User login
No survival dip with neoadjuvant letrozole-palbociclib in NeoPAL study
Three-year survival rates were similarly high among postmenopausal women with high-risk early luminal breast cancer who were treated with either the neoadjuvant combination of letrozole and palbociclib (Ibrance) or standard neoadjuvant chemotherapy in the phase 2 NeoPAL study.
Progression-free survival (PFS) was a respective 86.7% and 87.2%, with a hazard ratio (HR) of 1.01 (P = .98) comparing the endocrine therapy and cyclin-dependent kinase (CDK) 4/6 inhibitor combination versus FEC/taxane chemotherapy.
There were also no differences between the two treatment arms in terms of invasive disease-free survival (iDFS, HR = 0.83, P = .71) or breast cancer–specific survival (BCSS), although the latter was an exploratory endpoint alongside overall survival (OS).
“The lack of difference is impressive,” said Hope S. Rugo, MD, FASCO, who commented independently on the study’s findings after their presentation at the European Society for Medical Oncology: Breast Cancer virtual meeting.
“Overall survival in patients who received chemotherapy appears to be better, but the very small numbers here make interpretation of this difference impossible,” observed Dr. Rugo, professor of medicine at the University of California San Francisco’s Helen Diller Family Comprehensive Cancer Center.
“Unfortunately, this study is underpowered for definitive conclusions,” acknowledged study investigator Suzette Delaloge, MD, associate professor of medical oncology at Institut Gustave Roussy in Villejuif, France.
However, “it shows that the nonchemotherapy, preoperative letrozole/palbociclib approach deserves further exploration and could be an option for a chemotherapy-free regimen in some specific cases.”
Primary data already reported
The NeoPAL study was an open-label, randomized study conducted in 27 centers throughout France that compared the preoperative use of letrozole plus palbociclib to neoadjuvant chemotherapy in 106 postmenopausal patients with either luminal A or B node-positive disease.
Patients were considered for inclusion in the trial if they had been newly diagnosed with estrogen receptor (ER)-positive, HER2-negative stage I-III breast cancer and were not candidates for breast conservation. Genetic testing was used to confirm that only those with luminal B, or luminal A and who were node positive were recruited.
Neoadjuvant treatment consisted of either letrozole (2.5 mg/day) and palbociclib (125 mg daily for 3 weeks out of 4 weeks) for 19 weeks or three 21-day cycles of 5-fluorouracil (500 mg/m2), epirubicin (100 mg/m2), and cyclophosphamide (500 mg/m2), followed by three 21-day cycles of docetaxel (100 mg/m2).
The primary endpoint was the pathological complete response (pCR), defined as a residual cancer burden (RCB) of 0 to 1. Results, which have already been reported, showed equivalent, but perhaps disappointingly low, pathological responses in both the letrozole/palbociclib and chemotherapy arms (3.8% and 5.9%, respectively).
There were, however, identical clinical responses (at around 75%) and “encouraging biomarker responses in the Prosigna-defined high risk luminal breast cancer population,” Dr. Delaloge said.
The NeoPAL findings were on par with those of the CORALLEEN study, Dr. Delaloge suggested. That trial, as Dr. Rugo has also pointed out, was conducted in 106 patients with luminal B early breast cancer and used a combination of letrozole and the CDK 4/6 inhibitor ribociclib (Kisquali).
Future studies needed
NeoPAL “is a small study with relatively short follow-up even for hormone receptor-positive, high-risk disease,” Dr. Rugo observed. However, she qualified “this short follow-up can be very meaningful in high-risk disease.” as shown by other CDK 4/6 inhibitor trials.
Dr. Rugo also noted: “Short-term biologic endpoints are clearly more informative following and during neoadjuvant endocrine therapy than pCR and this trial, as well as the data from previous studies, indicates that this is the case.”
Further, Dr. Rugo said: “Antiproliferative response is enhanced with CDK 4/6 inhibitors, but this doesn’t seem to translate into a difference in pCR. The lack of impact on longer term, outcome to date, provides support for ongoing trials.”
Two such trials are already underway. The 200-patient CARABELA trial started recruitment in March last year and is comparing endocrine therapy with letrozole plus the CDK 4/6 inhibitor abemaciclib (Verzenio) to standard chemotherapy in patients with hormone receptor–positive, high-risk Ki67 disease.
Then there is the ADAPTcycle trial, a large open-label, phase 3 trial that is randomizing patients based on Ki67 and recurrence score after a short preoperative induction with endocrine therapy to postoperative chemotherapy or to 2 years of endocrine therapy plus ribociclib, with both arms receiving a standard course of 5 years of endocrine therapy.
“These two studies have provided interesting information that will help us design studies in the future,” said Dr. Rugo.
Not only that, but they will also help “investigate the subgroups of patients that benefit the most from CDK 4/6 inhibitors and better study neoadjuvant endocrine therapy which is an important option for patients that can be evaluated in terms of its efficacy by short term measures of antiproliferative response.”
NeoPAL was sponsored by UNICANCER with funding from Pfizer and NanoString Technologies. Dr. Delaloge disclosed receiving research grants or funding via her institution from Pfizer, AstraZeneca, Roche, Merck, Sanofi, Lilly, Novartis, BMS, Orion, Daiichi, Puma, and Pierre Fabre. Dr. Rugo reported receipt of grants via her institution to perform clinical trials from Pfizer and multiple other companies. She disclosed receiving honoraria from PUMA, Samsung, and Mylan.
Three-year survival rates were similarly high among postmenopausal women with high-risk early luminal breast cancer who were treated with either the neoadjuvant combination of letrozole and palbociclib (Ibrance) or standard neoadjuvant chemotherapy in the phase 2 NeoPAL study.
Progression-free survival (PFS) was a respective 86.7% and 87.2%, with a hazard ratio (HR) of 1.01 (P = .98) comparing the endocrine therapy and cyclin-dependent kinase (CDK) 4/6 inhibitor combination versus FEC/taxane chemotherapy.
There were also no differences between the two treatment arms in terms of invasive disease-free survival (iDFS, HR = 0.83, P = .71) or breast cancer–specific survival (BCSS), although the latter was an exploratory endpoint alongside overall survival (OS).
“The lack of difference is impressive,” said Hope S. Rugo, MD, FASCO, who commented independently on the study’s findings after their presentation at the European Society for Medical Oncology: Breast Cancer virtual meeting.
“Overall survival in patients who received chemotherapy appears to be better, but the very small numbers here make interpretation of this difference impossible,” observed Dr. Rugo, professor of medicine at the University of California San Francisco’s Helen Diller Family Comprehensive Cancer Center.
“Unfortunately, this study is underpowered for definitive conclusions,” acknowledged study investigator Suzette Delaloge, MD, associate professor of medical oncology at Institut Gustave Roussy in Villejuif, France.
However, “it shows that the nonchemotherapy, preoperative letrozole/palbociclib approach deserves further exploration and could be an option for a chemotherapy-free regimen in some specific cases.”
Primary data already reported
The NeoPAL study was an open-label, randomized study conducted in 27 centers throughout France that compared the preoperative use of letrozole plus palbociclib to neoadjuvant chemotherapy in 106 postmenopausal patients with either luminal A or B node-positive disease.
Patients were considered for inclusion in the trial if they had been newly diagnosed with estrogen receptor (ER)-positive, HER2-negative stage I-III breast cancer and were not candidates for breast conservation. Genetic testing was used to confirm that only those with luminal B, or luminal A and who were node positive were recruited.
Neoadjuvant treatment consisted of either letrozole (2.5 mg/day) and palbociclib (125 mg daily for 3 weeks out of 4 weeks) for 19 weeks or three 21-day cycles of 5-fluorouracil (500 mg/m2), epirubicin (100 mg/m2), and cyclophosphamide (500 mg/m2), followed by three 21-day cycles of docetaxel (100 mg/m2).
The primary endpoint was the pathological complete response (pCR), defined as a residual cancer burden (RCB) of 0 to 1. Results, which have already been reported, showed equivalent, but perhaps disappointingly low, pathological responses in both the letrozole/palbociclib and chemotherapy arms (3.8% and 5.9%, respectively).
There were, however, identical clinical responses (at around 75%) and “encouraging biomarker responses in the Prosigna-defined high risk luminal breast cancer population,” Dr. Delaloge said.
The NeoPAL findings were on par with those of the CORALLEEN study, Dr. Delaloge suggested. That trial, as Dr. Rugo has also pointed out, was conducted in 106 patients with luminal B early breast cancer and used a combination of letrozole and the CDK 4/6 inhibitor ribociclib (Kisquali).
Future studies needed
NeoPAL “is a small study with relatively short follow-up even for hormone receptor-positive, high-risk disease,” Dr. Rugo observed. However, she qualified “this short follow-up can be very meaningful in high-risk disease.” as shown by other CDK 4/6 inhibitor trials.
Dr. Rugo also noted: “Short-term biologic endpoints are clearly more informative following and during neoadjuvant endocrine therapy than pCR and this trial, as well as the data from previous studies, indicates that this is the case.”
Further, Dr. Rugo said: “Antiproliferative response is enhanced with CDK 4/6 inhibitors, but this doesn’t seem to translate into a difference in pCR. The lack of impact on longer term, outcome to date, provides support for ongoing trials.”
Two such trials are already underway. The 200-patient CARABELA trial started recruitment in March last year and is comparing endocrine therapy with letrozole plus the CDK 4/6 inhibitor abemaciclib (Verzenio) to standard chemotherapy in patients with hormone receptor–positive, high-risk Ki67 disease.
Then there is the ADAPTcycle trial, a large open-label, phase 3 trial that is randomizing patients based on Ki67 and recurrence score after a short preoperative induction with endocrine therapy to postoperative chemotherapy or to 2 years of endocrine therapy plus ribociclib, with both arms receiving a standard course of 5 years of endocrine therapy.
“These two studies have provided interesting information that will help us design studies in the future,” said Dr. Rugo.
Not only that, but they will also help “investigate the subgroups of patients that benefit the most from CDK 4/6 inhibitors and better study neoadjuvant endocrine therapy which is an important option for patients that can be evaluated in terms of its efficacy by short term measures of antiproliferative response.”
NeoPAL was sponsored by UNICANCER with funding from Pfizer and NanoString Technologies. Dr. Delaloge disclosed receiving research grants or funding via her institution from Pfizer, AstraZeneca, Roche, Merck, Sanofi, Lilly, Novartis, BMS, Orion, Daiichi, Puma, and Pierre Fabre. Dr. Rugo reported receipt of grants via her institution to perform clinical trials from Pfizer and multiple other companies. She disclosed receiving honoraria from PUMA, Samsung, and Mylan.
Three-year survival rates were similarly high among postmenopausal women with high-risk early luminal breast cancer who were treated with either the neoadjuvant combination of letrozole and palbociclib (Ibrance) or standard neoadjuvant chemotherapy in the phase 2 NeoPAL study.
Progression-free survival (PFS) was a respective 86.7% and 87.2%, with a hazard ratio (HR) of 1.01 (P = .98) comparing the endocrine therapy and cyclin-dependent kinase (CDK) 4/6 inhibitor combination versus FEC/taxane chemotherapy.
There were also no differences between the two treatment arms in terms of invasive disease-free survival (iDFS, HR = 0.83, P = .71) or breast cancer–specific survival (BCSS), although the latter was an exploratory endpoint alongside overall survival (OS).
“The lack of difference is impressive,” said Hope S. Rugo, MD, FASCO, who commented independently on the study’s findings after their presentation at the European Society for Medical Oncology: Breast Cancer virtual meeting.
“Overall survival in patients who received chemotherapy appears to be better, but the very small numbers here make interpretation of this difference impossible,” observed Dr. Rugo, professor of medicine at the University of California San Francisco’s Helen Diller Family Comprehensive Cancer Center.
“Unfortunately, this study is underpowered for definitive conclusions,” acknowledged study investigator Suzette Delaloge, MD, associate professor of medical oncology at Institut Gustave Roussy in Villejuif, France.
However, “it shows that the nonchemotherapy, preoperative letrozole/palbociclib approach deserves further exploration and could be an option for a chemotherapy-free regimen in some specific cases.”
Primary data already reported
The NeoPAL study was an open-label, randomized study conducted in 27 centers throughout France that compared the preoperative use of letrozole plus palbociclib to neoadjuvant chemotherapy in 106 postmenopausal patients with either luminal A or B node-positive disease.
Patients were considered for inclusion in the trial if they had been newly diagnosed with estrogen receptor (ER)-positive, HER2-negative stage I-III breast cancer and were not candidates for breast conservation. Genetic testing was used to confirm that only those with luminal B, or luminal A and who were node positive were recruited.
Neoadjuvant treatment consisted of either letrozole (2.5 mg/day) and palbociclib (125 mg daily for 3 weeks out of 4 weeks) for 19 weeks or three 21-day cycles of 5-fluorouracil (500 mg/m2), epirubicin (100 mg/m2), and cyclophosphamide (500 mg/m2), followed by three 21-day cycles of docetaxel (100 mg/m2).
The primary endpoint was the pathological complete response (pCR), defined as a residual cancer burden (RCB) of 0 to 1. Results, which have already been reported, showed equivalent, but perhaps disappointingly low, pathological responses in both the letrozole/palbociclib and chemotherapy arms (3.8% and 5.9%, respectively).
There were, however, identical clinical responses (at around 75%) and “encouraging biomarker responses in the Prosigna-defined high risk luminal breast cancer population,” Dr. Delaloge said.
The NeoPAL findings were on par with those of the CORALLEEN study, Dr. Delaloge suggested. That trial, as Dr. Rugo has also pointed out, was conducted in 106 patients with luminal B early breast cancer and used a combination of letrozole and the CDK 4/6 inhibitor ribociclib (Kisquali).
Future studies needed
NeoPAL “is a small study with relatively short follow-up even for hormone receptor-positive, high-risk disease,” Dr. Rugo observed. However, she qualified “this short follow-up can be very meaningful in high-risk disease.” as shown by other CDK 4/6 inhibitor trials.
Dr. Rugo also noted: “Short-term biologic endpoints are clearly more informative following and during neoadjuvant endocrine therapy than pCR and this trial, as well as the data from previous studies, indicates that this is the case.”
Further, Dr. Rugo said: “Antiproliferative response is enhanced with CDK 4/6 inhibitors, but this doesn’t seem to translate into a difference in pCR. The lack of impact on longer term, outcome to date, provides support for ongoing trials.”
Two such trials are already underway. The 200-patient CARABELA trial started recruitment in March last year and is comparing endocrine therapy with letrozole plus the CDK 4/6 inhibitor abemaciclib (Verzenio) to standard chemotherapy in patients with hormone receptor–positive, high-risk Ki67 disease.
Then there is the ADAPTcycle trial, a large open-label, phase 3 trial that is randomizing patients based on Ki67 and recurrence score after a short preoperative induction with endocrine therapy to postoperative chemotherapy or to 2 years of endocrine therapy plus ribociclib, with both arms receiving a standard course of 5 years of endocrine therapy.
“These two studies have provided interesting information that will help us design studies in the future,” said Dr. Rugo.
Not only that, but they will also help “investigate the subgroups of patients that benefit the most from CDK 4/6 inhibitors and better study neoadjuvant endocrine therapy which is an important option for patients that can be evaluated in terms of its efficacy by short term measures of antiproliferative response.”
NeoPAL was sponsored by UNICANCER with funding from Pfizer and NanoString Technologies. Dr. Delaloge disclosed receiving research grants or funding via her institution from Pfizer, AstraZeneca, Roche, Merck, Sanofi, Lilly, Novartis, BMS, Orion, Daiichi, Puma, and Pierre Fabre. Dr. Rugo reported receipt of grants via her institution to perform clinical trials from Pfizer and multiple other companies. She disclosed receiving honoraria from PUMA, Samsung, and Mylan.
FROM ESMO BREAST CANCER 2021
BERENICE: Further evidence of heart safety of dual HER2 blockade
Dual HER2 blockade with pertuzumab (Perjeta) and trastuzumab (Herceptin) on top of anthracycline-based neoadjuvant chemotherapy for early-stage breast cancer was associated with a low rate of clinically relevant cardiac events in the final follow-up of the BERENICE study.
After more than 5 years, 1.0%-1.5% of patients who had locally advanced, inflammatory, or early-stage breast cancer developed heart failure, and around 12%-13% showed any significant changes in left ventricular ejection fraction (LVEF).
Importantly, “there were no new safety concerns that arose during long-term follow-up,” study investigator Chau Dang, MD, said in presenting the findings at the European Society for Medical Oncology: Breast Cancer virtual meeting.
Dr. Dang, a medical oncologist at Memorial Sloan Kettering Cancer Centre in New York, reported that the most common cause of death was disease progression.
BERENICE was designed as a cardiac safety study and so not powered to look at long-term efficacy, which Dr. Dang was clear in reporting. Nevertheless event-free survival (EFS), invasive disease-free survival (IDFS), and overall survival (OS) rates at 5 years were all high, at least a respective 89.2%, 91%, and 93.8%, she said. “The medians have not been reached,” she observed.
“These data support the use of dual HER2 blockade with pertuzumab-trastuzumab–based regimens, including in combination with dose-dense, anthracycline-based chemotherapy, across the neoadjuvant and adjuvant treatment settings for the complete treatment of patients with HER2-positive early-stage breast cancer,” Dr. Dang said.
Evandro de Azambuja, MD, PhD, the invited discussant for the trial agreed that the regimens tested appeared “safe from a cardiac standpoint.” However, “you cannot forget that today we are using much less anthracyclines in our patient population.”
Patients in trials are also very different from those treated in clinical practice, often being younger and much fitter, he said. Therefore, it may be important to look at the baseline cardiac medications and comorbidities, Dr. de Azambuja, a medical oncologist at the Institut Jules Bordet in Brussels, Belgium, suggested.
That said, the BERENICE findings sit well with other trials that have been conducted, Dr. de Azambuja pointed out.
“If we look at other trials that have also tested dual HER2 blockade with anthracycline or nonanthracycline regimens, all of them reassure that dual blockade is not more cardiotoxic than single blockade,” he said. This includes trials such as TRYPHAENA, APHINITY, KRISTINE, NeoSphere and PEONY.
The 3-year IDFS rate of 91% in BERENICE also compares well to that seen in APHINITY (94%), Dr. de Azambuja said.
BERENICE study design
BERENICE was a multicenter, open-label, nonrandomized and noncomparative phase 2 trial that recruited 400 patients across 75 centers in 12 countries.
Eligibility criteria were that participants had to have been centrally confirmed HER2-positive locally advanced, inflammatory or early breast cancer, with the latter defined as tumors bigger than 2 cm or greater than 5 mm in size, and be node-positive. Patients also had to have a starting LVEF of 55% or higher.
Patients were allocated to one of two neoadjuvant chemotherapy regimens depending on the choice of their physician. One group received a regimen of dose-dense doxorubicin and cyclophosphamide (ddAC) given every 2 weeks for four cycles and then paclitaxel every week for 12 cycles. The other group received 5-fluorouracil, epirubicin, and cyclophosphamide (FEC) every 3 weeks for four cycles and then docetaxel every 3 weeks for four cycles.
Pertuzumab and trastuzumab were started at the same time as the taxanes in both groups and given every 3 weeks for four cycles. Patients then underwent surgery and continued pertuzumab/trastuzumab treatment alone for a further 13 cycles.
The co-primary endpoints were the incidence of New York Heart Association class III or IV heart failure and incidence of symptomatic and asymptomatic LVEF decline of 10% or more.
The primary analysis of the trial was published in 2018 and, at that time, it was reported that three patients in the ddAC cohort and none in the FEC cohort experienced heart failure. LVEF decline was observed in a respective 6.5% and 2% of patients.
Discussion points
Dr. de Azambuja noted that the contribution of the chemotherapy to the efficacy cannot be assessed because of the nonrandomized trial design. That should not matter, pointed out Sybille Loibl, MD, PhD, during discussion.
“I think it compares nicely to other trials that looked at dose-dense chemotherapy,” said Dr. Loibl, who is an associate professor at the University of Frankfurt in Germany. “It seems that, in the light of what we consider today probably one of the best anti-HER2 treatments, the chemotherapy is less relevant, and that’s why a dose-dense regimen doesn’t add so much on a standard anthracycline taxane-containing regimen.”
Dr. de Azambuja also commented on the assessment of cardiotoxicity and the use of reduced LVEF as a measure: LVEF decline is a late effect of cardiotoxicity, he observed, and he suggested a different approach in future trials.
“If you use Global Longitudinal Strain, this could be an optimal parameter to detect early subclinical LVEF dysfunction and you should consider it for the next trials looking for cardiac safety. Also, cardiac biomarkers. This was not implemented in this trial, and I strongly recommend this should be for the next trial.”
The BERENICE trial was funded by F. Hoffmann-La Roche. Dr. Dang disclosed receiving consultancy fees from F. Hoffmann-La Roche, Genentech, Daiichi Sankyo, Lilly, and Puma Biotechnology. Dr. de Azambuja was not involved in the study but disclosed receiving honoraria, travel grants, research grants from Roche and Genentech as well as from other companies. Dr. Loibl was one of the cochairs of the session and, among disclosures regarding many other companies, has been an invited speaker for Roche and received reimbursement via her institution for a writing engagement.
Dual HER2 blockade with pertuzumab (Perjeta) and trastuzumab (Herceptin) on top of anthracycline-based neoadjuvant chemotherapy for early-stage breast cancer was associated with a low rate of clinically relevant cardiac events in the final follow-up of the BERENICE study.
After more than 5 years, 1.0%-1.5% of patients who had locally advanced, inflammatory, or early-stage breast cancer developed heart failure, and around 12%-13% showed any significant changes in left ventricular ejection fraction (LVEF).
Importantly, “there were no new safety concerns that arose during long-term follow-up,” study investigator Chau Dang, MD, said in presenting the findings at the European Society for Medical Oncology: Breast Cancer virtual meeting.
Dr. Dang, a medical oncologist at Memorial Sloan Kettering Cancer Centre in New York, reported that the most common cause of death was disease progression.
BERENICE was designed as a cardiac safety study and so not powered to look at long-term efficacy, which Dr. Dang was clear in reporting. Nevertheless event-free survival (EFS), invasive disease-free survival (IDFS), and overall survival (OS) rates at 5 years were all high, at least a respective 89.2%, 91%, and 93.8%, she said. “The medians have not been reached,” she observed.
“These data support the use of dual HER2 blockade with pertuzumab-trastuzumab–based regimens, including in combination with dose-dense, anthracycline-based chemotherapy, across the neoadjuvant and adjuvant treatment settings for the complete treatment of patients with HER2-positive early-stage breast cancer,” Dr. Dang said.
Evandro de Azambuja, MD, PhD, the invited discussant for the trial agreed that the regimens tested appeared “safe from a cardiac standpoint.” However, “you cannot forget that today we are using much less anthracyclines in our patient population.”
Patients in trials are also very different from those treated in clinical practice, often being younger and much fitter, he said. Therefore, it may be important to look at the baseline cardiac medications and comorbidities, Dr. de Azambuja, a medical oncologist at the Institut Jules Bordet in Brussels, Belgium, suggested.
That said, the BERENICE findings sit well with other trials that have been conducted, Dr. de Azambuja pointed out.
“If we look at other trials that have also tested dual HER2 blockade with anthracycline or nonanthracycline regimens, all of them reassure that dual blockade is not more cardiotoxic than single blockade,” he said. This includes trials such as TRYPHAENA, APHINITY, KRISTINE, NeoSphere and PEONY.
The 3-year IDFS rate of 91% in BERENICE also compares well to that seen in APHINITY (94%), Dr. de Azambuja said.
BERENICE study design
BERENICE was a multicenter, open-label, nonrandomized and noncomparative phase 2 trial that recruited 400 patients across 75 centers in 12 countries.
Eligibility criteria were that participants had to have been centrally confirmed HER2-positive locally advanced, inflammatory or early breast cancer, with the latter defined as tumors bigger than 2 cm or greater than 5 mm in size, and be node-positive. Patients also had to have a starting LVEF of 55% or higher.
Patients were allocated to one of two neoadjuvant chemotherapy regimens depending on the choice of their physician. One group received a regimen of dose-dense doxorubicin and cyclophosphamide (ddAC) given every 2 weeks for four cycles and then paclitaxel every week for 12 cycles. The other group received 5-fluorouracil, epirubicin, and cyclophosphamide (FEC) every 3 weeks for four cycles and then docetaxel every 3 weeks for four cycles.
Pertuzumab and trastuzumab were started at the same time as the taxanes in both groups and given every 3 weeks for four cycles. Patients then underwent surgery and continued pertuzumab/trastuzumab treatment alone for a further 13 cycles.
The co-primary endpoints were the incidence of New York Heart Association class III or IV heart failure and incidence of symptomatic and asymptomatic LVEF decline of 10% or more.
The primary analysis of the trial was published in 2018 and, at that time, it was reported that three patients in the ddAC cohort and none in the FEC cohort experienced heart failure. LVEF decline was observed in a respective 6.5% and 2% of patients.
Discussion points
Dr. de Azambuja noted that the contribution of the chemotherapy to the efficacy cannot be assessed because of the nonrandomized trial design. That should not matter, pointed out Sybille Loibl, MD, PhD, during discussion.
“I think it compares nicely to other trials that looked at dose-dense chemotherapy,” said Dr. Loibl, who is an associate professor at the University of Frankfurt in Germany. “It seems that, in the light of what we consider today probably one of the best anti-HER2 treatments, the chemotherapy is less relevant, and that’s why a dose-dense regimen doesn’t add so much on a standard anthracycline taxane-containing regimen.”
Dr. de Azambuja also commented on the assessment of cardiotoxicity and the use of reduced LVEF as a measure: LVEF decline is a late effect of cardiotoxicity, he observed, and he suggested a different approach in future trials.
“If you use Global Longitudinal Strain, this could be an optimal parameter to detect early subclinical LVEF dysfunction and you should consider it for the next trials looking for cardiac safety. Also, cardiac biomarkers. This was not implemented in this trial, and I strongly recommend this should be for the next trial.”
The BERENICE trial was funded by F. Hoffmann-La Roche. Dr. Dang disclosed receiving consultancy fees from F. Hoffmann-La Roche, Genentech, Daiichi Sankyo, Lilly, and Puma Biotechnology. Dr. de Azambuja was not involved in the study but disclosed receiving honoraria, travel grants, research grants from Roche and Genentech as well as from other companies. Dr. Loibl was one of the cochairs of the session and, among disclosures regarding many other companies, has been an invited speaker for Roche and received reimbursement via her institution for a writing engagement.
Dual HER2 blockade with pertuzumab (Perjeta) and trastuzumab (Herceptin) on top of anthracycline-based neoadjuvant chemotherapy for early-stage breast cancer was associated with a low rate of clinically relevant cardiac events in the final follow-up of the BERENICE study.
After more than 5 years, 1.0%-1.5% of patients who had locally advanced, inflammatory, or early-stage breast cancer developed heart failure, and around 12%-13% showed any significant changes in left ventricular ejection fraction (LVEF).
Importantly, “there were no new safety concerns that arose during long-term follow-up,” study investigator Chau Dang, MD, said in presenting the findings at the European Society for Medical Oncology: Breast Cancer virtual meeting.
Dr. Dang, a medical oncologist at Memorial Sloan Kettering Cancer Centre in New York, reported that the most common cause of death was disease progression.
BERENICE was designed as a cardiac safety study and so not powered to look at long-term efficacy, which Dr. Dang was clear in reporting. Nevertheless event-free survival (EFS), invasive disease-free survival (IDFS), and overall survival (OS) rates at 5 years were all high, at least a respective 89.2%, 91%, and 93.8%, she said. “The medians have not been reached,” she observed.
