User login
Cancer Data Trends 2024: Breast Cancer
1. US Department of Veterans Affairs. Mammogram/breast health. March 28, 2022. Accessed January 10, 2024. https://www.womenshealth.va.gov/topics/mammogram-breast-health.asp
2. First anniversary of Mammography and Medical Options Act (MAMMO Act). VA News. June 6, 2023. Accessed January 10, 2024. https://news.va.gov/120476/anniversary-mammography-and-medicaloptions-act/
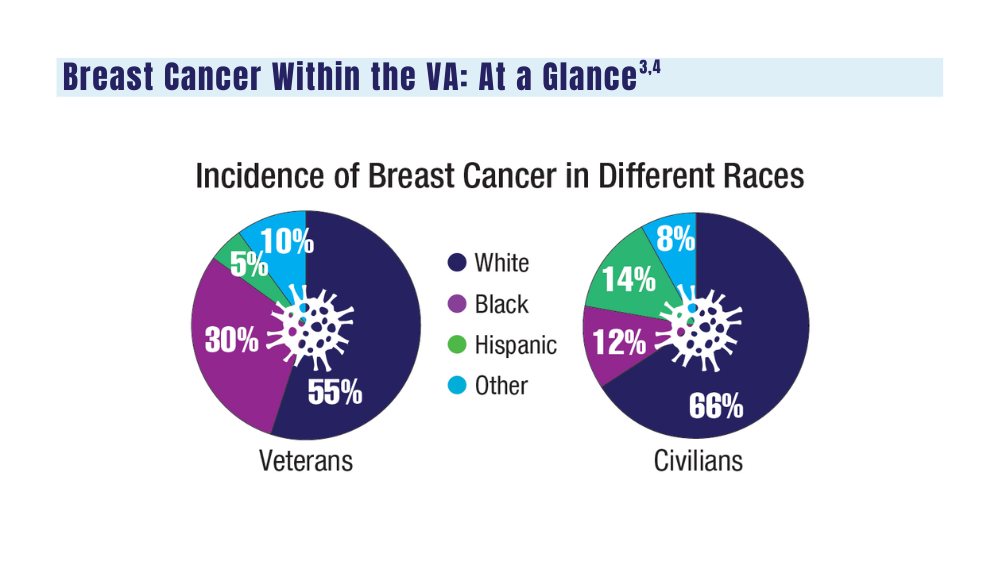
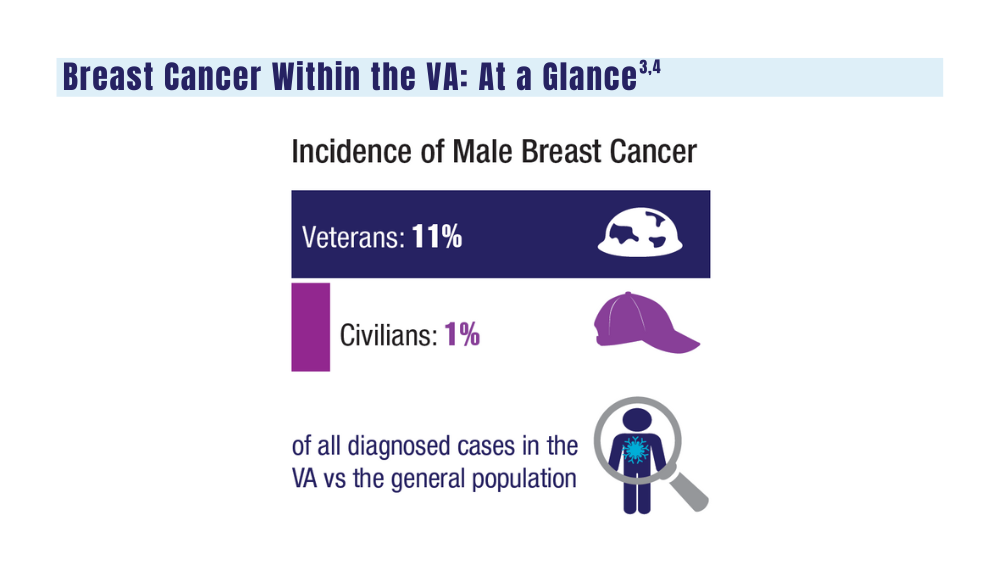
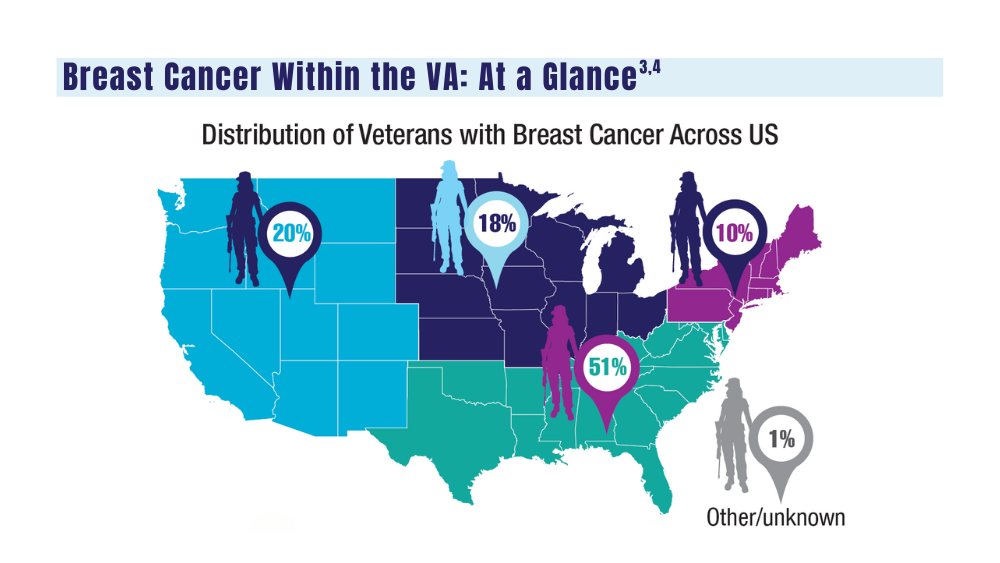
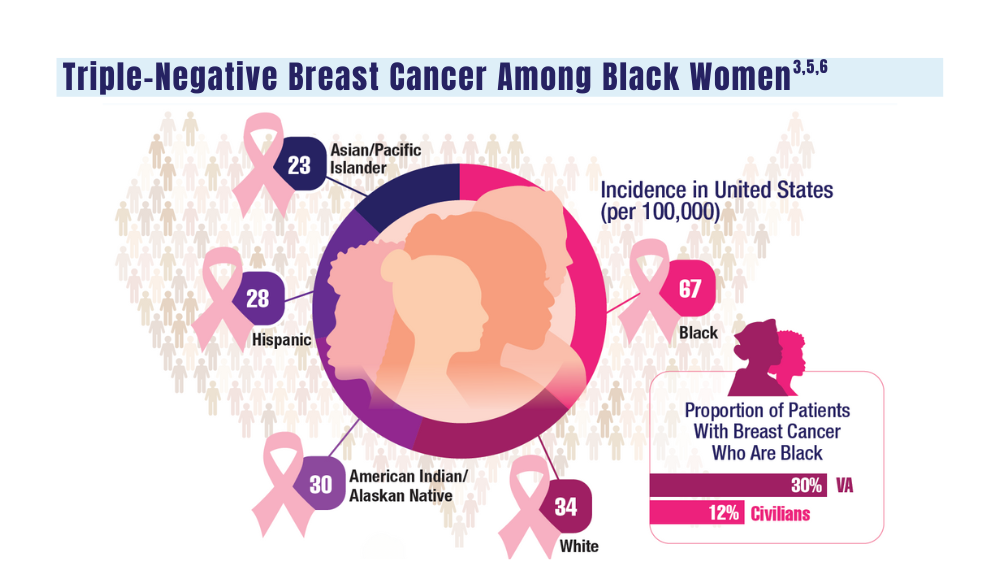
3. Moss HA, Rasmussen, KM, Patil, V, et al. Demographic characteristics of veterans diagnosed with breast and gynecologic cancers: a comparative analysis with the general population. Abstract presented at: Annual Meeting of the Association of VA Hematology/ Oncology (AVAHO); September 29–October 1, 2023; Chicago, IL. Abstract 47.
