User login
What’s the most likely cause of this man’s severe headaches?
He reports that these started 3 days ago. His headache is worse when he stands, and resolves when he lies down. Valsalva maneuver makes the headache much worse. The headaches are present in the occipital region. He also has noticed the onset of tinnitus. A physical exam reveals that his blood pressure is 110/70 mm Hg, his pulse is 60 beats per minute, and his temperature is 36.4° C. His standing BP is 105/60 mm Hg and standing pulse is 66 bpm. Both his neurologic exam and noncontrast head CT scan are normal.
Which of the following is the most likely diagnosis?
A) Subarachnoid hemorrhage
B) POTS (Postural orthostatic tachycardia syndrome)
C) Hypnic headache
D) Spontaneous intracranial hypotension (SIH)
E) Acoustic neuroma
The most likely cause for this patient’s headaches given his set of symptoms is spontaneous intracranial hypotension. Orthostatic headaches are common with POTS, but the absence of tachycardia with standing makes this diagnosis unlikely.
Spontaneous intracranial hypotension has symptoms that we are all familiar with in the post–lumbar puncture patient. In patients with post-LP headache, the positional nature makes it easy to diagnose. Patients who have had a lumbar puncture have a clear reason they have a cerebrospinal fluid (CSF) leak, leading to intracranial hypotension. Those with SIH do not.
Related research
Schievink summarized a lot of useful information in a review of patients with spontaneous intracranial hypotension.1 The incidence is about 5/100,000, with the most common age around 40 years old. The most common symptom is orthostatic headache. The headache usually occurs within 15 minutes upon standing, and many patients have the onset of headache rapidly upon standing.
Usually the headache improves with lying down, and it is often brought on with Valsalva maneuver. Many patients report headaches that are worse in the second half of the day.
Orthostatic headache occurs in almost all patients with spontaneous intracranial hypotension, but in one series it occurred only in 77% of patients with SIH.2 The patients who did not have typical headaches are more likely to have auditory symptoms such as tinnitus and muffled hearing.3
When you suspect SIH, appropriate workup is to start with brain MR imaging with contrast. Krantz and colleagues found dural enhancement was present in 83% of cases of SIH, venous distention sign in 75%, and brain sagging in 61%.4
About 10% of patients with SIH have normal brain imaging, so if the clinical features strongly suggest the diagnosis, moving on to spinal imaging with CT myelography or spinal MR are appropriate next steps.5
The causes of SIH are meningeal diverticula (usually in the thoracic or upper lumbar regions), ventral dural tears (usually from osteophytes), and cerebrospinal fluid–venous fistulas. Treatment of SIH has traditionally included a conservative approach of bed rest, oral hydration, and caffeine. The effectiveness of this is unknown, and, in one small series, 61% had headache symptoms at 6 months.6
Epidural blood patches are likely more rapidly effective than conservative therapy. In one study comparing the two treatments, Chung and colleagues found that 77% of the patients who received an epidural blood patch had complete headache relief at 4 weeks, compared with 40% of those who received conservative measures (P < .05).7
Clinical pearls
- Strongly consider SIH in patients with positional headache.
- Brain MR should be the first diagnostic test.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as 3rd-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at imnews@mdedge.com.
References
1. Schievink WI. Spontaneous spinal cerebrospinal fluid leaks and intracranial hypotension. JAMA. 2006;295:2286-96.
2. Mea E et al. Headache attributed to spontaneous intracranial hypotension. Neurol Sci. 2008;29:164-65.
3. Krantz PG et al. Spontaneous Intracranial Hypotension: 10 Myths and Misperceptions. Headache. 2018;58:948-59.
4. Krantz PG et. al. Imaging signs in spontaneous intracranial hypotension: prevalence and relationship to CSF pressure. AJNR Am J Neuroradiol. 2016;37:1374-8.
5. Krantz PG et al. Spontaneous intracranial hypotension: Pathogenesis, diagnosis, and treatment. Neuroimaging Clin N Am. 2019;29:581-94.
6. Kong D-S et. al. Clinical features and long-term results of spontaneous intracranial hypotension. Neurosurgery. 2005;57:91-6.
7. Chung SJ et al. Short- and long-term outcomes of spontaneous CSF hypovolemia. Eur Neurol. 2005;54:63-7.
He reports that these started 3 days ago. His headache is worse when he stands, and resolves when he lies down. Valsalva maneuver makes the headache much worse. The headaches are present in the occipital region. He also has noticed the onset of tinnitus. A physical exam reveals that his blood pressure is 110/70 mm Hg, his pulse is 60 beats per minute, and his temperature is 36.4° C. His standing BP is 105/60 mm Hg and standing pulse is 66 bpm. Both his neurologic exam and noncontrast head CT scan are normal.
Which of the following is the most likely diagnosis?
A) Subarachnoid hemorrhage
B) POTS (Postural orthostatic tachycardia syndrome)
C) Hypnic headache
D) Spontaneous intracranial hypotension (SIH)
E) Acoustic neuroma
The most likely cause for this patient’s headaches given his set of symptoms is spontaneous intracranial hypotension. Orthostatic headaches are common with POTS, but the absence of tachycardia with standing makes this diagnosis unlikely.
Spontaneous intracranial hypotension has symptoms that we are all familiar with in the post–lumbar puncture patient. In patients with post-LP headache, the positional nature makes it easy to diagnose. Patients who have had a lumbar puncture have a clear reason they have a cerebrospinal fluid (CSF) leak, leading to intracranial hypotension. Those with SIH do not.
Related research
Schievink summarized a lot of useful information in a review of patients with spontaneous intracranial hypotension.1 The incidence is about 5/100,000, with the most common age around 40 years old. The most common symptom is orthostatic headache. The headache usually occurs within 15 minutes upon standing, and many patients have the onset of headache rapidly upon standing.
Usually the headache improves with lying down, and it is often brought on with Valsalva maneuver. Many patients report headaches that are worse in the second half of the day.
Orthostatic headache occurs in almost all patients with spontaneous intracranial hypotension, but in one series it occurred only in 77% of patients with SIH.2 The patients who did not have typical headaches are more likely to have auditory symptoms such as tinnitus and muffled hearing.3
When you suspect SIH, appropriate workup is to start with brain MR imaging with contrast. Krantz and colleagues found dural enhancement was present in 83% of cases of SIH, venous distention sign in 75%, and brain sagging in 61%.4
About 10% of patients with SIH have normal brain imaging, so if the clinical features strongly suggest the diagnosis, moving on to spinal imaging with CT myelography or spinal MR are appropriate next steps.5
The causes of SIH are meningeal diverticula (usually in the thoracic or upper lumbar regions), ventral dural tears (usually from osteophytes), and cerebrospinal fluid–venous fistulas. Treatment of SIH has traditionally included a conservative approach of bed rest, oral hydration, and caffeine. The effectiveness of this is unknown, and, in one small series, 61% had headache symptoms at 6 months.6
Epidural blood patches are likely more rapidly effective than conservative therapy. In one study comparing the two treatments, Chung and colleagues found that 77% of the patients who received an epidural blood patch had complete headache relief at 4 weeks, compared with 40% of those who received conservative measures (P < .05).7
Clinical pearls
- Strongly consider SIH in patients with positional headache.
- Brain MR should be the first diagnostic test.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as 3rd-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at imnews@mdedge.com.
References
1. Schievink WI. Spontaneous spinal cerebrospinal fluid leaks and intracranial hypotension. JAMA. 2006;295:2286-96.
2. Mea E et al. Headache attributed to spontaneous intracranial hypotension. Neurol Sci. 2008;29:164-65.
3. Krantz PG et al. Spontaneous Intracranial Hypotension: 10 Myths and Misperceptions. Headache. 2018;58:948-59.
4. Krantz PG et. al. Imaging signs in spontaneous intracranial hypotension: prevalence and relationship to CSF pressure. AJNR Am J Neuroradiol. 2016;37:1374-8.
5. Krantz PG et al. Spontaneous intracranial hypotension: Pathogenesis, diagnosis, and treatment. Neuroimaging Clin N Am. 2019;29:581-94.
6. Kong D-S et. al. Clinical features and long-term results of spontaneous intracranial hypotension. Neurosurgery. 2005;57:91-6.
7. Chung SJ et al. Short- and long-term outcomes of spontaneous CSF hypovolemia. Eur Neurol. 2005;54:63-7.
He reports that these started 3 days ago. His headache is worse when he stands, and resolves when he lies down. Valsalva maneuver makes the headache much worse. The headaches are present in the occipital region. He also has noticed the onset of tinnitus. A physical exam reveals that his blood pressure is 110/70 mm Hg, his pulse is 60 beats per minute, and his temperature is 36.4° C. His standing BP is 105/60 mm Hg and standing pulse is 66 bpm. Both his neurologic exam and noncontrast head CT scan are normal.
Which of the following is the most likely diagnosis?
A) Subarachnoid hemorrhage
B) POTS (Postural orthostatic tachycardia syndrome)
C) Hypnic headache
D) Spontaneous intracranial hypotension (SIH)
E) Acoustic neuroma
The most likely cause for this patient’s headaches given his set of symptoms is spontaneous intracranial hypotension. Orthostatic headaches are common with POTS, but the absence of tachycardia with standing makes this diagnosis unlikely.
Spontaneous intracranial hypotension has symptoms that we are all familiar with in the post–lumbar puncture patient. In patients with post-LP headache, the positional nature makes it easy to diagnose. Patients who have had a lumbar puncture have a clear reason they have a cerebrospinal fluid (CSF) leak, leading to intracranial hypotension. Those with SIH do not.
Related research
Schievink summarized a lot of useful information in a review of patients with spontaneous intracranial hypotension.1 The incidence is about 5/100,000, with the most common age around 40 years old. The most common symptom is orthostatic headache. The headache usually occurs within 15 minutes upon standing, and many patients have the onset of headache rapidly upon standing.
Usually the headache improves with lying down, and it is often brought on with Valsalva maneuver. Many patients report headaches that are worse in the second half of the day.
Orthostatic headache occurs in almost all patients with spontaneous intracranial hypotension, but in one series it occurred only in 77% of patients with SIH.2 The patients who did not have typical headaches are more likely to have auditory symptoms such as tinnitus and muffled hearing.3
When you suspect SIH, appropriate workup is to start with brain MR imaging with contrast. Krantz and colleagues found dural enhancement was present in 83% of cases of SIH, venous distention sign in 75%, and brain sagging in 61%.4
About 10% of patients with SIH have normal brain imaging, so if the clinical features strongly suggest the diagnosis, moving on to spinal imaging with CT myelography or spinal MR are appropriate next steps.5
The causes of SIH are meningeal diverticula (usually in the thoracic or upper lumbar regions), ventral dural tears (usually from osteophytes), and cerebrospinal fluid–venous fistulas. Treatment of SIH has traditionally included a conservative approach of bed rest, oral hydration, and caffeine. The effectiveness of this is unknown, and, in one small series, 61% had headache symptoms at 6 months.6
Epidural blood patches are likely more rapidly effective than conservative therapy. In one study comparing the two treatments, Chung and colleagues found that 77% of the patients who received an epidural blood patch had complete headache relief at 4 weeks, compared with 40% of those who received conservative measures (P < .05).7
Clinical pearls
- Strongly consider SIH in patients with positional headache.
- Brain MR should be the first diagnostic test.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as 3rd-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at imnews@mdedge.com.
References
1. Schievink WI. Spontaneous spinal cerebrospinal fluid leaks and intracranial hypotension. JAMA. 2006;295:2286-96.
2. Mea E et al. Headache attributed to spontaneous intracranial hypotension. Neurol Sci. 2008;29:164-65.
3. Krantz PG et al. Spontaneous Intracranial Hypotension: 10 Myths and Misperceptions. Headache. 2018;58:948-59.
4. Krantz PG et. al. Imaging signs in spontaneous intracranial hypotension: prevalence and relationship to CSF pressure. AJNR Am J Neuroradiol. 2016;37:1374-8.
5. Krantz PG et al. Spontaneous intracranial hypotension: Pathogenesis, diagnosis, and treatment. Neuroimaging Clin N Am. 2019;29:581-94.
6. Kong D-S et. al. Clinical features and long-term results of spontaneous intracranial hypotension. Neurosurgery. 2005;57:91-6.
7. Chung SJ et al. Short- and long-term outcomes of spontaneous CSF hypovolemia. Eur Neurol. 2005;54:63-7.
Real-world data suggest coprescribing PDE5 inhibitors and nitrates may be safe
The authors of the new research specifically examined how frequently phosphodiesterase type 5 (PDE5) inhibitors, such as Viagra, were prescribed. The U.S. Food and Drug Administration and the European Medicines Agency have warned that these drugs for erectile dysfunction are contraindicated for use with nitrates because of concerns about cardiovascular risks.
“Small, randomized, pharmacologic studies have reported an amplified decrease in blood pressure during controlled coexposure with nitrates and [phosphodiesterase type 5 inhibitors], both in healthy participants and in participants with IHD,” wrote lead author Anders Holt, MD, of Copenhagen University Hospital–Herlev and Gentofte and colleagues, in Annals of Internal Medicine. “Potentially, this increases the risk for vascular ischemic events including myocardial infarction and stroke.”
But there is a scarcity of real-world data showing that using both types of drugs together increase these risks, the researchers noted.
To address this knowledge gap, Dr. Holt and colleagues conducted a retrospective study involving 249,541 Danish men with IHD. In this overall population, from 2000 to 2018, prescriptions for PDE5 inhibitors increased 10-fold, from 3.1 to 30.9 prescriptions per 100 persons per year. Within a subgroup of 42,073 patients continuously prescribed oral organic nitrates, PDE5-inhibitor prescriptions jumped twice that magnitude, from 0.9 to 19.7 prescriptions per 100 persons per year.
Despite this surge in coprescribing, the investigators did not observe a significant increase in either of two composite measures of cardiovascular adverse events. The first composite included ischemic stroke, shock, cardiac arrest, myocardial infarction, or acute coronary arteriography (odds ratio, 0.58; 95% confidence interval, 0.28-1.13). The second composite included drug-related adverse events, angina pectoris, or syncope (OR, 0.73; CI, 0.40-1.32).
Lead author speculates on reasons for findings
“I propose several explanations [for these findings],” Dr. Holt said in an interview, “but I want to emphasize that our study does not contain any data to back it up. It is just speculation. First, the observed drop in blood pressure may not cause a condition for which patients seek a hospital. A drop in blood pressure has been shown in pharmacologic trials, but it might not translate to a real-life risk for cardiovascular outcomes. Second, patients could be well informed and adherent to guidance that the prescribing physician has provided. For example, patients are aware of the recommended pause in nitrate treatment before PDE5-inhibitor use and follow these recommendations. Third, nitrates are often taken in the morning, and with the careful assumption that most PDE5-inhibitor activities take place in the evening, the nitrates could be metabolized to a degree such that the synergistic interaction is negligible.”
Dr. Holt went on to suggest a novel clinical approach based on the new findings.
“Coadministration should still be contraindicated due to the proven drop in blood pressure,” he said. “However, perhaps physicians can allow for coprescription if patients are adequately informed.”
A qualitative study is needed to determine how patients and physicians discuss coprescription, including avoidance of coadministration, Dr. Holt added.
Findings call for a reassessment of whether the contraindication is warranted
Robert A. Kloner, MD, PhD, chief science officer at the Huntington Medical Research Institutes in Pasadena, Calif., and professor of medicine at University of Southern California, Los Angeles, previously conducted research exploring drug interactions with PDE5 inhibitors, and in 2018, coauthored a literature review that concluded that PDE5 inhibitors and nitrates are contraindicated.
But now, considering these new findings, Dr. Kloner is offering a fresh perspective.
“This study is reassuring,” Dr. Kloner said in an interview. “I think that it’s time to reassess whether there should be an absolute contraindication, or this should be more of like a warning.”
He noted that in controlled studies, like the ones he previously conducted, PDE5 inhibitors and nitrates were administered “very close to each other, on purpose,” yet this probably doesn’t reflect typical practice, in which clinicians can guide usage based on durations of drug metabolism.
“I think that physicians might be more comfortable now prescribing the drugs at the same time, but then telling patients that they shouldn’t take the two drugs simultaneously; they should wait and take the nitrate 24 hours after the last Viagra, or the nitrate 48 hours after the last Cialis,” Dr. Kloner said. “I suspect that that is happening. I suspect also the fact that people would be more likely to take the nitrate in the morning and the PDE5 inhibitor at night probably also contributes to the safety findings.”
Dr. Kloner noted that blood pressures vary throughout the day based on circadian rhythm, and that the body can adapt to some fluctuations without negative effects.
There could still be some people who experience a drop in blood pressure and get sick from it from the two drugs interacting, but that’s probably not that common, he said.
The study was supported by several grants. The investigators disclosed relationships with Merck, BMS, Bayer, and others. Dr. Kloner consults for Sanofi.
The authors of the new research specifically examined how frequently phosphodiesterase type 5 (PDE5) inhibitors, such as Viagra, were prescribed. The U.S. Food and Drug Administration and the European Medicines Agency have warned that these drugs for erectile dysfunction are contraindicated for use with nitrates because of concerns about cardiovascular risks.