“These data support the use of dual HER2 blockade with pertuzumab-trastuzumab–based regimens, including in combination with dose-dense, anthracycline-based chemotherapy, across the neoadjuvant and adjuvant treatment settings for the complete treatment of patients with HER2-positive early-stage breast cancer,” Dr. Dang said.
Evandro de Azambuja, MD, PhD, the invited discussant for the trial agreed that the regimens tested appeared “safe from a cardiac standpoint.” However, “you cannot forget that today we are using much less anthracyclines in our patient population.”
Patients in trials are also very different from those treated in clinical practice, often being younger and much fitter, he said. Therefore, it may be important to look at the baseline cardiac medications and comorbidities, Dr. de Azambuja, a medical oncologist at the Institut Jules Bordet in Brussels, Belgium, suggested.
That said, the BERENICE findings sit well with other trials that have been conducted, Dr. de Azambuja pointed out.
“If we look at other trials that have also tested dual HER2 blockade with anthracycline or nonanthracycline regimens, all of them reassure that dual blockade is not more cardiotoxic than single blockade,” he said. This includes trials such as TRYPHAENA, APHINITY, KRISTINE, NeoSphere and PEONY.
The 3-year IDFS rate of 91% in BERENICE also compares well to that seen in APHINITY (94%), Dr. de Azambuja said.
BERENICE study design
BERENICE was a multicenter, open-label, nonrandomized and noncomparative phase 2 trial that recruited 400 patients across 75 centers in 12 countries.
Eligibility criteria were that participants had to have been centrally confirmed HER2-positive locally advanced, inflammatory or early breast cancer, with the latter defined as tumors bigger than 2 cm or greater than 5 mm in size, and be node-positive. Patients also had to have a starting LVEF of 55% or higher.
Patients were allocated to one of two neoadjuvant chemotherapy regimens depending on the choice of their physician. One group received a regimen of dose-dense doxorubicin and cyclophosphamide (ddAC) given every 2 weeks for four cycles and then paclitaxel every week for 12 cycles. The other group received 5-fluorouracil, epirubicin, and cyclophosphamide (FEC) every 3 weeks for four cycles and then docetaxel every 3 weeks for four cycles.
Pertuzumab and trastuzumab were started at the same time as the taxanes in both groups and given every 3 weeks for four cycles. Patients then underwent surgery and continued pertuzumab/trastuzumab treatment alone for a further 13 cycles.
The co-primary endpoints were the incidence of New York Heart Association class III or IV heart failure and incidence of symptomatic and asymptomatic LVEF decline of 10% or more.
The primary analysis of the trial was published in 2018 and, at that time, it was reported that three patients in the ddAC cohort and none in the FEC cohort experienced heart failure. LVEF decline was observed in a respective 6.5% and 2% of patients.
Discussion points
Dr. de Azambuja noted that the contribution of the chemotherapy to the efficacy cannot be assessed because of the nonrandomized trial design. That should not matter, pointed out Sybille Loibl, MD, PhD, during discussion.
“I think it compares nicely to other trials that looked at dose-dense chemotherapy,” said Dr. Loibl, who is an associate professor at the University of Frankfurt in Germany. “It seems that, in the light of what we consider today probably one of the best anti-HER2 treatments, the chemotherapy is less relevant, and that’s why a dose-dense regimen doesn’t add so much on a standard anthracycline taxane-containing regimen.”
Dr. de Azambuja also commented on the assessment of cardiotoxicity and the use of reduced LVEF as a measure: LVEF decline is a late effect of cardiotoxicity, he observed, and he suggested a different approach in future trials.
“If you use Global Longitudinal Strain, this could be an optimal parameter to detect early subclinical LVEF dysfunction and you should consider it for the next trials looking for cardiac safety. Also, cardiac biomarkers. This was not implemented in this trial, and I strongly recommend this should be for the next trial.”
The BERENICE trial was funded by F. Hoffmann-La Roche. Dr. Dang disclosed receiving consultancy fees from F. Hoffmann-La Roche, Genentech, Daiichi Sankyo, Lilly, and Puma Biotechnology. Dr. de Azambuja was not involved in the study but disclosed receiving honoraria, travel grants, research grants from Roche and Genentech as well as from other companies. Dr. Loibl was one of the cochairs of the session and, among disclosures regarding many other companies, has been an invited speaker for Roche and received reimbursement via her institution for a writing engagement.
FROM ESMO BREAST CANCER 2021
Screening High-Risk Women Veterans for Breast Cancer
The number of women seeking care from the Veterans Health Administration (VHA) is increasing.1 In 2015, there were 2 million women veterans in the United States, which is 9.4% of the total veteran population. This group is expected to increase at an average of about 18,000 women per year for the next 10 years.2 The percentage of women veterans who are US Department of Veterans Affairs (VA) users aged 45 to 64 years rose 46% from 2000 to 2015.1,3-4 It is estimated that 15% of veterans who used VA services in 2020 were women.1 Nineteen percent of women veterans are Black.1 The median age of women veterans in 2015 was 50 years.5 Breast cancer is the leading cancer affecting female veterans, and data suggest they have an increased risk of breast cancer based on unique service-related exposures.1,6-9
In the US, about 10 million women are eligible for breast cancer preventive therapy, including, but not limited to, medications, surgery, or lifestyle changes.10 Secondary prevention options include change in surveillance that can reduce their risk or identify cancer at an earlier stage when treatment is more effective. The United States Preventive Services Task Force, the National Comprehensive Cancer Network, the American Society for Clinical Oncology, the National Institute for Health and Care Excellence, and the Oncology Nursing Society recommend screening women aged ≥ 35 years to assess breast cancer risk.11-18 If a woman is at increased risk, she may be a candidate for chemoprevention, prozphylactic surgery, and possibly an enhanced screening regimen.
Urban and minority women are an understudied population. Most veterans (75%) live in urban or suburban settings.19,20 Urban veteran women constitute an important potential study population.
Chemoprevention measures have been underused because of factors involving both women and their health care providers. A large proportion of women are unaware of their higher risk status due to lack of adequate screening and risk assessment.21,22 In addition to patient lack of awareness of their high-risk status, primary care physicians are also reluctant to prescribe chemopreventive agents due to a lack of comfort or familiarity with the risks and benefits.23-26 The STAR2015, BCPT2005, IBIS2014, MAP3 2011, IBIS-I 2014, and IBIS II 2014 studies clearly demonstrate a 49 to 62% reduction in risk for women using chemoprevention such as selective estrogen receptor modulators or aromatase inhibitors, respectively.27-32 Yet only 4 to 9% of high-risk women not enrolled in a clinical trial are using chemoprevention.33-39
The possibility of developing breast cancer also may be increased because of a positive family history or being a member of a family in which there is a known susceptibility gene mutation.40 Based on these risk factors, women may be eligible for tailored follow-up and genetic counseling.41-44
Nationally, 7 to 10% of the civilian US population will experience posttraumatic stress disorder (PTSD).45 The rates are remarkably higher for women veterans, with roughly 20% diagnosed with PTSD.46,47 Anxiety and PTSD have been implicated in poor adherence to medical advice.48,49
In 2014, a national VA multidisciplinary group focused on breast cancer prevention, detection, treatment, and research to address breast health in the growing population of women veterans. High-risk breast cancer screenings are not routinely carried out by the VA in primary care, women’s health, or oncology services. Furthermore, the recording of screening questionnaire results was not synchronized until a standard questionnaire was created and approved as a template by this group in the VA electronic medical record (EMR) in 2015.
Several prediction models can identify which women are at an increased risk of developing breast cancer. The most commonly used risk assessment model, the Gail breast cancer risk assessment tool (BCRAT), has been refined to include women of additional ethnicities (https://www.cancer.gov/bcrisktool).
This pilot project was launched to identify an effective manner to screen women veterans regarding their risk of developing breast cancer and refer them for chemoprevention education or genetic counseling as appropriate.
Methods
A high-risk breast cancer screening questionnaire based on the Gail BCRAT and including lifestyle questions was developed and included as a note template in the VA EMR. The James J. Peters VA Medical Center, Bronx, NY (JJPVAMC) and the Washington DC VA Medical Center (DCVAMC) ran a pilot study between 2015 and 2018 using this breast cancer screening questionnaire to collect data from women veterans. Quality Executive Committee and institutional review board approvals were granted respectively.
Eligibility criteria included women aged ≥ 35 years with no personal history of breast cancer. Most patients were self-referred, but participants also were recruited during VA Breast Cancer Awareness month events, health fairs, or at informational tables in the hospital lobbies. After completing the 20 multiple choice questionnaire with a study team member, either in person or over the phone, a 5-year and lifetime risk of invasive breast cancer was calculated using the Gail BCRAT. A woman is considered high risk and eligible for chemoprevention if her 5-year risk is > 1.66% or her lifetime risk is ≥ 20%. Eligibility for genetic counseling is based on the Breast Cancer Referral Screening Tool, which includes a personal or family history of breast or ovarian cancer and Jewish ancestry.
All patients were notified of their average or high risk status by a clinician. Those who were deemed to be average risk received a follow-up letter in the mail with instructions (eg, to follow-up with a yearly mammogram). Those who were deemed to be high risk for developing breast cancer were asked to come in for an appointment with the study principal investigator (a VA oncologist/breast cancer specialist) to discuss prevention options, further screening, or referrals to genetic counseling. Depending on a patient’s other health factors, a woman at high risk for developing breast cancer also may be a candidate for chemoprevention with tamoxifen, raloxifene, exemestane, anastrozole, or letrozole.
Data on the participant’s lifestyle, including exercise, diet, and smoking, were evaluated to determine whether these factors had an impact on risk status.
Results
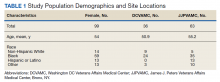
The JJP and DC VAMCs screened 103 women veterans between 2015 and 2018. Four patients were excluded for nonveteran (spousal) status, leaving 99 women veterans with a mean age of 54 years. The most common self-reported races were Black (60%), non-Hispanic White (14%), and Hispanic or Latino (13%) (Table 1).
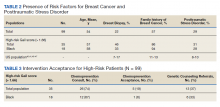
Women veterans in our study were nearly 3-times more likely than the general population were to receive a high-risk Gail Score/BCRAT (35% vs 13%, respectively).50,51 Of this subset, 46% had breast biopsies, and 86% had a positive family history. Thirty-one percent of Black women in our study were high risk, while nationally, 8.2 to 13.3% of Black women aged 50 to 59 years are considered high risk.50,51 Of the Black high-risk group with a high Gail/BCRAT score, 94% had a positive family history, and 33% had a history of breast biopsy (Table 2).
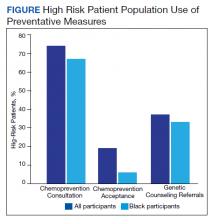
Of the 35 high-risk patients 26 (74%) patients accepted consultations for chemoprevention and 5 (19%) started chemoprevention. Of this high-risk group, 13 (37%) patients were referred for genetic counseling (Table 3).44 The prevalence of PTSD was present in 31% of high-risk women and 29% of the cohort (Figure).The lifestyle questions indicated that, among all participants, 79% had an overweight or obese body mass index; 58% exercised weekly; 51% consumed alcohol; 14% were smokers; and 21% consumed 3 to 4 servings of fruits/vegetables daily.
Discussion
Breast cancer is the most common cancer in women.52 The number of women with breast cancer in the VHA has more than tripled from 1995 to 2012.1 The lifetime risk of developing breast cancer in the general population is about 13%.50 This rate can be affected by risk factors including age, hormone exposure, family history, radiation exposure, and lifestyle factors, such as weight and alcohol use.6,52-56 In the United States, invasive breast cancer affects 1 in 8 women.50,52,57
Our screened population showed nearly 3 times as many women veterans were at an increased risk for breast cancer when compared with historical averages in US women. This difference may be based on a high rate of prior breast biopsies or positive family history, although a provocative study using the Surveillance, Epidemiology, and End Results database showed military women to have higher rates of breast cancer as well.9 Historically, Blacks are vastly understudied in clinical research with only 5% representation on a national level.5,58 The urban locations of both pilot sites (Washington, DC and Bronx, NY) allowed for the inclusion of minority patients in our study. We found that the rates of breast cancer in Black women veterans to be higher than seen nationally, possibly prompting further screening initiatives for this understudied population.
Our pilot study’s chemoprevention utilization (19%) was double the < 10% seen in the national population.33-35 The presence of a knowledgeable breast health practitioner to recruit study participants and offer personalized counseling to women veterans is a likely factor in overcoming barriers to chemopreventive acceptance. These participants may have been motivated to seek care for their high-risk status given a strong family history and prior breast biopsies.
Interestingly, a 3-fold higher PTSD rate was seen in this pilot population (29%) when compared with PTSD rates in the general female population (7-10%) and still one-third higher than the general population of women veterans (20%).45-47 Mental health, anxiety, and PTSD have been barriers to patients who sought treatment and have been implicated in poor adherence to medical advice.48,49 Cancer screening can induce anxiety in patients, and it may be amplified in patients with PTSD. It was remarkable that although adherence with screening recommendations is decreased when PTSD is present, our patient population demonstrated a higher rate of screening adherence.
Women who are seen at the VA often use multiple clinical specialties, and their EMR can be accessed across VA medical centers nationwide. Therefore, identifying women veterans who meet screening criteria is easily attainable within the VA.
When comparing high-risk with average risk women, the lifestyle results (BMI, smoking history, exercise and consumption of fruits, vegetables and alcohol) were essentially the same. Lifestyle factors were similar to national population rates and were unlikely to impact risk levels.
Limitations
Study limitations included a high number of self-referrals and the large percentage of patients with a family history of breast cancer, making them more likely to seek screening. The higher-than-average risk of breast cancer may be driven by a high rate of breast biopsies and a strong family history. Lifestyle metrics could not be accurately compared to other national assessments of lifestyle factors due to the difference in data points that we used or the format of our questions.
Conclusions
As the number of women veterans increases and the incidence of breast cancer in women veterans rise, chemoprevention options should follow national guidelines. To our knowledge, this is the only oncology study with 60% Black women veterans. This study had a higher participation rate for Black women veterans than is typically seen in national research studies and shows the VA to be a germane source for further understanding of an understudied population that may benefit from increased screening for breast cancer.
A team-based, multidisciplinary model that meets the unique healthcare needs of women veterans results in a patient-centric delivery of care for assessing breast cancer risk status and prevention options. This model can be replicated nationally by directing primary care physicians and women’s health practitioners to a risk-assessment questionnaire and referring high-risk women for appropriate preventative care. Given that these results show chemoprevention adherence rates doubled those seen nationally, perhaps techniques used within this VA pilot study may be adapted to decrease breast cancer incidence nationally.
Since the rate of PTSD among women veterans is triple the national average, we would expect adherence rates to be lower in our patient cohort. However, the multidisciplinary approach we used in this study (eg, 1:1 consultation with oncologist; genetic counseling referrals; mental health support available), may have improved adherence rates. Perhaps the high rates of PTSD seen in the VA patient population can be a useful way to explore patient adherence rates in those with mental illness and medical conditions.
Future research with a larger cohort may lead to greater insight into the correlation between PTSD and adherence to treatment. Exploring the connection between breast cancer, epigenetics, and specific military service-related exposures could be an area of analysis among this veteran population exhibiting increased breast cancer rates. VAMCs are situated in rural, suburban, and urban locations across the United States and offers a diverse socioeconomic and ethnic patient population for inclusion in clinical investigations. Women veterans make up a small subpopulation of women in the United States, but it is worth considering VA patients as an untapped resource for research collaboration.
Acknowledgements
The authors thank Steven Sanchez and Marissa Vallette, PhD, Breast Health Research Group. This research project was approved by the James J. Peters VA Medical Center Quality Executive Committee and the Washington, DC VA Medical Center Institutional Review Board. This work was supported by the US Department of Veterans Affairs. This work did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the US Government, or any of its agencies.
1. US Department of Veterans Affairs. National Center for Veterans Analysis and Statistics. The past, present and future of women veterans. Published February 2017. Accessed April 28, 2021. https://www.va.gov/vetdata/docs/specialreports/women_veterans_2015_final.pdf.
2. Frayne SM, Carney DV, Bastian L, et al. The VA Women’s Health Practice-Based Research Network: amplifying women veterans’ voices in VA research. J Gen Intern Med. 2013;28 Suppl 2(Suppl 2):S504-S509. doi:10.1007/s11606-013-2476-3
3. US Department of Veterans Affairs, Veterans Health Administration, Women’s Health Evaluation Initiative, Women Veterans Health Strategic Health Care Group. Sourcebook: women veterans in the Veterans Health Administration. Volume 1: Sociodemographic characteristics and use of VHA care. Published December 2010. Accessed April 12, 2021. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=2455
4. Bean-Mayberry B, Yano EM, Bayliss N, Navratil J, Weisman CS, Scholle SH. Federally funded comprehensive women’s health centers: leading innovation in women’s healthcare delivery. J Womens Health (Larchmt). 2007;16(9):1281-1290. doi:10.1089/jwh.2006.0284
5. US Department of Veterans Affairs. National Center for Veterans Analysis and Statistics.VA utilization profile FY 2016. Published November 2017. Accessed April 12, 2021. https://www.va.gov/vetdata/docs/QuickFacts/VA_Utilization_Profile.PDF
6. Ekenga CC, Parks CG, Sandler DP. Chemical exposures in the workplace and breast cancer risk: a prospective cohort study. Int J Cancer. 2015;137(7):1765-1774. doi:10.1002/ijc.29545
7. Rennix CP, Quinn MM, Amoroso PJ, Eisen EA, Wegman DH. Risk of breast cancer among enlisted Army women occupationally exposed to volatile organic compounds. Am J Ind Med. 2005;48(3):157-167. doi:10.1002/ajim.20201
8. Ritz B. Cancer mortality among workers exposed to chemicals during uranium processing. J Occup Environ Med. 1999;41(7):556-566. doi:10.1097/00043764-199907000-00004
9. Zhu K, Devesa SS, Wu H, et al. Cancer incidence in the U.S. military population: comparison with rates from the SEER program. Cancer Epidemiol Biomarkers Prev. 2009;18(6):1740-1745. doi:10.1158/1055-9965.EPI-09-0041
10. Freedman AN, Yu B, Gail MH, et al. Benefit/risk assessment for breast cancer chemoprevention with raloxifene or tamoxifen for women age 50 years or older [published correction appears in J Clin Oncol. 2013 Nov 10;31(32):4167]. J Clin Oncol. 2011;29(17):2327-2333. doi:10.1200/JCO.2010.33.0258
11. Greene, H. Cancer prevention, screening and early detection. In: Gobel BH, Triest-Robertson S, Vogel WH, eds. Advanced Oncology Nursing Certification Review and Resource Manual. 3rd ed. Oncology Nursing Society; 2016:1-34. https://www.ons.org/sites/default/files/publication_pdfs/2%20ADVPrac%20chapter%201.pdf
12. National Comprehensive Cancer Network. NCCN Breast Cancer Risk Reduction. Version 1.2021 NCCN Clinical Practice Guidelines in Oncology. Updated March 24, 2021 Accessed April 12, 2021. https://www.nccn.org/professionals/physician_gls/pdf/breast_risk.pdf
13. US Preventive Services Task Force. Breast cancer: Medications use to reduce risk. Updated September 3, 2019. Accessed April 12, 2021. https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/breast-cancer-medications-for-risk-reduction
14. Moyer VA; U.S. Preventive Services Task Force. Medications to decrease the risk for breast cancer in women: recommendations from the U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159(10):698-708. doi:10.7326/0003-4819-159-10-201311190-00717
15. Boucher JE. Chemoprevention: an overview of pharmacologic agents and nursing considerations. Clin J Oncol Nurs. 2018;22(3):350-353. doi:10.1188/18.CJON.350-353
16. Nichols HB, Stürmer T, Lee VS, et al. Breast cancer chemoprevention in an integrated health care setting. JCO Clin Cancer Inform. 2017;1:1-12. doi:10.1200/CCI.16.00059
17. Bevers TB, Helvie M, Bonaccio E, et al. Breast cancer screening and diagnosis, Version 3.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16(11):1362-1389. doi:10.6004/jnccn.2018.0083
18. Visvanathan K, Hurley P, Bantug E, et al. Use of pharmacologic interventions for breast cancer risk reduction: American Society of Clinical Oncology clinical practice guideline [published correction appears in J Clin Oncol. 2013 Dec 1;31(34):4383]. J Clin Oncol. 2013;31(23):2942-2962. doi:10.1200/JCO.2013.49.3122
19. Sealy-Jefferson S, Roseland ME, Cote ML, et al. rural-urban residence and stage at breast cancer diagnosis among postmenopausal women: The Women’s Health Initiative. J Womens Health (Larchmt). 2019;28(2):276-283. doi:10.1089/jwh.2017.6884
20. Holder KA. Veterans in rural America: 2011-2015. Published January 25, 2017. Accessed April 12, 2021. https://www.census.gov/library/publications/2017/acs/acs-36.html
21. Owens WL, Gallagher TJ, Kincheloe MJ, Ruetten VL. Implementation in a large health system of a program to identify women at high risk for breast cancer. J Oncol Pract. 2011;7(2):85-88. doi:10.1200/JOP.2010.000107
2. Pivot X, Viguier J, Touboul C, et al. Breast cancer screening controversy: too much or not enough?. Eur J Cancer Prev. 2015;24 Suppl:S73-S76. doi:10.1097/CEJ.0000000000000145
23. Bidassie B, Kovach A, Vallette MA, et al. Breast Cancer risk assessment and chemoprevention use among veterans affairs primary care providers: a national online survey. Mil Med. 2020;185(3-4):512-518. doi:10.1093/milmed/usz291
24. Brewster AM, Davidson NE, McCaskill-Stevens W. Chemoprevention for breast cancer: overcoming barriers to treatment. Am Soc Clin Oncol Educ Book. 2012;85-90. doi:10.14694/EdBook_AM.2012.32.152
25. Meyskens FL Jr, Curt GA, Brenner DE, et al. Regulatory approval of cancer risk-reducing (chemopreventive) drugs: moving what we have learned into the clinic. Cancer Prev Res (Phila). 2011;4(3):311-323. doi:10.1158/1940-6207.CAPR-09-0014
26. Tice JA, Kerlikowske K. Screening and prevention of breast cancer in primary care. Prim Care. 2009;36(3):533-558. doi:10.1016/j.pop.2009.04.003
27. Vogel VG. Selective estrogen receptor modulators and aromatase inhibitors for breast cancer chemoprevention. Curr Drug Targets. 2011;12(13):1874-1887. doi:10.2174/138945011798184164
28. Vogel VG, Costantino JP, Wickerham DL, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial [published correction appears in JAMA. 2006 Dec 27;296(24):2926] [published correction appears in JAMA. 2007 Sep 5;298(9):973]. JAMA. 2006;295(23):2727-2741. doi:10.1001/jama.295.23.joc60074
29. Pruthi S, Heisey RE, Bevers TB. Chemoprevention for breast cancer. Ann Surg Oncol. 2015;22(10):3230-3235. doi:10.1245/s10434-015-4715-9
30. Cuzick J, Sestak I, Forbes JF, et al. Anastrozole for prevention of breast cancer in high-risk postmenopausal women (IBIS-II): an international, double-blind, randomised placebo-controlled trial [published correction appears in Lancet. 2014 Mar 22;383(9922):1040] [published correction appears in Lancet. 2017 Mar 11;389(10073):1010]. Lancet. 2014;383(9922):1041-1048. doi:10.1016/S0140-6736(13)62292-8
31. Bozovic-Spasojevic I, Azambuja E, McCaskill-Stevens W, Dinh P, Cardoso F. Chemoprevention for breast cancer. Cancer Treat Rev. 2012;38(5):329-339. doi:10.1016/j.ctrv.2011.07.005
32. Gabriel EM, Jatoi I. Breast cancer chemoprevention. Expert Rev Anticancer Ther. 2012;12(2):223-228. doi:10.1586/era.11.206

33. Crew KD, Albain KS, Hershman DL, Unger JM, Lo SS. How do we increase uptake of tamoxifen and other anti-estrogens for breast cancer prevention?. NPJ Breast Cancer. 2017;3:20. Published 2017 May 19. doi:10.1038/s41523-017-0021-y
34. Ropka ME, Keim J, Philbrick JT. Patient decisions about breast cancer chemoprevention: a systematic review and meta-analysis. J Clin Oncol. 2010;28(18):3090-3095. doi:10.1200/JCO.2009.27.8077
35. Smith SG, Sestak I, Forster A, et al. Factors affecting uptake and adherence to breast cancer chemoprevention: a systematic review and meta-analysis. Ann Oncol. 2016;27(4):575-590. doi:10.1093/annonc/mdv590
36. Grann VR, Patel PR, Jacobson JS, et al. Comparative effectiveness of screening and prevention strategies among BRCA1/2-affected mutation carriers. Breast Cancer Res Treat. 2011 Feb;125(3):837-847. doi:10.1007/s10549-010-1043-4
37. Goss PE, Ingle JN, Alés-Martínez JE, et al. Exemestane for breast-cancer prevention in postmenopausal women [published correction appears in N Engl J Med. 2011 Oct 6;365(14):1361]. N Engl J Med. 2011;364(25):2381-2391. doi:10.1056/NEJMoa1103507
38. Kmietowicz Z. Five in six women reject drugs that could reduce their risk of breast cancer. BMJ. 2015;351:h6650. Published 2015 Dec 8. doi:10.1136/bmj.h6650
39. Nelson HD, Fu R, Griffin JC, Nygren P, Smith ME, Humphrey L. Systematic review: comparative effectiveness of medications to reduce risk for primary breast cancer. Ann Intern Med. 2009;151(10):703-235. doi:10.7326/0003-4819-151-10-200911170-00147
40. Dahabreh IJ, Wieland LS, Adam GP, Halladay C, Lau J, Trikalinos TA. Core needle and open surgery biopsy for diagnosis of breast lesions: an update to the 2009 report. Published September 2014. Accessed April 12, 2021. https://www.ncbi.nlm.nih.gov/books/NBK246878
41. National Cancer Institute. Genetics of breast and ovarian cancer (PDQ)—health profession version. Updated February 12, 2021. Accessed April 12, 2021. http://www.cancer.gov/cancertopics/pdq/genetics/breast-and-ovarian/HealthProfessional
42. US Department of Health and Human Services. National Institutes of Health, National Institute of Environmental Health Sciences The sister study. Accessed April 12, 2021. https://sisterstudy.niehs.nih.gov/english/NIEHS.htm
43. Tutt A, Ashworth A. Can genetic testing guide treatment in breast cancer?. Eur J Cancer. 2008;44(18):2774-2780. doi:10.1016/j.ejca.2008.10.009
44. Katz SJ, Ward KC, Hamilton AS, et al. Gaps in receipt of clinically indicated genetic counseling after diagnosis of breast cancer. J Clin Oncol. 2018;36(12):1218-1224. doi:10.1200/JCO.2017.76.2369
45. US Department of Veterans Affairs. PTSD: National Center for PTSD. How common is PTSD in adults? Updated October 17, 2019. Accessed April 12, 2021. https://www.ptsd.va.gov/understand/common/common_adults.asp
46. US Department of Veterans Affairs. PTSD: National Center for PTSD. How common is PTSD in women? Updated October 16, 2019. Accessed April 12, 2021. https://www.ptsd.va.gov/understand/common/common_women.asp
47. US Department of Veterans Affairs. PTSD: National Center for PTSD. How common is PTSD in veterans? Updated September 24, 2018. Accessed April 12, 2021. https://www.ptsd.va.gov/understand/common/common_veterans.asp
48. Lindberg NM, Wellisch D. Anxiety and compliance among women at high risk for breast cancer. Ann Behav Med. 2001;23(4):298-303. doi:10.1207/S15324796ABM2304_9
49. DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160(14):2101-2107. doi:10.1001/archinte.160.14.2101
50. Centers for Disease Control and Prevention. MMWR appendix: breast cancer rates among black women and white women. Updated October 13, 2016. Accessed April 12, 2021. https://www.cdc.gov/cancer/breast/statistics/trends_invasive.htm
51. Richardson LC, Henley SJ, Miller JW, Massetti G, Thomas CC. Patterns and trends in age-specific black-white differences in breast cancer incidence and mortality - United States, 1999-2014. MMWR Morb Mortal Wkly Rep. 2016;65(40):1093-1098. Published 2016 Oct 14. doi:10.15585/mmwr.mm6540a1
52. Brody JG, Moysich KB, Humblet O, Attfield KR, Beehler GP, Rudel RA. Environmental pollutants and breast cancer: epidemiologic studies. Cancer. 2007;109(12 Suppl):2667-2711. doi:10.1002/cncr.22655
53. Brophy JT, Keith MM, Watterson A, et al. Breast cancer risk in relation to occupations with exposure to carcinogens and endocrine disruptors: a Canadian case-control study. Environ Health. 2012;11:87. Published 2012 Nov 19. doi:10.1186/1476-069X-11-87
54. Labrèche F, Goldberg MS, Valois MF, Nadon L. Postmenopausal breast cancer and occupational exposures. Occup Environ Med. 2010;67(4):263-269. doi:10.1136/oem.2009.049817
55. National Institute of Environmental Health Sciences, Interagency Breast Cancer & Environmental Research Coordinating Committee. Breast cancer and the environment: prioritizing prevention. Updated March 8, 2013. Accessed April 12, 2021. https://www.niehs.nih.gov/about/boards/ibcercc/index.cfm
56. Gail MH, Costantino JP, Pee D, et al. Projecting individualized absolute invasive breast cancer risk in African American women [published correction appears in J Natl Cancer Inst. 2008 Aug 6;100(15):1118] [published correction appears in J Natl Cancer Inst. 2008 Mar 5;100(5):373]. J Natl Cancer Inst. 2007;99(23):1782-1792. doi:10.1093/jnci/djm223
57. Corbie-Smith G, Thomas SB, Williams MV, Moody-Ayers S. Attitudes and beliefs of African Americans toward participation in medical research. J Gen Intern Med. 1999;14(9):537-546. doi:10.1046/j.1525-1497.1999.07048.x
58. Braunstein JB, Sherber NS, Schulman SP, Ding EL, Powe NR. Race, medical researcher distrust, perceived harm, and willingness to participate in cardiovascular prevention trials. Medicine (Baltimore). 2008;87(1):1-9. doi:10.1097/MD.0b013e3181625d78
The number of women seeking care from the Veterans Health Administration (VHA) is increasing.1 In 2015, there were 2 million women veterans in the United States, which is 9.4% of the total veteran population. This group is expected to increase at an average of about 18,000 women per year for the next 10 years.2 The percentage of women veterans who are US Department of Veterans Affairs (VA) users aged 45 to 64 years rose 46% from 2000 to 2015.1,3-4 It is estimated that 15% of veterans who used VA services in 2020 were women.1 Nineteen percent of women veterans are Black.1 The median age of women veterans in 2015 was 50 years.5 Breast cancer is the leading cancer affecting female veterans, and data suggest they have an increased risk of breast cancer based on unique service-related exposures.1,6-9
In the US, about 10 million women are eligible for breast cancer preventive therapy, including, but not limited to, medications, surgery, or lifestyle changes.10 Secondary prevention options include change in surveillance that can reduce their risk or identify cancer at an earlier stage when treatment is more effective. The United States Preventive Services Task Force, the National Comprehensive Cancer Network, the American Society for Clinical Oncology, the National Institute for Health and Care Excellence, and the Oncology Nursing Society recommend screening women aged ≥ 35 years to assess breast cancer risk.11-18 If a woman is at increased risk, she may be a candidate for chemoprevention, prozphylactic surgery, and possibly an enhanced screening regimen.
