User login
Cannabinoids being studied for a variety of dermatologic conditions
.
“When you walk into places like CVS or Walgreens, you see lots of displays for CBD creams and oils,” Todd S. Anhalt, MD, said during the annual meeting of the Pacific Dermatologic Association. “The problem is, we don’t know what’s in them or who made them or how good they are. That’s going to be a problem for a while.”
According to Dr. Anhalt, clinical professor emeritus of dermatology at Stanford (Calif.) University, there are about 140 active cannabinoid compounds in cannabis, but the most important ones are THC and cannabidiol (CBD). There are three types of cannabinoids, based on where the cannabidiol is produced: endocannabinoids, which are produced in the human body; phytocannabinoids, which are derived from plants such as marijuana and hemp; and synthetic cannabinoids, which are derived in labs.
Dr. Anhalt described the endocannabinoid system as a conserved network of molecular signaling made of several components: signaling molecules (endocannabinoids), endocannabinoid receptors (CB-1 and CB-2), enzymes, and transporters. There is also overlap between cannabinoids and terpenes, which are responsible for flavor and aroma in plants and marijuana and can enhance the effects of CBD.
“For the most part, CB-1 receptors are in the central nervous system and CB-2 [receptors] are mostly in the periphery,” including the skin and digestive system, said Dr. Anhalt, who practices at the California Skin Institute in Los Altos, Calif. “This is interesting because one of the main conditions I recommend cannabidiol for is in patients with peripheral neuropathy, despite the fact they may be on all sorts of medications such as Neurontin and Lyrica or tricyclic antidepressants. Sometimes they don’t get much relief from those. I have had many patients tell me that they have had reduction of pain and increased functionality using the CBD creams.” CB-2 receptors, he noted, are located in keratinocytes, sensory receptors, sweat glands, fibroblasts, Langerhans cells, melanocytes, and sebaceous glands.
Recent research shows that the endocannabinoid system is involved in modulation of the CNS and in immune function, particularly skin homeostasis and barrier function. “We know that barrier function can be affected by the generation of oxidative species,” he said. “The stress that it causes can decrease barrier function and lead to cytokine release and itch. CBDs have been shown to enter cells, target and upregulate genes with decreased oxidation and inflammation, and protect membrane integrity in skin cells. Therefore, this might be helpful in atopic dermatitis.” Other potential uses in dermatology include wound healing, acne, hair growth modulation, skin and hair pigmentation, skin infections, psoriasis, and cutaneous malignancies, as well as neuropathic pain.
Evidence is strongest for neuropathic pain, he said, which is mediated by CB-1 receptors peripherally, followed by itch and atopic dermatitis. The authors of a 2017 systematic review concluded that “low-strength” evidence exists to suggest that cannabis alleviates neuropathic pain, with insufficient evidence for other types of pain.
Topical CBD comes in various forms: oils (usually hemp oil), creams, and lotions, Dr. Anhalt said. “I advise patients to apply it 2-4 times per day depending on how anxious or uncomfortable they are. It takes my patients 10 days to 2 weeks before they notice anything at all.”
For atopic dermatitis, it could be useful “not to use it instead of a moisturizer, but as a moisturizer,” Dr. Anhalt advised. “You can have a patient get big jars of CBD creams and lotions. They may have to try a few before they find one that they really like, but you can replace all of the other moisturizers that you’re using right now in patients who have a lot of itch.”
As for CBD’s effect on peripheral neuropathy, the medical literature is lacking, but some studies show low to moderate evidence of efficacy. For example, a Cochrane Review found that a 30% or greater pain reduction was achieved by 39% of patients who used cannabis-based treatments, vs. 33% of those on placebo.
“I would not suggest CBD as a first-line drug unless it’s very mild peripheral neuropathy, but for patients who are on gabapentin who are better but not better enough, this is an excellent adjunct,” Dr. Anhalt said. “It’s worth trying. It’s not too expensive and it’s really safe.”
The application of topical CBD to treat cutaneous malignancies has not yet shown evidence of significant efficacy, while using CBDs for acne holds promise. “The endogenous cannabinoid system is involved in the production of lipids,” he said. “Cannabinoids have an antilipogenic activity, so they decrease sebum production. CBD could help patients with mild acne who are reluctant to use other types of medications. For this and other potential dermatologic applications, lots more studies need to be done.”
Dr. Anhalt reported having no financial disclosures.
.
“When you walk into places like CVS or Walgreens, you see lots of displays for CBD creams and oils,” Todd S. Anhalt, MD, said during the annual meeting of the Pacific Dermatologic Association. “The problem is, we don’t know what’s in them or who made them or how good they are. That’s going to be a problem for a while.”
According to Dr. Anhalt, clinical professor emeritus of dermatology at Stanford (Calif.) University, there are about 140 active cannabinoid compounds in cannabis, but the most important ones are THC and cannabidiol (CBD). There are three types of cannabinoids, based on where the cannabidiol is produced: endocannabinoids, which are produced in the human body; phytocannabinoids, which are derived from plants such as marijuana and hemp; and synthetic cannabinoids, which are derived in labs.
Dr. Anhalt described the endocannabinoid system as a conserved network of molecular signaling made of several components: signaling molecules (endocannabinoids), endocannabinoid receptors (CB-1 and CB-2), enzymes, and transporters. There is also overlap between cannabinoids and terpenes, which are responsible for flavor and aroma in plants and marijuana and can enhance the effects of CBD.
“For the most part, CB-1 receptors are in the central nervous system and CB-2 [receptors] are mostly in the periphery,” including the skin and digestive system, said Dr. Anhalt, who practices at the California Skin Institute in Los Altos, Calif. “This is interesting because one of the main conditions I recommend cannabidiol for is in patients with peripheral neuropathy, despite the fact they may be on all sorts of medications such as Neurontin and Lyrica or tricyclic antidepressants. Sometimes they don’t get much relief from those. I have had many patients tell me that they have had reduction of pain and increased functionality using the CBD creams.” CB-2 receptors, he noted, are located in keratinocytes, sensory receptors, sweat glands, fibroblasts, Langerhans cells, melanocytes, and sebaceous glands.
Recent research shows that the endocannabinoid system is involved in modulation of the CNS and in immune function, particularly skin homeostasis and barrier function. “We know that barrier function can be affected by the generation of oxidative species,” he said. “The stress that it causes can decrease barrier function and lead to cytokine release and itch. CBDs have been shown to enter cells, target and upregulate genes with decreased oxidation and inflammation, and protect membrane integrity in skin cells. Therefore, this might be helpful in atopic dermatitis.” Other potential uses in dermatology include wound healing, acne, hair growth modulation, skin and hair pigmentation, skin infections, psoriasis, and cutaneous malignancies, as well as neuropathic pain.
Evidence is strongest for neuropathic pain, he said, which is mediated by CB-1 receptors peripherally, followed by itch and atopic dermatitis. The authors of a 2017 systematic review concluded that “low-strength” evidence exists to suggest that cannabis alleviates neuropathic pain, with insufficient evidence for other types of pain.
Topical CBD comes in various forms: oils (usually hemp oil), creams, and lotions, Dr. Anhalt said. “I advise patients to apply it 2-4 times per day depending on how anxious or uncomfortable they are. It takes my patients 10 days to 2 weeks before they notice anything at all.”
For atopic dermatitis, it could be useful “not to use it instead of a moisturizer, but as a moisturizer,” Dr. Anhalt advised. “You can have a patient get big jars of CBD creams and lotions. They may have to try a few before they find one that they really like, but you can replace all of the other moisturizers that you’re using right now in patients who have a lot of itch.”
As for CBD’s effect on peripheral neuropathy, the medical literature is lacking, but some studies show low to moderate evidence of efficacy. For example, a Cochrane Review found that a 30% or greater pain reduction was achieved by 39% of patients who used cannabis-based treatments, vs. 33% of those on placebo.
“I would not suggest CBD as a first-line drug unless it’s very mild peripheral neuropathy, but for patients who are on gabapentin who are better but not better enough, this is an excellent adjunct,” Dr. Anhalt said. “It’s worth trying. It’s not too expensive and it’s really safe.”
The application of topical CBD to treat cutaneous malignancies has not yet shown evidence of significant efficacy, while using CBDs for acne holds promise. “The endogenous cannabinoid system is involved in the production of lipids,” he said. “Cannabinoids have an antilipogenic activity, so they decrease sebum production. CBD could help patients with mild acne who are reluctant to use other types of medications. For this and other potential dermatologic applications, lots more studies need to be done.”
Dr. Anhalt reported having no financial disclosures.
.
“When you walk into places like CVS or Walgreens, you see lots of displays for CBD creams and oils,” Todd S. Anhalt, MD, said during the annual meeting of the Pacific Dermatologic Association. “The problem is, we don’t know what’s in them or who made them or how good they are. That’s going to be a problem for a while.”
According to Dr. Anhalt, clinical professor emeritus of dermatology at Stanford (Calif.) University, there are about 140 active cannabinoid compounds in cannabis, but the most important ones are THC and cannabidiol (CBD). There are three types of cannabinoids, based on where the cannabidiol is produced: endocannabinoids, which are produced in the human body; phytocannabinoids, which are derived from plants such as marijuana and hemp; and synthetic cannabinoids, which are derived in labs.
Dr. Anhalt described the endocannabinoid system as a conserved network of molecular signaling made of several components: signaling molecules (endocannabinoids), endocannabinoid receptors (CB-1 and CB-2), enzymes, and transporters. There is also overlap between cannabinoids and terpenes, which are responsible for flavor and aroma in plants and marijuana and can enhance the effects of CBD.
“For the most part, CB-1 receptors are in the central nervous system and CB-2 [receptors] are mostly in the periphery,” including the skin and digestive system, said Dr. Anhalt, who practices at the California Skin Institute in Los Altos, Calif. “This is interesting because one of the main conditions I recommend cannabidiol for is in patients with peripheral neuropathy, despite the fact they may be on all sorts of medications such as Neurontin and Lyrica or tricyclic antidepressants. Sometimes they don’t get much relief from those. I have had many patients tell me that they have had reduction of pain and increased functionality using the CBD creams.” CB-2 receptors, he noted, are located in keratinocytes, sensory receptors, sweat glands, fibroblasts, Langerhans cells, melanocytes, and sebaceous glands.
Recent research shows that the endocannabinoid system is involved in modulation of the CNS and in immune function, particularly skin homeostasis and barrier function. “We know that barrier function can be affected by the generation of oxidative species,” he said. “The stress that it causes can decrease barrier function and lead to cytokine release and itch. CBDs have been shown to enter cells, target and upregulate genes with decreased oxidation and inflammation, and protect membrane integrity in skin cells. Therefore, this might be helpful in atopic dermatitis.” Other potential uses in dermatology include wound healing, acne, hair growth modulation, skin and hair pigmentation, skin infections, psoriasis, and cutaneous malignancies, as well as neuropathic pain.
Evidence is strongest for neuropathic pain, he said, which is mediated by CB-1 receptors peripherally, followed by itch and atopic dermatitis. The authors of a 2017 systematic review concluded that “low-strength” evidence exists to suggest that cannabis alleviates neuropathic pain, with insufficient evidence for other types of pain.
Topical CBD comes in various forms: oils (usually hemp oil), creams, and lotions, Dr. Anhalt said. “I advise patients to apply it 2-4 times per day depending on how anxious or uncomfortable they are. It takes my patients 10 days to 2 weeks before they notice anything at all.”
For atopic dermatitis, it could be useful “not to use it instead of a moisturizer, but as a moisturizer,” Dr. Anhalt advised. “You can have a patient get big jars of CBD creams and lotions. They may have to try a few before they find one that they really like, but you can replace all of the other moisturizers that you’re using right now in patients who have a lot of itch.”
As for CBD’s effect on peripheral neuropathy, the medical literature is lacking, but some studies show low to moderate evidence of efficacy. For example, a Cochrane Review found that a 30% or greater pain reduction was achieved by 39% of patients who used cannabis-based treatments, vs. 33% of those on placebo.
“I would not suggest CBD as a first-line drug unless it’s very mild peripheral neuropathy, but for patients who are on gabapentin who are better but not better enough, this is an excellent adjunct,” Dr. Anhalt said. “It’s worth trying. It’s not too expensive and it’s really safe.”
The application of topical CBD to treat cutaneous malignancies has not yet shown evidence of significant efficacy, while using CBDs for acne holds promise. “The endogenous cannabinoid system is involved in the production of lipids,” he said. “Cannabinoids have an antilipogenic activity, so they decrease sebum production. CBD could help patients with mild acne who are reluctant to use other types of medications. For this and other potential dermatologic applications, lots more studies need to be done.”
Dr. Anhalt reported having no financial disclosures.
FROM PDA 2021
More eczema in children exposed to toxic metals in utero
published Oct. 27, 2021, in JAMA Network Open.
In this multicenter cohort study, led by epidemiologist Shu-Li Wang, PhD, of the National Institute of Environmental Health Sciences, in Taiwan, each twofold increase in prenatal arsenic level correlated with a 2.4-fold higher rate of atopic dermatitis in 4-year-olds.
Atopic diseases have been on the rise. Eczema (atopic dermatitis) is the first stage of the so-called atopic march, followed by food allergies, allergic rhinitis, and asthma later in childhood. Previous research has linked heavy metal exposure to allergic diseases in adults. In another study by Dr. Wang and colleagues that was published in 2021, prenatal and early-life arsenic exposure was found to correlate with higher rates of allergic rhinitis and asthma in children. In that study, the participants were followed every 2-3 years through the age of 14 as part of the Taiwan Maternal and Infant Cohort Study.
The new study included 370 mother and child pairs who were enrolled in that birth cohort study between October 2012 and May 2015. During their third trimester of pregnancy, women completed questionnaires about their lifestyle, diet, and living environment. In addition, their height, weight, and blood pressure were recorded, and urine samples were taken. In follow-up interviews 3-4 years later, the mothers were asked whether their child had ever been diagnosed with atopic dermatitis.
The researchers used an inductively coupled plasma mass spectrometer to analyze the participants’ urine samples. They assessed for exposures in utero to eight metals: arsenic, cadmium, lead, cobalt, copper, nickel, thallium, and zinc.
Each unit increase of an index that estimates the combined exposure to these metals during pregnancy was associated with 63% higher odds of atopic dermatitis in the children by age 4. The researchers adjusted for parental allergies (yes or no), mother’s educational level (<12 years, 13-16 years, or >16 years), geographic area (central or eastern Taiwan), exposure to tobacco smoke during pregnancy, and the child’s gender. Arsenic (40.1%) and cadmium (20.5%) accounted for most of the metal coexposure index.
A wealth of previous research links arsenic exposure during adulthood to skin disease and immune dysfunction. Early-life arsenic exposure has been linked with elevated risk for various adult disorders, including cancer, diabetes, and heart disease, years later. In light of such research, “the findings in this paper are not surprising,” J. Christopher States, PhD, director of the Center for Integrative Environmental Health Science at the University of Louisville (Ky.), told this news organization. “Low-level arsenic exposure does not cause disease immediately, but it does appear to have long-lasting effects, making individuals susceptible to ‘second hits’ with another environmental agent.”
Research into the molecular mechanisms for these links has shown that arsenic and cadmium exposure can promote allergic phenotypes in immune cells. “We think the toxic metals activate the alarmin pathway, thus inducing innate lymphoid cells, then activating T-helper 2 cells, which drive immunoglobulin E production and breakdown of the epithelium and promotion of allergies,” said Kari Nadeau, MD, PhD, director of the Sean N. Parker Center for Allergy and Asthma Research at Stanford University. Dr. Nadeau led that study, published in 2017 in PLOS One, along with epidemiologist Margaret Karagas, PhD, of Geisel School of Medicine at Dartmouth, Hanover, N.H.
As for what pregnant women can do to minimize their exposure to heavy metals, “that is a difficult problem and primarily a function of where one lives,” said Dr. States.
Drinking water and food are major sources of arsenic exposure. Groundwater is naturally contaminated with arsenic deposits that seep in from bedrock, said Dr. States. The U.S. Environmental Protection Agency regulates arsenic levels in public drinking water that is supplied to more than a few thousand people. However, small water supplies and private wells are unregulated, he said, and having these water sources tested for arsenic or fitted with systems to reduce arsenic can be very expensive.
Among foods, rice can have high concentrations of arsenic, Dr. Karagas told this news organization. To minimize arsenic exposure through the diet, women can limit rice-based foods, according to a web-based tool developed by her and coworkers.
In addition, tobacco smoke is a major source of cadmium exposure and a moderate source of arsenic exposure, Dr. States noted. Women can reduce their exposure to these metals by avoiding tobacco and secondhand smoke.
The study was supported by grants from the National Health Research Institutes, Chung Shan Medical University Hospital, Taiwan Ministry of Science and Technology, and the Taiwan Environmental Protection Administration. The authors and quoted experts report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
published Oct. 27, 2021, in JAMA Network Open.
In this multicenter cohort study, led by epidemiologist Shu-Li Wang, PhD, of the National Institute of Environmental Health Sciences, in Taiwan, each twofold increase in prenatal arsenic level correlated with a 2.4-fold higher rate of atopic dermatitis in 4-year-olds.
Atopic diseases have been on the rise. Eczema (atopic dermatitis) is the first stage of the so-called atopic march, followed by food allergies, allergic rhinitis, and asthma later in childhood. Previous research has linked heavy metal exposure to allergic diseases in adults. In another study by Dr. Wang and colleagues that was published in 2021, prenatal and early-life arsenic exposure was found to correlate with higher rates of allergic rhinitis and asthma in children. In that study, the participants were followed every 2-3 years through the age of 14 as part of the Taiwan Maternal and Infant Cohort Study.
The new study included 370 mother and child pairs who were enrolled in that birth cohort study between October 2012 and May 2015. During their third trimester of pregnancy, women completed questionnaires about their lifestyle, diet, and living environment. In addition, their height, weight, and blood pressure were recorded, and urine samples were taken. In follow-up interviews 3-4 years later, the mothers were asked whether their child had ever been diagnosed with atopic dermatitis.
The researchers used an inductively coupled plasma mass spectrometer to analyze the participants’ urine samples. They assessed for exposures in utero to eight metals: arsenic, cadmium, lead, cobalt, copper, nickel, thallium, and zinc.
Each unit increase of an index that estimates the combined exposure to these metals during pregnancy was associated with 63% higher odds of atopic dermatitis in the children by age 4. The researchers adjusted for parental allergies (yes or no), mother’s educational level (<12 years, 13-16 years, or >16 years), geographic area (central or eastern Taiwan), exposure to tobacco smoke during pregnancy, and the child’s gender. Arsenic (40.1%) and cadmium (20.5%) accounted for most of the metal coexposure index.