“Small, randomized, pharmacologic studies have reported an amplified decrease in blood pressure during controlled coexposure with nitrates and [phosphodiesterase type 5 inhibitors], both in healthy participants and in participants with IHD,” wrote lead author Anders Holt, MD, of Copenhagen University Hospital–Herlev and Gentofte and colleagues, in Annals of Internal Medicine. “Potentially, this increases the risk for vascular ischemic events including myocardial infarction and stroke.”
But there is a scarcity of real-world data showing that using both types of drugs together increase these risks, the researchers noted.
To address this knowledge gap, Dr. Holt and colleagues conducted a retrospective study involving 249,541 Danish men with IHD. In this overall population, from 2000 to 2018, prescriptions for PDE5 inhibitors increased 10-fold, from 3.1 to 30.9 prescriptions per 100 persons per year. Within a subgroup of 42,073 patients continuously prescribed oral organic nitrates, PDE5-inhibitor prescriptions jumped twice that magnitude, from 0.9 to 19.7 prescriptions per 100 persons per year.
Despite this surge in coprescribing, the investigators did not observe a significant increase in either of two composite measures of cardiovascular adverse events. The first composite included ischemic stroke, shock, cardiac arrest, myocardial infarction, or acute coronary arteriography (odds ratio, 0.58; 95% confidence interval, 0.28-1.13). The second composite included drug-related adverse events, angina pectoris, or syncope (OR, 0.73; CI, 0.40-1.32).
Lead author speculates on reasons for findings
“I propose several explanations [for these findings],” Dr. Holt said in an interview, “but I want to emphasize that our study does not contain any data to back it up. It is just speculation. First, the observed drop in blood pressure may not cause a condition for which patients seek a hospital. A drop in blood pressure has been shown in pharmacologic trials, but it might not translate to a real-life risk for cardiovascular outcomes. Second, patients could be well informed and adherent to guidance that the prescribing physician has provided. For example, patients are aware of the recommended pause in nitrate treatment before PDE5-inhibitor use and follow these recommendations. Third, nitrates are often taken in the morning, and with the careful assumption that most PDE5-inhibitor activities take place in the evening, the nitrates could be metabolized to a degree such that the synergistic interaction is negligible.”
Dr. Holt went on to suggest a novel clinical approach based on the new findings.
“Coadministration should still be contraindicated due to the proven drop in blood pressure,” he said. “However, perhaps physicians can allow for coprescription if patients are adequately informed.”
A qualitative study is needed to determine how patients and physicians discuss coprescription, including avoidance of coadministration, Dr. Holt added.
Findings call for a reassessment of whether the contraindication is warranted
Robert A. Kloner, MD, PhD, chief science officer at the Huntington Medical Research Institutes in Pasadena, Calif., and professor of medicine at University of Southern California, Los Angeles, previously conducted research exploring drug interactions with PDE5 inhibitors, and in 2018, coauthored a literature review that concluded that PDE5 inhibitors and nitrates are contraindicated.
But now, considering these new findings, Dr. Kloner is offering a fresh perspective.
“This study is reassuring,” Dr. Kloner said in an interview. “I think that it’s time to reassess whether there should be an absolute contraindication, or this should be more of like a warning.”
He noted that in controlled studies, like the ones he previously conducted, PDE5 inhibitors and nitrates were administered “very close to each other, on purpose,” yet this probably doesn’t reflect typical practice, in which clinicians can guide usage based on durations of drug metabolism.
“I think that physicians might be more comfortable now prescribing the drugs at the same time, but then telling patients that they shouldn’t take the two drugs simultaneously; they should wait and take the nitrate 24 hours after the last Viagra, or the nitrate 48 hours after the last Cialis,” Dr. Kloner said. “I suspect that that is happening. I suspect also the fact that people would be more likely to take the nitrate in the morning and the PDE5 inhibitor at night probably also contributes to the safety findings.”
Dr. Kloner noted that blood pressures vary throughout the day based on circadian rhythm, and that the body can adapt to some fluctuations without negative effects.
There could still be some people who experience a drop in blood pressure and get sick from it from the two drugs interacting, but that’s probably not that common, he said.
The study was supported by several grants. The investigators disclosed relationships with Merck, BMS, Bayer, and others. Dr. Kloner consults for Sanofi.
The authors of the new research specifically examined how frequently phosphodiesterase type 5 (PDE5) inhibitors, such as Viagra, were prescribed. The U.S. Food and Drug Administration and the European Medicines Agency have warned that these drugs for erectile dysfunction are contraindicated for use with nitrates because of concerns about cardiovascular risks.
“Small, randomized, pharmacologic studies have reported an amplified decrease in blood pressure during controlled coexposure with nitrates and [phosphodiesterase type 5 inhibitors], both in healthy participants and in participants with IHD,” wrote lead author Anders Holt, MD, of Copenhagen University Hospital–Herlev and Gentofte and colleagues, in Annals of Internal Medicine. “Potentially, this increases the risk for vascular ischemic events including myocardial infarction and stroke.”
But there is a scarcity of real-world data showing that using both types of drugs together increase these risks, the researchers noted.
To address this knowledge gap, Dr. Holt and colleagues conducted a retrospective study involving 249,541 Danish men with IHD. In this overall population, from 2000 to 2018, prescriptions for PDE5 inhibitors increased 10-fold, from 3.1 to 30.9 prescriptions per 100 persons per year. Within a subgroup of 42,073 patients continuously prescribed oral organic nitrates, PDE5-inhibitor prescriptions jumped twice that magnitude, from 0.9 to 19.7 prescriptions per 100 persons per year.
Despite this surge in coprescribing, the investigators did not observe a significant increase in either of two composite measures of cardiovascular adverse events. The first composite included ischemic stroke, shock, cardiac arrest, myocardial infarction, or acute coronary arteriography (odds ratio, 0.58; 95% confidence interval, 0.28-1.13). The second composite included drug-related adverse events, angina pectoris, or syncope (OR, 0.73; CI, 0.40-1.32).
Lead author speculates on reasons for findings
“I propose several explanations [for these findings],” Dr. Holt said in an interview, “but I want to emphasize that our study does not contain any data to back it up. It is just speculation. First, the observed drop in blood pressure may not cause a condition for which patients seek a hospital. A drop in blood pressure has been shown in pharmacologic trials, but it might not translate to a real-life risk for cardiovascular outcomes. Second, patients could be well informed and adherent to guidance that the prescribing physician has provided. For example, patients are aware of the recommended pause in nitrate treatment before PDE5-inhibitor use and follow these recommendations. Third, nitrates are often taken in the morning, and with the careful assumption that most PDE5-inhibitor activities take place in the evening, the nitrates could be metabolized to a degree such that the synergistic interaction is negligible.”
Dr. Holt went on to suggest a novel clinical approach based on the new findings.
“Coadministration should still be contraindicated due to the proven drop in blood pressure,” he said. “However, perhaps physicians can allow for coprescription if patients are adequately informed.”
A qualitative study is needed to determine how patients and physicians discuss coprescription, including avoidance of coadministration, Dr. Holt added.
Findings call for a reassessment of whether the contraindication is warranted
Robert A. Kloner, MD, PhD, chief science officer at the Huntington Medical Research Institutes in Pasadena, Calif., and professor of medicine at University of Southern California, Los Angeles, previously conducted research exploring drug interactions with PDE5 inhibitors, and in 2018, coauthored a literature review that concluded that PDE5 inhibitors and nitrates are contraindicated.
But now, considering these new findings, Dr. Kloner is offering a fresh perspective.
“This study is reassuring,” Dr. Kloner said in an interview. “I think that it’s time to reassess whether there should be an absolute contraindication, or this should be more of like a warning.”
He noted that in controlled studies, like the ones he previously conducted, PDE5 inhibitors and nitrates were administered “very close to each other, on purpose,” yet this probably doesn’t reflect typical practice, in which clinicians can guide usage based on durations of drug metabolism.
“I think that physicians might be more comfortable now prescribing the drugs at the same time, but then telling patients that they shouldn’t take the two drugs simultaneously; they should wait and take the nitrate 24 hours after the last Viagra, or the nitrate 48 hours after the last Cialis,” Dr. Kloner said. “I suspect that that is happening. I suspect also the fact that people would be more likely to take the nitrate in the morning and the PDE5 inhibitor at night probably also contributes to the safety findings.”
Dr. Kloner noted that blood pressures vary throughout the day based on circadian rhythm, and that the body can adapt to some fluctuations without negative effects.
There could still be some people who experience a drop in blood pressure and get sick from it from the two drugs interacting, but that’s probably not that common, he said.
The study was supported by several grants. The investigators disclosed relationships with Merck, BMS, Bayer, and others. Dr. Kloner consults for Sanofi.
FROM ANNALS OF INTERNAL MEDICINE
Bariatric surgery cuts cardiovascular events, even in seniors
Bariatric surgery can reduce the risk of long-term cardiovascular outcomes in older Medicare beneficiaries with obesity, a large new observational study in which a third of the patients were over age 65 years suggests.
Overall, patients who underwent bariatric surgery had 37% lower all-cause mortality and were significantly less likely to have admissions for new-onset heart failure (64% risk reduction), myocardial infarction (37% risk reduction), and ischemic stroke (29% risk reduction), compared with similar patients who received more conservative treatment, after a median of 4 years of follow-up, report Amgad Mentias, MD, MS, a clinical cardiologist at the Cleveland Clinic Foundation, Ohio, and colleagues.
The results were published in the Journal of the American College of Cardiology.
Previous studies on bariatric surgery outcomes have primarily focused on individuals from select health care networks or medical facilities with restricted coverage in the United States or on patients with diabetes, noted Tiffany M. Powell-Wiley, MD, MPH, of the National Institutes of Health’s National Heart, Lung, and Blood Institute, Bethesda, Maryland, and colleagues in an accompanying editorial.
Moreover, other long-term and observational studies have shown that bariatric surgery can decrease the risk of myocardial infarction, death, and stroke in young and middle-aged patients with obesity, but the evidence is less clear for older patients and those without diabetes, noted Dr. Mentias in a phone interview.
“To date, this is one of the first studies to support bariatric surgery for CVD risk reduction in patients older than 65 years, a population at highest risk for developing heart failure,” the editorial points out.
“We should consider referring patients who qualify for bariatric surgery based on BMI; it really should be considered as a treatment option for patients with class 3 obesity, especially with a body mass index over 40 kg/m2,” Dr. Powell-Wiley told this news organization.
“We know that patients are generally under-referred for bariatric surgery, and this highlights the need to refer patients for bariatric surgery,” she added.
“There should be discussion about expanding insurance coverage to include bariatric surgery for eligible patients,” Dr. Mentias added.
Contemporary cohort of patients
“A lot of the studies showed long-term outcomes outside of the U.S., specifically in Europe,” Dr. Mentias added.
The aim of this study was to evaluate the long-term association between bariatric surgery and risk of adverse cardiovascular outcomes in a contemporary large cohort from the United States.
Older patients (> 65 years) and those without diabetes were looked at as specific subgroups.
The researchers assessed 189,770 patients. There were 94,885 matched patients in each cohort. Mean age was 62.33 years. Female patients comprised 70% of the cohort. The study group had an average BMI of 44.7 kg/m2.
The study cohort was matched 1:1. Participants were either part of a control group with obesity or a group of Medicare beneficiaries who had bariatric surgery between 2013 and 2019. Sex, propensity score matching on 87 clinical variables, age, and BMI were used to match patients.
Myocardial infarction, new-onset heart failure, ischemic stroke, and all-cause mortality were all study outcomes. As a sensitivity analysis, the study team conducted an instrumental variable assessment.
More specifically, the findings showed that bariatric surgery was linked with the following after a median follow-up of 4.0 years:
- Myocardial infarction (hazard ratio, 0.63; 95% confidence interval, 0.59-0.68)
- Stroke (HR, 0.71; 95% CI, 0.65-0.79)
- New-onset heart failure (HR, 0.46; 95% CI, 0.44-0.49)
- Reduced risk of death (9.2 vs. 14.7 per 1000 person-years; HR, 0.63; 95% CI, 0.60-0.66)
Findings for those over the age of 65 were similar – lower risks of all-cause mortality (HR, 0.64), new-onset heart failure (HR, 0.52), myocardial infarction (HR, 0.70), and stroke (HR, 0.76; all P < .001). Similar findings were shown in subgroup analyses in men and women and in patients with and without diabetes.
The study cohort primarily consisted of Medicare patients, which limits the generalizability of the data. Lack of data on medications taken for cardiovascular and weight loss purposes and potential coding errors because the information was gathered from an administrative database were all limitations of the study, the researchers note.
An additional limitation was that residual unmeasured confounders, particularly patient-focused physical, social, and mental support factors, could play a role in whether a patient opted to have bariatric surgery, the study authors note.
“Additional studies are needed to compare cardiovascular outcomes after bariatric surgery with weight loss medications like glucagon-like peptide-1 (GLP-1) analogues,” the researchers add.
This study was partially funded by philanthropic contributions by the Khouri family, Bailey family, and Haslam family to the Cleveland Clinic for co-author Dr. Milind Y. Desai’s research. Dr. Mentias has disclosed no relevant financial relationships. Dr. Powell-Wiley disclosed relationships with the National Institute on Minority Health and Health Disparities and the Division of Intramural Research of the National, Heart, Lung, and Blood Institute of the National Institutes of Health.
A version of this article first appeared on Medscape.com.
Bariatric surgery can reduce the risk of long-term cardiovascular outcomes in older Medicare beneficiaries with obesity, a large new observational study in which a third of the patients were over age 65 years suggests.
Overall, patients who underwent bariatric surgery had 37% lower all-cause mortality and were significantly less likely to have admissions for new-onset heart failure (64% risk reduction), myocardial infarction (37% risk reduction), and ischemic stroke (29% risk reduction), compared with similar patients who received more conservative treatment, after a median of 4 years of follow-up, report Amgad Mentias, MD, MS, a clinical cardiologist at the Cleveland Clinic Foundation, Ohio, and colleagues.
The results were published in the Journal of the American College of Cardiology.
Previous studies on bariatric surgery outcomes have primarily focused on individuals from select health care networks or medical facilities with restricted coverage in the United States or on patients with diabetes, noted Tiffany M. Powell-Wiley, MD, MPH, of the National Institutes of Health’s National Heart, Lung, and Blood Institute, Bethesda, Maryland, and colleagues in an accompanying editorial.
Moreover, other long-term and observational studies have shown that bariatric surgery can decrease the risk of myocardial infarction, death, and stroke in young and middle-aged patients with obesity, but the evidence is less clear for older patients and those without diabetes, noted Dr. Mentias in a phone interview.
“To date, this is one of the first studies to support bariatric surgery for CVD risk reduction in patients older than 65 years, a population at highest risk for developing heart failure,” the editorial points out.
“We should consider referring patients who qualify for bariatric surgery based on BMI; it really should be considered as a treatment option for patients with class 3 obesity, especially with a body mass index over 40 kg/m2,” Dr. Powell-Wiley told this news organization.
“We know that patients are generally under-referred for bariatric surgery, and this highlights the need to refer patients for bariatric surgery,” she added.
“There should be discussion about expanding insurance coverage to include bariatric surgery for eligible patients,” Dr. Mentias added.
Contemporary cohort of patients
“A lot of the studies showed long-term outcomes outside of the U.S., specifically in Europe,” Dr. Mentias added.
The aim of this study was to evaluate the long-term association between bariatric surgery and risk of adverse cardiovascular outcomes in a contemporary large cohort from the United States.
Older patients (> 65 years) and those without diabetes were looked at as specific subgroups.
The researchers assessed 189,770 patients. There were 94,885 matched patients in each cohort. Mean age was 62.33 years. Female patients comprised 70% of the cohort. The study group had an average BMI of 44.7 kg/m2.
The study cohort was matched 1:1. Participants were either part of a control group with obesity or a group of Medicare beneficiaries who had bariatric surgery between 2013 and 2019. Sex, propensity score matching on 87 clinical variables, age, and BMI were used to match patients.
Myocardial infarction, new-onset heart failure, ischemic stroke, and all-cause mortality were all study outcomes. As a sensitivity analysis, the study team conducted an instrumental variable assessment.
More specifically, the findings showed that bariatric surgery was linked with the following after a median follow-up of 4.0 years:
- Myocardial infarction (hazard ratio, 0.63; 95% confidence interval, 0.59-0.68)
- Stroke (HR, 0.71; 95% CI, 0.65-0.79)
- New-onset heart failure (HR, 0.46; 95% CI, 0.44-0.49)
- Reduced risk of death (9.2 vs. 14.7 per 1000 person-years; HR, 0.63; 95% CI, 0.60-0.66)
Findings for those over the age of 65 were similar – lower risks of all-cause mortality (HR, 0.64), new-onset heart failure (HR, 0.52), myocardial infarction (HR, 0.70), and stroke (HR, 0.76; all P < .001). Similar findings were shown in subgroup analyses in men and women and in patients with and without diabetes.
The study cohort primarily consisted of Medicare patients, which limits the generalizability of the data. Lack of data on medications taken for cardiovascular and weight loss purposes and potential coding errors because the information was gathered from an administrative database were all limitations of the study, the researchers note.