Urban and minority women are an understudied population. Most veterans (75%) live in urban or suburban settings.19,20 Urban veteran women constitute an important potential study population.
Chemoprevention measures have been underused because of factors involving both women and their health care providers. A large proportion of women are unaware of their higher risk status due to lack of adequate screening and risk assessment.21,22 In addition to patient lack of awareness of their high-risk status, primary care physicians are also reluctant to prescribe chemopreventive agents due to a lack of comfort or familiarity with the risks and benefits.23-26 The STAR2015, BCPT2005, IBIS2014, MAP3 2011, IBIS-I 2014, and IBIS II 2014 studies clearly demonstrate a 49 to 62% reduction in risk for women using chemoprevention such as selective estrogen receptor modulators or aromatase inhibitors, respectively.27-32 Yet only 4 to 9% of high-risk women not enrolled in a clinical trial are using chemoprevention.33-39
The possibility of developing breast cancer also may be increased because of a positive family history or being a member of a family in which there is a known susceptibility gene mutation.40 Based on these risk factors, women may be eligible for tailored follow-up and genetic counseling.41-44
Nationally, 7 to 10% of the civilian US population will experience posttraumatic stress disorder (PTSD).45 The rates are remarkably higher for women veterans, with roughly 20% diagnosed with PTSD.46,47 Anxiety and PTSD have been implicated in poor adherence to medical advice.48,49
In 2014, a national VA multidisciplinary group focused on breast cancer prevention, detection, treatment, and research to address breast health in the growing population of women veterans. High-risk breast cancer screenings are not routinely carried out by the VA in primary care, women’s health, or oncology services. Furthermore, the recording of screening questionnaire results was not synchronized until a standard questionnaire was created and approved as a template by this group in the VA electronic medical record (EMR) in 2015.
Several prediction models can identify which women are at an increased risk of developing breast cancer. The most commonly used risk assessment model, the Gail breast cancer risk assessment tool (BCRAT), has been refined to include women of additional ethnicities (https://www.cancer.gov/bcrisktool).
This pilot project was launched to identify an effective manner to screen women veterans regarding their risk of developing breast cancer and refer them for chemoprevention education or genetic counseling as appropriate.
Methods
A high-risk breast cancer screening questionnaire based on the Gail BCRAT and including lifestyle questions was developed and included as a note template in the VA EMR. The James J. Peters VA Medical Center, Bronx, NY (JJPVAMC) and the Washington DC VA Medical Center (DCVAMC) ran a pilot study between 2015 and 2018 using this breast cancer screening questionnaire to collect data from women veterans. Quality Executive Committee and institutional review board approvals were granted respectively.
Eligibility criteria included women aged ≥ 35 years with no personal history of breast cancer. Most patients were self-referred, but participants also were recruited during VA Breast Cancer Awareness month events, health fairs, or at informational tables in the hospital lobbies. After completing the 20 multiple choice questionnaire with a study team member, either in person or over the phone, a 5-year and lifetime risk of invasive breast cancer was calculated using the Gail BCRAT. A woman is considered high risk and eligible for chemoprevention if her 5-year risk is > 1.66% or her lifetime risk is ≥ 20%. Eligibility for genetic counseling is based on the Breast Cancer Referral Screening Tool, which includes a personal or family history of breast or ovarian cancer and Jewish ancestry.
All patients were notified of their average or high risk status by a clinician. Those who were deemed to be average risk received a follow-up letter in the mail with instructions (eg, to follow-up with a yearly mammogram). Those who were deemed to be high risk for developing breast cancer were asked to come in for an appointment with the study principal investigator (a VA oncologist/breast cancer specialist) to discuss prevention options, further screening, or referrals to genetic counseling. Depending on a patient’s other health factors, a woman at high risk for developing breast cancer also may be a candidate for chemoprevention with tamoxifen, raloxifene, exemestane, anastrozole, or letrozole.
Data on the participant’s lifestyle, including exercise, diet, and smoking, were evaluated to determine whether these factors had an impact on risk status.
Results
The JJP and DC VAMCs screened 103 women veterans between 2015 and 2018. Four patients were excluded for nonveteran (spousal) status, leaving 99 women veterans with a mean age of 54 years. The most common self-reported races were Black (60%), non-Hispanic White (14%), and Hispanic or Latino (13%) (Table 1).
Women veterans in our study were nearly 3-times more likely than the general population were to receive a high-risk Gail Score/BCRAT (35% vs 13%, respectively).50,51 Of this subset, 46% had breast biopsies, and 86% had a positive family history. Thirty-one percent of Black women in our study were high risk, while nationally, 8.2 to 13.3% of Black women aged 50 to 59 years are considered high risk.50,51 Of the Black high-risk group with a high Gail/BCRAT score, 94% had a positive family history, and 33% had a history of breast biopsy (Table 2).
Of the 35 high-risk patients 26 (74%) patients accepted consultations for chemoprevention and 5 (19%) started chemoprevention. Of this high-risk group, 13 (37%) patients were referred for genetic counseling (Table 3).44 The prevalence of PTSD was present in 31% of high-risk women and 29% of the cohort (Figure).The lifestyle questions indicated that, among all participants, 79% had an overweight or obese body mass index; 58% exercised weekly; 51% consumed alcohol; 14% were smokers; and 21% consumed 3 to 4 servings of fruits/vegetables daily.
Discussion
Breast cancer is the most common cancer in women.52 The number of women with breast cancer in the VHA has more than tripled from 1995 to 2012.1 The lifetime risk of developing breast cancer in the general population is about 13%.50 This rate can be affected by risk factors including age, hormone exposure, family history, radiation exposure, and lifestyle factors, such as weight and alcohol use.6,52-56 In the United States, invasive breast cancer affects 1 in 8 women.50,52,57
Our screened population showed nearly 3 times as many women veterans were at an increased risk for breast cancer when compared with historical averages in US women. This difference may be based on a high rate of prior breast biopsies or positive family history, although a provocative study using the Surveillance, Epidemiology, and End Results database showed military women to have higher rates of breast cancer as well.9 Historically, Blacks are vastly understudied in clinical research with only 5% representation on a national level.5,58 The urban locations of both pilot sites (Washington, DC and Bronx, NY) allowed for the inclusion of minority patients in our study. We found that the rates of breast cancer in Black women veterans to be higher than seen nationally, possibly prompting further screening initiatives for this understudied population.
Our pilot study’s chemoprevention utilization (19%) was double the < 10% seen in the national population.33-35 The presence of a knowledgeable breast health practitioner to recruit study participants and offer personalized counseling to women veterans is a likely factor in overcoming barriers to chemopreventive acceptance. These participants may have been motivated to seek care for their high-risk status given a strong family history and prior breast biopsies.
Interestingly, a 3-fold higher PTSD rate was seen in this pilot population (29%) when compared with PTSD rates in the general female population (7-10%) and still one-third higher than the general population of women veterans (20%).45-47 Mental health, anxiety, and PTSD have been barriers to patients who sought treatment and have been implicated in poor adherence to medical advice.48,49 Cancer screening can induce anxiety in patients, and it may be amplified in patients with PTSD. It was remarkable that although adherence with screening recommendations is decreased when PTSD is present, our patient population demonstrated a higher rate of screening adherence.
Women who are seen at the VA often use multiple clinical specialties, and their EMR can be accessed across VA medical centers nationwide. Therefore, identifying women veterans who meet screening criteria is easily attainable within the VA.
When comparing high-risk with average risk women, the lifestyle results (BMI, smoking history, exercise and consumption of fruits, vegetables and alcohol) were essentially the same. Lifestyle factors were similar to national population rates and were unlikely to impact risk levels.
Limitations
Study limitations included a high number of self-referrals and the large percentage of patients with a family history of breast cancer, making them more likely to seek screening. The higher-than-average risk of breast cancer may be driven by a high rate of breast biopsies and a strong family history. Lifestyle metrics could not be accurately compared to other national assessments of lifestyle factors due to the difference in data points that we used or the format of our questions.
Conclusions
As the number of women veterans increases and the incidence of breast cancer in women veterans rise, chemoprevention options should follow national guidelines. To our knowledge, this is the only oncology study with 60% Black women veterans. This study had a higher participation rate for Black women veterans than is typically seen in national research studies and shows the VA to be a germane source for further understanding of an understudied population that may benefit from increased screening for breast cancer.
A team-based, multidisciplinary model that meets the unique healthcare needs of women veterans results in a patient-centric delivery of care for assessing breast cancer risk status and prevention options. This model can be replicated nationally by directing primary care physicians and women’s health practitioners to a risk-assessment questionnaire and referring high-risk women for appropriate preventative care. Given that these results show chemoprevention adherence rates doubled those seen nationally, perhaps techniques used within this VA pilot study may be adapted to decrease breast cancer incidence nationally.
Since the rate of PTSD among women veterans is triple the national average, we would expect adherence rates to be lower in our patient cohort. However, the multidisciplinary approach we used in this study (eg, 1:1 consultation with oncologist; genetic counseling referrals; mental health support available), may have improved adherence rates. Perhaps the high rates of PTSD seen in the VA patient population can be a useful way to explore patient adherence rates in those with mental illness and medical conditions.
Future research with a larger cohort may lead to greater insight into the correlation between PTSD and adherence to treatment. Exploring the connection between breast cancer, epigenetics, and specific military service-related exposures could be an area of analysis among this veteran population exhibiting increased breast cancer rates. VAMCs are situated in rural, suburban, and urban locations across the United States and offers a diverse socioeconomic and ethnic patient population for inclusion in clinical investigations. Women veterans make up a small subpopulation of women in the United States, but it is worth considering VA patients as an untapped resource for research collaboration.
Acknowledgements
The authors thank Steven Sanchez and Marissa Vallette, PhD, Breast Health Research Group. This research project was approved by the James J. Peters VA Medical Center Quality Executive Committee and the Washington, DC VA Medical Center Institutional Review Board. This work was supported by the US Department of Veterans Affairs. This work did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the US Government, or any of its agencies.
The number of women seeking care from the Veterans Health Administration (VHA) is increasing.1 In 2015, there were 2 million women veterans in the United States, which is 9.4% of the total veteran population. This group is expected to increase at an average of about 18,000 women per year for the next 10 years.2 The percentage of women veterans who are US Department of Veterans Affairs (VA) users aged 45 to 64 years rose 46% from 2000 to 2015.1,3-4 It is estimated that 15% of veterans who used VA services in 2020 were women.1 Nineteen percent of women veterans are Black.1 The median age of women veterans in 2015 was 50 years.5 Breast cancer is the leading cancer affecting female veterans, and data suggest they have an increased risk of breast cancer based on unique service-related exposures.1,6-9
In the US, about 10 million women are eligible for breast cancer preventive therapy, including, but not limited to, medications, surgery, or lifestyle changes.10 Secondary prevention options include change in surveillance that can reduce their risk or identify cancer at an earlier stage when treatment is more effective. The United States Preventive Services Task Force, the National Comprehensive Cancer Network, the American Society for Clinical Oncology, the National Institute for Health and Care Excellence, and the Oncology Nursing Society recommend screening women aged ≥ 35 years to assess breast cancer risk.11-18 If a woman is at increased risk, she may be a candidate for chemoprevention, prozphylactic surgery, and possibly an enhanced screening regimen.
Urban and minority women are an understudied population. Most veterans (75%) live in urban or suburban settings.19,20 Urban veteran women constitute an important potential study population.
Chemoprevention measures have been underused because of factors involving both women and their health care providers. A large proportion of women are unaware of their higher risk status due to lack of adequate screening and risk assessment.21,22 In addition to patient lack of awareness of their high-risk status, primary care physicians are also reluctant to prescribe chemopreventive agents due to a lack of comfort or familiarity with the risks and benefits.23-26 The STAR2015, BCPT2005, IBIS2014, MAP3 2011, IBIS-I 2014, and IBIS II 2014 studies clearly demonstrate a 49 to 62% reduction in risk for women using chemoprevention such as selective estrogen receptor modulators or aromatase inhibitors, respectively.27-32 Yet only 4 to 9% of high-risk women not enrolled in a clinical trial are using chemoprevention.33-39
The possibility of developing breast cancer also may be increased because of a positive family history or being a member of a family in which there is a known susceptibility gene mutation.40 Based on these risk factors, women may be eligible for tailored follow-up and genetic counseling.41-44
Nationally, 7 to 10% of the civilian US population will experience posttraumatic stress disorder (PTSD).45 The rates are remarkably higher for women veterans, with roughly 20% diagnosed with PTSD.46,47 Anxiety and PTSD have been implicated in poor adherence to medical advice.48,49
In 2014, a national VA multidisciplinary group focused on breast cancer prevention, detection, treatment, and research to address breast health in the growing population of women veterans. High-risk breast cancer screenings are not routinely carried out by the VA in primary care, women’s health, or oncology services. Furthermore, the recording of screening questionnaire results was not synchronized until a standard questionnaire was created and approved as a template by this group in the VA electronic medical record (EMR) in 2015.
Several prediction models can identify which women are at an increased risk of developing breast cancer. The most commonly used risk assessment model, the Gail breast cancer risk assessment tool (BCRAT), has been refined to include women of additional ethnicities (https://www.cancer.gov/bcrisktool).
This pilot project was launched to identify an effective manner to screen women veterans regarding their risk of developing breast cancer and refer them for chemoprevention education or genetic counseling as appropriate.
Methods
A high-risk breast cancer screening questionnaire based on the Gail BCRAT and including lifestyle questions was developed and included as a note template in the VA EMR. The James J. Peters VA Medical Center, Bronx, NY (JJPVAMC) and the Washington DC VA Medical Center (DCVAMC) ran a pilot study between 2015 and 2018 using this breast cancer screening questionnaire to collect data from women veterans. Quality Executive Committee and institutional review board approvals were granted respectively.
Eligibility criteria included women aged ≥ 35 years with no personal history of breast cancer. Most patients were self-referred, but participants also were recruited during VA Breast Cancer Awareness month events, health fairs, or at informational tables in the hospital lobbies. After completing the 20 multiple choice questionnaire with a study team member, either in person or over the phone, a 5-year and lifetime risk of invasive breast cancer was calculated using the Gail BCRAT. A woman is considered high risk and eligible for chemoprevention if her 5-year risk is > 1.66% or her lifetime risk is ≥ 20%. Eligibility for genetic counseling is based on the Breast Cancer Referral Screening Tool, which includes a personal or family history of breast or ovarian cancer and Jewish ancestry.
All patients were notified of their average or high risk status by a clinician. Those who were deemed to be average risk received a follow-up letter in the mail with instructions (eg, to follow-up with a yearly mammogram). Those who were deemed to be high risk for developing breast cancer were asked to come in for an appointment with the study principal investigator (a VA oncologist/breast cancer specialist) to discuss prevention options, further screening, or referrals to genetic counseling. Depending on a patient’s other health factors, a woman at high risk for developing breast cancer also may be a candidate for chemoprevention with tamoxifen, raloxifene, exemestane, anastrozole, or letrozole.
Data on the participant’s lifestyle, including exercise, diet, and smoking, were evaluated to determine whether these factors had an impact on risk status.
Results
The JJP and DC VAMCs screened 103 women veterans between 2015 and 2018. Four patients were excluded for nonveteran (spousal) status, leaving 99 women veterans with a mean age of 54 years. The most common self-reported races were Black (60%), non-Hispanic White (14%), and Hispanic or Latino (13%) (Table 1).
Women veterans in our study were nearly 3-times more likely than the general population were to receive a high-risk Gail Score/BCRAT (35% vs 13%, respectively).50,51 Of this subset, 46% had breast biopsies, and 86% had a positive family history. Thirty-one percent of Black women in our study were high risk, while nationally, 8.2 to 13.3% of Black women aged 50 to 59 years are considered high risk.50,51 Of the Black high-risk group with a high Gail/BCRAT score, 94% had a positive family history, and 33% had a history of breast biopsy (Table 2).
Of the 35 high-risk patients 26 (74%) patients accepted consultations for chemoprevention and 5 (19%) started chemoprevention. Of this high-risk group, 13 (37%) patients were referred for genetic counseling (Table 3).44 The prevalence of PTSD was present in 31% of high-risk women and 29% of the cohort (Figure).The lifestyle questions indicated that, among all participants, 79% had an overweight or obese body mass index; 58% exercised weekly; 51% consumed alcohol; 14% were smokers; and 21% consumed 3 to 4 servings of fruits/vegetables daily.
Discussion
Breast cancer is the most common cancer in women.52 The number of women with breast cancer in the VHA has more than tripled from 1995 to 2012.1 The lifetime risk of developing breast cancer in the general population is about 13%.50 This rate can be affected by risk factors including age, hormone exposure, family history, radiation exposure, and lifestyle factors, such as weight and alcohol use.6,52-56 In the United States, invasive breast cancer affects 1 in 8 women.50,52,57
Our screened population showed nearly 3 times as many women veterans were at an increased risk for breast cancer when compared with historical averages in US women. This difference may be based on a high rate of prior breast biopsies or positive family history, although a provocative study using the Surveillance, Epidemiology, and End Results database showed military women to have higher rates of breast cancer as well.9 Historically, Blacks are vastly understudied in clinical research with only 5% representation on a national level.5,58 The urban locations of both pilot sites (Washington, DC and Bronx, NY) allowed for the inclusion of minority patients in our study. We found that the rates of breast cancer in Black women veterans to be higher than seen nationally, possibly prompting further screening initiatives for this understudied population.
Our pilot study’s chemoprevention utilization (19%) was double the < 10% seen in the national population.33-35 The presence of a knowledgeable breast health practitioner to recruit study participants and offer personalized counseling to women veterans is a likely factor in overcoming barriers to chemopreventive acceptance. These participants may have been motivated to seek care for their high-risk status given a strong family history and prior breast biopsies.
Interestingly, a 3-fold higher PTSD rate was seen in this pilot population (29%) when compared with PTSD rates in the general female population (7-10%) and still one-third higher than the general population of women veterans (20%).45-47 Mental health, anxiety, and PTSD have been barriers to patients who sought treatment and have been implicated in poor adherence to medical advice.48,49 Cancer screening can induce anxiety in patients, and it may be amplified in patients with PTSD. It was remarkable that although adherence with screening recommendations is decreased when PTSD is present, our patient population demonstrated a higher rate of screening adherence.
Women who are seen at the VA often use multiple clinical specialties, and their EMR can be accessed across VA medical centers nationwide. Therefore, identifying women veterans who meet screening criteria is easily attainable within the VA.
When comparing high-risk with average risk women, the lifestyle results (BMI, smoking history, exercise and consumption of fruits, vegetables and alcohol) were essentially the same. Lifestyle factors were similar to national population rates and were unlikely to impact risk levels.
Limitations
Study limitations included a high number of self-referrals and the large percentage of patients with a family history of breast cancer, making them more likely to seek screening. The higher-than-average risk of breast cancer may be driven by a high rate of breast biopsies and a strong family history. Lifestyle metrics could not be accurately compared to other national assessments of lifestyle factors due to the difference in data points that we used or the format of our questions.
Conclusions
As the number of women veterans increases and the incidence of breast cancer in women veterans rise, chemoprevention options should follow national guidelines. To our knowledge, this is the only oncology study with 60% Black women veterans. This study had a higher participation rate for Black women veterans than is typically seen in national research studies and shows the VA to be a germane source for further understanding of an understudied population that may benefit from increased screening for breast cancer.
A team-based, multidisciplinary model that meets the unique healthcare needs of women veterans results in a patient-centric delivery of care for assessing breast cancer risk status and prevention options. This model can be replicated nationally by directing primary care physicians and women’s health practitioners to a risk-assessment questionnaire and referring high-risk women for appropriate preventative care. Given that these results show chemoprevention adherence rates doubled those seen nationally, perhaps techniques used within this VA pilot study may be adapted to decrease breast cancer incidence nationally.
Since the rate of PTSD among women veterans is triple the national average, we would expect adherence rates to be lower in our patient cohort. However, the multidisciplinary approach we used in this study (eg, 1:1 consultation with oncologist; genetic counseling referrals; mental health support available), may have improved adherence rates. Perhaps the high rates of PTSD seen in the VA patient population can be a useful way to explore patient adherence rates in those with mental illness and medical conditions.
Future research with a larger cohort may lead to greater insight into the correlation between PTSD and adherence to treatment. Exploring the connection between breast cancer, epigenetics, and specific military service-related exposures could be an area of analysis among this veteran population exhibiting increased breast cancer rates. VAMCs are situated in rural, suburban, and urban locations across the United States and offers a diverse socioeconomic and ethnic patient population for inclusion in clinical investigations. Women veterans make up a small subpopulation of women in the United States, but it is worth considering VA patients as an untapped resource for research collaboration.
Acknowledgements
The authors thank Steven Sanchez and Marissa Vallette, PhD, Breast Health Research Group. This research project was approved by the James J. Peters VA Medical Center Quality Executive Committee and the Washington, DC VA Medical Center Institutional Review Board. This work was supported by the US Department of Veterans Affairs. This work did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the US Government, or any of its agencies.
1. US Department of Veterans Affairs. National Center for Veterans Analysis and Statistics. The past, present and future of women veterans. Published February 2017. Accessed April 28, 2021. https://www.va.gov/vetdata/docs/specialreports/women_veterans_2015_final.pdf.
