advocating for your patients by second- or even third-level appeals to try and gain approval for children who are in need of treatment.”
The study was funded by Sanofi Genzyme and Regeneron Pharmaceuticals. The study authors reported various disclosures. Dr. Simpson reported investigator and consultant fee relationships from various pharmaceutical companies. Dr. Maguiness was an investigator for one of the initial pediatric dupilumab trials.
A version of this article first appeared on Medscape.com.
in a phase 3, open-label extension trial, researchers reported.
At 1 year, 86% of 50 remaining patients with weights under 60 kg (132 lb) had achieved 75% improvement on the Eczema Area and Severity Index (EASI-75, and 77% of 51 remaining patients with weights over 60 kg reached that level of clearance. Only 5 (1.7%) of 294 patients had serious treatment-emergent adverse events (TEAEs).
The findings back up a perception that patients can stay on dupilumab for some time instead of having to switch from one biologic to another after a few years, study coauthor Eric Simpson, MD, professor of dermatology, Oregon Health & Science University, Portland, said in an interview. He added that the drug’s long-term safety profile is “very reassuring.”
The industry-funded findings of the study were released in a poster at the 2021 meeting of the World Congress of Pediatric Dermatology.
The FDA approved dupilumab (Dupixent), an interleukin-4 receptor alpha antagonist, for treating AD in adults in 2017; it is now approved for treating patients ages 6 years and older with moderate to severe atopic dermatitis whose disease is not adequately controlled with topicals.
The new study tracked patients who received at least 300 mg dupilumab subcutaneously every 4 weeks. The dose could be increased if needed to improve clinical response to once every 2 weeks (200 mg if baseline weight was <60 kg; 300 mg if ≥60 kg).
At 52 weeks, 37% of 52 patients with weights under 60 kg reached an Investigator Global Assessment (IGA) of 0/1, a level that had been fairly steady since week 16 (n = 146). Among 51 heavier patients, 49% reached an IGA of 0/1 at 52 weeks; this percentage grew steadily since baseline.
The mean percentage change in EASI was –87% in the lower-weight group (n = 50) at 52 weeks and –80.1% in the larger-weight group (n = 51). The majority of the reduction in EASI occurred in the first 4 weeks of treatment.
At 52 weeks, the mean Children’s Dermatology Life Quality Index level, which judges the effect of AD on life, was judged as “small” (low) in 71 patients. At baseline, the mean level among 189 patients was “moderate.” The levels dipped below “moderate” at week 4 and never rose above “small” after that.
“Treatment-emergent adverse events reported in ≥5% of patients were nasopharyngitis (21.1%), AD (19.4%), upper respiratory tract infection (12.4%), headache (9.4%), and oropharyngeal pain (5.7%),” the investigators wrote in the poster. They add that 6.7% of patients experienced injection-site reactions, and 8.7% of patients experienced treatment-emergent “narrow conjunctivitis,” which includes conjunctivitis, allergic conjunctivitis, bacterial conjunctivitis, viral conjunctivitis, and atopic keratoconjunctivitis.
Dr. Simpson noted that cases of conjunctivitis fell over time. It’s not clear why this adverse effect appears, he said.
He said that the findings reflect his own experience in clinic. Many of his adolescent patients took part in early dupilumab trials, he said, and dozens have been taking the drug for more than 5 years. “They just seem to get better and better,” he said.
University of Minnesota, Minneapolis, dermatologist Sheilagh Maguiness, MD, who wasn’t involved with the study, said in an interview that dupilumab remains “the safest, most effective and evidence-based therapy we had for children with moderate to severe atopic dermatitis.”
The new study’s findings are “very reassuring,” she said, and similar to those in a 2021 report that tracked long-term use of the drug in children aged 6-11.
Like Dr. Simpson, Dr. Maguiness said many pediatric patients at her clinic have stayed on the drug for more than 5 years. They still have “sustained improvement in skin disease and in their quality of life as well”
There are, however, still questions about dupilumab treatment. “For children who have responded well, when could we consider dose reduction or discontinuation? I have done this successfully just a handful of times, but I would love to see data about what percentage of pediatric patients experience rebound disease after coming off the drug and after what duration of treatment,” she said. “Another mystery that will be very interesting to unravel is the question as to whether or not early treatment with dupilumab may attenuate other atopic diseases.”
Dr. Maguiness added that “another issue specific to pediatric use of dupilumab is the recommendation surrounding vaccinations. This is an issue that should be studied in terms of antibody response and safety surrounding vaccinations, particularly as we are eagerly awaiting a pediatric FDA approval for the COVID-19 vaccine in children.”
She also urged colleagues to push back against insurers who resist paying for dupilumab. “Whether prescribing this medication on or off label, insurance companies are often requiring patients to try and fail other traditional immunosuppressive medications such as methotrexate, cyclosporine, or to pursue phototherapy,” she said. “Oftentimes, these are not practical or even safe options for children for a multitude of reasons. Don’t be shy about advocating for your patients by second- or even third-level appeals to try and gain approval for children who are in need of treatment.”
The study was funded by Sanofi Genzyme and Regeneron Pharmaceuticals. The study authors reported various disclosures. Dr. Simpson reported investigator and consultant fee relationships from various pharmaceutical companies. Dr. Maguiness was an investigator for one of the initial pediatric dupilumab trials.
A version of this article first appeared on Medscape.com.
in a phase 3, open-label extension trial, researchers reported.
At 1 year, 86% of 50 remaining patients with weights under 60 kg (132 lb) had achieved 75% improvement on the Eczema Area and Severity Index (EASI-75, and 77% of 51 remaining patients with weights over 60 kg reached that level of clearance. Only 5 (1.7%) of 294 patients had serious treatment-emergent adverse events (TEAEs).
The findings back up a perception that patients can stay on dupilumab for some time instead of having to switch from one biologic to another after a few years, study coauthor Eric Simpson, MD, professor of dermatology, Oregon Health & Science University, Portland, said in an interview. He added that the drug’s long-term safety profile is “very reassuring.”
The industry-funded findings of the study were released in a poster at the 2021 meeting of the World Congress of Pediatric Dermatology.
The FDA approved dupilumab (Dupixent), an interleukin-4 receptor alpha antagonist, for treating AD in adults in 2017; it is now approved for treating patients ages 6 years and older with moderate to severe atopic dermatitis whose disease is not adequately controlled with topicals.
The new study tracked patients who received at least 300 mg dupilumab subcutaneously every 4 weeks. The dose could be increased if needed to improve clinical response to once every 2 weeks (200 mg if baseline weight was <60 kg; 300 mg if ≥60 kg).
At 52 weeks, 37% of 52 patients with weights under 60 kg reached an Investigator Global Assessment (IGA) of 0/1, a level that had been fairly steady since week 16 (n = 146). Among 51 heavier patients, 49% reached an IGA of 0/1 at 52 weeks; this percentage grew steadily since baseline.
The mean percentage change in EASI was –87% in the lower-weight group (n = 50) at 52 weeks and –80.1% in the larger-weight group (n = 51). The majority of the reduction in EASI occurred in the first 4 weeks of treatment.
At 52 weeks, the mean Children’s Dermatology Life Quality Index level, which judges the effect of AD on life, was judged as “small” (low) in 71 patients. At baseline, the mean level among 189 patients was “moderate.” The levels dipped below “moderate” at week 4 and never rose above “small” after that.
“Treatment-emergent adverse events reported in ≥5% of patients were nasopharyngitis (21.1%), AD (19.4%), upper respiratory tract infection (12.4%), headache (9.4%), and oropharyngeal pain (5.7%),” the investigators wrote in the poster. They add that 6.7% of patients experienced injection-site reactions, and 8.7% of patients experienced treatment-emergent “narrow conjunctivitis,” which includes conjunctivitis, allergic conjunctivitis, bacterial conjunctivitis, viral conjunctivitis, and atopic keratoconjunctivitis.
Dr. Simpson noted that cases of conjunctivitis fell over time. It’s not clear why this adverse effect appears, he said.
He said that the findings reflect his own experience in clinic. Many of his adolescent patients took part in early dupilumab trials, he said, and dozens have been taking the drug for more than 5 years. “They just seem to get better and better,” he said.