An additional limitation was that residual unmeasured confounders, particularly patient-focused physical, social, and mental support factors, could play a role in whether a patient opted to have bariatric surgery, the study authors note.
“Additional studies are needed to compare cardiovascular outcomes after bariatric surgery with weight loss medications like glucagon-like peptide-1 (GLP-1) analogues,” the researchers add.
This study was partially funded by philanthropic contributions by the Khouri family, Bailey family, and Haslam family to the Cleveland Clinic for co-author Dr. Milind Y. Desai’s research. Dr. Mentias has disclosed no relevant financial relationships. Dr. Powell-Wiley disclosed relationships with the National Institute on Minority Health and Health Disparities and the Division of Intramural Research of the National, Heart, Lung, and Blood Institute of the National Institutes of Health.
A version of this article first appeared on Medscape.com.
Bariatric surgery can reduce the risk of long-term cardiovascular outcomes in older Medicare beneficiaries with obesity, a large new observational study in which a third of the patients were over age 65 years suggests.
Overall, patients who underwent bariatric surgery had 37% lower all-cause mortality and were significantly less likely to have admissions for new-onset heart failure (64% risk reduction), myocardial infarction (37% risk reduction), and ischemic stroke (29% risk reduction), compared with similar patients who received more conservative treatment, after a median of 4 years of follow-up, report Amgad Mentias, MD, MS, a clinical cardiologist at the Cleveland Clinic Foundation, Ohio, and colleagues.
The results were published in the Journal of the American College of Cardiology.
Previous studies on bariatric surgery outcomes have primarily focused on individuals from select health care networks or medical facilities with restricted coverage in the United States or on patients with diabetes, noted Tiffany M. Powell-Wiley, MD, MPH, of the National Institutes of Health’s National Heart, Lung, and Blood Institute, Bethesda, Maryland, and colleagues in an accompanying editorial.
Moreover, other long-term and observational studies have shown that bariatric surgery can decrease the risk of myocardial infarction, death, and stroke in young and middle-aged patients with obesity, but the evidence is less clear for older patients and those without diabetes, noted Dr. Mentias in a phone interview.
“To date, this is one of the first studies to support bariatric surgery for CVD risk reduction in patients older than 65 years, a population at highest risk for developing heart failure,” the editorial points out.
“We should consider referring patients who qualify for bariatric surgery based on BMI; it really should be considered as a treatment option for patients with class 3 obesity, especially with a body mass index over 40 kg/m2,” Dr. Powell-Wiley told this news organization.
“We know that patients are generally under-referred for bariatric surgery, and this highlights the need to refer patients for bariatric surgery,” she added.
“There should be discussion about expanding insurance coverage to include bariatric surgery for eligible patients,” Dr. Mentias added.
Contemporary cohort of patients
“A lot of the studies showed long-term outcomes outside of the U.S., specifically in Europe,” Dr. Mentias added.
The aim of this study was to evaluate the long-term association between bariatric surgery and risk of adverse cardiovascular outcomes in a contemporary large cohort from the United States.
Older patients (> 65 years) and those without diabetes were looked at as specific subgroups.
The researchers assessed 189,770 patients. There were 94,885 matched patients in each cohort. Mean age was 62.33 years. Female patients comprised 70% of the cohort. The study group had an average BMI of 44.7 kg/m2.
The study cohort was matched 1:1. Participants were either part of a control group with obesity or a group of Medicare beneficiaries who had bariatric surgery between 2013 and 2019. Sex, propensity score matching on 87 clinical variables, age, and BMI were used to match patients.
Myocardial infarction, new-onset heart failure, ischemic stroke, and all-cause mortality were all study outcomes. As a sensitivity analysis, the study team conducted an instrumental variable assessment.
More specifically, the findings showed that bariatric surgery was linked with the following after a median follow-up of 4.0 years:
- Myocardial infarction (hazard ratio, 0.63; 95% confidence interval, 0.59-0.68)
- Stroke (HR, 0.71; 95% CI, 0.65-0.79)
- New-onset heart failure (HR, 0.46; 95% CI, 0.44-0.49)
- Reduced risk of death (9.2 vs. 14.7 per 1000 person-years; HR, 0.63; 95% CI, 0.60-0.66)
Findings for those over the age of 65 were similar – lower risks of all-cause mortality (HR, 0.64), new-onset heart failure (HR, 0.52), myocardial infarction (HR, 0.70), and stroke (HR, 0.76; all P < .001). Similar findings were shown in subgroup analyses in men and women and in patients with and without diabetes.
The study cohort primarily consisted of Medicare patients, which limits the generalizability of the data. Lack of data on medications taken for cardiovascular and weight loss purposes and potential coding errors because the information was gathered from an administrative database were all limitations of the study, the researchers note.
An additional limitation was that residual unmeasured confounders, particularly patient-focused physical, social, and mental support factors, could play a role in whether a patient opted to have bariatric surgery, the study authors note.
“Additional studies are needed to compare cardiovascular outcomes after bariatric surgery with weight loss medications like glucagon-like peptide-1 (GLP-1) analogues,” the researchers add.
This study was partially funded by philanthropic contributions by the Khouri family, Bailey family, and Haslam family to the Cleveland Clinic for co-author Dr. Milind Y. Desai’s research. Dr. Mentias has disclosed no relevant financial relationships. Dr. Powell-Wiley disclosed relationships with the National Institute on Minority Health and Health Disparities and the Division of Intramural Research of the National, Heart, Lung, and Blood Institute of the National Institutes of Health.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Fresh data confirm healthy plant foods link to lower diabetes risk
A scientific analysis of metabolites from plant-based-diets – especially those rich in whole grains, fruits, and vegetables – may in the future yield clues as to how such eating patterns lower the risk of type 2 diabetes, finds a new study of more than 8,000 people.
The research looked at healthy, unhealthy, and overall plant-based diets, but only metabolic profiles for the healthy and overall plant-based diets showed an inverse relationship with type 2 diabetes.
A primarily “unhealthy” plant-based diet was one including mainly refined grains (e.g., white bread and pasta), fruit juices, potatoes, sugar-sweetened beverages, and sweets/desserts.
“Individual metabolites from consumption of polyphenol-rich plant foods like fruits, vegetables, coffee, and legumes are all closely linked to healthy plant-based diet and lower risk of diabetes,” lead author Frank Hu, MD, said in a press release.
Dr. Hu, of the department of nutrition at Harvard T.H. Chan School of Public Health, Boston, and colleagues reported their findings in Diabetologia.
High-throughput profiling of the metabolome
Given that an individual’s metabolic profile reflects their diet, there is a growing trend in nutritional research to use a technique called high-throughput metabolomics to profile biological samples.
The team conducted an analysis of blood plasma samples and dietary intake using food frequency questionnaires of 10,684 participants from three prospective cohorts (Nurses’ Health Study, Nurses’ Health Study II, and Health Professionals Follow-Up Study). Participants were predominantly White and middle-aged (mean age 54 years), with a mean body mass index of 25.6 kg/m2.
Metabolite profile scores were generated from the blood samples, taken in the 1980s and 1990s, and matched to any cases of incident type 2 diabetes reported during follow-up, which ended in 2016-2017.
The team looked at three different plant-based diets – by definition, higher in plant foods and lower in animal foods – and further categorized them according to the actual foods consumed, to generate an overall plant diet index (PDI), a healthy PDI, or an unhealthy PDI.
In all, 8,827 participants completed the study, and 270 cases of diabetes were reported.
Multi-metabolite profiles were composed of 55 metabolites for the overall PDI, 93 metabolites for healthy PDI, and 75 metabolites for unhealthy PDI.
The findings are that metabolomics can be harnessed and “the identified metabolic profiles could be used to assess adherence to ... plant-based diets as part of type 2 diabetes prevention ... and provide new insights for future investigation,” the researchers concluded.
One coauthor received research support from the California Walnut Commission and Swiss ReManagement; another reported being a scientific consultant to LayerIV. The other authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A scientific analysis of metabolites from plant-based-diets – especially those rich in whole grains, fruits, and vegetables – may in the future yield clues as to how such eating patterns lower the risk of type 2 diabetes, finds a new study of more than 8,000 people.
The research looked at healthy, unhealthy, and overall plant-based diets, but only metabolic profiles for the healthy and overall plant-based diets showed an inverse relationship with type 2 diabetes.
A primarily “unhealthy” plant-based diet was one including mainly refined grains (e.g., white bread and pasta), fruit juices, potatoes, sugar-sweetened beverages, and sweets/desserts.
“Individual metabolites from consumption of polyphenol-rich plant foods like fruits, vegetables, coffee, and legumes are all closely linked to healthy plant-based diet and lower risk of diabetes,” lead author Frank Hu, MD, said in a press release.
Dr. Hu, of the department of nutrition at Harvard T.H. Chan School of Public Health, Boston, and colleagues reported their findings in Diabetologia.
High-throughput profiling of the metabolome
Given that an individual’s metabolic profile reflects their diet, there is a growing trend in nutritional research to use a technique called high-throughput metabolomics to profile biological samples.
The team conducted an analysis of blood plasma samples and dietary intake using food frequency questionnaires of 10,684 participants from three prospective cohorts (Nurses’ Health Study, Nurses’ Health Study II, and Health Professionals Follow-Up Study). Participants were predominantly White and middle-aged (mean age 54 years), with a mean body mass index of 25.6 kg/m2.
Metabolite profile scores were generated from the blood samples, taken in the 1980s and 1990s, and matched to any cases of incident type 2 diabetes reported during follow-up, which ended in 2016-2017.
The team looked at three different plant-based diets – by definition, higher in plant foods and lower in animal foods – and further categorized them according to the actual foods consumed, to generate an overall plant diet index (PDI), a healthy PDI, or an unhealthy PDI.
In all, 8,827 participants completed the study, and 270 cases of diabetes were reported.
Multi-metabolite profiles were composed of 55 metabolites for the overall PDI, 93 metabolites for healthy PDI, and 75 metabolites for unhealthy PDI.
The findings are that metabolomics can be harnessed and “the identified metabolic profiles could be used to assess adherence to ... plant-based diets as part of type 2 diabetes prevention ... and provide new insights for future investigation,” the researchers concluded.
One coauthor received research support from the California Walnut Commission and Swiss ReManagement; another reported being a scientific consultant to LayerIV. The other authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A scientific analysis of metabolites from plant-based-diets – especially those rich in whole grains, fruits, and vegetables – may in the future yield clues as to how such eating patterns lower the risk of type 2 diabetes, finds a new study of more than 8,000 people.
The research looked at healthy, unhealthy, and overall plant-based diets, but only metabolic profiles for the healthy and overall plant-based diets showed an inverse relationship with type 2 diabetes.
A primarily “unhealthy” plant-based diet was one including mainly refined grains (e.g., white bread and pasta), fruit juices, potatoes, sugar-sweetened beverages, and sweets/desserts.
“Individual metabolites from consumption of polyphenol-rich plant foods like fruits, vegetables, coffee, and legumes are all closely linked to healthy plant-based diet and lower risk of diabetes,” lead author Frank Hu, MD, said in a press release.
Dr. Hu, of the department of nutrition at Harvard T.H. Chan School of Public Health, Boston, and colleagues reported their findings in Diabetologia.
High-throughput profiling of the metabolome
Given that an individual’s metabolic profile reflects their diet, there is a growing trend in nutritional research to use a technique called high-throughput metabolomics to profile biological samples.
The team conducted an analysis of blood plasma samples and dietary intake using food frequency questionnaires of 10,684 participants from three prospective cohorts (Nurses’ Health Study, Nurses’ Health Study II, and Health Professionals Follow-Up Study). Participants were predominantly White and middle-aged (mean age 54 years), with a mean body mass index of 25.6 kg/m2.
Metabolite profile scores were generated from the blood samples, taken in the 1980s and 1990s, and matched to any cases of incident type 2 diabetes reported during follow-up, which ended in 2016-2017.
The team looked at three different plant-based diets – by definition, higher in plant foods and lower in animal foods – and further categorized them according to the actual foods consumed, to generate an overall plant diet index (PDI), a healthy PDI, or an unhealthy PDI.
In all, 8,827 participants completed the study, and 270 cases of diabetes were reported.
Multi-metabolite profiles were composed of 55 metabolites for the overall PDI, 93 metabolites for healthy PDI, and 75 metabolites for unhealthy PDI.
The findings are that metabolomics can be harnessed and “the identified metabolic profiles could be used to assess adherence to ... plant-based diets as part of type 2 diabetes prevention ... and provide new insights for future investigation,” the researchers concluded.
One coauthor received research support from the California Walnut Commission and Swiss ReManagement; another reported being a scientific consultant to LayerIV. The other authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM DIABETOLOGIA
Aged black garlic supplement may help lower BP
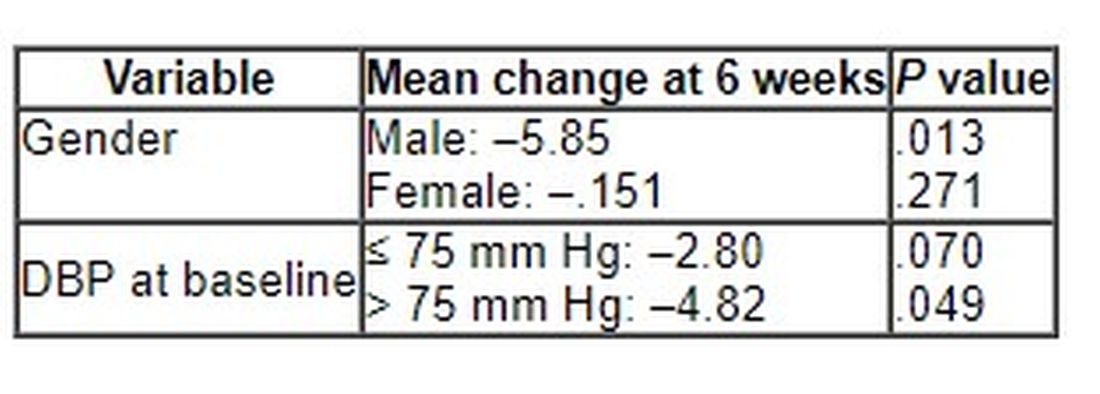
After 6 weeks, consumption of ABG with a high concentration of s-allyl-L-cystine (SAC) was associated with a nearly 6-mm Hg reduction in DBP in men. Other cardiovascular disease (CVD) risk factors were not significantly affected.
“The observed reduction in DBP by ABG extract was similar to the effects of dietary approaches, including the effects of the Dietary Approaches to Stop Hypertension(DASH) diet on BP,” say Rosa M. Valls, PhD, Universitat Rovira i Virgili, Reus, Spain, and colleagues.
“The potential beneficial effects of ABG may contribute to obtaining an optimal DBP” but were “better observed in men and in nonoptimal DBP populations,” they write in the study, published in Nutrients.
Pure SAC and aged garlics have shown healthy effects on multiple targets in in vitro and in vivo tests. However, previous studies in humans have not focused on ABG but rather on other types of aged garlic in patients with some type of CVD risk factor and suffered from methodologic or design weaknesses, the authors note.
To address this gap, Dr. Valls and colleagues randomly assigned 67 individuals with moderate hypercholesterolemia (defined as LDL levels of at least 115 mg/dL) to receive one ABG tablet (250 mg ABG extract/1.25 mg SAC) or placebo daily for 6 weeks. Following a 3-week washout, the groups were reversed and the new intervention continued for another 6 weeks.
Participants received dietary recommendations regarding CVD risk factors and had their dietary habits assessed through a 3-day food record at baseline and after 6 weeks during both treatments.
Individuals receiving lipid-lowering treatment or antihypertensives were excluded, as were those with a body mass index of 35 kg/m2 or higher, those with a fasting blood glucose of at least 126 mg/dL, or active smokers.
There were no differences in baseline characteristics between the two groups. The mean systolic and diastolic pressures at baseline were 124/75 mm Hg in the ABG group and 121/74 mm Hg in the placebo group. Their mean age was 53 years.
Adherence with the protocol was “high” at 96.5% in both groups, and no adverse effects were reported.
Reduced risk of death from stroke, ischemic heart disease
Although no significant differences between ABG and placebo were observed at 3 weeks, the decline in DBP after consumption of the ABG extract became significant at 6 weeks (mean change, –3.7 mm Hg vs. –0.10 mm Hg; P = .007).
When stratified by sex and categories of DBP, the mean change in DBP after 6 weeks of ABG consumption was particularly prominent in men and in those with a baseline DBP of at least 75 mm Hg.
The 6-week change in systolic blood pressure with ABG and placebo was 1.32 mm Hg and 2.84 mm Hg, respectively (P = .694).
At week 6, total cholesterol levels showed a “quadratic decreasing trend” after ABG treatment (P = .047), but no other significant differences between groups were observed for lipid profile, apolipoproteins, or other outcomes of interest, including serum insulin, waist circumference, and body mass index.
The authors note that although systolic BP elevation “has a greater effect on outcomes, both systolic and diastolic hypertension independently influence the risk of adverse cardiovascular events, regardless of the definition of hypertension” and that the risk of death from ischemic heart disease and stroke doubles with every 10 mm Hg increase in DBP in people between the ages of 40 and 89 years.
“Thus, reducing DBP by 5 mm Hg results in a 40% lower risk of death from stroke and a 30% lower risk of death from ischemic heart disease or other vascular death,” they state.