2. Frayne SM, Carney DV, Bastian L, et al. The VA Women’s Health Practice-Based Research Network: amplifying women veterans’ voices in VA research. J Gen Intern Med. 2013;28 Suppl 2(Suppl 2):S504-S509. doi:10.1007/s11606-013-2476-3
3. US Department of Veterans Affairs, Veterans Health Administration, Women’s Health Evaluation Initiative, Women Veterans Health Strategic Health Care Group. Sourcebook: women veterans in the Veterans Health Administration. Volume 1: Sociodemographic characteristics and use of VHA care. Published December 2010. Accessed April 12, 2021. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=2455
4. Bean-Mayberry B, Yano EM, Bayliss N, Navratil J, Weisman CS, Scholle SH. Federally funded comprehensive women’s health centers: leading innovation in women’s healthcare delivery. J Womens Health (Larchmt). 2007;16(9):1281-1290. doi:10.1089/jwh.2006.0284
5. US Department of Veterans Affairs. National Center for Veterans Analysis and Statistics.VA utilization profile FY 2016. Published November 2017. Accessed April 12, 2021. https://www.va.gov/vetdata/docs/QuickFacts/VA_Utilization_Profile.PDF
6. Ekenga CC, Parks CG, Sandler DP. Chemical exposures in the workplace and breast cancer risk: a prospective cohort study. Int J Cancer. 2015;137(7):1765-1774. doi:10.1002/ijc.29545
7. Rennix CP, Quinn MM, Amoroso PJ, Eisen EA, Wegman DH. Risk of breast cancer among enlisted Army women occupationally exposed to volatile organic compounds. Am J Ind Med. 2005;48(3):157-167. doi:10.1002/ajim.20201
8. Ritz B. Cancer mortality among workers exposed to chemicals during uranium processing. J Occup Environ Med. 1999;41(7):556-566. doi:10.1097/00043764-199907000-00004
9. Zhu K, Devesa SS, Wu H, et al. Cancer incidence in the U.S. military population: comparison with rates from the SEER program. Cancer Epidemiol Biomarkers Prev. 2009;18(6):1740-1745. doi:10.1158/1055-9965.EPI-09-0041
10. Freedman AN, Yu B, Gail MH, et al. Benefit/risk assessment for breast cancer chemoprevention with raloxifene or tamoxifen for women age 50 years or older [published correction appears in J Clin Oncol. 2013 Nov 10;31(32):4167]. J Clin Oncol. 2011;29(17):2327-2333. doi:10.1200/JCO.2010.33.0258
11. Greene, H. Cancer prevention, screening and early detection. In: Gobel BH, Triest-Robertson S, Vogel WH, eds. Advanced Oncology Nursing Certification Review and Resource Manual. 3rd ed. Oncology Nursing Society; 2016:1-34. https://www.ons.org/sites/default/files/publication_pdfs/2%20ADVPrac%20chapter%201.pdf
12. National Comprehensive Cancer Network. NCCN Breast Cancer Risk Reduction. Version 1.2021 NCCN Clinical Practice Guidelines in Oncology. Updated March 24, 2021 Accessed April 12, 2021. https://www.nccn.org/professionals/physician_gls/pdf/breast_risk.pdf
13. US Preventive Services Task Force. Breast cancer: Medications use to reduce risk. Updated September 3, 2019. Accessed April 12, 2021. https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/breast-cancer-medications-for-risk-reduction
14. Moyer VA; U.S. Preventive Services Task Force. Medications to decrease the risk for breast cancer in women: recommendations from the U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159(10):698-708. doi:10.7326/0003-4819-159-10-201311190-00717
15. Boucher JE. Chemoprevention: an overview of pharmacologic agents and nursing considerations. Clin J Oncol Nurs. 2018;22(3):350-353. doi:10.1188/18.CJON.350-353
16. Nichols HB, Stürmer T, Lee VS, et al. Breast cancer chemoprevention in an integrated health care setting. JCO Clin Cancer Inform. 2017;1:1-12. doi:10.1200/CCI.16.00059
17. Bevers TB, Helvie M, Bonaccio E, et al. Breast cancer screening and diagnosis, Version 3.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16(11):1362-1389. doi:10.6004/jnccn.2018.0083
18. Visvanathan K, Hurley P, Bantug E, et al. Use of pharmacologic interventions for breast cancer risk reduction: American Society of Clinical Oncology clinical practice guideline [published correction appears in J Clin Oncol. 2013 Dec 1;31(34):4383]. J Clin Oncol. 2013;31(23):2942-2962. doi:10.1200/JCO.2013.49.3122
19. Sealy-Jefferson S, Roseland ME, Cote ML, et al. rural-urban residence and stage at breast cancer diagnosis among postmenopausal women: The Women’s Health Initiative. J Womens Health (Larchmt). 2019;28(2):276-283. doi:10.1089/jwh.2017.6884
20. Holder KA. Veterans in rural America: 2011-2015. Published January 25, 2017. Accessed April 12, 2021. https://www.census.gov/library/publications/2017/acs/acs-36.html
21. Owens WL, Gallagher TJ, Kincheloe MJ, Ruetten VL. Implementation in a large health system of a program to identify women at high risk for breast cancer. J Oncol Pract. 2011;7(2):85-88. doi:10.1200/JOP.2010.000107
2. Pivot X, Viguier J, Touboul C, et al. Breast cancer screening controversy: too much or not enough?. Eur J Cancer Prev. 2015;24 Suppl:S73-S76. doi:10.1097/CEJ.0000000000000145
23. Bidassie B, Kovach A, Vallette MA, et al. Breast Cancer risk assessment and chemoprevention use among veterans affairs primary care providers: a national online survey. Mil Med. 2020;185(3-4):512-518. doi:10.1093/milmed/usz291
24. Brewster AM, Davidson NE, McCaskill-Stevens W. Chemoprevention for breast cancer: overcoming barriers to treatment. Am Soc Clin Oncol Educ Book. 2012;85-90. doi:10.14694/EdBook_AM.2012.32.152
25. Meyskens FL Jr, Curt GA, Brenner DE, et al. Regulatory approval of cancer risk-reducing (chemopreventive) drugs: moving what we have learned into the clinic. Cancer Prev Res (Phila). 2011;4(3):311-323. doi:10.1158/1940-6207.CAPR-09-0014
26. Tice JA, Kerlikowske K. Screening and prevention of breast cancer in primary care. Prim Care. 2009;36(3):533-558. doi:10.1016/j.pop.2009.04.003
27. Vogel VG. Selective estrogen receptor modulators and aromatase inhibitors for breast cancer chemoprevention. Curr Drug Targets. 2011;12(13):1874-1887. doi:10.2174/138945011798184164
28. Vogel VG, Costantino JP, Wickerham DL, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial [published correction appears in JAMA. 2006 Dec 27;296(24):2926] [published correction appears in JAMA. 2007 Sep 5;298(9):973]. JAMA. 2006;295(23):2727-2741. doi:10.1001/jama.295.23.joc60074
29. Pruthi S, Heisey RE, Bevers TB. Chemoprevention for breast cancer. Ann Surg Oncol. 2015;22(10):3230-3235. doi:10.1245/s10434-015-4715-9
30. Cuzick J, Sestak I, Forbes JF, et al. Anastrozole for prevention of breast cancer in high-risk postmenopausal women (IBIS-II): an international, double-blind, randomised placebo-controlled trial [published correction appears in Lancet. 2014 Mar 22;383(9922):1040] [published correction appears in Lancet. 2017 Mar 11;389(10073):1010]. Lancet. 2014;383(9922):1041-1048. doi:10.1016/S0140-6736(13)62292-8
31. Bozovic-Spasojevic I, Azambuja E, McCaskill-Stevens W, Dinh P, Cardoso F. Chemoprevention for breast cancer. Cancer Treat Rev. 2012;38(5):329-339. doi:10.1016/j.ctrv.2011.07.005
32. Gabriel EM, Jatoi I. Breast cancer chemoprevention. Expert Rev Anticancer Ther. 2012;12(2):223-228. doi:10.1586/era.11.206

33. Crew KD, Albain KS, Hershman DL, Unger JM, Lo SS. How do we increase uptake of tamoxifen and other anti-estrogens for breast cancer prevention?. NPJ Breast Cancer. 2017;3:20. Published 2017 May 19. doi:10.1038/s41523-017-0021-y
34. Ropka ME, Keim J, Philbrick JT. Patient decisions about breast cancer chemoprevention: a systematic review and meta-analysis. J Clin Oncol. 2010;28(18):3090-3095. doi:10.1200/JCO.2009.27.8077
35. Smith SG, Sestak I, Forster A, et al. Factors affecting uptake and adherence to breast cancer chemoprevention: a systematic review and meta-analysis. Ann Oncol. 2016;27(4):575-590. doi:10.1093/annonc/mdv590
36. Grann VR, Patel PR, Jacobson JS, et al. Comparative effectiveness of screening and prevention strategies among BRCA1/2-affected mutation carriers. Breast Cancer Res Treat. 2011 Feb;125(3):837-847. doi:10.1007/s10549-010-1043-4
37. Goss PE, Ingle JN, Alés-Martínez JE, et al. Exemestane for breast-cancer prevention in postmenopausal women [published correction appears in N Engl J Med. 2011 Oct 6;365(14):1361]. N Engl J Med. 2011;364(25):2381-2391. doi:10.1056/NEJMoa1103507
38. Kmietowicz Z. Five in six women reject drugs that could reduce their risk of breast cancer. BMJ. 2015;351:h6650. Published 2015 Dec 8. doi:10.1136/bmj.h6650
39. Nelson HD, Fu R, Griffin JC, Nygren P, Smith ME, Humphrey L. Systematic review: comparative effectiveness of medications to reduce risk for primary breast cancer. Ann Intern Med. 2009;151(10):703-235. doi:10.7326/0003-4819-151-10-200911170-00147
40. Dahabreh IJ, Wieland LS, Adam GP, Halladay C, Lau J, Trikalinos TA. Core needle and open surgery biopsy for diagnosis of breast lesions: an update to the 2009 report. Published September 2014. Accessed April 12, 2021. https://www.ncbi.nlm.nih.gov/books/NBK246878
41. National Cancer Institute. Genetics of breast and ovarian cancer (PDQ)—health profession version. Updated February 12, 2021. Accessed April 12, 2021. http://www.cancer.gov/cancertopics/pdq/genetics/breast-and-ovarian/HealthProfessional
42. US Department of Health and Human Services. National Institutes of Health, National Institute of Environmental Health Sciences The sister study. Accessed April 12, 2021. https://sisterstudy.niehs.nih.gov/english/NIEHS.htm
43. Tutt A, Ashworth A. Can genetic testing guide treatment in breast cancer?. Eur J Cancer. 2008;44(18):2774-2780. doi:10.1016/j.ejca.2008.10.009
44. Katz SJ, Ward KC, Hamilton AS, et al. Gaps in receipt of clinically indicated genetic counseling after diagnosis of breast cancer. J Clin Oncol. 2018;36(12):1218-1224. doi:10.1200/JCO.2017.76.2369
45. US Department of Veterans Affairs. PTSD: National Center for PTSD. How common is PTSD in adults? Updated October 17, 2019. Accessed April 12, 2021. https://www.ptsd.va.gov/understand/common/common_adults.asp
46. US Department of Veterans Affairs. PTSD: National Center for PTSD. How common is PTSD in women? Updated October 16, 2019. Accessed April 12, 2021. https://www.ptsd.va.gov/understand/common/common_women.asp
47. US Department of Veterans Affairs. PTSD: National Center for PTSD. How common is PTSD in veterans? Updated September 24, 2018. Accessed April 12, 2021. https://www.ptsd.va.gov/understand/common/common_veterans.asp
48. Lindberg NM, Wellisch D. Anxiety and compliance among women at high risk for breast cancer. Ann Behav Med. 2001;23(4):298-303. doi:10.1207/S15324796ABM2304_9
49. DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160(14):2101-2107. doi:10.1001/archinte.160.14.2101
50. Centers for Disease Control and Prevention. MMWR appendix: breast cancer rates among black women and white women. Updated October 13, 2016. Accessed April 12, 2021. https://www.cdc.gov/cancer/breast/statistics/trends_invasive.htm
51. Richardson LC, Henley SJ, Miller JW, Massetti G, Thomas CC. Patterns and trends in age-specific black-white differences in breast cancer incidence and mortality - United States, 1999-2014. MMWR Morb Mortal Wkly Rep. 2016;65(40):1093-1098. Published 2016 Oct 14. doi:10.15585/mmwr.mm6540a1
52. Brody JG, Moysich KB, Humblet O, Attfield KR, Beehler GP, Rudel RA. Environmental pollutants and breast cancer: epidemiologic studies. Cancer. 2007;109(12 Suppl):2667-2711. doi:10.1002/cncr.22655
53. Brophy JT, Keith MM, Watterson A, et al. Breast cancer risk in relation to occupations with exposure to carcinogens and endocrine disruptors: a Canadian case-control study. Environ Health. 2012;11:87. Published 2012 Nov 19. doi:10.1186/1476-069X-11-87
54. Labrèche F, Goldberg MS, Valois MF, Nadon L. Postmenopausal breast cancer and occupational exposures. Occup Environ Med. 2010;67(4):263-269. doi:10.1136/oem.2009.049817
55. National Institute of Environmental Health Sciences, Interagency Breast Cancer & Environmental Research Coordinating Committee. Breast cancer and the environment: prioritizing prevention. Updated March 8, 2013. Accessed April 12, 2021. https://www.niehs.nih.gov/about/boards/ibcercc/index.cfm
56. Gail MH, Costantino JP, Pee D, et al. Projecting individualized absolute invasive breast cancer risk in African American women [published correction appears in J Natl Cancer Inst. 2008 Aug 6;100(15):1118] [published correction appears in J Natl Cancer Inst. 2008 Mar 5;100(5):373]. J Natl Cancer Inst. 2007;99(23):1782-1792. doi:10.1093/jnci/djm223
57. Corbie-Smith G, Thomas SB, Williams MV, Moody-Ayers S. Attitudes and beliefs of African Americans toward participation in medical research. J Gen Intern Med. 1999;14(9):537-546. doi:10.1046/j.1525-1497.1999.07048.x
58. Braunstein JB, Sherber NS, Schulman SP, Ding EL, Powe NR. Race, medical researcher distrust, perceived harm, and willingness to participate in cardiovascular prevention trials. Medicine (Baltimore). 2008;87(1):1-9. doi:10.1097/MD.0b013e3181625d78
1. US Department of Veterans Affairs. National Center for Veterans Analysis and Statistics. The past, present and future of women veterans. Published February 2017. Accessed April 28, 2021. https://www.va.gov/vetdata/docs/specialreports/women_veterans_2015_final.pdf.
2. Frayne SM, Carney DV, Bastian L, et al. The VA Women’s Health Practice-Based Research Network: amplifying women veterans’ voices in VA research. J Gen Intern Med. 2013;28 Suppl 2(Suppl 2):S504-S509. doi:10.1007/s11606-013-2476-3
3. US Department of Veterans Affairs, Veterans Health Administration, Women’s Health Evaluation Initiative, Women Veterans Health Strategic Health Care Group. Sourcebook: women veterans in the Veterans Health Administration. Volume 1: Sociodemographic characteristics and use of VHA care. Published December 2010. Accessed April 12, 2021. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=2455
4. Bean-Mayberry B, Yano EM, Bayliss N, Navratil J, Weisman CS, Scholle SH. Federally funded comprehensive women’s health centers: leading innovation in women’s healthcare delivery. J Womens Health (Larchmt). 2007;16(9):1281-1290. doi:10.1089/jwh.2006.0284
5. US Department of Veterans Affairs. National Center for Veterans Analysis and Statistics.VA utilization profile FY 2016. Published November 2017. Accessed April 12, 2021. https://www.va.gov/vetdata/docs/QuickFacts/VA_Utilization_Profile.PDF
6. Ekenga CC, Parks CG, Sandler DP. Chemical exposures in the workplace and breast cancer risk: a prospective cohort study. Int J Cancer. 2015;137(7):1765-1774. doi:10.1002/ijc.29545
7. Rennix CP, Quinn MM, Amoroso PJ, Eisen EA, Wegman DH. Risk of breast cancer among enlisted Army women occupationally exposed to volatile organic compounds. Am J Ind Med. 2005;48(3):157-167. doi:10.1002/ajim.20201
8. Ritz B. Cancer mortality among workers exposed to chemicals during uranium processing. J Occup Environ Med. 1999;41(7):556-566. doi:10.1097/00043764-199907000-00004
9. Zhu K, Devesa SS, Wu H, et al. Cancer incidence in the U.S. military population: comparison with rates from the SEER program. Cancer Epidemiol Biomarkers Prev. 2009;18(6):1740-1745. doi:10.1158/1055-9965.EPI-09-0041
10. Freedman AN, Yu B, Gail MH, et al. Benefit/risk assessment for breast cancer chemoprevention with raloxifene or tamoxifen for women age 50 years or older [published correction appears in J Clin Oncol. 2013 Nov 10;31(32):4167]. J Clin Oncol. 2011;29(17):2327-2333. doi:10.1200/JCO.2010.33.0258
11. Greene, H. Cancer prevention, screening and early detection. In: Gobel BH, Triest-Robertson S, Vogel WH, eds. Advanced Oncology Nursing Certification Review and Resource Manual. 3rd ed. Oncology Nursing Society; 2016:1-34. https://www.ons.org/sites/default/files/publication_pdfs/2%20ADVPrac%20chapter%201.pdf
12. National Comprehensive Cancer Network. NCCN Breast Cancer Risk Reduction. Version 1.2021 NCCN Clinical Practice Guidelines in Oncology. Updated March 24, 2021 Accessed April 12, 2021. https://www.nccn.org/professionals/physician_gls/pdf/breast_risk.pdf
13. US Preventive Services Task Force. Breast cancer: Medications use to reduce risk. Updated September 3, 2019. Accessed April 12, 2021. https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/breast-cancer-medications-for-risk-reduction
14. Moyer VA; U.S. Preventive Services Task Force. Medications to decrease the risk for breast cancer in women: recommendations from the U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159(10):698-708. doi:10.7326/0003-4819-159-10-201311190-00717
15. Boucher JE. Chemoprevention: an overview of pharmacologic agents and nursing considerations. Clin J Oncol Nurs. 2018;22(3):350-353. doi:10.1188/18.CJON.350-353
16. Nichols HB, Stürmer T, Lee VS, et al. Breast cancer chemoprevention in an integrated health care setting. JCO Clin Cancer Inform. 2017;1:1-12. doi:10.1200/CCI.16.00059
17. Bevers TB, Helvie M, Bonaccio E, et al. Breast cancer screening and diagnosis, Version 3.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16(11):1362-1389. doi:10.6004/jnccn.2018.0083
18. Visvanathan K, Hurley P, Bantug E, et al. Use of pharmacologic interventions for breast cancer risk reduction: American Society of Clinical Oncology clinical practice guideline [published correction appears in J Clin Oncol. 2013 Dec 1;31(34):4383]. J Clin Oncol. 2013;31(23):2942-2962. doi:10.1200/JCO.2013.49.3122
19. Sealy-Jefferson S, Roseland ME, Cote ML, et al. rural-urban residence and stage at breast cancer diagnosis among postmenopausal women: The Women’s Health Initiative. J Womens Health (Larchmt). 2019;28(2):276-283. doi:10.1089/jwh.2017.6884
20. Holder KA. Veterans in rural America: 2011-2015. Published January 25, 2017. Accessed April 12, 2021. https://www.census.gov/library/publications/2017/acs/acs-36.html
21. Owens WL, Gallagher TJ, Kincheloe MJ, Ruetten VL. Implementation in a large health system of a program to identify women at high risk for breast cancer. J Oncol Pract. 2011;7(2):85-88. doi:10.1200/JOP.2010.000107
2. Pivot X, Viguier J, Touboul C, et al. Breast cancer screening controversy: too much or not enough?. Eur J Cancer Prev. 2015;24 Suppl:S73-S76. doi:10.1097/CEJ.0000000000000145
23. Bidassie B, Kovach A, Vallette MA, et al. Breast Cancer risk assessment and chemoprevention use among veterans affairs primary care providers: a national online survey. Mil Med. 2020;185(3-4):512-518. doi:10.1093/milmed/usz291
24. Brewster AM, Davidson NE, McCaskill-Stevens W. Chemoprevention for breast cancer: overcoming barriers to treatment. Am Soc Clin Oncol Educ Book. 2012;85-90. doi:10.14694/EdBook_AM.2012.32.152
25. Meyskens FL Jr, Curt GA, Brenner DE, et al. Regulatory approval of cancer risk-reducing (chemopreventive) drugs: moving what we have learned into the clinic. Cancer Prev Res (Phila). 2011;4(3):311-323. doi:10.1158/1940-6207.CAPR-09-0014
26. Tice JA, Kerlikowske K. Screening and prevention of breast cancer in primary care. Prim Care. 2009;36(3):533-558. doi:10.1016/j.pop.2009.04.003
27. Vogel VG. Selective estrogen receptor modulators and aromatase inhibitors for breast cancer chemoprevention. Curr Drug Targets. 2011;12(13):1874-1887. doi:10.2174/138945011798184164
28. Vogel VG, Costantino JP, Wickerham DL, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial [published correction appears in JAMA. 2006 Dec 27;296(24):2926] [published correction appears in JAMA. 2007 Sep 5;298(9):973]. JAMA. 2006;295(23):2727-2741. doi:10.1001/jama.295.23.joc60074
29. Pruthi S, Heisey RE, Bevers TB. Chemoprevention for breast cancer. Ann Surg Oncol. 2015;22(10):3230-3235. doi:10.1245/s10434-015-4715-9
30. Cuzick J, Sestak I, Forbes JF, et al. Anastrozole for prevention of breast cancer in high-risk postmenopausal women (IBIS-II): an international, double-blind, randomised placebo-controlled trial [published correction appears in Lancet. 2014 Mar 22;383(9922):1040] [published correction appears in Lancet. 2017 Mar 11;389(10073):1010]. Lancet. 2014;383(9922):1041-1048. doi:10.1016/S0140-6736(13)62292-8
31. Bozovic-Spasojevic I, Azambuja E, McCaskill-Stevens W, Dinh P, Cardoso F. Chemoprevention for breast cancer. Cancer Treat Rev. 2012;38(5):329-339. doi:10.1016/j.ctrv.2011.07.005
32. Gabriel EM, Jatoi I. Breast cancer chemoprevention. Expert Rev Anticancer Ther. 2012;12(2):223-228. doi:10.1586/era.11.206

33. Crew KD, Albain KS, Hershman DL, Unger JM, Lo SS. How do we increase uptake of tamoxifen and other anti-estrogens for breast cancer prevention?. NPJ Breast Cancer. 2017;3:20. Published 2017 May 19. doi:10.1038/s41523-017-0021-y
34. Ropka ME, Keim J, Philbrick JT. Patient decisions about breast cancer chemoprevention: a systematic review and meta-analysis. J Clin Oncol. 2010;28(18):3090-3095. doi:10.1200/JCO.2009.27.8077
35. Smith SG, Sestak I, Forster A, et al. Factors affecting uptake and adherence to breast cancer chemoprevention: a systematic review and meta-analysis. Ann Oncol. 2016;27(4):575-590. doi:10.1093/annonc/mdv590
36. Grann VR, Patel PR, Jacobson JS, et al. Comparative effectiveness of screening and prevention strategies among BRCA1/2-affected mutation carriers. Breast Cancer Res Treat. 2011 Feb;125(3):837-847. doi:10.1007/s10549-010-1043-4
37. Goss PE, Ingle JN, Alés-Martínez JE, et al. Exemestane for breast-cancer prevention in postmenopausal women [published correction appears in N Engl J Med. 2011 Oct 6;365(14):1361]. N Engl J Med. 2011;364(25):2381-2391. doi:10.1056/NEJMoa1103507
38. Kmietowicz Z. Five in six women reject drugs that could reduce their risk of breast cancer. BMJ. 2015;351:h6650. Published 2015 Dec 8. doi:10.1136/bmj.h6650
39. Nelson HD, Fu R, Griffin JC, Nygren P, Smith ME, Humphrey L. Systematic review: comparative effectiveness of medications to reduce risk for primary breast cancer. Ann Intern Med. 2009;151(10):703-235. doi:10.7326/0003-4819-151-10-200911170-00147
40. Dahabreh IJ, Wieland LS, Adam GP, Halladay C, Lau J, Trikalinos TA. Core needle and open surgery biopsy for diagnosis of breast lesions: an update to the 2009 report. Published September 2014. Accessed April 12, 2021. https://www.ncbi.nlm.nih.gov/books/NBK246878
41. National Cancer Institute. Genetics of breast and ovarian cancer (PDQ)—health profession version. Updated February 12, 2021. Accessed April 12, 2021. http://www.cancer.gov/cancertopics/pdq/genetics/breast-and-ovarian/HealthProfessional
42. US Department of Health and Human Services. National Institutes of Health, National Institute of Environmental Health Sciences The sister study. Accessed April 12, 2021. https://sisterstudy.niehs.nih.gov/english/NIEHS.htm
43. Tutt A, Ashworth A. Can genetic testing guide treatment in breast cancer?. Eur J Cancer. 2008;44(18):2774-2780. doi:10.1016/j.ejca.2008.10.009
44. Katz SJ, Ward KC, Hamilton AS, et al. Gaps in receipt of clinically indicated genetic counseling after diagnosis of breast cancer. J Clin Oncol. 2018;36(12):1218-1224. doi:10.1200/JCO.2017.76.2369
45. US Department of Veterans Affairs. PTSD: National Center for PTSD. How common is PTSD in adults? Updated October 17, 2019. Accessed April 12, 2021. https://www.ptsd.va.gov/understand/common/common_adults.asp
46. US Department of Veterans Affairs. PTSD: National Center for PTSD. How common is PTSD in women? Updated October 16, 2019. Accessed April 12, 2021. https://www.ptsd.va.gov/understand/common/common_women.asp
47. US Department of Veterans Affairs. PTSD: National Center for PTSD. How common is PTSD in veterans? Updated September 24, 2018. Accessed April 12, 2021. https://www.ptsd.va.gov/understand/common/common_veterans.asp
48. Lindberg NM, Wellisch D. Anxiety and compliance among women at high risk for breast cancer. Ann Behav Med. 2001;23(4):298-303. doi:10.1207/S15324796ABM2304_9
49. DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160(14):2101-2107. doi:10.1001/archinte.160.14.2101
50. Centers for Disease Control and Prevention. MMWR appendix: breast cancer rates among black women and white women. Updated October 13, 2016. Accessed April 12, 2021. https://www.cdc.gov/cancer/breast/statistics/trends_invasive.htm
51. Richardson LC, Henley SJ, Miller JW, Massetti G, Thomas CC. Patterns and trends in age-specific black-white differences in breast cancer incidence and mortality - United States, 1999-2014. MMWR Morb Mortal Wkly Rep. 2016;65(40):1093-1098. Published 2016 Oct 14. doi:10.15585/mmwr.mm6540a1
52. Brody JG, Moysich KB, Humblet O, Attfield KR, Beehler GP, Rudel RA. Environmental pollutants and breast cancer: epidemiologic studies. Cancer. 2007;109(12 Suppl):2667-2711. doi:10.1002/cncr.22655
53. Brophy JT, Keith MM, Watterson A, et al. Breast cancer risk in relation to occupations with exposure to carcinogens and endocrine disruptors: a Canadian case-control study. Environ Health. 2012;11:87. Published 2012 Nov 19. doi:10.1186/1476-069X-11-87
54. Labrèche F, Goldberg MS, Valois MF, Nadon L. Postmenopausal breast cancer and occupational exposures. Occup Environ Med. 2010;67(4):263-269. doi:10.1136/oem.2009.049817
55. National Institute of Environmental Health Sciences, Interagency Breast Cancer & Environmental Research Coordinating Committee. Breast cancer and the environment: prioritizing prevention. Updated March 8, 2013. Accessed April 12, 2021. https://www.niehs.nih.gov/about/boards/ibcercc/index.cfm
56. Gail MH, Costantino JP, Pee D, et al. Projecting individualized absolute invasive breast cancer risk in African American women [published correction appears in J Natl Cancer Inst. 2008 Aug 6;100(15):1118] [published correction appears in J Natl Cancer Inst. 2008 Mar 5;100(5):373]. J Natl Cancer Inst. 2007;99(23):1782-1792. doi:10.1093/jnci/djm223
57. Corbie-Smith G, Thomas SB, Williams MV, Moody-Ayers S. Attitudes and beliefs of African Americans toward participation in medical research. J Gen Intern Med. 1999;14(9):537-546. doi:10.1046/j.1525-1497.1999.07048.x
58. Braunstein JB, Sherber NS, Schulman SP, Ding EL, Powe NR. Race, medical researcher distrust, perceived harm, and willingness to participate in cardiovascular prevention trials. Medicine (Baltimore). 2008;87(1):1-9. doi:10.1097/MD.0b013e3181625d78
Breast cancer survivors have specific gynecological needs
Sexual dysfunction is a common problem among breast cancer survivors, but it’s also an issue inadequately addressed by either ob.gyns. or hematologists and oncologists, according to Erin Keyser, MD, the program director of the San Antonio Uniformed Services Health Education Consortium. Dr. Keyser discussed management of sexual dysfunction and a variety of other issues frequently faced by women who have survived breast cancer at the at the 2021 virtual meeting of the American College of Obstetricians and Gynecologists.