University of Minnesota, Minneapolis, dermatologist Sheilagh Maguiness, MD, who wasn’t involved with the study, said in an interview that dupilumab remains “the safest, most effective and evidence-based therapy we had for children with moderate to severe atopic dermatitis.”
The new study’s findings are “very reassuring,” she said, and similar to those in a 2021 report that tracked long-term use of the drug in children aged 6-11.
Like Dr. Simpson, Dr. Maguiness said many pediatric patients at her clinic have stayed on the drug for more than 5 years. They still have “sustained improvement in skin disease and in their quality of life as well”
There are, however, still questions about dupilumab treatment. “For children who have responded well, when could we consider dose reduction or discontinuation? I have done this successfully just a handful of times, but I would love to see data about what percentage of pediatric patients experience rebound disease after coming off the drug and after what duration of treatment,” she said. “Another mystery that will be very interesting to unravel is the question as to whether or not early treatment with dupilumab may attenuate other atopic diseases.”
Dr. Maguiness added that “another issue specific to pediatric use of dupilumab is the recommendation surrounding vaccinations. This is an issue that should be studied in terms of antibody response and safety surrounding vaccinations, particularly as we are eagerly awaiting a pediatric FDA approval for the COVID-19 vaccine in children.”
She also urged colleagues to push back against insurers who resist paying for dupilumab. “Whether prescribing this medication on or off label, insurance companies are often requiring patients to try and fail other traditional immunosuppressive medications such as methotrexate, cyclosporine, or to pursue phototherapy,” she said. “Oftentimes, these are not practical or even safe options for children for a multitude of reasons. Don’t be shy about advocating for your patients by second- or even third-level appeals to try and gain approval for children who are in need of treatment.”
The study was funded by Sanofi Genzyme and Regeneron Pharmaceuticals. The study authors reported various disclosures. Dr. Simpson reported investigator and consultant fee relationships from various pharmaceutical companies. Dr. Maguiness was an investigator for one of the initial pediatric dupilumab trials.
A version of this article first appeared on Medscape.com.
Clinical Edge Journal Scan Commentary: Atopic Dermatitis October 2021
George Washington University School of Medicine and Health Sciences
Washington, DC
Topical and oral Janus Kinase (JAK)-inhibitors are important new additions to the therapeutic armamentarium of atopic dermatitis (AD). I recently addressed some important treatment considerations regarding the JAK-inhibitors. In just two short months, there have already been a number of important new publications on JAK-inhibitors in AD that provide crucial data to guide treatment decisions.
Topical ruxolitinib 1.5% cream (a JAK1/2 inhibitor) was just approved by United States Food and Drug Administration for the treatment of mild-moderate AD. Clinicians always want to know about the comparative effectiveness of new agents compared to already approved agents. A previous phase 2B randomized controlled trial (RCT) compared multiple doses of ruxolitinib cream with a vehicle control and triamcinolone 0.1% cream active comparator1. Topical ruxolitinib 1.5% cream was significantly more effective than vehicle and numerically more effective than triamcinolone 0.1% cream.
Zhang et al. recently conducted a network meta-analysis of 10 RCT for topical JAK and phosphodiesterase E4 (PDE4)-inhibitors, mostly with mild-to-moderate AD. All included JAK inhibitors showed higher Investigators Global Assessment (IGA) response vs. vehicle, with ruxolitinib 1.5% once daily showing similar efficacy as tofacitinib 2% and delgocitinib 3% twice daily. Whereas, topical tacrolimus 0.1% and hydrocortisone butyrate 0.1% twice a day were not more effective than vehicle at achieving IGA response. These results suggest that topical ruxolitinib and other JAK-inhibitors are more effective at clearing AD lesions than currently used topical therapies.
There has been a recent flurry of publications regarding the efficacy and safety of abrocitinib (an oral, once daily, JAK1 inhibitor) in moderate-severe atopic dermatitis.
- Eichenfield et al. published the results of the JADE TEEN study 2, a phase 3 RCT of abrocitinib in adolescents. Abrocitinib 200 mg and 100 mg resulted in significant improvements of IGA, Eczema Area and Severity Index, and itch scores, etc. over a 12-week treatment period compared to placebo. These results support the efficacy of abrocitinib in adolescents with moderate-severe AD.
- Simpson et al. published the results from an integrated safety analysis of pooled data from 5 short-term and 1 long-term extension study of abrocitinib therapy 3. Abrocitinib 200 mg and 100 mg doses were well-tolerated during 12-week placebo controlled trials, with nausea, headache, and acne being the most common adverse-events. The incidence of different adverse-events did not consistently increase over time. However, there were some rare events reported for venous thromboembolism and deaths. These results indicate an overall good safety profile for abrocitinib, but proper patient and dose selection should be carefully considered.
- Additionally, strategies should be employed to potentially minimize risk of adverse-events. One such approach is flexible dosing in order to maintain long-term disease control using the lowest amount of medicine needed. Blauvelt et al. published findings from the JADE REGIMEN study 4. Patients who responded to 12 weeks of abrocitinib 200 mg open-label monotherapy were randomly assigned to abrocitinib 200 mg, abrocitinib 100 mg, or placebo maintenance therapy for 40-weeks. Flares occurred least commonly in patients maintained on abrocitinib 200 mg (18.9%), followed by abrocitinib 100 mg (42.6%), and most commonly for placebo (80.9%). These results indicate that a large subset of patients who achieve clinical response with abrocitinib 200 mg could be maintained on a lower dose of 100 mg and in some cases may even be able to have a drug holiday without flaring. While similar studies were not performed for other oral JAK-inhibitors, it may be that lower maintenance dosing may also be feasible and effective for other oral JAK-inhibitors. Future research is needed to identify patient subsets who will most likely maintain clinical response with lower maintenance dosing of oral JAK-inhibitors.
- Kim BS, Howell MD, Sun K, et al. Treatment of atopic dermatitis with ruxolitinib cream (JAK1/JAK2 inhibitor) or triamcinolone cream. The Journal of allergy and clinical immunology. 2020;145(2):572-582.
- Eichenfield LF, Flohr C, Sidbury R, et al. Efficacy and Safety of Abrocitinib in Combination With Topical Therapy in Adolescents With Moderate-to-Severe Atopic Dermatitis: The JADE TEEN Randomized Clinical Trial. JAMA dermatology. 2021.
- Simpson EL, Silverberg JI, Nosbaum A, et al. Integrated Safety Analysis of Abrocitinib for the Treatment of Moderate-to-Severe Atopic Dermatitis From the Phase II and Phase III Clinical Trial Program. American journal of clinical dermatology. 2021;22(5):693-707.
- Blauvelt A, Silverberg JI, Lynde CW, et al. Abrocitinib induction, randomized withdrawal, and retreatment in patients with moderate-to-severe atopic dermatitis: Results from the JAK1 Atopic Dermatitis Efficacy and Safety (JADE) REGIMEN phase 3 trial. Journal of the American Academy of Dermatology.
George Washington University School of Medicine and Health Sciences
Washington, DC
Topical and oral Janus Kinase (JAK)-inhibitors are important new additions to the therapeutic armamentarium of atopic dermatitis (AD). I recently addressed some important treatment considerations regarding the JAK-inhibitors. In just two short months, there have already been a number of important new publications on JAK-inhibitors in AD that provide crucial data to guide treatment decisions.
Topical ruxolitinib 1.5% cream (a JAK1/2 inhibitor) was just approved by United States Food and Drug Administration for the treatment of mild-moderate AD. Clinicians always want to know about the comparative effectiveness of new agents compared to already approved agents. A previous phase 2B randomized controlled trial (RCT) compared multiple doses of ruxolitinib cream with a vehicle control and triamcinolone 0.1% cream active comparator1. Topical ruxolitinib 1.5% cream was significantly more effective than vehicle and numerically more effective than triamcinolone 0.1% cream.
Zhang et al. recently conducted a network meta-analysis of 10 RCT for topical JAK and phosphodiesterase E4 (PDE4)-inhibitors, mostly with mild-to-moderate AD. All included JAK inhibitors showed higher Investigators Global Assessment (IGA) response vs. vehicle, with ruxolitinib 1.5% once daily showing similar efficacy as tofacitinib 2% and delgocitinib 3% twice daily. Whereas, topical tacrolimus 0.1% and hydrocortisone butyrate 0.1% twice a day were not more effective than vehicle at achieving IGA response. These results suggest that topical ruxolitinib and other JAK-inhibitors are more effective at clearing AD lesions than currently used topical therapies.