4. US Department of Veterans Affairs. Racial and ethnic minority veterans. Updated July 9, 2020. Accessed January 10, 2024. https:// www.va.gov/HEALTHEQUITY/Race_Ethnicity.asp
5. Stringer-Reasor EM, Elkhanany A, Khoury K, Simon MA, Newman LA. Disparities in breast cancer associated with African American identity. Am Soc Clin Oncol Educ Book. 2021;41:e29-e46. doi:10.1200/EDBK_319929
6. Landry I, Sumbly V, Vest M. Advancements in the treatment of triple- CANCER DATA TRENDS 2024 MARCH 2024 • FEDERAL PRACTITIONER negative breast cancer: a narrative review of the literature. Cureus. 2022;14(2):e21970. doi:10.7759/cureus.21970
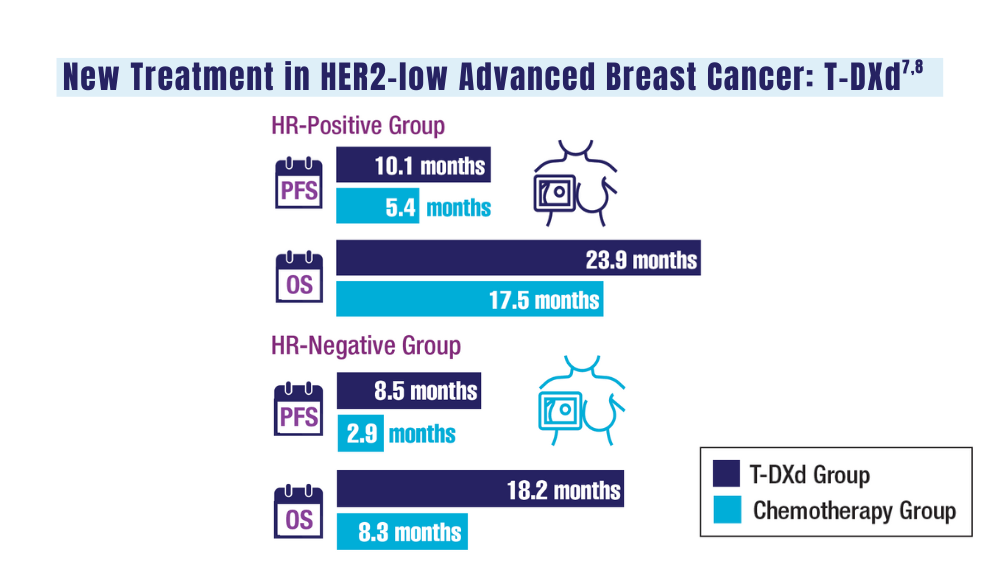
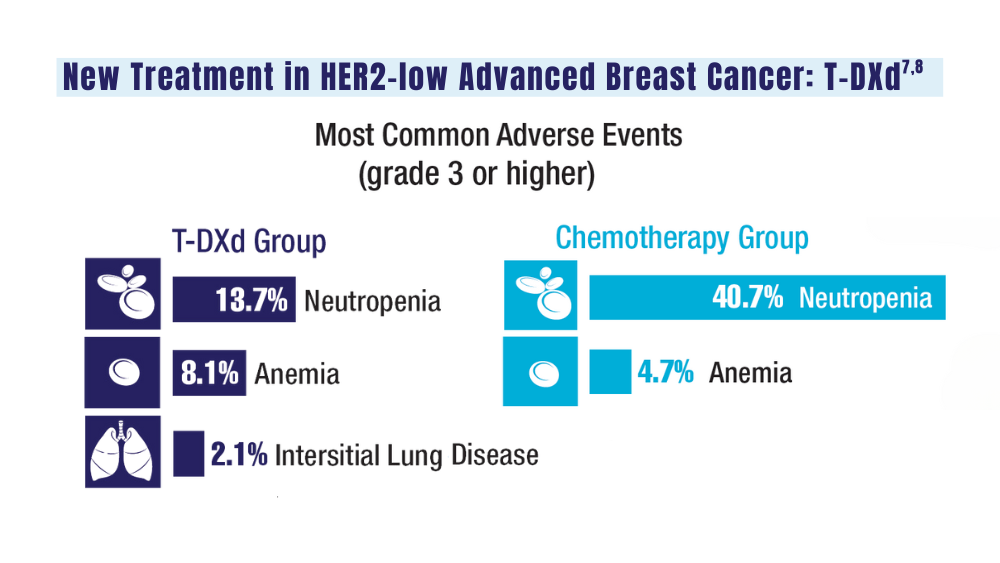
7. Schlam I, Tolaney SM, Tarantino P. How I treat HER2-low advanced breast cancer. Breast. 2023;67:116-123. doi:10.1016/j.breast.2023.01.005
8. Modi S, Jacot W, Yamashita T, et al; for the DESTINY-Breast04 Trial Investigators. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N Engl J Med. 2022;387(1):9-20. doi:10.1056/NEJMoa2203690
1. US Department of Veterans Affairs. Mammogram/breast health. March 28, 2022. Accessed January 10, 2024. https://www.womenshealth.va.gov/topics/mammogram-breast-health.asp
2. First anniversary of Mammography and Medical Options Act (MAMMO Act). VA News. June 6, 2023. Accessed January 10, 2024. https://news.va.gov/120476/anniversary-mammography-and-medicaloptions-act/
3. Moss HA, Rasmussen, KM, Patil, V, et al. Demographic characteristics of veterans diagnosed with breast and gynecologic cancers: a comparative analysis with the general population. Abstract presented at: Annual Meeting of the Association of VA Hematology/ Oncology (AVAHO); September 29–October 1, 2023; Chicago, IL. Abstract 47.