“Despite the fact that no specialty is better qualified to render care for this consequence of cancer treatments, many obstetrician-gynecologists feel uncomfortable or ill-equipped to address sexual pain in women affected by cancer,” Dr. Keyser quoted from a 2016 article in Obstetrics & Gynecology about the sexual health of women affected by cancer. As a breast cancer survivor herself, Dr. Keyser said hematologists and oncologists are even less equipped to discuss sexual health, “so oftentimes patients get punted between their hem-onc and their gyn,” with each telling the patient to ask the other specialist.
“There’s plenty of data in chronic health disease that maintaining sexual function for women is an indicator of the overall quality of life and that many women really don’t want to bring this up,” Dr. Keyser told attendees, so the onus is on the ob.gyn. to bring it up.
The effects of breast cancer treatment can impact women’s body image, fertility, menopause, sexual function, osteoporosis, and cardiovascular disease, but the bulk of Dr. Keyser’s talk focused on sexual health and bilateral salpingo-oophorectomy (BSO).
Lauren Streicher, MD, a clinical professor of obstetrics and gynecology at Northwestern University, Chicago, thought Dr. Keyser’s talk was useful for the general gynecologist but had some concerns about a few parts.
“She gave a very thoughtful analysis of whether someone should have their ovaries removed or not in a breast cancer diagnosis, ” Dr. Streicher said in an interview. “I would have liked to hear more about the consequences of an early menopause in women in terms of heart health, bone health, and cognitive function.”
Dr. Keyser noted that her talk pertained mostly to survivors of estrogen receptor (ER)–positive breast cancer since that population tends to struggle most with side effects of treatment. The most common medications used in this population are tamoxifen and aromatase inhibitors – such as anastrazole, letrozole, and exemestane – and these medications can affect management of different concerns.
Current guidance on ovarian removal
For women with a BRCA mutation, ACOG clinical guidance already exists regarding BSO. For other women, the complementary TEXT and SOFT trials changed the management of breast cancer treatment in premenopausal women, Dr. Keyser said.
Before these trials, postmenopausal hormone receptor–positive women began aromatase inhibitors and premenopausal HR-positive women began tamoxifen. These trials found that premenopausal women with HR-positive early breast cancer were less likely to experience recurrence when receiving adjuvant treatment with exemestane plus ovarian suppression compared to tamoxifen plus ovarian suppression. Ovarian suppression was achieved by either GnRH agonist injections, surgical removal of the ovaries, or radiation therapy to the ovaries.
The side effects of these treatments included hot flushes (92%), depression (87%), musculoskeletal symptoms (89%), vaginal dryness (52%), decreased libido (45%), dyspareunia (31%), osteoporosis (39%), insomnia (58%), and fatigue (61%). These are all quality of life concerns, Dr. Keyser said, and these findings raise questions about the consequences of long-term ovarian suppression. Findings from the Nurses’ Health Study showed that BSO before age 47.5 years resulted in lower mortality from ovarian cancer and breast cancer but was linked in women under 50 to increased all-cause mortality and mortality from coronary heart disease, lung cancer, and colorectal cancer, compared with ovarian conservation. Further, 74% of women who undergo risk-reducing BSO experience sexual dysfunction.
The bottom line, Dr. Keyser said, is that “premature removal of ovaries is not completely benign.” Her own recommendation is to follow ACOG guidance for women with BRCA mutations and, for women aged under 35 years, use ovarian suppression for 5-10 years, after which ovarian function may resume along with improved quality of life. In women aged over 40, remove ovaries since, after 5-10 years of treatment, there’s likely no benefit of retaining ovaries.
Addressing sexual health
Dyspareunia affects up to 45% of cancer survivors, Dr. Keyser said, and multiple treatment options exist for breast cancer survivors. The therapies she discussed included lubricants, moisturizers, local vaginal estrogen, DHEA, ospemifene, and CO2 laser therapy.
Though Dr. Keyser briefly touched on vaginal lubricants and moisturizers, Dr. Streicher was disappointed that Dr. Keyser did not clearly define and differentiate between lubricants and moisturizers or mention hyaluronic acid products. Dr. Streicher also disagreed with the way Dr. Keyser represented the benefits of coconut oil as a lubricant. “Oils are not condom compatible and are known to potentially increase the risk of infection, and not just from poor handwashing,” Dr. Streicher said.
Small retrospective studies support the safety of topical vaginal estrogen in breast cancer survivors, Dr. Keyser said, and the 10-mcg Vagifem tablet and vaginal estradiol ring appear to have the lowest systemic absorption. ACOG guidance recommends that women taking aromatase inhibitors who don’t respond to nonhormonal approaches may benefit from switching temporarily to tamoxifen with vaginal estrogen and then returning to aromatase inhibitors. However, Dr. Keyser said there’s plenty of data to support using vaginal estrogen in patients taking aromatase inhibitors.
“I do feel that it’s safe for patients, whether they’re on tamoxifen or aromatase inhibitors, to take vaginal estrogen,” Dr. Keyser said. “I usually stick with the estradiol vaginal ring or the estradiol tablet, and I base that on a patient’s comfort with placing and removing a ring.” She also, instead of asking the patient’s hematologist-oncologist, simply notifies them of the treatment since most hematologist-oncologists are less familiar with the data.
Another effective option is vaginal DHEA/prasterone, which can significantly improve sexual desire, arousal, pain, and overall sexual function. Although breast cancer patients were included in early studies on DHEA, Intrarosa manufacturers excluded breast cancer patients in their Food and Drug Administration application, resulting in a package stating that “use of exogenous estrogen is contraindicated in women with a known or suspected history of breast cancer” and that “Intrarosa has not been studied in women with a history of breast cancer.” While that’s true for Intrarosa specifically, DHEA has been studied in breast cancer patients, Dr. Keyser said, so she expects to see more research in this area.
Ospemifene is another option for improving vulvovaginal atrophy but cannot be taken at the same time as tamoxifen. It has similar chemopreventive effects as tamoxifen in rat studies, but it’s not as effective. It’s a reasonable option in women with refractory genitourinary syndrome of menopause (GSM) who have completed their 5-10 years of adjuvant therapy and have no history of venous thromboembolism.
Dr. Keyser said CO2 laser therapy is still being studied for treating GSM, and current data have shown benefits for dyspareunia and vaginal dryness without documented harms. There have now been randomized, controlled trials; however, since it’s not FDA approved, it’s not covered by insurance and costs approximately $5,000 for three treatments.
Dr. Streicher was glad to see Dr. Keyser’s discussion of the safety and types of local vaginal estrogen, “although she neglected to mention the 4-mcg vaginal suppository, Imvexxy, which has the lowest systemic absorption,” Dr. Streicher said. Dr. Streicher also felt the inclusion of DHEA/prasterone and ospemifene were also important, especially since the latter is “underutilized in breast cancer patients.”
The information provided on CO2 laser therapy, however, was problematic, Dr. Streicher said, given that long-term and randomized, controlled studies have now been published. Dr. Streicher also noted that two of the devices listed on the presentation slide, Thermiva and Voltiva, are radiofrequency, not laser devices.
Aside from these treatment options, the most consistent predictor of satisfying sexual experiences in women with breast cancer is the quality of their relationships, Dr. Keyser said, so couples counseling is recommended, and treatments in general are more effective with regularly sexual activity.
In discussing nonhormonal options for treating vasomotor symptoms, Dr. Keyser recommended venlafaxine, gabapentin, and low-dose paroxetine (though SSRIs and tamoxifen are contraindicated since they may reduce tamoxifen’s efficacy).
These are all off label, Dr. Streicher said it was important to note, and she would have liked to have seen a mention of the development of KNdy neurokinin disrupters along with a more in-depth discussion about which lifestyle modifications and botanicals have been shown in randomized, controlled trials to mitigate vasomotor symptoms.
Dr. Keyser wrapped up with a few additional notes and takeaways:
- The only safe reversible long-term option for contraception in HR-positive breast cancer survivors is the Paraguard IUD.
- It’s important to discuss fertility with breast cancer patients and survivors since a majority report unmet needs in this area.
- Patients taking tamoxifen need to be sure to report any vaginal spotting or bleeding since it increases risk of endometrial cancer in postmenopausal women.
- Screen for depression and anxiety.
- Ask women about sexual health and hot flashes.
- Ensure that they’re getting bone screening.
- A recommended resource is Living Beyond Breast Cancer.
Dr. Keyser had no disclosures. Dr. Streicher has consulted for Astellas Pharma and Church & Dwight, and she owns investments in InControl Medical and Sermonix Pharmaceuticals.
Sexual dysfunction is a common problem among breast cancer survivors, but it’s also an issue inadequately addressed by either ob.gyns. or hematologists and oncologists, according to Erin Keyser, MD, the program director of the San Antonio Uniformed Services Health Education Consortium. Dr. Keyser discussed management of sexual dysfunction and a variety of other issues frequently faced by women who have survived breast cancer at the at the 2021 virtual meeting of the American College of Obstetricians and Gynecologists.
“Despite the fact that no specialty is better qualified to render care for this consequence of cancer treatments, many obstetrician-gynecologists feel uncomfortable or ill-equipped to address sexual pain in women affected by cancer,” Dr. Keyser quoted from a 2016 article in Obstetrics & Gynecology about the sexual health of women affected by cancer. As a breast cancer survivor herself, Dr. Keyser said hematologists and oncologists are even less equipped to discuss sexual health, “so oftentimes patients get punted between their hem-onc and their gyn,” with each telling the patient to ask the other specialist.
“There’s plenty of data in chronic health disease that maintaining sexual function for women is an indicator of the overall quality of life and that many women really don’t want to bring this up,” Dr. Keyser told attendees, so the onus is on the ob.gyn. to bring it up.
The effects of breast cancer treatment can impact women’s body image, fertility, menopause, sexual function, osteoporosis, and cardiovascular disease, but the bulk of Dr. Keyser’s talk focused on sexual health and bilateral salpingo-oophorectomy (BSO).
Lauren Streicher, MD, a clinical professor of obstetrics and gynecology at Northwestern University, Chicago, thought Dr. Keyser’s talk was useful for the general gynecologist but had some concerns about a few parts.
“She gave a very thoughtful analysis of whether someone should have their ovaries removed or not in a breast cancer diagnosis, ” Dr. Streicher said in an interview. “I would have liked to hear more about the consequences of an early menopause in women in terms of heart health, bone health, and cognitive function.”
Dr. Keyser noted that her talk pertained mostly to survivors of estrogen receptor (ER)–positive breast cancer since that population tends to struggle most with side effects of treatment. The most common medications used in this population are tamoxifen and aromatase inhibitors – such as anastrazole, letrozole, and exemestane – and these medications can affect management of different concerns.
Current guidance on ovarian removal
For women with a BRCA mutation, ACOG clinical guidance already exists regarding BSO. For other women, the complementary TEXT and SOFT trials changed the management of breast cancer treatment in premenopausal women, Dr. Keyser said.
Before these trials, postmenopausal hormone receptor–positive women began aromatase inhibitors and premenopausal HR-positive women began tamoxifen. These trials found that premenopausal women with HR-positive early breast cancer were less likely to experience recurrence when receiving adjuvant treatment with exemestane plus ovarian suppression compared to tamoxifen plus ovarian suppression. Ovarian suppression was achieved by either GnRH agonist injections, surgical removal of the ovaries, or radiation therapy to the ovaries.
The side effects of these treatments included hot flushes (92%), depression (87%), musculoskeletal symptoms (89%), vaginal dryness (52%), decreased libido (45%), dyspareunia (31%), osteoporosis (39%), insomnia (58%), and fatigue (61%). These are all quality of life concerns, Dr. Keyser said, and these findings raise questions about the consequences of long-term ovarian suppression. Findings from the Nurses’ Health Study showed that BSO before age 47.5 years resulted in lower mortality from ovarian cancer and breast cancer but was linked in women under 50 to increased all-cause mortality and mortality from coronary heart disease, lung cancer, and colorectal cancer, compared with ovarian conservation. Further, 74% of women who undergo risk-reducing BSO experience sexual dysfunction.
The bottom line, Dr. Keyser said, is that “premature removal of ovaries is not completely benign.” Her own recommendation is to follow ACOG guidance for women with BRCA mutations and, for women aged under 35 years, use ovarian suppression for 5-10 years, after which ovarian function may resume along with improved quality of life. In women aged over 40, remove ovaries since, after 5-10 years of treatment, there’s likely no benefit of retaining ovaries.
Addressing sexual health
Dyspareunia affects up to 45% of cancer survivors, Dr. Keyser said, and multiple treatment options exist for breast cancer survivors. The therapies she discussed included lubricants, moisturizers, local vaginal estrogen, DHEA, ospemifene, and CO2 laser therapy.
Though Dr. Keyser briefly touched on vaginal lubricants and moisturizers, Dr. Streicher was disappointed that Dr. Keyser did not clearly define and differentiate between lubricants and moisturizers or mention hyaluronic acid products. Dr. Streicher also disagreed with the way Dr. Keyser represented the benefits of coconut oil as a lubricant. “Oils are not condom compatible and are known to potentially increase the risk of infection, and not just from poor handwashing,” Dr. Streicher said.
Small retrospective studies support the safety of topical vaginal estrogen in breast cancer survivors, Dr. Keyser said, and the 10-mcg Vagifem tablet and vaginal estradiol ring appear to have the lowest systemic absorption. ACOG guidance recommends that women taking aromatase inhibitors who don’t respond to nonhormonal approaches may benefit from switching temporarily to tamoxifen with vaginal estrogen and then returning to aromatase inhibitors. However, Dr. Keyser said there’s plenty of data to support using vaginal estrogen in patients taking aromatase inhibitors.
“I do feel that it’s safe for patients, whether they’re on tamoxifen or aromatase inhibitors, to take vaginal estrogen,” Dr. Keyser said. “I usually stick with the estradiol vaginal ring or the estradiol tablet, and I base that on a patient’s comfort with placing and removing a ring.” She also, instead of asking the patient’s hematologist-oncologist, simply notifies them of the treatment since most hematologist-oncologists are less familiar with the data.
Another effective option is vaginal DHEA/prasterone, which can significantly improve sexual desire, arousal, pain, and overall sexual function. Although breast cancer patients were included in early studies on DHEA, Intrarosa manufacturers excluded breast cancer patients in their Food and Drug Administration application, resulting in a package stating that “use of exogenous estrogen is contraindicated in women with a known or suspected history of breast cancer” and that “Intrarosa has not been studied in women with a history of breast cancer.” While that’s true for Intrarosa specifically, DHEA has been studied in breast cancer patients, Dr. Keyser said, so she expects to see more research in this area.
Ospemifene is another option for improving vulvovaginal atrophy but cannot be taken at the same time as tamoxifen. It has similar chemopreventive effects as tamoxifen in rat studies, but it’s not as effective. It’s a reasonable option in women with refractory genitourinary syndrome of menopause (GSM) who have completed their 5-10 years of adjuvant therapy and have no history of venous thromboembolism.
Dr. Keyser said CO2 laser therapy is still being studied for treating GSM, and current data have shown benefits for dyspareunia and vaginal dryness without documented harms. There have now been randomized, controlled trials; however, since it’s not FDA approved, it’s not covered by insurance and costs approximately $5,000 for three treatments.
Dr. Streicher was glad to see Dr. Keyser’s discussion of the safety and types of local vaginal estrogen, “although she neglected to mention the 4-mcg vaginal suppository, Imvexxy, which has the lowest systemic absorption,” Dr. Streicher said. Dr. Streicher also felt the inclusion of DHEA/prasterone and ospemifene were also important, especially since the latter is “underutilized in breast cancer patients.”
The information provided on CO2 laser therapy, however, was problematic, Dr. Streicher said, given that long-term and randomized, controlled studies have now been published. Dr. Streicher also noted that two of the devices listed on the presentation slide, Thermiva and Voltiva, are radiofrequency, not laser devices.
Aside from these treatment options, the most consistent predictor of satisfying sexual experiences in women with breast cancer is the quality of their relationships, Dr. Keyser said, so couples counseling is recommended, and treatments in general are more effective with regularly sexual activity.
In discussing nonhormonal options for treating vasomotor symptoms, Dr. Keyser recommended venlafaxine, gabapentin, and low-dose paroxetine (though SSRIs and tamoxifen are contraindicated since they may reduce tamoxifen’s efficacy).
These are all off label, Dr. Streicher said it was important to note, and she would have liked to have seen a mention of the development of KNdy neurokinin disrupters along with a more in-depth discussion about which lifestyle modifications and botanicals have been shown in randomized, controlled trials to mitigate vasomotor symptoms.
Dr. Keyser wrapped up with a few additional notes and takeaways:
- The only safe reversible long-term option for contraception in HR-positive breast cancer survivors is the Paraguard IUD.
- It’s important to discuss fertility with breast cancer patients and survivors since a majority report unmet needs in this area.
- Patients taking tamoxifen need to be sure to report any vaginal spotting or bleeding since it increases risk of endometrial cancer in postmenopausal women.
- Screen for depression and anxiety.
- Ask women about sexual health and hot flashes.
- Ensure that they’re getting bone screening.
- A recommended resource is Living Beyond Breast Cancer.
Dr. Keyser had no disclosures. Dr. Streicher has consulted for Astellas Pharma and Church & Dwight, and she owns investments in InControl Medical and Sermonix Pharmaceuticals.
Sexual dysfunction is a common problem among breast cancer survivors, but it’s also an issue inadequately addressed by either ob.gyns. or hematologists and oncologists, according to Erin Keyser, MD, the program director of the San Antonio Uniformed Services Health Education Consortium. Dr. Keyser discussed management of sexual dysfunction and a variety of other issues frequently faced by women who have survived breast cancer at the at the 2021 virtual meeting of the American College of Obstetricians and Gynecologists.
“Despite the fact that no specialty is better qualified to render care for this consequence of cancer treatments, many obstetrician-gynecologists feel uncomfortable or ill-equipped to address sexual pain in women affected by cancer,” Dr. Keyser quoted from a 2016 article in Obstetrics & Gynecology about the sexual health of women affected by cancer. As a breast cancer survivor herself, Dr. Keyser said hematologists and oncologists are even less equipped to discuss sexual health, “so oftentimes patients get punted between their hem-onc and their gyn,” with each telling the patient to ask the other specialist.
“There’s plenty of data in chronic health disease that maintaining sexual function for women is an indicator of the overall quality of life and that many women really don’t want to bring this up,” Dr. Keyser told attendees, so the onus is on the ob.gyn. to bring it up.
The effects of breast cancer treatment can impact women’s body image, fertility, menopause, sexual function, osteoporosis, and cardiovascular disease, but the bulk of Dr. Keyser’s talk focused on sexual health and bilateral salpingo-oophorectomy (BSO).
Lauren Streicher, MD, a clinical professor of obstetrics and gynecology at Northwestern University, Chicago, thought Dr. Keyser’s talk was useful for the general gynecologist but had some concerns about a few parts.
“She gave a very thoughtful analysis of whether someone should have their ovaries removed or not in a breast cancer diagnosis, ” Dr. Streicher said in an interview. “I would have liked to hear more about the consequences of an early menopause in women in terms of heart health, bone health, and cognitive function.”
Dr. Keyser noted that her talk pertained mostly to survivors of estrogen receptor (ER)–positive breast cancer since that population tends to struggle most with side effects of treatment. The most common medications used in this population are tamoxifen and aromatase inhibitors – such as anastrazole, letrozole, and exemestane – and these medications can affect management of different concerns.
Current guidance on ovarian removal
For women with a BRCA mutation, ACOG clinical guidance already exists regarding BSO. For other women, the complementary TEXT and SOFT trials changed the management of breast cancer treatment in premenopausal women, Dr. Keyser said.
Before these trials, postmenopausal hormone receptor–positive women began aromatase inhibitors and premenopausal HR-positive women began tamoxifen. These trials found that premenopausal women with HR-positive early breast cancer were less likely to experience recurrence when receiving adjuvant treatment with exemestane plus ovarian suppression compared to tamoxifen plus ovarian suppression. Ovarian suppression was achieved by either GnRH agonist injections, surgical removal of the ovaries, or radiation therapy to the ovaries.
The side effects of these treatments included hot flushes (92%), depression (87%), musculoskeletal symptoms (89%), vaginal dryness (52%), decreased libido (45%), dyspareunia (31%), osteoporosis (39%), insomnia (58%), and fatigue (61%). These are all quality of life concerns, Dr. Keyser said, and these findings raise questions about the consequences of long-term ovarian suppression. Findings from the Nurses’ Health Study showed that BSO before age 47.5 years resulted in lower mortality from ovarian cancer and breast cancer but was linked in women under 50 to increased all-cause mortality and mortality from coronary heart disease, lung cancer, and colorectal cancer, compared with ovarian conservation. Further, 74% of women who undergo risk-reducing BSO experience sexual dysfunction.
The bottom line, Dr. Keyser said, is that “premature removal of ovaries is not completely benign.” Her own recommendation is to follow ACOG guidance for women with BRCA mutations and, for women aged under 35 years, use ovarian suppression for 5-10 years, after which ovarian function may resume along with improved quality of life. In women aged over 40, remove ovaries since, after 5-10 years of treatment, there’s likely no benefit of retaining ovaries.
Addressing sexual health
Dyspareunia affects up to 45% of cancer survivors, Dr. Keyser said, and multiple treatment options exist for breast cancer survivors. The therapies she discussed included lubricants, moisturizers, local vaginal estrogen, DHEA, ospemifene, and CO2 laser therapy.
Though Dr. Keyser briefly touched on vaginal lubricants and moisturizers, Dr. Streicher was disappointed that Dr. Keyser did not clearly define and differentiate between lubricants and moisturizers or mention hyaluronic acid products. Dr. Streicher also disagreed with the way Dr. Keyser represented the benefits of coconut oil as a lubricant. “Oils are not condom compatible and are known to potentially increase the risk of infection, and not just from poor handwashing,” Dr. Streicher said.
Small retrospective studies support the safety of topical vaginal estrogen in breast cancer survivors, Dr. Keyser said, and the 10-mcg Vagifem tablet and vaginal estradiol ring appear to have the lowest systemic absorption. ACOG guidance recommends that women taking aromatase inhibitors who don’t respond to nonhormonal approaches may benefit from switching temporarily to tamoxifen with vaginal estrogen and then returning to aromatase inhibitors. However, Dr. Keyser said there’s plenty of data to support using vaginal estrogen in patients taking aromatase inhibitors.
“I do feel that it’s safe for patients, whether they’re on tamoxifen or aromatase inhibitors, to take vaginal estrogen,” Dr. Keyser said. “I usually stick with the estradiol vaginal ring or the estradiol tablet, and I base that on a patient’s comfort with placing and removing a ring.” She also, instead of asking the patient’s hematologist-oncologist, simply notifies them of the treatment since most hematologist-oncologists are less familiar with the data.
Another effective option is vaginal DHEA/prasterone, which can significantly improve sexual desire, arousal, pain, and overall sexual function. Although breast cancer patients were included in early studies on DHEA, Intrarosa manufacturers excluded breast cancer patients in their Food and Drug Administration application, resulting in a package stating that “use of exogenous estrogen is contraindicated in women with a known or suspected history of breast cancer” and that “Intrarosa has not been studied in women with a history of breast cancer.” While that’s true for Intrarosa specifically, DHEA has been studied in breast cancer patients, Dr. Keyser said, so she expects to see more research in this area.
Ospemifene is another option for improving vulvovaginal atrophy but cannot be taken at the same time as tamoxifen. It has similar chemopreventive effects as tamoxifen in rat studies, but it’s not as effective. It’s a reasonable option in women with refractory genitourinary syndrome of menopause (GSM) who have completed their 5-10 years of adjuvant therapy and have no history of venous thromboembolism.
Dr. Keyser said CO2 laser therapy is still being studied for treating GSM, and current data have shown benefits for dyspareunia and vaginal dryness without documented harms. There have now been randomized, controlled trials; however, since it’s not FDA approved, it’s not covered by insurance and costs approximately $5,000 for three treatments.
Dr. Streicher was glad to see Dr. Keyser’s discussion of the safety and types of local vaginal estrogen, “although she neglected to mention the 4-mcg vaginal suppository, Imvexxy, which has the lowest systemic absorption,” Dr. Streicher said. Dr. Streicher also felt the inclusion of DHEA/prasterone and ospemifene were also important, especially since the latter is “underutilized in breast cancer patients.”
The information provided on CO2 laser therapy, however, was problematic, Dr. Streicher said, given that long-term and randomized, controlled studies have now been published. Dr. Streicher also noted that two of the devices listed on the presentation slide, Thermiva and Voltiva, are radiofrequency, not laser devices.
Aside from these treatment options, the most consistent predictor of satisfying sexual experiences in women with breast cancer is the quality of their relationships, Dr. Keyser said, so couples counseling is recommended, and treatments in general are more effective with regularly sexual activity.
In discussing nonhormonal options for treating vasomotor symptoms, Dr. Keyser recommended venlafaxine, gabapentin, and low-dose paroxetine (though SSRIs and tamoxifen are contraindicated since they may reduce tamoxifen’s efficacy).
These are all off label, Dr. Streicher said it was important to note, and she would have liked to have seen a mention of the development of KNdy neurokinin disrupters along with a more in-depth discussion about which lifestyle modifications and botanicals have been shown in randomized, controlled trials to mitigate vasomotor symptoms.
Dr. Keyser wrapped up with a few additional notes and takeaways:
- The only safe reversible long-term option for contraception in HR-positive breast cancer survivors is the Paraguard IUD.
- It’s important to discuss fertility with breast cancer patients and survivors since a majority report unmet needs in this area.
- Patients taking tamoxifen need to be sure to report any vaginal spotting or bleeding since it increases risk of endometrial cancer in postmenopausal women.
- Screen for depression and anxiety.
- Ask women about sexual health and hot flashes.
- Ensure that they’re getting bone screening.
- A recommended resource is Living Beyond Breast Cancer.
Dr. Keyser had no disclosures. Dr. Streicher has consulted for Astellas Pharma and Church & Dwight, and she owns investments in InControl Medical and Sermonix Pharmaceuticals.
FROM ACOG 2021
Clinical Edge Journal Scan Commentary: Breast Cancer May 2021
Potential advantages of a neoadjuvant systemic therapy approach including downstaging of the primary breast tumor and axilla, as well the ability to assess tumor response which can have prognostic and adjuvant therapy implications. Samiei and colleagues performed a systematic review and meta-analysis of 33 studies (57,531 patients) in the neoadjuvant setting to assess axillary pathologic complete response (pCR) rates among clinically node-positive breast cancer of various subtypes. HR-negative/HER2-positive subtype was associated with the highest pCR rate (60%) followed by 59% for HER2-positive, 48% for triple-negative, 45% for HR+/HER2-positive, 35% for luminal B, 18% for HR+/HER2-negative, and 13% for luminal A. Achievement of axillary pCR after pre-operative chemotherapy has been associated with improvement in relapse-free survival and overall survival. Furthermore, this data stimulates consideration of less invasive axillary staging in certain patients pending chemotherapy response, and the contribution of breast cancer subtype and impact on outcomes deserves further investigation.
Chemotherapy-induced alopecia (CIA) during breast cancer treatment can affect an individual’s perception of their own appearance, body image, overall health and therefore may impact quality of life. Wang et al performed a meta-analysis including 27 studies with 2,202 participants and demonstrated a 61% effectiveness rate of scalp cooling to protect hair loss. The effectiveness rates of scalp cooling when taxanes and anthracyclines were used alone were higher compared to combination therapy (74% for taxanes, 66% for anthracyclines, and 54% for combination). A prospective study including 139 patients treated with anthracycline chemotherapy for breast cancer receiving scalp cooling found a 43% success rate (hair loss £50%). It is important to consider chemotherapy regimen, side effects (headache, dizziness, pain, nausea), resources and cost when counseling patients regarding scalp cooling. Future studies exploring ways to address these potential challenges will be beneficial to improve patient access and tolerance to scalp cooling.