There has been a recent flurry of publications regarding the efficacy and safety of abrocitinib (an oral, once daily, JAK1 inhibitor) in moderate-severe atopic dermatitis.
- Eichenfield et al. published the results of the JADE TEEN study 2, a phase 3 RCT of abrocitinib in adolescents. Abrocitinib 200 mg and 100 mg resulted in significant improvements of IGA, Eczema Area and Severity Index, and itch scores, etc. over a 12-week treatment period compared to placebo. These results support the efficacy of abrocitinib in adolescents with moderate-severe AD.
- Simpson et al. published the results from an integrated safety analysis of pooled data from 5 short-term and 1 long-term extension study of abrocitinib therapy 3. Abrocitinib 200 mg and 100 mg doses were well-tolerated during 12-week placebo controlled trials, with nausea, headache, and acne being the most common adverse-events. The incidence of different adverse-events did not consistently increase over time. However, there were some rare events reported for venous thromboembolism and deaths. These results indicate an overall good safety profile for abrocitinib, but proper patient and dose selection should be carefully considered.
- Additionally, strategies should be employed to potentially minimize risk of adverse-events. One such approach is flexible dosing in order to maintain long-term disease control using the lowest amount of medicine needed. Blauvelt et al. published findings from the JADE REGIMEN study 4. Patients who responded to 12 weeks of abrocitinib 200 mg open-label monotherapy were randomly assigned to abrocitinib 200 mg, abrocitinib 100 mg, or placebo maintenance therapy for 40-weeks. Flares occurred least commonly in patients maintained on abrocitinib 200 mg (18.9%), followed by abrocitinib 100 mg (42.6%), and most commonly for placebo (80.9%). These results indicate that a large subset of patients who achieve clinical response with abrocitinib 200 mg could be maintained on a lower dose of 100 mg and in some cases may even be able to have a drug holiday without flaring. While similar studies were not performed for other oral JAK-inhibitors, it may be that lower maintenance dosing may also be feasible and effective for other oral JAK-inhibitors. Future research is needed to identify patient subsets who will most likely maintain clinical response with lower maintenance dosing of oral JAK-inhibitors.
- Kim BS, Howell MD, Sun K, et al. Treatment of atopic dermatitis with ruxolitinib cream (JAK1/JAK2 inhibitor) or triamcinolone cream. The Journal of allergy and clinical immunology. 2020;145(2):572-582.
- Eichenfield LF, Flohr C, Sidbury R, et al. Efficacy and Safety of Abrocitinib in Combination With Topical Therapy in Adolescents With Moderate-to-Severe Atopic Dermatitis: The JADE TEEN Randomized Clinical Trial. JAMA dermatology. 2021.
- Simpson EL, Silverberg JI, Nosbaum A, et al. Integrated Safety Analysis of Abrocitinib for the Treatment of Moderate-to-Severe Atopic Dermatitis From the Phase II and Phase III Clinical Trial Program. American journal of clinical dermatology. 2021;22(5):693-707.
- Blauvelt A, Silverberg JI, Lynde CW, et al. Abrocitinib induction, randomized withdrawal, and retreatment in patients with moderate-to-severe atopic dermatitis: Results from the JAK1 Atopic Dermatitis Efficacy and Safety (JADE) REGIMEN phase 3 trial. Journal of the American Academy of Dermatology.
George Washington University School of Medicine and Health Sciences
Washington, DC
Topical and oral Janus Kinase (JAK)-inhibitors are important new additions to the therapeutic armamentarium of atopic dermatitis (AD). I recently addressed some important treatment considerations regarding the JAK-inhibitors. In just two short months, there have already been a number of important new publications on JAK-inhibitors in AD that provide crucial data to guide treatment decisions.
Topical ruxolitinib 1.5% cream (a JAK1/2 inhibitor) was just approved by United States Food and Drug Administration for the treatment of mild-moderate AD. Clinicians always want to know about the comparative effectiveness of new agents compared to already approved agents. A previous phase 2B randomized controlled trial (RCT) compared multiple doses of ruxolitinib cream with a vehicle control and triamcinolone 0.1% cream active comparator1. Topical ruxolitinib 1.5% cream was significantly more effective than vehicle and numerically more effective than triamcinolone 0.1% cream.
Zhang et al. recently conducted a network meta-analysis of 10 RCT for topical JAK and phosphodiesterase E4 (PDE4)-inhibitors, mostly with mild-to-moderate AD. All included JAK inhibitors showed higher Investigators Global Assessment (IGA) response vs. vehicle, with ruxolitinib 1.5% once daily showing similar efficacy as tofacitinib 2% and delgocitinib 3% twice daily. Whereas, topical tacrolimus 0.1% and hydrocortisone butyrate 0.1% twice a day were not more effective than vehicle at achieving IGA response. These results suggest that topical ruxolitinib and other JAK-inhibitors are more effective at clearing AD lesions than currently used topical therapies.
There has been a recent flurry of publications regarding the efficacy and safety of abrocitinib (an oral, once daily, JAK1 inhibitor) in moderate-severe atopic dermatitis.
- Eichenfield et al. published the results of the JADE TEEN study 2, a phase 3 RCT of abrocitinib in adolescents. Abrocitinib 200 mg and 100 mg resulted in significant improvements of IGA, Eczema Area and Severity Index, and itch scores, etc. over a 12-week treatment period compared to placebo. These results support the efficacy of abrocitinib in adolescents with moderate-severe AD.
- Simpson et al. published the results from an integrated safety analysis of pooled data from 5 short-term and 1 long-term extension study of abrocitinib therapy 3. Abrocitinib 200 mg and 100 mg doses were well-tolerated during 12-week placebo controlled trials, with nausea, headache, and acne being the most common adverse-events. The incidence of different adverse-events did not consistently increase over time. However, there were some rare events reported for venous thromboembolism and deaths. These results indicate an overall good safety profile for abrocitinib, but proper patient and dose selection should be carefully considered.
- Additionally, strategies should be employed to potentially minimize risk of adverse-events. One such approach is flexible dosing in order to maintain long-term disease control using the lowest amount of medicine needed. Blauvelt et al. published findings from the JADE REGIMEN study 4. Patients who responded to 12 weeks of abrocitinib 200 mg open-label monotherapy were randomly assigned to abrocitinib 200 mg, abrocitinib 100 mg, or placebo maintenance therapy for 40-weeks. Flares occurred least commonly in patients maintained on abrocitinib 200 mg (18.9%), followed by abrocitinib 100 mg (42.6%), and most commonly for placebo (80.9%). These results indicate that a large subset of patients who achieve clinical response with abrocitinib 200 mg could be maintained on a lower dose of 100 mg and in some cases may even be able to have a drug holiday without flaring. While similar studies were not performed for other oral JAK-inhibitors, it may be that lower maintenance dosing may also be feasible and effective for other oral JAK-inhibitors. Future research is needed to identify patient subsets who will most likely maintain clinical response with lower maintenance dosing of oral JAK-inhibitors.
- Kim BS, Howell MD, Sun K, et al. Treatment of atopic dermatitis with ruxolitinib cream (JAK1/JAK2 inhibitor) or triamcinolone cream. The Journal of allergy and clinical immunology. 2020;145(2):572-582.
- Eichenfield LF, Flohr C, Sidbury R, et al. Efficacy and Safety of Abrocitinib in Combination With Topical Therapy in Adolescents With Moderate-to-Severe Atopic Dermatitis: The JADE TEEN Randomized Clinical Trial. JAMA dermatology. 2021.
- Simpson EL, Silverberg JI, Nosbaum A, et al. Integrated Safety Analysis of Abrocitinib for the Treatment of Moderate-to-Severe Atopic Dermatitis From the Phase II and Phase III Clinical Trial Program. American journal of clinical dermatology. 2021;22(5):693-707.
- Blauvelt A, Silverberg JI, Lynde CW, et al. Abrocitinib induction, randomized withdrawal, and retreatment in patients with moderate-to-severe atopic dermatitis: Results from the JAK1 Atopic Dermatitis Efficacy and Safety (JADE) REGIMEN phase 3 trial. Journal of the American Academy of Dermatology.