Small study
Commenting for this news organization, Linda Van Horn, PhD, RDN, professor and chief of the department of preventive medicine’s nutrition division, Northwestern University, Chicago, said that for many years, garlic has been “reported to be an adjunct to the benefits of a healthy eating pattern, with inconclusive results.”
She noted that ABG is “literally aged for many months to years, and the resulting concentrate is found higher in many organosulfur compounds and phytochemicals that suggest enhanced response.”
Dr. Van Horn, a member of the American Heart Association’s Nutrition Committee, who was not involved with the study, continued: “The data suggest that ABG that is much more highly concentrated than fresh or processed garlic might be helpful in lowering BP in certain subgroups, in this case men with higher BP.”
However, she cautioned, “these results are limited in a small study, and ... potential other issues, such as sodium, potassium, or other nutrients known to be associated with blood pressure, were not reported, thereby raising questions about the exclusivity of the ABG over other accompanying dietary factors.”
The study was funded by the Center for the Development of Industrial Technology of the Spanish Ministry of Science and Innovation. Two authors are employees of Pharmactive Biotech Products, SL (Madrid), which manufactured the ABG product, but neither played a role in any result or conclusion. The other authors and Dr. Van Horn report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
After 6 weeks, consumption of ABG with a high concentration of s-allyl-L-cystine (SAC) was associated with a nearly 6-mm Hg reduction in DBP in men. Other cardiovascular disease (CVD) risk factors were not significantly affected.
“The observed reduction in DBP by ABG extract was similar to the effects of dietary approaches, including the effects of the Dietary Approaches to Stop Hypertension(DASH) diet on BP,” say Rosa M. Valls, PhD, Universitat Rovira i Virgili, Reus, Spain, and colleagues.
“The potential beneficial effects of ABG may contribute to obtaining an optimal DBP” but were “better observed in men and in nonoptimal DBP populations,” they write in the study, published in Nutrients.
Pure SAC and aged garlics have shown healthy effects on multiple targets in in vitro and in vivo tests. However, previous studies in humans have not focused on ABG but rather on other types of aged garlic in patients with some type of CVD risk factor and suffered from methodologic or design weaknesses, the authors note.
To address this gap, Dr. Valls and colleagues randomly assigned 67 individuals with moderate hypercholesterolemia (defined as LDL levels of at least 115 mg/dL) to receive one ABG tablet (250 mg ABG extract/1.25 mg SAC) or placebo daily for 6 weeks. Following a 3-week washout, the groups were reversed and the new intervention continued for another 6 weeks.
Participants received dietary recommendations regarding CVD risk factors and had their dietary habits assessed through a 3-day food record at baseline and after 6 weeks during both treatments.
Individuals receiving lipid-lowering treatment or antihypertensives were excluded, as were those with a body mass index of 35 kg/m2 or higher, those with a fasting blood glucose of at least 126 mg/dL, or active smokers.
There were no differences in baseline characteristics between the two groups. The mean systolic and diastolic pressures at baseline were 124/75 mm Hg in the ABG group and 121/74 mm Hg in the placebo group. Their mean age was 53 years.
Adherence with the protocol was “high” at 96.5% in both groups, and no adverse effects were reported.
Reduced risk of death from stroke, ischemic heart disease
Although no significant differences between ABG and placebo were observed at 3 weeks, the decline in DBP after consumption of the ABG extract became significant at 6 weeks (mean change, –3.7 mm Hg vs. –0.10 mm Hg; P = .007).
When stratified by sex and categories of DBP, the mean change in DBP after 6 weeks of ABG consumption was particularly prominent in men and in those with a baseline DBP of at least 75 mm Hg.
The 6-week change in systolic blood pressure with ABG and placebo was 1.32 mm Hg and 2.84 mm Hg, respectively (P = .694).
At week 6, total cholesterol levels showed a “quadratic decreasing trend” after ABG treatment (P = .047), but no other significant differences between groups were observed for lipid profile, apolipoproteins, or other outcomes of interest, including serum insulin, waist circumference, and body mass index.
The authors note that although systolic BP elevation “has a greater effect on outcomes, both systolic and diastolic hypertension independently influence the risk of adverse cardiovascular events, regardless of the definition of hypertension” and that the risk of death from ischemic heart disease and stroke doubles with every 10 mm Hg increase in DBP in people between the ages of 40 and 89 years.
“Thus, reducing DBP by 5 mm Hg results in a 40% lower risk of death from stroke and a 30% lower risk of death from ischemic heart disease or other vascular death,” they state.
Small study
Commenting for this news organization, Linda Van Horn, PhD, RDN, professor and chief of the department of preventive medicine’s nutrition division, Northwestern University, Chicago, said that for many years, garlic has been “reported to be an adjunct to the benefits of a healthy eating pattern, with inconclusive results.”
She noted that ABG is “literally aged for many months to years, and the resulting concentrate is found higher in many organosulfur compounds and phytochemicals that suggest enhanced response.”
Dr. Van Horn, a member of the American Heart Association’s Nutrition Committee, who was not involved with the study, continued: “The data suggest that ABG that is much more highly concentrated than fresh or processed garlic might be helpful in lowering BP in certain subgroups, in this case men with higher BP.”
However, she cautioned, “these results are limited in a small study, and ... potential other issues, such as sodium, potassium, or other nutrients known to be associated with blood pressure, were not reported, thereby raising questions about the exclusivity of the ABG over other accompanying dietary factors.”
The study was funded by the Center for the Development of Industrial Technology of the Spanish Ministry of Science and Innovation. Two authors are employees of Pharmactive Biotech Products, SL (Madrid), which manufactured the ABG product, but neither played a role in any result or conclusion. The other authors and Dr. Van Horn report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
After 6 weeks, consumption of ABG with a high concentration of s-allyl-L-cystine (SAC) was associated with a nearly 6-mm Hg reduction in DBP in men. Other cardiovascular disease (CVD) risk factors were not significantly affected.
“The observed reduction in DBP by ABG extract was similar to the effects of dietary approaches, including the effects of the Dietary Approaches to Stop Hypertension(DASH) diet on BP,” say Rosa M. Valls, PhD, Universitat Rovira i Virgili, Reus, Spain, and colleagues.
“The potential beneficial effects of ABG may contribute to obtaining an optimal DBP” but were “better observed in men and in nonoptimal DBP populations,” they write in the study, published in Nutrients.
Pure SAC and aged garlics have shown healthy effects on multiple targets in in vitro and in vivo tests. However, previous studies in humans have not focused on ABG but rather on other types of aged garlic in patients with some type of CVD risk factor and suffered from methodologic or design weaknesses, the authors note.
To address this gap, Dr. Valls and colleagues randomly assigned 67 individuals with moderate hypercholesterolemia (defined as LDL levels of at least 115 mg/dL) to receive one ABG tablet (250 mg ABG extract/1.25 mg SAC) or placebo daily for 6 weeks. Following a 3-week washout, the groups were reversed and the new intervention continued for another 6 weeks.
Participants received dietary recommendations regarding CVD risk factors and had their dietary habits assessed through a 3-day food record at baseline and after 6 weeks during both treatments.
Individuals receiving lipid-lowering treatment or antihypertensives were excluded, as were those with a body mass index of 35 kg/m2 or higher, those with a fasting blood glucose of at least 126 mg/dL, or active smokers.
There were no differences in baseline characteristics between the two groups. The mean systolic and diastolic pressures at baseline were 124/75 mm Hg in the ABG group and 121/74 mm Hg in the placebo group. Their mean age was 53 years.
Adherence with the protocol was “high” at 96.5% in both groups, and no adverse effects were reported.
Reduced risk of death from stroke, ischemic heart disease
Although no significant differences between ABG and placebo were observed at 3 weeks, the decline in DBP after consumption of the ABG extract became significant at 6 weeks (mean change, –3.7 mm Hg vs. –0.10 mm Hg; P = .007).
When stratified by sex and categories of DBP, the mean change in DBP after 6 weeks of ABG consumption was particularly prominent in men and in those with a baseline DBP of at least 75 mm Hg.
The 6-week change in systolic blood pressure with ABG and placebo was 1.32 mm Hg and 2.84 mm Hg, respectively (P = .694).
At week 6, total cholesterol levels showed a “quadratic decreasing trend” after ABG treatment (P = .047), but no other significant differences between groups were observed for lipid profile, apolipoproteins, or other outcomes of interest, including serum insulin, waist circumference, and body mass index.
The authors note that although systolic BP elevation “has a greater effect on outcomes, both systolic and diastolic hypertension independently influence the risk of adverse cardiovascular events, regardless of the definition of hypertension” and that the risk of death from ischemic heart disease and stroke doubles with every 10 mm Hg increase in DBP in people between the ages of 40 and 89 years.
“Thus, reducing DBP by 5 mm Hg results in a 40% lower risk of death from stroke and a 30% lower risk of death from ischemic heart disease or other vascular death,” they state.
Small study
Commenting for this news organization, Linda Van Horn, PhD, RDN, professor and chief of the department of preventive medicine’s nutrition division, Northwestern University, Chicago, said that for many years, garlic has been “reported to be an adjunct to the benefits of a healthy eating pattern, with inconclusive results.”
She noted that ABG is “literally aged for many months to years, and the resulting concentrate is found higher in many organosulfur compounds and phytochemicals that suggest enhanced response.”
Dr. Van Horn, a member of the American Heart Association’s Nutrition Committee, who was not involved with the study, continued: “The data suggest that ABG that is much more highly concentrated than fresh or processed garlic might be helpful in lowering BP in certain subgroups, in this case men with higher BP.”
However, she cautioned, “these results are limited in a small study, and ... potential other issues, such as sodium, potassium, or other nutrients known to be associated with blood pressure, were not reported, thereby raising questions about the exclusivity of the ABG over other accompanying dietary factors.”
The study was funded by the Center for the Development of Industrial Technology of the Spanish Ministry of Science and Innovation. Two authors are employees of Pharmactive Biotech Products, SL (Madrid), which manufactured the ABG product, but neither played a role in any result or conclusion. The other authors and Dr. Van Horn report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NUTRIENTS
The best statins to lower non-HDL cholesterol in diabetes?
A network meta-analysis of 42 clinical trials concludes that rosuvastatin, simvastatin, and atorvastatin are the statins most effective at lowering non-high-density-lipoprotein cholesterol (non-HDL-C) in people with diabetes and at risk for cardiovascular disease.
The analysis focused on the efficacy of statin treatment on reducing non-HDL-C, as opposed to reducing low-density-lipoprotein cholesterol (LDL-C), which has traditionally been used as a surrogate to determine cardiovascular disease risk from hypercholesterolemia.
“The National Cholesterol Education Program in the United States recommends that LDL-C values should be used to estimate the risk of cardiovascular disease related to lipoproteins,” lead author Alexander Hodkinson, MD, senior National Institute for Health Research fellow, University of Manchester, England, told this news organization.
“But we believe that non-high-density-lipoprotein cholesterol is more strongly associated with the risk of cardiovascular disease, because non-HDL-C combines all the bad types of cholesterol, which LDL-C misses, so it could be a better tool than LDL-C for assessing CVD risk and effects of treatment. We already knew which of the statins reduce LDL-C, but we wanted to know which ones reduced non-HDL-C; hence the reason for our study,” Dr. Hodkinson said.
The findings were published online in BMJ.
In April 2021, the National Institute for Health and Care Excellence (NICE) in the United Kingdom updated guidelines for adults with diabetes to recommend that non-HDL-C should replace LDL-C as the primary target for reducing the risk for cardiovascular disease with lipid-lowering treatment.
Currently, NICE is alone in its recommendation. Other international guidelines do not have a non-HDL-C target and use LDL-C reduction instead. These include guidelines from the European Society of Cardiology (ESC), the American College of Cardiology (ACC), the American Heart Association (AHA), and the National Lipid Association.
Non–HDL-C is simple to calculate and can easily be done by clinicians by subtracting HDL-C from the total cholesterol level, he added.
This analysis compared the effectiveness of different statins at different intensities in reducing levels of non-HDL-C in 42 randomized controlled trials that included 20,193 adults with diabetes.
Compared with placebo, rosuvastatin, given at moderate- and high-intensity doses, and simvastatin and atorvastatin at high-intensity doses, were the best at lowering levels of non-HDL-C over an average treatment period of 12 weeks.
High-intensity rosuvastatin led to a 2.31 mmol/L reduction in non-HDL-C (95% credible interval, –3.39 to –1.21). Moderate-intensity rosuvastatin led to a 2.27 mmol/L reduction in non-HDL-C (95% credible interval, –3.00 to –1.49).
High-intensity simvastatin led to a 2.26 mmol/L reduction in non-HDL-C (95% credible interval, –2.99 to –1.51).
High-intensity atorvastatin led to a 2.20 mmol/L reduction in non-HDL-C (95% credible interval, –2.69 to –1.70).
Atorvastatin and simvastatin at any intensity and pravastatin at low intensity were also effective in reducing levels of non-HDL-C, the researchers noted.
In 4,670 patients who were at great risk for a major cardiovascular event, atorvastatin at high intensity showed the largest reduction in levels of non-HDL-C (1.98 mmol/L; 95% credible interval, –4.16 to 0.26).
In addition, high-intensity simvastatin and rosuvastatin were the most effective in reducing LDL-C.
High-intensity simvastatin led to a 1.93 mmol/L reduction in LDL-C (95% credible interval, –2.63 to –1.21), and high-intensity rosuvastatin led to a 1.76 mmol/L reduction in LDL-C (95% credible interval, –2.37 to –1.15).
In four studies, significant reductions in nonfatal myocardial infarction were shown for atorvastatin at moderate intensity, compared with placebo (relative risk, 0.57; 95% confidence interval, 0.43-.76). No significant differences were seen for discontinuations, nonfatal stroke, or cardiovascular death.
“We hope our findings will help guide clinicians on statin selection itself, and what types of doses they should be giving patients. These results support using NICE’s new policy guidelines on cholesterol monitoring, using this non-HDL-C measure, which contains all the bad types of cholesterol for patients with diabetes,” Dr. Hodkinson said.
“This study further emphasizes what we have known about the benefit of statin therapy in patients with type 2 diabetes,” Prakash Deedwania, MD, professor of medicine, University of California, San Francisco, told this news organization.
Dr. Deedwania and others have published data on patients with diabetes that showed that treatment with high-intensity atorvastatin was associated with significant reductions in major adverse cardiovascular events.
“Here they use non-HDL cholesterol as a target. The NICE guidelines are the only guidelines looking at non-HDL cholesterol; however, all guidelines suggest an LDL to be less than 70 in all people with diabetes, and for those with recent acute coronary syndromes, the latest evidence suggests the LDL should actually be less than 50,” said Dr. Deedwania, spokesperson for the AHA and ACC.
As far as which measure to use, he believes both are useful. “It’s six of one and half a dozen of the other, in my opinion. The societies have not recommended non-HDL cholesterol and it’s easier to stay with what is readily available for clinicians, and using LDL cholesterol is still okay. The results of this analysis are confirmatory, in that looking at non-HDL cholesterol gives results very similar to what these statins have shown for their effect on LDL cholesterol,” he said.
Non-HDL cholesterol a better marker?
For Robert Rosenson, MD, director of metabolism and lipids at Mount Sinai Health System and professor of medicine and cardiology at the Icahn School of Medicine at Mount Sinai, New York, non-HDL cholesterol is becoming an important marker of risk for several reasons.
“The focus on LDL cholesterol has been due to the causal relationship of LDL with atherosclerotic cardiovascular disease, but in the last few decades, non-HDL has emerged because more people are overweight, have insulin resistance, and have diabetes,” Dr. Rosenson told this news organization. “In those situations, the LDL cholesterol underrepresents the risk of the LDL particles. With insulin resistance, the particles become more triglycerides and less cholesterol, so on a per-particle basis, you need to get more LDL particles to get to a certain LDL cholesterol concentration.”
Non-HDL cholesterol testing does not require fasting, another advantage of using it to monitor cholesterol, he added.
What is often forgotten is that moderate- to high-intensity statins have very good triglyceride-lowering effects, Dr. Rosenson said.
“This article highlights that, by using higher doses, you get more triglyceride-lowering. Hopefully, this will get practitioners to recognize that non-HDL cholesterol is a better predictor of risk in people with diabetes,” he said.
The study was funded by the National Institute for Health Research. Dr. Hodkinson, Dr. Rosenson, and Dr. Deedwania report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A network meta-analysis of 42 clinical trials concludes that rosuvastatin, simvastatin, and atorvastatin are the statins most effective at lowering non-high-density-lipoprotein cholesterol (non-HDL-C) in people with diabetes and at risk for cardiovascular disease.
The analysis focused on the efficacy of statin treatment on reducing non-HDL-C, as opposed to reducing low-density-lipoprotein cholesterol (LDL-C), which has traditionally been used as a surrogate to determine cardiovascular disease risk from hypercholesterolemia.
“The National Cholesterol Education Program in the United States recommends that LDL-C values should be used to estimate the risk of cardiovascular disease related to lipoproteins,” lead author Alexander Hodkinson, MD, senior National Institute for Health Research fellow, University of Manchester, England, told this news organization.