4. US Department of Veterans Affairs. Racial and ethnic minority veterans. Updated July 9, 2020. Accessed January 10, 2024. https:// www.va.gov/HEALTHEQUITY/Race_Ethnicity.asp
5. Stringer-Reasor EM, Elkhanany A, Khoury K, Simon MA, Newman LA. Disparities in breast cancer associated with African American identity. Am Soc Clin Oncol Educ Book. 2021;41:e29-e46. doi:10.1200/EDBK_319929
6. Landry I, Sumbly V, Vest M. Advancements in the treatment of triple- CANCER DATA TRENDS 2024 MARCH 2024 • FEDERAL PRACTITIONER negative breast cancer: a narrative review of the literature. Cureus. 2022;14(2):e21970. doi:10.7759/cureus.21970
7. Schlam I, Tolaney SM, Tarantino P. How I treat HER2-low advanced breast cancer. Breast. 2023;67:116-123. doi:10.1016/j.breast.2023.01.005
8. Modi S, Jacot W, Yamashita T, et al; for the DESTINY-Breast04 Trial Investigators. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N Engl J Med. 2022;387(1):9-20. doi:10.1056/NEJMoa2203690
1. US Department of Veterans Affairs. Mammogram/breast health. March 28, 2022. Accessed January 10, 2024. https://www.womenshealth.va.gov/topics/mammogram-breast-health.asp
2. First anniversary of Mammography and Medical Options Act (MAMMO Act). VA News. June 6, 2023. Accessed January 10, 2024. https://news.va.gov/120476/anniversary-mammography-and-medicaloptions-act/
3. Moss HA, Rasmussen, KM, Patil, V, et al. Demographic characteristics of veterans diagnosed with breast and gynecologic cancers: a comparative analysis with the general population. Abstract presented at: Annual Meeting of the Association of VA Hematology/ Oncology (AVAHO); September 29–October 1, 2023; Chicago, IL. Abstract 47.
4. US Department of Veterans Affairs. Racial and ethnic minority veterans. Updated July 9, 2020. Accessed January 10, 2024. https:// www.va.gov/HEALTHEQUITY/Race_Ethnicity.asp
5. Stringer-Reasor EM, Elkhanany A, Khoury K, Simon MA, Newman LA. Disparities in breast cancer associated with African American identity. Am Soc Clin Oncol Educ Book. 2021;41:e29-e46. doi:10.1200/EDBK_319929
6. Landry I, Sumbly V, Vest M. Advancements in the treatment of triple- CANCER DATA TRENDS 2024 MARCH 2024 • FEDERAL PRACTITIONER negative breast cancer: a narrative review of the literature. Cureus. 2022;14(2):e21970. doi:10.7759/cureus.21970
7. Schlam I, Tolaney SM, Tarantino P. How I treat HER2-low advanced breast cancer. Breast. 2023;67:116-123. doi:10.1016/j.breast.2023.01.005
8. Modi S, Jacot W, Yamashita T, et al; for the DESTINY-Breast04 Trial Investigators. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N Engl J Med. 2022;387(1):9-20. doi:10.1056/NEJMoa2203690
Cancer Data Trends 2024
The annual issue of Cancer Data Trends, produced in collaboration with the Association of VA Hematology/Oncology (AVAHO), highlights the latest research in some of the top cancers impacting US veterans.
Click to view the Digital Edition.
In this issue:
Hepatocellular Carcinoma
Special care for veterans, changes in staging, and biomarkers for early diagnosis
Lung Cancer
Guideline updates and racial disparities in veterans
Multiple Myeloma
Improving survival in the VA
Colorectal Cancer
Barriers to follow-up colonoscopies after FIT testing
B-Cell Lymphomas
Findings from the VA's National TeleOncology Program and recent therapy updates
Breast Cancer
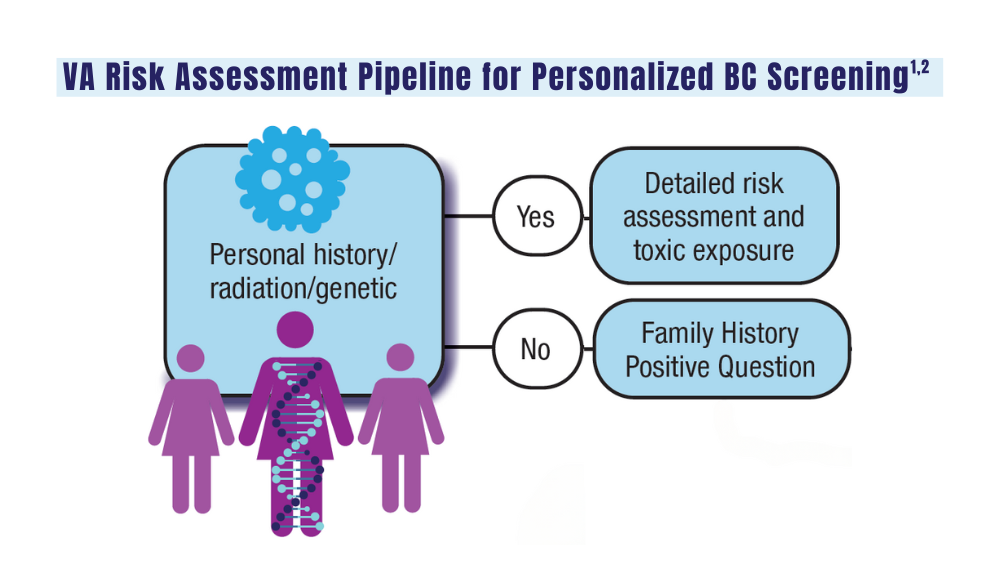
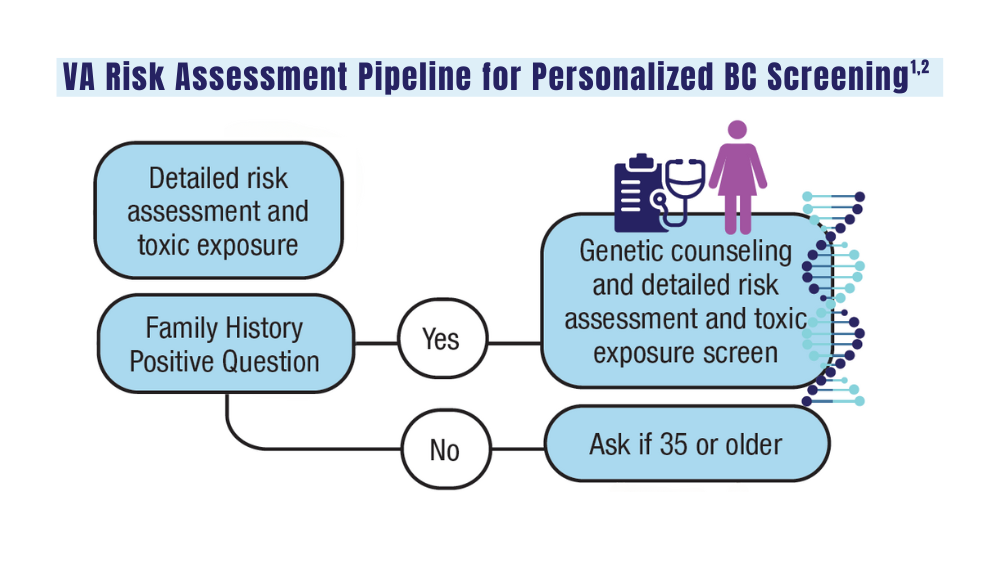
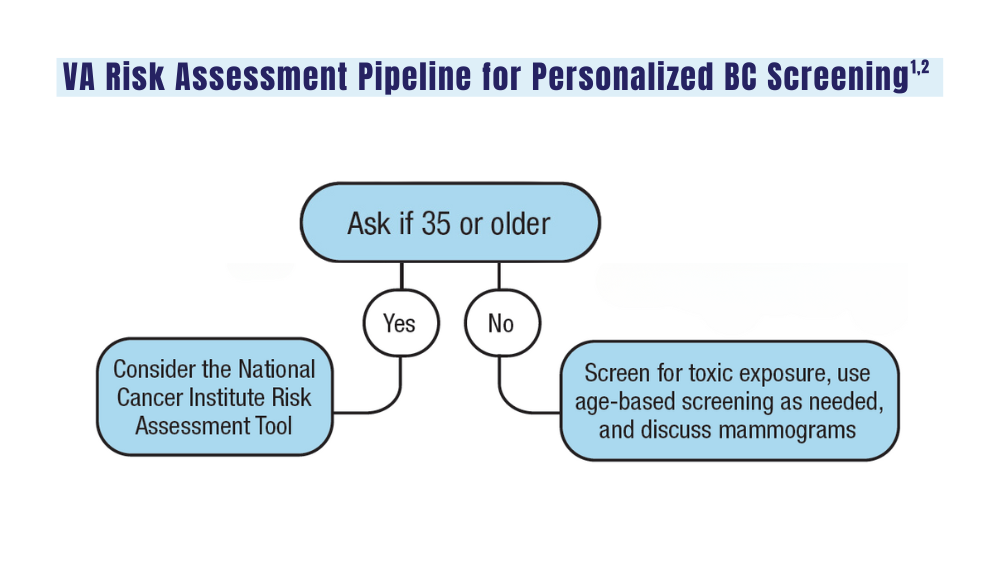
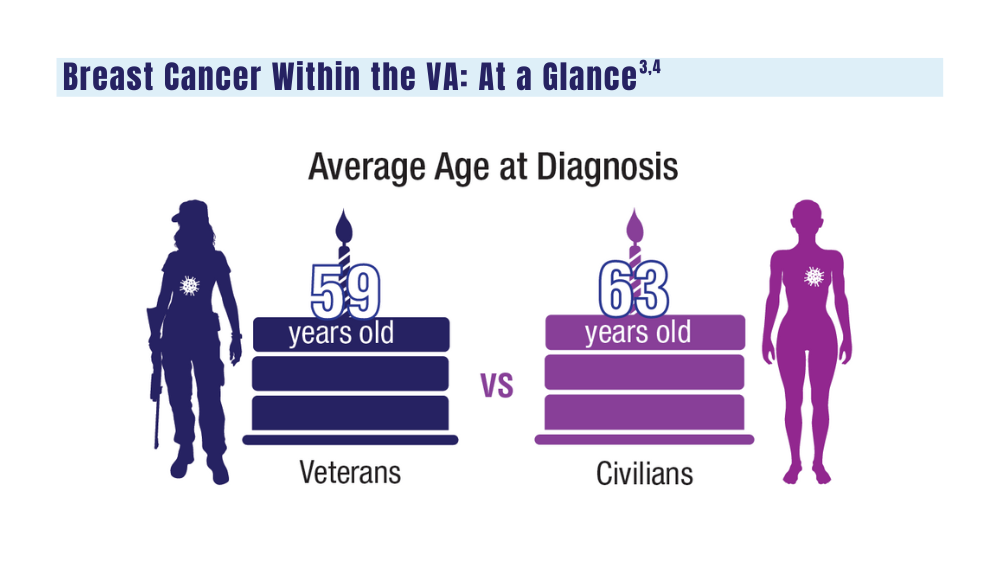
A look at the VA's Risk Assessment Pipeline and incidence among veterans vs the general population
Genitourinary Cancers
Molecular testing in prostate cancer, improving survival for metastatic RCC, and links between bladder cancer and Agent Orange exposure
The annual issue of Cancer Data Trends, produced in collaboration with the Association of VA Hematology/Oncology (AVAHO), highlights the latest research in some of the top cancers impacting US veterans.
Click to view the Digital Edition.
In this issue:
Hepatocellular Carcinoma
Special care for veterans, changes in staging, and biomarkers for early diagnosis
Lung Cancer
Guideline updates and racial disparities in veterans
Multiple Myeloma
Improving survival in the VA
Colorectal Cancer
Barriers to follow-up colonoscopies after FIT testing
B-Cell Lymphomas
Findings from the VA's National TeleOncology Program and recent therapy updates
Breast Cancer
A look at the VA's Risk Assessment Pipeline and incidence among veterans vs the general population