Management of pediatric food allergies evolving
The treatment of atopic dermatitis (AD) is undergoing a revolution thanks to biologics. Now, an allergist and a dietitian told pediatric dermatologists that the treatment of a related condition – food allergy – is also undergoing a dramatic transformation as the management approach evolves away from blanket avoidance of allergens.
“Over the past 15 years, we’ve seen a shift from a very passive approach where generally we just advised patients to avoid the things they’re allergic to,” said U.K. pediatric allergist Adam Fox, MBBS, MD, in a presentation at The World Congress of Pediatric Dermatology (WCPD) 2021 Annual Meeting. “Now, we have a much better understanding of how allergy develops and strategies to minimize the risk of allergy happening in the first place,” he said.
According to Carina Venter, PhD, RD, associate professor of pediatrics-allergy/immunology at the University of Colorado, Denver, who also spoke at the conference, an estimated 20% to 30% of patients with AD also have food allergies, and up to 90% of infants with cow’s milk allergy develop skin symptoms.
It may not be necessary for a breastfeeding mother to avoid food allergens if a child is allergic, said Dr. Fox, of Guy’s and St. Thomas’ NHS Foundation Trust, London. “A lot of parents will automatically assume that if their child has an egg or milk allergy, then it’s a good idea to completely eliminate that from their diet if they’re breastfeeding,” but it is “surprisingly uncommon” that this approach makes a difference, he said. “Less goes through the breast milk than people imagine,” he said.
He noted that eliminating foods from the breastfeeding mother’s diet may have negative consequences. “There’s always that risk that if you make life harder for the breastfeeding mom because they’re going to have to avoid all sorts of foods, they’ll be more likely to discontinue breastfeeding. You really need a compelling reason to stop the food.”
As for children themselves, Dr. Fox suggested that there’s often no connection between AD and food allergies. “What will commonly happen when you see and diagnose these kids is that their eczema has been quite significantly undertreated,” he said. “Once you just get them on the right [regimen], they don’t need to be cutting the food out of their diet. It’s just making their life unnecessarily harder.”
Dr. Venter said there may be little choice but to avoid a trigger food if a child develops AD with exposure. However, she noted, it’s important to understand that avoidance of certain foods could make the allergy – and AD – worse. “If you have a child or an adult with atopic dermatitis that’s not controlled by an optimal topical treatment, and you do consider avoidance, we need to be aware that development of more severe IgA-mediated symptoms can happen in a short period of time,” she said.
In a slide that Dr. Venter presented, the dilemma for physicians was expressed this way: “The potential benefit of food avoidance as a management strategy for some patients with AD must now be weighed against the strong evidence that unnecessarily avoiding a food in kids with AD increases the risk of developing anaphylaxis to that food.”
What should pediatric dermatologists do to balance the risks of allergen exposure to the risks that children will develop permanent allergies? Dr. Venter pointed to guidelines about AD that were developed by the U.K.’s National Institute for Health and Care Excellence. She also highlighted the International Milk Allergy in Primary Care recommendations.
She suggested considering creative ways to bypass complete avoidance and boost a child’s tolerance of allergens if possible. “If we’re going to keep a child with eczema on a mold-free diet for a longer period of time, is there perhaps a role for regularly introducing small amounts of yogurt or even small amounts of milk in the child’s diet to at least keep immune tolerance without necessarily aggravating eczema symptoms?”
Dr. Fox has consulted for DBV and Aimmune through his employer, NHS Trust. He serves as president of the British Society for Allergy and Clinical Immunology and as chair of the Allergy UK Health Advisory Board, both of which receive funding from drug companies. Dr. Venter has received support for allergy-related research from the National Peanut Board.
A version of this article first appeared on Medscape.com.
The treatment of atopic dermatitis (AD) is undergoing a revolution thanks to biologics. Now, an allergist and a dietitian told pediatric dermatologists that the treatment of a related condition – food allergy – is also undergoing a dramatic transformation as the management approach evolves away from blanket avoidance of allergens.
“Over the past 15 years, we’ve seen a shift from a very passive approach where generally we just advised patients to avoid the things they’re allergic to,” said U.K. pediatric allergist Adam Fox, MBBS, MD, in a presentation at The World Congress of Pediatric Dermatology (WCPD) 2021 Annual Meeting. “Now, we have a much better understanding of how allergy develops and strategies to minimize the risk of allergy happening in the first place,” he said.
According to Carina Venter, PhD, RD, associate professor of pediatrics-allergy/immunology at the University of Colorado, Denver, who also spoke at the conference, an estimated 20% to 30% of patients with AD also have food allergies, and up to 90% of infants with cow’s milk allergy develop skin symptoms.
It may not be necessary for a breastfeeding mother to avoid food allergens if a child is allergic, said Dr. Fox, of Guy’s and St. Thomas’ NHS Foundation Trust, London. “A lot of parents will automatically assume that if their child has an egg or milk allergy, then it’s a good idea to completely eliminate that from their diet if they’re breastfeeding,” but it is “surprisingly uncommon” that this approach makes a difference, he said. “Less goes through the breast milk than people imagine,” he said.
He noted that eliminating foods from the breastfeeding mother’s diet may have negative consequences. “There’s always that risk that if you make life harder for the breastfeeding mom because they’re going to have to avoid all sorts of foods, they’ll be more likely to discontinue breastfeeding. You really need a compelling reason to stop the food.”
As for children themselves, Dr. Fox suggested that there’s often no connection between AD and food allergies. “What will commonly happen when you see and diagnose these kids is that their eczema has been quite significantly undertreated,” he said. “Once you just get them on the right [regimen], they don’t need to be cutting the food out of their diet. It’s just making their life unnecessarily harder.”
Dr. Venter said there may be little choice but to avoid a trigger food if a child develops AD with exposure. However, she noted, it’s important to understand that avoidance of certain foods could make the allergy – and AD – worse. “If you have a child or an adult with atopic dermatitis that’s not controlled by an optimal topical treatment, and you do consider avoidance, we need to be aware that development of more severe IgA-mediated symptoms can happen in a short period of time,” she said.
In a slide that Dr. Venter presented, the dilemma for physicians was expressed this way: “The potential benefit of food avoidance as a management strategy for some patients with AD must now be weighed against the strong evidence that unnecessarily avoiding a food in kids with AD increases the risk of developing anaphylaxis to that food.”
What should pediatric dermatologists do to balance the risks of allergen exposure to the risks that children will develop permanent allergies? Dr. Venter pointed to guidelines about AD that were developed by the U.K.’s National Institute for Health and Care Excellence. She also highlighted the International Milk Allergy in Primary Care recommendations.
She suggested considering creative ways to bypass complete avoidance and boost a child’s tolerance of allergens if possible. “If we’re going to keep a child with eczema on a mold-free diet for a longer period of time, is there perhaps a role for regularly introducing small amounts of yogurt or even small amounts of milk in the child’s diet to at least keep immune tolerance without necessarily aggravating eczema symptoms?”
Dr. Fox has consulted for DBV and Aimmune through his employer, NHS Trust. He serves as president of the British Society for Allergy and Clinical Immunology and as chair of the Allergy UK Health Advisory Board, both of which receive funding from drug companies. Dr. Venter has received support for allergy-related research from the National Peanut Board.
A version of this article first appeared on Medscape.com.
The treatment of atopic dermatitis (AD) is undergoing a revolution thanks to biologics. Now, an allergist and a dietitian told pediatric dermatologists that the treatment of a related condition – food allergy – is also undergoing a dramatic transformation as the management approach evolves away from blanket avoidance of allergens.
“Over the past 15 years, we’ve seen a shift from a very passive approach where generally we just advised patients to avoid the things they’re allergic to,” said U.K. pediatric allergist Adam Fox, MBBS, MD, in a presentation at The World Congress of Pediatric Dermatology (WCPD) 2021 Annual Meeting. “Now, we have a much better understanding of how allergy develops and strategies to minimize the risk of allergy happening in the first place,” he said.
According to Carina Venter, PhD, RD, associate professor of pediatrics-allergy/immunology at the University of Colorado, Denver, who also spoke at the conference, an estimated 20% to 30% of patients with AD also have food allergies, and up to 90% of infants with cow’s milk allergy develop skin symptoms.