“But we believe that non-high-density-lipoprotein cholesterol is more strongly associated with the risk of cardiovascular disease, because non-HDL-C combines all the bad types of cholesterol, which LDL-C misses, so it could be a better tool than LDL-C for assessing CVD risk and effects of treatment. We already knew which of the statins reduce LDL-C, but we wanted to know which ones reduced non-HDL-C; hence the reason for our study,” Dr. Hodkinson said.
The findings were published online in BMJ.
In April 2021, the National Institute for Health and Care Excellence (NICE) in the United Kingdom updated guidelines for adults with diabetes to recommend that non-HDL-C should replace LDL-C as the primary target for reducing the risk for cardiovascular disease with lipid-lowering treatment.
Currently, NICE is alone in its recommendation. Other international guidelines do not have a non-HDL-C target and use LDL-C reduction instead. These include guidelines from the European Society of Cardiology (ESC), the American College of Cardiology (ACC), the American Heart Association (AHA), and the National Lipid Association.
Non–HDL-C is simple to calculate and can easily be done by clinicians by subtracting HDL-C from the total cholesterol level, he added.
This analysis compared the effectiveness of different statins at different intensities in reducing levels of non-HDL-C in 42 randomized controlled trials that included 20,193 adults with diabetes.
Compared with placebo, rosuvastatin, given at moderate- and high-intensity doses, and simvastatin and atorvastatin at high-intensity doses, were the best at lowering levels of non-HDL-C over an average treatment period of 12 weeks.
High-intensity rosuvastatin led to a 2.31 mmol/L reduction in non-HDL-C (95% credible interval, –3.39 to –1.21). Moderate-intensity rosuvastatin led to a 2.27 mmol/L reduction in non-HDL-C (95% credible interval, –3.00 to –1.49).
High-intensity simvastatin led to a 2.26 mmol/L reduction in non-HDL-C (95% credible interval, –2.99 to –1.51).
High-intensity atorvastatin led to a 2.20 mmol/L reduction in non-HDL-C (95% credible interval, –2.69 to –1.70).
Atorvastatin and simvastatin at any intensity and pravastatin at low intensity were also effective in reducing levels of non-HDL-C, the researchers noted.
In 4,670 patients who were at great risk for a major cardiovascular event, atorvastatin at high intensity showed the largest reduction in levels of non-HDL-C (1.98 mmol/L; 95% credible interval, –4.16 to 0.26).
In addition, high-intensity simvastatin and rosuvastatin were the most effective in reducing LDL-C.
High-intensity simvastatin led to a 1.93 mmol/L reduction in LDL-C (95% credible interval, –2.63 to –1.21), and high-intensity rosuvastatin led to a 1.76 mmol/L reduction in LDL-C (95% credible interval, –2.37 to –1.15).
In four studies, significant reductions in nonfatal myocardial infarction were shown for atorvastatin at moderate intensity, compared with placebo (relative risk, 0.57; 95% confidence interval, 0.43-.76). No significant differences were seen for discontinuations, nonfatal stroke, or cardiovascular death.
“We hope our findings will help guide clinicians on statin selection itself, and what types of doses they should be giving patients. These results support using NICE’s new policy guidelines on cholesterol monitoring, using this non-HDL-C measure, which contains all the bad types of cholesterol for patients with diabetes,” Dr. Hodkinson said.
“This study further emphasizes what we have known about the benefit of statin therapy in patients with type 2 diabetes,” Prakash Deedwania, MD, professor of medicine, University of California, San Francisco, told this news organization.
Dr. Deedwania and others have published data on patients with diabetes that showed that treatment with high-intensity atorvastatin was associated with significant reductions in major adverse cardiovascular events.
“Here they use non-HDL cholesterol as a target. The NICE guidelines are the only guidelines looking at non-HDL cholesterol; however, all guidelines suggest an LDL to be less than 70 in all people with diabetes, and for those with recent acute coronary syndromes, the latest evidence suggests the LDL should actually be less than 50,” said Dr. Deedwania, spokesperson for the AHA and ACC.
As far as which measure to use, he believes both are useful. “It’s six of one and half a dozen of the other, in my opinion. The societies have not recommended non-HDL cholesterol and it’s easier to stay with what is readily available for clinicians, and using LDL cholesterol is still okay. The results of this analysis are confirmatory, in that looking at non-HDL cholesterol gives results very similar to what these statins have shown for their effect on LDL cholesterol,” he said.
Non-HDL cholesterol a better marker?
For Robert Rosenson, MD, director of metabolism and lipids at Mount Sinai Health System and professor of medicine and cardiology at the Icahn School of Medicine at Mount Sinai, New York, non-HDL cholesterol is becoming an important marker of risk for several reasons.
“The focus on LDL cholesterol has been due to the causal relationship of LDL with atherosclerotic cardiovascular disease, but in the last few decades, non-HDL has emerged because more people are overweight, have insulin resistance, and have diabetes,” Dr. Rosenson told this news organization. “In those situations, the LDL cholesterol underrepresents the risk of the LDL particles. With insulin resistance, the particles become more triglycerides and less cholesterol, so on a per-particle basis, you need to get more LDL particles to get to a certain LDL cholesterol concentration.”
Non-HDL cholesterol testing does not require fasting, another advantage of using it to monitor cholesterol, he added.
What is often forgotten is that moderate- to high-intensity statins have very good triglyceride-lowering effects, Dr. Rosenson said.
“This article highlights that, by using higher doses, you get more triglyceride-lowering. Hopefully, this will get practitioners to recognize that non-HDL cholesterol is a better predictor of risk in people with diabetes,” he said.
The study was funded by the National Institute for Health Research. Dr. Hodkinson, Dr. Rosenson, and Dr. Deedwania report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A network meta-analysis of 42 clinical trials concludes that rosuvastatin, simvastatin, and atorvastatin are the statins most effective at lowering non-high-density-lipoprotein cholesterol (non-HDL-C) in people with diabetes and at risk for cardiovascular disease.
The analysis focused on the efficacy of statin treatment on reducing non-HDL-C, as opposed to reducing low-density-lipoprotein cholesterol (LDL-C), which has traditionally been used as a surrogate to determine cardiovascular disease risk from hypercholesterolemia.
“The National Cholesterol Education Program in the United States recommends that LDL-C values should be used to estimate the risk of cardiovascular disease related to lipoproteins,” lead author Alexander Hodkinson, MD, senior National Institute for Health Research fellow, University of Manchester, England, told this news organization.
“But we believe that non-high-density-lipoprotein cholesterol is more strongly associated with the risk of cardiovascular disease, because non-HDL-C combines all the bad types of cholesterol, which LDL-C misses, so it could be a better tool than LDL-C for assessing CVD risk and effects of treatment. We already knew which of the statins reduce LDL-C, but we wanted to know which ones reduced non-HDL-C; hence the reason for our study,” Dr. Hodkinson said.
The findings were published online in BMJ.
In April 2021, the National Institute for Health and Care Excellence (NICE) in the United Kingdom updated guidelines for adults with diabetes to recommend that non-HDL-C should replace LDL-C as the primary target for reducing the risk for cardiovascular disease with lipid-lowering treatment.
Currently, NICE is alone in its recommendation. Other international guidelines do not have a non-HDL-C target and use LDL-C reduction instead. These include guidelines from the European Society of Cardiology (ESC), the American College of Cardiology (ACC), the American Heart Association (AHA), and the National Lipid Association.
Non–HDL-C is simple to calculate and can easily be done by clinicians by subtracting HDL-C from the total cholesterol level, he added.
This analysis compared the effectiveness of different statins at different intensities in reducing levels of non-HDL-C in 42 randomized controlled trials that included 20,193 adults with diabetes.
Compared with placebo, rosuvastatin, given at moderate- and high-intensity doses, and simvastatin and atorvastatin at high-intensity doses, were the best at lowering levels of non-HDL-C over an average treatment period of 12 weeks.
High-intensity rosuvastatin led to a 2.31 mmol/L reduction in non-HDL-C (95% credible interval, –3.39 to –1.21). Moderate-intensity rosuvastatin led to a 2.27 mmol/L reduction in non-HDL-C (95% credible interval, –3.00 to –1.49).
High-intensity simvastatin led to a 2.26 mmol/L reduction in non-HDL-C (95% credible interval, –2.99 to –1.51).
High-intensity atorvastatin led to a 2.20 mmol/L reduction in non-HDL-C (95% credible interval, –2.69 to –1.70).
Atorvastatin and simvastatin at any intensity and pravastatin at low intensity were also effective in reducing levels of non-HDL-C, the researchers noted.
In 4,670 patients who were at great risk for a major cardiovascular event, atorvastatin at high intensity showed the largest reduction in levels of non-HDL-C (1.98 mmol/L; 95% credible interval, –4.16 to 0.26).
In addition, high-intensity simvastatin and rosuvastatin were the most effective in reducing LDL-C.
High-intensity simvastatin led to a 1.93 mmol/L reduction in LDL-C (95% credible interval, –2.63 to –1.21), and high-intensity rosuvastatin led to a 1.76 mmol/L reduction in LDL-C (95% credible interval, –2.37 to –1.15).
In four studies, significant reductions in nonfatal myocardial infarction were shown for atorvastatin at moderate intensity, compared with placebo (relative risk, 0.57; 95% confidence interval, 0.43-.76). No significant differences were seen for discontinuations, nonfatal stroke, or cardiovascular death.
“We hope our findings will help guide clinicians on statin selection itself, and what types of doses they should be giving patients. These results support using NICE’s new policy guidelines on cholesterol monitoring, using this non-HDL-C measure, which contains all the bad types of cholesterol for patients with diabetes,” Dr. Hodkinson said.
“This study further emphasizes what we have known about the benefit of statin therapy in patients with type 2 diabetes,” Prakash Deedwania, MD, professor of medicine, University of California, San Francisco, told this news organization.
Dr. Deedwania and others have published data on patients with diabetes that showed that treatment with high-intensity atorvastatin was associated with significant reductions in major adverse cardiovascular events.
“Here they use non-HDL cholesterol as a target. The NICE guidelines are the only guidelines looking at non-HDL cholesterol; however, all guidelines suggest an LDL to be less than 70 in all people with diabetes, and for those with recent acute coronary syndromes, the latest evidence suggests the LDL should actually be less than 50,” said Dr. Deedwania, spokesperson for the AHA and ACC.
As far as which measure to use, he believes both are useful. “It’s six of one and half a dozen of the other, in my opinion. The societies have not recommended non-HDL cholesterol and it’s easier to stay with what is readily available for clinicians, and using LDL cholesterol is still okay. The results of this analysis are confirmatory, in that looking at non-HDL cholesterol gives results very similar to what these statins have shown for their effect on LDL cholesterol,” he said.
Non-HDL cholesterol a better marker?
For Robert Rosenson, MD, director of metabolism and lipids at Mount Sinai Health System and professor of medicine and cardiology at the Icahn School of Medicine at Mount Sinai, New York, non-HDL cholesterol is becoming an important marker of risk for several reasons.
“The focus on LDL cholesterol has been due to the causal relationship of LDL with atherosclerotic cardiovascular disease, but in the last few decades, non-HDL has emerged because more people are overweight, have insulin resistance, and have diabetes,” Dr. Rosenson told this news organization. “In those situations, the LDL cholesterol underrepresents the risk of the LDL particles. With insulin resistance, the particles become more triglycerides and less cholesterol, so on a per-particle basis, you need to get more LDL particles to get to a certain LDL cholesterol concentration.”
Non-HDL cholesterol testing does not require fasting, another advantage of using it to monitor cholesterol, he added.
What is often forgotten is that moderate- to high-intensity statins have very good triglyceride-lowering effects, Dr. Rosenson said.
“This article highlights that, by using higher doses, you get more triglyceride-lowering. Hopefully, this will get practitioners to recognize that non-HDL cholesterol is a better predictor of risk in people with diabetes,” he said.
The study was funded by the National Institute for Health Research. Dr. Hodkinson, Dr. Rosenson, and Dr. Deedwania report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Aspirin exposure fails to reduce cardiovascular event risk
The benefits of aspirin use for the primary prevention of atherosclerotic cardiovascular disease (ASCVD) have been questioned in light of data showing neutral outcomes in low-risk patients and concerns about increased bleeding risk and mortality in healthy older adults, wrote Rita Del Pinto, MD, of University of L’Aquila (Italy) and colleagues in JAMA Network Open.
In the study, Dr. Del Pinto and colleagues conducted a post hoc analysis of data from more than 2,500 participants in SPRINT (Systolic Blood Pressure Intervention Trial), a multicenter, randomized trial conducted from 2010 to 2013.
The goal of SPRINT was to compare intensive and standard blood pressure–lowering strategies for hypertension patients. The primary outcome of the current study was risk of a first cardiovascular event, which included adjudicated myocardial infarction, non–myocardial infarction acute coronary syndrome, stroke, acute heart failure, and CVD death.“There has been considerable improvement in the management of cardiovascular risk factors since the first reports on aspirin use for cardiovascular prevention,” Dr. Del Pinto said in an interview.
“As for hypertension, not only have more effective antihypertensive medications become available, but also evidence has recently emerged in support of a downwards redefinition of blood pressure targets during treatment,” she said. “In this context, in an era when great attention is paid to the personalization of treatment, no specific studies had addressed the association of aspirin use as a primary prevention strategy in a cohort of relatively old, high-risk individuals with treated systolic blood pressure steadily below the recommended target,” she added.
The researchers assessed whether aspirin use in addition to standard blood pressure management (a target of less than 140 mm Hg) decreased risk and improved survival.
The study population included 2,664 adult patients; 29.3% were women, and 24.5% were aged 75 years and older. Half of the patients (1,332) received aspirin and 1,332 did not.
In a multivariate analysis, 42 cardiovascular events occurred in the aspirin group, compared with 20 events in those not exposed to aspirin (hazard ratio, 2.30). The findings were consistent in subgroup analyses of younger individuals, current and former smokers, and patients on statins.
An additional subgroup analysis of individuals randomized to standard care or intensive care in the SPRINT study showed no significant difference in primary outcome rates between individuals who received aspirin and those who did not. The rates for aspirin use vs. non–aspirin use were 5.85% vs. 3.60% in the standard treatment group and 4.66% vs. 2.56% in the intensive treatment group.
The study findings were limited by several factors, including the post hoc design, short follow-up period, and lack of data on the initiation of aspirin and bleeding events, the researchers wrote. However, the results suggest that modern management of hypertension may have redefined the potential benefits of aspirin in patients with hypertension, they concluded.
Findings confirm value of preventive care
“The study was conducted as a post-hoc analysis on an experimental cohort, which must be considered when interpreting the results,” Dr. Del Pinto said.
Despite the limitations, the study findings affirm that effective treatment of major cardiovascular risk factors, such as hypertension, with proven drugs is “a mainstay of the primary prevention of ASCVD,” she emphasized.
As for additional research, “Testing our findings in a dedicated setting with sufficiently long follow-up, where aspirin dose and indication, as well as any possible bleeding event, are reported could expand the clinical meaning of our observations,” said Dr. Del Pinto. “Also, the clinical impact of aspirin, even in combination with novel cardiovascular drugs such as direct oral anticoagulants, in populations exposed to combinations of risk factors, deserves further investigation.”
Data support shared decision-making
“While recent evidence has not shown a benefit of aspirin in the primary prevention of ASCVD in several populations, the subpopulation of patients with hypertension as an ASCVD risk factor is also of interest to the clinician,” Suman Pal, MD, of the University of New Mexico, Albuquerque, said in an interview. “The lack of benefit of aspirin in this study, despite its limitations, was surprising, and I would be eager to see how the role of aspirin in ASCVD prevention would continue to evolve in conjunction with improvement in other therapies for modification of risk factors.”
“The decision to continue aspirin in this subgroup of patients should warrant a discussion with patients and a reexamination of risks and benefits until further data are available,” Dr. Pal emphasized.
Larger studies with long-term follow-ups would be required to further clarify the role of aspirin in primary prevention of ASCVD in patients with hypertension without diabetes or chronic kidney disease, he added.
Data were supplied courtesy of BioLINCC. The study received no outside funding. The researchers and Dr. Pal had no financial conflicts to disclose.
The benefits of aspirin use for the primary prevention of atherosclerotic cardiovascular disease (ASCVD) have been questioned in light of data showing neutral outcomes in low-risk patients and concerns about increased bleeding risk and mortality in healthy older adults, wrote Rita Del Pinto, MD, of University of L’Aquila (Italy) and colleagues in JAMA Network Open.
In the study, Dr. Del Pinto and colleagues conducted a post hoc analysis of data from more than 2,500 participants in SPRINT (Systolic Blood Pressure Intervention Trial), a multicenter, randomized trial conducted from 2010 to 2013.
The goal of SPRINT was to compare intensive and standard blood pressure–lowering strategies for hypertension patients. The primary outcome of the current study was risk of a first cardiovascular event, which included adjudicated myocardial infarction, non–myocardial infarction acute coronary syndrome, stroke, acute heart failure, and CVD death.“There has been considerable improvement in the management of cardiovascular risk factors since the first reports on aspirin use for cardiovascular prevention,” Dr. Del Pinto said in an interview.