A wealth of previous research links arsenic exposure during adulthood to skin disease and immune dysfunction. Early-life arsenic exposure has been linked with elevated risk for various adult disorders, including cancer, diabetes, and heart disease, years later. In light of such research, “the findings in this paper are not surprising,” J. Christopher States, PhD, director of the Center for Integrative Environmental Health Science at the University of Louisville (Ky.), told this news organization. “Low-level arsenic exposure does not cause disease immediately, but it does appear to have long-lasting effects, making individuals susceptible to ‘second hits’ with another environmental agent.”
Research into the molecular mechanisms for these links has shown that arsenic and cadmium exposure can promote allergic phenotypes in immune cells. “We think the toxic metals activate the alarmin pathway, thus inducing innate lymphoid cells, then activating T-helper 2 cells, which drive immunoglobulin E production and breakdown of the epithelium and promotion of allergies,” said Kari Nadeau, MD, PhD, director of the Sean N. Parker Center for Allergy and Asthma Research at Stanford University. Dr. Nadeau led that study, published in 2017 in PLOS One, along with epidemiologist Margaret Karagas, PhD, of Geisel School of Medicine at Dartmouth, Hanover, N.H.
As for what pregnant women can do to minimize their exposure to heavy metals, “that is a difficult problem and primarily a function of where one lives,” said Dr. States.
Drinking water and food are major sources of arsenic exposure. Groundwater is naturally contaminated with arsenic deposits that seep in from bedrock, said Dr. States. The U.S. Environmental Protection Agency regulates arsenic levels in public drinking water that is supplied to more than a few thousand people. However, small water supplies and private wells are unregulated, he said, and having these water sources tested for arsenic or fitted with systems to reduce arsenic can be very expensive.
Among foods, rice can have high concentrations of arsenic, Dr. Karagas told this news organization. To minimize arsenic exposure through the diet, women can limit rice-based foods, according to a web-based tool developed by her and coworkers.
In addition, tobacco smoke is a major source of cadmium exposure and a moderate source of arsenic exposure, Dr. States noted. Women can reduce their exposure to these metals by avoiding tobacco and secondhand smoke.
The study was supported by grants from the National Health Research Institutes, Chung Shan Medical University Hospital, Taiwan Ministry of Science and Technology, and the Taiwan Environmental Protection Administration. The authors and quoted experts report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
published Oct. 27, 2021, in JAMA Network Open.
In this multicenter cohort study, led by epidemiologist Shu-Li Wang, PhD, of the National Institute of Environmental Health Sciences, in Taiwan, each twofold increase in prenatal arsenic level correlated with a 2.4-fold higher rate of atopic dermatitis in 4-year-olds.
Atopic diseases have been on the rise. Eczema (atopic dermatitis) is the first stage of the so-called atopic march, followed by food allergies, allergic rhinitis, and asthma later in childhood. Previous research has linked heavy metal exposure to allergic diseases in adults. In another study by Dr. Wang and colleagues that was published in 2021, prenatal and early-life arsenic exposure was found to correlate with higher rates of allergic rhinitis and asthma in children. In that study, the participants were followed every 2-3 years through the age of 14 as part of the Taiwan Maternal and Infant Cohort Study.
The new study included 370 mother and child pairs who were enrolled in that birth cohort study between October 2012 and May 2015. During their third trimester of pregnancy, women completed questionnaires about their lifestyle, diet, and living environment. In addition, their height, weight, and blood pressure were recorded, and urine samples were taken. In follow-up interviews 3-4 years later, the mothers were asked whether their child had ever been diagnosed with atopic dermatitis.
The researchers used an inductively coupled plasma mass spectrometer to analyze the participants’ urine samples. They assessed for exposures in utero to eight metals: arsenic, cadmium, lead, cobalt, copper, nickel, thallium, and zinc.
Each unit increase of an index that estimates the combined exposure to these metals during pregnancy was associated with 63% higher odds of atopic dermatitis in the children by age 4. The researchers adjusted for parental allergies (yes or no), mother’s educational level (<12 years, 13-16 years, or >16 years), geographic area (central or eastern Taiwan), exposure to tobacco smoke during pregnancy, and the child’s gender. Arsenic (40.1%) and cadmium (20.5%) accounted for most of the metal coexposure index.
A wealth of previous research links arsenic exposure during adulthood to skin disease and immune dysfunction. Early-life arsenic exposure has been linked with elevated risk for various adult disorders, including cancer, diabetes, and heart disease, years later. In light of such research, “the findings in this paper are not surprising,” J. Christopher States, PhD, director of the Center for Integrative Environmental Health Science at the University of Louisville (Ky.), told this news organization. “Low-level arsenic exposure does not cause disease immediately, but it does appear to have long-lasting effects, making individuals susceptible to ‘second hits’ with another environmental agent.”
Research into the molecular mechanisms for these links has shown that arsenic and cadmium exposure can promote allergic phenotypes in immune cells. “We think the toxic metals activate the alarmin pathway, thus inducing innate lymphoid cells, then activating T-helper 2 cells, which drive immunoglobulin E production and breakdown of the epithelium and promotion of allergies,” said Kari Nadeau, MD, PhD, director of the Sean N. Parker Center for Allergy and Asthma Research at Stanford University. Dr. Nadeau led that study, published in 2017 in PLOS One, along with epidemiologist Margaret Karagas, PhD, of Geisel School of Medicine at Dartmouth, Hanover, N.H.
As for what pregnant women can do to minimize their exposure to heavy metals, “that is a difficult problem and primarily a function of where one lives,” said Dr. States.
Drinking water and food are major sources of arsenic exposure. Groundwater is naturally contaminated with arsenic deposits that seep in from bedrock, said Dr. States. The U.S. Environmental Protection Agency regulates arsenic levels in public drinking water that is supplied to more than a few thousand people. However, small water supplies and private wells are unregulated, he said, and having these water sources tested for arsenic or fitted with systems to reduce arsenic can be very expensive.
Among foods, rice can have high concentrations of arsenic, Dr. Karagas told this news organization. To minimize arsenic exposure through the diet, women can limit rice-based foods, according to a web-based tool developed by her and coworkers.
In addition, tobacco smoke is a major source of cadmium exposure and a moderate source of arsenic exposure, Dr. States noted. Women can reduce their exposure to these metals by avoiding tobacco and secondhand smoke.
The study was supported by grants from the National Health Research Institutes, Chung Shan Medical University Hospital, Taiwan Ministry of Science and Technology, and the Taiwan Environmental Protection Administration. The authors and quoted experts report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Does the use of frankincense make sense in dermatology?
The Boswellia serrata exudate or gum (known in India as “guggulu”) that forms an aromatic resin traditionally used as incense – and known as frankincense (especially when retrieved from Boswellia species found in Eritrea and Somalia but also from the Indian variety) – has been considered for thousands of years to possess therapeutic properties. It is used in Ayurvedic medicine, as well as in traditional medicine in China and the Middle East, particularly for its anti-inflammatory effects to treat chronic conditions.1-8 In fact, such essential oils have been used since 2800 BC to treat various inflammatory conditions, including skin sores and wounds, as well as in perfumes and incense.2,9 In the West, use of frankincense dates back to thousands of years as well, more often found in the form of incense for religious and cultural ceremonies.7 Over the past 2 decades, .3 This column focuses on some of the emerging data on this ancient botanical agent.
Chemical constituents
Terpenoids and essential oils are the primary components of frankincense and are known to impart anti-inflammatory and anticancer activity. The same is true for myrrh, which has been combined with frankincense in traditional Chinese medicine as a single medication for millennia, with the two acting synergistically and considered still to be a potent combination in conferring various biological benefits.7
In 2010, in a systematic review of the anti-inflammatory and anticancer activities of Boswellia species and their chemical ingredients, Efferth and Oesch found that frankincense blocks the production of leukotrienes, cyclooxygenase (COX) 1 and 2, as well as 5-lipoxygenase; and oxidative stress. It also contributes to regulation of immune cells from the innate and acquired immune systems and exerts anticancer activity by influencing signaling transduction responsible for cell cycle arrest, as well as inhibition of proliferation, angiogenesis, invasion, and metastasis. The investigators also reported on clinical trial results that have found efficacy of frankincense and its constituents in ameliorating symptoms of psoriasis and erythematous eczema, among other disorders.3
Anti-inflammatory activity
Li et al. completed a study in 2016 to identify the active ingredients responsible for the anti-inflammatory and analgesic effects of frankincense. They found that alpha-pinene, linalool, and 1-octanol were key contributors. These constituents were noted for suppressing COX-2 overexpression in mice, as well as nociceptive stimulus-induced inflammatory infiltrates.10
Noting the increasing popularity of frankincense essential oil in skin care, despite a paucity of data, in 2017, Han et al. evaluated the biological activities of the essential oil in pre-inflamed human dermal fibroblasts using 17 key protein biomarkers. Frankincense essential oil displayed significant antiproliferative activity and suppressed collagen III, interferon gamma-induced protein 10, and intracellular adhesion molecule 1. The investigators referred to the overall encouraging potential of frankincense essential oil to exert influence over inflammation and tissue remodeling in human skin and called for additional research into its mechanisms of action and active constituents.11
Anticancer activity
The main active ingredient in frankincense, boswellic acid, has been shown to promote apoptosis, suppress matrix metalloproteinase secretion, and hinder migration in metastatic melanoma cell lines in mice.6,12
In 2019, Hakkim et al. demonstrated that frankincense essential oil yielded substantial antimelanoma activity in vitro and in vivo and ameliorated hepatotoxicity caused by acetaminophen.13
There is one case report in the literature on the use of frankincense as a treatment for skin cancer. A 56-year-old man received frankincense oil multiple times a day for 4 months to treat a nodular basal cell carcinoma on one arm (which resolved) and an infiltrative BCC on the chest (some focal residual tumor remained).6,14 Topical frankincense or boswellic acid has been given a grade D recommendation for treating skin cancer, however, because of only one level-of-evidence-5 study.6
Antimicrobial activity
In 2012, de Rapper et al. collected samples of three essential oils of frankincense (Boswellia rivae, Boswellia neglecta, and Boswellia papyrifera) and two essential oil samples of myrrh and sweet myrrh from different regions of Ethiopia to study their anti-infective properties alone and in combination. The investigators observed synergistic and additive effects, particularly between B. papyrifera and Commiphora myrrha. While noting the long history of the combined use of frankincense and myrrh essential oils since 1500 BC, the investigators highlighted their study as the first antimicrobial work to verify the effectiveness of this combination, validating the use of this combination to thwart particular pathogens.15
Just 2 years ago, Ljaljević Grbić et al. evaluated the in vitro antimicrobial potential of the liquid and vapor phases of B. carteri and C. myrrha (frankincense and myrrh, respectively) essential oils, finding that frankincense demonstrated marked capacity to act as a natural antimicrobial agent.9
Transdermal delivery
In 2017, Zhu et al. showed that frankincense and myrrh essential oils promoted the permeability of the Chinese herb Chuanxiong and may facilitate drug elimination from the epidermis via dermal capillaries by dint of improved cutaneous blood flow, thereby augmenting transdermal drug delivery.16 The same team also showed that frankincense and myrrh essential oils, by fostering permeation by enhancing drug delivery across the stratum corneum, can also alter the structure of the stratum corneum.17
Conclusion
The use of frankincense in traditional medicine has a long and impressive track record. Recent research provides reason for optimism, and further investigating the possible incorporation of this botanical agent into modern dermatologic therapies appears warranted. Clearly, however, much more research is needed.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Kimmatkar N et al. Phytomedicine. 2003 Jan;10(1):3-7.
2. Ammon HP. Wien Med Wochenschr. 2002;152(15-16):373-8.
3. Efferth T & Oesch F. Semin Cancer Biol. 2020 Feb 4;S1044-579X(20)30034-1.
4. Banno N et al. J Ethnopharmacol. 2006 Sep 19;107(2):249-53.
5. Poeckel D & Werz O. Curr Med Chem. 2006;13(28):3359-69.
6. Li JY, Kampp JT. Dermatol Surg. 2019 Jan;45(1):58-67.
7. Cao B et al. Molecules. 2019 Aug 24;24(17): 3076.
8. Mertens M et al. Flavour Fragr J. 2009;24:279-300.
9. Ljaljević Grbić M et al. J Ethnopharmacol. 2018 Jun 12;219:1-14.
10. Li XJ et al. J Ethnopharmacol. 2016 Feb 17;179:22-6.
11. Han X et al. Biochim Open. 2017 Feb 3;4:31-5.
12. Zhao W et al. Cancer Detect Prev. 2003;27:67-75.
13. Hakkim FL et al. Oncotarget. 2019 May 28;10(37):3472-90.
14. Fung K et al. OA Altern Med 2013;1:14.
15. de Rapper S et al. Lett Appl Microbiol. 2012 Apr;54(4):352-8.
16. Zhu XF et al. Zhongguo Zhong Yao Za Zhi. 2017 Feb;42(4):680-5.
17. Guan YM et al. Zhongguo Zhong Yao Za Zhi. 2017 Sep;42(17):3350-5.
The Boswellia serrata exudate or gum (known in India as “guggulu”) that forms an aromatic resin traditionally used as incense – and known as frankincense (especially when retrieved from Boswellia species found in Eritrea and Somalia but also from the Indian variety) – has been considered for thousands of years to possess therapeutic properties. It is used in Ayurvedic medicine, as well as in traditional medicine in China and the Middle East, particularly for its anti-inflammatory effects to treat chronic conditions.1-8 In fact, such essential oils have been used since 2800 BC to treat various inflammatory conditions, including skin sores and wounds, as well as in perfumes and incense.2,9 In the West, use of frankincense dates back to thousands of years as well, more often found in the form of incense for religious and cultural ceremonies.7 Over the past 2 decades, .3 This column focuses on some of the emerging data on this ancient botanical agent.
Chemical constituents
Terpenoids and essential oils are the primary components of frankincense and are known to impart anti-inflammatory and anticancer activity. The same is true for myrrh, which has been combined with frankincense in traditional Chinese medicine as a single medication for millennia, with the two acting synergistically and considered still to be a potent combination in conferring various biological benefits.7
In 2010, in a systematic review of the anti-inflammatory and anticancer activities of Boswellia species and their chemical ingredients, Efferth and Oesch found that frankincense blocks the production of leukotrienes, cyclooxygenase (COX) 1 and 2, as well as 5-lipoxygenase; and oxidative stress. It also contributes to regulation of immune cells from the innate and acquired immune systems and exerts anticancer activity by influencing signaling transduction responsible for cell cycle arrest, as well as inhibition of proliferation, angiogenesis, invasion, and metastasis. The investigators also reported on clinical trial results that have found efficacy of frankincense and its constituents in ameliorating symptoms of psoriasis and erythematous eczema, among other disorders.3
Anti-inflammatory activity
Li et al. completed a study in 2016 to identify the active ingredients responsible for the anti-inflammatory and analgesic effects of frankincense. They found that alpha-pinene, linalool, and 1-octanol were key contributors. These constituents were noted for suppressing COX-2 overexpression in mice, as well as nociceptive stimulus-induced inflammatory infiltrates.10
Noting the increasing popularity of frankincense essential oil in skin care, despite a paucity of data, in 2017, Han et al. evaluated the biological activities of the essential oil in pre-inflamed human dermal fibroblasts using 17 key protein biomarkers. Frankincense essential oil displayed significant antiproliferative activity and suppressed collagen III, interferon gamma-induced protein 10, and intracellular adhesion molecule 1. The investigators referred to the overall encouraging potential of frankincense essential oil to exert influence over inflammation and tissue remodeling in human skin and called for additional research into its mechanisms of action and active constituents.11
Anticancer activity
The main active ingredient in frankincense, boswellic acid, has been shown to promote apoptosis, suppress matrix metalloproteinase secretion, and hinder migration in metastatic melanoma cell lines in mice.6,12
In 2019, Hakkim et al. demonstrated that frankincense essential oil yielded substantial antimelanoma activity in vitro and in vivo and ameliorated hepatotoxicity caused by acetaminophen.13
There is one case report in the literature on the use of frankincense as a treatment for skin cancer. A 56-year-old man received frankincense oil multiple times a day for 4 months to treat a nodular basal cell carcinoma on one arm (which resolved) and an infiltrative BCC on the chest (some focal residual tumor remained).6,14 Topical frankincense or boswellic acid has been given a grade D recommendation for treating skin cancer, however, because of only one level-of-evidence-5 study.6
Antimicrobial activity
In 2012, de Rapper et al. collected samples of three essential oils of frankincense (Boswellia rivae, Boswellia neglecta, and Boswellia papyrifera) and two essential oil samples of myrrh and sweet myrrh from different regions of Ethiopia to study their anti-infective properties alone and in combination. The investigators observed synergistic and additive effects, particularly between B. papyrifera and Commiphora myrrha. While noting the long history of the combined use of frankincense and myrrh essential oils since 1500 BC, the investigators highlighted their study as the first antimicrobial work to verify the effectiveness of this combination, validating the use of this combination to thwart particular pathogens.15
Just 2 years ago, Ljaljević Grbić et al. evaluated the in vitro antimicrobial potential of the liquid and vapor phases of B. carteri and C. myrrha (frankincense and myrrh, respectively) essential oils, finding that frankincense demonstrated marked capacity to act as a natural antimicrobial agent.9
Transdermal delivery
In 2017, Zhu et al. showed that frankincense and myrrh essential oils promoted the permeability of the Chinese herb Chuanxiong and may facilitate drug elimination from the epidermis via dermal capillaries by dint of improved cutaneous blood flow, thereby augmenting transdermal drug delivery.16 The same team also showed that frankincense and myrrh essential oils, by fostering permeation by enhancing drug delivery across the stratum corneum, can also alter the structure of the stratum corneum.17
Conclusion
The use of frankincense in traditional medicine has a long and impressive track record. Recent research provides reason for optimism, and further investigating the possible incorporation of this botanical agent into modern dermatologic therapies appears warranted. Clearly, however, much more research is needed.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Kimmatkar N et al. Phytomedicine. 2003 Jan;10(1):3-7.
2. Ammon HP. Wien Med Wochenschr. 2002;152(15-16):373-8.
3. Efferth T & Oesch F. Semin Cancer Biol. 2020 Feb 4;S1044-579X(20)30034-1.
4. Banno N et al. J Ethnopharmacol. 2006 Sep 19;107(2):249-53.
5. Poeckel D & Werz O. Curr Med Chem. 2006;13(28):3359-69.
6. Li JY, Kampp JT. Dermatol Surg. 2019 Jan;45(1):58-67.
7. Cao B et al. Molecules. 2019 Aug 24;24(17): 3076.
8. Mertens M et al. Flavour Fragr J. 2009;24:279-300.
9. Ljaljević Grbić M et al. J Ethnopharmacol. 2018 Jun 12;219:1-14.
10. Li XJ et al. J Ethnopharmacol. 2016 Feb 17;179:22-6.
11. Han X et al. Biochim Open. 2017 Feb 3;4:31-5.
12. Zhao W et al. Cancer Detect Prev. 2003;27:67-75.
13. Hakkim FL et al. Oncotarget. 2019 May 28;10(37):3472-90.
14. Fung K et al. OA Altern Med 2013;1:14.
15. de Rapper S et al. Lett Appl Microbiol. 2012 Apr;54(4):352-8.