It may not be necessary for a breastfeeding mother to avoid food allergens if a child is allergic, said Dr. Fox, of Guy’s and St. Thomas’ NHS Foundation Trust, London. “A lot of parents will automatically assume that if their child has an egg or milk allergy, then it’s a good idea to completely eliminate that from their diet if they’re breastfeeding,” but it is “surprisingly uncommon” that this approach makes a difference, he said. “Less goes through the breast milk than people imagine,” he said.
He noted that eliminating foods from the breastfeeding mother’s diet may have negative consequences. “There’s always that risk that if you make life harder for the breastfeeding mom because they’re going to have to avoid all sorts of foods, they’ll be more likely to discontinue breastfeeding. You really need a compelling reason to stop the food.”
As for children themselves, Dr. Fox suggested that there’s often no connection between AD and food allergies. “What will commonly happen when you see and diagnose these kids is that their eczema has been quite significantly undertreated,” he said. “Once you just get them on the right [regimen], they don’t need to be cutting the food out of their diet. It’s just making their life unnecessarily harder.”
Dr. Venter said there may be little choice but to avoid a trigger food if a child develops AD with exposure. However, she noted, it’s important to understand that avoidance of certain foods could make the allergy – and AD – worse. “If you have a child or an adult with atopic dermatitis that’s not controlled by an optimal topical treatment, and you do consider avoidance, we need to be aware that development of more severe IgA-mediated symptoms can happen in a short period of time,” she said.
In a slide that Dr. Venter presented, the dilemma for physicians was expressed this way: “The potential benefit of food avoidance as a management strategy for some patients with AD must now be weighed against the strong evidence that unnecessarily avoiding a food in kids with AD increases the risk of developing anaphylaxis to that food.”
What should pediatric dermatologists do to balance the risks of allergen exposure to the risks that children will develop permanent allergies? Dr. Venter pointed to guidelines about AD that were developed by the U.K.’s National Institute for Health and Care Excellence. She also highlighted the International Milk Allergy in Primary Care recommendations.
She suggested considering creative ways to bypass complete avoidance and boost a child’s tolerance of allergens if possible. “If we’re going to keep a child with eczema on a mold-free diet for a longer period of time, is there perhaps a role for regularly introducing small amounts of yogurt or even small amounts of milk in the child’s diet to at least keep immune tolerance without necessarily aggravating eczema symptoms?”
Dr. Fox has consulted for DBV and Aimmune through his employer, NHS Trust. He serves as president of the British Society for Allergy and Clinical Immunology and as chair of the Allergy UK Health Advisory Board, both of which receive funding from drug companies. Dr. Venter has received support for allergy-related research from the National Peanut Board.
A version of this article first appeared on Medscape.com.
FDA approves topical ruxolitinib for atopic dermatitis, first JAK inhibitor for this indication in the U.S.
The , making it the first topical JAK inhibitor approved for AD – and the first JAK inhibitor approved for this indication – in the United States.
The approval is limited to patients whose AD is not adequately controlled with topical prescription therapies, or when those therapies are not advisable.
“Approval of topical ruxolitinib fills a major gap in the treatment of atopic dermatitis: a safe, effective, and tolerable non-steroidal topical therapy,” Eric L. Simpson, MD, professor of dermatology and director of the Oregon Health & Science University Dermatology Clinical Research Center, Portland, told this news organization. “This approval will allow for long-term treatment without the concern of steroid side effects. From earlier studies, ruxolitinib cream appears to be as effective as a medium-potency topical steroid. These efficacy levels and low incidence of burning will be a welcome addition to our current nonsteroidal therapies.”
The drug’s approval was based on results from two phase 3, randomized studies of identical design involving 1,249 patients aged 12 years and older with AD: TRuE-AD1 and TRuE-AD2. In these studies, ruxolitinib cream demonstrated anti-inflammatory activity, with rapid and sustained antipruritic action, compared with vehicle. In the trials, patients with an Investigator’s Global Assessment (IGA) score of 2 or 3 and 3%-20% of affected body surface area (BSA) were randomized (2:2:1) to twice-daily 0.75% ruxolitinib cream, 1.5% ruxolitinib cream, or vehicle cream for 8 continuous weeks. The 1.5% concentration was approved by the FDA.
A study first published in May of 2021 found that significantly more patients in TRuE-AD1 and TRuE-AD2 achieved IGA treatment success with 0.75% (50% vs. 39%, respectively) and 1.5% ruxolitinib cream (53.8% vs. 51.3%), compared with vehicle (15.1% vs. 7.6%; P < .0001) at week 8. In addition, significant reductions in itch, compared with vehicle, were reported within 12 hours of first applying 1.5% ruxolitinib cream (P < .05).
More key findings from TRuE-AD1 and TRuE-AD2 are scheduled to be presented during the upcoming European Academy of Dermatology and Venereology meeting Sept. 29-Oct. 2, but during the Revolutionizing Atopic Dermatitis Symposium on June 13, Kim Papp, MD, PhD, presented long-term safety data of ruxolitinib cream in patients who were followed for an additional 44 weeks. Dr. Papp, a dermatologist and founder of Probity Medical Research, Waterloo, Ont., reported that 543 patients from TRuE-AD1 and 530 from TRuE-AD2 entered the long-term analysis and that about 78% of these patients completed the study. From weeks 12 to 52, the proportion of patients with an IGA score of 0 or 1 with 0.75% and 1.5% ruxolitinib cream ranged from 62%-77% and 67%-77%, respectively, in TRuE-AD1, to 60%-77% and 72%-80% in TRuE-AD2.
The measured mean total affected BSA was less than 3% throughout the follow-up period in the 1.5% ruxolitinib cream arm in TRuE-AD1 and TRuE-AD2 and was less than 3% in the 0.75% ruxolitinib cream arm during most of the study period.
In a pooled safety analysis, treatment-emergent adverse events (TEAEs) were reported in 60% and 54% of patients who applied 0.75% and 1.5% ruxolitinib cream, respectively, over 44 weeks. The frequency of application-site reactions remained low. Specifically, treatment-related adverse events were reported in 5% of patients who applied 0.75% ruxolitinib cream and in 3% of patients who applied 1.5% ruxolitinib cream; none were serious. TEAEs led to discontinuation in 2% of patients in the 0.75% ruxolitinib cream group, and no patients in the 1.5% ruxolitinib cream group.
Dr. Papp and his colleagues observed that the most common treatment adverse events were upper respiratory tract infections and nasopharyngitis. According to Incyte’s press release, the most common treatment-emergent adverse reactions in patients treated with ruxolitinib during clinical trials were nasopharyngitis, diarrhea, bronchitis, ear infection, eosinophil count increases, urticaria, folliculitis, tonsillitis, and rhinorrhea. The labeling includes boxed warnings for serious infections, mortality, malignancy, major adverse cardiovascular events, and thrombosis, seen with oral JAK inhibitors for inflammatory conditions.
Incyte will market ruxolitinib under the trade name Opzelura.
Dr. Simpson disclosed that he is a consultant to and/or an investigator for several pharmaceutical companies, including Incyte, Regeneron/Sanofi, Eli Lilly and Company, AbbVie, and Pfizer.
Dr. Papp disclosed that he has received honoraria or clinical research grants as a consultant, speaker, scientific officer, advisory board member, and/or steering committee member for several pharmaceutical companies, including Incyte.
Commentary by Robert Sidbury, MD, MPH
Another nonsteroidal topical medication approved for atopic dermatitis (AD)? Thank goodness. Topical ruxolitinib 1.5% cream twice daily for mild to moderate AD demonstrated excellent efficacy vs. placebo in duplicative trials (53.8/51.3% vs. 15.1%/7.6%; P < .001), with a reassuring safety profile. Application site reactions were uncommon, though past experience with other new nonsteroidal agents suggests judgment be reserved on that score. More compelling was the fact that no patients discontinued therapy in the 1.5% arm, and adverse events were mild and self-limited such as nasopharyngitis and diarrhea. This stands in contradistinction to the boxed warning attached to JAK inhibitors (topical and systemic) against a daunting list of destructive possibilities: malignancy, infection, cardiovascular disease, and blood clots. None of these things was seen in these topical ruxolitinib trials.
Dr. Sidbury is chief of dermatology at Seattle Children's Hospital and professor, department of pediatrics, University of Washington, Seattle. He is a site principal investigator for dupilumab trials, for which the hospital has a contract with Regeneron.