“As for hypertension, not only have more effective antihypertensive medications become available, but also evidence has recently emerged in support of a downwards redefinition of blood pressure targets during treatment,” she said. “In this context, in an era when great attention is paid to the personalization of treatment, no specific studies had addressed the association of aspirin use as a primary prevention strategy in a cohort of relatively old, high-risk individuals with treated systolic blood pressure steadily below the recommended target,” she added.
The researchers assessed whether aspirin use in addition to standard blood pressure management (a target of less than 140 mm Hg) decreased risk and improved survival.
The study population included 2,664 adult patients; 29.3% were women, and 24.5% were aged 75 years and older. Half of the patients (1,332) received aspirin and 1,332 did not.
In a multivariate analysis, 42 cardiovascular events occurred in the aspirin group, compared with 20 events in those not exposed to aspirin (hazard ratio, 2.30). The findings were consistent in subgroup analyses of younger individuals, current and former smokers, and patients on statins.
An additional subgroup analysis of individuals randomized to standard care or intensive care in the SPRINT study showed no significant difference in primary outcome rates between individuals who received aspirin and those who did not. The rates for aspirin use vs. non–aspirin use were 5.85% vs. 3.60% in the standard treatment group and 4.66% vs. 2.56% in the intensive treatment group.
The study findings were limited by several factors, including the post hoc design, short follow-up period, and lack of data on the initiation of aspirin and bleeding events, the researchers wrote. However, the results suggest that modern management of hypertension may have redefined the potential benefits of aspirin in patients with hypertension, they concluded.
Findings confirm value of preventive care
“The study was conducted as a post-hoc analysis on an experimental cohort, which must be considered when interpreting the results,” Dr. Del Pinto said.
Despite the limitations, the study findings affirm that effective treatment of major cardiovascular risk factors, such as hypertension, with proven drugs is “a mainstay of the primary prevention of ASCVD,” she emphasized.
As for additional research, “Testing our findings in a dedicated setting with sufficiently long follow-up, where aspirin dose and indication, as well as any possible bleeding event, are reported could expand the clinical meaning of our observations,” said Dr. Del Pinto. “Also, the clinical impact of aspirin, even in combination with novel cardiovascular drugs such as direct oral anticoagulants, in populations exposed to combinations of risk factors, deserves further investigation.”
Data support shared decision-making
“While recent evidence has not shown a benefit of aspirin in the primary prevention of ASCVD in several populations, the subpopulation of patients with hypertension as an ASCVD risk factor is also of interest to the clinician,” Suman Pal, MD, of the University of New Mexico, Albuquerque, said in an interview. “The lack of benefit of aspirin in this study, despite its limitations, was surprising, and I would be eager to see how the role of aspirin in ASCVD prevention would continue to evolve in conjunction with improvement in other therapies for modification of risk factors.”
“The decision to continue aspirin in this subgroup of patients should warrant a discussion with patients and a reexamination of risks and benefits until further data are available,” Dr. Pal emphasized.
Larger studies with long-term follow-ups would be required to further clarify the role of aspirin in primary prevention of ASCVD in patients with hypertension without diabetes or chronic kidney disease, he added.
Data were supplied courtesy of BioLINCC. The study received no outside funding. The researchers and Dr. Pal had no financial conflicts to disclose.
The benefits of aspirin use for the primary prevention of atherosclerotic cardiovascular disease (ASCVD) have been questioned in light of data showing neutral outcomes in low-risk patients and concerns about increased bleeding risk and mortality in healthy older adults, wrote Rita Del Pinto, MD, of University of L’Aquila (Italy) and colleagues in JAMA Network Open.
In the study, Dr. Del Pinto and colleagues conducted a post hoc analysis of data from more than 2,500 participants in SPRINT (Systolic Blood Pressure Intervention Trial), a multicenter, randomized trial conducted from 2010 to 2013.
The goal of SPRINT was to compare intensive and standard blood pressure–lowering strategies for hypertension patients. The primary outcome of the current study was risk of a first cardiovascular event, which included adjudicated myocardial infarction, non–myocardial infarction acute coronary syndrome, stroke, acute heart failure, and CVD death.“There has been considerable improvement in the management of cardiovascular risk factors since the first reports on aspirin use for cardiovascular prevention,” Dr. Del Pinto said in an interview.
“As for hypertension, not only have more effective antihypertensive medications become available, but also evidence has recently emerged in support of a downwards redefinition of blood pressure targets during treatment,” she said. “In this context, in an era when great attention is paid to the personalization of treatment, no specific studies had addressed the association of aspirin use as a primary prevention strategy in a cohort of relatively old, high-risk individuals with treated systolic blood pressure steadily below the recommended target,” she added.
The researchers assessed whether aspirin use in addition to standard blood pressure management (a target of less than 140 mm Hg) decreased risk and improved survival.
The study population included 2,664 adult patients; 29.3% were women, and 24.5% were aged 75 years and older. Half of the patients (1,332) received aspirin and 1,332 did not.
In a multivariate analysis, 42 cardiovascular events occurred in the aspirin group, compared with 20 events in those not exposed to aspirin (hazard ratio, 2.30). The findings were consistent in subgroup analyses of younger individuals, current and former smokers, and patients on statins.
An additional subgroup analysis of individuals randomized to standard care or intensive care in the SPRINT study showed no significant difference in primary outcome rates between individuals who received aspirin and those who did not. The rates for aspirin use vs. non–aspirin use were 5.85% vs. 3.60% in the standard treatment group and 4.66% vs. 2.56% in the intensive treatment group.
The study findings were limited by several factors, including the post hoc design, short follow-up period, and lack of data on the initiation of aspirin and bleeding events, the researchers wrote. However, the results suggest that modern management of hypertension may have redefined the potential benefits of aspirin in patients with hypertension, they concluded.
Findings confirm value of preventive care
“The study was conducted as a post-hoc analysis on an experimental cohort, which must be considered when interpreting the results,” Dr. Del Pinto said.
Despite the limitations, the study findings affirm that effective treatment of major cardiovascular risk factors, such as hypertension, with proven drugs is “a mainstay of the primary prevention of ASCVD,” she emphasized.
As for additional research, “Testing our findings in a dedicated setting with sufficiently long follow-up, where aspirin dose and indication, as well as any possible bleeding event, are reported could expand the clinical meaning of our observations,” said Dr. Del Pinto. “Also, the clinical impact of aspirin, even in combination with novel cardiovascular drugs such as direct oral anticoagulants, in populations exposed to combinations of risk factors, deserves further investigation.”
Data support shared decision-making
“While recent evidence has not shown a benefit of aspirin in the primary prevention of ASCVD in several populations, the subpopulation of patients with hypertension as an ASCVD risk factor is also of interest to the clinician,” Suman Pal, MD, of the University of New Mexico, Albuquerque, said in an interview. “The lack of benefit of aspirin in this study, despite its limitations, was surprising, and I would be eager to see how the role of aspirin in ASCVD prevention would continue to evolve in conjunction with improvement in other therapies for modification of risk factors.”
“The decision to continue aspirin in this subgroup of patients should warrant a discussion with patients and a reexamination of risks and benefits until further data are available,” Dr. Pal emphasized.
Larger studies with long-term follow-ups would be required to further clarify the role of aspirin in primary prevention of ASCVD in patients with hypertension without diabetes or chronic kidney disease, he added.
Data were supplied courtesy of BioLINCC. The study received no outside funding. The researchers and Dr. Pal had no financial conflicts to disclose.
FROM JAMA NETWORK OPEN
Combo of SGLT2 inhibitor + GLP-1 RA boosts diabetes survival
WASHINGTON – Patients with type 2 diabetes and established atherosclerotic cardiovascular disease treated with both an sodium-glucose transporter 2 inhibitor and a glucagonlike peptide–1 receptor agonist had a significant 80% cut in their rate of all-cause death during 1-year follow-up, compared with matched patients treated with an agent from either class alone in an observational, retrospective study of more than 15,000 people in the U.S. Veterans Affairs health system.
For the study’s primary endpoint, the combined rate of all-cause death, nonfatal MI, or nonfatal stroke, combined treatment with both an agent from the sodium-glucose transporter 2 (SGLT2) inhibitor class and from the glucagonlike peptide–1 receptor agonist (GLP-1 RA) class linked with a significant, roughly 50% cut in events during 1-year follow-up, compared with patients treated with an agent from just one of these two classes, Persio D. Lopez, MD, reported at the annual scientific sessions of the American College of Cardiology.
This improvement in the combined endpoint outcome resulted entirely from reduced all-cause mortality. Dual treatment showed no significant association with the incidence of nonfatal MIs or strokes, compared with monotherapy, with rates that were nearly identical regardless of whether patients took one of the agents or both, said Dr. Lopez, a cardiologist at Mount Sinai Morningside and the James J. Peters VA Medical Center, both in New York.
Combining classes for hard-to-control diabetes
“We’re not sure what drives combined use” of agents from both drug classes in these types of patients, admitted Dr. Lopez during his talk. “Our hypothesis is that dual treatment is used in patients with harder-to-control diabetes.”
Salim S. Virani, MD, PhD, who practices in the VA system but was not involved with the study, agreed that this is the likely explanation for most instances of high-risk VA patients with diabetes who receive agents from both classes.
“I have a few patients” on both classes, usually “patients with higher starting A1c levels who need greater glycemic control,” said Dr. Virani, professor of medicine at Baylor College of Medicine and a cardiologist at the Michael E. DeBakey VA Medical Center, both in Houston.
U.S. use of either drug class, let alone both, in patients with type 2 diabetes is still struggling to gain traction in U.S. practice and remains limited to a minority of these patients, a prescribing pattern reflected in recent VA data. Analysis of more than half a million patients in the VA system with type 2 diabetes and atherosclerotic cardiovascular disease (ASCVD) who received treatment at any of 130 VA medical centers throughout 2020 showed that 11% had received an SGLT2 inhibitor, and 8% a GLP-1 RA.
The most frequently used antidiabetes drug classes in these patients were insulin in 36%, biguanides in 47%, and sulfonylureas in 22%.
These data also showed a striking level of variability among the 130 VA centers, with some of the sites prescribing either an SGLT2 inhibitor or a GLP-1 RA to as few as about 3% each of these patients, while other centers had a roughly 10-fold higher prescription rate for each of about 25%-30% of their patients with type 2 diabetes and ASCVD.
Despite the overall modest level of use of both classes in these types of patients as recently as 2020, no barriers exist at the VA to prescribing an agent from one or both classes “if you provide a good reason” for a patient to receive the drugs, Dr. Virani said in an interview. He also predicted that use of both classes in these patients, including combination treatment, will likely soon expand.
‘A lot of interest’ in combining an SGLT2 inhibitor and a GLP-1 RA
“There will be a lot of interest in combing the two classes. It makes intuitive sense [to treat with both classes] because most patients with diabetes need more than one drug” for glycemic control, he noted. “Why not use two classes that each reduce a patient’s risk” for adverse outcomes involving ASCVD, heart failure, and renal dysfunction, added Dr. Virani.
The study run by Dr. Lopez and his associates used data collected in the National VA Database and included 121,156 patients with both type 2 diabetes and established ASCVD. Using propensity-score matching the researchers compiled three subgroups that each included 5,277 matched patients. One subgroup had patients prescribed an SGLT2 inhibitor, a second subgroup included patients on a GLP-1 RA, and a third subgroup had patients on agents from both classes. Patient matching relied on age, sex, left ventricular ejection fraction, hemoglobin A1c level, systolic blood pressure, and the presence of coronary artery disease or peripheral artery disease.
Patients included in the analysis averaged about 67 years of age; 97% were men, their average body mass index was about 34 kg/m2, their average A1c was about 7.9%, their average estimated glomerular filtration rate was about 55-66 mL/min per 1.73 m2, and their average left ventricular ejection fraction was about 55%. The database provided a median follow-up of 902 days (about 2.5 years). The prespecified primary endpoint focused on events that occurred during the first year of follow-up, but the investigators also ran a 3-year follow-up analysis on a post hoc basis.
The most common SGLT2 inhibitor received by these patients was empagliflozin (Jardiance), used on virtually everyone who received an agent from this class. In contrast, the GLP-1 RA drugs that patients received split more widely. The most prescribed agent was liraglutide (Victoza), followed by semaglutide (Ozempic), and dulaglutide (Trulicity), with fewer than 5% receiving exenatide (Bydureon, Byetta).
Regarding other treatments, about 97% of all patients received a statin, about 94% were on a renin-angiotensin system inhibitor, about 90% were on metformin, and roughly 75% were on insulin, aspirin, and a beta-blocker, with smaller numbers on other types of agents.
For the study’s primary endpoint, the 1-year incidence of combined ASCVD events including all-cause death, patients on agents from both classes had a significant 46% reduced rate compared with those on an SGLT2 inhibitor only, and a significant 49% reduced rate, compared with those on a GLP-1 RA only. These between-group separations broadened slightly during 3-year follow-up. Dr. Lopez did not report results of a direct comparison between patients on just an SGLT2 inhibitor and those on just a GLP-1 RA.
For the endpoint of all-cause death, those on combined treatment had a 1-year rate that was 83% below the rate among patients on only an SGLT2 inhibitor, and 81% below the rate among patients who received a GLP-1 RA but not the other class.
Dr. Lopez cautioned that selection bias could have influenced the outcomes of patients who received both classes rather than one or the other, and he also highlighted that the analysis relied on administrative data rather than information gleaned from more detailed medical records or prospectively collected findings and was limited by only including a very small number of women.
“Our results need to be validated in prospective studies,” he declared.
Dr. Lopez and Dr. Virani had no commercial disclosures.
WASHINGTON – Patients with type 2 diabetes and established atherosclerotic cardiovascular disease treated with both an sodium-glucose transporter 2 inhibitor and a glucagonlike peptide–1 receptor agonist had a significant 80% cut in their rate of all-cause death during 1-year follow-up, compared with matched patients treated with an agent from either class alone in an observational, retrospective study of more than 15,000 people in the U.S. Veterans Affairs health system.
For the study’s primary endpoint, the combined rate of all-cause death, nonfatal MI, or nonfatal stroke, combined treatment with both an agent from the sodium-glucose transporter 2 (SGLT2) inhibitor class and from the glucagonlike peptide–1 receptor agonist (GLP-1 RA) class linked with a significant, roughly 50% cut in events during 1-year follow-up, compared with patients treated with an agent from just one of these two classes, Persio D. Lopez, MD, reported at the annual scientific sessions of the American College of Cardiology.
This improvement in the combined endpoint outcome resulted entirely from reduced all-cause mortality. Dual treatment showed no significant association with the incidence of nonfatal MIs or strokes, compared with monotherapy, with rates that were nearly identical regardless of whether patients took one of the agents or both, said Dr. Lopez, a cardiologist at Mount Sinai Morningside and the James J. Peters VA Medical Center, both in New York.
Combining classes for hard-to-control diabetes
“We’re not sure what drives combined use” of agents from both drug classes in these types of patients, admitted Dr. Lopez during his talk. “Our hypothesis is that dual treatment is used in patients with harder-to-control diabetes.”
Salim S. Virani, MD, PhD, who practices in the VA system but was not involved with the study, agreed that this is the likely explanation for most instances of high-risk VA patients with diabetes who receive agents from both classes.
“I have a few patients” on both classes, usually “patients with higher starting A1c levels who need greater glycemic control,” said Dr. Virani, professor of medicine at Baylor College of Medicine and a cardiologist at the Michael E. DeBakey VA Medical Center, both in Houston.
U.S. use of either drug class, let alone both, in patients with type 2 diabetes is still struggling to gain traction in U.S. practice and remains limited to a minority of these patients, a prescribing pattern reflected in recent VA data. Analysis of more than half a million patients in the VA system with type 2 diabetes and atherosclerotic cardiovascular disease (ASCVD) who received treatment at any of 130 VA medical centers throughout 2020 showed that 11% had received an SGLT2 inhibitor, and 8% a GLP-1 RA.
The most frequently used antidiabetes drug classes in these patients were insulin in 36%, biguanides in 47%, and sulfonylureas in 22%.
These data also showed a striking level of variability among the 130 VA centers, with some of the sites prescribing either an SGLT2 inhibitor or a GLP-1 RA to as few as about 3% each of these patients, while other centers had a roughly 10-fold higher prescription rate for each of about 25%-30% of their patients with type 2 diabetes and ASCVD.
Despite the overall modest level of use of both classes in these types of patients as recently as 2020, no barriers exist at the VA to prescribing an agent from one or both classes “if you provide a good reason” for a patient to receive the drugs, Dr. Virani said in an interview. He also predicted that use of both classes in these patients, including combination treatment, will likely soon expand.
‘A lot of interest’ in combining an SGLT2 inhibitor and a GLP-1 RA
“There will be a lot of interest in combing the two classes. It makes intuitive sense [to treat with both classes] because most patients with diabetes need more than one drug” for glycemic control, he noted. “Why not use two classes that each reduce a patient’s risk” for adverse outcomes involving ASCVD, heart failure, and renal dysfunction, added Dr. Virani.