16. Zhu XF et al. Zhongguo Zhong Yao Za Zhi. 2017 Feb;42(4):680-5.
17. Guan YM et al. Zhongguo Zhong Yao Za Zhi. 2017 Sep;42(17):3350-5.
The Boswellia serrata exudate or gum (known in India as “guggulu”) that forms an aromatic resin traditionally used as incense – and known as frankincense (especially when retrieved from Boswellia species found in Eritrea and Somalia but also from the Indian variety) – has been considered for thousands of years to possess therapeutic properties. It is used in Ayurvedic medicine, as well as in traditional medicine in China and the Middle East, particularly for its anti-inflammatory effects to treat chronic conditions.1-8 In fact, such essential oils have been used since 2800 BC to treat various inflammatory conditions, including skin sores and wounds, as well as in perfumes and incense.2,9 In the West, use of frankincense dates back to thousands of years as well, more often found in the form of incense for religious and cultural ceremonies.7 Over the past 2 decades, .3 This column focuses on some of the emerging data on this ancient botanical agent.
Chemical constituents
Terpenoids and essential oils are the primary components of frankincense and are known to impart anti-inflammatory and anticancer activity. The same is true for myrrh, which has been combined with frankincense in traditional Chinese medicine as a single medication for millennia, with the two acting synergistically and considered still to be a potent combination in conferring various biological benefits.7
In 2010, in a systematic review of the anti-inflammatory and anticancer activities of Boswellia species and their chemical ingredients, Efferth and Oesch found that frankincense blocks the production of leukotrienes, cyclooxygenase (COX) 1 and 2, as well as 5-lipoxygenase; and oxidative stress. It also contributes to regulation of immune cells from the innate and acquired immune systems and exerts anticancer activity by influencing signaling transduction responsible for cell cycle arrest, as well as inhibition of proliferation, angiogenesis, invasion, and metastasis. The investigators also reported on clinical trial results that have found efficacy of frankincense and its constituents in ameliorating symptoms of psoriasis and erythematous eczema, among other disorders.3
Anti-inflammatory activity
Li et al. completed a study in 2016 to identify the active ingredients responsible for the anti-inflammatory and analgesic effects of frankincense. They found that alpha-pinene, linalool, and 1-octanol were key contributors. These constituents were noted for suppressing COX-2 overexpression in mice, as well as nociceptive stimulus-induced inflammatory infiltrates.10
Noting the increasing popularity of frankincense essential oil in skin care, despite a paucity of data, in 2017, Han et al. evaluated the biological activities of the essential oil in pre-inflamed human dermal fibroblasts using 17 key protein biomarkers. Frankincense essential oil displayed significant antiproliferative activity and suppressed collagen III, interferon gamma-induced protein 10, and intracellular adhesion molecule 1. The investigators referred to the overall encouraging potential of frankincense essential oil to exert influence over inflammation and tissue remodeling in human skin and called for additional research into its mechanisms of action and active constituents.11
Anticancer activity
The main active ingredient in frankincense, boswellic acid, has been shown to promote apoptosis, suppress matrix metalloproteinase secretion, and hinder migration in metastatic melanoma cell lines in mice.6,12
In 2019, Hakkim et al. demonstrated that frankincense essential oil yielded substantial antimelanoma activity in vitro and in vivo and ameliorated hepatotoxicity caused by acetaminophen.13
There is one case report in the literature on the use of frankincense as a treatment for skin cancer. A 56-year-old man received frankincense oil multiple times a day for 4 months to treat a nodular basal cell carcinoma on one arm (which resolved) and an infiltrative BCC on the chest (some focal residual tumor remained).6,14 Topical frankincense or boswellic acid has been given a grade D recommendation for treating skin cancer, however, because of only one level-of-evidence-5 study.6
Antimicrobial activity
In 2012, de Rapper et al. collected samples of three essential oils of frankincense (Boswellia rivae, Boswellia neglecta, and Boswellia papyrifera) and two essential oil samples of myrrh and sweet myrrh from different regions of Ethiopia to study their anti-infective properties alone and in combination. The investigators observed synergistic and additive effects, particularly between B. papyrifera and Commiphora myrrha. While noting the long history of the combined use of frankincense and myrrh essential oils since 1500 BC, the investigators highlighted their study as the first antimicrobial work to verify the effectiveness of this combination, validating the use of this combination to thwart particular pathogens.15
Just 2 years ago, Ljaljević Grbić et al. evaluated the in vitro antimicrobial potential of the liquid and vapor phases of B. carteri and C. myrrha (frankincense and myrrh, respectively) essential oils, finding that frankincense demonstrated marked capacity to act as a natural antimicrobial agent.9
Transdermal delivery
In 2017, Zhu et al. showed that frankincense and myrrh essential oils promoted the permeability of the Chinese herb Chuanxiong and may facilitate drug elimination from the epidermis via dermal capillaries by dint of improved cutaneous blood flow, thereby augmenting transdermal drug delivery.16 The same team also showed that frankincense and myrrh essential oils, by fostering permeation by enhancing drug delivery across the stratum corneum, can also alter the structure of the stratum corneum.17
Conclusion
The use of frankincense in traditional medicine has a long and impressive track record. Recent research provides reason for optimism, and further investigating the possible incorporation of this botanical agent into modern dermatologic therapies appears warranted. Clearly, however, much more research is needed.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Kimmatkar N et al. Phytomedicine. 2003 Jan;10(1):3-7.
2. Ammon HP. Wien Med Wochenschr. 2002;152(15-16):373-8.
3. Efferth T & Oesch F. Semin Cancer Biol. 2020 Feb 4;S1044-579X(20)30034-1.
4. Banno N et al. J Ethnopharmacol. 2006 Sep 19;107(2):249-53.
5. Poeckel D & Werz O. Curr Med Chem. 2006;13(28):3359-69.
6. Li JY, Kampp JT. Dermatol Surg. 2019 Jan;45(1):58-67.
7. Cao B et al. Molecules. 2019 Aug 24;24(17): 3076.
8. Mertens M et al. Flavour Fragr J. 2009;24:279-300.
9. Ljaljević Grbić M et al. J Ethnopharmacol. 2018 Jun 12;219:1-14.
10. Li XJ et al. J Ethnopharmacol. 2016 Feb 17;179:22-6.
11. Han X et al. Biochim Open. 2017 Feb 3;4:31-5.
12. Zhao W et al. Cancer Detect Prev. 2003;27:67-75.
13. Hakkim FL et al. Oncotarget. 2019 May 28;10(37):3472-90.
14. Fung K et al. OA Altern Med 2013;1:14.
15. de Rapper S et al. Lett Appl Microbiol. 2012 Apr;54(4):352-8.
16. Zhu XF et al. Zhongguo Zhong Yao Za Zhi. 2017 Feb;42(4):680-5.
17. Guan YM et al. Zhongguo Zhong Yao Za Zhi. 2017 Sep;42(17):3350-5.
Early peanut feeding guidelines still not reaching families
Four years after new infant feeding guidelines were issued to prevent allergies to peanut and other foods, 70% of surveyed parents and caregivers in the United States said they had never heard about the new recommendation.
Food allergies in developed countries have doubled in each of the last decades and now affect 7.6% of U.S. children. About 1 in 50 are allergic to peanut. Data from the 2015 LEAP study and other research has convincingly shown that early, sustained feeding of peanuts, eggs, and other allergens can prevent babies from developing allergies to these foods.
Based on those findings, the National Institute of Allergy and Infectious Diseases (NIAID) updated its feeding guidelines in 2017, urging parents to introduce these foods to babies around 4-6 months of age rather than wait until 1-3 years of age, as previously recommended. The American Academy of Pediatrics approved those guidelines too, and in 2019 changed its own feeding recommendations.
To assess awareness of this new guidance and to what extent these recommendations are being translated into clinical practice, researchers surveyed a demographically representative U.S. sample of 3,062 parents and caregivers with children between 7 months and 3½ years old. The survey was conducted in English and Spanish over the web or by phone.
More than one-third reported that their child’s primary care physician never discussed when to start feeding peanut-containing foods. And among those whose doctors did offer guidance, fewer than 1 in 4 specifically recommended introducing peanut by 6 months of age.
These data show that “despite strong evidence that early introduction of peanut within the first year of life can prevent the development of peanut allergy, this evidence is simply not making its way to parents of infants,” said Christopher Warren, PhD, assistant professor of preventive medicine at the Northwestern University Feinberg School of Medicine, Chicago. Dr. Warren led the study and presented the findings on a poster at this year’s American College of Allergy, Asthma & Immunology annual meeting in New Orleans.
In addition to caregivers, the Northwestern team surveyed U.S. allergists and pediatricians about the new feeding guidelines. Uptake was fairly good among allergists, with 65% reporting full implementation. On the other hand, while most pediatricians seemed familiar with the 2017 recommendations, fewer than one-third said they were following them.
“What’s unique about this challenge is that it’s not just a guideline change – it’s a guideline reversal,” said Wendy Sue Swanson, MD, chief medical officer for SpoonfulONE, a company that makes mix-ins and other products for multi-allergen feeding. After telling families for years to avoid these allergens in early life because food allergies were rising, “it’s harder advice to say, actually, we were wrong. Not only should you not wait, you should get peanut in while your baby’s immune system has this critical moment to learn and develop, and you should keep getting it in,” Dr. Swanson said in an interview.
Making matters worse, pediatricians are time pressed. Typically, at 4- to 6-month-old well-check visits, “they’re talking about sleep and development and feeding and milestones,” said Ruchi Gupta, MD, MPH, professor of pediatrics and medicine at Northwestern Feinberg, who led the allergist and pediatrician analyses.
Another challenge: Guidelines differ depending on the child’s level of food allergy risk, so it’s hard to explain them clearly and quickly. Babies at highest risk – as judged by having severe eczema, egg allergy, or both – should get peanut IgE blood testing and, if negative, begin regular consumption of peanut by 4-6 months. Intermediate-risk babies who have mild-to-moderate eczema are recommended to start peanut-containing foods by 6 months. And for low-risk babies with no eczema or known food allergies, the guidance is simply to introduce peanut-containing foods “in accordance with family preferences and cultural practices.”
As for pediatricians who say it’s hard to distinguish mild-to-moderate from severe eczema, “any eczema puts you at some risk,” Dr. Gupta told this news organization. “If they’ve required steroid creams to clear up their skin, or if you look at their skin, and you think it’s severe, don’t hesitate. Go ahead and draw the IgE and send them to an allergist.”
Australia, which has the highest rate of confirmed food allergy, has had more success implementing early feeding guidelines, said Dr. Swanson. Unlike the United States’ tiered approach, she said, they “had a national guideline that very crisply, years ago, told parents what to do.” Australia also has nurse educators that follow up with new moms to make sure they understand and follow the recommendations.
Dr. Gupta receives research support from the National Institutes of Health, Food Allergy Research and Education, the Melchiorre Family Foundation, the Sunshine Charitable Foundation, the Walder Foundation, the UnitedHealth Group, Thermo Fisher Scientific, and Genentech. She serves as a medical consultant/advisor for Genentech, Novartis, and Food Allergy Research and Education. Dr. Swanson serves as chief medical officer for SpoonfulONE.
A version of this article first appeared on Medscape.com.
Four years after new infant feeding guidelines were issued to prevent allergies to peanut and other foods, 70% of surveyed parents and caregivers in the United States said they had never heard about the new recommendation.
Food allergies in developed countries have doubled in each of the last decades and now affect 7.6% of U.S. children. About 1 in 50 are allergic to peanut. Data from the 2015 LEAP study and other research has convincingly shown that early, sustained feeding of peanuts, eggs, and other allergens can prevent babies from developing allergies to these foods.
Based on those findings, the National Institute of Allergy and Infectious Diseases (NIAID) updated its feeding guidelines in 2017, urging parents to introduce these foods to babies around 4-6 months of age rather than wait until 1-3 years of age, as previously recommended. The American Academy of Pediatrics approved those guidelines too, and in 2019 changed its own feeding recommendations.
To assess awareness of this new guidance and to what extent these recommendations are being translated into clinical practice, researchers surveyed a demographically representative U.S. sample of 3,062 parents and caregivers with children between 7 months and 3½ years old. The survey was conducted in English and Spanish over the web or by phone.
More than one-third reported that their child’s primary care physician never discussed when to start feeding peanut-containing foods. And among those whose doctors did offer guidance, fewer than 1 in 4 specifically recommended introducing peanut by 6 months of age.
These data show that “despite strong evidence that early introduction of peanut within the first year of life can prevent the development of peanut allergy, this evidence is simply not making its way to parents of infants,” said Christopher Warren, PhD, assistant professor of preventive medicine at the Northwestern University Feinberg School of Medicine, Chicago. Dr. Warren led the study and presented the findings on a poster at this year’s American College of Allergy, Asthma & Immunology annual meeting in New Orleans.
In addition to caregivers, the Northwestern team surveyed U.S. allergists and pediatricians about the new feeding guidelines. Uptake was fairly good among allergists, with 65% reporting full implementation. On the other hand, while most pediatricians seemed familiar with the 2017 recommendations, fewer than one-third said they were following them.
“What’s unique about this challenge is that it’s not just a guideline change – it’s a guideline reversal,” said Wendy Sue Swanson, MD, chief medical officer for SpoonfulONE, a company that makes mix-ins and other products for multi-allergen feeding. After telling families for years to avoid these allergens in early life because food allergies were rising, “it’s harder advice to say, actually, we were wrong. Not only should you not wait, you should get peanut in while your baby’s immune system has this critical moment to learn and develop, and you should keep getting it in,” Dr. Swanson said in an interview.
Making matters worse, pediatricians are time pressed. Typically, at 4- to 6-month-old well-check visits, “they’re talking about sleep and development and feeding and milestones,” said Ruchi Gupta, MD, MPH, professor of pediatrics and medicine at Northwestern Feinberg, who led the allergist and pediatrician analyses.
Another challenge: Guidelines differ depending on the child’s level of food allergy risk, so it’s hard to explain them clearly and quickly. Babies at highest risk – as judged by having severe eczema, egg allergy, or both – should get peanut IgE blood testing and, if negative, begin regular consumption of peanut by 4-6 months. Intermediate-risk babies who have mild-to-moderate eczema are recommended to start peanut-containing foods by 6 months. And for low-risk babies with no eczema or known food allergies, the guidance is simply to introduce peanut-containing foods “in accordance with family preferences and cultural practices.”
As for pediatricians who say it’s hard to distinguish mild-to-moderate from severe eczema, “any eczema puts you at some risk,” Dr. Gupta told this news organization. “If they’ve required steroid creams to clear up their skin, or if you look at their skin, and you think it’s severe, don’t hesitate. Go ahead and draw the IgE and send them to an allergist.”
Australia, which has the highest rate of confirmed food allergy, has had more success implementing early feeding guidelines, said Dr. Swanson. Unlike the United States’ tiered approach, she said, they “had a national guideline that very crisply, years ago, told parents what to do.” Australia also has nurse educators that follow up with new moms to make sure they understand and follow the recommendations.
Dr. Gupta receives research support from the National Institutes of Health, Food Allergy Research and Education, the Melchiorre Family Foundation, the Sunshine Charitable Foundation, the Walder Foundation, the UnitedHealth Group, Thermo Fisher Scientific, and Genentech. She serves as a medical consultant/advisor for Genentech, Novartis, and Food Allergy Research and Education. Dr. Swanson serves as chief medical officer for SpoonfulONE.
A version of this article first appeared on Medscape.com.
Four years after new infant feeding guidelines were issued to prevent allergies to peanut and other foods, 70% of surveyed parents and caregivers in the United States said they had never heard about the new recommendation.
Food allergies in developed countries have doubled in each of the last decades and now affect 7.6% of U.S. children. About 1 in 50 are allergic to peanut. Data from the 2015 LEAP study and other research has convincingly shown that early, sustained feeding of peanuts, eggs, and other allergens can prevent babies from developing allergies to these foods.
Based on those findings, the National Institute of Allergy and Infectious Diseases (NIAID) updated its feeding guidelines in 2017, urging parents to introduce these foods to babies around 4-6 months of age rather than wait until 1-3 years of age, as previously recommended. The American Academy of Pediatrics approved those guidelines too, and in 2019 changed its own feeding recommendations.
To assess awareness of this new guidance and to what extent these recommendations are being translated into clinical practice, researchers surveyed a demographically representative U.S. sample of 3,062 parents and caregivers with children between 7 months and 3½ years old. The survey was conducted in English and Spanish over the web or by phone.
More than one-third reported that their child’s primary care physician never discussed when to start feeding peanut-containing foods. And among those whose doctors did offer guidance, fewer than 1 in 4 specifically recommended introducing peanut by 6 months of age.
These data show that “despite strong evidence that early introduction of peanut within the first year of life can prevent the development of peanut allergy, this evidence is simply not making its way to parents of infants,” said Christopher Warren, PhD, assistant professor of preventive medicine at the Northwestern University Feinberg School of Medicine, Chicago. Dr. Warren led the study and presented the findings on a poster at this year’s American College of Allergy, Asthma & Immunology annual meeting in New Orleans.
In addition to caregivers, the Northwestern team surveyed U.S. allergists and pediatricians about the new feeding guidelines. Uptake was fairly good among allergists, with 65% reporting full implementation. On the other hand, while most pediatricians seemed familiar with the 2017 recommendations, fewer than one-third said they were following them.
“What’s unique about this challenge is that it’s not just a guideline change – it’s a guideline reversal,” said Wendy Sue Swanson, MD, chief medical officer for SpoonfulONE, a company that makes mix-ins and other products for multi-allergen feeding. After telling families for years to avoid these allergens in early life because food allergies were rising, “it’s harder advice to say, actually, we were wrong. Not only should you not wait, you should get peanut in while your baby’s immune system has this critical moment to learn and develop, and you should keep getting it in,” Dr. Swanson said in an interview.
Making matters worse, pediatricians are time pressed. Typically, at 4- to 6-month-old well-check visits, “they’re talking about sleep and development and feeding and milestones,” said Ruchi Gupta, MD, MPH, professor of pediatrics and medicine at Northwestern Feinberg, who led the allergist and pediatrician analyses.