This article was updated 6/16/22.
The , making it the first topical JAK inhibitor approved for AD – and the first JAK inhibitor approved for this indication – in the United States.
The approval is limited to patients whose AD is not adequately controlled with topical prescription therapies, or when those therapies are not advisable.
“Approval of topical ruxolitinib fills a major gap in the treatment of atopic dermatitis: a safe, effective, and tolerable non-steroidal topical therapy,” Eric L. Simpson, MD, professor of dermatology and director of the Oregon Health & Science University Dermatology Clinical Research Center, Portland, told this news organization. “This approval will allow for long-term treatment without the concern of steroid side effects. From earlier studies, ruxolitinib cream appears to be as effective as a medium-potency topical steroid. These efficacy levels and low incidence of burning will be a welcome addition to our current nonsteroidal therapies.”
The drug’s approval was based on results from two phase 3, randomized studies of identical design involving 1,249 patients aged 12 years and older with AD: TRuE-AD1 and TRuE-AD2. In these studies, ruxolitinib cream demonstrated anti-inflammatory activity, with rapid and sustained antipruritic action, compared with vehicle. In the trials, patients with an Investigator’s Global Assessment (IGA) score of 2 or 3 and 3%-20% of affected body surface area (BSA) were randomized (2:2:1) to twice-daily 0.75% ruxolitinib cream, 1.5% ruxolitinib cream, or vehicle cream for 8 continuous weeks. The 1.5% concentration was approved by the FDA.
A study first published in May of 2021 found that significantly more patients in TRuE-AD1 and TRuE-AD2 achieved IGA treatment success with 0.75% (50% vs. 39%, respectively) and 1.5% ruxolitinib cream (53.8% vs. 51.3%), compared with vehicle (15.1% vs. 7.6%; P < .0001) at week 8. In addition, significant reductions in itch, compared with vehicle, were reported within 12 hours of first applying 1.5% ruxolitinib cream (P < .05).
More key findings from TRuE-AD1 and TRuE-AD2 are scheduled to be presented during the upcoming European Academy of Dermatology and Venereology meeting Sept. 29-Oct. 2, but during the Revolutionizing Atopic Dermatitis Symposium on June 13, Kim Papp, MD, PhD, presented long-term safety data of ruxolitinib cream in patients who were followed for an additional 44 weeks. Dr. Papp, a dermatologist and founder of Probity Medical Research, Waterloo, Ont., reported that 543 patients from TRuE-AD1 and 530 from TRuE-AD2 entered the long-term analysis and that about 78% of these patients completed the study. From weeks 12 to 52, the proportion of patients with an IGA score of 0 or 1 with 0.75% and 1.5% ruxolitinib cream ranged from 62%-77% and 67%-77%, respectively, in TRuE-AD1, to 60%-77% and 72%-80% in TRuE-AD2.
The measured mean total affected BSA was less than 3% throughout the follow-up period in the 1.5% ruxolitinib cream arm in TRuE-AD1 and TRuE-AD2 and was less than 3% in the 0.75% ruxolitinib cream arm during most of the study period.
In a pooled safety analysis, treatment-emergent adverse events (TEAEs) were reported in 60% and 54% of patients who applied 0.75% and 1.5% ruxolitinib cream, respectively, over 44 weeks. The frequency of application-site reactions remained low. Specifically, treatment-related adverse events were reported in 5% of patients who applied 0.75% ruxolitinib cream and in 3% of patients who applied 1.5% ruxolitinib cream; none were serious. TEAEs led to discontinuation in 2% of patients in the 0.75% ruxolitinib cream group, and no patients in the 1.5% ruxolitinib cream group.
Dr. Papp and his colleagues observed that the most common treatment adverse events were upper respiratory tract infections and nasopharyngitis. According to Incyte’s press release, the most common treatment-emergent adverse reactions in patients treated with ruxolitinib during clinical trials were nasopharyngitis, diarrhea, bronchitis, ear infection, eosinophil count increases, urticaria, folliculitis, tonsillitis, and rhinorrhea. The labeling includes boxed warnings for serious infections, mortality, malignancy, major adverse cardiovascular events, and thrombosis, seen with oral JAK inhibitors for inflammatory conditions.
Incyte will market ruxolitinib under the trade name Opzelura.
Dr. Simpson disclosed that he is a consultant to and/or an investigator for several pharmaceutical companies, including Incyte, Regeneron/Sanofi, Eli Lilly and Company, AbbVie, and Pfizer.
Dr. Papp disclosed that he has received honoraria or clinical research grants as a consultant, speaker, scientific officer, advisory board member, and/or steering committee member for several pharmaceutical companies, including Incyte.
Commentary by Robert Sidbury, MD, MPH
Another nonsteroidal topical medication approved for atopic dermatitis (AD)? Thank goodness. Topical ruxolitinib 1.5% cream twice daily for mild to moderate AD demonstrated excellent efficacy vs. placebo in duplicative trials (53.8/51.3% vs. 15.1%/7.6%; P < .001), with a reassuring safety profile. Application site reactions were uncommon, though past experience with other new nonsteroidal agents suggests judgment be reserved on that score. More compelling was the fact that no patients discontinued therapy in the 1.5% arm, and adverse events were mild and self-limited such as nasopharyngitis and diarrhea. This stands in contradistinction to the boxed warning attached to JAK inhibitors (topical and systemic) against a daunting list of destructive possibilities: malignancy, infection, cardiovascular disease, and blood clots. None of these things was seen in these topical ruxolitinib trials.
Dr. Sidbury is chief of dermatology at Seattle Children's Hospital and professor, department of pediatrics, University of Washington, Seattle. He is a site principal investigator for dupilumab trials, for which the hospital has a contract with Regeneron.
This article was updated 6/16/22.
The , making it the first topical JAK inhibitor approved for AD – and the first JAK inhibitor approved for this indication – in the United States.
The approval is limited to patients whose AD is not adequately controlled with topical prescription therapies, or when those therapies are not advisable.
“Approval of topical ruxolitinib fills a major gap in the treatment of atopic dermatitis: a safe, effective, and tolerable non-steroidal topical therapy,” Eric L. Simpson, MD, professor of dermatology and director of the Oregon Health & Science University Dermatology Clinical Research Center, Portland, told this news organization. “This approval will allow for long-term treatment without the concern of steroid side effects. From earlier studies, ruxolitinib cream appears to be as effective as a medium-potency topical steroid. These efficacy levels and low incidence of burning will be a welcome addition to our current nonsteroidal therapies.”
The drug’s approval was based on results from two phase 3, randomized studies of identical design involving 1,249 patients aged 12 years and older with AD: TRuE-AD1 and TRuE-AD2. In these studies, ruxolitinib cream demonstrated anti-inflammatory activity, with rapid and sustained antipruritic action, compared with vehicle. In the trials, patients with an Investigator’s Global Assessment (IGA) score of 2 or 3 and 3%-20% of affected body surface area (BSA) were randomized (2:2:1) to twice-daily 0.75% ruxolitinib cream, 1.5% ruxolitinib cream, or vehicle cream for 8 continuous weeks. The 1.5% concentration was approved by the FDA.
A study first published in May of 2021 found that significantly more patients in TRuE-AD1 and TRuE-AD2 achieved IGA treatment success with 0.75% (50% vs. 39%, respectively) and 1.5% ruxolitinib cream (53.8% vs. 51.3%), compared with vehicle (15.1% vs. 7.6%; P < .0001) at week 8. In addition, significant reductions in itch, compared with vehicle, were reported within 12 hours of first applying 1.5% ruxolitinib cream (P < .05).
More key findings from TRuE-AD1 and TRuE-AD2 are scheduled to be presented during the upcoming European Academy of Dermatology and Venereology meeting Sept. 29-Oct. 2, but during the Revolutionizing Atopic Dermatitis Symposium on June 13, Kim Papp, MD, PhD, presented long-term safety data of ruxolitinib cream in patients who were followed for an additional 44 weeks. Dr. Papp, a dermatologist and founder of Probity Medical Research, Waterloo, Ont., reported that 543 patients from TRuE-AD1 and 530 from TRuE-AD2 entered the long-term analysis and that about 78% of these patients completed the study. From weeks 12 to 52, the proportion of patients with an IGA score of 0 or 1 with 0.75% and 1.5% ruxolitinib cream ranged from 62%-77% and 67%-77%, respectively, in TRuE-AD1, to 60%-77% and 72%-80% in TRuE-AD2.