Obesity is associated with increased risk of various types of cancers, and can have a detrimental effect on cancer prognosis as well as treatment response and tolerance. Potential mechanisms to explain the relationship between obesity, physical activity and breast cancer prognosis include increased levels of sex and metabolic hormones, alteration in adipokine levels, and increased inflammation, oxidative stress and angiogenesis. A retrospective cohort study including 6,481 patients with an initial non-metastatic breast cancer diagnosis, majority of whom were overweight (33.4%) or obese (33.8%), observed increasing BMI (for every 5 kg/m2 BMI increase) was associated with an increased risk of second cancer development (7%, RR=1.07; p=0.01), obesity-related cancer (13%, RR=1.13; p<0.001), second breast cancer (11%, RR=1.11; p0.01) and second ER-positive breast cancer (15%, RR1.15; p0.008). There are several ongoing clinical trials that are examining the impact of diet and weight loss interventions on breast cancer outcomes (DIANA-5, B-AHEAD3, Breast Cancer Weight Loss Study). These studies will be key to counseling and empowering patients to address potentially modifiable variables that can positively impact their health.
References:
Kalinsky K, Diamond JR, Vahdat LT, Tolaney SM, Juric D, O’Shaughnessy J, Moroose RL, Mayer IA, Abramson VG, Goldengerg DM, Sharkey RM, Maliakel P, Hong Q, Goswami T, Wegener WA, Bardia A. Sacituzumab govitecan in previously treated hormone receptor-positive/ HER2-negative metastatic breast cancer: final results from a phase I/II, single-arm, basket trial. Ann Oncol. 2020;31:1709-1718.
Mougalian SS, Hernandez M, Lei X, Lynch S, Kuerer HM, Symmans WF, Theriault RL, Fornage BD, Hsu L, Buchholz TA, Sahin AA, Hunt KK, Yang WT, Hortobagyi GN, Valero V. Ten-year outcomes of patients with breast cancer with cytologically confirmed axillary lymph node metastases and pathologic complete response after primary systemic chemotherapy. JAMA Oncol. 2016;2:508-516.
Munzone M, Bagnardi V, Campennì G, Mazzocco K, Pagan E, Tramacere A, Masiero M, Iorfida M, Mazza M, Montagna E, Cancello G, Bianco N, Palazzo A, Cardillo A, Dellapasqua S, Sangalli C, Pettini G, Pravettoni G, Colleoni M, Veronesi P. Preventing chemotherapy-induced alopecia: a prospective clinical trial on the efficacy and safety of a scalp-cooling system in early breast cancer patients treated with anthracyclines. Br J Cancer. 2019;121:325–331.
McTiernan A. Weight, physical activity and breast cancer survival. Proc Nutr Soc. 2018;77:403–411.
Potential advantages of a neoadjuvant systemic therapy approach including downstaging of the primary breast tumor and axilla, as well the ability to assess tumor response which can have prognostic and adjuvant therapy implications. Samiei and colleagues performed a systematic review and meta-analysis of 33 studies (57,531 patients) in the neoadjuvant setting to assess axillary pathologic complete response (pCR) rates among clinically node-positive breast cancer of various subtypes. HR-negative/HER2-positive subtype was associated with the highest pCR rate (60%) followed by 59% for HER2-positive, 48% for triple-negative, 45% for HR+/HER2-positive, 35% for luminal B, 18% for HR+/HER2-negative, and 13% for luminal A. Achievement of axillary pCR after pre-operative chemotherapy has been associated with improvement in relapse-free survival and overall survival. Furthermore, this data stimulates consideration of less invasive axillary staging in certain patients pending chemotherapy response, and the contribution of breast cancer subtype and impact on outcomes deserves further investigation.
Chemotherapy-induced alopecia (CIA) during breast cancer treatment can affect an individual’s perception of their own appearance, body image, overall health and therefore may impact quality of life. Wang et al performed a meta-analysis including 27 studies with 2,202 participants and demonstrated a 61% effectiveness rate of scalp cooling to protect hair loss. The effectiveness rates of scalp cooling when taxanes and anthracyclines were used alone were higher compared to combination therapy (74% for taxanes, 66% for anthracyclines, and 54% for combination). A prospective study including 139 patients treated with anthracycline chemotherapy for breast cancer receiving scalp cooling found a 43% success rate (hair loss £50%). It is important to consider chemotherapy regimen, side effects (headache, dizziness, pain, nausea), resources and cost when counseling patients regarding scalp cooling. Future studies exploring ways to address these potential challenges will be beneficial to improve patient access and tolerance to scalp cooling.
Obesity is associated with increased risk of various types of cancers, and can have a detrimental effect on cancer prognosis as well as treatment response and tolerance. Potential mechanisms to explain the relationship between obesity, physical activity and breast cancer prognosis include increased levels of sex and metabolic hormones, alteration in adipokine levels, and increased inflammation, oxidative stress and angiogenesis. A retrospective cohort study including 6,481 patients with an initial non-metastatic breast cancer diagnosis, majority of whom were overweight (33.4%) or obese (33.8%), observed increasing BMI (for every 5 kg/m2 BMI increase) was associated with an increased risk of second cancer development (7%, RR=1.07; p=0.01), obesity-related cancer (13%, RR=1.13; p<0.001), second breast cancer (11%, RR=1.11; p0.01) and second ER-positive breast cancer (15%, RR1.15; p0.008). There are several ongoing clinical trials that are examining the impact of diet and weight loss interventions on breast cancer outcomes (DIANA-5, B-AHEAD3, Breast Cancer Weight Loss Study). These studies will be key to counseling and empowering patients to address potentially modifiable variables that can positively impact their health.
References:
Kalinsky K, Diamond JR, Vahdat LT, Tolaney SM, Juric D, O’Shaughnessy J, Moroose RL, Mayer IA, Abramson VG, Goldengerg DM, Sharkey RM, Maliakel P, Hong Q, Goswami T, Wegener WA, Bardia A. Sacituzumab govitecan in previously treated hormone receptor-positive/ HER2-negative metastatic breast cancer: final results from a phase I/II, single-arm, basket trial. Ann Oncol. 2020;31:1709-1718.
Mougalian SS, Hernandez M, Lei X, Lynch S, Kuerer HM, Symmans WF, Theriault RL, Fornage BD, Hsu L, Buchholz TA, Sahin AA, Hunt KK, Yang WT, Hortobagyi GN, Valero V. Ten-year outcomes of patients with breast cancer with cytologically confirmed axillary lymph node metastases and pathologic complete response after primary systemic chemotherapy. JAMA Oncol. 2016;2:508-516.
Munzone M, Bagnardi V, Campennì G, Mazzocco K, Pagan E, Tramacere A, Masiero M, Iorfida M, Mazza M, Montagna E, Cancello G, Bianco N, Palazzo A, Cardillo A, Dellapasqua S, Sangalli C, Pettini G, Pravettoni G, Colleoni M, Veronesi P. Preventing chemotherapy-induced alopecia: a prospective clinical trial on the efficacy and safety of a scalp-cooling system in early breast cancer patients treated with anthracyclines. Br J Cancer. 2019;121:325–331.
McTiernan A. Weight, physical activity and breast cancer survival. Proc Nutr Soc. 2018;77:403–411.
Potential advantages of a neoadjuvant systemic therapy approach including downstaging of the primary breast tumor and axilla, as well the ability to assess tumor response which can have prognostic and adjuvant therapy implications. Samiei and colleagues performed a systematic review and meta-analysis of 33 studies (57,531 patients) in the neoadjuvant setting to assess axillary pathologic complete response (pCR) rates among clinically node-positive breast cancer of various subtypes. HR-negative/HER2-positive subtype was associated with the highest pCR rate (60%) followed by 59% for HER2-positive, 48% for triple-negative, 45% for HR+/HER2-positive, 35% for luminal B, 18% for HR+/HER2-negative, and 13% for luminal A. Achievement of axillary pCR after pre-operative chemotherapy has been associated with improvement in relapse-free survival and overall survival. Furthermore, this data stimulates consideration of less invasive axillary staging in certain patients pending chemotherapy response, and the contribution of breast cancer subtype and impact on outcomes deserves further investigation.
Chemotherapy-induced alopecia (CIA) during breast cancer treatment can affect an individual’s perception of their own appearance, body image, overall health and therefore may impact quality of life. Wang et al performed a meta-analysis including 27 studies with 2,202 participants and demonstrated a 61% effectiveness rate of scalp cooling to protect hair loss. The effectiveness rates of scalp cooling when taxanes and anthracyclines were used alone were higher compared to combination therapy (74% for taxanes, 66% for anthracyclines, and 54% for combination). A prospective study including 139 patients treated with anthracycline chemotherapy for breast cancer receiving scalp cooling found a 43% success rate (hair loss £50%). It is important to consider chemotherapy regimen, side effects (headache, dizziness, pain, nausea), resources and cost when counseling patients regarding scalp cooling. Future studies exploring ways to address these potential challenges will be beneficial to improve patient access and tolerance to scalp cooling.
Obesity is associated with increased risk of various types of cancers, and can have a detrimental effect on cancer prognosis as well as treatment response and tolerance. Potential mechanisms to explain the relationship between obesity, physical activity and breast cancer prognosis include increased levels of sex and metabolic hormones, alteration in adipokine levels, and increased inflammation, oxidative stress and angiogenesis. A retrospective cohort study including 6,481 patients with an initial non-metastatic breast cancer diagnosis, majority of whom were overweight (33.4%) or obese (33.8%), observed increasing BMI (for every 5 kg/m2 BMI increase) was associated with an increased risk of second cancer development (7%, RR=1.07; p=0.01), obesity-related cancer (13%, RR=1.13; p<0.001), second breast cancer (11%, RR=1.11; p0.01) and second ER-positive breast cancer (15%, RR1.15; p0.008). There are several ongoing clinical trials that are examining the impact of diet and weight loss interventions on breast cancer outcomes (DIANA-5, B-AHEAD3, Breast Cancer Weight Loss Study). These studies will be key to counseling and empowering patients to address potentially modifiable variables that can positively impact their health.
References:
Kalinsky K, Diamond JR, Vahdat LT, Tolaney SM, Juric D, O’Shaughnessy J, Moroose RL, Mayer IA, Abramson VG, Goldengerg DM, Sharkey RM, Maliakel P, Hong Q, Goswami T, Wegener WA, Bardia A. Sacituzumab govitecan in previously treated hormone receptor-positive/ HER2-negative metastatic breast cancer: final results from a phase I/II, single-arm, basket trial. Ann Oncol. 2020;31:1709-1718.
Mougalian SS, Hernandez M, Lei X, Lynch S, Kuerer HM, Symmans WF, Theriault RL, Fornage BD, Hsu L, Buchholz TA, Sahin AA, Hunt KK, Yang WT, Hortobagyi GN, Valero V. Ten-year outcomes of patients with breast cancer with cytologically confirmed axillary lymph node metastases and pathologic complete response after primary systemic chemotherapy. JAMA Oncol. 2016;2:508-516.
Munzone M, Bagnardi V, Campennì G, Mazzocco K, Pagan E, Tramacere A, Masiero M, Iorfida M, Mazza M, Montagna E, Cancello G, Bianco N, Palazzo A, Cardillo A, Dellapasqua S, Sangalli C, Pettini G, Pravettoni G, Colleoni M, Veronesi P. Preventing chemotherapy-induced alopecia: a prospective clinical trial on the efficacy and safety of a scalp-cooling system in early breast cancer patients treated with anthracyclines. Br J Cancer. 2019;121:325–331.
McTiernan A. Weight, physical activity and breast cancer survival. Proc Nutr Soc. 2018;77:403–411.
The power and promise of social media in oncology
Mark A. Lewis, MD, explained to the COSMO meeting audience how storytelling on social media can educate and engage patients, advocates, and professional colleagues – advancing knowledge, dispelling misinformation, and promoting clinical research.
Dr. Lewis, an oncologist at Intermountain Healthcare in Salt Lake City, reflected on the bifid roles of oncologists as scientists engaged in life-long learning and humanists who can internalize and appreciate the unique character and circumstances of their patients.
Patients who have serious illnesses are necessarily aggregated by statistics. However, in an essay published in 2011, Dr. Lewis noted that “each individual patient partakes in a unique, irreproducible experiment where n = 1” (J Clin Oncol. 2011 Aug 1;29[22]:3103-4).
Dr. Lewis highlighted the duality of individual data points on a survival curve as descriptors of common disease trajectories and treatment effects. However, those data points also conceal important narratives regarding the most highly valued aspects of the doctor-patient relationship and the impact of cancer treatment on patients’ lives.
In referring to the futuristic essay “Ars Brevis,” Dr. Lewis contrasted the humanism of oncology specialists in the present day with the fictional image of data-regurgitating robots programmed to maximize the efficiency of each patient encounter (J Clin Oncol. 2013 May 10;31[14]:1792-4).
Dr. Lewis reminded attendees that to practice medicine without using both “head and heart” undermines the inherent nature of medical care.
Unfortunately, that perspective may not match the public perception of oncologists. Dr. Lewis described his experience of typing “oncologists are” into an Internet search engine and seeing the auto-complete function prompt words such as “criminals,” “evil,” “murderers,” and “confused.”
Obviously, it is hard to establish a trusting patient-doctor relationship if that is the prima facie perception of the oncology specialty.
Dispelling myths and creating community via social media
A primary goal of consultation with a newly-diagnosed cancer patient is for the patient to feel that the oncologist will be there to take care of them, regardless of what the future holds.
Dr. Lewis has found that social media can potentially extend that feeling to a global community of patients, caregivers, and others seeking information relevant to a cancer diagnosis. He believes that oncologists have an opportunity to dispel myths and fears by being attentive to the real-life concerns of patients.
Dr. Lewis took advantage of this opportunity when he underwent a Whipple procedure (pancreaticoduodenectomy) for a pancreatic neuroendocrine tumor. He and the hospital’s media services staff “live-tweeted” his surgery and recovery.
With those tweets, Dr. Lewis demystified each step of a major surgical procedure. From messages he received on social media, Dr. Lewis knows he made the decision to have a Whipple procedure more acceptable to other patients.
His personal medical experience notwithstanding, Dr. Lewis acknowledged that every patient’s circumstances are unique.
Oncologists cannot possibly empathize with every circumstance. However, when they show sensitivity to personal elements of the cancer experience, they shed light on the complicated role they play in patient care and can facilitate good decision-making among patients across the globe.
Social media for professional development and patient care
The publication of his 2011 essay was gratifying for Dr. Lewis, but the finite number of comments he received thereafter illustrated the rather limited audience that traditional academic publications have and the laborious process for subsequent interaction (J Clin Oncol. 2011 Aug 1;29[22]:3103-4).
First as an observer and later as a participant on social media, Dr. Lewis appreciated that teaching points and publications can be amplified by global distribution and the potential for informal bidirectional communication.
Social media platforms enable physicians to connect with a larger audience through participative communication, in which users develop, share, and react to content (N Engl J Med. 2009 Aug 13;361[7]:649-51).
Dr. Lewis reflected on how oncologists are challenged to sort through the thousands of oncology-focused publications annually. Through social media, one can see the studies on which the experts are commenting and appreciate the nuances that contextualize the results. Focused interactions with renowned doctors, at regular intervals, require little formality.
Online journal clubs enable the sharing of ideas, opinions, multimedia resources, and references across institutional and international borders (J Gen Intern Med. 2014 Oct;29[10]:1317-8).
Social media in oncology: Accomplishments and promise
The development of broadband Internet, wireless connectivity, and social media for peer-to-peer and general communication are among the major technological advances that have transformed medical communication.
As an organization, COSMO aims to describe, understand, and improve the use of social media to increase the penetration of evidence-based guidelines and research insights into clinical practice (Future Oncol. 2017 Jun;13[15]:1281-5).
At the inaugural COSMO meeting, areas of progress since COSMO’s inception in 2015 were highlighted, including:
- The involvement of cancer professionals and advocates in multiple distinctive platforms.
- The development of hashtag libraries to aggregate interest groups and topics.
- The refinement of strategies for engaging advocates with attention to inclusiveness.
- A steady trajectory of growth in tweeting at scientific conferences.
An overarching theme of the COSMO meeting was “authenticity,” a virtue that is easy to admire but requires conscious, consistent effort to achieve.
Disclosure of conflicts of interest and avoiding using social media simply as a recruitment tool for clinical trials are basic components of accurate self-representation.
In addition, Dr. Lewis advocated for sharing personal experiences in a component of social media posts so oncologists can show humanity as a feature of their professional online identity and inherent nature.
Dr. Lewis disclosed consultancy with Medscape/WebMD, which are owned by the same parent company as MDedge. He also disclosed relationships with Foundation Medicine, Natera, Exelixis, QED, HalioDX, and Ipsen.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
Mark A. Lewis, MD, explained to the COSMO meeting audience how storytelling on social media can educate and engage patients, advocates, and professional colleagues – advancing knowledge, dispelling misinformation, and promoting clinical research.
Dr. Lewis, an oncologist at Intermountain Healthcare in Salt Lake City, reflected on the bifid roles of oncologists as scientists engaged in life-long learning and humanists who can internalize and appreciate the unique character and circumstances of their patients.
Patients who have serious illnesses are necessarily aggregated by statistics. However, in an essay published in 2011, Dr. Lewis noted that “each individual patient partakes in a unique, irreproducible experiment where n = 1” (J Clin Oncol. 2011 Aug 1;29[22]:3103-4).
Dr. Lewis highlighted the duality of individual data points on a survival curve as descriptors of common disease trajectories and treatment effects. However, those data points also conceal important narratives regarding the most highly valued aspects of the doctor-patient relationship and the impact of cancer treatment on patients’ lives.
In referring to the futuristic essay “Ars Brevis,” Dr. Lewis contrasted the humanism of oncology specialists in the present day with the fictional image of data-regurgitating robots programmed to maximize the efficiency of each patient encounter (J Clin Oncol. 2013 May 10;31[14]:1792-4).
Dr. Lewis reminded attendees that to practice medicine without using both “head and heart” undermines the inherent nature of medical care.
Unfortunately, that perspective may not match the public perception of oncologists. Dr. Lewis described his experience of typing “oncologists are” into an Internet search engine and seeing the auto-complete function prompt words such as “criminals,” “evil,” “murderers,” and “confused.”
Obviously, it is hard to establish a trusting patient-doctor relationship if that is the prima facie perception of the oncology specialty.
Dispelling myths and creating community via social media
A primary goal of consultation with a newly-diagnosed cancer patient is for the patient to feel that the oncologist will be there to take care of them, regardless of what the future holds.
Dr. Lewis has found that social media can potentially extend that feeling to a global community of patients, caregivers, and others seeking information relevant to a cancer diagnosis. He believes that oncologists have an opportunity to dispel myths and fears by being attentive to the real-life concerns of patients.
Dr. Lewis took advantage of this opportunity when he underwent a Whipple procedure (pancreaticoduodenectomy) for a pancreatic neuroendocrine tumor. He and the hospital’s media services staff “live-tweeted” his surgery and recovery.
With those tweets, Dr. Lewis demystified each step of a major surgical procedure. From messages he received on social media, Dr. Lewis knows he made the decision to have a Whipple procedure more acceptable to other patients.
His personal medical experience notwithstanding, Dr. Lewis acknowledged that every patient’s circumstances are unique.
Oncologists cannot possibly empathize with every circumstance. However, when they show sensitivity to personal elements of the cancer experience, they shed light on the complicated role they play in patient care and can facilitate good decision-making among patients across the globe.
Social media for professional development and patient care
The publication of his 2011 essay was gratifying for Dr. Lewis, but the finite number of comments he received thereafter illustrated the rather limited audience that traditional academic publications have and the laborious process for subsequent interaction (J Clin Oncol. 2011 Aug 1;29[22]:3103-4).
First as an observer and later as a participant on social media, Dr. Lewis appreciated that teaching points and publications can be amplified by global distribution and the potential for informal bidirectional communication.
Social media platforms enable physicians to connect with a larger audience through participative communication, in which users develop, share, and react to content (N Engl J Med. 2009 Aug 13;361[7]:649-51).
Dr. Lewis reflected on how oncologists are challenged to sort through the thousands of oncology-focused publications annually. Through social media, one can see the studies on which the experts are commenting and appreciate the nuances that contextualize the results. Focused interactions with renowned doctors, at regular intervals, require little formality.
Online journal clubs enable the sharing of ideas, opinions, multimedia resources, and references across institutional and international borders (J Gen Intern Med. 2014 Oct;29[10]:1317-8).
Social media in oncology: Accomplishments and promise
The development of broadband Internet, wireless connectivity, and social media for peer-to-peer and general communication are among the major technological advances that have transformed medical communication.
As an organization, COSMO aims to describe, understand, and improve the use of social media to increase the penetration of evidence-based guidelines and research insights into clinical practice (Future Oncol. 2017 Jun;13[15]:1281-5).
At the inaugural COSMO meeting, areas of progress since COSMO’s inception in 2015 were highlighted, including:
- The involvement of cancer professionals and advocates in multiple distinctive platforms.
- The development of hashtag libraries to aggregate interest groups and topics.
- The refinement of strategies for engaging advocates with attention to inclusiveness.
- A steady trajectory of growth in tweeting at scientific conferences.
An overarching theme of the COSMO meeting was “authenticity,” a virtue that is easy to admire but requires conscious, consistent effort to achieve.
Disclosure of conflicts of interest and avoiding using social media simply as a recruitment tool for clinical trials are basic components of accurate self-representation.
In addition, Dr. Lewis advocated for sharing personal experiences in a component of social media posts so oncologists can show humanity as a feature of their professional online identity and inherent nature.
Dr. Lewis disclosed consultancy with Medscape/WebMD, which are owned by the same parent company as MDedge. He also disclosed relationships with Foundation Medicine, Natera, Exelixis, QED, HalioDX, and Ipsen.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
Mark A. Lewis, MD, explained to the COSMO meeting audience how storytelling on social media can educate and engage patients, advocates, and professional colleagues – advancing knowledge, dispelling misinformation, and promoting clinical research.
Dr. Lewis, an oncologist at Intermountain Healthcare in Salt Lake City, reflected on the bifid roles of oncologists as scientists engaged in life-long learning and humanists who can internalize and appreciate the unique character and circumstances of their patients.
Patients who have serious illnesses are necessarily aggregated by statistics. However, in an essay published in 2011, Dr. Lewis noted that “each individual patient partakes in a unique, irreproducible experiment where n = 1” (J Clin Oncol. 2011 Aug 1;29[22]:3103-4).
Dr. Lewis highlighted the duality of individual data points on a survival curve as descriptors of common disease trajectories and treatment effects. However, those data points also conceal important narratives regarding the most highly valued aspects of the doctor-patient relationship and the impact of cancer treatment on patients’ lives.
In referring to the futuristic essay “Ars Brevis,” Dr. Lewis contrasted the humanism of oncology specialists in the present day with the fictional image of data-regurgitating robots programmed to maximize the efficiency of each patient encounter (J Clin Oncol. 2013 May 10;31[14]:1792-4).
Dr. Lewis reminded attendees that to practice medicine without using both “head and heart” undermines the inherent nature of medical care.
Unfortunately, that perspective may not match the public perception of oncologists. Dr. Lewis described his experience of typing “oncologists are” into an Internet search engine and seeing the auto-complete function prompt words such as “criminals,” “evil,” “murderers,” and “confused.”
Obviously, it is hard to establish a trusting patient-doctor relationship if that is the prima facie perception of the oncology specialty.
Dispelling myths and creating community via social media
A primary goal of consultation with a newly-diagnosed cancer patient is for the patient to feel that the oncologist will be there to take care of them, regardless of what the future holds.
Dr. Lewis has found that social media can potentially extend that feeling to a global community of patients, caregivers, and others seeking information relevant to a cancer diagnosis. He believes that oncologists have an opportunity to dispel myths and fears by being attentive to the real-life concerns of patients.
Dr. Lewis took advantage of this opportunity when he underwent a Whipple procedure (pancreaticoduodenectomy) for a pancreatic neuroendocrine tumor. He and the hospital’s media services staff “live-tweeted” his surgery and recovery.
With those tweets, Dr. Lewis demystified each step of a major surgical procedure. From messages he received on social media, Dr. Lewis knows he made the decision to have a Whipple procedure more acceptable to other patients.
His personal medical experience notwithstanding, Dr. Lewis acknowledged that every patient’s circumstances are unique.
Oncologists cannot possibly empathize with every circumstance. However, when they show sensitivity to personal elements of the cancer experience, they shed light on the complicated role they play in patient care and can facilitate good decision-making among patients across the globe.
Social media for professional development and patient care
The publication of his 2011 essay was gratifying for Dr. Lewis, but the finite number of comments he received thereafter illustrated the rather limited audience that traditional academic publications have and the laborious process for subsequent interaction (J Clin Oncol. 2011 Aug 1;29[22]:3103-4).
First as an observer and later as a participant on social media, Dr. Lewis appreciated that teaching points and publications can be amplified by global distribution and the potential for informal bidirectional communication.
Social media platforms enable physicians to connect with a larger audience through participative communication, in which users develop, share, and react to content (N Engl J Med. 2009 Aug 13;361[7]:649-51).
Dr. Lewis reflected on how oncologists are challenged to sort through the thousands of oncology-focused publications annually. Through social media, one can see the studies on which the experts are commenting and appreciate the nuances that contextualize the results. Focused interactions with renowned doctors, at regular intervals, require little formality.
Online journal clubs enable the sharing of ideas, opinions, multimedia resources, and references across institutional and international borders (J Gen Intern Med. 2014 Oct;29[10]:1317-8).
Social media in oncology: Accomplishments and promise
The development of broadband Internet, wireless connectivity, and social media for peer-to-peer and general communication are among the major technological advances that have transformed medical communication.
As an organization, COSMO aims to describe, understand, and improve the use of social media to increase the penetration of evidence-based guidelines and research insights into clinical practice (Future Oncol. 2017 Jun;13[15]:1281-5).
At the inaugural COSMO meeting, areas of progress since COSMO’s inception in 2015 were highlighted, including:
- The involvement of cancer professionals and advocates in multiple distinctive platforms.
- The development of hashtag libraries to aggregate interest groups and topics.
- The refinement of strategies for engaging advocates with attention to inclusiveness.
- A steady trajectory of growth in tweeting at scientific conferences.
An overarching theme of the COSMO meeting was “authenticity,” a virtue that is easy to admire but requires conscious, consistent effort to achieve.
Disclosure of conflicts of interest and avoiding using social media simply as a recruitment tool for clinical trials are basic components of accurate self-representation.
In addition, Dr. Lewis advocated for sharing personal experiences in a component of social media posts so oncologists can show humanity as a feature of their professional online identity and inherent nature.
Dr. Lewis disclosed consultancy with Medscape/WebMD, which are owned by the same parent company as MDedge. He also disclosed relationships with Foundation Medicine, Natera, Exelixis, QED, HalioDX, and Ipsen.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
FROM COSMO 2021
Hyperprogression on immunotherapy: When outcomes are much worse
Immunotherapy with checkpoint inhibitors has ushered in a new era of cancer therapy, with some patients showing dramatic responses and significantly better outcomes than with other therapies across many cancer types. But some patients do worse, sometimes much worse.
A subset of patients who undergo immunotherapy experience unexpected, rapid disease progression, with a dramatic acceleration of disease trajectory. They also have a shorter progression-free survival and overall survival than would have been expected.