The study run by Dr. Lopez and his associates used data collected in the National VA Database and included 121,156 patients with both type 2 diabetes and established ASCVD. Using propensity-score matching the researchers compiled three subgroups that each included 5,277 matched patients. One subgroup had patients prescribed an SGLT2 inhibitor, a second subgroup included patients on a GLP-1 RA, and a third subgroup had patients on agents from both classes. Patient matching relied on age, sex, left ventricular ejection fraction, hemoglobin A1c level, systolic blood pressure, and the presence of coronary artery disease or peripheral artery disease.
Patients included in the analysis averaged about 67 years of age; 97% were men, their average body mass index was about 34 kg/m2, their average A1c was about 7.9%, their average estimated glomerular filtration rate was about 55-66 mL/min per 1.73 m2, and their average left ventricular ejection fraction was about 55%. The database provided a median follow-up of 902 days (about 2.5 years). The prespecified primary endpoint focused on events that occurred during the first year of follow-up, but the investigators also ran a 3-year follow-up analysis on a post hoc basis.
The most common SGLT2 inhibitor received by these patients was empagliflozin (Jardiance), used on virtually everyone who received an agent from this class. In contrast, the GLP-1 RA drugs that patients received split more widely. The most prescribed agent was liraglutide (Victoza), followed by semaglutide (Ozempic), and dulaglutide (Trulicity), with fewer than 5% receiving exenatide (Bydureon, Byetta).
Regarding other treatments, about 97% of all patients received a statin, about 94% were on a renin-angiotensin system inhibitor, about 90% were on metformin, and roughly 75% were on insulin, aspirin, and a beta-blocker, with smaller numbers on other types of agents.
For the study’s primary endpoint, the 1-year incidence of combined ASCVD events including all-cause death, patients on agents from both classes had a significant 46% reduced rate compared with those on an SGLT2 inhibitor only, and a significant 49% reduced rate, compared with those on a GLP-1 RA only. These between-group separations broadened slightly during 3-year follow-up. Dr. Lopez did not report results of a direct comparison between patients on just an SGLT2 inhibitor and those on just a GLP-1 RA.
For the endpoint of all-cause death, those on combined treatment had a 1-year rate that was 83% below the rate among patients on only an SGLT2 inhibitor, and 81% below the rate among patients who received a GLP-1 RA but not the other class.
Dr. Lopez cautioned that selection bias could have influenced the outcomes of patients who received both classes rather than one or the other, and he also highlighted that the analysis relied on administrative data rather than information gleaned from more detailed medical records or prospectively collected findings and was limited by only including a very small number of women.
“Our results need to be validated in prospective studies,” he declared.
Dr. Lopez and Dr. Virani had no commercial disclosures.
WASHINGTON – Patients with type 2 diabetes and established atherosclerotic cardiovascular disease treated with both an sodium-glucose transporter 2 inhibitor and a glucagonlike peptide–1 receptor agonist had a significant 80% cut in their rate of all-cause death during 1-year follow-up, compared with matched patients treated with an agent from either class alone in an observational, retrospective study of more than 15,000 people in the U.S. Veterans Affairs health system.
For the study’s primary endpoint, the combined rate of all-cause death, nonfatal MI, or nonfatal stroke, combined treatment with both an agent from the sodium-glucose transporter 2 (SGLT2) inhibitor class and from the glucagonlike peptide–1 receptor agonist (GLP-1 RA) class linked with a significant, roughly 50% cut in events during 1-year follow-up, compared with patients treated with an agent from just one of these two classes, Persio D. Lopez, MD, reported at the annual scientific sessions of the American College of Cardiology.
This improvement in the combined endpoint outcome resulted entirely from reduced all-cause mortality. Dual treatment showed no significant association with the incidence of nonfatal MIs or strokes, compared with monotherapy, with rates that were nearly identical regardless of whether patients took one of the agents or both, said Dr. Lopez, a cardiologist at Mount Sinai Morningside and the James J. Peters VA Medical Center, both in New York.
Combining classes for hard-to-control diabetes
“We’re not sure what drives combined use” of agents from both drug classes in these types of patients, admitted Dr. Lopez during his talk. “Our hypothesis is that dual treatment is used in patients with harder-to-control diabetes.”
Salim S. Virani, MD, PhD, who practices in the VA system but was not involved with the study, agreed that this is the likely explanation for most instances of high-risk VA patients with diabetes who receive agents from both classes.
“I have a few patients” on both classes, usually “patients with higher starting A1c levels who need greater glycemic control,” said Dr. Virani, professor of medicine at Baylor College of Medicine and a cardiologist at the Michael E. DeBakey VA Medical Center, both in Houston.
U.S. use of either drug class, let alone both, in patients with type 2 diabetes is still struggling to gain traction in U.S. practice and remains limited to a minority of these patients, a prescribing pattern reflected in recent VA data. Analysis of more than half a million patients in the VA system with type 2 diabetes and atherosclerotic cardiovascular disease (ASCVD) who received treatment at any of 130 VA medical centers throughout 2020 showed that 11% had received an SGLT2 inhibitor, and 8% a GLP-1 RA.
The most frequently used antidiabetes drug classes in these patients were insulin in 36%, biguanides in 47%, and sulfonylureas in 22%.
These data also showed a striking level of variability among the 130 VA centers, with some of the sites prescribing either an SGLT2 inhibitor or a GLP-1 RA to as few as about 3% each of these patients, while other centers had a roughly 10-fold higher prescription rate for each of about 25%-30% of their patients with type 2 diabetes and ASCVD.
Despite the overall modest level of use of both classes in these types of patients as recently as 2020, no barriers exist at the VA to prescribing an agent from one or both classes “if you provide a good reason” for a patient to receive the drugs, Dr. Virani said in an interview. He also predicted that use of both classes in these patients, including combination treatment, will likely soon expand.
‘A lot of interest’ in combining an SGLT2 inhibitor and a GLP-1 RA
“There will be a lot of interest in combing the two classes. It makes intuitive sense [to treat with both classes] because most patients with diabetes need more than one drug” for glycemic control, he noted. “Why not use two classes that each reduce a patient’s risk” for adverse outcomes involving ASCVD, heart failure, and renal dysfunction, added Dr. Virani.
The study run by Dr. Lopez and his associates used data collected in the National VA Database and included 121,156 patients with both type 2 diabetes and established ASCVD. Using propensity-score matching the researchers compiled three subgroups that each included 5,277 matched patients. One subgroup had patients prescribed an SGLT2 inhibitor, a second subgroup included patients on a GLP-1 RA, and a third subgroup had patients on agents from both classes. Patient matching relied on age, sex, left ventricular ejection fraction, hemoglobin A1c level, systolic blood pressure, and the presence of coronary artery disease or peripheral artery disease.
Patients included in the analysis averaged about 67 years of age; 97% were men, their average body mass index was about 34 kg/m2, their average A1c was about 7.9%, their average estimated glomerular filtration rate was about 55-66 mL/min per 1.73 m2, and their average left ventricular ejection fraction was about 55%. The database provided a median follow-up of 902 days (about 2.5 years). The prespecified primary endpoint focused on events that occurred during the first year of follow-up, but the investigators also ran a 3-year follow-up analysis on a post hoc basis.
The most common SGLT2 inhibitor received by these patients was empagliflozin (Jardiance), used on virtually everyone who received an agent from this class. In contrast, the GLP-1 RA drugs that patients received split more widely. The most prescribed agent was liraglutide (Victoza), followed by semaglutide (Ozempic), and dulaglutide (Trulicity), with fewer than 5% receiving exenatide (Bydureon, Byetta).
Regarding other treatments, about 97% of all patients received a statin, about 94% were on a renin-angiotensin system inhibitor, about 90% were on metformin, and roughly 75% were on insulin, aspirin, and a beta-blocker, with smaller numbers on other types of agents.
For the study’s primary endpoint, the 1-year incidence of combined ASCVD events including all-cause death, patients on agents from both classes had a significant 46% reduced rate compared with those on an SGLT2 inhibitor only, and a significant 49% reduced rate, compared with those on a GLP-1 RA only. These between-group separations broadened slightly during 3-year follow-up. Dr. Lopez did not report results of a direct comparison between patients on just an SGLT2 inhibitor and those on just a GLP-1 RA.
For the endpoint of all-cause death, those on combined treatment had a 1-year rate that was 83% below the rate among patients on only an SGLT2 inhibitor, and 81% below the rate among patients who received a GLP-1 RA but not the other class.
Dr. Lopez cautioned that selection bias could have influenced the outcomes of patients who received both classes rather than one or the other, and he also highlighted that the analysis relied on administrative data rather than information gleaned from more detailed medical records or prospectively collected findings and was limited by only including a very small number of women.
“Our results need to be validated in prospective studies,” he declared.
Dr. Lopez and Dr. Virani had no commercial disclosures.
AT ACC 2022
New smart device shows highly accurate AFib detection: mAFA II
Screening for heart rhythm disorders with a smartphone app and a wearable device had a high rate of correctly detecting atrial fibrillation (AFib) in a large new study.
The mAFA II study, conducted in a mass low-risk population in China, showed that more than 93% of possible AFib episodes detected by the smartphone app were confirmed to be AFib on further monitoring.
The study also used the app to screen for obstructive sleep apnea and found that sleep apnea was the most common risk factor associated with increased AFib susceptibility, and those identified as having the most severe sleep apnea were 1.5 times more likely to have AFib than those who did not have this condition.
This suggests that tools suitable for detecting both AFib and sleep apnea can work synergistically to further enhance health monitoring, said lead author, Yutao Guo, MD, professor of internal medicine at Chinese PLA General Hospital, Beijing.
Dr. Guo presented the mAFA II study at the American College of Cardiology (ACC) 2022 Scientific Session held in Washington, D.C., and online.
The trial, which involved more than 2.8 million participants, is the largest study to date to demonstrate how wearable consumer technologies can be used to screen for heart problems during everyday activities, Dr. Guo noted.
“Consumer-led screening with these technologies could increase early diagnosis of AFib and facilitate an integrated approach to fully implement clustered risk management to reduce AFib burden and its related complications,” she concluded.
Discussant of the study at the ACC session at which it was presented, Jodie Hurwitz, MD, Director of the Electrophysiology Lab at Medical City Hospital, Dallas, called this “a pretty impressive study. To get a 93.8% confirmation of AFib with these devices is great.”
But Dr. Hurwitz pointed out that the age of patients in the study was relatively young (average 37 years), and the group who really need to use such a device is much older than that.
“The take-home messages from this study are that AFib wearable detection algorithms have the ability to detect true AFib and that they might also be able to detect risk factors (such as sleep apnea) that predispose to AFib possibly even before AFib is present,” Dr. Hurwitz commented.
Moderator of the session, Edward Fry, MD, cardiologist at Ascension St. Vincent Heart Center, Indianapolis, and incoming president of the ACC, described the area of AFib screening with smart devices as “fascinating, especially with the perspective of the scalability of these types of studies.”
The mAFA II study tracked more than 2.8 million people who used a Huawei phone app together with Huawei and Honor smart devices incorporating photoplethysmography (PPG) technology, a light-based method to monitor blood flow and pulse. If an abnormal rhythm was detected, the wearer would be contacted by a clinician to set up an appointment for a clinical assessment.
Over the course of 4 years of the study, 12,244 (0.4%) of users received a notification of suspected AFib. Among 5,227 people who chose to follow up with a clinician, AFib was confirmed in 93.8% of patients using standard AFib diagnostic tools, including clinical evaluation, an electrocardiogram, and 24-hour Holter monitoring.
In this study, a subset of the individuals screened for AFib were also screened for signs of sleep apnea using the same PPG technology to detect physiological changes in parameters including oxygenation and respiratory rates. The app is also able to determine whether the individual is awake or asleep. Dr. Guo noted that the PPG algorithm for obstructive sleep apnea risk has been validated, compared with polysomnography or home sleep apnea tests.
Using measurements of apnea (signalled by a reduced respiratory rate) and hypopnea (when oxygenation would decrease), the apnea–hypopnea index (AHI) is calculated to determine the severity of the sleep apnea.
Of the 961,931 participants screened for sleep apnea, about 18,000 were notified they may have the condition.
Obstructive sleep apnea was the most reported common risk factor associated with increased AFib susceptibility, and those individuals with the highest risk sleep apnea (more than 80% monitoring measures with AHI greater than or equal to 30 during sleep) resulted in a 1.5-fold increase in prevalent AFib, Dr. Guo reported.
The mAFA II is the latest of several studies to show that AFib can be detected with various smartphone apps and wearable devices. Previous studies have included the Fitbit Heart Study and the Apple Heart Study.
Dr. Hurwitz told this news organization that the electrophysiologist community is enthusiastic about this new smart device technology.
“I sent my sister one so she could determine if she develops AFib: That’s a pretty good endorsement,” she commented, but added that there are still concerns about the rate of false-positive results.
Dr. Hurwitz said she suspected that there will probably be meaningful differences between the different apps and devices, but the algorithms are all proprietary, and the use of photoplethysmography seems to make a big difference.
She noted that the detection of sleep apnea in the current study was a novel approach. “This is important, as sleep apnea is felt to contribute to AFib, and treating it is felt to decrease the frequency of AFib. Perhaps if patients with sleep apnea were treated before they had documented AFib, the AFib burden could be reduced,” she said.
She added that further studies were needed to fine tune the algorithms and to try and identify other factors or heart rate variabilities that may predict future risk of AFib.
The study was funded by the National Natural Science Foundation of China. Dr. Guo reports no disclosures.
A version of this article first appeared on Medscape.com.
Screening for heart rhythm disorders with a smartphone app and a wearable device had a high rate of correctly detecting atrial fibrillation (AFib) in a large new study.
The mAFA II study, conducted in a mass low-risk population in China, showed that more than 93% of possible AFib episodes detected by the smartphone app were confirmed to be AFib on further monitoring.
The study also used the app to screen for obstructive sleep apnea and found that sleep apnea was the most common risk factor associated with increased AFib susceptibility, and those identified as having the most severe sleep apnea were 1.5 times more likely to have AFib than those who did not have this condition.
This suggests that tools suitable for detecting both AFib and sleep apnea can work synergistically to further enhance health monitoring, said lead author, Yutao Guo, MD, professor of internal medicine at Chinese PLA General Hospital, Beijing.
Dr. Guo presented the mAFA II study at the American College of Cardiology (ACC) 2022 Scientific Session held in Washington, D.C., and online.
The trial, which involved more than 2.8 million participants, is the largest study to date to demonstrate how wearable consumer technologies can be used to screen for heart problems during everyday activities, Dr. Guo noted.
“Consumer-led screening with these technologies could increase early diagnosis of AFib and facilitate an integrated approach to fully implement clustered risk management to reduce AFib burden and its related complications,” she concluded.
Discussant of the study at the ACC session at which it was presented, Jodie Hurwitz, MD, Director of the Electrophysiology Lab at Medical City Hospital, Dallas, called this “a pretty impressive study. To get a 93.8% confirmation of AFib with these devices is great.”
But Dr. Hurwitz pointed out that the age of patients in the study was relatively young (average 37 years), and the group who really need to use such a device is much older than that.
“The take-home messages from this study are that AFib wearable detection algorithms have the ability to detect true AFib and that they might also be able to detect risk factors (such as sleep apnea) that predispose to AFib possibly even before AFib is present,” Dr. Hurwitz commented.
Moderator of the session, Edward Fry, MD, cardiologist at Ascension St. Vincent Heart Center, Indianapolis, and incoming president of the ACC, described the area of AFib screening with smart devices as “fascinating, especially with the perspective of the scalability of these types of studies.”
The mAFA II study tracked more than 2.8 million people who used a Huawei phone app together with Huawei and Honor smart devices incorporating photoplethysmography (PPG) technology, a light-based method to monitor blood flow and pulse. If an abnormal rhythm was detected, the wearer would be contacted by a clinician to set up an appointment for a clinical assessment.
Over the course of 4 years of the study, 12,244 (0.4%) of users received a notification of suspected AFib. Among 5,227 people who chose to follow up with a clinician, AFib was confirmed in 93.8% of patients using standard AFib diagnostic tools, including clinical evaluation, an electrocardiogram, and 24-hour Holter monitoring.
In this study, a subset of the individuals screened for AFib were also screened for signs of sleep apnea using the same PPG technology to detect physiological changes in parameters including oxygenation and respiratory rates. The app is also able to determine whether the individual is awake or asleep. Dr. Guo noted that the PPG algorithm for obstructive sleep apnea risk has been validated, compared with polysomnography or home sleep apnea tests.
Using measurements of apnea (signalled by a reduced respiratory rate) and hypopnea (when oxygenation would decrease), the apnea–hypopnea index (AHI) is calculated to determine the severity of the sleep apnea.
Of the 961,931 participants screened for sleep apnea, about 18,000 were notified they may have the condition.
Obstructive sleep apnea was the most reported common risk factor associated with increased AFib susceptibility, and those individuals with the highest risk sleep apnea (more than 80% monitoring measures with AHI greater than or equal to 30 during sleep) resulted in a 1.5-fold increase in prevalent AFib, Dr. Guo reported.
The mAFA II is the latest of several studies to show that AFib can be detected with various smartphone apps and wearable devices. Previous studies have included the Fitbit Heart Study and the Apple Heart Study.
Dr. Hurwitz told this news organization that the electrophysiologist community is enthusiastic about this new smart device technology.
“I sent my sister one so she could determine if she develops AFib: That’s a pretty good endorsement,” she commented, but added that there are still concerns about the rate of false-positive results.