Genitourinary Cancers
Molecular testing in prostate cancer, improving survival for metastatic RCC, and links between bladder cancer and Agent Orange exposure
The annual issue of Cancer Data Trends, produced in collaboration with the Association of VA Hematology/Oncology (AVAHO), highlights the latest research in some of the top cancers impacting US veterans.
Click to view the Digital Edition.
In this issue:
Hepatocellular Carcinoma
Special care for veterans, changes in staging, and biomarkers for early diagnosis
Lung Cancer
Guideline updates and racial disparities in veterans
Multiple Myeloma
Improving survival in the VA
Colorectal Cancer
Barriers to follow-up colonoscopies after FIT testing
B-Cell Lymphomas
Findings from the VA's National TeleOncology Program and recent therapy updates
Breast Cancer
A look at the VA's Risk Assessment Pipeline and incidence among veterans vs the general population
Genitourinary Cancers
Molecular testing in prostate cancer, improving survival for metastatic RCC, and links between bladder cancer and Agent Orange exposure
Consider These Factors in an Academic Radiation Oncology Position
TOPLINE:
— and accept an offer if the practice is “great” in at least two of those areas and “good” in the third, experts say in a recent editorial.
METHODOLOGY:
- Many physicians choose to go into academic medicine because they want to stay involved in research and education while still treating patients.
- However, graduating radiation oncology residents often lack or have limited guidance on what to look for in a prospective job and how to assess their contract.
- This recent editorial provides guidance to radiation oncologists seeking academic positions. The authors advise prospective employees to evaluate three main factors — compensation, daily duties, and location — as well as provide tips for identifying red flags in each category.
TAKEAWAY:
- Compensation: Prospective faculty should assess both direct compensation, that is, salary, and indirect compensation, which typically includes retirement contributions and other perks. For direct compensation, what is the base salary? Is extra work compensated? How does the salary offer measure up to salary data reported by national agencies? Also: Don’t overlook uncompensated duties, such as time in tumor boards or in meetings, which may be time-consuming, and make sure compensation terms are clearly delineated in a contract and equitable among physicians in a specific rank.
- Daily duties: When it comes to daily life on the job, a prospective employee should consider many factors, including the cancer center’s excitement to hire you, the reputation of the faculty and leaders at the organization, employee turnover rates, diversity among faculty, and the time line of career advancement.
- Location: The location of the job encompasses the geography — such as distance from home to work, the number of practices covered, cost of living, and the area itself — as well as the atmosphere for conducting research and publishing.
- Finally, carefully review the job contract. All the key aspects of the job, including compensation and benefits, should be clearly stated in the contract to “improve communication of expectations.”
IN PRACTICE:
“A prospective faculty member can ask 100 questions, but they can’t make 100 demands; consideration of the three domains can help to focus negotiation efforts where the efforts are needed,” the authors noted.
SOURCE:
This editorial, led by Nicholas G. Zaorsky from the Department of Radiation Oncology, University Hospitals Seidman Cancer Center, Case Western Reserve School of Medicine, Cleveland, Ohio, was published online in Practical Radiation Oncology
DISCLOSURES:
The lead author declared being supported by the American Cancer Society and National Institutes of Health. He also reported having ties with many other sources.
A version of this article appeared on Medscape.com.
TOPLINE:
— and accept an offer if the practice is “great” in at least two of those areas and “good” in the third, experts say in a recent editorial.
METHODOLOGY:
- Many physicians choose to go into academic medicine because they want to stay involved in research and education while still treating patients.
- However, graduating radiation oncology residents often lack or have limited guidance on what to look for in a prospective job and how to assess their contract.