This has been described as hyperprogression and has been termed “hyperprogressive disease” (HPD). It has been seen in a variety of cancers; the incidence ranges from 4% to 29% in the studies reported to date.
There has been some debate over whether this is a real phenomenon or whether it is part of the natural course of disease.
HPD is a “provocative phenomenon,” wrote the authors of a recent commentary entitled “Hyperprogression and Immunotherapy: Fact, Fiction, or Alternative Fact?”
“This phenomenon has polarized oncologists who debate that this could still reflect the natural history of the disease,” said the author of another commentary.
But the tide is now turning toward acceptance of HPD, said Kartik Sehgal, MD, an oncologist at Dana-Farber Cancer Institute and Harvard University, both in Boston.
“With publication of multiple clinical reports of different cancer types worldwide, hyperprogression is now accepted by most oncologists to be a true phenomenon rather than natural progression of disease,” Dr. Sehgal said.
He authored an invited commentary in JAMA Network Openabout one of the latest meta-analyses (JAMA Netw Open. 2021;4[3]:e211136) to investigate HPD during immunotherapy. One of the biggest issues is that the studies that have reported on HPD have been retrospective, with a lack of comparator groups and a lack of a standardized definition of hyperprogression. Dr. Sehgal emphasized the need to study hyperprogression in well-designed prospective studies.
Existing data on HPD
HPD was described as “a new pattern of progression” seen in patients undergoing immune checkpoint inhibitor therapy in a 2017 article published in Clinical Cancer Research. Authors Stephane Champiat, MD, PhD, of Institut Gustave Roussy, Universite Paris Saclay, Villejuif, France, and colleagues cited “anecdotal occurrences” of HPD among patients in phase 1 trials of anti–PD-1/PD-L1 agents.
In that study, HPD was defined by tumor growth rate ratio. The incidence was 9% among 213 patients.
The findings raised concerns about treating elderly patients with anti–PD-1/PD-L1 monotherapy, according to the authors, who called for further study.
That same year, Roberto Ferrara, MD, and colleagues from the Insitut Gustave Roussy reported additional data indicating an incidence of HPD of 16% among 333 patients with non–small cell lung cancer who underwent immunotherapy at eight centers from 2012 to 2017. The findings, which were presented at the 2017 World Conference on Lung Cancer and reported at the time by this news organization, also showed that the incidence of HPD was higher with immunotherapy than with single-agent chemotherapy (5%).
Median overall survival (OS) was just 3.4 months among those with HPD, compared with 13 months in the overall study population – worse, even, than the median 5.4-month OS observed among patients with progressive disease who received immunotherapy.
In the wake of these findings, numerous researchers have attempted to better define HPD, its incidence, and patient factors associated with developing HPD while undergoing immunotherapy.
However, there is little so far to show for those efforts, Vivek Subbiah, MD, of the University of Texas MD Anderson Cancer Center, Houston, said in an interview.
“Many questions remain to be answered,” said Dr. Subbiah, clinical medical director of the Clinical Center for Targeted Therapy in the division of cancer medicine at MD Anderson. He was the senior author of the “Fact, Fiction, or Alternative Fact?” commentary.
Work is underway to elucidate biological mechanisms. Some groups have implicated the Fc region of antibodies. Another group has reported EGFR and MDM2/MDM4 amplifications in patients with HPD, Dr. Subbiah and colleagues noted.
Other “proposed contributing pathological mechanisms include modulation of tumor immune microenvironment through macrophages and regulatory T cells as well as activation of oncogenic signaling pathways,” noted Dr. Sehgal.
Both groups of authors emphasize the urgent need for prospective studies.
It is imperative to confirm underlying biology, predict which patients are at risk, and identify therapeutic directions for patients who experience HPD, Dr. Subbiah said.
The main challenge is defining HPD, he added. Definitions that have been proposed include tumor growth at least two times greater than in control persons, a 15% increase in tumor burden in a set period, and disease progression of 50% from the first evaluation before treatment, he said.
The recent meta-analysis by Hyo Jung Park, MD, PhD, and colleagues, which Dr. Sehgal addressed in his invited commentary, highlights the many approaches used for defining HPD.
Depending on the definition used, the incidence of HPD across 24 studies involving more than 3,100 patients ranged from 5.9% to 43.1%.
“Hyperprogressive disease could be overestimated or underestimated based on current assessment,” Dr. Park and colleagues concluded. They highlighted the importance of “establishing uniform and clinically relevant criteria based on currently available evidence.”
Steps for solving the HPD mystery
“I think we need to come up with consensus criteria for an HPD definition. We need a unified definition,” Dr. Subbiah said. “We also need to design prospective studies to prove or disprove the immunotherapy-HPD association.”
Prospective registries with independent review of patients with suspected immunotherapy-related HPD would be useful for assessing the true incidence and the biology of HPD among patients undergoing immunotherapy, he suggested.
“We need to know the immunologic signals of HPD. This can give us an idea if patients can be prospectively identified for being at risk,” he said. “We also need to know what to do if they are at risk.”
Dr. Sehgal also called for consensus on an HPD definition, with input from a multidisciplinary group that includes “colleagues from radiology, medical oncology, radiation oncology. Getting expertise from different disciplines would be helpful,” he said.
Dr. Park and colleagues suggested several key requirements for an optimal HP definition, such as the inclusion of multiple variables for measuring tumor growth acceleration, “sufficiently quantitative” criteria for determining time to failure, and establishment of a standardized measure of tumor growth acceleration.
The agreed-upon definition of HPD could be applied to patients in a prospective registry and to existing trial data, Dr. Sehgal said.
“Eventually, the goal of this exercise is to [determine] how we can help our patients the best, having a biomarker that can at least inform us in terms of being aware and being proactive in terms of looking for this ... so that interventions can be brought on earlier,” he said.
“If we know what may be a biological mechanism, we can design trials that are designed to look at how to overcome that HPD,” he said.
Dr. Sehgal said he believes HPD is triggered in some way by treatment, including immunotherapy, chemotherapy, and targeted therapy, but perhaps in different ways for each.
He estimated the true incidence of immunotherapy-related HPD will be in the 9%-10% range.
“This is a substantial number of patients, so it’s important that we try to understand this phenomenon, using, again, uniform criteria,” he said.
Current treatment decision-making
Until more is known, Dr. Sehgal said he considers the potential risk factors when treating patients with immunotherapy.
For example, the presence of MDM2 or MDM4 amplification on a genomic profile may factor into his treatment decision-making when it comes to using immunotherapy or immunotherapy in combination with chemotherapy, he said.
“Is that the only factor that is going to make me choose one thing or another? No,” Dr. Sehgal said. However, he said it would make him more “proactive in making sure the patient is doing clinically okay” and in determining when to obtain on-treatment imaging studies.
Dr. Subbiah emphasized the relative benefit of immunotherapy, noting that survival with chemotherapy for many difficult-to-treat cancers in the relapsed/refractory metastatic setting is less than 2 years.
Immunotherapy with checkpoint inhibitors has allowed some of these patients to live longer (with survival reported to be more than 10 years for patients with metastatic melanoma).
“Immunotherapy has been a game changer; it has been transformative in the lives of these patients,” Dr. Subbiah said. “So unless there is any other contraindication, the benefit of receiving immunotherapy for an approved indication far outweighs the risk of HPD.”
A version of this article first appeared on Medscape.com.
Immunotherapy with checkpoint inhibitors has ushered in a new era of cancer therapy, with some patients showing dramatic responses and significantly better outcomes than with other therapies across many cancer types. But some patients do worse, sometimes much worse.
A subset of patients who undergo immunotherapy experience unexpected, rapid disease progression, with a dramatic acceleration of disease trajectory. They also have a shorter progression-free survival and overall survival than would have been expected.
This has been described as hyperprogression and has been termed “hyperprogressive disease” (HPD). It has been seen in a variety of cancers; the incidence ranges from 4% to 29% in the studies reported to date.
There has been some debate over whether this is a real phenomenon or whether it is part of the natural course of disease.
HPD is a “provocative phenomenon,” wrote the authors of a recent commentary entitled “Hyperprogression and Immunotherapy: Fact, Fiction, or Alternative Fact?”
“This phenomenon has polarized oncologists who debate that this could still reflect the natural history of the disease,” said the author of another commentary.
But the tide is now turning toward acceptance of HPD, said Kartik Sehgal, MD, an oncologist at Dana-Farber Cancer Institute and Harvard University, both in Boston.
“With publication of multiple clinical reports of different cancer types worldwide, hyperprogression is now accepted by most oncologists to be a true phenomenon rather than natural progression of disease,” Dr. Sehgal said.
He authored an invited commentary in JAMA Network Openabout one of the latest meta-analyses (JAMA Netw Open. 2021;4[3]:e211136) to investigate HPD during immunotherapy. One of the biggest issues is that the studies that have reported on HPD have been retrospective, with a lack of comparator groups and a lack of a standardized definition of hyperprogression. Dr. Sehgal emphasized the need to study hyperprogression in well-designed prospective studies.
Existing data on HPD
HPD was described as “a new pattern of progression” seen in patients undergoing immune checkpoint inhibitor therapy in a 2017 article published in Clinical Cancer Research. Authors Stephane Champiat, MD, PhD, of Institut Gustave Roussy, Universite Paris Saclay, Villejuif, France, and colleagues cited “anecdotal occurrences” of HPD among patients in phase 1 trials of anti–PD-1/PD-L1 agents.
In that study, HPD was defined by tumor growth rate ratio. The incidence was 9% among 213 patients.
The findings raised concerns about treating elderly patients with anti–PD-1/PD-L1 monotherapy, according to the authors, who called for further study.
That same year, Roberto Ferrara, MD, and colleagues from the Insitut Gustave Roussy reported additional data indicating an incidence of HPD of 16% among 333 patients with non–small cell lung cancer who underwent immunotherapy at eight centers from 2012 to 2017. The findings, which were presented at the 2017 World Conference on Lung Cancer and reported at the time by this news organization, also showed that the incidence of HPD was higher with immunotherapy than with single-agent chemotherapy (5%).
Median overall survival (OS) was just 3.4 months among those with HPD, compared with 13 months in the overall study population – worse, even, than the median 5.4-month OS observed among patients with progressive disease who received immunotherapy.
In the wake of these findings, numerous researchers have attempted to better define HPD, its incidence, and patient factors associated with developing HPD while undergoing immunotherapy.
However, there is little so far to show for those efforts, Vivek Subbiah, MD, of the University of Texas MD Anderson Cancer Center, Houston, said in an interview.
“Many questions remain to be answered,” said Dr. Subbiah, clinical medical director of the Clinical Center for Targeted Therapy in the division of cancer medicine at MD Anderson. He was the senior author of the “Fact, Fiction, or Alternative Fact?” commentary.
Work is underway to elucidate biological mechanisms. Some groups have implicated the Fc region of antibodies. Another group has reported EGFR and MDM2/MDM4 amplifications in patients with HPD, Dr. Subbiah and colleagues noted.
Other “proposed contributing pathological mechanisms include modulation of tumor immune microenvironment through macrophages and regulatory T cells as well as activation of oncogenic signaling pathways,” noted Dr. Sehgal.
Both groups of authors emphasize the urgent need for prospective studies.
It is imperative to confirm underlying biology, predict which patients are at risk, and identify therapeutic directions for patients who experience HPD, Dr. Subbiah said.
The main challenge is defining HPD, he added. Definitions that have been proposed include tumor growth at least two times greater than in control persons, a 15% increase in tumor burden in a set period, and disease progression of 50% from the first evaluation before treatment, he said.
The recent meta-analysis by Hyo Jung Park, MD, PhD, and colleagues, which Dr. Sehgal addressed in his invited commentary, highlights the many approaches used for defining HPD.
Depending on the definition used, the incidence of HPD across 24 studies involving more than 3,100 patients ranged from 5.9% to 43.1%.
“Hyperprogressive disease could be overestimated or underestimated based on current assessment,” Dr. Park and colleagues concluded. They highlighted the importance of “establishing uniform and clinically relevant criteria based on currently available evidence.”
Steps for solving the HPD mystery
“I think we need to come up with consensus criteria for an HPD definition. We need a unified definition,” Dr. Subbiah said. “We also need to design prospective studies to prove or disprove the immunotherapy-HPD association.”
Prospective registries with independent review of patients with suspected immunotherapy-related HPD would be useful for assessing the true incidence and the biology of HPD among patients undergoing immunotherapy, he suggested.
“We need to know the immunologic signals of HPD. This can give us an idea if patients can be prospectively identified for being at risk,” he said. “We also need to know what to do if they are at risk.”
Dr. Sehgal also called for consensus on an HPD definition, with input from a multidisciplinary group that includes “colleagues from radiology, medical oncology, radiation oncology. Getting expertise from different disciplines would be helpful,” he said.
Dr. Park and colleagues suggested several key requirements for an optimal HP definition, such as the inclusion of multiple variables for measuring tumor growth acceleration, “sufficiently quantitative” criteria for determining time to failure, and establishment of a standardized measure of tumor growth acceleration.
The agreed-upon definition of HPD could be applied to patients in a prospective registry and to existing trial data, Dr. Sehgal said.
“Eventually, the goal of this exercise is to [determine] how we can help our patients the best, having a biomarker that can at least inform us in terms of being aware and being proactive in terms of looking for this ... so that interventions can be brought on earlier,” he said.
“If we know what may be a biological mechanism, we can design trials that are designed to look at how to overcome that HPD,” he said.
Dr. Sehgal said he believes HPD is triggered in some way by treatment, including immunotherapy, chemotherapy, and targeted therapy, but perhaps in different ways for each.
He estimated the true incidence of immunotherapy-related HPD will be in the 9%-10% range.
“This is a substantial number of patients, so it’s important that we try to understand this phenomenon, using, again, uniform criteria,” he said.
Current treatment decision-making
Until more is known, Dr. Sehgal said he considers the potential risk factors when treating patients with immunotherapy.
For example, the presence of MDM2 or MDM4 amplification on a genomic profile may factor into his treatment decision-making when it comes to using immunotherapy or immunotherapy in combination with chemotherapy, he said.
“Is that the only factor that is going to make me choose one thing or another? No,” Dr. Sehgal said. However, he said it would make him more “proactive in making sure the patient is doing clinically okay” and in determining when to obtain on-treatment imaging studies.
Dr. Subbiah emphasized the relative benefit of immunotherapy, noting that survival with chemotherapy for many difficult-to-treat cancers in the relapsed/refractory metastatic setting is less than 2 years.
Immunotherapy with checkpoint inhibitors has allowed some of these patients to live longer (with survival reported to be more than 10 years for patients with metastatic melanoma).
“Immunotherapy has been a game changer; it has been transformative in the lives of these patients,” Dr. Subbiah said. “So unless there is any other contraindication, the benefit of receiving immunotherapy for an approved indication far outweighs the risk of HPD.”
A version of this article first appeared on Medscape.com.
Immunotherapy with checkpoint inhibitors has ushered in a new era of cancer therapy, with some patients showing dramatic responses and significantly better outcomes than with other therapies across many cancer types. But some patients do worse, sometimes much worse.
A subset of patients who undergo immunotherapy experience unexpected, rapid disease progression, with a dramatic acceleration of disease trajectory. They also have a shorter progression-free survival and overall survival than would have been expected.
This has been described as hyperprogression and has been termed “hyperprogressive disease” (HPD). It has been seen in a variety of cancers; the incidence ranges from 4% to 29% in the studies reported to date.
There has been some debate over whether this is a real phenomenon or whether it is part of the natural course of disease.
HPD is a “provocative phenomenon,” wrote the authors of a recent commentary entitled “Hyperprogression and Immunotherapy: Fact, Fiction, or Alternative Fact?”
“This phenomenon has polarized oncologists who debate that this could still reflect the natural history of the disease,” said the author of another commentary.
But the tide is now turning toward acceptance of HPD, said Kartik Sehgal, MD, an oncologist at Dana-Farber Cancer Institute and Harvard University, both in Boston.
“With publication of multiple clinical reports of different cancer types worldwide, hyperprogression is now accepted by most oncologists to be a true phenomenon rather than natural progression of disease,” Dr. Sehgal said.
He authored an invited commentary in JAMA Network Openabout one of the latest meta-analyses (JAMA Netw Open. 2021;4[3]:e211136) to investigate HPD during immunotherapy. One of the biggest issues is that the studies that have reported on HPD have been retrospective, with a lack of comparator groups and a lack of a standardized definition of hyperprogression. Dr. Sehgal emphasized the need to study hyperprogression in well-designed prospective studies.
Existing data on HPD
HPD was described as “a new pattern of progression” seen in patients undergoing immune checkpoint inhibitor therapy in a 2017 article published in Clinical Cancer Research. Authors Stephane Champiat, MD, PhD, of Institut Gustave Roussy, Universite Paris Saclay, Villejuif, France, and colleagues cited “anecdotal occurrences” of HPD among patients in phase 1 trials of anti–PD-1/PD-L1 agents.
In that study, HPD was defined by tumor growth rate ratio. The incidence was 9% among 213 patients.
The findings raised concerns about treating elderly patients with anti–PD-1/PD-L1 monotherapy, according to the authors, who called for further study.
That same year, Roberto Ferrara, MD, and colleagues from the Insitut Gustave Roussy reported additional data indicating an incidence of HPD of 16% among 333 patients with non–small cell lung cancer who underwent immunotherapy at eight centers from 2012 to 2017. The findings, which were presented at the 2017 World Conference on Lung Cancer and reported at the time by this news organization, also showed that the incidence of HPD was higher with immunotherapy than with single-agent chemotherapy (5%).
Median overall survival (OS) was just 3.4 months among those with HPD, compared with 13 months in the overall study population – worse, even, than the median 5.4-month OS observed among patients with progressive disease who received immunotherapy.
In the wake of these findings, numerous researchers have attempted to better define HPD, its incidence, and patient factors associated with developing HPD while undergoing immunotherapy.
However, there is little so far to show for those efforts, Vivek Subbiah, MD, of the University of Texas MD Anderson Cancer Center, Houston, said in an interview.
“Many questions remain to be answered,” said Dr. Subbiah, clinical medical director of the Clinical Center for Targeted Therapy in the division of cancer medicine at MD Anderson. He was the senior author of the “Fact, Fiction, or Alternative Fact?” commentary.
Work is underway to elucidate biological mechanisms. Some groups have implicated the Fc region of antibodies. Another group has reported EGFR and MDM2/MDM4 amplifications in patients with HPD, Dr. Subbiah and colleagues noted.
Other “proposed contributing pathological mechanisms include modulation of tumor immune microenvironment through macrophages and regulatory T cells as well as activation of oncogenic signaling pathways,” noted Dr. Sehgal.
Both groups of authors emphasize the urgent need for prospective studies.
It is imperative to confirm underlying biology, predict which patients are at risk, and identify therapeutic directions for patients who experience HPD, Dr. Subbiah said.
The main challenge is defining HPD, he added. Definitions that have been proposed include tumor growth at least two times greater than in control persons, a 15% increase in tumor burden in a set period, and disease progression of 50% from the first evaluation before treatment, he said.
The recent meta-analysis by Hyo Jung Park, MD, PhD, and colleagues, which Dr. Sehgal addressed in his invited commentary, highlights the many approaches used for defining HPD.
Depending on the definition used, the incidence of HPD across 24 studies involving more than 3,100 patients ranged from 5.9% to 43.1%.
“Hyperprogressive disease could be overestimated or underestimated based on current assessment,” Dr. Park and colleagues concluded. They highlighted the importance of “establishing uniform and clinically relevant criteria based on currently available evidence.”
Steps for solving the HPD mystery
“I think we need to come up with consensus criteria for an HPD definition. We need a unified definition,” Dr. Subbiah said. “We also need to design prospective studies to prove or disprove the immunotherapy-HPD association.”
Prospective registries with independent review of patients with suspected immunotherapy-related HPD would be useful for assessing the true incidence and the biology of HPD among patients undergoing immunotherapy, he suggested.
“We need to know the immunologic signals of HPD. This can give us an idea if patients can be prospectively identified for being at risk,” he said. “We also need to know what to do if they are at risk.”
Dr. Sehgal also called for consensus on an HPD definition, with input from a multidisciplinary group that includes “colleagues from radiology, medical oncology, radiation oncology. Getting expertise from different disciplines would be helpful,” he said.
Dr. Park and colleagues suggested several key requirements for an optimal HP definition, such as the inclusion of multiple variables for measuring tumor growth acceleration, “sufficiently quantitative” criteria for determining time to failure, and establishment of a standardized measure of tumor growth acceleration.
The agreed-upon definition of HPD could be applied to patients in a prospective registry and to existing trial data, Dr. Sehgal said.
“Eventually, the goal of this exercise is to [determine] how we can help our patients the best, having a biomarker that can at least inform us in terms of being aware and being proactive in terms of looking for this ... so that interventions can be brought on earlier,” he said.
“If we know what may be a biological mechanism, we can design trials that are designed to look at how to overcome that HPD,” he said.
Dr. Sehgal said he believes HPD is triggered in some way by treatment, including immunotherapy, chemotherapy, and targeted therapy, but perhaps in different ways for each.
He estimated the true incidence of immunotherapy-related HPD will be in the 9%-10% range.
“This is a substantial number of patients, so it’s important that we try to understand this phenomenon, using, again, uniform criteria,” he said.
Current treatment decision-making
Until more is known, Dr. Sehgal said he considers the potential risk factors when treating patients with immunotherapy.
For example, the presence of MDM2 or MDM4 amplification on a genomic profile may factor into his treatment decision-making when it comes to using immunotherapy or immunotherapy in combination with chemotherapy, he said.
“Is that the only factor that is going to make me choose one thing or another? No,” Dr. Sehgal said. However, he said it would make him more “proactive in making sure the patient is doing clinically okay” and in determining when to obtain on-treatment imaging studies.
Dr. Subbiah emphasized the relative benefit of immunotherapy, noting that survival with chemotherapy for many difficult-to-treat cancers in the relapsed/refractory metastatic setting is less than 2 years.
Immunotherapy with checkpoint inhibitors has allowed some of these patients to live longer (with survival reported to be more than 10 years for patients with metastatic melanoma).
“Immunotherapy has been a game changer; it has been transformative in the lives of these patients,” Dr. Subbiah said. “So unless there is any other contraindication, the benefit of receiving immunotherapy for an approved indication far outweighs the risk of HPD.”
A version of this article first appeared on Medscape.com.
FDA panel votes against 2 cancer indications but backs 4 of 6
Federal advisers have supported the efforts of pharmaceutical companies in four of six cases in which these firms are fighting to maintain cancer indications for approved drugs. The advisers voted against the companies in two cases.
The staff of the Food and Drug Administration will now consider these votes as they decide what to do regarding the six cases of what they have termed “dangling” accelerated approvals.
“One of the reasons I think we’re convening today is to prevent these accelerated approvals from dangling ad infinitum,” commented one of the members of the advisory panel.
In these cases, companies have been unable to prove the expected benefits that led the FDA to grant accelerated approvals for these indications.
These accelerated approvals, which are often based on surrogate endpoints, such as overall response rates, are granted on the condition that further findings show a clinical benefit – such as in progression-free survival or overall survival – in larger trials.
The FDA tasked its Oncologic Drugs Advisory Committee (ODAC) with conducting the review of the six accelerated approvals for cancer indications at a 3-day meeting (April 27-29).
These reviews were only for specific cancer indications and will not lead to the removal of drugs from the market. These drugs have already been approved for several cancer indications. For example, one of the drugs that was reviewed, pembrolizumab (Keytruda), is approved in the United States for 28 indications.
The FDA is facing growing pains in its efforts to manage the rapidly changing landscape for these immune checkpoint inhibitors. This field of medicine has experienced an “unprecedented level of drug development” in recent years, FDA officials said in briefing materials, owing in part to the agency’s willingness to accept surrogate markers for accelerated approvals. Although some companies have struggled with these, others have built strong cases for the use of their checkpoint inhibitors for these indications.
The ODAC panelists, for example, noted the emergence of nivolumab (Opdivo) as an option for patients with gastric cancer as a reason for seeking to withdraw an indication for pembrolizumab (Keytruda) for this disease.
Just weeks before the meeting, on April 16, the FDA approved nivolumab plus chemotherapy as a first-line treatment for advanced or metastatic gastric cancer, gastroesophageal junction cancer, and esophageal adenocarcinoma. This was a full approval based on data showing an overall survival benefit from a phase 3 trial.
Votes by indication
On April 29, the last day of the meeting, the ODAC panel voted 6-2 against maintaining pembrolizumab’s indication as monotherapy for an advanced form of gastric cancer. This was an accelerated approval (granted in 2017) that was based on overall response rates from an open-label trial.
That last day of the meeting also saw another negative vote. On April 29, the ODAC panel voted 5-4 against maintaining an indication for nivolumab in patients with hepatocellular carcinoma (HCC) who were previously treated with sorafenib (Nexavar).
This accelerated approval for nivolumab was granted in 2017. The FDA said it had requested ODAC’s feedback on this indication because of the recent full approval of another checkpoint inhibitor for HCC, atezolizumab (Tecentriq), in combination with bevacizumab (Avastin) for patients with unresectable or metastatic diseases who have not received prior systemic therapy. This full approval (in May 2020) was based on an overall survival benefit.
There was one last vote on the third day of the meeting, and it was positive. The ODAC panel voted 8-0 in favor of maintaining the indication for the use of pembrolizumab as monotherapy for patients with HCC who have previously been treated with sorafenib.
The FDA altered the composition of the ODAC panel during the week, adding members in some cases who had expertise in particular cancers. That led to different totals for the week’s ODAC votes, as shown in the tallies summarized below.
On the first day of the meeting (April 27), the ODAC panel voted 7-2 in favor of maintaining a breast cancer indication for atezolizumab (Tecentriq). This covered use of the immunotherapy in combination with nab-paclitaxel for patients with unresectable locally advanced or metastatic triple-negative breast cancer whose tumors express PD-L1.
The second day of the meeting (April 28) also saw two positive votes. The ODAC panel voted 10-1 for maintaining the indication for atezolizumab for the first-line treatment of cisplatin-ineligible patients with advanced/metastatic urothelial carcinoma, pending final overall survival results from the IMvigor130 trial. The panel also voted 5-3 for maintaining the indication for pembrolizumab in patients with locally advanced or metastatic urothelial carcinoma who are not eligible for cisplatin-containing chemotherapy and whose tumors express PD-L1.
The FDA is not bound to follow the voting and recommendations of its advisory panels, but it usually does so.
Managing shifts in treatment
In both of the cases in which ODAC voted against maintaining indications, Richard Pazdur, MD, the FDA’s top regulator for cancer medicines, jumped into the debate. Dr. Pazdur countered arguments put forward by representatives of the manufacturers as they sought to maintain indications for their drugs.
Merck officials and representatives argued for pembrolizumab, saying that maintaining the gastric cancer indication might help patients whose disease has progressed despite earlier treatment.
Dr. Pazdur emphasized that the agency would help Merck and physicians to have access to pembrolizumab for these patients even if this one indication were to be withdrawn. But Dr. Pazdur and ODAC members also noted the recent shift in the landscape for gastric cancer, with the recent approval of a new indication for nivolumab.
“I want to emphasize to the patient community out there [that] we firmly believe in the role of checkpoint inhibitors in this disease,” Dr. Pazdur said during the discussion of the indication for pembrolizumab for gastric cancer. “We have to be cognizant of what is the appropriate setting for that, and it currently is in the first line.”
Dr. Pazdur noted that two studies had failed to confirm the expected benefit from pembrolizumab for patients with more advanced disease. Still, if “small numbers” of patients with advanced disease wanted access to Merck’s drug, the FDA and the company could accommodate them. The FDA could delay the removal of the gastric indication to allow patients to continue receiving it. The FDA also could work with physicians on other routes to provide the medicine, such as through single-patient investigational new drug applications or an expanded access program.