Another challenge: Guidelines differ depending on the child’s level of food allergy risk, so it’s hard to explain them clearly and quickly. Babies at highest risk – as judged by having severe eczema, egg allergy, or both – should get peanut IgE blood testing and, if negative, begin regular consumption of peanut by 4-6 months. Intermediate-risk babies who have mild-to-moderate eczema are recommended to start peanut-containing foods by 6 months. And for low-risk babies with no eczema or known food allergies, the guidance is simply to introduce peanut-containing foods “in accordance with family preferences and cultural practices.”
As for pediatricians who say it’s hard to distinguish mild-to-moderate from severe eczema, “any eczema puts you at some risk,” Dr. Gupta told this news organization. “If they’ve required steroid creams to clear up their skin, or if you look at their skin, and you think it’s severe, don’t hesitate. Go ahead and draw the IgE and send them to an allergist.”
Australia, which has the highest rate of confirmed food allergy, has had more success implementing early feeding guidelines, said Dr. Swanson. Unlike the United States’ tiered approach, she said, they “had a national guideline that very crisply, years ago, told parents what to do.” Australia also has nurse educators that follow up with new moms to make sure they understand and follow the recommendations.
Dr. Gupta receives research support from the National Institutes of Health, Food Allergy Research and Education, the Melchiorre Family Foundation, the Sunshine Charitable Foundation, the Walder Foundation, the UnitedHealth Group, Thermo Fisher Scientific, and Genentech. She serves as a medical consultant/advisor for Genentech, Novartis, and Food Allergy Research and Education. Dr. Swanson serves as chief medical officer for SpoonfulONE.
A version of this article first appeared on Medscape.com.
Atopic Dermatitis: Clinical Outcomes
European agency supports marketing of abrocitinib for atopic dermatitis
The
The full indication is for the treatment of moderate to severe AD in adults who are candidates for systemic therapy, according to a summary of the opinion, made on Oct. 14. It will be available as 50-mg, 100-mg, and 200-mg tablets, will be marketed under the name Cibinqo, and “should be prescribed by physicians experienced in the treatment of atopic dermatitis,” the statement said.
“The benefits of Cibinqo are its ability to improve the skin condition as measured by improvements in the Investigator’s Global Assessment 0/1 and Eczema Area and Severity Index 75 response and to reduce itching in patients with atopic dermatitis,” according to the opinion. The most common side effects of abrocitinib are nausea, headache, acne, herpes simplex, increased blood creatine phosphokinase, vomiting, dizziness, and upper abdominal pain, the statement said, and infections are the most serious.
Abrocitinib was first approved for AD in the United Kingdom and in Japan in September, and is under review at the Food and Drug Administration for this indication. The first JAK inhibitor approved for AD in the United States is topical ruxolitinib (Opzelura), approved in September, for the short-term, noncontinuous chronic treatment of mild to moderate AD in nonimmunocompromised patients aged 12 years and older whose disease is not adequately controlled with topical prescription treatments, “or when those therapies are not advisable.”
The
The full indication is for the treatment of moderate to severe AD in adults who are candidates for systemic therapy, according to a summary of the opinion, made on Oct. 14. It will be available as 50-mg, 100-mg, and 200-mg tablets, will be marketed under the name Cibinqo, and “should be prescribed by physicians experienced in the treatment of atopic dermatitis,” the statement said.
“The benefits of Cibinqo are its ability to improve the skin condition as measured by improvements in the Investigator’s Global Assessment 0/1 and Eczema Area and Severity Index 75 response and to reduce itching in patients with atopic dermatitis,” according to the opinion. The most common side effects of abrocitinib are nausea, headache, acne, herpes simplex, increased blood creatine phosphokinase, vomiting, dizziness, and upper abdominal pain, the statement said, and infections are the most serious.
Abrocitinib was first approved for AD in the United Kingdom and in Japan in September, and is under review at the Food and Drug Administration for this indication. The first JAK inhibitor approved for AD in the United States is topical ruxolitinib (Opzelura), approved in September, for the short-term, noncontinuous chronic treatment of mild to moderate AD in nonimmunocompromised patients aged 12 years and older whose disease is not adequately controlled with topical prescription treatments, “or when those therapies are not advisable.”
The
The full indication is for the treatment of moderate to severe AD in adults who are candidates for systemic therapy, according to a summary of the opinion, made on Oct. 14. It will be available as 50-mg, 100-mg, and 200-mg tablets, will be marketed under the name Cibinqo, and “should be prescribed by physicians experienced in the treatment of atopic dermatitis,” the statement said.
“The benefits of Cibinqo are its ability to improve the skin condition as measured by improvements in the Investigator’s Global Assessment 0/1 and Eczema Area and Severity Index 75 response and to reduce itching in patients with atopic dermatitis,” according to the opinion. The most common side effects of abrocitinib are nausea, headache, acne, herpes simplex, increased blood creatine phosphokinase, vomiting, dizziness, and upper abdominal pain, the statement said, and infections are the most serious.
Abrocitinib was first approved for AD in the United Kingdom and in Japan in September, and is under review at the Food and Drug Administration for this indication. The first JAK inhibitor approved for AD in the United States is topical ruxolitinib (Opzelura), approved in September, for the short-term, noncontinuous chronic treatment of mild to moderate AD in nonimmunocompromised patients aged 12 years and older whose disease is not adequately controlled with topical prescription treatments, “or when those therapies are not advisable.”
Woman with burning, itchy red eyes
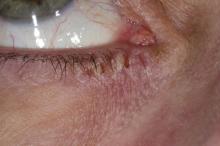
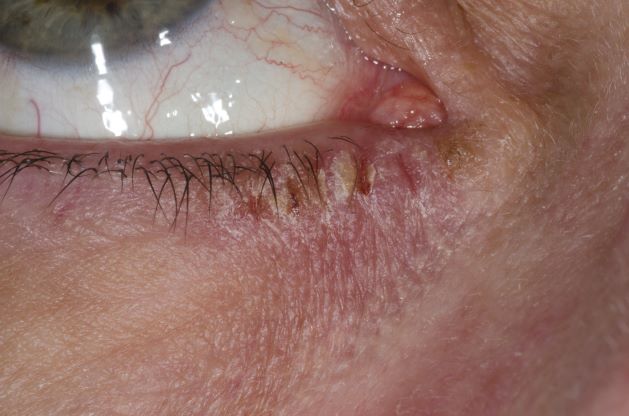
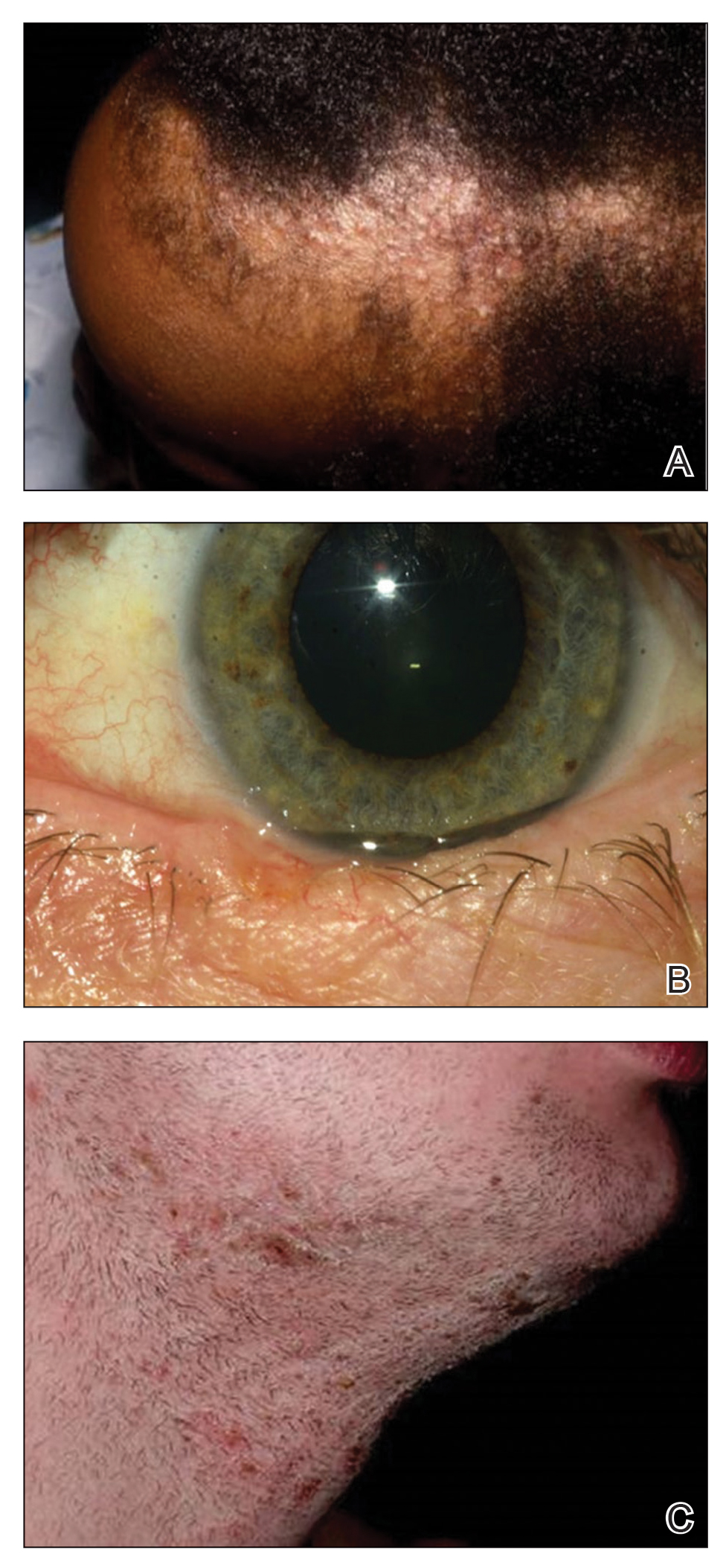
This patient has the “atopic triad” of allergies, asthma, and atopic dermatitis. Atopic dermatitis around the eyes and on the eyelids often develops in teenage years and adulthood but may also occur in older persons. Occasionally, it can be the only manifestation of atopic dermatitis. The upper eyelids may appear scaly and fissured. The so-called "allergic shiners" (symmetric, dark circles beneath the lower eyelid) and Dennie-Morgan lines (extra skin folds under the lower eyelid) are often present.
The thin skin of the eyelids is particularly sensitive to irritants and allergens and is thus prone to develop dermatitis. Contact with the same trigger may not lead to a rash on other areas of skin. Upper, lower or both eyelids on one or both sides can be affected. The patient may report itching, stinging or burning, and the lids are red and scaly. They may swell. With persistence of the dermatitis, the eyelids become thickened with increased skin markings (lichenification). The eyelid margins may become involved (blepharitis). The appearance is similar, whatever the cause.
The basis of treatment for atopic dermatitis is to provide moisturization for dryness, allay pruritus, and manage inflammation of the eczematous lesions. Conservative initial management of eyelid dermatitis also includes gentle skin care and avoidance of fragrance and other known irritants in personal care, hair, and facial skin care products. Bland, fragrance-free emollients, such as petrolatum, may be applied directly to the eyelids.
Topical corticosteroids are one therapeutic option for eyelid dermatitis. However, only low-potency topical corticosteroids are safe, and only for short-term use, on the eyelids. Typically, they are used twice daily for 2-4 weeks. However, even with low-potency topical corticosteroids, the eyelids remain vulnerable to thinning, even atrophy. Because of these issues, topical calcineurin inhibitors are often the preferred treatment.
Patients with atopic dermatitis have an increased risk of comorbid eye diseases, including keratitis, conjunctivitis, and keratoconus. A careful clinical examination for associated erythema, crusting, and blepharitis many prompt a referral to an ophthalmologist.
Brian S. Kim, MD, Associate Professor, Department of Medicine, Division of Dermatology, Washington University School of Medicine, St. Louis, Missouri
Brian S. Kim, MD, has disclosed no relevant financial relationships.
This patient has the “atopic triad” of allergies, asthma, and atopic dermatitis. Atopic dermatitis around the eyes and on the eyelids often develops in teenage years and adulthood but may also occur in older persons. Occasionally, it can be the only manifestation of atopic dermatitis. The upper eyelids may appear scaly and fissured. The so-called "allergic shiners" (symmetric, dark circles beneath the lower eyelid) and Dennie-Morgan lines (extra skin folds under the lower eyelid) are often present.
The thin skin of the eyelids is particularly sensitive to irritants and allergens and is thus prone to develop dermatitis. Contact with the same trigger may not lead to a rash on other areas of skin. Upper, lower or both eyelids on one or both sides can be affected. The patient may report itching, stinging or burning, and the lids are red and scaly. They may swell. With persistence of the dermatitis, the eyelids become thickened with increased skin markings (lichenification). The eyelid margins may become involved (blepharitis). The appearance is similar, whatever the cause.
The basis of treatment for atopic dermatitis is to provide moisturization for dryness, allay pruritus, and manage inflammation of the eczematous lesions. Conservative initial management of eyelid dermatitis also includes gentle skin care and avoidance of fragrance and other known irritants in personal care, hair, and facial skin care products. Bland, fragrance-free emollients, such as petrolatum, may be applied directly to the eyelids.
Topical corticosteroids are one therapeutic option for eyelid dermatitis. However, only low-potency topical corticosteroids are safe, and only for short-term use, on the eyelids. Typically, they are used twice daily for 2-4 weeks. However, even with low-potency topical corticosteroids, the eyelids remain vulnerable to thinning, even atrophy. Because of these issues, topical calcineurin inhibitors are often the preferred treatment.
Patients with atopic dermatitis have an increased risk of comorbid eye diseases, including keratitis, conjunctivitis, and keratoconus. A careful clinical examination for associated erythema, crusting, and blepharitis many prompt a referral to an ophthalmologist.
Brian S. Kim, MD, Associate Professor, Department of Medicine, Division of Dermatology, Washington University School of Medicine, St. Louis, Missouri
Brian S. Kim, MD, has disclosed no relevant financial relationships.
This patient has the “atopic triad” of allergies, asthma, and atopic dermatitis. Atopic dermatitis around the eyes and on the eyelids often develops in teenage years and adulthood but may also occur in older persons. Occasionally, it can be the only manifestation of atopic dermatitis. The upper eyelids may appear scaly and fissured. The so-called "allergic shiners" (symmetric, dark circles beneath the lower eyelid) and Dennie-Morgan lines (extra skin folds under the lower eyelid) are often present.
The thin skin of the eyelids is particularly sensitive to irritants and allergens and is thus prone to develop dermatitis. Contact with the same trigger may not lead to a rash on other areas of skin. Upper, lower or both eyelids on one or both sides can be affected. The patient may report itching, stinging or burning, and the lids are red and scaly. They may swell. With persistence of the dermatitis, the eyelids become thickened with increased skin markings (lichenification). The eyelid margins may become involved (blepharitis). The appearance is similar, whatever the cause.
The basis of treatment for atopic dermatitis is to provide moisturization for dryness, allay pruritus, and manage inflammation of the eczematous lesions. Conservative initial management of eyelid dermatitis also includes gentle skin care and avoidance of fragrance and other known irritants in personal care, hair, and facial skin care products. Bland, fragrance-free emollients, such as petrolatum, may be applied directly to the eyelids.
Topical corticosteroids are one therapeutic option for eyelid dermatitis. However, only low-potency topical corticosteroids are safe, and only for short-term use, on the eyelids. Typically, they are used twice daily for 2-4 weeks. However, even with low-potency topical corticosteroids, the eyelids remain vulnerable to thinning, even atrophy. Because of these issues, topical calcineurin inhibitors are often the preferred treatment.
Patients with atopic dermatitis have an increased risk of comorbid eye diseases, including keratitis, conjunctivitis, and keratoconus. A careful clinical examination for associated erythema, crusting, and blepharitis many prompt a referral to an ophthalmologist.
Brian S. Kim, MD, Associate Professor, Department of Medicine, Division of Dermatology, Washington University School of Medicine, St. Louis, Missouri
Brian S. Kim, MD, has disclosed no relevant financial relationships.
A 21-year-old woman presents with burning, itchy red eyes that she rubs incessantly. On examination, she has an erythematic, scaly, pruritic rash on the upper and lower eyelids and below her eyes. She has no other outbreaks on the rest of her skin except for mild acne. A moisturizer has provided minimal relief for the itching but has not helped with the rash. She has a history of asthma, for which she uses an inhaler, and of hay fever, for which she takes an antihistamine. She also reports that she has had two episodes of conjunctivitis within the past year, which were treated with antibiotic eye drops.
9-step ladder may kids with allergies return to eggs
For many children in the process of outgrowing egg allergy, the step-wise reintroduction of foods that contain eggs can be achieved at home using a nine-rung laddered approach, according to updated guidelines from the British Society for Allergy and Clinical Immunology (BSACI).
Attempts to reintroduce egg into the child’s diet can start at the age of 12 months or 6 months from the last reaction, as long as past reactions have been mild to moderate and the child does not have asthma, according to guidelines from the BSACI, which represents allergists, pediatricians, and other health care practitioners.
According to the guidelines, the reintroduction needs to be guided by a specialist allergy service for children who have had severe reactions to egg or who have asthma.
Susan C. Leech, MB BChir, DCH, first author of the guidelines and a consultant in pediatric allergy with the Department of Child Health at Kings College Hospital, London, told this news organization that home reintroduction should begin slowly with small amounts of baked egg, starting with a pea-sized piece of cake, and should proceed gradually.
“Parents can be reassured that it’s a relatively safe thing to do as long as it’s done with caution,” said Dr. Leech.
The expanded guidelines include a new nine-step reintroduction ladder. It builds on a three-stage classification of egg-containing foods that was first introduced in BSACI guidelines in 2010.
On the bottom four rungs, children work their way through small but increasing amounts of fairy cakes (cupcakes), biscuits (cookies), and other foods containing baked eggs.
The next three rungs involve hard-boiled eggs, quiche, and other well-cooked egg products.
At the eighth rung, children can have small mouthfuls of runny scrambled eggs, mayonnaise, and other less-cooked or raw egg-containing products. At the top rung, children can have increasing amounts of those products as well as licks of cake batter.
The guidelines were published online September 29 in Clinical and Experimental Allergy along with a supplement that includes a series of examples showing how the guidelines apply to specific patient cases.
“These are examples only,” the guideline authors caution in the appendix. “Clinical judgment of severity is important as risk assessment is not always easy.”
Anna Nowak-Wegrzyn, MD, PhD, a professor of pediatrics at NYU Grossman School of Medicine and chief of pediatric allergy and immunology for Hassenfeld Children’s Hospital at NYU Langone, who was not involved in the BSACI guidelines, described the egg ladder as a “proactive” strategy that deserves further study and consideration.
“I think that this may be a valid approach,” said Dr. Nowak-Wegrzyn in an interview. “Eggs have good nutritional value, and they are present in a lot of foods, so avoidance creates logistical challenges.”