Dr. Hurwitz said she suspected that there will probably be meaningful differences between the different apps and devices, but the algorithms are all proprietary, and the use of photoplethysmography seems to make a big difference.
She noted that the detection of sleep apnea in the current study was a novel approach. “This is important, as sleep apnea is felt to contribute to AFib, and treating it is felt to decrease the frequency of AFib. Perhaps if patients with sleep apnea were treated before they had documented AFib, the AFib burden could be reduced,” she said.
She added that further studies were needed to fine tune the algorithms and to try and identify other factors or heart rate variabilities that may predict future risk of AFib.
The study was funded by the National Natural Science Foundation of China. Dr. Guo reports no disclosures.
A version of this article first appeared on Medscape.com.
Screening for heart rhythm disorders with a smartphone app and a wearable device had a high rate of correctly detecting atrial fibrillation (AFib) in a large new study.
The mAFA II study, conducted in a mass low-risk population in China, showed that more than 93% of possible AFib episodes detected by the smartphone app were confirmed to be AFib on further monitoring.
The study also used the app to screen for obstructive sleep apnea and found that sleep apnea was the most common risk factor associated with increased AFib susceptibility, and those identified as having the most severe sleep apnea were 1.5 times more likely to have AFib than those who did not have this condition.
This suggests that tools suitable for detecting both AFib and sleep apnea can work synergistically to further enhance health monitoring, said lead author, Yutao Guo, MD, professor of internal medicine at Chinese PLA General Hospital, Beijing.
Dr. Guo presented the mAFA II study at the American College of Cardiology (ACC) 2022 Scientific Session held in Washington, D.C., and online.
The trial, which involved more than 2.8 million participants, is the largest study to date to demonstrate how wearable consumer technologies can be used to screen for heart problems during everyday activities, Dr. Guo noted.
“Consumer-led screening with these technologies could increase early diagnosis of AFib and facilitate an integrated approach to fully implement clustered risk management to reduce AFib burden and its related complications,” she concluded.
Discussant of the study at the ACC session at which it was presented, Jodie Hurwitz, MD, Director of the Electrophysiology Lab at Medical City Hospital, Dallas, called this “a pretty impressive study. To get a 93.8% confirmation of AFib with these devices is great.”
But Dr. Hurwitz pointed out that the age of patients in the study was relatively young (average 37 years), and the group who really need to use such a device is much older than that.
“The take-home messages from this study are that AFib wearable detection algorithms have the ability to detect true AFib and that they might also be able to detect risk factors (such as sleep apnea) that predispose to AFib possibly even before AFib is present,” Dr. Hurwitz commented.
Moderator of the session, Edward Fry, MD, cardiologist at Ascension St. Vincent Heart Center, Indianapolis, and incoming president of the ACC, described the area of AFib screening with smart devices as “fascinating, especially with the perspective of the scalability of these types of studies.”
The mAFA II study tracked more than 2.8 million people who used a Huawei phone app together with Huawei and Honor smart devices incorporating photoplethysmography (PPG) technology, a light-based method to monitor blood flow and pulse. If an abnormal rhythm was detected, the wearer would be contacted by a clinician to set up an appointment for a clinical assessment.
Over the course of 4 years of the study, 12,244 (0.4%) of users received a notification of suspected AFib. Among 5,227 people who chose to follow up with a clinician, AFib was confirmed in 93.8% of patients using standard AFib diagnostic tools, including clinical evaluation, an electrocardiogram, and 24-hour Holter monitoring.
In this study, a subset of the individuals screened for AFib were also screened for signs of sleep apnea using the same PPG technology to detect physiological changes in parameters including oxygenation and respiratory rates. The app is also able to determine whether the individual is awake or asleep. Dr. Guo noted that the PPG algorithm for obstructive sleep apnea risk has been validated, compared with polysomnography or home sleep apnea tests.
Using measurements of apnea (signalled by a reduced respiratory rate) and hypopnea (when oxygenation would decrease), the apnea–hypopnea index (AHI) is calculated to determine the severity of the sleep apnea.
Of the 961,931 participants screened for sleep apnea, about 18,000 were notified they may have the condition.
Obstructive sleep apnea was the most reported common risk factor associated with increased AFib susceptibility, and those individuals with the highest risk sleep apnea (more than 80% monitoring measures with AHI greater than or equal to 30 during sleep) resulted in a 1.5-fold increase in prevalent AFib, Dr. Guo reported.
The mAFA II is the latest of several studies to show that AFib can be detected with various smartphone apps and wearable devices. Previous studies have included the Fitbit Heart Study and the Apple Heart Study.
Dr. Hurwitz told this news organization that the electrophysiologist community is enthusiastic about this new smart device technology.
“I sent my sister one so she could determine if she develops AFib: That’s a pretty good endorsement,” she commented, but added that there are still concerns about the rate of false-positive results.
Dr. Hurwitz said she suspected that there will probably be meaningful differences between the different apps and devices, but the algorithms are all proprietary, and the use of photoplethysmography seems to make a big difference.
She noted that the detection of sleep apnea in the current study was a novel approach. “This is important, as sleep apnea is felt to contribute to AFib, and treating it is felt to decrease the frequency of AFib. Perhaps if patients with sleep apnea were treated before they had documented AFib, the AFib burden could be reduced,” she said.
She added that further studies were needed to fine tune the algorithms and to try and identify other factors or heart rate variabilities that may predict future risk of AFib.
The study was funded by the National Natural Science Foundation of China. Dr. Guo reports no disclosures.
A version of this article first appeared on Medscape.com.
AHA statement addresses CVD risk in NAFLD
At least one in four adults worldwide is thought to have nonalcoholic fatty liver disease, a major risk factor for cardiovascular disease (CVD), which is the leading cause of death in NAFLD, but the condition is widely underdiagnosed, according to a new American Heart Association scientific statement on NAFLD and cardiovascular risks.
The statement, published in Arteriosclerosis, Thrombosis, and Vascular Biology, aims to increase awareness of NAFLD among cardiologists and other clinicians treating vulnerable patients. It pulls together the existing evidence for using imaging to diagnose NAFLD as well as the role of current and emerging treatments for managing the disease.
“NAFLD is common, but most patients are undiagnosed,” statement writing committee chair P. Barton Duell, MD, said in an interview. “The identification of normal liver enzyme levels does not exclude the diagnosis of NAFLD. Early diagnosis and treatment are necessary to improve the health of patients with established NAFLD, as well as preventing the development of NAFLD in patients who are at risk for the condition.”
Dr. Duell is a professor at the Knight Cardiovascular Institute and division of endocrinology, diabetes and clinical nutrition at Oregon Health & Science University, Portland.
This is the AHA’s first scientific statement on NAFLD. In 2021, the association issued a statement on obesity and CVD). Also in 2021, a multiorganization group headed by the American Gastroenterological Association published a “Call to Action” on nonalcoholic steatohepatitis (NASH) , a form of NAFLD that’s characterized by inflammation and scarring of the liver, and typically requires a liver biopsy for diagnosis.
Key take-homes
The AHA statement on NAFLD is sweeping. Among its key take-home messages:
- Calling into question the effectiveness of AST and ALT testing for diagnosing NAFLD and NASH.
- Providing context to the role of insulin resistance – either with or without diabetes – as well as obesity (particularly visceral adiposity), metabolic syndrome, and dyslipidemia in NAFLD.
- Advocating for lifestyle interventions – diet, exercise, weight loss and alcohol avoidance – as the key therapeutic intervention for NAFLD.
- Asserting that glucagonlike peptide–1 receptor agonists may modestly improve NAFLD.
The statement also tackles the differences in terminology different organizations use to describe NAFLD. “The terminology section is important to ensure everyone is using the right terminology in assessing patients, as well as choosing appropriate treatment interventions,” Dr. Duell said.
The statement also explores genetic factors that can predispose people to NAFLD, Dr. Duell pointed out, and it goes into detail about strategies for screening NAFLD and NASH. “It is not possible to diagnose NAFLD without understanding the pros and cons of various screening modalities, as well as the lack of sensitivity of some tests for detection of NAFLD We hope this information will increase success in screening for and early identification of NAFLD.”
Dr. Duell explained the rationale for issuing the statement. “Rates of NAFLD are increasing worldwide in association with rising rates of elevated body mass index and the metabolic syndrome, but the condition is commonly undiagnosed,” he said. “This allows patients to experience progression of disease, leading to hepatic and cardiovascular complications.”
Avoiding NAFLD risk factors along with early diagnosis and treatment “may have the potential to mitigate long-term complications from NAFLD,” Dr. Duell said.
“This is one of first times where we really look at cardiovascular risks associated with NAFLD and pinpoint the risk factors, the imaging tools that can be used for diagnosing fatty liver disease, and ultimately what potential treatments we can consider,” Tiffany M. Powell-Wiley, MD, MPH, author of the AHA statement on obesity and CV risk, said in an interview.
“NAFLD has not been at the forefront of cardiologists’ minds, but this statement highlights the importance of liver fat as a fat depot,” said Dr. Powell-Wiley, chief of the Social Determinants of Obesity and Cardiovascular Risk Laboratory at the National Heart, Lung, and Blood Institute in Bethesda, Md.
“It does provide greater clarity for us as cardiologists, especially when thinking about what is required for diagnosis and ultimately how this relates to cardiovascular disease for people with fatty liver disease,” she said.
Dr. Duell and Dr. Powell-Wiley have no relevant relationships to disclose.
At least one in four adults worldwide is thought to have nonalcoholic fatty liver disease, a major risk factor for cardiovascular disease (CVD), which is the leading cause of death in NAFLD, but the condition is widely underdiagnosed, according to a new American Heart Association scientific statement on NAFLD and cardiovascular risks.
The statement, published in Arteriosclerosis, Thrombosis, and Vascular Biology, aims to increase awareness of NAFLD among cardiologists and other clinicians treating vulnerable patients. It pulls together the existing evidence for using imaging to diagnose NAFLD as well as the role of current and emerging treatments for managing the disease.
“NAFLD is common, but most patients are undiagnosed,” statement writing committee chair P. Barton Duell, MD, said in an interview. “The identification of normal liver enzyme levels does not exclude the diagnosis of NAFLD. Early diagnosis and treatment are necessary to improve the health of patients with established NAFLD, as well as preventing the development of NAFLD in patients who are at risk for the condition.”
Dr. Duell is a professor at the Knight Cardiovascular Institute and division of endocrinology, diabetes and clinical nutrition at Oregon Health & Science University, Portland.
This is the AHA’s first scientific statement on NAFLD. In 2021, the association issued a statement on obesity and CVD). Also in 2021, a multiorganization group headed by the American Gastroenterological Association published a “Call to Action” on nonalcoholic steatohepatitis (NASH) , a form of NAFLD that’s characterized by inflammation and scarring of the liver, and typically requires a liver biopsy for diagnosis.
Key take-homes
The AHA statement on NAFLD is sweeping. Among its key take-home messages:
- Calling into question the effectiveness of AST and ALT testing for diagnosing NAFLD and NASH.
- Providing context to the role of insulin resistance – either with or without diabetes – as well as obesity (particularly visceral adiposity), metabolic syndrome, and dyslipidemia in NAFLD.
- Advocating for lifestyle interventions – diet, exercise, weight loss and alcohol avoidance – as the key therapeutic intervention for NAFLD.
- Asserting that glucagonlike peptide–1 receptor agonists may modestly improve NAFLD.
The statement also tackles the differences in terminology different organizations use to describe NAFLD. “The terminology section is important to ensure everyone is using the right terminology in assessing patients, as well as choosing appropriate treatment interventions,” Dr. Duell said.
The statement also explores genetic factors that can predispose people to NAFLD, Dr. Duell pointed out, and it goes into detail about strategies for screening NAFLD and NASH. “It is not possible to diagnose NAFLD without understanding the pros and cons of various screening modalities, as well as the lack of sensitivity of some tests for detection of NAFLD We hope this information will increase success in screening for and early identification of NAFLD.”
Dr. Duell explained the rationale for issuing the statement. “Rates of NAFLD are increasing worldwide in association with rising rates of elevated body mass index and the metabolic syndrome, but the condition is commonly undiagnosed,” he said. “This allows patients to experience progression of disease, leading to hepatic and cardiovascular complications.”
Avoiding NAFLD risk factors along with early diagnosis and treatment “may have the potential to mitigate long-term complications from NAFLD,” Dr. Duell said.
“This is one of first times where we really look at cardiovascular risks associated with NAFLD and pinpoint the risk factors, the imaging tools that can be used for diagnosing fatty liver disease, and ultimately what potential treatments we can consider,” Tiffany M. Powell-Wiley, MD, MPH, author of the AHA statement on obesity and CV risk, said in an interview.
“NAFLD has not been at the forefront of cardiologists’ minds, but this statement highlights the importance of liver fat as a fat depot,” said Dr. Powell-Wiley, chief of the Social Determinants of Obesity and Cardiovascular Risk Laboratory at the National Heart, Lung, and Blood Institute in Bethesda, Md.
“It does provide greater clarity for us as cardiologists, especially when thinking about what is required for diagnosis and ultimately how this relates to cardiovascular disease for people with fatty liver disease,” she said.
Dr. Duell and Dr. Powell-Wiley have no relevant relationships to disclose.
At least one in four adults worldwide is thought to have nonalcoholic fatty liver disease, a major risk factor for cardiovascular disease (CVD), which is the leading cause of death in NAFLD, but the condition is widely underdiagnosed, according to a new American Heart Association scientific statement on NAFLD and cardiovascular risks.
The statement, published in Arteriosclerosis, Thrombosis, and Vascular Biology, aims to increase awareness of NAFLD among cardiologists and other clinicians treating vulnerable patients. It pulls together the existing evidence for using imaging to diagnose NAFLD as well as the role of current and emerging treatments for managing the disease.
“NAFLD is common, but most patients are undiagnosed,” statement writing committee chair P. Barton Duell, MD, said in an interview. “The identification of normal liver enzyme levels does not exclude the diagnosis of NAFLD. Early diagnosis and treatment are necessary to improve the health of patients with established NAFLD, as well as preventing the development of NAFLD in patients who are at risk for the condition.”
Dr. Duell is a professor at the Knight Cardiovascular Institute and division of endocrinology, diabetes and clinical nutrition at Oregon Health & Science University, Portland.
This is the AHA’s first scientific statement on NAFLD. In 2021, the association issued a statement on obesity and CVD). Also in 2021, a multiorganization group headed by the American Gastroenterological Association published a “Call to Action” on nonalcoholic steatohepatitis (NASH) , a form of NAFLD that’s characterized by inflammation and scarring of the liver, and typically requires a liver biopsy for diagnosis.
Key take-homes
The AHA statement on NAFLD is sweeping. Among its key take-home messages:
- Calling into question the effectiveness of AST and ALT testing for diagnosing NAFLD and NASH.
- Providing context to the role of insulin resistance – either with or without diabetes – as well as obesity (particularly visceral adiposity), metabolic syndrome, and dyslipidemia in NAFLD.
- Advocating for lifestyle interventions – diet, exercise, weight loss and alcohol avoidance – as the key therapeutic intervention for NAFLD.
- Asserting that glucagonlike peptide–1 receptor agonists may modestly improve NAFLD.
The statement also tackles the differences in terminology different organizations use to describe NAFLD. “The terminology section is important to ensure everyone is using the right terminology in assessing patients, as well as choosing appropriate treatment interventions,” Dr. Duell said.
The statement also explores genetic factors that can predispose people to NAFLD, Dr. Duell pointed out, and it goes into detail about strategies for screening NAFLD and NASH. “It is not possible to diagnose NAFLD without understanding the pros and cons of various screening modalities, as well as the lack of sensitivity of some tests for detection of NAFLD We hope this information will increase success in screening for and early identification of NAFLD.”
Dr. Duell explained the rationale for issuing the statement. “Rates of NAFLD are increasing worldwide in association with rising rates of elevated body mass index and the metabolic syndrome, but the condition is commonly undiagnosed,” he said. “This allows patients to experience progression of disease, leading to hepatic and cardiovascular complications.”
Avoiding NAFLD risk factors along with early diagnosis and treatment “may have the potential to mitigate long-term complications from NAFLD,” Dr. Duell said.
“This is one of first times where we really look at cardiovascular risks associated with NAFLD and pinpoint the risk factors, the imaging tools that can be used for diagnosing fatty liver disease, and ultimately what potential treatments we can consider,” Tiffany M. Powell-Wiley, MD, MPH, author of the AHA statement on obesity and CV risk, said in an interview.
“NAFLD has not been at the forefront of cardiologists’ minds, but this statement highlights the importance of liver fat as a fat depot,” said Dr. Powell-Wiley, chief of the Social Determinants of Obesity and Cardiovascular Risk Laboratory at the National Heart, Lung, and Blood Institute in Bethesda, Md.
“It does provide greater clarity for us as cardiologists, especially when thinking about what is required for diagnosis and ultimately how this relates to cardiovascular disease for people with fatty liver disease,” she said.
Dr. Duell and Dr. Powell-Wiley have no relevant relationships to disclose.
FROM ARTERIOSCLEROSIS, THROMBOSIS, AND VASCULAR BIOLOGY