- This recent editorial provides guidance to radiation oncologists seeking academic positions. The authors advise prospective employees to evaluate three main factors — compensation, daily duties, and location — as well as provide tips for identifying red flags in each category.
TAKEAWAY:
- Compensation: Prospective faculty should assess both direct compensation, that is, salary, and indirect compensation, which typically includes retirement contributions and other perks. For direct compensation, what is the base salary? Is extra work compensated? How does the salary offer measure up to salary data reported by national agencies? Also: Don’t overlook uncompensated duties, such as time in tumor boards or in meetings, which may be time-consuming, and make sure compensation terms are clearly delineated in a contract and equitable among physicians in a specific rank.
- Daily duties: When it comes to daily life on the job, a prospective employee should consider many factors, including the cancer center’s excitement to hire you, the reputation of the faculty and leaders at the organization, employee turnover rates, diversity among faculty, and the time line of career advancement.
- Location: The location of the job encompasses the geography — such as distance from home to work, the number of practices covered, cost of living, and the area itself — as well as the atmosphere for conducting research and publishing.
- Finally, carefully review the job contract. All the key aspects of the job, including compensation and benefits, should be clearly stated in the contract to “improve communication of expectations.”
IN PRACTICE:
“A prospective faculty member can ask 100 questions, but they can’t make 100 demands; consideration of the three domains can help to focus negotiation efforts where the efforts are needed,” the authors noted.
SOURCE:
This editorial, led by Nicholas G. Zaorsky from the Department of Radiation Oncology, University Hospitals Seidman Cancer Center, Case Western Reserve School of Medicine, Cleveland, Ohio, was published online in Practical Radiation Oncology
DISCLOSURES:
The lead author declared being supported by the American Cancer Society and National Institutes of Health. He also reported having ties with many other sources.
A version of this article appeared on Medscape.com.
TOPLINE:
— and accept an offer if the practice is “great” in at least two of those areas and “good” in the third, experts say in a recent editorial.
METHODOLOGY:
- Many physicians choose to go into academic medicine because they want to stay involved in research and education while still treating patients.
- However, graduating radiation oncology residents often lack or have limited guidance on what to look for in a prospective job and how to assess their contract.
- This recent editorial provides guidance to radiation oncologists seeking academic positions. The authors advise prospective employees to evaluate three main factors — compensation, daily duties, and location — as well as provide tips for identifying red flags in each category.
TAKEAWAY:
- Compensation: Prospective faculty should assess both direct compensation, that is, salary, and indirect compensation, which typically includes retirement contributions and other perks. For direct compensation, what is the base salary? Is extra work compensated? How does the salary offer measure up to salary data reported by national agencies? Also: Don’t overlook uncompensated duties, such as time in tumor boards or in meetings, which may be time-consuming, and make sure compensation terms are clearly delineated in a contract and equitable among physicians in a specific rank.
- Daily duties: When it comes to daily life on the job, a prospective employee should consider many factors, including the cancer center’s excitement to hire you, the reputation of the faculty and leaders at the organization, employee turnover rates, diversity among faculty, and the time line of career advancement.
- Location: The location of the job encompasses the geography — such as distance from home to work, the number of practices covered, cost of living, and the area itself — as well as the atmosphere for conducting research and publishing.
- Finally, carefully review the job contract. All the key aspects of the job, including compensation and benefits, should be clearly stated in the contract to “improve communication of expectations.”
IN PRACTICE:
“A prospective faculty member can ask 100 questions, but they can’t make 100 demands; consideration of the three domains can help to focus negotiation efforts where the efforts are needed,” the authors noted.
SOURCE:
This editorial, led by Nicholas G. Zaorsky from the Department of Radiation Oncology, University Hospitals Seidman Cancer Center, Case Western Reserve School of Medicine, Cleveland, Ohio, was published online in Practical Radiation Oncology
DISCLOSURES:
The lead author declared being supported by the American Cancer Society and National Institutes of Health. He also reported having ties with many other sources.
A version of this article appeared on Medscape.com.
Meta-analysis Identifies Unique Risk Factors of Triple-Negative Breast Cancer
Key clinical point: The risk factors for overall breast cancer (such as parity, menopausal hormone therapy use, and alcohol consumption) did not increase the risk for triple-negative breast cancer (TNBC), which had a distinct risk factor profile.
Major finding: Parity, menopausal hormone therapy use, alcohol consumption, smoking, and higher body mass index were not significantly associated with TNBC risk (all P > .05); instead, family history (odds ratio [OR] 1.55; P < .001), longer duration of oral contraceptive use (OR 1.29; P < .001), and higher breast density (OR 2.19; P < .001) were significantly associated with an increased risk for TNBC.
Study details: This meta-analysis evaluated the association between TNBC incidence and established BC risk factors using data from 33 studies.
Disclosures: This study was supported by grants from the American Cancer Society and Royal College of Surgeons in Ireland - Medical University of Bahrain (RCSI-MUB Bahrain). The authors declared no conflicts of interest.
Source: Kumar N, Ehsan S, Banerjee S, et al. The unique risk factor profile of triple negative breast cancer: A comprehensive meta-analysis. J Natl Cancer Inst. 2024 (Mar 5). Doi: 10.1093/jnci/djae056 Source
Key clinical point: The risk factors for overall breast cancer (such as parity, menopausal hormone therapy use, and alcohol consumption) did not increase the risk for triple-negative breast cancer (TNBC), which had a distinct risk factor profile.
Major finding: Parity, menopausal hormone therapy use, alcohol consumption, smoking, and higher body mass index were not significantly associated with TNBC risk (all P > .05); instead, family history (odds ratio [OR] 1.55; P < .001), longer duration of oral contraceptive use (OR 1.29; P < .001), and higher breast density (OR 2.19; P < .001) were significantly associated with an increased risk for TNBC.
Study details: This meta-analysis evaluated the association between TNBC incidence and established BC risk factors using data from 33 studies.
Disclosures: This study was supported by grants from the American Cancer Society and Royal College of Surgeons in Ireland - Medical University of Bahrain (RCSI-MUB Bahrain). The authors declared no conflicts of interest.
Source: Kumar N, Ehsan S, Banerjee S, et al. The unique risk factor profile of triple negative breast cancer: A comprehensive meta-analysis. J Natl Cancer Inst. 2024 (Mar 5). Doi: 10.1093/jnci/djae056 Source
Key clinical point: The risk factors for overall breast cancer (such as parity, menopausal hormone therapy use, and alcohol consumption) did not increase the risk for triple-negative breast cancer (TNBC), which had a distinct risk factor profile.
Major finding: Parity, menopausal hormone therapy use, alcohol consumption, smoking, and higher body mass index were not significantly associated with TNBC risk (all P > .05); instead, family history (odds ratio [OR] 1.55; P < .001), longer duration of oral contraceptive use (OR 1.29; P < .001), and higher breast density (OR 2.19; P < .001) were significantly associated with an increased risk for TNBC.
Study details: This meta-analysis evaluated the association between TNBC incidence and established BC risk factors using data from 33 studies.
Disclosures: This study was supported by grants from the American Cancer Society and Royal College of Surgeons in Ireland - Medical University of Bahrain (RCSI-MUB Bahrain). The authors declared no conflicts of interest.
Source: Kumar N, Ehsan S, Banerjee S, et al. The unique risk factor profile of triple negative breast cancer: A comprehensive meta-analysis. J Natl Cancer Inst. 2024 (Mar 5). Doi: 10.1093/jnci/djae056 Source
Pro-Vegetarian Diet May Lower Risk for Breast Cancer
Key clinical point: The pro-vegetarian dietary pattern (PDP) was associated with a significantly lower risk for breast cancer (BC) in women, particularly postmenopausal women.