The measured mean total affected BSA was less than 3% throughout the follow-up period in the 1.5% ruxolitinib cream arm in TRuE-AD1 and TRuE-AD2 and was less than 3% in the 0.75% ruxolitinib cream arm during most of the study period.
In a pooled safety analysis, treatment-emergent adverse events (TEAEs) were reported in 60% and 54% of patients who applied 0.75% and 1.5% ruxolitinib cream, respectively, over 44 weeks. The frequency of application-site reactions remained low. Specifically, treatment-related adverse events were reported in 5% of patients who applied 0.75% ruxolitinib cream and in 3% of patients who applied 1.5% ruxolitinib cream; none were serious. TEAEs led to discontinuation in 2% of patients in the 0.75% ruxolitinib cream group, and no patients in the 1.5% ruxolitinib cream group.
Dr. Papp and his colleagues observed that the most common treatment adverse events were upper respiratory tract infections and nasopharyngitis. According to Incyte’s press release, the most common treatment-emergent adverse reactions in patients treated with ruxolitinib during clinical trials were nasopharyngitis, diarrhea, bronchitis, ear infection, eosinophil count increases, urticaria, folliculitis, tonsillitis, and rhinorrhea. The labeling includes boxed warnings for serious infections, mortality, malignancy, major adverse cardiovascular events, and thrombosis, seen with oral JAK inhibitors for inflammatory conditions.
Incyte will market ruxolitinib under the trade name Opzelura.
Dr. Simpson disclosed that he is a consultant to and/or an investigator for several pharmaceutical companies, including Incyte, Regeneron/Sanofi, Eli Lilly and Company, AbbVie, and Pfizer.
Dr. Papp disclosed that he has received honoraria or clinical research grants as a consultant, speaker, scientific officer, advisory board member, and/or steering committee member for several pharmaceutical companies, including Incyte.
Commentary by Robert Sidbury, MD, MPH
Another nonsteroidal topical medication approved for atopic dermatitis (AD)? Thank goodness. Topical ruxolitinib 1.5% cream twice daily for mild to moderate AD demonstrated excellent efficacy vs. placebo in duplicative trials (53.8/51.3% vs. 15.1%/7.6%; P < .001), with a reassuring safety profile. Application site reactions were uncommon, though past experience with other new nonsteroidal agents suggests judgment be reserved on that score. More compelling was the fact that no patients discontinued therapy in the 1.5% arm, and adverse events were mild and self-limited such as nasopharyngitis and diarrhea. This stands in contradistinction to the boxed warning attached to JAK inhibitors (topical and systemic) against a daunting list of destructive possibilities: malignancy, infection, cardiovascular disease, and blood clots. None of these things was seen in these topical ruxolitinib trials.
Dr. Sidbury is chief of dermatology at Seattle Children's Hospital and professor, department of pediatrics, University of Washington, Seattle. He is a site principal investigator for dupilumab trials, for which the hospital has a contract with Regeneron.
This article was updated 6/16/22.
Atopic dermatitis: Meta-analysis confirms benefits of topical JAK and PDE4 inhibitors
Key clinical point: Findings from this network meta-analysis confirm efficacy and safety of topical Janus kinase (JAK) and phosphodiesterase-4 (PDE4) inhibitors for atopic dermatitis (AD).
Major finding: All included JAK and PDE4 inhibitors, specially tofacitinib 2% twice a day (BID; odds ratio [OR], 19.0; 95% confidence interval [CI], 5.6-77.0), delgocitinib 3% BID (OR, 19.2; 95% CI, 4.6-90.3), and ruxolitinib 1.5% once a day (QD; OR, 13.2; 95% CI, 7.5-25.0), showed higher Investigators Global Assessment response vs placebo. Tofacitinib 2% BID (OR, 0.4; 95% CI, 0.1-1.0) and ruxolitinib 1.5% QD (OR, 0.7; 95% CI, 0.5-1.0) had lower risk for adverse events vs placebo, whereas others showed a comparable safety profile.
Study details: Findings are from a network meta-analysis of 10 randomized controlled trials including 4,689 patients, mostly with mild-to-moderate AD treated with topical JAK and PDE4 inhibitors.
Disclosures: This study was supported by the National Natural Science Foundation of China and the Project for Disciplines of Excellence, West China Hospital. The authors declared no conflict of interests.
Source: Zhang L et al. J Dermatol. 2021 Sep 6. doi: 10.1111/1346-8138.16126.
Key clinical point: Findings from this network meta-analysis confirm efficacy and safety of topical Janus kinase (JAK) and phosphodiesterase-4 (PDE4) inhibitors for atopic dermatitis (AD).
Major finding: All included JAK and PDE4 inhibitors, specially tofacitinib 2% twice a day (BID; odds ratio [OR], 19.0; 95% confidence interval [CI], 5.6-77.0), delgocitinib 3% BID (OR, 19.2; 95% CI, 4.6-90.3), and ruxolitinib 1.5% once a day (QD; OR, 13.2; 95% CI, 7.5-25.0), showed higher Investigators Global Assessment response vs placebo. Tofacitinib 2% BID (OR, 0.4; 95% CI, 0.1-1.0) and ruxolitinib 1.5% QD (OR, 0.7; 95% CI, 0.5-1.0) had lower risk for adverse events vs placebo, whereas others showed a comparable safety profile.
Study details: Findings are from a network meta-analysis of 10 randomized controlled trials including 4,689 patients, mostly with mild-to-moderate AD treated with topical JAK and PDE4 inhibitors.
Disclosures: This study was supported by the National Natural Science Foundation of China and the Project for Disciplines of Excellence, West China Hospital. The authors declared no conflict of interests.
Source: Zhang L et al. J Dermatol. 2021 Sep 6. doi: 10.1111/1346-8138.16126.
Key clinical point: Findings from this network meta-analysis confirm efficacy and safety of topical Janus kinase (JAK) and phosphodiesterase-4 (PDE4) inhibitors for atopic dermatitis (AD).
Major finding: All included JAK and PDE4 inhibitors, specially tofacitinib 2% twice a day (BID; odds ratio [OR], 19.0; 95% confidence interval [CI], 5.6-77.0), delgocitinib 3% BID (OR, 19.2; 95% CI, 4.6-90.3), and ruxolitinib 1.5% once a day (QD; OR, 13.2; 95% CI, 7.5-25.0), showed higher Investigators Global Assessment response vs placebo. Tofacitinib 2% BID (OR, 0.4; 95% CI, 0.1-1.0) and ruxolitinib 1.5% QD (OR, 0.7; 95% CI, 0.5-1.0) had lower risk for adverse events vs placebo, whereas others showed a comparable safety profile.
Study details: Findings are from a network meta-analysis of 10 randomized controlled trials including 4,689 patients, mostly with mild-to-moderate AD treated with topical JAK and PDE4 inhibitors.
Disclosures: This study was supported by the National Natural Science Foundation of China and the Project for Disciplines of Excellence, West China Hospital. The authors declared no conflict of interests.
Source: Zhang L et al. J Dermatol. 2021 Sep 6. doi: 10.1111/1346-8138.16126.
Atopic dermatitis and cardiovascular diseases: Is there a link?
Key clinical point: Patients with atopic dermatitis (AD) were at a higher risk of developing cardiovascular diseases (CVD) and major adverse cardiovascular events (MACEs), independent of metabolic disorders.
Major finding: The risk for CVD (odds ratio [OR], 1.38; 95% confidence interval [CI], 1.34-1.41) and MACEs (OR, 1.40; 95% CI, 1.34-1.45) was significantly higher in patients with vs without AD. The risk for MACEs and CVD was higher in patients without (CVD: OR, 1.25; 95% CI, 1.13-1.39; MACE: OR, 1.22; 95% CI, 1.01-1.47) vs those with (CVD: OR, 1.09; 95% CI, 1.07-1.12; MACE: OR, 1.14; 95% CI, 1.09-1.18) metabolic disorders.
Study details: Findings are from a retrospective analysis of 1,32,460 adult patients with AD matched with 3,97,380 adults without AD.