Using the egg ladder for home-based reintroduction may be especially suited in resource-poor areas where access to an allergist may be difficult, she said. It may also be suited for families that can’t visit the office because of pandemic-related restrictions.
“If the child had a severe reaction or if they have asthma, then it’s a no-go,” she added, “but if you have a patient who has a really mild reaction and you think that overall the risk of a significant reaction or bad symptoms is low, then it may be worth doing.”
Dr. Leech and Dr. Nowak-Wegrzyn have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
For many children in the process of outgrowing egg allergy, the step-wise reintroduction of foods that contain eggs can be achieved at home using a nine-rung laddered approach, according to updated guidelines from the British Society for Allergy and Clinical Immunology (BSACI).
Attempts to reintroduce egg into the child’s diet can start at the age of 12 months or 6 months from the last reaction, as long as past reactions have been mild to moderate and the child does not have asthma, according to guidelines from the BSACI, which represents allergists, pediatricians, and other health care practitioners.
According to the guidelines, the reintroduction needs to be guided by a specialist allergy service for children who have had severe reactions to egg or who have asthma.
Susan C. Leech, MB BChir, DCH, first author of the guidelines and a consultant in pediatric allergy with the Department of Child Health at Kings College Hospital, London, told this news organization that home reintroduction should begin slowly with small amounts of baked egg, starting with a pea-sized piece of cake, and should proceed gradually.
“Parents can be reassured that it’s a relatively safe thing to do as long as it’s done with caution,” said Dr. Leech.
The expanded guidelines include a new nine-step reintroduction ladder. It builds on a three-stage classification of egg-containing foods that was first introduced in BSACI guidelines in 2010.
On the bottom four rungs, children work their way through small but increasing amounts of fairy cakes (cupcakes), biscuits (cookies), and other foods containing baked eggs.
The next three rungs involve hard-boiled eggs, quiche, and other well-cooked egg products.
At the eighth rung, children can have small mouthfuls of runny scrambled eggs, mayonnaise, and other less-cooked or raw egg-containing products. At the top rung, children can have increasing amounts of those products as well as licks of cake batter.
The guidelines were published online September 29 in Clinical and Experimental Allergy along with a supplement that includes a series of examples showing how the guidelines apply to specific patient cases.
“These are examples only,” the guideline authors caution in the appendix. “Clinical judgment of severity is important as risk assessment is not always easy.”
Anna Nowak-Wegrzyn, MD, PhD, a professor of pediatrics at NYU Grossman School of Medicine and chief of pediatric allergy and immunology for Hassenfeld Children’s Hospital at NYU Langone, who was not involved in the BSACI guidelines, described the egg ladder as a “proactive” strategy that deserves further study and consideration.
“I think that this may be a valid approach,” said Dr. Nowak-Wegrzyn in an interview. “Eggs have good nutritional value, and they are present in a lot of foods, so avoidance creates logistical challenges.”
Using the egg ladder for home-based reintroduction may be especially suited in resource-poor areas where access to an allergist may be difficult, she said. It may also be suited for families that can’t visit the office because of pandemic-related restrictions.
“If the child had a severe reaction or if they have asthma, then it’s a no-go,” she added, “but if you have a patient who has a really mild reaction and you think that overall the risk of a significant reaction or bad symptoms is low, then it may be worth doing.”
Dr. Leech and Dr. Nowak-Wegrzyn have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
For many children in the process of outgrowing egg allergy, the step-wise reintroduction of foods that contain eggs can be achieved at home using a nine-rung laddered approach, according to updated guidelines from the British Society for Allergy and Clinical Immunology (BSACI).
Attempts to reintroduce egg into the child’s diet can start at the age of 12 months or 6 months from the last reaction, as long as past reactions have been mild to moderate and the child does not have asthma, according to guidelines from the BSACI, which represents allergists, pediatricians, and other health care practitioners.
According to the guidelines, the reintroduction needs to be guided by a specialist allergy service for children who have had severe reactions to egg or who have asthma.
Susan C. Leech, MB BChir, DCH, first author of the guidelines and a consultant in pediatric allergy with the Department of Child Health at Kings College Hospital, London, told this news organization that home reintroduction should begin slowly with small amounts of baked egg, starting with a pea-sized piece of cake, and should proceed gradually.
“Parents can be reassured that it’s a relatively safe thing to do as long as it’s done with caution,” said Dr. Leech.
The expanded guidelines include a new nine-step reintroduction ladder. It builds on a three-stage classification of egg-containing foods that was first introduced in BSACI guidelines in 2010.
On the bottom four rungs, children work their way through small but increasing amounts of fairy cakes (cupcakes), biscuits (cookies), and other foods containing baked eggs.
The next three rungs involve hard-boiled eggs, quiche, and other well-cooked egg products.
At the eighth rung, children can have small mouthfuls of runny scrambled eggs, mayonnaise, and other less-cooked or raw egg-containing products. At the top rung, children can have increasing amounts of those products as well as licks of cake batter.
The guidelines were published online September 29 in Clinical and Experimental Allergy along with a supplement that includes a series of examples showing how the guidelines apply to specific patient cases.
“These are examples only,” the guideline authors caution in the appendix. “Clinical judgment of severity is important as risk assessment is not always easy.”
Anna Nowak-Wegrzyn, MD, PhD, a professor of pediatrics at NYU Grossman School of Medicine and chief of pediatric allergy and immunology for Hassenfeld Children’s Hospital at NYU Langone, who was not involved in the BSACI guidelines, described the egg ladder as a “proactive” strategy that deserves further study and consideration.
“I think that this may be a valid approach,” said Dr. Nowak-Wegrzyn in an interview. “Eggs have good nutritional value, and they are present in a lot of foods, so avoidance creates logistical challenges.”
Using the egg ladder for home-based reintroduction may be especially suited in resource-poor areas where access to an allergist may be difficult, she said. It may also be suited for families that can’t visit the office because of pandemic-related restrictions.
“If the child had a severe reaction or if they have asthma, then it’s a no-go,” she added, “but if you have a patient who has a really mild reaction and you think that overall the risk of a significant reaction or bad symptoms is low, then it may be worth doing.”
Dr. Leech and Dr. Nowak-Wegrzyn have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In atopic dermatitis trial, abrocitinib offers faster itch relief than dupilumab
), in a multicenter randomized trial presented as a late breaker at the annual meeting of the European Academy of Dermatology and Venereology.
The earlier onset of action with the JAK inhibitor was achieved even though most patients in both arms were on topical corticosteroids, a design element that “is clinically relevant” for a practical comparison of these two agents, according to Kristian Reich, MD, PhD, Center for Translational Research in Inflammatory Skin Diseases, University Medical Center, Hamburg-Eppendorf, Germany.
The goal of this phase 3b trial, called JADE DARE, was to compare relative safety and efficacy of these strategies over the early course of treatment, he said.
Over 700 patients randomized
JADE DARE enrolled 727 patients over age 18 years who previously had an inadequate response to conventional topical therapies. All had moderate to severe AD defined by criteria such as body surface area greater than or equal to 10% and Eczema Area Severity Index (EASI) greater than or equal to 16. They were randomly assigned to 200 mg oral abrocitinib once daily or 300 mg subcutaneous dupilumab (after a loading dose of 600 mg) every 2 weeks. A double-dummy design preserved blinding.
The coprimary endpoints were at least a 4-point improvement in pruritus as measured with the Peak Pruritus Numerical Rating Scale (PP-NRS) score at week 2 and at least a 90% improvement in the EASI (EASI 90) at week 4.
The primary endpoint for pruritus at 2 weeks was reached by nearly twice as many patients randomly assigned to abrocitinib (46.2% vs. 25.5%; P < .001). The proportion of those meeting the EASI 90 endpoint at week 4 was also superior on abrocitinib (28.5% vs. 14.6%; P < .001)
Advantage for pruritus control dissipates
For the pruritus endpoint, the advantage of abrocitinib slowly diminished over time after the peak difference observed at 2 weeks. Although the advantage at week 4 (58.1% vs. 40.8%) and week 8 (65.8% vs. 52.7%) remained sizable, there were very small differences thereafter. However, Dr. Reich pointed out that the percentages continued to favor abrocitinib at least numerically through the 26 weeks of follow-up completed so far.
The pattern of response on EASI 90 was not the same. After demonstrating superiority at the 4-week timepoint, the advantage of abrocitinib persisted. When compared at week 16, which was a secondary endpoint of the JADE DARE trial, the advantage of abrocitinib remained significant (54.3% vs. 41.9%; P < .001). The advantage of abrocitinib narrowed but remained numerically superior at 26 weeks (54.6% vs. 47.6%).
Based on the data collected to date, “abrocitinib is clearly superior early on,” Dr. Reich said. Moreover, he reiterated that topical corticosteroids were allowed as background therapy in both arms.
“It is difficult to show an advantage for one active therapy over the other in patients on background corticosteroids,” Dr. Reich maintained.
Both drugs are well tolerated
The drugs were similarly well tolerated. Serious adverse events were uncommon in either arm. The rate of study dropouts due to an adverse event potentially related to treatment assignment was 3% in each group.
Nausea (19% vs. 2%), acne (13.5% vs. 2%), and headache (13% vs. 7.5%) were all more common in patients randomly assigned to abrocitinib. Conjunctivitis was more common in the group randomly assigned to dupilumab (10% vs. 2%).
The two deaths that occurred during this study were in the abrocitinib arm, but one was the result of COVID-19 infection and the other was a cardiovascular event in a patient with risk factors. Neither was considered to be treatment-related.
Abrocitinib’s relative selectivity for the JAK1 inhibitor is a potential differentiator from other currently available JAK inhibitors, although direct comparisons of these therapies for clinical activity in AD as well as most other diseases remains limited.
The relatively rapid relief of pruritus with the JAK inhibitor relative to the monoclonal antibody in the JADE DARE trial is likely to be perceived as clinically significant by patients with AD, according to Sonja Ständer, MD, professor of dermatology and neurodermatology at the University Hospital Münster, Germany.
“One of the highest needs of patients with atopic dermatitis is a rapid and profound relief of itch,” Dr. Ständer, who wrote a review article on AD earlier this year, said in an interview.
Although several current therapies are effective against pruritus, Dr. Ständer believes that the higher proportion of patients achieving itch control at 2 weeks on abrocitinib “will attract the attention of affected patients.”
However, she added that patients need to take both benefits and risks into account, indicating that clinical utility cannot be judged on a single outcome. In selecting one drug over the others, she advised “a balanced use of therapies.”
Abrocitinib was first approved in the United Kingdom in early September, followed by Japan last Thursday, for the treatment of moderate to severe AD in patients ages 12 and older. It is under review elsewhere, including in the United States and the European Union for AD.
In September, the FDA approved the first JAK inhibitor for treating AD – a topical JAK inhibitor, ruxolitinib.
Dr. Reich reports financial relationships with 20 pharmaceutical companies, including Pfizer, which provided funding for the JADE DARE trial. Dr. Ständer reports financial relationships with Beiersdorf AG, Galderma, Kliniska, Lilly, Pfizer, and Sanofi.
A version of this article first appeared on Medscape.com.
), in a multicenter randomized trial presented as a late breaker at the annual meeting of the European Academy of Dermatology and Venereology.
The earlier onset of action with the JAK inhibitor was achieved even though most patients in both arms were on topical corticosteroids, a design element that “is clinically relevant” for a practical comparison of these two agents, according to Kristian Reich, MD, PhD, Center for Translational Research in Inflammatory Skin Diseases, University Medical Center, Hamburg-Eppendorf, Germany.
The goal of this phase 3b trial, called JADE DARE, was to compare relative safety and efficacy of these strategies over the early course of treatment, he said.
Over 700 patients randomized
JADE DARE enrolled 727 patients over age 18 years who previously had an inadequate response to conventional topical therapies. All had moderate to severe AD defined by criteria such as body surface area greater than or equal to 10% and Eczema Area Severity Index (EASI) greater than or equal to 16. They were randomly assigned to 200 mg oral abrocitinib once daily or 300 mg subcutaneous dupilumab (after a loading dose of 600 mg) every 2 weeks. A double-dummy design preserved blinding.
The coprimary endpoints were at least a 4-point improvement in pruritus as measured with the Peak Pruritus Numerical Rating Scale (PP-NRS) score at week 2 and at least a 90% improvement in the EASI (EASI 90) at week 4.
The primary endpoint for pruritus at 2 weeks was reached by nearly twice as many patients randomly assigned to abrocitinib (46.2% vs. 25.5%; P < .001). The proportion of those meeting the EASI 90 endpoint at week 4 was also superior on abrocitinib (28.5% vs. 14.6%; P < .001)
Advantage for pruritus control dissipates
For the pruritus endpoint, the advantage of abrocitinib slowly diminished over time after the peak difference observed at 2 weeks. Although the advantage at week 4 (58.1% vs. 40.8%) and week 8 (65.8% vs. 52.7%) remained sizable, there were very small differences thereafter. However, Dr. Reich pointed out that the percentages continued to favor abrocitinib at least numerically through the 26 weeks of follow-up completed so far.
The pattern of response on EASI 90 was not the same. After demonstrating superiority at the 4-week timepoint, the advantage of abrocitinib persisted. When compared at week 16, which was a secondary endpoint of the JADE DARE trial, the advantage of abrocitinib remained significant (54.3% vs. 41.9%; P < .001). The advantage of abrocitinib narrowed but remained numerically superior at 26 weeks (54.6% vs. 47.6%).
Based on the data collected to date, “abrocitinib is clearly superior early on,” Dr. Reich said. Moreover, he reiterated that topical corticosteroids were allowed as background therapy in both arms.
“It is difficult to show an advantage for one active therapy over the other in patients on background corticosteroids,” Dr. Reich maintained.
Both drugs are well tolerated
The drugs were similarly well tolerated. Serious adverse events were uncommon in either arm. The rate of study dropouts due to an adverse event potentially related to treatment assignment was 3% in each group.
Nausea (19% vs. 2%), acne (13.5% vs. 2%), and headache (13% vs. 7.5%) were all more common in patients randomly assigned to abrocitinib. Conjunctivitis was more common in the group randomly assigned to dupilumab (10% vs. 2%).
The two deaths that occurred during this study were in the abrocitinib arm, but one was the result of COVID-19 infection and the other was a cardiovascular event in a patient with risk factors. Neither was considered to be treatment-related.
Abrocitinib’s relative selectivity for the JAK1 inhibitor is a potential differentiator from other currently available JAK inhibitors, although direct comparisons of these therapies for clinical activity in AD as well as most other diseases remains limited.
The relatively rapid relief of pruritus with the JAK inhibitor relative to the monoclonal antibody in the JADE DARE trial is likely to be perceived as clinically significant by patients with AD, according to Sonja Ständer, MD, professor of dermatology and neurodermatology at the University Hospital Münster, Germany.
“One of the highest needs of patients with atopic dermatitis is a rapid and profound relief of itch,” Dr. Ständer, who wrote a review article on AD earlier this year, said in an interview.
Although several current therapies are effective against pruritus, Dr. Ständer believes that the higher proportion of patients achieving itch control at 2 weeks on abrocitinib “will attract the attention of affected patients.”
However, she added that patients need to take both benefits and risks into account, indicating that clinical utility cannot be judged on a single outcome. In selecting one drug over the others, she advised “a balanced use of therapies.”
Abrocitinib was first approved in the United Kingdom in early September, followed by Japan last Thursday, for the treatment of moderate to severe AD in patients ages 12 and older. It is under review elsewhere, including in the United States and the European Union for AD.
In September, the FDA approved the first JAK inhibitor for treating AD – a topical JAK inhibitor, ruxolitinib.
Dr. Reich reports financial relationships with 20 pharmaceutical companies, including Pfizer, which provided funding for the JADE DARE trial. Dr. Ständer reports financial relationships with Beiersdorf AG, Galderma, Kliniska, Lilly, Pfizer, and Sanofi.
A version of this article first appeared on Medscape.com.
), in a multicenter randomized trial presented as a late breaker at the annual meeting of the European Academy of Dermatology and Venereology.
The earlier onset of action with the JAK inhibitor was achieved even though most patients in both arms were on topical corticosteroids, a design element that “is clinically relevant” for a practical comparison of these two agents, according to Kristian Reich, MD, PhD, Center for Translational Research in Inflammatory Skin Diseases, University Medical Center, Hamburg-Eppendorf, Germany.
The goal of this phase 3b trial, called JADE DARE, was to compare relative safety and efficacy of these strategies over the early course of treatment, he said.
Over 700 patients randomized
JADE DARE enrolled 727 patients over age 18 years who previously had an inadequate response to conventional topical therapies. All had moderate to severe AD defined by criteria such as body surface area greater than or equal to 10% and Eczema Area Severity Index (EASI) greater than or equal to 16. They were randomly assigned to 200 mg oral abrocitinib once daily or 300 mg subcutaneous dupilumab (after a loading dose of 600 mg) every 2 weeks. A double-dummy design preserved blinding.
The coprimary endpoints were at least a 4-point improvement in pruritus as measured with the Peak Pruritus Numerical Rating Scale (PP-NRS) score at week 2 and at least a 90% improvement in the EASI (EASI 90) at week 4.
The primary endpoint for pruritus at 2 weeks was reached by nearly twice as many patients randomly assigned to abrocitinib (46.2% vs. 25.5%; P < .001). The proportion of those meeting the EASI 90 endpoint at week 4 was also superior on abrocitinib (28.5% vs. 14.6%; P < .001)
Advantage for pruritus control dissipates
For the pruritus endpoint, the advantage of abrocitinib slowly diminished over time after the peak difference observed at 2 weeks. Although the advantage at week 4 (58.1% vs. 40.8%) and week 8 (65.8% vs. 52.7%) remained sizable, there were very small differences thereafter. However, Dr. Reich pointed out that the percentages continued to favor abrocitinib at least numerically through the 26 weeks of follow-up completed so far.
The pattern of response on EASI 90 was not the same. After demonstrating superiority at the 4-week timepoint, the advantage of abrocitinib persisted. When compared at week 16, which was a secondary endpoint of the JADE DARE trial, the advantage of abrocitinib remained significant (54.3% vs. 41.9%; P < .001). The advantage of abrocitinib narrowed but remained numerically superior at 26 weeks (54.6% vs. 47.6%).
Based on the data collected to date, “abrocitinib is clearly superior early on,” Dr. Reich said. Moreover, he reiterated that topical corticosteroids were allowed as background therapy in both arms.
“It is difficult to show an advantage for one active therapy over the other in patients on background corticosteroids,” Dr. Reich maintained.
Both drugs are well tolerated
The drugs were similarly well tolerated. Serious adverse events were uncommon in either arm. The rate of study dropouts due to an adverse event potentially related to treatment assignment was 3% in each group.
Nausea (19% vs. 2%), acne (13.5% vs. 2%), and headache (13% vs. 7.5%) were all more common in patients randomly assigned to abrocitinib. Conjunctivitis was more common in the group randomly assigned to dupilumab (10% vs. 2%).