Major finding: Compared with women who had low adherence to PDP (score ≤ 33), the risk for BC was significantly lower among women with moderate adherence to PDP (score 34-38; adjusted odds ratio [aOR] 0.42; P = .003) and in those with high adherence to PDP (score ≥ 39; aOR 0.49; P = .017), with outcomes being similar in the subgroup of postmenopausal women.
Study details: Findings are from a case-control study including women with BC (n = 134) and those without cancer (n = 265).
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Hosseini Y, Hadi Sichani P, Moslemi E, et al. Pro-vegetarian dietary pattern and risk of breast cancer: A case-control study. Breast Cancer Res Treat. 2024 (Feb 28). doi: 10.1007/s10549-024-07243-8 Source
Key clinical point: The pro-vegetarian dietary pattern (PDP) was associated with a significantly lower risk for breast cancer (BC) in women, particularly postmenopausal women.
Major finding: Compared with women who had low adherence to PDP (score ≤ 33), the risk for BC was significantly lower among women with moderate adherence to PDP (score 34-38; adjusted odds ratio [aOR] 0.42; P = .003) and in those with high adherence to PDP (score ≥ 39; aOR 0.49; P = .017), with outcomes being similar in the subgroup of postmenopausal women.
Study details: Findings are from a case-control study including women with BC (n = 134) and those without cancer (n = 265).
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Hosseini Y, Hadi Sichani P, Moslemi E, et al. Pro-vegetarian dietary pattern and risk of breast cancer: A case-control study. Breast Cancer Res Treat. 2024 (Feb 28). doi: 10.1007/s10549-024-07243-8 Source
Key clinical point: The pro-vegetarian dietary pattern (PDP) was associated with a significantly lower risk for breast cancer (BC) in women, particularly postmenopausal women.
Major finding: Compared with women who had low adherence to PDP (score ≤ 33), the risk for BC was significantly lower among women with moderate adherence to PDP (score 34-38; adjusted odds ratio [aOR] 0.42; P = .003) and in those with high adherence to PDP (score ≥ 39; aOR 0.49; P = .017), with outcomes being similar in the subgroup of postmenopausal women.
Study details: Findings are from a case-control study including women with BC (n = 134) and those without cancer (n = 265).
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Hosseini Y, Hadi Sichani P, Moslemi E, et al. Pro-vegetarian dietary pattern and risk of breast cancer: A case-control study. Breast Cancer Res Treat. 2024 (Feb 28). doi: 10.1007/s10549-024-07243-8 Source
Real-World Study Supports Everolimus + Exemestane as HR+/HER2− BC Treatment
Key clinical point: Everolimus + exemestane demonstrated good efficacy and had a manageable safety profile in postmenopausal women with hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2−) breast cancer (BC).
Major finding: Everolimus + exemestane led to a median progression-free survival (PFS) of 6.6 months (95% CI 6.3-7.0 months). PFS was more favorable among patients with a greater vs lower body mass index (≥25 vs 20 to <25 kg/m2; P < .0001); however, the survival outcomes were worse among patients with vs without visceral metastases (hazard ratio 1.417; P < .0001). Stomatitis (42.6%) and fatigue (19.8%) were the most frequent adverse events.
Study details: Findings are from a prospective, non-interventional study including 2074 postmenopausal women with HR+/HER2− advanced BC who received everolimus + exemestane.
Disclosures: This study was funded by Novartis Deutschland GmbH, Germany. Two authors declared being employees of or holding stocks in Novartis. Some authors declared receiving honoraria or personal fees or having other ties with Novartis and various other sources. Eight authors declared no conflicts of interest.
Source: Lüftner D, Schuetz F, Schneeweiss A, et al. Efficacy and safety of everolimus plus exemestane in patients with hormone receptor-positive, HER-2-negative advanced breast cancer: Results from the open-label, multicentre, non-interventional BRAWO study. Int J Cancer. 2024 (Mar 6). doi: 10.1002/ijc.34912 Source
Key clinical point: Everolimus + exemestane demonstrated good efficacy and had a manageable safety profile in postmenopausal women with hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2−) breast cancer (BC).
Major finding: Everolimus + exemestane led to a median progression-free survival (PFS) of 6.6 months (95% CI 6.3-7.0 months). PFS was more favorable among patients with a greater vs lower body mass index (≥25 vs 20 to <25 kg/m2; P < .0001); however, the survival outcomes were worse among patients with vs without visceral metastases (hazard ratio 1.417; P < .0001). Stomatitis (42.6%) and fatigue (19.8%) were the most frequent adverse events.
Study details: Findings are from a prospective, non-interventional study including 2074 postmenopausal women with HR+/HER2− advanced BC who received everolimus + exemestane.
Disclosures: This study was funded by Novartis Deutschland GmbH, Germany. Two authors declared being employees of or holding stocks in Novartis. Some authors declared receiving honoraria or personal fees or having other ties with Novartis and various other sources. Eight authors declared no conflicts of interest.
Source: Lüftner D, Schuetz F, Schneeweiss A, et al. Efficacy and safety of everolimus plus exemestane in patients with hormone receptor-positive, HER-2-negative advanced breast cancer: Results from the open-label, multicentre, non-interventional BRAWO study. Int J Cancer. 2024 (Mar 6). doi: 10.1002/ijc.34912 Source
Key clinical point: Everolimus + exemestane demonstrated good efficacy and had a manageable safety profile in postmenopausal women with hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2−) breast cancer (BC).
Major finding: Everolimus + exemestane led to a median progression-free survival (PFS) of 6.6 months (95% CI 6.3-7.0 months). PFS was more favorable among patients with a greater vs lower body mass index (≥25 vs 20 to <25 kg/m2; P < .0001); however, the survival outcomes were worse among patients with vs without visceral metastases (hazard ratio 1.417; P < .0001). Stomatitis (42.6%) and fatigue (19.8%) were the most frequent adverse events.
Study details: Findings are from a prospective, non-interventional study including 2074 postmenopausal women with HR+/HER2− advanced BC who received everolimus + exemestane.
Disclosures: This study was funded by Novartis Deutschland GmbH, Germany. Two authors declared being employees of or holding stocks in Novartis. Some authors declared receiving honoraria or personal fees or having other ties with Novartis and various other sources. Eight authors declared no conflicts of interest.
Source: Lüftner D, Schuetz F, Schneeweiss A, et al. Efficacy and safety of everolimus plus exemestane in patients with hormone receptor-positive, HER-2-negative advanced breast cancer: Results from the open-label, multicentre, non-interventional BRAWO study. Int J Cancer. 2024 (Mar 6). doi: 10.1002/ijc.34912 Source
Axillary Lymph Node Dissection Can Be Safely Skipped in Breast Cancer Patients Undergoing Mastectomy
Key clinical point: Sentinel lymph node biopsy (SLNB), a less invasive strategy, resulted in comparable survival and regional disease control as axillary lymph node dissection (ALND) in patients with sentinel node-positive early breast cancer (BC) who underwent total mastectomy (TM).
Major finding: There were no significant differences in 5-year ipsilateral locoregional recurrence-free survival (LRRFS; P = .21), 5-year distant metastasis-free survival (P = .96), and disease-free survival (P > .05) between the SLNB-alone and ALND groups. However, receipt vs no receipt of radiation therapy improved local disease control in the SLNB group (5-year LRRFS; 100.0% vs 92.9%; P = .02).