“Or Merck can alternatively give the drug gratis to patients,” Dr. Pazdur said.
#ProjectFacilitate for expanded access
One of Merck’s speakers at the ODAC meeting, Peter Enzinger, MD, of the Dana-Farber Cancer Institute, Boston, objected to Dr. Pazdur’s plan.
A loss of the gastric indication for pembrolizumab would result in patients with advanced cancer missing out on a chance to try this therapy. Some patients will not have had a chance to try a checkpoint inhibitor earlier in their treatment, and a loss of the indication would cost them that opportunity, he said.
“An expanded-access program sounds very nice, but the reality is that our patients are incredibly sick and that weeks matter,” Dr. Enzinger said, citing administrative hurdles as a barrier to treatment.
“Our patients just don’t have the time for that, and therefore I don’t think an expanded access program is the way to go,” Dr. Enzinger said.
Dr. Pazdur responded to these objections by highlighting an initiative called Project Facilitate at the FDA’s Oncology Center for Excellence. During the meeting, Dr. Pazdur’s division used its @FDAOncology Twitter handle to draw attention to this project.
ODAC panelist Diane Reidy-Lagunes, MD, of Memorial Sloan Kettering Cancer Center, New York, said she had struggled with this vote. She was one of the two panelists to vote in favor of keeping the indication.
“This is also incredibly hard for me. I actually changed it at the last minute,” she said of her vote.
But Dr. Reidy-Lagunes said she was concerned that some patients with advanced disease might not be able to get a checkpoint inhibitor.
“With disparities in healthcare and differences in the way that patients are treated throughout our country, I was nervous that they may not be able to get treated,” she said, noting that she shared her fellow panelists’ doubts about use of pembrolizumab as third-line treatment, owing to negative results in trials.
ODAC member David Mitchell, who served as a consumer representative, also said he found the vote on the gastric indication for pembrolizumab to be a difficult decision.
“As a patient with incurable cancer who’s now being given all three major classes of drugs to treat my disease in combination, these issues really cut close to home,” Mr. Mitchell said.
He said the expectation that the FDA’s expanded access program could help patients with advanced disease try pembrolizumab helped him decide to vote with the 6-2 majority against maintaining this gastric cancer approval.
His vote was based on “the changing treatment landscape.” There is general agreement that the patients in question should receive checkpoint inhibitors as first-line treatment, not third-line treatment, Mr. Mitchell said. The FDA should delay a withdrawal of the approval for pembrolizumab in this case and should allow a transition for those who missed out on treatment with a checkpoint inhibitor earlier in the disease course, he suggested.
“To protect the safety and well-being of patients, we have to base decisions on data,” Mr. Mitchell said. “The data don’t support maintaining the indication” for pembrolizumab.
Close split on nivolumab
In contrast to the 6-2 vote against maintaining the pembrolizumab indication, the ODAC panel split more closely, 5-4, on the question of maintaining an indication for the use as monotherapy of nivolumab in HCC.
ODAC panelist Philip C. Hoffman, MD, of the University of Chicago was among those who supported keeping the indication.
“There’s still an unmet need for second-line immunotherapy because there will always be some patients who are poor candidates for bevacizumab or who are not tolerating or responding to sorafenib,” he said.
ODAC panelist Mark A. Lewis, MD, of Intermountain Healthcare, Salt Lake City, said he voted “no” in part because he doubted that Bristol-Myers Squibb would be able to soon produce data for nivolumab that was needed to support this indication.
A version of this article first appeared on Medscape.com.
Federal advisers have supported the efforts of pharmaceutical companies in four of six cases in which these firms are fighting to maintain cancer indications for approved drugs. The advisers voted against the companies in two cases.
The staff of the Food and Drug Administration will now consider these votes as they decide what to do regarding the six cases of what they have termed “dangling” accelerated approvals.
“One of the reasons I think we’re convening today is to prevent these accelerated approvals from dangling ad infinitum,” commented one of the members of the advisory panel.
In these cases, companies have been unable to prove the expected benefits that led the FDA to grant accelerated approvals for these indications.
These accelerated approvals, which are often based on surrogate endpoints, such as overall response rates, are granted on the condition that further findings show a clinical benefit – such as in progression-free survival or overall survival – in larger trials.
The FDA tasked its Oncologic Drugs Advisory Committee (ODAC) with conducting the review of the six accelerated approvals for cancer indications at a 3-day meeting (April 27-29).
These reviews were only for specific cancer indications and will not lead to the removal of drugs from the market. These drugs have already been approved for several cancer indications. For example, one of the drugs that was reviewed, pembrolizumab (Keytruda), is approved in the United States for 28 indications.
The FDA is facing growing pains in its efforts to manage the rapidly changing landscape for these immune checkpoint inhibitors. This field of medicine has experienced an “unprecedented level of drug development” in recent years, FDA officials said in briefing materials, owing in part to the agency’s willingness to accept surrogate markers for accelerated approvals. Although some companies have struggled with these, others have built strong cases for the use of their checkpoint inhibitors for these indications.
The ODAC panelists, for example, noted the emergence of nivolumab (Opdivo) as an option for patients with gastric cancer as a reason for seeking to withdraw an indication for pembrolizumab (Keytruda) for this disease.
Just weeks before the meeting, on April 16, the FDA approved nivolumab plus chemotherapy as a first-line treatment for advanced or metastatic gastric cancer, gastroesophageal junction cancer, and esophageal adenocarcinoma. This was a full approval based on data showing an overall survival benefit from a phase 3 trial.
Votes by indication
On April 29, the last day of the meeting, the ODAC panel voted 6-2 against maintaining pembrolizumab’s indication as monotherapy for an advanced form of gastric cancer. This was an accelerated approval (granted in 2017) that was based on overall response rates from an open-label trial.
That last day of the meeting also saw another negative vote. On April 29, the ODAC panel voted 5-4 against maintaining an indication for nivolumab in patients with hepatocellular carcinoma (HCC) who were previously treated with sorafenib (Nexavar).
This accelerated approval for nivolumab was granted in 2017. The FDA said it had requested ODAC’s feedback on this indication because of the recent full approval of another checkpoint inhibitor for HCC, atezolizumab (Tecentriq), in combination with bevacizumab (Avastin) for patients with unresectable or metastatic diseases who have not received prior systemic therapy. This full approval (in May 2020) was based on an overall survival benefit.
There was one last vote on the third day of the meeting, and it was positive. The ODAC panel voted 8-0 in favor of maintaining the indication for the use of pembrolizumab as monotherapy for patients with HCC who have previously been treated with sorafenib.
The FDA altered the composition of the ODAC panel during the week, adding members in some cases who had expertise in particular cancers. That led to different totals for the week’s ODAC votes, as shown in the tallies summarized below.
On the first day of the meeting (April 27), the ODAC panel voted 7-2 in favor of maintaining a breast cancer indication for atezolizumab (Tecentriq). This covered use of the immunotherapy in combination with nab-paclitaxel for patients with unresectable locally advanced or metastatic triple-negative breast cancer whose tumors express PD-L1.
The second day of the meeting (April 28) also saw two positive votes. The ODAC panel voted 10-1 for maintaining the indication for atezolizumab for the first-line treatment of cisplatin-ineligible patients with advanced/metastatic urothelial carcinoma, pending final overall survival results from the IMvigor130 trial. The panel also voted 5-3 for maintaining the indication for pembrolizumab in patients with locally advanced or metastatic urothelial carcinoma who are not eligible for cisplatin-containing chemotherapy and whose tumors express PD-L1.
The FDA is not bound to follow the voting and recommendations of its advisory panels, but it usually does so.
Managing shifts in treatment
In both of the cases in which ODAC voted against maintaining indications, Richard Pazdur, MD, the FDA’s top regulator for cancer medicines, jumped into the debate. Dr. Pazdur countered arguments put forward by representatives of the manufacturers as they sought to maintain indications for their drugs.
Merck officials and representatives argued for pembrolizumab, saying that maintaining the gastric cancer indication might help patients whose disease has progressed despite earlier treatment.
Dr. Pazdur emphasized that the agency would help Merck and physicians to have access to pembrolizumab for these patients even if this one indication were to be withdrawn. But Dr. Pazdur and ODAC members also noted the recent shift in the landscape for gastric cancer, with the recent approval of a new indication for nivolumab.
“I want to emphasize to the patient community out there [that] we firmly believe in the role of checkpoint inhibitors in this disease,” Dr. Pazdur said during the discussion of the indication for pembrolizumab for gastric cancer. “We have to be cognizant of what is the appropriate setting for that, and it currently is in the first line.”
Dr. Pazdur noted that two studies had failed to confirm the expected benefit from pembrolizumab for patients with more advanced disease. Still, if “small numbers” of patients with advanced disease wanted access to Merck’s drug, the FDA and the company could accommodate them. The FDA could delay the removal of the gastric indication to allow patients to continue receiving it. The FDA also could work with physicians on other routes to provide the medicine, such as through single-patient investigational new drug applications or an expanded access program.
“Or Merck can alternatively give the drug gratis to patients,” Dr. Pazdur said.
#ProjectFacilitate for expanded access
One of Merck’s speakers at the ODAC meeting, Peter Enzinger, MD, of the Dana-Farber Cancer Institute, Boston, objected to Dr. Pazdur’s plan.
A loss of the gastric indication for pembrolizumab would result in patients with advanced cancer missing out on a chance to try this therapy. Some patients will not have had a chance to try a checkpoint inhibitor earlier in their treatment, and a loss of the indication would cost them that opportunity, he said.
“An expanded-access program sounds very nice, but the reality is that our patients are incredibly sick and that weeks matter,” Dr. Enzinger said, citing administrative hurdles as a barrier to treatment.
“Our patients just don’t have the time for that, and therefore I don’t think an expanded access program is the way to go,” Dr. Enzinger said.
Dr. Pazdur responded to these objections by highlighting an initiative called Project Facilitate at the FDA’s Oncology Center for Excellence. During the meeting, Dr. Pazdur’s division used its @FDAOncology Twitter handle to draw attention to this project.
ODAC panelist Diane Reidy-Lagunes, MD, of Memorial Sloan Kettering Cancer Center, New York, said she had struggled with this vote. She was one of the two panelists to vote in favor of keeping the indication.
“This is also incredibly hard for me. I actually changed it at the last minute,” she said of her vote.
But Dr. Reidy-Lagunes said she was concerned that some patients with advanced disease might not be able to get a checkpoint inhibitor.
“With disparities in healthcare and differences in the way that patients are treated throughout our country, I was nervous that they may not be able to get treated,” she said, noting that she shared her fellow panelists’ doubts about use of pembrolizumab as third-line treatment, owing to negative results in trials.
ODAC member David Mitchell, who served as a consumer representative, also said he found the vote on the gastric indication for pembrolizumab to be a difficult decision.
“As a patient with incurable cancer who’s now being given all three major classes of drugs to treat my disease in combination, these issues really cut close to home,” Mr. Mitchell said.
He said the expectation that the FDA’s expanded access program could help patients with advanced disease try pembrolizumab helped him decide to vote with the 6-2 majority against maintaining this gastric cancer approval.
His vote was based on “the changing treatment landscape.” There is general agreement that the patients in question should receive checkpoint inhibitors as first-line treatment, not third-line treatment, Mr. Mitchell said. The FDA should delay a withdrawal of the approval for pembrolizumab in this case and should allow a transition for those who missed out on treatment with a checkpoint inhibitor earlier in the disease course, he suggested.
“To protect the safety and well-being of patients, we have to base decisions on data,” Mr. Mitchell said. “The data don’t support maintaining the indication” for pembrolizumab.
Close split on nivolumab
In contrast to the 6-2 vote against maintaining the pembrolizumab indication, the ODAC panel split more closely, 5-4, on the question of maintaining an indication for the use as monotherapy of nivolumab in HCC.
ODAC panelist Philip C. Hoffman, MD, of the University of Chicago was among those who supported keeping the indication.
“There’s still an unmet need for second-line immunotherapy because there will always be some patients who are poor candidates for bevacizumab or who are not tolerating or responding to sorafenib,” he said.
ODAC panelist Mark A. Lewis, MD, of Intermountain Healthcare, Salt Lake City, said he voted “no” in part because he doubted that Bristol-Myers Squibb would be able to soon produce data for nivolumab that was needed to support this indication.
A version of this article first appeared on Medscape.com.
Federal advisers have supported the efforts of pharmaceutical companies in four of six cases in which these firms are fighting to maintain cancer indications for approved drugs. The advisers voted against the companies in two cases.
The staff of the Food and Drug Administration will now consider these votes as they decide what to do regarding the six cases of what they have termed “dangling” accelerated approvals.
“One of the reasons I think we’re convening today is to prevent these accelerated approvals from dangling ad infinitum,” commented one of the members of the advisory panel.
In these cases, companies have been unable to prove the expected benefits that led the FDA to grant accelerated approvals for these indications.
These accelerated approvals, which are often based on surrogate endpoints, such as overall response rates, are granted on the condition that further findings show a clinical benefit – such as in progression-free survival or overall survival – in larger trials.
The FDA tasked its Oncologic Drugs Advisory Committee (ODAC) with conducting the review of the six accelerated approvals for cancer indications at a 3-day meeting (April 27-29).
These reviews were only for specific cancer indications and will not lead to the removal of drugs from the market. These drugs have already been approved for several cancer indications. For example, one of the drugs that was reviewed, pembrolizumab (Keytruda), is approved in the United States for 28 indications.
The FDA is facing growing pains in its efforts to manage the rapidly changing landscape for these immune checkpoint inhibitors. This field of medicine has experienced an “unprecedented level of drug development” in recent years, FDA officials said in briefing materials, owing in part to the agency’s willingness to accept surrogate markers for accelerated approvals. Although some companies have struggled with these, others have built strong cases for the use of their checkpoint inhibitors for these indications.
The ODAC panelists, for example, noted the emergence of nivolumab (Opdivo) as an option for patients with gastric cancer as a reason for seeking to withdraw an indication for pembrolizumab (Keytruda) for this disease.
Just weeks before the meeting, on April 16, the FDA approved nivolumab plus chemotherapy as a first-line treatment for advanced or metastatic gastric cancer, gastroesophageal junction cancer, and esophageal adenocarcinoma. This was a full approval based on data showing an overall survival benefit from a phase 3 trial.
Votes by indication
On April 29, the last day of the meeting, the ODAC panel voted 6-2 against maintaining pembrolizumab’s indication as monotherapy for an advanced form of gastric cancer. This was an accelerated approval (granted in 2017) that was based on overall response rates from an open-label trial.
That last day of the meeting also saw another negative vote. On April 29, the ODAC panel voted 5-4 against maintaining an indication for nivolumab in patients with hepatocellular carcinoma (HCC) who were previously treated with sorafenib (Nexavar).
This accelerated approval for nivolumab was granted in 2017. The FDA said it had requested ODAC’s feedback on this indication because of the recent full approval of another checkpoint inhibitor for HCC, atezolizumab (Tecentriq), in combination with bevacizumab (Avastin) for patients with unresectable or metastatic diseases who have not received prior systemic therapy. This full approval (in May 2020) was based on an overall survival benefit.
There was one last vote on the third day of the meeting, and it was positive. The ODAC panel voted 8-0 in favor of maintaining the indication for the use of pembrolizumab as monotherapy for patients with HCC who have previously been treated with sorafenib.
The FDA altered the composition of the ODAC panel during the week, adding members in some cases who had expertise in particular cancers. That led to different totals for the week’s ODAC votes, as shown in the tallies summarized below.
On the first day of the meeting (April 27), the ODAC panel voted 7-2 in favor of maintaining a breast cancer indication for atezolizumab (Tecentriq). This covered use of the immunotherapy in combination with nab-paclitaxel for patients with unresectable locally advanced or metastatic triple-negative breast cancer whose tumors express PD-L1.
The second day of the meeting (April 28) also saw two positive votes. The ODAC panel voted 10-1 for maintaining the indication for atezolizumab for the first-line treatment of cisplatin-ineligible patients with advanced/metastatic urothelial carcinoma, pending final overall survival results from the IMvigor130 trial. The panel also voted 5-3 for maintaining the indication for pembrolizumab in patients with locally advanced or metastatic urothelial carcinoma who are not eligible for cisplatin-containing chemotherapy and whose tumors express PD-L1.
The FDA is not bound to follow the voting and recommendations of its advisory panels, but it usually does so.
Managing shifts in treatment
In both of the cases in which ODAC voted against maintaining indications, Richard Pazdur, MD, the FDA’s top regulator for cancer medicines, jumped into the debate. Dr. Pazdur countered arguments put forward by representatives of the manufacturers as they sought to maintain indications for their drugs.
Merck officials and representatives argued for pembrolizumab, saying that maintaining the gastric cancer indication might help patients whose disease has progressed despite earlier treatment.
Dr. Pazdur emphasized that the agency would help Merck and physicians to have access to pembrolizumab for these patients even if this one indication were to be withdrawn. But Dr. Pazdur and ODAC members also noted the recent shift in the landscape for gastric cancer, with the recent approval of a new indication for nivolumab.
“I want to emphasize to the patient community out there [that] we firmly believe in the role of checkpoint inhibitors in this disease,” Dr. Pazdur said during the discussion of the indication for pembrolizumab for gastric cancer. “We have to be cognizant of what is the appropriate setting for that, and it currently is in the first line.”
Dr. Pazdur noted that two studies had failed to confirm the expected benefit from pembrolizumab for patients with more advanced disease. Still, if “small numbers” of patients with advanced disease wanted access to Merck’s drug, the FDA and the company could accommodate them. The FDA could delay the removal of the gastric indication to allow patients to continue receiving it. The FDA also could work with physicians on other routes to provide the medicine, such as through single-patient investigational new drug applications or an expanded access program.
“Or Merck can alternatively give the drug gratis to patients,” Dr. Pazdur said.
#ProjectFacilitate for expanded access
One of Merck’s speakers at the ODAC meeting, Peter Enzinger, MD, of the Dana-Farber Cancer Institute, Boston, objected to Dr. Pazdur’s plan.
A loss of the gastric indication for pembrolizumab would result in patients with advanced cancer missing out on a chance to try this therapy. Some patients will not have had a chance to try a checkpoint inhibitor earlier in their treatment, and a loss of the indication would cost them that opportunity, he said.
“An expanded-access program sounds very nice, but the reality is that our patients are incredibly sick and that weeks matter,” Dr. Enzinger said, citing administrative hurdles as a barrier to treatment.
“Our patients just don’t have the time for that, and therefore I don’t think an expanded access program is the way to go,” Dr. Enzinger said.
Dr. Pazdur responded to these objections by highlighting an initiative called Project Facilitate at the FDA’s Oncology Center for Excellence. During the meeting, Dr. Pazdur’s division used its @FDAOncology Twitter handle to draw attention to this project.
ODAC panelist Diane Reidy-Lagunes, MD, of Memorial Sloan Kettering Cancer Center, New York, said she had struggled with this vote. She was one of the two panelists to vote in favor of keeping the indication.
“This is also incredibly hard for me. I actually changed it at the last minute,” she said of her vote.
But Dr. Reidy-Lagunes said she was concerned that some patients with advanced disease might not be able to get a checkpoint inhibitor.
“With disparities in healthcare and differences in the way that patients are treated throughout our country, I was nervous that they may not be able to get treated,” she said, noting that she shared her fellow panelists’ doubts about use of pembrolizumab as third-line treatment, owing to negative results in trials.
ODAC member David Mitchell, who served as a consumer representative, also said he found the vote on the gastric indication for pembrolizumab to be a difficult decision.
“As a patient with incurable cancer who’s now being given all three major classes of drugs to treat my disease in combination, these issues really cut close to home,” Mr. Mitchell said.
He said the expectation that the FDA’s expanded access program could help patients with advanced disease try pembrolizumab helped him decide to vote with the 6-2 majority against maintaining this gastric cancer approval.
His vote was based on “the changing treatment landscape.” There is general agreement that the patients in question should receive checkpoint inhibitors as first-line treatment, not third-line treatment, Mr. Mitchell said. The FDA should delay a withdrawal of the approval for pembrolizumab in this case and should allow a transition for those who missed out on treatment with a checkpoint inhibitor earlier in the disease course, he suggested.
“To protect the safety and well-being of patients, we have to base decisions on data,” Mr. Mitchell said. “The data don’t support maintaining the indication” for pembrolizumab.
Close split on nivolumab
In contrast to the 6-2 vote against maintaining the pembrolizumab indication, the ODAC panel split more closely, 5-4, on the question of maintaining an indication for the use as monotherapy of nivolumab in HCC.
ODAC panelist Philip C. Hoffman, MD, of the University of Chicago was among those who supported keeping the indication.
“There’s still an unmet need for second-line immunotherapy because there will always be some patients who are poor candidates for bevacizumab or who are not tolerating or responding to sorafenib,” he said.
ODAC panelist Mark A. Lewis, MD, of Intermountain Healthcare, Salt Lake City, said he voted “no” in part because he doubted that Bristol-Myers Squibb would be able to soon produce data for nivolumab that was needed to support this indication.
A version of this article first appeared on Medscape.com.
Adjuvant palbociclib fails in early breast cancer
Key clinical point: The addition of palbociclib to estrogen therapy for 1 year does not improve invasive disease-free survival (DFS) in high-risk patients with hormone-receptor–positive (HR+), human epidermal growth factor receptor (HER)-negative early breast cancer who had residual invasive disease after neoadjuvant chemotherapy.
Major finding: Palbociclib did not improve invasive DFS vs. placebo (stratified hazard ratio, 0.93; P = .525) after a median follow-up of 42.8 months. Incidences of grade 3-4 neutropenia and leukopenia were significantly higher in the palbociclib group. Eight fatal serious adverse events were reported.
Study details: A phase 3, double-blind, randomized PENELOPE-B study evaluated 1,250 high-risk patients with HR+, HER-negative early breast cancer who have residual invasive disease after neoadjuvant chemotherapy. Patients received estrogen therapy with either palbociclib or placebo.
Disclosures: The study was supported by Pfizer. The authors declared receiving consulting, honoraria, travel/accomodation expenses and research funding outside the study work. Some of the authors declared being employee of and/or stocks/ownership interests of various sources including Pfizer.
Source: Loibl S et al. J Clin Oncol. 2021 Apr 1. doi: 10.1200/JCO.20.03639.
Key clinical point: The addition of palbociclib to estrogen therapy for 1 year does not improve invasive disease-free survival (DFS) in high-risk patients with hormone-receptor–positive (HR+), human epidermal growth factor receptor (HER)-negative early breast cancer who had residual invasive disease after neoadjuvant chemotherapy.
Major finding: Palbociclib did not improve invasive DFS vs. placebo (stratified hazard ratio, 0.93; P = .525) after a median follow-up of 42.8 months. Incidences of grade 3-4 neutropenia and leukopenia were significantly higher in the palbociclib group. Eight fatal serious adverse events were reported.
Study details: A phase 3, double-blind, randomized PENELOPE-B study evaluated 1,250 high-risk patients with HR+, HER-negative early breast cancer who have residual invasive disease after neoadjuvant chemotherapy. Patients received estrogen therapy with either palbociclib or placebo.
Disclosures: The study was supported by Pfizer. The authors declared receiving consulting, honoraria, travel/accomodation expenses and research funding outside the study work. Some of the authors declared being employee of and/or stocks/ownership interests of various sources including Pfizer.
Source: Loibl S et al. J Clin Oncol. 2021 Apr 1. doi: 10.1200/JCO.20.03639.
Key clinical point: The addition of palbociclib to estrogen therapy for 1 year does not improve invasive disease-free survival (DFS) in high-risk patients with hormone-receptor–positive (HR+), human epidermal growth factor receptor (HER)-negative early breast cancer who had residual invasive disease after neoadjuvant chemotherapy.
Major finding: Palbociclib did not improve invasive DFS vs. placebo (stratified hazard ratio, 0.93; P = .525) after a median follow-up of 42.8 months. Incidences of grade 3-4 neutropenia and leukopenia were significantly higher in the palbociclib group. Eight fatal serious adverse events were reported.
Study details: A phase 3, double-blind, randomized PENELOPE-B study evaluated 1,250 high-risk patients with HR+, HER-negative early breast cancer who have residual invasive disease after neoadjuvant chemotherapy. Patients received estrogen therapy with either palbociclib or placebo.
Disclosures: The study was supported by Pfizer. The authors declared receiving consulting, honoraria, travel/accomodation expenses and research funding outside the study work. Some of the authors declared being employee of and/or stocks/ownership interests of various sources including Pfizer.
Source: Loibl S et al. J Clin Oncol. 2021 Apr 1. doi: 10.1200/JCO.20.03639.
Breast cancer: Axillary complete pathologic response varies by subtype
Key clinical point: The hormone receptor (HR)-negative/ human epidermal growth factor receptor 2 (HER2)-positive subtype was associated with the highest axillary pathologic complete response (pCR) rate and luminal A subtype was associated with the lowest axillary pCR rate.
Major finding: The axillary pCR rates were 60% for HR-negative/HER2-positive, 59% for HER2-positive, 48% for triple-negative, 45% for HR-positive/HER2-positive, 35% for luminal B, 18% for HR-positive/HER2-negative, and 13% for luminal A breast cancer subtypes.
Study details: A meta-analysis of 33 studies including 57,531 patients with breast cancer who received neoadjuvant systemic therapy.
Disclosures: The study was supported by a grant from the Dutch Cancer Society and Alpe d’Huzes Foundation. The authors received grants and personal fees outside this work.
Source: Samiei S et al. JAMA Surg. 2021 Apr 21. doi: 10.1001/jamasurg.2021.0891.
Key clinical point: The hormone receptor (HR)-negative/ human epidermal growth factor receptor 2 (HER2)-positive subtype was associated with the highest axillary pathologic complete response (pCR) rate and luminal A subtype was associated with the lowest axillary pCR rate.
Major finding: The axillary pCR rates were 60% for HR-negative/HER2-positive, 59% for HER2-positive, 48% for triple-negative, 45% for HR-positive/HER2-positive, 35% for luminal B, 18% for HR-positive/HER2-negative, and 13% for luminal A breast cancer subtypes.
Study details: A meta-analysis of 33 studies including 57,531 patients with breast cancer who received neoadjuvant systemic therapy.
Disclosures: The study was supported by a grant from the Dutch Cancer Society and Alpe d’Huzes Foundation. The authors received grants and personal fees outside this work.
Source: Samiei S et al. JAMA Surg. 2021 Apr 21. doi: 10.1001/jamasurg.2021.0891.
Key clinical point: The hormone receptor (HR)-negative/ human epidermal growth factor receptor 2 (HER2)-positive subtype was associated with the highest axillary pathologic complete response (pCR) rate and luminal A subtype was associated with the lowest axillary pCR rate.
Major finding: The axillary pCR rates were 60% for HR-negative/HER2-positive, 59% for HER2-positive, 48% for triple-negative, 45% for HR-positive/HER2-positive, 35% for luminal B, 18% for HR-positive/HER2-negative, and 13% for luminal A breast cancer subtypes.
Study details: A meta-analysis of 33 studies including 57,531 patients with breast cancer who received neoadjuvant systemic therapy.
Disclosures: The study was supported by a grant from the Dutch Cancer Society and Alpe d’Huzes Foundation. The authors received grants and personal fees outside this work.
Source: Samiei S et al. JAMA Surg. 2021 Apr 21. doi: 10.1001/jamasurg.2021.0891.