The two deaths that occurred during this study were in the abrocitinib arm, but one was the result of COVID-19 infection and the other was a cardiovascular event in a patient with risk factors. Neither was considered to be treatment-related.
Abrocitinib’s relative selectivity for the JAK1 inhibitor is a potential differentiator from other currently available JAK inhibitors, although direct comparisons of these therapies for clinical activity in AD as well as most other diseases remains limited.
The relatively rapid relief of pruritus with the JAK inhibitor relative to the monoclonal antibody in the JADE DARE trial is likely to be perceived as clinically significant by patients with AD, according to Sonja Ständer, MD, professor of dermatology and neurodermatology at the University Hospital Münster, Germany.
“One of the highest needs of patients with atopic dermatitis is a rapid and profound relief of itch,” Dr. Ständer, who wrote a review article on AD earlier this year, said in an interview.
Although several current therapies are effective against pruritus, Dr. Ständer believes that the higher proportion of patients achieving itch control at 2 weeks on abrocitinib “will attract the attention of affected patients.”
However, she added that patients need to take both benefits and risks into account, indicating that clinical utility cannot be judged on a single outcome. In selecting one drug over the others, she advised “a balanced use of therapies.”
Abrocitinib was first approved in the United Kingdom in early September, followed by Japan last Thursday, for the treatment of moderate to severe AD in patients ages 12 and older. It is under review elsewhere, including in the United States and the European Union for AD.
In September, the FDA approved the first JAK inhibitor for treating AD – a topical JAK inhibitor, ruxolitinib.
Dr. Reich reports financial relationships with 20 pharmaceutical companies, including Pfizer, which provided funding for the JADE DARE trial. Dr. Ständer reports financial relationships with Beiersdorf AG, Galderma, Kliniska, Lilly, Pfizer, and Sanofi.
A version of this article first appeared on Medscape.com.
Skin of Color in Preclinical Medical Education: A Cross-Institutional Comparison and A Call to Action
A ccording to the US Census Bureau, more than half of all Americans are projected to belong to a minority group, defined as any group other than non-Hispanic White alone, by 2044. 1 Consequently, the United States rapidly is becoming a country in which the majority of citizens will have skin of color. Individuals with skin of color are of diverse ethnic backgrounds and include people of African, Latin American, Native American, Pacific Islander, and Asian descent, as well as interethnic backgrounds. 2 Throughout the country, dermatologists along with primary care practitioners may be confronted with certain cutaneous conditions that have varying disease presentations or processes in patients with skin of color. It also is important to note that racial categories are socially rather than biologically constructed, and the term skin of color includes a wide variety of diverse skin types. Nevertheless, the current literature thoroughly supports unique pathophysiologic differences in skin of color as well as variations in disease manifestation compared to White patients. 3-5 For example, the increased lability of melanosomes in skin of color patients, which increases their risk for postinflammatory hyperpigmentation, has been well documented. 5-7 There are various dermatologic conditions that also occur with higher frequency and manifest uniquely in people with darker, more pigmented skin, 7-9 and dermatologists, along with primary care physicians, should feel prepared to recognize and address them.
Extensive evidence also indicates that there are unique aspects to consider while managing certain skin diseases in patients with skin of color.8,10,11 Consequently, as noted on the Skin of Color Society (SOCS) website, “[a]n increase in the body of dermatological literature concerning skin of color as well as the advancement of both basic science and clinical investigational research is necessary to meet the needs of the expanding skin of color population.”2 In the meantime, current knowledge regarding cutaneous conditions that diversely or disproportionately affect skin of color should be actively disseminated to physicians in training. Although patients with skin of color should always have access to comprehensive care and knowledgeable practitioners, the current changes in national and regional demographics further underscore the need for a more thorough understanding of skin of color with regard to disease pathogenesis, diagnosis, and treatment.
Several studies have found that medical students in the United States are minimally exposed to dermatology in general compared to other clinical specialties,12-14 which can easily lead to the underrecognition of disorders that may uniquely or disproportionately affect individuals with pigmented skin. Recent data showed that medical schools typically required fewer than 10 hours of dermatology instruction,12 and on average, dermatologic training made up less than 1% of a medical student’s undergraduate medical education.13,15,16 Consequently, less than 40% of primary care residents felt that their medical school curriculum adequately prepared them to manage common skin conditions.14 Although not all physicians should be expected to fully grasp the complexities of skin of color and its diagnostic and therapeutic implications, both practicing and training dermatologists have acknowledged a lack of exposure to skin of color. In one study, approximately 47% of dermatologists and dermatology residents reported that their medical training (medical school and/or residency) was inadequate in training them on skin conditions in Black patients. Furthermore, many who felt their training was lacking in skin of color identified the need for greater exposure to Black patients and training materials.15 The absence of comprehensive medical education regarding skin of color ultimately can be a disadvantage for both practitioners and patients, resulting in poorer outcomes. Furthermore, underrepresentation of skin of color may persist beyond undergraduate and graduate medical education. There also is evidence to suggest that noninclusion of skin of color pervades foundational dermatologic educational resources, including commonly used textbooks as well as continuing medical education disseminated at national conferences and meetings.17 Taken together, these findings highlight the need for more diverse and representative exposure to skin of color throughout medical training, which begins with a diverse inclusive undergraduate medical education in dermatology.
The objective of this study was to determine if the preclinical dermatology curriculum at 3 US medical schools provided adequate representation of skin of color patients in their didactic presentation slides.
Methods
Participants—Three US medical schools, a blend of private and public medical schools located across different geographic boundaries, agreed to participate in the study. All 3 institutions were current members of the American Medical Association (AMA) Accelerating Change in Medical Education consortium, whose primary goal is to create the medical school of the future and transform physician training.18 All 32 member institutions of the AMA consortium were contacted to request their participation in the study. As part of the consortium, these institutions have vowed to collectively work to develop and share the best models for educational advancement to improve care for patients, populations, and communities18 and would expectedly provide a more racially and ethnically inclusive curriculum than an institution not accountable to a group dedicated to identifying the best ways to deliver care for increasingly diverse communities.
Data Collection—Lectures were included if they were presented during dermatology preclinical courses in the 2015 to 2016 academic year. An uninvolved third party removed the names and identities of instructors to preserve anonymity. Two independent coders from different institutions extracted the data—lecture title, total number of clinical and histologic images, and number of skin of color images—from each of the anonymized lectures using a standardized coding form. We documented differences in skin of color noted in lectures and the disease context for the discussed differences, such as variations in clinical presentation, disease process, epidemiology/risk, and treatment between different skin phenotypes or ethnic groups. Photographs in which the coders were unable to differentiate whether the patient had skin of color were designated as indeterminate or unclear. Photographs appearing to represent Fitzpatrick skin types IV, V, and VI19 were categorically designated as skin of color, and those appearing to represent Fitzpatrick skin types I and II were described as not skin of color; however, images appearing to represent Fitzpatrick skin type III often were classified as not skin of color or indeterminate and occasionally skin of color. The Figure shows examples of images classified as skin of color, indeterminate, and not skin of color. Photographs often were classified as indeterminate due to poor lighting, close-up view photographs, or highlighted pathology obscuring the surrounding skin. We excluded duplicate photographs and histologic images from the analyses.
We also reviewed 19 conditions previously highlighted by the SOCS as areas of importance to skin of color patients.20 The coders tracked how many of these conditions were noted in each lecture. Duplicate discussion of these conditions was not included in the analyses. Any discrepancies between coders were resolved through additional slide review and discussion. The final coded data with the agreed upon changes were used for statistical analyses. Recent national demographic data from the US Census Bureau in 2019 describe approximately 39.9% of the population as belonging to racial/ethnic groups other than non-Hispanic/Latinx White.21 Consequently, the standard for adequate representation for skin of color photographs was set at 35% for the purpose of this study.
Results
Across all 3 institutions included in the study, the proportion of the total number of clinical photographs showing skin of color was 16% (290/1812). Eight percent of the total photographs (145/1812) were noted to be indeterminate (Table). For institution 1, 23.6% of photographs (155/658) showed skin of color, and 12.6% (83/658) were indeterminate. For institution 2, 13.1% (76/578) showed skin of color and 7.8% (45/578) were indeterminate. For institution 3, 10.2% (59/576) showed skin of color and 3% (17/576) were indeterminate.
Institutions 1, 2, and 3 had 18, 8, and 17 total dermatology lectures, respectively. Of the 19 conditions designated as areas of importance to skin of color patients by the SOCS, 16 (84.2%) were discussed by institution 1, 11 (57.9%) by institution 2, and 9 (47.4%) by institution 3 (eTable 1). Institution 3 did not include photographs of skin of color patients in its acne, psoriasis, or cutaneous malignancy lectures. Institution 1 also did not include any skin of color patients in its malignancy lecture. Lectures that focused on pigmentary disorders, atopic dermatitis, infectious conditions, and benign cutaneous neoplasms were more likely to display photographs of skin of color patients; for example, lectures that discussed infectious conditions, such as superficial mycoses, herpes viruses, human papillomavirus, syphilis, and atypical mycobacterial infections, were consistently among those with higher proportions of photographs of skin of color patients.
Throughout the entire preclinical dermatology course at all 3 institutions, of 2945 lecture slides, only 24 (0.8%) unique differences were noted between skin color and non–skin of color patients, with 10 total differences noted by institution 1, 6 by institution 2, and 8 by institution 3 (Table). The majority of these differences (19/24) were related to epidemiologic differences in prevalence among varying racial/ethnic groups, with only 5 instances highlighting differences in clinical presentation. There was only a single instance that elaborated on the underlying pathophysiologic mechanisms of the discussed difference. Of all 24 unique differences discussed, 8 were related to skin cancer, 3 were related to dermatitis, and 2 were related to the difference in manifestation of erythema in patients with darker skin (eTable 2).
Comment
The results of this study demonstrated that skin of color is underrepresented in the preclinical dermatology curriculum at these 3 institutions. Although only 16% of all included clinical photographs were of skin of color, individuals with skin of color will soon represent more than half of the total US population within the next 2 decades.1 To increase representation of skin of color patients, teaching faculty should consciously and deliberately include more photographs of skin of color patients for a wider variety of common conditions, including atopic dermatitis and psoriasis, in addition to those that tend to disparately affect skin of color patients, such as pseudofolliculitis barbae or melasma. Furthermore, they also can incorporate more detailed discussions about important differences seen in skin of color patients.
More Skin of Color Photographs in Psoriasis Lectures—At institution 3, there were no skin of color patients included in the psoriasis lecture, even though there is considerable data in the literature indicating notable differences in the clinical presentation, quality-of-life impact, and treatment of psoriasis in skin of color patients.11,22 There are multiple nuances in psoriasis manifestation in patients with skin of color, including less-conspicuous erythema in darker skin, higher degrees of dyspigmentation, and greater body surface area involvement. For Black patients with scalp psoriasis, the impact of hair texture, styling practices, and washing frequency are additional considerations that may impact disease severity and selection of topical therapy.11 The lack of inclusion of any skin of color patients in the psoriasis lecture at one institution further underscores the pressing need to prioritize communities of color in medical education.
More Skin of Color Photographs in Cutaneous Malignancy Lectures—Similarly, while a lecturer at institution 2 noted that acral lentiginous melanoma accounts for a considerable proportion of melanoma among skin of color patients,23 there was no mention of how melanoma generally is substantially more deadly in this population, potentially due to decreased awareness and inconsistent screening.24 Furthermore, at institutions 1 and 3, there were no photographs or discussion of skin of color patients during the cutaneous malignancy lectures. Evidence shows that more emphasis is needed for melanoma screening and awareness in skin of color populations to improve survival outcomes,24 and this begins with educating not only future dermatologists but all future physicians as well. The failure to include photographs of skin of color patients in discussions or lectures regarding cutaneous malignancies may serve to further perpetuate the harmful misperception that individuals with skin of color are unaffected by skin cancer.25,26
Analysis of Skin of Color Photographs in Infectious Disease Lectures—In addition, lectures discussing infectious etiologies were among those with the highest proportion of skin of color photographs. This relatively disproportionate representation of skin of color compared to the other lectures may contribute to the development of harmful stereotypes or the stigmatization of skin of color patients. Although skin of color should continue to be represented in similar lectures, teaching faculty should remain mindful of the potential unintended impact from lectures including relatively disproportionate amounts of skin of color, particularly when other lectures may have sparse to absent representation of skin of color.
More Photographs Available for Education—Overall, our findings may help to inform changes to preclinical dermatology medical education at other institutions to create more inclusive and representative curricula for skin of color patients. The ability of instructors to provide visual representation of various dermatologic conditions may be limited by the photographs available in certain textbooks with few examples of patients with skin of color; however, concerns regarding the lack of skin of color representation in dermatology training is not a novel discussion.17 Although it is the responsibility of all dermatologists to advocate for the inclusion of skin of color, many dermatologists of color have been leading the way in this movement for decades, publishing several textbooks to document various skin conditions in those with darker skin types and discuss unique considerations for patients with skin of color.27-29 Images from these textbooks can be utilized by programs to increase representation of skin of color in dermatology training. There also are multiple expanding online dermatologic databases, such as VisualDx, with an increasing focus on skin of color patients, some of which allow users to filter images by degree of skin pigmentation.30 Moreover, instructors also can work to diversify their curricula by highlighting more of the SOCS conditions of importance to skin of color patients, which have since been renamed and highlighted on the Patient Dermatology Education section of the SOCS website.20 These conditions, while not completely comprehensive, provide a useful starting point for medical educators to reevaluate for potential areas of improvement and inclusion.
There are several potential strategies that can be used to better represent skin of color in dermatologic preclinical medical education, including increasing awareness, especially among dermatology teaching faculty, of existing disparities in the representation of skin of color in the preclinical curricula. Additionally, all dermatology teaching materials could be reviewed at the department level prior to being disseminated to medical students to assess for instances in which skin of color could be prioritized for discussion or varying disease presentations in skin of color could be demonstrated. Finally, teaching faculty may consider photographing more clinical images of their skin of color patients to further develop a catalog of diverse images that can be used to teach students.
Study Limitations—Our study was unable to account for verbal discussion of skin of color not otherwise denoted or captured in lecture slides. Additional limitations include the utilization of Fitzpatrick skin types to describe and differentiate varying skin tones, as the Fitzpatrick scale originally was developed as a method to describe an individual’s response to UV exposure.19 The inability to further delineate the representation of darker skin types, such as those that may be classified as Fitzpatrick skin types V or VI,19 compared to those with lighter skin of color also was a limiting factor. This study was unable to assess for discussion of other common conditions affecting skin of color patients that were not listed as one of the priority conditions by SOCS. Photographs that were designated as indeterminate were difficult to elucidate as skin of color; however, it is possible that instructors may have verbally described these images as skin of color during lectures. Nonetheless, it may be beneficial for learners if teaching faculty were to clearly label instances where skin of color patients are shown or when notable differences are present.
Conclusion
Future studies would benefit from the inclusion of audio data from lectures, syllabi, and small group teaching materials from preclinical courses to more accurately assess representation of skin of color in dermatology training. Additionally, future studies also may expand to include images from lectures of overlapping clinical specialties, particularly infectious disease and rheumatology, to provide a broader assessment of skin of color exposure. Furthermore, repeat assessment may be beneficial to assess the longitudinal effectiveness of curricular changes at the institutions included in this study, comparing older lectures to more recent, updated lectures. This study also may be replicated at other medical schools to allow for wider comparison of curricula.
Acknowledgment—The authors wish to thank the institutions that offered and agreed to participate in this study with the hopes of improving medical education.
- Colby SL, Ortman JM. Projections of the size and composition of the US population: 2014 to 2060. United States Census Bureau website. Published March 2015. Accessed September 14, 2021. https://www.census.gov/content/dam/Census/library/publications/2015/demo/p25-1143.pdf
- Learn more about SOCS. Skin of Color Society website. Accessed September 14, 2021. http://skinofcolorsociety.org/about-socs/
- Taylor SC. Skin of color: biology, structure, function, and implications for dermatologic disease. J Am Acad Dermatol. 2002;46(suppl 2):S41-S62.
- Berardesca E, Maibach H. Ethnic skin: overview of structure and function. J Am Acad Dermatol. 2003;48(suppl 6):S139-S142.
- Callender VD, Surin-Lord SS, Davis EC, et al. Postinflammatory hyperpigmentation. Am J Clin Dermatol. 2011;12:87-99.
- Davis EC, Callender VD. Postinflammatory hyperpigmentation: a review of the epidemiology, clinical features, and treatment options in skin of color. J Clin Aesthet Dermatol. 2010;3:20-31.
- Grimes PE, Stockton T. Pigmentary disorders in blacks. Dermatol Clin. 1988;6:271-281.
- Halder RM, Nootheti PK. Ethnic skin disorders overview. J Am Acad Dermatol. 2003;48(suppl 6):S143-S148.
- Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
- Callender VD. Acne in ethnic skin: special considerations for therapy. Dermatol Ther. 2004;17:184-195.
- Alexis AF, Blackcloud P. Psoriasis in skin of color: epidemiology, genetics, clinical presentation, and treatment nuances. J Clin Aesthet Dermatol. 2014;7:16-24.
- McCleskey PE, Gilson RT, DeVillez RL. Medical student core curriculum in dermatology survey. J Am Acad Dermatol. 2009;61:30-35.
- Ramsay DL, Mayer F. National survey of undergraduate dermatologic medical education. Arch Dermatol.1985;121:1529-1530.
- Hansra NK, O’Sullivan P, Chen CL, et al. Medical school dermatology curriculum: are we adequately preparing primary care physicians? J Am Acad Dermatol. 2009;61:23-29.
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59, viii.
- Knable A, Hood AF, Pearson TG. Undergraduate medical education in dermatology: report from the AAD Interdisciplinary Education Committee, Subcommittee on Undergraduate Medical Education. J Am Acad Dermatol. 1997;36:467-470.
- Ebede T, Papier A. Disparities in dermatology educational resources. J Am Acad Dermatol. 2006;55:687-690.
- Skochelak SE, Stack SJ. Creating the medical schools of the future. Acad Med. 2017;92:16-19.
- Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124:869-871.
- Skin of Color Society. Patient dermatology education. Accessed September 22, 2021. https://skinofcolorsociety.org/patient-dermatology-education
- QuickFacts: United States. US Census Bureau website. Updated July 1, 2019. Accessed September 14, 2021. https://www.census.gov/quickfacts/fact/table/US#
- Kaufman BP, Alexis AF. Psoriasis in skin of color: insights into the epidemiology, clinical presentation, genetics, quality-of-life impact, and treatment of psoriasis in non-white racial/ethnic groups. Am J Clin Dermatol. 2018;19:405-423.