Study details: Findings are from a retrospective study including 643 patients with early BC with 1-3 metastatic sentinel lymph nodes who underwent total mastectomy, of which 237 and 406 patients underwent SLNB alone and completion ALND, respectively.
Disclosures: The open access funding for this study was enabled and organized by Seoul National University. The authors declared no conflicts of interest.
Source: Chun JW, Kang E, Kim H-K, et al. Oncological safety of skipping axillary lymph node dissection in patients with clinical N0, sentinel node-positive breast cancer undergoing total mastectomy. Ann Surg Oncol. 2024 (Feb 17). doi: 10.1245/s10434-024-15049-7 Source
Key clinical point: Sentinel lymph node biopsy (SLNB), a less invasive strategy, resulted in comparable survival and regional disease control as axillary lymph node dissection (ALND) in patients with sentinel node-positive early breast cancer (BC) who underwent total mastectomy (TM).
Major finding: There were no significant differences in 5-year ipsilateral locoregional recurrence-free survival (LRRFS; P = .21), 5-year distant metastasis-free survival (P = .96), and disease-free survival (P > .05) between the SLNB-alone and ALND groups. However, receipt vs no receipt of radiation therapy improved local disease control in the SLNB group (5-year LRRFS; 100.0% vs 92.9%; P = .02).
Study details: Findings are from a retrospective study including 643 patients with early BC with 1-3 metastatic sentinel lymph nodes who underwent total mastectomy, of which 237 and 406 patients underwent SLNB alone and completion ALND, respectively.
Disclosures: The open access funding for this study was enabled and organized by Seoul National University. The authors declared no conflicts of interest.
Source: Chun JW, Kang E, Kim H-K, et al. Oncological safety of skipping axillary lymph node dissection in patients with clinical N0, sentinel node-positive breast cancer undergoing total mastectomy. Ann Surg Oncol. 2024 (Feb 17). doi: 10.1245/s10434-024-15049-7 Source
Key clinical point: Sentinel lymph node biopsy (SLNB), a less invasive strategy, resulted in comparable survival and regional disease control as axillary lymph node dissection (ALND) in patients with sentinel node-positive early breast cancer (BC) who underwent total mastectomy (TM).
Major finding: There were no significant differences in 5-year ipsilateral locoregional recurrence-free survival (LRRFS; P = .21), 5-year distant metastasis-free survival (P = .96), and disease-free survival (P > .05) between the SLNB-alone and ALND groups. However, receipt vs no receipt of radiation therapy improved local disease control in the SLNB group (5-year LRRFS; 100.0% vs 92.9%; P = .02).
Study details: Findings are from a retrospective study including 643 patients with early BC with 1-3 metastatic sentinel lymph nodes who underwent total mastectomy, of which 237 and 406 patients underwent SLNB alone and completion ALND, respectively.
Disclosures: The open access funding for this study was enabled and organized by Seoul National University. The authors declared no conflicts of interest.
Source: Chun JW, Kang E, Kim H-K, et al. Oncological safety of skipping axillary lymph node dissection in patients with clinical N0, sentinel node-positive breast cancer undergoing total mastectomy. Ann Surg Oncol. 2024 (Feb 17). doi: 10.1245/s10434-024-15049-7 Source
Obesity and Family History of Cancer Raise Breast Cancer Risk
Key clinical point: The coexistence of obesity and family history of cancer significantly increased the risk for breast cancer (BC) in women, suggesting that weight management is important in women with a family history of cancer.
Major finding: The risk for BC was significantly higher in women with vs without a family history of BC (adjusted hazard ratio [aHR] 1.63; 95% CI 1.22-2.49). The risk was further elevated in women with a body mass index (BMI) ≥ 24 kg/m2 and a family history of cancer vs women with BMI < 24 kg/m2 and no family history of cancer (adjusted hazard ratio 2.06; 95% CI 1.39-3.06).
Study details: Findings are from a population-based prospective cohort study that included 15,055 women, of which 4210 women had a family history of cancer.
Disclosures: This study was supported by the Nature Science Foundation of Minhang district, Shanghai, China. The authors declared no conflicts of interest.
Source: Cao J, Li J, Zhang Z, et al. Interaction between body mass index and family history of cancer on the risk of female breast cancer. Sci Rep. 2024;14:4927 (Feb 28). doi: 10.1038/s41598-024-54762-x Source
Key clinical point: The coexistence of obesity and family history of cancer significantly increased the risk for breast cancer (BC) in women, suggesting that weight management is important in women with a family history of cancer.
Major finding: The risk for BC was significantly higher in women with vs without a family history of BC (adjusted hazard ratio [aHR] 1.63; 95% CI 1.22-2.49). The risk was further elevated in women with a body mass index (BMI) ≥ 24 kg/m2 and a family history of cancer vs women with BMI < 24 kg/m2 and no family history of cancer (adjusted hazard ratio 2.06; 95% CI 1.39-3.06).
Study details: Findings are from a population-based prospective cohort study that included 15,055 women, of which 4210 women had a family history of cancer.
Disclosures: This study was supported by the Nature Science Foundation of Minhang district, Shanghai, China. The authors declared no conflicts of interest.
Source: Cao J, Li J, Zhang Z, et al. Interaction between body mass index and family history of cancer on the risk of female breast cancer. Sci Rep. 2024;14:4927 (Feb 28). doi: 10.1038/s41598-024-54762-x Source
Key clinical point: The coexistence of obesity and family history of cancer significantly increased the risk for breast cancer (BC) in women, suggesting that weight management is important in women with a family history of cancer.
Major finding: The risk for BC was significantly higher in women with vs without a family history of BC (adjusted hazard ratio [aHR] 1.63; 95% CI 1.22-2.49). The risk was further elevated in women with a body mass index (BMI) ≥ 24 kg/m2 and a family history of cancer vs women with BMI < 24 kg/m2 and no family history of cancer (adjusted hazard ratio 2.06; 95% CI 1.39-3.06).
Study details: Findings are from a population-based prospective cohort study that included 15,055 women, of which 4210 women had a family history of cancer.
Disclosures: This study was supported by the Nature Science Foundation of Minhang district, Shanghai, China. The authors declared no conflicts of interest.
Source: Cao J, Li J, Zhang Z, et al. Interaction between body mass index and family history of cancer on the risk of female breast cancer. Sci Rep. 2024;14:4927 (Feb 28). doi: 10.1038/s41598-024-54762-x Source
Breast-Conserving Surgery Does Not Increase Locoregional Recurrence in TNBC
Key clinical point: Compared with mastectomy, breast-conserving surgery (BCS) led to comparable locoregional recurrence (LRR; the first relapse site) events along with improved survival outcomes in patients with early-stage node-negative triple-negative breast cancer (TNBC) treated with neoadjuvant chemotherapy.
Major finding: BCS vs mastectomy following neoadjuvant chemotherapy did not increase LRR (P = .5209) and was significantly associated with improved disease-free survival (adjusted hazard ratio [aHR] 0.51; P < .001) and overall survival (aHR 0.43; P < .001). Absence of pathologic complete response was the only determinant for worsened LRR risk (HR 2.22; P = .001).
Study details: This retrospective analysis of eight prospective trials included 1074 neoadjuvant chemotherapy-treated patients with early-stage node-negative TNBC and available surgery data.