Disclosures: This study was funded by Sanofi and Regeneron Pharmaceuticals, Inc. The authors declared being investigator, consultant, speaker, current and/or former employees, and stockholders for various sources including Sanofi and/or Regeneron Pharmaceuticals, Inc.
Source: Wu JJ et al. Dermatol Ther (Heidelb). 2021 Aug 27. doi: 10.1007/s13555-021-00587-9.
Key clinical point: Patients with atopic dermatitis (AD) were at a higher risk of developing cardiovascular diseases (CVD) and major adverse cardiovascular events (MACEs), independent of metabolic disorders.
Major finding: The risk for CVD (odds ratio [OR], 1.38; 95% confidence interval [CI], 1.34-1.41) and MACEs (OR, 1.40; 95% CI, 1.34-1.45) was significantly higher in patients with vs without AD. The risk for MACEs and CVD was higher in patients without (CVD: OR, 1.25; 95% CI, 1.13-1.39; MACE: OR, 1.22; 95% CI, 1.01-1.47) vs those with (CVD: OR, 1.09; 95% CI, 1.07-1.12; MACE: OR, 1.14; 95% CI, 1.09-1.18) metabolic disorders.
Study details: Findings are from a retrospective analysis of 1,32,460 adult patients with AD matched with 3,97,380 adults without AD.
Disclosures: This study was funded by Sanofi and Regeneron Pharmaceuticals, Inc. The authors declared being investigator, consultant, speaker, current and/or former employees, and stockholders for various sources including Sanofi and/or Regeneron Pharmaceuticals, Inc.
Source: Wu JJ et al. Dermatol Ther (Heidelb). 2021 Aug 27. doi: 10.1007/s13555-021-00587-9.
Key clinical point: Patients with atopic dermatitis (AD) were at a higher risk of developing cardiovascular diseases (CVD) and major adverse cardiovascular events (MACEs), independent of metabolic disorders.
Major finding: The risk for CVD (odds ratio [OR], 1.38; 95% confidence interval [CI], 1.34-1.41) and MACEs (OR, 1.40; 95% CI, 1.34-1.45) was significantly higher in patients with vs without AD. The risk for MACEs and CVD was higher in patients without (CVD: OR, 1.25; 95% CI, 1.13-1.39; MACE: OR, 1.22; 95% CI, 1.01-1.47) vs those with (CVD: OR, 1.09; 95% CI, 1.07-1.12; MACE: OR, 1.14; 95% CI, 1.09-1.18) metabolic disorders.
Study details: Findings are from a retrospective analysis of 1,32,460 adult patients with AD matched with 3,97,380 adults without AD.
Disclosures: This study was funded by Sanofi and Regeneron Pharmaceuticals, Inc. The authors declared being investigator, consultant, speaker, current and/or former employees, and stockholders for various sources including Sanofi and/or Regeneron Pharmaceuticals, Inc.
Source: Wu JJ et al. Dermatol Ther (Heidelb). 2021 Aug 27. doi: 10.1007/s13555-021-00587-9.
Atopic dermatitis could be a risk factor for rheumatoid arthritis
Key clinical point: Patients with atopic dermatitis (AD) were at a significantly higher risk of developing rheumatoid arthritis (RA).
Major finding: Patients with AD had a significantly higher risk for incident RA than patients without AD (pooled odds ratio, 1.30; 95% confidence interval, 1.17-1.44).
Study details: Findings are from a meta-analysis of 4 cohorts and 9 case-control studies.
Disclosures: The study did not report any source of funding. No conflict of interests was reported.
Source: Rittiphairoj T et al. Dermatitis. 2021 Aug 16. doi: 10.1097/DER.0000000000000781.
Key clinical point: Patients with atopic dermatitis (AD) were at a significantly higher risk of developing rheumatoid arthritis (RA).
Major finding: Patients with AD had a significantly higher risk for incident RA than patients without AD (pooled odds ratio, 1.30; 95% confidence interval, 1.17-1.44).
Study details: Findings are from a meta-analysis of 4 cohorts and 9 case-control studies.
Disclosures: The study did not report any source of funding. No conflict of interests was reported.
Source: Rittiphairoj T et al. Dermatitis. 2021 Aug 16. doi: 10.1097/DER.0000000000000781.
Key clinical point: Patients with atopic dermatitis (AD) were at a significantly higher risk of developing rheumatoid arthritis (RA).
Major finding: Patients with AD had a significantly higher risk for incident RA than patients without AD (pooled odds ratio, 1.30; 95% confidence interval, 1.17-1.44).
Study details: Findings are from a meta-analysis of 4 cohorts and 9 case-control studies.
Disclosures: The study did not report any source of funding. No conflict of interests was reported.
Source: Rittiphairoj T et al. Dermatitis. 2021 Aug 16. doi: 10.1097/DER.0000000000000781.
Dupilumab is effective for pediatric atopic dermatitis across different anatomical regions
Key clinical point: Dupilumab improved signs of atopic dermatitis (AD) rapidly and consistently across all anatomical regions in pediatric patients.
Major finding: In children, dupilumab improved Eczema Area and Severity Index (EASI) score as early as week 1 in head and neck, trunk, and upper extremities and as early as week 2 in lower extremities. In adolescents, dupilumab improved the EASI score in all anatomical regions as early as week 2. All improvements were sustained through week 16 (all P less than .05).
Study details: Findings are a post hoc analysis of 2 phase 3 dupilumab therapy trials, LIBERTY AD ADOL and LIBERTY AD PEDS, including 167 adolescents with moderate-to-severe AD and 304 children with severe AD inadequately controlled by topical medication.
Disclosures: This study was funded by Sanofi and Regeneron Pharmaceuticals, Inc. Some of the authors declared receiving grants, honoraria, and/or serving as a consultant, speaker, advisory board member, and investigator for various sources. Some of the authors declared being employees and/or holding stocks/stock of Regeneron Pharmaceuticals or Sanofi Genzyme.
Source: Simpson EL et al. Dermatol Ther (Heidelb). 2021 Aug 24. doi: 10.1007/s13555-021-00568-y.
Key clinical point: Dupilumab improved signs of atopic dermatitis (AD) rapidly and consistently across all anatomical regions in pediatric patients.
Major finding: In children, dupilumab improved Eczema Area and Severity Index (EASI) score as early as week 1 in head and neck, trunk, and upper extremities and as early as week 2 in lower extremities. In adolescents, dupilumab improved the EASI score in all anatomical regions as early as week 2. All improvements were sustained through week 16 (all P less than .05).
Study details: Findings are a post hoc analysis of 2 phase 3 dupilumab therapy trials, LIBERTY AD ADOL and LIBERTY AD PEDS, including 167 adolescents with moderate-to-severe AD and 304 children with severe AD inadequately controlled by topical medication.
Disclosures: This study was funded by Sanofi and Regeneron Pharmaceuticals, Inc. Some of the authors declared receiving grants, honoraria, and/or serving as a consultant, speaker, advisory board member, and investigator for various sources. Some of the authors declared being employees and/or holding stocks/stock of Regeneron Pharmaceuticals or Sanofi Genzyme.
Source: Simpson EL et al. Dermatol Ther (Heidelb). 2021 Aug 24. doi: 10.1007/s13555-021-00568-y.
Key clinical point: Dupilumab improved signs of atopic dermatitis (AD) rapidly and consistently across all anatomical regions in pediatric patients.
Major finding: In children, dupilumab improved Eczema Area and Severity Index (EASI) score as early as week 1 in head and neck, trunk, and upper extremities and as early as week 2 in lower extremities. In adolescents, dupilumab improved the EASI score in all anatomical regions as early as week 2. All improvements were sustained through week 16 (all P less than .05).
Study details: Findings are a post hoc analysis of 2 phase 3 dupilumab therapy trials, LIBERTY AD ADOL and LIBERTY AD PEDS, including 167 adolescents with moderate-to-severe AD and 304 children with severe AD inadequately controlled by topical medication.
Disclosures: This study was funded by Sanofi and Regeneron Pharmaceuticals, Inc. Some of the authors declared receiving grants, honoraria, and/or serving as a consultant, speaker, advisory board member, and investigator for various sources. Some of the authors declared being employees and/or holding stocks/stock of Regeneron Pharmaceuticals or Sanofi Genzyme.
Source: Simpson EL et al. Dermatol Ther (Heidelb). 2021 Aug 24. doi: 10.1007/s13555-021-00568-y.