- Bradford PT, Goldstein AM, McMaster ML, et al. Acral lentiginous melanoma: incidence and survival patterns in the United States, 1986-2005. Arch Dermatol. 2009;145:427-434.
- Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival. J Am Acad Dermatol. 2016;75:983-991.
- Pipitone M, Robinson JK, Camara C, et al. Skin cancer awareness in suburban employees: a Hispanic perspective. J Am Acad Dermatol. 2002;47:118-123.
- Imahiyerobo-Ip J, Ip I, Jamal S, et al. Skin cancer awareness in communities of color. J Am Acad Dermatol. 2011;64:198-200.
- Taylor SSC, Serrano AMA, Kelly AP, et al, eds. Taylor and Kelly’s Dermatology for Skin of Color. 2nd ed. McGraw-Hill Education; 2016.
- Dadzie OE, Petit A, Alexis AF, eds. Ethnic Dermatology: Principles and Practice. Wiley-Blackwell; 2013.
- Jackson-Richards D, Pandya AG, eds. Dermatology Atlas for Skin of Color. Springer; 2014.
- VisualDx. New VisualDx feature: skin of color sort. Published October 14, 2020. Accessed September 22, 2021. https://www.visualdx.com/blog/new-visualdx-feature-skin-of-color-sort/
A ccording to the US Census Bureau, more than half of all Americans are projected to belong to a minority group, defined as any group other than non-Hispanic White alone, by 2044. 1 Consequently, the United States rapidly is becoming a country in which the majority of citizens will have skin of color. Individuals with skin of color are of diverse ethnic backgrounds and include people of African, Latin American, Native American, Pacific Islander, and Asian descent, as well as interethnic backgrounds. 2 Throughout the country, dermatologists along with primary care practitioners may be confronted with certain cutaneous conditions that have varying disease presentations or processes in patients with skin of color. It also is important to note that racial categories are socially rather than biologically constructed, and the term skin of color includes a wide variety of diverse skin types. Nevertheless, the current literature thoroughly supports unique pathophysiologic differences in skin of color as well as variations in disease manifestation compared to White patients. 3-5 For example, the increased lability of melanosomes in skin of color patients, which increases their risk for postinflammatory hyperpigmentation, has been well documented. 5-7 There are various dermatologic conditions that also occur with higher frequency and manifest uniquely in people with darker, more pigmented skin, 7-9 and dermatologists, along with primary care physicians, should feel prepared to recognize and address them.
Extensive evidence also indicates that there are unique aspects to consider while managing certain skin diseases in patients with skin of color.8,10,11 Consequently, as noted on the Skin of Color Society (SOCS) website, “[a]n increase in the body of dermatological literature concerning skin of color as well as the advancement of both basic science and clinical investigational research is necessary to meet the needs of the expanding skin of color population.”2 In the meantime, current knowledge regarding cutaneous conditions that diversely or disproportionately affect skin of color should be actively disseminated to physicians in training. Although patients with skin of color should always have access to comprehensive care and knowledgeable practitioners, the current changes in national and regional demographics further underscore the need for a more thorough understanding of skin of color with regard to disease pathogenesis, diagnosis, and treatment.
Several studies have found that medical students in the United States are minimally exposed to dermatology in general compared to other clinical specialties,12-14 which can easily lead to the underrecognition of disorders that may uniquely or disproportionately affect individuals with pigmented skin. Recent data showed that medical schools typically required fewer than 10 hours of dermatology instruction,12 and on average, dermatologic training made up less than 1% of a medical student’s undergraduate medical education.13,15,16 Consequently, less than 40% of primary care residents felt that their medical school curriculum adequately prepared them to manage common skin conditions.14 Although not all physicians should be expected to fully grasp the complexities of skin of color and its diagnostic and therapeutic implications, both practicing and training dermatologists have acknowledged a lack of exposure to skin of color. In one study, approximately 47% of dermatologists and dermatology residents reported that their medical training (medical school and/or residency) was inadequate in training them on skin conditions in Black patients. Furthermore, many who felt their training was lacking in skin of color identified the need for greater exposure to Black patients and training materials.15 The absence of comprehensive medical education regarding skin of color ultimately can be a disadvantage for both practitioners and patients, resulting in poorer outcomes. Furthermore, underrepresentation of skin of color may persist beyond undergraduate and graduate medical education. There also is evidence to suggest that noninclusion of skin of color pervades foundational dermatologic educational resources, including commonly used textbooks as well as continuing medical education disseminated at national conferences and meetings.17 Taken together, these findings highlight the need for more diverse and representative exposure to skin of color throughout medical training, which begins with a diverse inclusive undergraduate medical education in dermatology.
The objective of this study was to determine if the preclinical dermatology curriculum at 3 US medical schools provided adequate representation of skin of color patients in their didactic presentation slides.
Methods
Participants—Three US medical schools, a blend of private and public medical schools located across different geographic boundaries, agreed to participate in the study. All 3 institutions were current members of the American Medical Association (AMA) Accelerating Change in Medical Education consortium, whose primary goal is to create the medical school of the future and transform physician training.18 All 32 member institutions of the AMA consortium were contacted to request their participation in the study. As part of the consortium, these institutions have vowed to collectively work to develop and share the best models for educational advancement to improve care for patients, populations, and communities18 and would expectedly provide a more racially and ethnically inclusive curriculum than an institution not accountable to a group dedicated to identifying the best ways to deliver care for increasingly diverse communities.
Data Collection—Lectures were included if they were presented during dermatology preclinical courses in the 2015 to 2016 academic year. An uninvolved third party removed the names and identities of instructors to preserve anonymity. Two independent coders from different institutions extracted the data—lecture title, total number of clinical and histologic images, and number of skin of color images—from each of the anonymized lectures using a standardized coding form. We documented differences in skin of color noted in lectures and the disease context for the discussed differences, such as variations in clinical presentation, disease process, epidemiology/risk, and treatment between different skin phenotypes or ethnic groups. Photographs in which the coders were unable to differentiate whether the patient had skin of color were designated as indeterminate or unclear. Photographs appearing to represent Fitzpatrick skin types IV, V, and VI19 were categorically designated as skin of color, and those appearing to represent Fitzpatrick skin types I and II were described as not skin of color; however, images appearing to represent Fitzpatrick skin type III often were classified as not skin of color or indeterminate and occasionally skin of color. The Figure shows examples of images classified as skin of color, indeterminate, and not skin of color. Photographs often were classified as indeterminate due to poor lighting, close-up view photographs, or highlighted pathology obscuring the surrounding skin. We excluded duplicate photographs and histologic images from the analyses.
We also reviewed 19 conditions previously highlighted by the SOCS as areas of importance to skin of color patients.20 The coders tracked how many of these conditions were noted in each lecture. Duplicate discussion of these conditions was not included in the analyses. Any discrepancies between coders were resolved through additional slide review and discussion. The final coded data with the agreed upon changes were used for statistical analyses. Recent national demographic data from the US Census Bureau in 2019 describe approximately 39.9% of the population as belonging to racial/ethnic groups other than non-Hispanic/Latinx White.21 Consequently, the standard for adequate representation for skin of color photographs was set at 35% for the purpose of this study.
Results
Across all 3 institutions included in the study, the proportion of the total number of clinical photographs showing skin of color was 16% (290/1812). Eight percent of the total photographs (145/1812) were noted to be indeterminate (Table). For institution 1, 23.6% of photographs (155/658) showed skin of color, and 12.6% (83/658) were indeterminate. For institution 2, 13.1% (76/578) showed skin of color and 7.8% (45/578) were indeterminate. For institution 3, 10.2% (59/576) showed skin of color and 3% (17/576) were indeterminate.
Institutions 1, 2, and 3 had 18, 8, and 17 total dermatology lectures, respectively. Of the 19 conditions designated as areas of importance to skin of color patients by the SOCS, 16 (84.2%) were discussed by institution 1, 11 (57.9%) by institution 2, and 9 (47.4%) by institution 3 (eTable 1). Institution 3 did not include photographs of skin of color patients in its acne, psoriasis, or cutaneous malignancy lectures. Institution 1 also did not include any skin of color patients in its malignancy lecture. Lectures that focused on pigmentary disorders, atopic dermatitis, infectious conditions, and benign cutaneous neoplasms were more likely to display photographs of skin of color patients; for example, lectures that discussed infectious conditions, such as superficial mycoses, herpes viruses, human papillomavirus, syphilis, and atypical mycobacterial infections, were consistently among those with higher proportions of photographs of skin of color patients.
Throughout the entire preclinical dermatology course at all 3 institutions, of 2945 lecture slides, only 24 (0.8%) unique differences were noted between skin color and non–skin of color patients, with 10 total differences noted by institution 1, 6 by institution 2, and 8 by institution 3 (Table). The majority of these differences (19/24) were related to epidemiologic differences in prevalence among varying racial/ethnic groups, with only 5 instances highlighting differences in clinical presentation. There was only a single instance that elaborated on the underlying pathophysiologic mechanisms of the discussed difference. Of all 24 unique differences discussed, 8 were related to skin cancer, 3 were related to dermatitis, and 2 were related to the difference in manifestation of erythema in patients with darker skin (eTable 2).
Comment
The results of this study demonstrated that skin of color is underrepresented in the preclinical dermatology curriculum at these 3 institutions. Although only 16% of all included clinical photographs were of skin of color, individuals with skin of color will soon represent more than half of the total US population within the next 2 decades.1 To increase representation of skin of color patients, teaching faculty should consciously and deliberately include more photographs of skin of color patients for a wider variety of common conditions, including atopic dermatitis and psoriasis, in addition to those that tend to disparately affect skin of color patients, such as pseudofolliculitis barbae or melasma. Furthermore, they also can incorporate more detailed discussions about important differences seen in skin of color patients.
More Skin of Color Photographs in Psoriasis Lectures—At institution 3, there were no skin of color patients included in the psoriasis lecture, even though there is considerable data in the literature indicating notable differences in the clinical presentation, quality-of-life impact, and treatment of psoriasis in skin of color patients.11,22 There are multiple nuances in psoriasis manifestation in patients with skin of color, including less-conspicuous erythema in darker skin, higher degrees of dyspigmentation, and greater body surface area involvement. For Black patients with scalp psoriasis, the impact of hair texture, styling practices, and washing frequency are additional considerations that may impact disease severity and selection of topical therapy.11 The lack of inclusion of any skin of color patients in the psoriasis lecture at one institution further underscores the pressing need to prioritize communities of color in medical education.
More Skin of Color Photographs in Cutaneous Malignancy Lectures—Similarly, while a lecturer at institution 2 noted that acral lentiginous melanoma accounts for a considerable proportion of melanoma among skin of color patients,23 there was no mention of how melanoma generally is substantially more deadly in this population, potentially due to decreased awareness and inconsistent screening.24 Furthermore, at institutions 1 and 3, there were no photographs or discussion of skin of color patients during the cutaneous malignancy lectures. Evidence shows that more emphasis is needed for melanoma screening and awareness in skin of color populations to improve survival outcomes,24 and this begins with educating not only future dermatologists but all future physicians as well. The failure to include photographs of skin of color patients in discussions or lectures regarding cutaneous malignancies may serve to further perpetuate the harmful misperception that individuals with skin of color are unaffected by skin cancer.25,26
Analysis of Skin of Color Photographs in Infectious Disease Lectures—In addition, lectures discussing infectious etiologies were among those with the highest proportion of skin of color photographs. This relatively disproportionate representation of skin of color compared to the other lectures may contribute to the development of harmful stereotypes or the stigmatization of skin of color patients. Although skin of color should continue to be represented in similar lectures, teaching faculty should remain mindful of the potential unintended impact from lectures including relatively disproportionate amounts of skin of color, particularly when other lectures may have sparse to absent representation of skin of color.
More Photographs Available for Education—Overall, our findings may help to inform changes to preclinical dermatology medical education at other institutions to create more inclusive and representative curricula for skin of color patients. The ability of instructors to provide visual representation of various dermatologic conditions may be limited by the photographs available in certain textbooks with few examples of patients with skin of color; however, concerns regarding the lack of skin of color representation in dermatology training is not a novel discussion.17 Although it is the responsibility of all dermatologists to advocate for the inclusion of skin of color, many dermatologists of color have been leading the way in this movement for decades, publishing several textbooks to document various skin conditions in those with darker skin types and discuss unique considerations for patients with skin of color.27-29 Images from these textbooks can be utilized by programs to increase representation of skin of color in dermatology training. There also are multiple expanding online dermatologic databases, such as VisualDx, with an increasing focus on skin of color patients, some of which allow users to filter images by degree of skin pigmentation.30 Moreover, instructors also can work to diversify their curricula by highlighting more of the SOCS conditions of importance to skin of color patients, which have since been renamed and highlighted on the Patient Dermatology Education section of the SOCS website.20 These conditions, while not completely comprehensive, provide a useful starting point for medical educators to reevaluate for potential areas of improvement and inclusion.
There are several potential strategies that can be used to better represent skin of color in dermatologic preclinical medical education, including increasing awareness, especially among dermatology teaching faculty, of existing disparities in the representation of skin of color in the preclinical curricula. Additionally, all dermatology teaching materials could be reviewed at the department level prior to being disseminated to medical students to assess for instances in which skin of color could be prioritized for discussion or varying disease presentations in skin of color could be demonstrated. Finally, teaching faculty may consider photographing more clinical images of their skin of color patients to further develop a catalog of diverse images that can be used to teach students.
Study Limitations—Our study was unable to account for verbal discussion of skin of color not otherwise denoted or captured in lecture slides. Additional limitations include the utilization of Fitzpatrick skin types to describe and differentiate varying skin tones, as the Fitzpatrick scale originally was developed as a method to describe an individual’s response to UV exposure.19 The inability to further delineate the representation of darker skin types, such as those that may be classified as Fitzpatrick skin types V or VI,19 compared to those with lighter skin of color also was a limiting factor. This study was unable to assess for discussion of other common conditions affecting skin of color patients that were not listed as one of the priority conditions by SOCS. Photographs that were designated as indeterminate were difficult to elucidate as skin of color; however, it is possible that instructors may have verbally described these images as skin of color during lectures. Nonetheless, it may be beneficial for learners if teaching faculty were to clearly label instances where skin of color patients are shown or when notable differences are present.
Conclusion
Future studies would benefit from the inclusion of audio data from lectures, syllabi, and small group teaching materials from preclinical courses to more accurately assess representation of skin of color in dermatology training. Additionally, future studies also may expand to include images from lectures of overlapping clinical specialties, particularly infectious disease and rheumatology, to provide a broader assessment of skin of color exposure. Furthermore, repeat assessment may be beneficial to assess the longitudinal effectiveness of curricular changes at the institutions included in this study, comparing older lectures to more recent, updated lectures. This study also may be replicated at other medical schools to allow for wider comparison of curricula.
Acknowledgment—The authors wish to thank the institutions that offered and agreed to participate in this study with the hopes of improving medical education.
A ccording to the US Census Bureau, more than half of all Americans are projected to belong to a minority group, defined as any group other than non-Hispanic White alone, by 2044. 1 Consequently, the United States rapidly is becoming a country in which the majority of citizens will have skin of color. Individuals with skin of color are of diverse ethnic backgrounds and include people of African, Latin American, Native American, Pacific Islander, and Asian descent, as well as interethnic backgrounds. 2 Throughout the country, dermatologists along with primary care practitioners may be confronted with certain cutaneous conditions that have varying disease presentations or processes in patients with skin of color. It also is important to note that racial categories are socially rather than biologically constructed, and the term skin of color includes a wide variety of diverse skin types. Nevertheless, the current literature thoroughly supports unique pathophysiologic differences in skin of color as well as variations in disease manifestation compared to White patients. 3-5 For example, the increased lability of melanosomes in skin of color patients, which increases their risk for postinflammatory hyperpigmentation, has been well documented. 5-7 There are various dermatologic conditions that also occur with higher frequency and manifest uniquely in people with darker, more pigmented skin, 7-9 and dermatologists, along with primary care physicians, should feel prepared to recognize and address them.
Extensive evidence also indicates that there are unique aspects to consider while managing certain skin diseases in patients with skin of color.8,10,11 Consequently, as noted on the Skin of Color Society (SOCS) website, “[a]n increase in the body of dermatological literature concerning skin of color as well as the advancement of both basic science and clinical investigational research is necessary to meet the needs of the expanding skin of color population.”2 In the meantime, current knowledge regarding cutaneous conditions that diversely or disproportionately affect skin of color should be actively disseminated to physicians in training. Although patients with skin of color should always have access to comprehensive care and knowledgeable practitioners, the current changes in national and regional demographics further underscore the need for a more thorough understanding of skin of color with regard to disease pathogenesis, diagnosis, and treatment.
Several studies have found that medical students in the United States are minimally exposed to dermatology in general compared to other clinical specialties,12-14 which can easily lead to the underrecognition of disorders that may uniquely or disproportionately affect individuals with pigmented skin. Recent data showed that medical schools typically required fewer than 10 hours of dermatology instruction,12 and on average, dermatologic training made up less than 1% of a medical student’s undergraduate medical education.13,15,16 Consequently, less than 40% of primary care residents felt that their medical school curriculum adequately prepared them to manage common skin conditions.14 Although not all physicians should be expected to fully grasp the complexities of skin of color and its diagnostic and therapeutic implications, both practicing and training dermatologists have acknowledged a lack of exposure to skin of color. In one study, approximately 47% of dermatologists and dermatology residents reported that their medical training (medical school and/or residency) was inadequate in training them on skin conditions in Black patients. Furthermore, many who felt their training was lacking in skin of color identified the need for greater exposure to Black patients and training materials.15 The absence of comprehensive medical education regarding skin of color ultimately can be a disadvantage for both practitioners and patients, resulting in poorer outcomes. Furthermore, underrepresentation of skin of color may persist beyond undergraduate and graduate medical education. There also is evidence to suggest that noninclusion of skin of color pervades foundational dermatologic educational resources, including commonly used textbooks as well as continuing medical education disseminated at national conferences and meetings.17 Taken together, these findings highlight the need for more diverse and representative exposure to skin of color throughout medical training, which begins with a diverse inclusive undergraduate medical education in dermatology.
The objective of this study was to determine if the preclinical dermatology curriculum at 3 US medical schools provided adequate representation of skin of color patients in their didactic presentation slides.
Methods
Participants—Three US medical schools, a blend of private and public medical schools located across different geographic boundaries, agreed to participate in the study. All 3 institutions were current members of the American Medical Association (AMA) Accelerating Change in Medical Education consortium, whose primary goal is to create the medical school of the future and transform physician training.18 All 32 member institutions of the AMA consortium were contacted to request their participation in the study. As part of the consortium, these institutions have vowed to collectively work to develop and share the best models for educational advancement to improve care for patients, populations, and communities18 and would expectedly provide a more racially and ethnically inclusive curriculum than an institution not accountable to a group dedicated to identifying the best ways to deliver care for increasingly diverse communities.