Disclosures: This study received financial support from Deutschen Forschungsgemeinschaft (DFG) within the funding program Open Access Publikationskosten. Three authors declared being employees of GBG Forschungs GmbH. Ninel authors declared receiving honoraria, grants, consulting fees, or personal fees or having other ties with various sources. The other authors had no conflicts to declare.
Source: Krug D, Vladimirova V, Untch M, et al. Breast-conserving surgery is not associated with increased local recurrence in patients with early-stage node-negative triple-negative breast cancer treated with neoadjuvant chemotherapy. Breast. 2024;74:103701 (Feb 24). doi: 10.1016/j.breast.2024.103701 Source
Key clinical point: Compared with mastectomy, breast-conserving surgery (BCS) led to comparable locoregional recurrence (LRR; the first relapse site) events along with improved survival outcomes in patients with early-stage node-negative triple-negative breast cancer (TNBC) treated with neoadjuvant chemotherapy.
Major finding: BCS vs mastectomy following neoadjuvant chemotherapy did not increase LRR (P = .5209) and was significantly associated with improved disease-free survival (adjusted hazard ratio [aHR] 0.51; P < .001) and overall survival (aHR 0.43; P < .001). Absence of pathologic complete response was the only determinant for worsened LRR risk (HR 2.22; P = .001).
Study details: This retrospective analysis of eight prospective trials included 1074 neoadjuvant chemotherapy-treated patients with early-stage node-negative TNBC and available surgery data.
Disclosures: This study received financial support from Deutschen Forschungsgemeinschaft (DFG) within the funding program Open Access Publikationskosten. Three authors declared being employees of GBG Forschungs GmbH. Ninel authors declared receiving honoraria, grants, consulting fees, or personal fees or having other ties with various sources. The other authors had no conflicts to declare.
Source: Krug D, Vladimirova V, Untch M, et al. Breast-conserving surgery is not associated with increased local recurrence in patients with early-stage node-negative triple-negative breast cancer treated with neoadjuvant chemotherapy. Breast. 2024;74:103701 (Feb 24). doi: 10.1016/j.breast.2024.103701 Source
Key clinical point: Compared with mastectomy, breast-conserving surgery (BCS) led to comparable locoregional recurrence (LRR; the first relapse site) events along with improved survival outcomes in patients with early-stage node-negative triple-negative breast cancer (TNBC) treated with neoadjuvant chemotherapy.
Major finding: BCS vs mastectomy following neoadjuvant chemotherapy did not increase LRR (P = .5209) and was significantly associated with improved disease-free survival (adjusted hazard ratio [aHR] 0.51; P < .001) and overall survival (aHR 0.43; P < .001). Absence of pathologic complete response was the only determinant for worsened LRR risk (HR 2.22; P = .001).
Study details: This retrospective analysis of eight prospective trials included 1074 neoadjuvant chemotherapy-treated patients with early-stage node-negative TNBC and available surgery data.
Disclosures: This study received financial support from Deutschen Forschungsgemeinschaft (DFG) within the funding program Open Access Publikationskosten. Three authors declared being employees of GBG Forschungs GmbH. Ninel authors declared receiving honoraria, grants, consulting fees, or personal fees or having other ties with various sources. The other authors had no conflicts to declare.
Source: Krug D, Vladimirova V, Untch M, et al. Breast-conserving surgery is not associated with increased local recurrence in patients with early-stage node-negative triple-negative breast cancer treated with neoadjuvant chemotherapy. Breast. 2024;74:103701 (Feb 24). doi: 10.1016/j.breast.2024.103701 Source
Doxorubicin May Raise Breast Cancer Risk in Hodgkin Lymphoma Survivors
Key clinical point: The risk for breast cancer (BC) was significantly elevated in female 5-year survivors of Hodgkin lymphoma (HL) who were treated with doxorubicin between the ages of 15 and 50 years.
Major finding: The risk for BC was 1.4 times higher (95% CI 1.04-1.9) in survivors of HL who did vs did not receive doxorubicin, with the risk increasing further in survivors who received a cumulative doxorubicin dose > 200 mg/m2 (adjusted hazard ratio 1.5; 95% CI 1.08-2.1). In fact, every 100 mg/m2 increase in doxorubicin dose increased the risk for BC by 1.18 times (95% CI 1.05-1.32).
Study details: Findings are from a cohort study including 5-year survivors of HL (n = 1964) who received treatment between the ages of 15 and 50 years, of whom 1113 patients received doxorubicin.
Disclosures: This study was supported by grants from the Dutch Cancer Society, Netherlands. Five authors declared receiving honoraria, research funding, travel, accommodations, or other expenses from or serving in consulting or advisory roles or as speakers' bureau members for various sources.
Source: Neppelenbroek SIM, Geurts YM, Aleman BMP, et al. Doxorubicin exposure and breast cancer risk in survivors of adolescent and adult Hodgkin lymphoma. J Clin Oncol. 2024 (Feb 15). doi: 10.1200/JCO.23.01386 Source
Key clinical point: The risk for breast cancer (BC) was significantly elevated in female 5-year survivors of Hodgkin lymphoma (HL) who were treated with doxorubicin between the ages of 15 and 50 years.
Major finding: The risk for BC was 1.4 times higher (95% CI 1.04-1.9) in survivors of HL who did vs did not receive doxorubicin, with the risk increasing further in survivors who received a cumulative doxorubicin dose > 200 mg/m2 (adjusted hazard ratio 1.5; 95% CI 1.08-2.1). In fact, every 100 mg/m2 increase in doxorubicin dose increased the risk for BC by 1.18 times (95% CI 1.05-1.32).
Study details: Findings are from a cohort study including 5-year survivors of HL (n = 1964) who received treatment between the ages of 15 and 50 years, of whom 1113 patients received doxorubicin.
Disclosures: This study was supported by grants from the Dutch Cancer Society, Netherlands. Five authors declared receiving honoraria, research funding, travel, accommodations, or other expenses from or serving in consulting or advisory roles or as speakers' bureau members for various sources.
Source: Neppelenbroek SIM, Geurts YM, Aleman BMP, et al. Doxorubicin exposure and breast cancer risk in survivors of adolescent and adult Hodgkin lymphoma. J Clin Oncol. 2024 (Feb 15). doi: 10.1200/JCO.23.01386 Source
Key clinical point: The risk for breast cancer (BC) was significantly elevated in female 5-year survivors of Hodgkin lymphoma (HL) who were treated with doxorubicin between the ages of 15 and 50 years.
Major finding: The risk for BC was 1.4 times higher (95% CI 1.04-1.9) in survivors of HL who did vs did not receive doxorubicin, with the risk increasing further in survivors who received a cumulative doxorubicin dose > 200 mg/m2 (adjusted hazard ratio 1.5; 95% CI 1.08-2.1). In fact, every 100 mg/m2 increase in doxorubicin dose increased the risk for BC by 1.18 times (95% CI 1.05-1.32).
Study details: Findings are from a cohort study including 5-year survivors of HL (n = 1964) who received treatment between the ages of 15 and 50 years, of whom 1113 patients received doxorubicin.
Disclosures: This study was supported by grants from the Dutch Cancer Society, Netherlands. Five authors declared receiving honoraria, research funding, travel, accommodations, or other expenses from or serving in consulting or advisory roles or as speakers' bureau members for various sources.
Source: Neppelenbroek SIM, Geurts YM, Aleman BMP, et al. Doxorubicin exposure and breast cancer risk in survivors of adolescent and adult Hodgkin lymphoma. J Clin Oncol. 2024 (Feb 15). doi: 10.1200/JCO.23.01386 Source