Data Collection—Lectures were included if they were presented during dermatology preclinical courses in the 2015 to 2016 academic year. An uninvolved third party removed the names and identities of instructors to preserve anonymity. Two independent coders from different institutions extracted the data—lecture title, total number of clinical and histologic images, and number of skin of color images—from each of the anonymized lectures using a standardized coding form. We documented differences in skin of color noted in lectures and the disease context for the discussed differences, such as variations in clinical presentation, disease process, epidemiology/risk, and treatment between different skin phenotypes or ethnic groups. Photographs in which the coders were unable to differentiate whether the patient had skin of color were designated as indeterminate or unclear. Photographs appearing to represent Fitzpatrick skin types IV, V, and VI19 were categorically designated as skin of color, and those appearing to represent Fitzpatrick skin types I and II were described as not skin of color; however, images appearing to represent Fitzpatrick skin type III often were classified as not skin of color or indeterminate and occasionally skin of color. The Figure shows examples of images classified as skin of color, indeterminate, and not skin of color. Photographs often were classified as indeterminate due to poor lighting, close-up view photographs, or highlighted pathology obscuring the surrounding skin. We excluded duplicate photographs and histologic images from the analyses.
We also reviewed 19 conditions previously highlighted by the SOCS as areas of importance to skin of color patients.20 The coders tracked how many of these conditions were noted in each lecture. Duplicate discussion of these conditions was not included in the analyses. Any discrepancies between coders were resolved through additional slide review and discussion. The final coded data with the agreed upon changes were used for statistical analyses. Recent national demographic data from the US Census Bureau in 2019 describe approximately 39.9% of the population as belonging to racial/ethnic groups other than non-Hispanic/Latinx White.21 Consequently, the standard for adequate representation for skin of color photographs was set at 35% for the purpose of this study.
Results
Across all 3 institutions included in the study, the proportion of the total number of clinical photographs showing skin of color was 16% (290/1812). Eight percent of the total photographs (145/1812) were noted to be indeterminate (Table). For institution 1, 23.6% of photographs (155/658) showed skin of color, and 12.6% (83/658) were indeterminate. For institution 2, 13.1% (76/578) showed skin of color and 7.8% (45/578) were indeterminate. For institution 3, 10.2% (59/576) showed skin of color and 3% (17/576) were indeterminate.
Institutions 1, 2, and 3 had 18, 8, and 17 total dermatology lectures, respectively. Of the 19 conditions designated as areas of importance to skin of color patients by the SOCS, 16 (84.2%) were discussed by institution 1, 11 (57.9%) by institution 2, and 9 (47.4%) by institution 3 (eTable 1). Institution 3 did not include photographs of skin of color patients in its acne, psoriasis, or cutaneous malignancy lectures. Institution 1 also did not include any skin of color patients in its malignancy lecture. Lectures that focused on pigmentary disorders, atopic dermatitis, infectious conditions, and benign cutaneous neoplasms were more likely to display photographs of skin of color patients; for example, lectures that discussed infectious conditions, such as superficial mycoses, herpes viruses, human papillomavirus, syphilis, and atypical mycobacterial infections, were consistently among those with higher proportions of photographs of skin of color patients.
Throughout the entire preclinical dermatology course at all 3 institutions, of 2945 lecture slides, only 24 (0.8%) unique differences were noted between skin color and non–skin of color patients, with 10 total differences noted by institution 1, 6 by institution 2, and 8 by institution 3 (Table). The majority of these differences (19/24) were related to epidemiologic differences in prevalence among varying racial/ethnic groups, with only 5 instances highlighting differences in clinical presentation. There was only a single instance that elaborated on the underlying pathophysiologic mechanisms of the discussed difference. Of all 24 unique differences discussed, 8 were related to skin cancer, 3 were related to dermatitis, and 2 were related to the difference in manifestation of erythema in patients with darker skin (eTable 2).
Comment
The results of this study demonstrated that skin of color is underrepresented in the preclinical dermatology curriculum at these 3 institutions. Although only 16% of all included clinical photographs were of skin of color, individuals with skin of color will soon represent more than half of the total US population within the next 2 decades.1 To increase representation of skin of color patients, teaching faculty should consciously and deliberately include more photographs of skin of color patients for a wider variety of common conditions, including atopic dermatitis and psoriasis, in addition to those that tend to disparately affect skin of color patients, such as pseudofolliculitis barbae or melasma. Furthermore, they also can incorporate more detailed discussions about important differences seen in skin of color patients.
More Skin of Color Photographs in Psoriasis Lectures—At institution 3, there were no skin of color patients included in the psoriasis lecture, even though there is considerable data in the literature indicating notable differences in the clinical presentation, quality-of-life impact, and treatment of psoriasis in skin of color patients.11,22 There are multiple nuances in psoriasis manifestation in patients with skin of color, including less-conspicuous erythema in darker skin, higher degrees of dyspigmentation, and greater body surface area involvement. For Black patients with scalp psoriasis, the impact of hair texture, styling practices, and washing frequency are additional considerations that may impact disease severity and selection of topical therapy.11 The lack of inclusion of any skin of color patients in the psoriasis lecture at one institution further underscores the pressing need to prioritize communities of color in medical education.
More Skin of Color Photographs in Cutaneous Malignancy Lectures—Similarly, while a lecturer at institution 2 noted that acral lentiginous melanoma accounts for a considerable proportion of melanoma among skin of color patients,23 there was no mention of how melanoma generally is substantially more deadly in this population, potentially due to decreased awareness and inconsistent screening.24 Furthermore, at institutions 1 and 3, there were no photographs or discussion of skin of color patients during the cutaneous malignancy lectures. Evidence shows that more emphasis is needed for melanoma screening and awareness in skin of color populations to improve survival outcomes,24 and this begins with educating not only future dermatologists but all future physicians as well. The failure to include photographs of skin of color patients in discussions or lectures regarding cutaneous malignancies may serve to further perpetuate the harmful misperception that individuals with skin of color are unaffected by skin cancer.25,26
Analysis of Skin of Color Photographs in Infectious Disease Lectures—In addition, lectures discussing infectious etiologies were among those with the highest proportion of skin of color photographs. This relatively disproportionate representation of skin of color compared to the other lectures may contribute to the development of harmful stereotypes or the stigmatization of skin of color patients. Although skin of color should continue to be represented in similar lectures, teaching faculty should remain mindful of the potential unintended impact from lectures including relatively disproportionate amounts of skin of color, particularly when other lectures may have sparse to absent representation of skin of color.
More Photographs Available for Education—Overall, our findings may help to inform changes to preclinical dermatology medical education at other institutions to create more inclusive and representative curricula for skin of color patients. The ability of instructors to provide visual representation of various dermatologic conditions may be limited by the photographs available in certain textbooks with few examples of patients with skin of color; however, concerns regarding the lack of skin of color representation in dermatology training is not a novel discussion.17 Although it is the responsibility of all dermatologists to advocate for the inclusion of skin of color, many dermatologists of color have been leading the way in this movement for decades, publishing several textbooks to document various skin conditions in those with darker skin types and discuss unique considerations for patients with skin of color.27-29 Images from these textbooks can be utilized by programs to increase representation of skin of color in dermatology training. There also are multiple expanding online dermatologic databases, such as VisualDx, with an increasing focus on skin of color patients, some of which allow users to filter images by degree of skin pigmentation.30 Moreover, instructors also can work to diversify their curricula by highlighting more of the SOCS conditions of importance to skin of color patients, which have since been renamed and highlighted on the Patient Dermatology Education section of the SOCS website.20 These conditions, while not completely comprehensive, provide a useful starting point for medical educators to reevaluate for potential areas of improvement and inclusion.
There are several potential strategies that can be used to better represent skin of color in dermatologic preclinical medical education, including increasing awareness, especially among dermatology teaching faculty, of existing disparities in the representation of skin of color in the preclinical curricula. Additionally, all dermatology teaching materials could be reviewed at the department level prior to being disseminated to medical students to assess for instances in which skin of color could be prioritized for discussion or varying disease presentations in skin of color could be demonstrated. Finally, teaching faculty may consider photographing more clinical images of their skin of color patients to further develop a catalog of diverse images that can be used to teach students.
Study Limitations—Our study was unable to account for verbal discussion of skin of color not otherwise denoted or captured in lecture slides. Additional limitations include the utilization of Fitzpatrick skin types to describe and differentiate varying skin tones, as the Fitzpatrick scale originally was developed as a method to describe an individual’s response to UV exposure.19 The inability to further delineate the representation of darker skin types, such as those that may be classified as Fitzpatrick skin types V or VI,19 compared to those with lighter skin of color also was a limiting factor. This study was unable to assess for discussion of other common conditions affecting skin of color patients that were not listed as one of the priority conditions by SOCS. Photographs that were designated as indeterminate were difficult to elucidate as skin of color; however, it is possible that instructors may have verbally described these images as skin of color during lectures. Nonetheless, it may be beneficial for learners if teaching faculty were to clearly label instances where skin of color patients are shown or when notable differences are present.
Conclusion
Future studies would benefit from the inclusion of audio data from lectures, syllabi, and small group teaching materials from preclinical courses to more accurately assess representation of skin of color in dermatology training. Additionally, future studies also may expand to include images from lectures of overlapping clinical specialties, particularly infectious disease and rheumatology, to provide a broader assessment of skin of color exposure. Furthermore, repeat assessment may be beneficial to assess the longitudinal effectiveness of curricular changes at the institutions included in this study, comparing older lectures to more recent, updated lectures. This study also may be replicated at other medical schools to allow for wider comparison of curricula.
Acknowledgment—The authors wish to thank the institutions that offered and agreed to participate in this study with the hopes of improving medical education.
- Colby SL, Ortman JM. Projections of the size and composition of the US population: 2014 to 2060. United States Census Bureau website. Published March 2015. Accessed September 14, 2021. https://www.census.gov/content/dam/Census/library/publications/2015/demo/p25-1143.pdf
- Learn more about SOCS. Skin of Color Society website. Accessed September 14, 2021. http://skinofcolorsociety.org/about-socs/
- Taylor SC. Skin of color: biology, structure, function, and implications for dermatologic disease. J Am Acad Dermatol. 2002;46(suppl 2):S41-S62.
- Berardesca E, Maibach H. Ethnic skin: overview of structure and function. J Am Acad Dermatol. 2003;48(suppl 6):S139-S142.
- Callender VD, Surin-Lord SS, Davis EC, et al. Postinflammatory hyperpigmentation. Am J Clin Dermatol. 2011;12:87-99.
- Davis EC, Callender VD. Postinflammatory hyperpigmentation: a review of the epidemiology, clinical features, and treatment options in skin of color. J Clin Aesthet Dermatol. 2010;3:20-31.
- Grimes PE, Stockton T. Pigmentary disorders in blacks. Dermatol Clin. 1988;6:271-281.
- Halder RM, Nootheti PK. Ethnic skin disorders overview. J Am Acad Dermatol. 2003;48(suppl 6):S143-S148.
- Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
- Callender VD. Acne in ethnic skin: special considerations for therapy. Dermatol Ther. 2004;17:184-195.
- Alexis AF, Blackcloud P. Psoriasis in skin of color: epidemiology, genetics, clinical presentation, and treatment nuances. J Clin Aesthet Dermatol. 2014;7:16-24.
- McCleskey PE, Gilson RT, DeVillez RL. Medical student core curriculum in dermatology survey. J Am Acad Dermatol. 2009;61:30-35.
- Ramsay DL, Mayer F. National survey of undergraduate dermatologic medical education. Arch Dermatol.1985;121:1529-1530.
- Hansra NK, O’Sullivan P, Chen CL, et al. Medical school dermatology curriculum: are we adequately preparing primary care physicians? J Am Acad Dermatol. 2009;61:23-29.
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59, viii.
- Knable A, Hood AF, Pearson TG. Undergraduate medical education in dermatology: report from the AAD Interdisciplinary Education Committee, Subcommittee on Undergraduate Medical Education. J Am Acad Dermatol. 1997;36:467-470.
- Ebede T, Papier A. Disparities in dermatology educational resources. J Am Acad Dermatol. 2006;55:687-690.
- Skochelak SE, Stack SJ. Creating the medical schools of the future. Acad Med. 2017;92:16-19.
- Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124:869-871.
- Skin of Color Society. Patient dermatology education. Accessed September 22, 2021. https://skinofcolorsociety.org/patient-dermatology-education
- QuickFacts: United States. US Census Bureau website. Updated July 1, 2019. Accessed September 14, 2021. https://www.census.gov/quickfacts/fact/table/US#
- Kaufman BP, Alexis AF. Psoriasis in skin of color: insights into the epidemiology, clinical presentation, genetics, quality-of-life impact, and treatment of psoriasis in non-white racial/ethnic groups. Am J Clin Dermatol. 2018;19:405-423.
- Bradford PT, Goldstein AM, McMaster ML, et al. Acral lentiginous melanoma: incidence and survival patterns in the United States, 1986-2005. Arch Dermatol. 2009;145:427-434.
- Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival. J Am Acad Dermatol. 2016;75:983-991.
- Pipitone M, Robinson JK, Camara C, et al. Skin cancer awareness in suburban employees: a Hispanic perspective. J Am Acad Dermatol. 2002;47:118-123.
- Imahiyerobo-Ip J, Ip I, Jamal S, et al. Skin cancer awareness in communities of color. J Am Acad Dermatol. 2011;64:198-200.
- Taylor SSC, Serrano AMA, Kelly AP, et al, eds. Taylor and Kelly’s Dermatology for Skin of Color. 2nd ed. McGraw-Hill Education; 2016.
- Dadzie OE, Petit A, Alexis AF, eds. Ethnic Dermatology: Principles and Practice. Wiley-Blackwell; 2013.
- Jackson-Richards D, Pandya AG, eds. Dermatology Atlas for Skin of Color. Springer; 2014.
- VisualDx. New VisualDx feature: skin of color sort. Published October 14, 2020. Accessed September 22, 2021. https://www.visualdx.com/blog/new-visualdx-feature-skin-of-color-sort/
- Colby SL, Ortman JM. Projections of the size and composition of the US population: 2014 to 2060. United States Census Bureau website. Published March 2015. Accessed September 14, 2021. https://www.census.gov/content/dam/Census/library/publications/2015/demo/p25-1143.pdf
- Learn more about SOCS. Skin of Color Society website. Accessed September 14, 2021. http://skinofcolorsociety.org/about-socs/
- Taylor SC. Skin of color: biology, structure, function, and implications for dermatologic disease. J Am Acad Dermatol. 2002;46(suppl 2):S41-S62.
- Berardesca E, Maibach H. Ethnic skin: overview of structure and function. J Am Acad Dermatol. 2003;48(suppl 6):S139-S142.
- Callender VD, Surin-Lord SS, Davis EC, et al. Postinflammatory hyperpigmentation. Am J Clin Dermatol. 2011;12:87-99.
- Davis EC, Callender VD. Postinflammatory hyperpigmentation: a review of the epidemiology, clinical features, and treatment options in skin of color. J Clin Aesthet Dermatol. 2010;3:20-31.
- Grimes PE, Stockton T. Pigmentary disorders in blacks. Dermatol Clin. 1988;6:271-281.
- Halder RM, Nootheti PK. Ethnic skin disorders overview. J Am Acad Dermatol. 2003;48(suppl 6):S143-S148.
- Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
- Callender VD. Acne in ethnic skin: special considerations for therapy. Dermatol Ther. 2004;17:184-195.
- Alexis AF, Blackcloud P. Psoriasis in skin of color: epidemiology, genetics, clinical presentation, and treatment nuances. J Clin Aesthet Dermatol. 2014;7:16-24.
- McCleskey PE, Gilson RT, DeVillez RL. Medical student core curriculum in dermatology survey. J Am Acad Dermatol. 2009;61:30-35.
- Ramsay DL, Mayer F. National survey of undergraduate dermatologic medical education. Arch Dermatol.1985;121:1529-1530.
- Hansra NK, O’Sullivan P, Chen CL, et al. Medical school dermatology curriculum: are we adequately preparing primary care physicians? J Am Acad Dermatol. 2009;61:23-29.
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59, viii.
- Knable A, Hood AF, Pearson TG. Undergraduate medical education in dermatology: report from the AAD Interdisciplinary Education Committee, Subcommittee on Undergraduate Medical Education. J Am Acad Dermatol. 1997;36:467-470.
- Ebede T, Papier A. Disparities in dermatology educational resources. J Am Acad Dermatol. 2006;55:687-690.
- Skochelak SE, Stack SJ. Creating the medical schools of the future. Acad Med. 2017;92:16-19.
- Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124:869-871.
- Skin of Color Society. Patient dermatology education. Accessed September 22, 2021. https://skinofcolorsociety.org/patient-dermatology-education
- QuickFacts: United States. US Census Bureau website. Updated July 1, 2019. Accessed September 14, 2021. https://www.census.gov/quickfacts/fact/table/US#
- Kaufman BP, Alexis AF. Psoriasis in skin of color: insights into the epidemiology, clinical presentation, genetics, quality-of-life impact, and treatment of psoriasis in non-white racial/ethnic groups. Am J Clin Dermatol. 2018;19:405-423.
- Bradford PT, Goldstein AM, McMaster ML, et al. Acral lentiginous melanoma: incidence and survival patterns in the United States, 1986-2005. Arch Dermatol. 2009;145:427-434.
- Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival. J Am Acad Dermatol. 2016;75:983-991.
- Pipitone M, Robinson JK, Camara C, et al. Skin cancer awareness in suburban employees: a Hispanic perspective. J Am Acad Dermatol. 2002;47:118-123.
- Imahiyerobo-Ip J, Ip I, Jamal S, et al. Skin cancer awareness in communities of color. J Am Acad Dermatol. 2011;64:198-200.
- Taylor SSC, Serrano AMA, Kelly AP, et al, eds. Taylor and Kelly’s Dermatology for Skin of Color. 2nd ed. McGraw-Hill Education; 2016.
- Dadzie OE, Petit A, Alexis AF, eds. Ethnic Dermatology: Principles and Practice. Wiley-Blackwell; 2013.
- Jackson-Richards D, Pandya AG, eds. Dermatology Atlas for Skin of Color. Springer; 2014.
- VisualDx. New VisualDx feature: skin of color sort. Published October 14, 2020. Accessed September 22, 2021. https://www.visualdx.com/blog/new-visualdx-feature-skin-of-color-sort/
Practice Points
- The United States rapidly is becoming a country in which the majority of citizens will have skin of color.
- Our study results strongly suggest that skin of color may be seriously underrepresented in medical education and can guide modifications to preclinical dermatology medical education to develop a more comprehensive and inclusive curriculum.
- Efforts should be made to increase images and discussion of skin of color in preclinical didactics.